User login
Depression, medication, and ‘bad blood’
CASE: Sad and suicidal
Mr. G, age 44, has chronic depression with suicidality. At presentation he says he has felt sad and suicidal for 2 weeks. He also has no appetite and trouble sleeping at night.
Mr. G’s depression has left him unable to work and has led to 4 hospitalizations over 10 years. He first attempted suicide in 1984 after his ex-wife took their child and left him. He endorses no suicide plan and has been sober for 7 years after 12-plus years of alcohol abuse, but says he has been tempted lately to resume drinking.
The patient was taking an antidepressant but stopped while at a homeless shelter, where he had been staying for several weeks. For more than 20 years, he also has been taking phenytoin, 300 mg/d, and phenobarbital, 30 mg bid, for a seizure disorder.
Mr. G is admitted with a working diagnosis of recurrent major depressive disorder. White blood cell count (WBC) at admission is 5.12×109/L and neutrophils are 3.6×109/L—both low-normal readings. Other laboratory results are normal.
We continue phenytoin and phenobarbital at the same dosages and start the selective serotonin reuptake inhibitor (SSRI) citalopram, 20 mg/d, which interacts minimally with both anticonvulsants.
After 2 weeks, Mr. G’s seizures are well controlled and he is tolerating citalopram, but his depressive symptoms have not improved. We cross-taper citalopram to prevent SSRI-induced discontinuation syndrome and start the dopamine and norepinephrine reuptake inhibitor bupropion, 75 mg bid. We titrate bupropion over 2 weeks to 150 mg each morning and 300 mg at bedtime, and watch Mr. G closely for seizures. Although his seizure history contraindicates bupropion use, we think he can tolerate the medication because his seizure disorder is well controlled.
Mr. G’s affect, appetite, and energy are improving with bupropion, but a routine complete blood count (CBC) 5 days after the medication is started reveals leukopenia (WBC 3.04×109/L) without neutropenia (neutrophils 1.9×109/L). Repeat blood tests 18 and 32 days after the first blood draw show continued low WBC. The gastrointestinal medicine team tests Mr. G’s liver function but finds no abnormalities.
The author’s observations
A medical cause also is unlikely. Mr. G’s liver function is normal, and he shows no other signs or symptoms of a medical problem. Bone marrow biopsy and immunologic workup could rule out cancer, but the timing of Mr. G’s abnormal blood readings strongly suggests bupropion intolerance.
TREATMENT: Other medications
We immediately stop bupropion, start the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine at 37.5 mg bid, and titrate it over 5 days to 225 mg/d. Blood draws 3 and 5 days after bupropion discontinuation show slight increases in WBC.
Eleven days after venlafaxine is started, Mr. G’s WBC and neutrophils are normal. However, he has become increasingly irritable and volatile, often arguing with a staff nurse and other patients. We cross-taper venlafaxine over 5 days, start the SSRI sertraline at 50 mg/d, and titrate sertraline over 1 week to 150 mg/d. Mr. G’s irritability and depressive symptoms improve at the latter dosage.
Because Mr. G developed neutropenia while taking a medication not associated with this adverse effect, we start watching his WBC counts more closely than usual. WBC is 4.58×109/L 8 days after sertraline is started but falls to 3.4×109/L after another 8 days, with neutrophils at 1.5×109/L for both readings (Table).
We add lithium, 300 mg bid, to increase Mr. G’s neutrophils and augment sertraline’s antidepressant effects. Four days later, WBC is 5.8×109/L with neutrophils at 4.2×109/L.
We stop lithium briefly to see if WBC remains normal. After 3 days, WBC drops to 3.25×109/L with neutrophils at 1.5×109/L. We restart lithium, 300 mg/d, and Mr. G’s WBC increases to 4.18×109/L 4 days later, with neutrophils at 2.1×109/L.
Table
Mr G’s white blood cell (WBC) and neutrophil counts (NC)*
while taking bupropion and sertraline
| Antidepressant | When measurements were taken | WBC | NC |
| None for several weeks | Baseline, first hospital admission | 5.12×109/L | 3.6×109/L |
| Bupropion, 75 mg bid | 5 days after starting bupropion | 3.04×109/L | 1.9×109/L |
| Bupropion, 450 mg/d total | 23 days after starting bupropion | 3.14×109/L | 1.6×109/L |
| Bupropion, 450 mg/d total | 2 weeks after previous test | 2.73×109/L | 1.6×109/L |
| Sertraline, 150 mg/d | 8 days after starting sertraline (titration period) | 4.58×109/L | 1.5×109/L |
| Sertraline, 150 mg/d | 16 days after starting sertraline | 3.4×109/L | 1.5×109/L |
| Sertraline, 150 mg/d, and lithium, 300 mg bid | 4 days after lithium augmentation | 5.8×109/L | 4.2×109/L |
| None for 3 months | Baseline, second hospital admission | 3.7×109/L | 2.1×109/L |
| Sertraline, 150 mg/d | 12 days after restarting sertraline | 2.83×109/L | Not available |
| * Normal WBC values: 4.5 to 11×109/L; normal neutrophil values: 1.5 to 8×109/L | |||
The authors’ observations
For Mr. G, both bupropion and sertraline appear to have caused neutropenia on separate occasions.
To our knowledge, bupropion-induced leukopenia or neutropenia have not been reported in the literature. Neutropenia—a rare adverse effect of antidepressants2—and leukopenia were seen during bupropion’s pre-marketing trials but were not definitely attributed to the drug.1 According to pre- and post-marketing data, leukopenia was “infrequently” reported among 5,100 subjects who received bupropion.3
To our knowledge, sertraline-induced neutropenia has not been reported in nongeriatric patients, although sertraline-induced neutropenia4 and agranulocytosis5 have been reported in patients age >65. The Committee on Safety of Medicine in the United Kingdom has received 2 other reports of neutropenia and 1 report of leukopenia with sertraline.5
In one clinical trial, 2 of 1,304 patients taking unknown dosages of sertraline had low neutrophils (
Medication is the second most common cause of acquired neutropenia, with infection being most common.6 By definition, drug-induced neutropenia occurs within 4 weeks after starting the drug and usually resolves within 30 days after stopping it.
Neutropenia is an idiosyncratic reaction unrelated to pharmacologic action. Although overall neutropenia incidence is unknown, reported incidence of the rare, more severe agranulocytosis ranges from approximately 1 to 10 cases per million people annually, and medications have been implicated in 70% of these cases.6 Conversely, only 2 of 97 incidental neutropenia cases studied by Lima et al7 were medication-induced.
Drug-induced neutropenia can result from immune-mediated destruction of neutrophils by circulating antibodies or from direct toxic effects upon marrow granulocyte precursors. Whereas immune-mediated onset is acute and explosive, toxic effect is insidious (months to years) and asymptomatic.8 Clozapine is thought to deliver a direct toxic effect, whereas the thyroid-regulating drug propylthiouracil generates anti-neutrophil antibodies.9
Mr. G’s acute onset (within 5 to 16 days of starting bupropion or sertraline) and prompt return of neutropenia after stopping lithium suggest acute immune-mediated circulating neutrophil destruction.
Treating leukopenia
After 4 failed or intolerable antidepressant trials, lithium augmentation seemed reasonable and ultimately improved Mr. G’s neutrophil count and his mood.
Lithium has helped resolve clozapine-induced neutropenia in case reports.10-12 Well-controlled studies, however, have followed only patients with antineoplastic, drug-induced neutropenia.1
By acting on cyclic nucleotides, lithium prompts colony-stimulating factor production, which in turn stimulates neutrophil production by pluripotent stem cells. As with Mr. G, patients reach neutrophilia 3 to 7 days after starting lithium.
If the patient cannot tolerate lithium, try switching antidepressants or using growth factors to increase neutrophils.
Switching antidepressants.The SSRIs escitalopram or paroxetine, or the SNRI duloxetine are effective and do not necessarily cause neutropenia. Start at below-normal dosages to gauge tolerability, then titrate to normal dosages. Avoid tricyclics, which pose a higher risk of neutropenia than other antidepressant classes.
Case reports13,14 associate fluoxetine and mirtazapine with neutropenia. The patient who received mirtazapine, 30 mg/d, later responded well to sertraline, 50 mg/d.13
If the new antidepressant is ineffective, consider adding the mood-stabilizing anticonvulsant lamotrigine, 12.5 mg/d. Increase lamotrigine to 25 mg/d after 1 week, then titrate by 25 mg weekly to 100 to 400 mg/d depending on efficacy and tolerability.
Using growth factors.Although their efficacy is not proven, growth factors are minimally toxic and might have helped Mr. G. Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor resolved neutropenia in uncontrolled studies, but results of one randomized controlled trial were equivocal.8
TESTING: CT findings
Approximately 2 months after admission—shortly after a blood draw shows normal WBC and neutrophils—Mr. G complains of dizziness. He says he accidentally hit his head against a side table.
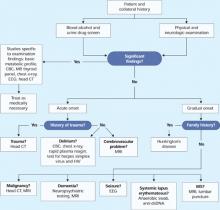
We order a full neurologic workup to check for traumatic brain injury or brain damage caused by long-term alcohol abuse:
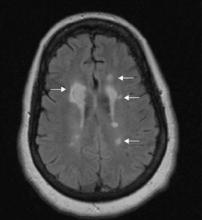
- Head CT shows evidence of previous cerebrovascular infarcts in the bilateral frontal and cerebellar lobes and basal ganglia.
- MRI shows atrophied mammillary bodies, fornix, and corpus callosum.
- Magnetic resonance angiography reveals small cerebral vessel disease.
FOLLOW-UP: Awaiting discharge
After 3 months of continuous hospitalization, Mr. G has become euthymic and nonsuicidal, though at times oversensitive and combative. We transfer him to an assisted-living center and continue sertraline, 150 mg/d; phenytoin, 300 mg/d; phenobarbital, 30 mg bid; lithium, 300 mg/d; and trazodone, 50 mg at night as needed for insomnia.
We also place Mr. G in a day treatment program for mentally ill chemical abusers. A psychiatrist sees him every 2 weeks, and staff supervise him daily.
The authors’ observations
Mr. G’s extended hospital stay allowed us to closely observe him and offered ready access to laboratory facilities while we cross-tapered medications. In outpatient treatment, however, a serious and life-threatening medication-induced complication could easily be missed.
For medically healthy outpatients, be sure CBC has been checked ≤6 months before presentation. Monitor CBC and urge the patient to see a primary care doctor if infection symptoms emerge. Watch for gingivitis, tooth abscess, and other oral cavity infections—which often are overlooked—and sore throat or fever.
Also check electrolytes and screen for SSRI-induced hyponatremia at baseline for all at-risk patients.
Stop the offending drug when WBC reaches 9/L or with absolute neutrophil count (ANC) 9/L, then take a peripheral smear to confirm neutropenia. If the patient is asymptomatic, check ANC 2 to 3 times weekly, particularly if he or she recently had an infection or started a medication that can cause neutropenia. Neutropenia should resolve within 6 to 8 weeks of stopping the offending drug.
If neutropenia persists, order bone marrow biopsy in collaboration with an internist or hematologist to test for cancer. If the biopsy is negative, test for:
- HIV infection
- antinuclear antibodies to check for collagen vascular disease
- antineutrophil antibody to rule out immune neutropenia
- serum folate and B12 deficiency secondary to low WBC.
FOLLOW-UP: Stressor and relapse
Seven months later, Mr. G is readmitted for depression. Three months earlier, he had stopped all medications and resumed drinking after a family member died. WBC at admission is 3.70×109/L
We refer Mr. G to an outpatient psychiatrist, who sees him monthly. Several months later, the psychiatrist reports a WBC of 4.58×109/L.
Nearly 1 year later, Mr. G still lives at the assisted-living facility. He has not been rehospitalized for depression, is functioning well, and has a girlfriend.
The authors’ observations
Mr. G’s abnormal blood counts after sertraline rechallenge confirms that the SSRI probably was causing leukopenia. If we had restarted bupropion and neutropenia recurred during that regimen, we could have more certainly established a bupropion-leukopenia connection.
- Neutropenia Support Association. www.neutropenia.ca.
- Baehner RL. Overview of neutropenia.UpToDate Online (version 15.1); March 30, 2006. www.uptodate.com.
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol, others
- Citalopram • Celexa
- Clozapine • Clozaril
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Lithium • various
- Mirtazapine • Remeron
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Phenobarbital • various
- Phenytoin • Dilantin
- Propylthiouracil • various
- Sertraline • Zoloft
- Trazodone • Desyrel
- Valproic acid • Depakene
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. McEvoy G, ed. AHFS drug information. Bethesda, MD: American Society of Health-System Pharmacists; 2005.
2. Nelson JC. Safety and tolerability of the new antidepressants. J Clin Psychiatry 1997;60:1101.-
3. Physicians desk reference, 61st ed. Montvale, NJ: Thomson PDR; 2007.
4. Cohn CK, Shrivastava R, Mendels J, et al. Double-blind, multicenter comparison of sertraline and amitriptyline in elderly depressed patients. J Clin Psychiatry 1990;51(suppl B):28-33.
5. Trescoli-Serrano C, Smith NK. Sertraline-induced agranulocytosis. Postgrad Med J 1996;72:446.-
6. Baehner RL. Overview of neutropenia. UpToDate Online (version 15.1); March 30, 2006. Available at: http://www.uptodate.com. Accessed April 16, 2007.
7. Lima CS, Paula EV, Takahashi T, et al. Causes of incidental neutropenia in adulthood. Ann Hematol 2006;85:705-9.
8. Holland SM, Gallin J. Disorders of granulocytes and monocytes. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison’s principles of internal medicine, 16th ed. New York: McGraw-Hill; 2005.
9. Baehner RL. Drug-induced neutropenia and agranulocytosis. UpToDate Online (version 15.1); June 8, 2005. Available at: http://www.uptodate.com. Accessed April 16, 2007.
10. Sporn A, Gogtay N, Ortiz-Aguayo R, et al. Clozapine-induced neutropenia in children: management with lithium carbonate. J Child Adolesc Psychopharmacol 2003;13:401-4.
11. Blier P, Slater S, Measham T, et al. Lithium and clozapine-induced neutropenia/agranulocytosis. Int Clin Psychopharmacol 1998;13:137-40.
12. Silverstone P. Prevention of clozapine-induced neutropenia by pretreatment with lithium. J Clin Psychopharmacol 1998;18:86-8.
13. Ozcanli T, Unsalver B, Ozdemir S, Ozmen M. Sertraline and mirtazapine-induced severe neutropenia. Am J Psych 2005;162:1386.-
14. Vilinsky FD, Lubin A. Severe neutropenia associated with fluoxetine hydrochloride. Ann Internal Med 1997;127:573-4.
CASE: Sad and suicidal
Mr. G, age 44, has chronic depression with suicidality. At presentation he says he has felt sad and suicidal for 2 weeks. He also has no appetite and trouble sleeping at night.
Mr. G’s depression has left him unable to work and has led to 4 hospitalizations over 10 years. He first attempted suicide in 1984 after his ex-wife took their child and left him. He endorses no suicide plan and has been sober for 7 years after 12-plus years of alcohol abuse, but says he has been tempted lately to resume drinking.
The patient was taking an antidepressant but stopped while at a homeless shelter, where he had been staying for several weeks. For more than 20 years, he also has been taking phenytoin, 300 mg/d, and phenobarbital, 30 mg bid, for a seizure disorder.
Mr. G is admitted with a working diagnosis of recurrent major depressive disorder. White blood cell count (WBC) at admission is 5.12×109/L and neutrophils are 3.6×109/L—both low-normal readings. Other laboratory results are normal.
We continue phenytoin and phenobarbital at the same dosages and start the selective serotonin reuptake inhibitor (SSRI) citalopram, 20 mg/d, which interacts minimally with both anticonvulsants.
After 2 weeks, Mr. G’s seizures are well controlled and he is tolerating citalopram, but his depressive symptoms have not improved. We cross-taper citalopram to prevent SSRI-induced discontinuation syndrome and start the dopamine and norepinephrine reuptake inhibitor bupropion, 75 mg bid. We titrate bupropion over 2 weeks to 150 mg each morning and 300 mg at bedtime, and watch Mr. G closely for seizures. Although his seizure history contraindicates bupropion use, we think he can tolerate the medication because his seizure disorder is well controlled.
Mr. G’s affect, appetite, and energy are improving with bupropion, but a routine complete blood count (CBC) 5 days after the medication is started reveals leukopenia (WBC 3.04×109/L) without neutropenia (neutrophils 1.9×109/L). Repeat blood tests 18 and 32 days after the first blood draw show continued low WBC. The gastrointestinal medicine team tests Mr. G’s liver function but finds no abnormalities.
The author’s observations
A medical cause also is unlikely. Mr. G’s liver function is normal, and he shows no other signs or symptoms of a medical problem. Bone marrow biopsy and immunologic workup could rule out cancer, but the timing of Mr. G’s abnormal blood readings strongly suggests bupropion intolerance.
TREATMENT: Other medications
We immediately stop bupropion, start the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine at 37.5 mg bid, and titrate it over 5 days to 225 mg/d. Blood draws 3 and 5 days after bupropion discontinuation show slight increases in WBC.
Eleven days after venlafaxine is started, Mr. G’s WBC and neutrophils are normal. However, he has become increasingly irritable and volatile, often arguing with a staff nurse and other patients. We cross-taper venlafaxine over 5 days, start the SSRI sertraline at 50 mg/d, and titrate sertraline over 1 week to 150 mg/d. Mr. G’s irritability and depressive symptoms improve at the latter dosage.
Because Mr. G developed neutropenia while taking a medication not associated with this adverse effect, we start watching his WBC counts more closely than usual. WBC is 4.58×109/L 8 days after sertraline is started but falls to 3.4×109/L after another 8 days, with neutrophils at 1.5×109/L for both readings (Table).
We add lithium, 300 mg bid, to increase Mr. G’s neutrophils and augment sertraline’s antidepressant effects. Four days later, WBC is 5.8×109/L with neutrophils at 4.2×109/L.
We stop lithium briefly to see if WBC remains normal. After 3 days, WBC drops to 3.25×109/L with neutrophils at 1.5×109/L. We restart lithium, 300 mg/d, and Mr. G’s WBC increases to 4.18×109/L 4 days later, with neutrophils at 2.1×109/L.
Table
Mr G’s white blood cell (WBC) and neutrophil counts (NC)*
while taking bupropion and sertraline
| Antidepressant | When measurements were taken | WBC | NC |
| None for several weeks | Baseline, first hospital admission | 5.12×109/L | 3.6×109/L |
| Bupropion, 75 mg bid | 5 days after starting bupropion | 3.04×109/L | 1.9×109/L |
| Bupropion, 450 mg/d total | 23 days after starting bupropion | 3.14×109/L | 1.6×109/L |
| Bupropion, 450 mg/d total | 2 weeks after previous test | 2.73×109/L | 1.6×109/L |
| Sertraline, 150 mg/d | 8 days after starting sertraline (titration period) | 4.58×109/L | 1.5×109/L |
| Sertraline, 150 mg/d | 16 days after starting sertraline | 3.4×109/L | 1.5×109/L |
| Sertraline, 150 mg/d, and lithium, 300 mg bid | 4 days after lithium augmentation | 5.8×109/L | 4.2×109/L |
| None for 3 months | Baseline, second hospital admission | 3.7×109/L | 2.1×109/L |
| Sertraline, 150 mg/d | 12 days after restarting sertraline | 2.83×109/L | Not available |
| * Normal WBC values: 4.5 to 11×109/L; normal neutrophil values: 1.5 to 8×109/L | |||
The authors’ observations
For Mr. G, both bupropion and sertraline appear to have caused neutropenia on separate occasions.
To our knowledge, bupropion-induced leukopenia or neutropenia have not been reported in the literature. Neutropenia—a rare adverse effect of antidepressants2—and leukopenia were seen during bupropion’s pre-marketing trials but were not definitely attributed to the drug.1 According to pre- and post-marketing data, leukopenia was “infrequently” reported among 5,100 subjects who received bupropion.3
To our knowledge, sertraline-induced neutropenia has not been reported in nongeriatric patients, although sertraline-induced neutropenia4 and agranulocytosis5 have been reported in patients age >65. The Committee on Safety of Medicine in the United Kingdom has received 2 other reports of neutropenia and 1 report of leukopenia with sertraline.5
In one clinical trial, 2 of 1,304 patients taking unknown dosages of sertraline had low neutrophils (
Medication is the second most common cause of acquired neutropenia, with infection being most common.6 By definition, drug-induced neutropenia occurs within 4 weeks after starting the drug and usually resolves within 30 days after stopping it.
Neutropenia is an idiosyncratic reaction unrelated to pharmacologic action. Although overall neutropenia incidence is unknown, reported incidence of the rare, more severe agranulocytosis ranges from approximately 1 to 10 cases per million people annually, and medications have been implicated in 70% of these cases.6 Conversely, only 2 of 97 incidental neutropenia cases studied by Lima et al7 were medication-induced.
Drug-induced neutropenia can result from immune-mediated destruction of neutrophils by circulating antibodies or from direct toxic effects upon marrow granulocyte precursors. Whereas immune-mediated onset is acute and explosive, toxic effect is insidious (months to years) and asymptomatic.8 Clozapine is thought to deliver a direct toxic effect, whereas the thyroid-regulating drug propylthiouracil generates anti-neutrophil antibodies.9
Mr. G’s acute onset (within 5 to 16 days of starting bupropion or sertraline) and prompt return of neutropenia after stopping lithium suggest acute immune-mediated circulating neutrophil destruction.
Treating leukopenia
After 4 failed or intolerable antidepressant trials, lithium augmentation seemed reasonable and ultimately improved Mr. G’s neutrophil count and his mood.
Lithium has helped resolve clozapine-induced neutropenia in case reports.10-12 Well-controlled studies, however, have followed only patients with antineoplastic, drug-induced neutropenia.1
By acting on cyclic nucleotides, lithium prompts colony-stimulating factor production, which in turn stimulates neutrophil production by pluripotent stem cells. As with Mr. G, patients reach neutrophilia 3 to 7 days after starting lithium.
If the patient cannot tolerate lithium, try switching antidepressants or using growth factors to increase neutrophils.
Switching antidepressants.The SSRIs escitalopram or paroxetine, or the SNRI duloxetine are effective and do not necessarily cause neutropenia. Start at below-normal dosages to gauge tolerability, then titrate to normal dosages. Avoid tricyclics, which pose a higher risk of neutropenia than other antidepressant classes.
Case reports13,14 associate fluoxetine and mirtazapine with neutropenia. The patient who received mirtazapine, 30 mg/d, later responded well to sertraline, 50 mg/d.13
If the new antidepressant is ineffective, consider adding the mood-stabilizing anticonvulsant lamotrigine, 12.5 mg/d. Increase lamotrigine to 25 mg/d after 1 week, then titrate by 25 mg weekly to 100 to 400 mg/d depending on efficacy and tolerability.
Using growth factors.Although their efficacy is not proven, growth factors are minimally toxic and might have helped Mr. G. Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor resolved neutropenia in uncontrolled studies, but results of one randomized controlled trial were equivocal.8
TESTING: CT findings
Approximately 2 months after admission—shortly after a blood draw shows normal WBC and neutrophils—Mr. G complains of dizziness. He says he accidentally hit his head against a side table.
We order a full neurologic workup to check for traumatic brain injury or brain damage caused by long-term alcohol abuse:
- Head CT shows evidence of previous cerebrovascular infarcts in the bilateral frontal and cerebellar lobes and basal ganglia.
- MRI shows atrophied mammillary bodies, fornix, and corpus callosum.
- Magnetic resonance angiography reveals small cerebral vessel disease.
FOLLOW-UP: Awaiting discharge
After 3 months of continuous hospitalization, Mr. G has become euthymic and nonsuicidal, though at times oversensitive and combative. We transfer him to an assisted-living center and continue sertraline, 150 mg/d; phenytoin, 300 mg/d; phenobarbital, 30 mg bid; lithium, 300 mg/d; and trazodone, 50 mg at night as needed for insomnia.
We also place Mr. G in a day treatment program for mentally ill chemical abusers. A psychiatrist sees him every 2 weeks, and staff supervise him daily.
The authors’ observations
Mr. G’s extended hospital stay allowed us to closely observe him and offered ready access to laboratory facilities while we cross-tapered medications. In outpatient treatment, however, a serious and life-threatening medication-induced complication could easily be missed.
For medically healthy outpatients, be sure CBC has been checked ≤6 months before presentation. Monitor CBC and urge the patient to see a primary care doctor if infection symptoms emerge. Watch for gingivitis, tooth abscess, and other oral cavity infections—which often are overlooked—and sore throat or fever.
Also check electrolytes and screen for SSRI-induced hyponatremia at baseline for all at-risk patients.
Stop the offending drug when WBC reaches 9/L or with absolute neutrophil count (ANC) 9/L, then take a peripheral smear to confirm neutropenia. If the patient is asymptomatic, check ANC 2 to 3 times weekly, particularly if he or she recently had an infection or started a medication that can cause neutropenia. Neutropenia should resolve within 6 to 8 weeks of stopping the offending drug.
If neutropenia persists, order bone marrow biopsy in collaboration with an internist or hematologist to test for cancer. If the biopsy is negative, test for:
- HIV infection
- antinuclear antibodies to check for collagen vascular disease
- antineutrophil antibody to rule out immune neutropenia
- serum folate and B12 deficiency secondary to low WBC.
FOLLOW-UP: Stressor and relapse
Seven months later, Mr. G is readmitted for depression. Three months earlier, he had stopped all medications and resumed drinking after a family member died. WBC at admission is 3.70×109/L
We refer Mr. G to an outpatient psychiatrist, who sees him monthly. Several months later, the psychiatrist reports a WBC of 4.58×109/L.
Nearly 1 year later, Mr. G still lives at the assisted-living facility. He has not been rehospitalized for depression, is functioning well, and has a girlfriend.
The authors’ observations
Mr. G’s abnormal blood counts after sertraline rechallenge confirms that the SSRI probably was causing leukopenia. If we had restarted bupropion and neutropenia recurred during that regimen, we could have more certainly established a bupropion-leukopenia connection.
- Neutropenia Support Association. www.neutropenia.ca.
- Baehner RL. Overview of neutropenia.UpToDate Online (version 15.1); March 30, 2006. www.uptodate.com.
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol, others
- Citalopram • Celexa
- Clozapine • Clozaril
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Lithium • various
- Mirtazapine • Remeron
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Phenobarbital • various
- Phenytoin • Dilantin
- Propylthiouracil • various
- Sertraline • Zoloft
- Trazodone • Desyrel
- Valproic acid • Depakene
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Sad and suicidal
Mr. G, age 44, has chronic depression with suicidality. At presentation he says he has felt sad and suicidal for 2 weeks. He also has no appetite and trouble sleeping at night.
Mr. G’s depression has left him unable to work and has led to 4 hospitalizations over 10 years. He first attempted suicide in 1984 after his ex-wife took their child and left him. He endorses no suicide plan and has been sober for 7 years after 12-plus years of alcohol abuse, but says he has been tempted lately to resume drinking.
The patient was taking an antidepressant but stopped while at a homeless shelter, where he had been staying for several weeks. For more than 20 years, he also has been taking phenytoin, 300 mg/d, and phenobarbital, 30 mg bid, for a seizure disorder.
Mr. G is admitted with a working diagnosis of recurrent major depressive disorder. White blood cell count (WBC) at admission is 5.12×109/L and neutrophils are 3.6×109/L—both low-normal readings. Other laboratory results are normal.
We continue phenytoin and phenobarbital at the same dosages and start the selective serotonin reuptake inhibitor (SSRI) citalopram, 20 mg/d, which interacts minimally with both anticonvulsants.
After 2 weeks, Mr. G’s seizures are well controlled and he is tolerating citalopram, but his depressive symptoms have not improved. We cross-taper citalopram to prevent SSRI-induced discontinuation syndrome and start the dopamine and norepinephrine reuptake inhibitor bupropion, 75 mg bid. We titrate bupropion over 2 weeks to 150 mg each morning and 300 mg at bedtime, and watch Mr. G closely for seizures. Although his seizure history contraindicates bupropion use, we think he can tolerate the medication because his seizure disorder is well controlled.
Mr. G’s affect, appetite, and energy are improving with bupropion, but a routine complete blood count (CBC) 5 days after the medication is started reveals leukopenia (WBC 3.04×109/L) without neutropenia (neutrophils 1.9×109/L). Repeat blood tests 18 and 32 days after the first blood draw show continued low WBC. The gastrointestinal medicine team tests Mr. G’s liver function but finds no abnormalities.
The author’s observations
A medical cause also is unlikely. Mr. G’s liver function is normal, and he shows no other signs or symptoms of a medical problem. Bone marrow biopsy and immunologic workup could rule out cancer, but the timing of Mr. G’s abnormal blood readings strongly suggests bupropion intolerance.
TREATMENT: Other medications
We immediately stop bupropion, start the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine at 37.5 mg bid, and titrate it over 5 days to 225 mg/d. Blood draws 3 and 5 days after bupropion discontinuation show slight increases in WBC.
Eleven days after venlafaxine is started, Mr. G’s WBC and neutrophils are normal. However, he has become increasingly irritable and volatile, often arguing with a staff nurse and other patients. We cross-taper venlafaxine over 5 days, start the SSRI sertraline at 50 mg/d, and titrate sertraline over 1 week to 150 mg/d. Mr. G’s irritability and depressive symptoms improve at the latter dosage.
Because Mr. G developed neutropenia while taking a medication not associated with this adverse effect, we start watching his WBC counts more closely than usual. WBC is 4.58×109/L 8 days after sertraline is started but falls to 3.4×109/L after another 8 days, with neutrophils at 1.5×109/L for both readings (Table).
We add lithium, 300 mg bid, to increase Mr. G’s neutrophils and augment sertraline’s antidepressant effects. Four days later, WBC is 5.8×109/L with neutrophils at 4.2×109/L.
We stop lithium briefly to see if WBC remains normal. After 3 days, WBC drops to 3.25×109/L with neutrophils at 1.5×109/L. We restart lithium, 300 mg/d, and Mr. G’s WBC increases to 4.18×109/L 4 days later, with neutrophils at 2.1×109/L.
Table
Mr G’s white blood cell (WBC) and neutrophil counts (NC)*
while taking bupropion and sertraline
| Antidepressant | When measurements were taken | WBC | NC |
| None for several weeks | Baseline, first hospital admission | 5.12×109/L | 3.6×109/L |
| Bupropion, 75 mg bid | 5 days after starting bupropion | 3.04×109/L | 1.9×109/L |
| Bupropion, 450 mg/d total | 23 days after starting bupropion | 3.14×109/L | 1.6×109/L |
| Bupropion, 450 mg/d total | 2 weeks after previous test | 2.73×109/L | 1.6×109/L |
| Sertraline, 150 mg/d | 8 days after starting sertraline (titration period) | 4.58×109/L | 1.5×109/L |
| Sertraline, 150 mg/d | 16 days after starting sertraline | 3.4×109/L | 1.5×109/L |
| Sertraline, 150 mg/d, and lithium, 300 mg bid | 4 days after lithium augmentation | 5.8×109/L | 4.2×109/L |
| None for 3 months | Baseline, second hospital admission | 3.7×109/L | 2.1×109/L |
| Sertraline, 150 mg/d | 12 days after restarting sertraline | 2.83×109/L | Not available |
| * Normal WBC values: 4.5 to 11×109/L; normal neutrophil values: 1.5 to 8×109/L | |||
The authors’ observations
For Mr. G, both bupropion and sertraline appear to have caused neutropenia on separate occasions.
To our knowledge, bupropion-induced leukopenia or neutropenia have not been reported in the literature. Neutropenia—a rare adverse effect of antidepressants2—and leukopenia were seen during bupropion’s pre-marketing trials but were not definitely attributed to the drug.1 According to pre- and post-marketing data, leukopenia was “infrequently” reported among 5,100 subjects who received bupropion.3
To our knowledge, sertraline-induced neutropenia has not been reported in nongeriatric patients, although sertraline-induced neutropenia4 and agranulocytosis5 have been reported in patients age >65. The Committee on Safety of Medicine in the United Kingdom has received 2 other reports of neutropenia and 1 report of leukopenia with sertraline.5
In one clinical trial, 2 of 1,304 patients taking unknown dosages of sertraline had low neutrophils (
Medication is the second most common cause of acquired neutropenia, with infection being most common.6 By definition, drug-induced neutropenia occurs within 4 weeks after starting the drug and usually resolves within 30 days after stopping it.
Neutropenia is an idiosyncratic reaction unrelated to pharmacologic action. Although overall neutropenia incidence is unknown, reported incidence of the rare, more severe agranulocytosis ranges from approximately 1 to 10 cases per million people annually, and medications have been implicated in 70% of these cases.6 Conversely, only 2 of 97 incidental neutropenia cases studied by Lima et al7 were medication-induced.
Drug-induced neutropenia can result from immune-mediated destruction of neutrophils by circulating antibodies or from direct toxic effects upon marrow granulocyte precursors. Whereas immune-mediated onset is acute and explosive, toxic effect is insidious (months to years) and asymptomatic.8 Clozapine is thought to deliver a direct toxic effect, whereas the thyroid-regulating drug propylthiouracil generates anti-neutrophil antibodies.9
Mr. G’s acute onset (within 5 to 16 days of starting bupropion or sertraline) and prompt return of neutropenia after stopping lithium suggest acute immune-mediated circulating neutrophil destruction.
Treating leukopenia
After 4 failed or intolerable antidepressant trials, lithium augmentation seemed reasonable and ultimately improved Mr. G’s neutrophil count and his mood.
Lithium has helped resolve clozapine-induced neutropenia in case reports.10-12 Well-controlled studies, however, have followed only patients with antineoplastic, drug-induced neutropenia.1
By acting on cyclic nucleotides, lithium prompts colony-stimulating factor production, which in turn stimulates neutrophil production by pluripotent stem cells. As with Mr. G, patients reach neutrophilia 3 to 7 days after starting lithium.
If the patient cannot tolerate lithium, try switching antidepressants or using growth factors to increase neutrophils.
Switching antidepressants.The SSRIs escitalopram or paroxetine, or the SNRI duloxetine are effective and do not necessarily cause neutropenia. Start at below-normal dosages to gauge tolerability, then titrate to normal dosages. Avoid tricyclics, which pose a higher risk of neutropenia than other antidepressant classes.
Case reports13,14 associate fluoxetine and mirtazapine with neutropenia. The patient who received mirtazapine, 30 mg/d, later responded well to sertraline, 50 mg/d.13
If the new antidepressant is ineffective, consider adding the mood-stabilizing anticonvulsant lamotrigine, 12.5 mg/d. Increase lamotrigine to 25 mg/d after 1 week, then titrate by 25 mg weekly to 100 to 400 mg/d depending on efficacy and tolerability.
Using growth factors.Although their efficacy is not proven, growth factors are minimally toxic and might have helped Mr. G. Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor resolved neutropenia in uncontrolled studies, but results of one randomized controlled trial were equivocal.8
TESTING: CT findings
Approximately 2 months after admission—shortly after a blood draw shows normal WBC and neutrophils—Mr. G complains of dizziness. He says he accidentally hit his head against a side table.
We order a full neurologic workup to check for traumatic brain injury or brain damage caused by long-term alcohol abuse:
- Head CT shows evidence of previous cerebrovascular infarcts in the bilateral frontal and cerebellar lobes and basal ganglia.
- MRI shows atrophied mammillary bodies, fornix, and corpus callosum.
- Magnetic resonance angiography reveals small cerebral vessel disease.
FOLLOW-UP: Awaiting discharge
After 3 months of continuous hospitalization, Mr. G has become euthymic and nonsuicidal, though at times oversensitive and combative. We transfer him to an assisted-living center and continue sertraline, 150 mg/d; phenytoin, 300 mg/d; phenobarbital, 30 mg bid; lithium, 300 mg/d; and trazodone, 50 mg at night as needed for insomnia.
We also place Mr. G in a day treatment program for mentally ill chemical abusers. A psychiatrist sees him every 2 weeks, and staff supervise him daily.
The authors’ observations
Mr. G’s extended hospital stay allowed us to closely observe him and offered ready access to laboratory facilities while we cross-tapered medications. In outpatient treatment, however, a serious and life-threatening medication-induced complication could easily be missed.
For medically healthy outpatients, be sure CBC has been checked ≤6 months before presentation. Monitor CBC and urge the patient to see a primary care doctor if infection symptoms emerge. Watch for gingivitis, tooth abscess, and other oral cavity infections—which often are overlooked—and sore throat or fever.
Also check electrolytes and screen for SSRI-induced hyponatremia at baseline for all at-risk patients.
Stop the offending drug when WBC reaches 9/L or with absolute neutrophil count (ANC) 9/L, then take a peripheral smear to confirm neutropenia. If the patient is asymptomatic, check ANC 2 to 3 times weekly, particularly if he or she recently had an infection or started a medication that can cause neutropenia. Neutropenia should resolve within 6 to 8 weeks of stopping the offending drug.
If neutropenia persists, order bone marrow biopsy in collaboration with an internist or hematologist to test for cancer. If the biopsy is negative, test for:
- HIV infection
- antinuclear antibodies to check for collagen vascular disease
- antineutrophil antibody to rule out immune neutropenia
- serum folate and B12 deficiency secondary to low WBC.
FOLLOW-UP: Stressor and relapse
Seven months later, Mr. G is readmitted for depression. Three months earlier, he had stopped all medications and resumed drinking after a family member died. WBC at admission is 3.70×109/L
We refer Mr. G to an outpatient psychiatrist, who sees him monthly. Several months later, the psychiatrist reports a WBC of 4.58×109/L.
Nearly 1 year later, Mr. G still lives at the assisted-living facility. He has not been rehospitalized for depression, is functioning well, and has a girlfriend.
The authors’ observations
Mr. G’s abnormal blood counts after sertraline rechallenge confirms that the SSRI probably was causing leukopenia. If we had restarted bupropion and neutropenia recurred during that regimen, we could have more certainly established a bupropion-leukopenia connection.
- Neutropenia Support Association. www.neutropenia.ca.
- Baehner RL. Overview of neutropenia.UpToDate Online (version 15.1); March 30, 2006. www.uptodate.com.
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol, others
- Citalopram • Celexa
- Clozapine • Clozaril
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Lithium • various
- Mirtazapine • Remeron
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Phenobarbital • various
- Phenytoin • Dilantin
- Propylthiouracil • various
- Sertraline • Zoloft
- Trazodone • Desyrel
- Valproic acid • Depakene
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. McEvoy G, ed. AHFS drug information. Bethesda, MD: American Society of Health-System Pharmacists; 2005.
2. Nelson JC. Safety and tolerability of the new antidepressants. J Clin Psychiatry 1997;60:1101.-
3. Physicians desk reference, 61st ed. Montvale, NJ: Thomson PDR; 2007.
4. Cohn CK, Shrivastava R, Mendels J, et al. Double-blind, multicenter comparison of sertraline and amitriptyline in elderly depressed patients. J Clin Psychiatry 1990;51(suppl B):28-33.
5. Trescoli-Serrano C, Smith NK. Sertraline-induced agranulocytosis. Postgrad Med J 1996;72:446.-
6. Baehner RL. Overview of neutropenia. UpToDate Online (version 15.1); March 30, 2006. Available at: http://www.uptodate.com. Accessed April 16, 2007.
7. Lima CS, Paula EV, Takahashi T, et al. Causes of incidental neutropenia in adulthood. Ann Hematol 2006;85:705-9.
8. Holland SM, Gallin J. Disorders of granulocytes and monocytes. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison’s principles of internal medicine, 16th ed. New York: McGraw-Hill; 2005.
9. Baehner RL. Drug-induced neutropenia and agranulocytosis. UpToDate Online (version 15.1); June 8, 2005. Available at: http://www.uptodate.com. Accessed April 16, 2007.
10. Sporn A, Gogtay N, Ortiz-Aguayo R, et al. Clozapine-induced neutropenia in children: management with lithium carbonate. J Child Adolesc Psychopharmacol 2003;13:401-4.
11. Blier P, Slater S, Measham T, et al. Lithium and clozapine-induced neutropenia/agranulocytosis. Int Clin Psychopharmacol 1998;13:137-40.
12. Silverstone P. Prevention of clozapine-induced neutropenia by pretreatment with lithium. J Clin Psychopharmacol 1998;18:86-8.
13. Ozcanli T, Unsalver B, Ozdemir S, Ozmen M. Sertraline and mirtazapine-induced severe neutropenia. Am J Psych 2005;162:1386.-
14. Vilinsky FD, Lubin A. Severe neutropenia associated with fluoxetine hydrochloride. Ann Internal Med 1997;127:573-4.
1. McEvoy G, ed. AHFS drug information. Bethesda, MD: American Society of Health-System Pharmacists; 2005.
2. Nelson JC. Safety and tolerability of the new antidepressants. J Clin Psychiatry 1997;60:1101.-
3. Physicians desk reference, 61st ed. Montvale, NJ: Thomson PDR; 2007.
4. Cohn CK, Shrivastava R, Mendels J, et al. Double-blind, multicenter comparison of sertraline and amitriptyline in elderly depressed patients. J Clin Psychiatry 1990;51(suppl B):28-33.
5. Trescoli-Serrano C, Smith NK. Sertraline-induced agranulocytosis. Postgrad Med J 1996;72:446.-
6. Baehner RL. Overview of neutropenia. UpToDate Online (version 15.1); March 30, 2006. Available at: http://www.uptodate.com. Accessed April 16, 2007.
7. Lima CS, Paula EV, Takahashi T, et al. Causes of incidental neutropenia in adulthood. Ann Hematol 2006;85:705-9.
8. Holland SM, Gallin J. Disorders of granulocytes and monocytes. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison’s principles of internal medicine, 16th ed. New York: McGraw-Hill; 2005.
9. Baehner RL. Drug-induced neutropenia and agranulocytosis. UpToDate Online (version 15.1); June 8, 2005. Available at: http://www.uptodate.com. Accessed April 16, 2007.
10. Sporn A, Gogtay N, Ortiz-Aguayo R, et al. Clozapine-induced neutropenia in children: management with lithium carbonate. J Child Adolesc Psychopharmacol 2003;13:401-4.
11. Blier P, Slater S, Measham T, et al. Lithium and clozapine-induced neutropenia/agranulocytosis. Int Clin Psychopharmacol 1998;13:137-40.
12. Silverstone P. Prevention of clozapine-induced neutropenia by pretreatment with lithium. J Clin Psychopharmacol 1998;18:86-8.
13. Ozcanli T, Unsalver B, Ozdemir S, Ozmen M. Sertraline and mirtazapine-induced severe neutropenia. Am J Psych 2005;162:1386.-
14. Vilinsky FD, Lubin A. Severe neutropenia associated with fluoxetine hydrochloride. Ann Internal Med 1997;127:573-4.
After 3 months, she’s still ‘mad’
History: ‘They want to kill me’
Police and security agents arrest Ms. A, age 64, at a metropolitan airport. She is extremely agitated and behaving bizarrely, yelling that “the Mafia” is trying to kill her. She has spent 3 days hiding in area hotels, fleeing her “assailants.”
Police arrange Ms. A’s return home; under court order, she is hospitalized in a psychiatric facility. She is diagnosed with paranoid schizophrenia and receives IM haloperidol, 2 mg bid, but shows minimal improvement after 2½ weeks. Her psychotic symptoms improve slightly after the psychiatrist switches her to risperidone, 2 mg bid, but she still cannot function normally. Three weeks after admission, she is transferred to a nursing home for long-term care. She continues risperidone but remains paranoid and delusional.
Three months later, Ms. A is rehospitalized. She is anxious, delusional, confused, and hallucinating at admission. The patient is verbally and physically combative, fearful that medical staff will harm her. She is too violent to be examined, but staff notice that her skin appears thickened, her eyes puffy, and her hair coarse. Her voice sounds low and raspy.
I speak with Ms. A’s son, who reports that before his mother’s arrest he found her in the kitchen wielding a knife, exclaiming she wanted to kill herself. He says she heard a “whoosh” or “ringing” in her right ear while a male voice in her left ear told her, “End it, end it.”
Ms. A is severely obese (weight 325 lbs, body mass index 49 kg/m2). Blood pressure is 140/90 mm Hg, and she is taking captopril, 50 mg bid, for hypertension. Pulse rate and temperature are normal.
Dr. Lachover’s observations
Ms. A’s hallucinatory experiences are atypical, and her psychotic symptoms show little response after 2 months of aggressive inpatient treatment. Three months after discharge, she is rehospitalized in a florid paranoid psychotic state.
The patient’s weight poses an additional obstacle. I avoided second-generation antipsychotics (SGAs) that can cause weight gain, such as clozapine or olanzapine. I tried the SGA risperidone after IM haloperidol, a first-generation antipsychotic, produced minimal response.
Ms. A’s physical symptoms (thickened skin, coarse hair, puffiness under her eyes, and vocal raspiness) suggest an underlying organic process that might be causing her psychosis.
TESTING: Telling results
I order laboratory and other tests to check for an underlying organic disorder:
- Brain MRI is normal, as are CBC, renal and liver function, and serum copper, ceruloplasmin, vitamin B12, and heavy metal levels.
- Slit lamp eye exam reveals no Kayser-Fleischer ring, which would have indicated Wilson’s disease.
- EEG shows a diffuse, nonspecific, abnormal pattern of slowing and decreased amplitude, suggesting diffuse cerebral dysfunction.
- ECG shows sinus bradycardia and a significantly prolonged corrected QT (QTc) interval, indicating delayed ventricular repolarization.
- Thyroid panel is abnormal with markedly elevated thyrotropin (31.07 mIU/L).
Across 3 weeks, Ms. A’s delusional perceptions and hallucination intensity decrease, and her reality testing and socialization skills improve. She is discharged, after which the internist and I see her weekly to monitor thyroid function and psychiatric symptoms, respectively. Thyroid function gradually returns to normal over 4 to 6 months, and she is maintained on levothyroxine, 0.025 mg/d. Her weight gradually decreases over 12 months to 229 lbs.
Six months after discharge, Ms. A is notably more adept at activities of daily living. Mental status exam shows progressively improved reality testing and decreased paranoia. She is more active, and her mood and affect have brightened. Risperidone is stopped 10 months after discharge, and she has not been rehospitalized for psychiatric problems.
Table 1
Ms. A’s thyroid panel values
| Component | Ms. A’s readings | Normal values |
| Serum cholesterol | 310 mg/dL | 100 to 199 mg/dL |
| TSH (thyrotropin) | 31.07 mIU/L | 0.25 to 4.30 mIU/L |
| Free T4 | 0.34 ng/dL | 0.80 to 1.80 ng/dL |
| Total T4 (serum thyroxine) | 1.5 µg/dL | 4.6 to 12 µg/dL |
| Total T3 (serum triiodothyronine) | 67 ng/dL | 70 to 180 ng/dL |
Dr. Lachover’s observations
Erroneously diagnosed with paranoid schizophrenia, Ms. A endured 2 extended hospitalizations. Her psychosis and mental state—both of which improved with thyroid replacement therapy—appear to have been a psychiatric manifestation of severe hypothyroidism, or “myxedema madness” (Box).1-3
Myxedema prevalence in the general public has been reported at 0.5% to 18%. It is roughly 10 times more common in women than in men,4 and 5% to 15% of patients with myxedema might develop signs of psychosis.4 Myxedema-induced psychosis usually occurs during middle age but has been reported between ages 18 and 73. Prevalence increases with age.4
Recognizing ‘myxedema madness’
Detecting and treating myxedema in patients with treatment-resistant psychosis can resolve psychiatric and medical symptoms and restore quality of life. Left untreated, it can impair cognitive function and cause lethargy, dysarthria, myopathy, neuropathy, status epilepticus, and coma.5-7
Myxedema can impair perception and intellectual functioning,9 and acute mania has been reported in some cases.10 Increasing delirium reduces integration of perceptual input, leading to misidentification and disorientation. Cognitive functioning may be impaired, and abnormal thyroid hormone levels might delay event-related brain potential.11
Physical signs also can be telling. The patient might show general psychomotor retardation and slowed speech. The tongue might be swollen, the voice hoarse and croaking. Hair is often coarse and brittle, with hair loss along the sides of the eyebrows. Body temperature often dips below normal.4
Dr. Lachover’s observations
Detecting Ms. A’s hypothyroidism early could have prevented needless hospitalizations and failed treatment. Order a baseline thyroid panel for every patient who presents with psychotic symptoms or depression, which is the primary affective disturbance seen in myxedema.
Researchers have proposed many potential causes for the psychotic and depressive symptoms seen in myxedema.
Psychotic symptoms. Tonks1 has attributed psychosis in myxedema to decreases in cerebral oxygenation and glucose metabolism, resulting in a relative cerebral hypoxia. Among patients with myxedema, Sheinberg et al2 reported markedly reduced cardiac output and found that:
- cerebral blood flow was reduced 38%
- oxygen and glucose absorption were decreased approximately 30%
- cerebrovascular resistance was notably increased.
Depressive symptoms. Catecholamine deficiency at the neuronal receptor sites might cause depression in hypothyroidism. Evidence suggests that thyroid hormone influences catecholamine function at the neuronal level.3
Monoamine oxidase, which is increased in myxedema, has also been implicated. This enzyme might lead to depression by helping to break down catecholamines at the neuronal axon-dendrite levels.3
Diffuse slowing of background activity is the most common EEG change found in myxedema.13 ECG might show slow, regular sinus rhythm or bradycardia, low voltage, prolonged QTc interval, and flattened T waves.14 Prolonged QRS complexes on ECG indicate delayed ventricular repolarization.11,15 Torsades de pointes, the potentially fatal ventricular tachycardia, can result from a prolonged QTc interval in rare myxedema cases.16
Table 2
Is it myxedema? Check the lab findings
| Component | Values that suggest myxedema |
| Serum cholesterol | >200 mg/dL |
| Free T4 | |
| Total T4 (serum thyroxine) | |
| Total T3 (serum triiodothyronine) | |
| TSH (thyrotropin) | >4.5 mIU/L |
| EEG | Diffuse slowing |
| EKG | Prolonged QTc interval |
Treating 2 sets of symptoms
Prescribe concomitant dessicated thyroid and low-dose antipsychotics over 4 to 6 months to treat both the thyroid dysfunction and psychosis. Because weight gain is common in myxedema, choose an antipsychotic that carries a relatively low risk of weight gain, such as risperidone, 2 mg bid, or aripiprazole, 5 to 10 mg/d.
Many patients reach euthyroidism and their psychosis improves gradually but notably over weeks or months after starting thyroid hormone replacement. Psychosis could recur if desiccated thyroid is stopped; restarting it will improve the patient’s mental state.17 Recovery takes about 3 months on average.4
Continue the SGA until delusion perception is gone and reality testing improves, then taper the medication until all psychotic symptoms have abated. Monitor thyroid function monthly.
For patients with myxedema-induced depression, supplement thyroid hormone replacement with a selective serotonin reuptake inhibitor such as sertraline at regular starting dosages.
Dr. Lachover’s observations
Consider contributing medical illness in any patient with psychosis, particularly with psychotic symptom onset after age 40 and lack of response to weeks of adequate antipsychotic therapy.
A meticulous search to rule out medical disorders in all patients with psychosis and/or depression is essential to planning treatment. Testing is especially urgent for elderly patients, as multiple medical comorbidities or medication side effects can mask hypothyroidism’s signs and symptoms and delay diagnosis.18
Check complete blood count, electrolytes, thyroid panel, urinalysis, urine drug screen, blood urea nitrogen, and creatinine to rule out an underlying metabolic or endocrinologic cause for psychosis. Watch for signs of anticholinergic syndrome during physical examination.
If any of the above results suggest a medical problem, test for the following as clinical suspicion warrants:
- serum copper/ceruloplasmin and liver function to rule out Wilson’s disease, a genetic disorder that causes copper to accumulate in the liver and brain
- systemic lupus erythematosus
- lead, magnesium, mercury, or manganese to rule out metal poisoning.
- Cronin AJ. The Citadel. Boston: Little, Brown & Co.;1937:399.
- Asher R. Myxoedamatous madness. BMJ 1949;2:555-62.
- Aripiprazole • Abilify
- Captopril • Capoten
- Clozapine • Clozaril
- Haloperidol • Haldol
- Levothyroxine • Synthroid
- Olanzapine • Zyprexa
- Risperidone • Risperdal
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Tonks CM. Mental illness and hypothyroid patients. Br J Psychiatry 1964;110:706-10.
2. Scheinberg P, et al. Cerebral metabolism and cardiac output in myxedema. J Clin Invest 1950;29:1139-46.
3. Whybrow PC, Prange AJ, Treadway CR. Mental changes accompanying thyroid gland dysfunction. Arch Gen Psychiatry 1969;20:48-63.
4. Heinrich TW, Grahm G. Hypothyroidism presenting as psychosis: myxedema madness revisited. Prim Care Companion J Clin Psychiatry 2003;5:260-6.
5. Jansen HJ, Doebe SR, Louwerse ES, et al. Status epilepticus caused by a myxoedema coma. Neth J Med 2006;64:202-5.
6. Pimental L, Hansen KN. Thyroid disease in the emergency department: a clinical and laboratory review. J Emerg Med 2005;28:201-9.
7. Wartofsky L. Myxedema coma. Endocrinol Metab Clin North Am 2006;35:687-98.
8. Roberts LM, Pattison H, Roalfe A, et al. Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Int Med 2006;145:573-81.
9. Adams CW. Electrocardiographic changes in hypothyroidism. Chest 1964;46:87-8.
10. Stowell CP, Barnhill JW. Acute mania in the setting of severe hypothyroidism. Psychosomatics 2005;46:259-61.
11. Strachan SR, Afolabi O, Brown N, Gray D. Chest pain, enzymes, and hypothyroidism. Postgrad Med J 2000;76:168-9.
12. Lolas F, de la Parra G, Gramegna G. Event-related slow potential (ERSP) correlates of thyroid gland function levels. Psychosom Med 1978;40:226-35.
13. Pinto A, Glick M. Management of patients with thyroid disease: oral health considerations. J Am Dent Assoc 2002;133:849-58.
14. Khedr EM, El Toony LF, Tarkhan MN, Abdella G. Peripheral and central nervous system alterations in hypothyroidism; electrophysiological findings. Neuropsychobiology 2000;41:88-94.
15. Bosch R, Wang Z, Li GR, Nattel S. Electrophysiological mechanisms by which hypothyroidism delays repolarization in guinea pig hearts. Am J Physiol 1999;277(1 Pt 2):H211-20.
16. Schenck JB, Rizvi AA, Lin T. Severe primary hypothyroidism manifesting with torsades de pointes. Am J Med Sci 2006;331:154-6.
17. McGaffee J, Barnes MA, Lippmann S. Psychiatric presentations of hypothyroidism. Am Fam Physicia 1981;23:129-33.
18. Rehman SU, Cope DW, Senseney AD, Brzezinski W. Thyroid disorders in elderly patients. South Med J 2005;98:543-9.
History: ‘They want to kill me’
Police and security agents arrest Ms. A, age 64, at a metropolitan airport. She is extremely agitated and behaving bizarrely, yelling that “the Mafia” is trying to kill her. She has spent 3 days hiding in area hotels, fleeing her “assailants.”
Police arrange Ms. A’s return home; under court order, she is hospitalized in a psychiatric facility. She is diagnosed with paranoid schizophrenia and receives IM haloperidol, 2 mg bid, but shows minimal improvement after 2½ weeks. Her psychotic symptoms improve slightly after the psychiatrist switches her to risperidone, 2 mg bid, but she still cannot function normally. Three weeks after admission, she is transferred to a nursing home for long-term care. She continues risperidone but remains paranoid and delusional.
Three months later, Ms. A is rehospitalized. She is anxious, delusional, confused, and hallucinating at admission. The patient is verbally and physically combative, fearful that medical staff will harm her. She is too violent to be examined, but staff notice that her skin appears thickened, her eyes puffy, and her hair coarse. Her voice sounds low and raspy.
I speak with Ms. A’s son, who reports that before his mother’s arrest he found her in the kitchen wielding a knife, exclaiming she wanted to kill herself. He says she heard a “whoosh” or “ringing” in her right ear while a male voice in her left ear told her, “End it, end it.”
Ms. A is severely obese (weight 325 lbs, body mass index 49 kg/m2). Blood pressure is 140/90 mm Hg, and she is taking captopril, 50 mg bid, for hypertension. Pulse rate and temperature are normal.
Dr. Lachover’s observations
Ms. A’s hallucinatory experiences are atypical, and her psychotic symptoms show little response after 2 months of aggressive inpatient treatment. Three months after discharge, she is rehospitalized in a florid paranoid psychotic state.
The patient’s weight poses an additional obstacle. I avoided second-generation antipsychotics (SGAs) that can cause weight gain, such as clozapine or olanzapine. I tried the SGA risperidone after IM haloperidol, a first-generation antipsychotic, produced minimal response.
Ms. A’s physical symptoms (thickened skin, coarse hair, puffiness under her eyes, and vocal raspiness) suggest an underlying organic process that might be causing her psychosis.
TESTING: Telling results
I order laboratory and other tests to check for an underlying organic disorder:
- Brain MRI is normal, as are CBC, renal and liver function, and serum copper, ceruloplasmin, vitamin B12, and heavy metal levels.
- Slit lamp eye exam reveals no Kayser-Fleischer ring, which would have indicated Wilson’s disease.
- EEG shows a diffuse, nonspecific, abnormal pattern of slowing and decreased amplitude, suggesting diffuse cerebral dysfunction.
- ECG shows sinus bradycardia and a significantly prolonged corrected QT (QTc) interval, indicating delayed ventricular repolarization.
- Thyroid panel is abnormal with markedly elevated thyrotropin (31.07 mIU/L).
Across 3 weeks, Ms. A’s delusional perceptions and hallucination intensity decrease, and her reality testing and socialization skills improve. She is discharged, after which the internist and I see her weekly to monitor thyroid function and psychiatric symptoms, respectively. Thyroid function gradually returns to normal over 4 to 6 months, and she is maintained on levothyroxine, 0.025 mg/d. Her weight gradually decreases over 12 months to 229 lbs.
Six months after discharge, Ms. A is notably more adept at activities of daily living. Mental status exam shows progressively improved reality testing and decreased paranoia. She is more active, and her mood and affect have brightened. Risperidone is stopped 10 months after discharge, and she has not been rehospitalized for psychiatric problems.
Table 1
Ms. A’s thyroid panel values
| Component | Ms. A’s readings | Normal values |
| Serum cholesterol | 310 mg/dL | 100 to 199 mg/dL |
| TSH (thyrotropin) | 31.07 mIU/L | 0.25 to 4.30 mIU/L |
| Free T4 | 0.34 ng/dL | 0.80 to 1.80 ng/dL |
| Total T4 (serum thyroxine) | 1.5 µg/dL | 4.6 to 12 µg/dL |
| Total T3 (serum triiodothyronine) | 67 ng/dL | 70 to 180 ng/dL |
Dr. Lachover’s observations
Erroneously diagnosed with paranoid schizophrenia, Ms. A endured 2 extended hospitalizations. Her psychosis and mental state—both of which improved with thyroid replacement therapy—appear to have been a psychiatric manifestation of severe hypothyroidism, or “myxedema madness” (Box).1-3
Myxedema prevalence in the general public has been reported at 0.5% to 18%. It is roughly 10 times more common in women than in men,4 and 5% to 15% of patients with myxedema might develop signs of psychosis.4 Myxedema-induced psychosis usually occurs during middle age but has been reported between ages 18 and 73. Prevalence increases with age.4
Recognizing ‘myxedema madness’
Detecting and treating myxedema in patients with treatment-resistant psychosis can resolve psychiatric and medical symptoms and restore quality of life. Left untreated, it can impair cognitive function and cause lethargy, dysarthria, myopathy, neuropathy, status epilepticus, and coma.5-7
Myxedema can impair perception and intellectual functioning,9 and acute mania has been reported in some cases.10 Increasing delirium reduces integration of perceptual input, leading to misidentification and disorientation. Cognitive functioning may be impaired, and abnormal thyroid hormone levels might delay event-related brain potential.11
Physical signs also can be telling. The patient might show general psychomotor retardation and slowed speech. The tongue might be swollen, the voice hoarse and croaking. Hair is often coarse and brittle, with hair loss along the sides of the eyebrows. Body temperature often dips below normal.4
Dr. Lachover’s observations
Detecting Ms. A’s hypothyroidism early could have prevented needless hospitalizations and failed treatment. Order a baseline thyroid panel for every patient who presents with psychotic symptoms or depression, which is the primary affective disturbance seen in myxedema.
Researchers have proposed many potential causes for the psychotic and depressive symptoms seen in myxedema.
Psychotic symptoms. Tonks1 has attributed psychosis in myxedema to decreases in cerebral oxygenation and glucose metabolism, resulting in a relative cerebral hypoxia. Among patients with myxedema, Sheinberg et al2 reported markedly reduced cardiac output and found that:
- cerebral blood flow was reduced 38%
- oxygen and glucose absorption were decreased approximately 30%
- cerebrovascular resistance was notably increased.
Depressive symptoms. Catecholamine deficiency at the neuronal receptor sites might cause depression in hypothyroidism. Evidence suggests that thyroid hormone influences catecholamine function at the neuronal level.3
Monoamine oxidase, which is increased in myxedema, has also been implicated. This enzyme might lead to depression by helping to break down catecholamines at the neuronal axon-dendrite levels.3
Diffuse slowing of background activity is the most common EEG change found in myxedema.13 ECG might show slow, regular sinus rhythm or bradycardia, low voltage, prolonged QTc interval, and flattened T waves.14 Prolonged QRS complexes on ECG indicate delayed ventricular repolarization.11,15 Torsades de pointes, the potentially fatal ventricular tachycardia, can result from a prolonged QTc interval in rare myxedema cases.16
Table 2
Is it myxedema? Check the lab findings
| Component | Values that suggest myxedema |
| Serum cholesterol | >200 mg/dL |
| Free T4 | |
| Total T4 (serum thyroxine) | |
| Total T3 (serum triiodothyronine) | |
| TSH (thyrotropin) | >4.5 mIU/L |
| EEG | Diffuse slowing |
| EKG | Prolonged QTc interval |
Treating 2 sets of symptoms
Prescribe concomitant dessicated thyroid and low-dose antipsychotics over 4 to 6 months to treat both the thyroid dysfunction and psychosis. Because weight gain is common in myxedema, choose an antipsychotic that carries a relatively low risk of weight gain, such as risperidone, 2 mg bid, or aripiprazole, 5 to 10 mg/d.
Many patients reach euthyroidism and their psychosis improves gradually but notably over weeks or months after starting thyroid hormone replacement. Psychosis could recur if desiccated thyroid is stopped; restarting it will improve the patient’s mental state.17 Recovery takes about 3 months on average.4
Continue the SGA until delusion perception is gone and reality testing improves, then taper the medication until all psychotic symptoms have abated. Monitor thyroid function monthly.
For patients with myxedema-induced depression, supplement thyroid hormone replacement with a selective serotonin reuptake inhibitor such as sertraline at regular starting dosages.
Dr. Lachover’s observations
Consider contributing medical illness in any patient with psychosis, particularly with psychotic symptom onset after age 40 and lack of response to weeks of adequate antipsychotic therapy.
A meticulous search to rule out medical disorders in all patients with psychosis and/or depression is essential to planning treatment. Testing is especially urgent for elderly patients, as multiple medical comorbidities or medication side effects can mask hypothyroidism’s signs and symptoms and delay diagnosis.18
Check complete blood count, electrolytes, thyroid panel, urinalysis, urine drug screen, blood urea nitrogen, and creatinine to rule out an underlying metabolic or endocrinologic cause for psychosis. Watch for signs of anticholinergic syndrome during physical examination.
If any of the above results suggest a medical problem, test for the following as clinical suspicion warrants:
- serum copper/ceruloplasmin and liver function to rule out Wilson’s disease, a genetic disorder that causes copper to accumulate in the liver and brain
- systemic lupus erythematosus
- lead, magnesium, mercury, or manganese to rule out metal poisoning.
- Cronin AJ. The Citadel. Boston: Little, Brown & Co.;1937:399.
- Asher R. Myxoedamatous madness. BMJ 1949;2:555-62.
- Aripiprazole • Abilify
- Captopril • Capoten
- Clozapine • Clozaril
- Haloperidol • Haldol
- Levothyroxine • Synthroid
- Olanzapine • Zyprexa
- Risperidone • Risperdal
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
History: ‘They want to kill me’
Police and security agents arrest Ms. A, age 64, at a metropolitan airport. She is extremely agitated and behaving bizarrely, yelling that “the Mafia” is trying to kill her. She has spent 3 days hiding in area hotels, fleeing her “assailants.”
Police arrange Ms. A’s return home; under court order, she is hospitalized in a psychiatric facility. She is diagnosed with paranoid schizophrenia and receives IM haloperidol, 2 mg bid, but shows minimal improvement after 2½ weeks. Her psychotic symptoms improve slightly after the psychiatrist switches her to risperidone, 2 mg bid, but she still cannot function normally. Three weeks after admission, she is transferred to a nursing home for long-term care. She continues risperidone but remains paranoid and delusional.
Three months later, Ms. A is rehospitalized. She is anxious, delusional, confused, and hallucinating at admission. The patient is verbally and physically combative, fearful that medical staff will harm her. She is too violent to be examined, but staff notice that her skin appears thickened, her eyes puffy, and her hair coarse. Her voice sounds low and raspy.
I speak with Ms. A’s son, who reports that before his mother’s arrest he found her in the kitchen wielding a knife, exclaiming she wanted to kill herself. He says she heard a “whoosh” or “ringing” in her right ear while a male voice in her left ear told her, “End it, end it.”
Ms. A is severely obese (weight 325 lbs, body mass index 49 kg/m2). Blood pressure is 140/90 mm Hg, and she is taking captopril, 50 mg bid, for hypertension. Pulse rate and temperature are normal.
Dr. Lachover’s observations
Ms. A’s hallucinatory experiences are atypical, and her psychotic symptoms show little response after 2 months of aggressive inpatient treatment. Three months after discharge, she is rehospitalized in a florid paranoid psychotic state.
The patient’s weight poses an additional obstacle. I avoided second-generation antipsychotics (SGAs) that can cause weight gain, such as clozapine or olanzapine. I tried the SGA risperidone after IM haloperidol, a first-generation antipsychotic, produced minimal response.
Ms. A’s physical symptoms (thickened skin, coarse hair, puffiness under her eyes, and vocal raspiness) suggest an underlying organic process that might be causing her psychosis.
TESTING: Telling results
I order laboratory and other tests to check for an underlying organic disorder:
- Brain MRI is normal, as are CBC, renal and liver function, and serum copper, ceruloplasmin, vitamin B12, and heavy metal levels.
- Slit lamp eye exam reveals no Kayser-Fleischer ring, which would have indicated Wilson’s disease.
- EEG shows a diffuse, nonspecific, abnormal pattern of slowing and decreased amplitude, suggesting diffuse cerebral dysfunction.
- ECG shows sinus bradycardia and a significantly prolonged corrected QT (QTc) interval, indicating delayed ventricular repolarization.
- Thyroid panel is abnormal with markedly elevated thyrotropin (31.07 mIU/L).
Across 3 weeks, Ms. A’s delusional perceptions and hallucination intensity decrease, and her reality testing and socialization skills improve. She is discharged, after which the internist and I see her weekly to monitor thyroid function and psychiatric symptoms, respectively. Thyroid function gradually returns to normal over 4 to 6 months, and she is maintained on levothyroxine, 0.025 mg/d. Her weight gradually decreases over 12 months to 229 lbs.
Six months after discharge, Ms. A is notably more adept at activities of daily living. Mental status exam shows progressively improved reality testing and decreased paranoia. She is more active, and her mood and affect have brightened. Risperidone is stopped 10 months after discharge, and she has not been rehospitalized for psychiatric problems.
Table 1
Ms. A’s thyroid panel values
| Component | Ms. A’s readings | Normal values |
| Serum cholesterol | 310 mg/dL | 100 to 199 mg/dL |
| TSH (thyrotropin) | 31.07 mIU/L | 0.25 to 4.30 mIU/L |
| Free T4 | 0.34 ng/dL | 0.80 to 1.80 ng/dL |
| Total T4 (serum thyroxine) | 1.5 µg/dL | 4.6 to 12 µg/dL |
| Total T3 (serum triiodothyronine) | 67 ng/dL | 70 to 180 ng/dL |
Dr. Lachover’s observations
Erroneously diagnosed with paranoid schizophrenia, Ms. A endured 2 extended hospitalizations. Her psychosis and mental state—both of which improved with thyroid replacement therapy—appear to have been a psychiatric manifestation of severe hypothyroidism, or “myxedema madness” (Box).1-3
Myxedema prevalence in the general public has been reported at 0.5% to 18%. It is roughly 10 times more common in women than in men,4 and 5% to 15% of patients with myxedema might develop signs of psychosis.4 Myxedema-induced psychosis usually occurs during middle age but has been reported between ages 18 and 73. Prevalence increases with age.4
Recognizing ‘myxedema madness’
Detecting and treating myxedema in patients with treatment-resistant psychosis can resolve psychiatric and medical symptoms and restore quality of life. Left untreated, it can impair cognitive function and cause lethargy, dysarthria, myopathy, neuropathy, status epilepticus, and coma.5-7
Myxedema can impair perception and intellectual functioning,9 and acute mania has been reported in some cases.10 Increasing delirium reduces integration of perceptual input, leading to misidentification and disorientation. Cognitive functioning may be impaired, and abnormal thyroid hormone levels might delay event-related brain potential.11
Physical signs also can be telling. The patient might show general psychomotor retardation and slowed speech. The tongue might be swollen, the voice hoarse and croaking. Hair is often coarse and brittle, with hair loss along the sides of the eyebrows. Body temperature often dips below normal.4
Dr. Lachover’s observations
Detecting Ms. A’s hypothyroidism early could have prevented needless hospitalizations and failed treatment. Order a baseline thyroid panel for every patient who presents with psychotic symptoms or depression, which is the primary affective disturbance seen in myxedema.
Researchers have proposed many potential causes for the psychotic and depressive symptoms seen in myxedema.
Psychotic symptoms. Tonks1 has attributed psychosis in myxedema to decreases in cerebral oxygenation and glucose metabolism, resulting in a relative cerebral hypoxia. Among patients with myxedema, Sheinberg et al2 reported markedly reduced cardiac output and found that:
- cerebral blood flow was reduced 38%
- oxygen and glucose absorption were decreased approximately 30%
- cerebrovascular resistance was notably increased.
Depressive symptoms. Catecholamine deficiency at the neuronal receptor sites might cause depression in hypothyroidism. Evidence suggests that thyroid hormone influences catecholamine function at the neuronal level.3
Monoamine oxidase, which is increased in myxedema, has also been implicated. This enzyme might lead to depression by helping to break down catecholamines at the neuronal axon-dendrite levels.3
Diffuse slowing of background activity is the most common EEG change found in myxedema.13 ECG might show slow, regular sinus rhythm or bradycardia, low voltage, prolonged QTc interval, and flattened T waves.14 Prolonged QRS complexes on ECG indicate delayed ventricular repolarization.11,15 Torsades de pointes, the potentially fatal ventricular tachycardia, can result from a prolonged QTc interval in rare myxedema cases.16
Table 2
Is it myxedema? Check the lab findings
| Component | Values that suggest myxedema |
| Serum cholesterol | >200 mg/dL |
| Free T4 | |
| Total T4 (serum thyroxine) | |
| Total T3 (serum triiodothyronine) | |
| TSH (thyrotropin) | >4.5 mIU/L |
| EEG | Diffuse slowing |
| EKG | Prolonged QTc interval |
Treating 2 sets of symptoms
Prescribe concomitant dessicated thyroid and low-dose antipsychotics over 4 to 6 months to treat both the thyroid dysfunction and psychosis. Because weight gain is common in myxedema, choose an antipsychotic that carries a relatively low risk of weight gain, such as risperidone, 2 mg bid, or aripiprazole, 5 to 10 mg/d.
Many patients reach euthyroidism and their psychosis improves gradually but notably over weeks or months after starting thyroid hormone replacement. Psychosis could recur if desiccated thyroid is stopped; restarting it will improve the patient’s mental state.17 Recovery takes about 3 months on average.4
Continue the SGA until delusion perception is gone and reality testing improves, then taper the medication until all psychotic symptoms have abated. Monitor thyroid function monthly.
For patients with myxedema-induced depression, supplement thyroid hormone replacement with a selective serotonin reuptake inhibitor such as sertraline at regular starting dosages.
Dr. Lachover’s observations
Consider contributing medical illness in any patient with psychosis, particularly with psychotic symptom onset after age 40 and lack of response to weeks of adequate antipsychotic therapy.
A meticulous search to rule out medical disorders in all patients with psychosis and/or depression is essential to planning treatment. Testing is especially urgent for elderly patients, as multiple medical comorbidities or medication side effects can mask hypothyroidism’s signs and symptoms and delay diagnosis.18
Check complete blood count, electrolytes, thyroid panel, urinalysis, urine drug screen, blood urea nitrogen, and creatinine to rule out an underlying metabolic or endocrinologic cause for psychosis. Watch for signs of anticholinergic syndrome during physical examination.
If any of the above results suggest a medical problem, test for the following as clinical suspicion warrants:
- serum copper/ceruloplasmin and liver function to rule out Wilson’s disease, a genetic disorder that causes copper to accumulate in the liver and brain
- systemic lupus erythematosus
- lead, magnesium, mercury, or manganese to rule out metal poisoning.
- Cronin AJ. The Citadel. Boston: Little, Brown & Co.;1937:399.
- Asher R. Myxoedamatous madness. BMJ 1949;2:555-62.
- Aripiprazole • Abilify
- Captopril • Capoten
- Clozapine • Clozaril
- Haloperidol • Haldol
- Levothyroxine • Synthroid
- Olanzapine • Zyprexa
- Risperidone • Risperdal
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Tonks CM. Mental illness and hypothyroid patients. Br J Psychiatry 1964;110:706-10.
2. Scheinberg P, et al. Cerebral metabolism and cardiac output in myxedema. J Clin Invest 1950;29:1139-46.
3. Whybrow PC, Prange AJ, Treadway CR. Mental changes accompanying thyroid gland dysfunction. Arch Gen Psychiatry 1969;20:48-63.
4. Heinrich TW, Grahm G. Hypothyroidism presenting as psychosis: myxedema madness revisited. Prim Care Companion J Clin Psychiatry 2003;5:260-6.
5. Jansen HJ, Doebe SR, Louwerse ES, et al. Status epilepticus caused by a myxoedema coma. Neth J Med 2006;64:202-5.
6. Pimental L, Hansen KN. Thyroid disease in the emergency department: a clinical and laboratory review. J Emerg Med 2005;28:201-9.
7. Wartofsky L. Myxedema coma. Endocrinol Metab Clin North Am 2006;35:687-98.
8. Roberts LM, Pattison H, Roalfe A, et al. Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Int Med 2006;145:573-81.
9. Adams CW. Electrocardiographic changes in hypothyroidism. Chest 1964;46:87-8.
10. Stowell CP, Barnhill JW. Acute mania in the setting of severe hypothyroidism. Psychosomatics 2005;46:259-61.
11. Strachan SR, Afolabi O, Brown N, Gray D. Chest pain, enzymes, and hypothyroidism. Postgrad Med J 2000;76:168-9.
12. Lolas F, de la Parra G, Gramegna G. Event-related slow potential (ERSP) correlates of thyroid gland function levels. Psychosom Med 1978;40:226-35.
13. Pinto A, Glick M. Management of patients with thyroid disease: oral health considerations. J Am Dent Assoc 2002;133:849-58.
14. Khedr EM, El Toony LF, Tarkhan MN, Abdella G. Peripheral and central nervous system alterations in hypothyroidism; electrophysiological findings. Neuropsychobiology 2000;41:88-94.
15. Bosch R, Wang Z, Li GR, Nattel S. Electrophysiological mechanisms by which hypothyroidism delays repolarization in guinea pig hearts. Am J Physiol 1999;277(1 Pt 2):H211-20.
16. Schenck JB, Rizvi AA, Lin T. Severe primary hypothyroidism manifesting with torsades de pointes. Am J Med Sci 2006;331:154-6.
17. McGaffee J, Barnes MA, Lippmann S. Psychiatric presentations of hypothyroidism. Am Fam Physicia 1981;23:129-33.
18. Rehman SU, Cope DW, Senseney AD, Brzezinski W. Thyroid disorders in elderly patients. South Med J 2005;98:543-9.
1. Tonks CM. Mental illness and hypothyroid patients. Br J Psychiatry 1964;110:706-10.
2. Scheinberg P, et al. Cerebral metabolism and cardiac output in myxedema. J Clin Invest 1950;29:1139-46.
3. Whybrow PC, Prange AJ, Treadway CR. Mental changes accompanying thyroid gland dysfunction. Arch Gen Psychiatry 1969;20:48-63.
4. Heinrich TW, Grahm G. Hypothyroidism presenting as psychosis: myxedema madness revisited. Prim Care Companion J Clin Psychiatry 2003;5:260-6.
5. Jansen HJ, Doebe SR, Louwerse ES, et al. Status epilepticus caused by a myxoedema coma. Neth J Med 2006;64:202-5.
6. Pimental L, Hansen KN. Thyroid disease in the emergency department: a clinical and laboratory review. J Emerg Med 2005;28:201-9.
7. Wartofsky L. Myxedema coma. Endocrinol Metab Clin North Am 2006;35:687-98.
8. Roberts LM, Pattison H, Roalfe A, et al. Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Int Med 2006;145:573-81.
9. Adams CW. Electrocardiographic changes in hypothyroidism. Chest 1964;46:87-8.
10. Stowell CP, Barnhill JW. Acute mania in the setting of severe hypothyroidism. Psychosomatics 2005;46:259-61.
11. Strachan SR, Afolabi O, Brown N, Gray D. Chest pain, enzymes, and hypothyroidism. Postgrad Med J 2000;76:168-9.
12. Lolas F, de la Parra G, Gramegna G. Event-related slow potential (ERSP) correlates of thyroid gland function levels. Psychosom Med 1978;40:226-35.
13. Pinto A, Glick M. Management of patients with thyroid disease: oral health considerations. J Am Dent Assoc 2002;133:849-58.
14. Khedr EM, El Toony LF, Tarkhan MN, Abdella G. Peripheral and central nervous system alterations in hypothyroidism; electrophysiological findings. Neuropsychobiology 2000;41:88-94.
15. Bosch R, Wang Z, Li GR, Nattel S. Electrophysiological mechanisms by which hypothyroidism delays repolarization in guinea pig hearts. Am J Physiol 1999;277(1 Pt 2):H211-20.
16. Schenck JB, Rizvi AA, Lin T. Severe primary hypothyroidism manifesting with torsades de pointes. Am J Med Sci 2006;331:154-6.
17. McGaffee J, Barnes MA, Lippmann S. Psychiatric presentations of hypothyroidism. Am Fam Physicia 1981;23:129-33.
18. Rehman SU, Cope DW, Senseney AD, Brzezinski W. Thyroid disorders in elderly patients. South Med J 2005;98:543-9.
‘Killer trolls’: One older man’s battle
History: bipolar for 30 years
Mr. B, age 66, was diagnosed 30 years ago with type I bipolar disorder and has type 2 diabetes, hypertension, alcohol abuse disorder, and cardiac disease. After repeated suicide attempts and hospitalizations in the past, he has been stable for 20 years on lithium, 600 mg bid, and nortriptyline, 50 mg at bedtime. He has had intermittent mania with little evidence of depression.
Two years ago, Mr. B called a local clinic to report that an intruder had him “holed up.” His speech was pressured and garbled, and his thoughts were tangential, irrational, and markedly paranoid. A clinic psychiatrist called Mr. B’s son, who said his father “built a bomb shelter” because “trolls and little people” were out to kill him. A family member called police, and Mr. B was brought to the ER and admitted for treatment.
A hospital psychiatrist stopped lithium in light of Mr. B’s history of cardiac problems and because the psychiatrist considered the medication ineffective, even though serum lithium was only 0.03 mEq/L. The psychiatrist then started:
- divalproex at 500 mg bid, titrated over 1 week to 500 mg each morning and 1,000 mg at bed-time to reach serum valproate of 80 mEq/L
- quetiapine at 200 mg at bedtime, titrated over 1 week to 400 mg at bedtime.
Two weeks later, Mr. B is brought to another psychiatric hospital, where a psychiatrist restarts unknown dosages of lithium, risperidone, and nortriptyline. From there, he is transferred to our in-patient unit. At presentation, he claims he has been drinking and that members of a drug cartel have recruited him. He says he has been skipping medications because he is “unclear which drugs to take.”
We stop lithium and restart divalproex, 500 mg each morning and 1,500 mg at bedtime, to try to treat his mania without causing cognitive problems.
We stop risperidone because of his hypotension and nortriptyline because it was not working, and restart quetiapine, 600 mg at bedtime, for his paranoia. He remains paranoid 1 week later but his mania improves, so we discharge him on the above regimen. We urge him to take his medications and follow up with his outpatient psychiatrist 1 week later.
Divorced, Mr. B lives alone with no family nearby. His son comes in from out of town to help him resettle after discharge, then leaves the next day.
Several months later, Mr. B’s paranoia returns. He is not taking his medications because “the doctors took away my lithium and these new drugs don’t work.” He tells staff he is a martial arts expert and has purchased 7 cars in recent weeks. We restart lithium at 600 mg bid; serum lithium reaches 1.1 mEq/L, but his mania persists. After 5 days, we add aripiprazole, 15 mg/d.
Nearly 2 weeks after admission, a county hearing officer recommends discharging Mr. B despite his severe mania and paranoia. We release him on the above regimen, arrange appointments with his outpatient psychiatrist and primary care physician, and urge medication adherence. We schedule a blood test 3 days after discharge to check serum lithium, but Mr. B does not keep the appointment.
The authors’ observations
Suspect delirium after rapid onset of mania or paranoia in any patient. Also consider dementia and cognitive deficits in older adults, although Mr. B’s symptoms resembled those of previous manic episodes. Although Mr. B’s psychosis was more severe than before, his case underscores the importance of a thorough patient history.
Late-life bipolar disorder. Little is known about diagnosing and treating bipolar disorder (BPD) in older patients. Gaps in empiric knowledge can confound diagnosis, treatment, and outcome. Also, patients age ≥65 with BPD often have severe medical illness and are difficult to treat.1
Keys to detecting late-life BPD include:
- recognizing clinical features of BPD unique to older persons
- differentiating the disorder from late-life schizophrenia (Table).1,2
Secondary cause. When an older patient’s mania has atypical features or doesn’t respond to conventional treatment, look for a nonpsychiatric process such as a general medical condition or substance abuse (see possible medical causes with this article at www.currentpsychiatry.com). Order laboratory and other tests as clinical suspicion warrants.
Cognitive deficits secondary to BPD can occur at any age and be persistent or progressive,4 although Depp et al1 found more-severe impairment in older patients. Cognitive impairment can endure after successful BPD treatment, although acute treatment might improve cognition in older patients.5
Lithium can cause dull affect, cognitive slowing, and depersonalization. Titrating to the lowest effective dosage might minimize these effects.
Dementia. Cognitive deficits that accompany mania in older adults could suggest dementia, which usually develops over years and is preceded by cognitive changes without manic-type symptoms. By contrast, bipolar mania emerges more abruptly and is accompanied by affective symptoms. Agitation and psychosis—both symptoms of late-stage dementia—can be early signs of geriatric BPD.2
Delirium. Restlessness, irritability, aggression, and changes in affect can accompany delirium, especially the hyperactive or hyperalert types. Symptoms of anxiety, depression, fear, and loose or tangential thinking also are common.
Mania shares some of these features but typically presents with an abnormally and persistently elevated or irritable mood lasting ≥1 week, usually without prominent cognitive impairment.6 Mania can also include:
- grandiosity
- decreased need for sleep
- flight of ideas
- distractibility
- pressured or increased rate of speech
- psychomotor agitation
- potentially harmful activities
- increased goal-directed activities.6
Frontal lobe lesions. Decreased prefrontal executive control could underlie mania’s cognitive and emotional symptoms. Decreased right rostral and orbital prefrontal cortex activation has been associated with impaired planning, judgment, and insight, as well as inappropriate conduct.7
Table
Clinical features of geriatric bipolar disorder (BPD)
| Psychotic features (delusions, hallucinations) |
|
| Family history |
|
| Compared with younger adults with BPD, older patients: |
|
| Compared with late-life schizophrenia, late-life BPD patients show: |
|
| Source: References 1,2 |
Continued treatment: depression emerges
Several months later, Mr. B presents with severe depression and continued medication nonadherence. He complains of hypersomnia, poor appetite, anhedonia, amotivation, and a leaden-like paresis in his hands and feet.
We readmit Mr. B to the psychiatric unit. He avoids contact with others, has lost 18 lbs over 6 weeks, and suffers hypotension caused by poor hydration before admission. Three weeks later, he complains that ants are crawling around his room and into his mouth.
Noncontrast brain CT shows no abnormalities. Laboratory tests performed at admission show a subtherapeutic lithium level (0.03 mEq/L), unremarkable thyroid panel, and normal B12 and folate, so we begin to rule out a medical cause for his psychiatric symptoms.
The authors’ observations
Check for these and other possible causes of depressive symptoms in older patients with a history of BPD. Mr. B’s depression likely resulted from multiple causes, including medical disease, functional impairment, loss of social and family contacts, and substance abuse—all late-life predictors of depression. BPD also predisposed him to depression.
Bipolar depression. Despite its profound morbidity and mortality, bipolar depression remains a mystery, especially in the elderly. Mr. B’s depression emerged after he was free of depressive symptoms for more than 20 years.
Some researchers believe that compared with other depressions, bipolar depression has a more acute onset, marked psychomotor retardation, and lessened response to antidepressants.6,8 Kraepelin associated bipolar depression with lethargy, mental slowing, and hypersomnia, whereas agitation and insomnia signal unipolar depression.9
To differentiate bipolar from unipolar or secondary depression in older patients, watch for:
- suicide risk, which is heightened during BPD’s depressive phase9
- secondary manias, for which underlying causes must be determined and treated if possible.
Depression caused by medication might be limited to somatic complaints such as fatigue or tiredness,9 and often lacks features seen with mood disorders such as depressed mood, anhedonia, guilt, and diminished interest in activities. Mr. B’s anhedonia and amotivation suggest his depression was not medication-induced.10
Disease-induced depression. Medical comorbidities are common among older persons with mood disorders and can complicate treatment response and outcome. Physical disease can cause or worsen depression:11
- Endocrine and immunologic diseases might cause depression or mania.
- Cardiovascular and cerebrovascular diseases; CNS disorders such as dementia, Parkinson’s disease, and multiple sclerosis; cancer; and connective tissue disease increase risk for comorbid depression.
11
Vascular depression. Comorbid depressive symptoms and vascular disease—or “vascular depression”—can cause ischemic brain lesions, cognitive impairment, increased apathy and retardation, and impaired fluency and naming.12
What defines vascular depression has been debated. Watch for clinical or laboratory evidence of vascular disease, depression, and neuropsychological impairment.13
Treatment: searching for evidence
Two days after admission, Mr. B is transferred to the ICU after suffering severe hypoglycemia and showing signs of medically induced delirium. Elevated creatinine (1.7 mg/dL) indicates acute renal failure, which could be related to his elevated serum lithium (1.7 mEq/L). Acting on the internist’s advice, the consulting psychiatrist stops lithium and restarts valproate, 500 mg bid.
Mr. B becomes medically stable after 3 days, mostly through acute IV hydration and by withholding oral diabetes medications, which normalizes his blood sugar. He is transferred back to the psychiatry unit. We try lithium again at 300 mg bid, but creatinine and serum lithium quickly rise.
Mr. B remains hospitalized for 3 months with severe, treatment-resistant depression. Trials of nearly every second-generation antipsychotic (SGA) cause symptomatic orthostatic hypotension, leading to several falls. He does not respond to divalproex, up to 2,000 mg/d; citalopram, 60 mg/d; mirtazapine, 30 mg at bedtime; venlafaxine, 100 mg tid; or bupropion, 100 mg tid.
We suggest electroconvulsive therapy (ECT) but Mr. B declines, saying this treatment caused his mother to decompensate. We try lamotrigine, 25 mg/d, and titrate it over 6 weeks to 200 mg bid. After we add haloperidol, 5 mg at bedtime, and bupropion, 300 mg/d, Mr. B becomes mentally stable.
The authors’ observations
Numerous clinical challenges—such as managing complicated/refractory BPD, medical comorbidity, and medication adherence (Box)14,15—complicate treatment of late-life BPD.16 Regular communication with providers and integrating health care services can minimize complication risk.16
Pharmacotherapy, a core element of BPD treatment, is challenging in older patients because of their:
- heightened threat of complications and sensitivity to side effects because of age-related pharmacokinetic changes
- increased risk of drug-drug interactions
- increased potential for age-related psychosocial problems (increased social isolation, financial difficulties, demoralization, increased stress, inability to work).
Between 40% and 60% of patients do not take medications as prescribed.14 That percentage probably is higher among cognitively impaired older adults because cognitive problems can compound other causes of nonadherence.
Few published controlled clinical trials have addressed adherence interventions for older adults. Educational approaches combined with cognitive supports are most likely to succeed. Ownby et al15 hypothesized that effective approaches usually employ multiple components including counseling, information reminders, and family therapy.
Techniques for improving adherence include:
- addressing the patient’s beliefs about his or her illness
- exploring how patient characteristics affect medication adherence
- use of memory aids, such as 7-day pill boxes
- working with caregivers
- prescribing lower-than-normal dosages to minimize side effects.
Consider side effects, medical and neurologic comorbidities, and treatment history before prescribing a mood stabilizer, antipsychotic, or antidepressant to an older patient. Avoid unwarranted discontinuation of a previously effective agent, such as when drug concentrations are elevated or inadequate—as happened with Mr. B.5 Also investigate the patient’s side-effect history before stopping a medication.5
Medications. Mr. B’s inability to tolerate lithium posed a treatment challenge. Adjusting lithium dosages to compensate for age-related pharmacokinetic, pharmacodynamic, and renal clearance changes can prevent toxicity.5 Avoid stopping lithium abruptly, as this can trigger recurrence of manic or depressive episodes.17
Lamotrigine, indicated for BPD maintenance therapy, appears to prevent depressive/mood relapse. Compared with other anticonvulsants, lamotrigine might cause fewer negative effects on cognition and less induction of hepatic enzymes. It is well tolerated by older patients but has not been studied adequately in this age group.16
Antipsychotics are widely used in BPD,16 especially when psychosis is present with mania or depression or the patient is agitated. Most studies of antipsychotics in BPD have followed younger adults, however, and most studies in older patients have followed those with dementia or schizophrenia.
Use of first-generation antipsychotics such as haloperidol is especially challenging in the elderly because these drugs increase risk of cardiovascular effects, extrapyramidal symptoms, and tardive dyskinesia and can cause depression in BPD.16 By comparison, SGAs carry a lower risk of involuntary motion18 but can increase risk of obesity, diabetes, and dyslipidemia. However:
- The need to manage psychosis usually overrides concerns about metabolic sequelae.
- Older patients might be less susceptible to metabolic effects,16 though this has not been confirmed.
SGAs can be used safely in patients with a history of diabetes. Start at lower-than-normal dosages and titrate slowly. Perform baseline and regular checks—including weight, blood glucose, lipid levels, and blood pressure—following American Psychiatric Association and American Diabetes Association consensus guidelines.19 Also check glycosylated hemoglobin every 3 to 6 months in patients with diabetes, and follow up with other providers to ensure proper diabetes management.
As with most aspects of late-life BPD, scant evidence guides SGA use. Avoid low-potency neuroleptics such as chlorpromazine, which can cause severe sedation and orthostatic hypotension. For Mr. B, a more-tolerable SGA such as aripiprazole or ziprasidone might be prudent, given his propensity for orthostatic hypotension and history of diabetes. Olanzapine or clozapine can cause anticholinergic effects and—in Mr. B’s case—lead to weight gain and worsen diabetes.
Antidepressant use in BPD usually is reserved for depressive symptoms that impair occupational or social functioning and exceed DSM-IV-TR diagnostic criteria.8,9 Consider later-generation antidepressants such as selective serotonin reuptake inhibitors (SSRIs), because tricyclics pose a greater risk of triggering a switch into hypomania or mania and can cause sedation and orthostatic, cardiac, anticholinergic, and anti-alpha 1 effects.
Among SSRIs, consider citalopram, escitalopram, or sertraline for older patients taking one or more other medications, as these antidepressants have less potential for drug-drug interactions than fluoxetine and paroxetine.11 In a recent comparison of newer antidepressants,20 venlafaxine showed the highest relative risk of mood polarity switching and bupropion the lowest.
Consider ECT for older patients with refractory mania or depression or who show evidence of suicidality or inadequate nutrition.5
Follow-up: ongoing issues
Three months after his admission, we discharge Mr. B to a board-and-care facility because family members will not take him in. Several weeks later, he again ignores his prescriptions and decompensates with worsening depression.
Family members have Mr. B admitted to an inpatient psychiatric facility closer to their home. He remains depressed, stays at the facility on and off for almost 1 year, and is eventually conserved by the county. Adverse side effects—mostly constipation and orthostatic hypotension—continue to complicate treatment.
Before Mr. B’s most recent discharge, another psychiatrist restarts lithium, 300 mg bid, and nortriptyline, 100 mg at bedtime—the combination that kept Mr. B relatively stable for more than 2 decades.
- National Institute of Mental Health—depression information. www.nimh.nih.gov/healthinformation/depressionmenu.cfm.
- Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). www.stepbd.org.
- Medicinenet.com. Medicines that cause depression. www.medicinenet.com/depression/index.htm. Click on “Medicines that cause depression.”
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Divalproex • Depakote
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lithium • Various
- Mirtazapine • Remeron
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Venlafaxine • Effexor
- Ziprasidone • Geodon
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Depp C, Jeste D. Bipolar disorder in older adults: a critical review. Bipolar Disord 2004;6:343-67.
2. Sajatovic M, Blow FC, Ignacio RV, Kales HC. New-onset bipolar disorder in later life. Am J Geriatr Psychiatry 2005;13:282-9.
3. Arciniegas DB. New-onset bipolar disorder in late life: a case of mistaken identity. Am J Psychiatry 2006;163:198-203.
4. Young RC. Bipolar disorder in older persons: perspectives and new findings. Am J Geriatr Psychiatry 2005;13:265-7.
5. Young RC. Evidence-based pharmacological treatment of geriatric bipolar disorder. Psychiatr Clin North Am 2005;28:837-69.
6. Diagnostic and statistical manual of mental disorders, 4 ed, text rev. Washington, DC; American Psychiatric Association; 2000.
7. Blumberg HP, Stern E, Ricketts S, et al. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry 1999;156:1986-8.
8. Gitlin M. Treatment-resistant bipolar disorder. Mol Psychiatry 2006;11:227-40.
9. Dubovsky SL. Treatment of bipolar depression. Psychiatr Clin N Am 2005;28:349-70.
10. Kroenke K. A 75-year-old man with depression. JAMA 2002;287:1568-76.
11. Shanmugham B, Karp J, Drayer R, et al. Evidence-based pharmacological interventions for geriatric depression. Psychiatr Clin N Am 2005;28:821-35.
12. Alexopoulos GS. In: Sadavoy J, Jarvik LF, Grossberg GT, Meyers BS, eds. Comprehensive textbook of geriatric psychiatry, 3 ed. New York: WW Norton and Co.; 2002:609-53.
13. Sneed JR, Roose SP, Sackeim HA. Vascular depression: a distinct diagnostic subtype? Biol Psychiatry 2006;60:1295-8.
14. Higgins N, Regan C. A systematic review of the effectiveness of interventions to help older people adhere to medication regimes. Age Ageing 2004;33:224-9.
15. Ownby RL, Hertzog C, Crocco E, Duara R. Factors related to medication adherence in memory disorder clinic patients. Aging & Mental Health 2006;10:378-85.
16. Sajatovic M, Madhusoodanan S, Coconcea N. Managing bipolar disorder in the elderly: defining the role of the newer agents. Drugs Aging 2005;22:39-54.
17. Cavanagh J, Smyth R, Goodwin GM. Relapse into mania or depression following lithium discontinuation: a 7-year follow up. Acta Psychiatr Scand 2004;109:91-5.
18. Dunner DL. Atypical antipsychotics: efficacy across bipolar disorder subpopulations. J Clin Psychiatry 2005;66(suppl 3):20-7.
19. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27:596-601.
20. Leverich GS, Altshuler LL, Frye MA, et al. Risk of switch in mood polarity to hypomania or mania in patient with bipolar depression during acute and continuation trials of venlafaxine, sertraline, and bupropion as adjuncts to mood stabilizers. Am J Psychiatry 2006;163:232-9.
History: bipolar for 30 years
Mr. B, age 66, was diagnosed 30 years ago with type I bipolar disorder and has type 2 diabetes, hypertension, alcohol abuse disorder, and cardiac disease. After repeated suicide attempts and hospitalizations in the past, he has been stable for 20 years on lithium, 600 mg bid, and nortriptyline, 50 mg at bedtime. He has had intermittent mania with little evidence of depression.
Two years ago, Mr. B called a local clinic to report that an intruder had him “holed up.” His speech was pressured and garbled, and his thoughts were tangential, irrational, and markedly paranoid. A clinic psychiatrist called Mr. B’s son, who said his father “built a bomb shelter” because “trolls and little people” were out to kill him. A family member called police, and Mr. B was brought to the ER and admitted for treatment.
A hospital psychiatrist stopped lithium in light of Mr. B’s history of cardiac problems and because the psychiatrist considered the medication ineffective, even though serum lithium was only 0.03 mEq/L. The psychiatrist then started:
- divalproex at 500 mg bid, titrated over 1 week to 500 mg each morning and 1,000 mg at bed-time to reach serum valproate of 80 mEq/L
- quetiapine at 200 mg at bedtime, titrated over 1 week to 400 mg at bedtime.
Two weeks later, Mr. B is brought to another psychiatric hospital, where a psychiatrist restarts unknown dosages of lithium, risperidone, and nortriptyline. From there, he is transferred to our in-patient unit. At presentation, he claims he has been drinking and that members of a drug cartel have recruited him. He says he has been skipping medications because he is “unclear which drugs to take.”
We stop lithium and restart divalproex, 500 mg each morning and 1,500 mg at bedtime, to try to treat his mania without causing cognitive problems.
We stop risperidone because of his hypotension and nortriptyline because it was not working, and restart quetiapine, 600 mg at bedtime, for his paranoia. He remains paranoid 1 week later but his mania improves, so we discharge him on the above regimen. We urge him to take his medications and follow up with his outpatient psychiatrist 1 week later.
Divorced, Mr. B lives alone with no family nearby. His son comes in from out of town to help him resettle after discharge, then leaves the next day.
Several months later, Mr. B’s paranoia returns. He is not taking his medications because “the doctors took away my lithium and these new drugs don’t work.” He tells staff he is a martial arts expert and has purchased 7 cars in recent weeks. We restart lithium at 600 mg bid; serum lithium reaches 1.1 mEq/L, but his mania persists. After 5 days, we add aripiprazole, 15 mg/d.
Nearly 2 weeks after admission, a county hearing officer recommends discharging Mr. B despite his severe mania and paranoia. We release him on the above regimen, arrange appointments with his outpatient psychiatrist and primary care physician, and urge medication adherence. We schedule a blood test 3 days after discharge to check serum lithium, but Mr. B does not keep the appointment.
The authors’ observations
Suspect delirium after rapid onset of mania or paranoia in any patient. Also consider dementia and cognitive deficits in older adults, although Mr. B’s symptoms resembled those of previous manic episodes. Although Mr. B’s psychosis was more severe than before, his case underscores the importance of a thorough patient history.
Late-life bipolar disorder. Little is known about diagnosing and treating bipolar disorder (BPD) in older patients. Gaps in empiric knowledge can confound diagnosis, treatment, and outcome. Also, patients age ≥65 with BPD often have severe medical illness and are difficult to treat.1
Keys to detecting late-life BPD include:
- recognizing clinical features of BPD unique to older persons
- differentiating the disorder from late-life schizophrenia (Table).1,2
Secondary cause. When an older patient’s mania has atypical features or doesn’t respond to conventional treatment, look for a nonpsychiatric process such as a general medical condition or substance abuse (see possible medical causes with this article at www.currentpsychiatry.com). Order laboratory and other tests as clinical suspicion warrants.
Cognitive deficits secondary to BPD can occur at any age and be persistent or progressive,4 although Depp et al1 found more-severe impairment in older patients. Cognitive impairment can endure after successful BPD treatment, although acute treatment might improve cognition in older patients.5
Lithium can cause dull affect, cognitive slowing, and depersonalization. Titrating to the lowest effective dosage might minimize these effects.
Dementia. Cognitive deficits that accompany mania in older adults could suggest dementia, which usually develops over years and is preceded by cognitive changes without manic-type symptoms. By contrast, bipolar mania emerges more abruptly and is accompanied by affective symptoms. Agitation and psychosis—both symptoms of late-stage dementia—can be early signs of geriatric BPD.2
Delirium. Restlessness, irritability, aggression, and changes in affect can accompany delirium, especially the hyperactive or hyperalert types. Symptoms of anxiety, depression, fear, and loose or tangential thinking also are common.
Mania shares some of these features but typically presents with an abnormally and persistently elevated or irritable mood lasting ≥1 week, usually without prominent cognitive impairment.6 Mania can also include:
- grandiosity
- decreased need for sleep
- flight of ideas
- distractibility
- pressured or increased rate of speech
- psychomotor agitation
- potentially harmful activities
- increased goal-directed activities.6
Frontal lobe lesions. Decreased prefrontal executive control could underlie mania’s cognitive and emotional symptoms. Decreased right rostral and orbital prefrontal cortex activation has been associated with impaired planning, judgment, and insight, as well as inappropriate conduct.7
Table
Clinical features of geriatric bipolar disorder (BPD)
| Psychotic features (delusions, hallucinations) |
|
| Family history |
|
| Compared with younger adults with BPD, older patients: |
|
| Compared with late-life schizophrenia, late-life BPD patients show: |
|
| Source: References 1,2 |
Continued treatment: depression emerges
Several months later, Mr. B presents with severe depression and continued medication nonadherence. He complains of hypersomnia, poor appetite, anhedonia, amotivation, and a leaden-like paresis in his hands and feet.
We readmit Mr. B to the psychiatric unit. He avoids contact with others, has lost 18 lbs over 6 weeks, and suffers hypotension caused by poor hydration before admission. Three weeks later, he complains that ants are crawling around his room and into his mouth.
Noncontrast brain CT shows no abnormalities. Laboratory tests performed at admission show a subtherapeutic lithium level (0.03 mEq/L), unremarkable thyroid panel, and normal B12 and folate, so we begin to rule out a medical cause for his psychiatric symptoms.
The authors’ observations
Check for these and other possible causes of depressive symptoms in older patients with a history of BPD. Mr. B’s depression likely resulted from multiple causes, including medical disease, functional impairment, loss of social and family contacts, and substance abuse—all late-life predictors of depression. BPD also predisposed him to depression.
Bipolar depression. Despite its profound morbidity and mortality, bipolar depression remains a mystery, especially in the elderly. Mr. B’s depression emerged after he was free of depressive symptoms for more than 20 years.
Some researchers believe that compared with other depressions, bipolar depression has a more acute onset, marked psychomotor retardation, and lessened response to antidepressants.6,8 Kraepelin associated bipolar depression with lethargy, mental slowing, and hypersomnia, whereas agitation and insomnia signal unipolar depression.9
To differentiate bipolar from unipolar or secondary depression in older patients, watch for:
- suicide risk, which is heightened during BPD’s depressive phase9
- secondary manias, for which underlying causes must be determined and treated if possible.
Depression caused by medication might be limited to somatic complaints such as fatigue or tiredness,9 and often lacks features seen with mood disorders such as depressed mood, anhedonia, guilt, and diminished interest in activities. Mr. B’s anhedonia and amotivation suggest his depression was not medication-induced.10
Disease-induced depression. Medical comorbidities are common among older persons with mood disorders and can complicate treatment response and outcome. Physical disease can cause or worsen depression:11
- Endocrine and immunologic diseases might cause depression or mania.
- Cardiovascular and cerebrovascular diseases; CNS disorders such as dementia, Parkinson’s disease, and multiple sclerosis; cancer; and connective tissue disease increase risk for comorbid depression.
11
Vascular depression. Comorbid depressive symptoms and vascular disease—or “vascular depression”—can cause ischemic brain lesions, cognitive impairment, increased apathy and retardation, and impaired fluency and naming.12
What defines vascular depression has been debated. Watch for clinical or laboratory evidence of vascular disease, depression, and neuropsychological impairment.13
Treatment: searching for evidence
Two days after admission, Mr. B is transferred to the ICU after suffering severe hypoglycemia and showing signs of medically induced delirium. Elevated creatinine (1.7 mg/dL) indicates acute renal failure, which could be related to his elevated serum lithium (1.7 mEq/L). Acting on the internist’s advice, the consulting psychiatrist stops lithium and restarts valproate, 500 mg bid.
Mr. B becomes medically stable after 3 days, mostly through acute IV hydration and by withholding oral diabetes medications, which normalizes his blood sugar. He is transferred back to the psychiatry unit. We try lithium again at 300 mg bid, but creatinine and serum lithium quickly rise.
Mr. B remains hospitalized for 3 months with severe, treatment-resistant depression. Trials of nearly every second-generation antipsychotic (SGA) cause symptomatic orthostatic hypotension, leading to several falls. He does not respond to divalproex, up to 2,000 mg/d; citalopram, 60 mg/d; mirtazapine, 30 mg at bedtime; venlafaxine, 100 mg tid; or bupropion, 100 mg tid.
We suggest electroconvulsive therapy (ECT) but Mr. B declines, saying this treatment caused his mother to decompensate. We try lamotrigine, 25 mg/d, and titrate it over 6 weeks to 200 mg bid. After we add haloperidol, 5 mg at bedtime, and bupropion, 300 mg/d, Mr. B becomes mentally stable.
The authors’ observations
Numerous clinical challenges—such as managing complicated/refractory BPD, medical comorbidity, and medication adherence (Box)14,15—complicate treatment of late-life BPD.16 Regular communication with providers and integrating health care services can minimize complication risk.16
Pharmacotherapy, a core element of BPD treatment, is challenging in older patients because of their:
- heightened threat of complications and sensitivity to side effects because of age-related pharmacokinetic changes
- increased risk of drug-drug interactions
- increased potential for age-related psychosocial problems (increased social isolation, financial difficulties, demoralization, increased stress, inability to work).
Between 40% and 60% of patients do not take medications as prescribed.14 That percentage probably is higher among cognitively impaired older adults because cognitive problems can compound other causes of nonadherence.
Few published controlled clinical trials have addressed adherence interventions for older adults. Educational approaches combined with cognitive supports are most likely to succeed. Ownby et al15 hypothesized that effective approaches usually employ multiple components including counseling, information reminders, and family therapy.
Techniques for improving adherence include:
- addressing the patient’s beliefs about his or her illness
- exploring how patient characteristics affect medication adherence
- use of memory aids, such as 7-day pill boxes
- working with caregivers
- prescribing lower-than-normal dosages to minimize side effects.
Consider side effects, medical and neurologic comorbidities, and treatment history before prescribing a mood stabilizer, antipsychotic, or antidepressant to an older patient. Avoid unwarranted discontinuation of a previously effective agent, such as when drug concentrations are elevated or inadequate—as happened with Mr. B.5 Also investigate the patient’s side-effect history before stopping a medication.5
Medications. Mr. B’s inability to tolerate lithium posed a treatment challenge. Adjusting lithium dosages to compensate for age-related pharmacokinetic, pharmacodynamic, and renal clearance changes can prevent toxicity.5 Avoid stopping lithium abruptly, as this can trigger recurrence of manic or depressive episodes.17
Lamotrigine, indicated for BPD maintenance therapy, appears to prevent depressive/mood relapse. Compared with other anticonvulsants, lamotrigine might cause fewer negative effects on cognition and less induction of hepatic enzymes. It is well tolerated by older patients but has not been studied adequately in this age group.16
Antipsychotics are widely used in BPD,16 especially when psychosis is present with mania or depression or the patient is agitated. Most studies of antipsychotics in BPD have followed younger adults, however, and most studies in older patients have followed those with dementia or schizophrenia.
Use of first-generation antipsychotics such as haloperidol is especially challenging in the elderly because these drugs increase risk of cardiovascular effects, extrapyramidal symptoms, and tardive dyskinesia and can cause depression in BPD.16 By comparison, SGAs carry a lower risk of involuntary motion18 but can increase risk of obesity, diabetes, and dyslipidemia. However:
- The need to manage psychosis usually overrides concerns about metabolic sequelae.
- Older patients might be less susceptible to metabolic effects,16 though this has not been confirmed.
SGAs can be used safely in patients with a history of diabetes. Start at lower-than-normal dosages and titrate slowly. Perform baseline and regular checks—including weight, blood glucose, lipid levels, and blood pressure—following American Psychiatric Association and American Diabetes Association consensus guidelines.19 Also check glycosylated hemoglobin every 3 to 6 months in patients with diabetes, and follow up with other providers to ensure proper diabetes management.
As with most aspects of late-life BPD, scant evidence guides SGA use. Avoid low-potency neuroleptics such as chlorpromazine, which can cause severe sedation and orthostatic hypotension. For Mr. B, a more-tolerable SGA such as aripiprazole or ziprasidone might be prudent, given his propensity for orthostatic hypotension and history of diabetes. Olanzapine or clozapine can cause anticholinergic effects and—in Mr. B’s case—lead to weight gain and worsen diabetes.
Antidepressant use in BPD usually is reserved for depressive symptoms that impair occupational or social functioning and exceed DSM-IV-TR diagnostic criteria.8,9 Consider later-generation antidepressants such as selective serotonin reuptake inhibitors (SSRIs), because tricyclics pose a greater risk of triggering a switch into hypomania or mania and can cause sedation and orthostatic, cardiac, anticholinergic, and anti-alpha 1 effects.
Among SSRIs, consider citalopram, escitalopram, or sertraline for older patients taking one or more other medications, as these antidepressants have less potential for drug-drug interactions than fluoxetine and paroxetine.11 In a recent comparison of newer antidepressants,20 venlafaxine showed the highest relative risk of mood polarity switching and bupropion the lowest.
Consider ECT for older patients with refractory mania or depression or who show evidence of suicidality or inadequate nutrition.5
Follow-up: ongoing issues
Three months after his admission, we discharge Mr. B to a board-and-care facility because family members will not take him in. Several weeks later, he again ignores his prescriptions and decompensates with worsening depression.
Family members have Mr. B admitted to an inpatient psychiatric facility closer to their home. He remains depressed, stays at the facility on and off for almost 1 year, and is eventually conserved by the county. Adverse side effects—mostly constipation and orthostatic hypotension—continue to complicate treatment.
Before Mr. B’s most recent discharge, another psychiatrist restarts lithium, 300 mg bid, and nortriptyline, 100 mg at bedtime—the combination that kept Mr. B relatively stable for more than 2 decades.
- National Institute of Mental Health—depression information. www.nimh.nih.gov/healthinformation/depressionmenu.cfm.
- Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). www.stepbd.org.
- Medicinenet.com. Medicines that cause depression. www.medicinenet.com/depression/index.htm. Click on “Medicines that cause depression.”
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Divalproex • Depakote
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lithium • Various
- Mirtazapine • Remeron
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Venlafaxine • Effexor
- Ziprasidone • Geodon
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
History: bipolar for 30 years
Mr. B, age 66, was diagnosed 30 years ago with type I bipolar disorder and has type 2 diabetes, hypertension, alcohol abuse disorder, and cardiac disease. After repeated suicide attempts and hospitalizations in the past, he has been stable for 20 years on lithium, 600 mg bid, and nortriptyline, 50 mg at bedtime. He has had intermittent mania with little evidence of depression.
Two years ago, Mr. B called a local clinic to report that an intruder had him “holed up.” His speech was pressured and garbled, and his thoughts were tangential, irrational, and markedly paranoid. A clinic psychiatrist called Mr. B’s son, who said his father “built a bomb shelter” because “trolls and little people” were out to kill him. A family member called police, and Mr. B was brought to the ER and admitted for treatment.
A hospital psychiatrist stopped lithium in light of Mr. B’s history of cardiac problems and because the psychiatrist considered the medication ineffective, even though serum lithium was only 0.03 mEq/L. The psychiatrist then started:
- divalproex at 500 mg bid, titrated over 1 week to 500 mg each morning and 1,000 mg at bed-time to reach serum valproate of 80 mEq/L
- quetiapine at 200 mg at bedtime, titrated over 1 week to 400 mg at bedtime.
Two weeks later, Mr. B is brought to another psychiatric hospital, where a psychiatrist restarts unknown dosages of lithium, risperidone, and nortriptyline. From there, he is transferred to our in-patient unit. At presentation, he claims he has been drinking and that members of a drug cartel have recruited him. He says he has been skipping medications because he is “unclear which drugs to take.”
We stop lithium and restart divalproex, 500 mg each morning and 1,500 mg at bedtime, to try to treat his mania without causing cognitive problems.
We stop risperidone because of his hypotension and nortriptyline because it was not working, and restart quetiapine, 600 mg at bedtime, for his paranoia. He remains paranoid 1 week later but his mania improves, so we discharge him on the above regimen. We urge him to take his medications and follow up with his outpatient psychiatrist 1 week later.
Divorced, Mr. B lives alone with no family nearby. His son comes in from out of town to help him resettle after discharge, then leaves the next day.
Several months later, Mr. B’s paranoia returns. He is not taking his medications because “the doctors took away my lithium and these new drugs don’t work.” He tells staff he is a martial arts expert and has purchased 7 cars in recent weeks. We restart lithium at 600 mg bid; serum lithium reaches 1.1 mEq/L, but his mania persists. After 5 days, we add aripiprazole, 15 mg/d.
Nearly 2 weeks after admission, a county hearing officer recommends discharging Mr. B despite his severe mania and paranoia. We release him on the above regimen, arrange appointments with his outpatient psychiatrist and primary care physician, and urge medication adherence. We schedule a blood test 3 days after discharge to check serum lithium, but Mr. B does not keep the appointment.
The authors’ observations
Suspect delirium after rapid onset of mania or paranoia in any patient. Also consider dementia and cognitive deficits in older adults, although Mr. B’s symptoms resembled those of previous manic episodes. Although Mr. B’s psychosis was more severe than before, his case underscores the importance of a thorough patient history.
Late-life bipolar disorder. Little is known about diagnosing and treating bipolar disorder (BPD) in older patients. Gaps in empiric knowledge can confound diagnosis, treatment, and outcome. Also, patients age ≥65 with BPD often have severe medical illness and are difficult to treat.1
Keys to detecting late-life BPD include:
- recognizing clinical features of BPD unique to older persons
- differentiating the disorder from late-life schizophrenia (Table).1,2
Secondary cause. When an older patient’s mania has atypical features or doesn’t respond to conventional treatment, look for a nonpsychiatric process such as a general medical condition or substance abuse (see possible medical causes with this article at www.currentpsychiatry.com). Order laboratory and other tests as clinical suspicion warrants.
Cognitive deficits secondary to BPD can occur at any age and be persistent or progressive,4 although Depp et al1 found more-severe impairment in older patients. Cognitive impairment can endure after successful BPD treatment, although acute treatment might improve cognition in older patients.5
Lithium can cause dull affect, cognitive slowing, and depersonalization. Titrating to the lowest effective dosage might minimize these effects.
Dementia. Cognitive deficits that accompany mania in older adults could suggest dementia, which usually develops over years and is preceded by cognitive changes without manic-type symptoms. By contrast, bipolar mania emerges more abruptly and is accompanied by affective symptoms. Agitation and psychosis—both symptoms of late-stage dementia—can be early signs of geriatric BPD.2
Delirium. Restlessness, irritability, aggression, and changes in affect can accompany delirium, especially the hyperactive or hyperalert types. Symptoms of anxiety, depression, fear, and loose or tangential thinking also are common.
Mania shares some of these features but typically presents with an abnormally and persistently elevated or irritable mood lasting ≥1 week, usually without prominent cognitive impairment.6 Mania can also include:
- grandiosity
- decreased need for sleep
- flight of ideas
- distractibility
- pressured or increased rate of speech
- psychomotor agitation
- potentially harmful activities
- increased goal-directed activities.6
Frontal lobe lesions. Decreased prefrontal executive control could underlie mania’s cognitive and emotional symptoms. Decreased right rostral and orbital prefrontal cortex activation has been associated with impaired planning, judgment, and insight, as well as inappropriate conduct.7
Table
Clinical features of geriatric bipolar disorder (BPD)
| Psychotic features (delusions, hallucinations) |
|
| Family history |
|
| Compared with younger adults with BPD, older patients: |
|
| Compared with late-life schizophrenia, late-life BPD patients show: |
|
| Source: References 1,2 |
Continued treatment: depression emerges
Several months later, Mr. B presents with severe depression and continued medication nonadherence. He complains of hypersomnia, poor appetite, anhedonia, amotivation, and a leaden-like paresis in his hands and feet.
We readmit Mr. B to the psychiatric unit. He avoids contact with others, has lost 18 lbs over 6 weeks, and suffers hypotension caused by poor hydration before admission. Three weeks later, he complains that ants are crawling around his room and into his mouth.
Noncontrast brain CT shows no abnormalities. Laboratory tests performed at admission show a subtherapeutic lithium level (0.03 mEq/L), unremarkable thyroid panel, and normal B12 and folate, so we begin to rule out a medical cause for his psychiatric symptoms.
The authors’ observations
Check for these and other possible causes of depressive symptoms in older patients with a history of BPD. Mr. B’s depression likely resulted from multiple causes, including medical disease, functional impairment, loss of social and family contacts, and substance abuse—all late-life predictors of depression. BPD also predisposed him to depression.
Bipolar depression. Despite its profound morbidity and mortality, bipolar depression remains a mystery, especially in the elderly. Mr. B’s depression emerged after he was free of depressive symptoms for more than 20 years.
Some researchers believe that compared with other depressions, bipolar depression has a more acute onset, marked psychomotor retardation, and lessened response to antidepressants.6,8 Kraepelin associated bipolar depression with lethargy, mental slowing, and hypersomnia, whereas agitation and insomnia signal unipolar depression.9
To differentiate bipolar from unipolar or secondary depression in older patients, watch for:
- suicide risk, which is heightened during BPD’s depressive phase9
- secondary manias, for which underlying causes must be determined and treated if possible.
Depression caused by medication might be limited to somatic complaints such as fatigue or tiredness,9 and often lacks features seen with mood disorders such as depressed mood, anhedonia, guilt, and diminished interest in activities. Mr. B’s anhedonia and amotivation suggest his depression was not medication-induced.10
Disease-induced depression. Medical comorbidities are common among older persons with mood disorders and can complicate treatment response and outcome. Physical disease can cause or worsen depression:11
- Endocrine and immunologic diseases might cause depression or mania.
- Cardiovascular and cerebrovascular diseases; CNS disorders such as dementia, Parkinson’s disease, and multiple sclerosis; cancer; and connective tissue disease increase risk for comorbid depression.
11
Vascular depression. Comorbid depressive symptoms and vascular disease—or “vascular depression”—can cause ischemic brain lesions, cognitive impairment, increased apathy and retardation, and impaired fluency and naming.12
What defines vascular depression has been debated. Watch for clinical or laboratory evidence of vascular disease, depression, and neuropsychological impairment.13
Treatment: searching for evidence
Two days after admission, Mr. B is transferred to the ICU after suffering severe hypoglycemia and showing signs of medically induced delirium. Elevated creatinine (1.7 mg/dL) indicates acute renal failure, which could be related to his elevated serum lithium (1.7 mEq/L). Acting on the internist’s advice, the consulting psychiatrist stops lithium and restarts valproate, 500 mg bid.
Mr. B becomes medically stable after 3 days, mostly through acute IV hydration and by withholding oral diabetes medications, which normalizes his blood sugar. He is transferred back to the psychiatry unit. We try lithium again at 300 mg bid, but creatinine and serum lithium quickly rise.
Mr. B remains hospitalized for 3 months with severe, treatment-resistant depression. Trials of nearly every second-generation antipsychotic (SGA) cause symptomatic orthostatic hypotension, leading to several falls. He does not respond to divalproex, up to 2,000 mg/d; citalopram, 60 mg/d; mirtazapine, 30 mg at bedtime; venlafaxine, 100 mg tid; or bupropion, 100 mg tid.
We suggest electroconvulsive therapy (ECT) but Mr. B declines, saying this treatment caused his mother to decompensate. We try lamotrigine, 25 mg/d, and titrate it over 6 weeks to 200 mg bid. After we add haloperidol, 5 mg at bedtime, and bupropion, 300 mg/d, Mr. B becomes mentally stable.
The authors’ observations
Numerous clinical challenges—such as managing complicated/refractory BPD, medical comorbidity, and medication adherence (Box)14,15—complicate treatment of late-life BPD.16 Regular communication with providers and integrating health care services can minimize complication risk.16
Pharmacotherapy, a core element of BPD treatment, is challenging in older patients because of their:
- heightened threat of complications and sensitivity to side effects because of age-related pharmacokinetic changes
- increased risk of drug-drug interactions
- increased potential for age-related psychosocial problems (increased social isolation, financial difficulties, demoralization, increased stress, inability to work).
Between 40% and 60% of patients do not take medications as prescribed.14 That percentage probably is higher among cognitively impaired older adults because cognitive problems can compound other causes of nonadherence.
Few published controlled clinical trials have addressed adherence interventions for older adults. Educational approaches combined with cognitive supports are most likely to succeed. Ownby et al15 hypothesized that effective approaches usually employ multiple components including counseling, information reminders, and family therapy.
Techniques for improving adherence include:
- addressing the patient’s beliefs about his or her illness
- exploring how patient characteristics affect medication adherence
- use of memory aids, such as 7-day pill boxes
- working with caregivers
- prescribing lower-than-normal dosages to minimize side effects.
Consider side effects, medical and neurologic comorbidities, and treatment history before prescribing a mood stabilizer, antipsychotic, or antidepressant to an older patient. Avoid unwarranted discontinuation of a previously effective agent, such as when drug concentrations are elevated or inadequate—as happened with Mr. B.5 Also investigate the patient’s side-effect history before stopping a medication.5
Medications. Mr. B’s inability to tolerate lithium posed a treatment challenge. Adjusting lithium dosages to compensate for age-related pharmacokinetic, pharmacodynamic, and renal clearance changes can prevent toxicity.5 Avoid stopping lithium abruptly, as this can trigger recurrence of manic or depressive episodes.17
Lamotrigine, indicated for BPD maintenance therapy, appears to prevent depressive/mood relapse. Compared with other anticonvulsants, lamotrigine might cause fewer negative effects on cognition and less induction of hepatic enzymes. It is well tolerated by older patients but has not been studied adequately in this age group.16
Antipsychotics are widely used in BPD,16 especially when psychosis is present with mania or depression or the patient is agitated. Most studies of antipsychotics in BPD have followed younger adults, however, and most studies in older patients have followed those with dementia or schizophrenia.
Use of first-generation antipsychotics such as haloperidol is especially challenging in the elderly because these drugs increase risk of cardiovascular effects, extrapyramidal symptoms, and tardive dyskinesia and can cause depression in BPD.16 By comparison, SGAs carry a lower risk of involuntary motion18 but can increase risk of obesity, diabetes, and dyslipidemia. However:
- The need to manage psychosis usually overrides concerns about metabolic sequelae.
- Older patients might be less susceptible to metabolic effects,16 though this has not been confirmed.
SGAs can be used safely in patients with a history of diabetes. Start at lower-than-normal dosages and titrate slowly. Perform baseline and regular checks—including weight, blood glucose, lipid levels, and blood pressure—following American Psychiatric Association and American Diabetes Association consensus guidelines.19 Also check glycosylated hemoglobin every 3 to 6 months in patients with diabetes, and follow up with other providers to ensure proper diabetes management.
As with most aspects of late-life BPD, scant evidence guides SGA use. Avoid low-potency neuroleptics such as chlorpromazine, which can cause severe sedation and orthostatic hypotension. For Mr. B, a more-tolerable SGA such as aripiprazole or ziprasidone might be prudent, given his propensity for orthostatic hypotension and history of diabetes. Olanzapine or clozapine can cause anticholinergic effects and—in Mr. B’s case—lead to weight gain and worsen diabetes.
Antidepressant use in BPD usually is reserved for depressive symptoms that impair occupational or social functioning and exceed DSM-IV-TR diagnostic criteria.8,9 Consider later-generation antidepressants such as selective serotonin reuptake inhibitors (SSRIs), because tricyclics pose a greater risk of triggering a switch into hypomania or mania and can cause sedation and orthostatic, cardiac, anticholinergic, and anti-alpha 1 effects.
Among SSRIs, consider citalopram, escitalopram, or sertraline for older patients taking one or more other medications, as these antidepressants have less potential for drug-drug interactions than fluoxetine and paroxetine.11 In a recent comparison of newer antidepressants,20 venlafaxine showed the highest relative risk of mood polarity switching and bupropion the lowest.
Consider ECT for older patients with refractory mania or depression or who show evidence of suicidality or inadequate nutrition.5
Follow-up: ongoing issues
Three months after his admission, we discharge Mr. B to a board-and-care facility because family members will not take him in. Several weeks later, he again ignores his prescriptions and decompensates with worsening depression.
Family members have Mr. B admitted to an inpatient psychiatric facility closer to their home. He remains depressed, stays at the facility on and off for almost 1 year, and is eventually conserved by the county. Adverse side effects—mostly constipation and orthostatic hypotension—continue to complicate treatment.
Before Mr. B’s most recent discharge, another psychiatrist restarts lithium, 300 mg bid, and nortriptyline, 100 mg at bedtime—the combination that kept Mr. B relatively stable for more than 2 decades.
- National Institute of Mental Health—depression information. www.nimh.nih.gov/healthinformation/depressionmenu.cfm.
- Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). www.stepbd.org.
- Medicinenet.com. Medicines that cause depression. www.medicinenet.com/depression/index.htm. Click on “Medicines that cause depression.”
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Divalproex • Depakote
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lithium • Various
- Mirtazapine • Remeron
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Venlafaxine • Effexor
- Ziprasidone • Geodon
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Depp C, Jeste D. Bipolar disorder in older adults: a critical review. Bipolar Disord 2004;6:343-67.
2. Sajatovic M, Blow FC, Ignacio RV, Kales HC. New-onset bipolar disorder in later life. Am J Geriatr Psychiatry 2005;13:282-9.
3. Arciniegas DB. New-onset bipolar disorder in late life: a case of mistaken identity. Am J Psychiatry 2006;163:198-203.
4. Young RC. Bipolar disorder in older persons: perspectives and new findings. Am J Geriatr Psychiatry 2005;13:265-7.
5. Young RC. Evidence-based pharmacological treatment of geriatric bipolar disorder. Psychiatr Clin North Am 2005;28:837-69.
6. Diagnostic and statistical manual of mental disorders, 4 ed, text rev. Washington, DC; American Psychiatric Association; 2000.
7. Blumberg HP, Stern E, Ricketts S, et al. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry 1999;156:1986-8.
8. Gitlin M. Treatment-resistant bipolar disorder. Mol Psychiatry 2006;11:227-40.
9. Dubovsky SL. Treatment of bipolar depression. Psychiatr Clin N Am 2005;28:349-70.
10. Kroenke K. A 75-year-old man with depression. JAMA 2002;287:1568-76.
11. Shanmugham B, Karp J, Drayer R, et al. Evidence-based pharmacological interventions for geriatric depression. Psychiatr Clin N Am 2005;28:821-35.
12. Alexopoulos GS. In: Sadavoy J, Jarvik LF, Grossberg GT, Meyers BS, eds. Comprehensive textbook of geriatric psychiatry, 3 ed. New York: WW Norton and Co.; 2002:609-53.
13. Sneed JR, Roose SP, Sackeim HA. Vascular depression: a distinct diagnostic subtype? Biol Psychiatry 2006;60:1295-8.
14. Higgins N, Regan C. A systematic review of the effectiveness of interventions to help older people adhere to medication regimes. Age Ageing 2004;33:224-9.
15. Ownby RL, Hertzog C, Crocco E, Duara R. Factors related to medication adherence in memory disorder clinic patients. Aging & Mental Health 2006;10:378-85.
16. Sajatovic M, Madhusoodanan S, Coconcea N. Managing bipolar disorder in the elderly: defining the role of the newer agents. Drugs Aging 2005;22:39-54.
17. Cavanagh J, Smyth R, Goodwin GM. Relapse into mania or depression following lithium discontinuation: a 7-year follow up. Acta Psychiatr Scand 2004;109:91-5.
18. Dunner DL. Atypical antipsychotics: efficacy across bipolar disorder subpopulations. J Clin Psychiatry 2005;66(suppl 3):20-7.
19. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27:596-601.
20. Leverich GS, Altshuler LL, Frye MA, et al. Risk of switch in mood polarity to hypomania or mania in patient with bipolar depression during acute and continuation trials of venlafaxine, sertraline, and bupropion as adjuncts to mood stabilizers. Am J Psychiatry 2006;163:232-9.
1. Depp C, Jeste D. Bipolar disorder in older adults: a critical review. Bipolar Disord 2004;6:343-67.
2. Sajatovic M, Blow FC, Ignacio RV, Kales HC. New-onset bipolar disorder in later life. Am J Geriatr Psychiatry 2005;13:282-9.
3. Arciniegas DB. New-onset bipolar disorder in late life: a case of mistaken identity. Am J Psychiatry 2006;163:198-203.
4. Young RC. Bipolar disorder in older persons: perspectives and new findings. Am J Geriatr Psychiatry 2005;13:265-7.
5. Young RC. Evidence-based pharmacological treatment of geriatric bipolar disorder. Psychiatr Clin North Am 2005;28:837-69.
6. Diagnostic and statistical manual of mental disorders, 4 ed, text rev. Washington, DC; American Psychiatric Association; 2000.
7. Blumberg HP, Stern E, Ricketts S, et al. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry 1999;156:1986-8.
8. Gitlin M. Treatment-resistant bipolar disorder. Mol Psychiatry 2006;11:227-40.
9. Dubovsky SL. Treatment of bipolar depression. Psychiatr Clin N Am 2005;28:349-70.
10. Kroenke K. A 75-year-old man with depression. JAMA 2002;287:1568-76.
11. Shanmugham B, Karp J, Drayer R, et al. Evidence-based pharmacological interventions for geriatric depression. Psychiatr Clin N Am 2005;28:821-35.
12. Alexopoulos GS. In: Sadavoy J, Jarvik LF, Grossberg GT, Meyers BS, eds. Comprehensive textbook of geriatric psychiatry, 3 ed. New York: WW Norton and Co.; 2002:609-53.
13. Sneed JR, Roose SP, Sackeim HA. Vascular depression: a distinct diagnostic subtype? Biol Psychiatry 2006;60:1295-8.
14. Higgins N, Regan C. A systematic review of the effectiveness of interventions to help older people adhere to medication regimes. Age Ageing 2004;33:224-9.
15. Ownby RL, Hertzog C, Crocco E, Duara R. Factors related to medication adherence in memory disorder clinic patients. Aging & Mental Health 2006;10:378-85.
16. Sajatovic M, Madhusoodanan S, Coconcea N. Managing bipolar disorder in the elderly: defining the role of the newer agents. Drugs Aging 2005;22:39-54.
17. Cavanagh J, Smyth R, Goodwin GM. Relapse into mania or depression following lithium discontinuation: a 7-year follow up. Acta Psychiatr Scand 2004;109:91-5.
18. Dunner DL. Atypical antipsychotics: efficacy across bipolar disorder subpopulations. J Clin Psychiatry 2005;66(suppl 3):20-7.
19. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27:596-601.
20. Leverich GS, Altshuler LL, Frye MA, et al. Risk of switch in mood polarity to hypomania or mania in patient with bipolar depression during acute and continuation trials of venlafaxine, sertraline, and bupropion as adjuncts to mood stabilizers. Am J Psychiatry 2006;163:232-9.
Did antismoking therapy make him sick?
Presentation: unconscious on the street
Emergency medical personnel bring Mr. M, age 66, to the ER after passers-by find him supine on the sidewalk. On arrival, he is comatose as confirmed by a Glasgow Coma Scale score of 8 (eye opening 3, verbal response 2, motor response 3). Systolic blood pressure is 108 mm Hg on palpation, pulse is 135 beats per minute, and temperature is 105 °F. Minor abrasions cover his face and arms, and his hands and feet are rigid.
Mr. M has lived at a board-and-care facility for 30 years. The facility’s operator tells us that Mr. M has had schizophrenia for 40 years and has been taking:
- olanzapine, 7.5 mg each morning and 10 mg at bedtime
- chlorpromazine, 50 mg nightly
- lithium carbonate, 300 mg tid
- and benztropine, 2 mg bid.
Three weeks ago, Mr. M was hospitalized for 6 days with pneumonia. In 3 months, he will undergo surgery for prostate cancer. He is taking no medication for the prostate cancer.
Creatine phosphokinase (CPK) is 2,939 IU/L, indicating neuroleptic malignant syndrome (NMS). Other laboratory test results suggest diabetes or renal failure (Table 1). Lumbar puncture shows protein at 91 mg/dL, glucose at 74 mg/dL, and red- and white-blood-cell counts at 0 and 1, respectively. CSF Gram’s stain and brain CT are unremarkable. ECG is normal except for sinus tachycardia. Serum lithium is normal (1.1 mmol/L).
Mr. M undergoes tracheal intubation and receives ceftazidime, dose unknown, because chest radiograph shows lower lung opacities, suggesting aspiration. He receives morphine, 2 to 4 mg hourly as needed, to calm him during intubation. He is then transferred to the intensive care unit.
Table 1
Diabetes, renal failure, or NMS? The story behind Mr. M’s laboratory values
| Mr. M’s reading | Normal range | Might suggest | |
|---|---|---|---|
| CPK | 2,939 IU/L | 8-150 IU/L | NMS |
| Serum creatinine | 1.9 mg/dL | 0.6-1.5 mg/dL | Renal failure, a complication from elevated CPK |
| Serum glucose | 143 mg/dL | 66-99 mg/dL | Diabetes mellitus |
| NMS: Neuroleptic malignant syndrome | |||
| CPK: Creatine phosphokinase | |||
The authors’ observations
NMS, a potentially fatal side effect of antipsychotics, is characterized by rigidity, hyperthermia, and autonomic instability1—as seen with Mr. M.
The patient’s rigidity, elevated creatine kinase, and face and arm abrasions could suggest a seizure. Mr. M’s EEG is negative, however, and he has no history of seizures or head trauma, so seizure is ruled out.
Researchers have associated bupropion with a small risk of developing seizures. Richmond and Zwar2 reported a 0.1% risk with bupropion, ≥300 mg/d, but Mr. M was taking 150 mg/d. Dunner et al3 estimated the risk of developing seizure while taking standard-release bupropion—the form Mr. M used—at 0.06%, but patients in this study who developed seizures typically had a past seizure disorder or head trauma.
The combination of hyperthermia, tachycardia, altered mental status, and positive chest X-ray suggest pneumonia, which was addressed with antibiotics. Pneumonia, however, does not solely account for Mr. M’s fever, rigidity, and profoundly increased CPK. These findings suggest NMS.
The Glasgow Coma Scale (GCS) is used to quantitatively rate degree of responsiveness in critically ill or injured patients (Table 2). Total scores range from 3 to 15 based on the patient’s best eye, motor, and verbal responses. Total score ≤8 indicates a probable coma. Serial GCS scores can measure clinical course in comatose patients.
Table 2
Using Glasgow Coma Scale to determine level of consciousness
| Component | Response | Score |
|---|---|---|
| Best eye response | No eye opening | 1 |
| Eye opening to pain | 2 | |
| Eye opening to verbal command | 3 | |
| Eyes open spontaneously | 4 | |
| Best verbal response | No verbal response | 1 |
| Incomprehensible sounds | 2 | |
| Inappropriate words | 3 | |
| Confused | 4 | |
| Oriented | 5 | |
| Best motor response | No motor response | 1 |
| Extension to pain | 2 | |
| Flexion to pain | 3 | |
| Withdrawal from pain | 4 | |
| Localizing pain | 5 | |
| Obeys commands | 6 | |
| Total score ≤8 is severe, and 90% of patients with scores ≤8 are in a coma). Coma is defined as not opening eyes, not obeying commands, and not saying understandable words. Composite scores listing eye, verbal, and motor responses (such as E3V3M5) are clinically more useful than totals. | ||
| Source: Reprinted from Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;304(7872):81-4, with permission from Elsevier. | ||
Treatment: slow progress
In the ICU, we diagnose NMS and stop all psychotropics, fearing that interactions between any of them might be causing NMS. We give midazolam, 1 to 2 mg hourly as needed for agitation, and continue morphine, 2 to 4 mg hourly as needed for pain. We stop ceftazidime after ruling out aspiration risk.
On day 2 of hospitalization, we call the neurology and consultation-liaison (C-L) psychiatry services. The C-L psychiatrist attempts a mental status examination, but Mr. M is too frail and sedated to communicate. Neurologic exam shows increased foot rigidity, and follow-up studies show negative EEG, normal head and neck MRIs and MRAs, a peak in CPK at 5,487 IU/L, and normal chest films.
We taper and discontinue midazolam and morphine, and Mr. M’s consciousness improves as the dosages decrease. We add lorazepam, 1 mg tid, to address Mr. M’s agitation. He also starts physical therapy to address potential movement problems caused by laying static for 3 days. By day 7, he is extubated and transferred to the general medical unit.
On day 9, Mr. M’s recall and concentration are diminished, and he cannot follow a 3-step command. His Mini-Mental State Examination (MMSE) score of 17 points to a cognitive impairment.
By day 12, residual psychosis is increasing Mr. M’s confusion, paranoia, and agitation. Despite this complication, he is able to work with his occupational and physical therapists.
By day 20, Mr. M becomes more paranoid, with tangential and loose associations. To address these symptoms, we stop lorazepam and start aripiprazole, 15 mg each morning. Because aripiprazole is a partial dopamine agonist and antagonist, it is less likely than other antipsychotics to cause recurrence of NMS symptoms.
Four days later, Mr. M is medically cleared for transfer to the county psychiatric hospital. Creatinine and CPK elevations, metabolic acidosis, and anemia have resolved.
Treatment: new facility, new drugs
On initial evaluation at the psychiatric hospital, Mr. M is cooperative and aware of person, place, and time. His thought processes range from tangential to disorganized, and his paranoia persists.
The attending psychiatrist stops aripiprazole and starts risperidone, 1 mg bid, possibly because he is less familiar with aripiprazole—a newer antipsychotic— than with risperidone. Laboratory results within 3 days of starting risperidone show normal serum levels, blood counts, liver enzymes, and CPK.
On day 2 at the psychiatric hospital, Mr. M’s behavior worsens; he frequently disrobes in front of others, yells at staff, and requires verbal redirection. His MMSE score has fallen to 15. The attending psychiatrist modifies risperidone to 2 mg nightly and adds donepezil, 10 mg each morning, to try to reverse his cognitive decline.
By day 8, Mr. M is more cooperative and his behavior improves. He is transferred back to his board-and-care facility on risperidone and donepezil at the above dosages.
The following month, Mr. M presents to his outpatient psychiatrist with improved cognitive function, but he is still delusional. The psychiatrist stops risperidone and donepezil and resumes olanzapine, 7.5 mg each morning and 10 mg nightly, and chlorpromazine, 50 mg nightly, to try to restore the patient’s pre-NMS function.
Mr. M undergoes successful prostate cancer surgery before his 3-month psychiatry follow-up, at which the psychiatrist adds lithium carbonate, 300 mg tid, for residual irritability. Serum lithium levels are normal; bupropion is not restarted.
One year after presentation, Mr. M is minimally delusional but functioning well. No symptoms suggesting NMS recurrence have been reported.
The authors’ observations
Though the precise mechanism is unknown, NMS has been linked with use of FGAs such as chlorpromazine, which can trigger excessive dopamine blockade.4 Studies increasingly associate SGAs such as olanzapine, risperidone, and aripiprazole with NMS onset.4-6 Mood stabilizers such as lithium carbonate also have been implicated, especially when used with antipsychotics.6-9 No association between antibiotics and NMS has been found.
For years, Mr. M has been taking FGAs and concomitant olanzapine and lithium carbonate without developing NMS symptoms until now. Since discharge, he has been free of NMS symptoms despite taking two SGAs (aripiprazole and risperidone) at different times and later resuming chlorpromazine, olanzapine, and lithium carbonate.
Of note, bupropion—the last psychotropic added before NMS onset—has not been restarted. The literature does not link bupropion to NMS, although one case report10 suggests an association between fluoxetine and NMS after the patient had taken several antipsychotic/antidepressant combinations.
As a dopamine agonist, bupropion should protect against NMS. Case reports,11,12 however, have described patients who developed NMS after antipsychotics were discontinued, and stopping an antipsychotic essentially mimics bupropion’s action by eliminating the dopamine blockade. Additionally, bupropion’s norepinephrine modulation could have precipitated NMS by dysregulating the sympathetic nervous system.13
Mr. M’s board-and-care operator indicated that the patient’s tobacco consumption decreased—from about a pack to a half-pack of cigarettes daily—after bupropion was added. Alternatively, the effects of pneumonia could have curtailed Mr. M’s smoking. Because nicotine increases metabolism of neuroleptics,14,15 decreased nicotine consumption might have increased dopamine blockade to the point of causing NMS.
Other possibilities. Mr. M’s pneumonia might have caused dehydration, which can also lead to NMS.
Bupropion also reportedly alters metabolism of chlorpromazine and other phenothiazine antipsychotics by inhibiting the cytochrome P-450 2D6 isoenzyme. This pharmacokinetic interaction could have precipitated Mr. M’s NMS episode independent of an antipsychotic dosage increase.16
Because this case is so complex, pinpointing a specific cause for Mr. M’s apparent NMS symptoms is difficult. Be aware that combining psychotropics can lead to NMS. Patients who present with mental status changes, hyperthermia, rigidity, and/or increased creatine kinase while taking psychotropics should be promptly evaluated and managed.
Treating NMS
A review of NMS treatment by Davis et al17 suggests that you:
- consider NMS in the differential diagnosis of an acutely delirious patient who has used antipsychotics, no matter how long he or she has been taking the medication(s) or how stable the dosage
- check for other signs of NMS—such as rigidity or autonomic instability—during the physical examination.
- consider NMS as a possible cause of dysarthria, diaphoresis, dysphagia, sialorrhea, and myoclonus, although these are less common signs of the disorder
- include CPK levels, chemistry panel, CBC, and liver enzyme assessment in the early evaluation of laboratory results. Consider performing a urine drug screen to check for illicit substance use. Head CT results might also help confirm NMS diagnosis.
If sedation becomes necessary, use benzodiazepines cautiously. Serial CPKs and daily reassessment of clinical degree of rigidity are essential; continued rigidity may indicate use of dopamine agonists and dantrolene.17
Related resources
- Neuroleptic Malignant Syndrome Information Service. Archive of articles addressing NMS diagnosis and treatment, and listing of psychotropics associated with NMS. www.nmsis.org.
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Bupropion SR • Wellbutrin, Zyban
- Ceftazidime • various
- Chlorpromazine • Thorazine
- Dantrolene • Dantrium
- Donepezil • Aricept
- Lithium carbonate • various
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Risperidone • Risperdal
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association, 2000.
2. Richmond R, Zwar N. Review of bupropion for smoking cessation. Drug Alcohol Rev 2003;22:203-20.
3. Dunner DL, Zisook S, Billow A, et al. A prospective safety study for bupropion sustained-release in the treatment of depression. J Clin Psychiatry 1998;59:366-73.
4. Caroff SN, Mann SC, Campbell EC. Atypical antipsychotics and neuroleptic malignant syndrome. Psychiatr Ann 2000;30:314-21.
5. Berry N, Pradhan S, Sagar R, Gupta SK. Neuroleptic malignant syndrome in an adolescent receiving olanzapine-lithium combination therapy. Pharmacotherapy 2003;23:255-9
6. Ananth J, Parameswaran S, Gunatilake S, et al. Neuroleptic malignant syndrome and atypical antipsychotic drugs. J Clin Psychiatry 2004;65:464-70.
7. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am 1993;77:185-202.
8. Bourgeois JA, Kahn DR. Neuroleptic malignant syndrome following administration of risperidone and lithium. J Clin Psychopharmacol 2003;23:315-6.
9. Gill J, Singh H, Nugent K. Acute lithium intoxication and neuroleptic malignant syndrome. Pharmacotherapy 2003;23:811-15.
10. Halman M, Goldbloom DS. Fluoxetine and neuroleptic malignant syndrome. Biol Psychiatry 1990;28:518-21.
11. Spivak B, Gonen N, Mester R, et al. Neuroleptic malignant syndrome associated with abrupt withdrawal of anticholinergic agents. Int Clin Psychopharmacol 1996;11:207-9.
12. Rosse R, Ciolino C. Dopamine agonists and neuroleptic malignant syndrome. Am J Psychiatry 1985;142:270-1.
13. Gurrera RJ. Sympathoadrenal hyperactivity and the etiology of neuroleptic malignant syndrome. Am J Psychiatry 1999;156:169-80.
14. Ereshefsky L, Jann MW, Saklad SR, et al. Effects of smoking on fluphenazine clearance in psychiatric inpatients. Biol Psychiatry 1985;20:329-32.
15. Jann MW, Saklad SR, Ereshefsky L, et al. Effects of smoking on haloperidol and reduced haloperidol plasma concentrations and haloperidol clearance. Psychopharmacology (Berl) 1986;90:468-70.
16. Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics 2005;46:464-94.
17. Davis JM, Caroff SN, Mann SC. Treatment of neuroleptic malignant syndrome. Psychiatr Ann 2000;30:325-31.
Presentation: unconscious on the street
Emergency medical personnel bring Mr. M, age 66, to the ER after passers-by find him supine on the sidewalk. On arrival, he is comatose as confirmed by a Glasgow Coma Scale score of 8 (eye opening 3, verbal response 2, motor response 3). Systolic blood pressure is 108 mm Hg on palpation, pulse is 135 beats per minute, and temperature is 105 °F. Minor abrasions cover his face and arms, and his hands and feet are rigid.
Mr. M has lived at a board-and-care facility for 30 years. The facility’s operator tells us that Mr. M has had schizophrenia for 40 years and has been taking:
- olanzapine, 7.5 mg each morning and 10 mg at bedtime
- chlorpromazine, 50 mg nightly
- lithium carbonate, 300 mg tid
- and benztropine, 2 mg bid.
Three weeks ago, Mr. M was hospitalized for 6 days with pneumonia. In 3 months, he will undergo surgery for prostate cancer. He is taking no medication for the prostate cancer.
Creatine phosphokinase (CPK) is 2,939 IU/L, indicating neuroleptic malignant syndrome (NMS). Other laboratory test results suggest diabetes or renal failure (Table 1). Lumbar puncture shows protein at 91 mg/dL, glucose at 74 mg/dL, and red- and white-blood-cell counts at 0 and 1, respectively. CSF Gram’s stain and brain CT are unremarkable. ECG is normal except for sinus tachycardia. Serum lithium is normal (1.1 mmol/L).
Mr. M undergoes tracheal intubation and receives ceftazidime, dose unknown, because chest radiograph shows lower lung opacities, suggesting aspiration. He receives morphine, 2 to 4 mg hourly as needed, to calm him during intubation. He is then transferred to the intensive care unit.
Table 1
Diabetes, renal failure, or NMS? The story behind Mr. M’s laboratory values
| Mr. M’s reading | Normal range | Might suggest | |
|---|---|---|---|
| CPK | 2,939 IU/L | 8-150 IU/L | NMS |
| Serum creatinine | 1.9 mg/dL | 0.6-1.5 mg/dL | Renal failure, a complication from elevated CPK |
| Serum glucose | 143 mg/dL | 66-99 mg/dL | Diabetes mellitus |
| NMS: Neuroleptic malignant syndrome | |||
| CPK: Creatine phosphokinase | |||
The authors’ observations
NMS, a potentially fatal side effect of antipsychotics, is characterized by rigidity, hyperthermia, and autonomic instability1—as seen with Mr. M.
The patient’s rigidity, elevated creatine kinase, and face and arm abrasions could suggest a seizure. Mr. M’s EEG is negative, however, and he has no history of seizures or head trauma, so seizure is ruled out.
Researchers have associated bupropion with a small risk of developing seizures. Richmond and Zwar2 reported a 0.1% risk with bupropion, ≥300 mg/d, but Mr. M was taking 150 mg/d. Dunner et al3 estimated the risk of developing seizure while taking standard-release bupropion—the form Mr. M used—at 0.06%, but patients in this study who developed seizures typically had a past seizure disorder or head trauma.
The combination of hyperthermia, tachycardia, altered mental status, and positive chest X-ray suggest pneumonia, which was addressed with antibiotics. Pneumonia, however, does not solely account for Mr. M’s fever, rigidity, and profoundly increased CPK. These findings suggest NMS.
The Glasgow Coma Scale (GCS) is used to quantitatively rate degree of responsiveness in critically ill or injured patients (Table 2). Total scores range from 3 to 15 based on the patient’s best eye, motor, and verbal responses. Total score ≤8 indicates a probable coma. Serial GCS scores can measure clinical course in comatose patients.
Table 2
Using Glasgow Coma Scale to determine level of consciousness
| Component | Response | Score |
|---|---|---|
| Best eye response | No eye opening | 1 |
| Eye opening to pain | 2 | |
| Eye opening to verbal command | 3 | |
| Eyes open spontaneously | 4 | |
| Best verbal response | No verbal response | 1 |
| Incomprehensible sounds | 2 | |
| Inappropriate words | 3 | |
| Confused | 4 | |
| Oriented | 5 | |
| Best motor response | No motor response | 1 |
| Extension to pain | 2 | |
| Flexion to pain | 3 | |
| Withdrawal from pain | 4 | |
| Localizing pain | 5 | |
| Obeys commands | 6 | |
| Total score ≤8 is severe, and 90% of patients with scores ≤8 are in a coma). Coma is defined as not opening eyes, not obeying commands, and not saying understandable words. Composite scores listing eye, verbal, and motor responses (such as E3V3M5) are clinically more useful than totals. | ||
| Source: Reprinted from Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;304(7872):81-4, with permission from Elsevier. | ||
Treatment: slow progress
In the ICU, we diagnose NMS and stop all psychotropics, fearing that interactions between any of them might be causing NMS. We give midazolam, 1 to 2 mg hourly as needed for agitation, and continue morphine, 2 to 4 mg hourly as needed for pain. We stop ceftazidime after ruling out aspiration risk.
On day 2 of hospitalization, we call the neurology and consultation-liaison (C-L) psychiatry services. The C-L psychiatrist attempts a mental status examination, but Mr. M is too frail and sedated to communicate. Neurologic exam shows increased foot rigidity, and follow-up studies show negative EEG, normal head and neck MRIs and MRAs, a peak in CPK at 5,487 IU/L, and normal chest films.
We taper and discontinue midazolam and morphine, and Mr. M’s consciousness improves as the dosages decrease. We add lorazepam, 1 mg tid, to address Mr. M’s agitation. He also starts physical therapy to address potential movement problems caused by laying static for 3 days. By day 7, he is extubated and transferred to the general medical unit.
On day 9, Mr. M’s recall and concentration are diminished, and he cannot follow a 3-step command. His Mini-Mental State Examination (MMSE) score of 17 points to a cognitive impairment.
By day 12, residual psychosis is increasing Mr. M’s confusion, paranoia, and agitation. Despite this complication, he is able to work with his occupational and physical therapists.
By day 20, Mr. M becomes more paranoid, with tangential and loose associations. To address these symptoms, we stop lorazepam and start aripiprazole, 15 mg each morning. Because aripiprazole is a partial dopamine agonist and antagonist, it is less likely than other antipsychotics to cause recurrence of NMS symptoms.
Four days later, Mr. M is medically cleared for transfer to the county psychiatric hospital. Creatinine and CPK elevations, metabolic acidosis, and anemia have resolved.
Treatment: new facility, new drugs
On initial evaluation at the psychiatric hospital, Mr. M is cooperative and aware of person, place, and time. His thought processes range from tangential to disorganized, and his paranoia persists.
The attending psychiatrist stops aripiprazole and starts risperidone, 1 mg bid, possibly because he is less familiar with aripiprazole—a newer antipsychotic— than with risperidone. Laboratory results within 3 days of starting risperidone show normal serum levels, blood counts, liver enzymes, and CPK.
On day 2 at the psychiatric hospital, Mr. M’s behavior worsens; he frequently disrobes in front of others, yells at staff, and requires verbal redirection. His MMSE score has fallen to 15. The attending psychiatrist modifies risperidone to 2 mg nightly and adds donepezil, 10 mg each morning, to try to reverse his cognitive decline.
By day 8, Mr. M is more cooperative and his behavior improves. He is transferred back to his board-and-care facility on risperidone and donepezil at the above dosages.
The following month, Mr. M presents to his outpatient psychiatrist with improved cognitive function, but he is still delusional. The psychiatrist stops risperidone and donepezil and resumes olanzapine, 7.5 mg each morning and 10 mg nightly, and chlorpromazine, 50 mg nightly, to try to restore the patient’s pre-NMS function.
Mr. M undergoes successful prostate cancer surgery before his 3-month psychiatry follow-up, at which the psychiatrist adds lithium carbonate, 300 mg tid, for residual irritability. Serum lithium levels are normal; bupropion is not restarted.
One year after presentation, Mr. M is minimally delusional but functioning well. No symptoms suggesting NMS recurrence have been reported.
The authors’ observations
Though the precise mechanism is unknown, NMS has been linked with use of FGAs such as chlorpromazine, which can trigger excessive dopamine blockade.4 Studies increasingly associate SGAs such as olanzapine, risperidone, and aripiprazole with NMS onset.4-6 Mood stabilizers such as lithium carbonate also have been implicated, especially when used with antipsychotics.6-9 No association between antibiotics and NMS has been found.
For years, Mr. M has been taking FGAs and concomitant olanzapine and lithium carbonate without developing NMS symptoms until now. Since discharge, he has been free of NMS symptoms despite taking two SGAs (aripiprazole and risperidone) at different times and later resuming chlorpromazine, olanzapine, and lithium carbonate.
Of note, bupropion—the last psychotropic added before NMS onset—has not been restarted. The literature does not link bupropion to NMS, although one case report10 suggests an association between fluoxetine and NMS after the patient had taken several antipsychotic/antidepressant combinations.
As a dopamine agonist, bupropion should protect against NMS. Case reports,11,12 however, have described patients who developed NMS after antipsychotics were discontinued, and stopping an antipsychotic essentially mimics bupropion’s action by eliminating the dopamine blockade. Additionally, bupropion’s norepinephrine modulation could have precipitated NMS by dysregulating the sympathetic nervous system.13
Mr. M’s board-and-care operator indicated that the patient’s tobacco consumption decreased—from about a pack to a half-pack of cigarettes daily—after bupropion was added. Alternatively, the effects of pneumonia could have curtailed Mr. M’s smoking. Because nicotine increases metabolism of neuroleptics,14,15 decreased nicotine consumption might have increased dopamine blockade to the point of causing NMS.
Other possibilities. Mr. M’s pneumonia might have caused dehydration, which can also lead to NMS.
Bupropion also reportedly alters metabolism of chlorpromazine and other phenothiazine antipsychotics by inhibiting the cytochrome P-450 2D6 isoenzyme. This pharmacokinetic interaction could have precipitated Mr. M’s NMS episode independent of an antipsychotic dosage increase.16
Because this case is so complex, pinpointing a specific cause for Mr. M’s apparent NMS symptoms is difficult. Be aware that combining psychotropics can lead to NMS. Patients who present with mental status changes, hyperthermia, rigidity, and/or increased creatine kinase while taking psychotropics should be promptly evaluated and managed.
Treating NMS
A review of NMS treatment by Davis et al17 suggests that you:
- consider NMS in the differential diagnosis of an acutely delirious patient who has used antipsychotics, no matter how long he or she has been taking the medication(s) or how stable the dosage
- check for other signs of NMS—such as rigidity or autonomic instability—during the physical examination.
- consider NMS as a possible cause of dysarthria, diaphoresis, dysphagia, sialorrhea, and myoclonus, although these are less common signs of the disorder
- include CPK levels, chemistry panel, CBC, and liver enzyme assessment in the early evaluation of laboratory results. Consider performing a urine drug screen to check for illicit substance use. Head CT results might also help confirm NMS diagnosis.
If sedation becomes necessary, use benzodiazepines cautiously. Serial CPKs and daily reassessment of clinical degree of rigidity are essential; continued rigidity may indicate use of dopamine agonists and dantrolene.17
Related resources
- Neuroleptic Malignant Syndrome Information Service. Archive of articles addressing NMS diagnosis and treatment, and listing of psychotropics associated with NMS. www.nmsis.org.
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Bupropion SR • Wellbutrin, Zyban
- Ceftazidime • various
- Chlorpromazine • Thorazine
- Dantrolene • Dantrium
- Donepezil • Aricept
- Lithium carbonate • various
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Risperidone • Risperdal
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Presentation: unconscious on the street
Emergency medical personnel bring Mr. M, age 66, to the ER after passers-by find him supine on the sidewalk. On arrival, he is comatose as confirmed by a Glasgow Coma Scale score of 8 (eye opening 3, verbal response 2, motor response 3). Systolic blood pressure is 108 mm Hg on palpation, pulse is 135 beats per minute, and temperature is 105 °F. Minor abrasions cover his face and arms, and his hands and feet are rigid.
Mr. M has lived at a board-and-care facility for 30 years. The facility’s operator tells us that Mr. M has had schizophrenia for 40 years and has been taking:
- olanzapine, 7.5 mg each morning and 10 mg at bedtime
- chlorpromazine, 50 mg nightly
- lithium carbonate, 300 mg tid
- and benztropine, 2 mg bid.
Three weeks ago, Mr. M was hospitalized for 6 days with pneumonia. In 3 months, he will undergo surgery for prostate cancer. He is taking no medication for the prostate cancer.
Creatine phosphokinase (CPK) is 2,939 IU/L, indicating neuroleptic malignant syndrome (NMS). Other laboratory test results suggest diabetes or renal failure (Table 1). Lumbar puncture shows protein at 91 mg/dL, glucose at 74 mg/dL, and red- and white-blood-cell counts at 0 and 1, respectively. CSF Gram’s stain and brain CT are unremarkable. ECG is normal except for sinus tachycardia. Serum lithium is normal (1.1 mmol/L).
Mr. M undergoes tracheal intubation and receives ceftazidime, dose unknown, because chest radiograph shows lower lung opacities, suggesting aspiration. He receives morphine, 2 to 4 mg hourly as needed, to calm him during intubation. He is then transferred to the intensive care unit.
Table 1
Diabetes, renal failure, or NMS? The story behind Mr. M’s laboratory values
| Mr. M’s reading | Normal range | Might suggest | |
|---|---|---|---|
| CPK | 2,939 IU/L | 8-150 IU/L | NMS |
| Serum creatinine | 1.9 mg/dL | 0.6-1.5 mg/dL | Renal failure, a complication from elevated CPK |
| Serum glucose | 143 mg/dL | 66-99 mg/dL | Diabetes mellitus |
| NMS: Neuroleptic malignant syndrome | |||
| CPK: Creatine phosphokinase | |||
The authors’ observations
NMS, a potentially fatal side effect of antipsychotics, is characterized by rigidity, hyperthermia, and autonomic instability1—as seen with Mr. M.
The patient’s rigidity, elevated creatine kinase, and face and arm abrasions could suggest a seizure. Mr. M’s EEG is negative, however, and he has no history of seizures or head trauma, so seizure is ruled out.
Researchers have associated bupropion with a small risk of developing seizures. Richmond and Zwar2 reported a 0.1% risk with bupropion, ≥300 mg/d, but Mr. M was taking 150 mg/d. Dunner et al3 estimated the risk of developing seizure while taking standard-release bupropion—the form Mr. M used—at 0.06%, but patients in this study who developed seizures typically had a past seizure disorder or head trauma.
The combination of hyperthermia, tachycardia, altered mental status, and positive chest X-ray suggest pneumonia, which was addressed with antibiotics. Pneumonia, however, does not solely account for Mr. M’s fever, rigidity, and profoundly increased CPK. These findings suggest NMS.
The Glasgow Coma Scale (GCS) is used to quantitatively rate degree of responsiveness in critically ill or injured patients (Table 2). Total scores range from 3 to 15 based on the patient’s best eye, motor, and verbal responses. Total score ≤8 indicates a probable coma. Serial GCS scores can measure clinical course in comatose patients.
Table 2
Using Glasgow Coma Scale to determine level of consciousness
| Component | Response | Score |
|---|---|---|
| Best eye response | No eye opening | 1 |
| Eye opening to pain | 2 | |
| Eye opening to verbal command | 3 | |
| Eyes open spontaneously | 4 | |
| Best verbal response | No verbal response | 1 |
| Incomprehensible sounds | 2 | |
| Inappropriate words | 3 | |
| Confused | 4 | |
| Oriented | 5 | |
| Best motor response | No motor response | 1 |
| Extension to pain | 2 | |
| Flexion to pain | 3 | |
| Withdrawal from pain | 4 | |
| Localizing pain | 5 | |
| Obeys commands | 6 | |
| Total score ≤8 is severe, and 90% of patients with scores ≤8 are in a coma). Coma is defined as not opening eyes, not obeying commands, and not saying understandable words. Composite scores listing eye, verbal, and motor responses (such as E3V3M5) are clinically more useful than totals. | ||
| Source: Reprinted from Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;304(7872):81-4, with permission from Elsevier. | ||
Treatment: slow progress
In the ICU, we diagnose NMS and stop all psychotropics, fearing that interactions between any of them might be causing NMS. We give midazolam, 1 to 2 mg hourly as needed for agitation, and continue morphine, 2 to 4 mg hourly as needed for pain. We stop ceftazidime after ruling out aspiration risk.
On day 2 of hospitalization, we call the neurology and consultation-liaison (C-L) psychiatry services. The C-L psychiatrist attempts a mental status examination, but Mr. M is too frail and sedated to communicate. Neurologic exam shows increased foot rigidity, and follow-up studies show negative EEG, normal head and neck MRIs and MRAs, a peak in CPK at 5,487 IU/L, and normal chest films.
We taper and discontinue midazolam and morphine, and Mr. M’s consciousness improves as the dosages decrease. We add lorazepam, 1 mg tid, to address Mr. M’s agitation. He also starts physical therapy to address potential movement problems caused by laying static for 3 days. By day 7, he is extubated and transferred to the general medical unit.
On day 9, Mr. M’s recall and concentration are diminished, and he cannot follow a 3-step command. His Mini-Mental State Examination (MMSE) score of 17 points to a cognitive impairment.
By day 12, residual psychosis is increasing Mr. M’s confusion, paranoia, and agitation. Despite this complication, he is able to work with his occupational and physical therapists.
By day 20, Mr. M becomes more paranoid, with tangential and loose associations. To address these symptoms, we stop lorazepam and start aripiprazole, 15 mg each morning. Because aripiprazole is a partial dopamine agonist and antagonist, it is less likely than other antipsychotics to cause recurrence of NMS symptoms.
Four days later, Mr. M is medically cleared for transfer to the county psychiatric hospital. Creatinine and CPK elevations, metabolic acidosis, and anemia have resolved.
Treatment: new facility, new drugs
On initial evaluation at the psychiatric hospital, Mr. M is cooperative and aware of person, place, and time. His thought processes range from tangential to disorganized, and his paranoia persists.
The attending psychiatrist stops aripiprazole and starts risperidone, 1 mg bid, possibly because he is less familiar with aripiprazole—a newer antipsychotic— than with risperidone. Laboratory results within 3 days of starting risperidone show normal serum levels, blood counts, liver enzymes, and CPK.
On day 2 at the psychiatric hospital, Mr. M’s behavior worsens; he frequently disrobes in front of others, yells at staff, and requires verbal redirection. His MMSE score has fallen to 15. The attending psychiatrist modifies risperidone to 2 mg nightly and adds donepezil, 10 mg each morning, to try to reverse his cognitive decline.
By day 8, Mr. M is more cooperative and his behavior improves. He is transferred back to his board-and-care facility on risperidone and donepezil at the above dosages.
The following month, Mr. M presents to his outpatient psychiatrist with improved cognitive function, but he is still delusional. The psychiatrist stops risperidone and donepezil and resumes olanzapine, 7.5 mg each morning and 10 mg nightly, and chlorpromazine, 50 mg nightly, to try to restore the patient’s pre-NMS function.
Mr. M undergoes successful prostate cancer surgery before his 3-month psychiatry follow-up, at which the psychiatrist adds lithium carbonate, 300 mg tid, for residual irritability. Serum lithium levels are normal; bupropion is not restarted.
One year after presentation, Mr. M is minimally delusional but functioning well. No symptoms suggesting NMS recurrence have been reported.
The authors’ observations
Though the precise mechanism is unknown, NMS has been linked with use of FGAs such as chlorpromazine, which can trigger excessive dopamine blockade.4 Studies increasingly associate SGAs such as olanzapine, risperidone, and aripiprazole with NMS onset.4-6 Mood stabilizers such as lithium carbonate also have been implicated, especially when used with antipsychotics.6-9 No association between antibiotics and NMS has been found.
For years, Mr. M has been taking FGAs and concomitant olanzapine and lithium carbonate without developing NMS symptoms until now. Since discharge, he has been free of NMS symptoms despite taking two SGAs (aripiprazole and risperidone) at different times and later resuming chlorpromazine, olanzapine, and lithium carbonate.
Of note, bupropion—the last psychotropic added before NMS onset—has not been restarted. The literature does not link bupropion to NMS, although one case report10 suggests an association between fluoxetine and NMS after the patient had taken several antipsychotic/antidepressant combinations.
As a dopamine agonist, bupropion should protect against NMS. Case reports,11,12 however, have described patients who developed NMS after antipsychotics were discontinued, and stopping an antipsychotic essentially mimics bupropion’s action by eliminating the dopamine blockade. Additionally, bupropion’s norepinephrine modulation could have precipitated NMS by dysregulating the sympathetic nervous system.13
Mr. M’s board-and-care operator indicated that the patient’s tobacco consumption decreased—from about a pack to a half-pack of cigarettes daily—after bupropion was added. Alternatively, the effects of pneumonia could have curtailed Mr. M’s smoking. Because nicotine increases metabolism of neuroleptics,14,15 decreased nicotine consumption might have increased dopamine blockade to the point of causing NMS.
Other possibilities. Mr. M’s pneumonia might have caused dehydration, which can also lead to NMS.
Bupropion also reportedly alters metabolism of chlorpromazine and other phenothiazine antipsychotics by inhibiting the cytochrome P-450 2D6 isoenzyme. This pharmacokinetic interaction could have precipitated Mr. M’s NMS episode independent of an antipsychotic dosage increase.16
Because this case is so complex, pinpointing a specific cause for Mr. M’s apparent NMS symptoms is difficult. Be aware that combining psychotropics can lead to NMS. Patients who present with mental status changes, hyperthermia, rigidity, and/or increased creatine kinase while taking psychotropics should be promptly evaluated and managed.
Treating NMS
A review of NMS treatment by Davis et al17 suggests that you:
- consider NMS in the differential diagnosis of an acutely delirious patient who has used antipsychotics, no matter how long he or she has been taking the medication(s) or how stable the dosage
- check for other signs of NMS—such as rigidity or autonomic instability—during the physical examination.
- consider NMS as a possible cause of dysarthria, diaphoresis, dysphagia, sialorrhea, and myoclonus, although these are less common signs of the disorder
- include CPK levels, chemistry panel, CBC, and liver enzyme assessment in the early evaluation of laboratory results. Consider performing a urine drug screen to check for illicit substance use. Head CT results might also help confirm NMS diagnosis.
If sedation becomes necessary, use benzodiazepines cautiously. Serial CPKs and daily reassessment of clinical degree of rigidity are essential; continued rigidity may indicate use of dopamine agonists and dantrolene.17
Related resources
- Neuroleptic Malignant Syndrome Information Service. Archive of articles addressing NMS diagnosis and treatment, and listing of psychotropics associated with NMS. www.nmsis.org.
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Bupropion SR • Wellbutrin, Zyban
- Ceftazidime • various
- Chlorpromazine • Thorazine
- Dantrolene • Dantrium
- Donepezil • Aricept
- Lithium carbonate • various
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Risperidone • Risperdal
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association, 2000.
2. Richmond R, Zwar N. Review of bupropion for smoking cessation. Drug Alcohol Rev 2003;22:203-20.
3. Dunner DL, Zisook S, Billow A, et al. A prospective safety study for bupropion sustained-release in the treatment of depression. J Clin Psychiatry 1998;59:366-73.
4. Caroff SN, Mann SC, Campbell EC. Atypical antipsychotics and neuroleptic malignant syndrome. Psychiatr Ann 2000;30:314-21.
5. Berry N, Pradhan S, Sagar R, Gupta SK. Neuroleptic malignant syndrome in an adolescent receiving olanzapine-lithium combination therapy. Pharmacotherapy 2003;23:255-9
6. Ananth J, Parameswaran S, Gunatilake S, et al. Neuroleptic malignant syndrome and atypical antipsychotic drugs. J Clin Psychiatry 2004;65:464-70.
7. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am 1993;77:185-202.
8. Bourgeois JA, Kahn DR. Neuroleptic malignant syndrome following administration of risperidone and lithium. J Clin Psychopharmacol 2003;23:315-6.
9. Gill J, Singh H, Nugent K. Acute lithium intoxication and neuroleptic malignant syndrome. Pharmacotherapy 2003;23:811-15.
10. Halman M, Goldbloom DS. Fluoxetine and neuroleptic malignant syndrome. Biol Psychiatry 1990;28:518-21.
11. Spivak B, Gonen N, Mester R, et al. Neuroleptic malignant syndrome associated with abrupt withdrawal of anticholinergic agents. Int Clin Psychopharmacol 1996;11:207-9.
12. Rosse R, Ciolino C. Dopamine agonists and neuroleptic malignant syndrome. Am J Psychiatry 1985;142:270-1.
13. Gurrera RJ. Sympathoadrenal hyperactivity and the etiology of neuroleptic malignant syndrome. Am J Psychiatry 1999;156:169-80.
14. Ereshefsky L, Jann MW, Saklad SR, et al. Effects of smoking on fluphenazine clearance in psychiatric inpatients. Biol Psychiatry 1985;20:329-32.
15. Jann MW, Saklad SR, Ereshefsky L, et al. Effects of smoking on haloperidol and reduced haloperidol plasma concentrations and haloperidol clearance. Psychopharmacology (Berl) 1986;90:468-70.
16. Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics 2005;46:464-94.
17. Davis JM, Caroff SN, Mann SC. Treatment of neuroleptic malignant syndrome. Psychiatr Ann 2000;30:325-31.
1. Diagnostic and statistical manual of mental disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association, 2000.
2. Richmond R, Zwar N. Review of bupropion for smoking cessation. Drug Alcohol Rev 2003;22:203-20.
3. Dunner DL, Zisook S, Billow A, et al. A prospective safety study for bupropion sustained-release in the treatment of depression. J Clin Psychiatry 1998;59:366-73.
4. Caroff SN, Mann SC, Campbell EC. Atypical antipsychotics and neuroleptic malignant syndrome. Psychiatr Ann 2000;30:314-21.
5. Berry N, Pradhan S, Sagar R, Gupta SK. Neuroleptic malignant syndrome in an adolescent receiving olanzapine-lithium combination therapy. Pharmacotherapy 2003;23:255-9
6. Ananth J, Parameswaran S, Gunatilake S, et al. Neuroleptic malignant syndrome and atypical antipsychotic drugs. J Clin Psychiatry 2004;65:464-70.
7. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am 1993;77:185-202.
8. Bourgeois JA, Kahn DR. Neuroleptic malignant syndrome following administration of risperidone and lithium. J Clin Psychopharmacol 2003;23:315-6.
9. Gill J, Singh H, Nugent K. Acute lithium intoxication and neuroleptic malignant syndrome. Pharmacotherapy 2003;23:811-15.
10. Halman M, Goldbloom DS. Fluoxetine and neuroleptic malignant syndrome. Biol Psychiatry 1990;28:518-21.
11. Spivak B, Gonen N, Mester R, et al. Neuroleptic malignant syndrome associated with abrupt withdrawal of anticholinergic agents. Int Clin Psychopharmacol 1996;11:207-9.
12. Rosse R, Ciolino C. Dopamine agonists and neuroleptic malignant syndrome. Am J Psychiatry 1985;142:270-1.
13. Gurrera RJ. Sympathoadrenal hyperactivity and the etiology of neuroleptic malignant syndrome. Am J Psychiatry 1999;156:169-80.
14. Ereshefsky L, Jann MW, Saklad SR, et al. Effects of smoking on fluphenazine clearance in psychiatric inpatients. Biol Psychiatry 1985;20:329-32.
15. Jann MW, Saklad SR, Ereshefsky L, et al. Effects of smoking on haloperidol and reduced haloperidol plasma concentrations and haloperidol clearance. Psychopharmacology (Berl) 1986;90:468-70.
16. Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics 2005;46:464-94.
17. Davis JM, Caroff SN, Mann SC. Treatment of neuroleptic malignant syndrome. Psychiatr Ann 2000;30:325-31.
Psychosis: Is it a medical problem?
History: shop talk
Ms. B, age 46, presents to the ER at her brother’s insistence. For about 6 months, she says, she has been “hearing voices”—including that of her boss—talking to each other about work.
Ms. B has no personal or family psychiatric history but notes that her sister died 6 months ago, and her father died the following month. At work, she is having trouble getting along with her boss. She adds that she has been skipping church lately because she believes her church is under investigation and the inquiry might be targeting her.
Ms. B has been a company manager for 20 years. She is divorced, has no children, and lives alone. She says she does not smoke or use illicit drugs and seldom drinks alcohol. She denies suicidal or homicidal thoughts, depressed mood, or visual hallucinations. She says she is sleeping only 3 to 4 hours nightly and feels fatigued in the afternoon. She denies loss of concentration or functioning.
Mental status. Ms. B is well groomed, maintains good eye contact, and is superficially cooperative but increasingly guarded with further questioning. She describes her mood as “OK,” but her affect is blunted. Thought process is logical but circumstantial at times, and her thoughts consist of auditory hallucinations, paranoid thinking, persecutory delusions, and ideas of reference. She has poor insight into her symptoms and does not want to be admitted.
Physical examination and laboratory tests are unremarkable. Negative ethanol and urine drug screens rule out substance abuse, and preliminary noncontrast head CT shows no acute changes.
The author’s observations
In women, schizophrenia typically emerges between ages 17 and 37;1 onset after age 45 is unusual.2 Ms. B’s age, family history, and lack of a formal thought disorder or negative symptoms make late-onset schizophrenia unlikely, though it cannot be ruled out.
Ms. B denies mood symptoms, but significant stressors—such as the recent deaths of her sister and father and difficulties at work—could precipitate a mood disorder. Of the possible diagnoses, major depressive disorder is most likely at this time.1,3 Because Ms. B’s symptoms do not clearly match any diagnosis, we speak with her brother and sister-in-law to seek collateral information.
Collateral history: beware of spies
Ms. B’s brother says his sister began behaving strangely about 8 months ago and has worsened lately. He says she suspects that her boss hired spies to watch her house, car, and her parent’s house. After work, she often parks in paid garages rather than at home to avoid being “followed.” When visiting, he says, she leaves her keys outside because she fears they contain a tracking device. Family members say Ms. B sometimes drops by at night—as late as 5 AM—complaining that she cannot sleep because she is being “watched.”
Ms. B’s family hired a private investigator 3 or 4 months ago to examine her house and car. Although no tracking devices were found, her brother says, Ms. B remains convinced she is being followed. He says she often speaks in “code” and whispers to herself.
According to her brother, Ms. B often hears voices while trying to sleep, saying such things as “Why won’t she turn over?” She reportedly wears a towel while showering because the “spies” are watching. During a conference she attended last week, she told her brother that a group of government investigators followed her there and arrested her boss.
Ms. B’s sister-in-law says the patient’s functioning has declined sharply, and that she has been helping Ms. B complete routine work. Neither she nor Ms. B’s brother have noticed a change in the patient’s energy, productivity, or speech production or speed, thus ruling out bipolar disorder. Ms. B’s brother confirms that there is no family history of mental illness.
The author’s observations
Collateral information about Ms. B points to psychosis rather than a mood disorder with psychotic features, but she lacks the formal thought disorder and negative symptoms common in primary psychotic disorders.
Because Ms. B’s presentation is atypical, we order brain MRI to check for a general medical condition (Figure 1). If brain MRI suggests a medical problem, we will follow with EEG, lumbar puncture, or other tests.
Figure 1 Clinical steps to rule out medical causes of late-onset psychosis
treatment, testing: what mri suggests
We admit Ms. B to the locked inpatient psychiatric unit—where she remains paranoid and guarded—and prescribe risperidone, 1 mg/d, to address her paranoia. She refuses medication at first because she feels she does not need psychiatric care, but we give her lorazepam, 0.5 mg/d for her anxiety, along with psychoeducation and family support. After 3 days, we stop lorazepam and Ms. B agrees to take risperidone.
Within 4 days of starting risperidone, Ms. B’s auditory hallucinations and paranoia have lessened and her insight is improved. We recommend increasing the dosage to 2 mg/d because we feel that 1 mg/d will not sufficiently control her symptoms. She remains paranoid but is reluctant to increase the dosage for fear of adverse effects, though she has reported none so far.
Brain MRI taken the night Ms. B was admitted shows:
- multiple focal, well-defined hyperintense periventricular lesions on fluid-attenuated inversion recovery (FLAIR)- and T2-weighted images (Figure 2). Some lesions are flame-shaped.
- a 1.5-cm lesion adjacent to the right frontal horn showing a hyperintense signal on T2-weighted images and a hypointense signal on T1-weighted images without contrast enhancement. White-matter edema surrounds this lesion.
- no gadolinium-enhancing lesions.
Two radiologists confirm possible demyelination, suggesting multiple sclerosis (MS). Final report of initial brain CT shows lowdensity, periventricular white matter changes consistent with the MRI findings.
Results of subsequent laboratory tests are normal. Erythrocyte sedimentation rate is slightly elevated at 35 mm/hr, suggesting a possible autoimmune disorder. ECG shows sinus bradycardia, and chest x-ray and MR angiogram are unremarkable, as are EEG and visual evoked potential results.
Lumbar puncture and CSF studies show increased immunoglobulin G to albumin ratio. CSF fluid is clear, blood counts and protein are normal, Gram’s stain and culture are negative, and cytologic findings show a marked increase in mature lymphocytes. These results suggest inflammation, but follow-up neurologic exam is unremarkable.
Figure 2 FLAIR-weighted image after Ms. B’s brain MRI
Right 1.5-cm lesion adjacent to right frontal horn and multiple left hyperintense lesions on fluid-attenuated inversion recovery (FLAIR)-weighted image.
The authors’ observations
Determining disease dissemination in time and space is key to diagnosing MS. Clinical presentation or MRI can determine both criteria (Table 1). Ms. B’s lesions and CSF results suggest that MS has disseminated throughout her body, but neurologic examination shows no objective clinical evidence of lesions.
Neuropsychological testing might help evaluate Ms. B’s cognition and executive functioning, but these deficits do not specifically suggest MS. The cortex, particularly the prefrontal cortex, is believed to coordinate organization, planning, and socially appropriate behavior. MS typically involves white matter rather than the cortex, but researchers have suggested that MS-related demyelination might disrupt the axonal circuits that connect the cortex to other brain areas.18
Increased lesion load has been correlated with decreased cognitive function. Neuropsychological testing could indirectly point to a lesion load increase by recording decreased cognitive function, but this decline cannot be attributed to MS without an MRI.
Ms. B’s psychotic symptoms could be clinical evidence of MS, but we cannot solidify the diagnosis until we establish dissemination in time. To do that, we need a second MRI 3 months after the first one. Concurrent late-onset paraphrenia and MS is possible but rare.
Table 1
Findings needed to determine MS diagnosis based on clinical presentation
| Clinical presentation | Findings needed for MS diagnosis |
|---|---|
| >2 clinical attacks* Objective clinical evidence of >2 lesions | None |
| >2 clinical attacks Objective clinical evidence of 1 lesion | Dissemination in space by MRI |
| or | |
| >2 MRI-detected lesions consistent with MS plus positive CSF | |
| or | |
| Await further attack implicating a different site | |
| 1 clinical attack >2 objective clinical lesions | Dissemination in time by MRI |
| or | |
| Second clinical attack | |
| 1 clinical attack 1 objective clinical lesion | Dissemination in space by MRI |
| or | |
| >2 MRI-detected lesions consistent with MS plus positive CSF | |
| and | |
| Dissemination in time by MRI | |
| or | |
| Second clinical attack | |
| * Clinical attack: neurologic disturbance defined by subjective report or objective observation lasting at least 24 hours. | |
| Source: Reference 5 | |
Follow-up: where is she?
Ms. B is discharged after 10 days. She denies hallucinations, and staff notices decreased paranoia, brighter affect, and improved insight. We tell her to continue taking risperidone, 1 mg/d.
Three weeks later, Ms. B sees an outpatient psychiatrist. She is paranoid, guarded, and has not been taking risperidone.
Because Ms. B’s previous MRI results are suspect, we ask the hospital’s neurology service to examine her. Findings are unremarkable, but the neurologist recommends a followup brain MRI in 3 months or sooner if symptoms emerge. More than 2 years later, she has not completed a second MRI or contacted her psychiatrist or neurologist.
The authors’ observations
Ms. B’s case highlights the importance of:
- recognizing an atypical presentation of a primary psychotic disorder
- checking for a medical cause of psychosis (Table 2)
- knowing which psychiatric symptoms are common in MS.
Despite absence of neurologic symptoms, Ms. B’s psychosis could have been the initial presentation of MS, which is more prevalent among psychiatric inpatients than in the general population.6,7 In a prospective study,8 95% of patients with MS had neuropsychiatric symptoms, and 79% had depressive symptoms. Hallucinations and delusions were reported in 10% and 7% of MS patients, respectively. These findings suggest that mood disturbances are considerably more common than psychosis among patients with MS.
Diagnosis of MSrelated psychosis has been addressed only in case reports or small studies, most of which have not clearly defined psychosis or adequately described the symptoms or confounding factors such as medications. Findings on prevalence of psychosis as the initial presentation in MS are more limited and confounded by instances in which neurologic symptoms might have been overlooked.9,11
Few studies have investigated whether lesion location correlates with specific neuropsychiatric symptoms. In one study,8 brain MRI taken within 9 months of presenting symptoms showed that MS was not significantly more severe among patients with psychosis compared with nonpsychotic MS patients. These data support psychosis as a possible early finding in MS.
At least two studies12,13 suggest a correlation between temporal lobe lesions and psychosis, but both study samples were small (8 and 10 patients) and used a combination of diagnoses. One case report also supports this correlation.14
Table 2
Medical conditions that can cause psychotic symptoms
| Cerebral malignancy (primary and metastases) | |
| Cerebral trauma | |
| Cerebral vascular accident | |
| Creutzfeldt-Jakob disease | |
| Delirium | |
| Dementia | |
| Epilepsy | |
| Huntington's disease | |
| Infection | |
| Multiple sclerosis | |
| Parkinson's disease | |
| Systemic lupus erythematosus | |
| Source: Reference 4 | |
Treating ms-related psychosis
MS-related psychosis should abate with MS treatment, but no systematized studies have verified this or determined which antipsychotics would be suitable. Single case reports suggest successful treatment with risperidone,13 haloperidol,15 clozapine,16 or ziprasidone.17 Ms. B showed initial improvement with risperidone, but because she was lost to follow-up we cannot say if this medication would work long-term.
Related resources
- Feinstein A. The clinical neuropsychiatry of multiple sclerosis. New York: Cambridge University Press; 1999.
- National Multiple Sclerosis Society. www.nationalmssociety.org.
Drug brand name
- Clozapine • Clozaril
- Risperidone • Risperdal
- Haloperidol • Haldol
- Ziprasidone • Geodon
- Lorazepam • Ativan
Disclosures
Dr. Higgins reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rafeyan is a speaker for AstraZeneca, BristolMyers Squibb Co., Eli Lilly and Co., GlaxoSmithKline, Pfizer, and Wyeth. He is also an advisor to Abbott Laboratories and Forest Pharmaceuticals.
1. Kaplan B, Sadock V, eds. Comprehensive textbook of psychiatry, 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2000:1107,1299.
2. Diagnostic and statistical manual of mental disorders 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Howard R, Rabins P, Seeman M, Jeste D. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. Am J Psychiatry 2000;157:172-8.
4. Lautenschlager NT, Forstl H. Organic psychosis: insight into the biology of psychosis. Curr Psychiatry Rep 2001;3:319-25.
5. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121-7.
6. Pine D, Douglas C, Charles E, et al. Patients with multiple sclerosis presenting to psychiatric hospitals. J Clin Psychiatry 1995;56:297-306.
7. Lyoo IK, Seol HY, Byun HS, Renshaw PF. Unsuspected multiple sclerosis in patients with psychiatric disorders: a magnetic resonance imaging study. J Neuropsychiatry Clin Neurosci 1996;8:54-9.
8. Diaz-Olavarrieta C, Cummings JL, Velazquez J, Garcia de la Cadena C. Neuropsychiatric manifestations of multiple sclerosis. J Neuropsychiatry Clin Neurosci 1999;11:51-7.
9. Felgenhauer K. Psychiatric disorders in the encephalitic form of multiple sclerosis. J Neurol 1990;237:11-8.
10. Skegg K, Corwin P, Skegg D. How often is multiple sclerosis mistaken for a psychiatric disorder? Psychol Med 1988;18:733-6.
11. Kohler J, Heilmeyer H, Volk B. Multiple sclerosis presenting as chronic atypical psychosis. J Neurol Neurosurg Psychiatry 1988;51:281-4.
12. Honer G, Hurwitz T, Li D, et al. Temporal lobe involvement in multiple sclerosis patients with psychiatric disorders. Arch Neurol 1987;44:187-90.
13. Feinstein A, du Boulay G, Ron M. Psychotic illness in multiple sclerosis: a clinical and magnetic resonance imaging study. Br J Psychiatry 1992;161:680-5.
14. Sirois F. Steroid psychosis: a review. Gen Hosp Psychiatry 2003;25:27-33.
15. Drake ME. Acute paranoid psychosis in multiple sclerosis. Psychosomatics 1984;25:60-3.
16. Chong SA, Ko SM. Clozapine treatment of psychosis associated with multiple sclerosis. Can J Psychiatry 1997;42:90-1.
17. Davids E, Hartwig U, Gastpar M. Antipsychotic treatment of psychosis associated with multiple sclerosis. Prog Neuropsychopharmacol Biol Psychiatry 2004;28:743-4.
18. Asghar-Ali A, Taber K, Hurley R, Hayman L. Pure neuropsychiatric presentation of multiple sclerosis. Am J Psychiatry 2004;161:226-31.
History: shop talk
Ms. B, age 46, presents to the ER at her brother’s insistence. For about 6 months, she says, she has been “hearing voices”—including that of her boss—talking to each other about work.
Ms. B has no personal or family psychiatric history but notes that her sister died 6 months ago, and her father died the following month. At work, she is having trouble getting along with her boss. She adds that she has been skipping church lately because she believes her church is under investigation and the inquiry might be targeting her.
Ms. B has been a company manager for 20 years. She is divorced, has no children, and lives alone. She says she does not smoke or use illicit drugs and seldom drinks alcohol. She denies suicidal or homicidal thoughts, depressed mood, or visual hallucinations. She says she is sleeping only 3 to 4 hours nightly and feels fatigued in the afternoon. She denies loss of concentration or functioning.
Mental status. Ms. B is well groomed, maintains good eye contact, and is superficially cooperative but increasingly guarded with further questioning. She describes her mood as “OK,” but her affect is blunted. Thought process is logical but circumstantial at times, and her thoughts consist of auditory hallucinations, paranoid thinking, persecutory delusions, and ideas of reference. She has poor insight into her symptoms and does not want to be admitted.
Physical examination and laboratory tests are unremarkable. Negative ethanol and urine drug screens rule out substance abuse, and preliminary noncontrast head CT shows no acute changes.
The author’s observations
In women, schizophrenia typically emerges between ages 17 and 37;1 onset after age 45 is unusual.2 Ms. B’s age, family history, and lack of a formal thought disorder or negative symptoms make late-onset schizophrenia unlikely, though it cannot be ruled out.
Ms. B denies mood symptoms, but significant stressors—such as the recent deaths of her sister and father and difficulties at work—could precipitate a mood disorder. Of the possible diagnoses, major depressive disorder is most likely at this time.1,3 Because Ms. B’s symptoms do not clearly match any diagnosis, we speak with her brother and sister-in-law to seek collateral information.
Collateral history: beware of spies
Ms. B’s brother says his sister began behaving strangely about 8 months ago and has worsened lately. He says she suspects that her boss hired spies to watch her house, car, and her parent’s house. After work, she often parks in paid garages rather than at home to avoid being “followed.” When visiting, he says, she leaves her keys outside because she fears they contain a tracking device. Family members say Ms. B sometimes drops by at night—as late as 5 AM—complaining that she cannot sleep because she is being “watched.”
Ms. B’s family hired a private investigator 3 or 4 months ago to examine her house and car. Although no tracking devices were found, her brother says, Ms. B remains convinced she is being followed. He says she often speaks in “code” and whispers to herself.
According to her brother, Ms. B often hears voices while trying to sleep, saying such things as “Why won’t she turn over?” She reportedly wears a towel while showering because the “spies” are watching. During a conference she attended last week, she told her brother that a group of government investigators followed her there and arrested her boss.
Ms. B’s sister-in-law says the patient’s functioning has declined sharply, and that she has been helping Ms. B complete routine work. Neither she nor Ms. B’s brother have noticed a change in the patient’s energy, productivity, or speech production or speed, thus ruling out bipolar disorder. Ms. B’s brother confirms that there is no family history of mental illness.
The author’s observations
Collateral information about Ms. B points to psychosis rather than a mood disorder with psychotic features, but she lacks the formal thought disorder and negative symptoms common in primary psychotic disorders.
Because Ms. B’s presentation is atypical, we order brain MRI to check for a general medical condition (Figure 1). If brain MRI suggests a medical problem, we will follow with EEG, lumbar puncture, or other tests.
Figure 1 Clinical steps to rule out medical causes of late-onset psychosis
treatment, testing: what mri suggests
We admit Ms. B to the locked inpatient psychiatric unit—where she remains paranoid and guarded—and prescribe risperidone, 1 mg/d, to address her paranoia. She refuses medication at first because she feels she does not need psychiatric care, but we give her lorazepam, 0.5 mg/d for her anxiety, along with psychoeducation and family support. After 3 days, we stop lorazepam and Ms. B agrees to take risperidone.
Within 4 days of starting risperidone, Ms. B’s auditory hallucinations and paranoia have lessened and her insight is improved. We recommend increasing the dosage to 2 mg/d because we feel that 1 mg/d will not sufficiently control her symptoms. She remains paranoid but is reluctant to increase the dosage for fear of adverse effects, though she has reported none so far.
Brain MRI taken the night Ms. B was admitted shows:
- multiple focal, well-defined hyperintense periventricular lesions on fluid-attenuated inversion recovery (FLAIR)- and T2-weighted images (Figure 2). Some lesions are flame-shaped.
- a 1.5-cm lesion adjacent to the right frontal horn showing a hyperintense signal on T2-weighted images and a hypointense signal on T1-weighted images without contrast enhancement. White-matter edema surrounds this lesion.
- no gadolinium-enhancing lesions.
Two radiologists confirm possible demyelination, suggesting multiple sclerosis (MS). Final report of initial brain CT shows lowdensity, periventricular white matter changes consistent with the MRI findings.
Results of subsequent laboratory tests are normal. Erythrocyte sedimentation rate is slightly elevated at 35 mm/hr, suggesting a possible autoimmune disorder. ECG shows sinus bradycardia, and chest x-ray and MR angiogram are unremarkable, as are EEG and visual evoked potential results.
Lumbar puncture and CSF studies show increased immunoglobulin G to albumin ratio. CSF fluid is clear, blood counts and protein are normal, Gram’s stain and culture are negative, and cytologic findings show a marked increase in mature lymphocytes. These results suggest inflammation, but follow-up neurologic exam is unremarkable.
Figure 2 FLAIR-weighted image after Ms. B’s brain MRI
Right 1.5-cm lesion adjacent to right frontal horn and multiple left hyperintense lesions on fluid-attenuated inversion recovery (FLAIR)-weighted image.
The authors’ observations
Determining disease dissemination in time and space is key to diagnosing MS. Clinical presentation or MRI can determine both criteria (Table 1). Ms. B’s lesions and CSF results suggest that MS has disseminated throughout her body, but neurologic examination shows no objective clinical evidence of lesions.
Neuropsychological testing might help evaluate Ms. B’s cognition and executive functioning, but these deficits do not specifically suggest MS. The cortex, particularly the prefrontal cortex, is believed to coordinate organization, planning, and socially appropriate behavior. MS typically involves white matter rather than the cortex, but researchers have suggested that MS-related demyelination might disrupt the axonal circuits that connect the cortex to other brain areas.18
Increased lesion load has been correlated with decreased cognitive function. Neuropsychological testing could indirectly point to a lesion load increase by recording decreased cognitive function, but this decline cannot be attributed to MS without an MRI.
Ms. B’s psychotic symptoms could be clinical evidence of MS, but we cannot solidify the diagnosis until we establish dissemination in time. To do that, we need a second MRI 3 months after the first one. Concurrent late-onset paraphrenia and MS is possible but rare.
Table 1
Findings needed to determine MS diagnosis based on clinical presentation
| Clinical presentation | Findings needed for MS diagnosis |
|---|---|
| >2 clinical attacks* Objective clinical evidence of >2 lesions | None |
| >2 clinical attacks Objective clinical evidence of 1 lesion | Dissemination in space by MRI |
| or | |
| >2 MRI-detected lesions consistent with MS plus positive CSF | |
| or | |
| Await further attack implicating a different site | |
| 1 clinical attack >2 objective clinical lesions | Dissemination in time by MRI |
| or | |
| Second clinical attack | |
| 1 clinical attack 1 objective clinical lesion | Dissemination in space by MRI |
| or | |
| >2 MRI-detected lesions consistent with MS plus positive CSF | |
| and | |
| Dissemination in time by MRI | |
| or | |
| Second clinical attack | |
| * Clinical attack: neurologic disturbance defined by subjective report or objective observation lasting at least 24 hours. | |
| Source: Reference 5 | |
Follow-up: where is she?
Ms. B is discharged after 10 days. She denies hallucinations, and staff notices decreased paranoia, brighter affect, and improved insight. We tell her to continue taking risperidone, 1 mg/d.
Three weeks later, Ms. B sees an outpatient psychiatrist. She is paranoid, guarded, and has not been taking risperidone.
Because Ms. B’s previous MRI results are suspect, we ask the hospital’s neurology service to examine her. Findings are unremarkable, but the neurologist recommends a followup brain MRI in 3 months or sooner if symptoms emerge. More than 2 years later, she has not completed a second MRI or contacted her psychiatrist or neurologist.
The authors’ observations
Ms. B’s case highlights the importance of:
- recognizing an atypical presentation of a primary psychotic disorder
- checking for a medical cause of psychosis (Table 2)
- knowing which psychiatric symptoms are common in MS.
Despite absence of neurologic symptoms, Ms. B’s psychosis could have been the initial presentation of MS, which is more prevalent among psychiatric inpatients than in the general population.6,7 In a prospective study,8 95% of patients with MS had neuropsychiatric symptoms, and 79% had depressive symptoms. Hallucinations and delusions were reported in 10% and 7% of MS patients, respectively. These findings suggest that mood disturbances are considerably more common than psychosis among patients with MS.
Diagnosis of MSrelated psychosis has been addressed only in case reports or small studies, most of which have not clearly defined psychosis or adequately described the symptoms or confounding factors such as medications. Findings on prevalence of psychosis as the initial presentation in MS are more limited and confounded by instances in which neurologic symptoms might have been overlooked.9,11
Few studies have investigated whether lesion location correlates with specific neuropsychiatric symptoms. In one study,8 brain MRI taken within 9 months of presenting symptoms showed that MS was not significantly more severe among patients with psychosis compared with nonpsychotic MS patients. These data support psychosis as a possible early finding in MS.
At least two studies12,13 suggest a correlation between temporal lobe lesions and psychosis, but both study samples were small (8 and 10 patients) and used a combination of diagnoses. One case report also supports this correlation.14
Table 2
Medical conditions that can cause psychotic symptoms
| Cerebral malignancy (primary and metastases) | |
| Cerebral trauma | |
| Cerebral vascular accident | |
| Creutzfeldt-Jakob disease | |
| Delirium | |
| Dementia | |
| Epilepsy | |
| Huntington's disease | |
| Infection | |
| Multiple sclerosis | |
| Parkinson's disease | |
| Systemic lupus erythematosus | |
| Source: Reference 4 | |
Treating ms-related psychosis
MS-related psychosis should abate with MS treatment, but no systematized studies have verified this or determined which antipsychotics would be suitable. Single case reports suggest successful treatment with risperidone,13 haloperidol,15 clozapine,16 or ziprasidone.17 Ms. B showed initial improvement with risperidone, but because she was lost to follow-up we cannot say if this medication would work long-term.
Related resources
- Feinstein A. The clinical neuropsychiatry of multiple sclerosis. New York: Cambridge University Press; 1999.
- National Multiple Sclerosis Society. www.nationalmssociety.org.
Drug brand name
- Clozapine • Clozaril
- Risperidone • Risperdal
- Haloperidol • Haldol
- Ziprasidone • Geodon
- Lorazepam • Ativan
Disclosures
Dr. Higgins reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rafeyan is a speaker for AstraZeneca, BristolMyers Squibb Co., Eli Lilly and Co., GlaxoSmithKline, Pfizer, and Wyeth. He is also an advisor to Abbott Laboratories and Forest Pharmaceuticals.
History: shop talk
Ms. B, age 46, presents to the ER at her brother’s insistence. For about 6 months, she says, she has been “hearing voices”—including that of her boss—talking to each other about work.
Ms. B has no personal or family psychiatric history but notes that her sister died 6 months ago, and her father died the following month. At work, she is having trouble getting along with her boss. She adds that she has been skipping church lately because she believes her church is under investigation and the inquiry might be targeting her.
Ms. B has been a company manager for 20 years. She is divorced, has no children, and lives alone. She says she does not smoke or use illicit drugs and seldom drinks alcohol. She denies suicidal or homicidal thoughts, depressed mood, or visual hallucinations. She says she is sleeping only 3 to 4 hours nightly and feels fatigued in the afternoon. She denies loss of concentration or functioning.
Mental status. Ms. B is well groomed, maintains good eye contact, and is superficially cooperative but increasingly guarded with further questioning. She describes her mood as “OK,” but her affect is blunted. Thought process is logical but circumstantial at times, and her thoughts consist of auditory hallucinations, paranoid thinking, persecutory delusions, and ideas of reference. She has poor insight into her symptoms and does not want to be admitted.
Physical examination and laboratory tests are unremarkable. Negative ethanol and urine drug screens rule out substance abuse, and preliminary noncontrast head CT shows no acute changes.
The author’s observations
In women, schizophrenia typically emerges between ages 17 and 37;1 onset after age 45 is unusual.2 Ms. B’s age, family history, and lack of a formal thought disorder or negative symptoms make late-onset schizophrenia unlikely, though it cannot be ruled out.
Ms. B denies mood symptoms, but significant stressors—such as the recent deaths of her sister and father and difficulties at work—could precipitate a mood disorder. Of the possible diagnoses, major depressive disorder is most likely at this time.1,3 Because Ms. B’s symptoms do not clearly match any diagnosis, we speak with her brother and sister-in-law to seek collateral information.
Collateral history: beware of spies
Ms. B’s brother says his sister began behaving strangely about 8 months ago and has worsened lately. He says she suspects that her boss hired spies to watch her house, car, and her parent’s house. After work, she often parks in paid garages rather than at home to avoid being “followed.” When visiting, he says, she leaves her keys outside because she fears they contain a tracking device. Family members say Ms. B sometimes drops by at night—as late as 5 AM—complaining that she cannot sleep because she is being “watched.”
Ms. B’s family hired a private investigator 3 or 4 months ago to examine her house and car. Although no tracking devices were found, her brother says, Ms. B remains convinced she is being followed. He says she often speaks in “code” and whispers to herself.
According to her brother, Ms. B often hears voices while trying to sleep, saying such things as “Why won’t she turn over?” She reportedly wears a towel while showering because the “spies” are watching. During a conference she attended last week, she told her brother that a group of government investigators followed her there and arrested her boss.
Ms. B’s sister-in-law says the patient’s functioning has declined sharply, and that she has been helping Ms. B complete routine work. Neither she nor Ms. B’s brother have noticed a change in the patient’s energy, productivity, or speech production or speed, thus ruling out bipolar disorder. Ms. B’s brother confirms that there is no family history of mental illness.
The author’s observations
Collateral information about Ms. B points to psychosis rather than a mood disorder with psychotic features, but she lacks the formal thought disorder and negative symptoms common in primary psychotic disorders.
Because Ms. B’s presentation is atypical, we order brain MRI to check for a general medical condition (Figure 1). If brain MRI suggests a medical problem, we will follow with EEG, lumbar puncture, or other tests.
Figure 1 Clinical steps to rule out medical causes of late-onset psychosis
treatment, testing: what mri suggests
We admit Ms. B to the locked inpatient psychiatric unit—where she remains paranoid and guarded—and prescribe risperidone, 1 mg/d, to address her paranoia. She refuses medication at first because she feels she does not need psychiatric care, but we give her lorazepam, 0.5 mg/d for her anxiety, along with psychoeducation and family support. After 3 days, we stop lorazepam and Ms. B agrees to take risperidone.
Within 4 days of starting risperidone, Ms. B’s auditory hallucinations and paranoia have lessened and her insight is improved. We recommend increasing the dosage to 2 mg/d because we feel that 1 mg/d will not sufficiently control her symptoms. She remains paranoid but is reluctant to increase the dosage for fear of adverse effects, though she has reported none so far.
Brain MRI taken the night Ms. B was admitted shows:
- multiple focal, well-defined hyperintense periventricular lesions on fluid-attenuated inversion recovery (FLAIR)- and T2-weighted images (Figure 2). Some lesions are flame-shaped.
- a 1.5-cm lesion adjacent to the right frontal horn showing a hyperintense signal on T2-weighted images and a hypointense signal on T1-weighted images without contrast enhancement. White-matter edema surrounds this lesion.
- no gadolinium-enhancing lesions.
Two radiologists confirm possible demyelination, suggesting multiple sclerosis (MS). Final report of initial brain CT shows lowdensity, periventricular white matter changes consistent with the MRI findings.
Results of subsequent laboratory tests are normal. Erythrocyte sedimentation rate is slightly elevated at 35 mm/hr, suggesting a possible autoimmune disorder. ECG shows sinus bradycardia, and chest x-ray and MR angiogram are unremarkable, as are EEG and visual evoked potential results.
Lumbar puncture and CSF studies show increased immunoglobulin G to albumin ratio. CSF fluid is clear, blood counts and protein are normal, Gram’s stain and culture are negative, and cytologic findings show a marked increase in mature lymphocytes. These results suggest inflammation, but follow-up neurologic exam is unremarkable.
Figure 2 FLAIR-weighted image after Ms. B’s brain MRI
Right 1.5-cm lesion adjacent to right frontal horn and multiple left hyperintense lesions on fluid-attenuated inversion recovery (FLAIR)-weighted image.
The authors’ observations
Determining disease dissemination in time and space is key to diagnosing MS. Clinical presentation or MRI can determine both criteria (Table 1). Ms. B’s lesions and CSF results suggest that MS has disseminated throughout her body, but neurologic examination shows no objective clinical evidence of lesions.
Neuropsychological testing might help evaluate Ms. B’s cognition and executive functioning, but these deficits do not specifically suggest MS. The cortex, particularly the prefrontal cortex, is believed to coordinate organization, planning, and socially appropriate behavior. MS typically involves white matter rather than the cortex, but researchers have suggested that MS-related demyelination might disrupt the axonal circuits that connect the cortex to other brain areas.18
Increased lesion load has been correlated with decreased cognitive function. Neuropsychological testing could indirectly point to a lesion load increase by recording decreased cognitive function, but this decline cannot be attributed to MS without an MRI.
Ms. B’s psychotic symptoms could be clinical evidence of MS, but we cannot solidify the diagnosis until we establish dissemination in time. To do that, we need a second MRI 3 months after the first one. Concurrent late-onset paraphrenia and MS is possible but rare.
Table 1
Findings needed to determine MS diagnosis based on clinical presentation
| Clinical presentation | Findings needed for MS diagnosis |
|---|---|
| >2 clinical attacks* Objective clinical evidence of >2 lesions | None |
| >2 clinical attacks Objective clinical evidence of 1 lesion | Dissemination in space by MRI |
| or | |
| >2 MRI-detected lesions consistent with MS plus positive CSF | |
| or | |
| Await further attack implicating a different site | |
| 1 clinical attack >2 objective clinical lesions | Dissemination in time by MRI |
| or | |
| Second clinical attack | |
| 1 clinical attack 1 objective clinical lesion | Dissemination in space by MRI |
| or | |
| >2 MRI-detected lesions consistent with MS plus positive CSF | |
| and | |
| Dissemination in time by MRI | |
| or | |
| Second clinical attack | |
| * Clinical attack: neurologic disturbance defined by subjective report or objective observation lasting at least 24 hours. | |
| Source: Reference 5 | |
Follow-up: where is she?
Ms. B is discharged after 10 days. She denies hallucinations, and staff notices decreased paranoia, brighter affect, and improved insight. We tell her to continue taking risperidone, 1 mg/d.
Three weeks later, Ms. B sees an outpatient psychiatrist. She is paranoid, guarded, and has not been taking risperidone.
Because Ms. B’s previous MRI results are suspect, we ask the hospital’s neurology service to examine her. Findings are unremarkable, but the neurologist recommends a followup brain MRI in 3 months or sooner if symptoms emerge. More than 2 years later, she has not completed a second MRI or contacted her psychiatrist or neurologist.
The authors’ observations
Ms. B’s case highlights the importance of:
- recognizing an atypical presentation of a primary psychotic disorder
- checking for a medical cause of psychosis (Table 2)
- knowing which psychiatric symptoms are common in MS.
Despite absence of neurologic symptoms, Ms. B’s psychosis could have been the initial presentation of MS, which is more prevalent among psychiatric inpatients than in the general population.6,7 In a prospective study,8 95% of patients with MS had neuropsychiatric symptoms, and 79% had depressive symptoms. Hallucinations and delusions were reported in 10% and 7% of MS patients, respectively. These findings suggest that mood disturbances are considerably more common than psychosis among patients with MS.
Diagnosis of MSrelated psychosis has been addressed only in case reports or small studies, most of which have not clearly defined psychosis or adequately described the symptoms or confounding factors such as medications. Findings on prevalence of psychosis as the initial presentation in MS are more limited and confounded by instances in which neurologic symptoms might have been overlooked.9,11
Few studies have investigated whether lesion location correlates with specific neuropsychiatric symptoms. In one study,8 brain MRI taken within 9 months of presenting symptoms showed that MS was not significantly more severe among patients with psychosis compared with nonpsychotic MS patients. These data support psychosis as a possible early finding in MS.
At least two studies12,13 suggest a correlation between temporal lobe lesions and psychosis, but both study samples were small (8 and 10 patients) and used a combination of diagnoses. One case report also supports this correlation.14
Table 2
Medical conditions that can cause psychotic symptoms
| Cerebral malignancy (primary and metastases) | |
| Cerebral trauma | |
| Cerebral vascular accident | |
| Creutzfeldt-Jakob disease | |
| Delirium | |
| Dementia | |
| Epilepsy | |
| Huntington's disease | |
| Infection | |
| Multiple sclerosis | |
| Parkinson's disease | |
| Systemic lupus erythematosus | |
| Source: Reference 4 | |
Treating ms-related psychosis
MS-related psychosis should abate with MS treatment, but no systematized studies have verified this or determined which antipsychotics would be suitable. Single case reports suggest successful treatment with risperidone,13 haloperidol,15 clozapine,16 or ziprasidone.17 Ms. B showed initial improvement with risperidone, but because she was lost to follow-up we cannot say if this medication would work long-term.
Related resources
- Feinstein A. The clinical neuropsychiatry of multiple sclerosis. New York: Cambridge University Press; 1999.
- National Multiple Sclerosis Society. www.nationalmssociety.org.
Drug brand name
- Clozapine • Clozaril
- Risperidone • Risperdal
- Haloperidol • Haldol
- Ziprasidone • Geodon
- Lorazepam • Ativan
Disclosures
Dr. Higgins reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rafeyan is a speaker for AstraZeneca, BristolMyers Squibb Co., Eli Lilly and Co., GlaxoSmithKline, Pfizer, and Wyeth. He is also an advisor to Abbott Laboratories and Forest Pharmaceuticals.
1. Kaplan B, Sadock V, eds. Comprehensive textbook of psychiatry, 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2000:1107,1299.
2. Diagnostic and statistical manual of mental disorders 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Howard R, Rabins P, Seeman M, Jeste D. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. Am J Psychiatry 2000;157:172-8.
4. Lautenschlager NT, Forstl H. Organic psychosis: insight into the biology of psychosis. Curr Psychiatry Rep 2001;3:319-25.
5. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121-7.
6. Pine D, Douglas C, Charles E, et al. Patients with multiple sclerosis presenting to psychiatric hospitals. J Clin Psychiatry 1995;56:297-306.
7. Lyoo IK, Seol HY, Byun HS, Renshaw PF. Unsuspected multiple sclerosis in patients with psychiatric disorders: a magnetic resonance imaging study. J Neuropsychiatry Clin Neurosci 1996;8:54-9.
8. Diaz-Olavarrieta C, Cummings JL, Velazquez J, Garcia de la Cadena C. Neuropsychiatric manifestations of multiple sclerosis. J Neuropsychiatry Clin Neurosci 1999;11:51-7.
9. Felgenhauer K. Psychiatric disorders in the encephalitic form of multiple sclerosis. J Neurol 1990;237:11-8.
10. Skegg K, Corwin P, Skegg D. How often is multiple sclerosis mistaken for a psychiatric disorder? Psychol Med 1988;18:733-6.
11. Kohler J, Heilmeyer H, Volk B. Multiple sclerosis presenting as chronic atypical psychosis. J Neurol Neurosurg Psychiatry 1988;51:281-4.
12. Honer G, Hurwitz T, Li D, et al. Temporal lobe involvement in multiple sclerosis patients with psychiatric disorders. Arch Neurol 1987;44:187-90.
13. Feinstein A, du Boulay G, Ron M. Psychotic illness in multiple sclerosis: a clinical and magnetic resonance imaging study. Br J Psychiatry 1992;161:680-5.
14. Sirois F. Steroid psychosis: a review. Gen Hosp Psychiatry 2003;25:27-33.
15. Drake ME. Acute paranoid psychosis in multiple sclerosis. Psychosomatics 1984;25:60-3.
16. Chong SA, Ko SM. Clozapine treatment of psychosis associated with multiple sclerosis. Can J Psychiatry 1997;42:90-1.
17. Davids E, Hartwig U, Gastpar M. Antipsychotic treatment of psychosis associated with multiple sclerosis. Prog Neuropsychopharmacol Biol Psychiatry 2004;28:743-4.
18. Asghar-Ali A, Taber K, Hurley R, Hayman L. Pure neuropsychiatric presentation of multiple sclerosis. Am J Psychiatry 2004;161:226-31.
1. Kaplan B, Sadock V, eds. Comprehensive textbook of psychiatry, 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2000:1107,1299.
2. Diagnostic and statistical manual of mental disorders 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Howard R, Rabins P, Seeman M, Jeste D. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. Am J Psychiatry 2000;157:172-8.
4. Lautenschlager NT, Forstl H. Organic psychosis: insight into the biology of psychosis. Curr Psychiatry Rep 2001;3:319-25.
5. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121-7.
6. Pine D, Douglas C, Charles E, et al. Patients with multiple sclerosis presenting to psychiatric hospitals. J Clin Psychiatry 1995;56:297-306.
7. Lyoo IK, Seol HY, Byun HS, Renshaw PF. Unsuspected multiple sclerosis in patients with psychiatric disorders: a magnetic resonance imaging study. J Neuropsychiatry Clin Neurosci 1996;8:54-9.
8. Diaz-Olavarrieta C, Cummings JL, Velazquez J, Garcia de la Cadena C. Neuropsychiatric manifestations of multiple sclerosis. J Neuropsychiatry Clin Neurosci 1999;11:51-7.
9. Felgenhauer K. Psychiatric disorders in the encephalitic form of multiple sclerosis. J Neurol 1990;237:11-8.
10. Skegg K, Corwin P, Skegg D. How often is multiple sclerosis mistaken for a psychiatric disorder? Psychol Med 1988;18:733-6.
11. Kohler J, Heilmeyer H, Volk B. Multiple sclerosis presenting as chronic atypical psychosis. J Neurol Neurosurg Psychiatry 1988;51:281-4.
12. Honer G, Hurwitz T, Li D, et al. Temporal lobe involvement in multiple sclerosis patients with psychiatric disorders. Arch Neurol 1987;44:187-90.
13. Feinstein A, du Boulay G, Ron M. Psychotic illness in multiple sclerosis: a clinical and magnetic resonance imaging study. Br J Psychiatry 1992;161:680-5.
14. Sirois F. Steroid psychosis: a review. Gen Hosp Psychiatry 2003;25:27-33.
15. Drake ME. Acute paranoid psychosis in multiple sclerosis. Psychosomatics 1984;25:60-3.
16. Chong SA, Ko SM. Clozapine treatment of psychosis associated with multiple sclerosis. Can J Psychiatry 1997;42:90-1.
17. Davids E, Hartwig U, Gastpar M. Antipsychotic treatment of psychosis associated with multiple sclerosis. Prog Neuropsychopharmacol Biol Psychiatry 2004;28:743-4.
18. Asghar-Ali A, Taber K, Hurley R, Hayman L. Pure neuropsychiatric presentation of multiple sclerosis. Am J Psychiatry 2004;161:226-31.
Gender dysphoria: ‘I’m a man, but…’
History: normal on paper
Mr. C, age 65, presents to an endocrinologist complaining of hot flashes and low libido. Initial testing shows low male testosterone, but a repeat test shows normal levels. No medical cause is found for his symptoms.
“My testosterone might be normal on paper,” Mr. C tells the endocrinologist, “but I’m not. I think I’m a woman.”
Mr. C requests referral to a female psychiatrist because he feels more comfortable discussing sexual issues with a woman. The endocrinologist refers him to me for evaluation.
Over 7 years, Mr. C’s other psychiatrist—a man—has been treating him for obsessive-compulsive disorder (OCD), anxiety disorder, and bipolar disorder type II. Mr. C takes paroxetine, 60 mg/d, for depressive symptoms and was taking divalproex, 1,500 mg/d, to stabilize his mood. He recently stopped divalproex because it was causing nausea and sedation.
During our initial visit, Mr. C says he’s “through pretending to be a man.” He says he first questioned his sexual identity in early childhood, when he sometimes dressed in his mother’s clothes for play. As an adult, he mostly cross-dresses in lingerie; he wears a woman’s tank top in public once or twice weekly underneath his polo dress shirt. Fifteen years ago, he suffered anorexia and bulimia while trying to look as svelte as a woman.
At 6 feet, 2 inches with good muscle tone and short, wavy black hair, Mr. C looks strikingly masculine. Now retired, he served in the Air Force and later worked as a commercial pilot and in construction. In private, however, he prefers gardening and cooking over sports and cars.
Mr. C is married but seldom has sexual intercourse with women. He gains sexual fulfillment by visualizing himself as a woman having sex with other women or with himself as a man. He denies interest in male-male sex.
The patient has been masturbating since age 5, mostly by rubbing his scrotum against a swing set pole he still keeps in his utility shed. He often tucks his penis to mimic female genitalia and makes believe his rectum is a vagina.
- Transsexualism
- Pure transvestism (having a firm gender identity but becoming sexually aroused by cross-dressing)
- Dual-role transvestism (cross-dressing solely to experience temporary membership in the opposite sex)
- Stress-related cross-dressing
- Men who desire penectomy or castration but no other gender-reassignment interventions
- Congenital intersex conditions, such as hermaphrodism
Mr. C’s Mini-Mental State Examination score of 30 indicates no underlying dementia. He shows stable affect with no evidence of derailment, paranoia, thought blocking, or auditory hallucinations.
Medical examination results are normal. Negative urine toxicology screen rules out substance abuse, and negative rapid plasma reagin rules out syphilis. Testosterone is not rechecked because levels were normal 2 days before.
The author’s observations
I suspect gender dysphoria, which describes a heterogeneous group of persons who express varying degrees of distress with their anatomic sex and sometimes desire secondary opposite-gender sexual characteristics (Box).
Sexual identity in gender dysphoria is often fluid. Symptoms might suggest transvestism, then evolve to transsexualism. Recognizing this heterogeneity and fluidity is crucial to diagnosis and treatment.
Primary transsexualism. The term “transsexualism” describes persons who want to live and be accepted within the opposite sex.1 The transsexual identity persists for ≥ 2 years and is not caused by another mental disorder or intersexed condition. Fetishism is classically absent and cross-dressing is not sexually gratifying. Most transsexuals want surgical and hormone treatment to make their bodies as congruent as possible with the preferred sex.
In 1994, DSM-IV recognized that some late-onset transsexuals showed features of comorbid transvestism and were sexually aroused by female dress and behaviors. Gender identity disorder (GID) replaced the term “transsexualism” and includes these individuals. A secondary diagnosis of transvestism is applied.
Secondary transsexualism. Case reports2 describe psychosis-induced transsexual desires in patients with schizophrenia. Gender dysphoria improved as their schizophrenia symptoms lessened.
The relationship between transsexualism and schizophrenia has been debated. Hyde and Kenna3 view transsexualism as a schizophrenia spectrum disorder, whereas sexologists consider transsexualism and schizophrenia distinct syndromes that can occur simultaneously.
Affective disorders might also alter contentment with gender role, but the relationship is unclear. Case reports of patients with bipolar disorder suggest that gender dysphoria intensity fluctuates with affective excursions.4 O’Gorman,5 however, described a bipolar patient whose gender dysphoria was mitigated during manic episodes.
Paraphilias are sexual disorders with recurrent intense urges and fantasies that do not follow normative arousal patterns and can diminish capacity for sexual intimacy.6 Manifestations include exhibitionism, fetishism, frotteurism, pedophilia, masochism/sadism, voyeurism, and transvestic fetishism.
Dividing transsexualism and pure transvestism paraphilia into discrete categories is simplistic, as transvestites can develop secondary components of transsexualism. Hoenig and Kenna7 assert that transsexualism—though not an anomalous erotic preference—is almost always preceded by transvestism or accompanied by cross-gender fetishism.
Nonparaphilic sexual addiction—included in DSM-IV-TR as sexual disorder not otherwise specified—describes culturally acceptable sexual interests and behaviors that are frequent or intense enough to reduce capacity for sexual intimacy. Such behaviors include compulsive masturbation, repetitive promiscuity, and dependence on anonymous sexual encounters.
An addiction model conceptualizes paraphilia as a form of pleasure seeking that has become habitual and self-destructive. Treatment involves directing patients to 12-step groups patterned after Alcoholics Anonymous.
Other models place paraphilias and related disorders within the OCD spectrum.8-13 Persons with OCD often are obsessed with sexual content and might grapple with religious and moral concerns about sexual issues. They typically consider their symptoms intrusive or senseless. Selective serotonin reuptake inhibitors—the standard medication for OCD—might alleviate paraphilia, but results are mixed.14
Mr. C’s symptoms. Mr. C shows features of GID and transvestism. His strong, persistent cross-gender identification and sense of inappropriateness with being a man indicate GID. His recurrent sexual urges and fantasies and impaired capacity for sexual intimacy suggest a paraphilia or transvestism.
The significance of Mr. C’s comorbid bipolar disorder and OCD is unclear. Both appeared controlled, but the potential for mania-induced hypersexuality cannot be ignored.
Diagnosing gender dysphoria
A thorough medical, psychiatric, and sexual history can reveal sexual identity symptoms’ source.
Consider a medical cause. Your medical workup may include a genital exam to check for irregularities such as hermaphrodism that can compound questions of sexual identity, and karyotyping to probe chromosomal anomalies, such as mosaicism or chimerism.
Consider schizophrenia or bipolar disorder, as mania or psychosis can cause aberrant sexual behavior. In gender-dysphoric patients with either disorder, treating the psychiatric comorbidity might alleviate the dysphoria. Watch for fluctuations in gender dysphoria intensity when you treat other psychopathologies.
Take a thorough sexual history. Being matter-of-fact while discussing unusual sexual acts will help the patient “open up” about his sexual problems. Ask him if he:
- showed gender-atypical behavior as a child, which can predict transsexualism or homosexuality has engaged in heterosexual, homosexual, or abnormal sexual acts; ask about frequency and preference
- is married or has a girlfriend. If so, are they getting along? How often do they have sex?
- cross-dresses. Does his partner cross-dress as well and, if so, do they cross-dress for sexual gratification or to identify with the opposite gender? Has this response changed over time? Where and how often do they cross-dress?
- is achieving sexual gratification. If so, how?
- has sexual fantasies involving breast-feeding, giving birth, or forced feminization through gender-changing surgeries or other means
- “tucks” his penis, urinates sitting down, or mimics other stereotypical feminine behavior.
The answers will uncover a motivation behind these behaviors, which is key to diagnosis. Sexual gratification as a motive suggests paraphilia, whereas a desire to live as a woman points to transsexualism. Because of the myriad presentations, multiple patient visits are necessary for a specific diagnosis.
Diagnosis: ‘i enjoy womanhood, but…’
I diagnose gender dysphoria, but because Mr. C’s mood is euthymic, I cannot discern how his mood instability might affect his dysphoria. His sexual fantasies are mood-congruent and evoke no shame.
Mr. C then states that he adamantly opposes living outwardly as a woman, and fears that an overt sex change would destroy his marriage and other relationships. Even so, he desires hormone therapy and surgical breast implants so he can more closely mimic physical womanhood and make masturbation more pleasurable. He says he would flatten his breasts with gauze while in public so he can continue to look like a man.
Though comfortable with his sexual fantasies, Mr. C laments that presenting himself as an “alpha male” drains his psychic energy.
The author’s observations
Mr. C meets criteria for GID and transvestism. Some transvestites also meet criteria for autogynephilia and report erotic arousal upon seeing oneself as a woman. Character pathology, specifically sexual fantasies associated with schizoid personality, might also contribute to unusual gender presentation. Sexologists also propose fluidity in gender identification across populations and over a person’s life span.
Autogynephilia—by which a man becomes sexually aroused by imagining or seeing himself as a woman15—usually is associated with transvestism. Autogynephiles often have sexual fantasies of possessing female anatomical structures, engaging in feminine behaviors, or performing female bodily functions such as lactation, menstruation, or childbirth.
Autogynephilia may be a misdirected heterosexuality and is more prevalent among male-to-female transsexuals who are attracted to women, both sexes, or neither sex than among those attracted only to men.16
Gender identity fluidity. Clinicians have recorded fluidity in gender identity (sense of masculinity or femininity) and sexual orientation (the sex to which one is attracted). Sexologists have tried to create scales that gauge these changes.
The Kinsey Heterosexual-Homosexual Rating Scale17 is based on Kinsey’s theory that men are not strictly heterosexual or homosexual. Scores between 0 and 6 indicate some degree of both (Table).
The Benjamin Gender Disorientation Scale, which measures gender identity variations, recognizes variability of gender dysphoria expression and underscores the difficulty of classifying patients who—like Mr. C—present with varied symptoms. The scale is available at www.wpath.org.
I did not administer the Kinsey or Benjamin scales to Mr. C. Although his case shows how innate sense of masculinity or femininity can vary among patients with gender dysphoria, his presentation has been stable, albeit unusual.
Mr. C’s symptoms. Mr. C shows autogynephilic features. He lacks the schizoid’s emotional inertness and his gender presentation is static, though dramatic. He appears to meet criteria for GID and transvestism, autogynephilic variant.
Schizoid and other personality disorders are associated with unusual sexual fantasies. Mr. C lacks primary schizoid features, such as flattened affectivity and indifference to close relationships.
Table
Kinsey Heterosexual-Homosexual Rating Scale
| Score | Indicates… |
|---|---|
| 0 | Exclusively heterosexual |
| 1 | Predominantly heterosexual, incidentally homosexual |
| 2 | Predominantly heterosexual, more than incidentally homosexual |
| 3 | Equally heterosexual and homosexual |
| 4 | Predominantly homosexual, more than incidentally heterosexual |
| 5 | Predominantly homosexual, incidentally heterosexual |
| 6 | Exclusively homosexual |
| Source: Reference 17. Reprinted by permission of The Kinsey Institute for Research in Sex, Gender, and Reproduction, Inc., Indiana University, Bloomington. | |
The author’s observations
Mr. C is a poor candidate for hormone therapy or gender reassignment surgery because of his circumscribed desire to live as a woman at home. Also, sexual gratification is his primary motivation for wanting to develop breasts.
Treating gender dysphoria
Serotonergic agents such as fluoxetine have shown effectiveness for treating paraphilias and nonparaphilic sexual addiction in case reports.18,19
Behavioral techniques, however, might have a more definite impact on gender dysphoria. Marks19 reported a 4-year remission of transsexualism in a patient after comorbid OCD improved with self-exposure therapy.
Psychotherapy will not resolve gender identity disorder but can promote a stable lifestyle and improve the patient’s chances for success in relationships, education, work, and gender identity expression.20 Psychotherapy can also help determine patients’ readiness for sexual reassignment surgery.
Treatment: learning to accept
I refer Mr. C back to his primary psychiatrist, who adds aripiprazole, 5 mg/d, to address grandiosity and hypomania that emerged months after my initial evaluation.
I also refer Mr. C to a gender disorder specialist for psychotherapy directed at examining his history, understanding his dilemmas, and identifying unrealistic ideas and maladaptive behaviors. The therapist has been teaching Mr. C coping skills and educating him on gender disorders and normal gender variations in activities and interests. He has been attending weekly sessions for 4 months.
To address his resentment over trying to look manly, I assure him that he doesn’t need to assume additional “masculine” behaviors and attitudes, and that his height and features make him appear masculine.
- World Professional Association for Transgender Health, formerly the Harry Benjamin International Gender Dysphoria Association (offers links to transgender resources, gender programs, and sexologists). www.hbigda.org.
- Divalproex sodium • Depakote
- Fluoxetine • Prozac
- Paroxetine • Paxil
Dr. Martin reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mental and behavioral disorders, diagnostic criteria for research. In: The ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research. Geneva: World Health Organization; 1993.
2. Caldwell C, Keshavan MS. Schizophrenia with secondary transsexualism. Can J Psychiatry 1991;36:300-1.
3. Hyde C, Kenna JC. A male MZ twin pair, concordant for transsexualism, discordant for schizophrenia. Acta Psychiatr Scand 1977;56:265-75.
4. Habermeyer E, Kamps I, Kawohl W. A case of bipolar psychosis and transsexualism. Psychopathology 2003;36:168-70.
5. O’Gorman EC. The effect of psychosis on gender identity. Br J Psychiatry 1980;136:314-5.
6. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000:566-76.
7. Hoenig J, Kenna JC. The nosolgical position of transsexualism. Arch Sex Behav 1974;3:273-87.
8. Jenike MA. Obsessive-compulsive and related disorders: a hidden epidemic. N Engl J Med 1989;24;321:539-41.
9. Stein DJ, Hollander E. The spectrum of obsessive-compulsive related disorders. In: Hollander E, ed. The obsessive-compulsive related disorders. Washington, DC: American Psychiatric Publishing. In press.
10. Hollander E. Serotonergic drugs and the treatment of disorders related to obsessive-compulsive disorder. In: Pato MT, Zohar J, eds. Current treatments of obsessive-compulsive disorder. Washington, DC: American Psychiatric Publishing; 1991:173-92.
11. Quadland MC. Compulsive sexual behavior: definition of a problem and an approach to treatment. J Sex Marital Ther 1985;11:121-32.
12. Coleman E. Sexual compulsivity: definition, etiology, and treatment considerations. In: Coleman E, ed. Chemical dependency and intimacy dysfunction. New York: Haworth Press; 1988.
13. Anthony DT, Hollander E. Sexual compulsions. In: Hollander E, ed. The obsessive-compulsive related disorders. Washington, DC: American Psychiatric Publishing. In press.
14. Perilstein RD, Lipper S, Friedman LJ. Three cases of paraphilias responsive to fluoxetine treatment. J Clin Psychiatry 1991;52:169-70.
15. Blanchard R. Nonmonotonic relation of autogynephilia and heterosexual attraction. J Abnorm Psychol 1992;101:271-6.
16. Hirschfeld M. Sexual anomalies. New York: Emerson Books; 1948.
17. Kinsey AC, Pomeroy WB, Martin CE. Sexual behavior in the human male. Philadelphia: WB Saunders; 1948;636-59.
18. Kafka MP. Successful antidepressant treatment of nonparaphilic sexual addictions and paraphilias in men. J Clin Psychiatry 1991;52:60-5.
19. Marks IM, Mataix-Cols D. Four-year remission of transsexualism after comorbid obsessive-compulsive disorder improved with self-exposure therapy. Case report. Br J Psychiatry 1997;171:389-90.
20. Harry Benjamin International Gender Dysphoria Association’s Standards of Care for Gender Identity Disorders. Int J Transgenderism 2001; 5(1). Available at: http://www.symposion.com/ijt/index.htm. Accessed November 3, 2006.
History: normal on paper
Mr. C, age 65, presents to an endocrinologist complaining of hot flashes and low libido. Initial testing shows low male testosterone, but a repeat test shows normal levels. No medical cause is found for his symptoms.
“My testosterone might be normal on paper,” Mr. C tells the endocrinologist, “but I’m not. I think I’m a woman.”
Mr. C requests referral to a female psychiatrist because he feels more comfortable discussing sexual issues with a woman. The endocrinologist refers him to me for evaluation.
Over 7 years, Mr. C’s other psychiatrist—a man—has been treating him for obsessive-compulsive disorder (OCD), anxiety disorder, and bipolar disorder type II. Mr. C takes paroxetine, 60 mg/d, for depressive symptoms and was taking divalproex, 1,500 mg/d, to stabilize his mood. He recently stopped divalproex because it was causing nausea and sedation.
During our initial visit, Mr. C says he’s “through pretending to be a man.” He says he first questioned his sexual identity in early childhood, when he sometimes dressed in his mother’s clothes for play. As an adult, he mostly cross-dresses in lingerie; he wears a woman’s tank top in public once or twice weekly underneath his polo dress shirt. Fifteen years ago, he suffered anorexia and bulimia while trying to look as svelte as a woman.
At 6 feet, 2 inches with good muscle tone and short, wavy black hair, Mr. C looks strikingly masculine. Now retired, he served in the Air Force and later worked as a commercial pilot and in construction. In private, however, he prefers gardening and cooking over sports and cars.
Mr. C is married but seldom has sexual intercourse with women. He gains sexual fulfillment by visualizing himself as a woman having sex with other women or with himself as a man. He denies interest in male-male sex.
The patient has been masturbating since age 5, mostly by rubbing his scrotum against a swing set pole he still keeps in his utility shed. He often tucks his penis to mimic female genitalia and makes believe his rectum is a vagina.
- Transsexualism
- Pure transvestism (having a firm gender identity but becoming sexually aroused by cross-dressing)
- Dual-role transvestism (cross-dressing solely to experience temporary membership in the opposite sex)
- Stress-related cross-dressing
- Men who desire penectomy or castration but no other gender-reassignment interventions
- Congenital intersex conditions, such as hermaphrodism
Mr. C’s Mini-Mental State Examination score of 30 indicates no underlying dementia. He shows stable affect with no evidence of derailment, paranoia, thought blocking, or auditory hallucinations.
Medical examination results are normal. Negative urine toxicology screen rules out substance abuse, and negative rapid plasma reagin rules out syphilis. Testosterone is not rechecked because levels were normal 2 days before.
The author’s observations
I suspect gender dysphoria, which describes a heterogeneous group of persons who express varying degrees of distress with their anatomic sex and sometimes desire secondary opposite-gender sexual characteristics (Box).
Sexual identity in gender dysphoria is often fluid. Symptoms might suggest transvestism, then evolve to transsexualism. Recognizing this heterogeneity and fluidity is crucial to diagnosis and treatment.
Primary transsexualism. The term “transsexualism” describes persons who want to live and be accepted within the opposite sex.1 The transsexual identity persists for ≥ 2 years and is not caused by another mental disorder or intersexed condition. Fetishism is classically absent and cross-dressing is not sexually gratifying. Most transsexuals want surgical and hormone treatment to make their bodies as congruent as possible with the preferred sex.
In 1994, DSM-IV recognized that some late-onset transsexuals showed features of comorbid transvestism and were sexually aroused by female dress and behaviors. Gender identity disorder (GID) replaced the term “transsexualism” and includes these individuals. A secondary diagnosis of transvestism is applied.
Secondary transsexualism. Case reports2 describe psychosis-induced transsexual desires in patients with schizophrenia. Gender dysphoria improved as their schizophrenia symptoms lessened.
The relationship between transsexualism and schizophrenia has been debated. Hyde and Kenna3 view transsexualism as a schizophrenia spectrum disorder, whereas sexologists consider transsexualism and schizophrenia distinct syndromes that can occur simultaneously.
Affective disorders might also alter contentment with gender role, but the relationship is unclear. Case reports of patients with bipolar disorder suggest that gender dysphoria intensity fluctuates with affective excursions.4 O’Gorman,5 however, described a bipolar patient whose gender dysphoria was mitigated during manic episodes.
Paraphilias are sexual disorders with recurrent intense urges and fantasies that do not follow normative arousal patterns and can diminish capacity for sexual intimacy.6 Manifestations include exhibitionism, fetishism, frotteurism, pedophilia, masochism/sadism, voyeurism, and transvestic fetishism.
Dividing transsexualism and pure transvestism paraphilia into discrete categories is simplistic, as transvestites can develop secondary components of transsexualism. Hoenig and Kenna7 assert that transsexualism—though not an anomalous erotic preference—is almost always preceded by transvestism or accompanied by cross-gender fetishism.
Nonparaphilic sexual addiction—included in DSM-IV-TR as sexual disorder not otherwise specified—describes culturally acceptable sexual interests and behaviors that are frequent or intense enough to reduce capacity for sexual intimacy. Such behaviors include compulsive masturbation, repetitive promiscuity, and dependence on anonymous sexual encounters.
An addiction model conceptualizes paraphilia as a form of pleasure seeking that has become habitual and self-destructive. Treatment involves directing patients to 12-step groups patterned after Alcoholics Anonymous.
Other models place paraphilias and related disorders within the OCD spectrum.8-13 Persons with OCD often are obsessed with sexual content and might grapple with religious and moral concerns about sexual issues. They typically consider their symptoms intrusive or senseless. Selective serotonin reuptake inhibitors—the standard medication for OCD—might alleviate paraphilia, but results are mixed.14
Mr. C’s symptoms. Mr. C shows features of GID and transvestism. His strong, persistent cross-gender identification and sense of inappropriateness with being a man indicate GID. His recurrent sexual urges and fantasies and impaired capacity for sexual intimacy suggest a paraphilia or transvestism.
The significance of Mr. C’s comorbid bipolar disorder and OCD is unclear. Both appeared controlled, but the potential for mania-induced hypersexuality cannot be ignored.
Diagnosing gender dysphoria
A thorough medical, psychiatric, and sexual history can reveal sexual identity symptoms’ source.
Consider a medical cause. Your medical workup may include a genital exam to check for irregularities such as hermaphrodism that can compound questions of sexual identity, and karyotyping to probe chromosomal anomalies, such as mosaicism or chimerism.
Consider schizophrenia or bipolar disorder, as mania or psychosis can cause aberrant sexual behavior. In gender-dysphoric patients with either disorder, treating the psychiatric comorbidity might alleviate the dysphoria. Watch for fluctuations in gender dysphoria intensity when you treat other psychopathologies.
Take a thorough sexual history. Being matter-of-fact while discussing unusual sexual acts will help the patient “open up” about his sexual problems. Ask him if he:
- showed gender-atypical behavior as a child, which can predict transsexualism or homosexuality has engaged in heterosexual, homosexual, or abnormal sexual acts; ask about frequency and preference
- is married or has a girlfriend. If so, are they getting along? How often do they have sex?
- cross-dresses. Does his partner cross-dress as well and, if so, do they cross-dress for sexual gratification or to identify with the opposite gender? Has this response changed over time? Where and how often do they cross-dress?
- is achieving sexual gratification. If so, how?
- has sexual fantasies involving breast-feeding, giving birth, or forced feminization through gender-changing surgeries or other means
- “tucks” his penis, urinates sitting down, or mimics other stereotypical feminine behavior.
The answers will uncover a motivation behind these behaviors, which is key to diagnosis. Sexual gratification as a motive suggests paraphilia, whereas a desire to live as a woman points to transsexualism. Because of the myriad presentations, multiple patient visits are necessary for a specific diagnosis.
Diagnosis: ‘i enjoy womanhood, but…’
I diagnose gender dysphoria, but because Mr. C’s mood is euthymic, I cannot discern how his mood instability might affect his dysphoria. His sexual fantasies are mood-congruent and evoke no shame.
Mr. C then states that he adamantly opposes living outwardly as a woman, and fears that an overt sex change would destroy his marriage and other relationships. Even so, he desires hormone therapy and surgical breast implants so he can more closely mimic physical womanhood and make masturbation more pleasurable. He says he would flatten his breasts with gauze while in public so he can continue to look like a man.
Though comfortable with his sexual fantasies, Mr. C laments that presenting himself as an “alpha male” drains his psychic energy.
The author’s observations
Mr. C meets criteria for GID and transvestism. Some transvestites also meet criteria for autogynephilia and report erotic arousal upon seeing oneself as a woman. Character pathology, specifically sexual fantasies associated with schizoid personality, might also contribute to unusual gender presentation. Sexologists also propose fluidity in gender identification across populations and over a person’s life span.
Autogynephilia—by which a man becomes sexually aroused by imagining or seeing himself as a woman15—usually is associated with transvestism. Autogynephiles often have sexual fantasies of possessing female anatomical structures, engaging in feminine behaviors, or performing female bodily functions such as lactation, menstruation, or childbirth.
Autogynephilia may be a misdirected heterosexuality and is more prevalent among male-to-female transsexuals who are attracted to women, both sexes, or neither sex than among those attracted only to men.16
Gender identity fluidity. Clinicians have recorded fluidity in gender identity (sense of masculinity or femininity) and sexual orientation (the sex to which one is attracted). Sexologists have tried to create scales that gauge these changes.
The Kinsey Heterosexual-Homosexual Rating Scale17 is based on Kinsey’s theory that men are not strictly heterosexual or homosexual. Scores between 0 and 6 indicate some degree of both (Table).
The Benjamin Gender Disorientation Scale, which measures gender identity variations, recognizes variability of gender dysphoria expression and underscores the difficulty of classifying patients who—like Mr. C—present with varied symptoms. The scale is available at www.wpath.org.
I did not administer the Kinsey or Benjamin scales to Mr. C. Although his case shows how innate sense of masculinity or femininity can vary among patients with gender dysphoria, his presentation has been stable, albeit unusual.
Mr. C’s symptoms. Mr. C shows autogynephilic features. He lacks the schizoid’s emotional inertness and his gender presentation is static, though dramatic. He appears to meet criteria for GID and transvestism, autogynephilic variant.
Schizoid and other personality disorders are associated with unusual sexual fantasies. Mr. C lacks primary schizoid features, such as flattened affectivity and indifference to close relationships.
Table
Kinsey Heterosexual-Homosexual Rating Scale
| Score | Indicates… |
|---|---|
| 0 | Exclusively heterosexual |
| 1 | Predominantly heterosexual, incidentally homosexual |
| 2 | Predominantly heterosexual, more than incidentally homosexual |
| 3 | Equally heterosexual and homosexual |
| 4 | Predominantly homosexual, more than incidentally heterosexual |
| 5 | Predominantly homosexual, incidentally heterosexual |
| 6 | Exclusively homosexual |
| Source: Reference 17. Reprinted by permission of The Kinsey Institute for Research in Sex, Gender, and Reproduction, Inc., Indiana University, Bloomington. | |
The author’s observations
Mr. C is a poor candidate for hormone therapy or gender reassignment surgery because of his circumscribed desire to live as a woman at home. Also, sexual gratification is his primary motivation for wanting to develop breasts.
Treating gender dysphoria
Serotonergic agents such as fluoxetine have shown effectiveness for treating paraphilias and nonparaphilic sexual addiction in case reports.18,19
Behavioral techniques, however, might have a more definite impact on gender dysphoria. Marks19 reported a 4-year remission of transsexualism in a patient after comorbid OCD improved with self-exposure therapy.
Psychotherapy will not resolve gender identity disorder but can promote a stable lifestyle and improve the patient’s chances for success in relationships, education, work, and gender identity expression.20 Psychotherapy can also help determine patients’ readiness for sexual reassignment surgery.
Treatment: learning to accept
I refer Mr. C back to his primary psychiatrist, who adds aripiprazole, 5 mg/d, to address grandiosity and hypomania that emerged months after my initial evaluation.
I also refer Mr. C to a gender disorder specialist for psychotherapy directed at examining his history, understanding his dilemmas, and identifying unrealistic ideas and maladaptive behaviors. The therapist has been teaching Mr. C coping skills and educating him on gender disorders and normal gender variations in activities and interests. He has been attending weekly sessions for 4 months.
To address his resentment over trying to look manly, I assure him that he doesn’t need to assume additional “masculine” behaviors and attitudes, and that his height and features make him appear masculine.
- World Professional Association for Transgender Health, formerly the Harry Benjamin International Gender Dysphoria Association (offers links to transgender resources, gender programs, and sexologists). www.hbigda.org.
- Divalproex sodium • Depakote
- Fluoxetine • Prozac
- Paroxetine • Paxil
Dr. Martin reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
History: normal on paper
Mr. C, age 65, presents to an endocrinologist complaining of hot flashes and low libido. Initial testing shows low male testosterone, but a repeat test shows normal levels. No medical cause is found for his symptoms.
“My testosterone might be normal on paper,” Mr. C tells the endocrinologist, “but I’m not. I think I’m a woman.”
Mr. C requests referral to a female psychiatrist because he feels more comfortable discussing sexual issues with a woman. The endocrinologist refers him to me for evaluation.
Over 7 years, Mr. C’s other psychiatrist—a man—has been treating him for obsessive-compulsive disorder (OCD), anxiety disorder, and bipolar disorder type II. Mr. C takes paroxetine, 60 mg/d, for depressive symptoms and was taking divalproex, 1,500 mg/d, to stabilize his mood. He recently stopped divalproex because it was causing nausea and sedation.
During our initial visit, Mr. C says he’s “through pretending to be a man.” He says he first questioned his sexual identity in early childhood, when he sometimes dressed in his mother’s clothes for play. As an adult, he mostly cross-dresses in lingerie; he wears a woman’s tank top in public once or twice weekly underneath his polo dress shirt. Fifteen years ago, he suffered anorexia and bulimia while trying to look as svelte as a woman.
At 6 feet, 2 inches with good muscle tone and short, wavy black hair, Mr. C looks strikingly masculine. Now retired, he served in the Air Force and later worked as a commercial pilot and in construction. In private, however, he prefers gardening and cooking over sports and cars.
Mr. C is married but seldom has sexual intercourse with women. He gains sexual fulfillment by visualizing himself as a woman having sex with other women or with himself as a man. He denies interest in male-male sex.
The patient has been masturbating since age 5, mostly by rubbing his scrotum against a swing set pole he still keeps in his utility shed. He often tucks his penis to mimic female genitalia and makes believe his rectum is a vagina.
- Transsexualism
- Pure transvestism (having a firm gender identity but becoming sexually aroused by cross-dressing)
- Dual-role transvestism (cross-dressing solely to experience temporary membership in the opposite sex)
- Stress-related cross-dressing
- Men who desire penectomy or castration but no other gender-reassignment interventions
- Congenital intersex conditions, such as hermaphrodism
Mr. C’s Mini-Mental State Examination score of 30 indicates no underlying dementia. He shows stable affect with no evidence of derailment, paranoia, thought blocking, or auditory hallucinations.
Medical examination results are normal. Negative urine toxicology screen rules out substance abuse, and negative rapid plasma reagin rules out syphilis. Testosterone is not rechecked because levels were normal 2 days before.
The author’s observations
I suspect gender dysphoria, which describes a heterogeneous group of persons who express varying degrees of distress with their anatomic sex and sometimes desire secondary opposite-gender sexual characteristics (Box).
Sexual identity in gender dysphoria is often fluid. Symptoms might suggest transvestism, then evolve to transsexualism. Recognizing this heterogeneity and fluidity is crucial to diagnosis and treatment.
Primary transsexualism. The term “transsexualism” describes persons who want to live and be accepted within the opposite sex.1 The transsexual identity persists for ≥ 2 years and is not caused by another mental disorder or intersexed condition. Fetishism is classically absent and cross-dressing is not sexually gratifying. Most transsexuals want surgical and hormone treatment to make their bodies as congruent as possible with the preferred sex.
In 1994, DSM-IV recognized that some late-onset transsexuals showed features of comorbid transvestism and were sexually aroused by female dress and behaviors. Gender identity disorder (GID) replaced the term “transsexualism” and includes these individuals. A secondary diagnosis of transvestism is applied.
Secondary transsexualism. Case reports2 describe psychosis-induced transsexual desires in patients with schizophrenia. Gender dysphoria improved as their schizophrenia symptoms lessened.
The relationship between transsexualism and schizophrenia has been debated. Hyde and Kenna3 view transsexualism as a schizophrenia spectrum disorder, whereas sexologists consider transsexualism and schizophrenia distinct syndromes that can occur simultaneously.
Affective disorders might also alter contentment with gender role, but the relationship is unclear. Case reports of patients with bipolar disorder suggest that gender dysphoria intensity fluctuates with affective excursions.4 O’Gorman,5 however, described a bipolar patient whose gender dysphoria was mitigated during manic episodes.
Paraphilias are sexual disorders with recurrent intense urges and fantasies that do not follow normative arousal patterns and can diminish capacity for sexual intimacy.6 Manifestations include exhibitionism, fetishism, frotteurism, pedophilia, masochism/sadism, voyeurism, and transvestic fetishism.
Dividing transsexualism and pure transvestism paraphilia into discrete categories is simplistic, as transvestites can develop secondary components of transsexualism. Hoenig and Kenna7 assert that transsexualism—though not an anomalous erotic preference—is almost always preceded by transvestism or accompanied by cross-gender fetishism.
Nonparaphilic sexual addiction—included in DSM-IV-TR as sexual disorder not otherwise specified—describes culturally acceptable sexual interests and behaviors that are frequent or intense enough to reduce capacity for sexual intimacy. Such behaviors include compulsive masturbation, repetitive promiscuity, and dependence on anonymous sexual encounters.
An addiction model conceptualizes paraphilia as a form of pleasure seeking that has become habitual and self-destructive. Treatment involves directing patients to 12-step groups patterned after Alcoholics Anonymous.
Other models place paraphilias and related disorders within the OCD spectrum.8-13 Persons with OCD often are obsessed with sexual content and might grapple with religious and moral concerns about sexual issues. They typically consider their symptoms intrusive or senseless. Selective serotonin reuptake inhibitors—the standard medication for OCD—might alleviate paraphilia, but results are mixed.14
Mr. C’s symptoms. Mr. C shows features of GID and transvestism. His strong, persistent cross-gender identification and sense of inappropriateness with being a man indicate GID. His recurrent sexual urges and fantasies and impaired capacity for sexual intimacy suggest a paraphilia or transvestism.
The significance of Mr. C’s comorbid bipolar disorder and OCD is unclear. Both appeared controlled, but the potential for mania-induced hypersexuality cannot be ignored.
Diagnosing gender dysphoria
A thorough medical, psychiatric, and sexual history can reveal sexual identity symptoms’ source.
Consider a medical cause. Your medical workup may include a genital exam to check for irregularities such as hermaphrodism that can compound questions of sexual identity, and karyotyping to probe chromosomal anomalies, such as mosaicism or chimerism.
Consider schizophrenia or bipolar disorder, as mania or psychosis can cause aberrant sexual behavior. In gender-dysphoric patients with either disorder, treating the psychiatric comorbidity might alleviate the dysphoria. Watch for fluctuations in gender dysphoria intensity when you treat other psychopathologies.
Take a thorough sexual history. Being matter-of-fact while discussing unusual sexual acts will help the patient “open up” about his sexual problems. Ask him if he:
- showed gender-atypical behavior as a child, which can predict transsexualism or homosexuality has engaged in heterosexual, homosexual, or abnormal sexual acts; ask about frequency and preference
- is married or has a girlfriend. If so, are they getting along? How often do they have sex?
- cross-dresses. Does his partner cross-dress as well and, if so, do they cross-dress for sexual gratification or to identify with the opposite gender? Has this response changed over time? Where and how often do they cross-dress?
- is achieving sexual gratification. If so, how?
- has sexual fantasies involving breast-feeding, giving birth, or forced feminization through gender-changing surgeries or other means
- “tucks” his penis, urinates sitting down, or mimics other stereotypical feminine behavior.
The answers will uncover a motivation behind these behaviors, which is key to diagnosis. Sexual gratification as a motive suggests paraphilia, whereas a desire to live as a woman points to transsexualism. Because of the myriad presentations, multiple patient visits are necessary for a specific diagnosis.
Diagnosis: ‘i enjoy womanhood, but…’
I diagnose gender dysphoria, but because Mr. C’s mood is euthymic, I cannot discern how his mood instability might affect his dysphoria. His sexual fantasies are mood-congruent and evoke no shame.
Mr. C then states that he adamantly opposes living outwardly as a woman, and fears that an overt sex change would destroy his marriage and other relationships. Even so, he desires hormone therapy and surgical breast implants so he can more closely mimic physical womanhood and make masturbation more pleasurable. He says he would flatten his breasts with gauze while in public so he can continue to look like a man.
Though comfortable with his sexual fantasies, Mr. C laments that presenting himself as an “alpha male” drains his psychic energy.
The author’s observations
Mr. C meets criteria for GID and transvestism. Some transvestites also meet criteria for autogynephilia and report erotic arousal upon seeing oneself as a woman. Character pathology, specifically sexual fantasies associated with schizoid personality, might also contribute to unusual gender presentation. Sexologists also propose fluidity in gender identification across populations and over a person’s life span.
Autogynephilia—by which a man becomes sexually aroused by imagining or seeing himself as a woman15—usually is associated with transvestism. Autogynephiles often have sexual fantasies of possessing female anatomical structures, engaging in feminine behaviors, or performing female bodily functions such as lactation, menstruation, or childbirth.
Autogynephilia may be a misdirected heterosexuality and is more prevalent among male-to-female transsexuals who are attracted to women, both sexes, or neither sex than among those attracted only to men.16
Gender identity fluidity. Clinicians have recorded fluidity in gender identity (sense of masculinity or femininity) and sexual orientation (the sex to which one is attracted). Sexologists have tried to create scales that gauge these changes.
The Kinsey Heterosexual-Homosexual Rating Scale17 is based on Kinsey’s theory that men are not strictly heterosexual or homosexual. Scores between 0 and 6 indicate some degree of both (Table).
The Benjamin Gender Disorientation Scale, which measures gender identity variations, recognizes variability of gender dysphoria expression and underscores the difficulty of classifying patients who—like Mr. C—present with varied symptoms. The scale is available at www.wpath.org.
I did not administer the Kinsey or Benjamin scales to Mr. C. Although his case shows how innate sense of masculinity or femininity can vary among patients with gender dysphoria, his presentation has been stable, albeit unusual.
Mr. C’s symptoms. Mr. C shows autogynephilic features. He lacks the schizoid’s emotional inertness and his gender presentation is static, though dramatic. He appears to meet criteria for GID and transvestism, autogynephilic variant.
Schizoid and other personality disorders are associated with unusual sexual fantasies. Mr. C lacks primary schizoid features, such as flattened affectivity and indifference to close relationships.
Table
Kinsey Heterosexual-Homosexual Rating Scale
| Score | Indicates… |
|---|---|
| 0 | Exclusively heterosexual |
| 1 | Predominantly heterosexual, incidentally homosexual |
| 2 | Predominantly heterosexual, more than incidentally homosexual |
| 3 | Equally heterosexual and homosexual |
| 4 | Predominantly homosexual, more than incidentally heterosexual |
| 5 | Predominantly homosexual, incidentally heterosexual |
| 6 | Exclusively homosexual |
| Source: Reference 17. Reprinted by permission of The Kinsey Institute for Research in Sex, Gender, and Reproduction, Inc., Indiana University, Bloomington. | |
The author’s observations
Mr. C is a poor candidate for hormone therapy or gender reassignment surgery because of his circumscribed desire to live as a woman at home. Also, sexual gratification is his primary motivation for wanting to develop breasts.
Treating gender dysphoria
Serotonergic agents such as fluoxetine have shown effectiveness for treating paraphilias and nonparaphilic sexual addiction in case reports.18,19
Behavioral techniques, however, might have a more definite impact on gender dysphoria. Marks19 reported a 4-year remission of transsexualism in a patient after comorbid OCD improved with self-exposure therapy.
Psychotherapy will not resolve gender identity disorder but can promote a stable lifestyle and improve the patient’s chances for success in relationships, education, work, and gender identity expression.20 Psychotherapy can also help determine patients’ readiness for sexual reassignment surgery.
Treatment: learning to accept
I refer Mr. C back to his primary psychiatrist, who adds aripiprazole, 5 mg/d, to address grandiosity and hypomania that emerged months after my initial evaluation.
I also refer Mr. C to a gender disorder specialist for psychotherapy directed at examining his history, understanding his dilemmas, and identifying unrealistic ideas and maladaptive behaviors. The therapist has been teaching Mr. C coping skills and educating him on gender disorders and normal gender variations in activities and interests. He has been attending weekly sessions for 4 months.
To address his resentment over trying to look manly, I assure him that he doesn’t need to assume additional “masculine” behaviors and attitudes, and that his height and features make him appear masculine.
- World Professional Association for Transgender Health, formerly the Harry Benjamin International Gender Dysphoria Association (offers links to transgender resources, gender programs, and sexologists). www.hbigda.org.
- Divalproex sodium • Depakote
- Fluoxetine • Prozac
- Paroxetine • Paxil
Dr. Martin reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mental and behavioral disorders, diagnostic criteria for research. In: The ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research. Geneva: World Health Organization; 1993.
2. Caldwell C, Keshavan MS. Schizophrenia with secondary transsexualism. Can J Psychiatry 1991;36:300-1.
3. Hyde C, Kenna JC. A male MZ twin pair, concordant for transsexualism, discordant for schizophrenia. Acta Psychiatr Scand 1977;56:265-75.
4. Habermeyer E, Kamps I, Kawohl W. A case of bipolar psychosis and transsexualism. Psychopathology 2003;36:168-70.
5. O’Gorman EC. The effect of psychosis on gender identity. Br J Psychiatry 1980;136:314-5.
6. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000:566-76.
7. Hoenig J, Kenna JC. The nosolgical position of transsexualism. Arch Sex Behav 1974;3:273-87.
8. Jenike MA. Obsessive-compulsive and related disorders: a hidden epidemic. N Engl J Med 1989;24;321:539-41.
9. Stein DJ, Hollander E. The spectrum of obsessive-compulsive related disorders. In: Hollander E, ed. The obsessive-compulsive related disorders. Washington, DC: American Psychiatric Publishing. In press.
10. Hollander E. Serotonergic drugs and the treatment of disorders related to obsessive-compulsive disorder. In: Pato MT, Zohar J, eds. Current treatments of obsessive-compulsive disorder. Washington, DC: American Psychiatric Publishing; 1991:173-92.
11. Quadland MC. Compulsive sexual behavior: definition of a problem and an approach to treatment. J Sex Marital Ther 1985;11:121-32.
12. Coleman E. Sexual compulsivity: definition, etiology, and treatment considerations. In: Coleman E, ed. Chemical dependency and intimacy dysfunction. New York: Haworth Press; 1988.
13. Anthony DT, Hollander E. Sexual compulsions. In: Hollander E, ed. The obsessive-compulsive related disorders. Washington, DC: American Psychiatric Publishing. In press.
14. Perilstein RD, Lipper S, Friedman LJ. Three cases of paraphilias responsive to fluoxetine treatment. J Clin Psychiatry 1991;52:169-70.
15. Blanchard R. Nonmonotonic relation of autogynephilia and heterosexual attraction. J Abnorm Psychol 1992;101:271-6.
16. Hirschfeld M. Sexual anomalies. New York: Emerson Books; 1948.
17. Kinsey AC, Pomeroy WB, Martin CE. Sexual behavior in the human male. Philadelphia: WB Saunders; 1948;636-59.
18. Kafka MP. Successful antidepressant treatment of nonparaphilic sexual addictions and paraphilias in men. J Clin Psychiatry 1991;52:60-5.
19. Marks IM, Mataix-Cols D. Four-year remission of transsexualism after comorbid obsessive-compulsive disorder improved with self-exposure therapy. Case report. Br J Psychiatry 1997;171:389-90.
20. Harry Benjamin International Gender Dysphoria Association’s Standards of Care for Gender Identity Disorders. Int J Transgenderism 2001; 5(1). Available at: http://www.symposion.com/ijt/index.htm. Accessed November 3, 2006.
1. Mental and behavioral disorders, diagnostic criteria for research. In: The ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research. Geneva: World Health Organization; 1993.
2. Caldwell C, Keshavan MS. Schizophrenia with secondary transsexualism. Can J Psychiatry 1991;36:300-1.
3. Hyde C, Kenna JC. A male MZ twin pair, concordant for transsexualism, discordant for schizophrenia. Acta Psychiatr Scand 1977;56:265-75.
4. Habermeyer E, Kamps I, Kawohl W. A case of bipolar psychosis and transsexualism. Psychopathology 2003;36:168-70.
5. O’Gorman EC. The effect of psychosis on gender identity. Br J Psychiatry 1980;136:314-5.
6. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000:566-76.
7. Hoenig J, Kenna JC. The nosolgical position of transsexualism. Arch Sex Behav 1974;3:273-87.
8. Jenike MA. Obsessive-compulsive and related disorders: a hidden epidemic. N Engl J Med 1989;24;321:539-41.
9. Stein DJ, Hollander E. The spectrum of obsessive-compulsive related disorders. In: Hollander E, ed. The obsessive-compulsive related disorders. Washington, DC: American Psychiatric Publishing. In press.
10. Hollander E. Serotonergic drugs and the treatment of disorders related to obsessive-compulsive disorder. In: Pato MT, Zohar J, eds. Current treatments of obsessive-compulsive disorder. Washington, DC: American Psychiatric Publishing; 1991:173-92.
11. Quadland MC. Compulsive sexual behavior: definition of a problem and an approach to treatment. J Sex Marital Ther 1985;11:121-32.
12. Coleman E. Sexual compulsivity: definition, etiology, and treatment considerations. In: Coleman E, ed. Chemical dependency and intimacy dysfunction. New York: Haworth Press; 1988.
13. Anthony DT, Hollander E. Sexual compulsions. In: Hollander E, ed. The obsessive-compulsive related disorders. Washington, DC: American Psychiatric Publishing. In press.
14. Perilstein RD, Lipper S, Friedman LJ. Three cases of paraphilias responsive to fluoxetine treatment. J Clin Psychiatry 1991;52:169-70.
15. Blanchard R. Nonmonotonic relation of autogynephilia and heterosexual attraction. J Abnorm Psychol 1992;101:271-6.
16. Hirschfeld M. Sexual anomalies. New York: Emerson Books; 1948.
17. Kinsey AC, Pomeroy WB, Martin CE. Sexual behavior in the human male. Philadelphia: WB Saunders; 1948;636-59.
18. Kafka MP. Successful antidepressant treatment of nonparaphilic sexual addictions and paraphilias in men. J Clin Psychiatry 1991;52:60-5.
19. Marks IM, Mataix-Cols D. Four-year remission of transsexualism after comorbid obsessive-compulsive disorder improved with self-exposure therapy. Case report. Br J Psychiatry 1997;171:389-90.
20. Harry Benjamin International Gender Dysphoria Association’s Standards of Care for Gender Identity Disorders. Int J Transgenderism 2001; 5(1). Available at: http://www.symposion.com/ijt/index.htm. Accessed November 3, 2006.
The consequences of sipping ‘tea’
History: a ‘negative’ view
Mr. J, a 50-year-old native of Fiji, has had depression and substance abuse disorder for more than 10 years, marked by irritability, poor sleep, hopelessness, and suicidality. He also suffered a traumatic brain injury in the military 25 years ago.
Police bring Mr. J to the ER after they find him wandering near traffic and speaking incoherently. His feet and hands jerk on the way to the hospital, leading police to suspect that Mr. J has suffered a grand mal seizure.
In the ER, Mr. J appears confused, has visual hallucinations, and moves his hands and feet involuntarily. His head and arms move erratically during the ER psychiatrist’s interview, and he says that his pelvis is arching forward and preventing him from walking steadily. The day before, he says, he saw frightening “visions” of a being who looked “like a photo negative.”
Mr. J has been seeing an outpatient psychiatrist, who has prescribed citalopram, 40 mg/d, for depression and clonazepam, 1 mg three times daily, for related anxiety symptoms.
The patient is disoriented and inattentive during the mental status examination. His cognitive deficits fluctuate in severity; at times he is aware of his surroundings, then suddenly loses this awareness.
Vital signs are stable. Physical exam shows Mr. J is approximately 30 lb underweight (97 lb) with a body mass index of 16.9 kg/m2—nearly 2 kg/m2 below normal. He says he has been skipping meals because of poor appetite. He also has strikingly lizard-like, scaly skin.
Urine drug screen shows no signs of recent alcohol or substance abuse. Complete metabolic profile shows elevated liver enzymes, suggesting alcohol or illicit substance toxicity, medication toxicity, hepatitis, thyroid disorder, muscle disease, or a rare liver condition. EEG shows mild encephalopathy but no ictal activity.
poll here
The authors’ observations
Our psychiatric differential diagnosis is broad:
- visual and auditory hallucinations are concurrent in numerous disorders, including schizophrenia and depression
- visual hallucinations alone suggest dementia, delirium, or psychosis resulting from a medical condition, medication, or substance(s) of abuse1
- Mr. J’s past head injury increases his risk of dementia and delirium
- his abrupt symptom onset and inattention suggest delirium.
Choreoathetosis can result from:
- medications such as stimulants and levodopa
- toxins
- systemic diseases such as systemic lupus erythematosus, thyrotoxicosis, or stroke
- degenerative brain diseases such as Huntington’s disease
- or focal brain diseases such as tumors.2
Although the test results narrow the differential diagnosis, we still have to consider numerous medical conditions that can cause delirium, such as trauma, cerebral vascular accident, intracerebral masses, CNS infection, and inflammatory disease.
poll here
History: collateral contributions
We refer Mr. J for lumbar puncture to rule out CNS infection and MRI to rule out tumor, abscess, or other structural brain abnormalities that could cause seizure. Results are unremarkable.
We then speak with Mr. J’s outpatient psychiatrist, who reports that Mr. J has had no residual cognitive impairment from his head injury. She adds, though, that he often develops cognitive problems after consuming large amounts of a traditional South Pacific beverage containing kava (Piper methysticum). She explains that Mr. J socializes with fellow Fijians who drink kava at gatherings, and that he often drinks kava to excess. She attributes his dry, scaly skin to excessive kava use.
Upon questioning, Mr. J says he consumes about a half-pound of kava root per day. He says he uses the root to make a tea-like beverage that, like alcohol, induces euphoria and relaxation. He says he began doing this in his youth back in Fiji, and now drinks “many cups” of kava per day.
Mr. J states that his current episode of strange movements and visual hallucinations began hours after he drank several cups of kava the day before police brought him to the ER. He considers his new psychiatric symptoms Jesus’ punishment for drinking kava.
The authors’ observations
Mr. J’s persecutory delusions suggest that he does not fully associate his symptoms with excessive kava use, but his abnormal movements, weight loss, skin changes, liver function abnormalities, and mental status changes are known adverse effects of kava.3 We diagnose substance-induced delirium rather than substance intoxication or substance-induced psychosis because:
- Mr. J’s cognitive symptoms are more severe than those caused by kava intoxication
- his psychotic symptoms occur only when he is delirious
- his disturbed consciousness, cognitive, and perceptual disturbances and the temporal relationship between symptom onset and massive kava use match DSM-IV-TR criteria for substance-induced delirium.4
Being aware of cultural customs and beliefs in your practice area can alert you to herbal substance use in various populations, such as kava by patients from the South Pacific or echinacea, goldenseal, and burdock by some Native Americans (see Related resources).
Medicinal use. Patients often use kava and other herbal supplements—including fatty acids, ginkgo biloba, ginseng, St. John’s wort, valerian, and others—with or instead of prescription drugs to alleviate psychiatric symptoms. Complementary and alternative medicine practitioners use kava to treat anxiety, for example (Box).
Anxiety and depression are among the most common reasons persons seek complementary or alternative treatment. In a national survey, 57% of respondents who suffered “anxiety attacks” and 54% of those with “severe depression” reported using such therapies.8 Nearly 1 in 5 persons who take prescription drugs also take herbs and/or high-dose vitamin supplements.9
Herbal products have been shown to cause adverse effects (Table 1).10 Kava, for example, has been associated with hepatotoxicity, dermopathy, movement disorders, GI disturbance, and weight loss. Standardized extracts such as capsules and tinctures appear more likely to cause adverse effects than traditional extractions, such as a beverage made by infusing kava root.5
Kava toxicity has been reported among heavy users. Although the dosage at which kava becomes dangerous is unknown, the FDA recommends that users not exceed typical dosages (50 to 280 mg/d) and use kava only under a physician’s supervision.11
Also, interactions between herbal products and allopathic medications can cause substantial morbidity (Table 2). St. John’s wort, for example, can lead to serotonin syndrome when combined with selective serotonin reuptake inhibitors (SSRIs)12 and can reduce blood levels of psychotropics metabolized by the cytochrome P-450 3A4 isoenzyme, such as alprazolam and carbamazepine.
Mr. J combined kava with clonazepam. Both substances affect gamma-aminobutyric (GABA) receptors, increasing the risk of sedation by depressing the CNS.
Table 1
Possible adverse effects of herbal supplements
used for psychiatric symptoms
| Medication | Psychiatric uses | Adverse effects |
|---|---|---|
| Fatty acids | Depression, mania | GI upset |
| 5-HTP (5-hydroxytryptophan) | Depression, anxiety | Agitation, ataxia, blurred vision, bradycardia, dyspnea, eosinophilia, headache, hypotension, insomnia, mania, psychosis, tremulousness |
| Ginkgo (Ginkgo biloba) | Cognitive enhancement | Bleeding, dizziness, GI upset, headache, palpitations, Stevens-Johnson syndrome |
| Ginseng (Panax ginseng) | Cognitive enhancement | Estrogenic effects, insomnia, mania |
| Kava (Piper methysticum) | Anxiety | Dermopathy, drowsiness, dry mouth, GI disturbance, hepatotoxicity, weight loss, movement disorders |
| SAM-e (S-adenosyl-L-methionine) | Depression, fibromyalgia | Constipation, diarrhea, increased salivation, headache, nausea, urinary frequency, mania in patients with bipolar disorder |
| St. John’s wort (Hypericum perforatum) | Depression | Anorexia, anorgasmia, anxiety, constipation, dizziness, dry mouth, fatigue, GI upset, mania, photosensitivity, pruritis, restlessness, urinary frequency |
| Valerian (Valeriana officinalis) | Insomnia | Drowsiness, GI upset, headache, hepatotoxicity |
| Source: Reference 10 | ||
Potential adverse interactions between psychotropics and complementary/alternative medications
| Herb | Interacts with… | Interaction can cause… |
|---|---|---|
| 5-HTP | carbidopa, MAOIs, SSRIs | delirium, serotonin syndrome |
| Ginseng | MAOIs | mania |
| Kava | first- and second-generation antipsychotics, benzodiazepines, MAOIs | sedation |
| SAM-E | TCAs | serotonin syndrome |
| St. John’s wort | benzodiazepines, beta blockers, buspirone, carbamazepine, clozapine, MAOIs, SSRIs, TCAs, trazodone | serotonin syndrome (w/SSRIs) |
| reduced plasma levels of cytochrome P-450 3A4 substrates, diminishing their effectiveness | ||
| Valerian | benzodiazepines | sedation |
| MAOIs: Monoamine oxidase inhibitors | ||
| SSRIs: Selective serotonin reuptake inhibitors | ||
| TCAs: Tricyclic antidepressants | ||
Kava, extracted from the roots of Piper methysticum, acts as a muscle relaxant, anesthetic, and anxiolytic.5 It is among the most commonly used alternative treatments for psychiatric symptoms, with sales estimated at $17 million in the United States in 2004.6
Kava lactones, the pharmacologically active components of kava, might act via several pathways, including GABA-A receptor binding and dopaminergic antagonism.7 This GABAergic CNS activity affects similar receptors as do benzodiazepines and produces kava’s anxiolytic effects.
Kava is available in health food stores as capsules, tinctures, and fluid extracts and can be obtained without a prescription. The amount of active ingredient varies greatly from preparation to preparation.
Ask about alternative medicine use
According to a national survey,13 many patients do not tell allopathic physicians they are using complementary or alternative medications because:
- “It wasn’t important for the doctor to know.”
- “The doctor never asked.”
- “It was none of the doctor’s business.”
- “The doctor would not understand.”
- or “The doctor would disapprove or discourage CAM use.”
- routinely question patients about use of alternative therapies
- discuss safety and efficacy of commonly used alternative treatments
- discuss merits of alternative treatments
- provide information on the effectiveness and risks of various treatments
- learn about alternative therapies by consulting the Physicians’ Desk Reference (PDR) for Herbal Medicines or similar references
- help patients make decisions about alternative treatments, such as finding a qualified, licensed alternative provider.
Treatment: quick resolution
We admit Mr. J to the inpatient psychiatry unit. There, we continue his outpatient prescription medications at the same dosages and block access to nonprescription substances. His symptoms begin to improve during the first day of hospitalization. His choreoathetosis, hallucinations, and confusion resolve within 48 hours, and he is medically stable.
We discharge Mr. J after 2 days and continue citalopram and clonazepam at the same dosages.
The authors’ observations
Kava reaches peak plasma levels 1.8 hours after oral dosing and has a short (9-hour) elimination half-life. As a result, kava intoxication symptoms tend to resolve rapidly, as in Mr. J’s case.
Although Mr. J is medically stable, liver damage associated with kava use can be irreversible, prompting some European countries to ban its sale. Make sure patients who report kava use are aware of its hepatotoxicity risk.
Follow-up: kicking the kava habit
Two weeks after Mr. J’s discharge, his psychiatrist notes that his mental status has returned to baseline and that his skin has improved dramatically. The patient is following his citalopram and clonazepam regimen, and he seems more aware of kava’s potential adverse effects.
Mr. J reports that he has not consumed kava since his hospitalization. He has been eating four meals per day and has gained 9 lb. He is pleased with his improved appetite and is motivated to continue abstaining from kava.
- National Center for Complementary and Alternative Medicine. http://nccam.nih.gov.
- Physicians Desk Reference (PDR) for herbal medicines, 3rd ed. Montvale, NJ: Thomson PDR; 2004.
- Ernst E, Pittler MH, Stevinson C, et al. The desktop guide to complementary and alternative medicine. Edinburgh, UK: Mosby; 2001.
- Alprazolam • Xanax
- Buspirone • BuSpar
- Carbamazepine • Equetro, others
- Carbidopa • Lodosyn
- Citalopram • Celexa
- Clonazepam • Klonopin
- Clozapine • Clozaril
- Trazodone • Desyrel
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Moore DA, Jefferson J. Handbook of medical psychiatry, 2nd ed. New York: Elsevier/Mosby; 2004:8.
2. Nagagopal V. Movement disorders. In: Noble J, Greene HL, Levinson W, eds. Textbook of primary care medicine, 3rd ed. New York: Elsevier/Mosby; 2001:1530-1.
3. De Smet PA. Health risks of herbal remedies: an update. Clin Pharmacol Ther 2004;76:1-17.
4. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000;143-5.
5. Whitton PA, Lau A, Salisbury J, et al. Kava lactones and the kavakava controversy. Phytochemistry 2003;64:673-9.
6. Nutrition Business Journal: Top 100 selling U.S. supplements sales & growth, 1999-2004. Available at: http://tdaf.nutrition4texas.org/meetings/files/2006TDA_Paul_Thomas_Top100.pdf. Accessed October 13, 2006.
7. Pittler MH, Ernst E. Efficacy of kava extract for treating anxiety: systematic review and meta-analysis. J Clin Psychopharmacol 2000;20:84-9.
8. Kessler RC, Soukup J, Davis RB, et al. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am J Psychiatry 2001;158:289-94.
9. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA 1998;280:1569-75.
10. Werneke U, Turner T, Priebe S. Complementary medicines in psychiatry: review of effectiveness and safety. Br J Psychiatry 2006;188:109-21.
11. Medline Plus. Kava (Piper methysticum G. Forst). Available at: http://www.nlm.nih.gov/medlineplus/druginfo/natural/patient-kava.html. Accessed October 13, 2006.
12. Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: a systematic review. Drugs 2001;61:2163-75.
13. Eisenberg DM, Kessler RC, Van Rompany MI, et al. Perceptions about complementary therapies relative to conventional therapies among adults who use both: results from a national survey. Ann Intern Med 2001;135:344-51.
14. Yager J, Siegfreid SL, DiMatteo TL. Use of alternative remedies by psychiatric patients: illustrative vignettes and a discussion of the issues. Am J Psychiatry 1999;156:1432-8.
History: a ‘negative’ view
Mr. J, a 50-year-old native of Fiji, has had depression and substance abuse disorder for more than 10 years, marked by irritability, poor sleep, hopelessness, and suicidality. He also suffered a traumatic brain injury in the military 25 years ago.
Police bring Mr. J to the ER after they find him wandering near traffic and speaking incoherently. His feet and hands jerk on the way to the hospital, leading police to suspect that Mr. J has suffered a grand mal seizure.
In the ER, Mr. J appears confused, has visual hallucinations, and moves his hands and feet involuntarily. His head and arms move erratically during the ER psychiatrist’s interview, and he says that his pelvis is arching forward and preventing him from walking steadily. The day before, he says, he saw frightening “visions” of a being who looked “like a photo negative.”
Mr. J has been seeing an outpatient psychiatrist, who has prescribed citalopram, 40 mg/d, for depression and clonazepam, 1 mg three times daily, for related anxiety symptoms.
The patient is disoriented and inattentive during the mental status examination. His cognitive deficits fluctuate in severity; at times he is aware of his surroundings, then suddenly loses this awareness.
Vital signs are stable. Physical exam shows Mr. J is approximately 30 lb underweight (97 lb) with a body mass index of 16.9 kg/m2—nearly 2 kg/m2 below normal. He says he has been skipping meals because of poor appetite. He also has strikingly lizard-like, scaly skin.
Urine drug screen shows no signs of recent alcohol or substance abuse. Complete metabolic profile shows elevated liver enzymes, suggesting alcohol or illicit substance toxicity, medication toxicity, hepatitis, thyroid disorder, muscle disease, or a rare liver condition. EEG shows mild encephalopathy but no ictal activity.
poll here
The authors’ observations
Our psychiatric differential diagnosis is broad:
- visual and auditory hallucinations are concurrent in numerous disorders, including schizophrenia and depression
- visual hallucinations alone suggest dementia, delirium, or psychosis resulting from a medical condition, medication, or substance(s) of abuse1
- Mr. J’s past head injury increases his risk of dementia and delirium
- his abrupt symptom onset and inattention suggest delirium.
Choreoathetosis can result from:
- medications such as stimulants and levodopa
- toxins
- systemic diseases such as systemic lupus erythematosus, thyrotoxicosis, or stroke
- degenerative brain diseases such as Huntington’s disease
- or focal brain diseases such as tumors.2
Although the test results narrow the differential diagnosis, we still have to consider numerous medical conditions that can cause delirium, such as trauma, cerebral vascular accident, intracerebral masses, CNS infection, and inflammatory disease.
poll here
History: collateral contributions
We refer Mr. J for lumbar puncture to rule out CNS infection and MRI to rule out tumor, abscess, or other structural brain abnormalities that could cause seizure. Results are unremarkable.
We then speak with Mr. J’s outpatient psychiatrist, who reports that Mr. J has had no residual cognitive impairment from his head injury. She adds, though, that he often develops cognitive problems after consuming large amounts of a traditional South Pacific beverage containing kava (Piper methysticum). She explains that Mr. J socializes with fellow Fijians who drink kava at gatherings, and that he often drinks kava to excess. She attributes his dry, scaly skin to excessive kava use.
Upon questioning, Mr. J says he consumes about a half-pound of kava root per day. He says he uses the root to make a tea-like beverage that, like alcohol, induces euphoria and relaxation. He says he began doing this in his youth back in Fiji, and now drinks “many cups” of kava per day.
Mr. J states that his current episode of strange movements and visual hallucinations began hours after he drank several cups of kava the day before police brought him to the ER. He considers his new psychiatric symptoms Jesus’ punishment for drinking kava.
The authors’ observations
Mr. J’s persecutory delusions suggest that he does not fully associate his symptoms with excessive kava use, but his abnormal movements, weight loss, skin changes, liver function abnormalities, and mental status changes are known adverse effects of kava.3 We diagnose substance-induced delirium rather than substance intoxication or substance-induced psychosis because:
- Mr. J’s cognitive symptoms are more severe than those caused by kava intoxication
- his psychotic symptoms occur only when he is delirious
- his disturbed consciousness, cognitive, and perceptual disturbances and the temporal relationship between symptom onset and massive kava use match DSM-IV-TR criteria for substance-induced delirium.4
Being aware of cultural customs and beliefs in your practice area can alert you to herbal substance use in various populations, such as kava by patients from the South Pacific or echinacea, goldenseal, and burdock by some Native Americans (see Related resources).
Medicinal use. Patients often use kava and other herbal supplements—including fatty acids, ginkgo biloba, ginseng, St. John’s wort, valerian, and others—with or instead of prescription drugs to alleviate psychiatric symptoms. Complementary and alternative medicine practitioners use kava to treat anxiety, for example (Box).
Anxiety and depression are among the most common reasons persons seek complementary or alternative treatment. In a national survey, 57% of respondents who suffered “anxiety attacks” and 54% of those with “severe depression” reported using such therapies.8 Nearly 1 in 5 persons who take prescription drugs also take herbs and/or high-dose vitamin supplements.9
Herbal products have been shown to cause adverse effects (Table 1).10 Kava, for example, has been associated with hepatotoxicity, dermopathy, movement disorders, GI disturbance, and weight loss. Standardized extracts such as capsules and tinctures appear more likely to cause adverse effects than traditional extractions, such as a beverage made by infusing kava root.5
Kava toxicity has been reported among heavy users. Although the dosage at which kava becomes dangerous is unknown, the FDA recommends that users not exceed typical dosages (50 to 280 mg/d) and use kava only under a physician’s supervision.11
Also, interactions between herbal products and allopathic medications can cause substantial morbidity (Table 2). St. John’s wort, for example, can lead to serotonin syndrome when combined with selective serotonin reuptake inhibitors (SSRIs)12 and can reduce blood levels of psychotropics metabolized by the cytochrome P-450 3A4 isoenzyme, such as alprazolam and carbamazepine.
Mr. J combined kava with clonazepam. Both substances affect gamma-aminobutyric (GABA) receptors, increasing the risk of sedation by depressing the CNS.
Table 1
Possible adverse effects of herbal supplements
used for psychiatric symptoms
| Medication | Psychiatric uses | Adverse effects |
|---|---|---|
| Fatty acids | Depression, mania | GI upset |
| 5-HTP (5-hydroxytryptophan) | Depression, anxiety | Agitation, ataxia, blurred vision, bradycardia, dyspnea, eosinophilia, headache, hypotension, insomnia, mania, psychosis, tremulousness |
| Ginkgo (Ginkgo biloba) | Cognitive enhancement | Bleeding, dizziness, GI upset, headache, palpitations, Stevens-Johnson syndrome |
| Ginseng (Panax ginseng) | Cognitive enhancement | Estrogenic effects, insomnia, mania |
| Kava (Piper methysticum) | Anxiety | Dermopathy, drowsiness, dry mouth, GI disturbance, hepatotoxicity, weight loss, movement disorders |
| SAM-e (S-adenosyl-L-methionine) | Depression, fibromyalgia | Constipation, diarrhea, increased salivation, headache, nausea, urinary frequency, mania in patients with bipolar disorder |
| St. John’s wort (Hypericum perforatum) | Depression | Anorexia, anorgasmia, anxiety, constipation, dizziness, dry mouth, fatigue, GI upset, mania, photosensitivity, pruritis, restlessness, urinary frequency |
| Valerian (Valeriana officinalis) | Insomnia | Drowsiness, GI upset, headache, hepatotoxicity |
| Source: Reference 10 | ||
Potential adverse interactions between psychotropics and complementary/alternative medications
| Herb | Interacts with… | Interaction can cause… |
|---|---|---|
| 5-HTP | carbidopa, MAOIs, SSRIs | delirium, serotonin syndrome |
| Ginseng | MAOIs | mania |
| Kava | first- and second-generation antipsychotics, benzodiazepines, MAOIs | sedation |
| SAM-E | TCAs | serotonin syndrome |
| St. John’s wort | benzodiazepines, beta blockers, buspirone, carbamazepine, clozapine, MAOIs, SSRIs, TCAs, trazodone | serotonin syndrome (w/SSRIs) |
| reduced plasma levels of cytochrome P-450 3A4 substrates, diminishing their effectiveness | ||
| Valerian | benzodiazepines | sedation |
| MAOIs: Monoamine oxidase inhibitors | ||
| SSRIs: Selective serotonin reuptake inhibitors | ||
| TCAs: Tricyclic antidepressants | ||
Kava, extracted from the roots of Piper methysticum, acts as a muscle relaxant, anesthetic, and anxiolytic.5 It is among the most commonly used alternative treatments for psychiatric symptoms, with sales estimated at $17 million in the United States in 2004.6
Kava lactones, the pharmacologically active components of kava, might act via several pathways, including GABA-A receptor binding and dopaminergic antagonism.7 This GABAergic CNS activity affects similar receptors as do benzodiazepines and produces kava’s anxiolytic effects.
Kava is available in health food stores as capsules, tinctures, and fluid extracts and can be obtained without a prescription. The amount of active ingredient varies greatly from preparation to preparation.
Ask about alternative medicine use
According to a national survey,13 many patients do not tell allopathic physicians they are using complementary or alternative medications because:
- “It wasn’t important for the doctor to know.”
- “The doctor never asked.”
- “It was none of the doctor’s business.”
- “The doctor would not understand.”
- or “The doctor would disapprove or discourage CAM use.”
- routinely question patients about use of alternative therapies
- discuss safety and efficacy of commonly used alternative treatments
- discuss merits of alternative treatments
- provide information on the effectiveness and risks of various treatments
- learn about alternative therapies by consulting the Physicians’ Desk Reference (PDR) for Herbal Medicines or similar references
- help patients make decisions about alternative treatments, such as finding a qualified, licensed alternative provider.
Treatment: quick resolution
We admit Mr. J to the inpatient psychiatry unit. There, we continue his outpatient prescription medications at the same dosages and block access to nonprescription substances. His symptoms begin to improve during the first day of hospitalization. His choreoathetosis, hallucinations, and confusion resolve within 48 hours, and he is medically stable.
We discharge Mr. J after 2 days and continue citalopram and clonazepam at the same dosages.
The authors’ observations
Kava reaches peak plasma levels 1.8 hours after oral dosing and has a short (9-hour) elimination half-life. As a result, kava intoxication symptoms tend to resolve rapidly, as in Mr. J’s case.
Although Mr. J is medically stable, liver damage associated with kava use can be irreversible, prompting some European countries to ban its sale. Make sure patients who report kava use are aware of its hepatotoxicity risk.
Follow-up: kicking the kava habit
Two weeks after Mr. J’s discharge, his psychiatrist notes that his mental status has returned to baseline and that his skin has improved dramatically. The patient is following his citalopram and clonazepam regimen, and he seems more aware of kava’s potential adverse effects.
Mr. J reports that he has not consumed kava since his hospitalization. He has been eating four meals per day and has gained 9 lb. He is pleased with his improved appetite and is motivated to continue abstaining from kava.
- National Center for Complementary and Alternative Medicine. http://nccam.nih.gov.
- Physicians Desk Reference (PDR) for herbal medicines, 3rd ed. Montvale, NJ: Thomson PDR; 2004.
- Ernst E, Pittler MH, Stevinson C, et al. The desktop guide to complementary and alternative medicine. Edinburgh, UK: Mosby; 2001.
- Alprazolam • Xanax
- Buspirone • BuSpar
- Carbamazepine • Equetro, others
- Carbidopa • Lodosyn
- Citalopram • Celexa
- Clonazepam • Klonopin
- Clozapine • Clozaril
- Trazodone • Desyrel
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
History: a ‘negative’ view
Mr. J, a 50-year-old native of Fiji, has had depression and substance abuse disorder for more than 10 years, marked by irritability, poor sleep, hopelessness, and suicidality. He also suffered a traumatic brain injury in the military 25 years ago.
Police bring Mr. J to the ER after they find him wandering near traffic and speaking incoherently. His feet and hands jerk on the way to the hospital, leading police to suspect that Mr. J has suffered a grand mal seizure.
In the ER, Mr. J appears confused, has visual hallucinations, and moves his hands and feet involuntarily. His head and arms move erratically during the ER psychiatrist’s interview, and he says that his pelvis is arching forward and preventing him from walking steadily. The day before, he says, he saw frightening “visions” of a being who looked “like a photo negative.”
Mr. J has been seeing an outpatient psychiatrist, who has prescribed citalopram, 40 mg/d, for depression and clonazepam, 1 mg three times daily, for related anxiety symptoms.
The patient is disoriented and inattentive during the mental status examination. His cognitive deficits fluctuate in severity; at times he is aware of his surroundings, then suddenly loses this awareness.
Vital signs are stable. Physical exam shows Mr. J is approximately 30 lb underweight (97 lb) with a body mass index of 16.9 kg/m2—nearly 2 kg/m2 below normal. He says he has been skipping meals because of poor appetite. He also has strikingly lizard-like, scaly skin.
Urine drug screen shows no signs of recent alcohol or substance abuse. Complete metabolic profile shows elevated liver enzymes, suggesting alcohol or illicit substance toxicity, medication toxicity, hepatitis, thyroid disorder, muscle disease, or a rare liver condition. EEG shows mild encephalopathy but no ictal activity.
poll here
The authors’ observations
Our psychiatric differential diagnosis is broad:
- visual and auditory hallucinations are concurrent in numerous disorders, including schizophrenia and depression
- visual hallucinations alone suggest dementia, delirium, or psychosis resulting from a medical condition, medication, or substance(s) of abuse1
- Mr. J’s past head injury increases his risk of dementia and delirium
- his abrupt symptom onset and inattention suggest delirium.
Choreoathetosis can result from:
- medications such as stimulants and levodopa
- toxins
- systemic diseases such as systemic lupus erythematosus, thyrotoxicosis, or stroke
- degenerative brain diseases such as Huntington’s disease
- or focal brain diseases such as tumors.2
Although the test results narrow the differential diagnosis, we still have to consider numerous medical conditions that can cause delirium, such as trauma, cerebral vascular accident, intracerebral masses, CNS infection, and inflammatory disease.
poll here
History: collateral contributions
We refer Mr. J for lumbar puncture to rule out CNS infection and MRI to rule out tumor, abscess, or other structural brain abnormalities that could cause seizure. Results are unremarkable.
We then speak with Mr. J’s outpatient psychiatrist, who reports that Mr. J has had no residual cognitive impairment from his head injury. She adds, though, that he often develops cognitive problems after consuming large amounts of a traditional South Pacific beverage containing kava (Piper methysticum). She explains that Mr. J socializes with fellow Fijians who drink kava at gatherings, and that he often drinks kava to excess. She attributes his dry, scaly skin to excessive kava use.
Upon questioning, Mr. J says he consumes about a half-pound of kava root per day. He says he uses the root to make a tea-like beverage that, like alcohol, induces euphoria and relaxation. He says he began doing this in his youth back in Fiji, and now drinks “many cups” of kava per day.
Mr. J states that his current episode of strange movements and visual hallucinations began hours after he drank several cups of kava the day before police brought him to the ER. He considers his new psychiatric symptoms Jesus’ punishment for drinking kava.
The authors’ observations
Mr. J’s persecutory delusions suggest that he does not fully associate his symptoms with excessive kava use, but his abnormal movements, weight loss, skin changes, liver function abnormalities, and mental status changes are known adverse effects of kava.3 We diagnose substance-induced delirium rather than substance intoxication or substance-induced psychosis because:
- Mr. J’s cognitive symptoms are more severe than those caused by kava intoxication
- his psychotic symptoms occur only when he is delirious
- his disturbed consciousness, cognitive, and perceptual disturbances and the temporal relationship between symptom onset and massive kava use match DSM-IV-TR criteria for substance-induced delirium.4
Being aware of cultural customs and beliefs in your practice area can alert you to herbal substance use in various populations, such as kava by patients from the South Pacific or echinacea, goldenseal, and burdock by some Native Americans (see Related resources).
Medicinal use. Patients often use kava and other herbal supplements—including fatty acids, ginkgo biloba, ginseng, St. John’s wort, valerian, and others—with or instead of prescription drugs to alleviate psychiatric symptoms. Complementary and alternative medicine practitioners use kava to treat anxiety, for example (Box).
Anxiety and depression are among the most common reasons persons seek complementary or alternative treatment. In a national survey, 57% of respondents who suffered “anxiety attacks” and 54% of those with “severe depression” reported using such therapies.8 Nearly 1 in 5 persons who take prescription drugs also take herbs and/or high-dose vitamin supplements.9
Herbal products have been shown to cause adverse effects (Table 1).10 Kava, for example, has been associated with hepatotoxicity, dermopathy, movement disorders, GI disturbance, and weight loss. Standardized extracts such as capsules and tinctures appear more likely to cause adverse effects than traditional extractions, such as a beverage made by infusing kava root.5
Kava toxicity has been reported among heavy users. Although the dosage at which kava becomes dangerous is unknown, the FDA recommends that users not exceed typical dosages (50 to 280 mg/d) and use kava only under a physician’s supervision.11
Also, interactions between herbal products and allopathic medications can cause substantial morbidity (Table 2). St. John’s wort, for example, can lead to serotonin syndrome when combined with selective serotonin reuptake inhibitors (SSRIs)12 and can reduce blood levels of psychotropics metabolized by the cytochrome P-450 3A4 isoenzyme, such as alprazolam and carbamazepine.
Mr. J combined kava with clonazepam. Both substances affect gamma-aminobutyric (GABA) receptors, increasing the risk of sedation by depressing the CNS.
Table 1
Possible adverse effects of herbal supplements
used for psychiatric symptoms
| Medication | Psychiatric uses | Adverse effects |
|---|---|---|
| Fatty acids | Depression, mania | GI upset |
| 5-HTP (5-hydroxytryptophan) | Depression, anxiety | Agitation, ataxia, blurred vision, bradycardia, dyspnea, eosinophilia, headache, hypotension, insomnia, mania, psychosis, tremulousness |
| Ginkgo (Ginkgo biloba) | Cognitive enhancement | Bleeding, dizziness, GI upset, headache, palpitations, Stevens-Johnson syndrome |
| Ginseng (Panax ginseng) | Cognitive enhancement | Estrogenic effects, insomnia, mania |
| Kava (Piper methysticum) | Anxiety | Dermopathy, drowsiness, dry mouth, GI disturbance, hepatotoxicity, weight loss, movement disorders |
| SAM-e (S-adenosyl-L-methionine) | Depression, fibromyalgia | Constipation, diarrhea, increased salivation, headache, nausea, urinary frequency, mania in patients with bipolar disorder |
| St. John’s wort (Hypericum perforatum) | Depression | Anorexia, anorgasmia, anxiety, constipation, dizziness, dry mouth, fatigue, GI upset, mania, photosensitivity, pruritis, restlessness, urinary frequency |
| Valerian (Valeriana officinalis) | Insomnia | Drowsiness, GI upset, headache, hepatotoxicity |
| Source: Reference 10 | ||
Potential adverse interactions between psychotropics and complementary/alternative medications
| Herb | Interacts with… | Interaction can cause… |
|---|---|---|
| 5-HTP | carbidopa, MAOIs, SSRIs | delirium, serotonin syndrome |
| Ginseng | MAOIs | mania |
| Kava | first- and second-generation antipsychotics, benzodiazepines, MAOIs | sedation |
| SAM-E | TCAs | serotonin syndrome |
| St. John’s wort | benzodiazepines, beta blockers, buspirone, carbamazepine, clozapine, MAOIs, SSRIs, TCAs, trazodone | serotonin syndrome (w/SSRIs) |
| reduced plasma levels of cytochrome P-450 3A4 substrates, diminishing their effectiveness | ||
| Valerian | benzodiazepines | sedation |
| MAOIs: Monoamine oxidase inhibitors | ||
| SSRIs: Selective serotonin reuptake inhibitors | ||
| TCAs: Tricyclic antidepressants | ||
Kava, extracted from the roots of Piper methysticum, acts as a muscle relaxant, anesthetic, and anxiolytic.5 It is among the most commonly used alternative treatments for psychiatric symptoms, with sales estimated at $17 million in the United States in 2004.6
Kava lactones, the pharmacologically active components of kava, might act via several pathways, including GABA-A receptor binding and dopaminergic antagonism.7 This GABAergic CNS activity affects similar receptors as do benzodiazepines and produces kava’s anxiolytic effects.
Kava is available in health food stores as capsules, tinctures, and fluid extracts and can be obtained without a prescription. The amount of active ingredient varies greatly from preparation to preparation.
Ask about alternative medicine use
According to a national survey,13 many patients do not tell allopathic physicians they are using complementary or alternative medications because:
- “It wasn’t important for the doctor to know.”
- “The doctor never asked.”
- “It was none of the doctor’s business.”
- “The doctor would not understand.”
- or “The doctor would disapprove or discourage CAM use.”
- routinely question patients about use of alternative therapies
- discuss safety and efficacy of commonly used alternative treatments
- discuss merits of alternative treatments
- provide information on the effectiveness and risks of various treatments
- learn about alternative therapies by consulting the Physicians’ Desk Reference (PDR) for Herbal Medicines or similar references
- help patients make decisions about alternative treatments, such as finding a qualified, licensed alternative provider.
Treatment: quick resolution
We admit Mr. J to the inpatient psychiatry unit. There, we continue his outpatient prescription medications at the same dosages and block access to nonprescription substances. His symptoms begin to improve during the first day of hospitalization. His choreoathetosis, hallucinations, and confusion resolve within 48 hours, and he is medically stable.
We discharge Mr. J after 2 days and continue citalopram and clonazepam at the same dosages.
The authors’ observations
Kava reaches peak plasma levels 1.8 hours after oral dosing and has a short (9-hour) elimination half-life. As a result, kava intoxication symptoms tend to resolve rapidly, as in Mr. J’s case.
Although Mr. J is medically stable, liver damage associated with kava use can be irreversible, prompting some European countries to ban its sale. Make sure patients who report kava use are aware of its hepatotoxicity risk.
Follow-up: kicking the kava habit
Two weeks after Mr. J’s discharge, his psychiatrist notes that his mental status has returned to baseline and that his skin has improved dramatically. The patient is following his citalopram and clonazepam regimen, and he seems more aware of kava’s potential adverse effects.
Mr. J reports that he has not consumed kava since his hospitalization. He has been eating four meals per day and has gained 9 lb. He is pleased with his improved appetite and is motivated to continue abstaining from kava.
- National Center for Complementary and Alternative Medicine. http://nccam.nih.gov.
- Physicians Desk Reference (PDR) for herbal medicines, 3rd ed. Montvale, NJ: Thomson PDR; 2004.
- Ernst E, Pittler MH, Stevinson C, et al. The desktop guide to complementary and alternative medicine. Edinburgh, UK: Mosby; 2001.
- Alprazolam • Xanax
- Buspirone • BuSpar
- Carbamazepine • Equetro, others
- Carbidopa • Lodosyn
- Citalopram • Celexa
- Clonazepam • Klonopin
- Clozapine • Clozaril
- Trazodone • Desyrel
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Moore DA, Jefferson J. Handbook of medical psychiatry, 2nd ed. New York: Elsevier/Mosby; 2004:8.
2. Nagagopal V. Movement disorders. In: Noble J, Greene HL, Levinson W, eds. Textbook of primary care medicine, 3rd ed. New York: Elsevier/Mosby; 2001:1530-1.
3. De Smet PA. Health risks of herbal remedies: an update. Clin Pharmacol Ther 2004;76:1-17.
4. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000;143-5.
5. Whitton PA, Lau A, Salisbury J, et al. Kava lactones and the kavakava controversy. Phytochemistry 2003;64:673-9.
6. Nutrition Business Journal: Top 100 selling U.S. supplements sales & growth, 1999-2004. Available at: http://tdaf.nutrition4texas.org/meetings/files/2006TDA_Paul_Thomas_Top100.pdf. Accessed October 13, 2006.
7. Pittler MH, Ernst E. Efficacy of kava extract for treating anxiety: systematic review and meta-analysis. J Clin Psychopharmacol 2000;20:84-9.
8. Kessler RC, Soukup J, Davis RB, et al. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am J Psychiatry 2001;158:289-94.
9. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA 1998;280:1569-75.
10. Werneke U, Turner T, Priebe S. Complementary medicines in psychiatry: review of effectiveness and safety. Br J Psychiatry 2006;188:109-21.
11. Medline Plus. Kava (Piper methysticum G. Forst). Available at: http://www.nlm.nih.gov/medlineplus/druginfo/natural/patient-kava.html. Accessed October 13, 2006.
12. Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: a systematic review. Drugs 2001;61:2163-75.
13. Eisenberg DM, Kessler RC, Van Rompany MI, et al. Perceptions about complementary therapies relative to conventional therapies among adults who use both: results from a national survey. Ann Intern Med 2001;135:344-51.
14. Yager J, Siegfreid SL, DiMatteo TL. Use of alternative remedies by psychiatric patients: illustrative vignettes and a discussion of the issues. Am J Psychiatry 1999;156:1432-8.
1. Moore DA, Jefferson J. Handbook of medical psychiatry, 2nd ed. New York: Elsevier/Mosby; 2004:8.
2. Nagagopal V. Movement disorders. In: Noble J, Greene HL, Levinson W, eds. Textbook of primary care medicine, 3rd ed. New York: Elsevier/Mosby; 2001:1530-1.
3. De Smet PA. Health risks of herbal remedies: an update. Clin Pharmacol Ther 2004;76:1-17.
4. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000;143-5.
5. Whitton PA, Lau A, Salisbury J, et al. Kava lactones and the kavakava controversy. Phytochemistry 2003;64:673-9.
6. Nutrition Business Journal: Top 100 selling U.S. supplements sales & growth, 1999-2004. Available at: http://tdaf.nutrition4texas.org/meetings/files/2006TDA_Paul_Thomas_Top100.pdf. Accessed October 13, 2006.
7. Pittler MH, Ernst E. Efficacy of kava extract for treating anxiety: systematic review and meta-analysis. J Clin Psychopharmacol 2000;20:84-9.
8. Kessler RC, Soukup J, Davis RB, et al. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am J Psychiatry 2001;158:289-94.
9. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA 1998;280:1569-75.
10. Werneke U, Turner T, Priebe S. Complementary medicines in psychiatry: review of effectiveness and safety. Br J Psychiatry 2006;188:109-21.
11. Medline Plus. Kava (Piper methysticum G. Forst). Available at: http://www.nlm.nih.gov/medlineplus/druginfo/natural/patient-kava.html. Accessed October 13, 2006.
12. Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: a systematic review. Drugs 2001;61:2163-75.
13. Eisenberg DM, Kessler RC, Van Rompany MI, et al. Perceptions about complementary therapies relative to conventional therapies among adults who use both: results from a national survey. Ann Intern Med 2001;135:344-51.
14. Yager J, Siegfreid SL, DiMatteo TL. Use of alternative remedies by psychiatric patients: illustrative vignettes and a discussion of the issues. Am J Psychiatry 1999;156:1432-8.
Anxiously looking for love
History: a lovelorn life
Ms. F, age 33, presents with one complaint: “I want to know how to maintain a relationship.” Problem is, social situations have made her feel anxious since childhood. She has trouble keeping a boyfriend; she left two intimate, extended relationships at different times.
She says she is too ashamed to invite people over because she cannot keep her apartment neat. She is also sick of her job as a filing clerk and wants a new career.
Ms. F reports no other anxiety symptoms or mood changes but often cannot concentrate. She denies impulsivity or poor judgment but admits that she makes decisions without getting important facts. For example, she enrolled at a community college without knowing what skills her new career would require. About 6 months ago, she left her boyfriend after realizing—18 months into the relationship—that he does not share her interests.
poll here
The authors’ observations
Information on all the above factors is crucial to diagnosing a socialization problem. Outline your differential diagnosis as the interview progresses.
Ask the patient:
How did you fare in school? A childhood history of pervasive inattention or impulsivity in at least two settings (at home and in school, for example) can signal attention-deficit/hyperactivity disorder (ADHD).fragile X syndrome). Boys with the fragile X premutation have a higher rate of ADHD symptoms and autism spectrum disorders than do boys without this premutation.3 Ms. F’s test showed two normal alleles, thus ruling out fragile X premutation.
Table 1
Mental status examination signs that suggest a PDD
| Little direct or sustained eye contact Eyes flit around the room Patient talks without looking at anyone |
| Few facial expressions Flat affect |
| Impaired speech production Although prosody (intonation) is normal, rate is rapid, with cluttered bursts followed by long pauses and occasional unusual emphasis on certain words |
| Tangential thought process Patient changes topics quickly without transition Non-sequitur responses |
| Brief responses to questions, offering little spontaneous information |
| Very detailed answers that include irrelevant information |
| Pedantic phrasing |
| Repetitive use of language |
| Does not pick up on nonquestions |
| Concrete answers to questions about emotion Patient cannot describe how emotions “feel” |
| Appears uncomfortable during conversation with examiner Rapport strained; patient does not seem to enjoy interaction |
| PDD: pervasive developmental disorder |
Treatment: medication and exploration
Ms. F agrees to an ADOS test. Her total score of 9 (7 in social, 2 in communication, and 0 in stereotyped/repetitive behavior) suggest a moderate PDD. We rule out autism based on the test score and Asperger’s syndrome because of her early language development delays (Table 2).
We start escitalopram, 10 mg/d, to address Ms. F’s anxiety. We see her weekly for medication management and start weekly psychotherapy to explore her two previous relationships and her desire to find a partner.
Ms. F, however, reacts anxiously to the therapist’s exploratory techniques. She has difficulty taking the lead and becomes extremely uncomfortable with silences in the conversation. The therapist tries cognitive-behavioral tactics to engage her, but Ms. F does not respond.
The therapist then conceptualizes her role as “coach” and tries a more-direct, problem-solving approach. She addresses specific challenges, such as an overwhelming class assignment, but Ms. F does not discuss or follow through on the problem.
After 6 months, Ms. F asks to stop psychotherapy because she has made little progress. She also asks to reduce medication checks to monthly, saying that weekly sessions interfere with her schoolwork. She says she would consider resuming psychotherapy.
At this point, Ms. F’s anxiety is significantly improved based on clinical impression. She continues to do well 6 months after stopping psychotherapy, though she is still without a boyfriend.
poll hereTable 2
Autism or Asperger’s? Watch for these distinguishing features
| Clinical feature | Autism | Asperger’s syndrome |
|---|---|---|
| Impaired nonverbal behavior | + | + |
| Language delay | + | – |
| Stereotyped behavior (routines, mannerisms) | + | + |
| Impaired social relationships | + | + |
| Cognitive delay | ± | – |
| +: Present –: absent ±: Might be present | ||
The authors’ observations
The ability to possess a theory of mind—or “mentalize”—helps us understand others’ beliefs, desires, thoughts, intentions, and knowledge. Attributing mental states to self and others helps explain and predict behavior, which is critical to social interaction.
A therapeutic relationship can help teach patients to handle social situations.4 In autism or PDD,5,6 however, theory of mind deficits typically frustrate relationship building.4 Because ability to mentalize is critical to psychodynamic psychotherapy,7 exploration does not help patients with PDD. By contrast, therapists can be more successful by being active in sessions and giving directions, suggestions, and information.
Which psychotherapy models work? Limited data address psychotherapy for adults with PDD; most studies have followed children.
CBT for persons with autism or PDD is directive, problem-focused, and targets automatic reactions.8 Social skills groups and CBT focusing on day-to-day problem solving can help older children and adolescents.9 A 20-week social skills intervention employing a CBT approach, paired with psychoeducation for parents, has helped boys ages 8 to 12 with autism, PDD, or Asperger’s syndrome.10
Other interventions use pictures, cartoons, and other visuals to help patients identify and correct misperceptions and determine how different responses might affect people’s thoughts and feelings.9,11 Role play allows the patient to practice social interaction but requires make-believe,11 so getting a PDD patient to participate can be challenging.
Medication can help manage comorbid anxiety, obsessive-compulsive, and mood symptoms in PDD. Limited data support using selective serotonin reuptake inhibitors for this purpose.12
Related resources
- Ozonoff S, Dawson G, McPartland J. A parent’s guide to Asperger syndrome & high-functioning autism: how to meet the challenges and help your child thrive. New York: Guilford Press; 2002.
- MAAP Services. A global information and support network for more advanced persons with autism and Asperger syndrome. www.asperger.org.
- Escitalopram • Lexapro
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
2. Lord C, Risi S, Lambrecht L, et al. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 2000;30:205-23.
3. Farzin F, Perry H, Hessl D, et al. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr 2006;27(S2):S137-S144.
4. Ramsay JR, Brodkin ES, Cohen MR, et al. “Better strangers:” using the relationship in psychotherapy for adult patients with Asperger syndrome. Psychotherapy: Theory, Research, Practice, Training 2005;42:483-93.
5. Hill E, Frith U. Understanding autism: insights from mind and brain. Philos Trans R Soc Lond B Biol Sci 2003;358:281-9.
6. Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain 2002;125:1839-49.
7. Gabbard GO. Psychodynamic psychiatry in clinical practice, 4th ed. Arlington, VA: American Psychiatric Publishing; 2005:60.
8. Beebe DW, Risi S. Treatment of adolescents and young adults with high-functioning autism or Asperger syndrome. In: Reinecke MA, Dattilio FM, Freeman A, eds. Cognitive therapy with children and adolescents. A casebook for clinical practice, 2nd ed. New York: Guilford Press; 2003.
9. Atwood T. Frameworks for behavioral interventions. Child Adolesc Psychiatr Clin N Am 2003;12:65-86.
10. Solomon M, Goodlin-Jones BL, Anders T. A social adjustment enhancement intervention for high functioning autism, Asperger’s syndrome, and pervasive developmental disorder NOS. J Autism Dev Disord 2004;34:649-68.
11. Rajendran G, Mitchell P, Rickards H. How do individuals with Asperger syndrome respond to nonliteral language and inappropriate requests in computer-mediated communication? J Autism Dev Disord 2005;35:429-43.
12. Namerow LB, Thomas P, Bostic JQ, et al. Use of citalopram in pervasive developmental disorders. J Dev Behav Pediatr 2003;24:104-8.
History: a lovelorn life
Ms. F, age 33, presents with one complaint: “I want to know how to maintain a relationship.” Problem is, social situations have made her feel anxious since childhood. She has trouble keeping a boyfriend; she left two intimate, extended relationships at different times.
She says she is too ashamed to invite people over because she cannot keep her apartment neat. She is also sick of her job as a filing clerk and wants a new career.
Ms. F reports no other anxiety symptoms or mood changes but often cannot concentrate. She denies impulsivity or poor judgment but admits that she makes decisions without getting important facts. For example, she enrolled at a community college without knowing what skills her new career would require. About 6 months ago, she left her boyfriend after realizing—18 months into the relationship—that he does not share her interests.
poll here
The authors’ observations
Information on all the above factors is crucial to diagnosing a socialization problem. Outline your differential diagnosis as the interview progresses.
Ask the patient:
How did you fare in school? A childhood history of pervasive inattention or impulsivity in at least two settings (at home and in school, for example) can signal attention-deficit/hyperactivity disorder (ADHD).fragile X syndrome). Boys with the fragile X premutation have a higher rate of ADHD symptoms and autism spectrum disorders than do boys without this premutation.3 Ms. F’s test showed two normal alleles, thus ruling out fragile X premutation.
Table 1
Mental status examination signs that suggest a PDD
| Little direct or sustained eye contact Eyes flit around the room Patient talks without looking at anyone |
| Few facial expressions Flat affect |
| Impaired speech production Although prosody (intonation) is normal, rate is rapid, with cluttered bursts followed by long pauses and occasional unusual emphasis on certain words |
| Tangential thought process Patient changes topics quickly without transition Non-sequitur responses |
| Brief responses to questions, offering little spontaneous information |
| Very detailed answers that include irrelevant information |
| Pedantic phrasing |
| Repetitive use of language |
| Does not pick up on nonquestions |
| Concrete answers to questions about emotion Patient cannot describe how emotions “feel” |
| Appears uncomfortable during conversation with examiner Rapport strained; patient does not seem to enjoy interaction |
| PDD: pervasive developmental disorder |
Treatment: medication and exploration
Ms. F agrees to an ADOS test. Her total score of 9 (7 in social, 2 in communication, and 0 in stereotyped/repetitive behavior) suggest a moderate PDD. We rule out autism based on the test score and Asperger’s syndrome because of her early language development delays (Table 2).
We start escitalopram, 10 mg/d, to address Ms. F’s anxiety. We see her weekly for medication management and start weekly psychotherapy to explore her two previous relationships and her desire to find a partner.
Ms. F, however, reacts anxiously to the therapist’s exploratory techniques. She has difficulty taking the lead and becomes extremely uncomfortable with silences in the conversation. The therapist tries cognitive-behavioral tactics to engage her, but Ms. F does not respond.
The therapist then conceptualizes her role as “coach” and tries a more-direct, problem-solving approach. She addresses specific challenges, such as an overwhelming class assignment, but Ms. F does not discuss or follow through on the problem.
After 6 months, Ms. F asks to stop psychotherapy because she has made little progress. She also asks to reduce medication checks to monthly, saying that weekly sessions interfere with her schoolwork. She says she would consider resuming psychotherapy.
At this point, Ms. F’s anxiety is significantly improved based on clinical impression. She continues to do well 6 months after stopping psychotherapy, though she is still without a boyfriend.
poll hereTable 2
Autism or Asperger’s? Watch for these distinguishing features
| Clinical feature | Autism | Asperger’s syndrome |
|---|---|---|
| Impaired nonverbal behavior | + | + |
| Language delay | + | – |
| Stereotyped behavior (routines, mannerisms) | + | + |
| Impaired social relationships | + | + |
| Cognitive delay | ± | – |
| +: Present –: absent ±: Might be present | ||
The authors’ observations
The ability to possess a theory of mind—or “mentalize”—helps us understand others’ beliefs, desires, thoughts, intentions, and knowledge. Attributing mental states to self and others helps explain and predict behavior, which is critical to social interaction.
A therapeutic relationship can help teach patients to handle social situations.4 In autism or PDD,5,6 however, theory of mind deficits typically frustrate relationship building.4 Because ability to mentalize is critical to psychodynamic psychotherapy,7 exploration does not help patients with PDD. By contrast, therapists can be more successful by being active in sessions and giving directions, suggestions, and information.
Which psychotherapy models work? Limited data address psychotherapy for adults with PDD; most studies have followed children.
CBT for persons with autism or PDD is directive, problem-focused, and targets automatic reactions.8 Social skills groups and CBT focusing on day-to-day problem solving can help older children and adolescents.9 A 20-week social skills intervention employing a CBT approach, paired with psychoeducation for parents, has helped boys ages 8 to 12 with autism, PDD, or Asperger’s syndrome.10
Other interventions use pictures, cartoons, and other visuals to help patients identify and correct misperceptions and determine how different responses might affect people’s thoughts and feelings.9,11 Role play allows the patient to practice social interaction but requires make-believe,11 so getting a PDD patient to participate can be challenging.
Medication can help manage comorbid anxiety, obsessive-compulsive, and mood symptoms in PDD. Limited data support using selective serotonin reuptake inhibitors for this purpose.12
Related resources
- Ozonoff S, Dawson G, McPartland J. A parent’s guide to Asperger syndrome & high-functioning autism: how to meet the challenges and help your child thrive. New York: Guilford Press; 2002.
- MAAP Services. A global information and support network for more advanced persons with autism and Asperger syndrome. www.asperger.org.
- Escitalopram • Lexapro
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
History: a lovelorn life
Ms. F, age 33, presents with one complaint: “I want to know how to maintain a relationship.” Problem is, social situations have made her feel anxious since childhood. She has trouble keeping a boyfriend; she left two intimate, extended relationships at different times.
She says she is too ashamed to invite people over because she cannot keep her apartment neat. She is also sick of her job as a filing clerk and wants a new career.
Ms. F reports no other anxiety symptoms or mood changes but often cannot concentrate. She denies impulsivity or poor judgment but admits that she makes decisions without getting important facts. For example, she enrolled at a community college without knowing what skills her new career would require. About 6 months ago, she left her boyfriend after realizing—18 months into the relationship—that he does not share her interests.
poll here
The authors’ observations
Information on all the above factors is crucial to diagnosing a socialization problem. Outline your differential diagnosis as the interview progresses.
Ask the patient:
How did you fare in school? A childhood history of pervasive inattention or impulsivity in at least two settings (at home and in school, for example) can signal attention-deficit/hyperactivity disorder (ADHD).fragile X syndrome). Boys with the fragile X premutation have a higher rate of ADHD symptoms and autism spectrum disorders than do boys without this premutation.3 Ms. F’s test showed two normal alleles, thus ruling out fragile X premutation.
Table 1
Mental status examination signs that suggest a PDD
| Little direct or sustained eye contact Eyes flit around the room Patient talks without looking at anyone |
| Few facial expressions Flat affect |
| Impaired speech production Although prosody (intonation) is normal, rate is rapid, with cluttered bursts followed by long pauses and occasional unusual emphasis on certain words |
| Tangential thought process Patient changes topics quickly without transition Non-sequitur responses |
| Brief responses to questions, offering little spontaneous information |
| Very detailed answers that include irrelevant information |
| Pedantic phrasing |
| Repetitive use of language |
| Does not pick up on nonquestions |
| Concrete answers to questions about emotion Patient cannot describe how emotions “feel” |
| Appears uncomfortable during conversation with examiner Rapport strained; patient does not seem to enjoy interaction |
| PDD: pervasive developmental disorder |
Treatment: medication and exploration
Ms. F agrees to an ADOS test. Her total score of 9 (7 in social, 2 in communication, and 0 in stereotyped/repetitive behavior) suggest a moderate PDD. We rule out autism based on the test score and Asperger’s syndrome because of her early language development delays (Table 2).
We start escitalopram, 10 mg/d, to address Ms. F’s anxiety. We see her weekly for medication management and start weekly psychotherapy to explore her two previous relationships and her desire to find a partner.
Ms. F, however, reacts anxiously to the therapist’s exploratory techniques. She has difficulty taking the lead and becomes extremely uncomfortable with silences in the conversation. The therapist tries cognitive-behavioral tactics to engage her, but Ms. F does not respond.
The therapist then conceptualizes her role as “coach” and tries a more-direct, problem-solving approach. She addresses specific challenges, such as an overwhelming class assignment, but Ms. F does not discuss or follow through on the problem.
After 6 months, Ms. F asks to stop psychotherapy because she has made little progress. She also asks to reduce medication checks to monthly, saying that weekly sessions interfere with her schoolwork. She says she would consider resuming psychotherapy.
At this point, Ms. F’s anxiety is significantly improved based on clinical impression. She continues to do well 6 months after stopping psychotherapy, though she is still without a boyfriend.
poll hereTable 2
Autism or Asperger’s? Watch for these distinguishing features
| Clinical feature | Autism | Asperger’s syndrome |
|---|---|---|
| Impaired nonverbal behavior | + | + |
| Language delay | + | – |
| Stereotyped behavior (routines, mannerisms) | + | + |
| Impaired social relationships | + | + |
| Cognitive delay | ± | – |
| +: Present –: absent ±: Might be present | ||
The authors’ observations
The ability to possess a theory of mind—or “mentalize”—helps us understand others’ beliefs, desires, thoughts, intentions, and knowledge. Attributing mental states to self and others helps explain and predict behavior, which is critical to social interaction.
A therapeutic relationship can help teach patients to handle social situations.4 In autism or PDD,5,6 however, theory of mind deficits typically frustrate relationship building.4 Because ability to mentalize is critical to psychodynamic psychotherapy,7 exploration does not help patients with PDD. By contrast, therapists can be more successful by being active in sessions and giving directions, suggestions, and information.
Which psychotherapy models work? Limited data address psychotherapy for adults with PDD; most studies have followed children.
CBT for persons with autism or PDD is directive, problem-focused, and targets automatic reactions.8 Social skills groups and CBT focusing on day-to-day problem solving can help older children and adolescents.9 A 20-week social skills intervention employing a CBT approach, paired with psychoeducation for parents, has helped boys ages 8 to 12 with autism, PDD, or Asperger’s syndrome.10
Other interventions use pictures, cartoons, and other visuals to help patients identify and correct misperceptions and determine how different responses might affect people’s thoughts and feelings.9,11 Role play allows the patient to practice social interaction but requires make-believe,11 so getting a PDD patient to participate can be challenging.
Medication can help manage comorbid anxiety, obsessive-compulsive, and mood symptoms in PDD. Limited data support using selective serotonin reuptake inhibitors for this purpose.12
Related resources
- Ozonoff S, Dawson G, McPartland J. A parent’s guide to Asperger syndrome & high-functioning autism: how to meet the challenges and help your child thrive. New York: Guilford Press; 2002.
- MAAP Services. A global information and support network for more advanced persons with autism and Asperger syndrome. www.asperger.org.
- Escitalopram • Lexapro
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
2. Lord C, Risi S, Lambrecht L, et al. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 2000;30:205-23.
3. Farzin F, Perry H, Hessl D, et al. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr 2006;27(S2):S137-S144.
4. Ramsay JR, Brodkin ES, Cohen MR, et al. “Better strangers:” using the relationship in psychotherapy for adult patients with Asperger syndrome. Psychotherapy: Theory, Research, Practice, Training 2005;42:483-93.
5. Hill E, Frith U. Understanding autism: insights from mind and brain. Philos Trans R Soc Lond B Biol Sci 2003;358:281-9.
6. Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain 2002;125:1839-49.
7. Gabbard GO. Psychodynamic psychiatry in clinical practice, 4th ed. Arlington, VA: American Psychiatric Publishing; 2005:60.
8. Beebe DW, Risi S. Treatment of adolescents and young adults with high-functioning autism or Asperger syndrome. In: Reinecke MA, Dattilio FM, Freeman A, eds. Cognitive therapy with children and adolescents. A casebook for clinical practice, 2nd ed. New York: Guilford Press; 2003.
9. Atwood T. Frameworks for behavioral interventions. Child Adolesc Psychiatr Clin N Am 2003;12:65-86.
10. Solomon M, Goodlin-Jones BL, Anders T. A social adjustment enhancement intervention for high functioning autism, Asperger’s syndrome, and pervasive developmental disorder NOS. J Autism Dev Disord 2004;34:649-68.
11. Rajendran G, Mitchell P, Rickards H. How do individuals with Asperger syndrome respond to nonliteral language and inappropriate requests in computer-mediated communication? J Autism Dev Disord 2005;35:429-43.
12. Namerow LB, Thomas P, Bostic JQ, et al. Use of citalopram in pervasive developmental disorders. J Dev Behav Pediatr 2003;24:104-8.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
2. Lord C, Risi S, Lambrecht L, et al. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 2000;30:205-23.
3. Farzin F, Perry H, Hessl D, et al. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr 2006;27(S2):S137-S144.
4. Ramsay JR, Brodkin ES, Cohen MR, et al. “Better strangers:” using the relationship in psychotherapy for adult patients with Asperger syndrome. Psychotherapy: Theory, Research, Practice, Training 2005;42:483-93.
5. Hill E, Frith U. Understanding autism: insights from mind and brain. Philos Trans R Soc Lond B Biol Sci 2003;358:281-9.
6. Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain 2002;125:1839-49.
7. Gabbard GO. Psychodynamic psychiatry in clinical practice, 4th ed. Arlington, VA: American Psychiatric Publishing; 2005:60.
8. Beebe DW, Risi S. Treatment of adolescents and young adults with high-functioning autism or Asperger syndrome. In: Reinecke MA, Dattilio FM, Freeman A, eds. Cognitive therapy with children and adolescents. A casebook for clinical practice, 2nd ed. New York: Guilford Press; 2003.
9. Atwood T. Frameworks for behavioral interventions. Child Adolesc Psychiatr Clin N Am 2003;12:65-86.
10. Solomon M, Goodlin-Jones BL, Anders T. A social adjustment enhancement intervention for high functioning autism, Asperger’s syndrome, and pervasive developmental disorder NOS. J Autism Dev Disord 2004;34:649-68.
11. Rajendran G, Mitchell P, Rickards H. How do individuals with Asperger syndrome respond to nonliteral language and inappropriate requests in computer-mediated communication? J Autism Dev Disord 2005;35:429-43.
12. Namerow LB, Thomas P, Bostic JQ, et al. Use of citalopram in pervasive developmental disorders. J Dev Behav Pediatr 2003;24:104-8.
When clozapine is not an option
History: ‘leaving town’
Mr. S, age 58, escaped repeatedly from his group home over 4 weeks. During one episode, he removed mail from neighbors’ mailboxes and tried to direct midday traffic. He would disappear for a few hours, sometimes overnight, before returning or being brought back by police.
The patient—who has had schizophrenia with catatonic features for 30 years—offered assorted explanations for escaping, most of them based on delusional beliefs, such as “I’m leaving town to get married” or “I’m late for engineering class.”
Since his last escape 3 weeks ago, Mr. S has remained in the group home without incident but has not been reporting for his usual outpatient psychiatric care. One day, he finally presents to us at the group home sponsor’s urging.
On evaluation, Mr. S shows stereotyped speech, staring, posturing, speech-prompt mutism, and odd mannerisms such as saluting. He has not been bathing or sleeping and smiles inappropriately. He speaks only when spoken to and answers with short phrases punctuated with ”By the grace of the good Lord.”
The authors’ observations
DSM-IV-TR requires at least two features to diagnose catatonic schizophrenia:
- peculiar voluntary movements
- extreme negativism
- excessive motor activity
- echolalia or echopraxia
- motoric immobility.1
Catatonia is common among the chronic mentally ill,2 yet it often goes undiagnosed.3 As a form of psychosis, catatonia might lead to greater functional impairment if not treated.
Treatment: time to try clozapine?
Over 10 years, numerous antipsychotic regimens plus adjunctive valproic acid, 500 mg tid, or lorazepam, up to 2 mg tid, have not lessened Mr. S’ psychosis and impulsivity. We start clozapine, 400 mg/d, and order twice-monthly blood tests to check for clozapine-induced agranulocytosis.
After nearly 6 months, some catatonic features improve gradually based on clinical interview. Serum clozapine is 363 ng/mL.
poll here
The authors’ observations
Second-generation antipsychotics (SGAs) are favored over first-generation antipsychotics to treat schizophrenia with catatonic features (Table),4,5 but no drug in either class has worked for Mr. S.
ECT can alleviate catatonic schizophrenia,4,6 but this option often is not available because the clinician fears a negative outcome would prompt legal action, or the guardian or next of kin do not consent to the procedure.3 We considered referring Mr. S to an ECT provider, but he has no legal guardian to provide consent. The group home sponsor also objected to ECT because Mr. S would have been sent out of town for treatment.
Catatonia patients who are immobile, physically compromised, and refuse food and drink typically are considered ECT candidates. Mr. S eats and drinks regularly and is physically able.
Lorazepam can produce rapid response, but it can be addictive.2 Also, an adjunctive 2 mg/d dosage showed no effect.
Clozapine monotherapy has shown effectiveness in catatonic schizophrenia7 and might be an option after other antipsychotics have failed.
Table 1
Treatments for catatonia: risks and benefits
| Medication | Use | Rationale | Benefits | Risks |
|---|---|---|---|---|
| First-generation antipsychotics (FGAs) | Often used for schizophrenia | Control positive symptoms | Well-established | Catatonia might be difficult to distinguish from NMS |
| Less expensive than other medications | ||||
| Second-generation antipsychotics (SGAs) | Beneficial in catatonia | Less likely than FGAs to worsen catatonia because of low D2 blockade | Some studies suggest greater efficacy than with FGAs | Metabolic syndrome, agranulocytosis with clozapine |
| Benzodiazepines | Lorazepam helpful in acute catatonia | Can be added to any antipsychotic | Safe, first-line treatment for catatonia | Respiratory compromise, incoordination, sedation, potential for abuse |
| Electroconvulsive therapy | ||||
| Electroconvulsive therapy | Beneficial in malignant catatonia | Effective in catatonia, NMS | Useful for treatment-refractory catatonia | Concerns with anesthesia, informed consent, availability |
| Rapid onset of action | ||||
| NMS: Neuroleptic malignant syndrome | ||||
Complication: agranulocytosis, then nms
Six months after starting clozapine, Mr. S starts having diaphoresis and night sweats, suggesting neutropenia. Blood testing shows a white blood cell count (WBC) of 3.6/μL, down from 4.6/μL 2 weeks before (normal range, 4.6 to 11/μL).
One week later, Mr. S’ WBC is 1.6/μL with a 46% relative neutrophil value (normal range, 50% to 70%) and an absolute neutrophil count of 736 (normal range, 2,500 to 7,000).
We diagnose agranulocytosis and stop clozapine, but Mr. S’ WBC continues to fall over 2 weeks to 0.8/μL with a 16% relative and 128 absolute neutrophil count. After 1 more week, his WBC increases to 2.6/μL and returns to normal 1 week later—4 weeks after stopping clozapine
We then target Mr. S’ catatonia with intramuscular haloperidol, 100 mg/d for 4 weeks, and ziprasidone, 80 mg bid with food. He tolerates this combination but gradually develops tremor and rigidity. Six weeks later, we add levodopa/carbidopa, 25/250 mg bid for his movement problems.
Two weeks later, Mr. S is sweating profusely, disoriented, rigid, and febrile (104.6°F). We diagnose neuroleptic malignant syndrome (NMS), stop both antipsychotics, and admit him for treatment. We start lorazepam, 1 mg tid for catatonia; bromocriptine, 250 mg bid for rigidity; and continue levodopa/carbidopa at the same dosage. We also add dantrolene, 25 mg tid for 5 days for fever and rigidity, and provide a cooling blanket for hyperthermia.
Mr. S’ fever, autonomic changes, and diaphoresis diminish within 3 days. Rigidity and mental status improve gradually over 2 weeks. We discharge him after 10 days.
poll here
The author’s observations
Catatonia is a recognized risk factor for NMS. White and Robins8 described 17 patients with a catatonic syndrome that developed into NMS within 5 to 96 hours of starting a neuroleptic. Sachdev developed an NMS rating scale that includes catatonic symptoms.9
Northoff,10 however, associates NMS with D2 receptor blockage in the basal ganglia and relates catatonia to a frontocortical gamma-aminobutyric acid (GABA) dysfunction. Based on this theory, haloperidol—which offers a higher D2 blockade than do SGAs such as ziprasidone—might have contributed to Mr. S’ NMS.
Some evidence suggests that lorazepam—which works on gamma-aminobutyric acid ionotropic type A (GABAA) receptors—helps treat catatonia in NMS and improves rigidity, hyperthermia, and autonomic signs.11
Treatment: which agents will work?
Three weeks after his discharge, we restart ziprasidone, 40 mg bid for Mr. S’ catatonic schizophrenia. He remains free of NMS symptoms but still has mannerisms (posturing, staring, immobility, stereotypic scratching on his face).
Over 1 year, Mr. S is hospitalized repeatedly because of persistent impulsivity and delusions. He has failed numerous antipsychotic regimens lasting 1 month or longer, including olanzapine, up to 30 mg/d; quetiapine, 300 mg tid; and risperidone, 2 mg tid. Adding a first-generation antipsychotic either does not help (as with perphenazine, 12 mg/d) or diminishes his memory (as with chlorpromazine, 250 mg/d). The anticholinergic benztropine, 2 mg bid, also is ineffective.
Combination quetiapine, 300 mg/d, and the antiviral amantadine, 100 mg tid, improve Mr. S’ stereotypy at first, but his delusions intensify within 1 week. His Bush-Francis Catatonia Rating Scale scores range from 9 (indicating moderate catatonia) to 16 (persistent catatonic features).12
poll here
The authors’ observations
Catatonic schizophrenia’s pathophysiology and response to medication might differ compared with other schizophrenia forms.13 Dopamine D2 hypoactivity, glutamate N-methyl-D-aspartate (NMDA) hyperactivity, or GABAA hypoactivity are believed to cause catatonia.3,6,7 GABA agonists, anticonvulsants, dopamine agonists, SGAs, and NMDA antagonists target these pathophysiologies, but patients with a catatonia subtype often respond to only one type of medication.
Lorazepam exerts an anticatatonic effect by binding to GABAA receptors and increasing GABA activity. Lorazepam can help some patients with schizophrenia but has not shown benefit when added to an antipsychotic for chronic catatonia.6,14
SGAs can provide marked improvement in patients with catatonic schizophrenia.5
Salokangas et al15 note that “atypicals” pass more dopamine to the D2 receptor when dopamine is low in the basal ganglia. This suggests that SGAs with low D2 binding—such as clozapine, olanzapine, and quetiapine—are more beneficial than other SGAs for catatonia. Serotonin binding or other mechanisms might add to these drugs’ anticatatonic effect.7
Anticonvulsants. Adjunctive anticonvulsant therapy might alleviate catatonia by increasing GABA activity or by causing a modest antiglutaminergic effect, as reported with carbamazepine or valproic acid.16 Anticholinergics also might help treat neuroleptic-induced catatonia.17
Amantadine—FDA-approved to treat Parkinson’s disease and extrapyramidal disease—can alleviate catatonia by blocking hyperglutamatergic excitotoxicity in neurons, thus blocking NMDA receptors.18 As with Mr. S, however, amantadine can worsen psychosis by increasing dopamine release.
Memantine—an NMDA receptor antagonist indicated for moderate to severe Alzheimer’s disease—also blocks hyperglutamatergic excitotoxicity in neurons. The medication has shown effectiveness for treating catatonic schizophrenia in case reports,19-21 but 3 patients have reported memantine-induced psychosis and seizures.21
Some might argue that Mr. S’ delusions are predominant and more compelling than his catatonia, but these did not hamper his ability to live in a group home. His catatonia-related negativism, impulsivity, and inability to cooperate are what led to frequent hospitalization.
Follow-up: treatment change
We stop amantadine, add memantine, 10 mg bid, and titrate quetiapine over 2 weeks to 900 mg/d. Mr. S’ catatonia improves but some delusions persist. We add olanzapine, 7.5 mg bid, and within 2 weeks Mr. S is less delusional and more cooperative.
We discharge Mr. S on the above medications, plus:
- lorazepam, 1 mg each morning and 2 mg nightly, which he has been taking for catatonia for about 1 year
- trazodone, 150 mg bid, which we added 6 months ago to help him sleep and reduce psychomotor excitement
- ranitidine, 150 mg bid, for gastroesophageal reflux disorder
- and levothyroxine, 0.5 mg/d, for comobrid hypothyroidism. His thyroid-stimulating hormone level is normal.
We see Mr. S monthly. He is still impulsive at times, occasionally collecting his neighbors’ newspapers and mail despite instructions from group home staff not to do so. Yet his sponsors say Mr. S is “like a new person.” He talks spontaneously, interacts, and is cooperative. He has not been hospitalized for more than 1 year.
The authors’ observations
Mr. S responded favorably to clozapine but cannot tolerate it. With a combination of two other SGAs, a patient might gain the benefits of clozapine without the need for frequent blood draws or the risk of agranulocytosis, other side effects, or interactions between clozapine and other drugs. Adding memantine was necessary to improve the catatonic features that prevented his return to the group home.
Related resources
- World Federation of Societies of Biological Psychiatry. www.wfsbp.com.
- Neuroleptic Malignant Syndrome Information Service. www.nmsis.org.
- Mann SC, Caroff SN, Keck PE Jr, Lazarus A. Neuroleptic malignant syndrome and related conditions, 2nd ed. Arlington, VA: American Psychiatric Press; 2003:1-44.
- Ungvari GS (ed). Catatonia-an anthology of classical contributions. Hong Kong: Scientific Communications International; 2006.
- Amantadine • Symmetrel
- Benztropine • Cogentin
- Bromocriptine • Parlodel
- Carbamazepine • Equetro, others
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Dantrolene • Dantrium
- Haloperidol • Haldol
- Levodopa/carbidopa • Sinemet
- Levothyroxine • Synthroid
- Lorazepam • Ativan
- Memantine • Namenda
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Ranitidine • Zantac
- Risperidone • Risperdal
- Trazodone • Desyrel
- Valproic acid • Depakene
- Ziprasidone • Geodon
Dr. Carroll is a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol Myers-Squibb Co., Forest Pharmaceuticals, Janssen Pharmaceutica, and Pfizer.
Dr. Thomas receives grant support from Pfizer and is a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, and Pfizer.
Dr. Tugrul is a consultant to and speaker for Bristol Myers-Squibb Co. and Eli Lilly and Co.
Dr. Jayanti reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Acknowledgment
The authors thank Francisco José Appiani, MD, chairman, psychiatry department, Military Hospital of Campo de Mayo, Buenos Aires, Argentina, and Vijay Jayanti, BS, medical student, The Ohio State University, Columbus, for their help with this article.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000:204.
2. Ungvari GS, Leung SK, Ng FS, et al. Schizophrenia with prominent catatonic features (“catatonic schizophrenia”) I. Demographic and clinical correlates in the chronic phase. Prog Neuropsychopharmacol Biol Psychiatry 2005;29:27-38.
3. Dhossche D, Wing L, Ohta M, Neumarker K (eds). Catatonia in autism spectrum disorders. International review of neurobiology, vol. 72. San Diego: Elsevier/Academic Press; 2006.
4. Falkai P, Wobrock T, Lieberman J. WFSBP guidelines for biological treatment of schizophrenia, part 1. Acute treatment of schizophrenia. World J Biol Psychiatry 2005;6:32-91.
5. Van Dalfsen F, Van Hecke J, Van Dalfsen A, et al. The use of atypical antipsychotics in the treatment of catatonia. Eur Psychiatry 2005;20:422-9.
6. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry 1990;51:357-62.
7. Dursun SM, Hallak JE, Haddad P, et al. Clozapine monotherapy for catatonic schizophrenia: should clozapine be the treatment of choice, with catatonia rather than psychosis as the main therapeutic index? J Psychopharmacol 2005;19:432-3.
8. White DAC, Robbins AH. An analysis of 17 catatonic patients diagnosed with neuroleptic malignant syndrome. CNS Spectrums 2000;5:58-65.
9. Sachdev PS. A rating scale for neuroleptic malignant syndrome. Psychiatry Res 2005;135:249-56.
10. Northoff G. Catatonia and neuroleptic malignant syndrome: psychopathology and pathophysiology. J Neural Transm 2002;109:1453-67.
11. Francis A, Chandragiri S, Rizvi S, et al. Is lorazepam a treatment for neuroleptic malignant syndrome? CNS Spectrums 2000;5:54-7.
12. Bush G, Fink M, Petrides, et al. Catatonia I: Rating scale and standardized examination. Acta Psychiatr Scand 1996;93:129-36.
13. Carroll BT, Thomas C, Jayanti K, et al. Schizophrenia with catatonic features deserves further study. World J Biol Psychiatry 2005;6(4):267-8.
14. Ungvari GS, Chie HFK, Chow LY, et al. Lorazepam for chronic catatonia: A random, double blind, placebo-controlled cross-over study. Psychopharmacology (Berl) 1999;142:393-8.
15. Salokangas R, Honkonen T, Stengard E, et al. Negative symptoms and neuroleptics in catatonic schizophrenia. Schizophr Res 2003;59:73-6.
16. Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge, UK: Cambridge University Press; 2003.
17. Franz M, Gallhofer B, Kanzow WT. Treatment of catatonia with intravenous biperidine. Br J Psychiatry 1994;164:847-8.
18. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry 1997;62:404-6.
19. Thomas C, Carroll BT, Maley JT, et al. Memantine in catatonic schizophrenia. Am J Psychiatry 2005;162:656.
20. Carroll BT, Thomas C, Jayanti K. Amantadine and memantine in catatonic schizophrenia. Ann Clin Psychiatry 2006;18:133-4.
21. Carpenter SS, Hatchett AD, Fuller MA. Catatonic schizophrenia and the use of memantine. Ann Pharmacother 2006;40:344-6.
History: ‘leaving town’
Mr. S, age 58, escaped repeatedly from his group home over 4 weeks. During one episode, he removed mail from neighbors’ mailboxes and tried to direct midday traffic. He would disappear for a few hours, sometimes overnight, before returning or being brought back by police.
The patient—who has had schizophrenia with catatonic features for 30 years—offered assorted explanations for escaping, most of them based on delusional beliefs, such as “I’m leaving town to get married” or “I’m late for engineering class.”
Since his last escape 3 weeks ago, Mr. S has remained in the group home without incident but has not been reporting for his usual outpatient psychiatric care. One day, he finally presents to us at the group home sponsor’s urging.
On evaluation, Mr. S shows stereotyped speech, staring, posturing, speech-prompt mutism, and odd mannerisms such as saluting. He has not been bathing or sleeping and smiles inappropriately. He speaks only when spoken to and answers with short phrases punctuated with ”By the grace of the good Lord.”
The authors’ observations
DSM-IV-TR requires at least two features to diagnose catatonic schizophrenia:
- peculiar voluntary movements
- extreme negativism
- excessive motor activity
- echolalia or echopraxia
- motoric immobility.1
Catatonia is common among the chronic mentally ill,2 yet it often goes undiagnosed.3 As a form of psychosis, catatonia might lead to greater functional impairment if not treated.
Treatment: time to try clozapine?
Over 10 years, numerous antipsychotic regimens plus adjunctive valproic acid, 500 mg tid, or lorazepam, up to 2 mg tid, have not lessened Mr. S’ psychosis and impulsivity. We start clozapine, 400 mg/d, and order twice-monthly blood tests to check for clozapine-induced agranulocytosis.
After nearly 6 months, some catatonic features improve gradually based on clinical interview. Serum clozapine is 363 ng/mL.
poll here
The authors’ observations
Second-generation antipsychotics (SGAs) are favored over first-generation antipsychotics to treat schizophrenia with catatonic features (Table),4,5 but no drug in either class has worked for Mr. S.
ECT can alleviate catatonic schizophrenia,4,6 but this option often is not available because the clinician fears a negative outcome would prompt legal action, or the guardian or next of kin do not consent to the procedure.3 We considered referring Mr. S to an ECT provider, but he has no legal guardian to provide consent. The group home sponsor also objected to ECT because Mr. S would have been sent out of town for treatment.
Catatonia patients who are immobile, physically compromised, and refuse food and drink typically are considered ECT candidates. Mr. S eats and drinks regularly and is physically able.
Lorazepam can produce rapid response, but it can be addictive.2 Also, an adjunctive 2 mg/d dosage showed no effect.
Clozapine monotherapy has shown effectiveness in catatonic schizophrenia7 and might be an option after other antipsychotics have failed.
Table 1
Treatments for catatonia: risks and benefits
| Medication | Use | Rationale | Benefits | Risks |
|---|---|---|---|---|
| First-generation antipsychotics (FGAs) | Often used for schizophrenia | Control positive symptoms | Well-established | Catatonia might be difficult to distinguish from NMS |
| Less expensive than other medications | ||||
| Second-generation antipsychotics (SGAs) | Beneficial in catatonia | Less likely than FGAs to worsen catatonia because of low D2 blockade | Some studies suggest greater efficacy than with FGAs | Metabolic syndrome, agranulocytosis with clozapine |
| Benzodiazepines | Lorazepam helpful in acute catatonia | Can be added to any antipsychotic | Safe, first-line treatment for catatonia | Respiratory compromise, incoordination, sedation, potential for abuse |
| Electroconvulsive therapy | ||||
| Electroconvulsive therapy | Beneficial in malignant catatonia | Effective in catatonia, NMS | Useful for treatment-refractory catatonia | Concerns with anesthesia, informed consent, availability |
| Rapid onset of action | ||||
| NMS: Neuroleptic malignant syndrome | ||||
Complication: agranulocytosis, then nms
Six months after starting clozapine, Mr. S starts having diaphoresis and night sweats, suggesting neutropenia. Blood testing shows a white blood cell count (WBC) of 3.6/μL, down from 4.6/μL 2 weeks before (normal range, 4.6 to 11/μL).
One week later, Mr. S’ WBC is 1.6/μL with a 46% relative neutrophil value (normal range, 50% to 70%) and an absolute neutrophil count of 736 (normal range, 2,500 to 7,000).
We diagnose agranulocytosis and stop clozapine, but Mr. S’ WBC continues to fall over 2 weeks to 0.8/μL with a 16% relative and 128 absolute neutrophil count. After 1 more week, his WBC increases to 2.6/μL and returns to normal 1 week later—4 weeks after stopping clozapine
We then target Mr. S’ catatonia with intramuscular haloperidol, 100 mg/d for 4 weeks, and ziprasidone, 80 mg bid with food. He tolerates this combination but gradually develops tremor and rigidity. Six weeks later, we add levodopa/carbidopa, 25/250 mg bid for his movement problems.
Two weeks later, Mr. S is sweating profusely, disoriented, rigid, and febrile (104.6°F). We diagnose neuroleptic malignant syndrome (NMS), stop both antipsychotics, and admit him for treatment. We start lorazepam, 1 mg tid for catatonia; bromocriptine, 250 mg bid for rigidity; and continue levodopa/carbidopa at the same dosage. We also add dantrolene, 25 mg tid for 5 days for fever and rigidity, and provide a cooling blanket for hyperthermia.
Mr. S’ fever, autonomic changes, and diaphoresis diminish within 3 days. Rigidity and mental status improve gradually over 2 weeks. We discharge him after 10 days.
poll here
The author’s observations
Catatonia is a recognized risk factor for NMS. White and Robins8 described 17 patients with a catatonic syndrome that developed into NMS within 5 to 96 hours of starting a neuroleptic. Sachdev developed an NMS rating scale that includes catatonic symptoms.9
Northoff,10 however, associates NMS with D2 receptor blockage in the basal ganglia and relates catatonia to a frontocortical gamma-aminobutyric acid (GABA) dysfunction. Based on this theory, haloperidol—which offers a higher D2 blockade than do SGAs such as ziprasidone—might have contributed to Mr. S’ NMS.
Some evidence suggests that lorazepam—which works on gamma-aminobutyric acid ionotropic type A (GABAA) receptors—helps treat catatonia in NMS and improves rigidity, hyperthermia, and autonomic signs.11
Treatment: which agents will work?
Three weeks after his discharge, we restart ziprasidone, 40 mg bid for Mr. S’ catatonic schizophrenia. He remains free of NMS symptoms but still has mannerisms (posturing, staring, immobility, stereotypic scratching on his face).
Over 1 year, Mr. S is hospitalized repeatedly because of persistent impulsivity and delusions. He has failed numerous antipsychotic regimens lasting 1 month or longer, including olanzapine, up to 30 mg/d; quetiapine, 300 mg tid; and risperidone, 2 mg tid. Adding a first-generation antipsychotic either does not help (as with perphenazine, 12 mg/d) or diminishes his memory (as with chlorpromazine, 250 mg/d). The anticholinergic benztropine, 2 mg bid, also is ineffective.
Combination quetiapine, 300 mg/d, and the antiviral amantadine, 100 mg tid, improve Mr. S’ stereotypy at first, but his delusions intensify within 1 week. His Bush-Francis Catatonia Rating Scale scores range from 9 (indicating moderate catatonia) to 16 (persistent catatonic features).12
poll here
The authors’ observations
Catatonic schizophrenia’s pathophysiology and response to medication might differ compared with other schizophrenia forms.13 Dopamine D2 hypoactivity, glutamate N-methyl-D-aspartate (NMDA) hyperactivity, or GABAA hypoactivity are believed to cause catatonia.3,6,7 GABA agonists, anticonvulsants, dopamine agonists, SGAs, and NMDA antagonists target these pathophysiologies, but patients with a catatonia subtype often respond to only one type of medication.
Lorazepam exerts an anticatatonic effect by binding to GABAA receptors and increasing GABA activity. Lorazepam can help some patients with schizophrenia but has not shown benefit when added to an antipsychotic for chronic catatonia.6,14
SGAs can provide marked improvement in patients with catatonic schizophrenia.5
Salokangas et al15 note that “atypicals” pass more dopamine to the D2 receptor when dopamine is low in the basal ganglia. This suggests that SGAs with low D2 binding—such as clozapine, olanzapine, and quetiapine—are more beneficial than other SGAs for catatonia. Serotonin binding or other mechanisms might add to these drugs’ anticatatonic effect.7
Anticonvulsants. Adjunctive anticonvulsant therapy might alleviate catatonia by increasing GABA activity or by causing a modest antiglutaminergic effect, as reported with carbamazepine or valproic acid.16 Anticholinergics also might help treat neuroleptic-induced catatonia.17
Amantadine—FDA-approved to treat Parkinson’s disease and extrapyramidal disease—can alleviate catatonia by blocking hyperglutamatergic excitotoxicity in neurons, thus blocking NMDA receptors.18 As with Mr. S, however, amantadine can worsen psychosis by increasing dopamine release.
Memantine—an NMDA receptor antagonist indicated for moderate to severe Alzheimer’s disease—also blocks hyperglutamatergic excitotoxicity in neurons. The medication has shown effectiveness for treating catatonic schizophrenia in case reports,19-21 but 3 patients have reported memantine-induced psychosis and seizures.21
Some might argue that Mr. S’ delusions are predominant and more compelling than his catatonia, but these did not hamper his ability to live in a group home. His catatonia-related negativism, impulsivity, and inability to cooperate are what led to frequent hospitalization.
Follow-up: treatment change
We stop amantadine, add memantine, 10 mg bid, and titrate quetiapine over 2 weeks to 900 mg/d. Mr. S’ catatonia improves but some delusions persist. We add olanzapine, 7.5 mg bid, and within 2 weeks Mr. S is less delusional and more cooperative.
We discharge Mr. S on the above medications, plus:
- lorazepam, 1 mg each morning and 2 mg nightly, which he has been taking for catatonia for about 1 year
- trazodone, 150 mg bid, which we added 6 months ago to help him sleep and reduce psychomotor excitement
- ranitidine, 150 mg bid, for gastroesophageal reflux disorder
- and levothyroxine, 0.5 mg/d, for comobrid hypothyroidism. His thyroid-stimulating hormone level is normal.
We see Mr. S monthly. He is still impulsive at times, occasionally collecting his neighbors’ newspapers and mail despite instructions from group home staff not to do so. Yet his sponsors say Mr. S is “like a new person.” He talks spontaneously, interacts, and is cooperative. He has not been hospitalized for more than 1 year.
The authors’ observations
Mr. S responded favorably to clozapine but cannot tolerate it. With a combination of two other SGAs, a patient might gain the benefits of clozapine without the need for frequent blood draws or the risk of agranulocytosis, other side effects, or interactions between clozapine and other drugs. Adding memantine was necessary to improve the catatonic features that prevented his return to the group home.
Related resources
- World Federation of Societies of Biological Psychiatry. www.wfsbp.com.
- Neuroleptic Malignant Syndrome Information Service. www.nmsis.org.
- Mann SC, Caroff SN, Keck PE Jr, Lazarus A. Neuroleptic malignant syndrome and related conditions, 2nd ed. Arlington, VA: American Psychiatric Press; 2003:1-44.
- Ungvari GS (ed). Catatonia-an anthology of classical contributions. Hong Kong: Scientific Communications International; 2006.
- Amantadine • Symmetrel
- Benztropine • Cogentin
- Bromocriptine • Parlodel
- Carbamazepine • Equetro, others
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Dantrolene • Dantrium
- Haloperidol • Haldol
- Levodopa/carbidopa • Sinemet
- Levothyroxine • Synthroid
- Lorazepam • Ativan
- Memantine • Namenda
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Ranitidine • Zantac
- Risperidone • Risperdal
- Trazodone • Desyrel
- Valproic acid • Depakene
- Ziprasidone • Geodon
Dr. Carroll is a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol Myers-Squibb Co., Forest Pharmaceuticals, Janssen Pharmaceutica, and Pfizer.
Dr. Thomas receives grant support from Pfizer and is a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, and Pfizer.
Dr. Tugrul is a consultant to and speaker for Bristol Myers-Squibb Co. and Eli Lilly and Co.
Dr. Jayanti reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Acknowledgment
The authors thank Francisco José Appiani, MD, chairman, psychiatry department, Military Hospital of Campo de Mayo, Buenos Aires, Argentina, and Vijay Jayanti, BS, medical student, The Ohio State University, Columbus, for their help with this article.
History: ‘leaving town’
Mr. S, age 58, escaped repeatedly from his group home over 4 weeks. During one episode, he removed mail from neighbors’ mailboxes and tried to direct midday traffic. He would disappear for a few hours, sometimes overnight, before returning or being brought back by police.
The patient—who has had schizophrenia with catatonic features for 30 years—offered assorted explanations for escaping, most of them based on delusional beliefs, such as “I’m leaving town to get married” or “I’m late for engineering class.”
Since his last escape 3 weeks ago, Mr. S has remained in the group home without incident but has not been reporting for his usual outpatient psychiatric care. One day, he finally presents to us at the group home sponsor’s urging.
On evaluation, Mr. S shows stereotyped speech, staring, posturing, speech-prompt mutism, and odd mannerisms such as saluting. He has not been bathing or sleeping and smiles inappropriately. He speaks only when spoken to and answers with short phrases punctuated with ”By the grace of the good Lord.”
The authors’ observations
DSM-IV-TR requires at least two features to diagnose catatonic schizophrenia:
- peculiar voluntary movements
- extreme negativism
- excessive motor activity
- echolalia or echopraxia
- motoric immobility.1
Catatonia is common among the chronic mentally ill,2 yet it often goes undiagnosed.3 As a form of psychosis, catatonia might lead to greater functional impairment if not treated.
Treatment: time to try clozapine?
Over 10 years, numerous antipsychotic regimens plus adjunctive valproic acid, 500 mg tid, or lorazepam, up to 2 mg tid, have not lessened Mr. S’ psychosis and impulsivity. We start clozapine, 400 mg/d, and order twice-monthly blood tests to check for clozapine-induced agranulocytosis.
After nearly 6 months, some catatonic features improve gradually based on clinical interview. Serum clozapine is 363 ng/mL.
poll here
The authors’ observations
Second-generation antipsychotics (SGAs) are favored over first-generation antipsychotics to treat schizophrenia with catatonic features (Table),4,5 but no drug in either class has worked for Mr. S.
ECT can alleviate catatonic schizophrenia,4,6 but this option often is not available because the clinician fears a negative outcome would prompt legal action, or the guardian or next of kin do not consent to the procedure.3 We considered referring Mr. S to an ECT provider, but he has no legal guardian to provide consent. The group home sponsor also objected to ECT because Mr. S would have been sent out of town for treatment.
Catatonia patients who are immobile, physically compromised, and refuse food and drink typically are considered ECT candidates. Mr. S eats and drinks regularly and is physically able.
Lorazepam can produce rapid response, but it can be addictive.2 Also, an adjunctive 2 mg/d dosage showed no effect.
Clozapine monotherapy has shown effectiveness in catatonic schizophrenia7 and might be an option after other antipsychotics have failed.
Table 1
Treatments for catatonia: risks and benefits
| Medication | Use | Rationale | Benefits | Risks |
|---|---|---|---|---|
| First-generation antipsychotics (FGAs) | Often used for schizophrenia | Control positive symptoms | Well-established | Catatonia might be difficult to distinguish from NMS |
| Less expensive than other medications | ||||
| Second-generation antipsychotics (SGAs) | Beneficial in catatonia | Less likely than FGAs to worsen catatonia because of low D2 blockade | Some studies suggest greater efficacy than with FGAs | Metabolic syndrome, agranulocytosis with clozapine |
| Benzodiazepines | Lorazepam helpful in acute catatonia | Can be added to any antipsychotic | Safe, first-line treatment for catatonia | Respiratory compromise, incoordination, sedation, potential for abuse |
| Electroconvulsive therapy | ||||
| Electroconvulsive therapy | Beneficial in malignant catatonia | Effective in catatonia, NMS | Useful for treatment-refractory catatonia | Concerns with anesthesia, informed consent, availability |
| Rapid onset of action | ||||
| NMS: Neuroleptic malignant syndrome | ||||
Complication: agranulocytosis, then nms
Six months after starting clozapine, Mr. S starts having diaphoresis and night sweats, suggesting neutropenia. Blood testing shows a white blood cell count (WBC) of 3.6/μL, down from 4.6/μL 2 weeks before (normal range, 4.6 to 11/μL).
One week later, Mr. S’ WBC is 1.6/μL with a 46% relative neutrophil value (normal range, 50% to 70%) and an absolute neutrophil count of 736 (normal range, 2,500 to 7,000).
We diagnose agranulocytosis and stop clozapine, but Mr. S’ WBC continues to fall over 2 weeks to 0.8/μL with a 16% relative and 128 absolute neutrophil count. After 1 more week, his WBC increases to 2.6/μL and returns to normal 1 week later—4 weeks after stopping clozapine
We then target Mr. S’ catatonia with intramuscular haloperidol, 100 mg/d for 4 weeks, and ziprasidone, 80 mg bid with food. He tolerates this combination but gradually develops tremor and rigidity. Six weeks later, we add levodopa/carbidopa, 25/250 mg bid for his movement problems.
Two weeks later, Mr. S is sweating profusely, disoriented, rigid, and febrile (104.6°F). We diagnose neuroleptic malignant syndrome (NMS), stop both antipsychotics, and admit him for treatment. We start lorazepam, 1 mg tid for catatonia; bromocriptine, 250 mg bid for rigidity; and continue levodopa/carbidopa at the same dosage. We also add dantrolene, 25 mg tid for 5 days for fever and rigidity, and provide a cooling blanket for hyperthermia.
Mr. S’ fever, autonomic changes, and diaphoresis diminish within 3 days. Rigidity and mental status improve gradually over 2 weeks. We discharge him after 10 days.
poll here
The author’s observations
Catatonia is a recognized risk factor for NMS. White and Robins8 described 17 patients with a catatonic syndrome that developed into NMS within 5 to 96 hours of starting a neuroleptic. Sachdev developed an NMS rating scale that includes catatonic symptoms.9
Northoff,10 however, associates NMS with D2 receptor blockage in the basal ganglia and relates catatonia to a frontocortical gamma-aminobutyric acid (GABA) dysfunction. Based on this theory, haloperidol—which offers a higher D2 blockade than do SGAs such as ziprasidone—might have contributed to Mr. S’ NMS.
Some evidence suggests that lorazepam—which works on gamma-aminobutyric acid ionotropic type A (GABAA) receptors—helps treat catatonia in NMS and improves rigidity, hyperthermia, and autonomic signs.11
Treatment: which agents will work?
Three weeks after his discharge, we restart ziprasidone, 40 mg bid for Mr. S’ catatonic schizophrenia. He remains free of NMS symptoms but still has mannerisms (posturing, staring, immobility, stereotypic scratching on his face).
Over 1 year, Mr. S is hospitalized repeatedly because of persistent impulsivity and delusions. He has failed numerous antipsychotic regimens lasting 1 month or longer, including olanzapine, up to 30 mg/d; quetiapine, 300 mg tid; and risperidone, 2 mg tid. Adding a first-generation antipsychotic either does not help (as with perphenazine, 12 mg/d) or diminishes his memory (as with chlorpromazine, 250 mg/d). The anticholinergic benztropine, 2 mg bid, also is ineffective.
Combination quetiapine, 300 mg/d, and the antiviral amantadine, 100 mg tid, improve Mr. S’ stereotypy at first, but his delusions intensify within 1 week. His Bush-Francis Catatonia Rating Scale scores range from 9 (indicating moderate catatonia) to 16 (persistent catatonic features).12
poll here
The authors’ observations
Catatonic schizophrenia’s pathophysiology and response to medication might differ compared with other schizophrenia forms.13 Dopamine D2 hypoactivity, glutamate N-methyl-D-aspartate (NMDA) hyperactivity, or GABAA hypoactivity are believed to cause catatonia.3,6,7 GABA agonists, anticonvulsants, dopamine agonists, SGAs, and NMDA antagonists target these pathophysiologies, but patients with a catatonia subtype often respond to only one type of medication.
Lorazepam exerts an anticatatonic effect by binding to GABAA receptors and increasing GABA activity. Lorazepam can help some patients with schizophrenia but has not shown benefit when added to an antipsychotic for chronic catatonia.6,14
SGAs can provide marked improvement in patients with catatonic schizophrenia.5
Salokangas et al15 note that “atypicals” pass more dopamine to the D2 receptor when dopamine is low in the basal ganglia. This suggests that SGAs with low D2 binding—such as clozapine, olanzapine, and quetiapine—are more beneficial than other SGAs for catatonia. Serotonin binding or other mechanisms might add to these drugs’ anticatatonic effect.7
Anticonvulsants. Adjunctive anticonvulsant therapy might alleviate catatonia by increasing GABA activity or by causing a modest antiglutaminergic effect, as reported with carbamazepine or valproic acid.16 Anticholinergics also might help treat neuroleptic-induced catatonia.17
Amantadine—FDA-approved to treat Parkinson’s disease and extrapyramidal disease—can alleviate catatonia by blocking hyperglutamatergic excitotoxicity in neurons, thus blocking NMDA receptors.18 As with Mr. S, however, amantadine can worsen psychosis by increasing dopamine release.
Memantine—an NMDA receptor antagonist indicated for moderate to severe Alzheimer’s disease—also blocks hyperglutamatergic excitotoxicity in neurons. The medication has shown effectiveness for treating catatonic schizophrenia in case reports,19-21 but 3 patients have reported memantine-induced psychosis and seizures.21
Some might argue that Mr. S’ delusions are predominant and more compelling than his catatonia, but these did not hamper his ability to live in a group home. His catatonia-related negativism, impulsivity, and inability to cooperate are what led to frequent hospitalization.
Follow-up: treatment change
We stop amantadine, add memantine, 10 mg bid, and titrate quetiapine over 2 weeks to 900 mg/d. Mr. S’ catatonia improves but some delusions persist. We add olanzapine, 7.5 mg bid, and within 2 weeks Mr. S is less delusional and more cooperative.
We discharge Mr. S on the above medications, plus:
- lorazepam, 1 mg each morning and 2 mg nightly, which he has been taking for catatonia for about 1 year
- trazodone, 150 mg bid, which we added 6 months ago to help him sleep and reduce psychomotor excitement
- ranitidine, 150 mg bid, for gastroesophageal reflux disorder
- and levothyroxine, 0.5 mg/d, for comobrid hypothyroidism. His thyroid-stimulating hormone level is normal.
We see Mr. S monthly. He is still impulsive at times, occasionally collecting his neighbors’ newspapers and mail despite instructions from group home staff not to do so. Yet his sponsors say Mr. S is “like a new person.” He talks spontaneously, interacts, and is cooperative. He has not been hospitalized for more than 1 year.
The authors’ observations
Mr. S responded favorably to clozapine but cannot tolerate it. With a combination of two other SGAs, a patient might gain the benefits of clozapine without the need for frequent blood draws or the risk of agranulocytosis, other side effects, or interactions between clozapine and other drugs. Adding memantine was necessary to improve the catatonic features that prevented his return to the group home.
Related resources
- World Federation of Societies of Biological Psychiatry. www.wfsbp.com.
- Neuroleptic Malignant Syndrome Information Service. www.nmsis.org.
- Mann SC, Caroff SN, Keck PE Jr, Lazarus A. Neuroleptic malignant syndrome and related conditions, 2nd ed. Arlington, VA: American Psychiatric Press; 2003:1-44.
- Ungvari GS (ed). Catatonia-an anthology of classical contributions. Hong Kong: Scientific Communications International; 2006.
- Amantadine • Symmetrel
- Benztropine • Cogentin
- Bromocriptine • Parlodel
- Carbamazepine • Equetro, others
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Dantrolene • Dantrium
- Haloperidol • Haldol
- Levodopa/carbidopa • Sinemet
- Levothyroxine • Synthroid
- Lorazepam • Ativan
- Memantine • Namenda
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Ranitidine • Zantac
- Risperidone • Risperdal
- Trazodone • Desyrel
- Valproic acid • Depakene
- Ziprasidone • Geodon
Dr. Carroll is a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol Myers-Squibb Co., Forest Pharmaceuticals, Janssen Pharmaceutica, and Pfizer.
Dr. Thomas receives grant support from Pfizer and is a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, and Pfizer.
Dr. Tugrul is a consultant to and speaker for Bristol Myers-Squibb Co. and Eli Lilly and Co.
Dr. Jayanti reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Acknowledgment
The authors thank Francisco José Appiani, MD, chairman, psychiatry department, Military Hospital of Campo de Mayo, Buenos Aires, Argentina, and Vijay Jayanti, BS, medical student, The Ohio State University, Columbus, for their help with this article.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000:204.
2. Ungvari GS, Leung SK, Ng FS, et al. Schizophrenia with prominent catatonic features (“catatonic schizophrenia”) I. Demographic and clinical correlates in the chronic phase. Prog Neuropsychopharmacol Biol Psychiatry 2005;29:27-38.
3. Dhossche D, Wing L, Ohta M, Neumarker K (eds). Catatonia in autism spectrum disorders. International review of neurobiology, vol. 72. San Diego: Elsevier/Academic Press; 2006.
4. Falkai P, Wobrock T, Lieberman J. WFSBP guidelines for biological treatment of schizophrenia, part 1. Acute treatment of schizophrenia. World J Biol Psychiatry 2005;6:32-91.
5. Van Dalfsen F, Van Hecke J, Van Dalfsen A, et al. The use of atypical antipsychotics in the treatment of catatonia. Eur Psychiatry 2005;20:422-9.
6. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry 1990;51:357-62.
7. Dursun SM, Hallak JE, Haddad P, et al. Clozapine monotherapy for catatonic schizophrenia: should clozapine be the treatment of choice, with catatonia rather than psychosis as the main therapeutic index? J Psychopharmacol 2005;19:432-3.
8. White DAC, Robbins AH. An analysis of 17 catatonic patients diagnosed with neuroleptic malignant syndrome. CNS Spectrums 2000;5:58-65.
9. Sachdev PS. A rating scale for neuroleptic malignant syndrome. Psychiatry Res 2005;135:249-56.
10. Northoff G. Catatonia and neuroleptic malignant syndrome: psychopathology and pathophysiology. J Neural Transm 2002;109:1453-67.
11. Francis A, Chandragiri S, Rizvi S, et al. Is lorazepam a treatment for neuroleptic malignant syndrome? CNS Spectrums 2000;5:54-7.
12. Bush G, Fink M, Petrides, et al. Catatonia I: Rating scale and standardized examination. Acta Psychiatr Scand 1996;93:129-36.
13. Carroll BT, Thomas C, Jayanti K, et al. Schizophrenia with catatonic features deserves further study. World J Biol Psychiatry 2005;6(4):267-8.
14. Ungvari GS, Chie HFK, Chow LY, et al. Lorazepam for chronic catatonia: A random, double blind, placebo-controlled cross-over study. Psychopharmacology (Berl) 1999;142:393-8.
15. Salokangas R, Honkonen T, Stengard E, et al. Negative symptoms and neuroleptics in catatonic schizophrenia. Schizophr Res 2003;59:73-6.
16. Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge, UK: Cambridge University Press; 2003.
17. Franz M, Gallhofer B, Kanzow WT. Treatment of catatonia with intravenous biperidine. Br J Psychiatry 1994;164:847-8.
18. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry 1997;62:404-6.
19. Thomas C, Carroll BT, Maley JT, et al. Memantine in catatonic schizophrenia. Am J Psychiatry 2005;162:656.
20. Carroll BT, Thomas C, Jayanti K. Amantadine and memantine in catatonic schizophrenia. Ann Clin Psychiatry 2006;18:133-4.
21. Carpenter SS, Hatchett AD, Fuller MA. Catatonic schizophrenia and the use of memantine. Ann Pharmacother 2006;40:344-6.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000:204.
2. Ungvari GS, Leung SK, Ng FS, et al. Schizophrenia with prominent catatonic features (“catatonic schizophrenia”) I. Demographic and clinical correlates in the chronic phase. Prog Neuropsychopharmacol Biol Psychiatry 2005;29:27-38.
3. Dhossche D, Wing L, Ohta M, Neumarker K (eds). Catatonia in autism spectrum disorders. International review of neurobiology, vol. 72. San Diego: Elsevier/Academic Press; 2006.
4. Falkai P, Wobrock T, Lieberman J. WFSBP guidelines for biological treatment of schizophrenia, part 1. Acute treatment of schizophrenia. World J Biol Psychiatry 2005;6:32-91.
5. Van Dalfsen F, Van Hecke J, Van Dalfsen A, et al. The use of atypical antipsychotics in the treatment of catatonia. Eur Psychiatry 2005;20:422-9.
6. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry 1990;51:357-62.
7. Dursun SM, Hallak JE, Haddad P, et al. Clozapine monotherapy for catatonic schizophrenia: should clozapine be the treatment of choice, with catatonia rather than psychosis as the main therapeutic index? J Psychopharmacol 2005;19:432-3.
8. White DAC, Robbins AH. An analysis of 17 catatonic patients diagnosed with neuroleptic malignant syndrome. CNS Spectrums 2000;5:58-65.
9. Sachdev PS. A rating scale for neuroleptic malignant syndrome. Psychiatry Res 2005;135:249-56.
10. Northoff G. Catatonia and neuroleptic malignant syndrome: psychopathology and pathophysiology. J Neural Transm 2002;109:1453-67.
11. Francis A, Chandragiri S, Rizvi S, et al. Is lorazepam a treatment for neuroleptic malignant syndrome? CNS Spectrums 2000;5:54-7.
12. Bush G, Fink M, Petrides, et al. Catatonia I: Rating scale and standardized examination. Acta Psychiatr Scand 1996;93:129-36.
13. Carroll BT, Thomas C, Jayanti K, et al. Schizophrenia with catatonic features deserves further study. World J Biol Psychiatry 2005;6(4):267-8.
14. Ungvari GS, Chie HFK, Chow LY, et al. Lorazepam for chronic catatonia: A random, double blind, placebo-controlled cross-over study. Psychopharmacology (Berl) 1999;142:393-8.
15. Salokangas R, Honkonen T, Stengard E, et al. Negative symptoms and neuroleptics in catatonic schizophrenia. Schizophr Res 2003;59:73-6.
16. Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge, UK: Cambridge University Press; 2003.
17. Franz M, Gallhofer B, Kanzow WT. Treatment of catatonia with intravenous biperidine. Br J Psychiatry 1994;164:847-8.
18. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry 1997;62:404-6.
19. Thomas C, Carroll BT, Maley JT, et al. Memantine in catatonic schizophrenia. Am J Psychiatry 2005;162:656.
20. Carroll BT, Thomas C, Jayanti K. Amantadine and memantine in catatonic schizophrenia. Ann Clin Psychiatry 2006;18:133-4.
21. Carpenter SS, Hatchett AD, Fuller MA. Catatonic schizophrenia and the use of memantine. Ann Pharmacother 2006;40:344-6.
Is it anxiety, depression, or bipolar disorder?
History: ‘A zillion racing thoughts’
Ms. R, age 44, is referred by her primary care physician. She complains of tenseness, irritability, avolition, and fatigue. She worries incessantly that her children will get sick, a catastrophe will befall her husband, or she’ll do something wrong. She says she sometimes feels as if she’s thinking “a zillion racing thoughts.”
Once fun-loving, outgoing, and energetic, Ms. R says she began feeling unusually anxious 3 years ago. A psychiatrist diagnosed bipolar disorder type II based on her racing thoughts, irritability, low energy, and history of mood swings. Over 2 years, the psychiatrist tried combining valproic acid with bupropion, citalopram, or extended-release venlafaxine, then tried lithium monotherapy. Nothing worked.
Frustrated, Ms. R left the psychiatrist and consulted her primary care physician, who prescribed gabapentin, 200 mg each morning and 300 mg at night; fluoxetine, 50 mg/d; and quetiapine, 12.5 mg/d. Ms. R noticed no improvement and stopped the medications after 6 weeks. The physician urged her to see another psychiatrist, and she presented to us 2 weeks after stopping the medications.
Ms. R also has been feeling depressed and irritable the past 4 months and has trouble falling and staying asleep at night. She sleeps 4 to 5 hours nightly, constantly feels tired, cannot concentrate, and overeats to try to alleviate her stress. She has gained 6 pounds over 2 to 3 months and weighs 160 lb; her body mass index of 26 indicates she is overweight.
She says her worries overwhelm her and cause heart palpitations and muscle tension in her neck and shoulders. She admits to feeling “worthless,” but denies suicidal thoughts.
Ms. R describes her husband and two teenage daughters as “very supportive,” but admits that her fatigue and irritability have strained these relationships; she says she snaps at them for minor things, such as coming to dinner 1 minute late. She misses her job, which she recently quit because of her decreasing ability to function.
At intake, Ms. R says she will not resume previous medications but will consider alternatives. She refuses psychotherapy because of time constraints and transportation problems but is willing to return every 2 weeks for medication checks. She says she adhered to every prescription over 3 years with no major side effects. She has never taken an antidepressant or anxiolytic without a mood stabilizer.
Ms. R reports no medical problems, past substance use, current or past psychotic symptoms, or psychiatric hospitalizations. Her family history shows depression in one first-degree relative and anxiety in others. Her Hamilton Anxiety Scale (HAM-A) score of 20 indicates moderate anxiety. Laboratory tests ordered by her primary care physician are normal.
poll here
The authors’ observations
Racing thoughts, irritable mood, decreased sleep, and concentration problems can point to GAD, mania associated with bipolar disorder type I, or hypomania suggesting bipolar disorder type I or II (Table 1).1
We suspect GAD because:
- Ms. R’s thoughts “race” only when she worries
- her irritability and concentration problems seem more sustained than episodic
- she has difficulty falling and staying asleep, but her need for sleep has not decreased
- she complains of constant fatigue, whereas abnormally high energy characterizes bipolar disorder’s manic or hypomanic phase.
Does Ms. R have depression? Determining if the patient’s depressive symptoms are secondary to GAD or warrant a separate diagnosis can be difficult (Table 1). With Ms. R’s permission, we talked to her family, because collateral information often helps clarify the diagnosis. Her husband and daughters offered no significant new insights, however.
Table 1
Overlap among symptoms that suggest GAD, mania, or major depression
| Symptom | GAD | Mania | MDD |
|---|---|---|---|
| Difficulty concentrating/distractibility | X | X | X |
| Mood irritability | X | X | X |
| ‘Racing’ thoughts | X | X | X |
| Sleep disturbance | X | X | X |
| Tiring easily/low energy | X | X | |
| Ecessive psychomotor activity/restlessness | X | X | X |
| GAD: generalized anxiety disorder | |||
| MDD: major depressive disorder | |||
| Source: Reference 1 | |||
Treatment: Targeting the anxiety
To address Ms. R’s anxiety symptoms, we start buspirone, 5 mg tid, and titrate to 15 mg bid over 2 weeks. We choose buspirone—which is FDA-approved to treat GAD—because it is unlikely to cause a mood switch if bipolar disorder is causing Ms. R’s depression. We discuss with her the drug’s indications, benefits, and potential side effects (such as vertigo, headache, lightheadedness, and nausea).
At the first 2-week follow-up, Ms. R reports no side effects but little improvement. After another 2 weeks, she says she feels less anxiety, irritability, pain, and fewer racing thoughts. She reports less difficulty falling asleep, though she’s still sleeping only about 6 hours nightly. Her HAM-A score falls to 12, indicating mild anxiety.
Ms. R, however, says she still feels depressed, tired, distracted, unmotivated, and worthless. Her Hamilton Rating Scale for Depression (HAM-D) score of 16 indicates moderate depression.
poll here
The authors’ observations
The persistence of Ms. R’s depressive symptoms suggests comorbid major depressive disorder (MDD). In fact, MDD and GAD are considered the most common mood-anxiety comorbidities.2
Determining whether Ms. R has unipolar depression or bipolar disorder is extremely important, considering the treatment implications. In patients with bipolar disorder, any antidepressant can trigger mania or hypomania if used without a mood stabilizer. Some studies also have associated rare cases of suicidal behavior with antidepressant use.3,4 Lifetime risk of suicide in bipolar disorder is approximately 20 times that in the general population.5
Although Ms. R’s history does not reveal a previous manic episode, ruling out hypomania is difficult because it usually does not impair work or social functioning. Hypomania often goes unreported because others hardly notice it. Collateral history can uncover clues to hypomania (See For Your Patient), but Ms. R’s husband and daughters say they have not seen such episodes.
On the other hand, normal behavior can be mistaken for hypomania. Ms. R’s previous psychiatrist and primary care physician might have misinterpreted Ms. R’s baseline extroverted personality as hypomanic behavior. Also, her over-whelming depressive and anxiety symptoms between depressive episodes made her normal moods appear hypomanic.
Compared with unipolar depression, bipolar depression is more frequently associated with psychomotor retardation, hypersomnia, early onset, and family history of bipolar disorder.6 Ms. R, however, suffered low energy, terminal insomnia, and late onset, and had no known family history of bipolar disorder.
The Mood Disorder Questionnaire, a scale of self-administered questions, can help screen for symptoms that suggest bipolar disorder. A positive questionnaire result demands further clinical evaluation.7
Depression, mania, or hypomania? Signs family, friends should not miss*
Patient could be depressed if he/she:
- is constantly sad or irritable
- seems lost, withdrawn, or isolated
- is preoccupied with negative ideas and concerns
- persistently feels guilty, hopeless, and helpless
- says he or she has considered suicide
- has not been showering regularly or is unkempt (indicates low mood)
- shows significant changes in sleep
- moves slowly or sparingly, as if “slowed down” (indicates depressed affect)
- is often restless (indicating agitated/anxious depression)
- talks in a low-tone or monotone voice
- no longer enjoys activities or hobbies he or she once found pleasurable
- shows significant changes in appetite
- no longer enjoys sex
- cannot concentrate or make decisions
Patient could be manic or hypomanic if he/she:
- seems abnormally hyperactive, restless, and energized, compared with normal self
- is inappropriately euphoric and jubilant or, on occasion, extremely irritable
- talks rapidly and excessively
- often wears clothes that are too bright or colorful
- seems unusually self-confident, grandiose, and highly distractible
- shows increased sexual desire
- is impulsive, increasingly daring, and shows seriously impaired judgment, such as by investing/spending large sums of money for ill-advised reasons
- seems energetic despite lack of sleep
*If a family member shows any of the above symptoms, get him or her to a mental health clinic as soon as possible. Take the family member to the nearest ER or call your local crisis unit or 911 if you suspect the family member might hurt him/herself or others.
Continued treatment: ‘normal’ again
In addition to the HAM-D, we also administer the Mood Disorder Questionnaire. Results suggest Ms. R does not have bipolar illness.
To address Ms. R’s depressive symptoms, we start the selective serotonin reuptake inhibitor escitalopram at a low dosage (5 mg/d) to avoid exacerbating her anxiety. We discuss the drug’s potential to induce mania, hypomania, or other adverse effects such as nausea, anxiety, sleep disturbance, headache, and sexual dysfunction. Buspirone is maintained at 15 mg bid.
Two weeks later, Ms. R reports some increase in energy and motivation. After another 2 weeks, she reports significantly improved mood and concentration. She consistently falls asleep at 10 PM and sleeps 8 hours each night. She also finds time to read and go out with her friends and she gets along more amicably with her daughters and husband.
One month later, we increase escitalopram to 10 mg/d, a normal therapeutic dosage. Ms. R continues to respond positively and reports no side effects. Two months after starting the antidepressant, her HAM-D score of 8 suggests normal mood. We decrease follow-up visits to once monthly.
At a subsequent visit, Ms. R tells us she wants to find a job. A month later, she says she is enjoying her new job in a department store. Over the next 6 months, she remains free of anxiety, depressive symptoms, hypomanic behavior, and side effects. She tells us it’s nice to feel “normal” again.
We reduce Ms. R’s appointments to every 3 months. After another year, we refer her back to her primary care physician at her request.
The authors’ observations
DSM-IV-TR1 divides bipolar disorder into three categories:
- type I, in which the patient has had at least one manic episode with or without major depression
- type II, characterized by one or more major depressive episodes and at least one hypomanic episode
- cyclothymia, which is defined as fluctuation between hypomanic and minor depressive episodes.
Much is said about how underdiagnosis of bipolar disorder8 delays or prevents proper therapy with mood stabilizers, leading to suboptimal symptom resolution. As with Ms. R, however, an incorrect bipolar disorder diagnosis can be just as harmful. Three years of unnecessary and ineffective treatment worsened her anxiety and depressive symptoms and quality of life.
A comprehensive clinical interview supplemented with insights from family and friends can minimize the risk of misdiagnosis when patients present with symptoms that suggest bipolar disorder, depression, or GAD.
Differentiating between the following clinical features can also help you reach a diagnosis:
Sleep/energy level. Mania/hypomania is characterized by decreased need for sleep; patients often feel energetic even after 2 to 4 hours of sleep. Both depression and GAD diminish energy level, although mood is more depressed in depression. Patients with GAD have trouble falling asleep, while those with depression awaken early or have hypersomnia.
Behavior. Patients in the manic phase of bipolar disorder engage in risky, pleasurable activities with high potential for painful consequences. This drastic behavior change is not seen in depression or GAD (Table 2).
Table 2
Differentiating symptoms common to GAD, major depression, and mania
| Symptom | GAD | Major depression | Mania |
|---|---|---|---|
| Concentration | Difficulty concentrating (mind goes blank) | Diminished ability to concentrate or think (indecisiveness) | Easily distracted (difficulty focusing on one task) |
| Energy | Tires easily | Constant fatigue or loss of energy | Subjective feeling of increased energy |
| Mood | Can be irritable | Irritable, though more depressed | Euphoric or extremely irritable |
| Behavior | Seems more keyed up | More withdrawn | Increase in risky behavior with potential for painful consequences |
| Sleep | Disturbed (mostly difficulty going to sleep) | Disturbed (hypersomnia or insomnia, more likely terminal insomnia) | Decreased need for sleep (energetic after sleeping 2 to 4 hours) |
Related resources
- Depression and Bipolar Support Alliance. www.dbsalliance.org.
- Anxiety Disorders Association of America. www.adaa.org.
Drug brand names
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Quetiapine • Seroquel
- Valproic acid • Depakene
- Venlafaxine • Effexor
Disclosures
Dr. Williams is a speaker for Wyeth.
Dr. Singh reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
2. Gorwood P. Generalized anxiety disorder and major depressive disorder comorbidity: an example of genetic pleiotropy? Eur Psychiatry 2004;19:27-33.
3. Aursnes I, Tvete IF, Gaasemyr J, Natvig B. Suicide attempts in clinical trials with paroxetine randomised against placebo. BMC Med 2005;3:14.-
4. Healy D, Aldred G. Antidepressant drug use & the risk of suicide. Int Rev Psychiatry 2005;17:163-72.
5. Osby U, Brandt L, Correia N, et al. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry 2001;58:844-50.
6. Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. J Affect Disord 2005;84:117-25.
7. Hirschfield RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry 2001;158:1743-4.
8. Matza LS, Rajagopalan KS, Thompson CL, Lissovoy G. Misdiagnosed patients with bipolar disorder: comorbidities, treatment patterns, and direct treatment costs. J Clin Psychiatry 2005;66:1432-40.
History: ‘A zillion racing thoughts’
Ms. R, age 44, is referred by her primary care physician. She complains of tenseness, irritability, avolition, and fatigue. She worries incessantly that her children will get sick, a catastrophe will befall her husband, or she’ll do something wrong. She says she sometimes feels as if she’s thinking “a zillion racing thoughts.”
Once fun-loving, outgoing, and energetic, Ms. R says she began feeling unusually anxious 3 years ago. A psychiatrist diagnosed bipolar disorder type II based on her racing thoughts, irritability, low energy, and history of mood swings. Over 2 years, the psychiatrist tried combining valproic acid with bupropion, citalopram, or extended-release venlafaxine, then tried lithium monotherapy. Nothing worked.
Frustrated, Ms. R left the psychiatrist and consulted her primary care physician, who prescribed gabapentin, 200 mg each morning and 300 mg at night; fluoxetine, 50 mg/d; and quetiapine, 12.5 mg/d. Ms. R noticed no improvement and stopped the medications after 6 weeks. The physician urged her to see another psychiatrist, and she presented to us 2 weeks after stopping the medications.
Ms. R also has been feeling depressed and irritable the past 4 months and has trouble falling and staying asleep at night. She sleeps 4 to 5 hours nightly, constantly feels tired, cannot concentrate, and overeats to try to alleviate her stress. She has gained 6 pounds over 2 to 3 months and weighs 160 lb; her body mass index of 26 indicates she is overweight.
She says her worries overwhelm her and cause heart palpitations and muscle tension in her neck and shoulders. She admits to feeling “worthless,” but denies suicidal thoughts.
Ms. R describes her husband and two teenage daughters as “very supportive,” but admits that her fatigue and irritability have strained these relationships; she says she snaps at them for minor things, such as coming to dinner 1 minute late. She misses her job, which she recently quit because of her decreasing ability to function.
At intake, Ms. R says she will not resume previous medications but will consider alternatives. She refuses psychotherapy because of time constraints and transportation problems but is willing to return every 2 weeks for medication checks. She says she adhered to every prescription over 3 years with no major side effects. She has never taken an antidepressant or anxiolytic without a mood stabilizer.
Ms. R reports no medical problems, past substance use, current or past psychotic symptoms, or psychiatric hospitalizations. Her family history shows depression in one first-degree relative and anxiety in others. Her Hamilton Anxiety Scale (HAM-A) score of 20 indicates moderate anxiety. Laboratory tests ordered by her primary care physician are normal.
poll here
The authors’ observations
Racing thoughts, irritable mood, decreased sleep, and concentration problems can point to GAD, mania associated with bipolar disorder type I, or hypomania suggesting bipolar disorder type I or II (Table 1).1
We suspect GAD because:
- Ms. R’s thoughts “race” only when she worries
- her irritability and concentration problems seem more sustained than episodic
- she has difficulty falling and staying asleep, but her need for sleep has not decreased
- she complains of constant fatigue, whereas abnormally high energy characterizes bipolar disorder’s manic or hypomanic phase.
Does Ms. R have depression? Determining if the patient’s depressive symptoms are secondary to GAD or warrant a separate diagnosis can be difficult (Table 1). With Ms. R’s permission, we talked to her family, because collateral information often helps clarify the diagnosis. Her husband and daughters offered no significant new insights, however.
Table 1
Overlap among symptoms that suggest GAD, mania, or major depression
| Symptom | GAD | Mania | MDD |
|---|---|---|---|
| Difficulty concentrating/distractibility | X | X | X |
| Mood irritability | X | X | X |
| ‘Racing’ thoughts | X | X | X |
| Sleep disturbance | X | X | X |
| Tiring easily/low energy | X | X | |
| Ecessive psychomotor activity/restlessness | X | X | X |
| GAD: generalized anxiety disorder | |||
| MDD: major depressive disorder | |||
| Source: Reference 1 | |||
Treatment: Targeting the anxiety
To address Ms. R’s anxiety symptoms, we start buspirone, 5 mg tid, and titrate to 15 mg bid over 2 weeks. We choose buspirone—which is FDA-approved to treat GAD—because it is unlikely to cause a mood switch if bipolar disorder is causing Ms. R’s depression. We discuss with her the drug’s indications, benefits, and potential side effects (such as vertigo, headache, lightheadedness, and nausea).
At the first 2-week follow-up, Ms. R reports no side effects but little improvement. After another 2 weeks, she says she feels less anxiety, irritability, pain, and fewer racing thoughts. She reports less difficulty falling asleep, though she’s still sleeping only about 6 hours nightly. Her HAM-A score falls to 12, indicating mild anxiety.
Ms. R, however, says she still feels depressed, tired, distracted, unmotivated, and worthless. Her Hamilton Rating Scale for Depression (HAM-D) score of 16 indicates moderate depression.
poll here
The authors’ observations
The persistence of Ms. R’s depressive symptoms suggests comorbid major depressive disorder (MDD). In fact, MDD and GAD are considered the most common mood-anxiety comorbidities.2
Determining whether Ms. R has unipolar depression or bipolar disorder is extremely important, considering the treatment implications. In patients with bipolar disorder, any antidepressant can trigger mania or hypomania if used without a mood stabilizer. Some studies also have associated rare cases of suicidal behavior with antidepressant use.3,4 Lifetime risk of suicide in bipolar disorder is approximately 20 times that in the general population.5
Although Ms. R’s history does not reveal a previous manic episode, ruling out hypomania is difficult because it usually does not impair work or social functioning. Hypomania often goes unreported because others hardly notice it. Collateral history can uncover clues to hypomania (See For Your Patient), but Ms. R’s husband and daughters say they have not seen such episodes.
On the other hand, normal behavior can be mistaken for hypomania. Ms. R’s previous psychiatrist and primary care physician might have misinterpreted Ms. R’s baseline extroverted personality as hypomanic behavior. Also, her over-whelming depressive and anxiety symptoms between depressive episodes made her normal moods appear hypomanic.
Compared with unipolar depression, bipolar depression is more frequently associated with psychomotor retardation, hypersomnia, early onset, and family history of bipolar disorder.6 Ms. R, however, suffered low energy, terminal insomnia, and late onset, and had no known family history of bipolar disorder.
The Mood Disorder Questionnaire, a scale of self-administered questions, can help screen for symptoms that suggest bipolar disorder. A positive questionnaire result demands further clinical evaluation.7
Depression, mania, or hypomania? Signs family, friends should not miss*
Patient could be depressed if he/she:
- is constantly sad or irritable
- seems lost, withdrawn, or isolated
- is preoccupied with negative ideas and concerns
- persistently feels guilty, hopeless, and helpless
- says he or she has considered suicide
- has not been showering regularly or is unkempt (indicates low mood)
- shows significant changes in sleep
- moves slowly or sparingly, as if “slowed down” (indicates depressed affect)
- is often restless (indicating agitated/anxious depression)
- talks in a low-tone or monotone voice
- no longer enjoys activities or hobbies he or she once found pleasurable
- shows significant changes in appetite
- no longer enjoys sex
- cannot concentrate or make decisions
Patient could be manic or hypomanic if he/she:
- seems abnormally hyperactive, restless, and energized, compared with normal self
- is inappropriately euphoric and jubilant or, on occasion, extremely irritable
- talks rapidly and excessively
- often wears clothes that are too bright or colorful
- seems unusually self-confident, grandiose, and highly distractible
- shows increased sexual desire
- is impulsive, increasingly daring, and shows seriously impaired judgment, such as by investing/spending large sums of money for ill-advised reasons
- seems energetic despite lack of sleep
*If a family member shows any of the above symptoms, get him or her to a mental health clinic as soon as possible. Take the family member to the nearest ER or call your local crisis unit or 911 if you suspect the family member might hurt him/herself or others.
Continued treatment: ‘normal’ again
In addition to the HAM-D, we also administer the Mood Disorder Questionnaire. Results suggest Ms. R does not have bipolar illness.
To address Ms. R’s depressive symptoms, we start the selective serotonin reuptake inhibitor escitalopram at a low dosage (5 mg/d) to avoid exacerbating her anxiety. We discuss the drug’s potential to induce mania, hypomania, or other adverse effects such as nausea, anxiety, sleep disturbance, headache, and sexual dysfunction. Buspirone is maintained at 15 mg bid.
Two weeks later, Ms. R reports some increase in energy and motivation. After another 2 weeks, she reports significantly improved mood and concentration. She consistently falls asleep at 10 PM and sleeps 8 hours each night. She also finds time to read and go out with her friends and she gets along more amicably with her daughters and husband.
One month later, we increase escitalopram to 10 mg/d, a normal therapeutic dosage. Ms. R continues to respond positively and reports no side effects. Two months after starting the antidepressant, her HAM-D score of 8 suggests normal mood. We decrease follow-up visits to once monthly.
At a subsequent visit, Ms. R tells us she wants to find a job. A month later, she says she is enjoying her new job in a department store. Over the next 6 months, she remains free of anxiety, depressive symptoms, hypomanic behavior, and side effects. She tells us it’s nice to feel “normal” again.
We reduce Ms. R’s appointments to every 3 months. After another year, we refer her back to her primary care physician at her request.
The authors’ observations
DSM-IV-TR1 divides bipolar disorder into three categories:
- type I, in which the patient has had at least one manic episode with or without major depression
- type II, characterized by one or more major depressive episodes and at least one hypomanic episode
- cyclothymia, which is defined as fluctuation between hypomanic and minor depressive episodes.
Much is said about how underdiagnosis of bipolar disorder8 delays or prevents proper therapy with mood stabilizers, leading to suboptimal symptom resolution. As with Ms. R, however, an incorrect bipolar disorder diagnosis can be just as harmful. Three years of unnecessary and ineffective treatment worsened her anxiety and depressive symptoms and quality of life.
A comprehensive clinical interview supplemented with insights from family and friends can minimize the risk of misdiagnosis when patients present with symptoms that suggest bipolar disorder, depression, or GAD.
Differentiating between the following clinical features can also help you reach a diagnosis:
Sleep/energy level. Mania/hypomania is characterized by decreased need for sleep; patients often feel energetic even after 2 to 4 hours of sleep. Both depression and GAD diminish energy level, although mood is more depressed in depression. Patients with GAD have trouble falling asleep, while those with depression awaken early or have hypersomnia.
Behavior. Patients in the manic phase of bipolar disorder engage in risky, pleasurable activities with high potential for painful consequences. This drastic behavior change is not seen in depression or GAD (Table 2).
Table 2
Differentiating symptoms common to GAD, major depression, and mania
| Symptom | GAD | Major depression | Mania |
|---|---|---|---|
| Concentration | Difficulty concentrating (mind goes blank) | Diminished ability to concentrate or think (indecisiveness) | Easily distracted (difficulty focusing on one task) |
| Energy | Tires easily | Constant fatigue or loss of energy | Subjective feeling of increased energy |
| Mood | Can be irritable | Irritable, though more depressed | Euphoric or extremely irritable |
| Behavior | Seems more keyed up | More withdrawn | Increase in risky behavior with potential for painful consequences |
| Sleep | Disturbed (mostly difficulty going to sleep) | Disturbed (hypersomnia or insomnia, more likely terminal insomnia) | Decreased need for sleep (energetic after sleeping 2 to 4 hours) |
Related resources
- Depression and Bipolar Support Alliance. www.dbsalliance.org.
- Anxiety Disorders Association of America. www.adaa.org.
Drug brand names
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Quetiapine • Seroquel
- Valproic acid • Depakene
- Venlafaxine • Effexor
Disclosures
Dr. Williams is a speaker for Wyeth.
Dr. Singh reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
History: ‘A zillion racing thoughts’
Ms. R, age 44, is referred by her primary care physician. She complains of tenseness, irritability, avolition, and fatigue. She worries incessantly that her children will get sick, a catastrophe will befall her husband, or she’ll do something wrong. She says she sometimes feels as if she’s thinking “a zillion racing thoughts.”
Once fun-loving, outgoing, and energetic, Ms. R says she began feeling unusually anxious 3 years ago. A psychiatrist diagnosed bipolar disorder type II based on her racing thoughts, irritability, low energy, and history of mood swings. Over 2 years, the psychiatrist tried combining valproic acid with bupropion, citalopram, or extended-release venlafaxine, then tried lithium monotherapy. Nothing worked.
Frustrated, Ms. R left the psychiatrist and consulted her primary care physician, who prescribed gabapentin, 200 mg each morning and 300 mg at night; fluoxetine, 50 mg/d; and quetiapine, 12.5 mg/d. Ms. R noticed no improvement and stopped the medications after 6 weeks. The physician urged her to see another psychiatrist, and she presented to us 2 weeks after stopping the medications.
Ms. R also has been feeling depressed and irritable the past 4 months and has trouble falling and staying asleep at night. She sleeps 4 to 5 hours nightly, constantly feels tired, cannot concentrate, and overeats to try to alleviate her stress. She has gained 6 pounds over 2 to 3 months and weighs 160 lb; her body mass index of 26 indicates she is overweight.
She says her worries overwhelm her and cause heart palpitations and muscle tension in her neck and shoulders. She admits to feeling “worthless,” but denies suicidal thoughts.
Ms. R describes her husband and two teenage daughters as “very supportive,” but admits that her fatigue and irritability have strained these relationships; she says she snaps at them for minor things, such as coming to dinner 1 minute late. She misses her job, which she recently quit because of her decreasing ability to function.
At intake, Ms. R says she will not resume previous medications but will consider alternatives. She refuses psychotherapy because of time constraints and transportation problems but is willing to return every 2 weeks for medication checks. She says she adhered to every prescription over 3 years with no major side effects. She has never taken an antidepressant or anxiolytic without a mood stabilizer.
Ms. R reports no medical problems, past substance use, current or past psychotic symptoms, or psychiatric hospitalizations. Her family history shows depression in one first-degree relative and anxiety in others. Her Hamilton Anxiety Scale (HAM-A) score of 20 indicates moderate anxiety. Laboratory tests ordered by her primary care physician are normal.
poll here
The authors’ observations
Racing thoughts, irritable mood, decreased sleep, and concentration problems can point to GAD, mania associated with bipolar disorder type I, or hypomania suggesting bipolar disorder type I or II (Table 1).1
We suspect GAD because:
- Ms. R’s thoughts “race” only when she worries
- her irritability and concentration problems seem more sustained than episodic
- she has difficulty falling and staying asleep, but her need for sleep has not decreased
- she complains of constant fatigue, whereas abnormally high energy characterizes bipolar disorder’s manic or hypomanic phase.
Does Ms. R have depression? Determining if the patient’s depressive symptoms are secondary to GAD or warrant a separate diagnosis can be difficult (Table 1). With Ms. R’s permission, we talked to her family, because collateral information often helps clarify the diagnosis. Her husband and daughters offered no significant new insights, however.
Table 1
Overlap among symptoms that suggest GAD, mania, or major depression
| Symptom | GAD | Mania | MDD |
|---|---|---|---|
| Difficulty concentrating/distractibility | X | X | X |
| Mood irritability | X | X | X |
| ‘Racing’ thoughts | X | X | X |
| Sleep disturbance | X | X | X |
| Tiring easily/low energy | X | X | |
| Ecessive psychomotor activity/restlessness | X | X | X |
| GAD: generalized anxiety disorder | |||
| MDD: major depressive disorder | |||
| Source: Reference 1 | |||
Treatment: Targeting the anxiety
To address Ms. R’s anxiety symptoms, we start buspirone, 5 mg tid, and titrate to 15 mg bid over 2 weeks. We choose buspirone—which is FDA-approved to treat GAD—because it is unlikely to cause a mood switch if bipolar disorder is causing Ms. R’s depression. We discuss with her the drug’s indications, benefits, and potential side effects (such as vertigo, headache, lightheadedness, and nausea).
At the first 2-week follow-up, Ms. R reports no side effects but little improvement. After another 2 weeks, she says she feels less anxiety, irritability, pain, and fewer racing thoughts. She reports less difficulty falling asleep, though she’s still sleeping only about 6 hours nightly. Her HAM-A score falls to 12, indicating mild anxiety.
Ms. R, however, says she still feels depressed, tired, distracted, unmotivated, and worthless. Her Hamilton Rating Scale for Depression (HAM-D) score of 16 indicates moderate depression.
poll here
The authors’ observations
The persistence of Ms. R’s depressive symptoms suggests comorbid major depressive disorder (MDD). In fact, MDD and GAD are considered the most common mood-anxiety comorbidities.2
Determining whether Ms. R has unipolar depression or bipolar disorder is extremely important, considering the treatment implications. In patients with bipolar disorder, any antidepressant can trigger mania or hypomania if used without a mood stabilizer. Some studies also have associated rare cases of suicidal behavior with antidepressant use.3,4 Lifetime risk of suicide in bipolar disorder is approximately 20 times that in the general population.5
Although Ms. R’s history does not reveal a previous manic episode, ruling out hypomania is difficult because it usually does not impair work or social functioning. Hypomania often goes unreported because others hardly notice it. Collateral history can uncover clues to hypomania (See For Your Patient), but Ms. R’s husband and daughters say they have not seen such episodes.
On the other hand, normal behavior can be mistaken for hypomania. Ms. R’s previous psychiatrist and primary care physician might have misinterpreted Ms. R’s baseline extroverted personality as hypomanic behavior. Also, her over-whelming depressive and anxiety symptoms between depressive episodes made her normal moods appear hypomanic.
Compared with unipolar depression, bipolar depression is more frequently associated with psychomotor retardation, hypersomnia, early onset, and family history of bipolar disorder.6 Ms. R, however, suffered low energy, terminal insomnia, and late onset, and had no known family history of bipolar disorder.
The Mood Disorder Questionnaire, a scale of self-administered questions, can help screen for symptoms that suggest bipolar disorder. A positive questionnaire result demands further clinical evaluation.7
Depression, mania, or hypomania? Signs family, friends should not miss*
Patient could be depressed if he/she:
- is constantly sad or irritable
- seems lost, withdrawn, or isolated
- is preoccupied with negative ideas and concerns
- persistently feels guilty, hopeless, and helpless
- says he or she has considered suicide
- has not been showering regularly or is unkempt (indicates low mood)
- shows significant changes in sleep
- moves slowly or sparingly, as if “slowed down” (indicates depressed affect)
- is often restless (indicating agitated/anxious depression)
- talks in a low-tone or monotone voice
- no longer enjoys activities or hobbies he or she once found pleasurable
- shows significant changes in appetite
- no longer enjoys sex
- cannot concentrate or make decisions
Patient could be manic or hypomanic if he/she:
- seems abnormally hyperactive, restless, and energized, compared with normal self
- is inappropriately euphoric and jubilant or, on occasion, extremely irritable
- talks rapidly and excessively
- often wears clothes that are too bright or colorful
- seems unusually self-confident, grandiose, and highly distractible
- shows increased sexual desire
- is impulsive, increasingly daring, and shows seriously impaired judgment, such as by investing/spending large sums of money for ill-advised reasons
- seems energetic despite lack of sleep
*If a family member shows any of the above symptoms, get him or her to a mental health clinic as soon as possible. Take the family member to the nearest ER or call your local crisis unit or 911 if you suspect the family member might hurt him/herself or others.
Continued treatment: ‘normal’ again
In addition to the HAM-D, we also administer the Mood Disorder Questionnaire. Results suggest Ms. R does not have bipolar illness.
To address Ms. R’s depressive symptoms, we start the selective serotonin reuptake inhibitor escitalopram at a low dosage (5 mg/d) to avoid exacerbating her anxiety. We discuss the drug’s potential to induce mania, hypomania, or other adverse effects such as nausea, anxiety, sleep disturbance, headache, and sexual dysfunction. Buspirone is maintained at 15 mg bid.
Two weeks later, Ms. R reports some increase in energy and motivation. After another 2 weeks, she reports significantly improved mood and concentration. She consistently falls asleep at 10 PM and sleeps 8 hours each night. She also finds time to read and go out with her friends and she gets along more amicably with her daughters and husband.
One month later, we increase escitalopram to 10 mg/d, a normal therapeutic dosage. Ms. R continues to respond positively and reports no side effects. Two months after starting the antidepressant, her HAM-D score of 8 suggests normal mood. We decrease follow-up visits to once monthly.
At a subsequent visit, Ms. R tells us she wants to find a job. A month later, she says she is enjoying her new job in a department store. Over the next 6 months, she remains free of anxiety, depressive symptoms, hypomanic behavior, and side effects. She tells us it’s nice to feel “normal” again.
We reduce Ms. R’s appointments to every 3 months. After another year, we refer her back to her primary care physician at her request.
The authors’ observations
DSM-IV-TR1 divides bipolar disorder into three categories:
- type I, in which the patient has had at least one manic episode with or without major depression
- type II, characterized by one or more major depressive episodes and at least one hypomanic episode
- cyclothymia, which is defined as fluctuation between hypomanic and minor depressive episodes.
Much is said about how underdiagnosis of bipolar disorder8 delays or prevents proper therapy with mood stabilizers, leading to suboptimal symptom resolution. As with Ms. R, however, an incorrect bipolar disorder diagnosis can be just as harmful. Three years of unnecessary and ineffective treatment worsened her anxiety and depressive symptoms and quality of life.
A comprehensive clinical interview supplemented with insights from family and friends can minimize the risk of misdiagnosis when patients present with symptoms that suggest bipolar disorder, depression, or GAD.
Differentiating between the following clinical features can also help you reach a diagnosis:
Sleep/energy level. Mania/hypomania is characterized by decreased need for sleep; patients often feel energetic even after 2 to 4 hours of sleep. Both depression and GAD diminish energy level, although mood is more depressed in depression. Patients with GAD have trouble falling asleep, while those with depression awaken early or have hypersomnia.
Behavior. Patients in the manic phase of bipolar disorder engage in risky, pleasurable activities with high potential for painful consequences. This drastic behavior change is not seen in depression or GAD (Table 2).
Table 2
Differentiating symptoms common to GAD, major depression, and mania
| Symptom | GAD | Major depression | Mania |
|---|---|---|---|
| Concentration | Difficulty concentrating (mind goes blank) | Diminished ability to concentrate or think (indecisiveness) | Easily distracted (difficulty focusing on one task) |
| Energy | Tires easily | Constant fatigue or loss of energy | Subjective feeling of increased energy |
| Mood | Can be irritable | Irritable, though more depressed | Euphoric or extremely irritable |
| Behavior | Seems more keyed up | More withdrawn | Increase in risky behavior with potential for painful consequences |
| Sleep | Disturbed (mostly difficulty going to sleep) | Disturbed (hypersomnia or insomnia, more likely terminal insomnia) | Decreased need for sleep (energetic after sleeping 2 to 4 hours) |
Related resources
- Depression and Bipolar Support Alliance. www.dbsalliance.org.
- Anxiety Disorders Association of America. www.adaa.org.
Drug brand names
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Quetiapine • Seroquel
- Valproic acid • Depakene
- Venlafaxine • Effexor
Disclosures
Dr. Williams is a speaker for Wyeth.
Dr. Singh reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
2. Gorwood P. Generalized anxiety disorder and major depressive disorder comorbidity: an example of genetic pleiotropy? Eur Psychiatry 2004;19:27-33.
3. Aursnes I, Tvete IF, Gaasemyr J, Natvig B. Suicide attempts in clinical trials with paroxetine randomised against placebo. BMC Med 2005;3:14.-
4. Healy D, Aldred G. Antidepressant drug use & the risk of suicide. Int Rev Psychiatry 2005;17:163-72.
5. Osby U, Brandt L, Correia N, et al. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry 2001;58:844-50.
6. Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. J Affect Disord 2005;84:117-25.
7. Hirschfield RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry 2001;158:1743-4.
8. Matza LS, Rajagopalan KS, Thompson CL, Lissovoy G. Misdiagnosed patients with bipolar disorder: comorbidities, treatment patterns, and direct treatment costs. J Clin Psychiatry 2005;66:1432-40.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
2. Gorwood P. Generalized anxiety disorder and major depressive disorder comorbidity: an example of genetic pleiotropy? Eur Psychiatry 2004;19:27-33.
3. Aursnes I, Tvete IF, Gaasemyr J, Natvig B. Suicide attempts in clinical trials with paroxetine randomised against placebo. BMC Med 2005;3:14.-
4. Healy D, Aldred G. Antidepressant drug use & the risk of suicide. Int Rev Psychiatry 2005;17:163-72.
5. Osby U, Brandt L, Correia N, et al. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry 2001;58:844-50.
6. Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. J Affect Disord 2005;84:117-25.
7. Hirschfield RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry 2001;158:1743-4.
8. Matza LS, Rajagopalan KS, Thompson CL, Lissovoy G. Misdiagnosed patients with bipolar disorder: comorbidities, treatment patterns, and direct treatment costs. J Clin Psychiatry 2005;66:1432-40.