User login
Superficial Vascular Anomaly of the Glabella Mimicking a Cutaneous Cyst
To the Editor:
Cutaneous cysts commonly are treated by dermatologists and typically are diagnosed clinically, followed by intraoperative or histologic confirmation; however, cyst mimickers can be misdiagnosed due to similar appearance and limited diagnostic guidelines.1 Vascular anomalies (VAs) of the face such as a facial aneurysm are rare.2 Preoperative assessment of findings suggestive of vascular etiology vs other common cutaneous tumors such as an epidermal inclusion cyst (EIC) and lipoma can help guide dermatologic management. We present a case of a VA of the glabella manifesting as a flesh-colored nodule that clinically mimicked a cyst and discuss the subsequent surgical management.
A 61-year-old man with a history of benign prostatic hyperplasia was evaluated at our dermatology clinic for an enlarging forehead mass of 1 year’s duration. Physical examination yielded a soft, flesh-colored, 2.5-cm nodule located superficially in the midline glabellar region without pulsation or palpable thrill. The differential diagnosis at the time included lipoma or EIC.
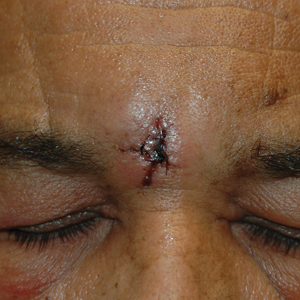
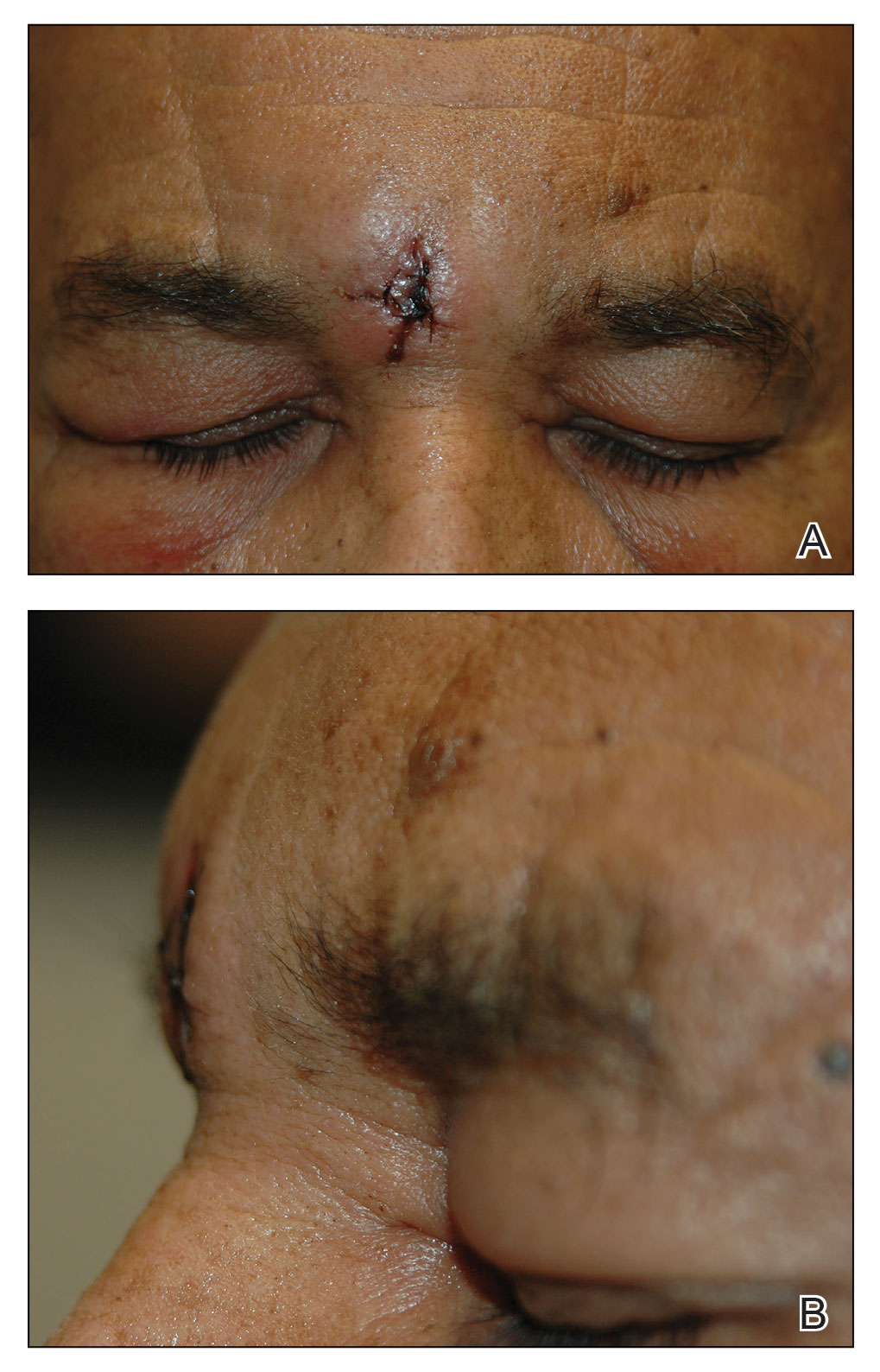
Excision of the lesion was performed utilizing superficial incisions with a descending depth of 1-mm increments to safely reach the target, identify the type of tumor, and prevent rupture of the suspected EIC. After the third incision to the level of the dermis, nonpulsatile bleeding was more than expected for a cyst. Digital pressure was applied, and the area was explored with blunt dissection to identify the source of bleeding. A fusiform, thin-walled aneurysm was identified in the dermal plane with additional tributaries coursing deep into the subcutaneous plane. The visualized tributaries were ligated with 3-0 polyglactin, figure-of-eight sutures resulting in hemostasis. The wound was closed with 5-0 nylon simple interrupted sutures. The patient was closely followed postoperatively for 1 week (Figure) and was referred for head imaging to evaluate for a possible associated intracranial aneurysm. Based on the thin vessel wall and continuous nonpulsatile hemorrhage, this VA was most consistent with venous aneurysm.
A VA can be encountered unexpectedly during dermatologic surgery. An aneurysm is a type of VA and is defined as an abnormal dilatation of a blood vessel that can be arterial, venous, or an arteriovenous malformation. Most reported aneurysms of the head and neck are cirsoid aneurysms or involve the superficial temporal artery.2,3 Reports of superficial venous aneurysms are rare.4 Preoperatively, cutaneous nodules can be evaluated for findings suggestive of a VA in the dermatologist’s office through physical examination. Arterial aneurysms may reveal palpable pulsation and audible bruit, while a venous aneurysm may exhibit a blue color, a size reduction with compression, and variable size with Valsalva maneuver.
The gold standard diagnostic tool for most dermatologic conditions is histopathology; however, dermatologic ultrasonography can provide noninvasive, real-time, important diagnostic characteristics of cutaneous pathologies as well as VA.5-7 Crisan et al6 outlined specific sonographic findings of lipomas, EICs, trichilemmal cysts, and other dermatologic conditions as well as the associated surgical pertinence. Ultrasonography of a venous aneurysm may show a heterogeneous, contiguous, echoic lesion with an adjacent superficial vein, which may be easily compressed by the probe.8 Advanced imaging such as computed tomography with contrast or magnetic resonance imaging may be performed, but these are more costly than ultrasonography. Additionally, point-of-care ultrasonography is becoming more popular and accessible for physicians to carry at bedside with portable tablet options available. Dermatologists may want to consider incorporating it into the outpatient setting to improve procedural planning.9
In conclusion, VAs should be included in the differential diagnosis of soft cutaneous nodules, as management differs from a cyst or lipoma. Dermatologists should use their clinical judgment preoperatively—including a comprehensive history, physical examination, and consideration of color Doppler ultrasonography to assess for findings of VA. We do not recommend intentional surgical exploration of cutaneous aneurysms in the ambulatory setting due to risk for hemorrhage. Furthermore, when clinical suspicion of EIC or lipoma is high, it still is preferable to descend the incision slowly at 1 to 2 mm per cut until the tumor is visualized.
- Ring CM, Kornreich DA, Lee JB. Clinical simulators of cysts. J Am Acad Dermatol. 2016;75:1255-1257.
- Evans CC, Larson MJ, Eichhorn PJ, et al. Traumatic pseudoaneurysm of the superficial temporal artery: two cases and review of the literature. J Am Acad Dermatol. 2003;49(5 suppl):S286-S288.
- Sofela A, Osunronbi T, Hettige S. Scalp cirsoid aneurysms: case illustration and systematic review of literature. Neurosurgery. 2020;86:E98-E107.
- McKesey J, Cohen PR. Spontaneous venous aneurysm: report of a non-traumatic superficial venous aneurysm on the distal arm. Cureus. 2018;10:E2641.
- Wortsman X, Alfageme F, Roustan G, et al. Guidelines for performing dermatologic ultrasound examinations by the DERMUS Group. J Ultrasound Med. 2016;35:577-580.
- Crisan D, Wortsman X, Alfageme F, et al. Ultrasonography in dermatologic surgery: revealing the unseen for improved surgical planning [published online May 26, 2022]. J Dtsch Dermatol Ges. doi:10.1111/ddg.14781
- Corvino A, Catalano O, Corvino F, et al. Superficial temporal artery pseudoaneurysm: what is the role of ultrasound. J Ultrasound. 2016;19:197-201.
- Lee HY, Lee W, Cho YK, et al. Superficial venous aneurysm: reports of 3 cases and literature review. J Ultrasound Med. 2006;25:771-776.
- Hadian Y, Link D, Dahle SE, et al. Ultrasound as a diagnostic and interventional aid at point-of-care in dermatology clinic: a case report. J Dermatolog Treat. 2020;31:74-76.
To the Editor:
Cutaneous cysts commonly are treated by dermatologists and typically are diagnosed clinically, followed by intraoperative or histologic confirmation; however, cyst mimickers can be misdiagnosed due to similar appearance and limited diagnostic guidelines.1 Vascular anomalies (VAs) of the face such as a facial aneurysm are rare.2 Preoperative assessment of findings suggestive of vascular etiology vs other common cutaneous tumors such as an epidermal inclusion cyst (EIC) and lipoma can help guide dermatologic management. We present a case of a VA of the glabella manifesting as a flesh-colored nodule that clinically mimicked a cyst and discuss the subsequent surgical management.
A 61-year-old man with a history of benign prostatic hyperplasia was evaluated at our dermatology clinic for an enlarging forehead mass of 1 year’s duration. Physical examination yielded a soft, flesh-colored, 2.5-cm nodule located superficially in the midline glabellar region without pulsation or palpable thrill. The differential diagnosis at the time included lipoma or EIC.
Excision of the lesion was performed utilizing superficial incisions with a descending depth of 1-mm increments to safely reach the target, identify the type of tumor, and prevent rupture of the suspected EIC. After the third incision to the level of the dermis, nonpulsatile bleeding was more than expected for a cyst. Digital pressure was applied, and the area was explored with blunt dissection to identify the source of bleeding. A fusiform, thin-walled aneurysm was identified in the dermal plane with additional tributaries coursing deep into the subcutaneous plane. The visualized tributaries were ligated with 3-0 polyglactin, figure-of-eight sutures resulting in hemostasis. The wound was closed with 5-0 nylon simple interrupted sutures. The patient was closely followed postoperatively for 1 week (Figure) and was referred for head imaging to evaluate for a possible associated intracranial aneurysm. Based on the thin vessel wall and continuous nonpulsatile hemorrhage, this VA was most consistent with venous aneurysm.

A VA can be encountered unexpectedly during dermatologic surgery. An aneurysm is a type of VA and is defined as an abnormal dilatation of a blood vessel that can be arterial, venous, or an arteriovenous malformation. Most reported aneurysms of the head and neck are cirsoid aneurysms or involve the superficial temporal artery.2,3 Reports of superficial venous aneurysms are rare.4 Preoperatively, cutaneous nodules can be evaluated for findings suggestive of a VA in the dermatologist’s office through physical examination. Arterial aneurysms may reveal palpable pulsation and audible bruit, while a venous aneurysm may exhibit a blue color, a size reduction with compression, and variable size with Valsalva maneuver.
The gold standard diagnostic tool for most dermatologic conditions is histopathology; however, dermatologic ultrasonography can provide noninvasive, real-time, important diagnostic characteristics of cutaneous pathologies as well as VA.5-7 Crisan et al6 outlined specific sonographic findings of lipomas, EICs, trichilemmal cysts, and other dermatologic conditions as well as the associated surgical pertinence. Ultrasonography of a venous aneurysm may show a heterogeneous, contiguous, echoic lesion with an adjacent superficial vein, which may be easily compressed by the probe.8 Advanced imaging such as computed tomography with contrast or magnetic resonance imaging may be performed, but these are more costly than ultrasonography. Additionally, point-of-care ultrasonography is becoming more popular and accessible for physicians to carry at bedside with portable tablet options available. Dermatologists may want to consider incorporating it into the outpatient setting to improve procedural planning.9
In conclusion, VAs should be included in the differential diagnosis of soft cutaneous nodules, as management differs from a cyst or lipoma. Dermatologists should use their clinical judgment preoperatively—including a comprehensive history, physical examination, and consideration of color Doppler ultrasonography to assess for findings of VA. We do not recommend intentional surgical exploration of cutaneous aneurysms in the ambulatory setting due to risk for hemorrhage. Furthermore, when clinical suspicion of EIC or lipoma is high, it still is preferable to descend the incision slowly at 1 to 2 mm per cut until the tumor is visualized.
To the Editor:
Cutaneous cysts commonly are treated by dermatologists and typically are diagnosed clinically, followed by intraoperative or histologic confirmation; however, cyst mimickers can be misdiagnosed due to similar appearance and limited diagnostic guidelines.1 Vascular anomalies (VAs) of the face such as a facial aneurysm are rare.2 Preoperative assessment of findings suggestive of vascular etiology vs other common cutaneous tumors such as an epidermal inclusion cyst (EIC) and lipoma can help guide dermatologic management. We present a case of a VA of the glabella manifesting as a flesh-colored nodule that clinically mimicked a cyst and discuss the subsequent surgical management.
A 61-year-old man with a history of benign prostatic hyperplasia was evaluated at our dermatology clinic for an enlarging forehead mass of 1 year’s duration. Physical examination yielded a soft, flesh-colored, 2.5-cm nodule located superficially in the midline glabellar region without pulsation or palpable thrill. The differential diagnosis at the time included lipoma or EIC.
Excision of the lesion was performed utilizing superficial incisions with a descending depth of 1-mm increments to safely reach the target, identify the type of tumor, and prevent rupture of the suspected EIC. After the third incision to the level of the dermis, nonpulsatile bleeding was more than expected for a cyst. Digital pressure was applied, and the area was explored with blunt dissection to identify the source of bleeding. A fusiform, thin-walled aneurysm was identified in the dermal plane with additional tributaries coursing deep into the subcutaneous plane. The visualized tributaries were ligated with 3-0 polyglactin, figure-of-eight sutures resulting in hemostasis. The wound was closed with 5-0 nylon simple interrupted sutures. The patient was closely followed postoperatively for 1 week (Figure) and was referred for head imaging to evaluate for a possible associated intracranial aneurysm. Based on the thin vessel wall and continuous nonpulsatile hemorrhage, this VA was most consistent with venous aneurysm.

A VA can be encountered unexpectedly during dermatologic surgery. An aneurysm is a type of VA and is defined as an abnormal dilatation of a blood vessel that can be arterial, venous, or an arteriovenous malformation. Most reported aneurysms of the head and neck are cirsoid aneurysms or involve the superficial temporal artery.2,3 Reports of superficial venous aneurysms are rare.4 Preoperatively, cutaneous nodules can be evaluated for findings suggestive of a VA in the dermatologist’s office through physical examination. Arterial aneurysms may reveal palpable pulsation and audible bruit, while a venous aneurysm may exhibit a blue color, a size reduction with compression, and variable size with Valsalva maneuver.
The gold standard diagnostic tool for most dermatologic conditions is histopathology; however, dermatologic ultrasonography can provide noninvasive, real-time, important diagnostic characteristics of cutaneous pathologies as well as VA.5-7 Crisan et al6 outlined specific sonographic findings of lipomas, EICs, trichilemmal cysts, and other dermatologic conditions as well as the associated surgical pertinence. Ultrasonography of a venous aneurysm may show a heterogeneous, contiguous, echoic lesion with an adjacent superficial vein, which may be easily compressed by the probe.8 Advanced imaging such as computed tomography with contrast or magnetic resonance imaging may be performed, but these are more costly than ultrasonography. Additionally, point-of-care ultrasonography is becoming more popular and accessible for physicians to carry at bedside with portable tablet options available. Dermatologists may want to consider incorporating it into the outpatient setting to improve procedural planning.9
In conclusion, VAs should be included in the differential diagnosis of soft cutaneous nodules, as management differs from a cyst or lipoma. Dermatologists should use their clinical judgment preoperatively—including a comprehensive history, physical examination, and consideration of color Doppler ultrasonography to assess for findings of VA. We do not recommend intentional surgical exploration of cutaneous aneurysms in the ambulatory setting due to risk for hemorrhage. Furthermore, when clinical suspicion of EIC or lipoma is high, it still is preferable to descend the incision slowly at 1 to 2 mm per cut until the tumor is visualized.
- Ring CM, Kornreich DA, Lee JB. Clinical simulators of cysts. J Am Acad Dermatol. 2016;75:1255-1257.
- Evans CC, Larson MJ, Eichhorn PJ, et al. Traumatic pseudoaneurysm of the superficial temporal artery: two cases and review of the literature. J Am Acad Dermatol. 2003;49(5 suppl):S286-S288.
- Sofela A, Osunronbi T, Hettige S. Scalp cirsoid aneurysms: case illustration and systematic review of literature. Neurosurgery. 2020;86:E98-E107.
- McKesey J, Cohen PR. Spontaneous venous aneurysm: report of a non-traumatic superficial venous aneurysm on the distal arm. Cureus. 2018;10:E2641.
- Wortsman X, Alfageme F, Roustan G, et al. Guidelines for performing dermatologic ultrasound examinations by the DERMUS Group. J Ultrasound Med. 2016;35:577-580.
- Crisan D, Wortsman X, Alfageme F, et al. Ultrasonography in dermatologic surgery: revealing the unseen for improved surgical planning [published online May 26, 2022]. J Dtsch Dermatol Ges. doi:10.1111/ddg.14781
- Corvino A, Catalano O, Corvino F, et al. Superficial temporal artery pseudoaneurysm: what is the role of ultrasound. J Ultrasound. 2016;19:197-201.
- Lee HY, Lee W, Cho YK, et al. Superficial venous aneurysm: reports of 3 cases and literature review. J Ultrasound Med. 2006;25:771-776.
- Hadian Y, Link D, Dahle SE, et al. Ultrasound as a diagnostic and interventional aid at point-of-care in dermatology clinic: a case report. J Dermatolog Treat. 2020;31:74-76.
- Ring CM, Kornreich DA, Lee JB. Clinical simulators of cysts. J Am Acad Dermatol. 2016;75:1255-1257.
- Evans CC, Larson MJ, Eichhorn PJ, et al. Traumatic pseudoaneurysm of the superficial temporal artery: two cases and review of the literature. J Am Acad Dermatol. 2003;49(5 suppl):S286-S288.
- Sofela A, Osunronbi T, Hettige S. Scalp cirsoid aneurysms: case illustration and systematic review of literature. Neurosurgery. 2020;86:E98-E107.
- McKesey J, Cohen PR. Spontaneous venous aneurysm: report of a non-traumatic superficial venous aneurysm on the distal arm. Cureus. 2018;10:E2641.
- Wortsman X, Alfageme F, Roustan G, et al. Guidelines for performing dermatologic ultrasound examinations by the DERMUS Group. J Ultrasound Med. 2016;35:577-580.
- Crisan D, Wortsman X, Alfageme F, et al. Ultrasonography in dermatologic surgery: revealing the unseen for improved surgical planning [published online May 26, 2022]. J Dtsch Dermatol Ges. doi:10.1111/ddg.14781
- Corvino A, Catalano O, Corvino F, et al. Superficial temporal artery pseudoaneurysm: what is the role of ultrasound. J Ultrasound. 2016;19:197-201.
- Lee HY, Lee W, Cho YK, et al. Superficial venous aneurysm: reports of 3 cases and literature review. J Ultrasound Med. 2006;25:771-776.
- Hadian Y, Link D, Dahle SE, et al. Ultrasound as a diagnostic and interventional aid at point-of-care in dermatology clinic: a case report. J Dermatolog Treat. 2020;31:74-76.
Practice Points
- Vascular anomalies should be included in the differential diagnosis of soft cutaneous nodules, as management differs from cysts or lipomas.
- Preoperative evaluation for a cutaneous cyst excision on the head and neck should include ruling out findings of a vascular lesion through history, physical examination, and consideration of color Doppler ultrasonography in unclear cases.
- Surgical technique should involve sequential superficial incisions, descending at 1 to 2 mm per cut, until the suspected capsule is identified to minimize the risk for inadvertent injury to a cyst mimicker such as a vascular anomaly.
Multiple New-Onset Pyogenic Granulomas During Treatment With Paclitaxel and Ramucirumab
To the Editor:
Pyogenic granuloma (PG) is a benign vascular tumor that clinically is characterized as a small eruptive friable papule.1 Lesions typically are solitary and most commonly occur in children but also are associated with pregnancy; trauma to the skin or mucosa; and use of certain medications such as isotretinoin, capecitabine, vemurafenib, or indinavir.1 Numerous antineoplastic medications have been associated with the development of solitary PGs, including the taxane mitotic inhibitor paclitaxel (PTX) and the vascular endothelial growth factor receptor 2 (VEGFR2) monoclonal antibody ramucirumab.2 We report a case of multiple PGs in a patient undergoing treatment with PTX and ramucirumab.

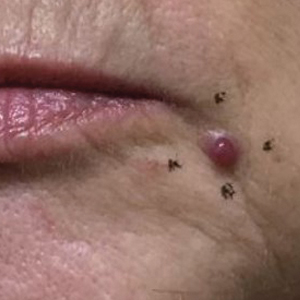
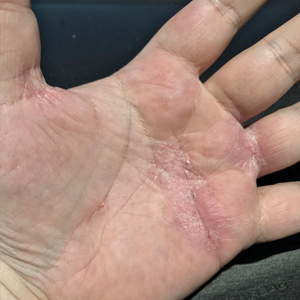
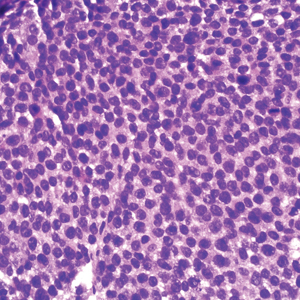
A 59-year-old woman presented to the dermatology clinic with red, itchy, bleeding skin lesions on the breast, superior chest, left cheek, and forearm of 1 month’s duration. She denied any preceding trauma to the areas. Her medical history was notable for gastroesophageal junction adenocarcinoma diagnosed more than 2 years prior to presentation. Her original treatment regimen included nivolumab, which was discontinued for unknown reasons 5 months prior to presentation, and she was started on combination therapy with PTX and ramucirumab at that time. She noted the formation of small red papules 2 months after the initiation of PTX-ramucirumab combination therapy, which grew larger over the course of the next month. Physical examination revealed 5 friable hemorrhagic papules and nodules ranging in size from 3 to 10 mm on the chest, cheek, and forearm consistent with PGs (Figure 1). Several scattered cherry angiomas were noted on the scalp and torso, but the patient reported these were not new. Biopsies of the PGs demonstrated lobular aggregates of small-caliber vessels set in an edematous inflamed stroma and partially enclosed by small collarettes of adnexal epithelium, confirming the clinical diagnosis of multiple PGs (Figure 2).
The first case of PTX-associated PG was reported in 2012.3 Based on a PubMed search of articles indexed for MEDLINE using the terms pyogenic granuloma, lobular capillary hemangioma, paclitaxel, taxane, and ramucirumab, there have been 9 cases of solitary PG development in the setting of PTX alone or in combination with ramucirumab since 2019 (Table).3-8 Pyogenic granulomas reported in patients who were treated exclusively with PTX were subungual, while the cases resulting from combined therapy were present on the scalp, face, oral mucosa, and surfaces of the hands sparing the nails. Ibe et al6 reported PG in a patient who received ramucirumab therapy without PTX but in combination with another taxane, docetaxel, which itself has been reported to cause subungual PG when used alone.9 Our case of the simultaneous development of multiple PGs in the setting of combined PTX and ramucirumab therapy added to the cutaneous distributions for which therapy-induced PGs have been observed (Table).

The development of PG, a vascular tumor, during treatment with the VEGFR2 inhibitor ramucirumab—whose mechanism of action is to inhibit angioneogenesis—is inherently paradoxical. In 2015, a rapidly expanding angioma with a mutation in the kinase domain receptor gene, KDR, that encodes VEGFR2 was identified in a patient undergoing ramucirumab therapy. The authors suggested that KDR mutation resulted in paradoxical activation of VEGFR2 in the setting of ramucirumab therapy.10 Since then, ramucirumab and PTX were suggested to have a synergistic effect in vascular proliferation,5 though an exact mechanism has not been proposed. Other authors have identified increased expression of VEGFR2 in biopsy specimens of PG during combined ramucirumab and taxane therapy.6 Although genetic studies have not been used to evaluate for the presence of KDR mutations specifically in our patient population, it is possible that patients who develop PG and other vascular tumors during combined taxane and ramucirumab therapy have a mutation that makes them more susceptible to VEGFR2 upregulation. UV exposure may have a role in the formation of PG in patients on combined ramucirumab and taxane therapy7; however, our patient’s lesions were distributed on both sun-exposed and unexposed areas. Although potential clinical implications have not yet been thoroughly investigated, following long-term outcomes for these patients may provide important information on the efficacy of the antineoplastic regimen in the subset of patients who develop cutaneous vascular tumors during antiangiogenic treatment.
Combination therapy with PTX and ramucirumab has been associated with the paradoxical development of cutaneous vascular tumors. We report a case of multiple new-onset PGs in a patient undergoing this treatment regimen.
- Elston D, Neuhaus I, James WD, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
- Pierson JC. Pyogenic granuloma (lobular capillary hemangioma) clinical presentation. Medscape. Updated February 21, 2020. Accessed December 26, 2023. https://emedicine.medscape.com/article/1084701-clinical#showall
- Paul LJ, Cohen PR. Paclitaxel-associated subungual pyogenic granuloma: report in a patient with breast cancer receiving paclitaxel and review of drug-induced pyogenic granulomas adjacent to and beneath the nail. J Drugs Dermatol. 2012;11:262-268.
- Alessandrini A, Starace M, Cerè G, et al. Management and outcome of taxane-induced nail side effects: experience of 79 patients from a single centre. Skin Appendage Disord. 2019;5:276-282.
- Watanabe R, Nakano E, Kawazoe A, et al. Four cases of paradoxical cephalocervical pyogenic granuloma during treatment with paclitaxel and ramucirumab. J Dermatol. 2019;46:E178-E180.
- Ibe T, Hamamoto Y, Takabatake M, et al. Development of pyogenic granuloma with strong vascular endothelial growth factor receptor-2 expression during ramucirumab treatment. BMJ Case Rep. 2019;12:E231464.
- Choi YH, Byun HJ, Lee JH, et al. Multiple cherry angiomas and pyogenic granuloma in a patient treated with ramucirumab and paclitaxel. Indian J Dermatol Venereol Leprol. 2020;86:199-202.
- Aragaki T, Tomomatsu N, Michi Y, et al. Ramucirumab-related oral pyogenic granuloma: a report of two cases [published online March 8, 2021]. Intern Med. 2021;60:2601-2605. doi:10.2169/internalmedicine.6650-20
- Devillers C, Vanhooteghem O, Henrijean A, et al. Subungual pyogenic granuloma secondary to docetaxel therapy. Clin Exp Dermatol. 2009;34:251-252.
- Lim YH, Odell ID, Ko CJ, et al. Somatic p.T771R KDR (VEGFR2) mutation arising in a sporadic angioma during ramucirumab therapy. JAMA Dermatol. 2015;151:1240-1243.
To the Editor:
Pyogenic granuloma (PG) is a benign vascular tumor that clinically is characterized as a small eruptive friable papule.1 Lesions typically are solitary and most commonly occur in children but also are associated with pregnancy; trauma to the skin or mucosa; and use of certain medications such as isotretinoin, capecitabine, vemurafenib, or indinavir.1 Numerous antineoplastic medications have been associated with the development of solitary PGs, including the taxane mitotic inhibitor paclitaxel (PTX) and the vascular endothelial growth factor receptor 2 (VEGFR2) monoclonal antibody ramucirumab.2 We report a case of multiple PGs in a patient undergoing treatment with PTX and ramucirumab.

A 59-year-old woman presented to the dermatology clinic with red, itchy, bleeding skin lesions on the breast, superior chest, left cheek, and forearm of 1 month’s duration. She denied any preceding trauma to the areas. Her medical history was notable for gastroesophageal junction adenocarcinoma diagnosed more than 2 years prior to presentation. Her original treatment regimen included nivolumab, which was discontinued for unknown reasons 5 months prior to presentation, and she was started on combination therapy with PTX and ramucirumab at that time. She noted the formation of small red papules 2 months after the initiation of PTX-ramucirumab combination therapy, which grew larger over the course of the next month. Physical examination revealed 5 friable hemorrhagic papules and nodules ranging in size from 3 to 10 mm on the chest, cheek, and forearm consistent with PGs (Figure 1). Several scattered cherry angiomas were noted on the scalp and torso, but the patient reported these were not new. Biopsies of the PGs demonstrated lobular aggregates of small-caliber vessels set in an edematous inflamed stroma and partially enclosed by small collarettes of adnexal epithelium, confirming the clinical diagnosis of multiple PGs (Figure 2).

The first case of PTX-associated PG was reported in 2012.3 Based on a PubMed search of articles indexed for MEDLINE using the terms pyogenic granuloma, lobular capillary hemangioma, paclitaxel, taxane, and ramucirumab, there have been 9 cases of solitary PG development in the setting of PTX alone or in combination with ramucirumab since 2019 (Table).3-8 Pyogenic granulomas reported in patients who were treated exclusively with PTX were subungual, while the cases resulting from combined therapy were present on the scalp, face, oral mucosa, and surfaces of the hands sparing the nails. Ibe et al6 reported PG in a patient who received ramucirumab therapy without PTX but in combination with another taxane, docetaxel, which itself has been reported to cause subungual PG when used alone.9 Our case of the simultaneous development of multiple PGs in the setting of combined PTX and ramucirumab therapy added to the cutaneous distributions for which therapy-induced PGs have been observed (Table).

The development of PG, a vascular tumor, during treatment with the VEGFR2 inhibitor ramucirumab—whose mechanism of action is to inhibit angioneogenesis—is inherently paradoxical. In 2015, a rapidly expanding angioma with a mutation in the kinase domain receptor gene, KDR, that encodes VEGFR2 was identified in a patient undergoing ramucirumab therapy. The authors suggested that KDR mutation resulted in paradoxical activation of VEGFR2 in the setting of ramucirumab therapy.10 Since then, ramucirumab and PTX were suggested to have a synergistic effect in vascular proliferation,5 though an exact mechanism has not been proposed. Other authors have identified increased expression of VEGFR2 in biopsy specimens of PG during combined ramucirumab and taxane therapy.6 Although genetic studies have not been used to evaluate for the presence of KDR mutations specifically in our patient population, it is possible that patients who develop PG and other vascular tumors during combined taxane and ramucirumab therapy have a mutation that makes them more susceptible to VEGFR2 upregulation. UV exposure may have a role in the formation of PG in patients on combined ramucirumab and taxane therapy7; however, our patient’s lesions were distributed on both sun-exposed and unexposed areas. Although potential clinical implications have not yet been thoroughly investigated, following long-term outcomes for these patients may provide important information on the efficacy of the antineoplastic regimen in the subset of patients who develop cutaneous vascular tumors during antiangiogenic treatment.
Combination therapy with PTX and ramucirumab has been associated with the paradoxical development of cutaneous vascular tumors. We report a case of multiple new-onset PGs in a patient undergoing this treatment regimen.
To the Editor:
Pyogenic granuloma (PG) is a benign vascular tumor that clinically is characterized as a small eruptive friable papule.1 Lesions typically are solitary and most commonly occur in children but also are associated with pregnancy; trauma to the skin or mucosa; and use of certain medications such as isotretinoin, capecitabine, vemurafenib, or indinavir.1 Numerous antineoplastic medications have been associated with the development of solitary PGs, including the taxane mitotic inhibitor paclitaxel (PTX) and the vascular endothelial growth factor receptor 2 (VEGFR2) monoclonal antibody ramucirumab.2 We report a case of multiple PGs in a patient undergoing treatment with PTX and ramucirumab.

A 59-year-old woman presented to the dermatology clinic with red, itchy, bleeding skin lesions on the breast, superior chest, left cheek, and forearm of 1 month’s duration. She denied any preceding trauma to the areas. Her medical history was notable for gastroesophageal junction adenocarcinoma diagnosed more than 2 years prior to presentation. Her original treatment regimen included nivolumab, which was discontinued for unknown reasons 5 months prior to presentation, and she was started on combination therapy with PTX and ramucirumab at that time. She noted the formation of small red papules 2 months after the initiation of PTX-ramucirumab combination therapy, which grew larger over the course of the next month. Physical examination revealed 5 friable hemorrhagic papules and nodules ranging in size from 3 to 10 mm on the chest, cheek, and forearm consistent with PGs (Figure 1). Several scattered cherry angiomas were noted on the scalp and torso, but the patient reported these were not new. Biopsies of the PGs demonstrated lobular aggregates of small-caliber vessels set in an edematous inflamed stroma and partially enclosed by small collarettes of adnexal epithelium, confirming the clinical diagnosis of multiple PGs (Figure 2).

The first case of PTX-associated PG was reported in 2012.3 Based on a PubMed search of articles indexed for MEDLINE using the terms pyogenic granuloma, lobular capillary hemangioma, paclitaxel, taxane, and ramucirumab, there have been 9 cases of solitary PG development in the setting of PTX alone or in combination with ramucirumab since 2019 (Table).3-8 Pyogenic granulomas reported in patients who were treated exclusively with PTX were subungual, while the cases resulting from combined therapy were present on the scalp, face, oral mucosa, and surfaces of the hands sparing the nails. Ibe et al6 reported PG in a patient who received ramucirumab therapy without PTX but in combination with another taxane, docetaxel, which itself has been reported to cause subungual PG when used alone.9 Our case of the simultaneous development of multiple PGs in the setting of combined PTX and ramucirumab therapy added to the cutaneous distributions for which therapy-induced PGs have been observed (Table).

The development of PG, a vascular tumor, during treatment with the VEGFR2 inhibitor ramucirumab—whose mechanism of action is to inhibit angioneogenesis—is inherently paradoxical. In 2015, a rapidly expanding angioma with a mutation in the kinase domain receptor gene, KDR, that encodes VEGFR2 was identified in a patient undergoing ramucirumab therapy. The authors suggested that KDR mutation resulted in paradoxical activation of VEGFR2 in the setting of ramucirumab therapy.10 Since then, ramucirumab and PTX were suggested to have a synergistic effect in vascular proliferation,5 though an exact mechanism has not been proposed. Other authors have identified increased expression of VEGFR2 in biopsy specimens of PG during combined ramucirumab and taxane therapy.6 Although genetic studies have not been used to evaluate for the presence of KDR mutations specifically in our patient population, it is possible that patients who develop PG and other vascular tumors during combined taxane and ramucirumab therapy have a mutation that makes them more susceptible to VEGFR2 upregulation. UV exposure may have a role in the formation of PG in patients on combined ramucirumab and taxane therapy7; however, our patient’s lesions were distributed on both sun-exposed and unexposed areas. Although potential clinical implications have not yet been thoroughly investigated, following long-term outcomes for these patients may provide important information on the efficacy of the antineoplastic regimen in the subset of patients who develop cutaneous vascular tumors during antiangiogenic treatment.
Combination therapy with PTX and ramucirumab has been associated with the paradoxical development of cutaneous vascular tumors. We report a case of multiple new-onset PGs in a patient undergoing this treatment regimen.
- Elston D, Neuhaus I, James WD, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
- Pierson JC. Pyogenic granuloma (lobular capillary hemangioma) clinical presentation. Medscape. Updated February 21, 2020. Accessed December 26, 2023. https://emedicine.medscape.com/article/1084701-clinical#showall
- Paul LJ, Cohen PR. Paclitaxel-associated subungual pyogenic granuloma: report in a patient with breast cancer receiving paclitaxel and review of drug-induced pyogenic granulomas adjacent to and beneath the nail. J Drugs Dermatol. 2012;11:262-268.
- Alessandrini A, Starace M, Cerè G, et al. Management and outcome of taxane-induced nail side effects: experience of 79 patients from a single centre. Skin Appendage Disord. 2019;5:276-282.
- Watanabe R, Nakano E, Kawazoe A, et al. Four cases of paradoxical cephalocervical pyogenic granuloma during treatment with paclitaxel and ramucirumab. J Dermatol. 2019;46:E178-E180.
- Ibe T, Hamamoto Y, Takabatake M, et al. Development of pyogenic granuloma with strong vascular endothelial growth factor receptor-2 expression during ramucirumab treatment. BMJ Case Rep. 2019;12:E231464.
- Choi YH, Byun HJ, Lee JH, et al. Multiple cherry angiomas and pyogenic granuloma in a patient treated with ramucirumab and paclitaxel. Indian J Dermatol Venereol Leprol. 2020;86:199-202.
- Aragaki T, Tomomatsu N, Michi Y, et al. Ramucirumab-related oral pyogenic granuloma: a report of two cases [published online March 8, 2021]. Intern Med. 2021;60:2601-2605. doi:10.2169/internalmedicine.6650-20
- Devillers C, Vanhooteghem O, Henrijean A, et al. Subungual pyogenic granuloma secondary to docetaxel therapy. Clin Exp Dermatol. 2009;34:251-252.
- Lim YH, Odell ID, Ko CJ, et al. Somatic p.T771R KDR (VEGFR2) mutation arising in a sporadic angioma during ramucirumab therapy. JAMA Dermatol. 2015;151:1240-1243.
- Elston D, Neuhaus I, James WD, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
- Pierson JC. Pyogenic granuloma (lobular capillary hemangioma) clinical presentation. Medscape. Updated February 21, 2020. Accessed December 26, 2023. https://emedicine.medscape.com/article/1084701-clinical#showall
- Paul LJ, Cohen PR. Paclitaxel-associated subungual pyogenic granuloma: report in a patient with breast cancer receiving paclitaxel and review of drug-induced pyogenic granulomas adjacent to and beneath the nail. J Drugs Dermatol. 2012;11:262-268.
- Alessandrini A, Starace M, Cerè G, et al. Management and outcome of taxane-induced nail side effects: experience of 79 patients from a single centre. Skin Appendage Disord. 2019;5:276-282.
- Watanabe R, Nakano E, Kawazoe A, et al. Four cases of paradoxical cephalocervical pyogenic granuloma during treatment with paclitaxel and ramucirumab. J Dermatol. 2019;46:E178-E180.
- Ibe T, Hamamoto Y, Takabatake M, et al. Development of pyogenic granuloma with strong vascular endothelial growth factor receptor-2 expression during ramucirumab treatment. BMJ Case Rep. 2019;12:E231464.
- Choi YH, Byun HJ, Lee JH, et al. Multiple cherry angiomas and pyogenic granuloma in a patient treated with ramucirumab and paclitaxel. Indian J Dermatol Venereol Leprol. 2020;86:199-202.
- Aragaki T, Tomomatsu N, Michi Y, et al. Ramucirumab-related oral pyogenic granuloma: a report of two cases [published online March 8, 2021]. Intern Med. 2021;60:2601-2605. doi:10.2169/internalmedicine.6650-20
- Devillers C, Vanhooteghem O, Henrijean A, et al. Subungual pyogenic granuloma secondary to docetaxel therapy. Clin Exp Dermatol. 2009;34:251-252.
- Lim YH, Odell ID, Ko CJ, et al. Somatic p.T771R KDR (VEGFR2) mutation arising in a sporadic angioma during ramucirumab therapy. JAMA Dermatol. 2015;151:1240-1243.
Practice Points
- Pyogenic granulomas (PGs) are benign vascular tumors that clinically are characterized as small, eruptive, friable papules.
- Ramucirumab is a monoclonal antibody against vascular endothelial growth factor receptor 2.
- Some patients experience paradoxical formation of vascular tumors such as PGs when treated with combination therapy with ramucirumab and a taxane such as paclitaxel.
Cemiplimab-Associated Eruption of Generalized Eruptive Keratoacanthoma of Grzybowski
To the Editor:
Treatment of cancer, including cutaneous malignancy, has been transformed by the use of immunotherapeutic agents such as immune checkpoint inhibitors (ICIs) that target cytotoxic T lymphocyte-associated antigen 4, programmed cell-death protein 1 (PD-1), or programmed cell-death ligand 1 (PD-L1). However, these drugs are associated with a distinct set of immune-related adverse events (IRAEs). We present a case of generalized eruptive keratoacanthoma of Grzybowski associated with the ICI cemiplimab.
A 94-year-old White woman presented to the dermatology clinic with acute onset of extensive, locally advanced cutaneous squamous cell carcinoma (cSCC) of the upper right posterolateral calf as well as multiple noninvasive cSCCs of the arms and legs. Her medical history was remarkable for widespread actinic keratoses and numerous cSCCs. The patient had no personal or family history of melanoma. Various cSCCs had required treatment with electrodesiccation and curettage, topical or intralesional 5-fluorouracil, and Mohs micrographic surgery. Approximately 1 year prior to presentation, oral acitretin was initiated to help control the cSCC. Given the extent of locally advanced disease, which was considered unresectable, she was referred to oncology but continued to follow up with dermatology. Positron emission tomography was remarkable for hypermetabolic cutaneous thickening in the upper right posterolateral calf with no evidence of visceral disease.

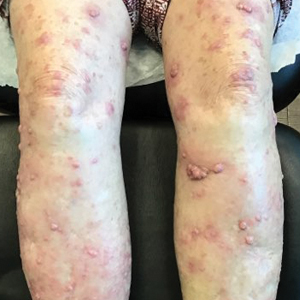
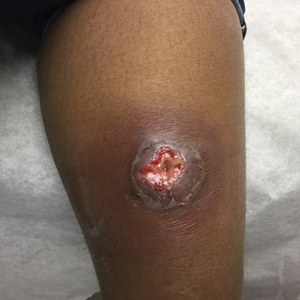
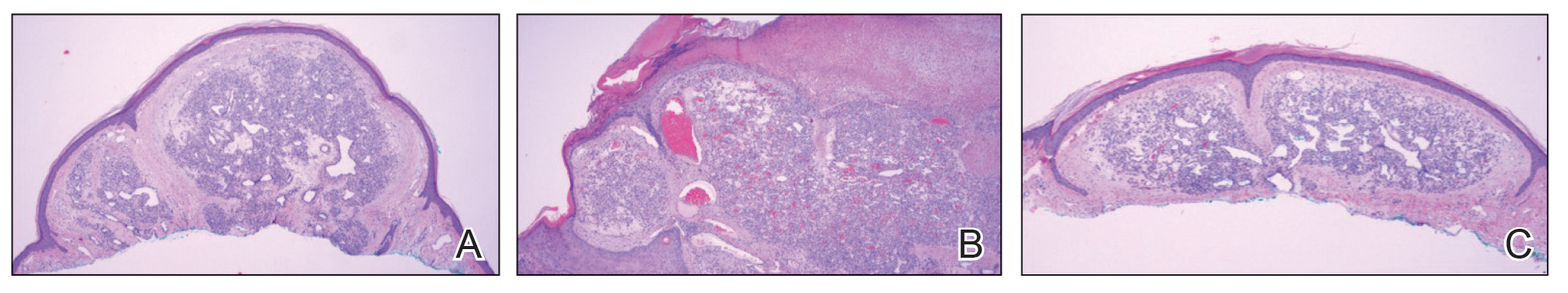
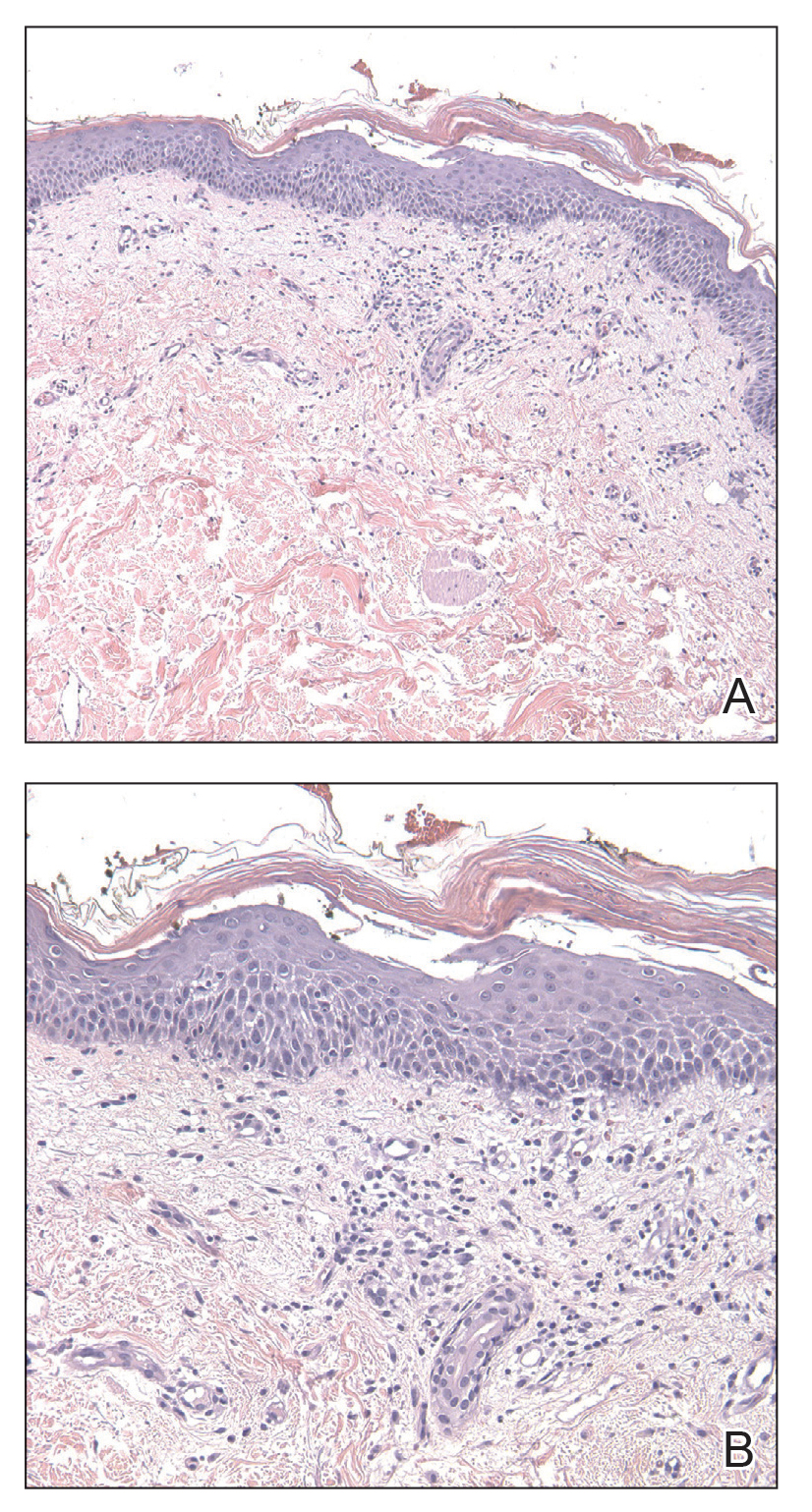
The patient was started on cemiplimab, an anti-PD-1 monoclonal antibody ICI indicated for the treatment of both metastatic and advanced cSCC. After 4 cycles of intravenous cemiplimab, the patient developed widespread nodules covering the arms and legs (Figure 1) as well as associated tenderness and pruritus. Biopsies of nodules revealed superficially invasive, well-differentiated cSCC consistent with keratoacanthoma. Although a lymphocytic infiltrate was present, no other specific reaction pattern, such as a lichenoid infiltrate, was present (Figure 2).

Positron emission tomography was repeated, demonstrating resolution of the right calf lesion; however, new diffuse cutaneous lesions and inguinal lymph node involvement were present, again without evidence of visceral disease. Given the clinical and histologic findings, a diagnosis of generalized eruptive keratoacanthoma of Grzybowski was made. Cemiplimab was discontinued after the fifth cycle. The patient declined further systemic treatment, instead choosing a regimen of topical steroids and an emollient.
Immunotherapeutics have transformed cancer therapy, which includes ICIs that target cytotoxic T lymphocyte-associated antigen 4, PD-1, or PD-L1. Increased activity of these checkpoints allows tumor cells to downregulate T-cell activation, thereby evading immune destruction. When PD-1 on T cells binds PD-L1 on tumor cells, T lymphocytes are inhibited from cytotoxic-mediated killing. Therefore, anti-PD-1 ICIs such as cemiplimab permit T-lymphocyte activation and destruction of malignant cells. However, this unique mechanism of immunotherapy is associated with an array of IRAEs, which often manifest in a delayed and prolonged fashion.1 Immune-related adverse events most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.2 Notably, patients with certain tumors who experience these adverse effects might be more likely to have superior overall survival; therefore, IRAEs are sometimes used as an indicator of favorable treatment response.2,3
Dermatologic IRAEs associated with the use of a PD-1 inhibitor include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.4,5 Eruptions of keratoacanthoma rarely have been reported following treatment with the PD-1 inhibitors nivolumab and pembrolizumab.3,6,7 In our patient, we believe the profound and generalized eruptive keratoacanthoma—a well-differentiated cSCC variant—was related to treatment of locally advanced cSCC with cemiplimab. The mechanism underlying the formation of anti-PD-1 eruptive keratoacanthoma is not well understood. In susceptible patients, it is plausible that the inflammatory environment permitted by ICIs paradoxically induces regression of tumors such as locally invasive cSCC and simultaneously promotes formation of keratoacanthoma.
The role of inflammation in the pathogenesis and progression of cSCC is complex and possibly involves contrasting roles of leukocyte subpopulations.8 The increased incidence of cSCC in the immunocompromised population,8 PD-L1 overexpression in cSCC,9,10 and successful treatment of cSCC with PD-1 inhibition10 all suggest that inhibition of specific inflammatory pathways is pivotal in tumor pathogenesis. However, increased inflammation, particularly inflammation driven by T lymphocytes and Langerhans cells, also is believed to play a key role in the formation of cSCCs, including the degeneration of actinic keratosis into cSCC. Moreover, because keratoacanthomas are believed to be a cSCC variant and also are associated with PD-L1 overexpression,9 it is perplexing that PD-1 blockade may result in eruptive keratoacanthoma in some patients while also treating locally advanced cSCC, as seen in our patient. Successful treatment of keratoacanthoma with anti-inflammatory intralesional or topical corticosteroids adds to this complicated picture.3
We hypothesize that the pathogenesis of invasive cSCC and keratoacanthoma shares certain immune-mediated mechanisms but also differs in distinct manners. To understand the relationship between systemic treatment of cSCC and eruptive keratoacanthoma, further research is required.
In addition, the RAS/BRAF/MEK oncogenic pathway may be involved in the development of cSCCs associated with anti-PD-1. It is hypothesized that BRAF and MEK inhibition increases T-cell infiltration and increases PD-L1 expression on tumor cells,11 thus increasing the susceptibility of those cells to PD-1 blockade. Further supporting a relationship between the RAS/BRAF/MEK and PD-1 pathways, BRAF inhibitors are associated with development of SCCs and verrucal keratosis by upregulation of the RAS pathway.12,13 Perhaps a common mechanism underlying these pathways results in their shared association for an increased risk for cSCC upon blockade. More research is needed to fully elucidate the underlying biochemical mechanism of immunotherapy and formation of SCCs, such as keratoacanthoma.
Treatment of solitary keratoacanthoma often involves surgical excision; however, the sheer number of lesions in eruptive keratoacanthoma presents a larger dilemma. Because oral systemic retinoids have been shown to be most effective for treating eruptive keratoacanthoma, they are considered first-line therapy as monotherapy or in combination with surgical excision.3 Other treatment options include intralesional or topical corticosteroids, cyclosporine, 5-fluorouracil, imiquimod, and cryotherapy.3,6
The development of ICIs has revolutionized the treatment of cutaneous malignancy, yet we have a great deal more to comprehend on the systemic effects of these medications. Although IRAEs may signal a better response to therapy, some of these effects regrettably can be dose limiting. In our patient, cemiplimab was successful in treating locally advanced cSCC, but treatment also resulted in devastating widespread eruptive keratoacanthoma. The mechanism of this kind of eruption has yet to be understood; we hypothesize that it likely involves T lymphocyte–driven inflammation and the interplay of molecular and immune-mediated pathways.
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. doi:10.1038/s41572-020-0160-6
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi:10.1186/s40425-019-0805-8
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Shen J, Chang J, Mendenhall M, et al. Diverse cutaneous adverse eruptions caused by anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) immunotherapies: clinicalfeatures and management. Ther Adv Med Oncol. 2018;10:1758834017751634. doi:10.1177/1758834017751634
- Bandino JP, Perry DM, Clarke CE, et al. Two cases of anti-programmed cell death 1-associated bullous pemphigoid-like disease and eruptive keratoacanthomas featuring combined histopathology. J Eur Acad Dermatol Venereol. 2017;31:E378-E380. doi:10.1111/jdv.14179
- Marsh RL, Kolodney JA, Iyengar S, et al. Formation of eruptive cutaneous squamous cell carcinomas after programmed cell death protein-1 blockade. JAAD Case Rep. 2020;6:390-393. doi:10.1016/j.jdcr.2020.02.024
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Bottomley MJ, Thomson J, Harwood C, et al. The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019;20:2009. doi:10.3390/ijms20082009
- Gambichler T, Gnielka M, Rüddel I, et al. Expression of PD-L1 in keratoacanthoma and different stages of progression in cutaneous squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:1199-1204. doi:10.1007/s00262-017-2015-x
- Patel R, Chang ALS. Immune checkpoint inhibitors for treating advanced cutaneous squamous cell carcinoma. Am J Clin Dermatol. 2019;20:477-482. doi:10.1007/s40257-019-00426-w
- Rozeman EA, Blank CU. Combining checkpoint inhibition and targeted therapy in melanoma. Nat Med. 2019;25:879-882. doi:10.1038/s41591-019-0482-7
- Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23. doi:10.3816/CGC.2009.n.003
- Chen P, Chen F, Zhou B. Systematic review and meta-analysis of prevalence of dermatological toxicities associated with vemurafenib treatment in patients with melanoma. Clin Exp Dermatol. 2019;44:243-251. doi:10.1111/ced.13751
To the Editor:
Treatment of cancer, including cutaneous malignancy, has been transformed by the use of immunotherapeutic agents such as immune checkpoint inhibitors (ICIs) that target cytotoxic T lymphocyte-associated antigen 4, programmed cell-death protein 1 (PD-1), or programmed cell-death ligand 1 (PD-L1). However, these drugs are associated with a distinct set of immune-related adverse events (IRAEs). We present a case of generalized eruptive keratoacanthoma of Grzybowski associated with the ICI cemiplimab.
A 94-year-old White woman presented to the dermatology clinic with acute onset of extensive, locally advanced cutaneous squamous cell carcinoma (cSCC) of the upper right posterolateral calf as well as multiple noninvasive cSCCs of the arms and legs. Her medical history was remarkable for widespread actinic keratoses and numerous cSCCs. The patient had no personal or family history of melanoma. Various cSCCs had required treatment with electrodesiccation and curettage, topical or intralesional 5-fluorouracil, and Mohs micrographic surgery. Approximately 1 year prior to presentation, oral acitretin was initiated to help control the cSCC. Given the extent of locally advanced disease, which was considered unresectable, she was referred to oncology but continued to follow up with dermatology. Positron emission tomography was remarkable for hypermetabolic cutaneous thickening in the upper right posterolateral calf with no evidence of visceral disease.

The patient was started on cemiplimab, an anti-PD-1 monoclonal antibody ICI indicated for the treatment of both metastatic and advanced cSCC. After 4 cycles of intravenous cemiplimab, the patient developed widespread nodules covering the arms and legs (Figure 1) as well as associated tenderness and pruritus. Biopsies of nodules revealed superficially invasive, well-differentiated cSCC consistent with keratoacanthoma. Although a lymphocytic infiltrate was present, no other specific reaction pattern, such as a lichenoid infiltrate, was present (Figure 2).

Positron emission tomography was repeated, demonstrating resolution of the right calf lesion; however, new diffuse cutaneous lesions and inguinal lymph node involvement were present, again without evidence of visceral disease. Given the clinical and histologic findings, a diagnosis of generalized eruptive keratoacanthoma of Grzybowski was made. Cemiplimab was discontinued after the fifth cycle. The patient declined further systemic treatment, instead choosing a regimen of topical steroids and an emollient.
Immunotherapeutics have transformed cancer therapy, which includes ICIs that target cytotoxic T lymphocyte-associated antigen 4, PD-1, or PD-L1. Increased activity of these checkpoints allows tumor cells to downregulate T-cell activation, thereby evading immune destruction. When PD-1 on T cells binds PD-L1 on tumor cells, T lymphocytes are inhibited from cytotoxic-mediated killing. Therefore, anti-PD-1 ICIs such as cemiplimab permit T-lymphocyte activation and destruction of malignant cells. However, this unique mechanism of immunotherapy is associated with an array of IRAEs, which often manifest in a delayed and prolonged fashion.1 Immune-related adverse events most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.2 Notably, patients with certain tumors who experience these adverse effects might be more likely to have superior overall survival; therefore, IRAEs are sometimes used as an indicator of favorable treatment response.2,3
Dermatologic IRAEs associated with the use of a PD-1 inhibitor include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.4,5 Eruptions of keratoacanthoma rarely have been reported following treatment with the PD-1 inhibitors nivolumab and pembrolizumab.3,6,7 In our patient, we believe the profound and generalized eruptive keratoacanthoma—a well-differentiated cSCC variant—was related to treatment of locally advanced cSCC with cemiplimab. The mechanism underlying the formation of anti-PD-1 eruptive keratoacanthoma is not well understood. In susceptible patients, it is plausible that the inflammatory environment permitted by ICIs paradoxically induces regression of tumors such as locally invasive cSCC and simultaneously promotes formation of keratoacanthoma.
The role of inflammation in the pathogenesis and progression of cSCC is complex and possibly involves contrasting roles of leukocyte subpopulations.8 The increased incidence of cSCC in the immunocompromised population,8 PD-L1 overexpression in cSCC,9,10 and successful treatment of cSCC with PD-1 inhibition10 all suggest that inhibition of specific inflammatory pathways is pivotal in tumor pathogenesis. However, increased inflammation, particularly inflammation driven by T lymphocytes and Langerhans cells, also is believed to play a key role in the formation of cSCCs, including the degeneration of actinic keratosis into cSCC. Moreover, because keratoacanthomas are believed to be a cSCC variant and also are associated with PD-L1 overexpression,9 it is perplexing that PD-1 blockade may result in eruptive keratoacanthoma in some patients while also treating locally advanced cSCC, as seen in our patient. Successful treatment of keratoacanthoma with anti-inflammatory intralesional or topical corticosteroids adds to this complicated picture.3
We hypothesize that the pathogenesis of invasive cSCC and keratoacanthoma shares certain immune-mediated mechanisms but also differs in distinct manners. To understand the relationship between systemic treatment of cSCC and eruptive keratoacanthoma, further research is required.
In addition, the RAS/BRAF/MEK oncogenic pathway may be involved in the development of cSCCs associated with anti-PD-1. It is hypothesized that BRAF and MEK inhibition increases T-cell infiltration and increases PD-L1 expression on tumor cells,11 thus increasing the susceptibility of those cells to PD-1 blockade. Further supporting a relationship between the RAS/BRAF/MEK and PD-1 pathways, BRAF inhibitors are associated with development of SCCs and verrucal keratosis by upregulation of the RAS pathway.12,13 Perhaps a common mechanism underlying these pathways results in their shared association for an increased risk for cSCC upon blockade. More research is needed to fully elucidate the underlying biochemical mechanism of immunotherapy and formation of SCCs, such as keratoacanthoma.
Treatment of solitary keratoacanthoma often involves surgical excision; however, the sheer number of lesions in eruptive keratoacanthoma presents a larger dilemma. Because oral systemic retinoids have been shown to be most effective for treating eruptive keratoacanthoma, they are considered first-line therapy as monotherapy or in combination with surgical excision.3 Other treatment options include intralesional or topical corticosteroids, cyclosporine, 5-fluorouracil, imiquimod, and cryotherapy.3,6
The development of ICIs has revolutionized the treatment of cutaneous malignancy, yet we have a great deal more to comprehend on the systemic effects of these medications. Although IRAEs may signal a better response to therapy, some of these effects regrettably can be dose limiting. In our patient, cemiplimab was successful in treating locally advanced cSCC, but treatment also resulted in devastating widespread eruptive keratoacanthoma. The mechanism of this kind of eruption has yet to be understood; we hypothesize that it likely involves T lymphocyte–driven inflammation and the interplay of molecular and immune-mediated pathways.
To the Editor:
Treatment of cancer, including cutaneous malignancy, has been transformed by the use of immunotherapeutic agents such as immune checkpoint inhibitors (ICIs) that target cytotoxic T lymphocyte-associated antigen 4, programmed cell-death protein 1 (PD-1), or programmed cell-death ligand 1 (PD-L1). However, these drugs are associated with a distinct set of immune-related adverse events (IRAEs). We present a case of generalized eruptive keratoacanthoma of Grzybowski associated with the ICI cemiplimab.
A 94-year-old White woman presented to the dermatology clinic with acute onset of extensive, locally advanced cutaneous squamous cell carcinoma (cSCC) of the upper right posterolateral calf as well as multiple noninvasive cSCCs of the arms and legs. Her medical history was remarkable for widespread actinic keratoses and numerous cSCCs. The patient had no personal or family history of melanoma. Various cSCCs had required treatment with electrodesiccation and curettage, topical or intralesional 5-fluorouracil, and Mohs micrographic surgery. Approximately 1 year prior to presentation, oral acitretin was initiated to help control the cSCC. Given the extent of locally advanced disease, which was considered unresectable, she was referred to oncology but continued to follow up with dermatology. Positron emission tomography was remarkable for hypermetabolic cutaneous thickening in the upper right posterolateral calf with no evidence of visceral disease.

The patient was started on cemiplimab, an anti-PD-1 monoclonal antibody ICI indicated for the treatment of both metastatic and advanced cSCC. After 4 cycles of intravenous cemiplimab, the patient developed widespread nodules covering the arms and legs (Figure 1) as well as associated tenderness and pruritus. Biopsies of nodules revealed superficially invasive, well-differentiated cSCC consistent with keratoacanthoma. Although a lymphocytic infiltrate was present, no other specific reaction pattern, such as a lichenoid infiltrate, was present (Figure 2).

Positron emission tomography was repeated, demonstrating resolution of the right calf lesion; however, new diffuse cutaneous lesions and inguinal lymph node involvement were present, again without evidence of visceral disease. Given the clinical and histologic findings, a diagnosis of generalized eruptive keratoacanthoma of Grzybowski was made. Cemiplimab was discontinued after the fifth cycle. The patient declined further systemic treatment, instead choosing a regimen of topical steroids and an emollient.
Immunotherapeutics have transformed cancer therapy, which includes ICIs that target cytotoxic T lymphocyte-associated antigen 4, PD-1, or PD-L1. Increased activity of these checkpoints allows tumor cells to downregulate T-cell activation, thereby evading immune destruction. When PD-1 on T cells binds PD-L1 on tumor cells, T lymphocytes are inhibited from cytotoxic-mediated killing. Therefore, anti-PD-1 ICIs such as cemiplimab permit T-lymphocyte activation and destruction of malignant cells. However, this unique mechanism of immunotherapy is associated with an array of IRAEs, which often manifest in a delayed and prolonged fashion.1 Immune-related adverse events most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.2 Notably, patients with certain tumors who experience these adverse effects might be more likely to have superior overall survival; therefore, IRAEs are sometimes used as an indicator of favorable treatment response.2,3
Dermatologic IRAEs associated with the use of a PD-1 inhibitor include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.4,5 Eruptions of keratoacanthoma rarely have been reported following treatment with the PD-1 inhibitors nivolumab and pembrolizumab.3,6,7 In our patient, we believe the profound and generalized eruptive keratoacanthoma—a well-differentiated cSCC variant—was related to treatment of locally advanced cSCC with cemiplimab. The mechanism underlying the formation of anti-PD-1 eruptive keratoacanthoma is not well understood. In susceptible patients, it is plausible that the inflammatory environment permitted by ICIs paradoxically induces regression of tumors such as locally invasive cSCC and simultaneously promotes formation of keratoacanthoma.
The role of inflammation in the pathogenesis and progression of cSCC is complex and possibly involves contrasting roles of leukocyte subpopulations.8 The increased incidence of cSCC in the immunocompromised population,8 PD-L1 overexpression in cSCC,9,10 and successful treatment of cSCC with PD-1 inhibition10 all suggest that inhibition of specific inflammatory pathways is pivotal in tumor pathogenesis. However, increased inflammation, particularly inflammation driven by T lymphocytes and Langerhans cells, also is believed to play a key role in the formation of cSCCs, including the degeneration of actinic keratosis into cSCC. Moreover, because keratoacanthomas are believed to be a cSCC variant and also are associated with PD-L1 overexpression,9 it is perplexing that PD-1 blockade may result in eruptive keratoacanthoma in some patients while also treating locally advanced cSCC, as seen in our patient. Successful treatment of keratoacanthoma with anti-inflammatory intralesional or topical corticosteroids adds to this complicated picture.3
We hypothesize that the pathogenesis of invasive cSCC and keratoacanthoma shares certain immune-mediated mechanisms but also differs in distinct manners. To understand the relationship between systemic treatment of cSCC and eruptive keratoacanthoma, further research is required.
In addition, the RAS/BRAF/MEK oncogenic pathway may be involved in the development of cSCCs associated with anti-PD-1. It is hypothesized that BRAF and MEK inhibition increases T-cell infiltration and increases PD-L1 expression on tumor cells,11 thus increasing the susceptibility of those cells to PD-1 blockade. Further supporting a relationship between the RAS/BRAF/MEK and PD-1 pathways, BRAF inhibitors are associated with development of SCCs and verrucal keratosis by upregulation of the RAS pathway.12,13 Perhaps a common mechanism underlying these pathways results in their shared association for an increased risk for cSCC upon blockade. More research is needed to fully elucidate the underlying biochemical mechanism of immunotherapy and formation of SCCs, such as keratoacanthoma.
Treatment of solitary keratoacanthoma often involves surgical excision; however, the sheer number of lesions in eruptive keratoacanthoma presents a larger dilemma. Because oral systemic retinoids have been shown to be most effective for treating eruptive keratoacanthoma, they are considered first-line therapy as monotherapy or in combination with surgical excision.3 Other treatment options include intralesional or topical corticosteroids, cyclosporine, 5-fluorouracil, imiquimod, and cryotherapy.3,6
The development of ICIs has revolutionized the treatment of cutaneous malignancy, yet we have a great deal more to comprehend on the systemic effects of these medications. Although IRAEs may signal a better response to therapy, some of these effects regrettably can be dose limiting. In our patient, cemiplimab was successful in treating locally advanced cSCC, but treatment also resulted in devastating widespread eruptive keratoacanthoma. The mechanism of this kind of eruption has yet to be understood; we hypothesize that it likely involves T lymphocyte–driven inflammation and the interplay of molecular and immune-mediated pathways.
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. doi:10.1038/s41572-020-0160-6
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi:10.1186/s40425-019-0805-8
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Shen J, Chang J, Mendenhall M, et al. Diverse cutaneous adverse eruptions caused by anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) immunotherapies: clinicalfeatures and management. Ther Adv Med Oncol. 2018;10:1758834017751634. doi:10.1177/1758834017751634
- Bandino JP, Perry DM, Clarke CE, et al. Two cases of anti-programmed cell death 1-associated bullous pemphigoid-like disease and eruptive keratoacanthomas featuring combined histopathology. J Eur Acad Dermatol Venereol. 2017;31:E378-E380. doi:10.1111/jdv.14179
- Marsh RL, Kolodney JA, Iyengar S, et al. Formation of eruptive cutaneous squamous cell carcinomas after programmed cell death protein-1 blockade. JAAD Case Rep. 2020;6:390-393. doi:10.1016/j.jdcr.2020.02.024
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Bottomley MJ, Thomson J, Harwood C, et al. The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019;20:2009. doi:10.3390/ijms20082009
- Gambichler T, Gnielka M, Rüddel I, et al. Expression of PD-L1 in keratoacanthoma and different stages of progression in cutaneous squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:1199-1204. doi:10.1007/s00262-017-2015-x
- Patel R, Chang ALS. Immune checkpoint inhibitors for treating advanced cutaneous squamous cell carcinoma. Am J Clin Dermatol. 2019;20:477-482. doi:10.1007/s40257-019-00426-w
- Rozeman EA, Blank CU. Combining checkpoint inhibition and targeted therapy in melanoma. Nat Med. 2019;25:879-882. doi:10.1038/s41591-019-0482-7
- Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23. doi:10.3816/CGC.2009.n.003
- Chen P, Chen F, Zhou B. Systematic review and meta-analysis of prevalence of dermatological toxicities associated with vemurafenib treatment in patients with melanoma. Clin Exp Dermatol. 2019;44:243-251. doi:10.1111/ced.13751
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. doi:10.1038/s41572-020-0160-6
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi:10.1186/s40425-019-0805-8
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Shen J, Chang J, Mendenhall M, et al. Diverse cutaneous adverse eruptions caused by anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) immunotherapies: clinicalfeatures and management. Ther Adv Med Oncol. 2018;10:1758834017751634. doi:10.1177/1758834017751634
- Bandino JP, Perry DM, Clarke CE, et al. Two cases of anti-programmed cell death 1-associated bullous pemphigoid-like disease and eruptive keratoacanthomas featuring combined histopathology. J Eur Acad Dermatol Venereol. 2017;31:E378-E380. doi:10.1111/jdv.14179
- Marsh RL, Kolodney JA, Iyengar S, et al. Formation of eruptive cutaneous squamous cell carcinomas after programmed cell death protein-1 blockade. JAAD Case Rep. 2020;6:390-393. doi:10.1016/j.jdcr.2020.02.024
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Bottomley MJ, Thomson J, Harwood C, et al. The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019;20:2009. doi:10.3390/ijms20082009
- Gambichler T, Gnielka M, Rüddel I, et al. Expression of PD-L1 in keratoacanthoma and different stages of progression in cutaneous squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:1199-1204. doi:10.1007/s00262-017-2015-x
- Patel R, Chang ALS. Immune checkpoint inhibitors for treating advanced cutaneous squamous cell carcinoma. Am J Clin Dermatol. 2019;20:477-482. doi:10.1007/s40257-019-00426-w
- Rozeman EA, Blank CU. Combining checkpoint inhibition and targeted therapy in melanoma. Nat Med. 2019;25:879-882. doi:10.1038/s41591-019-0482-7
- Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23. doi:10.3816/CGC.2009.n.003
- Chen P, Chen F, Zhou B. Systematic review and meta-analysis of prevalence of dermatological toxicities associated with vemurafenib treatment in patients with melanoma. Clin Exp Dermatol. 2019;44:243-251. doi:10.1111/ced.13751
Practice Points
- Immunotherapy, including immune checkpoint inhibitors such as programmed cell-death protein 1 (PD-1) inhibitors, is associated with an array of immune-related adverse events that often manifest in a delayed and prolonged manner. They most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.
- Dermatologic adverse effects associated with PD-1 inhibitors include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.
- Eruptions of keratoacanthoma rarely have been reported following treatment with PD-1 inhibitors such as cemiplimab, nivolumab, and pembrolizumab.
Thalidomide Analogue Drug Eruption Along the Lines of Blaschko
To the Editor:
Lenalidomide is a thalidomide analogue used to treat various hematologic malignancies, including non-Hodgkin lymphoma, myelodysplastic syndrome, and multiple myeloma (MM).1 Lenalidomide is referred to as a degrader therapeutic because it induces targeted protein degradation of disease-relevant proteins (eg, Ikaros family zinc finger protein 1 [IKZF1], Ikaros family zinc finger protein 3 [IKZF3], and casein kinase I isoform-α [CK1α]) as its primary mechanism of action.1,2 Although cutaneous adverse events are relatively common among thalidomide analogues, the morphologic and histopathologic descriptions of these drug eruptions have not been fully elucidated.3,4 We report a novel pityriasiform drug eruption followed by a clinical eruption suggestive of blaschkitis in a patient with MM who was being treated with lenalidomide.
A 76-year-old man presented to the dermatology clinic with a progressive, mildly pruritic eruption on the chest and axillae of 1 year’s duration. He had a medical history of chronic hepatitis B, malignant carcinoid tumor of the colon, prostate cancer, and MM. The eruption emerged 1 to 2 weeks after the patient started oral lenalidomide 10 mg/d and oral dexamethasone40 mg/wk following autologous stem cell transplantation for MM. The patient had not received any other therapy for MM.
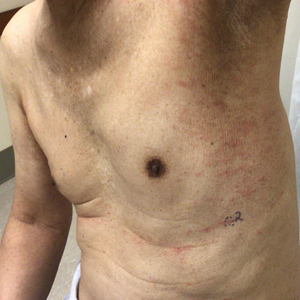
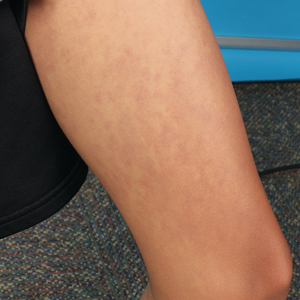
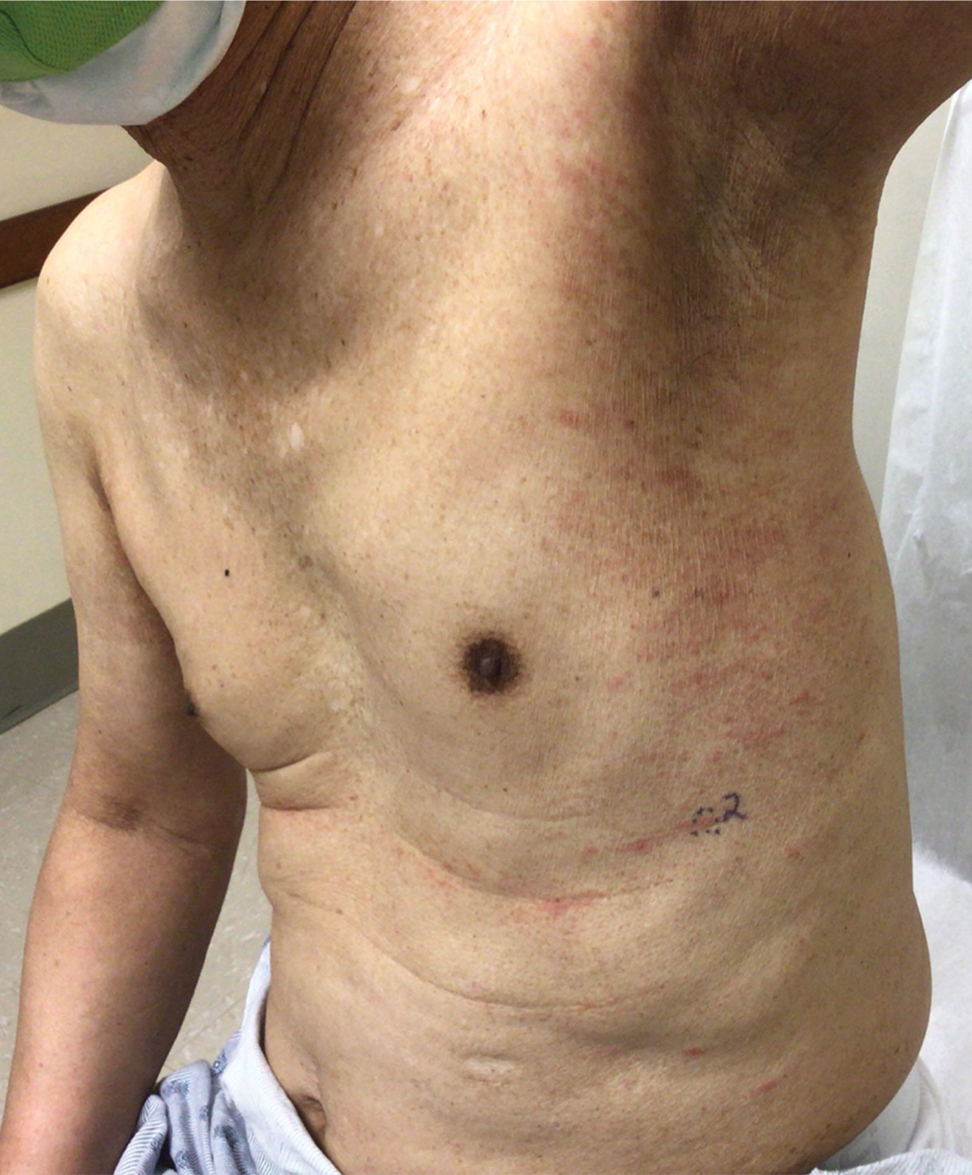
Physical examination revealed multiple erythematous, hyperpigmented, scaly papules and plaques on the lateral chest and within the axillae (Figure 1). A skin biopsy from the left axilla demonstrated a mild lichenoid and perivascular lymphocytic infiltrate with scattered eosinophils, neutrophils, and extravasated erythrocytes. The overlying epidermis showed spongiosis with parakeratosis in addition to lymphocytic exocytosis (Figure 2). No fungal organisms were highlighted on periodic acid–Schiff staining. After this evaluation, we recommended that the patient discontinue lenalidomide and start taking a topical over-the-counter corticosteroid for 2 weeks. Over time, he noted marked improvement in the eruption and associated pruritus.
After a drug holiday of 2 months, the patient resumed a maintenance dosage of oral lenalidomide 10 mg/d. Four or 5 days after restarting lenalidomide, a pruritic eruption appeared that involved the axillae and the left lower abdomen, circling around to the left lower back. The axillary eruption resolved with a topical over-the-counter corticosteroid; the abdominal eruption persisted.
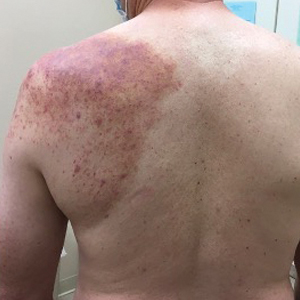
At the 3-month follow-up visit, physical examination revealed erythematous macules and papules that coalesced over a salmon-colored base along the lines of Blaschko extending from the left lower abdominal quadrant, crossing the left flank, and continuing to the left lower back without crossing the midline (Figure 3).
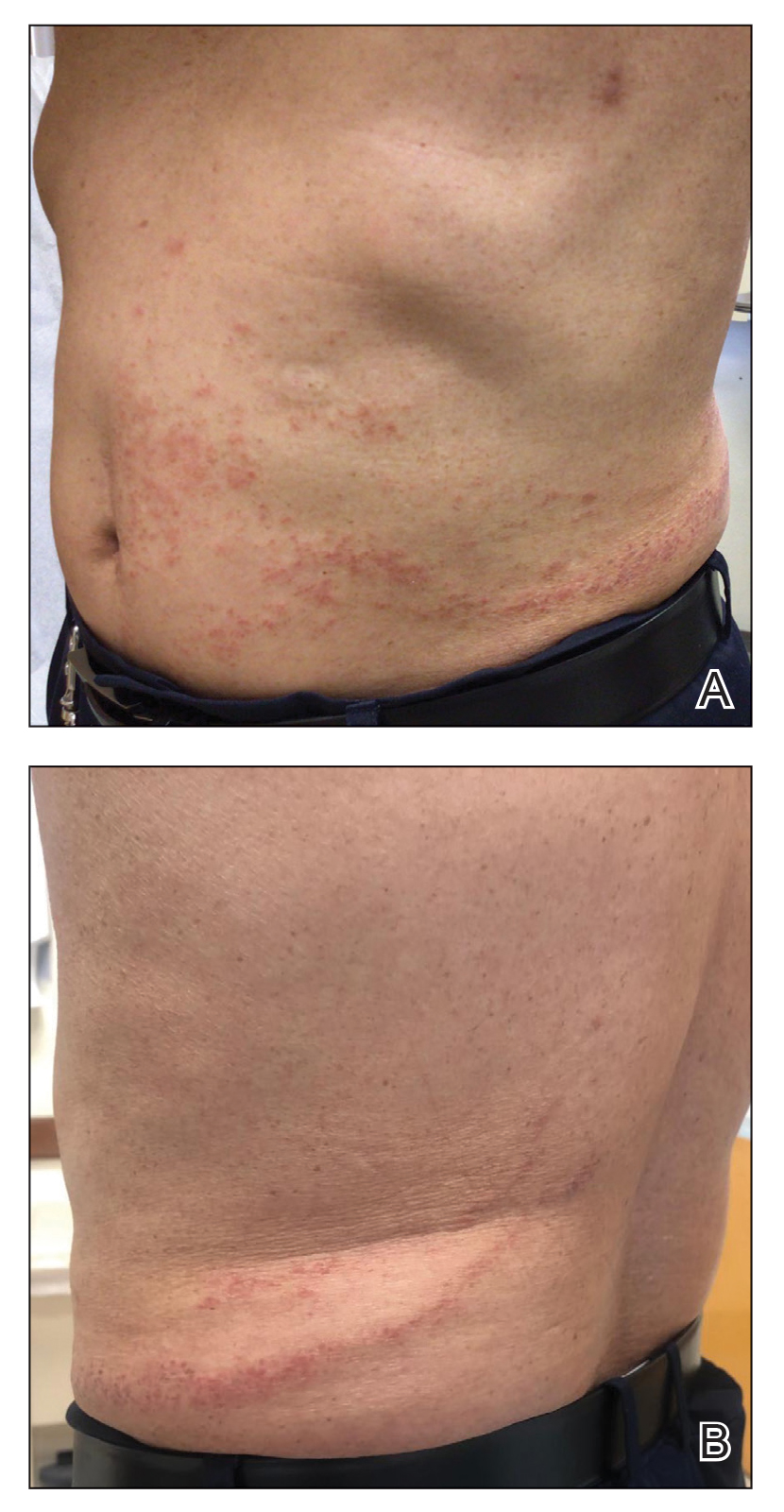
We recommended that the patient continue treatment through this eruption; he was instructed to apply a corticosteroid cream and resume lenalidomide at the maintenance dosage. A month later, he reported that the eruption and associated pruritus resolved with the corticosteroid cream and resumption of the maintenance dose of lenalidomide. The patient noted no further spread of the eruption.
Cutaneous adverse events are common following lenalidomide. In prior trials, the overall incidence of any-grade rash following lenalidomide exposure was 22% to 33%.5 A meta-analysis of 10 trials determined the overall incidence of all-grade and high-grade cutaneous adverse events after exposure to lenalidomide was 27.2% and 3.6%, respectively.6 Our case represents a pityriasiform eruption due to lenalidomide followed by a secondary eruption suggestive of blaschkitis.
The rash due to lenalidomide has been described as morbilliform, urticarial, dermatitic, acneform, and undefined.7 Lenalidomide-induced rash typically develops during the first month of therapy, similar to our patient’s presentation. It has even been observed in the first week of therapy.8 Severe reactions such as Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported.5,6 Risk factors associated with rash secondary to lenalidomide include advanced age (≥70 years), presence of Bence-Jones protein-type MM in urine, and no prior chemotherapy.8 Our patient had 2 of these risk factors: advanced age and no prior chemotherapy for MM. The exact pathogenesis by which lenalidomide leads to a pityriasiform eruption, as in our patient, or to a rash in general is unclear. Studies have hypothesized that a lenalidomide-induced rash could be attributable to a delayed hypersensitivity type IV reaction or to a reaction related to the molecular mechanism of action of the drug.9
At the molecular level, the antimyeloma effects of lenalidomide include promoting degradation of transcription factors IKZF1 and IKZF3, which subsequently increases production of IL-2.1,2,9 Recombinant IL-2 has been associated with an increased incidence of rash in other cancers.9 Overexpression of programmed death 1(PD-1) and its ligand (PD-L1) has been demonstrated in MM; lenalidomide has been shown to downregulate both PD-1 and PD-L1. Patients receiving PD-1 and PD-L1 inhibitors commonly have developed rash.9 However, the association between lenalidomide and its downregulation of PD-1 and PD-L1 leading to rash has not been fully elucidated. Given the multiple malignancies in our patient—MM, prostate cancer, malignant carcinoid tumor—an underlying paraneoplastic phenomenon may be possible. Additionally, because our patient initially received dexamethasone along with lenalidomide, the manifestation of the initial pityriasiform rash may have been less severe due to the steroid use. Although our patient underwent a 2-month drug holiday following the initial pityriasiform eruption, most lenalidomide-induced rashes do not necessitate discontinuation of the drug.5,7
Our patient’s secondary drug eruption was clinically suggestive of lenalidomide-induced blaschkitis. A report of a German patient with plasmacytoma described a unilateral papular exanthem that developed 4 months after lenalidomide was initiated.10 The papular exanthem following the lines of Blaschko lines extended from that patient’s posterior left foot to the calf and on to the thigh and flank,10 which was more extensive than our patient’s eruption. Blaschkitis in this patient resolved with a corticosteroid cream and UV light therapy10; lenalidomide was not discontinued, similar to our patient.
The pathogenesis of our patient’s secondary eruption that preferentially involved the lines of Blaschko is unclear. After the initial pityriasiform eruption, the secondary eruption was blaschkitis. Distinguishing dermatomes from the lines of Blaschko, which are thought to represent pathways of epidermal cell migration and proliferation during embryologic development, is important. Genodermatoses such as incontinentia pigmenti and hypomelanosis of Ito involve the lines of Blaschko11; other disorders in the differential diagnosis of linear configurations include linear lichen planus, linear cutaneous lupus erythematosus, linear morphea, and lichen striatus.11 Notably, drug-induced blaschkitis is rare.
Cutaneous adverse reactions from thalidomide analogues are relatively common. Our case of lenalidomide-associated blaschkitis that developed following an initial pityriasiform drug eruption in a patient with MM highlights that dermatologists need to collaborate with the oncologist regarding the severity of drug eruptions to determine if the patient should continue treatment through the cutaneous eruptions or discontinue a vital medication.
- Jan M, Sperling AS, Ebert BL. Cancer therapies based on targeted protein degradation—lessons learned with lenalidomide. Nat Rev Clin Oncol. 2021;18:401-417. doi:10.1038/s41571-021-00479-z
- Shah UA, Mailankody S. Emerging immunotherapies in multiple myeloma. BMJ. 2020;370:3176. doi:10.1136/BMJ.M3176
- Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458-3464. doi:10.1182/BLOOD-2006-04-015909
- Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906-917. doi:10.1056/NEJMOA1402551
- Tinsley SM, Kurtin SE, Ridgeway JA. Practical management of lenalidomide-related rash. Clin Lymphoma Myeloma Leuk. 2015;15(suppl):S64-S69. doi:10.1016/J.CLML.2015.02.008
- Nardone B, Wu S, Garden BC, et al. Risk of rash associated with lenalidomide in cancer patients: a systematic review of the literature and meta-analysis. Clin Lymphoma Myeloma Leuk. 2013;13:424-429. doi:10.1016/J.CLML.2013.03.006
- Sviggum HP, Davis MDP, Rajkumar SV, et al. Dermatologic adverse effects of lenalidomide therapy for amyloidosis and multiple myeloma. Arch Dermatol. 2006;142:1298-1302. doi:10.1001/ARCHDERM.142.10.1298
- Sugi T, Nishigami Y, Saigo H, et al. Analysis of risk factors for lenalidomide-associated skin rash in patients with multiple myeloma. Leuk Lymphoma. 2021;62:1405-1410. doi:10.1080/10428194.2021.1876867
- Barley K, He W, Agarwal S, et al. Outcomes and management of lenalidomide-associated rash in patients with multiple myeloma. Leuk Lymphoma. 2016;57:2510-2515. doi:10.3109/10428194.2016.1151507
- Grape J, Frosch P. Papular drug eruption along the lines of Blaschko caused by lenalidomide [in German]. Hautarzt. 2011;62:618-620. doi:10.1007/S00105-010-2121-6
- Bolognia JL, Orlow SJ, Glick SA. Lines of Blaschko. J Am Acad Dermatol. 1994;31(2 pt 1):157-190. doi:10.1016/S0190-9622(94)70143-1
To the Editor:
Lenalidomide is a thalidomide analogue used to treat various hematologic malignancies, including non-Hodgkin lymphoma, myelodysplastic syndrome, and multiple myeloma (MM).1 Lenalidomide is referred to as a degrader therapeutic because it induces targeted protein degradation of disease-relevant proteins (eg, Ikaros family zinc finger protein 1 [IKZF1], Ikaros family zinc finger protein 3 [IKZF3], and casein kinase I isoform-α [CK1α]) as its primary mechanism of action.1,2 Although cutaneous adverse events are relatively common among thalidomide analogues, the morphologic and histopathologic descriptions of these drug eruptions have not been fully elucidated.3,4 We report a novel pityriasiform drug eruption followed by a clinical eruption suggestive of blaschkitis in a patient with MM who was being treated with lenalidomide.
A 76-year-old man presented to the dermatology clinic with a progressive, mildly pruritic eruption on the chest and axillae of 1 year’s duration. He had a medical history of chronic hepatitis B, malignant carcinoid tumor of the colon, prostate cancer, and MM. The eruption emerged 1 to 2 weeks after the patient started oral lenalidomide 10 mg/d and oral dexamethasone40 mg/wk following autologous stem cell transplantation for MM. The patient had not received any other therapy for MM.
Physical examination revealed multiple erythematous, hyperpigmented, scaly papules and plaques on the lateral chest and within the axillae (Figure 1). A skin biopsy from the left axilla demonstrated a mild lichenoid and perivascular lymphocytic infiltrate with scattered eosinophils, neutrophils, and extravasated erythrocytes. The overlying epidermis showed spongiosis with parakeratosis in addition to lymphocytic exocytosis (Figure 2). No fungal organisms were highlighted on periodic acid–Schiff staining. After this evaluation, we recommended that the patient discontinue lenalidomide and start taking a topical over-the-counter corticosteroid for 2 weeks. Over time, he noted marked improvement in the eruption and associated pruritus.

After a drug holiday of 2 months, the patient resumed a maintenance dosage of oral lenalidomide 10 mg/d. Four or 5 days after restarting lenalidomide, a pruritic eruption appeared that involved the axillae and the left lower abdomen, circling around to the left lower back. The axillary eruption resolved with a topical over-the-counter corticosteroid; the abdominal eruption persisted.

At the 3-month follow-up visit, physical examination revealed erythematous macules and papules that coalesced over a salmon-colored base along the lines of Blaschko extending from the left lower abdominal quadrant, crossing the left flank, and continuing to the left lower back without crossing the midline (Figure 3).

We recommended that the patient continue treatment through this eruption; he was instructed to apply a corticosteroid cream and resume lenalidomide at the maintenance dosage. A month later, he reported that the eruption and associated pruritus resolved with the corticosteroid cream and resumption of the maintenance dose of lenalidomide. The patient noted no further spread of the eruption.
Cutaneous adverse events are common following lenalidomide. In prior trials, the overall incidence of any-grade rash following lenalidomide exposure was 22% to 33%.5 A meta-analysis of 10 trials determined the overall incidence of all-grade and high-grade cutaneous adverse events after exposure to lenalidomide was 27.2% and 3.6%, respectively.6 Our case represents a pityriasiform eruption due to lenalidomide followed by a secondary eruption suggestive of blaschkitis.
The rash due to lenalidomide has been described as morbilliform, urticarial, dermatitic, acneform, and undefined.7 Lenalidomide-induced rash typically develops during the first month of therapy, similar to our patient’s presentation. It has even been observed in the first week of therapy.8 Severe reactions such as Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported.5,6 Risk factors associated with rash secondary to lenalidomide include advanced age (≥70 years), presence of Bence-Jones protein-type MM in urine, and no prior chemotherapy.8 Our patient had 2 of these risk factors: advanced age and no prior chemotherapy for MM. The exact pathogenesis by which lenalidomide leads to a pityriasiform eruption, as in our patient, or to a rash in general is unclear. Studies have hypothesized that a lenalidomide-induced rash could be attributable to a delayed hypersensitivity type IV reaction or to a reaction related to the molecular mechanism of action of the drug.9
At the molecular level, the antimyeloma effects of lenalidomide include promoting degradation of transcription factors IKZF1 and IKZF3, which subsequently increases production of IL-2.1,2,9 Recombinant IL-2 has been associated with an increased incidence of rash in other cancers.9 Overexpression of programmed death 1(PD-1) and its ligand (PD-L1) has been demonstrated in MM; lenalidomide has been shown to downregulate both PD-1 and PD-L1. Patients receiving PD-1 and PD-L1 inhibitors commonly have developed rash.9 However, the association between lenalidomide and its downregulation of PD-1 and PD-L1 leading to rash has not been fully elucidated. Given the multiple malignancies in our patient—MM, prostate cancer, malignant carcinoid tumor—an underlying paraneoplastic phenomenon may be possible. Additionally, because our patient initially received dexamethasone along with lenalidomide, the manifestation of the initial pityriasiform rash may have been less severe due to the steroid use. Although our patient underwent a 2-month drug holiday following the initial pityriasiform eruption, most lenalidomide-induced rashes do not necessitate discontinuation of the drug.5,7
Our patient’s secondary drug eruption was clinically suggestive of lenalidomide-induced blaschkitis. A report of a German patient with plasmacytoma described a unilateral papular exanthem that developed 4 months after lenalidomide was initiated.10 The papular exanthem following the lines of Blaschko lines extended from that patient’s posterior left foot to the calf and on to the thigh and flank,10 which was more extensive than our patient’s eruption. Blaschkitis in this patient resolved with a corticosteroid cream and UV light therapy10; lenalidomide was not discontinued, similar to our patient.
The pathogenesis of our patient’s secondary eruption that preferentially involved the lines of Blaschko is unclear. After the initial pityriasiform eruption, the secondary eruption was blaschkitis. Distinguishing dermatomes from the lines of Blaschko, which are thought to represent pathways of epidermal cell migration and proliferation during embryologic development, is important. Genodermatoses such as incontinentia pigmenti and hypomelanosis of Ito involve the lines of Blaschko11; other disorders in the differential diagnosis of linear configurations include linear lichen planus, linear cutaneous lupus erythematosus, linear morphea, and lichen striatus.11 Notably, drug-induced blaschkitis is rare.
Cutaneous adverse reactions from thalidomide analogues are relatively common. Our case of lenalidomide-associated blaschkitis that developed following an initial pityriasiform drug eruption in a patient with MM highlights that dermatologists need to collaborate with the oncologist regarding the severity of drug eruptions to determine if the patient should continue treatment through the cutaneous eruptions or discontinue a vital medication.
To the Editor:
Lenalidomide is a thalidomide analogue used to treat various hematologic malignancies, including non-Hodgkin lymphoma, myelodysplastic syndrome, and multiple myeloma (MM).1 Lenalidomide is referred to as a degrader therapeutic because it induces targeted protein degradation of disease-relevant proteins (eg, Ikaros family zinc finger protein 1 [IKZF1], Ikaros family zinc finger protein 3 [IKZF3], and casein kinase I isoform-α [CK1α]) as its primary mechanism of action.1,2 Although cutaneous adverse events are relatively common among thalidomide analogues, the morphologic and histopathologic descriptions of these drug eruptions have not been fully elucidated.3,4 We report a novel pityriasiform drug eruption followed by a clinical eruption suggestive of blaschkitis in a patient with MM who was being treated with lenalidomide.
A 76-year-old man presented to the dermatology clinic with a progressive, mildly pruritic eruption on the chest and axillae of 1 year’s duration. He had a medical history of chronic hepatitis B, malignant carcinoid tumor of the colon, prostate cancer, and MM. The eruption emerged 1 to 2 weeks after the patient started oral lenalidomide 10 mg/d and oral dexamethasone40 mg/wk following autologous stem cell transplantation for MM. The patient had not received any other therapy for MM.
Physical examination revealed multiple erythematous, hyperpigmented, scaly papules and plaques on the lateral chest and within the axillae (Figure 1). A skin biopsy from the left axilla demonstrated a mild lichenoid and perivascular lymphocytic infiltrate with scattered eosinophils, neutrophils, and extravasated erythrocytes. The overlying epidermis showed spongiosis with parakeratosis in addition to lymphocytic exocytosis (Figure 2). No fungal organisms were highlighted on periodic acid–Schiff staining. After this evaluation, we recommended that the patient discontinue lenalidomide and start taking a topical over-the-counter corticosteroid for 2 weeks. Over time, he noted marked improvement in the eruption and associated pruritus.

After a drug holiday of 2 months, the patient resumed a maintenance dosage of oral lenalidomide 10 mg/d. Four or 5 days after restarting lenalidomide, a pruritic eruption appeared that involved the axillae and the left lower abdomen, circling around to the left lower back. The axillary eruption resolved with a topical over-the-counter corticosteroid; the abdominal eruption persisted.

At the 3-month follow-up visit, physical examination revealed erythematous macules and papules that coalesced over a salmon-colored base along the lines of Blaschko extending from the left lower abdominal quadrant, crossing the left flank, and continuing to the left lower back without crossing the midline (Figure 3).

We recommended that the patient continue treatment through this eruption; he was instructed to apply a corticosteroid cream and resume lenalidomide at the maintenance dosage. A month later, he reported that the eruption and associated pruritus resolved with the corticosteroid cream and resumption of the maintenance dose of lenalidomide. The patient noted no further spread of the eruption.
Cutaneous adverse events are common following lenalidomide. In prior trials, the overall incidence of any-grade rash following lenalidomide exposure was 22% to 33%.5 A meta-analysis of 10 trials determined the overall incidence of all-grade and high-grade cutaneous adverse events after exposure to lenalidomide was 27.2% and 3.6%, respectively.6 Our case represents a pityriasiform eruption due to lenalidomide followed by a secondary eruption suggestive of blaschkitis.
The rash due to lenalidomide has been described as morbilliform, urticarial, dermatitic, acneform, and undefined.7 Lenalidomide-induced rash typically develops during the first month of therapy, similar to our patient’s presentation. It has even been observed in the first week of therapy.8 Severe reactions such as Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported.5,6 Risk factors associated with rash secondary to lenalidomide include advanced age (≥70 years), presence of Bence-Jones protein-type MM in urine, and no prior chemotherapy.8 Our patient had 2 of these risk factors: advanced age and no prior chemotherapy for MM. The exact pathogenesis by which lenalidomide leads to a pityriasiform eruption, as in our patient, or to a rash in general is unclear. Studies have hypothesized that a lenalidomide-induced rash could be attributable to a delayed hypersensitivity type IV reaction or to a reaction related to the molecular mechanism of action of the drug.9
At the molecular level, the antimyeloma effects of lenalidomide include promoting degradation of transcription factors IKZF1 and IKZF3, which subsequently increases production of IL-2.1,2,9 Recombinant IL-2 has been associated with an increased incidence of rash in other cancers.9 Overexpression of programmed death 1(PD-1) and its ligand (PD-L1) has been demonstrated in MM; lenalidomide has been shown to downregulate both PD-1 and PD-L1. Patients receiving PD-1 and PD-L1 inhibitors commonly have developed rash.9 However, the association between lenalidomide and its downregulation of PD-1 and PD-L1 leading to rash has not been fully elucidated. Given the multiple malignancies in our patient—MM, prostate cancer, malignant carcinoid tumor—an underlying paraneoplastic phenomenon may be possible. Additionally, because our patient initially received dexamethasone along with lenalidomide, the manifestation of the initial pityriasiform rash may have been less severe due to the steroid use. Although our patient underwent a 2-month drug holiday following the initial pityriasiform eruption, most lenalidomide-induced rashes do not necessitate discontinuation of the drug.5,7
Our patient’s secondary drug eruption was clinically suggestive of lenalidomide-induced blaschkitis. A report of a German patient with plasmacytoma described a unilateral papular exanthem that developed 4 months after lenalidomide was initiated.10 The papular exanthem following the lines of Blaschko lines extended from that patient’s posterior left foot to the calf and on to the thigh and flank,10 which was more extensive than our patient’s eruption. Blaschkitis in this patient resolved with a corticosteroid cream and UV light therapy10; lenalidomide was not discontinued, similar to our patient.
The pathogenesis of our patient’s secondary eruption that preferentially involved the lines of Blaschko is unclear. After the initial pityriasiform eruption, the secondary eruption was blaschkitis. Distinguishing dermatomes from the lines of Blaschko, which are thought to represent pathways of epidermal cell migration and proliferation during embryologic development, is important. Genodermatoses such as incontinentia pigmenti and hypomelanosis of Ito involve the lines of Blaschko11; other disorders in the differential diagnosis of linear configurations include linear lichen planus, linear cutaneous lupus erythematosus, linear morphea, and lichen striatus.11 Notably, drug-induced blaschkitis is rare.
Cutaneous adverse reactions from thalidomide analogues are relatively common. Our case of lenalidomide-associated blaschkitis that developed following an initial pityriasiform drug eruption in a patient with MM highlights that dermatologists need to collaborate with the oncologist regarding the severity of drug eruptions to determine if the patient should continue treatment through the cutaneous eruptions or discontinue a vital medication.
- Jan M, Sperling AS, Ebert BL. Cancer therapies based on targeted protein degradation—lessons learned with lenalidomide. Nat Rev Clin Oncol. 2021;18:401-417. doi:10.1038/s41571-021-00479-z
- Shah UA, Mailankody S. Emerging immunotherapies in multiple myeloma. BMJ. 2020;370:3176. doi:10.1136/BMJ.M3176
- Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458-3464. doi:10.1182/BLOOD-2006-04-015909
- Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906-917. doi:10.1056/NEJMOA1402551
- Tinsley SM, Kurtin SE, Ridgeway JA. Practical management of lenalidomide-related rash. Clin Lymphoma Myeloma Leuk. 2015;15(suppl):S64-S69. doi:10.1016/J.CLML.2015.02.008
- Nardone B, Wu S, Garden BC, et al. Risk of rash associated with lenalidomide in cancer patients: a systematic review of the literature and meta-analysis. Clin Lymphoma Myeloma Leuk. 2013;13:424-429. doi:10.1016/J.CLML.2013.03.006
- Sviggum HP, Davis MDP, Rajkumar SV, et al. Dermatologic adverse effects of lenalidomide therapy for amyloidosis and multiple myeloma. Arch Dermatol. 2006;142:1298-1302. doi:10.1001/ARCHDERM.142.10.1298
- Sugi T, Nishigami Y, Saigo H, et al. Analysis of risk factors for lenalidomide-associated skin rash in patients with multiple myeloma. Leuk Lymphoma. 2021;62:1405-1410. doi:10.1080/10428194.2021.1876867
- Barley K, He W, Agarwal S, et al. Outcomes and management of lenalidomide-associated rash in patients with multiple myeloma. Leuk Lymphoma. 2016;57:2510-2515. doi:10.3109/10428194.2016.1151507
- Grape J, Frosch P. Papular drug eruption along the lines of Blaschko caused by lenalidomide [in German]. Hautarzt. 2011;62:618-620. doi:10.1007/S00105-010-2121-6
- Bolognia JL, Orlow SJ, Glick SA. Lines of Blaschko. J Am Acad Dermatol. 1994;31(2 pt 1):157-190. doi:10.1016/S0190-9622(94)70143-1
- Jan M, Sperling AS, Ebert BL. Cancer therapies based on targeted protein degradation—lessons learned with lenalidomide. Nat Rev Clin Oncol. 2021;18:401-417. doi:10.1038/s41571-021-00479-z
- Shah UA, Mailankody S. Emerging immunotherapies in multiple myeloma. BMJ. 2020;370:3176. doi:10.1136/BMJ.M3176
- Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458-3464. doi:10.1182/BLOOD-2006-04-015909
- Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906-917. doi:10.1056/NEJMOA1402551
- Tinsley SM, Kurtin SE, Ridgeway JA. Practical management of lenalidomide-related rash. Clin Lymphoma Myeloma Leuk. 2015;15(suppl):S64-S69. doi:10.1016/J.CLML.2015.02.008
- Nardone B, Wu S, Garden BC, et al. Risk of rash associated with lenalidomide in cancer patients: a systematic review of the literature and meta-analysis. Clin Lymphoma Myeloma Leuk. 2013;13:424-429. doi:10.1016/J.CLML.2013.03.006
- Sviggum HP, Davis MDP, Rajkumar SV, et al. Dermatologic adverse effects of lenalidomide therapy for amyloidosis and multiple myeloma. Arch Dermatol. 2006;142:1298-1302. doi:10.1001/ARCHDERM.142.10.1298
- Sugi T, Nishigami Y, Saigo H, et al. Analysis of risk factors for lenalidomide-associated skin rash in patients with multiple myeloma. Leuk Lymphoma. 2021;62:1405-1410. doi:10.1080/10428194.2021.1876867
- Barley K, He W, Agarwal S, et al. Outcomes and management of lenalidomide-associated rash in patients with multiple myeloma. Leuk Lymphoma. 2016;57:2510-2515. doi:10.3109/10428194.2016.1151507
- Grape J, Frosch P. Papular drug eruption along the lines of Blaschko caused by lenalidomide [in German]. Hautarzt. 2011;62:618-620. doi:10.1007/S00105-010-2121-6
- Bolognia JL, Orlow SJ, Glick SA. Lines of Blaschko. J Am Acad Dermatol. 1994;31(2 pt 1):157-190. doi:10.1016/S0190-9622(94)70143-1
Practice Points
- Dermatologists should be aware of the variety of cutaneous adverse events that can arise from the use of immunotherapeutic agents for hematologic malignancies.
- Some cutaneous reactions to immunotherapeutic medications, such as pityriasiform eruption and blaschkitis, generally are benign and may not necessitate halting an important therapy.
Reactive Angioendotheliomatosis Following Ad26.COV2.S Vaccination
To the Editor:
Reactive angioendotheliomatosis (RAE) is a rare self-limited cutaneous vascular proliferation of endothelial cells within blood vessels that manifests clinically as infiltrated red-blue patches and plaques with purpura that can progress to occlude vascular lumina. The etiology of RAE is mostly idiopathic; however, the disorder typically occurs in association with a range of systemic diseases, including infection, cryoglobulinemia, leukemia, antiphospholipid syndrome, peripheral vascular disease, and arteriovenous fistula. Histopathologic examination of these lesions shows marked proliferation of endothelial cells, including occlusion of the lumen of blood vessels over wide areas.
After ruling out malignancy, treatment of RAE focuses on targeting the underlying cause or disease, if any is present; 75% of reported cases occur in association with systemic disease.1 Onset can occur at any age without predilection for sex. Reactive angioendotheliomatosis commonly manifests on the extremities but may occur on the head and neck in rare instances.2
The rarity of the condition and its poorly defined clinical characteristics make it difficult to develop a treatment plan. There are no standardized treatment guidelines for the reactive form of angiomatosis. We report a case of RAE that developed 2 weeks after vaccination with the Ad26.COV2.S vaccine (Johnson & Johnson Innovative Medicine [formerly Janssen Pharmaceutical Companies of Johnson & Johnson]) that improved following 2 weeks of treatment with a topical corticosteroid and an oral antihistamine.
A 58-year-old man presented to an outpatient dermatology clinic with pruritus and occasional paresthesia associated with a rash over the left arm of 1 month’s duration. The patient suspected that the rash may have formed secondary to the bite of oak mites on the arms and chest while he was carrying milled wood. Further inquiry into the patient’s history revealed that he received the Ad26.COV2.S vaccine 2 weeks prior to the appearance of the rash. He denied mechanical trauma. His medical history included hypercholesterolemia and a mild COVID-19 infection 8 months prior to the appearance of the rash that did not require hospitalization. He denied fever or chills during the 2 weeks following vaccination. The pruritus was minimally relieved for short periods with over-the-counter calamine lotion. The patient’s medication regimen included daily pravastatin and loratadine at the time of the initial visit. He used acetaminophen as needed for knee pain.
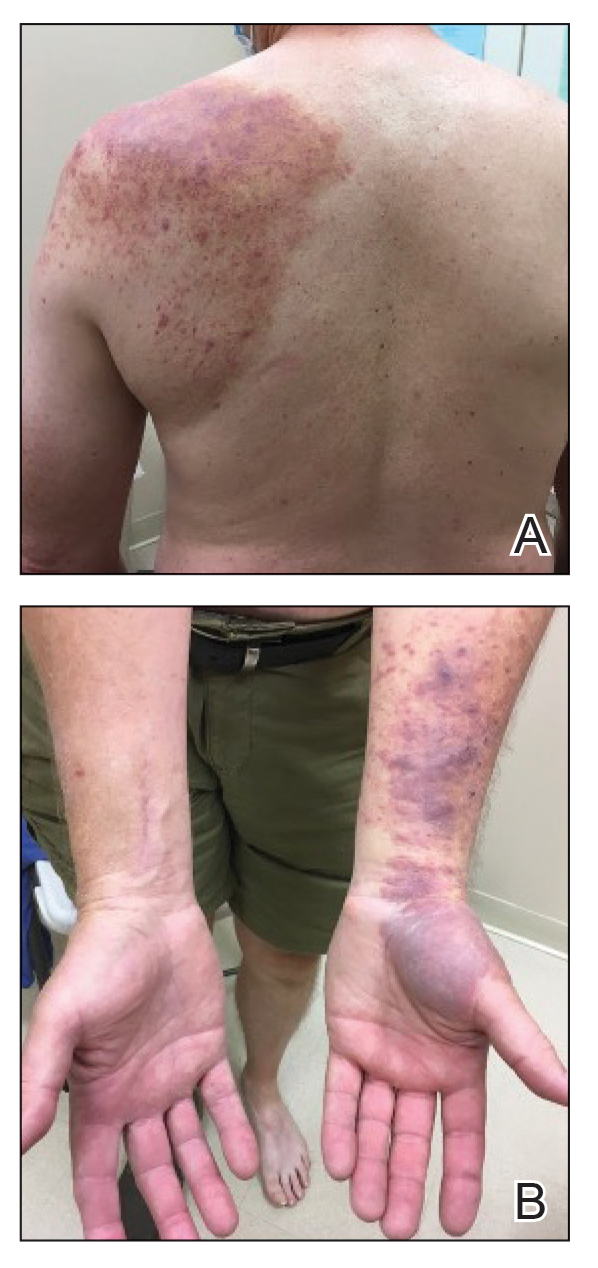
Physical examination revealed palpable purpura in a dermatomal distribution with nonpitting edema over the left scapula (Figure 1A), left anterolateral shoulder, left lateral volar forearm, and thenar eminence of the left hand (Figure 1B). Notably, the entire right arm, conjunctivae, tongue, lips, and bilateral fingernails were clear. Three 4-mm punch biopsies were performed at the initial presentation: 1 perilesional biopsy for direct immunofluorescence testing and 2 lesional biopsies for routine histologic evaluation. An extensive serologic workup failed to reveal abnormalities. An activated partial thromboplastin time, dilute Russell viper venom time, serum protein electrophoresis, and levels of rheumatoid factor and angiotensin-converting enzyme were within reference range. Anticardiolipin antibodies IgA, IgM, and IgG were negative. A cryoglobulin test was negative.
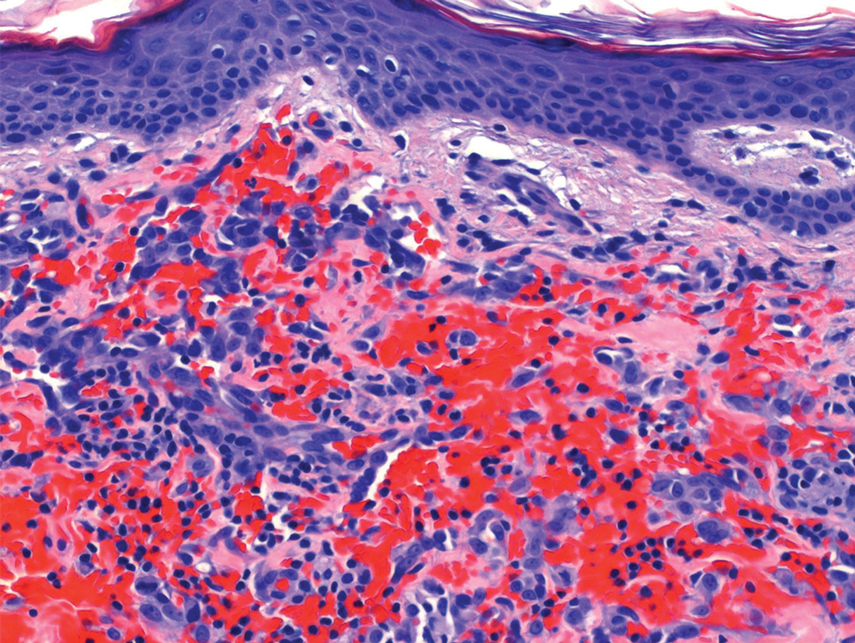
Histopathology revealed a proliferation of irregularly shaped vascular spaces with plump endothelium in the papillary dermis (Figure 2). Scattered leukocyte common antigen-positive lymphocytes were noted within lesions. The epidermis appeared normal, without evidence of spongiosis or alteration of the stratum corneum. Immunohistochemical studies of the perilesional skin biopsy revealed positivity for CD31 and D2-40 (Figure 3). Specimens were negative for CD20 and human herpesvirus 8. Direct immunofluorescence of the perilesional biopsy was negative.
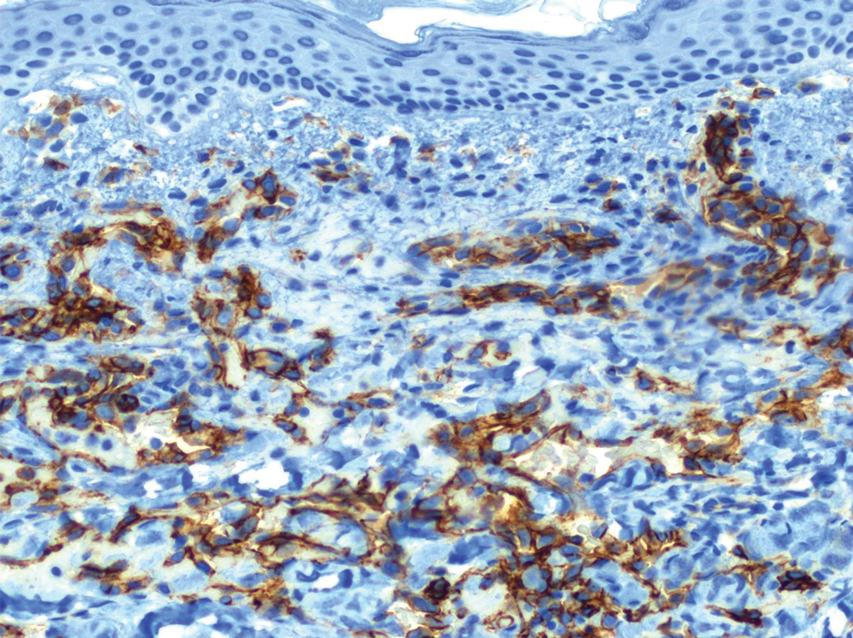
A diagnosis of RAE was made based on clinical and histologic findings. Treatment with triamcinolone ointment 0.1% twice daily and oral cetirizine 10 mg twice daily was initiated. Re-evaluation 2 weeks later revealed notable improvement in the affected areas, including decreased edema, improvement of the purpura, and absence of pruritus. The patient noted no further spread or blister formation while the active areas were being treated with the topical steroid. The treatment regimen was modified to triamcinolone ointment 0.1% once daily, and cetirizine was discontinued. At 3-month follow-up, active areas had completely resolved (Figure 4) and triamcinolone was discontinued. To date, the patient has not had recurrence of symptoms and remains healthy.
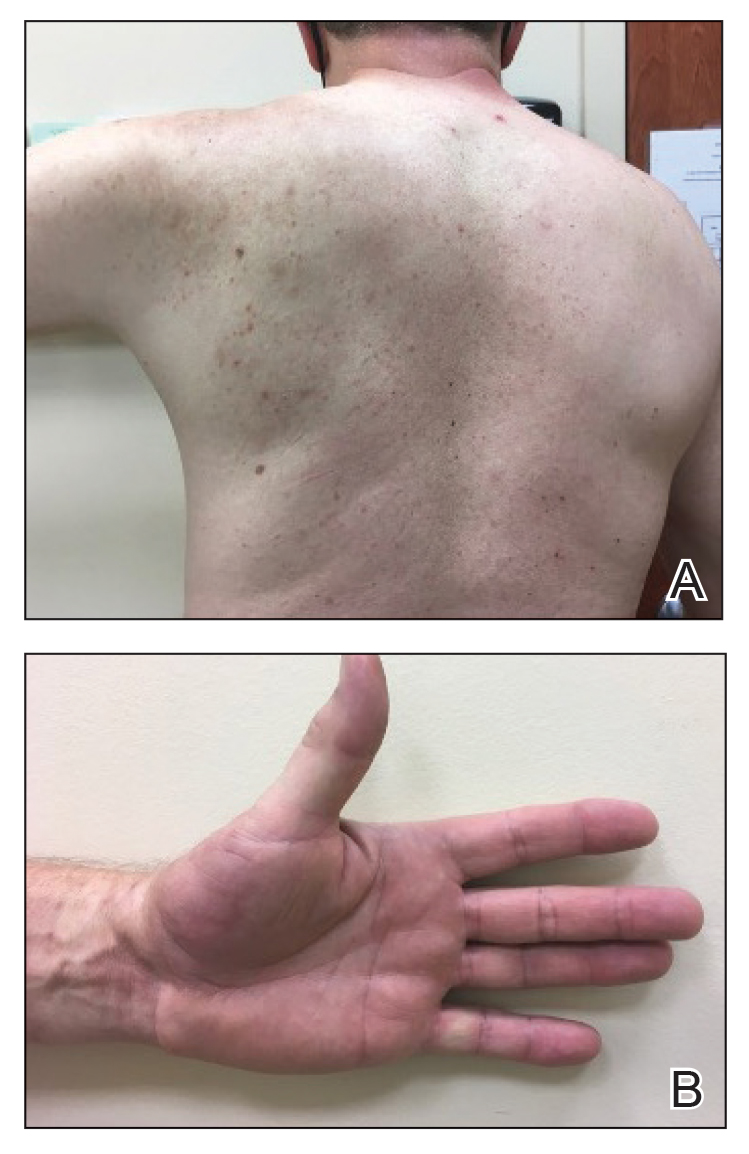
Gottron and Nikolowski3 reported the first case of RAE in an adult patient who presented with purpuric patches secondary to skin infarction. Current definitions use the umbrella term cutaneous reactive angiomatosis to cover 3 major subtypes: reactive angioendotheliomatosis, diffuse dermal angioendotheliomatosis, and acroangiodermatitis (pseudo-Kaposi sarcoma [KS]). The manifestation of these subgroups is clinically similar, and they must be differentiated through histologic evaluation.4
Reactive angioendotheliomatosis has an unknown pathogenesis and is poorly defined clinically. The exact pathophysiology is unknown but likely is linked to vaso-occlusion and hypoxia.1 A PubMed search of articles indexed for MEDLINE, as well as a review of Science Direct, Google Scholar, and Cochrane Library, using the terms reactive angioendotheliomatosis, COVID, vaccine, Ad26.COV2.S, and RAE in any combination revealed no prior cases of RAE in association with Ad26.COV2.S vaccination.
By the late 1980s, systemic angioendotheliomatosis was segregated into 2 distinct entities: malignant and reactive.4 The differential diagnosis of malignant systemic angioendotheliomatosis includes KS and angiosarcoma; nonmalignant causes are the variants of cutaneous reactive angiomatosis. It is important to rule out KS because of its malignant and deceptive nature. It is unknown if KS originates in blood vessels or lymphatic endothelial cells; however, evidence is strongly in favor of blood vessel origin using CD31 and CD34 endothelial markers.5 CD34 positivity is more reliable than CD31 in diagnosing KS, but the absence of both markers does not offer enough evidence to rule out KS on its own.6
In our patient, histopathology revealed cells positive for CD31 and D2-40; the latter is a lymphatic endothelial cell marker that stains the endothelium of lymphatic channels but not blood vessels.7 Positive D2-40 can be indicative of KS and non-KS lesions, each with a distinct staining pattern. D2-40 staining on non-KS lesions is confined to lymphatic vessels, as it was in our patient; in contrast, spindle-shaped cells also will be stained in KS lesions.8
Another cell marker, CD20, is a B cell–specific protein that can be measured to help diagnose malignant diseases such as B-cell lymphoma and leukemia. Human herpesvirus 8 (also known as KS-associated herpesvirus) is the infectious cause of KS and traditionally has been detected using methods such as the polymerase chain reaction.9,10
Most cases of RAE are idiopathic and occur in association with systemic disease, which was not the case in our patient. We speculated that his reaction was most likely triggered by vascular transfection of endothelial cells secondary to Ad26.COV2.S vaccination. Alternatively, vaccination may have caused vascular occlusion, though the lack of cyanosis, nail changes, and route of inoculant make this less likely.
All approved COVID-19 vaccines are designed solely for intramuscular injection. In comparison to other types of tissue, muscles have superior vascularity, allowing for enhanced mobilization of compounds, which results in faster systemic circulation.11 Alternative methods of injection, including intravascular, subcutaneous, and intradermal, may lead to decreased efficacy or adverse events, or both.
Prior cases of RAE have been treated with laser therapy, topical or systemic corticosteroids, excisional removal, or topical β-blockers, such as timolol.12 β-Blocking agents act on β-adrenergic receptors on endothelial cells to inhibit angiogenesis by reducing release of blood vessel growth-signaling molecules and triggering apoptosis. In this patient, topical steroids and oral antihistamines were sufficient treatment.
Vaccine-related adverse events have been reported but remain rare. The benefits of Ad26.COV2.S vaccination for protection against COVID-19 outweigh the extremely low risk for adverse events.13 For that reason, the Centers for Disease Control and Prevention recommends a booster for individuals who are eligible to maximize protection. Intramuscular injection of Ad26.COV2.S resulted in a lower incidence of moderate to severe COVID-19 cases in all age groups vs the placebo group. Hypersensitivity adverse events were reported in 0.4% of Ad26.COV2.S-vaccinated patients vs 0.4% of patients who received a placebo; the more common reactions were nonanaphylactic.13
There have been 12 reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, which sparked nationwide controversy over the safety of the Ad26.COV2.S vaccine.14 After further investigation into those reports, the US Food and Drug Administration and the Centers for Disease Control and Prevention concluded that the benefits of the Ad26.COV2.S vaccine outweigh the low risk for associated thrombosis.15
Although adverse reactions are rare, it is important that health care providers take proper safety measures before and while administering any COVID-19 vaccine. Patients should be screened for contraindications to the COVID-19 vaccine to mitigate adverse effects seen in the small percentage of patients who may need to take alternative precautions.
The broad tissue tropism and high transmissibility of SARS-CoV-2 are the main contributors to its infection having reached pandemic scale. The spike (S) protein on SARS-CoV-2 binds to ACE2, the most thoroughly studied SARS-CoV-2 receptor, which is found in a range of tissues, including arterial endothelial cells, leading to its transfection. Several studies have proposed that expression of the S protein causes endothelial dysfunction through cytokine release, activation of complement, and ultimately microvascular occlusion.16
Recent developments in the use of viral-like particles, such as vesicular stomatitis virus, may mitigate future cases of RAE that are associated with endothelial cell transfection. Vesicular stomatitis virus is a popular model virus for research applications due to its glycoprotein and matrix protein contributing to its broad tropism. Recent efforts to alter these proteins have successfully limited the broad tropism of vesicular stomatitis virus.17
The SARS-CoV-2 virus must be handled in a Biosafety Level 3 laboratory. Conversely, pseudoviruses can be handled in lower containment facilities due to their safe and efficacious nature, offering an avenue to expedite vaccine development against many viral outbreaks, including SARS-CoV-2.18
An increasing number of cutaneous manifestations have been associated with COVID-19 infection and vaccination. Eruptive pseudoangiomatosis, a rare self-limiting exanthem, has been reported in association with COVID-19 vaccination.19 Eruptive pseudoangiomatosis manifests as erythematous blanchable papules that resemble angiomas, typically in a widespread distribution. Eruptive pseudoangiomatosis has striking similarities to RAE histologically; both manifest as dilated dermal blood vessels with plump endothelial cells.
Our case is unique because of the vasculitic palpable nature of the lesions, which were localized to the left arm. Eruptive pseudoangiomatosis formation after COVID-19 infection or SARS-CoV-2 vaccination may suggest alteration of ACE2 by binding of S protein.20 Such alteration of the ACE2 pathway would lead to inflammation of angiotensin II, causing proliferation of endothelial cells in the formation of angiomalike lesions. This hypothesis suggests a paraviral eruption secondary to an immunologic reaction, not a classical virtual eruption from direct contact of the virus on blood vessels. Although EPA and RAE are harmless and self-limiting, these reports will spread awareness of the increasing number of skin manifestations related to COVID-19 and SARS-CoV-2 virus vaccination.
Acknowledgment—Thoughtful insights and comments on this manuscript were provided by Christine J. Ko, MD (New Haven, Connecticut); Christine L. Egan, MD (Glen Mills, Pennsylvania); Howard A. Bueller, MD (Delray Beach, Florida); and Juan Pablo Robles, PhD (Juriquilla, Mexico).
- McMenamin ME, Fletcher CDM. Reactive angioendotheliomatosis: a study of 15 cases demonstrating a wide clinicopathologic spectrum. Am J Surg Pathol. 2002;26:686-697. doi:10.1097/00000478-200206000-00001
- Khan S, Pujani M, Jetley S, et al. Angiomatosis: a rare vascular proliferation of head and neck region. J Cutan Aesthet Surg. 2015;8:108-110. doi:10.4103/0974-2077.158448
- Gottron HA, Nikolowski W. Extrarenal Lohlein focal nephritis of the skin in endocarditis. Arch Klin Exp Dermatol. 1958;207:156-176.
- Cooper PH. Angioendotheliomatosis: two separate diseases. J Cutan Pathol. 1988;15:259. doi:10.1111/j.1600-0560.1988.tb00556.x
- Cancian L, Hansen A, Boshoff C. Cellular origin of Kaposi’s sarcoma and Kaposi’s sarcoma-associated herpesvirus-induced cell reprogramming. Trends Cell Biol. Sep 2013;23:421-32. doi:10.1016/j.tcb.2013.04.001
- Russell Jones R, Orchard G, Zelger B, et al. Immunostaining for CD31 and CD34 in Kaposi sarcoma. J Clin Pathol. 1995;48:1011-1016. doi:10.1136/jcp.48.11.1011
- Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434-440. doi:10.1038/modpathol.3880543
- Genedy RM, Hamza AM, Abdel Latef AA, et al. Sensitivity and specificity of D2-40 in differentiating Kaposi sarcoma from its mimickers. J Egyptian Womens Dermatolog Soc. 2021;18:67-74. doi:10.4103/jewd.jewd_61_20
- Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707-719. doi:10.1038/nrc2888
- Patel RM, Goldblum JR, Hsi ED. Immunohistochemical detection of human herpes virus-8 latent nuclear antigen-1 is useful in the diagnosis of Kaposi sarcoma. Mod Pathol. 2004;17:456-460. doi:10.1038/modpathol.3800061
- Zuckerman JN. The importance of injecting vaccines into muscle. Different patients need different needle sizes. BMJ. 2000;321:1237-1238. doi:10.1136/bmj.321.7271.1237
- Bhatia R, Hazarika N, Chandrasekaran D, et al. Treatment of posttraumatic reactive angioendotheliomatosis with topical timolol maleate. JAMA Dermatol. 2021;157:1002-1004. doi:10.1001/jamadermatol.2021.1770
- Sadoff J, Gray G, Vandebosch A, et al; ENSEMBLE Study Group. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187-2201. doi:10.1056/NEJMoa2101544
- See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448-2456. doi:10.1001/jama.2021.7517
- Berry CT, Eliliwi M, Gallagher S, et al. Cutaneous small vessel vasculitis following single-dose Janssen Ad26.COV2.S vaccination. JAAD Case Rep. 2021;15:11-14. doi:10.1016/j.jdcr.2021.07.002
- Flaumenhaft R, Enjyoji K, Schmaier AA. Vasculopathy in COVID-19. Blood. 2022;140:222-235. doi:10.1182/blood.2021012250
- Hastie E, Cataldi M, Marriott I, et al. Understanding and altering cell tropism of vesicular stomatitis virus. Virus Res. 2013;176:16-32. doi:10.1016/j.virusres.2013.06.003
- Xiong H-L, Wu Y-T, Cao J-L, et al. Robust neutralization assay based on SARS-CoV-2 S-protein-bearing vesicular stomatitis virus (VSV) pseudovirus and ACE2-overexpressing BHK21 cells. Emerg Microbes Infect. 2020;9:2105-2113. doi:10.1080/22221751.2020.1815589
- Mohta A, Jain SK, Mehta RD, et al. Development of eruptive pseudoangiomatosis following COVID-19 immunization – apropos of 5 cases. J Eur Acad Dermatol Venereol. 2021;35:e722-e725. doi:10.1111/jdv.17499
- Angeli F, Spanevello A, Reboldi G, et al. SARS-CoV-2 vaccines: lights and shadows. Eur J Intern Med. 2021;88:1-8. doi:10.1016/j.ejim.2021.04.019
To the Editor:
Reactive angioendotheliomatosis (RAE) is a rare self-limited cutaneous vascular proliferation of endothelial cells within blood vessels that manifests clinically as infiltrated red-blue patches and plaques with purpura that can progress to occlude vascular lumina. The etiology of RAE is mostly idiopathic; however, the disorder typically occurs in association with a range of systemic diseases, including infection, cryoglobulinemia, leukemia, antiphospholipid syndrome, peripheral vascular disease, and arteriovenous fistula. Histopathologic examination of these lesions shows marked proliferation of endothelial cells, including occlusion of the lumen of blood vessels over wide areas.
After ruling out malignancy, treatment of RAE focuses on targeting the underlying cause or disease, if any is present; 75% of reported cases occur in association with systemic disease.1 Onset can occur at any age without predilection for sex. Reactive angioendotheliomatosis commonly manifests on the extremities but may occur on the head and neck in rare instances.2
The rarity of the condition and its poorly defined clinical characteristics make it difficult to develop a treatment plan. There are no standardized treatment guidelines for the reactive form of angiomatosis. We report a case of RAE that developed 2 weeks after vaccination with the Ad26.COV2.S vaccine (Johnson & Johnson Innovative Medicine [formerly Janssen Pharmaceutical Companies of Johnson & Johnson]) that improved following 2 weeks of treatment with a topical corticosteroid and an oral antihistamine.
A 58-year-old man presented to an outpatient dermatology clinic with pruritus and occasional paresthesia associated with a rash over the left arm of 1 month’s duration. The patient suspected that the rash may have formed secondary to the bite of oak mites on the arms and chest while he was carrying milled wood. Further inquiry into the patient’s history revealed that he received the Ad26.COV2.S vaccine 2 weeks prior to the appearance of the rash. He denied mechanical trauma. His medical history included hypercholesterolemia and a mild COVID-19 infection 8 months prior to the appearance of the rash that did not require hospitalization. He denied fever or chills during the 2 weeks following vaccination. The pruritus was minimally relieved for short periods with over-the-counter calamine lotion. The patient’s medication regimen included daily pravastatin and loratadine at the time of the initial visit. He used acetaminophen as needed for knee pain.

Physical examination revealed palpable purpura in a dermatomal distribution with nonpitting edema over the left scapula (Figure 1A), left anterolateral shoulder, left lateral volar forearm, and thenar eminence of the left hand (Figure 1B). Notably, the entire right arm, conjunctivae, tongue, lips, and bilateral fingernails were clear. Three 4-mm punch biopsies were performed at the initial presentation: 1 perilesional biopsy for direct immunofluorescence testing and 2 lesional biopsies for routine histologic evaluation. An extensive serologic workup failed to reveal abnormalities. An activated partial thromboplastin time, dilute Russell viper venom time, serum protein electrophoresis, and levels of rheumatoid factor and angiotensin-converting enzyme were within reference range. Anticardiolipin antibodies IgA, IgM, and IgG were negative. A cryoglobulin test was negative.

Histopathology revealed a proliferation of irregularly shaped vascular spaces with plump endothelium in the papillary dermis (Figure 2). Scattered leukocyte common antigen-positive lymphocytes were noted within lesions. The epidermis appeared normal, without evidence of spongiosis or alteration of the stratum corneum. Immunohistochemical studies of the perilesional skin biopsy revealed positivity for CD31 and D2-40 (Figure 3). Specimens were negative for CD20 and human herpesvirus 8. Direct immunofluorescence of the perilesional biopsy was negative.

A diagnosis of RAE was made based on clinical and histologic findings. Treatment with triamcinolone ointment 0.1% twice daily and oral cetirizine 10 mg twice daily was initiated. Re-evaluation 2 weeks later revealed notable improvement in the affected areas, including decreased edema, improvement of the purpura, and absence of pruritus. The patient noted no further spread or blister formation while the active areas were being treated with the topical steroid. The treatment regimen was modified to triamcinolone ointment 0.1% once daily, and cetirizine was discontinued. At 3-month follow-up, active areas had completely resolved (Figure 4) and triamcinolone was discontinued. To date, the patient has not had recurrence of symptoms and remains healthy.

Gottron and Nikolowski3 reported the first case of RAE in an adult patient who presented with purpuric patches secondary to skin infarction. Current definitions use the umbrella term cutaneous reactive angiomatosis to cover 3 major subtypes: reactive angioendotheliomatosis, diffuse dermal angioendotheliomatosis, and acroangiodermatitis (pseudo-Kaposi sarcoma [KS]). The manifestation of these subgroups is clinically similar, and they must be differentiated through histologic evaluation.4
Reactive angioendotheliomatosis has an unknown pathogenesis and is poorly defined clinically. The exact pathophysiology is unknown but likely is linked to vaso-occlusion and hypoxia.1 A PubMed search of articles indexed for MEDLINE, as well as a review of Science Direct, Google Scholar, and Cochrane Library, using the terms reactive angioendotheliomatosis, COVID, vaccine, Ad26.COV2.S, and RAE in any combination revealed no prior cases of RAE in association with Ad26.COV2.S vaccination.
By the late 1980s, systemic angioendotheliomatosis was segregated into 2 distinct entities: malignant and reactive.4 The differential diagnosis of malignant systemic angioendotheliomatosis includes KS and angiosarcoma; nonmalignant causes are the variants of cutaneous reactive angiomatosis. It is important to rule out KS because of its malignant and deceptive nature. It is unknown if KS originates in blood vessels or lymphatic endothelial cells; however, evidence is strongly in favor of blood vessel origin using CD31 and CD34 endothelial markers.5 CD34 positivity is more reliable than CD31 in diagnosing KS, but the absence of both markers does not offer enough evidence to rule out KS on its own.6
In our patient, histopathology revealed cells positive for CD31 and D2-40; the latter is a lymphatic endothelial cell marker that stains the endothelium of lymphatic channels but not blood vessels.7 Positive D2-40 can be indicative of KS and non-KS lesions, each with a distinct staining pattern. D2-40 staining on non-KS lesions is confined to lymphatic vessels, as it was in our patient; in contrast, spindle-shaped cells also will be stained in KS lesions.8
Another cell marker, CD20, is a B cell–specific protein that can be measured to help diagnose malignant diseases such as B-cell lymphoma and leukemia. Human herpesvirus 8 (also known as KS-associated herpesvirus) is the infectious cause of KS and traditionally has been detected using methods such as the polymerase chain reaction.9,10
Most cases of RAE are idiopathic and occur in association with systemic disease, which was not the case in our patient. We speculated that his reaction was most likely triggered by vascular transfection of endothelial cells secondary to Ad26.COV2.S vaccination. Alternatively, vaccination may have caused vascular occlusion, though the lack of cyanosis, nail changes, and route of inoculant make this less likely.
All approved COVID-19 vaccines are designed solely for intramuscular injection. In comparison to other types of tissue, muscles have superior vascularity, allowing for enhanced mobilization of compounds, which results in faster systemic circulation.11 Alternative methods of injection, including intravascular, subcutaneous, and intradermal, may lead to decreased efficacy or adverse events, or both.
Prior cases of RAE have been treated with laser therapy, topical or systemic corticosteroids, excisional removal, or topical β-blockers, such as timolol.12 β-Blocking agents act on β-adrenergic receptors on endothelial cells to inhibit angiogenesis by reducing release of blood vessel growth-signaling molecules and triggering apoptosis. In this patient, topical steroids and oral antihistamines were sufficient treatment.
Vaccine-related adverse events have been reported but remain rare. The benefits of Ad26.COV2.S vaccination for protection against COVID-19 outweigh the extremely low risk for adverse events.13 For that reason, the Centers for Disease Control and Prevention recommends a booster for individuals who are eligible to maximize protection. Intramuscular injection of Ad26.COV2.S resulted in a lower incidence of moderate to severe COVID-19 cases in all age groups vs the placebo group. Hypersensitivity adverse events were reported in 0.4% of Ad26.COV2.S-vaccinated patients vs 0.4% of patients who received a placebo; the more common reactions were nonanaphylactic.13
There have been 12 reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, which sparked nationwide controversy over the safety of the Ad26.COV2.S vaccine.14 After further investigation into those reports, the US Food and Drug Administration and the Centers for Disease Control and Prevention concluded that the benefits of the Ad26.COV2.S vaccine outweigh the low risk for associated thrombosis.15
Although adverse reactions are rare, it is important that health care providers take proper safety measures before and while administering any COVID-19 vaccine. Patients should be screened for contraindications to the COVID-19 vaccine to mitigate adverse effects seen in the small percentage of patients who may need to take alternative precautions.
The broad tissue tropism and high transmissibility of SARS-CoV-2 are the main contributors to its infection having reached pandemic scale. The spike (S) protein on SARS-CoV-2 binds to ACE2, the most thoroughly studied SARS-CoV-2 receptor, which is found in a range of tissues, including arterial endothelial cells, leading to its transfection. Several studies have proposed that expression of the S protein causes endothelial dysfunction through cytokine release, activation of complement, and ultimately microvascular occlusion.16
Recent developments in the use of viral-like particles, such as vesicular stomatitis virus, may mitigate future cases of RAE that are associated with endothelial cell transfection. Vesicular stomatitis virus is a popular model virus for research applications due to its glycoprotein and matrix protein contributing to its broad tropism. Recent efforts to alter these proteins have successfully limited the broad tropism of vesicular stomatitis virus.17
The SARS-CoV-2 virus must be handled in a Biosafety Level 3 laboratory. Conversely, pseudoviruses can be handled in lower containment facilities due to their safe and efficacious nature, offering an avenue to expedite vaccine development against many viral outbreaks, including SARS-CoV-2.18
An increasing number of cutaneous manifestations have been associated with COVID-19 infection and vaccination. Eruptive pseudoangiomatosis, a rare self-limiting exanthem, has been reported in association with COVID-19 vaccination.19 Eruptive pseudoangiomatosis manifests as erythematous blanchable papules that resemble angiomas, typically in a widespread distribution. Eruptive pseudoangiomatosis has striking similarities to RAE histologically; both manifest as dilated dermal blood vessels with plump endothelial cells.
Our case is unique because of the vasculitic palpable nature of the lesions, which were localized to the left arm. Eruptive pseudoangiomatosis formation after COVID-19 infection or SARS-CoV-2 vaccination may suggest alteration of ACE2 by binding of S protein.20 Such alteration of the ACE2 pathway would lead to inflammation of angiotensin II, causing proliferation of endothelial cells in the formation of angiomalike lesions. This hypothesis suggests a paraviral eruption secondary to an immunologic reaction, not a classical virtual eruption from direct contact of the virus on blood vessels. Although EPA and RAE are harmless and self-limiting, these reports will spread awareness of the increasing number of skin manifestations related to COVID-19 and SARS-CoV-2 virus vaccination.
Acknowledgment—Thoughtful insights and comments on this manuscript were provided by Christine J. Ko, MD (New Haven, Connecticut); Christine L. Egan, MD (Glen Mills, Pennsylvania); Howard A. Bueller, MD (Delray Beach, Florida); and Juan Pablo Robles, PhD (Juriquilla, Mexico).
To the Editor:
Reactive angioendotheliomatosis (RAE) is a rare self-limited cutaneous vascular proliferation of endothelial cells within blood vessels that manifests clinically as infiltrated red-blue patches and plaques with purpura that can progress to occlude vascular lumina. The etiology of RAE is mostly idiopathic; however, the disorder typically occurs in association with a range of systemic diseases, including infection, cryoglobulinemia, leukemia, antiphospholipid syndrome, peripheral vascular disease, and arteriovenous fistula. Histopathologic examination of these lesions shows marked proliferation of endothelial cells, including occlusion of the lumen of blood vessels over wide areas.
After ruling out malignancy, treatment of RAE focuses on targeting the underlying cause or disease, if any is present; 75% of reported cases occur in association with systemic disease.1 Onset can occur at any age without predilection for sex. Reactive angioendotheliomatosis commonly manifests on the extremities but may occur on the head and neck in rare instances.2
The rarity of the condition and its poorly defined clinical characteristics make it difficult to develop a treatment plan. There are no standardized treatment guidelines for the reactive form of angiomatosis. We report a case of RAE that developed 2 weeks after vaccination with the Ad26.COV2.S vaccine (Johnson & Johnson Innovative Medicine [formerly Janssen Pharmaceutical Companies of Johnson & Johnson]) that improved following 2 weeks of treatment with a topical corticosteroid and an oral antihistamine.
A 58-year-old man presented to an outpatient dermatology clinic with pruritus and occasional paresthesia associated with a rash over the left arm of 1 month’s duration. The patient suspected that the rash may have formed secondary to the bite of oak mites on the arms and chest while he was carrying milled wood. Further inquiry into the patient’s history revealed that he received the Ad26.COV2.S vaccine 2 weeks prior to the appearance of the rash. He denied mechanical trauma. His medical history included hypercholesterolemia and a mild COVID-19 infection 8 months prior to the appearance of the rash that did not require hospitalization. He denied fever or chills during the 2 weeks following vaccination. The pruritus was minimally relieved for short periods with over-the-counter calamine lotion. The patient’s medication regimen included daily pravastatin and loratadine at the time of the initial visit. He used acetaminophen as needed for knee pain.

Physical examination revealed palpable purpura in a dermatomal distribution with nonpitting edema over the left scapula (Figure 1A), left anterolateral shoulder, left lateral volar forearm, and thenar eminence of the left hand (Figure 1B). Notably, the entire right arm, conjunctivae, tongue, lips, and bilateral fingernails were clear. Three 4-mm punch biopsies were performed at the initial presentation: 1 perilesional biopsy for direct immunofluorescence testing and 2 lesional biopsies for routine histologic evaluation. An extensive serologic workup failed to reveal abnormalities. An activated partial thromboplastin time, dilute Russell viper venom time, serum protein electrophoresis, and levels of rheumatoid factor and angiotensin-converting enzyme were within reference range. Anticardiolipin antibodies IgA, IgM, and IgG were negative. A cryoglobulin test was negative.

Histopathology revealed a proliferation of irregularly shaped vascular spaces with plump endothelium in the papillary dermis (Figure 2). Scattered leukocyte common antigen-positive lymphocytes were noted within lesions. The epidermis appeared normal, without evidence of spongiosis or alteration of the stratum corneum. Immunohistochemical studies of the perilesional skin biopsy revealed positivity for CD31 and D2-40 (Figure 3). Specimens were negative for CD20 and human herpesvirus 8. Direct immunofluorescence of the perilesional biopsy was negative.

A diagnosis of RAE was made based on clinical and histologic findings. Treatment with triamcinolone ointment 0.1% twice daily and oral cetirizine 10 mg twice daily was initiated. Re-evaluation 2 weeks later revealed notable improvement in the affected areas, including decreased edema, improvement of the purpura, and absence of pruritus. The patient noted no further spread or blister formation while the active areas were being treated with the topical steroid. The treatment regimen was modified to triamcinolone ointment 0.1% once daily, and cetirizine was discontinued. At 3-month follow-up, active areas had completely resolved (Figure 4) and triamcinolone was discontinued. To date, the patient has not had recurrence of symptoms and remains healthy.

Gottron and Nikolowski3 reported the first case of RAE in an adult patient who presented with purpuric patches secondary to skin infarction. Current definitions use the umbrella term cutaneous reactive angiomatosis to cover 3 major subtypes: reactive angioendotheliomatosis, diffuse dermal angioendotheliomatosis, and acroangiodermatitis (pseudo-Kaposi sarcoma [KS]). The manifestation of these subgroups is clinically similar, and they must be differentiated through histologic evaluation.4
Reactive angioendotheliomatosis has an unknown pathogenesis and is poorly defined clinically. The exact pathophysiology is unknown but likely is linked to vaso-occlusion and hypoxia.1 A PubMed search of articles indexed for MEDLINE, as well as a review of Science Direct, Google Scholar, and Cochrane Library, using the terms reactive angioendotheliomatosis, COVID, vaccine, Ad26.COV2.S, and RAE in any combination revealed no prior cases of RAE in association with Ad26.COV2.S vaccination.
By the late 1980s, systemic angioendotheliomatosis was segregated into 2 distinct entities: malignant and reactive.4 The differential diagnosis of malignant systemic angioendotheliomatosis includes KS and angiosarcoma; nonmalignant causes are the variants of cutaneous reactive angiomatosis. It is important to rule out KS because of its malignant and deceptive nature. It is unknown if KS originates in blood vessels or lymphatic endothelial cells; however, evidence is strongly in favor of blood vessel origin using CD31 and CD34 endothelial markers.5 CD34 positivity is more reliable than CD31 in diagnosing KS, but the absence of both markers does not offer enough evidence to rule out KS on its own.6
In our patient, histopathology revealed cells positive for CD31 and D2-40; the latter is a lymphatic endothelial cell marker that stains the endothelium of lymphatic channels but not blood vessels.7 Positive D2-40 can be indicative of KS and non-KS lesions, each with a distinct staining pattern. D2-40 staining on non-KS lesions is confined to lymphatic vessels, as it was in our patient; in contrast, spindle-shaped cells also will be stained in KS lesions.8
Another cell marker, CD20, is a B cell–specific protein that can be measured to help diagnose malignant diseases such as B-cell lymphoma and leukemia. Human herpesvirus 8 (also known as KS-associated herpesvirus) is the infectious cause of KS and traditionally has been detected using methods such as the polymerase chain reaction.9,10
Most cases of RAE are idiopathic and occur in association with systemic disease, which was not the case in our patient. We speculated that his reaction was most likely triggered by vascular transfection of endothelial cells secondary to Ad26.COV2.S vaccination. Alternatively, vaccination may have caused vascular occlusion, though the lack of cyanosis, nail changes, and route of inoculant make this less likely.
All approved COVID-19 vaccines are designed solely for intramuscular injection. In comparison to other types of tissue, muscles have superior vascularity, allowing for enhanced mobilization of compounds, which results in faster systemic circulation.11 Alternative methods of injection, including intravascular, subcutaneous, and intradermal, may lead to decreased efficacy or adverse events, or both.
Prior cases of RAE have been treated with laser therapy, topical or systemic corticosteroids, excisional removal, or topical β-blockers, such as timolol.12 β-Blocking agents act on β-adrenergic receptors on endothelial cells to inhibit angiogenesis by reducing release of blood vessel growth-signaling molecules and triggering apoptosis. In this patient, topical steroids and oral antihistamines were sufficient treatment.
Vaccine-related adverse events have been reported but remain rare. The benefits of Ad26.COV2.S vaccination for protection against COVID-19 outweigh the extremely low risk for adverse events.13 For that reason, the Centers for Disease Control and Prevention recommends a booster for individuals who are eligible to maximize protection. Intramuscular injection of Ad26.COV2.S resulted in a lower incidence of moderate to severe COVID-19 cases in all age groups vs the placebo group. Hypersensitivity adverse events were reported in 0.4% of Ad26.COV2.S-vaccinated patients vs 0.4% of patients who received a placebo; the more common reactions were nonanaphylactic.13
There have been 12 reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, which sparked nationwide controversy over the safety of the Ad26.COV2.S vaccine.14 After further investigation into those reports, the US Food and Drug Administration and the Centers for Disease Control and Prevention concluded that the benefits of the Ad26.COV2.S vaccine outweigh the low risk for associated thrombosis.15
Although adverse reactions are rare, it is important that health care providers take proper safety measures before and while administering any COVID-19 vaccine. Patients should be screened for contraindications to the COVID-19 vaccine to mitigate adverse effects seen in the small percentage of patients who may need to take alternative precautions.
The broad tissue tropism and high transmissibility of SARS-CoV-2 are the main contributors to its infection having reached pandemic scale. The spike (S) protein on SARS-CoV-2 binds to ACE2, the most thoroughly studied SARS-CoV-2 receptor, which is found in a range of tissues, including arterial endothelial cells, leading to its transfection. Several studies have proposed that expression of the S protein causes endothelial dysfunction through cytokine release, activation of complement, and ultimately microvascular occlusion.16
Recent developments in the use of viral-like particles, such as vesicular stomatitis virus, may mitigate future cases of RAE that are associated with endothelial cell transfection. Vesicular stomatitis virus is a popular model virus for research applications due to its glycoprotein and matrix protein contributing to its broad tropism. Recent efforts to alter these proteins have successfully limited the broad tropism of vesicular stomatitis virus.17
The SARS-CoV-2 virus must be handled in a Biosafety Level 3 laboratory. Conversely, pseudoviruses can be handled in lower containment facilities due to their safe and efficacious nature, offering an avenue to expedite vaccine development against many viral outbreaks, including SARS-CoV-2.18
An increasing number of cutaneous manifestations have been associated with COVID-19 infection and vaccination. Eruptive pseudoangiomatosis, a rare self-limiting exanthem, has been reported in association with COVID-19 vaccination.19 Eruptive pseudoangiomatosis manifests as erythematous blanchable papules that resemble angiomas, typically in a widespread distribution. Eruptive pseudoangiomatosis has striking similarities to RAE histologically; both manifest as dilated dermal blood vessels with plump endothelial cells.
Our case is unique because of the vasculitic palpable nature of the lesions, which were localized to the left arm. Eruptive pseudoangiomatosis formation after COVID-19 infection or SARS-CoV-2 vaccination may suggest alteration of ACE2 by binding of S protein.20 Such alteration of the ACE2 pathway would lead to inflammation of angiotensin II, causing proliferation of endothelial cells in the formation of angiomalike lesions. This hypothesis suggests a paraviral eruption secondary to an immunologic reaction, not a classical virtual eruption from direct contact of the virus on blood vessels. Although EPA and RAE are harmless and self-limiting, these reports will spread awareness of the increasing number of skin manifestations related to COVID-19 and SARS-CoV-2 virus vaccination.
Acknowledgment—Thoughtful insights and comments on this manuscript were provided by Christine J. Ko, MD (New Haven, Connecticut); Christine L. Egan, MD (Glen Mills, Pennsylvania); Howard A. Bueller, MD (Delray Beach, Florida); and Juan Pablo Robles, PhD (Juriquilla, Mexico).
- McMenamin ME, Fletcher CDM. Reactive angioendotheliomatosis: a study of 15 cases demonstrating a wide clinicopathologic spectrum. Am J Surg Pathol. 2002;26:686-697. doi:10.1097/00000478-200206000-00001
- Khan S, Pujani M, Jetley S, et al. Angiomatosis: a rare vascular proliferation of head and neck region. J Cutan Aesthet Surg. 2015;8:108-110. doi:10.4103/0974-2077.158448
- Gottron HA, Nikolowski W. Extrarenal Lohlein focal nephritis of the skin in endocarditis. Arch Klin Exp Dermatol. 1958;207:156-176.
- Cooper PH. Angioendotheliomatosis: two separate diseases. J Cutan Pathol. 1988;15:259. doi:10.1111/j.1600-0560.1988.tb00556.x
- Cancian L, Hansen A, Boshoff C. Cellular origin of Kaposi’s sarcoma and Kaposi’s sarcoma-associated herpesvirus-induced cell reprogramming. Trends Cell Biol. Sep 2013;23:421-32. doi:10.1016/j.tcb.2013.04.001
- Russell Jones R, Orchard G, Zelger B, et al. Immunostaining for CD31 and CD34 in Kaposi sarcoma. J Clin Pathol. 1995;48:1011-1016. doi:10.1136/jcp.48.11.1011
- Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434-440. doi:10.1038/modpathol.3880543
- Genedy RM, Hamza AM, Abdel Latef AA, et al. Sensitivity and specificity of D2-40 in differentiating Kaposi sarcoma from its mimickers. J Egyptian Womens Dermatolog Soc. 2021;18:67-74. doi:10.4103/jewd.jewd_61_20
- Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707-719. doi:10.1038/nrc2888
- Patel RM, Goldblum JR, Hsi ED. Immunohistochemical detection of human herpes virus-8 latent nuclear antigen-1 is useful in the diagnosis of Kaposi sarcoma. Mod Pathol. 2004;17:456-460. doi:10.1038/modpathol.3800061
- Zuckerman JN. The importance of injecting vaccines into muscle. Different patients need different needle sizes. BMJ. 2000;321:1237-1238. doi:10.1136/bmj.321.7271.1237
- Bhatia R, Hazarika N, Chandrasekaran D, et al. Treatment of posttraumatic reactive angioendotheliomatosis with topical timolol maleate. JAMA Dermatol. 2021;157:1002-1004. doi:10.1001/jamadermatol.2021.1770
- Sadoff J, Gray G, Vandebosch A, et al; ENSEMBLE Study Group. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187-2201. doi:10.1056/NEJMoa2101544
- See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448-2456. doi:10.1001/jama.2021.7517
- Berry CT, Eliliwi M, Gallagher S, et al. Cutaneous small vessel vasculitis following single-dose Janssen Ad26.COV2.S vaccination. JAAD Case Rep. 2021;15:11-14. doi:10.1016/j.jdcr.2021.07.002
- Flaumenhaft R, Enjyoji K, Schmaier AA. Vasculopathy in COVID-19. Blood. 2022;140:222-235. doi:10.1182/blood.2021012250
- Hastie E, Cataldi M, Marriott I, et al. Understanding and altering cell tropism of vesicular stomatitis virus. Virus Res. 2013;176:16-32. doi:10.1016/j.virusres.2013.06.003
- Xiong H-L, Wu Y-T, Cao J-L, et al. Robust neutralization assay based on SARS-CoV-2 S-protein-bearing vesicular stomatitis virus (VSV) pseudovirus and ACE2-overexpressing BHK21 cells. Emerg Microbes Infect. 2020;9:2105-2113. doi:10.1080/22221751.2020.1815589
- Mohta A, Jain SK, Mehta RD, et al. Development of eruptive pseudoangiomatosis following COVID-19 immunization – apropos of 5 cases. J Eur Acad Dermatol Venereol. 2021;35:e722-e725. doi:10.1111/jdv.17499
- Angeli F, Spanevello A, Reboldi G, et al. SARS-CoV-2 vaccines: lights and shadows. Eur J Intern Med. 2021;88:1-8. doi:10.1016/j.ejim.2021.04.019
- McMenamin ME, Fletcher CDM. Reactive angioendotheliomatosis: a study of 15 cases demonstrating a wide clinicopathologic spectrum. Am J Surg Pathol. 2002;26:686-697. doi:10.1097/00000478-200206000-00001
- Khan S, Pujani M, Jetley S, et al. Angiomatosis: a rare vascular proliferation of head and neck region. J Cutan Aesthet Surg. 2015;8:108-110. doi:10.4103/0974-2077.158448
- Gottron HA, Nikolowski W. Extrarenal Lohlein focal nephritis of the skin in endocarditis. Arch Klin Exp Dermatol. 1958;207:156-176.
- Cooper PH. Angioendotheliomatosis: two separate diseases. J Cutan Pathol. 1988;15:259. doi:10.1111/j.1600-0560.1988.tb00556.x
- Cancian L, Hansen A, Boshoff C. Cellular origin of Kaposi’s sarcoma and Kaposi’s sarcoma-associated herpesvirus-induced cell reprogramming. Trends Cell Biol. Sep 2013;23:421-32. doi:10.1016/j.tcb.2013.04.001
- Russell Jones R, Orchard G, Zelger B, et al. Immunostaining for CD31 and CD34 in Kaposi sarcoma. J Clin Pathol. 1995;48:1011-1016. doi:10.1136/jcp.48.11.1011
- Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434-440. doi:10.1038/modpathol.3880543
- Genedy RM, Hamza AM, Abdel Latef AA, et al. Sensitivity and specificity of D2-40 in differentiating Kaposi sarcoma from its mimickers. J Egyptian Womens Dermatolog Soc. 2021;18:67-74. doi:10.4103/jewd.jewd_61_20
- Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707-719. doi:10.1038/nrc2888
- Patel RM, Goldblum JR, Hsi ED. Immunohistochemical detection of human herpes virus-8 latent nuclear antigen-1 is useful in the diagnosis of Kaposi sarcoma. Mod Pathol. 2004;17:456-460. doi:10.1038/modpathol.3800061
- Zuckerman JN. The importance of injecting vaccines into muscle. Different patients need different needle sizes. BMJ. 2000;321:1237-1238. doi:10.1136/bmj.321.7271.1237
- Bhatia R, Hazarika N, Chandrasekaran D, et al. Treatment of posttraumatic reactive angioendotheliomatosis with topical timolol maleate. JAMA Dermatol. 2021;157:1002-1004. doi:10.1001/jamadermatol.2021.1770
- Sadoff J, Gray G, Vandebosch A, et al; ENSEMBLE Study Group. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187-2201. doi:10.1056/NEJMoa2101544
- See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448-2456. doi:10.1001/jama.2021.7517
- Berry CT, Eliliwi M, Gallagher S, et al. Cutaneous small vessel vasculitis following single-dose Janssen Ad26.COV2.S vaccination. JAAD Case Rep. 2021;15:11-14. doi:10.1016/j.jdcr.2021.07.002
- Flaumenhaft R, Enjyoji K, Schmaier AA. Vasculopathy in COVID-19. Blood. 2022;140:222-235. doi:10.1182/blood.2021012250
- Hastie E, Cataldi M, Marriott I, et al. Understanding and altering cell tropism of vesicular stomatitis virus. Virus Res. 2013;176:16-32. doi:10.1016/j.virusres.2013.06.003
- Xiong H-L, Wu Y-T, Cao J-L, et al. Robust neutralization assay based on SARS-CoV-2 S-protein-bearing vesicular stomatitis virus (VSV) pseudovirus and ACE2-overexpressing BHK21 cells. Emerg Microbes Infect. 2020;9:2105-2113. doi:10.1080/22221751.2020.1815589
- Mohta A, Jain SK, Mehta RD, et al. Development of eruptive pseudoangiomatosis following COVID-19 immunization – apropos of 5 cases. J Eur Acad Dermatol Venereol. 2021;35:e722-e725. doi:10.1111/jdv.17499
- Angeli F, Spanevello A, Reboldi G, et al. SARS-CoV-2 vaccines: lights and shadows. Eur J Intern Med. 2021;88:1-8. doi:10.1016/j.ejim.2021.04.019
Practice points
- Reactive angioendotheliomatosis (RAE) is a rare benign vascular proliferation of endothelial cells lining blood vessels that clinically appears similar to Kaposi sarcoma and must be differentiated by microscopic evaluation.
- An increasing number of reports link SARS-CoV-2 viral infection or vaccination against this virus with various cutaneous manifestations. Our case offers a link between RAE and Ad26.COV2.S vaccination.
Diffuse Capillary Malformation With Undergrowth of a Limb in a Boy
To the Editor:
Capillary malformations (CMs), the most common vascular malformations that can affect the skin,1 present clinically as macules and patches of various colors, shapes, and sizes. Congenital structural abnormalities are associated with conditions such as Klippel-Trenaunay syndrome (KTS), cutis marmorata telangiectatica congenita (CMTC), and megalencephaly–capillary malformation syndrome.2 Diffuse CM with overgrowth (DCMO) of the soft tissue and bones is an established association of CMs; however, diffuse capillary malformation with undergrowth (DCMU) is a more recent term that describes the lesser-recognized counterpart to DCMO.3 Herein, we describe a case of CM with left-sided undergrowth.
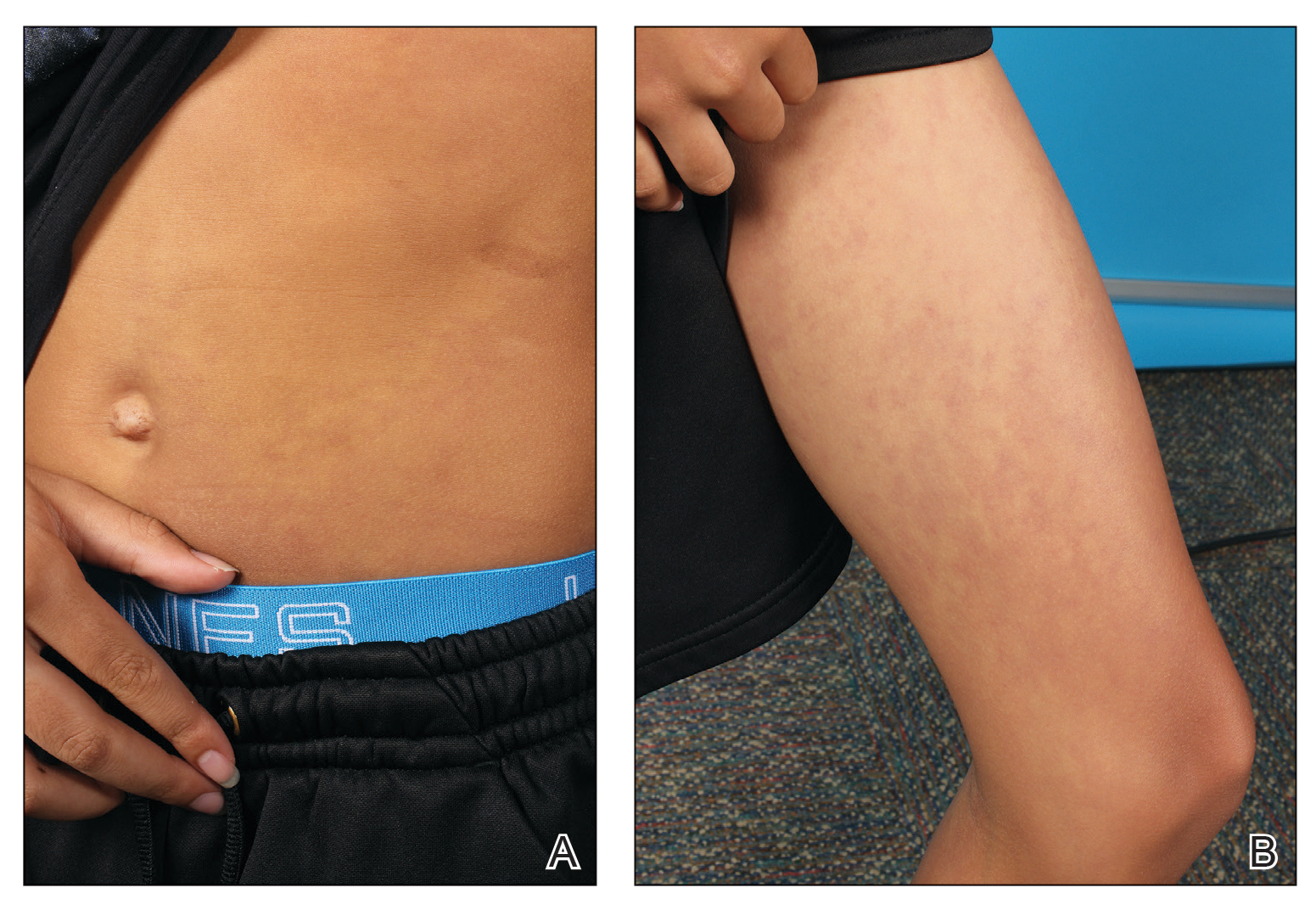
An 11-year-old boy presented to our clinic with asymptomatic vascular patterning on the left side of the body that had been present since birth. He previously was diagnosed with congenital right hemihypertrophy. He reported that the areas gradually lightened over time, and he denied any history of ulceration or venous or lymphatic malformations. Additionally, he explained how the left arm and leg have been noticeably smaller than the right extremities throughout his life. Physical examination revealed superficial, violaceous, reticulated patches along the left upper back tracking down the arm, abdomen (Figure 1A), and anterior thigh (Figure 1B) without crossing the midline. A few dilated veins were noted in the same region as the patches. There was no evidence of scarring or depression found in the skin. The right arms and legs were visibly larger compared to the left side (Figure 2A), and there also was macrodactyly of the third digit of the left hand (Figure 2B). Radiography confirmed the limb length discrepancy and showed the right and left legs to measure 73.2 cm and 71.3 cm, respectively. Given the patient’s multifocal reticulated CMs and ipsilateral undergrowth, a diagnosis of DCMU was rendered. The superficial vascular pattern is likely to fade over time, which will partially be hidden by his darker complexion. He also was advised to continue to see an orthopedist to monitor the limb length incongruity. Surgical intervention was not recommended.
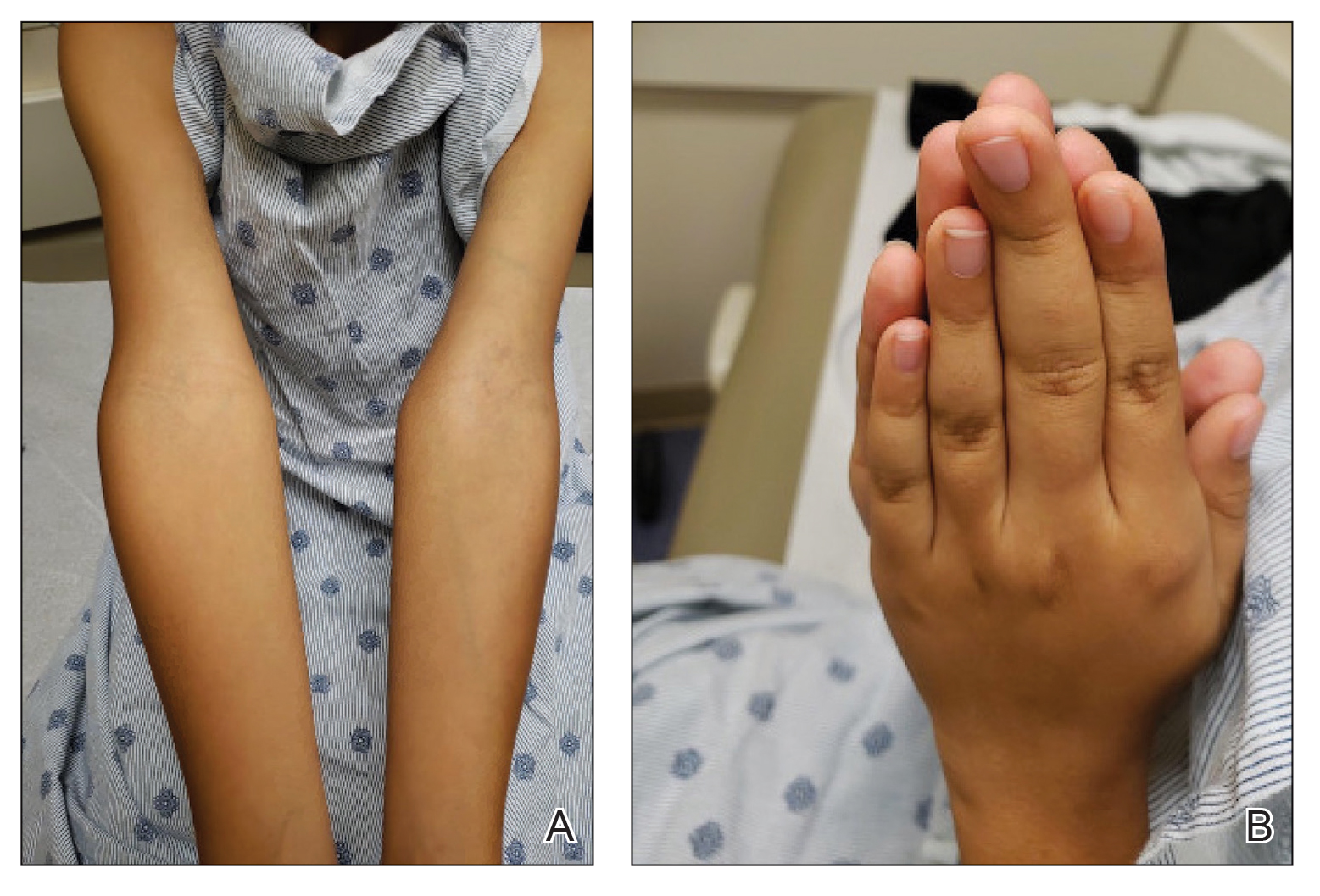
It ordinarily is thought that vascular anomalies of a limb may result in hypertrophy due to increased blood flow such as in KTS, but there are occasions where the affected limb(s) are inexplicably smaller.2,4 Cubiró et al3 observed that in 6 patients with unilateral CMs, all had ipsilateral limb undergrowth. They proposed the term diffuse capillary malformation with undergrowth as a distinct counterpart to DCMO. Diffuse capillary malformation with undergrowth is most similar to CMTC, as both can present with patchy or reticulated capillary staining with ipsilateral limb hypotrophy, but girth more often is affected than length; CMTC also may be associated with dermal atrophy and ulceration.2 The lesions of CMTC typically diminish within the first few years of life whereas those in DCMU tend to persist. Patients with KTS also can exhibit soft-tissue and bony undergrowth, which is termed inverse Klippel-Trenaunay syndrome3; however, the lack of the triad of capillary-lymphatic-venous malformation in our patient made this condition less likely. Additionally, it appears that our patient had left-sided undergrowth rather than the previously diagnosed right hemihypertrophy. The ipsilateral macrodactyly of the third digit of the left hand was an interesting observation and contrasted the undergrowth apparent in the rest of the left limb, which could be caused by increased blood flow specifically to the third digit resembling DCMO.4
Of note, genetic mutations have been implicated as a cause of vascular malformations and growth abnormalities. Specifically, mutations in the phosphoinositide-3-kinase–AKT pathway have been reported in these cases likely due its role in cell growth, proliferation, and angiogenesis.3,4 Future studies should investigate genetic associations in patients with DCMU to determine if there is a robust genotypic-phenotypic link.
Although CMs are a common occurrence in pediatric dermatology, CMs with concurrent limb undergrowth are rare. Our patient’s unique features included involvement of both an arm and leg as well as the presence of macrodactyly. We agree with the terminology for DCMU to describe multifocal reticulated vascular patterning with ipsilateral undergrowth.3
- Huang JT, Liang MG. Vascular malformations. Pediatr Clin North Am. 2010;57:1091-1110. doi:10.1016/j.pcl.2010.08.003
- Lee MS, Liang MG, Mulliken JB. Diffuse capillary malformation with overgrowth: a clinical subtype of vascular anomalies with hypertrophy. J Am Acad Dermatol. 2013;69:589-594. doi:10.1016/j.jaad.2013.05.030
- Cubiró X, Rozas‐Muñoz E, Castel P, et al. Clinical and genetic evaluation of six children with diffuse capillary malformation and undergrowth. Pediatr Dermatol. 2020;37:833-838. doi:10.1111/pde.14252
- Uihlein LC, Liang MG, Fishman SJ, et al. Capillary-venous malformation in the lower limb. Pediatr Dermatol. 2013;30:541-548. doi:10.1111/pde.12186
To the Editor:
Capillary malformations (CMs), the most common vascular malformations that can affect the skin,1 present clinically as macules and patches of various colors, shapes, and sizes. Congenital structural abnormalities are associated with conditions such as Klippel-Trenaunay syndrome (KTS), cutis marmorata telangiectatica congenita (CMTC), and megalencephaly–capillary malformation syndrome.2 Diffuse CM with overgrowth (DCMO) of the soft tissue and bones is an established association of CMs; however, diffuse capillary malformation with undergrowth (DCMU) is a more recent term that describes the lesser-recognized counterpart to DCMO.3 Herein, we describe a case of CM with left-sided undergrowth.

An 11-year-old boy presented to our clinic with asymptomatic vascular patterning on the left side of the body that had been present since birth. He previously was diagnosed with congenital right hemihypertrophy. He reported that the areas gradually lightened over time, and he denied any history of ulceration or venous or lymphatic malformations. Additionally, he explained how the left arm and leg have been noticeably smaller than the right extremities throughout his life. Physical examination revealed superficial, violaceous, reticulated patches along the left upper back tracking down the arm, abdomen (Figure 1A), and anterior thigh (Figure 1B) without crossing the midline. A few dilated veins were noted in the same region as the patches. There was no evidence of scarring or depression found in the skin. The right arms and legs were visibly larger compared to the left side (Figure 2A), and there also was macrodactyly of the third digit of the left hand (Figure 2B). Radiography confirmed the limb length discrepancy and showed the right and left legs to measure 73.2 cm and 71.3 cm, respectively. Given the patient’s multifocal reticulated CMs and ipsilateral undergrowth, a diagnosis of DCMU was rendered. The superficial vascular pattern is likely to fade over time, which will partially be hidden by his darker complexion. He also was advised to continue to see an orthopedist to monitor the limb length incongruity. Surgical intervention was not recommended.

It ordinarily is thought that vascular anomalies of a limb may result in hypertrophy due to increased blood flow such as in KTS, but there are occasions where the affected limb(s) are inexplicably smaller.2,4 Cubiró et al3 observed that in 6 patients with unilateral CMs, all had ipsilateral limb undergrowth. They proposed the term diffuse capillary malformation with undergrowth as a distinct counterpart to DCMO. Diffuse capillary malformation with undergrowth is most similar to CMTC, as both can present with patchy or reticulated capillary staining with ipsilateral limb hypotrophy, but girth more often is affected than length; CMTC also may be associated with dermal atrophy and ulceration.2 The lesions of CMTC typically diminish within the first few years of life whereas those in DCMU tend to persist. Patients with KTS also can exhibit soft-tissue and bony undergrowth, which is termed inverse Klippel-Trenaunay syndrome3; however, the lack of the triad of capillary-lymphatic-venous malformation in our patient made this condition less likely. Additionally, it appears that our patient had left-sided undergrowth rather than the previously diagnosed right hemihypertrophy. The ipsilateral macrodactyly of the third digit of the left hand was an interesting observation and contrasted the undergrowth apparent in the rest of the left limb, which could be caused by increased blood flow specifically to the third digit resembling DCMO.4
Of note, genetic mutations have been implicated as a cause of vascular malformations and growth abnormalities. Specifically, mutations in the phosphoinositide-3-kinase–AKT pathway have been reported in these cases likely due its role in cell growth, proliferation, and angiogenesis.3,4 Future studies should investigate genetic associations in patients with DCMU to determine if there is a robust genotypic-phenotypic link.
Although CMs are a common occurrence in pediatric dermatology, CMs with concurrent limb undergrowth are rare. Our patient’s unique features included involvement of both an arm and leg as well as the presence of macrodactyly. We agree with the terminology for DCMU to describe multifocal reticulated vascular patterning with ipsilateral undergrowth.3
To the Editor:
Capillary malformations (CMs), the most common vascular malformations that can affect the skin,1 present clinically as macules and patches of various colors, shapes, and sizes. Congenital structural abnormalities are associated with conditions such as Klippel-Trenaunay syndrome (KTS), cutis marmorata telangiectatica congenita (CMTC), and megalencephaly–capillary malformation syndrome.2 Diffuse CM with overgrowth (DCMO) of the soft tissue and bones is an established association of CMs; however, diffuse capillary malformation with undergrowth (DCMU) is a more recent term that describes the lesser-recognized counterpart to DCMO.3 Herein, we describe a case of CM with left-sided undergrowth.

An 11-year-old boy presented to our clinic with asymptomatic vascular patterning on the left side of the body that had been present since birth. He previously was diagnosed with congenital right hemihypertrophy. He reported that the areas gradually lightened over time, and he denied any history of ulceration or venous or lymphatic malformations. Additionally, he explained how the left arm and leg have been noticeably smaller than the right extremities throughout his life. Physical examination revealed superficial, violaceous, reticulated patches along the left upper back tracking down the arm, abdomen (Figure 1A), and anterior thigh (Figure 1B) without crossing the midline. A few dilated veins were noted in the same region as the patches. There was no evidence of scarring or depression found in the skin. The right arms and legs were visibly larger compared to the left side (Figure 2A), and there also was macrodactyly of the third digit of the left hand (Figure 2B). Radiography confirmed the limb length discrepancy and showed the right and left legs to measure 73.2 cm and 71.3 cm, respectively. Given the patient’s multifocal reticulated CMs and ipsilateral undergrowth, a diagnosis of DCMU was rendered. The superficial vascular pattern is likely to fade over time, which will partially be hidden by his darker complexion. He also was advised to continue to see an orthopedist to monitor the limb length incongruity. Surgical intervention was not recommended.

It ordinarily is thought that vascular anomalies of a limb may result in hypertrophy due to increased blood flow such as in KTS, but there are occasions where the affected limb(s) are inexplicably smaller.2,4 Cubiró et al3 observed that in 6 patients with unilateral CMs, all had ipsilateral limb undergrowth. They proposed the term diffuse capillary malformation with undergrowth as a distinct counterpart to DCMO. Diffuse capillary malformation with undergrowth is most similar to CMTC, as both can present with patchy or reticulated capillary staining with ipsilateral limb hypotrophy, but girth more often is affected than length; CMTC also may be associated with dermal atrophy and ulceration.2 The lesions of CMTC typically diminish within the first few years of life whereas those in DCMU tend to persist. Patients with KTS also can exhibit soft-tissue and bony undergrowth, which is termed inverse Klippel-Trenaunay syndrome3; however, the lack of the triad of capillary-lymphatic-venous malformation in our patient made this condition less likely. Additionally, it appears that our patient had left-sided undergrowth rather than the previously diagnosed right hemihypertrophy. The ipsilateral macrodactyly of the third digit of the left hand was an interesting observation and contrasted the undergrowth apparent in the rest of the left limb, which could be caused by increased blood flow specifically to the third digit resembling DCMO.4
Of note, genetic mutations have been implicated as a cause of vascular malformations and growth abnormalities. Specifically, mutations in the phosphoinositide-3-kinase–AKT pathway have been reported in these cases likely due its role in cell growth, proliferation, and angiogenesis.3,4 Future studies should investigate genetic associations in patients with DCMU to determine if there is a robust genotypic-phenotypic link.
Although CMs are a common occurrence in pediatric dermatology, CMs with concurrent limb undergrowth are rare. Our patient’s unique features included involvement of both an arm and leg as well as the presence of macrodactyly. We agree with the terminology for DCMU to describe multifocal reticulated vascular patterning with ipsilateral undergrowth.3
- Huang JT, Liang MG. Vascular malformations. Pediatr Clin North Am. 2010;57:1091-1110. doi:10.1016/j.pcl.2010.08.003
- Lee MS, Liang MG, Mulliken JB. Diffuse capillary malformation with overgrowth: a clinical subtype of vascular anomalies with hypertrophy. J Am Acad Dermatol. 2013;69:589-594. doi:10.1016/j.jaad.2013.05.030
- Cubiró X, Rozas‐Muñoz E, Castel P, et al. Clinical and genetic evaluation of six children with diffuse capillary malformation and undergrowth. Pediatr Dermatol. 2020;37:833-838. doi:10.1111/pde.14252
- Uihlein LC, Liang MG, Fishman SJ, et al. Capillary-venous malformation in the lower limb. Pediatr Dermatol. 2013;30:541-548. doi:10.1111/pde.12186
- Huang JT, Liang MG. Vascular malformations. Pediatr Clin North Am. 2010;57:1091-1110. doi:10.1016/j.pcl.2010.08.003
- Lee MS, Liang MG, Mulliken JB. Diffuse capillary malformation with overgrowth: a clinical subtype of vascular anomalies with hypertrophy. J Am Acad Dermatol. 2013;69:589-594. doi:10.1016/j.jaad.2013.05.030
- Cubiró X, Rozas‐Muñoz E, Castel P, et al. Clinical and genetic evaluation of six children with diffuse capillary malformation and undergrowth. Pediatr Dermatol. 2020;37:833-838. doi:10.1111/pde.14252
- Uihlein LC, Liang MG, Fishman SJ, et al. Capillary-venous malformation in the lower limb. Pediatr Dermatol. 2013;30:541-548. doi:10.1111/pde.12186
Practice Points
- The term diffuse capillary malformation with undergrowth (DCMU) describes a distinct counterpart to diffuse capillary malformation with overgrowth. It can be challenging to distinguish from other vascular malformations associated with congenital structural abnormalities.
- The vascular patterning of DCMU may fade over time, but patients should continue to be monitored for their structural incongruity.
Neutrophilic Dermatosis of the Dorsal Hand: A Distinctive Variant of Sweet Syndrome
To the Editor:
Neutrophilic dermatosis of the dorsal hand (NDDH) is an uncommon reactive neutrophilic dermatosis that presents as a painful, enlarging, ulcerative nodule. It often is misdiagnosed and initially treated as an infection. Similar to other neutrophilic dermatoses, it is associated with underlying infections, inflammatory conditions, and malignancies. Neutrophilic dermatosis of the dorsal hand is considered a subset of Sweet syndrome (SS); we highlight similarities and differences between NDDH and SS, reporting the case of a 66-year-old man without systemic symptoms who developed NDDH on the right hand.
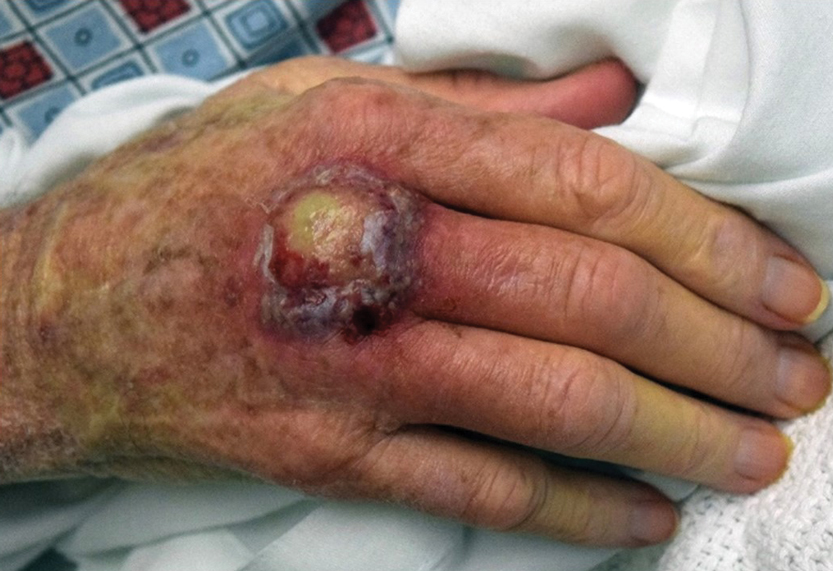
A 66-year-old man presented with a progressively enlarging, painful, ulcerative, 2-cm nodule on the right hand following mechanical trauma 2 weeks prior (Figure 1). He was afebrile with no remarkable medical history. Laboratory evaluation revealed an erythrocyte sedimentation rate (ESR) of 20 mm/h (reference range, 0-10 mm/h) and C-reactive protein (CRP) level of 3.52 mg/dL (reference range, 0-0.5 mg/dL) without leukocytosis; both were not remarkably elevated when adjusted for age.1,2 The clinical differential diagnosis was broad and included pyoderma with evolving cellulitis, neutrophilic dermatosis, atypical mycobacterial infection, subcutaneous or deep fungal infection, squamous cell carcinoma, cutaneous lymphoma, and metastasis. Due to the rapid development of the lesion, initial treatment focused on a bacterial infection, but there was no improvement on antibiotics and wound cultures were negative. The ulcerative nodule was biopsied, and histopathology demonstrated abundant neutrophilic inflammation, endothelial swelling, and leukocytoclasis without microorganisms (Figure 2). Tissue cultures for bacteria, fungi, and atypical mycobacteria were negative. A diagnosis of NDDH was made based on clinical and histologic findings. The wound improved with a 3-week course of oral prednisone.
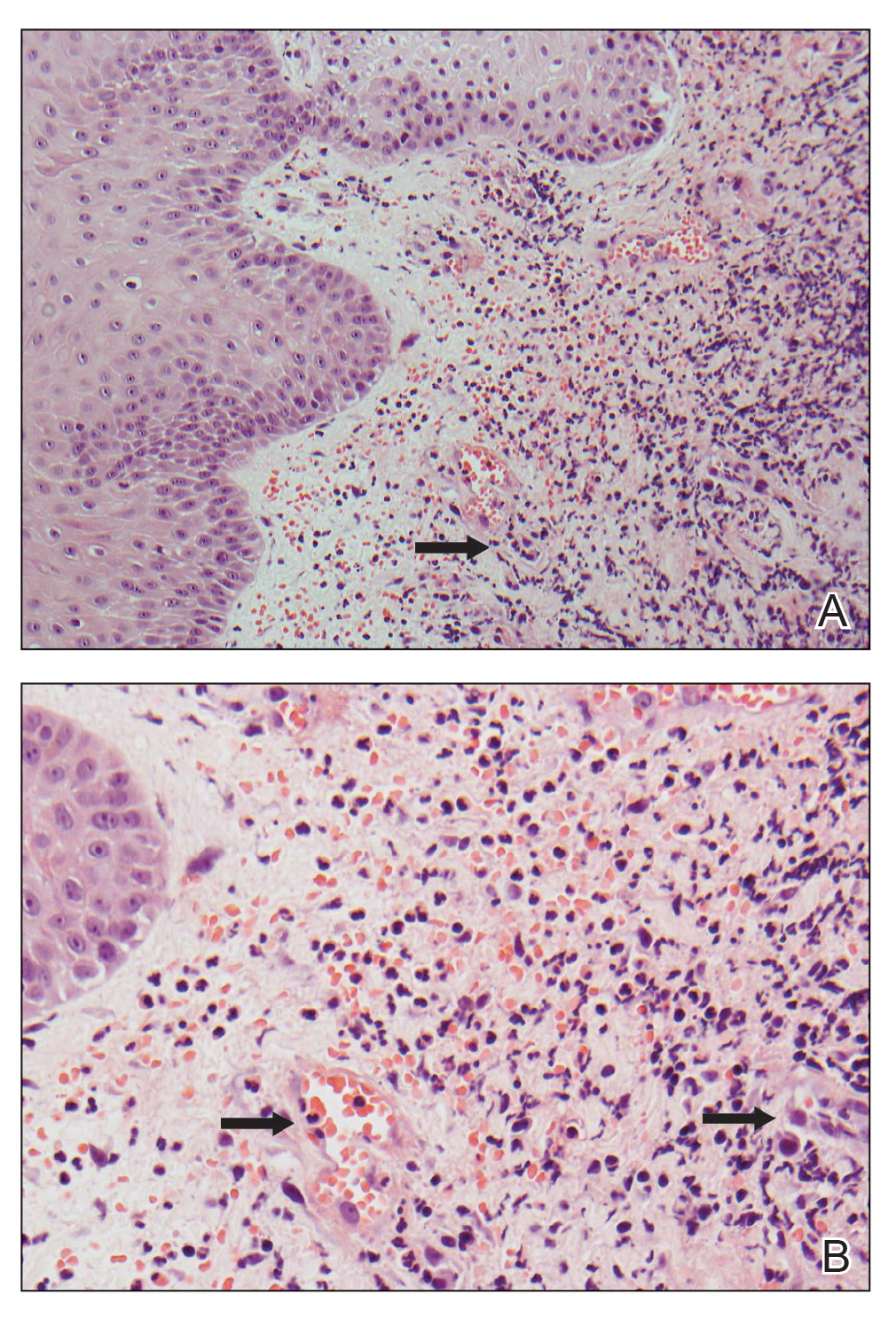
Neutrophilic dermatosis of the dorsal hand is a subset of reactive neutrophilic dermatoses, which includes SS (acute febrile neutrophilic dermatosis) and pyoderma gangrenosum. It is described as a localized variant of SS, with similar associated underlying inflammatory, neoplastic conditions and laboratory findings.3 However, NDDH has characteristic features that differ from classic SS. Neutrophilic dermatosis of the dorsal hand typically presents as painful papules, pustules, or ulcers that progress to become larger ulcers, plaques, and nodules. The clinical appearance may more closely resemble pyoderma gangrenosum or atypical SS, with ulceration frequently present. Pathergy also may be demonstrated in NDDH, similar to our patient. The average age of presentation for NDDH is 60 years, which is older than the average age for SS or pyoderma gangrenosum.3 Similar to other neutrophilic dermatoses, NDDH responds well to oral steroids or steroid-sparing immunosuppressants such as dapsone, colchicine, azathioprine, or tetracycline antibiotics.4
The criteria for SS are well established5,6 and may be used for the diagnosis of NDDH, taking into account the localization of lesions to the dorsal aspect of the hands. The diagnostic criteria for SS include fulfillment of both major and at least 2 of 4 minor criteria. The 2 major criteria include rapid presentation of skin lesions and neutrophilic dermal infiltrate on biopsy. Minor criteria are defined as the following: (1) preceding nonspecific respiratory or gastrointestinal tract infection, inflammatory conditions, underlying malignancy, or pregnancy; (2) fever; (3) excellent response to steroids; and (4) 3 of the 4 of the following laboratory abnormalities: elevated CRP, ESR, leukocytosis, or left shift in complete blood cell count. Our patient met both major criteria and only 1 minor criterion—excellent response to systemic corticosteroids. Nofal et al7 advocated for revised diagnostic criteria for SS, with one suggestion utilizing only the 2 major criteria being necessary for diagnosis. Given that serum inflammatory markers may not be as elevated in NDDH compared to SS,3,7,8 meeting the major criteria alone may be a better way to diagnose NDDH, as in our patient.
Our patient presented with an expanding ulcerating nodule on the hand that elicited a wide list of differential diagnoses to include infections and neoplasms. Rapid development, localization to the dorsal aspect of the hand, and treatment resistance to antibiotics may help the clinician consider a diagnosis of NDDH, which should be confirmed by a biopsy. Similar to other neutrophilic dermatoses, an underlying malignancy or inflammatory condition should be sought out. Neutrophilic dermatosis of the dorsal hand responds well to systemic steroids, though recurrences may occur.
- Miller A, Green M, Robinson D. Simple rule for calculating normal erythrocyte sedimentation rate. Br Med (Clinical Res Ed). 1983;286:226.
- Wyczalkowska-Tomasik A, Czarkowska-Paczek B, Zielenkiewicz M, et al. Inflammatory markers change with age, but do not fall beyond reported normal ranges. Arch Immunol Ther Exp (Warsz). 2016;64:249-254.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- Gaulding J, Kohen LL. Neutrophilic dermatosis of the dorsal hands. J Am Acad Dermatol. 2017; 76(6 suppl 1):AB178.
- Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Su WP, Liu HN. Diagnostic criteria for Sweet’s syndrome. Cutis. 1986;37:167-174.
- Nofal A, Abdelmaksoud A, Amer H, et al. Sweet’s syndrome: diagnostic criteria revisited. J Dtsch Dermatol Ges. 2017;15:1081-1088.
- Wolf R, Tüzün Y. Acral manifestations of Sweet syndrome (neutrophilic dermatosis of the hands). Clin Dermatol. 2017;35:81-84.
To the Editor:
Neutrophilic dermatosis of the dorsal hand (NDDH) is an uncommon reactive neutrophilic dermatosis that presents as a painful, enlarging, ulcerative nodule. It often is misdiagnosed and initially treated as an infection. Similar to other neutrophilic dermatoses, it is associated with underlying infections, inflammatory conditions, and malignancies. Neutrophilic dermatosis of the dorsal hand is considered a subset of Sweet syndrome (SS); we highlight similarities and differences between NDDH and SS, reporting the case of a 66-year-old man without systemic symptoms who developed NDDH on the right hand.

A 66-year-old man presented with a progressively enlarging, painful, ulcerative, 2-cm nodule on the right hand following mechanical trauma 2 weeks prior (Figure 1). He was afebrile with no remarkable medical history. Laboratory evaluation revealed an erythrocyte sedimentation rate (ESR) of 20 mm/h (reference range, 0-10 mm/h) and C-reactive protein (CRP) level of 3.52 mg/dL (reference range, 0-0.5 mg/dL) without leukocytosis; both were not remarkably elevated when adjusted for age.1,2 The clinical differential diagnosis was broad and included pyoderma with evolving cellulitis, neutrophilic dermatosis, atypical mycobacterial infection, subcutaneous or deep fungal infection, squamous cell carcinoma, cutaneous lymphoma, and metastasis. Due to the rapid development of the lesion, initial treatment focused on a bacterial infection, but there was no improvement on antibiotics and wound cultures were negative. The ulcerative nodule was biopsied, and histopathology demonstrated abundant neutrophilic inflammation, endothelial swelling, and leukocytoclasis without microorganisms (Figure 2). Tissue cultures for bacteria, fungi, and atypical mycobacteria were negative. A diagnosis of NDDH was made based on clinical and histologic findings. The wound improved with a 3-week course of oral prednisone.

Neutrophilic dermatosis of the dorsal hand is a subset of reactive neutrophilic dermatoses, which includes SS (acute febrile neutrophilic dermatosis) and pyoderma gangrenosum. It is described as a localized variant of SS, with similar associated underlying inflammatory, neoplastic conditions and laboratory findings.3 However, NDDH has characteristic features that differ from classic SS. Neutrophilic dermatosis of the dorsal hand typically presents as painful papules, pustules, or ulcers that progress to become larger ulcers, plaques, and nodules. The clinical appearance may more closely resemble pyoderma gangrenosum or atypical SS, with ulceration frequently present. Pathergy also may be demonstrated in NDDH, similar to our patient. The average age of presentation for NDDH is 60 years, which is older than the average age for SS or pyoderma gangrenosum.3 Similar to other neutrophilic dermatoses, NDDH responds well to oral steroids or steroid-sparing immunosuppressants such as dapsone, colchicine, azathioprine, or tetracycline antibiotics.4
The criteria for SS are well established5,6 and may be used for the diagnosis of NDDH, taking into account the localization of lesions to the dorsal aspect of the hands. The diagnostic criteria for SS include fulfillment of both major and at least 2 of 4 minor criteria. The 2 major criteria include rapid presentation of skin lesions and neutrophilic dermal infiltrate on biopsy. Minor criteria are defined as the following: (1) preceding nonspecific respiratory or gastrointestinal tract infection, inflammatory conditions, underlying malignancy, or pregnancy; (2) fever; (3) excellent response to steroids; and (4) 3 of the 4 of the following laboratory abnormalities: elevated CRP, ESR, leukocytosis, or left shift in complete blood cell count. Our patient met both major criteria and only 1 minor criterion—excellent response to systemic corticosteroids. Nofal et al7 advocated for revised diagnostic criteria for SS, with one suggestion utilizing only the 2 major criteria being necessary for diagnosis. Given that serum inflammatory markers may not be as elevated in NDDH compared to SS,3,7,8 meeting the major criteria alone may be a better way to diagnose NDDH, as in our patient.
Our patient presented with an expanding ulcerating nodule on the hand that elicited a wide list of differential diagnoses to include infections and neoplasms. Rapid development, localization to the dorsal aspect of the hand, and treatment resistance to antibiotics may help the clinician consider a diagnosis of NDDH, which should be confirmed by a biopsy. Similar to other neutrophilic dermatoses, an underlying malignancy or inflammatory condition should be sought out. Neutrophilic dermatosis of the dorsal hand responds well to systemic steroids, though recurrences may occur.
To the Editor:
Neutrophilic dermatosis of the dorsal hand (NDDH) is an uncommon reactive neutrophilic dermatosis that presents as a painful, enlarging, ulcerative nodule. It often is misdiagnosed and initially treated as an infection. Similar to other neutrophilic dermatoses, it is associated with underlying infections, inflammatory conditions, and malignancies. Neutrophilic dermatosis of the dorsal hand is considered a subset of Sweet syndrome (SS); we highlight similarities and differences between NDDH and SS, reporting the case of a 66-year-old man without systemic symptoms who developed NDDH on the right hand.

A 66-year-old man presented with a progressively enlarging, painful, ulcerative, 2-cm nodule on the right hand following mechanical trauma 2 weeks prior (Figure 1). He was afebrile with no remarkable medical history. Laboratory evaluation revealed an erythrocyte sedimentation rate (ESR) of 20 mm/h (reference range, 0-10 mm/h) and C-reactive protein (CRP) level of 3.52 mg/dL (reference range, 0-0.5 mg/dL) without leukocytosis; both were not remarkably elevated when adjusted for age.1,2 The clinical differential diagnosis was broad and included pyoderma with evolving cellulitis, neutrophilic dermatosis, atypical mycobacterial infection, subcutaneous or deep fungal infection, squamous cell carcinoma, cutaneous lymphoma, and metastasis. Due to the rapid development of the lesion, initial treatment focused on a bacterial infection, but there was no improvement on antibiotics and wound cultures were negative. The ulcerative nodule was biopsied, and histopathology demonstrated abundant neutrophilic inflammation, endothelial swelling, and leukocytoclasis without microorganisms (Figure 2). Tissue cultures for bacteria, fungi, and atypical mycobacteria were negative. A diagnosis of NDDH was made based on clinical and histologic findings. The wound improved with a 3-week course of oral prednisone.

Neutrophilic dermatosis of the dorsal hand is a subset of reactive neutrophilic dermatoses, which includes SS (acute febrile neutrophilic dermatosis) and pyoderma gangrenosum. It is described as a localized variant of SS, with similar associated underlying inflammatory, neoplastic conditions and laboratory findings.3 However, NDDH has characteristic features that differ from classic SS. Neutrophilic dermatosis of the dorsal hand typically presents as painful papules, pustules, or ulcers that progress to become larger ulcers, plaques, and nodules. The clinical appearance may more closely resemble pyoderma gangrenosum or atypical SS, with ulceration frequently present. Pathergy also may be demonstrated in NDDH, similar to our patient. The average age of presentation for NDDH is 60 years, which is older than the average age for SS or pyoderma gangrenosum.3 Similar to other neutrophilic dermatoses, NDDH responds well to oral steroids or steroid-sparing immunosuppressants such as dapsone, colchicine, azathioprine, or tetracycline antibiotics.4
The criteria for SS are well established5,6 and may be used for the diagnosis of NDDH, taking into account the localization of lesions to the dorsal aspect of the hands. The diagnostic criteria for SS include fulfillment of both major and at least 2 of 4 minor criteria. The 2 major criteria include rapid presentation of skin lesions and neutrophilic dermal infiltrate on biopsy. Minor criteria are defined as the following: (1) preceding nonspecific respiratory or gastrointestinal tract infection, inflammatory conditions, underlying malignancy, or pregnancy; (2) fever; (3) excellent response to steroids; and (4) 3 of the 4 of the following laboratory abnormalities: elevated CRP, ESR, leukocytosis, or left shift in complete blood cell count. Our patient met both major criteria and only 1 minor criterion—excellent response to systemic corticosteroids. Nofal et al7 advocated for revised diagnostic criteria for SS, with one suggestion utilizing only the 2 major criteria being necessary for diagnosis. Given that serum inflammatory markers may not be as elevated in NDDH compared to SS,3,7,8 meeting the major criteria alone may be a better way to diagnose NDDH, as in our patient.
Our patient presented with an expanding ulcerating nodule on the hand that elicited a wide list of differential diagnoses to include infections and neoplasms. Rapid development, localization to the dorsal aspect of the hand, and treatment resistance to antibiotics may help the clinician consider a diagnosis of NDDH, which should be confirmed by a biopsy. Similar to other neutrophilic dermatoses, an underlying malignancy or inflammatory condition should be sought out. Neutrophilic dermatosis of the dorsal hand responds well to systemic steroids, though recurrences may occur.
- Miller A, Green M, Robinson D. Simple rule for calculating normal erythrocyte sedimentation rate. Br Med (Clinical Res Ed). 1983;286:226.
- Wyczalkowska-Tomasik A, Czarkowska-Paczek B, Zielenkiewicz M, et al. Inflammatory markers change with age, but do not fall beyond reported normal ranges. Arch Immunol Ther Exp (Warsz). 2016;64:249-254.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- Gaulding J, Kohen LL. Neutrophilic dermatosis of the dorsal hands. J Am Acad Dermatol. 2017; 76(6 suppl 1):AB178.
- Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Su WP, Liu HN. Diagnostic criteria for Sweet’s syndrome. Cutis. 1986;37:167-174.
- Nofal A, Abdelmaksoud A, Amer H, et al. Sweet’s syndrome: diagnostic criteria revisited. J Dtsch Dermatol Ges. 2017;15:1081-1088.
- Wolf R, Tüzün Y. Acral manifestations of Sweet syndrome (neutrophilic dermatosis of the hands). Clin Dermatol. 2017;35:81-84.
- Miller A, Green M, Robinson D. Simple rule for calculating normal erythrocyte sedimentation rate. Br Med (Clinical Res Ed). 1983;286:226.
- Wyczalkowska-Tomasik A, Czarkowska-Paczek B, Zielenkiewicz M, et al. Inflammatory markers change with age, but do not fall beyond reported normal ranges. Arch Immunol Ther Exp (Warsz). 2016;64:249-254.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- Gaulding J, Kohen LL. Neutrophilic dermatosis of the dorsal hands. J Am Acad Dermatol. 2017; 76(6 suppl 1):AB178.
- Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Su WP, Liu HN. Diagnostic criteria for Sweet’s syndrome. Cutis. 1986;37:167-174.
- Nofal A, Abdelmaksoud A, Amer H, et al. Sweet’s syndrome: diagnostic criteria revisited. J Dtsch Dermatol Ges. 2017;15:1081-1088.
- Wolf R, Tüzün Y. Acral manifestations of Sweet syndrome (neutrophilic dermatosis of the hands). Clin Dermatol. 2017;35:81-84.
Practice Points
- Neutrophilic dermatosis of the dorsal hand (NDDH) is a reactive neutrophilic dermatosis that includes Sweet syndrome (SS) and pyoderma gangrenosum.
- Localization to the dorsal aspect of the hand, presence of ulcerative nodules, and older age at onset are characteristic features of NDDH.
- Meeting the major criteria alone for SS may be a more sensitive way to diagnose NDDH, as serum inflammatory markers may not be remarkably elevated in this condition.
Dupilumab for Dyshidrotic Eczema With Secondary Improvement in Eosinophilic Interstitial Lung Disease
To the Editor:
Biologic medications are increasingly utilized in adults with moderate to severe atopic dermatitis (AD) that is inadequately controlled with topical medication. By targeting the IL-4 receptor alpha subunit, dupilumab inhibits the biologic effects of IL-4 and IL-13, resulting in remarkable improvement in disease and quality of life for many patients with refractory AD.1
In 2017, the US Food and Drug Administration approved dupilumab for use in AD, asthma, and chronic rhinosinusitis. However, there is evidence of the drug’s off-label efficacy in conditions such as eosinophilic annular erythema.2 We present a patient with dyshidrotic eczema treated with dupilumab who experienced contemporaneous secondary improvement in chronic eosinophilic pneumonia (CEP) and interstitial lung disease (ILD).
A 45-year-old man was referred to our dermatology clinic for chronic hand dermatitis refractory to increasing strengths of topical corticosteroids. He had a history of progressive shortness of breath of unknown cause, which began 2 years prior, and he was being followed at our institution’s ILD clinic. Earlier pulmonary function testing revealed a restrictive pattern with interstitial infiltrates seen on chest computed tomography. A lung biopsy demonstrated features of fibrotic nonspecific interstitial pneumonitis with superimposed eosinophilic pneumonia. His pulmonary symptoms had progressively worsened; over a period of several months, the supplemental oxygen requirement had increased to 6 L at rest and 12 L upon exertion. Prednisone therapy was initiated, which alleviated respiratory symptoms; however, the patient was unable to tolerate a gradual wean of the medication, which rendered him steroid dependent at 30 mg/d.
Along with respiratory symptoms, the patient reported symptoms consistent with an autoimmune process, including dry eyes. Muscle weakness and tenderness also were noted. Ultimately, a diagnosis of anti–PL-7 (anti-threonyl-transfer RNA synthetase) antisynthetase syndrome was rendered by identification of anti–PL-7 antibodies and an elevated level of creatinine kinase.
Physical examination at our clinic revealed subtle palmar scaling on the hands and multiple small clear vesicles on the lateral aspects of the digits (Figure, A), consistent with dyshidrotic eczema. He initially was treated with clobetasol propionate ointment 0.05%. Despite adherence to this high-potency topical corticosteroid, he experienced only minimal improvement over a period of 3 months. Dupilumab was started at standard dosing—600 mg at initiation, followed by 300 mg every 2 weeks. The patient reported rapid improvement in dyshidrotic eczema over several months with near-complete resolution (Figure, B).
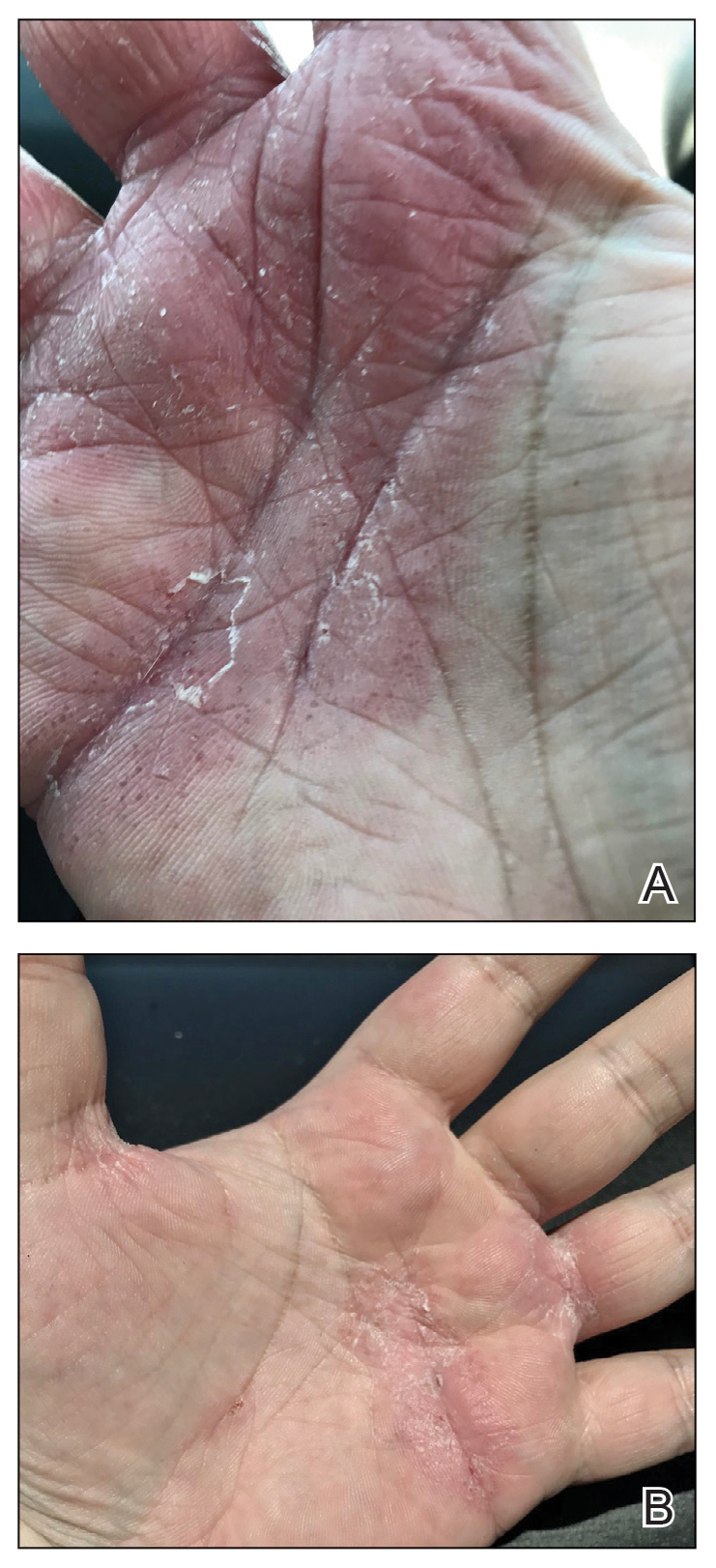
Concurrent with initiation and continued use of dupilumab, without other changes in his medication regimen, the patient noted gradual improvement in respiratory symptoms. At 6-month follow-up he reported notable improvement in respiratory function and quality of life. He then tolerated a gradual wean of prednisone to 10 mg/d, with a similar reduction in supplemental oxygen.
Off-label use of dupilumab for various eosinophilic conditions has shown promising efficacy. Our patient experienced improvement in CEP shortly after initiation of dupilumab, enabling weaning of prednisone, which has a well established adverse effect profile associated with long term use.3,4 In comparison, dupilumab generally is well tolerated, with rare ophthalmologic complications and injection-site reactions.5
One case report suggested that CEP may represent a potential rare adverse effect of dupilumab initiation.6 However, prior to initiation of dupilumab, that patient had poorly controlled asthma requiring frequent oral corticosteroid therapy. It is possible that CEP was subclinical prior to initiation of dupilumab and became more noticeable once the patient was weaned from corticosteroids, which had served as an indirect treatment.6 Nonetheless, more research is needed to definitively establish the efficacy of dupilumab in CEP prior to more widespread use.
Irrespective of the potential efficacy of dupilumab for the treatment of CEP, our case highlights the growing body of evidence that dupilumab should be considered in the treatment of dyshidrotic eczema, particularly in cases refractory to topical treatment.7 When a systemic medication is preferred, dupilumab likely represents an option with a relatively well-tolerated adverse effect profile compared to traditional systemic treatments for dyshidrotic eczema.
1. Barbarot S, Wollenberg A, Silverberg JI, et al. Dupilumab provides rapid and sustained improvement in SCORAD outcomes in adults with moderate-to-severe atopic dermatitis: combined results ofour randomized phase 3 trials. J Dermatolog Treat. 2022;33:266-277. doi:10.1080/09546634.2020.1750550
2. Gordon SC, Robinson SN, Abudu M, et al. Eosinophilic annular erythema treated with dupilumab. Pediatr Dermatol. 2018;35:E255-E256. doi:10.1111/pde.13533
3. Callaghan DJ 3rd. Use of Google Trends to examine interest in Mohs micrographic surgery: 2004 to 2016. Dermatol Surg. 2018;44:186-192. doi:10.1097/DSS.0000000000001270
4. Fowler C, Hoover W. Dupilumab for chronic eosinophilic pneumonia. Pediatr Pulmonol. 2020;55:3229-3230. doi:10.1002/ppul.25096
5. Simpson EL, Akinlade B, Ardeleanu M. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2017;376:1090-1091. doi:10.1056/NEJMc1700366
6. Menzella F, Montanari G, Patricelli G, et al. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Ther Clin Risk Manag. 2019;15:869-875. doi:10.2147/TCRM.S207402
7. Waldman RA, DeWane ME, Sloan B, et al. Dupilumab for the treatment of dyshidrotic eczema in 15 consecutive patients. J Am Acad Dermatol. 2020;82:1251-1252. doi:10.1016/j.jaad.2019.12.053
To the Editor:
Biologic medications are increasingly utilized in adults with moderate to severe atopic dermatitis (AD) that is inadequately controlled with topical medication. By targeting the IL-4 receptor alpha subunit, dupilumab inhibits the biologic effects of IL-4 and IL-13, resulting in remarkable improvement in disease and quality of life for many patients with refractory AD.1
In 2017, the US Food and Drug Administration approved dupilumab for use in AD, asthma, and chronic rhinosinusitis. However, there is evidence of the drug’s off-label efficacy in conditions such as eosinophilic annular erythema.2 We present a patient with dyshidrotic eczema treated with dupilumab who experienced contemporaneous secondary improvement in chronic eosinophilic pneumonia (CEP) and interstitial lung disease (ILD).
A 45-year-old man was referred to our dermatology clinic for chronic hand dermatitis refractory to increasing strengths of topical corticosteroids. He had a history of progressive shortness of breath of unknown cause, which began 2 years prior, and he was being followed at our institution’s ILD clinic. Earlier pulmonary function testing revealed a restrictive pattern with interstitial infiltrates seen on chest computed tomography. A lung biopsy demonstrated features of fibrotic nonspecific interstitial pneumonitis with superimposed eosinophilic pneumonia. His pulmonary symptoms had progressively worsened; over a period of several months, the supplemental oxygen requirement had increased to 6 L at rest and 12 L upon exertion. Prednisone therapy was initiated, which alleviated respiratory symptoms; however, the patient was unable to tolerate a gradual wean of the medication, which rendered him steroid dependent at 30 mg/d.
Along with respiratory symptoms, the patient reported symptoms consistent with an autoimmune process, including dry eyes. Muscle weakness and tenderness also were noted. Ultimately, a diagnosis of anti–PL-7 (anti-threonyl-transfer RNA synthetase) antisynthetase syndrome was rendered by identification of anti–PL-7 antibodies and an elevated level of creatinine kinase.
Physical examination at our clinic revealed subtle palmar scaling on the hands and multiple small clear vesicles on the lateral aspects of the digits (Figure, A), consistent with dyshidrotic eczema. He initially was treated with clobetasol propionate ointment 0.05%. Despite adherence to this high-potency topical corticosteroid, he experienced only minimal improvement over a period of 3 months. Dupilumab was started at standard dosing—600 mg at initiation, followed by 300 mg every 2 weeks. The patient reported rapid improvement in dyshidrotic eczema over several months with near-complete resolution (Figure, B).

Concurrent with initiation and continued use of dupilumab, without other changes in his medication regimen, the patient noted gradual improvement in respiratory symptoms. At 6-month follow-up he reported notable improvement in respiratory function and quality of life. He then tolerated a gradual wean of prednisone to 10 mg/d, with a similar reduction in supplemental oxygen.
Off-label use of dupilumab for various eosinophilic conditions has shown promising efficacy. Our patient experienced improvement in CEP shortly after initiation of dupilumab, enabling weaning of prednisone, which has a well established adverse effect profile associated with long term use.3,4 In comparison, dupilumab generally is well tolerated, with rare ophthalmologic complications and injection-site reactions.5
One case report suggested that CEP may represent a potential rare adverse effect of dupilumab initiation.6 However, prior to initiation of dupilumab, that patient had poorly controlled asthma requiring frequent oral corticosteroid therapy. It is possible that CEP was subclinical prior to initiation of dupilumab and became more noticeable once the patient was weaned from corticosteroids, which had served as an indirect treatment.6 Nonetheless, more research is needed to definitively establish the efficacy of dupilumab in CEP prior to more widespread use.
Irrespective of the potential efficacy of dupilumab for the treatment of CEP, our case highlights the growing body of evidence that dupilumab should be considered in the treatment of dyshidrotic eczema, particularly in cases refractory to topical treatment.7 When a systemic medication is preferred, dupilumab likely represents an option with a relatively well-tolerated adverse effect profile compared to traditional systemic treatments for dyshidrotic eczema.
To the Editor:
Biologic medications are increasingly utilized in adults with moderate to severe atopic dermatitis (AD) that is inadequately controlled with topical medication. By targeting the IL-4 receptor alpha subunit, dupilumab inhibits the biologic effects of IL-4 and IL-13, resulting in remarkable improvement in disease and quality of life for many patients with refractory AD.1
In 2017, the US Food and Drug Administration approved dupilumab for use in AD, asthma, and chronic rhinosinusitis. However, there is evidence of the drug’s off-label efficacy in conditions such as eosinophilic annular erythema.2 We present a patient with dyshidrotic eczema treated with dupilumab who experienced contemporaneous secondary improvement in chronic eosinophilic pneumonia (CEP) and interstitial lung disease (ILD).
A 45-year-old man was referred to our dermatology clinic for chronic hand dermatitis refractory to increasing strengths of topical corticosteroids. He had a history of progressive shortness of breath of unknown cause, which began 2 years prior, and he was being followed at our institution’s ILD clinic. Earlier pulmonary function testing revealed a restrictive pattern with interstitial infiltrates seen on chest computed tomography. A lung biopsy demonstrated features of fibrotic nonspecific interstitial pneumonitis with superimposed eosinophilic pneumonia. His pulmonary symptoms had progressively worsened; over a period of several months, the supplemental oxygen requirement had increased to 6 L at rest and 12 L upon exertion. Prednisone therapy was initiated, which alleviated respiratory symptoms; however, the patient was unable to tolerate a gradual wean of the medication, which rendered him steroid dependent at 30 mg/d.
Along with respiratory symptoms, the patient reported symptoms consistent with an autoimmune process, including dry eyes. Muscle weakness and tenderness also were noted. Ultimately, a diagnosis of anti–PL-7 (anti-threonyl-transfer RNA synthetase) antisynthetase syndrome was rendered by identification of anti–PL-7 antibodies and an elevated level of creatinine kinase.
Physical examination at our clinic revealed subtle palmar scaling on the hands and multiple small clear vesicles on the lateral aspects of the digits (Figure, A), consistent with dyshidrotic eczema. He initially was treated with clobetasol propionate ointment 0.05%. Despite adherence to this high-potency topical corticosteroid, he experienced only minimal improvement over a period of 3 months. Dupilumab was started at standard dosing—600 mg at initiation, followed by 300 mg every 2 weeks. The patient reported rapid improvement in dyshidrotic eczema over several months with near-complete resolution (Figure, B).

Concurrent with initiation and continued use of dupilumab, without other changes in his medication regimen, the patient noted gradual improvement in respiratory symptoms. At 6-month follow-up he reported notable improvement in respiratory function and quality of life. He then tolerated a gradual wean of prednisone to 10 mg/d, with a similar reduction in supplemental oxygen.
Off-label use of dupilumab for various eosinophilic conditions has shown promising efficacy. Our patient experienced improvement in CEP shortly after initiation of dupilumab, enabling weaning of prednisone, which has a well established adverse effect profile associated with long term use.3,4 In comparison, dupilumab generally is well tolerated, with rare ophthalmologic complications and injection-site reactions.5
One case report suggested that CEP may represent a potential rare adverse effect of dupilumab initiation.6 However, prior to initiation of dupilumab, that patient had poorly controlled asthma requiring frequent oral corticosteroid therapy. It is possible that CEP was subclinical prior to initiation of dupilumab and became more noticeable once the patient was weaned from corticosteroids, which had served as an indirect treatment.6 Nonetheless, more research is needed to definitively establish the efficacy of dupilumab in CEP prior to more widespread use.
Irrespective of the potential efficacy of dupilumab for the treatment of CEP, our case highlights the growing body of evidence that dupilumab should be considered in the treatment of dyshidrotic eczema, particularly in cases refractory to topical treatment.7 When a systemic medication is preferred, dupilumab likely represents an option with a relatively well-tolerated adverse effect profile compared to traditional systemic treatments for dyshidrotic eczema.
1. Barbarot S, Wollenberg A, Silverberg JI, et al. Dupilumab provides rapid and sustained improvement in SCORAD outcomes in adults with moderate-to-severe atopic dermatitis: combined results ofour randomized phase 3 trials. J Dermatolog Treat. 2022;33:266-277. doi:10.1080/09546634.2020.1750550
2. Gordon SC, Robinson SN, Abudu M, et al. Eosinophilic annular erythema treated with dupilumab. Pediatr Dermatol. 2018;35:E255-E256. doi:10.1111/pde.13533
3. Callaghan DJ 3rd. Use of Google Trends to examine interest in Mohs micrographic surgery: 2004 to 2016. Dermatol Surg. 2018;44:186-192. doi:10.1097/DSS.0000000000001270
4. Fowler C, Hoover W. Dupilumab for chronic eosinophilic pneumonia. Pediatr Pulmonol. 2020;55:3229-3230. doi:10.1002/ppul.25096
5. Simpson EL, Akinlade B, Ardeleanu M. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2017;376:1090-1091. doi:10.1056/NEJMc1700366
6. Menzella F, Montanari G, Patricelli G, et al. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Ther Clin Risk Manag. 2019;15:869-875. doi:10.2147/TCRM.S207402
7. Waldman RA, DeWane ME, Sloan B, et al. Dupilumab for the treatment of dyshidrotic eczema in 15 consecutive patients. J Am Acad Dermatol. 2020;82:1251-1252. doi:10.1016/j.jaad.2019.12.053
1. Barbarot S, Wollenberg A, Silverberg JI, et al. Dupilumab provides rapid and sustained improvement in SCORAD outcomes in adults with moderate-to-severe atopic dermatitis: combined results ofour randomized phase 3 trials. J Dermatolog Treat. 2022;33:266-277. doi:10.1080/09546634.2020.1750550
2. Gordon SC, Robinson SN, Abudu M, et al. Eosinophilic annular erythema treated with dupilumab. Pediatr Dermatol. 2018;35:E255-E256. doi:10.1111/pde.13533
3. Callaghan DJ 3rd. Use of Google Trends to examine interest in Mohs micrographic surgery: 2004 to 2016. Dermatol Surg. 2018;44:186-192. doi:10.1097/DSS.0000000000001270
4. Fowler C, Hoover W. Dupilumab for chronic eosinophilic pneumonia. Pediatr Pulmonol. 2020;55:3229-3230. doi:10.1002/ppul.25096
5. Simpson EL, Akinlade B, Ardeleanu M. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2017;376:1090-1091. doi:10.1056/NEJMc1700366
6. Menzella F, Montanari G, Patricelli G, et al. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Ther Clin Risk Manag. 2019;15:869-875. doi:10.2147/TCRM.S207402
7. Waldman RA, DeWane ME, Sloan B, et al. Dupilumab for the treatment of dyshidrotic eczema in 15 consecutive patients. J Am Acad Dermatol. 2020;82:1251-1252. doi:10.1016/j.jaad.2019.12.053
Practice Points
- Dupilumab can be considered for treatment of refractory dyshidrotic eczema.
- Dupilumab may provide secondary efficacy in patients with dyshidrotic eczema who also have an eosinophilic condition such as eosinophilic pneumonia.
Paradoxical Reaction to TNF-α Inhibitor Therapy in a Patient With Hidradenitis Suppurativa
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the pilosebaceous unit that occurs in concert with elevations of various cytokines, including tumor necrosis factor α (TNF-α), IL-1β, IL-10, and IL-17.1,2 Adalimumab is a TNF-α inhibitor approved by the US Food and Drug Administration for the treatment of HS. Although TNF-α inhibitors are effective for many immune-mediated inflammatory disorders, paradoxical drug reactions have been reported following treatment with these agents.3-6 True paradoxical drug reactions likely are immune mediated and directly lead to new onset of a pathologic condition that would otherwise respond to that drug. For example, there are reports of rheumatoid arthritis patients who were treated with a TNF-α inhibitor and developed psoriatic skin lesions.3,6 Paradoxical drug reactions also have been reported with acute-onset inflammatory bowel disease and HS or less commonly pyoderma gangrenosum (PG), uveitis, granulomatous reactions, and vasculitis.4,5 We present the case of a patient with HS who was treated with a TNF-α inhibitor and developed 2 distinct paradoxical drug reactions. We also provide an overview of paradoxical drug reactions associated with TNF-α inhibitors.
A 38-year-old woman developed a painful “boil” on the right leg that was previously treated in the emergency department with incision and drainage as well as oral clindamycin for 7 days, but the lesion spread and continued to worsen. She had a history of HS in the axillae and groin region that had been present since 12 years of age. The condition was poorly controlled despite multiple courses of oral antibiotics and surgical resections. An oral contraceptive also was attempted, but the patient discontinued treatment when liver enzyme levels became elevated. The patient had no other notable medical history, including skin disease. There was a family history of HS in her father and a sibling. Seeking more effective treatment, the patient was offered adalimumab approximately 4 months prior to clinical presentation and agreed to start a course of the drug. She received a loading dose of 160 mg on day 1 and 80 mg on day 15 followed by a maintenance dosage of 40 mg weekly. She experienced improvement in HS symptoms after 3 months on adalimumab; however, she developed scaly pruritic patches on the scalp, arms, and legs that were consistent with psoriasis. Because of the absence of a personal or family history of psoriasis, the patient was informed of the probability of paradoxical psoriasis resulting from adalimumab. She elected to continue adalimumab because of the improvement in HS symptoms, and the psoriatic lesions were mild and adequately controlled with a topical steroid.
At the current presentation 1 month later, physical examination revealed a large indurated and ulcerated area with jagged edges at the incision and drainage site (Figure 1). Pyoderma gangrenosum was clinically suspected; a biopsy was performed, and the patient was started on oral prednisone. At 2-week follow-up, the ulcer was found to be rapidly resolving with prednisone and healing with cribriform scarring (Figure 2). Histopathology revealed an undermining neutrophilic inflammatory process that was consistent with PG. A diagnosis of PG was made based on previously published criteria7 and the following major/minor criteria in the patient: pathology; absence of infection on histologic analysis; history of pathergy related to worsening ulceration at the site of incision and drainage of the initial boil; clinical findings of an ulcer with peripheral violaceous erythema; undermined borders and tenderness at the site; and rapid resolution of the ulcer with prednisone.
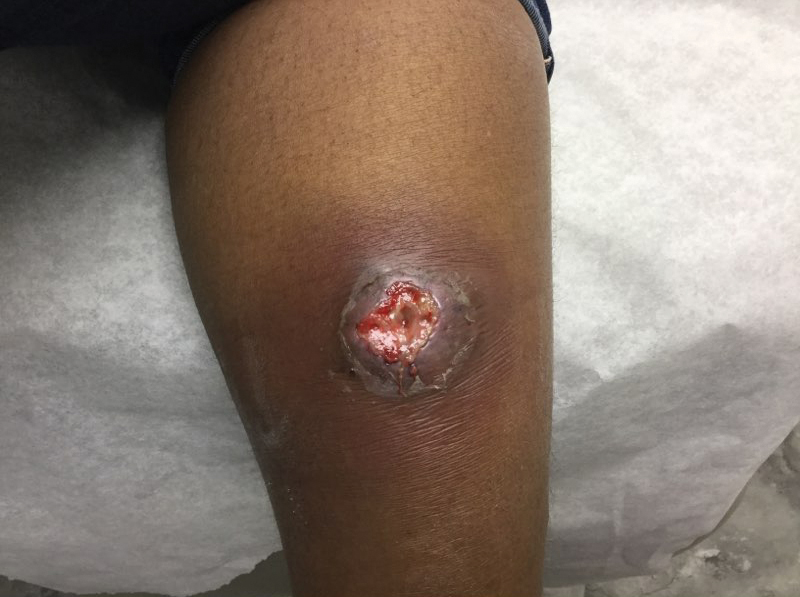
Cessation of adalimumab gradually led to clearance of both psoriasiform lesions and PG; however, HS lesions persisted.
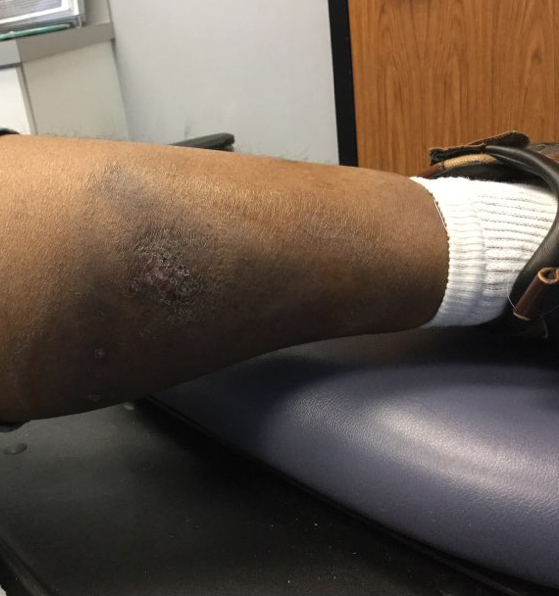
Although the precise pathogenesis of HS is unclear, both genetic abnormalities of the pilosebaceous unit and a dysregulated immune reaction appear to lead to the clinical characteristics of chronic inflammation and scarring seen in HS. A key effector appears to be helper T-cell (TH17) lymphocyte activation, with increased secretion of TNF-α, IL-1β, and IL-17.1,2 In turn, IL-17 induces higher expression of TNF-α, leading to a persistent cycle of inflammation. Peripheral recruitment of IL-17–producing neutrophils also may contribute to chronic inflammation.8
Adalimumab is the only US Food and Drug Administration–approved biologic indicated for the treatment of HS. Our patient initially responded to adalimumab with improvement of HS; however, treatment had to be discontinued because of the unusual occurrence of 2 distinct paradoxical reactions in a short span of time. Psoriasis and PG are both considered true paradoxical reactions because primary occurrences of both diseases usually are responsive to treatment with adalimumab.
Tumor necrosis factor α inhibitor–induced psoriasis arises de novo and is estimated to occur in approximately 5% of patients with rheumatoid arthritis.3,6 Palmoplantar pustular psoriasiform reactions are the most common form of paradoxical psoriasis. Topical medications can be used to treat skin lesions, but systemic treatment is required in many cases. Switching to an alternate class of a biologic, such as an IL-17, IL-12/23, or IL-23 inhibitor, can improve the skin reaction; however, such treatment is inconsistently successful, and paradoxical drug reactions also have been seen with these other classes of biologics.4,9
Recent studies support distinct immune causes for classical and paradoxical psoriasis. In classical psoriasis, plasmacytoid dendritic cells (pDCs) produce IFN-α, which stimulates conventional dendritic cells to produce TNF-α. However, TNF-α matures both pDCs and conventional dendritic cells; upon maturation, both types of dendritic cells lose the ability to produce IFN-α, thus allowing TNF-α to become dominant.10 The blockade of TNF-α prevents pDC maturation, leading to uninhibited IFN-α, which appears to drive inflammation in paradoxical psoriasis. In classical psoriasis, oligoclonal dermal CD4+ T cells and epidermal CD8+ T cells remain, even in resolved skin lesions, and can cause disease recurrence through reactivation of skin-resident memory T cells.11 No relapse of paradoxical psoriasis occurs with discontinuation of anti-TNF-α therapy, which supports the notion of an absence of memory T cells.
The incidence of paradoxical psoriasis in patients receiving a TNF-α inhibitor for HS is unclear.12 There are case series in which patients who had concurrent psoriasis and HS were successfully treated with a TNF-α inhibitor.13 A recently recognized condition—PASH syndrome—encompasses the clinical triad of PG, acne, and HS.10
Our patient had no history of acne or PG, only a long-standing history of HS. New-onset PG occurred only after a TNF-α inhibitor was initiated. Notably, PASH syndrome has been successfully treated with TNF-α inhibitors, highlighting the shared inflammatory etiology of HS and PG.14 In patients with concurrent PG and HS, TNF-α inhibitors were more effective for treating PG than for HS.
Pyoderma gangrenosum is an inflammatory disorder that often occurs concomitantly with other conditions, such as inflammatory bowel disease. The exact underlying cause of PG is unclear, but there appears to be both neutrophil and T-cell dysfunction in PG, with excess inflammatory cytokine production (eg, IL-1β, TNF-α, IL-17).15
The mainstay of treatment of PG is systemic corticosteroids and immunosuppressives, such as cyclosporine. Tumor necrosis factor α inhibitors as well as other interleukin inhibitors are increasingly utilized as potential therapeutic alternatives for PG.16,17
Unlike paradoxical psoriasis, the underlying cause of paradoxical PG is unclear.18,19 A similar mechanism may be postulated whereby inhibition of TNF-α leads to excessive activation of alternative inflammatory pathways that result in paradoxical PG. In one study, the prevalence of PG among 68,232 patients with HS was 0.18% compared with 0.01% among those without HS; therefore, patients with HS appear to be more predisposed to PG.20
This case illustrates the complex, often conflicting effects of cytokine inhibition in the paradoxical elicitation of alternative inflammatory disorders as an unintended consequence of the initial cytokine blockade. It is likely that genetic predisposition allows for paradoxical reactions in some patients when there is predominant inhibition of one cytokine in the inflammatory pathway. In rare cases, multiple paradoxical reactions are possible.
1. Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
2. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation and pathogenesis. J Am Acad Dermatol. 2020; 82:1045-1058. doi:10.1016/j.jaad.2019.08.090
3. Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341. doi:10.1016/j.jaad.2016.08.012
4. Puig L. Paradoxical reactions: anti-tumor necrosis factor alpha agents, ustekinumab, secukinumab, ixekizumab and others. Curr Prob Dermatol. 2018;53:49-63. doi:10.1159/000479475
5. Faivre C, Villani AP, Aubin F, et al; . Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
6. Ko JM, Gottlieb AB, Kerbleski JF. Induction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 cases. J Dermatolog Treat. 2009;20:100-108. doi:10.1080/09546630802441234
7. Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
8. Lima AL, Karl I, Giner T, et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol. 2016;174:514-521. doi:10.1111/bjd.14214
9. Li SJ, Perez-Chada LM, Merola JF. TNF inhibitor-induced psoriasis: proposed algorithm for treatment and management. J Psoriasis Psoriatic Arthritis. 2019;4:70-80. doi:10.1177/2475530318810851
10. Conrad C, Di Domizio J, Mylonas A, et al. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018;9:25. doi:10.1038/s41467-017-02466-4
11. Matos TR, O’Malley JT, Lowry EL, et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J Clin Invest. 2017;127:4031-4041. doi:10.1172/JCI93396
12. Faivre C, Villani AP, Aubin F, et al. Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
13. Marzano AV, Damiani G, Ceccherini I, et al. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br J Dermatol. 2017;176:1588-1598. doi:10.1111/bjd.15226
14. Cugno M, Borghi A, Marzano AV. PAPA, PASH, PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562. doi:10.1007/s40257-017-0265-1
15. Wang EA, Steel A, Luxardi G, et al. Classic ulcerative pyoderma gangrenosum is a T cell-mediated disease targeting follicular adnexal structures: a hypothesis based on molecular and clinicopathologic studies. Front Immunol. 2018;8:1980. doi:10.3389/fimmu.2017.01980
16. Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95:525-531. doi:10.2340/00015555-2008
17. Partridge ACR, Bai JW, Rosen CF, et al. Effectiveness of systemic treatments for pyoderma gangrenosum: a systematic review of observational studies and clinical trials. Br J Dermatol. 2018;179:290-295. doi:10.1111/bjd.16485
18. Benzaquen M, Monnier J, Beaussault Y, et al. Pyoderma gangrenosum arising during treatment of psoriasis with adalimumab: effectiveness of ustekinumab. Australas J Dermatol. 2017;58:e270-e271. doi:10.1111/ajd.12545
19. Fujimoto N, Yamasaki Y, Watanabe RJ. Paradoxical uveitis and pyoderma gangrenosum in a patient with psoriatic arthritis under infliximab treatment. J Dtsch Dermatol Ges. 2018;16:1139-1140. doi:10.1111/ddg.13632
20. Tannenbaum R, Strunk A, Garg A. Overall and subgroup prevalence of pyoderma gangrenosum among patients with hidradenitis suppurativa: a population-based analysis in the United States. J Am Acad Dermatol. 2019;80:1533-1537. doi:10.1016/j.jaad.2019.02.004
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the pilosebaceous unit that occurs in concert with elevations of various cytokines, including tumor necrosis factor α (TNF-α), IL-1β, IL-10, and IL-17.1,2 Adalimumab is a TNF-α inhibitor approved by the US Food and Drug Administration for the treatment of HS. Although TNF-α inhibitors are effective for many immune-mediated inflammatory disorders, paradoxical drug reactions have been reported following treatment with these agents.3-6 True paradoxical drug reactions likely are immune mediated and directly lead to new onset of a pathologic condition that would otherwise respond to that drug. For example, there are reports of rheumatoid arthritis patients who were treated with a TNF-α inhibitor and developed psoriatic skin lesions.3,6 Paradoxical drug reactions also have been reported with acute-onset inflammatory bowel disease and HS or less commonly pyoderma gangrenosum (PG), uveitis, granulomatous reactions, and vasculitis.4,5 We present the case of a patient with HS who was treated with a TNF-α inhibitor and developed 2 distinct paradoxical drug reactions. We also provide an overview of paradoxical drug reactions associated with TNF-α inhibitors.
A 38-year-old woman developed a painful “boil” on the right leg that was previously treated in the emergency department with incision and drainage as well as oral clindamycin for 7 days, but the lesion spread and continued to worsen. She had a history of HS in the axillae and groin region that had been present since 12 years of age. The condition was poorly controlled despite multiple courses of oral antibiotics and surgical resections. An oral contraceptive also was attempted, but the patient discontinued treatment when liver enzyme levels became elevated. The patient had no other notable medical history, including skin disease. There was a family history of HS in her father and a sibling. Seeking more effective treatment, the patient was offered adalimumab approximately 4 months prior to clinical presentation and agreed to start a course of the drug. She received a loading dose of 160 mg on day 1 and 80 mg on day 15 followed by a maintenance dosage of 40 mg weekly. She experienced improvement in HS symptoms after 3 months on adalimumab; however, she developed scaly pruritic patches on the scalp, arms, and legs that were consistent with psoriasis. Because of the absence of a personal or family history of psoriasis, the patient was informed of the probability of paradoxical psoriasis resulting from adalimumab. She elected to continue adalimumab because of the improvement in HS symptoms, and the psoriatic lesions were mild and adequately controlled with a topical steroid.
At the current presentation 1 month later, physical examination revealed a large indurated and ulcerated area with jagged edges at the incision and drainage site (Figure 1). Pyoderma gangrenosum was clinically suspected; a biopsy was performed, and the patient was started on oral prednisone. At 2-week follow-up, the ulcer was found to be rapidly resolving with prednisone and healing with cribriform scarring (Figure 2). Histopathology revealed an undermining neutrophilic inflammatory process that was consistent with PG. A diagnosis of PG was made based on previously published criteria7 and the following major/minor criteria in the patient: pathology; absence of infection on histologic analysis; history of pathergy related to worsening ulceration at the site of incision and drainage of the initial boil; clinical findings of an ulcer with peripheral violaceous erythema; undermined borders and tenderness at the site; and rapid resolution of the ulcer with prednisone.

Cessation of adalimumab gradually led to clearance of both psoriasiform lesions and PG; however, HS lesions persisted.

Although the precise pathogenesis of HS is unclear, both genetic abnormalities of the pilosebaceous unit and a dysregulated immune reaction appear to lead to the clinical characteristics of chronic inflammation and scarring seen in HS. A key effector appears to be helper T-cell (TH17) lymphocyte activation, with increased secretion of TNF-α, IL-1β, and IL-17.1,2 In turn, IL-17 induces higher expression of TNF-α, leading to a persistent cycle of inflammation. Peripheral recruitment of IL-17–producing neutrophils also may contribute to chronic inflammation.8
Adalimumab is the only US Food and Drug Administration–approved biologic indicated for the treatment of HS. Our patient initially responded to adalimumab with improvement of HS; however, treatment had to be discontinued because of the unusual occurrence of 2 distinct paradoxical reactions in a short span of time. Psoriasis and PG are both considered true paradoxical reactions because primary occurrences of both diseases usually are responsive to treatment with adalimumab.
Tumor necrosis factor α inhibitor–induced psoriasis arises de novo and is estimated to occur in approximately 5% of patients with rheumatoid arthritis.3,6 Palmoplantar pustular psoriasiform reactions are the most common form of paradoxical psoriasis. Topical medications can be used to treat skin lesions, but systemic treatment is required in many cases. Switching to an alternate class of a biologic, such as an IL-17, IL-12/23, or IL-23 inhibitor, can improve the skin reaction; however, such treatment is inconsistently successful, and paradoxical drug reactions also have been seen with these other classes of biologics.4,9
Recent studies support distinct immune causes for classical and paradoxical psoriasis. In classical psoriasis, plasmacytoid dendritic cells (pDCs) produce IFN-α, which stimulates conventional dendritic cells to produce TNF-α. However, TNF-α matures both pDCs and conventional dendritic cells; upon maturation, both types of dendritic cells lose the ability to produce IFN-α, thus allowing TNF-α to become dominant.10 The blockade of TNF-α prevents pDC maturation, leading to uninhibited IFN-α, which appears to drive inflammation in paradoxical psoriasis. In classical psoriasis, oligoclonal dermal CD4+ T cells and epidermal CD8+ T cells remain, even in resolved skin lesions, and can cause disease recurrence through reactivation of skin-resident memory T cells.11 No relapse of paradoxical psoriasis occurs with discontinuation of anti-TNF-α therapy, which supports the notion of an absence of memory T cells.
The incidence of paradoxical psoriasis in patients receiving a TNF-α inhibitor for HS is unclear.12 There are case series in which patients who had concurrent psoriasis and HS were successfully treated with a TNF-α inhibitor.13 A recently recognized condition—PASH syndrome—encompasses the clinical triad of PG, acne, and HS.10
Our patient had no history of acne or PG, only a long-standing history of HS. New-onset PG occurred only after a TNF-α inhibitor was initiated. Notably, PASH syndrome has been successfully treated with TNF-α inhibitors, highlighting the shared inflammatory etiology of HS and PG.14 In patients with concurrent PG and HS, TNF-α inhibitors were more effective for treating PG than for HS.
Pyoderma gangrenosum is an inflammatory disorder that often occurs concomitantly with other conditions, such as inflammatory bowel disease. The exact underlying cause of PG is unclear, but there appears to be both neutrophil and T-cell dysfunction in PG, with excess inflammatory cytokine production (eg, IL-1β, TNF-α, IL-17).15
The mainstay of treatment of PG is systemic corticosteroids and immunosuppressives, such as cyclosporine. Tumor necrosis factor α inhibitors as well as other interleukin inhibitors are increasingly utilized as potential therapeutic alternatives for PG.16,17
Unlike paradoxical psoriasis, the underlying cause of paradoxical PG is unclear.18,19 A similar mechanism may be postulated whereby inhibition of TNF-α leads to excessive activation of alternative inflammatory pathways that result in paradoxical PG. In one study, the prevalence of PG among 68,232 patients with HS was 0.18% compared with 0.01% among those without HS; therefore, patients with HS appear to be more predisposed to PG.20
This case illustrates the complex, often conflicting effects of cytokine inhibition in the paradoxical elicitation of alternative inflammatory disorders as an unintended consequence of the initial cytokine blockade. It is likely that genetic predisposition allows for paradoxical reactions in some patients when there is predominant inhibition of one cytokine in the inflammatory pathway. In rare cases, multiple paradoxical reactions are possible.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the pilosebaceous unit that occurs in concert with elevations of various cytokines, including tumor necrosis factor α (TNF-α), IL-1β, IL-10, and IL-17.1,2 Adalimumab is a TNF-α inhibitor approved by the US Food and Drug Administration for the treatment of HS. Although TNF-α inhibitors are effective for many immune-mediated inflammatory disorders, paradoxical drug reactions have been reported following treatment with these agents.3-6 True paradoxical drug reactions likely are immune mediated and directly lead to new onset of a pathologic condition that would otherwise respond to that drug. For example, there are reports of rheumatoid arthritis patients who were treated with a TNF-α inhibitor and developed psoriatic skin lesions.3,6 Paradoxical drug reactions also have been reported with acute-onset inflammatory bowel disease and HS or less commonly pyoderma gangrenosum (PG), uveitis, granulomatous reactions, and vasculitis.4,5 We present the case of a patient with HS who was treated with a TNF-α inhibitor and developed 2 distinct paradoxical drug reactions. We also provide an overview of paradoxical drug reactions associated with TNF-α inhibitors.
A 38-year-old woman developed a painful “boil” on the right leg that was previously treated in the emergency department with incision and drainage as well as oral clindamycin for 7 days, but the lesion spread and continued to worsen. She had a history of HS in the axillae and groin region that had been present since 12 years of age. The condition was poorly controlled despite multiple courses of oral antibiotics and surgical resections. An oral contraceptive also was attempted, but the patient discontinued treatment when liver enzyme levels became elevated. The patient had no other notable medical history, including skin disease. There was a family history of HS in her father and a sibling. Seeking more effective treatment, the patient was offered adalimumab approximately 4 months prior to clinical presentation and agreed to start a course of the drug. She received a loading dose of 160 mg on day 1 and 80 mg on day 15 followed by a maintenance dosage of 40 mg weekly. She experienced improvement in HS symptoms after 3 months on adalimumab; however, she developed scaly pruritic patches on the scalp, arms, and legs that were consistent with psoriasis. Because of the absence of a personal or family history of psoriasis, the patient was informed of the probability of paradoxical psoriasis resulting from adalimumab. She elected to continue adalimumab because of the improvement in HS symptoms, and the psoriatic lesions were mild and adequately controlled with a topical steroid.
At the current presentation 1 month later, physical examination revealed a large indurated and ulcerated area with jagged edges at the incision and drainage site (Figure 1). Pyoderma gangrenosum was clinically suspected; a biopsy was performed, and the patient was started on oral prednisone. At 2-week follow-up, the ulcer was found to be rapidly resolving with prednisone and healing with cribriform scarring (Figure 2). Histopathology revealed an undermining neutrophilic inflammatory process that was consistent with PG. A diagnosis of PG was made based on previously published criteria7 and the following major/minor criteria in the patient: pathology; absence of infection on histologic analysis; history of pathergy related to worsening ulceration at the site of incision and drainage of the initial boil; clinical findings of an ulcer with peripheral violaceous erythema; undermined borders and tenderness at the site; and rapid resolution of the ulcer with prednisone.

Cessation of adalimumab gradually led to clearance of both psoriasiform lesions and PG; however, HS lesions persisted.

Although the precise pathogenesis of HS is unclear, both genetic abnormalities of the pilosebaceous unit and a dysregulated immune reaction appear to lead to the clinical characteristics of chronic inflammation and scarring seen in HS. A key effector appears to be helper T-cell (TH17) lymphocyte activation, with increased secretion of TNF-α, IL-1β, and IL-17.1,2 In turn, IL-17 induces higher expression of TNF-α, leading to a persistent cycle of inflammation. Peripheral recruitment of IL-17–producing neutrophils also may contribute to chronic inflammation.8
Adalimumab is the only US Food and Drug Administration–approved biologic indicated for the treatment of HS. Our patient initially responded to adalimumab with improvement of HS; however, treatment had to be discontinued because of the unusual occurrence of 2 distinct paradoxical reactions in a short span of time. Psoriasis and PG are both considered true paradoxical reactions because primary occurrences of both diseases usually are responsive to treatment with adalimumab.
Tumor necrosis factor α inhibitor–induced psoriasis arises de novo and is estimated to occur in approximately 5% of patients with rheumatoid arthritis.3,6 Palmoplantar pustular psoriasiform reactions are the most common form of paradoxical psoriasis. Topical medications can be used to treat skin lesions, but systemic treatment is required in many cases. Switching to an alternate class of a biologic, such as an IL-17, IL-12/23, or IL-23 inhibitor, can improve the skin reaction; however, such treatment is inconsistently successful, and paradoxical drug reactions also have been seen with these other classes of biologics.4,9
Recent studies support distinct immune causes for classical and paradoxical psoriasis. In classical psoriasis, plasmacytoid dendritic cells (pDCs) produce IFN-α, which stimulates conventional dendritic cells to produce TNF-α. However, TNF-α matures both pDCs and conventional dendritic cells; upon maturation, both types of dendritic cells lose the ability to produce IFN-α, thus allowing TNF-α to become dominant.10 The blockade of TNF-α prevents pDC maturation, leading to uninhibited IFN-α, which appears to drive inflammation in paradoxical psoriasis. In classical psoriasis, oligoclonal dermal CD4+ T cells and epidermal CD8+ T cells remain, even in resolved skin lesions, and can cause disease recurrence through reactivation of skin-resident memory T cells.11 No relapse of paradoxical psoriasis occurs with discontinuation of anti-TNF-α therapy, which supports the notion of an absence of memory T cells.
The incidence of paradoxical psoriasis in patients receiving a TNF-α inhibitor for HS is unclear.12 There are case series in which patients who had concurrent psoriasis and HS were successfully treated with a TNF-α inhibitor.13 A recently recognized condition—PASH syndrome—encompasses the clinical triad of PG, acne, and HS.10
Our patient had no history of acne or PG, only a long-standing history of HS. New-onset PG occurred only after a TNF-α inhibitor was initiated. Notably, PASH syndrome has been successfully treated with TNF-α inhibitors, highlighting the shared inflammatory etiology of HS and PG.14 In patients with concurrent PG and HS, TNF-α inhibitors were more effective for treating PG than for HS.
Pyoderma gangrenosum is an inflammatory disorder that often occurs concomitantly with other conditions, such as inflammatory bowel disease. The exact underlying cause of PG is unclear, but there appears to be both neutrophil and T-cell dysfunction in PG, with excess inflammatory cytokine production (eg, IL-1β, TNF-α, IL-17).15
The mainstay of treatment of PG is systemic corticosteroids and immunosuppressives, such as cyclosporine. Tumor necrosis factor α inhibitors as well as other interleukin inhibitors are increasingly utilized as potential therapeutic alternatives for PG.16,17
Unlike paradoxical psoriasis, the underlying cause of paradoxical PG is unclear.18,19 A similar mechanism may be postulated whereby inhibition of TNF-α leads to excessive activation of alternative inflammatory pathways that result in paradoxical PG. In one study, the prevalence of PG among 68,232 patients with HS was 0.18% compared with 0.01% among those without HS; therefore, patients with HS appear to be more predisposed to PG.20
This case illustrates the complex, often conflicting effects of cytokine inhibition in the paradoxical elicitation of alternative inflammatory disorders as an unintended consequence of the initial cytokine blockade. It is likely that genetic predisposition allows for paradoxical reactions in some patients when there is predominant inhibition of one cytokine in the inflammatory pathway. In rare cases, multiple paradoxical reactions are possible.
1. Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
2. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation and pathogenesis. J Am Acad Dermatol. 2020; 82:1045-1058. doi:10.1016/j.jaad.2019.08.090
3. Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341. doi:10.1016/j.jaad.2016.08.012
4. Puig L. Paradoxical reactions: anti-tumor necrosis factor alpha agents, ustekinumab, secukinumab, ixekizumab and others. Curr Prob Dermatol. 2018;53:49-63. doi:10.1159/000479475
5. Faivre C, Villani AP, Aubin F, et al; . Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
6. Ko JM, Gottlieb AB, Kerbleski JF. Induction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 cases. J Dermatolog Treat. 2009;20:100-108. doi:10.1080/09546630802441234
7. Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
8. Lima AL, Karl I, Giner T, et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol. 2016;174:514-521. doi:10.1111/bjd.14214
9. Li SJ, Perez-Chada LM, Merola JF. TNF inhibitor-induced psoriasis: proposed algorithm for treatment and management. J Psoriasis Psoriatic Arthritis. 2019;4:70-80. doi:10.1177/2475530318810851
10. Conrad C, Di Domizio J, Mylonas A, et al. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018;9:25. doi:10.1038/s41467-017-02466-4
11. Matos TR, O’Malley JT, Lowry EL, et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J Clin Invest. 2017;127:4031-4041. doi:10.1172/JCI93396
12. Faivre C, Villani AP, Aubin F, et al. Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
13. Marzano AV, Damiani G, Ceccherini I, et al. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br J Dermatol. 2017;176:1588-1598. doi:10.1111/bjd.15226
14. Cugno M, Borghi A, Marzano AV. PAPA, PASH, PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562. doi:10.1007/s40257-017-0265-1
15. Wang EA, Steel A, Luxardi G, et al. Classic ulcerative pyoderma gangrenosum is a T cell-mediated disease targeting follicular adnexal structures: a hypothesis based on molecular and clinicopathologic studies. Front Immunol. 2018;8:1980. doi:10.3389/fimmu.2017.01980
16. Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95:525-531. doi:10.2340/00015555-2008
17. Partridge ACR, Bai JW, Rosen CF, et al. Effectiveness of systemic treatments for pyoderma gangrenosum: a systematic review of observational studies and clinical trials. Br J Dermatol. 2018;179:290-295. doi:10.1111/bjd.16485
18. Benzaquen M, Monnier J, Beaussault Y, et al. Pyoderma gangrenosum arising during treatment of psoriasis with adalimumab: effectiveness of ustekinumab. Australas J Dermatol. 2017;58:e270-e271. doi:10.1111/ajd.12545
19. Fujimoto N, Yamasaki Y, Watanabe RJ. Paradoxical uveitis and pyoderma gangrenosum in a patient with psoriatic arthritis under infliximab treatment. J Dtsch Dermatol Ges. 2018;16:1139-1140. doi:10.1111/ddg.13632
20. Tannenbaum R, Strunk A, Garg A. Overall and subgroup prevalence of pyoderma gangrenosum among patients with hidradenitis suppurativa: a population-based analysis in the United States. J Am Acad Dermatol. 2019;80:1533-1537. doi:10.1016/j.jaad.2019.02.004
1. Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
2. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation and pathogenesis. J Am Acad Dermatol. 2020; 82:1045-1058. doi:10.1016/j.jaad.2019.08.090
3. Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341. doi:10.1016/j.jaad.2016.08.012
4. Puig L. Paradoxical reactions: anti-tumor necrosis factor alpha agents, ustekinumab, secukinumab, ixekizumab and others. Curr Prob Dermatol. 2018;53:49-63. doi:10.1159/000479475
5. Faivre C, Villani AP, Aubin F, et al; . Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
6. Ko JM, Gottlieb AB, Kerbleski JF. Induction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 cases. J Dermatolog Treat. 2009;20:100-108. doi:10.1080/09546630802441234
7. Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
8. Lima AL, Karl I, Giner T, et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol. 2016;174:514-521. doi:10.1111/bjd.14214
9. Li SJ, Perez-Chada LM, Merola JF. TNF inhibitor-induced psoriasis: proposed algorithm for treatment and management. J Psoriasis Psoriatic Arthritis. 2019;4:70-80. doi:10.1177/2475530318810851
10. Conrad C, Di Domizio J, Mylonas A, et al. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018;9:25. doi:10.1038/s41467-017-02466-4
11. Matos TR, O’Malley JT, Lowry EL, et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J Clin Invest. 2017;127:4031-4041. doi:10.1172/JCI93396
12. Faivre C, Villani AP, Aubin F, et al. Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
13. Marzano AV, Damiani G, Ceccherini I, et al. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br J Dermatol. 2017;176:1588-1598. doi:10.1111/bjd.15226
14. Cugno M, Borghi A, Marzano AV. PAPA, PASH, PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562. doi:10.1007/s40257-017-0265-1
15. Wang EA, Steel A, Luxardi G, et al. Classic ulcerative pyoderma gangrenosum is a T cell-mediated disease targeting follicular adnexal structures: a hypothesis based on molecular and clinicopathologic studies. Front Immunol. 2018;8:1980. doi:10.3389/fimmu.2017.01980
16. Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95:525-531. doi:10.2340/00015555-2008
17. Partridge ACR, Bai JW, Rosen CF, et al. Effectiveness of systemic treatments for pyoderma gangrenosum: a systematic review of observational studies and clinical trials. Br J Dermatol. 2018;179:290-295. doi:10.1111/bjd.16485
18. Benzaquen M, Monnier J, Beaussault Y, et al. Pyoderma gangrenosum arising during treatment of psoriasis with adalimumab: effectiveness of ustekinumab. Australas J Dermatol. 2017;58:e270-e271. doi:10.1111/ajd.12545
19. Fujimoto N, Yamasaki Y, Watanabe RJ. Paradoxical uveitis and pyoderma gangrenosum in a patient with psoriatic arthritis under infliximab treatment. J Dtsch Dermatol Ges. 2018;16:1139-1140. doi:10.1111/ddg.13632
20. Tannenbaum R, Strunk A, Garg A. Overall and subgroup prevalence of pyoderma gangrenosum among patients with hidradenitis suppurativa: a population-based analysis in the United States. J Am Acad Dermatol. 2019;80:1533-1537. doi:10.1016/j.jaad.2019.02.004
Practice Points
- Clinicians need to be aware of the potential risk for a paradoxical reaction in patients receiving a tumor necrosis factor α (TNF-α) inhibitor for hidradenitis suppurativa.
- Although uncommon, developing more than 1 type of paradoxical skin reaction is possible with a TNF-α inhibitor.
- Early recognition and appropriate management of these paradoxical reactions are critical.
Aberrant Expression of CD56 in Metastatic Malignant Melanoma
To the Editor:
Many types of neoplasms can show aberrant immunoreactivity or unexpected expression of markers.1 Malignant melanoma is a tumor that can show not only aberrant immunohistochemical staining patterns but also notable histologic diversity,1,2 which often makes the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.2
The incidence of malignant melanoma continues to grow.3 Maintaining a high degree of suspicion for this disease, recognizing its heterogeneity and divergent differentiation, and knowing potential aberrant immunohistochemical staining patterns are imperative for accurate diagnosis.
A 36-year-old man presented to a primary care physician with right-sided chest pain, upper and lower back aches, bilateral hip pain, neck pain, headache, night sweats, chills, and nausea. After infectious causes were ruled out, he was placed on a steroid taper without improvement. He presented to the emergency department a few days later with muscle spasms and was found to also have diffuse abdominal tenderness and guarding. The patient’s medical history was noncontributory; he was a lifelong nonsmoker. Laboratory studies revealed elevated levels of alanine aminotransferase and C-reactive protein. Computed tomography of the chest and abdomen revealed innumerable liver and lung lesions that were suspicious for metastatic malignancy. A liver biopsy revealed nests and sheets of metastatic tumor with pleomorphic nuclei, inconspicuous nucleoli, and areas of intranuclear clearing (Figures 1 and 2). Immunohistochemical staining was performed to further characterize the tumor. Neoplastic cells were positive for MART-1 (also known as Melan-A and melanoma-associated antigen recognized by T cells)(Figure 3), SOX10, S-100, HMB-45, and vimentin. Nonspecific staining with CD56 (Figure 4), a neuroendocrine marker, also was noted; however, the neoplasm was negative for synaptophysin, another neuroendocrine marker. Other markers for which staining was negative included pan-keratin, CD138 (syndecan-1), desmin, placental alkaline phosphatase (PLAP), inhibin, OCT-4, cytokeratin 7, and cytokeratin 20. This staining pattern was compatible with metastatic melanoma with aberrant CD56 expression.
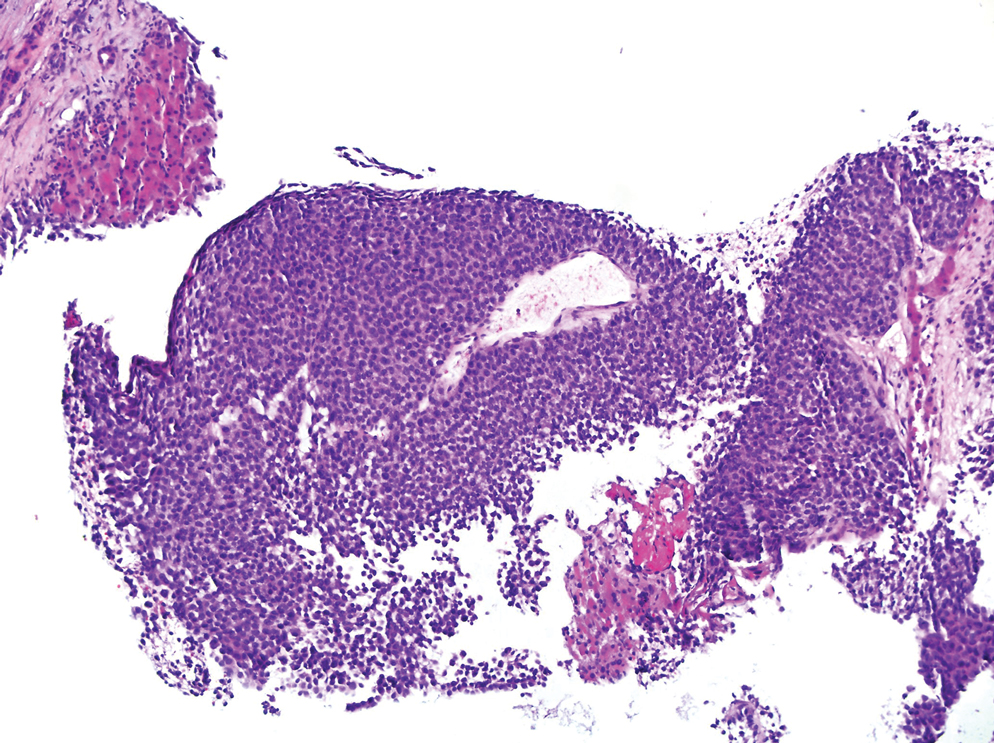
BRAF V600E immunohistochemical staining also was performed and showed strong and diffuse positivity within neoplastic cells. A subsequent positron emission tomography scan revealed widespread metastatic disease involving the lungs, liver, spleen, and bones. The patient did not have a history of an excised skin lesion; no primary cutaneous or mucosal lesions were identified.

The patient was started on targeted therapy with trametinib, a mitogen-activated extracellular signal-related kinase kinase (MEK) inhibitor, and dabrafenib, a BRAF inhibitor. The disease continued to progress; he developed extensive leptomeningeal metastatic disease for which palliative radiation therapy was administered. The patient died 4 months after the initial diagnosis.
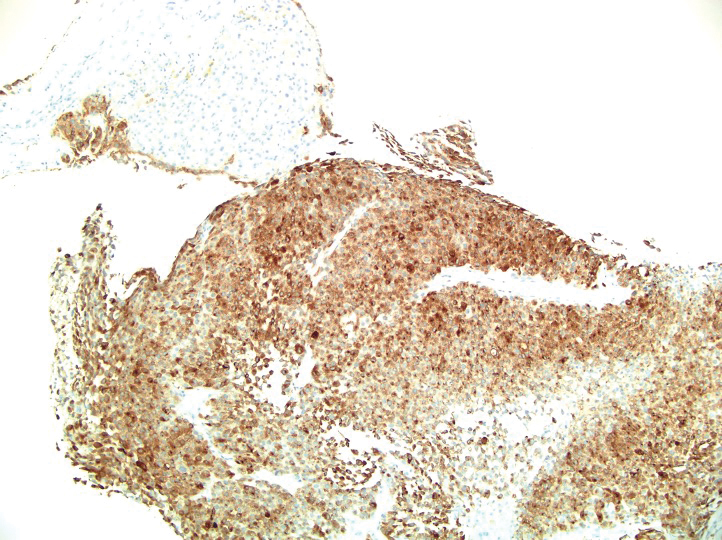
More than 90% of melanoma cases are of cutaneous origin; however, 4% to 8% of cases present as a metastatic lesion in the absence of an identified primary lesion,4 similar to our patient. The diagnosis of melanoma often is challenging; the tumor can show notable histologic diversity and has the potential to express aberrant immunophenotypes.1,2 The histologic diversity of melanoma includes a variety of architectural patterns (eg, nests, trabeculae, fascicular, pseudoglandular, pseudopapillary, or pseudorosette patterns), cytomorphologic features, and stromal changes. Cytomorphologic features of melanoma can be large pleomorphic cells; small cells; spindle cells; clear cells; signet-ring cells; and rhabdoid, plasmacytoid, and balloon cells.5
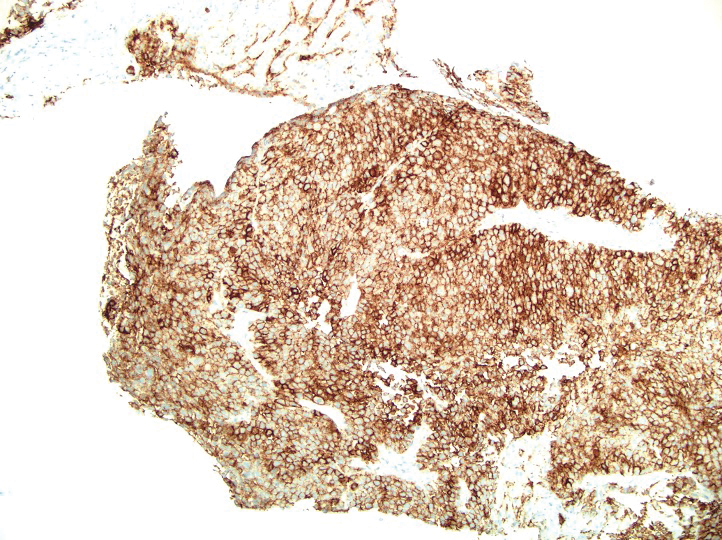
Melanoma can mimic carcinoma, sarcoma, lymphoma, benign stromal tumors, plasmacytoma, and germ-cell tumors.5 Nuclei can binucleated, multinucleated, or lobated and may contain inclusions or grooves. Stroma may become myxoid or desmoplastic in appearance or rarely show granulomatous inflammation or osteoclastic giant cells.5 These variations render the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.
Melanomas typically express MART-1, HMB-45, S-100, tyrosinase, NK1C3, vimentin, and neuron-specific enolase. However, melanoma is among the many neoplasms that sometimes exhibit aberrant immunoreactivity and differentiation toward nonmelanocytic elements.6 The most commonly expressed immunophenotypic aberration is cytokeratin, especially the low-molecular-weight keratin marker CAM5.2.5 CAM5.2 positivity also is seen more often in metastatic melanoma. Melanomas rarely express other intermediate filaments, including desmin, neurofilament protein, and glial fibrillary acidic protein; expression of smooth-muscle actin is rare.5
Only a few cases of melanoma showing expression of neuroendocrine markers have been reported. However, one study reported synaptophysin positivity in 29% (10/34) of cases of primary and metastatic melanoma, making the stain a relatively common finding.1
In contrast, expression of CD56 (also known as neural-cell adhesion molecule 1) in melanoma has been reported only rarely. CD56 is a nonspecific neuroendocrine marker that normally is expressed on neurons, glial tissue, skeletal muscle, and natural killer cells. Riddle and Bui7 reported a case of metastatic malignant melanoma with focal CD56 positivity and no expression of other neuroendocrine markers, similar to our patient. Suzuki and colleagues4 also reported a case of melanoma metastatic to bone marrow that showed CD56 expression in true nonhematologic tumor cells and negative immunoreactivity with synaptophysin and chromogranin A.
It is important to document cases of melanoma that express neuroendocrine markers to prevent an incorrect diagnosis of a neuroendocrine tumor.1 In some cases, distinguishing amelanotic melanoma from poorly differentiated squamous cell carcinoma, neuroendocrine tumor, and lymphoma can be difficult.5
The term neuroendocrine differentiation is reserved for cases of melanoma that show areas of ultrastructural change consistent with a neuroendocrine tumor.2 Neuroendocrine differentiation in melanoma is not common; its prognostic significance is unknown.8 We do not consider our case to be true neuroendocrine differentiation, as the tumor lacked the morphologic changes of a neuroendocrine tumor. Furthermore, CD56 is a nonspecific neuroendocrine marker, and the tumor was negative for synaptophysin.
Melanoma has the potential to show notable histologic diversity as well as aberrant immunohistochemical staining patterns.1,2 Our patient had metastatic melanoma with aberrant neuroendocrine expression of CD56, which could have been a potential diagnostic pitfall. Because expression of CD56 in melanoma is rare, it is imperative to recognize this potential aberrant staining pattern to ensure the accurate diagnosis of melanoma and appropriate provision of care.
1. Romano RC, Carter JM, Folpe AL. Aberrant intermediate filament and synaptophysin expression is a frequent event in malignant melanoma: an immunohistochemical study of 73 cases. Mod Pathol. 2015;28:1033-1042. doi:10.1038/modpathol.2015.62
2. Eyden B, Pandit D, Banerjee SS. Malignant melanoma with neuroendocrine differentiation: clinical, histological, immunohistochemical and ultrastructural features of three cases. Histopathology. 2005;47:402-409. doi:10.1111/j.1365-2559.2005.02240.x
3. Katerji H, Childs JM, Bratton LE, et al. Primary esophageal melanoma with aberrant CD56 expression: a potential diagnostic pitfall. Case Rep Pathol. 2017;2017:9052637. doi:10.1155/2017/9052637
4. Suzuki T, Kusumoto S, Iida S, et al. Amelanotic malignant melanoma of unknown primary origin metastasizing to the bone marrow: a case report and review of the literature. Intern Med. 2014;53:325-328. doi:10.2169/internalmedicine.53.1412
5. Banerjee SS, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology. 2000;36:387-402. doi:10.1046/j.1365-2559.2000.00894.x
6. Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52:119-129. doi:10.1111/j.1365-2559.2007.02823.x
7. Riddle ND, Bui MM. When melanoma is negative for S100: diagnostic pitfalls. Arch Pathol Lab Med. 2012;136:237-239. doi:10.5858/arpa.2011-0405-LE
8. Ilardi G, Caroppo D, Varricchio S, et al. Anal melanoma with neuroendocrine differentiation: report of a case. Int J Surg Pathol. 2015;23:329-332. doi:10.1177/1066896915573568
To the Editor:
Many types of neoplasms can show aberrant immunoreactivity or unexpected expression of markers.1 Malignant melanoma is a tumor that can show not only aberrant immunohistochemical staining patterns but also notable histologic diversity,1,2 which often makes the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.2
The incidence of malignant melanoma continues to grow.3 Maintaining a high degree of suspicion for this disease, recognizing its heterogeneity and divergent differentiation, and knowing potential aberrant immunohistochemical staining patterns are imperative for accurate diagnosis.
A 36-year-old man presented to a primary care physician with right-sided chest pain, upper and lower back aches, bilateral hip pain, neck pain, headache, night sweats, chills, and nausea. After infectious causes were ruled out, he was placed on a steroid taper without improvement. He presented to the emergency department a few days later with muscle spasms and was found to also have diffuse abdominal tenderness and guarding. The patient’s medical history was noncontributory; he was a lifelong nonsmoker. Laboratory studies revealed elevated levels of alanine aminotransferase and C-reactive protein. Computed tomography of the chest and abdomen revealed innumerable liver and lung lesions that were suspicious for metastatic malignancy. A liver biopsy revealed nests and sheets of metastatic tumor with pleomorphic nuclei, inconspicuous nucleoli, and areas of intranuclear clearing (Figures 1 and 2). Immunohistochemical staining was performed to further characterize the tumor. Neoplastic cells were positive for MART-1 (also known as Melan-A and melanoma-associated antigen recognized by T cells)(Figure 3), SOX10, S-100, HMB-45, and vimentin. Nonspecific staining with CD56 (Figure 4), a neuroendocrine marker, also was noted; however, the neoplasm was negative for synaptophysin, another neuroendocrine marker. Other markers for which staining was negative included pan-keratin, CD138 (syndecan-1), desmin, placental alkaline phosphatase (PLAP), inhibin, OCT-4, cytokeratin 7, and cytokeratin 20. This staining pattern was compatible with metastatic melanoma with aberrant CD56 expression.

BRAF V600E immunohistochemical staining also was performed and showed strong and diffuse positivity within neoplastic cells. A subsequent positron emission tomography scan revealed widespread metastatic disease involving the lungs, liver, spleen, and bones. The patient did not have a history of an excised skin lesion; no primary cutaneous or mucosal lesions were identified.

The patient was started on targeted therapy with trametinib, a mitogen-activated extracellular signal-related kinase kinase (MEK) inhibitor, and dabrafenib, a BRAF inhibitor. The disease continued to progress; he developed extensive leptomeningeal metastatic disease for which palliative radiation therapy was administered. The patient died 4 months after the initial diagnosis.

More than 90% of melanoma cases are of cutaneous origin; however, 4% to 8% of cases present as a metastatic lesion in the absence of an identified primary lesion,4 similar to our patient. The diagnosis of melanoma often is challenging; the tumor can show notable histologic diversity and has the potential to express aberrant immunophenotypes.1,2 The histologic diversity of melanoma includes a variety of architectural patterns (eg, nests, trabeculae, fascicular, pseudoglandular, pseudopapillary, or pseudorosette patterns), cytomorphologic features, and stromal changes. Cytomorphologic features of melanoma can be large pleomorphic cells; small cells; spindle cells; clear cells; signet-ring cells; and rhabdoid, plasmacytoid, and balloon cells.5

Melanoma can mimic carcinoma, sarcoma, lymphoma, benign stromal tumors, plasmacytoma, and germ-cell tumors.5 Nuclei can binucleated, multinucleated, or lobated and may contain inclusions or grooves. Stroma may become myxoid or desmoplastic in appearance or rarely show granulomatous inflammation or osteoclastic giant cells.5 These variations render the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.
Melanomas typically express MART-1, HMB-45, S-100, tyrosinase, NK1C3, vimentin, and neuron-specific enolase. However, melanoma is among the many neoplasms that sometimes exhibit aberrant immunoreactivity and differentiation toward nonmelanocytic elements.6 The most commonly expressed immunophenotypic aberration is cytokeratin, especially the low-molecular-weight keratin marker CAM5.2.5 CAM5.2 positivity also is seen more often in metastatic melanoma. Melanomas rarely express other intermediate filaments, including desmin, neurofilament protein, and glial fibrillary acidic protein; expression of smooth-muscle actin is rare.5
Only a few cases of melanoma showing expression of neuroendocrine markers have been reported. However, one study reported synaptophysin positivity in 29% (10/34) of cases of primary and metastatic melanoma, making the stain a relatively common finding.1
In contrast, expression of CD56 (also known as neural-cell adhesion molecule 1) in melanoma has been reported only rarely. CD56 is a nonspecific neuroendocrine marker that normally is expressed on neurons, glial tissue, skeletal muscle, and natural killer cells. Riddle and Bui7 reported a case of metastatic malignant melanoma with focal CD56 positivity and no expression of other neuroendocrine markers, similar to our patient. Suzuki and colleagues4 also reported a case of melanoma metastatic to bone marrow that showed CD56 expression in true nonhematologic tumor cells and negative immunoreactivity with synaptophysin and chromogranin A.
It is important to document cases of melanoma that express neuroendocrine markers to prevent an incorrect diagnosis of a neuroendocrine tumor.1 In some cases, distinguishing amelanotic melanoma from poorly differentiated squamous cell carcinoma, neuroendocrine tumor, and lymphoma can be difficult.5
The term neuroendocrine differentiation is reserved for cases of melanoma that show areas of ultrastructural change consistent with a neuroendocrine tumor.2 Neuroendocrine differentiation in melanoma is not common; its prognostic significance is unknown.8 We do not consider our case to be true neuroendocrine differentiation, as the tumor lacked the morphologic changes of a neuroendocrine tumor. Furthermore, CD56 is a nonspecific neuroendocrine marker, and the tumor was negative for synaptophysin.
Melanoma has the potential to show notable histologic diversity as well as aberrant immunohistochemical staining patterns.1,2 Our patient had metastatic melanoma with aberrant neuroendocrine expression of CD56, which could have been a potential diagnostic pitfall. Because expression of CD56 in melanoma is rare, it is imperative to recognize this potential aberrant staining pattern to ensure the accurate diagnosis of melanoma and appropriate provision of care.
To the Editor:
Many types of neoplasms can show aberrant immunoreactivity or unexpected expression of markers.1 Malignant melanoma is a tumor that can show not only aberrant immunohistochemical staining patterns but also notable histologic diversity,1,2 which often makes the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.2
The incidence of malignant melanoma continues to grow.3 Maintaining a high degree of suspicion for this disease, recognizing its heterogeneity and divergent differentiation, and knowing potential aberrant immunohistochemical staining patterns are imperative for accurate diagnosis.
A 36-year-old man presented to a primary care physician with right-sided chest pain, upper and lower back aches, bilateral hip pain, neck pain, headache, night sweats, chills, and nausea. After infectious causes were ruled out, he was placed on a steroid taper without improvement. He presented to the emergency department a few days later with muscle spasms and was found to also have diffuse abdominal tenderness and guarding. The patient’s medical history was noncontributory; he was a lifelong nonsmoker. Laboratory studies revealed elevated levels of alanine aminotransferase and C-reactive protein. Computed tomography of the chest and abdomen revealed innumerable liver and lung lesions that were suspicious for metastatic malignancy. A liver biopsy revealed nests and sheets of metastatic tumor with pleomorphic nuclei, inconspicuous nucleoli, and areas of intranuclear clearing (Figures 1 and 2). Immunohistochemical staining was performed to further characterize the tumor. Neoplastic cells were positive for MART-1 (also known as Melan-A and melanoma-associated antigen recognized by T cells)(Figure 3), SOX10, S-100, HMB-45, and vimentin. Nonspecific staining with CD56 (Figure 4), a neuroendocrine marker, also was noted; however, the neoplasm was negative for synaptophysin, another neuroendocrine marker. Other markers for which staining was negative included pan-keratin, CD138 (syndecan-1), desmin, placental alkaline phosphatase (PLAP), inhibin, OCT-4, cytokeratin 7, and cytokeratin 20. This staining pattern was compatible with metastatic melanoma with aberrant CD56 expression.

BRAF V600E immunohistochemical staining also was performed and showed strong and diffuse positivity within neoplastic cells. A subsequent positron emission tomography scan revealed widespread metastatic disease involving the lungs, liver, spleen, and bones. The patient did not have a history of an excised skin lesion; no primary cutaneous or mucosal lesions were identified.

The patient was started on targeted therapy with trametinib, a mitogen-activated extracellular signal-related kinase kinase (MEK) inhibitor, and dabrafenib, a BRAF inhibitor. The disease continued to progress; he developed extensive leptomeningeal metastatic disease for which palliative radiation therapy was administered. The patient died 4 months after the initial diagnosis.

More than 90% of melanoma cases are of cutaneous origin; however, 4% to 8% of cases present as a metastatic lesion in the absence of an identified primary lesion,4 similar to our patient. The diagnosis of melanoma often is challenging; the tumor can show notable histologic diversity and has the potential to express aberrant immunophenotypes.1,2 The histologic diversity of melanoma includes a variety of architectural patterns (eg, nests, trabeculae, fascicular, pseudoglandular, pseudopapillary, or pseudorosette patterns), cytomorphologic features, and stromal changes. Cytomorphologic features of melanoma can be large pleomorphic cells; small cells; spindle cells; clear cells; signet-ring cells; and rhabdoid, plasmacytoid, and balloon cells.5

Melanoma can mimic carcinoma, sarcoma, lymphoma, benign stromal tumors, plasmacytoma, and germ-cell tumors.5 Nuclei can binucleated, multinucleated, or lobated and may contain inclusions or grooves. Stroma may become myxoid or desmoplastic in appearance or rarely show granulomatous inflammation or osteoclastic giant cells.5 These variations render the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.
Melanomas typically express MART-1, HMB-45, S-100, tyrosinase, NK1C3, vimentin, and neuron-specific enolase. However, melanoma is among the many neoplasms that sometimes exhibit aberrant immunoreactivity and differentiation toward nonmelanocytic elements.6 The most commonly expressed immunophenotypic aberration is cytokeratin, especially the low-molecular-weight keratin marker CAM5.2.5 CAM5.2 positivity also is seen more often in metastatic melanoma. Melanomas rarely express other intermediate filaments, including desmin, neurofilament protein, and glial fibrillary acidic protein; expression of smooth-muscle actin is rare.5
Only a few cases of melanoma showing expression of neuroendocrine markers have been reported. However, one study reported synaptophysin positivity in 29% (10/34) of cases of primary and metastatic melanoma, making the stain a relatively common finding.1
In contrast, expression of CD56 (also known as neural-cell adhesion molecule 1) in melanoma has been reported only rarely. CD56 is a nonspecific neuroendocrine marker that normally is expressed on neurons, glial tissue, skeletal muscle, and natural killer cells. Riddle and Bui7 reported a case of metastatic malignant melanoma with focal CD56 positivity and no expression of other neuroendocrine markers, similar to our patient. Suzuki and colleagues4 also reported a case of melanoma metastatic to bone marrow that showed CD56 expression in true nonhematologic tumor cells and negative immunoreactivity with synaptophysin and chromogranin A.
It is important to document cases of melanoma that express neuroendocrine markers to prevent an incorrect diagnosis of a neuroendocrine tumor.1 In some cases, distinguishing amelanotic melanoma from poorly differentiated squamous cell carcinoma, neuroendocrine tumor, and lymphoma can be difficult.5
The term neuroendocrine differentiation is reserved for cases of melanoma that show areas of ultrastructural change consistent with a neuroendocrine tumor.2 Neuroendocrine differentiation in melanoma is not common; its prognostic significance is unknown.8 We do not consider our case to be true neuroendocrine differentiation, as the tumor lacked the morphologic changes of a neuroendocrine tumor. Furthermore, CD56 is a nonspecific neuroendocrine marker, and the tumor was negative for synaptophysin.
Melanoma has the potential to show notable histologic diversity as well as aberrant immunohistochemical staining patterns.1,2 Our patient had metastatic melanoma with aberrant neuroendocrine expression of CD56, which could have been a potential diagnostic pitfall. Because expression of CD56 in melanoma is rare, it is imperative to recognize this potential aberrant staining pattern to ensure the accurate diagnosis of melanoma and appropriate provision of care.
1. Romano RC, Carter JM, Folpe AL. Aberrant intermediate filament and synaptophysin expression is a frequent event in malignant melanoma: an immunohistochemical study of 73 cases. Mod Pathol. 2015;28:1033-1042. doi:10.1038/modpathol.2015.62
2. Eyden B, Pandit D, Banerjee SS. Malignant melanoma with neuroendocrine differentiation: clinical, histological, immunohistochemical and ultrastructural features of three cases. Histopathology. 2005;47:402-409. doi:10.1111/j.1365-2559.2005.02240.x
3. Katerji H, Childs JM, Bratton LE, et al. Primary esophageal melanoma with aberrant CD56 expression: a potential diagnostic pitfall. Case Rep Pathol. 2017;2017:9052637. doi:10.1155/2017/9052637
4. Suzuki T, Kusumoto S, Iida S, et al. Amelanotic malignant melanoma of unknown primary origin metastasizing to the bone marrow: a case report and review of the literature. Intern Med. 2014;53:325-328. doi:10.2169/internalmedicine.53.1412
5. Banerjee SS, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology. 2000;36:387-402. doi:10.1046/j.1365-2559.2000.00894.x
6. Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52:119-129. doi:10.1111/j.1365-2559.2007.02823.x
7. Riddle ND, Bui MM. When melanoma is negative for S100: diagnostic pitfalls. Arch Pathol Lab Med. 2012;136:237-239. doi:10.5858/arpa.2011-0405-LE
8. Ilardi G, Caroppo D, Varricchio S, et al. Anal melanoma with neuroendocrine differentiation: report of a case. Int J Surg Pathol. 2015;23:329-332. doi:10.1177/1066896915573568
1. Romano RC, Carter JM, Folpe AL. Aberrant intermediate filament and synaptophysin expression is a frequent event in malignant melanoma: an immunohistochemical study of 73 cases. Mod Pathol. 2015;28:1033-1042. doi:10.1038/modpathol.2015.62
2. Eyden B, Pandit D, Banerjee SS. Malignant melanoma with neuroendocrine differentiation: clinical, histological, immunohistochemical and ultrastructural features of three cases. Histopathology. 2005;47:402-409. doi:10.1111/j.1365-2559.2005.02240.x
3. Katerji H, Childs JM, Bratton LE, et al. Primary esophageal melanoma with aberrant CD56 expression: a potential diagnostic pitfall. Case Rep Pathol. 2017;2017:9052637. doi:10.1155/2017/9052637
4. Suzuki T, Kusumoto S, Iida S, et al. Amelanotic malignant melanoma of unknown primary origin metastasizing to the bone marrow: a case report and review of the literature. Intern Med. 2014;53:325-328. doi:10.2169/internalmedicine.53.1412
5. Banerjee SS, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology. 2000;36:387-402. doi:10.1046/j.1365-2559.2000.00894.x
6. Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52:119-129. doi:10.1111/j.1365-2559.2007.02823.x
7. Riddle ND, Bui MM. When melanoma is negative for S100: diagnostic pitfalls. Arch Pathol Lab Med. 2012;136:237-239. doi:10.5858/arpa.2011-0405-LE
8. Ilardi G, Caroppo D, Varricchio S, et al. Anal melanoma with neuroendocrine differentiation: report of a case. Int J Surg Pathol. 2015;23:329-332. doi:10.1177/1066896915573568
Practice Points
- The diagnosis of melanoma often is challenging as tumors can show notable histologic diversity and have the potential to express aberrant immunophenotypes including CD56 expression.
- Because expression of CD56 in melanoma is rare, it is important to be aware of this potential aberrant staining pattern.
- Recognizing this heterogeneity and divergent differentiation as well as knowing potential aberrant immunohistochemical staining patterns are imperative for accurate and timely diagnosis.