User login
Purpura Fulminans in the Setting of Escherichia coli Septicemia
To the Editor:
Purpura fulminans is a severe and rapidly fatal thrombotic disorder that can occur in association with either hereditary or acquired deficiencies of the natural anticoagulants protein C and protein S.1 It most commonly results from the acute inflammatory response and subsequent disseminated intravascular coagulation (DIC) seen in severe bacterial septicemia. Excessive bleeding, retiform purpura, and skin necrosis may develop as a result of the coagulopathies of typical DIC.1Neisseria meningitidis, Streptococcus, and Staphylococcus frequently are implicated as pathogens, but Escherichia coli–associated purpura fulminans in adults is rare.2,3 We report a case of purpura fulminans in the setting of E coli septicemia.
A 62-year-old woman with a history of end-stage liver disease secondary to alcoholic liver cirrhosis diagnosed 13 years prior complicated by ascites and esophageal varices presented to a primary care clinic for evaluation of a recent-onset nontender lesion on the left buttock. She was hypotensive with a blood pressure of 62/48 mmHg. The patient was prescribed ciprofloxacin 250 mg twice daily and hydrocodone/acetominophen 5 mg/325 mg twice daily as needed for pain management and was discharged. Six hours later, the patient presented to the emergency department with new onset symptoms of confusion and dark-colored spots on the abdomen and lower legs, which her family members noted had developed shortly after the patient took ciprofloxacin. In the emergency department, the patient was noted to be hypotensive and febrile with a severe metabolic acidosis. She was intubated for respiratory failure and received intravenous fluid resuscitation, broad-spectrum antibiotics, and vasopressors. Blood cultures were obtained, and the dermatology department was consulted.
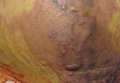
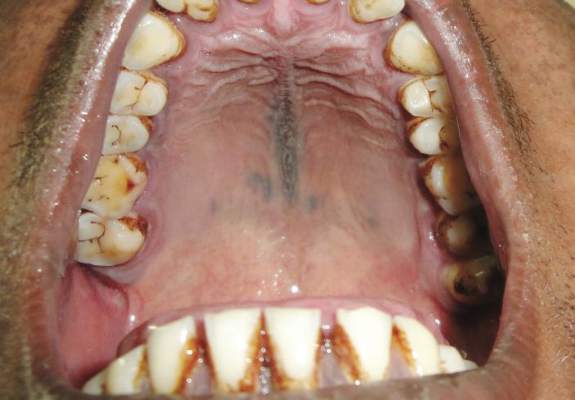
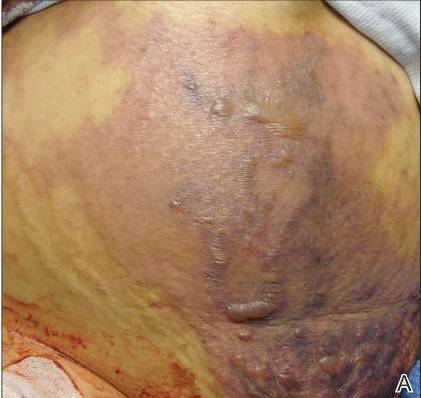
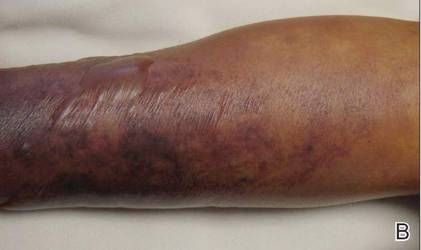
On physical examination, extensive purpuric, reticulated, and stellate plaques with central necrosis and hemorrhagic bullae were noted on the abdomen (Figure, A) and bilateral lower legs (Figure, B) extending onto the thighs. The patient was coagulopathic with persistent sanguineous oozing at intravenous sites and bilateral nares. A small erythematous ulcer with overlying black eschar was noted on the left medial buttock.
Laboratory test results showed new-onset thrombocytopenia, prolonged prothrombin time/international normalized ratio and partial thromboplastin time, and low fibrinogen levels, which confirmed a diagnosis of acute DIC. Blood cultures were positive for gram-negative rods in 4 out of 4 bottles within 12 hours of being drawn. Further testing identified the microorganism as E coli, and antibiotic susceptibility testing revealed it was sensitive to most antibiotics.
The patient was clinically diagnosed with purpura fulminans secondary to severe E coli septicemia and DIC. This life-threatening disorder is considered a medical emergency with a high mortality rate. Laboratory findings supporting DIC include the presence of schistocytes on a peripheral blood smear, thrombocytopenia, positive plasma protamine paracoagulation test, low fibrinogen levels, and positive fibrin degradation products. Reported cases of purpura fulminans in the setting of E coli septicemia are rare, and meningococcemia is the most common presentation.2,3 Bacterial components (eg, lipopolysaccharides found in the cell walls of gram-negative bacteria) may contribute to the progression of septicemia. Increased levels of endotoxin lipopolysaccharide can lead to septic shock and organ dysfunction.4 However, the release of lipooligosaccharides is associated with the development of meningococcal septicemia, and the lipopolysaccharide levels are directly correlated with prognosis in patients without meningitis.5-7
Human activated protein C concentrate (and its precursor, protein C concentrate) replacement therapy has been shown to improve outcomes in patients with meningococcemia-associated–purpura fulminans and severe sepsis, respectively.8 Heparin may be considered in the treatment of patients with purpura fulminans in addition to the replacement of any missing clotting factors or blood products.9 The international guidelines for the management of severe sepsis and septic shock include early quantitative resuscitation of the patient during the first 6 hours after recognition of sepsis, performing blood cultures before antibiotic therapy, and administering broad-spectrum antimicrobial therapy within 1 hour of recognition of septic shock.10 The elapsed time from triage to the actual administration of appropriate antimicrobials are primary determinants of patient mortality.11 Therefore, physicians must act quickly to stabilize the patient.
Gram-positive bacteria and gram-negative diplococci are common infectious agents implicated in purpura fulminans. Escherichia coli rarely has been identified as the inciting agent for purpura fulminans in adults. The increasing frequency of E coli strains that produce extended-spectrum β-lactamases—enzymes that mediate resistance to extended-spectrum (third generation) cephalosporins (eg, ceftazidime, cefotaxime, ceftriaxone) and monobactams (eg, aztreonam)—complicates matters further when deciding on appropriate antibiotics. Patients who have infections from extended-spectrum β-lactamase strains will require more potent carbapenems (eg, meropenem, imipenem) for treatment of infections. Despite undergoing treatment for septicemia, our patient went into cardiac arrest within 24 hours of presentation to the emergency department and died a few hours later. Physicians should consider E coli as an inciting agent of purpura fulminans and consider appropriate empiric antibiotics with gram-negative coverage to include E coli.
- Madden RM, Gill JC, Marlar RA. Protein C and protein S levels in two patients with acquired purpura fulminans. Br J Haematol. 1990;75:112-117.
- Nolan J, Sinclair R. Review of management of purpura fulminans and two case reports. Br J Anaesth. 2001;86:581-586.
- Huemer GM, Bonatti H, Dunst KM. Purpura fulminans due to E. coli septicemia. Wien Klin Wochenschr. 2004;116:82.
- Pugin J. Recognition of bacteria and bacterial products by host immune cells in sepsis. In: Vincent JL, ed. Yearbook of Intensive Care and Emergency Medicine. Berlin, Germany: Springer-Verlag; 1997:11-12.
- Brandtzaeg P, Oktedalen O, Kierulf P, et al. Elevated VIP and endotoxin plasma levels in human gram-negative septic shock. Regul Pept. 1989;24:37-44.
- Brandtzaeg P, Kierulf P, Gaustad P, et al. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J Infect Dis. 1989;159:195-204.
- Brandtzaeg P, Ovstebøo R, Kierulf P. Compartmentalization of lipopolysaccharide production correlates with clinical presentation in meningococcal disease. J Infect Dis. 1992;166:650-652.
- Hodgson A, Ryan T, Moriarty J, et al. Plasma exchange as a source of protein C for acute onset protein C pathway failure. Br J Haematol. 2002;116:905-908.
- Feinstein DI. Diagnosis and management of disseminated intravascular coagulation: the role of heparin therapy. Blood. 1982;60:284-287.
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign guidelines committee including the pediatric subgroup. Crit Care Med. 2013;41:580-637.
- Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38:1045-1053.
To the Editor:
Purpura fulminans is a severe and rapidly fatal thrombotic disorder that can occur in association with either hereditary or acquired deficiencies of the natural anticoagulants protein C and protein S.1 It most commonly results from the acute inflammatory response and subsequent disseminated intravascular coagulation (DIC) seen in severe bacterial septicemia. Excessive bleeding, retiform purpura, and skin necrosis may develop as a result of the coagulopathies of typical DIC.1Neisseria meningitidis, Streptococcus, and Staphylococcus frequently are implicated as pathogens, but Escherichia coli–associated purpura fulminans in adults is rare.2,3 We report a case of purpura fulminans in the setting of E coli septicemia.
A 62-year-old woman with a history of end-stage liver disease secondary to alcoholic liver cirrhosis diagnosed 13 years prior complicated by ascites and esophageal varices presented to a primary care clinic for evaluation of a recent-onset nontender lesion on the left buttock. She was hypotensive with a blood pressure of 62/48 mmHg. The patient was prescribed ciprofloxacin 250 mg twice daily and hydrocodone/acetominophen 5 mg/325 mg twice daily as needed for pain management and was discharged. Six hours later, the patient presented to the emergency department with new onset symptoms of confusion and dark-colored spots on the abdomen and lower legs, which her family members noted had developed shortly after the patient took ciprofloxacin. In the emergency department, the patient was noted to be hypotensive and febrile with a severe metabolic acidosis. She was intubated for respiratory failure and received intravenous fluid resuscitation, broad-spectrum antibiotics, and vasopressors. Blood cultures were obtained, and the dermatology department was consulted.
On physical examination, extensive purpuric, reticulated, and stellate plaques with central necrosis and hemorrhagic bullae were noted on the abdomen (Figure, A) and bilateral lower legs (Figure, B) extending onto the thighs. The patient was coagulopathic with persistent sanguineous oozing at intravenous sites and bilateral nares. A small erythematous ulcer with overlying black eschar was noted on the left medial buttock.


Laboratory test results showed new-onset thrombocytopenia, prolonged prothrombin time/international normalized ratio and partial thromboplastin time, and low fibrinogen levels, which confirmed a diagnosis of acute DIC. Blood cultures were positive for gram-negative rods in 4 out of 4 bottles within 12 hours of being drawn. Further testing identified the microorganism as E coli, and antibiotic susceptibility testing revealed it was sensitive to most antibiotics.
The patient was clinically diagnosed with purpura fulminans secondary to severe E coli septicemia and DIC. This life-threatening disorder is considered a medical emergency with a high mortality rate. Laboratory findings supporting DIC include the presence of schistocytes on a peripheral blood smear, thrombocytopenia, positive plasma protamine paracoagulation test, low fibrinogen levels, and positive fibrin degradation products. Reported cases of purpura fulminans in the setting of E coli septicemia are rare, and meningococcemia is the most common presentation.2,3 Bacterial components (eg, lipopolysaccharides found in the cell walls of gram-negative bacteria) may contribute to the progression of septicemia. Increased levels of endotoxin lipopolysaccharide can lead to septic shock and organ dysfunction.4 However, the release of lipooligosaccharides is associated with the development of meningococcal septicemia, and the lipopolysaccharide levels are directly correlated with prognosis in patients without meningitis.5-7
Human activated protein C concentrate (and its precursor, protein C concentrate) replacement therapy has been shown to improve outcomes in patients with meningococcemia-associated–purpura fulminans and severe sepsis, respectively.8 Heparin may be considered in the treatment of patients with purpura fulminans in addition to the replacement of any missing clotting factors or blood products.9 The international guidelines for the management of severe sepsis and septic shock include early quantitative resuscitation of the patient during the first 6 hours after recognition of sepsis, performing blood cultures before antibiotic therapy, and administering broad-spectrum antimicrobial therapy within 1 hour of recognition of septic shock.10 The elapsed time from triage to the actual administration of appropriate antimicrobials are primary determinants of patient mortality.11 Therefore, physicians must act quickly to stabilize the patient.
Gram-positive bacteria and gram-negative diplococci are common infectious agents implicated in purpura fulminans. Escherichia coli rarely has been identified as the inciting agent for purpura fulminans in adults. The increasing frequency of E coli strains that produce extended-spectrum β-lactamases—enzymes that mediate resistance to extended-spectrum (third generation) cephalosporins (eg, ceftazidime, cefotaxime, ceftriaxone) and monobactams (eg, aztreonam)—complicates matters further when deciding on appropriate antibiotics. Patients who have infections from extended-spectrum β-lactamase strains will require more potent carbapenems (eg, meropenem, imipenem) for treatment of infections. Despite undergoing treatment for septicemia, our patient went into cardiac arrest within 24 hours of presentation to the emergency department and died a few hours later. Physicians should consider E coli as an inciting agent of purpura fulminans and consider appropriate empiric antibiotics with gram-negative coverage to include E coli.
To the Editor:
Purpura fulminans is a severe and rapidly fatal thrombotic disorder that can occur in association with either hereditary or acquired deficiencies of the natural anticoagulants protein C and protein S.1 It most commonly results from the acute inflammatory response and subsequent disseminated intravascular coagulation (DIC) seen in severe bacterial septicemia. Excessive bleeding, retiform purpura, and skin necrosis may develop as a result of the coagulopathies of typical DIC.1Neisseria meningitidis, Streptococcus, and Staphylococcus frequently are implicated as pathogens, but Escherichia coli–associated purpura fulminans in adults is rare.2,3 We report a case of purpura fulminans in the setting of E coli septicemia.
A 62-year-old woman with a history of end-stage liver disease secondary to alcoholic liver cirrhosis diagnosed 13 years prior complicated by ascites and esophageal varices presented to a primary care clinic for evaluation of a recent-onset nontender lesion on the left buttock. She was hypotensive with a blood pressure of 62/48 mmHg. The patient was prescribed ciprofloxacin 250 mg twice daily and hydrocodone/acetominophen 5 mg/325 mg twice daily as needed for pain management and was discharged. Six hours later, the patient presented to the emergency department with new onset symptoms of confusion and dark-colored spots on the abdomen and lower legs, which her family members noted had developed shortly after the patient took ciprofloxacin. In the emergency department, the patient was noted to be hypotensive and febrile with a severe metabolic acidosis. She was intubated for respiratory failure and received intravenous fluid resuscitation, broad-spectrum antibiotics, and vasopressors. Blood cultures were obtained, and the dermatology department was consulted.
On physical examination, extensive purpuric, reticulated, and stellate plaques with central necrosis and hemorrhagic bullae were noted on the abdomen (Figure, A) and bilateral lower legs (Figure, B) extending onto the thighs. The patient was coagulopathic with persistent sanguineous oozing at intravenous sites and bilateral nares. A small erythematous ulcer with overlying black eschar was noted on the left medial buttock.


Laboratory test results showed new-onset thrombocytopenia, prolonged prothrombin time/international normalized ratio and partial thromboplastin time, and low fibrinogen levels, which confirmed a diagnosis of acute DIC. Blood cultures were positive for gram-negative rods in 4 out of 4 bottles within 12 hours of being drawn. Further testing identified the microorganism as E coli, and antibiotic susceptibility testing revealed it was sensitive to most antibiotics.
The patient was clinically diagnosed with purpura fulminans secondary to severe E coli septicemia and DIC. This life-threatening disorder is considered a medical emergency with a high mortality rate. Laboratory findings supporting DIC include the presence of schistocytes on a peripheral blood smear, thrombocytopenia, positive plasma protamine paracoagulation test, low fibrinogen levels, and positive fibrin degradation products. Reported cases of purpura fulminans in the setting of E coli septicemia are rare, and meningococcemia is the most common presentation.2,3 Bacterial components (eg, lipopolysaccharides found in the cell walls of gram-negative bacteria) may contribute to the progression of septicemia. Increased levels of endotoxin lipopolysaccharide can lead to septic shock and organ dysfunction.4 However, the release of lipooligosaccharides is associated with the development of meningococcal septicemia, and the lipopolysaccharide levels are directly correlated with prognosis in patients without meningitis.5-7
Human activated protein C concentrate (and its precursor, protein C concentrate) replacement therapy has been shown to improve outcomes in patients with meningococcemia-associated–purpura fulminans and severe sepsis, respectively.8 Heparin may be considered in the treatment of patients with purpura fulminans in addition to the replacement of any missing clotting factors or blood products.9 The international guidelines for the management of severe sepsis and septic shock include early quantitative resuscitation of the patient during the first 6 hours after recognition of sepsis, performing blood cultures before antibiotic therapy, and administering broad-spectrum antimicrobial therapy within 1 hour of recognition of septic shock.10 The elapsed time from triage to the actual administration of appropriate antimicrobials are primary determinants of patient mortality.11 Therefore, physicians must act quickly to stabilize the patient.
Gram-positive bacteria and gram-negative diplococci are common infectious agents implicated in purpura fulminans. Escherichia coli rarely has been identified as the inciting agent for purpura fulminans in adults. The increasing frequency of E coli strains that produce extended-spectrum β-lactamases—enzymes that mediate resistance to extended-spectrum (third generation) cephalosporins (eg, ceftazidime, cefotaxime, ceftriaxone) and monobactams (eg, aztreonam)—complicates matters further when deciding on appropriate antibiotics. Patients who have infections from extended-spectrum β-lactamase strains will require more potent carbapenems (eg, meropenem, imipenem) for treatment of infections. Despite undergoing treatment for septicemia, our patient went into cardiac arrest within 24 hours of presentation to the emergency department and died a few hours later. Physicians should consider E coli as an inciting agent of purpura fulminans and consider appropriate empiric antibiotics with gram-negative coverage to include E coli.
- Madden RM, Gill JC, Marlar RA. Protein C and protein S levels in two patients with acquired purpura fulminans. Br J Haematol. 1990;75:112-117.
- Nolan J, Sinclair R. Review of management of purpura fulminans and two case reports. Br J Anaesth. 2001;86:581-586.
- Huemer GM, Bonatti H, Dunst KM. Purpura fulminans due to E. coli septicemia. Wien Klin Wochenschr. 2004;116:82.
- Pugin J. Recognition of bacteria and bacterial products by host immune cells in sepsis. In: Vincent JL, ed. Yearbook of Intensive Care and Emergency Medicine. Berlin, Germany: Springer-Verlag; 1997:11-12.
- Brandtzaeg P, Oktedalen O, Kierulf P, et al. Elevated VIP and endotoxin plasma levels in human gram-negative septic shock. Regul Pept. 1989;24:37-44.
- Brandtzaeg P, Kierulf P, Gaustad P, et al. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J Infect Dis. 1989;159:195-204.
- Brandtzaeg P, Ovstebøo R, Kierulf P. Compartmentalization of lipopolysaccharide production correlates with clinical presentation in meningococcal disease. J Infect Dis. 1992;166:650-652.
- Hodgson A, Ryan T, Moriarty J, et al. Plasma exchange as a source of protein C for acute onset protein C pathway failure. Br J Haematol. 2002;116:905-908.
- Feinstein DI. Diagnosis and management of disseminated intravascular coagulation: the role of heparin therapy. Blood. 1982;60:284-287.
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign guidelines committee including the pediatric subgroup. Crit Care Med. 2013;41:580-637.
- Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38:1045-1053.
- Madden RM, Gill JC, Marlar RA. Protein C and protein S levels in two patients with acquired purpura fulminans. Br J Haematol. 1990;75:112-117.
- Nolan J, Sinclair R. Review of management of purpura fulminans and two case reports. Br J Anaesth. 2001;86:581-586.
- Huemer GM, Bonatti H, Dunst KM. Purpura fulminans due to E. coli septicemia. Wien Klin Wochenschr. 2004;116:82.
- Pugin J. Recognition of bacteria and bacterial products by host immune cells in sepsis. In: Vincent JL, ed. Yearbook of Intensive Care and Emergency Medicine. Berlin, Germany: Springer-Verlag; 1997:11-12.
- Brandtzaeg P, Oktedalen O, Kierulf P, et al. Elevated VIP and endotoxin plasma levels in human gram-negative septic shock. Regul Pept. 1989;24:37-44.
- Brandtzaeg P, Kierulf P, Gaustad P, et al. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J Infect Dis. 1989;159:195-204.
- Brandtzaeg P, Ovstebøo R, Kierulf P. Compartmentalization of lipopolysaccharide production correlates with clinical presentation in meningococcal disease. J Infect Dis. 1992;166:650-652.
- Hodgson A, Ryan T, Moriarty J, et al. Plasma exchange as a source of protein C for acute onset protein C pathway failure. Br J Haematol. 2002;116:905-908.
- Feinstein DI. Diagnosis and management of disseminated intravascular coagulation: the role of heparin therapy. Blood. 1982;60:284-287.
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign guidelines committee including the pediatric subgroup. Crit Care Med. 2013;41:580-637.
- Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38:1045-1053.
Factors Associated with Missed Dermatology Appointments
To the Editor:
Missed appointments are a major issue in every discipline of medicine1 and can be detrimental for dermatologists,2,3 whose clinics often have long wait times for referred patients and can lose up to $200 for each missed appointment.4 The purpose of this study was to quantify the rate of missed appointments at an academic dermatology clinic and identify factors associated with patient nonattendance.
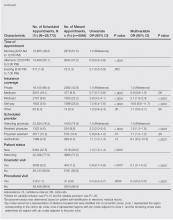
After approval by an institutional review board, appointment data was collected from the electronic medical record at the dermatology clinic at Wake Forest Baptist Health, Winston-Salem, North Carolina, for the period from May 1, 2013, to April 30, 2014. Variables that were evaluated included age, race, sex, primary language, employment status, zip code, appointment time, insurance coverage, scheduled provider, patient status (new vs returning), and the nature of the visit (cosmetic vs noncosmetic visits and procedural vs nonprocedural visits). Zip codes served as a representation of distance traveled and were stratified into 4 concentric zones: zone 1 represented the region corresponding to the clinic’s zip code; zone 2 represented regions with zip codes adjacent to zone 1; and the remaining zones were determined by regions with zip codes adjacent to the prior zone. Primary language spoken was categorized as English or non-English. Insurance coverage was categorized as private, Medicaid, Medicare, self-pay, and other. Using stepwise selection, both a univariate model and a multivariable logistic regression model were created (variable inclusion, P≤.10; variable exclusion, P>.05). Of the 28,772 appointments scheduled during the study period, 5584 (19.4%) were missed. Univariate and multivariable analyses of the factors associated with missed appointments are shown in Table 1.
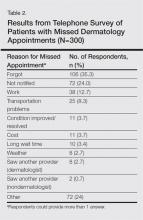
A telephone survey also was conducted to evaluate patient-reported factors associated with missed dermatology appointments. A list of patients who missed appointments during the period from January 1, 2014, to April 30, 2014, was extracted and every fourth patient was called within 6 weeks of the appointment to minimize recall bias. Patients were excluded from the study if they could not be reached after 3 attempts. Of the 799 patients contacted, 300 (38%) responded to the survey; 98 (12%) had phone numbers on record that were incorrect or were no longer in service; and 401 (50%) could not be reached after 3 attempts. The results of the telephone survey are provided in Table 2.
The demographic data suggested that characteristics associated with higher rates of missed appointments tended to reflect physical or financial barriers, such as dependency on others for transportation (eg, pediatric patients), longer distance traveled to the clinic, and lack of insurance coverage; however, only 4% and 8% of the survey respondents reported that they missed their appointment due to financial reasons or that they were unable to obtain transportation, respectively. Of the patients surveyed, 35% cited that the reason they missed their appointment was that they forgot about the appointment; additionally, 24% of respondents reported that they had not been reminded of the appointment.
Although physicians cannot directly address physical or financial barriers to attendance, we can introduce more effective methods of communication for patient reminders. Of the 799 patients who were called for the telephone survey, 12.3% had phone numbers on record that were either incorrect or no longer in service. As these patients’ phone numbers were listed in the electronic medical record for contact purposes, they likely did not receive telephone calls reminding them about their appointments. Although it was not formally evaluated in this study, many respondents expressed that they had other preferred methods of receiving appointment reminders (eg, e-mail, text message) than those that are currently considered commonplace (ie, telephone calls, voicemails).
This study was limited in that the appointment data came from a single academic dermatology clinic. There also were limitations in the data set for subgroup analysis; for example, to appropriately assess socioeconomic barriers to attendance of dermatology appointments, it would be valuable to stratify income within established factors of socioeconomic barriers (eg, race, employment status) to avoid research bias. Although many variables assessed were statistically significant (P<.05), the odds ratios often were close to 1, suggesting that they may not be clinically or practically relevant.
By identifying factors associated with missed dermatology appointments, interventions can be instituted to target high-risk groups and alter patient reminder protocols. If possible, identifying patients’ preferred contact methods (eg, telephone call, text message, etc) and verifying contact information may be cost-effective ways to reduce missed appointments in dermatology offices.
1. George A, Rubin G. Non-attendance in general practice: a systematic review and its implications for access to primary health care. Fam Pract. 2003;20:178-184.
2. Canizares MJ, Penneys NS. The incidence of nonattendance at an urgent care dermatology clinic. J Am Acad Dermatol. 2002;46:457-459.
3. Cronin PR, DeCoste L, Kimball AB. A multivariate analysis of dermatology missed appointment predictors. JAMA Dermatol. 2013;149:1435-1437.
4. Perez FD, Xie J, Sin A, et al. Characteristics and direct costs of academic pediatric subspecialty outpatient no-show events. J Healthc Qual. 2014;36:32-42.
To the Editor:
Missed appointments are a major issue in every discipline of medicine1 and can be detrimental for dermatologists,2,3 whose clinics often have long wait times for referred patients and can lose up to $200 for each missed appointment.4 The purpose of this study was to quantify the rate of missed appointments at an academic dermatology clinic and identify factors associated with patient nonattendance.
After approval by an institutional review board, appointment data was collected from the electronic medical record at the dermatology clinic at Wake Forest Baptist Health, Winston-Salem, North Carolina, for the period from May 1, 2013, to April 30, 2014. Variables that were evaluated included age, race, sex, primary language, employment status, zip code, appointment time, insurance coverage, scheduled provider, patient status (new vs returning), and the nature of the visit (cosmetic vs noncosmetic visits and procedural vs nonprocedural visits). Zip codes served as a representation of distance traveled and were stratified into 4 concentric zones: zone 1 represented the region corresponding to the clinic’s zip code; zone 2 represented regions with zip codes adjacent to zone 1; and the remaining zones were determined by regions with zip codes adjacent to the prior zone. Primary language spoken was categorized as English or non-English. Insurance coverage was categorized as private, Medicaid, Medicare, self-pay, and other. Using stepwise selection, both a univariate model and a multivariable logistic regression model were created (variable inclusion, P≤.10; variable exclusion, P>.05). Of the 28,772 appointments scheduled during the study period, 5584 (19.4%) were missed. Univariate and multivariable analyses of the factors associated with missed appointments are shown in Table 1.
A telephone survey also was conducted to evaluate patient-reported factors associated with missed dermatology appointments. A list of patients who missed appointments during the period from January 1, 2014, to April 30, 2014, was extracted and every fourth patient was called within 6 weeks of the appointment to minimize recall bias. Patients were excluded from the study if they could not be reached after 3 attempts. Of the 799 patients contacted, 300 (38%) responded to the survey; 98 (12%) had phone numbers on record that were incorrect or were no longer in service; and 401 (50%) could not be reached after 3 attempts. The results of the telephone survey are provided in Table 2.
The demographic data suggested that characteristics associated with higher rates of missed appointments tended to reflect physical or financial barriers, such as dependency on others for transportation (eg, pediatric patients), longer distance traveled to the clinic, and lack of insurance coverage; however, only 4% and 8% of the survey respondents reported that they missed their appointment due to financial reasons or that they were unable to obtain transportation, respectively. Of the patients surveyed, 35% cited that the reason they missed their appointment was that they forgot about the appointment; additionally, 24% of respondents reported that they had not been reminded of the appointment.
Although physicians cannot directly address physical or financial barriers to attendance, we can introduce more effective methods of communication for patient reminders. Of the 799 patients who were called for the telephone survey, 12.3% had phone numbers on record that were either incorrect or no longer in service. As these patients’ phone numbers were listed in the electronic medical record for contact purposes, they likely did not receive telephone calls reminding them about their appointments. Although it was not formally evaluated in this study, many respondents expressed that they had other preferred methods of receiving appointment reminders (eg, e-mail, text message) than those that are currently considered commonplace (ie, telephone calls, voicemails).
This study was limited in that the appointment data came from a single academic dermatology clinic. There also were limitations in the data set for subgroup analysis; for example, to appropriately assess socioeconomic barriers to attendance of dermatology appointments, it would be valuable to stratify income within established factors of socioeconomic barriers (eg, race, employment status) to avoid research bias. Although many variables assessed were statistically significant (P<.05), the odds ratios often were close to 1, suggesting that they may not be clinically or practically relevant.
By identifying factors associated with missed dermatology appointments, interventions can be instituted to target high-risk groups and alter patient reminder protocols. If possible, identifying patients’ preferred contact methods (eg, telephone call, text message, etc) and verifying contact information may be cost-effective ways to reduce missed appointments in dermatology offices.
To the Editor:
Missed appointments are a major issue in every discipline of medicine1 and can be detrimental for dermatologists,2,3 whose clinics often have long wait times for referred patients and can lose up to $200 for each missed appointment.4 The purpose of this study was to quantify the rate of missed appointments at an academic dermatology clinic and identify factors associated with patient nonattendance.
After approval by an institutional review board, appointment data was collected from the electronic medical record at the dermatology clinic at Wake Forest Baptist Health, Winston-Salem, North Carolina, for the period from May 1, 2013, to April 30, 2014. Variables that were evaluated included age, race, sex, primary language, employment status, zip code, appointment time, insurance coverage, scheduled provider, patient status (new vs returning), and the nature of the visit (cosmetic vs noncosmetic visits and procedural vs nonprocedural visits). Zip codes served as a representation of distance traveled and were stratified into 4 concentric zones: zone 1 represented the region corresponding to the clinic’s zip code; zone 2 represented regions with zip codes adjacent to zone 1; and the remaining zones were determined by regions with zip codes adjacent to the prior zone. Primary language spoken was categorized as English or non-English. Insurance coverage was categorized as private, Medicaid, Medicare, self-pay, and other. Using stepwise selection, both a univariate model and a multivariable logistic regression model were created (variable inclusion, P≤.10; variable exclusion, P>.05). Of the 28,772 appointments scheduled during the study period, 5584 (19.4%) were missed. Univariate and multivariable analyses of the factors associated with missed appointments are shown in Table 1.
A telephone survey also was conducted to evaluate patient-reported factors associated with missed dermatology appointments. A list of patients who missed appointments during the period from January 1, 2014, to April 30, 2014, was extracted and every fourth patient was called within 6 weeks of the appointment to minimize recall bias. Patients were excluded from the study if they could not be reached after 3 attempts. Of the 799 patients contacted, 300 (38%) responded to the survey; 98 (12%) had phone numbers on record that were incorrect or were no longer in service; and 401 (50%) could not be reached after 3 attempts. The results of the telephone survey are provided in Table 2.
The demographic data suggested that characteristics associated with higher rates of missed appointments tended to reflect physical or financial barriers, such as dependency on others for transportation (eg, pediatric patients), longer distance traveled to the clinic, and lack of insurance coverage; however, only 4% and 8% of the survey respondents reported that they missed their appointment due to financial reasons or that they were unable to obtain transportation, respectively. Of the patients surveyed, 35% cited that the reason they missed their appointment was that they forgot about the appointment; additionally, 24% of respondents reported that they had not been reminded of the appointment.
Although physicians cannot directly address physical or financial barriers to attendance, we can introduce more effective methods of communication for patient reminders. Of the 799 patients who were called for the telephone survey, 12.3% had phone numbers on record that were either incorrect or no longer in service. As these patients’ phone numbers were listed in the electronic medical record for contact purposes, they likely did not receive telephone calls reminding them about their appointments. Although it was not formally evaluated in this study, many respondents expressed that they had other preferred methods of receiving appointment reminders (eg, e-mail, text message) than those that are currently considered commonplace (ie, telephone calls, voicemails).
This study was limited in that the appointment data came from a single academic dermatology clinic. There also were limitations in the data set for subgroup analysis; for example, to appropriately assess socioeconomic barriers to attendance of dermatology appointments, it would be valuable to stratify income within established factors of socioeconomic barriers (eg, race, employment status) to avoid research bias. Although many variables assessed were statistically significant (P<.05), the odds ratios often were close to 1, suggesting that they may not be clinically or practically relevant.
By identifying factors associated with missed dermatology appointments, interventions can be instituted to target high-risk groups and alter patient reminder protocols. If possible, identifying patients’ preferred contact methods (eg, telephone call, text message, etc) and verifying contact information may be cost-effective ways to reduce missed appointments in dermatology offices.
1. George A, Rubin G. Non-attendance in general practice: a systematic review and its implications for access to primary health care. Fam Pract. 2003;20:178-184.
2. Canizares MJ, Penneys NS. The incidence of nonattendance at an urgent care dermatology clinic. J Am Acad Dermatol. 2002;46:457-459.
3. Cronin PR, DeCoste L, Kimball AB. A multivariate analysis of dermatology missed appointment predictors. JAMA Dermatol. 2013;149:1435-1437.
4. Perez FD, Xie J, Sin A, et al. Characteristics and direct costs of academic pediatric subspecialty outpatient no-show events. J Healthc Qual. 2014;36:32-42.
1. George A, Rubin G. Non-attendance in general practice: a systematic review and its implications for access to primary health care. Fam Pract. 2003;20:178-184.
2. Canizares MJ, Penneys NS. The incidence of nonattendance at an urgent care dermatology clinic. J Am Acad Dermatol. 2002;46:457-459.
3. Cronin PR, DeCoste L, Kimball AB. A multivariate analysis of dermatology missed appointment predictors. JAMA Dermatol. 2013;149:1435-1437.
4. Perez FD, Xie J, Sin A, et al. Characteristics and direct costs of academic pediatric subspecialty outpatient no-show events. J Healthc Qual. 2014;36:32-42.
Transient Reactive Papulotranslucent Acrokeratoderma: A Report of 3 Cases Showing Excellent Response to Topical Calcipotriene
To the Editor:
Transient reactive papulotranslucent acrokeratoderma (TRPA) is a rare disorder that also has been described using the terms aquagenic syringeal acrokeratoderma, aquagenic palmoplantar keratoderma, aquagenic acrokeratoderma, aquagenic papulotranslucent acrokeratoderma, and aquagenic wrinkling of the palms.1 It was initially described in 1996 by English and McCollough,2 and since then fewer than 100 cases have been reported.1-12
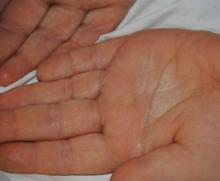
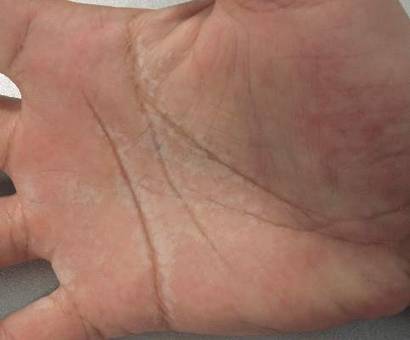
A 38-year-old man presented with prominent palmar hyperhidrosis with whitish papules on the palms of 10 days’ duration. The lesions were exacerbated following exposure to water but were asymptomatic aside from their unsightly cosmetic appearance. Dermatologic examination revealed translucent, whitish, pebbly papules confined to the central palmar creases (Figure 1) that were intensified following a 5-minute water immersion test.
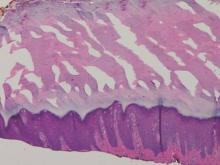
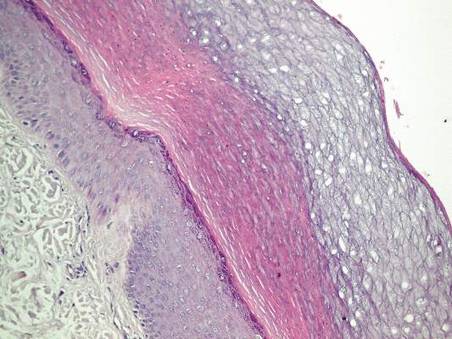
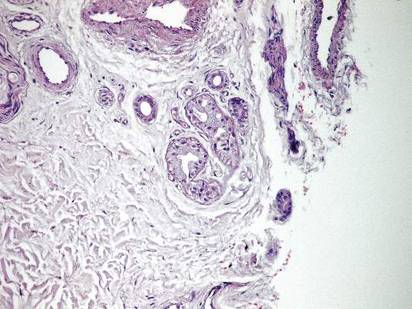
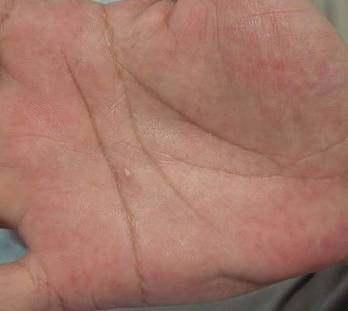
Histopathologic examination of a punch biopsy specimen from the right palm revealed orthokeratotic hyperkeratosis and slight hypergranulosis in the epidermis (Figure 2). Subtle eccrine glandular hyperplasia was evident in the dermis (Figure 3). Periodic acid–Schiff staining was negative. Based on the clinical findings and results of the water immersion test, a diagnosis of TRPA was made. A therapeutic trial of calcipotriene ointment 0.005% twice daily was initiated and resulted in dramatic clearance of the lesions within 2 weeks (Figure 4). At 1-month follow-up, the patient was virtually free of all symptoms and no disease recurrence was noted at 5-year follow-up.
| 
| ||
Figure 1. Whitish, pebbly papules confined to the central palmar creases in a 38-year-old man. | Figure 2. Orthokeratotic hyperkeratosis and mild hypergranulosis was noted in the epidermis (H&E, original magnification ×100). | ||
| 
| ||
| Figure 3. Luminal dilatation in the eccrine glands with a prominence of glandular epithelial cells, which displayed abundant cytoplasm with a granular appearance (H&E, original magnification ×100). | Figure 4. Remarkable response to calcipotriene ointment 0.005%. The white punctuate scar indicates the previous punch biopsy site. |
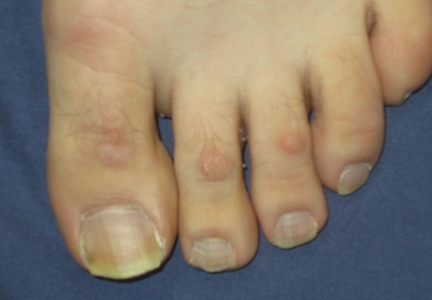
A 25-year-old woman presented with whitish plaques on the palms of 7 days’ duration. She reported frequent use of household cleansers in the month prior to presentation. The lesions were associated with prominent hyperhidrosis, pruritus, and a tingling sensation in the palms. Dermatologic examination revealed confluent, macerated, white, pavement stone–like papules with prominent puncta around the palmar flexures on both palms. Lesions were exacerbated after a 5-minute water immersion test (Figure 5).
The patient refused skin biopsy, and conservative treatment with a barrier cream and limited water exposure were of no benefit. Based on the clinical findings and results of the water immersion test, a diagnosis of TRPA was made. Due to the excellent outcome experienced in treating the previous patient, a trial of calcipotriene ointment 0.005% twice daily was initiated, and the patient reported complete resolution of signs and symptoms within the initial 2 weeks of treatment. Treatment was terminated at 1-month follow-up.
A 6-year-old boy presented with swollen, itchy palms of 2 months’ duration that the patient described as “wet” and “white.” Due to a recent epidemic of bird flu, the patient’s mother had advised him to use liquid cleansers and antiseptic gels on the hands for the past 2 months, which is when the symptoms on the palms started to develop. On dermatologic examination, whitish, cobblestonelike papules were noted near the palmar creases in association with profuse hyperhidrosis (Figure 6). Based on the clinical findings, a diagnosis of TRPA was made. Biopsy was not attempted and the patient was treated with calcipotriene ointment 0.005% twice daily. At 1-month follow-up, complete clearance of the lesions was noted.
Transient reactive papulotranslucent acrokeratoderma is an acquired and sporadic disorder that can occur in both sexes.2,4,6,8-11 Onset generally occurs during adolescence or young adulthood.1,3,8,9 Clinically, TRPA is characterized by edema and wrinkling of the palms following 5 to 10 minutes of contact with water that typically resolves within 1 hour after cessation of exposure.2,3,6-8,10 The “hand-in-the-bucket” sign refers to accentuation of physical findings upon immersion of the hand in water.6,10,11 Patients frequently report itching, burning, or tingling sensations in the affected areas.2,4,6,7,9,11 Transient reactive papulotranslucent acrokeratoderma usually affects the palms in a diffuse, bilateral, and symmetrical pattern,2,4,6-10 but cases showing involvement of the soles,6,7 marginal distribution of lesions,3 unilateral involvement,1 and prominence on the dorsal fingers5 also have been reported. The natural disease course involves reactive episodes and quiescent intervals.2,7,9 Spontaneous resolution of TRPA has been reported.4,6,8
The histological characteristics described in previous reports involve compact orthohyperkeratosis with dilated acrosyringia,2-6,9,11 hyperkeratosis and hypergranulosis in the epidermis,4,8,12 and eccrine glandular hyperplasia.5,12 Alternatively, the skin may appear completely normal on histology.1,7
Originally, it was proposed that TRPA is a variant of punctate keratoderma or hereditary papulotranslucent acrokeratoderma.2,3 However, its position within the keratoderma spectrum is unclear and the etiopathogenesis has not been fully elucidated. Some investigators believe that transient structural and functional alterations in the epidermal milieu prompt epidermal swelling and compensatory dilation of eccrine ducts.3,4,7,8,10 Other reports implicate the inherent structural weakness of eccrine duct walls3,4,11 or aberrations in eccrine glands.5,12 Whether the fundamental pathology lies within the epidermis, eccrine ducts, or the eccrine glands remains to be determined. Nevertheless, reports of TRPA in the setting of cystic fibrosis and its carrier state3,11 as well as the presence of hyperhidrosis in most affected patients and the accumulation of lesions along the palmar creases may implicate oversaturation of the epidermis (due to salt retention or abnormal water absorption by the stratum corneum) as the pivotal event in TRPA pathogenesis.1,10 Once the disease is expressed in susceptible individuals, episodes might be provoked by external factors such as friction, occlusion, sweating, liquid cleansers, antiseptic gels, gloves, topical preparations, and oral medications (eg, salicylic acid, cyclooxygenase 2 inhibitors).1,4
Treatment alternatives such as hydrophilic petrolatum and glycerin, ammonium lactate, salicylic acid (with or without urea), aluminum chloride hexahydrate, and topical corticosteroids are limited by unsuccessful or temporary outcomes.1,4,6,8-10 Botulinum toxin injections were effective in a patient with TRPA associated with hyperhidrosis.7 In the cases reported here, topical calcipotriene accomplished dramatic clearance of the lesions within the initial weeks of therapy. Spontaneous resolution was unlikely in these cases, as conservative therapies had not alleviated the signs and symptoms in any of the patients. However, we cannot exclude the possibility that improvement of the skin barrier function associated with other ingredients in the calcipotriene ointment (eg, petrolatum, mineral oil, α-tocopherol) may have led to the resolution of the lesions.
Calcipotriene has demonstrated efficacy in treating cutaneous disorders characterized by epidermal hyperproliferation and impaired terminal differentiation. Immunohistochemical and molecular biological evidence has indicated that topical calcipotriene exerts more pronounced inhibitory effects on epidermal proliferation than on dermal inflammation. It has been proposed that the bioavailability of calcipotriene in the dermal compartment may be markedly reduced compared to its availability in the epidermal compartment13; therefore it can be deduced that its penetration into the dermis is low in the thick skin of palms and its effect on eccrine sweat glands is negligible. Based on these factors, the clinical benefit of calcipotriene in TRPA could be ascribed directly to its antiproliferative and prodifferentiating effects on epidermal keratinocytes. We believe the primary pathology of TRPA lies in the epidermis and that changes in eccrine ducts and glands are secondary to the epidermal changes.
It is difficult to conduct large-scale studies of TRPA due to its rare presentation. Based on our encouraging preliminary observations in 3 patients, we recommend further therapeutic trials of topical calcipotriene in the treatment of TRPA.
1. Erkek E. Unilateral transient reactive papulotranslucent acrokeratoderma in a child. Pediatr Dermatol. 2007;24:564-566.
2. English JC 3rd, McCollough ML. Transient reactive papulotranslucent acrokeratoderma. J Am Acad Dermatol. 1996;34:686-687.
3. Lowes MA, Khaira GS, Holt D. Transient reactive papulotranslucent acrokeratoderma associated with cystic fibrosis. Australas J Dermatol. 2000;41:172-174.
4. MacCormack MA, Wiss K, Malhotra R. Aquagenic syringeal acrokeratoderma: report of two teenage cases. J Am Acad Dermatol. 2001;45:124-126.
5. Yoon TY, Kim KR, Lee JY, et al. Aquagenic syringeal acrokeratoderma: unusual prominence on the dorsal aspect of fingers [published online ahead of print May 22, 2008]. Br J Dermatol. 2008;159:486-488.
6. Yan AC, Aasi SZ, Alms WJ, et al. Aquagenic palmoplantar keratoderma. J Am Acad Dermatol. 2001;44:696-699.
7. Diba VC, Cormack GC, Burrows NP. Botulinum toxin is helpful in aquagenic palmoplantar keratoderma. Br J Dermatol. 2005;152:394-395.
8. Saray Y, Seckin D. Familial aquagenic acrokeratoderma: case reports and review of the literature. Int J Dermatol. 2005;44:906-909.
9. Yalcin B, Artuz F, Toy GG, et al. Acquired aquagenic papulotranslucent acrokeratoderma. J Eur Acad Dermatol Venereol. 2005;19:654-656.
10. Neri I, Bianchi F, Patrizi A. Transient aquagenic palmar hyperwrinkling: the first instance reported in a young boy. Pediatr Dermatol. 2006;23:39-42.
11. Katz KA, Yan AC, Turner ML. Aquagenic wrinkling of the palms in patients with cystic fibrosis homozygous for the delta F508 CFTR mutation. Arch Dermatol. 2005;141:621-624.
12. Kabashima K, Shimauchi T, Kobayashi M, et al. Aberrant aquaporin 5 expression in the sweat gland in aquagenic wrinkling of the palms. J Am Acad Dermatol. 2008;59(suppl 1):S28-S32.
13. Lehmann B, Querings K, Reichrath J. Vitamin D and skin: new aspects for dermatology. Exp Dermatol. 2004;13:11-15.
To the Editor:
Transient reactive papulotranslucent acrokeratoderma (TRPA) is a rare disorder that also has been described using the terms aquagenic syringeal acrokeratoderma, aquagenic palmoplantar keratoderma, aquagenic acrokeratoderma, aquagenic papulotranslucent acrokeratoderma, and aquagenic wrinkling of the palms.1 It was initially described in 1996 by English and McCollough,2 and since then fewer than 100 cases have been reported.1-12
A 38-year-old man presented with prominent palmar hyperhidrosis with whitish papules on the palms of 10 days’ duration. The lesions were exacerbated following exposure to water but were asymptomatic aside from their unsightly cosmetic appearance. Dermatologic examination revealed translucent, whitish, pebbly papules confined to the central palmar creases (Figure 1) that were intensified following a 5-minute water immersion test.
Histopathologic examination of a punch biopsy specimen from the right palm revealed orthokeratotic hyperkeratosis and slight hypergranulosis in the epidermis (Figure 2). Subtle eccrine glandular hyperplasia was evident in the dermis (Figure 3). Periodic acid–Schiff staining was negative. Based on the clinical findings and results of the water immersion test, a diagnosis of TRPA was made. A therapeutic trial of calcipotriene ointment 0.005% twice daily was initiated and resulted in dramatic clearance of the lesions within 2 weeks (Figure 4). At 1-month follow-up, the patient was virtually free of all symptoms and no disease recurrence was noted at 5-year follow-up.

| 
| ||
Figure 1. Whitish, pebbly papules confined to the central palmar creases in a 38-year-old man. | Figure 2. Orthokeratotic hyperkeratosis and mild hypergranulosis was noted in the epidermis (H&E, original magnification ×100). | ||

| 
| ||
| Figure 3. Luminal dilatation in the eccrine glands with a prominence of glandular epithelial cells, which displayed abundant cytoplasm with a granular appearance (H&E, original magnification ×100). | Figure 4. Remarkable response to calcipotriene ointment 0.005%. The white punctuate scar indicates the previous punch biopsy site. |
A 25-year-old woman presented with whitish plaques on the palms of 7 days’ duration. She reported frequent use of household cleansers in the month prior to presentation. The lesions were associated with prominent hyperhidrosis, pruritus, and a tingling sensation in the palms. Dermatologic examination revealed confluent, macerated, white, pavement stone–like papules with prominent puncta around the palmar flexures on both palms. Lesions were exacerbated after a 5-minute water immersion test (Figure 5).
The patient refused skin biopsy, and conservative treatment with a barrier cream and limited water exposure were of no benefit. Based on the clinical findings and results of the water immersion test, a diagnosis of TRPA was made. Due to the excellent outcome experienced in treating the previous patient, a trial of calcipotriene ointment 0.005% twice daily was initiated, and the patient reported complete resolution of signs and symptoms within the initial 2 weeks of treatment. Treatment was terminated at 1-month follow-up.
A 6-year-old boy presented with swollen, itchy palms of 2 months’ duration that the patient described as “wet” and “white.” Due to a recent epidemic of bird flu, the patient’s mother had advised him to use liquid cleansers and antiseptic gels on the hands for the past 2 months, which is when the symptoms on the palms started to develop. On dermatologic examination, whitish, cobblestonelike papules were noted near the palmar creases in association with profuse hyperhidrosis (Figure 6). Based on the clinical findings, a diagnosis of TRPA was made. Biopsy was not attempted and the patient was treated with calcipotriene ointment 0.005% twice daily. At 1-month follow-up, complete clearance of the lesions was noted.
Transient reactive papulotranslucent acrokeratoderma is an acquired and sporadic disorder that can occur in both sexes.2,4,6,8-11 Onset generally occurs during adolescence or young adulthood.1,3,8,9 Clinically, TRPA is characterized by edema and wrinkling of the palms following 5 to 10 minutes of contact with water that typically resolves within 1 hour after cessation of exposure.2,3,6-8,10 The “hand-in-the-bucket” sign refers to accentuation of physical findings upon immersion of the hand in water.6,10,11 Patients frequently report itching, burning, or tingling sensations in the affected areas.2,4,6,7,9,11 Transient reactive papulotranslucent acrokeratoderma usually affects the palms in a diffuse, bilateral, and symmetrical pattern,2,4,6-10 but cases showing involvement of the soles,6,7 marginal distribution of lesions,3 unilateral involvement,1 and prominence on the dorsal fingers5 also have been reported. The natural disease course involves reactive episodes and quiescent intervals.2,7,9 Spontaneous resolution of TRPA has been reported.4,6,8
The histological characteristics described in previous reports involve compact orthohyperkeratosis with dilated acrosyringia,2-6,9,11 hyperkeratosis and hypergranulosis in the epidermis,4,8,12 and eccrine glandular hyperplasia.5,12 Alternatively, the skin may appear completely normal on histology.1,7
Originally, it was proposed that TRPA is a variant of punctate keratoderma or hereditary papulotranslucent acrokeratoderma.2,3 However, its position within the keratoderma spectrum is unclear and the etiopathogenesis has not been fully elucidated. Some investigators believe that transient structural and functional alterations in the epidermal milieu prompt epidermal swelling and compensatory dilation of eccrine ducts.3,4,7,8,10 Other reports implicate the inherent structural weakness of eccrine duct walls3,4,11 or aberrations in eccrine glands.5,12 Whether the fundamental pathology lies within the epidermis, eccrine ducts, or the eccrine glands remains to be determined. Nevertheless, reports of TRPA in the setting of cystic fibrosis and its carrier state3,11 as well as the presence of hyperhidrosis in most affected patients and the accumulation of lesions along the palmar creases may implicate oversaturation of the epidermis (due to salt retention or abnormal water absorption by the stratum corneum) as the pivotal event in TRPA pathogenesis.1,10 Once the disease is expressed in susceptible individuals, episodes might be provoked by external factors such as friction, occlusion, sweating, liquid cleansers, antiseptic gels, gloves, topical preparations, and oral medications (eg, salicylic acid, cyclooxygenase 2 inhibitors).1,4
Treatment alternatives such as hydrophilic petrolatum and glycerin, ammonium lactate, salicylic acid (with or without urea), aluminum chloride hexahydrate, and topical corticosteroids are limited by unsuccessful or temporary outcomes.1,4,6,8-10 Botulinum toxin injections were effective in a patient with TRPA associated with hyperhidrosis.7 In the cases reported here, topical calcipotriene accomplished dramatic clearance of the lesions within the initial weeks of therapy. Spontaneous resolution was unlikely in these cases, as conservative therapies had not alleviated the signs and symptoms in any of the patients. However, we cannot exclude the possibility that improvement of the skin barrier function associated with other ingredients in the calcipotriene ointment (eg, petrolatum, mineral oil, α-tocopherol) may have led to the resolution of the lesions.
Calcipotriene has demonstrated efficacy in treating cutaneous disorders characterized by epidermal hyperproliferation and impaired terminal differentiation. Immunohistochemical and molecular biological evidence has indicated that topical calcipotriene exerts more pronounced inhibitory effects on epidermal proliferation than on dermal inflammation. It has been proposed that the bioavailability of calcipotriene in the dermal compartment may be markedly reduced compared to its availability in the epidermal compartment13; therefore it can be deduced that its penetration into the dermis is low in the thick skin of palms and its effect on eccrine sweat glands is negligible. Based on these factors, the clinical benefit of calcipotriene in TRPA could be ascribed directly to its antiproliferative and prodifferentiating effects on epidermal keratinocytes. We believe the primary pathology of TRPA lies in the epidermis and that changes in eccrine ducts and glands are secondary to the epidermal changes.
It is difficult to conduct large-scale studies of TRPA due to its rare presentation. Based on our encouraging preliminary observations in 3 patients, we recommend further therapeutic trials of topical calcipotriene in the treatment of TRPA.
To the Editor:
Transient reactive papulotranslucent acrokeratoderma (TRPA) is a rare disorder that also has been described using the terms aquagenic syringeal acrokeratoderma, aquagenic palmoplantar keratoderma, aquagenic acrokeratoderma, aquagenic papulotranslucent acrokeratoderma, and aquagenic wrinkling of the palms.1 It was initially described in 1996 by English and McCollough,2 and since then fewer than 100 cases have been reported.1-12
A 38-year-old man presented with prominent palmar hyperhidrosis with whitish papules on the palms of 10 days’ duration. The lesions were exacerbated following exposure to water but were asymptomatic aside from their unsightly cosmetic appearance. Dermatologic examination revealed translucent, whitish, pebbly papules confined to the central palmar creases (Figure 1) that were intensified following a 5-minute water immersion test.
Histopathologic examination of a punch biopsy specimen from the right palm revealed orthokeratotic hyperkeratosis and slight hypergranulosis in the epidermis (Figure 2). Subtle eccrine glandular hyperplasia was evident in the dermis (Figure 3). Periodic acid–Schiff staining was negative. Based on the clinical findings and results of the water immersion test, a diagnosis of TRPA was made. A therapeutic trial of calcipotriene ointment 0.005% twice daily was initiated and resulted in dramatic clearance of the lesions within 2 weeks (Figure 4). At 1-month follow-up, the patient was virtually free of all symptoms and no disease recurrence was noted at 5-year follow-up.

| 
| ||
Figure 1. Whitish, pebbly papules confined to the central palmar creases in a 38-year-old man. | Figure 2. Orthokeratotic hyperkeratosis and mild hypergranulosis was noted in the epidermis (H&E, original magnification ×100). | ||

| 
| ||
| Figure 3. Luminal dilatation in the eccrine glands with a prominence of glandular epithelial cells, which displayed abundant cytoplasm with a granular appearance (H&E, original magnification ×100). | Figure 4. Remarkable response to calcipotriene ointment 0.005%. The white punctuate scar indicates the previous punch biopsy site. |
A 25-year-old woman presented with whitish plaques on the palms of 7 days’ duration. She reported frequent use of household cleansers in the month prior to presentation. The lesions were associated with prominent hyperhidrosis, pruritus, and a tingling sensation in the palms. Dermatologic examination revealed confluent, macerated, white, pavement stone–like papules with prominent puncta around the palmar flexures on both palms. Lesions were exacerbated after a 5-minute water immersion test (Figure 5).
The patient refused skin biopsy, and conservative treatment with a barrier cream and limited water exposure were of no benefit. Based on the clinical findings and results of the water immersion test, a diagnosis of TRPA was made. Due to the excellent outcome experienced in treating the previous patient, a trial of calcipotriene ointment 0.005% twice daily was initiated, and the patient reported complete resolution of signs and symptoms within the initial 2 weeks of treatment. Treatment was terminated at 1-month follow-up.
A 6-year-old boy presented with swollen, itchy palms of 2 months’ duration that the patient described as “wet” and “white.” Due to a recent epidemic of bird flu, the patient’s mother had advised him to use liquid cleansers and antiseptic gels on the hands for the past 2 months, which is when the symptoms on the palms started to develop. On dermatologic examination, whitish, cobblestonelike papules were noted near the palmar creases in association with profuse hyperhidrosis (Figure 6). Based on the clinical findings, a diagnosis of TRPA was made. Biopsy was not attempted and the patient was treated with calcipotriene ointment 0.005% twice daily. At 1-month follow-up, complete clearance of the lesions was noted.
Transient reactive papulotranslucent acrokeratoderma is an acquired and sporadic disorder that can occur in both sexes.2,4,6,8-11 Onset generally occurs during adolescence or young adulthood.1,3,8,9 Clinically, TRPA is characterized by edema and wrinkling of the palms following 5 to 10 minutes of contact with water that typically resolves within 1 hour after cessation of exposure.2,3,6-8,10 The “hand-in-the-bucket” sign refers to accentuation of physical findings upon immersion of the hand in water.6,10,11 Patients frequently report itching, burning, or tingling sensations in the affected areas.2,4,6,7,9,11 Transient reactive papulotranslucent acrokeratoderma usually affects the palms in a diffuse, bilateral, and symmetrical pattern,2,4,6-10 but cases showing involvement of the soles,6,7 marginal distribution of lesions,3 unilateral involvement,1 and prominence on the dorsal fingers5 also have been reported. The natural disease course involves reactive episodes and quiescent intervals.2,7,9 Spontaneous resolution of TRPA has been reported.4,6,8
The histological characteristics described in previous reports involve compact orthohyperkeratosis with dilated acrosyringia,2-6,9,11 hyperkeratosis and hypergranulosis in the epidermis,4,8,12 and eccrine glandular hyperplasia.5,12 Alternatively, the skin may appear completely normal on histology.1,7
Originally, it was proposed that TRPA is a variant of punctate keratoderma or hereditary papulotranslucent acrokeratoderma.2,3 However, its position within the keratoderma spectrum is unclear and the etiopathogenesis has not been fully elucidated. Some investigators believe that transient structural and functional alterations in the epidermal milieu prompt epidermal swelling and compensatory dilation of eccrine ducts.3,4,7,8,10 Other reports implicate the inherent structural weakness of eccrine duct walls3,4,11 or aberrations in eccrine glands.5,12 Whether the fundamental pathology lies within the epidermis, eccrine ducts, or the eccrine glands remains to be determined. Nevertheless, reports of TRPA in the setting of cystic fibrosis and its carrier state3,11 as well as the presence of hyperhidrosis in most affected patients and the accumulation of lesions along the palmar creases may implicate oversaturation of the epidermis (due to salt retention or abnormal water absorption by the stratum corneum) as the pivotal event in TRPA pathogenesis.1,10 Once the disease is expressed in susceptible individuals, episodes might be provoked by external factors such as friction, occlusion, sweating, liquid cleansers, antiseptic gels, gloves, topical preparations, and oral medications (eg, salicylic acid, cyclooxygenase 2 inhibitors).1,4
Treatment alternatives such as hydrophilic petrolatum and glycerin, ammonium lactate, salicylic acid (with or without urea), aluminum chloride hexahydrate, and topical corticosteroids are limited by unsuccessful or temporary outcomes.1,4,6,8-10 Botulinum toxin injections were effective in a patient with TRPA associated with hyperhidrosis.7 In the cases reported here, topical calcipotriene accomplished dramatic clearance of the lesions within the initial weeks of therapy. Spontaneous resolution was unlikely in these cases, as conservative therapies had not alleviated the signs and symptoms in any of the patients. However, we cannot exclude the possibility that improvement of the skin barrier function associated with other ingredients in the calcipotriene ointment (eg, petrolatum, mineral oil, α-tocopherol) may have led to the resolution of the lesions.
Calcipotriene has demonstrated efficacy in treating cutaneous disorders characterized by epidermal hyperproliferation and impaired terminal differentiation. Immunohistochemical and molecular biological evidence has indicated that topical calcipotriene exerts more pronounced inhibitory effects on epidermal proliferation than on dermal inflammation. It has been proposed that the bioavailability of calcipotriene in the dermal compartment may be markedly reduced compared to its availability in the epidermal compartment13; therefore it can be deduced that its penetration into the dermis is low in the thick skin of palms and its effect on eccrine sweat glands is negligible. Based on these factors, the clinical benefit of calcipotriene in TRPA could be ascribed directly to its antiproliferative and prodifferentiating effects on epidermal keratinocytes. We believe the primary pathology of TRPA lies in the epidermis and that changes in eccrine ducts and glands are secondary to the epidermal changes.
It is difficult to conduct large-scale studies of TRPA due to its rare presentation. Based on our encouraging preliminary observations in 3 patients, we recommend further therapeutic trials of topical calcipotriene in the treatment of TRPA.
1. Erkek E. Unilateral transient reactive papulotranslucent acrokeratoderma in a child. Pediatr Dermatol. 2007;24:564-566.
2. English JC 3rd, McCollough ML. Transient reactive papulotranslucent acrokeratoderma. J Am Acad Dermatol. 1996;34:686-687.
3. Lowes MA, Khaira GS, Holt D. Transient reactive papulotranslucent acrokeratoderma associated with cystic fibrosis. Australas J Dermatol. 2000;41:172-174.
4. MacCormack MA, Wiss K, Malhotra R. Aquagenic syringeal acrokeratoderma: report of two teenage cases. J Am Acad Dermatol. 2001;45:124-126.
5. Yoon TY, Kim KR, Lee JY, et al. Aquagenic syringeal acrokeratoderma: unusual prominence on the dorsal aspect of fingers [published online ahead of print May 22, 2008]. Br J Dermatol. 2008;159:486-488.
6. Yan AC, Aasi SZ, Alms WJ, et al. Aquagenic palmoplantar keratoderma. J Am Acad Dermatol. 2001;44:696-699.
7. Diba VC, Cormack GC, Burrows NP. Botulinum toxin is helpful in aquagenic palmoplantar keratoderma. Br J Dermatol. 2005;152:394-395.
8. Saray Y, Seckin D. Familial aquagenic acrokeratoderma: case reports and review of the literature. Int J Dermatol. 2005;44:906-909.
9. Yalcin B, Artuz F, Toy GG, et al. Acquired aquagenic papulotranslucent acrokeratoderma. J Eur Acad Dermatol Venereol. 2005;19:654-656.
10. Neri I, Bianchi F, Patrizi A. Transient aquagenic palmar hyperwrinkling: the first instance reported in a young boy. Pediatr Dermatol. 2006;23:39-42.
11. Katz KA, Yan AC, Turner ML. Aquagenic wrinkling of the palms in patients with cystic fibrosis homozygous for the delta F508 CFTR mutation. Arch Dermatol. 2005;141:621-624.
12. Kabashima K, Shimauchi T, Kobayashi M, et al. Aberrant aquaporin 5 expression in the sweat gland in aquagenic wrinkling of the palms. J Am Acad Dermatol. 2008;59(suppl 1):S28-S32.
13. Lehmann B, Querings K, Reichrath J. Vitamin D and skin: new aspects for dermatology. Exp Dermatol. 2004;13:11-15.
1. Erkek E. Unilateral transient reactive papulotranslucent acrokeratoderma in a child. Pediatr Dermatol. 2007;24:564-566.
2. English JC 3rd, McCollough ML. Transient reactive papulotranslucent acrokeratoderma. J Am Acad Dermatol. 1996;34:686-687.
3. Lowes MA, Khaira GS, Holt D. Transient reactive papulotranslucent acrokeratoderma associated with cystic fibrosis. Australas J Dermatol. 2000;41:172-174.
4. MacCormack MA, Wiss K, Malhotra R. Aquagenic syringeal acrokeratoderma: report of two teenage cases. J Am Acad Dermatol. 2001;45:124-126.
5. Yoon TY, Kim KR, Lee JY, et al. Aquagenic syringeal acrokeratoderma: unusual prominence on the dorsal aspect of fingers [published online ahead of print May 22, 2008]. Br J Dermatol. 2008;159:486-488.
6. Yan AC, Aasi SZ, Alms WJ, et al. Aquagenic palmoplantar keratoderma. J Am Acad Dermatol. 2001;44:696-699.
7. Diba VC, Cormack GC, Burrows NP. Botulinum toxin is helpful in aquagenic palmoplantar keratoderma. Br J Dermatol. 2005;152:394-395.
8. Saray Y, Seckin D. Familial aquagenic acrokeratoderma: case reports and review of the literature. Int J Dermatol. 2005;44:906-909.
9. Yalcin B, Artuz F, Toy GG, et al. Acquired aquagenic papulotranslucent acrokeratoderma. J Eur Acad Dermatol Venereol. 2005;19:654-656.
10. Neri I, Bianchi F, Patrizi A. Transient aquagenic palmar hyperwrinkling: the first instance reported in a young boy. Pediatr Dermatol. 2006;23:39-42.
11. Katz KA, Yan AC, Turner ML. Aquagenic wrinkling of the palms in patients with cystic fibrosis homozygous for the delta F508 CFTR mutation. Arch Dermatol. 2005;141:621-624.
12. Kabashima K, Shimauchi T, Kobayashi M, et al. Aberrant aquaporin 5 expression in the sweat gland in aquagenic wrinkling of the palms. J Am Acad Dermatol. 2008;59(suppl 1):S28-S32.
13. Lehmann B, Querings K, Reichrath J. Vitamin D and skin: new aspects for dermatology. Exp Dermatol. 2004;13:11-15.
Recurrent Omphalitis Secondary to a Hair-Containing Umbilical Foreign Body
To the Editor:
We read with great interest the article, “Omphalith-Associated Relapsing Umbilical Cellulitis: Recurrent Omphalitis Secondary to a Hair-Containing Belly Button Bezoar” (Cutis. 2010;86:199-202), which introduced the terms omphalotrich and tricomphalith to describe the pilar composition of a hair-containing umbilical foreign body in an 18-year-old man. We report a similar case.
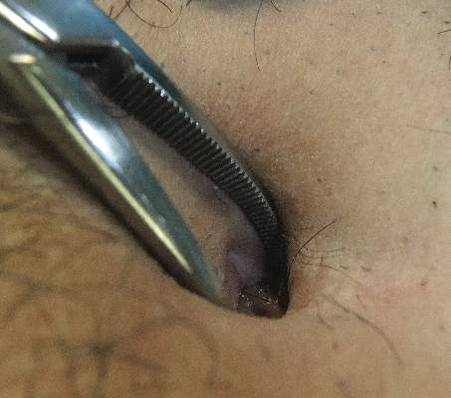
A 38-year-old man presented with a 10-year history of an unusual odor in the umbilical region with recurrent discharge. He diligently maintained proper hygiene of the umbilicus using cotton swabs and had received recurrent cycles of oral antibiotics prescribed by his general practitioner with temporary improvement of the odor and amount of discharge. Physical examination revealed a normal umbilicus with a deep and tight umbilical cleft that required the use of curved mosquito forceps for further examination (Figure 1). A bezoar comprised of a compact collection of terminal hair shafts was noted deep in the umbilicus (Figure 2). A considerable amount of terminal hairs also were noted on the skin of the abdominal area. Following removal of the bezoar, no umbilical fistula was observed, and the presence of embryologic abnormalities (eg, omphalomesenteric duct remnants) was ruled out on magnetic resonance imaging. A diagnosis of recurrent omphalitis secondary to a hair-containing bezoar was made. Following extraction of the bezoar, the odor and discharge promptly resolved, thereby avoiding the need for oral antibiotics; however, a smaller bezoar comprised of a collection of terminal hair shafts was removed 4 months later.
| 
| |
Figure 1. Deep and narrow umbilical cleft with serous exudate in the umbilicus after removal of the foreign body. | Figure 2. A section of the umbilical foreign body composed of a collection of terminal hair shafts. |
An omphalith is an umbilical foreign body that results from the accumulation of keratinous and amorphous sebaceous material.2 Several predisposing factors have been proposed for its pathogenesis, such as the anatomical disposition of the umbilicus and the patient’s hygiene. We hypothesize that a deep umbilicus and a large amount of terminal hairs in the abdominal area were predisposing factors in our patient. Cohen et al1 proposed the terms omphalotrich and trichomphalith to describe the pilar composition of a hair-containing umbilical foreign body that did not have the characteristic stonelike presentation of a traditional omphalith. The authors also referred to the umbilical foreign body in their patient as a trichobezoar, a term used to describe exogenous foreign bodies composed of ingested hair in the gastrointestinal tract, given the embryologic origin of the umbilicus and epithelium of the gastrointestinal tract. We agree that the terms omphalotrich and trichomphalith appropriately describe the current presentation; we also propose the terms omphalitrichia or thricomphalia to describe the findings seen in our patient, which should always be ruled out in patients with recurrent omphalitis that is unresponsive to antibiotics.
1. Cohen PR, Robinson FW, Gray JM. Omphalith-associated relapsing umbilical cellulitis: recurrent omphalitis secondary to a hair-containing belly button bezoar. Cutis. 2010;86:199-202.
2. Swanson SL, Woosley JT, Fleischer AB Jr, et al. Umbilical mass. omphalith. Arch Dermatol. 1992;128:1267, 1270.
To the Editor:
We read with great interest the article, “Omphalith-Associated Relapsing Umbilical Cellulitis: Recurrent Omphalitis Secondary to a Hair-Containing Belly Button Bezoar” (Cutis. 2010;86:199-202), which introduced the terms omphalotrich and tricomphalith to describe the pilar composition of a hair-containing umbilical foreign body in an 18-year-old man. We report a similar case.
A 38-year-old man presented with a 10-year history of an unusual odor in the umbilical region with recurrent discharge. He diligently maintained proper hygiene of the umbilicus using cotton swabs and had received recurrent cycles of oral antibiotics prescribed by his general practitioner with temporary improvement of the odor and amount of discharge. Physical examination revealed a normal umbilicus with a deep and tight umbilical cleft that required the use of curved mosquito forceps for further examination (Figure 1). A bezoar comprised of a compact collection of terminal hair shafts was noted deep in the umbilicus (Figure 2). A considerable amount of terminal hairs also were noted on the skin of the abdominal area. Following removal of the bezoar, no umbilical fistula was observed, and the presence of embryologic abnormalities (eg, omphalomesenteric duct remnants) was ruled out on magnetic resonance imaging. A diagnosis of recurrent omphalitis secondary to a hair-containing bezoar was made. Following extraction of the bezoar, the odor and discharge promptly resolved, thereby avoiding the need for oral antibiotics; however, a smaller bezoar comprised of a collection of terminal hair shafts was removed 4 months later.

| 
| |
Figure 1. Deep and narrow umbilical cleft with serous exudate in the umbilicus after removal of the foreign body. | Figure 2. A section of the umbilical foreign body composed of a collection of terminal hair shafts. |
An omphalith is an umbilical foreign body that results from the accumulation of keratinous and amorphous sebaceous material.2 Several predisposing factors have been proposed for its pathogenesis, such as the anatomical disposition of the umbilicus and the patient’s hygiene. We hypothesize that a deep umbilicus and a large amount of terminal hairs in the abdominal area were predisposing factors in our patient. Cohen et al1 proposed the terms omphalotrich and trichomphalith to describe the pilar composition of a hair-containing umbilical foreign body that did not have the characteristic stonelike presentation of a traditional omphalith. The authors also referred to the umbilical foreign body in their patient as a trichobezoar, a term used to describe exogenous foreign bodies composed of ingested hair in the gastrointestinal tract, given the embryologic origin of the umbilicus and epithelium of the gastrointestinal tract. We agree that the terms omphalotrich and trichomphalith appropriately describe the current presentation; we also propose the terms omphalitrichia or thricomphalia to describe the findings seen in our patient, which should always be ruled out in patients with recurrent omphalitis that is unresponsive to antibiotics.
To the Editor:
We read with great interest the article, “Omphalith-Associated Relapsing Umbilical Cellulitis: Recurrent Omphalitis Secondary to a Hair-Containing Belly Button Bezoar” (Cutis. 2010;86:199-202), which introduced the terms omphalotrich and tricomphalith to describe the pilar composition of a hair-containing umbilical foreign body in an 18-year-old man. We report a similar case.
A 38-year-old man presented with a 10-year history of an unusual odor in the umbilical region with recurrent discharge. He diligently maintained proper hygiene of the umbilicus using cotton swabs and had received recurrent cycles of oral antibiotics prescribed by his general practitioner with temporary improvement of the odor and amount of discharge. Physical examination revealed a normal umbilicus with a deep and tight umbilical cleft that required the use of curved mosquito forceps for further examination (Figure 1). A bezoar comprised of a compact collection of terminal hair shafts was noted deep in the umbilicus (Figure 2). A considerable amount of terminal hairs also were noted on the skin of the abdominal area. Following removal of the bezoar, no umbilical fistula was observed, and the presence of embryologic abnormalities (eg, omphalomesenteric duct remnants) was ruled out on magnetic resonance imaging. A diagnosis of recurrent omphalitis secondary to a hair-containing bezoar was made. Following extraction of the bezoar, the odor and discharge promptly resolved, thereby avoiding the need for oral antibiotics; however, a smaller bezoar comprised of a collection of terminal hair shafts was removed 4 months later.

| 
| |
Figure 1. Deep and narrow umbilical cleft with serous exudate in the umbilicus after removal of the foreign body. | Figure 2. A section of the umbilical foreign body composed of a collection of terminal hair shafts. |
An omphalith is an umbilical foreign body that results from the accumulation of keratinous and amorphous sebaceous material.2 Several predisposing factors have been proposed for its pathogenesis, such as the anatomical disposition of the umbilicus and the patient’s hygiene. We hypothesize that a deep umbilicus and a large amount of terminal hairs in the abdominal area were predisposing factors in our patient. Cohen et al1 proposed the terms omphalotrich and trichomphalith to describe the pilar composition of a hair-containing umbilical foreign body that did not have the characteristic stonelike presentation of a traditional omphalith. The authors also referred to the umbilical foreign body in their patient as a trichobezoar, a term used to describe exogenous foreign bodies composed of ingested hair in the gastrointestinal tract, given the embryologic origin of the umbilicus and epithelium of the gastrointestinal tract. We agree that the terms omphalotrich and trichomphalith appropriately describe the current presentation; we also propose the terms omphalitrichia or thricomphalia to describe the findings seen in our patient, which should always be ruled out in patients with recurrent omphalitis that is unresponsive to antibiotics.
1. Cohen PR, Robinson FW, Gray JM. Omphalith-associated relapsing umbilical cellulitis: recurrent omphalitis secondary to a hair-containing belly button bezoar. Cutis. 2010;86:199-202.
2. Swanson SL, Woosley JT, Fleischer AB Jr, et al. Umbilical mass. omphalith. Arch Dermatol. 1992;128:1267, 1270.
1. Cohen PR, Robinson FW, Gray JM. Omphalith-associated relapsing umbilical cellulitis: recurrent omphalitis secondary to a hair-containing belly button bezoar. Cutis. 2010;86:199-202.
2. Swanson SL, Woosley JT, Fleischer AB Jr, et al. Umbilical mass. omphalith. Arch Dermatol. 1992;128:1267, 1270.
Staphylococcal Scalded Skin Syndrome in Pregnancy
To the Editor:
Staphylococcal scalded skin syndrome (SSSS) is a superficial blistering disorder mediated by Staphylococcus aureus exfoliative toxins (ETs).1 It is rare in adults, but when diagnosed, it is often associated with renal failure, immunodeficiency, or overwhelming staphylococcal infection.2 We present a unique case of a pregnant woman with chronic atopic dermatitis (AD) who developed SSSS.
A 21-year-old gravida 3, para 2, aborta 0pregnant woman (29 weeks’ gestation) with a history of chronic AD who was hospitalized with facial edema, purulent ocular discharge, and substantial worsening of AD presented for a dermatology consultation. Her AD was previously managed with topical steroids but had been complicated by multiple methicillin-resistant Staphylococcus aureus (MRSA) infections. On physical examination, she had substantial periorbital edema with purulent discharge from both eyes (Figure 1A), perioral crust with radial fissures (Figure 2A), and mild generalized facial swelling and desquamation (Figure 3). However, the oral cavity was not involved. She had diffuse desquamation in addition to chronic lichenified plaques of the arms, legs, and trunk and SSSS was clinically diagnosed. Cultures of conjunctival discharge were positive for MRSA. The patient was treated with intravenous vancomycin and had a full recovery (Figures 1B and 2B). She delivered a healthy newborn with Apgar scores of 9 and 9 at 1 and 5 minutes, respectively, at 36 weeks and 6 days’ gestation by cesarean delivery; however, her postoperative care was complicated by preeclampsia, which was treated with magnesium sulfate. The newborn showed no evidence of infection or blistering at birth or during the hospital stay.
|
| ||
Figure 1. Periorbital edema with purulent ocular discharge before (A) and after (B) treatment. | Figure 2. Perioral desquamation and radial fissuring before (A) and after (B) treatment. |

Staphylococcal scalded skin syndrome is a superficial blistering disorder that ranges in severity from localized blisters to generalized exfoliation.1 Exfoliative toxin is the major virulence factor responsible for SSSS. Exfoliative toxin is a serine protease that targets desmoglein 1, resulting in intraepidermal separation of keratinocytes.3 Two serologically distinct exfoliative toxins—ETA and ETB—have been associated with human disease.4 Although ETA is encoded on a phage genome, ETB is encoded on a large plasmid.3 Initially it was thought that only strains of S aureus carrying lytic group II phages were responsible for ET production; however, it is now accepted that all phage groups are capable of producing ET and causing SSSS.1
Staphylococcal scalded skin syndrome is most common in infants and children and rare in adults. Although it has been occasionally described in otherwise healthy adults,5 it is most often diagnosed in patients with renal failure (decreased toxin excretion), immunodeficiency (lack of antibodies against toxins), and overwhelming staphylococcal infection (excessive toxin).2 Mortality in treated children is low, but it can reach almost 60% in adults1; therefore, defining risk factors that may aid in early diagnosis are exceedingly important.
We believe that both our patient’s history of AD and her pregnancy contributed to the development of SSSS. The patient had a history of multiple MRSA infections prior to this hospitalization, suggesting MRSA colonization, which is a common complication of AD with more than 75% of AD patients colonized with S aureus.6 Additionally, S aureus superantigen stimulation can result in the loss of regulatory T cells’ natural immunosuppression. Regulatory T cells are remarkably increased in patients with AD; therefore, the inflammatory response to S aureus is likely amplified in an atopic patient, as there is more native immunosuppressive capacity to be affected.4 Furthermore, we believe that pregnancy and its associated immunomodulation is a risk for SSSS. Immune changes in pregnancy are still not well understood; however, it is known that there are alterations to allow symbiosis between the mother and fetus. Anti-ET IgG antibodies are thought to play an important role in protecting against SSSS. Historically, studies on serum immunoglobulin levels during pregnancy have had conflicting findings. They have shown that IgG is either unchanged or decreased, while IgA, IgE, and IgM can be increased, decreased, or unchanged.7 In a study of immunoglobulins in pregnancy, Bahna et al7 showed that IgE is unchanged over the course of pregnancy, but their analysis did not address IgG levels. If IgG levels in fact decrease during pregnancy, the mother could be at risk for SSSS due to her inability to neutralize toxins. Even if total IgG levels remain unchanged, it is possible that specific antitoxin antibodies are decreased. Additionally, there is a documented suppression and alteration in T-cell response to prevent fetal rejection during pregnancy.8 Adult SSSS has been documented several times in human immunodeficiency virus–positive patients, suggesting there may be some association between T-cell suppression and SSSS susceptibility.9 Interestingly, pregnancy, similar to AD, results in an increase in immunosuppressive T cells,10 which, if deactivated by superantigens, could potentially contribute to an increased inflammatory response. All of these immune system alterations likely leave the mother vulnerable to toxin-mediated events such as SSSS.
We believe this case highlights the importance of considering SSSS in both atopic and pregnant patients with desquamating eruptions. In the case of pregnant patients, it is important to consider the risks and benefits of any medical treatments for both the mother and infant. Vancomycin is a pregnancy category B drug and was chosen for its known effectiveness and safety in pregnancy. One study compared 10 babies with mothers who were treated with vancomycin during the second and third trimesters for MRSA to 20 babies with mothers who did not receive vancomycin and did not find an increased risk for sensorineural hearing loss or nephrotoxicity.11 There is no known increased risk for preeclampsia with vancomycin, but some studies have suggested that maternal infection independently increases the risk for preeclampsia.12 Other treatment options were not as safe as vancomycin in this case: doxycycline is contraindicated (pregnancy category D) due to the potential for staining of deciduous teeth and skeletal growth impairment, trimethoprim-sulfamethoxazole is a pregnancy category D drug during the third trimester due to the risk of kernicterus, and linezolid is a pregnancy category C drug.13
1. Ladhani S. Recent developments in staphylococcal scalded skin syndrome. Clin Microbiol Infect. 2001;7:301-307.
2. Ladhani S, Joannou CL, Lochrie DP, et al. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin Microbiol Rev. 1999;12:224-242.
3. Kato F, Kadomoto N, Iwamoto Y, et al. Regulatory mechanism for exfoliative toxin production in Staphylococcus aureus. Infect Immun. 2011;79:1660-1670.
4. Iwatsuki K, Yamasaki O, Morizane S, et al. Staphylococcal cutaneous infections: invasion, evasion and aggression. J Dermatol Sci. 2006;42:203-214.
5. Opal SM, Johnson-Winegar AD, Cross AS. Staphylococcal scalded skin syndrome in two immunocompetent adults caused by exfoliation B-producing Staphylococcus aureus. J Clin Microbiol. 1988;26:1283-1286.
6. Hill SE, Yung A, Rademaker M. Prevalence of Staphylococcus aureus and antibiotic resistance in children with atopic dermatitis: a New Zealand experience. Australas J Dermatol. 2011;52:27-31.
7. Bahna SL, Woo CK, Manuel PV, et al. Serum total IgE level during pregnancy and postpartum. Allergol Immunopathol (Madr). 2011;39:291-294.
8. Poole JA, Claman HN. Immunology of pregnancy: implications for the mother. Clin Rev Allergy Immunol. 2004;26:161-170.
9. Farrell AM, Ross JS, Umasankar S, et al. Staphylococcal scalded skin syndrome in an HIV-1 seropositive man. Br J Dermatol. 1996;134:962-965.
10. Somerset DA, Zheng Y, Kilby MD, et al. Normal human pregnancy is associated with an elevation in the immune suppressive CD251 CD41 regulatory T-cell subset. Immunology. 2004;112:38-43.
11. Reyes MP, Ostrea EM Jr, Carbinian AE, et al. Vancomycin during pregnancy: does it cause hearing loss or nephrotoxicity in the infant? Am J Obstet Gynecol. 1989;161:977-981.
12. Rustveldt LO, Kelsey SF, Sharma, R. Associations between maternal infections and preeclampsia: a systemic review of epidemiologic studies. Matern Child Health J. 2008;12: 223-242.
13. Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Barcelona, Spain: Elsevier Limited; 2008.
To the Editor:
Staphylococcal scalded skin syndrome (SSSS) is a superficial blistering disorder mediated by Staphylococcus aureus exfoliative toxins (ETs).1 It is rare in adults, but when diagnosed, it is often associated with renal failure, immunodeficiency, or overwhelming staphylococcal infection.2 We present a unique case of a pregnant woman with chronic atopic dermatitis (AD) who developed SSSS.
A 21-year-old gravida 3, para 2, aborta 0pregnant woman (29 weeks’ gestation) with a history of chronic AD who was hospitalized with facial edema, purulent ocular discharge, and substantial worsening of AD presented for a dermatology consultation. Her AD was previously managed with topical steroids but had been complicated by multiple methicillin-resistant Staphylococcus aureus (MRSA) infections. On physical examination, she had substantial periorbital edema with purulent discharge from both eyes (Figure 1A), perioral crust with radial fissures (Figure 2A), and mild generalized facial swelling and desquamation (Figure 3). However, the oral cavity was not involved. She had diffuse desquamation in addition to chronic lichenified plaques of the arms, legs, and trunk and SSSS was clinically diagnosed. Cultures of conjunctival discharge were positive for MRSA. The patient was treated with intravenous vancomycin and had a full recovery (Figures 1B and 2B). She delivered a healthy newborn with Apgar scores of 9 and 9 at 1 and 5 minutes, respectively, at 36 weeks and 6 days’ gestation by cesarean delivery; however, her postoperative care was complicated by preeclampsia, which was treated with magnesium sulfate. The newborn showed no evidence of infection or blistering at birth or during the hospital stay.
|
| ||
Figure 1. Periorbital edema with purulent ocular discharge before (A) and after (B) treatment. | Figure 2. Perioral desquamation and radial fissuring before (A) and after (B) treatment. |

Staphylococcal scalded skin syndrome is a superficial blistering disorder that ranges in severity from localized blisters to generalized exfoliation.1 Exfoliative toxin is the major virulence factor responsible for SSSS. Exfoliative toxin is a serine protease that targets desmoglein 1, resulting in intraepidermal separation of keratinocytes.3 Two serologically distinct exfoliative toxins—ETA and ETB—have been associated with human disease.4 Although ETA is encoded on a phage genome, ETB is encoded on a large plasmid.3 Initially it was thought that only strains of S aureus carrying lytic group II phages were responsible for ET production; however, it is now accepted that all phage groups are capable of producing ET and causing SSSS.1
Staphylococcal scalded skin syndrome is most common in infants and children and rare in adults. Although it has been occasionally described in otherwise healthy adults,5 it is most often diagnosed in patients with renal failure (decreased toxin excretion), immunodeficiency (lack of antibodies against toxins), and overwhelming staphylococcal infection (excessive toxin).2 Mortality in treated children is low, but it can reach almost 60% in adults1; therefore, defining risk factors that may aid in early diagnosis are exceedingly important.
We believe that both our patient’s history of AD and her pregnancy contributed to the development of SSSS. The patient had a history of multiple MRSA infections prior to this hospitalization, suggesting MRSA colonization, which is a common complication of AD with more than 75% of AD patients colonized with S aureus.6 Additionally, S aureus superantigen stimulation can result in the loss of regulatory T cells’ natural immunosuppression. Regulatory T cells are remarkably increased in patients with AD; therefore, the inflammatory response to S aureus is likely amplified in an atopic patient, as there is more native immunosuppressive capacity to be affected.4 Furthermore, we believe that pregnancy and its associated immunomodulation is a risk for SSSS. Immune changes in pregnancy are still not well understood; however, it is known that there are alterations to allow symbiosis between the mother and fetus. Anti-ET IgG antibodies are thought to play an important role in protecting against SSSS. Historically, studies on serum immunoglobulin levels during pregnancy have had conflicting findings. They have shown that IgG is either unchanged or decreased, while IgA, IgE, and IgM can be increased, decreased, or unchanged.7 In a study of immunoglobulins in pregnancy, Bahna et al7 showed that IgE is unchanged over the course of pregnancy, but their analysis did not address IgG levels. If IgG levels in fact decrease during pregnancy, the mother could be at risk for SSSS due to her inability to neutralize toxins. Even if total IgG levels remain unchanged, it is possible that specific antitoxin antibodies are decreased. Additionally, there is a documented suppression and alteration in T-cell response to prevent fetal rejection during pregnancy.8 Adult SSSS has been documented several times in human immunodeficiency virus–positive patients, suggesting there may be some association between T-cell suppression and SSSS susceptibility.9 Interestingly, pregnancy, similar to AD, results in an increase in immunosuppressive T cells,10 which, if deactivated by superantigens, could potentially contribute to an increased inflammatory response. All of these immune system alterations likely leave the mother vulnerable to toxin-mediated events such as SSSS.
We believe this case highlights the importance of considering SSSS in both atopic and pregnant patients with desquamating eruptions. In the case of pregnant patients, it is important to consider the risks and benefits of any medical treatments for both the mother and infant. Vancomycin is a pregnancy category B drug and was chosen for its known effectiveness and safety in pregnancy. One study compared 10 babies with mothers who were treated with vancomycin during the second and third trimesters for MRSA to 20 babies with mothers who did not receive vancomycin and did not find an increased risk for sensorineural hearing loss or nephrotoxicity.11 There is no known increased risk for preeclampsia with vancomycin, but some studies have suggested that maternal infection independently increases the risk for preeclampsia.12 Other treatment options were not as safe as vancomycin in this case: doxycycline is contraindicated (pregnancy category D) due to the potential for staining of deciduous teeth and skeletal growth impairment, trimethoprim-sulfamethoxazole is a pregnancy category D drug during the third trimester due to the risk of kernicterus, and linezolid is a pregnancy category C drug.13
To the Editor:
Staphylococcal scalded skin syndrome (SSSS) is a superficial blistering disorder mediated by Staphylococcus aureus exfoliative toxins (ETs).1 It is rare in adults, but when diagnosed, it is often associated with renal failure, immunodeficiency, or overwhelming staphylococcal infection.2 We present a unique case of a pregnant woman with chronic atopic dermatitis (AD) who developed SSSS.
A 21-year-old gravida 3, para 2, aborta 0pregnant woman (29 weeks’ gestation) with a history of chronic AD who was hospitalized with facial edema, purulent ocular discharge, and substantial worsening of AD presented for a dermatology consultation. Her AD was previously managed with topical steroids but had been complicated by multiple methicillin-resistant Staphylococcus aureus (MRSA) infections. On physical examination, she had substantial periorbital edema with purulent discharge from both eyes (Figure 1A), perioral crust with radial fissures (Figure 2A), and mild generalized facial swelling and desquamation (Figure 3). However, the oral cavity was not involved. She had diffuse desquamation in addition to chronic lichenified plaques of the arms, legs, and trunk and SSSS was clinically diagnosed. Cultures of conjunctival discharge were positive for MRSA. The patient was treated with intravenous vancomycin and had a full recovery (Figures 1B and 2B). She delivered a healthy newborn with Apgar scores of 9 and 9 at 1 and 5 minutes, respectively, at 36 weeks and 6 days’ gestation by cesarean delivery; however, her postoperative care was complicated by preeclampsia, which was treated with magnesium sulfate. The newborn showed no evidence of infection or blistering at birth or during the hospital stay.
|
| ||
Figure 1. Periorbital edema with purulent ocular discharge before (A) and after (B) treatment. | Figure 2. Perioral desquamation and radial fissuring before (A) and after (B) treatment. |

Staphylococcal scalded skin syndrome is a superficial blistering disorder that ranges in severity from localized blisters to generalized exfoliation.1 Exfoliative toxin is the major virulence factor responsible for SSSS. Exfoliative toxin is a serine protease that targets desmoglein 1, resulting in intraepidermal separation of keratinocytes.3 Two serologically distinct exfoliative toxins—ETA and ETB—have been associated with human disease.4 Although ETA is encoded on a phage genome, ETB is encoded on a large plasmid.3 Initially it was thought that only strains of S aureus carrying lytic group II phages were responsible for ET production; however, it is now accepted that all phage groups are capable of producing ET and causing SSSS.1
Staphylococcal scalded skin syndrome is most common in infants and children and rare in adults. Although it has been occasionally described in otherwise healthy adults,5 it is most often diagnosed in patients with renal failure (decreased toxin excretion), immunodeficiency (lack of antibodies against toxins), and overwhelming staphylococcal infection (excessive toxin).2 Mortality in treated children is low, but it can reach almost 60% in adults1; therefore, defining risk factors that may aid in early diagnosis are exceedingly important.
We believe that both our patient’s history of AD and her pregnancy contributed to the development of SSSS. The patient had a history of multiple MRSA infections prior to this hospitalization, suggesting MRSA colonization, which is a common complication of AD with more than 75% of AD patients colonized with S aureus.6 Additionally, S aureus superantigen stimulation can result in the loss of regulatory T cells’ natural immunosuppression. Regulatory T cells are remarkably increased in patients with AD; therefore, the inflammatory response to S aureus is likely amplified in an atopic patient, as there is more native immunosuppressive capacity to be affected.4 Furthermore, we believe that pregnancy and its associated immunomodulation is a risk for SSSS. Immune changes in pregnancy are still not well understood; however, it is known that there are alterations to allow symbiosis between the mother and fetus. Anti-ET IgG antibodies are thought to play an important role in protecting against SSSS. Historically, studies on serum immunoglobulin levels during pregnancy have had conflicting findings. They have shown that IgG is either unchanged or decreased, while IgA, IgE, and IgM can be increased, decreased, or unchanged.7 In a study of immunoglobulins in pregnancy, Bahna et al7 showed that IgE is unchanged over the course of pregnancy, but their analysis did not address IgG levels. If IgG levels in fact decrease during pregnancy, the mother could be at risk for SSSS due to her inability to neutralize toxins. Even if total IgG levels remain unchanged, it is possible that specific antitoxin antibodies are decreased. Additionally, there is a documented suppression and alteration in T-cell response to prevent fetal rejection during pregnancy.8 Adult SSSS has been documented several times in human immunodeficiency virus–positive patients, suggesting there may be some association between T-cell suppression and SSSS susceptibility.9 Interestingly, pregnancy, similar to AD, results in an increase in immunosuppressive T cells,10 which, if deactivated by superantigens, could potentially contribute to an increased inflammatory response. All of these immune system alterations likely leave the mother vulnerable to toxin-mediated events such as SSSS.
We believe this case highlights the importance of considering SSSS in both atopic and pregnant patients with desquamating eruptions. In the case of pregnant patients, it is important to consider the risks and benefits of any medical treatments for both the mother and infant. Vancomycin is a pregnancy category B drug and was chosen for its known effectiveness and safety in pregnancy. One study compared 10 babies with mothers who were treated with vancomycin during the second and third trimesters for MRSA to 20 babies with mothers who did not receive vancomycin and did not find an increased risk for sensorineural hearing loss or nephrotoxicity.11 There is no known increased risk for preeclampsia with vancomycin, but some studies have suggested that maternal infection independently increases the risk for preeclampsia.12 Other treatment options were not as safe as vancomycin in this case: doxycycline is contraindicated (pregnancy category D) due to the potential for staining of deciduous teeth and skeletal growth impairment, trimethoprim-sulfamethoxazole is a pregnancy category D drug during the third trimester due to the risk of kernicterus, and linezolid is a pregnancy category C drug.13
1. Ladhani S. Recent developments in staphylococcal scalded skin syndrome. Clin Microbiol Infect. 2001;7:301-307.
2. Ladhani S, Joannou CL, Lochrie DP, et al. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin Microbiol Rev. 1999;12:224-242.
3. Kato F, Kadomoto N, Iwamoto Y, et al. Regulatory mechanism for exfoliative toxin production in Staphylococcus aureus. Infect Immun. 2011;79:1660-1670.
4. Iwatsuki K, Yamasaki O, Morizane S, et al. Staphylococcal cutaneous infections: invasion, evasion and aggression. J Dermatol Sci. 2006;42:203-214.
5. Opal SM, Johnson-Winegar AD, Cross AS. Staphylococcal scalded skin syndrome in two immunocompetent adults caused by exfoliation B-producing Staphylococcus aureus. J Clin Microbiol. 1988;26:1283-1286.
6. Hill SE, Yung A, Rademaker M. Prevalence of Staphylococcus aureus and antibiotic resistance in children with atopic dermatitis: a New Zealand experience. Australas J Dermatol. 2011;52:27-31.
7. Bahna SL, Woo CK, Manuel PV, et al. Serum total IgE level during pregnancy and postpartum. Allergol Immunopathol (Madr). 2011;39:291-294.
8. Poole JA, Claman HN. Immunology of pregnancy: implications for the mother. Clin Rev Allergy Immunol. 2004;26:161-170.
9. Farrell AM, Ross JS, Umasankar S, et al. Staphylococcal scalded skin syndrome in an HIV-1 seropositive man. Br J Dermatol. 1996;134:962-965.
10. Somerset DA, Zheng Y, Kilby MD, et al. Normal human pregnancy is associated with an elevation in the immune suppressive CD251 CD41 regulatory T-cell subset. Immunology. 2004;112:38-43.
11. Reyes MP, Ostrea EM Jr, Carbinian AE, et al. Vancomycin during pregnancy: does it cause hearing loss or nephrotoxicity in the infant? Am J Obstet Gynecol. 1989;161:977-981.
12. Rustveldt LO, Kelsey SF, Sharma, R. Associations between maternal infections and preeclampsia: a systemic review of epidemiologic studies. Matern Child Health J. 2008;12: 223-242.
13. Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Barcelona, Spain: Elsevier Limited; 2008.
1. Ladhani S. Recent developments in staphylococcal scalded skin syndrome. Clin Microbiol Infect. 2001;7:301-307.
2. Ladhani S, Joannou CL, Lochrie DP, et al. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin Microbiol Rev. 1999;12:224-242.
3. Kato F, Kadomoto N, Iwamoto Y, et al. Regulatory mechanism for exfoliative toxin production in Staphylococcus aureus. Infect Immun. 2011;79:1660-1670.
4. Iwatsuki K, Yamasaki O, Morizane S, et al. Staphylococcal cutaneous infections: invasion, evasion and aggression. J Dermatol Sci. 2006;42:203-214.
5. Opal SM, Johnson-Winegar AD, Cross AS. Staphylococcal scalded skin syndrome in two immunocompetent adults caused by exfoliation B-producing Staphylococcus aureus. J Clin Microbiol. 1988;26:1283-1286.
6. Hill SE, Yung A, Rademaker M. Prevalence of Staphylococcus aureus and antibiotic resistance in children with atopic dermatitis: a New Zealand experience. Australas J Dermatol. 2011;52:27-31.
7. Bahna SL, Woo CK, Manuel PV, et al. Serum total IgE level during pregnancy and postpartum. Allergol Immunopathol (Madr). 2011;39:291-294.
8. Poole JA, Claman HN. Immunology of pregnancy: implications for the mother. Clin Rev Allergy Immunol. 2004;26:161-170.
9. Farrell AM, Ross JS, Umasankar S, et al. Staphylococcal scalded skin syndrome in an HIV-1 seropositive man. Br J Dermatol. 1996;134:962-965.
10. Somerset DA, Zheng Y, Kilby MD, et al. Normal human pregnancy is associated with an elevation in the immune suppressive CD251 CD41 regulatory T-cell subset. Immunology. 2004;112:38-43.
11. Reyes MP, Ostrea EM Jr, Carbinian AE, et al. Vancomycin during pregnancy: does it cause hearing loss or nephrotoxicity in the infant? Am J Obstet Gynecol. 1989;161:977-981.
12. Rustveldt LO, Kelsey SF, Sharma, R. Associations between maternal infections and preeclampsia: a systemic review of epidemiologic studies. Matern Child Health J. 2008;12: 223-242.
13. Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Barcelona, Spain: Elsevier Limited; 2008.
Nevus of Ota/Oculodermal Melancytosis: A Rare Report of an Oral Mucosal Lesion Involving the Hard Palate
To the Editor:
Nevus of Ota, also known as oculodermal melanocytosis or nevus fuscoceruleus ophthalmomaxillaris, is a hamartoma of dermal melanocytes that is characterized by a unilateral or bilateral blue-brown, speckled patch usually involving the malar, periorbital, temple, and/or forehead regions of the face.1 It also may affect the sclera, conjunctiva, retinas, corneas, ocular muscles, periosteum, and retrobulbar fat corresponding to the distribution of the ophthalmic (V1) and maxillary (V2) divisions of the trigeminal nerve.
Examination of the oral cavity in the setting of nevus of Ota is imperative, as it can present as a developmental lesion of the oral mucosa.2 Involvement of the hard palate is rare but has been observed.3-5 We present a case of blue-pigmented macules in the upper right periorbital region with involvement of the hard palate that were diagnosed as nevus of Ota.
A 34-year-old Indian man presented with progressive, asymptomatic, ashy blue macules in the upper right periorbital region that had been present since birth. The pigmented macules had gradually increased to cover the infraorbital, maxillary, and temporal regions of the right side of the face with involvement of the conjunctiva and sclera (Figure 1).
Examination of the mucous membrane of the hard palate revealed several blue-pigmented macules with ill-defined borders merging into the surrounding mucosa (Figure 2). Ocular tension was normal and slit-lamp examination of the right eye did not reveal any abnormalities. Hematoxylin and eosin–stained sections prepared from a biopsy of the oral mucosa on the hard palate showed numerous elongated, fusiform, dendritic melanocytes in small aggregates scattered widely between the bundles of collagen in the papillary to midreticular dermis (Figure 3). On histology, the melanocytes stained positive for S100 protein (Figure 4) and human melanoma black 45. No evidence indicative of malignancy was found. The stratified squamous epithelium was unremarkable except for the presence of mild perivascular lymphocytic infiltrate in the subepithelial tissue. A diagnosis of nevus of Ota with involvement of the hard palate was made.
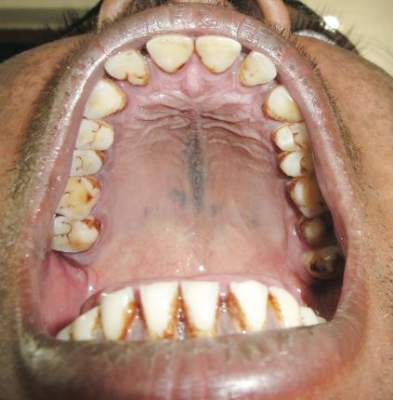
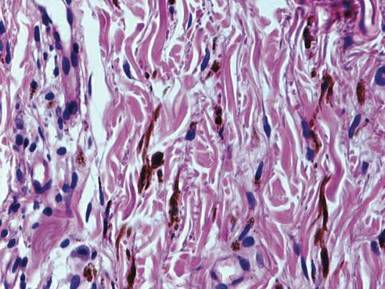
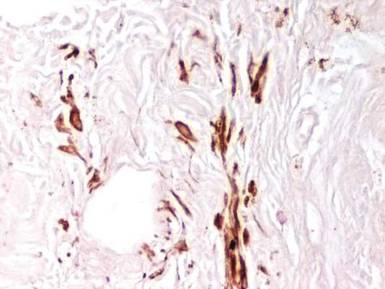
Cutaneous macules may enlarge slowly, become deeper in color, and persist throughout the patient’s life. Its pathogenesis is not known, but it is speculated that nevus of Ota is caused by faulty migration of melanoblasts from the neural crest to the skin. Nevus of Ito also is a dermal melanocytic aberration that exclusively affects the shoulders and often occurs in association with nevus of Ota.1
Ashy or slate-blue pigmentation in individuals with skin of color (eg, Fitzpatrick skin type V) is uncommon, as this discoloration usually is seen in fair-skinned individuals (eg, Fitzpatrick skin type II).6 Occasionally, blue-pigmented lesions of the oral mucosa may be seen in nevus of Ota (as in our patient) and are considered developmental; therefore, examination of the oral cavity is suggested when patients present with blue-pigmented lesions in the facial region. Although this finding is rare, several other cases of blue-pigmented macules on the palatal mucosa have been reported.3-5
The diagnosis of nevus of Ota should be confirmed by histopathology and can be classified into 5 types according to the distribution of melanocytes, including (1) superficial, (2) superficial dominant, (3) diffuse, (4) deep dominant, and (5) deep.7 The diagnosis of nevus of Ota can be made based on its characteristic morphology; however, nevus of Ito, Mongolian spots, melanoma, fixed drug eruptions,8 and lichen planus pigmantosus should also be ruled out.9
Nevus of Ota is a well-established entity that should be considered when ashy or slate-blue pigmentation is noted along the branches of the ophthalmic and maxillary divisions of the trigeminal nerve. Diagnosis is largely clinical, but should be confirmed on histopathology and immunohistochemistry. Possible concomitant involvement of the buccal mucosa and/or the hard palate warrants a thorough examination of the oral cavity in the setting of nevus of Ota to identify oral mucosal lesions. Histopathology is essential to confirm its status as well as to exclude melanoma.
- Ito M. Studies on melanin XXII. Nevus fuscocaeruleus acromio-deltoideus. Tohoku J Exper Med. 1954;60:10.
- Syed NH, Sehgal VN, Aggarwal A, et al. Oral mucosal lesions, institutional study of 200 consecutive patients in dermatologic practice. Int J Dermatol. In press.
- Rathi SK. Bilateral nevus of ota with oral mucosal involvement. Indian J Dermatol Venereol Leprol. 2002;68:104.
- Kannan SK. Oculodermal melanocytosis—nevus of Ota (with palatal pigmentation). Indian J Dent Res. 2003;14: 230-233.
- Shetty SR, Subhas BG, Rao KA, et al. Nevus of Ota with buccal mucosal pigmentation: a rare case. Dent Res J (Isfahan). 2011;8:52-55.
- Fitzpatrick TB, Pathak MA, Parrish JA. Protection of human skin against the effects of the sunburn ultraviolet (290–320 nm). In: Pathak MA, Harber LC, Seiji M, et al, eds. Sunlight and Man: Normal and Abnormal Photobiological Responses. Tokyo, Japan: University of Tokyo Press; 1974:751-765.
- Hirayama T, Suzuki T. A new classification of Ota’s nevus based on histopathological features. Dermatologica. 1991;183:169-172.
- Sehgal VN, Verma P, Bhattacharya SN, et al. Lichen planus pigmentosus. Skinmed. 2013;11:96-103.
- Sehgal VN, Srivastava G. Fixed drug eruption (FDE): changing scenario of incriminating drugs. Int J Dermatol. 2006;45:897-908.
To the Editor:
Nevus of Ota, also known as oculodermal melanocytosis or nevus fuscoceruleus ophthalmomaxillaris, is a hamartoma of dermal melanocytes that is characterized by a unilateral or bilateral blue-brown, speckled patch usually involving the malar, periorbital, temple, and/or forehead regions of the face.1 It also may affect the sclera, conjunctiva, retinas, corneas, ocular muscles, periosteum, and retrobulbar fat corresponding to the distribution of the ophthalmic (V1) and maxillary (V2) divisions of the trigeminal nerve.
Examination of the oral cavity in the setting of nevus of Ota is imperative, as it can present as a developmental lesion of the oral mucosa.2 Involvement of the hard palate is rare but has been observed.3-5 We present a case of blue-pigmented macules in the upper right periorbital region with involvement of the hard palate that were diagnosed as nevus of Ota.
A 34-year-old Indian man presented with progressive, asymptomatic, ashy blue macules in the upper right periorbital region that had been present since birth. The pigmented macules had gradually increased to cover the infraorbital, maxillary, and temporal regions of the right side of the face with involvement of the conjunctiva and sclera (Figure 1).

Examination of the mucous membrane of the hard palate revealed several blue-pigmented macules with ill-defined borders merging into the surrounding mucosa (Figure 2). Ocular tension was normal and slit-lamp examination of the right eye did not reveal any abnormalities. Hematoxylin and eosin–stained sections prepared from a biopsy of the oral mucosa on the hard palate showed numerous elongated, fusiform, dendritic melanocytes in small aggregates scattered widely between the bundles of collagen in the papillary to midreticular dermis (Figure 3). On histology, the melanocytes stained positive for S100 protein (Figure 4) and human melanoma black 45. No evidence indicative of malignancy was found. The stratified squamous epithelium was unremarkable except for the presence of mild perivascular lymphocytic infiltrate in the subepithelial tissue. A diagnosis of nevus of Ota with involvement of the hard palate was made.



Cutaneous macules may enlarge slowly, become deeper in color, and persist throughout the patient’s life. Its pathogenesis is not known, but it is speculated that nevus of Ota is caused by faulty migration of melanoblasts from the neural crest to the skin. Nevus of Ito also is a dermal melanocytic aberration that exclusively affects the shoulders and often occurs in association with nevus of Ota.1
Ashy or slate-blue pigmentation in individuals with skin of color (eg, Fitzpatrick skin type V) is uncommon, as this discoloration usually is seen in fair-skinned individuals (eg, Fitzpatrick skin type II).6 Occasionally, blue-pigmented lesions of the oral mucosa may be seen in nevus of Ota (as in our patient) and are considered developmental; therefore, examination of the oral cavity is suggested when patients present with blue-pigmented lesions in the facial region. Although this finding is rare, several other cases of blue-pigmented macules on the palatal mucosa have been reported.3-5
The diagnosis of nevus of Ota should be confirmed by histopathology and can be classified into 5 types according to the distribution of melanocytes, including (1) superficial, (2) superficial dominant, (3) diffuse, (4) deep dominant, and (5) deep.7 The diagnosis of nevus of Ota can be made based on its characteristic morphology; however, nevus of Ito, Mongolian spots, melanoma, fixed drug eruptions,8 and lichen planus pigmantosus should also be ruled out.9
Nevus of Ota is a well-established entity that should be considered when ashy or slate-blue pigmentation is noted along the branches of the ophthalmic and maxillary divisions of the trigeminal nerve. Diagnosis is largely clinical, but should be confirmed on histopathology and immunohistochemistry. Possible concomitant involvement of the buccal mucosa and/or the hard palate warrants a thorough examination of the oral cavity in the setting of nevus of Ota to identify oral mucosal lesions. Histopathology is essential to confirm its status as well as to exclude melanoma.
To the Editor:
Nevus of Ota, also known as oculodermal melanocytosis or nevus fuscoceruleus ophthalmomaxillaris, is a hamartoma of dermal melanocytes that is characterized by a unilateral or bilateral blue-brown, speckled patch usually involving the malar, periorbital, temple, and/or forehead regions of the face.1 It also may affect the sclera, conjunctiva, retinas, corneas, ocular muscles, periosteum, and retrobulbar fat corresponding to the distribution of the ophthalmic (V1) and maxillary (V2) divisions of the trigeminal nerve.
Examination of the oral cavity in the setting of nevus of Ota is imperative, as it can present as a developmental lesion of the oral mucosa.2 Involvement of the hard palate is rare but has been observed.3-5 We present a case of blue-pigmented macules in the upper right periorbital region with involvement of the hard palate that were diagnosed as nevus of Ota.
A 34-year-old Indian man presented with progressive, asymptomatic, ashy blue macules in the upper right periorbital region that had been present since birth. The pigmented macules had gradually increased to cover the infraorbital, maxillary, and temporal regions of the right side of the face with involvement of the conjunctiva and sclera (Figure 1).

Examination of the mucous membrane of the hard palate revealed several blue-pigmented macules with ill-defined borders merging into the surrounding mucosa (Figure 2). Ocular tension was normal and slit-lamp examination of the right eye did not reveal any abnormalities. Hematoxylin and eosin–stained sections prepared from a biopsy of the oral mucosa on the hard palate showed numerous elongated, fusiform, dendritic melanocytes in small aggregates scattered widely between the bundles of collagen in the papillary to midreticular dermis (Figure 3). On histology, the melanocytes stained positive for S100 protein (Figure 4) and human melanoma black 45. No evidence indicative of malignancy was found. The stratified squamous epithelium was unremarkable except for the presence of mild perivascular lymphocytic infiltrate in the subepithelial tissue. A diagnosis of nevus of Ota with involvement of the hard palate was made.



Cutaneous macules may enlarge slowly, become deeper in color, and persist throughout the patient’s life. Its pathogenesis is not known, but it is speculated that nevus of Ota is caused by faulty migration of melanoblasts from the neural crest to the skin. Nevus of Ito also is a dermal melanocytic aberration that exclusively affects the shoulders and often occurs in association with nevus of Ota.1
Ashy or slate-blue pigmentation in individuals with skin of color (eg, Fitzpatrick skin type V) is uncommon, as this discoloration usually is seen in fair-skinned individuals (eg, Fitzpatrick skin type II).6 Occasionally, blue-pigmented lesions of the oral mucosa may be seen in nevus of Ota (as in our patient) and are considered developmental; therefore, examination of the oral cavity is suggested when patients present with blue-pigmented lesions in the facial region. Although this finding is rare, several other cases of blue-pigmented macules on the palatal mucosa have been reported.3-5
The diagnosis of nevus of Ota should be confirmed by histopathology and can be classified into 5 types according to the distribution of melanocytes, including (1) superficial, (2) superficial dominant, (3) diffuse, (4) deep dominant, and (5) deep.7 The diagnosis of nevus of Ota can be made based on its characteristic morphology; however, nevus of Ito, Mongolian spots, melanoma, fixed drug eruptions,8 and lichen planus pigmantosus should also be ruled out.9
Nevus of Ota is a well-established entity that should be considered when ashy or slate-blue pigmentation is noted along the branches of the ophthalmic and maxillary divisions of the trigeminal nerve. Diagnosis is largely clinical, but should be confirmed on histopathology and immunohistochemistry. Possible concomitant involvement of the buccal mucosa and/or the hard palate warrants a thorough examination of the oral cavity in the setting of nevus of Ota to identify oral mucosal lesions. Histopathology is essential to confirm its status as well as to exclude melanoma.
- Ito M. Studies on melanin XXII. Nevus fuscocaeruleus acromio-deltoideus. Tohoku J Exper Med. 1954;60:10.
- Syed NH, Sehgal VN, Aggarwal A, et al. Oral mucosal lesions, institutional study of 200 consecutive patients in dermatologic practice. Int J Dermatol. In press.
- Rathi SK. Bilateral nevus of ota with oral mucosal involvement. Indian J Dermatol Venereol Leprol. 2002;68:104.
- Kannan SK. Oculodermal melanocytosis—nevus of Ota (with palatal pigmentation). Indian J Dent Res. 2003;14: 230-233.
- Shetty SR, Subhas BG, Rao KA, et al. Nevus of Ota with buccal mucosal pigmentation: a rare case. Dent Res J (Isfahan). 2011;8:52-55.
- Fitzpatrick TB, Pathak MA, Parrish JA. Protection of human skin against the effects of the sunburn ultraviolet (290–320 nm). In: Pathak MA, Harber LC, Seiji M, et al, eds. Sunlight and Man: Normal and Abnormal Photobiological Responses. Tokyo, Japan: University of Tokyo Press; 1974:751-765.
- Hirayama T, Suzuki T. A new classification of Ota’s nevus based on histopathological features. Dermatologica. 1991;183:169-172.
- Sehgal VN, Verma P, Bhattacharya SN, et al. Lichen planus pigmentosus. Skinmed. 2013;11:96-103.
- Sehgal VN, Srivastava G. Fixed drug eruption (FDE): changing scenario of incriminating drugs. Int J Dermatol. 2006;45:897-908.
- Ito M. Studies on melanin XXII. Nevus fuscocaeruleus acromio-deltoideus. Tohoku J Exper Med. 1954;60:10.
- Syed NH, Sehgal VN, Aggarwal A, et al. Oral mucosal lesions, institutional study of 200 consecutive patients in dermatologic practice. Int J Dermatol. In press.
- Rathi SK. Bilateral nevus of ota with oral mucosal involvement. Indian J Dermatol Venereol Leprol. 2002;68:104.
- Kannan SK. Oculodermal melanocytosis—nevus of Ota (with palatal pigmentation). Indian J Dent Res. 2003;14: 230-233.
- Shetty SR, Subhas BG, Rao KA, et al. Nevus of Ota with buccal mucosal pigmentation: a rare case. Dent Res J (Isfahan). 2011;8:52-55.
- Fitzpatrick TB, Pathak MA, Parrish JA. Protection of human skin against the effects of the sunburn ultraviolet (290–320 nm). In: Pathak MA, Harber LC, Seiji M, et al, eds. Sunlight and Man: Normal and Abnormal Photobiological Responses. Tokyo, Japan: University of Tokyo Press; 1974:751-765.
- Hirayama T, Suzuki T. A new classification of Ota’s nevus based on histopathological features. Dermatologica. 1991;183:169-172.
- Sehgal VN, Verma P, Bhattacharya SN, et al. Lichen planus pigmentosus. Skinmed. 2013;11:96-103.
- Sehgal VN, Srivastava G. Fixed drug eruption (FDE): changing scenario of incriminating drugs. Int J Dermatol. 2006;45:897-908.
Occupational Contact Dermatitis From Carbapenems
To the Editor:
Contact sensitivity to drugs that are systemically administered can occur among health care workers.1 We report the case of a 28-year-old nurse who developed eczema on the dorsal aspect of the hand (Figure 1A) and the face (Figure 1B) in the workplace. The nurse was working in the hematology department where she usually handled and administered antibiotics such as imipenem, ertapenem, piperacillin, vancomycin, anidulafungin, teicoplanin, and ciprofloxacin. She was moved to a different department where she did not have contact with the suspicious drugs and the dermatitis completely resolved.
One month after the resolution of the eczema she was referred to our allergy department for an allergological evaluation. A dermatologic evaluation was made and a skin biopsy was performed from a lesional area of the left hand. The patient underwent delayed skin test and patch tests with many β-lactam compounds including penicilloyl polylysine, minor determinant mixture, penicillin G, penicillin V, ampicillin, amoxicillin, bacampicillin, piperacillin, mezlocillin and ticarcillin, imipenem-cilastatin, aztreonam, meropenem, ertapenem, and cephalosporins (eg, cephalexin, cefaclor, cefalotin, cefadroxil, cephradine, cefuroxime, ceftriaxone, cefixime, cefoperazone, cefamandole, ceftazidime, cefotaxime). Undiluted solutions of commercial drugs (parenteral drugs when available were used) were used for skin prick test, and if negative, they were tested intradermally as described by Schiavino et al.2 The concentrations used for the skin test and for the patch test are reported in the Table. Histamine (10 mg/mL) and saline were employed as positive and negative controls, respectively. Immediate reactions of at least 3 mm greater in diameter compared to the control for the skin prick test and 5 mm greater for intradermal tests were considered positive. Immediate-type skin tests were read after 20 minutes and also after 48 hours should any delayed reaction occur. An infiltrated erythema with a diameter greater than 5 mm was considered a delayed positive reaction.
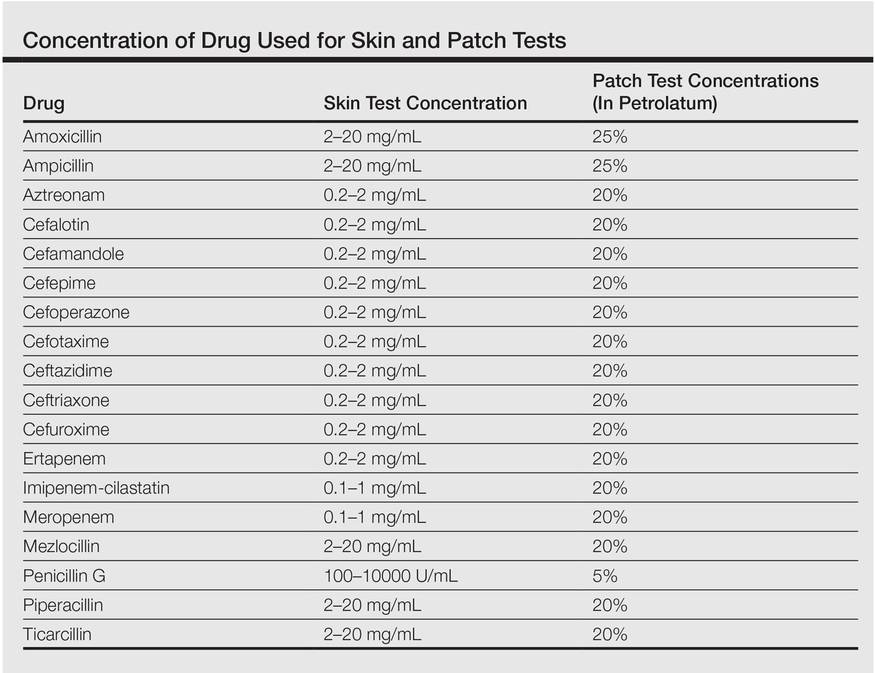
Patch tests were applied to the interscapular region using acrylate adhesive strips with small plates. They were evaluated at 48 and 72 hours. Positivity was assessed according to the indications of the European Network for Drug Allergy.3 Patch tests were carried out using the same drugs as the skin test. All drugs were mixed in petrolatum at 25% wt/wt for ampicillin and amoxicillin, 5% for penicillin G, and 20% for the other drugs as recommended by Schiavino et al.2 We also performed patch tests with ertapenem in 20 healthy controls.
A skin biopsy from lesional skin showed a perivascular infiltrate of the upper dermis with spongiosis of the lesional area similar to eczema. Patch tests and intradermal tests were positive for ertapenem after 48 hours (Figure 2). Imipenem-cilastatin, ampicillin, piperacillin, mezlocillin, and meropenem showed a positive reaction for patch tests. We concluded that the patient had delayed hypersensitivity to carbapenems (ertapenem, imipenem-cilastatin, and meropenem) and semisynthetic penicillins (piperacillin, mezlocillin, and ampicillin).

Drug sensitization in nurses and in health care workers can occur. Natural and semisynthetic penicillin can cause allergic contact dermatitis in health care workers. We report a case of occupational allergy to ertapenem, which is a 1-β-methyl-carbapenem that is administered as a single agent. It is highly active in vitro against bacteria that are generally associated with community-acquired and mixed aerobic and anaerobic infections.4 Occupational contact allergy to other carbapenems such as meropenem also was reported.5 The contact sensitization potential of imipenem has been confirmed in the guinea pig.6 Carbapenems have a bicyclic nucleus composed by a β-lactam ring with an associated 5-membered ring. In our patient, patch tests for ertapenem, imipenem, and meropenem were positive. Although the cross-reactivity between imipenem and penicillin has been demonstrated,2 data on the cross-reactivity between the carbapenems are not strong. Bauer et al7 reported a case of an allergy to imipenem-cilastatin that tolerated treatment with meropenem, but our case showed a complete cross-reactivity between carbapenems. Patch tests for ampicillin, mezlocillin, and piperacillin also were positive; therefore, it can be hypothesized that in our patient, the β-lactam ring was the main epitope recognized by T lymphocytes. Gielen and Goossens1 reported in a study on work-related dermatitis that the most common sensitizers were antibiotics such as penicillins, cephalosporins, and aminoglycosides.
Health care workers should protect their hands with gloves during the preparation of drugs because they have the risk for developing an occupational contact allergy. Detailed allergological and dermatological evaluation is mandatory to confirm or exclude occupational contact allergy.
- Gielen K, Goossens A. Occupational allergic contact dermatitis from drugs in healthcare workers. Contact Dermatitis. 2001;45:273-279.
- Schiavino D, Nucera E, Lombardo C, et al. Cross-reactivity and tolerability of imipenem in patients with delayed-type, cell-mediated hypersensitivity to beta-lactams. Allergy. 2009;64:1644-1648.
- Romano A, Blanca M, Torres MJ, et al. Diagnosis of nonimmediate reactions to beta-lactam antibiotics. Allergy. 2004;59:1153-1160.
- Teppler H, Gesser RM, Friedland IR, et al. Safety and tolerability of ertapenem. J Antimicrob Chemother. 2004;53(suppl 2):75-81.
- Yesudian PD, King CM. Occupational allergic contact dermatitis from meropenem. Contact Dermatitis. 2001;45:53.
- Nagakura N, Souma S, Shimizu T, et al. Comparison of cross-reactivities of imipenem and other beta-lactam antibiotics by delayed-type hypersensitivity reaction in guinea pigs. Chem Pharm Bull. 1991;39:765-768.
- Bauer SL, Wall GC, Skoglund K, et al. Lack of cross-reactivity to meropenem in a patient with an allergy to imipenem-cilastatin. J Allergy Clin Immunol. 2004;113:173-175.
To the Editor:
Contact sensitivity to drugs that are systemically administered can occur among health care workers.1 We report the case of a 28-year-old nurse who developed eczema on the dorsal aspect of the hand (Figure 1A) and the face (Figure 1B) in the workplace. The nurse was working in the hematology department where she usually handled and administered antibiotics such as imipenem, ertapenem, piperacillin, vancomycin, anidulafungin, teicoplanin, and ciprofloxacin. She was moved to a different department where she did not have contact with the suspicious drugs and the dermatitis completely resolved.

One month after the resolution of the eczema she was referred to our allergy department for an allergological evaluation. A dermatologic evaluation was made and a skin biopsy was performed from a lesional area of the left hand. The patient underwent delayed skin test and patch tests with many β-lactam compounds including penicilloyl polylysine, minor determinant mixture, penicillin G, penicillin V, ampicillin, amoxicillin, bacampicillin, piperacillin, mezlocillin and ticarcillin, imipenem-cilastatin, aztreonam, meropenem, ertapenem, and cephalosporins (eg, cephalexin, cefaclor, cefalotin, cefadroxil, cephradine, cefuroxime, ceftriaxone, cefixime, cefoperazone, cefamandole, ceftazidime, cefotaxime). Undiluted solutions of commercial drugs (parenteral drugs when available were used) were used for skin prick test, and if negative, they were tested intradermally as described by Schiavino et al.2 The concentrations used for the skin test and for the patch test are reported in the Table. Histamine (10 mg/mL) and saline were employed as positive and negative controls, respectively. Immediate reactions of at least 3 mm greater in diameter compared to the control for the skin prick test and 5 mm greater for intradermal tests were considered positive. Immediate-type skin tests were read after 20 minutes and also after 48 hours should any delayed reaction occur. An infiltrated erythema with a diameter greater than 5 mm was considered a delayed positive reaction.

Patch tests were applied to the interscapular region using acrylate adhesive strips with small plates. They were evaluated at 48 and 72 hours. Positivity was assessed according to the indications of the European Network for Drug Allergy.3 Patch tests were carried out using the same drugs as the skin test. All drugs were mixed in petrolatum at 25% wt/wt for ampicillin and amoxicillin, 5% for penicillin G, and 20% for the other drugs as recommended by Schiavino et al.2 We also performed patch tests with ertapenem in 20 healthy controls.
A skin biopsy from lesional skin showed a perivascular infiltrate of the upper dermis with spongiosis of the lesional area similar to eczema. Patch tests and intradermal tests were positive for ertapenem after 48 hours (Figure 2). Imipenem-cilastatin, ampicillin, piperacillin, mezlocillin, and meropenem showed a positive reaction for patch tests. We concluded that the patient had delayed hypersensitivity to carbapenems (ertapenem, imipenem-cilastatin, and meropenem) and semisynthetic penicillins (piperacillin, mezlocillin, and ampicillin).

Drug sensitization in nurses and in health care workers can occur. Natural and semisynthetic penicillin can cause allergic contact dermatitis in health care workers. We report a case of occupational allergy to ertapenem, which is a 1-β-methyl-carbapenem that is administered as a single agent. It is highly active in vitro against bacteria that are generally associated with community-acquired and mixed aerobic and anaerobic infections.4 Occupational contact allergy to other carbapenems such as meropenem also was reported.5 The contact sensitization potential of imipenem has been confirmed in the guinea pig.6 Carbapenems have a bicyclic nucleus composed by a β-lactam ring with an associated 5-membered ring. In our patient, patch tests for ertapenem, imipenem, and meropenem were positive. Although the cross-reactivity between imipenem and penicillin has been demonstrated,2 data on the cross-reactivity between the carbapenems are not strong. Bauer et al7 reported a case of an allergy to imipenem-cilastatin that tolerated treatment with meropenem, but our case showed a complete cross-reactivity between carbapenems. Patch tests for ampicillin, mezlocillin, and piperacillin also were positive; therefore, it can be hypothesized that in our patient, the β-lactam ring was the main epitope recognized by T lymphocytes. Gielen and Goossens1 reported in a study on work-related dermatitis that the most common sensitizers were antibiotics such as penicillins, cephalosporins, and aminoglycosides.
Health care workers should protect their hands with gloves during the preparation of drugs because they have the risk for developing an occupational contact allergy. Detailed allergological and dermatological evaluation is mandatory to confirm or exclude occupational contact allergy.
To the Editor:
Contact sensitivity to drugs that are systemically administered can occur among health care workers.1 We report the case of a 28-year-old nurse who developed eczema on the dorsal aspect of the hand (Figure 1A) and the face (Figure 1B) in the workplace. The nurse was working in the hematology department where she usually handled and administered antibiotics such as imipenem, ertapenem, piperacillin, vancomycin, anidulafungin, teicoplanin, and ciprofloxacin. She was moved to a different department where she did not have contact with the suspicious drugs and the dermatitis completely resolved.

One month after the resolution of the eczema she was referred to our allergy department for an allergological evaluation. A dermatologic evaluation was made and a skin biopsy was performed from a lesional area of the left hand. The patient underwent delayed skin test and patch tests with many β-lactam compounds including penicilloyl polylysine, minor determinant mixture, penicillin G, penicillin V, ampicillin, amoxicillin, bacampicillin, piperacillin, mezlocillin and ticarcillin, imipenem-cilastatin, aztreonam, meropenem, ertapenem, and cephalosporins (eg, cephalexin, cefaclor, cefalotin, cefadroxil, cephradine, cefuroxime, ceftriaxone, cefixime, cefoperazone, cefamandole, ceftazidime, cefotaxime). Undiluted solutions of commercial drugs (parenteral drugs when available were used) were used for skin prick test, and if negative, they were tested intradermally as described by Schiavino et al.2 The concentrations used for the skin test and for the patch test are reported in the Table. Histamine (10 mg/mL) and saline were employed as positive and negative controls, respectively. Immediate reactions of at least 3 mm greater in diameter compared to the control for the skin prick test and 5 mm greater for intradermal tests were considered positive. Immediate-type skin tests were read after 20 minutes and also after 48 hours should any delayed reaction occur. An infiltrated erythema with a diameter greater than 5 mm was considered a delayed positive reaction.

Patch tests were applied to the interscapular region using acrylate adhesive strips with small plates. They were evaluated at 48 and 72 hours. Positivity was assessed according to the indications of the European Network for Drug Allergy.3 Patch tests were carried out using the same drugs as the skin test. All drugs were mixed in petrolatum at 25% wt/wt for ampicillin and amoxicillin, 5% for penicillin G, and 20% for the other drugs as recommended by Schiavino et al.2 We also performed patch tests with ertapenem in 20 healthy controls.
A skin biopsy from lesional skin showed a perivascular infiltrate of the upper dermis with spongiosis of the lesional area similar to eczema. Patch tests and intradermal tests were positive for ertapenem after 48 hours (Figure 2). Imipenem-cilastatin, ampicillin, piperacillin, mezlocillin, and meropenem showed a positive reaction for patch tests. We concluded that the patient had delayed hypersensitivity to carbapenems (ertapenem, imipenem-cilastatin, and meropenem) and semisynthetic penicillins (piperacillin, mezlocillin, and ampicillin).

Drug sensitization in nurses and in health care workers can occur. Natural and semisynthetic penicillin can cause allergic contact dermatitis in health care workers. We report a case of occupational allergy to ertapenem, which is a 1-β-methyl-carbapenem that is administered as a single agent. It is highly active in vitro against bacteria that are generally associated with community-acquired and mixed aerobic and anaerobic infections.4 Occupational contact allergy to other carbapenems such as meropenem also was reported.5 The contact sensitization potential of imipenem has been confirmed in the guinea pig.6 Carbapenems have a bicyclic nucleus composed by a β-lactam ring with an associated 5-membered ring. In our patient, patch tests for ertapenem, imipenem, and meropenem were positive. Although the cross-reactivity between imipenem and penicillin has been demonstrated,2 data on the cross-reactivity between the carbapenems are not strong. Bauer et al7 reported a case of an allergy to imipenem-cilastatin that tolerated treatment with meropenem, but our case showed a complete cross-reactivity between carbapenems. Patch tests for ampicillin, mezlocillin, and piperacillin also were positive; therefore, it can be hypothesized that in our patient, the β-lactam ring was the main epitope recognized by T lymphocytes. Gielen and Goossens1 reported in a study on work-related dermatitis that the most common sensitizers were antibiotics such as penicillins, cephalosporins, and aminoglycosides.
Health care workers should protect their hands with gloves during the preparation of drugs because they have the risk for developing an occupational contact allergy. Detailed allergological and dermatological evaluation is mandatory to confirm or exclude occupational contact allergy.
- Gielen K, Goossens A. Occupational allergic contact dermatitis from drugs in healthcare workers. Contact Dermatitis. 2001;45:273-279.
- Schiavino D, Nucera E, Lombardo C, et al. Cross-reactivity and tolerability of imipenem in patients with delayed-type, cell-mediated hypersensitivity to beta-lactams. Allergy. 2009;64:1644-1648.
- Romano A, Blanca M, Torres MJ, et al. Diagnosis of nonimmediate reactions to beta-lactam antibiotics. Allergy. 2004;59:1153-1160.
- Teppler H, Gesser RM, Friedland IR, et al. Safety and tolerability of ertapenem. J Antimicrob Chemother. 2004;53(suppl 2):75-81.
- Yesudian PD, King CM. Occupational allergic contact dermatitis from meropenem. Contact Dermatitis. 2001;45:53.
- Nagakura N, Souma S, Shimizu T, et al. Comparison of cross-reactivities of imipenem and other beta-lactam antibiotics by delayed-type hypersensitivity reaction in guinea pigs. Chem Pharm Bull. 1991;39:765-768.
- Bauer SL, Wall GC, Skoglund K, et al. Lack of cross-reactivity to meropenem in a patient with an allergy to imipenem-cilastatin. J Allergy Clin Immunol. 2004;113:173-175.
- Gielen K, Goossens A. Occupational allergic contact dermatitis from drugs in healthcare workers. Contact Dermatitis. 2001;45:273-279.
- Schiavino D, Nucera E, Lombardo C, et al. Cross-reactivity and tolerability of imipenem in patients with delayed-type, cell-mediated hypersensitivity to beta-lactams. Allergy. 2009;64:1644-1648.
- Romano A, Blanca M, Torres MJ, et al. Diagnosis of nonimmediate reactions to beta-lactam antibiotics. Allergy. 2004;59:1153-1160.
- Teppler H, Gesser RM, Friedland IR, et al. Safety and tolerability of ertapenem. J Antimicrob Chemother. 2004;53(suppl 2):75-81.
- Yesudian PD, King CM. Occupational allergic contact dermatitis from meropenem. Contact Dermatitis. 2001;45:53.
- Nagakura N, Souma S, Shimizu T, et al. Comparison of cross-reactivities of imipenem and other beta-lactam antibiotics by delayed-type hypersensitivity reaction in guinea pigs. Chem Pharm Bull. 1991;39:765-768.
- Bauer SL, Wall GC, Skoglund K, et al. Lack of cross-reactivity to meropenem in a patient with an allergy to imipenem-cilastatin. J Allergy Clin Immunol. 2004;113:173-175.
Extensive Skin Necrosis From Suspected Levamisole-Contaminated Cocaine
To the Editor:
A 52-year-old man presented to the emergency department with skin pain. Although he felt well overall, he reported that he had developed skin sores 3 weeks prior to presentation that were progressively causing skin pain and sleep loss. He acknowledged smoking cigarettes and snorting cocaine but denied intravenous use of cocaine or using any other drugs. His usual medications were lisinopril and tramadol, and he had no known drug allergies. His history was remarkable for methicillin-resistant Staphylococcus aureus (MRSA) septic arthritis of the shoulder and MRSA prepatellar bursitis within the last 2 years. During examination in the emergency department he was alert, afebrile, nontoxic, generally healthy, and in no acute distress. Extensive necrotic skin lesions were present on the trunk, extremities, and both ears. The lesions were large necrotic patches with irregular, sharply angulated borders with thin or ulcerated epidermis surrounded by a bright halo of erythema (Figure 1). Ulcers were noted on the tongue (Figure 2).
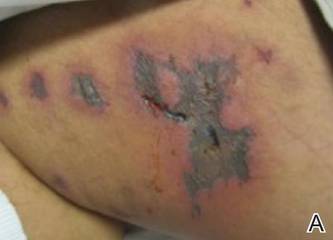
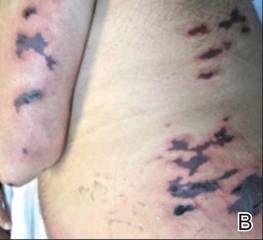

|
| Figure 1. Extensive skin necrosis on the leg from levamisole-contaminated cocaine (A). Necrotic skin lesions also were present on the trunk, arm (B), and ear (C). |
The clinical diagnosis was probable thrombosis of skin vessels with skin necrosis due to cocaine that was likely contaminated with levamisole. Pertinent laboratory results included the following: mild anemia and mild leukopenia; values within reference range for liver function, serum protein electrophoresis, hepatitis profile, human immunodeficiency virus 1 and 2, rapid plasma reagin, and antinuclear antibody; normal thrombotic studies for antithrombin III, protein C, protein S, factor V Leiden, prothrombin mutation G20210A, anticardiolipin IgG, IgM, and IgA; erythrocyte sedimentation rate of 26 mm/h (reference range, 0–15 mm/h); perinuclear antineutrophil cytoplasmic antibody greater than 1:320 (reference range, <1:20) with normal proteinase 3 and myeloperoxidase antibodies; urine positive for cocaine but blood negative for cocaine; normal chest radiograph; and normal electrocardiogram.
The patient was stable with good family support and was discharged from the emergency department to be followed in our dermatology office. The following day his skin biopsies were interpreted as neutrophilic vasculitis with extensive intravascular early and organizing thrombi involving all small- and medium-sized blood vessels consistent with levamisole-induced necrosis or septic vasculitis (Figure 3). With his history of MRSA septic arthritis and bursitis, he was hospitalized for treatment with intravenous vancomycin pending further studies. Skin biopsy for direct immunofluorescence revealed granular deposits of IgM and linear deposits of C3 at the dermoepidermal junction and in blood vessel walls. Two tissue cultures for bacteria and fungi were negative and 2 blood cultures were negative. An echocardiogram was normal and without evidence of emboli. The patient remained stable and antibiotics were discontinued. He was released from the hospital and his skin lesions healed satisfactorily with showering and mupirocin ointment.
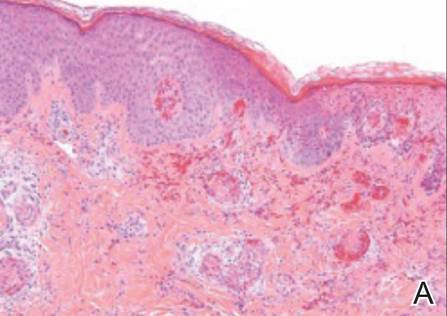
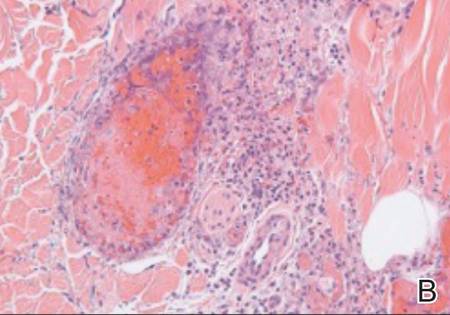
|
| Figure 3. Thrombotic occlusion of blood vessels was seen on histopathology (A and B)(H&E, original magnifications ×100 and ×400).
|
Cocaine is a white powder that is primarily derived from the leaves of the coca plant in South America. It is ingested orally; injected intravenously; snorted intranasally; chewed; eaten; used as a suppository; or dissolved in water and baking soda then heated to crystallization for smoking, which is the most addictive method and known as freebasing. When smoked, crack cocaine produces a crackling sound. Cocaine stimulates the central nervous system similar to amphetamine but may harm any body organ through vasoconstriction/vasospasm and cause skin necrosis without any additive. Perhaps less known is its ability to produce smooth muscle hyperplasia of small vessels and premature atherosclerosis.1
Levamisole has been used to treat worms, cancer, and stimulation of the immune system but currently is used only by veterinarians because of agranulocytosis and vasculitis in humans. As of July 2009, the Drug Enforcement Agency reported that 69% of seized cocaine lots coming into the United States contained levamisole.2 By January 2010, 73.2% of seized cocaine exhibits contained levamisole according to the California Poison Control System, with reports of contamination rates from across the country ranging from 40% to 90%.3 Levamisole is an inexpensive additive to cocaine and may increase the release of brain dopamine.4 It is difficult to detect levamisole in urine due to its short half-life of 5.6 hours and only 2% to 5% of the parent compound being found in the urine.5
Skin necrosis due to cocaine-contaminated levamisole usually occurs in younger individuals who have characteristic skin lesions and a history of cocaine use. Skin lesions usually are multiple, purpuric or necrotic with irregular angulated edges and a halo of erythema. Ear involvement is common but not invariable.6 Descriptive adjectives include branched, netlike, retiform, and stellate, all revealing the compromised underlying dermal and subcutaneous vascular anatomy. Supportive evidence includes a decreased white blood cell count (neutropenia in up to 50%),5 positive antineutrophilic cytoplasmic antibodies,5,7 and/or positive drug screen. Skin biopsy may reveal thrombosis,4 fibrin thrombi without vasculitis,8 or leukocytoclastic vasculitis,4,5 or may suggest septic vasculitis.9 Direct immunofluorescence may suggest an immune complex-mediated vasculitis.5
The differential diagnosis for a patient with purpuric/necrotic skin lesions should be broad and include vasculitis (eg, inflammatory, antineutrophil cytoplasmic antibody positive, septic), hypercoagulopathy (eg, antiphospholipid syndrome, antithrombin III, prothrombin mutation G20210A, factor V Leiden, protein C, protein S), drugs (eg, heparin, warfarin, cocaine with or without levamisole, intravenous drug use, hydroxyurea, ergotamine, propylthiouracil10), calciphylaxis, cold-induced thrombosis, emboli (eg, atheroma, cholesterol, endocarditis, myxoma, aortic angiosarcoma, marantic), febrile ulceronecrotic Mucha-Habermann disease, infection especially if immunosuppressed (eg, disseminated Acanthamoeba/Candida/histoplasmosis/strongyloides/varicella-zoster virus, S aureus, streptococcus, ecthyma gangrenosum, gas gangrene, hemorrhagic smallpox, lues maligna with human immunodeficiency virus, Meleney ulcer, Rocky Mountain spotted fever, Vibrio vulnificus), idiopathic thrombocytopenic purpura, thrombotic thrombocytopenic purpura, thrombocythemia, Waldenström hyperglobulinemic purpura, pyoderma gangrenosum, cancer (eg, paraneoplastic arterial thrombi), oxalosis, paraproteinemia (eg, multiple myeloma), and lupus with generalized coagulopathy. Less likely diagnoses might include Degos disease, factitial dermatitis, foreign bodies, multiple spider bites, paroxysmal nocturnal hemoglobinuria, sickle cell anemia, Buruli ulcer, or thromboangiitis obliterans. Branched, angulated, retiform lesions are an important finding, and some of these diagnostic possibilities are not classically retiform. However, clinical findings are not always classical, and astute physicians want to be circumspect. Had more ominous findings been present in our patient (eg, fever, hemodynamic instability, progressive skin lesions, systemic organ involvement), prompt hospitalization and additional considerations would have been necessary, such as septicemia (eg, meningococcemia, bubonic plague [Black Death], necrotizing fasciitis, purpura fulminans), catastrophic antiphospholipid syndrome, or disseminated intra- vascular coagulation.
The prognosis for skin necrosis caused by levamisole-contaminated cocaine generally is good without long-term sequelae.5 Autoantibody serologies normalize within weeks to months after stopping levamisole.5,8 Our patient recovered with conservative measures.
1. Dhawan SS, Wang BW. Four-extremity gangrene associated with crack cocaine abuse [published online ahead of print October 23, 2006]. Ann Emerg Med. 2007;49:186-189.
2. Centers for Disease Control and Prevention. Agranulocytosis associated with cocaine use—four states, March 2008–November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
3. Buchanan J; California Poison Control System. Levamisole-contaminated cocaine. Call Us… December 3, 2014. http://www.calpoison.org/hcp/2014/ callusvol12no3.htm. Accessed September 1, 2015.
4. Mouzakis J, Somboonwit C, Lakshmi S, et al. Levamisole induced necrosis of the skin and neutropenia following intranasal cocaine use: a newly recognized syndrome. J Drugs Dermatology. 2011;10:1204-1207.
5. Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine [published online ahead of print June 11, 2011]. J Am Acad Dermatol. 2011;65:722-725.
6. Farhat EK, Muirhead TT, Chaffins ML, et al. Levamisole-induced cutaneous necrosis mimicking coagulopathy. Arch Dermatol. 2010;46:1320-1321.
7. Geller L, Whang TB, Mercer SE. Retiform purpura: a new stigmata of illicit drug use? Dermatol Online J. 2011;17:7.
8. Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit [published online ahead of print March 20, 2010]? J Am Acad Dermatol. 2010;63:530-535.
9. Reutemann P, Grenier N, Telang GH. Occlusive vasculopathy with vascular and skin necrosis secondary to smoking crack cocaine. J Am Acad Dermatol. 2011;64:1004-1006.
10. Mahmood T, Delacerda A, Fiala K. Painful purpura on bilateral helices. JAMA Dermatol. 2015;151:551-552.
To the Editor:
A 52-year-old man presented to the emergency department with skin pain. Although he felt well overall, he reported that he had developed skin sores 3 weeks prior to presentation that were progressively causing skin pain and sleep loss. He acknowledged smoking cigarettes and snorting cocaine but denied intravenous use of cocaine or using any other drugs. His usual medications were lisinopril and tramadol, and he had no known drug allergies. His history was remarkable for methicillin-resistant Staphylococcus aureus (MRSA) septic arthritis of the shoulder and MRSA prepatellar bursitis within the last 2 years. During examination in the emergency department he was alert, afebrile, nontoxic, generally healthy, and in no acute distress. Extensive necrotic skin lesions were present on the trunk, extremities, and both ears. The lesions were large necrotic patches with irregular, sharply angulated borders with thin or ulcerated epidermis surrounded by a bright halo of erythema (Figure 1). Ulcers were noted on the tongue (Figure 2).



|
| Figure 1. Extensive skin necrosis on the leg from levamisole-contaminated cocaine (A). Necrotic skin lesions also were present on the trunk, arm (B), and ear (C). |
The clinical diagnosis was probable thrombosis of skin vessels with skin necrosis due to cocaine that was likely contaminated with levamisole. Pertinent laboratory results included the following: mild anemia and mild leukopenia; values within reference range for liver function, serum protein electrophoresis, hepatitis profile, human immunodeficiency virus 1 and 2, rapid plasma reagin, and antinuclear antibody; normal thrombotic studies for antithrombin III, protein C, protein S, factor V Leiden, prothrombin mutation G20210A, anticardiolipin IgG, IgM, and IgA; erythrocyte sedimentation rate of 26 mm/h (reference range, 0–15 mm/h); perinuclear antineutrophil cytoplasmic antibody greater than 1:320 (reference range, <1:20) with normal proteinase 3 and myeloperoxidase antibodies; urine positive for cocaine but blood negative for cocaine; normal chest radiograph; and normal electrocardiogram.
The patient was stable with good family support and was discharged from the emergency department to be followed in our dermatology office. The following day his skin biopsies were interpreted as neutrophilic vasculitis with extensive intravascular early and organizing thrombi involving all small- and medium-sized blood vessels consistent with levamisole-induced necrosis or septic vasculitis (Figure 3). With his history of MRSA septic arthritis and bursitis, he was hospitalized for treatment with intravenous vancomycin pending further studies. Skin biopsy for direct immunofluorescence revealed granular deposits of IgM and linear deposits of C3 at the dermoepidermal junction and in blood vessel walls. Two tissue cultures for bacteria and fungi were negative and 2 blood cultures were negative. An echocardiogram was normal and without evidence of emboli. The patient remained stable and antibiotics were discontinued. He was released from the hospital and his skin lesions healed satisfactorily with showering and mupirocin ointment.


|
| Figure 3. Thrombotic occlusion of blood vessels was seen on histopathology (A and B)(H&E, original magnifications ×100 and ×400).
|
Cocaine is a white powder that is primarily derived from the leaves of the coca plant in South America. It is ingested orally; injected intravenously; snorted intranasally; chewed; eaten; used as a suppository; or dissolved in water and baking soda then heated to crystallization for smoking, which is the most addictive method and known as freebasing. When smoked, crack cocaine produces a crackling sound. Cocaine stimulates the central nervous system similar to amphetamine but may harm any body organ through vasoconstriction/vasospasm and cause skin necrosis without any additive. Perhaps less known is its ability to produce smooth muscle hyperplasia of small vessels and premature atherosclerosis.1
Levamisole has been used to treat worms, cancer, and stimulation of the immune system but currently is used only by veterinarians because of agranulocytosis and vasculitis in humans. As of July 2009, the Drug Enforcement Agency reported that 69% of seized cocaine lots coming into the United States contained levamisole.2 By January 2010, 73.2% of seized cocaine exhibits contained levamisole according to the California Poison Control System, with reports of contamination rates from across the country ranging from 40% to 90%.3 Levamisole is an inexpensive additive to cocaine and may increase the release of brain dopamine.4 It is difficult to detect levamisole in urine due to its short half-life of 5.6 hours and only 2% to 5% of the parent compound being found in the urine.5
Skin necrosis due to cocaine-contaminated levamisole usually occurs in younger individuals who have characteristic skin lesions and a history of cocaine use. Skin lesions usually are multiple, purpuric or necrotic with irregular angulated edges and a halo of erythema. Ear involvement is common but not invariable.6 Descriptive adjectives include branched, netlike, retiform, and stellate, all revealing the compromised underlying dermal and subcutaneous vascular anatomy. Supportive evidence includes a decreased white blood cell count (neutropenia in up to 50%),5 positive antineutrophilic cytoplasmic antibodies,5,7 and/or positive drug screen. Skin biopsy may reveal thrombosis,4 fibrin thrombi without vasculitis,8 or leukocytoclastic vasculitis,4,5 or may suggest septic vasculitis.9 Direct immunofluorescence may suggest an immune complex-mediated vasculitis.5
The differential diagnosis for a patient with purpuric/necrotic skin lesions should be broad and include vasculitis (eg, inflammatory, antineutrophil cytoplasmic antibody positive, septic), hypercoagulopathy (eg, antiphospholipid syndrome, antithrombin III, prothrombin mutation G20210A, factor V Leiden, protein C, protein S), drugs (eg, heparin, warfarin, cocaine with or without levamisole, intravenous drug use, hydroxyurea, ergotamine, propylthiouracil10), calciphylaxis, cold-induced thrombosis, emboli (eg, atheroma, cholesterol, endocarditis, myxoma, aortic angiosarcoma, marantic), febrile ulceronecrotic Mucha-Habermann disease, infection especially if immunosuppressed (eg, disseminated Acanthamoeba/Candida/histoplasmosis/strongyloides/varicella-zoster virus, S aureus, streptococcus, ecthyma gangrenosum, gas gangrene, hemorrhagic smallpox, lues maligna with human immunodeficiency virus, Meleney ulcer, Rocky Mountain spotted fever, Vibrio vulnificus), idiopathic thrombocytopenic purpura, thrombotic thrombocytopenic purpura, thrombocythemia, Waldenström hyperglobulinemic purpura, pyoderma gangrenosum, cancer (eg, paraneoplastic arterial thrombi), oxalosis, paraproteinemia (eg, multiple myeloma), and lupus with generalized coagulopathy. Less likely diagnoses might include Degos disease, factitial dermatitis, foreign bodies, multiple spider bites, paroxysmal nocturnal hemoglobinuria, sickle cell anemia, Buruli ulcer, or thromboangiitis obliterans. Branched, angulated, retiform lesions are an important finding, and some of these diagnostic possibilities are not classically retiform. However, clinical findings are not always classical, and astute physicians want to be circumspect. Had more ominous findings been present in our patient (eg, fever, hemodynamic instability, progressive skin lesions, systemic organ involvement), prompt hospitalization and additional considerations would have been necessary, such as septicemia (eg, meningococcemia, bubonic plague [Black Death], necrotizing fasciitis, purpura fulminans), catastrophic antiphospholipid syndrome, or disseminated intra- vascular coagulation.
The prognosis for skin necrosis caused by levamisole-contaminated cocaine generally is good without long-term sequelae.5 Autoantibody serologies normalize within weeks to months after stopping levamisole.5,8 Our patient recovered with conservative measures.
To the Editor:
A 52-year-old man presented to the emergency department with skin pain. Although he felt well overall, he reported that he had developed skin sores 3 weeks prior to presentation that were progressively causing skin pain and sleep loss. He acknowledged smoking cigarettes and snorting cocaine but denied intravenous use of cocaine or using any other drugs. His usual medications were lisinopril and tramadol, and he had no known drug allergies. His history was remarkable for methicillin-resistant Staphylococcus aureus (MRSA) septic arthritis of the shoulder and MRSA prepatellar bursitis within the last 2 years. During examination in the emergency department he was alert, afebrile, nontoxic, generally healthy, and in no acute distress. Extensive necrotic skin lesions were present on the trunk, extremities, and both ears. The lesions were large necrotic patches with irregular, sharply angulated borders with thin or ulcerated epidermis surrounded by a bright halo of erythema (Figure 1). Ulcers were noted on the tongue (Figure 2).



|
| Figure 1. Extensive skin necrosis on the leg from levamisole-contaminated cocaine (A). Necrotic skin lesions also were present on the trunk, arm (B), and ear (C). |
The clinical diagnosis was probable thrombosis of skin vessels with skin necrosis due to cocaine that was likely contaminated with levamisole. Pertinent laboratory results included the following: mild anemia and mild leukopenia; values within reference range for liver function, serum protein electrophoresis, hepatitis profile, human immunodeficiency virus 1 and 2, rapid plasma reagin, and antinuclear antibody; normal thrombotic studies for antithrombin III, protein C, protein S, factor V Leiden, prothrombin mutation G20210A, anticardiolipin IgG, IgM, and IgA; erythrocyte sedimentation rate of 26 mm/h (reference range, 0–15 mm/h); perinuclear antineutrophil cytoplasmic antibody greater than 1:320 (reference range, <1:20) with normal proteinase 3 and myeloperoxidase antibodies; urine positive for cocaine but blood negative for cocaine; normal chest radiograph; and normal electrocardiogram.
The patient was stable with good family support and was discharged from the emergency department to be followed in our dermatology office. The following day his skin biopsies were interpreted as neutrophilic vasculitis with extensive intravascular early and organizing thrombi involving all small- and medium-sized blood vessels consistent with levamisole-induced necrosis or septic vasculitis (Figure 3). With his history of MRSA septic arthritis and bursitis, he was hospitalized for treatment with intravenous vancomycin pending further studies. Skin biopsy for direct immunofluorescence revealed granular deposits of IgM and linear deposits of C3 at the dermoepidermal junction and in blood vessel walls. Two tissue cultures for bacteria and fungi were negative and 2 blood cultures were negative. An echocardiogram was normal and without evidence of emboli. The patient remained stable and antibiotics were discontinued. He was released from the hospital and his skin lesions healed satisfactorily with showering and mupirocin ointment.


|
| Figure 3. Thrombotic occlusion of blood vessels was seen on histopathology (A and B)(H&E, original magnifications ×100 and ×400).
|
Cocaine is a white powder that is primarily derived from the leaves of the coca plant in South America. It is ingested orally; injected intravenously; snorted intranasally; chewed; eaten; used as a suppository; or dissolved in water and baking soda then heated to crystallization for smoking, which is the most addictive method and known as freebasing. When smoked, crack cocaine produces a crackling sound. Cocaine stimulates the central nervous system similar to amphetamine but may harm any body organ through vasoconstriction/vasospasm and cause skin necrosis without any additive. Perhaps less known is its ability to produce smooth muscle hyperplasia of small vessels and premature atherosclerosis.1
Levamisole has been used to treat worms, cancer, and stimulation of the immune system but currently is used only by veterinarians because of agranulocytosis and vasculitis in humans. As of July 2009, the Drug Enforcement Agency reported that 69% of seized cocaine lots coming into the United States contained levamisole.2 By January 2010, 73.2% of seized cocaine exhibits contained levamisole according to the California Poison Control System, with reports of contamination rates from across the country ranging from 40% to 90%.3 Levamisole is an inexpensive additive to cocaine and may increase the release of brain dopamine.4 It is difficult to detect levamisole in urine due to its short half-life of 5.6 hours and only 2% to 5% of the parent compound being found in the urine.5
Skin necrosis due to cocaine-contaminated levamisole usually occurs in younger individuals who have characteristic skin lesions and a history of cocaine use. Skin lesions usually are multiple, purpuric or necrotic with irregular angulated edges and a halo of erythema. Ear involvement is common but not invariable.6 Descriptive adjectives include branched, netlike, retiform, and stellate, all revealing the compromised underlying dermal and subcutaneous vascular anatomy. Supportive evidence includes a decreased white blood cell count (neutropenia in up to 50%),5 positive antineutrophilic cytoplasmic antibodies,5,7 and/or positive drug screen. Skin biopsy may reveal thrombosis,4 fibrin thrombi without vasculitis,8 or leukocytoclastic vasculitis,4,5 or may suggest septic vasculitis.9 Direct immunofluorescence may suggest an immune complex-mediated vasculitis.5
The differential diagnosis for a patient with purpuric/necrotic skin lesions should be broad and include vasculitis (eg, inflammatory, antineutrophil cytoplasmic antibody positive, septic), hypercoagulopathy (eg, antiphospholipid syndrome, antithrombin III, prothrombin mutation G20210A, factor V Leiden, protein C, protein S), drugs (eg, heparin, warfarin, cocaine with or without levamisole, intravenous drug use, hydroxyurea, ergotamine, propylthiouracil10), calciphylaxis, cold-induced thrombosis, emboli (eg, atheroma, cholesterol, endocarditis, myxoma, aortic angiosarcoma, marantic), febrile ulceronecrotic Mucha-Habermann disease, infection especially if immunosuppressed (eg, disseminated Acanthamoeba/Candida/histoplasmosis/strongyloides/varicella-zoster virus, S aureus, streptococcus, ecthyma gangrenosum, gas gangrene, hemorrhagic smallpox, lues maligna with human immunodeficiency virus, Meleney ulcer, Rocky Mountain spotted fever, Vibrio vulnificus), idiopathic thrombocytopenic purpura, thrombotic thrombocytopenic purpura, thrombocythemia, Waldenström hyperglobulinemic purpura, pyoderma gangrenosum, cancer (eg, paraneoplastic arterial thrombi), oxalosis, paraproteinemia (eg, multiple myeloma), and lupus with generalized coagulopathy. Less likely diagnoses might include Degos disease, factitial dermatitis, foreign bodies, multiple spider bites, paroxysmal nocturnal hemoglobinuria, sickle cell anemia, Buruli ulcer, or thromboangiitis obliterans. Branched, angulated, retiform lesions are an important finding, and some of these diagnostic possibilities are not classically retiform. However, clinical findings are not always classical, and astute physicians want to be circumspect. Had more ominous findings been present in our patient (eg, fever, hemodynamic instability, progressive skin lesions, systemic organ involvement), prompt hospitalization and additional considerations would have been necessary, such as septicemia (eg, meningococcemia, bubonic plague [Black Death], necrotizing fasciitis, purpura fulminans), catastrophic antiphospholipid syndrome, or disseminated intra- vascular coagulation.
The prognosis for skin necrosis caused by levamisole-contaminated cocaine generally is good without long-term sequelae.5 Autoantibody serologies normalize within weeks to months after stopping levamisole.5,8 Our patient recovered with conservative measures.
1. Dhawan SS, Wang BW. Four-extremity gangrene associated with crack cocaine abuse [published online ahead of print October 23, 2006]. Ann Emerg Med. 2007;49:186-189.
2. Centers for Disease Control and Prevention. Agranulocytosis associated with cocaine use—four states, March 2008–November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
3. Buchanan J; California Poison Control System. Levamisole-contaminated cocaine. Call Us… December 3, 2014. http://www.calpoison.org/hcp/2014/ callusvol12no3.htm. Accessed September 1, 2015.
4. Mouzakis J, Somboonwit C, Lakshmi S, et al. Levamisole induced necrosis of the skin and neutropenia following intranasal cocaine use: a newly recognized syndrome. J Drugs Dermatology. 2011;10:1204-1207.
5. Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine [published online ahead of print June 11, 2011]. J Am Acad Dermatol. 2011;65:722-725.
6. Farhat EK, Muirhead TT, Chaffins ML, et al. Levamisole-induced cutaneous necrosis mimicking coagulopathy. Arch Dermatol. 2010;46:1320-1321.
7. Geller L, Whang TB, Mercer SE. Retiform purpura: a new stigmata of illicit drug use? Dermatol Online J. 2011;17:7.
8. Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit [published online ahead of print March 20, 2010]? J Am Acad Dermatol. 2010;63:530-535.
9. Reutemann P, Grenier N, Telang GH. Occlusive vasculopathy with vascular and skin necrosis secondary to smoking crack cocaine. J Am Acad Dermatol. 2011;64:1004-1006.
10. Mahmood T, Delacerda A, Fiala K. Painful purpura on bilateral helices. JAMA Dermatol. 2015;151:551-552.
1. Dhawan SS, Wang BW. Four-extremity gangrene associated with crack cocaine abuse [published online ahead of print October 23, 2006]. Ann Emerg Med. 2007;49:186-189.
2. Centers for Disease Control and Prevention. Agranulocytosis associated with cocaine use—four states, March 2008–November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
3. Buchanan J; California Poison Control System. Levamisole-contaminated cocaine. Call Us… December 3, 2014. http://www.calpoison.org/hcp/2014/ callusvol12no3.htm. Accessed September 1, 2015.
4. Mouzakis J, Somboonwit C, Lakshmi S, et al. Levamisole induced necrosis of the skin and neutropenia following intranasal cocaine use: a newly recognized syndrome. J Drugs Dermatology. 2011;10:1204-1207.
5. Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine [published online ahead of print June 11, 2011]. J Am Acad Dermatol. 2011;65:722-725.
6. Farhat EK, Muirhead TT, Chaffins ML, et al. Levamisole-induced cutaneous necrosis mimicking coagulopathy. Arch Dermatol. 2010;46:1320-1321.
7. Geller L, Whang TB, Mercer SE. Retiform purpura: a new stigmata of illicit drug use? Dermatol Online J. 2011;17:7.
8. Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit [published online ahead of print March 20, 2010]? J Am Acad Dermatol. 2010;63:530-535.
9. Reutemann P, Grenier N, Telang GH. Occlusive vasculopathy with vascular and skin necrosis secondary to smoking crack cocaine. J Am Acad Dermatol. 2011;64:1004-1006.
10. Mahmood T, Delacerda A, Fiala K. Painful purpura on bilateral helices. JAMA Dermatol. 2015;151:551-552.
Characterization of Knuckle (Garrod) Pads Using Optical Coherence Tomography In Vivo
To the Editor:
Optical coherence tomography (OCT) is a noninvasive imaging technique that uses a low-power infrared laser light for cutaneous architecture visualization up to 2 mm in depth. Both malignant and nonmalignant lesions on OCT imaging have been correlated with histopathologic analysis.1 We describe the diagnostic features of knuckle pads on OCT.
A 43-year-old-man presented with warts on the right thumb and bilateral feet of several months’ duration with noncontributory medical and social history. Physical examination revealed nontender, well-demarcated, flesh-colored, verrucous papules on the dorsal interphalangeal joints of the right thumb and several toes (Figure 1). Pinpoint vessels were absent on dermoscopy (Figure 2). Histopathologic analysis of a shave biopsy of the lesion on the left second toe revealed dense orthokeratosis with compact keratin, suggestive of reactive hyperkeratosis or a knuckle pad (Figure 3). In situ hybridization failed to demonstrate staining for human papillomavirus types 6, 11, and 16.

| 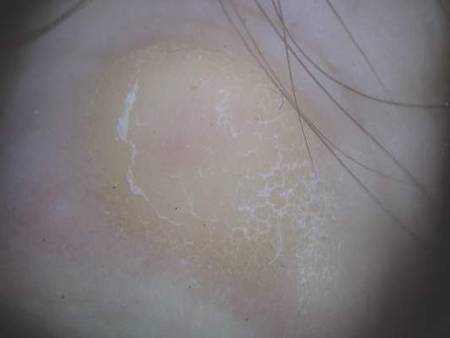
| |
| Figure 1. Verrucous papules on the left foot.
| Figure 2. Corresponding dermoscopy of the left second toe revealed an absence of pinpoint vessels. |
Optical coherence tomography demonstrated discrete thickening of the stratum corneum with distinctive granular and coarse textural appearance of the hyperkeratotic stratum corneum compared to normal adjacent skin. This textural difference was attributed to the alteration in collagen deposition of the knuckle pads, consistent with fibrous proliferation. Finally, OCT imaging provided further characterization of the lesion demonstrating the absence of any hair follicles and acrosyringium in areas resembling glabrous skin (Figure 4).
Knuckle pads, also known as Garrod pads, were first described by Garrod2 in 1893. They are benign, asymptomatic, fibrotic thickenings of the skin. Lesions are smooth, firm, flesh colored, and located on the dorsal aspect of the hands and feet along the metacarpophalangeal and interphalangeal joints. Knuckle pads are common, can develop at any age, and are observed more frequently in men than in women.3
Primary knuckle pads can be sporadic or associated with other conditions such as palmoplantar keratoderma, acrokeratoelastoidosis costa, fibrosing disorders, or Bart-Pumphrey syndrome.4 Secondary knuckle pads, which are more common, occur in sites of repetitive trauma or pressure. Certain occupations (eg, mechanics) or hobbies (eg, boxing) increase the risk for developing knuckle pads.3,4
The diagnosis of knuckle pads is usually made clinically, though several other conditions mimic knuckle pads, including scars, keloids, calluses, verruca vulgaris, fibromas, and rheumatoid nodules.3,5 We report a description of knuckle pads that was diagnosed with OCT imaging. Further characterization of both malignant and nonmalignant lesions on OCT imaging will contribute new insights to the role of OCT in the noninvasive diagnosis of skin diseases, pending future studies.
1. Forsea AM, Carstea EM, Ghervase L, et al. Clinical application of optical coherence tomography for the imaging of non-melanocytic cutaneous tumors: a pilot multi-modal study. J Med Life. 2010;3:381-389.
2. Garrod AE. On an unusual form of nodule upon joints of the fingers. St Bartholomew’s Hosp Rep. 1893;29:157-161.
3. Kodama BF, Gentry RH, Fitzpatrick JE. Papules and plaques over the joint spaces. knuckle pads (heloderma). Arch Dermatol. 1993;129:1044-1045, 1047.
4. Nenoff P, Woitek G. Images in clinical medicine. knuckle pads. N Engl J Med. 2011;364:2451.
5. Sehgal VN, Singh M, Saxena HM, et al. Primary knuckle pads. Clin Exp Dermatol. 1979;4:337-339.
To the Editor:
Optical coherence tomography (OCT) is a noninvasive imaging technique that uses a low-power infrared laser light for cutaneous architecture visualization up to 2 mm in depth. Both malignant and nonmalignant lesions on OCT imaging have been correlated with histopathologic analysis.1 We describe the diagnostic features of knuckle pads on OCT.
A 43-year-old-man presented with warts on the right thumb and bilateral feet of several months’ duration with noncontributory medical and social history. Physical examination revealed nontender, well-demarcated, flesh-colored, verrucous papules on the dorsal interphalangeal joints of the right thumb and several toes (Figure 1). Pinpoint vessels were absent on dermoscopy (Figure 2). Histopathologic analysis of a shave biopsy of the lesion on the left second toe revealed dense orthokeratosis with compact keratin, suggestive of reactive hyperkeratosis or a knuckle pad (Figure 3). In situ hybridization failed to demonstrate staining for human papillomavirus types 6, 11, and 16.

| 
| |
| Figure 1. Verrucous papules on the left foot.
| Figure 2. Corresponding dermoscopy of the left second toe revealed an absence of pinpoint vessels. |
Optical coherence tomography demonstrated discrete thickening of the stratum corneum with distinctive granular and coarse textural appearance of the hyperkeratotic stratum corneum compared to normal adjacent skin. This textural difference was attributed to the alteration in collagen deposition of the knuckle pads, consistent with fibrous proliferation. Finally, OCT imaging provided further characterization of the lesion demonstrating the absence of any hair follicles and acrosyringium in areas resembling glabrous skin (Figure 4).
Knuckle pads, also known as Garrod pads, were first described by Garrod2 in 1893. They are benign, asymptomatic, fibrotic thickenings of the skin. Lesions are smooth, firm, flesh colored, and located on the dorsal aspect of the hands and feet along the metacarpophalangeal and interphalangeal joints. Knuckle pads are common, can develop at any age, and are observed more frequently in men than in women.3
Primary knuckle pads can be sporadic or associated with other conditions such as palmoplantar keratoderma, acrokeratoelastoidosis costa, fibrosing disorders, or Bart-Pumphrey syndrome.4 Secondary knuckle pads, which are more common, occur in sites of repetitive trauma or pressure. Certain occupations (eg, mechanics) or hobbies (eg, boxing) increase the risk for developing knuckle pads.3,4
The diagnosis of knuckle pads is usually made clinically, though several other conditions mimic knuckle pads, including scars, keloids, calluses, verruca vulgaris, fibromas, and rheumatoid nodules.3,5 We report a description of knuckle pads that was diagnosed with OCT imaging. Further characterization of both malignant and nonmalignant lesions on OCT imaging will contribute new insights to the role of OCT in the noninvasive diagnosis of skin diseases, pending future studies.
To the Editor:
Optical coherence tomography (OCT) is a noninvasive imaging technique that uses a low-power infrared laser light for cutaneous architecture visualization up to 2 mm in depth. Both malignant and nonmalignant lesions on OCT imaging have been correlated with histopathologic analysis.1 We describe the diagnostic features of knuckle pads on OCT.
A 43-year-old-man presented with warts on the right thumb and bilateral feet of several months’ duration with noncontributory medical and social history. Physical examination revealed nontender, well-demarcated, flesh-colored, verrucous papules on the dorsal interphalangeal joints of the right thumb and several toes (Figure 1). Pinpoint vessels were absent on dermoscopy (Figure 2). Histopathologic analysis of a shave biopsy of the lesion on the left second toe revealed dense orthokeratosis with compact keratin, suggestive of reactive hyperkeratosis or a knuckle pad (Figure 3). In situ hybridization failed to demonstrate staining for human papillomavirus types 6, 11, and 16.

| 
| |
| Figure 1. Verrucous papules on the left foot.
| Figure 2. Corresponding dermoscopy of the left second toe revealed an absence of pinpoint vessels. |
Optical coherence tomography demonstrated discrete thickening of the stratum corneum with distinctive granular and coarse textural appearance of the hyperkeratotic stratum corneum compared to normal adjacent skin. This textural difference was attributed to the alteration in collagen deposition of the knuckle pads, consistent with fibrous proliferation. Finally, OCT imaging provided further characterization of the lesion demonstrating the absence of any hair follicles and acrosyringium in areas resembling glabrous skin (Figure 4).
Knuckle pads, also known as Garrod pads, were first described by Garrod2 in 1893. They are benign, asymptomatic, fibrotic thickenings of the skin. Lesions are smooth, firm, flesh colored, and located on the dorsal aspect of the hands and feet along the metacarpophalangeal and interphalangeal joints. Knuckle pads are common, can develop at any age, and are observed more frequently in men than in women.3
Primary knuckle pads can be sporadic or associated with other conditions such as palmoplantar keratoderma, acrokeratoelastoidosis costa, fibrosing disorders, or Bart-Pumphrey syndrome.4 Secondary knuckle pads, which are more common, occur in sites of repetitive trauma or pressure. Certain occupations (eg, mechanics) or hobbies (eg, boxing) increase the risk for developing knuckle pads.3,4
The diagnosis of knuckle pads is usually made clinically, though several other conditions mimic knuckle pads, including scars, keloids, calluses, verruca vulgaris, fibromas, and rheumatoid nodules.3,5 We report a description of knuckle pads that was diagnosed with OCT imaging. Further characterization of both malignant and nonmalignant lesions on OCT imaging will contribute new insights to the role of OCT in the noninvasive diagnosis of skin diseases, pending future studies.
1. Forsea AM, Carstea EM, Ghervase L, et al. Clinical application of optical coherence tomography for the imaging of non-melanocytic cutaneous tumors: a pilot multi-modal study. J Med Life. 2010;3:381-389.
2. Garrod AE. On an unusual form of nodule upon joints of the fingers. St Bartholomew’s Hosp Rep. 1893;29:157-161.
3. Kodama BF, Gentry RH, Fitzpatrick JE. Papules and plaques over the joint spaces. knuckle pads (heloderma). Arch Dermatol. 1993;129:1044-1045, 1047.
4. Nenoff P, Woitek G. Images in clinical medicine. knuckle pads. N Engl J Med. 2011;364:2451.
5. Sehgal VN, Singh M, Saxena HM, et al. Primary knuckle pads. Clin Exp Dermatol. 1979;4:337-339.
1. Forsea AM, Carstea EM, Ghervase L, et al. Clinical application of optical coherence tomography for the imaging of non-melanocytic cutaneous tumors: a pilot multi-modal study. J Med Life. 2010;3:381-389.
2. Garrod AE. On an unusual form of nodule upon joints of the fingers. St Bartholomew’s Hosp Rep. 1893;29:157-161.
3. Kodama BF, Gentry RH, Fitzpatrick JE. Papules and plaques over the joint spaces. knuckle pads (heloderma). Arch Dermatol. 1993;129:1044-1045, 1047.
4. Nenoff P, Woitek G. Images in clinical medicine. knuckle pads. N Engl J Med. 2011;364:2451.
5. Sehgal VN, Singh M, Saxena HM, et al. Primary knuckle pads. Clin Exp Dermatol. 1979;4:337-339.
Oral Lichen Planus With Malignant Transformation to Invasive Squamous Cell Carcinoma
To the Editor:
A 62-year-old woman with an extensive history of cutaneous and oral lichen planus (OLP) presented with gradual worsening of oral pain refractory to previously successful treatment regimens. The pains were described as sharp sensations originating in the right superior oral cavity, occurring almost constantly over the course of 2 months. On examination, the oral mucosa on the right side showed lacy, white, hyperkeratotic buccal lesions, as well as superficial erythematous erosion on the right upper alveolar ridge mucosa (Figure 1). On the left side, lacy, white, reticular patches were noted along the buccal mucosa. Gingival desquamation with superficial erosions were observed bilaterally, extending to the upper alveolar ridge in some locations. The skin examination revealed resolving, nonirritated, violaceous, flat-topped papules with a white-gray hue on the upper back and vulva.
The rest of the physical examination was benign, including a lack of appreciable lymphadenopathy, a cranial nerve examination without focal deficit, and the presence of fluent unaffected speech. On review of systems, the patient denied fevers, chills, weight loss, or night sweats. She had no history of skin cancer or oropharyngeal cancer. Family history revealed that her father had nonmelanoma skin cancer of the head and neck. She denied heavy alcohol use as well as history of smoking or other oral tobacco products. Laboratory tests revealed a complete blood cell count and comprehensive metabolic panel that was within reference range. Due to the refractory nature of the pain, which was out of character for OLP, the patient was referred to an oral maxillofacial surgeon who extracted right maxillary teeth adjacent to the erosion to obtain an adequate specimen for surgical biopsy of the lesion itself. Histopathology confirmed the diagnosis of chronic erosive OLP with malignant transformation to localized squamous cell carcinoma (SCC) of the right maxilla.
While awaiting treatment, she began to develop unremitting headaches and painful shooting sensations beginning in the right superior oral mucosa, radiating to the ipsilateral naris, nasolabial folds, malar cheek, and temple region. This clinical picture was consistent with neuralgia occurring along the maxillary nerve. A subsequent computed tomography scan revealed local bony destruction of the primary tumor and likely perineural involvement (Figure 2), without notable nodal involvement or metastasis (stage III: T4aN0M0). An otolaryngologist performed a wide alveolar and maxillary excision with lymph node dissection. Surgical margins were deemed as negative and there was no evidence of nodal disease. She was later seen by the oncology and radiation oncology teams and received several courses of chemoradiotherapy.
|
Figure 2. Coronal (A) and axial (B) computed tomography demonstrated right maxillary bony destruction. |
Seven months later, a new indurated ulcer was noted on the left lateral tongue. Biopsy revealed a new primary oral SCC (OSCC), which also was excised by an otolaryngologist. Recent computed tomography did not detect any recurrence or potential metastases, but the patient subsequently was lost to follow-up.
Lichen planus is an idiopathic inflammatory disease most commonly affecting the cutaneous skin as well as the oral mucosa, genital mucosa, nails, and scalp. Oral lichen planus is a relatively common manifestation, found in approximately 1% to 2% of individuals older than 15 years.1 Epidemiologic studies revealed that OLP is uncommon in children,2,3 it affects women more frequently than men (approximately 3:1 ratio),3 and its incidence peaks between 30 and 60 years of age.4 The literature on malignant transformation of OLP is varied and controversial, with some early investigations such as Krutchkoff et al5 concluding that the reported cases often fall short of supporting OLP as a premalignant source of OSCC due to insufficient evidence in claimed case reports supporting the diagnosis of OLP histopathologically, the occurrence of OSCC in sites where OLP lesions did not previously exist, and uncertainty regarding confounding factors such as carcinogen exposure.5 In contrast, a longitudinal cohort study reported malignant transformation in 2.4% of OLP cases (N=327), with a standardized incidence ratio of 17.7 (95% confidence interval, 8.8-35.3) when compared to a control group.6 Current literature has predominantly sided with the notion that OLP, especially the erosive variant, carries the risk for malignant potential6 as well as the World Health Organization’s classification of the disorder as precancerous.3
The pathophysiology of OLP and its potential for malignant transformation are unknown. It is believed that cell-mediated immunity, specifically CD8+ lymphocytes targeting stratum basale keratinocytes for apoptosis via the caspase cascade, plays a major role in the development of OLP, beginning with Langerhans cell recognition of an unknown basal cell antigen.3 Moreover, it is postulated that antigen expression is induced by certain drugs, infections, and contact allergens such as dental amalgams, explaining their known associations with OLP initiation and exacerbation. The etiology behind OLP developing into OSCC also is poorly understood and many different hypotheses have been suggested. Modified expression of p53, a 53-kd protein, in OLP patients has been demonstrated.6 Some investigators propose that a lack of the expected keratinocyte apoptotic response to the cell-mediated attack may be etiologic in cancerous transformation.3 Given their utility in treatment of OLP, there also has been apprehension over the potential for immunosuppressant medications leading to decreased expression of antitumor regulators and development of malignant cells, though it has not been substantiated by current literature.6 Finally, some cases of OSCC are believed to have been linked to N-nitrosobenzylmethylamine, a known carcinogen produced by colonized Candida albicans, which also may play a role in OLP treated with immunosuppressants.7
Clinically, OLP lesions are known to be more chronic in nature than cutaneous lichen planus.7 There are 6 classifications of OLP: reticular (lacy white with Wickham striae), plaquelike, papular, atrophic, bullous, and erosive. The latter 3 are known to be the more symptomatic manifestations.3,7 Of note, the atrophic and erosive forms are believed to account for the vast majority of cases of malignant transformation of OLP to OSCC. Approximately 90% of patients have involvement of multiple oral sites, with the most common affected areas being the buccal mucosa (90%), gingival margin (56%), and dorsal tongue (34%).7 Symptoms include increased sensitivity to foods, intense local pain, and coarse-feeling mucosa. The nature of the disease favors an active-quiescent-active course, with flares occurring after direct irritation (ie, dental procedures, Köbner phenomenon), emotional stress, medication use, and systemic illness.7 The differential diagnosis of OLP includes bite trauma, candidiasis, pemphigus, leukoplakia, lichenoid drug reaction, pemphigoid, and graft-versus-host disease.4 Red flags of malignant transformation include induration, worsening ulceration in the setting of previously effective therapy, and presence of constitutional symptoms.
Regarding the behavior of OSCC after malignant transformation, the literature seems to suggest a tendency for well-differentiated noninvasive tumors that most often occur on the buccal mucosa (43%), tongue (33%), gingiva (19%), and palate (4.8%).8 Interestingly, one study described that only 1 (4.8%) of 21 patients with OLP and OSCC was deemed as having stage II or higher disease at time of diagnosis. Likewise, 90% of the biopsied samples revealed well-differentiated carcinomas.8 These findings clearly contrast with our case in which the patient experienced rapid conversion of localized OSCC to more invasive disease. Also of consequence in this study was the finding that a relatively high proportion of patients (29% [6/21]) developed at least one other primary OSCC lesion over the course of follow-up.8 This finding is consistent with our patient.
Last, management of OLP lesions is most commonly accomplished with topical steroids such as fluocinolone acetonide or triamcinolone acetonide.3 Treatment of gingival disease may be enhanced with the use of form-fitting trays.2 For refractory erosive disease, tacrolimus ointment has been demonstrated as a useful backup therapy but may actually be associated with the development of OSCC through alteration of MAPK and p53.3 Some investigators suggest regular 4-month follow-up of OLP patients to detect if acute worsening and or refractoriness to treatment have signified early dysplastic change. Various scoring systems also have been suggested for following up on the severity of OLP lesions.3
The management of OSCC usually is accomplished via surgery, radiation, or both. The decision is dependent on tumor stage and the patient’s individual limitations. It is highly recommended that patients with OSCC arising from OLP be closely followed after diagnosis of cancer, with some sources suggesting follow-up every 2 months for the first 6 to 9 months after diagnosis due to the relatively high rate of discovery of nodal metastases and new primary lesions in that critical time span.8 Thereafter, an examination every 4 months is suggested as sufficient for detecting future complications.
1. van der Meij EH, Schepman KP, Smeele LE, et al. A review of the recent literature regarding malignant transformation of oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:307-310.
2. Scattarella A, Petruzzi M, Ballini A, et al. Oral lichen planus and dental hygiene: a case report [published online ahead of print September 1, 2010]. Int J Dent Hyg. 2011;9:163-166.
3. Ismail SB, Kumar SK, Zain RB. Oral lichen planus and lichenoid reactions: etiopathogenesis, diagnosis, management and malignant transformation. J Oral Sci. 2007;49:89-106.
4. Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
5. Krutchkoff DJ, Cutler L, Laskowski S. Oral lichen planus: the evidence regarding potential malignant transformation. J Oral Pathol. 1978;7:1-7.
6. Bombeccari GP, Guzzi G, Tettamanti M, et al. Oral lichen planus and malignant transformation: a longitudinal cohort study [published online ahead of print July 22, 2011]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:328-334.
7. Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. J Am Acad Dermatol. 2002;46:207-214.
8. Mignogna MD, Lo Russo L, Fedele S, et al. Clinical behaviour of malignant transforming oral lichen planus. Eur J Surg Oncol. 2002;28:838-843.
To the Editor:
A 62-year-old woman with an extensive history of cutaneous and oral lichen planus (OLP) presented with gradual worsening of oral pain refractory to previously successful treatment regimens. The pains were described as sharp sensations originating in the right superior oral cavity, occurring almost constantly over the course of 2 months. On examination, the oral mucosa on the right side showed lacy, white, hyperkeratotic buccal lesions, as well as superficial erythematous erosion on the right upper alveolar ridge mucosa (Figure 1). On the left side, lacy, white, reticular patches were noted along the buccal mucosa. Gingival desquamation with superficial erosions were observed bilaterally, extending to the upper alveolar ridge in some locations. The skin examination revealed resolving, nonirritated, violaceous, flat-topped papules with a white-gray hue on the upper back and vulva.
The rest of the physical examination was benign, including a lack of appreciable lymphadenopathy, a cranial nerve examination without focal deficit, and the presence of fluent unaffected speech. On review of systems, the patient denied fevers, chills, weight loss, or night sweats. She had no history of skin cancer or oropharyngeal cancer. Family history revealed that her father had nonmelanoma skin cancer of the head and neck. She denied heavy alcohol use as well as history of smoking or other oral tobacco products. Laboratory tests revealed a complete blood cell count and comprehensive metabolic panel that was within reference range. Due to the refractory nature of the pain, which was out of character for OLP, the patient was referred to an oral maxillofacial surgeon who extracted right maxillary teeth adjacent to the erosion to obtain an adequate specimen for surgical biopsy of the lesion itself. Histopathology confirmed the diagnosis of chronic erosive OLP with malignant transformation to localized squamous cell carcinoma (SCC) of the right maxilla.
While awaiting treatment, she began to develop unremitting headaches and painful shooting sensations beginning in the right superior oral mucosa, radiating to the ipsilateral naris, nasolabial folds, malar cheek, and temple region. This clinical picture was consistent with neuralgia occurring along the maxillary nerve. A subsequent computed tomography scan revealed local bony destruction of the primary tumor and likely perineural involvement (Figure 2), without notable nodal involvement or metastasis (stage III: T4aN0M0). An otolaryngologist performed a wide alveolar and maxillary excision with lymph node dissection. Surgical margins were deemed as negative and there was no evidence of nodal disease. She was later seen by the oncology and radiation oncology teams and received several courses of chemoradiotherapy.


|
Figure 2. Coronal (A) and axial (B) computed tomography demonstrated right maxillary bony destruction. |
Seven months later, a new indurated ulcer was noted on the left lateral tongue. Biopsy revealed a new primary oral SCC (OSCC), which also was excised by an otolaryngologist. Recent computed tomography did not detect any recurrence or potential metastases, but the patient subsequently was lost to follow-up.
Lichen planus is an idiopathic inflammatory disease most commonly affecting the cutaneous skin as well as the oral mucosa, genital mucosa, nails, and scalp. Oral lichen planus is a relatively common manifestation, found in approximately 1% to 2% of individuals older than 15 years.1 Epidemiologic studies revealed that OLP is uncommon in children,2,3 it affects women more frequently than men (approximately 3:1 ratio),3 and its incidence peaks between 30 and 60 years of age.4 The literature on malignant transformation of OLP is varied and controversial, with some early investigations such as Krutchkoff et al5 concluding that the reported cases often fall short of supporting OLP as a premalignant source of OSCC due to insufficient evidence in claimed case reports supporting the diagnosis of OLP histopathologically, the occurrence of OSCC in sites where OLP lesions did not previously exist, and uncertainty regarding confounding factors such as carcinogen exposure.5 In contrast, a longitudinal cohort study reported malignant transformation in 2.4% of OLP cases (N=327), with a standardized incidence ratio of 17.7 (95% confidence interval, 8.8-35.3) when compared to a control group.6 Current literature has predominantly sided with the notion that OLP, especially the erosive variant, carries the risk for malignant potential6 as well as the World Health Organization’s classification of the disorder as precancerous.3
The pathophysiology of OLP and its potential for malignant transformation are unknown. It is believed that cell-mediated immunity, specifically CD8+ lymphocytes targeting stratum basale keratinocytes for apoptosis via the caspase cascade, plays a major role in the development of OLP, beginning with Langerhans cell recognition of an unknown basal cell antigen.3 Moreover, it is postulated that antigen expression is induced by certain drugs, infections, and contact allergens such as dental amalgams, explaining their known associations with OLP initiation and exacerbation. The etiology behind OLP developing into OSCC also is poorly understood and many different hypotheses have been suggested. Modified expression of p53, a 53-kd protein, in OLP patients has been demonstrated.6 Some investigators propose that a lack of the expected keratinocyte apoptotic response to the cell-mediated attack may be etiologic in cancerous transformation.3 Given their utility in treatment of OLP, there also has been apprehension over the potential for immunosuppressant medications leading to decreased expression of antitumor regulators and development of malignant cells, though it has not been substantiated by current literature.6 Finally, some cases of OSCC are believed to have been linked to N-nitrosobenzylmethylamine, a known carcinogen produced by colonized Candida albicans, which also may play a role in OLP treated with immunosuppressants.7
Clinically, OLP lesions are known to be more chronic in nature than cutaneous lichen planus.7 There are 6 classifications of OLP: reticular (lacy white with Wickham striae), plaquelike, papular, atrophic, bullous, and erosive. The latter 3 are known to be the more symptomatic manifestations.3,7 Of note, the atrophic and erosive forms are believed to account for the vast majority of cases of malignant transformation of OLP to OSCC. Approximately 90% of patients have involvement of multiple oral sites, with the most common affected areas being the buccal mucosa (90%), gingival margin (56%), and dorsal tongue (34%).7 Symptoms include increased sensitivity to foods, intense local pain, and coarse-feeling mucosa. The nature of the disease favors an active-quiescent-active course, with flares occurring after direct irritation (ie, dental procedures, Köbner phenomenon), emotional stress, medication use, and systemic illness.7 The differential diagnosis of OLP includes bite trauma, candidiasis, pemphigus, leukoplakia, lichenoid drug reaction, pemphigoid, and graft-versus-host disease.4 Red flags of malignant transformation include induration, worsening ulceration in the setting of previously effective therapy, and presence of constitutional symptoms.
Regarding the behavior of OSCC after malignant transformation, the literature seems to suggest a tendency for well-differentiated noninvasive tumors that most often occur on the buccal mucosa (43%), tongue (33%), gingiva (19%), and palate (4.8%).8 Interestingly, one study described that only 1 (4.8%) of 21 patients with OLP and OSCC was deemed as having stage II or higher disease at time of diagnosis. Likewise, 90% of the biopsied samples revealed well-differentiated carcinomas.8 These findings clearly contrast with our case in which the patient experienced rapid conversion of localized OSCC to more invasive disease. Also of consequence in this study was the finding that a relatively high proportion of patients (29% [6/21]) developed at least one other primary OSCC lesion over the course of follow-up.8 This finding is consistent with our patient.
Last, management of OLP lesions is most commonly accomplished with topical steroids such as fluocinolone acetonide or triamcinolone acetonide.3 Treatment of gingival disease may be enhanced with the use of form-fitting trays.2 For refractory erosive disease, tacrolimus ointment has been demonstrated as a useful backup therapy but may actually be associated with the development of OSCC through alteration of MAPK and p53.3 Some investigators suggest regular 4-month follow-up of OLP patients to detect if acute worsening and or refractoriness to treatment have signified early dysplastic change. Various scoring systems also have been suggested for following up on the severity of OLP lesions.3
The management of OSCC usually is accomplished via surgery, radiation, or both. The decision is dependent on tumor stage and the patient’s individual limitations. It is highly recommended that patients with OSCC arising from OLP be closely followed after diagnosis of cancer, with some sources suggesting follow-up every 2 months for the first 6 to 9 months after diagnosis due to the relatively high rate of discovery of nodal metastases and new primary lesions in that critical time span.8 Thereafter, an examination every 4 months is suggested as sufficient for detecting future complications.
To the Editor:
A 62-year-old woman with an extensive history of cutaneous and oral lichen planus (OLP) presented with gradual worsening of oral pain refractory to previously successful treatment regimens. The pains were described as sharp sensations originating in the right superior oral cavity, occurring almost constantly over the course of 2 months. On examination, the oral mucosa on the right side showed lacy, white, hyperkeratotic buccal lesions, as well as superficial erythematous erosion on the right upper alveolar ridge mucosa (Figure 1). On the left side, lacy, white, reticular patches were noted along the buccal mucosa. Gingival desquamation with superficial erosions were observed bilaterally, extending to the upper alveolar ridge in some locations. The skin examination revealed resolving, nonirritated, violaceous, flat-topped papules with a white-gray hue on the upper back and vulva.
The rest of the physical examination was benign, including a lack of appreciable lymphadenopathy, a cranial nerve examination without focal deficit, and the presence of fluent unaffected speech. On review of systems, the patient denied fevers, chills, weight loss, or night sweats. She had no history of skin cancer or oropharyngeal cancer. Family history revealed that her father had nonmelanoma skin cancer of the head and neck. She denied heavy alcohol use as well as history of smoking or other oral tobacco products. Laboratory tests revealed a complete blood cell count and comprehensive metabolic panel that was within reference range. Due to the refractory nature of the pain, which was out of character for OLP, the patient was referred to an oral maxillofacial surgeon who extracted right maxillary teeth adjacent to the erosion to obtain an adequate specimen for surgical biopsy of the lesion itself. Histopathology confirmed the diagnosis of chronic erosive OLP with malignant transformation to localized squamous cell carcinoma (SCC) of the right maxilla.
While awaiting treatment, she began to develop unremitting headaches and painful shooting sensations beginning in the right superior oral mucosa, radiating to the ipsilateral naris, nasolabial folds, malar cheek, and temple region. This clinical picture was consistent with neuralgia occurring along the maxillary nerve. A subsequent computed tomography scan revealed local bony destruction of the primary tumor and likely perineural involvement (Figure 2), without notable nodal involvement or metastasis (stage III: T4aN0M0). An otolaryngologist performed a wide alveolar and maxillary excision with lymph node dissection. Surgical margins were deemed as negative and there was no evidence of nodal disease. She was later seen by the oncology and radiation oncology teams and received several courses of chemoradiotherapy.


|
Figure 2. Coronal (A) and axial (B) computed tomography demonstrated right maxillary bony destruction. |
Seven months later, a new indurated ulcer was noted on the left lateral tongue. Biopsy revealed a new primary oral SCC (OSCC), which also was excised by an otolaryngologist. Recent computed tomography did not detect any recurrence or potential metastases, but the patient subsequently was lost to follow-up.
Lichen planus is an idiopathic inflammatory disease most commonly affecting the cutaneous skin as well as the oral mucosa, genital mucosa, nails, and scalp. Oral lichen planus is a relatively common manifestation, found in approximately 1% to 2% of individuals older than 15 years.1 Epidemiologic studies revealed that OLP is uncommon in children,2,3 it affects women more frequently than men (approximately 3:1 ratio),3 and its incidence peaks between 30 and 60 years of age.4 The literature on malignant transformation of OLP is varied and controversial, with some early investigations such as Krutchkoff et al5 concluding that the reported cases often fall short of supporting OLP as a premalignant source of OSCC due to insufficient evidence in claimed case reports supporting the diagnosis of OLP histopathologically, the occurrence of OSCC in sites where OLP lesions did not previously exist, and uncertainty regarding confounding factors such as carcinogen exposure.5 In contrast, a longitudinal cohort study reported malignant transformation in 2.4% of OLP cases (N=327), with a standardized incidence ratio of 17.7 (95% confidence interval, 8.8-35.3) when compared to a control group.6 Current literature has predominantly sided with the notion that OLP, especially the erosive variant, carries the risk for malignant potential6 as well as the World Health Organization’s classification of the disorder as precancerous.3
The pathophysiology of OLP and its potential for malignant transformation are unknown. It is believed that cell-mediated immunity, specifically CD8+ lymphocytes targeting stratum basale keratinocytes for apoptosis via the caspase cascade, plays a major role in the development of OLP, beginning with Langerhans cell recognition of an unknown basal cell antigen.3 Moreover, it is postulated that antigen expression is induced by certain drugs, infections, and contact allergens such as dental amalgams, explaining their known associations with OLP initiation and exacerbation. The etiology behind OLP developing into OSCC also is poorly understood and many different hypotheses have been suggested. Modified expression of p53, a 53-kd protein, in OLP patients has been demonstrated.6 Some investigators propose that a lack of the expected keratinocyte apoptotic response to the cell-mediated attack may be etiologic in cancerous transformation.3 Given their utility in treatment of OLP, there also has been apprehension over the potential for immunosuppressant medications leading to decreased expression of antitumor regulators and development of malignant cells, though it has not been substantiated by current literature.6 Finally, some cases of OSCC are believed to have been linked to N-nitrosobenzylmethylamine, a known carcinogen produced by colonized Candida albicans, which also may play a role in OLP treated with immunosuppressants.7
Clinically, OLP lesions are known to be more chronic in nature than cutaneous lichen planus.7 There are 6 classifications of OLP: reticular (lacy white with Wickham striae), plaquelike, papular, atrophic, bullous, and erosive. The latter 3 are known to be the more symptomatic manifestations.3,7 Of note, the atrophic and erosive forms are believed to account for the vast majority of cases of malignant transformation of OLP to OSCC. Approximately 90% of patients have involvement of multiple oral sites, with the most common affected areas being the buccal mucosa (90%), gingival margin (56%), and dorsal tongue (34%).7 Symptoms include increased sensitivity to foods, intense local pain, and coarse-feeling mucosa. The nature of the disease favors an active-quiescent-active course, with flares occurring after direct irritation (ie, dental procedures, Köbner phenomenon), emotional stress, medication use, and systemic illness.7 The differential diagnosis of OLP includes bite trauma, candidiasis, pemphigus, leukoplakia, lichenoid drug reaction, pemphigoid, and graft-versus-host disease.4 Red flags of malignant transformation include induration, worsening ulceration in the setting of previously effective therapy, and presence of constitutional symptoms.
Regarding the behavior of OSCC after malignant transformation, the literature seems to suggest a tendency for well-differentiated noninvasive tumors that most often occur on the buccal mucosa (43%), tongue (33%), gingiva (19%), and palate (4.8%).8 Interestingly, one study described that only 1 (4.8%) of 21 patients with OLP and OSCC was deemed as having stage II or higher disease at time of diagnosis. Likewise, 90% of the biopsied samples revealed well-differentiated carcinomas.8 These findings clearly contrast with our case in which the patient experienced rapid conversion of localized OSCC to more invasive disease. Also of consequence in this study was the finding that a relatively high proportion of patients (29% [6/21]) developed at least one other primary OSCC lesion over the course of follow-up.8 This finding is consistent with our patient.
Last, management of OLP lesions is most commonly accomplished with topical steroids such as fluocinolone acetonide or triamcinolone acetonide.3 Treatment of gingival disease may be enhanced with the use of form-fitting trays.2 For refractory erosive disease, tacrolimus ointment has been demonstrated as a useful backup therapy but may actually be associated with the development of OSCC through alteration of MAPK and p53.3 Some investigators suggest regular 4-month follow-up of OLP patients to detect if acute worsening and or refractoriness to treatment have signified early dysplastic change. Various scoring systems also have been suggested for following up on the severity of OLP lesions.3
The management of OSCC usually is accomplished via surgery, radiation, or both. The decision is dependent on tumor stage and the patient’s individual limitations. It is highly recommended that patients with OSCC arising from OLP be closely followed after diagnosis of cancer, with some sources suggesting follow-up every 2 months for the first 6 to 9 months after diagnosis due to the relatively high rate of discovery of nodal metastases and new primary lesions in that critical time span.8 Thereafter, an examination every 4 months is suggested as sufficient for detecting future complications.
1. van der Meij EH, Schepman KP, Smeele LE, et al. A review of the recent literature regarding malignant transformation of oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:307-310.
2. Scattarella A, Petruzzi M, Ballini A, et al. Oral lichen planus and dental hygiene: a case report [published online ahead of print September 1, 2010]. Int J Dent Hyg. 2011;9:163-166.
3. Ismail SB, Kumar SK, Zain RB. Oral lichen planus and lichenoid reactions: etiopathogenesis, diagnosis, management and malignant transformation. J Oral Sci. 2007;49:89-106.
4. Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
5. Krutchkoff DJ, Cutler L, Laskowski S. Oral lichen planus: the evidence regarding potential malignant transformation. J Oral Pathol. 1978;7:1-7.
6. Bombeccari GP, Guzzi G, Tettamanti M, et al. Oral lichen planus and malignant transformation: a longitudinal cohort study [published online ahead of print July 22, 2011]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:328-334.
7. Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. J Am Acad Dermatol. 2002;46:207-214.
8. Mignogna MD, Lo Russo L, Fedele S, et al. Clinical behaviour of malignant transforming oral lichen planus. Eur J Surg Oncol. 2002;28:838-843.
1. van der Meij EH, Schepman KP, Smeele LE, et al. A review of the recent literature regarding malignant transformation of oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:307-310.
2. Scattarella A, Petruzzi M, Ballini A, et al. Oral lichen planus and dental hygiene: a case report [published online ahead of print September 1, 2010]. Int J Dent Hyg. 2011;9:163-166.
3. Ismail SB, Kumar SK, Zain RB. Oral lichen planus and lichenoid reactions: etiopathogenesis, diagnosis, management and malignant transformation. J Oral Sci. 2007;49:89-106.
4. Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
5. Krutchkoff DJ, Cutler L, Laskowski S. Oral lichen planus: the evidence regarding potential malignant transformation. J Oral Pathol. 1978;7:1-7.
6. Bombeccari GP, Guzzi G, Tettamanti M, et al. Oral lichen planus and malignant transformation: a longitudinal cohort study [published online ahead of print July 22, 2011]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:328-334.
7. Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. J Am Acad Dermatol. 2002;46:207-214.
8. Mignogna MD, Lo Russo L, Fedele S, et al. Clinical behaviour of malignant transforming oral lichen planus. Eur J Surg Oncol. 2002;28:838-843.