User login
The woman who couldn’t stop eating
CASE Uncontrollable eating and weight gain
Ms. C, age 33, presents to an outpatient clinic with complaints of weight gain and “uncontrollable eating.” Ms. C says she’s gained >50 lb over the last year. She describes progressively frequent episodes of overeating during which she feels that she has no control over the amount of food she consumes. She reports eating as often as 10 times a day, and overeating to the point of physical discomfort during most meals. She gives an example of having recently consumed a large pizza, several portions of Chinese food, approximately 20 chicken wings, and half a chocolate cake for dinner. Ms. C admits that on several occasions she has vomited after meals due to feeling extremely full; however, she denies having done so intentionally. She also denies restricting her food intake, misusing laxatives or diuretics, or exercising excessively.
Ms. C expresses frustration and embarrassment with her eating and resulting weight gain. She says she has poor self-esteem, low energy and motivation, and poor concentration. She feels that her condition has significantly impacted her social life, romantic relationships, and family life. She admits she’s been avoiding dating and seeing friends due to her weight gain, and has been irritable with her teenage daughter.
During her initial evaluation, Ms. C is alert and oriented, with a linear and goal-directed thought process. She is somewhat irritable and guarded, wearing large sunglasses that cover most of her face, but is not overtly paranoid. Although she appears frustrated when discussing her condition, she denies feeling hopeless or helpless.
HISTORY Thyroid cancer and mood swings
Ms. C, who is single and unemployed, lives in an apartment with her teenage daughter, with whom she describes having a good relationship. She has been receiving disability benefits for the past 2 years after a motor vehicle accident resulted in multiple fractures of her arm and elbow, and subsequent chronic pain. Ms. C reports a distant history of “problems with alcohol,” but denies drinking any alcohol since being charged with driving under the influence several years ago. She has a 10 pack-year history of smoking and denies any history of illicit drug use.
Two years ago, Ms. C was diagnosed with thyroid carcinoma, and treated with surgical resection and a course of radiation. She has regular visits with her endocrinologist and has been prescribed oral levothyroxine, 150 mcg/d.
Ms. C reports a history of “mood swings” characterized by “snapping at people” and becoming irritable in response to stressful situations, but denies any past symptoms consistent with a manic or hypomanic episode. Ms. C has not been admitted to a psychiatric hospital, nor has she received any prior psychiatric treatment. She reluctantly discloses that approximately 3 years ago she had a less severe episode of uncontrollable eating and weight gain (20 to 30 lb). At that time, she was able to regain her desired physical appearance by going on the “Subway diet” and undergoing liposuction and plastic surgery.
At her current outpatient clinic visit, Ms. C expresses an interest in exploring bariatric surgery as a potential solution to her weight gain.
[polldaddy:10446186]
Continue to: EVALUATION Obese; stable thyroid function
EVALUATION Obese; stable thyroid function
We refer Ms. C for a physical examination and routine blood analysis to rule out any medical contributors to her condition. Her physical examination is reported as normal, with no signs of skin changes, goiter, or exophthalmos. Ms. C is noted to be obese, with a body mass index of 37.2 kg/m2, and an abdominal circumference of 38.5 in.
A blood analysis shows that Ms. C has elevated triglyceride levels (202 mg/dL) and elevated cholesterol levels (210 mg/dL). Her thyroid function tests are within normal limits based on the dose of levothyroxine she’s been receiving. A pregnancy test is negative.
Ms. C gives the team at the clinic permission to contact her endocrinologist, who reports that he does not suspect that Ms. C’s drastic weight gain and abnormal eating patterns are attributable to her history of thyroid carcinoma because her thyroid function tests have been stable on her current regimen.
The authors’ observations
Based on Ms. C’s initial presentation, we strongly suspected a diagnosis of binge eating disorder (BED). Several differential diagnoses were considered and carefully ruled out; Ms. C’s medical workup did not suggest that her weight gain was due to an active medical condition, and she did not meet DSM-5 criteria for a mood or psychotic disorder or anorexia nervosa or bulimia nervosa.
With an estimated lifetime prevalence in the United States of 2.6%, BED is the most prevalent eating disorder (compared with 0.6% for anorexia nervosa and 1% for bulimia nervosa).1 BED is more prevalent in women than in men, and the mean age of onset is mid-20s.
Continue to: BED may be difficult...
BED may be difficult to detect because patients may feel ashamed or guilty and are often hesitant to disclose and discuss their symptoms. Furthermore, they are frequently frustrated by the subjective loss of control over their behaviors. Patients with BED often present to medical facilities seeking weight loss solutions rather than to psychiatric clinics.
Screening for eating disorders
Several screening instruments have been developed to help clinicians identify patients who may need further evaluation for possible diagnosis of an eating disorder, including anorexia nervosa, bulimia nervosa, and BED.2 The SCOFF questionnaire is composed of 5 brief clinician-administered questions to screen for eating disorders.2 The 7-item Binge Eating Disorder Screener (BED-7) is a screening instrument specific for BED that examines a patient’s eating patterns and behaviors during the past 3 months.3
In general, suspect BED in patients who have significant weight dissatisfaction, fluctuation in weight, and depressive symptoms. The DSM-5 criteria for binge eating disorder are shown in Table 14.
BED and comorbid psychiatric disorders
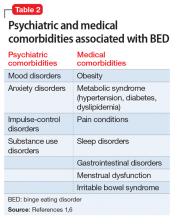
Patients with BED are more likely than the general population to have comorbid psychiatric disorders, including mood and anxiety disorders, attention-deficit/hyperactivity disorder, posttraumatic stress disorder, and substance use disorders. Swanson et al5 found that 83.5% of adolescents who met criteria for BED also met criteria for at least 1 other psychiatric disorder, and 37% endorsed >3 concurrent psychiatric conditions. Once BED is confirmed, it is important to screen for other psychiatric and medical comorbidities that are often present in individuals with BED (Table 21,6).
The rates of diagnosis and treatment of BED remain low. This is likely due to patient factors such as shame and fear of stigma and clinician factors such as lack of awareness, ineffective communication, hesitation to discuss the sensitive topic, or insufficient knowledge about treatment options once BED is diagnosed.
[polldaddy:10446187]
Continue to: TREATMENT Combination therapy
TREATMENT Combination therapy
Ms. C is ambivalent about her BED diagnosis, and becomes angry about it when the proposed treatments do not involve bariatric surgery or cosmetic procedures. Ms. C is enrolled in weekly individual psychotherapy, where she receives a combination of CBT and psychodynamic therapy; however, her attendance is inconsistent. Ms. C is offered a trial of fluoxetine, but adamantly refuses, citing a relative who experienced adverse effects while receiving this type of antidepressant. Ms. C also refuses a trial of topiramate due to concerns of feeling sedated. Finally, she is offered a trial of lisdexamfetamine, 30 mg/d, which was FDA-approved in 2015 to treat moderate to severe BED. We discuss the risks, benefits, and adverse effects of lisdexamfetamine with Ms. C; however, she is hesitant to start this medication and expresses increasing interest in obtaining a consultation for bariatric surgery. Ms. C is provided with extensive education about the risks and dangers of surgery before addressing her eating patterns, and the clinician provides validation, verbal support, and counseling. Ms. C eventually agrees to a trial of lisdexamfetamine, but her insurance denies coverage of this medication.
The authors’ observations
When developing an individualized treatment plan for a patient with BED, the patient’s psychiatric and medical comorbidities should be considered. Treatment goals for patients with BED include:
- abstinence from binge eating
- sustainable weight loss and metabolic health
- reduction in symptoms associated with comorbid conditions
- improvement in self-esteem and overall quality of life.
A 2015 comparative effectiveness review of management and outcomes for patients with BED evaluated pharmacologic, psychologic, behavioral, and combined approaches for treating patients with BED.7 The results suggested that second-generation antidepressants, topiramate, and lisdexamfetamine were superior to placebo in reducing binge-eating episodes and achieving abstinence from binge-eating. Weight reduction was also achieved with topiramate and lisdexamfetamine, and antidepressants helped relieve symptoms of comorbid depression.
Various formats of CBT, including therapist-led and guided self-help, were also superior to placebo in reducing the frequency of binge-eating and promoting abstinence; however, they were generally not effective in treating depression or reducing patients’ weight.7
OUTCOME Fixated on surgery
We appeal the decision of Ms. C’s insurance company; however, during the appeals process, Ms. C becomes increasingly irritable and informs us that she has changed her mind and, with the reported support of her medical doctors, wishes to undergo bariatric surgery. Although we made multiple attempts to engage Ms. C in further treatment, she is lost to follow-up.
Continue to: Bottom Line
Bottom Line
Diagnosing and managing patients with binge eating disorder (BED) can be challenging because patients may hesitate to seek help, and/or have psychiatric and medical comorbidities. They often present to medical facilities seeking weight loss solutions rather than to psychiatric clinics. Once BED is confirmed, screen for other psychiatric and medical comorbidities. A combination of pharmacologic and psychotherapeutic interventions can benefit some patients with BED, but treatment should be individualized.
Related Resources
- National Eating Disorders Association. NEDA. www.nationaleatingdisorders.org/.
- Safer D, Telch C, Chen EY. Dialectical behavior therapy for binge eating and bulimia. New York, NY: Guilford Press; 2017.
Drug Brand Names
Fluoxetine • Prozac
Levothyroxine • Synthroid
Lisdexamfetamine • Vyvanse
Topiramate • Topamax
1. Hudson JI, Hiripi E, Pope HG Jr, et al. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348-358.
2. Morgan JF, Reid F, Lacey JH. The SCOFF questionnaire: assessment of a new screening tool for eating disorders. BMJ. 1999;319(7223):1467-1468.
3. Herman BK, Deal LS, DiBenedetti DB, et al. Development of the 7-Item Binge-Eating Disorder screener (BEDS-7). Prim Care Companion CNS Disord. 2016;18(2):10.4088/PCC.15m01896. doi:10.4088/PCC.15m01896.
4. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
5. Swanson SA, Crow SJ, Le Grange D, et al. Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement. Arch Gen Psychiatry. 2011;68(7):714.
6. Guerdjikova AI, Mori N, Casuto LS, et al. Binge eating disorder. Psychiatric Clinics of North America. 2017;40(2):255-266.
7. Berkman ND, Brownley KA, Peat CM, et al. Management and outcomes of binge-eating disorder. Comparative Effectiveness Reviews, No. 160. Agency for Healthcare Research and Quality (US). https://www.ncbi.nlm.nih.gov/books/NBK338312/. Published December 2015. Accessed July 29, 2019.
CASE Uncontrollable eating and weight gain
Ms. C, age 33, presents to an outpatient clinic with complaints of weight gain and “uncontrollable eating.” Ms. C says she’s gained >50 lb over the last year. She describes progressively frequent episodes of overeating during which she feels that she has no control over the amount of food she consumes. She reports eating as often as 10 times a day, and overeating to the point of physical discomfort during most meals. She gives an example of having recently consumed a large pizza, several portions of Chinese food, approximately 20 chicken wings, and half a chocolate cake for dinner. Ms. C admits that on several occasions she has vomited after meals due to feeling extremely full; however, she denies having done so intentionally. She also denies restricting her food intake, misusing laxatives or diuretics, or exercising excessively.
Ms. C expresses frustration and embarrassment with her eating and resulting weight gain. She says she has poor self-esteem, low energy and motivation, and poor concentration. She feels that her condition has significantly impacted her social life, romantic relationships, and family life. She admits she’s been avoiding dating and seeing friends due to her weight gain, and has been irritable with her teenage daughter.
During her initial evaluation, Ms. C is alert and oriented, with a linear and goal-directed thought process. She is somewhat irritable and guarded, wearing large sunglasses that cover most of her face, but is not overtly paranoid. Although she appears frustrated when discussing her condition, she denies feeling hopeless or helpless.
HISTORY Thyroid cancer and mood swings
Ms. C, who is single and unemployed, lives in an apartment with her teenage daughter, with whom she describes having a good relationship. She has been receiving disability benefits for the past 2 years after a motor vehicle accident resulted in multiple fractures of her arm and elbow, and subsequent chronic pain. Ms. C reports a distant history of “problems with alcohol,” but denies drinking any alcohol since being charged with driving under the influence several years ago. She has a 10 pack-year history of smoking and denies any history of illicit drug use.
Two years ago, Ms. C was diagnosed with thyroid carcinoma, and treated with surgical resection and a course of radiation. She has regular visits with her endocrinologist and has been prescribed oral levothyroxine, 150 mcg/d.
Ms. C reports a history of “mood swings” characterized by “snapping at people” and becoming irritable in response to stressful situations, but denies any past symptoms consistent with a manic or hypomanic episode. Ms. C has not been admitted to a psychiatric hospital, nor has she received any prior psychiatric treatment. She reluctantly discloses that approximately 3 years ago she had a less severe episode of uncontrollable eating and weight gain (20 to 30 lb). At that time, she was able to regain her desired physical appearance by going on the “Subway diet” and undergoing liposuction and plastic surgery.
At her current outpatient clinic visit, Ms. C expresses an interest in exploring bariatric surgery as a potential solution to her weight gain.
[polldaddy:10446186]
Continue to: EVALUATION Obese; stable thyroid function
EVALUATION Obese; stable thyroid function
We refer Ms. C for a physical examination and routine blood analysis to rule out any medical contributors to her condition. Her physical examination is reported as normal, with no signs of skin changes, goiter, or exophthalmos. Ms. C is noted to be obese, with a body mass index of 37.2 kg/m2, and an abdominal circumference of 38.5 in.
A blood analysis shows that Ms. C has elevated triglyceride levels (202 mg/dL) and elevated cholesterol levels (210 mg/dL). Her thyroid function tests are within normal limits based on the dose of levothyroxine she’s been receiving. A pregnancy test is negative.
Ms. C gives the team at the clinic permission to contact her endocrinologist, who reports that he does not suspect that Ms. C’s drastic weight gain and abnormal eating patterns are attributable to her history of thyroid carcinoma because her thyroid function tests have been stable on her current regimen.
The authors’ observations
Based on Ms. C’s initial presentation, we strongly suspected a diagnosis of binge eating disorder (BED). Several differential diagnoses were considered and carefully ruled out; Ms. C’s medical workup did not suggest that her weight gain was due to an active medical condition, and she did not meet DSM-5 criteria for a mood or psychotic disorder or anorexia nervosa or bulimia nervosa.
With an estimated lifetime prevalence in the United States of 2.6%, BED is the most prevalent eating disorder (compared with 0.6% for anorexia nervosa and 1% for bulimia nervosa).1 BED is more prevalent in women than in men, and the mean age of onset is mid-20s.
Continue to: BED may be difficult...
BED may be difficult to detect because patients may feel ashamed or guilty and are often hesitant to disclose and discuss their symptoms. Furthermore, they are frequently frustrated by the subjective loss of control over their behaviors. Patients with BED often present to medical facilities seeking weight loss solutions rather than to psychiatric clinics.
Screening for eating disorders
Several screening instruments have been developed to help clinicians identify patients who may need further evaluation for possible diagnosis of an eating disorder, including anorexia nervosa, bulimia nervosa, and BED.2 The SCOFF questionnaire is composed of 5 brief clinician-administered questions to screen for eating disorders.2 The 7-item Binge Eating Disorder Screener (BED-7) is a screening instrument specific for BED that examines a patient’s eating patterns and behaviors during the past 3 months.3
In general, suspect BED in patients who have significant weight dissatisfaction, fluctuation in weight, and depressive symptoms. The DSM-5 criteria for binge eating disorder are shown in Table 14.
BED and comorbid psychiatric disorders
Patients with BED are more likely than the general population to have comorbid psychiatric disorders, including mood and anxiety disorders, attention-deficit/hyperactivity disorder, posttraumatic stress disorder, and substance use disorders. Swanson et al5 found that 83.5% of adolescents who met criteria for BED also met criteria for at least 1 other psychiatric disorder, and 37% endorsed >3 concurrent psychiatric conditions. Once BED is confirmed, it is important to screen for other psychiatric and medical comorbidities that are often present in individuals with BED (Table 21,6).
The rates of diagnosis and treatment of BED remain low. This is likely due to patient factors such as shame and fear of stigma and clinician factors such as lack of awareness, ineffective communication, hesitation to discuss the sensitive topic, or insufficient knowledge about treatment options once BED is diagnosed.
[polldaddy:10446187]
Continue to: TREATMENT Combination therapy
TREATMENT Combination therapy
Ms. C is ambivalent about her BED diagnosis, and becomes angry about it when the proposed treatments do not involve bariatric surgery or cosmetic procedures. Ms. C is enrolled in weekly individual psychotherapy, where she receives a combination of CBT and psychodynamic therapy; however, her attendance is inconsistent. Ms. C is offered a trial of fluoxetine, but adamantly refuses, citing a relative who experienced adverse effects while receiving this type of antidepressant. Ms. C also refuses a trial of topiramate due to concerns of feeling sedated. Finally, she is offered a trial of lisdexamfetamine, 30 mg/d, which was FDA-approved in 2015 to treat moderate to severe BED. We discuss the risks, benefits, and adverse effects of lisdexamfetamine with Ms. C; however, she is hesitant to start this medication and expresses increasing interest in obtaining a consultation for bariatric surgery. Ms. C is provided with extensive education about the risks and dangers of surgery before addressing her eating patterns, and the clinician provides validation, verbal support, and counseling. Ms. C eventually agrees to a trial of lisdexamfetamine, but her insurance denies coverage of this medication.
The authors’ observations
When developing an individualized treatment plan for a patient with BED, the patient’s psychiatric and medical comorbidities should be considered. Treatment goals for patients with BED include:
- abstinence from binge eating
- sustainable weight loss and metabolic health
- reduction in symptoms associated with comorbid conditions
- improvement in self-esteem and overall quality of life.
A 2015 comparative effectiveness review of management and outcomes for patients with BED evaluated pharmacologic, psychologic, behavioral, and combined approaches for treating patients with BED.7 The results suggested that second-generation antidepressants, topiramate, and lisdexamfetamine were superior to placebo in reducing binge-eating episodes and achieving abstinence from binge-eating. Weight reduction was also achieved with topiramate and lisdexamfetamine, and antidepressants helped relieve symptoms of comorbid depression.
Various formats of CBT, including therapist-led and guided self-help, were also superior to placebo in reducing the frequency of binge-eating and promoting abstinence; however, they were generally not effective in treating depression or reducing patients’ weight.7
OUTCOME Fixated on surgery
We appeal the decision of Ms. C’s insurance company; however, during the appeals process, Ms. C becomes increasingly irritable and informs us that she has changed her mind and, with the reported support of her medical doctors, wishes to undergo bariatric surgery. Although we made multiple attempts to engage Ms. C in further treatment, she is lost to follow-up.
Continue to: Bottom Line
Bottom Line
Diagnosing and managing patients with binge eating disorder (BED) can be challenging because patients may hesitate to seek help, and/or have psychiatric and medical comorbidities. They often present to medical facilities seeking weight loss solutions rather than to psychiatric clinics. Once BED is confirmed, screen for other psychiatric and medical comorbidities. A combination of pharmacologic and psychotherapeutic interventions can benefit some patients with BED, but treatment should be individualized.
Related Resources
- National Eating Disorders Association. NEDA. www.nationaleatingdisorders.org/.
- Safer D, Telch C, Chen EY. Dialectical behavior therapy for binge eating and bulimia. New York, NY: Guilford Press; 2017.
Drug Brand Names
Fluoxetine • Prozac
Levothyroxine • Synthroid
Lisdexamfetamine • Vyvanse
Topiramate • Topamax
CASE Uncontrollable eating and weight gain
Ms. C, age 33, presents to an outpatient clinic with complaints of weight gain and “uncontrollable eating.” Ms. C says she’s gained >50 lb over the last year. She describes progressively frequent episodes of overeating during which she feels that she has no control over the amount of food she consumes. She reports eating as often as 10 times a day, and overeating to the point of physical discomfort during most meals. She gives an example of having recently consumed a large pizza, several portions of Chinese food, approximately 20 chicken wings, and half a chocolate cake for dinner. Ms. C admits that on several occasions she has vomited after meals due to feeling extremely full; however, she denies having done so intentionally. She also denies restricting her food intake, misusing laxatives or diuretics, or exercising excessively.
Ms. C expresses frustration and embarrassment with her eating and resulting weight gain. She says she has poor self-esteem, low energy and motivation, and poor concentration. She feels that her condition has significantly impacted her social life, romantic relationships, and family life. She admits she’s been avoiding dating and seeing friends due to her weight gain, and has been irritable with her teenage daughter.
During her initial evaluation, Ms. C is alert and oriented, with a linear and goal-directed thought process. She is somewhat irritable and guarded, wearing large sunglasses that cover most of her face, but is not overtly paranoid. Although she appears frustrated when discussing her condition, she denies feeling hopeless or helpless.
HISTORY Thyroid cancer and mood swings
Ms. C, who is single and unemployed, lives in an apartment with her teenage daughter, with whom she describes having a good relationship. She has been receiving disability benefits for the past 2 years after a motor vehicle accident resulted in multiple fractures of her arm and elbow, and subsequent chronic pain. Ms. C reports a distant history of “problems with alcohol,” but denies drinking any alcohol since being charged with driving under the influence several years ago. She has a 10 pack-year history of smoking and denies any history of illicit drug use.
Two years ago, Ms. C was diagnosed with thyroid carcinoma, and treated with surgical resection and a course of radiation. She has regular visits with her endocrinologist and has been prescribed oral levothyroxine, 150 mcg/d.
Ms. C reports a history of “mood swings” characterized by “snapping at people” and becoming irritable in response to stressful situations, but denies any past symptoms consistent with a manic or hypomanic episode. Ms. C has not been admitted to a psychiatric hospital, nor has she received any prior psychiatric treatment. She reluctantly discloses that approximately 3 years ago she had a less severe episode of uncontrollable eating and weight gain (20 to 30 lb). At that time, she was able to regain her desired physical appearance by going on the “Subway diet” and undergoing liposuction and plastic surgery.
At her current outpatient clinic visit, Ms. C expresses an interest in exploring bariatric surgery as a potential solution to her weight gain.
[polldaddy:10446186]
Continue to: EVALUATION Obese; stable thyroid function
EVALUATION Obese; stable thyroid function
We refer Ms. C for a physical examination and routine blood analysis to rule out any medical contributors to her condition. Her physical examination is reported as normal, with no signs of skin changes, goiter, or exophthalmos. Ms. C is noted to be obese, with a body mass index of 37.2 kg/m2, and an abdominal circumference of 38.5 in.
A blood analysis shows that Ms. C has elevated triglyceride levels (202 mg/dL) and elevated cholesterol levels (210 mg/dL). Her thyroid function tests are within normal limits based on the dose of levothyroxine she’s been receiving. A pregnancy test is negative.
Ms. C gives the team at the clinic permission to contact her endocrinologist, who reports that he does not suspect that Ms. C’s drastic weight gain and abnormal eating patterns are attributable to her history of thyroid carcinoma because her thyroid function tests have been stable on her current regimen.
The authors’ observations
Based on Ms. C’s initial presentation, we strongly suspected a diagnosis of binge eating disorder (BED). Several differential diagnoses were considered and carefully ruled out; Ms. C’s medical workup did not suggest that her weight gain was due to an active medical condition, and she did not meet DSM-5 criteria for a mood or psychotic disorder or anorexia nervosa or bulimia nervosa.
With an estimated lifetime prevalence in the United States of 2.6%, BED is the most prevalent eating disorder (compared with 0.6% for anorexia nervosa and 1% for bulimia nervosa).1 BED is more prevalent in women than in men, and the mean age of onset is mid-20s.
Continue to: BED may be difficult...
BED may be difficult to detect because patients may feel ashamed or guilty and are often hesitant to disclose and discuss their symptoms. Furthermore, they are frequently frustrated by the subjective loss of control over their behaviors. Patients with BED often present to medical facilities seeking weight loss solutions rather than to psychiatric clinics.
Screening for eating disorders
Several screening instruments have been developed to help clinicians identify patients who may need further evaluation for possible diagnosis of an eating disorder, including anorexia nervosa, bulimia nervosa, and BED.2 The SCOFF questionnaire is composed of 5 brief clinician-administered questions to screen for eating disorders.2 The 7-item Binge Eating Disorder Screener (BED-7) is a screening instrument specific for BED that examines a patient’s eating patterns and behaviors during the past 3 months.3
In general, suspect BED in patients who have significant weight dissatisfaction, fluctuation in weight, and depressive symptoms. The DSM-5 criteria for binge eating disorder are shown in Table 14.
BED and comorbid psychiatric disorders
Patients with BED are more likely than the general population to have comorbid psychiatric disorders, including mood and anxiety disorders, attention-deficit/hyperactivity disorder, posttraumatic stress disorder, and substance use disorders. Swanson et al5 found that 83.5% of adolescents who met criteria for BED also met criteria for at least 1 other psychiatric disorder, and 37% endorsed >3 concurrent psychiatric conditions. Once BED is confirmed, it is important to screen for other psychiatric and medical comorbidities that are often present in individuals with BED (Table 21,6).
The rates of diagnosis and treatment of BED remain low. This is likely due to patient factors such as shame and fear of stigma and clinician factors such as lack of awareness, ineffective communication, hesitation to discuss the sensitive topic, or insufficient knowledge about treatment options once BED is diagnosed.
[polldaddy:10446187]
Continue to: TREATMENT Combination therapy
TREATMENT Combination therapy
Ms. C is ambivalent about her BED diagnosis, and becomes angry about it when the proposed treatments do not involve bariatric surgery or cosmetic procedures. Ms. C is enrolled in weekly individual psychotherapy, where she receives a combination of CBT and psychodynamic therapy; however, her attendance is inconsistent. Ms. C is offered a trial of fluoxetine, but adamantly refuses, citing a relative who experienced adverse effects while receiving this type of antidepressant. Ms. C also refuses a trial of topiramate due to concerns of feeling sedated. Finally, she is offered a trial of lisdexamfetamine, 30 mg/d, which was FDA-approved in 2015 to treat moderate to severe BED. We discuss the risks, benefits, and adverse effects of lisdexamfetamine with Ms. C; however, she is hesitant to start this medication and expresses increasing interest in obtaining a consultation for bariatric surgery. Ms. C is provided with extensive education about the risks and dangers of surgery before addressing her eating patterns, and the clinician provides validation, verbal support, and counseling. Ms. C eventually agrees to a trial of lisdexamfetamine, but her insurance denies coverage of this medication.
The authors’ observations
When developing an individualized treatment plan for a patient with BED, the patient’s psychiatric and medical comorbidities should be considered. Treatment goals for patients with BED include:
- abstinence from binge eating
- sustainable weight loss and metabolic health
- reduction in symptoms associated with comorbid conditions
- improvement in self-esteem and overall quality of life.
A 2015 comparative effectiveness review of management and outcomes for patients with BED evaluated pharmacologic, psychologic, behavioral, and combined approaches for treating patients with BED.7 The results suggested that second-generation antidepressants, topiramate, and lisdexamfetamine were superior to placebo in reducing binge-eating episodes and achieving abstinence from binge-eating. Weight reduction was also achieved with topiramate and lisdexamfetamine, and antidepressants helped relieve symptoms of comorbid depression.
Various formats of CBT, including therapist-led and guided self-help, were also superior to placebo in reducing the frequency of binge-eating and promoting abstinence; however, they were generally not effective in treating depression or reducing patients’ weight.7
OUTCOME Fixated on surgery
We appeal the decision of Ms. C’s insurance company; however, during the appeals process, Ms. C becomes increasingly irritable and informs us that she has changed her mind and, with the reported support of her medical doctors, wishes to undergo bariatric surgery. Although we made multiple attempts to engage Ms. C in further treatment, she is lost to follow-up.
Continue to: Bottom Line
Bottom Line
Diagnosing and managing patients with binge eating disorder (BED) can be challenging because patients may hesitate to seek help, and/or have psychiatric and medical comorbidities. They often present to medical facilities seeking weight loss solutions rather than to psychiatric clinics. Once BED is confirmed, screen for other psychiatric and medical comorbidities. A combination of pharmacologic and psychotherapeutic interventions can benefit some patients with BED, but treatment should be individualized.
Related Resources
- National Eating Disorders Association. NEDA. www.nationaleatingdisorders.org/.
- Safer D, Telch C, Chen EY. Dialectical behavior therapy for binge eating and bulimia. New York, NY: Guilford Press; 2017.
Drug Brand Names
Fluoxetine • Prozac
Levothyroxine • Synthroid
Lisdexamfetamine • Vyvanse
Topiramate • Topamax
1. Hudson JI, Hiripi E, Pope HG Jr, et al. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348-358.
2. Morgan JF, Reid F, Lacey JH. The SCOFF questionnaire: assessment of a new screening tool for eating disorders. BMJ. 1999;319(7223):1467-1468.
3. Herman BK, Deal LS, DiBenedetti DB, et al. Development of the 7-Item Binge-Eating Disorder screener (BEDS-7). Prim Care Companion CNS Disord. 2016;18(2):10.4088/PCC.15m01896. doi:10.4088/PCC.15m01896.
4. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
5. Swanson SA, Crow SJ, Le Grange D, et al. Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement. Arch Gen Psychiatry. 2011;68(7):714.
6. Guerdjikova AI, Mori N, Casuto LS, et al. Binge eating disorder. Psychiatric Clinics of North America. 2017;40(2):255-266.
7. Berkman ND, Brownley KA, Peat CM, et al. Management and outcomes of binge-eating disorder. Comparative Effectiveness Reviews, No. 160. Agency for Healthcare Research and Quality (US). https://www.ncbi.nlm.nih.gov/books/NBK338312/. Published December 2015. Accessed July 29, 2019.
1. Hudson JI, Hiripi E, Pope HG Jr, et al. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348-358.
2. Morgan JF, Reid F, Lacey JH. The SCOFF questionnaire: assessment of a new screening tool for eating disorders. BMJ. 1999;319(7223):1467-1468.
3. Herman BK, Deal LS, DiBenedetti DB, et al. Development of the 7-Item Binge-Eating Disorder screener (BEDS-7). Prim Care Companion CNS Disord. 2016;18(2):10.4088/PCC.15m01896. doi:10.4088/PCC.15m01896.
4. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
5. Swanson SA, Crow SJ, Le Grange D, et al. Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement. Arch Gen Psychiatry. 2011;68(7):714.
6. Guerdjikova AI, Mori N, Casuto LS, et al. Binge eating disorder. Psychiatric Clinics of North America. 2017;40(2):255-266.
7. Berkman ND, Brownley KA, Peat CM, et al. Management and outcomes of binge-eating disorder. Comparative Effectiveness Reviews, No. 160. Agency for Healthcare Research and Quality (US). https://www.ncbi.nlm.nih.gov/books/NBK338312/. Published December 2015. Accessed July 29, 2019.
Effects of psychotropic medications on thyroid function
Ms. L, age 53, presents to an inpatient psychiatric unit with depression, difficulty concentrating, fatigue, cognitive blunting, loss of appetite, increased alcohol intake, and recent suicidal ideation. Her symptoms began 3 months ago and gradually worsened. Her medical and psychiatric history is significant for hypertension, fibromyalgia, and chronic pain (back and neck), major depressive disorder (MDD; recurrent, severe), and generalized anxiety disorder (GAD). Ms. L’s current medication regimen includes lisinopril, 40 mg daily; fluoxetine, 60 mg daily; mirtazapine, 30 mg at bedtime; gabapentin, 300 mg twice daily; alprazolam, 0.5 mg twice daily as needed for anxiety; and oral docusate, 100 mg twice daily as needed. Her blood pressure is 124/85 mm Hg, heart rate is 66 beats per minute, and an electrocardiogram is normal. Laboratory workup reveals a potassium level of 4.4 mEq/L, blood urea nitrogen level of 20 mg/dL, serum creatinine level of 0.8 mg/dL, estimated creatinine clearance of 89.6 mL/min, free triiodothyronine (T3) levels of 2.7 pg/mL, thyroid-stimulating hormone (TSH) level of 7.68 mIU/L, free thyroxine (T4) level of 1.3 ng/dL, and blood ethanol level <10 mg/dL. In addition to the symptoms Ms. L initially described, a review of systems reveals word-finding difficulty, cold intolerance, constipation, hair loss, brittle nails, and dry skin.
To target Ms. L’s MDD, GAD, fibromyalgia, and chronic pain, fluoxetine, 60 mg daily is cross titrated beginning on Day 1 to duloxetine, 60 mg twice daily, over 4 days. Mirtazapine is decreased on Day 3 to 7.5 mg at bedtime to target Ms. L’s sleep and appetite. Due to the presence of several symptoms associated with hypothyroidism and a slightly elevated TSH level, on Day 6 we initiate adjunctive levothyroxine, 50 mcg daily each morning to target symptomatic subclinical hypothyroidism, and to potentially augment the other medications prescribed to address Ms. L’s MDD.
Thyroid hormone function is a complex physiological process controlled through the hypothalamic-pituitary-thyroid (HPT) axis. Psychotropic medications can impact thyroid hormone function and contribute to aberrations in thyroid physiology.1 Because patients with mental illness may require multiple psychotropic medications, it is imperative to understand the potential effects of these agents.
Antidepressants can induce hypothyroidism along multiple points of hormonal synthesis and iodine utilization. Tricyclic antidepressants have been implicated in the development of drug-iodide complexes, thus reducing biologically active iodine.2 Tricyclic antidepressants also can bind thyroid peroxidase, an enzyme necessary in the production of T4 and T3, altering hormonal production, resulting in a hypothyroid state.1 Non-tricyclic antidepressants (ie, selective serotonin reuptake inhibitors [SSRIs] and non-SSRIs [including serotonin-norepinephrine reuptake inhibitors and mirtazapine]) have also been implicated in thyroid dysfunction. Selective serotonin reuptake inhibitors have the propensity to induce hypothyroidism through inhibition of thyroid hormones T4 and T3.1,3 This inhibition is not always seen with concurrent reductions in TSH levels. Conversely, non-SSRIs can influence thyroid hormone levels with great variation, leading to thyroid hormone levels that are increased, decreased, or unchanged.1 Patients with a history of thyroid dysfunction should receive close thyroid function monitoring, especially while taking antidepressants.
Antipsychotics have a proclivity to induce hypothyroidism by means similar to antidepressants via hormonal manipulation and immunogenicity. Phenothiazines impact thyroid function through hormonal activation and degradation, and induction of autoimmunity.1 Autoimmunity may develop by means of antibody production or antigen immunization through the major histocompatibility complex.2 Other first-generation antipsychotics (FGAs) (eg, haloperidol and loxapine) are known to antagonize dopamine receptors in the tuberoinfundibular pathway, resulting in increased prolactin levels. Hyperprolactinemia may result in increased TSH levels through HPT axis activation.1 Additionally, FGAs can induce an immunogenic effect through production of antithyroid antibodies.1 Similar to FGAs, second-generation antipsychotics (SGAs) can increase TSH levels through hyperprolactinemia. Further research focused on SGAs is needed to determine how profound this effect may be.
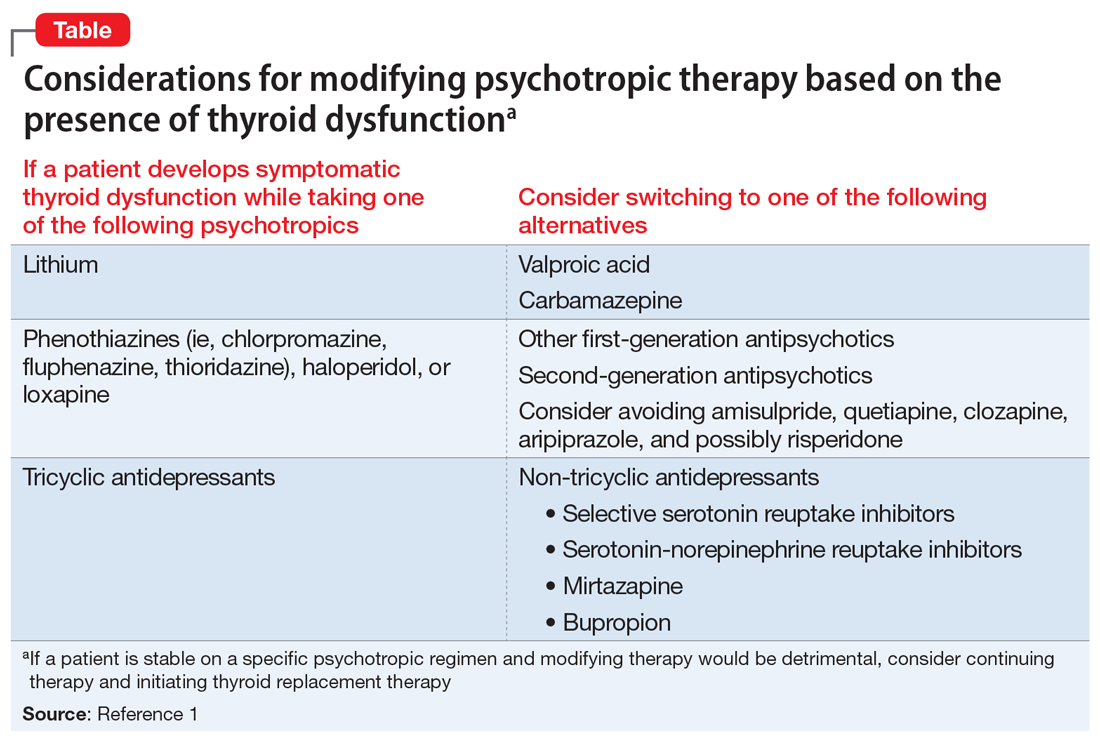
The Table1 outlines considerations for modifying psychotropic therapy based on the presence of concurrent thyroid dysfunction. Thyroid function should be routinely assessed in patients treated with antipsychotics.
Mood stabilizers are capable of altering thyroid function and inducing a hypothyroid state. Lithium has been implicated in both hypothyroidism and hyperthyroidism due to its inhibition of hormonal secretion, and toxicity to thyroid cells with chronic use, respectively.1,4 Hypothyroidism can develop shortly after initiating lithium; women tend to have a greater predilection for thyroid dysfunction than men.1 Carbamazepine (CBZ) can reduce thyroid hormone levels without having a direct effect on TSH or thyroid dysfunction.1 As with lithium, women tend to be more susceptible to this effect. Valproic acid (VPA) has been shown to either increase, decrease, or have no impact on thyroid hormone levels, with little effect on TSH.1 When VPA is given in combination with CBZ, significant reductions in thyroid levels with a concurrent increase in TSH can occur.1 In patients with preexisting thyroid dysfunction, the combination of VPA and CBZ should be used with caution.
Continue to: CASE
CASE CONTINUED
By Day 8, Ms. L reports less fatigue, clearer thinking, improved concentration, and less pain. She also no longer reports suicidal ideation, and demonstrates improved appetite and mood. She is discharged on Day 9 of her hospitalization.
The treatment team refers Ms. L for outpatient follow-up in 4 weeks, with a goal TSH level <3.0. Unfortunately, the effects of levothyroxine on Ms. L’s TSH level could not be determined during her hospital stay, and she has not returned to the facility since the initial presentation.
Thyroid function and mood
Ms. L’s case illustrates how thyroid function, pain, cognition, and mood may be interconnected. It is important to address all potential underlying comorbidities and establish appropriate outpatient care and follow-up so that patients may experience a more robust recovery. Further, this case highlights the importance of ruling out other potential medical causes of MDD during the initial diagnosis, and during times of recurrence or relapse, especially when a recent stressor, medication changes, or medication nonadherence cannot be identified as potential contributors.
Related Resources
- Cojić M, Cvejanov-Kezunović L. Subclinical hypothyroidism – whether and when to start treatment? Open Access Maced J Med Sci. 2017;5(7):1042-1046.
- Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22(12):1200-1235.
- Iosifescu DV. ‘Supercharge’ antidepressants by adding thyroid hormones. Current Psychiatry. 2006;5(7):15-20,25.
Drug Brand Names
Alprazolam • Xanax
Aripiprazole • Abilify
Bupropion • Wellbutrin
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Clozapine • Clozaril
Duloxetine • Cymbalta
Fluoxetine • Prozac
Fluphenazine • Prolixin
Gabapentin • Neurontin
Haloperidol • Haldol
Levothyroxine • Synthroid
Lisinopril • Prinivil, Zestril
Lithium • Eskalith, Lithobid
Loxapine • Loxitane
Mirtazapine • Remeron
Quetiapine • Seroquel
Risperidone • Risperdal
Thioridazine • Mellaril
Valproic acid • Depakote
1. Bou Khalil R, Richa S. Thyroid adverse effect of psychotropic drugs: a review. Clin Neuropharm. 2001;34(6):248-255.
2. Sauvage MF, Marquet P, Rousseau A, et al. Relationship between psychotropic drugs and thyroid function: a review. Toxicol Appl Pharmacol. 1998;149(2):127-135.
3. Shelton RC, Winn S, Ekhatore N, et al. The effects of antidepressants on the thyroid axis in depression. Biol Psychiatry. 1993;33(2):120-126.
4. Kundra P, Burman KD. The effect of medications on thyroid function tests. Med Clin North Am. 2012;96(2):283-295.
Ms. L, age 53, presents to an inpatient psychiatric unit with depression, difficulty concentrating, fatigue, cognitive blunting, loss of appetite, increased alcohol intake, and recent suicidal ideation. Her symptoms began 3 months ago and gradually worsened. Her medical and psychiatric history is significant for hypertension, fibromyalgia, and chronic pain (back and neck), major depressive disorder (MDD; recurrent, severe), and generalized anxiety disorder (GAD). Ms. L’s current medication regimen includes lisinopril, 40 mg daily; fluoxetine, 60 mg daily; mirtazapine, 30 mg at bedtime; gabapentin, 300 mg twice daily; alprazolam, 0.5 mg twice daily as needed for anxiety; and oral docusate, 100 mg twice daily as needed. Her blood pressure is 124/85 mm Hg, heart rate is 66 beats per minute, and an electrocardiogram is normal. Laboratory workup reveals a potassium level of 4.4 mEq/L, blood urea nitrogen level of 20 mg/dL, serum creatinine level of 0.8 mg/dL, estimated creatinine clearance of 89.6 mL/min, free triiodothyronine (T3) levels of 2.7 pg/mL, thyroid-stimulating hormone (TSH) level of 7.68 mIU/L, free thyroxine (T4) level of 1.3 ng/dL, and blood ethanol level <10 mg/dL. In addition to the symptoms Ms. L initially described, a review of systems reveals word-finding difficulty, cold intolerance, constipation, hair loss, brittle nails, and dry skin.
To target Ms. L’s MDD, GAD, fibromyalgia, and chronic pain, fluoxetine, 60 mg daily is cross titrated beginning on Day 1 to duloxetine, 60 mg twice daily, over 4 days. Mirtazapine is decreased on Day 3 to 7.5 mg at bedtime to target Ms. L’s sleep and appetite. Due to the presence of several symptoms associated with hypothyroidism and a slightly elevated TSH level, on Day 6 we initiate adjunctive levothyroxine, 50 mcg daily each morning to target symptomatic subclinical hypothyroidism, and to potentially augment the other medications prescribed to address Ms. L’s MDD.
Thyroid hormone function is a complex physiological process controlled through the hypothalamic-pituitary-thyroid (HPT) axis. Psychotropic medications can impact thyroid hormone function and contribute to aberrations in thyroid physiology.1 Because patients with mental illness may require multiple psychotropic medications, it is imperative to understand the potential effects of these agents.
Antidepressants can induce hypothyroidism along multiple points of hormonal synthesis and iodine utilization. Tricyclic antidepressants have been implicated in the development of drug-iodide complexes, thus reducing biologically active iodine.2 Tricyclic antidepressants also can bind thyroid peroxidase, an enzyme necessary in the production of T4 and T3, altering hormonal production, resulting in a hypothyroid state.1 Non-tricyclic antidepressants (ie, selective serotonin reuptake inhibitors [SSRIs] and non-SSRIs [including serotonin-norepinephrine reuptake inhibitors and mirtazapine]) have also been implicated in thyroid dysfunction. Selective serotonin reuptake inhibitors have the propensity to induce hypothyroidism through inhibition of thyroid hormones T4 and T3.1,3 This inhibition is not always seen with concurrent reductions in TSH levels. Conversely, non-SSRIs can influence thyroid hormone levels with great variation, leading to thyroid hormone levels that are increased, decreased, or unchanged.1 Patients with a history of thyroid dysfunction should receive close thyroid function monitoring, especially while taking antidepressants.
Antipsychotics have a proclivity to induce hypothyroidism by means similar to antidepressants via hormonal manipulation and immunogenicity. Phenothiazines impact thyroid function through hormonal activation and degradation, and induction of autoimmunity.1 Autoimmunity may develop by means of antibody production or antigen immunization through the major histocompatibility complex.2 Other first-generation antipsychotics (FGAs) (eg, haloperidol and loxapine) are known to antagonize dopamine receptors in the tuberoinfundibular pathway, resulting in increased prolactin levels. Hyperprolactinemia may result in increased TSH levels through HPT axis activation.1 Additionally, FGAs can induce an immunogenic effect through production of antithyroid antibodies.1 Similar to FGAs, second-generation antipsychotics (SGAs) can increase TSH levels through hyperprolactinemia. Further research focused on SGAs is needed to determine how profound this effect may be.
The Table1 outlines considerations for modifying psychotropic therapy based on the presence of concurrent thyroid dysfunction. Thyroid function should be routinely assessed in patients treated with antipsychotics.
Mood stabilizers are capable of altering thyroid function and inducing a hypothyroid state. Lithium has been implicated in both hypothyroidism and hyperthyroidism due to its inhibition of hormonal secretion, and toxicity to thyroid cells with chronic use, respectively.1,4 Hypothyroidism can develop shortly after initiating lithium; women tend to have a greater predilection for thyroid dysfunction than men.1 Carbamazepine (CBZ) can reduce thyroid hormone levels without having a direct effect on TSH or thyroid dysfunction.1 As with lithium, women tend to be more susceptible to this effect. Valproic acid (VPA) has been shown to either increase, decrease, or have no impact on thyroid hormone levels, with little effect on TSH.1 When VPA is given in combination with CBZ, significant reductions in thyroid levels with a concurrent increase in TSH can occur.1 In patients with preexisting thyroid dysfunction, the combination of VPA and CBZ should be used with caution.
Continue to: CASE
CASE CONTINUED
By Day 8, Ms. L reports less fatigue, clearer thinking, improved concentration, and less pain. She also no longer reports suicidal ideation, and demonstrates improved appetite and mood. She is discharged on Day 9 of her hospitalization.
The treatment team refers Ms. L for outpatient follow-up in 4 weeks, with a goal TSH level <3.0. Unfortunately, the effects of levothyroxine on Ms. L’s TSH level could not be determined during her hospital stay, and she has not returned to the facility since the initial presentation.
Thyroid function and mood
Ms. L’s case illustrates how thyroid function, pain, cognition, and mood may be interconnected. It is important to address all potential underlying comorbidities and establish appropriate outpatient care and follow-up so that patients may experience a more robust recovery. Further, this case highlights the importance of ruling out other potential medical causes of MDD during the initial diagnosis, and during times of recurrence or relapse, especially when a recent stressor, medication changes, or medication nonadherence cannot be identified as potential contributors.
Related Resources
- Cojić M, Cvejanov-Kezunović L. Subclinical hypothyroidism – whether and when to start treatment? Open Access Maced J Med Sci. 2017;5(7):1042-1046.
- Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22(12):1200-1235.
- Iosifescu DV. ‘Supercharge’ antidepressants by adding thyroid hormones. Current Psychiatry. 2006;5(7):15-20,25.
Drug Brand Names
Alprazolam • Xanax
Aripiprazole • Abilify
Bupropion • Wellbutrin
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Clozapine • Clozaril
Duloxetine • Cymbalta
Fluoxetine • Prozac
Fluphenazine • Prolixin
Gabapentin • Neurontin
Haloperidol • Haldol
Levothyroxine • Synthroid
Lisinopril • Prinivil, Zestril
Lithium • Eskalith, Lithobid
Loxapine • Loxitane
Mirtazapine • Remeron
Quetiapine • Seroquel
Risperidone • Risperdal
Thioridazine • Mellaril
Valproic acid • Depakote
Ms. L, age 53, presents to an inpatient psychiatric unit with depression, difficulty concentrating, fatigue, cognitive blunting, loss of appetite, increased alcohol intake, and recent suicidal ideation. Her symptoms began 3 months ago and gradually worsened. Her medical and psychiatric history is significant for hypertension, fibromyalgia, and chronic pain (back and neck), major depressive disorder (MDD; recurrent, severe), and generalized anxiety disorder (GAD). Ms. L’s current medication regimen includes lisinopril, 40 mg daily; fluoxetine, 60 mg daily; mirtazapine, 30 mg at bedtime; gabapentin, 300 mg twice daily; alprazolam, 0.5 mg twice daily as needed for anxiety; and oral docusate, 100 mg twice daily as needed. Her blood pressure is 124/85 mm Hg, heart rate is 66 beats per minute, and an electrocardiogram is normal. Laboratory workup reveals a potassium level of 4.4 mEq/L, blood urea nitrogen level of 20 mg/dL, serum creatinine level of 0.8 mg/dL, estimated creatinine clearance of 89.6 mL/min, free triiodothyronine (T3) levels of 2.7 pg/mL, thyroid-stimulating hormone (TSH) level of 7.68 mIU/L, free thyroxine (T4) level of 1.3 ng/dL, and blood ethanol level <10 mg/dL. In addition to the symptoms Ms. L initially described, a review of systems reveals word-finding difficulty, cold intolerance, constipation, hair loss, brittle nails, and dry skin.
To target Ms. L’s MDD, GAD, fibromyalgia, and chronic pain, fluoxetine, 60 mg daily is cross titrated beginning on Day 1 to duloxetine, 60 mg twice daily, over 4 days. Mirtazapine is decreased on Day 3 to 7.5 mg at bedtime to target Ms. L’s sleep and appetite. Due to the presence of several symptoms associated with hypothyroidism and a slightly elevated TSH level, on Day 6 we initiate adjunctive levothyroxine, 50 mcg daily each morning to target symptomatic subclinical hypothyroidism, and to potentially augment the other medications prescribed to address Ms. L’s MDD.
Thyroid hormone function is a complex physiological process controlled through the hypothalamic-pituitary-thyroid (HPT) axis. Psychotropic medications can impact thyroid hormone function and contribute to aberrations in thyroid physiology.1 Because patients with mental illness may require multiple psychotropic medications, it is imperative to understand the potential effects of these agents.
Antidepressants can induce hypothyroidism along multiple points of hormonal synthesis and iodine utilization. Tricyclic antidepressants have been implicated in the development of drug-iodide complexes, thus reducing biologically active iodine.2 Tricyclic antidepressants also can bind thyroid peroxidase, an enzyme necessary in the production of T4 and T3, altering hormonal production, resulting in a hypothyroid state.1 Non-tricyclic antidepressants (ie, selective serotonin reuptake inhibitors [SSRIs] and non-SSRIs [including serotonin-norepinephrine reuptake inhibitors and mirtazapine]) have also been implicated in thyroid dysfunction. Selective serotonin reuptake inhibitors have the propensity to induce hypothyroidism through inhibition of thyroid hormones T4 and T3.1,3 This inhibition is not always seen with concurrent reductions in TSH levels. Conversely, non-SSRIs can influence thyroid hormone levels with great variation, leading to thyroid hormone levels that are increased, decreased, or unchanged.1 Patients with a history of thyroid dysfunction should receive close thyroid function monitoring, especially while taking antidepressants.
Antipsychotics have a proclivity to induce hypothyroidism by means similar to antidepressants via hormonal manipulation and immunogenicity. Phenothiazines impact thyroid function through hormonal activation and degradation, and induction of autoimmunity.1 Autoimmunity may develop by means of antibody production or antigen immunization through the major histocompatibility complex.2 Other first-generation antipsychotics (FGAs) (eg, haloperidol and loxapine) are known to antagonize dopamine receptors in the tuberoinfundibular pathway, resulting in increased prolactin levels. Hyperprolactinemia may result in increased TSH levels through HPT axis activation.1 Additionally, FGAs can induce an immunogenic effect through production of antithyroid antibodies.1 Similar to FGAs, second-generation antipsychotics (SGAs) can increase TSH levels through hyperprolactinemia. Further research focused on SGAs is needed to determine how profound this effect may be.
The Table1 outlines considerations for modifying psychotropic therapy based on the presence of concurrent thyroid dysfunction. Thyroid function should be routinely assessed in patients treated with antipsychotics.
Mood stabilizers are capable of altering thyroid function and inducing a hypothyroid state. Lithium has been implicated in both hypothyroidism and hyperthyroidism due to its inhibition of hormonal secretion, and toxicity to thyroid cells with chronic use, respectively.1,4 Hypothyroidism can develop shortly after initiating lithium; women tend to have a greater predilection for thyroid dysfunction than men.1 Carbamazepine (CBZ) can reduce thyroid hormone levels without having a direct effect on TSH or thyroid dysfunction.1 As with lithium, women tend to be more susceptible to this effect. Valproic acid (VPA) has been shown to either increase, decrease, or have no impact on thyroid hormone levels, with little effect on TSH.1 When VPA is given in combination with CBZ, significant reductions in thyroid levels with a concurrent increase in TSH can occur.1 In patients with preexisting thyroid dysfunction, the combination of VPA and CBZ should be used with caution.
Continue to: CASE
CASE CONTINUED
By Day 8, Ms. L reports less fatigue, clearer thinking, improved concentration, and less pain. She also no longer reports suicidal ideation, and demonstrates improved appetite and mood. She is discharged on Day 9 of her hospitalization.
The treatment team refers Ms. L for outpatient follow-up in 4 weeks, with a goal TSH level <3.0. Unfortunately, the effects of levothyroxine on Ms. L’s TSH level could not be determined during her hospital stay, and she has not returned to the facility since the initial presentation.
Thyroid function and mood
Ms. L’s case illustrates how thyroid function, pain, cognition, and mood may be interconnected. It is important to address all potential underlying comorbidities and establish appropriate outpatient care and follow-up so that patients may experience a more robust recovery. Further, this case highlights the importance of ruling out other potential medical causes of MDD during the initial diagnosis, and during times of recurrence or relapse, especially when a recent stressor, medication changes, or medication nonadherence cannot be identified as potential contributors.
Related Resources
- Cojić M, Cvejanov-Kezunović L. Subclinical hypothyroidism – whether and when to start treatment? Open Access Maced J Med Sci. 2017;5(7):1042-1046.
- Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22(12):1200-1235.
- Iosifescu DV. ‘Supercharge’ antidepressants by adding thyroid hormones. Current Psychiatry. 2006;5(7):15-20,25.
Drug Brand Names
Alprazolam • Xanax
Aripiprazole • Abilify
Bupropion • Wellbutrin
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Clozapine • Clozaril
Duloxetine • Cymbalta
Fluoxetine • Prozac
Fluphenazine • Prolixin
Gabapentin • Neurontin
Haloperidol • Haldol
Levothyroxine • Synthroid
Lisinopril • Prinivil, Zestril
Lithium • Eskalith, Lithobid
Loxapine • Loxitane
Mirtazapine • Remeron
Quetiapine • Seroquel
Risperidone • Risperdal
Thioridazine • Mellaril
Valproic acid • Depakote
1. Bou Khalil R, Richa S. Thyroid adverse effect of psychotropic drugs: a review. Clin Neuropharm. 2001;34(6):248-255.
2. Sauvage MF, Marquet P, Rousseau A, et al. Relationship between psychotropic drugs and thyroid function: a review. Toxicol Appl Pharmacol. 1998;149(2):127-135.
3. Shelton RC, Winn S, Ekhatore N, et al. The effects of antidepressants on the thyroid axis in depression. Biol Psychiatry. 1993;33(2):120-126.
4. Kundra P, Burman KD. The effect of medications on thyroid function tests. Med Clin North Am. 2012;96(2):283-295.
1. Bou Khalil R, Richa S. Thyroid adverse effect of psychotropic drugs: a review. Clin Neuropharm. 2001;34(6):248-255.
2. Sauvage MF, Marquet P, Rousseau A, et al. Relationship between psychotropic drugs and thyroid function: a review. Toxicol Appl Pharmacol. 1998;149(2):127-135.
3. Shelton RC, Winn S, Ekhatore N, et al. The effects of antidepressants on the thyroid axis in depression. Biol Psychiatry. 1993;33(2):120-126.
4. Kundra P, Burman KD. The effect of medications on thyroid function tests. Med Clin North Am. 2012;96(2):283-295.
Strategies to reduce and prevent polypharmacy in older patients
CASE
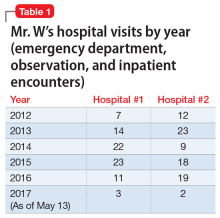
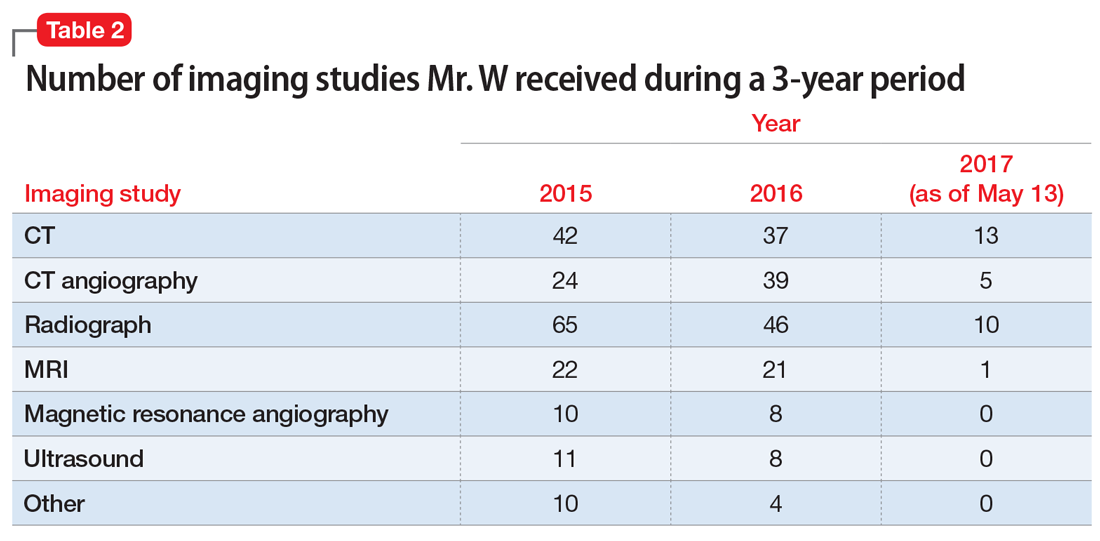
Ronald Wa is a 74-year old man with an extensive medical history: diabetes, hypertension, heart failure, atrial fibrillation, pancreatitis, hyperlipidemia, gout, depression, generalized anxiety, obstructive sleep apnea, and benign prostatic hypertrophy. He arrives at the emergency department (ED) of the hospital by nonemergent ambulance from home for evaluation of lethargy and confusion over the past week.
In the ED, Mr. W is afebrile, normotensive, and oxygenating on room air. Mucous membranes are dry. On physical examination, he appears pale, fatigued, and modestly confused but is able to state his name and birthday, although not the location or date.
Laboratory testing reveals: blood glucose, 107 mg/dL; serum creatinine, 2.3 mg/dL; sodium, 127 mEq/L; and hemoglobin level and hematocrit, within normal limits. Urinalysis is negative. Renal ultrasonography is unremarkable, without evidence of urinary tract obstruction.
Mr. W is admitted to the general medical unit with hyponatremia. The pharmacy admission specialist begins reconciliation of the long list of the patient’s home medications.
Overprescribing: Often, more is not better
Some experts consider prescribing medication to be the most common form of medical intervention; beyond that, polypharmacy—often defined as the use of more medications than are medically necessary (see the next section on terminology)—is recognized as an increasingly serious problem in many medical specialties.1 Here are specifics about the extent of, and harm caused by, the problem2,3:
- The US General Accounting Office reports that inappropriate polypharmacy is associated with significant morbidity and mortality.2 Research has established a strong relationship between polypharmacy and harmful clinical consequences,3 to which the older patient population is most susceptible.
- Polypharmacy is also recognized as an expensive practice; the US Center for Medicare and Medicaid Services estimates that polypharmacy cost US health insurers more than $50 billion annually.2
- Worldwide, with more and more people older than 65 years, polypharmacy is becoming more prevalent, and a growing concern, in older adults; approximately 50% of them take ≥ 1 medications that are medically unnecessary.3
Despite many programs to help with deprescribing, drug–drug interactions and the so-called prescribing cascade (ie, when signs and symptoms of an adverse drug effect are misdiagnosed as a new medical condition) continue to affect patients, leading to comorbidities. It is important, therefore, for physicians to be aware of commonly used tools to prevent polypharmacy and its consequences.
What is “polypharmacy” understood to mean?
Despite the compelling association of polypharmacy with the presence of multiple morbidities in the older patient population, there is no consensus on its definition:
- Starting with the dictionary, “polypharmacy” derives from 2 words in Ancient Greek: poly, “more than one,” and “pharmakon, “drug.”3
- The definition can vary based on the number of drugs a patient has been prescribed, their safety, and the appropriateness of their use.1
- Another definition is the use of more medications than are medically necessary; such a grouping includes agents that are not indicated, are ineffective, or constitute a therapeutic duplication. Although this definition is more clinically relevant than the others, it is premised on undertaking a clinical review of a medication regimen.3
- A numerical definition is the most commonly reported category, a number that varies from study to study—from ≥ 2 to ≥ 11 medications. When applied to health care settings, accepted definitions are ≥ 5 medications at hospital discharge and ≥ 10 during a hospital stay.4 Numerical definitions of polypharmacy do not ascertain the clinical appropriateness of therapy nor the process of rationalizing those medications.1
aA composite, hypothetical patient, based on the authors' clinical experience.
Continue to: Appropriateness
Appropriateness
Polypharmacy is classified as appropriate or inappropriate:
- Appropriate polypharmacy is the optimization of medications for patients with complex or multiple conditions, when the use of medicine is in agreement with best evidence.
- Inappropriate polypharmacy can increase the risk of adverse drug effects and drug–drug interactions and can be characterized by medication underuse and duplication.4
There are subdefinitions of “appropriateness,” but these are beyond the scope of this article.
What variables contribute to polypharmacy?
Multimorbidity is common in the older population. The presence of multiple chronic conditions increases the complexity of therapeutic management for health professionals and patients; such complexity can have a harmful impact on health outcomes. Combinations of medications to treat chronic diseases automatically push many patients into polypharmacy. Few treatment guidelines provide recommendations on when to stop medications.
Consequences of polypharmacy, some of which are masked as syndromes in the older patient, include delirium and dementia, urinary incontinence, dizziness, falls, adverse drug reactions, increased length of hospital stay, readmission soon after discharge, and death.3-5 Relatively high rates of drug consumption and other variables (eg, decreased renal and hepatic function, decreased total body water and lean body mass, cognitive impairment, age-related decline in vision and hearing, frequency of chronic diseases and medical comorbidities, communication barriers, prescribing cascades, and health care delivery involving multiple prescribers) can contribute to an increased prevalence of medication-associated morbidity and mortality as the result of polypharmacy.
In a descriptive study6 that examined these variables, researchers explored whether general practitioners experience barriers to medication review in multimorbid patients with polypharmacy. They concluded that the primary barriers were (1) lack of communication and teamwork with specialists and (2) the challenge of handling polypharmacy in a culture that encourages adding medications and inhibits conversations about medication withdrawal.6
Continue to: Reducing consequences of polypharmacy
Reducing consequences of polypharmacy
Collaborative medication review
Interventions to help physicians reduce polypharmacy include reviewing medications with older patients at every office visit and during transitions of care into and out of the hospital or other care facility. A 2016 Cochrane review of 5 randomized trials of inpatient medication reviews led by pharmacists, physicians, and other health care professionals showed a 36% reduction in ED visits 30 days to 1 year after discharge.7
Patients can collaborate in this effort by bringing all medications to each appointment or upon hospital admission—not just a list but the actual supply, to ensure that a correct medication list is compiled and a thorough review conducted.8 Explicitly ask open-ended questions of the patient about over-the-counter medications, herbal products, and other home remedies that have not been prescribed; many patients may have trouble with recall or are uncertain what fits the definition of a nonprescription medication.8,9
Compare the medication list with the patient’s current problem list; consider removing medications that do not have a pertinent indication. (Physicians can help in this regard when prescribing by making note in the medical record of the indication for each medication they prescribe.)
Evaluate the patient’s signs and symptoms as a possible drug-related adverse effect, thus making an effort to minimize the chance of a prescribing cascade.9
Use Beers criteria,10 which list potentially inappropriate medications to be avoided in older adults. The criteria serve as a filter when considering starting a new medication and aiding in the review process.8
Continue to: The NO TEARS tool...
The NO TEARS tool11 can be useful for simplifying the medication review process. Components of this tool are:
- Need and indication: Does the patient still require each of his medications? Was long-term treatment anticipated?
- Open questions: Ask the patient for his views about his medications; for example, “Do you think the drugs you take work?”
- Tests and monitoring: Are any of the patient’s conditions undertreated, based on laboratory and clinical findings?
- Evidence and guidelines: Has the base of evidence been updated for each of the patient’s medications since they were started?
- Adverse events: Is the patient experiencing adverse effects of medication? Have possible adverse drug interactions been noted?
- Risk reduction or prevention: Does the patient face risks of treatment (eg, loss of appetite, urinary incontinence) that can be reduced by optimizing the medication plan?
- Simplification and switches: Can treatment be simplified while maintaining effectiveness?
There are strategies to promote patient advocacy, as well. Encourage patients to use a holistic approach by asking you, their other physicians, and their pharmacist about how their condition is being treated:
- What other treatment options exist, including nonpharmacotherapeutic options?
- What are the possible benefits and harms of medical therapy?
- Under what circumstances would discontinuing a medication be appropriate?12
CASE
Medication reconciliation identifies > 20 medications that had been prescribed for the patient to take at home (TABLE 1). A clinical pharmacist then performs a home medication review as part of routine patient care upon transition of care into the hospital.
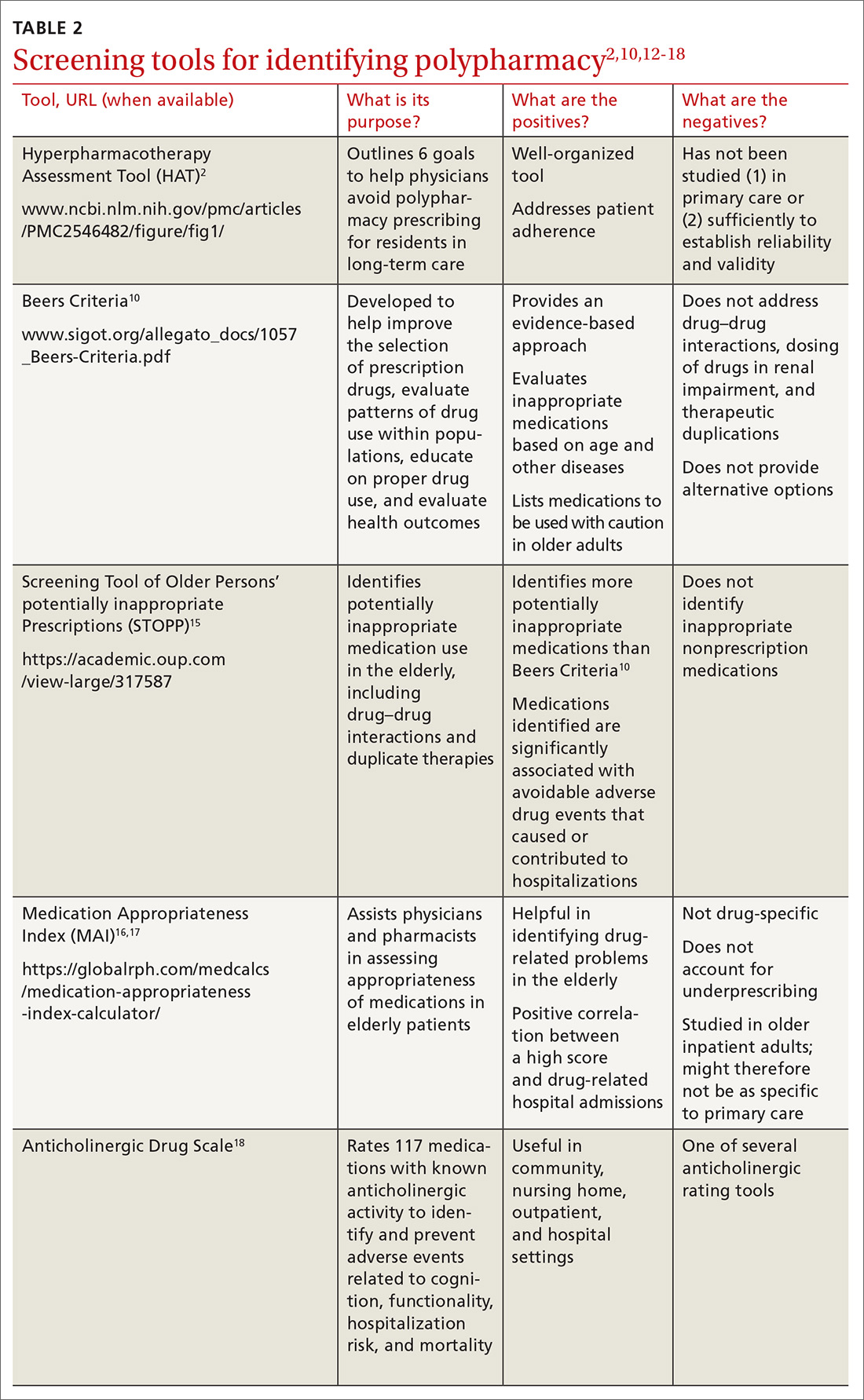
Identifying polypharmacy
Implementing polypharmacy identification tools is a necessary first step in the process of mitigating the risk of multiple concurrent medications (TABLE 22,10,12-18). In addition to tools that are used to identify polypharmacy, there are steps that physicians and pharmacists can take to decrease the risk of polypharmacy.
For example, in a longitudinal, time-series cohort study measuring polypharmacy events, a pharmacist intervention was used as the means to decrease polypharmacy.19 Pharmacists intervened twice (each intervention separated by 1 year) to identify and manage 5 categories of high-risk drugs in patients whose care was provided by a managed care plan.19 During that time, pharmacists provided drug therapy reviews, education to physicians and patients about drug safety, and information for physicians on ways to correct problems with polypharmacy.19
Continue to: Over the course of the 2 interventions...
Over the course of the 2 interventions, the overall rate of polypharmacy events decreased 67% after the first intervention and 39% after the second. The practice of having pharmacists spearhead this task was shown to reduce the cost and number of prescriptions in patients at risk for polypharmacy. (In fact, some general practitioners report that they deem multidisciplinary decision-making with pharmacists a necessary component of managing polypharmacy effectively.6)
Screening for medications as a cause of signs and symptoms
As noted earlier, a prescribing cascade arises when a drug administered to a patient causes an adverse event that is then mistakenly identified as a new condition, resulting in a new medication being prescribed.9 The pattern of a cascade then repeats itself, resulting in inappropriate polypharmacy.
Erroneous treatment of an adverse drug event as a medical condition is often the result of a lack of pharmacologic knowledge—which is why it is necessary to evaluate each new symptom with the mindset that a medication might, in fact, be causing the sign or symptom and with the aim of reducing the risk of a prescribing cascade.8,9 Routinely update a patient’s medication list in the event that a medication no longer has an indication aligned with the patient’s problem list; then, ideally, the initial therapy can be adjusted instead of starting additional medications.9
CASE
A review of Mr. W’s home medications reveals 1 therapeutic duplication and 2 drugs that lacked an indication. Application of the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP)15 and Beers criteria10 helped the pharmacist identify additional elements of inappropriate polypharmacy, including inappropriate medication use, drug–disease interactions, contraindications, and recommendations for dosage adjustment based on kidney function. Specifically:
- Aripiprazole and quetiapine: Present an increased risk of falls. (General recommendation: Avoid using Frutiger LT Std≥ 3 drugs that act on the central nervous system [CNS], due to an increased risk of falls.)
- Fluoxetine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Gabapentin: Presents an increased risk of CNS adverse effects. Reduce the dosage when the estimated creatinine clearance is < 60 mL/min.
- Hydrocodone–acetaminophen: Presents an increased risk of falls. (Again, avoid or minimize the number of drugs that act on the CNS.)
- Lorazepam: Indication is missing. Avoid use of this drug due to an increased risk of cognitive impairment and decreased metabolism of medication.
- Mirtazapine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Pantoprazole: Avoid scheduled use for > 8 weeks, except in high-risk patients, due to the risk of Clostridium difficile infection and bone loss and fractures.
- Prazosin: Indication is missing. Avoid use of this drug as an antihypertensive due to the high risk of orthostatic hypotension.
- Ranitidine: Duplicates concurrent treatment with pantoprazole. Reduce the dosage when the estimated creatinine clearance is < 50 mL/min.
The value of deprescribing
Direct evidence of the efficacy and safety of deprescribing, and strategies for deprescribing, have been documented in the literature:
Observational study. Cessation of inappropriate antihypertensive agents was associated with fewer cardiovascular events and deaths over a 5-year follow-up period.20
Continue to: Deprescribing protocol
Deprescribing protocol. A method developed by Scott and co-workers21 is an additional resource to consider. Appropriate times to consider deprescribing are (1) when new symptoms suggest an adverse drug effect; (2) in the presence of end-stage disease, terminal illness, dementia, extreme frailty, or full dependence on others for all care; (3) upon receipt of high-risk medications or combinations; and (4) upon receipt of preventive medications for which risk outweighs benefit.21
This suggested method of deprescribing comprises several steps: (1) collecting all medications that the patient is taking and identifying the indication for each; (2) considering the overall risk of drug-induced harm to determine necessary intensity of deprescribing; (3) assessing each drug for its eligibility to be discontinued, such as no indication, part of a prescribing cascade, or lack of benefit; (4) prioritizing drugs for discontinuation; and (5) implementing and monitoring the drug discontinuation regimen.21
Drug-by-drug elimination trial. Reducing the dosage of, or stopping, only 1 medication at a time has been shown to be paramount to assessing development of medication-associated problems and then identifying a likely cause.14
Good Palliative-Geriatric Practice algorithm. This algorithm22 can be used to guide discontinuation of inappropriate medications and improve drug therapy in community-dwelling older adults. The algorithm has been shown to improve the overall well-being of patients studied; however, it has been tested only in patients in long-term care settings and community-dwelling palliative care patients, limiting its generalizability to a larger population. The algorithm is also difficult to apply to patients who have multiple comorbidities.
Risk vs. benefit of discontinuing chronic medical therapy. A systematic review of the effects of discontinuing chronic medication reveals that the risk of doing so might outweigh benefit14; this finding is thought to be due to potential relapse in the disease state being treated.11 The risks of discontinuation should be contemplated before removing the medication or reducing the dosage. Medications that can be considered to present a risk when discontinued include, but are not limited to, benzodiazepines, oral corticosteroids, antidepressants, acid suppressants, bisphosphonates, statins, and transdermal opioids.1
Continue to: CASE
CASE
After applying Beers criteria10 and STOPP15, the pharmacist makes several recommendations:
- Use aripiprazole and quetiapine with caution.
- Consider discontinuing fluoxetine, hydrocodone–acetaminophen, lorazepam, pantoprazole, and ranitidine.
- Reduce the dosage of gabapentin.
- Clarify the indication for prazosin. Consider discontinuing if being used as an antihypertensive.
In addition, the pharmacist recommends holding metformin because lactic acidosis can develop (however rarely) when a person taking metformin experiences acute kidney injury.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected]
1. All Wales Medicines Strategy Group. Polypharmacy: Guidance for Prescribing. July 2014. http://awmsg.org/docs/awmsg/medman/Polypharmacy%20-%20Guidance%20for%20Prescribing.pdf. Accessed October 3, 2019.
2. Bushardt RL, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3:383-389.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
4. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
5. Milton JC, Hill-Smith I, Jackson SH. Prescribing for older people. BMJ. 2008;336:606-609.
6. Laursen J, Kornholt J, Betzer C, et al. General practitioners’ barriers toward medication reviews in polymedicated multimorbid patients: How can a focus on the pharmacotherapy in an outpatient clinic support GPs? Health Serv Res Manag Epidemiol. 2018;5:2333392818792169.
7. Christensen M, Lundh A. Medication review in hospitalized patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986.
8. Zurakowski T. The practicalities and pitfalls of polypharmacy. Nurse Pract. 2009;34:36-41.
9. Ponte ML, Wachs L, Wachs A, et al. Prescribing cascade. A proposed new way to evaluate it. Medicina (B Aires). 2017;77:13-16.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Lewis T. Using the NO TEARS tool for medication review. BMJ. 2004;329:434.
12. Hamilton HJ, Gallagher PF, O’Mahony D. Inappropriate prescribing and adverse events in older people. BMC Geriatr. 2009;9:5.
13. Skinner M. A literature review: polypharmacy protocol for primary care. Geriatr Nurs. 2015;36:367-371.
14. Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31.
15. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers criteria. Age Ageing. 2008;37:673-679.
16. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045-1051.
17. Samsa G, Hanlon JT, Schmader KE, et al. A summated score for the Medication Appropriateness Index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47:891-896.
18. Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481-1486.
19. Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25:1636-1645.
20. Thio SL, Nam J, van Driel ML, et al. Effects of discontinuation of chronic medication in primary care: a systematic review of deprescribing trials. Br J Gen Pract. 2018;68:e663-e672.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
22. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
CASE
Ronald Wa is a 74-year old man with an extensive medical history: diabetes, hypertension, heart failure, atrial fibrillation, pancreatitis, hyperlipidemia, gout, depression, generalized anxiety, obstructive sleep apnea, and benign prostatic hypertrophy. He arrives at the emergency department (ED) of the hospital by nonemergent ambulance from home for evaluation of lethargy and confusion over the past week.
In the ED, Mr. W is afebrile, normotensive, and oxygenating on room air. Mucous membranes are dry. On physical examination, he appears pale, fatigued, and modestly confused but is able to state his name and birthday, although not the location or date.
Laboratory testing reveals: blood glucose, 107 mg/dL; serum creatinine, 2.3 mg/dL; sodium, 127 mEq/L; and hemoglobin level and hematocrit, within normal limits. Urinalysis is negative. Renal ultrasonography is unremarkable, without evidence of urinary tract obstruction.
Mr. W is admitted to the general medical unit with hyponatremia. The pharmacy admission specialist begins reconciliation of the long list of the patient’s home medications.
Overprescribing: Often, more is not better
Some experts consider prescribing medication to be the most common form of medical intervention; beyond that, polypharmacy—often defined as the use of more medications than are medically necessary (see the next section on terminology)—is recognized as an increasingly serious problem in many medical specialties.1 Here are specifics about the extent of, and harm caused by, the problem2,3:
- The US General Accounting Office reports that inappropriate polypharmacy is associated with significant morbidity and mortality.2 Research has established a strong relationship between polypharmacy and harmful clinical consequences,3 to which the older patient population is most susceptible.
- Polypharmacy is also recognized as an expensive practice; the US Center for Medicare and Medicaid Services estimates that polypharmacy cost US health insurers more than $50 billion annually.2
- Worldwide, with more and more people older than 65 years, polypharmacy is becoming more prevalent, and a growing concern, in older adults; approximately 50% of them take ≥ 1 medications that are medically unnecessary.3
Despite many programs to help with deprescribing, drug–drug interactions and the so-called prescribing cascade (ie, when signs and symptoms of an adverse drug effect are misdiagnosed as a new medical condition) continue to affect patients, leading to comorbidities. It is important, therefore, for physicians to be aware of commonly used tools to prevent polypharmacy and its consequences.
What is “polypharmacy” understood to mean?
Despite the compelling association of polypharmacy with the presence of multiple morbidities in the older patient population, there is no consensus on its definition:
- Starting with the dictionary, “polypharmacy” derives from 2 words in Ancient Greek: poly, “more than one,” and “pharmakon, “drug.”3
- The definition can vary based on the number of drugs a patient has been prescribed, their safety, and the appropriateness of their use.1
- Another definition is the use of more medications than are medically necessary; such a grouping includes agents that are not indicated, are ineffective, or constitute a therapeutic duplication. Although this definition is more clinically relevant than the others, it is premised on undertaking a clinical review of a medication regimen.3
- A numerical definition is the most commonly reported category, a number that varies from study to study—from ≥ 2 to ≥ 11 medications. When applied to health care settings, accepted definitions are ≥ 5 medications at hospital discharge and ≥ 10 during a hospital stay.4 Numerical definitions of polypharmacy do not ascertain the clinical appropriateness of therapy nor the process of rationalizing those medications.1
aA composite, hypothetical patient, based on the authors' clinical experience.
Continue to: Appropriateness
Appropriateness
Polypharmacy is classified as appropriate or inappropriate:
- Appropriate polypharmacy is the optimization of medications for patients with complex or multiple conditions, when the use of medicine is in agreement with best evidence.
- Inappropriate polypharmacy can increase the risk of adverse drug effects and drug–drug interactions and can be characterized by medication underuse and duplication.4
There are subdefinitions of “appropriateness,” but these are beyond the scope of this article.
What variables contribute to polypharmacy?
Multimorbidity is common in the older population. The presence of multiple chronic conditions increases the complexity of therapeutic management for health professionals and patients; such complexity can have a harmful impact on health outcomes. Combinations of medications to treat chronic diseases automatically push many patients into polypharmacy. Few treatment guidelines provide recommendations on when to stop medications.
Consequences of polypharmacy, some of which are masked as syndromes in the older patient, include delirium and dementia, urinary incontinence, dizziness, falls, adverse drug reactions, increased length of hospital stay, readmission soon after discharge, and death.3-5 Relatively high rates of drug consumption and other variables (eg, decreased renal and hepatic function, decreased total body water and lean body mass, cognitive impairment, age-related decline in vision and hearing, frequency of chronic diseases and medical comorbidities, communication barriers, prescribing cascades, and health care delivery involving multiple prescribers) can contribute to an increased prevalence of medication-associated morbidity and mortality as the result of polypharmacy.
In a descriptive study6 that examined these variables, researchers explored whether general practitioners experience barriers to medication review in multimorbid patients with polypharmacy. They concluded that the primary barriers were (1) lack of communication and teamwork with specialists and (2) the challenge of handling polypharmacy in a culture that encourages adding medications and inhibits conversations about medication withdrawal.6
Continue to: Reducing consequences of polypharmacy
Reducing consequences of polypharmacy
Collaborative medication review
Interventions to help physicians reduce polypharmacy include reviewing medications with older patients at every office visit and during transitions of care into and out of the hospital or other care facility. A 2016 Cochrane review of 5 randomized trials of inpatient medication reviews led by pharmacists, physicians, and other health care professionals showed a 36% reduction in ED visits 30 days to 1 year after discharge.7
Patients can collaborate in this effort by bringing all medications to each appointment or upon hospital admission—not just a list but the actual supply, to ensure that a correct medication list is compiled and a thorough review conducted.8 Explicitly ask open-ended questions of the patient about over-the-counter medications, herbal products, and other home remedies that have not been prescribed; many patients may have trouble with recall or are uncertain what fits the definition of a nonprescription medication.8,9
Compare the medication list with the patient’s current problem list; consider removing medications that do not have a pertinent indication. (Physicians can help in this regard when prescribing by making note in the medical record of the indication for each medication they prescribe.)
Evaluate the patient’s signs and symptoms as a possible drug-related adverse effect, thus making an effort to minimize the chance of a prescribing cascade.9
Use Beers criteria,10 which list potentially inappropriate medications to be avoided in older adults. The criteria serve as a filter when considering starting a new medication and aiding in the review process.8
Continue to: The NO TEARS tool...
The NO TEARS tool11 can be useful for simplifying the medication review process. Components of this tool are:
- Need and indication: Does the patient still require each of his medications? Was long-term treatment anticipated?
- Open questions: Ask the patient for his views about his medications; for example, “Do you think the drugs you take work?”
- Tests and monitoring: Are any of the patient’s conditions undertreated, based on laboratory and clinical findings?
- Evidence and guidelines: Has the base of evidence been updated for each of the patient’s medications since they were started?
- Adverse events: Is the patient experiencing adverse effects of medication? Have possible adverse drug interactions been noted?
- Risk reduction or prevention: Does the patient face risks of treatment (eg, loss of appetite, urinary incontinence) that can be reduced by optimizing the medication plan?
- Simplification and switches: Can treatment be simplified while maintaining effectiveness?
There are strategies to promote patient advocacy, as well. Encourage patients to use a holistic approach by asking you, their other physicians, and their pharmacist about how their condition is being treated:
- What other treatment options exist, including nonpharmacotherapeutic options?
- What are the possible benefits and harms of medical therapy?
- Under what circumstances would discontinuing a medication be appropriate?12
CASE
Medication reconciliation identifies > 20 medications that had been prescribed for the patient to take at home (TABLE 1). A clinical pharmacist then performs a home medication review as part of routine patient care upon transition of care into the hospital.
Identifying polypharmacy
Implementing polypharmacy identification tools is a necessary first step in the process of mitigating the risk of multiple concurrent medications (TABLE 22,10,12-18). In addition to tools that are used to identify polypharmacy, there are steps that physicians and pharmacists can take to decrease the risk of polypharmacy.
For example, in a longitudinal, time-series cohort study measuring polypharmacy events, a pharmacist intervention was used as the means to decrease polypharmacy.19 Pharmacists intervened twice (each intervention separated by 1 year) to identify and manage 5 categories of high-risk drugs in patients whose care was provided by a managed care plan.19 During that time, pharmacists provided drug therapy reviews, education to physicians and patients about drug safety, and information for physicians on ways to correct problems with polypharmacy.19
Continue to: Over the course of the 2 interventions...
Over the course of the 2 interventions, the overall rate of polypharmacy events decreased 67% after the first intervention and 39% after the second. The practice of having pharmacists spearhead this task was shown to reduce the cost and number of prescriptions in patients at risk for polypharmacy. (In fact, some general practitioners report that they deem multidisciplinary decision-making with pharmacists a necessary component of managing polypharmacy effectively.6)
Screening for medications as a cause of signs and symptoms
As noted earlier, a prescribing cascade arises when a drug administered to a patient causes an adverse event that is then mistakenly identified as a new condition, resulting in a new medication being prescribed.9 The pattern of a cascade then repeats itself, resulting in inappropriate polypharmacy.
Erroneous treatment of an adverse drug event as a medical condition is often the result of a lack of pharmacologic knowledge—which is why it is necessary to evaluate each new symptom with the mindset that a medication might, in fact, be causing the sign or symptom and with the aim of reducing the risk of a prescribing cascade.8,9 Routinely update a patient’s medication list in the event that a medication no longer has an indication aligned with the patient’s problem list; then, ideally, the initial therapy can be adjusted instead of starting additional medications.9
CASE
A review of Mr. W’s home medications reveals 1 therapeutic duplication and 2 drugs that lacked an indication. Application of the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP)15 and Beers criteria10 helped the pharmacist identify additional elements of inappropriate polypharmacy, including inappropriate medication use, drug–disease interactions, contraindications, and recommendations for dosage adjustment based on kidney function. Specifically:
- Aripiprazole and quetiapine: Present an increased risk of falls. (General recommendation: Avoid using Frutiger LT Std≥ 3 drugs that act on the central nervous system [CNS], due to an increased risk of falls.)
- Fluoxetine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Gabapentin: Presents an increased risk of CNS adverse effects. Reduce the dosage when the estimated creatinine clearance is < 60 mL/min.
- Hydrocodone–acetaminophen: Presents an increased risk of falls. (Again, avoid or minimize the number of drugs that act on the CNS.)
- Lorazepam: Indication is missing. Avoid use of this drug due to an increased risk of cognitive impairment and decreased metabolism of medication.
- Mirtazapine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Pantoprazole: Avoid scheduled use for > 8 weeks, except in high-risk patients, due to the risk of Clostridium difficile infection and bone loss and fractures.
- Prazosin: Indication is missing. Avoid use of this drug as an antihypertensive due to the high risk of orthostatic hypotension.
- Ranitidine: Duplicates concurrent treatment with pantoprazole. Reduce the dosage when the estimated creatinine clearance is < 50 mL/min.
The value of deprescribing
Direct evidence of the efficacy and safety of deprescribing, and strategies for deprescribing, have been documented in the literature:
Observational study. Cessation of inappropriate antihypertensive agents was associated with fewer cardiovascular events and deaths over a 5-year follow-up period.20
Continue to: Deprescribing protocol
Deprescribing protocol. A method developed by Scott and co-workers21 is an additional resource to consider. Appropriate times to consider deprescribing are (1) when new symptoms suggest an adverse drug effect; (2) in the presence of end-stage disease, terminal illness, dementia, extreme frailty, or full dependence on others for all care; (3) upon receipt of high-risk medications or combinations; and (4) upon receipt of preventive medications for which risk outweighs benefit.21
This suggested method of deprescribing comprises several steps: (1) collecting all medications that the patient is taking and identifying the indication for each; (2) considering the overall risk of drug-induced harm to determine necessary intensity of deprescribing; (3) assessing each drug for its eligibility to be discontinued, such as no indication, part of a prescribing cascade, or lack of benefit; (4) prioritizing drugs for discontinuation; and (5) implementing and monitoring the drug discontinuation regimen.21
Drug-by-drug elimination trial. Reducing the dosage of, or stopping, only 1 medication at a time has been shown to be paramount to assessing development of medication-associated problems and then identifying a likely cause.14
Good Palliative-Geriatric Practice algorithm. This algorithm22 can be used to guide discontinuation of inappropriate medications and improve drug therapy in community-dwelling older adults. The algorithm has been shown to improve the overall well-being of patients studied; however, it has been tested only in patients in long-term care settings and community-dwelling palliative care patients, limiting its generalizability to a larger population. The algorithm is also difficult to apply to patients who have multiple comorbidities.
Risk vs. benefit of discontinuing chronic medical therapy. A systematic review of the effects of discontinuing chronic medication reveals that the risk of doing so might outweigh benefit14; this finding is thought to be due to potential relapse in the disease state being treated.11 The risks of discontinuation should be contemplated before removing the medication or reducing the dosage. Medications that can be considered to present a risk when discontinued include, but are not limited to, benzodiazepines, oral corticosteroids, antidepressants, acid suppressants, bisphosphonates, statins, and transdermal opioids.1
Continue to: CASE
CASE
After applying Beers criteria10 and STOPP15, the pharmacist makes several recommendations:
- Use aripiprazole and quetiapine with caution.
- Consider discontinuing fluoxetine, hydrocodone–acetaminophen, lorazepam, pantoprazole, and ranitidine.
- Reduce the dosage of gabapentin.
- Clarify the indication for prazosin. Consider discontinuing if being used as an antihypertensive.
In addition, the pharmacist recommends holding metformin because lactic acidosis can develop (however rarely) when a person taking metformin experiences acute kidney injury.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected]
CASE
Ronald Wa is a 74-year old man with an extensive medical history: diabetes, hypertension, heart failure, atrial fibrillation, pancreatitis, hyperlipidemia, gout, depression, generalized anxiety, obstructive sleep apnea, and benign prostatic hypertrophy. He arrives at the emergency department (ED) of the hospital by nonemergent ambulance from home for evaluation of lethargy and confusion over the past week.
In the ED, Mr. W is afebrile, normotensive, and oxygenating on room air. Mucous membranes are dry. On physical examination, he appears pale, fatigued, and modestly confused but is able to state his name and birthday, although not the location or date.
Laboratory testing reveals: blood glucose, 107 mg/dL; serum creatinine, 2.3 mg/dL; sodium, 127 mEq/L; and hemoglobin level and hematocrit, within normal limits. Urinalysis is negative. Renal ultrasonography is unremarkable, without evidence of urinary tract obstruction.
Mr. W is admitted to the general medical unit with hyponatremia. The pharmacy admission specialist begins reconciliation of the long list of the patient’s home medications.
Overprescribing: Often, more is not better
Some experts consider prescribing medication to be the most common form of medical intervention; beyond that, polypharmacy—often defined as the use of more medications than are medically necessary (see the next section on terminology)—is recognized as an increasingly serious problem in many medical specialties.1 Here are specifics about the extent of, and harm caused by, the problem2,3:
- The US General Accounting Office reports that inappropriate polypharmacy is associated with significant morbidity and mortality.2 Research has established a strong relationship between polypharmacy and harmful clinical consequences,3 to which the older patient population is most susceptible.
- Polypharmacy is also recognized as an expensive practice; the US Center for Medicare and Medicaid Services estimates that polypharmacy cost US health insurers more than $50 billion annually.2
- Worldwide, with more and more people older than 65 years, polypharmacy is becoming more prevalent, and a growing concern, in older adults; approximately 50% of them take ≥ 1 medications that are medically unnecessary.3
Despite many programs to help with deprescribing, drug–drug interactions and the so-called prescribing cascade (ie, when signs and symptoms of an adverse drug effect are misdiagnosed as a new medical condition) continue to affect patients, leading to comorbidities. It is important, therefore, for physicians to be aware of commonly used tools to prevent polypharmacy and its consequences.
What is “polypharmacy” understood to mean?
Despite the compelling association of polypharmacy with the presence of multiple morbidities in the older patient population, there is no consensus on its definition:
- Starting with the dictionary, “polypharmacy” derives from 2 words in Ancient Greek: poly, “more than one,” and “pharmakon, “drug.”3
- The definition can vary based on the number of drugs a patient has been prescribed, their safety, and the appropriateness of their use.1
- Another definition is the use of more medications than are medically necessary; such a grouping includes agents that are not indicated, are ineffective, or constitute a therapeutic duplication. Although this definition is more clinically relevant than the others, it is premised on undertaking a clinical review of a medication regimen.3
- A numerical definition is the most commonly reported category, a number that varies from study to study—from ≥ 2 to ≥ 11 medications. When applied to health care settings, accepted definitions are ≥ 5 medications at hospital discharge and ≥ 10 during a hospital stay.4 Numerical definitions of polypharmacy do not ascertain the clinical appropriateness of therapy nor the process of rationalizing those medications.1
aA composite, hypothetical patient, based on the authors' clinical experience.
Continue to: Appropriateness
Appropriateness
Polypharmacy is classified as appropriate or inappropriate:
- Appropriate polypharmacy is the optimization of medications for patients with complex or multiple conditions, when the use of medicine is in agreement with best evidence.
- Inappropriate polypharmacy can increase the risk of adverse drug effects and drug–drug interactions and can be characterized by medication underuse and duplication.4
There are subdefinitions of “appropriateness,” but these are beyond the scope of this article.
What variables contribute to polypharmacy?
Multimorbidity is common in the older population. The presence of multiple chronic conditions increases the complexity of therapeutic management for health professionals and patients; such complexity can have a harmful impact on health outcomes. Combinations of medications to treat chronic diseases automatically push many patients into polypharmacy. Few treatment guidelines provide recommendations on when to stop medications.
Consequences of polypharmacy, some of which are masked as syndromes in the older patient, include delirium and dementia, urinary incontinence, dizziness, falls, adverse drug reactions, increased length of hospital stay, readmission soon after discharge, and death.3-5 Relatively high rates of drug consumption and other variables (eg, decreased renal and hepatic function, decreased total body water and lean body mass, cognitive impairment, age-related decline in vision and hearing, frequency of chronic diseases and medical comorbidities, communication barriers, prescribing cascades, and health care delivery involving multiple prescribers) can contribute to an increased prevalence of medication-associated morbidity and mortality as the result of polypharmacy.
In a descriptive study6 that examined these variables, researchers explored whether general practitioners experience barriers to medication review in multimorbid patients with polypharmacy. They concluded that the primary barriers were (1) lack of communication and teamwork with specialists and (2) the challenge of handling polypharmacy in a culture that encourages adding medications and inhibits conversations about medication withdrawal.6
Continue to: Reducing consequences of polypharmacy
Reducing consequences of polypharmacy
Collaborative medication review
Interventions to help physicians reduce polypharmacy include reviewing medications with older patients at every office visit and during transitions of care into and out of the hospital or other care facility. A 2016 Cochrane review of 5 randomized trials of inpatient medication reviews led by pharmacists, physicians, and other health care professionals showed a 36% reduction in ED visits 30 days to 1 year after discharge.7
Patients can collaborate in this effort by bringing all medications to each appointment or upon hospital admission—not just a list but the actual supply, to ensure that a correct medication list is compiled and a thorough review conducted.8 Explicitly ask open-ended questions of the patient about over-the-counter medications, herbal products, and other home remedies that have not been prescribed; many patients may have trouble with recall or are uncertain what fits the definition of a nonprescription medication.8,9
Compare the medication list with the patient’s current problem list; consider removing medications that do not have a pertinent indication. (Physicians can help in this regard when prescribing by making note in the medical record of the indication for each medication they prescribe.)
Evaluate the patient’s signs and symptoms as a possible drug-related adverse effect, thus making an effort to minimize the chance of a prescribing cascade.9
Use Beers criteria,10 which list potentially inappropriate medications to be avoided in older adults. The criteria serve as a filter when considering starting a new medication and aiding in the review process.8
Continue to: The NO TEARS tool...
The NO TEARS tool11 can be useful for simplifying the medication review process. Components of this tool are:
- Need and indication: Does the patient still require each of his medications? Was long-term treatment anticipated?
- Open questions: Ask the patient for his views about his medications; for example, “Do you think the drugs you take work?”
- Tests and monitoring: Are any of the patient’s conditions undertreated, based on laboratory and clinical findings?
- Evidence and guidelines: Has the base of evidence been updated for each of the patient’s medications since they were started?
- Adverse events: Is the patient experiencing adverse effects of medication? Have possible adverse drug interactions been noted?
- Risk reduction or prevention: Does the patient face risks of treatment (eg, loss of appetite, urinary incontinence) that can be reduced by optimizing the medication plan?
- Simplification and switches: Can treatment be simplified while maintaining effectiveness?
There are strategies to promote patient advocacy, as well. Encourage patients to use a holistic approach by asking you, their other physicians, and their pharmacist about how their condition is being treated:
- What other treatment options exist, including nonpharmacotherapeutic options?
- What are the possible benefits and harms of medical therapy?
- Under what circumstances would discontinuing a medication be appropriate?12
CASE
Medication reconciliation identifies > 20 medications that had been prescribed for the patient to take at home (TABLE 1). A clinical pharmacist then performs a home medication review as part of routine patient care upon transition of care into the hospital.
Identifying polypharmacy
Implementing polypharmacy identification tools is a necessary first step in the process of mitigating the risk of multiple concurrent medications (TABLE 22,10,12-18). In addition to tools that are used to identify polypharmacy, there are steps that physicians and pharmacists can take to decrease the risk of polypharmacy.
For example, in a longitudinal, time-series cohort study measuring polypharmacy events, a pharmacist intervention was used as the means to decrease polypharmacy.19 Pharmacists intervened twice (each intervention separated by 1 year) to identify and manage 5 categories of high-risk drugs in patients whose care was provided by a managed care plan.19 During that time, pharmacists provided drug therapy reviews, education to physicians and patients about drug safety, and information for physicians on ways to correct problems with polypharmacy.19
Continue to: Over the course of the 2 interventions...
Over the course of the 2 interventions, the overall rate of polypharmacy events decreased 67% after the first intervention and 39% after the second. The practice of having pharmacists spearhead this task was shown to reduce the cost and number of prescriptions in patients at risk for polypharmacy. (In fact, some general practitioners report that they deem multidisciplinary decision-making with pharmacists a necessary component of managing polypharmacy effectively.6)
Screening for medications as a cause of signs and symptoms
As noted earlier, a prescribing cascade arises when a drug administered to a patient causes an adverse event that is then mistakenly identified as a new condition, resulting in a new medication being prescribed.9 The pattern of a cascade then repeats itself, resulting in inappropriate polypharmacy.
Erroneous treatment of an adverse drug event as a medical condition is often the result of a lack of pharmacologic knowledge—which is why it is necessary to evaluate each new symptom with the mindset that a medication might, in fact, be causing the sign or symptom and with the aim of reducing the risk of a prescribing cascade.8,9 Routinely update a patient’s medication list in the event that a medication no longer has an indication aligned with the patient’s problem list; then, ideally, the initial therapy can be adjusted instead of starting additional medications.9
CASE
A review of Mr. W’s home medications reveals 1 therapeutic duplication and 2 drugs that lacked an indication. Application of the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP)15 and Beers criteria10 helped the pharmacist identify additional elements of inappropriate polypharmacy, including inappropriate medication use, drug–disease interactions, contraindications, and recommendations for dosage adjustment based on kidney function. Specifically:
- Aripiprazole and quetiapine: Present an increased risk of falls. (General recommendation: Avoid using Frutiger LT Std≥ 3 drugs that act on the central nervous system [CNS], due to an increased risk of falls.)
- Fluoxetine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Gabapentin: Presents an increased risk of CNS adverse effects. Reduce the dosage when the estimated creatinine clearance is < 60 mL/min.
- Hydrocodone–acetaminophen: Presents an increased risk of falls. (Again, avoid or minimize the number of drugs that act on the CNS.)
- Lorazepam: Indication is missing. Avoid use of this drug due to an increased risk of cognitive impairment and decreased metabolism of medication.
- Mirtazapine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Pantoprazole: Avoid scheduled use for > 8 weeks, except in high-risk patients, due to the risk of Clostridium difficile infection and bone loss and fractures.
- Prazosin: Indication is missing. Avoid use of this drug as an antihypertensive due to the high risk of orthostatic hypotension.
- Ranitidine: Duplicates concurrent treatment with pantoprazole. Reduce the dosage when the estimated creatinine clearance is < 50 mL/min.
The value of deprescribing
Direct evidence of the efficacy and safety of deprescribing, and strategies for deprescribing, have been documented in the literature:
Observational study. Cessation of inappropriate antihypertensive agents was associated with fewer cardiovascular events and deaths over a 5-year follow-up period.20
Continue to: Deprescribing protocol
Deprescribing protocol. A method developed by Scott and co-workers21 is an additional resource to consider. Appropriate times to consider deprescribing are (1) when new symptoms suggest an adverse drug effect; (2) in the presence of end-stage disease, terminal illness, dementia, extreme frailty, or full dependence on others for all care; (3) upon receipt of high-risk medications or combinations; and (4) upon receipt of preventive medications for which risk outweighs benefit.21
This suggested method of deprescribing comprises several steps: (1) collecting all medications that the patient is taking and identifying the indication for each; (2) considering the overall risk of drug-induced harm to determine necessary intensity of deprescribing; (3) assessing each drug for its eligibility to be discontinued, such as no indication, part of a prescribing cascade, or lack of benefit; (4) prioritizing drugs for discontinuation; and (5) implementing and monitoring the drug discontinuation regimen.21
Drug-by-drug elimination trial. Reducing the dosage of, or stopping, only 1 medication at a time has been shown to be paramount to assessing development of medication-associated problems and then identifying a likely cause.14
Good Palliative-Geriatric Practice algorithm. This algorithm22 can be used to guide discontinuation of inappropriate medications and improve drug therapy in community-dwelling older adults. The algorithm has been shown to improve the overall well-being of patients studied; however, it has been tested only in patients in long-term care settings and community-dwelling palliative care patients, limiting its generalizability to a larger population. The algorithm is also difficult to apply to patients who have multiple comorbidities.
Risk vs. benefit of discontinuing chronic medical therapy. A systematic review of the effects of discontinuing chronic medication reveals that the risk of doing so might outweigh benefit14; this finding is thought to be due to potential relapse in the disease state being treated.11 The risks of discontinuation should be contemplated before removing the medication or reducing the dosage. Medications that can be considered to present a risk when discontinued include, but are not limited to, benzodiazepines, oral corticosteroids, antidepressants, acid suppressants, bisphosphonates, statins, and transdermal opioids.1
Continue to: CASE
CASE
After applying Beers criteria10 and STOPP15, the pharmacist makes several recommendations:
- Use aripiprazole and quetiapine with caution.
- Consider discontinuing fluoxetine, hydrocodone–acetaminophen, lorazepam, pantoprazole, and ranitidine.
- Reduce the dosage of gabapentin.
- Clarify the indication for prazosin. Consider discontinuing if being used as an antihypertensive.
In addition, the pharmacist recommends holding metformin because lactic acidosis can develop (however rarely) when a person taking metformin experiences acute kidney injury.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected]
1. All Wales Medicines Strategy Group. Polypharmacy: Guidance for Prescribing. July 2014. http://awmsg.org/docs/awmsg/medman/Polypharmacy%20-%20Guidance%20for%20Prescribing.pdf. Accessed October 3, 2019.
2. Bushardt RL, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3:383-389.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
4. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
5. Milton JC, Hill-Smith I, Jackson SH. Prescribing for older people. BMJ. 2008;336:606-609.
6. Laursen J, Kornholt J, Betzer C, et al. General practitioners’ barriers toward medication reviews in polymedicated multimorbid patients: How can a focus on the pharmacotherapy in an outpatient clinic support GPs? Health Serv Res Manag Epidemiol. 2018;5:2333392818792169.
7. Christensen M, Lundh A. Medication review in hospitalized patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986.
8. Zurakowski T. The practicalities and pitfalls of polypharmacy. Nurse Pract. 2009;34:36-41.
9. Ponte ML, Wachs L, Wachs A, et al. Prescribing cascade. A proposed new way to evaluate it. Medicina (B Aires). 2017;77:13-16.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Lewis T. Using the NO TEARS tool for medication review. BMJ. 2004;329:434.
12. Hamilton HJ, Gallagher PF, O’Mahony D. Inappropriate prescribing and adverse events in older people. BMC Geriatr. 2009;9:5.
13. Skinner M. A literature review: polypharmacy protocol for primary care. Geriatr Nurs. 2015;36:367-371.
14. Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31.
15. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers criteria. Age Ageing. 2008;37:673-679.
16. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045-1051.
17. Samsa G, Hanlon JT, Schmader KE, et al. A summated score for the Medication Appropriateness Index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47:891-896.
18. Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481-1486.
19. Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25:1636-1645.
20. Thio SL, Nam J, van Driel ML, et al. Effects of discontinuation of chronic medication in primary care: a systematic review of deprescribing trials. Br J Gen Pract. 2018;68:e663-e672.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
22. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
1. All Wales Medicines Strategy Group. Polypharmacy: Guidance for Prescribing. July 2014. http://awmsg.org/docs/awmsg/medman/Polypharmacy%20-%20Guidance%20for%20Prescribing.pdf. Accessed October 3, 2019.
2. Bushardt RL, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3:383-389.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
4. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
5. Milton JC, Hill-Smith I, Jackson SH. Prescribing for older people. BMJ. 2008;336:606-609.
6. Laursen J, Kornholt J, Betzer C, et al. General practitioners’ barriers toward medication reviews in polymedicated multimorbid patients: How can a focus on the pharmacotherapy in an outpatient clinic support GPs? Health Serv Res Manag Epidemiol. 2018;5:2333392818792169.
7. Christensen M, Lundh A. Medication review in hospitalized patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986.
8. Zurakowski T. The practicalities and pitfalls of polypharmacy. Nurse Pract. 2009;34:36-41.
9. Ponte ML, Wachs L, Wachs A, et al. Prescribing cascade. A proposed new way to evaluate it. Medicina (B Aires). 2017;77:13-16.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Lewis T. Using the NO TEARS tool for medication review. BMJ. 2004;329:434.
12. Hamilton HJ, Gallagher PF, O’Mahony D. Inappropriate prescribing and adverse events in older people. BMC Geriatr. 2009;9:5.
13. Skinner M. A literature review: polypharmacy protocol for primary care. Geriatr Nurs. 2015;36:367-371.
14. Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31.
15. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers criteria. Age Ageing. 2008;37:673-679.
16. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045-1051.
17. Samsa G, Hanlon JT, Schmader KE, et al. A summated score for the Medication Appropriateness Index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47:891-896.
18. Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481-1486.
19. Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25:1636-1645.
20. Thio SL, Nam J, van Driel ML, et al. Effects of discontinuation of chronic medication in primary care: a systematic review of deprescribing trials. Br J Gen Pract. 2018;68:e663-e672.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
22. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
PRACTICE RECOMMENDATIONS
› Use one of the available tested and recommended screening tools to identify polypharmacy. C
› Engage in collaborative medication review to reduce the incidence of polypharmacy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The 84-year-old state boxing champ: Bipolar disorder, or something else?
CASE Agitated, uncooperative, and irritable
Mr. X, age 84, presents to the emergency department with agitation, mania-like symptoms, and mood-congruent psychotic symptoms that started 2 weeks ago. Mr. X, who is accompanied by his wife, has no psychiatric history.
On examination, Mr. X is easily agitated and uncooperative. His speech is fast, but not pressured, with increased volume and tone. He states, “My mood is fantastic” with mood-congruent affect. His thought process reveals circumstantiality and loose association. Mr. X’s thought content includes flight of ideas and delusions of grandeur; he claims to be a state boxing champion and a psychologist. He also claims that he will run for Congress in the near future. He reports that he’s started knocking on his neighbors’ doors, pitched the idea to buy their house, and convinced them to vote for him as their congressman. He denies any suicidal or homicidal ideations. There is no evidence of perceptual disturbance. Mr. X undergoes a Mini-Mental State Examination (MMSE) and scores 26/30, which suggests no cognitive impairment. However, his insight and judgment are poor.
Mr. X’s physical examination is unremarkable. His laboratory workup includes a complete blood count, comprehensive metabolic panel, urinalysis, thyroid function test, vitamin B12 and folate levels, urine drug screen, and blood alcohol level. All results are within normal limits. He has no history of alcohol or recreational drug use as evident by the laboratory results and collateral information from his wife. Further, a non-contrast CT scan of his head shows no abnormality.
Approximately 1 month ago, Mr. X was diagnosed with restless leg syndrome (RLS). Mr. X’s medication regimen consists of gabapentin, 300 mg 3 times daily, prescribed years ago by his neurologist for neuropathic pain; and ropinirole, 3 mg/d, for RLS. His neurologist had prescribed him ropinirole, which was started at 1 mg/d and titrated to 3 mg/d within a 1-week span. Two weeks after Mr. X started this medication regimen, his wife reports that she noticed changes in his behavior, including severe agitation, irritability, delusions of grandeur, decreased need for sleep, and racing of thoughts.
[polldaddy:10417490]
The authors’ observations
Mr. X was diagnosed with medication (ropinirole)-induced bipolar and related disorder with mood-congruent psychotic features.
To determine this diagnosis, we initially considered Mr. X’s age and medical conditions, including stroke and space-occupying lesions of the brain. However, the laboratory and neuroimaging studies, which included a CT scan of the head and MRI of the brain, were negative. Next, because Mr. X had sudden onset manic symptoms after ropinirole was initiated, we considered the possibility of a substance/medication-induced bipolar and related disorder. Further, ropinirole is capable of producing the symptoms in criterion A of DSM-5 criteria for substance/medication-induced bipolar and related disorder. Mr. X met all DSM-5 criteria for substance/medication-induced bipolar and related disorder (Table1).
[polldaddy:10417494]
TREATMENT Medication adjustments and improvement
The admitting clinician discontinues ropinirole and initiates divalproex sodium, 500 mg twice a day. By Day 4, Mr. X shows significant improvement, including no irritable mood and regression of delusions of grandeur, and his sleep cycle returns to normal. At this time, the divalproex sodium is also discontinued.
Continue to: The authors' observations
The authors’ observations
Dopamine agonist agents are a standard treatment in the management of Parkinson’s disease and RLS.2-5 Ropinirole, a dopamine receptor agonist, has a high affinity for dopamine D2 and D3 receptor subtypes.4 Published reports have linked dopamine agonists to mania with psychotic features.6,7 In a study by Stoner et al,8 of 95 patients treated with ropinirole, 13 patients developed psychotic features that necessitated the use of antipsychotic medications or a lower dose of ropinirole.
The recommended starting dose for ropinirole is 0.25 mg/d. The dose can be increased to 0.5 mg in the next 2 days, and to 1 mg/d at the end of the first week.9 The mean effective daily dose is 2 mg/d, and maximum recommended dose is 4 mg/d.9 For Mr. X, ropinirole was quickly titrated to 3 mg/d over 1 week, which resulted in mania and psychosis. We suggest that when treating geriatric patients, clinicians should consider prescribing the lowest effective dose of psychotropic medications, such as ropinirole, to prevent adverse effects. Higher doses of dopamine agonists, especially in geriatric patients, increase the risk of common adverse effects, such as nausea (25% to 50%), headache (7% to 22%), fatigue (1% to 19%), dizziness (6% to 18%), and vomiting (5% to 11%).10 When prescribing dopamine agonists, clinicians should educate patients and their caregivers about the rare but potential risk of medication-induced mania and psychosis.
Mr. X’s case emphasizes the importance of a comprehensive psychiatric evaluation and medical workup to rule out a wide differential diagnosis when approaching new-onset mania and psychosis in geriatric patients.11 Our case contributes to the evidence that dopamine agonist medications are associated with mania and psychotic symptoms.
OUTCOME A return to baseline
On Day 12, Mr. X is discharged home in a stable condition. Two weeks later, at an outpatient follow-up visit, Mr. X is asymptomatic and has returned to his baseline functioning.
Bottom Line
When approaching new-onset mania and psychosis in geriatric patients, a comprehensive psychiatric evaluation and medical workup are necessary to rule out a wide differential diagnosis. Ropinirole use can lead to mania and psychotic symptoms, especially in geriatric patients. As should be done with all other dopaminergic agents, increase the dose of ropinirole with caution, and be vigilant for the emergence of signs of mania and/or psychosis.
Continue to: Related Resources
Related Resources
- Adabie A, Jackson JC, Torrence CL. Older-age bipolar disorder: A case series. Current Psychiatry. 2019;18(2):24-29.
- Chen P, Dols A, Rej S, et al. Update on the epidemiology, diagnosis, and treatment of mania in older-age bipolar disorder. Curr Psychiatry Rep. 2017;19(8):46.
Drug Brand Names
Divalproex sodium • Depakote
Gabapentin • Neurontin
Ropinirole • Requip
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Singh A, Althoff R, Martineau RJ, et al. Pramipexole, ropinirole, and mania in Parkinson’s disease. Am J Psychiatry. 2005;162(4):814-815.
3. Weiss HD, Pontone GM. Dopamine receptor agonist drugs and impulse control disorders. JAMA Intern Med. 2014;174(12):1935-1937.
4 Shill HA, Stacy M. Update on ropinirole in the treatment of Parkinson’s disease. Neuropsychiatr Dis Treat. 2009;5:33-36.
5. Borovac JA. Side effects of a dopamine agonist therapy for Parkinson’s disease: a mini-review of clinical pharmacology. Yale J Biol Med. 2016;89(1):37-47.
6. Yüksel RN, Elyas Kaya Z, Dilbaz N, et al. Cabergoline-induced manic episode: case report. Ther Adv Psychopharmacol. 2016;6(3):229-231.
7. Perea E, Robbins BV, Hutto B. Psychosis related to ropinirole. Am J Psychiatry. 2006;163(3):547-548.
8. Stoner SC, Dahmen MM, Makos M, et al. An exploratory retrospective evaluation of ropinirole-associated psychotic symptoms in an outpatient population treated for restless legs syndrome or Parkinson’s disease. Ann Pharmacother. 2009;43(9):1426-1432.
9. Trenkwalder C, Hening WA, Montagna P, et al. Treatment of restless legs syndrome: an evidence-based review and implications for clinical practice. Mov Disord. 2008;23(16):2267-2302.
10. Garcia-Borreguero D, Kohnen R, Silber MH, et al. The long-term treatment of restless legs syndrome/Willis-Ekbom disease: evidence-based guidelines and clinical consensus best practice guidance: a report from the International Restless Legs Syndrome Study Group. Sleep Med. 2013;14(7):675-684.
11. Dols A, Beekman A. Older age bipolar disorder. Psychiatr Clin North Am. 2018;41(1):95-110.
CASE Agitated, uncooperative, and irritable
Mr. X, age 84, presents to the emergency department with agitation, mania-like symptoms, and mood-congruent psychotic symptoms that started 2 weeks ago. Mr. X, who is accompanied by his wife, has no psychiatric history.
On examination, Mr. X is easily agitated and uncooperative. His speech is fast, but not pressured, with increased volume and tone. He states, “My mood is fantastic” with mood-congruent affect. His thought process reveals circumstantiality and loose association. Mr. X’s thought content includes flight of ideas and delusions of grandeur; he claims to be a state boxing champion and a psychologist. He also claims that he will run for Congress in the near future. He reports that he’s started knocking on his neighbors’ doors, pitched the idea to buy their house, and convinced them to vote for him as their congressman. He denies any suicidal or homicidal ideations. There is no evidence of perceptual disturbance. Mr. X undergoes a Mini-Mental State Examination (MMSE) and scores 26/30, which suggests no cognitive impairment. However, his insight and judgment are poor.
Mr. X’s physical examination is unremarkable. His laboratory workup includes a complete blood count, comprehensive metabolic panel, urinalysis, thyroid function test, vitamin B12 and folate levels, urine drug screen, and blood alcohol level. All results are within normal limits. He has no history of alcohol or recreational drug use as evident by the laboratory results and collateral information from his wife. Further, a non-contrast CT scan of his head shows no abnormality.
Approximately 1 month ago, Mr. X was diagnosed with restless leg syndrome (RLS). Mr. X’s medication regimen consists of gabapentin, 300 mg 3 times daily, prescribed years ago by his neurologist for neuropathic pain; and ropinirole, 3 mg/d, for RLS. His neurologist had prescribed him ropinirole, which was started at 1 mg/d and titrated to 3 mg/d within a 1-week span. Two weeks after Mr. X started this medication regimen, his wife reports that she noticed changes in his behavior, including severe agitation, irritability, delusions of grandeur, decreased need for sleep, and racing of thoughts.
[polldaddy:10417490]
The authors’ observations
Mr. X was diagnosed with medication (ropinirole)-induced bipolar and related disorder with mood-congruent psychotic features.
To determine this diagnosis, we initially considered Mr. X’s age and medical conditions, including stroke and space-occupying lesions of the brain. However, the laboratory and neuroimaging studies, which included a CT scan of the head and MRI of the brain, were negative. Next, because Mr. X had sudden onset manic symptoms after ropinirole was initiated, we considered the possibility of a substance/medication-induced bipolar and related disorder. Further, ropinirole is capable of producing the symptoms in criterion A of DSM-5 criteria for substance/medication-induced bipolar and related disorder. Mr. X met all DSM-5 criteria for substance/medication-induced bipolar and related disorder (Table1).
[polldaddy:10417494]
TREATMENT Medication adjustments and improvement
The admitting clinician discontinues ropinirole and initiates divalproex sodium, 500 mg twice a day. By Day 4, Mr. X shows significant improvement, including no irritable mood and regression of delusions of grandeur, and his sleep cycle returns to normal. At this time, the divalproex sodium is also discontinued.
Continue to: The authors' observations
The authors’ observations
Dopamine agonist agents are a standard treatment in the management of Parkinson’s disease and RLS.2-5 Ropinirole, a dopamine receptor agonist, has a high affinity for dopamine D2 and D3 receptor subtypes.4 Published reports have linked dopamine agonists to mania with psychotic features.6,7 In a study by Stoner et al,8 of 95 patients treated with ropinirole, 13 patients developed psychotic features that necessitated the use of antipsychotic medications or a lower dose of ropinirole.
The recommended starting dose for ropinirole is 0.25 mg/d. The dose can be increased to 0.5 mg in the next 2 days, and to 1 mg/d at the end of the first week.9 The mean effective daily dose is 2 mg/d, and maximum recommended dose is 4 mg/d.9 For Mr. X, ropinirole was quickly titrated to 3 mg/d over 1 week, which resulted in mania and psychosis. We suggest that when treating geriatric patients, clinicians should consider prescribing the lowest effective dose of psychotropic medications, such as ropinirole, to prevent adverse effects. Higher doses of dopamine agonists, especially in geriatric patients, increase the risk of common adverse effects, such as nausea (25% to 50%), headache (7% to 22%), fatigue (1% to 19%), dizziness (6% to 18%), and vomiting (5% to 11%).10 When prescribing dopamine agonists, clinicians should educate patients and their caregivers about the rare but potential risk of medication-induced mania and psychosis.
Mr. X’s case emphasizes the importance of a comprehensive psychiatric evaluation and medical workup to rule out a wide differential diagnosis when approaching new-onset mania and psychosis in geriatric patients.11 Our case contributes to the evidence that dopamine agonist medications are associated with mania and psychotic symptoms.
OUTCOME A return to baseline
On Day 12, Mr. X is discharged home in a stable condition. Two weeks later, at an outpatient follow-up visit, Mr. X is asymptomatic and has returned to his baseline functioning.
Bottom Line
When approaching new-onset mania and psychosis in geriatric patients, a comprehensive psychiatric evaluation and medical workup are necessary to rule out a wide differential diagnosis. Ropinirole use can lead to mania and psychotic symptoms, especially in geriatric patients. As should be done with all other dopaminergic agents, increase the dose of ropinirole with caution, and be vigilant for the emergence of signs of mania and/or psychosis.
Continue to: Related Resources
Related Resources
- Adabie A, Jackson JC, Torrence CL. Older-age bipolar disorder: A case series. Current Psychiatry. 2019;18(2):24-29.
- Chen P, Dols A, Rej S, et al. Update on the epidemiology, diagnosis, and treatment of mania in older-age bipolar disorder. Curr Psychiatry Rep. 2017;19(8):46.
Drug Brand Names
Divalproex sodium • Depakote
Gabapentin • Neurontin
Ropinirole • Requip
CASE Agitated, uncooperative, and irritable
Mr. X, age 84, presents to the emergency department with agitation, mania-like symptoms, and mood-congruent psychotic symptoms that started 2 weeks ago. Mr. X, who is accompanied by his wife, has no psychiatric history.
On examination, Mr. X is easily agitated and uncooperative. His speech is fast, but not pressured, with increased volume and tone. He states, “My mood is fantastic” with mood-congruent affect. His thought process reveals circumstantiality and loose association. Mr. X’s thought content includes flight of ideas and delusions of grandeur; he claims to be a state boxing champion and a psychologist. He also claims that he will run for Congress in the near future. He reports that he’s started knocking on his neighbors’ doors, pitched the idea to buy their house, and convinced them to vote for him as their congressman. He denies any suicidal or homicidal ideations. There is no evidence of perceptual disturbance. Mr. X undergoes a Mini-Mental State Examination (MMSE) and scores 26/30, which suggests no cognitive impairment. However, his insight and judgment are poor.
Mr. X’s physical examination is unremarkable. His laboratory workup includes a complete blood count, comprehensive metabolic panel, urinalysis, thyroid function test, vitamin B12 and folate levels, urine drug screen, and blood alcohol level. All results are within normal limits. He has no history of alcohol or recreational drug use as evident by the laboratory results and collateral information from his wife. Further, a non-contrast CT scan of his head shows no abnormality.
Approximately 1 month ago, Mr. X was diagnosed with restless leg syndrome (RLS). Mr. X’s medication regimen consists of gabapentin, 300 mg 3 times daily, prescribed years ago by his neurologist for neuropathic pain; and ropinirole, 3 mg/d, for RLS. His neurologist had prescribed him ropinirole, which was started at 1 mg/d and titrated to 3 mg/d within a 1-week span. Two weeks after Mr. X started this medication regimen, his wife reports that she noticed changes in his behavior, including severe agitation, irritability, delusions of grandeur, decreased need for sleep, and racing of thoughts.
[polldaddy:10417490]
The authors’ observations
Mr. X was diagnosed with medication (ropinirole)-induced bipolar and related disorder with mood-congruent psychotic features.
To determine this diagnosis, we initially considered Mr. X’s age and medical conditions, including stroke and space-occupying lesions of the brain. However, the laboratory and neuroimaging studies, which included a CT scan of the head and MRI of the brain, were negative. Next, because Mr. X had sudden onset manic symptoms after ropinirole was initiated, we considered the possibility of a substance/medication-induced bipolar and related disorder. Further, ropinirole is capable of producing the symptoms in criterion A of DSM-5 criteria for substance/medication-induced bipolar and related disorder. Mr. X met all DSM-5 criteria for substance/medication-induced bipolar and related disorder (Table1).
[polldaddy:10417494]
TREATMENT Medication adjustments and improvement
The admitting clinician discontinues ropinirole and initiates divalproex sodium, 500 mg twice a day. By Day 4, Mr. X shows significant improvement, including no irritable mood and regression of delusions of grandeur, and his sleep cycle returns to normal. At this time, the divalproex sodium is also discontinued.
Continue to: The authors' observations
The authors’ observations
Dopamine agonist agents are a standard treatment in the management of Parkinson’s disease and RLS.2-5 Ropinirole, a dopamine receptor agonist, has a high affinity for dopamine D2 and D3 receptor subtypes.4 Published reports have linked dopamine agonists to mania with psychotic features.6,7 In a study by Stoner et al,8 of 95 patients treated with ropinirole, 13 patients developed psychotic features that necessitated the use of antipsychotic medications or a lower dose of ropinirole.
The recommended starting dose for ropinirole is 0.25 mg/d. The dose can be increased to 0.5 mg in the next 2 days, and to 1 mg/d at the end of the first week.9 The mean effective daily dose is 2 mg/d, and maximum recommended dose is 4 mg/d.9 For Mr. X, ropinirole was quickly titrated to 3 mg/d over 1 week, which resulted in mania and psychosis. We suggest that when treating geriatric patients, clinicians should consider prescribing the lowest effective dose of psychotropic medications, such as ropinirole, to prevent adverse effects. Higher doses of dopamine agonists, especially in geriatric patients, increase the risk of common adverse effects, such as nausea (25% to 50%), headache (7% to 22%), fatigue (1% to 19%), dizziness (6% to 18%), and vomiting (5% to 11%).10 When prescribing dopamine agonists, clinicians should educate patients and their caregivers about the rare but potential risk of medication-induced mania and psychosis.
Mr. X’s case emphasizes the importance of a comprehensive psychiatric evaluation and medical workup to rule out a wide differential diagnosis when approaching new-onset mania and psychosis in geriatric patients.11 Our case contributes to the evidence that dopamine agonist medications are associated with mania and psychotic symptoms.
OUTCOME A return to baseline
On Day 12, Mr. X is discharged home in a stable condition. Two weeks later, at an outpatient follow-up visit, Mr. X is asymptomatic and has returned to his baseline functioning.
Bottom Line
When approaching new-onset mania and psychosis in geriatric patients, a comprehensive psychiatric evaluation and medical workup are necessary to rule out a wide differential diagnosis. Ropinirole use can lead to mania and psychotic symptoms, especially in geriatric patients. As should be done with all other dopaminergic agents, increase the dose of ropinirole with caution, and be vigilant for the emergence of signs of mania and/or psychosis.
Continue to: Related Resources
Related Resources
- Adabie A, Jackson JC, Torrence CL. Older-age bipolar disorder: A case series. Current Psychiatry. 2019;18(2):24-29.
- Chen P, Dols A, Rej S, et al. Update on the epidemiology, diagnosis, and treatment of mania in older-age bipolar disorder. Curr Psychiatry Rep. 2017;19(8):46.
Drug Brand Names
Divalproex sodium • Depakote
Gabapentin • Neurontin
Ropinirole • Requip
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Singh A, Althoff R, Martineau RJ, et al. Pramipexole, ropinirole, and mania in Parkinson’s disease. Am J Psychiatry. 2005;162(4):814-815.
3. Weiss HD, Pontone GM. Dopamine receptor agonist drugs and impulse control disorders. JAMA Intern Med. 2014;174(12):1935-1937.
4 Shill HA, Stacy M. Update on ropinirole in the treatment of Parkinson’s disease. Neuropsychiatr Dis Treat. 2009;5:33-36.
5. Borovac JA. Side effects of a dopamine agonist therapy for Parkinson’s disease: a mini-review of clinical pharmacology. Yale J Biol Med. 2016;89(1):37-47.
6. Yüksel RN, Elyas Kaya Z, Dilbaz N, et al. Cabergoline-induced manic episode: case report. Ther Adv Psychopharmacol. 2016;6(3):229-231.
7. Perea E, Robbins BV, Hutto B. Psychosis related to ropinirole. Am J Psychiatry. 2006;163(3):547-548.
8. Stoner SC, Dahmen MM, Makos M, et al. An exploratory retrospective evaluation of ropinirole-associated psychotic symptoms in an outpatient population treated for restless legs syndrome or Parkinson’s disease. Ann Pharmacother. 2009;43(9):1426-1432.
9. Trenkwalder C, Hening WA, Montagna P, et al. Treatment of restless legs syndrome: an evidence-based review and implications for clinical practice. Mov Disord. 2008;23(16):2267-2302.
10. Garcia-Borreguero D, Kohnen R, Silber MH, et al. The long-term treatment of restless legs syndrome/Willis-Ekbom disease: evidence-based guidelines and clinical consensus best practice guidance: a report from the International Restless Legs Syndrome Study Group. Sleep Med. 2013;14(7):675-684.
11. Dols A, Beekman A. Older age bipolar disorder. Psychiatr Clin North Am. 2018;41(1):95-110.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Singh A, Althoff R, Martineau RJ, et al. Pramipexole, ropinirole, and mania in Parkinson’s disease. Am J Psychiatry. 2005;162(4):814-815.
3. Weiss HD, Pontone GM. Dopamine receptor agonist drugs and impulse control disorders. JAMA Intern Med. 2014;174(12):1935-1937.
4 Shill HA, Stacy M. Update on ropinirole in the treatment of Parkinson’s disease. Neuropsychiatr Dis Treat. 2009;5:33-36.
5. Borovac JA. Side effects of a dopamine agonist therapy for Parkinson’s disease: a mini-review of clinical pharmacology. Yale J Biol Med. 2016;89(1):37-47.
6. Yüksel RN, Elyas Kaya Z, Dilbaz N, et al. Cabergoline-induced manic episode: case report. Ther Adv Psychopharmacol. 2016;6(3):229-231.
7. Perea E, Robbins BV, Hutto B. Psychosis related to ropinirole. Am J Psychiatry. 2006;163(3):547-548.
8. Stoner SC, Dahmen MM, Makos M, et al. An exploratory retrospective evaluation of ropinirole-associated psychotic symptoms in an outpatient population treated for restless legs syndrome or Parkinson’s disease. Ann Pharmacother. 2009;43(9):1426-1432.
9. Trenkwalder C, Hening WA, Montagna P, et al. Treatment of restless legs syndrome: an evidence-based review and implications for clinical practice. Mov Disord. 2008;23(16):2267-2302.
10. Garcia-Borreguero D, Kohnen R, Silber MH, et al. The long-term treatment of restless legs syndrome/Willis-Ekbom disease: evidence-based guidelines and clinical consensus best practice guidance: a report from the International Restless Legs Syndrome Study Group. Sleep Med. 2013;14(7):675-684.
11. Dols A, Beekman A. Older age bipolar disorder. Psychiatr Clin North Am. 2018;41(1):95-110.
Medical Cannabis: A guide to the clinical and legal landscapes
CASE
Barry S, a 45-year-old man with a new diagnosis of non-Hodgkin’s lymphoma, recently started induction chemotherapy. He has struggled with nausea, profound gustatory changes, and poor appetite; various antiemetics have provided only minimal relief. He tells you that he is hesitant to try “yet another pill” but has heard and read that marijuana (genus Cannabis) is used to alleviate disruptive chemotherapy-induced adverse effects. He asks if this is a treatment you’d recommend for him.
As Mr. S’s physician, how do you respond?
Understandably, some family physicians are hesitant to recommend an unregulated, federally illegal substance characterized by conflicting or absent evidence of safety and effectiveness.1 Nevertheless, throughout history and in the current court of public opinion, medical Cannabis has overwhelming support,2 leading to legalization in most of the United States.
As with many traditionally accepted therapies (whether they are or are not supported by substantial evidence), physicians are expected to provide individualized guidance regarding minimizing risk and maximizing benefit of the therapeutic use of Cannabis. The rapidly growing scientific and commercial fields of medical Cannabis guarantee that information on this topic will constantly be changing—and will often be contradictory. In this article, we review the most common concerns about medical Cannabis and provide up-to-date evidence on its use.
The pharmacology of cannabis
Cannabis sativa was among the earliest plants cultivated by man, with the first evidence of its use in China, approximately 4000 BC, to make twine and rope from its fibers.3 Records of medicinal Cannabis date back to the world’s oldest pharmacopoeia, a written summary of what was known about herbal medicine through the late 16th century.4
The 2 principal species of Cannabis are sativa and indica. There is no good medical evidence to separate the impacts of either strain; however, a staggering amount of lay information exists about the reported differing effects of each strain.5
Chemical constituents. Phytocannabinoids derived from C sativa are the plant’s best-known proteins, constituting a complex lipid-signaling network involved in numerous physiological processes. There are more than 100 known phytocannabinoids, the most well-recognized being Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Additional sources of cannabinoids include endogenous cannabinoids, or endocannabinoids, and synthetic cannabinoids.
The endocannabinoid system, comprising cannabinoid receptors, endocannabinoids, and their specific enzymes, is a potential therapeutic target for a variety of pathologic processes.6,7 The 2 most well-studied targets for cannabinoids in the human body are the cannabinoid receptors CB1 and CB2, found throughout the body: CB1, predominantly in the central and peripheral nervous system, and CB2 in a more limited distribution in the immune and hematopoietic systems. Other pathways activated or antagonized by THC and CBD exist, but are less well-mapped than CB1 and CB2.
[polldaddy:10402702]
Continue to: Botanical or synthetic?
Botanical or synthetic? It is important to distinguish between synthetic and plant-based cannabinoids, for you and your patients' benefit. Pharmaceutical (synthetic) THC is just that: THC alone. Whole-plant Cannabis, on the other hand, has hundreds of additional chemicals—most notably, phytocannabinoids and terpenoids. Data on the mechanisms of action and interactions of these additional chemicals are limited.
Although clinical trials have been undertaken with synthetic cannabinoids, there is increasing understanding and interest in the medical community of whole-plant Cannabis as a distinct entity. For example, nabiximols is a novel development in plant-based Cannabis products. Available as an oromucosal spray, a dose provides THC and CBD at 2.7 mg/100 mcL. Nabiximols is not approved by the US Food and Drug Administration (FDA) but is widely used
A third class of Cannabis comprises nonregulated synthetic cannabinoids that have no medically recognized benefit. They are solely a drug of abuse; common names include “K2” and “Spice.” These cannabinoids are outside of the scope of our discussion, but patients and providers should be aware of these cannabinoids because they are street-available. Unsuspecting patients might not know the difference between abusive and therapeutic formulations.8
Delivery and strength. Common forms of plant-based Cannabis include leaf that is smoked or vaporized, oral tincture, pill, and oil concentrate that can be vaporized. All forms come in a range of THC:CBD ratios—from as high as 90% THC content to 0% THC and all CBD-based content. Patients who are naïve to Cannabis might be concerned about formulations with a high THC concentration because of the psychoactive effects of this substance. Given the minimal CNS activity of CBD, a tolerable therapeutic starting point often is a THC:CBD ratio of 1:1, which contains a lower percentage of THC.4
Physiologic effects. THC is a partial agonist of CB1 and CB2 receptors; CBD functions as an antagonist at both receptors. The primary effects of THC result from activation of CB1 receptors, which exist in various areas of the cerebrum and cerebellum, as well as in the spinal cord.7 THC exerts its psychotropic effects at CB1 sites in the central nervous system; CBD can antagonize these THC effects at CB1 receptors. CBD also has anti-inflammatory and other effects that are mediated through peripherally distributed CB2 receptors.9
Continue to: THC has tremendously...
THC has tremendously complex capacity for activation and inhibition within various neuronal circuits, resulting in effects on mood, appetite, and movement.1,7 Adverse effects associated with Cannabis are wide-ranging: Most commonly, nausea, drowsiness, fatigue, dry mouth, and dizziness are reported alongside cognitive effects. Rarely, tachycardia, hypotension, hyperemesis, and depression can be seen.
Clinical implications and indications
Clinical indications for legal medical Cannabis vary by state; typically, indications include human immunodeficiency virus (HIV) infection and acquired immune deficiency syndrome (AIDS), cachexia, cancer, glaucoma, epilepsy and other seizure disorders, severe and chronic pain, spasticity from neurodegenerative disorders, and irritable bowel syndrome and Crohn’s disease, as well as a wide range of less-universal diagnoses. A patient may have a so-called qualifying diagnosis (ie, having the potential to allow the patient to be certified to purchase and use Cannabis) in one state but not have the same standing in a neighboring state, posing a complex legal issue. Given the significant complexities of performing medical research with plant-based Cannabis in the United States, little research has been done. The result? Policymakers are grappling with questions that only scientific research can answer:
- For which conditions does Cannabis provide medicinal benefit equal to or superior to alternatives?
- What are the appropriate dosages (or CBD:THC ratios), formulations (plant-derived or synthetic), and routes of administration (smoked, ingested, or topical) for various conditions?
Bird’s-eye view of clinical research. A meta-analysis of isolated synthetic and plant-based cannabinoids for medical use was published in 2015.10 The analysis included more than 6000 patients in 79 trials, most of which assessed whether dronabinol or nabilone (both synthetic isolates) were effective compared to placebo or alternative non-Cannabis-based therapy. The studies examined chemotherapy-induced nausea and vomiting, appetite stimulation in HIV and AIDS, chronic pain, spasticity, depression and anxiety, sleep disorders, and psychosis.
Twenty-eight studies assessed chemotherapy-induced nausea and vomiting. All of these studies indicated a greater benefit from cannabinoids than from alternative antiemetic regimens and placebo; however, that finding did not reach statistical significance across all studies.
There was moderate evidence to suggest the use of Cannabis for neuropathic and nonneuropathic cancer-related pain. However, there is an increased short-term risk of adverse events with synthetic isolates dronabinol (when used for pain) and nabilone (when used for nausea and vomiting).
Continue to: The primary conclusion...
The primary conclusion of the meta-analysis is that further study is required because little evidence exists on the effects and the adverse events of plant-based Cannabis.
HIV infection. Data on Cannabis for the treatment of refractory neuropathy and appetite stimulation in HIV infection is mixed.10,11 Smoked Cannabis for medically refractory neuropathy was examined in several trials:
- In a randomized crossover trial, researchers found statistically significant subjective improvement in neuropathic pain, with minimal intolerable adverse effects, in the 28 HIV-infected participants who completed the trial.11
- In another study,Cannabis ingested in various forms resulted in appetite stimulation in late-stage HIV infection but did not produce statistically significant weight gain.10
Pediatric epilepsy. Research on pediatric patients who have epilepsy characterized by refractory seizures has shown that the impact of Cannabis on their disease is promising. Specifically, CBD has shown tremendous potential impact: Patients experienced a statistically significant reduction in the number of seizures.9 In 2018, the FDA approved the first plant-based derivative of Cannabis: an oral cannabidiol (marketed as Epidiolex [Greenwich Biosciences, Inc.]) for the treatment of intractable seizures associated with Lennox-Gastaut syndrome and Dravet syndrome, rare and severe forms of epilepsy. Epidiolex is the first FDA-approved drug that contains a purified drug substance derived from marijuana.
CASE
Mr. S’s diagnosis of cancer is broadly included in the list of Cannabis-qualifying illnesses in all 34 states that certify patients for medical Cannabis. He qualifies both because (1) he is a cancer patient and (2) he has not found relief from chemotherapy-induced nausea and vomiting with several targeted therapies, including 5-hydroxytryptamine-receptor antagonists, steroids, and antipsychotics. Evidence supports CB1 and CB2 as potential targets for antiemetic treatment.
Given Mr. S’s consequent anorexia, his frustration with taking an increasing number of medications, and possible adverse effects of additional therapy, Cannabis is a reasonable course of action to treat nausea and vomiting. He would be able to use oral tincture or vaporization of oil to further limit his pill burden—likely, with a THC:CBD ratio of 1:1 or similar.
Continue to: Based on recent observational data...
Based on recent observational data from New York Cannabis dispensaries, cancer patients pursing Cannabis to treat chemotherapy-induced symptoms report that (1) either products with a high concentration of THC or products that contain THC and CBD in a 1:1 ratio are most effective and (2) products in 1:1 ratio of THC and CBD are most tolerable.
A legal system at oddsover the status of medical Cannabis
The core legal issue underlying medical Cannabis is a contradiction between federal and state laws.
At the federal level. The federal government regulates the lawful production, possession, and distribution of controlled substances through the Controlled Substances Act (CSA).12 The CSA is the basis for categorizing certain plants, drugs, and chemicals into 5 schedules, based on the substance’s medical use, potential for abuse, and safety or dependence liability.13 Under the CSA, marijuana (along with substances such as heroin and methamphetamine) is categorized as Schedule I14; ie, the substance
- has high potential for abuse,
- has no accepted therapeutic medical use in the United States, and
- lacks acceptable safety for use under medical supervision.
Despite waxing and waning efforts to protect states from federal prosecution, any use of a Schedule-1 substance violates federal law.15
In June 2018, a bipartisan group of federal lawmakers introduced a bill designed to amend the CSA and guarantee the rights of states and territories to self-determine marijuana regulation. The bill established a so-called STATES (Strengthening the Tenth Amendment Through Entrusting States) Act that “amends the Controlled Substances Act (21 U.S.C. § 801 et seq.) so that—as states and tribes comply with a few basic protections—its provisions no longer apply to any person acting in compliance with state or tribal laws relating to the manufacture, production, possession, distribution, dispensation, administration, or delivery of marijuana.”15
Continue to: The bill was referred to the Senate...
The bill was referred to the Senate and House Judiciary Committees but, ultimately, the STATES Act was blocked from debate in 2018.
On April 4, 2019, the Act was reintroduced in the House (H.R. 2093) and Senate (S. 1028) of the 116th Congress. Although there is bipartisan support for this bill, the timeline for moving it forward is unclear.16,17
At the state level. Thirty-four states have comprehensive public medical marijuana and Cannabis programs. The National Conference of State Legislatures18 (www.ncsl.org) designates a program “comprehensive” if it
- includes protection from criminal penalties for using marijuana for a medical purpose,
- allows access to marijuana through home cultivation, dispensaries, or other system,
- permits a variety of strains, including those more potent than what is labeled “low-THC,” and
- allows smoking or vaporization of marijuana products, plant-based material, or extract.
An additional 14 states allow for “low-THC, high-CBD” products for medical reasons, in limited situations, or as a legal defense. Regulation in these states varies widely, however: Some states allow industrialized hemp products only; others do not provide for any in-state production.18
Last, many states have some form of so-called “affirmative-defense” statutes that allow people charged with marijuana possession to mention use of marijuana for medical purposes as a possible defense.
Continue to: Physician shield
Physician shield. Despite inconsistent and evolving state and federal laws, physicians are protected, based on the Conant v Walters decision, from prosecution or revocation of their prescriptive authority for the professional “recommendation” of the use of medical marijuana.19 In 2002, the US Ninth Circuit Court of Appeals upheld the permanent injunction, based on a physician’s First Amendment right to discuss medical marijuana with patients.
CASE
Mr. S is amenable to trial of Cannabis to relieve nausea and anorexia. He asks you if he is allowed to use Cannabis at work, were he to return to an office-based desk job—even part-time—during treatment for cancer.
How would you answer Mr. S? Patients are legally protected from workplace penalties and dismissal for using and consuming Cannabis in states with a medical Cannabis law (including the state in which Mr. S resides). However, all employers have some variability in corporate policy, especially if a person works in a federally supported or regulated occupation. It’s always helpful to advise patients who will be using medical Cannabis to be proactive and speak with a human resources or employee health department staff member before beginning a course of medical Cannabis. Additionally, Cannabis with any amount of THC has the ability to alter focus, concentration, and perceptions of time. Thus, if a patient using medical Cannabis with THC asks about driving to work, he should be given the same advice one would offer about driving after consuming alcohol or ingesting opioids.
Common concerns
Ignorance of legal status. Theoretically, the Conant v Walters decision protects physicians from investigation for recommending medical Cannabis even in states where it is illegal. However, you should adhere closely to procedures set out by your state. The National Council of State Legislatures provides up-to-date information on each state’s procedures and programs,18 and the American Society of Addiction Medicine (www.asam.org) has established standards of professionalism for physicians who discuss medical Cannabis with patients (TABLE).20
Exposure to smoke. Cannabis smoke carries many of the same carcinogens found in tobacco smoke; furthermore, use of Cannabis and tobacco are highly correlated, confounding many population-based studies. The manner of inhalation of Cannabis can result in significantly higher levels of tar and carbon dioxide than with tobacco smoking. Because the effects of Cannabis last longer, however, people who smoke Cannabis may smoke it less often than tobacco smokers smoke tobacco.21
Continue to: Large cross-sectional...
Large cross-sectional and longitudinal studies have not found a link between Cannabis smoking and long-term pulmonary consequences, such as chronic obstructive pulmonary disease and lung cancer.22,23 The technology of Cannabis delivery systems has progressed far more rapidly than the clinical evidence for or against such technology.
“Vaping” is an informal term for inhalation of aerosolized Cannabis components and water vapor. Vaporizers do not heat Cannabis to the point of combustion; therefore, they provide less exposure to smoke-related toxicants while providing similar time of onset.
Neuropsychiatric adverse effects. Data regarding the relationship between Cannabis use and psychiatric disorders are incompletely understood, in conflict, and related to cannabinoid type. Consider Pennsylvania’s addition of anxiety disorder as a “serious medical condition” covered under the Pennsylvania Medical Marijuana Act.24 Although patients often report the use of medical Cannabis to treat anxiety,25 panic attacks are often associated with Cannabis use.26
While there is a clear association between Cannabis use and psychotic disorder, a causal link has yet to be unequivocally established. However, the rate of psychiatric hospitalization is increased in bipolar disorder and schizophrenia patients who use Cannabis heavily.27
We recommend, therefore, that physicians screen patients for serious mental health concerns before recommending or certifying them to use medical Cannabis.
Continue to: Overconsumption of edibles
Overconsumption of edibles. Cannabis edibles (ie, food products infused with Cannabis extract) are distinct from inhaled Cannabis in regard to onset, duration, and potential for adverse effects. Cannabis edibles might be more popular than inhaled products among older medical Cannabis users.28
Edible Cannabis has a reported onset of 1 to 3 hours (compared to 5-10 minutes with inhaled Cannabis) and a duration of effect of 6 to 8 hours (compared with 2-4 hours for inhaled products).29 These qualities might render Cannabis edibles preferable to inhaled formulations for controlling chronic symptoms and conditions. However, delayed onset of edible products and wide variation in the concentration of THC also increase the risk of overconsumption, which can lead to overdose and self-limited Cannabis-induced psychosis. We recommend providing patient education about the effects of the physiologically active therapeutic compounds tetrahydrocannabinol and cannabidiol, to prevent overconsumption of high-THC products.30
CASE
Mr. S returns to your office after a trial of Cannabis as vaporized oil and reports some relief of nausea and a mild increase in appetite, but no weight gain. He is concerned about overconsumption or overdose, and asks you what the risks of these problems are.
How should you counsel Mr. S? Explain that ingestion of Cannabis has a prolonged onset of action; vaporization has a more rapid onset of action; therefore, he could more easily self-regulate ingestion with the vehicle he has chosen. In states where edible Cannabis products are legal, education is necessary so that patients know how much of the edible to consume and how long they will wait to feel the full impact of the effects of THC.30
Cannabis use disorder in the context of medical marijuana
Cannabis use disorder (CUD) incorporates general diagnostic features of a substance use disorder, including behavioral, cognitive, and physiologic symptoms such as cravings, tolerance, and withdrawal, in the setting of persistent use despite significant substance-related problems.31 Features of Cannabis withdrawal syndrome include irritability, anger or aggression, anxiety, depressed mood, restlessness, sleep difficulty, and decreased appetite or weight loss.31 Cannabis use disorder can develop in people who use medical Cannabis; however, physiologic symptoms of tolerance and withdrawal can also develop in the setting of appropriate medical use and do not, in isolation, represent CUD.
Continue to: A recent study...
A recent study considered nationwide cross-sectional survey data from the US National Survey of Drug Use and Health to examine the relationship between medical marijuana laws and CUD.32 Study findings did not show an increase in the prevalence of CUD or marijuana use among adults in states with a legalized medical marijuana program. Importantly, when researchers looked at marijuana use among adolescents and young adults, they found no increase in measured outcomes (eg, active [ie, past-month] marijuana use, heavy [> 300 d/yr] use, and a diagnosis of CUD) after medical marijuana laws were passed.32
A paucity of pediatric data
The adolescent brain might be more vulnerable to the adverse long-term effects of Cannabis; there is potential significant harm associated with Cannabis in children and adolescence. However, accurate data concerning risk and benefit are limited.
The most recent policy statement of the American Academy of Pediatrics (AAP) reflects this paucity of data.33 The AAP opposes the use of medical Cannabis outside regulation by the FDA, although the organization allows for consideration of compassionate use of medical Cannabis for children who have life-threatening or severely disabling conditions. The AAP does support (1) additional research into pharmaceutical cannabinoids and (2) changing Cannabis from Schedule I to Schedule II to facilitate this process. Since the publication of the policy statement, Pediatrics, the official journal of the AAP, has published a review of medical cannabinoids and found (1) strong evidence for benefit in chemotherapy-induced nausea and vomiting and (2) accumulating evidence of benefit in epilepsy.34
Recognized risk: Not supporting medical Cannabis
As with all medical decisions, the risks and benefits of certifying patients for medical Cannabis must be balanced against the risks and benefits of not doing so. The risks that accompany failure to certify a patient for medical marijuana fall into 3 categories:
Blocking access to a substance that has potential therapeutic benefit. More data regarding the potential benefits and risks of medical Cannabis will, undoubtedly, dispel some of the uncertainty regarding the decision to certify a patient for medical Cannabis. When you recommend medical Cannabis and certify patients for its use, you do so with the certainty that the Cannabis safety index (ie, risk of overdose or serious adverse effects) is exceedingly low.35
Continue to: Limiting patients to other medications
Limiting patients to other medications that, potentially, carry a risk of more or greater harmful effects. An example is the decision to prescribe an opioid for chronic pain instead of certifying a patient for medical Cannabis. For certain other conditions, including chemotherapy-induced nausea and vomiting, FDA-approved pharmaceuticals might have more reported serious adverse events and interactions than medical Cannabis.36
Resigning patients to obtain Cannabis from an illegal source. This speaks to harm reduction and social justice, because obtaining Cannabis from an illegal source carries health and legal risks:
- Increased health risks result from lacing or cutting botanical or synthetic Cannabis products with potentially toxic substances. Cocaine, the rodenticide brodifacoum, methamphetamine, and phencyclidine are all known, or have been reported, to be added to botanical and synthetic Cannabis.37
- Legal repercussions of Cannabis possession are disproportionately racially based, with a significantly higher arrest rate among people of color, even in states where medical Cannabis has been legalized.38
CORRESPONDENCE
Lara Carson Weinstein, MD, MPH, DrPH, Department of Family and Community Medicine, Sidney Kimmel Medical College at Thomas Jefferson University, 1015 Walnut Street, Suite 401, Philadelphia, PA 19107; [email protected].
1. College of Family Physicians of Canada. Authorizing Dried Cannabis for Chronic Pain or Anxiety: Preliminary Guidance from the College of Family Physicians of Canada. Mississauga, Ontario: College of Family Physicians of Canada; 2014. www.cfpc.ca/uploadedFiles/Resources/_PDFs/Authorizing%20Dried%20Cannabis%20for%20Chronic%20Pain%20or%20Anxiety.pdf. Accessed July 10, 2019.
2. Hartig H, Geiger AW. About six-in-ten Americans support marijuana legalization. Pew Research Center Web site. www.pewresearch.org/fact-tank/2018/10/08/americans-support-marijuana-legalization/. Published October 8, 2018. Accessed July 10, 2019.
3. Li H-L. An archaeological and historical account of cannabis in China. Econ Bot. 1974:28:437-448.
4. Zuardi AW. History of cannabis as a medicine: a review. Braz J Psychiatry. 2006;28:153-157.
5. Marijuana strains and infused products. Leafly Web site. www.leafly.com/start-exploring. Accessed July 10, 2019.
6. Fraguas-Sánchez AI, Torres-Suárez AI. Medical use of cannabinoids. Drugs. 2018;78:1665-1703.
7. Maurya N, Velmurugan BK. Therapeutic applications of cannabinoids. Chem Biol Interact. 2018;293:77-88.
8. Kelkar AH, Smith NA, Martial A, et al. An outbreak of synthetic cannabinoid-associated coagulopathy in Illinois. N Engl J Med. 2018;379:1216-1223.
9. Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
10. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
11. Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2008;34:672-680.
12. US Department of Justice, Drug Enforcement Administration, Diversion Control Division. Title 21 United States Code (USC) Controlled Substances Act. Subchapter I—Control and Enforcement. Part A—Introductory Provisions. §801. Congressional findings and declarations: controlled substances. www.deadiversion.usdoj.gov/21cfr/21usc/801.htm. Accessed July 10, 2019.
13. Yeh BT. The Controlled Substances Act: regulatory requirements. Congressional Research Service 7-5700. https://fas.org/sgp/crs/misc/RL34635.pdf. Published December 13, 2012. Accessed July 10, 2019.
14. US Department of Justice, Drug Enforcement Administration, Diversion Control Division. Title 21 United States Code (USC) Controlled Substances Act. Subchapter I—Control and Enforcement. Part B—Authority to Control; Standards and Schedules. §812. Schedules of controlled substances. www.deadiversion.usdoj.gov/21cfr/21usc/812.htm. Accessed July 10, 2019.
15. United States Senate. The STATES Act. Senator Elizabeth Warren and Senator Cory Gardner. 2018. www.warren.senate.gov/imo/media/doc/STATES%20Act%20One%20Pager.pdf. Accessed July 10, 2019.
16. Strengthening the Tenth Amendment Through Entrusting States (STATES) Act of 2019, HR 2093. 116th Cong, 1st Session (2019). www.congress.gov/bill/116th-congress/house-bill/2093/text. Accessed July 20, 2019.
17. Strengthening the Tenth Amendment Through Entrusting States (STATES) Act of 2019, S 1028. 116th Cong, 1st Session (2019). www.congress.gov/bill/116th-congress/senate-bill/1028/all-info?r=3&s=6. Accessed August 8, 2019.
18. State medical marijuana laws. National Conference of State Legislatures Web site. www.ncsl.org/research/health/state-medical-marijuana-laws.aspx#3. Published July 2, 2019. Accessed July 10, 2019.
19. Conant v Walters. 309 F.3d 629 (9th cir. 2002).
20. American Society of Addiction Medicine. The role of the physician in “medical” marijuana. www.asam.org/docs/publicy-policy-statements/1role_of_phys_in_med_mj_9-10.pdf?sfvrsn=0. Published September 2010. Accessed July 12, 2019.
21. What are marijuana’s effects on lung health? National Institute on Drug Abuse Web site. www.drugabuse.gov/publications/research-reports/marijuana/what-are-marijuanas-effects-lung-health. Updated July 2019. Accessed July 10, 2019.
22. Tashkin DP. Effects of marijuana smoking on the lung. Ann Am Thorac Soc. 2013;10:239-247.
23. Zhang LR, Morgenstern H, Greenland S, et al. Cannabis smoking and lung cancer risk: pooled analysis in the International Lung Cancer Consortium. Int J Cancer. 2015;136:894-903.
24. Getting medical marijuana. Commonwealth of Pennsylvania Web site. www.pa.gov/guides/pennsylvania-medical-marijuana-program/. Accessed July 20, 2019.
25. Kosiba JD, Maisto SA, Ditre JW. Patient-reported use of medical cannabis for pain, anxiety, and depression symptoms: systematic review and meta-analysis. Soc Sci Med. 2019;233:181-192.
26. Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24:515-523.
27. Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319-328.
28. Barrus DG, Capogrossi KL, Cates S, et al. Tasty THC: Promises and Challenges of Cannabis Edibles. Publication No. OP-0035-1611. Research Triangle Park, NC: RTI Press; 2016. www.rti.org/sites/default/files/resources/rti-publication-file-6ff047d7-3fa4-41ad-90ed-9fb11663bc89.pdf. Accessed July 10, 2019.
29. MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med. 2018;49:12-19.
30. MacCoun RJ, Mello MM. Half-baked—the retail promotion of marijuana edibles. N Engl J Med. 2015;372:989-991.
31. Cannabis use disorder [305.20, 304.30]. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013:509-516.
32. Williams AR, Santaella-Tenorio J, Mauro CM, et al. Loose regulation of medical marijuana programs associated with higher rates of adult marijuana use but not cannabis use disorder. Addiction. 2017;112:1985-1991.
33. American Academy of Pediatrics Committee on Substance Abuse, American Academy of Pediatrics Committee on Adolescents. The impact of marijuana policies on youth: clinical, research, and legal update. Pediatrics. 2015;135:584-587.
34. Wong SS, Wilens TE. Medical cannabinoids in children and adolescents: a systematic review. Pediatrics. 2017;140. pii: e20171818.
35. Drug Enforcement Administration. Drugs of abuse: a DEA resource guide. www.dea.gov/sites/default/files/drug_of_abuse.pdf. Published 2017. Accessed July 10, 2019.
36. National Academies of Science, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press; 2017. www.nap.edu/read/24625/chapter/12017:2017-2019. Published 2017. Accessed July 10, 2019.
37. Emerging trend and alerts. National Institute on Drug Abuse Web site. www.drugabuse.gov/drugs-abuse/emerging-trends-alerts. Accessed July 10, 2019.
38. Drug Policy Alliance. From prohibition to progress: a status report on marijuana legalization. www.drugpolicy.org/sites/default/files/dpa_marijuana_legalization_report_feb14_2018_0.pdf. Published January 2018. Accessed July 10, 2019.
CASE
Barry S, a 45-year-old man with a new diagnosis of non-Hodgkin’s lymphoma, recently started induction chemotherapy. He has struggled with nausea, profound gustatory changes, and poor appetite; various antiemetics have provided only minimal relief. He tells you that he is hesitant to try “yet another pill” but has heard and read that marijuana (genus Cannabis) is used to alleviate disruptive chemotherapy-induced adverse effects. He asks if this is a treatment you’d recommend for him.
As Mr. S’s physician, how do you respond?
Understandably, some family physicians are hesitant to recommend an unregulated, federally illegal substance characterized by conflicting or absent evidence of safety and effectiveness.1 Nevertheless, throughout history and in the current court of public opinion, medical Cannabis has overwhelming support,2 leading to legalization in most of the United States.
As with many traditionally accepted therapies (whether they are or are not supported by substantial evidence), physicians are expected to provide individualized guidance regarding minimizing risk and maximizing benefit of the therapeutic use of Cannabis. The rapidly growing scientific and commercial fields of medical Cannabis guarantee that information on this topic will constantly be changing—and will often be contradictory. In this article, we review the most common concerns about medical Cannabis and provide up-to-date evidence on its use.
The pharmacology of cannabis
Cannabis sativa was among the earliest plants cultivated by man, with the first evidence of its use in China, approximately 4000 BC, to make twine and rope from its fibers.3 Records of medicinal Cannabis date back to the world’s oldest pharmacopoeia, a written summary of what was known about herbal medicine through the late 16th century.4
The 2 principal species of Cannabis are sativa and indica. There is no good medical evidence to separate the impacts of either strain; however, a staggering amount of lay information exists about the reported differing effects of each strain.5
Chemical constituents. Phytocannabinoids derived from C sativa are the plant’s best-known proteins, constituting a complex lipid-signaling network involved in numerous physiological processes. There are more than 100 known phytocannabinoids, the most well-recognized being Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Additional sources of cannabinoids include endogenous cannabinoids, or endocannabinoids, and synthetic cannabinoids.
The endocannabinoid system, comprising cannabinoid receptors, endocannabinoids, and their specific enzymes, is a potential therapeutic target for a variety of pathologic processes.6,7 The 2 most well-studied targets for cannabinoids in the human body are the cannabinoid receptors CB1 and CB2, found throughout the body: CB1, predominantly in the central and peripheral nervous system, and CB2 in a more limited distribution in the immune and hematopoietic systems. Other pathways activated or antagonized by THC and CBD exist, but are less well-mapped than CB1 and CB2.
[polldaddy:10402702]
Continue to: Botanical or synthetic?
Botanical or synthetic? It is important to distinguish between synthetic and plant-based cannabinoids, for you and your patients' benefit. Pharmaceutical (synthetic) THC is just that: THC alone. Whole-plant Cannabis, on the other hand, has hundreds of additional chemicals—most notably, phytocannabinoids and terpenoids. Data on the mechanisms of action and interactions of these additional chemicals are limited.
Although clinical trials have been undertaken with synthetic cannabinoids, there is increasing understanding and interest in the medical community of whole-plant Cannabis as a distinct entity. For example, nabiximols is a novel development in plant-based Cannabis products. Available as an oromucosal spray, a dose provides THC and CBD at 2.7 mg/100 mcL. Nabiximols is not approved by the US Food and Drug Administration (FDA) but is widely used
A third class of Cannabis comprises nonregulated synthetic cannabinoids that have no medically recognized benefit. They are solely a drug of abuse; common names include “K2” and “Spice.” These cannabinoids are outside of the scope of our discussion, but patients and providers should be aware of these cannabinoids because they are street-available. Unsuspecting patients might not know the difference between abusive and therapeutic formulations.8
Delivery and strength. Common forms of plant-based Cannabis include leaf that is smoked or vaporized, oral tincture, pill, and oil concentrate that can be vaporized. All forms come in a range of THC:CBD ratios—from as high as 90% THC content to 0% THC and all CBD-based content. Patients who are naïve to Cannabis might be concerned about formulations with a high THC concentration because of the psychoactive effects of this substance. Given the minimal CNS activity of CBD, a tolerable therapeutic starting point often is a THC:CBD ratio of 1:1, which contains a lower percentage of THC.4
Physiologic effects. THC is a partial agonist of CB1 and CB2 receptors; CBD functions as an antagonist at both receptors. The primary effects of THC result from activation of CB1 receptors, which exist in various areas of the cerebrum and cerebellum, as well as in the spinal cord.7 THC exerts its psychotropic effects at CB1 sites in the central nervous system; CBD can antagonize these THC effects at CB1 receptors. CBD also has anti-inflammatory and other effects that are mediated through peripherally distributed CB2 receptors.9
Continue to: THC has tremendously...
THC has tremendously complex capacity for activation and inhibition within various neuronal circuits, resulting in effects on mood, appetite, and movement.1,7 Adverse effects associated with Cannabis are wide-ranging: Most commonly, nausea, drowsiness, fatigue, dry mouth, and dizziness are reported alongside cognitive effects. Rarely, tachycardia, hypotension, hyperemesis, and depression can be seen.
Clinical implications and indications
Clinical indications for legal medical Cannabis vary by state; typically, indications include human immunodeficiency virus (HIV) infection and acquired immune deficiency syndrome (AIDS), cachexia, cancer, glaucoma, epilepsy and other seizure disorders, severe and chronic pain, spasticity from neurodegenerative disorders, and irritable bowel syndrome and Crohn’s disease, as well as a wide range of less-universal diagnoses. A patient may have a so-called qualifying diagnosis (ie, having the potential to allow the patient to be certified to purchase and use Cannabis) in one state but not have the same standing in a neighboring state, posing a complex legal issue. Given the significant complexities of performing medical research with plant-based Cannabis in the United States, little research has been done. The result? Policymakers are grappling with questions that only scientific research can answer:
- For which conditions does Cannabis provide medicinal benefit equal to or superior to alternatives?
- What are the appropriate dosages (or CBD:THC ratios), formulations (plant-derived or synthetic), and routes of administration (smoked, ingested, or topical) for various conditions?
Bird’s-eye view of clinical research. A meta-analysis of isolated synthetic and plant-based cannabinoids for medical use was published in 2015.10 The analysis included more than 6000 patients in 79 trials, most of which assessed whether dronabinol or nabilone (both synthetic isolates) were effective compared to placebo or alternative non-Cannabis-based therapy. The studies examined chemotherapy-induced nausea and vomiting, appetite stimulation in HIV and AIDS, chronic pain, spasticity, depression and anxiety, sleep disorders, and psychosis.
Twenty-eight studies assessed chemotherapy-induced nausea and vomiting. All of these studies indicated a greater benefit from cannabinoids than from alternative antiemetic regimens and placebo; however, that finding did not reach statistical significance across all studies.
There was moderate evidence to suggest the use of Cannabis for neuropathic and nonneuropathic cancer-related pain. However, there is an increased short-term risk of adverse events with synthetic isolates dronabinol (when used for pain) and nabilone (when used for nausea and vomiting).
Continue to: The primary conclusion...
The primary conclusion of the meta-analysis is that further study is required because little evidence exists on the effects and the adverse events of plant-based Cannabis.
HIV infection. Data on Cannabis for the treatment of refractory neuropathy and appetite stimulation in HIV infection is mixed.10,11 Smoked Cannabis for medically refractory neuropathy was examined in several trials:
- In a randomized crossover trial, researchers found statistically significant subjective improvement in neuropathic pain, with minimal intolerable adverse effects, in the 28 HIV-infected participants who completed the trial.11
- In another study,Cannabis ingested in various forms resulted in appetite stimulation in late-stage HIV infection but did not produce statistically significant weight gain.10
Pediatric epilepsy. Research on pediatric patients who have epilepsy characterized by refractory seizures has shown that the impact of Cannabis on their disease is promising. Specifically, CBD has shown tremendous potential impact: Patients experienced a statistically significant reduction in the number of seizures.9 In 2018, the FDA approved the first plant-based derivative of Cannabis: an oral cannabidiol (marketed as Epidiolex [Greenwich Biosciences, Inc.]) for the treatment of intractable seizures associated with Lennox-Gastaut syndrome and Dravet syndrome, rare and severe forms of epilepsy. Epidiolex is the first FDA-approved drug that contains a purified drug substance derived from marijuana.
CASE
Mr. S’s diagnosis of cancer is broadly included in the list of Cannabis-qualifying illnesses in all 34 states that certify patients for medical Cannabis. He qualifies both because (1) he is a cancer patient and (2) he has not found relief from chemotherapy-induced nausea and vomiting with several targeted therapies, including 5-hydroxytryptamine-receptor antagonists, steroids, and antipsychotics. Evidence supports CB1 and CB2 as potential targets for antiemetic treatment.
Given Mr. S’s consequent anorexia, his frustration with taking an increasing number of medications, and possible adverse effects of additional therapy, Cannabis is a reasonable course of action to treat nausea and vomiting. He would be able to use oral tincture or vaporization of oil to further limit his pill burden—likely, with a THC:CBD ratio of 1:1 or similar.
Continue to: Based on recent observational data...
Based on recent observational data from New York Cannabis dispensaries, cancer patients pursing Cannabis to treat chemotherapy-induced symptoms report that (1) either products with a high concentration of THC or products that contain THC and CBD in a 1:1 ratio are most effective and (2) products in 1:1 ratio of THC and CBD are most tolerable.
A legal system at oddsover the status of medical Cannabis
The core legal issue underlying medical Cannabis is a contradiction between federal and state laws.
At the federal level. The federal government regulates the lawful production, possession, and distribution of controlled substances through the Controlled Substances Act (CSA).12 The CSA is the basis for categorizing certain plants, drugs, and chemicals into 5 schedules, based on the substance’s medical use, potential for abuse, and safety or dependence liability.13 Under the CSA, marijuana (along with substances such as heroin and methamphetamine) is categorized as Schedule I14; ie, the substance
- has high potential for abuse,
- has no accepted therapeutic medical use in the United States, and
- lacks acceptable safety for use under medical supervision.
Despite waxing and waning efforts to protect states from federal prosecution, any use of a Schedule-1 substance violates federal law.15
In June 2018, a bipartisan group of federal lawmakers introduced a bill designed to amend the CSA and guarantee the rights of states and territories to self-determine marijuana regulation. The bill established a so-called STATES (Strengthening the Tenth Amendment Through Entrusting States) Act that “amends the Controlled Substances Act (21 U.S.C. § 801 et seq.) so that—as states and tribes comply with a few basic protections—its provisions no longer apply to any person acting in compliance with state or tribal laws relating to the manufacture, production, possession, distribution, dispensation, administration, or delivery of marijuana.”15
Continue to: The bill was referred to the Senate...
The bill was referred to the Senate and House Judiciary Committees but, ultimately, the STATES Act was blocked from debate in 2018.
On April 4, 2019, the Act was reintroduced in the House (H.R. 2093) and Senate (S. 1028) of the 116th Congress. Although there is bipartisan support for this bill, the timeline for moving it forward is unclear.16,17
At the state level. Thirty-four states have comprehensive public medical marijuana and Cannabis programs. The National Conference of State Legislatures18 (www.ncsl.org) designates a program “comprehensive” if it
- includes protection from criminal penalties for using marijuana for a medical purpose,
- allows access to marijuana through home cultivation, dispensaries, or other system,
- permits a variety of strains, including those more potent than what is labeled “low-THC,” and
- allows smoking or vaporization of marijuana products, plant-based material, or extract.
An additional 14 states allow for “low-THC, high-CBD” products for medical reasons, in limited situations, or as a legal defense. Regulation in these states varies widely, however: Some states allow industrialized hemp products only; others do not provide for any in-state production.18
Last, many states have some form of so-called “affirmative-defense” statutes that allow people charged with marijuana possession to mention use of marijuana for medical purposes as a possible defense.
Continue to: Physician shield
Physician shield. Despite inconsistent and evolving state and federal laws, physicians are protected, based on the Conant v Walters decision, from prosecution or revocation of their prescriptive authority for the professional “recommendation” of the use of medical marijuana.19 In 2002, the US Ninth Circuit Court of Appeals upheld the permanent injunction, based on a physician’s First Amendment right to discuss medical marijuana with patients.
CASE
Mr. S is amenable to trial of Cannabis to relieve nausea and anorexia. He asks you if he is allowed to use Cannabis at work, were he to return to an office-based desk job—even part-time—during treatment for cancer.
How would you answer Mr. S? Patients are legally protected from workplace penalties and dismissal for using and consuming Cannabis in states with a medical Cannabis law (including the state in which Mr. S resides). However, all employers have some variability in corporate policy, especially if a person works in a federally supported or regulated occupation. It’s always helpful to advise patients who will be using medical Cannabis to be proactive and speak with a human resources or employee health department staff member before beginning a course of medical Cannabis. Additionally, Cannabis with any amount of THC has the ability to alter focus, concentration, and perceptions of time. Thus, if a patient using medical Cannabis with THC asks about driving to work, he should be given the same advice one would offer about driving after consuming alcohol or ingesting opioids.
Common concerns
Ignorance of legal status. Theoretically, the Conant v Walters decision protects physicians from investigation for recommending medical Cannabis even in states where it is illegal. However, you should adhere closely to procedures set out by your state. The National Council of State Legislatures provides up-to-date information on each state’s procedures and programs,18 and the American Society of Addiction Medicine (www.asam.org) has established standards of professionalism for physicians who discuss medical Cannabis with patients (TABLE).20
Exposure to smoke. Cannabis smoke carries many of the same carcinogens found in tobacco smoke; furthermore, use of Cannabis and tobacco are highly correlated, confounding many population-based studies. The manner of inhalation of Cannabis can result in significantly higher levels of tar and carbon dioxide than with tobacco smoking. Because the effects of Cannabis last longer, however, people who smoke Cannabis may smoke it less often than tobacco smokers smoke tobacco.21
Continue to: Large cross-sectional...
Large cross-sectional and longitudinal studies have not found a link between Cannabis smoking and long-term pulmonary consequences, such as chronic obstructive pulmonary disease and lung cancer.22,23 The technology of Cannabis delivery systems has progressed far more rapidly than the clinical evidence for or against such technology.
“Vaping” is an informal term for inhalation of aerosolized Cannabis components and water vapor. Vaporizers do not heat Cannabis to the point of combustion; therefore, they provide less exposure to smoke-related toxicants while providing similar time of onset.
Neuropsychiatric adverse effects. Data regarding the relationship between Cannabis use and psychiatric disorders are incompletely understood, in conflict, and related to cannabinoid type. Consider Pennsylvania’s addition of anxiety disorder as a “serious medical condition” covered under the Pennsylvania Medical Marijuana Act.24 Although patients often report the use of medical Cannabis to treat anxiety,25 panic attacks are often associated with Cannabis use.26
While there is a clear association between Cannabis use and psychotic disorder, a causal link has yet to be unequivocally established. However, the rate of psychiatric hospitalization is increased in bipolar disorder and schizophrenia patients who use Cannabis heavily.27
We recommend, therefore, that physicians screen patients for serious mental health concerns before recommending or certifying them to use medical Cannabis.
Continue to: Overconsumption of edibles
Overconsumption of edibles. Cannabis edibles (ie, food products infused with Cannabis extract) are distinct from inhaled Cannabis in regard to onset, duration, and potential for adverse effects. Cannabis edibles might be more popular than inhaled products among older medical Cannabis users.28
Edible Cannabis has a reported onset of 1 to 3 hours (compared to 5-10 minutes with inhaled Cannabis) and a duration of effect of 6 to 8 hours (compared with 2-4 hours for inhaled products).29 These qualities might render Cannabis edibles preferable to inhaled formulations for controlling chronic symptoms and conditions. However, delayed onset of edible products and wide variation in the concentration of THC also increase the risk of overconsumption, which can lead to overdose and self-limited Cannabis-induced psychosis. We recommend providing patient education about the effects of the physiologically active therapeutic compounds tetrahydrocannabinol and cannabidiol, to prevent overconsumption of high-THC products.30
CASE
Mr. S returns to your office after a trial of Cannabis as vaporized oil and reports some relief of nausea and a mild increase in appetite, but no weight gain. He is concerned about overconsumption or overdose, and asks you what the risks of these problems are.
How should you counsel Mr. S? Explain that ingestion of Cannabis has a prolonged onset of action; vaporization has a more rapid onset of action; therefore, he could more easily self-regulate ingestion with the vehicle he has chosen. In states where edible Cannabis products are legal, education is necessary so that patients know how much of the edible to consume and how long they will wait to feel the full impact of the effects of THC.30
Cannabis use disorder in the context of medical marijuana
Cannabis use disorder (CUD) incorporates general diagnostic features of a substance use disorder, including behavioral, cognitive, and physiologic symptoms such as cravings, tolerance, and withdrawal, in the setting of persistent use despite significant substance-related problems.31 Features of Cannabis withdrawal syndrome include irritability, anger or aggression, anxiety, depressed mood, restlessness, sleep difficulty, and decreased appetite or weight loss.31 Cannabis use disorder can develop in people who use medical Cannabis; however, physiologic symptoms of tolerance and withdrawal can also develop in the setting of appropriate medical use and do not, in isolation, represent CUD.
Continue to: A recent study...
A recent study considered nationwide cross-sectional survey data from the US National Survey of Drug Use and Health to examine the relationship between medical marijuana laws and CUD.32 Study findings did not show an increase in the prevalence of CUD or marijuana use among adults in states with a legalized medical marijuana program. Importantly, when researchers looked at marijuana use among adolescents and young adults, they found no increase in measured outcomes (eg, active [ie, past-month] marijuana use, heavy [> 300 d/yr] use, and a diagnosis of CUD) after medical marijuana laws were passed.32
A paucity of pediatric data
The adolescent brain might be more vulnerable to the adverse long-term effects of Cannabis; there is potential significant harm associated with Cannabis in children and adolescence. However, accurate data concerning risk and benefit are limited.
The most recent policy statement of the American Academy of Pediatrics (AAP) reflects this paucity of data.33 The AAP opposes the use of medical Cannabis outside regulation by the FDA, although the organization allows for consideration of compassionate use of medical Cannabis for children who have life-threatening or severely disabling conditions. The AAP does support (1) additional research into pharmaceutical cannabinoids and (2) changing Cannabis from Schedule I to Schedule II to facilitate this process. Since the publication of the policy statement, Pediatrics, the official journal of the AAP, has published a review of medical cannabinoids and found (1) strong evidence for benefit in chemotherapy-induced nausea and vomiting and (2) accumulating evidence of benefit in epilepsy.34
Recognized risk: Not supporting medical Cannabis
As with all medical decisions, the risks and benefits of certifying patients for medical Cannabis must be balanced against the risks and benefits of not doing so. The risks that accompany failure to certify a patient for medical marijuana fall into 3 categories:
Blocking access to a substance that has potential therapeutic benefit. More data regarding the potential benefits and risks of medical Cannabis will, undoubtedly, dispel some of the uncertainty regarding the decision to certify a patient for medical Cannabis. When you recommend medical Cannabis and certify patients for its use, you do so with the certainty that the Cannabis safety index (ie, risk of overdose or serious adverse effects) is exceedingly low.35
Continue to: Limiting patients to other medications
Limiting patients to other medications that, potentially, carry a risk of more or greater harmful effects. An example is the decision to prescribe an opioid for chronic pain instead of certifying a patient for medical Cannabis. For certain other conditions, including chemotherapy-induced nausea and vomiting, FDA-approved pharmaceuticals might have more reported serious adverse events and interactions than medical Cannabis.36
Resigning patients to obtain Cannabis from an illegal source. This speaks to harm reduction and social justice, because obtaining Cannabis from an illegal source carries health and legal risks:
- Increased health risks result from lacing or cutting botanical or synthetic Cannabis products with potentially toxic substances. Cocaine, the rodenticide brodifacoum, methamphetamine, and phencyclidine are all known, or have been reported, to be added to botanical and synthetic Cannabis.37
- Legal repercussions of Cannabis possession are disproportionately racially based, with a significantly higher arrest rate among people of color, even in states where medical Cannabis has been legalized.38
CORRESPONDENCE
Lara Carson Weinstein, MD, MPH, DrPH, Department of Family and Community Medicine, Sidney Kimmel Medical College at Thomas Jefferson University, 1015 Walnut Street, Suite 401, Philadelphia, PA 19107; [email protected].
CASE
Barry S, a 45-year-old man with a new diagnosis of non-Hodgkin’s lymphoma, recently started induction chemotherapy. He has struggled with nausea, profound gustatory changes, and poor appetite; various antiemetics have provided only minimal relief. He tells you that he is hesitant to try “yet another pill” but has heard and read that marijuana (genus Cannabis) is used to alleviate disruptive chemotherapy-induced adverse effects. He asks if this is a treatment you’d recommend for him.
As Mr. S’s physician, how do you respond?
Understandably, some family physicians are hesitant to recommend an unregulated, federally illegal substance characterized by conflicting or absent evidence of safety and effectiveness.1 Nevertheless, throughout history and in the current court of public opinion, medical Cannabis has overwhelming support,2 leading to legalization in most of the United States.
As with many traditionally accepted therapies (whether they are or are not supported by substantial evidence), physicians are expected to provide individualized guidance regarding minimizing risk and maximizing benefit of the therapeutic use of Cannabis. The rapidly growing scientific and commercial fields of medical Cannabis guarantee that information on this topic will constantly be changing—and will often be contradictory. In this article, we review the most common concerns about medical Cannabis and provide up-to-date evidence on its use.
The pharmacology of cannabis
Cannabis sativa was among the earliest plants cultivated by man, with the first evidence of its use in China, approximately 4000 BC, to make twine and rope from its fibers.3 Records of medicinal Cannabis date back to the world’s oldest pharmacopoeia, a written summary of what was known about herbal medicine through the late 16th century.4
The 2 principal species of Cannabis are sativa and indica. There is no good medical evidence to separate the impacts of either strain; however, a staggering amount of lay information exists about the reported differing effects of each strain.5
Chemical constituents. Phytocannabinoids derived from C sativa are the plant’s best-known proteins, constituting a complex lipid-signaling network involved in numerous physiological processes. There are more than 100 known phytocannabinoids, the most well-recognized being Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Additional sources of cannabinoids include endogenous cannabinoids, or endocannabinoids, and synthetic cannabinoids.
The endocannabinoid system, comprising cannabinoid receptors, endocannabinoids, and their specific enzymes, is a potential therapeutic target for a variety of pathologic processes.6,7 The 2 most well-studied targets for cannabinoids in the human body are the cannabinoid receptors CB1 and CB2, found throughout the body: CB1, predominantly in the central and peripheral nervous system, and CB2 in a more limited distribution in the immune and hematopoietic systems. Other pathways activated or antagonized by THC and CBD exist, but are less well-mapped than CB1 and CB2.
[polldaddy:10402702]
Continue to: Botanical or synthetic?
Botanical or synthetic? It is important to distinguish between synthetic and plant-based cannabinoids, for you and your patients' benefit. Pharmaceutical (synthetic) THC is just that: THC alone. Whole-plant Cannabis, on the other hand, has hundreds of additional chemicals—most notably, phytocannabinoids and terpenoids. Data on the mechanisms of action and interactions of these additional chemicals are limited.
Although clinical trials have been undertaken with synthetic cannabinoids, there is increasing understanding and interest in the medical community of whole-plant Cannabis as a distinct entity. For example, nabiximols is a novel development in plant-based Cannabis products. Available as an oromucosal spray, a dose provides THC and CBD at 2.7 mg/100 mcL. Nabiximols is not approved by the US Food and Drug Administration (FDA) but is widely used
A third class of Cannabis comprises nonregulated synthetic cannabinoids that have no medically recognized benefit. They are solely a drug of abuse; common names include “K2” and “Spice.” These cannabinoids are outside of the scope of our discussion, but patients and providers should be aware of these cannabinoids because they are street-available. Unsuspecting patients might not know the difference between abusive and therapeutic formulations.8
Delivery and strength. Common forms of plant-based Cannabis include leaf that is smoked or vaporized, oral tincture, pill, and oil concentrate that can be vaporized. All forms come in a range of THC:CBD ratios—from as high as 90% THC content to 0% THC and all CBD-based content. Patients who are naïve to Cannabis might be concerned about formulations with a high THC concentration because of the psychoactive effects of this substance. Given the minimal CNS activity of CBD, a tolerable therapeutic starting point often is a THC:CBD ratio of 1:1, which contains a lower percentage of THC.4
Physiologic effects. THC is a partial agonist of CB1 and CB2 receptors; CBD functions as an antagonist at both receptors. The primary effects of THC result from activation of CB1 receptors, which exist in various areas of the cerebrum and cerebellum, as well as in the spinal cord.7 THC exerts its psychotropic effects at CB1 sites in the central nervous system; CBD can antagonize these THC effects at CB1 receptors. CBD also has anti-inflammatory and other effects that are mediated through peripherally distributed CB2 receptors.9
Continue to: THC has tremendously...
THC has tremendously complex capacity for activation and inhibition within various neuronal circuits, resulting in effects on mood, appetite, and movement.1,7 Adverse effects associated with Cannabis are wide-ranging: Most commonly, nausea, drowsiness, fatigue, dry mouth, and dizziness are reported alongside cognitive effects. Rarely, tachycardia, hypotension, hyperemesis, and depression can be seen.
Clinical implications and indications
Clinical indications for legal medical Cannabis vary by state; typically, indications include human immunodeficiency virus (HIV) infection and acquired immune deficiency syndrome (AIDS), cachexia, cancer, glaucoma, epilepsy and other seizure disorders, severe and chronic pain, spasticity from neurodegenerative disorders, and irritable bowel syndrome and Crohn’s disease, as well as a wide range of less-universal diagnoses. A patient may have a so-called qualifying diagnosis (ie, having the potential to allow the patient to be certified to purchase and use Cannabis) in one state but not have the same standing in a neighboring state, posing a complex legal issue. Given the significant complexities of performing medical research with plant-based Cannabis in the United States, little research has been done. The result? Policymakers are grappling with questions that only scientific research can answer:
- For which conditions does Cannabis provide medicinal benefit equal to or superior to alternatives?
- What are the appropriate dosages (or CBD:THC ratios), formulations (plant-derived or synthetic), and routes of administration (smoked, ingested, or topical) for various conditions?
Bird’s-eye view of clinical research. A meta-analysis of isolated synthetic and plant-based cannabinoids for medical use was published in 2015.10 The analysis included more than 6000 patients in 79 trials, most of which assessed whether dronabinol or nabilone (both synthetic isolates) were effective compared to placebo or alternative non-Cannabis-based therapy. The studies examined chemotherapy-induced nausea and vomiting, appetite stimulation in HIV and AIDS, chronic pain, spasticity, depression and anxiety, sleep disorders, and psychosis.
Twenty-eight studies assessed chemotherapy-induced nausea and vomiting. All of these studies indicated a greater benefit from cannabinoids than from alternative antiemetic regimens and placebo; however, that finding did not reach statistical significance across all studies.
There was moderate evidence to suggest the use of Cannabis for neuropathic and nonneuropathic cancer-related pain. However, there is an increased short-term risk of adverse events with synthetic isolates dronabinol (when used for pain) and nabilone (when used for nausea and vomiting).
Continue to: The primary conclusion...
The primary conclusion of the meta-analysis is that further study is required because little evidence exists on the effects and the adverse events of plant-based Cannabis.
HIV infection. Data on Cannabis for the treatment of refractory neuropathy and appetite stimulation in HIV infection is mixed.10,11 Smoked Cannabis for medically refractory neuropathy was examined in several trials:
- In a randomized crossover trial, researchers found statistically significant subjective improvement in neuropathic pain, with minimal intolerable adverse effects, in the 28 HIV-infected participants who completed the trial.11
- In another study,Cannabis ingested in various forms resulted in appetite stimulation in late-stage HIV infection but did not produce statistically significant weight gain.10
Pediatric epilepsy. Research on pediatric patients who have epilepsy characterized by refractory seizures has shown that the impact of Cannabis on their disease is promising. Specifically, CBD has shown tremendous potential impact: Patients experienced a statistically significant reduction in the number of seizures.9 In 2018, the FDA approved the first plant-based derivative of Cannabis: an oral cannabidiol (marketed as Epidiolex [Greenwich Biosciences, Inc.]) for the treatment of intractable seizures associated with Lennox-Gastaut syndrome and Dravet syndrome, rare and severe forms of epilepsy. Epidiolex is the first FDA-approved drug that contains a purified drug substance derived from marijuana.
CASE
Mr. S’s diagnosis of cancer is broadly included in the list of Cannabis-qualifying illnesses in all 34 states that certify patients for medical Cannabis. He qualifies both because (1) he is a cancer patient and (2) he has not found relief from chemotherapy-induced nausea and vomiting with several targeted therapies, including 5-hydroxytryptamine-receptor antagonists, steroids, and antipsychotics. Evidence supports CB1 and CB2 as potential targets for antiemetic treatment.
Given Mr. S’s consequent anorexia, his frustration with taking an increasing number of medications, and possible adverse effects of additional therapy, Cannabis is a reasonable course of action to treat nausea and vomiting. He would be able to use oral tincture or vaporization of oil to further limit his pill burden—likely, with a THC:CBD ratio of 1:1 or similar.
Continue to: Based on recent observational data...
Based on recent observational data from New York Cannabis dispensaries, cancer patients pursing Cannabis to treat chemotherapy-induced symptoms report that (1) either products with a high concentration of THC or products that contain THC and CBD in a 1:1 ratio are most effective and (2) products in 1:1 ratio of THC and CBD are most tolerable.
A legal system at oddsover the status of medical Cannabis
The core legal issue underlying medical Cannabis is a contradiction between federal and state laws.
At the federal level. The federal government regulates the lawful production, possession, and distribution of controlled substances through the Controlled Substances Act (CSA).12 The CSA is the basis for categorizing certain plants, drugs, and chemicals into 5 schedules, based on the substance’s medical use, potential for abuse, and safety or dependence liability.13 Under the CSA, marijuana (along with substances such as heroin and methamphetamine) is categorized as Schedule I14; ie, the substance
- has high potential for abuse,
- has no accepted therapeutic medical use in the United States, and
- lacks acceptable safety for use under medical supervision.
Despite waxing and waning efforts to protect states from federal prosecution, any use of a Schedule-1 substance violates federal law.15
In June 2018, a bipartisan group of federal lawmakers introduced a bill designed to amend the CSA and guarantee the rights of states and territories to self-determine marijuana regulation. The bill established a so-called STATES (Strengthening the Tenth Amendment Through Entrusting States) Act that “amends the Controlled Substances Act (21 U.S.C. § 801 et seq.) so that—as states and tribes comply with a few basic protections—its provisions no longer apply to any person acting in compliance with state or tribal laws relating to the manufacture, production, possession, distribution, dispensation, administration, or delivery of marijuana.”15
Continue to: The bill was referred to the Senate...
The bill was referred to the Senate and House Judiciary Committees but, ultimately, the STATES Act was blocked from debate in 2018.
On April 4, 2019, the Act was reintroduced in the House (H.R. 2093) and Senate (S. 1028) of the 116th Congress. Although there is bipartisan support for this bill, the timeline for moving it forward is unclear.16,17
At the state level. Thirty-four states have comprehensive public medical marijuana and Cannabis programs. The National Conference of State Legislatures18 (www.ncsl.org) designates a program “comprehensive” if it
- includes protection from criminal penalties for using marijuana for a medical purpose,
- allows access to marijuana through home cultivation, dispensaries, or other system,
- permits a variety of strains, including those more potent than what is labeled “low-THC,” and
- allows smoking or vaporization of marijuana products, plant-based material, or extract.
An additional 14 states allow for “low-THC, high-CBD” products for medical reasons, in limited situations, or as a legal defense. Regulation in these states varies widely, however: Some states allow industrialized hemp products only; others do not provide for any in-state production.18
Last, many states have some form of so-called “affirmative-defense” statutes that allow people charged with marijuana possession to mention use of marijuana for medical purposes as a possible defense.
Continue to: Physician shield
Physician shield. Despite inconsistent and evolving state and federal laws, physicians are protected, based on the Conant v Walters decision, from prosecution or revocation of their prescriptive authority for the professional “recommendation” of the use of medical marijuana.19 In 2002, the US Ninth Circuit Court of Appeals upheld the permanent injunction, based on a physician’s First Amendment right to discuss medical marijuana with patients.
CASE
Mr. S is amenable to trial of Cannabis to relieve nausea and anorexia. He asks you if he is allowed to use Cannabis at work, were he to return to an office-based desk job—even part-time—during treatment for cancer.
How would you answer Mr. S? Patients are legally protected from workplace penalties and dismissal for using and consuming Cannabis in states with a medical Cannabis law (including the state in which Mr. S resides). However, all employers have some variability in corporate policy, especially if a person works in a federally supported or regulated occupation. It’s always helpful to advise patients who will be using medical Cannabis to be proactive and speak with a human resources or employee health department staff member before beginning a course of medical Cannabis. Additionally, Cannabis with any amount of THC has the ability to alter focus, concentration, and perceptions of time. Thus, if a patient using medical Cannabis with THC asks about driving to work, he should be given the same advice one would offer about driving after consuming alcohol or ingesting opioids.
Common concerns
Ignorance of legal status. Theoretically, the Conant v Walters decision protects physicians from investigation for recommending medical Cannabis even in states where it is illegal. However, you should adhere closely to procedures set out by your state. The National Council of State Legislatures provides up-to-date information on each state’s procedures and programs,18 and the American Society of Addiction Medicine (www.asam.org) has established standards of professionalism for physicians who discuss medical Cannabis with patients (TABLE).20
Exposure to smoke. Cannabis smoke carries many of the same carcinogens found in tobacco smoke; furthermore, use of Cannabis and tobacco are highly correlated, confounding many population-based studies. The manner of inhalation of Cannabis can result in significantly higher levels of tar and carbon dioxide than with tobacco smoking. Because the effects of Cannabis last longer, however, people who smoke Cannabis may smoke it less often than tobacco smokers smoke tobacco.21
Continue to: Large cross-sectional...
Large cross-sectional and longitudinal studies have not found a link between Cannabis smoking and long-term pulmonary consequences, such as chronic obstructive pulmonary disease and lung cancer.22,23 The technology of Cannabis delivery systems has progressed far more rapidly than the clinical evidence for or against such technology.
“Vaping” is an informal term for inhalation of aerosolized Cannabis components and water vapor. Vaporizers do not heat Cannabis to the point of combustion; therefore, they provide less exposure to smoke-related toxicants while providing similar time of onset.
Neuropsychiatric adverse effects. Data regarding the relationship between Cannabis use and psychiatric disorders are incompletely understood, in conflict, and related to cannabinoid type. Consider Pennsylvania’s addition of anxiety disorder as a “serious medical condition” covered under the Pennsylvania Medical Marijuana Act.24 Although patients often report the use of medical Cannabis to treat anxiety,25 panic attacks are often associated with Cannabis use.26
While there is a clear association between Cannabis use and psychotic disorder, a causal link has yet to be unequivocally established. However, the rate of psychiatric hospitalization is increased in bipolar disorder and schizophrenia patients who use Cannabis heavily.27
We recommend, therefore, that physicians screen patients for serious mental health concerns before recommending or certifying them to use medical Cannabis.
Continue to: Overconsumption of edibles
Overconsumption of edibles. Cannabis edibles (ie, food products infused with Cannabis extract) are distinct from inhaled Cannabis in regard to onset, duration, and potential for adverse effects. Cannabis edibles might be more popular than inhaled products among older medical Cannabis users.28
Edible Cannabis has a reported onset of 1 to 3 hours (compared to 5-10 minutes with inhaled Cannabis) and a duration of effect of 6 to 8 hours (compared with 2-4 hours for inhaled products).29 These qualities might render Cannabis edibles preferable to inhaled formulations for controlling chronic symptoms and conditions. However, delayed onset of edible products and wide variation in the concentration of THC also increase the risk of overconsumption, which can lead to overdose and self-limited Cannabis-induced psychosis. We recommend providing patient education about the effects of the physiologically active therapeutic compounds tetrahydrocannabinol and cannabidiol, to prevent overconsumption of high-THC products.30
CASE
Mr. S returns to your office after a trial of Cannabis as vaporized oil and reports some relief of nausea and a mild increase in appetite, but no weight gain. He is concerned about overconsumption or overdose, and asks you what the risks of these problems are.
How should you counsel Mr. S? Explain that ingestion of Cannabis has a prolonged onset of action; vaporization has a more rapid onset of action; therefore, he could more easily self-regulate ingestion with the vehicle he has chosen. In states where edible Cannabis products are legal, education is necessary so that patients know how much of the edible to consume and how long they will wait to feel the full impact of the effects of THC.30
Cannabis use disorder in the context of medical marijuana
Cannabis use disorder (CUD) incorporates general diagnostic features of a substance use disorder, including behavioral, cognitive, and physiologic symptoms such as cravings, tolerance, and withdrawal, in the setting of persistent use despite significant substance-related problems.31 Features of Cannabis withdrawal syndrome include irritability, anger or aggression, anxiety, depressed mood, restlessness, sleep difficulty, and decreased appetite or weight loss.31 Cannabis use disorder can develop in people who use medical Cannabis; however, physiologic symptoms of tolerance and withdrawal can also develop in the setting of appropriate medical use and do not, in isolation, represent CUD.
Continue to: A recent study...
A recent study considered nationwide cross-sectional survey data from the US National Survey of Drug Use and Health to examine the relationship between medical marijuana laws and CUD.32 Study findings did not show an increase in the prevalence of CUD or marijuana use among adults in states with a legalized medical marijuana program. Importantly, when researchers looked at marijuana use among adolescents and young adults, they found no increase in measured outcomes (eg, active [ie, past-month] marijuana use, heavy [> 300 d/yr] use, and a diagnosis of CUD) after medical marijuana laws were passed.32
A paucity of pediatric data
The adolescent brain might be more vulnerable to the adverse long-term effects of Cannabis; there is potential significant harm associated with Cannabis in children and adolescence. However, accurate data concerning risk and benefit are limited.
The most recent policy statement of the American Academy of Pediatrics (AAP) reflects this paucity of data.33 The AAP opposes the use of medical Cannabis outside regulation by the FDA, although the organization allows for consideration of compassionate use of medical Cannabis for children who have life-threatening or severely disabling conditions. The AAP does support (1) additional research into pharmaceutical cannabinoids and (2) changing Cannabis from Schedule I to Schedule II to facilitate this process. Since the publication of the policy statement, Pediatrics, the official journal of the AAP, has published a review of medical cannabinoids and found (1) strong evidence for benefit in chemotherapy-induced nausea and vomiting and (2) accumulating evidence of benefit in epilepsy.34
Recognized risk: Not supporting medical Cannabis
As with all medical decisions, the risks and benefits of certifying patients for medical Cannabis must be balanced against the risks and benefits of not doing so. The risks that accompany failure to certify a patient for medical marijuana fall into 3 categories:
Blocking access to a substance that has potential therapeutic benefit. More data regarding the potential benefits and risks of medical Cannabis will, undoubtedly, dispel some of the uncertainty regarding the decision to certify a patient for medical Cannabis. When you recommend medical Cannabis and certify patients for its use, you do so with the certainty that the Cannabis safety index (ie, risk of overdose or serious adverse effects) is exceedingly low.35
Continue to: Limiting patients to other medications
Limiting patients to other medications that, potentially, carry a risk of more or greater harmful effects. An example is the decision to prescribe an opioid for chronic pain instead of certifying a patient for medical Cannabis. For certain other conditions, including chemotherapy-induced nausea and vomiting, FDA-approved pharmaceuticals might have more reported serious adverse events and interactions than medical Cannabis.36
Resigning patients to obtain Cannabis from an illegal source. This speaks to harm reduction and social justice, because obtaining Cannabis from an illegal source carries health and legal risks:
- Increased health risks result from lacing or cutting botanical or synthetic Cannabis products with potentially toxic substances. Cocaine, the rodenticide brodifacoum, methamphetamine, and phencyclidine are all known, or have been reported, to be added to botanical and synthetic Cannabis.37
- Legal repercussions of Cannabis possession are disproportionately racially based, with a significantly higher arrest rate among people of color, even in states where medical Cannabis has been legalized.38
CORRESPONDENCE
Lara Carson Weinstein, MD, MPH, DrPH, Department of Family and Community Medicine, Sidney Kimmel Medical College at Thomas Jefferson University, 1015 Walnut Street, Suite 401, Philadelphia, PA 19107; [email protected].
1. College of Family Physicians of Canada. Authorizing Dried Cannabis for Chronic Pain or Anxiety: Preliminary Guidance from the College of Family Physicians of Canada. Mississauga, Ontario: College of Family Physicians of Canada; 2014. www.cfpc.ca/uploadedFiles/Resources/_PDFs/Authorizing%20Dried%20Cannabis%20for%20Chronic%20Pain%20or%20Anxiety.pdf. Accessed July 10, 2019.
2. Hartig H, Geiger AW. About six-in-ten Americans support marijuana legalization. Pew Research Center Web site. www.pewresearch.org/fact-tank/2018/10/08/americans-support-marijuana-legalization/. Published October 8, 2018. Accessed July 10, 2019.
3. Li H-L. An archaeological and historical account of cannabis in China. Econ Bot. 1974:28:437-448.
4. Zuardi AW. History of cannabis as a medicine: a review. Braz J Psychiatry. 2006;28:153-157.
5. Marijuana strains and infused products. Leafly Web site. www.leafly.com/start-exploring. Accessed July 10, 2019.
6. Fraguas-Sánchez AI, Torres-Suárez AI. Medical use of cannabinoids. Drugs. 2018;78:1665-1703.
7. Maurya N, Velmurugan BK. Therapeutic applications of cannabinoids. Chem Biol Interact. 2018;293:77-88.
8. Kelkar AH, Smith NA, Martial A, et al. An outbreak of synthetic cannabinoid-associated coagulopathy in Illinois. N Engl J Med. 2018;379:1216-1223.
9. Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
10. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
11. Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2008;34:672-680.
12. US Department of Justice, Drug Enforcement Administration, Diversion Control Division. Title 21 United States Code (USC) Controlled Substances Act. Subchapter I—Control and Enforcement. Part A—Introductory Provisions. §801. Congressional findings and declarations: controlled substances. www.deadiversion.usdoj.gov/21cfr/21usc/801.htm. Accessed July 10, 2019.
13. Yeh BT. The Controlled Substances Act: regulatory requirements. Congressional Research Service 7-5700. https://fas.org/sgp/crs/misc/RL34635.pdf. Published December 13, 2012. Accessed July 10, 2019.
14. US Department of Justice, Drug Enforcement Administration, Diversion Control Division. Title 21 United States Code (USC) Controlled Substances Act. Subchapter I—Control and Enforcement. Part B—Authority to Control; Standards and Schedules. §812. Schedules of controlled substances. www.deadiversion.usdoj.gov/21cfr/21usc/812.htm. Accessed July 10, 2019.
15. United States Senate. The STATES Act. Senator Elizabeth Warren and Senator Cory Gardner. 2018. www.warren.senate.gov/imo/media/doc/STATES%20Act%20One%20Pager.pdf. Accessed July 10, 2019.
16. Strengthening the Tenth Amendment Through Entrusting States (STATES) Act of 2019, HR 2093. 116th Cong, 1st Session (2019). www.congress.gov/bill/116th-congress/house-bill/2093/text. Accessed July 20, 2019.
17. Strengthening the Tenth Amendment Through Entrusting States (STATES) Act of 2019, S 1028. 116th Cong, 1st Session (2019). www.congress.gov/bill/116th-congress/senate-bill/1028/all-info?r=3&s=6. Accessed August 8, 2019.
18. State medical marijuana laws. National Conference of State Legislatures Web site. www.ncsl.org/research/health/state-medical-marijuana-laws.aspx#3. Published July 2, 2019. Accessed July 10, 2019.
19. Conant v Walters. 309 F.3d 629 (9th cir. 2002).
20. American Society of Addiction Medicine. The role of the physician in “medical” marijuana. www.asam.org/docs/publicy-policy-statements/1role_of_phys_in_med_mj_9-10.pdf?sfvrsn=0. Published September 2010. Accessed July 12, 2019.
21. What are marijuana’s effects on lung health? National Institute on Drug Abuse Web site. www.drugabuse.gov/publications/research-reports/marijuana/what-are-marijuanas-effects-lung-health. Updated July 2019. Accessed July 10, 2019.
22. Tashkin DP. Effects of marijuana smoking on the lung. Ann Am Thorac Soc. 2013;10:239-247.
23. Zhang LR, Morgenstern H, Greenland S, et al. Cannabis smoking and lung cancer risk: pooled analysis in the International Lung Cancer Consortium. Int J Cancer. 2015;136:894-903.
24. Getting medical marijuana. Commonwealth of Pennsylvania Web site. www.pa.gov/guides/pennsylvania-medical-marijuana-program/. Accessed July 20, 2019.
25. Kosiba JD, Maisto SA, Ditre JW. Patient-reported use of medical cannabis for pain, anxiety, and depression symptoms: systematic review and meta-analysis. Soc Sci Med. 2019;233:181-192.
26. Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24:515-523.
27. Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319-328.
28. Barrus DG, Capogrossi KL, Cates S, et al. Tasty THC: Promises and Challenges of Cannabis Edibles. Publication No. OP-0035-1611. Research Triangle Park, NC: RTI Press; 2016. www.rti.org/sites/default/files/resources/rti-publication-file-6ff047d7-3fa4-41ad-90ed-9fb11663bc89.pdf. Accessed July 10, 2019.
29. MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med. 2018;49:12-19.
30. MacCoun RJ, Mello MM. Half-baked—the retail promotion of marijuana edibles. N Engl J Med. 2015;372:989-991.
31. Cannabis use disorder [305.20, 304.30]. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013:509-516.
32. Williams AR, Santaella-Tenorio J, Mauro CM, et al. Loose regulation of medical marijuana programs associated with higher rates of adult marijuana use but not cannabis use disorder. Addiction. 2017;112:1985-1991.
33. American Academy of Pediatrics Committee on Substance Abuse, American Academy of Pediatrics Committee on Adolescents. The impact of marijuana policies on youth: clinical, research, and legal update. Pediatrics. 2015;135:584-587.
34. Wong SS, Wilens TE. Medical cannabinoids in children and adolescents: a systematic review. Pediatrics. 2017;140. pii: e20171818.
35. Drug Enforcement Administration. Drugs of abuse: a DEA resource guide. www.dea.gov/sites/default/files/drug_of_abuse.pdf. Published 2017. Accessed July 10, 2019.
36. National Academies of Science, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press; 2017. www.nap.edu/read/24625/chapter/12017:2017-2019. Published 2017. Accessed July 10, 2019.
37. Emerging trend and alerts. National Institute on Drug Abuse Web site. www.drugabuse.gov/drugs-abuse/emerging-trends-alerts. Accessed July 10, 2019.
38. Drug Policy Alliance. From prohibition to progress: a status report on marijuana legalization. www.drugpolicy.org/sites/default/files/dpa_marijuana_legalization_report_feb14_2018_0.pdf. Published January 2018. Accessed July 10, 2019.
1. College of Family Physicians of Canada. Authorizing Dried Cannabis for Chronic Pain or Anxiety: Preliminary Guidance from the College of Family Physicians of Canada. Mississauga, Ontario: College of Family Physicians of Canada; 2014. www.cfpc.ca/uploadedFiles/Resources/_PDFs/Authorizing%20Dried%20Cannabis%20for%20Chronic%20Pain%20or%20Anxiety.pdf. Accessed July 10, 2019.
2. Hartig H, Geiger AW. About six-in-ten Americans support marijuana legalization. Pew Research Center Web site. www.pewresearch.org/fact-tank/2018/10/08/americans-support-marijuana-legalization/. Published October 8, 2018. Accessed July 10, 2019.
3. Li H-L. An archaeological and historical account of cannabis in China. Econ Bot. 1974:28:437-448.
4. Zuardi AW. History of cannabis as a medicine: a review. Braz J Psychiatry. 2006;28:153-157.
5. Marijuana strains and infused products. Leafly Web site. www.leafly.com/start-exploring. Accessed July 10, 2019.
6. Fraguas-Sánchez AI, Torres-Suárez AI. Medical use of cannabinoids. Drugs. 2018;78:1665-1703.
7. Maurya N, Velmurugan BK. Therapeutic applications of cannabinoids. Chem Biol Interact. 2018;293:77-88.
8. Kelkar AH, Smith NA, Martial A, et al. An outbreak of synthetic cannabinoid-associated coagulopathy in Illinois. N Engl J Med. 2018;379:1216-1223.
9. Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
10. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
11. Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2008;34:672-680.
12. US Department of Justice, Drug Enforcement Administration, Diversion Control Division. Title 21 United States Code (USC) Controlled Substances Act. Subchapter I—Control and Enforcement. Part A—Introductory Provisions. §801. Congressional findings and declarations: controlled substances. www.deadiversion.usdoj.gov/21cfr/21usc/801.htm. Accessed July 10, 2019.
13. Yeh BT. The Controlled Substances Act: regulatory requirements. Congressional Research Service 7-5700. https://fas.org/sgp/crs/misc/RL34635.pdf. Published December 13, 2012. Accessed July 10, 2019.
14. US Department of Justice, Drug Enforcement Administration, Diversion Control Division. Title 21 United States Code (USC) Controlled Substances Act. Subchapter I—Control and Enforcement. Part B—Authority to Control; Standards and Schedules. §812. Schedules of controlled substances. www.deadiversion.usdoj.gov/21cfr/21usc/812.htm. Accessed July 10, 2019.
15. United States Senate. The STATES Act. Senator Elizabeth Warren and Senator Cory Gardner. 2018. www.warren.senate.gov/imo/media/doc/STATES%20Act%20One%20Pager.pdf. Accessed July 10, 2019.
16. Strengthening the Tenth Amendment Through Entrusting States (STATES) Act of 2019, HR 2093. 116th Cong, 1st Session (2019). www.congress.gov/bill/116th-congress/house-bill/2093/text. Accessed July 20, 2019.
17. Strengthening the Tenth Amendment Through Entrusting States (STATES) Act of 2019, S 1028. 116th Cong, 1st Session (2019). www.congress.gov/bill/116th-congress/senate-bill/1028/all-info?r=3&s=6. Accessed August 8, 2019.
18. State medical marijuana laws. National Conference of State Legislatures Web site. www.ncsl.org/research/health/state-medical-marijuana-laws.aspx#3. Published July 2, 2019. Accessed July 10, 2019.
19. Conant v Walters. 309 F.3d 629 (9th cir. 2002).
20. American Society of Addiction Medicine. The role of the physician in “medical” marijuana. www.asam.org/docs/publicy-policy-statements/1role_of_phys_in_med_mj_9-10.pdf?sfvrsn=0. Published September 2010. Accessed July 12, 2019.
21. What are marijuana’s effects on lung health? National Institute on Drug Abuse Web site. www.drugabuse.gov/publications/research-reports/marijuana/what-are-marijuanas-effects-lung-health. Updated July 2019. Accessed July 10, 2019.
22. Tashkin DP. Effects of marijuana smoking on the lung. Ann Am Thorac Soc. 2013;10:239-247.
23. Zhang LR, Morgenstern H, Greenland S, et al. Cannabis smoking and lung cancer risk: pooled analysis in the International Lung Cancer Consortium. Int J Cancer. 2015;136:894-903.
24. Getting medical marijuana. Commonwealth of Pennsylvania Web site. www.pa.gov/guides/pennsylvania-medical-marijuana-program/. Accessed July 20, 2019.
25. Kosiba JD, Maisto SA, Ditre JW. Patient-reported use of medical cannabis for pain, anxiety, and depression symptoms: systematic review and meta-analysis. Soc Sci Med. 2019;233:181-192.
26. Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24:515-523.
27. Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319-328.
28. Barrus DG, Capogrossi KL, Cates S, et al. Tasty THC: Promises and Challenges of Cannabis Edibles. Publication No. OP-0035-1611. Research Triangle Park, NC: RTI Press; 2016. www.rti.org/sites/default/files/resources/rti-publication-file-6ff047d7-3fa4-41ad-90ed-9fb11663bc89.pdf. Accessed July 10, 2019.
29. MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med. 2018;49:12-19.
30. MacCoun RJ, Mello MM. Half-baked—the retail promotion of marijuana edibles. N Engl J Med. 2015;372:989-991.
31. Cannabis use disorder [305.20, 304.30]. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013:509-516.
32. Williams AR, Santaella-Tenorio J, Mauro CM, et al. Loose regulation of medical marijuana programs associated with higher rates of adult marijuana use but not cannabis use disorder. Addiction. 2017;112:1985-1991.
33. American Academy of Pediatrics Committee on Substance Abuse, American Academy of Pediatrics Committee on Adolescents. The impact of marijuana policies on youth: clinical, research, and legal update. Pediatrics. 2015;135:584-587.
34. Wong SS, Wilens TE. Medical cannabinoids in children and adolescents: a systematic review. Pediatrics. 2017;140. pii: e20171818.
35. Drug Enforcement Administration. Drugs of abuse: a DEA resource guide. www.dea.gov/sites/default/files/drug_of_abuse.pdf. Published 2017. Accessed July 10, 2019.
36. National Academies of Science, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press; 2017. www.nap.edu/read/24625/chapter/12017:2017-2019. Published 2017. Accessed July 10, 2019.
37. Emerging trend and alerts. National Institute on Drug Abuse Web site. www.drugabuse.gov/drugs-abuse/emerging-trends-alerts. Accessed July 10, 2019.
38. Drug Policy Alliance. From prohibition to progress: a status report on marijuana legalization. www.drugpolicy.org/sites/default/files/dpa_marijuana_legalization_report_feb14_2018_0.pdf. Published January 2018. Accessed July 10, 2019.
PRACTICE RECOMMENDATIONS
› Educate patients about the effects of the physiologically active therapeutic compounds in Cannabis; this is critical to prevent overconsumption of products with high levels of tetrahydrocannabinol. B
› Screen patients for serious mental health concerns before recommending or certifying medical Cannabis; this is essential because the rate of psychiatric hospitalization is increased in bipolar disorder and schizophrenia patients who use Cannabis heavily. B
› You can recommend medical Cannabis and certify patients for its use with the certainty that the risk of overdose or serious adverse effects is exceedingly low. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Sick, or faking it?
CASE Vague symptoms; no clear etiology
Mr. W, age 53, presents to the emergency department (ED) describing acute mid-sternal chest pain (severity: 8 out of 10). His medical history is significant for pulmonary embolism and ascending aortic aneurysm in the context of Takayasu’s arteritis, an inflammatory condition of the large arterial blood vessels characterized by lesions that can lead to vascular stenosis, occlusion, or aneurysm. Takayasu’s arteritis is also known as pulseless disease due to the weak or absent pulses the condition produces.
A review of Mr. W’s medical records reveals that this is his 23rd visit to this hospital within a year; the year before that, he had 22 visits. At each of these previous visits, he had similar vague symptoms, including dizziness, chest pain, lightheadedness, fainting, bilateral knee weakness, and left-arm numbness/weakness, and no clear acute etiology for his reported symptoms. Each time, after the treating clinicians ruled out possible acute complications of a flare-up of Takayasu’s arteritis through a physical examination, laboratory tests, and imaging studies, Mr. W was discharged with recommendations that he follow-up with his primary care physician and specialists. At each discharge, he would leave the hospital with hesitation.
At this present visit, the ED physician recognizes Mr. W as someone who visits the ED often with no profound acute issues, and reviews the substantial medical records available to the hospital. He suspects Mr. W is feigning symptoms, and orders a psychiatric consultation.
EVALUATION Psychiatric interview and mental status exam
On examination, Mr. W is not in acute distress. Despite reporting an 8 out of 10 for chest pain severity, he displays no psychomotor agitation, and his pulse rate and blood pressure are within normal limits. He makes appropriate eye contact and describes his mood as “great.” He reports no problems with sleep, appetite, or disinterest in pleasurable activities, and denies being depressed or having any symptoms consistent with a mood disorder, anxiety disorder, or psychosis. He denies a history of panic attacks or excessive worrying that interferes with his sleep or activities of daily living. Additionally, Mr. W describes a stable, peaceful, and stress-free life within the limitations of his Takayasu’s arteritis, which he has been managing well since his diagnosis 6 years earlier.
Mr. W denies having any psychiatric symptoms, apprehensive feelings, or beliefs/fears that would be considered delusional, and he has no previous legal issues aside from an occasional driving citation. During the assessment, his affect remains broad and he denies having thoughts of suicide or homicide, or auditory or visual hallucinations.
Mr. W’s drug screen results are negative, and he denies using any illicit drugs. He uses only the medications that are prescribed by his clinicians. Overall, he seems to be a well-functioning individual. Mr. W reports that work is generally not stressful.
When the psychiatric team asks him about his frequent hospitalizations and ED visits, Mr. W is insistent that he is “just doing what my doctors said for me to do.” He repeats that he does not have any mental illness and did not see the point of seeing a psychiatrist.
In pursuit of collateral information, the psychiatry team accesses a regional medical record database that allows registered medical institutions and practices to track patients’ medical encounters within the region. According to this database, within approximately 5.5 years, Mr. W had 163 clinical encounters (ED visits and inpatient admissions) and 376 radiological studies in our region (Table 1 and Table 2).
[polldaddy:10394110]
Continue to: The authors' observations
The authors’ observations
The psychiatry team’s investigation of Mr. W’s medical records revealed the extent of his care-seeking behavior, and provided evidence for a diagnosis of factitious disorder.
Factitious disorder is an elusive psychiatric condition in which an individual chronically stimulates, induces, or aggravates illnesses to gain the status of being a patient. Although its exact cause has not been fully deciphered, it is seen mostly among individuals with knowledge of the workings of the medical field, such as a health care worker.1 Factitious disorder is taxing on the health care system, with an estimated cost in the thousands of dollars per patient visit.2 The condition has an estimated prevalence of 0.8% to 1.0% of patients seen by psychiatric consult services3 and is reported to be more prevalent among women than men.1 Its cardinal features include health care site hopping and hospital shopping, vagueness about the patient’s history and symptoms, and discrepancy among reported symptoms, the patient’s behaviors, and objective clinical findings.4,5 Although not all patients with factitious disorder have a legitimate medical reason for seeking care, some individuals with an established medical diagnosis use their condition as a tool to chronically seek care and play the sick role.
Factitious disorder should not be confused with malingering, which is differentiated by the patient’s search for a secondary gain, such as financial reward or avoiding jail; or conversion disorder, which is marked by true physical or neurologic symptoms and clinical findings triggered by psychological stressors. Patients with factitious disorder usually are cooperative during hospital stays and resume their normal daily routine shortly after discharge.4 In this case, Mr. W denied any psychiatric symptoms, apprehensive feelings, or beliefs or fears that would be considered delusional. He had no previous or pending legal issues, which ruled out malingering to avoid legal repercussions.
Mr. W’s presentation was complicated by his Takayasu’s arteritis diagnosis. Because Takayasu’s arteritis has a serious list of potential complications, ED physicians have a low threshold for ordering diagnostic studies for a patient with Takayasu’s arteritis who presents with a chief complaint of chest pain. In other words, when a patient with this condition presents to an acute setting (such as the ED) with chest pain, his/her chief complaint is taken with extreme seriousness. Conventional angiography is the standard diagnostic tool for Takayasu’s arteritis; CT angiography and magnetic resonance angiography are used for monitoring the disease’s progression.6
[polldaddy:10394113]
The authors’ observations
Currently, there are no FDA-approved treatments for factitious disorder, and patients with this condition generally are resistant to psychiatric and/or psychological care when discovered and offered treatment.7 Among those who consent to psychiatric care, psychoeducation, or psychotherapy, which have shown some efficacy for the condition, the dropout rate is high.8
Continue to: Although the instinctive approach...
Although the instinctive approach is to confront the patient once the deception has been uncovered, expert recommendations are contradictory. Some recommend confrontation as part of a treatment protocol,8 while others advise against such an approach.9
Because of how often patients with factitious disorder seek medical care, secondary iatrogenic consequences are possible. For example, for years, Mr. W has been unknowingly exposing himself to the iatrogenic consequences of the cumulative effect of diagnostic imaging for years. In 3 years alone, Mr. W had undergone an average of 125 diagnostic imaging studies per year—with and without contrast—and many unnecessary rounds of treatment with steroids and other interventions known to have secondary iatrogenic consequences.10 Excessive radiation exposure is known to be carcinogenic over time,10 and excessive use of steroids is associated with weight gain, physical habitus changes, and increased risk of infections.11 In addition, the renal effects of the contrast materials from repeated imaging studies over so many years on Mr. W’s future kidney function are unknown.
TREATMENT Psychoeducation and referral for psychotherapy
We counsel Mr. W about factitious disorder and the risks of excessive hospitalizations, and refer him for follow-up at our local psychiatric clinic, as well as for individual psychotherapy. Mr. W is discharged because his medical work-up does not reveal any significant acute medical issues.
The authors’ observations
Because of the poor insight associated with factitious disorder and the limited treatment options available, a patient with factitious disorder is unlikely to enter psychiatric treatment on his/her own. The prognosis for a patient with factitious disorder remains poor unless the patient is forced into treatment. More intervention-focused research is needed to help improve outcomes for patients with factitious disorder.
OUTCOME Failure to follow up
Mr. W fails to attend individual psychotherapy as recommended. According to our regional record database, Mr. W continues to present to other EDs regularly.
Continue to: Bottom Line
Bottom Line
A patient with factitious disorder stimulates, induces, or aggravates illnesses to gain the status of being a patient. Treatment options include psychiatric care, psychoeducation, or psychotherapy. However, due to poor insight, a patient with factitious disorder is unlikely to enter psychiatric treatment on his/her own.
Related Resources
- Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
- Galli S, Tatu L, Bogousslavsky J, et al. Conversion, factitious disorder and malingering: a distinct pattern or a continuum? Front Neurol Neurosci. 2018;42:72-80.
1. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
2. Hoertel N, Lavaud P, Le Strat Y, et al. Estimated cost of a factitious disorder with 6-year follow-up. Psychiatry Res. 2012;200(2):1077-1078.
3. Sadock BJ, Sadock VA, Ruiz P. Psychosomatic medicine; factitious disorder. In: Pataki CS, Sussman N, eds. Synopsis of psychiatry: Behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015:34-45.
4 . Savino AC, Fordtran JS. Factitious disease: clinical lessons from case studies at Baylor University Medical Center. Proc (Bayl Univ Med Cent). 2006;19(3):195-208.
5. Burnel A. Recognition and management of factitious disorder. Prescriber. 2015;26(21):37-39.
6. Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4(1):e000612. doi: 10.1136/rmdopen-2017-000612.
7. Jafferany M, Khalid Z, McDonald KA, et al. Psychological aspects of factitious disorder. Prim Care Companion CNS Disord. 2018;20(1). doi: 10.4088/PCC.17nr02229.
8. Bolat N, Yalçin O. Factitious disorder presenting with stuttering in two adolescents: the importance of psychoeducation. Noro Psikiyatri Arsivi. 2017;54(1):87-89.
9. Eisendrath SJ. Factitious physical disorders. West J Med. 1994;160(2):177-179.
10. Sodickson A, Baeyens PF, Andriole KP, et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology. 2009;251(1):175-184.
11. Oray M, Abu Samra K, Ebrahimiadib N, et al. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15(4):457-465.
CASE Vague symptoms; no clear etiology
Mr. W, age 53, presents to the emergency department (ED) describing acute mid-sternal chest pain (severity: 8 out of 10). His medical history is significant for pulmonary embolism and ascending aortic aneurysm in the context of Takayasu’s arteritis, an inflammatory condition of the large arterial blood vessels characterized by lesions that can lead to vascular stenosis, occlusion, or aneurysm. Takayasu’s arteritis is also known as pulseless disease due to the weak or absent pulses the condition produces.
A review of Mr. W’s medical records reveals that this is his 23rd visit to this hospital within a year; the year before that, he had 22 visits. At each of these previous visits, he had similar vague symptoms, including dizziness, chest pain, lightheadedness, fainting, bilateral knee weakness, and left-arm numbness/weakness, and no clear acute etiology for his reported symptoms. Each time, after the treating clinicians ruled out possible acute complications of a flare-up of Takayasu’s arteritis through a physical examination, laboratory tests, and imaging studies, Mr. W was discharged with recommendations that he follow-up with his primary care physician and specialists. At each discharge, he would leave the hospital with hesitation.
At this present visit, the ED physician recognizes Mr. W as someone who visits the ED often with no profound acute issues, and reviews the substantial medical records available to the hospital. He suspects Mr. W is feigning symptoms, and orders a psychiatric consultation.
EVALUATION Psychiatric interview and mental status exam
On examination, Mr. W is not in acute distress. Despite reporting an 8 out of 10 for chest pain severity, he displays no psychomotor agitation, and his pulse rate and blood pressure are within normal limits. He makes appropriate eye contact and describes his mood as “great.” He reports no problems with sleep, appetite, or disinterest in pleasurable activities, and denies being depressed or having any symptoms consistent with a mood disorder, anxiety disorder, or psychosis. He denies a history of panic attacks or excessive worrying that interferes with his sleep or activities of daily living. Additionally, Mr. W describes a stable, peaceful, and stress-free life within the limitations of his Takayasu’s arteritis, which he has been managing well since his diagnosis 6 years earlier.
Mr. W denies having any psychiatric symptoms, apprehensive feelings, or beliefs/fears that would be considered delusional, and he has no previous legal issues aside from an occasional driving citation. During the assessment, his affect remains broad and he denies having thoughts of suicide or homicide, or auditory or visual hallucinations.
Mr. W’s drug screen results are negative, and he denies using any illicit drugs. He uses only the medications that are prescribed by his clinicians. Overall, he seems to be a well-functioning individual. Mr. W reports that work is generally not stressful.
When the psychiatric team asks him about his frequent hospitalizations and ED visits, Mr. W is insistent that he is “just doing what my doctors said for me to do.” He repeats that he does not have any mental illness and did not see the point of seeing a psychiatrist.
In pursuit of collateral information, the psychiatry team accesses a regional medical record database that allows registered medical institutions and practices to track patients’ medical encounters within the region. According to this database, within approximately 5.5 years, Mr. W had 163 clinical encounters (ED visits and inpatient admissions) and 376 radiological studies in our region (Table 1 and Table 2).
[polldaddy:10394110]
Continue to: The authors' observations
The authors’ observations
The psychiatry team’s investigation of Mr. W’s medical records revealed the extent of his care-seeking behavior, and provided evidence for a diagnosis of factitious disorder.
Factitious disorder is an elusive psychiatric condition in which an individual chronically stimulates, induces, or aggravates illnesses to gain the status of being a patient. Although its exact cause has not been fully deciphered, it is seen mostly among individuals with knowledge of the workings of the medical field, such as a health care worker.1 Factitious disorder is taxing on the health care system, with an estimated cost in the thousands of dollars per patient visit.2 The condition has an estimated prevalence of 0.8% to 1.0% of patients seen by psychiatric consult services3 and is reported to be more prevalent among women than men.1 Its cardinal features include health care site hopping and hospital shopping, vagueness about the patient’s history and symptoms, and discrepancy among reported symptoms, the patient’s behaviors, and objective clinical findings.4,5 Although not all patients with factitious disorder have a legitimate medical reason for seeking care, some individuals with an established medical diagnosis use their condition as a tool to chronically seek care and play the sick role.
Factitious disorder should not be confused with malingering, which is differentiated by the patient’s search for a secondary gain, such as financial reward or avoiding jail; or conversion disorder, which is marked by true physical or neurologic symptoms and clinical findings triggered by psychological stressors. Patients with factitious disorder usually are cooperative during hospital stays and resume their normal daily routine shortly after discharge.4 In this case, Mr. W denied any psychiatric symptoms, apprehensive feelings, or beliefs or fears that would be considered delusional. He had no previous or pending legal issues, which ruled out malingering to avoid legal repercussions.
Mr. W’s presentation was complicated by his Takayasu’s arteritis diagnosis. Because Takayasu’s arteritis has a serious list of potential complications, ED physicians have a low threshold for ordering diagnostic studies for a patient with Takayasu’s arteritis who presents with a chief complaint of chest pain. In other words, when a patient with this condition presents to an acute setting (such as the ED) with chest pain, his/her chief complaint is taken with extreme seriousness. Conventional angiography is the standard diagnostic tool for Takayasu’s arteritis; CT angiography and magnetic resonance angiography are used for monitoring the disease’s progression.6
[polldaddy:10394113]
The authors’ observations
Currently, there are no FDA-approved treatments for factitious disorder, and patients with this condition generally are resistant to psychiatric and/or psychological care when discovered and offered treatment.7 Among those who consent to psychiatric care, psychoeducation, or psychotherapy, which have shown some efficacy for the condition, the dropout rate is high.8
Continue to: Although the instinctive approach...
Although the instinctive approach is to confront the patient once the deception has been uncovered, expert recommendations are contradictory. Some recommend confrontation as part of a treatment protocol,8 while others advise against such an approach.9
Because of how often patients with factitious disorder seek medical care, secondary iatrogenic consequences are possible. For example, for years, Mr. W has been unknowingly exposing himself to the iatrogenic consequences of the cumulative effect of diagnostic imaging for years. In 3 years alone, Mr. W had undergone an average of 125 diagnostic imaging studies per year—with and without contrast—and many unnecessary rounds of treatment with steroids and other interventions known to have secondary iatrogenic consequences.10 Excessive radiation exposure is known to be carcinogenic over time,10 and excessive use of steroids is associated with weight gain, physical habitus changes, and increased risk of infections.11 In addition, the renal effects of the contrast materials from repeated imaging studies over so many years on Mr. W’s future kidney function are unknown.
TREATMENT Psychoeducation and referral for psychotherapy
We counsel Mr. W about factitious disorder and the risks of excessive hospitalizations, and refer him for follow-up at our local psychiatric clinic, as well as for individual psychotherapy. Mr. W is discharged because his medical work-up does not reveal any significant acute medical issues.
The authors’ observations
Because of the poor insight associated with factitious disorder and the limited treatment options available, a patient with factitious disorder is unlikely to enter psychiatric treatment on his/her own. The prognosis for a patient with factitious disorder remains poor unless the patient is forced into treatment. More intervention-focused research is needed to help improve outcomes for patients with factitious disorder.
OUTCOME Failure to follow up
Mr. W fails to attend individual psychotherapy as recommended. According to our regional record database, Mr. W continues to present to other EDs regularly.
Continue to: Bottom Line
Bottom Line
A patient with factitious disorder stimulates, induces, or aggravates illnesses to gain the status of being a patient. Treatment options include psychiatric care, psychoeducation, or psychotherapy. However, due to poor insight, a patient with factitious disorder is unlikely to enter psychiatric treatment on his/her own.
Related Resources
- Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
- Galli S, Tatu L, Bogousslavsky J, et al. Conversion, factitious disorder and malingering: a distinct pattern or a continuum? Front Neurol Neurosci. 2018;42:72-80.
CASE Vague symptoms; no clear etiology
Mr. W, age 53, presents to the emergency department (ED) describing acute mid-sternal chest pain (severity: 8 out of 10). His medical history is significant for pulmonary embolism and ascending aortic aneurysm in the context of Takayasu’s arteritis, an inflammatory condition of the large arterial blood vessels characterized by lesions that can lead to vascular stenosis, occlusion, or aneurysm. Takayasu’s arteritis is also known as pulseless disease due to the weak or absent pulses the condition produces.
A review of Mr. W’s medical records reveals that this is his 23rd visit to this hospital within a year; the year before that, he had 22 visits. At each of these previous visits, he had similar vague symptoms, including dizziness, chest pain, lightheadedness, fainting, bilateral knee weakness, and left-arm numbness/weakness, and no clear acute etiology for his reported symptoms. Each time, after the treating clinicians ruled out possible acute complications of a flare-up of Takayasu’s arteritis through a physical examination, laboratory tests, and imaging studies, Mr. W was discharged with recommendations that he follow-up with his primary care physician and specialists. At each discharge, he would leave the hospital with hesitation.
At this present visit, the ED physician recognizes Mr. W as someone who visits the ED often with no profound acute issues, and reviews the substantial medical records available to the hospital. He suspects Mr. W is feigning symptoms, and orders a psychiatric consultation.
EVALUATION Psychiatric interview and mental status exam
On examination, Mr. W is not in acute distress. Despite reporting an 8 out of 10 for chest pain severity, he displays no psychomotor agitation, and his pulse rate and blood pressure are within normal limits. He makes appropriate eye contact and describes his mood as “great.” He reports no problems with sleep, appetite, or disinterest in pleasurable activities, and denies being depressed or having any symptoms consistent with a mood disorder, anxiety disorder, or psychosis. He denies a history of panic attacks or excessive worrying that interferes with his sleep or activities of daily living. Additionally, Mr. W describes a stable, peaceful, and stress-free life within the limitations of his Takayasu’s arteritis, which he has been managing well since his diagnosis 6 years earlier.
Mr. W denies having any psychiatric symptoms, apprehensive feelings, or beliefs/fears that would be considered delusional, and he has no previous legal issues aside from an occasional driving citation. During the assessment, his affect remains broad and he denies having thoughts of suicide or homicide, or auditory or visual hallucinations.
Mr. W’s drug screen results are negative, and he denies using any illicit drugs. He uses only the medications that are prescribed by his clinicians. Overall, he seems to be a well-functioning individual. Mr. W reports that work is generally not stressful.
When the psychiatric team asks him about his frequent hospitalizations and ED visits, Mr. W is insistent that he is “just doing what my doctors said for me to do.” He repeats that he does not have any mental illness and did not see the point of seeing a psychiatrist.
In pursuit of collateral information, the psychiatry team accesses a regional medical record database that allows registered medical institutions and practices to track patients’ medical encounters within the region. According to this database, within approximately 5.5 years, Mr. W had 163 clinical encounters (ED visits and inpatient admissions) and 376 radiological studies in our region (Table 1 and Table 2).
[polldaddy:10394110]
Continue to: The authors' observations
The authors’ observations
The psychiatry team’s investigation of Mr. W’s medical records revealed the extent of his care-seeking behavior, and provided evidence for a diagnosis of factitious disorder.
Factitious disorder is an elusive psychiatric condition in which an individual chronically stimulates, induces, or aggravates illnesses to gain the status of being a patient. Although its exact cause has not been fully deciphered, it is seen mostly among individuals with knowledge of the workings of the medical field, such as a health care worker.1 Factitious disorder is taxing on the health care system, with an estimated cost in the thousands of dollars per patient visit.2 The condition has an estimated prevalence of 0.8% to 1.0% of patients seen by psychiatric consult services3 and is reported to be more prevalent among women than men.1 Its cardinal features include health care site hopping and hospital shopping, vagueness about the patient’s history and symptoms, and discrepancy among reported symptoms, the patient’s behaviors, and objective clinical findings.4,5 Although not all patients with factitious disorder have a legitimate medical reason for seeking care, some individuals with an established medical diagnosis use their condition as a tool to chronically seek care and play the sick role.
Factitious disorder should not be confused with malingering, which is differentiated by the patient’s search for a secondary gain, such as financial reward or avoiding jail; or conversion disorder, which is marked by true physical or neurologic symptoms and clinical findings triggered by psychological stressors. Patients with factitious disorder usually are cooperative during hospital stays and resume their normal daily routine shortly after discharge.4 In this case, Mr. W denied any psychiatric symptoms, apprehensive feelings, or beliefs or fears that would be considered delusional. He had no previous or pending legal issues, which ruled out malingering to avoid legal repercussions.
Mr. W’s presentation was complicated by his Takayasu’s arteritis diagnosis. Because Takayasu’s arteritis has a serious list of potential complications, ED physicians have a low threshold for ordering diagnostic studies for a patient with Takayasu’s arteritis who presents with a chief complaint of chest pain. In other words, when a patient with this condition presents to an acute setting (such as the ED) with chest pain, his/her chief complaint is taken with extreme seriousness. Conventional angiography is the standard diagnostic tool for Takayasu’s arteritis; CT angiography and magnetic resonance angiography are used for monitoring the disease’s progression.6
[polldaddy:10394113]
The authors’ observations
Currently, there are no FDA-approved treatments for factitious disorder, and patients with this condition generally are resistant to psychiatric and/or psychological care when discovered and offered treatment.7 Among those who consent to psychiatric care, psychoeducation, or psychotherapy, which have shown some efficacy for the condition, the dropout rate is high.8
Continue to: Although the instinctive approach...
Although the instinctive approach is to confront the patient once the deception has been uncovered, expert recommendations are contradictory. Some recommend confrontation as part of a treatment protocol,8 while others advise against such an approach.9
Because of how often patients with factitious disorder seek medical care, secondary iatrogenic consequences are possible. For example, for years, Mr. W has been unknowingly exposing himself to the iatrogenic consequences of the cumulative effect of diagnostic imaging for years. In 3 years alone, Mr. W had undergone an average of 125 diagnostic imaging studies per year—with and without contrast—and many unnecessary rounds of treatment with steroids and other interventions known to have secondary iatrogenic consequences.10 Excessive radiation exposure is known to be carcinogenic over time,10 and excessive use of steroids is associated with weight gain, physical habitus changes, and increased risk of infections.11 In addition, the renal effects of the contrast materials from repeated imaging studies over so many years on Mr. W’s future kidney function are unknown.
TREATMENT Psychoeducation and referral for psychotherapy
We counsel Mr. W about factitious disorder and the risks of excessive hospitalizations, and refer him for follow-up at our local psychiatric clinic, as well as for individual psychotherapy. Mr. W is discharged because his medical work-up does not reveal any significant acute medical issues.
The authors’ observations
Because of the poor insight associated with factitious disorder and the limited treatment options available, a patient with factitious disorder is unlikely to enter psychiatric treatment on his/her own. The prognosis for a patient with factitious disorder remains poor unless the patient is forced into treatment. More intervention-focused research is needed to help improve outcomes for patients with factitious disorder.
OUTCOME Failure to follow up
Mr. W fails to attend individual psychotherapy as recommended. According to our regional record database, Mr. W continues to present to other EDs regularly.
Continue to: Bottom Line
Bottom Line
A patient with factitious disorder stimulates, induces, or aggravates illnesses to gain the status of being a patient. Treatment options include psychiatric care, psychoeducation, or psychotherapy. However, due to poor insight, a patient with factitious disorder is unlikely to enter psychiatric treatment on his/her own.
Related Resources
- Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
- Galli S, Tatu L, Bogousslavsky J, et al. Conversion, factitious disorder and malingering: a distinct pattern or a continuum? Front Neurol Neurosci. 2018;42:72-80.
1. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
2. Hoertel N, Lavaud P, Le Strat Y, et al. Estimated cost of a factitious disorder with 6-year follow-up. Psychiatry Res. 2012;200(2):1077-1078.
3. Sadock BJ, Sadock VA, Ruiz P. Psychosomatic medicine; factitious disorder. In: Pataki CS, Sussman N, eds. Synopsis of psychiatry: Behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015:34-45.
4 . Savino AC, Fordtran JS. Factitious disease: clinical lessons from case studies at Baylor University Medical Center. Proc (Bayl Univ Med Cent). 2006;19(3):195-208.
5. Burnel A. Recognition and management of factitious disorder. Prescriber. 2015;26(21):37-39.
6. Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4(1):e000612. doi: 10.1136/rmdopen-2017-000612.
7. Jafferany M, Khalid Z, McDonald KA, et al. Psychological aspects of factitious disorder. Prim Care Companion CNS Disord. 2018;20(1). doi: 10.4088/PCC.17nr02229.
8. Bolat N, Yalçin O. Factitious disorder presenting with stuttering in two adolescents: the importance of psychoeducation. Noro Psikiyatri Arsivi. 2017;54(1):87-89.
9. Eisendrath SJ. Factitious physical disorders. West J Med. 1994;160(2):177-179.
10. Sodickson A, Baeyens PF, Andriole KP, et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology. 2009;251(1):175-184.
11. Oray M, Abu Samra K, Ebrahimiadib N, et al. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15(4):457-465.
1. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
2. Hoertel N, Lavaud P, Le Strat Y, et al. Estimated cost of a factitious disorder with 6-year follow-up. Psychiatry Res. 2012;200(2):1077-1078.
3. Sadock BJ, Sadock VA, Ruiz P. Psychosomatic medicine; factitious disorder. In: Pataki CS, Sussman N, eds. Synopsis of psychiatry: Behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015:34-45.
4 . Savino AC, Fordtran JS. Factitious disease: clinical lessons from case studies at Baylor University Medical Center. Proc (Bayl Univ Med Cent). 2006;19(3):195-208.
5. Burnel A. Recognition and management of factitious disorder. Prescriber. 2015;26(21):37-39.
6. Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4(1):e000612. doi: 10.1136/rmdopen-2017-000612.
7. Jafferany M, Khalid Z, McDonald KA, et al. Psychological aspects of factitious disorder. Prim Care Companion CNS Disord. 2018;20(1). doi: 10.4088/PCC.17nr02229.
8. Bolat N, Yalçin O. Factitious disorder presenting with stuttering in two adolescents: the importance of psychoeducation. Noro Psikiyatri Arsivi. 2017;54(1):87-89.
9. Eisendrath SJ. Factitious physical disorders. West J Med. 1994;160(2):177-179.
10. Sodickson A, Baeyens PF, Andriole KP, et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology. 2009;251(1):175-184.
11. Oray M, Abu Samra K, Ebrahimiadib N, et al. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15(4):457-465.
Rheumatoid Arthritis: Therapeutic Strategies After Inadequate Response to Initial TNF Inhibitor Therapy
From the University of Iowa Hospitals and Clinics, Iowa City, IA.
Abstract
- Objective: To discuss the variability in response to tumor necrosis factor inhibitors (TNFis) observed in patients with rheumatoid arthritis (RA) and discuss therapeutic options for patients who do not respond to initial TNFi therapy.
- Methods: Review of the literature.
- Results: Optimal treatment of RA aims at achieving and then maintaining remission or low disease activity. In a patient with an inadequate response to initial biologic therapy, several therapeutic options exist. Current evidence supports TNFi dose escalation for only infliximab; optimization of concurrent conventional synthetic disease-modifying antirheumatic drug (csDMARD) or switching to a different csDMARD are other options. Cycling (switching to an alternative TNFi) and swapping (switching to a therapy with a different mode of action) strategies are other alternate approaches supported by many observational studies. While no head-to-head trials exist directly comparing the 2 strategies, data suggest superiority of the swapping strategy over the cycling approach. Also, several studies have shown that switching to a drug with a different mechanism of action is associated with higher treatment persistence and lower health care costs than TNFi cycling.
- Conclusion: Physicians have a growing list of treatment options to help their patients with RA achieve disease remission. The choice of best treatment for a given patient needs to be individualized, keeping in mind other factors, including comorbidities.
Keywords: biologics; rheumatoid arthritis; swapping strategy; cycling strategy; TNF inhibitors.
Following the discovery of tumor necrosis factor (TNF) as a proinflammatory cytokine 30 years ago, the use of TNF antagonists has revolutionized the treatment of rheumatoid arthritis (RA). Although TNF inhibitors (TNFIs) are frequently used as a first-line biologic disease-modifying antirheumatic drug (bDMARD), they are not uniformly efficacious in achieving remission in all patients with RA. This article highlights the reasons for such variability in observed response and discusses therapeutic options for patients who do not respond to TNFi therapy.
Case Presentation
A 60-year-old woman is evaluated in the clinic for complaints of pain in her hands, morning stiffness lasting 2 hours, and swelling in her wrists, all of which have been ongoing for 3 months. Physical exam reveals evidence of active inflammation, with synovitis in her second, third, and fourth metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints bilaterally, swelling over both wrists, and a weak grip. Inflammatory markers are elevated, and rheumatoid factor and anti-cyclic citrullinated peptide (anti-CCP) are both positive at high titer. Radiographs reveal evidence of small erosions at the third and fourth MCPs and PIPs bilaterally and periarticular osteopenia. The patient is diagnosed with seropositive, erosive RA based on history, physical exam, laboratory studies, and imaging. She is started on 20 mg of prednisone for acute treatment of her symptoms along with methotrexate, and, initially, her symptoms are well controlled. A few months after starting treatment, she develops voluminous diarrhea that necessitates cessation of methotrexate. Leflunomide also causes similar symptoms. The combination of sulfasalazine and hydroxychloroquine does not adequately control her symptoms, and ongoing use of low-dose glucocorticoids is required to improve functionality in all joints. Using the treat-to-target (T2T) strategy, adalimumab is initiated. However, she continues to report persistent swelling and pain and still requests oral glucocorticoids to help decrease inflammation. The 28-joint Disease Activity Score (DAS28) is 4.8, suggestive of moderate disease activity.
Why are TNFi agents sometimes ineffective?
The introduction of monoclonal antibodies and fusion proteins to block TNF and other cytokines was a remarkable development in the treatment of RA that revolutionized patient care. Despite the efficacy of TNFis, clinical response to these agents is not universal and only some patients achieve complete remission. In targeting the eventual goal of remission or low disease activity in patients with RA, the concept of “TNF failure” becomes extremely relevant. These inadequate responses to anti-TNF therapy may be due to primary failures, or complete lack of clinical response after initiation of the bDMARD, and secondary failures, or the loss of initially achieved clinical response to therapy. Other reasons for discontinuation of a given TNFi include partial disease control and intolerance to the medication (possible injection-site or infusion reactions). Keystone and Kavanaugh1 divided causes of failure of TNF agents into 2 broad categories: perceptual (related to natural variations in disease course like hormonal variation and physical and emotional stress) and pathophysiological failures (genetic variations, high body mass index, concomitant cigarette use).
Another important consideration in patients treated with a TNFi is the consequent formation of anti-drug antibodies (ADAs). TNFi agents are immunogenic and normally elicit an immune response. The appearance of such ADAs may reduce the bioavailability of free drug, resulting in a decreased clinical response,2 or may lead to serious adverse effects.
How common is discontinuation of the first TNFi?
Several studies have reported that the prevalence of primary failure, secondary failure, and intolerance to TNFis ranges from 30% to 40%.3-6 Female sex,7 concurrent prednisone use,8 high disease activity scores,6,8,9 and the absence of treatment with low-dose methotrexate7,8 have all been shown to be negative predictors of bDMARD retention and response.10
Are there any factors that predict TNFi failure?
There are no specific parameters to accurately predict responses to TNFI therapy.11 Several clinical and molecular biomarkers in synovium (initial TNF levels, macrophages, T cells)12 and peripheral blood (serum myeloid-related protein 8 and 14 complex levels,13 prealbumin, platelet factor 4, and S100A12)14 have been described as predictors of clinical response to TNFis, but their utility in clinical practice has not been established and the use of these markers has not yet been incorporated into clinical guidelines.
How is disease activity measured in patients with RA?
In 2010 an international expert consensus panel published treatment recommendations for RA that emphasized a T2T strategy of individualizing and escalating treatment to achieve the lowest disease activity or remission. In clinical practice, numerous tools are available to measure RA disease activity. Herein, we mention several that are most commonly used in clinical practice.
DAS28 combines single activity measures into an overall continuous measure of disease activity and has been endorsed by both the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR). It includes a 28-swollen joint count (SJC), 28-tender joint count (TJC), erythrocyte sedimentation rate (ESR; can also be calculated using C-reactive protein [CRP]), and a patient global assessment (PtGA). The cut-offs used for DAS28 interpretation are as follows: remission (< 2.6), low (≥ 2.6 but ≤ 3.2), moderate (> 3.2 but ≤ 5.1), or high (> 5.1).15 Some of the difficulties in using DAS28 in daily clinical practice include the need for a lab value and the time needed to perform the joint counts. Note also that due to the inclusion of ESR, which is influenced by age and other factors, DAS28 may underestimate remission in the elderly.
Another measure of RA disease activity is the Simplified Disease Activity Index (SDAI), which includes 28 SJC, 28 TJC, PtGA, provider global assessment (PrGA), and CRP in mg/dL. The level of disease activity using the SDAI is interpreted as: remission (SDAI ≤ 3.3), low (≥ 3.4 but ≤ 11), moderate (> 11 but ≤ 26), or high (> 26). The advantage of the SDAI is that a calculator or computer is not required for calculations. Another measure, the Clinical Disease Activity Index (CDAI), includes a 28 SJC, 28 TJC, PtGA, and PrGA. Because a laboratory value is not needed to calculate the CDAI, it is well-suited for use in clinical practice. When using the CDAI, the level of disease activity can be defined as remission (CDAI ≤ 2.8), low (> 2.8 but ≤ 10), moderate (> 10 but ≤ 22), or high (> 22). Again, as with the SDAI, a calculator or computer is not needed for calculations.
What are the alternative treatment options after first biologic failure?
In patients who have failed treatment with an initial biologic, usually a TNFi, the treating rheumatologist has the following options (Figure), with the best treatment strategy being driven by individualized patient and disease-related factors (Table 1 and Table 2):
- TNFi dose escalation
- Trial of an alternate TNFi agent (the “cycling” strategy)
- Optimization of therapy conjoined with a conventional synthetic DMARD (csDMARD)
- Use of a non-TNF biologic or targeted synthetic DMARD (the “swapping” strategy)
If all the listed strategies fail, the next step can be the addition of short-term, low-dose glucocorticoid therapy.
TNFi Dose Escalation
The available data have demonstrated the safety, efficacy, and cost-effectiveness of dose escalation in patients with RA receiving infliximab.16-18 The ATTRACT trial first demonstrated this, with greater clinical and radiographic improvements in those with higher trough serum concentrations, suggesting that doses higher than 3 mg/kg or more frequent than every 8 weeks may be needed for full response in some patients.19
There is a lack of studies in RA patients to determine the most effective dose escalation strategy. A study in patients with Crohn disease showed that intensification to 10 mg/kg every 8 weeks (dose doubling) was at least as effective as 5 mg/kg every 4 weeks (halving interval) at 12 months.16 Due to greater patient and administration convenience of dose-doubling, this strategy may be preferred.17 A starting dose of 10 mg/kg every 8 weeks is not routinely recommended due to an increased risk of serious infection; these adverse events were not found when the dose was gradually increased, as clinically indicated, starting at 3 mg/kg.19,20 Further studies are needed to explore this approach in RA patients.
These results, however, have not been replicated with other TNFi agents. No significant clinical improvements were identified with etanercept 50 mg twice weekly,21 adalimumab 40 mg every week in the PREMIER trial,18 or certolizumab 400 mg every other week in an open-label extension phase of the RAPID 1 study.22 A Japanese study found significantly worse clinical outcomes with dose escalation of golimumab.23 Conversely, 2 studies found clinical benefits after escalating the tocilizumab dose, the first a real-world review from the Consortium of Rheumatology Researchers of North America (CORRONA) registry using the intravenous formulation,24 and the other the BREVACTA study utilizing subcutaneous tocilizumab.25 No studies to date have been published on dose escalation of abatacept in patients with RA who respond poorly. Overall, previous studies support dose escalation in individuals being treated with infliximab to improve clinical outcomes, but additional studies are needed for other bDMARDs.
Trial of an Alternate TNF Agent: The “Cycling” Strategy
Per the ACR/EULAR26,27 guidelines, all approved bDMARDs may be used without hierarchical positioning. However, after the failure of a TNFi agent, these guidelines do not provide specific advice about a preference between the “cycling” strategy (switching to an alternative TNFi) and “swapping” strategy (switching to a therapy with a different mode of action). Cycling might work for several reasons, including differences in the agents’ molecular structure, immunological mechanism of action, immunogenicity, and pharmacokinetics.28-30 The cycling strategy is a well-established approach adopted by more than 94% of practicing rheumatologists, according to a national survey,31 and its efficacy is supported by trials and additional observational studies.32-35
The greater clinical effectiveness of switching to infliximab compared with continuing with etanercept in patients with inadequate response to etanercept (n = 28) was suggested in the open-label OPPOSITE trial.36 Data from the GO-AFTER trial37 suggests that a greater proportion of patients with RA refractory to adalimumab, etanercept, or infliximab who were treated with golimumab achieved an ACR20 and ACR50 response compared with patients who received placebo, and this response persisted through 5 years.38 More recently, certolizumab pegol and adalimumab were compared head-to-head in the EXXELERATE trial.39 The results of this trial revealed the adequate efficacy of cycling to another TNFi after primary insufficient response to the first.
In studies from Finland and Sweden,35,40 it has been observed that a better response is achieved in patients in whom TNF failure was initially due to secondary failure or intolerance rather than primary failure. A post-hoc analysis of the results of the GO-AFTER trial41 and from a few observational studies35,40,42 revealed that switching from one TNFi to another, especially from a monoclonal antibody to a soluble receptor, was often more beneficial for RA patients than switching from a soluble receptor to a monoclonal antibody.
Optimization of Therapy Conjoined with csDMARDs
Methotrexate is one of the oldest and most effective csDMARDs available for the treatment of RA.43 The 2016 EULAR guidelines recommend the addition of methotrexate and/or other csDMARDs to potentiate the effect of bDMARDs.26 In the case of TNFi therapy, the observed synergistic effect between the monoclonal antibody and methotrexate may be explained by sustained suppression of ADA formation.44 In the TEMPO,45 PREMIER,18 and GO-BEFORE46 trials, the addition of methotrexate led to improved clinical and radiological outcomes in patients treated with etanercept, adalimumab, and golimumab,47 respectively. These findings were also demonstrated in several registries, where significant improvement in clinical response and retention rate of the TNFi agents was noted. Results have been replicated with non-TNFi bDMARDs, including abatacept48,49 and rituximab.50 Patients treated with interleukin (IL)-6 inhibitors in combination with methotrexate have shown significantly less radiographic progression compared to those treated with tocilizumab alone and those treated with monotherapy tocilizumab versus monotherapy methotrexate.51,52 Results possibly favor the use of IL-6 inhibitors alone in those who cannot tolerate or have contraindications to methotrexate.
An open prospective study by Cohen et al added methotrexate to the treatment regimens of individuals on bDMARD monotherapy with a primary failure and found favorable changes in ACR20 and DAS28 scores at 3 and 12 months and therapeutic biological response (ESR, CRP) at 3 months.53 Unlike monotherapy, in these situations methotrexate is known to be efficacious even at a lower dose, possibly at 7.5 mg to 10 mg per week. Some studies have shown that methotrexate administered parenterally may be more efficacious than when given orally.54-58
In clinical trials and observational studies, leflunomide, sulfasalazine, and hydroxychloroquine have been used as alternate csDMARDs added to the treatment regimen.59-62 There are, however, only 2 trials comparing the efficacy of methotrexate with that of other csDMARDs as concomitant treatment in patients with inadequate response to TNFi therapy. The RABBIT trial found a slight decrease in effectiveness with concomitant TNFi and leflunomide compared to TNFi/methotrexate, but overall each group had similar EULAR responses at 24 months.63 A study by De Stefano et al found comparable ACR20 and DAS28 responses among individuals receiving TNFis with methotrexate or leflunomide.61
The “Swapping” Strategy
The efficacy of the swapping strategy has been shown in 3 randomized clinical trials demonstrating the superiority of abatacept, tocilizumab, and rituximab in the treatment of individuals with RA refractory to TNFis. Tocilizumab was studied in the RADIATE64 trial, which involved 499 patients with inadequate response to 1 or more TNFi agents. The primary endpoint (24-week ACR20) was achieved by 50.0%, 30.4%, and 10.1% of patients in the 8 mg/kg, 4 mg/kg, and control groups, respectively (P < 0.001 for both tocilizumab groups versus placebo). The utility of abatacept as second-line therapy after initial TNF failure was evaluated in the ATTAIN65 study. Participants with an inadequate response to etanercept or infliximab were randomly assigned to receive either abatacept or placebo. ACR50 response rates after 6 months of treatment were 20.3% with abatacept and 3.8% with placebo (P < 0.001). The SWITCH-RA study,66 an observational study, compared rituximab to TNFis in 1112 participants with inadequate response to initial anti-TNF therapy. At 6 months, mean change in DAS28 was small but significantly greater for the rituximab group (–1.5 vs –1.1; P = 0.007). The difference in response rates was greatest among seropositive patients. These data suggest that rituximab has efficacy following TNFi failure, particularly for seropositive patients. Additionally, REFLEX67 is the sole randomized controlled trial in patients with insufficient response to TNFis that showed significant prevention of radiographic progression at week 56 in patients on rituximab compared to placebo (mean change from baseline in total Genant-modified Sharp score, 1.00 vs 2.31, respectively; P = 0.005).
One study randomly assigned 399 patients with active RA who had inadequate response to prior TNFi therapy to tofacitinib68 (5 mg twice daily or 10 mg twice daily) or placebo, both with methotrexate.6 After 3 months of treatment, ACR20 response rates (41.7% for 5 mg, 28.1% for 10 mg, 24.4% for placebo) and DAS28 remission rates (6.7% for 5 mg, 8.8% for 10 mg, 1.7% for placebo) were significantly greater among patients treated with tofacitinib compared to those treated with placebo. More recently, the RA-BEACON trial69 demonstrated a consistent, beneficial treatment effect of baricitinib in patients with insufficient response to 1 or more TNFis. In this trial, 527 patients with an inadequate response to bDMARDs were randomly assigned to receive baricitinib 2 mg or 4 mg daily or placebo for 24 weeks. A higher proportion of patients receiving baricitinib 4 mg had an ACR20 response at week 12 compared with those treated with placebo (55% vs 27%, P < 0.001), and patients receiving the 4-mg dose had significant improvements from baseline in DAS28 and Health Assessment Questionnaire–Disability Index scores (P < 0.001 for both comparisons).
To Cycle or to Swap?
Several observational studies (SCQM-RA,70 STURE,71 BSRBR,72 Favalli,43 MIRAR,73 SWITCH-RA,74 ROC72) have clearly demonstrated that the swapping strategy is favored over the cycling strategy. In the ROC study,72 patients were randomly assigned (based on physician discretion) to receive a non-TNF biologic or a TNFi. More patients in the non-TNF group than in the TNFi group showed low disease activity at week 24 (45% vs 28%; odds ratio [OR], 2.09; 95% confidence interval [CI], 1.27-3.43; P = 0.004) and at week 52 (41% vs 23%; OR, 2.26; 95% CI, 1.33-3.86; P = 0.003). The authors concluded that in patients having an insufficient response to TNFi therapy, a non-TNF biologic agent may be more effective than a second TNFi drug. Only a few studies75-77 have demonstrated similar results between the 2 strategies. Overall, the available evidence seems to suggest the superiority of the swapping over the cycling strategy.
An important clinical pearl to keep in mind is that both swapping and cycling strategies might theoretically increase the risk of infection; however, limited evidence is reported in the literature. In a large retrospective analysis78 of data on 4332 RA patients from a large US claims database, patients who had cycled between TNFi agents had a 30% to 40% increased risk of infection compared to patients treated with rituximab. Patients on infliximab had a 62% higher hazard of severe infections, and this has also been reported in an observational study.79 In another study,70 41% of 201 patients with RA followed between 1999 and 2013 who swapped to abatacept/rituximab or tocilizumab developed adverse events, as compared to 59% of those who switched to a second TNFi.
What are recent trends in the use of bDMARDs?
Currently, there are no specific guidelines or biomarkers available to facilitate selection of specific treatment from among the classes of biologics. With the development of several new drugs and regulatory approval of baricitinib, physicians now have several biologic options to treat patients. A recent large time-trend study80 deriving data from more than 200,000 patients with RA showed that etanercept remains the most frequently used agent for the treatment of RA; it also showed that the use of adalimumab and infliximab is decreasing, and that the use of newer agents, especially abatacept, golimumab, and certolizumab, has considerably risen in recent years. In this study, abatacept, rituximab, certolizumab, golimumab, tocilizumab, and tofacitinib accounted for 13.2%, 13.8%, 6.9%, 11.9%, and 7.5% switches from first TNFi therapy.
Jin et al81 studied factors associated with the choice of bDMARD for initial and subsequent use. They found that patients with commercial insurance had an 87% higher likelihood of initiating a bDMARD. In the Medicaid subgroup, African Americans had lower odds of initiating and switching bDMARDs than non-Hispanic whites. Prior use of steroids and nonbiologic DMARDs predicted both bDMARD initiation and subsequent switching. Etanercept, adalimumab, and infliximab were the most commonly used first- and second-line bDMARDS; patients on anakinra and golimumab were most likely to be switched to other bDMARDs.
Which treatment strategy is the most cost-effective?
Several studies have reported better treatment persistence rates among patients who are treated with the swapping strategy compared to the cycling strategy. In a retrospective analysis of claims data,82 the authors examined treatment persistence and health care costs in patients switching to biologics with a different mechanism of action or cycling to another TNFi. The mean cost was significantly lower among patients treated using the swapping strategy than among the TNFi cyclers, both for the total cost of care for RA and for the total cost of the targeted DMARDs in the first year after the change in therapy. The authors concluded that switching to a drug with a different mechanism of action is associated with higher treatment persistence and lower health care costs than TNFi cycling.
What about biosimilars?
Biosimilars are copies of already licensed biologics that are very similar to the biologics, but are made by different sponsors using independently derived cell lines and separately developed manufacturing processes.83 Regarding biosimilar use, EULAR26 states that biosimilar bDMARDs approved by the European Medicines Agency or US Food and Drug Administration have similar efficacy and safety as the originator bDMARDs, and recommends them as preferred agents if they are indeed appreciably cheaper than originator or other bDMARDs.
What are the novel treatment targets in RA?
New therapeutics for RA continue to be developed. One of the new agents is peficitinib (ASP015K), an oral, once-daily Janus kinase (Jak) inhibitor targeting Jak-1, Jak-2, and tyrosine kinase-2, with moderate selectivity for Jak-3. In a phase 2b trial, 100-mg and 150-mg doses of peficitinib achieved a statistically significant ACR20 response (48.3% and 56.3%) compared to placebo (29.4%) at 12 weeks.84
Given the benefit of targeting TNF-α and IL-17 in RA, a novel molecule (ABT-122) that targets both human TNF and IL-17 has been developed. Two phase 1 studies85 showed that dual neutralization of TNF and IL-17 with ABT-122 has characteristics acceptable for further exploration of therapeutic potential of this agent in TNF- and IL-17A–driven immune-mediated inflammatory diseases. Another novel drug is mavrilimumab, a human monoclonal antibody that targets granulocyte–macrophage colony-stimulating factor receptor α. A recent studyshowed that long-term treatment with mavrilimumab maintained response and was well-tolerated, with no increased incidence of treatment-emergent adverse events.86
Namilumab (AMG203) is an immunoglobulin G1 monoclonal antibody that binds with high affinity to the GM-CSF ligand. In a phase 1b, randomized, double-blind study (PRIORA)87 to assess namilumab in treating active, mild-to-moderate RA, significant improvement was seen in the DAS28-CRP score with namilumab (150 and 300 mg groups combined) compared with placebo at day 43 (P = 0.0117) and also 8 weeks after last dosing at day 99 (P = 0.0154). Adverse events were similar across different doses of namilumab and placebo, and included nasopharyngitis and exacerbation/worsening of RA. Another drug showing promise in RA is fosdagrocorat (PF-04171327), a potential dissociated agonist of the glucocorticoid receptor. A multicenter, double-blind, parallel-group, active- and placebo-controlled phase 2 study randomly assigned 86 patients to receive fosdagrocorat 10 mg, fosdagrocorat 25 mg, prednisone 5 mg, or placebo, all with stable background methotrexate therapy.88 Both fosdagrocorat doses demonstrated efficacy in improving signs and symptoms in RA patients, with manageable adverse events.
Case Conclusion
There are several available treatment options for the case patient. Based on the PREMIER trial, solely increasing the dose of adalimumab is unlikely to provide a therapeutic benefit. Adding low-dose methotrexate (possibly via a parenteral route because of patient-reported gastrointestinal discomfort) might provide some synergistic and therapeutic effect. However, because of primary failure with TNFi therapy, she may benefit from the initiation of a biologic with a different mechanism of action (ie, swapping strategy). Therapeutic options include tocilizumab, abatacept, rituximab, and the Jak inhibitors (tofacitinib and baricitinib).
Summary
The optimal treatment of RA aims at achieving, and then maintaining, remission or a low disease activity. The choice of best treatment must be individualized to the patient, keeping in mind other factors, including comorbidities like fibromyalgia, history of diverticulitis (prior to use of tocilizumab), history of chronic obstructive pulmonary disease (prior to the use of abatacept), malignancy, and the presence of risk factors for infections (age, diabetes, chronic bronchitis). In a patient with inadequate response to initial biologic therapy, several options exist for the rheumatologist. Current evidence supports TNFi dose escalation for only infliximab; optimization of concurrent csDMARD or switching to a different csDMARD are other options. Cycling and swapping are other alternate approaches supported by many observational studies. While no head-to-head trials exist comparing the 2 strategies, data suggest superiority of the swapping strategy over the cycling approach. With the continuing development of novel therapeutics in RA, physicians have a growing list of treatment options to help their patients achieve disease remission.
Corresponding author: Namrata Singh, MD, 200 Hawkins Drive, Iowa City, IA 52242.
Financial disclosures: None.
1. Keystone ED, Kavanaugh KA. What to do with TNF failures. Expert Opin Drug Saf. 2005;4:149-155.
2. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707-718.
3. Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253-259.
4. Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50(5):1400-1411.
5. Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594-1602.
6. Finckh A, Simard JF, Gabay C, et al. Evidence for differential acquired drug resistance to anti-tumour necrosis factor agents in rheumatoid arthritis. Ann Rheum Dis. 2006;65:746-752.
7. Souto A, Maneiro JR, Gomez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology. 2016;55:523-534.
8. Hetland ML, Christensen IJ, Tarp U, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010;62:22-32.
9. Gabay C, Riek M, Scherer A, et al. Effectiveness of biologic DMARDs in monotherapy versus in combination with synthetic DMARDs in rheumatoid arthritis: data from the Swiss Clinical Quality Management Registry. Rheumatology. 2015;54(9):1664-1672.
10. Ebina K, Hashimoto M, Yamamoto W, et al. Drug retention and discontinuation reasons between seven biologics in patients with rheumatoid arthritis-The ANSWER cohort study. PloS One. 2018;13:e0194130.
11. Wijbrandts CA, Tak PP. Prediction of response to targeted treatment in rheumatoid arthritis. Mayo Clin Proc. 2017;92:1129-1143.
12. Ulfgren AK, Andersson U, Engstrom M, et al. Systemic anti-tumor necrosis factor alpha therapy in rheumatoid arthritis down-regulates synovial tumor necrosis factor alpha synthesis. Arthritis Rheum. 2000;43:2391-2396.
13. Choi IY, Gerlag DM, Herenius MJ, et al. MRP8/14 serum levels as a strong predictor of response to biological treatments in patients with rheumatoid arthritis. Ann Rheum Dis. 2015;74:499-505.
14. Nguyen MVC, Baillet A, Romand X, et al. Prealbumin, platelet factor 4 and S100A12 combination at baseline predicts good response to TNF alpha inhibitors in rheumatoid arthritis. Joint Bone Spine. 2019;86:195-201.
15. Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28-Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score-II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), Chronic Arthritis Systemic Index (CASI), Patient-Based Disease Activity Score With ESR (PDAS1) and Patient-Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI-RA). Arthritis Care Res. 2011;63(suppl 11):S14-S36.
16. Katz L, Gisbert JP, Manoogian B, et al. Doubling the infliximab dose versus halving the infusion intervals in Crohn’s disease patients with loss of response. Inflamm Bowel Dis. 2012;18:2026-2033.
17. Durez P, Van den Bosch F, Corluy L, et al. A dose adjustment in patients with rheumatoid arthritis not optimally responding to a standard dose of infliximab of 3 mg/kg every 8 weeks can be effective: a Belgian prospective study. Rheumatology. 2005;44:465-468.
18. Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26-37.
19. St Clair EW, Wagner CL, Fasanmade AA, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:1451-1459.
20. Rahman MU, Strusberg I, Geusens P, et al. Double-blinded infliximab dose escalation in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:1233-1238.
21. Weinblatt ME, Schiff MH, Ruderman EM, et al. Efficacy and safety of etanercept 50 mg twice a week in patients with rheumatoid arthritis who had a suboptimal response to etanercept 50 mg once a week: results of a multicenter, randomized, double-blind, active drug-controlled study. Arthritis Rheum. 2008;58:1921-1930.
22. Curtis JR, Chen L, Luijtens K, et al. Dose escalation of certolizumab pegol from 200 mg to 400 mg every other week provides no additional efficacy in rheumatoid arthritis: an analysis of individual patient-level data. Arthritis Rheum. 2011;63:2203-2208.
23. Okazaki M, Kobayashi H, Ishii Y, et al. Real-world treatment patterns for golimumab and concomitant medications in Japanese rheumatoid arthritis patients. Rheumatol Ther. 2018;5:185-201.
24. Pappas DA, John A, Curtis JR, et al. Dosing of intravenous tocilizumab in a real-world setting of rheumatoid arthritis: analyses from the Corrona Registry. Rheumatol Ther. 2016;3:103-115.
25. Kivitz A, Olech E, Borofsky MA, et al. Two-year efficacy and safety of subcutaneous tocilizumab in combination with disease-modifying antirheumatic drugs including escalation to weekly dosing in rheumatoid arthritis. J Rheumatol. 2018;45:456-464.
26. Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960-977.
27. Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2016;68:1-25.
28. Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11:81-88.
29. Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:493-507.
30. Navarro Coy NC, Brown S, Bosworth A, et al. The ‘Switch’ study protocol: a randomised-controlled trial of switching to an alternative tumour-necrosis factor (TNF)-inhibitor drug or abatacept or rituximab in patients with rheumatoid arthritis who have failed an initial TNF-inhibitor drug. BMC Musculoskelet Disord. 2014;15:452.
31. Kamal KM, Madhavan SS, Hornsby JA, et al. Use of tumor necrosis factor inhibitors in rheumatoid arthritis: a national survey of practicing United States rheumatologists. Joint Bone Spine. 2006;73:718-724.
32. Gomez-Reino JJ, Carmona L, BIOBADASER Group. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006;8:R29.
33. Caporali R, Sarzi-Puttini P, Atzeni F, et al. Switching TNF-alpha antagonists in rheumatoid arthritis: The experience of the LORHEN registry. Autoimmun Rev. 2010;9:465-469.
34. Iannone F, Trotta F, Monteccuco C, et al. Etanercept maintains the clinical benefit achieved by infliximab in patients with rheumatoid arthritis who discontinued infliximab because of side effects. Ann Rheum Dis. 2007;66:249-252.
35. Virkki LM, Valleala H, Takakubo Y, et al. Outcomes of switching anti-TNF drugs in rheumatoid arthritis—a study based on observational data from the Finnish Register of Biological Treatment (ROB-FIN). Clin Rheumatol. 2011;30:1447-1454.
36. Furst DE, Gaylis N, Bray V, et al. Open-label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: the opposite study. Ann Rheum Dis. 2007;66:893-899.
37. Smolen JS, Kay J, Doyle MK, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2009;374:210-221.
38. Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor α inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.
39. Smolen JS, Burmester G-R, Combe B, et al. Head-to-head comparison of certolizumab pegol versus adalimumab in rheumatoid arthritis: 2-year efficacy and safety results from the randomised EXXELERATE study. Lancet. 2016;388:2763-2774.
40. Chatzidionysiou K, Askling J, Eriksson J, et al. Effectiveness of TNF inhibitor switch in RA: results from the national Swedish register. Ann Rheum Dis. 2015;74:890.
41. Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor alpha inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.
42. Lequerré T, Farran É, Ménard J-F, et al. Switching from an anti-TNF monoclonal antibody to soluble TNF-receptor yields better results than vice versa: An observational retrospective study of 72 rheumatoid arthritis switchers. Joint Bone Spine. 2015;82:330-337.
43. Favalli EG, Biggioggero M, Meroni PL. Methotrexate for the treatment of rheumatoid arthritis in the biologic era: Still an “anchor” drug? Autoimmun Rev. 2014;13:1102-1108.
44. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707-718.
45. Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675-681.
46. Emery P, Fleischmann RM, Strusberg I, et al. Efficacy and safety of subcutaneous golimumab in methotrexate-naive patients with rheumatoid arthritis: five-year results of a randomized clinical trial. Arthritis Care Res. 2016;68:744-752.
47. Emery P, Fleischmann RM, Moreland LW, et al. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 2009;60:2272-2283.
48. Emery P, Burmester GR, Bykerk VP, et al. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis. 2015;74:19-26.
49. Westhovens R, Robles M, Ximenes AC, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis. 2009;68:1870-1877.
50. Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793-2806.
51. Burmester GR, Rigby WF, van Vollenhoven RF, et al. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann Rheum Dis. 2016;75:1081-1091.
52. Bijlsma JWJ, Welsing PMJ, Woodworth TG, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet. 2016;388:343-355.
53. Cohen JD, Zaltni S, Kaiser MJ, et al. Secondary addition of methotrexate to partial responders to etanercept alone is effective in severe rheumatoid arthritis. Ann Rheum Dis. 2004;63:209-210.
54. Hamilton RA, Kremer JM. Why intramuscular methotrexate may be more efficacious than oral dosing in patients with rheumatoid arthritis. Br J Rheumatol. 1997;36:86-90.
55. Hoekstra M, Haagsma C, Neef C, et al. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J Rheumatol. 2004;31:645-648.
56. Herman RA, Veng-Pedersen P, Hoffman J, et al. Pharmacokinetics of low-dose methotrexate in rheumatoid arthritis patients. J Pharm Sci. 1989;78:165-171.
57. Schiff MH, Jaffe JS, Freundlich B. Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses ± 15 mg may be overcome with subcutaneous administration. Ann Rheum Dis. 2014;73:1549-1551.
58. Hazlewood GS, Thorne JC, Pope JE, et al. The comparative effectiveness of oral versus subcutaneous methotrexate for the treatment of early rheumatoid arthritis. Ann Rheum Dis. 2016;75:1003-1008.
59. O’Dell JR, Petersen K, Leff R, et al. Etanercept in combination with sulfasalazine, hydroxychloroquine, or gold in the treatment of rheumatoid arthritis. J Rheumatol. 2006;33:213-218.
60. Finckh A, Dehler S, Gabay C. The effectiveness of leflunomide as a co-therapy of tumour necrosis factor inhibitors in rheumatoid arthritis: a population-based study. Ann Rheum Dis. 2009;68:33-39.
61. De Stefano R, Frati E, Nargi F, et al. Comparison of combination therapies in the treatment of rheumatoid arthritis: leflunomide-anti-TNF-alpha versus methotrexate-anti-TNF-alpha. Clin Rheumatol. 2010;29:517-524.
62. Combe B, Codreanu C, Fiocco U, et al. Etanercept and sulfasalazine, alone and combined, in patients with active rheumatoid arthritis despite receiving sulfasalazine: a double-blind comparison. Ann Rheum Dis. 2006;65:1357-1362.
63. Strangfeld A, Hierse F, Kekow J, et al. Comparative effectiveness of tumour necrosis factor α inhibitors in combination with either methotrexate or leflunomide. Ann Rheum Dis. 2009;68:1856.
64. Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516.
65. Genovese MC, Becker J-C, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor α inhibition. N Engl J Med. 2005;353:1114-1123.
66. Emery P, Gottenberg JE, Rubbert-Roth A, et al. Rituximab versus an alternative TNF inhibitor in patients with rheumatoid arthritis who failed to respond to a single previous TNF inhibitor: SWITCH-RA, a global, observational, comparative effectiveness study. Ann Rheum Dis. 2015;74:979-984.
67. Keystone E, Emery P, Peterfy CG, et al. Rituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapies. Ann Rheum Dis. 2009;68:216.
68. Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451-460.
69. Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374:1243-1252.
70. Favalli EG, Biggioggero M, Marchesoni A, Meroni PL. Survival on treatment with second-line biologic therapy: a cohort study comparing cycling and swap strategies. Rheumatology. 2014;53:1664-1668.
71. Harrold LR, Reed GW, Solomon DH, et al. Comparative effectiveness of abatacept versus tocilizumab in rheumatoid arthritis patients with prior TNFi exposure in the US Corrona registry. Arthritis Res Ther. 2016;18:280.
72. Gottenberg J, Brocq O, Perdriger A, et al. Non–TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug: A randomized clinical trial. JAMA. 2016;316:1172-1180.
73. Pascart T, Philippe P, Drumez E, et al. Comparative efficacy of tocilizumab, abatacept and rituximab after non-TNF inhibitor failure: results from a multicentre study. Int J Rheum Dis. 2016;19:1093-1102.
74. Akiyama M, Kaneko Y, Kondo H, Takeuchi T. Comparison of the clinical effectiveness of tumour necrosis factor inhibitors and abatacept after insufficient response to tocilizumab in patients with rheumatoid arthritis. Clin Rheumatol. 2016;35:2829-2834.
75. Schoels M, Aletaha D, Smolen JS, Wong JB. Comparative effectiveness and safety of biological treatment options after tumour necrosis factor α inhibitor failure in rheumatoid arthritis: systematic review and indirect pairwise meta-analysis. Ann Rheum Dis. 2012;71:1303.
76. Soliman MM, Hyrich KL, Lunt M, et al. Rituximab or a second anti-tumor necrosis factor therapy for rheumatoid arthritis patients who have failed their first anti-tumor necrosis factor therapy? Comparative analysis from the British Society for Rheumatology Biologics Register. Arthritis Care Res. 2012;64:1108-1115.
77. Chatzidionysiou K, Vollenhoven RF. Rituximab versus anti-TNF in patients who previously failed one TNF inhibitor in an observational cohort. Scand J Rheumatol. 2013;42:190-195.
78. Johnston SS, Turpcu A, Shi N, et al. Risk of infections in rheumatoid arthritis patients switching from anti-TNF agents to rituximab, abatacept, or another anti-TNF agent, a retrospective administrative claims analysis. Semim Arthritis Rheum. 2013;43:39-47.
79. Curtis JR, Xie F, Chen L, et al. The comparative risk of serious infections among rheumatoid arthritis patients starting or switching biological agents. Ann Rheum Dis. 2011;70:1401.
80. Desai RJ, Solomon DH, Jin Y, et al. Temporal trends in use of biologic DMARDs for rheumatoid arthritis in the United States: a cohort study of publicly and privately insured patients. J Manag Care Spec Pharm. 2017;23:809-814.
81. Jin Y, Desai RJ, Liu J, et al. Factors associated with initial or subsequent choice of biologic disease-modifying antirheumatic drugs for treatment of rheumatoid arthritis. Arthritis Res Ther. 2017;19:159.
82. Bonafede MMK, McMorrow D, Proudfoot C, et al. Treatment persistence and healthcare costs among patients with rheumatoid arthritis after a change in targeted therapy. Am Health Drug Benefits. 2018;11:192-202.
83. US Food and Drug Administration. Biosimilars are safe, effective treatment options. www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/biosimilars/. Accessed November 9, 2018.
84. Genovese MC, Greenwald M, Codding C, et al. Peficitinib, a JAK inhibitor, in combination with limited conventional synthetic disease-modifying antirheumatic drugs in the treatment of moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2017;69:932-942.
85. Fleischmann RM, Wagner F, Kivitz AJ, et al. Safety, tolerability, and pharmacodynamics of ABT-122, a tumor necrosis factor- and interleukin-17-targeted dual variable domain immunoglobulin, in patients with rheumatoid arthritis. Arthritis Rheumatol. 2017;69:2283-2291.
86. Burmester GR, McInnes IB, Kremer JM, et al. Mavrilimumab, a fully human granulocyte-macrophage colony-stimulating factor receptor alpha monoclonal antibody: long-term safety and efficacy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2018;70:679-689.
87. Huizinga TW, Batalov A, Stoilov R, et al. Phase 1b randomized, double-blind study of namilumab, an anti-granulocyte macrophage colony-stimulating factor monoclonal antibody, in mild-to-moderate rheumatoid arthritis. Arthritis Res Ther. 2017;19:53.
88. Stock T, Fleishaker D, Wang X, et al. Improved disease activity with fosdagrocorat (PF-04171327), a partial agonist of the glucocorticoid receptor, in patients with rheumatoid arthritis: a Phase 2 randomized study. Int J Rheum Dis. 2017;20:960-970.
89. Orencia [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2013.
90. Humira[package insert]. North Chicago, IL: AbbVie; 2012.
91. Kineret [package insert]. Stockholm, Sweden: Sobi; 2012.
92. Olumiant [package insert]. Indianapolis, IN: Lilly USA, LLC; 2018.
93. Cimzia [package insert]. Smyrna, GA: UCB, Inc; 2008.
94. Enbrel [package insert]. Thousand Oaks, CA: Immunex Corporation; 1998.
95. Simponi [package insert]. Horsham, PA: Janssen Biotech, Inc; 2009.
96. Remicade [package insert]. Horsham, PA: Janssen Biotech, Inc; 1998.
97. Rituxan [package insert]. South San Francisco, CA: Genetech, Inc; 1997.
98. Kevzara [package insert]. Bridgewater, NJ: Sanofi-Aventis US LLC; 2018.
99. Actemra [package insert]. South San Francisco, CA: Genentech, Inc; 2013.
100. Xeljanz [package insert]. New York, NY: Pfizer Inc; 2016.
From the University of Iowa Hospitals and Clinics, Iowa City, IA.
Abstract
- Objective: To discuss the variability in response to tumor necrosis factor inhibitors (TNFis) observed in patients with rheumatoid arthritis (RA) and discuss therapeutic options for patients who do not respond to initial TNFi therapy.
- Methods: Review of the literature.
- Results: Optimal treatment of RA aims at achieving and then maintaining remission or low disease activity. In a patient with an inadequate response to initial biologic therapy, several therapeutic options exist. Current evidence supports TNFi dose escalation for only infliximab; optimization of concurrent conventional synthetic disease-modifying antirheumatic drug (csDMARD) or switching to a different csDMARD are other options. Cycling (switching to an alternative TNFi) and swapping (switching to a therapy with a different mode of action) strategies are other alternate approaches supported by many observational studies. While no head-to-head trials exist directly comparing the 2 strategies, data suggest superiority of the swapping strategy over the cycling approach. Also, several studies have shown that switching to a drug with a different mechanism of action is associated with higher treatment persistence and lower health care costs than TNFi cycling.
- Conclusion: Physicians have a growing list of treatment options to help their patients with RA achieve disease remission. The choice of best treatment for a given patient needs to be individualized, keeping in mind other factors, including comorbidities.
Keywords: biologics; rheumatoid arthritis; swapping strategy; cycling strategy; TNF inhibitors.
Following the discovery of tumor necrosis factor (TNF) as a proinflammatory cytokine 30 years ago, the use of TNF antagonists has revolutionized the treatment of rheumatoid arthritis (RA). Although TNF inhibitors (TNFIs) are frequently used as a first-line biologic disease-modifying antirheumatic drug (bDMARD), they are not uniformly efficacious in achieving remission in all patients with RA. This article highlights the reasons for such variability in observed response and discusses therapeutic options for patients who do not respond to TNFi therapy.
Case Presentation
A 60-year-old woman is evaluated in the clinic for complaints of pain in her hands, morning stiffness lasting 2 hours, and swelling in her wrists, all of which have been ongoing for 3 months. Physical exam reveals evidence of active inflammation, with synovitis in her second, third, and fourth metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints bilaterally, swelling over both wrists, and a weak grip. Inflammatory markers are elevated, and rheumatoid factor and anti-cyclic citrullinated peptide (anti-CCP) are both positive at high titer. Radiographs reveal evidence of small erosions at the third and fourth MCPs and PIPs bilaterally and periarticular osteopenia. The patient is diagnosed with seropositive, erosive RA based on history, physical exam, laboratory studies, and imaging. She is started on 20 mg of prednisone for acute treatment of her symptoms along with methotrexate, and, initially, her symptoms are well controlled. A few months after starting treatment, she develops voluminous diarrhea that necessitates cessation of methotrexate. Leflunomide also causes similar symptoms. The combination of sulfasalazine and hydroxychloroquine does not adequately control her symptoms, and ongoing use of low-dose glucocorticoids is required to improve functionality in all joints. Using the treat-to-target (T2T) strategy, adalimumab is initiated. However, she continues to report persistent swelling and pain and still requests oral glucocorticoids to help decrease inflammation. The 28-joint Disease Activity Score (DAS28) is 4.8, suggestive of moderate disease activity.
Why are TNFi agents sometimes ineffective?
The introduction of monoclonal antibodies and fusion proteins to block TNF and other cytokines was a remarkable development in the treatment of RA that revolutionized patient care. Despite the efficacy of TNFis, clinical response to these agents is not universal and only some patients achieve complete remission. In targeting the eventual goal of remission or low disease activity in patients with RA, the concept of “TNF failure” becomes extremely relevant. These inadequate responses to anti-TNF therapy may be due to primary failures, or complete lack of clinical response after initiation of the bDMARD, and secondary failures, or the loss of initially achieved clinical response to therapy. Other reasons for discontinuation of a given TNFi include partial disease control and intolerance to the medication (possible injection-site or infusion reactions). Keystone and Kavanaugh1 divided causes of failure of TNF agents into 2 broad categories: perceptual (related to natural variations in disease course like hormonal variation and physical and emotional stress) and pathophysiological failures (genetic variations, high body mass index, concomitant cigarette use).
Another important consideration in patients treated with a TNFi is the consequent formation of anti-drug antibodies (ADAs). TNFi agents are immunogenic and normally elicit an immune response. The appearance of such ADAs may reduce the bioavailability of free drug, resulting in a decreased clinical response,2 or may lead to serious adverse effects.
How common is discontinuation of the first TNFi?
Several studies have reported that the prevalence of primary failure, secondary failure, and intolerance to TNFis ranges from 30% to 40%.3-6 Female sex,7 concurrent prednisone use,8 high disease activity scores,6,8,9 and the absence of treatment with low-dose methotrexate7,8 have all been shown to be negative predictors of bDMARD retention and response.10
Are there any factors that predict TNFi failure?
There are no specific parameters to accurately predict responses to TNFI therapy.11 Several clinical and molecular biomarkers in synovium (initial TNF levels, macrophages, T cells)12 and peripheral blood (serum myeloid-related protein 8 and 14 complex levels,13 prealbumin, platelet factor 4, and S100A12)14 have been described as predictors of clinical response to TNFis, but their utility in clinical practice has not been established and the use of these markers has not yet been incorporated into clinical guidelines.
How is disease activity measured in patients with RA?
In 2010 an international expert consensus panel published treatment recommendations for RA that emphasized a T2T strategy of individualizing and escalating treatment to achieve the lowest disease activity or remission. In clinical practice, numerous tools are available to measure RA disease activity. Herein, we mention several that are most commonly used in clinical practice.
DAS28 combines single activity measures into an overall continuous measure of disease activity and has been endorsed by both the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR). It includes a 28-swollen joint count (SJC), 28-tender joint count (TJC), erythrocyte sedimentation rate (ESR; can also be calculated using C-reactive protein [CRP]), and a patient global assessment (PtGA). The cut-offs used for DAS28 interpretation are as follows: remission (< 2.6), low (≥ 2.6 but ≤ 3.2), moderate (> 3.2 but ≤ 5.1), or high (> 5.1).15 Some of the difficulties in using DAS28 in daily clinical practice include the need for a lab value and the time needed to perform the joint counts. Note also that due to the inclusion of ESR, which is influenced by age and other factors, DAS28 may underestimate remission in the elderly.
Another measure of RA disease activity is the Simplified Disease Activity Index (SDAI), which includes 28 SJC, 28 TJC, PtGA, provider global assessment (PrGA), and CRP in mg/dL. The level of disease activity using the SDAI is interpreted as: remission (SDAI ≤ 3.3), low (≥ 3.4 but ≤ 11), moderate (> 11 but ≤ 26), or high (> 26). The advantage of the SDAI is that a calculator or computer is not required for calculations. Another measure, the Clinical Disease Activity Index (CDAI), includes a 28 SJC, 28 TJC, PtGA, and PrGA. Because a laboratory value is not needed to calculate the CDAI, it is well-suited for use in clinical practice. When using the CDAI, the level of disease activity can be defined as remission (CDAI ≤ 2.8), low (> 2.8 but ≤ 10), moderate (> 10 but ≤ 22), or high (> 22). Again, as with the SDAI, a calculator or computer is not needed for calculations.
What are the alternative treatment options after first biologic failure?
In patients who have failed treatment with an initial biologic, usually a TNFi, the treating rheumatologist has the following options (Figure), with the best treatment strategy being driven by individualized patient and disease-related factors (Table 1 and Table 2):
- TNFi dose escalation
- Trial of an alternate TNFi agent (the “cycling” strategy)
- Optimization of therapy conjoined with a conventional synthetic DMARD (csDMARD)
- Use of a non-TNF biologic or targeted synthetic DMARD (the “swapping” strategy)
If all the listed strategies fail, the next step can be the addition of short-term, low-dose glucocorticoid therapy.
TNFi Dose Escalation
The available data have demonstrated the safety, efficacy, and cost-effectiveness of dose escalation in patients with RA receiving infliximab.16-18 The ATTRACT trial first demonstrated this, with greater clinical and radiographic improvements in those with higher trough serum concentrations, suggesting that doses higher than 3 mg/kg or more frequent than every 8 weeks may be needed for full response in some patients.19
There is a lack of studies in RA patients to determine the most effective dose escalation strategy. A study in patients with Crohn disease showed that intensification to 10 mg/kg every 8 weeks (dose doubling) was at least as effective as 5 mg/kg every 4 weeks (halving interval) at 12 months.16 Due to greater patient and administration convenience of dose-doubling, this strategy may be preferred.17 A starting dose of 10 mg/kg every 8 weeks is not routinely recommended due to an increased risk of serious infection; these adverse events were not found when the dose was gradually increased, as clinically indicated, starting at 3 mg/kg.19,20 Further studies are needed to explore this approach in RA patients.
These results, however, have not been replicated with other TNFi agents. No significant clinical improvements were identified with etanercept 50 mg twice weekly,21 adalimumab 40 mg every week in the PREMIER trial,18 or certolizumab 400 mg every other week in an open-label extension phase of the RAPID 1 study.22 A Japanese study found significantly worse clinical outcomes with dose escalation of golimumab.23 Conversely, 2 studies found clinical benefits after escalating the tocilizumab dose, the first a real-world review from the Consortium of Rheumatology Researchers of North America (CORRONA) registry using the intravenous formulation,24 and the other the BREVACTA study utilizing subcutaneous tocilizumab.25 No studies to date have been published on dose escalation of abatacept in patients with RA who respond poorly. Overall, previous studies support dose escalation in individuals being treated with infliximab to improve clinical outcomes, but additional studies are needed for other bDMARDs.
Trial of an Alternate TNF Agent: The “Cycling” Strategy
Per the ACR/EULAR26,27 guidelines, all approved bDMARDs may be used without hierarchical positioning. However, after the failure of a TNFi agent, these guidelines do not provide specific advice about a preference between the “cycling” strategy (switching to an alternative TNFi) and “swapping” strategy (switching to a therapy with a different mode of action). Cycling might work for several reasons, including differences in the agents’ molecular structure, immunological mechanism of action, immunogenicity, and pharmacokinetics.28-30 The cycling strategy is a well-established approach adopted by more than 94% of practicing rheumatologists, according to a national survey,31 and its efficacy is supported by trials and additional observational studies.32-35
The greater clinical effectiveness of switching to infliximab compared with continuing with etanercept in patients with inadequate response to etanercept (n = 28) was suggested in the open-label OPPOSITE trial.36 Data from the GO-AFTER trial37 suggests that a greater proportion of patients with RA refractory to adalimumab, etanercept, or infliximab who were treated with golimumab achieved an ACR20 and ACR50 response compared with patients who received placebo, and this response persisted through 5 years.38 More recently, certolizumab pegol and adalimumab were compared head-to-head in the EXXELERATE trial.39 The results of this trial revealed the adequate efficacy of cycling to another TNFi after primary insufficient response to the first.
In studies from Finland and Sweden,35,40 it has been observed that a better response is achieved in patients in whom TNF failure was initially due to secondary failure or intolerance rather than primary failure. A post-hoc analysis of the results of the GO-AFTER trial41 and from a few observational studies35,40,42 revealed that switching from one TNFi to another, especially from a monoclonal antibody to a soluble receptor, was often more beneficial for RA patients than switching from a soluble receptor to a monoclonal antibody.
Optimization of Therapy Conjoined with csDMARDs
Methotrexate is one of the oldest and most effective csDMARDs available for the treatment of RA.43 The 2016 EULAR guidelines recommend the addition of methotrexate and/or other csDMARDs to potentiate the effect of bDMARDs.26 In the case of TNFi therapy, the observed synergistic effect between the monoclonal antibody and methotrexate may be explained by sustained suppression of ADA formation.44 In the TEMPO,45 PREMIER,18 and GO-BEFORE46 trials, the addition of methotrexate led to improved clinical and radiological outcomes in patients treated with etanercept, adalimumab, and golimumab,47 respectively. These findings were also demonstrated in several registries, where significant improvement in clinical response and retention rate of the TNFi agents was noted. Results have been replicated with non-TNFi bDMARDs, including abatacept48,49 and rituximab.50 Patients treated with interleukin (IL)-6 inhibitors in combination with methotrexate have shown significantly less radiographic progression compared to those treated with tocilizumab alone and those treated with monotherapy tocilizumab versus monotherapy methotrexate.51,52 Results possibly favor the use of IL-6 inhibitors alone in those who cannot tolerate or have contraindications to methotrexate.
An open prospective study by Cohen et al added methotrexate to the treatment regimens of individuals on bDMARD monotherapy with a primary failure and found favorable changes in ACR20 and DAS28 scores at 3 and 12 months and therapeutic biological response (ESR, CRP) at 3 months.53 Unlike monotherapy, in these situations methotrexate is known to be efficacious even at a lower dose, possibly at 7.5 mg to 10 mg per week. Some studies have shown that methotrexate administered parenterally may be more efficacious than when given orally.54-58
In clinical trials and observational studies, leflunomide, sulfasalazine, and hydroxychloroquine have been used as alternate csDMARDs added to the treatment regimen.59-62 There are, however, only 2 trials comparing the efficacy of methotrexate with that of other csDMARDs as concomitant treatment in patients with inadequate response to TNFi therapy. The RABBIT trial found a slight decrease in effectiveness with concomitant TNFi and leflunomide compared to TNFi/methotrexate, but overall each group had similar EULAR responses at 24 months.63 A study by De Stefano et al found comparable ACR20 and DAS28 responses among individuals receiving TNFis with methotrexate or leflunomide.61
The “Swapping” Strategy
The efficacy of the swapping strategy has been shown in 3 randomized clinical trials demonstrating the superiority of abatacept, tocilizumab, and rituximab in the treatment of individuals with RA refractory to TNFis. Tocilizumab was studied in the RADIATE64 trial, which involved 499 patients with inadequate response to 1 or more TNFi agents. The primary endpoint (24-week ACR20) was achieved by 50.0%, 30.4%, and 10.1% of patients in the 8 mg/kg, 4 mg/kg, and control groups, respectively (P < 0.001 for both tocilizumab groups versus placebo). The utility of abatacept as second-line therapy after initial TNF failure was evaluated in the ATTAIN65 study. Participants with an inadequate response to etanercept or infliximab were randomly assigned to receive either abatacept or placebo. ACR50 response rates after 6 months of treatment were 20.3% with abatacept and 3.8% with placebo (P < 0.001). The SWITCH-RA study,66 an observational study, compared rituximab to TNFis in 1112 participants with inadequate response to initial anti-TNF therapy. At 6 months, mean change in DAS28 was small but significantly greater for the rituximab group (–1.5 vs –1.1; P = 0.007). The difference in response rates was greatest among seropositive patients. These data suggest that rituximab has efficacy following TNFi failure, particularly for seropositive patients. Additionally, REFLEX67 is the sole randomized controlled trial in patients with insufficient response to TNFis that showed significant prevention of radiographic progression at week 56 in patients on rituximab compared to placebo (mean change from baseline in total Genant-modified Sharp score, 1.00 vs 2.31, respectively; P = 0.005).
One study randomly assigned 399 patients with active RA who had inadequate response to prior TNFi therapy to tofacitinib68 (5 mg twice daily or 10 mg twice daily) or placebo, both with methotrexate.6 After 3 months of treatment, ACR20 response rates (41.7% for 5 mg, 28.1% for 10 mg, 24.4% for placebo) and DAS28 remission rates (6.7% for 5 mg, 8.8% for 10 mg, 1.7% for placebo) were significantly greater among patients treated with tofacitinib compared to those treated with placebo. More recently, the RA-BEACON trial69 demonstrated a consistent, beneficial treatment effect of baricitinib in patients with insufficient response to 1 or more TNFis. In this trial, 527 patients with an inadequate response to bDMARDs were randomly assigned to receive baricitinib 2 mg or 4 mg daily or placebo for 24 weeks. A higher proportion of patients receiving baricitinib 4 mg had an ACR20 response at week 12 compared with those treated with placebo (55% vs 27%, P < 0.001), and patients receiving the 4-mg dose had significant improvements from baseline in DAS28 and Health Assessment Questionnaire–Disability Index scores (P < 0.001 for both comparisons).
To Cycle or to Swap?
Several observational studies (SCQM-RA,70 STURE,71 BSRBR,72 Favalli,43 MIRAR,73 SWITCH-RA,74 ROC72) have clearly demonstrated that the swapping strategy is favored over the cycling strategy. In the ROC study,72 patients were randomly assigned (based on physician discretion) to receive a non-TNF biologic or a TNFi. More patients in the non-TNF group than in the TNFi group showed low disease activity at week 24 (45% vs 28%; odds ratio [OR], 2.09; 95% confidence interval [CI], 1.27-3.43; P = 0.004) and at week 52 (41% vs 23%; OR, 2.26; 95% CI, 1.33-3.86; P = 0.003). The authors concluded that in patients having an insufficient response to TNFi therapy, a non-TNF biologic agent may be more effective than a second TNFi drug. Only a few studies75-77 have demonstrated similar results between the 2 strategies. Overall, the available evidence seems to suggest the superiority of the swapping over the cycling strategy.
An important clinical pearl to keep in mind is that both swapping and cycling strategies might theoretically increase the risk of infection; however, limited evidence is reported in the literature. In a large retrospective analysis78 of data on 4332 RA patients from a large US claims database, patients who had cycled between TNFi agents had a 30% to 40% increased risk of infection compared to patients treated with rituximab. Patients on infliximab had a 62% higher hazard of severe infections, and this has also been reported in an observational study.79 In another study,70 41% of 201 patients with RA followed between 1999 and 2013 who swapped to abatacept/rituximab or tocilizumab developed adverse events, as compared to 59% of those who switched to a second TNFi.
What are recent trends in the use of bDMARDs?
Currently, there are no specific guidelines or biomarkers available to facilitate selection of specific treatment from among the classes of biologics. With the development of several new drugs and regulatory approval of baricitinib, physicians now have several biologic options to treat patients. A recent large time-trend study80 deriving data from more than 200,000 patients with RA showed that etanercept remains the most frequently used agent for the treatment of RA; it also showed that the use of adalimumab and infliximab is decreasing, and that the use of newer agents, especially abatacept, golimumab, and certolizumab, has considerably risen in recent years. In this study, abatacept, rituximab, certolizumab, golimumab, tocilizumab, and tofacitinib accounted for 13.2%, 13.8%, 6.9%, 11.9%, and 7.5% switches from first TNFi therapy.
Jin et al81 studied factors associated with the choice of bDMARD for initial and subsequent use. They found that patients with commercial insurance had an 87% higher likelihood of initiating a bDMARD. In the Medicaid subgroup, African Americans had lower odds of initiating and switching bDMARDs than non-Hispanic whites. Prior use of steroids and nonbiologic DMARDs predicted both bDMARD initiation and subsequent switching. Etanercept, adalimumab, and infliximab were the most commonly used first- and second-line bDMARDS; patients on anakinra and golimumab were most likely to be switched to other bDMARDs.
Which treatment strategy is the most cost-effective?
Several studies have reported better treatment persistence rates among patients who are treated with the swapping strategy compared to the cycling strategy. In a retrospective analysis of claims data,82 the authors examined treatment persistence and health care costs in patients switching to biologics with a different mechanism of action or cycling to another TNFi. The mean cost was significantly lower among patients treated using the swapping strategy than among the TNFi cyclers, both for the total cost of care for RA and for the total cost of the targeted DMARDs in the first year after the change in therapy. The authors concluded that switching to a drug with a different mechanism of action is associated with higher treatment persistence and lower health care costs than TNFi cycling.
What about biosimilars?
Biosimilars are copies of already licensed biologics that are very similar to the biologics, but are made by different sponsors using independently derived cell lines and separately developed manufacturing processes.83 Regarding biosimilar use, EULAR26 states that biosimilar bDMARDs approved by the European Medicines Agency or US Food and Drug Administration have similar efficacy and safety as the originator bDMARDs, and recommends them as preferred agents if they are indeed appreciably cheaper than originator or other bDMARDs.
What are the novel treatment targets in RA?
New therapeutics for RA continue to be developed. One of the new agents is peficitinib (ASP015K), an oral, once-daily Janus kinase (Jak) inhibitor targeting Jak-1, Jak-2, and tyrosine kinase-2, with moderate selectivity for Jak-3. In a phase 2b trial, 100-mg and 150-mg doses of peficitinib achieved a statistically significant ACR20 response (48.3% and 56.3%) compared to placebo (29.4%) at 12 weeks.84
Given the benefit of targeting TNF-α and IL-17 in RA, a novel molecule (ABT-122) that targets both human TNF and IL-17 has been developed. Two phase 1 studies85 showed that dual neutralization of TNF and IL-17 with ABT-122 has characteristics acceptable for further exploration of therapeutic potential of this agent in TNF- and IL-17A–driven immune-mediated inflammatory diseases. Another novel drug is mavrilimumab, a human monoclonal antibody that targets granulocyte–macrophage colony-stimulating factor receptor α. A recent studyshowed that long-term treatment with mavrilimumab maintained response and was well-tolerated, with no increased incidence of treatment-emergent adverse events.86
Namilumab (AMG203) is an immunoglobulin G1 monoclonal antibody that binds with high affinity to the GM-CSF ligand. In a phase 1b, randomized, double-blind study (PRIORA)87 to assess namilumab in treating active, mild-to-moderate RA, significant improvement was seen in the DAS28-CRP score with namilumab (150 and 300 mg groups combined) compared with placebo at day 43 (P = 0.0117) and also 8 weeks after last dosing at day 99 (P = 0.0154). Adverse events were similar across different doses of namilumab and placebo, and included nasopharyngitis and exacerbation/worsening of RA. Another drug showing promise in RA is fosdagrocorat (PF-04171327), a potential dissociated agonist of the glucocorticoid receptor. A multicenter, double-blind, parallel-group, active- and placebo-controlled phase 2 study randomly assigned 86 patients to receive fosdagrocorat 10 mg, fosdagrocorat 25 mg, prednisone 5 mg, or placebo, all with stable background methotrexate therapy.88 Both fosdagrocorat doses demonstrated efficacy in improving signs and symptoms in RA patients, with manageable adverse events.
Case Conclusion
There are several available treatment options for the case patient. Based on the PREMIER trial, solely increasing the dose of adalimumab is unlikely to provide a therapeutic benefit. Adding low-dose methotrexate (possibly via a parenteral route because of patient-reported gastrointestinal discomfort) might provide some synergistic and therapeutic effect. However, because of primary failure with TNFi therapy, she may benefit from the initiation of a biologic with a different mechanism of action (ie, swapping strategy). Therapeutic options include tocilizumab, abatacept, rituximab, and the Jak inhibitors (tofacitinib and baricitinib).
Summary
The optimal treatment of RA aims at achieving, and then maintaining, remission or a low disease activity. The choice of best treatment must be individualized to the patient, keeping in mind other factors, including comorbidities like fibromyalgia, history of diverticulitis (prior to use of tocilizumab), history of chronic obstructive pulmonary disease (prior to the use of abatacept), malignancy, and the presence of risk factors for infections (age, diabetes, chronic bronchitis). In a patient with inadequate response to initial biologic therapy, several options exist for the rheumatologist. Current evidence supports TNFi dose escalation for only infliximab; optimization of concurrent csDMARD or switching to a different csDMARD are other options. Cycling and swapping are other alternate approaches supported by many observational studies. While no head-to-head trials exist comparing the 2 strategies, data suggest superiority of the swapping strategy over the cycling approach. With the continuing development of novel therapeutics in RA, physicians have a growing list of treatment options to help their patients achieve disease remission.
Corresponding author: Namrata Singh, MD, 200 Hawkins Drive, Iowa City, IA 52242.
Financial disclosures: None.
From the University of Iowa Hospitals and Clinics, Iowa City, IA.
Abstract
- Objective: To discuss the variability in response to tumor necrosis factor inhibitors (TNFis) observed in patients with rheumatoid arthritis (RA) and discuss therapeutic options for patients who do not respond to initial TNFi therapy.
- Methods: Review of the literature.
- Results: Optimal treatment of RA aims at achieving and then maintaining remission or low disease activity. In a patient with an inadequate response to initial biologic therapy, several therapeutic options exist. Current evidence supports TNFi dose escalation for only infliximab; optimization of concurrent conventional synthetic disease-modifying antirheumatic drug (csDMARD) or switching to a different csDMARD are other options. Cycling (switching to an alternative TNFi) and swapping (switching to a therapy with a different mode of action) strategies are other alternate approaches supported by many observational studies. While no head-to-head trials exist directly comparing the 2 strategies, data suggest superiority of the swapping strategy over the cycling approach. Also, several studies have shown that switching to a drug with a different mechanism of action is associated with higher treatment persistence and lower health care costs than TNFi cycling.
- Conclusion: Physicians have a growing list of treatment options to help their patients with RA achieve disease remission. The choice of best treatment for a given patient needs to be individualized, keeping in mind other factors, including comorbidities.
Keywords: biologics; rheumatoid arthritis; swapping strategy; cycling strategy; TNF inhibitors.
Following the discovery of tumor necrosis factor (TNF) as a proinflammatory cytokine 30 years ago, the use of TNF antagonists has revolutionized the treatment of rheumatoid arthritis (RA). Although TNF inhibitors (TNFIs) are frequently used as a first-line biologic disease-modifying antirheumatic drug (bDMARD), they are not uniformly efficacious in achieving remission in all patients with RA. This article highlights the reasons for such variability in observed response and discusses therapeutic options for patients who do not respond to TNFi therapy.
Case Presentation
A 60-year-old woman is evaluated in the clinic for complaints of pain in her hands, morning stiffness lasting 2 hours, and swelling in her wrists, all of which have been ongoing for 3 months. Physical exam reveals evidence of active inflammation, with synovitis in her second, third, and fourth metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints bilaterally, swelling over both wrists, and a weak grip. Inflammatory markers are elevated, and rheumatoid factor and anti-cyclic citrullinated peptide (anti-CCP) are both positive at high titer. Radiographs reveal evidence of small erosions at the third and fourth MCPs and PIPs bilaterally and periarticular osteopenia. The patient is diagnosed with seropositive, erosive RA based on history, physical exam, laboratory studies, and imaging. She is started on 20 mg of prednisone for acute treatment of her symptoms along with methotrexate, and, initially, her symptoms are well controlled. A few months after starting treatment, she develops voluminous diarrhea that necessitates cessation of methotrexate. Leflunomide also causes similar symptoms. The combination of sulfasalazine and hydroxychloroquine does not adequately control her symptoms, and ongoing use of low-dose glucocorticoids is required to improve functionality in all joints. Using the treat-to-target (T2T) strategy, adalimumab is initiated. However, she continues to report persistent swelling and pain and still requests oral glucocorticoids to help decrease inflammation. The 28-joint Disease Activity Score (DAS28) is 4.8, suggestive of moderate disease activity.
Why are TNFi agents sometimes ineffective?
The introduction of monoclonal antibodies and fusion proteins to block TNF and other cytokines was a remarkable development in the treatment of RA that revolutionized patient care. Despite the efficacy of TNFis, clinical response to these agents is not universal and only some patients achieve complete remission. In targeting the eventual goal of remission or low disease activity in patients with RA, the concept of “TNF failure” becomes extremely relevant. These inadequate responses to anti-TNF therapy may be due to primary failures, or complete lack of clinical response after initiation of the bDMARD, and secondary failures, or the loss of initially achieved clinical response to therapy. Other reasons for discontinuation of a given TNFi include partial disease control and intolerance to the medication (possible injection-site or infusion reactions). Keystone and Kavanaugh1 divided causes of failure of TNF agents into 2 broad categories: perceptual (related to natural variations in disease course like hormonal variation and physical and emotional stress) and pathophysiological failures (genetic variations, high body mass index, concomitant cigarette use).
Another important consideration in patients treated with a TNFi is the consequent formation of anti-drug antibodies (ADAs). TNFi agents are immunogenic and normally elicit an immune response. The appearance of such ADAs may reduce the bioavailability of free drug, resulting in a decreased clinical response,2 or may lead to serious adverse effects.
How common is discontinuation of the first TNFi?
Several studies have reported that the prevalence of primary failure, secondary failure, and intolerance to TNFis ranges from 30% to 40%.3-6 Female sex,7 concurrent prednisone use,8 high disease activity scores,6,8,9 and the absence of treatment with low-dose methotrexate7,8 have all been shown to be negative predictors of bDMARD retention and response.10
Are there any factors that predict TNFi failure?
There are no specific parameters to accurately predict responses to TNFI therapy.11 Several clinical and molecular biomarkers in synovium (initial TNF levels, macrophages, T cells)12 and peripheral blood (serum myeloid-related protein 8 and 14 complex levels,13 prealbumin, platelet factor 4, and S100A12)14 have been described as predictors of clinical response to TNFis, but their utility in clinical practice has not been established and the use of these markers has not yet been incorporated into clinical guidelines.
How is disease activity measured in patients with RA?
In 2010 an international expert consensus panel published treatment recommendations for RA that emphasized a T2T strategy of individualizing and escalating treatment to achieve the lowest disease activity or remission. In clinical practice, numerous tools are available to measure RA disease activity. Herein, we mention several that are most commonly used in clinical practice.
DAS28 combines single activity measures into an overall continuous measure of disease activity and has been endorsed by both the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR). It includes a 28-swollen joint count (SJC), 28-tender joint count (TJC), erythrocyte sedimentation rate (ESR; can also be calculated using C-reactive protein [CRP]), and a patient global assessment (PtGA). The cut-offs used for DAS28 interpretation are as follows: remission (< 2.6), low (≥ 2.6 but ≤ 3.2), moderate (> 3.2 but ≤ 5.1), or high (> 5.1).15 Some of the difficulties in using DAS28 in daily clinical practice include the need for a lab value and the time needed to perform the joint counts. Note also that due to the inclusion of ESR, which is influenced by age and other factors, DAS28 may underestimate remission in the elderly.
Another measure of RA disease activity is the Simplified Disease Activity Index (SDAI), which includes 28 SJC, 28 TJC, PtGA, provider global assessment (PrGA), and CRP in mg/dL. The level of disease activity using the SDAI is interpreted as: remission (SDAI ≤ 3.3), low (≥ 3.4 but ≤ 11), moderate (> 11 but ≤ 26), or high (> 26). The advantage of the SDAI is that a calculator or computer is not required for calculations. Another measure, the Clinical Disease Activity Index (CDAI), includes a 28 SJC, 28 TJC, PtGA, and PrGA. Because a laboratory value is not needed to calculate the CDAI, it is well-suited for use in clinical practice. When using the CDAI, the level of disease activity can be defined as remission (CDAI ≤ 2.8), low (> 2.8 but ≤ 10), moderate (> 10 but ≤ 22), or high (> 22). Again, as with the SDAI, a calculator or computer is not needed for calculations.
What are the alternative treatment options after first biologic failure?
In patients who have failed treatment with an initial biologic, usually a TNFi, the treating rheumatologist has the following options (Figure), with the best treatment strategy being driven by individualized patient and disease-related factors (Table 1 and Table 2):
- TNFi dose escalation
- Trial of an alternate TNFi agent (the “cycling” strategy)
- Optimization of therapy conjoined with a conventional synthetic DMARD (csDMARD)
- Use of a non-TNF biologic or targeted synthetic DMARD (the “swapping” strategy)
If all the listed strategies fail, the next step can be the addition of short-term, low-dose glucocorticoid therapy.
TNFi Dose Escalation
The available data have demonstrated the safety, efficacy, and cost-effectiveness of dose escalation in patients with RA receiving infliximab.16-18 The ATTRACT trial first demonstrated this, with greater clinical and radiographic improvements in those with higher trough serum concentrations, suggesting that doses higher than 3 mg/kg or more frequent than every 8 weeks may be needed for full response in some patients.19
There is a lack of studies in RA patients to determine the most effective dose escalation strategy. A study in patients with Crohn disease showed that intensification to 10 mg/kg every 8 weeks (dose doubling) was at least as effective as 5 mg/kg every 4 weeks (halving interval) at 12 months.16 Due to greater patient and administration convenience of dose-doubling, this strategy may be preferred.17 A starting dose of 10 mg/kg every 8 weeks is not routinely recommended due to an increased risk of serious infection; these adverse events were not found when the dose was gradually increased, as clinically indicated, starting at 3 mg/kg.19,20 Further studies are needed to explore this approach in RA patients.
These results, however, have not been replicated with other TNFi agents. No significant clinical improvements were identified with etanercept 50 mg twice weekly,21 adalimumab 40 mg every week in the PREMIER trial,18 or certolizumab 400 mg every other week in an open-label extension phase of the RAPID 1 study.22 A Japanese study found significantly worse clinical outcomes with dose escalation of golimumab.23 Conversely, 2 studies found clinical benefits after escalating the tocilizumab dose, the first a real-world review from the Consortium of Rheumatology Researchers of North America (CORRONA) registry using the intravenous formulation,24 and the other the BREVACTA study utilizing subcutaneous tocilizumab.25 No studies to date have been published on dose escalation of abatacept in patients with RA who respond poorly. Overall, previous studies support dose escalation in individuals being treated with infliximab to improve clinical outcomes, but additional studies are needed for other bDMARDs.
Trial of an Alternate TNF Agent: The “Cycling” Strategy
Per the ACR/EULAR26,27 guidelines, all approved bDMARDs may be used without hierarchical positioning. However, after the failure of a TNFi agent, these guidelines do not provide specific advice about a preference between the “cycling” strategy (switching to an alternative TNFi) and “swapping” strategy (switching to a therapy with a different mode of action). Cycling might work for several reasons, including differences in the agents’ molecular structure, immunological mechanism of action, immunogenicity, and pharmacokinetics.28-30 The cycling strategy is a well-established approach adopted by more than 94% of practicing rheumatologists, according to a national survey,31 and its efficacy is supported by trials and additional observational studies.32-35
The greater clinical effectiveness of switching to infliximab compared with continuing with etanercept in patients with inadequate response to etanercept (n = 28) was suggested in the open-label OPPOSITE trial.36 Data from the GO-AFTER trial37 suggests that a greater proportion of patients with RA refractory to adalimumab, etanercept, or infliximab who were treated with golimumab achieved an ACR20 and ACR50 response compared with patients who received placebo, and this response persisted through 5 years.38 More recently, certolizumab pegol and adalimumab were compared head-to-head in the EXXELERATE trial.39 The results of this trial revealed the adequate efficacy of cycling to another TNFi after primary insufficient response to the first.
In studies from Finland and Sweden,35,40 it has been observed that a better response is achieved in patients in whom TNF failure was initially due to secondary failure or intolerance rather than primary failure. A post-hoc analysis of the results of the GO-AFTER trial41 and from a few observational studies35,40,42 revealed that switching from one TNFi to another, especially from a monoclonal antibody to a soluble receptor, was often more beneficial for RA patients than switching from a soluble receptor to a monoclonal antibody.
Optimization of Therapy Conjoined with csDMARDs
Methotrexate is one of the oldest and most effective csDMARDs available for the treatment of RA.43 The 2016 EULAR guidelines recommend the addition of methotrexate and/or other csDMARDs to potentiate the effect of bDMARDs.26 In the case of TNFi therapy, the observed synergistic effect between the monoclonal antibody and methotrexate may be explained by sustained suppression of ADA formation.44 In the TEMPO,45 PREMIER,18 and GO-BEFORE46 trials, the addition of methotrexate led to improved clinical and radiological outcomes in patients treated with etanercept, adalimumab, and golimumab,47 respectively. These findings were also demonstrated in several registries, where significant improvement in clinical response and retention rate of the TNFi agents was noted. Results have been replicated with non-TNFi bDMARDs, including abatacept48,49 and rituximab.50 Patients treated with interleukin (IL)-6 inhibitors in combination with methotrexate have shown significantly less radiographic progression compared to those treated with tocilizumab alone and those treated with monotherapy tocilizumab versus monotherapy methotrexate.51,52 Results possibly favor the use of IL-6 inhibitors alone in those who cannot tolerate or have contraindications to methotrexate.
An open prospective study by Cohen et al added methotrexate to the treatment regimens of individuals on bDMARD monotherapy with a primary failure and found favorable changes in ACR20 and DAS28 scores at 3 and 12 months and therapeutic biological response (ESR, CRP) at 3 months.53 Unlike monotherapy, in these situations methotrexate is known to be efficacious even at a lower dose, possibly at 7.5 mg to 10 mg per week. Some studies have shown that methotrexate administered parenterally may be more efficacious than when given orally.54-58
In clinical trials and observational studies, leflunomide, sulfasalazine, and hydroxychloroquine have been used as alternate csDMARDs added to the treatment regimen.59-62 There are, however, only 2 trials comparing the efficacy of methotrexate with that of other csDMARDs as concomitant treatment in patients with inadequate response to TNFi therapy. The RABBIT trial found a slight decrease in effectiveness with concomitant TNFi and leflunomide compared to TNFi/methotrexate, but overall each group had similar EULAR responses at 24 months.63 A study by De Stefano et al found comparable ACR20 and DAS28 responses among individuals receiving TNFis with methotrexate or leflunomide.61
The “Swapping” Strategy
The efficacy of the swapping strategy has been shown in 3 randomized clinical trials demonstrating the superiority of abatacept, tocilizumab, and rituximab in the treatment of individuals with RA refractory to TNFis. Tocilizumab was studied in the RADIATE64 trial, which involved 499 patients with inadequate response to 1 or more TNFi agents. The primary endpoint (24-week ACR20) was achieved by 50.0%, 30.4%, and 10.1% of patients in the 8 mg/kg, 4 mg/kg, and control groups, respectively (P < 0.001 for both tocilizumab groups versus placebo). The utility of abatacept as second-line therapy after initial TNF failure was evaluated in the ATTAIN65 study. Participants with an inadequate response to etanercept or infliximab were randomly assigned to receive either abatacept or placebo. ACR50 response rates after 6 months of treatment were 20.3% with abatacept and 3.8% with placebo (P < 0.001). The SWITCH-RA study,66 an observational study, compared rituximab to TNFis in 1112 participants with inadequate response to initial anti-TNF therapy. At 6 months, mean change in DAS28 was small but significantly greater for the rituximab group (–1.5 vs –1.1; P = 0.007). The difference in response rates was greatest among seropositive patients. These data suggest that rituximab has efficacy following TNFi failure, particularly for seropositive patients. Additionally, REFLEX67 is the sole randomized controlled trial in patients with insufficient response to TNFis that showed significant prevention of radiographic progression at week 56 in patients on rituximab compared to placebo (mean change from baseline in total Genant-modified Sharp score, 1.00 vs 2.31, respectively; P = 0.005).
One study randomly assigned 399 patients with active RA who had inadequate response to prior TNFi therapy to tofacitinib68 (5 mg twice daily or 10 mg twice daily) or placebo, both with methotrexate.6 After 3 months of treatment, ACR20 response rates (41.7% for 5 mg, 28.1% for 10 mg, 24.4% for placebo) and DAS28 remission rates (6.7% for 5 mg, 8.8% for 10 mg, 1.7% for placebo) were significantly greater among patients treated with tofacitinib compared to those treated with placebo. More recently, the RA-BEACON trial69 demonstrated a consistent, beneficial treatment effect of baricitinib in patients with insufficient response to 1 or more TNFis. In this trial, 527 patients with an inadequate response to bDMARDs were randomly assigned to receive baricitinib 2 mg or 4 mg daily or placebo for 24 weeks. A higher proportion of patients receiving baricitinib 4 mg had an ACR20 response at week 12 compared with those treated with placebo (55% vs 27%, P < 0.001), and patients receiving the 4-mg dose had significant improvements from baseline in DAS28 and Health Assessment Questionnaire–Disability Index scores (P < 0.001 for both comparisons).
To Cycle or to Swap?
Several observational studies (SCQM-RA,70 STURE,71 BSRBR,72 Favalli,43 MIRAR,73 SWITCH-RA,74 ROC72) have clearly demonstrated that the swapping strategy is favored over the cycling strategy. In the ROC study,72 patients were randomly assigned (based on physician discretion) to receive a non-TNF biologic or a TNFi. More patients in the non-TNF group than in the TNFi group showed low disease activity at week 24 (45% vs 28%; odds ratio [OR], 2.09; 95% confidence interval [CI], 1.27-3.43; P = 0.004) and at week 52 (41% vs 23%; OR, 2.26; 95% CI, 1.33-3.86; P = 0.003). The authors concluded that in patients having an insufficient response to TNFi therapy, a non-TNF biologic agent may be more effective than a second TNFi drug. Only a few studies75-77 have demonstrated similar results between the 2 strategies. Overall, the available evidence seems to suggest the superiority of the swapping over the cycling strategy.
An important clinical pearl to keep in mind is that both swapping and cycling strategies might theoretically increase the risk of infection; however, limited evidence is reported in the literature. In a large retrospective analysis78 of data on 4332 RA patients from a large US claims database, patients who had cycled between TNFi agents had a 30% to 40% increased risk of infection compared to patients treated with rituximab. Patients on infliximab had a 62% higher hazard of severe infections, and this has also been reported in an observational study.79 In another study,70 41% of 201 patients with RA followed between 1999 and 2013 who swapped to abatacept/rituximab or tocilizumab developed adverse events, as compared to 59% of those who switched to a second TNFi.
What are recent trends in the use of bDMARDs?
Currently, there are no specific guidelines or biomarkers available to facilitate selection of specific treatment from among the classes of biologics. With the development of several new drugs and regulatory approval of baricitinib, physicians now have several biologic options to treat patients. A recent large time-trend study80 deriving data from more than 200,000 patients with RA showed that etanercept remains the most frequently used agent for the treatment of RA; it also showed that the use of adalimumab and infliximab is decreasing, and that the use of newer agents, especially abatacept, golimumab, and certolizumab, has considerably risen in recent years. In this study, abatacept, rituximab, certolizumab, golimumab, tocilizumab, and tofacitinib accounted for 13.2%, 13.8%, 6.9%, 11.9%, and 7.5% switches from first TNFi therapy.
Jin et al81 studied factors associated with the choice of bDMARD for initial and subsequent use. They found that patients with commercial insurance had an 87% higher likelihood of initiating a bDMARD. In the Medicaid subgroup, African Americans had lower odds of initiating and switching bDMARDs than non-Hispanic whites. Prior use of steroids and nonbiologic DMARDs predicted both bDMARD initiation and subsequent switching. Etanercept, adalimumab, and infliximab were the most commonly used first- and second-line bDMARDS; patients on anakinra and golimumab were most likely to be switched to other bDMARDs.
Which treatment strategy is the most cost-effective?
Several studies have reported better treatment persistence rates among patients who are treated with the swapping strategy compared to the cycling strategy. In a retrospective analysis of claims data,82 the authors examined treatment persistence and health care costs in patients switching to biologics with a different mechanism of action or cycling to another TNFi. The mean cost was significantly lower among patients treated using the swapping strategy than among the TNFi cyclers, both for the total cost of care for RA and for the total cost of the targeted DMARDs in the first year after the change in therapy. The authors concluded that switching to a drug with a different mechanism of action is associated with higher treatment persistence and lower health care costs than TNFi cycling.
What about biosimilars?
Biosimilars are copies of already licensed biologics that are very similar to the biologics, but are made by different sponsors using independently derived cell lines and separately developed manufacturing processes.83 Regarding biosimilar use, EULAR26 states that biosimilar bDMARDs approved by the European Medicines Agency or US Food and Drug Administration have similar efficacy and safety as the originator bDMARDs, and recommends them as preferred agents if they are indeed appreciably cheaper than originator or other bDMARDs.
What are the novel treatment targets in RA?
New therapeutics for RA continue to be developed. One of the new agents is peficitinib (ASP015K), an oral, once-daily Janus kinase (Jak) inhibitor targeting Jak-1, Jak-2, and tyrosine kinase-2, with moderate selectivity for Jak-3. In a phase 2b trial, 100-mg and 150-mg doses of peficitinib achieved a statistically significant ACR20 response (48.3% and 56.3%) compared to placebo (29.4%) at 12 weeks.84
Given the benefit of targeting TNF-α and IL-17 in RA, a novel molecule (ABT-122) that targets both human TNF and IL-17 has been developed. Two phase 1 studies85 showed that dual neutralization of TNF and IL-17 with ABT-122 has characteristics acceptable for further exploration of therapeutic potential of this agent in TNF- and IL-17A–driven immune-mediated inflammatory diseases. Another novel drug is mavrilimumab, a human monoclonal antibody that targets granulocyte–macrophage colony-stimulating factor receptor α. A recent studyshowed that long-term treatment with mavrilimumab maintained response and was well-tolerated, with no increased incidence of treatment-emergent adverse events.86
Namilumab (AMG203) is an immunoglobulin G1 monoclonal antibody that binds with high affinity to the GM-CSF ligand. In a phase 1b, randomized, double-blind study (PRIORA)87 to assess namilumab in treating active, mild-to-moderate RA, significant improvement was seen in the DAS28-CRP score with namilumab (150 and 300 mg groups combined) compared with placebo at day 43 (P = 0.0117) and also 8 weeks after last dosing at day 99 (P = 0.0154). Adverse events were similar across different doses of namilumab and placebo, and included nasopharyngitis and exacerbation/worsening of RA. Another drug showing promise in RA is fosdagrocorat (PF-04171327), a potential dissociated agonist of the glucocorticoid receptor. A multicenter, double-blind, parallel-group, active- and placebo-controlled phase 2 study randomly assigned 86 patients to receive fosdagrocorat 10 mg, fosdagrocorat 25 mg, prednisone 5 mg, or placebo, all with stable background methotrexate therapy.88 Both fosdagrocorat doses demonstrated efficacy in improving signs and symptoms in RA patients, with manageable adverse events.
Case Conclusion
There are several available treatment options for the case patient. Based on the PREMIER trial, solely increasing the dose of adalimumab is unlikely to provide a therapeutic benefit. Adding low-dose methotrexate (possibly via a parenteral route because of patient-reported gastrointestinal discomfort) might provide some synergistic and therapeutic effect. However, because of primary failure with TNFi therapy, she may benefit from the initiation of a biologic with a different mechanism of action (ie, swapping strategy). Therapeutic options include tocilizumab, abatacept, rituximab, and the Jak inhibitors (tofacitinib and baricitinib).
Summary
The optimal treatment of RA aims at achieving, and then maintaining, remission or a low disease activity. The choice of best treatment must be individualized to the patient, keeping in mind other factors, including comorbidities like fibromyalgia, history of diverticulitis (prior to use of tocilizumab), history of chronic obstructive pulmonary disease (prior to the use of abatacept), malignancy, and the presence of risk factors for infections (age, diabetes, chronic bronchitis). In a patient with inadequate response to initial biologic therapy, several options exist for the rheumatologist. Current evidence supports TNFi dose escalation for only infliximab; optimization of concurrent csDMARD or switching to a different csDMARD are other options. Cycling and swapping are other alternate approaches supported by many observational studies. While no head-to-head trials exist comparing the 2 strategies, data suggest superiority of the swapping strategy over the cycling approach. With the continuing development of novel therapeutics in RA, physicians have a growing list of treatment options to help their patients achieve disease remission.
Corresponding author: Namrata Singh, MD, 200 Hawkins Drive, Iowa City, IA 52242.
Financial disclosures: None.
1. Keystone ED, Kavanaugh KA. What to do with TNF failures. Expert Opin Drug Saf. 2005;4:149-155.
2. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707-718.
3. Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253-259.
4. Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50(5):1400-1411.
5. Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594-1602.
6. Finckh A, Simard JF, Gabay C, et al. Evidence for differential acquired drug resistance to anti-tumour necrosis factor agents in rheumatoid arthritis. Ann Rheum Dis. 2006;65:746-752.
7. Souto A, Maneiro JR, Gomez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology. 2016;55:523-534.
8. Hetland ML, Christensen IJ, Tarp U, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010;62:22-32.
9. Gabay C, Riek M, Scherer A, et al. Effectiveness of biologic DMARDs in monotherapy versus in combination with synthetic DMARDs in rheumatoid arthritis: data from the Swiss Clinical Quality Management Registry. Rheumatology. 2015;54(9):1664-1672.
10. Ebina K, Hashimoto M, Yamamoto W, et al. Drug retention and discontinuation reasons between seven biologics in patients with rheumatoid arthritis-The ANSWER cohort study. PloS One. 2018;13:e0194130.
11. Wijbrandts CA, Tak PP. Prediction of response to targeted treatment in rheumatoid arthritis. Mayo Clin Proc. 2017;92:1129-1143.
12. Ulfgren AK, Andersson U, Engstrom M, et al. Systemic anti-tumor necrosis factor alpha therapy in rheumatoid arthritis down-regulates synovial tumor necrosis factor alpha synthesis. Arthritis Rheum. 2000;43:2391-2396.
13. Choi IY, Gerlag DM, Herenius MJ, et al. MRP8/14 serum levels as a strong predictor of response to biological treatments in patients with rheumatoid arthritis. Ann Rheum Dis. 2015;74:499-505.
14. Nguyen MVC, Baillet A, Romand X, et al. Prealbumin, platelet factor 4 and S100A12 combination at baseline predicts good response to TNF alpha inhibitors in rheumatoid arthritis. Joint Bone Spine. 2019;86:195-201.
15. Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28-Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score-II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), Chronic Arthritis Systemic Index (CASI), Patient-Based Disease Activity Score With ESR (PDAS1) and Patient-Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI-RA). Arthritis Care Res. 2011;63(suppl 11):S14-S36.
16. Katz L, Gisbert JP, Manoogian B, et al. Doubling the infliximab dose versus halving the infusion intervals in Crohn’s disease patients with loss of response. Inflamm Bowel Dis. 2012;18:2026-2033.
17. Durez P, Van den Bosch F, Corluy L, et al. A dose adjustment in patients with rheumatoid arthritis not optimally responding to a standard dose of infliximab of 3 mg/kg every 8 weeks can be effective: a Belgian prospective study. Rheumatology. 2005;44:465-468.
18. Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26-37.
19. St Clair EW, Wagner CL, Fasanmade AA, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:1451-1459.
20. Rahman MU, Strusberg I, Geusens P, et al. Double-blinded infliximab dose escalation in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:1233-1238.
21. Weinblatt ME, Schiff MH, Ruderman EM, et al. Efficacy and safety of etanercept 50 mg twice a week in patients with rheumatoid arthritis who had a suboptimal response to etanercept 50 mg once a week: results of a multicenter, randomized, double-blind, active drug-controlled study. Arthritis Rheum. 2008;58:1921-1930.
22. Curtis JR, Chen L, Luijtens K, et al. Dose escalation of certolizumab pegol from 200 mg to 400 mg every other week provides no additional efficacy in rheumatoid arthritis: an analysis of individual patient-level data. Arthritis Rheum. 2011;63:2203-2208.
23. Okazaki M, Kobayashi H, Ishii Y, et al. Real-world treatment patterns for golimumab and concomitant medications in Japanese rheumatoid arthritis patients. Rheumatol Ther. 2018;5:185-201.
24. Pappas DA, John A, Curtis JR, et al. Dosing of intravenous tocilizumab in a real-world setting of rheumatoid arthritis: analyses from the Corrona Registry. Rheumatol Ther. 2016;3:103-115.
25. Kivitz A, Olech E, Borofsky MA, et al. Two-year efficacy and safety of subcutaneous tocilizumab in combination with disease-modifying antirheumatic drugs including escalation to weekly dosing in rheumatoid arthritis. J Rheumatol. 2018;45:456-464.
26. Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960-977.
27. Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2016;68:1-25.
28. Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11:81-88.
29. Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:493-507.
30. Navarro Coy NC, Brown S, Bosworth A, et al. The ‘Switch’ study protocol: a randomised-controlled trial of switching to an alternative tumour-necrosis factor (TNF)-inhibitor drug or abatacept or rituximab in patients with rheumatoid arthritis who have failed an initial TNF-inhibitor drug. BMC Musculoskelet Disord. 2014;15:452.
31. Kamal KM, Madhavan SS, Hornsby JA, et al. Use of tumor necrosis factor inhibitors in rheumatoid arthritis: a national survey of practicing United States rheumatologists. Joint Bone Spine. 2006;73:718-724.
32. Gomez-Reino JJ, Carmona L, BIOBADASER Group. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006;8:R29.
33. Caporali R, Sarzi-Puttini P, Atzeni F, et al. Switching TNF-alpha antagonists in rheumatoid arthritis: The experience of the LORHEN registry. Autoimmun Rev. 2010;9:465-469.
34. Iannone F, Trotta F, Monteccuco C, et al. Etanercept maintains the clinical benefit achieved by infliximab in patients with rheumatoid arthritis who discontinued infliximab because of side effects. Ann Rheum Dis. 2007;66:249-252.
35. Virkki LM, Valleala H, Takakubo Y, et al. Outcomes of switching anti-TNF drugs in rheumatoid arthritis—a study based on observational data from the Finnish Register of Biological Treatment (ROB-FIN). Clin Rheumatol. 2011;30:1447-1454.
36. Furst DE, Gaylis N, Bray V, et al. Open-label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: the opposite study. Ann Rheum Dis. 2007;66:893-899.
37. Smolen JS, Kay J, Doyle MK, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2009;374:210-221.
38. Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor α inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.
39. Smolen JS, Burmester G-R, Combe B, et al. Head-to-head comparison of certolizumab pegol versus adalimumab in rheumatoid arthritis: 2-year efficacy and safety results from the randomised EXXELERATE study. Lancet. 2016;388:2763-2774.
40. Chatzidionysiou K, Askling J, Eriksson J, et al. Effectiveness of TNF inhibitor switch in RA: results from the national Swedish register. Ann Rheum Dis. 2015;74:890.
41. Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor alpha inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.
42. Lequerré T, Farran É, Ménard J-F, et al. Switching from an anti-TNF monoclonal antibody to soluble TNF-receptor yields better results than vice versa: An observational retrospective study of 72 rheumatoid arthritis switchers. Joint Bone Spine. 2015;82:330-337.
43. Favalli EG, Biggioggero M, Meroni PL. Methotrexate for the treatment of rheumatoid arthritis in the biologic era: Still an “anchor” drug? Autoimmun Rev. 2014;13:1102-1108.
44. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707-718.
45. Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675-681.
46. Emery P, Fleischmann RM, Strusberg I, et al. Efficacy and safety of subcutaneous golimumab in methotrexate-naive patients with rheumatoid arthritis: five-year results of a randomized clinical trial. Arthritis Care Res. 2016;68:744-752.
47. Emery P, Fleischmann RM, Moreland LW, et al. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 2009;60:2272-2283.
48. Emery P, Burmester GR, Bykerk VP, et al. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis. 2015;74:19-26.
49. Westhovens R, Robles M, Ximenes AC, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis. 2009;68:1870-1877.
50. Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793-2806.
51. Burmester GR, Rigby WF, van Vollenhoven RF, et al. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann Rheum Dis. 2016;75:1081-1091.
52. Bijlsma JWJ, Welsing PMJ, Woodworth TG, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet. 2016;388:343-355.
53. Cohen JD, Zaltni S, Kaiser MJ, et al. Secondary addition of methotrexate to partial responders to etanercept alone is effective in severe rheumatoid arthritis. Ann Rheum Dis. 2004;63:209-210.
54. Hamilton RA, Kremer JM. Why intramuscular methotrexate may be more efficacious than oral dosing in patients with rheumatoid arthritis. Br J Rheumatol. 1997;36:86-90.
55. Hoekstra M, Haagsma C, Neef C, et al. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J Rheumatol. 2004;31:645-648.
56. Herman RA, Veng-Pedersen P, Hoffman J, et al. Pharmacokinetics of low-dose methotrexate in rheumatoid arthritis patients. J Pharm Sci. 1989;78:165-171.
57. Schiff MH, Jaffe JS, Freundlich B. Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses ± 15 mg may be overcome with subcutaneous administration. Ann Rheum Dis. 2014;73:1549-1551.
58. Hazlewood GS, Thorne JC, Pope JE, et al. The comparative effectiveness of oral versus subcutaneous methotrexate for the treatment of early rheumatoid arthritis. Ann Rheum Dis. 2016;75:1003-1008.
59. O’Dell JR, Petersen K, Leff R, et al. Etanercept in combination with sulfasalazine, hydroxychloroquine, or gold in the treatment of rheumatoid arthritis. J Rheumatol. 2006;33:213-218.
60. Finckh A, Dehler S, Gabay C. The effectiveness of leflunomide as a co-therapy of tumour necrosis factor inhibitors in rheumatoid arthritis: a population-based study. Ann Rheum Dis. 2009;68:33-39.
61. De Stefano R, Frati E, Nargi F, et al. Comparison of combination therapies in the treatment of rheumatoid arthritis: leflunomide-anti-TNF-alpha versus methotrexate-anti-TNF-alpha. Clin Rheumatol. 2010;29:517-524.
62. Combe B, Codreanu C, Fiocco U, et al. Etanercept and sulfasalazine, alone and combined, in patients with active rheumatoid arthritis despite receiving sulfasalazine: a double-blind comparison. Ann Rheum Dis. 2006;65:1357-1362.
63. Strangfeld A, Hierse F, Kekow J, et al. Comparative effectiveness of tumour necrosis factor α inhibitors in combination with either methotrexate or leflunomide. Ann Rheum Dis. 2009;68:1856.
64. Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516.
65. Genovese MC, Becker J-C, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor α inhibition. N Engl J Med. 2005;353:1114-1123.
66. Emery P, Gottenberg JE, Rubbert-Roth A, et al. Rituximab versus an alternative TNF inhibitor in patients with rheumatoid arthritis who failed to respond to a single previous TNF inhibitor: SWITCH-RA, a global, observational, comparative effectiveness study. Ann Rheum Dis. 2015;74:979-984.
67. Keystone E, Emery P, Peterfy CG, et al. Rituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapies. Ann Rheum Dis. 2009;68:216.
68. Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451-460.
69. Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374:1243-1252.
70. Favalli EG, Biggioggero M, Marchesoni A, Meroni PL. Survival on treatment with second-line biologic therapy: a cohort study comparing cycling and swap strategies. Rheumatology. 2014;53:1664-1668.
71. Harrold LR, Reed GW, Solomon DH, et al. Comparative effectiveness of abatacept versus tocilizumab in rheumatoid arthritis patients with prior TNFi exposure in the US Corrona registry. Arthritis Res Ther. 2016;18:280.
72. Gottenberg J, Brocq O, Perdriger A, et al. Non–TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug: A randomized clinical trial. JAMA. 2016;316:1172-1180.
73. Pascart T, Philippe P, Drumez E, et al. Comparative efficacy of tocilizumab, abatacept and rituximab after non-TNF inhibitor failure: results from a multicentre study. Int J Rheum Dis. 2016;19:1093-1102.
74. Akiyama M, Kaneko Y, Kondo H, Takeuchi T. Comparison of the clinical effectiveness of tumour necrosis factor inhibitors and abatacept after insufficient response to tocilizumab in patients with rheumatoid arthritis. Clin Rheumatol. 2016;35:2829-2834.
75. Schoels M, Aletaha D, Smolen JS, Wong JB. Comparative effectiveness and safety of biological treatment options after tumour necrosis factor α inhibitor failure in rheumatoid arthritis: systematic review and indirect pairwise meta-analysis. Ann Rheum Dis. 2012;71:1303.
76. Soliman MM, Hyrich KL, Lunt M, et al. Rituximab or a second anti-tumor necrosis factor therapy for rheumatoid arthritis patients who have failed their first anti-tumor necrosis factor therapy? Comparative analysis from the British Society for Rheumatology Biologics Register. Arthritis Care Res. 2012;64:1108-1115.
77. Chatzidionysiou K, Vollenhoven RF. Rituximab versus anti-TNF in patients who previously failed one TNF inhibitor in an observational cohort. Scand J Rheumatol. 2013;42:190-195.
78. Johnston SS, Turpcu A, Shi N, et al. Risk of infections in rheumatoid arthritis patients switching from anti-TNF agents to rituximab, abatacept, or another anti-TNF agent, a retrospective administrative claims analysis. Semim Arthritis Rheum. 2013;43:39-47.
79. Curtis JR, Xie F, Chen L, et al. The comparative risk of serious infections among rheumatoid arthritis patients starting or switching biological agents. Ann Rheum Dis. 2011;70:1401.
80. Desai RJ, Solomon DH, Jin Y, et al. Temporal trends in use of biologic DMARDs for rheumatoid arthritis in the United States: a cohort study of publicly and privately insured patients. J Manag Care Spec Pharm. 2017;23:809-814.
81. Jin Y, Desai RJ, Liu J, et al. Factors associated with initial or subsequent choice of biologic disease-modifying antirheumatic drugs for treatment of rheumatoid arthritis. Arthritis Res Ther. 2017;19:159.
82. Bonafede MMK, McMorrow D, Proudfoot C, et al. Treatment persistence and healthcare costs among patients with rheumatoid arthritis after a change in targeted therapy. Am Health Drug Benefits. 2018;11:192-202.
83. US Food and Drug Administration. Biosimilars are safe, effective treatment options. www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/biosimilars/. Accessed November 9, 2018.
84. Genovese MC, Greenwald M, Codding C, et al. Peficitinib, a JAK inhibitor, in combination with limited conventional synthetic disease-modifying antirheumatic drugs in the treatment of moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2017;69:932-942.
85. Fleischmann RM, Wagner F, Kivitz AJ, et al. Safety, tolerability, and pharmacodynamics of ABT-122, a tumor necrosis factor- and interleukin-17-targeted dual variable domain immunoglobulin, in patients with rheumatoid arthritis. Arthritis Rheumatol. 2017;69:2283-2291.
86. Burmester GR, McInnes IB, Kremer JM, et al. Mavrilimumab, a fully human granulocyte-macrophage colony-stimulating factor receptor alpha monoclonal antibody: long-term safety and efficacy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2018;70:679-689.
87. Huizinga TW, Batalov A, Stoilov R, et al. Phase 1b randomized, double-blind study of namilumab, an anti-granulocyte macrophage colony-stimulating factor monoclonal antibody, in mild-to-moderate rheumatoid arthritis. Arthritis Res Ther. 2017;19:53.
88. Stock T, Fleishaker D, Wang X, et al. Improved disease activity with fosdagrocorat (PF-04171327), a partial agonist of the glucocorticoid receptor, in patients with rheumatoid arthritis: a Phase 2 randomized study. Int J Rheum Dis. 2017;20:960-970.
89. Orencia [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2013.
90. Humira[package insert]. North Chicago, IL: AbbVie; 2012.
91. Kineret [package insert]. Stockholm, Sweden: Sobi; 2012.
92. Olumiant [package insert]. Indianapolis, IN: Lilly USA, LLC; 2018.
93. Cimzia [package insert]. Smyrna, GA: UCB, Inc; 2008.
94. Enbrel [package insert]. Thousand Oaks, CA: Immunex Corporation; 1998.
95. Simponi [package insert]. Horsham, PA: Janssen Biotech, Inc; 2009.
96. Remicade [package insert]. Horsham, PA: Janssen Biotech, Inc; 1998.
97. Rituxan [package insert]. South San Francisco, CA: Genetech, Inc; 1997.
98. Kevzara [package insert]. Bridgewater, NJ: Sanofi-Aventis US LLC; 2018.
99. Actemra [package insert]. South San Francisco, CA: Genentech, Inc; 2013.
100. Xeljanz [package insert]. New York, NY: Pfizer Inc; 2016.
1. Keystone ED, Kavanaugh KA. What to do with TNF failures. Expert Opin Drug Saf. 2005;4:149-155.
2. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707-718.
3. Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253-259.
4. Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50(5):1400-1411.
5. Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594-1602.
6. Finckh A, Simard JF, Gabay C, et al. Evidence for differential acquired drug resistance to anti-tumour necrosis factor agents in rheumatoid arthritis. Ann Rheum Dis. 2006;65:746-752.
7. Souto A, Maneiro JR, Gomez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology. 2016;55:523-534.
8. Hetland ML, Christensen IJ, Tarp U, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010;62:22-32.
9. Gabay C, Riek M, Scherer A, et al. Effectiveness of biologic DMARDs in monotherapy versus in combination with synthetic DMARDs in rheumatoid arthritis: data from the Swiss Clinical Quality Management Registry. Rheumatology. 2015;54(9):1664-1672.
10. Ebina K, Hashimoto M, Yamamoto W, et al. Drug retention and discontinuation reasons between seven biologics in patients with rheumatoid arthritis-The ANSWER cohort study. PloS One. 2018;13:e0194130.
11. Wijbrandts CA, Tak PP. Prediction of response to targeted treatment in rheumatoid arthritis. Mayo Clin Proc. 2017;92:1129-1143.
12. Ulfgren AK, Andersson U, Engstrom M, et al. Systemic anti-tumor necrosis factor alpha therapy in rheumatoid arthritis down-regulates synovial tumor necrosis factor alpha synthesis. Arthritis Rheum. 2000;43:2391-2396.
13. Choi IY, Gerlag DM, Herenius MJ, et al. MRP8/14 serum levels as a strong predictor of response to biological treatments in patients with rheumatoid arthritis. Ann Rheum Dis. 2015;74:499-505.
14. Nguyen MVC, Baillet A, Romand X, et al. Prealbumin, platelet factor 4 and S100A12 combination at baseline predicts good response to TNF alpha inhibitors in rheumatoid arthritis. Joint Bone Spine. 2019;86:195-201.
15. Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28-Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score-II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), Chronic Arthritis Systemic Index (CASI), Patient-Based Disease Activity Score With ESR (PDAS1) and Patient-Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI-RA). Arthritis Care Res. 2011;63(suppl 11):S14-S36.
16. Katz L, Gisbert JP, Manoogian B, et al. Doubling the infliximab dose versus halving the infusion intervals in Crohn’s disease patients with loss of response. Inflamm Bowel Dis. 2012;18:2026-2033.
17. Durez P, Van den Bosch F, Corluy L, et al. A dose adjustment in patients with rheumatoid arthritis not optimally responding to a standard dose of infliximab of 3 mg/kg every 8 weeks can be effective: a Belgian prospective study. Rheumatology. 2005;44:465-468.
18. Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26-37.
19. St Clair EW, Wagner CL, Fasanmade AA, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:1451-1459.
20. Rahman MU, Strusberg I, Geusens P, et al. Double-blinded infliximab dose escalation in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:1233-1238.
21. Weinblatt ME, Schiff MH, Ruderman EM, et al. Efficacy and safety of etanercept 50 mg twice a week in patients with rheumatoid arthritis who had a suboptimal response to etanercept 50 mg once a week: results of a multicenter, randomized, double-blind, active drug-controlled study. Arthritis Rheum. 2008;58:1921-1930.
22. Curtis JR, Chen L, Luijtens K, et al. Dose escalation of certolizumab pegol from 200 mg to 400 mg every other week provides no additional efficacy in rheumatoid arthritis: an analysis of individual patient-level data. Arthritis Rheum. 2011;63:2203-2208.
23. Okazaki M, Kobayashi H, Ishii Y, et al. Real-world treatment patterns for golimumab and concomitant medications in Japanese rheumatoid arthritis patients. Rheumatol Ther. 2018;5:185-201.
24. Pappas DA, John A, Curtis JR, et al. Dosing of intravenous tocilizumab in a real-world setting of rheumatoid arthritis: analyses from the Corrona Registry. Rheumatol Ther. 2016;3:103-115.
25. Kivitz A, Olech E, Borofsky MA, et al. Two-year efficacy and safety of subcutaneous tocilizumab in combination with disease-modifying antirheumatic drugs including escalation to weekly dosing in rheumatoid arthritis. J Rheumatol. 2018;45:456-464.
26. Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960-977.
27. Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2016;68:1-25.
28. Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11:81-88.
29. Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:493-507.
30. Navarro Coy NC, Brown S, Bosworth A, et al. The ‘Switch’ study protocol: a randomised-controlled trial of switching to an alternative tumour-necrosis factor (TNF)-inhibitor drug or abatacept or rituximab in patients with rheumatoid arthritis who have failed an initial TNF-inhibitor drug. BMC Musculoskelet Disord. 2014;15:452.
31. Kamal KM, Madhavan SS, Hornsby JA, et al. Use of tumor necrosis factor inhibitors in rheumatoid arthritis: a national survey of practicing United States rheumatologists. Joint Bone Spine. 2006;73:718-724.
32. Gomez-Reino JJ, Carmona L, BIOBADASER Group. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006;8:R29.
33. Caporali R, Sarzi-Puttini P, Atzeni F, et al. Switching TNF-alpha antagonists in rheumatoid arthritis: The experience of the LORHEN registry. Autoimmun Rev. 2010;9:465-469.
34. Iannone F, Trotta F, Monteccuco C, et al. Etanercept maintains the clinical benefit achieved by infliximab in patients with rheumatoid arthritis who discontinued infliximab because of side effects. Ann Rheum Dis. 2007;66:249-252.
35. Virkki LM, Valleala H, Takakubo Y, et al. Outcomes of switching anti-TNF drugs in rheumatoid arthritis—a study based on observational data from the Finnish Register of Biological Treatment (ROB-FIN). Clin Rheumatol. 2011;30:1447-1454.
36. Furst DE, Gaylis N, Bray V, et al. Open-label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: the opposite study. Ann Rheum Dis. 2007;66:893-899.
37. Smolen JS, Kay J, Doyle MK, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2009;374:210-221.
38. Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor α inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.
39. Smolen JS, Burmester G-R, Combe B, et al. Head-to-head comparison of certolizumab pegol versus adalimumab in rheumatoid arthritis: 2-year efficacy and safety results from the randomised EXXELERATE study. Lancet. 2016;388:2763-2774.
40. Chatzidionysiou K, Askling J, Eriksson J, et al. Effectiveness of TNF inhibitor switch in RA: results from the national Swedish register. Ann Rheum Dis. 2015;74:890.
41. Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor alpha inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.
42. Lequerré T, Farran É, Ménard J-F, et al. Switching from an anti-TNF monoclonal antibody to soluble TNF-receptor yields better results than vice versa: An observational retrospective study of 72 rheumatoid arthritis switchers. Joint Bone Spine. 2015;82:330-337.
43. Favalli EG, Biggioggero M, Meroni PL. Methotrexate for the treatment of rheumatoid arthritis in the biologic era: Still an “anchor” drug? Autoimmun Rev. 2014;13:1102-1108.
44. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707-718.
45. Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675-681.
46. Emery P, Fleischmann RM, Strusberg I, et al. Efficacy and safety of subcutaneous golimumab in methotrexate-naive patients with rheumatoid arthritis: five-year results of a randomized clinical trial. Arthritis Care Res. 2016;68:744-752.
47. Emery P, Fleischmann RM, Moreland LW, et al. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 2009;60:2272-2283.
48. Emery P, Burmester GR, Bykerk VP, et al. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis. 2015;74:19-26.
49. Westhovens R, Robles M, Ximenes AC, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis. 2009;68:1870-1877.
50. Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793-2806.
51. Burmester GR, Rigby WF, van Vollenhoven RF, et al. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann Rheum Dis. 2016;75:1081-1091.
52. Bijlsma JWJ, Welsing PMJ, Woodworth TG, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet. 2016;388:343-355.
53. Cohen JD, Zaltni S, Kaiser MJ, et al. Secondary addition of methotrexate to partial responders to etanercept alone is effective in severe rheumatoid arthritis. Ann Rheum Dis. 2004;63:209-210.
54. Hamilton RA, Kremer JM. Why intramuscular methotrexate may be more efficacious than oral dosing in patients with rheumatoid arthritis. Br J Rheumatol. 1997;36:86-90.
55. Hoekstra M, Haagsma C, Neef C, et al. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J Rheumatol. 2004;31:645-648.
56. Herman RA, Veng-Pedersen P, Hoffman J, et al. Pharmacokinetics of low-dose methotrexate in rheumatoid arthritis patients. J Pharm Sci. 1989;78:165-171.
57. Schiff MH, Jaffe JS, Freundlich B. Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses ± 15 mg may be overcome with subcutaneous administration. Ann Rheum Dis. 2014;73:1549-1551.
58. Hazlewood GS, Thorne JC, Pope JE, et al. The comparative effectiveness of oral versus subcutaneous methotrexate for the treatment of early rheumatoid arthritis. Ann Rheum Dis. 2016;75:1003-1008.
59. O’Dell JR, Petersen K, Leff R, et al. Etanercept in combination with sulfasalazine, hydroxychloroquine, or gold in the treatment of rheumatoid arthritis. J Rheumatol. 2006;33:213-218.
60. Finckh A, Dehler S, Gabay C. The effectiveness of leflunomide as a co-therapy of tumour necrosis factor inhibitors in rheumatoid arthritis: a population-based study. Ann Rheum Dis. 2009;68:33-39.
61. De Stefano R, Frati E, Nargi F, et al. Comparison of combination therapies in the treatment of rheumatoid arthritis: leflunomide-anti-TNF-alpha versus methotrexate-anti-TNF-alpha. Clin Rheumatol. 2010;29:517-524.
62. Combe B, Codreanu C, Fiocco U, et al. Etanercept and sulfasalazine, alone and combined, in patients with active rheumatoid arthritis despite receiving sulfasalazine: a double-blind comparison. Ann Rheum Dis. 2006;65:1357-1362.
63. Strangfeld A, Hierse F, Kekow J, et al. Comparative effectiveness of tumour necrosis factor α inhibitors in combination with either methotrexate or leflunomide. Ann Rheum Dis. 2009;68:1856.
64. Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516.
65. Genovese MC, Becker J-C, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor α inhibition. N Engl J Med. 2005;353:1114-1123.
66. Emery P, Gottenberg JE, Rubbert-Roth A, et al. Rituximab versus an alternative TNF inhibitor in patients with rheumatoid arthritis who failed to respond to a single previous TNF inhibitor: SWITCH-RA, a global, observational, comparative effectiveness study. Ann Rheum Dis. 2015;74:979-984.
67. Keystone E, Emery P, Peterfy CG, et al. Rituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapies. Ann Rheum Dis. 2009;68:216.
68. Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451-460.
69. Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374:1243-1252.
70. Favalli EG, Biggioggero M, Marchesoni A, Meroni PL. Survival on treatment with second-line biologic therapy: a cohort study comparing cycling and swap strategies. Rheumatology. 2014;53:1664-1668.
71. Harrold LR, Reed GW, Solomon DH, et al. Comparative effectiveness of abatacept versus tocilizumab in rheumatoid arthritis patients with prior TNFi exposure in the US Corrona registry. Arthritis Res Ther. 2016;18:280.
72. Gottenberg J, Brocq O, Perdriger A, et al. Non–TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug: A randomized clinical trial. JAMA. 2016;316:1172-1180.
73. Pascart T, Philippe P, Drumez E, et al. Comparative efficacy of tocilizumab, abatacept and rituximab after non-TNF inhibitor failure: results from a multicentre study. Int J Rheum Dis. 2016;19:1093-1102.
74. Akiyama M, Kaneko Y, Kondo H, Takeuchi T. Comparison of the clinical effectiveness of tumour necrosis factor inhibitors and abatacept after insufficient response to tocilizumab in patients with rheumatoid arthritis. Clin Rheumatol. 2016;35:2829-2834.
75. Schoels M, Aletaha D, Smolen JS, Wong JB. Comparative effectiveness and safety of biological treatment options after tumour necrosis factor α inhibitor failure in rheumatoid arthritis: systematic review and indirect pairwise meta-analysis. Ann Rheum Dis. 2012;71:1303.
76. Soliman MM, Hyrich KL, Lunt M, et al. Rituximab or a second anti-tumor necrosis factor therapy for rheumatoid arthritis patients who have failed their first anti-tumor necrosis factor therapy? Comparative analysis from the British Society for Rheumatology Biologics Register. Arthritis Care Res. 2012;64:1108-1115.
77. Chatzidionysiou K, Vollenhoven RF. Rituximab versus anti-TNF in patients who previously failed one TNF inhibitor in an observational cohort. Scand J Rheumatol. 2013;42:190-195.
78. Johnston SS, Turpcu A, Shi N, et al. Risk of infections in rheumatoid arthritis patients switching from anti-TNF agents to rituximab, abatacept, or another anti-TNF agent, a retrospective administrative claims analysis. Semim Arthritis Rheum. 2013;43:39-47.
79. Curtis JR, Xie F, Chen L, et al. The comparative risk of serious infections among rheumatoid arthritis patients starting or switching biological agents. Ann Rheum Dis. 2011;70:1401.
80. Desai RJ, Solomon DH, Jin Y, et al. Temporal trends in use of biologic DMARDs for rheumatoid arthritis in the United States: a cohort study of publicly and privately insured patients. J Manag Care Spec Pharm. 2017;23:809-814.
81. Jin Y, Desai RJ, Liu J, et al. Factors associated with initial or subsequent choice of biologic disease-modifying antirheumatic drugs for treatment of rheumatoid arthritis. Arthritis Res Ther. 2017;19:159.
82. Bonafede MMK, McMorrow D, Proudfoot C, et al. Treatment persistence and healthcare costs among patients with rheumatoid arthritis after a change in targeted therapy. Am Health Drug Benefits. 2018;11:192-202.
83. US Food and Drug Administration. Biosimilars are safe, effective treatment options. www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/biosimilars/. Accessed November 9, 2018.
84. Genovese MC, Greenwald M, Codding C, et al. Peficitinib, a JAK inhibitor, in combination with limited conventional synthetic disease-modifying antirheumatic drugs in the treatment of moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2017;69:932-942.
85. Fleischmann RM, Wagner F, Kivitz AJ, et al. Safety, tolerability, and pharmacodynamics of ABT-122, a tumor necrosis factor- and interleukin-17-targeted dual variable domain immunoglobulin, in patients with rheumatoid arthritis. Arthritis Rheumatol. 2017;69:2283-2291.
86. Burmester GR, McInnes IB, Kremer JM, et al. Mavrilimumab, a fully human granulocyte-macrophage colony-stimulating factor receptor alpha monoclonal antibody: long-term safety and efficacy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2018;70:679-689.
87. Huizinga TW, Batalov A, Stoilov R, et al. Phase 1b randomized, double-blind study of namilumab, an anti-granulocyte macrophage colony-stimulating factor monoclonal antibody, in mild-to-moderate rheumatoid arthritis. Arthritis Res Ther. 2017;19:53.
88. Stock T, Fleishaker D, Wang X, et al. Improved disease activity with fosdagrocorat (PF-04171327), a partial agonist of the glucocorticoid receptor, in patients with rheumatoid arthritis: a Phase 2 randomized study. Int J Rheum Dis. 2017;20:960-970.
89. Orencia [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2013.
90. Humira[package insert]. North Chicago, IL: AbbVie; 2012.
91. Kineret [package insert]. Stockholm, Sweden: Sobi; 2012.
92. Olumiant [package insert]. Indianapolis, IN: Lilly USA, LLC; 2018.
93. Cimzia [package insert]. Smyrna, GA: UCB, Inc; 2008.
94. Enbrel [package insert]. Thousand Oaks, CA: Immunex Corporation; 1998.
95. Simponi [package insert]. Horsham, PA: Janssen Biotech, Inc; 2009.
96. Remicade [package insert]. Horsham, PA: Janssen Biotech, Inc; 1998.
97. Rituxan [package insert]. South San Francisco, CA: Genetech, Inc; 1997.
98. Kevzara [package insert]. Bridgewater, NJ: Sanofi-Aventis US LLC; 2018.
99. Actemra [package insert]. South San Francisco, CA: Genentech, Inc; 2013.
100. Xeljanz [package insert]. New York, NY: Pfizer Inc; 2016.
Suicidal, violent, and treatment-resistant
CASE Violent, then catatonic
Mr. T, age 52, has a long history of schizoaffective disorder, depressed type; several suicide attempts; and violent episodes. He is admitted to a mental health rehabilitation center under a forensic commitment.
Several years earlier, Mr. T had been charged with first-degree attempted murder, assault with a deadly weapon, and abuse of a dependent/geriatric adult after allegedly stabbing his mother in the upper chest and neck. At that time, Mr. T was not in psychiatric treatment and was drinking heavily. He had become obsessed with John F. Kennedy’s assassination and believed the Central Intelligence Agency (CIA), not Lee Harvey Oswald, was responsible. He feared the CIA wanted to kill him because of his knowledge, and he heard voices from his television he believed were threatening him. He acquired knives for self-protection. When his mother arrived at his apartment to take him to a psychiatric appointment, he believed she was conspiring with the CIA and attacked her. Mr. T’s mother survived her injuries. He was taken to the county jail, where psychiatric staff noted that Mr. T was psychotic.
The court found Mr. T incompetent to stand trial and sent him to a state hospital for psychiatric treatment and competency restoration. After 3 years, he was declared unable to be restored because of repeated decompensations, placed on a conservatorship, and sent back to county jail.
In the jail, Mr. T began to show signs of catatonia. He refused medications, food, and water, and became mute. He was admitted to a medical center after a 45-minute episode that appeared similar to a seizure; however, all laboratory evaluations were within normal limits, head CT was negative, and an EEG was unremarkable.
Mr. T’s catatonic state gradually resolved with increasing dosages of lorazepam, as well as clozapine. He showed improved mobility and oral intake. A month later, his train of thought was rambling and difficult to follow, circumstantial, and perseverating. However, at times he could be directed and respond to questions in a linear and logical fashion. Lorazepam was tapered, discontinued, and replaced with gabapentin because Mr. T viewed taking lorazepam as a threat to his sobriety.
Recently, Mr. T was transferred to our mental health rehabilitation center, where he expresses that he is grateful to be in a therapeutic environment. Upon admission, his medication regimen consists of clozapine, 300 mg by mouth at bedtime, duloxetine, 60 mg/d by mouth, gabapentin 600 mg by mouth 3 times a day, and docusate sodium, 250 mg/d by mouth. Our team has a discussion about the growing recognition of the pro-inflammatory state present in many patients who experience serious mental illness and the importance of augmenting standard evidence-based psychopharmacotherapy with agents that have neuroprotective properties.1,2 We offer Mr. T
[polldaddy:10375843]
The authors’ observations
Several studies have found that acute psychosis is associated with an inflammatory state, and interleukin-6 (IL-6) is a crucial biomarker. A recent meta-analysis of serum cytokines in patients with schizophrenia found that IL-6 levels were significantly increased among acutely ill patients compared with controls.3 IL-6 levels significantly decreased after treating acute episodes of schizophrenia.3 Further, levels of peripheral IL-6 mRNA levels in individuals with schizophrenia are directly correlated with severity of positive symptoms.4
Continue to: A meta-analyis reported...
A meta-analysis reported that tumor necrosis factor-alpha and IL-6 are elevated during acute psychosis3; however, IL-6 normalized with treatment, whereas tumor necrosis factor-alpha did not. This means that IL-6 is a more clinically meaningful biomarker to help gauge treatment response.
EVALUATION Elevated markers of inflammation
Laboratory testing reveals that Mr. T’s IL-6 level is 56.64 pg/mL, which is significantly elevated (reference range: 0.31 to 5.00 pg/mL). After reviewing the IL-6 results with Mr. T and explaining that there is “too much inflammation” in his brain, he agrees to take minocycline and complete follow-up IL-6 level tests to monitor his progress during treatment.
HISTORY Alcohol abuse, treatment resistance
According to Mr. T’s mother, he had met all developmental milestones and graduated from high school with plans to enter culinary school. At age 20, Mr. T began to experience psychotic symptoms, telling family members that he was being followed by FBI agents and was receiving messages from televisions. He began drinking heavily and was arrested twice for driving under the influence. In his mid-20s, he attempted suicide by overdose after his father died. Mr. T required inpatient hospitalization nearly every year thereafter. His mother, a registered nurse, was significantly involved in his care and carefully documented his treatment history.
Mr. T has had numerous medication trials, including oral and long-acting injectable risperidone, olanzapine, aripiprazole, ziprasidone, lithium, gabapentin, buspirone, quetiapine, trazodone, bupropion, and paroxetine. None of these medications were effective.
In his mid-40s, Mr. T attempted suicide by wandering into traffic and being struck by a motor vehicle. A year later, he attempted suicide by driving his car at high speed into a concrete highway median. Mr. T told first responders that he was “possessed,” and a demonic entity “forced” him to crash his car. He begged law enforcement officers at the scene to give him a gun so he could shoot himself.
Continue to: Mr. T entered an intensive outpatient treatment program...
Mr. T entered an intensive outpatient treatment program and was switched from long-acting injectable risperidone to oral aripiprazole. After taking aripiprazole for several weeks, he began to gamble compulsively at a nearby casino. Frustrated by the lack of response to psychotropic medications and his idiosyncratic response to aripiprazole, he stopped psychiatric treatment, relapsed to alcohol use, and isolated himself in his apartment shortly before stabbing his mother.
EVALUATION Pharmacogenomics testing
At the mental health rehabilitation center, Mr. T agrees to undergo pharmacogenomics testing, which suggests that he will have a normal response to selective serotonin reuptake inhibitors and is unlikely to experience adverse reactions. He does not carry the 2 alleles that place him at higher risk of serious dermatologic reactions when taking certain mood stabilizers. He is heterozygous for the C677T allele polymorphism in the MTHFR gene that is associated with reduced folic acid metabolism, moderately decreased serum folate levels, and moderately increased homocysteine levels. On the pharmacokinetic genes tested, Mr. T has the normal metabolism genotype on 5 of 6 cytochrome P450 (CYP) enzymes; he has the ultrarapid metabolizer genotype on CYP1A2. He also has normal activity and intermediate metabolizer phenotype on the 2 UGT enzymes tested, which are responsible for the glucuronidation process, a major part of phase II metabolism.
Based on these results, Mr. T’s clozapine dosage is decreased by 50% (from 300 to 150 mg/d) and he is started on fluvoxamine, 50 mg/d, because it is a strong inhibitor of CYP1A2. The reduced conversion of clozapine to norclozapine results in an average serum clozapine level of 527 ng/mL (a level of 350 ng/mL is usually therapeutic in patients with schizophrenia) and norclozapine level of 140 ng/mL (clozapine:norclozapine ratio = 3.8), which is to be expected because fluvoxamine can increase serum clozapine levels.
Due to accumulating evidence in the literature suggesting that latent infections in the CNS play a role in serious mental illnesses such as schizophrenia, Mr. T undergoes further laboratory testing.
[polldaddy:10375845]
The authors’ observations
Mr. T tested positive for TG and CMV and negative for HSV-1. We were aware of accumulating evidence that latent infections in the CNS play a role in serious mental illnesses such as schizophrenia, specifically TG5—a parasite transmitted by cats—and CMV and HSV-1,6 which are transmitted by humans. The theory that TG infection could be a factor in schizophrenia emerged in the 1990s but only in recent years received mainstream scientific attention. Toxoplasma gondii, the infectious parasite that causes toxoplasmosis, infects more than 30 million people in the United States; however, most individuals are asymptomatic because of the body’s immune response to the parasite.7
Continue to: A study of 162 individuals...
A study of 162 individuals with schizophrenia, bipolar disorder, or major depressive disorder found that this immunologic profile is associated with suicide attempts,8 which is consistent with Mr. T’s history. Research suggests that individuals with schizophrenia who have latent TG infection have a more severe form of the illness compared with patients without the infection.9-12 Many of these factors were present in Mr. T’s case (Table 18-12).
TREATMENT Improvement, then setback
Mr. T’s medication regimen at the rehabilitation center includes clozapine, 100 mg/d; minocycline, 200 mg/d; fluvoxamine, 200 mg/d; and N-acetylcysteine, 1,200 mg/d. N-acetylcysteine is an antioxidant that could ease negative symptoms of schizophrenia by reducing oxidative stress caused by free radicals.13 Mr. T makes slow but steady improvement, and his IL-6 levels drop steadily (Figure 1).
After 6 months in the rehabilitation center, Mr. T no longer experiences catatonic symptoms and is able to participate in the therapeutic program. He is permitted to leave the facility on day passes with family members. However, approximately every 8 weeks, he continues to cycle through periods of intense anxiety, perseverates on topics, and exhibits fragmented thinking and speech. During these episodes, he has difficulty receiving and processing information.
During one of these periods, Mr. T eats 4 oleander leaves he gathered while on day pass outside of the facility. After he experiences stomach pain, nausea, and vomiting, he informs nursing staff that he ate oleander. He is brought to the emergency department, receives activated charcoal and a digoxin antidote, and is placed on continuous electrocardiogram monitoring. When asked why he made the suicide attempt, he said “I realized things will never be the same because of what happened. I felt trapped.” He later expresses regret and wants to return to the mental health rehabilitation center.
At the facility, Mr. T agrees to take 2 more agents—valproic acid and ginger root extract—that specifically target latent toxoplasmosis infection before pursuing electroconvulsive therapy. We offer valproic acid because it inhibits replication of TG in an in vitro model.14 Mr. T is started on extended-release valproic acid, 1,500 mg/d, which results in a therapeutic serum level of 74.8 µg/mL.
Continue to: Additionally, Mr. T expresses interest...
Additionally, Mr. T expresses interest in taking “natural” agents in addition to psychotropics. After reviewing the quality of available ginger root extract products, Mr. T is started on a supplement that contains 22.4 mg of gingerols and 6.7 mg of shogaols, titrated to 4 capsules twice daily.
The authors’ observations
A retrospective cross-sectional analysis reported that patients with bipolar disorder who received medications with anti-toxoplasmic activity (Table 215), specifically valproic acid, had significantly fewer lifetime depressive episodes compared with patients who received medications without anti-toxoplasmic activity.15
Alternative medicine options
Research has demonstrated the beneficial effects of Chinese herbal plants for toxoplasmosis16,17 and ginger root extract has potent anti-toxoplasmic activity. A mouse model found that ginger root extract (Zingiber officinale) reduced the number of TG-infected cells by suppressing activation of apoptotic proteins the parasite induces, which prevents programmed cell death.18
Table 3 presents a stepwise approach to identifying and treating inflammation in patients with treatment-resistant psychosis.
OUTCOME Immune response, improvement
One month after the valproic acid and ginger root extract therapy is initiated, Mr. T’s toxoplasma antibody immunoglobulin G increases by 15.2 IU/mL, indicating that his immune system is mounting an enhanced response against the parasite (Figure 2). Mr. T continues to make progress while receiving the new regimen of clozapine, minocycline, valproic acid, and ginger root extract. He no longer cycles into periods of intense anxiety, perseverative thought, and fragmented thought and speech. He participates meaningfully in weekly psychotherapy and hopes to live independently and obtain gainful employment.
The District Attorney’s office dismisses his criminal charges, and Mr. T is discharged to a less restrictive level of care.
Continue to: Bottom Line
Bottom Line
Several studies have shown that neuroinflammation increases the severity of mental illness. Consider adjunct anti-inflammatory agents for patients who have elevated levels of inflammatory biomarkers and for whom standard treatment approaches do not adequately control psychiatric symptoms. Also consider testing for the presence of latent infections in the CNS, which could reveal the underlying cause of treatment resistance or the genesis of disabling psychiatric symptoms.
Related Resources
- Fond G, Macgregor A, Tamouza R, et al. Comparative analysis of anti-toxoplasmic activity of antipsychotic drugs and valproate. Eur Arch Psychiatry Clin Neurosci. 2014;264(2):179-183.
- Hamdani N, Daban-Huard C, Lajnef M, et al. Cognitive deterioration among bipolar disorder patients infected by Toxoplasma gondii is correlated to interleukin 6 levels. J Affect Disord. 2015;179:161-166.
- Monroe JM, Buckley PF, Miller BJ. Meta-analysis of antitoxoplasma gondii IgM antibodies in acute psychosis. Schizophr Bull. 2015;41(4):989-998.
Drug Brand Names
Acyclovir • Zovirax
Aripiprazole • Abilify
Bupropion • Wellbutrin
Buspirone • Buspar
Clozapine • Clozaril
Duloxetine • Cymbalta
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Gabapentin • Neurontin
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Loxapine • Loxitane
Minocycline • Minocin
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone • Risperdal, Risperdal Consta
Thioridazine • Mellaril
Trifluoperazine • Stelazine
Trazodone • Desyrel
Valproic acid • Depakote
Ziprasidone • Geodon
1. Koola MM, Raines JK, Hamilton RG, et al. Can anti-inflammatory medications improve symptoms and reduce mortality in schizophrenia? Current Psychiatry. 2016;15(5):52-57.
2. Nasrallah HA. Are you neuroprotecting your patients? 10 Adjunctive therapies to consider. Current Psychiatry. 2016;15(12):12-14.
3. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21(12):1696-1709.
4. Chase KA, Cone JJ, Rosen C, et al. The value of interleukin 6 as a peripheral diagnostic marker in schizophrenia. BMC Psychiatry. 2016;16:152.
5. Torrey EF, Bartko JJ, Lun ZR, et al. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33(3):729-736.
6. Shirts BH, Prasad KM, Pogue-Geile MF, et al. Antibodies to cytomegalovirus and herpes simplex virus 1 associated with cognitive function in schizophrenia. Schizophr Res. 2008;106(2-3):268-274.
7. Centers for Disease Control and Prevention. Parasites - Toxoplasmosis (Toxoplasma infection). https://www.cdc.gov/parasites/toxoplasmosis/index.html. Accessed February 26, 2019.
8. Dickerson F, Wilcox HC, Adamos M, et al. Suicide attempts and markers of immune response in individuals with serious mental illness. J Psychiatr Res. 2017;87:37-43.
9. Celik T, Kartalci S, Aytas O, et al. Association between latent toxoplasmosis and clinical course of schizophrenia - continuous course of the disease is characteristic for Toxoplasma gondii-infected patients. Folia Parasitol (Praha). 2015;62. doi: 10.14411/fp.2015.015.
10. Dickerson F, Boronow J, Stallings C, et al. Toxoplasma gondii in individuals with schizophrenia: association with clinical and demographic factors and with mortality. Schizophr Bull. 2007;33(3):737-740.
11. Esshili A, Thabet S, Jemli A, et al. Toxoplasma gondii infection in schizophrenia and associated clinical features. Psychiatry Res. 2016;245:327-332.
12. Holub D, Flegr J, Dragomirecka E, et al. Differences in onset of disease and severity of psychopathology between toxoplasmosis-related and toxoplasmosis-unrelated schizophrenia. Acta Psychiatr Scand. 2013;127(3):227-238.
13. Chen AT, Chibnall JT, Nasrallah HA. Placebo-controlled augmentation trials of the antioxidant NAC in schizophrenia: a review. Ann Clin Psychiatry. 2016;28(3):190-196.
14. Jones-Brando L, Torrey EF, Yolken R. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr Res. 2003;62(3):237-244.
15. Fond G, Boyer L, Gaman A, et al. Treatment with anti-toxoplasmic activity (TATA) for toxoplasma positive patients with bipolar disorders or schizophrenia: a cross-sectional study. J Psychiatr Res. 2015;63:58-64.
16. Wei HX, Wei SS, Lindsay DS, et al. A systematic review and meta-analysis of the efficacy of anti-Toxoplasma gondii medicines in humans. PLoS One. 2015;10(9):e0138204.
17. Zhuo XH, Sun HC, Huang B, et al. Evaluation of potential anti-toxoplasmosis efficiency of combined traditional herbs in a mouse model. J Zhejiang Univ Sci B. 2017;18(6):453-461.
18. Choi WH, Jiang MH, Chu JP. Antiparasitic effects of Zingiber officinale (Ginger) extract against Toxoplasma gondii. Journal of Applied Biomedicine. 2013;11:15-26.
CASE Violent, then catatonic
Mr. T, age 52, has a long history of schizoaffective disorder, depressed type; several suicide attempts; and violent episodes. He is admitted to a mental health rehabilitation center under a forensic commitment.
Several years earlier, Mr. T had been charged with first-degree attempted murder, assault with a deadly weapon, and abuse of a dependent/geriatric adult after allegedly stabbing his mother in the upper chest and neck. At that time, Mr. T was not in psychiatric treatment and was drinking heavily. He had become obsessed with John F. Kennedy’s assassination and believed the Central Intelligence Agency (CIA), not Lee Harvey Oswald, was responsible. He feared the CIA wanted to kill him because of his knowledge, and he heard voices from his television he believed were threatening him. He acquired knives for self-protection. When his mother arrived at his apartment to take him to a psychiatric appointment, he believed she was conspiring with the CIA and attacked her. Mr. T’s mother survived her injuries. He was taken to the county jail, where psychiatric staff noted that Mr. T was psychotic.
The court found Mr. T incompetent to stand trial and sent him to a state hospital for psychiatric treatment and competency restoration. After 3 years, he was declared unable to be restored because of repeated decompensations, placed on a conservatorship, and sent back to county jail.
In the jail, Mr. T began to show signs of catatonia. He refused medications, food, and water, and became mute. He was admitted to a medical center after a 45-minute episode that appeared similar to a seizure; however, all laboratory evaluations were within normal limits, head CT was negative, and an EEG was unremarkable.
Mr. T’s catatonic state gradually resolved with increasing dosages of lorazepam, as well as clozapine. He showed improved mobility and oral intake. A month later, his train of thought was rambling and difficult to follow, circumstantial, and perseverating. However, at times he could be directed and respond to questions in a linear and logical fashion. Lorazepam was tapered, discontinued, and replaced with gabapentin because Mr. T viewed taking lorazepam as a threat to his sobriety.
Recently, Mr. T was transferred to our mental health rehabilitation center, where he expresses that he is grateful to be in a therapeutic environment. Upon admission, his medication regimen consists of clozapine, 300 mg by mouth at bedtime, duloxetine, 60 mg/d by mouth, gabapentin 600 mg by mouth 3 times a day, and docusate sodium, 250 mg/d by mouth. Our team has a discussion about the growing recognition of the pro-inflammatory state present in many patients who experience serious mental illness and the importance of augmenting standard evidence-based psychopharmacotherapy with agents that have neuroprotective properties.1,2 We offer Mr. T
[polldaddy:10375843]
The authors’ observations
Several studies have found that acute psychosis is associated with an inflammatory state, and interleukin-6 (IL-6) is a crucial biomarker. A recent meta-analysis of serum cytokines in patients with schizophrenia found that IL-6 levels were significantly increased among acutely ill patients compared with controls.3 IL-6 levels significantly decreased after treating acute episodes of schizophrenia.3 Further, levels of peripheral IL-6 mRNA levels in individuals with schizophrenia are directly correlated with severity of positive symptoms.4
Continue to: A meta-analyis reported...
A meta-analysis reported that tumor necrosis factor-alpha and IL-6 are elevated during acute psychosis3; however, IL-6 normalized with treatment, whereas tumor necrosis factor-alpha did not. This means that IL-6 is a more clinically meaningful biomarker to help gauge treatment response.
EVALUATION Elevated markers of inflammation
Laboratory testing reveals that Mr. T’s IL-6 level is 56.64 pg/mL, which is significantly elevated (reference range: 0.31 to 5.00 pg/mL). After reviewing the IL-6 results with Mr. T and explaining that there is “too much inflammation” in his brain, he agrees to take minocycline and complete follow-up IL-6 level tests to monitor his progress during treatment.
HISTORY Alcohol abuse, treatment resistance
According to Mr. T’s mother, he had met all developmental milestones and graduated from high school with plans to enter culinary school. At age 20, Mr. T began to experience psychotic symptoms, telling family members that he was being followed by FBI agents and was receiving messages from televisions. He began drinking heavily and was arrested twice for driving under the influence. In his mid-20s, he attempted suicide by overdose after his father died. Mr. T required inpatient hospitalization nearly every year thereafter. His mother, a registered nurse, was significantly involved in his care and carefully documented his treatment history.
Mr. T has had numerous medication trials, including oral and long-acting injectable risperidone, olanzapine, aripiprazole, ziprasidone, lithium, gabapentin, buspirone, quetiapine, trazodone, bupropion, and paroxetine. None of these medications were effective.
In his mid-40s, Mr. T attempted suicide by wandering into traffic and being struck by a motor vehicle. A year later, he attempted suicide by driving his car at high speed into a concrete highway median. Mr. T told first responders that he was “possessed,” and a demonic entity “forced” him to crash his car. He begged law enforcement officers at the scene to give him a gun so he could shoot himself.
Continue to: Mr. T entered an intensive outpatient treatment program...
Mr. T entered an intensive outpatient treatment program and was switched from long-acting injectable risperidone to oral aripiprazole. After taking aripiprazole for several weeks, he began to gamble compulsively at a nearby casino. Frustrated by the lack of response to psychotropic medications and his idiosyncratic response to aripiprazole, he stopped psychiatric treatment, relapsed to alcohol use, and isolated himself in his apartment shortly before stabbing his mother.
EVALUATION Pharmacogenomics testing
At the mental health rehabilitation center, Mr. T agrees to undergo pharmacogenomics testing, which suggests that he will have a normal response to selective serotonin reuptake inhibitors and is unlikely to experience adverse reactions. He does not carry the 2 alleles that place him at higher risk of serious dermatologic reactions when taking certain mood stabilizers. He is heterozygous for the C677T allele polymorphism in the MTHFR gene that is associated with reduced folic acid metabolism, moderately decreased serum folate levels, and moderately increased homocysteine levels. On the pharmacokinetic genes tested, Mr. T has the normal metabolism genotype on 5 of 6 cytochrome P450 (CYP) enzymes; he has the ultrarapid metabolizer genotype on CYP1A2. He also has normal activity and intermediate metabolizer phenotype on the 2 UGT enzymes tested, which are responsible for the glucuronidation process, a major part of phase II metabolism.
Based on these results, Mr. T’s clozapine dosage is decreased by 50% (from 300 to 150 mg/d) and he is started on fluvoxamine, 50 mg/d, because it is a strong inhibitor of CYP1A2. The reduced conversion of clozapine to norclozapine results in an average serum clozapine level of 527 ng/mL (a level of 350 ng/mL is usually therapeutic in patients with schizophrenia) and norclozapine level of 140 ng/mL (clozapine:norclozapine ratio = 3.8), which is to be expected because fluvoxamine can increase serum clozapine levels.
Due to accumulating evidence in the literature suggesting that latent infections in the CNS play a role in serious mental illnesses such as schizophrenia, Mr. T undergoes further laboratory testing.
[polldaddy:10375845]
The authors’ observations
Mr. T tested positive for TG and CMV and negative for HSV-1. We were aware of accumulating evidence that latent infections in the CNS play a role in serious mental illnesses such as schizophrenia, specifically TG5—a parasite transmitted by cats—and CMV and HSV-1,6 which are transmitted by humans. The theory that TG infection could be a factor in schizophrenia emerged in the 1990s but only in recent years received mainstream scientific attention. Toxoplasma gondii, the infectious parasite that causes toxoplasmosis, infects more than 30 million people in the United States; however, most individuals are asymptomatic because of the body’s immune response to the parasite.7
Continue to: A study of 162 individuals...
A study of 162 individuals with schizophrenia, bipolar disorder, or major depressive disorder found that this immunologic profile is associated with suicide attempts,8 which is consistent with Mr. T’s history. Research suggests that individuals with schizophrenia who have latent TG infection have a more severe form of the illness compared with patients without the infection.9-12 Many of these factors were present in Mr. T’s case (Table 18-12).
TREATMENT Improvement, then setback
Mr. T’s medication regimen at the rehabilitation center includes clozapine, 100 mg/d; minocycline, 200 mg/d; fluvoxamine, 200 mg/d; and N-acetylcysteine, 1,200 mg/d. N-acetylcysteine is an antioxidant that could ease negative symptoms of schizophrenia by reducing oxidative stress caused by free radicals.13 Mr. T makes slow but steady improvement, and his IL-6 levels drop steadily (Figure 1).
After 6 months in the rehabilitation center, Mr. T no longer experiences catatonic symptoms and is able to participate in the therapeutic program. He is permitted to leave the facility on day passes with family members. However, approximately every 8 weeks, he continues to cycle through periods of intense anxiety, perseverates on topics, and exhibits fragmented thinking and speech. During these episodes, he has difficulty receiving and processing information.
During one of these periods, Mr. T eats 4 oleander leaves he gathered while on day pass outside of the facility. After he experiences stomach pain, nausea, and vomiting, he informs nursing staff that he ate oleander. He is brought to the emergency department, receives activated charcoal and a digoxin antidote, and is placed on continuous electrocardiogram monitoring. When asked why he made the suicide attempt, he said “I realized things will never be the same because of what happened. I felt trapped.” He later expresses regret and wants to return to the mental health rehabilitation center.
At the facility, Mr. T agrees to take 2 more agents—valproic acid and ginger root extract—that specifically target latent toxoplasmosis infection before pursuing electroconvulsive therapy. We offer valproic acid because it inhibits replication of TG in an in vitro model.14 Mr. T is started on extended-release valproic acid, 1,500 mg/d, which results in a therapeutic serum level of 74.8 µg/mL.
Continue to: Additionally, Mr. T expresses interest...
Additionally, Mr. T expresses interest in taking “natural” agents in addition to psychotropics. After reviewing the quality of available ginger root extract products, Mr. T is started on a supplement that contains 22.4 mg of gingerols and 6.7 mg of shogaols, titrated to 4 capsules twice daily.
The authors’ observations
A retrospective cross-sectional analysis reported that patients with bipolar disorder who received medications with anti-toxoplasmic activity (Table 215), specifically valproic acid, had significantly fewer lifetime depressive episodes compared with patients who received medications without anti-toxoplasmic activity.15
Alternative medicine options
Research has demonstrated the beneficial effects of Chinese herbal plants for toxoplasmosis16,17 and ginger root extract has potent anti-toxoplasmic activity. A mouse model found that ginger root extract (Zingiber officinale) reduced the number of TG-infected cells by suppressing activation of apoptotic proteins the parasite induces, which prevents programmed cell death.18
Table 3 presents a stepwise approach to identifying and treating inflammation in patients with treatment-resistant psychosis.
OUTCOME Immune response, improvement
One month after the valproic acid and ginger root extract therapy is initiated, Mr. T’s toxoplasma antibody immunoglobulin G increases by 15.2 IU/mL, indicating that his immune system is mounting an enhanced response against the parasite (Figure 2). Mr. T continues to make progress while receiving the new regimen of clozapine, minocycline, valproic acid, and ginger root extract. He no longer cycles into periods of intense anxiety, perseverative thought, and fragmented thought and speech. He participates meaningfully in weekly psychotherapy and hopes to live independently and obtain gainful employment.
The District Attorney’s office dismisses his criminal charges, and Mr. T is discharged to a less restrictive level of care.
Continue to: Bottom Line
Bottom Line
Several studies have shown that neuroinflammation increases the severity of mental illness. Consider adjunct anti-inflammatory agents for patients who have elevated levels of inflammatory biomarkers and for whom standard treatment approaches do not adequately control psychiatric symptoms. Also consider testing for the presence of latent infections in the CNS, which could reveal the underlying cause of treatment resistance or the genesis of disabling psychiatric symptoms.
Related Resources
- Fond G, Macgregor A, Tamouza R, et al. Comparative analysis of anti-toxoplasmic activity of antipsychotic drugs and valproate. Eur Arch Psychiatry Clin Neurosci. 2014;264(2):179-183.
- Hamdani N, Daban-Huard C, Lajnef M, et al. Cognitive deterioration among bipolar disorder patients infected by Toxoplasma gondii is correlated to interleukin 6 levels. J Affect Disord. 2015;179:161-166.
- Monroe JM, Buckley PF, Miller BJ. Meta-analysis of antitoxoplasma gondii IgM antibodies in acute psychosis. Schizophr Bull. 2015;41(4):989-998.
Drug Brand Names
Acyclovir • Zovirax
Aripiprazole • Abilify
Bupropion • Wellbutrin
Buspirone • Buspar
Clozapine • Clozaril
Duloxetine • Cymbalta
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Gabapentin • Neurontin
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Loxapine • Loxitane
Minocycline • Minocin
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone • Risperdal, Risperdal Consta
Thioridazine • Mellaril
Trifluoperazine • Stelazine
Trazodone • Desyrel
Valproic acid • Depakote
Ziprasidone • Geodon
CASE Violent, then catatonic
Mr. T, age 52, has a long history of schizoaffective disorder, depressed type; several suicide attempts; and violent episodes. He is admitted to a mental health rehabilitation center under a forensic commitment.
Several years earlier, Mr. T had been charged with first-degree attempted murder, assault with a deadly weapon, and abuse of a dependent/geriatric adult after allegedly stabbing his mother in the upper chest and neck. At that time, Mr. T was not in psychiatric treatment and was drinking heavily. He had become obsessed with John F. Kennedy’s assassination and believed the Central Intelligence Agency (CIA), not Lee Harvey Oswald, was responsible. He feared the CIA wanted to kill him because of his knowledge, and he heard voices from his television he believed were threatening him. He acquired knives for self-protection. When his mother arrived at his apartment to take him to a psychiatric appointment, he believed she was conspiring with the CIA and attacked her. Mr. T’s mother survived her injuries. He was taken to the county jail, where psychiatric staff noted that Mr. T was psychotic.
The court found Mr. T incompetent to stand trial and sent him to a state hospital for psychiatric treatment and competency restoration. After 3 years, he was declared unable to be restored because of repeated decompensations, placed on a conservatorship, and sent back to county jail.
In the jail, Mr. T began to show signs of catatonia. He refused medications, food, and water, and became mute. He was admitted to a medical center after a 45-minute episode that appeared similar to a seizure; however, all laboratory evaluations were within normal limits, head CT was negative, and an EEG was unremarkable.
Mr. T’s catatonic state gradually resolved with increasing dosages of lorazepam, as well as clozapine. He showed improved mobility and oral intake. A month later, his train of thought was rambling and difficult to follow, circumstantial, and perseverating. However, at times he could be directed and respond to questions in a linear and logical fashion. Lorazepam was tapered, discontinued, and replaced with gabapentin because Mr. T viewed taking lorazepam as a threat to his sobriety.
Recently, Mr. T was transferred to our mental health rehabilitation center, where he expresses that he is grateful to be in a therapeutic environment. Upon admission, his medication regimen consists of clozapine, 300 mg by mouth at bedtime, duloxetine, 60 mg/d by mouth, gabapentin 600 mg by mouth 3 times a day, and docusate sodium, 250 mg/d by mouth. Our team has a discussion about the growing recognition of the pro-inflammatory state present in many patients who experience serious mental illness and the importance of augmenting standard evidence-based psychopharmacotherapy with agents that have neuroprotective properties.1,2 We offer Mr. T
[polldaddy:10375843]
The authors’ observations
Several studies have found that acute psychosis is associated with an inflammatory state, and interleukin-6 (IL-6) is a crucial biomarker. A recent meta-analysis of serum cytokines in patients with schizophrenia found that IL-6 levels were significantly increased among acutely ill patients compared with controls.3 IL-6 levels significantly decreased after treating acute episodes of schizophrenia.3 Further, levels of peripheral IL-6 mRNA levels in individuals with schizophrenia are directly correlated with severity of positive symptoms.4
Continue to: A meta-analyis reported...
A meta-analysis reported that tumor necrosis factor-alpha and IL-6 are elevated during acute psychosis3; however, IL-6 normalized with treatment, whereas tumor necrosis factor-alpha did not. This means that IL-6 is a more clinically meaningful biomarker to help gauge treatment response.
EVALUATION Elevated markers of inflammation
Laboratory testing reveals that Mr. T’s IL-6 level is 56.64 pg/mL, which is significantly elevated (reference range: 0.31 to 5.00 pg/mL). After reviewing the IL-6 results with Mr. T and explaining that there is “too much inflammation” in his brain, he agrees to take minocycline and complete follow-up IL-6 level tests to monitor his progress during treatment.
HISTORY Alcohol abuse, treatment resistance
According to Mr. T’s mother, he had met all developmental milestones and graduated from high school with plans to enter culinary school. At age 20, Mr. T began to experience psychotic symptoms, telling family members that he was being followed by FBI agents and was receiving messages from televisions. He began drinking heavily and was arrested twice for driving under the influence. In his mid-20s, he attempted suicide by overdose after his father died. Mr. T required inpatient hospitalization nearly every year thereafter. His mother, a registered nurse, was significantly involved in his care and carefully documented his treatment history.
Mr. T has had numerous medication trials, including oral and long-acting injectable risperidone, olanzapine, aripiprazole, ziprasidone, lithium, gabapentin, buspirone, quetiapine, trazodone, bupropion, and paroxetine. None of these medications were effective.
In his mid-40s, Mr. T attempted suicide by wandering into traffic and being struck by a motor vehicle. A year later, he attempted suicide by driving his car at high speed into a concrete highway median. Mr. T told first responders that he was “possessed,” and a demonic entity “forced” him to crash his car. He begged law enforcement officers at the scene to give him a gun so he could shoot himself.
Continue to: Mr. T entered an intensive outpatient treatment program...
Mr. T entered an intensive outpatient treatment program and was switched from long-acting injectable risperidone to oral aripiprazole. After taking aripiprazole for several weeks, he began to gamble compulsively at a nearby casino. Frustrated by the lack of response to psychotropic medications and his idiosyncratic response to aripiprazole, he stopped psychiatric treatment, relapsed to alcohol use, and isolated himself in his apartment shortly before stabbing his mother.
EVALUATION Pharmacogenomics testing
At the mental health rehabilitation center, Mr. T agrees to undergo pharmacogenomics testing, which suggests that he will have a normal response to selective serotonin reuptake inhibitors and is unlikely to experience adverse reactions. He does not carry the 2 alleles that place him at higher risk of serious dermatologic reactions when taking certain mood stabilizers. He is heterozygous for the C677T allele polymorphism in the MTHFR gene that is associated with reduced folic acid metabolism, moderately decreased serum folate levels, and moderately increased homocysteine levels. On the pharmacokinetic genes tested, Mr. T has the normal metabolism genotype on 5 of 6 cytochrome P450 (CYP) enzymes; he has the ultrarapid metabolizer genotype on CYP1A2. He also has normal activity and intermediate metabolizer phenotype on the 2 UGT enzymes tested, which are responsible for the glucuronidation process, a major part of phase II metabolism.
Based on these results, Mr. T’s clozapine dosage is decreased by 50% (from 300 to 150 mg/d) and he is started on fluvoxamine, 50 mg/d, because it is a strong inhibitor of CYP1A2. The reduced conversion of clozapine to norclozapine results in an average serum clozapine level of 527 ng/mL (a level of 350 ng/mL is usually therapeutic in patients with schizophrenia) and norclozapine level of 140 ng/mL (clozapine:norclozapine ratio = 3.8), which is to be expected because fluvoxamine can increase serum clozapine levels.
Due to accumulating evidence in the literature suggesting that latent infections in the CNS play a role in serious mental illnesses such as schizophrenia, Mr. T undergoes further laboratory testing.
[polldaddy:10375845]
The authors’ observations
Mr. T tested positive for TG and CMV and negative for HSV-1. We were aware of accumulating evidence that latent infections in the CNS play a role in serious mental illnesses such as schizophrenia, specifically TG5—a parasite transmitted by cats—and CMV and HSV-1,6 which are transmitted by humans. The theory that TG infection could be a factor in schizophrenia emerged in the 1990s but only in recent years received mainstream scientific attention. Toxoplasma gondii, the infectious parasite that causes toxoplasmosis, infects more than 30 million people in the United States; however, most individuals are asymptomatic because of the body’s immune response to the parasite.7
Continue to: A study of 162 individuals...
A study of 162 individuals with schizophrenia, bipolar disorder, or major depressive disorder found that this immunologic profile is associated with suicide attempts,8 which is consistent with Mr. T’s history. Research suggests that individuals with schizophrenia who have latent TG infection have a more severe form of the illness compared with patients without the infection.9-12 Many of these factors were present in Mr. T’s case (Table 18-12).
TREATMENT Improvement, then setback
Mr. T’s medication regimen at the rehabilitation center includes clozapine, 100 mg/d; minocycline, 200 mg/d; fluvoxamine, 200 mg/d; and N-acetylcysteine, 1,200 mg/d. N-acetylcysteine is an antioxidant that could ease negative symptoms of schizophrenia by reducing oxidative stress caused by free radicals.13 Mr. T makes slow but steady improvement, and his IL-6 levels drop steadily (Figure 1).
After 6 months in the rehabilitation center, Mr. T no longer experiences catatonic symptoms and is able to participate in the therapeutic program. He is permitted to leave the facility on day passes with family members. However, approximately every 8 weeks, he continues to cycle through periods of intense anxiety, perseverates on topics, and exhibits fragmented thinking and speech. During these episodes, he has difficulty receiving and processing information.
During one of these periods, Mr. T eats 4 oleander leaves he gathered while on day pass outside of the facility. After he experiences stomach pain, nausea, and vomiting, he informs nursing staff that he ate oleander. He is brought to the emergency department, receives activated charcoal and a digoxin antidote, and is placed on continuous electrocardiogram monitoring. When asked why he made the suicide attempt, he said “I realized things will never be the same because of what happened. I felt trapped.” He later expresses regret and wants to return to the mental health rehabilitation center.
At the facility, Mr. T agrees to take 2 more agents—valproic acid and ginger root extract—that specifically target latent toxoplasmosis infection before pursuing electroconvulsive therapy. We offer valproic acid because it inhibits replication of TG in an in vitro model.14 Mr. T is started on extended-release valproic acid, 1,500 mg/d, which results in a therapeutic serum level of 74.8 µg/mL.
Continue to: Additionally, Mr. T expresses interest...
Additionally, Mr. T expresses interest in taking “natural” agents in addition to psychotropics. After reviewing the quality of available ginger root extract products, Mr. T is started on a supplement that contains 22.4 mg of gingerols and 6.7 mg of shogaols, titrated to 4 capsules twice daily.
The authors’ observations
A retrospective cross-sectional analysis reported that patients with bipolar disorder who received medications with anti-toxoplasmic activity (Table 215), specifically valproic acid, had significantly fewer lifetime depressive episodes compared with patients who received medications without anti-toxoplasmic activity.15
Alternative medicine options
Research has demonstrated the beneficial effects of Chinese herbal plants for toxoplasmosis16,17 and ginger root extract has potent anti-toxoplasmic activity. A mouse model found that ginger root extract (Zingiber officinale) reduced the number of TG-infected cells by suppressing activation of apoptotic proteins the parasite induces, which prevents programmed cell death.18
Table 3 presents a stepwise approach to identifying and treating inflammation in patients with treatment-resistant psychosis.
OUTCOME Immune response, improvement
One month after the valproic acid and ginger root extract therapy is initiated, Mr. T’s toxoplasma antibody immunoglobulin G increases by 15.2 IU/mL, indicating that his immune system is mounting an enhanced response against the parasite (Figure 2). Mr. T continues to make progress while receiving the new regimen of clozapine, minocycline, valproic acid, and ginger root extract. He no longer cycles into periods of intense anxiety, perseverative thought, and fragmented thought and speech. He participates meaningfully in weekly psychotherapy and hopes to live independently and obtain gainful employment.
The District Attorney’s office dismisses his criminal charges, and Mr. T is discharged to a less restrictive level of care.
Continue to: Bottom Line
Bottom Line
Several studies have shown that neuroinflammation increases the severity of mental illness. Consider adjunct anti-inflammatory agents for patients who have elevated levels of inflammatory biomarkers and for whom standard treatment approaches do not adequately control psychiatric symptoms. Also consider testing for the presence of latent infections in the CNS, which could reveal the underlying cause of treatment resistance or the genesis of disabling psychiatric symptoms.
Related Resources
- Fond G, Macgregor A, Tamouza R, et al. Comparative analysis of anti-toxoplasmic activity of antipsychotic drugs and valproate. Eur Arch Psychiatry Clin Neurosci. 2014;264(2):179-183.
- Hamdani N, Daban-Huard C, Lajnef M, et al. Cognitive deterioration among bipolar disorder patients infected by Toxoplasma gondii is correlated to interleukin 6 levels. J Affect Disord. 2015;179:161-166.
- Monroe JM, Buckley PF, Miller BJ. Meta-analysis of antitoxoplasma gondii IgM antibodies in acute psychosis. Schizophr Bull. 2015;41(4):989-998.
Drug Brand Names
Acyclovir • Zovirax
Aripiprazole • Abilify
Bupropion • Wellbutrin
Buspirone • Buspar
Clozapine • Clozaril
Duloxetine • Cymbalta
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Gabapentin • Neurontin
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Loxapine • Loxitane
Minocycline • Minocin
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone • Risperdal, Risperdal Consta
Thioridazine • Mellaril
Trifluoperazine • Stelazine
Trazodone • Desyrel
Valproic acid • Depakote
Ziprasidone • Geodon
1. Koola MM, Raines JK, Hamilton RG, et al. Can anti-inflammatory medications improve symptoms and reduce mortality in schizophrenia? Current Psychiatry. 2016;15(5):52-57.
2. Nasrallah HA. Are you neuroprotecting your patients? 10 Adjunctive therapies to consider. Current Psychiatry. 2016;15(12):12-14.
3. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21(12):1696-1709.
4. Chase KA, Cone JJ, Rosen C, et al. The value of interleukin 6 as a peripheral diagnostic marker in schizophrenia. BMC Psychiatry. 2016;16:152.
5. Torrey EF, Bartko JJ, Lun ZR, et al. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33(3):729-736.
6. Shirts BH, Prasad KM, Pogue-Geile MF, et al. Antibodies to cytomegalovirus and herpes simplex virus 1 associated with cognitive function in schizophrenia. Schizophr Res. 2008;106(2-3):268-274.
7. Centers for Disease Control and Prevention. Parasites - Toxoplasmosis (Toxoplasma infection). https://www.cdc.gov/parasites/toxoplasmosis/index.html. Accessed February 26, 2019.
8. Dickerson F, Wilcox HC, Adamos M, et al. Suicide attempts and markers of immune response in individuals with serious mental illness. J Psychiatr Res. 2017;87:37-43.
9. Celik T, Kartalci S, Aytas O, et al. Association between latent toxoplasmosis and clinical course of schizophrenia - continuous course of the disease is characteristic for Toxoplasma gondii-infected patients. Folia Parasitol (Praha). 2015;62. doi: 10.14411/fp.2015.015.
10. Dickerson F, Boronow J, Stallings C, et al. Toxoplasma gondii in individuals with schizophrenia: association with clinical and demographic factors and with mortality. Schizophr Bull. 2007;33(3):737-740.
11. Esshili A, Thabet S, Jemli A, et al. Toxoplasma gondii infection in schizophrenia and associated clinical features. Psychiatry Res. 2016;245:327-332.
12. Holub D, Flegr J, Dragomirecka E, et al. Differences in onset of disease and severity of psychopathology between toxoplasmosis-related and toxoplasmosis-unrelated schizophrenia. Acta Psychiatr Scand. 2013;127(3):227-238.
13. Chen AT, Chibnall JT, Nasrallah HA. Placebo-controlled augmentation trials of the antioxidant NAC in schizophrenia: a review. Ann Clin Psychiatry. 2016;28(3):190-196.
14. Jones-Brando L, Torrey EF, Yolken R. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr Res. 2003;62(3):237-244.
15. Fond G, Boyer L, Gaman A, et al. Treatment with anti-toxoplasmic activity (TATA) for toxoplasma positive patients with bipolar disorders or schizophrenia: a cross-sectional study. J Psychiatr Res. 2015;63:58-64.
16. Wei HX, Wei SS, Lindsay DS, et al. A systematic review and meta-analysis of the efficacy of anti-Toxoplasma gondii medicines in humans. PLoS One. 2015;10(9):e0138204.
17. Zhuo XH, Sun HC, Huang B, et al. Evaluation of potential anti-toxoplasmosis efficiency of combined traditional herbs in a mouse model. J Zhejiang Univ Sci B. 2017;18(6):453-461.
18. Choi WH, Jiang MH, Chu JP. Antiparasitic effects of Zingiber officinale (Ginger) extract against Toxoplasma gondii. Journal of Applied Biomedicine. 2013;11:15-26.
1. Koola MM, Raines JK, Hamilton RG, et al. Can anti-inflammatory medications improve symptoms and reduce mortality in schizophrenia? Current Psychiatry. 2016;15(5):52-57.
2. Nasrallah HA. Are you neuroprotecting your patients? 10 Adjunctive therapies to consider. Current Psychiatry. 2016;15(12):12-14.
3. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21(12):1696-1709.
4. Chase KA, Cone JJ, Rosen C, et al. The value of interleukin 6 as a peripheral diagnostic marker in schizophrenia. BMC Psychiatry. 2016;16:152.
5. Torrey EF, Bartko JJ, Lun ZR, et al. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33(3):729-736.
6. Shirts BH, Prasad KM, Pogue-Geile MF, et al. Antibodies to cytomegalovirus and herpes simplex virus 1 associated with cognitive function in schizophrenia. Schizophr Res. 2008;106(2-3):268-274.
7. Centers for Disease Control and Prevention. Parasites - Toxoplasmosis (Toxoplasma infection). https://www.cdc.gov/parasites/toxoplasmosis/index.html. Accessed February 26, 2019.
8. Dickerson F, Wilcox HC, Adamos M, et al. Suicide attempts and markers of immune response in individuals with serious mental illness. J Psychiatr Res. 2017;87:37-43.
9. Celik T, Kartalci S, Aytas O, et al. Association between latent toxoplasmosis and clinical course of schizophrenia - continuous course of the disease is characteristic for Toxoplasma gondii-infected patients. Folia Parasitol (Praha). 2015;62. doi: 10.14411/fp.2015.015.
10. Dickerson F, Boronow J, Stallings C, et al. Toxoplasma gondii in individuals with schizophrenia: association with clinical and demographic factors and with mortality. Schizophr Bull. 2007;33(3):737-740.
11. Esshili A, Thabet S, Jemli A, et al. Toxoplasma gondii infection in schizophrenia and associated clinical features. Psychiatry Res. 2016;245:327-332.
12. Holub D, Flegr J, Dragomirecka E, et al. Differences in onset of disease and severity of psychopathology between toxoplasmosis-related and toxoplasmosis-unrelated schizophrenia. Acta Psychiatr Scand. 2013;127(3):227-238.
13. Chen AT, Chibnall JT, Nasrallah HA. Placebo-controlled augmentation trials of the antioxidant NAC in schizophrenia: a review. Ann Clin Psychiatry. 2016;28(3):190-196.
14. Jones-Brando L, Torrey EF, Yolken R. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr Res. 2003;62(3):237-244.
15. Fond G, Boyer L, Gaman A, et al. Treatment with anti-toxoplasmic activity (TATA) for toxoplasma positive patients with bipolar disorders or schizophrenia: a cross-sectional study. J Psychiatr Res. 2015;63:58-64.
16. Wei HX, Wei SS, Lindsay DS, et al. A systematic review and meta-analysis of the efficacy of anti-Toxoplasma gondii medicines in humans. PLoS One. 2015;10(9):e0138204.
17. Zhuo XH, Sun HC, Huang B, et al. Evaluation of potential anti-toxoplasmosis efficiency of combined traditional herbs in a mouse model. J Zhejiang Univ Sci B. 2017;18(6):453-461.
18. Choi WH, Jiang MH, Chu JP. Antiparasitic effects of Zingiber officinale (Ginger) extract against Toxoplasma gondii. Journal of Applied Biomedicine. 2013;11:15-26.
Seizure-like episodes, but is it really epilepsy?
CASE Increasingly frequent paroxysmal episodes
Ms. N, age 12, comes to the hospital for evaluation of paroxysmal episodes of pain, weakness, and muscle spasms. A neurologist who evaluated her as an outpatient had recommended a routine electroencephalogram (EEG); after those results were inconclusive, Ms. N’s mother brought her to the hospital for a 24-hour video EEG.
Ms. N has a history of asthma. She has no history of seizures or headache, but her mother has an unspecified seizure disorder that has been stable with antiepileptic medication for many years. Ms. N has no other family history of autoimmune or neurologic disorders.
Ms. N’s episodes began 6 months ago and have progressively increased in frequency from 5 to 12 episodes a day. She says that before she has an episode, she “ feels tingling in her fingers and mouth, and butterflies in her belly,” and then her “whole body clenches up.” She denies experiencing tongue biting, facial or extremity weakness, incontinence, or loss of consciousness during these episodes.
Shortly before her hospitalization, Ms. N had won a scholarship to attend an overnight art camp. Because her episodes were becoming more frequent and their etiology remained unclear, Ms. N and her mother decided it would be unsafe for her to attend, and that she should go to the hospital for evaluation instead.
EVALUATION Tough questions reveal answers
The pediatric team evaluates Ms. N. Her physical exam, laboratory values, and imaging are all within normal limits. Her neurologic exam demonstrates full strength, tone, and sensation in all extremities. All cranial nerves and reflexes are intact. No dysmorphic features or gait abnormalities are noted. All laboratory and imaging tests are normal, including complete blood cell count, electrolytes, calcium, magnesium, phosphorus, glucose, creatine kinase, liver enzymes, urine drug screen, human chorionic gonadotropin (hCG) urine test, and h
After the initial workup, the pediatric team consults the child and adolescent psychiatry team for a complete assessment of Ms. N due to concerns that a psychological component is contributing to her episodes. According to the psychosocial history obtained from Ms. N and her mother, Ms. N had experienced disrupted attachment, trauma, and loss. At age 5, Ms. N was temporarily removed from her mother’s custody after a fight between her mother and brother. At age 9, Ms. N’s stepfather, her primary father figure, died of a brain tumor.
Ms. N also has significant trauma stemming from her relationship with her biological father. Ms. N’s mother reports that her daughter was conceived during nonconsensual sexual intercourse. Ms. N did not have much contact with her biological father until 6 months ago, when he started picking her up at school and taking her to his home for several hours without permission or supervision. Afterwards, Ms. N confided to her mother and a teacher that her father sexually assaulted her during those visits.
Continue to: Ms. N and her mother...
Ms. N and her mother reported the assault to the police and were awaiting legal action.
During the interview with the psychiatry team, Ms. N denies that any thoughts or actions trigger the episodes and reports that she cannot control when they happen. Because she cannot anticipate the episodes, she says she is afraid to leave her house. She does not know why the episodes are happening and feels frustrated that they are getting worse. Ms. N says, “I have been feeling down lately,” but she denies hopelessness, worthlessness, suicidal ideation, homicidal ideation, delusions, or hallucinations.
In the hospital, when the psychiatry team asks Ms. N about her visits with her father, she says that they are “too painful to talk about,” and fears that discussing them will trigger an episode. However, her mother suggests that her daughter’s sexual trauma, as well as ongoing frustrations with the legal system, are influencing her mood; she has had low energy, poor appetite, and is spending more time in bed. Her mother also reports that Ms. N “avoids going out in the sun and spending time with her friends outside. She doesn’t seem to enjoy shopping and art like she used to.” Ms. N told her mother that she was having nightmares about the trauma and “could not stop thinking about some of the bad stuff that happened during the day.”
Ten minutes into the interview, while being questioned about her father, Ms. N experiences a spastic episode. She curls up in bed on her left side, clenches her entire body, and shuts her eyes. Her mother quickly runs to her bedside and counts the seconds until the end of the episode. After 25 seconds, Ms. N awakes with full recollection of the episode. On review of the video EEG during the episode, no ictal patterns are seen.
[polldaddy:10375873]
The authors’ observations
Paroxysmal episodes of weakness, numbness, and muscle spasms in a young female are suggestive of either epilepsy or nonepileptic seizure (NES).1,2 The negative EEG and physical features are inconsistent with epileptiform seizure, and Ms. N’s history and evaluation are suggestive of NES. Nonepileptic seizures are a type of a conversion disorder, or functional neurologic symptom disorder, in which a patient experiences weakness, abnormal movements, or seizure-like episodes that are inconsistent with organic neurologic disease.3 When a diagnosis of conversion disorder is suspected, a clinician must always consider other pathology that can explain the symptoms, such as migraine, vasovagal syncope, or intracranial mass. If a patient has focal neurologic deficits, head imaging should be pursued. Additionally, the clinician must screen for malingering and factitious disorder before establishing a definitive diagnosis. However, conversion disorder is not a diagnosis of exclusion. For example, a negative EEG does not rule out epilepsy, and patients can have both epilepsy and concomitant NES.
Continue to: Although NES is a common...
Although NES is a common type of conversion disorder, it is often difficult to diagnose, manage, and treat. Patients often receive antiepileptic medications but continue to have worsening events that are refractory to treatment. Various clinical features can suggest NES instead of epilepsy. Forced eye closure on video recording is a specific finding suggestive of NES, yet this feature is not sufficient to make the diagnosis.4 A video EEG must be performed to assess for epilepsy. The diagnosis of NES does not exclude the possibility that a patient has epilepsy, as NES can occur in up to 40% of patients with epilepsy.5 A video EEG without ictal patterns before, during, and after an observed episode is diagnostic of NES.6
[polldaddy:10375874]
The authors’ observations
Conversion disorders such as NES are a presentation of neurologic symptoms that cannot be readily accounted for by other conditions and are often associated with antecedent trauma. Multiple factors in Ms. N’s history increase her risk of NES, including loss of multiple loved ones, ongoing legal involvement, and alleged sexual abuse by her father.
Victims of sexual abuse are more likely than the general population to demonstrate symptoms of conversion disorder, especially NES.7,8 The onset of paroxysmal episodes after incestuous abuse in a teenage girl is characteristic of NES. Compared with patients with complex partial epilepsy (CPE), patients with NES are 3 times more likely to report sexual trauma.9,10 Children who report sexual abuse that precedes NES are more likely to have been victimized by a first-degree relative than patients with CPE who report sexual abuse.11 Risk factors for victims developing NES may be related to the severity of adversity, stress sensitivity, and decreased hippocampal volume.12,13
Ms. N endorsed many psychiatric symptoms that accompany her paroxysmal episodes; this is similar to findings in other patients with NES.14 One study found that depression is 3 times more prevalent and PTSD is 8 times more prevalent in patients with NES.12 During the evaluation, Ms. N’s mother said her daughter had low energy, poor appetite, lethargy, and anhedonia for the preceding 5 months, which is consistent with adjustment disorder.3 Her flashbacks, nightmares, difficulty sleeping, and agoraphobia, along with her trouble engaging with the people and activities that used to bring her joy, are symptoms of PTSD. Nonepileptic seizure is often associated with PTSD and can be viewed as an expression of a dissociated subtype.15
In a literature review, Durrant et al16 isolated prognostic indicators for NES (Table16). This study found that 70% of children and 40% of adults achieve remission from NES. Ms. N’s case has multiple concerning features, such as her comorbid psychiatric conditions, ongoing involvement in a legal case, and sexual trauma; this last factor is associated with the most severe symptoms and worse outcomes.16,17 Despite this somber reality, Ms. N has the support of her mother and is relatively young, which play a vital role in recovery.
Continue to: TREATMENT A strategy for minimizing the episodes
TREATMENT A strategy for minimizing the episodes
Ms. N’s medical workup remains unremarkable throughout the rest of her hospital stay. The psychiatry and pediatric teams discuss their assessments and agree that NES is the most likely diagnosis. The psychiatry team counsels Ms. N and her mother on the diagnosis and etiology of NES.
[polldaddy:10375876]
The authors’ observations
Cognitive-behavioral therapy is currently the treatment of choice for reducing seizure frequency in patients with NES.18,19 The use of CBT was suggested due to the theory that NES represents a dissociative response to trauma. Therapy focuses on changing a patient’s beliefs and perceptions associated with attacks.5 A randomized study of 66 patients with NES compared the use of CBT plus standard medical care with standard medical care alone.18 The standard medical care consisted of supportive treatment, an explanation of NES from a neuropsychiatrist, and supervised withdrawal of antiepileptic drugs. The CBT treatment group was offered weekly hour-long sessions for 12 weeks, accompanied by CBT homework and journaling the frequency and nature of seizure episodes (the CBT techniques are outlined in the Figure18). After 4 months, the CBT treatment group had fewer seizures, and after a 6-month follow-up, they were more likely to be seizure-free. However, in this study, CBT treatment did not improve mood or employment status.
A later investigation looked at using selective serotonin reuptake inhibitors to treat NES in adults.19 This study divided participants into 4 treatment groups: CBT with informed psychotherapy (CBT-ip), CBT-ip plus sertraline, sertraline alone, and treatment as usual. Sertraline was titrated up to a dose of 200 mg/d as tolerated. After 16 weeks of sertraline alone, seizure frequency did not decrease. Although both CBT groups showed a reduction in symptoms of up to 60%, the CBT-ip group reported fewer psychiatric symptoms with better social interactions, quality of life, and global functioning compared with patients treated with CBT-ip plus sertraline. The authors suggested that this may be due to the somatic adverse effects associated with sertraline. This study suggests that CBT without medication is the treatment of choice.
In addition to CBT, studies of psychodynamic psychotherapy for NES have had promising findings.20 Psychodynamic psychotherapy focuses on addressing conscious and unconscious anger, loss, feelings of isolation, and trauma. Through improving emotional processing, insight, coping skills and self-regulation, patients often benefit from an improvement in seizures, psychosocial functioning and health care utilization.
Metin et al21 found that group therapy alongside a family-centered approach elicited a strong and durable reduction in seizures in patients with NES. At enrollment, investigators distributed information on NES to patients and families. Psychoeducation and psychoanalysis with behavior modification techniques were provided in 90-minute weekly group sessions over 3 months. Participants also underwent monthly individualized sessions for standard psychiatric care for 9 months. During the group sessions, operant conditioning techniques were used to prevent secondary gain from seizure-like activity. Families met 4 times for 1 hour each to discuss seizures, receive psychoeducation on a subconscious etiology of NES, and learn behavior modification techniques. All 9 participants who completed group and individual therapy reported a significant and sustained reduction in seizure frequency by at least 50% at 12-month follow-up. Patients also demonstrated improvements in mood, anxiety, and quality of life.
Continue to: A meta-analysis...
A meta-analysis by Carlson and Perry22 that included 13 studies and 228 participants, examined different treatment modalities and their effectiveness for NES. They found that patients who received psychological intervention had a 47% remission rate and 82% improvement in seizure frequency compared with only 14% to 23% of those who did not receive therapy. They postulated that therapy for this illness must be flexible to properly address the socially, psychologically, and functionally heterogenous patient population. Although there are few randomized controlled trials for NES to determine the best evidence-based intervention, there is now consensus that NES has a favorable prognosis when barriers to psychological care are eliminated.
OUTCOME Referral for CBT
The treatment team advises Ms. N to engage in outpatient therapy after discharge from the hospital. Ms. N and her mother agree to the treatment plan, and leave the hospital with a referral for CBT the next day.
Bottom Line
Nonepileptic seizure (NES) is a type of conversion disorder characterized by seizure-like episodes without ictal qualities. Risk factors for NES include concomitant epilepsy, psychiatric disorders, unstable psychosocial situations, and antecedent trauma. Patients with a history of incestuous sexual abuse are most at risk for developing NES. A normal EEG that fully captures a seizure-like episode is diagnostic of NES. Cognitive-behavioral therapy can minimize seizure frequency and intensity.
Related Resources
- Marsh P, Benbadis S, Fernandez F. Psychogenic nonepileptic seizures: ways to win over skeptical patients. Current Psychiatry. 2008;7(1):21-24, 32-35.
- LaFrance WC Jr. Eye-opening behaviors help diagnose nonepileptic seizures. Current Psychiatry. 2006;5(11):121-122, 124, 130.
- LaFrance WC Jr, Kanner AM, Barry JJ. Treating patients with psychological nonepileptic seizures. In: Ettinger AB, Kanner AM, eds. Psychiatric issues in epilepsy: a practical guide to diagnosis and treatment. 2nd ed. Philadelphia, PA: Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2007:461-488.
Drug Brand Name
Sertraline • Zoloft
1. Lesser R. Psychogenic seizures. Neurology. 1996;46(6):1499-1507.
2. Stone J, LaFrance W, Brown R, et al. Conversion disorder: current problems and potential solutions for DSM-5. J Psychosom Res. 2011;71(6):369-376.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Syed T, Arozullah A, Suciu G, et al. Do observer and self-reports of ictal eye closure predict psychogenic nonepileptic seizures? Epilepsia. 2008;49(5):898-904.
5. Vega-Zelaya L, Alvarez M, Ezquiaga E, et al. Psychogenic non-epileptic seizures in a surgical epilepsy unit: experience and a comprehensive review. Epilepsy Topics. 2014. doi: 10.5772/57439.
6. LaFrance W, Baker G, Duncan R, et al. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach. Epilepsia. 2013;54(11):2005-2018.
7. Roeloes K, Pasman J. Stress, childhood trauma, and cognitive functions in functional neurologic disorders. In: Hallett M, Stone J, Carson A, eds. Handbook of clinical neurology: functional neurologic disorders. 3rd ed. New York, NY: Elsevier; 2017:139-155.
8. Paras M, Murad M, Chen L, et al. Sexual abuse and lifetime diagnosis of somatic disorders. JAMA. 2009;302(5):550.
9. Fiszman A, Alves-Leon SV, Nunes RG, et al. Traumatic events and posttraumatic stress disorder in patients with psychogenic nonepileptic seizures: a critical review. Epilepsy Behav. 2004;5(6):818-825.
10. Sharpe D, Faye C. Non-epileptic seizures and child sexual abuse: a critical review of the literature. Clin Psychol Rev. 2006;26(8):1020-1040.
11. Alper K, Devinsky O, Perrine K, et al. Nonepileptic seizures and childhood sexual and physical abuse. Neurology. 1993;43(10):1950-1953.
12. Plioplys S, Doss J, Siddarth P et al. A multisite controlled study of risk factors in pediatric psychogenic nonepileptic seizures. Epilepsia. 2014;55(11):1739-1747.
13. Andersen S, Tomada A, Vincow E, et al. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20(3):292-301.
14. Sar V. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
15. Rosenberg HJ, Rosenberg SD, Williamson PD, et al. A comparative study of trauma and posttraumatic stress disorder prevalence in epilepsy patients and psychogenic nonepileptic seizure patients. Epilepsia. 2000;41(4):447-452.
16. Durrant J, Rickards H, Cavanna A. Prognosis and outcome predictors in psychogenic nonepileptic seizures. Epilepsy Res Treat. 2011;2011:1-7.
17. Selkirk M, Duncan R, Oto M, et al. Clinical differences between patients with nonepileptic seizures who report antecedent sexual abuse and those who do not. Epilepsia. 2008;49(8):1446-1450.
18. Goldstein L, Chalder T, Chigwedere C, et al. Cognitive-behavioral therapy for psychogenic nonepileptic seizures: a pilot RCT. Neurology. 2010;74(24):1986-1994.
19. LaFrance W, Baird G, Barry J, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures. JAMA Psychiatry. 2014;71(9):997.
20. Howlett S, Reuber M. An augmented model of brief psychodynamic interpersonal therapy for patients with nonepileptic seizures. Psychotherapy (Chic). 2009;46(1):125-138.
21. Metin SZ, Ozmen M, Metin B, et al. Treatment with group psychotherapy for chronic psychogenic nonepileptic seizures. Epilepsy Behav. 2013;28(1):91-94.
22. Carlson P, Perry KN. Psychological interventions for psychogenic non-epileptic seizures: a meta-analysis. Seizure. 2017;45:142-150.
CASE Increasingly frequent paroxysmal episodes
Ms. N, age 12, comes to the hospital for evaluation of paroxysmal episodes of pain, weakness, and muscle spasms. A neurologist who evaluated her as an outpatient had recommended a routine electroencephalogram (EEG); after those results were inconclusive, Ms. N’s mother brought her to the hospital for a 24-hour video EEG.
Ms. N has a history of asthma. She has no history of seizures or headache, but her mother has an unspecified seizure disorder that has been stable with antiepileptic medication for many years. Ms. N has no other family history of autoimmune or neurologic disorders.
Ms. N’s episodes began 6 months ago and have progressively increased in frequency from 5 to 12 episodes a day. She says that before she has an episode, she “ feels tingling in her fingers and mouth, and butterflies in her belly,” and then her “whole body clenches up.” She denies experiencing tongue biting, facial or extremity weakness, incontinence, or loss of consciousness during these episodes.
Shortly before her hospitalization, Ms. N had won a scholarship to attend an overnight art camp. Because her episodes were becoming more frequent and their etiology remained unclear, Ms. N and her mother decided it would be unsafe for her to attend, and that she should go to the hospital for evaluation instead.
EVALUATION Tough questions reveal answers
The pediatric team evaluates Ms. N. Her physical exam, laboratory values, and imaging are all within normal limits. Her neurologic exam demonstrates full strength, tone, and sensation in all extremities. All cranial nerves and reflexes are intact. No dysmorphic features or gait abnormalities are noted. All laboratory and imaging tests are normal, including complete blood cell count, electrolytes, calcium, magnesium, phosphorus, glucose, creatine kinase, liver enzymes, urine drug screen, human chorionic gonadotropin (hCG) urine test, and h
After the initial workup, the pediatric team consults the child and adolescent psychiatry team for a complete assessment of Ms. N due to concerns that a psychological component is contributing to her episodes. According to the psychosocial history obtained from Ms. N and her mother, Ms. N had experienced disrupted attachment, trauma, and loss. At age 5, Ms. N was temporarily removed from her mother’s custody after a fight between her mother and brother. At age 9, Ms. N’s stepfather, her primary father figure, died of a brain tumor.
Ms. N also has significant trauma stemming from her relationship with her biological father. Ms. N’s mother reports that her daughter was conceived during nonconsensual sexual intercourse. Ms. N did not have much contact with her biological father until 6 months ago, when he started picking her up at school and taking her to his home for several hours without permission or supervision. Afterwards, Ms. N confided to her mother and a teacher that her father sexually assaulted her during those visits.
Continue to: Ms. N and her mother...
Ms. N and her mother reported the assault to the police and were awaiting legal action.
During the interview with the psychiatry team, Ms. N denies that any thoughts or actions trigger the episodes and reports that she cannot control when they happen. Because she cannot anticipate the episodes, she says she is afraid to leave her house. She does not know why the episodes are happening and feels frustrated that they are getting worse. Ms. N says, “I have been feeling down lately,” but she denies hopelessness, worthlessness, suicidal ideation, homicidal ideation, delusions, or hallucinations.
In the hospital, when the psychiatry team asks Ms. N about her visits with her father, she says that they are “too painful to talk about,” and fears that discussing them will trigger an episode. However, her mother suggests that her daughter’s sexual trauma, as well as ongoing frustrations with the legal system, are influencing her mood; she has had low energy, poor appetite, and is spending more time in bed. Her mother also reports that Ms. N “avoids going out in the sun and spending time with her friends outside. She doesn’t seem to enjoy shopping and art like she used to.” Ms. N told her mother that she was having nightmares about the trauma and “could not stop thinking about some of the bad stuff that happened during the day.”
Ten minutes into the interview, while being questioned about her father, Ms. N experiences a spastic episode. She curls up in bed on her left side, clenches her entire body, and shuts her eyes. Her mother quickly runs to her bedside and counts the seconds until the end of the episode. After 25 seconds, Ms. N awakes with full recollection of the episode. On review of the video EEG during the episode, no ictal patterns are seen.
[polldaddy:10375873]
The authors’ observations
Paroxysmal episodes of weakness, numbness, and muscle spasms in a young female are suggestive of either epilepsy or nonepileptic seizure (NES).1,2 The negative EEG and physical features are inconsistent with epileptiform seizure, and Ms. N’s history and evaluation are suggestive of NES. Nonepileptic seizures are a type of a conversion disorder, or functional neurologic symptom disorder, in which a patient experiences weakness, abnormal movements, or seizure-like episodes that are inconsistent with organic neurologic disease.3 When a diagnosis of conversion disorder is suspected, a clinician must always consider other pathology that can explain the symptoms, such as migraine, vasovagal syncope, or intracranial mass. If a patient has focal neurologic deficits, head imaging should be pursued. Additionally, the clinician must screen for malingering and factitious disorder before establishing a definitive diagnosis. However, conversion disorder is not a diagnosis of exclusion. For example, a negative EEG does not rule out epilepsy, and patients can have both epilepsy and concomitant NES.
Continue to: Although NES is a common...
Although NES is a common type of conversion disorder, it is often difficult to diagnose, manage, and treat. Patients often receive antiepileptic medications but continue to have worsening events that are refractory to treatment. Various clinical features can suggest NES instead of epilepsy. Forced eye closure on video recording is a specific finding suggestive of NES, yet this feature is not sufficient to make the diagnosis.4 A video EEG must be performed to assess for epilepsy. The diagnosis of NES does not exclude the possibility that a patient has epilepsy, as NES can occur in up to 40% of patients with epilepsy.5 A video EEG without ictal patterns before, during, and after an observed episode is diagnostic of NES.6
[polldaddy:10375874]
The authors’ observations
Conversion disorders such as NES are a presentation of neurologic symptoms that cannot be readily accounted for by other conditions and are often associated with antecedent trauma. Multiple factors in Ms. N’s history increase her risk of NES, including loss of multiple loved ones, ongoing legal involvement, and alleged sexual abuse by her father.
Victims of sexual abuse are more likely than the general population to demonstrate symptoms of conversion disorder, especially NES.7,8 The onset of paroxysmal episodes after incestuous abuse in a teenage girl is characteristic of NES. Compared with patients with complex partial epilepsy (CPE), patients with NES are 3 times more likely to report sexual trauma.9,10 Children who report sexual abuse that precedes NES are more likely to have been victimized by a first-degree relative than patients with CPE who report sexual abuse.11 Risk factors for victims developing NES may be related to the severity of adversity, stress sensitivity, and decreased hippocampal volume.12,13
Ms. N endorsed many psychiatric symptoms that accompany her paroxysmal episodes; this is similar to findings in other patients with NES.14 One study found that depression is 3 times more prevalent and PTSD is 8 times more prevalent in patients with NES.12 During the evaluation, Ms. N’s mother said her daughter had low energy, poor appetite, lethargy, and anhedonia for the preceding 5 months, which is consistent with adjustment disorder.3 Her flashbacks, nightmares, difficulty sleeping, and agoraphobia, along with her trouble engaging with the people and activities that used to bring her joy, are symptoms of PTSD. Nonepileptic seizure is often associated with PTSD and can be viewed as an expression of a dissociated subtype.15
In a literature review, Durrant et al16 isolated prognostic indicators for NES (Table16). This study found that 70% of children and 40% of adults achieve remission from NES. Ms. N’s case has multiple concerning features, such as her comorbid psychiatric conditions, ongoing involvement in a legal case, and sexual trauma; this last factor is associated with the most severe symptoms and worse outcomes.16,17 Despite this somber reality, Ms. N has the support of her mother and is relatively young, which play a vital role in recovery.
Continue to: TREATMENT A strategy for minimizing the episodes
TREATMENT A strategy for minimizing the episodes
Ms. N’s medical workup remains unremarkable throughout the rest of her hospital stay. The psychiatry and pediatric teams discuss their assessments and agree that NES is the most likely diagnosis. The psychiatry team counsels Ms. N and her mother on the diagnosis and etiology of NES.
[polldaddy:10375876]
The authors’ observations
Cognitive-behavioral therapy is currently the treatment of choice for reducing seizure frequency in patients with NES.18,19 The use of CBT was suggested due to the theory that NES represents a dissociative response to trauma. Therapy focuses on changing a patient’s beliefs and perceptions associated with attacks.5 A randomized study of 66 patients with NES compared the use of CBT plus standard medical care with standard medical care alone.18 The standard medical care consisted of supportive treatment, an explanation of NES from a neuropsychiatrist, and supervised withdrawal of antiepileptic drugs. The CBT treatment group was offered weekly hour-long sessions for 12 weeks, accompanied by CBT homework and journaling the frequency and nature of seizure episodes (the CBT techniques are outlined in the Figure18). After 4 months, the CBT treatment group had fewer seizures, and after a 6-month follow-up, they were more likely to be seizure-free. However, in this study, CBT treatment did not improve mood or employment status.
A later investigation looked at using selective serotonin reuptake inhibitors to treat NES in adults.19 This study divided participants into 4 treatment groups: CBT with informed psychotherapy (CBT-ip), CBT-ip plus sertraline, sertraline alone, and treatment as usual. Sertraline was titrated up to a dose of 200 mg/d as tolerated. After 16 weeks of sertraline alone, seizure frequency did not decrease. Although both CBT groups showed a reduction in symptoms of up to 60%, the CBT-ip group reported fewer psychiatric symptoms with better social interactions, quality of life, and global functioning compared with patients treated with CBT-ip plus sertraline. The authors suggested that this may be due to the somatic adverse effects associated with sertraline. This study suggests that CBT without medication is the treatment of choice.
In addition to CBT, studies of psychodynamic psychotherapy for NES have had promising findings.20 Psychodynamic psychotherapy focuses on addressing conscious and unconscious anger, loss, feelings of isolation, and trauma. Through improving emotional processing, insight, coping skills and self-regulation, patients often benefit from an improvement in seizures, psychosocial functioning and health care utilization.
Metin et al21 found that group therapy alongside a family-centered approach elicited a strong and durable reduction in seizures in patients with NES. At enrollment, investigators distributed information on NES to patients and families. Psychoeducation and psychoanalysis with behavior modification techniques were provided in 90-minute weekly group sessions over 3 months. Participants also underwent monthly individualized sessions for standard psychiatric care for 9 months. During the group sessions, operant conditioning techniques were used to prevent secondary gain from seizure-like activity. Families met 4 times for 1 hour each to discuss seizures, receive psychoeducation on a subconscious etiology of NES, and learn behavior modification techniques. All 9 participants who completed group and individual therapy reported a significant and sustained reduction in seizure frequency by at least 50% at 12-month follow-up. Patients also demonstrated improvements in mood, anxiety, and quality of life.
Continue to: A meta-analysis...
A meta-analysis by Carlson and Perry22 that included 13 studies and 228 participants, examined different treatment modalities and their effectiveness for NES. They found that patients who received psychological intervention had a 47% remission rate and 82% improvement in seizure frequency compared with only 14% to 23% of those who did not receive therapy. They postulated that therapy for this illness must be flexible to properly address the socially, psychologically, and functionally heterogenous patient population. Although there are few randomized controlled trials for NES to determine the best evidence-based intervention, there is now consensus that NES has a favorable prognosis when barriers to psychological care are eliminated.
OUTCOME Referral for CBT
The treatment team advises Ms. N to engage in outpatient therapy after discharge from the hospital. Ms. N and her mother agree to the treatment plan, and leave the hospital with a referral for CBT the next day.
Bottom Line
Nonepileptic seizure (NES) is a type of conversion disorder characterized by seizure-like episodes without ictal qualities. Risk factors for NES include concomitant epilepsy, psychiatric disorders, unstable psychosocial situations, and antecedent trauma. Patients with a history of incestuous sexual abuse are most at risk for developing NES. A normal EEG that fully captures a seizure-like episode is diagnostic of NES. Cognitive-behavioral therapy can minimize seizure frequency and intensity.
Related Resources
- Marsh P, Benbadis S, Fernandez F. Psychogenic nonepileptic seizures: ways to win over skeptical patients. Current Psychiatry. 2008;7(1):21-24, 32-35.
- LaFrance WC Jr. Eye-opening behaviors help diagnose nonepileptic seizures. Current Psychiatry. 2006;5(11):121-122, 124, 130.
- LaFrance WC Jr, Kanner AM, Barry JJ. Treating patients with psychological nonepileptic seizures. In: Ettinger AB, Kanner AM, eds. Psychiatric issues in epilepsy: a practical guide to diagnosis and treatment. 2nd ed. Philadelphia, PA: Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2007:461-488.
Drug Brand Name
Sertraline • Zoloft
CASE Increasingly frequent paroxysmal episodes
Ms. N, age 12, comes to the hospital for evaluation of paroxysmal episodes of pain, weakness, and muscle spasms. A neurologist who evaluated her as an outpatient had recommended a routine electroencephalogram (EEG); after those results were inconclusive, Ms. N’s mother brought her to the hospital for a 24-hour video EEG.
Ms. N has a history of asthma. She has no history of seizures or headache, but her mother has an unspecified seizure disorder that has been stable with antiepileptic medication for many years. Ms. N has no other family history of autoimmune or neurologic disorders.
Ms. N’s episodes began 6 months ago and have progressively increased in frequency from 5 to 12 episodes a day. She says that before she has an episode, she “ feels tingling in her fingers and mouth, and butterflies in her belly,” and then her “whole body clenches up.” She denies experiencing tongue biting, facial or extremity weakness, incontinence, or loss of consciousness during these episodes.
Shortly before her hospitalization, Ms. N had won a scholarship to attend an overnight art camp. Because her episodes were becoming more frequent and their etiology remained unclear, Ms. N and her mother decided it would be unsafe for her to attend, and that she should go to the hospital for evaluation instead.
EVALUATION Tough questions reveal answers
The pediatric team evaluates Ms. N. Her physical exam, laboratory values, and imaging are all within normal limits. Her neurologic exam demonstrates full strength, tone, and sensation in all extremities. All cranial nerves and reflexes are intact. No dysmorphic features or gait abnormalities are noted. All laboratory and imaging tests are normal, including complete blood cell count, electrolytes, calcium, magnesium, phosphorus, glucose, creatine kinase, liver enzymes, urine drug screen, human chorionic gonadotropin (hCG) urine test, and h
After the initial workup, the pediatric team consults the child and adolescent psychiatry team for a complete assessment of Ms. N due to concerns that a psychological component is contributing to her episodes. According to the psychosocial history obtained from Ms. N and her mother, Ms. N had experienced disrupted attachment, trauma, and loss. At age 5, Ms. N was temporarily removed from her mother’s custody after a fight between her mother and brother. At age 9, Ms. N’s stepfather, her primary father figure, died of a brain tumor.
Ms. N also has significant trauma stemming from her relationship with her biological father. Ms. N’s mother reports that her daughter was conceived during nonconsensual sexual intercourse. Ms. N did not have much contact with her biological father until 6 months ago, when he started picking her up at school and taking her to his home for several hours without permission or supervision. Afterwards, Ms. N confided to her mother and a teacher that her father sexually assaulted her during those visits.
Continue to: Ms. N and her mother...
Ms. N and her mother reported the assault to the police and were awaiting legal action.
During the interview with the psychiatry team, Ms. N denies that any thoughts or actions trigger the episodes and reports that she cannot control when they happen. Because she cannot anticipate the episodes, she says she is afraid to leave her house. She does not know why the episodes are happening and feels frustrated that they are getting worse. Ms. N says, “I have been feeling down lately,” but she denies hopelessness, worthlessness, suicidal ideation, homicidal ideation, delusions, or hallucinations.
In the hospital, when the psychiatry team asks Ms. N about her visits with her father, she says that they are “too painful to talk about,” and fears that discussing them will trigger an episode. However, her mother suggests that her daughter’s sexual trauma, as well as ongoing frustrations with the legal system, are influencing her mood; she has had low energy, poor appetite, and is spending more time in bed. Her mother also reports that Ms. N “avoids going out in the sun and spending time with her friends outside. She doesn’t seem to enjoy shopping and art like she used to.” Ms. N told her mother that she was having nightmares about the trauma and “could not stop thinking about some of the bad stuff that happened during the day.”
Ten minutes into the interview, while being questioned about her father, Ms. N experiences a spastic episode. She curls up in bed on her left side, clenches her entire body, and shuts her eyes. Her mother quickly runs to her bedside and counts the seconds until the end of the episode. After 25 seconds, Ms. N awakes with full recollection of the episode. On review of the video EEG during the episode, no ictal patterns are seen.
[polldaddy:10375873]
The authors’ observations
Paroxysmal episodes of weakness, numbness, and muscle spasms in a young female are suggestive of either epilepsy or nonepileptic seizure (NES).1,2 The negative EEG and physical features are inconsistent with epileptiform seizure, and Ms. N’s history and evaluation are suggestive of NES. Nonepileptic seizures are a type of a conversion disorder, or functional neurologic symptom disorder, in which a patient experiences weakness, abnormal movements, or seizure-like episodes that are inconsistent with organic neurologic disease.3 When a diagnosis of conversion disorder is suspected, a clinician must always consider other pathology that can explain the symptoms, such as migraine, vasovagal syncope, or intracranial mass. If a patient has focal neurologic deficits, head imaging should be pursued. Additionally, the clinician must screen for malingering and factitious disorder before establishing a definitive diagnosis. However, conversion disorder is not a diagnosis of exclusion. For example, a negative EEG does not rule out epilepsy, and patients can have both epilepsy and concomitant NES.
Continue to: Although NES is a common...
Although NES is a common type of conversion disorder, it is often difficult to diagnose, manage, and treat. Patients often receive antiepileptic medications but continue to have worsening events that are refractory to treatment. Various clinical features can suggest NES instead of epilepsy. Forced eye closure on video recording is a specific finding suggestive of NES, yet this feature is not sufficient to make the diagnosis.4 A video EEG must be performed to assess for epilepsy. The diagnosis of NES does not exclude the possibility that a patient has epilepsy, as NES can occur in up to 40% of patients with epilepsy.5 A video EEG without ictal patterns before, during, and after an observed episode is diagnostic of NES.6
[polldaddy:10375874]
The authors’ observations
Conversion disorders such as NES are a presentation of neurologic symptoms that cannot be readily accounted for by other conditions and are often associated with antecedent trauma. Multiple factors in Ms. N’s history increase her risk of NES, including loss of multiple loved ones, ongoing legal involvement, and alleged sexual abuse by her father.
Victims of sexual abuse are more likely than the general population to demonstrate symptoms of conversion disorder, especially NES.7,8 The onset of paroxysmal episodes after incestuous abuse in a teenage girl is characteristic of NES. Compared with patients with complex partial epilepsy (CPE), patients with NES are 3 times more likely to report sexual trauma.9,10 Children who report sexual abuse that precedes NES are more likely to have been victimized by a first-degree relative than patients with CPE who report sexual abuse.11 Risk factors for victims developing NES may be related to the severity of adversity, stress sensitivity, and decreased hippocampal volume.12,13
Ms. N endorsed many psychiatric symptoms that accompany her paroxysmal episodes; this is similar to findings in other patients with NES.14 One study found that depression is 3 times more prevalent and PTSD is 8 times more prevalent in patients with NES.12 During the evaluation, Ms. N’s mother said her daughter had low energy, poor appetite, lethargy, and anhedonia for the preceding 5 months, which is consistent with adjustment disorder.3 Her flashbacks, nightmares, difficulty sleeping, and agoraphobia, along with her trouble engaging with the people and activities that used to bring her joy, are symptoms of PTSD. Nonepileptic seizure is often associated with PTSD and can be viewed as an expression of a dissociated subtype.15
In a literature review, Durrant et al16 isolated prognostic indicators for NES (Table16). This study found that 70% of children and 40% of adults achieve remission from NES. Ms. N’s case has multiple concerning features, such as her comorbid psychiatric conditions, ongoing involvement in a legal case, and sexual trauma; this last factor is associated with the most severe symptoms and worse outcomes.16,17 Despite this somber reality, Ms. N has the support of her mother and is relatively young, which play a vital role in recovery.
Continue to: TREATMENT A strategy for minimizing the episodes
TREATMENT A strategy for minimizing the episodes
Ms. N’s medical workup remains unremarkable throughout the rest of her hospital stay. The psychiatry and pediatric teams discuss their assessments and agree that NES is the most likely diagnosis. The psychiatry team counsels Ms. N and her mother on the diagnosis and etiology of NES.
[polldaddy:10375876]
The authors’ observations
Cognitive-behavioral therapy is currently the treatment of choice for reducing seizure frequency in patients with NES.18,19 The use of CBT was suggested due to the theory that NES represents a dissociative response to trauma. Therapy focuses on changing a patient’s beliefs and perceptions associated with attacks.5 A randomized study of 66 patients with NES compared the use of CBT plus standard medical care with standard medical care alone.18 The standard medical care consisted of supportive treatment, an explanation of NES from a neuropsychiatrist, and supervised withdrawal of antiepileptic drugs. The CBT treatment group was offered weekly hour-long sessions for 12 weeks, accompanied by CBT homework and journaling the frequency and nature of seizure episodes (the CBT techniques are outlined in the Figure18). After 4 months, the CBT treatment group had fewer seizures, and after a 6-month follow-up, they were more likely to be seizure-free. However, in this study, CBT treatment did not improve mood or employment status.
A later investigation looked at using selective serotonin reuptake inhibitors to treat NES in adults.19 This study divided participants into 4 treatment groups: CBT with informed psychotherapy (CBT-ip), CBT-ip plus sertraline, sertraline alone, and treatment as usual. Sertraline was titrated up to a dose of 200 mg/d as tolerated. After 16 weeks of sertraline alone, seizure frequency did not decrease. Although both CBT groups showed a reduction in symptoms of up to 60%, the CBT-ip group reported fewer psychiatric symptoms with better social interactions, quality of life, and global functioning compared with patients treated with CBT-ip plus sertraline. The authors suggested that this may be due to the somatic adverse effects associated with sertraline. This study suggests that CBT without medication is the treatment of choice.
In addition to CBT, studies of psychodynamic psychotherapy for NES have had promising findings.20 Psychodynamic psychotherapy focuses on addressing conscious and unconscious anger, loss, feelings of isolation, and trauma. Through improving emotional processing, insight, coping skills and self-regulation, patients often benefit from an improvement in seizures, psychosocial functioning and health care utilization.
Metin et al21 found that group therapy alongside a family-centered approach elicited a strong and durable reduction in seizures in patients with NES. At enrollment, investigators distributed information on NES to patients and families. Psychoeducation and psychoanalysis with behavior modification techniques were provided in 90-minute weekly group sessions over 3 months. Participants also underwent monthly individualized sessions for standard psychiatric care for 9 months. During the group sessions, operant conditioning techniques were used to prevent secondary gain from seizure-like activity. Families met 4 times for 1 hour each to discuss seizures, receive psychoeducation on a subconscious etiology of NES, and learn behavior modification techniques. All 9 participants who completed group and individual therapy reported a significant and sustained reduction in seizure frequency by at least 50% at 12-month follow-up. Patients also demonstrated improvements in mood, anxiety, and quality of life.
Continue to: A meta-analysis...
A meta-analysis by Carlson and Perry22 that included 13 studies and 228 participants, examined different treatment modalities and their effectiveness for NES. They found that patients who received psychological intervention had a 47% remission rate and 82% improvement in seizure frequency compared with only 14% to 23% of those who did not receive therapy. They postulated that therapy for this illness must be flexible to properly address the socially, psychologically, and functionally heterogenous patient population. Although there are few randomized controlled trials for NES to determine the best evidence-based intervention, there is now consensus that NES has a favorable prognosis when barriers to psychological care are eliminated.
OUTCOME Referral for CBT
The treatment team advises Ms. N to engage in outpatient therapy after discharge from the hospital. Ms. N and her mother agree to the treatment plan, and leave the hospital with a referral for CBT the next day.
Bottom Line
Nonepileptic seizure (NES) is a type of conversion disorder characterized by seizure-like episodes without ictal qualities. Risk factors for NES include concomitant epilepsy, psychiatric disorders, unstable psychosocial situations, and antecedent trauma. Patients with a history of incestuous sexual abuse are most at risk for developing NES. A normal EEG that fully captures a seizure-like episode is diagnostic of NES. Cognitive-behavioral therapy can minimize seizure frequency and intensity.
Related Resources
- Marsh P, Benbadis S, Fernandez F. Psychogenic nonepileptic seizures: ways to win over skeptical patients. Current Psychiatry. 2008;7(1):21-24, 32-35.
- LaFrance WC Jr. Eye-opening behaviors help diagnose nonepileptic seizures. Current Psychiatry. 2006;5(11):121-122, 124, 130.
- LaFrance WC Jr, Kanner AM, Barry JJ. Treating patients with psychological nonepileptic seizures. In: Ettinger AB, Kanner AM, eds. Psychiatric issues in epilepsy: a practical guide to diagnosis and treatment. 2nd ed. Philadelphia, PA: Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2007:461-488.
Drug Brand Name
Sertraline • Zoloft
1. Lesser R. Psychogenic seizures. Neurology. 1996;46(6):1499-1507.
2. Stone J, LaFrance W, Brown R, et al. Conversion disorder: current problems and potential solutions for DSM-5. J Psychosom Res. 2011;71(6):369-376.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Syed T, Arozullah A, Suciu G, et al. Do observer and self-reports of ictal eye closure predict psychogenic nonepileptic seizures? Epilepsia. 2008;49(5):898-904.
5. Vega-Zelaya L, Alvarez M, Ezquiaga E, et al. Psychogenic non-epileptic seizures in a surgical epilepsy unit: experience and a comprehensive review. Epilepsy Topics. 2014. doi: 10.5772/57439.
6. LaFrance W, Baker G, Duncan R, et al. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach. Epilepsia. 2013;54(11):2005-2018.
7. Roeloes K, Pasman J. Stress, childhood trauma, and cognitive functions in functional neurologic disorders. In: Hallett M, Stone J, Carson A, eds. Handbook of clinical neurology: functional neurologic disorders. 3rd ed. New York, NY: Elsevier; 2017:139-155.
8. Paras M, Murad M, Chen L, et al. Sexual abuse and lifetime diagnosis of somatic disorders. JAMA. 2009;302(5):550.
9. Fiszman A, Alves-Leon SV, Nunes RG, et al. Traumatic events and posttraumatic stress disorder in patients with psychogenic nonepileptic seizures: a critical review. Epilepsy Behav. 2004;5(6):818-825.
10. Sharpe D, Faye C. Non-epileptic seizures and child sexual abuse: a critical review of the literature. Clin Psychol Rev. 2006;26(8):1020-1040.
11. Alper K, Devinsky O, Perrine K, et al. Nonepileptic seizures and childhood sexual and physical abuse. Neurology. 1993;43(10):1950-1953.
12. Plioplys S, Doss J, Siddarth P et al. A multisite controlled study of risk factors in pediatric psychogenic nonepileptic seizures. Epilepsia. 2014;55(11):1739-1747.
13. Andersen S, Tomada A, Vincow E, et al. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20(3):292-301.
14. Sar V. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
15. Rosenberg HJ, Rosenberg SD, Williamson PD, et al. A comparative study of trauma and posttraumatic stress disorder prevalence in epilepsy patients and psychogenic nonepileptic seizure patients. Epilepsia. 2000;41(4):447-452.
16. Durrant J, Rickards H, Cavanna A. Prognosis and outcome predictors in psychogenic nonepileptic seizures. Epilepsy Res Treat. 2011;2011:1-7.
17. Selkirk M, Duncan R, Oto M, et al. Clinical differences between patients with nonepileptic seizures who report antecedent sexual abuse and those who do not. Epilepsia. 2008;49(8):1446-1450.
18. Goldstein L, Chalder T, Chigwedere C, et al. Cognitive-behavioral therapy for psychogenic nonepileptic seizures: a pilot RCT. Neurology. 2010;74(24):1986-1994.
19. LaFrance W, Baird G, Barry J, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures. JAMA Psychiatry. 2014;71(9):997.
20. Howlett S, Reuber M. An augmented model of brief psychodynamic interpersonal therapy for patients with nonepileptic seizures. Psychotherapy (Chic). 2009;46(1):125-138.
21. Metin SZ, Ozmen M, Metin B, et al. Treatment with group psychotherapy for chronic psychogenic nonepileptic seizures. Epilepsy Behav. 2013;28(1):91-94.
22. Carlson P, Perry KN. Psychological interventions for psychogenic non-epileptic seizures: a meta-analysis. Seizure. 2017;45:142-150.
1. Lesser R. Psychogenic seizures. Neurology. 1996;46(6):1499-1507.
2. Stone J, LaFrance W, Brown R, et al. Conversion disorder: current problems and potential solutions for DSM-5. J Psychosom Res. 2011;71(6):369-376.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Syed T, Arozullah A, Suciu G, et al. Do observer and self-reports of ictal eye closure predict psychogenic nonepileptic seizures? Epilepsia. 2008;49(5):898-904.
5. Vega-Zelaya L, Alvarez M, Ezquiaga E, et al. Psychogenic non-epileptic seizures in a surgical epilepsy unit: experience and a comprehensive review. Epilepsy Topics. 2014. doi: 10.5772/57439.
6. LaFrance W, Baker G, Duncan R, et al. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach. Epilepsia. 2013;54(11):2005-2018.
7. Roeloes K, Pasman J. Stress, childhood trauma, and cognitive functions in functional neurologic disorders. In: Hallett M, Stone J, Carson A, eds. Handbook of clinical neurology: functional neurologic disorders. 3rd ed. New York, NY: Elsevier; 2017:139-155.
8. Paras M, Murad M, Chen L, et al. Sexual abuse and lifetime diagnosis of somatic disorders. JAMA. 2009;302(5):550.
9. Fiszman A, Alves-Leon SV, Nunes RG, et al. Traumatic events and posttraumatic stress disorder in patients with psychogenic nonepileptic seizures: a critical review. Epilepsy Behav. 2004;5(6):818-825.
10. Sharpe D, Faye C. Non-epileptic seizures and child sexual abuse: a critical review of the literature. Clin Psychol Rev. 2006;26(8):1020-1040.
11. Alper K, Devinsky O, Perrine K, et al. Nonepileptic seizures and childhood sexual and physical abuse. Neurology. 1993;43(10):1950-1953.
12. Plioplys S, Doss J, Siddarth P et al. A multisite controlled study of risk factors in pediatric psychogenic nonepileptic seizures. Epilepsia. 2014;55(11):1739-1747.
13. Andersen S, Tomada A, Vincow E, et al. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20(3):292-301.
14. Sar V. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
15. Rosenberg HJ, Rosenberg SD, Williamson PD, et al. A comparative study of trauma and posttraumatic stress disorder prevalence in epilepsy patients and psychogenic nonepileptic seizure patients. Epilepsia. 2000;41(4):447-452.
16. Durrant J, Rickards H, Cavanna A. Prognosis and outcome predictors in psychogenic nonepileptic seizures. Epilepsy Res Treat. 2011;2011:1-7.
17. Selkirk M, Duncan R, Oto M, et al. Clinical differences between patients with nonepileptic seizures who report antecedent sexual abuse and those who do not. Epilepsia. 2008;49(8):1446-1450.
18. Goldstein L, Chalder T, Chigwedere C, et al. Cognitive-behavioral therapy for psychogenic nonepileptic seizures: a pilot RCT. Neurology. 2010;74(24):1986-1994.
19. LaFrance W, Baird G, Barry J, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures. JAMA Psychiatry. 2014;71(9):997.
20. Howlett S, Reuber M. An augmented model of brief psychodynamic interpersonal therapy for patients with nonepileptic seizures. Psychotherapy (Chic). 2009;46(1):125-138.
21. Metin SZ, Ozmen M, Metin B, et al. Treatment with group psychotherapy for chronic psychogenic nonepileptic seizures. Epilepsy Behav. 2013;28(1):91-94.
22. Carlson P, Perry KN. Psychological interventions for psychogenic non-epileptic seizures: a meta-analysis. Seizure. 2017;45:142-150.
Testicular Cancer: Diagnosis and Treatment
Malignant testicular neoplasms can arise from either the germ cells or sex-cord stromal cells, with the former comprising approximately 95% of all testicular cancers (Table 1). Germ cell tumors may contain a single histology or a mix of multiple histologies. For clinical decision making, testicular tumors are categorized as either pure seminoma (no nonseminomatous elements present) or nonseminomatous germ cell tumors (NSGCT). The prevalence of seminoma and NSGCT is roughly equal. If a testicular tumor contains both seminomatous and nonseminomatous components, it is called a mixed germ cell tumor. Because of similarities in biological behavior, the approach to treatment of mixed germ cell tumors is similar to that for NSGCT.
The key points to remember for testicular cancer are:
- With early diagnosis and aggressive multidisciplinary therapy, the overwhelming majority of patients can be cured;
- Specialized care is often critical and affects outcomes; and
- Survivorship, or post-treatment care, is very important for these patients, as they often have lifespan of several decades and a unique set of short- and long-term treatment-related complications.
Developmental Biology and Genetics
The developmental biology of germ cells and germ cell neoplasms is beyond the scope of this review, and interested readers are recommended to refer to pertinent articles on the topic.1,2 A characteristic genetic marker of all germ cell tumors is an isochromosome of the short arm of chromosome 12, i(12p). This is present in testicular tumors regardless of histologic subtype as well as in carcinoma-in-situ. In germ cell tumors without i(12p) karyotype, excess 12p genetic material consisting of repetitive segments has been found, suggesting that this is an early and potentially critical change in oncogenesis.3 Several recent studies have revealed a diverse genomic landscape in testicular cancers, including KIT, KRAS and NRAS mutations in addition to a hyperdiploid karyotype.4,5
Evaluation and Diagnosis
Case Presentation
A 23-year-old Caucasian man presents to a primary care clinic for a pre-employment history and physical exam. He reports testicular pain on the sexually transmitted infections screening questionnaire. On examination, the physician finds a firm, mobile, minimally-tender, 1.5-cm mass in the inferior aspect of left testicle. No contralateral testicular mass or inguinal lymphadenopathy is noted, and a detailed physical exam is otherwise unremarkable. The physician immediately orders an ultrasound of the testicles, which shows a 1.5-cm hypoechoic mass in the inferior aspect of the left testicle, with an unremarkable contralateral testicle. After discussion of the results, the patient is referred a urologic oncologist with expertise in testicular cancer for further care.
The urologic oncologist orders a computed tomography (CT) abdomen and pelvis with and without contrast, which shows a 1.8-cm pathologic-appearing retroperitoneal lymph node at the level of the left renal vein. Chest radiograph with anteroposterior and lateral views is unremarkable. Tumor markers are as follows: beta human chorionic gonadotropin (beta-HCG) 8 mIU/mL (normal range, 0–4 mIU/mL), alpha-fetoprotein (AFP) 2 ng/mL (normal range, 0–8.5 ng/mL), and lactate dehydrogenase (LDH) 195 U/L (normal range, 119–213 U/L).
What is the approach to the initial workup and diagnosis of testicular cancer?
Clinical Presentation and Physical Exam
The majority of testicular cancers are diagnosed on work-up of a nodule or painless swelling of one testicle, usually noted incidentally by the patient. Approximately 30% to 40% of patients complain of a dull ache or heavy sensation in the lower abdomen, perianal area, or scrotum, while acute pain is the presenting symptom in 10%.3
In approximately 10% of patients, the presenting symptom is a result of distant metastatic involvement, such as cough and dyspnea on exertion (pulmonary or mediastinal metastasis), intractable bone pain (skeletal metastasis), intractable back/flank pain, presence of psoas sign or unexplained lower extremity deep vein thrombosis (bulky retroperitoneal metastasis), or central nervous system symptoms (vertebral, spinal or brain metastasis). Constitutional symptoms (unexplained weight loss, anorexia, fatigue) often accompany these symptoms.3
Rarely (5% or less), testicular cancer may present with systemic endocrine symptoms or paraneoplastic symptoms. Gynecomastia is the most common in this category, occurring in approximately 2% of germ cell tumors and more commonly (20%–30%) in Leydig cell tumors of testis.6 Classically, these patients are either 6- to 10-year-old boys with precocious puberty or young men (mid 20s-mid 30s) with a combination of testicular mass, gynecomastia, loss of libido, and impotence. Workup typically reveals increased beta-HCG levels in blood.
Anti-Ma2-antibody-associated limbic encephalitis is the most common (and still quite rare) paraneoplastic complication associated with testicular germ cell tumors. The Ma2 antigen is selectively expressed in the neuronal nucleoli of normal brain tissue and the testicular tumor of the patient. Importantly, in a subset of these patients, the treatment of testicular cancer may result in improvement of symptoms of encephalitis.7
The first step in the diagnosis of testicular neoplasm is a physical exam. This should include a bimanual examination of the scrotal contents, starting with the normal contralateral testis. Normal testicle has a homogeneous texture and consistency, is freely movable, and is separable from the epididymis. Any firm, hard, or fixed mass within the substance of the tunica albuginea should be considered suspicious until proven otherwise. Spread to the epididymis or spermatic cord occurs in 10% to 15% of patients and examination should include these structures as well.3 A comprehensive system-wise examination for features of metastatic spread as discussed above should then be performed. If the patient has cryptorchidism, ultrasound is a mandatory part of the diagnostic workup.
If clinical evaluation suggests a possibility of testicular cancer, the patient must be counseled to undergo an expedited diagnostic workup and specialist evaluation, as a prompt diagnosis and treatment is key to not only improving the likelihood of cure, but also minimizing the treatments needed to achieve it.
Role of Imaging
Scrotal Ultrasound
Scrotal ultrasound is the first imaging modality used in the diagnostic workup of patient with suspected testicular cancer. Bilateral scrotal ultrasound can detect lesions as small as 1 to 2 mm in diameter and help differentiate intratesticular lesions from extrinsic masses. A cystic mass on ultrasound is unlikely to be malignant. Seminomas appear as well-defined hypoechoic lesions without cystic areas, while NSGCTs are typically inhomogeneous with calcifications, cystic areas, and indistinct margins. However, this distinction is not always apparent or reliable. Ultrasound alone is also insufficient for tumor staging.8 For these reasons, a radical inguinal orchiectomy must be pursued for accurate determination of histology and local stage.
If testicular ultrasound shows a suspicious intratesticular mass, the following workup is typically done:
- Measurement of serum tumor markers (beta-HCG, AFP and LDH);
- CT abdomen and pelvis with and without contrast;
- Chest radiograph anteroposterior and lateral views, or CT chest with and without contrast if clinically indicated;
- Any additional focal imaging based on symptoms (eg, magnetic resonance imaging [MRI] scan with and without contrast to evaluate the brain if the patient has CNS symptoms).
CT Scan
CT scan is the preferred imaging modality for staging of testicular cancers, specifically for evaluation of the retroperitoneum, as it is the predominant site for metastases.9 CT scan should encompass the abdomen and pelvis, and contrast-enhanced sequences should be obtained unless medically contraindicated. CT scan of the chest (if not initially done) is compulsory should a CT of abdomen and pelvis and/or a chest radiograph show abnormal findings.
The sensitivity and specificity of CT scans for detection of nodal metastases can vary significantly based on the cutoff. For example, in a series of 70 patients using a cutoff of 10 mm, the sensitivity and specificity of CT scans for patients undergoing retroperitoneal lymph node dissection were 37% and 100%, respectively.10 In the same study, a cutoff of 4 mm increased the sensitivity to 93% and decreased the specificity to 58%. The current general consensus for this cutoff value is 8 to 10 mm measured in the short axis in the transverse (axial) plane.
Approximately 20% of men with clinical stage I testicular cancer (ie, those with non-enlarged retroperitoneal lymph nodes) who do not undergo any adjuvant therapy will have disease relapse in the retroperitoneum, suggesting that they had occult micrometastases that were missed on the initial CT scans.11,12
MRI/Radionuclide Bone Scan/PET Scan
Abdominal or pelvic MRI, whole-body radionuclide bone scan, and positron emission tomography (PET) scans are almost never needed as part of the initial staging workup for testicular cancers due to several limitations, including a high false-negative rate, specifically for the PET scans, and lack of any additional value compared with CT and testicular ultrasound alone.9,13,14 If necessary, these should only be ordered after a multidisciplinary oncology consultation to prevent unnecessary delays in treatment, inappropriate changes to treatment, and unnecessary increases in cost of care.
Tumor Markers, Biopsy, and Staging
What is the role of tumor markers in the management of testicular cancers?
Serum AFP, beta-hCG, and LDH have a well-established role as tumor markers in testicular cancer. The alpha subunit of hCG is shared between multiple pituitary hormones and hence does not serve as a specific marker for testicular cancer. Serum levels of AFP and/or beta-hCG are elevated in approximately 80% percent of men with NSGCTs, even in absence of metastatic spread. On the other hand, serum beta-hCG is elevated in less than 20% and AFP is not elevated in pure seminomas.3
Tumor markers by themselves are not sufficiently sensitive or specific for the diagnosis of testicular cancer, in general, or to differentiate among its subtypes. Despite this limitation, marked elevations in these markers are rarely due to causes other than germ cell tumor. For example, serum beta-hCG concentrations greater than 10,000 mIU/mL occur only in germ cell tumors, trophoblastic differentiation of a primary lung or gastric cancer, gestational trophoblastic disease, or pregnancy. Serum AFP concentrations greater than 10,000 ng/mL occur almost exclusively in germ cell tumors and hepatocellular carcinoma.15
The pattern of marker elevation may play an important role in management of testicular cancer patients. For example, in our practice, several patients have had discordant serum tumor markers and pathology results (eg, elevated AFP with pure seminoma on orchiectomy). One of these patients was treated with adjuvant retroperitoneal lymph node dissection, which confirmed that he had a NSGCT with a seminoma, choriocarcinoma, and teratoma on pathology evaluation of retroperitoneal lymph nodes.
Serum tumor markers have 2 additional critical roles—(1) in the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) staging16 and International Germ Cell Cancer Collaboration Group (IGCCCG) risk stratification of testicular cancer,17 and (2) in post-treatment disease monitoring.
Is a testicular biopsy necessary for diagnosis?
A testicular biopsy is almost never pursued to confirm the diagnosis of testicular cancer. There is a concern that percutaneous testicular biopsy, which is associated with scrotal skin violation, can adversely affect outcomes due to tumor seeding of scrotal sac or metastatic spread into the inguinal nodes via scrotal skin lymphatics.
Tissue diagnosis is made by radical orchiectomy in a majority of cases. Rarely in our practice, we obtain a biopsy of metastatic lesion for a tissue diagnosis. This is only done in cases where chemotherapy must be started urgently to prevent worsening of complications from metastatic spread. This decision should be made only after a multidisciplinary consultation with urologic and medical oncology teams.
How is testicular cancer staged?
Both seminomatous and nonseminomatous germ cell tumors of the testis are staged using the AJCC/UICC staging system, which incorporates assessments of the primary tumor (T), lymph nodes (N), and distant metastases (M) and serum tumor marker values (S). Details of this staging system are beyond the scope of this review and further information can be obtained through the AJCC website (www.cancerstaging.org). This TNMS staging enables a prognostic assessment and helps with the therapeutic approach.
For patients with advanced germ cell tumors, a risk group classification developed by the IGCCCG is used to classify patients into good-risk, intermediate-risk, and poor-risk category (Table 2). This classification has been extensively validated for the past 2 decades, provides important prognostic information, and helps inform therapy decisions.
Treatment
Case 1 Continued
Based on the patient’s imaging and biomarker results, the patient undergoes a left radical inguinal orchiectomy. The physician’s operative note mentions that the left testicle was delivered without violation of scrotal integrity. A pathology report shows pure spermatocytic seminoma (unifocal, 1.4 cm size) with negative margins and no evidence of lymphovascular invasion. No lymph nodes are identified in the resection specimen. Post-orchiectomy markers are “negative,” meaning within normal range. After discussions with medical and radiation oncology physicians, the patient opts to pursue active surveillance.
Surgery alone followed by active surveillance is an appropriate option for this patient, as the likelihood of recurrence is low and most recurrences can be subsequently salvaged using treatment options detailed below.
What are the therapeutic options for testicular cancer?
An overview of management for most testicular cancers is presented in Table 3. Note that the actual treatments are significantly more complex and need a comprehensive multidisciplinary consultation (urologic, medical and radiation oncology) at centers with specialized testicular cancer teams, if possible.
Fertility Preservation
All patients initiating treatment for testicular cancer must be offered options for fertility preservation and consultation with a reproductive health team, if available. At the time of diagnosis, approximately 50% patients have some degree of impairment in spermatogenesis, but with effective fertility preservation, successful pregnancy can occur for as many as 30% to 60% of patients.18,19
Orchiectomy
Radical inguinal orchiectomy with high ligation of the spermatic cord at the level of the internal ring is the procedure of choice for suspected testicular cancer. The goal is to provide a definitive tissue diagnosis and local tumor control with minimal morbidity. It can be performed under general, regional, or local anesthesia. Depending on the complexity and surgical expertise, it can be done in an inpatient or outpatient setting. During the procedure, the testicle is delivered from the scrotum through an incision in the inguinal region and then resected. A testicular prosthesis is usually inserted, with resultant excellent cosmetic and patient satisfaction outcomes.20
Testicular sparing surgery (TSS) has been explored as an alternative to radical orchiectomy but is not considered a standard-of-care option at this time. Small studies have shown evidence for comparable short-term oncologic outcomes in a very select group of patients, generally with solitary tumors < 2 cm in size and solitary testicle. If this is being considered as an option, we recommended obtaining a consultation from a urologist at a high-volume center. For a majority of patients, the value of a TSS is diminished due to excellent anatomic/cosmetic outcomes with a testicular prosthesis implanted during the radical orchiectomy, and resumption of sexual functions by the unaffected contralateral testicle.
Retroperitoneal Lymph Node Dissection
As discussed, conventional cross-sectional imaging has a high false-negative rate for detection of retroperitoneal involvement. General indications for RPLND in various stages and histologies of testicular cancer germ cell tumors are outlined in Table 3. Seminoma tends to most commonly metastasize to retroperitoneum, but RPLND for seminoma is generally reserved for a very small subset of these patients. Patterns of metastases of NSGCT (except choriocarcinoma) are considered to be well-defined. In a series of patients with stage II NSGCTs, left-sided tumors metastasized to the pre- and para-aortic nodes in 88% and 86% of cases, respectively (drainage basin of left testicular vein); and right-sided tumors involved the interaortocaval nodes in 93% of patients.3 Inguinal and pelvic nodal metastases may rarely be seen and should not be used to rule out the diagnosis of testicular cancer.
Choriocarcinoma is an exception to this pattern of retroperitoneal spread, as it tends to have a higher likelihood of hematogenous metastases to distant organs. Compared with NSGCTs, pure seminomas are either localized to the testis (80% of all cases) or limited to the retroperitoneum (an additional 15% of all cases) at presentation.3
Depending on the case and expertise of the surgical team, robotic or open RPLND can be performed.21 Regardless of the approach used, RPLND remains a technically challenging surgery. The retroperitoneal “landing zone” lymph nodes lie in close proximity to, and are often densely adherent to, the abdominal great vessels. Complication rates vary widely in the reported literature, but can be as high as 50%.21-23 As detailed in Table 2, the number and size of involved retroperitoneal lymph nodes have prognostic importance.
In summary, RPLND is considered to be a viable option for a subset of early-stage NSGCT (T1-3, N0-2, M0) and for those with advanced seminoma, NSGCT, or mixed germ cell tumors with post-chemotherapy residual disease.
Systemic Chemotherapy
Except for the single-agent carboplatin, most chemotherapy regimens used to treat testicular cancer are combinations of 2 or more chemotherapy agents. For this review, we will focus on the 3 most commonly used regimens: bleomycin, etoposide, and cisplatin (BEP), etoposide and cisplatin (EP), and etoposide, ifosfamide, and cisplatin (VIP).
The core principles of testicular cancer chemotherapy are:
- Minimize dose interruptions, delays, or reductions, as these adversely affect outcomes without clearly improving side effect profile;
- Do not substitute carboplatin for cisplatin in combination regimens because carboplatin-containing combination regimens have been shown to result in significantly poorer outcomes in multiple trials of adults with germ cell tumors;24-27 and
- Give myeloid growth factor support, if necessary.
BEP
The standard BEP regimen comprises a 21-day cycle with bleomycin 30 units on days 1, 8, and 15; etoposide 100 mg/m2 on days 1 to 5; and cisplatin 20 mg/m2 on days 1 to 5. Number of cycles varies based on histology and stage (Table 3). A strong justification to maintain treatment intensity comes from the Australian and New Zealand Germ Cell Trial Group trial. In this study, 166 men were randomly assigned to treatment using 3 cycles of standard BEP or 4 cycles of a modified BEP regimen (bleomycin 30 units day 1; etoposide 120 mg/m2 days 1 to 3; cisplatin 100 mg/m2 day 1) every 21 days. This trial was stopped at interim analyses because the modified BEP arm was inferior to the standard BEP arm. With a median follow-up of 8.5 years, 8-year overall survival was 92% with standard BEP and 83% with modified BEP (P = 0.037).28
Bleomycin used in the BEP regimen has been associated with uncommon but potentially fatal pulmonary toxicity that tends to present as interstitial pneumonitis, which may ultimately progress to fibrosis or bronchiolitis obliterans with organizing pneumonia.29 This has led to evaluation of EP as an alternative to BEP.
EP
The standard EP regimen consists of a 21-day cycle with etoposide 100 mg/m2 on days 1 to 5, and cisplatin 20 mg/m2 on days 1 to 5. Due to conflicting data from multiple randomized trials, there is considerable debate in the field regarding whether 4 cycles of EP are equivalent to 3 cycles of BEP.30,31 The benefit of the EP regimen is that it avoids the higher rates of pulmonary, cutaneous, and neurologic toxicities associated bleomycin, but it does result in the patient receiving an up to 33% higher cumulative dose of cisplatin and etoposide due to the extra cycle of treatment. This has important implications in terms of tolerability and side effects, including delayed toxicities such as second malignancies, which increase with a higher cumulative dose of these agents (etoposide in particular).
VIP
The standard VIP regimen consists of a 21-day cycle with etoposide 75 mg/m2 on days 1 to 5; cisplatin 20 mg/m2 on days 1 to 5; ifosfamide 1200 mg/m2 on days 1 to 5; and mesna 120 mg/m2 IV push on day 1 followed by 1200 mg/m2 on days 1 to 5. For patients with intermediate- or poor-risk disease, 4 cycles of VIP has demonstrated comparable efficacy but higher rates of hematologic toxicities compared with 4 cycles of BEP.32-34 It remains an option for upfront treatment of patients who are not good candidates for a bleomycin-based regimen, and for patients who need salvage chemotherapy.
Adverse Effects of Chemotherapy
Acute and late chemotherapy toxicities vary significantly between regimens depending on the chemotherapy drugs used. Bleomycin-induced pneumonitis may masquerade as a “pneumonia,” which can lead to a delay in diagnosis or institution of treatment, as well as institution of an incorrect treatment (for example, there is a concern that bleomycin toxicity can be precipitated or worsened by a high fraction of inspired oxygen). Chemotherapy-associated neutropenia tends to occur a few days (7–10 days) after initiation of chemotherapy, and neutrophil counts recover without intervention in most patients after an additional 7 to 10 days. Myeloid growth factor support (eg, filgrastim, pegfilgrastim) can be given to patients either prophylactically (if they had an episode of febrile or prolonged neutropenia with the preceding cycle) or secondarily if they present with neutropenia (an absolute neutrophil count ≤ 500 cells/µL) with fever or active infection. Such interventions tend to shorten the duration of neutropenia but does not affect overall survival. Patients with asymptomatic neutropenia do not benefit from growth factor use.35
Stem Cell Transplant
Autologous stem cell transplant (SCT) is the preferred type of SCT for patients with testicular cancer and involves delivery of high doses of chemotherapy followed by infusion of patient-derived myeloid stem cells. While the details of this treatment are outside the scope of this review, decades of experience has shown that this is an effective curative option for a subset of patients with poor prognosis, such as those with platinum-refractory or relapsed disease.36
Clinical Trials
Due to excellent clinical outcomes with front-line therapy, as described, and the relatively low incidence of testicular and other germ cell tumors, clinical trial options for patients with testicular cancer are limited. The TIGER trial is an ongoing international, randomized, phase 3 trial comparing conventional TIP (paclitaxel, ifosfamide, and cisplatin) chemotherapy with high-dose chemotherapy with SCT as the first salvage treatment for relapsed/refractory germ cell tumors (NCT02375204). It is enrolling at multiple centers in the United States and results are expected in 2022. At least 2 ongoing trials are evaluating the role of immunotherapy in patients with relapsed/refractory germ cell tumors (NCT03081923 and NCT03726281). Cluster of differentiation antigen-30 (CD30) has emerged as a potential target of interest in germ cell tumors, and brentuximab vedotin, an anti-CD30 monoclonal antibody, is undergoing evaluation in a phase 2 trial of CD-30–expressing germ cell tumors (NCT01851200). This trial has completed enrollment and results are expected to be available in late 2019 or early 2020.
When possible, patients with relapsed/refractory germ cell tumors should be referred to centers of excellence with access to either testicular/germ-cell tumor specific clinical trials or phase 1 clinical trials.
Radiation Therapy
Adjuvant radiation to the retroperitoneum has a role in the management of stage I and IIA seminomas (Table 3). In a randomized noninferiority trial of radiation therapy versus single-dose carboplatin in stage I seminoma patients, 5-year recurrence-free survival was comparable at approximately 95% in either arm.37,38 In a retrospective database review of 2437 patients receiving either radiation therapy or multi-agent chemotherapy for stage II seminoma, the 5-year survival exceeded 90% in both treatment groups.39 Typically, a total of 30 to 36 Gy of radiation is delivered to para-aortic and ipsilateral external iliac lymph nodes (“dog-leg” field), followed by an optional boost to the involved nodal areas.40 Radiation is associated with acute side effects such as fatigue, gastrointestinal effects, myelosuppression as well as late side effects such as second cancers in the irradiated field (eg, sarcoma, bladder cancer).
Evaluation of Treatment Response
Monitoring of treatment response is fairly straightforward for patients with testicular cancer. Our practice is the following:
- Measure tumor markers on day 1 of each chemotherapy cycle and 3 to 4 weeks after completion of treatment.
- CT of the chest, abdomen, and pelvis with intravenous contrast prior to chemotherapy and upon completion of chemotherapy. Interim imaging is only needed for a small subset of patients with additional clinical indications (eg, new symptoms, lack of improvement in existing symptoms).
- For patients with stage II/III seminoma who have a residual mass ≥ 3 cm on post-treatment CT scan, a PET-CT scan is indicated 6 to 8 weeks after the completion of chemotherapy to determine the need for further treatment.
Active Surveillance
Because testicular cancer has high cure rates even when patients have disease relapse after primary therapy, and additional therapies have significant short- and long-term side effects in these generally young patients, active surveillance is a critical option used in the management of testicular cancer.41
Patients must be counseled that active surveillance is a form of treatment itself in that it involves close clinical and radiographic monitoring. Because there is a risk of disease relapse, patients opting to undergo active surveillance must fully understand the risks of disease recurrence and be willing to abide by the recommended follow-up schedule.
Surveillance is necessary for a minimum of 5 years and possibly 10 years following orchiectomy, and most relapses tend to occur within the first 2 years. Late relapses such as skeletal metastatic disease from seminoma have been reported to occur more than 15 years after orchiectomy, but are generally rare and unpredictable.
The general guidelines for active surveillance are as follows:
For patients with seminoma, history and physical exam and tumor marker assessment should be performed every 3 to 6 months for the first year, then every 6 to 12 months in years 2 and 3, and then annually. CT of the abdomen and pelvis should be done at 3, 6, and 12 months, every 6 to 12 months in years 2 and 3, and then every 12 to 24 months in years 4 and 5. A chest radiograph is performed only if clinically indicated, as the likelihood of distant metastatic recurrence is low.
For patients with nonseminoma, history and physical exam and tumor markers assessment should be performed every 2 to 3 months for first 2 years, every 4 to 6 months in years 3 and 4, and then annually. CT of the abdomen and pelvis should be obtained every 4 to 6 months in year 1, gradually decreasing to annually in year 3 or 4. Chest radiograph is indicated at 4 and 12 months and annually thereafter for stage IA disease. For those with stage IB disease, chest radiograph is indicated every 2 months during the first year and then gradually decreasing to annually beginning year 5.
These recommendations are expected to change over time, and treating physicians are recommended to exercise discretion and consider the patient and tumor characteristics to develop the optimal surveillance plan.
Conclusion
Testicular cancer is the most common cancer afflicting young men. Prompt diagnostic workup initiated in a primary care or hospital setting followed by a referral to a multidisciplinary team of urologists, medical oncologists, and radiation oncologists enables cure in a majority of patients. For patients with stage I seminoma, a radical inguinal orchiectomy followed by active surveillance may offer the best long-term outcome with minimal side effects. For patients with relapsed/refractory testicular cancers, clinical trial participation is strongly encouraged. Patients with a history of testicular cancer benefit from robust survivorship care tailored to their prior therapies. This can be safely delivered through their primary care providers in collaboration with the multidisciplinary oncology team.
1. van der Zwan YG, Biermann K, Wolffenbuttel KP, et al. Gonadal maldevelopment as risk factor for germ cell cancer: towards a clinical decision model. Eur Urol. 2015; 67:692–701.
2. Pierce JL, Frazier AL, Amatruda JF. Pediatric germ cell tumors: a developmental perspective. Adv Urol. 2018 Feb 4;2018.
3. Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med. 1997;337:242-253.
4. Pyle LC, Nathanson KL. Genetic changes associated with testicular cancer susceptibility. Semin Oncol. 2016;43:575-581.
5. Shen H, Shih J, Hollern DP, et al. Integrated molecular characterization of testicular germ cell tumors. Cell Rep. 2018;23:3392-3406.
6. Barry M, Rao A, Lauer R. Sex cord-stromal tumors of the testis. In: Pagliaro L, ed. Rare Genitourinary Tumors. Cham: Springer International Publishing; 2016: 231-251.
7. Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain J Neurol. 2004;127:1831-1844.
8. Coursey Moreno C, Small WC, Camacho JC, et al. Testicular tumors: what radiologists need to know—differential diagnosis, staging, and management. RadioGraphics. 2015;35:400-415.
9. Kreydin EI, Barrisford GW, Feldman AS, Preston MA. Testicular cancer: what the radiologist needs to know. Am J Roentgenol. 2013;200:1215-1225.
10. Hilton S, Herr HW, Teitcher JB, et al. CT detection of retroperitoneal lymph node metastases in patients with clinical stage I testicular nonseminomatous germ cell cancer: assessment of size and distribution criteria. Am J Roentgenol. 1997;169:521-525.
11. Thompson PI, Nixon J, Harvey VJ. Disease relapse in patients with stage I nonseminomatous germ cell tumor of the testis on active surveillance. J Clin Oncol. 1988;6:1597-1603.
12. Nicolai N, Pizzocaro G. A surveillance study of clinical stage I nonseminomatous germ cell tumors of the testis: 10-year followup. J Urol. 1995;154:1045-1049.
13. Kok HK, Leong S, Torreggiani WC. Is magnetic resonance imaging comparable with computed tomography in the diagnosis of retroperitoneal metastasis in patients with testicular cancer? Can Assoc Radiol J. 2014;65:196-198.
14. Hale GR, Teplitsky S, Truong H, et al. Lymph node imaging in testicular cancer. Transl Androl Urol. 2018;7:864-874.
15. Honecker F, Aparicio J, Berney D, et al. ESMO Consensus Conference on testicular germ cell cancer: diagnosis, treatment and follow-up. Ann Oncol. 2018;29:1658-1686.
16. Paner GP, Stadler WM, Hansel DE, et al. Updates in the Eighth Edition of the Tumor-Node-Metastasis Staging Classification for Urologic Cancers. Eur Urol. 2018;73:560-569.
17. International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997;15:594-603.
18. Lopategui DM, Ibrahim E, Aballa TC, et al. Effect of a formal oncofertility program on fertility preservation rates-first year experience. Transl Androl Urol. 2018;7:S271-S275.
19. Moody JA, Ahmed K, Horsfield C, et al. Fertility preservation in testicular cancer - predictors of spermatogenesis. BJU Int. 2018;122:236-242.
20. Dieckmann KP, Anheuser P, Schmidt S, et al. Testicular prostheses in patients with testicular cancer - acceptance rate and patient satisfaction. BMC Urol. 2015;15:16.
21. Schwen ZR, Gupta M, Pierorazio PM. A review of outcomes and technique for the robotic-assisted laparoscopic retroperitoneal lymph node dissection for testicular cancer. Adv Urol. 2018;2146080.
22. Singh P, Yadav S, Mahapatra S, Seth A. Outcomes following retroperitoneal lymph node dissection in postchemotherapy residual masses in advanced testicular germ cell tumors. Indian J Urol. 2016;32:40-44.
23. Heidenreich A, Thüer D, Polyakov S. Postchemotherapy retroperitoneal lymph node dissection in advanced germ cell tumours of the testis. Eur Urol. 2008;53:260-272.
24. Bajorin DF, Sarosdy MF, Pfister DG, et al. Randomized trial of etoposide and cisplatin versus etoposide and carboplatin in patients with good-risk germ cell tumors: a multiinstitutional study. J Clin Oncol. 1993;11:598-606.
25. Bokemeyer C, Köhrmann O, Tischler J, et al. A randomized trial of cisplatin, etoposide and bleomycin (PEB) versus carboplatin, etoposide and bleomycin (CEB) for patients with “good-risk” metastatic non-seminomatous germ cell tumors. Ann Oncol. 1996;7:1015-1021.
26. Horwich A, Sleijfer DT, Fosså SD, et al. Randomized trial of bleomycin, etoposide, and cisplatin compared with bleomycin, etoposide, and carboplatin in good-prognosis metastatic nonseminomatous germ cell cancer: a Multiinstitutional Medical Research Council/European Organization for Research and Treatment of Cancer Trial. J Clin Oncol. 1997;15:1844-1852.
27. Shaikh F, Nathan PC, Hale J, et al. Is there a role for carboplatin in the treatment of malignant germ cell tumors? A systematic review of adult and pediatric trials. Pediatr Blood Cancer. 2013;60:587-592.
28. Grimison PS, Stockler MR, Thomson DB, et al. Comparison of two standard chemotherapy regimens for good-prognosis germ cell tumors: updated analysis of a randomized trial. J Natl Cancer Inst. 2010;102:1253-1262.
29. Reinert T, da Rocha Baldotto CS, Nunes FAP, de Souza Scheliga AA. Bleomycin-induced lung injury. J Cancer Res. 2013;480608.
30. Jones RH, Vasey PA. Part II: Testicular cancer—management of advanced disease. Lancet Oncol. 2003;4:738-747.
31. Jankilevich G. BEP versus EP for treatment of metastatic germ-cell tumours. Lancet Oncol. 2004;5, 146.
32. Nichols CR, Catalano PJ, Crawford ED, et al. Randomized comparison of cisplatin and etoposide and either bleomycin or ifosfamide in treatment of advanced disseminated germ cell tumors: an Eastern Cooperative Oncology Group, Southwest Oncology Group, and Cancer and Leukemia Group B Study. J Clin Oncol. 1998;16:12871293.
33. Hinton S, Catalano PJ, Einhorn LH, et al. Cisplatin, etoposide and either bleomycin or ifosfamide in the treatment of disseminated germ cell tumors: final analysis of an intergroup trial. Cancer. 2003;97: 1869-1875.
34. de Wit R, Stoter G, Sleijfer DT, et al. Four cycles of BEP vs four cycles of VIP in patients with intermediate-prognosis metastatic testicular non-seminoma: a randomized study of the EORTC Genitourinary Tract Cancer Cooperative Group. European Organization for Research and Treatment of Cancer. Br J Cancer. 1998;78:828-832.
35. Mhaskar R, Clark OA, Lyman G, et al. Colony-stimulating factors for chemotherapy-induced febrile neutropenia. Cochrane Database Syst. Rev. 2014;CD003039.
36. Adra N, Abonour R, Althouse SK, et al. High-dose chemotherapy and autologous peripheral-blood stem-cell transplantation for relapsed metastatic germ cell tumors: The Indiana University experience. J Clin Oncol. 2017;35:1096-1102.
37. Oliver RT, Mason MD, Mead GM, et al. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet. 2005;366:293-300.
38. Oliver RT, Mead GM, Rustin GJ, et al. Randomized trial of carboplatin versus radiotherapy for stage I seminoma: mature results on relapse and contralateral testis cancer rates in MRC TE19/EORTC 30982 study (ISRCTN27163214). J Clin Oncol. 2011;29:957-962.
39. Glaser SM, Vargo JA, Balasubramani GK, Beriwal S. Stage II testicular seminoma: patterns of care and survival by treatment strategy. Clin Oncol. 2016;28:513-521.
40. Boujelbene N, Cosinschi A, Boujelbene N, et al. Pure seminoma: A review and update. Radiat Oncol. 2011;6:90.
41. Nichols CR, Roth B, Albers P, et al. Active surveillance is the preferred approach to clinical stage I testicular cancer. J Clin Oncol. 2013;31;3490-3493.
Malignant testicular neoplasms can arise from either the germ cells or sex-cord stromal cells, with the former comprising approximately 95% of all testicular cancers (Table 1). Germ cell tumors may contain a single histology or a mix of multiple histologies. For clinical decision making, testicular tumors are categorized as either pure seminoma (no nonseminomatous elements present) or nonseminomatous germ cell tumors (NSGCT). The prevalence of seminoma and NSGCT is roughly equal. If a testicular tumor contains both seminomatous and nonseminomatous components, it is called a mixed germ cell tumor. Because of similarities in biological behavior, the approach to treatment of mixed germ cell tumors is similar to that for NSGCT.
The key points to remember for testicular cancer are:
- With early diagnosis and aggressive multidisciplinary therapy, the overwhelming majority of patients can be cured;
- Specialized care is often critical and affects outcomes; and
- Survivorship, or post-treatment care, is very important for these patients, as they often have lifespan of several decades and a unique set of short- and long-term treatment-related complications.
Developmental Biology and Genetics
The developmental biology of germ cells and germ cell neoplasms is beyond the scope of this review, and interested readers are recommended to refer to pertinent articles on the topic.1,2 A characteristic genetic marker of all germ cell tumors is an isochromosome of the short arm of chromosome 12, i(12p). This is present in testicular tumors regardless of histologic subtype as well as in carcinoma-in-situ. In germ cell tumors without i(12p) karyotype, excess 12p genetic material consisting of repetitive segments has been found, suggesting that this is an early and potentially critical change in oncogenesis.3 Several recent studies have revealed a diverse genomic landscape in testicular cancers, including KIT, KRAS and NRAS mutations in addition to a hyperdiploid karyotype.4,5
Evaluation and Diagnosis
Case Presentation
A 23-year-old Caucasian man presents to a primary care clinic for a pre-employment history and physical exam. He reports testicular pain on the sexually transmitted infections screening questionnaire. On examination, the physician finds a firm, mobile, minimally-tender, 1.5-cm mass in the inferior aspect of left testicle. No contralateral testicular mass or inguinal lymphadenopathy is noted, and a detailed physical exam is otherwise unremarkable. The physician immediately orders an ultrasound of the testicles, which shows a 1.5-cm hypoechoic mass in the inferior aspect of the left testicle, with an unremarkable contralateral testicle. After discussion of the results, the patient is referred a urologic oncologist with expertise in testicular cancer for further care.
The urologic oncologist orders a computed tomography (CT) abdomen and pelvis with and without contrast, which shows a 1.8-cm pathologic-appearing retroperitoneal lymph node at the level of the left renal vein. Chest radiograph with anteroposterior and lateral views is unremarkable. Tumor markers are as follows: beta human chorionic gonadotropin (beta-HCG) 8 mIU/mL (normal range, 0–4 mIU/mL), alpha-fetoprotein (AFP) 2 ng/mL (normal range, 0–8.5 ng/mL), and lactate dehydrogenase (LDH) 195 U/L (normal range, 119–213 U/L).
What is the approach to the initial workup and diagnosis of testicular cancer?
Clinical Presentation and Physical Exam
The majority of testicular cancers are diagnosed on work-up of a nodule or painless swelling of one testicle, usually noted incidentally by the patient. Approximately 30% to 40% of patients complain of a dull ache or heavy sensation in the lower abdomen, perianal area, or scrotum, while acute pain is the presenting symptom in 10%.3
In approximately 10% of patients, the presenting symptom is a result of distant metastatic involvement, such as cough and dyspnea on exertion (pulmonary or mediastinal metastasis), intractable bone pain (skeletal metastasis), intractable back/flank pain, presence of psoas sign or unexplained lower extremity deep vein thrombosis (bulky retroperitoneal metastasis), or central nervous system symptoms (vertebral, spinal or brain metastasis). Constitutional symptoms (unexplained weight loss, anorexia, fatigue) often accompany these symptoms.3
Rarely (5% or less), testicular cancer may present with systemic endocrine symptoms or paraneoplastic symptoms. Gynecomastia is the most common in this category, occurring in approximately 2% of germ cell tumors and more commonly (20%–30%) in Leydig cell tumors of testis.6 Classically, these patients are either 6- to 10-year-old boys with precocious puberty or young men (mid 20s-mid 30s) with a combination of testicular mass, gynecomastia, loss of libido, and impotence. Workup typically reveals increased beta-HCG levels in blood.
Anti-Ma2-antibody-associated limbic encephalitis is the most common (and still quite rare) paraneoplastic complication associated with testicular germ cell tumors. The Ma2 antigen is selectively expressed in the neuronal nucleoli of normal brain tissue and the testicular tumor of the patient. Importantly, in a subset of these patients, the treatment of testicular cancer may result in improvement of symptoms of encephalitis.7
The first step in the diagnosis of testicular neoplasm is a physical exam. This should include a bimanual examination of the scrotal contents, starting with the normal contralateral testis. Normal testicle has a homogeneous texture and consistency, is freely movable, and is separable from the epididymis. Any firm, hard, or fixed mass within the substance of the tunica albuginea should be considered suspicious until proven otherwise. Spread to the epididymis or spermatic cord occurs in 10% to 15% of patients and examination should include these structures as well.3 A comprehensive system-wise examination for features of metastatic spread as discussed above should then be performed. If the patient has cryptorchidism, ultrasound is a mandatory part of the diagnostic workup.
If clinical evaluation suggests a possibility of testicular cancer, the patient must be counseled to undergo an expedited diagnostic workup and specialist evaluation, as a prompt diagnosis and treatment is key to not only improving the likelihood of cure, but also minimizing the treatments needed to achieve it.
Role of Imaging
Scrotal Ultrasound
Scrotal ultrasound is the first imaging modality used in the diagnostic workup of patient with suspected testicular cancer. Bilateral scrotal ultrasound can detect lesions as small as 1 to 2 mm in diameter and help differentiate intratesticular lesions from extrinsic masses. A cystic mass on ultrasound is unlikely to be malignant. Seminomas appear as well-defined hypoechoic lesions without cystic areas, while NSGCTs are typically inhomogeneous with calcifications, cystic areas, and indistinct margins. However, this distinction is not always apparent or reliable. Ultrasound alone is also insufficient for tumor staging.8 For these reasons, a radical inguinal orchiectomy must be pursued for accurate determination of histology and local stage.
If testicular ultrasound shows a suspicious intratesticular mass, the following workup is typically done:
- Measurement of serum tumor markers (beta-HCG, AFP and LDH);
- CT abdomen and pelvis with and without contrast;
- Chest radiograph anteroposterior and lateral views, or CT chest with and without contrast if clinically indicated;
- Any additional focal imaging based on symptoms (eg, magnetic resonance imaging [MRI] scan with and without contrast to evaluate the brain if the patient has CNS symptoms).
CT Scan
CT scan is the preferred imaging modality for staging of testicular cancers, specifically for evaluation of the retroperitoneum, as it is the predominant site for metastases.9 CT scan should encompass the abdomen and pelvis, and contrast-enhanced sequences should be obtained unless medically contraindicated. CT scan of the chest (if not initially done) is compulsory should a CT of abdomen and pelvis and/or a chest radiograph show abnormal findings.
The sensitivity and specificity of CT scans for detection of nodal metastases can vary significantly based on the cutoff. For example, in a series of 70 patients using a cutoff of 10 mm, the sensitivity and specificity of CT scans for patients undergoing retroperitoneal lymph node dissection were 37% and 100%, respectively.10 In the same study, a cutoff of 4 mm increased the sensitivity to 93% and decreased the specificity to 58%. The current general consensus for this cutoff value is 8 to 10 mm measured in the short axis in the transverse (axial) plane.
Approximately 20% of men with clinical stage I testicular cancer (ie, those with non-enlarged retroperitoneal lymph nodes) who do not undergo any adjuvant therapy will have disease relapse in the retroperitoneum, suggesting that they had occult micrometastases that were missed on the initial CT scans.11,12
MRI/Radionuclide Bone Scan/PET Scan
Abdominal or pelvic MRI, whole-body radionuclide bone scan, and positron emission tomography (PET) scans are almost never needed as part of the initial staging workup for testicular cancers due to several limitations, including a high false-negative rate, specifically for the PET scans, and lack of any additional value compared with CT and testicular ultrasound alone.9,13,14 If necessary, these should only be ordered after a multidisciplinary oncology consultation to prevent unnecessary delays in treatment, inappropriate changes to treatment, and unnecessary increases in cost of care.
Tumor Markers, Biopsy, and Staging
What is the role of tumor markers in the management of testicular cancers?
Serum AFP, beta-hCG, and LDH have a well-established role as tumor markers in testicular cancer. The alpha subunit of hCG is shared between multiple pituitary hormones and hence does not serve as a specific marker for testicular cancer. Serum levels of AFP and/or beta-hCG are elevated in approximately 80% percent of men with NSGCTs, even in absence of metastatic spread. On the other hand, serum beta-hCG is elevated in less than 20% and AFP is not elevated in pure seminomas.3
Tumor markers by themselves are not sufficiently sensitive or specific for the diagnosis of testicular cancer, in general, or to differentiate among its subtypes. Despite this limitation, marked elevations in these markers are rarely due to causes other than germ cell tumor. For example, serum beta-hCG concentrations greater than 10,000 mIU/mL occur only in germ cell tumors, trophoblastic differentiation of a primary lung or gastric cancer, gestational trophoblastic disease, or pregnancy. Serum AFP concentrations greater than 10,000 ng/mL occur almost exclusively in germ cell tumors and hepatocellular carcinoma.15
The pattern of marker elevation may play an important role in management of testicular cancer patients. For example, in our practice, several patients have had discordant serum tumor markers and pathology results (eg, elevated AFP with pure seminoma on orchiectomy). One of these patients was treated with adjuvant retroperitoneal lymph node dissection, which confirmed that he had a NSGCT with a seminoma, choriocarcinoma, and teratoma on pathology evaluation of retroperitoneal lymph nodes.
Serum tumor markers have 2 additional critical roles—(1) in the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) staging16 and International Germ Cell Cancer Collaboration Group (IGCCCG) risk stratification of testicular cancer,17 and (2) in post-treatment disease monitoring.
Is a testicular biopsy necessary for diagnosis?
A testicular biopsy is almost never pursued to confirm the diagnosis of testicular cancer. There is a concern that percutaneous testicular biopsy, which is associated with scrotal skin violation, can adversely affect outcomes due to tumor seeding of scrotal sac or metastatic spread into the inguinal nodes via scrotal skin lymphatics.
Tissue diagnosis is made by radical orchiectomy in a majority of cases. Rarely in our practice, we obtain a biopsy of metastatic lesion for a tissue diagnosis. This is only done in cases where chemotherapy must be started urgently to prevent worsening of complications from metastatic spread. This decision should be made only after a multidisciplinary consultation with urologic and medical oncology teams.
How is testicular cancer staged?
Both seminomatous and nonseminomatous germ cell tumors of the testis are staged using the AJCC/UICC staging system, which incorporates assessments of the primary tumor (T), lymph nodes (N), and distant metastases (M) and serum tumor marker values (S). Details of this staging system are beyond the scope of this review and further information can be obtained through the AJCC website (www.cancerstaging.org). This TNMS staging enables a prognostic assessment and helps with the therapeutic approach.
For patients with advanced germ cell tumors, a risk group classification developed by the IGCCCG is used to classify patients into good-risk, intermediate-risk, and poor-risk category (Table 2). This classification has been extensively validated for the past 2 decades, provides important prognostic information, and helps inform therapy decisions.
Treatment
Case 1 Continued
Based on the patient’s imaging and biomarker results, the patient undergoes a left radical inguinal orchiectomy. The physician’s operative note mentions that the left testicle was delivered without violation of scrotal integrity. A pathology report shows pure spermatocytic seminoma (unifocal, 1.4 cm size) with negative margins and no evidence of lymphovascular invasion. No lymph nodes are identified in the resection specimen. Post-orchiectomy markers are “negative,” meaning within normal range. After discussions with medical and radiation oncology physicians, the patient opts to pursue active surveillance.
Surgery alone followed by active surveillance is an appropriate option for this patient, as the likelihood of recurrence is low and most recurrences can be subsequently salvaged using treatment options detailed below.
What are the therapeutic options for testicular cancer?
An overview of management for most testicular cancers is presented in Table 3. Note that the actual treatments are significantly more complex and need a comprehensive multidisciplinary consultation (urologic, medical and radiation oncology) at centers with specialized testicular cancer teams, if possible.
Fertility Preservation
All patients initiating treatment for testicular cancer must be offered options for fertility preservation and consultation with a reproductive health team, if available. At the time of diagnosis, approximately 50% patients have some degree of impairment in spermatogenesis, but with effective fertility preservation, successful pregnancy can occur for as many as 30% to 60% of patients.18,19
Orchiectomy
Radical inguinal orchiectomy with high ligation of the spermatic cord at the level of the internal ring is the procedure of choice for suspected testicular cancer. The goal is to provide a definitive tissue diagnosis and local tumor control with minimal morbidity. It can be performed under general, regional, or local anesthesia. Depending on the complexity and surgical expertise, it can be done in an inpatient or outpatient setting. During the procedure, the testicle is delivered from the scrotum through an incision in the inguinal region and then resected. A testicular prosthesis is usually inserted, with resultant excellent cosmetic and patient satisfaction outcomes.20
Testicular sparing surgery (TSS) has been explored as an alternative to radical orchiectomy but is not considered a standard-of-care option at this time. Small studies have shown evidence for comparable short-term oncologic outcomes in a very select group of patients, generally with solitary tumors < 2 cm in size and solitary testicle. If this is being considered as an option, we recommended obtaining a consultation from a urologist at a high-volume center. For a majority of patients, the value of a TSS is diminished due to excellent anatomic/cosmetic outcomes with a testicular prosthesis implanted during the radical orchiectomy, and resumption of sexual functions by the unaffected contralateral testicle.
Retroperitoneal Lymph Node Dissection
As discussed, conventional cross-sectional imaging has a high false-negative rate for detection of retroperitoneal involvement. General indications for RPLND in various stages and histologies of testicular cancer germ cell tumors are outlined in Table 3. Seminoma tends to most commonly metastasize to retroperitoneum, but RPLND for seminoma is generally reserved for a very small subset of these patients. Patterns of metastases of NSGCT (except choriocarcinoma) are considered to be well-defined. In a series of patients with stage II NSGCTs, left-sided tumors metastasized to the pre- and para-aortic nodes in 88% and 86% of cases, respectively (drainage basin of left testicular vein); and right-sided tumors involved the interaortocaval nodes in 93% of patients.3 Inguinal and pelvic nodal metastases may rarely be seen and should not be used to rule out the diagnosis of testicular cancer.
Choriocarcinoma is an exception to this pattern of retroperitoneal spread, as it tends to have a higher likelihood of hematogenous metastases to distant organs. Compared with NSGCTs, pure seminomas are either localized to the testis (80% of all cases) or limited to the retroperitoneum (an additional 15% of all cases) at presentation.3
Depending on the case and expertise of the surgical team, robotic or open RPLND can be performed.21 Regardless of the approach used, RPLND remains a technically challenging surgery. The retroperitoneal “landing zone” lymph nodes lie in close proximity to, and are often densely adherent to, the abdominal great vessels. Complication rates vary widely in the reported literature, but can be as high as 50%.21-23 As detailed in Table 2, the number and size of involved retroperitoneal lymph nodes have prognostic importance.
In summary, RPLND is considered to be a viable option for a subset of early-stage NSGCT (T1-3, N0-2, M0) and for those with advanced seminoma, NSGCT, or mixed germ cell tumors with post-chemotherapy residual disease.
Systemic Chemotherapy
Except for the single-agent carboplatin, most chemotherapy regimens used to treat testicular cancer are combinations of 2 or more chemotherapy agents. For this review, we will focus on the 3 most commonly used regimens: bleomycin, etoposide, and cisplatin (BEP), etoposide and cisplatin (EP), and etoposide, ifosfamide, and cisplatin (VIP).
The core principles of testicular cancer chemotherapy are:
- Minimize dose interruptions, delays, or reductions, as these adversely affect outcomes without clearly improving side effect profile;
- Do not substitute carboplatin for cisplatin in combination regimens because carboplatin-containing combination regimens have been shown to result in significantly poorer outcomes in multiple trials of adults with germ cell tumors;24-27 and
- Give myeloid growth factor support, if necessary.
BEP
The standard BEP regimen comprises a 21-day cycle with bleomycin 30 units on days 1, 8, and 15; etoposide 100 mg/m2 on days 1 to 5; and cisplatin 20 mg/m2 on days 1 to 5. Number of cycles varies based on histology and stage (Table 3). A strong justification to maintain treatment intensity comes from the Australian and New Zealand Germ Cell Trial Group trial. In this study, 166 men were randomly assigned to treatment using 3 cycles of standard BEP or 4 cycles of a modified BEP regimen (bleomycin 30 units day 1; etoposide 120 mg/m2 days 1 to 3; cisplatin 100 mg/m2 day 1) every 21 days. This trial was stopped at interim analyses because the modified BEP arm was inferior to the standard BEP arm. With a median follow-up of 8.5 years, 8-year overall survival was 92% with standard BEP and 83% with modified BEP (P = 0.037).28
Bleomycin used in the BEP regimen has been associated with uncommon but potentially fatal pulmonary toxicity that tends to present as interstitial pneumonitis, which may ultimately progress to fibrosis or bronchiolitis obliterans with organizing pneumonia.29 This has led to evaluation of EP as an alternative to BEP.
EP
The standard EP regimen consists of a 21-day cycle with etoposide 100 mg/m2 on days 1 to 5, and cisplatin 20 mg/m2 on days 1 to 5. Due to conflicting data from multiple randomized trials, there is considerable debate in the field regarding whether 4 cycles of EP are equivalent to 3 cycles of BEP.30,31 The benefit of the EP regimen is that it avoids the higher rates of pulmonary, cutaneous, and neurologic toxicities associated bleomycin, but it does result in the patient receiving an up to 33% higher cumulative dose of cisplatin and etoposide due to the extra cycle of treatment. This has important implications in terms of tolerability and side effects, including delayed toxicities such as second malignancies, which increase with a higher cumulative dose of these agents (etoposide in particular).
VIP
The standard VIP regimen consists of a 21-day cycle with etoposide 75 mg/m2 on days 1 to 5; cisplatin 20 mg/m2 on days 1 to 5; ifosfamide 1200 mg/m2 on days 1 to 5; and mesna 120 mg/m2 IV push on day 1 followed by 1200 mg/m2 on days 1 to 5. For patients with intermediate- or poor-risk disease, 4 cycles of VIP has demonstrated comparable efficacy but higher rates of hematologic toxicities compared with 4 cycles of BEP.32-34 It remains an option for upfront treatment of patients who are not good candidates for a bleomycin-based regimen, and for patients who need salvage chemotherapy.
Adverse Effects of Chemotherapy
Acute and late chemotherapy toxicities vary significantly between regimens depending on the chemotherapy drugs used. Bleomycin-induced pneumonitis may masquerade as a “pneumonia,” which can lead to a delay in diagnosis or institution of treatment, as well as institution of an incorrect treatment (for example, there is a concern that bleomycin toxicity can be precipitated or worsened by a high fraction of inspired oxygen). Chemotherapy-associated neutropenia tends to occur a few days (7–10 days) after initiation of chemotherapy, and neutrophil counts recover without intervention in most patients after an additional 7 to 10 days. Myeloid growth factor support (eg, filgrastim, pegfilgrastim) can be given to patients either prophylactically (if they had an episode of febrile or prolonged neutropenia with the preceding cycle) or secondarily if they present with neutropenia (an absolute neutrophil count ≤ 500 cells/µL) with fever or active infection. Such interventions tend to shorten the duration of neutropenia but does not affect overall survival. Patients with asymptomatic neutropenia do not benefit from growth factor use.35
Stem Cell Transplant
Autologous stem cell transplant (SCT) is the preferred type of SCT for patients with testicular cancer and involves delivery of high doses of chemotherapy followed by infusion of patient-derived myeloid stem cells. While the details of this treatment are outside the scope of this review, decades of experience has shown that this is an effective curative option for a subset of patients with poor prognosis, such as those with platinum-refractory or relapsed disease.36
Clinical Trials
Due to excellent clinical outcomes with front-line therapy, as described, and the relatively low incidence of testicular and other germ cell tumors, clinical trial options for patients with testicular cancer are limited. The TIGER trial is an ongoing international, randomized, phase 3 trial comparing conventional TIP (paclitaxel, ifosfamide, and cisplatin) chemotherapy with high-dose chemotherapy with SCT as the first salvage treatment for relapsed/refractory germ cell tumors (NCT02375204). It is enrolling at multiple centers in the United States and results are expected in 2022. At least 2 ongoing trials are evaluating the role of immunotherapy in patients with relapsed/refractory germ cell tumors (NCT03081923 and NCT03726281). Cluster of differentiation antigen-30 (CD30) has emerged as a potential target of interest in germ cell tumors, and brentuximab vedotin, an anti-CD30 monoclonal antibody, is undergoing evaluation in a phase 2 trial of CD-30–expressing germ cell tumors (NCT01851200). This trial has completed enrollment and results are expected to be available in late 2019 or early 2020.
When possible, patients with relapsed/refractory germ cell tumors should be referred to centers of excellence with access to either testicular/germ-cell tumor specific clinical trials or phase 1 clinical trials.
Radiation Therapy
Adjuvant radiation to the retroperitoneum has a role in the management of stage I and IIA seminomas (Table 3). In a randomized noninferiority trial of radiation therapy versus single-dose carboplatin in stage I seminoma patients, 5-year recurrence-free survival was comparable at approximately 95% in either arm.37,38 In a retrospective database review of 2437 patients receiving either radiation therapy or multi-agent chemotherapy for stage II seminoma, the 5-year survival exceeded 90% in both treatment groups.39 Typically, a total of 30 to 36 Gy of radiation is delivered to para-aortic and ipsilateral external iliac lymph nodes (“dog-leg” field), followed by an optional boost to the involved nodal areas.40 Radiation is associated with acute side effects such as fatigue, gastrointestinal effects, myelosuppression as well as late side effects such as second cancers in the irradiated field (eg, sarcoma, bladder cancer).
Evaluation of Treatment Response
Monitoring of treatment response is fairly straightforward for patients with testicular cancer. Our practice is the following:
- Measure tumor markers on day 1 of each chemotherapy cycle and 3 to 4 weeks after completion of treatment.
- CT of the chest, abdomen, and pelvis with intravenous contrast prior to chemotherapy and upon completion of chemotherapy. Interim imaging is only needed for a small subset of patients with additional clinical indications (eg, new symptoms, lack of improvement in existing symptoms).
- For patients with stage II/III seminoma who have a residual mass ≥ 3 cm on post-treatment CT scan, a PET-CT scan is indicated 6 to 8 weeks after the completion of chemotherapy to determine the need for further treatment.
Active Surveillance
Because testicular cancer has high cure rates even when patients have disease relapse after primary therapy, and additional therapies have significant short- and long-term side effects in these generally young patients, active surveillance is a critical option used in the management of testicular cancer.41
Patients must be counseled that active surveillance is a form of treatment itself in that it involves close clinical and radiographic monitoring. Because there is a risk of disease relapse, patients opting to undergo active surveillance must fully understand the risks of disease recurrence and be willing to abide by the recommended follow-up schedule.
Surveillance is necessary for a minimum of 5 years and possibly 10 years following orchiectomy, and most relapses tend to occur within the first 2 years. Late relapses such as skeletal metastatic disease from seminoma have been reported to occur more than 15 years after orchiectomy, but are generally rare and unpredictable.
The general guidelines for active surveillance are as follows:
For patients with seminoma, history and physical exam and tumor marker assessment should be performed every 3 to 6 months for the first year, then every 6 to 12 months in years 2 and 3, and then annually. CT of the abdomen and pelvis should be done at 3, 6, and 12 months, every 6 to 12 months in years 2 and 3, and then every 12 to 24 months in years 4 and 5. A chest radiograph is performed only if clinically indicated, as the likelihood of distant metastatic recurrence is low.
For patients with nonseminoma, history and physical exam and tumor markers assessment should be performed every 2 to 3 months for first 2 years, every 4 to 6 months in years 3 and 4, and then annually. CT of the abdomen and pelvis should be obtained every 4 to 6 months in year 1, gradually decreasing to annually in year 3 or 4. Chest radiograph is indicated at 4 and 12 months and annually thereafter for stage IA disease. For those with stage IB disease, chest radiograph is indicated every 2 months during the first year and then gradually decreasing to annually beginning year 5.
These recommendations are expected to change over time, and treating physicians are recommended to exercise discretion and consider the patient and tumor characteristics to develop the optimal surveillance plan.
Conclusion
Testicular cancer is the most common cancer afflicting young men. Prompt diagnostic workup initiated in a primary care or hospital setting followed by a referral to a multidisciplinary team of urologists, medical oncologists, and radiation oncologists enables cure in a majority of patients. For patients with stage I seminoma, a radical inguinal orchiectomy followed by active surveillance may offer the best long-term outcome with minimal side effects. For patients with relapsed/refractory testicular cancers, clinical trial participation is strongly encouraged. Patients with a history of testicular cancer benefit from robust survivorship care tailored to their prior therapies. This can be safely delivered through their primary care providers in collaboration with the multidisciplinary oncology team.
Malignant testicular neoplasms can arise from either the germ cells or sex-cord stromal cells, with the former comprising approximately 95% of all testicular cancers (Table 1). Germ cell tumors may contain a single histology or a mix of multiple histologies. For clinical decision making, testicular tumors are categorized as either pure seminoma (no nonseminomatous elements present) or nonseminomatous germ cell tumors (NSGCT). The prevalence of seminoma and NSGCT is roughly equal. If a testicular tumor contains both seminomatous and nonseminomatous components, it is called a mixed germ cell tumor. Because of similarities in biological behavior, the approach to treatment of mixed germ cell tumors is similar to that for NSGCT.
The key points to remember for testicular cancer are:
- With early diagnosis and aggressive multidisciplinary therapy, the overwhelming majority of patients can be cured;
- Specialized care is often critical and affects outcomes; and
- Survivorship, or post-treatment care, is very important for these patients, as they often have lifespan of several decades and a unique set of short- and long-term treatment-related complications.
Developmental Biology and Genetics
The developmental biology of germ cells and germ cell neoplasms is beyond the scope of this review, and interested readers are recommended to refer to pertinent articles on the topic.1,2 A characteristic genetic marker of all germ cell tumors is an isochromosome of the short arm of chromosome 12, i(12p). This is present in testicular tumors regardless of histologic subtype as well as in carcinoma-in-situ. In germ cell tumors without i(12p) karyotype, excess 12p genetic material consisting of repetitive segments has been found, suggesting that this is an early and potentially critical change in oncogenesis.3 Several recent studies have revealed a diverse genomic landscape in testicular cancers, including KIT, KRAS and NRAS mutations in addition to a hyperdiploid karyotype.4,5
Evaluation and Diagnosis
Case Presentation
A 23-year-old Caucasian man presents to a primary care clinic for a pre-employment history and physical exam. He reports testicular pain on the sexually transmitted infections screening questionnaire. On examination, the physician finds a firm, mobile, minimally-tender, 1.5-cm mass in the inferior aspect of left testicle. No contralateral testicular mass or inguinal lymphadenopathy is noted, and a detailed physical exam is otherwise unremarkable. The physician immediately orders an ultrasound of the testicles, which shows a 1.5-cm hypoechoic mass in the inferior aspect of the left testicle, with an unremarkable contralateral testicle. After discussion of the results, the patient is referred a urologic oncologist with expertise in testicular cancer for further care.
The urologic oncologist orders a computed tomography (CT) abdomen and pelvis with and without contrast, which shows a 1.8-cm pathologic-appearing retroperitoneal lymph node at the level of the left renal vein. Chest radiograph with anteroposterior and lateral views is unremarkable. Tumor markers are as follows: beta human chorionic gonadotropin (beta-HCG) 8 mIU/mL (normal range, 0–4 mIU/mL), alpha-fetoprotein (AFP) 2 ng/mL (normal range, 0–8.5 ng/mL), and lactate dehydrogenase (LDH) 195 U/L (normal range, 119–213 U/L).
What is the approach to the initial workup and diagnosis of testicular cancer?
Clinical Presentation and Physical Exam
The majority of testicular cancers are diagnosed on work-up of a nodule or painless swelling of one testicle, usually noted incidentally by the patient. Approximately 30% to 40% of patients complain of a dull ache or heavy sensation in the lower abdomen, perianal area, or scrotum, while acute pain is the presenting symptom in 10%.3
In approximately 10% of patients, the presenting symptom is a result of distant metastatic involvement, such as cough and dyspnea on exertion (pulmonary or mediastinal metastasis), intractable bone pain (skeletal metastasis), intractable back/flank pain, presence of psoas sign or unexplained lower extremity deep vein thrombosis (bulky retroperitoneal metastasis), or central nervous system symptoms (vertebral, spinal or brain metastasis). Constitutional symptoms (unexplained weight loss, anorexia, fatigue) often accompany these symptoms.3
Rarely (5% or less), testicular cancer may present with systemic endocrine symptoms or paraneoplastic symptoms. Gynecomastia is the most common in this category, occurring in approximately 2% of germ cell tumors and more commonly (20%–30%) in Leydig cell tumors of testis.6 Classically, these patients are either 6- to 10-year-old boys with precocious puberty or young men (mid 20s-mid 30s) with a combination of testicular mass, gynecomastia, loss of libido, and impotence. Workup typically reveals increased beta-HCG levels in blood.
Anti-Ma2-antibody-associated limbic encephalitis is the most common (and still quite rare) paraneoplastic complication associated with testicular germ cell tumors. The Ma2 antigen is selectively expressed in the neuronal nucleoli of normal brain tissue and the testicular tumor of the patient. Importantly, in a subset of these patients, the treatment of testicular cancer may result in improvement of symptoms of encephalitis.7
The first step in the diagnosis of testicular neoplasm is a physical exam. This should include a bimanual examination of the scrotal contents, starting with the normal contralateral testis. Normal testicle has a homogeneous texture and consistency, is freely movable, and is separable from the epididymis. Any firm, hard, or fixed mass within the substance of the tunica albuginea should be considered suspicious until proven otherwise. Spread to the epididymis or spermatic cord occurs in 10% to 15% of patients and examination should include these structures as well.3 A comprehensive system-wise examination for features of metastatic spread as discussed above should then be performed. If the patient has cryptorchidism, ultrasound is a mandatory part of the diagnostic workup.
If clinical evaluation suggests a possibility of testicular cancer, the patient must be counseled to undergo an expedited diagnostic workup and specialist evaluation, as a prompt diagnosis and treatment is key to not only improving the likelihood of cure, but also minimizing the treatments needed to achieve it.
Role of Imaging
Scrotal Ultrasound
Scrotal ultrasound is the first imaging modality used in the diagnostic workup of patient with suspected testicular cancer. Bilateral scrotal ultrasound can detect lesions as small as 1 to 2 mm in diameter and help differentiate intratesticular lesions from extrinsic masses. A cystic mass on ultrasound is unlikely to be malignant. Seminomas appear as well-defined hypoechoic lesions without cystic areas, while NSGCTs are typically inhomogeneous with calcifications, cystic areas, and indistinct margins. However, this distinction is not always apparent or reliable. Ultrasound alone is also insufficient for tumor staging.8 For these reasons, a radical inguinal orchiectomy must be pursued for accurate determination of histology and local stage.
If testicular ultrasound shows a suspicious intratesticular mass, the following workup is typically done:
- Measurement of serum tumor markers (beta-HCG, AFP and LDH);
- CT abdomen and pelvis with and without contrast;
- Chest radiograph anteroposterior and lateral views, or CT chest with and without contrast if clinically indicated;
- Any additional focal imaging based on symptoms (eg, magnetic resonance imaging [MRI] scan with and without contrast to evaluate the brain if the patient has CNS symptoms).
CT Scan
CT scan is the preferred imaging modality for staging of testicular cancers, specifically for evaluation of the retroperitoneum, as it is the predominant site for metastases.9 CT scan should encompass the abdomen and pelvis, and contrast-enhanced sequences should be obtained unless medically contraindicated. CT scan of the chest (if not initially done) is compulsory should a CT of abdomen and pelvis and/or a chest radiograph show abnormal findings.
The sensitivity and specificity of CT scans for detection of nodal metastases can vary significantly based on the cutoff. For example, in a series of 70 patients using a cutoff of 10 mm, the sensitivity and specificity of CT scans for patients undergoing retroperitoneal lymph node dissection were 37% and 100%, respectively.10 In the same study, a cutoff of 4 mm increased the sensitivity to 93% and decreased the specificity to 58%. The current general consensus for this cutoff value is 8 to 10 mm measured in the short axis in the transverse (axial) plane.
Approximately 20% of men with clinical stage I testicular cancer (ie, those with non-enlarged retroperitoneal lymph nodes) who do not undergo any adjuvant therapy will have disease relapse in the retroperitoneum, suggesting that they had occult micrometastases that were missed on the initial CT scans.11,12
MRI/Radionuclide Bone Scan/PET Scan
Abdominal or pelvic MRI, whole-body radionuclide bone scan, and positron emission tomography (PET) scans are almost never needed as part of the initial staging workup for testicular cancers due to several limitations, including a high false-negative rate, specifically for the PET scans, and lack of any additional value compared with CT and testicular ultrasound alone.9,13,14 If necessary, these should only be ordered after a multidisciplinary oncology consultation to prevent unnecessary delays in treatment, inappropriate changes to treatment, and unnecessary increases in cost of care.
Tumor Markers, Biopsy, and Staging
What is the role of tumor markers in the management of testicular cancers?
Serum AFP, beta-hCG, and LDH have a well-established role as tumor markers in testicular cancer. The alpha subunit of hCG is shared between multiple pituitary hormones and hence does not serve as a specific marker for testicular cancer. Serum levels of AFP and/or beta-hCG are elevated in approximately 80% percent of men with NSGCTs, even in absence of metastatic spread. On the other hand, serum beta-hCG is elevated in less than 20% and AFP is not elevated in pure seminomas.3
Tumor markers by themselves are not sufficiently sensitive or specific for the diagnosis of testicular cancer, in general, or to differentiate among its subtypes. Despite this limitation, marked elevations in these markers are rarely due to causes other than germ cell tumor. For example, serum beta-hCG concentrations greater than 10,000 mIU/mL occur only in germ cell tumors, trophoblastic differentiation of a primary lung or gastric cancer, gestational trophoblastic disease, or pregnancy. Serum AFP concentrations greater than 10,000 ng/mL occur almost exclusively in germ cell tumors and hepatocellular carcinoma.15
The pattern of marker elevation may play an important role in management of testicular cancer patients. For example, in our practice, several patients have had discordant serum tumor markers and pathology results (eg, elevated AFP with pure seminoma on orchiectomy). One of these patients was treated with adjuvant retroperitoneal lymph node dissection, which confirmed that he had a NSGCT with a seminoma, choriocarcinoma, and teratoma on pathology evaluation of retroperitoneal lymph nodes.
Serum tumor markers have 2 additional critical roles—(1) in the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) staging16 and International Germ Cell Cancer Collaboration Group (IGCCCG) risk stratification of testicular cancer,17 and (2) in post-treatment disease monitoring.
Is a testicular biopsy necessary for diagnosis?
A testicular biopsy is almost never pursued to confirm the diagnosis of testicular cancer. There is a concern that percutaneous testicular biopsy, which is associated with scrotal skin violation, can adversely affect outcomes due to tumor seeding of scrotal sac or metastatic spread into the inguinal nodes via scrotal skin lymphatics.
Tissue diagnosis is made by radical orchiectomy in a majority of cases. Rarely in our practice, we obtain a biopsy of metastatic lesion for a tissue diagnosis. This is only done in cases where chemotherapy must be started urgently to prevent worsening of complications from metastatic spread. This decision should be made only after a multidisciplinary consultation with urologic and medical oncology teams.
How is testicular cancer staged?
Both seminomatous and nonseminomatous germ cell tumors of the testis are staged using the AJCC/UICC staging system, which incorporates assessments of the primary tumor (T), lymph nodes (N), and distant metastases (M) and serum tumor marker values (S). Details of this staging system are beyond the scope of this review and further information can be obtained through the AJCC website (www.cancerstaging.org). This TNMS staging enables a prognostic assessment and helps with the therapeutic approach.
For patients with advanced germ cell tumors, a risk group classification developed by the IGCCCG is used to classify patients into good-risk, intermediate-risk, and poor-risk category (Table 2). This classification has been extensively validated for the past 2 decades, provides important prognostic information, and helps inform therapy decisions.
Treatment
Case 1 Continued
Based on the patient’s imaging and biomarker results, the patient undergoes a left radical inguinal orchiectomy. The physician’s operative note mentions that the left testicle was delivered without violation of scrotal integrity. A pathology report shows pure spermatocytic seminoma (unifocal, 1.4 cm size) with negative margins and no evidence of lymphovascular invasion. No lymph nodes are identified in the resection specimen. Post-orchiectomy markers are “negative,” meaning within normal range. After discussions with medical and radiation oncology physicians, the patient opts to pursue active surveillance.
Surgery alone followed by active surveillance is an appropriate option for this patient, as the likelihood of recurrence is low and most recurrences can be subsequently salvaged using treatment options detailed below.
What are the therapeutic options for testicular cancer?
An overview of management for most testicular cancers is presented in Table 3. Note that the actual treatments are significantly more complex and need a comprehensive multidisciplinary consultation (urologic, medical and radiation oncology) at centers with specialized testicular cancer teams, if possible.
Fertility Preservation
All patients initiating treatment for testicular cancer must be offered options for fertility preservation and consultation with a reproductive health team, if available. At the time of diagnosis, approximately 50% patients have some degree of impairment in spermatogenesis, but with effective fertility preservation, successful pregnancy can occur for as many as 30% to 60% of patients.18,19
Orchiectomy
Radical inguinal orchiectomy with high ligation of the spermatic cord at the level of the internal ring is the procedure of choice for suspected testicular cancer. The goal is to provide a definitive tissue diagnosis and local tumor control with minimal morbidity. It can be performed under general, regional, or local anesthesia. Depending on the complexity and surgical expertise, it can be done in an inpatient or outpatient setting. During the procedure, the testicle is delivered from the scrotum through an incision in the inguinal region and then resected. A testicular prosthesis is usually inserted, with resultant excellent cosmetic and patient satisfaction outcomes.20
Testicular sparing surgery (TSS) has been explored as an alternative to radical orchiectomy but is not considered a standard-of-care option at this time. Small studies have shown evidence for comparable short-term oncologic outcomes in a very select group of patients, generally with solitary tumors < 2 cm in size and solitary testicle. If this is being considered as an option, we recommended obtaining a consultation from a urologist at a high-volume center. For a majority of patients, the value of a TSS is diminished due to excellent anatomic/cosmetic outcomes with a testicular prosthesis implanted during the radical orchiectomy, and resumption of sexual functions by the unaffected contralateral testicle.
Retroperitoneal Lymph Node Dissection
As discussed, conventional cross-sectional imaging has a high false-negative rate for detection of retroperitoneal involvement. General indications for RPLND in various stages and histologies of testicular cancer germ cell tumors are outlined in Table 3. Seminoma tends to most commonly metastasize to retroperitoneum, but RPLND for seminoma is generally reserved for a very small subset of these patients. Patterns of metastases of NSGCT (except choriocarcinoma) are considered to be well-defined. In a series of patients with stage II NSGCTs, left-sided tumors metastasized to the pre- and para-aortic nodes in 88% and 86% of cases, respectively (drainage basin of left testicular vein); and right-sided tumors involved the interaortocaval nodes in 93% of patients.3 Inguinal and pelvic nodal metastases may rarely be seen and should not be used to rule out the diagnosis of testicular cancer.
Choriocarcinoma is an exception to this pattern of retroperitoneal spread, as it tends to have a higher likelihood of hematogenous metastases to distant organs. Compared with NSGCTs, pure seminomas are either localized to the testis (80% of all cases) or limited to the retroperitoneum (an additional 15% of all cases) at presentation.3
Depending on the case and expertise of the surgical team, robotic or open RPLND can be performed.21 Regardless of the approach used, RPLND remains a technically challenging surgery. The retroperitoneal “landing zone” lymph nodes lie in close proximity to, and are often densely adherent to, the abdominal great vessels. Complication rates vary widely in the reported literature, but can be as high as 50%.21-23 As detailed in Table 2, the number and size of involved retroperitoneal lymph nodes have prognostic importance.
In summary, RPLND is considered to be a viable option for a subset of early-stage NSGCT (T1-3, N0-2, M0) and for those with advanced seminoma, NSGCT, or mixed germ cell tumors with post-chemotherapy residual disease.
Systemic Chemotherapy
Except for the single-agent carboplatin, most chemotherapy regimens used to treat testicular cancer are combinations of 2 or more chemotherapy agents. For this review, we will focus on the 3 most commonly used regimens: bleomycin, etoposide, and cisplatin (BEP), etoposide and cisplatin (EP), and etoposide, ifosfamide, and cisplatin (VIP).
The core principles of testicular cancer chemotherapy are:
- Minimize dose interruptions, delays, or reductions, as these adversely affect outcomes without clearly improving side effect profile;
- Do not substitute carboplatin for cisplatin in combination regimens because carboplatin-containing combination regimens have been shown to result in significantly poorer outcomes in multiple trials of adults with germ cell tumors;24-27 and
- Give myeloid growth factor support, if necessary.
BEP
The standard BEP regimen comprises a 21-day cycle with bleomycin 30 units on days 1, 8, and 15; etoposide 100 mg/m2 on days 1 to 5; and cisplatin 20 mg/m2 on days 1 to 5. Number of cycles varies based on histology and stage (Table 3). A strong justification to maintain treatment intensity comes from the Australian and New Zealand Germ Cell Trial Group trial. In this study, 166 men were randomly assigned to treatment using 3 cycles of standard BEP or 4 cycles of a modified BEP regimen (bleomycin 30 units day 1; etoposide 120 mg/m2 days 1 to 3; cisplatin 100 mg/m2 day 1) every 21 days. This trial was stopped at interim analyses because the modified BEP arm was inferior to the standard BEP arm. With a median follow-up of 8.5 years, 8-year overall survival was 92% with standard BEP and 83% with modified BEP (P = 0.037).28
Bleomycin used in the BEP regimen has been associated with uncommon but potentially fatal pulmonary toxicity that tends to present as interstitial pneumonitis, which may ultimately progress to fibrosis or bronchiolitis obliterans with organizing pneumonia.29 This has led to evaluation of EP as an alternative to BEP.
EP
The standard EP regimen consists of a 21-day cycle with etoposide 100 mg/m2 on days 1 to 5, and cisplatin 20 mg/m2 on days 1 to 5. Due to conflicting data from multiple randomized trials, there is considerable debate in the field regarding whether 4 cycles of EP are equivalent to 3 cycles of BEP.30,31 The benefit of the EP regimen is that it avoids the higher rates of pulmonary, cutaneous, and neurologic toxicities associated bleomycin, but it does result in the patient receiving an up to 33% higher cumulative dose of cisplatin and etoposide due to the extra cycle of treatment. This has important implications in terms of tolerability and side effects, including delayed toxicities such as second malignancies, which increase with a higher cumulative dose of these agents (etoposide in particular).
VIP
The standard VIP regimen consists of a 21-day cycle with etoposide 75 mg/m2 on days 1 to 5; cisplatin 20 mg/m2 on days 1 to 5; ifosfamide 1200 mg/m2 on days 1 to 5; and mesna 120 mg/m2 IV push on day 1 followed by 1200 mg/m2 on days 1 to 5. For patients with intermediate- or poor-risk disease, 4 cycles of VIP has demonstrated comparable efficacy but higher rates of hematologic toxicities compared with 4 cycles of BEP.32-34 It remains an option for upfront treatment of patients who are not good candidates for a bleomycin-based regimen, and for patients who need salvage chemotherapy.
Adverse Effects of Chemotherapy
Acute and late chemotherapy toxicities vary significantly between regimens depending on the chemotherapy drugs used. Bleomycin-induced pneumonitis may masquerade as a “pneumonia,” which can lead to a delay in diagnosis or institution of treatment, as well as institution of an incorrect treatment (for example, there is a concern that bleomycin toxicity can be precipitated or worsened by a high fraction of inspired oxygen). Chemotherapy-associated neutropenia tends to occur a few days (7–10 days) after initiation of chemotherapy, and neutrophil counts recover without intervention in most patients after an additional 7 to 10 days. Myeloid growth factor support (eg, filgrastim, pegfilgrastim) can be given to patients either prophylactically (if they had an episode of febrile or prolonged neutropenia with the preceding cycle) or secondarily if they present with neutropenia (an absolute neutrophil count ≤ 500 cells/µL) with fever or active infection. Such interventions tend to shorten the duration of neutropenia but does not affect overall survival. Patients with asymptomatic neutropenia do not benefit from growth factor use.35
Stem Cell Transplant
Autologous stem cell transplant (SCT) is the preferred type of SCT for patients with testicular cancer and involves delivery of high doses of chemotherapy followed by infusion of patient-derived myeloid stem cells. While the details of this treatment are outside the scope of this review, decades of experience has shown that this is an effective curative option for a subset of patients with poor prognosis, such as those with platinum-refractory or relapsed disease.36
Clinical Trials
Due to excellent clinical outcomes with front-line therapy, as described, and the relatively low incidence of testicular and other germ cell tumors, clinical trial options for patients with testicular cancer are limited. The TIGER trial is an ongoing international, randomized, phase 3 trial comparing conventional TIP (paclitaxel, ifosfamide, and cisplatin) chemotherapy with high-dose chemotherapy with SCT as the first salvage treatment for relapsed/refractory germ cell tumors (NCT02375204). It is enrolling at multiple centers in the United States and results are expected in 2022. At least 2 ongoing trials are evaluating the role of immunotherapy in patients with relapsed/refractory germ cell tumors (NCT03081923 and NCT03726281). Cluster of differentiation antigen-30 (CD30) has emerged as a potential target of interest in germ cell tumors, and brentuximab vedotin, an anti-CD30 monoclonal antibody, is undergoing evaluation in a phase 2 trial of CD-30–expressing germ cell tumors (NCT01851200). This trial has completed enrollment and results are expected to be available in late 2019 or early 2020.
When possible, patients with relapsed/refractory germ cell tumors should be referred to centers of excellence with access to either testicular/germ-cell tumor specific clinical trials or phase 1 clinical trials.
Radiation Therapy
Adjuvant radiation to the retroperitoneum has a role in the management of stage I and IIA seminomas (Table 3). In a randomized noninferiority trial of radiation therapy versus single-dose carboplatin in stage I seminoma patients, 5-year recurrence-free survival was comparable at approximately 95% in either arm.37,38 In a retrospective database review of 2437 patients receiving either radiation therapy or multi-agent chemotherapy for stage II seminoma, the 5-year survival exceeded 90% in both treatment groups.39 Typically, a total of 30 to 36 Gy of radiation is delivered to para-aortic and ipsilateral external iliac lymph nodes (“dog-leg” field), followed by an optional boost to the involved nodal areas.40 Radiation is associated with acute side effects such as fatigue, gastrointestinal effects, myelosuppression as well as late side effects such as second cancers in the irradiated field (eg, sarcoma, bladder cancer).
Evaluation of Treatment Response
Monitoring of treatment response is fairly straightforward for patients with testicular cancer. Our practice is the following:
- Measure tumor markers on day 1 of each chemotherapy cycle and 3 to 4 weeks after completion of treatment.
- CT of the chest, abdomen, and pelvis with intravenous contrast prior to chemotherapy and upon completion of chemotherapy. Interim imaging is only needed for a small subset of patients with additional clinical indications (eg, new symptoms, lack of improvement in existing symptoms).
- For patients with stage II/III seminoma who have a residual mass ≥ 3 cm on post-treatment CT scan, a PET-CT scan is indicated 6 to 8 weeks after the completion of chemotherapy to determine the need for further treatment.
Active Surveillance
Because testicular cancer has high cure rates even when patients have disease relapse after primary therapy, and additional therapies have significant short- and long-term side effects in these generally young patients, active surveillance is a critical option used in the management of testicular cancer.41
Patients must be counseled that active surveillance is a form of treatment itself in that it involves close clinical and radiographic monitoring. Because there is a risk of disease relapse, patients opting to undergo active surveillance must fully understand the risks of disease recurrence and be willing to abide by the recommended follow-up schedule.
Surveillance is necessary for a minimum of 5 years and possibly 10 years following orchiectomy, and most relapses tend to occur within the first 2 years. Late relapses such as skeletal metastatic disease from seminoma have been reported to occur more than 15 years after orchiectomy, but are generally rare and unpredictable.
The general guidelines for active surveillance are as follows:
For patients with seminoma, history and physical exam and tumor marker assessment should be performed every 3 to 6 months for the first year, then every 6 to 12 months in years 2 and 3, and then annually. CT of the abdomen and pelvis should be done at 3, 6, and 12 months, every 6 to 12 months in years 2 and 3, and then every 12 to 24 months in years 4 and 5. A chest radiograph is performed only if clinically indicated, as the likelihood of distant metastatic recurrence is low.
For patients with nonseminoma, history and physical exam and tumor markers assessment should be performed every 2 to 3 months for first 2 years, every 4 to 6 months in years 3 and 4, and then annually. CT of the abdomen and pelvis should be obtained every 4 to 6 months in year 1, gradually decreasing to annually in year 3 or 4. Chest radiograph is indicated at 4 and 12 months and annually thereafter for stage IA disease. For those with stage IB disease, chest radiograph is indicated every 2 months during the first year and then gradually decreasing to annually beginning year 5.
These recommendations are expected to change over time, and treating physicians are recommended to exercise discretion and consider the patient and tumor characteristics to develop the optimal surveillance plan.
Conclusion
Testicular cancer is the most common cancer afflicting young men. Prompt diagnostic workup initiated in a primary care or hospital setting followed by a referral to a multidisciplinary team of urologists, medical oncologists, and radiation oncologists enables cure in a majority of patients. For patients with stage I seminoma, a radical inguinal orchiectomy followed by active surveillance may offer the best long-term outcome with minimal side effects. For patients with relapsed/refractory testicular cancers, clinical trial participation is strongly encouraged. Patients with a history of testicular cancer benefit from robust survivorship care tailored to their prior therapies. This can be safely delivered through their primary care providers in collaboration with the multidisciplinary oncology team.
1. van der Zwan YG, Biermann K, Wolffenbuttel KP, et al. Gonadal maldevelopment as risk factor for germ cell cancer: towards a clinical decision model. Eur Urol. 2015; 67:692–701.
2. Pierce JL, Frazier AL, Amatruda JF. Pediatric germ cell tumors: a developmental perspective. Adv Urol. 2018 Feb 4;2018.
3. Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med. 1997;337:242-253.
4. Pyle LC, Nathanson KL. Genetic changes associated with testicular cancer susceptibility. Semin Oncol. 2016;43:575-581.
5. Shen H, Shih J, Hollern DP, et al. Integrated molecular characterization of testicular germ cell tumors. Cell Rep. 2018;23:3392-3406.
6. Barry M, Rao A, Lauer R. Sex cord-stromal tumors of the testis. In: Pagliaro L, ed. Rare Genitourinary Tumors. Cham: Springer International Publishing; 2016: 231-251.
7. Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain J Neurol. 2004;127:1831-1844.
8. Coursey Moreno C, Small WC, Camacho JC, et al. Testicular tumors: what radiologists need to know—differential diagnosis, staging, and management. RadioGraphics. 2015;35:400-415.
9. Kreydin EI, Barrisford GW, Feldman AS, Preston MA. Testicular cancer: what the radiologist needs to know. Am J Roentgenol. 2013;200:1215-1225.
10. Hilton S, Herr HW, Teitcher JB, et al. CT detection of retroperitoneal lymph node metastases in patients with clinical stage I testicular nonseminomatous germ cell cancer: assessment of size and distribution criteria. Am J Roentgenol. 1997;169:521-525.
11. Thompson PI, Nixon J, Harvey VJ. Disease relapse in patients with stage I nonseminomatous germ cell tumor of the testis on active surveillance. J Clin Oncol. 1988;6:1597-1603.
12. Nicolai N, Pizzocaro G. A surveillance study of clinical stage I nonseminomatous germ cell tumors of the testis: 10-year followup. J Urol. 1995;154:1045-1049.
13. Kok HK, Leong S, Torreggiani WC. Is magnetic resonance imaging comparable with computed tomography in the diagnosis of retroperitoneal metastasis in patients with testicular cancer? Can Assoc Radiol J. 2014;65:196-198.
14. Hale GR, Teplitsky S, Truong H, et al. Lymph node imaging in testicular cancer. Transl Androl Urol. 2018;7:864-874.
15. Honecker F, Aparicio J, Berney D, et al. ESMO Consensus Conference on testicular germ cell cancer: diagnosis, treatment and follow-up. Ann Oncol. 2018;29:1658-1686.
16. Paner GP, Stadler WM, Hansel DE, et al. Updates in the Eighth Edition of the Tumor-Node-Metastasis Staging Classification for Urologic Cancers. Eur Urol. 2018;73:560-569.
17. International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997;15:594-603.
18. Lopategui DM, Ibrahim E, Aballa TC, et al. Effect of a formal oncofertility program on fertility preservation rates-first year experience. Transl Androl Urol. 2018;7:S271-S275.
19. Moody JA, Ahmed K, Horsfield C, et al. Fertility preservation in testicular cancer - predictors of spermatogenesis. BJU Int. 2018;122:236-242.
20. Dieckmann KP, Anheuser P, Schmidt S, et al. Testicular prostheses in patients with testicular cancer - acceptance rate and patient satisfaction. BMC Urol. 2015;15:16.
21. Schwen ZR, Gupta M, Pierorazio PM. A review of outcomes and technique for the robotic-assisted laparoscopic retroperitoneal lymph node dissection for testicular cancer. Adv Urol. 2018;2146080.
22. Singh P, Yadav S, Mahapatra S, Seth A. Outcomes following retroperitoneal lymph node dissection in postchemotherapy residual masses in advanced testicular germ cell tumors. Indian J Urol. 2016;32:40-44.
23. Heidenreich A, Thüer D, Polyakov S. Postchemotherapy retroperitoneal lymph node dissection in advanced germ cell tumours of the testis. Eur Urol. 2008;53:260-272.
24. Bajorin DF, Sarosdy MF, Pfister DG, et al. Randomized trial of etoposide and cisplatin versus etoposide and carboplatin in patients with good-risk germ cell tumors: a multiinstitutional study. J Clin Oncol. 1993;11:598-606.
25. Bokemeyer C, Köhrmann O, Tischler J, et al. A randomized trial of cisplatin, etoposide and bleomycin (PEB) versus carboplatin, etoposide and bleomycin (CEB) for patients with “good-risk” metastatic non-seminomatous germ cell tumors. Ann Oncol. 1996;7:1015-1021.
26. Horwich A, Sleijfer DT, Fosså SD, et al. Randomized trial of bleomycin, etoposide, and cisplatin compared with bleomycin, etoposide, and carboplatin in good-prognosis metastatic nonseminomatous germ cell cancer: a Multiinstitutional Medical Research Council/European Organization for Research and Treatment of Cancer Trial. J Clin Oncol. 1997;15:1844-1852.
27. Shaikh F, Nathan PC, Hale J, et al. Is there a role for carboplatin in the treatment of malignant germ cell tumors? A systematic review of adult and pediatric trials. Pediatr Blood Cancer. 2013;60:587-592.
28. Grimison PS, Stockler MR, Thomson DB, et al. Comparison of two standard chemotherapy regimens for good-prognosis germ cell tumors: updated analysis of a randomized trial. J Natl Cancer Inst. 2010;102:1253-1262.
29. Reinert T, da Rocha Baldotto CS, Nunes FAP, de Souza Scheliga AA. Bleomycin-induced lung injury. J Cancer Res. 2013;480608.
30. Jones RH, Vasey PA. Part II: Testicular cancer—management of advanced disease. Lancet Oncol. 2003;4:738-747.
31. Jankilevich G. BEP versus EP for treatment of metastatic germ-cell tumours. Lancet Oncol. 2004;5, 146.
32. Nichols CR, Catalano PJ, Crawford ED, et al. Randomized comparison of cisplatin and etoposide and either bleomycin or ifosfamide in treatment of advanced disseminated germ cell tumors: an Eastern Cooperative Oncology Group, Southwest Oncology Group, and Cancer and Leukemia Group B Study. J Clin Oncol. 1998;16:12871293.
33. Hinton S, Catalano PJ, Einhorn LH, et al. Cisplatin, etoposide and either bleomycin or ifosfamide in the treatment of disseminated germ cell tumors: final analysis of an intergroup trial. Cancer. 2003;97: 1869-1875.
34. de Wit R, Stoter G, Sleijfer DT, et al. Four cycles of BEP vs four cycles of VIP in patients with intermediate-prognosis metastatic testicular non-seminoma: a randomized study of the EORTC Genitourinary Tract Cancer Cooperative Group. European Organization for Research and Treatment of Cancer. Br J Cancer. 1998;78:828-832.
35. Mhaskar R, Clark OA, Lyman G, et al. Colony-stimulating factors for chemotherapy-induced febrile neutropenia. Cochrane Database Syst. Rev. 2014;CD003039.
36. Adra N, Abonour R, Althouse SK, et al. High-dose chemotherapy and autologous peripheral-blood stem-cell transplantation for relapsed metastatic germ cell tumors: The Indiana University experience. J Clin Oncol. 2017;35:1096-1102.
37. Oliver RT, Mason MD, Mead GM, et al. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet. 2005;366:293-300.
38. Oliver RT, Mead GM, Rustin GJ, et al. Randomized trial of carboplatin versus radiotherapy for stage I seminoma: mature results on relapse and contralateral testis cancer rates in MRC TE19/EORTC 30982 study (ISRCTN27163214). J Clin Oncol. 2011;29:957-962.
39. Glaser SM, Vargo JA, Balasubramani GK, Beriwal S. Stage II testicular seminoma: patterns of care and survival by treatment strategy. Clin Oncol. 2016;28:513-521.
40. Boujelbene N, Cosinschi A, Boujelbene N, et al. Pure seminoma: A review and update. Radiat Oncol. 2011;6:90.
41. Nichols CR, Roth B, Albers P, et al. Active surveillance is the preferred approach to clinical stage I testicular cancer. J Clin Oncol. 2013;31;3490-3493.
1. van der Zwan YG, Biermann K, Wolffenbuttel KP, et al. Gonadal maldevelopment as risk factor for germ cell cancer: towards a clinical decision model. Eur Urol. 2015; 67:692–701.
2. Pierce JL, Frazier AL, Amatruda JF. Pediatric germ cell tumors: a developmental perspective. Adv Urol. 2018 Feb 4;2018.
3. Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med. 1997;337:242-253.
4. Pyle LC, Nathanson KL. Genetic changes associated with testicular cancer susceptibility. Semin Oncol. 2016;43:575-581.
5. Shen H, Shih J, Hollern DP, et al. Integrated molecular characterization of testicular germ cell tumors. Cell Rep. 2018;23:3392-3406.
6. Barry M, Rao A, Lauer R. Sex cord-stromal tumors of the testis. In: Pagliaro L, ed. Rare Genitourinary Tumors. Cham: Springer International Publishing; 2016: 231-251.
7. Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain J Neurol. 2004;127:1831-1844.
8. Coursey Moreno C, Small WC, Camacho JC, et al. Testicular tumors: what radiologists need to know—differential diagnosis, staging, and management. RadioGraphics. 2015;35:400-415.
9. Kreydin EI, Barrisford GW, Feldman AS, Preston MA. Testicular cancer: what the radiologist needs to know. Am J Roentgenol. 2013;200:1215-1225.
10. Hilton S, Herr HW, Teitcher JB, et al. CT detection of retroperitoneal lymph node metastases in patients with clinical stage I testicular nonseminomatous germ cell cancer: assessment of size and distribution criteria. Am J Roentgenol. 1997;169:521-525.
11. Thompson PI, Nixon J, Harvey VJ. Disease relapse in patients with stage I nonseminomatous germ cell tumor of the testis on active surveillance. J Clin Oncol. 1988;6:1597-1603.
12. Nicolai N, Pizzocaro G. A surveillance study of clinical stage I nonseminomatous germ cell tumors of the testis: 10-year followup. J Urol. 1995;154:1045-1049.
13. Kok HK, Leong S, Torreggiani WC. Is magnetic resonance imaging comparable with computed tomography in the diagnosis of retroperitoneal metastasis in patients with testicular cancer? Can Assoc Radiol J. 2014;65:196-198.
14. Hale GR, Teplitsky S, Truong H, et al. Lymph node imaging in testicular cancer. Transl Androl Urol. 2018;7:864-874.
15. Honecker F, Aparicio J, Berney D, et al. ESMO Consensus Conference on testicular germ cell cancer: diagnosis, treatment and follow-up. Ann Oncol. 2018;29:1658-1686.
16. Paner GP, Stadler WM, Hansel DE, et al. Updates in the Eighth Edition of the Tumor-Node-Metastasis Staging Classification for Urologic Cancers. Eur Urol. 2018;73:560-569.
17. International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997;15:594-603.
18. Lopategui DM, Ibrahim E, Aballa TC, et al. Effect of a formal oncofertility program on fertility preservation rates-first year experience. Transl Androl Urol. 2018;7:S271-S275.
19. Moody JA, Ahmed K, Horsfield C, et al. Fertility preservation in testicular cancer - predictors of spermatogenesis. BJU Int. 2018;122:236-242.
20. Dieckmann KP, Anheuser P, Schmidt S, et al. Testicular prostheses in patients with testicular cancer - acceptance rate and patient satisfaction. BMC Urol. 2015;15:16.
21. Schwen ZR, Gupta M, Pierorazio PM. A review of outcomes and technique for the robotic-assisted laparoscopic retroperitoneal lymph node dissection for testicular cancer. Adv Urol. 2018;2146080.
22. Singh P, Yadav S, Mahapatra S, Seth A. Outcomes following retroperitoneal lymph node dissection in postchemotherapy residual masses in advanced testicular germ cell tumors. Indian J Urol. 2016;32:40-44.
23. Heidenreich A, Thüer D, Polyakov S. Postchemotherapy retroperitoneal lymph node dissection in advanced germ cell tumours of the testis. Eur Urol. 2008;53:260-272.
24. Bajorin DF, Sarosdy MF, Pfister DG, et al. Randomized trial of etoposide and cisplatin versus etoposide and carboplatin in patients with good-risk germ cell tumors: a multiinstitutional study. J Clin Oncol. 1993;11:598-606.
25. Bokemeyer C, Köhrmann O, Tischler J, et al. A randomized trial of cisplatin, etoposide and bleomycin (PEB) versus carboplatin, etoposide and bleomycin (CEB) for patients with “good-risk” metastatic non-seminomatous germ cell tumors. Ann Oncol. 1996;7:1015-1021.
26. Horwich A, Sleijfer DT, Fosså SD, et al. Randomized trial of bleomycin, etoposide, and cisplatin compared with bleomycin, etoposide, and carboplatin in good-prognosis metastatic nonseminomatous germ cell cancer: a Multiinstitutional Medical Research Council/European Organization for Research and Treatment of Cancer Trial. J Clin Oncol. 1997;15:1844-1852.
27. Shaikh F, Nathan PC, Hale J, et al. Is there a role for carboplatin in the treatment of malignant germ cell tumors? A systematic review of adult and pediatric trials. Pediatr Blood Cancer. 2013;60:587-592.
28. Grimison PS, Stockler MR, Thomson DB, et al. Comparison of two standard chemotherapy regimens for good-prognosis germ cell tumors: updated analysis of a randomized trial. J Natl Cancer Inst. 2010;102:1253-1262.
29. Reinert T, da Rocha Baldotto CS, Nunes FAP, de Souza Scheliga AA. Bleomycin-induced lung injury. J Cancer Res. 2013;480608.
30. Jones RH, Vasey PA. Part II: Testicular cancer—management of advanced disease. Lancet Oncol. 2003;4:738-747.
31. Jankilevich G. BEP versus EP for treatment of metastatic germ-cell tumours. Lancet Oncol. 2004;5, 146.
32. Nichols CR, Catalano PJ, Crawford ED, et al. Randomized comparison of cisplatin and etoposide and either bleomycin or ifosfamide in treatment of advanced disseminated germ cell tumors: an Eastern Cooperative Oncology Group, Southwest Oncology Group, and Cancer and Leukemia Group B Study. J Clin Oncol. 1998;16:12871293.
33. Hinton S, Catalano PJ, Einhorn LH, et al. Cisplatin, etoposide and either bleomycin or ifosfamide in the treatment of disseminated germ cell tumors: final analysis of an intergroup trial. Cancer. 2003;97: 1869-1875.
34. de Wit R, Stoter G, Sleijfer DT, et al. Four cycles of BEP vs four cycles of VIP in patients with intermediate-prognosis metastatic testicular non-seminoma: a randomized study of the EORTC Genitourinary Tract Cancer Cooperative Group. European Organization for Research and Treatment of Cancer. Br J Cancer. 1998;78:828-832.
35. Mhaskar R, Clark OA, Lyman G, et al. Colony-stimulating factors for chemotherapy-induced febrile neutropenia. Cochrane Database Syst. Rev. 2014;CD003039.
36. Adra N, Abonour R, Althouse SK, et al. High-dose chemotherapy and autologous peripheral-blood stem-cell transplantation for relapsed metastatic germ cell tumors: The Indiana University experience. J Clin Oncol. 2017;35:1096-1102.
37. Oliver RT, Mason MD, Mead GM, et al. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet. 2005;366:293-300.
38. Oliver RT, Mead GM, Rustin GJ, et al. Randomized trial of carboplatin versus radiotherapy for stage I seminoma: mature results on relapse and contralateral testis cancer rates in MRC TE19/EORTC 30982 study (ISRCTN27163214). J Clin Oncol. 2011;29:957-962.
39. Glaser SM, Vargo JA, Balasubramani GK, Beriwal S. Stage II testicular seminoma: patterns of care and survival by treatment strategy. Clin Oncol. 2016;28:513-521.
40. Boujelbene N, Cosinschi A, Boujelbene N, et al. Pure seminoma: A review and update. Radiat Oncol. 2011;6:90.
41. Nichols CR, Roth B, Albers P, et al. Active surveillance is the preferred approach to clinical stage I testicular cancer. J Clin Oncol. 2013;31;3490-3493.