User login
When clozapine is not an option
History: ‘leaving town’
Mr. S, age 58, escaped repeatedly from his group home over 4 weeks. During one episode, he removed mail from neighbors’ mailboxes and tried to direct midday traffic. He would disappear for a few hours, sometimes overnight, before returning or being brought back by police.
The patient—who has had schizophrenia with catatonic features for 30 years—offered assorted explanations for escaping, most of them based on delusional beliefs, such as “I’m leaving town to get married” or “I’m late for engineering class.”
Since his last escape 3 weeks ago, Mr. S has remained in the group home without incident but has not been reporting for his usual outpatient psychiatric care. One day, he finally presents to us at the group home sponsor’s urging.
On evaluation, Mr. S shows stereotyped speech, staring, posturing, speech-prompt mutism, and odd mannerisms such as saluting. He has not been bathing or sleeping and smiles inappropriately. He speaks only when spoken to and answers with short phrases punctuated with ”By the grace of the good Lord.”
The authors’ observations
DSM-IV-TR requires at least two features to diagnose catatonic schizophrenia:
- peculiar voluntary movements
- extreme negativism
- excessive motor activity
- echolalia or echopraxia
- motoric immobility.1
Catatonia is common among the chronic mentally ill,2 yet it often goes undiagnosed.3 As a form of psychosis, catatonia might lead to greater functional impairment if not treated.
Treatment: time to try clozapine?
Over 10 years, numerous antipsychotic regimens plus adjunctive valproic acid, 500 mg tid, or lorazepam, up to 2 mg tid, have not lessened Mr. S’ psychosis and impulsivity. We start clozapine, 400 mg/d, and order twice-monthly blood tests to check for clozapine-induced agranulocytosis.
After nearly 6 months, some catatonic features improve gradually based on clinical interview. Serum clozapine is 363 ng/mL.
poll here
The authors’ observations
Second-generation antipsychotics (SGAs) are favored over first-generation antipsychotics to treat schizophrenia with catatonic features (Table),4,5 but no drug in either class has worked for Mr. S.
ECT can alleviate catatonic schizophrenia,4,6 but this option often is not available because the clinician fears a negative outcome would prompt legal action, or the guardian or next of kin do not consent to the procedure.3 We considered referring Mr. S to an ECT provider, but he has no legal guardian to provide consent. The group home sponsor also objected to ECT because Mr. S would have been sent out of town for treatment.
Catatonia patients who are immobile, physically compromised, and refuse food and drink typically are considered ECT candidates. Mr. S eats and drinks regularly and is physically able.
Lorazepam can produce rapid response, but it can be addictive.2 Also, an adjunctive 2 mg/d dosage showed no effect.
Clozapine monotherapy has shown effectiveness in catatonic schizophrenia7 and might be an option after other antipsychotics have failed.
Table 1
Treatments for catatonia: risks and benefits
| Medication | Use | Rationale | Benefits | Risks |
|---|---|---|---|---|
| First-generation antipsychotics (FGAs) | Often used for schizophrenia | Control positive symptoms | Well-established | Catatonia might be difficult to distinguish from NMS |
| Less expensive than other medications | ||||
| Second-generation antipsychotics (SGAs) | Beneficial in catatonia | Less likely than FGAs to worsen catatonia because of low D2 blockade | Some studies suggest greater efficacy than with FGAs | Metabolic syndrome, agranulocytosis with clozapine |
| Benzodiazepines | Lorazepam helpful in acute catatonia | Can be added to any antipsychotic | Safe, first-line treatment for catatonia | Respiratory compromise, incoordination, sedation, potential for abuse |
| Electroconvulsive therapy | ||||
| Electroconvulsive therapy | Beneficial in malignant catatonia | Effective in catatonia, NMS | Useful for treatment-refractory catatonia | Concerns with anesthesia, informed consent, availability |
| Rapid onset of action | ||||
| NMS: Neuroleptic malignant syndrome | ||||
Complication: agranulocytosis, then nms
Six months after starting clozapine, Mr. S starts having diaphoresis and night sweats, suggesting neutropenia. Blood testing shows a white blood cell count (WBC) of 3.6/μL, down from 4.6/μL 2 weeks before (normal range, 4.6 to 11/μL).
One week later, Mr. S’ WBC is 1.6/μL with a 46% relative neutrophil value (normal range, 50% to 70%) and an absolute neutrophil count of 736 (normal range, 2,500 to 7,000).
We diagnose agranulocytosis and stop clozapine, but Mr. S’ WBC continues to fall over 2 weeks to 0.8/μL with a 16% relative and 128 absolute neutrophil count. After 1 more week, his WBC increases to 2.6/μL and returns to normal 1 week later—4 weeks after stopping clozapine
We then target Mr. S’ catatonia with intramuscular haloperidol, 100 mg/d for 4 weeks, and ziprasidone, 80 mg bid with food. He tolerates this combination but gradually develops tremor and rigidity. Six weeks later, we add levodopa/carbidopa, 25/250 mg bid for his movement problems.
Two weeks later, Mr. S is sweating profusely, disoriented, rigid, and febrile (104.6°F). We diagnose neuroleptic malignant syndrome (NMS), stop both antipsychotics, and admit him for treatment. We start lorazepam, 1 mg tid for catatonia; bromocriptine, 250 mg bid for rigidity; and continue levodopa/carbidopa at the same dosage. We also add dantrolene, 25 mg tid for 5 days for fever and rigidity, and provide a cooling blanket for hyperthermia.
Mr. S’ fever, autonomic changes, and diaphoresis diminish within 3 days. Rigidity and mental status improve gradually over 2 weeks. We discharge him after 10 days.
poll here
The author’s observations
Catatonia is a recognized risk factor for NMS. White and Robins8 described 17 patients with a catatonic syndrome that developed into NMS within 5 to 96 hours of starting a neuroleptic. Sachdev developed an NMS rating scale that includes catatonic symptoms.9
Northoff,10 however, associates NMS with D2 receptor blockage in the basal ganglia and relates catatonia to a frontocortical gamma-aminobutyric acid (GABA) dysfunction. Based on this theory, haloperidol—which offers a higher D2 blockade than do SGAs such as ziprasidone—might have contributed to Mr. S’ NMS.
Some evidence suggests that lorazepam—which works on gamma-aminobutyric acid ionotropic type A (GABAA) receptors—helps treat catatonia in NMS and improves rigidity, hyperthermia, and autonomic signs.11
Treatment: which agents will work?
Three weeks after his discharge, we restart ziprasidone, 40 mg bid for Mr. S’ catatonic schizophrenia. He remains free of NMS symptoms but still has mannerisms (posturing, staring, immobility, stereotypic scratching on his face).
Over 1 year, Mr. S is hospitalized repeatedly because of persistent impulsivity and delusions. He has failed numerous antipsychotic regimens lasting 1 month or longer, including olanzapine, up to 30 mg/d; quetiapine, 300 mg tid; and risperidone, 2 mg tid. Adding a first-generation antipsychotic either does not help (as with perphenazine, 12 mg/d) or diminishes his memory (as with chlorpromazine, 250 mg/d). The anticholinergic benztropine, 2 mg bid, also is ineffective.
Combination quetiapine, 300 mg/d, and the antiviral amantadine, 100 mg tid, improve Mr. S’ stereotypy at first, but his delusions intensify within 1 week. His Bush-Francis Catatonia Rating Scale scores range from 9 (indicating moderate catatonia) to 16 (persistent catatonic features).12
poll here
The authors’ observations
Catatonic schizophrenia’s pathophysiology and response to medication might differ compared with other schizophrenia forms.13 Dopamine D2 hypoactivity, glutamate N-methyl-D-aspartate (NMDA) hyperactivity, or GABAA hypoactivity are believed to cause catatonia.3,6,7 GABA agonists, anticonvulsants, dopamine agonists, SGAs, and NMDA antagonists target these pathophysiologies, but patients with a catatonia subtype often respond to only one type of medication.
Lorazepam exerts an anticatatonic effect by binding to GABAA receptors and increasing GABA activity. Lorazepam can help some patients with schizophrenia but has not shown benefit when added to an antipsychotic for chronic catatonia.6,14
SGAs can provide marked improvement in patients with catatonic schizophrenia.5
Salokangas et al15 note that “atypicals” pass more dopamine to the D2 receptor when dopamine is low in the basal ganglia. This suggests that SGAs with low D2 binding—such as clozapine, olanzapine, and quetiapine—are more beneficial than other SGAs for catatonia. Serotonin binding or other mechanisms might add to these drugs’ anticatatonic effect.7
Anticonvulsants. Adjunctive anticonvulsant therapy might alleviate catatonia by increasing GABA activity or by causing a modest antiglutaminergic effect, as reported with carbamazepine or valproic acid.16 Anticholinergics also might help treat neuroleptic-induced catatonia.17
Amantadine—FDA-approved to treat Parkinson’s disease and extrapyramidal disease—can alleviate catatonia by blocking hyperglutamatergic excitotoxicity in neurons, thus blocking NMDA receptors.18 As with Mr. S, however, amantadine can worsen psychosis by increasing dopamine release.
Memantine—an NMDA receptor antagonist indicated for moderate to severe Alzheimer’s disease—also blocks hyperglutamatergic excitotoxicity in neurons. The medication has shown effectiveness for treating catatonic schizophrenia in case reports,19-21 but 3 patients have reported memantine-induced psychosis and seizures.21
Some might argue that Mr. S’ delusions are predominant and more compelling than his catatonia, but these did not hamper his ability to live in a group home. His catatonia-related negativism, impulsivity, and inability to cooperate are what led to frequent hospitalization.
Follow-up: treatment change
We stop amantadine, add memantine, 10 mg bid, and titrate quetiapine over 2 weeks to 900 mg/d. Mr. S’ catatonia improves but some delusions persist. We add olanzapine, 7.5 mg bid, and within 2 weeks Mr. S is less delusional and more cooperative.
We discharge Mr. S on the above medications, plus:
- lorazepam, 1 mg each morning and 2 mg nightly, which he has been taking for catatonia for about 1 year
- trazodone, 150 mg bid, which we added 6 months ago to help him sleep and reduce psychomotor excitement
- ranitidine, 150 mg bid, for gastroesophageal reflux disorder
- and levothyroxine, 0.5 mg/d, for comobrid hypothyroidism. His thyroid-stimulating hormone level is normal.
We see Mr. S monthly. He is still impulsive at times, occasionally collecting his neighbors’ newspapers and mail despite instructions from group home staff not to do so. Yet his sponsors say Mr. S is “like a new person.” He talks spontaneously, interacts, and is cooperative. He has not been hospitalized for more than 1 year.
The authors’ observations
Mr. S responded favorably to clozapine but cannot tolerate it. With a combination of two other SGAs, a patient might gain the benefits of clozapine without the need for frequent blood draws or the risk of agranulocytosis, other side effects, or interactions between clozapine and other drugs. Adding memantine was necessary to improve the catatonic features that prevented his return to the group home.
Related resources
- World Federation of Societies of Biological Psychiatry. www.wfsbp.com.
- Neuroleptic Malignant Syndrome Information Service. www.nmsis.org.
- Mann SC, Caroff SN, Keck PE Jr, Lazarus A. Neuroleptic malignant syndrome and related conditions, 2nd ed. Arlington, VA: American Psychiatric Press; 2003:1-44.
- Ungvari GS (ed). Catatonia-an anthology of classical contributions. Hong Kong: Scientific Communications International; 2006.
- Amantadine • Symmetrel
- Benztropine • Cogentin
- Bromocriptine • Parlodel
- Carbamazepine • Equetro, others
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Dantrolene • Dantrium
- Haloperidol • Haldol
- Levodopa/carbidopa • Sinemet
- Levothyroxine • Synthroid
- Lorazepam • Ativan
- Memantine • Namenda
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Ranitidine • Zantac
- Risperidone • Risperdal
- Trazodone • Desyrel
- Valproic acid • Depakene
- Ziprasidone • Geodon
Dr. Carroll is a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol Myers-Squibb Co., Forest Pharmaceuticals, Janssen Pharmaceutica, and Pfizer.
Dr. Thomas receives grant support from Pfizer and is a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, and Pfizer.
Dr. Tugrul is a consultant to and speaker for Bristol Myers-Squibb Co. and Eli Lilly and Co.
Dr. Jayanti reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Acknowledgment
The authors thank Francisco José Appiani, MD, chairman, psychiatry department, Military Hospital of Campo de Mayo, Buenos Aires, Argentina, and Vijay Jayanti, BS, medical student, The Ohio State University, Columbus, for their help with this article.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000:204.
2. Ungvari GS, Leung SK, Ng FS, et al. Schizophrenia with prominent catatonic features (“catatonic schizophrenia”) I. Demographic and clinical correlates in the chronic phase. Prog Neuropsychopharmacol Biol Psychiatry 2005;29:27-38.
3. Dhossche D, Wing L, Ohta M, Neumarker K (eds). Catatonia in autism spectrum disorders. International review of neurobiology, vol. 72. San Diego: Elsevier/Academic Press; 2006.
4. Falkai P, Wobrock T, Lieberman J. WFSBP guidelines for biological treatment of schizophrenia, part 1. Acute treatment of schizophrenia. World J Biol Psychiatry 2005;6:32-91.
5. Van Dalfsen F, Van Hecke J, Van Dalfsen A, et al. The use of atypical antipsychotics in the treatment of catatonia. Eur Psychiatry 2005;20:422-9.
6. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry 1990;51:357-62.
7. Dursun SM, Hallak JE, Haddad P, et al. Clozapine monotherapy for catatonic schizophrenia: should clozapine be the treatment of choice, with catatonia rather than psychosis as the main therapeutic index? J Psychopharmacol 2005;19:432-3.
8. White DAC, Robbins AH. An analysis of 17 catatonic patients diagnosed with neuroleptic malignant syndrome. CNS Spectrums 2000;5:58-65.
9. Sachdev PS. A rating scale for neuroleptic malignant syndrome. Psychiatry Res 2005;135:249-56.
10. Northoff G. Catatonia and neuroleptic malignant syndrome: psychopathology and pathophysiology. J Neural Transm 2002;109:1453-67.
11. Francis A, Chandragiri S, Rizvi S, et al. Is lorazepam a treatment for neuroleptic malignant syndrome? CNS Spectrums 2000;5:54-7.
12. Bush G, Fink M, Petrides, et al. Catatonia I: Rating scale and standardized examination. Acta Psychiatr Scand 1996;93:129-36.
13. Carroll BT, Thomas C, Jayanti K, et al. Schizophrenia with catatonic features deserves further study. World J Biol Psychiatry 2005;6(4):267-8.
14. Ungvari GS, Chie HFK, Chow LY, et al. Lorazepam for chronic catatonia: A random, double blind, placebo-controlled cross-over study. Psychopharmacology (Berl) 1999;142:393-8.
15. Salokangas R, Honkonen T, Stengard E, et al. Negative symptoms and neuroleptics in catatonic schizophrenia. Schizophr Res 2003;59:73-6.
16. Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge, UK: Cambridge University Press; 2003.
17. Franz M, Gallhofer B, Kanzow WT. Treatment of catatonia with intravenous biperidine. Br J Psychiatry 1994;164:847-8.
18. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry 1997;62:404-6.
19. Thomas C, Carroll BT, Maley JT, et al. Memantine in catatonic schizophrenia. Am J Psychiatry 2005;162:656.
20. Carroll BT, Thomas C, Jayanti K. Amantadine and memantine in catatonic schizophrenia. Ann Clin Psychiatry 2006;18:133-4.
21. Carpenter SS, Hatchett AD, Fuller MA. Catatonic schizophrenia and the use of memantine. Ann Pharmacother 2006;40:344-6.
History: ‘leaving town’
Mr. S, age 58, escaped repeatedly from his group home over 4 weeks. During one episode, he removed mail from neighbors’ mailboxes and tried to direct midday traffic. He would disappear for a few hours, sometimes overnight, before returning or being brought back by police.
The patient—who has had schizophrenia with catatonic features for 30 years—offered assorted explanations for escaping, most of them based on delusional beliefs, such as “I’m leaving town to get married” or “I’m late for engineering class.”
Since his last escape 3 weeks ago, Mr. S has remained in the group home without incident but has not been reporting for his usual outpatient psychiatric care. One day, he finally presents to us at the group home sponsor’s urging.
On evaluation, Mr. S shows stereotyped speech, staring, posturing, speech-prompt mutism, and odd mannerisms such as saluting. He has not been bathing or sleeping and smiles inappropriately. He speaks only when spoken to and answers with short phrases punctuated with ”By the grace of the good Lord.”
The authors’ observations
DSM-IV-TR requires at least two features to diagnose catatonic schizophrenia:
- peculiar voluntary movements
- extreme negativism
- excessive motor activity
- echolalia or echopraxia
- motoric immobility.1
Catatonia is common among the chronic mentally ill,2 yet it often goes undiagnosed.3 As a form of psychosis, catatonia might lead to greater functional impairment if not treated.
Treatment: time to try clozapine?
Over 10 years, numerous antipsychotic regimens plus adjunctive valproic acid, 500 mg tid, or lorazepam, up to 2 mg tid, have not lessened Mr. S’ psychosis and impulsivity. We start clozapine, 400 mg/d, and order twice-monthly blood tests to check for clozapine-induced agranulocytosis.
After nearly 6 months, some catatonic features improve gradually based on clinical interview. Serum clozapine is 363 ng/mL.
poll here
The authors’ observations
Second-generation antipsychotics (SGAs) are favored over first-generation antipsychotics to treat schizophrenia with catatonic features (Table),4,5 but no drug in either class has worked for Mr. S.
ECT can alleviate catatonic schizophrenia,4,6 but this option often is not available because the clinician fears a negative outcome would prompt legal action, or the guardian or next of kin do not consent to the procedure.3 We considered referring Mr. S to an ECT provider, but he has no legal guardian to provide consent. The group home sponsor also objected to ECT because Mr. S would have been sent out of town for treatment.
Catatonia patients who are immobile, physically compromised, and refuse food and drink typically are considered ECT candidates. Mr. S eats and drinks regularly and is physically able.
Lorazepam can produce rapid response, but it can be addictive.2 Also, an adjunctive 2 mg/d dosage showed no effect.
Clozapine monotherapy has shown effectiveness in catatonic schizophrenia7 and might be an option after other antipsychotics have failed.
Table 1
Treatments for catatonia: risks and benefits
| Medication | Use | Rationale | Benefits | Risks |
|---|---|---|---|---|
| First-generation antipsychotics (FGAs) | Often used for schizophrenia | Control positive symptoms | Well-established | Catatonia might be difficult to distinguish from NMS |
| Less expensive than other medications | ||||
| Second-generation antipsychotics (SGAs) | Beneficial in catatonia | Less likely than FGAs to worsen catatonia because of low D2 blockade | Some studies suggest greater efficacy than with FGAs | Metabolic syndrome, agranulocytosis with clozapine |
| Benzodiazepines | Lorazepam helpful in acute catatonia | Can be added to any antipsychotic | Safe, first-line treatment for catatonia | Respiratory compromise, incoordination, sedation, potential for abuse |
| Electroconvulsive therapy | ||||
| Electroconvulsive therapy | Beneficial in malignant catatonia | Effective in catatonia, NMS | Useful for treatment-refractory catatonia | Concerns with anesthesia, informed consent, availability |
| Rapid onset of action | ||||
| NMS: Neuroleptic malignant syndrome | ||||
Complication: agranulocytosis, then nms
Six months after starting clozapine, Mr. S starts having diaphoresis and night sweats, suggesting neutropenia. Blood testing shows a white blood cell count (WBC) of 3.6/μL, down from 4.6/μL 2 weeks before (normal range, 4.6 to 11/μL).
One week later, Mr. S’ WBC is 1.6/μL with a 46% relative neutrophil value (normal range, 50% to 70%) and an absolute neutrophil count of 736 (normal range, 2,500 to 7,000).
We diagnose agranulocytosis and stop clozapine, but Mr. S’ WBC continues to fall over 2 weeks to 0.8/μL with a 16% relative and 128 absolute neutrophil count. After 1 more week, his WBC increases to 2.6/μL and returns to normal 1 week later—4 weeks after stopping clozapine
We then target Mr. S’ catatonia with intramuscular haloperidol, 100 mg/d for 4 weeks, and ziprasidone, 80 mg bid with food. He tolerates this combination but gradually develops tremor and rigidity. Six weeks later, we add levodopa/carbidopa, 25/250 mg bid for his movement problems.
Two weeks later, Mr. S is sweating profusely, disoriented, rigid, and febrile (104.6°F). We diagnose neuroleptic malignant syndrome (NMS), stop both antipsychotics, and admit him for treatment. We start lorazepam, 1 mg tid for catatonia; bromocriptine, 250 mg bid for rigidity; and continue levodopa/carbidopa at the same dosage. We also add dantrolene, 25 mg tid for 5 days for fever and rigidity, and provide a cooling blanket for hyperthermia.
Mr. S’ fever, autonomic changes, and diaphoresis diminish within 3 days. Rigidity and mental status improve gradually over 2 weeks. We discharge him after 10 days.
poll here
The author’s observations
Catatonia is a recognized risk factor for NMS. White and Robins8 described 17 patients with a catatonic syndrome that developed into NMS within 5 to 96 hours of starting a neuroleptic. Sachdev developed an NMS rating scale that includes catatonic symptoms.9
Northoff,10 however, associates NMS with D2 receptor blockage in the basal ganglia and relates catatonia to a frontocortical gamma-aminobutyric acid (GABA) dysfunction. Based on this theory, haloperidol—which offers a higher D2 blockade than do SGAs such as ziprasidone—might have contributed to Mr. S’ NMS.
Some evidence suggests that lorazepam—which works on gamma-aminobutyric acid ionotropic type A (GABAA) receptors—helps treat catatonia in NMS and improves rigidity, hyperthermia, and autonomic signs.11
Treatment: which agents will work?
Three weeks after his discharge, we restart ziprasidone, 40 mg bid for Mr. S’ catatonic schizophrenia. He remains free of NMS symptoms but still has mannerisms (posturing, staring, immobility, stereotypic scratching on his face).
Over 1 year, Mr. S is hospitalized repeatedly because of persistent impulsivity and delusions. He has failed numerous antipsychotic regimens lasting 1 month or longer, including olanzapine, up to 30 mg/d; quetiapine, 300 mg tid; and risperidone, 2 mg tid. Adding a first-generation antipsychotic either does not help (as with perphenazine, 12 mg/d) or diminishes his memory (as with chlorpromazine, 250 mg/d). The anticholinergic benztropine, 2 mg bid, also is ineffective.
Combination quetiapine, 300 mg/d, and the antiviral amantadine, 100 mg tid, improve Mr. S’ stereotypy at first, but his delusions intensify within 1 week. His Bush-Francis Catatonia Rating Scale scores range from 9 (indicating moderate catatonia) to 16 (persistent catatonic features).12
poll here
The authors’ observations
Catatonic schizophrenia’s pathophysiology and response to medication might differ compared with other schizophrenia forms.13 Dopamine D2 hypoactivity, glutamate N-methyl-D-aspartate (NMDA) hyperactivity, or GABAA hypoactivity are believed to cause catatonia.3,6,7 GABA agonists, anticonvulsants, dopamine agonists, SGAs, and NMDA antagonists target these pathophysiologies, but patients with a catatonia subtype often respond to only one type of medication.
Lorazepam exerts an anticatatonic effect by binding to GABAA receptors and increasing GABA activity. Lorazepam can help some patients with schizophrenia but has not shown benefit when added to an antipsychotic for chronic catatonia.6,14
SGAs can provide marked improvement in patients with catatonic schizophrenia.5
Salokangas et al15 note that “atypicals” pass more dopamine to the D2 receptor when dopamine is low in the basal ganglia. This suggests that SGAs with low D2 binding—such as clozapine, olanzapine, and quetiapine—are more beneficial than other SGAs for catatonia. Serotonin binding or other mechanisms might add to these drugs’ anticatatonic effect.7
Anticonvulsants. Adjunctive anticonvulsant therapy might alleviate catatonia by increasing GABA activity or by causing a modest antiglutaminergic effect, as reported with carbamazepine or valproic acid.16 Anticholinergics also might help treat neuroleptic-induced catatonia.17
Amantadine—FDA-approved to treat Parkinson’s disease and extrapyramidal disease—can alleviate catatonia by blocking hyperglutamatergic excitotoxicity in neurons, thus blocking NMDA receptors.18 As with Mr. S, however, amantadine can worsen psychosis by increasing dopamine release.
Memantine—an NMDA receptor antagonist indicated for moderate to severe Alzheimer’s disease—also blocks hyperglutamatergic excitotoxicity in neurons. The medication has shown effectiveness for treating catatonic schizophrenia in case reports,19-21 but 3 patients have reported memantine-induced psychosis and seizures.21
Some might argue that Mr. S’ delusions are predominant and more compelling than his catatonia, but these did not hamper his ability to live in a group home. His catatonia-related negativism, impulsivity, and inability to cooperate are what led to frequent hospitalization.
Follow-up: treatment change
We stop amantadine, add memantine, 10 mg bid, and titrate quetiapine over 2 weeks to 900 mg/d. Mr. S’ catatonia improves but some delusions persist. We add olanzapine, 7.5 mg bid, and within 2 weeks Mr. S is less delusional and more cooperative.
We discharge Mr. S on the above medications, plus:
- lorazepam, 1 mg each morning and 2 mg nightly, which he has been taking for catatonia for about 1 year
- trazodone, 150 mg bid, which we added 6 months ago to help him sleep and reduce psychomotor excitement
- ranitidine, 150 mg bid, for gastroesophageal reflux disorder
- and levothyroxine, 0.5 mg/d, for comobrid hypothyroidism. His thyroid-stimulating hormone level is normal.
We see Mr. S monthly. He is still impulsive at times, occasionally collecting his neighbors’ newspapers and mail despite instructions from group home staff not to do so. Yet his sponsors say Mr. S is “like a new person.” He talks spontaneously, interacts, and is cooperative. He has not been hospitalized for more than 1 year.
The authors’ observations
Mr. S responded favorably to clozapine but cannot tolerate it. With a combination of two other SGAs, a patient might gain the benefits of clozapine without the need for frequent blood draws or the risk of agranulocytosis, other side effects, or interactions between clozapine and other drugs. Adding memantine was necessary to improve the catatonic features that prevented his return to the group home.
Related resources
- World Federation of Societies of Biological Psychiatry. www.wfsbp.com.
- Neuroleptic Malignant Syndrome Information Service. www.nmsis.org.
- Mann SC, Caroff SN, Keck PE Jr, Lazarus A. Neuroleptic malignant syndrome and related conditions, 2nd ed. Arlington, VA: American Psychiatric Press; 2003:1-44.
- Ungvari GS (ed). Catatonia-an anthology of classical contributions. Hong Kong: Scientific Communications International; 2006.
- Amantadine • Symmetrel
- Benztropine • Cogentin
- Bromocriptine • Parlodel
- Carbamazepine • Equetro, others
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Dantrolene • Dantrium
- Haloperidol • Haldol
- Levodopa/carbidopa • Sinemet
- Levothyroxine • Synthroid
- Lorazepam • Ativan
- Memantine • Namenda
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Ranitidine • Zantac
- Risperidone • Risperdal
- Trazodone • Desyrel
- Valproic acid • Depakene
- Ziprasidone • Geodon
Dr. Carroll is a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol Myers-Squibb Co., Forest Pharmaceuticals, Janssen Pharmaceutica, and Pfizer.
Dr. Thomas receives grant support from Pfizer and is a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, and Pfizer.
Dr. Tugrul is a consultant to and speaker for Bristol Myers-Squibb Co. and Eli Lilly and Co.
Dr. Jayanti reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Acknowledgment
The authors thank Francisco José Appiani, MD, chairman, psychiatry department, Military Hospital of Campo de Mayo, Buenos Aires, Argentina, and Vijay Jayanti, BS, medical student, The Ohio State University, Columbus, for their help with this article.
History: ‘leaving town’
Mr. S, age 58, escaped repeatedly from his group home over 4 weeks. During one episode, he removed mail from neighbors’ mailboxes and tried to direct midday traffic. He would disappear for a few hours, sometimes overnight, before returning or being brought back by police.
The patient—who has had schizophrenia with catatonic features for 30 years—offered assorted explanations for escaping, most of them based on delusional beliefs, such as “I’m leaving town to get married” or “I’m late for engineering class.”
Since his last escape 3 weeks ago, Mr. S has remained in the group home without incident but has not been reporting for his usual outpatient psychiatric care. One day, he finally presents to us at the group home sponsor’s urging.
On evaluation, Mr. S shows stereotyped speech, staring, posturing, speech-prompt mutism, and odd mannerisms such as saluting. He has not been bathing or sleeping and smiles inappropriately. He speaks only when spoken to and answers with short phrases punctuated with ”By the grace of the good Lord.”
The authors’ observations
DSM-IV-TR requires at least two features to diagnose catatonic schizophrenia:
- peculiar voluntary movements
- extreme negativism
- excessive motor activity
- echolalia or echopraxia
- motoric immobility.1
Catatonia is common among the chronic mentally ill,2 yet it often goes undiagnosed.3 As a form of psychosis, catatonia might lead to greater functional impairment if not treated.
Treatment: time to try clozapine?
Over 10 years, numerous antipsychotic regimens plus adjunctive valproic acid, 500 mg tid, or lorazepam, up to 2 mg tid, have not lessened Mr. S’ psychosis and impulsivity. We start clozapine, 400 mg/d, and order twice-monthly blood tests to check for clozapine-induced agranulocytosis.
After nearly 6 months, some catatonic features improve gradually based on clinical interview. Serum clozapine is 363 ng/mL.
poll here
The authors’ observations
Second-generation antipsychotics (SGAs) are favored over first-generation antipsychotics to treat schizophrenia with catatonic features (Table),4,5 but no drug in either class has worked for Mr. S.
ECT can alleviate catatonic schizophrenia,4,6 but this option often is not available because the clinician fears a negative outcome would prompt legal action, or the guardian or next of kin do not consent to the procedure.3 We considered referring Mr. S to an ECT provider, but he has no legal guardian to provide consent. The group home sponsor also objected to ECT because Mr. S would have been sent out of town for treatment.
Catatonia patients who are immobile, physically compromised, and refuse food and drink typically are considered ECT candidates. Mr. S eats and drinks regularly and is physically able.
Lorazepam can produce rapid response, but it can be addictive.2 Also, an adjunctive 2 mg/d dosage showed no effect.
Clozapine monotherapy has shown effectiveness in catatonic schizophrenia7 and might be an option after other antipsychotics have failed.
Table 1
Treatments for catatonia: risks and benefits
| Medication | Use | Rationale | Benefits | Risks |
|---|---|---|---|---|
| First-generation antipsychotics (FGAs) | Often used for schizophrenia | Control positive symptoms | Well-established | Catatonia might be difficult to distinguish from NMS |
| Less expensive than other medications | ||||
| Second-generation antipsychotics (SGAs) | Beneficial in catatonia | Less likely than FGAs to worsen catatonia because of low D2 blockade | Some studies suggest greater efficacy than with FGAs | Metabolic syndrome, agranulocytosis with clozapine |
| Benzodiazepines | Lorazepam helpful in acute catatonia | Can be added to any antipsychotic | Safe, first-line treatment for catatonia | Respiratory compromise, incoordination, sedation, potential for abuse |
| Electroconvulsive therapy | ||||
| Electroconvulsive therapy | Beneficial in malignant catatonia | Effective in catatonia, NMS | Useful for treatment-refractory catatonia | Concerns with anesthesia, informed consent, availability |
| Rapid onset of action | ||||
| NMS: Neuroleptic malignant syndrome | ||||
Complication: agranulocytosis, then nms
Six months after starting clozapine, Mr. S starts having diaphoresis and night sweats, suggesting neutropenia. Blood testing shows a white blood cell count (WBC) of 3.6/μL, down from 4.6/μL 2 weeks before (normal range, 4.6 to 11/μL).
One week later, Mr. S’ WBC is 1.6/μL with a 46% relative neutrophil value (normal range, 50% to 70%) and an absolute neutrophil count of 736 (normal range, 2,500 to 7,000).
We diagnose agranulocytosis and stop clozapine, but Mr. S’ WBC continues to fall over 2 weeks to 0.8/μL with a 16% relative and 128 absolute neutrophil count. After 1 more week, his WBC increases to 2.6/μL and returns to normal 1 week later—4 weeks after stopping clozapine
We then target Mr. S’ catatonia with intramuscular haloperidol, 100 mg/d for 4 weeks, and ziprasidone, 80 mg bid with food. He tolerates this combination but gradually develops tremor and rigidity. Six weeks later, we add levodopa/carbidopa, 25/250 mg bid for his movement problems.
Two weeks later, Mr. S is sweating profusely, disoriented, rigid, and febrile (104.6°F). We diagnose neuroleptic malignant syndrome (NMS), stop both antipsychotics, and admit him for treatment. We start lorazepam, 1 mg tid for catatonia; bromocriptine, 250 mg bid for rigidity; and continue levodopa/carbidopa at the same dosage. We also add dantrolene, 25 mg tid for 5 days for fever and rigidity, and provide a cooling blanket for hyperthermia.
Mr. S’ fever, autonomic changes, and diaphoresis diminish within 3 days. Rigidity and mental status improve gradually over 2 weeks. We discharge him after 10 days.
poll here
The author’s observations
Catatonia is a recognized risk factor for NMS. White and Robins8 described 17 patients with a catatonic syndrome that developed into NMS within 5 to 96 hours of starting a neuroleptic. Sachdev developed an NMS rating scale that includes catatonic symptoms.9
Northoff,10 however, associates NMS with D2 receptor blockage in the basal ganglia and relates catatonia to a frontocortical gamma-aminobutyric acid (GABA) dysfunction. Based on this theory, haloperidol—which offers a higher D2 blockade than do SGAs such as ziprasidone—might have contributed to Mr. S’ NMS.
Some evidence suggests that lorazepam—which works on gamma-aminobutyric acid ionotropic type A (GABAA) receptors—helps treat catatonia in NMS and improves rigidity, hyperthermia, and autonomic signs.11
Treatment: which agents will work?
Three weeks after his discharge, we restart ziprasidone, 40 mg bid for Mr. S’ catatonic schizophrenia. He remains free of NMS symptoms but still has mannerisms (posturing, staring, immobility, stereotypic scratching on his face).
Over 1 year, Mr. S is hospitalized repeatedly because of persistent impulsivity and delusions. He has failed numerous antipsychotic regimens lasting 1 month or longer, including olanzapine, up to 30 mg/d; quetiapine, 300 mg tid; and risperidone, 2 mg tid. Adding a first-generation antipsychotic either does not help (as with perphenazine, 12 mg/d) or diminishes his memory (as with chlorpromazine, 250 mg/d). The anticholinergic benztropine, 2 mg bid, also is ineffective.
Combination quetiapine, 300 mg/d, and the antiviral amantadine, 100 mg tid, improve Mr. S’ stereotypy at first, but his delusions intensify within 1 week. His Bush-Francis Catatonia Rating Scale scores range from 9 (indicating moderate catatonia) to 16 (persistent catatonic features).12
poll here
The authors’ observations
Catatonic schizophrenia’s pathophysiology and response to medication might differ compared with other schizophrenia forms.13 Dopamine D2 hypoactivity, glutamate N-methyl-D-aspartate (NMDA) hyperactivity, or GABAA hypoactivity are believed to cause catatonia.3,6,7 GABA agonists, anticonvulsants, dopamine agonists, SGAs, and NMDA antagonists target these pathophysiologies, but patients with a catatonia subtype often respond to only one type of medication.
Lorazepam exerts an anticatatonic effect by binding to GABAA receptors and increasing GABA activity. Lorazepam can help some patients with schizophrenia but has not shown benefit when added to an antipsychotic for chronic catatonia.6,14
SGAs can provide marked improvement in patients with catatonic schizophrenia.5
Salokangas et al15 note that “atypicals” pass more dopamine to the D2 receptor when dopamine is low in the basal ganglia. This suggests that SGAs with low D2 binding—such as clozapine, olanzapine, and quetiapine—are more beneficial than other SGAs for catatonia. Serotonin binding or other mechanisms might add to these drugs’ anticatatonic effect.7
Anticonvulsants. Adjunctive anticonvulsant therapy might alleviate catatonia by increasing GABA activity or by causing a modest antiglutaminergic effect, as reported with carbamazepine or valproic acid.16 Anticholinergics also might help treat neuroleptic-induced catatonia.17
Amantadine—FDA-approved to treat Parkinson’s disease and extrapyramidal disease—can alleviate catatonia by blocking hyperglutamatergic excitotoxicity in neurons, thus blocking NMDA receptors.18 As with Mr. S, however, amantadine can worsen psychosis by increasing dopamine release.
Memantine—an NMDA receptor antagonist indicated for moderate to severe Alzheimer’s disease—also blocks hyperglutamatergic excitotoxicity in neurons. The medication has shown effectiveness for treating catatonic schizophrenia in case reports,19-21 but 3 patients have reported memantine-induced psychosis and seizures.21
Some might argue that Mr. S’ delusions are predominant and more compelling than his catatonia, but these did not hamper his ability to live in a group home. His catatonia-related negativism, impulsivity, and inability to cooperate are what led to frequent hospitalization.
Follow-up: treatment change
We stop amantadine, add memantine, 10 mg bid, and titrate quetiapine over 2 weeks to 900 mg/d. Mr. S’ catatonia improves but some delusions persist. We add olanzapine, 7.5 mg bid, and within 2 weeks Mr. S is less delusional and more cooperative.
We discharge Mr. S on the above medications, plus:
- lorazepam, 1 mg each morning and 2 mg nightly, which he has been taking for catatonia for about 1 year
- trazodone, 150 mg bid, which we added 6 months ago to help him sleep and reduce psychomotor excitement
- ranitidine, 150 mg bid, for gastroesophageal reflux disorder
- and levothyroxine, 0.5 mg/d, for comobrid hypothyroidism. His thyroid-stimulating hormone level is normal.
We see Mr. S monthly. He is still impulsive at times, occasionally collecting his neighbors’ newspapers and mail despite instructions from group home staff not to do so. Yet his sponsors say Mr. S is “like a new person.” He talks spontaneously, interacts, and is cooperative. He has not been hospitalized for more than 1 year.
The authors’ observations
Mr. S responded favorably to clozapine but cannot tolerate it. With a combination of two other SGAs, a patient might gain the benefits of clozapine without the need for frequent blood draws or the risk of agranulocytosis, other side effects, or interactions between clozapine and other drugs. Adding memantine was necessary to improve the catatonic features that prevented his return to the group home.
Related resources
- World Federation of Societies of Biological Psychiatry. www.wfsbp.com.
- Neuroleptic Malignant Syndrome Information Service. www.nmsis.org.
- Mann SC, Caroff SN, Keck PE Jr, Lazarus A. Neuroleptic malignant syndrome and related conditions, 2nd ed. Arlington, VA: American Psychiatric Press; 2003:1-44.
- Ungvari GS (ed). Catatonia-an anthology of classical contributions. Hong Kong: Scientific Communications International; 2006.
- Amantadine • Symmetrel
- Benztropine • Cogentin
- Bromocriptine • Parlodel
- Carbamazepine • Equetro, others
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Dantrolene • Dantrium
- Haloperidol • Haldol
- Levodopa/carbidopa • Sinemet
- Levothyroxine • Synthroid
- Lorazepam • Ativan
- Memantine • Namenda
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Ranitidine • Zantac
- Risperidone • Risperdal
- Trazodone • Desyrel
- Valproic acid • Depakene
- Ziprasidone • Geodon
Dr. Carroll is a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol Myers-Squibb Co., Forest Pharmaceuticals, Janssen Pharmaceutica, and Pfizer.
Dr. Thomas receives grant support from Pfizer and is a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, and Pfizer.
Dr. Tugrul is a consultant to and speaker for Bristol Myers-Squibb Co. and Eli Lilly and Co.
Dr. Jayanti reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Acknowledgment
The authors thank Francisco José Appiani, MD, chairman, psychiatry department, Military Hospital of Campo de Mayo, Buenos Aires, Argentina, and Vijay Jayanti, BS, medical student, The Ohio State University, Columbus, for their help with this article.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000:204.
2. Ungvari GS, Leung SK, Ng FS, et al. Schizophrenia with prominent catatonic features (“catatonic schizophrenia”) I. Demographic and clinical correlates in the chronic phase. Prog Neuropsychopharmacol Biol Psychiatry 2005;29:27-38.
3. Dhossche D, Wing L, Ohta M, Neumarker K (eds). Catatonia in autism spectrum disorders. International review of neurobiology, vol. 72. San Diego: Elsevier/Academic Press; 2006.
4. Falkai P, Wobrock T, Lieberman J. WFSBP guidelines for biological treatment of schizophrenia, part 1. Acute treatment of schizophrenia. World J Biol Psychiatry 2005;6:32-91.
5. Van Dalfsen F, Van Hecke J, Van Dalfsen A, et al. The use of atypical antipsychotics in the treatment of catatonia. Eur Psychiatry 2005;20:422-9.
6. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry 1990;51:357-62.
7. Dursun SM, Hallak JE, Haddad P, et al. Clozapine monotherapy for catatonic schizophrenia: should clozapine be the treatment of choice, with catatonia rather than psychosis as the main therapeutic index? J Psychopharmacol 2005;19:432-3.
8. White DAC, Robbins AH. An analysis of 17 catatonic patients diagnosed with neuroleptic malignant syndrome. CNS Spectrums 2000;5:58-65.
9. Sachdev PS. A rating scale for neuroleptic malignant syndrome. Psychiatry Res 2005;135:249-56.
10. Northoff G. Catatonia and neuroleptic malignant syndrome: psychopathology and pathophysiology. J Neural Transm 2002;109:1453-67.
11. Francis A, Chandragiri S, Rizvi S, et al. Is lorazepam a treatment for neuroleptic malignant syndrome? CNS Spectrums 2000;5:54-7.
12. Bush G, Fink M, Petrides, et al. Catatonia I: Rating scale and standardized examination. Acta Psychiatr Scand 1996;93:129-36.
13. Carroll BT, Thomas C, Jayanti K, et al. Schizophrenia with catatonic features deserves further study. World J Biol Psychiatry 2005;6(4):267-8.
14. Ungvari GS, Chie HFK, Chow LY, et al. Lorazepam for chronic catatonia: A random, double blind, placebo-controlled cross-over study. Psychopharmacology (Berl) 1999;142:393-8.
15. Salokangas R, Honkonen T, Stengard E, et al. Negative symptoms and neuroleptics in catatonic schizophrenia. Schizophr Res 2003;59:73-6.
16. Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge, UK: Cambridge University Press; 2003.
17. Franz M, Gallhofer B, Kanzow WT. Treatment of catatonia with intravenous biperidine. Br J Psychiatry 1994;164:847-8.
18. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry 1997;62:404-6.
19. Thomas C, Carroll BT, Maley JT, et al. Memantine in catatonic schizophrenia. Am J Psychiatry 2005;162:656.
20. Carroll BT, Thomas C, Jayanti K. Amantadine and memantine in catatonic schizophrenia. Ann Clin Psychiatry 2006;18:133-4.
21. Carpenter SS, Hatchett AD, Fuller MA. Catatonic schizophrenia and the use of memantine. Ann Pharmacother 2006;40:344-6.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000:204.
2. Ungvari GS, Leung SK, Ng FS, et al. Schizophrenia with prominent catatonic features (“catatonic schizophrenia”) I. Demographic and clinical correlates in the chronic phase. Prog Neuropsychopharmacol Biol Psychiatry 2005;29:27-38.
3. Dhossche D, Wing L, Ohta M, Neumarker K (eds). Catatonia in autism spectrum disorders. International review of neurobiology, vol. 72. San Diego: Elsevier/Academic Press; 2006.
4. Falkai P, Wobrock T, Lieberman J. WFSBP guidelines for biological treatment of schizophrenia, part 1. Acute treatment of schizophrenia. World J Biol Psychiatry 2005;6:32-91.
5. Van Dalfsen F, Van Hecke J, Van Dalfsen A, et al. The use of atypical antipsychotics in the treatment of catatonia. Eur Psychiatry 2005;20:422-9.
6. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry 1990;51:357-62.
7. Dursun SM, Hallak JE, Haddad P, et al. Clozapine monotherapy for catatonic schizophrenia: should clozapine be the treatment of choice, with catatonia rather than psychosis as the main therapeutic index? J Psychopharmacol 2005;19:432-3.
8. White DAC, Robbins AH. An analysis of 17 catatonic patients diagnosed with neuroleptic malignant syndrome. CNS Spectrums 2000;5:58-65.
9. Sachdev PS. A rating scale for neuroleptic malignant syndrome. Psychiatry Res 2005;135:249-56.
10. Northoff G. Catatonia and neuroleptic malignant syndrome: psychopathology and pathophysiology. J Neural Transm 2002;109:1453-67.
11. Francis A, Chandragiri S, Rizvi S, et al. Is lorazepam a treatment for neuroleptic malignant syndrome? CNS Spectrums 2000;5:54-7.
12. Bush G, Fink M, Petrides, et al. Catatonia I: Rating scale and standardized examination. Acta Psychiatr Scand 1996;93:129-36.
13. Carroll BT, Thomas C, Jayanti K, et al. Schizophrenia with catatonic features deserves further study. World J Biol Psychiatry 2005;6(4):267-8.
14. Ungvari GS, Chie HFK, Chow LY, et al. Lorazepam for chronic catatonia: A random, double blind, placebo-controlled cross-over study. Psychopharmacology (Berl) 1999;142:393-8.
15. Salokangas R, Honkonen T, Stengard E, et al. Negative symptoms and neuroleptics in catatonic schizophrenia. Schizophr Res 2003;59:73-6.
16. Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge, UK: Cambridge University Press; 2003.
17. Franz M, Gallhofer B, Kanzow WT. Treatment of catatonia with intravenous biperidine. Br J Psychiatry 1994;164:847-8.
18. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry 1997;62:404-6.
19. Thomas C, Carroll BT, Maley JT, et al. Memantine in catatonic schizophrenia. Am J Psychiatry 2005;162:656.
20. Carroll BT, Thomas C, Jayanti K. Amantadine and memantine in catatonic schizophrenia. Ann Clin Psychiatry 2006;18:133-4.
21. Carpenter SS, Hatchett AD, Fuller MA. Catatonic schizophrenia and the use of memantine. Ann Pharmacother 2006;40:344-6.
Treating persistent catatonia when benzodiazepines fail
Many catatonia cases respond to benzodiazepines—especially lorazepam—but up to 30% do not. Electroconvulsive therapy (ECT) can be effective, but what’s the next step when ECT is unavailable or inappropriate for your patient?
To help you solve this dilemma, we describe our diagnosis and treatment decisions for a patient we call Mr. C. We explain how our process was guided by recent understandings of an abnormal neural circuit that appears to cause catatonia’s complex motor and behavioral symptoms.
This article describes that neurologic pathology and answers common questions about the clinical workup and treatment of catatonia.
CASE: TROUBLE IN TV LAND
Mr. C, age 69, caused a disturbance at a local TV station, demanding that they broadcast a manuscript he had written. Police took him to a local hospital, where he was stabilized and then transferred to a neuropsychiatric hospital for evaluation.
The psychiatric interview revealed that he had developed insomnia, excessive activity, and delusional thinking 2 weeks before admission. His medical history included coronary artery disease (CAD), hypertension, and hypothyroidism. Medications included thyroid hormone replacement therapy, furosemide, potassium, ranitidine, simvastatin, metoprolol, and lisinopril. CAD treatment included stent placement and nitroglycerin as needed.
He had been hospitalized in his 30s and treated with ECT for what he called “bad thoughts.” He said he improved after 1 month and had no subsequent psychiatric history. He denied drug or alcohol abuse.
Shortly after admission, he refused to eat or drink and after 1 week became dehydrated. He also showed mutism, immobility, and stupor. He was transferred to the medical service for IV rehydration.
MANY SCENARIOS AND SIGNS
Mr. C’s symptoms suggest possible catatonia, a neuropsychiatric syndrome of motor dysregulation found in up to 10% of acutely ill psychiatric inpatients.1,2 A movement disorder,1,2 catatonia occurs with general medical conditions and psychiatric disorders (Table 1).
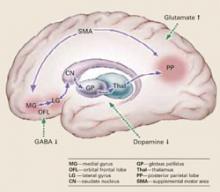
Pathophysiology. Catatonic signs develop when aberrant signals from neurochemical abnormalities trigger a neural circuit that affects the medial gyrus of the orbital frontal lobe, the lateral gyrus, caudate nucleus, globus pallidus, and thalamus (Box).3-5
Presentation. A focused exam is required because patients with catatonia often do not provide a comprehensive or reliable history.2 They show mutism, characteristic postures, rigidity, aberrant speech, negativism, and stereotyped behaviors.1,2 They may present in an excited or retarded state:
- Excited patients may injure themselves or others and develop hyperthermia, tachycardia, and elevated blood pressure from excessive motor activity.
- Patients in a retarded state may present with bradykinesia and poor self-care. They may be unresponsive to external stimuli, develop catatonic stupor, and refuse to eat or drink.
Mr. C’s earlier insomnia, excessive activity, and delusional thinking (such as the TV station incident) may have signaled an excited catatonia. On admission to the medical service, however, he presented in a retarded state.
Signs. Part of the challenge with detecting catatonia’s signs is that there are so many; some rating scales list more than 20. Not all signs need to be present to make the diagnosis, however, and if you find one, others usually turn up in the examination.
A mnemonic from the Bush-Francis Catatonia Screening Instrument (Table 2) represents diagnostic signs in patients with the excited or retarded forms.2 We recommend that you review an authoritative text (see Related resources) to understand catatonia’s psychopathology.2
Table 1
Common diagnoses of patients with catatonia
| Psychiatric |
|
| Organic |
|
Catatonia is caused by neurochemical abnormalities including low GABA activity in the frontal cortex, low dopamine (D2) activity in the basal ganglia, high glutamate—N-methyl-D-aspartate (NMDA)—activity in the parietal cortex, or a combination of these.3-5 Catatonic signs occur when these neurochemical changes cause aberrant signals and trigger a neural circuit affecting the medial gyrus of the orbital frontal lobe, the lateral gyrus, caudate nucleus, globus pallidus, and thalamus (Figure).
Posturing occurs when the aberrant signal reaches the posterior parietal lobe. Patients’ bizarre and mundane postures in catatonia are maintained by “anosognosia of position.” For example, an individual does not know the position of rest for his arm, and it remains in an unusual position as if at rest.3
The PP goes on to influence the supplemental motor area (SMA), causing bradykinesia, rigidity, and other motor phenomena that catatonia shares with Parkinson’s disease. The SMA feeds back to the medial orbital gyrus, completing the neural circuit.3
Regions such as the anterior cingulate area (ACA) and amygdala (1AMG) — also may be recruited into the expanded circuit. ACA recruitment may cause akinetic mutism, and fear is a symptom of AMG recruitment. If the anterior hypothalamus is affected, malignant catatonia or neuroleptic malignant syndrome may occur.3,5
This neural loop demonstrates an integrated model of psychosis. It may help explain why catatonia responds to treatment with lorazepam, ECT, and other agents such as antipsychotics and NMDA antagonists.
Illustration for CURRENT PSYCHIATRY by Marcia Hartsock, CMI
Table 2
WIRED `N MIRED: Mnemonic for detecting catatonia
| Waxy flexibility/catalepsy |
| Immobility/stupor |
| Refusal to eat or drink |
| Excitement |
| Deadpan staring |
| Negativism/negative symptoms |
| Mutism |
| Impulsivity |
| Rigidity |
| Echolalia/echopraxia |
| Direct observation |
CASE CONTINUED: MAKING THE DIAGNOSIS
In the medical unit, Mr. C was found to be in a catatonic stupor, with immobility, mutism (monosyllabic speech), catalepsy, intermittent waxy flexibility, withdrawal (refusal to eat and drink), automatic obedience, and mitgehen (exaggerated movements in response to light finger pressure, despite instructions to stay still). ECT workup was started, along with a trial of lorazepam, 1 mg tid.
Laboratory studies revealed high BUN/creatinine (80/2.0) that returned to normal range (BUN 7 to 21 mg/dL; creatinine 0.5 to 1.2 mg/dL) after 3 days of hydration. Because of Mr. C’s earlier excited symptoms and delusional thinking, we considered a diagnosis of bipolar disorder with catatonia. However, his symptoms did not improve with a trial of valproic acid (serum level 64 mcg/mL).
Head CT showed generalized atrophy and EEG showed delta slowing. Single-photo emission computer tomography (SPECT) showed areas of decreased perfusion in the cortex, with no perfusion in the left posterior parietal area (PP).
Mental status exam found Mr. C disoriented with poor short-term memory and unable to complete the Mini Mental State Examination (MMSE). His Bush-Francis Catatonia Rating Scale score was 28 and included many catatonic signs that would not be seen a patient with simple dehydration.
The workup supported a diagnosis of catatonia due to general medical condition (vascular dementia) and ruled out schizophrenia with catatonic features, bipolar disorder, or major depression with catatonia.
EVALUATION AND DIAGNOSIS
Medical causes. A careful history and thorough physical examination are essential for making an accurate diagnosis and ruling out medical conditions that could present with or mimic catatonia (Table 3). Medications that can induce catatonia include antipsychotics, corticosteroids, and disulfiram at therapeutic doses. Drug abuse (such as with phencyclidine), use of the general anesthetic ketamine, and benzodiazepine withdrawal may also lead to catatonia.
Head CT or MRI is indicated for patients being considered for ECT or for localizing neurologic findings. EEG can be useful when patients present with features of seizure activity—such as tongue biting, incontinence, or stupor—or with catatonia as a manifestation of delirium or dementia.
A history of head injury or neurologic disease warrants further neurologic investigation. Also consider a neurology consult when the patient has prolonged stupor or does not respond to initial drug therapy.
Psychiatric causes. The clinical setting may suggest the most likely primary psychiatric disorders to consider, such as:
- bipolar or major depression in acute inpatient psychiatric units
- autism and pervasive developmental disorders (PDD) in pediatric or PDD units
- catatonic schizophrenia in chronic psychotic patients
- somatoform or factitious disorders in forensic settings.
These generalizations are not clinically exclusive, of course, but may provide a starting point for the treatment team confronted with limited history and exam information.
Table 3
Catatonia workup: Recommended lab tests
| Test | Recommendation |
|---|---|
| Complete blood count with WBC differential | Look for leukocytosis |
| Serum chemistries | Look for electrolyte imbalances |
| Serum iron | May be low in NMS |
| Serum creatine kinase | If NMS is suspected |
| Brain MRI or CT | If structural lesion is suspected |
| Electroencephalography | If seizure disorder or brain abnormality is suspected |
| Lumbar puncture | If encephalitis or meningits is suspected |
| NMS: neuroleptic malignant syndrome | |
Initial treatment. Catatonia related to medical and psychiatric causes has been shown to respond to lorazepam and to ECT.6,7 Lorazepam is preferred because of its specificity for the GABAa receptor and ease of administration (oral, IM, or IV). Other agents that act on GABA—including amobarbital and zolpidem—have also been used. Catatonia’s hallmark features such as mutism and immobility have been shown to respond to lorazepam.8,9
ECT is a first-line treatment for catatonia with life-threatening conditions and should be considered for refractory cases.
Lorazepam. The starting dosage is usually 1 mg tid for healthy adults; 0.5 mg tid can be used for children and the elderly. Observe the patient for improvement in catatonic signs after the first dose and before giving the second. Dosages of up to 16 mg/d have been used.
In many cases, lorazepam can be tapered off after adequate treatment of the primary psychiatric condition. In severe cases, however—such as when patients refuse to eat or drink—lorazepam may be continued for as long as 1 year. Weigh the risk of benzodiazepine tolerance, dependence, and misuse versus the possibility of relapse and rehospitalization.
Medical catatonias and neuroleptic malignant syndrome (NMS) have responded favorably to ECT.8 Addressing the medical cause itself usually does not resolve catatonia, with the possible exception of seizure-induced (“ictal”) catatonia, which may respond to anticonvulsants and lorazepam.6,7
ECT. An ECT workup can begin as soon as a patient presents with catatonia. If lorazepam produces no response within 24 hours, consider ECT.
CASE CONTINUED: PERSISTENT SYMPTOMS
After three 1-mg doses of lorazepam, Mr. C became more alert and oriented but his catatonia symptoms persisted, as indicated by a Bush-Francis score of 23, significant grasp reflex, and gegenhalten (automatic rather than willful resistance to passive limb movement in proportion to the strength of the stimulus). An attempt to gradually increase lorazepam to 2 mg tid produced delirium. He remained confused even when lorazepam was reduced to 0.5 mg tid, so the drug was discontinued.
Mr. C’s neurologist added amantadine, 100 mg tid, and carbidopa/levodopa, 10/100 mg tid, to treat his parkinsonian rigidity.
WHAT NEXT? OTHER OPTIONS
Antipsychotics have been investigated as a possible treatment for catatonia. The literature suggests that conventional antipsychotics may cause catatonia and atypical antipsychotics may improve it. Conventional antipsychotics are best avoided in catatonia because they:
- appear less effective than other treatments in resolving catatonic symptoms8,10
- are associated with catatonic-like side effects, such as rigidity, akinesia, and staring10
- appear to increase NMS risk in patients with catatonic symptoms.11,12
Atypicals appear more effective in treating catatonia and less likely to cause NMS. Case reports13,14 indicate many of these agents can be effective and well tolerated in treating catatonic symptoms, although this was not the case for Mr. C.
Anticonvulsants such as valproate15 and carbamazepine, 600 to 1200 mg/d,16 may take longer to work than lorazepam but may be options for patients who do not respond to benzodiazepines.8,9
Amantadine, an N-methyl-D-aspartate (NMDA) antagonist, has been used with some success for catatonia that does not unrespond to lorazepam.17 However, amantidine’s dopamine agonist activity could worsen underlying psychosis.
Memantine—another NMDA antagonist—differs from amantadine despite having a similar chemical structure. Memantine is a noncompetitive antagonist at the NMDA receptor, without affinity for dopamine, norepinephrine, serotonin, or muscarinic receptors.18
Although no published data support using memantine in patients with catatonia, it might be considered for those who are not candidates for lorazepam or ECT. For instance, a double-blind, placebo-controlled study found that lorazepam was not effective for catatonic schizophrenia.19 We have found memantine to help in some patients with catatonic schizophrenia.
CASE CONTINUED: TRIAL OF MEMANTINE
Mr. C remained in a catatonic stupor, but we decided against ECT because he resumed eating and drinking and was not medically at risk. Quetiapine, 100 to 300 mg/d, was tried to address his dementia symptoms, confusion, and poor mentation. This trial was discontinued after Mr. C fell and was readmitted to the medical unit. We then added memantine, 5 mg bid.
In the first week after beginning memantine, Mr. C’s MMSE score was 21, consistent with vascular dementia, but he remained immobile and staring. Motor signs also persisted, including automatic obedience, ambitendency, and a grasp reflex.
The next week, we increased memantine to 10 mg bid. Mr. C was oriented to person, place, and time, and his affect was blunted. His MMSE score increased to 25, showing improved cognition and memory. His Bush-Francis scale score was 6, showing reduced catatonic signs, with remaining mild immobility, bradykinesia, speech-prompt mutism, staring, and grasp reflex.
He maintained this improvement on carbidopa/levodopa, 10/100 tid; amantadine, 100 mg tid; and memantine, 10 mg bid, and was discharged from the nursing home unit.
IMPROVEMENT WITH MEMANTINE
Memantine may reduce excess glutamate at the NMDA receptor in the parietal-SMA-frontal cortical circuit. It may help to increase GABA and dopamine, which are deficient in catatonia. Our patient with vascular dementia had a severe ischemic deficit in the posterior parietal area, as seen on SPECT.
Amantadine, another NMDA receptor antagonist, acts on dopamine neurons and may have anticholinergic-like side effects, whereas memantine does not. Although both drugs share antagonism at the NMDA glutamate receptor, noncompetitive binding is weak for amantadine and moderate for memantine. Memantine has some serotonin (5-HT3) antagonism, but neither agent has direct GABA activity.
Memantine can improve function in vascular dementia.20 Thus, Mr. C’s improvement may have been caused by the drug’s effect on his vascular dementia, the primary neuropsychiatric illness. However, his catatonic signs improved without antipsychotics, cholinesterase inhibitors, benzodiazepines, or ECT. No anticoagulation treatment or cerebral perfusion procedures account for his improved mental status.
CASE CONCLUSION
Mr. C went to live with his son’s family. Although he has problems with calculation, he shows good selfcare. When asked why he did not respond during his catatonic stupor, Mr. C stated that he believed the physician was an Internal Revenue Service agent asking him about serious tax problems. Upon reflection, he said he no longer believes this.
- Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge, UK: Cambridge University Press, 2003.
- Caroff SN, Mann SC, Francis A, Fricchione GE. Catatonia: from psychopathology to neurobiology. Washington, DC: American Psychiatric Publishing, 2004.
- Mann SC, Caroff SN, Keck PE Jr, Lazarus A. Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing, 2003.
- Neuroleptic Malignant Syndrome Information Service. www.NMSIS.org.
Drug brand names
- Amantadine • Symmetrel
- Amobarbital • Amytal sodium
- Carbamazepine • Carbatrol, Equetro
- Carbidopa/levodopa • Sinemet
- Disulfiram • Antabuse
- Divalproex • Depakote
- Furosemide • Lasix
- Lisinopril • Prinivil, Zestril
- Lorazepam • Ativan
- Memantine • Namenda
- Metoprolol • Lopressor
- Ranitidine • Zantac
- Simvastatin • Zocor
- Valproic acid • Depakene
- Zolpidem • Ambien
Disclosure
Dr. Carroll and Dr. Hawkins are speakers for Forest Laboratories. The other authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The authors thank Dr. Niraj Ahuja, consultant psychiatrist and honorary clinical lecturer (psychiatry), Newcastle, North Tyneside and Northumberland Mental Health Trust, UK, for assistance with the figure.
1. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry 2003;160:1233-41.
2. Bush G, Fink M, Petrides G, et al. Catatonia I: Rating scale and standardized examination. Acta Psychiatr Scand 1996;93:129-36.
3. Northoff G. What catatonia can tell us about “top-down” modulation:” a neuropsychiatric hypothesis. Brain Behav Sci 2002;25:555-604.
4. Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectrums 2000;5(7):26-33.
5. Carroll BT. Catatonia is the rosetta stone of psychosis (poster presentation). New York: American Psychiatric Association annual meeting, 2004.
6. Barnes MP, Saunders M, Walls TJ, et al. The syndrome of Karl Ludwig Kahlbaum. J Neurol Neurosurg Psychiatry 1986;49:991-6.
7. Carroll BT, Anfinson TJ, Kennedy JC, et al. Catatonic disorder due to general medical conditions. J Neuropsychiatry Clin Neurosci 1994;6:122-33.
8. Hawkins JM, Archer KJ, Strakowski SM, Keck PE. Somatic treatments of catatonia. Int J Psychiatry Med 1995;25:345-69.
9. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry 1990;51:357-62.
10. Dose M. Neuroleptic-induced pseudo-catatonia. Pharmacopsychiatry 2001;34:262-4.
11. Keck PE, Jr, Pope HG, Jr, Cohen BM, et al. Risk factors for neuroleptic malignant syndrome. Arch Gen Psychiatry 1989;46:914-18.
12. White DAC. 17 catatonic patients diagnosed as neuroleptic malignant syndrome. CNS Spectrums 2000;5:58-65.
13. Levy WO, Nunez CY. Use of ziprasidone to treat bipolar-associated catatonia. Bipolar Disord 2004;6:166-7.
14. Hesslinger B, Walden J, Normann C. Acute and long-term treatment of catatonia with risperidone. Pharmacopsychiatry 2001;34:25-6.
15. Kruger S, Braunig P. Intravenous valproic acid in the treatment of severe catatonia. J Neuropsychiatry Clin Neurosci 2001;13:303-4.
16. Kritzinger PR, Jordaan GP. Catatonia: an open prospective series with carbamazepine. Int J Neuropsychopharmacol 2001;4:251-7.
17. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry 1997;62:404-6.
18. Namenda (memantine) Package labeling. Forest Laboratories, 2004.
19. Ungvari GS, Chie HFK, Chow LY, et al. Lorazepam for chronic catatonia: a random, double-blind, placebo-controlled, cross-over study. Psychopharmacol 1999;142:393-8.
20. Mobius HJ. Pharmacologic rationale for memantine in chronic cerebral hypoperfusion, especially vascular dementia. Alz Dis Assoc Disord 1999;13(suppl 3):172-8.
Many catatonia cases respond to benzodiazepines—especially lorazepam—but up to 30% do not. Electroconvulsive therapy (ECT) can be effective, but what’s the next step when ECT is unavailable or inappropriate for your patient?
To help you solve this dilemma, we describe our diagnosis and treatment decisions for a patient we call Mr. C. We explain how our process was guided by recent understandings of an abnormal neural circuit that appears to cause catatonia’s complex motor and behavioral symptoms.
This article describes that neurologic pathology and answers common questions about the clinical workup and treatment of catatonia.
CASE: TROUBLE IN TV LAND
Mr. C, age 69, caused a disturbance at a local TV station, demanding that they broadcast a manuscript he had written. Police took him to a local hospital, where he was stabilized and then transferred to a neuropsychiatric hospital for evaluation.
The psychiatric interview revealed that he had developed insomnia, excessive activity, and delusional thinking 2 weeks before admission. His medical history included coronary artery disease (CAD), hypertension, and hypothyroidism. Medications included thyroid hormone replacement therapy, furosemide, potassium, ranitidine, simvastatin, metoprolol, and lisinopril. CAD treatment included stent placement and nitroglycerin as needed.
He had been hospitalized in his 30s and treated with ECT for what he called “bad thoughts.” He said he improved after 1 month and had no subsequent psychiatric history. He denied drug or alcohol abuse.
Shortly after admission, he refused to eat or drink and after 1 week became dehydrated. He also showed mutism, immobility, and stupor. He was transferred to the medical service for IV rehydration.
MANY SCENARIOS AND SIGNS
Mr. C’s symptoms suggest possible catatonia, a neuropsychiatric syndrome of motor dysregulation found in up to 10% of acutely ill psychiatric inpatients.1,2 A movement disorder,1,2 catatonia occurs with general medical conditions and psychiatric disorders (Table 1).
Pathophysiology. Catatonic signs develop when aberrant signals from neurochemical abnormalities trigger a neural circuit that affects the medial gyrus of the orbital frontal lobe, the lateral gyrus, caudate nucleus, globus pallidus, and thalamus (Box).3-5
Presentation. A focused exam is required because patients with catatonia often do not provide a comprehensive or reliable history.2 They show mutism, characteristic postures, rigidity, aberrant speech, negativism, and stereotyped behaviors.1,2 They may present in an excited or retarded state:
- Excited patients may injure themselves or others and develop hyperthermia, tachycardia, and elevated blood pressure from excessive motor activity.
- Patients in a retarded state may present with bradykinesia and poor self-care. They may be unresponsive to external stimuli, develop catatonic stupor, and refuse to eat or drink.
Mr. C’s earlier insomnia, excessive activity, and delusional thinking (such as the TV station incident) may have signaled an excited catatonia. On admission to the medical service, however, he presented in a retarded state.
Signs. Part of the challenge with detecting catatonia’s signs is that there are so many; some rating scales list more than 20. Not all signs need to be present to make the diagnosis, however, and if you find one, others usually turn up in the examination.
A mnemonic from the Bush-Francis Catatonia Screening Instrument (Table 2) represents diagnostic signs in patients with the excited or retarded forms.2 We recommend that you review an authoritative text (see Related resources) to understand catatonia’s psychopathology.2
Table 1
Common diagnoses of patients with catatonia
| Psychiatric |
|
| Organic |
|
Catatonia is caused by neurochemical abnormalities including low GABA activity in the frontal cortex, low dopamine (D2) activity in the basal ganglia, high glutamate—N-methyl-D-aspartate (NMDA)—activity in the parietal cortex, or a combination of these.3-5 Catatonic signs occur when these neurochemical changes cause aberrant signals and trigger a neural circuit affecting the medial gyrus of the orbital frontal lobe, the lateral gyrus, caudate nucleus, globus pallidus, and thalamus (Figure).
Posturing occurs when the aberrant signal reaches the posterior parietal lobe. Patients’ bizarre and mundane postures in catatonia are maintained by “anosognosia of position.” For example, an individual does not know the position of rest for his arm, and it remains in an unusual position as if at rest.3
The PP goes on to influence the supplemental motor area (SMA), causing bradykinesia, rigidity, and other motor phenomena that catatonia shares with Parkinson’s disease. The SMA feeds back to the medial orbital gyrus, completing the neural circuit.3
Regions such as the anterior cingulate area (ACA) and amygdala (1AMG) — also may be recruited into the expanded circuit. ACA recruitment may cause akinetic mutism, and fear is a symptom of AMG recruitment. If the anterior hypothalamus is affected, malignant catatonia or neuroleptic malignant syndrome may occur.3,5
This neural loop demonstrates an integrated model of psychosis. It may help explain why catatonia responds to treatment with lorazepam, ECT, and other agents such as antipsychotics and NMDA antagonists.
Illustration for CURRENT PSYCHIATRY by Marcia Hartsock, CMI
Table 2
WIRED `N MIRED: Mnemonic for detecting catatonia
| Waxy flexibility/catalepsy |
| Immobility/stupor |
| Refusal to eat or drink |
| Excitement |
| Deadpan staring |
| Negativism/negative symptoms |
| Mutism |
| Impulsivity |
| Rigidity |
| Echolalia/echopraxia |
| Direct observation |
CASE CONTINUED: MAKING THE DIAGNOSIS
In the medical unit, Mr. C was found to be in a catatonic stupor, with immobility, mutism (monosyllabic speech), catalepsy, intermittent waxy flexibility, withdrawal (refusal to eat and drink), automatic obedience, and mitgehen (exaggerated movements in response to light finger pressure, despite instructions to stay still). ECT workup was started, along with a trial of lorazepam, 1 mg tid.
Laboratory studies revealed high BUN/creatinine (80/2.0) that returned to normal range (BUN 7 to 21 mg/dL; creatinine 0.5 to 1.2 mg/dL) after 3 days of hydration. Because of Mr. C’s earlier excited symptoms and delusional thinking, we considered a diagnosis of bipolar disorder with catatonia. However, his symptoms did not improve with a trial of valproic acid (serum level 64 mcg/mL).
Head CT showed generalized atrophy and EEG showed delta slowing. Single-photo emission computer tomography (SPECT) showed areas of decreased perfusion in the cortex, with no perfusion in the left posterior parietal area (PP).
Mental status exam found Mr. C disoriented with poor short-term memory and unable to complete the Mini Mental State Examination (MMSE). His Bush-Francis Catatonia Rating Scale score was 28 and included many catatonic signs that would not be seen a patient with simple dehydration.
The workup supported a diagnosis of catatonia due to general medical condition (vascular dementia) and ruled out schizophrenia with catatonic features, bipolar disorder, or major depression with catatonia.
EVALUATION AND DIAGNOSIS
Medical causes. A careful history and thorough physical examination are essential for making an accurate diagnosis and ruling out medical conditions that could present with or mimic catatonia (Table 3). Medications that can induce catatonia include antipsychotics, corticosteroids, and disulfiram at therapeutic doses. Drug abuse (such as with phencyclidine), use of the general anesthetic ketamine, and benzodiazepine withdrawal may also lead to catatonia.
Head CT or MRI is indicated for patients being considered for ECT or for localizing neurologic findings. EEG can be useful when patients present with features of seizure activity—such as tongue biting, incontinence, or stupor—or with catatonia as a manifestation of delirium or dementia.
A history of head injury or neurologic disease warrants further neurologic investigation. Also consider a neurology consult when the patient has prolonged stupor or does not respond to initial drug therapy.
Psychiatric causes. The clinical setting may suggest the most likely primary psychiatric disorders to consider, such as:
- bipolar or major depression in acute inpatient psychiatric units
- autism and pervasive developmental disorders (PDD) in pediatric or PDD units
- catatonic schizophrenia in chronic psychotic patients
- somatoform or factitious disorders in forensic settings.
These generalizations are not clinically exclusive, of course, but may provide a starting point for the treatment team confronted with limited history and exam information.
Table 3
Catatonia workup: Recommended lab tests
| Test | Recommendation |
|---|---|
| Complete blood count with WBC differential | Look for leukocytosis |
| Serum chemistries | Look for electrolyte imbalances |
| Serum iron | May be low in NMS |
| Serum creatine kinase | If NMS is suspected |
| Brain MRI or CT | If structural lesion is suspected |
| Electroencephalography | If seizure disorder or brain abnormality is suspected |
| Lumbar puncture | If encephalitis or meningits is suspected |
| NMS: neuroleptic malignant syndrome | |
Initial treatment. Catatonia related to medical and psychiatric causes has been shown to respond to lorazepam and to ECT.6,7 Lorazepam is preferred because of its specificity for the GABAa receptor and ease of administration (oral, IM, or IV). Other agents that act on GABA—including amobarbital and zolpidem—have also been used. Catatonia’s hallmark features such as mutism and immobility have been shown to respond to lorazepam.8,9
ECT is a first-line treatment for catatonia with life-threatening conditions and should be considered for refractory cases.
Lorazepam. The starting dosage is usually 1 mg tid for healthy adults; 0.5 mg tid can be used for children and the elderly. Observe the patient for improvement in catatonic signs after the first dose and before giving the second. Dosages of up to 16 mg/d have been used.
In many cases, lorazepam can be tapered off after adequate treatment of the primary psychiatric condition. In severe cases, however—such as when patients refuse to eat or drink—lorazepam may be continued for as long as 1 year. Weigh the risk of benzodiazepine tolerance, dependence, and misuse versus the possibility of relapse and rehospitalization.
Medical catatonias and neuroleptic malignant syndrome (NMS) have responded favorably to ECT.8 Addressing the medical cause itself usually does not resolve catatonia, with the possible exception of seizure-induced (“ictal”) catatonia, which may respond to anticonvulsants and lorazepam.6,7
ECT. An ECT workup can begin as soon as a patient presents with catatonia. If lorazepam produces no response within 24 hours, consider ECT.
CASE CONTINUED: PERSISTENT SYMPTOMS
After three 1-mg doses of lorazepam, Mr. C became more alert and oriented but his catatonia symptoms persisted, as indicated by a Bush-Francis score of 23, significant grasp reflex, and gegenhalten (automatic rather than willful resistance to passive limb movement in proportion to the strength of the stimulus). An attempt to gradually increase lorazepam to 2 mg tid produced delirium. He remained confused even when lorazepam was reduced to 0.5 mg tid, so the drug was discontinued.
Mr. C’s neurologist added amantadine, 100 mg tid, and carbidopa/levodopa, 10/100 mg tid, to treat his parkinsonian rigidity.
WHAT NEXT? OTHER OPTIONS
Antipsychotics have been investigated as a possible treatment for catatonia. The literature suggests that conventional antipsychotics may cause catatonia and atypical antipsychotics may improve it. Conventional antipsychotics are best avoided in catatonia because they:
- appear less effective than other treatments in resolving catatonic symptoms8,10
- are associated with catatonic-like side effects, such as rigidity, akinesia, and staring10
- appear to increase NMS risk in patients with catatonic symptoms.11,12
Atypicals appear more effective in treating catatonia and less likely to cause NMS. Case reports13,14 indicate many of these agents can be effective and well tolerated in treating catatonic symptoms, although this was not the case for Mr. C.
Anticonvulsants such as valproate15 and carbamazepine, 600 to 1200 mg/d,16 may take longer to work than lorazepam but may be options for patients who do not respond to benzodiazepines.8,9
Amantadine, an N-methyl-D-aspartate (NMDA) antagonist, has been used with some success for catatonia that does not unrespond to lorazepam.17 However, amantidine’s dopamine agonist activity could worsen underlying psychosis.
Memantine—another NMDA antagonist—differs from amantadine despite having a similar chemical structure. Memantine is a noncompetitive antagonist at the NMDA receptor, without affinity for dopamine, norepinephrine, serotonin, or muscarinic receptors.18
Although no published data support using memantine in patients with catatonia, it might be considered for those who are not candidates for lorazepam or ECT. For instance, a double-blind, placebo-controlled study found that lorazepam was not effective for catatonic schizophrenia.19 We have found memantine to help in some patients with catatonic schizophrenia.
CASE CONTINUED: TRIAL OF MEMANTINE
Mr. C remained in a catatonic stupor, but we decided against ECT because he resumed eating and drinking and was not medically at risk. Quetiapine, 100 to 300 mg/d, was tried to address his dementia symptoms, confusion, and poor mentation. This trial was discontinued after Mr. C fell and was readmitted to the medical unit. We then added memantine, 5 mg bid.
In the first week after beginning memantine, Mr. C’s MMSE score was 21, consistent with vascular dementia, but he remained immobile and staring. Motor signs also persisted, including automatic obedience, ambitendency, and a grasp reflex.
The next week, we increased memantine to 10 mg bid. Mr. C was oriented to person, place, and time, and his affect was blunted. His MMSE score increased to 25, showing improved cognition and memory. His Bush-Francis scale score was 6, showing reduced catatonic signs, with remaining mild immobility, bradykinesia, speech-prompt mutism, staring, and grasp reflex.
He maintained this improvement on carbidopa/levodopa, 10/100 tid; amantadine, 100 mg tid; and memantine, 10 mg bid, and was discharged from the nursing home unit.
IMPROVEMENT WITH MEMANTINE
Memantine may reduce excess glutamate at the NMDA receptor in the parietal-SMA-frontal cortical circuit. It may help to increase GABA and dopamine, which are deficient in catatonia. Our patient with vascular dementia had a severe ischemic deficit in the posterior parietal area, as seen on SPECT.
Amantadine, another NMDA receptor antagonist, acts on dopamine neurons and may have anticholinergic-like side effects, whereas memantine does not. Although both drugs share antagonism at the NMDA glutamate receptor, noncompetitive binding is weak for amantadine and moderate for memantine. Memantine has some serotonin (5-HT3) antagonism, but neither agent has direct GABA activity.
Memantine can improve function in vascular dementia.20 Thus, Mr. C’s improvement may have been caused by the drug’s effect on his vascular dementia, the primary neuropsychiatric illness. However, his catatonic signs improved without antipsychotics, cholinesterase inhibitors, benzodiazepines, or ECT. No anticoagulation treatment or cerebral perfusion procedures account for his improved mental status.
CASE CONCLUSION
Mr. C went to live with his son’s family. Although he has problems with calculation, he shows good selfcare. When asked why he did not respond during his catatonic stupor, Mr. C stated that he believed the physician was an Internal Revenue Service agent asking him about serious tax problems. Upon reflection, he said he no longer believes this.
- Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge, UK: Cambridge University Press, 2003.
- Caroff SN, Mann SC, Francis A, Fricchione GE. Catatonia: from psychopathology to neurobiology. Washington, DC: American Psychiatric Publishing, 2004.
- Mann SC, Caroff SN, Keck PE Jr, Lazarus A. Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing, 2003.
- Neuroleptic Malignant Syndrome Information Service. www.NMSIS.org.
Drug brand names
- Amantadine • Symmetrel
- Amobarbital • Amytal sodium
- Carbamazepine • Carbatrol, Equetro
- Carbidopa/levodopa • Sinemet
- Disulfiram • Antabuse
- Divalproex • Depakote
- Furosemide • Lasix
- Lisinopril • Prinivil, Zestril
- Lorazepam • Ativan
- Memantine • Namenda
- Metoprolol • Lopressor
- Ranitidine • Zantac
- Simvastatin • Zocor
- Valproic acid • Depakene
- Zolpidem • Ambien
Disclosure
Dr. Carroll and Dr. Hawkins are speakers for Forest Laboratories. The other authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The authors thank Dr. Niraj Ahuja, consultant psychiatrist and honorary clinical lecturer (psychiatry), Newcastle, North Tyneside and Northumberland Mental Health Trust, UK, for assistance with the figure.
Many catatonia cases respond to benzodiazepines—especially lorazepam—but up to 30% do not. Electroconvulsive therapy (ECT) can be effective, but what’s the next step when ECT is unavailable or inappropriate for your patient?
To help you solve this dilemma, we describe our diagnosis and treatment decisions for a patient we call Mr. C. We explain how our process was guided by recent understandings of an abnormal neural circuit that appears to cause catatonia’s complex motor and behavioral symptoms.
This article describes that neurologic pathology and answers common questions about the clinical workup and treatment of catatonia.
CASE: TROUBLE IN TV LAND
Mr. C, age 69, caused a disturbance at a local TV station, demanding that they broadcast a manuscript he had written. Police took him to a local hospital, where he was stabilized and then transferred to a neuropsychiatric hospital for evaluation.
The psychiatric interview revealed that he had developed insomnia, excessive activity, and delusional thinking 2 weeks before admission. His medical history included coronary artery disease (CAD), hypertension, and hypothyroidism. Medications included thyroid hormone replacement therapy, furosemide, potassium, ranitidine, simvastatin, metoprolol, and lisinopril. CAD treatment included stent placement and nitroglycerin as needed.
He had been hospitalized in his 30s and treated with ECT for what he called “bad thoughts.” He said he improved after 1 month and had no subsequent psychiatric history. He denied drug or alcohol abuse.
Shortly after admission, he refused to eat or drink and after 1 week became dehydrated. He also showed mutism, immobility, and stupor. He was transferred to the medical service for IV rehydration.
MANY SCENARIOS AND SIGNS
Mr. C’s symptoms suggest possible catatonia, a neuropsychiatric syndrome of motor dysregulation found in up to 10% of acutely ill psychiatric inpatients.1,2 A movement disorder,1,2 catatonia occurs with general medical conditions and psychiatric disorders (Table 1).
Pathophysiology. Catatonic signs develop when aberrant signals from neurochemical abnormalities trigger a neural circuit that affects the medial gyrus of the orbital frontal lobe, the lateral gyrus, caudate nucleus, globus pallidus, and thalamus (Box).3-5
Presentation. A focused exam is required because patients with catatonia often do not provide a comprehensive or reliable history.2 They show mutism, characteristic postures, rigidity, aberrant speech, negativism, and stereotyped behaviors.1,2 They may present in an excited or retarded state:
- Excited patients may injure themselves or others and develop hyperthermia, tachycardia, and elevated blood pressure from excessive motor activity.
- Patients in a retarded state may present with bradykinesia and poor self-care. They may be unresponsive to external stimuli, develop catatonic stupor, and refuse to eat or drink.
Mr. C’s earlier insomnia, excessive activity, and delusional thinking (such as the TV station incident) may have signaled an excited catatonia. On admission to the medical service, however, he presented in a retarded state.
Signs. Part of the challenge with detecting catatonia’s signs is that there are so many; some rating scales list more than 20. Not all signs need to be present to make the diagnosis, however, and if you find one, others usually turn up in the examination.
A mnemonic from the Bush-Francis Catatonia Screening Instrument (Table 2) represents diagnostic signs in patients with the excited or retarded forms.2 We recommend that you review an authoritative text (see Related resources) to understand catatonia’s psychopathology.2
Table 1
Common diagnoses of patients with catatonia
| Psychiatric |
|
| Organic |
|
Catatonia is caused by neurochemical abnormalities including low GABA activity in the frontal cortex, low dopamine (D2) activity in the basal ganglia, high glutamate—N-methyl-D-aspartate (NMDA)—activity in the parietal cortex, or a combination of these.3-5 Catatonic signs occur when these neurochemical changes cause aberrant signals and trigger a neural circuit affecting the medial gyrus of the orbital frontal lobe, the lateral gyrus, caudate nucleus, globus pallidus, and thalamus (Figure).
Posturing occurs when the aberrant signal reaches the posterior parietal lobe. Patients’ bizarre and mundane postures in catatonia are maintained by “anosognosia of position.” For example, an individual does not know the position of rest for his arm, and it remains in an unusual position as if at rest.3
The PP goes on to influence the supplemental motor area (SMA), causing bradykinesia, rigidity, and other motor phenomena that catatonia shares with Parkinson’s disease. The SMA feeds back to the medial orbital gyrus, completing the neural circuit.3
Regions such as the anterior cingulate area (ACA) and amygdala (1AMG) — also may be recruited into the expanded circuit. ACA recruitment may cause akinetic mutism, and fear is a symptom of AMG recruitment. If the anterior hypothalamus is affected, malignant catatonia or neuroleptic malignant syndrome may occur.3,5
This neural loop demonstrates an integrated model of psychosis. It may help explain why catatonia responds to treatment with lorazepam, ECT, and other agents such as antipsychotics and NMDA antagonists.
Illustration for CURRENT PSYCHIATRY by Marcia Hartsock, CMI
Table 2
WIRED `N MIRED: Mnemonic for detecting catatonia
| Waxy flexibility/catalepsy |
| Immobility/stupor |
| Refusal to eat or drink |
| Excitement |
| Deadpan staring |
| Negativism/negative symptoms |
| Mutism |
| Impulsivity |
| Rigidity |
| Echolalia/echopraxia |
| Direct observation |
CASE CONTINUED: MAKING THE DIAGNOSIS
In the medical unit, Mr. C was found to be in a catatonic stupor, with immobility, mutism (monosyllabic speech), catalepsy, intermittent waxy flexibility, withdrawal (refusal to eat and drink), automatic obedience, and mitgehen (exaggerated movements in response to light finger pressure, despite instructions to stay still). ECT workup was started, along with a trial of lorazepam, 1 mg tid.
Laboratory studies revealed high BUN/creatinine (80/2.0) that returned to normal range (BUN 7 to 21 mg/dL; creatinine 0.5 to 1.2 mg/dL) after 3 days of hydration. Because of Mr. C’s earlier excited symptoms and delusional thinking, we considered a diagnosis of bipolar disorder with catatonia. However, his symptoms did not improve with a trial of valproic acid (serum level 64 mcg/mL).
Head CT showed generalized atrophy and EEG showed delta slowing. Single-photo emission computer tomography (SPECT) showed areas of decreased perfusion in the cortex, with no perfusion in the left posterior parietal area (PP).
Mental status exam found Mr. C disoriented with poor short-term memory and unable to complete the Mini Mental State Examination (MMSE). His Bush-Francis Catatonia Rating Scale score was 28 and included many catatonic signs that would not be seen a patient with simple dehydration.
The workup supported a diagnosis of catatonia due to general medical condition (vascular dementia) and ruled out schizophrenia with catatonic features, bipolar disorder, or major depression with catatonia.
EVALUATION AND DIAGNOSIS
Medical causes. A careful history and thorough physical examination are essential for making an accurate diagnosis and ruling out medical conditions that could present with or mimic catatonia (Table 3). Medications that can induce catatonia include antipsychotics, corticosteroids, and disulfiram at therapeutic doses. Drug abuse (such as with phencyclidine), use of the general anesthetic ketamine, and benzodiazepine withdrawal may also lead to catatonia.
Head CT or MRI is indicated for patients being considered for ECT or for localizing neurologic findings. EEG can be useful when patients present with features of seizure activity—such as tongue biting, incontinence, or stupor—or with catatonia as a manifestation of delirium or dementia.
A history of head injury or neurologic disease warrants further neurologic investigation. Also consider a neurology consult when the patient has prolonged stupor or does not respond to initial drug therapy.
Psychiatric causes. The clinical setting may suggest the most likely primary psychiatric disorders to consider, such as:
- bipolar or major depression in acute inpatient psychiatric units
- autism and pervasive developmental disorders (PDD) in pediatric or PDD units
- catatonic schizophrenia in chronic psychotic patients
- somatoform or factitious disorders in forensic settings.
These generalizations are not clinically exclusive, of course, but may provide a starting point for the treatment team confronted with limited history and exam information.
Table 3
Catatonia workup: Recommended lab tests
| Test | Recommendation |
|---|---|
| Complete blood count with WBC differential | Look for leukocytosis |
| Serum chemistries | Look for electrolyte imbalances |
| Serum iron | May be low in NMS |
| Serum creatine kinase | If NMS is suspected |
| Brain MRI or CT | If structural lesion is suspected |
| Electroencephalography | If seizure disorder or brain abnormality is suspected |
| Lumbar puncture | If encephalitis or meningits is suspected |
| NMS: neuroleptic malignant syndrome | |
Initial treatment. Catatonia related to medical and psychiatric causes has been shown to respond to lorazepam and to ECT.6,7 Lorazepam is preferred because of its specificity for the GABAa receptor and ease of administration (oral, IM, or IV). Other agents that act on GABA—including amobarbital and zolpidem—have also been used. Catatonia’s hallmark features such as mutism and immobility have been shown to respond to lorazepam.8,9
ECT is a first-line treatment for catatonia with life-threatening conditions and should be considered for refractory cases.
Lorazepam. The starting dosage is usually 1 mg tid for healthy adults; 0.5 mg tid can be used for children and the elderly. Observe the patient for improvement in catatonic signs after the first dose and before giving the second. Dosages of up to 16 mg/d have been used.
In many cases, lorazepam can be tapered off after adequate treatment of the primary psychiatric condition. In severe cases, however—such as when patients refuse to eat or drink—lorazepam may be continued for as long as 1 year. Weigh the risk of benzodiazepine tolerance, dependence, and misuse versus the possibility of relapse and rehospitalization.
Medical catatonias and neuroleptic malignant syndrome (NMS) have responded favorably to ECT.8 Addressing the medical cause itself usually does not resolve catatonia, with the possible exception of seizure-induced (“ictal”) catatonia, which may respond to anticonvulsants and lorazepam.6,7
ECT. An ECT workup can begin as soon as a patient presents with catatonia. If lorazepam produces no response within 24 hours, consider ECT.
CASE CONTINUED: PERSISTENT SYMPTOMS
After three 1-mg doses of lorazepam, Mr. C became more alert and oriented but his catatonia symptoms persisted, as indicated by a Bush-Francis score of 23, significant grasp reflex, and gegenhalten (automatic rather than willful resistance to passive limb movement in proportion to the strength of the stimulus). An attempt to gradually increase lorazepam to 2 mg tid produced delirium. He remained confused even when lorazepam was reduced to 0.5 mg tid, so the drug was discontinued.
Mr. C’s neurologist added amantadine, 100 mg tid, and carbidopa/levodopa, 10/100 mg tid, to treat his parkinsonian rigidity.
WHAT NEXT? OTHER OPTIONS
Antipsychotics have been investigated as a possible treatment for catatonia. The literature suggests that conventional antipsychotics may cause catatonia and atypical antipsychotics may improve it. Conventional antipsychotics are best avoided in catatonia because they:
- appear less effective than other treatments in resolving catatonic symptoms8,10
- are associated with catatonic-like side effects, such as rigidity, akinesia, and staring10
- appear to increase NMS risk in patients with catatonic symptoms.11,12
Atypicals appear more effective in treating catatonia and less likely to cause NMS. Case reports13,14 indicate many of these agents can be effective and well tolerated in treating catatonic symptoms, although this was not the case for Mr. C.
Anticonvulsants such as valproate15 and carbamazepine, 600 to 1200 mg/d,16 may take longer to work than lorazepam but may be options for patients who do not respond to benzodiazepines.8,9
Amantadine, an N-methyl-D-aspartate (NMDA) antagonist, has been used with some success for catatonia that does not unrespond to lorazepam.17 However, amantidine’s dopamine agonist activity could worsen underlying psychosis.
Memantine—another NMDA antagonist—differs from amantadine despite having a similar chemical structure. Memantine is a noncompetitive antagonist at the NMDA receptor, without affinity for dopamine, norepinephrine, serotonin, or muscarinic receptors.18
Although no published data support using memantine in patients with catatonia, it might be considered for those who are not candidates for lorazepam or ECT. For instance, a double-blind, placebo-controlled study found that lorazepam was not effective for catatonic schizophrenia.19 We have found memantine to help in some patients with catatonic schizophrenia.
CASE CONTINUED: TRIAL OF MEMANTINE
Mr. C remained in a catatonic stupor, but we decided against ECT because he resumed eating and drinking and was not medically at risk. Quetiapine, 100 to 300 mg/d, was tried to address his dementia symptoms, confusion, and poor mentation. This trial was discontinued after Mr. C fell and was readmitted to the medical unit. We then added memantine, 5 mg bid.
In the first week after beginning memantine, Mr. C’s MMSE score was 21, consistent with vascular dementia, but he remained immobile and staring. Motor signs also persisted, including automatic obedience, ambitendency, and a grasp reflex.
The next week, we increased memantine to 10 mg bid. Mr. C was oriented to person, place, and time, and his affect was blunted. His MMSE score increased to 25, showing improved cognition and memory. His Bush-Francis scale score was 6, showing reduced catatonic signs, with remaining mild immobility, bradykinesia, speech-prompt mutism, staring, and grasp reflex.
He maintained this improvement on carbidopa/levodopa, 10/100 tid; amantadine, 100 mg tid; and memantine, 10 mg bid, and was discharged from the nursing home unit.
IMPROVEMENT WITH MEMANTINE
Memantine may reduce excess glutamate at the NMDA receptor in the parietal-SMA-frontal cortical circuit. It may help to increase GABA and dopamine, which are deficient in catatonia. Our patient with vascular dementia had a severe ischemic deficit in the posterior parietal area, as seen on SPECT.
Amantadine, another NMDA receptor antagonist, acts on dopamine neurons and may have anticholinergic-like side effects, whereas memantine does not. Although both drugs share antagonism at the NMDA glutamate receptor, noncompetitive binding is weak for amantadine and moderate for memantine. Memantine has some serotonin (5-HT3) antagonism, but neither agent has direct GABA activity.
Memantine can improve function in vascular dementia.20 Thus, Mr. C’s improvement may have been caused by the drug’s effect on his vascular dementia, the primary neuropsychiatric illness. However, his catatonic signs improved without antipsychotics, cholinesterase inhibitors, benzodiazepines, or ECT. No anticoagulation treatment or cerebral perfusion procedures account for his improved mental status.
CASE CONCLUSION
Mr. C went to live with his son’s family. Although he has problems with calculation, he shows good selfcare. When asked why he did not respond during his catatonic stupor, Mr. C stated that he believed the physician was an Internal Revenue Service agent asking him about serious tax problems. Upon reflection, he said he no longer believes this.
- Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge, UK: Cambridge University Press, 2003.
- Caroff SN, Mann SC, Francis A, Fricchione GE. Catatonia: from psychopathology to neurobiology. Washington, DC: American Psychiatric Publishing, 2004.
- Mann SC, Caroff SN, Keck PE Jr, Lazarus A. Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing, 2003.
- Neuroleptic Malignant Syndrome Information Service. www.NMSIS.org.
Drug brand names
- Amantadine • Symmetrel
- Amobarbital • Amytal sodium
- Carbamazepine • Carbatrol, Equetro
- Carbidopa/levodopa • Sinemet
- Disulfiram • Antabuse
- Divalproex • Depakote
- Furosemide • Lasix
- Lisinopril • Prinivil, Zestril
- Lorazepam • Ativan
- Memantine • Namenda
- Metoprolol • Lopressor
- Ranitidine • Zantac
- Simvastatin • Zocor
- Valproic acid • Depakene
- Zolpidem • Ambien
Disclosure
Dr. Carroll and Dr. Hawkins are speakers for Forest Laboratories. The other authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The authors thank Dr. Niraj Ahuja, consultant psychiatrist and honorary clinical lecturer (psychiatry), Newcastle, North Tyneside and Northumberland Mental Health Trust, UK, for assistance with the figure.
1. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry 2003;160:1233-41.
2. Bush G, Fink M, Petrides G, et al. Catatonia I: Rating scale and standardized examination. Acta Psychiatr Scand 1996;93:129-36.
3. Northoff G. What catatonia can tell us about “top-down” modulation:” a neuropsychiatric hypothesis. Brain Behav Sci 2002;25:555-604.
4. Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectrums 2000;5(7):26-33.
5. Carroll BT. Catatonia is the rosetta stone of psychosis (poster presentation). New York: American Psychiatric Association annual meeting, 2004.
6. Barnes MP, Saunders M, Walls TJ, et al. The syndrome of Karl Ludwig Kahlbaum. J Neurol Neurosurg Psychiatry 1986;49:991-6.
7. Carroll BT, Anfinson TJ, Kennedy JC, et al. Catatonic disorder due to general medical conditions. J Neuropsychiatry Clin Neurosci 1994;6:122-33.
8. Hawkins JM, Archer KJ, Strakowski SM, Keck PE. Somatic treatments of catatonia. Int J Psychiatry Med 1995;25:345-69.
9. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry 1990;51:357-62.
10. Dose M. Neuroleptic-induced pseudo-catatonia. Pharmacopsychiatry 2001;34:262-4.
11. Keck PE, Jr, Pope HG, Jr, Cohen BM, et al. Risk factors for neuroleptic malignant syndrome. Arch Gen Psychiatry 1989;46:914-18.
12. White DAC. 17 catatonic patients diagnosed as neuroleptic malignant syndrome. CNS Spectrums 2000;5:58-65.
13. Levy WO, Nunez CY. Use of ziprasidone to treat bipolar-associated catatonia. Bipolar Disord 2004;6:166-7.
14. Hesslinger B, Walden J, Normann C. Acute and long-term treatment of catatonia with risperidone. Pharmacopsychiatry 2001;34:25-6.
15. Kruger S, Braunig P. Intravenous valproic acid in the treatment of severe catatonia. J Neuropsychiatry Clin Neurosci 2001;13:303-4.
16. Kritzinger PR, Jordaan GP. Catatonia: an open prospective series with carbamazepine. Int J Neuropsychopharmacol 2001;4:251-7.
17. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry 1997;62:404-6.
18. Namenda (memantine) Package labeling. Forest Laboratories, 2004.
19. Ungvari GS, Chie HFK, Chow LY, et al. Lorazepam for chronic catatonia: a random, double-blind, placebo-controlled, cross-over study. Psychopharmacol 1999;142:393-8.
20. Mobius HJ. Pharmacologic rationale for memantine in chronic cerebral hypoperfusion, especially vascular dementia. Alz Dis Assoc Disord 1999;13(suppl 3):172-8.
1. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry 2003;160:1233-41.
2. Bush G, Fink M, Petrides G, et al. Catatonia I: Rating scale and standardized examination. Acta Psychiatr Scand 1996;93:129-36.
3. Northoff G. What catatonia can tell us about “top-down” modulation:” a neuropsychiatric hypothesis. Brain Behav Sci 2002;25:555-604.
4. Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectrums 2000;5(7):26-33.
5. Carroll BT. Catatonia is the rosetta stone of psychosis (poster presentation). New York: American Psychiatric Association annual meeting, 2004.
6. Barnes MP, Saunders M, Walls TJ, et al. The syndrome of Karl Ludwig Kahlbaum. J Neurol Neurosurg Psychiatry 1986;49:991-6.
7. Carroll BT, Anfinson TJ, Kennedy JC, et al. Catatonic disorder due to general medical conditions. J Neuropsychiatry Clin Neurosci 1994;6:122-33.
8. Hawkins JM, Archer KJ, Strakowski SM, Keck PE. Somatic treatments of catatonia. Int J Psychiatry Med 1995;25:345-69.
9. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry 1990;51:357-62.
10. Dose M. Neuroleptic-induced pseudo-catatonia. Pharmacopsychiatry 2001;34:262-4.
11. Keck PE, Jr, Pope HG, Jr, Cohen BM, et al. Risk factors for neuroleptic malignant syndrome. Arch Gen Psychiatry 1989;46:914-18.
12. White DAC. 17 catatonic patients diagnosed as neuroleptic malignant syndrome. CNS Spectrums 2000;5:58-65.
13. Levy WO, Nunez CY. Use of ziprasidone to treat bipolar-associated catatonia. Bipolar Disord 2004;6:166-7.
14. Hesslinger B, Walden J, Normann C. Acute and long-term treatment of catatonia with risperidone. Pharmacopsychiatry 2001;34:25-6.
15. Kruger S, Braunig P. Intravenous valproic acid in the treatment of severe catatonia. J Neuropsychiatry Clin Neurosci 2001;13:303-4.
16. Kritzinger PR, Jordaan GP. Catatonia: an open prospective series with carbamazepine. Int J Neuropsychopharmacol 2001;4:251-7.
17. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry 1997;62:404-6.
18. Namenda (memantine) Package labeling. Forest Laboratories, 2004.
19. Ungvari GS, Chie HFK, Chow LY, et al. Lorazepam for chronic catatonia: a random, double-blind, placebo-controlled, cross-over study. Psychopharmacol 1999;142:393-8.
20. Mobius HJ. Pharmacologic rationale for memantine in chronic cerebral hypoperfusion, especially vascular dementia. Alz Dis Assoc Disord 1999;13(suppl 3):172-8.
Acute bipolar mania: Aggressive initial dosing provides faster symptom relief
Evidence on aggressive initial dosing of mood stabilizers and antipsychotics is changing the way acute bipolar mania is treated. To help you apply this information, we searched the literature and meeting abstracts for aggressive strategies tested to date. This article discusses how to:
- identify patients who may benefit from loading or aggressive initial dosing
- calculate mood stabilizer dosages
- dose each atypical antipsychotic
- manage potential antipsychotic side effects during maintenance therapy.
PATIENT SELECTION
Rapidly achieving therapeutic blood levels relieves patient suffering faster than standard titration. It quickly calms the hyperactivity, impulsivity, tension, hostility, and uncooperativeness that distress patients and increase the risk of harm to themselves and others.
The challenge of using higher dosages is to minimize side effects. Loading is not a one-size-fits-all approach, as antimanic drugs’ unique pharmacokinetic and pharmacotherapeutic properties influence how each agent is used.
An interesting body of literature advocates using mood stabilizers plus antipsychotics to treat mania, suggesting greater efficacy than with either agent alone.16 This strategy raises important questions, such as:
- Can two drugs be loaded simultaneously?
- Can patients taking mood stabilizers be treated with antipsychotic loading, and can those taking antipsychotics receive loading dosages of mood stabilizers?
- Would “double loading” improve bipolar mania treatment?
Answers to these questions are needed because of increased demands on clinicians to control hospital costs by rapidly and effectively treating patients with bipolar mania.
Loading doses cannot be standardized but are calculated by multiplying target steady-state plasma concentration by volume of distribution. We suggest aggressive initial schedules for divalproex sodium and atypical antipsychotics in this article with the understanding that practitioners will adjust them based on each patient’s tolerance and response.
Hospitalization. Patients with acute bipolar mania should be supervised closely in the hospital during loading or aggressive initial dosing. Monitor for cardiovascular changes, neurologic disturbances, sensorium changes, and response.
Precautions. Not all patients are candidates for aggressive initial dosing. Contraindications include age <18 or >65 years, pregnancy, breast-feeding, medical illness, and known sensitivity to the medication being given.
Higher-than-usual dosing increases the risk of excessive drug concentrations in sensitive individuals—such as those with a history of sensitivity to lower dosages of similar medications—and toxic levels of drugs with long half-lives can persist. When in doubt, consider giving a smaller amount of the loading dose early in the day, followed later by a larger amount.
DRUG SELECTION
Loading and aggressive initial dosing strategies for bipolar mania were first advanced for divalproex sodium.1 Investigators then examined loading strategies for lithium and carbamazepine, as well as the antipsychotics olanzapine, risperidone, quetiapine, ziprasidone, and aripiprazole, which are known to have antimanic properties.
Olanzapine, quetiapine, and risperidone are FDA-approved for short-term treatment of acute manic episodes associated with bipolar I disorder, and similar indications were being considered for aripiprazole and ziprasidone as this article went to press. We discuss evidence on loading and aggressive initial dosing strategies for each agent.
No studies have compared one loading strategy with another. Thus, when we choose drugs for loading, we consider what each patient needs, available formulations, tolerability, and efficacy for long-term stabilization and maintenance treatment.
LITHIUM
Lithium loading targets the therapeutic range (<1.4 mEq/L), without crossing the toxic threshold (>1.5 mEq/L). Lithium loading has shown antimanic effects, although using >30 mg/kg/d causes severe nausea and vomiting.
Moscovich et al2 reported a case series of 9 adults with acute mania who received lithium loading dosages of 4,050 mg/d. Patients tolerated lithium well at plasma drug levels of approximately 1.2 to 1.4 mEq/L. Their manic symptoms declined significantly within 4 to 5 days, as measured by Clinical Global Impression (CGI) severity of illness, Biegel-Murphy Mania State Rating Scale, and Brief Psychiatric Rating Scale scores.
Table 1
How to calculate divalproex loading for acute bipolar mania*
| Days 1 and 2 |
| Patient weight in pounds x 15 = dosage (mg/d) (Example: 150 lbs x 15 = 1,750 mg/d) |
| Days 3 to 10 |
| Patient weight in pounds x 10 = dosage (mg/d) (Example: 150 lbs. x 10 = 1,500 mg/d) |
| To avoid splitting tablets, make dosage divisible by 125 (round up for young adults, round down for older adults). Divide into bid or tid doses for improved tolerability. |
| Days 4 and 7 |
| Draw blood to monitor valproic acid levels and for other values such as liver function studies. |
| * For use with oral divalproex sodium (delayed-release or extended-release formulations). Do not use valproic acid preparations, as loading is unlikely to be well tolerated. |
| Source: Reference 5 |
Kook et al3 attempted a 30-mg/kg loading dose of slow-release lithium carbonate in 38 patients to evaluate the safety of achieving a therapeutic level in 12 hours. No patient experienced adverse effects during loading or in the 12 hours after loading was completed.
Patients who develop a manic episode while taking lithium pose a therapeutic dilemma. If they stop taking lithium—especially abruptly—manic symptoms can return in as few as 2 days. For these patients, consider increasing the usual dosage by 50% to 100% on the first day of mania treatment,4 then continue a dosage that maintains efficacy and achieves a therapeutic blood level.
To avoid GI upset, avoid any single dose of >1,500 mg; if a greater dosage is needed, divide it for improved tolerance. Obtain lithium blood levels at baseline, after 4 days, and thereafter as clinically relevant to monitor for drug interactions that may affect serum levels.
Side effects—GI irritation, tremor, muscle weakness, thirst, polyuria—are common in the first week, and weight gain may occur after prolonged treatment.
DIVALPROEX
In a study by Keck et al1 of 19 adults with acute bipolar mania, aggressive initial dosing of divalproex sodium, 20 mg/kg/d, was well-tolerated and shortened hospitalization. Side effects including sedation and nausea occurred at a rate similar to that seen with more-gradual titration.
To calculate the initial dose, the authors used a conversion factor of 20 mg/kg/d or added a zero to the patient’s weight in pounds. This amount was given in single or divided doses the first day, then continued for 4 to 7 days. Blood levels were measured on day 4, and all 15 patients who completed the study achieved serum valproate levels >50 mcg/mL.
In a double-blind study by Hirschfeld et al,5 59 adults with Young Mania Rating Scale (YMRS) scores >14 were randomly assigned to receive divalproex oral loading; divalproex nonloading (250 mg tid on days 1 and 2, followed by standard titration on days 3 to 10); or lithium carbonate (300 mg tid, followed by standard titration on days 3 to 10).
Divalproex loading—30 mg/kg/d on days 1 and 2, followed by 20 mg/kg on days 3 to 10— yielded valproate levels >50 mcg/mL in 84% of patients by day 3. Rapid loading appeared to increase antimanic efficacy, as measured by YMRS endpoint scores, without increasing adverse effects. For Table 1, we converted this study’s metric values to patient weight in pounds.
Side effects. Divalproex can inhibit metabolism of other drugs (including anticonvulsants such as lamotrigine) and increase their blood levels. Side effects such as sedation, alopecia, abdominal pain, diarrhea, and tremor may require observation and treatment. Pancreatitis, hepatitis, and allergic reactions are rare but may require discontinuation.
Recommendation. Aggressive initial dosing and loading for patients with acute mania has been reported with enteric-coated delayed-release and extended-release6 divalproex sodium tablets. With identical dosages, the extended-release form produces 11% lower serum levels than the delayed-release form.7
Aggressive dosing of oral valproic acid preparations is not recommended and is likely to be poorly tolerated.8 A pilot study evaluating IV valproate loading in acute mania found no changes in mania in the 2 hours that followed loading.9
CARBAMAZEPINE
Carbamazepine is used off-label to treat mania and mixed phases of bipolar disorder.4,9,10 Excessive absorption rates are associated with dizziness, ataxia, and nausea. Side effects may occur when the plasma level is therapeutic for epilepsy (4 to 12 mcg/mL).11
In mania, the relationship between carbamazepine levels and clinical response is not always clear. Lerer et al12 found a correlation between acute mania response and a serum level of 8.8 mcg/mL (range 4.7 to 14.0 mcg/mL).
Patients with acute mania may require 600 to 1,600 mg/d. Carbamazepine is available in immediate-release and controlled-release preparations. Because generic preparations might not be bio-equivalent,13 use the same formulation throughout treatment to maintain consistent serum levels.
Enzymatic auto-induction—in which carbamazepine gradually increases the activity of its metabolic enzyme—is likely to occur at 3 to 5 weeks of administration, often after hospital discharge. An aggressive initial rescue adjustment can be used if a patient develops mania after having been stabilized on carbamazepine for >6 weeks. Because these patients are past the point of auto-induction, a target blood level of 8.8 mcg/mL can be used and the dosage adjusted proportionally.
For example, if mania recurs in a patient who is stabilized on carbamazepine, 400 mg/d (plasma level 4.5 mcg/mL), carbamazepine can be loaded up to 800 mg/d. Measure the plasma level within 4 days; the target level would be 9.0 mcg/mL (2 times 4.5 mcg/mL), which closely approximates steady state and sets up a ratio to reach the target level of 8.8 mcg/mL.
Side effects include ataxia, diplopia, and dizziness. Complete blood counts, liver function studies, plasma levels, and serum chemistries require regular monitoring.
Recommendation. Carbamazepine is not recommended for oral loading in patients who have never taken it or for those with hyponatremia, hepatic dysfunction, or history of intolerance or agranulocytosis.
OLANZAPINE
Olanzapine has pharmacologic properties favorable for loading.4 The recommended dosage for acute mania is 15 mg/d with standard titration; Karagianis et al14 showed that initial loading doses of >20 mg/d resulted in good control of agitated patients with psychosis. Side effects were uncommon, with sedation occurring in 14% of patients in this case series. The loading dose reduced agitation more effectively than did dosages <20 mg/d given to similar patients.
A multicenter study of 148 acutely agitated inpatients with a variety of psychiatric disorders compared olanzapine rapid initial dose escalation with usual clinical practice.15 Mean aggressive dosages were 28.8 mg/d on day 1, 30.3 mg/d on day 2, and 16.1 mg/d on day 5. Usual-practice dosages were 10 mg/d, plus lorazepam, 0 to 4 mg/d for the first 2 days and 0 to 2 mg/d on days 3 to 4. Based on Positive and Negative Syndrome Scale excited component subscale scores, olanzapine loading controlled agitation more effectively than did usual practice, with similar side-effect rates.
IM olanzapine or the orally dissolving form are bioequivalent to the tablets and may be used for acute agitation associated with bipolar mania in certain clinical settings.14
Table 2
Suggested antipsychotic loading for acute bipolar mania and mixed states*
| Drug | Day(s) | Aggressive initial dosing schedule | Comment |
|---|---|---|---|
| Aripiprazole24,25 | 1 2 to 3 4+ | 30 mg once daily with food 30 mg/d with food Reduce dosage by 10 to 15 mg/d, based on tolerance and response | Nausea and vomiting may occur in first few days; adjust dosage based on tolerance and response |
| Olanzapine14,15 | 1 and 2 3 and 4 5 to 10 | 40 mg in single or divided dosage 20 to 30 mg at bedtime 15 mg once daily (may reduce to 5, 7.5, or 10 mg/d) | Adjust dosage based on tolerance and response; oral or IM formulations may be used |
| Quetiapine19,21 | 1 2 and 3 4 to 10 | 100 mg upon admission and 100 mg at bedtime 100 mg bid (or tid to qid) plus 100 to 200 mg at bedtime 200 mg bid plus 200 to 300 mg at bedtime; may adjust to 400 to 800 mg/d) | Adjust dosage based on tolerance and response |
| Risperidone16,18 | 1 2 3 and 4 | 3 mg in single or divided dosage 4 mg in single or divided dosage 5 mg in single or divided dosage | Adjust dosage by 1 mg up or down, based on tolerance and response; use tablet, rapid-dissolving tablet, or liquid form, but not long-acting IM form |
| Ziprasidone22,23 | 1 2 | 20 mg IM in single dose (may repeat for severe agitation) or 40 mg po bid with food 60 to 80 mg po bid with food | Adjust dosage based on tolerance and response |
| * For hospitalized or partially hospitalized patients, ages18. Not recommended for patients who are pregnant, breastfeeding, medically ill, age >65, or with known sensitivity to the antipsychotic being given. | |||
RISPERIDONE
Sachs et al16 studied 156 inpatients who developed an acute manic or mixed episode while receiving lithium or divalproex. These patients were randomly assigned to begin adjunctive risperidone, 2 mg/d, haloperidol, or placebo. Dosing was flexible, increasing or decreasing by 1 mg/d. Risperidone’s mean modal dosage was 3.8 mg/d across 3 weeks, with mean exposure of 17 days. Risperidone plus a mood stabilizer was more effective than a mood stabilizer alone, and the combination provided rapid, well-tolerated control of manic symptoms.
In a double-blind trial, Hirschfeld et al17 randomly assigned 279 patients with acute bipolar mania to risperidone, 1 to 6 mg/d, or placebo for 3 weeks. As early as day 3, YMRS scores were reduced significantly more with risperidone than with placebo. Somnolence was the most common side effect, and mean modal dosage was 4.1 mg/d.
Table 3
Screening schedule for antipsychotic side effects during bipolar maintenance treatment
| Baseline | |
| Side effect | Recommended screening |
| Weight gain | Weight and body mass index (BMI) monthly for first 3 months; waist circumference |
| Hypertension | Blood pressure |
| Hyperglycemia | Fasting plasma glucose, with glycosylated hemoglobin (Hb A 1c ) if hyperglycemia is detected |
| Hyperlipidemia | Fasting lipid profile |
| Tardive dyskinesia | Abnormal Involuntary Movement Scale (AIMS) or other screen |
| Ophthalmic changes | Ophthalmologic examination for patients taking quetiapine and for all with diabetes mellitus |
| Follow-up schedules | |
| 3 months | |
| Weight and BMI | |
| Blood pressure | |
| Fasting plasma glucose, with Hb A 1c if hyperglycemia is detected; Hb A 1c values may be used to measure interval changes in glucose tolerance | |
| Fasting lipid profile | |
| 6 months | |
| AIMS or other tardive dyskinesia screen | |
| Ophthalmologic examination | |
| Source: Adapted from reference 26. | |
In a multicenter, randomized, double-blind, placebo-controlled study of patients with acute bipolar mania, Khanna et al18 assigned patients to receive risperidone monotherapy (mean modal dosage 5.6 mg/d) or placebo for 3 weeks. Mania scores of patients receiving risperidone were significantly lower at weeks 1 and 2, compared with the placebo group. Risperidone was well-tolerated, with no unexpected adverse events.
Recommendation. Because of a risk of extrapyramidal symptoms (EPS) and orthostatic hypotension, initial risperidone loading dosages >4 mg on day 1 are not recommended.
QUETIAPINE
Quetiapine has shown antimanic efficacy as monotherapy and as adjunctive therapy to mood stabilizers.19,20 The effective dosage was a mean 600 mg/d (range 400 to 800 mg/d) in monotherapy and adjunctive treatment. These studies achieved 400 mg/d within the first 4 days (100 mg on day 1, 200 mg on day 2, 300 mg on day 3, and 400 mg on day 4).
These data combined with revised prescribing information suggest aggressive initial dose escalation of quetiapine within the first 4 days for selected patients. A titration study in patients with schizophrenia used a more-rapid escalation rate of 400 mg within 2 days.21 Dizziness, orthostatic hypotension, and sedation were not more frequent in this high-dose group than in the two lower-dose titration groups. In our experience, 200 to 400 mg can be given the first day of treatment.
ZIPRASIDONE
In a randomized, double-blind, controlled trial, Keck et al22 assigned 210 patients with a manic or mixed bipolar episode to 3 weeks of ziprasidone, 40 to 80 mg bid, or placebo. Ziprasidone produced rapid, sustained improvement in manic symptoms on all primary and most secondary efficacy measures, such as the YMRS and CGI.
Significant improvements seen within 2 days were maintained. Ziprasidone was well tolerated and was associated with a low EPS rate; neither weight gain nor clinically significant changes in vital signs were seen.
IM ziprasidone, which is approved for use in schizophrenia, may also have efficacy in bipolar mania.23 The recommended dose of 20 mg IM is equivalent to 120 to 160 mg orally, so a single injection may reach the target antimanic dosage.
Recommendation. Ziprasidone could be an option for aggressive initial dosing for a patient who has previously received ziprasidone IM and is not at risk for QTc prolongation.
ARIPIPRAZOLE
In a randomized controlled trial, Keck et al24 assigned 262 patients with acute bipolar mania or mixed states to aripiprazole, 30 mg/d, or placebo for 3 weeks. By day 4, manic symptoms were improved significantly more in patients receiving aripiprazole, and discontinuation rates were similar.
Similarly, in a randomized, controlled multi-center study, Sachs et al25 used 30 mg/d in 272 patients with bipolar mania or mixed states. Compared with placebo, aripiprazole produced significant improvement by day 4, with similar discontinuation rates.
Recommendation. Aggressive initial dosing of aripiprazole could be useful for a patient who does not require an IM or rapidly dissolvable medication.
Table 4
Suggested response to metabolic changes during bipolar maintenance therapy
| Metabolic change | Therapeutic action |
|---|---|
| ≥5% increase in total body weight | Consider weight-reduction strategies or medication adjustment |
| Fasting glucose: ≥126 mg/mL ≥300 mg/mL or ≤60 mg/mL | Consider evaluation for diabetes mellitus Seek immediate consultation |
| Total cholesterol ≥200 mg/dL or triglycerides ≥165 mg/dL | Consider lipid-lowering with dietary and/or medication changes |
| Source: Adapted from reference 26. | |
MAINTENANCE THERAPY
Ideally, if a medication stabilizes a patient’s acute bipolar mania, that medication is continued for further stabilization and maintenance. Aggressive initial dosing befits this approach because it establishes a therapeutic blood level and usually reveals any side effects within days. Moreover, patients often prefer to continue the medication that provided relief when they felt most distressed.
Weight gain. Long-term use of atypical antipsychotics may be associated with weight gain, dyslipidemia, and the development of metabolic syndromes and diabetes mellitus. Weight gain risk may be further elevated in patients taking both antipsychotics and lithium or valproic acid.26 When atypical antipsychotics are used for bipolar maintenance therapy, the American Diabetes Association and American Psychiatric Association recommend close monitoring (Tables 3 and 4).
Abnormal movements. Though tardive dyskinesia risk is very low with atypical antipsychotics, we recommend screening during the first year of treatment. The development of diabetes mellitus may precipitate or worsen abnormal movements.
Related resources
- American Diabetes Association. www.diabetes.org
- American Obesity Association. www.obesity.org
- Expert Consensus Treatment Guidelines for Bipolar Disorder: A Guide for Patients and Families. Task Force for the APA Practice Guideline for the Treatment of Patients with Bipolar Disorder. www.psychguides.com/pfg3.php
Drug brand names
- Aripiprazole • Abilify
- Carbamazepine • Tegretol
- Divalproex sodium • Depakote
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Valproic acid • Depakene
- Ziprasidone • Geodon
Disclosures
Dr. Carroll is a consultant to Abbott Laboratories, Bristol-Myers Squibb Co., AstraZeneca Pharmaceuticals, Eli Lilly and Co., Janssen Pharmaceutica, and Pfizer Inc.
Dr. Fawver is a consultant to Eli Lilly and Co. and Pfizer Inc.
Dr. Thalassinos is a consultant for AstraZeneca Pharamaceuticals, Eli Lilly and Co., and Pfizer Inc.
Acknowledgment
The authors thank Donald R. Schmitt, PharmD, Christopher Thomas, PharmD, and Tina Fore, Library Service, Chillicothe VA Medical Center, for their help in identifying articles used in this review.
1. Keck PE, Jr, McElroy SL, Tugrul KC, et al. Valproate oral loading in the treatment of acute mania. J Clin Psychiatry. 1993;54:305-8.
2. Moscovich DG, Shapira B, Lerer B, et al. Rapid lithiumization in acute manic patients. Hum Psychopharmacol. 1992;7:343-5.
3. Kook KA, Stimmel GL, Wilkins JN, et al. Accuracy and safety of a priori lithium loading. J Clin Psychiatry. 1985;46:49-51.
4. Carroll BT, Thalassinos A, Fawver JD. Loading strategies in acute mania. CNS Spectrums. 2001;6:919-30.
5. Hirschfeld RMA, Allen MH, McEvoy J, et al. Safety and tolerability of oral loading divalproex sodium in acutely manic bipolar patients. J Clin Psychiatry. 1999;60:815-18.
6. Miller BP, et al. Oral loading of extended-release divalproex in acute mania (presentation). New Orleans: American Psychiatric Association annual meeting, 2001.
7. Thibault M, Blume WT, Saint-Hilaire JM, et al. Divalproex extended-release versus the original divalproex tablet: results of a randomized, crossover study of well-controlled epileptic patients with primary generalized seizures. Epilepsy Res. 2002;50:243-9.
8. Hirschfeld RMA, Baker JD, Wozniak P, et al. The safety and early efficacy of oral-loaded divalproex versus standard-titration divalproex, lithium, olanzapine, and placebo in the treatment of acute mania associated with bipolar disorder. J Clin Psychiatry. 2003;64:841-6.
9. Phrolov K, Applebaum J, Levine J, et al. Single-dose intravenous valproate in acute mania. J Clin Psychiatry. 2004;65:68-70.
10. Keck PE, Jr, McElroy SL, Bennet TA. Pharmacologic loading in the treatment of acute mania. Bipolar Disord. 2000;2:42-6.
11. Dunn RT, Frye MS, Tim KA, et al. The efficacy and use of anticonvulsants in mood disorders. Clin Neuropharmacol. 1998;21:215-35.
12. Lerer B, Moore N, Meyendor E, et al. Carbamazepine versus lithium in mania a double-blind study. J Clin Psychiatry. 1987;48:89-93.
13. Brown B. The use of generic mood stabilizers: carbamazepine (monograph). J Clin Psychiatry. 1997;15(4):11-17.
14. Karagianis JL, Dawe IC, Thakur A, et al. Rapid tranquilization with olanzapine in acute psychosis: a case series. J Clin Psychiatry. 2001;62(suppl 2):12-16.
15. Baker RW, Kinon BJ, Maguire GA, et al. Effectiveness of rapid initial dose escalation of up to 40 milligrams per day of oral olanzapine in acute agitation. J Clin Psychopharmacol. 2003;23(4):342-8.
16. Sachs GS, Grossman F, Ghaemi SN, et al. Combination of mood stabilizer with risperidone or haloperidol for the treatment of acute mania: a double-blind, placebo controlled comparison of efficacy and safety. Am J Psychiatry. 2002;159:1146-54.
17. Hirschfeld RMA, Keck PE, Jr, Karcher K, et al. Rapid antimanic effect of risperidone monotherapy: a 3-week multicenter, randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2004;161:1057-65.
18. Khanna S, Vieta E, Lyons B, et al. Risperidone monotherapy in acute bipolar mania (abstract P219). Pittsburgh: Fifth International Conference on Bipolar Disorder, 2003.
19. Jones MW, Huizar K, et al. Quetiapine monotherapy for acute mania associated with bipolar disorder (poster presentation). San Francisco: American Psychiatric Association annual meeting, 2003.
20. Sachs G, Mullen JA, Devine NA, Sweitzer DE. Quetiapine versus placebo as adjunct to mood stabilizer for the treatment of acute mania (abstract). Bipolar Disord. 2002;4(suppl 1):133.-
21. Smith MA, McCoy R, Hamer J, Brecher M. Optimal titration for quetiapine (poster presentation): Boca Raton, FL: National Clinical Drug Evaluation Unit annual meeting, 2002.
22. Keck PE, Jr, Versiani M, Potkin S, et al. Ziprasidone in the treatment of acute bipolar mania: a three-week, placebo-controlled, double-blind, randomized trial. Am J Psychiatry. 2003;160:741-8.
23. Holtzheimer PE, Neumaier JF. Treatment of acute mania. CNS Spectrums. 2003;8(12):917-28.
24. Keck PE, Jr, Marcus R, Tourkodimitris S, et al. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatry. 2003;160(9):1651-8.
25. Sachs G, Sanchez R, Marcus R, et al. Aripiprazole vs placebo with an acute manic or mixed episode. New York: American Psychiatric Association annual meeting, 2004.
26. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes. Diabetes Care. 2004;27:596-601.
Evidence on aggressive initial dosing of mood stabilizers and antipsychotics is changing the way acute bipolar mania is treated. To help you apply this information, we searched the literature and meeting abstracts for aggressive strategies tested to date. This article discusses how to:
- identify patients who may benefit from loading or aggressive initial dosing
- calculate mood stabilizer dosages
- dose each atypical antipsychotic
- manage potential antipsychotic side effects during maintenance therapy.
PATIENT SELECTION
Rapidly achieving therapeutic blood levels relieves patient suffering faster than standard titration. It quickly calms the hyperactivity, impulsivity, tension, hostility, and uncooperativeness that distress patients and increase the risk of harm to themselves and others.
The challenge of using higher dosages is to minimize side effects. Loading is not a one-size-fits-all approach, as antimanic drugs’ unique pharmacokinetic and pharmacotherapeutic properties influence how each agent is used.
An interesting body of literature advocates using mood stabilizers plus antipsychotics to treat mania, suggesting greater efficacy than with either agent alone.16 This strategy raises important questions, such as:
- Can two drugs be loaded simultaneously?
- Can patients taking mood stabilizers be treated with antipsychotic loading, and can those taking antipsychotics receive loading dosages of mood stabilizers?
- Would “double loading” improve bipolar mania treatment?
Answers to these questions are needed because of increased demands on clinicians to control hospital costs by rapidly and effectively treating patients with bipolar mania.
Loading doses cannot be standardized but are calculated by multiplying target steady-state plasma concentration by volume of distribution. We suggest aggressive initial schedules for divalproex sodium and atypical antipsychotics in this article with the understanding that practitioners will adjust them based on each patient’s tolerance and response.
Hospitalization. Patients with acute bipolar mania should be supervised closely in the hospital during loading or aggressive initial dosing. Monitor for cardiovascular changes, neurologic disturbances, sensorium changes, and response.
Precautions. Not all patients are candidates for aggressive initial dosing. Contraindications include age <18 or >65 years, pregnancy, breast-feeding, medical illness, and known sensitivity to the medication being given.
Higher-than-usual dosing increases the risk of excessive drug concentrations in sensitive individuals—such as those with a history of sensitivity to lower dosages of similar medications—and toxic levels of drugs with long half-lives can persist. When in doubt, consider giving a smaller amount of the loading dose early in the day, followed later by a larger amount.
DRUG SELECTION
Loading and aggressive initial dosing strategies for bipolar mania were first advanced for divalproex sodium.1 Investigators then examined loading strategies for lithium and carbamazepine, as well as the antipsychotics olanzapine, risperidone, quetiapine, ziprasidone, and aripiprazole, which are known to have antimanic properties.
Olanzapine, quetiapine, and risperidone are FDA-approved for short-term treatment of acute manic episodes associated with bipolar I disorder, and similar indications were being considered for aripiprazole and ziprasidone as this article went to press. We discuss evidence on loading and aggressive initial dosing strategies for each agent.
No studies have compared one loading strategy with another. Thus, when we choose drugs for loading, we consider what each patient needs, available formulations, tolerability, and efficacy for long-term stabilization and maintenance treatment.
LITHIUM
Lithium loading targets the therapeutic range (<1.4 mEq/L), without crossing the toxic threshold (>1.5 mEq/L). Lithium loading has shown antimanic effects, although using >30 mg/kg/d causes severe nausea and vomiting.
Moscovich et al2 reported a case series of 9 adults with acute mania who received lithium loading dosages of 4,050 mg/d. Patients tolerated lithium well at plasma drug levels of approximately 1.2 to 1.4 mEq/L. Their manic symptoms declined significantly within 4 to 5 days, as measured by Clinical Global Impression (CGI) severity of illness, Biegel-Murphy Mania State Rating Scale, and Brief Psychiatric Rating Scale scores.
Table 1
How to calculate divalproex loading for acute bipolar mania*
| Days 1 and 2 |
| Patient weight in pounds x 15 = dosage (mg/d) (Example: 150 lbs x 15 = 1,750 mg/d) |
| Days 3 to 10 |
| Patient weight in pounds x 10 = dosage (mg/d) (Example: 150 lbs. x 10 = 1,500 mg/d) |
| To avoid splitting tablets, make dosage divisible by 125 (round up for young adults, round down for older adults). Divide into bid or tid doses for improved tolerability. |
| Days 4 and 7 |
| Draw blood to monitor valproic acid levels and for other values such as liver function studies. |
| * For use with oral divalproex sodium (delayed-release or extended-release formulations). Do not use valproic acid preparations, as loading is unlikely to be well tolerated. |
| Source: Reference 5 |
Kook et al3 attempted a 30-mg/kg loading dose of slow-release lithium carbonate in 38 patients to evaluate the safety of achieving a therapeutic level in 12 hours. No patient experienced adverse effects during loading or in the 12 hours after loading was completed.
Patients who develop a manic episode while taking lithium pose a therapeutic dilemma. If they stop taking lithium—especially abruptly—manic symptoms can return in as few as 2 days. For these patients, consider increasing the usual dosage by 50% to 100% on the first day of mania treatment,4 then continue a dosage that maintains efficacy and achieves a therapeutic blood level.
To avoid GI upset, avoid any single dose of >1,500 mg; if a greater dosage is needed, divide it for improved tolerance. Obtain lithium blood levels at baseline, after 4 days, and thereafter as clinically relevant to monitor for drug interactions that may affect serum levels.
Side effects—GI irritation, tremor, muscle weakness, thirst, polyuria—are common in the first week, and weight gain may occur after prolonged treatment.
DIVALPROEX
In a study by Keck et al1 of 19 adults with acute bipolar mania, aggressive initial dosing of divalproex sodium, 20 mg/kg/d, was well-tolerated and shortened hospitalization. Side effects including sedation and nausea occurred at a rate similar to that seen with more-gradual titration.
To calculate the initial dose, the authors used a conversion factor of 20 mg/kg/d or added a zero to the patient’s weight in pounds. This amount was given in single or divided doses the first day, then continued for 4 to 7 days. Blood levels were measured on day 4, and all 15 patients who completed the study achieved serum valproate levels >50 mcg/mL.
In a double-blind study by Hirschfeld et al,5 59 adults with Young Mania Rating Scale (YMRS) scores >14 were randomly assigned to receive divalproex oral loading; divalproex nonloading (250 mg tid on days 1 and 2, followed by standard titration on days 3 to 10); or lithium carbonate (300 mg tid, followed by standard titration on days 3 to 10).
Divalproex loading—30 mg/kg/d on days 1 and 2, followed by 20 mg/kg on days 3 to 10— yielded valproate levels >50 mcg/mL in 84% of patients by day 3. Rapid loading appeared to increase antimanic efficacy, as measured by YMRS endpoint scores, without increasing adverse effects. For Table 1, we converted this study’s metric values to patient weight in pounds.
Side effects. Divalproex can inhibit metabolism of other drugs (including anticonvulsants such as lamotrigine) and increase their blood levels. Side effects such as sedation, alopecia, abdominal pain, diarrhea, and tremor may require observation and treatment. Pancreatitis, hepatitis, and allergic reactions are rare but may require discontinuation.
Recommendation. Aggressive initial dosing and loading for patients with acute mania has been reported with enteric-coated delayed-release and extended-release6 divalproex sodium tablets. With identical dosages, the extended-release form produces 11% lower serum levels than the delayed-release form.7
Aggressive dosing of oral valproic acid preparations is not recommended and is likely to be poorly tolerated.8 A pilot study evaluating IV valproate loading in acute mania found no changes in mania in the 2 hours that followed loading.9
CARBAMAZEPINE
Carbamazepine is used off-label to treat mania and mixed phases of bipolar disorder.4,9,10 Excessive absorption rates are associated with dizziness, ataxia, and nausea. Side effects may occur when the plasma level is therapeutic for epilepsy (4 to 12 mcg/mL).11
In mania, the relationship between carbamazepine levels and clinical response is not always clear. Lerer et al12 found a correlation between acute mania response and a serum level of 8.8 mcg/mL (range 4.7 to 14.0 mcg/mL).
Patients with acute mania may require 600 to 1,600 mg/d. Carbamazepine is available in immediate-release and controlled-release preparations. Because generic preparations might not be bio-equivalent,13 use the same formulation throughout treatment to maintain consistent serum levels.
Enzymatic auto-induction—in which carbamazepine gradually increases the activity of its metabolic enzyme—is likely to occur at 3 to 5 weeks of administration, often after hospital discharge. An aggressive initial rescue adjustment can be used if a patient develops mania after having been stabilized on carbamazepine for >6 weeks. Because these patients are past the point of auto-induction, a target blood level of 8.8 mcg/mL can be used and the dosage adjusted proportionally.
For example, if mania recurs in a patient who is stabilized on carbamazepine, 400 mg/d (plasma level 4.5 mcg/mL), carbamazepine can be loaded up to 800 mg/d. Measure the plasma level within 4 days; the target level would be 9.0 mcg/mL (2 times 4.5 mcg/mL), which closely approximates steady state and sets up a ratio to reach the target level of 8.8 mcg/mL.
Side effects include ataxia, diplopia, and dizziness. Complete blood counts, liver function studies, plasma levels, and serum chemistries require regular monitoring.
Recommendation. Carbamazepine is not recommended for oral loading in patients who have never taken it or for those with hyponatremia, hepatic dysfunction, or history of intolerance or agranulocytosis.
OLANZAPINE
Olanzapine has pharmacologic properties favorable for loading.4 The recommended dosage for acute mania is 15 mg/d with standard titration; Karagianis et al14 showed that initial loading doses of >20 mg/d resulted in good control of agitated patients with psychosis. Side effects were uncommon, with sedation occurring in 14% of patients in this case series. The loading dose reduced agitation more effectively than did dosages <20 mg/d given to similar patients.
A multicenter study of 148 acutely agitated inpatients with a variety of psychiatric disorders compared olanzapine rapid initial dose escalation with usual clinical practice.15 Mean aggressive dosages were 28.8 mg/d on day 1, 30.3 mg/d on day 2, and 16.1 mg/d on day 5. Usual-practice dosages were 10 mg/d, plus lorazepam, 0 to 4 mg/d for the first 2 days and 0 to 2 mg/d on days 3 to 4. Based on Positive and Negative Syndrome Scale excited component subscale scores, olanzapine loading controlled agitation more effectively than did usual practice, with similar side-effect rates.
IM olanzapine or the orally dissolving form are bioequivalent to the tablets and may be used for acute agitation associated with bipolar mania in certain clinical settings.14
Table 2
Suggested antipsychotic loading for acute bipolar mania and mixed states*
| Drug | Day(s) | Aggressive initial dosing schedule | Comment |
|---|---|---|---|
| Aripiprazole24,25 | 1 2 to 3 4+ | 30 mg once daily with food 30 mg/d with food Reduce dosage by 10 to 15 mg/d, based on tolerance and response | Nausea and vomiting may occur in first few days; adjust dosage based on tolerance and response |
| Olanzapine14,15 | 1 and 2 3 and 4 5 to 10 | 40 mg in single or divided dosage 20 to 30 mg at bedtime 15 mg once daily (may reduce to 5, 7.5, or 10 mg/d) | Adjust dosage based on tolerance and response; oral or IM formulations may be used |
| Quetiapine19,21 | 1 2 and 3 4 to 10 | 100 mg upon admission and 100 mg at bedtime 100 mg bid (or tid to qid) plus 100 to 200 mg at bedtime 200 mg bid plus 200 to 300 mg at bedtime; may adjust to 400 to 800 mg/d) | Adjust dosage based on tolerance and response |
| Risperidone16,18 | 1 2 3 and 4 | 3 mg in single or divided dosage 4 mg in single or divided dosage 5 mg in single or divided dosage | Adjust dosage by 1 mg up or down, based on tolerance and response; use tablet, rapid-dissolving tablet, or liquid form, but not long-acting IM form |
| Ziprasidone22,23 | 1 2 | 20 mg IM in single dose (may repeat for severe agitation) or 40 mg po bid with food 60 to 80 mg po bid with food | Adjust dosage based on tolerance and response |
| * For hospitalized or partially hospitalized patients, ages18. Not recommended for patients who are pregnant, breastfeeding, medically ill, age >65, or with known sensitivity to the antipsychotic being given. | |||
RISPERIDONE
Sachs et al16 studied 156 inpatients who developed an acute manic or mixed episode while receiving lithium or divalproex. These patients were randomly assigned to begin adjunctive risperidone, 2 mg/d, haloperidol, or placebo. Dosing was flexible, increasing or decreasing by 1 mg/d. Risperidone’s mean modal dosage was 3.8 mg/d across 3 weeks, with mean exposure of 17 days. Risperidone plus a mood stabilizer was more effective than a mood stabilizer alone, and the combination provided rapid, well-tolerated control of manic symptoms.
In a double-blind trial, Hirschfeld et al17 randomly assigned 279 patients with acute bipolar mania to risperidone, 1 to 6 mg/d, or placebo for 3 weeks. As early as day 3, YMRS scores were reduced significantly more with risperidone than with placebo. Somnolence was the most common side effect, and mean modal dosage was 4.1 mg/d.
Table 3
Screening schedule for antipsychotic side effects during bipolar maintenance treatment
| Baseline | |
| Side effect | Recommended screening |
| Weight gain | Weight and body mass index (BMI) monthly for first 3 months; waist circumference |
| Hypertension | Blood pressure |
| Hyperglycemia | Fasting plasma glucose, with glycosylated hemoglobin (Hb A 1c ) if hyperglycemia is detected |
| Hyperlipidemia | Fasting lipid profile |
| Tardive dyskinesia | Abnormal Involuntary Movement Scale (AIMS) or other screen |
| Ophthalmic changes | Ophthalmologic examination for patients taking quetiapine and for all with diabetes mellitus |
| Follow-up schedules | |
| 3 months | |
| Weight and BMI | |
| Blood pressure | |
| Fasting plasma glucose, with Hb A 1c if hyperglycemia is detected; Hb A 1c values may be used to measure interval changes in glucose tolerance | |
| Fasting lipid profile | |
| 6 months | |
| AIMS or other tardive dyskinesia screen | |
| Ophthalmologic examination | |
| Source: Adapted from reference 26. | |
In a multicenter, randomized, double-blind, placebo-controlled study of patients with acute bipolar mania, Khanna et al18 assigned patients to receive risperidone monotherapy (mean modal dosage 5.6 mg/d) or placebo for 3 weeks. Mania scores of patients receiving risperidone were significantly lower at weeks 1 and 2, compared with the placebo group. Risperidone was well-tolerated, with no unexpected adverse events.
Recommendation. Because of a risk of extrapyramidal symptoms (EPS) and orthostatic hypotension, initial risperidone loading dosages >4 mg on day 1 are not recommended.
QUETIAPINE
Quetiapine has shown antimanic efficacy as monotherapy and as adjunctive therapy to mood stabilizers.19,20 The effective dosage was a mean 600 mg/d (range 400 to 800 mg/d) in monotherapy and adjunctive treatment. These studies achieved 400 mg/d within the first 4 days (100 mg on day 1, 200 mg on day 2, 300 mg on day 3, and 400 mg on day 4).
These data combined with revised prescribing information suggest aggressive initial dose escalation of quetiapine within the first 4 days for selected patients. A titration study in patients with schizophrenia used a more-rapid escalation rate of 400 mg within 2 days.21 Dizziness, orthostatic hypotension, and sedation were not more frequent in this high-dose group than in the two lower-dose titration groups. In our experience, 200 to 400 mg can be given the first day of treatment.
ZIPRASIDONE
In a randomized, double-blind, controlled trial, Keck et al22 assigned 210 patients with a manic or mixed bipolar episode to 3 weeks of ziprasidone, 40 to 80 mg bid, or placebo. Ziprasidone produced rapid, sustained improvement in manic symptoms on all primary and most secondary efficacy measures, such as the YMRS and CGI.
Significant improvements seen within 2 days were maintained. Ziprasidone was well tolerated and was associated with a low EPS rate; neither weight gain nor clinically significant changes in vital signs were seen.
IM ziprasidone, which is approved for use in schizophrenia, may also have efficacy in bipolar mania.23 The recommended dose of 20 mg IM is equivalent to 120 to 160 mg orally, so a single injection may reach the target antimanic dosage.
Recommendation. Ziprasidone could be an option for aggressive initial dosing for a patient who has previously received ziprasidone IM and is not at risk for QTc prolongation.
ARIPIPRAZOLE
In a randomized controlled trial, Keck et al24 assigned 262 patients with acute bipolar mania or mixed states to aripiprazole, 30 mg/d, or placebo for 3 weeks. By day 4, manic symptoms were improved significantly more in patients receiving aripiprazole, and discontinuation rates were similar.
Similarly, in a randomized, controlled multi-center study, Sachs et al25 used 30 mg/d in 272 patients with bipolar mania or mixed states. Compared with placebo, aripiprazole produced significant improvement by day 4, with similar discontinuation rates.
Recommendation. Aggressive initial dosing of aripiprazole could be useful for a patient who does not require an IM or rapidly dissolvable medication.
Table 4
Suggested response to metabolic changes during bipolar maintenance therapy
| Metabolic change | Therapeutic action |
|---|---|
| ≥5% increase in total body weight | Consider weight-reduction strategies or medication adjustment |
| Fasting glucose: ≥126 mg/mL ≥300 mg/mL or ≤60 mg/mL | Consider evaluation for diabetes mellitus Seek immediate consultation |
| Total cholesterol ≥200 mg/dL or triglycerides ≥165 mg/dL | Consider lipid-lowering with dietary and/or medication changes |
| Source: Adapted from reference 26. | |
MAINTENANCE THERAPY
Ideally, if a medication stabilizes a patient’s acute bipolar mania, that medication is continued for further stabilization and maintenance. Aggressive initial dosing befits this approach because it establishes a therapeutic blood level and usually reveals any side effects within days. Moreover, patients often prefer to continue the medication that provided relief when they felt most distressed.
Weight gain. Long-term use of atypical antipsychotics may be associated with weight gain, dyslipidemia, and the development of metabolic syndromes and diabetes mellitus. Weight gain risk may be further elevated in patients taking both antipsychotics and lithium or valproic acid.26 When atypical antipsychotics are used for bipolar maintenance therapy, the American Diabetes Association and American Psychiatric Association recommend close monitoring (Tables 3 and 4).
Abnormal movements. Though tardive dyskinesia risk is very low with atypical antipsychotics, we recommend screening during the first year of treatment. The development of diabetes mellitus may precipitate or worsen abnormal movements.
Related resources
- American Diabetes Association. www.diabetes.org
- American Obesity Association. www.obesity.org
- Expert Consensus Treatment Guidelines for Bipolar Disorder: A Guide for Patients and Families. Task Force for the APA Practice Guideline for the Treatment of Patients with Bipolar Disorder. www.psychguides.com/pfg3.php
Drug brand names
- Aripiprazole • Abilify
- Carbamazepine • Tegretol
- Divalproex sodium • Depakote
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Valproic acid • Depakene
- Ziprasidone • Geodon
Disclosures
Dr. Carroll is a consultant to Abbott Laboratories, Bristol-Myers Squibb Co., AstraZeneca Pharmaceuticals, Eli Lilly and Co., Janssen Pharmaceutica, and Pfizer Inc.
Dr. Fawver is a consultant to Eli Lilly and Co. and Pfizer Inc.
Dr. Thalassinos is a consultant for AstraZeneca Pharamaceuticals, Eli Lilly and Co., and Pfizer Inc.
Acknowledgment
The authors thank Donald R. Schmitt, PharmD, Christopher Thomas, PharmD, and Tina Fore, Library Service, Chillicothe VA Medical Center, for their help in identifying articles used in this review.
Evidence on aggressive initial dosing of mood stabilizers and antipsychotics is changing the way acute bipolar mania is treated. To help you apply this information, we searched the literature and meeting abstracts for aggressive strategies tested to date. This article discusses how to:
- identify patients who may benefit from loading or aggressive initial dosing
- calculate mood stabilizer dosages
- dose each atypical antipsychotic
- manage potential antipsychotic side effects during maintenance therapy.
PATIENT SELECTION
Rapidly achieving therapeutic blood levels relieves patient suffering faster than standard titration. It quickly calms the hyperactivity, impulsivity, tension, hostility, and uncooperativeness that distress patients and increase the risk of harm to themselves and others.
The challenge of using higher dosages is to minimize side effects. Loading is not a one-size-fits-all approach, as antimanic drugs’ unique pharmacokinetic and pharmacotherapeutic properties influence how each agent is used.
An interesting body of literature advocates using mood stabilizers plus antipsychotics to treat mania, suggesting greater efficacy than with either agent alone.16 This strategy raises important questions, such as:
- Can two drugs be loaded simultaneously?
- Can patients taking mood stabilizers be treated with antipsychotic loading, and can those taking antipsychotics receive loading dosages of mood stabilizers?
- Would “double loading” improve bipolar mania treatment?
Answers to these questions are needed because of increased demands on clinicians to control hospital costs by rapidly and effectively treating patients with bipolar mania.
Loading doses cannot be standardized but are calculated by multiplying target steady-state plasma concentration by volume of distribution. We suggest aggressive initial schedules for divalproex sodium and atypical antipsychotics in this article with the understanding that practitioners will adjust them based on each patient’s tolerance and response.
Hospitalization. Patients with acute bipolar mania should be supervised closely in the hospital during loading or aggressive initial dosing. Monitor for cardiovascular changes, neurologic disturbances, sensorium changes, and response.
Precautions. Not all patients are candidates for aggressive initial dosing. Contraindications include age <18 or >65 years, pregnancy, breast-feeding, medical illness, and known sensitivity to the medication being given.
Higher-than-usual dosing increases the risk of excessive drug concentrations in sensitive individuals—such as those with a history of sensitivity to lower dosages of similar medications—and toxic levels of drugs with long half-lives can persist. When in doubt, consider giving a smaller amount of the loading dose early in the day, followed later by a larger amount.
DRUG SELECTION
Loading and aggressive initial dosing strategies for bipolar mania were first advanced for divalproex sodium.1 Investigators then examined loading strategies for lithium and carbamazepine, as well as the antipsychotics olanzapine, risperidone, quetiapine, ziprasidone, and aripiprazole, which are known to have antimanic properties.
Olanzapine, quetiapine, and risperidone are FDA-approved for short-term treatment of acute manic episodes associated with bipolar I disorder, and similar indications were being considered for aripiprazole and ziprasidone as this article went to press. We discuss evidence on loading and aggressive initial dosing strategies for each agent.
No studies have compared one loading strategy with another. Thus, when we choose drugs for loading, we consider what each patient needs, available formulations, tolerability, and efficacy for long-term stabilization and maintenance treatment.
LITHIUM
Lithium loading targets the therapeutic range (<1.4 mEq/L), without crossing the toxic threshold (>1.5 mEq/L). Lithium loading has shown antimanic effects, although using >30 mg/kg/d causes severe nausea and vomiting.
Moscovich et al2 reported a case series of 9 adults with acute mania who received lithium loading dosages of 4,050 mg/d. Patients tolerated lithium well at plasma drug levels of approximately 1.2 to 1.4 mEq/L. Their manic symptoms declined significantly within 4 to 5 days, as measured by Clinical Global Impression (CGI) severity of illness, Biegel-Murphy Mania State Rating Scale, and Brief Psychiatric Rating Scale scores.
Table 1
How to calculate divalproex loading for acute bipolar mania*
| Days 1 and 2 |
| Patient weight in pounds x 15 = dosage (mg/d) (Example: 150 lbs x 15 = 1,750 mg/d) |
| Days 3 to 10 |
| Patient weight in pounds x 10 = dosage (mg/d) (Example: 150 lbs. x 10 = 1,500 mg/d) |
| To avoid splitting tablets, make dosage divisible by 125 (round up for young adults, round down for older adults). Divide into bid or tid doses for improved tolerability. |
| Days 4 and 7 |
| Draw blood to monitor valproic acid levels and for other values such as liver function studies. |
| * For use with oral divalproex sodium (delayed-release or extended-release formulations). Do not use valproic acid preparations, as loading is unlikely to be well tolerated. |
| Source: Reference 5 |
Kook et al3 attempted a 30-mg/kg loading dose of slow-release lithium carbonate in 38 patients to evaluate the safety of achieving a therapeutic level in 12 hours. No patient experienced adverse effects during loading or in the 12 hours after loading was completed.
Patients who develop a manic episode while taking lithium pose a therapeutic dilemma. If they stop taking lithium—especially abruptly—manic symptoms can return in as few as 2 days. For these patients, consider increasing the usual dosage by 50% to 100% on the first day of mania treatment,4 then continue a dosage that maintains efficacy and achieves a therapeutic blood level.
To avoid GI upset, avoid any single dose of >1,500 mg; if a greater dosage is needed, divide it for improved tolerance. Obtain lithium blood levels at baseline, after 4 days, and thereafter as clinically relevant to monitor for drug interactions that may affect serum levels.
Side effects—GI irritation, tremor, muscle weakness, thirst, polyuria—are common in the first week, and weight gain may occur after prolonged treatment.
DIVALPROEX
In a study by Keck et al1 of 19 adults with acute bipolar mania, aggressive initial dosing of divalproex sodium, 20 mg/kg/d, was well-tolerated and shortened hospitalization. Side effects including sedation and nausea occurred at a rate similar to that seen with more-gradual titration.
To calculate the initial dose, the authors used a conversion factor of 20 mg/kg/d or added a zero to the patient’s weight in pounds. This amount was given in single or divided doses the first day, then continued for 4 to 7 days. Blood levels were measured on day 4, and all 15 patients who completed the study achieved serum valproate levels >50 mcg/mL.
In a double-blind study by Hirschfeld et al,5 59 adults with Young Mania Rating Scale (YMRS) scores >14 were randomly assigned to receive divalproex oral loading; divalproex nonloading (250 mg tid on days 1 and 2, followed by standard titration on days 3 to 10); or lithium carbonate (300 mg tid, followed by standard titration on days 3 to 10).
Divalproex loading—30 mg/kg/d on days 1 and 2, followed by 20 mg/kg on days 3 to 10— yielded valproate levels >50 mcg/mL in 84% of patients by day 3. Rapid loading appeared to increase antimanic efficacy, as measured by YMRS endpoint scores, without increasing adverse effects. For Table 1, we converted this study’s metric values to patient weight in pounds.
Side effects. Divalproex can inhibit metabolism of other drugs (including anticonvulsants such as lamotrigine) and increase their blood levels. Side effects such as sedation, alopecia, abdominal pain, diarrhea, and tremor may require observation and treatment. Pancreatitis, hepatitis, and allergic reactions are rare but may require discontinuation.
Recommendation. Aggressive initial dosing and loading for patients with acute mania has been reported with enteric-coated delayed-release and extended-release6 divalproex sodium tablets. With identical dosages, the extended-release form produces 11% lower serum levels than the delayed-release form.7
Aggressive dosing of oral valproic acid preparations is not recommended and is likely to be poorly tolerated.8 A pilot study evaluating IV valproate loading in acute mania found no changes in mania in the 2 hours that followed loading.9
CARBAMAZEPINE
Carbamazepine is used off-label to treat mania and mixed phases of bipolar disorder.4,9,10 Excessive absorption rates are associated with dizziness, ataxia, and nausea. Side effects may occur when the plasma level is therapeutic for epilepsy (4 to 12 mcg/mL).11
In mania, the relationship between carbamazepine levels and clinical response is not always clear. Lerer et al12 found a correlation between acute mania response and a serum level of 8.8 mcg/mL (range 4.7 to 14.0 mcg/mL).
Patients with acute mania may require 600 to 1,600 mg/d. Carbamazepine is available in immediate-release and controlled-release preparations. Because generic preparations might not be bio-equivalent,13 use the same formulation throughout treatment to maintain consistent serum levels.
Enzymatic auto-induction—in which carbamazepine gradually increases the activity of its metabolic enzyme—is likely to occur at 3 to 5 weeks of administration, often after hospital discharge. An aggressive initial rescue adjustment can be used if a patient develops mania after having been stabilized on carbamazepine for >6 weeks. Because these patients are past the point of auto-induction, a target blood level of 8.8 mcg/mL can be used and the dosage adjusted proportionally.
For example, if mania recurs in a patient who is stabilized on carbamazepine, 400 mg/d (plasma level 4.5 mcg/mL), carbamazepine can be loaded up to 800 mg/d. Measure the plasma level within 4 days; the target level would be 9.0 mcg/mL (2 times 4.5 mcg/mL), which closely approximates steady state and sets up a ratio to reach the target level of 8.8 mcg/mL.
Side effects include ataxia, diplopia, and dizziness. Complete blood counts, liver function studies, plasma levels, and serum chemistries require regular monitoring.
Recommendation. Carbamazepine is not recommended for oral loading in patients who have never taken it or for those with hyponatremia, hepatic dysfunction, or history of intolerance or agranulocytosis.
OLANZAPINE
Olanzapine has pharmacologic properties favorable for loading.4 The recommended dosage for acute mania is 15 mg/d with standard titration; Karagianis et al14 showed that initial loading doses of >20 mg/d resulted in good control of agitated patients with psychosis. Side effects were uncommon, with sedation occurring in 14% of patients in this case series. The loading dose reduced agitation more effectively than did dosages <20 mg/d given to similar patients.
A multicenter study of 148 acutely agitated inpatients with a variety of psychiatric disorders compared olanzapine rapid initial dose escalation with usual clinical practice.15 Mean aggressive dosages were 28.8 mg/d on day 1, 30.3 mg/d on day 2, and 16.1 mg/d on day 5. Usual-practice dosages were 10 mg/d, plus lorazepam, 0 to 4 mg/d for the first 2 days and 0 to 2 mg/d on days 3 to 4. Based on Positive and Negative Syndrome Scale excited component subscale scores, olanzapine loading controlled agitation more effectively than did usual practice, with similar side-effect rates.
IM olanzapine or the orally dissolving form are bioequivalent to the tablets and may be used for acute agitation associated with bipolar mania in certain clinical settings.14
Table 2
Suggested antipsychotic loading for acute bipolar mania and mixed states*
| Drug | Day(s) | Aggressive initial dosing schedule | Comment |
|---|---|---|---|
| Aripiprazole24,25 | 1 2 to 3 4+ | 30 mg once daily with food 30 mg/d with food Reduce dosage by 10 to 15 mg/d, based on tolerance and response | Nausea and vomiting may occur in first few days; adjust dosage based on tolerance and response |
| Olanzapine14,15 | 1 and 2 3 and 4 5 to 10 | 40 mg in single or divided dosage 20 to 30 mg at bedtime 15 mg once daily (may reduce to 5, 7.5, or 10 mg/d) | Adjust dosage based on tolerance and response; oral or IM formulations may be used |
| Quetiapine19,21 | 1 2 and 3 4 to 10 | 100 mg upon admission and 100 mg at bedtime 100 mg bid (or tid to qid) plus 100 to 200 mg at bedtime 200 mg bid plus 200 to 300 mg at bedtime; may adjust to 400 to 800 mg/d) | Adjust dosage based on tolerance and response |
| Risperidone16,18 | 1 2 3 and 4 | 3 mg in single or divided dosage 4 mg in single or divided dosage 5 mg in single or divided dosage | Adjust dosage by 1 mg up or down, based on tolerance and response; use tablet, rapid-dissolving tablet, or liquid form, but not long-acting IM form |
| Ziprasidone22,23 | 1 2 | 20 mg IM in single dose (may repeat for severe agitation) or 40 mg po bid with food 60 to 80 mg po bid with food | Adjust dosage based on tolerance and response |
| * For hospitalized or partially hospitalized patients, ages18. Not recommended for patients who are pregnant, breastfeeding, medically ill, age >65, or with known sensitivity to the antipsychotic being given. | |||
RISPERIDONE
Sachs et al16 studied 156 inpatients who developed an acute manic or mixed episode while receiving lithium or divalproex. These patients were randomly assigned to begin adjunctive risperidone, 2 mg/d, haloperidol, or placebo. Dosing was flexible, increasing or decreasing by 1 mg/d. Risperidone’s mean modal dosage was 3.8 mg/d across 3 weeks, with mean exposure of 17 days. Risperidone plus a mood stabilizer was more effective than a mood stabilizer alone, and the combination provided rapid, well-tolerated control of manic symptoms.
In a double-blind trial, Hirschfeld et al17 randomly assigned 279 patients with acute bipolar mania to risperidone, 1 to 6 mg/d, or placebo for 3 weeks. As early as day 3, YMRS scores were reduced significantly more with risperidone than with placebo. Somnolence was the most common side effect, and mean modal dosage was 4.1 mg/d.
Table 3
Screening schedule for antipsychotic side effects during bipolar maintenance treatment
| Baseline | |
| Side effect | Recommended screening |
| Weight gain | Weight and body mass index (BMI) monthly for first 3 months; waist circumference |
| Hypertension | Blood pressure |
| Hyperglycemia | Fasting plasma glucose, with glycosylated hemoglobin (Hb A 1c ) if hyperglycemia is detected |
| Hyperlipidemia | Fasting lipid profile |
| Tardive dyskinesia | Abnormal Involuntary Movement Scale (AIMS) or other screen |
| Ophthalmic changes | Ophthalmologic examination for patients taking quetiapine and for all with diabetes mellitus |
| Follow-up schedules | |
| 3 months | |
| Weight and BMI | |
| Blood pressure | |
| Fasting plasma glucose, with Hb A 1c if hyperglycemia is detected; Hb A 1c values may be used to measure interval changes in glucose tolerance | |
| Fasting lipid profile | |
| 6 months | |
| AIMS or other tardive dyskinesia screen | |
| Ophthalmologic examination | |
| Source: Adapted from reference 26. | |
In a multicenter, randomized, double-blind, placebo-controlled study of patients with acute bipolar mania, Khanna et al18 assigned patients to receive risperidone monotherapy (mean modal dosage 5.6 mg/d) or placebo for 3 weeks. Mania scores of patients receiving risperidone were significantly lower at weeks 1 and 2, compared with the placebo group. Risperidone was well-tolerated, with no unexpected adverse events.
Recommendation. Because of a risk of extrapyramidal symptoms (EPS) and orthostatic hypotension, initial risperidone loading dosages >4 mg on day 1 are not recommended.
QUETIAPINE
Quetiapine has shown antimanic efficacy as monotherapy and as adjunctive therapy to mood stabilizers.19,20 The effective dosage was a mean 600 mg/d (range 400 to 800 mg/d) in monotherapy and adjunctive treatment. These studies achieved 400 mg/d within the first 4 days (100 mg on day 1, 200 mg on day 2, 300 mg on day 3, and 400 mg on day 4).
These data combined with revised prescribing information suggest aggressive initial dose escalation of quetiapine within the first 4 days for selected patients. A titration study in patients with schizophrenia used a more-rapid escalation rate of 400 mg within 2 days.21 Dizziness, orthostatic hypotension, and sedation were not more frequent in this high-dose group than in the two lower-dose titration groups. In our experience, 200 to 400 mg can be given the first day of treatment.
ZIPRASIDONE
In a randomized, double-blind, controlled trial, Keck et al22 assigned 210 patients with a manic or mixed bipolar episode to 3 weeks of ziprasidone, 40 to 80 mg bid, or placebo. Ziprasidone produced rapid, sustained improvement in manic symptoms on all primary and most secondary efficacy measures, such as the YMRS and CGI.
Significant improvements seen within 2 days were maintained. Ziprasidone was well tolerated and was associated with a low EPS rate; neither weight gain nor clinically significant changes in vital signs were seen.
IM ziprasidone, which is approved for use in schizophrenia, may also have efficacy in bipolar mania.23 The recommended dose of 20 mg IM is equivalent to 120 to 160 mg orally, so a single injection may reach the target antimanic dosage.
Recommendation. Ziprasidone could be an option for aggressive initial dosing for a patient who has previously received ziprasidone IM and is not at risk for QTc prolongation.
ARIPIPRAZOLE
In a randomized controlled trial, Keck et al24 assigned 262 patients with acute bipolar mania or mixed states to aripiprazole, 30 mg/d, or placebo for 3 weeks. By day 4, manic symptoms were improved significantly more in patients receiving aripiprazole, and discontinuation rates were similar.
Similarly, in a randomized, controlled multi-center study, Sachs et al25 used 30 mg/d in 272 patients with bipolar mania or mixed states. Compared with placebo, aripiprazole produced significant improvement by day 4, with similar discontinuation rates.
Recommendation. Aggressive initial dosing of aripiprazole could be useful for a patient who does not require an IM or rapidly dissolvable medication.
Table 4
Suggested response to metabolic changes during bipolar maintenance therapy
| Metabolic change | Therapeutic action |
|---|---|
| ≥5% increase in total body weight | Consider weight-reduction strategies or medication adjustment |
| Fasting glucose: ≥126 mg/mL ≥300 mg/mL or ≤60 mg/mL | Consider evaluation for diabetes mellitus Seek immediate consultation |
| Total cholesterol ≥200 mg/dL or triglycerides ≥165 mg/dL | Consider lipid-lowering with dietary and/or medication changes |
| Source: Adapted from reference 26. | |
MAINTENANCE THERAPY
Ideally, if a medication stabilizes a patient’s acute bipolar mania, that medication is continued for further stabilization and maintenance. Aggressive initial dosing befits this approach because it establishes a therapeutic blood level and usually reveals any side effects within days. Moreover, patients often prefer to continue the medication that provided relief when they felt most distressed.
Weight gain. Long-term use of atypical antipsychotics may be associated with weight gain, dyslipidemia, and the development of metabolic syndromes and diabetes mellitus. Weight gain risk may be further elevated in patients taking both antipsychotics and lithium or valproic acid.26 When atypical antipsychotics are used for bipolar maintenance therapy, the American Diabetes Association and American Psychiatric Association recommend close monitoring (Tables 3 and 4).
Abnormal movements. Though tardive dyskinesia risk is very low with atypical antipsychotics, we recommend screening during the first year of treatment. The development of diabetes mellitus may precipitate or worsen abnormal movements.
Related resources
- American Diabetes Association. www.diabetes.org
- American Obesity Association. www.obesity.org
- Expert Consensus Treatment Guidelines for Bipolar Disorder: A Guide for Patients and Families. Task Force for the APA Practice Guideline for the Treatment of Patients with Bipolar Disorder. www.psychguides.com/pfg3.php
Drug brand names
- Aripiprazole • Abilify
- Carbamazepine • Tegretol
- Divalproex sodium • Depakote
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Valproic acid • Depakene
- Ziprasidone • Geodon
Disclosures
Dr. Carroll is a consultant to Abbott Laboratories, Bristol-Myers Squibb Co., AstraZeneca Pharmaceuticals, Eli Lilly and Co., Janssen Pharmaceutica, and Pfizer Inc.
Dr. Fawver is a consultant to Eli Lilly and Co. and Pfizer Inc.
Dr. Thalassinos is a consultant for AstraZeneca Pharamaceuticals, Eli Lilly and Co., and Pfizer Inc.
Acknowledgment
The authors thank Donald R. Schmitt, PharmD, Christopher Thomas, PharmD, and Tina Fore, Library Service, Chillicothe VA Medical Center, for their help in identifying articles used in this review.
1. Keck PE, Jr, McElroy SL, Tugrul KC, et al. Valproate oral loading in the treatment of acute mania. J Clin Psychiatry. 1993;54:305-8.
2. Moscovich DG, Shapira B, Lerer B, et al. Rapid lithiumization in acute manic patients. Hum Psychopharmacol. 1992;7:343-5.
3. Kook KA, Stimmel GL, Wilkins JN, et al. Accuracy and safety of a priori lithium loading. J Clin Psychiatry. 1985;46:49-51.
4. Carroll BT, Thalassinos A, Fawver JD. Loading strategies in acute mania. CNS Spectrums. 2001;6:919-30.
5. Hirschfeld RMA, Allen MH, McEvoy J, et al. Safety and tolerability of oral loading divalproex sodium in acutely manic bipolar patients. J Clin Psychiatry. 1999;60:815-18.
6. Miller BP, et al. Oral loading of extended-release divalproex in acute mania (presentation). New Orleans: American Psychiatric Association annual meeting, 2001.
7. Thibault M, Blume WT, Saint-Hilaire JM, et al. Divalproex extended-release versus the original divalproex tablet: results of a randomized, crossover study of well-controlled epileptic patients with primary generalized seizures. Epilepsy Res. 2002;50:243-9.
8. Hirschfeld RMA, Baker JD, Wozniak P, et al. The safety and early efficacy of oral-loaded divalproex versus standard-titration divalproex, lithium, olanzapine, and placebo in the treatment of acute mania associated with bipolar disorder. J Clin Psychiatry. 2003;64:841-6.
9. Phrolov K, Applebaum J, Levine J, et al. Single-dose intravenous valproate in acute mania. J Clin Psychiatry. 2004;65:68-70.
10. Keck PE, Jr, McElroy SL, Bennet TA. Pharmacologic loading in the treatment of acute mania. Bipolar Disord. 2000;2:42-6.
11. Dunn RT, Frye MS, Tim KA, et al. The efficacy and use of anticonvulsants in mood disorders. Clin Neuropharmacol. 1998;21:215-35.
12. Lerer B, Moore N, Meyendor E, et al. Carbamazepine versus lithium in mania a double-blind study. J Clin Psychiatry. 1987;48:89-93.
13. Brown B. The use of generic mood stabilizers: carbamazepine (monograph). J Clin Psychiatry. 1997;15(4):11-17.
14. Karagianis JL, Dawe IC, Thakur A, et al. Rapid tranquilization with olanzapine in acute psychosis: a case series. J Clin Psychiatry. 2001;62(suppl 2):12-16.
15. Baker RW, Kinon BJ, Maguire GA, et al. Effectiveness of rapid initial dose escalation of up to 40 milligrams per day of oral olanzapine in acute agitation. J Clin Psychopharmacol. 2003;23(4):342-8.
16. Sachs GS, Grossman F, Ghaemi SN, et al. Combination of mood stabilizer with risperidone or haloperidol for the treatment of acute mania: a double-blind, placebo controlled comparison of efficacy and safety. Am J Psychiatry. 2002;159:1146-54.
17. Hirschfeld RMA, Keck PE, Jr, Karcher K, et al. Rapid antimanic effect of risperidone monotherapy: a 3-week multicenter, randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2004;161:1057-65.
18. Khanna S, Vieta E, Lyons B, et al. Risperidone monotherapy in acute bipolar mania (abstract P219). Pittsburgh: Fifth International Conference on Bipolar Disorder, 2003.
19. Jones MW, Huizar K, et al. Quetiapine monotherapy for acute mania associated with bipolar disorder (poster presentation). San Francisco: American Psychiatric Association annual meeting, 2003.
20. Sachs G, Mullen JA, Devine NA, Sweitzer DE. Quetiapine versus placebo as adjunct to mood stabilizer for the treatment of acute mania (abstract). Bipolar Disord. 2002;4(suppl 1):133.-
21. Smith MA, McCoy R, Hamer J, Brecher M. Optimal titration for quetiapine (poster presentation): Boca Raton, FL: National Clinical Drug Evaluation Unit annual meeting, 2002.
22. Keck PE, Jr, Versiani M, Potkin S, et al. Ziprasidone in the treatment of acute bipolar mania: a three-week, placebo-controlled, double-blind, randomized trial. Am J Psychiatry. 2003;160:741-8.
23. Holtzheimer PE, Neumaier JF. Treatment of acute mania. CNS Spectrums. 2003;8(12):917-28.
24. Keck PE, Jr, Marcus R, Tourkodimitris S, et al. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatry. 2003;160(9):1651-8.
25. Sachs G, Sanchez R, Marcus R, et al. Aripiprazole vs placebo with an acute manic or mixed episode. New York: American Psychiatric Association annual meeting, 2004.
26. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes. Diabetes Care. 2004;27:596-601.
1. Keck PE, Jr, McElroy SL, Tugrul KC, et al. Valproate oral loading in the treatment of acute mania. J Clin Psychiatry. 1993;54:305-8.
2. Moscovich DG, Shapira B, Lerer B, et al. Rapid lithiumization in acute manic patients. Hum Psychopharmacol. 1992;7:343-5.
3. Kook KA, Stimmel GL, Wilkins JN, et al. Accuracy and safety of a priori lithium loading. J Clin Psychiatry. 1985;46:49-51.
4. Carroll BT, Thalassinos A, Fawver JD. Loading strategies in acute mania. CNS Spectrums. 2001;6:919-30.
5. Hirschfeld RMA, Allen MH, McEvoy J, et al. Safety and tolerability of oral loading divalproex sodium in acutely manic bipolar patients. J Clin Psychiatry. 1999;60:815-18.
6. Miller BP, et al. Oral loading of extended-release divalproex in acute mania (presentation). New Orleans: American Psychiatric Association annual meeting, 2001.
7. Thibault M, Blume WT, Saint-Hilaire JM, et al. Divalproex extended-release versus the original divalproex tablet: results of a randomized, crossover study of well-controlled epileptic patients with primary generalized seizures. Epilepsy Res. 2002;50:243-9.
8. Hirschfeld RMA, Baker JD, Wozniak P, et al. The safety and early efficacy of oral-loaded divalproex versus standard-titration divalproex, lithium, olanzapine, and placebo in the treatment of acute mania associated with bipolar disorder. J Clin Psychiatry. 2003;64:841-6.
9. Phrolov K, Applebaum J, Levine J, et al. Single-dose intravenous valproate in acute mania. J Clin Psychiatry. 2004;65:68-70.
10. Keck PE, Jr, McElroy SL, Bennet TA. Pharmacologic loading in the treatment of acute mania. Bipolar Disord. 2000;2:42-6.
11. Dunn RT, Frye MS, Tim KA, et al. The efficacy and use of anticonvulsants in mood disorders. Clin Neuropharmacol. 1998;21:215-35.
12. Lerer B, Moore N, Meyendor E, et al. Carbamazepine versus lithium in mania a double-blind study. J Clin Psychiatry. 1987;48:89-93.
13. Brown B. The use of generic mood stabilizers: carbamazepine (monograph). J Clin Psychiatry. 1997;15(4):11-17.
14. Karagianis JL, Dawe IC, Thakur A, et al. Rapid tranquilization with olanzapine in acute psychosis: a case series. J Clin Psychiatry. 2001;62(suppl 2):12-16.
15. Baker RW, Kinon BJ, Maguire GA, et al. Effectiveness of rapid initial dose escalation of up to 40 milligrams per day of oral olanzapine in acute agitation. J Clin Psychopharmacol. 2003;23(4):342-8.
16. Sachs GS, Grossman F, Ghaemi SN, et al. Combination of mood stabilizer with risperidone or haloperidol for the treatment of acute mania: a double-blind, placebo controlled comparison of efficacy and safety. Am J Psychiatry. 2002;159:1146-54.
17. Hirschfeld RMA, Keck PE, Jr, Karcher K, et al. Rapid antimanic effect of risperidone monotherapy: a 3-week multicenter, randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2004;161:1057-65.
18. Khanna S, Vieta E, Lyons B, et al. Risperidone monotherapy in acute bipolar mania (abstract P219). Pittsburgh: Fifth International Conference on Bipolar Disorder, 2003.
19. Jones MW, Huizar K, et al. Quetiapine monotherapy for acute mania associated with bipolar disorder (poster presentation). San Francisco: American Psychiatric Association annual meeting, 2003.
20. Sachs G, Mullen JA, Devine NA, Sweitzer DE. Quetiapine versus placebo as adjunct to mood stabilizer for the treatment of acute mania (abstract). Bipolar Disord. 2002;4(suppl 1):133.-
21. Smith MA, McCoy R, Hamer J, Brecher M. Optimal titration for quetiapine (poster presentation): Boca Raton, FL: National Clinical Drug Evaluation Unit annual meeting, 2002.
22. Keck PE, Jr, Versiani M, Potkin S, et al. Ziprasidone in the treatment of acute bipolar mania: a three-week, placebo-controlled, double-blind, randomized trial. Am J Psychiatry. 2003;160:741-8.
23. Holtzheimer PE, Neumaier JF. Treatment of acute mania. CNS Spectrums. 2003;8(12):917-28.
24. Keck PE, Jr, Marcus R, Tourkodimitris S, et al. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatry. 2003;160(9):1651-8.
25. Sachs G, Sanchez R, Marcus R, et al. Aripiprazole vs placebo with an acute manic or mixed episode. New York: American Psychiatric Association annual meeting, 2004.
26. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes. Diabetes Care. 2004;27:596-601.