User login
An insidious onset of symptoms
CASE Tremors, increasing anxiety
Ms. S, age 56, has a history of depression and anxiety.
The authors’ observations
The incidence of serotonin syndrome has increased because of increasing use of serotonergic agents.1-3 Although the severity could range from benign to life-threatening, the potential lethality combined with difficulty of diagnosis makes this condition of continued interest. Stimulation of the 5-hydroxytryptamine (5-HT) receptor subtypes, specifically 5-HT1A and 5-HT2, are implicated in this syndrome.4,5
Serotonin syndrome is a clinical diagnosis with a triad of symptoms that includes mental status changes, autonomic hyperactivity, and neuromuscular abnormalities.1,2 However, because of the varied presentation and similarity to other syndromes such as NMS, serotonin syndrome often is undiagnosed.5
TREATMENT Discontinue fluoxetine
Several months after the fluoxetine increase, Ms. S's physical symptoms emerge. Several weeks later, she notices significant hypertension of 162/102 mm Hg by checking her blood pressure at home. She had no history of hypertension before taking fluoxetine. She then tells her psychiatrist she has been experiencing confusion, shakiness, loss of balance, forgetfulness, joint pain, sweating, and fatigue, along with worsening anxiety. The treatment team makes a diagnosis of serotonin syndrome and recommends discontinuing fluoxetine and starting cyproheptadine, 4 mg initially, and then repeating the cyproheptadine dose in several hours if her symptoms do not resolve. Approximately, 2.5 months after the serotonin syndrome reaction, Ms. S receives hydroxyzine, 10 mg every 8 hours, as needed for anxiety.
The authors’ observations
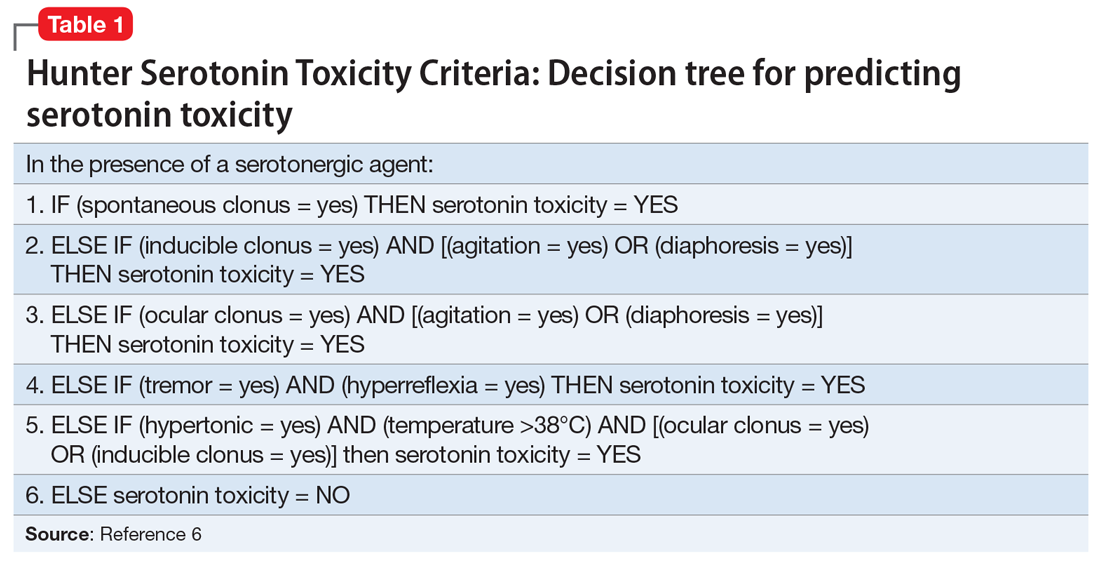
The diagnosis of serotonin syndrome is most accurately made using Hunter Serotonin Toxicity Criteria (Table 16). Because Ms. S had an insidious onset of symptoms, and treatment was initiated before full evaluation, it is unknown if she met Hunter criteria. To meet these criteria, a patient must have ≥1 of the following6:
- spontaneous clonus
- inducible clonus plus agitation or diaphoresis
- ocular clonus plus agitation or diaphoresis
- tremor plus hyperreflexia
- hypertonia plus temperature >38°C plus ocular or inducible clonus.
The Sternbach diagnostic criteria for serotonin syndrome (Table 23) is another commonly used tool.3 These criteria include the addition or increase of a serotonin agent and absence of substances or metabolic derangements that could account for symptoms and at least 3 of the following 10 symptoms3:
- mental status changes (confusion, hypomania)
- agitation
- myoclonus
- hyperreflexia
- diaphoresis
- shivering
- tremor
- diarrhea
- incoordination
- fever.
Continue to: Ms. S met the Sternbach diagnostic criteria...
Ms. S met the Sternbach diagnostic criteria for serotonin syndrome.
Ms. S was taking a single serotonin agent and initially had mild symptoms. More commonly, a patient who presents with serotonin syndrome has been receiving ≥2 serotonergic agents or toxic levels of a single agent, and these agents usually include a psychotropic medication such as a monoamine oxidase inhibitor, tricyclic antidepressant, or SSRI, as well as a medication from a different class, such as dextromethorphan, linezolid, tramadol, methylene blue, and/or St. John’s wort.1,7-13 However, in this case, Ms. S also was taking bupropion, a known inhibitor of cytochrome P450 2D6. Bupropion might have increased Ms. S’s fluoxetine levels.
Ms. S was a healthy, middle-age patient who took no medications other than those listed, had no medical comorbidities, and had a straightforward psychiatric history, which makes the diagnosis of serotonin syndrome clearer. However, other potential differential diagnoses, such as NMS, delirium tremens, and anticholinergic toxicity, might cloud the clinical picture. When differentiating NMS and serotonin syndrome, it is helpful to note whether a patient shows tremor, diarrhea, and myoclonus present in the absence of muscular, “lead-pipe” rigidity, which suggests a diagnosis of serotonin syndrome.2,3,5
[polldaddy:10279173]
The authors’ observations
Treating serotonin syndrome includes supportive care, discontinuing offending agents, administering benzodiazepines, and using a serotonin antagonist as an antidote for patients with moderate-to-severe cases. Cyproheptadine is an antihistaminergic medication with non-specific 5-HT1A and 5-HT2 antagonism. It is FDA-approved for specific allergic reactions, urticaria, and anaphylaxis adjunctive therapy, but not for serotonin syndrome. Case series support the use of cyproheptadine for acute management of serotonin syndrome, with rapid symptom improvement.4,7,14-18 We observed a similar outcome with Ms. S. Her significant autonomic symptoms resolved rapidly, although she experienced some residual, mild symptoms that took weeks to resolve.
Continue to: Because serotonergic agents...
Because serotonergic agents are used frequently and readily by primary care clinicians as well as psychiatrists, the ability to properly diagnose this syndrome is vital, particularly because severe cases can rapidly deteriorate.1,9,16,17 This presentation of a single serotonergic agent causing significant symptoms that worsened over months is not typical, but important to recognize as a patient begins to experience autonomic instability. As was the case with Ms. S, it is important to remain vigilant when changing dosages or adding medications. Symptoms of serotonin syndrome might be vague and difficult to diagnose, especially if the clinician is not aware of the variability of presentation of this syndrome. Cyproheptadine can be used safely and rapidly and should be considered a treatment option for serotonin syndrome.
OUTCOME Hypertension resolves
After her first dose of cyproheptadine, Ms. S’s blood pressure drops to 146/86 mm Hg. Three hours later, she repeats the cyproheptadine dose and her blood pressure drops to 106/60 mm Hg. She reports that her anxiety has lessened, although she is still tremulous. Overall, she says she feels better. She experiences improvement of her condition with a pharmacologic regimen of bupropion, gabapentin, and hydroxyzine.
Several weeks later, her health returns to baseline, with complete resolution of hypertension.
Bottom Line
Although serotonin syndrome is most commonly associated with co-administered serotonergic medications, symptoms can emerge with a single, moderately dosed agent. Treatment includes withdrawing the offending agent, and administering a serotonin antagonist. Mild cases of serotonin syndrome usually resolve.
Related Resources
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
- Iqbal MM, Basil MJ, Kaplan J, et al. Overview of serotonin syndrome. Ann Clin Psychiatry. 2012;24(4):310-318.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Cyproheptadine • Periactin
Dextromethorphan • Benylin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Gabapentin • Neurontin
Hydroxyzine • Atarax
Linezolid • Zyvox
Tramadol • Ultram, Ryzolt
1. Mason PJ, Morris VA, Balcezak TJ. Serotonin syndrome: presentation of 2 cases and review of literature. Medicine (Baltimore). 2000;79(4):201-209.
2. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
3. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):705-713.
4. Graudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. J Emerg Med. 1998;16(4):615-619.
5. Mills KC. Serotonin syndrome a clinical update. Crit Care Clin. 1997;13(4):763-783.
6. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96:635-642.
7. Horowitz BZ, Mullins ME. Cyproheptadine for serotonin syndrome in an accidental pediatric sertraline ingestion. Pediatr Emerg Care. 1999;15(5):325-327.
8. Kolecki P. Isolated venlafaxine-induced serotonin syndrome. J Emerg Med. 1996;15:491-493.
9. Pan J, Shen W. Serotonin syndrome induced by low-dose venlafaxine. Ann Pharmacother. 2003;37(2):209-211.
10. Hernández JL, Ramos FJ, Infante J, et al. Severe serotonin syndrome induced by mirtazapine monotherapy. Ann Pharmacother. 2002;36(4):641-643.
11. Patel DD, Galarneau D. Serotonin syndrome with fluoxetine: two case reports. Ochsner J. 2016;16(4):554-557.
12. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
13. Zuschlag ZD, Warren MW, K Schultz S. Serotonin toxicity and urinary analgesics: a case report and systematic literature review of methylene blue-induced serotonin syndrome. Psychosomatics. 2018;59(6):539-546.
14. Kapur S, Zipursky RB, Jones C, et al. Cyproheptadine: a potent in vivo serotonin antagonist. Am J Psychiatry. 1997;154(6):884.
15. Baigel GD. Cyproheptadine and the treatment of an unconscious patient with the serotonin syndrome. Eur J Anaesthesiol. 2003;20(7):586-588.
16. Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med. 1994;331:1021-1022.
17. McDaniel WW. Serotonin syndrome: early management with cyproheptadine. Ann Pharmacother. 2001;35(7-8):870-873.
18. Kolecki P. Venlafaxine induced serotonin syndrome occurring after abstinence from phenelzine for more than two weeks. J Toxicol Clin Toxicol. 1997;35(2):211-212.
CASE Tremors, increasing anxiety
Ms. S, age 56, has a history of depression and anxiety.
The authors’ observations
The incidence of serotonin syndrome has increased because of increasing use of serotonergic agents.1-3 Although the severity could range from benign to life-threatening, the potential lethality combined with difficulty of diagnosis makes this condition of continued interest. Stimulation of the 5-hydroxytryptamine (5-HT) receptor subtypes, specifically 5-HT1A and 5-HT2, are implicated in this syndrome.4,5
Serotonin syndrome is a clinical diagnosis with a triad of symptoms that includes mental status changes, autonomic hyperactivity, and neuromuscular abnormalities.1,2 However, because of the varied presentation and similarity to other syndromes such as NMS, serotonin syndrome often is undiagnosed.5
TREATMENT Discontinue fluoxetine
Several months after the fluoxetine increase, Ms. S's physical symptoms emerge. Several weeks later, she notices significant hypertension of 162/102 mm Hg by checking her blood pressure at home. She had no history of hypertension before taking fluoxetine. She then tells her psychiatrist she has been experiencing confusion, shakiness, loss of balance, forgetfulness, joint pain, sweating, and fatigue, along with worsening anxiety. The treatment team makes a diagnosis of serotonin syndrome and recommends discontinuing fluoxetine and starting cyproheptadine, 4 mg initially, and then repeating the cyproheptadine dose in several hours if her symptoms do not resolve. Approximately, 2.5 months after the serotonin syndrome reaction, Ms. S receives hydroxyzine, 10 mg every 8 hours, as needed for anxiety.
The authors’ observations
The diagnosis of serotonin syndrome is most accurately made using Hunter Serotonin Toxicity Criteria (Table 16). Because Ms. S had an insidious onset of symptoms, and treatment was initiated before full evaluation, it is unknown if she met Hunter criteria. To meet these criteria, a patient must have ≥1 of the following6:
- spontaneous clonus
- inducible clonus plus agitation or diaphoresis
- ocular clonus plus agitation or diaphoresis
- tremor plus hyperreflexia
- hypertonia plus temperature >38°C plus ocular or inducible clonus.
The Sternbach diagnostic criteria for serotonin syndrome (Table 23) is another commonly used tool.3 These criteria include the addition or increase of a serotonin agent and absence of substances or metabolic derangements that could account for symptoms and at least 3 of the following 10 symptoms3:
- mental status changes (confusion, hypomania)
- agitation
- myoclonus
- hyperreflexia
- diaphoresis
- shivering
- tremor
- diarrhea
- incoordination
- fever.
Continue to: Ms. S met the Sternbach diagnostic criteria...
Ms. S met the Sternbach diagnostic criteria for serotonin syndrome.
Ms. S was taking a single serotonin agent and initially had mild symptoms. More commonly, a patient who presents with serotonin syndrome has been receiving ≥2 serotonergic agents or toxic levels of a single agent, and these agents usually include a psychotropic medication such as a monoamine oxidase inhibitor, tricyclic antidepressant, or SSRI, as well as a medication from a different class, such as dextromethorphan, linezolid, tramadol, methylene blue, and/or St. John’s wort.1,7-13 However, in this case, Ms. S also was taking bupropion, a known inhibitor of cytochrome P450 2D6. Bupropion might have increased Ms. S’s fluoxetine levels.
Ms. S was a healthy, middle-age patient who took no medications other than those listed, had no medical comorbidities, and had a straightforward psychiatric history, which makes the diagnosis of serotonin syndrome clearer. However, other potential differential diagnoses, such as NMS, delirium tremens, and anticholinergic toxicity, might cloud the clinical picture. When differentiating NMS and serotonin syndrome, it is helpful to note whether a patient shows tremor, diarrhea, and myoclonus present in the absence of muscular, “lead-pipe” rigidity, which suggests a diagnosis of serotonin syndrome.2,3,5
[polldaddy:10279173]
The authors’ observations
Treating serotonin syndrome includes supportive care, discontinuing offending agents, administering benzodiazepines, and using a serotonin antagonist as an antidote for patients with moderate-to-severe cases. Cyproheptadine is an antihistaminergic medication with non-specific 5-HT1A and 5-HT2 antagonism. It is FDA-approved for specific allergic reactions, urticaria, and anaphylaxis adjunctive therapy, but not for serotonin syndrome. Case series support the use of cyproheptadine for acute management of serotonin syndrome, with rapid symptom improvement.4,7,14-18 We observed a similar outcome with Ms. S. Her significant autonomic symptoms resolved rapidly, although she experienced some residual, mild symptoms that took weeks to resolve.
Continue to: Because serotonergic agents...
Because serotonergic agents are used frequently and readily by primary care clinicians as well as psychiatrists, the ability to properly diagnose this syndrome is vital, particularly because severe cases can rapidly deteriorate.1,9,16,17 This presentation of a single serotonergic agent causing significant symptoms that worsened over months is not typical, but important to recognize as a patient begins to experience autonomic instability. As was the case with Ms. S, it is important to remain vigilant when changing dosages or adding medications. Symptoms of serotonin syndrome might be vague and difficult to diagnose, especially if the clinician is not aware of the variability of presentation of this syndrome. Cyproheptadine can be used safely and rapidly and should be considered a treatment option for serotonin syndrome.
OUTCOME Hypertension resolves
After her first dose of cyproheptadine, Ms. S’s blood pressure drops to 146/86 mm Hg. Three hours later, she repeats the cyproheptadine dose and her blood pressure drops to 106/60 mm Hg. She reports that her anxiety has lessened, although she is still tremulous. Overall, she says she feels better. She experiences improvement of her condition with a pharmacologic regimen of bupropion, gabapentin, and hydroxyzine.
Several weeks later, her health returns to baseline, with complete resolution of hypertension.
Bottom Line
Although serotonin syndrome is most commonly associated with co-administered serotonergic medications, symptoms can emerge with a single, moderately dosed agent. Treatment includes withdrawing the offending agent, and administering a serotonin antagonist. Mild cases of serotonin syndrome usually resolve.
Related Resources
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
- Iqbal MM, Basil MJ, Kaplan J, et al. Overview of serotonin syndrome. Ann Clin Psychiatry. 2012;24(4):310-318.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Cyproheptadine • Periactin
Dextromethorphan • Benylin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Gabapentin • Neurontin
Hydroxyzine • Atarax
Linezolid • Zyvox
Tramadol • Ultram, Ryzolt
CASE Tremors, increasing anxiety
Ms. S, age 56, has a history of depression and anxiety.
The authors’ observations
The incidence of serotonin syndrome has increased because of increasing use of serotonergic agents.1-3 Although the severity could range from benign to life-threatening, the potential lethality combined with difficulty of diagnosis makes this condition of continued interest. Stimulation of the 5-hydroxytryptamine (5-HT) receptor subtypes, specifically 5-HT1A and 5-HT2, are implicated in this syndrome.4,5
Serotonin syndrome is a clinical diagnosis with a triad of symptoms that includes mental status changes, autonomic hyperactivity, and neuromuscular abnormalities.1,2 However, because of the varied presentation and similarity to other syndromes such as NMS, serotonin syndrome often is undiagnosed.5
TREATMENT Discontinue fluoxetine
Several months after the fluoxetine increase, Ms. S's physical symptoms emerge. Several weeks later, she notices significant hypertension of 162/102 mm Hg by checking her blood pressure at home. She had no history of hypertension before taking fluoxetine. She then tells her psychiatrist she has been experiencing confusion, shakiness, loss of balance, forgetfulness, joint pain, sweating, and fatigue, along with worsening anxiety. The treatment team makes a diagnosis of serotonin syndrome and recommends discontinuing fluoxetine and starting cyproheptadine, 4 mg initially, and then repeating the cyproheptadine dose in several hours if her symptoms do not resolve. Approximately, 2.5 months after the serotonin syndrome reaction, Ms. S receives hydroxyzine, 10 mg every 8 hours, as needed for anxiety.
The authors’ observations
The diagnosis of serotonin syndrome is most accurately made using Hunter Serotonin Toxicity Criteria (Table 16). Because Ms. S had an insidious onset of symptoms, and treatment was initiated before full evaluation, it is unknown if she met Hunter criteria. To meet these criteria, a patient must have ≥1 of the following6:
- spontaneous clonus
- inducible clonus plus agitation or diaphoresis
- ocular clonus plus agitation or diaphoresis
- tremor plus hyperreflexia
- hypertonia plus temperature >38°C plus ocular or inducible clonus.
The Sternbach diagnostic criteria for serotonin syndrome (Table 23) is another commonly used tool.3 These criteria include the addition or increase of a serotonin agent and absence of substances or metabolic derangements that could account for symptoms and at least 3 of the following 10 symptoms3:
- mental status changes (confusion, hypomania)
- agitation
- myoclonus
- hyperreflexia
- diaphoresis
- shivering
- tremor
- diarrhea
- incoordination
- fever.
Continue to: Ms. S met the Sternbach diagnostic criteria...
Ms. S met the Sternbach diagnostic criteria for serotonin syndrome.
Ms. S was taking a single serotonin agent and initially had mild symptoms. More commonly, a patient who presents with serotonin syndrome has been receiving ≥2 serotonergic agents or toxic levels of a single agent, and these agents usually include a psychotropic medication such as a monoamine oxidase inhibitor, tricyclic antidepressant, or SSRI, as well as a medication from a different class, such as dextromethorphan, linezolid, tramadol, methylene blue, and/or St. John’s wort.1,7-13 However, in this case, Ms. S also was taking bupropion, a known inhibitor of cytochrome P450 2D6. Bupropion might have increased Ms. S’s fluoxetine levels.
Ms. S was a healthy, middle-age patient who took no medications other than those listed, had no medical comorbidities, and had a straightforward psychiatric history, which makes the diagnosis of serotonin syndrome clearer. However, other potential differential diagnoses, such as NMS, delirium tremens, and anticholinergic toxicity, might cloud the clinical picture. When differentiating NMS and serotonin syndrome, it is helpful to note whether a patient shows tremor, diarrhea, and myoclonus present in the absence of muscular, “lead-pipe” rigidity, which suggests a diagnosis of serotonin syndrome.2,3,5
[polldaddy:10279173]
The authors’ observations
Treating serotonin syndrome includes supportive care, discontinuing offending agents, administering benzodiazepines, and using a serotonin antagonist as an antidote for patients with moderate-to-severe cases. Cyproheptadine is an antihistaminergic medication with non-specific 5-HT1A and 5-HT2 antagonism. It is FDA-approved for specific allergic reactions, urticaria, and anaphylaxis adjunctive therapy, but not for serotonin syndrome. Case series support the use of cyproheptadine for acute management of serotonin syndrome, with rapid symptom improvement.4,7,14-18 We observed a similar outcome with Ms. S. Her significant autonomic symptoms resolved rapidly, although she experienced some residual, mild symptoms that took weeks to resolve.
Continue to: Because serotonergic agents...
Because serotonergic agents are used frequently and readily by primary care clinicians as well as psychiatrists, the ability to properly diagnose this syndrome is vital, particularly because severe cases can rapidly deteriorate.1,9,16,17 This presentation of a single serotonergic agent causing significant symptoms that worsened over months is not typical, but important to recognize as a patient begins to experience autonomic instability. As was the case with Ms. S, it is important to remain vigilant when changing dosages or adding medications. Symptoms of serotonin syndrome might be vague and difficult to diagnose, especially if the clinician is not aware of the variability of presentation of this syndrome. Cyproheptadine can be used safely and rapidly and should be considered a treatment option for serotonin syndrome.
OUTCOME Hypertension resolves
After her first dose of cyproheptadine, Ms. S’s blood pressure drops to 146/86 mm Hg. Three hours later, she repeats the cyproheptadine dose and her blood pressure drops to 106/60 mm Hg. She reports that her anxiety has lessened, although she is still tremulous. Overall, she says she feels better. She experiences improvement of her condition with a pharmacologic regimen of bupropion, gabapentin, and hydroxyzine.
Several weeks later, her health returns to baseline, with complete resolution of hypertension.
Bottom Line
Although serotonin syndrome is most commonly associated with co-administered serotonergic medications, symptoms can emerge with a single, moderately dosed agent. Treatment includes withdrawing the offending agent, and administering a serotonin antagonist. Mild cases of serotonin syndrome usually resolve.
Related Resources
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
- Iqbal MM, Basil MJ, Kaplan J, et al. Overview of serotonin syndrome. Ann Clin Psychiatry. 2012;24(4):310-318.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Cyproheptadine • Periactin
Dextromethorphan • Benylin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Gabapentin • Neurontin
Hydroxyzine • Atarax
Linezolid • Zyvox
Tramadol • Ultram, Ryzolt
1. Mason PJ, Morris VA, Balcezak TJ. Serotonin syndrome: presentation of 2 cases and review of literature. Medicine (Baltimore). 2000;79(4):201-209.
2. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
3. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):705-713.
4. Graudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. J Emerg Med. 1998;16(4):615-619.
5. Mills KC. Serotonin syndrome a clinical update. Crit Care Clin. 1997;13(4):763-783.
6. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96:635-642.
7. Horowitz BZ, Mullins ME. Cyproheptadine for serotonin syndrome in an accidental pediatric sertraline ingestion. Pediatr Emerg Care. 1999;15(5):325-327.
8. Kolecki P. Isolated venlafaxine-induced serotonin syndrome. J Emerg Med. 1996;15:491-493.
9. Pan J, Shen W. Serotonin syndrome induced by low-dose venlafaxine. Ann Pharmacother. 2003;37(2):209-211.
10. Hernández JL, Ramos FJ, Infante J, et al. Severe serotonin syndrome induced by mirtazapine monotherapy. Ann Pharmacother. 2002;36(4):641-643.
11. Patel DD, Galarneau D. Serotonin syndrome with fluoxetine: two case reports. Ochsner J. 2016;16(4):554-557.
12. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
13. Zuschlag ZD, Warren MW, K Schultz S. Serotonin toxicity and urinary analgesics: a case report and systematic literature review of methylene blue-induced serotonin syndrome. Psychosomatics. 2018;59(6):539-546.
14. Kapur S, Zipursky RB, Jones C, et al. Cyproheptadine: a potent in vivo serotonin antagonist. Am J Psychiatry. 1997;154(6):884.
15. Baigel GD. Cyproheptadine and the treatment of an unconscious patient with the serotonin syndrome. Eur J Anaesthesiol. 2003;20(7):586-588.
16. Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med. 1994;331:1021-1022.
17. McDaniel WW. Serotonin syndrome: early management with cyproheptadine. Ann Pharmacother. 2001;35(7-8):870-873.
18. Kolecki P. Venlafaxine induced serotonin syndrome occurring after abstinence from phenelzine for more than two weeks. J Toxicol Clin Toxicol. 1997;35(2):211-212.
1. Mason PJ, Morris VA, Balcezak TJ. Serotonin syndrome: presentation of 2 cases and review of literature. Medicine (Baltimore). 2000;79(4):201-209.
2. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
3. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):705-713.
4. Graudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. J Emerg Med. 1998;16(4):615-619.
5. Mills KC. Serotonin syndrome a clinical update. Crit Care Clin. 1997;13(4):763-783.
6. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96:635-642.
7. Horowitz BZ, Mullins ME. Cyproheptadine for serotonin syndrome in an accidental pediatric sertraline ingestion. Pediatr Emerg Care. 1999;15(5):325-327.
8. Kolecki P. Isolated venlafaxine-induced serotonin syndrome. J Emerg Med. 1996;15:491-493.
9. Pan J, Shen W. Serotonin syndrome induced by low-dose venlafaxine. Ann Pharmacother. 2003;37(2):209-211.
10. Hernández JL, Ramos FJ, Infante J, et al. Severe serotonin syndrome induced by mirtazapine monotherapy. Ann Pharmacother. 2002;36(4):641-643.
11. Patel DD, Galarneau D. Serotonin syndrome with fluoxetine: two case reports. Ochsner J. 2016;16(4):554-557.
12. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
13. Zuschlag ZD, Warren MW, K Schultz S. Serotonin toxicity and urinary analgesics: a case report and systematic literature review of methylene blue-induced serotonin syndrome. Psychosomatics. 2018;59(6):539-546.
14. Kapur S, Zipursky RB, Jones C, et al. Cyproheptadine: a potent in vivo serotonin antagonist. Am J Psychiatry. 1997;154(6):884.
15. Baigel GD. Cyproheptadine and the treatment of an unconscious patient with the serotonin syndrome. Eur J Anaesthesiol. 2003;20(7):586-588.
16. Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med. 1994;331:1021-1022.
17. McDaniel WW. Serotonin syndrome: early management with cyproheptadine. Ann Pharmacother. 2001;35(7-8):870-873.
18. Kolecki P. Venlafaxine induced serotonin syndrome occurring after abstinence from phenelzine for more than two weeks. J Toxicol Clin Toxicol. 1997;35(2):211-212.
Young, angry, and in need of a liver transplant
CASE Rash, fever, extreme lethargy; multiple hospital visits
Ms. L, age 21, a single woman with a history of major depressive disorder (MDD), is directly admitted from an outside community hospital to our tertiary care academic hospital with acute liver failure.
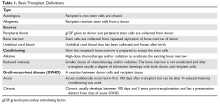
One month earlier, Ms. L had an argument with her family and punched a wall, fracturing her hand. Following the episode, Ms. L’s primary care physician (PCP) prescribed valproic acid, 500 mg/d, to address “mood swings,” which included angry outbursts and irritability. According to her PCP, no baseline laboratory tests were ordered for Ms. L when she started valproic acid because she was young and otherwise healthy.
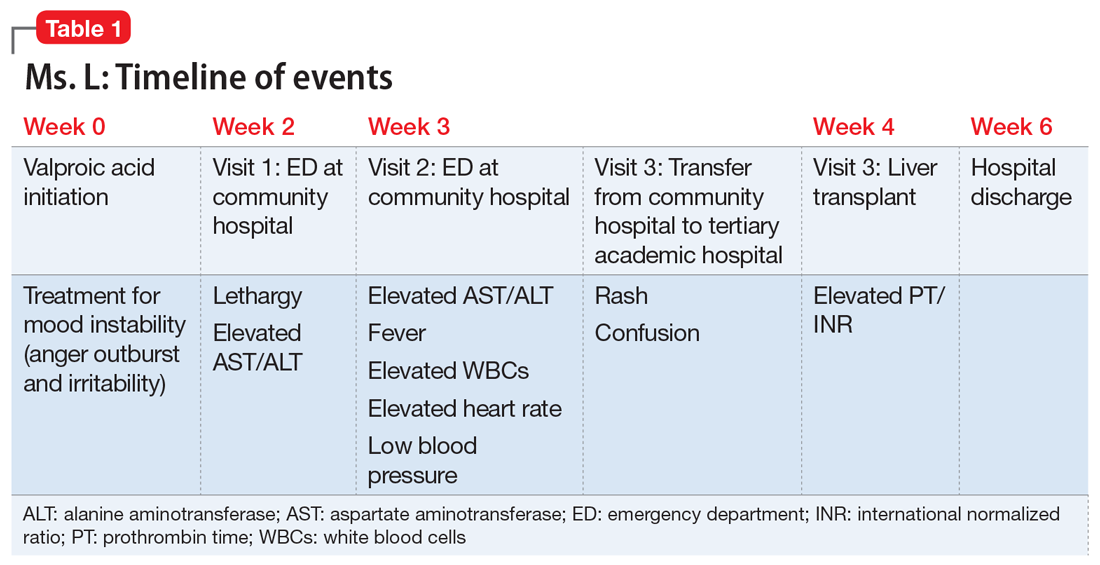
After Ms. L had been taking valproic acid for approximately 2 weeks, her mother noticed she became extremely lethargic and took her to the emergency department (ED) of a community hospital (Visit 1) (Table 1). At this time, her laboratory results were notable for an aspartate aminotransferase (AST) level of 303 IU/L (reference range: 8 to 40 IU/L) and an alanine aminotransferase (ALT) level of 241 IU/L (reference range: 20 to 60 IU/L). She also underwent a liver ultrasound, urine toxicology screen, blood alcohol level, and acetaminophen level; the results of all of these tests were unremarkable. Her valproic acid level was within therapeutic limits, consistent with patient adherence; her ammonia level was within normal limits. At Visit 1, Ms. L’s transaminitis was presumed to be secondary to valproic acid. The ED clinicians told her to stop taking valproic acid and discharged her. Her PCP did not give her any follow-up instructions for further laboratory tests or any other recommendations.
During the next week, even though she stopped taking the valproic acid as instructed, Ms. L developed a rash and fever, and continued to have lethargy and general malaise. When she returned to the ED (Visit 2) (Table 1), she was febrile, tachycardic, and hypotensive, with an elevated white blood cell count, eosinophilia, low platelets, and elevated liver function tests. At Visit 2, she was alert and oriented to person, place, time, and situation. Ms. L insisted that she had not overdosed on any medications, or used illicit drugs or alcohol. A test for hepatitis C was negative. Her ammonia level was 58 µmol/L (reference range: 11 to 32 µmol/L). Ms. L received N-acetylcysteine (NAC), prednisone, diphenhydramine, famotidine, and ibuprofen before she was transferred to our tertiary care hospital.
When she arrives at our facility (Visit 3) (Table 1), Ms. L is admitted with acute liver failure. She has an ALT level of 4,091 IU/L, and an AST level of 2,049 IU/L. Ms. L’s mother says that her daughter had been taking sertraline for depression for “some time” with no adverse effects, although she is not clear on the dose or frequency. Her mother says that Ms. L generally likes to spend most of her time at home, and does not believe her daughter is a danger to herself or others. Ms. L’s mother could not describe any episodes of mania or recurrent, dangerous anger episodes. Ms. L has no other medical history and has otherwise been healthy.
On hospital Day 2, Ms. L’s ammonia level is 72 µmol/L, which is slightly elevated. The hepatology team confirms that Ms. L may require a liver transplantation. The primary team consults the inpatient psychiatry consultation-liaison (C-L) team for a pre-transplant psychiatric evaluation.
[polldaddy:10307646]
The authors’ observations
The differential diagnosis for Ms. L was broad and included both accidental and intentional medication overdose. The primary team consulted the inpatient psychiatry C-L team not only for a pre-transplant evaluation, but also to assess for possible overdose.
Continue to: A review of the records...
A review of the records from Visit 1 and Visit 2 at the outside hospital found no acetaminophen in Ms. L’s system and verified that there was no evidence of a current valproic acid overdose. Ms. L had stated that she had not overdosed on any other medications or used any illicit drugs or alcohol. Ms. L’s complex symptoms—namely fever, acute liver failure, and rash—were more consistent with an adverse effect of valproic acid or possibly an inherent autoimmune process.
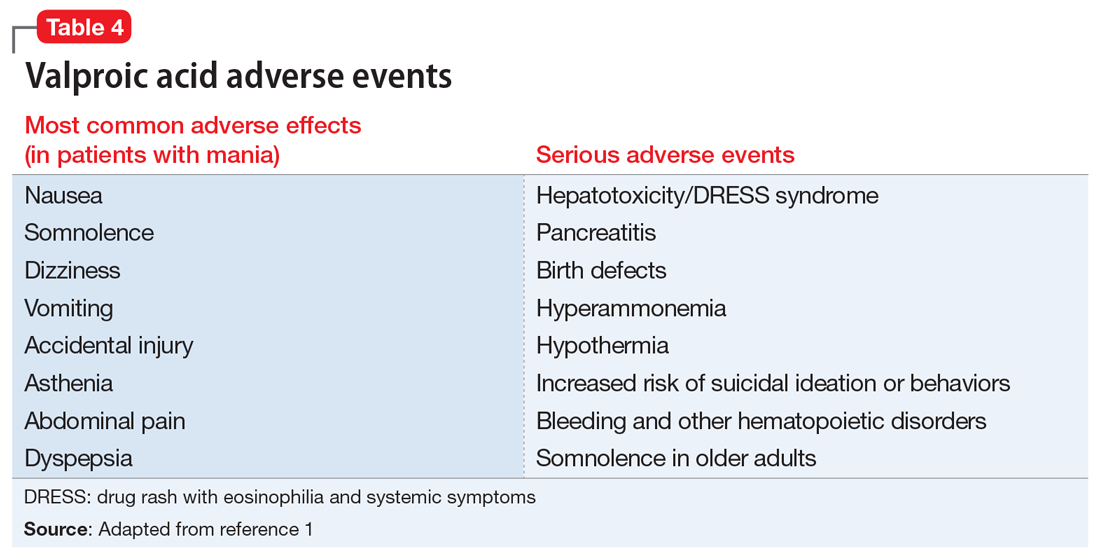
Liver damage from valproic acid
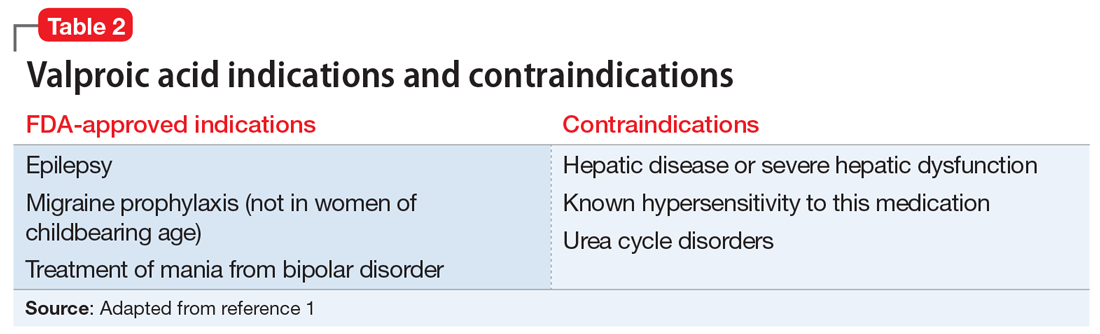
Valproic acid is FDA-approved for treating bipolar disorder, epilepsy, and migraine headaches1 (Table 21). Common adverse effects include nausea, vomiting, sleepiness, and dry mouth. Rarely, valproic acid can impair liver function. While receiving valproic acid, 5% to 10% of patients develop elevated ALT levels, but most are asymptomatic and resolve with time, even if the patient continues taking valproic acid.2 Valproic acid hepatotoxicity resulting in liver transplantation for a healthy patient is extremely rare (Table 31). Liver failure, both fatal and non-fatal, is more prevalent in patients concurrently taking other medications, such as antiepileptics, benzodiazepines, and antipsychotics, as compared with patients receiving only valproic acid.3
There are 3 clinically distinguishable forms of hepatotoxicity due to valproic acid2:
- hyperammonemia
- acute liver failure and jaundice
- Myriad ProReye-like syndrome, which is generally seen in children.
In case reports of hyperammonemia due to valproic acid, previously healthy patients experience confusion, lethargy, and eventual coma in the context of elevated serum ammonia levels; these symptoms resolved upon discontinuing valproic acid.4,5 Liver function remained normal, with normal to near-normal liver enzymes and bilirubin.3 Hyperammonemia and resulting encephalopathy generally occurred within 1 to 3 weeks after initiation of valproate therapy, with resolution of hyperammonemia and resulting symptoms within a few days after stopping valproic acid.2-4
At Visit 2, Ms. L’s presentation was not initially consistent with hepatic encephalopathy. She was alert and oriented to person, place, time, and situation. Additionally, Ms. L’s presenting problem was elevated liver function tests, not elevated ammonia levels. At Visit 2, her ammonia level was 58 µmol/L; on Day 2 (Visit 3) of her hospital stay, her ammonia level was 72 µmol/L (slightly elevated).
Continue to: At Visit 2 in the ED...
At Visit 2 in the ED, Ms. L was started on NAC because the team suspected she was experiencing drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. This syndrome is characterized by extensive rash, fever, and involvement of at least 1 internal organ. It is a variation of a drug-induced hypersensitivity syndrome. Ms. L’s unremarkable valproic acid levels combined with the psychiatry assessment ruled out valproic hepatotoxicity due to overdose, either intentional or accidental.
In case reports, patients who developed acute liver failure due to valproic acid typically had a hepatitis-like syndrome consisting of moderate elevation in liver enzymes, jaundice, and liver failure necessitating transplantation after at least 1 month of treatment with valproic acid.2 In addition to the typical hepatitis-like syndrome resulting from valproic acid, case reports have also linked treatment with valproic acid to DRESS syndrome.2 This syndrome is known to occur with anticonvulsants such as phenobarbital, lamotrigine, and phenytoin, but there are only a few reported cases of DRESS syndrome due to valproic acid therapy alone.6 Drug rash with eosinophilia and systemic symptoms syndrome differs from other acute liver failure cases in that patients also develop lymphadenopathy, fever, and rash.2,6,7 Patients with DRESS syndrome typically respond to corticosteroid therapy and discontinuation of valproic acid, and the liver damage resolves after several weeks, without a need for transplantation.2,6,7
Ms. L seemed to have similarities to DRESS syndrome. However, the severity of her liver damage, which might require transplantation even after only 2 weeks of valproic acid therapy, initially led the hepatology and C-L teams to consider her presentation similar to severe hepatitis-like cases.
EVALUATION Consent for transplantation
As an inpatient, Ms. L undergoes further laboratory testing. Her hepatic function panel demonstrates a total protein level of 4.8 g/dL, an albumin level of 2.0 g/dL, total bilirubin level of 12.2 mg/dL, and alkaline phosphatase of 166 IU/L. Her laboratory results indicate a prothrombin time (PT) of 77.4 seconds, partial thromboplastin time of 61.5 seconds, and PT international normalized ratio (INR) of 9.6. Ms. L’s basic metabolic panel is within normal limits except for a blood urea nitrogen level of 6 mg/dL, glucose level of 136 mg/dL, and calcium level of 7.0 mg/dL. Her complete blood count indicates a white blood cell count of 12.1, hemoglobin of 10.3 g/dL, hematocrit of 30.4%, mean corpuscular volume of 85.9 fL, and platelet count of 84. Her lipase level is normal at 49 U/L. Her serum acetaminophen concentration is <3.0 mcg/mL, valproic acid level was <2 µg/mL, and she is negative for hepatitis A, B, and C. A urine toxicology screen and testing for herpes simplex, rapid plasma reagin, and human immunodeficiency virus are all negative. Results from several auto-antibodies tests are negative and within normal limits, except filamentous actin (F-actin) antibody, which is slightly higher than normal at 21.4 ELISA units. Based on these results, Ms. L’s liver failure seemed most likely secondary to a reaction to valproic acid.
During her pre-transplant psychiatric evaluation, Ms. L is found to be a poor historian with minimal speech production, flat affect, and clouded sensorium. She denies overdosing on her prescribed valproic acid or sertraline, reports no current suicidal ideation, and does not want to die. She accurately recalls her correct daily dosing of each medication, and verifies that she stopped taking valproic acid 2 weeks ago after being advised to do so by the ED clinicians at Visit 2. She continued to take sertraline until Visit 2. She denied any past or present episodes consistent with mania, which was consistent with her mother’s report.
Continue to: Ms. L becomes agitated...
Ms. L becomes agitated upon further questioning, and requests immediate discharge so that she can return to her family. The evaluation is postponed briefly.
When they reconvene, the C-L team performs a decision-making capacity evaluation, which reveals that Ms. L’s mood and affect are consistent with fear of her impending liver transplant and being alone and approximately 2 hours from her family. This is likely complicated by delirium due to hepatotoxicity. Further discussion between Ms. L and the multidisciplinary team focuses on the risks, benefits, adverse effects of, and alternatives to her current treatment; the possibility of needing a liver transplantation; and how to help her family with transportation to the hospital. Following the discussion, Ms. L is fully cooperative with further treatment, and the pre-transplant psychiatric evaluation is completed.
On physical examination, Ms. L is noted to have a widespread morbilliform rash covering 50% to 60% of her body.
[polldaddy:10307651]
The authors’ observations
L-carnitine supplementation
Multiple studies have shown that supplementation with L-carnitine may increase survival from severe hepatotoxicity due to valproic acid.8,9 Valproic acid may contribute to carnitine deficiency due to its inhibition of carnitine biosynthesis via a decrease in alpha-ketoglutarate concentration.8 Hepatotoxicity or hyperammonemia due to valproic acid may be potentiated by a carnitine deficiency, either pre-existing or resulting from valproic acid.8 L-carnitine supplementation has hastened the decrease of valproic acid–induced ammonemia in a dose-dependent manner,10 and it is currently recommended in cases of valproic acid toxicity, especially in children.8 Children at high risk for developing carnitine deficiency who need to receive valproic acid can be given carnitine supplementation.11 It is not known whether L-carnitine is clinically effective in protecting the liver or hastening liver recovery,8 but it is believed that it might prevent adverse effects of hepatotoxicity and hyperammonemia, especially in patients who receive long-term valproic acid therapy.12
TREATMENT Decompensation and transplantation
Ms. L’s treatment regimen includes NAC, lactulose, and L-carnitine supplementation. During the course of Ms. L’s hospital stay, her liver enzymes begin to trend downward, but her INR and PT remain elevated.
Continue to: On hospital Day 6...
On hospital Day 6, she develops more severe symptoms of hepatic encephalopathy, with significant altered mental status and inattention. Ms. L is transferred to the ICU, intubated, and placed on the liver transplant list.
On hospital Day 9, she undergoes a liver transplantation.
[polldaddy:10307652]
The authors’ observations
Baseline laboratory testing should have been conducted prior to initiating valproic acid. As Ms. L’s symptoms worsened, better communication with her PCP and closer monitoring after starting valproic acid might have resulted in more immediate care. Early recognition of her symptoms and decompensation may have triggered earlier inpatient admission and/or transfer to a tertiary care facility for observation and treatment. Additionally, repeat laboratory testing and instructions on when to return to the ED should have been provided at Visit 1.
This case demonstrates the need for all clinicians who prescribe valproic acid to remain diligent about the accurate diagnosis of mood and behavioral symptoms, knowing when psychotropic medications are indicated, and carefully considering and discussing even rare, potentially life-threatening adverse effects of all medications with patients.
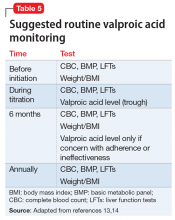
Although rare, after starting valproic acid, a patient may experience a rapid decompensation and life-threatening illness. Ideally, clinicians should closely monitor patients after initiating valproic acid (Table 41). Clinicians must have a clear knowledge of the recommended monitoring and indications for hospitalization and treatment when they note adverse effects such as elevated liver enzymes or transaminitis (Table 513,14). Even after stopping valproic acid, patients who have experienced adverse events should be closely monitored to ensure complete resolution.
Continue to: Consider patient-specific factors
Consider patient-specific factors
Consider the mental state, intellectual capacity, and social support of each patient before initiating valproic acid. Its use as a mood stabilizer for “mood swings” outside of the context of bipolar disorder is questionable. Valproic acid is FDA-approved for treating bipolar disorder and seizures, but not for anger outbursts/irritability. Prior to starting valproic acid, Ms. L may have benefited from alternative nonpharmacologic treatments, such as psychotherapy, for her anger outbursts and poor coping skills. Therapeutic techniques that focused on helping her acquire better coping mechanisms may have been useful, especially because her mood symptoms did not meet criteria for bipolar disorder, and her depression had long been controlled with sertraline monotherapy.
OUTCOME Discharged after 20 days
Ms. L stays in the hospital for 10 days after receiving her liver transplant. She has low appetite and some difficulty with sleep after the transplant; therefore, the C-L team recommends mirtazapine, 15 mg/d. She has no behavioral problems during her stay, and is set up with home health, case management, and psychiatry follow-up. On hospital Day 20, she is discharged.
Bottom Line
Use caution when prescribing valproic acid, even in young, otherwise healthy patients. Although rare, some patients may experience a rapid decompensation and life-threatening illness after starting valproic acid. When prescribing valproic acid, ensure close follow-up after initiation, including mental status examinations, physical examinations, and laboratory testing.
Related Resource
- Doroudgar S, Chou TI. How to modify psychotropic therapy for patients who have liver dysfunction. Current Psychiatry. 2014;13(12):46-49.
Drug Brand Names
Diphenhydramine • Benadryl
Famotidine • Fluxid, Pepcid
Lamotrigine • Lamictal
Mirtazapine • Remeron
N-acetylcysteine • Mucomyst
Phenobarbital • Luminal
Phenytoin • Dilantin
Prednisone • Cortan, Deltasone
Sertraline • Zoloft
Valproic acid • Depakene
1. Depakote [package insert]. North Chicago, IL: AbbVie, Inc.; 2019.
2. National Institutes of Health. U.S. Department of Health and Human Services. Drug Record: Valproate. https://livertox.nlm.nih.gov/Valproate.htm. Updated October 30, 2018. Accessed March 21, 2019.
3. Schmid MM, Freudenmann RW, Keller F, et al. Non-fatal and fatal liver failure associated with valproic acid. Pharmacopsychiatry. 2013;46(2):63-68.
4. Patel N, Landry KB, Fargason RE, et al. Reversible encephalopathy due to valproic acid induced hyperammonemia in a patient with Bipolar I disorder: a cautionary report. Psychopharmacol Bull. 2017;47(1):40-44.
5. Eze E, Workman M, Donley B. Hyperammonemia and coma developed by a woman treated with valproic acid for affective disorder. Psychiatr Serv. 1998;49(10):1358-1359.
6. Darban M and Bagheri B. Drug reaction with eosinophilia and systemic symptoms induced by valproic acid: a case report. Iran Red Crescent Med J. 2016;18(9): e35825.
7. van Zoelen MA, de Graaf M, van Dijk MR, et al. Valproic acid-induced DRESS syndrome with acute liver failure. Neth J Med. 2012;70(3):155.
8. Lheureux PE, Hantson P. Carnitine in the treatment of valproic acid-induced toxicity. Clin Toxicol (Phila). 2009;47(2):101-111.
9. Bohan TP, Helton E, McDonald I, et al. Effect of L-carnitine treatment for valproate-induced hepatotoxicity. Neurology. 2001;56(10):1405-1409.
10. Böhles H, Sewell AC, Wenzel D. The effect of carnitine supplementation in valproate-induced hyperammonaemia. Acta Paediatr. 1996;85(4):446-449.
11. Raskind JY, El-Chaar GM. The role of carnitine supplementation during valproic acid therapy. Ann Pharmacother. 2000;34(5):630-638.
12. Romero-Falcón A, de la Santa-Belda E, García-Contreras R, et al. A case of valproate-associated hepatotoxicity treated with L-carnitine. Eur J Intern Med. 2003;14(5):338-340.
13. National Institute for Health and Clinical Excellence. Bipolar disorder: the management of bipolar disorder in adults, children, and adolescents, in primary and secondary care. https://www.nice.org.uk/guidance/cg185. Updated April 2018. Accessed March 21, 2019.
14 . Hirschfeld RMA, Bowden CL, Gitlin MJ, et al. Practice guideline for the treatment of patients with biopolar disorder: second edition. American Psychiatric Association. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/bipolar.pdf. Published 2002. Accessed March 21, 2019.
CASE Rash, fever, extreme lethargy; multiple hospital visits
Ms. L, age 21, a single woman with a history of major depressive disorder (MDD), is directly admitted from an outside community hospital to our tertiary care academic hospital with acute liver failure.
One month earlier, Ms. L had an argument with her family and punched a wall, fracturing her hand. Following the episode, Ms. L’s primary care physician (PCP) prescribed valproic acid, 500 mg/d, to address “mood swings,” which included angry outbursts and irritability. According to her PCP, no baseline laboratory tests were ordered for Ms. L when she started valproic acid because she was young and otherwise healthy.
After Ms. L had been taking valproic acid for approximately 2 weeks, her mother noticed she became extremely lethargic and took her to the emergency department (ED) of a community hospital (Visit 1) (Table 1). At this time, her laboratory results were notable for an aspartate aminotransferase (AST) level of 303 IU/L (reference range: 8 to 40 IU/L) and an alanine aminotransferase (ALT) level of 241 IU/L (reference range: 20 to 60 IU/L). She also underwent a liver ultrasound, urine toxicology screen, blood alcohol level, and acetaminophen level; the results of all of these tests were unremarkable. Her valproic acid level was within therapeutic limits, consistent with patient adherence; her ammonia level was within normal limits. At Visit 1, Ms. L’s transaminitis was presumed to be secondary to valproic acid. The ED clinicians told her to stop taking valproic acid and discharged her. Her PCP did not give her any follow-up instructions for further laboratory tests or any other recommendations.
During the next week, even though she stopped taking the valproic acid as instructed, Ms. L developed a rash and fever, and continued to have lethargy and general malaise. When she returned to the ED (Visit 2) (Table 1), she was febrile, tachycardic, and hypotensive, with an elevated white blood cell count, eosinophilia, low platelets, and elevated liver function tests. At Visit 2, she was alert and oriented to person, place, time, and situation. Ms. L insisted that she had not overdosed on any medications, or used illicit drugs or alcohol. A test for hepatitis C was negative. Her ammonia level was 58 µmol/L (reference range: 11 to 32 µmol/L). Ms. L received N-acetylcysteine (NAC), prednisone, diphenhydramine, famotidine, and ibuprofen before she was transferred to our tertiary care hospital.
When she arrives at our facility (Visit 3) (Table 1), Ms. L is admitted with acute liver failure. She has an ALT level of 4,091 IU/L, and an AST level of 2,049 IU/L. Ms. L’s mother says that her daughter had been taking sertraline for depression for “some time” with no adverse effects, although she is not clear on the dose or frequency. Her mother says that Ms. L generally likes to spend most of her time at home, and does not believe her daughter is a danger to herself or others. Ms. L’s mother could not describe any episodes of mania or recurrent, dangerous anger episodes. Ms. L has no other medical history and has otherwise been healthy.
On hospital Day 2, Ms. L’s ammonia level is 72 µmol/L, which is slightly elevated. The hepatology team confirms that Ms. L may require a liver transplantation. The primary team consults the inpatient psychiatry consultation-liaison (C-L) team for a pre-transplant psychiatric evaluation.
[polldaddy:10307646]
The authors’ observations
The differential diagnosis for Ms. L was broad and included both accidental and intentional medication overdose. The primary team consulted the inpatient psychiatry C-L team not only for a pre-transplant evaluation, but also to assess for possible overdose.
Continue to: A review of the records...
A review of the records from Visit 1 and Visit 2 at the outside hospital found no acetaminophen in Ms. L’s system and verified that there was no evidence of a current valproic acid overdose. Ms. L had stated that she had not overdosed on any other medications or used any illicit drugs or alcohol. Ms. L’s complex symptoms—namely fever, acute liver failure, and rash—were more consistent with an adverse effect of valproic acid or possibly an inherent autoimmune process.
Liver damage from valproic acid
Valproic acid is FDA-approved for treating bipolar disorder, epilepsy, and migraine headaches1 (Table 21). Common adverse effects include nausea, vomiting, sleepiness, and dry mouth. Rarely, valproic acid can impair liver function. While receiving valproic acid, 5% to 10% of patients develop elevated ALT levels, but most are asymptomatic and resolve with time, even if the patient continues taking valproic acid.2 Valproic acid hepatotoxicity resulting in liver transplantation for a healthy patient is extremely rare (Table 31). Liver failure, both fatal and non-fatal, is more prevalent in patients concurrently taking other medications, such as antiepileptics, benzodiazepines, and antipsychotics, as compared with patients receiving only valproic acid.3
There are 3 clinically distinguishable forms of hepatotoxicity due to valproic acid2:
- hyperammonemia
- acute liver failure and jaundice
- Myriad ProReye-like syndrome, which is generally seen in children.
In case reports of hyperammonemia due to valproic acid, previously healthy patients experience confusion, lethargy, and eventual coma in the context of elevated serum ammonia levels; these symptoms resolved upon discontinuing valproic acid.4,5 Liver function remained normal, with normal to near-normal liver enzymes and bilirubin.3 Hyperammonemia and resulting encephalopathy generally occurred within 1 to 3 weeks after initiation of valproate therapy, with resolution of hyperammonemia and resulting symptoms within a few days after stopping valproic acid.2-4
At Visit 2, Ms. L’s presentation was not initially consistent with hepatic encephalopathy. She was alert and oriented to person, place, time, and situation. Additionally, Ms. L’s presenting problem was elevated liver function tests, not elevated ammonia levels. At Visit 2, her ammonia level was 58 µmol/L; on Day 2 (Visit 3) of her hospital stay, her ammonia level was 72 µmol/L (slightly elevated).
Continue to: At Visit 2 in the ED...
At Visit 2 in the ED, Ms. L was started on NAC because the team suspected she was experiencing drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. This syndrome is characterized by extensive rash, fever, and involvement of at least 1 internal organ. It is a variation of a drug-induced hypersensitivity syndrome. Ms. L’s unremarkable valproic acid levels combined with the psychiatry assessment ruled out valproic hepatotoxicity due to overdose, either intentional or accidental.
In case reports, patients who developed acute liver failure due to valproic acid typically had a hepatitis-like syndrome consisting of moderate elevation in liver enzymes, jaundice, and liver failure necessitating transplantation after at least 1 month of treatment with valproic acid.2 In addition to the typical hepatitis-like syndrome resulting from valproic acid, case reports have also linked treatment with valproic acid to DRESS syndrome.2 This syndrome is known to occur with anticonvulsants such as phenobarbital, lamotrigine, and phenytoin, but there are only a few reported cases of DRESS syndrome due to valproic acid therapy alone.6 Drug rash with eosinophilia and systemic symptoms syndrome differs from other acute liver failure cases in that patients also develop lymphadenopathy, fever, and rash.2,6,7 Patients with DRESS syndrome typically respond to corticosteroid therapy and discontinuation of valproic acid, and the liver damage resolves after several weeks, without a need for transplantation.2,6,7
Ms. L seemed to have similarities to DRESS syndrome. However, the severity of her liver damage, which might require transplantation even after only 2 weeks of valproic acid therapy, initially led the hepatology and C-L teams to consider her presentation similar to severe hepatitis-like cases.
EVALUATION Consent for transplantation
As an inpatient, Ms. L undergoes further laboratory testing. Her hepatic function panel demonstrates a total protein level of 4.8 g/dL, an albumin level of 2.0 g/dL, total bilirubin level of 12.2 mg/dL, and alkaline phosphatase of 166 IU/L. Her laboratory results indicate a prothrombin time (PT) of 77.4 seconds, partial thromboplastin time of 61.5 seconds, and PT international normalized ratio (INR) of 9.6. Ms. L’s basic metabolic panel is within normal limits except for a blood urea nitrogen level of 6 mg/dL, glucose level of 136 mg/dL, and calcium level of 7.0 mg/dL. Her complete blood count indicates a white blood cell count of 12.1, hemoglobin of 10.3 g/dL, hematocrit of 30.4%, mean corpuscular volume of 85.9 fL, and platelet count of 84. Her lipase level is normal at 49 U/L. Her serum acetaminophen concentration is <3.0 mcg/mL, valproic acid level was <2 µg/mL, and she is negative for hepatitis A, B, and C. A urine toxicology screen and testing for herpes simplex, rapid plasma reagin, and human immunodeficiency virus are all negative. Results from several auto-antibodies tests are negative and within normal limits, except filamentous actin (F-actin) antibody, which is slightly higher than normal at 21.4 ELISA units. Based on these results, Ms. L’s liver failure seemed most likely secondary to a reaction to valproic acid.
During her pre-transplant psychiatric evaluation, Ms. L is found to be a poor historian with minimal speech production, flat affect, and clouded sensorium. She denies overdosing on her prescribed valproic acid or sertraline, reports no current suicidal ideation, and does not want to die. She accurately recalls her correct daily dosing of each medication, and verifies that she stopped taking valproic acid 2 weeks ago after being advised to do so by the ED clinicians at Visit 2. She continued to take sertraline until Visit 2. She denied any past or present episodes consistent with mania, which was consistent with her mother’s report.
Continue to: Ms. L becomes agitated...
Ms. L becomes agitated upon further questioning, and requests immediate discharge so that she can return to her family. The evaluation is postponed briefly.
When they reconvene, the C-L team performs a decision-making capacity evaluation, which reveals that Ms. L’s mood and affect are consistent with fear of her impending liver transplant and being alone and approximately 2 hours from her family. This is likely complicated by delirium due to hepatotoxicity. Further discussion between Ms. L and the multidisciplinary team focuses on the risks, benefits, adverse effects of, and alternatives to her current treatment; the possibility of needing a liver transplantation; and how to help her family with transportation to the hospital. Following the discussion, Ms. L is fully cooperative with further treatment, and the pre-transplant psychiatric evaluation is completed.
On physical examination, Ms. L is noted to have a widespread morbilliform rash covering 50% to 60% of her body.
[polldaddy:10307651]
The authors’ observations
L-carnitine supplementation
Multiple studies have shown that supplementation with L-carnitine may increase survival from severe hepatotoxicity due to valproic acid.8,9 Valproic acid may contribute to carnitine deficiency due to its inhibition of carnitine biosynthesis via a decrease in alpha-ketoglutarate concentration.8 Hepatotoxicity or hyperammonemia due to valproic acid may be potentiated by a carnitine deficiency, either pre-existing or resulting from valproic acid.8 L-carnitine supplementation has hastened the decrease of valproic acid–induced ammonemia in a dose-dependent manner,10 and it is currently recommended in cases of valproic acid toxicity, especially in children.8 Children at high risk for developing carnitine deficiency who need to receive valproic acid can be given carnitine supplementation.11 It is not known whether L-carnitine is clinically effective in protecting the liver or hastening liver recovery,8 but it is believed that it might prevent adverse effects of hepatotoxicity and hyperammonemia, especially in patients who receive long-term valproic acid therapy.12
TREATMENT Decompensation and transplantation
Ms. L’s treatment regimen includes NAC, lactulose, and L-carnitine supplementation. During the course of Ms. L’s hospital stay, her liver enzymes begin to trend downward, but her INR and PT remain elevated.
Continue to: On hospital Day 6...
On hospital Day 6, she develops more severe symptoms of hepatic encephalopathy, with significant altered mental status and inattention. Ms. L is transferred to the ICU, intubated, and placed on the liver transplant list.
On hospital Day 9, she undergoes a liver transplantation.
[polldaddy:10307652]
The authors’ observations
Baseline laboratory testing should have been conducted prior to initiating valproic acid. As Ms. L’s symptoms worsened, better communication with her PCP and closer monitoring after starting valproic acid might have resulted in more immediate care. Early recognition of her symptoms and decompensation may have triggered earlier inpatient admission and/or transfer to a tertiary care facility for observation and treatment. Additionally, repeat laboratory testing and instructions on when to return to the ED should have been provided at Visit 1.
This case demonstrates the need for all clinicians who prescribe valproic acid to remain diligent about the accurate diagnosis of mood and behavioral symptoms, knowing when psychotropic medications are indicated, and carefully considering and discussing even rare, potentially life-threatening adverse effects of all medications with patients.
Although rare, after starting valproic acid, a patient may experience a rapid decompensation and life-threatening illness. Ideally, clinicians should closely monitor patients after initiating valproic acid (Table 41). Clinicians must have a clear knowledge of the recommended monitoring and indications for hospitalization and treatment when they note adverse effects such as elevated liver enzymes or transaminitis (Table 513,14). Even after stopping valproic acid, patients who have experienced adverse events should be closely monitored to ensure complete resolution.
Continue to: Consider patient-specific factors
Consider patient-specific factors
Consider the mental state, intellectual capacity, and social support of each patient before initiating valproic acid. Its use as a mood stabilizer for “mood swings” outside of the context of bipolar disorder is questionable. Valproic acid is FDA-approved for treating bipolar disorder and seizures, but not for anger outbursts/irritability. Prior to starting valproic acid, Ms. L may have benefited from alternative nonpharmacologic treatments, such as psychotherapy, for her anger outbursts and poor coping skills. Therapeutic techniques that focused on helping her acquire better coping mechanisms may have been useful, especially because her mood symptoms did not meet criteria for bipolar disorder, and her depression had long been controlled with sertraline monotherapy.
OUTCOME Discharged after 20 days
Ms. L stays in the hospital for 10 days after receiving her liver transplant. She has low appetite and some difficulty with sleep after the transplant; therefore, the C-L team recommends mirtazapine, 15 mg/d. She has no behavioral problems during her stay, and is set up with home health, case management, and psychiatry follow-up. On hospital Day 20, she is discharged.
Bottom Line
Use caution when prescribing valproic acid, even in young, otherwise healthy patients. Although rare, some patients may experience a rapid decompensation and life-threatening illness after starting valproic acid. When prescribing valproic acid, ensure close follow-up after initiation, including mental status examinations, physical examinations, and laboratory testing.
Related Resource
- Doroudgar S, Chou TI. How to modify psychotropic therapy for patients who have liver dysfunction. Current Psychiatry. 2014;13(12):46-49.
Drug Brand Names
Diphenhydramine • Benadryl
Famotidine • Fluxid, Pepcid
Lamotrigine • Lamictal
Mirtazapine • Remeron
N-acetylcysteine • Mucomyst
Phenobarbital • Luminal
Phenytoin • Dilantin
Prednisone • Cortan, Deltasone
Sertraline • Zoloft
Valproic acid • Depakene
CASE Rash, fever, extreme lethargy; multiple hospital visits
Ms. L, age 21, a single woman with a history of major depressive disorder (MDD), is directly admitted from an outside community hospital to our tertiary care academic hospital with acute liver failure.
One month earlier, Ms. L had an argument with her family and punched a wall, fracturing her hand. Following the episode, Ms. L’s primary care physician (PCP) prescribed valproic acid, 500 mg/d, to address “mood swings,” which included angry outbursts and irritability. According to her PCP, no baseline laboratory tests were ordered for Ms. L when she started valproic acid because she was young and otherwise healthy.
After Ms. L had been taking valproic acid for approximately 2 weeks, her mother noticed she became extremely lethargic and took her to the emergency department (ED) of a community hospital (Visit 1) (Table 1). At this time, her laboratory results were notable for an aspartate aminotransferase (AST) level of 303 IU/L (reference range: 8 to 40 IU/L) and an alanine aminotransferase (ALT) level of 241 IU/L (reference range: 20 to 60 IU/L). She also underwent a liver ultrasound, urine toxicology screen, blood alcohol level, and acetaminophen level; the results of all of these tests were unremarkable. Her valproic acid level was within therapeutic limits, consistent with patient adherence; her ammonia level was within normal limits. At Visit 1, Ms. L’s transaminitis was presumed to be secondary to valproic acid. The ED clinicians told her to stop taking valproic acid and discharged her. Her PCP did not give her any follow-up instructions for further laboratory tests or any other recommendations.
During the next week, even though she stopped taking the valproic acid as instructed, Ms. L developed a rash and fever, and continued to have lethargy and general malaise. When she returned to the ED (Visit 2) (Table 1), she was febrile, tachycardic, and hypotensive, with an elevated white blood cell count, eosinophilia, low platelets, and elevated liver function tests. At Visit 2, she was alert and oriented to person, place, time, and situation. Ms. L insisted that she had not overdosed on any medications, or used illicit drugs or alcohol. A test for hepatitis C was negative. Her ammonia level was 58 µmol/L (reference range: 11 to 32 µmol/L). Ms. L received N-acetylcysteine (NAC), prednisone, diphenhydramine, famotidine, and ibuprofen before she was transferred to our tertiary care hospital.
When she arrives at our facility (Visit 3) (Table 1), Ms. L is admitted with acute liver failure. She has an ALT level of 4,091 IU/L, and an AST level of 2,049 IU/L. Ms. L’s mother says that her daughter had been taking sertraline for depression for “some time” with no adverse effects, although she is not clear on the dose or frequency. Her mother says that Ms. L generally likes to spend most of her time at home, and does not believe her daughter is a danger to herself or others. Ms. L’s mother could not describe any episodes of mania or recurrent, dangerous anger episodes. Ms. L has no other medical history and has otherwise been healthy.
On hospital Day 2, Ms. L’s ammonia level is 72 µmol/L, which is slightly elevated. The hepatology team confirms that Ms. L may require a liver transplantation. The primary team consults the inpatient psychiatry consultation-liaison (C-L) team for a pre-transplant psychiatric evaluation.
[polldaddy:10307646]
The authors’ observations
The differential diagnosis for Ms. L was broad and included both accidental and intentional medication overdose. The primary team consulted the inpatient psychiatry C-L team not only for a pre-transplant evaluation, but also to assess for possible overdose.
Continue to: A review of the records...
A review of the records from Visit 1 and Visit 2 at the outside hospital found no acetaminophen in Ms. L’s system and verified that there was no evidence of a current valproic acid overdose. Ms. L had stated that she had not overdosed on any other medications or used any illicit drugs or alcohol. Ms. L’s complex symptoms—namely fever, acute liver failure, and rash—were more consistent with an adverse effect of valproic acid or possibly an inherent autoimmune process.
Liver damage from valproic acid
Valproic acid is FDA-approved for treating bipolar disorder, epilepsy, and migraine headaches1 (Table 21). Common adverse effects include nausea, vomiting, sleepiness, and dry mouth. Rarely, valproic acid can impair liver function. While receiving valproic acid, 5% to 10% of patients develop elevated ALT levels, but most are asymptomatic and resolve with time, even if the patient continues taking valproic acid.2 Valproic acid hepatotoxicity resulting in liver transplantation for a healthy patient is extremely rare (Table 31). Liver failure, both fatal and non-fatal, is more prevalent in patients concurrently taking other medications, such as antiepileptics, benzodiazepines, and antipsychotics, as compared with patients receiving only valproic acid.3
There are 3 clinically distinguishable forms of hepatotoxicity due to valproic acid2:
- hyperammonemia
- acute liver failure and jaundice
- Myriad ProReye-like syndrome, which is generally seen in children.
In case reports of hyperammonemia due to valproic acid, previously healthy patients experience confusion, lethargy, and eventual coma in the context of elevated serum ammonia levels; these symptoms resolved upon discontinuing valproic acid.4,5 Liver function remained normal, with normal to near-normal liver enzymes and bilirubin.3 Hyperammonemia and resulting encephalopathy generally occurred within 1 to 3 weeks after initiation of valproate therapy, with resolution of hyperammonemia and resulting symptoms within a few days after stopping valproic acid.2-4
At Visit 2, Ms. L’s presentation was not initially consistent with hepatic encephalopathy. She was alert and oriented to person, place, time, and situation. Additionally, Ms. L’s presenting problem was elevated liver function tests, not elevated ammonia levels. At Visit 2, her ammonia level was 58 µmol/L; on Day 2 (Visit 3) of her hospital stay, her ammonia level was 72 µmol/L (slightly elevated).
Continue to: At Visit 2 in the ED...
At Visit 2 in the ED, Ms. L was started on NAC because the team suspected she was experiencing drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. This syndrome is characterized by extensive rash, fever, and involvement of at least 1 internal organ. It is a variation of a drug-induced hypersensitivity syndrome. Ms. L’s unremarkable valproic acid levels combined with the psychiatry assessment ruled out valproic hepatotoxicity due to overdose, either intentional or accidental.
In case reports, patients who developed acute liver failure due to valproic acid typically had a hepatitis-like syndrome consisting of moderate elevation in liver enzymes, jaundice, and liver failure necessitating transplantation after at least 1 month of treatment with valproic acid.2 In addition to the typical hepatitis-like syndrome resulting from valproic acid, case reports have also linked treatment with valproic acid to DRESS syndrome.2 This syndrome is known to occur with anticonvulsants such as phenobarbital, lamotrigine, and phenytoin, but there are only a few reported cases of DRESS syndrome due to valproic acid therapy alone.6 Drug rash with eosinophilia and systemic symptoms syndrome differs from other acute liver failure cases in that patients also develop lymphadenopathy, fever, and rash.2,6,7 Patients with DRESS syndrome typically respond to corticosteroid therapy and discontinuation of valproic acid, and the liver damage resolves after several weeks, without a need for transplantation.2,6,7
Ms. L seemed to have similarities to DRESS syndrome. However, the severity of her liver damage, which might require transplantation even after only 2 weeks of valproic acid therapy, initially led the hepatology and C-L teams to consider her presentation similar to severe hepatitis-like cases.
EVALUATION Consent for transplantation
As an inpatient, Ms. L undergoes further laboratory testing. Her hepatic function panel demonstrates a total protein level of 4.8 g/dL, an albumin level of 2.0 g/dL, total bilirubin level of 12.2 mg/dL, and alkaline phosphatase of 166 IU/L. Her laboratory results indicate a prothrombin time (PT) of 77.4 seconds, partial thromboplastin time of 61.5 seconds, and PT international normalized ratio (INR) of 9.6. Ms. L’s basic metabolic panel is within normal limits except for a blood urea nitrogen level of 6 mg/dL, glucose level of 136 mg/dL, and calcium level of 7.0 mg/dL. Her complete blood count indicates a white blood cell count of 12.1, hemoglobin of 10.3 g/dL, hematocrit of 30.4%, mean corpuscular volume of 85.9 fL, and platelet count of 84. Her lipase level is normal at 49 U/L. Her serum acetaminophen concentration is <3.0 mcg/mL, valproic acid level was <2 µg/mL, and she is negative for hepatitis A, B, and C. A urine toxicology screen and testing for herpes simplex, rapid plasma reagin, and human immunodeficiency virus are all negative. Results from several auto-antibodies tests are negative and within normal limits, except filamentous actin (F-actin) antibody, which is slightly higher than normal at 21.4 ELISA units. Based on these results, Ms. L’s liver failure seemed most likely secondary to a reaction to valproic acid.
During her pre-transplant psychiatric evaluation, Ms. L is found to be a poor historian with minimal speech production, flat affect, and clouded sensorium. She denies overdosing on her prescribed valproic acid or sertraline, reports no current suicidal ideation, and does not want to die. She accurately recalls her correct daily dosing of each medication, and verifies that she stopped taking valproic acid 2 weeks ago after being advised to do so by the ED clinicians at Visit 2. She continued to take sertraline until Visit 2. She denied any past or present episodes consistent with mania, which was consistent with her mother’s report.
Continue to: Ms. L becomes agitated...
Ms. L becomes agitated upon further questioning, and requests immediate discharge so that she can return to her family. The evaluation is postponed briefly.
When they reconvene, the C-L team performs a decision-making capacity evaluation, which reveals that Ms. L’s mood and affect are consistent with fear of her impending liver transplant and being alone and approximately 2 hours from her family. This is likely complicated by delirium due to hepatotoxicity. Further discussion between Ms. L and the multidisciplinary team focuses on the risks, benefits, adverse effects of, and alternatives to her current treatment; the possibility of needing a liver transplantation; and how to help her family with transportation to the hospital. Following the discussion, Ms. L is fully cooperative with further treatment, and the pre-transplant psychiatric evaluation is completed.
On physical examination, Ms. L is noted to have a widespread morbilliform rash covering 50% to 60% of her body.
[polldaddy:10307651]
The authors’ observations
L-carnitine supplementation
Multiple studies have shown that supplementation with L-carnitine may increase survival from severe hepatotoxicity due to valproic acid.8,9 Valproic acid may contribute to carnitine deficiency due to its inhibition of carnitine biosynthesis via a decrease in alpha-ketoglutarate concentration.8 Hepatotoxicity or hyperammonemia due to valproic acid may be potentiated by a carnitine deficiency, either pre-existing or resulting from valproic acid.8 L-carnitine supplementation has hastened the decrease of valproic acid–induced ammonemia in a dose-dependent manner,10 and it is currently recommended in cases of valproic acid toxicity, especially in children.8 Children at high risk for developing carnitine deficiency who need to receive valproic acid can be given carnitine supplementation.11 It is not known whether L-carnitine is clinically effective in protecting the liver or hastening liver recovery,8 but it is believed that it might prevent adverse effects of hepatotoxicity and hyperammonemia, especially in patients who receive long-term valproic acid therapy.12
TREATMENT Decompensation and transplantation
Ms. L’s treatment regimen includes NAC, lactulose, and L-carnitine supplementation. During the course of Ms. L’s hospital stay, her liver enzymes begin to trend downward, but her INR and PT remain elevated.
Continue to: On hospital Day 6...
On hospital Day 6, she develops more severe symptoms of hepatic encephalopathy, with significant altered mental status and inattention. Ms. L is transferred to the ICU, intubated, and placed on the liver transplant list.
On hospital Day 9, she undergoes a liver transplantation.
[polldaddy:10307652]
The authors’ observations
Baseline laboratory testing should have been conducted prior to initiating valproic acid. As Ms. L’s symptoms worsened, better communication with her PCP and closer monitoring after starting valproic acid might have resulted in more immediate care. Early recognition of her symptoms and decompensation may have triggered earlier inpatient admission and/or transfer to a tertiary care facility for observation and treatment. Additionally, repeat laboratory testing and instructions on when to return to the ED should have been provided at Visit 1.
This case demonstrates the need for all clinicians who prescribe valproic acid to remain diligent about the accurate diagnosis of mood and behavioral symptoms, knowing when psychotropic medications are indicated, and carefully considering and discussing even rare, potentially life-threatening adverse effects of all medications with patients.
Although rare, after starting valproic acid, a patient may experience a rapid decompensation and life-threatening illness. Ideally, clinicians should closely monitor patients after initiating valproic acid (Table 41). Clinicians must have a clear knowledge of the recommended monitoring and indications for hospitalization and treatment when they note adverse effects such as elevated liver enzymes or transaminitis (Table 513,14). Even after stopping valproic acid, patients who have experienced adverse events should be closely monitored to ensure complete resolution.
Continue to: Consider patient-specific factors
Consider patient-specific factors
Consider the mental state, intellectual capacity, and social support of each patient before initiating valproic acid. Its use as a mood stabilizer for “mood swings” outside of the context of bipolar disorder is questionable. Valproic acid is FDA-approved for treating bipolar disorder and seizures, but not for anger outbursts/irritability. Prior to starting valproic acid, Ms. L may have benefited from alternative nonpharmacologic treatments, such as psychotherapy, for her anger outbursts and poor coping skills. Therapeutic techniques that focused on helping her acquire better coping mechanisms may have been useful, especially because her mood symptoms did not meet criteria for bipolar disorder, and her depression had long been controlled with sertraline monotherapy.
OUTCOME Discharged after 20 days
Ms. L stays in the hospital for 10 days after receiving her liver transplant. She has low appetite and some difficulty with sleep after the transplant; therefore, the C-L team recommends mirtazapine, 15 mg/d. She has no behavioral problems during her stay, and is set up with home health, case management, and psychiatry follow-up. On hospital Day 20, she is discharged.
Bottom Line
Use caution when prescribing valproic acid, even in young, otherwise healthy patients. Although rare, some patients may experience a rapid decompensation and life-threatening illness after starting valproic acid. When prescribing valproic acid, ensure close follow-up after initiation, including mental status examinations, physical examinations, and laboratory testing.
Related Resource
- Doroudgar S, Chou TI. How to modify psychotropic therapy for patients who have liver dysfunction. Current Psychiatry. 2014;13(12):46-49.
Drug Brand Names
Diphenhydramine • Benadryl
Famotidine • Fluxid, Pepcid
Lamotrigine • Lamictal
Mirtazapine • Remeron
N-acetylcysteine • Mucomyst
Phenobarbital • Luminal
Phenytoin • Dilantin
Prednisone • Cortan, Deltasone
Sertraline • Zoloft
Valproic acid • Depakene
1. Depakote [package insert]. North Chicago, IL: AbbVie, Inc.; 2019.
2. National Institutes of Health. U.S. Department of Health and Human Services. Drug Record: Valproate. https://livertox.nlm.nih.gov/Valproate.htm. Updated October 30, 2018. Accessed March 21, 2019.
3. Schmid MM, Freudenmann RW, Keller F, et al. Non-fatal and fatal liver failure associated with valproic acid. Pharmacopsychiatry. 2013;46(2):63-68.
4. Patel N, Landry KB, Fargason RE, et al. Reversible encephalopathy due to valproic acid induced hyperammonemia in a patient with Bipolar I disorder: a cautionary report. Psychopharmacol Bull. 2017;47(1):40-44.
5. Eze E, Workman M, Donley B. Hyperammonemia and coma developed by a woman treated with valproic acid for affective disorder. Psychiatr Serv. 1998;49(10):1358-1359.
6. Darban M and Bagheri B. Drug reaction with eosinophilia and systemic symptoms induced by valproic acid: a case report. Iran Red Crescent Med J. 2016;18(9): e35825.
7. van Zoelen MA, de Graaf M, van Dijk MR, et al. Valproic acid-induced DRESS syndrome with acute liver failure. Neth J Med. 2012;70(3):155.
8. Lheureux PE, Hantson P. Carnitine in the treatment of valproic acid-induced toxicity. Clin Toxicol (Phila). 2009;47(2):101-111.
9. Bohan TP, Helton E, McDonald I, et al. Effect of L-carnitine treatment for valproate-induced hepatotoxicity. Neurology. 2001;56(10):1405-1409.
10. Böhles H, Sewell AC, Wenzel D. The effect of carnitine supplementation in valproate-induced hyperammonaemia. Acta Paediatr. 1996;85(4):446-449.
11. Raskind JY, El-Chaar GM. The role of carnitine supplementation during valproic acid therapy. Ann Pharmacother. 2000;34(5):630-638.
12. Romero-Falcón A, de la Santa-Belda E, García-Contreras R, et al. A case of valproate-associated hepatotoxicity treated with L-carnitine. Eur J Intern Med. 2003;14(5):338-340.
13. National Institute for Health and Clinical Excellence. Bipolar disorder: the management of bipolar disorder in adults, children, and adolescents, in primary and secondary care. https://www.nice.org.uk/guidance/cg185. Updated April 2018. Accessed March 21, 2019.
14 . Hirschfeld RMA, Bowden CL, Gitlin MJ, et al. Practice guideline for the treatment of patients with biopolar disorder: second edition. American Psychiatric Association. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/bipolar.pdf. Published 2002. Accessed March 21, 2019.
1. Depakote [package insert]. North Chicago, IL: AbbVie, Inc.; 2019.
2. National Institutes of Health. U.S. Department of Health and Human Services. Drug Record: Valproate. https://livertox.nlm.nih.gov/Valproate.htm. Updated October 30, 2018. Accessed March 21, 2019.
3. Schmid MM, Freudenmann RW, Keller F, et al. Non-fatal and fatal liver failure associated with valproic acid. Pharmacopsychiatry. 2013;46(2):63-68.
4. Patel N, Landry KB, Fargason RE, et al. Reversible encephalopathy due to valproic acid induced hyperammonemia in a patient with Bipolar I disorder: a cautionary report. Psychopharmacol Bull. 2017;47(1):40-44.
5. Eze E, Workman M, Donley B. Hyperammonemia and coma developed by a woman treated with valproic acid for affective disorder. Psychiatr Serv. 1998;49(10):1358-1359.
6. Darban M and Bagheri B. Drug reaction with eosinophilia and systemic symptoms induced by valproic acid: a case report. Iran Red Crescent Med J. 2016;18(9): e35825.
7. van Zoelen MA, de Graaf M, van Dijk MR, et al. Valproic acid-induced DRESS syndrome with acute liver failure. Neth J Med. 2012;70(3):155.
8. Lheureux PE, Hantson P. Carnitine in the treatment of valproic acid-induced toxicity. Clin Toxicol (Phila). 2009;47(2):101-111.
9. Bohan TP, Helton E, McDonald I, et al. Effect of L-carnitine treatment for valproate-induced hepatotoxicity. Neurology. 2001;56(10):1405-1409.
10. Böhles H, Sewell AC, Wenzel D. The effect of carnitine supplementation in valproate-induced hyperammonaemia. Acta Paediatr. 1996;85(4):446-449.
11. Raskind JY, El-Chaar GM. The role of carnitine supplementation during valproic acid therapy. Ann Pharmacother. 2000;34(5):630-638.
12. Romero-Falcón A, de la Santa-Belda E, García-Contreras R, et al. A case of valproate-associated hepatotoxicity treated with L-carnitine. Eur J Intern Med. 2003;14(5):338-340.
13. National Institute for Health and Clinical Excellence. Bipolar disorder: the management of bipolar disorder in adults, children, and adolescents, in primary and secondary care. https://www.nice.org.uk/guidance/cg185. Updated April 2018. Accessed March 21, 2019.
14 . Hirschfeld RMA, Bowden CL, Gitlin MJ, et al. Practice guideline for the treatment of patients with biopolar disorder: second edition. American Psychiatric Association. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/bipolar.pdf. Published 2002. Accessed March 21, 2019.
Management of Early Pulmonary Complications After Hematopoietic Stem Cell Transplantation
Hematopoietic stem cell transplantation (HSCT) is widely used in the economically developed world to treat a variety of hematologic malignancies as well as nonmalignant diseases and solid tumors. An estimated 17,900 HSCTs were performed in 2011, and survival rates continue to increase.1 Pulmonary complications post HSCT are common, with rates ranging from 40% to 60%, and are associated with increased morbidity and mortality.2
Clinical diagnosis of pulmonary complications in the HSCT population has been aided by a previously well-defined chronology of the most common diseases.3 Historically, early pulmonary complications were defined as pulmonary complications occurring within 100 days of HSCT (corresponding to the acute graft-versus-host disease [GVHD] period). Late pulmonary complications are those that occur thereafter. This timeline, however, is now more variable given the increasing indications for HSCT, the use of reduced-intensity conditioning strategies, and varied individual immune reconstitution. This article discusses the management of early post-HSCT pulmonary complications; late post-HSCT pulmonary complications will be discussed in a separate follow-up article.
Transplant Basics
The development of pulmonary complications is affected by many factors associated with the transplant. Autologous transplantation involves the collection of a patient’s own stem cells, appropriate storage and processing, and re-implantation after induction therapy. During induction therapy, the patient undergoes high-dose chemotherapy or radiation therapy that ablates the bone marrow. The stem cells are then transfused back into the patient to repopulate the bone marrow. Allogeneic transplants involve the collection of stem cells from a donor. Donors are matched as closely as possible to the recipient’s histocompatibility antigen (HLA) haplotypes to prevent graft failure and rejection. The donor can be related or unrelated to the recipient. If there is not a possibility of a related match (from a sibling), then a national search is undertaken to look for a match through the National Marrow Donor Program. There are fewer transplant reactions and occurrences of GVHD if the major HLAs of the donor and recipient match. Table 1 reviews basic definitions pertaining to HSCT.
How the cells for transplantation are obtained is also an important factor in the rate of complications. There are 3 main sources: peripheral blood, bone marrow, and umbilical cord. Peripheral stem cell harvesting involves exposing the donor to granulocyte-colony stimulating factor (gCSF), which increases peripheral circulation of stem cells. These cells are then collected and infused into the recipient after the recipient has completed an induction regimen involving chemotherapy and/or radiation, depending on the protocol. This procedure is called peripheral blood stem cell transplant (PBSCT). Stem cells can also be directly harvested from bone marrow cells, which are collected from repeated aspiration of bone marrow from the posterior iliac crest.4 This technique is most common in children, whereas in adults peripheral blood stem cells are the most common source. Overall mortality does not differ based on the source of the stem cells. It is postulated that GVHD may be more common in patients undergoing PBSCT, but the graft failure rate may be lower.5
The third option is umbilical cord blood (UCB) as the source of stem cells. This involves the collection of umbilical cord blood that is prepared and frozen after birth. It has a smaller volume of cells, and although fewer cells are needed when using UCB, 2 separate donors may be required for a single adult recipient. The engraftment of the stem cells is slower and infections in the post-transplant period are more common. Prior reports indicate GVHD rates may be lower.4 While the use of UCB is not common in adults, the incidence has doubled over the past decade, increasing from 3% to 6%.
The conditioning regimen can influence pulmonary complications. Traditionally, an ablative transplant involves high-dose chemotherapy or radiation to eradicate the recipient’s bone marrow. This regimen can lead to many complications, especially in the immediate post-transplant period. In the past 10 years, there has been increasing interest in non-myeloablative, or reduced-intensity, conditioning transplants.6 These “mini transplants” involve smaller doses of chemotherapy or radiation, which do not totally eradicate the bone marrow; after the transplant a degree of chimerism develops where the donor and recipient stem cells coexist. The medications in the preparative regimen also should be considered because they can affect pulmonary complications after transplant. Certain chemotherapeutic agents such as carmustine, bleomycin, and many others can lead to acute and chronic presentations of pulmonary diseases such as hypersensitivity pneumonitis, pulmonary fibrosis, acute respiratory distress syndrome, and abnormal pulmonary function testing.
After the HSCT, GVHD can develop in more than 50% of allogeneic recipients.3 The incidence of GVHD has been reported to be increasing over the past 12 years.It is divided into acute GVHD (which traditionally happens in the first 100 days after transplant) and chronic GVHD (after day 100). This calendar-day–based system has been augmented based on a 2006 National Institutes of Health working group report emphasizing the importance of organ-specific features of chronic GVHD in the clinical presentation of GVHD.7 Histologic changes in chronic organ GVHD tend to include more fibrotic features, whereas in acute GVHD more inflammatory changes are seen. The NIH working group report also stressed the importance of obtaining a biopsy specimen for histopathologic review and interdisciplinary collaboration to arrive at a consensus diagnosis, and noted the limitations of using histologic changes as the sole determinant of a “gold standard” diagnosis.7 GVHD can directly predispose patients to pulmonary GVHD and indirectly predispose them to infectious complications because the mainstay of therapy for GVHD is increased immunosuppression.
Pretransplant Evaluation
Case Patient 1
A 56-year-old man is diagnosed with acute myeloid leukemia (AML) after presenting with signs and symptoms consistent with pancytopenia. He has a past medical history of chronic sinus congestion, arthritis, depression, chronic pain, and carpal tunnel surgery. He is employed as an oilfield worker and has a 40-pack-year smoking history, but he recently cut back to half a pack per day. He is being evaluated for allogeneic transplant with his brother as the donor and the planned conditioning regimen is total body irradiation (TBI), thiotepa, cyclophosphamide, and antithymocyte globulin with T-cell depletion. Routine pretransplant pulmonary function testing (PFT) reveals a restrictive pattern and he is sent for pretransplant pulmonary evaluation.
Physical exam reveals a chronically ill appearing man. He is afebrile, the respiratory rate is 16 breaths/min, blood pressure is 145/88 mm Hg, heart rate is 92 beats/min, and oxygen saturation is 95%. He is in no distress. Auscultation of the chest reveals slightly diminished breath sounds bilaterally but is clear and without wheezes, rhonchi, or rales. Heart exam shows regular rate and rhythm without murmurs, rubs, or gallops. Extremities reveal no edema or rashes. Otherwise, the remainder of the exam is normal. The patient’s PFT results are shown in Table 2.
- What aspects of this patient’s history put him at risk for pulmonary complications after transplantation?
Risk Factors for Pulmonary Complications
Predicting who is at risk for pulmonary complications is difficult. Complications are generally divided into infectious and noninfectious categories. Regardless of category, allogeneic HSCT recipients are at increased risk compared with autologous recipients, but even in autologous transplants, more than 25% of patients will develop pulmonary complications in the first year.8 Prior to transplant, patients undergo full PFT. Early on, many studies attempted to show relationships between various factors and post-transplant pulmonary complications. Factors that were implicated were forced expiratory volume in 1 second (FEV1), diffusing capacity of the lung for carbon monoxide (D
Another sometimes overlooked risk before transplantation is restrictive lung disease. One study showed a twofold increase in respiratory failure and mortality if there was pretransplant restriction based on TLC < 80%.16
An interesting study by one group in pretransplant evaluation found decreased muscle strength by maximal inspiratory muscle strength (PImax), maximal expiratory muscle strength (PEmax), dominant hand grip strength, and 6-minute walk test (6MWT) distance prior to allogeneic transplant, but did not find a relationship between these variables and mortality.17 While this study had a small sample size, these findings likely deserve continued investigation.18
- What methods are used to calculate risk for complications?
Risk Scoring Systems
Several pretransplantation risk scores have been developed. In a study that looked at more than 2500 allogeneic transplants, Parimon et al showed that risk of mortality and respiratory failure could be estimated prior to transplant using a scoring system—the Lung Function Score (LFS)—that combines the FEV1 and D
The Pretransplantation Assessment of Mortality score, initially developed in 2006, predicts mortality within the first 2 years after HSCT based on 8 clinical factors: disease risk, age at transplant, donor type, conditioning regimen, and markers of organ function (percentage of predicted FEV1, percentage of predicted D
- What other preoperative testing or interventions should be considered in this patient?
Since there is a high risk of infectious complications after transplant, the question of whether pretransplantation patients should undergo screening imaging may arise. There is no evidence that routine chest computed tomography (CT) reduces the risk of infectious complications after transplantation.26 An area that may be insufficiently addressed in the pretransplantation evaluation is smoking cessation counseling.27 Studies have shown an elevated risk of mortality in smokers.28-30 Others have found a higher incidence of respiratory failure but not an increased mortality.31 Overall, with the good rates of smoking cessation that can be accomplished, smokers should be counseled to quit before transplantation.
In summary, patients should undergo full PFTs prior to transplantation to help stratify risk for pulmonary complications and mortality and to establish a clinical baseline. The LFS (using FEV1 and D
Case Patient 1 Conclusion
The patient undergoes transplantation due to his lack of other treatment options. Evaluation prior to transplant, however, shows that he is at high risk for pulmonary complications. He has a LFS of 7 prior to transplant (using the D
Early Infectious Pulmonary Complications
Case Patient 2
A 27-year-old man with a medical history significant for AML and allogeneic HSCT presents with cough productive of a small amount of clear to white sputum, dyspnea on exertion, and fevers for 1 week. He also has mild nausea and a decrease in appetite. He underwent HSCT 2.5 months prior to admission, which was a matched unrelated bone marrow transplant with TBI and cyclophosphamide conditioning. His past medical history is significant only for exercise-induced asthma for which he takes a rescue inhaler infrequently prior to transplantation. His pretransplant PFTs showed normal spirometry with an FEV1 of 106% of predicted and D
Physical exam is notable for fever of 101.0°F, heart rate 80 beats/min, respiratory rate 16 breaths/ min, and blood pressure 142/78 mm Hg; an admission oxygen saturation is 93% on room air. Lungs show bibasilar crackles and the remainder of the exam is normal. Laboratory testing shows a white blood cell count of 2400 cells/μL, hemoglobin 7.6 g/dL, and platelet count 66 × 103/μL. Creatinine is 1.0 mg/dL. Chest radiograph shows ill-defined bilateral lower-lobe infiltrates. CT scans are shown in the Figure.
- For which infectious complications is this patient most at risk?
Pneumonia
A prospective trial in the HSCT population reported a pneumonia incidence rate of 68%, and pneumonia is more common in allogeneic HSCT with prolonged immunosuppressive therapy.32 Development of pneumonia within 100 days of transplant directly correlates with nonrelapsed mortality.33 Early detection is key, and bronchoscopy within the first 5 days of symptoms has been shown to change therapy in approximately 40% of cases but has not been shown to affect mortality.34 The clinical presentation of pneumonia in the HSCT population can be variable because of the presence of neutropenia and profound immunosuppression. Traditionally accepted diagnostic criteria of fevers, sputum production, and new infiltrates should be used with caution, and an appropriately high index of suspicion should be maintained. Progression to respiratory failure, regardless of causative organism of infection, portends a poor prognosis, with mortality rates estimated at 70% to 90%.35,36 Several transplant-specific factors may affect early infections. For instance, UCB transplants have been found to have a higher incidence of invasive aspergillosis and cytomegalovirus (CMV) infections but without higher mortality attributed to the infections.37
Bacterial Pneumonia
Bacterial pneumonia accounts for 20% to 50% of pneumonia cases in HSCT recipients.38 Gram-negative organisms, specifically Pseudomonas aeruginosa and Escherichia coli, were reported to be the most common pathologic bacteria in recent prospective trials, whereas previous retrospective trials showed that common community-acquired organisms were the most common cause of pneumonia in HSCT recipients.32,39 This underscores the importance of being aware of the clinical prevalence of microorganisms and local antibiograms, along with associated institutional susceptibility profiles. Initiation of immediate empiric broad-spectrum antibiotics is essential when bacterial pneumonia is suspected.
Viral Pneumonia
The prevalence of viral pneumonia in stem cell transplant recipients is estimated at 28%,32 with most cases being caused by community viral pathogens such as rhinovirus, respiratory syncytial virus (RSV), influenza A and B, and parainfluenza.39 The prevention, prophylaxis, and early treatment of viral pneumonias, specifically CMV infection, have decreased the mortality associated with early pneumonia after HSCT. Co-infection with bacterial organisms must be considered and has been associated with increased mortality in the intensive care unit setting.40
Supportive treatment with rhinovirus infection is sufficient as the disease is usually self-limited in immunocompromised patients. In contrast, infection with RSV in the lower respiratory tract is associated with increased mortality in prior reports, and recent studies suggest that further exploration of prophylaxis strategies is warranted.41 Treatment with ribavirin remains the backbone of therapy, but drug toxicity continues to limit its use. The addition of immunomodulators such as RSV immune globulin or palivizumab to ribavirin remains controversial, but a retrospective review suggests that early treatment may prevent progression to lower respiratory tract infection and lead to improved mortality.42 Infection with influenza A/B must be considered during influenza season. Treatment with oseltamivir may shorten the duration of disease when influenza A/B or parainfluenza are detected. Reactivation of latent herpes simplex virus during the pre-engraftment phase should also be considered. Treatment is similar to that in nonimmunocompromised hosts. When CMV pneumonia is suspected, careful history regarding compliance with prophylactic antivirals and CMV status of both the recipient and donor are key. A presumptive diagnosis can be made with the presence of appropriate clinical scenario, supportive radiographic images showing areas of ground-glass opacification or consolidation, and positive CMV polymerase chain reaction (PCR) assay. Visualization of inclusion bodies on lung biopsy tissue remains the gold standard for diagnosis. Treatment consists of CMV immunoglobulin and ganciclovir.
Fungal Pneumonia
Early fungal pneumonias have been associated with increased mortality in the HSCT population.43 Clinical suspicion should remain high and compliance with antifungal prophylaxis should be questioned thoroughly. Invasive aspergillosis (IA) remains the most common fungal infection. A bimodal distribution of onset of infection peaking on day 16 and again on day 96 has been described in the literature.44 Patients often present with classic pneumonia symptoms, but these may be accompanied by hemoptysis. Proven IA diagnosis requires visualization of fungal forms from biopsy or needle aspiration or a positive culture obtained in a sterile fashion.45 Most clinical data comes from experience with probable and possible diagnosis of IA. Bronchoalveolar lavage with testing with Aspergillus galactomannan assay has been shown to be clinically useful in establishing the clinical diagnosis in the HSCT population.46 Classic air-crescent findings on chest CT are helpful in establishing a possible diagnosis, but retrospective analysis reveals CT findings such as focal infiltrates and pulmonary nodular patterns are more common.47 First-line treatment with voriconazole has been shown to decrease short-term mortality attributable to IA but has not had an effect on long-term, all-cause mortality.48 Surgical resection is reserved for patients with refractory disease or patients presenting with massive hemoptysis.
Mucormycosis is an emerging disease with ever increasing prevalence in the HSCT population, reflecting the improved prophylaxis and treatment of IA. Initial clinical presentation is similar to IA, most commonly affecting the lung, although craniofacial involvement is classic for mucormycosis, especially in HSCT patients with diabetes.49Mucor infections can present with massive hemoptysis due to tissue invasion and disregard for tissue and fascial planes. Diagnosis of mucormycosis is associated with as much as a six-fold increase in risk for death. Diagnosis requires identification of the organism by examination or culture and biopsy is often necessary.50,51 Amphotericin B remains first-line therapy as mucormycosis is resistant to azole antifungals, with higher doses recommended for cerebral involvement.52
Candida pulmonary infections during the early HSCT period are becoming increasingly rare due to widespread use of fluconazole prophylaxis and early treatment of mucosal involvement during neutropenia. Endemic fungal infections such as blastomycosis, coccidioidomycosis, and histoplasmosis should be considered in patients inhabiting specific geographic areas or with recent travel to these areas.
- What test should be performed to evaluate for infectious causes of pneumonia?
Role of Flexible Fiberoptic Bronchoscopy
The utility of flexible fiberoptic bronchoscopy (FOB) in immune-compromised patients for the evaluation of pulmonary infiltrates is a frequently debated topic. Current studies suggest a diagnosis can be made in approximately 80% of cases in the immune-compromised population.32,53 Noninvasive testing such as urine and serum antigens, sputum cultures, Aspergillus galactomannan assays, viral nasal swabs, and PCR studies often lead to a diagnosis in appropriate clinical scenarios. Conservative management would dictate the use of noninvasive testing whenever possible, and randomized controlled trials have shown noninvasive testing to be noninferior to FOB in preventing need for mechanical ventilation, with no difference in overall mortality.54 FOB has been shown to be most useful in establishing a diagnosis when an infectious etiology is suspected.55 In multivariate analysis, a delay in the identification of the etiology of pulmonary infiltrate was associated with increased mortality.56 Additionally, early FOB was found to be superior to late FOB in revealing a diagnosis. 32,57 Despite its ability to detect the cause of pulmonary disease, direct antibiotic therapy, and possibly change therapy, FOB with diagnostic maneuvers has not been shown to affect mortality.58 In a large case series, FOB with bronchoalveolar lavage (BAL) revealed a diagnosis in approximately 30% to 50% of cases. The addition of transbronchial biopsy did not improve diagnostic utility.58 More recent studies have confirmed that the addition of transbronchial biopsy does not add to diagnostic yield and is associated with increased adverse events.59 The appropriate use of advanced techniques such as endobronchial ultrasound–guided transbronchial needle aspirations, endobronchial biopsy, and CT-guided navigational bronchoscopy has not been established and should be considered on a case-by-case basis. In summary, routine early BAL is the diagnostic test of choice, especially when infectious pulmonary complications are suspected.
Contraindications for FOB in this population mirror those in the general population. These include acute severe hypoxemic respiratory failure, myocardial ischemia or acute coronary syndrome within 2 weeks of procedure, severe thrombocytopenia, and inability to provide or obtain informed consent from patient or health care power of attorney. Coagulopathy and thrombocytopenia are common comorbid conditions in the HSCT population. A platelet count of < 20 × 103/µL has generally been used as a cut-off for routine FOB with BAL.60 Risks of the procedures should be discussed clearly with the patient, but simple FOB for airway evaluation and BAL is generally well tolerated even under these conditions.
Early Nonifectious Pulmonary Complications
Case Patient 2 Continued
Bronchoscopy with BAL performed the day after admission is unremarkable and stains and cultures are negative for viral, bacterial, and fungal organisms. The patient is initially started on broad-spectrum antibiotics, but his oxygenation continues to worsen to the point that he is placed on noninvasive positive pressure ventilation. He is started empirically on amphotericin B and eventually is intubated. VATS lung biopsy is ultimately performed and pathology is consistent with diffuse alveolar damage.
- Based on these biopsy findings, what is the diagnosis?
Based on the pathology consistent with diffuse alveolar damage, a diagnosis of idiopathic pneumonia syndrome (IPS) is made.
- What noninfectious pulmonary complications occur in the early post-transplant period?
The overall incidence of noninfectious pulmonary complications after HSCT is generally estimated at 20% to 30%.32 Acute pulmonary edema is a common very early noninfectious pulmonary complication and clinically the most straightforward to treat. Three distinct clinical syndromes—peri-engraftment respiratory distress syndrome (PERDS), diffuse alveolar hemorrhage (DAH), and IPS—comprise the remainder of the pertinent early noninfectious complications. Clinical presentation differs based upon the disease entity. Recent studies have evaluated the role of angiotensin-converting enzyme polymorphisms as a predictive marker for risk of developing early noninfectious pulmonary complications.61
Peri-Engraftment Respiratory Distress Syndrome
PERDS is a clinical syndrome comprising the cardinal features of erythematous rash and fever along with noncardiogenic pulmonary infiltrates and hypoxemia that occur in the peri-engraftment period, defined as recovery of absolute neutrophil count to > 500/μL on 2 consecutive days.62 PERDS occurs in the autologous HSCT population and may be a clinical correlate to early GVHD in the allogeneic HSCT population. It is hypothesized that the pathophysiology underlying PERDS is an autoimmune-related capillary leak caused by pro-inflammatory cytokine release.63 Treatment remains anecdotal and currently consists of supportive care and high-dose corticosteroids. Some have favored limiting the use of gCSF given its role in stimulating rapid white blood cell recovery.33 Prognosis is favorable, but progression to fulminant respiratory failure requiring mechanical ventilation portends a poor prognosis.
Diffuse Alveolar Hemorrhage
DAH is clinical syndrome consisting of diffuse alveolar infiltrates on pulmonary imaging combined with progressively bloodier return per aliquot during BAL in 3 different subsegments or more than 20% hemosiderin-laden macrophages on BAL fluid evaluation. Classically, DAH is defined in the absence of pulmonary infection or cardiac dysfunction. The pathophysiology is thought to be related to inflammation of pulmonary vasculature within the alveolar walls leading to alveolitis. Although no prospective trials exist, early use of high-dose corticosteroid therapy is thought to improve outcomes;64,65 a recent study, however, showed low-dose steroids may be associated with the lowest mortality.66 Mortality is directly linked to the presence of superimposed infection, need for mechanical ventilation, late onset, and development of multiorgan failure.67
Idiopathic Pneumonia Syndrome
IPS is a complex clinical syndrome whose pathology is felt to stem from a variety of possible lung insults such as direct myeloablative drug toxicity, occult pulmonary infection, or cytokine-driven inflammation. The ATS published an article further subcategorizing IPS as different clinical entities based upon whether the primary insult involves the vascular endothelium, interstitial tissue, and airway tissue, truly idiopathic, or unclassified.68 In clinical practice, IPS is defined as widespread alveolar injury in the absence of evidence of renal failure, heart failure, and excessive fluid resuscitation. In addition, negative testing for a variety of bacterial, viral, and fungal causes is also necessary.69 Clinical syndromes included within the IPS definition are ARDS, acute interstitial pneumonia, DAH, cryptogenic organizing pneumonia, and BOS.70 Risk factors for developing IPS include TBI, older age of recipient, acute GVHD, and underlying diagnosis of AML or myelodysplastic syndrome.12 In addition, it has been shown that risk for developing IPS is lower in patients undergoing allogeneic HSCT who receive non-myeloablative conditioning regimens.71 The pathologic finding in IPS is diffuse alveolar damage. A 2006 study in which investigators reviewed BAL samples from patients with IPS found that 3% of the patients had PCR evidence of human metapneumovirus infection, and a study in 2015 found PCR evidence of infection in 53% of BAL samples from patients diagnosed with IPS.72,73 This fuels the debate on whether IPS is truly an infection-driven process where the source of infection, pulmonary or otherwise, simply escapes detection. Various surfactant proteins, which play a role in decreasing surface tension within the alveolar interface and function as mediators within the innate immunity of the lung, have been studied in regard to development of IPS. Small retrospective studies have shown a trend toward lower pre-transplant serum protein surfactant D and the development of IPS.74
The diagnosis of IPS does not require pathologic diagnosis in most circumstances. The correct clinical findings in association with a negative infectious workup lead to a presumptive diagnosis of IPS. The extent of the infectious workup that must be completed to adequately rule out infection is often a difficult clinical question. Recent recommendations include BAL fluid evaluation for routine bacterial cultures, appropriate viral culture, and consideration of PCR testing to evaluate for Mycoplasma, Chlamydia, and Aspergillus antigens.75 Transbronchial biopsy continues to appear in recommendations, but is not routinely performed and should be completed as the patient’s clinical status permits.8,68 Table 3 reviews basic features of early noninfectious pulmonary complications.
Treatment of IPS centers around moderate to high doses of corticosteroids. Based on IPS experimental modes, tumor necrosis factor (TNF)-α has been implicated as an important mediator. Unfortunately, several studies evaluating etanercept have produced conflicting results, and this agent’s clinical effects on morbidity and mortality remain in question.76
- What treatment should be offered to the patient with diffuse alveolar damage on biopsy?
Treatment consists of supportive care and empiric broad-spectrum antibiotics with consideration of high-dose corticosteroids. Based upon early studies in murine models implicating TNF, pilot studies were performed evaluating etanercept as a possible safe and effective addition to high-dose systemic corticosteroids.77 Although these results were promising, data from a truncated randomized control clinical trial failed to show improvement in patient response in the adult population.76 More recent data from the same author suggests that pediatric populations with IPS are, however, responsive to etanercept and high-dose corticosteroid therapy.78 When IPS develops as a late complication, treatment with high-dose corticosteroids (2 mg/kg/day) and etanercept (0.4 mg/kg twice weekly) has been shown to improve 2-year survival.79
Case Patient 2 Conclusion
The patient is started on steroids and makes a speedy recovery. He is successfully extubated 5 days later.
Conclusion
Careful pretransplant evaluation, including a full set of pulmonary function tests, can help predict a patient’s risk for pulmonary complications after transplant, allowing risk factor modification strategies to be implemented prior to transplant, including smoking cessation. It also helps identify patients at high risk for complications who will require closer monitoring after transplantation. Early posttransplant complications include infectious and noninfectious entities. Bacterial, viral, and fungal pneumonias are in the differential of infectious pneumonia, and bronchoscopy can be helpful in establishing a diagnosis. A common, important noninfectious cause of early pulmonary complications is IPS, which is treated with steroids and sometimes anti-TNF therapy.
1. Gratwohl A, Baldomero H, Aljurf M, et al. Hematopoietic stem cell transplantation: a global perspective. JAMA 2010;303:1617–24.
2. Kotloff RM, Ahya VN, Crawford SW. Pulmonary complications of solid organ and hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2004;170:22–48.
4. Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med 2006;354:1813–26.
5. Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med 2012;367:1487–96.
6. Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant 2009;15:367–9.
7. Shulman HM, Kleiner D, Lee SJ, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant 2006;12:31–47.
8. Afessa B, Abdulai RM, Kremers WK, et al. Risk factors and outcome of pulmonary complications after autologous hematopoietic stem cell transplant. Chest 2012;141:442–50.
9. Bolwell BJ. Are predictive factors clinically useful in bone marrow transplantation? Bone Marrow Transplant 2003;32:853–61.
10. Carlson K, Backlund L, Smedmyr B, et al. Pulmonary function and complications subsequent to autologous bone marrow transplantation. Bone Marrow Transplant 1994;14:805–11.
11. Clark JG, Schwartz DA, Flournoy N, et al. Risk factors for airflow obstruction in recipients of bone marrow transplants. Ann Intern Med 1987;107:648–56.
12. Crawford SW, Fisher L. Predictive value of pulmonary function tests before marrow transplantation. Chest 1992; 101:1257–64.
13. Ghalie R, Szidon JP, Thompson L, et al. Evaluation of pulmonary complications after bone marrow transplantation: the role of pretransplant pulmonary function tests. Bone Marrow Transplant 1992;10:359–65.
14. Ho VT, Weller E, Lee SJ, et al. Prognostic factors for early severe pulmonary complications after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2001;7:223–9.
15. Horak DA, Schmidt GM, Zaia JA, et al. Pretransplant pulmonary function predicts cytomegalovirus-associated interstitial pneumonia following bone marrow transplantation. Chest 1992;102:1484–90.
16. Ramirez-Sarmiento A, Orozco-Levi M, Walter EC, et al. Influence of pretransplantation restrictive lung disease on allogeneic hematopoietic cell transplantation outcomes. Biol Blood Marrow Transplant 2010;16:199–206.
17. White AC, Terrin N, Miller KB, Ryan HF. Impaired respiratory and skeletal muscle strength in patients prior to hematopoietic stem-cell transplantation. Chest 2005;128145–52.
18. Afessa B. Pretransplant pulmonary evaluation of the blood and marrow transplant recipient. Chest 2005;128:8–10.
19. Parimon T, Madtes DK, Au DH, et al. Pretransplant lung function, respiratory failure, and mortality after stem cell transplantation. Am J Respir Crit Care Med 2005;172:384–90.
20. Pavletic SZ, Martin P, Lee SJ, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant 2006;12:252–66.
21. Parimon T, Au DH, Martin PJ, Chien JW. A risk score for mortality after allogeneic hematopoietic cell transplantation. Ann Intern Med 2006;144:407–14.
22. Au BK, Gooley TA, Armand P, et al. Reevaluation of the pretransplant assessment of mortality score after allogeneic hematopoietic transplantation. Biol Blood Marrow Transplant 2015;21:848–54.
23. Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005;106:2912–9.
24. Chien JW, Sullivan KM. Carbon monoxide diffusion capacity: how low can you go for hematopoietic cell transplantation eligibility? Biol Blood Marrow Transplant 2009;15: 447–53.
25. Coffey DG, Pollyea DA, Myint H, et al. Adjusting DLCO for Hb and its effects on the Hematopoietic Cell Transplantation-specific Comorbidity Index. Bone Marrow Transplant 2013;48:1253–6.
26. Kasow KA, Krueger J, Srivastava DK, et al. Clinical utility of computed tomography screening of chest, abdomen, and sinuses before hematopoietic stem cell transplantation: the St. Jude experience. Biol Blood Marrow Transplant 2009;15:490–5.
27. Hamadani M, Craig M, Awan FT, Devine SM. How we approach patient evaluation for hematopoietic stem cell transplantation. Bone Marrow Transplant 2010;45: 1259–68.
28. Savani BN, Montero A, Wu C, et al. Prediction and prevention of transplant-related mortality from pulmonary causes after total body irradiation and allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2005;11:223–30.
29. Ehlers SL, Gastineau DA, Patten CA, et al. The impact of smoking on outcomes among patients undergoing hematopoietic SCT for the treatment of acute leukemia. Bone Marrow Transplant 2011;46:285–90.
30. Marks DI, Ballen K, Logan BR, et al. The effect of smoking on allogeneic transplant outcomes. Biol Blood Marrow Transplant 2009;15:1277–87.
31. Tran BT, Halperin A, Chien JW. Cigarette smoking and outcomes after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2011;17:1004–11.
32. Lucena CM, Torres A, Rovira M, et al. Pulmonary complications in hematopoietic SCT: a prospective study. Bone Marrow Transplant 2014;49:1293–9.
33. Chi AK, Soubani AO, White AC, Miller KB. An update on pulmonary complications of hematopoietic stem cell transplantation. Chest 2013;144:1913–22.
34. Dunagan DP, Baker AM, Hurd DD, Haponik EF. Bronchoscopic evaluation of pulmonary infiltrates following bone marrow transplantation. Chest 1997;111:135–41.
35. Naeem N, Reed MD, Creger RJ, et al. Transfer of the hematopoietic stem cell transplant patient to the intensive care unit: does it really matter? Bone Marrow Transplant 2006;37:119–33.
36. Afessa B, Tefferi A, Hoagland HC, et al. Outcome of recipients of bone marrow transplants who require intensive care unit support. Mayo Clin Proc 1992;67:117–22.
37. Parody R, Martino R, de la Camara R, et al. Fungal and viral infections after allogeneic hematopoietic transplantation from unrelated donors in adults: improving outcomes over time. Bone Marrow Transplant 2015;50:274–81.
38. Orasch C, Weisser M, Mertz D, et al. Comparison of infectious complications during induction/consolidation chemotherapy versus allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2010;45:521–6.
39. Aguilar-Guisado M, Jimenez-Jambrina M, Espigado I, et al. Pneumonia in allogeneic stem cell transplantation recipients: a multicenter prospective study. Clin Transplant 2011;25:E629–38.
40. Palacios G, Hornig M, Cisterna D, et al. Streptococcus pneumoniae coinfection is correlated with the severity of H1N1 pandemic influenza. PLoS One 2009;4:e8540.
41. Hynicka LM, Ensor CR. Prophylaxis and treatment of respiratory syncytial virus in adult immunocompromised patients. Ann Pharmacother 2012;46:558–66.
42. Shah JN, Chemaly RF. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood 2011;2755–63.
43. Marr KA, Bowden RA. Fungal infections in patients undergoing blood and marrow transplantation. Transpl Infect Dis 1999;1:237–46.
44. Wald A, Leisenring W, van Burik JA, Bowden RA. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis 1997;175:1459–66.
45. Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 2002;34:7–14.
46. Fisher CE, Stevens AM, Leisenring W, et al. Independent contribution of bronchoalveolar lavage and serum galactomannan in the diagnosis of invasive pulmonary aspergillosis. Transpl Infect Dis 2014;16:505–10.
47. Kojima R, Tateishi U, Kami M, et al. Chest computed tomography of late invasive aspergillosis after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2005;11:506–11.
48. Salmeron G, Porcher R, Bergeron A, et al. Persistent poor long-term prognosis of allogeneic hematopoietic stem cell transplant recipients surviving invasive aspergillosis. Haematologica 2012;97:1357–63.
49. McNulty JS. Rhinocerebral mucormycosis: predisposing factors. Laryngoscope 1982;92(10 Pt 1):1140.
50. Walsh TJ, Gamaletsou MN, McGinnis MR, et al. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin Infect Dis 2012;54 Suppl 1:S55–60.
51. Klingspor L, Saaedi B, Ljungman P, Szakos A. Epidemiology and outcomes of patients with invasive mould infections: a retrospective observational study from a single centre (2005-2009). Mycoses 2015;58:470–7.
52. Danion F, Aguilar C, Catherinot E, et al. Mucormycosis: new developments in a persistently devastating infection. Semin Respir Crit Care Med 2015;36:692–70.
53. Rano A, Agusti C, Jimenez P, et al. Pulmonary infiltrates in non-HIV immunocompromised patients: a diagnostic approach using non-invasive and bronchoscopic procedures. Thorax 2001;56:379–87.
54. Azoulay E, Mokart D, Rabbat A, et al. Diagnostic bronchoscopy in hematology and oncology patients with acute respiratory failure: prospective multicenter data. Crit Care Med 2008;36:100–7.
55. Jain P, Sandur S, Meli Y, et al. Role of flexible bronchoscopy in immunocompromised patients with lung infiltrates. Chest 2004;125:712–22.
56. Rano A, Agusti C, Benito N, et al. Prognostic factors of non-HIV immunocompromised patients with pulmonary infiltrates. Chest 2002;122:253–61.
57. Shannon VR, Andersson BS, Lei X, et al. Utility of early versus late fiberoptic bronchoscopy in the evaluation of new pulmonary infiltrates following hematopoietic stem cell transplantation. Bone Marrow Transplant 2010;45:647–55.
58. Patel NR, Lee PS, Kim JH, et al. The influence of diagnostic bronchoscopy on clinical outcomes comparing adult autologous and allogeneic bone marrow transplant patients. Chest 2005;127:1388–96.
59. Chellapandian D, Lehrnbecher T, Phillips B, et al. Bronchoalveolar lavage and lung biopsy in patients with cancer and hematopoietic stem-cell transplantation recipients: a systematic review and meta-analysis. J Clin Oncol 2015;33:501–9.
60. Carr IM, Koegelenberg CF, von Groote-Bidlingmaier F, et al. Blood loss during flexible bronchoscopy: a prospective observational study. Respiration 2012;84:312–8.
61. Miyamoto M, Onizuka M, Machida S, et al. ACE deletion polymorphism is associated with a high risk of non-infectious pulmonary complications after stem cell transplantation. Int J Hematol 2014;99:175–83.
62. Capizzi SA, Kumar S, Huneke NE, et al. Peri-engraftment respiratory distress syndrome during autologous hematopoietic stem cell transplantation. Bone Marrow Transplant 2001;27:1299–303.
63. Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant 2001;27:893–8.
64. Wanko SO, Broadwater G, Folz RJ, Chao NJ. Diffuse alveolar hemorrhage: retrospective review of clinical outcome in allogeneic transplant recipients treated with aminocaproic acid. Biol Blood Marrow Transplant 2006;12:949–53.
65. Metcalf JP, Rennard SI, Reed EC, et al. Corticosteroids as adjunctive therapy for diffuse alveolar hemorrhage associated with bone marrow transplantation. University of Nebraska Medical Center Bone Marrow Transplant Group. Am J Med 1994;96:327–34.
66. Rathi NK, Tanner AR, Dinh A, et al. Low-, medium- and high-dose steroids with or without aminocaproic acid in adult hematopoietic SCT patients with diffuse alveolar hemorrhage. Bone Marrow Transplant 2015;50:420–6.
67. Afessa B, Tefferi A, Litzow MR, Peters SG. Outcome of diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipients. Am J Respir Crit Care Med 2002;166:1364–8.
68. Panoskaltsis-Mortari A, Griese M, Madtes DK, et al. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med 2011;183:1262–79.
69. Clark JG, Hansen JA, Hertz MI, Pet al. NHLBI workshop summary. Idiopathic pneumonia syndrome after bone marrow transplantation. Am Rev Resp Dis 1993;147:1601–6.
70. Vande Vusse LK, Madtes DK. Early onset noninfectious pulmonary syndromes after hematopoietic cell transplantation. Clin Chest Med 2017;38:233–48.
71. Fukuda T, Hackman RC, Guthrie KA, et al. Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventional conditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood 2003;102:2777–85.
72. Englund JA, Boeckh M, Kuypers J, et al. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med 2006;144:344–9.
73. Seo S, Renaud C, Kuypers JM, et al. Idiopathic pneumonia syndrome after hematopoietic cell transplantation: evidence of occult infectious etiologies. Blood 2015;125:3789–97.
74. Nakane T, Nakamae H, Kamoi H, et al. Prognostic value of serum surfactant protein D level prior to transplant for the development of bronchiolitis obliterans syndrome and idiopathic pneumonia syndrome following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2008;42:43–9.
75. Gilbert CR, Lerner A, Baram M, Awsare BK. Utility of flexible bronchoscopy in the evaluation of pulmonary infiltrates in the hematopoietic stem cell transplant population—a single center fourteen year experience. Arch Bronconeumol 2013;49:189–95.
76. Yanik GA, Horowitz MM, Weisdorf DJ, et al. Randomized, double-blind, placebo-controlled trial of soluble tumor necrosis factor receptor: enbrel (etanercept) for the treatment of idiopathic pneumonia syndrome after allogeneic stem cell transplantation: blood and marrow transplant clinical trials network protocol. Biol Blood Marrow Transplant 2014;20:858–64.
77. Levine JE, Paczesny S, Mineishi S, et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood 2008;111:2470–5.
78. Yanik GA, Grupp SA, Pulsipher MA, et al. TNF-receptor inhibitor therapy for the treatment of children with idiopathic pneumonia syndrome. A joint Pediatric Blood and Marrow Transplant Consortium and Children’s Oncology Group Study (ASCT0521). Biol Blood Marrow Transplant 2015;21:67–73.
79. Thompson J, Yin Z, D’Souza A, et al. Etanercept and corticosteroid therapy for the treatment of late-onset idiopathic pneumonia syndrome. Biol Blood Marrow Transplant J 2017; 23:1955–60.
Hematopoietic stem cell transplantation (HSCT) is widely used in the economically developed world to treat a variety of hematologic malignancies as well as nonmalignant diseases and solid tumors. An estimated 17,900 HSCTs were performed in 2011, and survival rates continue to increase.1 Pulmonary complications post HSCT are common, with rates ranging from 40% to 60%, and are associated with increased morbidity and mortality.2
Clinical diagnosis of pulmonary complications in the HSCT population has been aided by a previously well-defined chronology of the most common diseases.3 Historically, early pulmonary complications were defined as pulmonary complications occurring within 100 days of HSCT (corresponding to the acute graft-versus-host disease [GVHD] period). Late pulmonary complications are those that occur thereafter. This timeline, however, is now more variable given the increasing indications for HSCT, the use of reduced-intensity conditioning strategies, and varied individual immune reconstitution. This article discusses the management of early post-HSCT pulmonary complications; late post-HSCT pulmonary complications will be discussed in a separate follow-up article.
Transplant Basics
The development of pulmonary complications is affected by many factors associated with the transplant. Autologous transplantation involves the collection of a patient’s own stem cells, appropriate storage and processing, and re-implantation after induction therapy. During induction therapy, the patient undergoes high-dose chemotherapy or radiation therapy that ablates the bone marrow. The stem cells are then transfused back into the patient to repopulate the bone marrow. Allogeneic transplants involve the collection of stem cells from a donor. Donors are matched as closely as possible to the recipient’s histocompatibility antigen (HLA) haplotypes to prevent graft failure and rejection. The donor can be related or unrelated to the recipient. If there is not a possibility of a related match (from a sibling), then a national search is undertaken to look for a match through the National Marrow Donor Program. There are fewer transplant reactions and occurrences of GVHD if the major HLAs of the donor and recipient match. Table 1 reviews basic definitions pertaining to HSCT.
How the cells for transplantation are obtained is also an important factor in the rate of complications. There are 3 main sources: peripheral blood, bone marrow, and umbilical cord. Peripheral stem cell harvesting involves exposing the donor to granulocyte-colony stimulating factor (gCSF), which increases peripheral circulation of stem cells. These cells are then collected and infused into the recipient after the recipient has completed an induction regimen involving chemotherapy and/or radiation, depending on the protocol. This procedure is called peripheral blood stem cell transplant (PBSCT). Stem cells can also be directly harvested from bone marrow cells, which are collected from repeated aspiration of bone marrow from the posterior iliac crest.4 This technique is most common in children, whereas in adults peripheral blood stem cells are the most common source. Overall mortality does not differ based on the source of the stem cells. It is postulated that GVHD may be more common in patients undergoing PBSCT, but the graft failure rate may be lower.5
The third option is umbilical cord blood (UCB) as the source of stem cells. This involves the collection of umbilical cord blood that is prepared and frozen after birth. It has a smaller volume of cells, and although fewer cells are needed when using UCB, 2 separate donors may be required for a single adult recipient. The engraftment of the stem cells is slower and infections in the post-transplant period are more common. Prior reports indicate GVHD rates may be lower.4 While the use of UCB is not common in adults, the incidence has doubled over the past decade, increasing from 3% to 6%.
The conditioning regimen can influence pulmonary complications. Traditionally, an ablative transplant involves high-dose chemotherapy or radiation to eradicate the recipient’s bone marrow. This regimen can lead to many complications, especially in the immediate post-transplant period. In the past 10 years, there has been increasing interest in non-myeloablative, or reduced-intensity, conditioning transplants.6 These “mini transplants” involve smaller doses of chemotherapy or radiation, which do not totally eradicate the bone marrow; after the transplant a degree of chimerism develops where the donor and recipient stem cells coexist. The medications in the preparative regimen also should be considered because they can affect pulmonary complications after transplant. Certain chemotherapeutic agents such as carmustine, bleomycin, and many others can lead to acute and chronic presentations of pulmonary diseases such as hypersensitivity pneumonitis, pulmonary fibrosis, acute respiratory distress syndrome, and abnormal pulmonary function testing.
After the HSCT, GVHD can develop in more than 50% of allogeneic recipients.3 The incidence of GVHD has been reported to be increasing over the past 12 years.It is divided into acute GVHD (which traditionally happens in the first 100 days after transplant) and chronic GVHD (after day 100). This calendar-day–based system has been augmented based on a 2006 National Institutes of Health working group report emphasizing the importance of organ-specific features of chronic GVHD in the clinical presentation of GVHD.7 Histologic changes in chronic organ GVHD tend to include more fibrotic features, whereas in acute GVHD more inflammatory changes are seen. The NIH working group report also stressed the importance of obtaining a biopsy specimen for histopathologic review and interdisciplinary collaboration to arrive at a consensus diagnosis, and noted the limitations of using histologic changes as the sole determinant of a “gold standard” diagnosis.7 GVHD can directly predispose patients to pulmonary GVHD and indirectly predispose them to infectious complications because the mainstay of therapy for GVHD is increased immunosuppression.
Pretransplant Evaluation
Case Patient 1
A 56-year-old man is diagnosed with acute myeloid leukemia (AML) after presenting with signs and symptoms consistent with pancytopenia. He has a past medical history of chronic sinus congestion, arthritis, depression, chronic pain, and carpal tunnel surgery. He is employed as an oilfield worker and has a 40-pack-year smoking history, but he recently cut back to half a pack per day. He is being evaluated for allogeneic transplant with his brother as the donor and the planned conditioning regimen is total body irradiation (TBI), thiotepa, cyclophosphamide, and antithymocyte globulin with T-cell depletion. Routine pretransplant pulmonary function testing (PFT) reveals a restrictive pattern and he is sent for pretransplant pulmonary evaluation.
Physical exam reveals a chronically ill appearing man. He is afebrile, the respiratory rate is 16 breaths/min, blood pressure is 145/88 mm Hg, heart rate is 92 beats/min, and oxygen saturation is 95%. He is in no distress. Auscultation of the chest reveals slightly diminished breath sounds bilaterally but is clear and without wheezes, rhonchi, or rales. Heart exam shows regular rate and rhythm without murmurs, rubs, or gallops. Extremities reveal no edema or rashes. Otherwise, the remainder of the exam is normal. The patient’s PFT results are shown in Table 2.
- What aspects of this patient’s history put him at risk for pulmonary complications after transplantation?
Risk Factors for Pulmonary Complications
Predicting who is at risk for pulmonary complications is difficult. Complications are generally divided into infectious and noninfectious categories. Regardless of category, allogeneic HSCT recipients are at increased risk compared with autologous recipients, but even in autologous transplants, more than 25% of patients will develop pulmonary complications in the first year.8 Prior to transplant, patients undergo full PFT. Early on, many studies attempted to show relationships between various factors and post-transplant pulmonary complications. Factors that were implicated were forced expiratory volume in 1 second (FEV1), diffusing capacity of the lung for carbon monoxide (D
Another sometimes overlooked risk before transplantation is restrictive lung disease. One study showed a twofold increase in respiratory failure and mortality if there was pretransplant restriction based on TLC < 80%.16
An interesting study by one group in pretransplant evaluation found decreased muscle strength by maximal inspiratory muscle strength (PImax), maximal expiratory muscle strength (PEmax), dominant hand grip strength, and 6-minute walk test (6MWT) distance prior to allogeneic transplant, but did not find a relationship between these variables and mortality.17 While this study had a small sample size, these findings likely deserve continued investigation.18
- What methods are used to calculate risk for complications?
Risk Scoring Systems
Several pretransplantation risk scores have been developed. In a study that looked at more than 2500 allogeneic transplants, Parimon et al showed that risk of mortality and respiratory failure could be estimated prior to transplant using a scoring system—the Lung Function Score (LFS)—that combines the FEV1 and D
The Pretransplantation Assessment of Mortality score, initially developed in 2006, predicts mortality within the first 2 years after HSCT based on 8 clinical factors: disease risk, age at transplant, donor type, conditioning regimen, and markers of organ function (percentage of predicted FEV1, percentage of predicted D
- What other preoperative testing or interventions should be considered in this patient?
Since there is a high risk of infectious complications after transplant, the question of whether pretransplantation patients should undergo screening imaging may arise. There is no evidence that routine chest computed tomography (CT) reduces the risk of infectious complications after transplantation.26 An area that may be insufficiently addressed in the pretransplantation evaluation is smoking cessation counseling.27 Studies have shown an elevated risk of mortality in smokers.28-30 Others have found a higher incidence of respiratory failure but not an increased mortality.31 Overall, with the good rates of smoking cessation that can be accomplished, smokers should be counseled to quit before transplantation.
In summary, patients should undergo full PFTs prior to transplantation to help stratify risk for pulmonary complications and mortality and to establish a clinical baseline. The LFS (using FEV1 and D
Case Patient 1 Conclusion
The patient undergoes transplantation due to his lack of other treatment options. Evaluation prior to transplant, however, shows that he is at high risk for pulmonary complications. He has a LFS of 7 prior to transplant (using the D
Early Infectious Pulmonary Complications
Case Patient 2
A 27-year-old man with a medical history significant for AML and allogeneic HSCT presents with cough productive of a small amount of clear to white sputum, dyspnea on exertion, and fevers for 1 week. He also has mild nausea and a decrease in appetite. He underwent HSCT 2.5 months prior to admission, which was a matched unrelated bone marrow transplant with TBI and cyclophosphamide conditioning. His past medical history is significant only for exercise-induced asthma for which he takes a rescue inhaler infrequently prior to transplantation. His pretransplant PFTs showed normal spirometry with an FEV1 of 106% of predicted and D
Physical exam is notable for fever of 101.0°F, heart rate 80 beats/min, respiratory rate 16 breaths/ min, and blood pressure 142/78 mm Hg; an admission oxygen saturation is 93% on room air. Lungs show bibasilar crackles and the remainder of the exam is normal. Laboratory testing shows a white blood cell count of 2400 cells/μL, hemoglobin 7.6 g/dL, and platelet count 66 × 103/μL. Creatinine is 1.0 mg/dL. Chest radiograph shows ill-defined bilateral lower-lobe infiltrates. CT scans are shown in the Figure.
- For which infectious complications is this patient most at risk?
Pneumonia
A prospective trial in the HSCT population reported a pneumonia incidence rate of 68%, and pneumonia is more common in allogeneic HSCT with prolonged immunosuppressive therapy.32 Development of pneumonia within 100 days of transplant directly correlates with nonrelapsed mortality.33 Early detection is key, and bronchoscopy within the first 5 days of symptoms has been shown to change therapy in approximately 40% of cases but has not been shown to affect mortality.34 The clinical presentation of pneumonia in the HSCT population can be variable because of the presence of neutropenia and profound immunosuppression. Traditionally accepted diagnostic criteria of fevers, sputum production, and new infiltrates should be used with caution, and an appropriately high index of suspicion should be maintained. Progression to respiratory failure, regardless of causative organism of infection, portends a poor prognosis, with mortality rates estimated at 70% to 90%.35,36 Several transplant-specific factors may affect early infections. For instance, UCB transplants have been found to have a higher incidence of invasive aspergillosis and cytomegalovirus (CMV) infections but without higher mortality attributed to the infections.37
Bacterial Pneumonia
Bacterial pneumonia accounts for 20% to 50% of pneumonia cases in HSCT recipients.38 Gram-negative organisms, specifically Pseudomonas aeruginosa and Escherichia coli, were reported to be the most common pathologic bacteria in recent prospective trials, whereas previous retrospective trials showed that common community-acquired organisms were the most common cause of pneumonia in HSCT recipients.32,39 This underscores the importance of being aware of the clinical prevalence of microorganisms and local antibiograms, along with associated institutional susceptibility profiles. Initiation of immediate empiric broad-spectrum antibiotics is essential when bacterial pneumonia is suspected.
Viral Pneumonia
The prevalence of viral pneumonia in stem cell transplant recipients is estimated at 28%,32 with most cases being caused by community viral pathogens such as rhinovirus, respiratory syncytial virus (RSV), influenza A and B, and parainfluenza.39 The prevention, prophylaxis, and early treatment of viral pneumonias, specifically CMV infection, have decreased the mortality associated with early pneumonia after HSCT. Co-infection with bacterial organisms must be considered and has been associated with increased mortality in the intensive care unit setting.40
Supportive treatment with rhinovirus infection is sufficient as the disease is usually self-limited in immunocompromised patients. In contrast, infection with RSV in the lower respiratory tract is associated with increased mortality in prior reports, and recent studies suggest that further exploration of prophylaxis strategies is warranted.41 Treatment with ribavirin remains the backbone of therapy, but drug toxicity continues to limit its use. The addition of immunomodulators such as RSV immune globulin or palivizumab to ribavirin remains controversial, but a retrospective review suggests that early treatment may prevent progression to lower respiratory tract infection and lead to improved mortality.42 Infection with influenza A/B must be considered during influenza season. Treatment with oseltamivir may shorten the duration of disease when influenza A/B or parainfluenza are detected. Reactivation of latent herpes simplex virus during the pre-engraftment phase should also be considered. Treatment is similar to that in nonimmunocompromised hosts. When CMV pneumonia is suspected, careful history regarding compliance with prophylactic antivirals and CMV status of both the recipient and donor are key. A presumptive diagnosis can be made with the presence of appropriate clinical scenario, supportive radiographic images showing areas of ground-glass opacification or consolidation, and positive CMV polymerase chain reaction (PCR) assay. Visualization of inclusion bodies on lung biopsy tissue remains the gold standard for diagnosis. Treatment consists of CMV immunoglobulin and ganciclovir.
Fungal Pneumonia
Early fungal pneumonias have been associated with increased mortality in the HSCT population.43 Clinical suspicion should remain high and compliance with antifungal prophylaxis should be questioned thoroughly. Invasive aspergillosis (IA) remains the most common fungal infection. A bimodal distribution of onset of infection peaking on day 16 and again on day 96 has been described in the literature.44 Patients often present with classic pneumonia symptoms, but these may be accompanied by hemoptysis. Proven IA diagnosis requires visualization of fungal forms from biopsy or needle aspiration or a positive culture obtained in a sterile fashion.45 Most clinical data comes from experience with probable and possible diagnosis of IA. Bronchoalveolar lavage with testing with Aspergillus galactomannan assay has been shown to be clinically useful in establishing the clinical diagnosis in the HSCT population.46 Classic air-crescent findings on chest CT are helpful in establishing a possible diagnosis, but retrospective analysis reveals CT findings such as focal infiltrates and pulmonary nodular patterns are more common.47 First-line treatment with voriconazole has been shown to decrease short-term mortality attributable to IA but has not had an effect on long-term, all-cause mortality.48 Surgical resection is reserved for patients with refractory disease or patients presenting with massive hemoptysis.
Mucormycosis is an emerging disease with ever increasing prevalence in the HSCT population, reflecting the improved prophylaxis and treatment of IA. Initial clinical presentation is similar to IA, most commonly affecting the lung, although craniofacial involvement is classic for mucormycosis, especially in HSCT patients with diabetes.49Mucor infections can present with massive hemoptysis due to tissue invasion and disregard for tissue and fascial planes. Diagnosis of mucormycosis is associated with as much as a six-fold increase in risk for death. Diagnosis requires identification of the organism by examination or culture and biopsy is often necessary.50,51 Amphotericin B remains first-line therapy as mucormycosis is resistant to azole antifungals, with higher doses recommended for cerebral involvement.52
Candida pulmonary infections during the early HSCT period are becoming increasingly rare due to widespread use of fluconazole prophylaxis and early treatment of mucosal involvement during neutropenia. Endemic fungal infections such as blastomycosis, coccidioidomycosis, and histoplasmosis should be considered in patients inhabiting specific geographic areas or with recent travel to these areas.
- What test should be performed to evaluate for infectious causes of pneumonia?
Role of Flexible Fiberoptic Bronchoscopy
The utility of flexible fiberoptic bronchoscopy (FOB) in immune-compromised patients for the evaluation of pulmonary infiltrates is a frequently debated topic. Current studies suggest a diagnosis can be made in approximately 80% of cases in the immune-compromised population.32,53 Noninvasive testing such as urine and serum antigens, sputum cultures, Aspergillus galactomannan assays, viral nasal swabs, and PCR studies often lead to a diagnosis in appropriate clinical scenarios. Conservative management would dictate the use of noninvasive testing whenever possible, and randomized controlled trials have shown noninvasive testing to be noninferior to FOB in preventing need for mechanical ventilation, with no difference in overall mortality.54 FOB has been shown to be most useful in establishing a diagnosis when an infectious etiology is suspected.55 In multivariate analysis, a delay in the identification of the etiology of pulmonary infiltrate was associated with increased mortality.56 Additionally, early FOB was found to be superior to late FOB in revealing a diagnosis. 32,57 Despite its ability to detect the cause of pulmonary disease, direct antibiotic therapy, and possibly change therapy, FOB with diagnostic maneuvers has not been shown to affect mortality.58 In a large case series, FOB with bronchoalveolar lavage (BAL) revealed a diagnosis in approximately 30% to 50% of cases. The addition of transbronchial biopsy did not improve diagnostic utility.58 More recent studies have confirmed that the addition of transbronchial biopsy does not add to diagnostic yield and is associated with increased adverse events.59 The appropriate use of advanced techniques such as endobronchial ultrasound–guided transbronchial needle aspirations, endobronchial biopsy, and CT-guided navigational bronchoscopy has not been established and should be considered on a case-by-case basis. In summary, routine early BAL is the diagnostic test of choice, especially when infectious pulmonary complications are suspected.
Contraindications for FOB in this population mirror those in the general population. These include acute severe hypoxemic respiratory failure, myocardial ischemia or acute coronary syndrome within 2 weeks of procedure, severe thrombocytopenia, and inability to provide or obtain informed consent from patient or health care power of attorney. Coagulopathy and thrombocytopenia are common comorbid conditions in the HSCT population. A platelet count of < 20 × 103/µL has generally been used as a cut-off for routine FOB with BAL.60 Risks of the procedures should be discussed clearly with the patient, but simple FOB for airway evaluation and BAL is generally well tolerated even under these conditions.
Early Nonifectious Pulmonary Complications
Case Patient 2 Continued
Bronchoscopy with BAL performed the day after admission is unremarkable and stains and cultures are negative for viral, bacterial, and fungal organisms. The patient is initially started on broad-spectrum antibiotics, but his oxygenation continues to worsen to the point that he is placed on noninvasive positive pressure ventilation. He is started empirically on amphotericin B and eventually is intubated. VATS lung biopsy is ultimately performed and pathology is consistent with diffuse alveolar damage.
- Based on these biopsy findings, what is the diagnosis?
Based on the pathology consistent with diffuse alveolar damage, a diagnosis of idiopathic pneumonia syndrome (IPS) is made.
- What noninfectious pulmonary complications occur in the early post-transplant period?
The overall incidence of noninfectious pulmonary complications after HSCT is generally estimated at 20% to 30%.32 Acute pulmonary edema is a common very early noninfectious pulmonary complication and clinically the most straightforward to treat. Three distinct clinical syndromes—peri-engraftment respiratory distress syndrome (PERDS), diffuse alveolar hemorrhage (DAH), and IPS—comprise the remainder of the pertinent early noninfectious complications. Clinical presentation differs based upon the disease entity. Recent studies have evaluated the role of angiotensin-converting enzyme polymorphisms as a predictive marker for risk of developing early noninfectious pulmonary complications.61
Peri-Engraftment Respiratory Distress Syndrome
PERDS is a clinical syndrome comprising the cardinal features of erythematous rash and fever along with noncardiogenic pulmonary infiltrates and hypoxemia that occur in the peri-engraftment period, defined as recovery of absolute neutrophil count to > 500/μL on 2 consecutive days.62 PERDS occurs in the autologous HSCT population and may be a clinical correlate to early GVHD in the allogeneic HSCT population. It is hypothesized that the pathophysiology underlying PERDS is an autoimmune-related capillary leak caused by pro-inflammatory cytokine release.63 Treatment remains anecdotal and currently consists of supportive care and high-dose corticosteroids. Some have favored limiting the use of gCSF given its role in stimulating rapid white blood cell recovery.33 Prognosis is favorable, but progression to fulminant respiratory failure requiring mechanical ventilation portends a poor prognosis.
Diffuse Alveolar Hemorrhage
DAH is clinical syndrome consisting of diffuse alveolar infiltrates on pulmonary imaging combined with progressively bloodier return per aliquot during BAL in 3 different subsegments or more than 20% hemosiderin-laden macrophages on BAL fluid evaluation. Classically, DAH is defined in the absence of pulmonary infection or cardiac dysfunction. The pathophysiology is thought to be related to inflammation of pulmonary vasculature within the alveolar walls leading to alveolitis. Although no prospective trials exist, early use of high-dose corticosteroid therapy is thought to improve outcomes;64,65 a recent study, however, showed low-dose steroids may be associated with the lowest mortality.66 Mortality is directly linked to the presence of superimposed infection, need for mechanical ventilation, late onset, and development of multiorgan failure.67
Idiopathic Pneumonia Syndrome
IPS is a complex clinical syndrome whose pathology is felt to stem from a variety of possible lung insults such as direct myeloablative drug toxicity, occult pulmonary infection, or cytokine-driven inflammation. The ATS published an article further subcategorizing IPS as different clinical entities based upon whether the primary insult involves the vascular endothelium, interstitial tissue, and airway tissue, truly idiopathic, or unclassified.68 In clinical practice, IPS is defined as widespread alveolar injury in the absence of evidence of renal failure, heart failure, and excessive fluid resuscitation. In addition, negative testing for a variety of bacterial, viral, and fungal causes is also necessary.69 Clinical syndromes included within the IPS definition are ARDS, acute interstitial pneumonia, DAH, cryptogenic organizing pneumonia, and BOS.70 Risk factors for developing IPS include TBI, older age of recipient, acute GVHD, and underlying diagnosis of AML or myelodysplastic syndrome.12 In addition, it has been shown that risk for developing IPS is lower in patients undergoing allogeneic HSCT who receive non-myeloablative conditioning regimens.71 The pathologic finding in IPS is diffuse alveolar damage. A 2006 study in which investigators reviewed BAL samples from patients with IPS found that 3% of the patients had PCR evidence of human metapneumovirus infection, and a study in 2015 found PCR evidence of infection in 53% of BAL samples from patients diagnosed with IPS.72,73 This fuels the debate on whether IPS is truly an infection-driven process where the source of infection, pulmonary or otherwise, simply escapes detection. Various surfactant proteins, which play a role in decreasing surface tension within the alveolar interface and function as mediators within the innate immunity of the lung, have been studied in regard to development of IPS. Small retrospective studies have shown a trend toward lower pre-transplant serum protein surfactant D and the development of IPS.74
The diagnosis of IPS does not require pathologic diagnosis in most circumstances. The correct clinical findings in association with a negative infectious workup lead to a presumptive diagnosis of IPS. The extent of the infectious workup that must be completed to adequately rule out infection is often a difficult clinical question. Recent recommendations include BAL fluid evaluation for routine bacterial cultures, appropriate viral culture, and consideration of PCR testing to evaluate for Mycoplasma, Chlamydia, and Aspergillus antigens.75 Transbronchial biopsy continues to appear in recommendations, but is not routinely performed and should be completed as the patient’s clinical status permits.8,68 Table 3 reviews basic features of early noninfectious pulmonary complications.
Treatment of IPS centers around moderate to high doses of corticosteroids. Based on IPS experimental modes, tumor necrosis factor (TNF)-α has been implicated as an important mediator. Unfortunately, several studies evaluating etanercept have produced conflicting results, and this agent’s clinical effects on morbidity and mortality remain in question.76
- What treatment should be offered to the patient with diffuse alveolar damage on biopsy?
Treatment consists of supportive care and empiric broad-spectrum antibiotics with consideration of high-dose corticosteroids. Based upon early studies in murine models implicating TNF, pilot studies were performed evaluating etanercept as a possible safe and effective addition to high-dose systemic corticosteroids.77 Although these results were promising, data from a truncated randomized control clinical trial failed to show improvement in patient response in the adult population.76 More recent data from the same author suggests that pediatric populations with IPS are, however, responsive to etanercept and high-dose corticosteroid therapy.78 When IPS develops as a late complication, treatment with high-dose corticosteroids (2 mg/kg/day) and etanercept (0.4 mg/kg twice weekly) has been shown to improve 2-year survival.79
Case Patient 2 Conclusion
The patient is started on steroids and makes a speedy recovery. He is successfully extubated 5 days later.
Conclusion
Careful pretransplant evaluation, including a full set of pulmonary function tests, can help predict a patient’s risk for pulmonary complications after transplant, allowing risk factor modification strategies to be implemented prior to transplant, including smoking cessation. It also helps identify patients at high risk for complications who will require closer monitoring after transplantation. Early posttransplant complications include infectious and noninfectious entities. Bacterial, viral, and fungal pneumonias are in the differential of infectious pneumonia, and bronchoscopy can be helpful in establishing a diagnosis. A common, important noninfectious cause of early pulmonary complications is IPS, which is treated with steroids and sometimes anti-TNF therapy.
Hematopoietic stem cell transplantation (HSCT) is widely used in the economically developed world to treat a variety of hematologic malignancies as well as nonmalignant diseases and solid tumors. An estimated 17,900 HSCTs were performed in 2011, and survival rates continue to increase.1 Pulmonary complications post HSCT are common, with rates ranging from 40% to 60%, and are associated with increased morbidity and mortality.2
Clinical diagnosis of pulmonary complications in the HSCT population has been aided by a previously well-defined chronology of the most common diseases.3 Historically, early pulmonary complications were defined as pulmonary complications occurring within 100 days of HSCT (corresponding to the acute graft-versus-host disease [GVHD] period). Late pulmonary complications are those that occur thereafter. This timeline, however, is now more variable given the increasing indications for HSCT, the use of reduced-intensity conditioning strategies, and varied individual immune reconstitution. This article discusses the management of early post-HSCT pulmonary complications; late post-HSCT pulmonary complications will be discussed in a separate follow-up article.
Transplant Basics
The development of pulmonary complications is affected by many factors associated with the transplant. Autologous transplantation involves the collection of a patient’s own stem cells, appropriate storage and processing, and re-implantation after induction therapy. During induction therapy, the patient undergoes high-dose chemotherapy or radiation therapy that ablates the bone marrow. The stem cells are then transfused back into the patient to repopulate the bone marrow. Allogeneic transplants involve the collection of stem cells from a donor. Donors are matched as closely as possible to the recipient’s histocompatibility antigen (HLA) haplotypes to prevent graft failure and rejection. The donor can be related or unrelated to the recipient. If there is not a possibility of a related match (from a sibling), then a national search is undertaken to look for a match through the National Marrow Donor Program. There are fewer transplant reactions and occurrences of GVHD if the major HLAs of the donor and recipient match. Table 1 reviews basic definitions pertaining to HSCT.
How the cells for transplantation are obtained is also an important factor in the rate of complications. There are 3 main sources: peripheral blood, bone marrow, and umbilical cord. Peripheral stem cell harvesting involves exposing the donor to granulocyte-colony stimulating factor (gCSF), which increases peripheral circulation of stem cells. These cells are then collected and infused into the recipient after the recipient has completed an induction regimen involving chemotherapy and/or radiation, depending on the protocol. This procedure is called peripheral blood stem cell transplant (PBSCT). Stem cells can also be directly harvested from bone marrow cells, which are collected from repeated aspiration of bone marrow from the posterior iliac crest.4 This technique is most common in children, whereas in adults peripheral blood stem cells are the most common source. Overall mortality does not differ based on the source of the stem cells. It is postulated that GVHD may be more common in patients undergoing PBSCT, but the graft failure rate may be lower.5
The third option is umbilical cord blood (UCB) as the source of stem cells. This involves the collection of umbilical cord blood that is prepared and frozen after birth. It has a smaller volume of cells, and although fewer cells are needed when using UCB, 2 separate donors may be required for a single adult recipient. The engraftment of the stem cells is slower and infections in the post-transplant period are more common. Prior reports indicate GVHD rates may be lower.4 While the use of UCB is not common in adults, the incidence has doubled over the past decade, increasing from 3% to 6%.
The conditioning regimen can influence pulmonary complications. Traditionally, an ablative transplant involves high-dose chemotherapy or radiation to eradicate the recipient’s bone marrow. This regimen can lead to many complications, especially in the immediate post-transplant period. In the past 10 years, there has been increasing interest in non-myeloablative, or reduced-intensity, conditioning transplants.6 These “mini transplants” involve smaller doses of chemotherapy or radiation, which do not totally eradicate the bone marrow; after the transplant a degree of chimerism develops where the donor and recipient stem cells coexist. The medications in the preparative regimen also should be considered because they can affect pulmonary complications after transplant. Certain chemotherapeutic agents such as carmustine, bleomycin, and many others can lead to acute and chronic presentations of pulmonary diseases such as hypersensitivity pneumonitis, pulmonary fibrosis, acute respiratory distress syndrome, and abnormal pulmonary function testing.
After the HSCT, GVHD can develop in more than 50% of allogeneic recipients.3 The incidence of GVHD has been reported to be increasing over the past 12 years.It is divided into acute GVHD (which traditionally happens in the first 100 days after transplant) and chronic GVHD (after day 100). This calendar-day–based system has been augmented based on a 2006 National Institutes of Health working group report emphasizing the importance of organ-specific features of chronic GVHD in the clinical presentation of GVHD.7 Histologic changes in chronic organ GVHD tend to include more fibrotic features, whereas in acute GVHD more inflammatory changes are seen. The NIH working group report also stressed the importance of obtaining a biopsy specimen for histopathologic review and interdisciplinary collaboration to arrive at a consensus diagnosis, and noted the limitations of using histologic changes as the sole determinant of a “gold standard” diagnosis.7 GVHD can directly predispose patients to pulmonary GVHD and indirectly predispose them to infectious complications because the mainstay of therapy for GVHD is increased immunosuppression.
Pretransplant Evaluation
Case Patient 1
A 56-year-old man is diagnosed with acute myeloid leukemia (AML) after presenting with signs and symptoms consistent with pancytopenia. He has a past medical history of chronic sinus congestion, arthritis, depression, chronic pain, and carpal tunnel surgery. He is employed as an oilfield worker and has a 40-pack-year smoking history, but he recently cut back to half a pack per day. He is being evaluated for allogeneic transplant with his brother as the donor and the planned conditioning regimen is total body irradiation (TBI), thiotepa, cyclophosphamide, and antithymocyte globulin with T-cell depletion. Routine pretransplant pulmonary function testing (PFT) reveals a restrictive pattern and he is sent for pretransplant pulmonary evaluation.
Physical exam reveals a chronically ill appearing man. He is afebrile, the respiratory rate is 16 breaths/min, blood pressure is 145/88 mm Hg, heart rate is 92 beats/min, and oxygen saturation is 95%. He is in no distress. Auscultation of the chest reveals slightly diminished breath sounds bilaterally but is clear and without wheezes, rhonchi, or rales. Heart exam shows regular rate and rhythm without murmurs, rubs, or gallops. Extremities reveal no edema or rashes. Otherwise, the remainder of the exam is normal. The patient’s PFT results are shown in Table 2.
- What aspects of this patient’s history put him at risk for pulmonary complications after transplantation?
Risk Factors for Pulmonary Complications
Predicting who is at risk for pulmonary complications is difficult. Complications are generally divided into infectious and noninfectious categories. Regardless of category, allogeneic HSCT recipients are at increased risk compared with autologous recipients, but even in autologous transplants, more than 25% of patients will develop pulmonary complications in the first year.8 Prior to transplant, patients undergo full PFT. Early on, many studies attempted to show relationships between various factors and post-transplant pulmonary complications. Factors that were implicated were forced expiratory volume in 1 second (FEV1), diffusing capacity of the lung for carbon monoxide (D
Another sometimes overlooked risk before transplantation is restrictive lung disease. One study showed a twofold increase in respiratory failure and mortality if there was pretransplant restriction based on TLC < 80%.16
An interesting study by one group in pretransplant evaluation found decreased muscle strength by maximal inspiratory muscle strength (PImax), maximal expiratory muscle strength (PEmax), dominant hand grip strength, and 6-minute walk test (6MWT) distance prior to allogeneic transplant, but did not find a relationship between these variables and mortality.17 While this study had a small sample size, these findings likely deserve continued investigation.18
- What methods are used to calculate risk for complications?
Risk Scoring Systems
Several pretransplantation risk scores have been developed. In a study that looked at more than 2500 allogeneic transplants, Parimon et al showed that risk of mortality and respiratory failure could be estimated prior to transplant using a scoring system—the Lung Function Score (LFS)—that combines the FEV1 and D
The Pretransplantation Assessment of Mortality score, initially developed in 2006, predicts mortality within the first 2 years after HSCT based on 8 clinical factors: disease risk, age at transplant, donor type, conditioning regimen, and markers of organ function (percentage of predicted FEV1, percentage of predicted D
- What other preoperative testing or interventions should be considered in this patient?
Since there is a high risk of infectious complications after transplant, the question of whether pretransplantation patients should undergo screening imaging may arise. There is no evidence that routine chest computed tomography (CT) reduces the risk of infectious complications after transplantation.26 An area that may be insufficiently addressed in the pretransplantation evaluation is smoking cessation counseling.27 Studies have shown an elevated risk of mortality in smokers.28-30 Others have found a higher incidence of respiratory failure but not an increased mortality.31 Overall, with the good rates of smoking cessation that can be accomplished, smokers should be counseled to quit before transplantation.
In summary, patients should undergo full PFTs prior to transplantation to help stratify risk for pulmonary complications and mortality and to establish a clinical baseline. The LFS (using FEV1 and D
Case Patient 1 Conclusion
The patient undergoes transplantation due to his lack of other treatment options. Evaluation prior to transplant, however, shows that he is at high risk for pulmonary complications. He has a LFS of 7 prior to transplant (using the D
Early Infectious Pulmonary Complications
Case Patient 2
A 27-year-old man with a medical history significant for AML and allogeneic HSCT presents with cough productive of a small amount of clear to white sputum, dyspnea on exertion, and fevers for 1 week. He also has mild nausea and a decrease in appetite. He underwent HSCT 2.5 months prior to admission, which was a matched unrelated bone marrow transplant with TBI and cyclophosphamide conditioning. His past medical history is significant only for exercise-induced asthma for which he takes a rescue inhaler infrequently prior to transplantation. His pretransplant PFTs showed normal spirometry with an FEV1 of 106% of predicted and D
Physical exam is notable for fever of 101.0°F, heart rate 80 beats/min, respiratory rate 16 breaths/ min, and blood pressure 142/78 mm Hg; an admission oxygen saturation is 93% on room air. Lungs show bibasilar crackles and the remainder of the exam is normal. Laboratory testing shows a white blood cell count of 2400 cells/μL, hemoglobin 7.6 g/dL, and platelet count 66 × 103/μL. Creatinine is 1.0 mg/dL. Chest radiograph shows ill-defined bilateral lower-lobe infiltrates. CT scans are shown in the Figure.
- For which infectious complications is this patient most at risk?
Pneumonia
A prospective trial in the HSCT population reported a pneumonia incidence rate of 68%, and pneumonia is more common in allogeneic HSCT with prolonged immunosuppressive therapy.32 Development of pneumonia within 100 days of transplant directly correlates with nonrelapsed mortality.33 Early detection is key, and bronchoscopy within the first 5 days of symptoms has been shown to change therapy in approximately 40% of cases but has not been shown to affect mortality.34 The clinical presentation of pneumonia in the HSCT population can be variable because of the presence of neutropenia and profound immunosuppression. Traditionally accepted diagnostic criteria of fevers, sputum production, and new infiltrates should be used with caution, and an appropriately high index of suspicion should be maintained. Progression to respiratory failure, regardless of causative organism of infection, portends a poor prognosis, with mortality rates estimated at 70% to 90%.35,36 Several transplant-specific factors may affect early infections. For instance, UCB transplants have been found to have a higher incidence of invasive aspergillosis and cytomegalovirus (CMV) infections but without higher mortality attributed to the infections.37
Bacterial Pneumonia
Bacterial pneumonia accounts for 20% to 50% of pneumonia cases in HSCT recipients.38 Gram-negative organisms, specifically Pseudomonas aeruginosa and Escherichia coli, were reported to be the most common pathologic bacteria in recent prospective trials, whereas previous retrospective trials showed that common community-acquired organisms were the most common cause of pneumonia in HSCT recipients.32,39 This underscores the importance of being aware of the clinical prevalence of microorganisms and local antibiograms, along with associated institutional susceptibility profiles. Initiation of immediate empiric broad-spectrum antibiotics is essential when bacterial pneumonia is suspected.
Viral Pneumonia
The prevalence of viral pneumonia in stem cell transplant recipients is estimated at 28%,32 with most cases being caused by community viral pathogens such as rhinovirus, respiratory syncytial virus (RSV), influenza A and B, and parainfluenza.39 The prevention, prophylaxis, and early treatment of viral pneumonias, specifically CMV infection, have decreased the mortality associated with early pneumonia after HSCT. Co-infection with bacterial organisms must be considered and has been associated with increased mortality in the intensive care unit setting.40
Supportive treatment with rhinovirus infection is sufficient as the disease is usually self-limited in immunocompromised patients. In contrast, infection with RSV in the lower respiratory tract is associated with increased mortality in prior reports, and recent studies suggest that further exploration of prophylaxis strategies is warranted.41 Treatment with ribavirin remains the backbone of therapy, but drug toxicity continues to limit its use. The addition of immunomodulators such as RSV immune globulin or palivizumab to ribavirin remains controversial, but a retrospective review suggests that early treatment may prevent progression to lower respiratory tract infection and lead to improved mortality.42 Infection with influenza A/B must be considered during influenza season. Treatment with oseltamivir may shorten the duration of disease when influenza A/B or parainfluenza are detected. Reactivation of latent herpes simplex virus during the pre-engraftment phase should also be considered. Treatment is similar to that in nonimmunocompromised hosts. When CMV pneumonia is suspected, careful history regarding compliance with prophylactic antivirals and CMV status of both the recipient and donor are key. A presumptive diagnosis can be made with the presence of appropriate clinical scenario, supportive radiographic images showing areas of ground-glass opacification or consolidation, and positive CMV polymerase chain reaction (PCR) assay. Visualization of inclusion bodies on lung biopsy tissue remains the gold standard for diagnosis. Treatment consists of CMV immunoglobulin and ganciclovir.
Fungal Pneumonia
Early fungal pneumonias have been associated with increased mortality in the HSCT population.43 Clinical suspicion should remain high and compliance with antifungal prophylaxis should be questioned thoroughly. Invasive aspergillosis (IA) remains the most common fungal infection. A bimodal distribution of onset of infection peaking on day 16 and again on day 96 has been described in the literature.44 Patients often present with classic pneumonia symptoms, but these may be accompanied by hemoptysis. Proven IA diagnosis requires visualization of fungal forms from biopsy or needle aspiration or a positive culture obtained in a sterile fashion.45 Most clinical data comes from experience with probable and possible diagnosis of IA. Bronchoalveolar lavage with testing with Aspergillus galactomannan assay has been shown to be clinically useful in establishing the clinical diagnosis in the HSCT population.46 Classic air-crescent findings on chest CT are helpful in establishing a possible diagnosis, but retrospective analysis reveals CT findings such as focal infiltrates and pulmonary nodular patterns are more common.47 First-line treatment with voriconazole has been shown to decrease short-term mortality attributable to IA but has not had an effect on long-term, all-cause mortality.48 Surgical resection is reserved for patients with refractory disease or patients presenting with massive hemoptysis.
Mucormycosis is an emerging disease with ever increasing prevalence in the HSCT population, reflecting the improved prophylaxis and treatment of IA. Initial clinical presentation is similar to IA, most commonly affecting the lung, although craniofacial involvement is classic for mucormycosis, especially in HSCT patients with diabetes.49Mucor infections can present with massive hemoptysis due to tissue invasion and disregard for tissue and fascial planes. Diagnosis of mucormycosis is associated with as much as a six-fold increase in risk for death. Diagnosis requires identification of the organism by examination or culture and biopsy is often necessary.50,51 Amphotericin B remains first-line therapy as mucormycosis is resistant to azole antifungals, with higher doses recommended for cerebral involvement.52
Candida pulmonary infections during the early HSCT period are becoming increasingly rare due to widespread use of fluconazole prophylaxis and early treatment of mucosal involvement during neutropenia. Endemic fungal infections such as blastomycosis, coccidioidomycosis, and histoplasmosis should be considered in patients inhabiting specific geographic areas or with recent travel to these areas.
- What test should be performed to evaluate for infectious causes of pneumonia?
Role of Flexible Fiberoptic Bronchoscopy
The utility of flexible fiberoptic bronchoscopy (FOB) in immune-compromised patients for the evaluation of pulmonary infiltrates is a frequently debated topic. Current studies suggest a diagnosis can be made in approximately 80% of cases in the immune-compromised population.32,53 Noninvasive testing such as urine and serum antigens, sputum cultures, Aspergillus galactomannan assays, viral nasal swabs, and PCR studies often lead to a diagnosis in appropriate clinical scenarios. Conservative management would dictate the use of noninvasive testing whenever possible, and randomized controlled trials have shown noninvasive testing to be noninferior to FOB in preventing need for mechanical ventilation, with no difference in overall mortality.54 FOB has been shown to be most useful in establishing a diagnosis when an infectious etiology is suspected.55 In multivariate analysis, a delay in the identification of the etiology of pulmonary infiltrate was associated with increased mortality.56 Additionally, early FOB was found to be superior to late FOB in revealing a diagnosis. 32,57 Despite its ability to detect the cause of pulmonary disease, direct antibiotic therapy, and possibly change therapy, FOB with diagnostic maneuvers has not been shown to affect mortality.58 In a large case series, FOB with bronchoalveolar lavage (BAL) revealed a diagnosis in approximately 30% to 50% of cases. The addition of transbronchial biopsy did not improve diagnostic utility.58 More recent studies have confirmed that the addition of transbronchial biopsy does not add to diagnostic yield and is associated with increased adverse events.59 The appropriate use of advanced techniques such as endobronchial ultrasound–guided transbronchial needle aspirations, endobronchial biopsy, and CT-guided navigational bronchoscopy has not been established and should be considered on a case-by-case basis. In summary, routine early BAL is the diagnostic test of choice, especially when infectious pulmonary complications are suspected.
Contraindications for FOB in this population mirror those in the general population. These include acute severe hypoxemic respiratory failure, myocardial ischemia or acute coronary syndrome within 2 weeks of procedure, severe thrombocytopenia, and inability to provide or obtain informed consent from patient or health care power of attorney. Coagulopathy and thrombocytopenia are common comorbid conditions in the HSCT population. A platelet count of < 20 × 103/µL has generally been used as a cut-off for routine FOB with BAL.60 Risks of the procedures should be discussed clearly with the patient, but simple FOB for airway evaluation and BAL is generally well tolerated even under these conditions.
Early Nonifectious Pulmonary Complications
Case Patient 2 Continued
Bronchoscopy with BAL performed the day after admission is unremarkable and stains and cultures are negative for viral, bacterial, and fungal organisms. The patient is initially started on broad-spectrum antibiotics, but his oxygenation continues to worsen to the point that he is placed on noninvasive positive pressure ventilation. He is started empirically on amphotericin B and eventually is intubated. VATS lung biopsy is ultimately performed and pathology is consistent with diffuse alveolar damage.
- Based on these biopsy findings, what is the diagnosis?
Based on the pathology consistent with diffuse alveolar damage, a diagnosis of idiopathic pneumonia syndrome (IPS) is made.
- What noninfectious pulmonary complications occur in the early post-transplant period?
The overall incidence of noninfectious pulmonary complications after HSCT is generally estimated at 20% to 30%.32 Acute pulmonary edema is a common very early noninfectious pulmonary complication and clinically the most straightforward to treat. Three distinct clinical syndromes—peri-engraftment respiratory distress syndrome (PERDS), diffuse alveolar hemorrhage (DAH), and IPS—comprise the remainder of the pertinent early noninfectious complications. Clinical presentation differs based upon the disease entity. Recent studies have evaluated the role of angiotensin-converting enzyme polymorphisms as a predictive marker for risk of developing early noninfectious pulmonary complications.61
Peri-Engraftment Respiratory Distress Syndrome
PERDS is a clinical syndrome comprising the cardinal features of erythematous rash and fever along with noncardiogenic pulmonary infiltrates and hypoxemia that occur in the peri-engraftment period, defined as recovery of absolute neutrophil count to > 500/μL on 2 consecutive days.62 PERDS occurs in the autologous HSCT population and may be a clinical correlate to early GVHD in the allogeneic HSCT population. It is hypothesized that the pathophysiology underlying PERDS is an autoimmune-related capillary leak caused by pro-inflammatory cytokine release.63 Treatment remains anecdotal and currently consists of supportive care and high-dose corticosteroids. Some have favored limiting the use of gCSF given its role in stimulating rapid white blood cell recovery.33 Prognosis is favorable, but progression to fulminant respiratory failure requiring mechanical ventilation portends a poor prognosis.
Diffuse Alveolar Hemorrhage
DAH is clinical syndrome consisting of diffuse alveolar infiltrates on pulmonary imaging combined with progressively bloodier return per aliquot during BAL in 3 different subsegments or more than 20% hemosiderin-laden macrophages on BAL fluid evaluation. Classically, DAH is defined in the absence of pulmonary infection or cardiac dysfunction. The pathophysiology is thought to be related to inflammation of pulmonary vasculature within the alveolar walls leading to alveolitis. Although no prospective trials exist, early use of high-dose corticosteroid therapy is thought to improve outcomes;64,65 a recent study, however, showed low-dose steroids may be associated with the lowest mortality.66 Mortality is directly linked to the presence of superimposed infection, need for mechanical ventilation, late onset, and development of multiorgan failure.67
Idiopathic Pneumonia Syndrome
IPS is a complex clinical syndrome whose pathology is felt to stem from a variety of possible lung insults such as direct myeloablative drug toxicity, occult pulmonary infection, or cytokine-driven inflammation. The ATS published an article further subcategorizing IPS as different clinical entities based upon whether the primary insult involves the vascular endothelium, interstitial tissue, and airway tissue, truly idiopathic, or unclassified.68 In clinical practice, IPS is defined as widespread alveolar injury in the absence of evidence of renal failure, heart failure, and excessive fluid resuscitation. In addition, negative testing for a variety of bacterial, viral, and fungal causes is also necessary.69 Clinical syndromes included within the IPS definition are ARDS, acute interstitial pneumonia, DAH, cryptogenic organizing pneumonia, and BOS.70 Risk factors for developing IPS include TBI, older age of recipient, acute GVHD, and underlying diagnosis of AML or myelodysplastic syndrome.12 In addition, it has been shown that risk for developing IPS is lower in patients undergoing allogeneic HSCT who receive non-myeloablative conditioning regimens.71 The pathologic finding in IPS is diffuse alveolar damage. A 2006 study in which investigators reviewed BAL samples from patients with IPS found that 3% of the patients had PCR evidence of human metapneumovirus infection, and a study in 2015 found PCR evidence of infection in 53% of BAL samples from patients diagnosed with IPS.72,73 This fuels the debate on whether IPS is truly an infection-driven process where the source of infection, pulmonary or otherwise, simply escapes detection. Various surfactant proteins, which play a role in decreasing surface tension within the alveolar interface and function as mediators within the innate immunity of the lung, have been studied in regard to development of IPS. Small retrospective studies have shown a trend toward lower pre-transplant serum protein surfactant D and the development of IPS.74
The diagnosis of IPS does not require pathologic diagnosis in most circumstances. The correct clinical findings in association with a negative infectious workup lead to a presumptive diagnosis of IPS. The extent of the infectious workup that must be completed to adequately rule out infection is often a difficult clinical question. Recent recommendations include BAL fluid evaluation for routine bacterial cultures, appropriate viral culture, and consideration of PCR testing to evaluate for Mycoplasma, Chlamydia, and Aspergillus antigens.75 Transbronchial biopsy continues to appear in recommendations, but is not routinely performed and should be completed as the patient’s clinical status permits.8,68 Table 3 reviews basic features of early noninfectious pulmonary complications.
Treatment of IPS centers around moderate to high doses of corticosteroids. Based on IPS experimental modes, tumor necrosis factor (TNF)-α has been implicated as an important mediator. Unfortunately, several studies evaluating etanercept have produced conflicting results, and this agent’s clinical effects on morbidity and mortality remain in question.76
- What treatment should be offered to the patient with diffuse alveolar damage on biopsy?
Treatment consists of supportive care and empiric broad-spectrum antibiotics with consideration of high-dose corticosteroids. Based upon early studies in murine models implicating TNF, pilot studies were performed evaluating etanercept as a possible safe and effective addition to high-dose systemic corticosteroids.77 Although these results were promising, data from a truncated randomized control clinical trial failed to show improvement in patient response in the adult population.76 More recent data from the same author suggests that pediatric populations with IPS are, however, responsive to etanercept and high-dose corticosteroid therapy.78 When IPS develops as a late complication, treatment with high-dose corticosteroids (2 mg/kg/day) and etanercept (0.4 mg/kg twice weekly) has been shown to improve 2-year survival.79
Case Patient 2 Conclusion
The patient is started on steroids and makes a speedy recovery. He is successfully extubated 5 days later.
Conclusion
Careful pretransplant evaluation, including a full set of pulmonary function tests, can help predict a patient’s risk for pulmonary complications after transplant, allowing risk factor modification strategies to be implemented prior to transplant, including smoking cessation. It also helps identify patients at high risk for complications who will require closer monitoring after transplantation. Early posttransplant complications include infectious and noninfectious entities. Bacterial, viral, and fungal pneumonias are in the differential of infectious pneumonia, and bronchoscopy can be helpful in establishing a diagnosis. A common, important noninfectious cause of early pulmonary complications is IPS, which is treated with steroids and sometimes anti-TNF therapy.
1. Gratwohl A, Baldomero H, Aljurf M, et al. Hematopoietic stem cell transplantation: a global perspective. JAMA 2010;303:1617–24.
2. Kotloff RM, Ahya VN, Crawford SW. Pulmonary complications of solid organ and hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2004;170:22–48.
4. Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med 2006;354:1813–26.
5. Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med 2012;367:1487–96.
6. Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant 2009;15:367–9.
7. Shulman HM, Kleiner D, Lee SJ, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant 2006;12:31–47.
8. Afessa B, Abdulai RM, Kremers WK, et al. Risk factors and outcome of pulmonary complications after autologous hematopoietic stem cell transplant. Chest 2012;141:442–50.
9. Bolwell BJ. Are predictive factors clinically useful in bone marrow transplantation? Bone Marrow Transplant 2003;32:853–61.
10. Carlson K, Backlund L, Smedmyr B, et al. Pulmonary function and complications subsequent to autologous bone marrow transplantation. Bone Marrow Transplant 1994;14:805–11.
11. Clark JG, Schwartz DA, Flournoy N, et al. Risk factors for airflow obstruction in recipients of bone marrow transplants. Ann Intern Med 1987;107:648–56.
12. Crawford SW, Fisher L. Predictive value of pulmonary function tests before marrow transplantation. Chest 1992; 101:1257–64.
13. Ghalie R, Szidon JP, Thompson L, et al. Evaluation of pulmonary complications after bone marrow transplantation: the role of pretransplant pulmonary function tests. Bone Marrow Transplant 1992;10:359–65.
14. Ho VT, Weller E, Lee SJ, et al. Prognostic factors for early severe pulmonary complications after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2001;7:223–9.
15. Horak DA, Schmidt GM, Zaia JA, et al. Pretransplant pulmonary function predicts cytomegalovirus-associated interstitial pneumonia following bone marrow transplantation. Chest 1992;102:1484–90.
16. Ramirez-Sarmiento A, Orozco-Levi M, Walter EC, et al. Influence of pretransplantation restrictive lung disease on allogeneic hematopoietic cell transplantation outcomes. Biol Blood Marrow Transplant 2010;16:199–206.
17. White AC, Terrin N, Miller KB, Ryan HF. Impaired respiratory and skeletal muscle strength in patients prior to hematopoietic stem-cell transplantation. Chest 2005;128145–52.
18. Afessa B. Pretransplant pulmonary evaluation of the blood and marrow transplant recipient. Chest 2005;128:8–10.
19. Parimon T, Madtes DK, Au DH, et al. Pretransplant lung function, respiratory failure, and mortality after stem cell transplantation. Am J Respir Crit Care Med 2005;172:384–90.
20. Pavletic SZ, Martin P, Lee SJ, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant 2006;12:252–66.
21. Parimon T, Au DH, Martin PJ, Chien JW. A risk score for mortality after allogeneic hematopoietic cell transplantation. Ann Intern Med 2006;144:407–14.
22. Au BK, Gooley TA, Armand P, et al. Reevaluation of the pretransplant assessment of mortality score after allogeneic hematopoietic transplantation. Biol Blood Marrow Transplant 2015;21:848–54.
23. Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005;106:2912–9.
24. Chien JW, Sullivan KM. Carbon monoxide diffusion capacity: how low can you go for hematopoietic cell transplantation eligibility? Biol Blood Marrow Transplant 2009;15: 447–53.
25. Coffey DG, Pollyea DA, Myint H, et al. Adjusting DLCO for Hb and its effects on the Hematopoietic Cell Transplantation-specific Comorbidity Index. Bone Marrow Transplant 2013;48:1253–6.
26. Kasow KA, Krueger J, Srivastava DK, et al. Clinical utility of computed tomography screening of chest, abdomen, and sinuses before hematopoietic stem cell transplantation: the St. Jude experience. Biol Blood Marrow Transplant 2009;15:490–5.
27. Hamadani M, Craig M, Awan FT, Devine SM. How we approach patient evaluation for hematopoietic stem cell transplantation. Bone Marrow Transplant 2010;45: 1259–68.
28. Savani BN, Montero A, Wu C, et al. Prediction and prevention of transplant-related mortality from pulmonary causes after total body irradiation and allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2005;11:223–30.
29. Ehlers SL, Gastineau DA, Patten CA, et al. The impact of smoking on outcomes among patients undergoing hematopoietic SCT for the treatment of acute leukemia. Bone Marrow Transplant 2011;46:285–90.
30. Marks DI, Ballen K, Logan BR, et al. The effect of smoking on allogeneic transplant outcomes. Biol Blood Marrow Transplant 2009;15:1277–87.
31. Tran BT, Halperin A, Chien JW. Cigarette smoking and outcomes after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2011;17:1004–11.
32. Lucena CM, Torres A, Rovira M, et al. Pulmonary complications in hematopoietic SCT: a prospective study. Bone Marrow Transplant 2014;49:1293–9.
33. Chi AK, Soubani AO, White AC, Miller KB. An update on pulmonary complications of hematopoietic stem cell transplantation. Chest 2013;144:1913–22.
34. Dunagan DP, Baker AM, Hurd DD, Haponik EF. Bronchoscopic evaluation of pulmonary infiltrates following bone marrow transplantation. Chest 1997;111:135–41.
35. Naeem N, Reed MD, Creger RJ, et al. Transfer of the hematopoietic stem cell transplant patient to the intensive care unit: does it really matter? Bone Marrow Transplant 2006;37:119–33.
36. Afessa B, Tefferi A, Hoagland HC, et al. Outcome of recipients of bone marrow transplants who require intensive care unit support. Mayo Clin Proc 1992;67:117–22.
37. Parody R, Martino R, de la Camara R, et al. Fungal and viral infections after allogeneic hematopoietic transplantation from unrelated donors in adults: improving outcomes over time. Bone Marrow Transplant 2015;50:274–81.
38. Orasch C, Weisser M, Mertz D, et al. Comparison of infectious complications during induction/consolidation chemotherapy versus allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2010;45:521–6.
39. Aguilar-Guisado M, Jimenez-Jambrina M, Espigado I, et al. Pneumonia in allogeneic stem cell transplantation recipients: a multicenter prospective study. Clin Transplant 2011;25:E629–38.
40. Palacios G, Hornig M, Cisterna D, et al. Streptococcus pneumoniae coinfection is correlated with the severity of H1N1 pandemic influenza. PLoS One 2009;4:e8540.
41. Hynicka LM, Ensor CR. Prophylaxis and treatment of respiratory syncytial virus in adult immunocompromised patients. Ann Pharmacother 2012;46:558–66.
42. Shah JN, Chemaly RF. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood 2011;2755–63.
43. Marr KA, Bowden RA. Fungal infections in patients undergoing blood and marrow transplantation. Transpl Infect Dis 1999;1:237–46.
44. Wald A, Leisenring W, van Burik JA, Bowden RA. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis 1997;175:1459–66.
45. Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 2002;34:7–14.
46. Fisher CE, Stevens AM, Leisenring W, et al. Independent contribution of bronchoalveolar lavage and serum galactomannan in the diagnosis of invasive pulmonary aspergillosis. Transpl Infect Dis 2014;16:505–10.
47. Kojima R, Tateishi U, Kami M, et al. Chest computed tomography of late invasive aspergillosis after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2005;11:506–11.
48. Salmeron G, Porcher R, Bergeron A, et al. Persistent poor long-term prognosis of allogeneic hematopoietic stem cell transplant recipients surviving invasive aspergillosis. Haematologica 2012;97:1357–63.
49. McNulty JS. Rhinocerebral mucormycosis: predisposing factors. Laryngoscope 1982;92(10 Pt 1):1140.
50. Walsh TJ, Gamaletsou MN, McGinnis MR, et al. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin Infect Dis 2012;54 Suppl 1:S55–60.
51. Klingspor L, Saaedi B, Ljungman P, Szakos A. Epidemiology and outcomes of patients with invasive mould infections: a retrospective observational study from a single centre (2005-2009). Mycoses 2015;58:470–7.
52. Danion F, Aguilar C, Catherinot E, et al. Mucormycosis: new developments in a persistently devastating infection. Semin Respir Crit Care Med 2015;36:692–70.
53. Rano A, Agusti C, Jimenez P, et al. Pulmonary infiltrates in non-HIV immunocompromised patients: a diagnostic approach using non-invasive and bronchoscopic procedures. Thorax 2001;56:379–87.
54. Azoulay E, Mokart D, Rabbat A, et al. Diagnostic bronchoscopy in hematology and oncology patients with acute respiratory failure: prospective multicenter data. Crit Care Med 2008;36:100–7.
55. Jain P, Sandur S, Meli Y, et al. Role of flexible bronchoscopy in immunocompromised patients with lung infiltrates. Chest 2004;125:712–22.
56. Rano A, Agusti C, Benito N, et al. Prognostic factors of non-HIV immunocompromised patients with pulmonary infiltrates. Chest 2002;122:253–61.
57. Shannon VR, Andersson BS, Lei X, et al. Utility of early versus late fiberoptic bronchoscopy in the evaluation of new pulmonary infiltrates following hematopoietic stem cell transplantation. Bone Marrow Transplant 2010;45:647–55.
58. Patel NR, Lee PS, Kim JH, et al. The influence of diagnostic bronchoscopy on clinical outcomes comparing adult autologous and allogeneic bone marrow transplant patients. Chest 2005;127:1388–96.
59. Chellapandian D, Lehrnbecher T, Phillips B, et al. Bronchoalveolar lavage and lung biopsy in patients with cancer and hematopoietic stem-cell transplantation recipients: a systematic review and meta-analysis. J Clin Oncol 2015;33:501–9.
60. Carr IM, Koegelenberg CF, von Groote-Bidlingmaier F, et al. Blood loss during flexible bronchoscopy: a prospective observational study. Respiration 2012;84:312–8.
61. Miyamoto M, Onizuka M, Machida S, et al. ACE deletion polymorphism is associated with a high risk of non-infectious pulmonary complications after stem cell transplantation. Int J Hematol 2014;99:175–83.
62. Capizzi SA, Kumar S, Huneke NE, et al. Peri-engraftment respiratory distress syndrome during autologous hematopoietic stem cell transplantation. Bone Marrow Transplant 2001;27:1299–303.
63. Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant 2001;27:893–8.
64. Wanko SO, Broadwater G, Folz RJ, Chao NJ. Diffuse alveolar hemorrhage: retrospective review of clinical outcome in allogeneic transplant recipients treated with aminocaproic acid. Biol Blood Marrow Transplant 2006;12:949–53.
65. Metcalf JP, Rennard SI, Reed EC, et al. Corticosteroids as adjunctive therapy for diffuse alveolar hemorrhage associated with bone marrow transplantation. University of Nebraska Medical Center Bone Marrow Transplant Group. Am J Med 1994;96:327–34.
66. Rathi NK, Tanner AR, Dinh A, et al. Low-, medium- and high-dose steroids with or without aminocaproic acid in adult hematopoietic SCT patients with diffuse alveolar hemorrhage. Bone Marrow Transplant 2015;50:420–6.
67. Afessa B, Tefferi A, Litzow MR, Peters SG. Outcome of diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipients. Am J Respir Crit Care Med 2002;166:1364–8.
68. Panoskaltsis-Mortari A, Griese M, Madtes DK, et al. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med 2011;183:1262–79.
69. Clark JG, Hansen JA, Hertz MI, Pet al. NHLBI workshop summary. Idiopathic pneumonia syndrome after bone marrow transplantation. Am Rev Resp Dis 1993;147:1601–6.
70. Vande Vusse LK, Madtes DK. Early onset noninfectious pulmonary syndromes after hematopoietic cell transplantation. Clin Chest Med 2017;38:233–48.
71. Fukuda T, Hackman RC, Guthrie KA, et al. Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventional conditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood 2003;102:2777–85.
72. Englund JA, Boeckh M, Kuypers J, et al. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med 2006;144:344–9.
73. Seo S, Renaud C, Kuypers JM, et al. Idiopathic pneumonia syndrome after hematopoietic cell transplantation: evidence of occult infectious etiologies. Blood 2015;125:3789–97.
74. Nakane T, Nakamae H, Kamoi H, et al. Prognostic value of serum surfactant protein D level prior to transplant for the development of bronchiolitis obliterans syndrome and idiopathic pneumonia syndrome following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2008;42:43–9.
75. Gilbert CR, Lerner A, Baram M, Awsare BK. Utility of flexible bronchoscopy in the evaluation of pulmonary infiltrates in the hematopoietic stem cell transplant population—a single center fourteen year experience. Arch Bronconeumol 2013;49:189–95.
76. Yanik GA, Horowitz MM, Weisdorf DJ, et al. Randomized, double-blind, placebo-controlled trial of soluble tumor necrosis factor receptor: enbrel (etanercept) for the treatment of idiopathic pneumonia syndrome after allogeneic stem cell transplantation: blood and marrow transplant clinical trials network protocol. Biol Blood Marrow Transplant 2014;20:858–64.
77. Levine JE, Paczesny S, Mineishi S, et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood 2008;111:2470–5.
78. Yanik GA, Grupp SA, Pulsipher MA, et al. TNF-receptor inhibitor therapy for the treatment of children with idiopathic pneumonia syndrome. A joint Pediatric Blood and Marrow Transplant Consortium and Children’s Oncology Group Study (ASCT0521). Biol Blood Marrow Transplant 2015;21:67–73.
79. Thompson J, Yin Z, D’Souza A, et al. Etanercept and corticosteroid therapy for the treatment of late-onset idiopathic pneumonia syndrome. Biol Blood Marrow Transplant J 2017; 23:1955–60.
1. Gratwohl A, Baldomero H, Aljurf M, et al. Hematopoietic stem cell transplantation: a global perspective. JAMA 2010;303:1617–24.
2. Kotloff RM, Ahya VN, Crawford SW. Pulmonary complications of solid organ and hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2004;170:22–48.
4. Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med 2006;354:1813–26.
5. Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med 2012;367:1487–96.
6. Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant 2009;15:367–9.
7. Shulman HM, Kleiner D, Lee SJ, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant 2006;12:31–47.
8. Afessa B, Abdulai RM, Kremers WK, et al. Risk factors and outcome of pulmonary complications after autologous hematopoietic stem cell transplant. Chest 2012;141:442–50.
9. Bolwell BJ. Are predictive factors clinically useful in bone marrow transplantation? Bone Marrow Transplant 2003;32:853–61.
10. Carlson K, Backlund L, Smedmyr B, et al. Pulmonary function and complications subsequent to autologous bone marrow transplantation. Bone Marrow Transplant 1994;14:805–11.
11. Clark JG, Schwartz DA, Flournoy N, et al. Risk factors for airflow obstruction in recipients of bone marrow transplants. Ann Intern Med 1987;107:648–56.
12. Crawford SW, Fisher L. Predictive value of pulmonary function tests before marrow transplantation. Chest 1992; 101:1257–64.
13. Ghalie R, Szidon JP, Thompson L, et al. Evaluation of pulmonary complications after bone marrow transplantation: the role of pretransplant pulmonary function tests. Bone Marrow Transplant 1992;10:359–65.
14. Ho VT, Weller E, Lee SJ, et al. Prognostic factors for early severe pulmonary complications after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2001;7:223–9.
15. Horak DA, Schmidt GM, Zaia JA, et al. Pretransplant pulmonary function predicts cytomegalovirus-associated interstitial pneumonia following bone marrow transplantation. Chest 1992;102:1484–90.
16. Ramirez-Sarmiento A, Orozco-Levi M, Walter EC, et al. Influence of pretransplantation restrictive lung disease on allogeneic hematopoietic cell transplantation outcomes. Biol Blood Marrow Transplant 2010;16:199–206.
17. White AC, Terrin N, Miller KB, Ryan HF. Impaired respiratory and skeletal muscle strength in patients prior to hematopoietic stem-cell transplantation. Chest 2005;128145–52.
18. Afessa B. Pretransplant pulmonary evaluation of the blood and marrow transplant recipient. Chest 2005;128:8–10.
19. Parimon T, Madtes DK, Au DH, et al. Pretransplant lung function, respiratory failure, and mortality after stem cell transplantation. Am J Respir Crit Care Med 2005;172:384–90.
20. Pavletic SZ, Martin P, Lee SJ, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant 2006;12:252–66.
21. Parimon T, Au DH, Martin PJ, Chien JW. A risk score for mortality after allogeneic hematopoietic cell transplantation. Ann Intern Med 2006;144:407–14.
22. Au BK, Gooley TA, Armand P, et al. Reevaluation of the pretransplant assessment of mortality score after allogeneic hematopoietic transplantation. Biol Blood Marrow Transplant 2015;21:848–54.
23. Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005;106:2912–9.
24. Chien JW, Sullivan KM. Carbon monoxide diffusion capacity: how low can you go for hematopoietic cell transplantation eligibility? Biol Blood Marrow Transplant 2009;15: 447–53.
25. Coffey DG, Pollyea DA, Myint H, et al. Adjusting DLCO for Hb and its effects on the Hematopoietic Cell Transplantation-specific Comorbidity Index. Bone Marrow Transplant 2013;48:1253–6.
26. Kasow KA, Krueger J, Srivastava DK, et al. Clinical utility of computed tomography screening of chest, abdomen, and sinuses before hematopoietic stem cell transplantation: the St. Jude experience. Biol Blood Marrow Transplant 2009;15:490–5.
27. Hamadani M, Craig M, Awan FT, Devine SM. How we approach patient evaluation for hematopoietic stem cell transplantation. Bone Marrow Transplant 2010;45: 1259–68.
28. Savani BN, Montero A, Wu C, et al. Prediction and prevention of transplant-related mortality from pulmonary causes after total body irradiation and allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2005;11:223–30.
29. Ehlers SL, Gastineau DA, Patten CA, et al. The impact of smoking on outcomes among patients undergoing hematopoietic SCT for the treatment of acute leukemia. Bone Marrow Transplant 2011;46:285–90.
30. Marks DI, Ballen K, Logan BR, et al. The effect of smoking on allogeneic transplant outcomes. Biol Blood Marrow Transplant 2009;15:1277–87.
31. Tran BT, Halperin A, Chien JW. Cigarette smoking and outcomes after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2011;17:1004–11.
32. Lucena CM, Torres A, Rovira M, et al. Pulmonary complications in hematopoietic SCT: a prospective study. Bone Marrow Transplant 2014;49:1293–9.
33. Chi AK, Soubani AO, White AC, Miller KB. An update on pulmonary complications of hematopoietic stem cell transplantation. Chest 2013;144:1913–22.
34. Dunagan DP, Baker AM, Hurd DD, Haponik EF. Bronchoscopic evaluation of pulmonary infiltrates following bone marrow transplantation. Chest 1997;111:135–41.
35. Naeem N, Reed MD, Creger RJ, et al. Transfer of the hematopoietic stem cell transplant patient to the intensive care unit: does it really matter? Bone Marrow Transplant 2006;37:119–33.
36. Afessa B, Tefferi A, Hoagland HC, et al. Outcome of recipients of bone marrow transplants who require intensive care unit support. Mayo Clin Proc 1992;67:117–22.
37. Parody R, Martino R, de la Camara R, et al. Fungal and viral infections after allogeneic hematopoietic transplantation from unrelated donors in adults: improving outcomes over time. Bone Marrow Transplant 2015;50:274–81.
38. Orasch C, Weisser M, Mertz D, et al. Comparison of infectious complications during induction/consolidation chemotherapy versus allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2010;45:521–6.
39. Aguilar-Guisado M, Jimenez-Jambrina M, Espigado I, et al. Pneumonia in allogeneic stem cell transplantation recipients: a multicenter prospective study. Clin Transplant 2011;25:E629–38.
40. Palacios G, Hornig M, Cisterna D, et al. Streptococcus pneumoniae coinfection is correlated with the severity of H1N1 pandemic influenza. PLoS One 2009;4:e8540.
41. Hynicka LM, Ensor CR. Prophylaxis and treatment of respiratory syncytial virus in adult immunocompromised patients. Ann Pharmacother 2012;46:558–66.
42. Shah JN, Chemaly RF. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood 2011;2755–63.
43. Marr KA, Bowden RA. Fungal infections in patients undergoing blood and marrow transplantation. Transpl Infect Dis 1999;1:237–46.
44. Wald A, Leisenring W, van Burik JA, Bowden RA. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis 1997;175:1459–66.
45. Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 2002;34:7–14.
46. Fisher CE, Stevens AM, Leisenring W, et al. Independent contribution of bronchoalveolar lavage and serum galactomannan in the diagnosis of invasive pulmonary aspergillosis. Transpl Infect Dis 2014;16:505–10.
47. Kojima R, Tateishi U, Kami M, et al. Chest computed tomography of late invasive aspergillosis after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2005;11:506–11.
48. Salmeron G, Porcher R, Bergeron A, et al. Persistent poor long-term prognosis of allogeneic hematopoietic stem cell transplant recipients surviving invasive aspergillosis. Haematologica 2012;97:1357–63.
49. McNulty JS. Rhinocerebral mucormycosis: predisposing factors. Laryngoscope 1982;92(10 Pt 1):1140.
50. Walsh TJ, Gamaletsou MN, McGinnis MR, et al. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin Infect Dis 2012;54 Suppl 1:S55–60.
51. Klingspor L, Saaedi B, Ljungman P, Szakos A. Epidemiology and outcomes of patients with invasive mould infections: a retrospective observational study from a single centre (2005-2009). Mycoses 2015;58:470–7.
52. Danion F, Aguilar C, Catherinot E, et al. Mucormycosis: new developments in a persistently devastating infection. Semin Respir Crit Care Med 2015;36:692–70.
53. Rano A, Agusti C, Jimenez P, et al. Pulmonary infiltrates in non-HIV immunocompromised patients: a diagnostic approach using non-invasive and bronchoscopic procedures. Thorax 2001;56:379–87.
54. Azoulay E, Mokart D, Rabbat A, et al. Diagnostic bronchoscopy in hematology and oncology patients with acute respiratory failure: prospective multicenter data. Crit Care Med 2008;36:100–7.
55. Jain P, Sandur S, Meli Y, et al. Role of flexible bronchoscopy in immunocompromised patients with lung infiltrates. Chest 2004;125:712–22.
56. Rano A, Agusti C, Benito N, et al. Prognostic factors of non-HIV immunocompromised patients with pulmonary infiltrates. Chest 2002;122:253–61.
57. Shannon VR, Andersson BS, Lei X, et al. Utility of early versus late fiberoptic bronchoscopy in the evaluation of new pulmonary infiltrates following hematopoietic stem cell transplantation. Bone Marrow Transplant 2010;45:647–55.
58. Patel NR, Lee PS, Kim JH, et al. The influence of diagnostic bronchoscopy on clinical outcomes comparing adult autologous and allogeneic bone marrow transplant patients. Chest 2005;127:1388–96.
59. Chellapandian D, Lehrnbecher T, Phillips B, et al. Bronchoalveolar lavage and lung biopsy in patients with cancer and hematopoietic stem-cell transplantation recipients: a systematic review and meta-analysis. J Clin Oncol 2015;33:501–9.
60. Carr IM, Koegelenberg CF, von Groote-Bidlingmaier F, et al. Blood loss during flexible bronchoscopy: a prospective observational study. Respiration 2012;84:312–8.
61. Miyamoto M, Onizuka M, Machida S, et al. ACE deletion polymorphism is associated with a high risk of non-infectious pulmonary complications after stem cell transplantation. Int J Hematol 2014;99:175–83.
62. Capizzi SA, Kumar S, Huneke NE, et al. Peri-engraftment respiratory distress syndrome during autologous hematopoietic stem cell transplantation. Bone Marrow Transplant 2001;27:1299–303.
63. Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant 2001;27:893–8.
64. Wanko SO, Broadwater G, Folz RJ, Chao NJ. Diffuse alveolar hemorrhage: retrospective review of clinical outcome in allogeneic transplant recipients treated with aminocaproic acid. Biol Blood Marrow Transplant 2006;12:949–53.
65. Metcalf JP, Rennard SI, Reed EC, et al. Corticosteroids as adjunctive therapy for diffuse alveolar hemorrhage associated with bone marrow transplantation. University of Nebraska Medical Center Bone Marrow Transplant Group. Am J Med 1994;96:327–34.
66. Rathi NK, Tanner AR, Dinh A, et al. Low-, medium- and high-dose steroids with or without aminocaproic acid in adult hematopoietic SCT patients with diffuse alveolar hemorrhage. Bone Marrow Transplant 2015;50:420–6.
67. Afessa B, Tefferi A, Litzow MR, Peters SG. Outcome of diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipients. Am J Respir Crit Care Med 2002;166:1364–8.
68. Panoskaltsis-Mortari A, Griese M, Madtes DK, et al. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med 2011;183:1262–79.
69. Clark JG, Hansen JA, Hertz MI, Pet al. NHLBI workshop summary. Idiopathic pneumonia syndrome after bone marrow transplantation. Am Rev Resp Dis 1993;147:1601–6.
70. Vande Vusse LK, Madtes DK. Early onset noninfectious pulmonary syndromes after hematopoietic cell transplantation. Clin Chest Med 2017;38:233–48.
71. Fukuda T, Hackman RC, Guthrie KA, et al. Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventional conditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood 2003;102:2777–85.
72. Englund JA, Boeckh M, Kuypers J, et al. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med 2006;144:344–9.
73. Seo S, Renaud C, Kuypers JM, et al. Idiopathic pneumonia syndrome after hematopoietic cell transplantation: evidence of occult infectious etiologies. Blood 2015;125:3789–97.
74. Nakane T, Nakamae H, Kamoi H, et al. Prognostic value of serum surfactant protein D level prior to transplant for the development of bronchiolitis obliterans syndrome and idiopathic pneumonia syndrome following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2008;42:43–9.
75. Gilbert CR, Lerner A, Baram M, Awsare BK. Utility of flexible bronchoscopy in the evaluation of pulmonary infiltrates in the hematopoietic stem cell transplant population—a single center fourteen year experience. Arch Bronconeumol 2013;49:189–95.
76. Yanik GA, Horowitz MM, Weisdorf DJ, et al. Randomized, double-blind, placebo-controlled trial of soluble tumor necrosis factor receptor: enbrel (etanercept) for the treatment of idiopathic pneumonia syndrome after allogeneic stem cell transplantation: blood and marrow transplant clinical trials network protocol. Biol Blood Marrow Transplant 2014;20:858–64.
77. Levine JE, Paczesny S, Mineishi S, et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood 2008;111:2470–5.
78. Yanik GA, Grupp SA, Pulsipher MA, et al. TNF-receptor inhibitor therapy for the treatment of children with idiopathic pneumonia syndrome. A joint Pediatric Blood and Marrow Transplant Consortium and Children’s Oncology Group Study (ASCT0521). Biol Blood Marrow Transplant 2015;21:67–73.
79. Thompson J, Yin Z, D’Souza A, et al. Etanercept and corticosteroid therapy for the treatment of late-onset idiopathic pneumonia syndrome. Biol Blood Marrow Transplant J 2017; 23:1955–60.
The angry disciple
CASE Disorganized thoughts and grandiose delusions
Mr. J, age 54, presents to the psychiatric emergency department (ED) with agitation and disruptive behavior. He claims that he is “the son of Jesus Christ” and has to travel to the Middle East to be baptized. Mr. J is irritable, shouting, and threatening staff members. He receives
The next day, he is calm and cooperative, but continues to express the same religious delusions. Mr. J is admitted to the psychiatric inpatient unit for further evaluation.
On the unit, Mr. J is pleasant and cooperative, but tangential in thought process. He reports he was “saved” by God 4 years ago, and that God communicates with him through music. Despite this, he denies having auditory or visual hallucinations.
Approximately 3 months earlier, Mr. J had stopped working and left his home and family in another state to pursue his “mission” of being baptized in the Middle East. Mr. J has been homeless since then. Despite that, he reports that his mood is “great” and denies any recent changes in mood, sleep, appetite, energy level, or psychomotor agitation. Although no formal cognitive testing is performed, Mr. J is alert and oriented to person, place, and time with intact remote and recent memory, no language deficits, and no lapses in concentration or attention throughout interview.
Mr. J says he has been drinking alcohol regularly throughout his adult life, often a few times per week, up to “a case and a half” of beer at times. He claims he’s had multiple periods of sobriety but denies having experienced withdrawal symptoms during those times. Mr. J reports 1 prior psychiatric hospitalization 25 years ago after attempting suicide by overdose following the loss of a loved one. At that time, he was diagnosed with posttraumatic stress disorder (PTSD). During this admission, he denies having any symptoms of PTSD or periods of mania or depression, and he has not undergone psychiatric treatment since he had been diagnosed with PTSD. He denies any family history of psychiatric illness as well as any medical comorbidities or medication use.
[polldaddy:10279202]
The authors’ observations
Mr. J’s presentation had a wide differential diagnosis (Table 1). The initial agitation Mr. J displayed in the psychiatric ED was likely secondary to acute alcohol intoxication, given that he was subsequently pleasant, calm, and cooperative after the alcohol was metabolized. Despite this, Mr. J continued to demonstrate delusions of a religious and somewhat grandiose nature with tangential thought processes, which made substance-induced psychosis less likely to be the sole diagnosis. Although it is possible to develop psychotic symptoms due to severe alcohol withdrawal (alcoholic hallucinosis), Mr. J’s vital signs remained stable, and he demonstrated no other signs or symptoms of withdrawal throughout his hospitalization. His presentation also did not fit that of delirium tremens because he was not confused or disoriented, and did not demonstrate perceptual disturbance.
While delusions were the most prominent feature of Mr. J’s apparent psychosis, the presence of disorganized thought processes and impaired functioning, as evidenced by Mr. J’s unemployment and recent homelessness, were more consistent with a primary psychotic disorder than a delusional disorder.1
Continue to: Mr. J began to exhibit...
Mr. J began to exhibit these psychotic symptoms in his early 50s; because the average age of onset of schizophrenia for males is approximately age 20 to 25, the likelihood of his presentation being the result of a primary psychotic disorder was low.1 Although less common, it was possible that Mr. J had developed late-onset schizophrenia, where the first episode typically occurs after approximately age 40 to 45. Mr. J also described that he was in a “great” mood but had grandiose delusions and had made recent impulsive decisions, which suggests there was a possible mood component to his presentation and a potential diagnosis of schizoaffective disorder or bipolar disorder with psychotic symptoms. However, before any of these diagnoses could be made, a medical or neurologic condition that could cause his symptoms needed to be investigated and ruled out. Further collateral information regarding Mr. J’s history and timeline of symptoms was required.
EVALUATION Family history reveals clues
All laboratory studies completed during Mr. J’s hospitalization are unremarkable, including complete blood count, basic metabolic panel, hepatic function panel, gamma-glutamyl transferase test, magnesium, phosphate, thyroid-stimulating hormone, vitamin B12, thiamine, folate, urinalysis, and urine drug screen. Mr. J does not undergo any head imaging.
Mr. J has not been in touch with his family since leaving his home approximately 3 months before he presented to the ED, and he gives consent for the inpatient team to attempt to contact them. One week into hospitalization, Mr. J’s sibling informs the team of a family history of genetically confirmed Huntington’s disease (HD), with psychiatric symptoms preceding the onset of motor symptoms in multiple first-degree relatives. His family says that before Mr. J first developed delusions 4 years ago, he had not exhibited any psychotic symptoms during periods of alcohol use or sobriety.
Mr. J does not demonstrate any overt movement symptoms on the unit and denies noting any rigidity, change in gait, or abnormal/uncontrolled movements. The inpatient psychiatric team consults neurology and a full neurologic evaluation is performed. The results are unremarkable outside of his psychiatric symptoms; specifically, Mr. J does not demonstrate even subtle motor signs or cognitive impairment. Given Mr. J’s family history, unremarkable lab findings, and age at presentation, the neurology team and inpatient psychiatry team suspect that his psychosis is likely an early presentation of HD.
[polldaddy:10279212]
The authors’ observations
Genetics of Huntington’s disease
Huntington’s disease is an autosomal dominant neurodegenerative disorder caused by expansion of cytosine-adenine-guanine (CAG) trinucleotide repeats within the Huntingtin (HTT) gene on chromosome 4, which codes for the huntingtin protein.2,3 While the function of “normal” huntingtin protein is not fully understood, it is known that CAG repeat expansion in the HTT gene of >35 repeats codes for a mutant huntingtin protein.2,3 The mutant huntingtin protein causes progressive neuronal loss in the basal ganglia and striatum, resulting in the clinical Huntington’s phenotype.3 Notably, the patient’s age at disease onset is inversely correlated with the number of repeats. For example, expansions of approximately 40 to 50 CAG repeats often result in adult-onset HD, while expansions of >60 repeats are typically associated with juvenile-onset HD (before age 20). CAG repeat lengths of approximately 36 to 39 demonstrate reduced penetrance, with some individuals developing symptomatic HD while others do not.2 Instability of the CAG repeat expansion can result in genetic “anticipation,” wherein repeat length increases between generations, causing earlier age of onset in affected offspring. Genetic anticipation in HD occurs more frequently in paternal transmission—approximately 80% to 90% of juvenile HD cases are inherited paternally, at times with the number of CAG repeats exceeding 200.3
Continue to: Psychiatric manifestations of Huntington's disease
Psychiatric manifestations of Huntington’s disease
Huntington’s disease is characterized by motor, cognitive, and behavioral disturbances (Table 22,4). Motor symptoms include a characteristic and well-recognized chorea, often predominating earlier in HD, that progresses to rigidity, spasticity, and bradykinesia later in the disease course.2 Cognitive impairments develop in a similar progressive manner and can often precede the onset of motor symptoms, beginning with early executive dysfunction. Thinking often becomes more rigid and less efficient, causing difficulty with multi-tasking and concentration, and often progressing to subcortical dementia.2
Psychiatric symptoms have long been recognized as a feature of HD; the estimated lifetime prevalence in patients with HD ranges from approximately 33% to 76%.4 Depressed mood, anxiety, irritability, and apathy are the most commonly reported symptoms, while a smaller percentage of patients with HD can experience obsessive-compulsive disorder (10% to 52%) or psychotic symptoms (3% to 11%).4 A more specific schizophrenia-like psychosis occurs in approximately 3% to 6% of patients, and often is a paranoid type.5,6 Positive psychotic symptoms, such as hallucinations and delusions, typically become less overt as HD progresses and cognitive impairments worsen.7
Although the onset of motor symptoms leads to diagnosis in the majority of patients with HD, many patients present with psychiatric symptoms—most commonly depression—prior to motor symptoms.8 An increasing body of literature details instances of psychosis preceding motor symptom onset by up to 10 years.6,9-12 In many of these cases, the patient has a family history of HD-associated psychosis. Family history is a major risk factor for HD-associated psychosis, as is early-onset HD.7,9
TREATMENT Antipsychotics result in some improvement
On Day 1 or 2, Mr. J is started on risperidone, 1 mg twice daily, to manage his symptoms. He shows incremental improvement in thought organization. Although his religious and grandiose delusions persist, they become less fixed, and he is able to take the team’s suggestion that he reconnect with his family.
Mr. J is aware of his family history of HD and acknowledges that multiple relatives had early psychiatric manifestations of HD. Despite this, he still has difficulty recognizing any connection between other family members’ presentation and his own. The psychiatry and neurology teams discuss the process, ethics, and implications of genetic testing for HD with Mr. J; however, he is ambivalent regarding genetic testing, and states he would consider it after discussing it with his family.
Continue to: The neurology team recommends...
The neurology team recommends against imaging for Mr. J because HD-related changes are not typically seen until later in the disease progression. On Day 9, they recommend changing from risperidone to quetiapine (50 mg every night at bedtime) due to evidence of its effectiveness specifically for treating behavioral symptoms of HD.13
While receiving quetiapine, Mr. J experiences significant drowsiness. Because he had experienced improvement in thought organization while he was receiving risperidone, he is switched back to risperidone.
[polldaddy:10279220]
The authors’ observations
Currently, no treatments are available to prevent the development or progression of HD. However, symptomatic treatment of motor and behavioral disturbances can lead to functional improvement and improved quality of life for individuals affected by HD.
There are no extensive clinical trials to date, but multiple case reports and studies have shown second-generation antipsychotics (SGAs), including quetiapine, olanzapine,
OUTCOME Discharge despite persistent delusions
Mr. J’s religious and grandiose delusions continue throughout hospitalization despite treatment with antipsychotics. However, because he remains calm and cooperative and demonstrates improvement in thought organization, he is deemed safe for discharge and instructed to continue risperidone. The team coordinates with Mr. J’s family to arrange transportation home and outpatient neurology follow-up.
Bottom Line
Psychiatric manifestations, including psychosis, are prominent symptoms of Huntington’s disease (HD) and may precede the onset of more readily recognized motor symptoms. This poses a diagnostic challenge, and clinicians should remain cognizant of this possibility, especially in patients with a family history of HD-associated psychosis.
Related Resources
- Huntington’s Disease Society of America. http://hdsa.org.
- National Institute of Neurological Disorders and Stroke. Huntington’s disease information page: What research is being done? https://www.ninds.nih.gov/Disorders/All-Disorders/Huntingtons-Disease-Information-Page.
- Scher LM. How to target psychiatric symptoms of Huntington’s disease. Current Psychiatry. 2012;11(9):34-39.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
2. Novak MJ, Tabrizi SJ. Huntington’s disease: clinical presentation and treatment. Int Rev Neurobiol. 2011;98:297-323.
3. Reiner A, Dragatsis I, Dietrich P. Genetics and neuropathology of Huntington’s disease. Int Rev Neurobiol. 2011;98:325-372.
4. van Duijn E, Kingma EM, Van der mast RC. Psychopathology in verified Huntington’s disease gene carriers. J Neuropsychiatry Clin Neurosci. 2007;19(4):441-448.
5. Naarding P, Kremer HP, Zitman FG. Huntington’s disease: a review of the literature on prevalence and treatment of neuropsychiatric phenomena. Eur Psychiatry. 2001;16(8):439-445.
6. Xu C, Yogaratnam J, Tan N, et al. Psychosis, treatment emergent extrapyramidal events, and subsequent onset of Huntington’s disease: a case report and review of the literature. Clin Psychopharmacol Neurosci. 2016;14(3):302-304.
7. Mendez MF. Huntington’s disease: update and review of neuropsychiatric aspects. Int J Psychiatry Med. 1994;24(3):189-208.
8. Di Maio L, Squitieri F, Napolitano G, et al. Onset symptoms in 510 patients with Huntington’s disease. J Med Genet. 1993;30(4):289-292.
9. Jauhar S, Ritchie S. Psychiatric and behavioural manifestations of Huntington’s disease. Adv Psychiatr Treat. 2010;16(3):168-175.
10. Nagel M, Rumpf HJ, Kasten M. Acute psychosis in a verified Huntington disease gene carrier with subtle motor signs: psychiatric criteria should be considered for the diagnosis. Gen Hosp Psychiatry. 2014;36(3):361.e3-e4. doi: 10.1016/j.genhosppsych.2014.01.008.
11. Corrêa BB, Xavier M, Guimarães J. Association of Huntington’s disease and schizophrenia-like psychosis in a Huntington’s disease pedigree. Clin Pract Epidemiol Ment Health. 2006;2:1.
12. Ding J, Gadit AM. Psychosis with Huntington’s disease: role of antipsychotic medications. BMJ Case Rep. 2014: bcr2013202625. doi: 10.1136/bcr-2013-202625.
13. Alpay M, Koroshetz WJ. Quetiapine in the treatment of behavioral disturbances in patients with Huntington’s disease. Psychosomatics. 2006;47(1):70-72.
14. Duff K, Beglinger LJ, O’Rourke ME, et al. Risperidone and the treatment of psychiatric, motor, and cognitive symptoms in Huntington’s disease. Ann Clin Psychiatry. 2008;20(1):1-3.
15. Paleacu D, Anca M, Giladi N. Olanzapine in Huntington’s disease. Acta Neurol Scand. 2002;105(6):441-444.
16. Lin W, Chou Y. Aripiprazole effects on psychosis and chorea in a patient with Huntington’s disease. Am J Psychiatry. 2008;165(9):1207-1208.
17. van Vugt JP, Siesling S, Vergeer M, et al. Clozapine versus placebo in Huntington’s disease: a double blind randomized comparative study. J Neurol Neurosurg Psychiatr. 1997;63(1):35-39.
CASE Disorganized thoughts and grandiose delusions
Mr. J, age 54, presents to the psychiatric emergency department (ED) with agitation and disruptive behavior. He claims that he is “the son of Jesus Christ” and has to travel to the Middle East to be baptized. Mr. J is irritable, shouting, and threatening staff members. He receives
The next day, he is calm and cooperative, but continues to express the same religious delusions. Mr. J is admitted to the psychiatric inpatient unit for further evaluation.
On the unit, Mr. J is pleasant and cooperative, but tangential in thought process. He reports he was “saved” by God 4 years ago, and that God communicates with him through music. Despite this, he denies having auditory or visual hallucinations.
Approximately 3 months earlier, Mr. J had stopped working and left his home and family in another state to pursue his “mission” of being baptized in the Middle East. Mr. J has been homeless since then. Despite that, he reports that his mood is “great” and denies any recent changes in mood, sleep, appetite, energy level, or psychomotor agitation. Although no formal cognitive testing is performed, Mr. J is alert and oriented to person, place, and time with intact remote and recent memory, no language deficits, and no lapses in concentration or attention throughout interview.
Mr. J says he has been drinking alcohol regularly throughout his adult life, often a few times per week, up to “a case and a half” of beer at times. He claims he’s had multiple periods of sobriety but denies having experienced withdrawal symptoms during those times. Mr. J reports 1 prior psychiatric hospitalization 25 years ago after attempting suicide by overdose following the loss of a loved one. At that time, he was diagnosed with posttraumatic stress disorder (PTSD). During this admission, he denies having any symptoms of PTSD or periods of mania or depression, and he has not undergone psychiatric treatment since he had been diagnosed with PTSD. He denies any family history of psychiatric illness as well as any medical comorbidities or medication use.
[polldaddy:10279202]
The authors’ observations
Mr. J’s presentation had a wide differential diagnosis (Table 1). The initial agitation Mr. J displayed in the psychiatric ED was likely secondary to acute alcohol intoxication, given that he was subsequently pleasant, calm, and cooperative after the alcohol was metabolized. Despite this, Mr. J continued to demonstrate delusions of a religious and somewhat grandiose nature with tangential thought processes, which made substance-induced psychosis less likely to be the sole diagnosis. Although it is possible to develop psychotic symptoms due to severe alcohol withdrawal (alcoholic hallucinosis), Mr. J’s vital signs remained stable, and he demonstrated no other signs or symptoms of withdrawal throughout his hospitalization. His presentation also did not fit that of delirium tremens because he was not confused or disoriented, and did not demonstrate perceptual disturbance.
While delusions were the most prominent feature of Mr. J’s apparent psychosis, the presence of disorganized thought processes and impaired functioning, as evidenced by Mr. J’s unemployment and recent homelessness, were more consistent with a primary psychotic disorder than a delusional disorder.1
Continue to: Mr. J began to exhibit...
Mr. J began to exhibit these psychotic symptoms in his early 50s; because the average age of onset of schizophrenia for males is approximately age 20 to 25, the likelihood of his presentation being the result of a primary psychotic disorder was low.1 Although less common, it was possible that Mr. J had developed late-onset schizophrenia, where the first episode typically occurs after approximately age 40 to 45. Mr. J also described that he was in a “great” mood but had grandiose delusions and had made recent impulsive decisions, which suggests there was a possible mood component to his presentation and a potential diagnosis of schizoaffective disorder or bipolar disorder with psychotic symptoms. However, before any of these diagnoses could be made, a medical or neurologic condition that could cause his symptoms needed to be investigated and ruled out. Further collateral information regarding Mr. J’s history and timeline of symptoms was required.
EVALUATION Family history reveals clues
All laboratory studies completed during Mr. J’s hospitalization are unremarkable, including complete blood count, basic metabolic panel, hepatic function panel, gamma-glutamyl transferase test, magnesium, phosphate, thyroid-stimulating hormone, vitamin B12, thiamine, folate, urinalysis, and urine drug screen. Mr. J does not undergo any head imaging.
Mr. J has not been in touch with his family since leaving his home approximately 3 months before he presented to the ED, and he gives consent for the inpatient team to attempt to contact them. One week into hospitalization, Mr. J’s sibling informs the team of a family history of genetically confirmed Huntington’s disease (HD), with psychiatric symptoms preceding the onset of motor symptoms in multiple first-degree relatives. His family says that before Mr. J first developed delusions 4 years ago, he had not exhibited any psychotic symptoms during periods of alcohol use or sobriety.
Mr. J does not demonstrate any overt movement symptoms on the unit and denies noting any rigidity, change in gait, or abnormal/uncontrolled movements. The inpatient psychiatric team consults neurology and a full neurologic evaluation is performed. The results are unremarkable outside of his psychiatric symptoms; specifically, Mr. J does not demonstrate even subtle motor signs or cognitive impairment. Given Mr. J’s family history, unremarkable lab findings, and age at presentation, the neurology team and inpatient psychiatry team suspect that his psychosis is likely an early presentation of HD.
[polldaddy:10279212]
The authors’ observations
Genetics of Huntington’s disease
Huntington’s disease is an autosomal dominant neurodegenerative disorder caused by expansion of cytosine-adenine-guanine (CAG) trinucleotide repeats within the Huntingtin (HTT) gene on chromosome 4, which codes for the huntingtin protein.2,3 While the function of “normal” huntingtin protein is not fully understood, it is known that CAG repeat expansion in the HTT gene of >35 repeats codes for a mutant huntingtin protein.2,3 The mutant huntingtin protein causes progressive neuronal loss in the basal ganglia and striatum, resulting in the clinical Huntington’s phenotype.3 Notably, the patient’s age at disease onset is inversely correlated with the number of repeats. For example, expansions of approximately 40 to 50 CAG repeats often result in adult-onset HD, while expansions of >60 repeats are typically associated with juvenile-onset HD (before age 20). CAG repeat lengths of approximately 36 to 39 demonstrate reduced penetrance, with some individuals developing symptomatic HD while others do not.2 Instability of the CAG repeat expansion can result in genetic “anticipation,” wherein repeat length increases between generations, causing earlier age of onset in affected offspring. Genetic anticipation in HD occurs more frequently in paternal transmission—approximately 80% to 90% of juvenile HD cases are inherited paternally, at times with the number of CAG repeats exceeding 200.3
Continue to: Psychiatric manifestations of Huntington's disease
Psychiatric manifestations of Huntington’s disease
Huntington’s disease is characterized by motor, cognitive, and behavioral disturbances (Table 22,4). Motor symptoms include a characteristic and well-recognized chorea, often predominating earlier in HD, that progresses to rigidity, spasticity, and bradykinesia later in the disease course.2 Cognitive impairments develop in a similar progressive manner and can often precede the onset of motor symptoms, beginning with early executive dysfunction. Thinking often becomes more rigid and less efficient, causing difficulty with multi-tasking and concentration, and often progressing to subcortical dementia.2
Psychiatric symptoms have long been recognized as a feature of HD; the estimated lifetime prevalence in patients with HD ranges from approximately 33% to 76%.4 Depressed mood, anxiety, irritability, and apathy are the most commonly reported symptoms, while a smaller percentage of patients with HD can experience obsessive-compulsive disorder (10% to 52%) or psychotic symptoms (3% to 11%).4 A more specific schizophrenia-like psychosis occurs in approximately 3% to 6% of patients, and often is a paranoid type.5,6 Positive psychotic symptoms, such as hallucinations and delusions, typically become less overt as HD progresses and cognitive impairments worsen.7
Although the onset of motor symptoms leads to diagnosis in the majority of patients with HD, many patients present with psychiatric symptoms—most commonly depression—prior to motor symptoms.8 An increasing body of literature details instances of psychosis preceding motor symptom onset by up to 10 years.6,9-12 In many of these cases, the patient has a family history of HD-associated psychosis. Family history is a major risk factor for HD-associated psychosis, as is early-onset HD.7,9
TREATMENT Antipsychotics result in some improvement
On Day 1 or 2, Mr. J is started on risperidone, 1 mg twice daily, to manage his symptoms. He shows incremental improvement in thought organization. Although his religious and grandiose delusions persist, they become less fixed, and he is able to take the team’s suggestion that he reconnect with his family.
Mr. J is aware of his family history of HD and acknowledges that multiple relatives had early psychiatric manifestations of HD. Despite this, he still has difficulty recognizing any connection between other family members’ presentation and his own. The psychiatry and neurology teams discuss the process, ethics, and implications of genetic testing for HD with Mr. J; however, he is ambivalent regarding genetic testing, and states he would consider it after discussing it with his family.
Continue to: The neurology team recommends...
The neurology team recommends against imaging for Mr. J because HD-related changes are not typically seen until later in the disease progression. On Day 9, they recommend changing from risperidone to quetiapine (50 mg every night at bedtime) due to evidence of its effectiveness specifically for treating behavioral symptoms of HD.13
While receiving quetiapine, Mr. J experiences significant drowsiness. Because he had experienced improvement in thought organization while he was receiving risperidone, he is switched back to risperidone.
[polldaddy:10279220]
The authors’ observations
Currently, no treatments are available to prevent the development or progression of HD. However, symptomatic treatment of motor and behavioral disturbances can lead to functional improvement and improved quality of life for individuals affected by HD.
There are no extensive clinical trials to date, but multiple case reports and studies have shown second-generation antipsychotics (SGAs), including quetiapine, olanzapine,
OUTCOME Discharge despite persistent delusions
Mr. J’s religious and grandiose delusions continue throughout hospitalization despite treatment with antipsychotics. However, because he remains calm and cooperative and demonstrates improvement in thought organization, he is deemed safe for discharge and instructed to continue risperidone. The team coordinates with Mr. J’s family to arrange transportation home and outpatient neurology follow-up.
Bottom Line
Psychiatric manifestations, including psychosis, are prominent symptoms of Huntington’s disease (HD) and may precede the onset of more readily recognized motor symptoms. This poses a diagnostic challenge, and clinicians should remain cognizant of this possibility, especially in patients with a family history of HD-associated psychosis.
Related Resources
- Huntington’s Disease Society of America. http://hdsa.org.
- National Institute of Neurological Disorders and Stroke. Huntington’s disease information page: What research is being done? https://www.ninds.nih.gov/Disorders/All-Disorders/Huntingtons-Disease-Information-Page.
- Scher LM. How to target psychiatric symptoms of Huntington’s disease. Current Psychiatry. 2012;11(9):34-39.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
CASE Disorganized thoughts and grandiose delusions
Mr. J, age 54, presents to the psychiatric emergency department (ED) with agitation and disruptive behavior. He claims that he is “the son of Jesus Christ” and has to travel to the Middle East to be baptized. Mr. J is irritable, shouting, and threatening staff members. He receives
The next day, he is calm and cooperative, but continues to express the same religious delusions. Mr. J is admitted to the psychiatric inpatient unit for further evaluation.
On the unit, Mr. J is pleasant and cooperative, but tangential in thought process. He reports he was “saved” by God 4 years ago, and that God communicates with him through music. Despite this, he denies having auditory or visual hallucinations.
Approximately 3 months earlier, Mr. J had stopped working and left his home and family in another state to pursue his “mission” of being baptized in the Middle East. Mr. J has been homeless since then. Despite that, he reports that his mood is “great” and denies any recent changes in mood, sleep, appetite, energy level, or psychomotor agitation. Although no formal cognitive testing is performed, Mr. J is alert and oriented to person, place, and time with intact remote and recent memory, no language deficits, and no lapses in concentration or attention throughout interview.
Mr. J says he has been drinking alcohol regularly throughout his adult life, often a few times per week, up to “a case and a half” of beer at times. He claims he’s had multiple periods of sobriety but denies having experienced withdrawal symptoms during those times. Mr. J reports 1 prior psychiatric hospitalization 25 years ago after attempting suicide by overdose following the loss of a loved one. At that time, he was diagnosed with posttraumatic stress disorder (PTSD). During this admission, he denies having any symptoms of PTSD or periods of mania or depression, and he has not undergone psychiatric treatment since he had been diagnosed with PTSD. He denies any family history of psychiatric illness as well as any medical comorbidities or medication use.
[polldaddy:10279202]
The authors’ observations
Mr. J’s presentation had a wide differential diagnosis (Table 1). The initial agitation Mr. J displayed in the psychiatric ED was likely secondary to acute alcohol intoxication, given that he was subsequently pleasant, calm, and cooperative after the alcohol was metabolized. Despite this, Mr. J continued to demonstrate delusions of a religious and somewhat grandiose nature with tangential thought processes, which made substance-induced psychosis less likely to be the sole diagnosis. Although it is possible to develop psychotic symptoms due to severe alcohol withdrawal (alcoholic hallucinosis), Mr. J’s vital signs remained stable, and he demonstrated no other signs or symptoms of withdrawal throughout his hospitalization. His presentation also did not fit that of delirium tremens because he was not confused or disoriented, and did not demonstrate perceptual disturbance.
While delusions were the most prominent feature of Mr. J’s apparent psychosis, the presence of disorganized thought processes and impaired functioning, as evidenced by Mr. J’s unemployment and recent homelessness, were more consistent with a primary psychotic disorder than a delusional disorder.1
Continue to: Mr. J began to exhibit...
Mr. J began to exhibit these psychotic symptoms in his early 50s; because the average age of onset of schizophrenia for males is approximately age 20 to 25, the likelihood of his presentation being the result of a primary psychotic disorder was low.1 Although less common, it was possible that Mr. J had developed late-onset schizophrenia, where the first episode typically occurs after approximately age 40 to 45. Mr. J also described that he was in a “great” mood but had grandiose delusions and had made recent impulsive decisions, which suggests there was a possible mood component to his presentation and a potential diagnosis of schizoaffective disorder or bipolar disorder with psychotic symptoms. However, before any of these diagnoses could be made, a medical or neurologic condition that could cause his symptoms needed to be investigated and ruled out. Further collateral information regarding Mr. J’s history and timeline of symptoms was required.
EVALUATION Family history reveals clues
All laboratory studies completed during Mr. J’s hospitalization are unremarkable, including complete blood count, basic metabolic panel, hepatic function panel, gamma-glutamyl transferase test, magnesium, phosphate, thyroid-stimulating hormone, vitamin B12, thiamine, folate, urinalysis, and urine drug screen. Mr. J does not undergo any head imaging.
Mr. J has not been in touch with his family since leaving his home approximately 3 months before he presented to the ED, and he gives consent for the inpatient team to attempt to contact them. One week into hospitalization, Mr. J’s sibling informs the team of a family history of genetically confirmed Huntington’s disease (HD), with psychiatric symptoms preceding the onset of motor symptoms in multiple first-degree relatives. His family says that before Mr. J first developed delusions 4 years ago, he had not exhibited any psychotic symptoms during periods of alcohol use or sobriety.
Mr. J does not demonstrate any overt movement symptoms on the unit and denies noting any rigidity, change in gait, or abnormal/uncontrolled movements. The inpatient psychiatric team consults neurology and a full neurologic evaluation is performed. The results are unremarkable outside of his psychiatric symptoms; specifically, Mr. J does not demonstrate even subtle motor signs or cognitive impairment. Given Mr. J’s family history, unremarkable lab findings, and age at presentation, the neurology team and inpatient psychiatry team suspect that his psychosis is likely an early presentation of HD.
[polldaddy:10279212]
The authors’ observations
Genetics of Huntington’s disease
Huntington’s disease is an autosomal dominant neurodegenerative disorder caused by expansion of cytosine-adenine-guanine (CAG) trinucleotide repeats within the Huntingtin (HTT) gene on chromosome 4, which codes for the huntingtin protein.2,3 While the function of “normal” huntingtin protein is not fully understood, it is known that CAG repeat expansion in the HTT gene of >35 repeats codes for a mutant huntingtin protein.2,3 The mutant huntingtin protein causes progressive neuronal loss in the basal ganglia and striatum, resulting in the clinical Huntington’s phenotype.3 Notably, the patient’s age at disease onset is inversely correlated with the number of repeats. For example, expansions of approximately 40 to 50 CAG repeats often result in adult-onset HD, while expansions of >60 repeats are typically associated with juvenile-onset HD (before age 20). CAG repeat lengths of approximately 36 to 39 demonstrate reduced penetrance, with some individuals developing symptomatic HD while others do not.2 Instability of the CAG repeat expansion can result in genetic “anticipation,” wherein repeat length increases between generations, causing earlier age of onset in affected offspring. Genetic anticipation in HD occurs more frequently in paternal transmission—approximately 80% to 90% of juvenile HD cases are inherited paternally, at times with the number of CAG repeats exceeding 200.3
Continue to: Psychiatric manifestations of Huntington's disease
Psychiatric manifestations of Huntington’s disease
Huntington’s disease is characterized by motor, cognitive, and behavioral disturbances (Table 22,4). Motor symptoms include a characteristic and well-recognized chorea, often predominating earlier in HD, that progresses to rigidity, spasticity, and bradykinesia later in the disease course.2 Cognitive impairments develop in a similar progressive manner and can often precede the onset of motor symptoms, beginning with early executive dysfunction. Thinking often becomes more rigid and less efficient, causing difficulty with multi-tasking and concentration, and often progressing to subcortical dementia.2
Psychiatric symptoms have long been recognized as a feature of HD; the estimated lifetime prevalence in patients with HD ranges from approximately 33% to 76%.4 Depressed mood, anxiety, irritability, and apathy are the most commonly reported symptoms, while a smaller percentage of patients with HD can experience obsessive-compulsive disorder (10% to 52%) or psychotic symptoms (3% to 11%).4 A more specific schizophrenia-like psychosis occurs in approximately 3% to 6% of patients, and often is a paranoid type.5,6 Positive psychotic symptoms, such as hallucinations and delusions, typically become less overt as HD progresses and cognitive impairments worsen.7
Although the onset of motor symptoms leads to diagnosis in the majority of patients with HD, many patients present with psychiatric symptoms—most commonly depression—prior to motor symptoms.8 An increasing body of literature details instances of psychosis preceding motor symptom onset by up to 10 years.6,9-12 In many of these cases, the patient has a family history of HD-associated psychosis. Family history is a major risk factor for HD-associated psychosis, as is early-onset HD.7,9
TREATMENT Antipsychotics result in some improvement
On Day 1 or 2, Mr. J is started on risperidone, 1 mg twice daily, to manage his symptoms. He shows incremental improvement in thought organization. Although his religious and grandiose delusions persist, they become less fixed, and he is able to take the team’s suggestion that he reconnect with his family.
Mr. J is aware of his family history of HD and acknowledges that multiple relatives had early psychiatric manifestations of HD. Despite this, he still has difficulty recognizing any connection between other family members’ presentation and his own. The psychiatry and neurology teams discuss the process, ethics, and implications of genetic testing for HD with Mr. J; however, he is ambivalent regarding genetic testing, and states he would consider it after discussing it with his family.
Continue to: The neurology team recommends...
The neurology team recommends against imaging for Mr. J because HD-related changes are not typically seen until later in the disease progression. On Day 9, they recommend changing from risperidone to quetiapine (50 mg every night at bedtime) due to evidence of its effectiveness specifically for treating behavioral symptoms of HD.13
While receiving quetiapine, Mr. J experiences significant drowsiness. Because he had experienced improvement in thought organization while he was receiving risperidone, he is switched back to risperidone.
[polldaddy:10279220]
The authors’ observations
Currently, no treatments are available to prevent the development or progression of HD. However, symptomatic treatment of motor and behavioral disturbances can lead to functional improvement and improved quality of life for individuals affected by HD.
There are no extensive clinical trials to date, but multiple case reports and studies have shown second-generation antipsychotics (SGAs), including quetiapine, olanzapine,
OUTCOME Discharge despite persistent delusions
Mr. J’s religious and grandiose delusions continue throughout hospitalization despite treatment with antipsychotics. However, because he remains calm and cooperative and demonstrates improvement in thought organization, he is deemed safe for discharge and instructed to continue risperidone. The team coordinates with Mr. J’s family to arrange transportation home and outpatient neurology follow-up.
Bottom Line
Psychiatric manifestations, including psychosis, are prominent symptoms of Huntington’s disease (HD) and may precede the onset of more readily recognized motor symptoms. This poses a diagnostic challenge, and clinicians should remain cognizant of this possibility, especially in patients with a family history of HD-associated psychosis.
Related Resources
- Huntington’s Disease Society of America. http://hdsa.org.
- National Institute of Neurological Disorders and Stroke. Huntington’s disease information page: What research is being done? https://www.ninds.nih.gov/Disorders/All-Disorders/Huntingtons-Disease-Information-Page.
- Scher LM. How to target psychiatric symptoms of Huntington’s disease. Current Psychiatry. 2012;11(9):34-39.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
2. Novak MJ, Tabrizi SJ. Huntington’s disease: clinical presentation and treatment. Int Rev Neurobiol. 2011;98:297-323.
3. Reiner A, Dragatsis I, Dietrich P. Genetics and neuropathology of Huntington’s disease. Int Rev Neurobiol. 2011;98:325-372.
4. van Duijn E, Kingma EM, Van der mast RC. Psychopathology in verified Huntington’s disease gene carriers. J Neuropsychiatry Clin Neurosci. 2007;19(4):441-448.
5. Naarding P, Kremer HP, Zitman FG. Huntington’s disease: a review of the literature on prevalence and treatment of neuropsychiatric phenomena. Eur Psychiatry. 2001;16(8):439-445.
6. Xu C, Yogaratnam J, Tan N, et al. Psychosis, treatment emergent extrapyramidal events, and subsequent onset of Huntington’s disease: a case report and review of the literature. Clin Psychopharmacol Neurosci. 2016;14(3):302-304.
7. Mendez MF. Huntington’s disease: update and review of neuropsychiatric aspects. Int J Psychiatry Med. 1994;24(3):189-208.
8. Di Maio L, Squitieri F, Napolitano G, et al. Onset symptoms in 510 patients with Huntington’s disease. J Med Genet. 1993;30(4):289-292.
9. Jauhar S, Ritchie S. Psychiatric and behavioural manifestations of Huntington’s disease. Adv Psychiatr Treat. 2010;16(3):168-175.
10. Nagel M, Rumpf HJ, Kasten M. Acute psychosis in a verified Huntington disease gene carrier with subtle motor signs: psychiatric criteria should be considered for the diagnosis. Gen Hosp Psychiatry. 2014;36(3):361.e3-e4. doi: 10.1016/j.genhosppsych.2014.01.008.
11. Corrêa BB, Xavier M, Guimarães J. Association of Huntington’s disease and schizophrenia-like psychosis in a Huntington’s disease pedigree. Clin Pract Epidemiol Ment Health. 2006;2:1.
12. Ding J, Gadit AM. Psychosis with Huntington’s disease: role of antipsychotic medications. BMJ Case Rep. 2014: bcr2013202625. doi: 10.1136/bcr-2013-202625.
13. Alpay M, Koroshetz WJ. Quetiapine in the treatment of behavioral disturbances in patients with Huntington’s disease. Psychosomatics. 2006;47(1):70-72.
14. Duff K, Beglinger LJ, O’Rourke ME, et al. Risperidone and the treatment of psychiatric, motor, and cognitive symptoms in Huntington’s disease. Ann Clin Psychiatry. 2008;20(1):1-3.
15. Paleacu D, Anca M, Giladi N. Olanzapine in Huntington’s disease. Acta Neurol Scand. 2002;105(6):441-444.
16. Lin W, Chou Y. Aripiprazole effects on psychosis and chorea in a patient with Huntington’s disease. Am J Psychiatry. 2008;165(9):1207-1208.
17. van Vugt JP, Siesling S, Vergeer M, et al. Clozapine versus placebo in Huntington’s disease: a double blind randomized comparative study. J Neurol Neurosurg Psychiatr. 1997;63(1):35-39.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
2. Novak MJ, Tabrizi SJ. Huntington’s disease: clinical presentation and treatment. Int Rev Neurobiol. 2011;98:297-323.
3. Reiner A, Dragatsis I, Dietrich P. Genetics and neuropathology of Huntington’s disease. Int Rev Neurobiol. 2011;98:325-372.
4. van Duijn E, Kingma EM, Van der mast RC. Psychopathology in verified Huntington’s disease gene carriers. J Neuropsychiatry Clin Neurosci. 2007;19(4):441-448.
5. Naarding P, Kremer HP, Zitman FG. Huntington’s disease: a review of the literature on prevalence and treatment of neuropsychiatric phenomena. Eur Psychiatry. 2001;16(8):439-445.
6. Xu C, Yogaratnam J, Tan N, et al. Psychosis, treatment emergent extrapyramidal events, and subsequent onset of Huntington’s disease: a case report and review of the literature. Clin Psychopharmacol Neurosci. 2016;14(3):302-304.
7. Mendez MF. Huntington’s disease: update and review of neuropsychiatric aspects. Int J Psychiatry Med. 1994;24(3):189-208.
8. Di Maio L, Squitieri F, Napolitano G, et al. Onset symptoms in 510 patients with Huntington’s disease. J Med Genet. 1993;30(4):289-292.
9. Jauhar S, Ritchie S. Psychiatric and behavioural manifestations of Huntington’s disease. Adv Psychiatr Treat. 2010;16(3):168-175.
10. Nagel M, Rumpf HJ, Kasten M. Acute psychosis in a verified Huntington disease gene carrier with subtle motor signs: psychiatric criteria should be considered for the diagnosis. Gen Hosp Psychiatry. 2014;36(3):361.e3-e4. doi: 10.1016/j.genhosppsych.2014.01.008.
11. Corrêa BB, Xavier M, Guimarães J. Association of Huntington’s disease and schizophrenia-like psychosis in a Huntington’s disease pedigree. Clin Pract Epidemiol Ment Health. 2006;2:1.
12. Ding J, Gadit AM. Psychosis with Huntington’s disease: role of antipsychotic medications. BMJ Case Rep. 2014: bcr2013202625. doi: 10.1136/bcr-2013-202625.
13. Alpay M, Koroshetz WJ. Quetiapine in the treatment of behavioral disturbances in patients with Huntington’s disease. Psychosomatics. 2006;47(1):70-72.
14. Duff K, Beglinger LJ, O’Rourke ME, et al. Risperidone and the treatment of psychiatric, motor, and cognitive symptoms in Huntington’s disease. Ann Clin Psychiatry. 2008;20(1):1-3.
15. Paleacu D, Anca M, Giladi N. Olanzapine in Huntington’s disease. Acta Neurol Scand. 2002;105(6):441-444.
16. Lin W, Chou Y. Aripiprazole effects on psychosis and chorea in a patient with Huntington’s disease. Am J Psychiatry. 2008;165(9):1207-1208.
17. van Vugt JP, Siesling S, Vergeer M, et al. Clozapine versus placebo in Huntington’s disease: a double blind randomized comparative study. J Neurol Neurosurg Psychiatr. 1997;63(1):35-39.
Pharmacogenomics testing: What the FDA says
Mr. R, age 30, is referred to you by his primary care physician, who diagnosed him with depression approximately 2 years ago. When he was first diagnosed, Mr. R was prescribed
Mr. R says that based on his primary care physician’s recommendation, he had undergone pharmacogenomics testing to help guide therapy. He presents the results to you, and you notice that he has the cytochrome P450 (CYP) 2C19 *2/*3 genotype and a CYP2D6*4/*5 genotype. Both are associated with a poor metabolism phenotype. Should you use these findings to determine which medication Mr. R should be treated with next?
While the field of pharmacogenomics is not new, within the last few years this science has begun to transition into clinical practice. A recent meta-analysis found support for using pharmacogenomics testing results in clinical practice.1 This study included more than 1,700 patients who took part in 5 controlled trials that randomized participants to either pharmacogenetics-guided or unguided (ie, standard) treatment. Each participant was assessed using the Hamilton Depression Rating Scale-17 (HDRS-17) a minimum of 3 times over a minimum of 8 weeks.1 While the exact inclusion and exclusion criteria for each trial differed, they all defined remission of depression as achieving an HDRS-17 score ≤7. Overall, the authors concluded that based on the random-effects pooled risk ratio, there was a significant association between pharmacogenetics-guided prescribing and remission (relative risk = 1.71, 95% confidence interval [CI], 1.17 to 2.48; P = .005). The results of this meta-analysis are controversial, however, because all 5 studies were industry-funded, and interpretation of the testing results was based on proprietary algorithms.
Experts in the field and professional societies, such as the International Society of Psychiatric Genetics (ISPG), have issued policy statements on genetic testing within psychiatry.2,3 While the ISPG did not necessarily endorse use of pharmacogenomics in practice, they recommended that clinicians follow good medical practice and stay current on changes to drug labeling and adverse event reports.3 The ISPG also noted that useful but not exhaustive lists of pharmacogenetic tests are maintained by the Clinical Pharmacogenetics Implementation Consortium (CPIC) and the US FDA.3
Laboratory developed vs direct-to-consumer tests
In a previous Savvy Psychopharmacology article,4 we had discussed the role of CPIC, but not the role of the FDA. This issue is key because there is a lack of clarity regarding pharmacogenomics tests and whether they are considered Class II devices by the FDA, which would require their review and approval. Until recently, the FDA was fairly quiet regarding pharmacogenomics tests because most of these tests were considered laboratory developed tests, which were regulated under the Clinic Laboratory Improvements Amendments program. The critical distinction of a laboratory developed test is that it is developed and performed in a single laboratory and is offered to patients only when prescribed by a clinician. Due to this distinction, laboratory developed pharmacogenomics tests did not need FDA 510(k) clearance, which is a premarket submission common for medical devices.
Direct-to-consumer pharmacogenomics tests are different in that the FDA has classified these platforms as medical devices; however, they are reviewed by the FDA only if they are being used for moderate- to high-risk medical purposes, or if the results of the testing may have a higher impact on medical care. As part of its review, the FDA examines test accuracy and reliably measures to determine if the measurement is predictive of a certain state of health and supported by what the company claims about the test and how well it works. Additionally, the FDA examines the company-provided descriptive information to ensure that consumers can easily understand it without the help of a clinician.5
Conflicting FDA statements
Recently the FDA issued 2 statements—one a policy statement and the other a safety communication—about laboratory developed tests and direct-to-consumer tests. The statements appear to contradict themselves, despite focusing on using pharmacogenomics testing in practice.
Continue to: The FDA's first statement
The FDA’s first statement. On October 31, 2018, the FDA released a policy statement that they had “permitted marketing, with special controls,” of the Personal Genome Service Pharmacogenetic Reports test through 23andMe (a direct-to-consumer genetic testing company) for 33 different variants within specific pharmacogenomic genes (CYP2C19, CYP2C9, CYP3A5, UGT1A1, DPYD, TPMT, SLC01B1, and CYP2D6) that may impact drug metabolism or response.6 As part of its review of this Personal Genome Service Pharmacogenetic Reports test, the FDA found that the company-provided data showed that the test is accurate and can correctly identify the 33 specific genetic variants. The FDA review also showed that the testing results were reproducible, and the test instructions and reports could be understood by consumers.
While the specific reports related to this testing are not yet available within 23andMe, this approval allows for greater oversight by the FDA with regard to the pharmacogenomics information provided through this company’s Personal Genome Service Pharmacogenetic Reports test. The FDA noted that this approval was only for adults age >185 and that consumers “should not use the test results to stop or change any medication.”6 Further, the FDA stated that the results of the direct-to-consumer test should be confirmed with independent pharmacogenomics testing before making any medical decision. Unfortunately, the FDA did not offer guidance on what would be an appropriate independent pharmacogenomics test, but it did provide a link to a list of FDA-approved nucleic acid–based tests, on which 23andMe’s Personal Genome Service Pharmacogenetic Reports test is included.7
The FDA’s second statement. On November 1, 2018, the FDA issued a separate safety communication that cautioned clinicians and patients that most of the current commercially available testing platforms for pharmacogenomics have not been FDA-reviewed, meaning that they may lack clinical evidence supporting their use.8 Further, the FDA safety communication stated, “Changing drug treatment based on the results from such a genetic test could lead to inappropriate treatment decisions and potentially serious health consequences for the patient.”8
Taken together, these FDA statements appear to support pharmacogenomics testing with approval of the 23andMe’s Personal Genome Service Pharmacogenetic Reports test but warn that the testing results should not be used to make treatment decisions, and that they should be verified. However, the FDA does not offer any guidance on what an appropriate testing platform would be
What the FDA advises
The FDA has provided some guidance to clinicians and patients regarding next steps for patients who are interested in having pharmacogenomics testing or who have already undergone testing. The FDA’s first point is that both clinicians and patients need to be aware that pharmacogenomics testing is not FDA-reviewed, that patients should discuss the results of their testing with their clinicians, and that they should not stop their medication based on the results of the testing. Additionally, the FDA recommends that clinicians and patients should be aware that any claims made by the testing companies regarding the specific effect of a medication may not be supported by evidence. Furthermore, the FDA strongly recommends that clinicians consult the FDA-approved drug label, or the label of the FDA-cleared or FDA-approved genetic test, for information regarding how genetic information should be used in making treatment decisions. The FDA recommends reviewing the Warning section, as well as the Indications and Usage, Dosage and Administration, or Use in Specific Populations sections of the FDA-approved drug labeling.
Continue to: Unfortunately, this information...
Unfortunately, this information might be difficult to locate due to the lack of consistency regarding where it is placed in the FDA-approved drug labeling. The Pharmacogenomics Knowledgebase (https://www.pharmgkb.org/) can help clinicians quickly identify information regarding medications, their metabolic pathways, CPIC dosing guidelines, and the FDA-approved drug labeling information.9 By searching for specific medications within the Pharmacogenomic Knowledge Base, information regarding the FDA-approved drug labeling can be easily found, which is important because currently >120 medications contain pharmacogenomics information in their FDA-approved drug labeling.10
CASE CONTINUED
Overall Mr. R’s pharmacogenomics testing results indicate that he has 2 genotypes that are associated with poor metabolism phenotypes and could result in reduced metabolism of medications that are metabolized by these CYP enzymes, leading to higher blood levels and an increased risk of adverse effects. The Table11 lists pharmacogenomics information from the FDA-approved drug labeling and from the Pharmacogenomics Knowledgebase for both the medications Mr. R has previously been prescribed and for several potential medications to consider.
It would be prudent to first discuss with Mr. R the FDA’s recent policy statement and safety communication. While you could recommend that he pursue additional pharmacogenomics testing, it is unclear which specific laboratory is available to conduct this confirmatory analysis.
Because Mr. R has had unsuccessful trials of several medications that primarily fall in the selective serotonin reuptake inhibitors class, it might be time to consider a medication from a different class. A quick review of the FDA-approved drug labeling for
Related Resources
- Gammal RS, Gardner KN, Burghardt KJ. Where to find guidance on using pharmacogenomics in psychiatric practice. Current Psychiatry. 2016;15(9):93-94.
- Clinical Pharmacogenomics Implementation Consortium. What is CPIC? https://www.pharmgkb.org/page/cpic.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Citalopram • Celexa
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
1. Bousman CA, Arandjelovic K, Mancuso SG, et al. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenomics. 2019;20(1):37-47.
2. Zubenko GS, Sommer BR, Cohen BM. Pharmacogenetics in psychiatry: a companion, rather than competitor, to protocol-based care-reply. JAMA Psychiatry. 2018;75(10):1090-1091.
3. International Society for Psychiatric Genetics. Genetic testing statement: genetic testing and psychiatric disorders: a statement from the International Society of Psychiatric Genetics. https://ispg.net/genetic-testing-statement/. Revised January 26, 2017. Accessed January 1, 2019.
4. Ellingrod VL, Ward KM. Using pharmacogenetics guidelines when prescribing: what’s available. Current Psychiatry. 2018;17(1):43-46.
5. U.S. Food and Drug Administration. Medical devices: direct-to-consumer tests. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm624726.htm. Published November 1, 2018. Accessed January 1, 2019.
6. U.S. Food and Drug Administration. FDA news releases: FDA authorizes first direct-to consumer test for detecting variants that may be associated with medication metabolism. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm624753.htm. Published October 31, 2018. Accessed January 1, 2019.
7. U.S. Food and Drug Administration. Medical devices: nucleic acid based tests. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm330711.htm. Published February 5, 2019. Accessed March 1, 2019.
8. U.S. Food and Drug Administration. Medical devices. The FDA warns against the use of many genetic tests with unapproved claims to predict patient response to specific medications: FDA Safety Communications. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm624725.htm. Published November 1, 2018. Accessed January 1, 2019.
9. Whirl-Carrillo EM, McDonagh JM, Hebert L, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92(4):414-417.
10. U.S. Food and Drug Administration. Drugs. Table of pharmacogenomic biomarkers in drug labeling. https://www.fda.gov/Drugs/ScienceResearch/ucm572698.htm. Published August 3, 2018. Accessed January 1, 2019.
11. U.S. Food and Drug Administration. Drugs@FDA: FDA approved drug products. https://www.accessdata.fda.gov/scripts/cder/daf. Accessed March 4, 2019.
Mr. R, age 30, is referred to you by his primary care physician, who diagnosed him with depression approximately 2 years ago. When he was first diagnosed, Mr. R was prescribed
Mr. R says that based on his primary care physician’s recommendation, he had undergone pharmacogenomics testing to help guide therapy. He presents the results to you, and you notice that he has the cytochrome P450 (CYP) 2C19 *2/*3 genotype and a CYP2D6*4/*5 genotype. Both are associated with a poor metabolism phenotype. Should you use these findings to determine which medication Mr. R should be treated with next?
While the field of pharmacogenomics is not new, within the last few years this science has begun to transition into clinical practice. A recent meta-analysis found support for using pharmacogenomics testing results in clinical practice.1 This study included more than 1,700 patients who took part in 5 controlled trials that randomized participants to either pharmacogenetics-guided or unguided (ie, standard) treatment. Each participant was assessed using the Hamilton Depression Rating Scale-17 (HDRS-17) a minimum of 3 times over a minimum of 8 weeks.1 While the exact inclusion and exclusion criteria for each trial differed, they all defined remission of depression as achieving an HDRS-17 score ≤7. Overall, the authors concluded that based on the random-effects pooled risk ratio, there was a significant association between pharmacogenetics-guided prescribing and remission (relative risk = 1.71, 95% confidence interval [CI], 1.17 to 2.48; P = .005). The results of this meta-analysis are controversial, however, because all 5 studies were industry-funded, and interpretation of the testing results was based on proprietary algorithms.
Experts in the field and professional societies, such as the International Society of Psychiatric Genetics (ISPG), have issued policy statements on genetic testing within psychiatry.2,3 While the ISPG did not necessarily endorse use of pharmacogenomics in practice, they recommended that clinicians follow good medical practice and stay current on changes to drug labeling and adverse event reports.3 The ISPG also noted that useful but not exhaustive lists of pharmacogenetic tests are maintained by the Clinical Pharmacogenetics Implementation Consortium (CPIC) and the US FDA.3
Laboratory developed vs direct-to-consumer tests
In a previous Savvy Psychopharmacology article,4 we had discussed the role of CPIC, but not the role of the FDA. This issue is key because there is a lack of clarity regarding pharmacogenomics tests and whether they are considered Class II devices by the FDA, which would require their review and approval. Until recently, the FDA was fairly quiet regarding pharmacogenomics tests because most of these tests were considered laboratory developed tests, which were regulated under the Clinic Laboratory Improvements Amendments program. The critical distinction of a laboratory developed test is that it is developed and performed in a single laboratory and is offered to patients only when prescribed by a clinician. Due to this distinction, laboratory developed pharmacogenomics tests did not need FDA 510(k) clearance, which is a premarket submission common for medical devices.
Direct-to-consumer pharmacogenomics tests are different in that the FDA has classified these platforms as medical devices; however, they are reviewed by the FDA only if they are being used for moderate- to high-risk medical purposes, or if the results of the testing may have a higher impact on medical care. As part of its review, the FDA examines test accuracy and reliably measures to determine if the measurement is predictive of a certain state of health and supported by what the company claims about the test and how well it works. Additionally, the FDA examines the company-provided descriptive information to ensure that consumers can easily understand it without the help of a clinician.5
Conflicting FDA statements
Recently the FDA issued 2 statements—one a policy statement and the other a safety communication—about laboratory developed tests and direct-to-consumer tests. The statements appear to contradict themselves, despite focusing on using pharmacogenomics testing in practice.
Continue to: The FDA's first statement
The FDA’s first statement. On October 31, 2018, the FDA released a policy statement that they had “permitted marketing, with special controls,” of the Personal Genome Service Pharmacogenetic Reports test through 23andMe (a direct-to-consumer genetic testing company) for 33 different variants within specific pharmacogenomic genes (CYP2C19, CYP2C9, CYP3A5, UGT1A1, DPYD, TPMT, SLC01B1, and CYP2D6) that may impact drug metabolism or response.6 As part of its review of this Personal Genome Service Pharmacogenetic Reports test, the FDA found that the company-provided data showed that the test is accurate and can correctly identify the 33 specific genetic variants. The FDA review also showed that the testing results were reproducible, and the test instructions and reports could be understood by consumers.
While the specific reports related to this testing are not yet available within 23andMe, this approval allows for greater oversight by the FDA with regard to the pharmacogenomics information provided through this company’s Personal Genome Service Pharmacogenetic Reports test. The FDA noted that this approval was only for adults age >185 and that consumers “should not use the test results to stop or change any medication.”6 Further, the FDA stated that the results of the direct-to-consumer test should be confirmed with independent pharmacogenomics testing before making any medical decision. Unfortunately, the FDA did not offer guidance on what would be an appropriate independent pharmacogenomics test, but it did provide a link to a list of FDA-approved nucleic acid–based tests, on which 23andMe’s Personal Genome Service Pharmacogenetic Reports test is included.7
The FDA’s second statement. On November 1, 2018, the FDA issued a separate safety communication that cautioned clinicians and patients that most of the current commercially available testing platforms for pharmacogenomics have not been FDA-reviewed, meaning that they may lack clinical evidence supporting their use.8 Further, the FDA safety communication stated, “Changing drug treatment based on the results from such a genetic test could lead to inappropriate treatment decisions and potentially serious health consequences for the patient.”8
Taken together, these FDA statements appear to support pharmacogenomics testing with approval of the 23andMe’s Personal Genome Service Pharmacogenetic Reports test but warn that the testing results should not be used to make treatment decisions, and that they should be verified. However, the FDA does not offer any guidance on what an appropriate testing platform would be
What the FDA advises
The FDA has provided some guidance to clinicians and patients regarding next steps for patients who are interested in having pharmacogenomics testing or who have already undergone testing. The FDA’s first point is that both clinicians and patients need to be aware that pharmacogenomics testing is not FDA-reviewed, that patients should discuss the results of their testing with their clinicians, and that they should not stop their medication based on the results of the testing. Additionally, the FDA recommends that clinicians and patients should be aware that any claims made by the testing companies regarding the specific effect of a medication may not be supported by evidence. Furthermore, the FDA strongly recommends that clinicians consult the FDA-approved drug label, or the label of the FDA-cleared or FDA-approved genetic test, for information regarding how genetic information should be used in making treatment decisions. The FDA recommends reviewing the Warning section, as well as the Indications and Usage, Dosage and Administration, or Use in Specific Populations sections of the FDA-approved drug labeling.
Continue to: Unfortunately, this information...
Unfortunately, this information might be difficult to locate due to the lack of consistency regarding where it is placed in the FDA-approved drug labeling. The Pharmacogenomics Knowledgebase (https://www.pharmgkb.org/) can help clinicians quickly identify information regarding medications, their metabolic pathways, CPIC dosing guidelines, and the FDA-approved drug labeling information.9 By searching for specific medications within the Pharmacogenomic Knowledge Base, information regarding the FDA-approved drug labeling can be easily found, which is important because currently >120 medications contain pharmacogenomics information in their FDA-approved drug labeling.10
CASE CONTINUED
Overall Mr. R’s pharmacogenomics testing results indicate that he has 2 genotypes that are associated with poor metabolism phenotypes and could result in reduced metabolism of medications that are metabolized by these CYP enzymes, leading to higher blood levels and an increased risk of adverse effects. The Table11 lists pharmacogenomics information from the FDA-approved drug labeling and from the Pharmacogenomics Knowledgebase for both the medications Mr. R has previously been prescribed and for several potential medications to consider.
It would be prudent to first discuss with Mr. R the FDA’s recent policy statement and safety communication. While you could recommend that he pursue additional pharmacogenomics testing, it is unclear which specific laboratory is available to conduct this confirmatory analysis.
Because Mr. R has had unsuccessful trials of several medications that primarily fall in the selective serotonin reuptake inhibitors class, it might be time to consider a medication from a different class. A quick review of the FDA-approved drug labeling for
Related Resources
- Gammal RS, Gardner KN, Burghardt KJ. Where to find guidance on using pharmacogenomics in psychiatric practice. Current Psychiatry. 2016;15(9):93-94.
- Clinical Pharmacogenomics Implementation Consortium. What is CPIC? https://www.pharmgkb.org/page/cpic.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Citalopram • Celexa
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
Mr. R, age 30, is referred to you by his primary care physician, who diagnosed him with depression approximately 2 years ago. When he was first diagnosed, Mr. R was prescribed
Mr. R says that based on his primary care physician’s recommendation, he had undergone pharmacogenomics testing to help guide therapy. He presents the results to you, and you notice that he has the cytochrome P450 (CYP) 2C19 *2/*3 genotype and a CYP2D6*4/*5 genotype. Both are associated with a poor metabolism phenotype. Should you use these findings to determine which medication Mr. R should be treated with next?
While the field of pharmacogenomics is not new, within the last few years this science has begun to transition into clinical practice. A recent meta-analysis found support for using pharmacogenomics testing results in clinical practice.1 This study included more than 1,700 patients who took part in 5 controlled trials that randomized participants to either pharmacogenetics-guided or unguided (ie, standard) treatment. Each participant was assessed using the Hamilton Depression Rating Scale-17 (HDRS-17) a minimum of 3 times over a minimum of 8 weeks.1 While the exact inclusion and exclusion criteria for each trial differed, they all defined remission of depression as achieving an HDRS-17 score ≤7. Overall, the authors concluded that based on the random-effects pooled risk ratio, there was a significant association between pharmacogenetics-guided prescribing and remission (relative risk = 1.71, 95% confidence interval [CI], 1.17 to 2.48; P = .005). The results of this meta-analysis are controversial, however, because all 5 studies were industry-funded, and interpretation of the testing results was based on proprietary algorithms.
Experts in the field and professional societies, such as the International Society of Psychiatric Genetics (ISPG), have issued policy statements on genetic testing within psychiatry.2,3 While the ISPG did not necessarily endorse use of pharmacogenomics in practice, they recommended that clinicians follow good medical practice and stay current on changes to drug labeling and adverse event reports.3 The ISPG also noted that useful but not exhaustive lists of pharmacogenetic tests are maintained by the Clinical Pharmacogenetics Implementation Consortium (CPIC) and the US FDA.3
Laboratory developed vs direct-to-consumer tests
In a previous Savvy Psychopharmacology article,4 we had discussed the role of CPIC, but not the role of the FDA. This issue is key because there is a lack of clarity regarding pharmacogenomics tests and whether they are considered Class II devices by the FDA, which would require their review and approval. Until recently, the FDA was fairly quiet regarding pharmacogenomics tests because most of these tests were considered laboratory developed tests, which were regulated under the Clinic Laboratory Improvements Amendments program. The critical distinction of a laboratory developed test is that it is developed and performed in a single laboratory and is offered to patients only when prescribed by a clinician. Due to this distinction, laboratory developed pharmacogenomics tests did not need FDA 510(k) clearance, which is a premarket submission common for medical devices.
Direct-to-consumer pharmacogenomics tests are different in that the FDA has classified these platforms as medical devices; however, they are reviewed by the FDA only if they are being used for moderate- to high-risk medical purposes, or if the results of the testing may have a higher impact on medical care. As part of its review, the FDA examines test accuracy and reliably measures to determine if the measurement is predictive of a certain state of health and supported by what the company claims about the test and how well it works. Additionally, the FDA examines the company-provided descriptive information to ensure that consumers can easily understand it without the help of a clinician.5
Conflicting FDA statements
Recently the FDA issued 2 statements—one a policy statement and the other a safety communication—about laboratory developed tests and direct-to-consumer tests. The statements appear to contradict themselves, despite focusing on using pharmacogenomics testing in practice.
Continue to: The FDA's first statement
The FDA’s first statement. On October 31, 2018, the FDA released a policy statement that they had “permitted marketing, with special controls,” of the Personal Genome Service Pharmacogenetic Reports test through 23andMe (a direct-to-consumer genetic testing company) for 33 different variants within specific pharmacogenomic genes (CYP2C19, CYP2C9, CYP3A5, UGT1A1, DPYD, TPMT, SLC01B1, and CYP2D6) that may impact drug metabolism or response.6 As part of its review of this Personal Genome Service Pharmacogenetic Reports test, the FDA found that the company-provided data showed that the test is accurate and can correctly identify the 33 specific genetic variants. The FDA review also showed that the testing results were reproducible, and the test instructions and reports could be understood by consumers.
While the specific reports related to this testing are not yet available within 23andMe, this approval allows for greater oversight by the FDA with regard to the pharmacogenomics information provided through this company’s Personal Genome Service Pharmacogenetic Reports test. The FDA noted that this approval was only for adults age >185 and that consumers “should not use the test results to stop or change any medication.”6 Further, the FDA stated that the results of the direct-to-consumer test should be confirmed with independent pharmacogenomics testing before making any medical decision. Unfortunately, the FDA did not offer guidance on what would be an appropriate independent pharmacogenomics test, but it did provide a link to a list of FDA-approved nucleic acid–based tests, on which 23andMe’s Personal Genome Service Pharmacogenetic Reports test is included.7
The FDA’s second statement. On November 1, 2018, the FDA issued a separate safety communication that cautioned clinicians and patients that most of the current commercially available testing platforms for pharmacogenomics have not been FDA-reviewed, meaning that they may lack clinical evidence supporting their use.8 Further, the FDA safety communication stated, “Changing drug treatment based on the results from such a genetic test could lead to inappropriate treatment decisions and potentially serious health consequences for the patient.”8
Taken together, these FDA statements appear to support pharmacogenomics testing with approval of the 23andMe’s Personal Genome Service Pharmacogenetic Reports test but warn that the testing results should not be used to make treatment decisions, and that they should be verified. However, the FDA does not offer any guidance on what an appropriate testing platform would be
What the FDA advises
The FDA has provided some guidance to clinicians and patients regarding next steps for patients who are interested in having pharmacogenomics testing or who have already undergone testing. The FDA’s first point is that both clinicians and patients need to be aware that pharmacogenomics testing is not FDA-reviewed, that patients should discuss the results of their testing with their clinicians, and that they should not stop their medication based on the results of the testing. Additionally, the FDA recommends that clinicians and patients should be aware that any claims made by the testing companies regarding the specific effect of a medication may not be supported by evidence. Furthermore, the FDA strongly recommends that clinicians consult the FDA-approved drug label, or the label of the FDA-cleared or FDA-approved genetic test, for information regarding how genetic information should be used in making treatment decisions. The FDA recommends reviewing the Warning section, as well as the Indications and Usage, Dosage and Administration, or Use in Specific Populations sections of the FDA-approved drug labeling.
Continue to: Unfortunately, this information...
Unfortunately, this information might be difficult to locate due to the lack of consistency regarding where it is placed in the FDA-approved drug labeling. The Pharmacogenomics Knowledgebase (https://www.pharmgkb.org/) can help clinicians quickly identify information regarding medications, their metabolic pathways, CPIC dosing guidelines, and the FDA-approved drug labeling information.9 By searching for specific medications within the Pharmacogenomic Knowledge Base, information regarding the FDA-approved drug labeling can be easily found, which is important because currently >120 medications contain pharmacogenomics information in their FDA-approved drug labeling.10
CASE CONTINUED
Overall Mr. R’s pharmacogenomics testing results indicate that he has 2 genotypes that are associated with poor metabolism phenotypes and could result in reduced metabolism of medications that are metabolized by these CYP enzymes, leading to higher blood levels and an increased risk of adverse effects. The Table11 lists pharmacogenomics information from the FDA-approved drug labeling and from the Pharmacogenomics Knowledgebase for both the medications Mr. R has previously been prescribed and for several potential medications to consider.
It would be prudent to first discuss with Mr. R the FDA’s recent policy statement and safety communication. While you could recommend that he pursue additional pharmacogenomics testing, it is unclear which specific laboratory is available to conduct this confirmatory analysis.
Because Mr. R has had unsuccessful trials of several medications that primarily fall in the selective serotonin reuptake inhibitors class, it might be time to consider a medication from a different class. A quick review of the FDA-approved drug labeling for
Related Resources
- Gammal RS, Gardner KN, Burghardt KJ. Where to find guidance on using pharmacogenomics in psychiatric practice. Current Psychiatry. 2016;15(9):93-94.
- Clinical Pharmacogenomics Implementation Consortium. What is CPIC? https://www.pharmgkb.org/page/cpic.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Citalopram • Celexa
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
1. Bousman CA, Arandjelovic K, Mancuso SG, et al. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenomics. 2019;20(1):37-47.
2. Zubenko GS, Sommer BR, Cohen BM. Pharmacogenetics in psychiatry: a companion, rather than competitor, to protocol-based care-reply. JAMA Psychiatry. 2018;75(10):1090-1091.
3. International Society for Psychiatric Genetics. Genetic testing statement: genetic testing and psychiatric disorders: a statement from the International Society of Psychiatric Genetics. https://ispg.net/genetic-testing-statement/. Revised January 26, 2017. Accessed January 1, 2019.
4. Ellingrod VL, Ward KM. Using pharmacogenetics guidelines when prescribing: what’s available. Current Psychiatry. 2018;17(1):43-46.
5. U.S. Food and Drug Administration. Medical devices: direct-to-consumer tests. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm624726.htm. Published November 1, 2018. Accessed January 1, 2019.
6. U.S. Food and Drug Administration. FDA news releases: FDA authorizes first direct-to consumer test for detecting variants that may be associated with medication metabolism. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm624753.htm. Published October 31, 2018. Accessed January 1, 2019.
7. U.S. Food and Drug Administration. Medical devices: nucleic acid based tests. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm330711.htm. Published February 5, 2019. Accessed March 1, 2019.
8. U.S. Food and Drug Administration. Medical devices. The FDA warns against the use of many genetic tests with unapproved claims to predict patient response to specific medications: FDA Safety Communications. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm624725.htm. Published November 1, 2018. Accessed January 1, 2019.
9. Whirl-Carrillo EM, McDonagh JM, Hebert L, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92(4):414-417.
10. U.S. Food and Drug Administration. Drugs. Table of pharmacogenomic biomarkers in drug labeling. https://www.fda.gov/Drugs/ScienceResearch/ucm572698.htm. Published August 3, 2018. Accessed January 1, 2019.
11. U.S. Food and Drug Administration. Drugs@FDA: FDA approved drug products. https://www.accessdata.fda.gov/scripts/cder/daf. Accessed March 4, 2019.
1. Bousman CA, Arandjelovic K, Mancuso SG, et al. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenomics. 2019;20(1):37-47.
2. Zubenko GS, Sommer BR, Cohen BM. Pharmacogenetics in psychiatry: a companion, rather than competitor, to protocol-based care-reply. JAMA Psychiatry. 2018;75(10):1090-1091.
3. International Society for Psychiatric Genetics. Genetic testing statement: genetic testing and psychiatric disorders: a statement from the International Society of Psychiatric Genetics. https://ispg.net/genetic-testing-statement/. Revised January 26, 2017. Accessed January 1, 2019.
4. Ellingrod VL, Ward KM. Using pharmacogenetics guidelines when prescribing: what’s available. Current Psychiatry. 2018;17(1):43-46.
5. U.S. Food and Drug Administration. Medical devices: direct-to-consumer tests. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm624726.htm. Published November 1, 2018. Accessed January 1, 2019.
6. U.S. Food and Drug Administration. FDA news releases: FDA authorizes first direct-to consumer test for detecting variants that may be associated with medication metabolism. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm624753.htm. Published October 31, 2018. Accessed January 1, 2019.
7. U.S. Food and Drug Administration. Medical devices: nucleic acid based tests. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm330711.htm. Published February 5, 2019. Accessed March 1, 2019.
8. U.S. Food and Drug Administration. Medical devices. The FDA warns against the use of many genetic tests with unapproved claims to predict patient response to specific medications: FDA Safety Communications. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm624725.htm. Published November 1, 2018. Accessed January 1, 2019.
9. Whirl-Carrillo EM, McDonagh JM, Hebert L, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92(4):414-417.
10. U.S. Food and Drug Administration. Drugs. Table of pharmacogenomic biomarkers in drug labeling. https://www.fda.gov/Drugs/ScienceResearch/ucm572698.htm. Published August 3, 2018. Accessed January 1, 2019.
11. U.S. Food and Drug Administration. Drugs@FDA: FDA approved drug products. https://www.accessdata.fda.gov/scripts/cder/daf. Accessed March 4, 2019.
Bariatric surgery + medical therapy: Effective Tx for T2DM?
ILLUSTRATIVE CASE
A 46-year-old woman presents with a body mass index (BMI) of 28 kg/m2, a 4-year history of type 2 diabetes mellitus (T2DM), and a glycated hemoglobin (HgbA1c) of 9.8%. The patient is currently being treated with intensive medical therapy (IMT), including metformin 2000 mg/d, sitagliptin 100 mg/d, and insulin glargine 12 units/d, with minimal change in HgbA1c. Should you recommend bariatric surgery as an option for the treatment of diabetes?
One in 11 Americans has diabetes and at least 95% of those have type 2.2,3 The treatment of T2DM is generally multimodal in order to target the various mechanisms that cause hyperglycemia. Treatment strategies may include lifestyle modifications, decreasing insulin resistance, increasing secretion of insulin, insulin replacement, and targeting incretin-hormonal pathways.
The American Diabetes Association (ADA) currently recommends diet, exercise, and behavioral modifications as first-line therapy for the management of diabetes,2 but these by themselves are often inadequate. In addition to various pharmacotherapeutic strategies for other populations with T2DM (see the PURL, “How do these 3 diabetes agents compare in reducing mortality?”), the ADA recommends bariatric surgery for the treatment of patients with T2DM, a BMI ≥35 kg/m2, and uncontrolled hyperglycemia.2,4 However, this recommendation from the ADA supporting bariatric surgery is based only on short-term studies.
For example, one single-center nonblinded randomized controlled trial (RCT) involving 60 patients with a BMI ≥35 kg/m2 found reductions in HgbA1C levels from the average baseline of 8.65±1.45% to 7.7±0.6% in the IMT group and to 6.4±1.4% in the gastric-bypass group at 2 years.5 In another study, a randomized double-blind trial involving 60 moderately obese patients (BMI, 25-35 kg/m2), gastric bypass had better outcomes than sleeve gastrectomy, with 93% of patients in the gastric bypass group achieving remission of T2DM vs 47% of patients in the sleeve gastrectomy group (P=.02) over a 12-month period.6
The current study sought to examine the long-term outcomes of IMT alone vs bariatric surgery with IMT for the treatment of T2DM in patients who are overweight or obese.1
STUDY SUMMARY
5-year follow-up shows surgery + intensive medical therapy works
This study by Schauer et al was a 5-year follow-up of a nonblinded, single-center RCT comparing IMT alone to IMT with Roux-en-Y gastric bypass or sleeve gastrectomy in 150 patients with T2DM.1 Patients were included if they were 20 to 60 years of age, had a BMI of 27 to 43 kg/m2, and had an HgbA1C >7%. Patients with previous bariatric surgery, complex abdominal surgery, or uncontrolled medical or psychiatric disorders were excluded.
Each patient was randomly placed in a 1:1:1 fashion into 3 groups: IMT only, IMT and gastric bypass, or IMT and sleeve gastrectomy. All patients underwent IMT as defined by the ADA. The primary outcome was the number of patients with an HgbA1c ≤6%. Secondary outcomes included weight loss, glucose control, lipid levels, blood pressure, medication use, renal function, adverse effects, ophthalmologic outcomes, and quality of life.
Continue to: Of the 150 patients...
Of the 150 patients, 1 died during the follow-up period leaving 149; 134 completed the 5-year follow-up; 8 patients in the IMT group and 1 patient in the sleeve gastrectomy group never initiated assigned treatment; an additional 6 patients were lost to follow-up. One patient from the IMT group and 1 patient from the sleeve gastrectomy group crossed over to the gastric bypass group.
Results. More patients in the bariatric surgery and sleeve gastrectomy groups achieved an HgbA1c of ≤6% compared with the IMT group (14 of 49 gastric bypass patients vs 2 of 38 IMT patients; P=.01; 11 of 47 sleeve gastrectomy patients vs 2 of 38 IMT patients; P=.03). Compared with those in the IMT group, the patients in the bariatric surgery and sleeve gastrectomy groups showed greater reductions from baseline in body weight and triglyceride levels, and greater increases from baseline in high-density lipoprotein (HDL) cholesterol levels; they also required less diabetic medication for glycemic control (see TABLE 11). However, when data were imputed for the intention-to-treat analysis, P-values were P=0.08 for gastric bypass and P=0.17 for sleeve gastrectomy compared with the IMT group for lowering HgbA1c.
WHAT’S NEW?
Adding surgery has big benefits with minimal adverse effects
Prior studies that evaluated the effect of gastric bypass surgery on diabetes were observational or had a shorter follow-up duration. This study demonstrates bariatric surgery plus IMT has long-term benefits with minimal adverse events compared with IMT alone.1,5 Additionally, this study supports recommendations for bariatric surgery as treatment for T2DM for patients with a BMI ≥27 kg/m2, which is below the starting BMI (35 kg/m2) recommended by the ADA.1,4
CAVEATS
Surgery is not without risks
The risk for surgical complications, such as gastrointestinal bleeding, severe hypoglycemia requiring intervention, and ketoacidosis, in this patient population is significant.1 Complications can include gastrointestinal leak, stroke, and infection.1 Additionally, long-term complications from bariatric surgery are emerging and include choledocholithiasis, intestinal obstruction, and esophageal pathology.7 Extensive patient counseling regarding the possible complications is necessary to ensure that patients make an informed decision regarding surgery.
This study utilized surrogate markers (A1c, lipid levels, and body weight) as disease-oriented outcome measures. Patient-oriented outcomes, such as morbidity and mortality, were not explored in this study.
Continue to: Due to the small sample size of the study...
Due to the small sample size of the study, it is unclear if the outcomes of the 2 surgery groups were significantly different. Patients who received gastric bypass surgery had more weight loss and used less diabetes medication at the end of follow-up compared with the patients who received sleeve gastrectomy. More information is needed to determine which gastric surgery is preferable for the treatment of T2DM while minimizing adverse effects. However, both of the procedures had outcomes superior to that with IMT, and selection of a particular type of surgery should be a joint decision between the patient and provider.
CHALLENGES TO IMPLEMENTATION
Access and cost may be barriers
The major barriers to implementation are access to, and the cost of, bariatric surgery.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
2. American Diabetes Asssociation. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42 (suppl 1):S81-S89.
3. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 1, 2019.
4. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861-877.
5. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585.
6. Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146:143-148.
7. Schulman AR, Thompson CC. Complications of bariatric surgery: what you can expect to see in your GI practice. Am J Gastroenterol. 2017;112:1640-1655.
ILLUSTRATIVE CASE
A 46-year-old woman presents with a body mass index (BMI) of 28 kg/m2, a 4-year history of type 2 diabetes mellitus (T2DM), and a glycated hemoglobin (HgbA1c) of 9.8%. The patient is currently being treated with intensive medical therapy (IMT), including metformin 2000 mg/d, sitagliptin 100 mg/d, and insulin glargine 12 units/d, with minimal change in HgbA1c. Should you recommend bariatric surgery as an option for the treatment of diabetes?
One in 11 Americans has diabetes and at least 95% of those have type 2.2,3 The treatment of T2DM is generally multimodal in order to target the various mechanisms that cause hyperglycemia. Treatment strategies may include lifestyle modifications, decreasing insulin resistance, increasing secretion of insulin, insulin replacement, and targeting incretin-hormonal pathways.
The American Diabetes Association (ADA) currently recommends diet, exercise, and behavioral modifications as first-line therapy for the management of diabetes,2 but these by themselves are often inadequate. In addition to various pharmacotherapeutic strategies for other populations with T2DM (see the PURL, “How do these 3 diabetes agents compare in reducing mortality?”), the ADA recommends bariatric surgery for the treatment of patients with T2DM, a BMI ≥35 kg/m2, and uncontrolled hyperglycemia.2,4 However, this recommendation from the ADA supporting bariatric surgery is based only on short-term studies.
For example, one single-center nonblinded randomized controlled trial (RCT) involving 60 patients with a BMI ≥35 kg/m2 found reductions in HgbA1C levels from the average baseline of 8.65±1.45% to 7.7±0.6% in the IMT group and to 6.4±1.4% in the gastric-bypass group at 2 years.5 In another study, a randomized double-blind trial involving 60 moderately obese patients (BMI, 25-35 kg/m2), gastric bypass had better outcomes than sleeve gastrectomy, with 93% of patients in the gastric bypass group achieving remission of T2DM vs 47% of patients in the sleeve gastrectomy group (P=.02) over a 12-month period.6
The current study sought to examine the long-term outcomes of IMT alone vs bariatric surgery with IMT for the treatment of T2DM in patients who are overweight or obese.1
STUDY SUMMARY
5-year follow-up shows surgery + intensive medical therapy works
This study by Schauer et al was a 5-year follow-up of a nonblinded, single-center RCT comparing IMT alone to IMT with Roux-en-Y gastric bypass or sleeve gastrectomy in 150 patients with T2DM.1 Patients were included if they were 20 to 60 years of age, had a BMI of 27 to 43 kg/m2, and had an HgbA1C >7%. Patients with previous bariatric surgery, complex abdominal surgery, or uncontrolled medical or psychiatric disorders were excluded.
Each patient was randomly placed in a 1:1:1 fashion into 3 groups: IMT only, IMT and gastric bypass, or IMT and sleeve gastrectomy. All patients underwent IMT as defined by the ADA. The primary outcome was the number of patients with an HgbA1c ≤6%. Secondary outcomes included weight loss, glucose control, lipid levels, blood pressure, medication use, renal function, adverse effects, ophthalmologic outcomes, and quality of life.
Continue to: Of the 150 patients...
Of the 150 patients, 1 died during the follow-up period leaving 149; 134 completed the 5-year follow-up; 8 patients in the IMT group and 1 patient in the sleeve gastrectomy group never initiated assigned treatment; an additional 6 patients were lost to follow-up. One patient from the IMT group and 1 patient from the sleeve gastrectomy group crossed over to the gastric bypass group.
Results. More patients in the bariatric surgery and sleeve gastrectomy groups achieved an HgbA1c of ≤6% compared with the IMT group (14 of 49 gastric bypass patients vs 2 of 38 IMT patients; P=.01; 11 of 47 sleeve gastrectomy patients vs 2 of 38 IMT patients; P=.03). Compared with those in the IMT group, the patients in the bariatric surgery and sleeve gastrectomy groups showed greater reductions from baseline in body weight and triglyceride levels, and greater increases from baseline in high-density lipoprotein (HDL) cholesterol levels; they also required less diabetic medication for glycemic control (see TABLE 11). However, when data were imputed for the intention-to-treat analysis, P-values were P=0.08 for gastric bypass and P=0.17 for sleeve gastrectomy compared with the IMT group for lowering HgbA1c.
WHAT’S NEW?
Adding surgery has big benefits with minimal adverse effects
Prior studies that evaluated the effect of gastric bypass surgery on diabetes were observational or had a shorter follow-up duration. This study demonstrates bariatric surgery plus IMT has long-term benefits with minimal adverse events compared with IMT alone.1,5 Additionally, this study supports recommendations for bariatric surgery as treatment for T2DM for patients with a BMI ≥27 kg/m2, which is below the starting BMI (35 kg/m2) recommended by the ADA.1,4
CAVEATS
Surgery is not without risks
The risk for surgical complications, such as gastrointestinal bleeding, severe hypoglycemia requiring intervention, and ketoacidosis, in this patient population is significant.1 Complications can include gastrointestinal leak, stroke, and infection.1 Additionally, long-term complications from bariatric surgery are emerging and include choledocholithiasis, intestinal obstruction, and esophageal pathology.7 Extensive patient counseling regarding the possible complications is necessary to ensure that patients make an informed decision regarding surgery.
This study utilized surrogate markers (A1c, lipid levels, and body weight) as disease-oriented outcome measures. Patient-oriented outcomes, such as morbidity and mortality, were not explored in this study.
Continue to: Due to the small sample size of the study...
Due to the small sample size of the study, it is unclear if the outcomes of the 2 surgery groups were significantly different. Patients who received gastric bypass surgery had more weight loss and used less diabetes medication at the end of follow-up compared with the patients who received sleeve gastrectomy. More information is needed to determine which gastric surgery is preferable for the treatment of T2DM while minimizing adverse effects. However, both of the procedures had outcomes superior to that with IMT, and selection of a particular type of surgery should be a joint decision between the patient and provider.
CHALLENGES TO IMPLEMENTATION
Access and cost may be barriers
The major barriers to implementation are access to, and the cost of, bariatric surgery.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 46-year-old woman presents with a body mass index (BMI) of 28 kg/m2, a 4-year history of type 2 diabetes mellitus (T2DM), and a glycated hemoglobin (HgbA1c) of 9.8%. The patient is currently being treated with intensive medical therapy (IMT), including metformin 2000 mg/d, sitagliptin 100 mg/d, and insulin glargine 12 units/d, with minimal change in HgbA1c. Should you recommend bariatric surgery as an option for the treatment of diabetes?
One in 11 Americans has diabetes and at least 95% of those have type 2.2,3 The treatment of T2DM is generally multimodal in order to target the various mechanisms that cause hyperglycemia. Treatment strategies may include lifestyle modifications, decreasing insulin resistance, increasing secretion of insulin, insulin replacement, and targeting incretin-hormonal pathways.
The American Diabetes Association (ADA) currently recommends diet, exercise, and behavioral modifications as first-line therapy for the management of diabetes,2 but these by themselves are often inadequate. In addition to various pharmacotherapeutic strategies for other populations with T2DM (see the PURL, “How do these 3 diabetes agents compare in reducing mortality?”), the ADA recommends bariatric surgery for the treatment of patients with T2DM, a BMI ≥35 kg/m2, and uncontrolled hyperglycemia.2,4 However, this recommendation from the ADA supporting bariatric surgery is based only on short-term studies.
For example, one single-center nonblinded randomized controlled trial (RCT) involving 60 patients with a BMI ≥35 kg/m2 found reductions in HgbA1C levels from the average baseline of 8.65±1.45% to 7.7±0.6% in the IMT group and to 6.4±1.4% in the gastric-bypass group at 2 years.5 In another study, a randomized double-blind trial involving 60 moderately obese patients (BMI, 25-35 kg/m2), gastric bypass had better outcomes than sleeve gastrectomy, with 93% of patients in the gastric bypass group achieving remission of T2DM vs 47% of patients in the sleeve gastrectomy group (P=.02) over a 12-month period.6
The current study sought to examine the long-term outcomes of IMT alone vs bariatric surgery with IMT for the treatment of T2DM in patients who are overweight or obese.1
STUDY SUMMARY
5-year follow-up shows surgery + intensive medical therapy works
This study by Schauer et al was a 5-year follow-up of a nonblinded, single-center RCT comparing IMT alone to IMT with Roux-en-Y gastric bypass or sleeve gastrectomy in 150 patients with T2DM.1 Patients were included if they were 20 to 60 years of age, had a BMI of 27 to 43 kg/m2, and had an HgbA1C >7%. Patients with previous bariatric surgery, complex abdominal surgery, or uncontrolled medical or psychiatric disorders were excluded.
Each patient was randomly placed in a 1:1:1 fashion into 3 groups: IMT only, IMT and gastric bypass, or IMT and sleeve gastrectomy. All patients underwent IMT as defined by the ADA. The primary outcome was the number of patients with an HgbA1c ≤6%. Secondary outcomes included weight loss, glucose control, lipid levels, blood pressure, medication use, renal function, adverse effects, ophthalmologic outcomes, and quality of life.
Continue to: Of the 150 patients...
Of the 150 patients, 1 died during the follow-up period leaving 149; 134 completed the 5-year follow-up; 8 patients in the IMT group and 1 patient in the sleeve gastrectomy group never initiated assigned treatment; an additional 6 patients were lost to follow-up. One patient from the IMT group and 1 patient from the sleeve gastrectomy group crossed over to the gastric bypass group.
Results. More patients in the bariatric surgery and sleeve gastrectomy groups achieved an HgbA1c of ≤6% compared with the IMT group (14 of 49 gastric bypass patients vs 2 of 38 IMT patients; P=.01; 11 of 47 sleeve gastrectomy patients vs 2 of 38 IMT patients; P=.03). Compared with those in the IMT group, the patients in the bariatric surgery and sleeve gastrectomy groups showed greater reductions from baseline in body weight and triglyceride levels, and greater increases from baseline in high-density lipoprotein (HDL) cholesterol levels; they also required less diabetic medication for glycemic control (see TABLE 11). However, when data were imputed for the intention-to-treat analysis, P-values were P=0.08 for gastric bypass and P=0.17 for sleeve gastrectomy compared with the IMT group for lowering HgbA1c.
WHAT’S NEW?
Adding surgery has big benefits with minimal adverse effects
Prior studies that evaluated the effect of gastric bypass surgery on diabetes were observational or had a shorter follow-up duration. This study demonstrates bariatric surgery plus IMT has long-term benefits with minimal adverse events compared with IMT alone.1,5 Additionally, this study supports recommendations for bariatric surgery as treatment for T2DM for patients with a BMI ≥27 kg/m2, which is below the starting BMI (35 kg/m2) recommended by the ADA.1,4
CAVEATS
Surgery is not without risks
The risk for surgical complications, such as gastrointestinal bleeding, severe hypoglycemia requiring intervention, and ketoacidosis, in this patient population is significant.1 Complications can include gastrointestinal leak, stroke, and infection.1 Additionally, long-term complications from bariatric surgery are emerging and include choledocholithiasis, intestinal obstruction, and esophageal pathology.7 Extensive patient counseling regarding the possible complications is necessary to ensure that patients make an informed decision regarding surgery.
This study utilized surrogate markers (A1c, lipid levels, and body weight) as disease-oriented outcome measures. Patient-oriented outcomes, such as morbidity and mortality, were not explored in this study.
Continue to: Due to the small sample size of the study...
Due to the small sample size of the study, it is unclear if the outcomes of the 2 surgery groups were significantly different. Patients who received gastric bypass surgery had more weight loss and used less diabetes medication at the end of follow-up compared with the patients who received sleeve gastrectomy. More information is needed to determine which gastric surgery is preferable for the treatment of T2DM while minimizing adverse effects. However, both of the procedures had outcomes superior to that with IMT, and selection of a particular type of surgery should be a joint decision between the patient and provider.
CHALLENGES TO IMPLEMENTATION
Access and cost may be barriers
The major barriers to implementation are access to, and the cost of, bariatric surgery.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
2. American Diabetes Asssociation. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42 (suppl 1):S81-S89.
3. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 1, 2019.
4. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861-877.
5. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585.
6. Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146:143-148.
7. Schulman AR, Thompson CC. Complications of bariatric surgery: what you can expect to see in your GI practice. Am J Gastroenterol. 2017;112:1640-1655.
1. Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
2. American Diabetes Asssociation. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42 (suppl 1):S81-S89.
3. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 1, 2019.
4. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861-877.
5. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585.
6. Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146:143-148.
7. Schulman AR, Thompson CC. Complications of bariatric surgery: what you can expect to see in your GI practice. Am J Gastroenterol. 2017;112:1640-1655.
PRACTICE CHANGER
Consider bariatric surgery with medical therapy as a treatment option for adults with uncontrolled type 2 diabetes and a body mass index ≥27 kg/m2.1
STRENGTH OF RECOMMENDATION
B: Based on a nonblinded, single-center, randomized controlled trial.
Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
A suicide attempt, or something else?
CASE Unexplained hypoglycemia
Ms. A, age 12, is brought to the emergency department (ED) via ambulance with altered mentation and life-threatening hypoglycemia for management of a hypoglycemic seizure. Earlier that day, Ms. A’s parents had found her unresponsive and incontinent of urine. In the ED, Ms. A is minimally responsive. Her blood glucose level measurements are in the range of 30 to 39 mg/dL (reference range: 70 to 99 mg/dL), despite having received IV dextrose first from paramedics, and then in the ED. Ms. A has no history of hypoglycemia or diabetes. Her parents say that the night before coming to the ED, Ms. A had experienced flu-like symptoms, including nausea, vomiting, and diarrhea, that continued overnight and resulted in minimal food intake for 24 hours (Table 1).
A physical exam demonstrates left-sided weakness of face, arm, and leg, rightward gaze, and left-sided neglect. However, the results of CT angiography and an MRI of the brain rule out a stroke. An EEG shows right hemispheric slowing consistent with postictal paralysis, but no ongoing seizure activity. Ms. A is transferred to the pediatric intensive care unit (PICU).
Although Ms. A has no psychiatric diagnoses, she has a history of depressive symptoms, self-harm by cutting, and a suicide attempt by ingestion of an over-the-counter (OTC) medication 1 year ago. She had reported the suicide attempt to her parents several months after the fact, and asked them to find her a therapist, which her parents arranged. She also has a history of asthma, which is well-controlled with montelukast, 5 mg/d.
EVALUATION Elevated insulin levels
Subsequent investigations for organic causes of hypoglycemia are negative for adrenal insufficiency, fatty acid oxidation defect, and sepsis. Blood results demonstrate significantly elevated insulin levels of 92.4 mcIU/mL (reference range: 2.6 to 24.9 mcIU/mL) and a C-peptide level of 9.5 ng/mL (reference range: 1.1 to 4.4 ng/mL).
On Day 1 of admission to the PICU, Ms. A’s blood glucose level normalizes, and her mentation improves. Her parents report that one of them has diabetes and takes oral hypoglycemic agents at home, including glipizide immediate release (IR) tablets, 10 mg, and long-acting insulin glargine. The treatment team suspects that Ms. A may have ingested one or both of these agents, and orders a toxicologic screening for oral hypoglycemic agents.
On Day 2, the toxicology results are returned and are positive for glipizide, which Ms. A had not been prescribed. Ms. A states that she had taken only her montelukast tablet on the day of admission and adamantly denies deliberately ingesting her parent’s diabetes medications. Her parents check the home medications and state there are no missing glipizide IR tablets or insulin vials. They also report that Ms. A had no access to extended-release glipizide.
The treatment team discuss Ms. A’s clinical condition and toxicology results with the pediatric endocrinology team. The endocrinology team states that with no history of hypoglycemic episodes, it is unlikely that Ms. A had an endogenous etiology that would present so catastrophically. In their experience, inexplicable hypoglycemia in a healthy individual who lives in a household with someone who has diabetes is due to ingestion of a hypoglycemic agent until proven otherwise.
[polldaddy:10252689]
Continue to: The authors' observations
The authors’ observations
In the context of Ms. A’s prior suicide attempt and history of self-harm, the pediatric team was concerned that her presentation was consistent with a suicide attempt and consulted the psychiatry service.
Glipizide is a second-generation sulfonylurea used to treat type 2 diabetes. It lowers blood glucose by stimulating pancreatic insulin secretion. It is a rare drug of overdose.1 Although pediatric glipizide overdoses have been documented, there are currently no pediatric or adolescent glipizide pharmacokinetic studies in the literature.1-4 In adults, the immediate-release formulation has 100% oral bioavailability, with a maximum plasma concentration (Tmax) of approximately 2 hours.5 The half-life typically ranges from 4 to 6 hours in adults.6 Patients who do not have diabetes are much more susceptible to the hypoglycemic effects of glipizide because the medication simulates their fully functional pancreas to produce a vigorous insulin response.
Ms. A’s significantly elevated insulin was consistent with normal glipizide effects in a healthy child, while the elevated C-peptide was consistent with insulin being endogenously produced, which ruled out ingestion of her parent’s insulin. Importantly, the pediatric endocrinology team noted that, in their experience, a single 5- to 10-mg dose of glipizide IR was sufficient to lower blood glucose levels to the low 30s mg/dL in the context of a functional pancreas, which suggested that Ms. A might have accidentally ingested a single glipizide IR tablet, and might be telling the truth when she denies deliberately ingesting it to hurt herself.
The clinical value of pharmacokinetics
The screen of Ms. A’s toxicology sample detected glipizide. The laboratory used a semi-quantitative serum screen of several hypoglycemic agents. A positive result for each agent is based on a quantitative cut-off value, which is 3 ng/mL for glipizide. The clinical chemist on call was asked to assist in interpreting the results. The serum specimen collected on Day 1 had a significantly positive glipizide result of 86 to 130 ng/mL. The maximum effective glipizide concentration for adult patients with diabetes is 100 ng/mL.7 Thus, the glipizide level of 86 to 130 ng/mL (20.5 hours after initial symptoms) is consistent with the clinical presentation of persistent hypoglycemia requiring ongoing glucose replacement therapy.
Due to the lack of pediatric pharmacokinetic data for glipizide and only a single serum measurement, it is not possible to estimate the glipizide concentration at the time of maximal symptoms (loss of consciousness at 2:30
Continue to: Clinicians need to be aware that...
Clinicians need to be aware that although hypoglycemia usually presents rapidly, in children glipizide IR can rarely cause delayed hypoglycemia up to 16 hours after ingestion,2 and a delay of 45 hours was reported in a case of ingestion of extended-release glipizide.8 Hypoglycemia can last up to nearly 24 hours and is exacerbated if the patient has not eaten.1,2 Importantly, Ms. A’s parents reported that she had no access to extended-release glipizide. When detailed pharmacokinetic data are not available, the information provided by the patient and parents becomes extremely important, especially in distinguishing between single and multiple overdoses prior to presentation, or co-ingestions, or decreased food intake that could exacerbate hypoglycemia.
EVALUATION Safety assessment
On Day 2, Ms. A and her parents are interviewed separately, and they all are consistent in their recollection that Ms. A had been feverish with flu-like symptoms throughout the night before coming to the ED, and had still seemed mildly confused on the morning of admission.
During the interview, her parents wonder when Ms. A took her daily dose of a single montelukast tablet for asthma, and whether she had accidentally confused it with their glipizide. They report that on the morning of admission, both the glipizide and montelukast medication vials were in the same room. The vials are the same color, the same size, and labeled from the same pharmacy, and contain white, scored, round tablets that look very similar.
During the interview, Ms. A consistently denies having thoughts of hurting or killing herself on the day of admission or before that.
[polldaddy:10252690]
Continue to: The authors' observations
The authors’ observations
This case was ultimately an accidental ingestion of glipizide, rather than a suicide attempt. The initial suspicion for a suicide attempt had been reasonable in the context of Ms. A’s depressive symptoms, remote history of a prior suicide attempt by ingesting an OTC medication, and toxicologic evidence of ingesting a drug not prescribed to her. Additionally, because of the life-threatening presentation, it was easy to make the erroneous assumption that the ingestion of glipizide must have involved many tablets, and thus must have been deliberate. However, through multidisciplinary teamwork, we were able to demonstrate that this was likely an accidental ingestion by a patient who had an acute febrile illness. Her illness had caused confusion, which contributed to the accidental ingestion, and also caused reduced food intake, which enhanced the hypoglycemic effects of glipizide. Additionally, a lack of awareness of medication safety in the home had facilitated the confusion between the two medication vials.
A single tablet of glipizide IR is sufficient to produce profound clinical effects that could be mistaken by medical and psychiatric teams for a much larger and/or deliberate overdose, especially in patients with a psychiatric history. The inappropriate psychiatric hospitalization of a patient, especially a child, who has been mistakenly diagnosed as having attempted suicide, can have negative therapeutic consequences (Table 2). A psychiatric admission would have been misguided if it attempted to address safety and reduce suicidality when no such concerns were present. Additionally, it could have damaged relationships with the patient and the family, especially in a child who had historically not sought psychiatric care despite depressive symptoms and a previous suicide attempt. When assessing for suicidality, consider accidental ingestion in the differential and use specialty expertise and confirmatory testing in the evaluation, taking the pharmacokinetics of the suspected agent into account.
OUTCOME Outpatient treatment
Ms. A’s neurologic symptoms resolve within 24 hours of admission. She is offered psychiatric inpatient hospitalization to address her depressive symptoms; however, her parents prefer that she receive outpatient care. Ms. A’s parents also state that after Ms. A’s admission, they locked up all household medications and will be more mindful with medication in the home. Because her parents are arranging appropriate outpatient treatment for Ms. A’s depression and maintenance of her safety, an involuntary hospitalization is not deemed necessary.
On Day 2, Ms. A is eating normally, her blood glucose levels remain stable, and she is discharged home.
Bottom Line
Oral hypoglycemic agents can cause life-threatening syndromes in healthy patients and can clinically mimic large, intentional overdoses. Clinicians must be aware of the differential of accidental ingestion when assessing for suicidality, and can use toxicology results in their assessment.
Related Resources
- Kidemergencies.com. Emergencies: One pill can kill. http://kidemergencies.com/onepill1.html.
- Safe Kids Worldwide. Medication safety. https://www.safekids.org/medicinesafety.
- American Association of Poison Control Centers. http://www.aapcc.org/.
Drug Brand Names
Glipizide • Glucotrol
Insulin glargine • Lantus
Montelukast • Singulair
1. Spiller HA, Villalobos D, Krenzelok EP, et al. Prospective multicenter study of sulfonylurea ingestion in children. J Pediatr. 1997;131(1):141-146.
2. Quadrani DA, Spiller HA, Widder P. Five year retrospective evaluation of sulfonylurea ingestion in children. J Toxicol Clin Toxicol. 1996;34(3):267-270.
3. Borowski H, Caraccio T, Mofenson H. Sulfonylurea ingestion in children: is an 8-hour observation period sufficient? J Pediatr. 1998;133(4):584-585.
4. Little GL, Boniface KS. Are one or two dangerous? Sulfony-lurea exposure in toddlers. J Emerg Med. 2005;28(3):305-310.
5. Huupponen R, Seppala P, Iisalo E. Glipizide pharma-cokinetics and response in diabetics. Int J Clin Pharmacol Ther Toxicol. 1982;20(9):417-422.
6. Baselt RC. Disposition of toxic drugs and chemicals in man. 10th ed. Seal Beach, California: Biomedical Publications; 2014.
7. Simonson DC, Kourides IA, Feinglos M, et al; the Glipizide Gastrointestinal Therapeutic System Study Group. Efficacy, safety, and dose-response characteristics of glipizide gastrointestinal therapeutic system on glycemic control and insulin secretion in NIDDM. Results of two multicenter, randomized, placebo-controlled clinical trials. Diabetes Care. 1997;20(4):597-606.
8. Pelavin PI, Abramson E, Pon S, et al. Extended-release glipizide overdose presenting with delayed hypoglycemia and treated with subcutaneous octreotide. J Pediatr Endocrinol Metab. 2009;22(2):171-175.
CASE Unexplained hypoglycemia
Ms. A, age 12, is brought to the emergency department (ED) via ambulance with altered mentation and life-threatening hypoglycemia for management of a hypoglycemic seizure. Earlier that day, Ms. A’s parents had found her unresponsive and incontinent of urine. In the ED, Ms. A is minimally responsive. Her blood glucose level measurements are in the range of 30 to 39 mg/dL (reference range: 70 to 99 mg/dL), despite having received IV dextrose first from paramedics, and then in the ED. Ms. A has no history of hypoglycemia or diabetes. Her parents say that the night before coming to the ED, Ms. A had experienced flu-like symptoms, including nausea, vomiting, and diarrhea, that continued overnight and resulted in minimal food intake for 24 hours (Table 1).
A physical exam demonstrates left-sided weakness of face, arm, and leg, rightward gaze, and left-sided neglect. However, the results of CT angiography and an MRI of the brain rule out a stroke. An EEG shows right hemispheric slowing consistent with postictal paralysis, but no ongoing seizure activity. Ms. A is transferred to the pediatric intensive care unit (PICU).
Although Ms. A has no psychiatric diagnoses, she has a history of depressive symptoms, self-harm by cutting, and a suicide attempt by ingestion of an over-the-counter (OTC) medication 1 year ago. She had reported the suicide attempt to her parents several months after the fact, and asked them to find her a therapist, which her parents arranged. She also has a history of asthma, which is well-controlled with montelukast, 5 mg/d.
EVALUATION Elevated insulin levels
Subsequent investigations for organic causes of hypoglycemia are negative for adrenal insufficiency, fatty acid oxidation defect, and sepsis. Blood results demonstrate significantly elevated insulin levels of 92.4 mcIU/mL (reference range: 2.6 to 24.9 mcIU/mL) and a C-peptide level of 9.5 ng/mL (reference range: 1.1 to 4.4 ng/mL).
On Day 1 of admission to the PICU, Ms. A’s blood glucose level normalizes, and her mentation improves. Her parents report that one of them has diabetes and takes oral hypoglycemic agents at home, including glipizide immediate release (IR) tablets, 10 mg, and long-acting insulin glargine. The treatment team suspects that Ms. A may have ingested one or both of these agents, and orders a toxicologic screening for oral hypoglycemic agents.
On Day 2, the toxicology results are returned and are positive for glipizide, which Ms. A had not been prescribed. Ms. A states that she had taken only her montelukast tablet on the day of admission and adamantly denies deliberately ingesting her parent’s diabetes medications. Her parents check the home medications and state there are no missing glipizide IR tablets or insulin vials. They also report that Ms. A had no access to extended-release glipizide.
The treatment team discuss Ms. A’s clinical condition and toxicology results with the pediatric endocrinology team. The endocrinology team states that with no history of hypoglycemic episodes, it is unlikely that Ms. A had an endogenous etiology that would present so catastrophically. In their experience, inexplicable hypoglycemia in a healthy individual who lives in a household with someone who has diabetes is due to ingestion of a hypoglycemic agent until proven otherwise.
[polldaddy:10252689]
Continue to: The authors' observations
The authors’ observations
In the context of Ms. A’s prior suicide attempt and history of self-harm, the pediatric team was concerned that her presentation was consistent with a suicide attempt and consulted the psychiatry service.
Glipizide is a second-generation sulfonylurea used to treat type 2 diabetes. It lowers blood glucose by stimulating pancreatic insulin secretion. It is a rare drug of overdose.1 Although pediatric glipizide overdoses have been documented, there are currently no pediatric or adolescent glipizide pharmacokinetic studies in the literature.1-4 In adults, the immediate-release formulation has 100% oral bioavailability, with a maximum plasma concentration (Tmax) of approximately 2 hours.5 The half-life typically ranges from 4 to 6 hours in adults.6 Patients who do not have diabetes are much more susceptible to the hypoglycemic effects of glipizide because the medication simulates their fully functional pancreas to produce a vigorous insulin response.
Ms. A’s significantly elevated insulin was consistent with normal glipizide effects in a healthy child, while the elevated C-peptide was consistent with insulin being endogenously produced, which ruled out ingestion of her parent’s insulin. Importantly, the pediatric endocrinology team noted that, in their experience, a single 5- to 10-mg dose of glipizide IR was sufficient to lower blood glucose levels to the low 30s mg/dL in the context of a functional pancreas, which suggested that Ms. A might have accidentally ingested a single glipizide IR tablet, and might be telling the truth when she denies deliberately ingesting it to hurt herself.
The clinical value of pharmacokinetics
The screen of Ms. A’s toxicology sample detected glipizide. The laboratory used a semi-quantitative serum screen of several hypoglycemic agents. A positive result for each agent is based on a quantitative cut-off value, which is 3 ng/mL for glipizide. The clinical chemist on call was asked to assist in interpreting the results. The serum specimen collected on Day 1 had a significantly positive glipizide result of 86 to 130 ng/mL. The maximum effective glipizide concentration for adult patients with diabetes is 100 ng/mL.7 Thus, the glipizide level of 86 to 130 ng/mL (20.5 hours after initial symptoms) is consistent with the clinical presentation of persistent hypoglycemia requiring ongoing glucose replacement therapy.
Due to the lack of pediatric pharmacokinetic data for glipizide and only a single serum measurement, it is not possible to estimate the glipizide concentration at the time of maximal symptoms (loss of consciousness at 2:30
Continue to: Clinicians need to be aware that...
Clinicians need to be aware that although hypoglycemia usually presents rapidly, in children glipizide IR can rarely cause delayed hypoglycemia up to 16 hours after ingestion,2 and a delay of 45 hours was reported in a case of ingestion of extended-release glipizide.8 Hypoglycemia can last up to nearly 24 hours and is exacerbated if the patient has not eaten.1,2 Importantly, Ms. A’s parents reported that she had no access to extended-release glipizide. When detailed pharmacokinetic data are not available, the information provided by the patient and parents becomes extremely important, especially in distinguishing between single and multiple overdoses prior to presentation, or co-ingestions, or decreased food intake that could exacerbate hypoglycemia.
EVALUATION Safety assessment
On Day 2, Ms. A and her parents are interviewed separately, and they all are consistent in their recollection that Ms. A had been feverish with flu-like symptoms throughout the night before coming to the ED, and had still seemed mildly confused on the morning of admission.
During the interview, her parents wonder when Ms. A took her daily dose of a single montelukast tablet for asthma, and whether she had accidentally confused it with their glipizide. They report that on the morning of admission, both the glipizide and montelukast medication vials were in the same room. The vials are the same color, the same size, and labeled from the same pharmacy, and contain white, scored, round tablets that look very similar.
During the interview, Ms. A consistently denies having thoughts of hurting or killing herself on the day of admission or before that.
[polldaddy:10252690]
Continue to: The authors' observations
The authors’ observations
This case was ultimately an accidental ingestion of glipizide, rather than a suicide attempt. The initial suspicion for a suicide attempt had been reasonable in the context of Ms. A’s depressive symptoms, remote history of a prior suicide attempt by ingesting an OTC medication, and toxicologic evidence of ingesting a drug not prescribed to her. Additionally, because of the life-threatening presentation, it was easy to make the erroneous assumption that the ingestion of glipizide must have involved many tablets, and thus must have been deliberate. However, through multidisciplinary teamwork, we were able to demonstrate that this was likely an accidental ingestion by a patient who had an acute febrile illness. Her illness had caused confusion, which contributed to the accidental ingestion, and also caused reduced food intake, which enhanced the hypoglycemic effects of glipizide. Additionally, a lack of awareness of medication safety in the home had facilitated the confusion between the two medication vials.
A single tablet of glipizide IR is sufficient to produce profound clinical effects that could be mistaken by medical and psychiatric teams for a much larger and/or deliberate overdose, especially in patients with a psychiatric history. The inappropriate psychiatric hospitalization of a patient, especially a child, who has been mistakenly diagnosed as having attempted suicide, can have negative therapeutic consequences (Table 2). A psychiatric admission would have been misguided if it attempted to address safety and reduce suicidality when no such concerns were present. Additionally, it could have damaged relationships with the patient and the family, especially in a child who had historically not sought psychiatric care despite depressive symptoms and a previous suicide attempt. When assessing for suicidality, consider accidental ingestion in the differential and use specialty expertise and confirmatory testing in the evaluation, taking the pharmacokinetics of the suspected agent into account.
OUTCOME Outpatient treatment
Ms. A’s neurologic symptoms resolve within 24 hours of admission. She is offered psychiatric inpatient hospitalization to address her depressive symptoms; however, her parents prefer that she receive outpatient care. Ms. A’s parents also state that after Ms. A’s admission, they locked up all household medications and will be more mindful with medication in the home. Because her parents are arranging appropriate outpatient treatment for Ms. A’s depression and maintenance of her safety, an involuntary hospitalization is not deemed necessary.
On Day 2, Ms. A is eating normally, her blood glucose levels remain stable, and she is discharged home.
Bottom Line
Oral hypoglycemic agents can cause life-threatening syndromes in healthy patients and can clinically mimic large, intentional overdoses. Clinicians must be aware of the differential of accidental ingestion when assessing for suicidality, and can use toxicology results in their assessment.
Related Resources
- Kidemergencies.com. Emergencies: One pill can kill. http://kidemergencies.com/onepill1.html.
- Safe Kids Worldwide. Medication safety. https://www.safekids.org/medicinesafety.
- American Association of Poison Control Centers. http://www.aapcc.org/.
Drug Brand Names
Glipizide • Glucotrol
Insulin glargine • Lantus
Montelukast • Singulair
CASE Unexplained hypoglycemia
Ms. A, age 12, is brought to the emergency department (ED) via ambulance with altered mentation and life-threatening hypoglycemia for management of a hypoglycemic seizure. Earlier that day, Ms. A’s parents had found her unresponsive and incontinent of urine. In the ED, Ms. A is minimally responsive. Her blood glucose level measurements are in the range of 30 to 39 mg/dL (reference range: 70 to 99 mg/dL), despite having received IV dextrose first from paramedics, and then in the ED. Ms. A has no history of hypoglycemia or diabetes. Her parents say that the night before coming to the ED, Ms. A had experienced flu-like symptoms, including nausea, vomiting, and diarrhea, that continued overnight and resulted in minimal food intake for 24 hours (Table 1).
A physical exam demonstrates left-sided weakness of face, arm, and leg, rightward gaze, and left-sided neglect. However, the results of CT angiography and an MRI of the brain rule out a stroke. An EEG shows right hemispheric slowing consistent with postictal paralysis, but no ongoing seizure activity. Ms. A is transferred to the pediatric intensive care unit (PICU).
Although Ms. A has no psychiatric diagnoses, she has a history of depressive symptoms, self-harm by cutting, and a suicide attempt by ingestion of an over-the-counter (OTC) medication 1 year ago. She had reported the suicide attempt to her parents several months after the fact, and asked them to find her a therapist, which her parents arranged. She also has a history of asthma, which is well-controlled with montelukast, 5 mg/d.
EVALUATION Elevated insulin levels
Subsequent investigations for organic causes of hypoglycemia are negative for adrenal insufficiency, fatty acid oxidation defect, and sepsis. Blood results demonstrate significantly elevated insulin levels of 92.4 mcIU/mL (reference range: 2.6 to 24.9 mcIU/mL) and a C-peptide level of 9.5 ng/mL (reference range: 1.1 to 4.4 ng/mL).
On Day 1 of admission to the PICU, Ms. A’s blood glucose level normalizes, and her mentation improves. Her parents report that one of them has diabetes and takes oral hypoglycemic agents at home, including glipizide immediate release (IR) tablets, 10 mg, and long-acting insulin glargine. The treatment team suspects that Ms. A may have ingested one or both of these agents, and orders a toxicologic screening for oral hypoglycemic agents.
On Day 2, the toxicology results are returned and are positive for glipizide, which Ms. A had not been prescribed. Ms. A states that she had taken only her montelukast tablet on the day of admission and adamantly denies deliberately ingesting her parent’s diabetes medications. Her parents check the home medications and state there are no missing glipizide IR tablets or insulin vials. They also report that Ms. A had no access to extended-release glipizide.
The treatment team discuss Ms. A’s clinical condition and toxicology results with the pediatric endocrinology team. The endocrinology team states that with no history of hypoglycemic episodes, it is unlikely that Ms. A had an endogenous etiology that would present so catastrophically. In their experience, inexplicable hypoglycemia in a healthy individual who lives in a household with someone who has diabetes is due to ingestion of a hypoglycemic agent until proven otherwise.
[polldaddy:10252689]
Continue to: The authors' observations
The authors’ observations
In the context of Ms. A’s prior suicide attempt and history of self-harm, the pediatric team was concerned that her presentation was consistent with a suicide attempt and consulted the psychiatry service.
Glipizide is a second-generation sulfonylurea used to treat type 2 diabetes. It lowers blood glucose by stimulating pancreatic insulin secretion. It is a rare drug of overdose.1 Although pediatric glipizide overdoses have been documented, there are currently no pediatric or adolescent glipizide pharmacokinetic studies in the literature.1-4 In adults, the immediate-release formulation has 100% oral bioavailability, with a maximum plasma concentration (Tmax) of approximately 2 hours.5 The half-life typically ranges from 4 to 6 hours in adults.6 Patients who do not have diabetes are much more susceptible to the hypoglycemic effects of glipizide because the medication simulates their fully functional pancreas to produce a vigorous insulin response.
Ms. A’s significantly elevated insulin was consistent with normal glipizide effects in a healthy child, while the elevated C-peptide was consistent with insulin being endogenously produced, which ruled out ingestion of her parent’s insulin. Importantly, the pediatric endocrinology team noted that, in their experience, a single 5- to 10-mg dose of glipizide IR was sufficient to lower blood glucose levels to the low 30s mg/dL in the context of a functional pancreas, which suggested that Ms. A might have accidentally ingested a single glipizide IR tablet, and might be telling the truth when she denies deliberately ingesting it to hurt herself.
The clinical value of pharmacokinetics
The screen of Ms. A’s toxicology sample detected glipizide. The laboratory used a semi-quantitative serum screen of several hypoglycemic agents. A positive result for each agent is based on a quantitative cut-off value, which is 3 ng/mL for glipizide. The clinical chemist on call was asked to assist in interpreting the results. The serum specimen collected on Day 1 had a significantly positive glipizide result of 86 to 130 ng/mL. The maximum effective glipizide concentration for adult patients with diabetes is 100 ng/mL.7 Thus, the glipizide level of 86 to 130 ng/mL (20.5 hours after initial symptoms) is consistent with the clinical presentation of persistent hypoglycemia requiring ongoing glucose replacement therapy.
Due to the lack of pediatric pharmacokinetic data for glipizide and only a single serum measurement, it is not possible to estimate the glipizide concentration at the time of maximal symptoms (loss of consciousness at 2:30
Continue to: Clinicians need to be aware that...
Clinicians need to be aware that although hypoglycemia usually presents rapidly, in children glipizide IR can rarely cause delayed hypoglycemia up to 16 hours after ingestion,2 and a delay of 45 hours was reported in a case of ingestion of extended-release glipizide.8 Hypoglycemia can last up to nearly 24 hours and is exacerbated if the patient has not eaten.1,2 Importantly, Ms. A’s parents reported that she had no access to extended-release glipizide. When detailed pharmacokinetic data are not available, the information provided by the patient and parents becomes extremely important, especially in distinguishing between single and multiple overdoses prior to presentation, or co-ingestions, or decreased food intake that could exacerbate hypoglycemia.
EVALUATION Safety assessment
On Day 2, Ms. A and her parents are interviewed separately, and they all are consistent in their recollection that Ms. A had been feverish with flu-like symptoms throughout the night before coming to the ED, and had still seemed mildly confused on the morning of admission.
During the interview, her parents wonder when Ms. A took her daily dose of a single montelukast tablet for asthma, and whether she had accidentally confused it with their glipizide. They report that on the morning of admission, both the glipizide and montelukast medication vials were in the same room. The vials are the same color, the same size, and labeled from the same pharmacy, and contain white, scored, round tablets that look very similar.
During the interview, Ms. A consistently denies having thoughts of hurting or killing herself on the day of admission or before that.
[polldaddy:10252690]
Continue to: The authors' observations
The authors’ observations
This case was ultimately an accidental ingestion of glipizide, rather than a suicide attempt. The initial suspicion for a suicide attempt had been reasonable in the context of Ms. A’s depressive symptoms, remote history of a prior suicide attempt by ingesting an OTC medication, and toxicologic evidence of ingesting a drug not prescribed to her. Additionally, because of the life-threatening presentation, it was easy to make the erroneous assumption that the ingestion of glipizide must have involved many tablets, and thus must have been deliberate. However, through multidisciplinary teamwork, we were able to demonstrate that this was likely an accidental ingestion by a patient who had an acute febrile illness. Her illness had caused confusion, which contributed to the accidental ingestion, and also caused reduced food intake, which enhanced the hypoglycemic effects of glipizide. Additionally, a lack of awareness of medication safety in the home had facilitated the confusion between the two medication vials.
A single tablet of glipizide IR is sufficient to produce profound clinical effects that could be mistaken by medical and psychiatric teams for a much larger and/or deliberate overdose, especially in patients with a psychiatric history. The inappropriate psychiatric hospitalization of a patient, especially a child, who has been mistakenly diagnosed as having attempted suicide, can have negative therapeutic consequences (Table 2). A psychiatric admission would have been misguided if it attempted to address safety and reduce suicidality when no such concerns were present. Additionally, it could have damaged relationships with the patient and the family, especially in a child who had historically not sought psychiatric care despite depressive symptoms and a previous suicide attempt. When assessing for suicidality, consider accidental ingestion in the differential and use specialty expertise and confirmatory testing in the evaluation, taking the pharmacokinetics of the suspected agent into account.
OUTCOME Outpatient treatment
Ms. A’s neurologic symptoms resolve within 24 hours of admission. She is offered psychiatric inpatient hospitalization to address her depressive symptoms; however, her parents prefer that she receive outpatient care. Ms. A’s parents also state that after Ms. A’s admission, they locked up all household medications and will be more mindful with medication in the home. Because her parents are arranging appropriate outpatient treatment for Ms. A’s depression and maintenance of her safety, an involuntary hospitalization is not deemed necessary.
On Day 2, Ms. A is eating normally, her blood glucose levels remain stable, and she is discharged home.
Bottom Line
Oral hypoglycemic agents can cause life-threatening syndromes in healthy patients and can clinically mimic large, intentional overdoses. Clinicians must be aware of the differential of accidental ingestion when assessing for suicidality, and can use toxicology results in their assessment.
Related Resources
- Kidemergencies.com. Emergencies: One pill can kill. http://kidemergencies.com/onepill1.html.
- Safe Kids Worldwide. Medication safety. https://www.safekids.org/medicinesafety.
- American Association of Poison Control Centers. http://www.aapcc.org/.
Drug Brand Names
Glipizide • Glucotrol
Insulin glargine • Lantus
Montelukast • Singulair
1. Spiller HA, Villalobos D, Krenzelok EP, et al. Prospective multicenter study of sulfonylurea ingestion in children. J Pediatr. 1997;131(1):141-146.
2. Quadrani DA, Spiller HA, Widder P. Five year retrospective evaluation of sulfonylurea ingestion in children. J Toxicol Clin Toxicol. 1996;34(3):267-270.
3. Borowski H, Caraccio T, Mofenson H. Sulfonylurea ingestion in children: is an 8-hour observation period sufficient? J Pediatr. 1998;133(4):584-585.
4. Little GL, Boniface KS. Are one or two dangerous? Sulfony-lurea exposure in toddlers. J Emerg Med. 2005;28(3):305-310.
5. Huupponen R, Seppala P, Iisalo E. Glipizide pharma-cokinetics and response in diabetics. Int J Clin Pharmacol Ther Toxicol. 1982;20(9):417-422.
6. Baselt RC. Disposition of toxic drugs and chemicals in man. 10th ed. Seal Beach, California: Biomedical Publications; 2014.
7. Simonson DC, Kourides IA, Feinglos M, et al; the Glipizide Gastrointestinal Therapeutic System Study Group. Efficacy, safety, and dose-response characteristics of glipizide gastrointestinal therapeutic system on glycemic control and insulin secretion in NIDDM. Results of two multicenter, randomized, placebo-controlled clinical trials. Diabetes Care. 1997;20(4):597-606.
8. Pelavin PI, Abramson E, Pon S, et al. Extended-release glipizide overdose presenting with delayed hypoglycemia and treated with subcutaneous octreotide. J Pediatr Endocrinol Metab. 2009;22(2):171-175.
1. Spiller HA, Villalobos D, Krenzelok EP, et al. Prospective multicenter study of sulfonylurea ingestion in children. J Pediatr. 1997;131(1):141-146.
2. Quadrani DA, Spiller HA, Widder P. Five year retrospective evaluation of sulfonylurea ingestion in children. J Toxicol Clin Toxicol. 1996;34(3):267-270.
3. Borowski H, Caraccio T, Mofenson H. Sulfonylurea ingestion in children: is an 8-hour observation period sufficient? J Pediatr. 1998;133(4):584-585.
4. Little GL, Boniface KS. Are one or two dangerous? Sulfony-lurea exposure in toddlers. J Emerg Med. 2005;28(3):305-310.
5. Huupponen R, Seppala P, Iisalo E. Glipizide pharma-cokinetics and response in diabetics. Int J Clin Pharmacol Ther Toxicol. 1982;20(9):417-422.
6. Baselt RC. Disposition of toxic drugs and chemicals in man. 10th ed. Seal Beach, California: Biomedical Publications; 2014.
7. Simonson DC, Kourides IA, Feinglos M, et al; the Glipizide Gastrointestinal Therapeutic System Study Group. Efficacy, safety, and dose-response characteristics of glipizide gastrointestinal therapeutic system on glycemic control and insulin secretion in NIDDM. Results of two multicenter, randomized, placebo-controlled clinical trials. Diabetes Care. 1997;20(4):597-606.
8. Pelavin PI, Abramson E, Pon S, et al. Extended-release glipizide overdose presenting with delayed hypoglycemia and treated with subcutaneous octreotide. J Pediatr Endocrinol Metab. 2009;22(2):171-175.
Antipsychotics and seizures: What are the risks?
Antipsychotics, especially second-generation antipsychotics (SGAs), have been proven effective for treating psychosis as well as mood disorders.1,2 Because antipsychotics can lower the epileptogenic threshold, seizures are a serious potential adverse effect. Antipsychotics can cause isolated EEG abnormalities in 7% of patients with no history of epilepsy, and clinical seizures in .5% to 1.2% of such patients.3 Additionally, the neuropathophysiology underlying epilepsy can predispose patients to psychiatric disorders4; the estimated prevalence of psychosis in patients with epilepsy is approximately 7%.5 This review will shed light on the risk of clinical seizures related to antipsychotics.
Comparing seizure risk among antipsychotics
In a review of the World Health Organization’s adverse drug reactions database, Kumlien and Lundberg6 calculated the ratio of the number of reports of seizures to the total number of reports for each drug. They found that approximately 9% of all adverse drug reaction reports involving clozapine were due to seizures. Equivalent ratios were 5.90% for quetiapine, 4.91% for olanzapine, 3.68% for risperidone, 3.27% for haloperidol, and 2.59% for aripiprazole. Using the database of the Pharmacovigilance Unit of the Basque Country, Lertxundi et al7 reported a 3.2-fold increased risk of seizure with SGAs in comparison with first-generation antipsychotics (FGAs) (95% confidence interval [CI], 2.21 to 4.63), which went down to 2.08 (CI, 1.39 to 3.12) once clozapine was excluded. However, as the authors of both studies noted, the quality and relevance of this data are limited because it relies on spontaneous reporting.
Overall, the evidence regarding the seizure risk associated with antipsychotics is scarce. To the best of our knowledge, only 2 large observational studies have compared the seizure risks associated with different antipsychotics.
Using data from the UK-based Clinical Practice Research Datalink between 1998 and 2013, Bloechlinger et al8 examined the incidence rates of seizures among patients newly diagnosed with schizophrenia, affective disorders, or dementia who were prescribed antipsychotics. They excluded patients with a history of seizures or antiepileptic use. In the cohort of 60,121 patients, the incidence rates of seizures per 10,000 person-years were 11.7 (CI, 10.0 to 13.4) for those who did not use antipsychotics, 12.4 (CI, 10.9 to 13.8) for past users, 115.4 (CI, 50.1 to 180.7) for current users of haloperidol, 48.8 (CI, 30.7 to 66.9) for current users of quetiapine, 25.9 (CI, 11.8 to 40.0) for current users of risperidone, and 19.0 (CI, 8.7 to 29.3) for current users of olanzapine. No data were available about clozapine use.
In subsequent analyses, the authors found that among patients with affective disorders, only current use of medium- to high-potency FGAs (haloperidol, prochlorperazine, and trifluoperazine) was associated with a significantly increased risk of seizures (adjusted odds ratio: 2.51, CI, 1.51 to 4.18) compared with non-users.8 Among patients with dementia, current use of olanzapine or quetiapine and current use of any FGAs were associated with significantly increased odds of seizures. This study suggests that the underlying mental illness might modulate the seizure risk associated with antipsychotics.8
Wu et al9 conducted a study based on the National Health Insurance Research Database in Taiwan. They examined the 1-year incidence of new-onset seizures among patients diagnosed with schizophrenia or mood disorders who were new to antipsychotic treatment, and calculated the risk of seizure associated with each antipsychotic in reference to risperidone. They found that those receiving clozapine, thioridazine, and haloperidol were 2 to 3 times more likely to develop seizures than those treated with risperidone; risks associated with the rest of the FGAs were similar to that of risperidone.
The results of these 2 large cohort studies are somewhat concurrent in indicating that, other than clozapine, SGAs incur similar risks of seizures; furthermore, they specify that, contrary to earlier studies,10 haloperidol is associated with significantly higher odds of seizures. While both of these cohort studies controlled for several sociodemographic and clinical confounders, they have several limitations. First, diagnoses of seizures were based on information available in databases, which might be subject to inaccuracies. Second, neither study evaluated the effect of drug dosage and duration of exposure on new-onset seizures.
Continue to: Most evidence is from case reports
Most evidence is from case reports
Other than these 2 large studies, most of the evidence addressing the relationship between the use of antipsychotics and incidence of seizures is low quality and relies on case reports or expert opinions. Older studies found that, among FGAs, seizure risk is highest with chlorpromazine and promazine, and lowest with thioridazine and haloperidol.10 As for SGAs, case reports have described seizuresassociated with the use of quetiapine, aripiprazole, risperidone, paliperidone, and olanzapine.
Quetiapine. Three case reports published between 2002 and 2010 describe generalized
Aripiprazole. Five case reports described staring spells and tonic-clonic seizures in patients receiving 10 to 15 mg of aripiprazole.15-19 In the New Drug Application (NDA) for aripiprazole, the incidence of seizures was estimated to be .11% (1 of 926 patients) in placebo-controlled trials and .46% (3 of 859 patients) in haloperidol-controlled trials.20
Risperidone’s product labeling suggests the drug should be used with caution in patients with a history of seizures or conditions that could result in a lower seizure threshold. In Phase III placebo-controlled trials, seizures occurred in .3% of patients treated with risperidone, although in some cases, the seizures were induced by electrolyte disturbances such as hyponatremia.21 Gonzalez-Heydrich et al22 and Holzhausen et al23 found no increase in seizure activity among patients with epilepsy who were receiving risperidone. Lane et al24 published a case report of a geriatric woman who presented with a generalized tonic-clonic seizure related to rapid titration of risperidone; however, with slower titration and lower doses, she stopped having seizures without adding any antiepileptic drugs. Komossa et al25 found that risperidone is less epileptogenic than clozapine, with a relative risk of .22.
Paliperidone is the active metabolite of risperidone and does not have pharmacokinetic interactions with drugs metabolized by the cytochrome P450 (CYP) enzymes. Its labeling indicates that the drug should be used with caution in patients with a history of seizures.26 In Phase III placebo-controlled trials of paliperidone, the rate of seizures was .22%.27 Two case reports suggest close monitoring of seizure risk in patients receiving paliperidone.28,29 Liang et al29 reported that co-administration of valproic acid could mask an underlying decrease of the seizure threshold caused by antipsychotics such as paliperidone.
Continue to: Olanzapine
Olanzapine is a thienobenzodiazepine derivative and is chemically related to clozapine.30 The olanzapine NDA31 shows that 23 of 3,139 patients developed seizures, mainly tonic-clonic, with evidence suggesting that the seizures may have been due to confounding factors such as a history of seizures or metabolic abnormalities. There were no statistically significant differences in the rate of seizures associated with olanzapine compared with placebo or haloperidol (P = .252 and .168, respectively).
A literature review for olanzapine yielded 1 case report of repetitive focal seizures and lingual dystonia,32 5 case reports of generalized tonic-clonic seizures and myoclonus,33-37 and 2 case reports of status epilepticus.38,39 Olanzapine’s clearance is 25% to 30% lower in women, and most of these case reports occurred women.40
Details of the above case reports are summarized in Table 1 (aripiprazole15-19), Table 2 (olanzapine32-39), and Table 3 (paliperidone,28,29 quetiapine,11-13 and risperidone22-24).
Ziprasidone. According to the NDA safety database, the seizure rate attributed to ziprasidone was 1.8 per 100 subject-years or 0.54% of participants (12 of 2,588).41 No additional studies have been published regarding its seizure risk.
Clozapine has a black-box warning
To the best of our knowledge, clozapine is the only antipsychotic that carries an FDA “black-box” warning regarding its risk of inducing seizures.42 Devinsky and Pacia43 reported a cumulative risk of 10% after 3.8 years of treatment. The literature has described clozapine-induced generalized tonic-clonic, myoclonic, simple and complex partial, and absence seizures.44 Table 445 lists the estimated frequency of each seizure type based on 101 cases of clozapine-induced seizures. Myoclonic seizures and drop attacks could be precursors/warning signs of grand mal tonic-clonic seizures.46,47 Seizures have been observed at all stages of treatment, but were more common during initiation of clozapine, which emphasizes the importance of a progressive and slow titration.43,48 The incidence of seizures was estimated to be 6% in a sample of 216 patients with schizophrenia with no history of epilepsy who were prescribed clozapine.49
Continue to: Regarding a possible association between...
Regarding a possible association between clozapine dose or clozapine plasma levels and seizure risk, there is a positive linear relationship between the dose of clozapine and its serum concentration over a dosing range of 25 to 800 mg/d.50 However, the plasma concentration is also significantly affected by factors such as smoking, gender, age, drug interactions, and CYP genotypes. Therefore, the same clozapine dose will yield a lower serum concentration in an older male who smokes compared with a younger, non-smoking female.51 Perry et al52 suggested a dosing nomogram to calculate the influence of gender and smoking. Seizure risk, especially for tonic-clonic seizures, has been reported to increase with clozapine doses >600 mg/d,53 and with plasma concentrations exceeding 1,000 to 1,300 mg/L.54 However, in a 2011 regression analysis, Varma et al55 found no statistically significant relationship between seizure risk and clozapine oral dose; there was not enough data to test a correlation between clozapine plasma levels and the incidence of seizures.
How antipsychotics might lower the seizure threshold
Researchers have suggested several possible mechanisms to explain how antipsychotics might lower the seizure threshold. Antagonism of dopamine D4, histamine H1, and acetylcholine-muscarinic receptors seems to induce EEG alterations and increase the risk of seizures.56 Additionally, modulation of the N-methyl-
Watch for pharmacokinetic interactions
The CYP enzymes involved in drug metabolism include CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. Most commonly used antiepileptics and antipsychotics are metabolized by CYP enzymes, and may also act as inhibitors or inducers of these enzymes.61 Drug interactions may impair seizure control, which is why monotherapy is preferable to combination treatment in patients with epilepsy.62 Carbamazepine and phenytoin are inducers of both CYP1A2 (which metabolizes olanzapine and clozapine), and CYP3A4 (which metabolizes haloperidol, risperidone, quetiapine, ziprasidone and clozapine). Paliperidone is not metabolized by CYP enzymes.62 Discontinuing an enzyme-inducing agent may result in increased antipsychotic plasma concentrations, which might lead to an increased risk of seizures.
Valproic acid, which is often used to prevent or treat clozapine-induced seizures, has an unclear effect on clozapine plasma concentrations.63 Although valproic acid is known to inhibit clozapine metabolism, 2 reports have suggested that the plasma concentrations of clozapine and its metabolites may decrease after adding valproic acid.64,65 Other studies have found that valproic acid increases plasma concentrations of clozapine while it decreases plasma concentrations of norclozapine; norclozapine is the main clozapine metabolite responsible for inducing seizures.66,67
Steps for minimizing seizure risk
Determining the seizure risk for a patient taking an antipsychotic is challenging because doing so depends not only on the seizurogenic potential of each drug but also on individualized predisposing factors.11,57,68 Choosing the “best” antipsychotic therefore largely depends on each patient’s profile. The predisposing factors consist mainly of the individually inherited seizure threshold (personal history of febrile convulsions or a family history of seizures) and other comorbid seizurogenic conditions, such as a history of head trauma, brain injury, intellectual disability, cerebral arteriosclerosis, neurodegenerative diseases, encephalopathy, chronic renal insufficiency, and hyponatremia. Furthermore, seizure risk depends on the antipsychotic dose administered and the rate of titration.11
Continue to: There is not enough evidence...
There is not enough evidence to recommend performing an EEG in all patients taking antipsychotics. Such testing is recommended only for patients who have predisposing factors for seizures. If an EEG shows any abnormality in a patient taking clozapine, consider decreasing the clozapine dose69,70 or adding an antiepileptic drug such as valproic acid or lamotrigine.44,70
Although clozapine carries a black-box warning of increased risk of causing seizures, there is no consensus regarding the efficacy of co-prescribing an antiepileptic. Some studies have suggested prescribing valproic acid prophylactically,71 after the occurrence of 1 seizure,59 or after 2 seizures.54,72 Others have recommended prescribing prophylactic valproic acid for patients taking ≥600 mg/d of clozapine or whose clozapine plasma levels are >500 mg/L.73 Varma et al55 recommended starting an antiepileptic medication if there are clear epileptiform discharges on EEG, if the patient develops stuttering or speech difficulties, or if seizures occur. Liukkonen et al72 advised initiating an antiepileptic at the start of clozapine treatment in patients who are taking other epileptogenic medications, patients with pre-existing seizure disorder, and patients with neurologic abnormalities. On the other hand, Caetano51 argued against primary prevention of seizures for patients receiving >600 mg/d of clozapine, suggesting that “the risk of seizures would be better managed by close clinical monitoring and measures of clozapine serum concentration rather than adding an anticonvulsant drug.”
Current recommendations for primary and secondary prevention of clozapine-induced seizures are detailed in Table 5.42,44,45,51,55,57,69,74,75
Studies addressing the seizurogenic potential of SGAs other than clozapine have a low level of evidence and include patients who had comorbid conditions and were taking other medications that could cause seizures. Additionally, clinical trials of SGAs rarely include patients with seizure disorders; this might underestimate the risk of seizures.4
The effect of the mental illness itself on the seizure threshold needs to be considered.43 Bloechlinger et al8 found that dementia might be inherently associated with a higher risk of antipsychotic-related seizures. Moreover, numerous qualitative EEG studies have found abnormalities in 20% to 60% of patients with schizophrenia.56 Other quantitative studies have reported mild and nonspecific EEG abnormalities, such as increased delta and/or theta activity, in many non-medicated patients with schizophrenia.10,76 Additionally, brain tissue analysis of deceased patients who had schizophrenia has shown a significant increase in dopamine concentrations in the left amygdala compared with controls, and this might be responsible for enhanced electrical activity in this region.10 Some studies have described EEG slowing in the frontal brain regions of patients with schizophrenia,77 and was selectively normalized in these areas with antipsychotics.78
As always, start low, go slow
Mounting evidence suggests that antipsychotic medications decrease the seizure threshold. Practitioners should thus be cautious in prescribing antipsychotics and should target reaching the minimal effective dose with slow titration, especially in patients with predisposing factors for epilepsy.
Continue to: Although evidence suggests...
Although evidence suggests antipsychotics can induce different types of epileptic seizures, the quality of this evidence is low. Randomized controlled trials are needed to determine which antipsychotics increase seizure risk and whether there is a dose-effect relationship.
Bottom Line
Among second-generation antipsychotics, clozapine appears to increase the risk of clinical seizure the most. Correlations with dosage and/or plasma levels have not been proven. Psychiatrists should be vigilant for pharmacokinetic interactions between antipsychotics and antiepileptics, notably via CYP1A2 and CYP3A4.
Related Resources
- Druschky K, Bleich S, Grohmann R, et al. Seizure rates under treatment with antipsychotic drugs: Data from the AMSP project. World J Biol Psychiatry. 2018;15:1-10.
- Epilepsy Foundation. For professionals: Antipsychotics. https://www.epilepsy.com/learn/professionals/diagnosistreatment/psychotropic-drugs-developmental-disabilities/comorbid-5.
Drug Brand Names
Aripiprazole • Abilify
Benztropine • Cogentin
Bethanechol • Duvoid
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Cimetidine • Tagamet
Ciprofloxacin • Cipro
Citalopram • Celexa
Clonazepam • Klonopin
Clozapine • Clozaril
Donepezil • Aricept
Enalapril • Vasotec
Erythromycin • Erythrocin
Escitalopram • Lexapro
Flunitrazepam • Rohypnol
Fluvoxamine • Luvox
Gabapentin • Neurontin
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Mirtazapine • Remeron
Nitrofurantoin • Furadantin
Olanzapine • Zyprexa
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Prochlorperazine • Compazine
Procyclidine • Kemadrin
Propranolol • Inderal
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Simvastatin • Zocor
Sulfamethoxazole/trimethoprim • Bactrim, Sulfatrim
Topiramate • Topamax
Trifluoperazine • Stelazine
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
1. Bruijnzeel D, Suryadevara U, Tandon R. Antipsychotic treatment of schizophrenia: an update. Asian J Psychiatr. 2014;11:3-7.
2. Hrdlicka M, Dudova I. Atypical antipsychotics in the treatment of early-onset schizophrenia. Neuropsychiatr Dis Treat. 2015;11:907-913.
3. Koch-Stoecker S. Antipsychotic drugs and epilepsy: indications and treatment guidelines. Epilepsia. 2002;43(suppl 2):19-24.
4. Alper K, Schwartz KA, Kolts RL, et al. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biol Psychiatry. 2007;62(4):345-354.
5. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 1999;40(suppl 10):S2-S20.
6. Kumlien E, Lundberg PO. Seizure risk associated with neuroactive drugs: data from the WHO adverse drug reactions database. Seizure. 2010;19(2):69-73.
7. Lertxundi U, Hernandez R, Medrano J, et al. Antipsychotics and seizures: higher risk with atypicals? Seizure. 2013;22(2):141-143.
8. Bloechliger M, Rüegg S, Jick SS, et al. Antipsychotic drug use and the risk of seizures: follow-up study with a nested case-control analysis. CNS Drugs. 2015;29(7):591-603.
9. Wu CS, Wang SC, Yeh IJ, et al. Comparative risk of seizure with use of first- and second-generation antipsychotics in patients with schizophrenia and mood disorders. J Clin Psychiatry. 2016;77(5):e573-e579.
10. Cold JA, Wells BG, Froemming JH. Seizure activity associated with antipsychotic therapy. [Erratum in DICP. 1990;24(10):1012.] DICP. 1990;24(6):601-606.
11. Hedges DW, Jeppson KG. New-onset seizure associated with quetiapine and olanzapine. Ann Pharmacother. 2002;36(3):437-439.
12. Dogu O, Sevim S, Kaleagasi HS. Seizures associated with quetiapine treatment. Ann Pharmacother. 2003;37(9):1224-1227.
13. Young AC, Kleinschmidt KC, Wax PM. Late-onset seizures associated with quetiapine poisoning. J Med Toxicol. 2009;5(1):24-26.
14. US Food and Drug Administration. Recommendation of approvable action for quetiapine fumarate extended release (Seroquel® XR) for the treatment of schizophrenia. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022047Orig1s000MedR.pdf. April 24, 2007. Accessed January 28, 2019.
15. Malik AR, Ravasia S. Aripiprazole-induced seizure. Can J Psychiatry. 2005;50(3):186.
16. Tsai JF. Aripiprazole-associated seizure. J Clin Psychiatry. 2006;67(6):995-996.
17. Arora M, Arndorfer L. EEG abnormalities in a patient taking aripiprazole. Psychiatry (Edgmont). 2007;4(7):18-19.
18. Yueh CL, Yu SL, Chen HM, et al. Aripiprazole-induced seizure: a second case report. BMJ case reports. 2009;2009:bcr03.2009.1693. doi: 10.1136/bcr.03.2009.1693.
19. Thabet FI, Sweis RT, Joseph SA. Aripiprazole-induced seizure in a 3-year-old child: a case report and literature review. Clin Neuropharmacol. 2013;36(1):29-30.
20. US Food and Drug Administration. Abilify (Aripiprazole) tablets. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-436_Abilify_medr_P2.pdf. Published March 07, 2003. Accessed January 28, 2019.
21. US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Risperdal tablets, Risperdal oral solution & Risperdal M-tab orally disintegrating tablets. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/021444_S004_RISPERDAL_TABLETS.pdf. Published September 10, 2003. Accessed January 28, 2019.
22. Gonzalez-Heydrich J, Pandina GJ, Fleisher CA, et al. No seizure exacerbation from risperidone in youth with comorbid epilepsy and psychiatric disorders: a case series. J Child Adolesc Psychopharmacol. 2004;14(2):295-310.
23. Holzhausen SPF, Guerreiro MM, Baccin CE, et al. Use of risperidone in children with epilepsy. Epilepsy Behav. 2007;10(3):412-416.
24. Lane HY, Chang WH, Chou JC. Seizure during risperidone treatment in an elderly woman treated with concomitant medications. J Clinl Psychiatry. 1998;59(2):81-82.
25. Komossa K, Rummel-Kluge C, Schwarz S, et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2011;(1):19:CD006626.
26. Paliperidone [package insert]. Mountainville, CA: Janssen Pharmaceuticals, Inc.; 2007.
27. Brugge, MD; US Food and Drug Administration. Paliperidone OROS oral formulation. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021999s000_MedR_Part4.pdf. Accessed January 28, 2019.
28. Schneider RA, Lizer MH. Apparent seizure and atrial fibrillation associated with paliperidone. Am J Health System Pharm. 2008;65(22):2122-2125.
29. Liang CS, Yang FW, Chiang KT. Paliperidone-associated seizure after discontinuation of sodium valproate: a case report. J Clin Psychopharmacol. 2011;31(2):246-247.
30. Fulton B, Goa KL. Olanzapine. A review of its pharmacological properties and therapeutic efficacy in the management of schizophrenia and related psychoses. Drugs. 1997;53(2):281-298.
31. US Food and Drug Administration. Drugs@FDA: FDA approved drug products: Zyprexa (olanzapine). ORIG-1. http://www.accessdata.fda.gov/drugsatfda_docs/nda/96/020592_Original_Approval_Pkg%20.pdf. Published September 30, 1996. Accessed January 28, 2019.
32. Anzellotti F, Capasso M, Frazzini V, et al. Olanzapine-related repetitive focal seizures with lingual dystonia. Epileptic Disord. 2016;18(1):83-86.
33. Lee JW, Crismon ML, Dorson PG. Seizure associated with olanzapine. Ann Pharmac. 1999;33(5):554-556.
34. Woolley J, Smith S. Lowered seizure threshold on olanzapine. Br J Psychiatry. 2001;178(1):85-86.
35. Behere RV, Anjith D, Rao NP, et al. Olanzapine-induced clinical seizure: a case report. Clin Neuropharmacol. 2009;32(5):297-298.
36. Camacho A, García-Navarro M, Martínez B, et al. Olanzapine-induced myoclonic status. Clin Neuropharmacol. 2005;28(3):145-147.
37. Rosen JB, Milstein MJ, Haut SR. Olanzapine-associated myoclonus. Epilepsy Res. 2012;98(2-3):247-250.
38. Wyderski RJ, Starrett WG, Abou-Saif A. Fatal status epilepticus associated with olanzapine therapy. Ann Pharmacother. 1999;33(7-8):787-789.
39. Spyridi S, Sokolaki S, Nimatoudis J, et al. Status epilepticus in a patient treated with olanzapine and mirtazapine. Int J Clin Pharmacol Ther. 2009;47(2):120-123.
40. Schatzberg AF, Nemeroff CB. Essentials of clinical psychopharmacology. 2nd ed. Arlington, Virginia: American Psychiatric Publishing; 2006.
41. US Food and Drug Administration. Drug approval package: Geodon (Ziprasidone HCI) Capsules. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/20-825_Geodan_medr_P2.pdf. Published February 5, 2001. Accessed January 29, 2019.
42. Clozaril [package insert]. East Hanover, NJ: Novartis; 2008.
43. Devinsky O, Pacia SV. Seizures during clozapine therapy. J Clin Psychiatry. 1994;55(suppl B):153-156.
44. Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29(2):101-111.
45. Wong J, Delva N. Clozapine-induced seizures: recognition and treatment. Can J Psychiatry. 2007;52(7):457-463.
46. Berman I, Zalma A, DuRand CJ, et al. Clozapine-induced myoclonic jerks and drop attacks. J Clin Psychiatry. 1992;53(9):329-330.
47. Gouzoulis E, Ozdaglar A, Kasper J. Myoclonic seizures followed by grand mal seizures during clozapine treatment. Am J Psychiatry. 1993;150(7):1128.
48. Sajatovic M, Meltzer HY. Clozapine-induced myoclonus and generalized seizures. Biol Psychiatry. 1996;39(5):367-370.
49. Grover S, Hazari N, Chakrabarti S, et al. Association of clozapine with seizures: a brief report involving 222 patients prescribed clozapine. East Asian Arch Psychiatry. 2015;25(2):73-78.
50. Byerly MJ, DeVane CL. Pharmacokinetics of clozapine and risperidone: a review of recent literature. J Clin Psychopharmacol. 1996;16(2):177-187.
51. Caetano D. Use of anticonvulsants as prophylaxis for seizures in patients on clozapine. Australas Psychiatry. 2014;22(1):78-83.
52. Perry PJ, Bever KA, Arndt S, et al. Relationship between patient variables and plasma clozapine concentrations: a dosing nomogram. Biol Psychiatry.1998;44(8):733-738.
53. Dumortier G, Mahé V, Pons D, et al. Clonic seizure associated with high clozapine plasma level. J Neuropsychiatry Clin Neurosci. 2001;13(2):302-303.
54. Funderburg LG, Vertrees JE, True JE, et al. Seizure following addition of erythromycin to clozapine treatment. Am J Psychiatry. 1994;151(12):1840-1841.
55. Varma S, Bishara D, Besag FMC, et al. Clozapine-related EEG changes and seizures: dose and plasma-level relationships. Ther Adv Psychopharmacol. 2011;1(2):47-66.
56. Amann BL, Pogarell O, Mergl R, et al. EEG abnormalities associated with antipsychotics: a comparison of quetiapine, olanzapine, haloperidol and healthy subjects. Hum Psychopharmacol. 2003;18(8):641-646.
57. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
58. Maurice T, Phan VL, Urani A, et al. Neuroactive neurosteroids as endogenous effectors for the sigma1 (sigma1) receptor: pharmacological evidence and therapeutic opportunities. Jpn J Pharmacol. 1999;81(2):125-155.
59. Haller E, Binder RL. Clozapine and seizures. Am J Psychiatry. 1990;147(8):1069-1071.
60. Torta R, Monaco F. Atypical antipsychotics and serotoninergic antidepressants in patients with epilepsy: pharmacodynamic considerations. Epilepsia. 2002;43(suppl 2):8-13.
61. Spina E. Drug interactions. In: Shorvon S, Perucca E, Engel J Jr, eds. The treatment of epilepsy. 3rd ed. Oxford, UK: Blackwell Publishing; 2009:361-377.
62. Spina E, Perucca E. Clinical significance of pharmacokinetic interactions between antiepileptic and psychotropic drugs. Epilepsia. 2002;43(suppl 2):37-44.
63. de Leon J, Santoro V, D’Arrigo C, et al. Interactions between antiepileptics and second-generation antipsychotics. Expert Opin Drug Metab Toxicol. 2012;8(3):311-334.
64. Finley P, Warner D. Potential impact of valproic acid therapy on clozapine disposition. Biol Psychiatry. 1994;36(7):487-488.
65. Longo LP, Salzman C. Valproic acid effects on serum concentrations of clozapine and norclozapine. Am J Psychiatry. 1995;152(4):650.
66. Centorrino F, Baldessarini RJ, Kando J, et al. Serum concentrations of clozapine and its major metabolites: effects of cotreatment with fluoxetine or valproate. Am J Psychiatry. 1994;151(1):123-125.
67. Facciolà G, Avenoso A, Scordo MG, et al. Small effects of valproic acid on the plasma concentrations of clozapine and its major metabolites in patients with schizophrenic or affective disorders. Ther Drug Monit. 1999;21(3):341-345.
68. Hyde TM, Weinberger DR. Seizures and schizophrenia. Schizophr Bull. 1997;23(4):611-622.
69. Muzyk A, Gala G, Kahn DA. Use of lamotrigine in a patient with a clozapine-related seizure. J Psychiatr Pract. 2010;16(2):125-128.
70. Kikuchi YS, Sato W, Ataka K, et al. Clozapine-induced seizures, electroencephalography abnormalities, and clinical responses in Japanese patients with schizophrenia. Neuropsychiatr Dis Treat. 2014;10:1973-1978.
71. Taner E, Coşar B, Işik E. Clozapine-induced myoclonic seizures and valproic acid. Int J Psychiatry Clin Pract. 1998;2(1):53-55.
72. Liukkonen J, Koponen HJ, Nousiainen U. Clinical picture and long-term course of epileptic seizures that occur during clozapine treatment. Psychiatry Res. 1992;44(2):107-112.
73. Devinsky O, Honigfeld G, Patin J. Clozapine-related seizures. Neurology. 1991;41(3):369-371.
74. Foster R, Olajide D. A case of clozapine-induced tonic-clonic seizures managed with valproate: implications for clinical care. J Psychopharmacol. 2005;19(1):93-96.
75. Gandelman-Marton R, Theitler J, Klein C, et al. Phenytoin intoxication in a clozapine-related prolonged seizure. J Emerg Med. 2008;35(4):407-409.
76. Primavera A, Giberti L, Scotto P, et al. Nonconvulsive status epilepticus as a cause of confusion in later life: a report of 5 cases. Neuropsychobiology. 1994;30(2-3):148-152.
77. Boutros NN, Arfken C, Galderisi S, et al. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophrenia Res. 2008;99(1-3):225-237.
78. Takahashi T, Cho RY, Mizuno T, et al. Antipsychotics reverse abnormal EEG complexity in drug-naïve schizophrenia: a multiscale entropy analysis. Neuroimage. 2010;51(1):173-182.
Antipsychotics, especially second-generation antipsychotics (SGAs), have been proven effective for treating psychosis as well as mood disorders.1,2 Because antipsychotics can lower the epileptogenic threshold, seizures are a serious potential adverse effect. Antipsychotics can cause isolated EEG abnormalities in 7% of patients with no history of epilepsy, and clinical seizures in .5% to 1.2% of such patients.3 Additionally, the neuropathophysiology underlying epilepsy can predispose patients to psychiatric disorders4; the estimated prevalence of psychosis in patients with epilepsy is approximately 7%.5 This review will shed light on the risk of clinical seizures related to antipsychotics.
Comparing seizure risk among antipsychotics
In a review of the World Health Organization’s adverse drug reactions database, Kumlien and Lundberg6 calculated the ratio of the number of reports of seizures to the total number of reports for each drug. They found that approximately 9% of all adverse drug reaction reports involving clozapine were due to seizures. Equivalent ratios were 5.90% for quetiapine, 4.91% for olanzapine, 3.68% for risperidone, 3.27% for haloperidol, and 2.59% for aripiprazole. Using the database of the Pharmacovigilance Unit of the Basque Country, Lertxundi et al7 reported a 3.2-fold increased risk of seizure with SGAs in comparison with first-generation antipsychotics (FGAs) (95% confidence interval [CI], 2.21 to 4.63), which went down to 2.08 (CI, 1.39 to 3.12) once clozapine was excluded. However, as the authors of both studies noted, the quality and relevance of this data are limited because it relies on spontaneous reporting.
Overall, the evidence regarding the seizure risk associated with antipsychotics is scarce. To the best of our knowledge, only 2 large observational studies have compared the seizure risks associated with different antipsychotics.
Using data from the UK-based Clinical Practice Research Datalink between 1998 and 2013, Bloechlinger et al8 examined the incidence rates of seizures among patients newly diagnosed with schizophrenia, affective disorders, or dementia who were prescribed antipsychotics. They excluded patients with a history of seizures or antiepileptic use. In the cohort of 60,121 patients, the incidence rates of seizures per 10,000 person-years were 11.7 (CI, 10.0 to 13.4) for those who did not use antipsychotics, 12.4 (CI, 10.9 to 13.8) for past users, 115.4 (CI, 50.1 to 180.7) for current users of haloperidol, 48.8 (CI, 30.7 to 66.9) for current users of quetiapine, 25.9 (CI, 11.8 to 40.0) for current users of risperidone, and 19.0 (CI, 8.7 to 29.3) for current users of olanzapine. No data were available about clozapine use.
In subsequent analyses, the authors found that among patients with affective disorders, only current use of medium- to high-potency FGAs (haloperidol, prochlorperazine, and trifluoperazine) was associated with a significantly increased risk of seizures (adjusted odds ratio: 2.51, CI, 1.51 to 4.18) compared with non-users.8 Among patients with dementia, current use of olanzapine or quetiapine and current use of any FGAs were associated with significantly increased odds of seizures. This study suggests that the underlying mental illness might modulate the seizure risk associated with antipsychotics.8
Wu et al9 conducted a study based on the National Health Insurance Research Database in Taiwan. They examined the 1-year incidence of new-onset seizures among patients diagnosed with schizophrenia or mood disorders who were new to antipsychotic treatment, and calculated the risk of seizure associated with each antipsychotic in reference to risperidone. They found that those receiving clozapine, thioridazine, and haloperidol were 2 to 3 times more likely to develop seizures than those treated with risperidone; risks associated with the rest of the FGAs were similar to that of risperidone.
The results of these 2 large cohort studies are somewhat concurrent in indicating that, other than clozapine, SGAs incur similar risks of seizures; furthermore, they specify that, contrary to earlier studies,10 haloperidol is associated with significantly higher odds of seizures. While both of these cohort studies controlled for several sociodemographic and clinical confounders, they have several limitations. First, diagnoses of seizures were based on information available in databases, which might be subject to inaccuracies. Second, neither study evaluated the effect of drug dosage and duration of exposure on new-onset seizures.
Continue to: Most evidence is from case reports
Most evidence is from case reports
Other than these 2 large studies, most of the evidence addressing the relationship between the use of antipsychotics and incidence of seizures is low quality and relies on case reports or expert opinions. Older studies found that, among FGAs, seizure risk is highest with chlorpromazine and promazine, and lowest with thioridazine and haloperidol.10 As for SGAs, case reports have described seizuresassociated with the use of quetiapine, aripiprazole, risperidone, paliperidone, and olanzapine.
Quetiapine. Three case reports published between 2002 and 2010 describe generalized
Aripiprazole. Five case reports described staring spells and tonic-clonic seizures in patients receiving 10 to 15 mg of aripiprazole.15-19 In the New Drug Application (NDA) for aripiprazole, the incidence of seizures was estimated to be .11% (1 of 926 patients) in placebo-controlled trials and .46% (3 of 859 patients) in haloperidol-controlled trials.20
Risperidone’s product labeling suggests the drug should be used with caution in patients with a history of seizures or conditions that could result in a lower seizure threshold. In Phase III placebo-controlled trials, seizures occurred in .3% of patients treated with risperidone, although in some cases, the seizures were induced by electrolyte disturbances such as hyponatremia.21 Gonzalez-Heydrich et al22 and Holzhausen et al23 found no increase in seizure activity among patients with epilepsy who were receiving risperidone. Lane et al24 published a case report of a geriatric woman who presented with a generalized tonic-clonic seizure related to rapid titration of risperidone; however, with slower titration and lower doses, she stopped having seizures without adding any antiepileptic drugs. Komossa et al25 found that risperidone is less epileptogenic than clozapine, with a relative risk of .22.
Paliperidone is the active metabolite of risperidone and does not have pharmacokinetic interactions with drugs metabolized by the cytochrome P450 (CYP) enzymes. Its labeling indicates that the drug should be used with caution in patients with a history of seizures.26 In Phase III placebo-controlled trials of paliperidone, the rate of seizures was .22%.27 Two case reports suggest close monitoring of seizure risk in patients receiving paliperidone.28,29 Liang et al29 reported that co-administration of valproic acid could mask an underlying decrease of the seizure threshold caused by antipsychotics such as paliperidone.
Continue to: Olanzapine
Olanzapine is a thienobenzodiazepine derivative and is chemically related to clozapine.30 The olanzapine NDA31 shows that 23 of 3,139 patients developed seizures, mainly tonic-clonic, with evidence suggesting that the seizures may have been due to confounding factors such as a history of seizures or metabolic abnormalities. There were no statistically significant differences in the rate of seizures associated with olanzapine compared with placebo or haloperidol (P = .252 and .168, respectively).
A literature review for olanzapine yielded 1 case report of repetitive focal seizures and lingual dystonia,32 5 case reports of generalized tonic-clonic seizures and myoclonus,33-37 and 2 case reports of status epilepticus.38,39 Olanzapine’s clearance is 25% to 30% lower in women, and most of these case reports occurred women.40
Details of the above case reports are summarized in Table 1 (aripiprazole15-19), Table 2 (olanzapine32-39), and Table 3 (paliperidone,28,29 quetiapine,11-13 and risperidone22-24).
Ziprasidone. According to the NDA safety database, the seizure rate attributed to ziprasidone was 1.8 per 100 subject-years or 0.54% of participants (12 of 2,588).41 No additional studies have been published regarding its seizure risk.
Clozapine has a black-box warning
To the best of our knowledge, clozapine is the only antipsychotic that carries an FDA “black-box” warning regarding its risk of inducing seizures.42 Devinsky and Pacia43 reported a cumulative risk of 10% after 3.8 years of treatment. The literature has described clozapine-induced generalized tonic-clonic, myoclonic, simple and complex partial, and absence seizures.44 Table 445 lists the estimated frequency of each seizure type based on 101 cases of clozapine-induced seizures. Myoclonic seizures and drop attacks could be precursors/warning signs of grand mal tonic-clonic seizures.46,47 Seizures have been observed at all stages of treatment, but were more common during initiation of clozapine, which emphasizes the importance of a progressive and slow titration.43,48 The incidence of seizures was estimated to be 6% in a sample of 216 patients with schizophrenia with no history of epilepsy who were prescribed clozapine.49
Continue to: Regarding a possible association between...
Regarding a possible association between clozapine dose or clozapine plasma levels and seizure risk, there is a positive linear relationship between the dose of clozapine and its serum concentration over a dosing range of 25 to 800 mg/d.50 However, the plasma concentration is also significantly affected by factors such as smoking, gender, age, drug interactions, and CYP genotypes. Therefore, the same clozapine dose will yield a lower serum concentration in an older male who smokes compared with a younger, non-smoking female.51 Perry et al52 suggested a dosing nomogram to calculate the influence of gender and smoking. Seizure risk, especially for tonic-clonic seizures, has been reported to increase with clozapine doses >600 mg/d,53 and with plasma concentrations exceeding 1,000 to 1,300 mg/L.54 However, in a 2011 regression analysis, Varma et al55 found no statistically significant relationship between seizure risk and clozapine oral dose; there was not enough data to test a correlation between clozapine plasma levels and the incidence of seizures.
How antipsychotics might lower the seizure threshold
Researchers have suggested several possible mechanisms to explain how antipsychotics might lower the seizure threshold. Antagonism of dopamine D4, histamine H1, and acetylcholine-muscarinic receptors seems to induce EEG alterations and increase the risk of seizures.56 Additionally, modulation of the N-methyl-
Watch for pharmacokinetic interactions
The CYP enzymes involved in drug metabolism include CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. Most commonly used antiepileptics and antipsychotics are metabolized by CYP enzymes, and may also act as inhibitors or inducers of these enzymes.61 Drug interactions may impair seizure control, which is why monotherapy is preferable to combination treatment in patients with epilepsy.62 Carbamazepine and phenytoin are inducers of both CYP1A2 (which metabolizes olanzapine and clozapine), and CYP3A4 (which metabolizes haloperidol, risperidone, quetiapine, ziprasidone and clozapine). Paliperidone is not metabolized by CYP enzymes.62 Discontinuing an enzyme-inducing agent may result in increased antipsychotic plasma concentrations, which might lead to an increased risk of seizures.
Valproic acid, which is often used to prevent or treat clozapine-induced seizures, has an unclear effect on clozapine plasma concentrations.63 Although valproic acid is known to inhibit clozapine metabolism, 2 reports have suggested that the plasma concentrations of clozapine and its metabolites may decrease after adding valproic acid.64,65 Other studies have found that valproic acid increases plasma concentrations of clozapine while it decreases plasma concentrations of norclozapine; norclozapine is the main clozapine metabolite responsible for inducing seizures.66,67
Steps for minimizing seizure risk
Determining the seizure risk for a patient taking an antipsychotic is challenging because doing so depends not only on the seizurogenic potential of each drug but also on individualized predisposing factors.11,57,68 Choosing the “best” antipsychotic therefore largely depends on each patient’s profile. The predisposing factors consist mainly of the individually inherited seizure threshold (personal history of febrile convulsions or a family history of seizures) and other comorbid seizurogenic conditions, such as a history of head trauma, brain injury, intellectual disability, cerebral arteriosclerosis, neurodegenerative diseases, encephalopathy, chronic renal insufficiency, and hyponatremia. Furthermore, seizure risk depends on the antipsychotic dose administered and the rate of titration.11
Continue to: There is not enough evidence...
There is not enough evidence to recommend performing an EEG in all patients taking antipsychotics. Such testing is recommended only for patients who have predisposing factors for seizures. If an EEG shows any abnormality in a patient taking clozapine, consider decreasing the clozapine dose69,70 or adding an antiepileptic drug such as valproic acid or lamotrigine.44,70
Although clozapine carries a black-box warning of increased risk of causing seizures, there is no consensus regarding the efficacy of co-prescribing an antiepileptic. Some studies have suggested prescribing valproic acid prophylactically,71 after the occurrence of 1 seizure,59 or after 2 seizures.54,72 Others have recommended prescribing prophylactic valproic acid for patients taking ≥600 mg/d of clozapine or whose clozapine plasma levels are >500 mg/L.73 Varma et al55 recommended starting an antiepileptic medication if there are clear epileptiform discharges on EEG, if the patient develops stuttering or speech difficulties, or if seizures occur. Liukkonen et al72 advised initiating an antiepileptic at the start of clozapine treatment in patients who are taking other epileptogenic medications, patients with pre-existing seizure disorder, and patients with neurologic abnormalities. On the other hand, Caetano51 argued against primary prevention of seizures for patients receiving >600 mg/d of clozapine, suggesting that “the risk of seizures would be better managed by close clinical monitoring and measures of clozapine serum concentration rather than adding an anticonvulsant drug.”
Current recommendations for primary and secondary prevention of clozapine-induced seizures are detailed in Table 5.42,44,45,51,55,57,69,74,75
Studies addressing the seizurogenic potential of SGAs other than clozapine have a low level of evidence and include patients who had comorbid conditions and were taking other medications that could cause seizures. Additionally, clinical trials of SGAs rarely include patients with seizure disorders; this might underestimate the risk of seizures.4
The effect of the mental illness itself on the seizure threshold needs to be considered.43 Bloechlinger et al8 found that dementia might be inherently associated with a higher risk of antipsychotic-related seizures. Moreover, numerous qualitative EEG studies have found abnormalities in 20% to 60% of patients with schizophrenia.56 Other quantitative studies have reported mild and nonspecific EEG abnormalities, such as increased delta and/or theta activity, in many non-medicated patients with schizophrenia.10,76 Additionally, brain tissue analysis of deceased patients who had schizophrenia has shown a significant increase in dopamine concentrations in the left amygdala compared with controls, and this might be responsible for enhanced electrical activity in this region.10 Some studies have described EEG slowing in the frontal brain regions of patients with schizophrenia,77 and was selectively normalized in these areas with antipsychotics.78
As always, start low, go slow
Mounting evidence suggests that antipsychotic medications decrease the seizure threshold. Practitioners should thus be cautious in prescribing antipsychotics and should target reaching the minimal effective dose with slow titration, especially in patients with predisposing factors for epilepsy.
Continue to: Although evidence suggests...
Although evidence suggests antipsychotics can induce different types of epileptic seizures, the quality of this evidence is low. Randomized controlled trials are needed to determine which antipsychotics increase seizure risk and whether there is a dose-effect relationship.
Bottom Line
Among second-generation antipsychotics, clozapine appears to increase the risk of clinical seizure the most. Correlations with dosage and/or plasma levels have not been proven. Psychiatrists should be vigilant for pharmacokinetic interactions between antipsychotics and antiepileptics, notably via CYP1A2 and CYP3A4.
Related Resources
- Druschky K, Bleich S, Grohmann R, et al. Seizure rates under treatment with antipsychotic drugs: Data from the AMSP project. World J Biol Psychiatry. 2018;15:1-10.
- Epilepsy Foundation. For professionals: Antipsychotics. https://www.epilepsy.com/learn/professionals/diagnosistreatment/psychotropic-drugs-developmental-disabilities/comorbid-5.
Drug Brand Names
Aripiprazole • Abilify
Benztropine • Cogentin
Bethanechol • Duvoid
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Cimetidine • Tagamet
Ciprofloxacin • Cipro
Citalopram • Celexa
Clonazepam • Klonopin
Clozapine • Clozaril
Donepezil • Aricept
Enalapril • Vasotec
Erythromycin • Erythrocin
Escitalopram • Lexapro
Flunitrazepam • Rohypnol
Fluvoxamine • Luvox
Gabapentin • Neurontin
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Mirtazapine • Remeron
Nitrofurantoin • Furadantin
Olanzapine • Zyprexa
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Prochlorperazine • Compazine
Procyclidine • Kemadrin
Propranolol • Inderal
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Simvastatin • Zocor
Sulfamethoxazole/trimethoprim • Bactrim, Sulfatrim
Topiramate • Topamax
Trifluoperazine • Stelazine
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
Antipsychotics, especially second-generation antipsychotics (SGAs), have been proven effective for treating psychosis as well as mood disorders.1,2 Because antipsychotics can lower the epileptogenic threshold, seizures are a serious potential adverse effect. Antipsychotics can cause isolated EEG abnormalities in 7% of patients with no history of epilepsy, and clinical seizures in .5% to 1.2% of such patients.3 Additionally, the neuropathophysiology underlying epilepsy can predispose patients to psychiatric disorders4; the estimated prevalence of psychosis in patients with epilepsy is approximately 7%.5 This review will shed light on the risk of clinical seizures related to antipsychotics.
Comparing seizure risk among antipsychotics
In a review of the World Health Organization’s adverse drug reactions database, Kumlien and Lundberg6 calculated the ratio of the number of reports of seizures to the total number of reports for each drug. They found that approximately 9% of all adverse drug reaction reports involving clozapine were due to seizures. Equivalent ratios were 5.90% for quetiapine, 4.91% for olanzapine, 3.68% for risperidone, 3.27% for haloperidol, and 2.59% for aripiprazole. Using the database of the Pharmacovigilance Unit of the Basque Country, Lertxundi et al7 reported a 3.2-fold increased risk of seizure with SGAs in comparison with first-generation antipsychotics (FGAs) (95% confidence interval [CI], 2.21 to 4.63), which went down to 2.08 (CI, 1.39 to 3.12) once clozapine was excluded. However, as the authors of both studies noted, the quality and relevance of this data are limited because it relies on spontaneous reporting.
Overall, the evidence regarding the seizure risk associated with antipsychotics is scarce. To the best of our knowledge, only 2 large observational studies have compared the seizure risks associated with different antipsychotics.
Using data from the UK-based Clinical Practice Research Datalink between 1998 and 2013, Bloechlinger et al8 examined the incidence rates of seizures among patients newly diagnosed with schizophrenia, affective disorders, or dementia who were prescribed antipsychotics. They excluded patients with a history of seizures or antiepileptic use. In the cohort of 60,121 patients, the incidence rates of seizures per 10,000 person-years were 11.7 (CI, 10.0 to 13.4) for those who did not use antipsychotics, 12.4 (CI, 10.9 to 13.8) for past users, 115.4 (CI, 50.1 to 180.7) for current users of haloperidol, 48.8 (CI, 30.7 to 66.9) for current users of quetiapine, 25.9 (CI, 11.8 to 40.0) for current users of risperidone, and 19.0 (CI, 8.7 to 29.3) for current users of olanzapine. No data were available about clozapine use.
In subsequent analyses, the authors found that among patients with affective disorders, only current use of medium- to high-potency FGAs (haloperidol, prochlorperazine, and trifluoperazine) was associated with a significantly increased risk of seizures (adjusted odds ratio: 2.51, CI, 1.51 to 4.18) compared with non-users.8 Among patients with dementia, current use of olanzapine or quetiapine and current use of any FGAs were associated with significantly increased odds of seizures. This study suggests that the underlying mental illness might modulate the seizure risk associated with antipsychotics.8
Wu et al9 conducted a study based on the National Health Insurance Research Database in Taiwan. They examined the 1-year incidence of new-onset seizures among patients diagnosed with schizophrenia or mood disorders who were new to antipsychotic treatment, and calculated the risk of seizure associated with each antipsychotic in reference to risperidone. They found that those receiving clozapine, thioridazine, and haloperidol were 2 to 3 times more likely to develop seizures than those treated with risperidone; risks associated with the rest of the FGAs were similar to that of risperidone.
The results of these 2 large cohort studies are somewhat concurrent in indicating that, other than clozapine, SGAs incur similar risks of seizures; furthermore, they specify that, contrary to earlier studies,10 haloperidol is associated with significantly higher odds of seizures. While both of these cohort studies controlled for several sociodemographic and clinical confounders, they have several limitations. First, diagnoses of seizures were based on information available in databases, which might be subject to inaccuracies. Second, neither study evaluated the effect of drug dosage and duration of exposure on new-onset seizures.
Continue to: Most evidence is from case reports
Most evidence is from case reports
Other than these 2 large studies, most of the evidence addressing the relationship between the use of antipsychotics and incidence of seizures is low quality and relies on case reports or expert opinions. Older studies found that, among FGAs, seizure risk is highest with chlorpromazine and promazine, and lowest with thioridazine and haloperidol.10 As for SGAs, case reports have described seizuresassociated with the use of quetiapine, aripiprazole, risperidone, paliperidone, and olanzapine.
Quetiapine. Three case reports published between 2002 and 2010 describe generalized
Aripiprazole. Five case reports described staring spells and tonic-clonic seizures in patients receiving 10 to 15 mg of aripiprazole.15-19 In the New Drug Application (NDA) for aripiprazole, the incidence of seizures was estimated to be .11% (1 of 926 patients) in placebo-controlled trials and .46% (3 of 859 patients) in haloperidol-controlled trials.20
Risperidone’s product labeling suggests the drug should be used with caution in patients with a history of seizures or conditions that could result in a lower seizure threshold. In Phase III placebo-controlled trials, seizures occurred in .3% of patients treated with risperidone, although in some cases, the seizures were induced by electrolyte disturbances such as hyponatremia.21 Gonzalez-Heydrich et al22 and Holzhausen et al23 found no increase in seizure activity among patients with epilepsy who were receiving risperidone. Lane et al24 published a case report of a geriatric woman who presented with a generalized tonic-clonic seizure related to rapid titration of risperidone; however, with slower titration and lower doses, she stopped having seizures without adding any antiepileptic drugs. Komossa et al25 found that risperidone is less epileptogenic than clozapine, with a relative risk of .22.
Paliperidone is the active metabolite of risperidone and does not have pharmacokinetic interactions with drugs metabolized by the cytochrome P450 (CYP) enzymes. Its labeling indicates that the drug should be used with caution in patients with a history of seizures.26 In Phase III placebo-controlled trials of paliperidone, the rate of seizures was .22%.27 Two case reports suggest close monitoring of seizure risk in patients receiving paliperidone.28,29 Liang et al29 reported that co-administration of valproic acid could mask an underlying decrease of the seizure threshold caused by antipsychotics such as paliperidone.
Continue to: Olanzapine
Olanzapine is a thienobenzodiazepine derivative and is chemically related to clozapine.30 The olanzapine NDA31 shows that 23 of 3,139 patients developed seizures, mainly tonic-clonic, with evidence suggesting that the seizures may have been due to confounding factors such as a history of seizures or metabolic abnormalities. There were no statistically significant differences in the rate of seizures associated with olanzapine compared with placebo or haloperidol (P = .252 and .168, respectively).
A literature review for olanzapine yielded 1 case report of repetitive focal seizures and lingual dystonia,32 5 case reports of generalized tonic-clonic seizures and myoclonus,33-37 and 2 case reports of status epilepticus.38,39 Olanzapine’s clearance is 25% to 30% lower in women, and most of these case reports occurred women.40
Details of the above case reports are summarized in Table 1 (aripiprazole15-19), Table 2 (olanzapine32-39), and Table 3 (paliperidone,28,29 quetiapine,11-13 and risperidone22-24).
Ziprasidone. According to the NDA safety database, the seizure rate attributed to ziprasidone was 1.8 per 100 subject-years or 0.54% of participants (12 of 2,588).41 No additional studies have been published regarding its seizure risk.
Clozapine has a black-box warning
To the best of our knowledge, clozapine is the only antipsychotic that carries an FDA “black-box” warning regarding its risk of inducing seizures.42 Devinsky and Pacia43 reported a cumulative risk of 10% after 3.8 years of treatment. The literature has described clozapine-induced generalized tonic-clonic, myoclonic, simple and complex partial, and absence seizures.44 Table 445 lists the estimated frequency of each seizure type based on 101 cases of clozapine-induced seizures. Myoclonic seizures and drop attacks could be precursors/warning signs of grand mal tonic-clonic seizures.46,47 Seizures have been observed at all stages of treatment, but were more common during initiation of clozapine, which emphasizes the importance of a progressive and slow titration.43,48 The incidence of seizures was estimated to be 6% in a sample of 216 patients with schizophrenia with no history of epilepsy who were prescribed clozapine.49
Continue to: Regarding a possible association between...
Regarding a possible association between clozapine dose or clozapine plasma levels and seizure risk, there is a positive linear relationship between the dose of clozapine and its serum concentration over a dosing range of 25 to 800 mg/d.50 However, the plasma concentration is also significantly affected by factors such as smoking, gender, age, drug interactions, and CYP genotypes. Therefore, the same clozapine dose will yield a lower serum concentration in an older male who smokes compared with a younger, non-smoking female.51 Perry et al52 suggested a dosing nomogram to calculate the influence of gender and smoking. Seizure risk, especially for tonic-clonic seizures, has been reported to increase with clozapine doses >600 mg/d,53 and with plasma concentrations exceeding 1,000 to 1,300 mg/L.54 However, in a 2011 regression analysis, Varma et al55 found no statistically significant relationship between seizure risk and clozapine oral dose; there was not enough data to test a correlation between clozapine plasma levels and the incidence of seizures.
How antipsychotics might lower the seizure threshold
Researchers have suggested several possible mechanisms to explain how antipsychotics might lower the seizure threshold. Antagonism of dopamine D4, histamine H1, and acetylcholine-muscarinic receptors seems to induce EEG alterations and increase the risk of seizures.56 Additionally, modulation of the N-methyl-
Watch for pharmacokinetic interactions
The CYP enzymes involved in drug metabolism include CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. Most commonly used antiepileptics and antipsychotics are metabolized by CYP enzymes, and may also act as inhibitors or inducers of these enzymes.61 Drug interactions may impair seizure control, which is why monotherapy is preferable to combination treatment in patients with epilepsy.62 Carbamazepine and phenytoin are inducers of both CYP1A2 (which metabolizes olanzapine and clozapine), and CYP3A4 (which metabolizes haloperidol, risperidone, quetiapine, ziprasidone and clozapine). Paliperidone is not metabolized by CYP enzymes.62 Discontinuing an enzyme-inducing agent may result in increased antipsychotic plasma concentrations, which might lead to an increased risk of seizures.
Valproic acid, which is often used to prevent or treat clozapine-induced seizures, has an unclear effect on clozapine plasma concentrations.63 Although valproic acid is known to inhibit clozapine metabolism, 2 reports have suggested that the plasma concentrations of clozapine and its metabolites may decrease after adding valproic acid.64,65 Other studies have found that valproic acid increases plasma concentrations of clozapine while it decreases plasma concentrations of norclozapine; norclozapine is the main clozapine metabolite responsible for inducing seizures.66,67
Steps for minimizing seizure risk
Determining the seizure risk for a patient taking an antipsychotic is challenging because doing so depends not only on the seizurogenic potential of each drug but also on individualized predisposing factors.11,57,68 Choosing the “best” antipsychotic therefore largely depends on each patient’s profile. The predisposing factors consist mainly of the individually inherited seizure threshold (personal history of febrile convulsions or a family history of seizures) and other comorbid seizurogenic conditions, such as a history of head trauma, brain injury, intellectual disability, cerebral arteriosclerosis, neurodegenerative diseases, encephalopathy, chronic renal insufficiency, and hyponatremia. Furthermore, seizure risk depends on the antipsychotic dose administered and the rate of titration.11
Continue to: There is not enough evidence...
There is not enough evidence to recommend performing an EEG in all patients taking antipsychotics. Such testing is recommended only for patients who have predisposing factors for seizures. If an EEG shows any abnormality in a patient taking clozapine, consider decreasing the clozapine dose69,70 or adding an antiepileptic drug such as valproic acid or lamotrigine.44,70
Although clozapine carries a black-box warning of increased risk of causing seizures, there is no consensus regarding the efficacy of co-prescribing an antiepileptic. Some studies have suggested prescribing valproic acid prophylactically,71 after the occurrence of 1 seizure,59 or after 2 seizures.54,72 Others have recommended prescribing prophylactic valproic acid for patients taking ≥600 mg/d of clozapine or whose clozapine plasma levels are >500 mg/L.73 Varma et al55 recommended starting an antiepileptic medication if there are clear epileptiform discharges on EEG, if the patient develops stuttering or speech difficulties, or if seizures occur. Liukkonen et al72 advised initiating an antiepileptic at the start of clozapine treatment in patients who are taking other epileptogenic medications, patients with pre-existing seizure disorder, and patients with neurologic abnormalities. On the other hand, Caetano51 argued against primary prevention of seizures for patients receiving >600 mg/d of clozapine, suggesting that “the risk of seizures would be better managed by close clinical monitoring and measures of clozapine serum concentration rather than adding an anticonvulsant drug.”
Current recommendations for primary and secondary prevention of clozapine-induced seizures are detailed in Table 5.42,44,45,51,55,57,69,74,75
Studies addressing the seizurogenic potential of SGAs other than clozapine have a low level of evidence and include patients who had comorbid conditions and were taking other medications that could cause seizures. Additionally, clinical trials of SGAs rarely include patients with seizure disorders; this might underestimate the risk of seizures.4
The effect of the mental illness itself on the seizure threshold needs to be considered.43 Bloechlinger et al8 found that dementia might be inherently associated with a higher risk of antipsychotic-related seizures. Moreover, numerous qualitative EEG studies have found abnormalities in 20% to 60% of patients with schizophrenia.56 Other quantitative studies have reported mild and nonspecific EEG abnormalities, such as increased delta and/or theta activity, in many non-medicated patients with schizophrenia.10,76 Additionally, brain tissue analysis of deceased patients who had schizophrenia has shown a significant increase in dopamine concentrations in the left amygdala compared with controls, and this might be responsible for enhanced electrical activity in this region.10 Some studies have described EEG slowing in the frontal brain regions of patients with schizophrenia,77 and was selectively normalized in these areas with antipsychotics.78
As always, start low, go slow
Mounting evidence suggests that antipsychotic medications decrease the seizure threshold. Practitioners should thus be cautious in prescribing antipsychotics and should target reaching the minimal effective dose with slow titration, especially in patients with predisposing factors for epilepsy.
Continue to: Although evidence suggests...
Although evidence suggests antipsychotics can induce different types of epileptic seizures, the quality of this evidence is low. Randomized controlled trials are needed to determine which antipsychotics increase seizure risk and whether there is a dose-effect relationship.
Bottom Line
Among second-generation antipsychotics, clozapine appears to increase the risk of clinical seizure the most. Correlations with dosage and/or plasma levels have not been proven. Psychiatrists should be vigilant for pharmacokinetic interactions between antipsychotics and antiepileptics, notably via CYP1A2 and CYP3A4.
Related Resources
- Druschky K, Bleich S, Grohmann R, et al. Seizure rates under treatment with antipsychotic drugs: Data from the AMSP project. World J Biol Psychiatry. 2018;15:1-10.
- Epilepsy Foundation. For professionals: Antipsychotics. https://www.epilepsy.com/learn/professionals/diagnosistreatment/psychotropic-drugs-developmental-disabilities/comorbid-5.
Drug Brand Names
Aripiprazole • Abilify
Benztropine • Cogentin
Bethanechol • Duvoid
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Cimetidine • Tagamet
Ciprofloxacin • Cipro
Citalopram • Celexa
Clonazepam • Klonopin
Clozapine • Clozaril
Donepezil • Aricept
Enalapril • Vasotec
Erythromycin • Erythrocin
Escitalopram • Lexapro
Flunitrazepam • Rohypnol
Fluvoxamine • Luvox
Gabapentin • Neurontin
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Mirtazapine • Remeron
Nitrofurantoin • Furadantin
Olanzapine • Zyprexa
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Prochlorperazine • Compazine
Procyclidine • Kemadrin
Propranolol • Inderal
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Simvastatin • Zocor
Sulfamethoxazole/trimethoprim • Bactrim, Sulfatrim
Topiramate • Topamax
Trifluoperazine • Stelazine
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
1. Bruijnzeel D, Suryadevara U, Tandon R. Antipsychotic treatment of schizophrenia: an update. Asian J Psychiatr. 2014;11:3-7.
2. Hrdlicka M, Dudova I. Atypical antipsychotics in the treatment of early-onset schizophrenia. Neuropsychiatr Dis Treat. 2015;11:907-913.
3. Koch-Stoecker S. Antipsychotic drugs and epilepsy: indications and treatment guidelines. Epilepsia. 2002;43(suppl 2):19-24.
4. Alper K, Schwartz KA, Kolts RL, et al. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biol Psychiatry. 2007;62(4):345-354.
5. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 1999;40(suppl 10):S2-S20.
6. Kumlien E, Lundberg PO. Seizure risk associated with neuroactive drugs: data from the WHO adverse drug reactions database. Seizure. 2010;19(2):69-73.
7. Lertxundi U, Hernandez R, Medrano J, et al. Antipsychotics and seizures: higher risk with atypicals? Seizure. 2013;22(2):141-143.
8. Bloechliger M, Rüegg S, Jick SS, et al. Antipsychotic drug use and the risk of seizures: follow-up study with a nested case-control analysis. CNS Drugs. 2015;29(7):591-603.
9. Wu CS, Wang SC, Yeh IJ, et al. Comparative risk of seizure with use of first- and second-generation antipsychotics in patients with schizophrenia and mood disorders. J Clin Psychiatry. 2016;77(5):e573-e579.
10. Cold JA, Wells BG, Froemming JH. Seizure activity associated with antipsychotic therapy. [Erratum in DICP. 1990;24(10):1012.] DICP. 1990;24(6):601-606.
11. Hedges DW, Jeppson KG. New-onset seizure associated with quetiapine and olanzapine. Ann Pharmacother. 2002;36(3):437-439.
12. Dogu O, Sevim S, Kaleagasi HS. Seizures associated with quetiapine treatment. Ann Pharmacother. 2003;37(9):1224-1227.
13. Young AC, Kleinschmidt KC, Wax PM. Late-onset seizures associated with quetiapine poisoning. J Med Toxicol. 2009;5(1):24-26.
14. US Food and Drug Administration. Recommendation of approvable action for quetiapine fumarate extended release (Seroquel® XR) for the treatment of schizophrenia. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022047Orig1s000MedR.pdf. April 24, 2007. Accessed January 28, 2019.
15. Malik AR, Ravasia S. Aripiprazole-induced seizure. Can J Psychiatry. 2005;50(3):186.
16. Tsai JF. Aripiprazole-associated seizure. J Clin Psychiatry. 2006;67(6):995-996.
17. Arora M, Arndorfer L. EEG abnormalities in a patient taking aripiprazole. Psychiatry (Edgmont). 2007;4(7):18-19.
18. Yueh CL, Yu SL, Chen HM, et al. Aripiprazole-induced seizure: a second case report. BMJ case reports. 2009;2009:bcr03.2009.1693. doi: 10.1136/bcr.03.2009.1693.
19. Thabet FI, Sweis RT, Joseph SA. Aripiprazole-induced seizure in a 3-year-old child: a case report and literature review. Clin Neuropharmacol. 2013;36(1):29-30.
20. US Food and Drug Administration. Abilify (Aripiprazole) tablets. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-436_Abilify_medr_P2.pdf. Published March 07, 2003. Accessed January 28, 2019.
21. US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Risperdal tablets, Risperdal oral solution & Risperdal M-tab orally disintegrating tablets. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/021444_S004_RISPERDAL_TABLETS.pdf. Published September 10, 2003. Accessed January 28, 2019.
22. Gonzalez-Heydrich J, Pandina GJ, Fleisher CA, et al. No seizure exacerbation from risperidone in youth with comorbid epilepsy and psychiatric disorders: a case series. J Child Adolesc Psychopharmacol. 2004;14(2):295-310.
23. Holzhausen SPF, Guerreiro MM, Baccin CE, et al. Use of risperidone in children with epilepsy. Epilepsy Behav. 2007;10(3):412-416.
24. Lane HY, Chang WH, Chou JC. Seizure during risperidone treatment in an elderly woman treated with concomitant medications. J Clinl Psychiatry. 1998;59(2):81-82.
25. Komossa K, Rummel-Kluge C, Schwarz S, et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2011;(1):19:CD006626.
26. Paliperidone [package insert]. Mountainville, CA: Janssen Pharmaceuticals, Inc.; 2007.
27. Brugge, MD; US Food and Drug Administration. Paliperidone OROS oral formulation. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021999s000_MedR_Part4.pdf. Accessed January 28, 2019.
28. Schneider RA, Lizer MH. Apparent seizure and atrial fibrillation associated with paliperidone. Am J Health System Pharm. 2008;65(22):2122-2125.
29. Liang CS, Yang FW, Chiang KT. Paliperidone-associated seizure after discontinuation of sodium valproate: a case report. J Clin Psychopharmacol. 2011;31(2):246-247.
30. Fulton B, Goa KL. Olanzapine. A review of its pharmacological properties and therapeutic efficacy in the management of schizophrenia and related psychoses. Drugs. 1997;53(2):281-298.
31. US Food and Drug Administration. Drugs@FDA: FDA approved drug products: Zyprexa (olanzapine). ORIG-1. http://www.accessdata.fda.gov/drugsatfda_docs/nda/96/020592_Original_Approval_Pkg%20.pdf. Published September 30, 1996. Accessed January 28, 2019.
32. Anzellotti F, Capasso M, Frazzini V, et al. Olanzapine-related repetitive focal seizures with lingual dystonia. Epileptic Disord. 2016;18(1):83-86.
33. Lee JW, Crismon ML, Dorson PG. Seizure associated with olanzapine. Ann Pharmac. 1999;33(5):554-556.
34. Woolley J, Smith S. Lowered seizure threshold on olanzapine. Br J Psychiatry. 2001;178(1):85-86.
35. Behere RV, Anjith D, Rao NP, et al. Olanzapine-induced clinical seizure: a case report. Clin Neuropharmacol. 2009;32(5):297-298.
36. Camacho A, García-Navarro M, Martínez B, et al. Olanzapine-induced myoclonic status. Clin Neuropharmacol. 2005;28(3):145-147.
37. Rosen JB, Milstein MJ, Haut SR. Olanzapine-associated myoclonus. Epilepsy Res. 2012;98(2-3):247-250.
38. Wyderski RJ, Starrett WG, Abou-Saif A. Fatal status epilepticus associated with olanzapine therapy. Ann Pharmacother. 1999;33(7-8):787-789.
39. Spyridi S, Sokolaki S, Nimatoudis J, et al. Status epilepticus in a patient treated with olanzapine and mirtazapine. Int J Clin Pharmacol Ther. 2009;47(2):120-123.
40. Schatzberg AF, Nemeroff CB. Essentials of clinical psychopharmacology. 2nd ed. Arlington, Virginia: American Psychiatric Publishing; 2006.
41. US Food and Drug Administration. Drug approval package: Geodon (Ziprasidone HCI) Capsules. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/20-825_Geodan_medr_P2.pdf. Published February 5, 2001. Accessed January 29, 2019.
42. Clozaril [package insert]. East Hanover, NJ: Novartis; 2008.
43. Devinsky O, Pacia SV. Seizures during clozapine therapy. J Clin Psychiatry. 1994;55(suppl B):153-156.
44. Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29(2):101-111.
45. Wong J, Delva N. Clozapine-induced seizures: recognition and treatment. Can J Psychiatry. 2007;52(7):457-463.
46. Berman I, Zalma A, DuRand CJ, et al. Clozapine-induced myoclonic jerks and drop attacks. J Clin Psychiatry. 1992;53(9):329-330.
47. Gouzoulis E, Ozdaglar A, Kasper J. Myoclonic seizures followed by grand mal seizures during clozapine treatment. Am J Psychiatry. 1993;150(7):1128.
48. Sajatovic M, Meltzer HY. Clozapine-induced myoclonus and generalized seizures. Biol Psychiatry. 1996;39(5):367-370.
49. Grover S, Hazari N, Chakrabarti S, et al. Association of clozapine with seizures: a brief report involving 222 patients prescribed clozapine. East Asian Arch Psychiatry. 2015;25(2):73-78.
50. Byerly MJ, DeVane CL. Pharmacokinetics of clozapine and risperidone: a review of recent literature. J Clin Psychopharmacol. 1996;16(2):177-187.
51. Caetano D. Use of anticonvulsants as prophylaxis for seizures in patients on clozapine. Australas Psychiatry. 2014;22(1):78-83.
52. Perry PJ, Bever KA, Arndt S, et al. Relationship between patient variables and plasma clozapine concentrations: a dosing nomogram. Biol Psychiatry.1998;44(8):733-738.
53. Dumortier G, Mahé V, Pons D, et al. Clonic seizure associated with high clozapine plasma level. J Neuropsychiatry Clin Neurosci. 2001;13(2):302-303.
54. Funderburg LG, Vertrees JE, True JE, et al. Seizure following addition of erythromycin to clozapine treatment. Am J Psychiatry. 1994;151(12):1840-1841.
55. Varma S, Bishara D, Besag FMC, et al. Clozapine-related EEG changes and seizures: dose and plasma-level relationships. Ther Adv Psychopharmacol. 2011;1(2):47-66.
56. Amann BL, Pogarell O, Mergl R, et al. EEG abnormalities associated with antipsychotics: a comparison of quetiapine, olanzapine, haloperidol and healthy subjects. Hum Psychopharmacol. 2003;18(8):641-646.
57. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
58. Maurice T, Phan VL, Urani A, et al. Neuroactive neurosteroids as endogenous effectors for the sigma1 (sigma1) receptor: pharmacological evidence and therapeutic opportunities. Jpn J Pharmacol. 1999;81(2):125-155.
59. Haller E, Binder RL. Clozapine and seizures. Am J Psychiatry. 1990;147(8):1069-1071.
60. Torta R, Monaco F. Atypical antipsychotics and serotoninergic antidepressants in patients with epilepsy: pharmacodynamic considerations. Epilepsia. 2002;43(suppl 2):8-13.
61. Spina E. Drug interactions. In: Shorvon S, Perucca E, Engel J Jr, eds. The treatment of epilepsy. 3rd ed. Oxford, UK: Blackwell Publishing; 2009:361-377.
62. Spina E, Perucca E. Clinical significance of pharmacokinetic interactions between antiepileptic and psychotropic drugs. Epilepsia. 2002;43(suppl 2):37-44.
63. de Leon J, Santoro V, D’Arrigo C, et al. Interactions between antiepileptics and second-generation antipsychotics. Expert Opin Drug Metab Toxicol. 2012;8(3):311-334.
64. Finley P, Warner D. Potential impact of valproic acid therapy on clozapine disposition. Biol Psychiatry. 1994;36(7):487-488.
65. Longo LP, Salzman C. Valproic acid effects on serum concentrations of clozapine and norclozapine. Am J Psychiatry. 1995;152(4):650.
66. Centorrino F, Baldessarini RJ, Kando J, et al. Serum concentrations of clozapine and its major metabolites: effects of cotreatment with fluoxetine or valproate. Am J Psychiatry. 1994;151(1):123-125.
67. Facciolà G, Avenoso A, Scordo MG, et al. Small effects of valproic acid on the plasma concentrations of clozapine and its major metabolites in patients with schizophrenic or affective disorders. Ther Drug Monit. 1999;21(3):341-345.
68. Hyde TM, Weinberger DR. Seizures and schizophrenia. Schizophr Bull. 1997;23(4):611-622.
69. Muzyk A, Gala G, Kahn DA. Use of lamotrigine in a patient with a clozapine-related seizure. J Psychiatr Pract. 2010;16(2):125-128.
70. Kikuchi YS, Sato W, Ataka K, et al. Clozapine-induced seizures, electroencephalography abnormalities, and clinical responses in Japanese patients with schizophrenia. Neuropsychiatr Dis Treat. 2014;10:1973-1978.
71. Taner E, Coşar B, Işik E. Clozapine-induced myoclonic seizures and valproic acid. Int J Psychiatry Clin Pract. 1998;2(1):53-55.
72. Liukkonen J, Koponen HJ, Nousiainen U. Clinical picture and long-term course of epileptic seizures that occur during clozapine treatment. Psychiatry Res. 1992;44(2):107-112.
73. Devinsky O, Honigfeld G, Patin J. Clozapine-related seizures. Neurology. 1991;41(3):369-371.
74. Foster R, Olajide D. A case of clozapine-induced tonic-clonic seizures managed with valproate: implications for clinical care. J Psychopharmacol. 2005;19(1):93-96.
75. Gandelman-Marton R, Theitler J, Klein C, et al. Phenytoin intoxication in a clozapine-related prolonged seizure. J Emerg Med. 2008;35(4):407-409.
76. Primavera A, Giberti L, Scotto P, et al. Nonconvulsive status epilepticus as a cause of confusion in later life: a report of 5 cases. Neuropsychobiology. 1994;30(2-3):148-152.
77. Boutros NN, Arfken C, Galderisi S, et al. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophrenia Res. 2008;99(1-3):225-237.
78. Takahashi T, Cho RY, Mizuno T, et al. Antipsychotics reverse abnormal EEG complexity in drug-naïve schizophrenia: a multiscale entropy analysis. Neuroimage. 2010;51(1):173-182.
1. Bruijnzeel D, Suryadevara U, Tandon R. Antipsychotic treatment of schizophrenia: an update. Asian J Psychiatr. 2014;11:3-7.
2. Hrdlicka M, Dudova I. Atypical antipsychotics in the treatment of early-onset schizophrenia. Neuropsychiatr Dis Treat. 2015;11:907-913.
3. Koch-Stoecker S. Antipsychotic drugs and epilepsy: indications and treatment guidelines. Epilepsia. 2002;43(suppl 2):19-24.
4. Alper K, Schwartz KA, Kolts RL, et al. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biol Psychiatry. 2007;62(4):345-354.
5. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 1999;40(suppl 10):S2-S20.
6. Kumlien E, Lundberg PO. Seizure risk associated with neuroactive drugs: data from the WHO adverse drug reactions database. Seizure. 2010;19(2):69-73.
7. Lertxundi U, Hernandez R, Medrano J, et al. Antipsychotics and seizures: higher risk with atypicals? Seizure. 2013;22(2):141-143.
8. Bloechliger M, Rüegg S, Jick SS, et al. Antipsychotic drug use and the risk of seizures: follow-up study with a nested case-control analysis. CNS Drugs. 2015;29(7):591-603.
9. Wu CS, Wang SC, Yeh IJ, et al. Comparative risk of seizure with use of first- and second-generation antipsychotics in patients with schizophrenia and mood disorders. J Clin Psychiatry. 2016;77(5):e573-e579.
10. Cold JA, Wells BG, Froemming JH. Seizure activity associated with antipsychotic therapy. [Erratum in DICP. 1990;24(10):1012.] DICP. 1990;24(6):601-606.
11. Hedges DW, Jeppson KG. New-onset seizure associated with quetiapine and olanzapine. Ann Pharmacother. 2002;36(3):437-439.
12. Dogu O, Sevim S, Kaleagasi HS. Seizures associated with quetiapine treatment. Ann Pharmacother. 2003;37(9):1224-1227.
13. Young AC, Kleinschmidt KC, Wax PM. Late-onset seizures associated with quetiapine poisoning. J Med Toxicol. 2009;5(1):24-26.
14. US Food and Drug Administration. Recommendation of approvable action for quetiapine fumarate extended release (Seroquel® XR) for the treatment of schizophrenia. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022047Orig1s000MedR.pdf. April 24, 2007. Accessed January 28, 2019.
15. Malik AR, Ravasia S. Aripiprazole-induced seizure. Can J Psychiatry. 2005;50(3):186.
16. Tsai JF. Aripiprazole-associated seizure. J Clin Psychiatry. 2006;67(6):995-996.
17. Arora M, Arndorfer L. EEG abnormalities in a patient taking aripiprazole. Psychiatry (Edgmont). 2007;4(7):18-19.
18. Yueh CL, Yu SL, Chen HM, et al. Aripiprazole-induced seizure: a second case report. BMJ case reports. 2009;2009:bcr03.2009.1693. doi: 10.1136/bcr.03.2009.1693.
19. Thabet FI, Sweis RT, Joseph SA. Aripiprazole-induced seizure in a 3-year-old child: a case report and literature review. Clin Neuropharmacol. 2013;36(1):29-30.
20. US Food and Drug Administration. Abilify (Aripiprazole) tablets. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-436_Abilify_medr_P2.pdf. Published March 07, 2003. Accessed January 28, 2019.
21. US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Risperdal tablets, Risperdal oral solution & Risperdal M-tab orally disintegrating tablets. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/021444_S004_RISPERDAL_TABLETS.pdf. Published September 10, 2003. Accessed January 28, 2019.
22. Gonzalez-Heydrich J, Pandina GJ, Fleisher CA, et al. No seizure exacerbation from risperidone in youth with comorbid epilepsy and psychiatric disorders: a case series. J Child Adolesc Psychopharmacol. 2004;14(2):295-310.
23. Holzhausen SPF, Guerreiro MM, Baccin CE, et al. Use of risperidone in children with epilepsy. Epilepsy Behav. 2007;10(3):412-416.
24. Lane HY, Chang WH, Chou JC. Seizure during risperidone treatment in an elderly woman treated with concomitant medications. J Clinl Psychiatry. 1998;59(2):81-82.
25. Komossa K, Rummel-Kluge C, Schwarz S, et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2011;(1):19:CD006626.
26. Paliperidone [package insert]. Mountainville, CA: Janssen Pharmaceuticals, Inc.; 2007.
27. Brugge, MD; US Food and Drug Administration. Paliperidone OROS oral formulation. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021999s000_MedR_Part4.pdf. Accessed January 28, 2019.
28. Schneider RA, Lizer MH. Apparent seizure and atrial fibrillation associated with paliperidone. Am J Health System Pharm. 2008;65(22):2122-2125.
29. Liang CS, Yang FW, Chiang KT. Paliperidone-associated seizure after discontinuation of sodium valproate: a case report. J Clin Psychopharmacol. 2011;31(2):246-247.
30. Fulton B, Goa KL. Olanzapine. A review of its pharmacological properties and therapeutic efficacy in the management of schizophrenia and related psychoses. Drugs. 1997;53(2):281-298.
31. US Food and Drug Administration. Drugs@FDA: FDA approved drug products: Zyprexa (olanzapine). ORIG-1. http://www.accessdata.fda.gov/drugsatfda_docs/nda/96/020592_Original_Approval_Pkg%20.pdf. Published September 30, 1996. Accessed January 28, 2019.
32. Anzellotti F, Capasso M, Frazzini V, et al. Olanzapine-related repetitive focal seizures with lingual dystonia. Epileptic Disord. 2016;18(1):83-86.
33. Lee JW, Crismon ML, Dorson PG. Seizure associated with olanzapine. Ann Pharmac. 1999;33(5):554-556.
34. Woolley J, Smith S. Lowered seizure threshold on olanzapine. Br J Psychiatry. 2001;178(1):85-86.
35. Behere RV, Anjith D, Rao NP, et al. Olanzapine-induced clinical seizure: a case report. Clin Neuropharmacol. 2009;32(5):297-298.
36. Camacho A, García-Navarro M, Martínez B, et al. Olanzapine-induced myoclonic status. Clin Neuropharmacol. 2005;28(3):145-147.
37. Rosen JB, Milstein MJ, Haut SR. Olanzapine-associated myoclonus. Epilepsy Res. 2012;98(2-3):247-250.
38. Wyderski RJ, Starrett WG, Abou-Saif A. Fatal status epilepticus associated with olanzapine therapy. Ann Pharmacother. 1999;33(7-8):787-789.
39. Spyridi S, Sokolaki S, Nimatoudis J, et al. Status epilepticus in a patient treated with olanzapine and mirtazapine. Int J Clin Pharmacol Ther. 2009;47(2):120-123.
40. Schatzberg AF, Nemeroff CB. Essentials of clinical psychopharmacology. 2nd ed. Arlington, Virginia: American Psychiatric Publishing; 2006.
41. US Food and Drug Administration. Drug approval package: Geodon (Ziprasidone HCI) Capsules. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/20-825_Geodan_medr_P2.pdf. Published February 5, 2001. Accessed January 29, 2019.
42. Clozaril [package insert]. East Hanover, NJ: Novartis; 2008.
43. Devinsky O, Pacia SV. Seizures during clozapine therapy. J Clin Psychiatry. 1994;55(suppl B):153-156.
44. Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29(2):101-111.
45. Wong J, Delva N. Clozapine-induced seizures: recognition and treatment. Can J Psychiatry. 2007;52(7):457-463.
46. Berman I, Zalma A, DuRand CJ, et al. Clozapine-induced myoclonic jerks and drop attacks. J Clin Psychiatry. 1992;53(9):329-330.
47. Gouzoulis E, Ozdaglar A, Kasper J. Myoclonic seizures followed by grand mal seizures during clozapine treatment. Am J Psychiatry. 1993;150(7):1128.
48. Sajatovic M, Meltzer HY. Clozapine-induced myoclonus and generalized seizures. Biol Psychiatry. 1996;39(5):367-370.
49. Grover S, Hazari N, Chakrabarti S, et al. Association of clozapine with seizures: a brief report involving 222 patients prescribed clozapine. East Asian Arch Psychiatry. 2015;25(2):73-78.
50. Byerly MJ, DeVane CL. Pharmacokinetics of clozapine and risperidone: a review of recent literature. J Clin Psychopharmacol. 1996;16(2):177-187.
51. Caetano D. Use of anticonvulsants as prophylaxis for seizures in patients on clozapine. Australas Psychiatry. 2014;22(1):78-83.
52. Perry PJ, Bever KA, Arndt S, et al. Relationship between patient variables and plasma clozapine concentrations: a dosing nomogram. Biol Psychiatry.1998;44(8):733-738.
53. Dumortier G, Mahé V, Pons D, et al. Clonic seizure associated with high clozapine plasma level. J Neuropsychiatry Clin Neurosci. 2001;13(2):302-303.
54. Funderburg LG, Vertrees JE, True JE, et al. Seizure following addition of erythromycin to clozapine treatment. Am J Psychiatry. 1994;151(12):1840-1841.
55. Varma S, Bishara D, Besag FMC, et al. Clozapine-related EEG changes and seizures: dose and plasma-level relationships. Ther Adv Psychopharmacol. 2011;1(2):47-66.
56. Amann BL, Pogarell O, Mergl R, et al. EEG abnormalities associated with antipsychotics: a comparison of quetiapine, olanzapine, haloperidol and healthy subjects. Hum Psychopharmacol. 2003;18(8):641-646.
57. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
58. Maurice T, Phan VL, Urani A, et al. Neuroactive neurosteroids as endogenous effectors for the sigma1 (sigma1) receptor: pharmacological evidence and therapeutic opportunities. Jpn J Pharmacol. 1999;81(2):125-155.
59. Haller E, Binder RL. Clozapine and seizures. Am J Psychiatry. 1990;147(8):1069-1071.
60. Torta R, Monaco F. Atypical antipsychotics and serotoninergic antidepressants in patients with epilepsy: pharmacodynamic considerations. Epilepsia. 2002;43(suppl 2):8-13.
61. Spina E. Drug interactions. In: Shorvon S, Perucca E, Engel J Jr, eds. The treatment of epilepsy. 3rd ed. Oxford, UK: Blackwell Publishing; 2009:361-377.
62. Spina E, Perucca E. Clinical significance of pharmacokinetic interactions between antiepileptic and psychotropic drugs. Epilepsia. 2002;43(suppl 2):37-44.
63. de Leon J, Santoro V, D’Arrigo C, et al. Interactions between antiepileptics and second-generation antipsychotics. Expert Opin Drug Metab Toxicol. 2012;8(3):311-334.
64. Finley P, Warner D. Potential impact of valproic acid therapy on clozapine disposition. Biol Psychiatry. 1994;36(7):487-488.
65. Longo LP, Salzman C. Valproic acid effects on serum concentrations of clozapine and norclozapine. Am J Psychiatry. 1995;152(4):650.
66. Centorrino F, Baldessarini RJ, Kando J, et al. Serum concentrations of clozapine and its major metabolites: effects of cotreatment with fluoxetine or valproate. Am J Psychiatry. 1994;151(1):123-125.
67. Facciolà G, Avenoso A, Scordo MG, et al. Small effects of valproic acid on the plasma concentrations of clozapine and its major metabolites in patients with schizophrenic or affective disorders. Ther Drug Monit. 1999;21(3):341-345.
68. Hyde TM, Weinberger DR. Seizures and schizophrenia. Schizophr Bull. 1997;23(4):611-622.
69. Muzyk A, Gala G, Kahn DA. Use of lamotrigine in a patient with a clozapine-related seizure. J Psychiatr Pract. 2010;16(2):125-128.
70. Kikuchi YS, Sato W, Ataka K, et al. Clozapine-induced seizures, electroencephalography abnormalities, and clinical responses in Japanese patients with schizophrenia. Neuropsychiatr Dis Treat. 2014;10:1973-1978.
71. Taner E, Coşar B, Işik E. Clozapine-induced myoclonic seizures and valproic acid. Int J Psychiatry Clin Pract. 1998;2(1):53-55.
72. Liukkonen J, Koponen HJ, Nousiainen U. Clinical picture and long-term course of epileptic seizures that occur during clozapine treatment. Psychiatry Res. 1992;44(2):107-112.
73. Devinsky O, Honigfeld G, Patin J. Clozapine-related seizures. Neurology. 1991;41(3):369-371.
74. Foster R, Olajide D. A case of clozapine-induced tonic-clonic seizures managed with valproate: implications for clinical care. J Psychopharmacol. 2005;19(1):93-96.
75. Gandelman-Marton R, Theitler J, Klein C, et al. Phenytoin intoxication in a clozapine-related prolonged seizure. J Emerg Med. 2008;35(4):407-409.
76. Primavera A, Giberti L, Scotto P, et al. Nonconvulsive status epilepticus as a cause of confusion in later life: a report of 5 cases. Neuropsychobiology. 1994;30(2-3):148-152.
77. Boutros NN, Arfken C, Galderisi S, et al. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophrenia Res. 2008;99(1-3):225-237.
78. Takahashi T, Cho RY, Mizuno T, et al. Antipsychotics reverse abnormal EEG complexity in drug-naïve schizophrenia: a multiscale entropy analysis. Neuroimage. 2010;51(1):173-182.
Chronic Myeloid Leukemia: A Review of TKI Therapy
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm that arises from a reciprocal translocation between the Abelson (ABL) region on chromosome 9 and the breakpoint cluster region (BCR) of chromosome 22, t(9;22)(q34;q11.2) (the Philadelphia chromosome), resulting in the generation of the BCR-ABL1 fusion gene and its protein product, BCR-ABL tyrosine kinase. BCR-ABL is a constitutively active fusion kinase that confers proliferative and survival advantage to hematopoietic cells through activation of downstream pathways.
CML is divided into 3 phases based on the number of myeloblasts observed in the blood or bone marrow: chronic, accelerated, and blast. Most cases of CML are diagnosed in the chronic phase (CP), which is marked by proliferation of primarily the myeloid element.
The advent of tyrosine kinase inhibitors (TKIs), a class of small molecules targeting the tyrosine kinases, particularly the BCR-ABL tyrosine kinase, led to rapid changes in the management of CML and improved survival for patients. Patients diagnosed with CP-CML now a have life-expectancy that is similar to that of the general population, as long as they receive the appropriate TKI therapy and adhere to treatment. As such, it is crucial to identify patients with CML, ensure they receive a complete, appropriate diagnostic work-up, and select the best therapy for each individual patient. The diagnosis and work-up of CML are reviewed in a separate article; here, the selection of TKI therapy for a patient with newly diagnosed CP-CML is reviewed.
Case Presentation
A 53-year-old woman who recently was diagnosed with CML presents to review her treatment options. The diagnosis was made after she presented to her primary care physician with fatigue, early satiety, left upper quadrant abdominal pain, and an 8-lb unintentional weight loss over the prior month. On physical exam her spleen was palpated 8 cm below the left costal margin. Laboratory evaluation showed a total white blood cell (WBC) count of 124,000/μL with a left-shifted differential including 6% basophils, 3% eosinophils, and 3% blasts; hemoglobin and platelet count were 12.4 g/dL and 801 × 103/µL, respectively. Fluorescent in-situ hybridization for BCR-ABL gene rearrangement using peripheral blood was positive in 87% of cells. Bone marrow biopsy and aspiration showed a 95% cellular bone marrow with granulocytic hyperplasia and 1% blasts. Cytogenetics were 46,XX,t(9;22)(q34;q11.2), and quantitative real-time polymerase chain reaction (RQ-PCR) to measure BCR-ABL1 transcripts in the peripheral blood showed a value of 98% international standard (IS). Her Sokal risk score was 1.42 (high risk). In addition, prior review of her past medical history revealed uncontrolled diabetes, coronary artery disease requiring placement of 3 cardiac stents 2 years prior, and chronic obstructive pulmonary disease (COPD) related to a 30-pack-year history of smoking.
- What factors must be considered when selecting first-line therapy for this patient?
Selection of the most appropriate first-line TKI for newly diagnosed CP-CML patients requires incorporation of many patient-specific factors. These factors include baseline karyotype and confirmation of CP-CML through bone marrow biopsy, Sokal or EURO risk score, and a thorough patient history, including a clear understanding of the patient's comorbidities. In this case, the patient's high Sokal risk score along with her history of diabetes, coronary artery disease, and COPD are all factors that must be accounted for when choosing the most appropriate TKI. The adverse effect profile of all TKIs must be considered in conjunction with the patient's ongoing medical issues in order to decrease the likelihood of worsening her current symptoms or causing a severe complication from TKI therapy.
Imatinib
The management of CML was revolutionized by the development and ultimate regulatory approval of imatinib mesylate in 2001. Imatinib was the first small-molecule cancer therapy developed and approved. It acts by binding to the adenosine triphosphate (ATP) binding site in the catalytic domain of BCR-ABL, thus inhibiting the oncoprotein's tyrosine kinase activity.1
The International Randomized Study of Interferon versus STI571 (IRIS) trial was a randomized phase 3 study that compared imatinib 400 mg daily to interferon α (IFNα) plus cytarabine. More than 1000 CP-CML patients were randomly assigned 1:1 to either imatinib or IFNα plus cytarabine and were assessed for event-free survival, hematologic and cytogenetic responses, freedom from progression to accelerated phase (AP) or blast phase (BP), and toxicity. Imatinib was superior to the prior standard of care for all these outcomes.2 The long-term follow up of the IRIS trial reported an 83% estimated 10-year overall survival (OS) and 79% estimated event-free survival for patients on the imatinib arm of this study.3 The cumulative rate of complete cytogenetic response (CCyR) was 82.8%. Of the 204 imatinib-treated patients who could undergo a molecular response evaluation at 10 years, 93.1% had a major molecular response (MMR) and 63.2% had a molecular response 4.5 (MR4.5), suggesting durable, deep molecular responses for many patients (see Chronic Myeloid Leukemia: Evaluation and Diagnosis for discussion of the hematologic parameters, cytogenetic results, and molecular responses ussed in monitoring response to TKI therapy). The estimated 10-year rate of freedom from progression to AP or BP was 92.1%.
Higher doses of imatinib (600-800 mg daily) have been studied in an attempt to overcome resistance and improve cytogenetic and molecular response rates. The Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) trial was a randomized phase 3 study that compared imatinib 800 mg daily to imatinib 400 mg daily. Although the 6-month assessments found increased rates of CCyR and a MMR in the higher-dose imatinib arm, these differences were no longer present at the 12-month assessment. Furthermore, the higher dose of imatinib led to a significantly higher incidence of grade 3/4 hematologic adverse events, and approximately 50% of patients on imatinib 800 mg daily required a dose reduction to less than 600 mg daily because of toxicity.4
The Therapeutic Intensification in De Novo Leukaemia (TIDEL) -II study used plasma trough levels of imatinib on day 22 of treatment with imatinib 600 mg daily to determine if patients should escalate the imatinib dose to 800 mg daily. In patients who did not meet molecular milestones at 3, 6, or 12 months, cohort 1 was dose escalated to imatinib 800 mg daily and subsequently switched to nilotinib 400 mg twice daily for failing the same target 3 months later, and cohort 2 was switched to nilotinib. At 2 years, 73% of patients achieved MMR and 34% achieved MR4.5, suggesting that initial treatment with higher-dose imatinib subsequently followed by a switch to nilotinib in those failing to achieve desired milestones could be an effective strategy for managing newly diagnosed CP-CML.5
Toxicity
Imatinib 400 mg is considered the standard starting dose in CP-CML patients. The safety profile of imatinib has been very well established. In the IRIS trial, the most common adverse events (all grades in decreasing order of frequency) were peripheral and periorbital edema (60%), nausea (50%), muscle cramps (49%), musculoskeletal pain (47%), diarrhea (45%), rash (40%), fatigue (39%), abdominal pain (37%), headache (37%), and joint pain (31%). Grade 3/4 liver enzyme elevation can occur in 5% of patients.6 In the event of severe liver toxicity or fluid retention, imatinib should be held until the event resolves. At that time, imatinib can be restarted if deemed appropriate, but this is dependent on the severity of the inciting event. Fluid retention can be managed by the use of supportive care, diuretics, imatinib dose reduction, dose interruption, or imatinib discontinuation if the fluid retention is severe. Muscle cramps can be managed by the use of a calcium supplements or tonic water. Management of rash can include topical or systemic steroids, or in some cases imatinib dose reduction, interruption, or discontinuation.7
Grade 3/4 imatinib-induced hematologic toxicity is not uncommon, with 17% of patients experiencing neutropenia, 9% thrombocytopenia, and 4% anemia. These adverse events occurred most commonly during the first year of therapy, and the frequency decreased over time.3,6 Depending on the degree of cytopenias, imatinib dosing should be interrupted until recovery of the absolute neutrophil count or platelet count, and can often be resumed at 400 mg daily. However, if cytopenias recur, imatinib should be held and subsequently restarted at 300 mg daily.7
Dasatinib
Dasatinib is a second-generation TKI that has regulatory approval for treatment of adult patients with newly diagnosed CP-CML or CP-CML in patients with resistance or intolerance to prior TKIs. In addition to dasatinib's ability to inhibit ABL kinases, it is also known to be a potent inhibitor of Src family kinases. Dasatinib has shown efficacy in patients who have developed imatinib-resistant ABL kinase domain mutations.
Dasatinib was initially approved as second-line therapy in patients with resistance or intolerance to imatinib. This indication was based on the results of the phase 3 CA180-034 trial which ultimately identified dasatinib 100 mg daily as the optimal dose. In this trial, 74% of patients enrolled had resistance to imatinib and the remainder were intolerant. The 7-year follow-up of patients randomized to dasatinib 100 mg (n = 167) daily indicated that 46% achieved MMR while on study. Of the 124 imatinib-resistant patients on dasatinib 100 mg daily, the 7-year progression-free survival (PFS) was 39% and OS was 63%. In the 43 imatinib-intolerant patients, the 7-year PFS was 51% and OS was 70%.8
Dasatinib 100 mg daily was compared to imatinib 400 mg daily in newly diagnosed CP-CML patients in the randomized phase 3 DASISION trial. More patients on the dasatinib arm achieved an early molecular response of BCR-ABL1 transcripts ≤10% IS after 3 months on treatment compared to imatinib (84% versus 64%). Furthermore, the 5-year follow-up reports that the cumulative incidence of MMR and MR4.5 in dasatinib-treated patients was 76% and 42%, and was 64% and 33%, with imatinib (P = 0.0022 and P = 0.0251, respectively). Fewer patients treated with dasatinib progressed to AP or BP (4.6%) compared to imatinib (7.3%), but the estimated 5-year OS was similar between the 2 arms (91% for dasatinib versus 90% for imatinib).9 Regulatory approval for dasatinib as first-line therapy in newly diagnosed CML patients was based on results of the DASISION trial.
Toxicity
Most dasatinib-related toxicities are reported as grade 1 or grade 2, but grade 3/4 hematologic adverse events are fairly common. In the DASISION trial, grade 3/4 neutropenia, anemia, and thrombocytopenia occurred in 29%, 13%, and 22% of dasatinib-treated patients, respectively. Cytopenias can generally be managed with temporary dose interruptions or dose reductions.
During the 5-year follow-up of the DASISION trial, pleural effusions were reported in 28% of patients, most of which were grade 1/2. This occurred at a rate of approximately ≤ 8% per year, suggesting a stable incidence over time, and the effusions appear to be dose-dependent.9 Depending on the severity of the effusion, this may be treated with diuretics, dose interruption, and in some instances, steroids or a thoracentesis. Typically, dasatinib can be restarted at 1 dose level lower than the previous dose once the effusion has resolved.7 Other, less common side effects of dasatinib include pulmonary hypertension (5% of patients), as well as abdominal pain, fluid retention, headaches, fatigue, musculoskeletal pain, rash, nausea, and diarrhea. Pulmonary hypertension is typically reversible after cessation of dasatinib, and thus dasatinib should be permanently discontinued once the diagnosis is confirmed. Fluid retention is often treated with diuretics and supportive care. Nausea and diarrhea are generally manageable and occur less frequently when dasatinib is taken with food and a large glass of water. Antiemetics and antidiarrheals can be used as needed. Troublesome rash can be best managed with topical or systemic steroids as well as possible dose reduction or dose interruption.7,9 In the DASISION trial, adverse events led to therapy discontinuation more often in the dasatinib group than in the imatinib group (16% versus 7%).9 Bleeding, particularly in the setting of thrombocytopenia, has been reported in patients being treated with dasatinib as a result of the drug-induced reversible inhibition of platelet aggregation.10
Nilotinib
The structure of nilotinib is similar to that of imatinib; however, it has a markedly increased affinity for the ATP‐binding site on the BCR-ABL1 protein. It was initially given regulatory approval in the setting of imatinib failure. Nilotinib was studied at a dose of 400 mg twice daily in 321 patients who were imatinib-resistant or -intolerant. It proved to be highly effective at inducing cytogenetic remissions in the second-line setting, with 59% of patients achieving a major cytogenetic response (MCyR) and 45% achieving CCyR. With a median follow-up time of 4 years, the OS was 78%.11
Nilotinib gained regulatory approval for use as a first-line TKI after completion of the randomized phase 3 ENESTnd (Evaluating Nilotinib Efficacy and Safety in Clinical Trials-Newly Diagnosed Patients) trial. ENESTnd was a 3-arm study comparing nilotinib 300 mg twice daily versus nilotinib 400 mg twice daily versus imatinib 400 mg daily in newly diagnosed, previously untreated patients diagnosed with CP-CML. The primary endpoint of this clinical trial was rate of MMR at 12 months.12 Nilotinib surpassed imatinib in this regard, with 44% of patients on nilotinib 300 mg twice daily achieving MMR at 12 months versus 43% of nilotinib 400 mg twice daily patients versus 22% of the imatinib-treated patients (P < 0.001 for both comparisons). Furthermore, the rate of CCyR by 12 months was significantly higher for both nilotinib arms compared with imatinib (80% for nilotinib 300 mg, 78% for nilotinib 400 mg, and 65% for imatinib) (P < 0.001).12 Based on this data, nilotinib 300 mg twice daily was chosen as the standard dose of nilotinib in the first-line setting. After 5 years of follow-up on the ENESTnd study, there were fewer progressions to AP/BP CML in nilotinib-treated patients compared with imatinib. MMR was achieved in 77% of nilotinib 300 mg patients compared with 60.4% of patients on the imatinib arm. MR4.5 was also more common in patients treated with nilotinib 300 mg twice daily, with a rate of 53.5% at 5 years versus 31.4% in the imatinib arm.13 In spite of the deeper cytogenetic and molecular responses achieved with nilotinib, this did not translate into a significant improvement in OS. The 5-year OS rate was 93.7% in nilotinib 300 mg patients versus 91.7% in imatinib-treated patients, and this difference lacked statistical significance.13
Toxicity
Although some similarities exist between the toxicity profiles of nilotinib and imatinib, each drug has some distinct adverse events. On the ENESTnd trial, the rate of any grade 3/4 non-hematologic adverse event was fairly low; however, lower-grade toxicities were not uncommon. Patients treated with nilotinib 300 mg twice daily experienced rash (31%), headache (14%), pruritis (15%), and fatigue (11%) most commonly. The most frequently reported laboratory abnormalities included increased total bilirubin (53%), hypophosphatemia (32%), hyperglycemia (36%), elevated lipase (24%), increased alanine aminotransferase (ALT; 66%), and increased aspartate aminotransferase (AST; 40%). Any grade of neutropenia, thrombocytopenia, or anemia occurred at rates of 43%, 48%, and 38%, respectively.12 Although nilotinib has a Black Box Warning from the US Food and Drug Administration for QT interval prolongation, no patients on the ENESTnd trial experienced a QT interval corrected for heart rate greater than 500 msec.12
More recent concerns have emerged regarding the potential for cardiovascular toxicity after long-term use of nilotinib. The 5-year update of ENESTnd reports cardiovascular events, including ischemic heart disease, ischemic cerebrovascular events, or peripheral arterial disease occurring in 7.5% of patients treated with nilotinib 300 mg twice daily compared with a rate of 2.1% in imatinib-treated patients. The frequency of these cardiovascular events increased linearly over time in both arms. Elevations in total cholesterol from baseline occurred in 27.6% of nilotinib patients compared with 3.9% of imatinib patients. Furthermore, clinically meaningful increases in low-density lipoprotein cholesterol and glycated hemoglobin occurred more frequently with nilotinib therapy.12
Nilotinib should be taken on an empty stomach; therefore, patients should be made aware of the need to fast for 2 hours prior to each dose and 1 hour after each dose. Given the potential risk of QT interval prolongation, a baseline electrocardiogram (ECG) is recommended prior to initiating treatment to ensure the QT interval is within a normal range. A repeat ECG should be done approximately 7 days after nilotinib initiation to ensure no prolongation of the QT interval after starting. Close monitoring of potassium and magnesium levels is important to decrease the risk of cardiac arrhythmias, and concomitant use of drugs considered strong CYP3A4 inhibitors should be avoided.7
If the patient experiences any grade 3 or higher laboratory abnormalities, nilotinib should be held until resolution of the toxicity, and then restarted at a lower dose. Similarly, if patients develop significant neutropenia or thrombocytopenia, nilotinib doses should be interrupted until resolution of the cytopenias. At that point, nilotinib can be reinitiated at either the same or a lower dose. Rash can be managed by the use of topical or systemic steroids as well as potential dose reduction, interruption, or discontinuation.
Given the concerns for potential cardiovascular events with long-term use of nilotinib, caution is advised when prescribing it to any patient with a history of cardiovascular disease or peripheral arterial occlusive disease. At the first sign of new occlusive disease, nilotinib should be discontinued.7
Bosutinib
Bosutinib is a second-generation BCR-ABL1 TKI with activity against the Src family of kinases that was initially approved to treat patients with CP-, AP-, or BP-CML after resistance or intolerance to imatinib. Long-term data has been reported from the phase 1/2 trial of bosutinib therapy in patients with CP-CML who developed resistance or intolerance to imatinib plus dasatinib and/or nilotinib. A total of 119 patients were included in the 4-year follow-up; 38 were resistant/intolerant to imatinib and resistant to dasatinib, 50 were resistant/intolerant to imatinib and intolerant to dasatinib, 26 were resistant/intolerant to imatinib and resistant to nilotinib, and 5 were resistant/intolerant to imatinib and intolerant to nilotinib or resistant/intolerant to dasatinib and nilotinib. Bosutinib 400 mg daily was studied in this setting. Of the 38 patients with imatinib resistance/intolerance and dasatinib resistance, 39% achieved MCyR, 22% achieved CCyR, and the OS was 67%. Of the 50 patients with imatinib resistance/intolerance and dasatinib intolerance, 42% achieved MCyR, 40% achieved CCyR, and the OS was 80%. Finally, in the 26 patients with imatinib resistance/intolerance and nilotinib resistance, 38% achieved MCyR, 31% achieved CcyR, and the OS was 87%.14
Five-year follow-up from the phase 1/2 clinical trial which studied bosutinib 500 mg daily in CP-CML patients after imatinib failure reported data on 284 patients. By 5 years on study, 60% of patients had achieved MCyR and 50% achieved CCyR with a 71% and 69% probability, respectively, of maintaining these responses at 5 years. The 5-year OS was 84%.15 These data led to the regulatory approval of bosutinib 500 mg daily as second-line or later therapy.
Bosutinib was initially studied in the first-line setting in the randomized phase 3 BELA (Bosutinib Efficacy and Safety in Newly Diagnosed Chronic Myeloid Leukemia) trial. This trial compared bosutinib 500 mg daily to imatinib 400 mg daily in newly diagnosed, previously untreated CP-CML patients. This trial failed to meet its primary endpoint of increased rate of CCyR at 12 months, with 70% of bosutinib patients achieving this response compared to 68% of imatinib-treated patients (P = 0.601). In spite of this, the rate of MMR at 12 months was significantly higher in the bosutinib arm (41%) compared to the imatinib arm (27%; P = 0.001).16
A second phase 3 trial (BFORE) was designed to study bosutinib 400 mg daily versus imatinib in newly diagnosed, previously untreated CP-CML patients. This study enrolled 536 patients who were randomly assigned 1:1 to bosutinib versus imatinib. The primary endpoint of this trial was rate of MMR at 12 months. A significantly higher number of bosutinib-treated patients achieved this response (47.2%) compared with imatinib-treated patients (36.9%, P = 0.02). Furthermore, by 12 months 77.2% of patients on the bosutinib arm had achieved CCyR compared with 66.4% on the imatinib arm, and this difference did meet statistical significance (P = 0.0075). A lower rate of progression to AP- or BP-CML was noted in bosutinib-treated patients as well (1.6% versus 2.5%). Based on this data, bosutinib gained regulatory approval for first-line therapy in CP-CML at a dose of 400 mg daily.17
Toxicity
On the BFORE trial, the most common treatment-emergent adverse events of any grade reported in the bosutinib-treated patients were diarrhea (70.1%), nausea (35.1%), increased ALT (30.6%), and increased AST (22.8%). Musculoskeletal pain or spasms occurred in 29.5% of patients, rash in 19.8%, fatigue in 19.4%, and headache in 18.7%. Hematologic toxicity was also reported, but most was grade 1/2. Thrombocytopenia was reported in 35.1%, anemia in 18.7%, and neutropenia in 11.2%.17
Cardiovascular events occurred in 5.2% of patients on the bosutinib arm of the BFORE trial, which was similar to the rate observed in imatinib patients. The most common cardiovascular event was QT interval prolongation, which occurred in 1.5% of patients. Pleural effusions were reported in 1.9% of patients treated with bosutinib, and none were grade 3 or higher.17
If liver enzyme elevation occurs at a value greater than 5 times the institutional upper limit of normal, bosutinib should be held until the level recovers to ≤2.5 times the upper limit of normal, at which point bosutinib can be restarted at a lower dose. If recovery takes longer than 4 weeks, bosutinib should be permanently discontinued. Liver enzymes elevated greater than 3 times the institutional upper limit of normal and a concurrent elevation in total bilirubin to 2 times the upper limit of normal is consistent with Hy's law, and bosutinib should be discontinued. Although diarrhea is the most common toxicity associated with bosutinib, it is commonly low grade and transient. Diarrhea occurs most frequently in the first few days after initiating bosutinib. It can often be managed with over-the-counter antidiarrheal medications, but if the diarrhea is grade or higher, bosutinib should be held until recovery to grade 1 or lower. Gastrointestinal side effects may be improved by taking bosutinib with a meal and a large glass of water. Fluid retention can be managed with diuretics and supportive care. Finally, if rash occurs, this can be addressed with topical or systemic steroids as well as bosutinib dose reduction, interruption, or discontinuation.7
Similar to other TKIs, if bosutinib-induced cytopenias occur, treatment should be held and restarted at the same or a lower dose upon blood count recovery.7
Ponatinib
The most common cause of TKI resistance in CP-CML is the development of ABL kinase domain mutations. The majority of imatinib-resistant mutations can be overcome by the use of second-generation TKIs including dasatinib, nilotinib, or bosutinib. However, ponatinib is the only BCR-ABL1 TKI able to overcome a T315I mutation. The phase 2 PACE (Ponatinib Ph-positive ALL and CML Evaluation) trial enrolled patients with CP-, AP-, or BP-CML as well as patients with Ph-positive acute lymphoblastic leukemia who were resistant or intolerant to nilotinib or dasatinib, or who had evidence of a T315I mutation. The starting dose of ponatinib on this trial was 45 mg daily.18 The PACE trial enrolled 267 patients with CP-CML: 203 with resistance or intolerance to nilotinib or dasatinib, and 64 with a T315I mutation. The primary endpoint in the CP cohort was rate of MCyR at any time within 12 months of starting ponatinib. The overall rate of MCyR by 12 months in the CP-CML patients was 56%. In those with a T315I mutation, 70% achieved MCyR, which compared favorably with those with resistance or intolerance to nilotinib or dasatinib, 51% of whom achieved MCyR. CCyR was achieved in 46% of CP-CML patients (40% in the resistant/intolerant cohort and 66% in the T315I cohort). In general, patients with T315I mutations received fewer prior therapies than those in the resistant/intolerant cohort, which likely contributed to the higher response rates in the T315I patients. MR4.5 was achieved in 15% of CP-CML patients by 12 months on the PACE trial.18 The 5-year update to this study reported that 60%, 40%, and 24% of CP-CML patients achieved MCyR, MMR, and MR4.5, respectively. In the patients who achieved MCyR, the probability of maintaining this response for 5 years was 82% and the estimated 5-year OS was 73%.19
Toxicity
In 2013, after the regulatory approval of ponatinib, reports became available that the drug can cause an increase in arterial occlusive events including fatal myocardial infarctions and cerebral vascular accidents. For this reason, dose reductions were implemented in patients who were deriving clinical benefit from ponatinib. In spite of these dose reductions, ≥90% of responders maintained their response for up to 40 months.19 Although the likelihood of developing an arterial occlusive event appears higher in the first year after starting ponatinib than in later years, the cumulative incidence of events continues to increase. The 5-year follow-up to the PACE trial reports 31% of patients experiencing any grade of arterial occlusive event while on ponatinib. Aside from these events, the most common treatment-emergent adverse events in ponatinib-treated patients on the PACE trial included rash (47%), abdominal pain (46%), headache (43%), dry skin (42%), constipation (41%), and hypertension (37%). Hematologic toxicity was also common, with 46% of patients experiencing any grade of thrombocytopenia, 20% experiencing neutropenia, and 20% anemia.19
Patients receiving ponatinib therapy should be monitored closely for any evidence of arterial or venous thrombosis. In the event of an occlusive event, ponatinib should be discontinued. Similarly, in the setting of any new or worsening heart failure symptoms, ponatinib should be promptly discontinued. Management of any underlying cardiovascular risk factors including hypertension, hyperlipidemia, diabetes, or smoking history is recommended, and these patients should be referred to a cardiologist for a full evaluation. In the absence of any contraindications to aspirin, low-dose aspirin should be considered as a means of decreasing cardiovascular risks associated with ponatinib. In patients with known risk factors, a ponatinib starting dose of 30 mg daily rather than the standard 45 mg daily may be a safer option resulting in fewer arterial occlusive events, although the efficacy of this dose is still being studied in comparison to 45 mg daily.7
In the event of ponatinib-induced transaminitis greater than 3 times the upper limit of normal, ponatinib should be held until resolution to less than 3 times the upper limit of normal, at which point it should be resumed at a lower dose. Similarly, in the setting of elevated serum lipase or symptomatic pancreatitis, ponatinib should be held and restarted at a lower dose after resolution of symptoms.7
In the event of neutropenia or thrombocytopenia, ponatinib should be held until blood count recovery and then restarted at the same dose. If cytopenias occur for a second time, the dose of ponatinib should be lowered at the time of treatment reinitiation. If rash occurs, it can be addressed with topical or systemic steroids as well as dose reduction, interruption, or discontinuation.7
Case Conclusion
Given the patient's high-risk Sokal score, ideal first-line treatment is a second-generation TKI in order to increase the likelihood of achieving the desired treatment milestones and improving long-term outcomes. Her history of uncontrolled diabetes and coronary artery disease raises concerns for using nilotinib. Furthermore, her history of COPD makes dasatinib suboptimal because she would have little pulmonary reserve if she were to develop a pleural effusion. For this reason, bosutinib 400 mg daily is chosen as her first-line TKI. Shortly after starting bosutinib, she experiences diarrhea that occurs approximately 3 or 4 times daily during the first week on treatment. She is able to manage this with over-the-counter loperamide and the diarrhea resolves shortly thereafter.
After 3 months of bosutinib therapy, quantitative real-time PCR (RQ-PCR) assay on peripheral blood is done to measure BCR-ABL1 transcripts, and the result is reported at 1.2% IS. This indicates that the patient has achieved an early molecular response, which is defined as a RQ-PCR value of ≤10% IS. She undergoes RQ-PCR monitoring every 3 months, and at 12 months her results indicate a value of 0.07% IS, suggesting she has achieved a MMR.
Conclusion
With the development of imatinib and the subsequent TKIs, dasatinib, nilotinib, bosutinib, and ponatinib, CP-CML has become a chronic disease with a life-expectancy that is similar to the general population. Given the successful treatments available for these patients, it is crucial to identify patients with this diagnosis, ensure they receive a complete, appropriate diagnostic workup including a bone marrow biopsy and aspiration with cytogenetic testing, and select the best therapy for each individual patient. Once on treatment, the importance of frequent monitoring cannot be overstated. This is the only way to be certain patients are achieving the desired treatment milestones that correlate with the favorable long-term outcomes that have been observed with TKI-based treatment of CP-CML.
1. Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037.
2. O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994-1004.
3. Hochhaus A, Larson RA, Guilhot F, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917-927.
4. Baccarani M, Druker BJ, Branford S, et al. Long-term response to imatinib is not affected by the initial dose in patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: final update from the Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) study. Int J Hematol. 2014;99:616-624.
5. Yeung DT, Osborn MP, White DL, et al. TIDEL-II: first-line use of imatinib in CML with early switch to nilotinib for failure to achieve time-dependent molecular targets. Blood. 2015;125:915-923.
6. Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408-2417.
7. Radich JP, Deininger M, Abboud CN, et al. Chronic Myeloid Leukemia, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1108-1135.
8. Shah NP, Rousselot P, Schiffer C, et al. Dasatinib in imatinib-resistant or -intolerant chronic-phase, chronic myeloid leukemia patients: 7-year follow-up of study CA180-034. Am J Hematol. 2016;91:869-874.
9. Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients trial. J Clin Oncol. 2016;34:2333-3340.
10. Quintas-Cardama A, Han X, Kantarjian H, Cortes J. Tyrosine kinase inhibitor-induced platelet dysfunction in patients with chronic myeloid leukemia. Blood. 2009;114:261-263.
11. Giles FJ, le Coutre PD, Pinilla-Ibarz J, et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia. 2013;27:107-112.
12. Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251-2259.
13. Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044-1054.
14. Cortes JE, Khoury HJ, Kantarjian HM, et al. Long-term bosutinib for chronic phase chronic myeloid leukemia after failure of imatinib plus dasatinib and/or nilotinib. Am J Hematol. 2016;91:1206-1214.
15. Gambacorti-Passerini C, Cortes JE, Lipton JH, et al. Safety and efficacy of second-line bosutinib for chronic phase chronic myeloid leukemia over a five-year period: final results of a phase I/II study. Haematologica. 2018;103:1298-1307.
16. Cortes JE, Kim DW, Kantarjian HM, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol. 2012;30:3486-3492.
17. Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36:231-237.
18. Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369:1783-1796.
19. Cortes JE, Kim DW, Pinilla-Ibarz J, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132:393-404.
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm that arises from a reciprocal translocation between the Abelson (ABL) region on chromosome 9 and the breakpoint cluster region (BCR) of chromosome 22, t(9;22)(q34;q11.2) (the Philadelphia chromosome), resulting in the generation of the BCR-ABL1 fusion gene and its protein product, BCR-ABL tyrosine kinase. BCR-ABL is a constitutively active fusion kinase that confers proliferative and survival advantage to hematopoietic cells through activation of downstream pathways.
CML is divided into 3 phases based on the number of myeloblasts observed in the blood or bone marrow: chronic, accelerated, and blast. Most cases of CML are diagnosed in the chronic phase (CP), which is marked by proliferation of primarily the myeloid element.
The advent of tyrosine kinase inhibitors (TKIs), a class of small molecules targeting the tyrosine kinases, particularly the BCR-ABL tyrosine kinase, led to rapid changes in the management of CML and improved survival for patients. Patients diagnosed with CP-CML now a have life-expectancy that is similar to that of the general population, as long as they receive the appropriate TKI therapy and adhere to treatment. As such, it is crucial to identify patients with CML, ensure they receive a complete, appropriate diagnostic work-up, and select the best therapy for each individual patient. The diagnosis and work-up of CML are reviewed in a separate article; here, the selection of TKI therapy for a patient with newly diagnosed CP-CML is reviewed.
Case Presentation
A 53-year-old woman who recently was diagnosed with CML presents to review her treatment options. The diagnosis was made after she presented to her primary care physician with fatigue, early satiety, left upper quadrant abdominal pain, and an 8-lb unintentional weight loss over the prior month. On physical exam her spleen was palpated 8 cm below the left costal margin. Laboratory evaluation showed a total white blood cell (WBC) count of 124,000/μL with a left-shifted differential including 6% basophils, 3% eosinophils, and 3% blasts; hemoglobin and platelet count were 12.4 g/dL and 801 × 103/µL, respectively. Fluorescent in-situ hybridization for BCR-ABL gene rearrangement using peripheral blood was positive in 87% of cells. Bone marrow biopsy and aspiration showed a 95% cellular bone marrow with granulocytic hyperplasia and 1% blasts. Cytogenetics were 46,XX,t(9;22)(q34;q11.2), and quantitative real-time polymerase chain reaction (RQ-PCR) to measure BCR-ABL1 transcripts in the peripheral blood showed a value of 98% international standard (IS). Her Sokal risk score was 1.42 (high risk). In addition, prior review of her past medical history revealed uncontrolled diabetes, coronary artery disease requiring placement of 3 cardiac stents 2 years prior, and chronic obstructive pulmonary disease (COPD) related to a 30-pack-year history of smoking.
- What factors must be considered when selecting first-line therapy for this patient?
Selection of the most appropriate first-line TKI for newly diagnosed CP-CML patients requires incorporation of many patient-specific factors. These factors include baseline karyotype and confirmation of CP-CML through bone marrow biopsy, Sokal or EURO risk score, and a thorough patient history, including a clear understanding of the patient's comorbidities. In this case, the patient's high Sokal risk score along with her history of diabetes, coronary artery disease, and COPD are all factors that must be accounted for when choosing the most appropriate TKI. The adverse effect profile of all TKIs must be considered in conjunction with the patient's ongoing medical issues in order to decrease the likelihood of worsening her current symptoms or causing a severe complication from TKI therapy.
Imatinib
The management of CML was revolutionized by the development and ultimate regulatory approval of imatinib mesylate in 2001. Imatinib was the first small-molecule cancer therapy developed and approved. It acts by binding to the adenosine triphosphate (ATP) binding site in the catalytic domain of BCR-ABL, thus inhibiting the oncoprotein's tyrosine kinase activity.1
The International Randomized Study of Interferon versus STI571 (IRIS) trial was a randomized phase 3 study that compared imatinib 400 mg daily to interferon α (IFNα) plus cytarabine. More than 1000 CP-CML patients were randomly assigned 1:1 to either imatinib or IFNα plus cytarabine and were assessed for event-free survival, hematologic and cytogenetic responses, freedom from progression to accelerated phase (AP) or blast phase (BP), and toxicity. Imatinib was superior to the prior standard of care for all these outcomes.2 The long-term follow up of the IRIS trial reported an 83% estimated 10-year overall survival (OS) and 79% estimated event-free survival for patients on the imatinib arm of this study.3 The cumulative rate of complete cytogenetic response (CCyR) was 82.8%. Of the 204 imatinib-treated patients who could undergo a molecular response evaluation at 10 years, 93.1% had a major molecular response (MMR) and 63.2% had a molecular response 4.5 (MR4.5), suggesting durable, deep molecular responses for many patients (see Chronic Myeloid Leukemia: Evaluation and Diagnosis for discussion of the hematologic parameters, cytogenetic results, and molecular responses ussed in monitoring response to TKI therapy). The estimated 10-year rate of freedom from progression to AP or BP was 92.1%.
Higher doses of imatinib (600-800 mg daily) have been studied in an attempt to overcome resistance and improve cytogenetic and molecular response rates. The Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) trial was a randomized phase 3 study that compared imatinib 800 mg daily to imatinib 400 mg daily. Although the 6-month assessments found increased rates of CCyR and a MMR in the higher-dose imatinib arm, these differences were no longer present at the 12-month assessment. Furthermore, the higher dose of imatinib led to a significantly higher incidence of grade 3/4 hematologic adverse events, and approximately 50% of patients on imatinib 800 mg daily required a dose reduction to less than 600 mg daily because of toxicity.4
The Therapeutic Intensification in De Novo Leukaemia (TIDEL) -II study used plasma trough levels of imatinib on day 22 of treatment with imatinib 600 mg daily to determine if patients should escalate the imatinib dose to 800 mg daily. In patients who did not meet molecular milestones at 3, 6, or 12 months, cohort 1 was dose escalated to imatinib 800 mg daily and subsequently switched to nilotinib 400 mg twice daily for failing the same target 3 months later, and cohort 2 was switched to nilotinib. At 2 years, 73% of patients achieved MMR and 34% achieved MR4.5, suggesting that initial treatment with higher-dose imatinib subsequently followed by a switch to nilotinib in those failing to achieve desired milestones could be an effective strategy for managing newly diagnosed CP-CML.5
Toxicity
Imatinib 400 mg is considered the standard starting dose in CP-CML patients. The safety profile of imatinib has been very well established. In the IRIS trial, the most common adverse events (all grades in decreasing order of frequency) were peripheral and periorbital edema (60%), nausea (50%), muscle cramps (49%), musculoskeletal pain (47%), diarrhea (45%), rash (40%), fatigue (39%), abdominal pain (37%), headache (37%), and joint pain (31%). Grade 3/4 liver enzyme elevation can occur in 5% of patients.6 In the event of severe liver toxicity or fluid retention, imatinib should be held until the event resolves. At that time, imatinib can be restarted if deemed appropriate, but this is dependent on the severity of the inciting event. Fluid retention can be managed by the use of supportive care, diuretics, imatinib dose reduction, dose interruption, or imatinib discontinuation if the fluid retention is severe. Muscle cramps can be managed by the use of a calcium supplements or tonic water. Management of rash can include topical or systemic steroids, or in some cases imatinib dose reduction, interruption, or discontinuation.7
Grade 3/4 imatinib-induced hematologic toxicity is not uncommon, with 17% of patients experiencing neutropenia, 9% thrombocytopenia, and 4% anemia. These adverse events occurred most commonly during the first year of therapy, and the frequency decreased over time.3,6 Depending on the degree of cytopenias, imatinib dosing should be interrupted until recovery of the absolute neutrophil count or platelet count, and can often be resumed at 400 mg daily. However, if cytopenias recur, imatinib should be held and subsequently restarted at 300 mg daily.7
Dasatinib
Dasatinib is a second-generation TKI that has regulatory approval for treatment of adult patients with newly diagnosed CP-CML or CP-CML in patients with resistance or intolerance to prior TKIs. In addition to dasatinib's ability to inhibit ABL kinases, it is also known to be a potent inhibitor of Src family kinases. Dasatinib has shown efficacy in patients who have developed imatinib-resistant ABL kinase domain mutations.
Dasatinib was initially approved as second-line therapy in patients with resistance or intolerance to imatinib. This indication was based on the results of the phase 3 CA180-034 trial which ultimately identified dasatinib 100 mg daily as the optimal dose. In this trial, 74% of patients enrolled had resistance to imatinib and the remainder were intolerant. The 7-year follow-up of patients randomized to dasatinib 100 mg (n = 167) daily indicated that 46% achieved MMR while on study. Of the 124 imatinib-resistant patients on dasatinib 100 mg daily, the 7-year progression-free survival (PFS) was 39% and OS was 63%. In the 43 imatinib-intolerant patients, the 7-year PFS was 51% and OS was 70%.8
Dasatinib 100 mg daily was compared to imatinib 400 mg daily in newly diagnosed CP-CML patients in the randomized phase 3 DASISION trial. More patients on the dasatinib arm achieved an early molecular response of BCR-ABL1 transcripts ≤10% IS after 3 months on treatment compared to imatinib (84% versus 64%). Furthermore, the 5-year follow-up reports that the cumulative incidence of MMR and MR4.5 in dasatinib-treated patients was 76% and 42%, and was 64% and 33%, with imatinib (P = 0.0022 and P = 0.0251, respectively). Fewer patients treated with dasatinib progressed to AP or BP (4.6%) compared to imatinib (7.3%), but the estimated 5-year OS was similar between the 2 arms (91% for dasatinib versus 90% for imatinib).9 Regulatory approval for dasatinib as first-line therapy in newly diagnosed CML patients was based on results of the DASISION trial.
Toxicity
Most dasatinib-related toxicities are reported as grade 1 or grade 2, but grade 3/4 hematologic adverse events are fairly common. In the DASISION trial, grade 3/4 neutropenia, anemia, and thrombocytopenia occurred in 29%, 13%, and 22% of dasatinib-treated patients, respectively. Cytopenias can generally be managed with temporary dose interruptions or dose reductions.
During the 5-year follow-up of the DASISION trial, pleural effusions were reported in 28% of patients, most of which were grade 1/2. This occurred at a rate of approximately ≤ 8% per year, suggesting a stable incidence over time, and the effusions appear to be dose-dependent.9 Depending on the severity of the effusion, this may be treated with diuretics, dose interruption, and in some instances, steroids or a thoracentesis. Typically, dasatinib can be restarted at 1 dose level lower than the previous dose once the effusion has resolved.7 Other, less common side effects of dasatinib include pulmonary hypertension (5% of patients), as well as abdominal pain, fluid retention, headaches, fatigue, musculoskeletal pain, rash, nausea, and diarrhea. Pulmonary hypertension is typically reversible after cessation of dasatinib, and thus dasatinib should be permanently discontinued once the diagnosis is confirmed. Fluid retention is often treated with diuretics and supportive care. Nausea and diarrhea are generally manageable and occur less frequently when dasatinib is taken with food and a large glass of water. Antiemetics and antidiarrheals can be used as needed. Troublesome rash can be best managed with topical or systemic steroids as well as possible dose reduction or dose interruption.7,9 In the DASISION trial, adverse events led to therapy discontinuation more often in the dasatinib group than in the imatinib group (16% versus 7%).9 Bleeding, particularly in the setting of thrombocytopenia, has been reported in patients being treated with dasatinib as a result of the drug-induced reversible inhibition of platelet aggregation.10
Nilotinib
The structure of nilotinib is similar to that of imatinib; however, it has a markedly increased affinity for the ATP‐binding site on the BCR-ABL1 protein. It was initially given regulatory approval in the setting of imatinib failure. Nilotinib was studied at a dose of 400 mg twice daily in 321 patients who were imatinib-resistant or -intolerant. It proved to be highly effective at inducing cytogenetic remissions in the second-line setting, with 59% of patients achieving a major cytogenetic response (MCyR) and 45% achieving CCyR. With a median follow-up time of 4 years, the OS was 78%.11
Nilotinib gained regulatory approval for use as a first-line TKI after completion of the randomized phase 3 ENESTnd (Evaluating Nilotinib Efficacy and Safety in Clinical Trials-Newly Diagnosed Patients) trial. ENESTnd was a 3-arm study comparing nilotinib 300 mg twice daily versus nilotinib 400 mg twice daily versus imatinib 400 mg daily in newly diagnosed, previously untreated patients diagnosed with CP-CML. The primary endpoint of this clinical trial was rate of MMR at 12 months.12 Nilotinib surpassed imatinib in this regard, with 44% of patients on nilotinib 300 mg twice daily achieving MMR at 12 months versus 43% of nilotinib 400 mg twice daily patients versus 22% of the imatinib-treated patients (P < 0.001 for both comparisons). Furthermore, the rate of CCyR by 12 months was significantly higher for both nilotinib arms compared with imatinib (80% for nilotinib 300 mg, 78% for nilotinib 400 mg, and 65% for imatinib) (P < 0.001).12 Based on this data, nilotinib 300 mg twice daily was chosen as the standard dose of nilotinib in the first-line setting. After 5 years of follow-up on the ENESTnd study, there were fewer progressions to AP/BP CML in nilotinib-treated patients compared with imatinib. MMR was achieved in 77% of nilotinib 300 mg patients compared with 60.4% of patients on the imatinib arm. MR4.5 was also more common in patients treated with nilotinib 300 mg twice daily, with a rate of 53.5% at 5 years versus 31.4% in the imatinib arm.13 In spite of the deeper cytogenetic and molecular responses achieved with nilotinib, this did not translate into a significant improvement in OS. The 5-year OS rate was 93.7% in nilotinib 300 mg patients versus 91.7% in imatinib-treated patients, and this difference lacked statistical significance.13
Toxicity
Although some similarities exist between the toxicity profiles of nilotinib and imatinib, each drug has some distinct adverse events. On the ENESTnd trial, the rate of any grade 3/4 non-hematologic adverse event was fairly low; however, lower-grade toxicities were not uncommon. Patients treated with nilotinib 300 mg twice daily experienced rash (31%), headache (14%), pruritis (15%), and fatigue (11%) most commonly. The most frequently reported laboratory abnormalities included increased total bilirubin (53%), hypophosphatemia (32%), hyperglycemia (36%), elevated lipase (24%), increased alanine aminotransferase (ALT; 66%), and increased aspartate aminotransferase (AST; 40%). Any grade of neutropenia, thrombocytopenia, or anemia occurred at rates of 43%, 48%, and 38%, respectively.12 Although nilotinib has a Black Box Warning from the US Food and Drug Administration for QT interval prolongation, no patients on the ENESTnd trial experienced a QT interval corrected for heart rate greater than 500 msec.12
More recent concerns have emerged regarding the potential for cardiovascular toxicity after long-term use of nilotinib. The 5-year update of ENESTnd reports cardiovascular events, including ischemic heart disease, ischemic cerebrovascular events, or peripheral arterial disease occurring in 7.5% of patients treated with nilotinib 300 mg twice daily compared with a rate of 2.1% in imatinib-treated patients. The frequency of these cardiovascular events increased linearly over time in both arms. Elevations in total cholesterol from baseline occurred in 27.6% of nilotinib patients compared with 3.9% of imatinib patients. Furthermore, clinically meaningful increases in low-density lipoprotein cholesterol and glycated hemoglobin occurred more frequently with nilotinib therapy.12
Nilotinib should be taken on an empty stomach; therefore, patients should be made aware of the need to fast for 2 hours prior to each dose and 1 hour after each dose. Given the potential risk of QT interval prolongation, a baseline electrocardiogram (ECG) is recommended prior to initiating treatment to ensure the QT interval is within a normal range. A repeat ECG should be done approximately 7 days after nilotinib initiation to ensure no prolongation of the QT interval after starting. Close monitoring of potassium and magnesium levels is important to decrease the risk of cardiac arrhythmias, and concomitant use of drugs considered strong CYP3A4 inhibitors should be avoided.7
If the patient experiences any grade 3 or higher laboratory abnormalities, nilotinib should be held until resolution of the toxicity, and then restarted at a lower dose. Similarly, if patients develop significant neutropenia or thrombocytopenia, nilotinib doses should be interrupted until resolution of the cytopenias. At that point, nilotinib can be reinitiated at either the same or a lower dose. Rash can be managed by the use of topical or systemic steroids as well as potential dose reduction, interruption, or discontinuation.
Given the concerns for potential cardiovascular events with long-term use of nilotinib, caution is advised when prescribing it to any patient with a history of cardiovascular disease or peripheral arterial occlusive disease. At the first sign of new occlusive disease, nilotinib should be discontinued.7
Bosutinib
Bosutinib is a second-generation BCR-ABL1 TKI with activity against the Src family of kinases that was initially approved to treat patients with CP-, AP-, or BP-CML after resistance or intolerance to imatinib. Long-term data has been reported from the phase 1/2 trial of bosutinib therapy in patients with CP-CML who developed resistance or intolerance to imatinib plus dasatinib and/or nilotinib. A total of 119 patients were included in the 4-year follow-up; 38 were resistant/intolerant to imatinib and resistant to dasatinib, 50 were resistant/intolerant to imatinib and intolerant to dasatinib, 26 were resistant/intolerant to imatinib and resistant to nilotinib, and 5 were resistant/intolerant to imatinib and intolerant to nilotinib or resistant/intolerant to dasatinib and nilotinib. Bosutinib 400 mg daily was studied in this setting. Of the 38 patients with imatinib resistance/intolerance and dasatinib resistance, 39% achieved MCyR, 22% achieved CCyR, and the OS was 67%. Of the 50 patients with imatinib resistance/intolerance and dasatinib intolerance, 42% achieved MCyR, 40% achieved CCyR, and the OS was 80%. Finally, in the 26 patients with imatinib resistance/intolerance and nilotinib resistance, 38% achieved MCyR, 31% achieved CcyR, and the OS was 87%.14
Five-year follow-up from the phase 1/2 clinical trial which studied bosutinib 500 mg daily in CP-CML patients after imatinib failure reported data on 284 patients. By 5 years on study, 60% of patients had achieved MCyR and 50% achieved CCyR with a 71% and 69% probability, respectively, of maintaining these responses at 5 years. The 5-year OS was 84%.15 These data led to the regulatory approval of bosutinib 500 mg daily as second-line or later therapy.
Bosutinib was initially studied in the first-line setting in the randomized phase 3 BELA (Bosutinib Efficacy and Safety in Newly Diagnosed Chronic Myeloid Leukemia) trial. This trial compared bosutinib 500 mg daily to imatinib 400 mg daily in newly diagnosed, previously untreated CP-CML patients. This trial failed to meet its primary endpoint of increased rate of CCyR at 12 months, with 70% of bosutinib patients achieving this response compared to 68% of imatinib-treated patients (P = 0.601). In spite of this, the rate of MMR at 12 months was significantly higher in the bosutinib arm (41%) compared to the imatinib arm (27%; P = 0.001).16
A second phase 3 trial (BFORE) was designed to study bosutinib 400 mg daily versus imatinib in newly diagnosed, previously untreated CP-CML patients. This study enrolled 536 patients who were randomly assigned 1:1 to bosutinib versus imatinib. The primary endpoint of this trial was rate of MMR at 12 months. A significantly higher number of bosutinib-treated patients achieved this response (47.2%) compared with imatinib-treated patients (36.9%, P = 0.02). Furthermore, by 12 months 77.2% of patients on the bosutinib arm had achieved CCyR compared with 66.4% on the imatinib arm, and this difference did meet statistical significance (P = 0.0075). A lower rate of progression to AP- or BP-CML was noted in bosutinib-treated patients as well (1.6% versus 2.5%). Based on this data, bosutinib gained regulatory approval for first-line therapy in CP-CML at a dose of 400 mg daily.17
Toxicity
On the BFORE trial, the most common treatment-emergent adverse events of any grade reported in the bosutinib-treated patients were diarrhea (70.1%), nausea (35.1%), increased ALT (30.6%), and increased AST (22.8%). Musculoskeletal pain or spasms occurred in 29.5% of patients, rash in 19.8%, fatigue in 19.4%, and headache in 18.7%. Hematologic toxicity was also reported, but most was grade 1/2. Thrombocytopenia was reported in 35.1%, anemia in 18.7%, and neutropenia in 11.2%.17
Cardiovascular events occurred in 5.2% of patients on the bosutinib arm of the BFORE trial, which was similar to the rate observed in imatinib patients. The most common cardiovascular event was QT interval prolongation, which occurred in 1.5% of patients. Pleural effusions were reported in 1.9% of patients treated with bosutinib, and none were grade 3 or higher.17
If liver enzyme elevation occurs at a value greater than 5 times the institutional upper limit of normal, bosutinib should be held until the level recovers to ≤2.5 times the upper limit of normal, at which point bosutinib can be restarted at a lower dose. If recovery takes longer than 4 weeks, bosutinib should be permanently discontinued. Liver enzymes elevated greater than 3 times the institutional upper limit of normal and a concurrent elevation in total bilirubin to 2 times the upper limit of normal is consistent with Hy's law, and bosutinib should be discontinued. Although diarrhea is the most common toxicity associated with bosutinib, it is commonly low grade and transient. Diarrhea occurs most frequently in the first few days after initiating bosutinib. It can often be managed with over-the-counter antidiarrheal medications, but if the diarrhea is grade or higher, bosutinib should be held until recovery to grade 1 or lower. Gastrointestinal side effects may be improved by taking bosutinib with a meal and a large glass of water. Fluid retention can be managed with diuretics and supportive care. Finally, if rash occurs, this can be addressed with topical or systemic steroids as well as bosutinib dose reduction, interruption, or discontinuation.7
Similar to other TKIs, if bosutinib-induced cytopenias occur, treatment should be held and restarted at the same or a lower dose upon blood count recovery.7
Ponatinib
The most common cause of TKI resistance in CP-CML is the development of ABL kinase domain mutations. The majority of imatinib-resistant mutations can be overcome by the use of second-generation TKIs including dasatinib, nilotinib, or bosutinib. However, ponatinib is the only BCR-ABL1 TKI able to overcome a T315I mutation. The phase 2 PACE (Ponatinib Ph-positive ALL and CML Evaluation) trial enrolled patients with CP-, AP-, or BP-CML as well as patients with Ph-positive acute lymphoblastic leukemia who were resistant or intolerant to nilotinib or dasatinib, or who had evidence of a T315I mutation. The starting dose of ponatinib on this trial was 45 mg daily.18 The PACE trial enrolled 267 patients with CP-CML: 203 with resistance or intolerance to nilotinib or dasatinib, and 64 with a T315I mutation. The primary endpoint in the CP cohort was rate of MCyR at any time within 12 months of starting ponatinib. The overall rate of MCyR by 12 months in the CP-CML patients was 56%. In those with a T315I mutation, 70% achieved MCyR, which compared favorably with those with resistance or intolerance to nilotinib or dasatinib, 51% of whom achieved MCyR. CCyR was achieved in 46% of CP-CML patients (40% in the resistant/intolerant cohort and 66% in the T315I cohort). In general, patients with T315I mutations received fewer prior therapies than those in the resistant/intolerant cohort, which likely contributed to the higher response rates in the T315I patients. MR4.5 was achieved in 15% of CP-CML patients by 12 months on the PACE trial.18 The 5-year update to this study reported that 60%, 40%, and 24% of CP-CML patients achieved MCyR, MMR, and MR4.5, respectively. In the patients who achieved MCyR, the probability of maintaining this response for 5 years was 82% and the estimated 5-year OS was 73%.19
Toxicity
In 2013, after the regulatory approval of ponatinib, reports became available that the drug can cause an increase in arterial occlusive events including fatal myocardial infarctions and cerebral vascular accidents. For this reason, dose reductions were implemented in patients who were deriving clinical benefit from ponatinib. In spite of these dose reductions, ≥90% of responders maintained their response for up to 40 months.19 Although the likelihood of developing an arterial occlusive event appears higher in the first year after starting ponatinib than in later years, the cumulative incidence of events continues to increase. The 5-year follow-up to the PACE trial reports 31% of patients experiencing any grade of arterial occlusive event while on ponatinib. Aside from these events, the most common treatment-emergent adverse events in ponatinib-treated patients on the PACE trial included rash (47%), abdominal pain (46%), headache (43%), dry skin (42%), constipation (41%), and hypertension (37%). Hematologic toxicity was also common, with 46% of patients experiencing any grade of thrombocytopenia, 20% experiencing neutropenia, and 20% anemia.19
Patients receiving ponatinib therapy should be monitored closely for any evidence of arterial or venous thrombosis. In the event of an occlusive event, ponatinib should be discontinued. Similarly, in the setting of any new or worsening heart failure symptoms, ponatinib should be promptly discontinued. Management of any underlying cardiovascular risk factors including hypertension, hyperlipidemia, diabetes, or smoking history is recommended, and these patients should be referred to a cardiologist for a full evaluation. In the absence of any contraindications to aspirin, low-dose aspirin should be considered as a means of decreasing cardiovascular risks associated with ponatinib. In patients with known risk factors, a ponatinib starting dose of 30 mg daily rather than the standard 45 mg daily may be a safer option resulting in fewer arterial occlusive events, although the efficacy of this dose is still being studied in comparison to 45 mg daily.7
In the event of ponatinib-induced transaminitis greater than 3 times the upper limit of normal, ponatinib should be held until resolution to less than 3 times the upper limit of normal, at which point it should be resumed at a lower dose. Similarly, in the setting of elevated serum lipase or symptomatic pancreatitis, ponatinib should be held and restarted at a lower dose after resolution of symptoms.7
In the event of neutropenia or thrombocytopenia, ponatinib should be held until blood count recovery and then restarted at the same dose. If cytopenias occur for a second time, the dose of ponatinib should be lowered at the time of treatment reinitiation. If rash occurs, it can be addressed with topical or systemic steroids as well as dose reduction, interruption, or discontinuation.7
Case Conclusion
Given the patient's high-risk Sokal score, ideal first-line treatment is a second-generation TKI in order to increase the likelihood of achieving the desired treatment milestones and improving long-term outcomes. Her history of uncontrolled diabetes and coronary artery disease raises concerns for using nilotinib. Furthermore, her history of COPD makes dasatinib suboptimal because she would have little pulmonary reserve if she were to develop a pleural effusion. For this reason, bosutinib 400 mg daily is chosen as her first-line TKI. Shortly after starting bosutinib, she experiences diarrhea that occurs approximately 3 or 4 times daily during the first week on treatment. She is able to manage this with over-the-counter loperamide and the diarrhea resolves shortly thereafter.
After 3 months of bosutinib therapy, quantitative real-time PCR (RQ-PCR) assay on peripheral blood is done to measure BCR-ABL1 transcripts, and the result is reported at 1.2% IS. This indicates that the patient has achieved an early molecular response, which is defined as a RQ-PCR value of ≤10% IS. She undergoes RQ-PCR monitoring every 3 months, and at 12 months her results indicate a value of 0.07% IS, suggesting she has achieved a MMR.
Conclusion
With the development of imatinib and the subsequent TKIs, dasatinib, nilotinib, bosutinib, and ponatinib, CP-CML has become a chronic disease with a life-expectancy that is similar to the general population. Given the successful treatments available for these patients, it is crucial to identify patients with this diagnosis, ensure they receive a complete, appropriate diagnostic workup including a bone marrow biopsy and aspiration with cytogenetic testing, and select the best therapy for each individual patient. Once on treatment, the importance of frequent monitoring cannot be overstated. This is the only way to be certain patients are achieving the desired treatment milestones that correlate with the favorable long-term outcomes that have been observed with TKI-based treatment of CP-CML.
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm that arises from a reciprocal translocation between the Abelson (ABL) region on chromosome 9 and the breakpoint cluster region (BCR) of chromosome 22, t(9;22)(q34;q11.2) (the Philadelphia chromosome), resulting in the generation of the BCR-ABL1 fusion gene and its protein product, BCR-ABL tyrosine kinase. BCR-ABL is a constitutively active fusion kinase that confers proliferative and survival advantage to hematopoietic cells through activation of downstream pathways.
CML is divided into 3 phases based on the number of myeloblasts observed in the blood or bone marrow: chronic, accelerated, and blast. Most cases of CML are diagnosed in the chronic phase (CP), which is marked by proliferation of primarily the myeloid element.
The advent of tyrosine kinase inhibitors (TKIs), a class of small molecules targeting the tyrosine kinases, particularly the BCR-ABL tyrosine kinase, led to rapid changes in the management of CML and improved survival for patients. Patients diagnosed with CP-CML now a have life-expectancy that is similar to that of the general population, as long as they receive the appropriate TKI therapy and adhere to treatment. As such, it is crucial to identify patients with CML, ensure they receive a complete, appropriate diagnostic work-up, and select the best therapy for each individual patient. The diagnosis and work-up of CML are reviewed in a separate article; here, the selection of TKI therapy for a patient with newly diagnosed CP-CML is reviewed.
Case Presentation
A 53-year-old woman who recently was diagnosed with CML presents to review her treatment options. The diagnosis was made after she presented to her primary care physician with fatigue, early satiety, left upper quadrant abdominal pain, and an 8-lb unintentional weight loss over the prior month. On physical exam her spleen was palpated 8 cm below the left costal margin. Laboratory evaluation showed a total white blood cell (WBC) count of 124,000/μL with a left-shifted differential including 6% basophils, 3% eosinophils, and 3% blasts; hemoglobin and platelet count were 12.4 g/dL and 801 × 103/µL, respectively. Fluorescent in-situ hybridization for BCR-ABL gene rearrangement using peripheral blood was positive in 87% of cells. Bone marrow biopsy and aspiration showed a 95% cellular bone marrow with granulocytic hyperplasia and 1% blasts. Cytogenetics were 46,XX,t(9;22)(q34;q11.2), and quantitative real-time polymerase chain reaction (RQ-PCR) to measure BCR-ABL1 transcripts in the peripheral blood showed a value of 98% international standard (IS). Her Sokal risk score was 1.42 (high risk). In addition, prior review of her past medical history revealed uncontrolled diabetes, coronary artery disease requiring placement of 3 cardiac stents 2 years prior, and chronic obstructive pulmonary disease (COPD) related to a 30-pack-year history of smoking.
- What factors must be considered when selecting first-line therapy for this patient?
Selection of the most appropriate first-line TKI for newly diagnosed CP-CML patients requires incorporation of many patient-specific factors. These factors include baseline karyotype and confirmation of CP-CML through bone marrow biopsy, Sokal or EURO risk score, and a thorough patient history, including a clear understanding of the patient's comorbidities. In this case, the patient's high Sokal risk score along with her history of diabetes, coronary artery disease, and COPD are all factors that must be accounted for when choosing the most appropriate TKI. The adverse effect profile of all TKIs must be considered in conjunction with the patient's ongoing medical issues in order to decrease the likelihood of worsening her current symptoms or causing a severe complication from TKI therapy.
Imatinib
The management of CML was revolutionized by the development and ultimate regulatory approval of imatinib mesylate in 2001. Imatinib was the first small-molecule cancer therapy developed and approved. It acts by binding to the adenosine triphosphate (ATP) binding site in the catalytic domain of BCR-ABL, thus inhibiting the oncoprotein's tyrosine kinase activity.1
The International Randomized Study of Interferon versus STI571 (IRIS) trial was a randomized phase 3 study that compared imatinib 400 mg daily to interferon α (IFNα) plus cytarabine. More than 1000 CP-CML patients were randomly assigned 1:1 to either imatinib or IFNα plus cytarabine and were assessed for event-free survival, hematologic and cytogenetic responses, freedom from progression to accelerated phase (AP) or blast phase (BP), and toxicity. Imatinib was superior to the prior standard of care for all these outcomes.2 The long-term follow up of the IRIS trial reported an 83% estimated 10-year overall survival (OS) and 79% estimated event-free survival for patients on the imatinib arm of this study.3 The cumulative rate of complete cytogenetic response (CCyR) was 82.8%. Of the 204 imatinib-treated patients who could undergo a molecular response evaluation at 10 years, 93.1% had a major molecular response (MMR) and 63.2% had a molecular response 4.5 (MR4.5), suggesting durable, deep molecular responses for many patients (see Chronic Myeloid Leukemia: Evaluation and Diagnosis for discussion of the hematologic parameters, cytogenetic results, and molecular responses ussed in monitoring response to TKI therapy). The estimated 10-year rate of freedom from progression to AP or BP was 92.1%.
Higher doses of imatinib (600-800 mg daily) have been studied in an attempt to overcome resistance and improve cytogenetic and molecular response rates. The Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) trial was a randomized phase 3 study that compared imatinib 800 mg daily to imatinib 400 mg daily. Although the 6-month assessments found increased rates of CCyR and a MMR in the higher-dose imatinib arm, these differences were no longer present at the 12-month assessment. Furthermore, the higher dose of imatinib led to a significantly higher incidence of grade 3/4 hematologic adverse events, and approximately 50% of patients on imatinib 800 mg daily required a dose reduction to less than 600 mg daily because of toxicity.4
The Therapeutic Intensification in De Novo Leukaemia (TIDEL) -II study used plasma trough levels of imatinib on day 22 of treatment with imatinib 600 mg daily to determine if patients should escalate the imatinib dose to 800 mg daily. In patients who did not meet molecular milestones at 3, 6, or 12 months, cohort 1 was dose escalated to imatinib 800 mg daily and subsequently switched to nilotinib 400 mg twice daily for failing the same target 3 months later, and cohort 2 was switched to nilotinib. At 2 years, 73% of patients achieved MMR and 34% achieved MR4.5, suggesting that initial treatment with higher-dose imatinib subsequently followed by a switch to nilotinib in those failing to achieve desired milestones could be an effective strategy for managing newly diagnosed CP-CML.5
Toxicity
Imatinib 400 mg is considered the standard starting dose in CP-CML patients. The safety profile of imatinib has been very well established. In the IRIS trial, the most common adverse events (all grades in decreasing order of frequency) were peripheral and periorbital edema (60%), nausea (50%), muscle cramps (49%), musculoskeletal pain (47%), diarrhea (45%), rash (40%), fatigue (39%), abdominal pain (37%), headache (37%), and joint pain (31%). Grade 3/4 liver enzyme elevation can occur in 5% of patients.6 In the event of severe liver toxicity or fluid retention, imatinib should be held until the event resolves. At that time, imatinib can be restarted if deemed appropriate, but this is dependent on the severity of the inciting event. Fluid retention can be managed by the use of supportive care, diuretics, imatinib dose reduction, dose interruption, or imatinib discontinuation if the fluid retention is severe. Muscle cramps can be managed by the use of a calcium supplements or tonic water. Management of rash can include topical or systemic steroids, or in some cases imatinib dose reduction, interruption, or discontinuation.7
Grade 3/4 imatinib-induced hematologic toxicity is not uncommon, with 17% of patients experiencing neutropenia, 9% thrombocytopenia, and 4% anemia. These adverse events occurred most commonly during the first year of therapy, and the frequency decreased over time.3,6 Depending on the degree of cytopenias, imatinib dosing should be interrupted until recovery of the absolute neutrophil count or platelet count, and can often be resumed at 400 mg daily. However, if cytopenias recur, imatinib should be held and subsequently restarted at 300 mg daily.7
Dasatinib
Dasatinib is a second-generation TKI that has regulatory approval for treatment of adult patients with newly diagnosed CP-CML or CP-CML in patients with resistance or intolerance to prior TKIs. In addition to dasatinib's ability to inhibit ABL kinases, it is also known to be a potent inhibitor of Src family kinases. Dasatinib has shown efficacy in patients who have developed imatinib-resistant ABL kinase domain mutations.
Dasatinib was initially approved as second-line therapy in patients with resistance or intolerance to imatinib. This indication was based on the results of the phase 3 CA180-034 trial which ultimately identified dasatinib 100 mg daily as the optimal dose. In this trial, 74% of patients enrolled had resistance to imatinib and the remainder were intolerant. The 7-year follow-up of patients randomized to dasatinib 100 mg (n = 167) daily indicated that 46% achieved MMR while on study. Of the 124 imatinib-resistant patients on dasatinib 100 mg daily, the 7-year progression-free survival (PFS) was 39% and OS was 63%. In the 43 imatinib-intolerant patients, the 7-year PFS was 51% and OS was 70%.8
Dasatinib 100 mg daily was compared to imatinib 400 mg daily in newly diagnosed CP-CML patients in the randomized phase 3 DASISION trial. More patients on the dasatinib arm achieved an early molecular response of BCR-ABL1 transcripts ≤10% IS after 3 months on treatment compared to imatinib (84% versus 64%). Furthermore, the 5-year follow-up reports that the cumulative incidence of MMR and MR4.5 in dasatinib-treated patients was 76% and 42%, and was 64% and 33%, with imatinib (P = 0.0022 and P = 0.0251, respectively). Fewer patients treated with dasatinib progressed to AP or BP (4.6%) compared to imatinib (7.3%), but the estimated 5-year OS was similar between the 2 arms (91% for dasatinib versus 90% for imatinib).9 Regulatory approval for dasatinib as first-line therapy in newly diagnosed CML patients was based on results of the DASISION trial.
Toxicity
Most dasatinib-related toxicities are reported as grade 1 or grade 2, but grade 3/4 hematologic adverse events are fairly common. In the DASISION trial, grade 3/4 neutropenia, anemia, and thrombocytopenia occurred in 29%, 13%, and 22% of dasatinib-treated patients, respectively. Cytopenias can generally be managed with temporary dose interruptions or dose reductions.
During the 5-year follow-up of the DASISION trial, pleural effusions were reported in 28% of patients, most of which were grade 1/2. This occurred at a rate of approximately ≤ 8% per year, suggesting a stable incidence over time, and the effusions appear to be dose-dependent.9 Depending on the severity of the effusion, this may be treated with diuretics, dose interruption, and in some instances, steroids or a thoracentesis. Typically, dasatinib can be restarted at 1 dose level lower than the previous dose once the effusion has resolved.7 Other, less common side effects of dasatinib include pulmonary hypertension (5% of patients), as well as abdominal pain, fluid retention, headaches, fatigue, musculoskeletal pain, rash, nausea, and diarrhea. Pulmonary hypertension is typically reversible after cessation of dasatinib, and thus dasatinib should be permanently discontinued once the diagnosis is confirmed. Fluid retention is often treated with diuretics and supportive care. Nausea and diarrhea are generally manageable and occur less frequently when dasatinib is taken with food and a large glass of water. Antiemetics and antidiarrheals can be used as needed. Troublesome rash can be best managed with topical or systemic steroids as well as possible dose reduction or dose interruption.7,9 In the DASISION trial, adverse events led to therapy discontinuation more often in the dasatinib group than in the imatinib group (16% versus 7%).9 Bleeding, particularly in the setting of thrombocytopenia, has been reported in patients being treated with dasatinib as a result of the drug-induced reversible inhibition of platelet aggregation.10
Nilotinib
The structure of nilotinib is similar to that of imatinib; however, it has a markedly increased affinity for the ATP‐binding site on the BCR-ABL1 protein. It was initially given regulatory approval in the setting of imatinib failure. Nilotinib was studied at a dose of 400 mg twice daily in 321 patients who were imatinib-resistant or -intolerant. It proved to be highly effective at inducing cytogenetic remissions in the second-line setting, with 59% of patients achieving a major cytogenetic response (MCyR) and 45% achieving CCyR. With a median follow-up time of 4 years, the OS was 78%.11
Nilotinib gained regulatory approval for use as a first-line TKI after completion of the randomized phase 3 ENESTnd (Evaluating Nilotinib Efficacy and Safety in Clinical Trials-Newly Diagnosed Patients) trial. ENESTnd was a 3-arm study comparing nilotinib 300 mg twice daily versus nilotinib 400 mg twice daily versus imatinib 400 mg daily in newly diagnosed, previously untreated patients diagnosed with CP-CML. The primary endpoint of this clinical trial was rate of MMR at 12 months.12 Nilotinib surpassed imatinib in this regard, with 44% of patients on nilotinib 300 mg twice daily achieving MMR at 12 months versus 43% of nilotinib 400 mg twice daily patients versus 22% of the imatinib-treated patients (P < 0.001 for both comparisons). Furthermore, the rate of CCyR by 12 months was significantly higher for both nilotinib arms compared with imatinib (80% for nilotinib 300 mg, 78% for nilotinib 400 mg, and 65% for imatinib) (P < 0.001).12 Based on this data, nilotinib 300 mg twice daily was chosen as the standard dose of nilotinib in the first-line setting. After 5 years of follow-up on the ENESTnd study, there were fewer progressions to AP/BP CML in nilotinib-treated patients compared with imatinib. MMR was achieved in 77% of nilotinib 300 mg patients compared with 60.4% of patients on the imatinib arm. MR4.5 was also more common in patients treated with nilotinib 300 mg twice daily, with a rate of 53.5% at 5 years versus 31.4% in the imatinib arm.13 In spite of the deeper cytogenetic and molecular responses achieved with nilotinib, this did not translate into a significant improvement in OS. The 5-year OS rate was 93.7% in nilotinib 300 mg patients versus 91.7% in imatinib-treated patients, and this difference lacked statistical significance.13
Toxicity
Although some similarities exist between the toxicity profiles of nilotinib and imatinib, each drug has some distinct adverse events. On the ENESTnd trial, the rate of any grade 3/4 non-hematologic adverse event was fairly low; however, lower-grade toxicities were not uncommon. Patients treated with nilotinib 300 mg twice daily experienced rash (31%), headache (14%), pruritis (15%), and fatigue (11%) most commonly. The most frequently reported laboratory abnormalities included increased total bilirubin (53%), hypophosphatemia (32%), hyperglycemia (36%), elevated lipase (24%), increased alanine aminotransferase (ALT; 66%), and increased aspartate aminotransferase (AST; 40%). Any grade of neutropenia, thrombocytopenia, or anemia occurred at rates of 43%, 48%, and 38%, respectively.12 Although nilotinib has a Black Box Warning from the US Food and Drug Administration for QT interval prolongation, no patients on the ENESTnd trial experienced a QT interval corrected for heart rate greater than 500 msec.12
More recent concerns have emerged regarding the potential for cardiovascular toxicity after long-term use of nilotinib. The 5-year update of ENESTnd reports cardiovascular events, including ischemic heart disease, ischemic cerebrovascular events, or peripheral arterial disease occurring in 7.5% of patients treated with nilotinib 300 mg twice daily compared with a rate of 2.1% in imatinib-treated patients. The frequency of these cardiovascular events increased linearly over time in both arms. Elevations in total cholesterol from baseline occurred in 27.6% of nilotinib patients compared with 3.9% of imatinib patients. Furthermore, clinically meaningful increases in low-density lipoprotein cholesterol and glycated hemoglobin occurred more frequently with nilotinib therapy.12
Nilotinib should be taken on an empty stomach; therefore, patients should be made aware of the need to fast for 2 hours prior to each dose and 1 hour after each dose. Given the potential risk of QT interval prolongation, a baseline electrocardiogram (ECG) is recommended prior to initiating treatment to ensure the QT interval is within a normal range. A repeat ECG should be done approximately 7 days after nilotinib initiation to ensure no prolongation of the QT interval after starting. Close monitoring of potassium and magnesium levels is important to decrease the risk of cardiac arrhythmias, and concomitant use of drugs considered strong CYP3A4 inhibitors should be avoided.7
If the patient experiences any grade 3 or higher laboratory abnormalities, nilotinib should be held until resolution of the toxicity, and then restarted at a lower dose. Similarly, if patients develop significant neutropenia or thrombocytopenia, nilotinib doses should be interrupted until resolution of the cytopenias. At that point, nilotinib can be reinitiated at either the same or a lower dose. Rash can be managed by the use of topical or systemic steroids as well as potential dose reduction, interruption, or discontinuation.
Given the concerns for potential cardiovascular events with long-term use of nilotinib, caution is advised when prescribing it to any patient with a history of cardiovascular disease or peripheral arterial occlusive disease. At the first sign of new occlusive disease, nilotinib should be discontinued.7
Bosutinib
Bosutinib is a second-generation BCR-ABL1 TKI with activity against the Src family of kinases that was initially approved to treat patients with CP-, AP-, or BP-CML after resistance or intolerance to imatinib. Long-term data has been reported from the phase 1/2 trial of bosutinib therapy in patients with CP-CML who developed resistance or intolerance to imatinib plus dasatinib and/or nilotinib. A total of 119 patients were included in the 4-year follow-up; 38 were resistant/intolerant to imatinib and resistant to dasatinib, 50 were resistant/intolerant to imatinib and intolerant to dasatinib, 26 were resistant/intolerant to imatinib and resistant to nilotinib, and 5 were resistant/intolerant to imatinib and intolerant to nilotinib or resistant/intolerant to dasatinib and nilotinib. Bosutinib 400 mg daily was studied in this setting. Of the 38 patients with imatinib resistance/intolerance and dasatinib resistance, 39% achieved MCyR, 22% achieved CCyR, and the OS was 67%. Of the 50 patients with imatinib resistance/intolerance and dasatinib intolerance, 42% achieved MCyR, 40% achieved CCyR, and the OS was 80%. Finally, in the 26 patients with imatinib resistance/intolerance and nilotinib resistance, 38% achieved MCyR, 31% achieved CcyR, and the OS was 87%.14
Five-year follow-up from the phase 1/2 clinical trial which studied bosutinib 500 mg daily in CP-CML patients after imatinib failure reported data on 284 patients. By 5 years on study, 60% of patients had achieved MCyR and 50% achieved CCyR with a 71% and 69% probability, respectively, of maintaining these responses at 5 years. The 5-year OS was 84%.15 These data led to the regulatory approval of bosutinib 500 mg daily as second-line or later therapy.
Bosutinib was initially studied in the first-line setting in the randomized phase 3 BELA (Bosutinib Efficacy and Safety in Newly Diagnosed Chronic Myeloid Leukemia) trial. This trial compared bosutinib 500 mg daily to imatinib 400 mg daily in newly diagnosed, previously untreated CP-CML patients. This trial failed to meet its primary endpoint of increased rate of CCyR at 12 months, with 70% of bosutinib patients achieving this response compared to 68% of imatinib-treated patients (P = 0.601). In spite of this, the rate of MMR at 12 months was significantly higher in the bosutinib arm (41%) compared to the imatinib arm (27%; P = 0.001).16
A second phase 3 trial (BFORE) was designed to study bosutinib 400 mg daily versus imatinib in newly diagnosed, previously untreated CP-CML patients. This study enrolled 536 patients who were randomly assigned 1:1 to bosutinib versus imatinib. The primary endpoint of this trial was rate of MMR at 12 months. A significantly higher number of bosutinib-treated patients achieved this response (47.2%) compared with imatinib-treated patients (36.9%, P = 0.02). Furthermore, by 12 months 77.2% of patients on the bosutinib arm had achieved CCyR compared with 66.4% on the imatinib arm, and this difference did meet statistical significance (P = 0.0075). A lower rate of progression to AP- or BP-CML was noted in bosutinib-treated patients as well (1.6% versus 2.5%). Based on this data, bosutinib gained regulatory approval for first-line therapy in CP-CML at a dose of 400 mg daily.17
Toxicity
On the BFORE trial, the most common treatment-emergent adverse events of any grade reported in the bosutinib-treated patients were diarrhea (70.1%), nausea (35.1%), increased ALT (30.6%), and increased AST (22.8%). Musculoskeletal pain or spasms occurred in 29.5% of patients, rash in 19.8%, fatigue in 19.4%, and headache in 18.7%. Hematologic toxicity was also reported, but most was grade 1/2. Thrombocytopenia was reported in 35.1%, anemia in 18.7%, and neutropenia in 11.2%.17
Cardiovascular events occurred in 5.2% of patients on the bosutinib arm of the BFORE trial, which was similar to the rate observed in imatinib patients. The most common cardiovascular event was QT interval prolongation, which occurred in 1.5% of patients. Pleural effusions were reported in 1.9% of patients treated with bosutinib, and none were grade 3 or higher.17
If liver enzyme elevation occurs at a value greater than 5 times the institutional upper limit of normal, bosutinib should be held until the level recovers to ≤2.5 times the upper limit of normal, at which point bosutinib can be restarted at a lower dose. If recovery takes longer than 4 weeks, bosutinib should be permanently discontinued. Liver enzymes elevated greater than 3 times the institutional upper limit of normal and a concurrent elevation in total bilirubin to 2 times the upper limit of normal is consistent with Hy's law, and bosutinib should be discontinued. Although diarrhea is the most common toxicity associated with bosutinib, it is commonly low grade and transient. Diarrhea occurs most frequently in the first few days after initiating bosutinib. It can often be managed with over-the-counter antidiarrheal medications, but if the diarrhea is grade or higher, bosutinib should be held until recovery to grade 1 or lower. Gastrointestinal side effects may be improved by taking bosutinib with a meal and a large glass of water. Fluid retention can be managed with diuretics and supportive care. Finally, if rash occurs, this can be addressed with topical or systemic steroids as well as bosutinib dose reduction, interruption, or discontinuation.7
Similar to other TKIs, if bosutinib-induced cytopenias occur, treatment should be held and restarted at the same or a lower dose upon blood count recovery.7
Ponatinib
The most common cause of TKI resistance in CP-CML is the development of ABL kinase domain mutations. The majority of imatinib-resistant mutations can be overcome by the use of second-generation TKIs including dasatinib, nilotinib, or bosutinib. However, ponatinib is the only BCR-ABL1 TKI able to overcome a T315I mutation. The phase 2 PACE (Ponatinib Ph-positive ALL and CML Evaluation) trial enrolled patients with CP-, AP-, or BP-CML as well as patients with Ph-positive acute lymphoblastic leukemia who were resistant or intolerant to nilotinib or dasatinib, or who had evidence of a T315I mutation. The starting dose of ponatinib on this trial was 45 mg daily.18 The PACE trial enrolled 267 patients with CP-CML: 203 with resistance or intolerance to nilotinib or dasatinib, and 64 with a T315I mutation. The primary endpoint in the CP cohort was rate of MCyR at any time within 12 months of starting ponatinib. The overall rate of MCyR by 12 months in the CP-CML patients was 56%. In those with a T315I mutation, 70% achieved MCyR, which compared favorably with those with resistance or intolerance to nilotinib or dasatinib, 51% of whom achieved MCyR. CCyR was achieved in 46% of CP-CML patients (40% in the resistant/intolerant cohort and 66% in the T315I cohort). In general, patients with T315I mutations received fewer prior therapies than those in the resistant/intolerant cohort, which likely contributed to the higher response rates in the T315I patients. MR4.5 was achieved in 15% of CP-CML patients by 12 months on the PACE trial.18 The 5-year update to this study reported that 60%, 40%, and 24% of CP-CML patients achieved MCyR, MMR, and MR4.5, respectively. In the patients who achieved MCyR, the probability of maintaining this response for 5 years was 82% and the estimated 5-year OS was 73%.19
Toxicity
In 2013, after the regulatory approval of ponatinib, reports became available that the drug can cause an increase in arterial occlusive events including fatal myocardial infarctions and cerebral vascular accidents. For this reason, dose reductions were implemented in patients who were deriving clinical benefit from ponatinib. In spite of these dose reductions, ≥90% of responders maintained their response for up to 40 months.19 Although the likelihood of developing an arterial occlusive event appears higher in the first year after starting ponatinib than in later years, the cumulative incidence of events continues to increase. The 5-year follow-up to the PACE trial reports 31% of patients experiencing any grade of arterial occlusive event while on ponatinib. Aside from these events, the most common treatment-emergent adverse events in ponatinib-treated patients on the PACE trial included rash (47%), abdominal pain (46%), headache (43%), dry skin (42%), constipation (41%), and hypertension (37%). Hematologic toxicity was also common, with 46% of patients experiencing any grade of thrombocytopenia, 20% experiencing neutropenia, and 20% anemia.19
Patients receiving ponatinib therapy should be monitored closely for any evidence of arterial or venous thrombosis. In the event of an occlusive event, ponatinib should be discontinued. Similarly, in the setting of any new or worsening heart failure symptoms, ponatinib should be promptly discontinued. Management of any underlying cardiovascular risk factors including hypertension, hyperlipidemia, diabetes, or smoking history is recommended, and these patients should be referred to a cardiologist for a full evaluation. In the absence of any contraindications to aspirin, low-dose aspirin should be considered as a means of decreasing cardiovascular risks associated with ponatinib. In patients with known risk factors, a ponatinib starting dose of 30 mg daily rather than the standard 45 mg daily may be a safer option resulting in fewer arterial occlusive events, although the efficacy of this dose is still being studied in comparison to 45 mg daily.7
In the event of ponatinib-induced transaminitis greater than 3 times the upper limit of normal, ponatinib should be held until resolution to less than 3 times the upper limit of normal, at which point it should be resumed at a lower dose. Similarly, in the setting of elevated serum lipase or symptomatic pancreatitis, ponatinib should be held and restarted at a lower dose after resolution of symptoms.7
In the event of neutropenia or thrombocytopenia, ponatinib should be held until blood count recovery and then restarted at the same dose. If cytopenias occur for a second time, the dose of ponatinib should be lowered at the time of treatment reinitiation. If rash occurs, it can be addressed with topical or systemic steroids as well as dose reduction, interruption, or discontinuation.7
Case Conclusion
Given the patient's high-risk Sokal score, ideal first-line treatment is a second-generation TKI in order to increase the likelihood of achieving the desired treatment milestones and improving long-term outcomes. Her history of uncontrolled diabetes and coronary artery disease raises concerns for using nilotinib. Furthermore, her history of COPD makes dasatinib suboptimal because she would have little pulmonary reserve if she were to develop a pleural effusion. For this reason, bosutinib 400 mg daily is chosen as her first-line TKI. Shortly after starting bosutinib, she experiences diarrhea that occurs approximately 3 or 4 times daily during the first week on treatment. She is able to manage this with over-the-counter loperamide and the diarrhea resolves shortly thereafter.
After 3 months of bosutinib therapy, quantitative real-time PCR (RQ-PCR) assay on peripheral blood is done to measure BCR-ABL1 transcripts, and the result is reported at 1.2% IS. This indicates that the patient has achieved an early molecular response, which is defined as a RQ-PCR value of ≤10% IS. She undergoes RQ-PCR monitoring every 3 months, and at 12 months her results indicate a value of 0.07% IS, suggesting she has achieved a MMR.
Conclusion
With the development of imatinib and the subsequent TKIs, dasatinib, nilotinib, bosutinib, and ponatinib, CP-CML has become a chronic disease with a life-expectancy that is similar to the general population. Given the successful treatments available for these patients, it is crucial to identify patients with this diagnosis, ensure they receive a complete, appropriate diagnostic workup including a bone marrow biopsy and aspiration with cytogenetic testing, and select the best therapy for each individual patient. Once on treatment, the importance of frequent monitoring cannot be overstated. This is the only way to be certain patients are achieving the desired treatment milestones that correlate with the favorable long-term outcomes that have been observed with TKI-based treatment of CP-CML.
1. Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037.
2. O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994-1004.
3. Hochhaus A, Larson RA, Guilhot F, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917-927.
4. Baccarani M, Druker BJ, Branford S, et al. Long-term response to imatinib is not affected by the initial dose in patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: final update from the Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) study. Int J Hematol. 2014;99:616-624.
5. Yeung DT, Osborn MP, White DL, et al. TIDEL-II: first-line use of imatinib in CML with early switch to nilotinib for failure to achieve time-dependent molecular targets. Blood. 2015;125:915-923.
6. Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408-2417.
7. Radich JP, Deininger M, Abboud CN, et al. Chronic Myeloid Leukemia, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1108-1135.
8. Shah NP, Rousselot P, Schiffer C, et al. Dasatinib in imatinib-resistant or -intolerant chronic-phase, chronic myeloid leukemia patients: 7-year follow-up of study CA180-034. Am J Hematol. 2016;91:869-874.
9. Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients trial. J Clin Oncol. 2016;34:2333-3340.
10. Quintas-Cardama A, Han X, Kantarjian H, Cortes J. Tyrosine kinase inhibitor-induced platelet dysfunction in patients with chronic myeloid leukemia. Blood. 2009;114:261-263.
11. Giles FJ, le Coutre PD, Pinilla-Ibarz J, et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia. 2013;27:107-112.
12. Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251-2259.
13. Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044-1054.
14. Cortes JE, Khoury HJ, Kantarjian HM, et al. Long-term bosutinib for chronic phase chronic myeloid leukemia after failure of imatinib plus dasatinib and/or nilotinib. Am J Hematol. 2016;91:1206-1214.
15. Gambacorti-Passerini C, Cortes JE, Lipton JH, et al. Safety and efficacy of second-line bosutinib for chronic phase chronic myeloid leukemia over a five-year period: final results of a phase I/II study. Haematologica. 2018;103:1298-1307.
16. Cortes JE, Kim DW, Kantarjian HM, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol. 2012;30:3486-3492.
17. Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36:231-237.
18. Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369:1783-1796.
19. Cortes JE, Kim DW, Pinilla-Ibarz J, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132:393-404.
1. Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037.
2. O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994-1004.
3. Hochhaus A, Larson RA, Guilhot F, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917-927.
4. Baccarani M, Druker BJ, Branford S, et al. Long-term response to imatinib is not affected by the initial dose in patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: final update from the Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) study. Int J Hematol. 2014;99:616-624.
5. Yeung DT, Osborn MP, White DL, et al. TIDEL-II: first-line use of imatinib in CML with early switch to nilotinib for failure to achieve time-dependent molecular targets. Blood. 2015;125:915-923.
6. Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408-2417.
7. Radich JP, Deininger M, Abboud CN, et al. Chronic Myeloid Leukemia, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1108-1135.
8. Shah NP, Rousselot P, Schiffer C, et al. Dasatinib in imatinib-resistant or -intolerant chronic-phase, chronic myeloid leukemia patients: 7-year follow-up of study CA180-034. Am J Hematol. 2016;91:869-874.
9. Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients trial. J Clin Oncol. 2016;34:2333-3340.
10. Quintas-Cardama A, Han X, Kantarjian H, Cortes J. Tyrosine kinase inhibitor-induced platelet dysfunction in patients with chronic myeloid leukemia. Blood. 2009;114:261-263.
11. Giles FJ, le Coutre PD, Pinilla-Ibarz J, et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia. 2013;27:107-112.
12. Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251-2259.
13. Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044-1054.
14. Cortes JE, Khoury HJ, Kantarjian HM, et al. Long-term bosutinib for chronic phase chronic myeloid leukemia after failure of imatinib plus dasatinib and/or nilotinib. Am J Hematol. 2016;91:1206-1214.
15. Gambacorti-Passerini C, Cortes JE, Lipton JH, et al. Safety and efficacy of second-line bosutinib for chronic phase chronic myeloid leukemia over a five-year period: final results of a phase I/II study. Haematologica. 2018;103:1298-1307.
16. Cortes JE, Kim DW, Kantarjian HM, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol. 2012;30:3486-3492.
17. Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36:231-237.
18. Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369:1783-1796.
19. Cortes JE, Kim DW, Pinilla-Ibarz J, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132:393-404.
Chronic Myeloid Leukemia: Evaluation and Diagnosis
Chronic myeloid leukemia (CML) is a rare myeloproliferative neoplasm that is characterized by the presence of the Philadelphia (Ph) chromosome and uninhibited expansion of bone marrow stem cells. The Ph chromosome arises from a reciprocal translocation between the Abelson (ABL) region on chromosome 9 and the breakpoint cluster region (BCR) of chromosome 22 (t(9;22)(q34;q11.2), resulting in the BCR-ABL1 fusion gene.1BCR-ABL1 encodes an oncoprotein with constitutive tyrosine kinase activity that promotes growth and replication through downstream pathways, which is the driving factor in the pathogenesis of CML.1
Typical treatment for CML involves life-long use of oral BCR-ABL tyrosine kinase inhibitors (TKI). Currently, 5 TKIs have regulatory approval for treatment of this disease. With the introduction of imatinib in 2001 and the subsequent development of second- (dasatinib, nilotinib, bosutinib) and third-generation (ponatinib) TKIs, CML has become a chronic disease with a life-expectancy that is similar to that of the general population. This article reviews the diagnosis of CML and the parameters used for monitoring response to TKI therapy; the selection of initial TKI therapy is reviewed in a separate follow-up article.
Epidemiology
According to SEER data estimates, 8430 new cases of CML were diagnosed in the United States in 2018. CML is a disease of older adults, with a median age of 65 years at diagnosis, and there is a slight male predominance. Between 2011 and 2015, the number of new CML cases was 1.8 per 100,000 persons. The median overall survival (OS) in patients with newly diagnosed chronic-phase CML (CP-CML) has not been reached.2 Given the effective treatments available for managing CML, it is estimated that the prevalence of CML in the United States will plateau at 180,000 patients by 2050.3
Diagnosis
Case Presentation
A 53-year-old woman presents to her primary care physician with complaints of fatigue, early satiety, left upper quadrant abdominal pain, and an 8-lb unintentional weight loss over the prior month. Her past medical history is significant for uncontrolled diabetes, coronary artery disease requiring placement of 3 cardiac stents 2 years prior, and chronic obstructive pulmonary disease (COPD) related to a 30-pack-year history of smoking. On physicial exam her spleen is palpated 8 cm below the left costal margin. A complete blood count (CBC) with differential identifies a total white blood cell (WBC) count of 124,000/μL, with a left-shifted differential including 6% basophils, 3% eosinophils, and 3% blasts; hemoglobin is 12.4 g/dL and platelet count is 801 × 103/µL.
- How is the diagnosis of CML made?
Clinical Features
The diagnosis of CML is often suspected based on an incidental finding of leukocytosis and, in some cases, thrombocytosis. In many cases, this is an incidental finding on routine blood work, but approximately 50% of patients will present with constitutional symptoms associated with the disease. Characteristic features of the WBC differential include left-shifted maturation with neutrophilia and immature circulating myeloid cells. Basophilia and eosinophilia are often present as well. Splenomegaly is a common sign, present in 50% to 90% of patients at diagnosis. In those patients with symptoms related to CML at diagnosis, the most common presentation includes increasing fatigue, fevers, night sweats, early satiety, and weight loss. The diagnosis is confirmed by cytogenetic studies showing the Ph chromosome abnormality, t(9; 22)(q3.4;q1.1), and/or reverse transcriptase polymerase chain reaction (PCR) showing BCR-ABL1 transcripts.
- What further testing is needed when evaluating a patient for CML?
There are 3 distinct phases of CML: chronic phase (CP), accelerated phase (AP), and blast phase (BP). Bone marrow biopsy and aspiration at diagnosis are mandatory in order to determine the phase of the disease at diagnosis. This distinction is based on the percentage of blasts, promyelocytes, and basophils present as well as the platelet count and presence or absence of extramedullary disease.4 The vast majority of patients at diagnosis have CML that is in the chronic phase. The typical appearance in CP-CML is a hypercellular marrow with granulocytic and occasionally megakaryocytic hyperplasia. In many cases, basophilia and/or eosinophilia are noted as well. Dysplasia is not a typical finding in CML.5 Bone marrow fibrosis can be seen in up to one-third of patients at diagnosis, and may indicate a slightly worse prognosis.6 Although a diagnosis of CML can be made without a bone marrow biopsy, complete staging and prognostication are only possible with information gained from this test, including baseline karyotype and confirmation of CP versus a more advanced phase of CML.
The criteria for diagnosing AP-CML has not been agreed upon by various groups, but the modified MD Anderson Cancer Center (MDACC) criteria are used in the majority of clinical trials evaluating the efficacy of TKIs in preventing progression to advanced phases of CML. MDACC criteria define AP-CML as the presence of one of the following: 15% to 29% blasts in the peripheral blood or bone marrow, ≥ 30% peripheral blasts plus promyelocytes, ≥ 20% basophils in the blood or bone marrow, platelet count ≤ 100 × 103/μL unrelated to therapy, and clonal cytogenetic evolution in Ph-positive metaphases (Table).7
BP-CML is typically defined using the criteria developed by the International Bone Marrow Transplant Registry (IBMTR): ≥ 30% blasts in the peripheral blood and/or the bone marrow or the presence of extramedullary disease.8 Although not typically used in clinical trials, the revised World Health Organization (WHO) criteria for BP-CML include ≥ 20% blasts in the peripheral blood or bone marrow, extramedullary blast proliferation, and large foci or clusters of blasts in the bone marrow biopsy (Table).9 The defining feature of CML is the presence of the Ph chromosome abnormality. In a small subset of patients, additional chromosomal abnormalities (ACA) in the Ph-positive cells may be identified at diagnosis. Some reports indicate that the presence of “major route” ACA (trisomy 8, isochromosome 17q, a second Ph chromosome, or trisomy 19) at diagnosis may negatively impact prognosis, but other reports contradict these findings.10,11
The typical BCR breakpoint in CML is the major breakpoint cluster region (M-BCR), which results in a 210-kDa protein (p210). Alternate breakpoints that are less frequently identified are the minor BCR (mBCR or p190), which is more commonly found in Ph-positive acute lymphoblastic leukemia (ALL), and the micro BCR (µBCR or p230), which is much less common and is often characterized by chronic neutrophilia.12 Identifying which BCR-ABL1 transcript is present in each patient using qualitative PCR is crucial in order to ensure proper monitoring during treatment.
The most sensitive method for detecting BCR-ABL1 mRNA transcripts is the quantitative real-time PCR (RQ-PCR) assay, which is typically done on peripheral blood. RQ-PCR is capable of detecting a single CML cell in the presence of ≥ 100,000 normal cells. This test should be done during the initial diagnostic workup in order to confirm the presence of BCR-ABL1 transcripts, and it is used as a standard method for monitoring response to TKI therapy.13 The International Scale (IS) is a standardized approach to reporting RQ-PCR results that was developed to allow comparison of results across various laboratories and has become the gold standard for reporting BCR-ABL1 transcript values.14
Determining Risk Scores
Calculating a patient’s Sokal score or EURO risk score at diagnosis remains an important component of the diagnostic workup in CP-CML, as this information has prognostic and therapeutic implications (an online calculator is available through European LeukemiaNet [ELN]). The risk for disease progression to the accelerated or blast phases is higher in patients with intermediate- or high-risk scores compared to those with a low-risk score at diagnosis. The risk of progression in intermediate- or high-risk patients is lower when a second-generation TKI (dasatinib, nilotinib, or bosutinib) is used as frontline therapy compared to imatinib, and therefore, the National Comprehensive Cancer Network (NCCN) CML Panel recommends starting with a second-generation TKI in these patients.15-19
Monitoring Response to Therapy
Case Continued
Fluorescent in-situ hybridization using a peripheral blood sample to detect the BCR-ABL gene rearrangement is performed and is positive in 87% of cells. Bone marrow biopsy and aspiration show a 95% cellular bone marrow with granulocytic hyperplasia and 1% blasts. Cytogenetics are 46,XX,t(9;22)(q34;q11.2).20 RQ-PCR assay performed to measure BCR-ABL1 transcripts in the peripheral blood shows a value of 98% IS. The patient is ultimately given a diagnosis of CP-CML. Her Sokal risk score is 1.42, making her disease high risk.
How is response to TKI therapy measured and monitored?
After confirming a diagnosis of CML and selecting the most appropriate TKI for first-line therapy, the successful management of a CML patient relies on close monitoring and follow-up to ensure patients are meeting the desired treatment milestones. Responses in CML can be assessed based on hematologic parameters, cytogenetic results, and molecular responses. A complete hematologic response (CHR) implies complete normalization of peripheral blood counts (with the exception of TKI-induced cytopenias) and resolution of any palpable splenomegaly. The majority of patients will achieve a CHR within 4 to 6 weeks after initiating CML-directed therapy.21
Cytogenetic Response
Cytogenetic responses are defined by the decrease in the number of Ph chromosome–positive metaphases when assessed on bone marrow cytogenetics. A partial cytogenetic response (PCyR) is defined as having 1% to 35% Ph-positive metaphases, a major cytogenetic response (MCyR) as having 0% to 35% Ph-positive metaphases, and a CCyR implies that no Ph-positive metaphases are identified on bone marrow cytogenetics. An ideal response is the achievement of PCyR after 3 months on a TKI and a CCyR after 12 months on a TKI.22
Molecular Response
Once a patient has achieved a CCyR, monitoring their response to therapy can only be done using RQ-PCR to measure BCR-ABL1 transcripts in the peripheral blood. The NCCN and the ELN recommend monitoring RQ-PCR from the peripheral blood every 3 months in order to assess response to TKIs.19,23 As noted, the International Scale (IS) has become the gold standard reporting system for all BCR-ABL1 transcript levels in the majority of laboratories worldwide.14,24 Molecular responses are based on a log-reduction in BCR-ABL1 transcripts from a standardized baseline. Many molecular responses can be correlated with cytogenetic responses such that if reliable RQ-PCR testing is available, monitoring can be done using only peripheral blood RQ-PCR rather than repeat bone marrow biopsies. For example, an early molecular response (EMR) is defined as a RQ-PCR value of ≤ 10% IS, which is approximately equivalent to a PCyR.25 A value of 1% IS is approximately equivalent to CCyR. A major molecular response (MMR) is a ≥ 3-log reduction in BCR-ABL1 transcripts from baseline and is a value of ≤ 0.1% IS. Deeper levels of molecular response are best described by the log-reduction in BCR-ABL1 transcripts, with a 4-log reduction denoted as MR4.0, a 4.5-log reduction as MR4.5, and so forth. Complete molecular response (CMR) is defined by the level of sensitivity of the RQ-PCR assay being used.14
The definition of relapsed disease in CML is dependent on the type of response the patient had previously achieved. Relapse could be the loss of a hematologic or cytogenetic response, but fluctuations in BCR-ABL1 transcripts on routine RQ-PCR do not necessarily indicate relapsed CML. A 1-log increase in the level of BCR-ABL1 transcripts with a concurrent loss of MMR should prompt a bone marrow biopsy in order to assess for the loss of CCyR, and thus a cytogenetic relapse; however, this loss of MMR does not define relapse in and of itself. In the setting of relapsed disease, testing should be done to look for possible ABL kinase domain mutations, and alternate therapy should be selected.19
Multiple reports have identified the prognostic relevance of achieving an EMR at 3 and 6 months after starting TKI therapy. Marin and colleagues reported that in 282 imatinib-treated patients, there was a significant improvement in 8-year OS, progression-free survival, and cumulative incidence of CCyR and CMR in patients who had BCR-ABL1 transcripts < 9.84% IS after 3 months on treatment.25 This data highlights the importance of early molecular monitoring in order to ensure the best outcomes for patients with CP-CML.
The NCCN CML guidelines and ELN recommendations both agree that an ideal response after 3 months on a TKI is BCR-ABL1 transcripts < 10% IS, but treatment is not considered to be failing at this point if the patient marginally misses this milestone. After 6 months on treatment, an ideal response is considered BCR-ABL1 transcripts < 1%–10% IS. Ideally, patients will have BCR-ABL1 transcripts < 0.1%–1% IS by the time they complete 12 months of TKI therapy, suggesting that these patients have at least achieved a CCyR.19,23 Even after patients achieve these early milestones, frequent monitoring by RQ-PCR is required to ensure that they are maintaining their response to treatment. This will help to ensure patient compliance with treatment and will also help to identify a select subset of patients who could potentially be considered for an attempt at TKI cessation (not discussed in detail here) after a minimum of 3 years on therapy.19,26
Conclusion
Given the successful treatments available for patients with CML, it is crucial to identify patients with this disease, ensure they receive a complete, appropriate diagnostic workup including a bone marrow biopsy and aspiration with cytogenetic testing, and select the best therapy for each individual patient. Once on treatment, the importance of frequent monitoring cannot be overstated.
1. Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164-172.
2. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Leukemia - Chronic Myeloid Leukemia (CML). 2018.
3. Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer. 2012;118:3123-3127.
4. Savage DG, Szydlo RM, Chase A, et al. Bone marrow transplantation for chronic myeloid leukaemia: the effects of differing criteria for defining chronic phase on probabilities of survival and relapse. Br J Haematol. 1997;99:30-35.
5. Knox WF, Bhavnani M, Davson J, Geary CG. Histological classification of chronic granulocytic leukaemia. Clin Lab Haematol. 1984;6:171-175.
6. Kvasnicka HM, Thiele J, Schmitt-Graeff A, et al. Impact of bone marrow morphology on multivariate risk classification in chronic myelogenous leukemia. Acta Haematol. 2003;109:53-56.
7. Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306-1315.
8. Druker BJ. Chronic myeloid leukemia In: DeVita VT, Lawrence TS, Rosenburg SA, eds. DeVita, Hellman, and Rosenberg’s Cancer Principles & Practice of Oncology. 8th ed. Philadelphia, PA: Lippincott, Williams and Wilkins; 2007:2267-2304.
9. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405.
10. Fabarius A, Leitner A, Hochhaus A, et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118:6760-6768.
11. Alhuraiji A, Kantarjian H, Boddu P, et al. Prognostic significance of additional chromosomal abnormalities at the time of diagnosis in patients with chronic myeloid leukemia treated with frontline tyrosine kinase inhibitors. Am J Hematol. 2018;93:84-90.
12. Melo JV. BCR-ABL gene variants. Baillieres Clin Haematol. 1997;10:203-222.
13. Kantarjian HM, Talpaz M, Cortes J, et al. Quantitative polymerase chain reaction monitoring of BCR-ABL during therapy with imatinib mesylate (STI571; gleevec) in chronic-phase chronic myelogenous leukemia. Clin Cancer Res. 2003;9:160-166.
14. Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28-37.
15. Hochhaus A, Larson RA, Guilhot F, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917-927.
16. Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients trial. J Clin Oncol. 2016;34:2333-3340.
17. Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044-1054.
18. Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36:231-237.
19. Radich JP, Deininger M, Abboud CN, et al. Chronic Myeloid Leukemia, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1108-1135.
20. Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037.
21. Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Ann Intern Med. 1999;131:207-219.
22. O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994-1004.
23. Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872-884.
24. Larripa I, Ruiz MS, Gutierrez M, Bianchini M. [Guidelines for molecular monitoring of BCR-ABL1 in chronic myeloid leukemia patients by RT-qPCR.] Medicina (B Aires). 2017;77:61-72.
25. Marin D, Ibrahim AR, Lucas C, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012;30:232-238.
26. Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128:17-23.
Chronic myeloid leukemia (CML) is a rare myeloproliferative neoplasm that is characterized by the presence of the Philadelphia (Ph) chromosome and uninhibited expansion of bone marrow stem cells. The Ph chromosome arises from a reciprocal translocation between the Abelson (ABL) region on chromosome 9 and the breakpoint cluster region (BCR) of chromosome 22 (t(9;22)(q34;q11.2), resulting in the BCR-ABL1 fusion gene.1BCR-ABL1 encodes an oncoprotein with constitutive tyrosine kinase activity that promotes growth and replication through downstream pathways, which is the driving factor in the pathogenesis of CML.1
Typical treatment for CML involves life-long use of oral BCR-ABL tyrosine kinase inhibitors (TKI). Currently, 5 TKIs have regulatory approval for treatment of this disease. With the introduction of imatinib in 2001 and the subsequent development of second- (dasatinib, nilotinib, bosutinib) and third-generation (ponatinib) TKIs, CML has become a chronic disease with a life-expectancy that is similar to that of the general population. This article reviews the diagnosis of CML and the parameters used for monitoring response to TKI therapy; the selection of initial TKI therapy is reviewed in a separate follow-up article.
Epidemiology
According to SEER data estimates, 8430 new cases of CML were diagnosed in the United States in 2018. CML is a disease of older adults, with a median age of 65 years at diagnosis, and there is a slight male predominance. Between 2011 and 2015, the number of new CML cases was 1.8 per 100,000 persons. The median overall survival (OS) in patients with newly diagnosed chronic-phase CML (CP-CML) has not been reached.2 Given the effective treatments available for managing CML, it is estimated that the prevalence of CML in the United States will plateau at 180,000 patients by 2050.3
Diagnosis
Case Presentation
A 53-year-old woman presents to her primary care physician with complaints of fatigue, early satiety, left upper quadrant abdominal pain, and an 8-lb unintentional weight loss over the prior month. Her past medical history is significant for uncontrolled diabetes, coronary artery disease requiring placement of 3 cardiac stents 2 years prior, and chronic obstructive pulmonary disease (COPD) related to a 30-pack-year history of smoking. On physicial exam her spleen is palpated 8 cm below the left costal margin. A complete blood count (CBC) with differential identifies a total white blood cell (WBC) count of 124,000/μL, with a left-shifted differential including 6% basophils, 3% eosinophils, and 3% blasts; hemoglobin is 12.4 g/dL and platelet count is 801 × 103/µL.
- How is the diagnosis of CML made?
Clinical Features
The diagnosis of CML is often suspected based on an incidental finding of leukocytosis and, in some cases, thrombocytosis. In many cases, this is an incidental finding on routine blood work, but approximately 50% of patients will present with constitutional symptoms associated with the disease. Characteristic features of the WBC differential include left-shifted maturation with neutrophilia and immature circulating myeloid cells. Basophilia and eosinophilia are often present as well. Splenomegaly is a common sign, present in 50% to 90% of patients at diagnosis. In those patients with symptoms related to CML at diagnosis, the most common presentation includes increasing fatigue, fevers, night sweats, early satiety, and weight loss. The diagnosis is confirmed by cytogenetic studies showing the Ph chromosome abnormality, t(9; 22)(q3.4;q1.1), and/or reverse transcriptase polymerase chain reaction (PCR) showing BCR-ABL1 transcripts.
- What further testing is needed when evaluating a patient for CML?
There are 3 distinct phases of CML: chronic phase (CP), accelerated phase (AP), and blast phase (BP). Bone marrow biopsy and aspiration at diagnosis are mandatory in order to determine the phase of the disease at diagnosis. This distinction is based on the percentage of blasts, promyelocytes, and basophils present as well as the platelet count and presence or absence of extramedullary disease.4 The vast majority of patients at diagnosis have CML that is in the chronic phase. The typical appearance in CP-CML is a hypercellular marrow with granulocytic and occasionally megakaryocytic hyperplasia. In many cases, basophilia and/or eosinophilia are noted as well. Dysplasia is not a typical finding in CML.5 Bone marrow fibrosis can be seen in up to one-third of patients at diagnosis, and may indicate a slightly worse prognosis.6 Although a diagnosis of CML can be made without a bone marrow biopsy, complete staging and prognostication are only possible with information gained from this test, including baseline karyotype and confirmation of CP versus a more advanced phase of CML.
The criteria for diagnosing AP-CML has not been agreed upon by various groups, but the modified MD Anderson Cancer Center (MDACC) criteria are used in the majority of clinical trials evaluating the efficacy of TKIs in preventing progression to advanced phases of CML. MDACC criteria define AP-CML as the presence of one of the following: 15% to 29% blasts in the peripheral blood or bone marrow, ≥ 30% peripheral blasts plus promyelocytes, ≥ 20% basophils in the blood or bone marrow, platelet count ≤ 100 × 103/μL unrelated to therapy, and clonal cytogenetic evolution in Ph-positive metaphases (Table).7
BP-CML is typically defined using the criteria developed by the International Bone Marrow Transplant Registry (IBMTR): ≥ 30% blasts in the peripheral blood and/or the bone marrow or the presence of extramedullary disease.8 Although not typically used in clinical trials, the revised World Health Organization (WHO) criteria for BP-CML include ≥ 20% blasts in the peripheral blood or bone marrow, extramedullary blast proliferation, and large foci or clusters of blasts in the bone marrow biopsy (Table).9 The defining feature of CML is the presence of the Ph chromosome abnormality. In a small subset of patients, additional chromosomal abnormalities (ACA) in the Ph-positive cells may be identified at diagnosis. Some reports indicate that the presence of “major route” ACA (trisomy 8, isochromosome 17q, a second Ph chromosome, or trisomy 19) at diagnosis may negatively impact prognosis, but other reports contradict these findings.10,11
The typical BCR breakpoint in CML is the major breakpoint cluster region (M-BCR), which results in a 210-kDa protein (p210). Alternate breakpoints that are less frequently identified are the minor BCR (mBCR or p190), which is more commonly found in Ph-positive acute lymphoblastic leukemia (ALL), and the micro BCR (µBCR or p230), which is much less common and is often characterized by chronic neutrophilia.12 Identifying which BCR-ABL1 transcript is present in each patient using qualitative PCR is crucial in order to ensure proper monitoring during treatment.
The most sensitive method for detecting BCR-ABL1 mRNA transcripts is the quantitative real-time PCR (RQ-PCR) assay, which is typically done on peripheral blood. RQ-PCR is capable of detecting a single CML cell in the presence of ≥ 100,000 normal cells. This test should be done during the initial diagnostic workup in order to confirm the presence of BCR-ABL1 transcripts, and it is used as a standard method for monitoring response to TKI therapy.13 The International Scale (IS) is a standardized approach to reporting RQ-PCR results that was developed to allow comparison of results across various laboratories and has become the gold standard for reporting BCR-ABL1 transcript values.14
Determining Risk Scores
Calculating a patient’s Sokal score or EURO risk score at diagnosis remains an important component of the diagnostic workup in CP-CML, as this information has prognostic and therapeutic implications (an online calculator is available through European LeukemiaNet [ELN]). The risk for disease progression to the accelerated or blast phases is higher in patients with intermediate- or high-risk scores compared to those with a low-risk score at diagnosis. The risk of progression in intermediate- or high-risk patients is lower when a second-generation TKI (dasatinib, nilotinib, or bosutinib) is used as frontline therapy compared to imatinib, and therefore, the National Comprehensive Cancer Network (NCCN) CML Panel recommends starting with a second-generation TKI in these patients.15-19
Monitoring Response to Therapy
Case Continued
Fluorescent in-situ hybridization using a peripheral blood sample to detect the BCR-ABL gene rearrangement is performed and is positive in 87% of cells. Bone marrow biopsy and aspiration show a 95% cellular bone marrow with granulocytic hyperplasia and 1% blasts. Cytogenetics are 46,XX,t(9;22)(q34;q11.2).20 RQ-PCR assay performed to measure BCR-ABL1 transcripts in the peripheral blood shows a value of 98% IS. The patient is ultimately given a diagnosis of CP-CML. Her Sokal risk score is 1.42, making her disease high risk.
How is response to TKI therapy measured and monitored?
After confirming a diagnosis of CML and selecting the most appropriate TKI for first-line therapy, the successful management of a CML patient relies on close monitoring and follow-up to ensure patients are meeting the desired treatment milestones. Responses in CML can be assessed based on hematologic parameters, cytogenetic results, and molecular responses. A complete hematologic response (CHR) implies complete normalization of peripheral blood counts (with the exception of TKI-induced cytopenias) and resolution of any palpable splenomegaly. The majority of patients will achieve a CHR within 4 to 6 weeks after initiating CML-directed therapy.21
Cytogenetic Response
Cytogenetic responses are defined by the decrease in the number of Ph chromosome–positive metaphases when assessed on bone marrow cytogenetics. A partial cytogenetic response (PCyR) is defined as having 1% to 35% Ph-positive metaphases, a major cytogenetic response (MCyR) as having 0% to 35% Ph-positive metaphases, and a CCyR implies that no Ph-positive metaphases are identified on bone marrow cytogenetics. An ideal response is the achievement of PCyR after 3 months on a TKI and a CCyR after 12 months on a TKI.22
Molecular Response
Once a patient has achieved a CCyR, monitoring their response to therapy can only be done using RQ-PCR to measure BCR-ABL1 transcripts in the peripheral blood. The NCCN and the ELN recommend monitoring RQ-PCR from the peripheral blood every 3 months in order to assess response to TKIs.19,23 As noted, the International Scale (IS) has become the gold standard reporting system for all BCR-ABL1 transcript levels in the majority of laboratories worldwide.14,24 Molecular responses are based on a log-reduction in BCR-ABL1 transcripts from a standardized baseline. Many molecular responses can be correlated with cytogenetic responses such that if reliable RQ-PCR testing is available, monitoring can be done using only peripheral blood RQ-PCR rather than repeat bone marrow biopsies. For example, an early molecular response (EMR) is defined as a RQ-PCR value of ≤ 10% IS, which is approximately equivalent to a PCyR.25 A value of 1% IS is approximately equivalent to CCyR. A major molecular response (MMR) is a ≥ 3-log reduction in BCR-ABL1 transcripts from baseline and is a value of ≤ 0.1% IS. Deeper levels of molecular response are best described by the log-reduction in BCR-ABL1 transcripts, with a 4-log reduction denoted as MR4.0, a 4.5-log reduction as MR4.5, and so forth. Complete molecular response (CMR) is defined by the level of sensitivity of the RQ-PCR assay being used.14
The definition of relapsed disease in CML is dependent on the type of response the patient had previously achieved. Relapse could be the loss of a hematologic or cytogenetic response, but fluctuations in BCR-ABL1 transcripts on routine RQ-PCR do not necessarily indicate relapsed CML. A 1-log increase in the level of BCR-ABL1 transcripts with a concurrent loss of MMR should prompt a bone marrow biopsy in order to assess for the loss of CCyR, and thus a cytogenetic relapse; however, this loss of MMR does not define relapse in and of itself. In the setting of relapsed disease, testing should be done to look for possible ABL kinase domain mutations, and alternate therapy should be selected.19
Multiple reports have identified the prognostic relevance of achieving an EMR at 3 and 6 months after starting TKI therapy. Marin and colleagues reported that in 282 imatinib-treated patients, there was a significant improvement in 8-year OS, progression-free survival, and cumulative incidence of CCyR and CMR in patients who had BCR-ABL1 transcripts < 9.84% IS after 3 months on treatment.25 This data highlights the importance of early molecular monitoring in order to ensure the best outcomes for patients with CP-CML.
The NCCN CML guidelines and ELN recommendations both agree that an ideal response after 3 months on a TKI is BCR-ABL1 transcripts < 10% IS, but treatment is not considered to be failing at this point if the patient marginally misses this milestone. After 6 months on treatment, an ideal response is considered BCR-ABL1 transcripts < 1%–10% IS. Ideally, patients will have BCR-ABL1 transcripts < 0.1%–1% IS by the time they complete 12 months of TKI therapy, suggesting that these patients have at least achieved a CCyR.19,23 Even after patients achieve these early milestones, frequent monitoring by RQ-PCR is required to ensure that they are maintaining their response to treatment. This will help to ensure patient compliance with treatment and will also help to identify a select subset of patients who could potentially be considered for an attempt at TKI cessation (not discussed in detail here) after a minimum of 3 years on therapy.19,26
Conclusion
Given the successful treatments available for patients with CML, it is crucial to identify patients with this disease, ensure they receive a complete, appropriate diagnostic workup including a bone marrow biopsy and aspiration with cytogenetic testing, and select the best therapy for each individual patient. Once on treatment, the importance of frequent monitoring cannot be overstated.
Chronic myeloid leukemia (CML) is a rare myeloproliferative neoplasm that is characterized by the presence of the Philadelphia (Ph) chromosome and uninhibited expansion of bone marrow stem cells. The Ph chromosome arises from a reciprocal translocation between the Abelson (ABL) region on chromosome 9 and the breakpoint cluster region (BCR) of chromosome 22 (t(9;22)(q34;q11.2), resulting in the BCR-ABL1 fusion gene.1BCR-ABL1 encodes an oncoprotein with constitutive tyrosine kinase activity that promotes growth and replication through downstream pathways, which is the driving factor in the pathogenesis of CML.1
Typical treatment for CML involves life-long use of oral BCR-ABL tyrosine kinase inhibitors (TKI). Currently, 5 TKIs have regulatory approval for treatment of this disease. With the introduction of imatinib in 2001 and the subsequent development of second- (dasatinib, nilotinib, bosutinib) and third-generation (ponatinib) TKIs, CML has become a chronic disease with a life-expectancy that is similar to that of the general population. This article reviews the diagnosis of CML and the parameters used for monitoring response to TKI therapy; the selection of initial TKI therapy is reviewed in a separate follow-up article.
Epidemiology
According to SEER data estimates, 8430 new cases of CML were diagnosed in the United States in 2018. CML is a disease of older adults, with a median age of 65 years at diagnosis, and there is a slight male predominance. Between 2011 and 2015, the number of new CML cases was 1.8 per 100,000 persons. The median overall survival (OS) in patients with newly diagnosed chronic-phase CML (CP-CML) has not been reached.2 Given the effective treatments available for managing CML, it is estimated that the prevalence of CML in the United States will plateau at 180,000 patients by 2050.3
Diagnosis
Case Presentation
A 53-year-old woman presents to her primary care physician with complaints of fatigue, early satiety, left upper quadrant abdominal pain, and an 8-lb unintentional weight loss over the prior month. Her past medical history is significant for uncontrolled diabetes, coronary artery disease requiring placement of 3 cardiac stents 2 years prior, and chronic obstructive pulmonary disease (COPD) related to a 30-pack-year history of smoking. On physicial exam her spleen is palpated 8 cm below the left costal margin. A complete blood count (CBC) with differential identifies a total white blood cell (WBC) count of 124,000/μL, with a left-shifted differential including 6% basophils, 3% eosinophils, and 3% blasts; hemoglobin is 12.4 g/dL and platelet count is 801 × 103/µL.
- How is the diagnosis of CML made?
Clinical Features
The diagnosis of CML is often suspected based on an incidental finding of leukocytosis and, in some cases, thrombocytosis. In many cases, this is an incidental finding on routine blood work, but approximately 50% of patients will present with constitutional symptoms associated with the disease. Characteristic features of the WBC differential include left-shifted maturation with neutrophilia and immature circulating myeloid cells. Basophilia and eosinophilia are often present as well. Splenomegaly is a common sign, present in 50% to 90% of patients at diagnosis. In those patients with symptoms related to CML at diagnosis, the most common presentation includes increasing fatigue, fevers, night sweats, early satiety, and weight loss. The diagnosis is confirmed by cytogenetic studies showing the Ph chromosome abnormality, t(9; 22)(q3.4;q1.1), and/or reverse transcriptase polymerase chain reaction (PCR) showing BCR-ABL1 transcripts.
- What further testing is needed when evaluating a patient for CML?
There are 3 distinct phases of CML: chronic phase (CP), accelerated phase (AP), and blast phase (BP). Bone marrow biopsy and aspiration at diagnosis are mandatory in order to determine the phase of the disease at diagnosis. This distinction is based on the percentage of blasts, promyelocytes, and basophils present as well as the platelet count and presence or absence of extramedullary disease.4 The vast majority of patients at diagnosis have CML that is in the chronic phase. The typical appearance in CP-CML is a hypercellular marrow with granulocytic and occasionally megakaryocytic hyperplasia. In many cases, basophilia and/or eosinophilia are noted as well. Dysplasia is not a typical finding in CML.5 Bone marrow fibrosis can be seen in up to one-third of patients at diagnosis, and may indicate a slightly worse prognosis.6 Although a diagnosis of CML can be made without a bone marrow biopsy, complete staging and prognostication are only possible with information gained from this test, including baseline karyotype and confirmation of CP versus a more advanced phase of CML.
The criteria for diagnosing AP-CML has not been agreed upon by various groups, but the modified MD Anderson Cancer Center (MDACC) criteria are used in the majority of clinical trials evaluating the efficacy of TKIs in preventing progression to advanced phases of CML. MDACC criteria define AP-CML as the presence of one of the following: 15% to 29% blasts in the peripheral blood or bone marrow, ≥ 30% peripheral blasts plus promyelocytes, ≥ 20% basophils in the blood or bone marrow, platelet count ≤ 100 × 103/μL unrelated to therapy, and clonal cytogenetic evolution in Ph-positive metaphases (Table).7
BP-CML is typically defined using the criteria developed by the International Bone Marrow Transplant Registry (IBMTR): ≥ 30% blasts in the peripheral blood and/or the bone marrow or the presence of extramedullary disease.8 Although not typically used in clinical trials, the revised World Health Organization (WHO) criteria for BP-CML include ≥ 20% blasts in the peripheral blood or bone marrow, extramedullary blast proliferation, and large foci or clusters of blasts in the bone marrow biopsy (Table).9 The defining feature of CML is the presence of the Ph chromosome abnormality. In a small subset of patients, additional chromosomal abnormalities (ACA) in the Ph-positive cells may be identified at diagnosis. Some reports indicate that the presence of “major route” ACA (trisomy 8, isochromosome 17q, a second Ph chromosome, or trisomy 19) at diagnosis may negatively impact prognosis, but other reports contradict these findings.10,11
The typical BCR breakpoint in CML is the major breakpoint cluster region (M-BCR), which results in a 210-kDa protein (p210). Alternate breakpoints that are less frequently identified are the minor BCR (mBCR or p190), which is more commonly found in Ph-positive acute lymphoblastic leukemia (ALL), and the micro BCR (µBCR or p230), which is much less common and is often characterized by chronic neutrophilia.12 Identifying which BCR-ABL1 transcript is present in each patient using qualitative PCR is crucial in order to ensure proper monitoring during treatment.
The most sensitive method for detecting BCR-ABL1 mRNA transcripts is the quantitative real-time PCR (RQ-PCR) assay, which is typically done on peripheral blood. RQ-PCR is capable of detecting a single CML cell in the presence of ≥ 100,000 normal cells. This test should be done during the initial diagnostic workup in order to confirm the presence of BCR-ABL1 transcripts, and it is used as a standard method for monitoring response to TKI therapy.13 The International Scale (IS) is a standardized approach to reporting RQ-PCR results that was developed to allow comparison of results across various laboratories and has become the gold standard for reporting BCR-ABL1 transcript values.14
Determining Risk Scores
Calculating a patient’s Sokal score or EURO risk score at diagnosis remains an important component of the diagnostic workup in CP-CML, as this information has prognostic and therapeutic implications (an online calculator is available through European LeukemiaNet [ELN]). The risk for disease progression to the accelerated or blast phases is higher in patients with intermediate- or high-risk scores compared to those with a low-risk score at diagnosis. The risk of progression in intermediate- or high-risk patients is lower when a second-generation TKI (dasatinib, nilotinib, or bosutinib) is used as frontline therapy compared to imatinib, and therefore, the National Comprehensive Cancer Network (NCCN) CML Panel recommends starting with a second-generation TKI in these patients.15-19
Monitoring Response to Therapy
Case Continued
Fluorescent in-situ hybridization using a peripheral blood sample to detect the BCR-ABL gene rearrangement is performed and is positive in 87% of cells. Bone marrow biopsy and aspiration show a 95% cellular bone marrow with granulocytic hyperplasia and 1% blasts. Cytogenetics are 46,XX,t(9;22)(q34;q11.2).20 RQ-PCR assay performed to measure BCR-ABL1 transcripts in the peripheral blood shows a value of 98% IS. The patient is ultimately given a diagnosis of CP-CML. Her Sokal risk score is 1.42, making her disease high risk.
How is response to TKI therapy measured and monitored?
After confirming a diagnosis of CML and selecting the most appropriate TKI for first-line therapy, the successful management of a CML patient relies on close monitoring and follow-up to ensure patients are meeting the desired treatment milestones. Responses in CML can be assessed based on hematologic parameters, cytogenetic results, and molecular responses. A complete hematologic response (CHR) implies complete normalization of peripheral blood counts (with the exception of TKI-induced cytopenias) and resolution of any palpable splenomegaly. The majority of patients will achieve a CHR within 4 to 6 weeks after initiating CML-directed therapy.21
Cytogenetic Response
Cytogenetic responses are defined by the decrease in the number of Ph chromosome–positive metaphases when assessed on bone marrow cytogenetics. A partial cytogenetic response (PCyR) is defined as having 1% to 35% Ph-positive metaphases, a major cytogenetic response (MCyR) as having 0% to 35% Ph-positive metaphases, and a CCyR implies that no Ph-positive metaphases are identified on bone marrow cytogenetics. An ideal response is the achievement of PCyR after 3 months on a TKI and a CCyR after 12 months on a TKI.22
Molecular Response
Once a patient has achieved a CCyR, monitoring their response to therapy can only be done using RQ-PCR to measure BCR-ABL1 transcripts in the peripheral blood. The NCCN and the ELN recommend monitoring RQ-PCR from the peripheral blood every 3 months in order to assess response to TKIs.19,23 As noted, the International Scale (IS) has become the gold standard reporting system for all BCR-ABL1 transcript levels in the majority of laboratories worldwide.14,24 Molecular responses are based on a log-reduction in BCR-ABL1 transcripts from a standardized baseline. Many molecular responses can be correlated with cytogenetic responses such that if reliable RQ-PCR testing is available, monitoring can be done using only peripheral blood RQ-PCR rather than repeat bone marrow biopsies. For example, an early molecular response (EMR) is defined as a RQ-PCR value of ≤ 10% IS, which is approximately equivalent to a PCyR.25 A value of 1% IS is approximately equivalent to CCyR. A major molecular response (MMR) is a ≥ 3-log reduction in BCR-ABL1 transcripts from baseline and is a value of ≤ 0.1% IS. Deeper levels of molecular response are best described by the log-reduction in BCR-ABL1 transcripts, with a 4-log reduction denoted as MR4.0, a 4.5-log reduction as MR4.5, and so forth. Complete molecular response (CMR) is defined by the level of sensitivity of the RQ-PCR assay being used.14
The definition of relapsed disease in CML is dependent on the type of response the patient had previously achieved. Relapse could be the loss of a hematologic or cytogenetic response, but fluctuations in BCR-ABL1 transcripts on routine RQ-PCR do not necessarily indicate relapsed CML. A 1-log increase in the level of BCR-ABL1 transcripts with a concurrent loss of MMR should prompt a bone marrow biopsy in order to assess for the loss of CCyR, and thus a cytogenetic relapse; however, this loss of MMR does not define relapse in and of itself. In the setting of relapsed disease, testing should be done to look for possible ABL kinase domain mutations, and alternate therapy should be selected.19
Multiple reports have identified the prognostic relevance of achieving an EMR at 3 and 6 months after starting TKI therapy. Marin and colleagues reported that in 282 imatinib-treated patients, there was a significant improvement in 8-year OS, progression-free survival, and cumulative incidence of CCyR and CMR in patients who had BCR-ABL1 transcripts < 9.84% IS after 3 months on treatment.25 This data highlights the importance of early molecular monitoring in order to ensure the best outcomes for patients with CP-CML.
The NCCN CML guidelines and ELN recommendations both agree that an ideal response after 3 months on a TKI is BCR-ABL1 transcripts < 10% IS, but treatment is not considered to be failing at this point if the patient marginally misses this milestone. After 6 months on treatment, an ideal response is considered BCR-ABL1 transcripts < 1%–10% IS. Ideally, patients will have BCR-ABL1 transcripts < 0.1%–1% IS by the time they complete 12 months of TKI therapy, suggesting that these patients have at least achieved a CCyR.19,23 Even after patients achieve these early milestones, frequent monitoring by RQ-PCR is required to ensure that they are maintaining their response to treatment. This will help to ensure patient compliance with treatment and will also help to identify a select subset of patients who could potentially be considered for an attempt at TKI cessation (not discussed in detail here) after a minimum of 3 years on therapy.19,26
Conclusion
Given the successful treatments available for patients with CML, it is crucial to identify patients with this disease, ensure they receive a complete, appropriate diagnostic workup including a bone marrow biopsy and aspiration with cytogenetic testing, and select the best therapy for each individual patient. Once on treatment, the importance of frequent monitoring cannot be overstated.
1. Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164-172.
2. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Leukemia - Chronic Myeloid Leukemia (CML). 2018.
3. Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer. 2012;118:3123-3127.
4. Savage DG, Szydlo RM, Chase A, et al. Bone marrow transplantation for chronic myeloid leukaemia: the effects of differing criteria for defining chronic phase on probabilities of survival and relapse. Br J Haematol. 1997;99:30-35.
5. Knox WF, Bhavnani M, Davson J, Geary CG. Histological classification of chronic granulocytic leukaemia. Clin Lab Haematol. 1984;6:171-175.
6. Kvasnicka HM, Thiele J, Schmitt-Graeff A, et al. Impact of bone marrow morphology on multivariate risk classification in chronic myelogenous leukemia. Acta Haematol. 2003;109:53-56.
7. Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306-1315.
8. Druker BJ. Chronic myeloid leukemia In: DeVita VT, Lawrence TS, Rosenburg SA, eds. DeVita, Hellman, and Rosenberg’s Cancer Principles & Practice of Oncology. 8th ed. Philadelphia, PA: Lippincott, Williams and Wilkins; 2007:2267-2304.
9. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405.
10. Fabarius A, Leitner A, Hochhaus A, et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118:6760-6768.
11. Alhuraiji A, Kantarjian H, Boddu P, et al. Prognostic significance of additional chromosomal abnormalities at the time of diagnosis in patients with chronic myeloid leukemia treated with frontline tyrosine kinase inhibitors. Am J Hematol. 2018;93:84-90.
12. Melo JV. BCR-ABL gene variants. Baillieres Clin Haematol. 1997;10:203-222.
13. Kantarjian HM, Talpaz M, Cortes J, et al. Quantitative polymerase chain reaction monitoring of BCR-ABL during therapy with imatinib mesylate (STI571; gleevec) in chronic-phase chronic myelogenous leukemia. Clin Cancer Res. 2003;9:160-166.
14. Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28-37.
15. Hochhaus A, Larson RA, Guilhot F, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917-927.
16. Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients trial. J Clin Oncol. 2016;34:2333-3340.
17. Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044-1054.
18. Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36:231-237.
19. Radich JP, Deininger M, Abboud CN, et al. Chronic Myeloid Leukemia, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1108-1135.
20. Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037.
21. Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Ann Intern Med. 1999;131:207-219.
22. O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994-1004.
23. Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872-884.
24. Larripa I, Ruiz MS, Gutierrez M, Bianchini M. [Guidelines for molecular monitoring of BCR-ABL1 in chronic myeloid leukemia patients by RT-qPCR.] Medicina (B Aires). 2017;77:61-72.
25. Marin D, Ibrahim AR, Lucas C, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012;30:232-238.
26. Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128:17-23.
1. Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164-172.
2. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Leukemia - Chronic Myeloid Leukemia (CML). 2018.
3. Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer. 2012;118:3123-3127.
4. Savage DG, Szydlo RM, Chase A, et al. Bone marrow transplantation for chronic myeloid leukaemia: the effects of differing criteria for defining chronic phase on probabilities of survival and relapse. Br J Haematol. 1997;99:30-35.
5. Knox WF, Bhavnani M, Davson J, Geary CG. Histological classification of chronic granulocytic leukaemia. Clin Lab Haematol. 1984;6:171-175.
6. Kvasnicka HM, Thiele J, Schmitt-Graeff A, et al. Impact of bone marrow morphology on multivariate risk classification in chronic myelogenous leukemia. Acta Haematol. 2003;109:53-56.
7. Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306-1315.
8. Druker BJ. Chronic myeloid leukemia In: DeVita VT, Lawrence TS, Rosenburg SA, eds. DeVita, Hellman, and Rosenberg’s Cancer Principles & Practice of Oncology. 8th ed. Philadelphia, PA: Lippincott, Williams and Wilkins; 2007:2267-2304.
9. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405.
10. Fabarius A, Leitner A, Hochhaus A, et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118:6760-6768.
11. Alhuraiji A, Kantarjian H, Boddu P, et al. Prognostic significance of additional chromosomal abnormalities at the time of diagnosis in patients with chronic myeloid leukemia treated with frontline tyrosine kinase inhibitors. Am J Hematol. 2018;93:84-90.
12. Melo JV. BCR-ABL gene variants. Baillieres Clin Haematol. 1997;10:203-222.
13. Kantarjian HM, Talpaz M, Cortes J, et al. Quantitative polymerase chain reaction monitoring of BCR-ABL during therapy with imatinib mesylate (STI571; gleevec) in chronic-phase chronic myelogenous leukemia. Clin Cancer Res. 2003;9:160-166.
14. Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28-37.
15. Hochhaus A, Larson RA, Guilhot F, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917-927.
16. Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients trial. J Clin Oncol. 2016;34:2333-3340.
17. Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044-1054.
18. Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36:231-237.
19. Radich JP, Deininger M, Abboud CN, et al. Chronic Myeloid Leukemia, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1108-1135.
20. Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037.
21. Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Ann Intern Med. 1999;131:207-219.
22. O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994-1004.
23. Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872-884.
24. Larripa I, Ruiz MS, Gutierrez M, Bianchini M. [Guidelines for molecular monitoring of BCR-ABL1 in chronic myeloid leukemia patients by RT-qPCR.] Medicina (B Aires). 2017;77:61-72.
25. Marin D, Ibrahim AR, Lucas C, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012;30:232-238.
26. Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128:17-23.