User login
Opioid use disorder in adolescents: An overview
Ms. L, age 17, seeks treatment because she has an ongoing struggle with multiple substances, including benzodiazepines, heroin, alcohol, cannabis, and prescription opioids.
She reports that she was 13 when she first used a prescription opioid that was not prescribed for her. She also reports engaging in unsafe sexual practices while using these substances, and has been diagnosed and treated for a sexually transmitted disease. She dropped out of school and is estranged from her family. She says that for a long time she has felt depressed and that she uses drugs to “self-medicate my emotions.” She endorses high anxiety and lack of motivation. Ms. L also reports having several criminal charges for theft, assault, and exchanging sex for drugs. She has undergone 3 admissions for detoxification, but promptly resumed using drugs, primarily heroin and oxycodone, immediately after discharge. Ms. L meets DSM-5 criteria for opioid use disorder (OUD).
Ms. L’s case illustrates a disturbing trend in the current opioid epidemic in the United States. Nearly 11.8 million individuals age ≥12 reported misuse of opioids in the last year.1 Adolescents who misuse prescription or illicit opioids are more likely to be involved with the legal system due to truancy, running away from home, physical altercations, prostitution, exchanging sex for drugs, robbery, and gang involvement. Adolescents who use opioids may also struggle with academic decline, drop out of school early, be unable to maintain a job, and have relationship difficulties, especially with family members.
In this article, I describe the scope of OUD among adolescents, including epidemiology, clinical manifestations, screening tools, and treatment approaches.
Scope of the problem
According to the most recent Monitoring the Future survey of more than 42,500 8th, 10th, and 12th grade students, 2.7% of 12th graders reported prescription opioid misuse (reported in the survey as “narcotics other than heroin”) in the past year.2 In addition, 0.4% of 12th graders reported heroin use over the same period.2 Although the prevalence of opioid use among adolescents has been declining over the past 5 years,2 it still represents a serious health crisis.
Part of the issue may relate to easier access to more potent opioids. For example, heroin available today can be >4 times purer than it was in the past. In 2002, t
Between 1997 and 2012, the annual incidence of youth (age 15 to 19) hospitalizations for prescription opioid poisoning increased >170%.5 Approximately 6% to 9% of youth involved in risky opioid use develop OUD 6 to 12 months after s
Continue to: In recent years...
In recent years, deaths from drug overdose have increased for all age groups; however, limited data is available regarding adolescent overdose deaths. According to the Centers for Disease Control and Prevention (CDC), from 2015 to 2016, drug overdose death rates for persons age 15 to 24 increased to 28%.9
How opioids work
Opioids activate specific transmembrane neurotransmitter receptors, including mu, kappa, and delta, in the CNS and peripheral nervous system (PNS). This leads to activation of G protein–mediated intracellular signal transduction. Mainly it is activation of endogenous mu opioid receptors that mediates the reward, withdrawal, and analgesic effects of opioids. These effects depend on the location of mu receptors. In the CNS, activation of mu opioid receptors may cause miosis, respiratory depression, euphoria, and analgesia.10
Different opioids vary in terms of their half-life; for most opioids, the half-life ranges from 2 to 4 hours.10 Heroin has a half-life of 30 minutes, but due to active metabolites its duration of action is 4 to 5 hours. Opioid metabolites can be detected in urine toxicology within approximately 1 to 2 days since last use.10
Chronic opioid use is associated with neurologic effects that change the function of areas of the brain that control pleasure/reward, stress, decision-making, and more. This leads to cravings, continued substance use, and dependence.11 After continued long-term use, patients report decreased euphoria, but typically they continue to use opioids to avoid withdrawal symptoms or worsening mood.
Criteria for opioid use disorder
In DSM-5, substance use disorders (SUDs)are no longer categorized as abuse or dependence.12 For opioids, the diagnosis is OUD. The Table12 outlines the DSM-5 criteria for OUD. Craving opioids is included for the first time in the OUD diagnosis. Having problems with the legal system is no longer considered a diagnostic criterion for OUD.
Continue to: A vulnerable population
A vulnerable population
As defined by Erik Erikson’s psychosocial stages of development, adolescents struggle between establishing their own identity vs role confusion.13 In an attempt to relate to peers or give in to peer pressure, some adolescents start by experimenting with nicotine, alcohol, and/or marijuana; however, some may move on to using other illicit drugs.14 Risk factors for the development of SUDs include early onset of substance use and a rapid progression through stages of substance use from experimentation to regular use, risky use, and dependence.15 In our case study, Ms. L’s substance use followed a similar pattern. Further, the comorbidity of SUDs and other psychiatric disorders may add a layer of complexity when caring for adolescents. Box 116-20 describes the relationship between comorbid psychiatric disorders and SUDs in adolescents.
Box 1
Disruptive behavior disorders are the most common coexisting psychiatric disorders in an adolescent with a substance use disorder (SUD), including opioid use disorder. These individuals typically present with aggression and other conduct disorder symptoms, and have early involvement with the legal system. Conversely, patients with conduct disorder are at high risk of early initiation of illicit substance use, including opioids. Early onset of substance use is a strong risk factor for developing an SUD.16
Mood disorders, particularly depression, can either precede or occur as a result of heavy and prolonged substance use.17 The estimated prevalence of major depressive disorder in individuals with an SUD is 24% to 50%. Among adolescents, an SUD is also a risk factor for suicidal ideation, suicide attempts, and completed suicide.18-20
Anxiety disorders, especially social phobia, and posttraumatic stress disorder are common in individuals with SUD.
Adolescents with SUD should be carefully evaluated for comorbid psychiatric disorders and treated accordingly.
Clinical manifestations
Common clinical manifestations of opioid use vary depending on when the patient is seen. An individual with OUD may appear acutely intoxicated, be in withdrawal, or show no effects. Chronic/prolonged use can lead to tolerance, such that a user needs to ingest larger amounts of the opioid to produce the same effects.
Acute intoxication can cause sedation, slurring of speech, and pinpoint pupils. Fresh injection sites may be visible on physical examination of IV users. The effects of acute intoxication usually depend on the half-life of the specific opioid and the individual’s tolerance.10 Tolerance to heroin can occur in 10 days and withdrawal can manifest in 3 to 7 hours after last use, depending on dose and purity.3 Tolerance can lead to unintentional overdose and death.
Withdrawal. Individuals experiencing withdrawal from opioids present with flu-like physical symptoms, including generalized body ache, rhinorrhea, diarrhea, goose bumps, lacrimation, and vomiting. Individuals also may experience irritability, restlessness, insomnia, anxiety, and depression during withdrawal.
Other manifestations. Excessive and chronic/prolonged opioid use can adversely impact socio-occupational functioning and cause academic decline in adolescents and youth. Personal relationships are significantly affected. Opioid users may have legal difficulties as a result of committing crimes such as theft, prostitution, or robbery in order to obtain opioids.
Continue to: Screening for OUD
Screening for OUD
Several screening tools are available to assess adolescents for SUDs, including OUD.
CRAFFT is a 6-item, clinician-administered screening tool that has been approved by American Academy of Pediatrics’ Committee on Substance Abuse for adolescents and young adults age <21.21-23 This commonly used tool can assess for alcohol, cannabis, and other drug use. A score ≥2 is considered positive for drug use, indicating that the individual would require further evaluation and assessment22,23 (Figure). There is also a self-administered CRAFFT questionnaire that can be completed by the patient.
NIDA-modified ASSIST. The American Psychiatric Association has adapted the National Institute on Drug Abuse (NIDA)-modified ASSIST. One version is designated for parents/guardians to administer to their children (age 6 to 17), and one is designated for adolescents (age 11 to 17) to self-administer.24,25 Each screening tool has 2 levels: Level 1 screens for substance use and other mental health symptoms, and Level 2 is more specific for substance use alone.
Drug Use Screening Inventory (DUSI) is a self-report questionnaire that has 149 items that assess the use of numerous drugs. It is designed to quantify the severity of consequences associated with drug and alcohol use.26,27
Problem-Oriented Screening Instrument for Teenagers (PO
Continue to: Personal Experience Screening Questionnaire (PESQ)...
Personal Experience Screening Questionnaire (PESQ) is a brief, 40-item, cost-effective, self-report questionnaire that can help identify adolescents (age 12 to 18) who should be referred for further evaluation.30
Addressing treatment expectations
For an adolescent with OUD, treatment should begin in the least restrictive environment that is perceived as safe for the patient. An adolescent’s readiness and motivation to achieve and maintain abstinence are crucial. Treatment planning should include the adolescent as well as his/her family to ensure they are able to verbalize their expectations. Start with a definitive treatment plan that addresses an individual’s needs. The plan should provide structure and an understanding of treatment expectations. The treatment team should clarify the realistic plan and goals based on empirical and clinical evidence. Treatment goals should include interventions to strengthen interpersonal relationships and assist with rehabilitation, such as establishing academic and/or vocational goals. Addressing readiness and working on a patient’s motivation is extremely important for most of these interventions.
In order for any intervention to be successful, clinicians need to establish and foster rapport with the adolescent. By law, substance use or behaviors related to substance use are not allowed to be shared outside the patient-clinician relationship, unless the adolescent gives consent or there are concerns that such behaviors might put the patient or others at risk. It is important to prime the adolescent and help them understand that any information pertaining to their safety or the safety of others may need to be shared outside the patient-clinician relationship.
Choosing an intervention
Less than 50% of a nationally representative sample of 345 addiction treatment programs serving adolescents and adults offer medications for treating OUD.31 Even in programs that offer pharmacotherapy, medications are significantly underutilized. Fewer than 30% of patients in addiction treatment programs receive medication, compared with 74% of patients receiving treatment for other mental health disorders.31 A
Psychotherapy may be used to treat OUD in adolescents. Several family therapies have been studied and are considered as critical psychotherapeutic interventions for treating SUDs, including structural family treatment and functional family therapy approaches.34 An integrated behavioral and family therapy model is also recommended for adolescent patients with SUDs. Cognitive distortions and use of self-deprecatory statements are common among adolescents.35 Therefore, using approaches of cognitive-behavioral therapy (CBT), or CBT plus motivational enhancement therapy, also might be effective for this population.36 The adolescent community reinforcement approach (A-CRA) is a behavioral treatment designed to help adolescents and their families learn how to lead a healthy and happy life without the use of drugs or alcohol by increasing access to social, familial, and educational/vocational reinforcers. Support groups and peer and family support should be encouraged as adjuncts to other interventions. In some areas, sober housing options for adolescents are also available.
Continue to: Harm-reduction strategies
Harm-reduction strategies. Although the primary goal of treatment for adolescents with OUD is to achieve and maintain abstinence from opioid use, implicit and explicit goals can be set. Short-term implicit goals may include harm-reduction strategies that emphasize decreasing the duration, frequency, and amount of substance use and limiting the chances of adverse effects, while the long-term explicit goal should be abstinence from opioid use.
Naloxone nasal spray is used as a harm-reduction strategy. It is an FDA-approved formulation that can reverse the effects of unintentional opioid overdoses and potentially prevent death from respiratory depression.37 Other harm-reduction strategies include needle exchange programs, which provide sterile needles to individuals who inject drugs in an effort to prevent or reduce the transmission of human immunodeficiency virus and other bloodborne viruses that can be spread via shared injection equipment. Fentanyl testing strips allow opioid users to test for the presence fentanyl and fentanyl analogs in the unregulated “street” opioid supply.
Pharmacologic interventions. Because there is limited empirical evidence on the efficacy of medication-assisted treatment (MAT) for adolescents with OUD, clinicians need to rely on evidence from research and experience with adults. Unfortunately, MAT is offered to adolescents considerably less often than it is to adults. Feder et al38 reported that only 2.4% of adolescents received MAT for heroin use and only 0.4% of adolescents received MAT for prescription opioid use, compared with 26.3% and 12% of adults, respectively.
Detoxification. Medications available for detoxification from opioids include opiates (such as methadone or buprenorphine) and clonidine (a central sympathomimetic). If the patient has used heroin for a short period (<1 year) and has no history of detoxification, consider a detoxification strategy with a longer-term taper (90 to 180 days) to allow for stabilization.
Maintenance treatment. Consider maintenance treatment for adolescents with a history of long-term opioid use and at least 2 prior short-term detoxification attempts or nonpharmacotherapy-based treatment within 12 months. Be sure to receive consent from a legal guardian and the patient. Maintenance treatment is usually recommended to continue for 1 to 6 years. Maintenance programs with longer durations have shown higher rates of abstinence, improved engagement, and retention in treatment.39
Continue to: According to guidelines from...
According to guidelines from the American Society of Addiction Medicine (ASAM), adolescents age >16 should be offered MAT; the first-line treatment is buprenorphine.40 To avoid risks of abuse and diversion, a combination of buprenorphine/naloxone may be administered.
Maintenance with buprenorphine
In order to prescribe and dispense buprenorphine, clinicians need to obtain a waiver from the Substance Abuse and Mental Health Services Administration. Before initiating buprenorphine, consider the type of opioid the individual used (short- or long-acting), the severity of the OUD, and the last reported use. The 3 phases of buprenorphine treatment are41:
- Induction phase. Buprenorphine can be initiated at 2 to 4 mg/d. Some patients may require up to 8 mg/d on the first day, which can be administered in divided doses.42 Evaluate and monitor patients carefully during the first few hours after the first dose. Patients should be in early withdrawal; otherwise, the buprenorphine might precipitate withdrawal. The induction phase can be completed in 2 to 4 days by titrating the dose so that the signs and symptoms of opioid withdrawal are minimal, and the patient is able to continue treatment. It may be helpful to have the patient’s legal guardian nearby in case the patient does not tolerate the medication or experiences withdrawal. The initial target dose for buprenorphine is approximately 12 to 16 mg/d.
- Stabilization phase. Patients no longer experience withdrawal symptoms and no longer have cravings. This phase can last 6 to 8 weeks. During this phase, patients should be seen weekly and doses should be adjusted if necessary. As a partial mu agonist, buprenorphine does not activate mu receptors fully and reaches a ceiling effect. Hence, doses >24 mg/d have limited added agonist properties.
- Maintenance phase. Because discontinuation of buprenorphine is associated with high relapse rates, patients may need to be maintained long-term on their stabilization dose, and for some patients, the length of time could be indefinite.39 During this phase, patients continue to undergo follow-up, but do so less frequently.
Methadone maintenance is generally not recommended for individuals age <18.
Preventing opioid diversion
Prescription medications that are kept in the home are a substantial source of opioids for adolescents. In 2014, 56% of 12th graders who did not need medications for medical purposes were able to acquire them from their friends or relatives; 36% of 12th graders used their own prescriptions.21 Limiting adolescents’ access to prescription opioids is the first line of prevention. Box 2 describes interventions and strategies to limit adolescents’ access to opioids.
Box 2
Many adolescents obtain opioids for recreational use from medications that were legitimately prescribed to family or friends. Both clinicians and parents/ guardians can take steps to reduce or prevent this type of diversion
Health care facilities. Regulating the number of pills dispensed to patients is crucial. It is highly recommended to prescribe only the minimal number of opioids necessary. In most cases, 3 to 7 days’ worth of opioids at a time might be sufficient, especially after surgical procedures.
Home. Families can limit adolescents’ access to prescription opioids in the home by keeping all medications in a lock box.
Proper disposal. Various entities offer locations for patients to drop off their unused opioids and other medications for safe disposal. These include police or fire departments and retail pharmacies. The US Drug Enforcement Administration sponsors a National Prescription Drug Take Back Day; see https://www.deadiversion.usdoj.gov/drug_disposal/takeback/index.html. The FDA also offers information on where and how to dispose of unused medicines at https://www.fda.gov/consumers/consumer-updates/where-and-how-dispose-unused-medicines.
CASE CONTINUED
Ms. L is initially prescribed, clonidine, 0.1 mg every 6 hours, to address opioid withdrawal. Clonidine is then tapered and maintained at 0.1 mg twice a day for irritability and impulse control. She is also prescribed sertraline, 100 mg/d, for depression and anxiety, and trazodone, 75 mg as needed at night, to assist with sleep.
Continue to: Following inpatient hospitalization...
Following inpatient hospitalization, during 12 weeks of partial hospital treatment, Ms. L participates in individual psychotherapy sessions 5 days/week; family therapy sessions once a week; and experiential therapy along with group sessions with other peers. She undergoes medication evaluations and adjustments on a weekly basis. Ms. L is now working at a store and is pursuing a high school equivalency certificate. She manages to avoid high-risk behaviors, although she reports having occasional cravings. Ms. L is actively involved in Narcotics Anonymous and has a sponsor. She has reconciled with her mother and moved back home, so she can stay away from her former acquaintances who are still using.
Bottom Line
Adolescents with opioid use disorder can benefit from an individualized treatment plan that includes psychosocial interventions, pharmacotherapy, or a combination of the two. Treatment planning should include the adolescent and his/her family to ensure they are able to verbalize their expectations. Treatment should focus on interventions that strengthen interpersonal relationships and assist with rehabilitation. Ongoing follow-up care is necessary for maintaining abstinence.
Related Resource
- Patkar AA, Weisler RH. Opioid abuse and overdose: Keep your patients safe. Current Psychiatry. 2017;16(8):8-12,14-16.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone
Clonidine • Clorpres
Methadone • Methadose
Naloxone • Narcan
Oxycodone • OxyContin
Sertraline • Zoloft
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
1. Davis JP, Prindle JJ, Eddie D, et al. Addressing the opioid epidemic with behavioral interventions for adolescents and young adults: a quasi-experimental design. J Consult Clin Psychol. 2019;87(10):941-951.
2. National Institute on Drug Abuse; National Institutes of Health; U.S. Department of Health and Human Services. Monitoring the Future Survey: High School and Youth Trends. https://www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends. Updated December 2019. Accessed January 13, 2020.
3. Hopfer CJ, Khuri E, Crowley TJ. Treating adolescent heroin use. J Am Acad Child Adolesc Psychiatry. 2003;42(5):609-611.
4. US Department of Justice, Drug Enforcement Agency, Diversion Control Division. https://www.deadiversion.usdoj.gov/. Accessed January 21, 2020.
5. Gaither JR, Leventhal JM, Ryan SA, et al. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997-2012. JAMA Pediatr. 2016;170(12):1195-1201.
6. Parker MA, Anthony JC. Epidemiological evidence on extra-medical use of prescription pain relievers: transitions from newly incident use to dependence among 12-21 year olds in United States using meta-analysis, 2002-13. Peer J. 2015;3:e1340. doi: 10.7717/peerj.1340. eCollection 2015.
7. Subramaniam GA, Fishman MJ, Woody G. Treatment of opioid-dependent adolescents and young adults with buprenorphine. Curr Psychiatry Rep. 2009;11(5):360-363.
8. Borodovsky JT, Levy S, Fishman M. Buprenorphine treatment for adolescents and young adults with opioid use disorders: a narrative review. J Addict Med. 2018;12(3):170-183.
9. Centers for Disease Control and Prevention: National Center for Health Statistics. Drug overdose deaths in the United States, 1999-2016. https://www.cdc.gov/nchs/products/databriefs/db294.htm. Published December 2017. Accessed January 15, 2020.
10. Strain E. Opioid use disorder: epidemiology, pharmacology, clinical manifestation, course, screening, assessment, diagnosis. https://www.uptodate.com/contents/opioid-use-disorder-epidemiology-pharmacology-clinical-manifestations-course-screening-assessment-and-diagnosis. Updated August 15, 2019. Accessed January 21, 2020.
11. American Academy of Pediatrics Committee on Substance Use and Prevention. Policy statement: medication-assisted treatment of adolescents with opioid use disorder. Pediatrics. 2016;138(3):e20161893. doi: https://doi.org/10.1542/peds.2016-1893.
12. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:514.
13. Sadock BJ, Sadock VA. Chapter 6: Theories of personality and psychopathology. In: Sadock BJ, Sadock VA, eds. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:209.
14. Kandel DB. Stages and pathways of drug involvement: examining the gateway hypothesis. Cambridge, United Kingdom: Cambridge University Press; 2002.
15. Robins LN, McEvoy L. Conduct problems as predictors of substance abuse. In: Robins LN, Rutter M, eds. Straight and devious pathways from childhood to adulthood. Cambridge, United Kingdom: Cambridge University Press; 1990;182-204.
16. Hopfer C, Salomonsen-Sautel S, Mikulich-Gilbertson S, et al. Conduct disorder and initiation of substance use: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2013;52(5):511-518.e4.
17. Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol. 2002;70(6):1224-1239.
18. Crumley FE. Substance abuse and adolescent suicidal behavior. JAMA. 1990;263(22):3051-3056.
19. Lewinsohn PM, Rohde P, Seeley JR. Adolescent suicidal ideation and attempts: prevalence, risk factors, and clinical implications. Clinical Psychology: Science and Practice. 1996;3(1):25-46.
20. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorder in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
21. Yule AM, Wilens TE, Rausch PK. The opioid epidemic: what a child psychiatrist is to do? J Am Acad Child Adolesc Psychiatry. 2017;56(7);541-543.
22. CRAFFT. https://crafft.org. Accessed January 21, 2020.
23. Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27(1):67-73.
24. American Psychiatric Association. Online assessment measures. https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures. Accessed January 15, 2020.
25. National Institute of Drug Abuse. American Psychiatric Association adapted NIDA modified ASSIST tools. https://www.drugabuse.gov/nidamed-medical-health-professionals/tool-resources-your-practice/screening-assessment-drug-testing-resources/american-psychiatric-association-adapted-nida. Updated November 15, 2015. Accessed January 21, 2020.
26. Canada’s Mental Health & Addiction Network. Drug Use Screening Inventory (DUSI). https://www.porticonetwork.ca/web/knowledgex-archive/amh-specialists/screening-for-cd-in-youth/screening-both-mh-sud/dusi. Published 2009. Accessed January 21, 2020.
27. Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am J Drug Alcohol Abuse. 1990;16(1-2):1-46.
28. Klitzner M, Gruenwald PJ, Taff GA, et al. The adolescent assessment referral system-final report. National Institute on Drug Abuse; Rockville, MD: 1993. NIDA Contract No. 271-89-8252.
29. Slesnick N, Tonigan JS. Assessment of alcohol and other drug use by runaway youths: a test-retest study of the Form 90. Alcohol Treat Q. 2004;22(2):21-34.
30. Winters KC, Kaminer Y. Screening and assessing adolescent substance use disorders in clinical populations. J Am Acad Child Adolesc Psychiatry. 2008;47(7):740-744.
31. Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011;5(1):21-27.
32. Deas D, Thomas SE. An overview of controlled study of adolescent substance abuse treatment. Am J Addiction. 2001;10(2):178-189.
33. William RJ, Chang, SY. A comprehensive and comparative review of adolescent substance abuse treatment outcome. Clinical Psychology: Science and Practice. 2000;7(2):138-166.
34. Bukstein OG, Work Group on Quality Issues. Practice parameters for the assessment and treatment of children and adolescents with substance use disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(6):609-621.
35. Van Hasselt VB, Null JA, Kempton T, et al. Social skills and depression in adolescent substance abusers. Addict Behav. 1993;18(1):9-18.
36. Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27(3):197-213.
37. US Food and Drug Administration. Information about naloxone. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-about-naloxone. Updated December 19, 2019. Accessed January 21, 2020.
38. Feder KA, Krawcyzk N, Saloner, B. Medication-assisted treatment for adolescents in specialty treatment for opioid use disorder. J Adolesc Health. 2018;60(6):747-750.
39. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003-2011.
40. US Department of Health and Human Services. Substance Abuse and Mental Health Ser-vices Administration. Medication-assisted treatment for opioid addiction in opioid treatment programs: a treatment improvement protocol TIP 43. https://www.asam.org/docs/advocacy/samhsa_tip43_matforopioidaddiction.pdf?sfvrsn=0. Published 2005. Accessed January 15, 2020.
41. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated September 9, 2019. Accessed January 21, 2020.
42. Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(suppl 2):S59-S77.
Ms. L, age 17, seeks treatment because she has an ongoing struggle with multiple substances, including benzodiazepines, heroin, alcohol, cannabis, and prescription opioids.
She reports that she was 13 when she first used a prescription opioid that was not prescribed for her. She also reports engaging in unsafe sexual practices while using these substances, and has been diagnosed and treated for a sexually transmitted disease. She dropped out of school and is estranged from her family. She says that for a long time she has felt depressed and that she uses drugs to “self-medicate my emotions.” She endorses high anxiety and lack of motivation. Ms. L also reports having several criminal charges for theft, assault, and exchanging sex for drugs. She has undergone 3 admissions for detoxification, but promptly resumed using drugs, primarily heroin and oxycodone, immediately after discharge. Ms. L meets DSM-5 criteria for opioid use disorder (OUD).
Ms. L’s case illustrates a disturbing trend in the current opioid epidemic in the United States. Nearly 11.8 million individuals age ≥12 reported misuse of opioids in the last year.1 Adolescents who misuse prescription or illicit opioids are more likely to be involved with the legal system due to truancy, running away from home, physical altercations, prostitution, exchanging sex for drugs, robbery, and gang involvement. Adolescents who use opioids may also struggle with academic decline, drop out of school early, be unable to maintain a job, and have relationship difficulties, especially with family members.
In this article, I describe the scope of OUD among adolescents, including epidemiology, clinical manifestations, screening tools, and treatment approaches.
Scope of the problem
According to the most recent Monitoring the Future survey of more than 42,500 8th, 10th, and 12th grade students, 2.7% of 12th graders reported prescription opioid misuse (reported in the survey as “narcotics other than heroin”) in the past year.2 In addition, 0.4% of 12th graders reported heroin use over the same period.2 Although the prevalence of opioid use among adolescents has been declining over the past 5 years,2 it still represents a serious health crisis.
Part of the issue may relate to easier access to more potent opioids. For example, heroin available today can be >4 times purer than it was in the past. In 2002, t
Between 1997 and 2012, the annual incidence of youth (age 15 to 19) hospitalizations for prescription opioid poisoning increased >170%.5 Approximately 6% to 9% of youth involved in risky opioid use develop OUD 6 to 12 months after s
Continue to: In recent years...
In recent years, deaths from drug overdose have increased for all age groups; however, limited data is available regarding adolescent overdose deaths. According to the Centers for Disease Control and Prevention (CDC), from 2015 to 2016, drug overdose death rates for persons age 15 to 24 increased to 28%.9
How opioids work
Opioids activate specific transmembrane neurotransmitter receptors, including mu, kappa, and delta, in the CNS and peripheral nervous system (PNS). This leads to activation of G protein–mediated intracellular signal transduction. Mainly it is activation of endogenous mu opioid receptors that mediates the reward, withdrawal, and analgesic effects of opioids. These effects depend on the location of mu receptors. In the CNS, activation of mu opioid receptors may cause miosis, respiratory depression, euphoria, and analgesia.10
Different opioids vary in terms of their half-life; for most opioids, the half-life ranges from 2 to 4 hours.10 Heroin has a half-life of 30 minutes, but due to active metabolites its duration of action is 4 to 5 hours. Opioid metabolites can be detected in urine toxicology within approximately 1 to 2 days since last use.10
Chronic opioid use is associated with neurologic effects that change the function of areas of the brain that control pleasure/reward, stress, decision-making, and more. This leads to cravings, continued substance use, and dependence.11 After continued long-term use, patients report decreased euphoria, but typically they continue to use opioids to avoid withdrawal symptoms or worsening mood.
Criteria for opioid use disorder
In DSM-5, substance use disorders (SUDs)are no longer categorized as abuse or dependence.12 For opioids, the diagnosis is OUD. The Table12 outlines the DSM-5 criteria for OUD. Craving opioids is included for the first time in the OUD diagnosis. Having problems with the legal system is no longer considered a diagnostic criterion for OUD.
Continue to: A vulnerable population
A vulnerable population
As defined by Erik Erikson’s psychosocial stages of development, adolescents struggle between establishing their own identity vs role confusion.13 In an attempt to relate to peers or give in to peer pressure, some adolescents start by experimenting with nicotine, alcohol, and/or marijuana; however, some may move on to using other illicit drugs.14 Risk factors for the development of SUDs include early onset of substance use and a rapid progression through stages of substance use from experimentation to regular use, risky use, and dependence.15 In our case study, Ms. L’s substance use followed a similar pattern. Further, the comorbidity of SUDs and other psychiatric disorders may add a layer of complexity when caring for adolescents. Box 116-20 describes the relationship between comorbid psychiatric disorders and SUDs in adolescents.
Box 1
Disruptive behavior disorders are the most common coexisting psychiatric disorders in an adolescent with a substance use disorder (SUD), including opioid use disorder. These individuals typically present with aggression and other conduct disorder symptoms, and have early involvement with the legal system. Conversely, patients with conduct disorder are at high risk of early initiation of illicit substance use, including opioids. Early onset of substance use is a strong risk factor for developing an SUD.16
Mood disorders, particularly depression, can either precede or occur as a result of heavy and prolonged substance use.17 The estimated prevalence of major depressive disorder in individuals with an SUD is 24% to 50%. Among adolescents, an SUD is also a risk factor for suicidal ideation, suicide attempts, and completed suicide.18-20
Anxiety disorders, especially social phobia, and posttraumatic stress disorder are common in individuals with SUD.
Adolescents with SUD should be carefully evaluated for comorbid psychiatric disorders and treated accordingly.
Clinical manifestations
Common clinical manifestations of opioid use vary depending on when the patient is seen. An individual with OUD may appear acutely intoxicated, be in withdrawal, or show no effects. Chronic/prolonged use can lead to tolerance, such that a user needs to ingest larger amounts of the opioid to produce the same effects.
Acute intoxication can cause sedation, slurring of speech, and pinpoint pupils. Fresh injection sites may be visible on physical examination of IV users. The effects of acute intoxication usually depend on the half-life of the specific opioid and the individual’s tolerance.10 Tolerance to heroin can occur in 10 days and withdrawal can manifest in 3 to 7 hours after last use, depending on dose and purity.3 Tolerance can lead to unintentional overdose and death.
Withdrawal. Individuals experiencing withdrawal from opioids present with flu-like physical symptoms, including generalized body ache, rhinorrhea, diarrhea, goose bumps, lacrimation, and vomiting. Individuals also may experience irritability, restlessness, insomnia, anxiety, and depression during withdrawal.
Other manifestations. Excessive and chronic/prolonged opioid use can adversely impact socio-occupational functioning and cause academic decline in adolescents and youth. Personal relationships are significantly affected. Opioid users may have legal difficulties as a result of committing crimes such as theft, prostitution, or robbery in order to obtain opioids.
Continue to: Screening for OUD
Screening for OUD
Several screening tools are available to assess adolescents for SUDs, including OUD.
CRAFFT is a 6-item, clinician-administered screening tool that has been approved by American Academy of Pediatrics’ Committee on Substance Abuse for adolescents and young adults age <21.21-23 This commonly used tool can assess for alcohol, cannabis, and other drug use. A score ≥2 is considered positive for drug use, indicating that the individual would require further evaluation and assessment22,23 (Figure). There is also a self-administered CRAFFT questionnaire that can be completed by the patient.
NIDA-modified ASSIST. The American Psychiatric Association has adapted the National Institute on Drug Abuse (NIDA)-modified ASSIST. One version is designated for parents/guardians to administer to their children (age 6 to 17), and one is designated for adolescents (age 11 to 17) to self-administer.24,25 Each screening tool has 2 levels: Level 1 screens for substance use and other mental health symptoms, and Level 2 is more specific for substance use alone.
Drug Use Screening Inventory (DUSI) is a self-report questionnaire that has 149 items that assess the use of numerous drugs. It is designed to quantify the severity of consequences associated with drug and alcohol use.26,27
Problem-Oriented Screening Instrument for Teenagers (PO
Continue to: Personal Experience Screening Questionnaire (PESQ)...
Personal Experience Screening Questionnaire (PESQ) is a brief, 40-item, cost-effective, self-report questionnaire that can help identify adolescents (age 12 to 18) who should be referred for further evaluation.30
Addressing treatment expectations
For an adolescent with OUD, treatment should begin in the least restrictive environment that is perceived as safe for the patient. An adolescent’s readiness and motivation to achieve and maintain abstinence are crucial. Treatment planning should include the adolescent as well as his/her family to ensure they are able to verbalize their expectations. Start with a definitive treatment plan that addresses an individual’s needs. The plan should provide structure and an understanding of treatment expectations. The treatment team should clarify the realistic plan and goals based on empirical and clinical evidence. Treatment goals should include interventions to strengthen interpersonal relationships and assist with rehabilitation, such as establishing academic and/or vocational goals. Addressing readiness and working on a patient’s motivation is extremely important for most of these interventions.
In order for any intervention to be successful, clinicians need to establish and foster rapport with the adolescent. By law, substance use or behaviors related to substance use are not allowed to be shared outside the patient-clinician relationship, unless the adolescent gives consent or there are concerns that such behaviors might put the patient or others at risk. It is important to prime the adolescent and help them understand that any information pertaining to their safety or the safety of others may need to be shared outside the patient-clinician relationship.
Choosing an intervention
Less than 50% of a nationally representative sample of 345 addiction treatment programs serving adolescents and adults offer medications for treating OUD.31 Even in programs that offer pharmacotherapy, medications are significantly underutilized. Fewer than 30% of patients in addiction treatment programs receive medication, compared with 74% of patients receiving treatment for other mental health disorders.31 A
Psychotherapy may be used to treat OUD in adolescents. Several family therapies have been studied and are considered as critical psychotherapeutic interventions for treating SUDs, including structural family treatment and functional family therapy approaches.34 An integrated behavioral and family therapy model is also recommended for adolescent patients with SUDs. Cognitive distortions and use of self-deprecatory statements are common among adolescents.35 Therefore, using approaches of cognitive-behavioral therapy (CBT), or CBT plus motivational enhancement therapy, also might be effective for this population.36 The adolescent community reinforcement approach (A-CRA) is a behavioral treatment designed to help adolescents and their families learn how to lead a healthy and happy life without the use of drugs or alcohol by increasing access to social, familial, and educational/vocational reinforcers. Support groups and peer and family support should be encouraged as adjuncts to other interventions. In some areas, sober housing options for adolescents are also available.
Continue to: Harm-reduction strategies
Harm-reduction strategies. Although the primary goal of treatment for adolescents with OUD is to achieve and maintain abstinence from opioid use, implicit and explicit goals can be set. Short-term implicit goals may include harm-reduction strategies that emphasize decreasing the duration, frequency, and amount of substance use and limiting the chances of adverse effects, while the long-term explicit goal should be abstinence from opioid use.
Naloxone nasal spray is used as a harm-reduction strategy. It is an FDA-approved formulation that can reverse the effects of unintentional opioid overdoses and potentially prevent death from respiratory depression.37 Other harm-reduction strategies include needle exchange programs, which provide sterile needles to individuals who inject drugs in an effort to prevent or reduce the transmission of human immunodeficiency virus and other bloodborne viruses that can be spread via shared injection equipment. Fentanyl testing strips allow opioid users to test for the presence fentanyl and fentanyl analogs in the unregulated “street” opioid supply.
Pharmacologic interventions. Because there is limited empirical evidence on the efficacy of medication-assisted treatment (MAT) for adolescents with OUD, clinicians need to rely on evidence from research and experience with adults. Unfortunately, MAT is offered to adolescents considerably less often than it is to adults. Feder et al38 reported that only 2.4% of adolescents received MAT for heroin use and only 0.4% of adolescents received MAT for prescription opioid use, compared with 26.3% and 12% of adults, respectively.
Detoxification. Medications available for detoxification from opioids include opiates (such as methadone or buprenorphine) and clonidine (a central sympathomimetic). If the patient has used heroin for a short period (<1 year) and has no history of detoxification, consider a detoxification strategy with a longer-term taper (90 to 180 days) to allow for stabilization.
Maintenance treatment. Consider maintenance treatment for adolescents with a history of long-term opioid use and at least 2 prior short-term detoxification attempts or nonpharmacotherapy-based treatment within 12 months. Be sure to receive consent from a legal guardian and the patient. Maintenance treatment is usually recommended to continue for 1 to 6 years. Maintenance programs with longer durations have shown higher rates of abstinence, improved engagement, and retention in treatment.39
Continue to: According to guidelines from...
According to guidelines from the American Society of Addiction Medicine (ASAM), adolescents age >16 should be offered MAT; the first-line treatment is buprenorphine.40 To avoid risks of abuse and diversion, a combination of buprenorphine/naloxone may be administered.
Maintenance with buprenorphine
In order to prescribe and dispense buprenorphine, clinicians need to obtain a waiver from the Substance Abuse and Mental Health Services Administration. Before initiating buprenorphine, consider the type of opioid the individual used (short- or long-acting), the severity of the OUD, and the last reported use. The 3 phases of buprenorphine treatment are41:
- Induction phase. Buprenorphine can be initiated at 2 to 4 mg/d. Some patients may require up to 8 mg/d on the first day, which can be administered in divided doses.42 Evaluate and monitor patients carefully during the first few hours after the first dose. Patients should be in early withdrawal; otherwise, the buprenorphine might precipitate withdrawal. The induction phase can be completed in 2 to 4 days by titrating the dose so that the signs and symptoms of opioid withdrawal are minimal, and the patient is able to continue treatment. It may be helpful to have the patient’s legal guardian nearby in case the patient does not tolerate the medication or experiences withdrawal. The initial target dose for buprenorphine is approximately 12 to 16 mg/d.
- Stabilization phase. Patients no longer experience withdrawal symptoms and no longer have cravings. This phase can last 6 to 8 weeks. During this phase, patients should be seen weekly and doses should be adjusted if necessary. As a partial mu agonist, buprenorphine does not activate mu receptors fully and reaches a ceiling effect. Hence, doses >24 mg/d have limited added agonist properties.
- Maintenance phase. Because discontinuation of buprenorphine is associated with high relapse rates, patients may need to be maintained long-term on their stabilization dose, and for some patients, the length of time could be indefinite.39 During this phase, patients continue to undergo follow-up, but do so less frequently.
Methadone maintenance is generally not recommended for individuals age <18.
Preventing opioid diversion
Prescription medications that are kept in the home are a substantial source of opioids for adolescents. In 2014, 56% of 12th graders who did not need medications for medical purposes were able to acquire them from their friends or relatives; 36% of 12th graders used their own prescriptions.21 Limiting adolescents’ access to prescription opioids is the first line of prevention. Box 2 describes interventions and strategies to limit adolescents’ access to opioids.
Box 2
Many adolescents obtain opioids for recreational use from medications that were legitimately prescribed to family or friends. Both clinicians and parents/ guardians can take steps to reduce or prevent this type of diversion
Health care facilities. Regulating the number of pills dispensed to patients is crucial. It is highly recommended to prescribe only the minimal number of opioids necessary. In most cases, 3 to 7 days’ worth of opioids at a time might be sufficient, especially after surgical procedures.
Home. Families can limit adolescents’ access to prescription opioids in the home by keeping all medications in a lock box.
Proper disposal. Various entities offer locations for patients to drop off their unused opioids and other medications for safe disposal. These include police or fire departments and retail pharmacies. The US Drug Enforcement Administration sponsors a National Prescription Drug Take Back Day; see https://www.deadiversion.usdoj.gov/drug_disposal/takeback/index.html. The FDA also offers information on where and how to dispose of unused medicines at https://www.fda.gov/consumers/consumer-updates/where-and-how-dispose-unused-medicines.
CASE CONTINUED
Ms. L is initially prescribed, clonidine, 0.1 mg every 6 hours, to address opioid withdrawal. Clonidine is then tapered and maintained at 0.1 mg twice a day for irritability and impulse control. She is also prescribed sertraline, 100 mg/d, for depression and anxiety, and trazodone, 75 mg as needed at night, to assist with sleep.
Continue to: Following inpatient hospitalization...
Following inpatient hospitalization, during 12 weeks of partial hospital treatment, Ms. L participates in individual psychotherapy sessions 5 days/week; family therapy sessions once a week; and experiential therapy along with group sessions with other peers. She undergoes medication evaluations and adjustments on a weekly basis. Ms. L is now working at a store and is pursuing a high school equivalency certificate. She manages to avoid high-risk behaviors, although she reports having occasional cravings. Ms. L is actively involved in Narcotics Anonymous and has a sponsor. She has reconciled with her mother and moved back home, so she can stay away from her former acquaintances who are still using.
Bottom Line
Adolescents with opioid use disorder can benefit from an individualized treatment plan that includes psychosocial interventions, pharmacotherapy, or a combination of the two. Treatment planning should include the adolescent and his/her family to ensure they are able to verbalize their expectations. Treatment should focus on interventions that strengthen interpersonal relationships and assist with rehabilitation. Ongoing follow-up care is necessary for maintaining abstinence.
Related Resource
- Patkar AA, Weisler RH. Opioid abuse and overdose: Keep your patients safe. Current Psychiatry. 2017;16(8):8-12,14-16.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone
Clonidine • Clorpres
Methadone • Methadose
Naloxone • Narcan
Oxycodone • OxyContin
Sertraline • Zoloft
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
Ms. L, age 17, seeks treatment because she has an ongoing struggle with multiple substances, including benzodiazepines, heroin, alcohol, cannabis, and prescription opioids.
She reports that she was 13 when she first used a prescription opioid that was not prescribed for her. She also reports engaging in unsafe sexual practices while using these substances, and has been diagnosed and treated for a sexually transmitted disease. She dropped out of school and is estranged from her family. She says that for a long time she has felt depressed and that she uses drugs to “self-medicate my emotions.” She endorses high anxiety and lack of motivation. Ms. L also reports having several criminal charges for theft, assault, and exchanging sex for drugs. She has undergone 3 admissions for detoxification, but promptly resumed using drugs, primarily heroin and oxycodone, immediately after discharge. Ms. L meets DSM-5 criteria for opioid use disorder (OUD).
Ms. L’s case illustrates a disturbing trend in the current opioid epidemic in the United States. Nearly 11.8 million individuals age ≥12 reported misuse of opioids in the last year.1 Adolescents who misuse prescription or illicit opioids are more likely to be involved with the legal system due to truancy, running away from home, physical altercations, prostitution, exchanging sex for drugs, robbery, and gang involvement. Adolescents who use opioids may also struggle with academic decline, drop out of school early, be unable to maintain a job, and have relationship difficulties, especially with family members.
In this article, I describe the scope of OUD among adolescents, including epidemiology, clinical manifestations, screening tools, and treatment approaches.
Scope of the problem
According to the most recent Monitoring the Future survey of more than 42,500 8th, 10th, and 12th grade students, 2.7% of 12th graders reported prescription opioid misuse (reported in the survey as “narcotics other than heroin”) in the past year.2 In addition, 0.4% of 12th graders reported heroin use over the same period.2 Although the prevalence of opioid use among adolescents has been declining over the past 5 years,2 it still represents a serious health crisis.
Part of the issue may relate to easier access to more potent opioids. For example, heroin available today can be >4 times purer than it was in the past. In 2002, t
Between 1997 and 2012, the annual incidence of youth (age 15 to 19) hospitalizations for prescription opioid poisoning increased >170%.5 Approximately 6% to 9% of youth involved in risky opioid use develop OUD 6 to 12 months after s
Continue to: In recent years...
In recent years, deaths from drug overdose have increased for all age groups; however, limited data is available regarding adolescent overdose deaths. According to the Centers for Disease Control and Prevention (CDC), from 2015 to 2016, drug overdose death rates for persons age 15 to 24 increased to 28%.9
How opioids work
Opioids activate specific transmembrane neurotransmitter receptors, including mu, kappa, and delta, in the CNS and peripheral nervous system (PNS). This leads to activation of G protein–mediated intracellular signal transduction. Mainly it is activation of endogenous mu opioid receptors that mediates the reward, withdrawal, and analgesic effects of opioids. These effects depend on the location of mu receptors. In the CNS, activation of mu opioid receptors may cause miosis, respiratory depression, euphoria, and analgesia.10
Different opioids vary in terms of their half-life; for most opioids, the half-life ranges from 2 to 4 hours.10 Heroin has a half-life of 30 minutes, but due to active metabolites its duration of action is 4 to 5 hours. Opioid metabolites can be detected in urine toxicology within approximately 1 to 2 days since last use.10
Chronic opioid use is associated with neurologic effects that change the function of areas of the brain that control pleasure/reward, stress, decision-making, and more. This leads to cravings, continued substance use, and dependence.11 After continued long-term use, patients report decreased euphoria, but typically they continue to use opioids to avoid withdrawal symptoms or worsening mood.
Criteria for opioid use disorder
In DSM-5, substance use disorders (SUDs)are no longer categorized as abuse or dependence.12 For opioids, the diagnosis is OUD. The Table12 outlines the DSM-5 criteria for OUD. Craving opioids is included for the first time in the OUD diagnosis. Having problems with the legal system is no longer considered a diagnostic criterion for OUD.
Continue to: A vulnerable population
A vulnerable population
As defined by Erik Erikson’s psychosocial stages of development, adolescents struggle between establishing their own identity vs role confusion.13 In an attempt to relate to peers or give in to peer pressure, some adolescents start by experimenting with nicotine, alcohol, and/or marijuana; however, some may move on to using other illicit drugs.14 Risk factors for the development of SUDs include early onset of substance use and a rapid progression through stages of substance use from experimentation to regular use, risky use, and dependence.15 In our case study, Ms. L’s substance use followed a similar pattern. Further, the comorbidity of SUDs and other psychiatric disorders may add a layer of complexity when caring for adolescents. Box 116-20 describes the relationship between comorbid psychiatric disorders and SUDs in adolescents.
Box 1
Disruptive behavior disorders are the most common coexisting psychiatric disorders in an adolescent with a substance use disorder (SUD), including opioid use disorder. These individuals typically present with aggression and other conduct disorder symptoms, and have early involvement with the legal system. Conversely, patients with conduct disorder are at high risk of early initiation of illicit substance use, including opioids. Early onset of substance use is a strong risk factor for developing an SUD.16
Mood disorders, particularly depression, can either precede or occur as a result of heavy and prolonged substance use.17 The estimated prevalence of major depressive disorder in individuals with an SUD is 24% to 50%. Among adolescents, an SUD is also a risk factor for suicidal ideation, suicide attempts, and completed suicide.18-20
Anxiety disorders, especially social phobia, and posttraumatic stress disorder are common in individuals with SUD.
Adolescents with SUD should be carefully evaluated for comorbid psychiatric disorders and treated accordingly.
Clinical manifestations
Common clinical manifestations of opioid use vary depending on when the patient is seen. An individual with OUD may appear acutely intoxicated, be in withdrawal, or show no effects. Chronic/prolonged use can lead to tolerance, such that a user needs to ingest larger amounts of the opioid to produce the same effects.
Acute intoxication can cause sedation, slurring of speech, and pinpoint pupils. Fresh injection sites may be visible on physical examination of IV users. The effects of acute intoxication usually depend on the half-life of the specific opioid and the individual’s tolerance.10 Tolerance to heroin can occur in 10 days and withdrawal can manifest in 3 to 7 hours after last use, depending on dose and purity.3 Tolerance can lead to unintentional overdose and death.
Withdrawal. Individuals experiencing withdrawal from opioids present with flu-like physical symptoms, including generalized body ache, rhinorrhea, diarrhea, goose bumps, lacrimation, and vomiting. Individuals also may experience irritability, restlessness, insomnia, anxiety, and depression during withdrawal.
Other manifestations. Excessive and chronic/prolonged opioid use can adversely impact socio-occupational functioning and cause academic decline in adolescents and youth. Personal relationships are significantly affected. Opioid users may have legal difficulties as a result of committing crimes such as theft, prostitution, or robbery in order to obtain opioids.
Continue to: Screening for OUD
Screening for OUD
Several screening tools are available to assess adolescents for SUDs, including OUD.
CRAFFT is a 6-item, clinician-administered screening tool that has been approved by American Academy of Pediatrics’ Committee on Substance Abuse for adolescents and young adults age <21.21-23 This commonly used tool can assess for alcohol, cannabis, and other drug use. A score ≥2 is considered positive for drug use, indicating that the individual would require further evaluation and assessment22,23 (Figure). There is also a self-administered CRAFFT questionnaire that can be completed by the patient.
NIDA-modified ASSIST. The American Psychiatric Association has adapted the National Institute on Drug Abuse (NIDA)-modified ASSIST. One version is designated for parents/guardians to administer to their children (age 6 to 17), and one is designated for adolescents (age 11 to 17) to self-administer.24,25 Each screening tool has 2 levels: Level 1 screens for substance use and other mental health symptoms, and Level 2 is more specific for substance use alone.
Drug Use Screening Inventory (DUSI) is a self-report questionnaire that has 149 items that assess the use of numerous drugs. It is designed to quantify the severity of consequences associated with drug and alcohol use.26,27
Problem-Oriented Screening Instrument for Teenagers (PO
Continue to: Personal Experience Screening Questionnaire (PESQ)...
Personal Experience Screening Questionnaire (PESQ) is a brief, 40-item, cost-effective, self-report questionnaire that can help identify adolescents (age 12 to 18) who should be referred for further evaluation.30
Addressing treatment expectations
For an adolescent with OUD, treatment should begin in the least restrictive environment that is perceived as safe for the patient. An adolescent’s readiness and motivation to achieve and maintain abstinence are crucial. Treatment planning should include the adolescent as well as his/her family to ensure they are able to verbalize their expectations. Start with a definitive treatment plan that addresses an individual’s needs. The plan should provide structure and an understanding of treatment expectations. The treatment team should clarify the realistic plan and goals based on empirical and clinical evidence. Treatment goals should include interventions to strengthen interpersonal relationships and assist with rehabilitation, such as establishing academic and/or vocational goals. Addressing readiness and working on a patient’s motivation is extremely important for most of these interventions.
In order for any intervention to be successful, clinicians need to establish and foster rapport with the adolescent. By law, substance use or behaviors related to substance use are not allowed to be shared outside the patient-clinician relationship, unless the adolescent gives consent or there are concerns that such behaviors might put the patient or others at risk. It is important to prime the adolescent and help them understand that any information pertaining to their safety or the safety of others may need to be shared outside the patient-clinician relationship.
Choosing an intervention
Less than 50% of a nationally representative sample of 345 addiction treatment programs serving adolescents and adults offer medications for treating OUD.31 Even in programs that offer pharmacotherapy, medications are significantly underutilized. Fewer than 30% of patients in addiction treatment programs receive medication, compared with 74% of patients receiving treatment for other mental health disorders.31 A
Psychotherapy may be used to treat OUD in adolescents. Several family therapies have been studied and are considered as critical psychotherapeutic interventions for treating SUDs, including structural family treatment and functional family therapy approaches.34 An integrated behavioral and family therapy model is also recommended for adolescent patients with SUDs. Cognitive distortions and use of self-deprecatory statements are common among adolescents.35 Therefore, using approaches of cognitive-behavioral therapy (CBT), or CBT plus motivational enhancement therapy, also might be effective for this population.36 The adolescent community reinforcement approach (A-CRA) is a behavioral treatment designed to help adolescents and their families learn how to lead a healthy and happy life without the use of drugs or alcohol by increasing access to social, familial, and educational/vocational reinforcers. Support groups and peer and family support should be encouraged as adjuncts to other interventions. In some areas, sober housing options for adolescents are also available.
Continue to: Harm-reduction strategies
Harm-reduction strategies. Although the primary goal of treatment for adolescents with OUD is to achieve and maintain abstinence from opioid use, implicit and explicit goals can be set. Short-term implicit goals may include harm-reduction strategies that emphasize decreasing the duration, frequency, and amount of substance use and limiting the chances of adverse effects, while the long-term explicit goal should be abstinence from opioid use.
Naloxone nasal spray is used as a harm-reduction strategy. It is an FDA-approved formulation that can reverse the effects of unintentional opioid overdoses and potentially prevent death from respiratory depression.37 Other harm-reduction strategies include needle exchange programs, which provide sterile needles to individuals who inject drugs in an effort to prevent or reduce the transmission of human immunodeficiency virus and other bloodborne viruses that can be spread via shared injection equipment. Fentanyl testing strips allow opioid users to test for the presence fentanyl and fentanyl analogs in the unregulated “street” opioid supply.
Pharmacologic interventions. Because there is limited empirical evidence on the efficacy of medication-assisted treatment (MAT) for adolescents with OUD, clinicians need to rely on evidence from research and experience with adults. Unfortunately, MAT is offered to adolescents considerably less often than it is to adults. Feder et al38 reported that only 2.4% of adolescents received MAT for heroin use and only 0.4% of adolescents received MAT for prescription opioid use, compared with 26.3% and 12% of adults, respectively.
Detoxification. Medications available for detoxification from opioids include opiates (such as methadone or buprenorphine) and clonidine (a central sympathomimetic). If the patient has used heroin for a short period (<1 year) and has no history of detoxification, consider a detoxification strategy with a longer-term taper (90 to 180 days) to allow for stabilization.
Maintenance treatment. Consider maintenance treatment for adolescents with a history of long-term opioid use and at least 2 prior short-term detoxification attempts or nonpharmacotherapy-based treatment within 12 months. Be sure to receive consent from a legal guardian and the patient. Maintenance treatment is usually recommended to continue for 1 to 6 years. Maintenance programs with longer durations have shown higher rates of abstinence, improved engagement, and retention in treatment.39
Continue to: According to guidelines from...
According to guidelines from the American Society of Addiction Medicine (ASAM), adolescents age >16 should be offered MAT; the first-line treatment is buprenorphine.40 To avoid risks of abuse and diversion, a combination of buprenorphine/naloxone may be administered.
Maintenance with buprenorphine
In order to prescribe and dispense buprenorphine, clinicians need to obtain a waiver from the Substance Abuse and Mental Health Services Administration. Before initiating buprenorphine, consider the type of opioid the individual used (short- or long-acting), the severity of the OUD, and the last reported use. The 3 phases of buprenorphine treatment are41:
- Induction phase. Buprenorphine can be initiated at 2 to 4 mg/d. Some patients may require up to 8 mg/d on the first day, which can be administered in divided doses.42 Evaluate and monitor patients carefully during the first few hours after the first dose. Patients should be in early withdrawal; otherwise, the buprenorphine might precipitate withdrawal. The induction phase can be completed in 2 to 4 days by titrating the dose so that the signs and symptoms of opioid withdrawal are minimal, and the patient is able to continue treatment. It may be helpful to have the patient’s legal guardian nearby in case the patient does not tolerate the medication or experiences withdrawal. The initial target dose for buprenorphine is approximately 12 to 16 mg/d.
- Stabilization phase. Patients no longer experience withdrawal symptoms and no longer have cravings. This phase can last 6 to 8 weeks. During this phase, patients should be seen weekly and doses should be adjusted if necessary. As a partial mu agonist, buprenorphine does not activate mu receptors fully and reaches a ceiling effect. Hence, doses >24 mg/d have limited added agonist properties.
- Maintenance phase. Because discontinuation of buprenorphine is associated with high relapse rates, patients may need to be maintained long-term on their stabilization dose, and for some patients, the length of time could be indefinite.39 During this phase, patients continue to undergo follow-up, but do so less frequently.
Methadone maintenance is generally not recommended for individuals age <18.
Preventing opioid diversion
Prescription medications that are kept in the home are a substantial source of opioids for adolescents. In 2014, 56% of 12th graders who did not need medications for medical purposes were able to acquire them from their friends or relatives; 36% of 12th graders used their own prescriptions.21 Limiting adolescents’ access to prescription opioids is the first line of prevention. Box 2 describes interventions and strategies to limit adolescents’ access to opioids.
Box 2
Many adolescents obtain opioids for recreational use from medications that were legitimately prescribed to family or friends. Both clinicians and parents/ guardians can take steps to reduce or prevent this type of diversion
Health care facilities. Regulating the number of pills dispensed to patients is crucial. It is highly recommended to prescribe only the minimal number of opioids necessary. In most cases, 3 to 7 days’ worth of opioids at a time might be sufficient, especially after surgical procedures.
Home. Families can limit adolescents’ access to prescription opioids in the home by keeping all medications in a lock box.
Proper disposal. Various entities offer locations for patients to drop off their unused opioids and other medications for safe disposal. These include police or fire departments and retail pharmacies. The US Drug Enforcement Administration sponsors a National Prescription Drug Take Back Day; see https://www.deadiversion.usdoj.gov/drug_disposal/takeback/index.html. The FDA also offers information on where and how to dispose of unused medicines at https://www.fda.gov/consumers/consumer-updates/where-and-how-dispose-unused-medicines.
CASE CONTINUED
Ms. L is initially prescribed, clonidine, 0.1 mg every 6 hours, to address opioid withdrawal. Clonidine is then tapered and maintained at 0.1 mg twice a day for irritability and impulse control. She is also prescribed sertraline, 100 mg/d, for depression and anxiety, and trazodone, 75 mg as needed at night, to assist with sleep.
Continue to: Following inpatient hospitalization...
Following inpatient hospitalization, during 12 weeks of partial hospital treatment, Ms. L participates in individual psychotherapy sessions 5 days/week; family therapy sessions once a week; and experiential therapy along with group sessions with other peers. She undergoes medication evaluations and adjustments on a weekly basis. Ms. L is now working at a store and is pursuing a high school equivalency certificate. She manages to avoid high-risk behaviors, although she reports having occasional cravings. Ms. L is actively involved in Narcotics Anonymous and has a sponsor. She has reconciled with her mother and moved back home, so she can stay away from her former acquaintances who are still using.
Bottom Line
Adolescents with opioid use disorder can benefit from an individualized treatment plan that includes psychosocial interventions, pharmacotherapy, or a combination of the two. Treatment planning should include the adolescent and his/her family to ensure they are able to verbalize their expectations. Treatment should focus on interventions that strengthen interpersonal relationships and assist with rehabilitation. Ongoing follow-up care is necessary for maintaining abstinence.
Related Resource
- Patkar AA, Weisler RH. Opioid abuse and overdose: Keep your patients safe. Current Psychiatry. 2017;16(8):8-12,14-16.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone
Clonidine • Clorpres
Methadone • Methadose
Naloxone • Narcan
Oxycodone • OxyContin
Sertraline • Zoloft
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
1. Davis JP, Prindle JJ, Eddie D, et al. Addressing the opioid epidemic with behavioral interventions for adolescents and young adults: a quasi-experimental design. J Consult Clin Psychol. 2019;87(10):941-951.
2. National Institute on Drug Abuse; National Institutes of Health; U.S. Department of Health and Human Services. Monitoring the Future Survey: High School and Youth Trends. https://www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends. Updated December 2019. Accessed January 13, 2020.
3. Hopfer CJ, Khuri E, Crowley TJ. Treating adolescent heroin use. J Am Acad Child Adolesc Psychiatry. 2003;42(5):609-611.
4. US Department of Justice, Drug Enforcement Agency, Diversion Control Division. https://www.deadiversion.usdoj.gov/. Accessed January 21, 2020.
5. Gaither JR, Leventhal JM, Ryan SA, et al. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997-2012. JAMA Pediatr. 2016;170(12):1195-1201.
6. Parker MA, Anthony JC. Epidemiological evidence on extra-medical use of prescription pain relievers: transitions from newly incident use to dependence among 12-21 year olds in United States using meta-analysis, 2002-13. Peer J. 2015;3:e1340. doi: 10.7717/peerj.1340. eCollection 2015.
7. Subramaniam GA, Fishman MJ, Woody G. Treatment of opioid-dependent adolescents and young adults with buprenorphine. Curr Psychiatry Rep. 2009;11(5):360-363.
8. Borodovsky JT, Levy S, Fishman M. Buprenorphine treatment for adolescents and young adults with opioid use disorders: a narrative review. J Addict Med. 2018;12(3):170-183.
9. Centers for Disease Control and Prevention: National Center for Health Statistics. Drug overdose deaths in the United States, 1999-2016. https://www.cdc.gov/nchs/products/databriefs/db294.htm. Published December 2017. Accessed January 15, 2020.
10. Strain E. Opioid use disorder: epidemiology, pharmacology, clinical manifestation, course, screening, assessment, diagnosis. https://www.uptodate.com/contents/opioid-use-disorder-epidemiology-pharmacology-clinical-manifestations-course-screening-assessment-and-diagnosis. Updated August 15, 2019. Accessed January 21, 2020.
11. American Academy of Pediatrics Committee on Substance Use and Prevention. Policy statement: medication-assisted treatment of adolescents with opioid use disorder. Pediatrics. 2016;138(3):e20161893. doi: https://doi.org/10.1542/peds.2016-1893.
12. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:514.
13. Sadock BJ, Sadock VA. Chapter 6: Theories of personality and psychopathology. In: Sadock BJ, Sadock VA, eds. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:209.
14. Kandel DB. Stages and pathways of drug involvement: examining the gateway hypothesis. Cambridge, United Kingdom: Cambridge University Press; 2002.
15. Robins LN, McEvoy L. Conduct problems as predictors of substance abuse. In: Robins LN, Rutter M, eds. Straight and devious pathways from childhood to adulthood. Cambridge, United Kingdom: Cambridge University Press; 1990;182-204.
16. Hopfer C, Salomonsen-Sautel S, Mikulich-Gilbertson S, et al. Conduct disorder and initiation of substance use: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2013;52(5):511-518.e4.
17. Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol. 2002;70(6):1224-1239.
18. Crumley FE. Substance abuse and adolescent suicidal behavior. JAMA. 1990;263(22):3051-3056.
19. Lewinsohn PM, Rohde P, Seeley JR. Adolescent suicidal ideation and attempts: prevalence, risk factors, and clinical implications. Clinical Psychology: Science and Practice. 1996;3(1):25-46.
20. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorder in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
21. Yule AM, Wilens TE, Rausch PK. The opioid epidemic: what a child psychiatrist is to do? J Am Acad Child Adolesc Psychiatry. 2017;56(7);541-543.
22. CRAFFT. https://crafft.org. Accessed January 21, 2020.
23. Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27(1):67-73.
24. American Psychiatric Association. Online assessment measures. https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures. Accessed January 15, 2020.
25. National Institute of Drug Abuse. American Psychiatric Association adapted NIDA modified ASSIST tools. https://www.drugabuse.gov/nidamed-medical-health-professionals/tool-resources-your-practice/screening-assessment-drug-testing-resources/american-psychiatric-association-adapted-nida. Updated November 15, 2015. Accessed January 21, 2020.
26. Canada’s Mental Health & Addiction Network. Drug Use Screening Inventory (DUSI). https://www.porticonetwork.ca/web/knowledgex-archive/amh-specialists/screening-for-cd-in-youth/screening-both-mh-sud/dusi. Published 2009. Accessed January 21, 2020.
27. Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am J Drug Alcohol Abuse. 1990;16(1-2):1-46.
28. Klitzner M, Gruenwald PJ, Taff GA, et al. The adolescent assessment referral system-final report. National Institute on Drug Abuse; Rockville, MD: 1993. NIDA Contract No. 271-89-8252.
29. Slesnick N, Tonigan JS. Assessment of alcohol and other drug use by runaway youths: a test-retest study of the Form 90. Alcohol Treat Q. 2004;22(2):21-34.
30. Winters KC, Kaminer Y. Screening and assessing adolescent substance use disorders in clinical populations. J Am Acad Child Adolesc Psychiatry. 2008;47(7):740-744.
31. Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011;5(1):21-27.
32. Deas D, Thomas SE. An overview of controlled study of adolescent substance abuse treatment. Am J Addiction. 2001;10(2):178-189.
33. William RJ, Chang, SY. A comprehensive and comparative review of adolescent substance abuse treatment outcome. Clinical Psychology: Science and Practice. 2000;7(2):138-166.
34. Bukstein OG, Work Group on Quality Issues. Practice parameters for the assessment and treatment of children and adolescents with substance use disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(6):609-621.
35. Van Hasselt VB, Null JA, Kempton T, et al. Social skills and depression in adolescent substance abusers. Addict Behav. 1993;18(1):9-18.
36. Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27(3):197-213.
37. US Food and Drug Administration. Information about naloxone. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-about-naloxone. Updated December 19, 2019. Accessed January 21, 2020.
38. Feder KA, Krawcyzk N, Saloner, B. Medication-assisted treatment for adolescents in specialty treatment for opioid use disorder. J Adolesc Health. 2018;60(6):747-750.
39. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003-2011.
40. US Department of Health and Human Services. Substance Abuse and Mental Health Ser-vices Administration. Medication-assisted treatment for opioid addiction in opioid treatment programs: a treatment improvement protocol TIP 43. https://www.asam.org/docs/advocacy/samhsa_tip43_matforopioidaddiction.pdf?sfvrsn=0. Published 2005. Accessed January 15, 2020.
41. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated September 9, 2019. Accessed January 21, 2020.
42. Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(suppl 2):S59-S77.
1. Davis JP, Prindle JJ, Eddie D, et al. Addressing the opioid epidemic with behavioral interventions for adolescents and young adults: a quasi-experimental design. J Consult Clin Psychol. 2019;87(10):941-951.
2. National Institute on Drug Abuse; National Institutes of Health; U.S. Department of Health and Human Services. Monitoring the Future Survey: High School and Youth Trends. https://www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends. Updated December 2019. Accessed January 13, 2020.
3. Hopfer CJ, Khuri E, Crowley TJ. Treating adolescent heroin use. J Am Acad Child Adolesc Psychiatry. 2003;42(5):609-611.
4. US Department of Justice, Drug Enforcement Agency, Diversion Control Division. https://www.deadiversion.usdoj.gov/. Accessed January 21, 2020.
5. Gaither JR, Leventhal JM, Ryan SA, et al. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997-2012. JAMA Pediatr. 2016;170(12):1195-1201.
6. Parker MA, Anthony JC. Epidemiological evidence on extra-medical use of prescription pain relievers: transitions from newly incident use to dependence among 12-21 year olds in United States using meta-analysis, 2002-13. Peer J. 2015;3:e1340. doi: 10.7717/peerj.1340. eCollection 2015.
7. Subramaniam GA, Fishman MJ, Woody G. Treatment of opioid-dependent adolescents and young adults with buprenorphine. Curr Psychiatry Rep. 2009;11(5):360-363.
8. Borodovsky JT, Levy S, Fishman M. Buprenorphine treatment for adolescents and young adults with opioid use disorders: a narrative review. J Addict Med. 2018;12(3):170-183.
9. Centers for Disease Control and Prevention: National Center for Health Statistics. Drug overdose deaths in the United States, 1999-2016. https://www.cdc.gov/nchs/products/databriefs/db294.htm. Published December 2017. Accessed January 15, 2020.
10. Strain E. Opioid use disorder: epidemiology, pharmacology, clinical manifestation, course, screening, assessment, diagnosis. https://www.uptodate.com/contents/opioid-use-disorder-epidemiology-pharmacology-clinical-manifestations-course-screening-assessment-and-diagnosis. Updated August 15, 2019. Accessed January 21, 2020.
11. American Academy of Pediatrics Committee on Substance Use and Prevention. Policy statement: medication-assisted treatment of adolescents with opioid use disorder. Pediatrics. 2016;138(3):e20161893. doi: https://doi.org/10.1542/peds.2016-1893.
12. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:514.
13. Sadock BJ, Sadock VA. Chapter 6: Theories of personality and psychopathology. In: Sadock BJ, Sadock VA, eds. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:209.
14. Kandel DB. Stages and pathways of drug involvement: examining the gateway hypothesis. Cambridge, United Kingdom: Cambridge University Press; 2002.
15. Robins LN, McEvoy L. Conduct problems as predictors of substance abuse. In: Robins LN, Rutter M, eds. Straight and devious pathways from childhood to adulthood. Cambridge, United Kingdom: Cambridge University Press; 1990;182-204.
16. Hopfer C, Salomonsen-Sautel S, Mikulich-Gilbertson S, et al. Conduct disorder and initiation of substance use: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2013;52(5):511-518.e4.
17. Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol. 2002;70(6):1224-1239.
18. Crumley FE. Substance abuse and adolescent suicidal behavior. JAMA. 1990;263(22):3051-3056.
19. Lewinsohn PM, Rohde P, Seeley JR. Adolescent suicidal ideation and attempts: prevalence, risk factors, and clinical implications. Clinical Psychology: Science and Practice. 1996;3(1):25-46.
20. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorder in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
21. Yule AM, Wilens TE, Rausch PK. The opioid epidemic: what a child psychiatrist is to do? J Am Acad Child Adolesc Psychiatry. 2017;56(7);541-543.
22. CRAFFT. https://crafft.org. Accessed January 21, 2020.
23. Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27(1):67-73.
24. American Psychiatric Association. Online assessment measures. https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures. Accessed January 15, 2020.
25. National Institute of Drug Abuse. American Psychiatric Association adapted NIDA modified ASSIST tools. https://www.drugabuse.gov/nidamed-medical-health-professionals/tool-resources-your-practice/screening-assessment-drug-testing-resources/american-psychiatric-association-adapted-nida. Updated November 15, 2015. Accessed January 21, 2020.
26. Canada’s Mental Health & Addiction Network. Drug Use Screening Inventory (DUSI). https://www.porticonetwork.ca/web/knowledgex-archive/amh-specialists/screening-for-cd-in-youth/screening-both-mh-sud/dusi. Published 2009. Accessed January 21, 2020.
27. Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am J Drug Alcohol Abuse. 1990;16(1-2):1-46.
28. Klitzner M, Gruenwald PJ, Taff GA, et al. The adolescent assessment referral system-final report. National Institute on Drug Abuse; Rockville, MD: 1993. NIDA Contract No. 271-89-8252.
29. Slesnick N, Tonigan JS. Assessment of alcohol and other drug use by runaway youths: a test-retest study of the Form 90. Alcohol Treat Q. 2004;22(2):21-34.
30. Winters KC, Kaminer Y. Screening and assessing adolescent substance use disorders in clinical populations. J Am Acad Child Adolesc Psychiatry. 2008;47(7):740-744.
31. Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011;5(1):21-27.
32. Deas D, Thomas SE. An overview of controlled study of adolescent substance abuse treatment. Am J Addiction. 2001;10(2):178-189.
33. William RJ, Chang, SY. A comprehensive and comparative review of adolescent substance abuse treatment outcome. Clinical Psychology: Science and Practice. 2000;7(2):138-166.
34. Bukstein OG, Work Group on Quality Issues. Practice parameters for the assessment and treatment of children and adolescents with substance use disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(6):609-621.
35. Van Hasselt VB, Null JA, Kempton T, et al. Social skills and depression in adolescent substance abusers. Addict Behav. 1993;18(1):9-18.
36. Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27(3):197-213.
37. US Food and Drug Administration. Information about naloxone. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-about-naloxone. Updated December 19, 2019. Accessed January 21, 2020.
38. Feder KA, Krawcyzk N, Saloner, B. Medication-assisted treatment for adolescents in specialty treatment for opioid use disorder. J Adolesc Health. 2018;60(6):747-750.
39. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003-2011.
40. US Department of Health and Human Services. Substance Abuse and Mental Health Ser-vices Administration. Medication-assisted treatment for opioid addiction in opioid treatment programs: a treatment improvement protocol TIP 43. https://www.asam.org/docs/advocacy/samhsa_tip43_matforopioidaddiction.pdf?sfvrsn=0. Published 2005. Accessed January 15, 2020.
41. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated September 9, 2019. Accessed January 21, 2020.
42. Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(suppl 2):S59-S77.
Therapeutic drug monitoring of antipsychotics
Mr. Q, age 36, has a history of schizophrenia. He is brought to the hospital due to persistent auditory hallucinations and paranoid delusions. His history documents a trial and failure of risperidone, 4 mg twice daily, and aripiprazole, 20 mg/d. Based on this, the treatment team initiates haloperidol, 5 mg twice daily. Because he experiences persistent auditory hallucinations and paranoid delusions, Mr. Q is titrated to increasing doses of haloperidol over 2 weeks during the course of the hospitalization. Once Mr. Q is receiving a total haloperidol dose of 30 mg/d, the team decides to obtain a serum haloperidol level due to his persistent psychotic symptoms and the development of drug-induced parkinsonism. His serum haloperidol level is 24 ng/mL, which is within the expected range for his dose, but above the therapeutic window for efficacy. The team decides that the severity of Mr. Q’s illness and documented treatment resistance (failing at least 2 adequate trials of antipsychotics) warrant a trial of clozapine.
Despite a long history of therapeutic drug monitoring (TDM) within psychiatry, routine monitoring of antipsychotic serum levels has not been unanimously adopted as standard practice. Clinical practice typically results in mostly a subjective assessment of the safety and efficacy of antipsychotics. This practice is in contrast to agents such as valproic acid and lithium, which are routinely monitored for safety and efficacy using both subjective and laboratory measures. Clinicians may adhere to these monitoring practices for lithium and valproic acid because of these agents’ narrow “therapeutic window” between toxicity and efficacy. However, antipsychotics can be viewed in a similar fashion.
To help conceptualize the therapeutic window for antipsychotics, it is important to understand that in most cases, the pharmacologic target for antipsychotics is dopamine (D2)receptor antagonism between 60% to 80%.1 Total drug exposure would thus determine a patient’s likelihood of minimizing positive symptoms, or exposure to adverse effects related to total dopamine antagonism. Serum drug concentrations are a better metric than total daily dose for determining drug exposure and achieving the pharmacologic target.2 Evaluating serum antipsychotic levels also is a better method of determining true treatment failure than relying on the clinical judgment of the treating psychiatrist.3
Pros and cons of TDM
Benefits of using TDM for patients being treated with antipsychotics include4:
- ensuring adherence
- quantitatively adjusting dosages for medication interactions or genetic variations
- ensuring an adequate trial of a medication before considering it a treatment failure.
Potential drawbacks to TDM include:
- Delayed results. Access to expeditious testing may not be possible in certain laboratories, and this may require send-out testing, which could result in a delay in obtaining results. Continued advocacy and research on the value of TDM in antipsychotics may improve access to these resources in the future. Nonetheless, obtaining antipsychotic serum levels will still give clinicians insight into the antipsychotic exposure at a given dose. Further, obtaining antipsychotic serum levels may strengthen decisions about treatment resistance and the assessment of interactions, adherence, or the likelihood of adverse effects.
- Lack of guidance. Unfortunately, there is no established guidance outlining what to do once antipsychotic serum levels are obtained. The correlation of serum levels of commonly used second-generation antipsychotics with clinical efficacy needs to be more closely investigated; however, certain agents do have more data associated with appropriate ranges for efficacy/toxicity. While researchers should continue to study the precise relationship between antipsychotic serum levels and effect, clinicians still have resources available to help determine what the expected serum value for a given patient may be. Knowing an expected serum level may help clinicians determine whether there is an unknown interaction or genetic variation that is causing lower- or higher-than-expected levels. This may also help determine whether a patient is adhering to their medication regimen.
Growing evidence for TDM
In recent years, evidence supporting the use of TDM in patients receiving antipsychotics has been increasing, and recommendations from consensus groups have been strengthened. One of the most comprehensive assessments of these practices was published by the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie (AGNP), a German-based psychopharmacology group consisting of researchers and clinicians. In 2018, the AGNP published consensus guidelines for TDM in various neuropsychiatric medication classes and recommendations for collecting, interpreting, and decision-making elements of the process.5 The Table5 lists the AGNP-recommended therapeutic serum range for several commonly used second-generation antipsychotics.
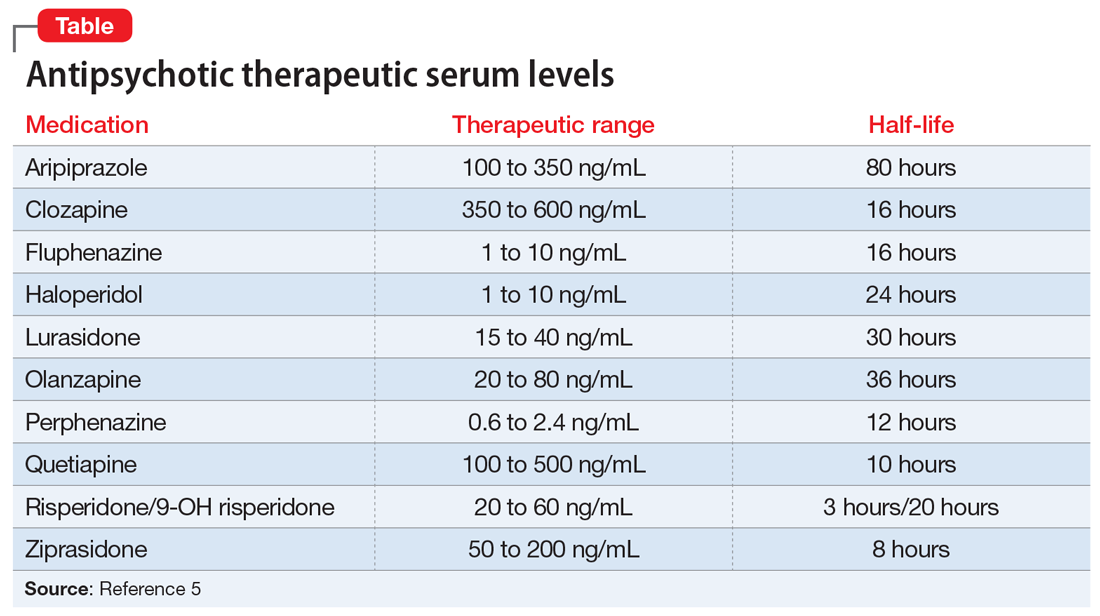
Researchers should be encouraged to contribute to the body of knowledge on the correlation of clinical response to serum level. However, there is compelling evidence for the use of TDM across many first- and second-generation antipsychotics. Of the most common, haloperidol and clozapine have evidence of a therapeutic range that is better correlated with serum level than daily dose. Specifically, haloperidol appears to lose benefit with dose increases beyond serum levels of approximately 10 ng/mL.6
Continue to: Clozapine levels may be...
Clozapine levels may be reported by measuring the metabolite norclozapine, which is not correlated with efficacy, or as a total level (combination of clozapine and norclozapine). While norclozapine is not associated with efficacy, the ratio of clozapine to norclozapine may indicate adherence to the medication, or any enzymatic modulation (genetic or drug–drug interaction) that may increase or decrease total exposure. A ratio of 1.5 to 2.0 (clozapine to norclozapine) is optimal; a ratio <0.5 may indicate nonadherence; and a ratio >2.0 may indicate inhibited drug clearance. A 12-hour serum clozapine level of ≥350 ng/mL is more likely to predict treatment response.7
CASE CONTINUED
Mr. Q is carefully tapered from haloperidol while initiating clozapine at 25 mg/d. As he is titrated on clozapine, Mr. Q’s serum levels are periodically checked and compared with expected levels and levels associated with efficacy. Eventually, Mr. Q is titrated to a clozapine dose of 400 mg/d at bedtime.
While receiving clozapine for 4 weeks, Mr. Q’s psychotic symptoms resolve, and he is scheduled for follow-up in the outpatient clozapine clinic.
Related Resources
- De Leon J. A critical commentary on the 2017 AGNP consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology. Pharmacopsychiatry. 2018; 51(1-02):63-68.
- Meyer JM. Is monitoring of plasma antipsychotic levels useful? Current Psychiatry. 2015;14(11):16,19-20.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Fluphenazine • Prolixin
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Perphenazine • Trilafon
Quetiapine • Seroquel
Risperidone • Risperdal
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
1. Stahl SM. Stahl’s essential psychopharmacology, neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013:129-236.
2. Potkin SG, Keator DB, Kesler-West ML, et al. D2 receptor occupancy following lurasidone treatment in patients with schizophrenia or schizoaffective disorder. CNS Spectr. 2014;19(2):176-181.
3. McCutcheon R, Beck K, D’Ambrosio E, et al. Antipsychotic plasma levels in the assessment of poor treatment response in schizophrenia. Acta Psychiatr Scand. 2018;137(1):39-46.
4. Horvitz-Lennon M, Mattke S, Predmore Z, et al. The role of antipsychotic plasma levels in the treatment of schizophrenia. Am J Psychiatry. 2017;174(5):421-426.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Van Putten T, Marder SR, Mintz J, et al. Haloperidol plasma levels and clinical response: a therapeutic window relationship. Am J Psychiatry. 1992;149(4):500-505.
7. Couchman L, Morgan PE, Spencer EP, et al. Plasma clozapine, norclozapine, and the clozapine:norclozapine ratio in relation to prescribed dose and other factors: data from a therapeutic drug monitoring service, 1993-2007. Ther Drug Monit. 2010;32(4):438-447.
Mr. Q, age 36, has a history of schizophrenia. He is brought to the hospital due to persistent auditory hallucinations and paranoid delusions. His history documents a trial and failure of risperidone, 4 mg twice daily, and aripiprazole, 20 mg/d. Based on this, the treatment team initiates haloperidol, 5 mg twice daily. Because he experiences persistent auditory hallucinations and paranoid delusions, Mr. Q is titrated to increasing doses of haloperidol over 2 weeks during the course of the hospitalization. Once Mr. Q is receiving a total haloperidol dose of 30 mg/d, the team decides to obtain a serum haloperidol level due to his persistent psychotic symptoms and the development of drug-induced parkinsonism. His serum haloperidol level is 24 ng/mL, which is within the expected range for his dose, but above the therapeutic window for efficacy. The team decides that the severity of Mr. Q’s illness and documented treatment resistance (failing at least 2 adequate trials of antipsychotics) warrant a trial of clozapine.
Despite a long history of therapeutic drug monitoring (TDM) within psychiatry, routine monitoring of antipsychotic serum levels has not been unanimously adopted as standard practice. Clinical practice typically results in mostly a subjective assessment of the safety and efficacy of antipsychotics. This practice is in contrast to agents such as valproic acid and lithium, which are routinely monitored for safety and efficacy using both subjective and laboratory measures. Clinicians may adhere to these monitoring practices for lithium and valproic acid because of these agents’ narrow “therapeutic window” between toxicity and efficacy. However, antipsychotics can be viewed in a similar fashion.
To help conceptualize the therapeutic window for antipsychotics, it is important to understand that in most cases, the pharmacologic target for antipsychotics is dopamine (D2)receptor antagonism between 60% to 80%.1 Total drug exposure would thus determine a patient’s likelihood of minimizing positive symptoms, or exposure to adverse effects related to total dopamine antagonism. Serum drug concentrations are a better metric than total daily dose for determining drug exposure and achieving the pharmacologic target.2 Evaluating serum antipsychotic levels also is a better method of determining true treatment failure than relying on the clinical judgment of the treating psychiatrist.3
Pros and cons of TDM
Benefits of using TDM for patients being treated with antipsychotics include4:
- ensuring adherence
- quantitatively adjusting dosages for medication interactions or genetic variations
- ensuring an adequate trial of a medication before considering it a treatment failure.
Potential drawbacks to TDM include:
- Delayed results. Access to expeditious testing may not be possible in certain laboratories, and this may require send-out testing, which could result in a delay in obtaining results. Continued advocacy and research on the value of TDM in antipsychotics may improve access to these resources in the future. Nonetheless, obtaining antipsychotic serum levels will still give clinicians insight into the antipsychotic exposure at a given dose. Further, obtaining antipsychotic serum levels may strengthen decisions about treatment resistance and the assessment of interactions, adherence, or the likelihood of adverse effects.
- Lack of guidance. Unfortunately, there is no established guidance outlining what to do once antipsychotic serum levels are obtained. The correlation of serum levels of commonly used second-generation antipsychotics with clinical efficacy needs to be more closely investigated; however, certain agents do have more data associated with appropriate ranges for efficacy/toxicity. While researchers should continue to study the precise relationship between antipsychotic serum levels and effect, clinicians still have resources available to help determine what the expected serum value for a given patient may be. Knowing an expected serum level may help clinicians determine whether there is an unknown interaction or genetic variation that is causing lower- or higher-than-expected levels. This may also help determine whether a patient is adhering to their medication regimen.
Growing evidence for TDM
In recent years, evidence supporting the use of TDM in patients receiving antipsychotics has been increasing, and recommendations from consensus groups have been strengthened. One of the most comprehensive assessments of these practices was published by the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie (AGNP), a German-based psychopharmacology group consisting of researchers and clinicians. In 2018, the AGNP published consensus guidelines for TDM in various neuropsychiatric medication classes and recommendations for collecting, interpreting, and decision-making elements of the process.5 The Table5 lists the AGNP-recommended therapeutic serum range for several commonly used second-generation antipsychotics.

Researchers should be encouraged to contribute to the body of knowledge on the correlation of clinical response to serum level. However, there is compelling evidence for the use of TDM across many first- and second-generation antipsychotics. Of the most common, haloperidol and clozapine have evidence of a therapeutic range that is better correlated with serum level than daily dose. Specifically, haloperidol appears to lose benefit with dose increases beyond serum levels of approximately 10 ng/mL.6
Continue to: Clozapine levels may be...
Clozapine levels may be reported by measuring the metabolite norclozapine, which is not correlated with efficacy, or as a total level (combination of clozapine and norclozapine). While norclozapine is not associated with efficacy, the ratio of clozapine to norclozapine may indicate adherence to the medication, or any enzymatic modulation (genetic or drug–drug interaction) that may increase or decrease total exposure. A ratio of 1.5 to 2.0 (clozapine to norclozapine) is optimal; a ratio <0.5 may indicate nonadherence; and a ratio >2.0 may indicate inhibited drug clearance. A 12-hour serum clozapine level of ≥350 ng/mL is more likely to predict treatment response.7
CASE CONTINUED
Mr. Q is carefully tapered from haloperidol while initiating clozapine at 25 mg/d. As he is titrated on clozapine, Mr. Q’s serum levels are periodically checked and compared with expected levels and levels associated with efficacy. Eventually, Mr. Q is titrated to a clozapine dose of 400 mg/d at bedtime.
While receiving clozapine for 4 weeks, Mr. Q’s psychotic symptoms resolve, and he is scheduled for follow-up in the outpatient clozapine clinic.
Related Resources
- De Leon J. A critical commentary on the 2017 AGNP consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology. Pharmacopsychiatry. 2018; 51(1-02):63-68.
- Meyer JM. Is monitoring of plasma antipsychotic levels useful? Current Psychiatry. 2015;14(11):16,19-20.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Fluphenazine • Prolixin
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Perphenazine • Trilafon
Quetiapine • Seroquel
Risperidone • Risperdal
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
Mr. Q, age 36, has a history of schizophrenia. He is brought to the hospital due to persistent auditory hallucinations and paranoid delusions. His history documents a trial and failure of risperidone, 4 mg twice daily, and aripiprazole, 20 mg/d. Based on this, the treatment team initiates haloperidol, 5 mg twice daily. Because he experiences persistent auditory hallucinations and paranoid delusions, Mr. Q is titrated to increasing doses of haloperidol over 2 weeks during the course of the hospitalization. Once Mr. Q is receiving a total haloperidol dose of 30 mg/d, the team decides to obtain a serum haloperidol level due to his persistent psychotic symptoms and the development of drug-induced parkinsonism. His serum haloperidol level is 24 ng/mL, which is within the expected range for his dose, but above the therapeutic window for efficacy. The team decides that the severity of Mr. Q’s illness and documented treatment resistance (failing at least 2 adequate trials of antipsychotics) warrant a trial of clozapine.
Despite a long history of therapeutic drug monitoring (TDM) within psychiatry, routine monitoring of antipsychotic serum levels has not been unanimously adopted as standard practice. Clinical practice typically results in mostly a subjective assessment of the safety and efficacy of antipsychotics. This practice is in contrast to agents such as valproic acid and lithium, which are routinely monitored for safety and efficacy using both subjective and laboratory measures. Clinicians may adhere to these monitoring practices for lithium and valproic acid because of these agents’ narrow “therapeutic window” between toxicity and efficacy. However, antipsychotics can be viewed in a similar fashion.
To help conceptualize the therapeutic window for antipsychotics, it is important to understand that in most cases, the pharmacologic target for antipsychotics is dopamine (D2)receptor antagonism between 60% to 80%.1 Total drug exposure would thus determine a patient’s likelihood of minimizing positive symptoms, or exposure to adverse effects related to total dopamine antagonism. Serum drug concentrations are a better metric than total daily dose for determining drug exposure and achieving the pharmacologic target.2 Evaluating serum antipsychotic levels also is a better method of determining true treatment failure than relying on the clinical judgment of the treating psychiatrist.3
Pros and cons of TDM
Benefits of using TDM for patients being treated with antipsychotics include4:
- ensuring adherence
- quantitatively adjusting dosages for medication interactions or genetic variations
- ensuring an adequate trial of a medication before considering it a treatment failure.
Potential drawbacks to TDM include:
- Delayed results. Access to expeditious testing may not be possible in certain laboratories, and this may require send-out testing, which could result in a delay in obtaining results. Continued advocacy and research on the value of TDM in antipsychotics may improve access to these resources in the future. Nonetheless, obtaining antipsychotic serum levels will still give clinicians insight into the antipsychotic exposure at a given dose. Further, obtaining antipsychotic serum levels may strengthen decisions about treatment resistance and the assessment of interactions, adherence, or the likelihood of adverse effects.
- Lack of guidance. Unfortunately, there is no established guidance outlining what to do once antipsychotic serum levels are obtained. The correlation of serum levels of commonly used second-generation antipsychotics with clinical efficacy needs to be more closely investigated; however, certain agents do have more data associated with appropriate ranges for efficacy/toxicity. While researchers should continue to study the precise relationship between antipsychotic serum levels and effect, clinicians still have resources available to help determine what the expected serum value for a given patient may be. Knowing an expected serum level may help clinicians determine whether there is an unknown interaction or genetic variation that is causing lower- or higher-than-expected levels. This may also help determine whether a patient is adhering to their medication regimen.
Growing evidence for TDM
In recent years, evidence supporting the use of TDM in patients receiving antipsychotics has been increasing, and recommendations from consensus groups have been strengthened. One of the most comprehensive assessments of these practices was published by the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie (AGNP), a German-based psychopharmacology group consisting of researchers and clinicians. In 2018, the AGNP published consensus guidelines for TDM in various neuropsychiatric medication classes and recommendations for collecting, interpreting, and decision-making elements of the process.5 The Table5 lists the AGNP-recommended therapeutic serum range for several commonly used second-generation antipsychotics.

Researchers should be encouraged to contribute to the body of knowledge on the correlation of clinical response to serum level. However, there is compelling evidence for the use of TDM across many first- and second-generation antipsychotics. Of the most common, haloperidol and clozapine have evidence of a therapeutic range that is better correlated with serum level than daily dose. Specifically, haloperidol appears to lose benefit with dose increases beyond serum levels of approximately 10 ng/mL.6
Continue to: Clozapine levels may be...
Clozapine levels may be reported by measuring the metabolite norclozapine, which is not correlated with efficacy, or as a total level (combination of clozapine and norclozapine). While norclozapine is not associated with efficacy, the ratio of clozapine to norclozapine may indicate adherence to the medication, or any enzymatic modulation (genetic or drug–drug interaction) that may increase or decrease total exposure. A ratio of 1.5 to 2.0 (clozapine to norclozapine) is optimal; a ratio <0.5 may indicate nonadherence; and a ratio >2.0 may indicate inhibited drug clearance. A 12-hour serum clozapine level of ≥350 ng/mL is more likely to predict treatment response.7
CASE CONTINUED
Mr. Q is carefully tapered from haloperidol while initiating clozapine at 25 mg/d. As he is titrated on clozapine, Mr. Q’s serum levels are periodically checked and compared with expected levels and levels associated with efficacy. Eventually, Mr. Q is titrated to a clozapine dose of 400 mg/d at bedtime.
While receiving clozapine for 4 weeks, Mr. Q’s psychotic symptoms resolve, and he is scheduled for follow-up in the outpatient clozapine clinic.
Related Resources
- De Leon J. A critical commentary on the 2017 AGNP consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology. Pharmacopsychiatry. 2018; 51(1-02):63-68.
- Meyer JM. Is monitoring of plasma antipsychotic levels useful? Current Psychiatry. 2015;14(11):16,19-20.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Fluphenazine • Prolixin
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Perphenazine • Trilafon
Quetiapine • Seroquel
Risperidone • Risperdal
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
1. Stahl SM. Stahl’s essential psychopharmacology, neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013:129-236.
2. Potkin SG, Keator DB, Kesler-West ML, et al. D2 receptor occupancy following lurasidone treatment in patients with schizophrenia or schizoaffective disorder. CNS Spectr. 2014;19(2):176-181.
3. McCutcheon R, Beck K, D’Ambrosio E, et al. Antipsychotic plasma levels in the assessment of poor treatment response in schizophrenia. Acta Psychiatr Scand. 2018;137(1):39-46.
4. Horvitz-Lennon M, Mattke S, Predmore Z, et al. The role of antipsychotic plasma levels in the treatment of schizophrenia. Am J Psychiatry. 2017;174(5):421-426.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Van Putten T, Marder SR, Mintz J, et al. Haloperidol plasma levels and clinical response: a therapeutic window relationship. Am J Psychiatry. 1992;149(4):500-505.
7. Couchman L, Morgan PE, Spencer EP, et al. Plasma clozapine, norclozapine, and the clozapine:norclozapine ratio in relation to prescribed dose and other factors: data from a therapeutic drug monitoring service, 1993-2007. Ther Drug Monit. 2010;32(4):438-447.
1. Stahl SM. Stahl’s essential psychopharmacology, neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013:129-236.
2. Potkin SG, Keator DB, Kesler-West ML, et al. D2 receptor occupancy following lurasidone treatment in patients with schizophrenia or schizoaffective disorder. CNS Spectr. 2014;19(2):176-181.
3. McCutcheon R, Beck K, D’Ambrosio E, et al. Antipsychotic plasma levels in the assessment of poor treatment response in schizophrenia. Acta Psychiatr Scand. 2018;137(1):39-46.
4. Horvitz-Lennon M, Mattke S, Predmore Z, et al. The role of antipsychotic plasma levels in the treatment of schizophrenia. Am J Psychiatry. 2017;174(5):421-426.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Van Putten T, Marder SR, Mintz J, et al. Haloperidol plasma levels and clinical response: a therapeutic window relationship. Am J Psychiatry. 1992;149(4):500-505.
7. Couchman L, Morgan PE, Spencer EP, et al. Plasma clozapine, norclozapine, and the clozapine:norclozapine ratio in relation to prescribed dose and other factors: data from a therapeutic drug monitoring service, 1993-2007. Ther Drug Monit. 2010;32(4):438-447.
Suicidal while receiving treatment for breast cancer
CASE Worsening mood symptoms and suicidal ideation
On a recent visit to the oncology clinic, where she has been receiving treatment for breast cancer for 11 months, Mrs. L, age 46, reports the abrupt onset of sadness, irritability, difficulty sleeping, and negative self-thoughts.
Eleven months ago, Mrs. L was diagnosed with invasive lobular carcinoma of the right breast that was classified as T2N0MX, representing relatively early-stage disease. Shortly after her diagnosis, Mrs. L completed 4 cycles of neoadjuvant chemotherapy with doxorubicin and cyclophosphamide, followed by treatment with trastuzumab. Subsequently, she underwent a right segmental mastectomy with bilateral mastopexy and radiation therapy. Recently, Mrs. L’s oncology team prescribed tamoxifen, 20 mg/d, and trastuzumab, 420 mg IV every 3 weeks; however, within 3 weeks after starting tamoxifen, Mrs. L’s mood symptoms worsened to the point where she says she is considering suicide—with a plan to use her husband’s gun to kill herself.
Mrs. L has no other pertinent medical history and no reported history of psychiatric disease.
The primary oncology team discontinues tamoxifen (after 5 weeks of treatment) and refers Mrs. L to psychiatry for further mood evaluation.
[polldaddy:10497042]
The authors’ observations
The prevalence of depression is higher in patients with cancer than in the general population.1 The etiology of depression is often multifactorial.2 In Mrs. L’s case, we hypothesized that the possible cause of her depressive symptoms included concerns about her self-image after mastectomy and the adverse effects of chemotherapy and tamoxifen.
Among these possible causes, estrogen level is particularly important. Estrogen affects the brain in numerous ways, including by modulating different neurotransmitters,3,4 regulating neuroplasticity, providing neuroprotection by preventing formation of oxidative free radicals and of beta amyloid, and possibly avoiding inflammation. From a behavioral standpoint, estrogen acts as an antidepressant while enhancing memory and modulating maternal behavior.4 Therefore, decreased estrogen levels could result in depression and other neuropsychiatric problems. This is illustrated in Mrs. L’s case, where tamoxifen administered after breast cancer treatment coincided with the abrupt onset of depression with suicidal ideation.
Depression in patients receiving tamoxifen might be explained by the fact that tamoxifen is a selective estrogen receptor blocker with dual properties. Specifically, while it has antagonistic action in breast tissue, diminishing the growth-promoting action of estrogen on breast cancer cells, it additionally crosses the blood-brain barrier, so it may block the neuroprotective action of estrogen in the brain.
EXAMINATION Improvement in depression but slightly anxious
During her psychiatric examination, Mrs. L is fairly well-groomed and cooperative. Her speech is normal, thought process is organized, and she has fair insight into her medical situation, with fair judgment. She is alert, attentive, and oriented to time, place, as well as person. She confirms that she has no prior psychiatric history, including no prior suicide attempts. She lives with her husband, who has been supportive. Mrs. L has no children, and she continues to work.
Continue to: Mrs. L reports that per her oncology...
Mrs. L reports that per her oncology team’s instruction, she has not taken tamoxifen for almost 1 week, and notes improvement in her mood. She describes her mood as “fine now,” but appears slightly anxious. She adamantly denies suicidal ideation since stopping tamoxifen; however, she confirms that prior to stopping tamoxifen, she experienced low mood, suicidal thoughts, and a decreased interest in activities. Mrs. L’s Patient Health Questionnaire–9 score is 13, indicating moderate depression. She says she is constantly preoccupied with thoughts about the adverse effects of hormone therapy, and specifically about the oncology team’s suggestion of a retrial of tamoxifen. Due to her constant worry, she has difficulty relaxing; her Generalized Anxiety Disorder–7 item scale score is 12, indicating moderate anxiety. She has a history of cigarette smoking but stopped after her breast cancer diagnosis. She also reports gaining weight since beginning cancer treatment (body mass index: 28.0 kg/m2) and experiencing breast pain.
Mrs. L’s vital signs are normal. Results of her laboratory workup reveal a thyroid-stimulating hormone level of 1.40 µU/mL (reference range: 0.27 to 4.20 µU/mL); a follicle-stimulating hormone (FSH) level of 78.4 mIU/mL (postmenopausal reference range: 25.8 to 134.8 mIU/mL); and an estradiol level of <12.0 pg/mL (postmenopausal range: <55 pg/mL).
The authors’ observations
Studies investigating the effects of tamoxifen on mood have produced varying results (Table5-16). Some researchers have found a significant relationship between depression and tamoxifen in patients with breast cancer. In a case-control study, 42 postmenopausal women with breast cancer who received tamoxifen reported statistically significant elevated depression scores.5 Similarly, in a prospective trial that assessed mood symptoms in 21 pre- and postmenopausal women who developed estrogen deficiency during breast cancer treatment (including treatment with tamoxifen and chemotherapy), 38% of patients met the criteria for major depressive disorder (MDD) in the first 6 months of treatment. Sixty-six percent of these patients were postmenopausal, and 38% were premenopausal. Twenty-five percent of the premenopausal women who experienced MDD symptoms had been treated with tamoxifen and chemotherapy.6
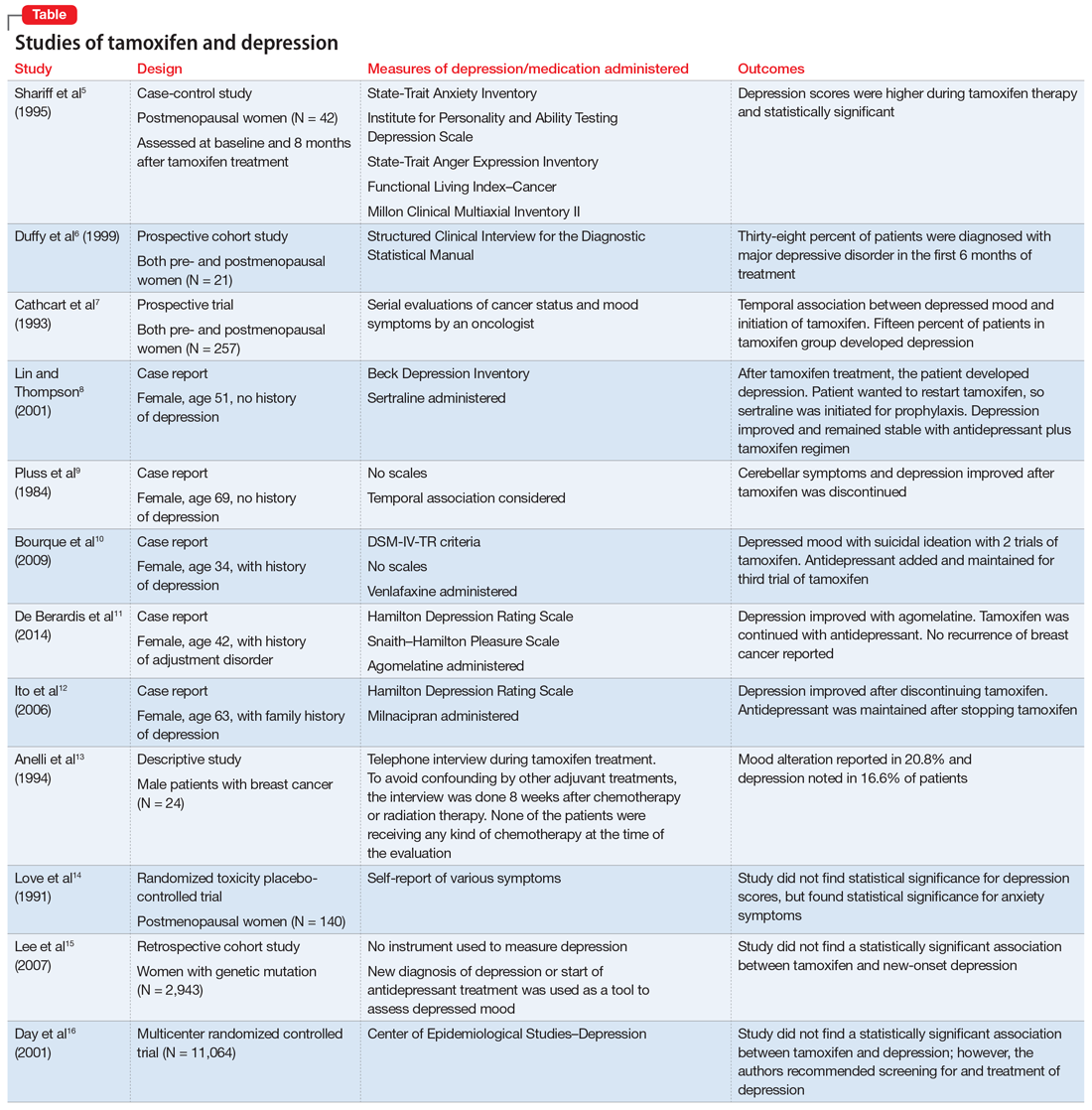
In a larger prospective trial (N = 257), an oncologist assessed mood symptoms in 2 groups of patients with breast cancer: individuals who received tamoxifen, and those who did not receive tamoxifen.7 They found that 15% of patients who received tamoxifen experienced depression, compared with 3% of patients who did not receive tamoxifen; this difference was statistically significant.7 Overall, 31% of the patients had “significant depression” and 27% discontinued tamoxifen because of adverse effects.7 There have been 2 case reports of tamoxifen use and severe depression in patients with no prior psychiatry history8,9 and 3 case reports of tamoxifen use and severe depression in patients who had a psychiatric history.10-12
One study that examined 24 men with breast cancer found that 62.5% of these patients experienced adverse effects related to tamoxifen, and 25% discontinued tamoxifen because of its adverse effects.13 Among the various adverse effects related to tamoxifen, mood alteration was reported in 20.8% of cases, and depressed feelings were reported in 16.6%.13
Continue to: Despite the evidence...
Despite this evidence, other studies have not found an association between tamoxifen and depressed mood in patients with breast cancer. One group of researchers who assessed various symptoms self-reported by postmenopausal women who were breast cancer survivors found that the depression scores were not significant.14 A retrospective cohort study assessed the onset of depression in patients with breast cancer with positive hormone receptor status (who received tamoxifen) vs negative hormone receptor status (who did not receive tamoxifen). These researchers did not find a statistically significant hazard ratio for “new-onset depression.”15 Unfortunately, the criteria for “new-onset depression” used in this study was the diagnosis of depression or use of an antidepressant given or ordered by a clinician, which is not a sensitive assessment of depressed mood.15
A multicenter randomized, placebo-controlled trial (the National Surgical Adjuvant Breast and Bowel Project) assessed the incidence of negative health outcomes, including depression, in a secondary outcome analysis.16 These researchers did not find a statistically significantassociation between tamoxifen and depression. However, in this study, assessment of depression was based on self-report using the Center of Epidemiologic Studies Depression (CES-D) scale, which does not clinically categorize depression. Furthermore, these researchers strongly recommended screening for mood disorders in routine clinical practice. In this study, 3 women completed suicide, 2 of whom were in the tamoxifen arm.16
[polldaddy:10497045]
The authors’ observations
Tamoxifen is a prodrug that converts to the active metabolite, endoxifen, via cytochrome P450 2D6 (CYP2D6) activity. Antidepressants with strong 2D6-inhibiting properties, such as fluoxetine, duloxetine, bupropion, and paroxetine, should be avoided in patients receiving tamoxifen because they interfere with the formation of the active metabolite and could reduce the effectiveness of tamoxifen and its ability to reduce the risk of cancer recurrence.17 Antidepressants can help treat psychological distress, especially depression, which is common in patients with cancer, and vasomotor symptoms, which may impair quality of life and adherence to long-term endocrine therapy. Because tamoxifen can decrease cancer recurrence and associated mortality,18 adherence with treatment is crucial.
TREATMENT Starting an antidepressant
The psychiatry team initiates venlafaxine, 37.5 mg/d, to treat Mrs. L’s anxiety and help prevent the recurrence of severe depression. They prescribe venlafaxine because they anticipate that, based on Mrs. L’s age, the oncology team might reconsider treatment with tamoxifen. Venlafaxine is preferred because it has a favorable pharmacodynamic profile and does not interfere with the metabolism of tamoxifen, as is the case with many selective serotonin reuptake inhibitors.17
Although Mrs. L’s depression had abated once she stopped receiving tamoxifen, she continues to experience anxiety and tearfulness, primarily due to fear of adverse effects of hormone therapy, and due to family as well as work stressors. Therefore, venlafaxine is gradually titrated up to 150 mg/d.
Continue to: The oncology team proposes...
The oncology team proposes a trial of leuprolide, a gonadotropin-releasing hormone agonist that downregulates pituitary receptors, subsequently suppressing female reproductive hormones, which in turn stops the ovaries from producing estrogen so there is a minimal amount of estrogen to promote the growth of estrogen–receptor-positive breast cancer. Mrs. L declines this agent because she is concerned that she will gain weight. Instead, Mrs. L expresses interest in undergoing an oophorectomy to reduce her estrogen level. In the meantime, based on her reproductive hormone levels (FSH and estradiol levels) which are indicative of postmenopausal status, the oncology team prescribes the aromatase inhibitor (AI) exemestane 25 mg/d. The AI helps to decrease the amount of estrogen the body makes peripherally, which is the main source of estrogen in postmenopausal women.
The authors’ observations
Estrogen originates in the ovaries in premenopausal women; it is also produced by peripheral conversion of androgens to estrogen in adipose tissues and muscle in postmenopausal women.19 Aromatase inhibitors block the enzyme aromatase that converts androgen to estrogen, which leads to estrogen deficiency in postmenopausal women and possibly to neuropsychiatric effects.19
The results of studies assessing the adverse psychiatric effects of AIs are mixed. When the results of studies evaluating tamoxifen are compared with those evaluating AIs, overall patients who received AIs had less severe or less frequent mood symptoms. One possible explanation could be that AIs are relatively new compared with tamoxifen. Second, AIs are more commonly used in postmenopausal women with breast cancer, and these patients’ overall estrogen level is significantly lower than that of premenopausal women with breast cancer. Therefore, the degree of hormone fluctuation is less intense in postmenopausal breast cancer survivors.
OUTCOME
After starting exemestane, and while still receiving venlafaxine, Mrs. L no longer experiences severe depressive symptoms. After 8 months, venlafaxine is discontinued. She continues to deny depressive symptoms but has intermittent anxiety, which she is able to manage without psychiatric medication. She continues to remain adherent with ongoing exemestane treatment, with no evidence of disease progression or recurrence.
The authors’ observations
For patients with estrogen-positive breast cancer, the decision to discontinue tamoxifen because of unacceptable adverse effects is an important one because it may increase the risk of cancer recurrence. Psychiatrists have an important role in supporting the patient through this process, helping patients understand alternatives, and working with the oncology team to formulate a plan that is acceptable to everyone.
Continue to: Bottom Line
Bottom Line
For patients with estrogen–positive breast cancer, anti-estrogen treatment can reduce the risk of cancer recurrence. However, it can cause adverse effects, including depression, that might impair quality of life and treatment adherence. For patients with severe depression, stopping estrogen blockers may be warranted. Initiating an antidepressant that does not interfere with the metabolism of tamoxifen may help treat depression and vasomotor symptoms.
Related Resource
- Agarwala P. Tailoring depression treatment for women with breast cancer. Current Psychiatry. 2010;9(11):39-40,45-46,48-49.
Drug Brand Names
Agomelatine • Valdoxan
Bupropion • Wellbutrin, Zyban
Cyclophosphamide • Cytoxan
Doxorubicin • Adriamycin
Duloxetine • Cymbalta
Exemestane • Aromasin
Fluoxetine • Prozac
Leuprolide • Eligard, Lupron
Milnacipran • Savella
Paroxetine • Paxil
Sertraline • Zoloft
Tamoxifen • Soltamox
Trastuzumab • Herceptin
Venlafaxine • Effexor
1. Zabora J, BrintzenhofeSzoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19-28.
2. Thompson DS, Spanier CA, Vogel VG. The relationship between tamoxifen, estrogen, and depressive symptoms. Breast J. 1999;5(6):375-382.
3. Halbreich U. Role of estrogen in postmenopausal depression. Neurology. 1997;48(5 suppl 7):S16-S19.
4. Schiller CE, Johnson SL, Abate AC, et al. Reproductive steroid regulation of mood and behavior. Compr Physiol. 2016;6(3):1135-1160.
5. Shariff S, Cumming CE, Lees A, et al. Mood disorder in women with early breast cancer taking tamoxifen, an estradiol receptor antagonist. An expected or unexpected effect? Ann N Y Acad Sci. 1995;761:365-368.
6. Duffy LS, Greenberg DB, Younger J, et al. Iatrogenic acute estrogen deficiency and psychiatric syndromes in breast cancer patients. Psychosomatics. 1999;40(4):304-308.
7. Cathcart CK, Jones SE, Pumroy CS, et al. Clinical recognition and management of depression in node negative breast cancer patients treated with tamoxifen. Breast Cancer Res Treat. 1993;27(3):277-281.
8. Lin J, Thompson DS. Case report: tamoxifen-induced depression. Primary Care Update for Ob/Gyns. 2001;8(5):207-208.
9. Pluss JL, DiBella NJ. Reversible central nervous system dysfunction due to tamoxifen in a patient with breast cancer. Ann Intern Med. 1984;101(5):652.
10. Bourque F, Karama S, Looper K, et al. Acute tamoxifen-induced depression and its prevention with venlafaxine. Psychosomatics. 2009;50(2):162-165.
11. De Berardis D, Brucchi M, Serroni N, et al. Successful use of agomelatine in the treatment of major depression in a woman taking tamoxifen: a case report. Clin Neuropharmacol. 2014;37(1):31-33.
12. Ito M, Baba H, Kawashima R, et al. A case of prolonged depression with tamoxifen. Japan Med Assoc J. 2006;49(4):167-172.
13. Anelli TF, Anelli A, Tran KN, et al. Tamoxifen administration is associated with a high rate of treatment-limiting symptoms in male breast cancer patients. Cancer. 1994;74(1):74-77.
14. Love RR, Cameron L, Connell BL, et al. Symptoms associated with tamoxifen treatment in postmenopausal women. Arch Intern Med. 1991;151(9):1842-1847.
15. Lee KC, Ray GT, Hunkeler EM, et al. Tamoxifen treatment and new-onset depression in breast cancer patients. Psychosomatics. 2007;48(3):205-210.
16. Day R, Ganz PA, Costantino JP. Tamoxifen and depression: more evidence from the National Surgical Adjuvant Breast and Bowel Project’s Breast Cancer Prevention (P-1) Randomized Study. J Natl Cancer Inst. 2001;93(21):1615-1623.
17. Juurlink D. Revisiting the drug interaction between tamoxifen and SSRI antidepressants. BMJ. 2016;354:i5309.
18. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771-784.
19. Buijs C, de Vries EGE, Mourits MJE, et al. The influence of endocrine treatments for breast cancer on health-related quality of life. Cancer Treat Rev. 2008;34(7):640-655.
CASE Worsening mood symptoms and suicidal ideation
On a recent visit to the oncology clinic, where she has been receiving treatment for breast cancer for 11 months, Mrs. L, age 46, reports the abrupt onset of sadness, irritability, difficulty sleeping, and negative self-thoughts.
Eleven months ago, Mrs. L was diagnosed with invasive lobular carcinoma of the right breast that was classified as T2N0MX, representing relatively early-stage disease. Shortly after her diagnosis, Mrs. L completed 4 cycles of neoadjuvant chemotherapy with doxorubicin and cyclophosphamide, followed by treatment with trastuzumab. Subsequently, she underwent a right segmental mastectomy with bilateral mastopexy and radiation therapy. Recently, Mrs. L’s oncology team prescribed tamoxifen, 20 mg/d, and trastuzumab, 420 mg IV every 3 weeks; however, within 3 weeks after starting tamoxifen, Mrs. L’s mood symptoms worsened to the point where she says she is considering suicide—with a plan to use her husband’s gun to kill herself.
Mrs. L has no other pertinent medical history and no reported history of psychiatric disease.
The primary oncology team discontinues tamoxifen (after 5 weeks of treatment) and refers Mrs. L to psychiatry for further mood evaluation.
[polldaddy:10497042]
The authors’ observations
The prevalence of depression is higher in patients with cancer than in the general population.1 The etiology of depression is often multifactorial.2 In Mrs. L’s case, we hypothesized that the possible cause of her depressive symptoms included concerns about her self-image after mastectomy and the adverse effects of chemotherapy and tamoxifen.
Among these possible causes, estrogen level is particularly important. Estrogen affects the brain in numerous ways, including by modulating different neurotransmitters,3,4 regulating neuroplasticity, providing neuroprotection by preventing formation of oxidative free radicals and of beta amyloid, and possibly avoiding inflammation. From a behavioral standpoint, estrogen acts as an antidepressant while enhancing memory and modulating maternal behavior.4 Therefore, decreased estrogen levels could result in depression and other neuropsychiatric problems. This is illustrated in Mrs. L’s case, where tamoxifen administered after breast cancer treatment coincided with the abrupt onset of depression with suicidal ideation.
Depression in patients receiving tamoxifen might be explained by the fact that tamoxifen is a selective estrogen receptor blocker with dual properties. Specifically, while it has antagonistic action in breast tissue, diminishing the growth-promoting action of estrogen on breast cancer cells, it additionally crosses the blood-brain barrier, so it may block the neuroprotective action of estrogen in the brain.
EXAMINATION Improvement in depression but slightly anxious
During her psychiatric examination, Mrs. L is fairly well-groomed and cooperative. Her speech is normal, thought process is organized, and she has fair insight into her medical situation, with fair judgment. She is alert, attentive, and oriented to time, place, as well as person. She confirms that she has no prior psychiatric history, including no prior suicide attempts. She lives with her husband, who has been supportive. Mrs. L has no children, and she continues to work.
Continue to: Mrs. L reports that per her oncology...
Mrs. L reports that per her oncology team’s instruction, she has not taken tamoxifen for almost 1 week, and notes improvement in her mood. She describes her mood as “fine now,” but appears slightly anxious. She adamantly denies suicidal ideation since stopping tamoxifen; however, she confirms that prior to stopping tamoxifen, she experienced low mood, suicidal thoughts, and a decreased interest in activities. Mrs. L’s Patient Health Questionnaire–9 score is 13, indicating moderate depression. She says she is constantly preoccupied with thoughts about the adverse effects of hormone therapy, and specifically about the oncology team’s suggestion of a retrial of tamoxifen. Due to her constant worry, she has difficulty relaxing; her Generalized Anxiety Disorder–7 item scale score is 12, indicating moderate anxiety. She has a history of cigarette smoking but stopped after her breast cancer diagnosis. She also reports gaining weight since beginning cancer treatment (body mass index: 28.0 kg/m2) and experiencing breast pain.
Mrs. L’s vital signs are normal. Results of her laboratory workup reveal a thyroid-stimulating hormone level of 1.40 µU/mL (reference range: 0.27 to 4.20 µU/mL); a follicle-stimulating hormone (FSH) level of 78.4 mIU/mL (postmenopausal reference range: 25.8 to 134.8 mIU/mL); and an estradiol level of <12.0 pg/mL (postmenopausal range: <55 pg/mL).
The authors’ observations
Studies investigating the effects of tamoxifen on mood have produced varying results (Table5-16). Some researchers have found a significant relationship between depression and tamoxifen in patients with breast cancer. In a case-control study, 42 postmenopausal women with breast cancer who received tamoxifen reported statistically significant elevated depression scores.5 Similarly, in a prospective trial that assessed mood symptoms in 21 pre- and postmenopausal women who developed estrogen deficiency during breast cancer treatment (including treatment with tamoxifen and chemotherapy), 38% of patients met the criteria for major depressive disorder (MDD) in the first 6 months of treatment. Sixty-six percent of these patients were postmenopausal, and 38% were premenopausal. Twenty-five percent of the premenopausal women who experienced MDD symptoms had been treated with tamoxifen and chemotherapy.6

In a larger prospective trial (N = 257), an oncologist assessed mood symptoms in 2 groups of patients with breast cancer: individuals who received tamoxifen, and those who did not receive tamoxifen.7 They found that 15% of patients who received tamoxifen experienced depression, compared with 3% of patients who did not receive tamoxifen; this difference was statistically significant.7 Overall, 31% of the patients had “significant depression” and 27% discontinued tamoxifen because of adverse effects.7 There have been 2 case reports of tamoxifen use and severe depression in patients with no prior psychiatry history8,9 and 3 case reports of tamoxifen use and severe depression in patients who had a psychiatric history.10-12
One study that examined 24 men with breast cancer found that 62.5% of these patients experienced adverse effects related to tamoxifen, and 25% discontinued tamoxifen because of its adverse effects.13 Among the various adverse effects related to tamoxifen, mood alteration was reported in 20.8% of cases, and depressed feelings were reported in 16.6%.13
Continue to: Despite the evidence...
Despite this evidence, other studies have not found an association between tamoxifen and depressed mood in patients with breast cancer. One group of researchers who assessed various symptoms self-reported by postmenopausal women who were breast cancer survivors found that the depression scores were not significant.14 A retrospective cohort study assessed the onset of depression in patients with breast cancer with positive hormone receptor status (who received tamoxifen) vs negative hormone receptor status (who did not receive tamoxifen). These researchers did not find a statistically significant hazard ratio for “new-onset depression.”15 Unfortunately, the criteria for “new-onset depression” used in this study was the diagnosis of depression or use of an antidepressant given or ordered by a clinician, which is not a sensitive assessment of depressed mood.15
A multicenter randomized, placebo-controlled trial (the National Surgical Adjuvant Breast and Bowel Project) assessed the incidence of negative health outcomes, including depression, in a secondary outcome analysis.16 These researchers did not find a statistically significantassociation between tamoxifen and depression. However, in this study, assessment of depression was based on self-report using the Center of Epidemiologic Studies Depression (CES-D) scale, which does not clinically categorize depression. Furthermore, these researchers strongly recommended screening for mood disorders in routine clinical practice. In this study, 3 women completed suicide, 2 of whom were in the tamoxifen arm.16
[polldaddy:10497045]
The authors’ observations
Tamoxifen is a prodrug that converts to the active metabolite, endoxifen, via cytochrome P450 2D6 (CYP2D6) activity. Antidepressants with strong 2D6-inhibiting properties, such as fluoxetine, duloxetine, bupropion, and paroxetine, should be avoided in patients receiving tamoxifen because they interfere with the formation of the active metabolite and could reduce the effectiveness of tamoxifen and its ability to reduce the risk of cancer recurrence.17 Antidepressants can help treat psychological distress, especially depression, which is common in patients with cancer, and vasomotor symptoms, which may impair quality of life and adherence to long-term endocrine therapy. Because tamoxifen can decrease cancer recurrence and associated mortality,18 adherence with treatment is crucial.
TREATMENT Starting an antidepressant
The psychiatry team initiates venlafaxine, 37.5 mg/d, to treat Mrs. L’s anxiety and help prevent the recurrence of severe depression. They prescribe venlafaxine because they anticipate that, based on Mrs. L’s age, the oncology team might reconsider treatment with tamoxifen. Venlafaxine is preferred because it has a favorable pharmacodynamic profile and does not interfere with the metabolism of tamoxifen, as is the case with many selective serotonin reuptake inhibitors.17
Although Mrs. L’s depression had abated once she stopped receiving tamoxifen, she continues to experience anxiety and tearfulness, primarily due to fear of adverse effects of hormone therapy, and due to family as well as work stressors. Therefore, venlafaxine is gradually titrated up to 150 mg/d.
Continue to: The oncology team proposes...
The oncology team proposes a trial of leuprolide, a gonadotropin-releasing hormone agonist that downregulates pituitary receptors, subsequently suppressing female reproductive hormones, which in turn stops the ovaries from producing estrogen so there is a minimal amount of estrogen to promote the growth of estrogen–receptor-positive breast cancer. Mrs. L declines this agent because she is concerned that she will gain weight. Instead, Mrs. L expresses interest in undergoing an oophorectomy to reduce her estrogen level. In the meantime, based on her reproductive hormone levels (FSH and estradiol levels) which are indicative of postmenopausal status, the oncology team prescribes the aromatase inhibitor (AI) exemestane 25 mg/d. The AI helps to decrease the amount of estrogen the body makes peripherally, which is the main source of estrogen in postmenopausal women.
The authors’ observations
Estrogen originates in the ovaries in premenopausal women; it is also produced by peripheral conversion of androgens to estrogen in adipose tissues and muscle in postmenopausal women.19 Aromatase inhibitors block the enzyme aromatase that converts androgen to estrogen, which leads to estrogen deficiency in postmenopausal women and possibly to neuropsychiatric effects.19
The results of studies assessing the adverse psychiatric effects of AIs are mixed. When the results of studies evaluating tamoxifen are compared with those evaluating AIs, overall patients who received AIs had less severe or less frequent mood symptoms. One possible explanation could be that AIs are relatively new compared with tamoxifen. Second, AIs are more commonly used in postmenopausal women with breast cancer, and these patients’ overall estrogen level is significantly lower than that of premenopausal women with breast cancer. Therefore, the degree of hormone fluctuation is less intense in postmenopausal breast cancer survivors.
OUTCOME
After starting exemestane, and while still receiving venlafaxine, Mrs. L no longer experiences severe depressive symptoms. After 8 months, venlafaxine is discontinued. She continues to deny depressive symptoms but has intermittent anxiety, which she is able to manage without psychiatric medication. She continues to remain adherent with ongoing exemestane treatment, with no evidence of disease progression or recurrence.
The authors’ observations
For patients with estrogen-positive breast cancer, the decision to discontinue tamoxifen because of unacceptable adverse effects is an important one because it may increase the risk of cancer recurrence. Psychiatrists have an important role in supporting the patient through this process, helping patients understand alternatives, and working with the oncology team to formulate a plan that is acceptable to everyone.
Continue to: Bottom Line
Bottom Line
For patients with estrogen–positive breast cancer, anti-estrogen treatment can reduce the risk of cancer recurrence. However, it can cause adverse effects, including depression, that might impair quality of life and treatment adherence. For patients with severe depression, stopping estrogen blockers may be warranted. Initiating an antidepressant that does not interfere with the metabolism of tamoxifen may help treat depression and vasomotor symptoms.
Related Resource
- Agarwala P. Tailoring depression treatment for women with breast cancer. Current Psychiatry. 2010;9(11):39-40,45-46,48-49.
Drug Brand Names
Agomelatine • Valdoxan
Bupropion • Wellbutrin, Zyban
Cyclophosphamide • Cytoxan
Doxorubicin • Adriamycin
Duloxetine • Cymbalta
Exemestane • Aromasin
Fluoxetine • Prozac
Leuprolide • Eligard, Lupron
Milnacipran • Savella
Paroxetine • Paxil
Sertraline • Zoloft
Tamoxifen • Soltamox
Trastuzumab • Herceptin
Venlafaxine • Effexor
CASE Worsening mood symptoms and suicidal ideation
On a recent visit to the oncology clinic, where she has been receiving treatment for breast cancer for 11 months, Mrs. L, age 46, reports the abrupt onset of sadness, irritability, difficulty sleeping, and negative self-thoughts.
Eleven months ago, Mrs. L was diagnosed with invasive lobular carcinoma of the right breast that was classified as T2N0MX, representing relatively early-stage disease. Shortly after her diagnosis, Mrs. L completed 4 cycles of neoadjuvant chemotherapy with doxorubicin and cyclophosphamide, followed by treatment with trastuzumab. Subsequently, she underwent a right segmental mastectomy with bilateral mastopexy and radiation therapy. Recently, Mrs. L’s oncology team prescribed tamoxifen, 20 mg/d, and trastuzumab, 420 mg IV every 3 weeks; however, within 3 weeks after starting tamoxifen, Mrs. L’s mood symptoms worsened to the point where she says she is considering suicide—with a plan to use her husband’s gun to kill herself.
Mrs. L has no other pertinent medical history and no reported history of psychiatric disease.
The primary oncology team discontinues tamoxifen (after 5 weeks of treatment) and refers Mrs. L to psychiatry for further mood evaluation.
[polldaddy:10497042]
The authors’ observations
The prevalence of depression is higher in patients with cancer than in the general population.1 The etiology of depression is often multifactorial.2 In Mrs. L’s case, we hypothesized that the possible cause of her depressive symptoms included concerns about her self-image after mastectomy and the adverse effects of chemotherapy and tamoxifen.
Among these possible causes, estrogen level is particularly important. Estrogen affects the brain in numerous ways, including by modulating different neurotransmitters,3,4 regulating neuroplasticity, providing neuroprotection by preventing formation of oxidative free radicals and of beta amyloid, and possibly avoiding inflammation. From a behavioral standpoint, estrogen acts as an antidepressant while enhancing memory and modulating maternal behavior.4 Therefore, decreased estrogen levels could result in depression and other neuropsychiatric problems. This is illustrated in Mrs. L’s case, where tamoxifen administered after breast cancer treatment coincided with the abrupt onset of depression with suicidal ideation.
Depression in patients receiving tamoxifen might be explained by the fact that tamoxifen is a selective estrogen receptor blocker with dual properties. Specifically, while it has antagonistic action in breast tissue, diminishing the growth-promoting action of estrogen on breast cancer cells, it additionally crosses the blood-brain barrier, so it may block the neuroprotective action of estrogen in the brain.
EXAMINATION Improvement in depression but slightly anxious
During her psychiatric examination, Mrs. L is fairly well-groomed and cooperative. Her speech is normal, thought process is organized, and she has fair insight into her medical situation, with fair judgment. She is alert, attentive, and oriented to time, place, as well as person. She confirms that she has no prior psychiatric history, including no prior suicide attempts. She lives with her husband, who has been supportive. Mrs. L has no children, and she continues to work.
Continue to: Mrs. L reports that per her oncology...
Mrs. L reports that per her oncology team’s instruction, she has not taken tamoxifen for almost 1 week, and notes improvement in her mood. She describes her mood as “fine now,” but appears slightly anxious. She adamantly denies suicidal ideation since stopping tamoxifen; however, she confirms that prior to stopping tamoxifen, she experienced low mood, suicidal thoughts, and a decreased interest in activities. Mrs. L’s Patient Health Questionnaire–9 score is 13, indicating moderate depression. She says she is constantly preoccupied with thoughts about the adverse effects of hormone therapy, and specifically about the oncology team’s suggestion of a retrial of tamoxifen. Due to her constant worry, she has difficulty relaxing; her Generalized Anxiety Disorder–7 item scale score is 12, indicating moderate anxiety. She has a history of cigarette smoking but stopped after her breast cancer diagnosis. She also reports gaining weight since beginning cancer treatment (body mass index: 28.0 kg/m2) and experiencing breast pain.
Mrs. L’s vital signs are normal. Results of her laboratory workup reveal a thyroid-stimulating hormone level of 1.40 µU/mL (reference range: 0.27 to 4.20 µU/mL); a follicle-stimulating hormone (FSH) level of 78.4 mIU/mL (postmenopausal reference range: 25.8 to 134.8 mIU/mL); and an estradiol level of <12.0 pg/mL (postmenopausal range: <55 pg/mL).
The authors’ observations
Studies investigating the effects of tamoxifen on mood have produced varying results (Table5-16). Some researchers have found a significant relationship between depression and tamoxifen in patients with breast cancer. In a case-control study, 42 postmenopausal women with breast cancer who received tamoxifen reported statistically significant elevated depression scores.5 Similarly, in a prospective trial that assessed mood symptoms in 21 pre- and postmenopausal women who developed estrogen deficiency during breast cancer treatment (including treatment with tamoxifen and chemotherapy), 38% of patients met the criteria for major depressive disorder (MDD) in the first 6 months of treatment. Sixty-six percent of these patients were postmenopausal, and 38% were premenopausal. Twenty-five percent of the premenopausal women who experienced MDD symptoms had been treated with tamoxifen and chemotherapy.6

In a larger prospective trial (N = 257), an oncologist assessed mood symptoms in 2 groups of patients with breast cancer: individuals who received tamoxifen, and those who did not receive tamoxifen.7 They found that 15% of patients who received tamoxifen experienced depression, compared with 3% of patients who did not receive tamoxifen; this difference was statistically significant.7 Overall, 31% of the patients had “significant depression” and 27% discontinued tamoxifen because of adverse effects.7 There have been 2 case reports of tamoxifen use and severe depression in patients with no prior psychiatry history8,9 and 3 case reports of tamoxifen use and severe depression in patients who had a psychiatric history.10-12
One study that examined 24 men with breast cancer found that 62.5% of these patients experienced adverse effects related to tamoxifen, and 25% discontinued tamoxifen because of its adverse effects.13 Among the various adverse effects related to tamoxifen, mood alteration was reported in 20.8% of cases, and depressed feelings were reported in 16.6%.13
Continue to: Despite the evidence...
Despite this evidence, other studies have not found an association between tamoxifen and depressed mood in patients with breast cancer. One group of researchers who assessed various symptoms self-reported by postmenopausal women who were breast cancer survivors found that the depression scores were not significant.14 A retrospective cohort study assessed the onset of depression in patients with breast cancer with positive hormone receptor status (who received tamoxifen) vs negative hormone receptor status (who did not receive tamoxifen). These researchers did not find a statistically significant hazard ratio for “new-onset depression.”15 Unfortunately, the criteria for “new-onset depression” used in this study was the diagnosis of depression or use of an antidepressant given or ordered by a clinician, which is not a sensitive assessment of depressed mood.15
A multicenter randomized, placebo-controlled trial (the National Surgical Adjuvant Breast and Bowel Project) assessed the incidence of negative health outcomes, including depression, in a secondary outcome analysis.16 These researchers did not find a statistically significantassociation between tamoxifen and depression. However, in this study, assessment of depression was based on self-report using the Center of Epidemiologic Studies Depression (CES-D) scale, which does not clinically categorize depression. Furthermore, these researchers strongly recommended screening for mood disorders in routine clinical practice. In this study, 3 women completed suicide, 2 of whom were in the tamoxifen arm.16
[polldaddy:10497045]
The authors’ observations
Tamoxifen is a prodrug that converts to the active metabolite, endoxifen, via cytochrome P450 2D6 (CYP2D6) activity. Antidepressants with strong 2D6-inhibiting properties, such as fluoxetine, duloxetine, bupropion, and paroxetine, should be avoided in patients receiving tamoxifen because they interfere with the formation of the active metabolite and could reduce the effectiveness of tamoxifen and its ability to reduce the risk of cancer recurrence.17 Antidepressants can help treat psychological distress, especially depression, which is common in patients with cancer, and vasomotor symptoms, which may impair quality of life and adherence to long-term endocrine therapy. Because tamoxifen can decrease cancer recurrence and associated mortality,18 adherence with treatment is crucial.
TREATMENT Starting an antidepressant
The psychiatry team initiates venlafaxine, 37.5 mg/d, to treat Mrs. L’s anxiety and help prevent the recurrence of severe depression. They prescribe venlafaxine because they anticipate that, based on Mrs. L’s age, the oncology team might reconsider treatment with tamoxifen. Venlafaxine is preferred because it has a favorable pharmacodynamic profile and does not interfere with the metabolism of tamoxifen, as is the case with many selective serotonin reuptake inhibitors.17
Although Mrs. L’s depression had abated once she stopped receiving tamoxifen, she continues to experience anxiety and tearfulness, primarily due to fear of adverse effects of hormone therapy, and due to family as well as work stressors. Therefore, venlafaxine is gradually titrated up to 150 mg/d.
Continue to: The oncology team proposes...
The oncology team proposes a trial of leuprolide, a gonadotropin-releasing hormone agonist that downregulates pituitary receptors, subsequently suppressing female reproductive hormones, which in turn stops the ovaries from producing estrogen so there is a minimal amount of estrogen to promote the growth of estrogen–receptor-positive breast cancer. Mrs. L declines this agent because she is concerned that she will gain weight. Instead, Mrs. L expresses interest in undergoing an oophorectomy to reduce her estrogen level. In the meantime, based on her reproductive hormone levels (FSH and estradiol levels) which are indicative of postmenopausal status, the oncology team prescribes the aromatase inhibitor (AI) exemestane 25 mg/d. The AI helps to decrease the amount of estrogen the body makes peripherally, which is the main source of estrogen in postmenopausal women.
The authors’ observations
Estrogen originates in the ovaries in premenopausal women; it is also produced by peripheral conversion of androgens to estrogen in adipose tissues and muscle in postmenopausal women.19 Aromatase inhibitors block the enzyme aromatase that converts androgen to estrogen, which leads to estrogen deficiency in postmenopausal women and possibly to neuropsychiatric effects.19
The results of studies assessing the adverse psychiatric effects of AIs are mixed. When the results of studies evaluating tamoxifen are compared with those evaluating AIs, overall patients who received AIs had less severe or less frequent mood symptoms. One possible explanation could be that AIs are relatively new compared with tamoxifen. Second, AIs are more commonly used in postmenopausal women with breast cancer, and these patients’ overall estrogen level is significantly lower than that of premenopausal women with breast cancer. Therefore, the degree of hormone fluctuation is less intense in postmenopausal breast cancer survivors.
OUTCOME
After starting exemestane, and while still receiving venlafaxine, Mrs. L no longer experiences severe depressive symptoms. After 8 months, venlafaxine is discontinued. She continues to deny depressive symptoms but has intermittent anxiety, which she is able to manage without psychiatric medication. She continues to remain adherent with ongoing exemestane treatment, with no evidence of disease progression or recurrence.
The authors’ observations
For patients with estrogen-positive breast cancer, the decision to discontinue tamoxifen because of unacceptable adverse effects is an important one because it may increase the risk of cancer recurrence. Psychiatrists have an important role in supporting the patient through this process, helping patients understand alternatives, and working with the oncology team to formulate a plan that is acceptable to everyone.
Continue to: Bottom Line
Bottom Line
For patients with estrogen–positive breast cancer, anti-estrogen treatment can reduce the risk of cancer recurrence. However, it can cause adverse effects, including depression, that might impair quality of life and treatment adherence. For patients with severe depression, stopping estrogen blockers may be warranted. Initiating an antidepressant that does not interfere with the metabolism of tamoxifen may help treat depression and vasomotor symptoms.
Related Resource
- Agarwala P. Tailoring depression treatment for women with breast cancer. Current Psychiatry. 2010;9(11):39-40,45-46,48-49.
Drug Brand Names
Agomelatine • Valdoxan
Bupropion • Wellbutrin, Zyban
Cyclophosphamide • Cytoxan
Doxorubicin • Adriamycin
Duloxetine • Cymbalta
Exemestane • Aromasin
Fluoxetine • Prozac
Leuprolide • Eligard, Lupron
Milnacipran • Savella
Paroxetine • Paxil
Sertraline • Zoloft
Tamoxifen • Soltamox
Trastuzumab • Herceptin
Venlafaxine • Effexor
1. Zabora J, BrintzenhofeSzoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19-28.
2. Thompson DS, Spanier CA, Vogel VG. The relationship between tamoxifen, estrogen, and depressive symptoms. Breast J. 1999;5(6):375-382.
3. Halbreich U. Role of estrogen in postmenopausal depression. Neurology. 1997;48(5 suppl 7):S16-S19.
4. Schiller CE, Johnson SL, Abate AC, et al. Reproductive steroid regulation of mood and behavior. Compr Physiol. 2016;6(3):1135-1160.
5. Shariff S, Cumming CE, Lees A, et al. Mood disorder in women with early breast cancer taking tamoxifen, an estradiol receptor antagonist. An expected or unexpected effect? Ann N Y Acad Sci. 1995;761:365-368.
6. Duffy LS, Greenberg DB, Younger J, et al. Iatrogenic acute estrogen deficiency and psychiatric syndromes in breast cancer patients. Psychosomatics. 1999;40(4):304-308.
7. Cathcart CK, Jones SE, Pumroy CS, et al. Clinical recognition and management of depression in node negative breast cancer patients treated with tamoxifen. Breast Cancer Res Treat. 1993;27(3):277-281.
8. Lin J, Thompson DS. Case report: tamoxifen-induced depression. Primary Care Update for Ob/Gyns. 2001;8(5):207-208.
9. Pluss JL, DiBella NJ. Reversible central nervous system dysfunction due to tamoxifen in a patient with breast cancer. Ann Intern Med. 1984;101(5):652.
10. Bourque F, Karama S, Looper K, et al. Acute tamoxifen-induced depression and its prevention with venlafaxine. Psychosomatics. 2009;50(2):162-165.
11. De Berardis D, Brucchi M, Serroni N, et al. Successful use of agomelatine in the treatment of major depression in a woman taking tamoxifen: a case report. Clin Neuropharmacol. 2014;37(1):31-33.
12. Ito M, Baba H, Kawashima R, et al. A case of prolonged depression with tamoxifen. Japan Med Assoc J. 2006;49(4):167-172.
13. Anelli TF, Anelli A, Tran KN, et al. Tamoxifen administration is associated with a high rate of treatment-limiting symptoms in male breast cancer patients. Cancer. 1994;74(1):74-77.
14. Love RR, Cameron L, Connell BL, et al. Symptoms associated with tamoxifen treatment in postmenopausal women. Arch Intern Med. 1991;151(9):1842-1847.
15. Lee KC, Ray GT, Hunkeler EM, et al. Tamoxifen treatment and new-onset depression in breast cancer patients. Psychosomatics. 2007;48(3):205-210.
16. Day R, Ganz PA, Costantino JP. Tamoxifen and depression: more evidence from the National Surgical Adjuvant Breast and Bowel Project’s Breast Cancer Prevention (P-1) Randomized Study. J Natl Cancer Inst. 2001;93(21):1615-1623.
17. Juurlink D. Revisiting the drug interaction between tamoxifen and SSRI antidepressants. BMJ. 2016;354:i5309.
18. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771-784.
19. Buijs C, de Vries EGE, Mourits MJE, et al. The influence of endocrine treatments for breast cancer on health-related quality of life. Cancer Treat Rev. 2008;34(7):640-655.
1. Zabora J, BrintzenhofeSzoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19-28.
2. Thompson DS, Spanier CA, Vogel VG. The relationship between tamoxifen, estrogen, and depressive symptoms. Breast J. 1999;5(6):375-382.
3. Halbreich U. Role of estrogen in postmenopausal depression. Neurology. 1997;48(5 suppl 7):S16-S19.
4. Schiller CE, Johnson SL, Abate AC, et al. Reproductive steroid regulation of mood and behavior. Compr Physiol. 2016;6(3):1135-1160.
5. Shariff S, Cumming CE, Lees A, et al. Mood disorder in women with early breast cancer taking tamoxifen, an estradiol receptor antagonist. An expected or unexpected effect? Ann N Y Acad Sci. 1995;761:365-368.
6. Duffy LS, Greenberg DB, Younger J, et al. Iatrogenic acute estrogen deficiency and psychiatric syndromes in breast cancer patients. Psychosomatics. 1999;40(4):304-308.
7. Cathcart CK, Jones SE, Pumroy CS, et al. Clinical recognition and management of depression in node negative breast cancer patients treated with tamoxifen. Breast Cancer Res Treat. 1993;27(3):277-281.
8. Lin J, Thompson DS. Case report: tamoxifen-induced depression. Primary Care Update for Ob/Gyns. 2001;8(5):207-208.
9. Pluss JL, DiBella NJ. Reversible central nervous system dysfunction due to tamoxifen in a patient with breast cancer. Ann Intern Med. 1984;101(5):652.
10. Bourque F, Karama S, Looper K, et al. Acute tamoxifen-induced depression and its prevention with venlafaxine. Psychosomatics. 2009;50(2):162-165.
11. De Berardis D, Brucchi M, Serroni N, et al. Successful use of agomelatine in the treatment of major depression in a woman taking tamoxifen: a case report. Clin Neuropharmacol. 2014;37(1):31-33.
12. Ito M, Baba H, Kawashima R, et al. A case of prolonged depression with tamoxifen. Japan Med Assoc J. 2006;49(4):167-172.
13. Anelli TF, Anelli A, Tran KN, et al. Tamoxifen administration is associated with a high rate of treatment-limiting symptoms in male breast cancer patients. Cancer. 1994;74(1):74-77.
14. Love RR, Cameron L, Connell BL, et al. Symptoms associated with tamoxifen treatment in postmenopausal women. Arch Intern Med. 1991;151(9):1842-1847.
15. Lee KC, Ray GT, Hunkeler EM, et al. Tamoxifen treatment and new-onset depression in breast cancer patients. Psychosomatics. 2007;48(3):205-210.
16. Day R, Ganz PA, Costantino JP. Tamoxifen and depression: more evidence from the National Surgical Adjuvant Breast and Bowel Project’s Breast Cancer Prevention (P-1) Randomized Study. J Natl Cancer Inst. 2001;93(21):1615-1623.
17. Juurlink D. Revisiting the drug interaction between tamoxifen and SSRI antidepressants. BMJ. 2016;354:i5309.
18. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771-784.
19. Buijs C, de Vries EGE, Mourits MJE, et al. The influence of endocrine treatments for breast cancer on health-related quality of life. Cancer Treat Rev. 2008;34(7):640-655.
Evidence-based tools for premenstrual disorders
CASE
A 30-year-old G2P2 woman presents for a well-woman visit and reports 6 months of premenstrual symptoms including irritability, depression, breast pain, and headaches. She is not taking any medications or hormonal contraceptives. She is sexually active and currently not interested in becoming pregnant. She asks what you can do for her symptoms, as they are affecting her life at home and at work.
Symptoms and definitions vary
Although more than 150 premenstrual symptoms have been reported, the most common psychological and behavioral ones are mood swings, depression, anxiety, irritability, crying, social withdrawal, forgetfulness, and problems concentrating.1-3 The most common physical symptoms are fatigue, abdominal bloating, weight gain, breast tenderness, acne, change in appetite or food cravings, edema, headache, and gastrointestinal upset. The etiology of these symptoms is usually multifactorial, with some combination of hormonal, neurotransmitter, lifestyle, environmental, and psychosocial factors playing a role.
Premenstrual disorder. In reviewing diagnostic criteria for the various premenstrual syndromes and disorders from different organizations (eg, the International Society for Premenstrual Disorders; the American College of Obstetricians and Gynecologists; the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition), there is agreement on the following criteria for premenstrual syndrome (PMS)4-6:
- The woman must be ovulating. (Women who no longer menstruate [eg, because of hysterectomy or endometrial ablation] can have premenstrual disorders as long as ovarian function remains intact.)
- The woman experiences a constellation of disabling physical and/or psychological symptoms that appears in the luteal phase of her menstrual cycle.
- The symptoms improve soon after the onset of menses.
- There is a symptom-free interval before ovulation.
- There is prospective documentation of symptoms for at least 2 consecutive cycles.
- The symptoms are sufficient in severity to affect activities of daily living and/or important relationships.
Premenstrual dysphoric disorder. PMDD is another common premenstrual disorder. It is distinguished by significant premenstrual psychological symptoms and requires the presence of marked affective lability, marked irritability or anger, markedly depressed mood, and/or marked anxiety (TABLE 1).7
Exacerbation of other ailments. Another premenstrual disorder is the premenstrual exacerbation of underlying chronic medical or psychological problems such as migraines, seizures, asthma, diabetes, irritable bowel syndrome, fibromyalgia, anxiety, or depression.
Differences in interpretation lead to variations in prevalence
Differences in the interpretation of significant premenstrual symptoms have led to variations in estimated prevalence. For example, 80% to 95% of women report premenstrual symptoms, but only 30% to 40% meet criteria for PMS and only 3% to 8% meet criteria for PMDD.8 Many women who report premenstrual symptoms in a retrospective questionnaire do not meet criteria for PMS or PMDD based on prospective symptom charting. The Daily Record of Severity of Problems (DRSP), a prospective tracking tool for premenstrual symptoms, is sensitive and specific for diagnosing PMS and PMDD if administered on the first day of menstruation.9
Ask about symptoms and use a tracking tool
When you see a woman for a well-woman visit or a gynecologic problem, inquire about physical/emotional symptoms and their severity during the week that precedes menstruation. If a patient reports few symptoms of a mild nature, then no further work-up is needed.
Continue to: If patients report significant...
If patients report significant premenstrual symptoms, recommend the use of a tool to track the symptoms. Older tools such as the DRSP and the Premenstrual Symptoms Screening Tool (PSST), newer symptom diaries that can be used for both PMS and PMDD,and questionnaires that have been used in research situations can be time consuming and difficult for patients to complete.10-12 Instead, physicians can easily construct their own charting tool, as we did for patients to use when tracking their most bothersome symptoms (FIGURE 1). Tracking helps to confirm the diagnosis and helps you and the patient focus on treatment goals.
Keep in mind other diagnoses (eg, anemia, thyroid disorders, perimenopause, anxiety, depression, eating disorders, substance abuse) that can cause or exacerbate the psychological/physical symptoms the patient is reporting. If you suspect any of these other diagnoses, laboratory evaluation (eg, complete blood count, thyroid-stimulating hormone level or other hormonal testing, urine drug screen, etc) may be warranted to rule out other etiologies for the reported symptoms.
Develop a Tx plan that considers symptoms, family-planning needs
Focus treatment on the patient’s predominant symptoms whether they are physical, psychological, or mixed (FIGURE 2). The patient’s preferences regarding family planning are another important consideration. Women who are using a fertility awareness
Although the definitions for PMS and PMDD require at least 2 cycles of prospective documentation of symptoms, dietary and lifestyle changes can begin immediately. Regular follow-up to document improvement of symptoms is important; using the patient’s symptoms charting tool can help with this.
Focus on diet and lifestyle right away
Experts in the field of PMS/PMDD suggest that simple dietary changes may be a reasonable first step to help improve symptoms. Researchers have found that diets high in fiber, vegetables, and whole grains are inversely related to PMS.13 Older studies have suggested an increased prevalence and severity of PMS with increased caffeine intake; however, a newer study found no such association.14
Continue to: A case-control study nested...
A case-control study nested within the Nurses’ Health Study II cohort showed that a high intake of both dietary calcium and vitamin D prevented the development of PMS in women ages 27 to 44.15 B vitamins, such as thiamine and riboflavin, from food sources have been associated with a lower risk of PMS.16 A variety of older clinical studies showed benefit from aerobic exercise on PMS symptoms,17-19 but a newer cross-sectional study of young adult women found no association between physical activity and the prevalence of PMS.20 Acupuncture has demonstrated efficacy for the treatment of the physical symptoms of PMS and PMDD, but more rigorous studies are needed.21,22 Cognitive behavioral therapy has been studied as a treatment, but data to support this approach are limited so it cannot be recommended at this time.23
Make the most of supplements—especially calcium
Calcium is the nutritional supplement with the most evidence to support its use to relieve symptoms of PMS and PMDD (TABLE 221,22,24-45). Research indicates that disturbances in calcium regulation and calcium deficiency may be responsible for various premenstrual symptoms. One study showed that, compared with placebo, women who took 1200 mg/d calcium carbonate for 3 menstrual cycles had a 48% decrease in both somatic and affective symptoms.24 Another trial demonstrated improvement in PMS symptoms of early tiredness, appetite changes, and depression with calcium therapy.25
Pyridoxine (vitamin B6) has potential benefit in treating PMS due to its ability to increase levels of serotonin, norepinephrine, histamine, dopamine, and taurine.26 An older systematic review showed benefit for symptoms associated with PMS, but the authors concluded that larger randomized controlled trials (RCTs) were needed before definitive recommendations could be made.27
Chasteberry. A number of studies have evaluated the effect of vitex agnus castus (VAC), commonly referred to as chasteberry, on PMS and PMDD symptoms. The exact mechanism of VAC is unknown, but in vitro studies show binding of VAC extracts to dopamine-2 receptors and opioid receptors, and an affinity for estrogen receptors.28
A recent meta-analysis concluded that VAC extracts are not superior to selective serotonin reuptake inhibitors (SSRIs) or oral contraceptives (OCs) for PMS/PMDD.28 The authors suggested a possible benefit of VAC compared with placebo or other nutritional supplements; however, the studies supporting its use are limited by small sample size and potential bias.
Continue to: Magnesium
Magnesium. Many small studies have evaluated the role of other herbal and nutritional supplements for the treatment of PMS/PMDD. A systematic review of studies on the effect of magnesium supplementation on anxiety and stress showed that magnesium may have a potential role in the treatment of the premenstrual symptom of anxiety.29 Other studies have demonstrated a potential role in the treatment of premenstrual migraine.30,31
Vitamin E has demonstrated benefit in the treatment of cyclic mastalgia; however, evidence for using vitamin E for mood and depressive symptoms associated with PMS and PMDD is inconsistent.32-34 Other studies involving vitamin D, St. John’s wort, black cohosh, evening primrose oil, saffron, and ginkgo biloba either showed these agents to be nonefficacious in relieving PMS/PMDD symptoms or to require more data before they can be recommended for use.34,46
Patient doesn’t respond? Start an SSRI
Pharmacotherapy with antidepressants is typically reserved for those who do not respond to nonpharmacologic therapies and are experiencing more moderate to severe symptoms of PMS or PMDD. Reduced levels of serotonin and serotonergic activity in the brain may be linked to symptoms of PMS and PMDD.47 Studies have shown SSRIs to be effective in reducing many psychological symptoms (eg, depression, anxiety, lethargy, irritability) and some physical symptoms (eg, headache, breast tenderness, muscle or joint pain) associated with PMS and PMDD.
A Cochrane review of 31 RCTs compared various SSRIs to placebo. When taken either continuously or intermittently (administration during luteal phase), SSRIs were similarly effective in relieving symptoms when compared with placebo.35 Psychological symptoms are more likely to improve with both low and moderate doses of SSRIs, while physical symptoms may only improve with moderate or higher doses. A direct comparison of the various SSRIs for the treatment of PMS or PMDD is lacking; therefore, the selection of SSRI may be based on patient characteristics and preference.
The benefits of SSRIs are noted much earlier in the treatment of PMS/PMDD than they are observed in their use for depression or anxiety.36 This suggests that the mechanism by which SSRIs relieve PMS/PMDD symptoms is different than that for depression or anxiety. Intermittent dosing capitalizes upon the rapid effect seen with these medications and the cyclical nature of these disorders. In most studies, the benefit of intermittent dosing is similar to continuous dosing; however, one meta-analysis did note that continuous dosing had a larger effect.37
Continue to: The doses of SSRIs...
The doses of SSRIs used in most PMS/PMDD trials were lower than those typically used for the treatment of depression and anxiety. The withdrawal effect that can be seen with abrupt cessation of SSRIs has not been reported in the intermittent-dosing studies for PMS/PMDD.38 While this might imply a more tolerable safety profile, the most common adverse effects reported in trials were still as expected: sleep disturbances, headache, nausea, and sexual dysfunction. It is important to note that SSRIs should be used with caution during pregnancy, and paroxetine should be avoided in women considering pregnancy in the near future.
Other antidepressant classes have been studied to a lesser extent than SSRIs. Continuously dosed venlafaxine, a serotonin and norepinephrine reuptake inhibitor, demonstrated efficacy in PMS/PMDD treatment when compared with placebo within the first cycle of therapy.39 The response seen was comparable to that associated with SSRI treatments in other trials.
Buspirone, an anxiolytic with serotonin receptor activity that is different from that of the SSRIs, demonstrated efficacy in reducing the symptom of irritability.48 Buspirone may have a role to play in those presenting with irritability as a primary symptom or in those who are unable to tolerate the adverse effects of SSRIs. Tricyclic antidepressants, bupropion, and alprazolam have either limited data regarding efficacy or are associated with adverse effects that limit their use.38
Hormonal treatments may be worth considering
One commonly prescribed hormonal therapy for PMS and PMDD is continuous OCs. A 2012 Cochrane review of OCs containing drospirenone evaluated 5 trials and a total of 1920 women.40 Two placebo-controlled trials of women with severe premenstrual symptoms (PMDD) showed improvement after 3 months of taking daily drospirenone 3 mg with ethinyl estradiol 20 mcg, compared with placebo.
While experiencing greater benefit, these groups also experienced significantly more adverse effects including nausea, intermenstrual bleeding, and breast pain. The respective odds ratios for the 3 adverse effects were 3.15 (95% confidence interval [CI], 1.90-5.22), 4.92 (95% CI, 3.03-7.96), and 2.67 (95% CI, 1.50-4.78). The review concluded that drospirenone 3 mg with ethinyl estradiol 20 mcg may help in the treatment of severe premenstrual symptoms (PMDD) but that it is unknown whether this treatment is appropriate for patients with less severe premenstrual symptoms.
Continue to: Another multicenter RCT
Another multicenter RCT evaluated women with PMDD who received levonorgestrel 90 mcg with ethinyl estradiol 20 mcg or placebo daily for 112 days.41 Symptoms were recorded utilizing the DRSP. Significantly more women taking the daily combination hormone (52%) than placebo (40%) had a positive response (≥ 50% improvement in the DRSP 7-day late luteal phase score and Clinical Global Impression of Severity score of ≥ 1 improvement, evaluated at the last “on-therapy” cycle [P = .025]). Twenty-three of 186 patients in the treatment arm dropped out because of adverse effects.
Noncontraceptive estrogen-containing preparations. Hormone therapy preparations containing lower doses of estrogen than seen in OC preparations have also been studied for PMS management. A 2017 Cochrane review of noncontraceptive estrogen-containing preparations found very low-quality evidence to support the effectiveness of continuous estrogen (transdermal patches or subcutaneous implants) plus progestogen.49
Progesterone. The cyclic use of progesterone in the luteal phase has been reviewed as a hormonal treatment for PMS. A 2012 Cochrane review of the efficacy of progesterone for PMS was inconclusive; however, route of administration, dose, and duration differed across studies.42
Another systematic review of 10 trials involving 531 women concluded that progesterone was no better than placebo in the treatment of PMS.43 However, it should be noted that each trial evaluated a different dose of progesterone, and all but 1 of the trials administered progesterone by using the calendar method to predict the beginning of the luteal phase. The only trial to use an objective confirmation of ovulation prior to beginning progesterone therapy did demonstrate significant improvement in premenstrual symptoms.
This 1985 study by Dennerstein et al44 prescribed progesterone for 10 days of each menstrual cycle starting 3 days after ovulation. In each cycle, ovulation was confirmed by determinations of urinary 24-hour pregnanediol and total estrogen concentrations. Progesterone was then prescribed during the objectively identified luteal phase, resulting in significant improvement in symptoms.
Continue to: Another study evaluated...
Another study evaluated the post-ovulatory progesterone profiles of 77 women with symptoms of PMS and found lower levels of progesterone and a sharper rate of decline in the women with PMS vs the control group.45 Subsequent progesterone treatment during the objectively identified luteal phase significantly improved PMS symptoms. These studies would seem to suggest that progesterone replacement when administered during an objectively identified luteal phase may offer some benefit in the treatment of PMS, but larger RCTs are needed to confirm this.
CASE
You provide the patient with diet and lifestyle education as well as a recommendation for calcium supplementation. The patient agrees to prospectively chart her most significant premenstrual symptoms. You review additional treatment options including SSRI medications and hormonal approaches. She is using a fertility awareness–based method of family planning that allows her to confidently identify her luteal phase. She agrees to take sertraline 50 mg/d during the luteal phase of her cycle. At her follow-up office visit 3 months later, she reports improvement in her premenstrual symptoms. Her charting of symptoms confirms this.
CORRESPONDENCE
Peter Danis, MD, Mercy Family Medicine St. Louis, 12680 Olive Boulevard, St. Louis, MO 63141; [email protected].
1. Woods NF, Most A, Dery GK. Prevalence of perimenstrual symptoms. Am J Public Health. 1982;72:1257-1264.
2. Johnson SR, McChesney C, Bean JA. Epidemiology of premenstrual symptoms in a nonclinical sample. 1. Prevalence, natural history and help-seeking behavior. J Repro Med. 1988;33:340-346.
3. Campbell EM, Peterkin D, O’Grady K, et al. Premenstrual symptoms in general practice patients. Prevalence and treatment. J Reprod Med. 1997;42:637-646.
4. O’Brien PM, Bäckström T, Brown C, et al. Towards a consensus on diagnostic criteria, measurement, and trial design of the premenstrual disorders: the ISPMD Montreal consensus. Arch Womens Ment Health. 2011;14:13-21.
5. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169:465-475.
6. American College of Obstetricians and Gynecologists. Guidelines for Women’s Health Care: A Resource Manual. 4th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014:607-613.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association, 2013.
8. Dennerstein L, Lehert P, Heinemann K. Epidemiology of premenstrual symptoms and disorders. Menopause Int. 2012;18:48-51.
9. Borenstein JE, Dean BB, Yonkers KA, et al. Using the daily record of severity of problems as a screening instrument for premenstrual syndrome. Obstet Gynecol. 2007;109:1068-1075.
10. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203-209.
11. Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9:41-49.
12. Janda C, Kues JN, Andersson G, et al. A symptom diary to assess severe premenstrual syndrome and premenstrual dysphoric disorder. Women Health. 2017;57:837-854.
13. Farasati N, Siassi F, Koohdani F, et al. Western dietary pattern is related to premenstrual syndrome: a case-control study. Brit J Nutr. 2015;114:2016-2021.
14. Purdue-Smithe AC, Manson JE, Hankinson SE, et al. A prospective study of caffeine and coffee intake and premenstrual syndrome. Am J Clin Nutr. 2016;104:499-507.
15. Bertone-Johnson ER, Hankinson SE, Bendich A, et al. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch Intern Med. 2005;165:1246-1252.
16. Chocano-Bedoya PO, Manson JE, Hankinson SE, et al. Dietary B vitamin intake and incident premenstrual syndrome. Am J Clin Nutr. 2011;93:1080-1086.
17. Prior JC, Vigna Y. Conditioning exercise and premenstrual symptoms. J Reprod Med. 1987;32:423-428.
18. Aganoff JA, Boyle GJ. Aerobic exercise, mood states, and menstrual cycle symptoms. J Psychosom Res. 1994;38:183-192.
19. El-Lithy A, El-Mazny A, Sabbour A, et al. Effect of aerobic exercise on premenstrual symptoms, haematological and hormonal parameters in young women. J Obstet Gynaecol. 2015;35:389-392.
20. Kroll-Desrosiers AR, Ronnenberg AG, Zagarins SE, et al. Recreational physical activity and premenstrual syndrome in young adult women: a cross-sectional study. PLoS One. 2017;12:1-13.
21. Jang SH, Kim DI, Choi MS. Effects and treatment methods of acupuncture and herbal medicine for premenstrual syndrome/premenstrual dysphoric disorder: systematic review. BMC Complement Altern Med. 2014;14:11.
22. Kim SY, Park HJ, Lee H, et al. Acupuncture for premenstrual syndrome: a systematic review and meta-analysis of randomized controlled trials. BJOG. 2011;118:899-915.
23. Lustyk MK, Gerrish WG, Shaver S, et al. Cognitive-behavioral therapy for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Womens Ment Health. 2009;12:85-96.
24. Thys-Jacob S, Starkey P, Bernstein D, et al. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual syndromes. Am J Obstet Gynecol. 1998;179:444-452.
25. Ghanbari Z, Haghollahi F, Shariat M, et al. Effects of calcium supplement therapy in women with premenstrual syndrome. Taiwan J Obstet Gynecol. 2009;48:124-129.
26. Girman A, Lee R, Kligler B. An integrative medicine approach to premenstrual syndrome. Am J Obstet Gynecol. 2003;188(5 suppl):s56-s65.
27. Wyatt KM, Dimmock PW, Jones PW, et al. Efficacy of vitamin B-6 in the treatment of premenstrual syndrome: systematic review. BMJ. 1999;318:1375-1381.
28. Verkaik S, Kamperman AM, van Westrhenen R, et al. The treatment of premenstrual syndrome with preparations of vitex agnus castus: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:150-166.
29. Boyle NB, Lawton C, Dye L. The effects of magnesium supplementation on subjective anxiety and stress—a systematic review. Nutrients. 2017;9:429-450.
30. Mauskop A, Altura BT, Altura BM. Serum ionized magnesium levels and serum ionized calcium/ionized magnesium ratios in women with menstrual migraine. Headache. 2002;42:242-248.
31. Facchinetti F, Sances C, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
32. Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
33. London RS, Murphy L, Kitlowski KE, et al. Efficacy of alpha-tocopherol in the treatment of the premenstrual syndrome. J Reprod Med. 1987;32:400-404.
34. Whelan AM, Jurgens TM, Naylor H. Herbs, vitamins, and minerals in the treatment of premenstrual syndrome: a systematic review. Can J Clin Pharmacol. 2009;16:e407-e429.
, , , . Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6): CD001396.
36. Dimmock P, Wyatt K, Jones P, et al. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet. 2000;356:1131-1136.
37. Shah NR, Jones JB, Aperi J, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder. Obstet Gynecol. 2008;111:1175-1182.
38. Freeman EW. Luteal phase administration of agents for the treatment of premenstrual dysphoric disorder. CNS Drugs. 2004;18:453-468.
39. Freeman EW, Rickels K, Yonkers KA, et al. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet Gynecol. 2001;98:737-744.
40. Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(2):CD006586.
41. Halbreich U, Freeman EW, Rapkin AJ, et al. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. 2012;85:19-27.
, , , . Progesterone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(3):CD003415.
43. Wyatt K, Dimmock P, Jones P, et al. Efficacy of progesterone and progestogens in management of premenstrual syndrome: systematic review. BMJ. 2001;323: 776-780.
44. Dennerstein L, Spencer-Gardner C, Gotts G, et al. Progesterone and the premenstrual syndrome: a double-blind crossover trial. Br Med J (Clin Res Ed). 1985;290:1617-1621.
45. NaProTECHNOLOGY. The Medical and Surgical Practice of NaProTECHNOLOGY. Premenstrual Syndrome: Evaluation and Treatment. Omaha, NE: Pope Paul VI Institute Press. 2004;29:345-368. https://www.naprotechnology.com/naprotext.htm. Accessed January 23, 2020.
46. Dante G, Facchinetti F. Herbal treatments for alleviating premenstrual symptoms: a systematic review. J Psychosom Obstet Gynaecol. 2011;32:42-51.
47. Jarvis CI, Lynch AM, Morin AK. Management strategies for premenstrual syndrome/premenstrual dysphoric disorder. Ann Pharmacother. 2008;42:967-978.
48. Landen M, Eriksson O, Sundblad C, et al. Compounds with affinity for serotonergic receptors in the treatment of premenstrual dysphoria: a comparison of buspirone, nefazodone and placebo. Psychopharmacology (Berl). 2001;155:292-298.
, , , . Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome . Cochrane Database Syst Rev . 2017 ;( 3) :CD010503.
CASE
A 30-year-old G2P2 woman presents for a well-woman visit and reports 6 months of premenstrual symptoms including irritability, depression, breast pain, and headaches. She is not taking any medications or hormonal contraceptives. She is sexually active and currently not interested in becoming pregnant. She asks what you can do for her symptoms, as they are affecting her life at home and at work.
Symptoms and definitions vary
Although more than 150 premenstrual symptoms have been reported, the most common psychological and behavioral ones are mood swings, depression, anxiety, irritability, crying, social withdrawal, forgetfulness, and problems concentrating.1-3 The most common physical symptoms are fatigue, abdominal bloating, weight gain, breast tenderness, acne, change in appetite or food cravings, edema, headache, and gastrointestinal upset. The etiology of these symptoms is usually multifactorial, with some combination of hormonal, neurotransmitter, lifestyle, environmental, and psychosocial factors playing a role.
Premenstrual disorder. In reviewing diagnostic criteria for the various premenstrual syndromes and disorders from different organizations (eg, the International Society for Premenstrual Disorders; the American College of Obstetricians and Gynecologists; the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition), there is agreement on the following criteria for premenstrual syndrome (PMS)4-6:
- The woman must be ovulating. (Women who no longer menstruate [eg, because of hysterectomy or endometrial ablation] can have premenstrual disorders as long as ovarian function remains intact.)
- The woman experiences a constellation of disabling physical and/or psychological symptoms that appears in the luteal phase of her menstrual cycle.
- The symptoms improve soon after the onset of menses.
- There is a symptom-free interval before ovulation.
- There is prospective documentation of symptoms for at least 2 consecutive cycles.
- The symptoms are sufficient in severity to affect activities of daily living and/or important relationships.
Premenstrual dysphoric disorder. PMDD is another common premenstrual disorder. It is distinguished by significant premenstrual psychological symptoms and requires the presence of marked affective lability, marked irritability or anger, markedly depressed mood, and/or marked anxiety (TABLE 1).7
Exacerbation of other ailments. Another premenstrual disorder is the premenstrual exacerbation of underlying chronic medical or psychological problems such as migraines, seizures, asthma, diabetes, irritable bowel syndrome, fibromyalgia, anxiety, or depression.
Differences in interpretation lead to variations in prevalence
Differences in the interpretation of significant premenstrual symptoms have led to variations in estimated prevalence. For example, 80% to 95% of women report premenstrual symptoms, but only 30% to 40% meet criteria for PMS and only 3% to 8% meet criteria for PMDD.8 Many women who report premenstrual symptoms in a retrospective questionnaire do not meet criteria for PMS or PMDD based on prospective symptom charting. The Daily Record of Severity of Problems (DRSP), a prospective tracking tool for premenstrual symptoms, is sensitive and specific for diagnosing PMS and PMDD if administered on the first day of menstruation.9
Ask about symptoms and use a tracking tool
When you see a woman for a well-woman visit or a gynecologic problem, inquire about physical/emotional symptoms and their severity during the week that precedes menstruation. If a patient reports few symptoms of a mild nature, then no further work-up is needed.
Continue to: If patients report significant...
If patients report significant premenstrual symptoms, recommend the use of a tool to track the symptoms. Older tools such as the DRSP and the Premenstrual Symptoms Screening Tool (PSST), newer symptom diaries that can be used for both PMS and PMDD,and questionnaires that have been used in research situations can be time consuming and difficult for patients to complete.10-12 Instead, physicians can easily construct their own charting tool, as we did for patients to use when tracking their most bothersome symptoms (FIGURE 1). Tracking helps to confirm the diagnosis and helps you and the patient focus on treatment goals.
Keep in mind other diagnoses (eg, anemia, thyroid disorders, perimenopause, anxiety, depression, eating disorders, substance abuse) that can cause or exacerbate the psychological/physical symptoms the patient is reporting. If you suspect any of these other diagnoses, laboratory evaluation (eg, complete blood count, thyroid-stimulating hormone level or other hormonal testing, urine drug screen, etc) may be warranted to rule out other etiologies for the reported symptoms.
Develop a Tx plan that considers symptoms, family-planning needs
Focus treatment on the patient’s predominant symptoms whether they are physical, psychological, or mixed (FIGURE 2). The patient’s preferences regarding family planning are another important consideration. Women who are using a fertility awareness
Although the definitions for PMS and PMDD require at least 2 cycles of prospective documentation of symptoms, dietary and lifestyle changes can begin immediately. Regular follow-up to document improvement of symptoms is important; using the patient’s symptoms charting tool can help with this.
Focus on diet and lifestyle right away
Experts in the field of PMS/PMDD suggest that simple dietary changes may be a reasonable first step to help improve symptoms. Researchers have found that diets high in fiber, vegetables, and whole grains are inversely related to PMS.13 Older studies have suggested an increased prevalence and severity of PMS with increased caffeine intake; however, a newer study found no such association.14
Continue to: A case-control study nested...
A case-control study nested within the Nurses’ Health Study II cohort showed that a high intake of both dietary calcium and vitamin D prevented the development of PMS in women ages 27 to 44.15 B vitamins, such as thiamine and riboflavin, from food sources have been associated with a lower risk of PMS.16 A variety of older clinical studies showed benefit from aerobic exercise on PMS symptoms,17-19 but a newer cross-sectional study of young adult women found no association between physical activity and the prevalence of PMS.20 Acupuncture has demonstrated efficacy for the treatment of the physical symptoms of PMS and PMDD, but more rigorous studies are needed.21,22 Cognitive behavioral therapy has been studied as a treatment, but data to support this approach are limited so it cannot be recommended at this time.23
Make the most of supplements—especially calcium
Calcium is the nutritional supplement with the most evidence to support its use to relieve symptoms of PMS and PMDD (TABLE 221,22,24-45). Research indicates that disturbances in calcium regulation and calcium deficiency may be responsible for various premenstrual symptoms. One study showed that, compared with placebo, women who took 1200 mg/d calcium carbonate for 3 menstrual cycles had a 48% decrease in both somatic and affective symptoms.24 Another trial demonstrated improvement in PMS symptoms of early tiredness, appetite changes, and depression with calcium therapy.25
Pyridoxine (vitamin B6) has potential benefit in treating PMS due to its ability to increase levels of serotonin, norepinephrine, histamine, dopamine, and taurine.26 An older systematic review showed benefit for symptoms associated with PMS, but the authors concluded that larger randomized controlled trials (RCTs) were needed before definitive recommendations could be made.27
Chasteberry. A number of studies have evaluated the effect of vitex agnus castus (VAC), commonly referred to as chasteberry, on PMS and PMDD symptoms. The exact mechanism of VAC is unknown, but in vitro studies show binding of VAC extracts to dopamine-2 receptors and opioid receptors, and an affinity for estrogen receptors.28
A recent meta-analysis concluded that VAC extracts are not superior to selective serotonin reuptake inhibitors (SSRIs) or oral contraceptives (OCs) for PMS/PMDD.28 The authors suggested a possible benefit of VAC compared with placebo or other nutritional supplements; however, the studies supporting its use are limited by small sample size and potential bias.
Continue to: Magnesium
Magnesium. Many small studies have evaluated the role of other herbal and nutritional supplements for the treatment of PMS/PMDD. A systematic review of studies on the effect of magnesium supplementation on anxiety and stress showed that magnesium may have a potential role in the treatment of the premenstrual symptom of anxiety.29 Other studies have demonstrated a potential role in the treatment of premenstrual migraine.30,31
Vitamin E has demonstrated benefit in the treatment of cyclic mastalgia; however, evidence for using vitamin E for mood and depressive symptoms associated with PMS and PMDD is inconsistent.32-34 Other studies involving vitamin D, St. John’s wort, black cohosh, evening primrose oil, saffron, and ginkgo biloba either showed these agents to be nonefficacious in relieving PMS/PMDD symptoms or to require more data before they can be recommended for use.34,46
Patient doesn’t respond? Start an SSRI
Pharmacotherapy with antidepressants is typically reserved for those who do not respond to nonpharmacologic therapies and are experiencing more moderate to severe symptoms of PMS or PMDD. Reduced levels of serotonin and serotonergic activity in the brain may be linked to symptoms of PMS and PMDD.47 Studies have shown SSRIs to be effective in reducing many psychological symptoms (eg, depression, anxiety, lethargy, irritability) and some physical symptoms (eg, headache, breast tenderness, muscle or joint pain) associated with PMS and PMDD.
A Cochrane review of 31 RCTs compared various SSRIs to placebo. When taken either continuously or intermittently (administration during luteal phase), SSRIs were similarly effective in relieving symptoms when compared with placebo.35 Psychological symptoms are more likely to improve with both low and moderate doses of SSRIs, while physical symptoms may only improve with moderate or higher doses. A direct comparison of the various SSRIs for the treatment of PMS or PMDD is lacking; therefore, the selection of SSRI may be based on patient characteristics and preference.
The benefits of SSRIs are noted much earlier in the treatment of PMS/PMDD than they are observed in their use for depression or anxiety.36 This suggests that the mechanism by which SSRIs relieve PMS/PMDD symptoms is different than that for depression or anxiety. Intermittent dosing capitalizes upon the rapid effect seen with these medications and the cyclical nature of these disorders. In most studies, the benefit of intermittent dosing is similar to continuous dosing; however, one meta-analysis did note that continuous dosing had a larger effect.37
Continue to: The doses of SSRIs...
The doses of SSRIs used in most PMS/PMDD trials were lower than those typically used for the treatment of depression and anxiety. The withdrawal effect that can be seen with abrupt cessation of SSRIs has not been reported in the intermittent-dosing studies for PMS/PMDD.38 While this might imply a more tolerable safety profile, the most common adverse effects reported in trials were still as expected: sleep disturbances, headache, nausea, and sexual dysfunction. It is important to note that SSRIs should be used with caution during pregnancy, and paroxetine should be avoided in women considering pregnancy in the near future.
Other antidepressant classes have been studied to a lesser extent than SSRIs. Continuously dosed venlafaxine, a serotonin and norepinephrine reuptake inhibitor, demonstrated efficacy in PMS/PMDD treatment when compared with placebo within the first cycle of therapy.39 The response seen was comparable to that associated with SSRI treatments in other trials.
Buspirone, an anxiolytic with serotonin receptor activity that is different from that of the SSRIs, demonstrated efficacy in reducing the symptom of irritability.48 Buspirone may have a role to play in those presenting with irritability as a primary symptom or in those who are unable to tolerate the adverse effects of SSRIs. Tricyclic antidepressants, bupropion, and alprazolam have either limited data regarding efficacy or are associated with adverse effects that limit their use.38
Hormonal treatments may be worth considering
One commonly prescribed hormonal therapy for PMS and PMDD is continuous OCs. A 2012 Cochrane review of OCs containing drospirenone evaluated 5 trials and a total of 1920 women.40 Two placebo-controlled trials of women with severe premenstrual symptoms (PMDD) showed improvement after 3 months of taking daily drospirenone 3 mg with ethinyl estradiol 20 mcg, compared with placebo.
While experiencing greater benefit, these groups also experienced significantly more adverse effects including nausea, intermenstrual bleeding, and breast pain. The respective odds ratios for the 3 adverse effects were 3.15 (95% confidence interval [CI], 1.90-5.22), 4.92 (95% CI, 3.03-7.96), and 2.67 (95% CI, 1.50-4.78). The review concluded that drospirenone 3 mg with ethinyl estradiol 20 mcg may help in the treatment of severe premenstrual symptoms (PMDD) but that it is unknown whether this treatment is appropriate for patients with less severe premenstrual symptoms.
Continue to: Another multicenter RCT
Another multicenter RCT evaluated women with PMDD who received levonorgestrel 90 mcg with ethinyl estradiol 20 mcg or placebo daily for 112 days.41 Symptoms were recorded utilizing the DRSP. Significantly more women taking the daily combination hormone (52%) than placebo (40%) had a positive response (≥ 50% improvement in the DRSP 7-day late luteal phase score and Clinical Global Impression of Severity score of ≥ 1 improvement, evaluated at the last “on-therapy” cycle [P = .025]). Twenty-three of 186 patients in the treatment arm dropped out because of adverse effects.
Noncontraceptive estrogen-containing preparations. Hormone therapy preparations containing lower doses of estrogen than seen in OC preparations have also been studied for PMS management. A 2017 Cochrane review of noncontraceptive estrogen-containing preparations found very low-quality evidence to support the effectiveness of continuous estrogen (transdermal patches or subcutaneous implants) plus progestogen.49
Progesterone. The cyclic use of progesterone in the luteal phase has been reviewed as a hormonal treatment for PMS. A 2012 Cochrane review of the efficacy of progesterone for PMS was inconclusive; however, route of administration, dose, and duration differed across studies.42
Another systematic review of 10 trials involving 531 women concluded that progesterone was no better than placebo in the treatment of PMS.43 However, it should be noted that each trial evaluated a different dose of progesterone, and all but 1 of the trials administered progesterone by using the calendar method to predict the beginning of the luteal phase. The only trial to use an objective confirmation of ovulation prior to beginning progesterone therapy did demonstrate significant improvement in premenstrual symptoms.
This 1985 study by Dennerstein et al44 prescribed progesterone for 10 days of each menstrual cycle starting 3 days after ovulation. In each cycle, ovulation was confirmed by determinations of urinary 24-hour pregnanediol and total estrogen concentrations. Progesterone was then prescribed during the objectively identified luteal phase, resulting in significant improvement in symptoms.
Continue to: Another study evaluated...
Another study evaluated the post-ovulatory progesterone profiles of 77 women with symptoms of PMS and found lower levels of progesterone and a sharper rate of decline in the women with PMS vs the control group.45 Subsequent progesterone treatment during the objectively identified luteal phase significantly improved PMS symptoms. These studies would seem to suggest that progesterone replacement when administered during an objectively identified luteal phase may offer some benefit in the treatment of PMS, but larger RCTs are needed to confirm this.
CASE
You provide the patient with diet and lifestyle education as well as a recommendation for calcium supplementation. The patient agrees to prospectively chart her most significant premenstrual symptoms. You review additional treatment options including SSRI medications and hormonal approaches. She is using a fertility awareness–based method of family planning that allows her to confidently identify her luteal phase. She agrees to take sertraline 50 mg/d during the luteal phase of her cycle. At her follow-up office visit 3 months later, she reports improvement in her premenstrual symptoms. Her charting of symptoms confirms this.
CORRESPONDENCE
Peter Danis, MD, Mercy Family Medicine St. Louis, 12680 Olive Boulevard, St. Louis, MO 63141; [email protected].
CASE
A 30-year-old G2P2 woman presents for a well-woman visit and reports 6 months of premenstrual symptoms including irritability, depression, breast pain, and headaches. She is not taking any medications or hormonal contraceptives. She is sexually active and currently not interested in becoming pregnant. She asks what you can do for her symptoms, as they are affecting her life at home and at work.
Symptoms and definitions vary
Although more than 150 premenstrual symptoms have been reported, the most common psychological and behavioral ones are mood swings, depression, anxiety, irritability, crying, social withdrawal, forgetfulness, and problems concentrating.1-3 The most common physical symptoms are fatigue, abdominal bloating, weight gain, breast tenderness, acne, change in appetite or food cravings, edema, headache, and gastrointestinal upset. The etiology of these symptoms is usually multifactorial, with some combination of hormonal, neurotransmitter, lifestyle, environmental, and psychosocial factors playing a role.
Premenstrual disorder. In reviewing diagnostic criteria for the various premenstrual syndromes and disorders from different organizations (eg, the International Society for Premenstrual Disorders; the American College of Obstetricians and Gynecologists; the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition), there is agreement on the following criteria for premenstrual syndrome (PMS)4-6:
- The woman must be ovulating. (Women who no longer menstruate [eg, because of hysterectomy or endometrial ablation] can have premenstrual disorders as long as ovarian function remains intact.)
- The woman experiences a constellation of disabling physical and/or psychological symptoms that appears in the luteal phase of her menstrual cycle.
- The symptoms improve soon after the onset of menses.
- There is a symptom-free interval before ovulation.
- There is prospective documentation of symptoms for at least 2 consecutive cycles.
- The symptoms are sufficient in severity to affect activities of daily living and/or important relationships.
Premenstrual dysphoric disorder. PMDD is another common premenstrual disorder. It is distinguished by significant premenstrual psychological symptoms and requires the presence of marked affective lability, marked irritability or anger, markedly depressed mood, and/or marked anxiety (TABLE 1).7
Exacerbation of other ailments. Another premenstrual disorder is the premenstrual exacerbation of underlying chronic medical or psychological problems such as migraines, seizures, asthma, diabetes, irritable bowel syndrome, fibromyalgia, anxiety, or depression.
Differences in interpretation lead to variations in prevalence
Differences in the interpretation of significant premenstrual symptoms have led to variations in estimated prevalence. For example, 80% to 95% of women report premenstrual symptoms, but only 30% to 40% meet criteria for PMS and only 3% to 8% meet criteria for PMDD.8 Many women who report premenstrual symptoms in a retrospective questionnaire do not meet criteria for PMS or PMDD based on prospective symptom charting. The Daily Record of Severity of Problems (DRSP), a prospective tracking tool for premenstrual symptoms, is sensitive and specific for diagnosing PMS and PMDD if administered on the first day of menstruation.9
Ask about symptoms and use a tracking tool
When you see a woman for a well-woman visit or a gynecologic problem, inquire about physical/emotional symptoms and their severity during the week that precedes menstruation. If a patient reports few symptoms of a mild nature, then no further work-up is needed.
Continue to: If patients report significant...
If patients report significant premenstrual symptoms, recommend the use of a tool to track the symptoms. Older tools such as the DRSP and the Premenstrual Symptoms Screening Tool (PSST), newer symptom diaries that can be used for both PMS and PMDD,and questionnaires that have been used in research situations can be time consuming and difficult for patients to complete.10-12 Instead, physicians can easily construct their own charting tool, as we did for patients to use when tracking their most bothersome symptoms (FIGURE 1). Tracking helps to confirm the diagnosis and helps you and the patient focus on treatment goals.
Keep in mind other diagnoses (eg, anemia, thyroid disorders, perimenopause, anxiety, depression, eating disorders, substance abuse) that can cause or exacerbate the psychological/physical symptoms the patient is reporting. If you suspect any of these other diagnoses, laboratory evaluation (eg, complete blood count, thyroid-stimulating hormone level or other hormonal testing, urine drug screen, etc) may be warranted to rule out other etiologies for the reported symptoms.
Develop a Tx plan that considers symptoms, family-planning needs
Focus treatment on the patient’s predominant symptoms whether they are physical, psychological, or mixed (FIGURE 2). The patient’s preferences regarding family planning are another important consideration. Women who are using a fertility awareness
Although the definitions for PMS and PMDD require at least 2 cycles of prospective documentation of symptoms, dietary and lifestyle changes can begin immediately. Regular follow-up to document improvement of symptoms is important; using the patient’s symptoms charting tool can help with this.
Focus on diet and lifestyle right away
Experts in the field of PMS/PMDD suggest that simple dietary changes may be a reasonable first step to help improve symptoms. Researchers have found that diets high in fiber, vegetables, and whole grains are inversely related to PMS.13 Older studies have suggested an increased prevalence and severity of PMS with increased caffeine intake; however, a newer study found no such association.14
Continue to: A case-control study nested...
A case-control study nested within the Nurses’ Health Study II cohort showed that a high intake of both dietary calcium and vitamin D prevented the development of PMS in women ages 27 to 44.15 B vitamins, such as thiamine and riboflavin, from food sources have been associated with a lower risk of PMS.16 A variety of older clinical studies showed benefit from aerobic exercise on PMS symptoms,17-19 but a newer cross-sectional study of young adult women found no association between physical activity and the prevalence of PMS.20 Acupuncture has demonstrated efficacy for the treatment of the physical symptoms of PMS and PMDD, but more rigorous studies are needed.21,22 Cognitive behavioral therapy has been studied as a treatment, but data to support this approach are limited so it cannot be recommended at this time.23
Make the most of supplements—especially calcium
Calcium is the nutritional supplement with the most evidence to support its use to relieve symptoms of PMS and PMDD (TABLE 221,22,24-45). Research indicates that disturbances in calcium regulation and calcium deficiency may be responsible for various premenstrual symptoms. One study showed that, compared with placebo, women who took 1200 mg/d calcium carbonate for 3 menstrual cycles had a 48% decrease in both somatic and affective symptoms.24 Another trial demonstrated improvement in PMS symptoms of early tiredness, appetite changes, and depression with calcium therapy.25
Pyridoxine (vitamin B6) has potential benefit in treating PMS due to its ability to increase levels of serotonin, norepinephrine, histamine, dopamine, and taurine.26 An older systematic review showed benefit for symptoms associated with PMS, but the authors concluded that larger randomized controlled trials (RCTs) were needed before definitive recommendations could be made.27
Chasteberry. A number of studies have evaluated the effect of vitex agnus castus (VAC), commonly referred to as chasteberry, on PMS and PMDD symptoms. The exact mechanism of VAC is unknown, but in vitro studies show binding of VAC extracts to dopamine-2 receptors and opioid receptors, and an affinity for estrogen receptors.28
A recent meta-analysis concluded that VAC extracts are not superior to selective serotonin reuptake inhibitors (SSRIs) or oral contraceptives (OCs) for PMS/PMDD.28 The authors suggested a possible benefit of VAC compared with placebo or other nutritional supplements; however, the studies supporting its use are limited by small sample size and potential bias.
Continue to: Magnesium
Magnesium. Many small studies have evaluated the role of other herbal and nutritional supplements for the treatment of PMS/PMDD. A systematic review of studies on the effect of magnesium supplementation on anxiety and stress showed that magnesium may have a potential role in the treatment of the premenstrual symptom of anxiety.29 Other studies have demonstrated a potential role in the treatment of premenstrual migraine.30,31
Vitamin E has demonstrated benefit in the treatment of cyclic mastalgia; however, evidence for using vitamin E for mood and depressive symptoms associated with PMS and PMDD is inconsistent.32-34 Other studies involving vitamin D, St. John’s wort, black cohosh, evening primrose oil, saffron, and ginkgo biloba either showed these agents to be nonefficacious in relieving PMS/PMDD symptoms or to require more data before they can be recommended for use.34,46
Patient doesn’t respond? Start an SSRI
Pharmacotherapy with antidepressants is typically reserved for those who do not respond to nonpharmacologic therapies and are experiencing more moderate to severe symptoms of PMS or PMDD. Reduced levels of serotonin and serotonergic activity in the brain may be linked to symptoms of PMS and PMDD.47 Studies have shown SSRIs to be effective in reducing many psychological symptoms (eg, depression, anxiety, lethargy, irritability) and some physical symptoms (eg, headache, breast tenderness, muscle or joint pain) associated with PMS and PMDD.
A Cochrane review of 31 RCTs compared various SSRIs to placebo. When taken either continuously or intermittently (administration during luteal phase), SSRIs were similarly effective in relieving symptoms when compared with placebo.35 Psychological symptoms are more likely to improve with both low and moderate doses of SSRIs, while physical symptoms may only improve with moderate or higher doses. A direct comparison of the various SSRIs for the treatment of PMS or PMDD is lacking; therefore, the selection of SSRI may be based on patient characteristics and preference.
The benefits of SSRIs are noted much earlier in the treatment of PMS/PMDD than they are observed in their use for depression or anxiety.36 This suggests that the mechanism by which SSRIs relieve PMS/PMDD symptoms is different than that for depression or anxiety. Intermittent dosing capitalizes upon the rapid effect seen with these medications and the cyclical nature of these disorders. In most studies, the benefit of intermittent dosing is similar to continuous dosing; however, one meta-analysis did note that continuous dosing had a larger effect.37
Continue to: The doses of SSRIs...
The doses of SSRIs used in most PMS/PMDD trials were lower than those typically used for the treatment of depression and anxiety. The withdrawal effect that can be seen with abrupt cessation of SSRIs has not been reported in the intermittent-dosing studies for PMS/PMDD.38 While this might imply a more tolerable safety profile, the most common adverse effects reported in trials were still as expected: sleep disturbances, headache, nausea, and sexual dysfunction. It is important to note that SSRIs should be used with caution during pregnancy, and paroxetine should be avoided in women considering pregnancy in the near future.
Other antidepressant classes have been studied to a lesser extent than SSRIs. Continuously dosed venlafaxine, a serotonin and norepinephrine reuptake inhibitor, demonstrated efficacy in PMS/PMDD treatment when compared with placebo within the first cycle of therapy.39 The response seen was comparable to that associated with SSRI treatments in other trials.
Buspirone, an anxiolytic with serotonin receptor activity that is different from that of the SSRIs, demonstrated efficacy in reducing the symptom of irritability.48 Buspirone may have a role to play in those presenting with irritability as a primary symptom or in those who are unable to tolerate the adverse effects of SSRIs. Tricyclic antidepressants, bupropion, and alprazolam have either limited data regarding efficacy or are associated with adverse effects that limit their use.38
Hormonal treatments may be worth considering
One commonly prescribed hormonal therapy for PMS and PMDD is continuous OCs. A 2012 Cochrane review of OCs containing drospirenone evaluated 5 trials and a total of 1920 women.40 Two placebo-controlled trials of women with severe premenstrual symptoms (PMDD) showed improvement after 3 months of taking daily drospirenone 3 mg with ethinyl estradiol 20 mcg, compared with placebo.
While experiencing greater benefit, these groups also experienced significantly more adverse effects including nausea, intermenstrual bleeding, and breast pain. The respective odds ratios for the 3 adverse effects were 3.15 (95% confidence interval [CI], 1.90-5.22), 4.92 (95% CI, 3.03-7.96), and 2.67 (95% CI, 1.50-4.78). The review concluded that drospirenone 3 mg with ethinyl estradiol 20 mcg may help in the treatment of severe premenstrual symptoms (PMDD) but that it is unknown whether this treatment is appropriate for patients with less severe premenstrual symptoms.
Continue to: Another multicenter RCT
Another multicenter RCT evaluated women with PMDD who received levonorgestrel 90 mcg with ethinyl estradiol 20 mcg or placebo daily for 112 days.41 Symptoms were recorded utilizing the DRSP. Significantly more women taking the daily combination hormone (52%) than placebo (40%) had a positive response (≥ 50% improvement in the DRSP 7-day late luteal phase score and Clinical Global Impression of Severity score of ≥ 1 improvement, evaluated at the last “on-therapy” cycle [P = .025]). Twenty-three of 186 patients in the treatment arm dropped out because of adverse effects.
Noncontraceptive estrogen-containing preparations. Hormone therapy preparations containing lower doses of estrogen than seen in OC preparations have also been studied for PMS management. A 2017 Cochrane review of noncontraceptive estrogen-containing preparations found very low-quality evidence to support the effectiveness of continuous estrogen (transdermal patches or subcutaneous implants) plus progestogen.49
Progesterone. The cyclic use of progesterone in the luteal phase has been reviewed as a hormonal treatment for PMS. A 2012 Cochrane review of the efficacy of progesterone for PMS was inconclusive; however, route of administration, dose, and duration differed across studies.42
Another systematic review of 10 trials involving 531 women concluded that progesterone was no better than placebo in the treatment of PMS.43 However, it should be noted that each trial evaluated a different dose of progesterone, and all but 1 of the trials administered progesterone by using the calendar method to predict the beginning of the luteal phase. The only trial to use an objective confirmation of ovulation prior to beginning progesterone therapy did demonstrate significant improvement in premenstrual symptoms.
This 1985 study by Dennerstein et al44 prescribed progesterone for 10 days of each menstrual cycle starting 3 days after ovulation. In each cycle, ovulation was confirmed by determinations of urinary 24-hour pregnanediol and total estrogen concentrations. Progesterone was then prescribed during the objectively identified luteal phase, resulting in significant improvement in symptoms.
Continue to: Another study evaluated...
Another study evaluated the post-ovulatory progesterone profiles of 77 women with symptoms of PMS and found lower levels of progesterone and a sharper rate of decline in the women with PMS vs the control group.45 Subsequent progesterone treatment during the objectively identified luteal phase significantly improved PMS symptoms. These studies would seem to suggest that progesterone replacement when administered during an objectively identified luteal phase may offer some benefit in the treatment of PMS, but larger RCTs are needed to confirm this.
CASE
You provide the patient with diet and lifestyle education as well as a recommendation for calcium supplementation. The patient agrees to prospectively chart her most significant premenstrual symptoms. You review additional treatment options including SSRI medications and hormonal approaches. She is using a fertility awareness–based method of family planning that allows her to confidently identify her luteal phase. She agrees to take sertraline 50 mg/d during the luteal phase of her cycle. At her follow-up office visit 3 months later, she reports improvement in her premenstrual symptoms. Her charting of symptoms confirms this.
CORRESPONDENCE
Peter Danis, MD, Mercy Family Medicine St. Louis, 12680 Olive Boulevard, St. Louis, MO 63141; [email protected].
1. Woods NF, Most A, Dery GK. Prevalence of perimenstrual symptoms. Am J Public Health. 1982;72:1257-1264.
2. Johnson SR, McChesney C, Bean JA. Epidemiology of premenstrual symptoms in a nonclinical sample. 1. Prevalence, natural history and help-seeking behavior. J Repro Med. 1988;33:340-346.
3. Campbell EM, Peterkin D, O’Grady K, et al. Premenstrual symptoms in general practice patients. Prevalence and treatment. J Reprod Med. 1997;42:637-646.
4. O’Brien PM, Bäckström T, Brown C, et al. Towards a consensus on diagnostic criteria, measurement, and trial design of the premenstrual disorders: the ISPMD Montreal consensus. Arch Womens Ment Health. 2011;14:13-21.
5. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169:465-475.
6. American College of Obstetricians and Gynecologists. Guidelines for Women’s Health Care: A Resource Manual. 4th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014:607-613.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association, 2013.
8. Dennerstein L, Lehert P, Heinemann K. Epidemiology of premenstrual symptoms and disorders. Menopause Int. 2012;18:48-51.
9. Borenstein JE, Dean BB, Yonkers KA, et al. Using the daily record of severity of problems as a screening instrument for premenstrual syndrome. Obstet Gynecol. 2007;109:1068-1075.
10. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203-209.
11. Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9:41-49.
12. Janda C, Kues JN, Andersson G, et al. A symptom diary to assess severe premenstrual syndrome and premenstrual dysphoric disorder. Women Health. 2017;57:837-854.
13. Farasati N, Siassi F, Koohdani F, et al. Western dietary pattern is related to premenstrual syndrome: a case-control study. Brit J Nutr. 2015;114:2016-2021.
14. Purdue-Smithe AC, Manson JE, Hankinson SE, et al. A prospective study of caffeine and coffee intake and premenstrual syndrome. Am J Clin Nutr. 2016;104:499-507.
15. Bertone-Johnson ER, Hankinson SE, Bendich A, et al. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch Intern Med. 2005;165:1246-1252.
16. Chocano-Bedoya PO, Manson JE, Hankinson SE, et al. Dietary B vitamin intake and incident premenstrual syndrome. Am J Clin Nutr. 2011;93:1080-1086.
17. Prior JC, Vigna Y. Conditioning exercise and premenstrual symptoms. J Reprod Med. 1987;32:423-428.
18. Aganoff JA, Boyle GJ. Aerobic exercise, mood states, and menstrual cycle symptoms. J Psychosom Res. 1994;38:183-192.
19. El-Lithy A, El-Mazny A, Sabbour A, et al. Effect of aerobic exercise on premenstrual symptoms, haematological and hormonal parameters in young women. J Obstet Gynaecol. 2015;35:389-392.
20. Kroll-Desrosiers AR, Ronnenberg AG, Zagarins SE, et al. Recreational physical activity and premenstrual syndrome in young adult women: a cross-sectional study. PLoS One. 2017;12:1-13.
21. Jang SH, Kim DI, Choi MS. Effects and treatment methods of acupuncture and herbal medicine for premenstrual syndrome/premenstrual dysphoric disorder: systematic review. BMC Complement Altern Med. 2014;14:11.
22. Kim SY, Park HJ, Lee H, et al. Acupuncture for premenstrual syndrome: a systematic review and meta-analysis of randomized controlled trials. BJOG. 2011;118:899-915.
23. Lustyk MK, Gerrish WG, Shaver S, et al. Cognitive-behavioral therapy for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Womens Ment Health. 2009;12:85-96.
24. Thys-Jacob S, Starkey P, Bernstein D, et al. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual syndromes. Am J Obstet Gynecol. 1998;179:444-452.
25. Ghanbari Z, Haghollahi F, Shariat M, et al. Effects of calcium supplement therapy in women with premenstrual syndrome. Taiwan J Obstet Gynecol. 2009;48:124-129.
26. Girman A, Lee R, Kligler B. An integrative medicine approach to premenstrual syndrome. Am J Obstet Gynecol. 2003;188(5 suppl):s56-s65.
27. Wyatt KM, Dimmock PW, Jones PW, et al. Efficacy of vitamin B-6 in the treatment of premenstrual syndrome: systematic review. BMJ. 1999;318:1375-1381.
28. Verkaik S, Kamperman AM, van Westrhenen R, et al. The treatment of premenstrual syndrome with preparations of vitex agnus castus: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:150-166.
29. Boyle NB, Lawton C, Dye L. The effects of magnesium supplementation on subjective anxiety and stress—a systematic review. Nutrients. 2017;9:429-450.
30. Mauskop A, Altura BT, Altura BM. Serum ionized magnesium levels and serum ionized calcium/ionized magnesium ratios in women with menstrual migraine. Headache. 2002;42:242-248.
31. Facchinetti F, Sances C, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
32. Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
33. London RS, Murphy L, Kitlowski KE, et al. Efficacy of alpha-tocopherol in the treatment of the premenstrual syndrome. J Reprod Med. 1987;32:400-404.
34. Whelan AM, Jurgens TM, Naylor H. Herbs, vitamins, and minerals in the treatment of premenstrual syndrome: a systematic review. Can J Clin Pharmacol. 2009;16:e407-e429.
, , , . Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6): CD001396.
36. Dimmock P, Wyatt K, Jones P, et al. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet. 2000;356:1131-1136.
37. Shah NR, Jones JB, Aperi J, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder. Obstet Gynecol. 2008;111:1175-1182.
38. Freeman EW. Luteal phase administration of agents for the treatment of premenstrual dysphoric disorder. CNS Drugs. 2004;18:453-468.
39. Freeman EW, Rickels K, Yonkers KA, et al. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet Gynecol. 2001;98:737-744.
40. Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(2):CD006586.
41. Halbreich U, Freeman EW, Rapkin AJ, et al. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. 2012;85:19-27.
, , , . Progesterone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(3):CD003415.
43. Wyatt K, Dimmock P, Jones P, et al. Efficacy of progesterone and progestogens in management of premenstrual syndrome: systematic review. BMJ. 2001;323: 776-780.
44. Dennerstein L, Spencer-Gardner C, Gotts G, et al. Progesterone and the premenstrual syndrome: a double-blind crossover trial. Br Med J (Clin Res Ed). 1985;290:1617-1621.
45. NaProTECHNOLOGY. The Medical and Surgical Practice of NaProTECHNOLOGY. Premenstrual Syndrome: Evaluation and Treatment. Omaha, NE: Pope Paul VI Institute Press. 2004;29:345-368. https://www.naprotechnology.com/naprotext.htm. Accessed January 23, 2020.
46. Dante G, Facchinetti F. Herbal treatments for alleviating premenstrual symptoms: a systematic review. J Psychosom Obstet Gynaecol. 2011;32:42-51.
47. Jarvis CI, Lynch AM, Morin AK. Management strategies for premenstrual syndrome/premenstrual dysphoric disorder. Ann Pharmacother. 2008;42:967-978.
48. Landen M, Eriksson O, Sundblad C, et al. Compounds with affinity for serotonergic receptors in the treatment of premenstrual dysphoria: a comparison of buspirone, nefazodone and placebo. Psychopharmacology (Berl). 2001;155:292-298.
, , , . Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome . Cochrane Database Syst Rev . 2017 ;( 3) :CD010503.
1. Woods NF, Most A, Dery GK. Prevalence of perimenstrual symptoms. Am J Public Health. 1982;72:1257-1264.
2. Johnson SR, McChesney C, Bean JA. Epidemiology of premenstrual symptoms in a nonclinical sample. 1. Prevalence, natural history and help-seeking behavior. J Repro Med. 1988;33:340-346.
3. Campbell EM, Peterkin D, O’Grady K, et al. Premenstrual symptoms in general practice patients. Prevalence and treatment. J Reprod Med. 1997;42:637-646.
4. O’Brien PM, Bäckström T, Brown C, et al. Towards a consensus on diagnostic criteria, measurement, and trial design of the premenstrual disorders: the ISPMD Montreal consensus. Arch Womens Ment Health. 2011;14:13-21.
5. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169:465-475.
6. American College of Obstetricians and Gynecologists. Guidelines for Women’s Health Care: A Resource Manual. 4th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014:607-613.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association, 2013.
8. Dennerstein L, Lehert P, Heinemann K. Epidemiology of premenstrual symptoms and disorders. Menopause Int. 2012;18:48-51.
9. Borenstein JE, Dean BB, Yonkers KA, et al. Using the daily record of severity of problems as a screening instrument for premenstrual syndrome. Obstet Gynecol. 2007;109:1068-1075.
10. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203-209.
11. Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9:41-49.
12. Janda C, Kues JN, Andersson G, et al. A symptom diary to assess severe premenstrual syndrome and premenstrual dysphoric disorder. Women Health. 2017;57:837-854.
13. Farasati N, Siassi F, Koohdani F, et al. Western dietary pattern is related to premenstrual syndrome: a case-control study. Brit J Nutr. 2015;114:2016-2021.
14. Purdue-Smithe AC, Manson JE, Hankinson SE, et al. A prospective study of caffeine and coffee intake and premenstrual syndrome. Am J Clin Nutr. 2016;104:499-507.
15. Bertone-Johnson ER, Hankinson SE, Bendich A, et al. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch Intern Med. 2005;165:1246-1252.
16. Chocano-Bedoya PO, Manson JE, Hankinson SE, et al. Dietary B vitamin intake and incident premenstrual syndrome. Am J Clin Nutr. 2011;93:1080-1086.
17. Prior JC, Vigna Y. Conditioning exercise and premenstrual symptoms. J Reprod Med. 1987;32:423-428.
18. Aganoff JA, Boyle GJ. Aerobic exercise, mood states, and menstrual cycle symptoms. J Psychosom Res. 1994;38:183-192.
19. El-Lithy A, El-Mazny A, Sabbour A, et al. Effect of aerobic exercise on premenstrual symptoms, haematological and hormonal parameters in young women. J Obstet Gynaecol. 2015;35:389-392.
20. Kroll-Desrosiers AR, Ronnenberg AG, Zagarins SE, et al. Recreational physical activity and premenstrual syndrome in young adult women: a cross-sectional study. PLoS One. 2017;12:1-13.
21. Jang SH, Kim DI, Choi MS. Effects and treatment methods of acupuncture and herbal medicine for premenstrual syndrome/premenstrual dysphoric disorder: systematic review. BMC Complement Altern Med. 2014;14:11.
22. Kim SY, Park HJ, Lee H, et al. Acupuncture for premenstrual syndrome: a systematic review and meta-analysis of randomized controlled trials. BJOG. 2011;118:899-915.
23. Lustyk MK, Gerrish WG, Shaver S, et al. Cognitive-behavioral therapy for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Womens Ment Health. 2009;12:85-96.
24. Thys-Jacob S, Starkey P, Bernstein D, et al. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual syndromes. Am J Obstet Gynecol. 1998;179:444-452.
25. Ghanbari Z, Haghollahi F, Shariat M, et al. Effects of calcium supplement therapy in women with premenstrual syndrome. Taiwan J Obstet Gynecol. 2009;48:124-129.
26. Girman A, Lee R, Kligler B. An integrative medicine approach to premenstrual syndrome. Am J Obstet Gynecol. 2003;188(5 suppl):s56-s65.
27. Wyatt KM, Dimmock PW, Jones PW, et al. Efficacy of vitamin B-6 in the treatment of premenstrual syndrome: systematic review. BMJ. 1999;318:1375-1381.
28. Verkaik S, Kamperman AM, van Westrhenen R, et al. The treatment of premenstrual syndrome with preparations of vitex agnus castus: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:150-166.
29. Boyle NB, Lawton C, Dye L. The effects of magnesium supplementation on subjective anxiety and stress—a systematic review. Nutrients. 2017;9:429-450.
30. Mauskop A, Altura BT, Altura BM. Serum ionized magnesium levels and serum ionized calcium/ionized magnesium ratios in women with menstrual migraine. Headache. 2002;42:242-248.
31. Facchinetti F, Sances C, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
32. Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
33. London RS, Murphy L, Kitlowski KE, et al. Efficacy of alpha-tocopherol in the treatment of the premenstrual syndrome. J Reprod Med. 1987;32:400-404.
34. Whelan AM, Jurgens TM, Naylor H. Herbs, vitamins, and minerals in the treatment of premenstrual syndrome: a systematic review. Can J Clin Pharmacol. 2009;16:e407-e429.
, , , . Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6): CD001396.
36. Dimmock P, Wyatt K, Jones P, et al. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet. 2000;356:1131-1136.
37. Shah NR, Jones JB, Aperi J, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder. Obstet Gynecol. 2008;111:1175-1182.
38. Freeman EW. Luteal phase administration of agents for the treatment of premenstrual dysphoric disorder. CNS Drugs. 2004;18:453-468.
39. Freeman EW, Rickels K, Yonkers KA, et al. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet Gynecol. 2001;98:737-744.
40. Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(2):CD006586.
41. Halbreich U, Freeman EW, Rapkin AJ, et al. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. 2012;85:19-27.
, , , . Progesterone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(3):CD003415.
43. Wyatt K, Dimmock P, Jones P, et al. Efficacy of progesterone and progestogens in management of premenstrual syndrome: systematic review. BMJ. 2001;323: 776-780.
44. Dennerstein L, Spencer-Gardner C, Gotts G, et al. Progesterone and the premenstrual syndrome: a double-blind crossover trial. Br Med J (Clin Res Ed). 1985;290:1617-1621.
45. NaProTECHNOLOGY. The Medical and Surgical Practice of NaProTECHNOLOGY. Premenstrual Syndrome: Evaluation and Treatment. Omaha, NE: Pope Paul VI Institute Press. 2004;29:345-368. https://www.naprotechnology.com/naprotext.htm. Accessed January 23, 2020.
46. Dante G, Facchinetti F. Herbal treatments for alleviating premenstrual symptoms: a systematic review. J Psychosom Obstet Gynaecol. 2011;32:42-51.
47. Jarvis CI, Lynch AM, Morin AK. Management strategies for premenstrual syndrome/premenstrual dysphoric disorder. Ann Pharmacother. 2008;42:967-978.
48. Landen M, Eriksson O, Sundblad C, et al. Compounds with affinity for serotonergic receptors in the treatment of premenstrual dysphoria: a comparison of buspirone, nefazodone and placebo. Psychopharmacology (Berl). 2001;155:292-298.
, , , . Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome . Cochrane Database Syst Rev . 2017 ;( 3) :CD010503.
PRACTICE RECOMMENDATIONS
› Start calcium supplementation in all patients who report significant premenstrual symptoms. A
› Add a selective serotonin reuptake inhibitor (SSRI) to calcium supplementationfor patients who have more severe premenstrual psychological symptoms. A
› Consider hormonal treatment options for patients who require treatment beyond calcium and an SSRI. B
› Provide nutrition and exercise information to all patients who report significant premenstrual symptoms. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The paranoid business executive
CASE Bipolar-like symptoms
Mr. R, age 48, presents to the psychiatric emergency department (ED) for the third time in 4 days after a change in his behavior over the last 2.5 weeks. He exhibits heightened extroversion, pressured speech, and uncharacteristic irritability. Mr. R’s wife reports that her husband normally is reserved.
Mr. R’s wife first became concerned when she noticed he was not sleeping and spending his nights changing the locks on their home. Mr. R, who is a business executive, occupied his time by taking notes on ways to protect his identity from the senior partners at his company.
Three weeks before his first ED visit, Mr. R had been treated for a neck abscess with incision and drainage. He was sent home with a 10-day course of amoxicillin/clavulanate, 875/125 mg by mouth twice daily. There were no reports of steroid use during or after the procedure. Four days after starting the antibiotic, he stopped taking it because he and his wife felt it was contributing to his mood changes and bizarre behavior.
During his first visit to the ED, Mr. R received a 1-time dose of olanzapine, 5 mg by mouth, which helped temporarily reduce his anxiety; however, he returned the following day with the same anxiety symptoms and was discharged with a 30-day prescription for olanzapine, 5 mg/d, to manage symptoms until he could establish care with an outpatient psychiatrist. Two days later, he returned to the ED yet again convinced people were spying on him and that his coworkers were plotting to have him fired. He was not taking his phone to work due to fears that it would be hacked.
Mr. R’s only home medication is clomiphene citrate, 100 mg/d by mouth, which he’s received for the past 7 months to treat low testosterone. He has no personal or family history of psychiatric illness and no prior signs of mania or hypomania.
At the current ED visit, Mr. R’s testosterone level is checked and is within normal limits. His urine drug screen, head CT, and standard laboratory test results are unremarkable, except for mild transaminitis that does not warrant acute management.
The clinicians in the ED establish a diagnosis of mania, unspecified, and psychotic disorder, unspecified. They recommend that Mr. R be admitted for mood stabilization.
[polldaddy:10485725]
Continue to: The authors' observations
The authors’ observations
Our initial impression was that Mr. R was experiencing a manic episode from undiagnosed bipolar I disorder. The diagnosis was equivocal considering his age, lack of family history, and absence of prior psychiatric symptoms. In most cases, the mean age of onset for mania is late adolescence to early adulthood. It would be less common for a patient to experience a first manic episode at age 48, although mania may emerge at any age. Results from a large British study showed that the incidence of a first manic episode drops from 13.81% in men age 16 to 25 to 2.62% in men age 46 to 55.1 However, some estimates suggest that the prevalence of late-onset mania is much higher than previously expected; medical comorbidities, such as dementia and delirium, may play a significant role in posing as manic-type symptoms in these patients.2
In Mr. R’s case, he remained fully alert and oriented without waxing and waning attentional deficits, which made delirium less likely. His affective symptoms included a reduced need for sleep, anxiety, irritability, rapid speech, and grandiosity lasting at least 2 weeks. He also exhibited psychotic symptoms in the form of paranoia. Altogether, he fit diagnostic criteria for bipolar I disorder well.
At the time of his manic episode, Mr. R was taking clomiphene. Clomiphene-induced mania and psychosis has been reported scarcely in the literature.3 In these cases, behavioral changes occurred within the first month of clomiphene initiation, which is dissimilar from Mr. R’s timeline.4 However, there appeared to be a temporal relationship between Mr. R’s use of amoxicillin/clavulanate and his manic episode.
This led us to consider whether medication-induced bipolar disorder would be a more appropriate diagnosis. There are documented associations between mania and antibiotics5; however, to our knowledge, mania secondary specifically to amoxicillin/clavulanate has not been reported extensively in the American literature. We found 1 case of suspected amoxicillin-induced psychosis,6 as well as a case report from the Netherlands of possible amoxicillin/clavulanate-induced mania.7
EVALUATION Ongoing paranoia
During his psychiatric hospitalization, Mr. R remains cooperative and polite, but exhibits ongoing paranoia, pressured speech, and poor reality testing. He remains convinced that “people are out to get me,” and routinely scans the room for safety during daily evaluations. He reports that he feels safe in the hospital, but does not feel safe to leave. Mr. R does not recall if in the past he had taken any products containing amoxicillin, but he is able to appreciate changes in his mood after being prescribed the antibiotic. He reports that starting the antibiotic made him feel confident in social interactions.
Continue to: During Mr. R's psychiatric hospitalization...
During Mr. R’s psychiatric hospitalization, olanzapine is titrated to 10 mg at bedtime. Clomiphene citrate is discontinued to limit any potential precipitants of mania, and amoxicillin/clavulanate is not restarted.
Mr. R gradually shows improvement in sleep quality and duration and becomes less irritable. His speech returns to a regular rate and rhythm. He eventually begins to question whether his fears were reality-based. After 4 days, Mr. R is ready to be discharged home and return to work.
[polldaddy:10485726]
The authors’ observations
The term “antibiomania” is used to describe manic episodes that coincide with antibiotic usage.8 Clarithromycin and ciprofloxacin are the agents most frequently implicated in antibiomania.9 While numerous reports exist in the literature, antibiomania is still considered a rare or unusual adverse event.
The link between infections and neuropsychiatric symptoms is well documented, which makes it challenging to tease apart the role of the acute infection from the use of antibiotics in precipitating psychiatric symptoms. However, in most reported cases of antibiomania, the onset of manic symptoms typically occurs within the first week of antibiotic initiation and resolves 1 to 3 days after medication discontinuation. The temporal relationship between antibiotic initiation and onset of neuropsychiatric symptoms has been best highlighted in cases where clarithromycin is used to treat a chronic Helicobacter pylori infection.10
While reports of antibiomania date back more than 6 decades, the exact mechanism by which antibiotics cause psychiatric symptoms is mostly unknown, although there are several hypotheses.5 Many hypotheses suggest some antibiotics play a role in reducing gamma-aminobutyric acid (GABA) neurotransmission. Quinolones, for example, have been found to cross the blood–brain barrier and can inhibit GABA from binding to the receptor sites. This can result in hyper-excitability in the CNS. Several quinolones have been implicated in antibiomania (Table 15). Penicillins are also thought to interfere with GABA neurotransmission in a similar fashion; however, amoxicillin-clavulanate has poor CNS penetration in the absence of blood–brain barrier disruption,11 which makes this theory a less plausible explanation for Mr. R’s case.
Continue to: Another possible mechanism...
Another possible mechanism of antibiotic-induced CNS excitability is through the glutamatergic system. Cycloserine, an antitubercular agent, is an N-methyl-D-aspartate receptor (NMDA) partial agonist and has reported neuropsychiatric adverse effects.12 It has been proposed that quinolones may also have NMDA agonist activity.
The prostaglandin hypothesis suggests that a decrease in GABA may increase concentrations of steroid hormones in the rat CNS.13 Steroids have been implicated in the breakdown of prostaglandin E1 (PGE1).13 A disruption in steroid regulation may prevent PGE1 breakdown. Lithium’s antimanic properties are thought to be caused at least in part by limiting prostaglandin production.14 Thus, a shift in PGE1 may lead to mood dysregulation.
Bipolar disorder has been linked with mitochondrial function abnormalities.15 Antibiotics that target ribosomal RNA may disrupt normal mitochondrial function and increase risk for mania precipitation.15 However, amoxicillin exerts its antibiotic effects through binding to penicillin-binding proteins, which leads to inhibition of the cell wall biosynthesis.
Lastly, research into the microbiome has elucidated the gut-brain axis. In animal studies, the microbiome has been found to play a role in immunity, cognitive function, and behavior. Dysbiosis in the microbiome is currently being investigated for its role in schizophrenia and bipolar disorder.16 Both the microbiome and changes in mitochondrial function are thought to develop over time, so while these are plausible explanations, an onset within 4 days of antibiotic initiation is likely too short of an exposure time to produce these changes.
The most likely causes of Mr. R’s manic episode were clomiphene or amoxicillin-clavulanate, and the time course seems to indicate the antibiotic was the most likely culprit. Table 2 lists things to consider if you suspect your patient may be experiencing antibiomania.

Continue to: TREATMENT Stable on olanzapine
TREATMENT Stable on olanzapine
During his first visit to the outpatient clinic 4 weeks after being discharged, Mr. R reports that he has successfully returned to work, and his paranoia has completely resolved. He continues to take olanzapine, 10 mg nightly, and has restarted clomiphene, 100 mg/d.
During this outpatient follow-up visit, Mr. R attributes his manic episode to an adverse reaction to amoxicillin/clavulanate, and requests to be tapered off olanzapine. After he and his psychiatrist discuss the risk of relapse in untreated bipolar disorder, olanzapine is reduced to 7.5 mg at bedtime with a plan to taper to discontinuation.
At his second follow-up visit 1 month later, Mr. R has also stopped clomiphene and is taking a herbal supplement instead, which he reports is helpful for his fatigue.
[polldaddy:10485727]
OUTCOME Lasting euthymic mood
Mr. R agrees to our recommendation of continuing to monitor him every 3 months for at least 1 year. We provide him and his wife with education about early warning signs of mood instability. Eight months after his manic episode, Mr. R no longer receives any psychotropic medications and shows no signs of mood instability. His mood remains euthymic and he is able to function well at work and in his personal life.
Bottom Line
‘Antibiomania’ describes manic episodes that coincide with antibiotic usage. This adverse effect is rare but should be considered in patients who present with unexplained first-episode mania, particularly those with an initial onset of mania after early adulthood.
Continue to: Related Resources
Related Resources
- Rakofsky JJ, Dunlop BW. Nothing to sneeze at: Upper respiratory infections and mood disorders. Current Psychiatry. 2019;18(7):29-34.
- Adiba A, Jackson JC, Torrence CL. Older-age bipolar disorder: A case series. Current Psychiatry. 2019;18(2):24-29
Drug Brand Names
Amoxicillin • Amoxil
Amoxicillin/clavulanate • Augmentin
Ampicillin • Omnipen-N, Polycillin-N
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Cycloserine • Seromycin
Dapsone • Dapsone
Erythromycin • Erythrocin, Pediamycin
Ethambutol • Myambutol
Ethionamide • Trecator-SC
Gentamicin • Garamycin
Isoniazid • Hyzyd, Nydrazid
Lithium • Eskalith, Lithobid
Metronidazole • Flagyl
Minocycline • Dynacin, Solodyn
Norfloxacin • Noroxin
Ofloxacin • Floxin
Olanzapine • Zyprexa
Penicillin G procaine • Duracillin A-S, Pfizerpen
Sulfamethoxazole/trimethoprim • Bactrim, Septra
1. Kennedy M, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results for a 35-year study. Psychol Med. 2005;35(6):855-863.
2. Dols A, Kupka RW, van Lammeren A, et al. The prevalence of late-life mania: a review. Bipolar Disord. 2014;16:113-118.
3. Siedontopf F, Horstkamp B, Stief G, et al. Clomiphene citrate as a possible cause of a psychotic reaction during infertility treatment. Hum Reprod. 1997;12(4):706-707.
4. Oyffe T, Lerner A, Isaacs G, et al. Clomiphene-induced psychosis. Am J Psychiatry. 1997;154(8):1169-1170.
5. Lambrichts S, Van Oudenhove L, Sienaert P. Antibiotics and mania: a systematic review. J Affect Disord. 2017;219:149-156.
6. Beal DM, Hudson B, Zaiac M. Amoxicillin-induced psychosis? Am J Psychiatry. 1986;143(2):255-256.
7. Klain V, Timmerman L. Antibiomania, acute manic psychosis following the use of antibiotics. European Psychiatry. 2013;28(suppl 1):1.
8. Abouesh A, Stone C, Hobbs WR. Antimicrobial-induced mania (antibiomania): a review of spontaneous reports. J Clin Psychopharmacol. 2002;22(1):71-81.
9. Lally L, Mannion L. The potential for antimicrobials to adversely affect mental state. BMJ Case Rep. 2013. pii: bcr2013009659. doi: 10.1136/bcr-2013-009659.
10. Neufeld NH, Mohamed NS, Grujich N, et al. Acute neuropsychiatric symptoms associated with antibiotic treatment of Helicobactor Pylori infections: a review. J Psychiatr Pract. 2017;23(1):25-35.
11. Sutter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;85(15):1332-1341.
12. Bakhla A, Gore P, Srivastava S. Cycloserine induced mania. Ind Psychiatry J. 2013;22(1):69-70.
13. Barbaccia ML, Roscetti G, Trabucchi M, et al. Isoniazid-induced inhibition of GABAergic transmission enhances neurosteroid content in the rat brain. Neuropharmacology. 1996;35(9-10):1299-1305.
14. Murphy D, Donnelly C, Moskowitz J. Inhibition by lithium of prostaglandin E1 and norepinephrine effects on cyclic adenosine monophosphate production in human platelets. Clin Pharmacol Ther. 1973;14(5):810-814.
15. Clay H, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29(3):311-324.
16. Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun. 2017;62:46-52.
CASE Bipolar-like symptoms
Mr. R, age 48, presents to the psychiatric emergency department (ED) for the third time in 4 days after a change in his behavior over the last 2.5 weeks. He exhibits heightened extroversion, pressured speech, and uncharacteristic irritability. Mr. R’s wife reports that her husband normally is reserved.
Mr. R’s wife first became concerned when she noticed he was not sleeping and spending his nights changing the locks on their home. Mr. R, who is a business executive, occupied his time by taking notes on ways to protect his identity from the senior partners at his company.
Three weeks before his first ED visit, Mr. R had been treated for a neck abscess with incision and drainage. He was sent home with a 10-day course of amoxicillin/clavulanate, 875/125 mg by mouth twice daily. There were no reports of steroid use during or after the procedure. Four days after starting the antibiotic, he stopped taking it because he and his wife felt it was contributing to his mood changes and bizarre behavior.
During his first visit to the ED, Mr. R received a 1-time dose of olanzapine, 5 mg by mouth, which helped temporarily reduce his anxiety; however, he returned the following day with the same anxiety symptoms and was discharged with a 30-day prescription for olanzapine, 5 mg/d, to manage symptoms until he could establish care with an outpatient psychiatrist. Two days later, he returned to the ED yet again convinced people were spying on him and that his coworkers were plotting to have him fired. He was not taking his phone to work due to fears that it would be hacked.
Mr. R’s only home medication is clomiphene citrate, 100 mg/d by mouth, which he’s received for the past 7 months to treat low testosterone. He has no personal or family history of psychiatric illness and no prior signs of mania or hypomania.
At the current ED visit, Mr. R’s testosterone level is checked and is within normal limits. His urine drug screen, head CT, and standard laboratory test results are unremarkable, except for mild transaminitis that does not warrant acute management.
The clinicians in the ED establish a diagnosis of mania, unspecified, and psychotic disorder, unspecified. They recommend that Mr. R be admitted for mood stabilization.
[polldaddy:10485725]
Continue to: The authors' observations
The authors’ observations
Our initial impression was that Mr. R was experiencing a manic episode from undiagnosed bipolar I disorder. The diagnosis was equivocal considering his age, lack of family history, and absence of prior psychiatric symptoms. In most cases, the mean age of onset for mania is late adolescence to early adulthood. It would be less common for a patient to experience a first manic episode at age 48, although mania may emerge at any age. Results from a large British study showed that the incidence of a first manic episode drops from 13.81% in men age 16 to 25 to 2.62% in men age 46 to 55.1 However, some estimates suggest that the prevalence of late-onset mania is much higher than previously expected; medical comorbidities, such as dementia and delirium, may play a significant role in posing as manic-type symptoms in these patients.2
In Mr. R’s case, he remained fully alert and oriented without waxing and waning attentional deficits, which made delirium less likely. His affective symptoms included a reduced need for sleep, anxiety, irritability, rapid speech, and grandiosity lasting at least 2 weeks. He also exhibited psychotic symptoms in the form of paranoia. Altogether, he fit diagnostic criteria for bipolar I disorder well.
At the time of his manic episode, Mr. R was taking clomiphene. Clomiphene-induced mania and psychosis has been reported scarcely in the literature.3 In these cases, behavioral changes occurred within the first month of clomiphene initiation, which is dissimilar from Mr. R’s timeline.4 However, there appeared to be a temporal relationship between Mr. R’s use of amoxicillin/clavulanate and his manic episode.
This led us to consider whether medication-induced bipolar disorder would be a more appropriate diagnosis. There are documented associations between mania and antibiotics5; however, to our knowledge, mania secondary specifically to amoxicillin/clavulanate has not been reported extensively in the American literature. We found 1 case of suspected amoxicillin-induced psychosis,6 as well as a case report from the Netherlands of possible amoxicillin/clavulanate-induced mania.7
EVALUATION Ongoing paranoia
During his psychiatric hospitalization, Mr. R remains cooperative and polite, but exhibits ongoing paranoia, pressured speech, and poor reality testing. He remains convinced that “people are out to get me,” and routinely scans the room for safety during daily evaluations. He reports that he feels safe in the hospital, but does not feel safe to leave. Mr. R does not recall if in the past he had taken any products containing amoxicillin, but he is able to appreciate changes in his mood after being prescribed the antibiotic. He reports that starting the antibiotic made him feel confident in social interactions.
Continue to: During Mr. R's psychiatric hospitalization...
During Mr. R’s psychiatric hospitalization, olanzapine is titrated to 10 mg at bedtime. Clomiphene citrate is discontinued to limit any potential precipitants of mania, and amoxicillin/clavulanate is not restarted.
Mr. R gradually shows improvement in sleep quality and duration and becomes less irritable. His speech returns to a regular rate and rhythm. He eventually begins to question whether his fears were reality-based. After 4 days, Mr. R is ready to be discharged home and return to work.
[polldaddy:10485726]
The authors’ observations
The term “antibiomania” is used to describe manic episodes that coincide with antibiotic usage.8 Clarithromycin and ciprofloxacin are the agents most frequently implicated in antibiomania.9 While numerous reports exist in the literature, antibiomania is still considered a rare or unusual adverse event.
The link between infections and neuropsychiatric symptoms is well documented, which makes it challenging to tease apart the role of the acute infection from the use of antibiotics in precipitating psychiatric symptoms. However, in most reported cases of antibiomania, the onset of manic symptoms typically occurs within the first week of antibiotic initiation and resolves 1 to 3 days after medication discontinuation. The temporal relationship between antibiotic initiation and onset of neuropsychiatric symptoms has been best highlighted in cases where clarithromycin is used to treat a chronic Helicobacter pylori infection.10
While reports of antibiomania date back more than 6 decades, the exact mechanism by which antibiotics cause psychiatric symptoms is mostly unknown, although there are several hypotheses.5 Many hypotheses suggest some antibiotics play a role in reducing gamma-aminobutyric acid (GABA) neurotransmission. Quinolones, for example, have been found to cross the blood–brain barrier and can inhibit GABA from binding to the receptor sites. This can result in hyper-excitability in the CNS. Several quinolones have been implicated in antibiomania (Table 15). Penicillins are also thought to interfere with GABA neurotransmission in a similar fashion; however, amoxicillin-clavulanate has poor CNS penetration in the absence of blood–brain barrier disruption,11 which makes this theory a less plausible explanation for Mr. R’s case.
Continue to: Another possible mechanism...
Another possible mechanism of antibiotic-induced CNS excitability is through the glutamatergic system. Cycloserine, an antitubercular agent, is an N-methyl-D-aspartate receptor (NMDA) partial agonist and has reported neuropsychiatric adverse effects.12 It has been proposed that quinolones may also have NMDA agonist activity.
The prostaglandin hypothesis suggests that a decrease in GABA may increase concentrations of steroid hormones in the rat CNS.13 Steroids have been implicated in the breakdown of prostaglandin E1 (PGE1).13 A disruption in steroid regulation may prevent PGE1 breakdown. Lithium’s antimanic properties are thought to be caused at least in part by limiting prostaglandin production.14 Thus, a shift in PGE1 may lead to mood dysregulation.
Bipolar disorder has been linked with mitochondrial function abnormalities.15 Antibiotics that target ribosomal RNA may disrupt normal mitochondrial function and increase risk for mania precipitation.15 However, amoxicillin exerts its antibiotic effects through binding to penicillin-binding proteins, which leads to inhibition of the cell wall biosynthesis.
Lastly, research into the microbiome has elucidated the gut-brain axis. In animal studies, the microbiome has been found to play a role in immunity, cognitive function, and behavior. Dysbiosis in the microbiome is currently being investigated for its role in schizophrenia and bipolar disorder.16 Both the microbiome and changes in mitochondrial function are thought to develop over time, so while these are plausible explanations, an onset within 4 days of antibiotic initiation is likely too short of an exposure time to produce these changes.
The most likely causes of Mr. R’s manic episode were clomiphene or amoxicillin-clavulanate, and the time course seems to indicate the antibiotic was the most likely culprit. Table 2 lists things to consider if you suspect your patient may be experiencing antibiomania.

Continue to: TREATMENT Stable on olanzapine
TREATMENT Stable on olanzapine
During his first visit to the outpatient clinic 4 weeks after being discharged, Mr. R reports that he has successfully returned to work, and his paranoia has completely resolved. He continues to take olanzapine, 10 mg nightly, and has restarted clomiphene, 100 mg/d.
During this outpatient follow-up visit, Mr. R attributes his manic episode to an adverse reaction to amoxicillin/clavulanate, and requests to be tapered off olanzapine. After he and his psychiatrist discuss the risk of relapse in untreated bipolar disorder, olanzapine is reduced to 7.5 mg at bedtime with a plan to taper to discontinuation.
At his second follow-up visit 1 month later, Mr. R has also stopped clomiphene and is taking a herbal supplement instead, which he reports is helpful for his fatigue.
[polldaddy:10485727]
OUTCOME Lasting euthymic mood
Mr. R agrees to our recommendation of continuing to monitor him every 3 months for at least 1 year. We provide him and his wife with education about early warning signs of mood instability. Eight months after his manic episode, Mr. R no longer receives any psychotropic medications and shows no signs of mood instability. His mood remains euthymic and he is able to function well at work and in his personal life.
Bottom Line
‘Antibiomania’ describes manic episodes that coincide with antibiotic usage. This adverse effect is rare but should be considered in patients who present with unexplained first-episode mania, particularly those with an initial onset of mania after early adulthood.
Continue to: Related Resources
Related Resources
- Rakofsky JJ, Dunlop BW. Nothing to sneeze at: Upper respiratory infections and mood disorders. Current Psychiatry. 2019;18(7):29-34.
- Adiba A, Jackson JC, Torrence CL. Older-age bipolar disorder: A case series. Current Psychiatry. 2019;18(2):24-29
Drug Brand Names
Amoxicillin • Amoxil
Amoxicillin/clavulanate • Augmentin
Ampicillin • Omnipen-N, Polycillin-N
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Cycloserine • Seromycin
Dapsone • Dapsone
Erythromycin • Erythrocin, Pediamycin
Ethambutol • Myambutol
Ethionamide • Trecator-SC
Gentamicin • Garamycin
Isoniazid • Hyzyd, Nydrazid
Lithium • Eskalith, Lithobid
Metronidazole • Flagyl
Minocycline • Dynacin, Solodyn
Norfloxacin • Noroxin
Ofloxacin • Floxin
Olanzapine • Zyprexa
Penicillin G procaine • Duracillin A-S, Pfizerpen
Sulfamethoxazole/trimethoprim • Bactrim, Septra
CASE Bipolar-like symptoms
Mr. R, age 48, presents to the psychiatric emergency department (ED) for the third time in 4 days after a change in his behavior over the last 2.5 weeks. He exhibits heightened extroversion, pressured speech, and uncharacteristic irritability. Mr. R’s wife reports that her husband normally is reserved.
Mr. R’s wife first became concerned when she noticed he was not sleeping and spending his nights changing the locks on their home. Mr. R, who is a business executive, occupied his time by taking notes on ways to protect his identity from the senior partners at his company.
Three weeks before his first ED visit, Mr. R had been treated for a neck abscess with incision and drainage. He was sent home with a 10-day course of amoxicillin/clavulanate, 875/125 mg by mouth twice daily. There were no reports of steroid use during or after the procedure. Four days after starting the antibiotic, he stopped taking it because he and his wife felt it was contributing to his mood changes and bizarre behavior.
During his first visit to the ED, Mr. R received a 1-time dose of olanzapine, 5 mg by mouth, which helped temporarily reduce his anxiety; however, he returned the following day with the same anxiety symptoms and was discharged with a 30-day prescription for olanzapine, 5 mg/d, to manage symptoms until he could establish care with an outpatient psychiatrist. Two days later, he returned to the ED yet again convinced people were spying on him and that his coworkers were plotting to have him fired. He was not taking his phone to work due to fears that it would be hacked.
Mr. R’s only home medication is clomiphene citrate, 100 mg/d by mouth, which he’s received for the past 7 months to treat low testosterone. He has no personal or family history of psychiatric illness and no prior signs of mania or hypomania.
At the current ED visit, Mr. R’s testosterone level is checked and is within normal limits. His urine drug screen, head CT, and standard laboratory test results are unremarkable, except for mild transaminitis that does not warrant acute management.
The clinicians in the ED establish a diagnosis of mania, unspecified, and psychotic disorder, unspecified. They recommend that Mr. R be admitted for mood stabilization.
[polldaddy:10485725]
Continue to: The authors' observations
The authors’ observations
Our initial impression was that Mr. R was experiencing a manic episode from undiagnosed bipolar I disorder. The diagnosis was equivocal considering his age, lack of family history, and absence of prior psychiatric symptoms. In most cases, the mean age of onset for mania is late adolescence to early adulthood. It would be less common for a patient to experience a first manic episode at age 48, although mania may emerge at any age. Results from a large British study showed that the incidence of a first manic episode drops from 13.81% in men age 16 to 25 to 2.62% in men age 46 to 55.1 However, some estimates suggest that the prevalence of late-onset mania is much higher than previously expected; medical comorbidities, such as dementia and delirium, may play a significant role in posing as manic-type symptoms in these patients.2
In Mr. R’s case, he remained fully alert and oriented without waxing and waning attentional deficits, which made delirium less likely. His affective symptoms included a reduced need for sleep, anxiety, irritability, rapid speech, and grandiosity lasting at least 2 weeks. He also exhibited psychotic symptoms in the form of paranoia. Altogether, he fit diagnostic criteria for bipolar I disorder well.
At the time of his manic episode, Mr. R was taking clomiphene. Clomiphene-induced mania and psychosis has been reported scarcely in the literature.3 In these cases, behavioral changes occurred within the first month of clomiphene initiation, which is dissimilar from Mr. R’s timeline.4 However, there appeared to be a temporal relationship between Mr. R’s use of amoxicillin/clavulanate and his manic episode.
This led us to consider whether medication-induced bipolar disorder would be a more appropriate diagnosis. There are documented associations between mania and antibiotics5; however, to our knowledge, mania secondary specifically to amoxicillin/clavulanate has not been reported extensively in the American literature. We found 1 case of suspected amoxicillin-induced psychosis,6 as well as a case report from the Netherlands of possible amoxicillin/clavulanate-induced mania.7
EVALUATION Ongoing paranoia
During his psychiatric hospitalization, Mr. R remains cooperative and polite, but exhibits ongoing paranoia, pressured speech, and poor reality testing. He remains convinced that “people are out to get me,” and routinely scans the room for safety during daily evaluations. He reports that he feels safe in the hospital, but does not feel safe to leave. Mr. R does not recall if in the past he had taken any products containing amoxicillin, but he is able to appreciate changes in his mood after being prescribed the antibiotic. He reports that starting the antibiotic made him feel confident in social interactions.
Continue to: During Mr. R's psychiatric hospitalization...
During Mr. R’s psychiatric hospitalization, olanzapine is titrated to 10 mg at bedtime. Clomiphene citrate is discontinued to limit any potential precipitants of mania, and amoxicillin/clavulanate is not restarted.
Mr. R gradually shows improvement in sleep quality and duration and becomes less irritable. His speech returns to a regular rate and rhythm. He eventually begins to question whether his fears were reality-based. After 4 days, Mr. R is ready to be discharged home and return to work.
[polldaddy:10485726]
The authors’ observations
The term “antibiomania” is used to describe manic episodes that coincide with antibiotic usage.8 Clarithromycin and ciprofloxacin are the agents most frequently implicated in antibiomania.9 While numerous reports exist in the literature, antibiomania is still considered a rare or unusual adverse event.
The link between infections and neuropsychiatric symptoms is well documented, which makes it challenging to tease apart the role of the acute infection from the use of antibiotics in precipitating psychiatric symptoms. However, in most reported cases of antibiomania, the onset of manic symptoms typically occurs within the first week of antibiotic initiation and resolves 1 to 3 days after medication discontinuation. The temporal relationship between antibiotic initiation and onset of neuropsychiatric symptoms has been best highlighted in cases where clarithromycin is used to treat a chronic Helicobacter pylori infection.10
While reports of antibiomania date back more than 6 decades, the exact mechanism by which antibiotics cause psychiatric symptoms is mostly unknown, although there are several hypotheses.5 Many hypotheses suggest some antibiotics play a role in reducing gamma-aminobutyric acid (GABA) neurotransmission. Quinolones, for example, have been found to cross the blood–brain barrier and can inhibit GABA from binding to the receptor sites. This can result in hyper-excitability in the CNS. Several quinolones have been implicated in antibiomania (Table 15). Penicillins are also thought to interfere with GABA neurotransmission in a similar fashion; however, amoxicillin-clavulanate has poor CNS penetration in the absence of blood–brain barrier disruption,11 which makes this theory a less plausible explanation for Mr. R’s case.
Continue to: Another possible mechanism...
Another possible mechanism of antibiotic-induced CNS excitability is through the glutamatergic system. Cycloserine, an antitubercular agent, is an N-methyl-D-aspartate receptor (NMDA) partial agonist and has reported neuropsychiatric adverse effects.12 It has been proposed that quinolones may also have NMDA agonist activity.
The prostaglandin hypothesis suggests that a decrease in GABA may increase concentrations of steroid hormones in the rat CNS.13 Steroids have been implicated in the breakdown of prostaglandin E1 (PGE1).13 A disruption in steroid regulation may prevent PGE1 breakdown. Lithium’s antimanic properties are thought to be caused at least in part by limiting prostaglandin production.14 Thus, a shift in PGE1 may lead to mood dysregulation.
Bipolar disorder has been linked with mitochondrial function abnormalities.15 Antibiotics that target ribosomal RNA may disrupt normal mitochondrial function and increase risk for mania precipitation.15 However, amoxicillin exerts its antibiotic effects through binding to penicillin-binding proteins, which leads to inhibition of the cell wall biosynthesis.
Lastly, research into the microbiome has elucidated the gut-brain axis. In animal studies, the microbiome has been found to play a role in immunity, cognitive function, and behavior. Dysbiosis in the microbiome is currently being investigated for its role in schizophrenia and bipolar disorder.16 Both the microbiome and changes in mitochondrial function are thought to develop over time, so while these are plausible explanations, an onset within 4 days of antibiotic initiation is likely too short of an exposure time to produce these changes.
The most likely causes of Mr. R’s manic episode were clomiphene or amoxicillin-clavulanate, and the time course seems to indicate the antibiotic was the most likely culprit. Table 2 lists things to consider if you suspect your patient may be experiencing antibiomania.

Continue to: TREATMENT Stable on olanzapine
TREATMENT Stable on olanzapine
During his first visit to the outpatient clinic 4 weeks after being discharged, Mr. R reports that he has successfully returned to work, and his paranoia has completely resolved. He continues to take olanzapine, 10 mg nightly, and has restarted clomiphene, 100 mg/d.
During this outpatient follow-up visit, Mr. R attributes his manic episode to an adverse reaction to amoxicillin/clavulanate, and requests to be tapered off olanzapine. After he and his psychiatrist discuss the risk of relapse in untreated bipolar disorder, olanzapine is reduced to 7.5 mg at bedtime with a plan to taper to discontinuation.
At his second follow-up visit 1 month later, Mr. R has also stopped clomiphene and is taking a herbal supplement instead, which he reports is helpful for his fatigue.
[polldaddy:10485727]
OUTCOME Lasting euthymic mood
Mr. R agrees to our recommendation of continuing to monitor him every 3 months for at least 1 year. We provide him and his wife with education about early warning signs of mood instability. Eight months after his manic episode, Mr. R no longer receives any psychotropic medications and shows no signs of mood instability. His mood remains euthymic and he is able to function well at work and in his personal life.
Bottom Line
‘Antibiomania’ describes manic episodes that coincide with antibiotic usage. This adverse effect is rare but should be considered in patients who present with unexplained first-episode mania, particularly those with an initial onset of mania after early adulthood.
Continue to: Related Resources
Related Resources
- Rakofsky JJ, Dunlop BW. Nothing to sneeze at: Upper respiratory infections and mood disorders. Current Psychiatry. 2019;18(7):29-34.
- Adiba A, Jackson JC, Torrence CL. Older-age bipolar disorder: A case series. Current Psychiatry. 2019;18(2):24-29
Drug Brand Names
Amoxicillin • Amoxil
Amoxicillin/clavulanate • Augmentin
Ampicillin • Omnipen-N, Polycillin-N
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Cycloserine • Seromycin
Dapsone • Dapsone
Erythromycin • Erythrocin, Pediamycin
Ethambutol • Myambutol
Ethionamide • Trecator-SC
Gentamicin • Garamycin
Isoniazid • Hyzyd, Nydrazid
Lithium • Eskalith, Lithobid
Metronidazole • Flagyl
Minocycline • Dynacin, Solodyn
Norfloxacin • Noroxin
Ofloxacin • Floxin
Olanzapine • Zyprexa
Penicillin G procaine • Duracillin A-S, Pfizerpen
Sulfamethoxazole/trimethoprim • Bactrim, Septra
1. Kennedy M, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results for a 35-year study. Psychol Med. 2005;35(6):855-863.
2. Dols A, Kupka RW, van Lammeren A, et al. The prevalence of late-life mania: a review. Bipolar Disord. 2014;16:113-118.
3. Siedontopf F, Horstkamp B, Stief G, et al. Clomiphene citrate as a possible cause of a psychotic reaction during infertility treatment. Hum Reprod. 1997;12(4):706-707.
4. Oyffe T, Lerner A, Isaacs G, et al. Clomiphene-induced psychosis. Am J Psychiatry. 1997;154(8):1169-1170.
5. Lambrichts S, Van Oudenhove L, Sienaert P. Antibiotics and mania: a systematic review. J Affect Disord. 2017;219:149-156.
6. Beal DM, Hudson B, Zaiac M. Amoxicillin-induced psychosis? Am J Psychiatry. 1986;143(2):255-256.
7. Klain V, Timmerman L. Antibiomania, acute manic psychosis following the use of antibiotics. European Psychiatry. 2013;28(suppl 1):1.
8. Abouesh A, Stone C, Hobbs WR. Antimicrobial-induced mania (antibiomania): a review of spontaneous reports. J Clin Psychopharmacol. 2002;22(1):71-81.
9. Lally L, Mannion L. The potential for antimicrobials to adversely affect mental state. BMJ Case Rep. 2013. pii: bcr2013009659. doi: 10.1136/bcr-2013-009659.
10. Neufeld NH, Mohamed NS, Grujich N, et al. Acute neuropsychiatric symptoms associated with antibiotic treatment of Helicobactor Pylori infections: a review. J Psychiatr Pract. 2017;23(1):25-35.
11. Sutter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;85(15):1332-1341.
12. Bakhla A, Gore P, Srivastava S. Cycloserine induced mania. Ind Psychiatry J. 2013;22(1):69-70.
13. Barbaccia ML, Roscetti G, Trabucchi M, et al. Isoniazid-induced inhibition of GABAergic transmission enhances neurosteroid content in the rat brain. Neuropharmacology. 1996;35(9-10):1299-1305.
14. Murphy D, Donnelly C, Moskowitz J. Inhibition by lithium of prostaglandin E1 and norepinephrine effects on cyclic adenosine monophosphate production in human platelets. Clin Pharmacol Ther. 1973;14(5):810-814.
15. Clay H, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29(3):311-324.
16. Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun. 2017;62:46-52.
1. Kennedy M, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results for a 35-year study. Psychol Med. 2005;35(6):855-863.
2. Dols A, Kupka RW, van Lammeren A, et al. The prevalence of late-life mania: a review. Bipolar Disord. 2014;16:113-118.
3. Siedontopf F, Horstkamp B, Stief G, et al. Clomiphene citrate as a possible cause of a psychotic reaction during infertility treatment. Hum Reprod. 1997;12(4):706-707.
4. Oyffe T, Lerner A, Isaacs G, et al. Clomiphene-induced psychosis. Am J Psychiatry. 1997;154(8):1169-1170.
5. Lambrichts S, Van Oudenhove L, Sienaert P. Antibiotics and mania: a systematic review. J Affect Disord. 2017;219:149-156.
6. Beal DM, Hudson B, Zaiac M. Amoxicillin-induced psychosis? Am J Psychiatry. 1986;143(2):255-256.
7. Klain V, Timmerman L. Antibiomania, acute manic psychosis following the use of antibiotics. European Psychiatry. 2013;28(suppl 1):1.
8. Abouesh A, Stone C, Hobbs WR. Antimicrobial-induced mania (antibiomania): a review of spontaneous reports. J Clin Psychopharmacol. 2002;22(1):71-81.
9. Lally L, Mannion L. The potential for antimicrobials to adversely affect mental state. BMJ Case Rep. 2013. pii: bcr2013009659. doi: 10.1136/bcr-2013-009659.
10. Neufeld NH, Mohamed NS, Grujich N, et al. Acute neuropsychiatric symptoms associated with antibiotic treatment of Helicobactor Pylori infections: a review. J Psychiatr Pract. 2017;23(1):25-35.
11. Sutter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;85(15):1332-1341.
12. Bakhla A, Gore P, Srivastava S. Cycloserine induced mania. Ind Psychiatry J. 2013;22(1):69-70.
13. Barbaccia ML, Roscetti G, Trabucchi M, et al. Isoniazid-induced inhibition of GABAergic transmission enhances neurosteroid content in the rat brain. Neuropharmacology. 1996;35(9-10):1299-1305.
14. Murphy D, Donnelly C, Moskowitz J. Inhibition by lithium of prostaglandin E1 and norepinephrine effects on cyclic adenosine monophosphate production in human platelets. Clin Pharmacol Ther. 1973;14(5):810-814.
15. Clay H, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29(3):311-324.
16. Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun. 2017;62:46-52.
Valproic acid-induced hyperammonemic encephalopathy
Mrs. C, age 75, is transferred to our inpatient medical/surgical hospital from a psychiatric hospital after presenting with shortness of breath and altered mental status.
Eight days earlier, Mrs. C had been admitted to the psychiatric hospital for bipolar mania with psychotic features. While there, Mrs. C received quetiapine, 400 mg nightly, and an initial valproic acid (VPA) dosage of 500 mg 2 times daily. While receiving VPA 500 mg 2 times daily, her VPA total level was 62 µg/mL, which is on the lower end of the therapeutic range (50 to 125 µg/mL). This prompted the team at the psychiatric hospital to increase her VPA dosage to 500 mg 3 times daily the day before she was transferred to our hospital.
At our hospital, she is found to be in hypoxic respiratory failure secondary to pneumonia. Upon admission, her laboratory data show evidence of infection and anemia and she also has an
From hospital Day 3 to Day 6, Mrs. C experiences gradual improvement in her respiratory and mental status. However, on hospital Day 7, she has extreme somnolence and altered mental status without respiratory involvement. Our team suspects VPA toxicity and/or VPA-induced hyperammonemic encephalopathy (VHE).
VPA-induced hyperammonemia
Hyperammonemia can occur in individuals receiving VPA and is most often asymptomatic. However, elevations in ammonia may lead to VHE, which is a rare but serious adverse effect. VHE has been reported early in treatment, in acute VPA overdose, and in chronic VPA use despite normal doses and levels.1 It also can occur in the absence of clinical and laboratory evidence of hepatotoxicity. VHE is associated with significant morbidity and CNS damage. Symptoms of VHE include vomiting, lethargy, and confusion. If left untreated, VHE can lead to coma and death.
Mechanism of VHE. The exact mechanism of VHE is unknown.1-3 Ammonia is a toxic base produced by deamination of amino acids. The liver eliminates ammonia via the urea cycle.2 Valproic acid metabolites, propionate and 4-en-VPA, can directly inhibit N-acetyl glutamate, which can disrupt the urea cycle, leading to elevated ammonia levels.3 Long-term or high-dose VPA can lead to carnitine deficiency, primarily by inhibiting its biosynthesis and depleting stores.4 Carnitine deficiency leads to disturbances in mitochondrial function, causing inhibition of the urea cycle and increasing ammonia. CNS toxicity due to hyperammonemia is thought to be due to activation of glutamate receptors.3
Risk factors. Co-administration of other antiepileptic drugs (AEDs) with VPA is a risk factor for VHE.1,5 This happens because enzyme-inducing AEDs such as phenytoin, phenobarbital, and carbamazepine can increase toxic metabolites of VPA, which can lead to hyperammonemia. Topiramate can also inhibit the urea cycle, leading to increased ammonia levels. Additionally, co-administration of VPA with quetiapine, paliperidone, risperidone, or aripiprazole has been reported to increase the risk of VHE.1,5 Intellectual disability, carnitine deficiency, low albumin, and abnormal liver function have also been reported to increase the risk of VHE.1,5
Continue to: Diagnosis and management
Diagnosis and management. If a patient receiving VPA is experiencing nausea, fatigue, or somnolence, it is important to check the patient’s ammonia level (normal range: 11 to 32 µmol/L) and VPA total levels (therapeutic range: 50 to 125 µg/mL). Consider checking a VPA free level, especially in geriatric patients or patients who have low albumin; the therapeutic range of VPA free is 6 to 22 µg/mL.3 If the ammonia level is elevated, discontinue VPA immediately (Table).1-3 Clinicians may also elect to prescribe lactulose until ammonia levels return to normal range. Adding levocarnitine may also help, although evidence is limited to small case series or retrospective studies.3 Currently, there is no known advantage in combining lactulose and levocarnitine to address VHE. Severe cases of VHE (ammonia levels >400 µmol/L) may require hemodialysis.1
Prevention. Strategies to prevent VHE include avoiding polypharmacy, especially concurrent use of enzyme-inducing AEDs and possibly second-generation antipsychotics. Additionally, VPA should not be used in individuals with urea cycle disorders. It is unknown if levocarnitine supplementation is preventive, but this approach has been suggested.3
CASE CONTINUED
Mrs. C has several possible risk factors for VHE, including co-administration of quetiapine and VPA, and a low albumin level. A further laboratory workup for Mrs. C reveals a VPA free level of 19 µg/mL (21.1% free), a VPA total level of 90 µg/mL, and an ammonia level of 79 µmol/L, confirming our suspicions regarding VHE. We determine that Mrs. C’s altered mental status is likely due her elevated ammonia levels, because the infection had been improving in the days leading up to the sudden, extreme somnolence.
VPA is immediately stopped and Mrs. C receives 1 dose of lactulose. The following day, Mrs. C’s mental status improves, and her ammonia levels return to normal. On hospital Day 9, she is transferred back to the psychiatric facility for management of manic and psychotic symptoms.
Related Resources
- Brown LM, Cupples N, Moore TA. Levocarnitine for valproate-induced hyperammonemia in the psychiatric setting: a case series and literature review. Ment Health Clin. 2018;8(3):148-154.
- Aires CCP, van Cruchten A, Ijlat L, et al. New insights on the mechanisms of valproate-induced hyperammonemia: inhibition of hepatic N-acetylglutamate synthase activity by valproyl-CoA. J Hepatol. 2011;55(2):426-434.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Lactulose • Enulose
Levocarnitine • Carnitine, Carnitor
Levofloxacin • Levaquin IV
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Quetiapine • Seroquel
Risperidone • Risperdal
Topiramate • Topamax
Valproic acid • Depakene
1. Chopra A, Kolla BP, Mansukhani MP, et al. Valproate-induced hyperammonemic encephalopathy: an update on risk factors, clinical correlates, and management. Gen Hosp Psychiatry. 2012;34(3):290-298.
2. Kowalski PC, Dowben JS, Keltner NL. Ammonium: the deadly toxin you don’t want to miss when using mood stabilizers. Perspect Psychiatr Care. 2013;49(4):221-225.
3. Baddour E, Tewksbury A, Stauner N. Valproic acid-induced hyper ammonemia: incidence, clinical significance, and treatment management. Ment Health Clin. 2018;8(2):73-77.
4. Raskind JY, El-Chaar GM. The role of carnitine supplementation during valproic acid therapy. Ann Pharmacother. 2000;34(5):630-638. 5. Tseng YL, Huang CR, Lin CH, et al. Risk factors of hyperammonemia in patients with epilepsy. Medicine (Baltimore). 2014;93(11):e66. doi: 10.1097/MD.0000000000000066.
Mrs. C, age 75, is transferred to our inpatient medical/surgical hospital from a psychiatric hospital after presenting with shortness of breath and altered mental status.
Eight days earlier, Mrs. C had been admitted to the psychiatric hospital for bipolar mania with psychotic features. While there, Mrs. C received quetiapine, 400 mg nightly, and an initial valproic acid (VPA) dosage of 500 mg 2 times daily. While receiving VPA 500 mg 2 times daily, her VPA total level was 62 µg/mL, which is on the lower end of the therapeutic range (50 to 125 µg/mL). This prompted the team at the psychiatric hospital to increase her VPA dosage to 500 mg 3 times daily the day before she was transferred to our hospital.
At our hospital, she is found to be in hypoxic respiratory failure secondary to pneumonia. Upon admission, her laboratory data show evidence of infection and anemia and she also has an
From hospital Day 3 to Day 6, Mrs. C experiences gradual improvement in her respiratory and mental status. However, on hospital Day 7, she has extreme somnolence and altered mental status without respiratory involvement. Our team suspects VPA toxicity and/or VPA-induced hyperammonemic encephalopathy (VHE).
VPA-induced hyperammonemia
Hyperammonemia can occur in individuals receiving VPA and is most often asymptomatic. However, elevations in ammonia may lead to VHE, which is a rare but serious adverse effect. VHE has been reported early in treatment, in acute VPA overdose, and in chronic VPA use despite normal doses and levels.1 It also can occur in the absence of clinical and laboratory evidence of hepatotoxicity. VHE is associated with significant morbidity and CNS damage. Symptoms of VHE include vomiting, lethargy, and confusion. If left untreated, VHE can lead to coma and death.
Mechanism of VHE. The exact mechanism of VHE is unknown.1-3 Ammonia is a toxic base produced by deamination of amino acids. The liver eliminates ammonia via the urea cycle.2 Valproic acid metabolites, propionate and 4-en-VPA, can directly inhibit N-acetyl glutamate, which can disrupt the urea cycle, leading to elevated ammonia levels.3 Long-term or high-dose VPA can lead to carnitine deficiency, primarily by inhibiting its biosynthesis and depleting stores.4 Carnitine deficiency leads to disturbances in mitochondrial function, causing inhibition of the urea cycle and increasing ammonia. CNS toxicity due to hyperammonemia is thought to be due to activation of glutamate receptors.3
Risk factors. Co-administration of other antiepileptic drugs (AEDs) with VPA is a risk factor for VHE.1,5 This happens because enzyme-inducing AEDs such as phenytoin, phenobarbital, and carbamazepine can increase toxic metabolites of VPA, which can lead to hyperammonemia. Topiramate can also inhibit the urea cycle, leading to increased ammonia levels. Additionally, co-administration of VPA with quetiapine, paliperidone, risperidone, or aripiprazole has been reported to increase the risk of VHE.1,5 Intellectual disability, carnitine deficiency, low albumin, and abnormal liver function have also been reported to increase the risk of VHE.1,5
Continue to: Diagnosis and management
Diagnosis and management. If a patient receiving VPA is experiencing nausea, fatigue, or somnolence, it is important to check the patient’s ammonia level (normal range: 11 to 32 µmol/L) and VPA total levels (therapeutic range: 50 to 125 µg/mL). Consider checking a VPA free level, especially in geriatric patients or patients who have low albumin; the therapeutic range of VPA free is 6 to 22 µg/mL.3 If the ammonia level is elevated, discontinue VPA immediately (Table).1-3 Clinicians may also elect to prescribe lactulose until ammonia levels return to normal range. Adding levocarnitine may also help, although evidence is limited to small case series or retrospective studies.3 Currently, there is no known advantage in combining lactulose and levocarnitine to address VHE. Severe cases of VHE (ammonia levels >400 µmol/L) may require hemodialysis.1
Prevention. Strategies to prevent VHE include avoiding polypharmacy, especially concurrent use of enzyme-inducing AEDs and possibly second-generation antipsychotics. Additionally, VPA should not be used in individuals with urea cycle disorders. It is unknown if levocarnitine supplementation is preventive, but this approach has been suggested.3
CASE CONTINUED
Mrs. C has several possible risk factors for VHE, including co-administration of quetiapine and VPA, and a low albumin level. A further laboratory workup for Mrs. C reveals a VPA free level of 19 µg/mL (21.1% free), a VPA total level of 90 µg/mL, and an ammonia level of 79 µmol/L, confirming our suspicions regarding VHE. We determine that Mrs. C’s altered mental status is likely due her elevated ammonia levels, because the infection had been improving in the days leading up to the sudden, extreme somnolence.
VPA is immediately stopped and Mrs. C receives 1 dose of lactulose. The following day, Mrs. C’s mental status improves, and her ammonia levels return to normal. On hospital Day 9, she is transferred back to the psychiatric facility for management of manic and psychotic symptoms.
Related Resources
- Brown LM, Cupples N, Moore TA. Levocarnitine for valproate-induced hyperammonemia in the psychiatric setting: a case series and literature review. Ment Health Clin. 2018;8(3):148-154.
- Aires CCP, van Cruchten A, Ijlat L, et al. New insights on the mechanisms of valproate-induced hyperammonemia: inhibition of hepatic N-acetylglutamate synthase activity by valproyl-CoA. J Hepatol. 2011;55(2):426-434.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Lactulose • Enulose
Levocarnitine • Carnitine, Carnitor
Levofloxacin • Levaquin IV
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Quetiapine • Seroquel
Risperidone • Risperdal
Topiramate • Topamax
Valproic acid • Depakene
Mrs. C, age 75, is transferred to our inpatient medical/surgical hospital from a psychiatric hospital after presenting with shortness of breath and altered mental status.
Eight days earlier, Mrs. C had been admitted to the psychiatric hospital for bipolar mania with psychotic features. While there, Mrs. C received quetiapine, 400 mg nightly, and an initial valproic acid (VPA) dosage of 500 mg 2 times daily. While receiving VPA 500 mg 2 times daily, her VPA total level was 62 µg/mL, which is on the lower end of the therapeutic range (50 to 125 µg/mL). This prompted the team at the psychiatric hospital to increase her VPA dosage to 500 mg 3 times daily the day before she was transferred to our hospital.
At our hospital, she is found to be in hypoxic respiratory failure secondary to pneumonia. Upon admission, her laboratory data show evidence of infection and anemia and she also has an
From hospital Day 3 to Day 6, Mrs. C experiences gradual improvement in her respiratory and mental status. However, on hospital Day 7, she has extreme somnolence and altered mental status without respiratory involvement. Our team suspects VPA toxicity and/or VPA-induced hyperammonemic encephalopathy (VHE).
VPA-induced hyperammonemia
Hyperammonemia can occur in individuals receiving VPA and is most often asymptomatic. However, elevations in ammonia may lead to VHE, which is a rare but serious adverse effect. VHE has been reported early in treatment, in acute VPA overdose, and in chronic VPA use despite normal doses and levels.1 It also can occur in the absence of clinical and laboratory evidence of hepatotoxicity. VHE is associated with significant morbidity and CNS damage. Symptoms of VHE include vomiting, lethargy, and confusion. If left untreated, VHE can lead to coma and death.
Mechanism of VHE. The exact mechanism of VHE is unknown.1-3 Ammonia is a toxic base produced by deamination of amino acids. The liver eliminates ammonia via the urea cycle.2 Valproic acid metabolites, propionate and 4-en-VPA, can directly inhibit N-acetyl glutamate, which can disrupt the urea cycle, leading to elevated ammonia levels.3 Long-term or high-dose VPA can lead to carnitine deficiency, primarily by inhibiting its biosynthesis and depleting stores.4 Carnitine deficiency leads to disturbances in mitochondrial function, causing inhibition of the urea cycle and increasing ammonia. CNS toxicity due to hyperammonemia is thought to be due to activation of glutamate receptors.3
Risk factors. Co-administration of other antiepileptic drugs (AEDs) with VPA is a risk factor for VHE.1,5 This happens because enzyme-inducing AEDs such as phenytoin, phenobarbital, and carbamazepine can increase toxic metabolites of VPA, which can lead to hyperammonemia. Topiramate can also inhibit the urea cycle, leading to increased ammonia levels. Additionally, co-administration of VPA with quetiapine, paliperidone, risperidone, or aripiprazole has been reported to increase the risk of VHE.1,5 Intellectual disability, carnitine deficiency, low albumin, and abnormal liver function have also been reported to increase the risk of VHE.1,5
Continue to: Diagnosis and management
Diagnosis and management. If a patient receiving VPA is experiencing nausea, fatigue, or somnolence, it is important to check the patient’s ammonia level (normal range: 11 to 32 µmol/L) and VPA total levels (therapeutic range: 50 to 125 µg/mL). Consider checking a VPA free level, especially in geriatric patients or patients who have low albumin; the therapeutic range of VPA free is 6 to 22 µg/mL.3 If the ammonia level is elevated, discontinue VPA immediately (Table).1-3 Clinicians may also elect to prescribe lactulose until ammonia levels return to normal range. Adding levocarnitine may also help, although evidence is limited to small case series or retrospective studies.3 Currently, there is no known advantage in combining lactulose and levocarnitine to address VHE. Severe cases of VHE (ammonia levels >400 µmol/L) may require hemodialysis.1
Prevention. Strategies to prevent VHE include avoiding polypharmacy, especially concurrent use of enzyme-inducing AEDs and possibly second-generation antipsychotics. Additionally, VPA should not be used in individuals with urea cycle disorders. It is unknown if levocarnitine supplementation is preventive, but this approach has been suggested.3
CASE CONTINUED
Mrs. C has several possible risk factors for VHE, including co-administration of quetiapine and VPA, and a low albumin level. A further laboratory workup for Mrs. C reveals a VPA free level of 19 µg/mL (21.1% free), a VPA total level of 90 µg/mL, and an ammonia level of 79 µmol/L, confirming our suspicions regarding VHE. We determine that Mrs. C’s altered mental status is likely due her elevated ammonia levels, because the infection had been improving in the days leading up to the sudden, extreme somnolence.
VPA is immediately stopped and Mrs. C receives 1 dose of lactulose. The following day, Mrs. C’s mental status improves, and her ammonia levels return to normal. On hospital Day 9, she is transferred back to the psychiatric facility for management of manic and psychotic symptoms.
Related Resources
- Brown LM, Cupples N, Moore TA. Levocarnitine for valproate-induced hyperammonemia in the psychiatric setting: a case series and literature review. Ment Health Clin. 2018;8(3):148-154.
- Aires CCP, van Cruchten A, Ijlat L, et al. New insights on the mechanisms of valproate-induced hyperammonemia: inhibition of hepatic N-acetylglutamate synthase activity by valproyl-CoA. J Hepatol. 2011;55(2):426-434.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Lactulose • Enulose
Levocarnitine • Carnitine, Carnitor
Levofloxacin • Levaquin IV
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Quetiapine • Seroquel
Risperidone • Risperdal
Topiramate • Topamax
Valproic acid • Depakene
1. Chopra A, Kolla BP, Mansukhani MP, et al. Valproate-induced hyperammonemic encephalopathy: an update on risk factors, clinical correlates, and management. Gen Hosp Psychiatry. 2012;34(3):290-298.
2. Kowalski PC, Dowben JS, Keltner NL. Ammonium: the deadly toxin you don’t want to miss when using mood stabilizers. Perspect Psychiatr Care. 2013;49(4):221-225.
3. Baddour E, Tewksbury A, Stauner N. Valproic acid-induced hyper ammonemia: incidence, clinical significance, and treatment management. Ment Health Clin. 2018;8(2):73-77.
4. Raskind JY, El-Chaar GM. The role of carnitine supplementation during valproic acid therapy. Ann Pharmacother. 2000;34(5):630-638. 5. Tseng YL, Huang CR, Lin CH, et al. Risk factors of hyperammonemia in patients with epilepsy. Medicine (Baltimore). 2014;93(11):e66. doi: 10.1097/MD.0000000000000066.
1. Chopra A, Kolla BP, Mansukhani MP, et al. Valproate-induced hyperammonemic encephalopathy: an update on risk factors, clinical correlates, and management. Gen Hosp Psychiatry. 2012;34(3):290-298.
2. Kowalski PC, Dowben JS, Keltner NL. Ammonium: the deadly toxin you don’t want to miss when using mood stabilizers. Perspect Psychiatr Care. 2013;49(4):221-225.
3. Baddour E, Tewksbury A, Stauner N. Valproic acid-induced hyper ammonemia: incidence, clinical significance, and treatment management. Ment Health Clin. 2018;8(2):73-77.
4. Raskind JY, El-Chaar GM. The role of carnitine supplementation during valproic acid therapy. Ann Pharmacother. 2000;34(5):630-638. 5. Tseng YL, Huang CR, Lin CH, et al. Risk factors of hyperammonemia in patients with epilepsy. Medicine (Baltimore). 2014;93(11):e66. doi: 10.1097/MD.0000000000000066.
When guideline treatment of asthma fails, consider a macrolide antibiotic
In vitro laboratory and in vivo animal models support the biologic plausibility that chronic infection is a potential cause of asthma.1,2 Arising from that hypothesis, macrolide antibiotics have been the subject of clinical trials and other studies to determine whether these drugs are efficacious in the long-term management of asthma in adults and children. Macrolides might also have immunomodulatory and antiviral properties that can benefit patients with asthma.3
This article looks at the evidence and clinical scenarios for the use of macrolides in asthma, provides proposed dosing schedules, and reviews associated concerns, including adverse effects, risk of bacterial resistance, and cost.
3 cases to consider
CASE 1 Paul D developed severe, refractory asthma at 30 years of age after an acute respiratory illness. At age 40, he was treated with 14 weekly doses of azithromycin. His asthma resolved slowly over 12 months.
Outcome. Mr. D has remained free of symptoms of asthma for more than 20 years.
CASE 2 Casey K developed severe wheezing at 18 months of age after an acute respiratory illness. Refractory asthma symptoms persisted until 6 years of age, at which time he was given 12 weekly doses of azithromycin. Asthma symptoms gradually resolved.
Outcome. Casey was able to resume normal physical activities, including competitive swimming.
CASE 3 Amy S, who had no history of respiratory problems, presented at 30 years of age with a 3-month history of wheezing and dyspnea after an acute respiratory illness. She was treated symptomatically with bronchodilators; wheezing failed to resolve. After 6 months of persistent wheezing that significantly affected her exercise capacity, Ms. S was given a diagnosis of persistent asthma and received 12 weekly doses of azithromycin.
[polldaddy:10475438]
Continue to: Outcome...
Outcome. Ms. S’s symptoms resolved completely within months.
Evidence of benefit of macrolides in asthma
These 3 cases, taken from my practice (but with names changed), demonstrate the therapeutic potential of macrolide antibiotics for patients with asthma under specific clinical circumstances. The cases are referenced again in the following examination of the literature on macrolides for asthma
SIDEBAR
Macrolides for Asthma: Registry of Clinical Experience
More information is needed about the “real world” effectiveness of antibiotic treatment for severe refractory and new-onset asthma. If you are a prescribing clinician who cares for patients with asthma and you are considering prescribing antibiotics for asthma, you are invited to document your outcomes by entering prospective, de-identified patient data into a human subjects committee-approved online registry. To gain access to the registry, and for more information, contact the author at [email protected] or visit https://www.fammed.wisc.edu/wren/resources/macrolides-for-asthma/ .
Meta-analysis. Reiter et al4 performed a meta-analysis of 12 randomized clinical trials of macrolides for long-term management of asthma in children and adults. Prolonged treatment was defined as > 3 weeks of continuous administration of a macrolide. The pooled effect of macrolides on forced expiratory volume in 1 second (FEV1) was not significant; however, a significant effect on peak expiratory flow, symptom scores, quality of life, and airway hyperreactivity was observed.
Comment: The study’s authors concluded: “Macrolides may therefore be beneficial as adjunct asthma therapy. Future trials, focusing on long-term safety and effectiveness, should use standardized outcomes and procedures.”
Cochrane meta-analysis. Kew et al5 performed a meta-analysis of 23 studies of macrolides for managing chronic asthma for the Cochrane Database of Systematic Reviews. In their review, they reported
- no significant effects of macrolides on asthma exacerbations, asthma control, quality of life, and rescue medication use; and
- significant effects of macrolides for asthma symptoms and FEV1.
Continue to: Two within-study subgroup...
Two within-study subgroup analyses showed a possible benefit of macrolides for non-eosinophilic asthma, defined by a predominance of neutrophils in a bronchoalveolar lavage specimen. Kew et al5 noted that (1) most of the evidence examined in the review was of low quality and (2) inclusion criteria, interventions, and outcomes were highly variable.
Comment: The validity of a meta-analysis depends on the validity and similarity of underlying trials. Both meta-analyses just described were characterized by (1) grouping trials of older and newer macrolides and (2) significant selection bias in the underlying trials.
Selection bias is prevalent in asthma research and is a major contributor to uncertainty: Randomized controlled trials upon which guideline treatments are based have systematically excluded > 90% of people with asthma.6 Exclusions include past or current smoking, the asthma–chronic obstructive pulmonary disease (COPD) overlap syndrome, severe asthma, and acute respiratory illness; these exclusion criteria have also been applied to studies of macrolides. Importantly, patients in the excluded groups are probably those most likely to respond to a macrolide.2 Pragmatic effectiveness studies (broad eligibility criteria, adequate duration of azithromycin treatment, a posttreatment observation period, and pre-specified biomarker subgroup analyses) have been recommended to address the hypothesis of what has been termed infectious asthma.2
Inconsistent evidence, the generally poor quality of underlying studies, and uncertainty about which subgroup(s) of asthma patients might benefit all contribute to a strength of recommendation of “B” for treating asthma with macrolides. Two recent randomized trials7,8 that were not included in the cited meta-analyses, along with other evidence,2 point to 2 groups of patients who are candidates for a trial of azithromycin: those with severe refractory asthma and those with new-onset asthma.
Clinical trial in adults. Gibson et al7 conducted a randomized, double-blind, placebo-controlled trial of azithromycin 500 mg 3 times a week or placebo for 1 year in 420 adults who had uncontrolled persistent asthma despite taking medium-to-high doses of an inhaled corticosteroid (ICS) plus a long-acting β agonist (LABA) (the AMAZES [Asthma and Macrolides: The Azithromycin Efficacy and Safety] trial; Level 1 study). The mean baseline asthma control questionnaire score was 1.5, equivalent to an Asthma Control Test (ACT) score* of 15.9
Continue to: Azithromycin reduced the frequency...
Azithromycin reduced the frequency of asthma exacerbations (to 1.07 per patient–year for azithromycin, compared with 1.86 per patient–year for placebo [incidence rate ratio = 0.59; 95% confidence interval (CI), 0.47-0.74]). The percentage of patients experiencing at least 1 exacerbation was reduced with azithromycin treatment (61% of patients in the placebo group experienced ≥ 1 exacerbation, compared with 44% in the azithromycin group [P < .0001; number needed to treat = 6]). Asthma quality of life was also improved by azithromycin (P = .001).
There was no significant difference between azithromycin and placebo in the overall rate of serious adverse events. Diarrhea that did not require treatment discontinuation was more common in patients treated with azithromycin (34%) than in the placebo group (19%). There was no posttreatment observation period to assess whether these azithromycin benefits waned or persisted after treatment was stopped.
Other evidence10 indicates that at least some patients who respond to azithromycin will experience persistent improvement after antibiotic treatment is completed (see CASE 1).
Pediatric clinical trial. Stokholm et al8 performed a randomized, double-blind, placebo-controlled trial of azithromycin in children 1 to 3 years of age who had been given a diagnosis of recurrent asthma-like symptoms (Level 1 study). Treatment was a 3-day course of azithromycin oral solution, 10 mg/kg/d, or placebo. Random allocation was performed for 158 asthma-like episodes in 72 children.
Azithromycin reduced the wheezing episode to a mean duration of 3.4 days, compared with 7.7 days for placebo (risk reduction = 63.3%; 95% CI, 56%-69.3% [P < .0001]). Effect size increased with early initiation of treatment: ie, an 83% reduction in episode duration was seen when treatment was initiated before Day 6 of the episode, compared with a 36% reduction if treatment was initiated on or after Day 6 (P < .0001).
Continue to: No differences between...
No differences between the randomized groups were observed in clinical adverse effects.
Comment: The brief course of azithromycin provided to patients in this trial did not have a significant impact on time to next episode of troublesome lung symptoms in individual children. Previous clinical observations have suggested that a longer duration of treatment (3-6 months) might be required to achieve lasting improvement or remission in selected patients with asthma (see CASE 2).10,11 The short-term benefit of azithromycin for acute wheezing is limited to children: Two comparable acute dosing trials in adults have shown little12 or no13 short-term benefit; however, these negative findings have been hypothesized to be the result of selection bias.14
Other evidence is worth examining
Other studies not included in the meta-analyses of randomized controlled trials provide additional evidence to support a recommendation of a trial of azithromycin in patients with severe, refractory, or new-onset asthma.
Nonrandomized controlled evidence. AZMATICS (AZithroMycin/Asthma Trial In Community Settings)15 is the sole randomized, double-blind, placebo-controlled trial of long-term azithromycin that included a 9-month posttreatment observation period. Seventy-five participants were randomized to receive a loading dose of 600 mg of azithromycin or placebo once daily for 3 days in Week 1. They then received either azithromycin 600 mg or placebo once weekly for 11 weeks. Posttreatment observation was performed until 48 weeks after randomization.
However, many eligible subjects, whom the principal investigator believed were ideal candidates for randomization, declined randomization because they did not want to risk receiving placebo. To accommodate those patients, the protocol was amended to include an open-label (OL) azithromycin arm, in which each participant’s personal physician prescribed azithromycin 750 mg for 11 weeks after a loading dose16 (OL cohort only, Level 2 study: controlled, nonrandomized, nonblinded). The OL group had (1) a higher baseline prevalence of severe, persistent asthma (32%) than the randomized group (8%) (P = .012); and (2) worse asthma quality of life than the randomized patients (P = .023). The OL group represented selection bias attributable to patient preference.
Continue to: The less severely...
The less severely affected randomized group of the trial did not exhibit significant effects attributable to azithromycin. The more severely affected OL cohort demonstrated significant, and large, azithromycin treatment effects for asthma symptoms, asthma quality of life, and asthma control (P < .05 for both groups; number needed to treat [NNT] = 3) that persisted during the posttreatment observation period.
Comment: The authors concluded: “Pending further randomized trials and given the relative safety of azithromycin and the significant disease burden from severe, refractory asthma, prescribing prolonged azithromycin therapy to patients with uncontrolled asthma may be considered by managing clinicians, particularly for patients who have failed to respond to conventional treatment and as an alternative to instituting immunomodulatory agents.”15
Before-and-after trial. Forty-six patients with moderate or severe chronic, persistent, stable asthma were selected as a cohort unlikely to experience spontaneous remission (ie, patients in exacerbation were excluded) (Level 2 study: prospective cohort).17 Subjects were treated for a median of 4 weeks (range, 3 to 9 weeks) with oral doxycycline, 100 mg bid; azithromycin, 1000 mg, once weekly; or erythromycin, 1000 mg/d in divided doses. Average duration of posttreatment follow-up was 6 months. All subjects were positive for antibodies to Chlamydia pneumoniae.
Four patients with diagnosed acuteC pneumoniae respiratory infection developed chronic asthma, which disappeared in each case after treatment. Of the other 42 seroreactive patients who were treated a mean of 6 years after they developed chronic asthma, 21 had either complete remission of asthma symptoms (n = 3) or major persistent clinical improvement (n = 18). Clinical improvement was more likely to occur in patients with early disease (P = .01) and before development of fixed airway obstruction (P < .01).
These results are consistent with the hypothesis that chronic infection of the lower respiratory tract contributes to the development and progression of asthma.17 Although clinical improvement was more likely in early asthma compared with asthma with fixed airway obstruction, improvement was nevertheless noted in the latter group.
Continue to: Physicians should also note...
Physicians should also note the landmark trial of azithromycin in severe, smoking-associated COPD, which found a clinically significant benefit in reducing exacerbations and improving quality of life (NNT = 3, to prevent 1 exacerbation).18
Case series. In a prospective case series (Level 2 study: prospective cohort), 163 primary care outpatients (adolescents and adults) who had acute wheezing illnesses or chronic asthma were evaluated for C pneumoniae infection by serologic testing.19 A subgroup of this cohort also had nasopharyngeal cultures tested for C pneumoniae.
Twenty patients (12%) were given a diagnosis of C pneumoniae infection defined by serology (n = 15), culture isolation (n = 3), or both (n = 2). Of the 20, 10 wheezed for the first time—6 of whom subsequently developed chronic asthma (n = 5) or chronic bronchitis (n = 1), with a serologic profile suggesting chronic infection. The other 10 patients who had a diagnosis of C pneumoniae infection already had a diagnosis of chronic asthma. In patients with established chronic asthma, initial serologic findings suggested chronic, rather than acute, C pneumoniae infection.
Tx recommendations: When to consider azithromycin
Randomized7 and nonrandomized15 evidence supports treating severely uncontrolled or refractory asthma (strength of recommendation [SOR], B); no comparable randomized trials of azithromycin have been conducted for new-onset asthma (SOR, C). Consider prescribing empiric azithromycin for patients with new-onset asthma in the context of shared decision making about potential benefits, harms, and consequences of chronic asthma (SOR, C).
It is important to note that wheezing is frequently associated with uncomplicated acute bronchitis that resolves spontaneously without antibiotic treatment.11 Azithromycin treatment for new-onset asthma should therefore be reserved for patients in whom apparent uncomplicated acute bronchitis fails to resolve after 3 to 6 months, and whose illness is diagnosable as asthma (see CASE 3).10
Continue to: Do biomarkers predict response?
Do biomarkers predict response?
Confirming C pneumoniae infection by bronchoscopy before beginning treatment has been recommended20 but might be impractical; also, diagnostic testing for C pneumoniae is limited in availability and has potentially low sensitivity for diagnosing chronic deep lung infection.
So should you test for C pneumoniae biomarkers (or for biomarkers of Mycoplasma pneumoniae, another atypical infection implicated in the pathogenesis of asthma21) before initiating treatment? Azithromycin has antimicrobial, immunomodulatory, and potential antiviral properties.3 The body of evidence reviewed here indicates that the effects of macrolides on asthma might be, at least in part, antimicrobial. However, there is no direct evidence that the benefit of azithromycin in asthma is limited to patients who have positive infection biomarkers.22 Therefore, infection biomarker testing as a decision aid cannot be recommended at this time (although future research might alter this recommendation).
Acute bronchitis and asthma-onset associated with an acute lower respiratory tract infection have been statistically associated with biomarkers of C pneumoniae infection.23 However, C pneumoniae biomarkers are also prevalent in patients who have asthma that is not associated with an infectious onset.23 Several other matters are worth noting:
- C pneumoniae-specific IgA23 and IgE24 are promising biomarkers that deserve further investigation.
- M pneumoniae infection has also been associated with asthma and a response to antibiotic therapy.21,25
- Noneosinophilic severe asthma is another potential predictive characteristic.26 The applicability of this biomarker to primary care practice is limited, however, by the invasive nature of bronchoscopy and by the uncertain validity of the diagnostic concept: There is no guarantee that dynamic inflammatory infiltrates remain stable over a lifetime. Furthermore, the AMAZES Trial7 reported that azithromycin benefit was comparable in eosinophilic and noneosinophilic asthma.
Potential for harm withlong-term macrolide use?
Controversies about the role of macrolides in asthma involve uncertainty about who might benefit from treatment and the potential harms of macrolides use (TABLE 127,28 and discussed below).29
Adverse effects. The newer macrolides azithromycin and clarithromycin offer favorable safety and tolerability profiles, compared with those of older agents.30 In clinical trials of azithromycin, gastrointestinal symptoms (nausea, vomiting, abdominal pain, and diarrhea) were usually mild or moderate and rarely (< 2% of subjects) required discontinuation of study medication.31,32Clostridium difficile diarrhea has not been reported in any of the large clinical trials, in which thousands of patients received azithromycin for 3 to 12 months.31,32 The major clinical “side effects” attributable to azithromycin are a significant reduction, compared to placebo, in acute respiratory illness, bronchitis, pneumonia, and sinusitis.31,32
Continue to: Antibiotic resistance
Antibiotic resistance. Exposure of populations to macrolides can increase the percentage of macrolide-resistant bacterial respiratory pathogens33; policies aimed at decreasing inappropriate macrolide prescribing can significantly lower that percentage.34 There is no evidence, however, of any detrimental effects of macrolide resistance in individual patients receiving azithromycin.33
In trials of azithromycin for the treatment of trachoma in Africa, significantly fewer deaths occurred in villages where subjects were treated with azithromycin than in villages where azithromycin therapy was not provided.35 In the United States, weekly azithromycin treatment for 3 to 12 months in adults with heart disease resulted in fewer cases of acute bronchitis and pneumonia, compared with the placebo-treated groups31,32; similar benefit for azithromycin was seen in children who had recurrent lung infection.8,36
Nevertheless, concern over the spread of macrolide-resistant bacteria to the surrounding community is a concern and a possibility—and should be the subject of future research.
Sudden cardiac death. In a Medicaid population, the risk of sudden cardiac death from taking a macrolide among patients at high risk of cardiovascular disease was 1 in every 4000 administrations.27 Compare that level of risk with the 1 in 167 risk of an acute cardiovascular event in patients with COPD who start taking a LABA.37 There is no detectable increase in the risk of sudden cardiac death when taking azithromycin in the general (ie, average cardiovascular risk) population38,39 or when azithromycin is coadministered with a LABA.3
Hearing loss. An excess of 18 (< 1%) patients affected by hearing loss, 7 of whom sought medical attention, was reported among 2004 patients who had stable coronary artery disease and had been treated once weekly with azithromycin for 12 months (P = .02, compared with placebo).32 In another study, hearing test changes leading to discontinuation of azithromycin were detected in an excess of 32
Continue to: Physicians who prescribe...
Physicians who prescribe long-term azithromycin should instruct patients to report any hearing loss.
Drug–drug interactions. Azithromycin is free of the drug–drug interactions characteristic of conventional macrolides, such as clarithromycin.40 Nevertheless:
- Caution is advised when giving azithromycin in conjunction with coumadin or theophylline.
- Giving azithromycin with antacids that contain aluminum or magnesium salts can reduce the rate, although not the extent, of the absorption of azithromycin.
- The serum concentration of azithromycin is markedly increased when it is given with nelfinavir.40
Microbiome effects. The host microbiome can have a significant effect on the risk of asthma.2 A cross-sectional study indicated that lower respiratory bacterial burden is greater in patients with asthma, compared with that of healthy control subjects, and correlates with bronchial hyperresponsiveness.41 Early colonization of the infant nasopharynx, particularly with Streptococcus spp, is a predictor of asthma risk.42,43 Bacterial pathogens in the nasopharyngeal biome at the time of upper respiratory viral infection are significant determinants of risk for the spread of infection to the lower airways, suggesting that these microorganisms contribute to the risk of persistent asthma.41
Investigators have speculated that, rather than increasing the risk of asthma by disrupting the “healthy” microbiome, azithromycin might be helpful in treating an “unhealthy” microbiome.42,43 Recently, it was shown in a randomized trial that azithromycin induced a perturbation in the gut microbiota of children 14 days after randomization, although the drug did not have a long-lasting effect on the composition of gut microbiota.44
What about cost?
Inhaled corticosteroids and combination formulations of an ICS and a LABA are expensive and must be taken for the long term. A 3-month course of generic azithromycin—comparable to what was used in the OL subgroup of AZMATICS15—costs about as much as 1 ICS and LABA combination inhaler. Using published results,15,45 a pilot cost-effectiveness analysis in patients with persistent asthma compared doubling the ICS dosage, adding salmeterol, adding tiotropium, or prescribing 3 months of azithromycin. In the long run, azithromycin was 10 to 20 times as cost-effective as the other 3 therapeutic options for improving asthma quality-of-life outcomes.* However, reliable cost-effectiveness analyses require more, and better, evidence.
Continue to: Recommendations to reflect on for your practice
Recommendations to reflect on for your practice
Table 27,15 outlines selected long-term (≥ 3 months) macrolide dosing schedules in the management of asthma. Consider a trial of azithromycin for your patients
- whose asthma is refractory (poorly controlled persistent asthma), despite treatment with either an ICS and LABA combination or an ICS and long-acting muscarinic antagonist combination; and
- who have new-onset asthma.
Last, there is no evidence for or against prescribing azithromycin for patients who have chronic asthma that is not refractory but is uncontrolled because they are not being treated according to guidelines.
*Data available from the author upon request. See “Correspondence,” at end of article.
CORRESPONDENCE
David L. Hahn, MD, MS, Department of Family Medicine & Community Health, University of Wisconsin School of Medicine & Public Health, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
1. Hahn DL. Role of Chlamydia pneumoniae as an inducer of asthma. In: Friedman H, Yamamoto Y, Bendinelli M, eds. Chlamydia Pneumoniae: Infection and Disease. New York: Kluwer Academic/Plenum Publishers; 2004:239-262.
2. Webley WC, Hahn DL. Infection-mediated asthma: etiology, mechanisms and treatment options, with focus on Chlamydia pneumoniae and macrolides. Respir Res. 2017;18:98.
3. Wong EH, Porter JD, Edwards MR, et al. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med. 2014;2:657-670.
4. Reiter J, Demirel N, Mendy A, et al. Macrolides for the long-term management of asthma—a meta-analysis of randomized clinical trials. Allergy. 2013;68:1040-1049.
5. Kew KM, Undela K, Kotortsi I, et al. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015(9):CD002997.
6. Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62:219-223.
7. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
8. Stokholm J, Chawes BL, Vissing NH, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:19-26.
9. Korn S, Both J, Jung M, et al. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol. 2011;107:474-479.
10. Hahn DL. A Cure for Asthma? What Your Doctor Isn’t Telling You—and Why. Durham, North Carolina: Peoples Pharmacy Press; 2013.
11. Hahn DL. Acute asthmatic bronchitis: a new twist to an old problem. J Fam Pract. 1994;39:431-435.
12. Johnston SL, Blasi F, Black PN, et al; TELICAST Investigators. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589-1600.
13. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma: the AZALEA Randomized Clinical Trial. JAMA Intern Med. 2016;176:1630-1637.
14. Brusselle GG, Van Braeckel E. AZALEA trial highlights antibiotic overuse in acute asthma attacks. JAMA Intern Med. 2016;176:1637-1638.
15. Hahn DL, Grasmick M, Hetzel S, et al; AZMATICS (AZithroMycinAsthma Trial In Community Settings) Study Group. Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med. 2012;25:442-459.
16. Hahn DL. An unanticipated effect of clinical trial registration. BMJ.com. November 2, 2007. https://www.bmj.com/rapid-response/2011/11/01/unanticipated-effect-clinical-trial-registration. Accessed November 2, 2019.
17. Hahn DL. Treatment of Chlamydia pneumoniae infection in adult asthma: a before-after trial. J Fam Pract. 1995;41:345-351.
18. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
19. Hahn DL, McDonald R. Can acute Chlamydia pneumoniae infection initiate chronic asthma? Ann Allergy Asthma Immunol. 1998;81:339-344.
20. Rollins DR, Beuther DA, Martin RJ. Update on infection and antibiotics in asthma. Curr Allergy Asthma Rep. 2010;10:67-73.
21. Martin RJ, Kraft M, Chu HW, et al. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595-601.
22. Hahn DL, Plane MB, Mahdi OS, et al. Secondary outcomes of a pilot randomized trial of azithromycin treatment for asthma. PLoS Clin Trials. 2006;1:e11.
23. Hahn DL, Peeling RW, Dillon E, et al. Serologic markers for Chlamydia pneumoniae in asthma. Ann Allergy Asthma Immunol. 2000;84: 227-233.
24. Hahn DL, Schure A, Patel K, et al. Chlamydia pneumoniae-specific IgE is prevalent in asthma and is associated with disease severity. PLoS One. 2012;7:e35945.
25. Kraft M, Cassell GH, Pak J, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121:1782-1788.
26. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
27. Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
28. Jespersen CM, Als-Nielsen B, Damgaard M, et al. Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. BMJ. 2006;332:22-27.
29. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343-373.
30. Jackson LA, Stewart DK, Wang SP, et al. Safety and effect on antiChlamydia pneumoniae antibody titres of a 1 month course of daily azithromycin in adults with coronary artery disease. J Antimicrob Chemother. 1999;44:411-414.
31. O’Connor CM, Dunne MW, Pfeffer MA, et al; Investigators in the WIZARD Study. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA. 2003;290:1459-1466.
32. Grayston JT, Kronmal RA, Jackson LA, et al; ACES Investigators. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637-1645.
33. Skalet AH, Cevallos V, Ayele B, et al. Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med. 2010;7:e1000377.
34. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441-446.
35. Keenan JD, Emerson PM, Gaynor BD, et al. Adult mortality in a randomized trial of mass azithromycin for trachoma. JAMA Intern Med. 2013;173:821-833.
36. Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314:2034-2044.
37. Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med. 2018;178:229-238.
38. Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med. 2013;368:1704-1712.
39. Khosropour CM, Capizzi JD, Schafer SD, et al. Lack of association between azithromycin and death from cardiovascular causes. N Engl J Med. 2014;370:1961-1962.
40. Bakheit AH, Al-Hadiya BM, Abd-Elgalil AA. Azithromycin. Profiles Drug Subst Excip Relat Methodol. 2014;39:1-40.
41. Huang YJ, Nelson CE, Brodie EL, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372-381.e1-3.
42. Bisgaard H, Hermansen MN, Bønnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978.
43. Teo SM, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704-715.
44. Wei S, Mortensen MS, Stokholm J, et al. Short- and long-term impacts of azithromycin treatment on the gut microbiota in children: a double-blind, randomized, placebo-controlled trial. EBioMedicine. 2018;38:265-272.
45. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. New Engl J Med. 2010;363:1715-1726.
In vitro laboratory and in vivo animal models support the biologic plausibility that chronic infection is a potential cause of asthma.1,2 Arising from that hypothesis, macrolide antibiotics have been the subject of clinical trials and other studies to determine whether these drugs are efficacious in the long-term management of asthma in adults and children. Macrolides might also have immunomodulatory and antiviral properties that can benefit patients with asthma.3
This article looks at the evidence and clinical scenarios for the use of macrolides in asthma, provides proposed dosing schedules, and reviews associated concerns, including adverse effects, risk of bacterial resistance, and cost.
3 cases to consider
CASE 1 Paul D developed severe, refractory asthma at 30 years of age after an acute respiratory illness. At age 40, he was treated with 14 weekly doses of azithromycin. His asthma resolved slowly over 12 months.
Outcome. Mr. D has remained free of symptoms of asthma for more than 20 years.
CASE 2 Casey K developed severe wheezing at 18 months of age after an acute respiratory illness. Refractory asthma symptoms persisted until 6 years of age, at which time he was given 12 weekly doses of azithromycin. Asthma symptoms gradually resolved.
Outcome. Casey was able to resume normal physical activities, including competitive swimming.
CASE 3 Amy S, who had no history of respiratory problems, presented at 30 years of age with a 3-month history of wheezing and dyspnea after an acute respiratory illness. She was treated symptomatically with bronchodilators; wheezing failed to resolve. After 6 months of persistent wheezing that significantly affected her exercise capacity, Ms. S was given a diagnosis of persistent asthma and received 12 weekly doses of azithromycin.
[polldaddy:10475438]
Continue to: Outcome...
Outcome. Ms. S’s symptoms resolved completely within months.
Evidence of benefit of macrolides in asthma
These 3 cases, taken from my practice (but with names changed), demonstrate the therapeutic potential of macrolide antibiotics for patients with asthma under specific clinical circumstances. The cases are referenced again in the following examination of the literature on macrolides for asthma
SIDEBAR
Macrolides for Asthma: Registry of Clinical Experience
More information is needed about the “real world” effectiveness of antibiotic treatment for severe refractory and new-onset asthma. If you are a prescribing clinician who cares for patients with asthma and you are considering prescribing antibiotics for asthma, you are invited to document your outcomes by entering prospective, de-identified patient data into a human subjects committee-approved online registry. To gain access to the registry, and for more information, contact the author at [email protected] or visit https://www.fammed.wisc.edu/wren/resources/macrolides-for-asthma/ .
Meta-analysis. Reiter et al4 performed a meta-analysis of 12 randomized clinical trials of macrolides for long-term management of asthma in children and adults. Prolonged treatment was defined as > 3 weeks of continuous administration of a macrolide. The pooled effect of macrolides on forced expiratory volume in 1 second (FEV1) was not significant; however, a significant effect on peak expiratory flow, symptom scores, quality of life, and airway hyperreactivity was observed.
Comment: The study’s authors concluded: “Macrolides may therefore be beneficial as adjunct asthma therapy. Future trials, focusing on long-term safety and effectiveness, should use standardized outcomes and procedures.”
Cochrane meta-analysis. Kew et al5 performed a meta-analysis of 23 studies of macrolides for managing chronic asthma for the Cochrane Database of Systematic Reviews. In their review, they reported
- no significant effects of macrolides on asthma exacerbations, asthma control, quality of life, and rescue medication use; and
- significant effects of macrolides for asthma symptoms and FEV1.
Continue to: Two within-study subgroup...
Two within-study subgroup analyses showed a possible benefit of macrolides for non-eosinophilic asthma, defined by a predominance of neutrophils in a bronchoalveolar lavage specimen. Kew et al5 noted that (1) most of the evidence examined in the review was of low quality and (2) inclusion criteria, interventions, and outcomes were highly variable.
Comment: The validity of a meta-analysis depends on the validity and similarity of underlying trials. Both meta-analyses just described were characterized by (1) grouping trials of older and newer macrolides and (2) significant selection bias in the underlying trials.
Selection bias is prevalent in asthma research and is a major contributor to uncertainty: Randomized controlled trials upon which guideline treatments are based have systematically excluded > 90% of people with asthma.6 Exclusions include past or current smoking, the asthma–chronic obstructive pulmonary disease (COPD) overlap syndrome, severe asthma, and acute respiratory illness; these exclusion criteria have also been applied to studies of macrolides. Importantly, patients in the excluded groups are probably those most likely to respond to a macrolide.2 Pragmatic effectiveness studies (broad eligibility criteria, adequate duration of azithromycin treatment, a posttreatment observation period, and pre-specified biomarker subgroup analyses) have been recommended to address the hypothesis of what has been termed infectious asthma.2
Inconsistent evidence, the generally poor quality of underlying studies, and uncertainty about which subgroup(s) of asthma patients might benefit all contribute to a strength of recommendation of “B” for treating asthma with macrolides. Two recent randomized trials7,8 that were not included in the cited meta-analyses, along with other evidence,2 point to 2 groups of patients who are candidates for a trial of azithromycin: those with severe refractory asthma and those with new-onset asthma.
Clinical trial in adults. Gibson et al7 conducted a randomized, double-blind, placebo-controlled trial of azithromycin 500 mg 3 times a week or placebo for 1 year in 420 adults who had uncontrolled persistent asthma despite taking medium-to-high doses of an inhaled corticosteroid (ICS) plus a long-acting β agonist (LABA) (the AMAZES [Asthma and Macrolides: The Azithromycin Efficacy and Safety] trial; Level 1 study). The mean baseline asthma control questionnaire score was 1.5, equivalent to an Asthma Control Test (ACT) score* of 15.9
Continue to: Azithromycin reduced the frequency...
Azithromycin reduced the frequency of asthma exacerbations (to 1.07 per patient–year for azithromycin, compared with 1.86 per patient–year for placebo [incidence rate ratio = 0.59; 95% confidence interval (CI), 0.47-0.74]). The percentage of patients experiencing at least 1 exacerbation was reduced with azithromycin treatment (61% of patients in the placebo group experienced ≥ 1 exacerbation, compared with 44% in the azithromycin group [P < .0001; number needed to treat = 6]). Asthma quality of life was also improved by azithromycin (P = .001).
There was no significant difference between azithromycin and placebo in the overall rate of serious adverse events. Diarrhea that did not require treatment discontinuation was more common in patients treated with azithromycin (34%) than in the placebo group (19%). There was no posttreatment observation period to assess whether these azithromycin benefits waned or persisted after treatment was stopped.
Other evidence10 indicates that at least some patients who respond to azithromycin will experience persistent improvement after antibiotic treatment is completed (see CASE 1).
Pediatric clinical trial. Stokholm et al8 performed a randomized, double-blind, placebo-controlled trial of azithromycin in children 1 to 3 years of age who had been given a diagnosis of recurrent asthma-like symptoms (Level 1 study). Treatment was a 3-day course of azithromycin oral solution, 10 mg/kg/d, or placebo. Random allocation was performed for 158 asthma-like episodes in 72 children.
Azithromycin reduced the wheezing episode to a mean duration of 3.4 days, compared with 7.7 days for placebo (risk reduction = 63.3%; 95% CI, 56%-69.3% [P < .0001]). Effect size increased with early initiation of treatment: ie, an 83% reduction in episode duration was seen when treatment was initiated before Day 6 of the episode, compared with a 36% reduction if treatment was initiated on or after Day 6 (P < .0001).
Continue to: No differences between...
No differences between the randomized groups were observed in clinical adverse effects.
Comment: The brief course of azithromycin provided to patients in this trial did not have a significant impact on time to next episode of troublesome lung symptoms in individual children. Previous clinical observations have suggested that a longer duration of treatment (3-6 months) might be required to achieve lasting improvement or remission in selected patients with asthma (see CASE 2).10,11 The short-term benefit of azithromycin for acute wheezing is limited to children: Two comparable acute dosing trials in adults have shown little12 or no13 short-term benefit; however, these negative findings have been hypothesized to be the result of selection bias.14
Other evidence is worth examining
Other studies not included in the meta-analyses of randomized controlled trials provide additional evidence to support a recommendation of a trial of azithromycin in patients with severe, refractory, or new-onset asthma.
Nonrandomized controlled evidence. AZMATICS (AZithroMycin/Asthma Trial In Community Settings)15 is the sole randomized, double-blind, placebo-controlled trial of long-term azithromycin that included a 9-month posttreatment observation period. Seventy-five participants were randomized to receive a loading dose of 600 mg of azithromycin or placebo once daily for 3 days in Week 1. They then received either azithromycin 600 mg or placebo once weekly for 11 weeks. Posttreatment observation was performed until 48 weeks after randomization.
However, many eligible subjects, whom the principal investigator believed were ideal candidates for randomization, declined randomization because they did not want to risk receiving placebo. To accommodate those patients, the protocol was amended to include an open-label (OL) azithromycin arm, in which each participant’s personal physician prescribed azithromycin 750 mg for 11 weeks after a loading dose16 (OL cohort only, Level 2 study: controlled, nonrandomized, nonblinded). The OL group had (1) a higher baseline prevalence of severe, persistent asthma (32%) than the randomized group (8%) (P = .012); and (2) worse asthma quality of life than the randomized patients (P = .023). The OL group represented selection bias attributable to patient preference.
Continue to: The less severely...
The less severely affected randomized group of the trial did not exhibit significant effects attributable to azithromycin. The more severely affected OL cohort demonstrated significant, and large, azithromycin treatment effects for asthma symptoms, asthma quality of life, and asthma control (P < .05 for both groups; number needed to treat [NNT] = 3) that persisted during the posttreatment observation period.
Comment: The authors concluded: “Pending further randomized trials and given the relative safety of azithromycin and the significant disease burden from severe, refractory asthma, prescribing prolonged azithromycin therapy to patients with uncontrolled asthma may be considered by managing clinicians, particularly for patients who have failed to respond to conventional treatment and as an alternative to instituting immunomodulatory agents.”15
Before-and-after trial. Forty-six patients with moderate or severe chronic, persistent, stable asthma were selected as a cohort unlikely to experience spontaneous remission (ie, patients in exacerbation were excluded) (Level 2 study: prospective cohort).17 Subjects were treated for a median of 4 weeks (range, 3 to 9 weeks) with oral doxycycline, 100 mg bid; azithromycin, 1000 mg, once weekly; or erythromycin, 1000 mg/d in divided doses. Average duration of posttreatment follow-up was 6 months. All subjects were positive for antibodies to Chlamydia pneumoniae.
Four patients with diagnosed acuteC pneumoniae respiratory infection developed chronic asthma, which disappeared in each case after treatment. Of the other 42 seroreactive patients who were treated a mean of 6 years after they developed chronic asthma, 21 had either complete remission of asthma symptoms (n = 3) or major persistent clinical improvement (n = 18). Clinical improvement was more likely to occur in patients with early disease (P = .01) and before development of fixed airway obstruction (P < .01).
These results are consistent with the hypothesis that chronic infection of the lower respiratory tract contributes to the development and progression of asthma.17 Although clinical improvement was more likely in early asthma compared with asthma with fixed airway obstruction, improvement was nevertheless noted in the latter group.
Continue to: Physicians should also note...
Physicians should also note the landmark trial of azithromycin in severe, smoking-associated COPD, which found a clinically significant benefit in reducing exacerbations and improving quality of life (NNT = 3, to prevent 1 exacerbation).18
Case series. In a prospective case series (Level 2 study: prospective cohort), 163 primary care outpatients (adolescents and adults) who had acute wheezing illnesses or chronic asthma were evaluated for C pneumoniae infection by serologic testing.19 A subgroup of this cohort also had nasopharyngeal cultures tested for C pneumoniae.
Twenty patients (12%) were given a diagnosis of C pneumoniae infection defined by serology (n = 15), culture isolation (n = 3), or both (n = 2). Of the 20, 10 wheezed for the first time—6 of whom subsequently developed chronic asthma (n = 5) or chronic bronchitis (n = 1), with a serologic profile suggesting chronic infection. The other 10 patients who had a diagnosis of C pneumoniae infection already had a diagnosis of chronic asthma. In patients with established chronic asthma, initial serologic findings suggested chronic, rather than acute, C pneumoniae infection.
Tx recommendations: When to consider azithromycin
Randomized7 and nonrandomized15 evidence supports treating severely uncontrolled or refractory asthma (strength of recommendation [SOR], B); no comparable randomized trials of azithromycin have been conducted for new-onset asthma (SOR, C). Consider prescribing empiric azithromycin for patients with new-onset asthma in the context of shared decision making about potential benefits, harms, and consequences of chronic asthma (SOR, C).
It is important to note that wheezing is frequently associated with uncomplicated acute bronchitis that resolves spontaneously without antibiotic treatment.11 Azithromycin treatment for new-onset asthma should therefore be reserved for patients in whom apparent uncomplicated acute bronchitis fails to resolve after 3 to 6 months, and whose illness is diagnosable as asthma (see CASE 3).10
Continue to: Do biomarkers predict response?
Do biomarkers predict response?
Confirming C pneumoniae infection by bronchoscopy before beginning treatment has been recommended20 but might be impractical; also, diagnostic testing for C pneumoniae is limited in availability and has potentially low sensitivity for diagnosing chronic deep lung infection.
So should you test for C pneumoniae biomarkers (or for biomarkers of Mycoplasma pneumoniae, another atypical infection implicated in the pathogenesis of asthma21) before initiating treatment? Azithromycin has antimicrobial, immunomodulatory, and potential antiviral properties.3 The body of evidence reviewed here indicates that the effects of macrolides on asthma might be, at least in part, antimicrobial. However, there is no direct evidence that the benefit of azithromycin in asthma is limited to patients who have positive infection biomarkers.22 Therefore, infection biomarker testing as a decision aid cannot be recommended at this time (although future research might alter this recommendation).
Acute bronchitis and asthma-onset associated with an acute lower respiratory tract infection have been statistically associated with biomarkers of C pneumoniae infection.23 However, C pneumoniae biomarkers are also prevalent in patients who have asthma that is not associated with an infectious onset.23 Several other matters are worth noting:
- C pneumoniae-specific IgA23 and IgE24 are promising biomarkers that deserve further investigation.
- M pneumoniae infection has also been associated with asthma and a response to antibiotic therapy.21,25
- Noneosinophilic severe asthma is another potential predictive characteristic.26 The applicability of this biomarker to primary care practice is limited, however, by the invasive nature of bronchoscopy and by the uncertain validity of the diagnostic concept: There is no guarantee that dynamic inflammatory infiltrates remain stable over a lifetime. Furthermore, the AMAZES Trial7 reported that azithromycin benefit was comparable in eosinophilic and noneosinophilic asthma.
Potential for harm withlong-term macrolide use?
Controversies about the role of macrolides in asthma involve uncertainty about who might benefit from treatment and the potential harms of macrolides use (TABLE 127,28 and discussed below).29
Adverse effects. The newer macrolides azithromycin and clarithromycin offer favorable safety and tolerability profiles, compared with those of older agents.30 In clinical trials of azithromycin, gastrointestinal symptoms (nausea, vomiting, abdominal pain, and diarrhea) were usually mild or moderate and rarely (< 2% of subjects) required discontinuation of study medication.31,32Clostridium difficile diarrhea has not been reported in any of the large clinical trials, in which thousands of patients received azithromycin for 3 to 12 months.31,32 The major clinical “side effects” attributable to azithromycin are a significant reduction, compared to placebo, in acute respiratory illness, bronchitis, pneumonia, and sinusitis.31,32
Continue to: Antibiotic resistance
Antibiotic resistance. Exposure of populations to macrolides can increase the percentage of macrolide-resistant bacterial respiratory pathogens33; policies aimed at decreasing inappropriate macrolide prescribing can significantly lower that percentage.34 There is no evidence, however, of any detrimental effects of macrolide resistance in individual patients receiving azithromycin.33
In trials of azithromycin for the treatment of trachoma in Africa, significantly fewer deaths occurred in villages where subjects were treated with azithromycin than in villages where azithromycin therapy was not provided.35 In the United States, weekly azithromycin treatment for 3 to 12 months in adults with heart disease resulted in fewer cases of acute bronchitis and pneumonia, compared with the placebo-treated groups31,32; similar benefit for azithromycin was seen in children who had recurrent lung infection.8,36
Nevertheless, concern over the spread of macrolide-resistant bacteria to the surrounding community is a concern and a possibility—and should be the subject of future research.
Sudden cardiac death. In a Medicaid population, the risk of sudden cardiac death from taking a macrolide among patients at high risk of cardiovascular disease was 1 in every 4000 administrations.27 Compare that level of risk with the 1 in 167 risk of an acute cardiovascular event in patients with COPD who start taking a LABA.37 There is no detectable increase in the risk of sudden cardiac death when taking azithromycin in the general (ie, average cardiovascular risk) population38,39 or when azithromycin is coadministered with a LABA.3
Hearing loss. An excess of 18 (< 1%) patients affected by hearing loss, 7 of whom sought medical attention, was reported among 2004 patients who had stable coronary artery disease and had been treated once weekly with azithromycin for 12 months (P = .02, compared with placebo).32 In another study, hearing test changes leading to discontinuation of azithromycin were detected in an excess of 32
Continue to: Physicians who prescribe...
Physicians who prescribe long-term azithromycin should instruct patients to report any hearing loss.
Drug–drug interactions. Azithromycin is free of the drug–drug interactions characteristic of conventional macrolides, such as clarithromycin.40 Nevertheless:
- Caution is advised when giving azithromycin in conjunction with coumadin or theophylline.
- Giving azithromycin with antacids that contain aluminum or magnesium salts can reduce the rate, although not the extent, of the absorption of azithromycin.
- The serum concentration of azithromycin is markedly increased when it is given with nelfinavir.40
Microbiome effects. The host microbiome can have a significant effect on the risk of asthma.2 A cross-sectional study indicated that lower respiratory bacterial burden is greater in patients with asthma, compared with that of healthy control subjects, and correlates with bronchial hyperresponsiveness.41 Early colonization of the infant nasopharynx, particularly with Streptococcus spp, is a predictor of asthma risk.42,43 Bacterial pathogens in the nasopharyngeal biome at the time of upper respiratory viral infection are significant determinants of risk for the spread of infection to the lower airways, suggesting that these microorganisms contribute to the risk of persistent asthma.41
Investigators have speculated that, rather than increasing the risk of asthma by disrupting the “healthy” microbiome, azithromycin might be helpful in treating an “unhealthy” microbiome.42,43 Recently, it was shown in a randomized trial that azithromycin induced a perturbation in the gut microbiota of children 14 days after randomization, although the drug did not have a long-lasting effect on the composition of gut microbiota.44
What about cost?
Inhaled corticosteroids and combination formulations of an ICS and a LABA are expensive and must be taken for the long term. A 3-month course of generic azithromycin—comparable to what was used in the OL subgroup of AZMATICS15—costs about as much as 1 ICS and LABA combination inhaler. Using published results,15,45 a pilot cost-effectiveness analysis in patients with persistent asthma compared doubling the ICS dosage, adding salmeterol, adding tiotropium, or prescribing 3 months of azithromycin. In the long run, azithromycin was 10 to 20 times as cost-effective as the other 3 therapeutic options for improving asthma quality-of-life outcomes.* However, reliable cost-effectiveness analyses require more, and better, evidence.
Continue to: Recommendations to reflect on for your practice
Recommendations to reflect on for your practice
Table 27,15 outlines selected long-term (≥ 3 months) macrolide dosing schedules in the management of asthma. Consider a trial of azithromycin for your patients
- whose asthma is refractory (poorly controlled persistent asthma), despite treatment with either an ICS and LABA combination or an ICS and long-acting muscarinic antagonist combination; and
- who have new-onset asthma.
Last, there is no evidence for or against prescribing azithromycin for patients who have chronic asthma that is not refractory but is uncontrolled because they are not being treated according to guidelines.
*Data available from the author upon request. See “Correspondence,” at end of article.
CORRESPONDENCE
David L. Hahn, MD, MS, Department of Family Medicine & Community Health, University of Wisconsin School of Medicine & Public Health, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
In vitro laboratory and in vivo animal models support the biologic plausibility that chronic infection is a potential cause of asthma.1,2 Arising from that hypothesis, macrolide antibiotics have been the subject of clinical trials and other studies to determine whether these drugs are efficacious in the long-term management of asthma in adults and children. Macrolides might also have immunomodulatory and antiviral properties that can benefit patients with asthma.3
This article looks at the evidence and clinical scenarios for the use of macrolides in asthma, provides proposed dosing schedules, and reviews associated concerns, including adverse effects, risk of bacterial resistance, and cost.
3 cases to consider
CASE 1 Paul D developed severe, refractory asthma at 30 years of age after an acute respiratory illness. At age 40, he was treated with 14 weekly doses of azithromycin. His asthma resolved slowly over 12 months.
Outcome. Mr. D has remained free of symptoms of asthma for more than 20 years.
CASE 2 Casey K developed severe wheezing at 18 months of age after an acute respiratory illness. Refractory asthma symptoms persisted until 6 years of age, at which time he was given 12 weekly doses of azithromycin. Asthma symptoms gradually resolved.
Outcome. Casey was able to resume normal physical activities, including competitive swimming.
CASE 3 Amy S, who had no history of respiratory problems, presented at 30 years of age with a 3-month history of wheezing and dyspnea after an acute respiratory illness. She was treated symptomatically with bronchodilators; wheezing failed to resolve. After 6 months of persistent wheezing that significantly affected her exercise capacity, Ms. S was given a diagnosis of persistent asthma and received 12 weekly doses of azithromycin.
[polldaddy:10475438]
Continue to: Outcome...
Outcome. Ms. S’s symptoms resolved completely within months.
Evidence of benefit of macrolides in asthma
These 3 cases, taken from my practice (but with names changed), demonstrate the therapeutic potential of macrolide antibiotics for patients with asthma under specific clinical circumstances. The cases are referenced again in the following examination of the literature on macrolides for asthma
SIDEBAR
Macrolides for Asthma: Registry of Clinical Experience
More information is needed about the “real world” effectiveness of antibiotic treatment for severe refractory and new-onset asthma. If you are a prescribing clinician who cares for patients with asthma and you are considering prescribing antibiotics for asthma, you are invited to document your outcomes by entering prospective, de-identified patient data into a human subjects committee-approved online registry. To gain access to the registry, and for more information, contact the author at [email protected] or visit https://www.fammed.wisc.edu/wren/resources/macrolides-for-asthma/ .
Meta-analysis. Reiter et al4 performed a meta-analysis of 12 randomized clinical trials of macrolides for long-term management of asthma in children and adults. Prolonged treatment was defined as > 3 weeks of continuous administration of a macrolide. The pooled effect of macrolides on forced expiratory volume in 1 second (FEV1) was not significant; however, a significant effect on peak expiratory flow, symptom scores, quality of life, and airway hyperreactivity was observed.
Comment: The study’s authors concluded: “Macrolides may therefore be beneficial as adjunct asthma therapy. Future trials, focusing on long-term safety and effectiveness, should use standardized outcomes and procedures.”
Cochrane meta-analysis. Kew et al5 performed a meta-analysis of 23 studies of macrolides for managing chronic asthma for the Cochrane Database of Systematic Reviews. In their review, they reported
- no significant effects of macrolides on asthma exacerbations, asthma control, quality of life, and rescue medication use; and
- significant effects of macrolides for asthma symptoms and FEV1.
Continue to: Two within-study subgroup...
Two within-study subgroup analyses showed a possible benefit of macrolides for non-eosinophilic asthma, defined by a predominance of neutrophils in a bronchoalveolar lavage specimen. Kew et al5 noted that (1) most of the evidence examined in the review was of low quality and (2) inclusion criteria, interventions, and outcomes were highly variable.
Comment: The validity of a meta-analysis depends on the validity and similarity of underlying trials. Both meta-analyses just described were characterized by (1) grouping trials of older and newer macrolides and (2) significant selection bias in the underlying trials.
Selection bias is prevalent in asthma research and is a major contributor to uncertainty: Randomized controlled trials upon which guideline treatments are based have systematically excluded > 90% of people with asthma.6 Exclusions include past or current smoking, the asthma–chronic obstructive pulmonary disease (COPD) overlap syndrome, severe asthma, and acute respiratory illness; these exclusion criteria have also been applied to studies of macrolides. Importantly, patients in the excluded groups are probably those most likely to respond to a macrolide.2 Pragmatic effectiveness studies (broad eligibility criteria, adequate duration of azithromycin treatment, a posttreatment observation period, and pre-specified biomarker subgroup analyses) have been recommended to address the hypothesis of what has been termed infectious asthma.2
Inconsistent evidence, the generally poor quality of underlying studies, and uncertainty about which subgroup(s) of asthma patients might benefit all contribute to a strength of recommendation of “B” for treating asthma with macrolides. Two recent randomized trials7,8 that were not included in the cited meta-analyses, along with other evidence,2 point to 2 groups of patients who are candidates for a trial of azithromycin: those with severe refractory asthma and those with new-onset asthma.
Clinical trial in adults. Gibson et al7 conducted a randomized, double-blind, placebo-controlled trial of azithromycin 500 mg 3 times a week or placebo for 1 year in 420 adults who had uncontrolled persistent asthma despite taking medium-to-high doses of an inhaled corticosteroid (ICS) plus a long-acting β agonist (LABA) (the AMAZES [Asthma and Macrolides: The Azithromycin Efficacy and Safety] trial; Level 1 study). The mean baseline asthma control questionnaire score was 1.5, equivalent to an Asthma Control Test (ACT) score* of 15.9
Continue to: Azithromycin reduced the frequency...
Azithromycin reduced the frequency of asthma exacerbations (to 1.07 per patient–year for azithromycin, compared with 1.86 per patient–year for placebo [incidence rate ratio = 0.59; 95% confidence interval (CI), 0.47-0.74]). The percentage of patients experiencing at least 1 exacerbation was reduced with azithromycin treatment (61% of patients in the placebo group experienced ≥ 1 exacerbation, compared with 44% in the azithromycin group [P < .0001; number needed to treat = 6]). Asthma quality of life was also improved by azithromycin (P = .001).
There was no significant difference between azithromycin and placebo in the overall rate of serious adverse events. Diarrhea that did not require treatment discontinuation was more common in patients treated with azithromycin (34%) than in the placebo group (19%). There was no posttreatment observation period to assess whether these azithromycin benefits waned or persisted after treatment was stopped.
Other evidence10 indicates that at least some patients who respond to azithromycin will experience persistent improvement after antibiotic treatment is completed (see CASE 1).
Pediatric clinical trial. Stokholm et al8 performed a randomized, double-blind, placebo-controlled trial of azithromycin in children 1 to 3 years of age who had been given a diagnosis of recurrent asthma-like symptoms (Level 1 study). Treatment was a 3-day course of azithromycin oral solution, 10 mg/kg/d, or placebo. Random allocation was performed for 158 asthma-like episodes in 72 children.
Azithromycin reduced the wheezing episode to a mean duration of 3.4 days, compared with 7.7 days for placebo (risk reduction = 63.3%; 95% CI, 56%-69.3% [P < .0001]). Effect size increased with early initiation of treatment: ie, an 83% reduction in episode duration was seen when treatment was initiated before Day 6 of the episode, compared with a 36% reduction if treatment was initiated on or after Day 6 (P < .0001).
Continue to: No differences between...
No differences between the randomized groups were observed in clinical adverse effects.
Comment: The brief course of azithromycin provided to patients in this trial did not have a significant impact on time to next episode of troublesome lung symptoms in individual children. Previous clinical observations have suggested that a longer duration of treatment (3-6 months) might be required to achieve lasting improvement or remission in selected patients with asthma (see CASE 2).10,11 The short-term benefit of azithromycin for acute wheezing is limited to children: Two comparable acute dosing trials in adults have shown little12 or no13 short-term benefit; however, these negative findings have been hypothesized to be the result of selection bias.14
Other evidence is worth examining
Other studies not included in the meta-analyses of randomized controlled trials provide additional evidence to support a recommendation of a trial of azithromycin in patients with severe, refractory, or new-onset asthma.
Nonrandomized controlled evidence. AZMATICS (AZithroMycin/Asthma Trial In Community Settings)15 is the sole randomized, double-blind, placebo-controlled trial of long-term azithromycin that included a 9-month posttreatment observation period. Seventy-five participants were randomized to receive a loading dose of 600 mg of azithromycin or placebo once daily for 3 days in Week 1. They then received either azithromycin 600 mg or placebo once weekly for 11 weeks. Posttreatment observation was performed until 48 weeks after randomization.
However, many eligible subjects, whom the principal investigator believed were ideal candidates for randomization, declined randomization because they did not want to risk receiving placebo. To accommodate those patients, the protocol was amended to include an open-label (OL) azithromycin arm, in which each participant’s personal physician prescribed azithromycin 750 mg for 11 weeks after a loading dose16 (OL cohort only, Level 2 study: controlled, nonrandomized, nonblinded). The OL group had (1) a higher baseline prevalence of severe, persistent asthma (32%) than the randomized group (8%) (P = .012); and (2) worse asthma quality of life than the randomized patients (P = .023). The OL group represented selection bias attributable to patient preference.
Continue to: The less severely...
The less severely affected randomized group of the trial did not exhibit significant effects attributable to azithromycin. The more severely affected OL cohort demonstrated significant, and large, azithromycin treatment effects for asthma symptoms, asthma quality of life, and asthma control (P < .05 for both groups; number needed to treat [NNT] = 3) that persisted during the posttreatment observation period.
Comment: The authors concluded: “Pending further randomized trials and given the relative safety of azithromycin and the significant disease burden from severe, refractory asthma, prescribing prolonged azithromycin therapy to patients with uncontrolled asthma may be considered by managing clinicians, particularly for patients who have failed to respond to conventional treatment and as an alternative to instituting immunomodulatory agents.”15
Before-and-after trial. Forty-six patients with moderate or severe chronic, persistent, stable asthma were selected as a cohort unlikely to experience spontaneous remission (ie, patients in exacerbation were excluded) (Level 2 study: prospective cohort).17 Subjects were treated for a median of 4 weeks (range, 3 to 9 weeks) with oral doxycycline, 100 mg bid; azithromycin, 1000 mg, once weekly; or erythromycin, 1000 mg/d in divided doses. Average duration of posttreatment follow-up was 6 months. All subjects were positive for antibodies to Chlamydia pneumoniae.
Four patients with diagnosed acuteC pneumoniae respiratory infection developed chronic asthma, which disappeared in each case after treatment. Of the other 42 seroreactive patients who were treated a mean of 6 years after they developed chronic asthma, 21 had either complete remission of asthma symptoms (n = 3) or major persistent clinical improvement (n = 18). Clinical improvement was more likely to occur in patients with early disease (P = .01) and before development of fixed airway obstruction (P < .01).
These results are consistent with the hypothesis that chronic infection of the lower respiratory tract contributes to the development and progression of asthma.17 Although clinical improvement was more likely in early asthma compared with asthma with fixed airway obstruction, improvement was nevertheless noted in the latter group.
Continue to: Physicians should also note...
Physicians should also note the landmark trial of azithromycin in severe, smoking-associated COPD, which found a clinically significant benefit in reducing exacerbations and improving quality of life (NNT = 3, to prevent 1 exacerbation).18
Case series. In a prospective case series (Level 2 study: prospective cohort), 163 primary care outpatients (adolescents and adults) who had acute wheezing illnesses or chronic asthma were evaluated for C pneumoniae infection by serologic testing.19 A subgroup of this cohort also had nasopharyngeal cultures tested for C pneumoniae.
Twenty patients (12%) were given a diagnosis of C pneumoniae infection defined by serology (n = 15), culture isolation (n = 3), or both (n = 2). Of the 20, 10 wheezed for the first time—6 of whom subsequently developed chronic asthma (n = 5) or chronic bronchitis (n = 1), with a serologic profile suggesting chronic infection. The other 10 patients who had a diagnosis of C pneumoniae infection already had a diagnosis of chronic asthma. In patients with established chronic asthma, initial serologic findings suggested chronic, rather than acute, C pneumoniae infection.
Tx recommendations: When to consider azithromycin
Randomized7 and nonrandomized15 evidence supports treating severely uncontrolled or refractory asthma (strength of recommendation [SOR], B); no comparable randomized trials of azithromycin have been conducted for new-onset asthma (SOR, C). Consider prescribing empiric azithromycin for patients with new-onset asthma in the context of shared decision making about potential benefits, harms, and consequences of chronic asthma (SOR, C).
It is important to note that wheezing is frequently associated with uncomplicated acute bronchitis that resolves spontaneously without antibiotic treatment.11 Azithromycin treatment for new-onset asthma should therefore be reserved for patients in whom apparent uncomplicated acute bronchitis fails to resolve after 3 to 6 months, and whose illness is diagnosable as asthma (see CASE 3).10
Continue to: Do biomarkers predict response?
Do biomarkers predict response?
Confirming C pneumoniae infection by bronchoscopy before beginning treatment has been recommended20 but might be impractical; also, diagnostic testing for C pneumoniae is limited in availability and has potentially low sensitivity for diagnosing chronic deep lung infection.
So should you test for C pneumoniae biomarkers (or for biomarkers of Mycoplasma pneumoniae, another atypical infection implicated in the pathogenesis of asthma21) before initiating treatment? Azithromycin has antimicrobial, immunomodulatory, and potential antiviral properties.3 The body of evidence reviewed here indicates that the effects of macrolides on asthma might be, at least in part, antimicrobial. However, there is no direct evidence that the benefit of azithromycin in asthma is limited to patients who have positive infection biomarkers.22 Therefore, infection biomarker testing as a decision aid cannot be recommended at this time (although future research might alter this recommendation).
Acute bronchitis and asthma-onset associated with an acute lower respiratory tract infection have been statistically associated with biomarkers of C pneumoniae infection.23 However, C pneumoniae biomarkers are also prevalent in patients who have asthma that is not associated with an infectious onset.23 Several other matters are worth noting:
- C pneumoniae-specific IgA23 and IgE24 are promising biomarkers that deserve further investigation.
- M pneumoniae infection has also been associated with asthma and a response to antibiotic therapy.21,25
- Noneosinophilic severe asthma is another potential predictive characteristic.26 The applicability of this biomarker to primary care practice is limited, however, by the invasive nature of bronchoscopy and by the uncertain validity of the diagnostic concept: There is no guarantee that dynamic inflammatory infiltrates remain stable over a lifetime. Furthermore, the AMAZES Trial7 reported that azithromycin benefit was comparable in eosinophilic and noneosinophilic asthma.
Potential for harm withlong-term macrolide use?
Controversies about the role of macrolides in asthma involve uncertainty about who might benefit from treatment and the potential harms of macrolides use (TABLE 127,28 and discussed below).29
Adverse effects. The newer macrolides azithromycin and clarithromycin offer favorable safety and tolerability profiles, compared with those of older agents.30 In clinical trials of azithromycin, gastrointestinal symptoms (nausea, vomiting, abdominal pain, and diarrhea) were usually mild or moderate and rarely (< 2% of subjects) required discontinuation of study medication.31,32Clostridium difficile diarrhea has not been reported in any of the large clinical trials, in which thousands of patients received azithromycin for 3 to 12 months.31,32 The major clinical “side effects” attributable to azithromycin are a significant reduction, compared to placebo, in acute respiratory illness, bronchitis, pneumonia, and sinusitis.31,32
Continue to: Antibiotic resistance
Antibiotic resistance. Exposure of populations to macrolides can increase the percentage of macrolide-resistant bacterial respiratory pathogens33; policies aimed at decreasing inappropriate macrolide prescribing can significantly lower that percentage.34 There is no evidence, however, of any detrimental effects of macrolide resistance in individual patients receiving azithromycin.33
In trials of azithromycin for the treatment of trachoma in Africa, significantly fewer deaths occurred in villages where subjects were treated with azithromycin than in villages where azithromycin therapy was not provided.35 In the United States, weekly azithromycin treatment for 3 to 12 months in adults with heart disease resulted in fewer cases of acute bronchitis and pneumonia, compared with the placebo-treated groups31,32; similar benefit for azithromycin was seen in children who had recurrent lung infection.8,36
Nevertheless, concern over the spread of macrolide-resistant bacteria to the surrounding community is a concern and a possibility—and should be the subject of future research.
Sudden cardiac death. In a Medicaid population, the risk of sudden cardiac death from taking a macrolide among patients at high risk of cardiovascular disease was 1 in every 4000 administrations.27 Compare that level of risk with the 1 in 167 risk of an acute cardiovascular event in patients with COPD who start taking a LABA.37 There is no detectable increase in the risk of sudden cardiac death when taking azithromycin in the general (ie, average cardiovascular risk) population38,39 or when azithromycin is coadministered with a LABA.3
Hearing loss. An excess of 18 (< 1%) patients affected by hearing loss, 7 of whom sought medical attention, was reported among 2004 patients who had stable coronary artery disease and had been treated once weekly with azithromycin for 12 months (P = .02, compared with placebo).32 In another study, hearing test changes leading to discontinuation of azithromycin were detected in an excess of 32
Continue to: Physicians who prescribe...
Physicians who prescribe long-term azithromycin should instruct patients to report any hearing loss.
Drug–drug interactions. Azithromycin is free of the drug–drug interactions characteristic of conventional macrolides, such as clarithromycin.40 Nevertheless:
- Caution is advised when giving azithromycin in conjunction with coumadin or theophylline.
- Giving azithromycin with antacids that contain aluminum or magnesium salts can reduce the rate, although not the extent, of the absorption of azithromycin.
- The serum concentration of azithromycin is markedly increased when it is given with nelfinavir.40
Microbiome effects. The host microbiome can have a significant effect on the risk of asthma.2 A cross-sectional study indicated that lower respiratory bacterial burden is greater in patients with asthma, compared with that of healthy control subjects, and correlates with bronchial hyperresponsiveness.41 Early colonization of the infant nasopharynx, particularly with Streptococcus spp, is a predictor of asthma risk.42,43 Bacterial pathogens in the nasopharyngeal biome at the time of upper respiratory viral infection are significant determinants of risk for the spread of infection to the lower airways, suggesting that these microorganisms contribute to the risk of persistent asthma.41
Investigators have speculated that, rather than increasing the risk of asthma by disrupting the “healthy” microbiome, azithromycin might be helpful in treating an “unhealthy” microbiome.42,43 Recently, it was shown in a randomized trial that azithromycin induced a perturbation in the gut microbiota of children 14 days after randomization, although the drug did not have a long-lasting effect on the composition of gut microbiota.44
What about cost?
Inhaled corticosteroids and combination formulations of an ICS and a LABA are expensive and must be taken for the long term. A 3-month course of generic azithromycin—comparable to what was used in the OL subgroup of AZMATICS15—costs about as much as 1 ICS and LABA combination inhaler. Using published results,15,45 a pilot cost-effectiveness analysis in patients with persistent asthma compared doubling the ICS dosage, adding salmeterol, adding tiotropium, or prescribing 3 months of azithromycin. In the long run, azithromycin was 10 to 20 times as cost-effective as the other 3 therapeutic options for improving asthma quality-of-life outcomes.* However, reliable cost-effectiveness analyses require more, and better, evidence.
Continue to: Recommendations to reflect on for your practice
Recommendations to reflect on for your practice
Table 27,15 outlines selected long-term (≥ 3 months) macrolide dosing schedules in the management of asthma. Consider a trial of azithromycin for your patients
- whose asthma is refractory (poorly controlled persistent asthma), despite treatment with either an ICS and LABA combination or an ICS and long-acting muscarinic antagonist combination; and
- who have new-onset asthma.
Last, there is no evidence for or against prescribing azithromycin for patients who have chronic asthma that is not refractory but is uncontrolled because they are not being treated according to guidelines.
*Data available from the author upon request. See “Correspondence,” at end of article.
CORRESPONDENCE
David L. Hahn, MD, MS, Department of Family Medicine & Community Health, University of Wisconsin School of Medicine & Public Health, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
1. Hahn DL. Role of Chlamydia pneumoniae as an inducer of asthma. In: Friedman H, Yamamoto Y, Bendinelli M, eds. Chlamydia Pneumoniae: Infection and Disease. New York: Kluwer Academic/Plenum Publishers; 2004:239-262.
2. Webley WC, Hahn DL. Infection-mediated asthma: etiology, mechanisms and treatment options, with focus on Chlamydia pneumoniae and macrolides. Respir Res. 2017;18:98.
3. Wong EH, Porter JD, Edwards MR, et al. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med. 2014;2:657-670.
4. Reiter J, Demirel N, Mendy A, et al. Macrolides for the long-term management of asthma—a meta-analysis of randomized clinical trials. Allergy. 2013;68:1040-1049.
5. Kew KM, Undela K, Kotortsi I, et al. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015(9):CD002997.
6. Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62:219-223.
7. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
8. Stokholm J, Chawes BL, Vissing NH, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:19-26.
9. Korn S, Both J, Jung M, et al. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol. 2011;107:474-479.
10. Hahn DL. A Cure for Asthma? What Your Doctor Isn’t Telling You—and Why. Durham, North Carolina: Peoples Pharmacy Press; 2013.
11. Hahn DL. Acute asthmatic bronchitis: a new twist to an old problem. J Fam Pract. 1994;39:431-435.
12. Johnston SL, Blasi F, Black PN, et al; TELICAST Investigators. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589-1600.
13. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma: the AZALEA Randomized Clinical Trial. JAMA Intern Med. 2016;176:1630-1637.
14. Brusselle GG, Van Braeckel E. AZALEA trial highlights antibiotic overuse in acute asthma attacks. JAMA Intern Med. 2016;176:1637-1638.
15. Hahn DL, Grasmick M, Hetzel S, et al; AZMATICS (AZithroMycinAsthma Trial In Community Settings) Study Group. Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med. 2012;25:442-459.
16. Hahn DL. An unanticipated effect of clinical trial registration. BMJ.com. November 2, 2007. https://www.bmj.com/rapid-response/2011/11/01/unanticipated-effect-clinical-trial-registration. Accessed November 2, 2019.
17. Hahn DL. Treatment of Chlamydia pneumoniae infection in adult asthma: a before-after trial. J Fam Pract. 1995;41:345-351.
18. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
19. Hahn DL, McDonald R. Can acute Chlamydia pneumoniae infection initiate chronic asthma? Ann Allergy Asthma Immunol. 1998;81:339-344.
20. Rollins DR, Beuther DA, Martin RJ. Update on infection and antibiotics in asthma. Curr Allergy Asthma Rep. 2010;10:67-73.
21. Martin RJ, Kraft M, Chu HW, et al. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595-601.
22. Hahn DL, Plane MB, Mahdi OS, et al. Secondary outcomes of a pilot randomized trial of azithromycin treatment for asthma. PLoS Clin Trials. 2006;1:e11.
23. Hahn DL, Peeling RW, Dillon E, et al. Serologic markers for Chlamydia pneumoniae in asthma. Ann Allergy Asthma Immunol. 2000;84: 227-233.
24. Hahn DL, Schure A, Patel K, et al. Chlamydia pneumoniae-specific IgE is prevalent in asthma and is associated with disease severity. PLoS One. 2012;7:e35945.
25. Kraft M, Cassell GH, Pak J, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121:1782-1788.
26. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
27. Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
28. Jespersen CM, Als-Nielsen B, Damgaard M, et al. Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. BMJ. 2006;332:22-27.
29. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343-373.
30. Jackson LA, Stewart DK, Wang SP, et al. Safety and effect on antiChlamydia pneumoniae antibody titres of a 1 month course of daily azithromycin in adults with coronary artery disease. J Antimicrob Chemother. 1999;44:411-414.
31. O’Connor CM, Dunne MW, Pfeffer MA, et al; Investigators in the WIZARD Study. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA. 2003;290:1459-1466.
32. Grayston JT, Kronmal RA, Jackson LA, et al; ACES Investigators. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637-1645.
33. Skalet AH, Cevallos V, Ayele B, et al. Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med. 2010;7:e1000377.
34. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441-446.
35. Keenan JD, Emerson PM, Gaynor BD, et al. Adult mortality in a randomized trial of mass azithromycin for trachoma. JAMA Intern Med. 2013;173:821-833.
36. Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314:2034-2044.
37. Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med. 2018;178:229-238.
38. Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med. 2013;368:1704-1712.
39. Khosropour CM, Capizzi JD, Schafer SD, et al. Lack of association between azithromycin and death from cardiovascular causes. N Engl J Med. 2014;370:1961-1962.
40. Bakheit AH, Al-Hadiya BM, Abd-Elgalil AA. Azithromycin. Profiles Drug Subst Excip Relat Methodol. 2014;39:1-40.
41. Huang YJ, Nelson CE, Brodie EL, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372-381.e1-3.
42. Bisgaard H, Hermansen MN, Bønnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978.
43. Teo SM, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704-715.
44. Wei S, Mortensen MS, Stokholm J, et al. Short- and long-term impacts of azithromycin treatment on the gut microbiota in children: a double-blind, randomized, placebo-controlled trial. EBioMedicine. 2018;38:265-272.
45. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. New Engl J Med. 2010;363:1715-1726.
1. Hahn DL. Role of Chlamydia pneumoniae as an inducer of asthma. In: Friedman H, Yamamoto Y, Bendinelli M, eds. Chlamydia Pneumoniae: Infection and Disease. New York: Kluwer Academic/Plenum Publishers; 2004:239-262.
2. Webley WC, Hahn DL. Infection-mediated asthma: etiology, mechanisms and treatment options, with focus on Chlamydia pneumoniae and macrolides. Respir Res. 2017;18:98.
3. Wong EH, Porter JD, Edwards MR, et al. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med. 2014;2:657-670.
4. Reiter J, Demirel N, Mendy A, et al. Macrolides for the long-term management of asthma—a meta-analysis of randomized clinical trials. Allergy. 2013;68:1040-1049.
5. Kew KM, Undela K, Kotortsi I, et al. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015(9):CD002997.
6. Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62:219-223.
7. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
8. Stokholm J, Chawes BL, Vissing NH, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:19-26.
9. Korn S, Both J, Jung M, et al. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol. 2011;107:474-479.
10. Hahn DL. A Cure for Asthma? What Your Doctor Isn’t Telling You—and Why. Durham, North Carolina: Peoples Pharmacy Press; 2013.
11. Hahn DL. Acute asthmatic bronchitis: a new twist to an old problem. J Fam Pract. 1994;39:431-435.
12. Johnston SL, Blasi F, Black PN, et al; TELICAST Investigators. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589-1600.
13. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma: the AZALEA Randomized Clinical Trial. JAMA Intern Med. 2016;176:1630-1637.
14. Brusselle GG, Van Braeckel E. AZALEA trial highlights antibiotic overuse in acute asthma attacks. JAMA Intern Med. 2016;176:1637-1638.
15. Hahn DL, Grasmick M, Hetzel S, et al; AZMATICS (AZithroMycinAsthma Trial In Community Settings) Study Group. Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med. 2012;25:442-459.
16. Hahn DL. An unanticipated effect of clinical trial registration. BMJ.com. November 2, 2007. https://www.bmj.com/rapid-response/2011/11/01/unanticipated-effect-clinical-trial-registration. Accessed November 2, 2019.
17. Hahn DL. Treatment of Chlamydia pneumoniae infection in adult asthma: a before-after trial. J Fam Pract. 1995;41:345-351.
18. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
19. Hahn DL, McDonald R. Can acute Chlamydia pneumoniae infection initiate chronic asthma? Ann Allergy Asthma Immunol. 1998;81:339-344.
20. Rollins DR, Beuther DA, Martin RJ. Update on infection and antibiotics in asthma. Curr Allergy Asthma Rep. 2010;10:67-73.
21. Martin RJ, Kraft M, Chu HW, et al. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595-601.
22. Hahn DL, Plane MB, Mahdi OS, et al. Secondary outcomes of a pilot randomized trial of azithromycin treatment for asthma. PLoS Clin Trials. 2006;1:e11.
23. Hahn DL, Peeling RW, Dillon E, et al. Serologic markers for Chlamydia pneumoniae in asthma. Ann Allergy Asthma Immunol. 2000;84: 227-233.
24. Hahn DL, Schure A, Patel K, et al. Chlamydia pneumoniae-specific IgE is prevalent in asthma and is associated with disease severity. PLoS One. 2012;7:e35945.
25. Kraft M, Cassell GH, Pak J, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121:1782-1788.
26. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
27. Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
28. Jespersen CM, Als-Nielsen B, Damgaard M, et al. Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. BMJ. 2006;332:22-27.
29. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343-373.
30. Jackson LA, Stewart DK, Wang SP, et al. Safety and effect on antiChlamydia pneumoniae antibody titres of a 1 month course of daily azithromycin in adults with coronary artery disease. J Antimicrob Chemother. 1999;44:411-414.
31. O’Connor CM, Dunne MW, Pfeffer MA, et al; Investigators in the WIZARD Study. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA. 2003;290:1459-1466.
32. Grayston JT, Kronmal RA, Jackson LA, et al; ACES Investigators. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637-1645.
33. Skalet AH, Cevallos V, Ayele B, et al. Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med. 2010;7:e1000377.
34. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441-446.
35. Keenan JD, Emerson PM, Gaynor BD, et al. Adult mortality in a randomized trial of mass azithromycin for trachoma. JAMA Intern Med. 2013;173:821-833.
36. Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314:2034-2044.
37. Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med. 2018;178:229-238.
38. Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med. 2013;368:1704-1712.
39. Khosropour CM, Capizzi JD, Schafer SD, et al. Lack of association between azithromycin and death from cardiovascular causes. N Engl J Med. 2014;370:1961-1962.
40. Bakheit AH, Al-Hadiya BM, Abd-Elgalil AA. Azithromycin. Profiles Drug Subst Excip Relat Methodol. 2014;39:1-40.
41. Huang YJ, Nelson CE, Brodie EL, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372-381.e1-3.
42. Bisgaard H, Hermansen MN, Bønnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978.
43. Teo SM, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704-715.
44. Wei S, Mortensen MS, Stokholm J, et al. Short- and long-term impacts of azithromycin treatment on the gut microbiota in children: a double-blind, randomized, placebo-controlled trial. EBioMedicine. 2018;38:265-272.
45. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. New Engl J Med. 2010;363:1715-1726.
PRACTICE RECOMMENDATIONS
› Consider a trial of azithromycin for patients who have poorly controlled persistent asthma and are not responding to guideline treatment with the combination of an inhaled corticosteroid and either a long-acting bronchodilator or long-acting muscarinic antagonist. B
› Consider a trial of azithromycin in addition to first-line guideline therapy for patients who have new-onset asthma. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Patient-Reported Outcomes in Multiple Sclerosis: An Overview
From the Dartmouth Institute for Health Policy & Clinical Practice, Geisel School of Medicine, Hanover, NH (Ms. Manohar and Dr. Oliver), the Department of Community & Family Medicine, Dartmouth-Hitchcock Medical Center, Lebanon, NH (Ms. Perkins, Ms. Laurion, and Dr. Oliver), and the Multiple Sclerosis Specialty Care Program, Concord Hospital, Concord, NH (Dr. Oliver).
Abstract
- Background: Patient-reported outcomes (PROs), including patient-reported outcome measures (PROMs) and patient-reported experience measures (PREMs), can be used to assess perceived health status, functioning, quality of life, and experience of care. Complex chronic illnesses such as multiple sclerosis (MS) affect multiple aspects of health, and PROs can be applied in assessment and decision-making in MS care as well as in research pertaining to MS.
- Objective: To provide a general review of PROs, with a specific focus on implications for MS care.
- Methods: Evidence synthesis of available literature on PROs in MS care.
- Results: PROs (including PROMs and PREMs) have historically been utilized in research and are now being applied in clinical, improvement, and population health settings using learning health system approaches in many disease populations, including MS. Many challenges complicate the use of PROs in MS care, including reliability, validity, and interpretability of PROMs, as well as feasibility barriers due to time and financial constraints in clinical settings.
- Conclusion: PROs have the potential to better inform clinical care, empower patient-centered care, inform health care improvement efforts, and create the conditions for coproduction of health care services.
Keywords: PRO; PROM; patient-reported outcome measure; patient-reported experience measure; quality of life; patient-centered care.
Multiple sclerosis (MS) is a disabling, complex, chronic, immune-mediated disorder of the central nervous system (CNS). MS causes inflammatory and degenerative damage in the CNS, which disrupts signaling pathways.1 It is most commonly diagnosed in young adults and affects 2.3 million people worldwide.2 People with MS experience very different disease courses and a wide range of neurological symptoms, including visual, somatic, mental health, sensory, motor, and cognitive problems.1-3 Relapsing-remitting MS, the most common form, affects 85% of those with MS and is characterized by periods of relapse (exacerbation) and remission.1 Other forms of MS (primary progressive and secondary progressive MS) are characterized by progressive deterioration and worsening symptom severity without exacerbations. Disease-modifying therapies (DMTs) can reduce the frequency of exacerbations and disability progression, but unfortunately there is no cure for MS. Treatment is focused on increasing quality of life, minimizing disability, and maximizing wellness.
Patient-reported outcomes (PROs) describe the perceived health status, function, and/or experience of a person as obtained by direct self-report. Patient-reported outcome measures (PROMs) are validated PROs that can be used to inform clinical care,4 and have demonstrated effectiveness in improving patient-provider communication and decision-making.5-7 PROMs are currently used in some MS clinical trials to determine the impact of experimental interventions,8-10 and are also being used to inform and improve clinical care in some settings. Especially for persons with MS, they can provide individualized perspectives about health experience and outcomes.11 In more advanced applications, PROMs can be used to improve face-to-face collaborations between clinicians and patients and to inform patient-centered systems of care.12-14 PROMs can also be used to inform systems-level improvement for entire patient populations.15,16
In this article, we review current applications of PROs and PROMs in the care of persons with MS, as well as current limitations and barriers to their use.
At a recent visit to her neurologist, Marion reviews her health diary, in which she has been tracking her fatigue levels throughout the day and when she has to visit the bathroom. The PRO diary also helps her remember details that she might not otherwise be able to recall at the time of her clinic visit. They review the diary entrees together to develop a shared understanding of what Marion has been experiencing and identify trends in the PRO data. They discuss symptom management and use the PRO information from the diary to help guide adjustments to her physical therapy routine and medication regimen.
Part of Marion’s “PRO package” includes the Center for Epidemiologic Studies Depression Scale (CES-D), a validated depression screening and symptom severity questionnaire that she completes every 3 months. Although she denies being depressed, she has noticed that her CES-D scores in recent months have been consistently increasing. This prompts a discussion about mental health in MS and a referral to work on depression with the MS mental health specialist. Marion and the mental health specialist use CES-D measures at baseline and during treatment to set a remission target and to track progress during treatment. Marion finds this helpful because she says it is hard for her to “wrap my hands around depression… it’s not something that there is a blood test or a MRI for.” Marion is encouraged by being able to see her CES-D scores change as her depression severity decreases, and this helps motivate her to keep engaged in treatment.
PROs and PROMs: General Applications
PROs are measures obtained directly from an individual without a priori interpretation by a clinician.9,17 PROs capture individual perspectives on symptoms, capability, disability, and health-related quality of life.9 With increasing emphasis on patient-centered care,18 individual perspectives and preferences elicited using PROMs may be able to inform better quality of care and patient-centered disease treatment and management.19-21
PROMs are standardized, validated questionnaires used to assess PROs and can be generic or condition-specific. Generic PROMs can be used in any patient population. The SF-3622 is a set of quality of life measures that assess perceived ability to complete physical tasks and routine activities, general health status, fatigue, social functioning, pain, and emotional and mental health.23 Condition-specific PROMs can be used for particular patient populations and are helpful in identifying changes in health status for a specific disease, disability, or surgery. For example, the PDQ-39 assesses 8 dimensions of daily living, functioning, and well-being for people with Parkinson’s disease.24
PROMs have been used in some MS clinical trials and research studies to determine the effectiveness of experimental treatments from the viewpoint of study participants.9,25,26 PROs can also be utilized in clinical care to facilitate communication of needs and track health outcomes,27 and can inform improvement in outcomes for health systems and populations. They can also be used to assess experience of care,28 encouraging a focus on high-quality outcomes through PRO-connected reimbursement mechanisms,29 and provide aggregate data to evaluate clinical practice, population health outcomes, and the effectiveness of public policies.27
Patient-reported experience measures (PREMs) assess patient satisfaction and experience of health care.30,31 CollaboRATE32 is a PREM that assesses the degree of shared decision-making occurring between patients and clinicians during clinical care. PREMs are currently used for assessing self-efficacy and in shared decision-making and health care improvement applications. PREMs have yet to be developed specifically for persons with MS.
PROMs in MS Care
Generic PROMs have shown that persons with MS are disproportionately burdened by poor quality of life.33-35 Other generic PROMs, like the SF-36,36 the Sickness Impact Profile,37 and versions of the Health Utilities Index,38 can be used to gather information on dysfunction and to determine quality and duration of life modified by MS-related dysfunction and disability. MS-specific PROMs are used to assess MS impairments, including pain, fatigue, cognition, sexual dysfunction, and depression.12,39-42 PROMs have also been used in MS clinical trials, including the Multiple Sclerosis Impact Scale-29 (MSIS-29),43,44 the Leeds MS QoL (LMSQoL),45,46 the Functional Assessment of MS (FAMS),47 the Hamburg Quality of Life Questionnaire in MS (HAQUAMS),48 the MS Quality of Life-54 (MSQoL-54),49 the MS International QoL (MUSIQoL),50 and the Patient-Reported Indices for MS Activity Limitations Scale (PRIMUS).51
Condition-specific PROMs are more sensitive to changes in health status and functioning for persons with MS compared to generic PROMs. They are also more reliable during MS remission and relapse periods.44,52 For example, the SF-36 has floor and ceiling effects in MS populations—a high proportion of persons with MS are scored at the maximum or minimum levels of the scale, limiting discriminant capability.22 As a result, a “combined approach” using both generic and MS-specific measures is often recommended.53 Some MS PROMs (eg, MSQoL-54) include generic questions found in the SF-36 as well as additional MS-specific questions or scales.
The variety of PROMs available (see Table for a selected listing) introduces a significant challenge to using them—limited generalizability and difficulty comparing PROs across MS studies. Efforts to establish common PROMs have been undertaken to address this problem.54 The National Institute of Neurologicical Disorders and Stroke (NINDS) sponsored the development of a neurological quality of life battery, the Neuro-QOL.55 Neuro-QOL measures the physical, mental, and social effects of neurological conditions in adults and children with neurological disorders and has the capability to facilitate comparisons across different neurological conditions. Additionally, the Patient-Reported Outcomes Measure Information System (PROMIS) has been developed to assess physical, mental, and social effects of chronic disease. PROMIS has a hybrid design that includes generic and MS-specific measures (such as PROMIS FatigueMS).56 PROMIS can be used to assess persons with MS as well as to compare the MS population with other populations with chronic illness.
PROMs have varying levels of reliability and validity. The Evaluating the Measure of Patient-Reported Outcomes57 study evaluated the development process of MS PROMs,43 and found that the MSIS-29 and LMSQoL had the highest overall reliability among the most common MS PROMs. However, both scored poorly on validity due to lack of patient involvement during development. This questions the overall capability of existing MS PROMs to accurately and consistently assess PROs in persons with MS.
“Feed-Forward” PROMs
Oliver and colleagues16 have described “feed-forward” PROM applications in MS care in a community hospital setting using a learning health system approach. This MS clinic uses feed-forward PROs to inform clinical care—PRO data are gathered before the clinic visit and analyzed ahead of or during the clinic visit by the clinician. Patients are asked to arrive early and complete a questionnaire comprised of PROMs measuring disability, functioning, quality of life, cognitive ability, pain, fatigue, sleep quality, anxiety, and depression. Clinicians score the PROMs and input scores into the electronic health record before the clinical encounter. During the clinic visit, PROM data is visually displayed so that the clinician and patient can discuss results and use the data to better inform decision-making. The visual data display contains longitudinal information, displaying trends in health status across multiple domains, and includes specified thresholds for clinically active symptom levels (Figure).16 Longitudinal monitoring of PROM data allows for real-time assessment of goal-related progress throughout treatment. As illustrated previously by Marion’s case study, the use of real-time feed-forward PROM data can strengthen the partnership between patient and clinician as well as improve empowerment, engagement, self-monitoring, and adherence.
PRO Dashboards
Performance dashboards are increasingly used in health care to visually display clinical and PRO data for individual patients, systems, and populations over time. Dashboards display a parsimonious group of critically important measures to give clinicians and patients a longitudinal view of PRO status. They can inform decision-making in clinical care, operations, health care improvement efforts, and population health initiatives.58 Effective dashboards allow for user customization with meaningful measures, knowledge discovery for analysis of health problems, accessibility of health information, clear visualization, alerts for unexpected data values, and system connectivity.59,60 Appropriate development of PRO dashboards requires meaningful patient and clinician involvement via focus groups and key informant interviews, Delphi process approaches to prioritize and finalize selection of priority measures, iterative building of the interface with design input from key informants and stakeholders (co-design), and pilot testing to assess feasibility and acceptability of use.61-63
Other Applications of PROs/PROMs in MS
Learning Health Systems
The National Quality Forum (NQF) and the Centers for Medicaid and Medicare Services have adopted PROs for use in quality measurement.64-66 This includes a movement towards the use of LHS, defined as a health system in which information from patients and clinicians is systematically collected and synthesized with external evidence to inform clinical care, improvement, and research.67-70 Often a LHS is undertaken as a collaborative effort between multiple health care centers to improve quality and outcomes of care.70 The MS Continuous Quality Improvement Collaborative (MS-CQI), the first multi-center systems-level health care improvement research collaborative for MS,71 as well as IBD Qorus and the Cystic Fibrosis Care Center Network utilize LHS approaches.72-77
IBD Qorus is a LHS developed by the Crohn’s and Colitis Foundation that uses performance dashboards to better inform clinical care for people with inflammatory bowel disease. It also employs system-level dashboards for performance benchmarking in quality improvement initiatives and aggregate-level dashboards to assess population health status.78,79 MS-CQI uses a LHS approach to inform the improvement of MS care across multiple centers using a comprehensive dashboard, including PROMs, for benchmarking and to monitor system and population health status. MS-CQI collects PROMs using a secure online platform that can be accessed by persons with MS and their clinicians and also includes a journaling feature for collecting qualitative information and for reference and self-monitoring.71
MS Research
PROMs are used in clinical and epidemiological research to evaluate many aspects of MS, including the FAMS, the PDSS, the Fatigue Impact Scale (FIS), and others.80-82 For example, the PROMIS FatigueMS and the Fatigue Performance Scale have been used to assess the impact of MS-related fatigue on social participation.83 Generic and MS-specific PROMs have been used to assess pain levels for people with MS,84-87 and multiple MS-specific PROMs, like PRIMUS and MSQoL-54,43 as well as the SF-3639 include pain assessment scales. PROMs have also been used to assess MS-related bladder, bowel, and sexual dysfunction. Urgency, frequency, and incontinence affect up to 75% of patients with MS,88 and many PROMs, such as the LMSQoL, MUSIQoL, and the MSQoL-54, are able to evaluate bladder control and sexual functioning.43,89
PROMs are employed in MS clinical trials to help assess the tolerability and effectiveness of DMTs.90,91 PROs have been used as secondary endpoints to understand the global experience of a DMT from the patient perspective.92-94 There are 15 FDA-approved DMTs for MS, and clinical trials for 6 of these have used PROMs as an effectiveness end point.54,91,95,96 However, most DMT clinical trials are powered for MRI, relapse rate, or disease progression primary outcomes rather than PROMs, often resulting in underpowered PROM analyses.97 In addition, many PROMs are not appropriate for use in DMT clinical trials.98,99
In order to bridge the gap between clinical research and practice, some industry entities are championing “patient-focused drug development” approaches. The Accelerated Cure Project for MS has launched iConquerMS, which collects PROMs from persons with MS to further PRO research in MS and follows 4700 individuals with MS worldwide.100 In 2018, the American College of Physicians announced a collaboration with an industry partner to share data to inform DMT clinical trials and develop and validate PROMs specifically designed for DMT clinical trials.101
Population Health
Registries following large cohorts of people with MS have the potential to develop knowledge about disease progression, treatment patterns, and outcomes.102 The Swedish EIMS study has identified associations between pre-disease body mass index and MS prognosis,102 alcohol and tobacco consumption affecting MS risk,103,104 and exposure to shift work at a young age and increased MS risk.105 The North American Research Committee on MS83,106,107 and iConquerMS registries are “PROM-driven” and have been useful in identifying reductions in disease progression in people using DMTs.107,108 The New York State MS Consortium has identified important demographic characteristics that influence MS progression.109,110 PROs can also be used to determine risk of MS-related mortality111 and decline in quality of life.112,113 Limitations of these approaches include use of different PROMs, inconsistencies in data collection processes, and different follow-up intervals used across registries.102
Patient-Centered Care
The Institute of Medicine defines patient-centeredness as “care that is respectful and responsive to individual patient preferences, needs, and values and ensures that patient values guide all clinical decisions.”114 PROs are useful for identifying a patient’s individual health concerns and preferences, something that is needed when treating a highly variable chronic health condition like MS. The use of PROs can help clinicicans visualize the lived experience of persons with MS and identify personal preferences,115 as well as improve self-monitoring, self-management, self-efficacy, adherence, wellness, and coping ability.116 At the system level, PROs can inform improvement initiatives and patient-centered care design efforts.117-120
Selecting PROMs
Initiatives from groups like the COnsensus-based Standards for the Selection of health Measurement INstruments (COSMIN)121 and the International Society for Quality of Life Research (ISOQOL)108 offer guidance on selecting PROs. The NINDS has promoted common data collection between clinical studies of the brain and nervous system.122 General guidance from these sources recommends first considering the outcome and target population, selecting PROMs to measure the outcome through a synthesis of the available evidence, assessing validity and reliability of selected PROMs, and using standard measures that can be compared across studies or populations.108,121 Other factors include feasibility, acceptability, and burden of use for patients, clinicians, and systems, as well as literacy, cultural, and linguistic factors.123
The NQF recommends that consideration be given to individual patient needs, insurance factors, clinical setting constraints, and available resources when selecting PROMs.124 To maximize response rate, PROMs that are sensitive, reliable, valid, and developed in a comparative demographic of patients are advised.125 ISOQOL has released a User’s Guide and several companion guides on implementing and utilizing PROMs.108,126,127 Finally, PRO-Performance Measures (PRO-PMs) are sometimes used to assess whether PROMs are appropriately contributing to performance improvement and accountability.124
The Cons of PROs
PROs can disrupt busy clinical care environments and overextend clinical staff.125 Online collection of PROs outside of clinical encounters can relieve PRO-related burden, but this requires finding and funding appropriate secure online networks to effectively collect PROs.128 In 2015, only 60% of people seen for primary care visits could access or view their records online, and of those, only 57% used messaging for medical questions or concerns.129 Ideally, online patient portal or mobile health apps could synchronize directly to electronic health records or virtual scribes to transfer patient communications into clinical documentation.130 There has been limited success with this approach in European countries131 and with some chronic illness conditions in the United States.74
Electronic health technologies, including mobile health (mHealth) solutions, have improved the self-monitoring and self-management capability of patients with MS via information sharing in patient networks, assistive technologies, smartphone applications, and wearable devices.132,133 A recent study found that communication modes included secure online patient portal use (29%) and email use (21%), and among those who owned tablets or smartphones, 46% used mHealth apps.134 Social media use has been associated with increased peer/social/emotional support and increased access to health information, as well as clinical monitoring and behavior change.134,135 Individuals using mHealth apps are younger, have comorbidities, and have higher socioeconomic and education levels,135,136 suggesting that inequities in mHealth access exist.
Questionnaires can be time-consuming and cause mental distress if not appropriately facilitated.137 Decreasing questionnaire length and providing the option for PROMs to be delivered and completed online or outside of the clinic context can reduce burden.138 Additionally, while some people are consistent in sharing their PROs, others struggle with using computers, especially while experiencing severe symptoms, forget to complete PROMs, or simply do not have internet access due to financial or geographic constraints.139 A group of disabled and elderly persons with MS reported barriers to internet use due to visual deficits, small website font sizes, and distracting color schemes.140
Interpretability
Interpreting PROMs and displays of longitudinal PROM data can be a challenge for persons with MS and their clinicians. There is little standardization in how PROMs are scored and presented, and there is often confusion about thresholds for clinical significance and how PROM scores can be compared to other PROMs.141,142 While guidelines exist for implementing PRO scores in clinical settings,126,143 there are few that aid PROM interpretation. As a result, clinicians often seek research evidence for PROMs used in other similar patient populations as a benchmark,142-144 or compare them to other patients seen in their clinical practice.
Longitudinal PRO data are usually displayed in simple line graphs.145,146 Overall, line graphs have been found to have the highest ease of understanding by both patients and clinicians, but sometimes can be confusing.147 For example, upward trending lines are usually viewed as improvement and downward trending lines as decline; however, upward trending scores on a PROM can indicate decline, such as increasing fatigue severity. Annotation of visual displays can help. Patients and clinicians find that employing thresholds and color coding is useful, and better than “stoplight” red-yellow-green shading schemes or red-circle formats to indicate data that warrant attention.142
PROs are not free of risk for error, especially if they are used independently of other information sources, such as clinical interview, examination, and diagnostic testing, or if they are utilized too frequently, too infrequently, or are duplicated in practice. If a PRO instrument is employed too frequently, score changes may reflect learning effects rather than actual clinical status. Conversely, if used too infrequently, PRO information will not be timely enough to inform real-time clinical practice. Duplication of PRO assessments (eg, multiple measures of the same PRO for the same patient on the same day) or use of multiple PRO measures to assess the same aspect (eg, 2 measures used to assess fatigue) could introduce unnecessary complexity and confusion to interpretation of PRO results.
PRO measures also can be biased or modified by clinical status and/or perceptions of people with MS at the time of assessment. For example, cognitive impairment, whether at baseline state or due to a cognitive MS relapse event, could impact patients’ ability to understand and respond to PRO assessments, producing erroneous results. However, when used appropriately, PROs targeting cognitive dysfunction may be able to detect onset of cognitive events or help to measure recovery from them. Finally, PROs measure perceived (self-reported) status, which may not be an accurate depiction of actual status.
All of these potential pitfalls support the argument that PROs should be utilized to augment the clinical interview, examination, and diagnostic (objective) testing aspects of comprehensive MS care. In this way, PROs can be correlated with other information sources to deepen the shared understanding of health status between a person with MS and her clinician, increasing the potential to make better treatment decisions and care plans together in partnership.
Value and Cost
National groups such as the Patient-Centered Outcomes Research Institute (PCORI) are working with regulatory bodies, funding agencies, insurance providers, patient advocacy groups, researchers, providers, and specialty groups to investigate how PROMs can be implemented into value-based health care reforms, including value-based reimbursement.148 However, practical PRO implementation requires considerable time and resources, and many methodological and operational questions must be addressed before widespread adoption and reimbursement for PROMs will be feasible.148,149
Summary
PROs can generate valuable information about perceived health status, function, quality of life, and experience of care using self-reported sources. Validated PRO assessment tools include PROMs and PREMs. PROs are currently utilized in research settings (especially PROMs) but are also being used in clinical practice, quality improvement initiatives, and population health applications using LHS approaches. PROs have the advantages of empowering and informing persons with MS and clinicians to optimize patient-centered care, improve systems of care, and study population health outcomes. Barriers include PROM validity, reliability, comparability, specificity, interpretability, equity, time, and cost. Generic PROMs and PREMs, and some MS-specific PROMs, can be used for persons with MS. Unfortunately, no PREMs have been developed specifically for persons with MS, and this is an area for future research. With appropriate development and utilization in LHS applications, PROs can inform patient-centered clinical care, system-level improvement initiatives, and population health research, and have the potential to help facilitate coproduction of health care services.
Acknowledgments: The authors thank Ann Cabot, DO, of the MS Specialty Care Program at Concord Hospital and (especially) peer mentors from a peer outreach wellness program for people with MS (who have asked to remain anonymous) for interviews conducted with their permission to inform the case study described in this article. The case study used in this manuscript has been de-identified, with some aspects modified from actual, and the person in the case study is fictitiously named.
Corresponding author: Brant Oliver, PhD, MS, MPH, APRN-BC, Department of Community & Family Medicine, Dartmouth-Hitchcock Medical Center, Lebanon, NH 03756; [email protected].
Financial disclosures: None.
1. Goldenberg MM. Multiple sclerosis review. PT. 2012;37:175-184.
2. Doshi A, Chataway J. Multiple sclerosis, a treatable disease. Clin Med. 2016;16(Suppl 6):s53-s9.
3. Perrin Ross A. Management of multiple sclerosis. Am J Managed Care. 2013;19(16 Suppl):s301-s306.
4. Batalden M, Batalden P, Margolis P, et al. Coproduction of healthcare service. BMJ Qual Saf. 2016;25:509-517.
5. Chen J, Ou L, Hollis SJ. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res. 2013;13:211.
6. Knaup C, Koesters M, Schoefer D, et al. Effect of feedback of treatment outcome in specialist mental healthcare: meta-analysis. Br J Psychiatr. 2009;195:15-22.
7. Dudgeon D. The impact of measuring patient-reported outcome measures on quality of and access to palliative care. J Palliat Med. 2018;21(S1):S76-s80.
8. Snyder CF, Herman JM, White SM, et al. When using patient-reported outcomes in clinical practice, the measure matters: a randomized controlled trial. J Oncol Pract. 2014;10:e299-e306.
9. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79.
10. de Jong MJ, Huibregtse R, Masclee AAM, et al. Patient-reported outcome measures for use in clinical trials and clinical practice in inflammatory bowel diseases: a systematic review. Clin Gastroenterol Hepatol. 2018;16:648-663.
11. Roman D, Osborne-Stafsnes J, Amy CH, et al. Early lessons from four ‘aligning forces for quality’ communities bolster the case for patient-centered care. Health Affairs. 2013;32:232-241.
12. Ehde DM, Alschuler KN, Sullivan MD, et al. Improving the quality of depression and pain care in multiple sclerosis using collaborative care: The MS-care trial protocol. Contemporary Clinical Trials. 2018;64:219-229.
13. Lavallee DC, Chenok KE, Love RM, et al. Incorporating patient-reported outcomes into health care to engage patients and enhance care. Health Affairs. 2016;35:575-582.
14. Schick-Makaroff K, Thummapol O, Thompson S, et al. Strategies for incorporating patient-reported outcomes in the care of people with chronic kidney disease (PRO kidney): a protocol for a realist synthesis. Clin Gastroenterol Hepatol. 2018;16:648-663.
15. Nelson EC, Dixon-Woods M, Batalden PB, et al. Patient focused registries can improve health, care, and science. BMJ. 2016;354:i3319.
16. Oliver BJ, Nelson EC, Kerrigan CL. Turning feed-forward and feedback processes on patient-reported data into intelligent action and informed decision-making: case studies and principles. Med Care. 2019;57 Suppl 1:S31-s37.
17. Deshpande PR, Rajan S, Sudeepthi BL, Abdul Nazir CP. Patient-reported outcomes: A new era in clinical research. Perspect Clin Res. 2011;2:137-144.
18. Epstein RM, Street RL, Jr. The values and value of patient-centered care. Ann Fam Med. 2011;9:100-103.
19. Payne SA. A study of quality of life in cancer patients receiving palliative chemotherapy. Soc Sci Med. 1992;35:1505-1509.
20. Borgaonkar MR, Irvine EJ. Quality of life measurement in gastrointestinal and liver disorders. Gut. 2000;47:444-454.
21. Macdonell R, Nagels G, Laplaud DA, et al. Improved patient-reported health impact of multiple sclerosis: The ENABLE study of PR-fampridine. Multiple Sclerosis. 2016;22:944-954.
22. Hobart J, Freeman J, Lamping D, et al. The SF-36 in multiple sclerosis: why basic assumptions must be tested. J Neurol Neurosurg Psychiatry. 2001;71:363-370.
23. Lins L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: Scoping review. SAGE Open Med. 2016;4:2050312116671725.
24. Parkinson’s Disease Society of the United Kingdom. The Parkinson’s Disease Questionnaire (PDQ-39). https://www.parkinsons.org.uk/professionals/resources/parkinsons-disease-questionnaire-pdq-39. Accessed October 20, 2019.
25. Basch E. The missing voice of patients in drug-safety reporting. N Engl J Med. 2010;362:865-869.
26. Calvert M, Brundage M, Jacobsen PB, et al. The CONSORT Patient-Reported Outcome (PRO) extension: implications for clinical trials and practice. Health Qual Life Outcomes. 2013;11:184.
27. Black N. Patient reported outcome measures could help transform healthcare. BMJ. 2013;346:f167.
28. Franks P, Fiscella K, Shields CG, et al. Are patients’ ratings of their physicians related to health outcomes? Ann Fam Med. 2005;3:229-234.
29. Hostetter M, Klein S. Using patient-reported outcomes to improve health care quality. The Commonwealth Fund 2019. www.commonwealthfund.org/publications/newsletter-article/using-patient-reported-outcomes-improve-health-care-quality. Accessed October 24, 2019.
30. Charlotte Kingsley SP. Patient-reported outcome measures and patient-reported experience measures. BJA Education. 2017;17:137-144.
31. Weldring T, Smith SM. Patient-Reported outcomes (PROs) and patient-reported outcome measures (PROMs). Health Services Insights. 2013;6:61-68.
32. Elwyn G, Barr PJ, Grande SW, et al. Developing CollaboRATE: A fast and frugal patient-reported measure of shared decision making in clinical encounters. Patient Educ Couns. 2013;93:102-107.
33. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33:1444-1452.
34. Rudick RA, Miller D, Clough JD, et al. Quality of life in multiple sclerosis. Comparison with inflammatory bowel disease and rheumatoid arthritis. Arch Neurol. 1992;49:1237-1242.
35. Benito-Leon J, Morales JM, Rivera-Navarro J, Mitchell A. A review about the impact of multiple sclerosis on health-related quality of life. Disabil Rehabil. 2003;25:1291-1303.
36. RAND Corporation. 36-Item Short Form Survey (SF-36). [https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html. Accessed October 20, 2019.
37. Bergner M, Bobbitt RA, Carter WB, Gilson BS. The sickness impact profile: development and final revision of a health status measure. Medical Care. 1981;19:787-805.
38. Horsman J, Furlong W, Feeny D, Torrance G. The Health Utilities Index (HUI): concepts, measurement properties and applications. Health Qual Life Outcomes. 2003;1:54.
39. Kinkel RP, Laforet G, You X. Disease-related determinants of quality of life 10 years after clinically isolated syndrome. Int J MS Care. 2015;17:26-34.
40. Baumstarck-Barrau K, Simeoni MC, Reuter F, et al. Cognitive function and quality of life in multiple sclerosis patients: a cross-sectional study. BMC Neurology. 2011;11:17.
41. Samartzis L, Gavala E, Zoukos Y, et al. Perceived cognitive decline in multiple sclerosis impacts quality of life independently of depression. Rehabil Res Pract. 2014;2014:128751.
42. Vitkova M, Rosenberger J, Krokavcova M, et al. Health-related quality of life in multiple sclerosis patients with bladder, bowel and sexual dysfunction. Disabil Rehabil. 2014;36:987-992.
43. Khurana V, Sharma H, Afroz N, et al. Patient-reported outcomes in multiple sclerosis: a systematic comparison of available measures. Eur J Neurol. 2017;24:1099-1107.
44. Hobart J, Lamping D, Fitzpatrick R, et al. The Multiple Sclerosis Impact Scale (MSIS-29): a new patient-based outcome measure. Brain. 2001;124(Pt 5):962-973.
45. Lily O, McFadden E, Hensor E, et al. Disease-specific quality of life in multiple sclerosis: the effect of disease modifying treatment. Multiple Sclerosis. 2006;12:808-813.
46. Ford HL, Gerry E, Tennant A, et al. Developing a disease-specific quality of life measure for people with multiple sclerosis. Clin Rehabil. 2001;15:247-258.
47. Cella DF, Dineen K, Arnason B, et al. Validation of the functional assessment of multiple sclerosis quality of life instrument. Neurology. 1996;47:129-139.
48. Gold SM, Heesen C, Schulz H, et al. Disease specific quality of life instruments in multiple sclerosis: validation of the Hamburg Quality of Life Questionnaire in Multiple Sclerosis (HAQUAMS). Multiple Sclerosis. 2001;7:119-130.
49. Vickrey BG, Hays RD, Harooni R, et al. A health-related quality of life measure for multiple sclerosis. Qual Life Res. 1995;4:187-206.
50. Simeoni M, Auquier P, Fernandez O, et al. Validation of the Multiple Sclerosis International Quality of Life questionnaire. Multiple Sclerosis. 2008;14:219-230.
51. Doward LC, McKenna SP, Meads DM, et al. The development of patient-reported outcome indices for multiple sclerosis (PRIMUS). Multiple Sclerosis. 2009;15:1092-1102.
52. Ozakbas S, Akdede BB, Kosehasanogullari G, et al. Difference between generic and multiple sclerosis-specific quality of life instruments regarding the assessment of treatment efficacy. J Neurol Sci. 2007;256:30-34.
53. Cella DF, Wiklund I, Shumaker SA, Aaronson NK. Integrating health-related quality of life into cross-national clinical trials. Qual Life Res. 1993;2:433-440.
54. Nowinski CJ, Miller DM, Cella D. Evolution of patient-reported outcomes and their role in multiple sclerosis clinical trials. Neurotherapeutics. 2017;14:934-944.
55. Cella D, Nowinski C, Peterman A, et al. The neurology quality-of-life measurement initiative. Arch Phys Med Rehabil. 2011;92(10 Suppl):S28-S36.
56. HealthMeasures: PROMIS. http://www.healthmeasures.net/explore-measurement-systems/promis. Accessed October 20, 2019.
57. Valderas JM, Ferrer M, Mendivil J, et al. Development of EMPRO: a tool for the standardized assessment of patient-reported outcome measures. Value Health. 2008;11:700-708.
58. Bach K, Martling C, Mork PJ, et al. Design of a clinician dashboard to facilitate co-decision making in the management of non-specific low back pain. J Intelligent Helath Syst. 2019;52:269-284.
59. Karami M, Safdari R, Rahimi A. Effective radiology dashboards: key research findings. Radiology Management. 2013;35:42-45.
60. Dowding D, Randell R, Gardner P, et al. Dashboards for improving patient care: review of the literature. Int J Med Informatic. 2015;84:87-100.
61. Mlaver E, Schnipper JL, Boxer RB, et al. User-centered collaborative design and development of an inpatient safety dashboard. Jt Comm J Qual Patient Saf. 2017;43:676-685.
62. Dowding D, Merrill J, Russell D. Using feedback intervention theory to guide clinical dashboard design. AMIA Annu Symp Proc. 2018;2018:395-403.
63. Hartzler AL, Izard JP, Dalkin BL, et al. Design and feasibility of integrating personalized PRO dashboards into prostate cancer care. J Am Med Inform Assoc. 2016;23:38-47.
64. Lavallee DC, Chenok KE, Love RM, et al. Incorporating patient-reported outcomes into health care to engage patients and enhance care. Health Affairs. 2016;35:575-582.
65. U.S. Centers for Medicare & Medicaid Services. Comprehensive Care for Joint Replacement Model. https://innovation.cms.gov/initiatives/CJR. Accessed August 3, 2019.
66. National Quality Forum. Patient-reported outcomes: Accessed August 3, 2019. http://www.qualityforum.org/Patient-Reported_Outcomes.aspx. Accessed October 20, 2019.
67. Institute of Medicine Roundtable on Evidence-Based Medicine. The National Academies Collection: Reports funded by National Institutes of Health. In: Olsen LA, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US).National Academy of Sciences; 2007.
68. About Learning Health Systems: Agency for Healthcare Research and Quality. https://www.ahrq.gov/learning-health-systems/about.html. Accessed August 3, 2019.
69. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington (DC): National Academies Press; 2013.
70. Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
71. Oliver BJ, for the MS-CQI Investigators. The Multiple Sclerosis Continuous improvement collaborative: the first coproduction learning health system improvement science research collaborative for multiple sclerosis. The International Society for Quality in Health Care (ISQua) Annual International Conference; October 2019; Cape Town, South Africa.
72. Britto MT, Fuller SC, Kaplan HC, et al. Using a network organisational architecture to support the development of Learning Healthcare Systems. BMJ Qual Saf. 2018;27:937-946.
73. Care Centers: Cystic Fibrosis Foundation. https://www.cff.org/Care/Care-Centers/. Accessed August 3, 2019.
74. Schechter MS, Fink AK, Homa K, Goss CH. The Cystic Fibrosis Foundation patient registry as a tool for use in quality improvement. BMJ Qual Saf. 2014;23(Suppl 1):i9-i14.
75. Mogayzel PJ, Dunitz J, Marrow LC, Hazle LA. Improving chronic care delivery and outcomes: the impact of the cystic fibrosis Care Center Network. BMJ Qual Saf. 2014;23(Suppl 1):i3-i8.
76. Godfrey MM, Oliver BJ. Accelerating the rate of improvement in cystic fibrosis care: contributions and insights of the learning and leadership collaborative. BMJ Qual Saf. 2014;23(Suppl 1):i23-i32.
77. Nelson EC, Godfrey MM. Quality by Design: A Clinical Microsystems Approach. San Francisco, California: Jossey-Bass; 2007.
78. Crohn’s & Colitis Foundation.Quality of Care: IBD Qorus: https://www.crohnscolitisfoundation.org/research/ibd-qorus. Accessed October 23, 2019.
79. Johnson LC, Melmed GY, Nelson EC, et al. Fostering collaboration through creation of an ibd learning health system. Am J Gastroenterol. 2017;112:406-468.
80. Fernandez-Munoz JJ, Moron-Verdasco A, Cigaran-Mendez M, et al. Disability, quality of life, personality, cognitive and psychological variables associated with fatigue in patients with multiple sclerosis. Acta Neurologica Scandinavica. 2015;132:118-124.
81. Flachenecker P, Kumpfel T, Kallmann B, et al. Fatigue in multiple sclerosis: a comparison of different rating scales and correlation to clinical parameters. Multiple Sclerosis. 2002;8:523-526.
82. Tellez N, Rio J, Tintore M, et al. Does the Modified Fatigue Impact Scale offer a more comprehensive assessment of fatigue in MS? Multiple Sclerosis. 2005;11:198-202.
83. Salter A, Fox RJ, Tyry T, et al. The association of fatigue and social participation in multiple sclerosis as assessed using two different instruments. Mult Scler Relat Disord. 2019;31:165-72.
84. Solaro C, Trabucco E, Messmer Uccelli M. Pain and multiple sclerosis: pathophysiology and treatment. Curr Neurol Neurosci Rep. 2013;13:320.
85. Jawahar R, Oh U, Yang S, Lapane KL. A systematic review of pharmacological pain management in multiple sclerosis. Drugs. 2013;73:1711-1722.
86. Rintala A, Hakkinen A, Paltamaa J. Ten-year follow-up of health-related quality of life among ambulatory persons with multiple sclerosis at baseline. Qual Life Res. 2016;25:3119-3127.
87. Tepavcevic DK, Pekmezovic T, Stojsavljevic N, et al. Change in quality of life and predictors of change among patients with multiple sclerosis: a prospective cohort study. Qual Life Res. 2014;23:1027-1037.
88. DasGupta R, Fowler CJ. Bladder, bowel and sexual dysfunction in multiple sclerosis: management strategies. Drugs. 2003;63:153-166.
89. Marck CH, Jelinek PL, Weiland TJ, et al. Sexual function in multiple sclerosis and associations with demographic, disease and lifestyle characteristics: an international cross-sectional study. BMC Neurology. 2016;16:210.
90. Gajofatto A, Benedetti MD. Treatment strategies for multiple sclerosis: When to start, when to change, when to stop? World J Clinical Cases. 2015;3:545-555.
91. National Multiple Sclerosis Society. Disease-modifying therapies for MS. 2018.
92. Rudick RA, Panzara MA. Natalizumab for the treatment of relapsing multiple sclerosis. Biologics. 2008;2:189-199.
93. Cohen JA, Cutter GR, Fischer JS, et al. Benefit of interferon beta-1a on MSFC progression in secondary progressive MS. Neurology. 2002;59:679-687.
94. Patti F, Pappalardo A, Montanari E, et al. Interferon-beta-1a treatment has a positive effect on quality of life of relapsing-remitting multiple sclerosis: results from a longitudinal study. J Neurol Sci. 2014;337:180-185.
95. Novartis. Exploring the efficacy and safety of Siponimod in patients with secondary progressive multiple sclerosis (EXPAND). Available from clinicaltrials.gov/ct2/show/NCT01665144. NLM identifier: NCT01665144.
96. Singal AG, Higgins PD, Waljee AK. A primer on effectiveness and efficacy trials. Clin Transl Gastroenterol. 2014;5:e45.
97. Jongen PJ. Health-related quality of life in patients with multiple sclerosis: impact of disease-modifying drugs. CNS Drugs. 2017;31:585-602.
98. Coretti S, Ruggeri M, McNamee P. The minimum clinically important difference for EQ-5D index: a critical review. Expert Rev Pharmacoecon Outcomes Res. 2014;14:221-233.
99. Clinical Review Report: Ocrelizumab (Ocrevus). CADTH Common Drug Review. 2018:Appendix 4, Validity of Outcome Measures.
100. iCounquer MS; Empowering patients: how individuals with ms are contributing to the fight to find a cure: 2018. https://www.iconquerms.org/empowering-patients-how-individuals-ms-are-contributing-fight-find-cure. Accessed October 23, 2019.
101. K M. Accelerated Cure Project Announces Collaboration with EMD Serono to Advance Patient-Focused Drug Development in Multiple Sclerosis2018. https://www.iconquerms.org/accelerated-cure-project-announces-collaboration-emd-serono-advance-patient-focused-drug-development. Accessed October 24, 2019.
102. Bebo BF Jr, Fox RJ, Lee K, et al. Landscape of MS patient cohorts and registries: Recommendations for maximizing impact. Multiple Sclerosis. 2018;24:579-586.
103. Hedstrom AK, Hillert J, Olsson T, Alfredsson L. Alcohol as a modifiable lifestyle factor affecting multiple sclerosis risk. JAMA Neurology. 2014;71:300-305.
104. Hedstrom AK, Baarnhielm M, Olsson T, Alfredsson L. Tobacco smoking, but not Swedish snuff use, increases the risk of multiple sclerosis. Neurology. 2009;73:696-701.
105. Hedstrom AK, Akerstedt T, Hillert J, et al. Shift work at young age is associated with increased risk for multiple sclerosis. Ann Neurol. 2011;70:733-741.
106. Hadjimichael O, Vollmer T, Oleen-Burkey M. Fatigue characteristics in multiple sclerosis: the North American Research Committee on Multiple Sclerosis (NARCOMS) survey. Health Qual Life Outcomes. 2008;6:100.
107. Fox RJ, Salter AR, Tyry T, et al. Treatment discontinuation and disease progression with injectable disease-modifying therapies: findings from the North American research committee on multiple sclerosis database. Int J MS Care. 2013;15:194-201.
108. Reeve BB, Wyrwich KW, Wu AW, et al. ISOQOL recommends minimum standards for patient-reported outcome measures used in patient-centered outcomes and comparative effectiveness research. Qual Life Res. 2013;22:1889-1905.
109. Jacobs LD, Wende KE, Brownscheidle CM, et al. A profile of multiple sclerosis: the New York State Multiple Sclerosis Consortium. Multiple Sclerosis. 1999;5:369-376.
110. Weinstock-Guttman B, Jacobs LD, Brownscheidle CM, et al. Multiple sclerosis characteristics in African American patients in the New York State Multiple Sclerosis Consortium. Multiple Sclerosis. 2003;9:293-298.
111. Raffel J, Wallace A, Gveric D, et al. Patient-reported outcomes and survival in multiple sclerosis: A 10-year retrospective cohort study using the Multiple Sclerosis Impact Scale-29. PLoS Med. 2017;14:e1002346.
112. Vaughn CB, Kavak KS, Dwyer MG, et al. Fatigue at enrollment predicts EDSS worsening in the New York State Multiple Sclerosis Consortium. Multiple Sclerosis. 2018:1352458518816619.
113. Alroughani RA, Akhtar S, Ahmed SF, Al-Hashel JY. Clinical predictors of disease progression in multiple sclerosis patients with relapsing onset in a nation-wide cohort. Int J Neuroscience. 2015;125:831-837.
114. Dolan JG, Veazie PJ, Russ AJ. Development and initial evaluation of a treatment decision dashboard. BMC Med Inform Decision Mak. 2013;13:51.
115. Stuifbergen AK, Becker H, Blozis S, et al. A randomized clinical trial of a wellness intervention for women with multiple sclerosis. Arch Phys Med Rehabil. 2003;84:467-476.
116. Finkelstein J, Martin C, Bhushan A, et al. Feasibility of computer-assisted education in patients with multiple sclerosis. In: Proceedings of the IEEE Symposium on Computer-Based Medical Systems. Bethesda, MD; 2004.
117. Batalden P. Getting more health from healthcare: quality improvement must acknowledge patient coproduction—an essay by Paul Batalden. BMJ. 2018;362:k3617.
118. Batalden PB, Davidoff F. What is “quality improvement” and how can it transform healthcare? Qual Saf Health Care. 2007;16:2-3.
119. Ovretveit J, Zubkoff L, Nelson EC, et al. Using patient-reported outcome measurement to improve patient care. Int J Qual Health Care. 2017;29:874-879.
120. Prodinger B, Taylor P. Improving quality of care through patient-reported outcome measures (PROMs): expert interviews using the NHS PROMs Programme and the Swedish quality registers for knee and hip arthroplasty as examples. BMC Health Services Res. 2018;18:87.
121. Prinsen CA, Vohra S, Rose MR, et al. How to select outcome measurement instruments for outcomes included in a “Core Outcome Set” – a practical guideline. Trials. 2016;17:449.
122. NINDS. NINDS Common Data Elements: Streamline Your Neuroscience Clinical Research National Institute of Health. https://www.commondataelements.ninds.nih.gov/#page=Default. Accessed October 23, 2019.
123. Francis DO, McPheeters ML, Noud M, et al. Checklist to operationalize measurement characteristics of patient-reported outcome measures. Systematic Rev. 2016;5:129.
124. National Quality Forum. Patient-reported outcomes (PROs) in performance measurement. 2013. https://www.qualityforum.org/Publications/2012/12/Patient-Reported_Outcomes_in_Performance_Measurement.aspx. Accessed October 15, 2019.
125. Dawson J, Doll H, Fitzpatrick R, et al. The routine use of patient reported outcome measures in healthcare settings. BMJ. 2010;340:c186.
126. Aaronson N ET, Greenlhalgh J, Halyard M, et al. User’s guide to implementation of patient reported outcomes assessment in clinical practice. International Society for Quality of Life. 2015.
127. Chan EKH, Edwards TC, Haywood K, et al. Implementing patient-reported outcomes mesaures in clinical practice: a companion guide to the ISOQOL user’s guide. Qual Life Res. 2018;28:621-627.
128. Snyder CF, Wu AW, Miller RS, et al. The role of informatics in promoting patient-centered care. Cancer J. 2011;17:211-218
129. Osborn R, Moulds D, Schneider EC, et al. Primary care physicians in ten countries report challenges caring for patients with complex health needs. Health Affairs. 2015;34:2104-2112.
130. Wu AW, Kharrazi H, Boulware LE, Snyder CF. Measure once, cut 0wice--adding patient-reported outcome measures to the electronic health record for comparative effectiveness research. J Clin Epidemiol. 2013;66(8 Suppl):S12-S20.
131. Trojano M, Bergamaschi R, Amato MP, et al. The Italian multiple sclerosis register. Neurol Sci. 2019;40:155-165.
132. Moorhead SA, Hazlett DE, Harrison L, et al. A new dimension of health care: systematic review of the uses, benefits, and limitations of social media for health communication. J Med Internet Res. 2013;15:e85.
133. Luigi L, Francesco B, Marcello M, et al. e-Health and multiple sclerosis: An update. Multiple Sclerosis J. 2018;24:1657-1664.
134. Marrie RA, Leung S, Tyry T, et al. Use of eHealth and mHealth technology by persons with multiple sclerosis. Mult Scler Relat Dis. 2019;27:13-19.
135. Giunti G, Kool J, Rivera Romero O, Dorronzoro Zubiete E. Exploring the specific needs of persons with multiple sclerosis for mhealth solutions for physical activity: mixed-methods study. JMIR Mhealth Uhealth. 2018;6:e37.
136. Spooner KK, Salemi JL, Salihu HM, Zoorob RJ. eHealth patient-provider communication in the United States: interest, inequalities, and predictors. J Am Med Inform Assoc. 2017;24(e1):e18-e27.
137. Safdar N, Abbo LM, Knobloch MJ, Seo SK. Research methods in healthcare epidemiology: survey and qualitative research. Infect Control Hosp Epidemiol. 2016;37:1272-1277.
138. Rolstad S, Adler J, Rydén A. Response burden and questionnaire length: is shorter better? A review and meta-analysis. Value Health. 2011;14:1101-1108.
139. Chiu C, Bishop M, Pionke JJ, et al. Barriers to the accessibility and continuity of health-care services in people with multiple sclerosis: a literature review. Int J MS Care. 2017;19:313-321.
140. Atreja A, Mehta N, Miller D, et al. One size does not fit all: using qualitative methods to inform the development of an Internet portal for multiple sclerosis patients. AMIA Annu Symp Proc Symp. 2005:16-20.
141. Snyder CF, Blackford AL, Brahmer JR, et al. Needs assessments can identify scores on HRQOL questionnaires that represent problems for patients: an illustration with the Supportive Care Needs Survey and the QLQ-C30. Qual Life Res. 2010;19:837-845.
142. de Vet HC, Terwee CB. The minimal detectable change should not replace the minimal important difference. J Clin Epidemiol. 2010;63:804-805.
143. Snyder CF, Aaronson NK, Choucair AK, et al. Implementing patient-reported outcomes assessment in clinical practice: a review of the options and considerations. Qual Life. 2012;21:1305-1314.
144. Myla DG, Melanie DW, Robert WM, et al. Identification and validation of clinically meaningful benchmarks in the 12-item Multiple Sclerosis Walking Scale. Multiple Sclerosis J. 2017;23:1405-1414.
145. van Munster CEP, Uitdehaag BMJ. Outcome measures in clinical trials for multiple sclerosis. CNS Drugs. 2017;31:217-236.
146. Snyder CF, Jensen R, Courtin SO, Wu AW. PatientViewpoint: a website for patient-reported outcomes assessment. Quality Life Res. 2009;18:793-800.
147. Snyder CF, Smith KC, Bantug ET, et al. What do these scores mean? Presenting patient-reported outcomes data to patients and clinicians to improve interpretability. Cancer. 2017;123:1848-1859.
148. Squitieri L, Bozic KJ, Pusic AL. The role of patient-reported outcome measures in value-based payment reform. Value Health. 2017;20:834-836.
149. Boyce MB, Browne JP. The effectiveness of providing peer benchmarked feedback to hip replacement surgeons based on patient-reported outcome measures—results from the PROFILE trial: a cluster randomised controlled study. BMJ Open. 2015;5:e008325.
From the Dartmouth Institute for Health Policy & Clinical Practice, Geisel School of Medicine, Hanover, NH (Ms. Manohar and Dr. Oliver), the Department of Community & Family Medicine, Dartmouth-Hitchcock Medical Center, Lebanon, NH (Ms. Perkins, Ms. Laurion, and Dr. Oliver), and the Multiple Sclerosis Specialty Care Program, Concord Hospital, Concord, NH (Dr. Oliver).
Abstract
- Background: Patient-reported outcomes (PROs), including patient-reported outcome measures (PROMs) and patient-reported experience measures (PREMs), can be used to assess perceived health status, functioning, quality of life, and experience of care. Complex chronic illnesses such as multiple sclerosis (MS) affect multiple aspects of health, and PROs can be applied in assessment and decision-making in MS care as well as in research pertaining to MS.
- Objective: To provide a general review of PROs, with a specific focus on implications for MS care.
- Methods: Evidence synthesis of available literature on PROs in MS care.
- Results: PROs (including PROMs and PREMs) have historically been utilized in research and are now being applied in clinical, improvement, and population health settings using learning health system approaches in many disease populations, including MS. Many challenges complicate the use of PROs in MS care, including reliability, validity, and interpretability of PROMs, as well as feasibility barriers due to time and financial constraints in clinical settings.
- Conclusion: PROs have the potential to better inform clinical care, empower patient-centered care, inform health care improvement efforts, and create the conditions for coproduction of health care services.
Keywords: PRO; PROM; patient-reported outcome measure; patient-reported experience measure; quality of life; patient-centered care.
Multiple sclerosis (MS) is a disabling, complex, chronic, immune-mediated disorder of the central nervous system (CNS). MS causes inflammatory and degenerative damage in the CNS, which disrupts signaling pathways.1 It is most commonly diagnosed in young adults and affects 2.3 million people worldwide.2 People with MS experience very different disease courses and a wide range of neurological symptoms, including visual, somatic, mental health, sensory, motor, and cognitive problems.1-3 Relapsing-remitting MS, the most common form, affects 85% of those with MS and is characterized by periods of relapse (exacerbation) and remission.1 Other forms of MS (primary progressive and secondary progressive MS) are characterized by progressive deterioration and worsening symptom severity without exacerbations. Disease-modifying therapies (DMTs) can reduce the frequency of exacerbations and disability progression, but unfortunately there is no cure for MS. Treatment is focused on increasing quality of life, minimizing disability, and maximizing wellness.
Patient-reported outcomes (PROs) describe the perceived health status, function, and/or experience of a person as obtained by direct self-report. Patient-reported outcome measures (PROMs) are validated PROs that can be used to inform clinical care,4 and have demonstrated effectiveness in improving patient-provider communication and decision-making.5-7 PROMs are currently used in some MS clinical trials to determine the impact of experimental interventions,8-10 and are also being used to inform and improve clinical care in some settings. Especially for persons with MS, they can provide individualized perspectives about health experience and outcomes.11 In more advanced applications, PROMs can be used to improve face-to-face collaborations between clinicians and patients and to inform patient-centered systems of care.12-14 PROMs can also be used to inform systems-level improvement for entire patient populations.15,16
In this article, we review current applications of PROs and PROMs in the care of persons with MS, as well as current limitations and barriers to their use.
At a recent visit to her neurologist, Marion reviews her health diary, in which she has been tracking her fatigue levels throughout the day and when she has to visit the bathroom. The PRO diary also helps her remember details that she might not otherwise be able to recall at the time of her clinic visit. They review the diary entrees together to develop a shared understanding of what Marion has been experiencing and identify trends in the PRO data. They discuss symptom management and use the PRO information from the diary to help guide adjustments to her physical therapy routine and medication regimen.
Part of Marion’s “PRO package” includes the Center for Epidemiologic Studies Depression Scale (CES-D), a validated depression screening and symptom severity questionnaire that she completes every 3 months. Although she denies being depressed, she has noticed that her CES-D scores in recent months have been consistently increasing. This prompts a discussion about mental health in MS and a referral to work on depression with the MS mental health specialist. Marion and the mental health specialist use CES-D measures at baseline and during treatment to set a remission target and to track progress during treatment. Marion finds this helpful because she says it is hard for her to “wrap my hands around depression… it’s not something that there is a blood test or a MRI for.” Marion is encouraged by being able to see her CES-D scores change as her depression severity decreases, and this helps motivate her to keep engaged in treatment.
PROs and PROMs: General Applications
PROs are measures obtained directly from an individual without a priori interpretation by a clinician.9,17 PROs capture individual perspectives on symptoms, capability, disability, and health-related quality of life.9 With increasing emphasis on patient-centered care,18 individual perspectives and preferences elicited using PROMs may be able to inform better quality of care and patient-centered disease treatment and management.19-21
PROMs are standardized, validated questionnaires used to assess PROs and can be generic or condition-specific. Generic PROMs can be used in any patient population. The SF-3622 is a set of quality of life measures that assess perceived ability to complete physical tasks and routine activities, general health status, fatigue, social functioning, pain, and emotional and mental health.23 Condition-specific PROMs can be used for particular patient populations and are helpful in identifying changes in health status for a specific disease, disability, or surgery. For example, the PDQ-39 assesses 8 dimensions of daily living, functioning, and well-being for people with Parkinson’s disease.24
PROMs have been used in some MS clinical trials and research studies to determine the effectiveness of experimental treatments from the viewpoint of study participants.9,25,26 PROs can also be utilized in clinical care to facilitate communication of needs and track health outcomes,27 and can inform improvement in outcomes for health systems and populations. They can also be used to assess experience of care,28 encouraging a focus on high-quality outcomes through PRO-connected reimbursement mechanisms,29 and provide aggregate data to evaluate clinical practice, population health outcomes, and the effectiveness of public policies.27
Patient-reported experience measures (PREMs) assess patient satisfaction and experience of health care.30,31 CollaboRATE32 is a PREM that assesses the degree of shared decision-making occurring between patients and clinicians during clinical care. PREMs are currently used for assessing self-efficacy and in shared decision-making and health care improvement applications. PREMs have yet to be developed specifically for persons with MS.
PROMs in MS Care
Generic PROMs have shown that persons with MS are disproportionately burdened by poor quality of life.33-35 Other generic PROMs, like the SF-36,36 the Sickness Impact Profile,37 and versions of the Health Utilities Index,38 can be used to gather information on dysfunction and to determine quality and duration of life modified by MS-related dysfunction and disability. MS-specific PROMs are used to assess MS impairments, including pain, fatigue, cognition, sexual dysfunction, and depression.12,39-42 PROMs have also been used in MS clinical trials, including the Multiple Sclerosis Impact Scale-29 (MSIS-29),43,44 the Leeds MS QoL (LMSQoL),45,46 the Functional Assessment of MS (FAMS),47 the Hamburg Quality of Life Questionnaire in MS (HAQUAMS),48 the MS Quality of Life-54 (MSQoL-54),49 the MS International QoL (MUSIQoL),50 and the Patient-Reported Indices for MS Activity Limitations Scale (PRIMUS).51
Condition-specific PROMs are more sensitive to changes in health status and functioning for persons with MS compared to generic PROMs. They are also more reliable during MS remission and relapse periods.44,52 For example, the SF-36 has floor and ceiling effects in MS populations—a high proportion of persons with MS are scored at the maximum or minimum levels of the scale, limiting discriminant capability.22 As a result, a “combined approach” using both generic and MS-specific measures is often recommended.53 Some MS PROMs (eg, MSQoL-54) include generic questions found in the SF-36 as well as additional MS-specific questions or scales.
The variety of PROMs available (see Table for a selected listing) introduces a significant challenge to using them—limited generalizability and difficulty comparing PROs across MS studies. Efforts to establish common PROMs have been undertaken to address this problem.54 The National Institute of Neurologicical Disorders and Stroke (NINDS) sponsored the development of a neurological quality of life battery, the Neuro-QOL.55 Neuro-QOL measures the physical, mental, and social effects of neurological conditions in adults and children with neurological disorders and has the capability to facilitate comparisons across different neurological conditions. Additionally, the Patient-Reported Outcomes Measure Information System (PROMIS) has been developed to assess physical, mental, and social effects of chronic disease. PROMIS has a hybrid design that includes generic and MS-specific measures (such as PROMIS FatigueMS).56 PROMIS can be used to assess persons with MS as well as to compare the MS population with other populations with chronic illness.
PROMs have varying levels of reliability and validity. The Evaluating the Measure of Patient-Reported Outcomes57 study evaluated the development process of MS PROMs,43 and found that the MSIS-29 and LMSQoL had the highest overall reliability among the most common MS PROMs. However, both scored poorly on validity due to lack of patient involvement during development. This questions the overall capability of existing MS PROMs to accurately and consistently assess PROs in persons with MS.
“Feed-Forward” PROMs
Oliver and colleagues16 have described “feed-forward” PROM applications in MS care in a community hospital setting using a learning health system approach. This MS clinic uses feed-forward PROs to inform clinical care—PRO data are gathered before the clinic visit and analyzed ahead of or during the clinic visit by the clinician. Patients are asked to arrive early and complete a questionnaire comprised of PROMs measuring disability, functioning, quality of life, cognitive ability, pain, fatigue, sleep quality, anxiety, and depression. Clinicians score the PROMs and input scores into the electronic health record before the clinical encounter. During the clinic visit, PROM data is visually displayed so that the clinician and patient can discuss results and use the data to better inform decision-making. The visual data display contains longitudinal information, displaying trends in health status across multiple domains, and includes specified thresholds for clinically active symptom levels (Figure).16 Longitudinal monitoring of PROM data allows for real-time assessment of goal-related progress throughout treatment. As illustrated previously by Marion’s case study, the use of real-time feed-forward PROM data can strengthen the partnership between patient and clinician as well as improve empowerment, engagement, self-monitoring, and adherence.
PRO Dashboards
Performance dashboards are increasingly used in health care to visually display clinical and PRO data for individual patients, systems, and populations over time. Dashboards display a parsimonious group of critically important measures to give clinicians and patients a longitudinal view of PRO status. They can inform decision-making in clinical care, operations, health care improvement efforts, and population health initiatives.58 Effective dashboards allow for user customization with meaningful measures, knowledge discovery for analysis of health problems, accessibility of health information, clear visualization, alerts for unexpected data values, and system connectivity.59,60 Appropriate development of PRO dashboards requires meaningful patient and clinician involvement via focus groups and key informant interviews, Delphi process approaches to prioritize and finalize selection of priority measures, iterative building of the interface with design input from key informants and stakeholders (co-design), and pilot testing to assess feasibility and acceptability of use.61-63
Other Applications of PROs/PROMs in MS
Learning Health Systems
The National Quality Forum (NQF) and the Centers for Medicaid and Medicare Services have adopted PROs for use in quality measurement.64-66 This includes a movement towards the use of LHS, defined as a health system in which information from patients and clinicians is systematically collected and synthesized with external evidence to inform clinical care, improvement, and research.67-70 Often a LHS is undertaken as a collaborative effort between multiple health care centers to improve quality and outcomes of care.70 The MS Continuous Quality Improvement Collaborative (MS-CQI), the first multi-center systems-level health care improvement research collaborative for MS,71 as well as IBD Qorus and the Cystic Fibrosis Care Center Network utilize LHS approaches.72-77
IBD Qorus is a LHS developed by the Crohn’s and Colitis Foundation that uses performance dashboards to better inform clinical care for people with inflammatory bowel disease. It also employs system-level dashboards for performance benchmarking in quality improvement initiatives and aggregate-level dashboards to assess population health status.78,79 MS-CQI uses a LHS approach to inform the improvement of MS care across multiple centers using a comprehensive dashboard, including PROMs, for benchmarking and to monitor system and population health status. MS-CQI collects PROMs using a secure online platform that can be accessed by persons with MS and their clinicians and also includes a journaling feature for collecting qualitative information and for reference and self-monitoring.71
MS Research
PROMs are used in clinical and epidemiological research to evaluate many aspects of MS, including the FAMS, the PDSS, the Fatigue Impact Scale (FIS), and others.80-82 For example, the PROMIS FatigueMS and the Fatigue Performance Scale have been used to assess the impact of MS-related fatigue on social participation.83 Generic and MS-specific PROMs have been used to assess pain levels for people with MS,84-87 and multiple MS-specific PROMs, like PRIMUS and MSQoL-54,43 as well as the SF-3639 include pain assessment scales. PROMs have also been used to assess MS-related bladder, bowel, and sexual dysfunction. Urgency, frequency, and incontinence affect up to 75% of patients with MS,88 and many PROMs, such as the LMSQoL, MUSIQoL, and the MSQoL-54, are able to evaluate bladder control and sexual functioning.43,89
PROMs are employed in MS clinical trials to help assess the tolerability and effectiveness of DMTs.90,91 PROs have been used as secondary endpoints to understand the global experience of a DMT from the patient perspective.92-94 There are 15 FDA-approved DMTs for MS, and clinical trials for 6 of these have used PROMs as an effectiveness end point.54,91,95,96 However, most DMT clinical trials are powered for MRI, relapse rate, or disease progression primary outcomes rather than PROMs, often resulting in underpowered PROM analyses.97 In addition, many PROMs are not appropriate for use in DMT clinical trials.98,99
In order to bridge the gap between clinical research and practice, some industry entities are championing “patient-focused drug development” approaches. The Accelerated Cure Project for MS has launched iConquerMS, which collects PROMs from persons with MS to further PRO research in MS and follows 4700 individuals with MS worldwide.100 In 2018, the American College of Physicians announced a collaboration with an industry partner to share data to inform DMT clinical trials and develop and validate PROMs specifically designed for DMT clinical trials.101
Population Health
Registries following large cohorts of people with MS have the potential to develop knowledge about disease progression, treatment patterns, and outcomes.102 The Swedish EIMS study has identified associations between pre-disease body mass index and MS prognosis,102 alcohol and tobacco consumption affecting MS risk,103,104 and exposure to shift work at a young age and increased MS risk.105 The North American Research Committee on MS83,106,107 and iConquerMS registries are “PROM-driven” and have been useful in identifying reductions in disease progression in people using DMTs.107,108 The New York State MS Consortium has identified important demographic characteristics that influence MS progression.109,110 PROs can also be used to determine risk of MS-related mortality111 and decline in quality of life.112,113 Limitations of these approaches include use of different PROMs, inconsistencies in data collection processes, and different follow-up intervals used across registries.102
Patient-Centered Care
The Institute of Medicine defines patient-centeredness as “care that is respectful and responsive to individual patient preferences, needs, and values and ensures that patient values guide all clinical decisions.”114 PROs are useful for identifying a patient’s individual health concerns and preferences, something that is needed when treating a highly variable chronic health condition like MS. The use of PROs can help clinicicans visualize the lived experience of persons with MS and identify personal preferences,115 as well as improve self-monitoring, self-management, self-efficacy, adherence, wellness, and coping ability.116 At the system level, PROs can inform improvement initiatives and patient-centered care design efforts.117-120
Selecting PROMs
Initiatives from groups like the COnsensus-based Standards for the Selection of health Measurement INstruments (COSMIN)121 and the International Society for Quality of Life Research (ISOQOL)108 offer guidance on selecting PROs. The NINDS has promoted common data collection between clinical studies of the brain and nervous system.122 General guidance from these sources recommends first considering the outcome and target population, selecting PROMs to measure the outcome through a synthesis of the available evidence, assessing validity and reliability of selected PROMs, and using standard measures that can be compared across studies or populations.108,121 Other factors include feasibility, acceptability, and burden of use for patients, clinicians, and systems, as well as literacy, cultural, and linguistic factors.123
The NQF recommends that consideration be given to individual patient needs, insurance factors, clinical setting constraints, and available resources when selecting PROMs.124 To maximize response rate, PROMs that are sensitive, reliable, valid, and developed in a comparative demographic of patients are advised.125 ISOQOL has released a User’s Guide and several companion guides on implementing and utilizing PROMs.108,126,127 Finally, PRO-Performance Measures (PRO-PMs) are sometimes used to assess whether PROMs are appropriately contributing to performance improvement and accountability.124
The Cons of PROs
PROs can disrupt busy clinical care environments and overextend clinical staff.125 Online collection of PROs outside of clinical encounters can relieve PRO-related burden, but this requires finding and funding appropriate secure online networks to effectively collect PROs.128 In 2015, only 60% of people seen for primary care visits could access or view their records online, and of those, only 57% used messaging for medical questions or concerns.129 Ideally, online patient portal or mobile health apps could synchronize directly to electronic health records or virtual scribes to transfer patient communications into clinical documentation.130 There has been limited success with this approach in European countries131 and with some chronic illness conditions in the United States.74
Electronic health technologies, including mobile health (mHealth) solutions, have improved the self-monitoring and self-management capability of patients with MS via information sharing in patient networks, assistive technologies, smartphone applications, and wearable devices.132,133 A recent study found that communication modes included secure online patient portal use (29%) and email use (21%), and among those who owned tablets or smartphones, 46% used mHealth apps.134 Social media use has been associated with increased peer/social/emotional support and increased access to health information, as well as clinical monitoring and behavior change.134,135 Individuals using mHealth apps are younger, have comorbidities, and have higher socioeconomic and education levels,135,136 suggesting that inequities in mHealth access exist.
Questionnaires can be time-consuming and cause mental distress if not appropriately facilitated.137 Decreasing questionnaire length and providing the option for PROMs to be delivered and completed online or outside of the clinic context can reduce burden.138 Additionally, while some people are consistent in sharing their PROs, others struggle with using computers, especially while experiencing severe symptoms, forget to complete PROMs, or simply do not have internet access due to financial or geographic constraints.139 A group of disabled and elderly persons with MS reported barriers to internet use due to visual deficits, small website font sizes, and distracting color schemes.140
Interpretability
Interpreting PROMs and displays of longitudinal PROM data can be a challenge for persons with MS and their clinicians. There is little standardization in how PROMs are scored and presented, and there is often confusion about thresholds for clinical significance and how PROM scores can be compared to other PROMs.141,142 While guidelines exist for implementing PRO scores in clinical settings,126,143 there are few that aid PROM interpretation. As a result, clinicians often seek research evidence for PROMs used in other similar patient populations as a benchmark,142-144 or compare them to other patients seen in their clinical practice.
Longitudinal PRO data are usually displayed in simple line graphs.145,146 Overall, line graphs have been found to have the highest ease of understanding by both patients and clinicians, but sometimes can be confusing.147 For example, upward trending lines are usually viewed as improvement and downward trending lines as decline; however, upward trending scores on a PROM can indicate decline, such as increasing fatigue severity. Annotation of visual displays can help. Patients and clinicians find that employing thresholds and color coding is useful, and better than “stoplight” red-yellow-green shading schemes or red-circle formats to indicate data that warrant attention.142
PROs are not free of risk for error, especially if they are used independently of other information sources, such as clinical interview, examination, and diagnostic testing, or if they are utilized too frequently, too infrequently, or are duplicated in practice. If a PRO instrument is employed too frequently, score changes may reflect learning effects rather than actual clinical status. Conversely, if used too infrequently, PRO information will not be timely enough to inform real-time clinical practice. Duplication of PRO assessments (eg, multiple measures of the same PRO for the same patient on the same day) or use of multiple PRO measures to assess the same aspect (eg, 2 measures used to assess fatigue) could introduce unnecessary complexity and confusion to interpretation of PRO results.
PRO measures also can be biased or modified by clinical status and/or perceptions of people with MS at the time of assessment. For example, cognitive impairment, whether at baseline state or due to a cognitive MS relapse event, could impact patients’ ability to understand and respond to PRO assessments, producing erroneous results. However, when used appropriately, PROs targeting cognitive dysfunction may be able to detect onset of cognitive events or help to measure recovery from them. Finally, PROs measure perceived (self-reported) status, which may not be an accurate depiction of actual status.
All of these potential pitfalls support the argument that PROs should be utilized to augment the clinical interview, examination, and diagnostic (objective) testing aspects of comprehensive MS care. In this way, PROs can be correlated with other information sources to deepen the shared understanding of health status between a person with MS and her clinician, increasing the potential to make better treatment decisions and care plans together in partnership.
Value and Cost
National groups such as the Patient-Centered Outcomes Research Institute (PCORI) are working with regulatory bodies, funding agencies, insurance providers, patient advocacy groups, researchers, providers, and specialty groups to investigate how PROMs can be implemented into value-based health care reforms, including value-based reimbursement.148 However, practical PRO implementation requires considerable time and resources, and many methodological and operational questions must be addressed before widespread adoption and reimbursement for PROMs will be feasible.148,149
Summary
PROs can generate valuable information about perceived health status, function, quality of life, and experience of care using self-reported sources. Validated PRO assessment tools include PROMs and PREMs. PROs are currently utilized in research settings (especially PROMs) but are also being used in clinical practice, quality improvement initiatives, and population health applications using LHS approaches. PROs have the advantages of empowering and informing persons with MS and clinicians to optimize patient-centered care, improve systems of care, and study population health outcomes. Barriers include PROM validity, reliability, comparability, specificity, interpretability, equity, time, and cost. Generic PROMs and PREMs, and some MS-specific PROMs, can be used for persons with MS. Unfortunately, no PREMs have been developed specifically for persons with MS, and this is an area for future research. With appropriate development and utilization in LHS applications, PROs can inform patient-centered clinical care, system-level improvement initiatives, and population health research, and have the potential to help facilitate coproduction of health care services.
Acknowledgments: The authors thank Ann Cabot, DO, of the MS Specialty Care Program at Concord Hospital and (especially) peer mentors from a peer outreach wellness program for people with MS (who have asked to remain anonymous) for interviews conducted with their permission to inform the case study described in this article. The case study used in this manuscript has been de-identified, with some aspects modified from actual, and the person in the case study is fictitiously named.
Corresponding author: Brant Oliver, PhD, MS, MPH, APRN-BC, Department of Community & Family Medicine, Dartmouth-Hitchcock Medical Center, Lebanon, NH 03756; [email protected].
Financial disclosures: None.
From the Dartmouth Institute for Health Policy & Clinical Practice, Geisel School of Medicine, Hanover, NH (Ms. Manohar and Dr. Oliver), the Department of Community & Family Medicine, Dartmouth-Hitchcock Medical Center, Lebanon, NH (Ms. Perkins, Ms. Laurion, and Dr. Oliver), and the Multiple Sclerosis Specialty Care Program, Concord Hospital, Concord, NH (Dr. Oliver).
Abstract
- Background: Patient-reported outcomes (PROs), including patient-reported outcome measures (PROMs) and patient-reported experience measures (PREMs), can be used to assess perceived health status, functioning, quality of life, and experience of care. Complex chronic illnesses such as multiple sclerosis (MS) affect multiple aspects of health, and PROs can be applied in assessment and decision-making in MS care as well as in research pertaining to MS.
- Objective: To provide a general review of PROs, with a specific focus on implications for MS care.
- Methods: Evidence synthesis of available literature on PROs in MS care.
- Results: PROs (including PROMs and PREMs) have historically been utilized in research and are now being applied in clinical, improvement, and population health settings using learning health system approaches in many disease populations, including MS. Many challenges complicate the use of PROs in MS care, including reliability, validity, and interpretability of PROMs, as well as feasibility barriers due to time and financial constraints in clinical settings.
- Conclusion: PROs have the potential to better inform clinical care, empower patient-centered care, inform health care improvement efforts, and create the conditions for coproduction of health care services.
Keywords: PRO; PROM; patient-reported outcome measure; patient-reported experience measure; quality of life; patient-centered care.
Multiple sclerosis (MS) is a disabling, complex, chronic, immune-mediated disorder of the central nervous system (CNS). MS causes inflammatory and degenerative damage in the CNS, which disrupts signaling pathways.1 It is most commonly diagnosed in young adults and affects 2.3 million people worldwide.2 People with MS experience very different disease courses and a wide range of neurological symptoms, including visual, somatic, mental health, sensory, motor, and cognitive problems.1-3 Relapsing-remitting MS, the most common form, affects 85% of those with MS and is characterized by periods of relapse (exacerbation) and remission.1 Other forms of MS (primary progressive and secondary progressive MS) are characterized by progressive deterioration and worsening symptom severity without exacerbations. Disease-modifying therapies (DMTs) can reduce the frequency of exacerbations and disability progression, but unfortunately there is no cure for MS. Treatment is focused on increasing quality of life, minimizing disability, and maximizing wellness.
Patient-reported outcomes (PROs) describe the perceived health status, function, and/or experience of a person as obtained by direct self-report. Patient-reported outcome measures (PROMs) are validated PROs that can be used to inform clinical care,4 and have demonstrated effectiveness in improving patient-provider communication and decision-making.5-7 PROMs are currently used in some MS clinical trials to determine the impact of experimental interventions,8-10 and are also being used to inform and improve clinical care in some settings. Especially for persons with MS, they can provide individualized perspectives about health experience and outcomes.11 In more advanced applications, PROMs can be used to improve face-to-face collaborations between clinicians and patients and to inform patient-centered systems of care.12-14 PROMs can also be used to inform systems-level improvement for entire patient populations.15,16
In this article, we review current applications of PROs and PROMs in the care of persons with MS, as well as current limitations and barriers to their use.
At a recent visit to her neurologist, Marion reviews her health diary, in which she has been tracking her fatigue levels throughout the day and when she has to visit the bathroom. The PRO diary also helps her remember details that she might not otherwise be able to recall at the time of her clinic visit. They review the diary entrees together to develop a shared understanding of what Marion has been experiencing and identify trends in the PRO data. They discuss symptom management and use the PRO information from the diary to help guide adjustments to her physical therapy routine and medication regimen.
Part of Marion’s “PRO package” includes the Center for Epidemiologic Studies Depression Scale (CES-D), a validated depression screening and symptom severity questionnaire that she completes every 3 months. Although she denies being depressed, she has noticed that her CES-D scores in recent months have been consistently increasing. This prompts a discussion about mental health in MS and a referral to work on depression with the MS mental health specialist. Marion and the mental health specialist use CES-D measures at baseline and during treatment to set a remission target and to track progress during treatment. Marion finds this helpful because she says it is hard for her to “wrap my hands around depression… it’s not something that there is a blood test or a MRI for.” Marion is encouraged by being able to see her CES-D scores change as her depression severity decreases, and this helps motivate her to keep engaged in treatment.
PROs and PROMs: General Applications
PROs are measures obtained directly from an individual without a priori interpretation by a clinician.9,17 PROs capture individual perspectives on symptoms, capability, disability, and health-related quality of life.9 With increasing emphasis on patient-centered care,18 individual perspectives and preferences elicited using PROMs may be able to inform better quality of care and patient-centered disease treatment and management.19-21
PROMs are standardized, validated questionnaires used to assess PROs and can be generic or condition-specific. Generic PROMs can be used in any patient population. The SF-3622 is a set of quality of life measures that assess perceived ability to complete physical tasks and routine activities, general health status, fatigue, social functioning, pain, and emotional and mental health.23 Condition-specific PROMs can be used for particular patient populations and are helpful in identifying changes in health status for a specific disease, disability, or surgery. For example, the PDQ-39 assesses 8 dimensions of daily living, functioning, and well-being for people with Parkinson’s disease.24
PROMs have been used in some MS clinical trials and research studies to determine the effectiveness of experimental treatments from the viewpoint of study participants.9,25,26 PROs can also be utilized in clinical care to facilitate communication of needs and track health outcomes,27 and can inform improvement in outcomes for health systems and populations. They can also be used to assess experience of care,28 encouraging a focus on high-quality outcomes through PRO-connected reimbursement mechanisms,29 and provide aggregate data to evaluate clinical practice, population health outcomes, and the effectiveness of public policies.27
Patient-reported experience measures (PREMs) assess patient satisfaction and experience of health care.30,31 CollaboRATE32 is a PREM that assesses the degree of shared decision-making occurring between patients and clinicians during clinical care. PREMs are currently used for assessing self-efficacy and in shared decision-making and health care improvement applications. PREMs have yet to be developed specifically for persons with MS.
PROMs in MS Care
Generic PROMs have shown that persons with MS are disproportionately burdened by poor quality of life.33-35 Other generic PROMs, like the SF-36,36 the Sickness Impact Profile,37 and versions of the Health Utilities Index,38 can be used to gather information on dysfunction and to determine quality and duration of life modified by MS-related dysfunction and disability. MS-specific PROMs are used to assess MS impairments, including pain, fatigue, cognition, sexual dysfunction, and depression.12,39-42 PROMs have also been used in MS clinical trials, including the Multiple Sclerosis Impact Scale-29 (MSIS-29),43,44 the Leeds MS QoL (LMSQoL),45,46 the Functional Assessment of MS (FAMS),47 the Hamburg Quality of Life Questionnaire in MS (HAQUAMS),48 the MS Quality of Life-54 (MSQoL-54),49 the MS International QoL (MUSIQoL),50 and the Patient-Reported Indices for MS Activity Limitations Scale (PRIMUS).51
Condition-specific PROMs are more sensitive to changes in health status and functioning for persons with MS compared to generic PROMs. They are also more reliable during MS remission and relapse periods.44,52 For example, the SF-36 has floor and ceiling effects in MS populations—a high proportion of persons with MS are scored at the maximum or minimum levels of the scale, limiting discriminant capability.22 As a result, a “combined approach” using both generic and MS-specific measures is often recommended.53 Some MS PROMs (eg, MSQoL-54) include generic questions found in the SF-36 as well as additional MS-specific questions or scales.
The variety of PROMs available (see Table for a selected listing) introduces a significant challenge to using them—limited generalizability and difficulty comparing PROs across MS studies. Efforts to establish common PROMs have been undertaken to address this problem.54 The National Institute of Neurologicical Disorders and Stroke (NINDS) sponsored the development of a neurological quality of life battery, the Neuro-QOL.55 Neuro-QOL measures the physical, mental, and social effects of neurological conditions in adults and children with neurological disorders and has the capability to facilitate comparisons across different neurological conditions. Additionally, the Patient-Reported Outcomes Measure Information System (PROMIS) has been developed to assess physical, mental, and social effects of chronic disease. PROMIS has a hybrid design that includes generic and MS-specific measures (such as PROMIS FatigueMS).56 PROMIS can be used to assess persons with MS as well as to compare the MS population with other populations with chronic illness.
PROMs have varying levels of reliability and validity. The Evaluating the Measure of Patient-Reported Outcomes57 study evaluated the development process of MS PROMs,43 and found that the MSIS-29 and LMSQoL had the highest overall reliability among the most common MS PROMs. However, both scored poorly on validity due to lack of patient involvement during development. This questions the overall capability of existing MS PROMs to accurately and consistently assess PROs in persons with MS.
“Feed-Forward” PROMs
Oliver and colleagues16 have described “feed-forward” PROM applications in MS care in a community hospital setting using a learning health system approach. This MS clinic uses feed-forward PROs to inform clinical care—PRO data are gathered before the clinic visit and analyzed ahead of or during the clinic visit by the clinician. Patients are asked to arrive early and complete a questionnaire comprised of PROMs measuring disability, functioning, quality of life, cognitive ability, pain, fatigue, sleep quality, anxiety, and depression. Clinicians score the PROMs and input scores into the electronic health record before the clinical encounter. During the clinic visit, PROM data is visually displayed so that the clinician and patient can discuss results and use the data to better inform decision-making. The visual data display contains longitudinal information, displaying trends in health status across multiple domains, and includes specified thresholds for clinically active symptom levels (Figure).16 Longitudinal monitoring of PROM data allows for real-time assessment of goal-related progress throughout treatment. As illustrated previously by Marion’s case study, the use of real-time feed-forward PROM data can strengthen the partnership between patient and clinician as well as improve empowerment, engagement, self-monitoring, and adherence.
PRO Dashboards
Performance dashboards are increasingly used in health care to visually display clinical and PRO data for individual patients, systems, and populations over time. Dashboards display a parsimonious group of critically important measures to give clinicians and patients a longitudinal view of PRO status. They can inform decision-making in clinical care, operations, health care improvement efforts, and population health initiatives.58 Effective dashboards allow for user customization with meaningful measures, knowledge discovery for analysis of health problems, accessibility of health information, clear visualization, alerts for unexpected data values, and system connectivity.59,60 Appropriate development of PRO dashboards requires meaningful patient and clinician involvement via focus groups and key informant interviews, Delphi process approaches to prioritize and finalize selection of priority measures, iterative building of the interface with design input from key informants and stakeholders (co-design), and pilot testing to assess feasibility and acceptability of use.61-63
Other Applications of PROs/PROMs in MS
Learning Health Systems
The National Quality Forum (NQF) and the Centers for Medicaid and Medicare Services have adopted PROs for use in quality measurement.64-66 This includes a movement towards the use of LHS, defined as a health system in which information from patients and clinicians is systematically collected and synthesized with external evidence to inform clinical care, improvement, and research.67-70 Often a LHS is undertaken as a collaborative effort between multiple health care centers to improve quality and outcomes of care.70 The MS Continuous Quality Improvement Collaborative (MS-CQI), the first multi-center systems-level health care improvement research collaborative for MS,71 as well as IBD Qorus and the Cystic Fibrosis Care Center Network utilize LHS approaches.72-77
IBD Qorus is a LHS developed by the Crohn’s and Colitis Foundation that uses performance dashboards to better inform clinical care for people with inflammatory bowel disease. It also employs system-level dashboards for performance benchmarking in quality improvement initiatives and aggregate-level dashboards to assess population health status.78,79 MS-CQI uses a LHS approach to inform the improvement of MS care across multiple centers using a comprehensive dashboard, including PROMs, for benchmarking and to monitor system and population health status. MS-CQI collects PROMs using a secure online platform that can be accessed by persons with MS and their clinicians and also includes a journaling feature for collecting qualitative information and for reference and self-monitoring.71
MS Research
PROMs are used in clinical and epidemiological research to evaluate many aspects of MS, including the FAMS, the PDSS, the Fatigue Impact Scale (FIS), and others.80-82 For example, the PROMIS FatigueMS and the Fatigue Performance Scale have been used to assess the impact of MS-related fatigue on social participation.83 Generic and MS-specific PROMs have been used to assess pain levels for people with MS,84-87 and multiple MS-specific PROMs, like PRIMUS and MSQoL-54,43 as well as the SF-3639 include pain assessment scales. PROMs have also been used to assess MS-related bladder, bowel, and sexual dysfunction. Urgency, frequency, and incontinence affect up to 75% of patients with MS,88 and many PROMs, such as the LMSQoL, MUSIQoL, and the MSQoL-54, are able to evaluate bladder control and sexual functioning.43,89
PROMs are employed in MS clinical trials to help assess the tolerability and effectiveness of DMTs.90,91 PROs have been used as secondary endpoints to understand the global experience of a DMT from the patient perspective.92-94 There are 15 FDA-approved DMTs for MS, and clinical trials for 6 of these have used PROMs as an effectiveness end point.54,91,95,96 However, most DMT clinical trials are powered for MRI, relapse rate, or disease progression primary outcomes rather than PROMs, often resulting in underpowered PROM analyses.97 In addition, many PROMs are not appropriate for use in DMT clinical trials.98,99
In order to bridge the gap between clinical research and practice, some industry entities are championing “patient-focused drug development” approaches. The Accelerated Cure Project for MS has launched iConquerMS, which collects PROMs from persons with MS to further PRO research in MS and follows 4700 individuals with MS worldwide.100 In 2018, the American College of Physicians announced a collaboration with an industry partner to share data to inform DMT clinical trials and develop and validate PROMs specifically designed for DMT clinical trials.101
Population Health
Registries following large cohorts of people with MS have the potential to develop knowledge about disease progression, treatment patterns, and outcomes.102 The Swedish EIMS study has identified associations between pre-disease body mass index and MS prognosis,102 alcohol and tobacco consumption affecting MS risk,103,104 and exposure to shift work at a young age and increased MS risk.105 The North American Research Committee on MS83,106,107 and iConquerMS registries are “PROM-driven” and have been useful in identifying reductions in disease progression in people using DMTs.107,108 The New York State MS Consortium has identified important demographic characteristics that influence MS progression.109,110 PROs can also be used to determine risk of MS-related mortality111 and decline in quality of life.112,113 Limitations of these approaches include use of different PROMs, inconsistencies in data collection processes, and different follow-up intervals used across registries.102
Patient-Centered Care
The Institute of Medicine defines patient-centeredness as “care that is respectful and responsive to individual patient preferences, needs, and values and ensures that patient values guide all clinical decisions.”114 PROs are useful for identifying a patient’s individual health concerns and preferences, something that is needed when treating a highly variable chronic health condition like MS. The use of PROs can help clinicicans visualize the lived experience of persons with MS and identify personal preferences,115 as well as improve self-monitoring, self-management, self-efficacy, adherence, wellness, and coping ability.116 At the system level, PROs can inform improvement initiatives and patient-centered care design efforts.117-120
Selecting PROMs
Initiatives from groups like the COnsensus-based Standards for the Selection of health Measurement INstruments (COSMIN)121 and the International Society for Quality of Life Research (ISOQOL)108 offer guidance on selecting PROs. The NINDS has promoted common data collection between clinical studies of the brain and nervous system.122 General guidance from these sources recommends first considering the outcome and target population, selecting PROMs to measure the outcome through a synthesis of the available evidence, assessing validity and reliability of selected PROMs, and using standard measures that can be compared across studies or populations.108,121 Other factors include feasibility, acceptability, and burden of use for patients, clinicians, and systems, as well as literacy, cultural, and linguistic factors.123
The NQF recommends that consideration be given to individual patient needs, insurance factors, clinical setting constraints, and available resources when selecting PROMs.124 To maximize response rate, PROMs that are sensitive, reliable, valid, and developed in a comparative demographic of patients are advised.125 ISOQOL has released a User’s Guide and several companion guides on implementing and utilizing PROMs.108,126,127 Finally, PRO-Performance Measures (PRO-PMs) are sometimes used to assess whether PROMs are appropriately contributing to performance improvement and accountability.124
The Cons of PROs
PROs can disrupt busy clinical care environments and overextend clinical staff.125 Online collection of PROs outside of clinical encounters can relieve PRO-related burden, but this requires finding and funding appropriate secure online networks to effectively collect PROs.128 In 2015, only 60% of people seen for primary care visits could access or view their records online, and of those, only 57% used messaging for medical questions or concerns.129 Ideally, online patient portal or mobile health apps could synchronize directly to electronic health records or virtual scribes to transfer patient communications into clinical documentation.130 There has been limited success with this approach in European countries131 and with some chronic illness conditions in the United States.74
Electronic health technologies, including mobile health (mHealth) solutions, have improved the self-monitoring and self-management capability of patients with MS via information sharing in patient networks, assistive technologies, smartphone applications, and wearable devices.132,133 A recent study found that communication modes included secure online patient portal use (29%) and email use (21%), and among those who owned tablets or smartphones, 46% used mHealth apps.134 Social media use has been associated with increased peer/social/emotional support and increased access to health information, as well as clinical monitoring and behavior change.134,135 Individuals using mHealth apps are younger, have comorbidities, and have higher socioeconomic and education levels,135,136 suggesting that inequities in mHealth access exist.
Questionnaires can be time-consuming and cause mental distress if not appropriately facilitated.137 Decreasing questionnaire length and providing the option for PROMs to be delivered and completed online or outside of the clinic context can reduce burden.138 Additionally, while some people are consistent in sharing their PROs, others struggle with using computers, especially while experiencing severe symptoms, forget to complete PROMs, or simply do not have internet access due to financial or geographic constraints.139 A group of disabled and elderly persons with MS reported barriers to internet use due to visual deficits, small website font sizes, and distracting color schemes.140
Interpretability
Interpreting PROMs and displays of longitudinal PROM data can be a challenge for persons with MS and their clinicians. There is little standardization in how PROMs are scored and presented, and there is often confusion about thresholds for clinical significance and how PROM scores can be compared to other PROMs.141,142 While guidelines exist for implementing PRO scores in clinical settings,126,143 there are few that aid PROM interpretation. As a result, clinicians often seek research evidence for PROMs used in other similar patient populations as a benchmark,142-144 or compare them to other patients seen in their clinical practice.
Longitudinal PRO data are usually displayed in simple line graphs.145,146 Overall, line graphs have been found to have the highest ease of understanding by both patients and clinicians, but sometimes can be confusing.147 For example, upward trending lines are usually viewed as improvement and downward trending lines as decline; however, upward trending scores on a PROM can indicate decline, such as increasing fatigue severity. Annotation of visual displays can help. Patients and clinicians find that employing thresholds and color coding is useful, and better than “stoplight” red-yellow-green shading schemes or red-circle formats to indicate data that warrant attention.142
PROs are not free of risk for error, especially if they are used independently of other information sources, such as clinical interview, examination, and diagnostic testing, or if they are utilized too frequently, too infrequently, or are duplicated in practice. If a PRO instrument is employed too frequently, score changes may reflect learning effects rather than actual clinical status. Conversely, if used too infrequently, PRO information will not be timely enough to inform real-time clinical practice. Duplication of PRO assessments (eg, multiple measures of the same PRO for the same patient on the same day) or use of multiple PRO measures to assess the same aspect (eg, 2 measures used to assess fatigue) could introduce unnecessary complexity and confusion to interpretation of PRO results.
PRO measures also can be biased or modified by clinical status and/or perceptions of people with MS at the time of assessment. For example, cognitive impairment, whether at baseline state or due to a cognitive MS relapse event, could impact patients’ ability to understand and respond to PRO assessments, producing erroneous results. However, when used appropriately, PROs targeting cognitive dysfunction may be able to detect onset of cognitive events or help to measure recovery from them. Finally, PROs measure perceived (self-reported) status, which may not be an accurate depiction of actual status.
All of these potential pitfalls support the argument that PROs should be utilized to augment the clinical interview, examination, and diagnostic (objective) testing aspects of comprehensive MS care. In this way, PROs can be correlated with other information sources to deepen the shared understanding of health status between a person with MS and her clinician, increasing the potential to make better treatment decisions and care plans together in partnership.
Value and Cost
National groups such as the Patient-Centered Outcomes Research Institute (PCORI) are working with regulatory bodies, funding agencies, insurance providers, patient advocacy groups, researchers, providers, and specialty groups to investigate how PROMs can be implemented into value-based health care reforms, including value-based reimbursement.148 However, practical PRO implementation requires considerable time and resources, and many methodological and operational questions must be addressed before widespread adoption and reimbursement for PROMs will be feasible.148,149
Summary
PROs can generate valuable information about perceived health status, function, quality of life, and experience of care using self-reported sources. Validated PRO assessment tools include PROMs and PREMs. PROs are currently utilized in research settings (especially PROMs) but are also being used in clinical practice, quality improvement initiatives, and population health applications using LHS approaches. PROs have the advantages of empowering and informing persons with MS and clinicians to optimize patient-centered care, improve systems of care, and study population health outcomes. Barriers include PROM validity, reliability, comparability, specificity, interpretability, equity, time, and cost. Generic PROMs and PREMs, and some MS-specific PROMs, can be used for persons with MS. Unfortunately, no PREMs have been developed specifically for persons with MS, and this is an area for future research. With appropriate development and utilization in LHS applications, PROs can inform patient-centered clinical care, system-level improvement initiatives, and population health research, and have the potential to help facilitate coproduction of health care services.
Acknowledgments: The authors thank Ann Cabot, DO, of the MS Specialty Care Program at Concord Hospital and (especially) peer mentors from a peer outreach wellness program for people with MS (who have asked to remain anonymous) for interviews conducted with their permission to inform the case study described in this article. The case study used in this manuscript has been de-identified, with some aspects modified from actual, and the person in the case study is fictitiously named.
Corresponding author: Brant Oliver, PhD, MS, MPH, APRN-BC, Department of Community & Family Medicine, Dartmouth-Hitchcock Medical Center, Lebanon, NH 03756; [email protected].
Financial disclosures: None.
1. Goldenberg MM. Multiple sclerosis review. PT. 2012;37:175-184.
2. Doshi A, Chataway J. Multiple sclerosis, a treatable disease. Clin Med. 2016;16(Suppl 6):s53-s9.
3. Perrin Ross A. Management of multiple sclerosis. Am J Managed Care. 2013;19(16 Suppl):s301-s306.
4. Batalden M, Batalden P, Margolis P, et al. Coproduction of healthcare service. BMJ Qual Saf. 2016;25:509-517.
5. Chen J, Ou L, Hollis SJ. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res. 2013;13:211.
6. Knaup C, Koesters M, Schoefer D, et al. Effect of feedback of treatment outcome in specialist mental healthcare: meta-analysis. Br J Psychiatr. 2009;195:15-22.
7. Dudgeon D. The impact of measuring patient-reported outcome measures on quality of and access to palliative care. J Palliat Med. 2018;21(S1):S76-s80.
8. Snyder CF, Herman JM, White SM, et al. When using patient-reported outcomes in clinical practice, the measure matters: a randomized controlled trial. J Oncol Pract. 2014;10:e299-e306.
9. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79.
10. de Jong MJ, Huibregtse R, Masclee AAM, et al. Patient-reported outcome measures for use in clinical trials and clinical practice in inflammatory bowel diseases: a systematic review. Clin Gastroenterol Hepatol. 2018;16:648-663.
11. Roman D, Osborne-Stafsnes J, Amy CH, et al. Early lessons from four ‘aligning forces for quality’ communities bolster the case for patient-centered care. Health Affairs. 2013;32:232-241.
12. Ehde DM, Alschuler KN, Sullivan MD, et al. Improving the quality of depression and pain care in multiple sclerosis using collaborative care: The MS-care trial protocol. Contemporary Clinical Trials. 2018;64:219-229.
13. Lavallee DC, Chenok KE, Love RM, et al. Incorporating patient-reported outcomes into health care to engage patients and enhance care. Health Affairs. 2016;35:575-582.
14. Schick-Makaroff K, Thummapol O, Thompson S, et al. Strategies for incorporating patient-reported outcomes in the care of people with chronic kidney disease (PRO kidney): a protocol for a realist synthesis. Clin Gastroenterol Hepatol. 2018;16:648-663.
15. Nelson EC, Dixon-Woods M, Batalden PB, et al. Patient focused registries can improve health, care, and science. BMJ. 2016;354:i3319.
16. Oliver BJ, Nelson EC, Kerrigan CL. Turning feed-forward and feedback processes on patient-reported data into intelligent action and informed decision-making: case studies and principles. Med Care. 2019;57 Suppl 1:S31-s37.
17. Deshpande PR, Rajan S, Sudeepthi BL, Abdul Nazir CP. Patient-reported outcomes: A new era in clinical research. Perspect Clin Res. 2011;2:137-144.
18. Epstein RM, Street RL, Jr. The values and value of patient-centered care. Ann Fam Med. 2011;9:100-103.
19. Payne SA. A study of quality of life in cancer patients receiving palliative chemotherapy. Soc Sci Med. 1992;35:1505-1509.
20. Borgaonkar MR, Irvine EJ. Quality of life measurement in gastrointestinal and liver disorders. Gut. 2000;47:444-454.
21. Macdonell R, Nagels G, Laplaud DA, et al. Improved patient-reported health impact of multiple sclerosis: The ENABLE study of PR-fampridine. Multiple Sclerosis. 2016;22:944-954.
22. Hobart J, Freeman J, Lamping D, et al. The SF-36 in multiple sclerosis: why basic assumptions must be tested. J Neurol Neurosurg Psychiatry. 2001;71:363-370.
23. Lins L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: Scoping review. SAGE Open Med. 2016;4:2050312116671725.
24. Parkinson’s Disease Society of the United Kingdom. The Parkinson’s Disease Questionnaire (PDQ-39). https://www.parkinsons.org.uk/professionals/resources/parkinsons-disease-questionnaire-pdq-39. Accessed October 20, 2019.
25. Basch E. The missing voice of patients in drug-safety reporting. N Engl J Med. 2010;362:865-869.
26. Calvert M, Brundage M, Jacobsen PB, et al. The CONSORT Patient-Reported Outcome (PRO) extension: implications for clinical trials and practice. Health Qual Life Outcomes. 2013;11:184.
27. Black N. Patient reported outcome measures could help transform healthcare. BMJ. 2013;346:f167.
28. Franks P, Fiscella K, Shields CG, et al. Are patients’ ratings of their physicians related to health outcomes? Ann Fam Med. 2005;3:229-234.
29. Hostetter M, Klein S. Using patient-reported outcomes to improve health care quality. The Commonwealth Fund 2019. www.commonwealthfund.org/publications/newsletter-article/using-patient-reported-outcomes-improve-health-care-quality. Accessed October 24, 2019.
30. Charlotte Kingsley SP. Patient-reported outcome measures and patient-reported experience measures. BJA Education. 2017;17:137-144.
31. Weldring T, Smith SM. Patient-Reported outcomes (PROs) and patient-reported outcome measures (PROMs). Health Services Insights. 2013;6:61-68.
32. Elwyn G, Barr PJ, Grande SW, et al. Developing CollaboRATE: A fast and frugal patient-reported measure of shared decision making in clinical encounters. Patient Educ Couns. 2013;93:102-107.
33. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33:1444-1452.
34. Rudick RA, Miller D, Clough JD, et al. Quality of life in multiple sclerosis. Comparison with inflammatory bowel disease and rheumatoid arthritis. Arch Neurol. 1992;49:1237-1242.
35. Benito-Leon J, Morales JM, Rivera-Navarro J, Mitchell A. A review about the impact of multiple sclerosis on health-related quality of life. Disabil Rehabil. 2003;25:1291-1303.
36. RAND Corporation. 36-Item Short Form Survey (SF-36). [https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html. Accessed October 20, 2019.
37. Bergner M, Bobbitt RA, Carter WB, Gilson BS. The sickness impact profile: development and final revision of a health status measure. Medical Care. 1981;19:787-805.
38. Horsman J, Furlong W, Feeny D, Torrance G. The Health Utilities Index (HUI): concepts, measurement properties and applications. Health Qual Life Outcomes. 2003;1:54.
39. Kinkel RP, Laforet G, You X. Disease-related determinants of quality of life 10 years after clinically isolated syndrome. Int J MS Care. 2015;17:26-34.
40. Baumstarck-Barrau K, Simeoni MC, Reuter F, et al. Cognitive function and quality of life in multiple sclerosis patients: a cross-sectional study. BMC Neurology. 2011;11:17.
41. Samartzis L, Gavala E, Zoukos Y, et al. Perceived cognitive decline in multiple sclerosis impacts quality of life independently of depression. Rehabil Res Pract. 2014;2014:128751.
42. Vitkova M, Rosenberger J, Krokavcova M, et al. Health-related quality of life in multiple sclerosis patients with bladder, bowel and sexual dysfunction. Disabil Rehabil. 2014;36:987-992.
43. Khurana V, Sharma H, Afroz N, et al. Patient-reported outcomes in multiple sclerosis: a systematic comparison of available measures. Eur J Neurol. 2017;24:1099-1107.
44. Hobart J, Lamping D, Fitzpatrick R, et al. The Multiple Sclerosis Impact Scale (MSIS-29): a new patient-based outcome measure. Brain. 2001;124(Pt 5):962-973.
45. Lily O, McFadden E, Hensor E, et al. Disease-specific quality of life in multiple sclerosis: the effect of disease modifying treatment. Multiple Sclerosis. 2006;12:808-813.
46. Ford HL, Gerry E, Tennant A, et al. Developing a disease-specific quality of life measure for people with multiple sclerosis. Clin Rehabil. 2001;15:247-258.
47. Cella DF, Dineen K, Arnason B, et al. Validation of the functional assessment of multiple sclerosis quality of life instrument. Neurology. 1996;47:129-139.
48. Gold SM, Heesen C, Schulz H, et al. Disease specific quality of life instruments in multiple sclerosis: validation of the Hamburg Quality of Life Questionnaire in Multiple Sclerosis (HAQUAMS). Multiple Sclerosis. 2001;7:119-130.
49. Vickrey BG, Hays RD, Harooni R, et al. A health-related quality of life measure for multiple sclerosis. Qual Life Res. 1995;4:187-206.
50. Simeoni M, Auquier P, Fernandez O, et al. Validation of the Multiple Sclerosis International Quality of Life questionnaire. Multiple Sclerosis. 2008;14:219-230.
51. Doward LC, McKenna SP, Meads DM, et al. The development of patient-reported outcome indices for multiple sclerosis (PRIMUS). Multiple Sclerosis. 2009;15:1092-1102.
52. Ozakbas S, Akdede BB, Kosehasanogullari G, et al. Difference between generic and multiple sclerosis-specific quality of life instruments regarding the assessment of treatment efficacy. J Neurol Sci. 2007;256:30-34.
53. Cella DF, Wiklund I, Shumaker SA, Aaronson NK. Integrating health-related quality of life into cross-national clinical trials. Qual Life Res. 1993;2:433-440.
54. Nowinski CJ, Miller DM, Cella D. Evolution of patient-reported outcomes and their role in multiple sclerosis clinical trials. Neurotherapeutics. 2017;14:934-944.
55. Cella D, Nowinski C, Peterman A, et al. The neurology quality-of-life measurement initiative. Arch Phys Med Rehabil. 2011;92(10 Suppl):S28-S36.
56. HealthMeasures: PROMIS. http://www.healthmeasures.net/explore-measurement-systems/promis. Accessed October 20, 2019.
57. Valderas JM, Ferrer M, Mendivil J, et al. Development of EMPRO: a tool for the standardized assessment of patient-reported outcome measures. Value Health. 2008;11:700-708.
58. Bach K, Martling C, Mork PJ, et al. Design of a clinician dashboard to facilitate co-decision making in the management of non-specific low back pain. J Intelligent Helath Syst. 2019;52:269-284.
59. Karami M, Safdari R, Rahimi A. Effective radiology dashboards: key research findings. Radiology Management. 2013;35:42-45.
60. Dowding D, Randell R, Gardner P, et al. Dashboards for improving patient care: review of the literature. Int J Med Informatic. 2015;84:87-100.
61. Mlaver E, Schnipper JL, Boxer RB, et al. User-centered collaborative design and development of an inpatient safety dashboard. Jt Comm J Qual Patient Saf. 2017;43:676-685.
62. Dowding D, Merrill J, Russell D. Using feedback intervention theory to guide clinical dashboard design. AMIA Annu Symp Proc. 2018;2018:395-403.
63. Hartzler AL, Izard JP, Dalkin BL, et al. Design and feasibility of integrating personalized PRO dashboards into prostate cancer care. J Am Med Inform Assoc. 2016;23:38-47.
64. Lavallee DC, Chenok KE, Love RM, et al. Incorporating patient-reported outcomes into health care to engage patients and enhance care. Health Affairs. 2016;35:575-582.
65. U.S. Centers for Medicare & Medicaid Services. Comprehensive Care for Joint Replacement Model. https://innovation.cms.gov/initiatives/CJR. Accessed August 3, 2019.
66. National Quality Forum. Patient-reported outcomes: Accessed August 3, 2019. http://www.qualityforum.org/Patient-Reported_Outcomes.aspx. Accessed October 20, 2019.
67. Institute of Medicine Roundtable on Evidence-Based Medicine. The National Academies Collection: Reports funded by National Institutes of Health. In: Olsen LA, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US).National Academy of Sciences; 2007.
68. About Learning Health Systems: Agency for Healthcare Research and Quality. https://www.ahrq.gov/learning-health-systems/about.html. Accessed August 3, 2019.
69. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington (DC): National Academies Press; 2013.
70. Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
71. Oliver BJ, for the MS-CQI Investigators. The Multiple Sclerosis Continuous improvement collaborative: the first coproduction learning health system improvement science research collaborative for multiple sclerosis. The International Society for Quality in Health Care (ISQua) Annual International Conference; October 2019; Cape Town, South Africa.
72. Britto MT, Fuller SC, Kaplan HC, et al. Using a network organisational architecture to support the development of Learning Healthcare Systems. BMJ Qual Saf. 2018;27:937-946.
73. Care Centers: Cystic Fibrosis Foundation. https://www.cff.org/Care/Care-Centers/. Accessed August 3, 2019.
74. Schechter MS, Fink AK, Homa K, Goss CH. The Cystic Fibrosis Foundation patient registry as a tool for use in quality improvement. BMJ Qual Saf. 2014;23(Suppl 1):i9-i14.
75. Mogayzel PJ, Dunitz J, Marrow LC, Hazle LA. Improving chronic care delivery and outcomes: the impact of the cystic fibrosis Care Center Network. BMJ Qual Saf. 2014;23(Suppl 1):i3-i8.
76. Godfrey MM, Oliver BJ. Accelerating the rate of improvement in cystic fibrosis care: contributions and insights of the learning and leadership collaborative. BMJ Qual Saf. 2014;23(Suppl 1):i23-i32.
77. Nelson EC, Godfrey MM. Quality by Design: A Clinical Microsystems Approach. San Francisco, California: Jossey-Bass; 2007.
78. Crohn’s & Colitis Foundation.Quality of Care: IBD Qorus: https://www.crohnscolitisfoundation.org/research/ibd-qorus. Accessed October 23, 2019.
79. Johnson LC, Melmed GY, Nelson EC, et al. Fostering collaboration through creation of an ibd learning health system. Am J Gastroenterol. 2017;112:406-468.
80. Fernandez-Munoz JJ, Moron-Verdasco A, Cigaran-Mendez M, et al. Disability, quality of life, personality, cognitive and psychological variables associated with fatigue in patients with multiple sclerosis. Acta Neurologica Scandinavica. 2015;132:118-124.
81. Flachenecker P, Kumpfel T, Kallmann B, et al. Fatigue in multiple sclerosis: a comparison of different rating scales and correlation to clinical parameters. Multiple Sclerosis. 2002;8:523-526.
82. Tellez N, Rio J, Tintore M, et al. Does the Modified Fatigue Impact Scale offer a more comprehensive assessment of fatigue in MS? Multiple Sclerosis. 2005;11:198-202.
83. Salter A, Fox RJ, Tyry T, et al. The association of fatigue and social participation in multiple sclerosis as assessed using two different instruments. Mult Scler Relat Disord. 2019;31:165-72.
84. Solaro C, Trabucco E, Messmer Uccelli M. Pain and multiple sclerosis: pathophysiology and treatment. Curr Neurol Neurosci Rep. 2013;13:320.
85. Jawahar R, Oh U, Yang S, Lapane KL. A systematic review of pharmacological pain management in multiple sclerosis. Drugs. 2013;73:1711-1722.
86. Rintala A, Hakkinen A, Paltamaa J. Ten-year follow-up of health-related quality of life among ambulatory persons with multiple sclerosis at baseline. Qual Life Res. 2016;25:3119-3127.
87. Tepavcevic DK, Pekmezovic T, Stojsavljevic N, et al. Change in quality of life and predictors of change among patients with multiple sclerosis: a prospective cohort study. Qual Life Res. 2014;23:1027-1037.
88. DasGupta R, Fowler CJ. Bladder, bowel and sexual dysfunction in multiple sclerosis: management strategies. Drugs. 2003;63:153-166.
89. Marck CH, Jelinek PL, Weiland TJ, et al. Sexual function in multiple sclerosis and associations with demographic, disease and lifestyle characteristics: an international cross-sectional study. BMC Neurology. 2016;16:210.
90. Gajofatto A, Benedetti MD. Treatment strategies for multiple sclerosis: When to start, when to change, when to stop? World J Clinical Cases. 2015;3:545-555.
91. National Multiple Sclerosis Society. Disease-modifying therapies for MS. 2018.
92. Rudick RA, Panzara MA. Natalizumab for the treatment of relapsing multiple sclerosis. Biologics. 2008;2:189-199.
93. Cohen JA, Cutter GR, Fischer JS, et al. Benefit of interferon beta-1a on MSFC progression in secondary progressive MS. Neurology. 2002;59:679-687.
94. Patti F, Pappalardo A, Montanari E, et al. Interferon-beta-1a treatment has a positive effect on quality of life of relapsing-remitting multiple sclerosis: results from a longitudinal study. J Neurol Sci. 2014;337:180-185.
95. Novartis. Exploring the efficacy and safety of Siponimod in patients with secondary progressive multiple sclerosis (EXPAND). Available from clinicaltrials.gov/ct2/show/NCT01665144. NLM identifier: NCT01665144.
96. Singal AG, Higgins PD, Waljee AK. A primer on effectiveness and efficacy trials. Clin Transl Gastroenterol. 2014;5:e45.
97. Jongen PJ. Health-related quality of life in patients with multiple sclerosis: impact of disease-modifying drugs. CNS Drugs. 2017;31:585-602.
98. Coretti S, Ruggeri M, McNamee P. The minimum clinically important difference for EQ-5D index: a critical review. Expert Rev Pharmacoecon Outcomes Res. 2014;14:221-233.
99. Clinical Review Report: Ocrelizumab (Ocrevus). CADTH Common Drug Review. 2018:Appendix 4, Validity of Outcome Measures.
100. iCounquer MS; Empowering patients: how individuals with ms are contributing to the fight to find a cure: 2018. https://www.iconquerms.org/empowering-patients-how-individuals-ms-are-contributing-fight-find-cure. Accessed October 23, 2019.
101. K M. Accelerated Cure Project Announces Collaboration with EMD Serono to Advance Patient-Focused Drug Development in Multiple Sclerosis2018. https://www.iconquerms.org/accelerated-cure-project-announces-collaboration-emd-serono-advance-patient-focused-drug-development. Accessed October 24, 2019.
102. Bebo BF Jr, Fox RJ, Lee K, et al. Landscape of MS patient cohorts and registries: Recommendations for maximizing impact. Multiple Sclerosis. 2018;24:579-586.
103. Hedstrom AK, Hillert J, Olsson T, Alfredsson L. Alcohol as a modifiable lifestyle factor affecting multiple sclerosis risk. JAMA Neurology. 2014;71:300-305.
104. Hedstrom AK, Baarnhielm M, Olsson T, Alfredsson L. Tobacco smoking, but not Swedish snuff use, increases the risk of multiple sclerosis. Neurology. 2009;73:696-701.
105. Hedstrom AK, Akerstedt T, Hillert J, et al. Shift work at young age is associated with increased risk for multiple sclerosis. Ann Neurol. 2011;70:733-741.
106. Hadjimichael O, Vollmer T, Oleen-Burkey M. Fatigue characteristics in multiple sclerosis: the North American Research Committee on Multiple Sclerosis (NARCOMS) survey. Health Qual Life Outcomes. 2008;6:100.
107. Fox RJ, Salter AR, Tyry T, et al. Treatment discontinuation and disease progression with injectable disease-modifying therapies: findings from the North American research committee on multiple sclerosis database. Int J MS Care. 2013;15:194-201.
108. Reeve BB, Wyrwich KW, Wu AW, et al. ISOQOL recommends minimum standards for patient-reported outcome measures used in patient-centered outcomes and comparative effectiveness research. Qual Life Res. 2013;22:1889-1905.
109. Jacobs LD, Wende KE, Brownscheidle CM, et al. A profile of multiple sclerosis: the New York State Multiple Sclerosis Consortium. Multiple Sclerosis. 1999;5:369-376.
110. Weinstock-Guttman B, Jacobs LD, Brownscheidle CM, et al. Multiple sclerosis characteristics in African American patients in the New York State Multiple Sclerosis Consortium. Multiple Sclerosis. 2003;9:293-298.
111. Raffel J, Wallace A, Gveric D, et al. Patient-reported outcomes and survival in multiple sclerosis: A 10-year retrospective cohort study using the Multiple Sclerosis Impact Scale-29. PLoS Med. 2017;14:e1002346.
112. Vaughn CB, Kavak KS, Dwyer MG, et al. Fatigue at enrollment predicts EDSS worsening in the New York State Multiple Sclerosis Consortium. Multiple Sclerosis. 2018:1352458518816619.
113. Alroughani RA, Akhtar S, Ahmed SF, Al-Hashel JY. Clinical predictors of disease progression in multiple sclerosis patients with relapsing onset in a nation-wide cohort. Int J Neuroscience. 2015;125:831-837.
114. Dolan JG, Veazie PJ, Russ AJ. Development and initial evaluation of a treatment decision dashboard. BMC Med Inform Decision Mak. 2013;13:51.
115. Stuifbergen AK, Becker H, Blozis S, et al. A randomized clinical trial of a wellness intervention for women with multiple sclerosis. Arch Phys Med Rehabil. 2003;84:467-476.
116. Finkelstein J, Martin C, Bhushan A, et al. Feasibility of computer-assisted education in patients with multiple sclerosis. In: Proceedings of the IEEE Symposium on Computer-Based Medical Systems. Bethesda, MD; 2004.
117. Batalden P. Getting more health from healthcare: quality improvement must acknowledge patient coproduction—an essay by Paul Batalden. BMJ. 2018;362:k3617.
118. Batalden PB, Davidoff F. What is “quality improvement” and how can it transform healthcare? Qual Saf Health Care. 2007;16:2-3.
119. Ovretveit J, Zubkoff L, Nelson EC, et al. Using patient-reported outcome measurement to improve patient care. Int J Qual Health Care. 2017;29:874-879.
120. Prodinger B, Taylor P. Improving quality of care through patient-reported outcome measures (PROMs): expert interviews using the NHS PROMs Programme and the Swedish quality registers for knee and hip arthroplasty as examples. BMC Health Services Res. 2018;18:87.
121. Prinsen CA, Vohra S, Rose MR, et al. How to select outcome measurement instruments for outcomes included in a “Core Outcome Set” – a practical guideline. Trials. 2016;17:449.
122. NINDS. NINDS Common Data Elements: Streamline Your Neuroscience Clinical Research National Institute of Health. https://www.commondataelements.ninds.nih.gov/#page=Default. Accessed October 23, 2019.
123. Francis DO, McPheeters ML, Noud M, et al. Checklist to operationalize measurement characteristics of patient-reported outcome measures. Systematic Rev. 2016;5:129.
124. National Quality Forum. Patient-reported outcomes (PROs) in performance measurement. 2013. https://www.qualityforum.org/Publications/2012/12/Patient-Reported_Outcomes_in_Performance_Measurement.aspx. Accessed October 15, 2019.
125. Dawson J, Doll H, Fitzpatrick R, et al. The routine use of patient reported outcome measures in healthcare settings. BMJ. 2010;340:c186.
126. Aaronson N ET, Greenlhalgh J, Halyard M, et al. User’s guide to implementation of patient reported outcomes assessment in clinical practice. International Society for Quality of Life. 2015.
127. Chan EKH, Edwards TC, Haywood K, et al. Implementing patient-reported outcomes mesaures in clinical practice: a companion guide to the ISOQOL user’s guide. Qual Life Res. 2018;28:621-627.
128. Snyder CF, Wu AW, Miller RS, et al. The role of informatics in promoting patient-centered care. Cancer J. 2011;17:211-218
129. Osborn R, Moulds D, Schneider EC, et al. Primary care physicians in ten countries report challenges caring for patients with complex health needs. Health Affairs. 2015;34:2104-2112.
130. Wu AW, Kharrazi H, Boulware LE, Snyder CF. Measure once, cut 0wice--adding patient-reported outcome measures to the electronic health record for comparative effectiveness research. J Clin Epidemiol. 2013;66(8 Suppl):S12-S20.
131. Trojano M, Bergamaschi R, Amato MP, et al. The Italian multiple sclerosis register. Neurol Sci. 2019;40:155-165.
132. Moorhead SA, Hazlett DE, Harrison L, et al. A new dimension of health care: systematic review of the uses, benefits, and limitations of social media for health communication. J Med Internet Res. 2013;15:e85.
133. Luigi L, Francesco B, Marcello M, et al. e-Health and multiple sclerosis: An update. Multiple Sclerosis J. 2018;24:1657-1664.
134. Marrie RA, Leung S, Tyry T, et al. Use of eHealth and mHealth technology by persons with multiple sclerosis. Mult Scler Relat Dis. 2019;27:13-19.
135. Giunti G, Kool J, Rivera Romero O, Dorronzoro Zubiete E. Exploring the specific needs of persons with multiple sclerosis for mhealth solutions for physical activity: mixed-methods study. JMIR Mhealth Uhealth. 2018;6:e37.
136. Spooner KK, Salemi JL, Salihu HM, Zoorob RJ. eHealth patient-provider communication in the United States: interest, inequalities, and predictors. J Am Med Inform Assoc. 2017;24(e1):e18-e27.
137. Safdar N, Abbo LM, Knobloch MJ, Seo SK. Research methods in healthcare epidemiology: survey and qualitative research. Infect Control Hosp Epidemiol. 2016;37:1272-1277.
138. Rolstad S, Adler J, Rydén A. Response burden and questionnaire length: is shorter better? A review and meta-analysis. Value Health. 2011;14:1101-1108.
139. Chiu C, Bishop M, Pionke JJ, et al. Barriers to the accessibility and continuity of health-care services in people with multiple sclerosis: a literature review. Int J MS Care. 2017;19:313-321.
140. Atreja A, Mehta N, Miller D, et al. One size does not fit all: using qualitative methods to inform the development of an Internet portal for multiple sclerosis patients. AMIA Annu Symp Proc Symp. 2005:16-20.
141. Snyder CF, Blackford AL, Brahmer JR, et al. Needs assessments can identify scores on HRQOL questionnaires that represent problems for patients: an illustration with the Supportive Care Needs Survey and the QLQ-C30. Qual Life Res. 2010;19:837-845.
142. de Vet HC, Terwee CB. The minimal detectable change should not replace the minimal important difference. J Clin Epidemiol. 2010;63:804-805.
143. Snyder CF, Aaronson NK, Choucair AK, et al. Implementing patient-reported outcomes assessment in clinical practice: a review of the options and considerations. Qual Life. 2012;21:1305-1314.
144. Myla DG, Melanie DW, Robert WM, et al. Identification and validation of clinically meaningful benchmarks in the 12-item Multiple Sclerosis Walking Scale. Multiple Sclerosis J. 2017;23:1405-1414.
145. van Munster CEP, Uitdehaag BMJ. Outcome measures in clinical trials for multiple sclerosis. CNS Drugs. 2017;31:217-236.
146. Snyder CF, Jensen R, Courtin SO, Wu AW. PatientViewpoint: a website for patient-reported outcomes assessment. Quality Life Res. 2009;18:793-800.
147. Snyder CF, Smith KC, Bantug ET, et al. What do these scores mean? Presenting patient-reported outcomes data to patients and clinicians to improve interpretability. Cancer. 2017;123:1848-1859.
148. Squitieri L, Bozic KJ, Pusic AL. The role of patient-reported outcome measures in value-based payment reform. Value Health. 2017;20:834-836.
149. Boyce MB, Browne JP. The effectiveness of providing peer benchmarked feedback to hip replacement surgeons based on patient-reported outcome measures—results from the PROFILE trial: a cluster randomised controlled study. BMJ Open. 2015;5:e008325.
1. Goldenberg MM. Multiple sclerosis review. PT. 2012;37:175-184.
2. Doshi A, Chataway J. Multiple sclerosis, a treatable disease. Clin Med. 2016;16(Suppl 6):s53-s9.
3. Perrin Ross A. Management of multiple sclerosis. Am J Managed Care. 2013;19(16 Suppl):s301-s306.
4. Batalden M, Batalden P, Margolis P, et al. Coproduction of healthcare service. BMJ Qual Saf. 2016;25:509-517.
5. Chen J, Ou L, Hollis SJ. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res. 2013;13:211.
6. Knaup C, Koesters M, Schoefer D, et al. Effect of feedback of treatment outcome in specialist mental healthcare: meta-analysis. Br J Psychiatr. 2009;195:15-22.
7. Dudgeon D. The impact of measuring patient-reported outcome measures on quality of and access to palliative care. J Palliat Med. 2018;21(S1):S76-s80.
8. Snyder CF, Herman JM, White SM, et al. When using patient-reported outcomes in clinical practice, the measure matters: a randomized controlled trial. J Oncol Pract. 2014;10:e299-e306.
9. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79.
10. de Jong MJ, Huibregtse R, Masclee AAM, et al. Patient-reported outcome measures for use in clinical trials and clinical practice in inflammatory bowel diseases: a systematic review. Clin Gastroenterol Hepatol. 2018;16:648-663.
11. Roman D, Osborne-Stafsnes J, Amy CH, et al. Early lessons from four ‘aligning forces for quality’ communities bolster the case for patient-centered care. Health Affairs. 2013;32:232-241.
12. Ehde DM, Alschuler KN, Sullivan MD, et al. Improving the quality of depression and pain care in multiple sclerosis using collaborative care: The MS-care trial protocol. Contemporary Clinical Trials. 2018;64:219-229.
13. Lavallee DC, Chenok KE, Love RM, et al. Incorporating patient-reported outcomes into health care to engage patients and enhance care. Health Affairs. 2016;35:575-582.
14. Schick-Makaroff K, Thummapol O, Thompson S, et al. Strategies for incorporating patient-reported outcomes in the care of people with chronic kidney disease (PRO kidney): a protocol for a realist synthesis. Clin Gastroenterol Hepatol. 2018;16:648-663.
15. Nelson EC, Dixon-Woods M, Batalden PB, et al. Patient focused registries can improve health, care, and science. BMJ. 2016;354:i3319.
16. Oliver BJ, Nelson EC, Kerrigan CL. Turning feed-forward and feedback processes on patient-reported data into intelligent action and informed decision-making: case studies and principles. Med Care. 2019;57 Suppl 1:S31-s37.
17. Deshpande PR, Rajan S, Sudeepthi BL, Abdul Nazir CP. Patient-reported outcomes: A new era in clinical research. Perspect Clin Res. 2011;2:137-144.
18. Epstein RM, Street RL, Jr. The values and value of patient-centered care. Ann Fam Med. 2011;9:100-103.
19. Payne SA. A study of quality of life in cancer patients receiving palliative chemotherapy. Soc Sci Med. 1992;35:1505-1509.
20. Borgaonkar MR, Irvine EJ. Quality of life measurement in gastrointestinal and liver disorders. Gut. 2000;47:444-454.
21. Macdonell R, Nagels G, Laplaud DA, et al. Improved patient-reported health impact of multiple sclerosis: The ENABLE study of PR-fampridine. Multiple Sclerosis. 2016;22:944-954.
22. Hobart J, Freeman J, Lamping D, et al. The SF-36 in multiple sclerosis: why basic assumptions must be tested. J Neurol Neurosurg Psychiatry. 2001;71:363-370.
23. Lins L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: Scoping review. SAGE Open Med. 2016;4:2050312116671725.
24. Parkinson’s Disease Society of the United Kingdom. The Parkinson’s Disease Questionnaire (PDQ-39). https://www.parkinsons.org.uk/professionals/resources/parkinsons-disease-questionnaire-pdq-39. Accessed October 20, 2019.
25. Basch E. The missing voice of patients in drug-safety reporting. N Engl J Med. 2010;362:865-869.
26. Calvert M, Brundage M, Jacobsen PB, et al. The CONSORT Patient-Reported Outcome (PRO) extension: implications for clinical trials and practice. Health Qual Life Outcomes. 2013;11:184.
27. Black N. Patient reported outcome measures could help transform healthcare. BMJ. 2013;346:f167.
28. Franks P, Fiscella K, Shields CG, et al. Are patients’ ratings of their physicians related to health outcomes? Ann Fam Med. 2005;3:229-234.
29. Hostetter M, Klein S. Using patient-reported outcomes to improve health care quality. The Commonwealth Fund 2019. www.commonwealthfund.org/publications/newsletter-article/using-patient-reported-outcomes-improve-health-care-quality. Accessed October 24, 2019.
30. Charlotte Kingsley SP. Patient-reported outcome measures and patient-reported experience measures. BJA Education. 2017;17:137-144.
31. Weldring T, Smith SM. Patient-Reported outcomes (PROs) and patient-reported outcome measures (PROMs). Health Services Insights. 2013;6:61-68.
32. Elwyn G, Barr PJ, Grande SW, et al. Developing CollaboRATE: A fast and frugal patient-reported measure of shared decision making in clinical encounters. Patient Educ Couns. 2013;93:102-107.
33. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33:1444-1452.
34. Rudick RA, Miller D, Clough JD, et al. Quality of life in multiple sclerosis. Comparison with inflammatory bowel disease and rheumatoid arthritis. Arch Neurol. 1992;49:1237-1242.
35. Benito-Leon J, Morales JM, Rivera-Navarro J, Mitchell A. A review about the impact of multiple sclerosis on health-related quality of life. Disabil Rehabil. 2003;25:1291-1303.
36. RAND Corporation. 36-Item Short Form Survey (SF-36). [https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html. Accessed October 20, 2019.
37. Bergner M, Bobbitt RA, Carter WB, Gilson BS. The sickness impact profile: development and final revision of a health status measure. Medical Care. 1981;19:787-805.
38. Horsman J, Furlong W, Feeny D, Torrance G. The Health Utilities Index (HUI): concepts, measurement properties and applications. Health Qual Life Outcomes. 2003;1:54.
39. Kinkel RP, Laforet G, You X. Disease-related determinants of quality of life 10 years after clinically isolated syndrome. Int J MS Care. 2015;17:26-34.
40. Baumstarck-Barrau K, Simeoni MC, Reuter F, et al. Cognitive function and quality of life in multiple sclerosis patients: a cross-sectional study. BMC Neurology. 2011;11:17.
41. Samartzis L, Gavala E, Zoukos Y, et al. Perceived cognitive decline in multiple sclerosis impacts quality of life independently of depression. Rehabil Res Pract. 2014;2014:128751.
42. Vitkova M, Rosenberger J, Krokavcova M, et al. Health-related quality of life in multiple sclerosis patients with bladder, bowel and sexual dysfunction. Disabil Rehabil. 2014;36:987-992.
43. Khurana V, Sharma H, Afroz N, et al. Patient-reported outcomes in multiple sclerosis: a systematic comparison of available measures. Eur J Neurol. 2017;24:1099-1107.
44. Hobart J, Lamping D, Fitzpatrick R, et al. The Multiple Sclerosis Impact Scale (MSIS-29): a new patient-based outcome measure. Brain. 2001;124(Pt 5):962-973.
45. Lily O, McFadden E, Hensor E, et al. Disease-specific quality of life in multiple sclerosis: the effect of disease modifying treatment. Multiple Sclerosis. 2006;12:808-813.
46. Ford HL, Gerry E, Tennant A, et al. Developing a disease-specific quality of life measure for people with multiple sclerosis. Clin Rehabil. 2001;15:247-258.
47. Cella DF, Dineen K, Arnason B, et al. Validation of the functional assessment of multiple sclerosis quality of life instrument. Neurology. 1996;47:129-139.
48. Gold SM, Heesen C, Schulz H, et al. Disease specific quality of life instruments in multiple sclerosis: validation of the Hamburg Quality of Life Questionnaire in Multiple Sclerosis (HAQUAMS). Multiple Sclerosis. 2001;7:119-130.
49. Vickrey BG, Hays RD, Harooni R, et al. A health-related quality of life measure for multiple sclerosis. Qual Life Res. 1995;4:187-206.
50. Simeoni M, Auquier P, Fernandez O, et al. Validation of the Multiple Sclerosis International Quality of Life questionnaire. Multiple Sclerosis. 2008;14:219-230.
51. Doward LC, McKenna SP, Meads DM, et al. The development of patient-reported outcome indices for multiple sclerosis (PRIMUS). Multiple Sclerosis. 2009;15:1092-1102.
52. Ozakbas S, Akdede BB, Kosehasanogullari G, et al. Difference between generic and multiple sclerosis-specific quality of life instruments regarding the assessment of treatment efficacy. J Neurol Sci. 2007;256:30-34.
53. Cella DF, Wiklund I, Shumaker SA, Aaronson NK. Integrating health-related quality of life into cross-national clinical trials. Qual Life Res. 1993;2:433-440.
54. Nowinski CJ, Miller DM, Cella D. Evolution of patient-reported outcomes and their role in multiple sclerosis clinical trials. Neurotherapeutics. 2017;14:934-944.
55. Cella D, Nowinski C, Peterman A, et al. The neurology quality-of-life measurement initiative. Arch Phys Med Rehabil. 2011;92(10 Suppl):S28-S36.
56. HealthMeasures: PROMIS. http://www.healthmeasures.net/explore-measurement-systems/promis. Accessed October 20, 2019.
57. Valderas JM, Ferrer M, Mendivil J, et al. Development of EMPRO: a tool for the standardized assessment of patient-reported outcome measures. Value Health. 2008;11:700-708.
58. Bach K, Martling C, Mork PJ, et al. Design of a clinician dashboard to facilitate co-decision making in the management of non-specific low back pain. J Intelligent Helath Syst. 2019;52:269-284.
59. Karami M, Safdari R, Rahimi A. Effective radiology dashboards: key research findings. Radiology Management. 2013;35:42-45.
60. Dowding D, Randell R, Gardner P, et al. Dashboards for improving patient care: review of the literature. Int J Med Informatic. 2015;84:87-100.
61. Mlaver E, Schnipper JL, Boxer RB, et al. User-centered collaborative design and development of an inpatient safety dashboard. Jt Comm J Qual Patient Saf. 2017;43:676-685.
62. Dowding D, Merrill J, Russell D. Using feedback intervention theory to guide clinical dashboard design. AMIA Annu Symp Proc. 2018;2018:395-403.
63. Hartzler AL, Izard JP, Dalkin BL, et al. Design and feasibility of integrating personalized PRO dashboards into prostate cancer care. J Am Med Inform Assoc. 2016;23:38-47.
64. Lavallee DC, Chenok KE, Love RM, et al. Incorporating patient-reported outcomes into health care to engage patients and enhance care. Health Affairs. 2016;35:575-582.
65. U.S. Centers for Medicare & Medicaid Services. Comprehensive Care for Joint Replacement Model. https://innovation.cms.gov/initiatives/CJR. Accessed August 3, 2019.
66. National Quality Forum. Patient-reported outcomes: Accessed August 3, 2019. http://www.qualityforum.org/Patient-Reported_Outcomes.aspx. Accessed October 20, 2019.
67. Institute of Medicine Roundtable on Evidence-Based Medicine. The National Academies Collection: Reports funded by National Institutes of Health. In: Olsen LA, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US).National Academy of Sciences; 2007.
68. About Learning Health Systems: Agency for Healthcare Research and Quality. https://www.ahrq.gov/learning-health-systems/about.html. Accessed August 3, 2019.
69. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington (DC): National Academies Press; 2013.
70. Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
71. Oliver BJ, for the MS-CQI Investigators. The Multiple Sclerosis Continuous improvement collaborative: the first coproduction learning health system improvement science research collaborative for multiple sclerosis. The International Society for Quality in Health Care (ISQua) Annual International Conference; October 2019; Cape Town, South Africa.
72. Britto MT, Fuller SC, Kaplan HC, et al. Using a network organisational architecture to support the development of Learning Healthcare Systems. BMJ Qual Saf. 2018;27:937-946.
73. Care Centers: Cystic Fibrosis Foundation. https://www.cff.org/Care/Care-Centers/. Accessed August 3, 2019.
74. Schechter MS, Fink AK, Homa K, Goss CH. The Cystic Fibrosis Foundation patient registry as a tool for use in quality improvement. BMJ Qual Saf. 2014;23(Suppl 1):i9-i14.
75. Mogayzel PJ, Dunitz J, Marrow LC, Hazle LA. Improving chronic care delivery and outcomes: the impact of the cystic fibrosis Care Center Network. BMJ Qual Saf. 2014;23(Suppl 1):i3-i8.
76. Godfrey MM, Oliver BJ. Accelerating the rate of improvement in cystic fibrosis care: contributions and insights of the learning and leadership collaborative. BMJ Qual Saf. 2014;23(Suppl 1):i23-i32.
77. Nelson EC, Godfrey MM. Quality by Design: A Clinical Microsystems Approach. San Francisco, California: Jossey-Bass; 2007.
78. Crohn’s & Colitis Foundation.Quality of Care: IBD Qorus: https://www.crohnscolitisfoundation.org/research/ibd-qorus. Accessed October 23, 2019.
79. Johnson LC, Melmed GY, Nelson EC, et al. Fostering collaboration through creation of an ibd learning health system. Am J Gastroenterol. 2017;112:406-468.
80. Fernandez-Munoz JJ, Moron-Verdasco A, Cigaran-Mendez M, et al. Disability, quality of life, personality, cognitive and psychological variables associated with fatigue in patients with multiple sclerosis. Acta Neurologica Scandinavica. 2015;132:118-124.
81. Flachenecker P, Kumpfel T, Kallmann B, et al. Fatigue in multiple sclerosis: a comparison of different rating scales and correlation to clinical parameters. Multiple Sclerosis. 2002;8:523-526.
82. Tellez N, Rio J, Tintore M, et al. Does the Modified Fatigue Impact Scale offer a more comprehensive assessment of fatigue in MS? Multiple Sclerosis. 2005;11:198-202.
83. Salter A, Fox RJ, Tyry T, et al. The association of fatigue and social participation in multiple sclerosis as assessed using two different instruments. Mult Scler Relat Disord. 2019;31:165-72.
84. Solaro C, Trabucco E, Messmer Uccelli M. Pain and multiple sclerosis: pathophysiology and treatment. Curr Neurol Neurosci Rep. 2013;13:320.
85. Jawahar R, Oh U, Yang S, Lapane KL. A systematic review of pharmacological pain management in multiple sclerosis. Drugs. 2013;73:1711-1722.
86. Rintala A, Hakkinen A, Paltamaa J. Ten-year follow-up of health-related quality of life among ambulatory persons with multiple sclerosis at baseline. Qual Life Res. 2016;25:3119-3127.
87. Tepavcevic DK, Pekmezovic T, Stojsavljevic N, et al. Change in quality of life and predictors of change among patients with multiple sclerosis: a prospective cohort study. Qual Life Res. 2014;23:1027-1037.
88. DasGupta R, Fowler CJ. Bladder, bowel and sexual dysfunction in multiple sclerosis: management strategies. Drugs. 2003;63:153-166.
89. Marck CH, Jelinek PL, Weiland TJ, et al. Sexual function in multiple sclerosis and associations with demographic, disease and lifestyle characteristics: an international cross-sectional study. BMC Neurology. 2016;16:210.
90. Gajofatto A, Benedetti MD. Treatment strategies for multiple sclerosis: When to start, when to change, when to stop? World J Clinical Cases. 2015;3:545-555.
91. National Multiple Sclerosis Society. Disease-modifying therapies for MS. 2018.
92. Rudick RA, Panzara MA. Natalizumab for the treatment of relapsing multiple sclerosis. Biologics. 2008;2:189-199.
93. Cohen JA, Cutter GR, Fischer JS, et al. Benefit of interferon beta-1a on MSFC progression in secondary progressive MS. Neurology. 2002;59:679-687.
94. Patti F, Pappalardo A, Montanari E, et al. Interferon-beta-1a treatment has a positive effect on quality of life of relapsing-remitting multiple sclerosis: results from a longitudinal study. J Neurol Sci. 2014;337:180-185.
95. Novartis. Exploring the efficacy and safety of Siponimod in patients with secondary progressive multiple sclerosis (EXPAND). Available from clinicaltrials.gov/ct2/show/NCT01665144. NLM identifier: NCT01665144.
96. Singal AG, Higgins PD, Waljee AK. A primer on effectiveness and efficacy trials. Clin Transl Gastroenterol. 2014;5:e45.
97. Jongen PJ. Health-related quality of life in patients with multiple sclerosis: impact of disease-modifying drugs. CNS Drugs. 2017;31:585-602.
98. Coretti S, Ruggeri M, McNamee P. The minimum clinically important difference for EQ-5D index: a critical review. Expert Rev Pharmacoecon Outcomes Res. 2014;14:221-233.
99. Clinical Review Report: Ocrelizumab (Ocrevus). CADTH Common Drug Review. 2018:Appendix 4, Validity of Outcome Measures.
100. iCounquer MS; Empowering patients: how individuals with ms are contributing to the fight to find a cure: 2018. https://www.iconquerms.org/empowering-patients-how-individuals-ms-are-contributing-fight-find-cure. Accessed October 23, 2019.
101. K M. Accelerated Cure Project Announces Collaboration with EMD Serono to Advance Patient-Focused Drug Development in Multiple Sclerosis2018. https://www.iconquerms.org/accelerated-cure-project-announces-collaboration-emd-serono-advance-patient-focused-drug-development. Accessed October 24, 2019.
102. Bebo BF Jr, Fox RJ, Lee K, et al. Landscape of MS patient cohorts and registries: Recommendations for maximizing impact. Multiple Sclerosis. 2018;24:579-586.
103. Hedstrom AK, Hillert J, Olsson T, Alfredsson L. Alcohol as a modifiable lifestyle factor affecting multiple sclerosis risk. JAMA Neurology. 2014;71:300-305.
104. Hedstrom AK, Baarnhielm M, Olsson T, Alfredsson L. Tobacco smoking, but not Swedish snuff use, increases the risk of multiple sclerosis. Neurology. 2009;73:696-701.
105. Hedstrom AK, Akerstedt T, Hillert J, et al. Shift work at young age is associated with increased risk for multiple sclerosis. Ann Neurol. 2011;70:733-741.
106. Hadjimichael O, Vollmer T, Oleen-Burkey M. Fatigue characteristics in multiple sclerosis: the North American Research Committee on Multiple Sclerosis (NARCOMS) survey. Health Qual Life Outcomes. 2008;6:100.
107. Fox RJ, Salter AR, Tyry T, et al. Treatment discontinuation and disease progression with injectable disease-modifying therapies: findings from the North American research committee on multiple sclerosis database. Int J MS Care. 2013;15:194-201.
108. Reeve BB, Wyrwich KW, Wu AW, et al. ISOQOL recommends minimum standards for patient-reported outcome measures used in patient-centered outcomes and comparative effectiveness research. Qual Life Res. 2013;22:1889-1905.
109. Jacobs LD, Wende KE, Brownscheidle CM, et al. A profile of multiple sclerosis: the New York State Multiple Sclerosis Consortium. Multiple Sclerosis. 1999;5:369-376.
110. Weinstock-Guttman B, Jacobs LD, Brownscheidle CM, et al. Multiple sclerosis characteristics in African American patients in the New York State Multiple Sclerosis Consortium. Multiple Sclerosis. 2003;9:293-298.
111. Raffel J, Wallace A, Gveric D, et al. Patient-reported outcomes and survival in multiple sclerosis: A 10-year retrospective cohort study using the Multiple Sclerosis Impact Scale-29. PLoS Med. 2017;14:e1002346.
112. Vaughn CB, Kavak KS, Dwyer MG, et al. Fatigue at enrollment predicts EDSS worsening in the New York State Multiple Sclerosis Consortium. Multiple Sclerosis. 2018:1352458518816619.
113. Alroughani RA, Akhtar S, Ahmed SF, Al-Hashel JY. Clinical predictors of disease progression in multiple sclerosis patients with relapsing onset in a nation-wide cohort. Int J Neuroscience. 2015;125:831-837.
114. Dolan JG, Veazie PJ, Russ AJ. Development and initial evaluation of a treatment decision dashboard. BMC Med Inform Decision Mak. 2013;13:51.
115. Stuifbergen AK, Becker H, Blozis S, et al. A randomized clinical trial of a wellness intervention for women with multiple sclerosis. Arch Phys Med Rehabil. 2003;84:467-476.
116. Finkelstein J, Martin C, Bhushan A, et al. Feasibility of computer-assisted education in patients with multiple sclerosis. In: Proceedings of the IEEE Symposium on Computer-Based Medical Systems. Bethesda, MD; 2004.
117. Batalden P. Getting more health from healthcare: quality improvement must acknowledge patient coproduction—an essay by Paul Batalden. BMJ. 2018;362:k3617.
118. Batalden PB, Davidoff F. What is “quality improvement” and how can it transform healthcare? Qual Saf Health Care. 2007;16:2-3.
119. Ovretveit J, Zubkoff L, Nelson EC, et al. Using patient-reported outcome measurement to improve patient care. Int J Qual Health Care. 2017;29:874-879.
120. Prodinger B, Taylor P. Improving quality of care through patient-reported outcome measures (PROMs): expert interviews using the NHS PROMs Programme and the Swedish quality registers for knee and hip arthroplasty as examples. BMC Health Services Res. 2018;18:87.
121. Prinsen CA, Vohra S, Rose MR, et al. How to select outcome measurement instruments for outcomes included in a “Core Outcome Set” – a practical guideline. Trials. 2016;17:449.
122. NINDS. NINDS Common Data Elements: Streamline Your Neuroscience Clinical Research National Institute of Health. https://www.commondataelements.ninds.nih.gov/#page=Default. Accessed October 23, 2019.
123. Francis DO, McPheeters ML, Noud M, et al. Checklist to operationalize measurement characteristics of patient-reported outcome measures. Systematic Rev. 2016;5:129.
124. National Quality Forum. Patient-reported outcomes (PROs) in performance measurement. 2013. https://www.qualityforum.org/Publications/2012/12/Patient-Reported_Outcomes_in_Performance_Measurement.aspx. Accessed October 15, 2019.
125. Dawson J, Doll H, Fitzpatrick R, et al. The routine use of patient reported outcome measures in healthcare settings. BMJ. 2010;340:c186.
126. Aaronson N ET, Greenlhalgh J, Halyard M, et al. User’s guide to implementation of patient reported outcomes assessment in clinical practice. International Society for Quality of Life. 2015.
127. Chan EKH, Edwards TC, Haywood K, et al. Implementing patient-reported outcomes mesaures in clinical practice: a companion guide to the ISOQOL user’s guide. Qual Life Res. 2018;28:621-627.
128. Snyder CF, Wu AW, Miller RS, et al. The role of informatics in promoting patient-centered care. Cancer J. 2011;17:211-218
129. Osborn R, Moulds D, Schneider EC, et al. Primary care physicians in ten countries report challenges caring for patients with complex health needs. Health Affairs. 2015;34:2104-2112.
130. Wu AW, Kharrazi H, Boulware LE, Snyder CF. Measure once, cut 0wice--adding patient-reported outcome measures to the electronic health record for comparative effectiveness research. J Clin Epidemiol. 2013;66(8 Suppl):S12-S20.
131. Trojano M, Bergamaschi R, Amato MP, et al. The Italian multiple sclerosis register. Neurol Sci. 2019;40:155-165.
132. Moorhead SA, Hazlett DE, Harrison L, et al. A new dimension of health care: systematic review of the uses, benefits, and limitations of social media for health communication. J Med Internet Res. 2013;15:e85.
133. Luigi L, Francesco B, Marcello M, et al. e-Health and multiple sclerosis: An update. Multiple Sclerosis J. 2018;24:1657-1664.
134. Marrie RA, Leung S, Tyry T, et al. Use of eHealth and mHealth technology by persons with multiple sclerosis. Mult Scler Relat Dis. 2019;27:13-19.
135. Giunti G, Kool J, Rivera Romero O, Dorronzoro Zubiete E. Exploring the specific needs of persons with multiple sclerosis for mhealth solutions for physical activity: mixed-methods study. JMIR Mhealth Uhealth. 2018;6:e37.
136. Spooner KK, Salemi JL, Salihu HM, Zoorob RJ. eHealth patient-provider communication in the United States: interest, inequalities, and predictors. J Am Med Inform Assoc. 2017;24(e1):e18-e27.
137. Safdar N, Abbo LM, Knobloch MJ, Seo SK. Research methods in healthcare epidemiology: survey and qualitative research. Infect Control Hosp Epidemiol. 2016;37:1272-1277.
138. Rolstad S, Adler J, Rydén A. Response burden and questionnaire length: is shorter better? A review and meta-analysis. Value Health. 2011;14:1101-1108.
139. Chiu C, Bishop M, Pionke JJ, et al. Barriers to the accessibility and continuity of health-care services in people with multiple sclerosis: a literature review. Int J MS Care. 2017;19:313-321.
140. Atreja A, Mehta N, Miller D, et al. One size does not fit all: using qualitative methods to inform the development of an Internet portal for multiple sclerosis patients. AMIA Annu Symp Proc Symp. 2005:16-20.
141. Snyder CF, Blackford AL, Brahmer JR, et al. Needs assessments can identify scores on HRQOL questionnaires that represent problems for patients: an illustration with the Supportive Care Needs Survey and the QLQ-C30. Qual Life Res. 2010;19:837-845.
142. de Vet HC, Terwee CB. The minimal detectable change should not replace the minimal important difference. J Clin Epidemiol. 2010;63:804-805.
143. Snyder CF, Aaronson NK, Choucair AK, et al. Implementing patient-reported outcomes assessment in clinical practice: a review of the options and considerations. Qual Life. 2012;21:1305-1314.
144. Myla DG, Melanie DW, Robert WM, et al. Identification and validation of clinically meaningful benchmarks in the 12-item Multiple Sclerosis Walking Scale. Multiple Sclerosis J. 2017;23:1405-1414.
145. van Munster CEP, Uitdehaag BMJ. Outcome measures in clinical trials for multiple sclerosis. CNS Drugs. 2017;31:217-236.
146. Snyder CF, Jensen R, Courtin SO, Wu AW. PatientViewpoint: a website for patient-reported outcomes assessment. Quality Life Res. 2009;18:793-800.
147. Snyder CF, Smith KC, Bantug ET, et al. What do these scores mean? Presenting patient-reported outcomes data to patients and clinicians to improve interpretability. Cancer. 2017;123:1848-1859.
148. Squitieri L, Bozic KJ, Pusic AL. The role of patient-reported outcome measures in value-based payment reform. Value Health. 2017;20:834-836.
149. Boyce MB, Browne JP. The effectiveness of providing peer benchmarked feedback to hip replacement surgeons based on patient-reported outcome measures—results from the PROFILE trial: a cluster randomised controlled study. BMJ Open. 2015;5:e008325.
Seeing snakes that aren’t there
CASE Disruptive and inattentive
R, age 9, is brought by his mother to our child/adolescent psychiatry clinic, where he has been receiving treatment for attention-deficit/hyperactivity disorder (ADHD), because he is experiencing visual hallucinations and exhibiting aggressive behavior. R had initially been prescribed (and had been taking) short-acting methylphenidate, 5 mg every morning for weeks. During this time, he responded well to the medication; he had reduced hyperactivity, talked less in class, and was able to give increased attention to his academic work. After 2 weeks, because R did not want to take short-acting methylphenidate in school, we switched him to osmotic-controlled release oral delivery system (OROS) methylphenidate, 18 mg every morning.
Two days after starting the OROS methylphenidate formulation, R develops visual hallucinations and aggressive behavior. His visual hallucinations—which occur both at home and at school—involve seeing snakes circling him. When hallucinating, he hits and pushes family members and throws objects at them. He refuses to go to school because he fears the snakes. The hallucinations continue throughout the day and persist for the next 3 to 4 days.
R does not have any comorbid medical or psychiatric illnesses; however, his father has a history of schizophrenia, polysubstance abuse, and multiple prior psychiatric hospitalizations due to medication noncompliance.
R undergoes laboratory workup, which includes a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone level, and urine drug screening. All results are within normal limits.
[polldaddy:10468215]
The authors’ observations
We ruled out delirium by ordering a basic laboratory workup. We considered the possibility of a new mood or psychotic disorder, but began to suspect the OROS methylphenidate might be causing R’s symptoms.
Attention-deficit/hyperactivity disorder is an increasingly prevalent diagnosis in the United States, affecting up to 6.4 million children age 4 to 17. While symptoms of ADHD often first appear in preschool-age children, the average age at which a child receives a diagnosis of ADHD is 7.
Stimulants are a clinically effective treatment for ADHD. In general, their use is safe and well tolerated, especially in pediatric patients. Some common adverse effects of stimulant medications include reduced appetite, headache, and insomnia.1 Psychotic symptoms such as paranoid delusions, visual hallucinations, auditory hallucinations, and tactile hallucinations are rare. In some cases, these psychotic symptoms can be accompanied by increased aggression.2-4
Continue to: Methylphenidate is one of the most...
Methylphenidate is one of the most commonly prescribed stimulants for treating ADHD. Methylphenidate has 2 known mechanisms of action: 1) inhibition of catecholamine reuptake at the presynaptic dopamine reuptake inhibitor, and 2) binding to and blocking intracellular dopamine transporters, inhibiting both dopamine and norepinephrine reuptake.5,6 Because increased levels of synaptic dopamine are implicated in the generation of psychotic symptoms, the pharmacologic mechanism of methylphenidate also implies a potential to induce psychotic symptoms.7
How common is this problem?
On the population level, there is no detectable difference in the event rate (incidence) of psychosis in children treated with stimulants or children not taking stimulants.8 However, there are reports that individual patients can experience psychosis due to treatment with stimulants as an unusual adverse medication reaction. In 1971, Lucas and Weiss9 were among the first to describe 3 cases of methylphenidate-induced psychosis. Since then, many articles in the scientific literature have reported cases of psychosis related to stimulant medications.
A brief review of the literature between 2002 and 2010 revealed 14 cases of stimulant-related psychosis, in patients ranging from age 7 to 45. Six of the patients were children, age 7 to 12; 1 patient was an adolescent, age 15; 4 were young adults, age 18 to 25; and 3 were older adults. Of all 14 individuals, 7 reported visual hallucinations, 4 had tactile hallucinations, 4 had auditory hallucinations, and 3 displayed paranoid delusions.10 With the aim of exploring possible etiologic factors associated with psychotic symptoms, such as type of drug and dosage, it was found that 9 patients received methylphenidate, with total daily doses ranging from 7.5 to 74 mg (3 patients received short-acting methylphenidate; 1 patient received methylphenidate extended release (ER); 1 patient received both; 4 patients received dextroamphetamine, with doses of 30 to 50 mg/d; and 1 patient received amphetamine, 10 mg/d). In terms of family history, 1 patient had a positive family history of schizophrenia; 1 patient had a family history of bipolar disorder; and 6 patients were negative for family history of any psychotic disorder.10
In 2006, due to growing concerns about adverse psychiatric effects of ADHD medications, the FDA Center for Drug Evaluation and Research Office of Surveillance and Epidemiology requested the electronic clinical trial databases of manufacturers of drugs approved for the treatment of ADHD, or those with active clinical development programs for the same indication.11 In that study, Mosholder et al11 analyzed data from 49 randomized, controlled clinical trials that were in pediatric development programs and found that there were psychotic or manic adverse events in 11 individuals in the pooled active drug group. These were observed with methylphenidate, dexmethylphenidate, and atomoxetine. There were no events in the placebo group, which reinforced the causality between the ADHD medication and these symptoms, as participants with untreated ADHD did not develop them.11
It is important to note that ADHD medications taken in excessive doses are much more likely to provoke psychotic adverse effects than when taken at therapeutic doses. However, as seen in our clinical case, patients such as R could develop acute psychosis even with a lower dosage of stimulant medications. An article by Ross2 suggested rates of .25% for this psychiatric adverse effect (1 in 400 children treated with therapeutic doses of stimulants will develop psychosis), which is consistent with the data from the Mosholder et al11 study.
Continue to: TREATMENT Discontinuation and re-challenge
TREATMENT Discontinuation and re-challenge
After 3 days, we discontinue OROS methylphenidate. Five days after discontinuation, R’s visual hallucinations and aggressive behaviors completely resolve. After not receiving stimulants for 2 weeks, R is restarted on short-acting methylphenidate, 5 mg/d, because he had a relatively good clinical response to short-acting methylphenidate previously. After 14 days, the short-acting methylphenidate dosage is increased to 5 mg twice daily without the re-emergence of psychosis or aggressive behaviors.
The authors’ observations
Although stimulant-induced psychosis can be a disturbing adverse effect, severe ADHD greatly affects a person’s functioning at school and at home and can lead to several comorbidities, including depression, anxiety, and substance abuse. For these reasons, most patients with ADHD who experience psychotic symptoms are re-challenged with stimulants.10 Out of the 14 cases discussed above, 4 patients were restarted on the same stimulant or a different ADHD medication; 2 of them had the same psychotic symptoms days after the reintroduction of the drug and the other 2 had no recurrence.10,12,13
Stimulant-induced hallucinations
The emergence of hallucinations with methylphenidate or amphetamines has been attributed to a chronic increase of dopamine levels in the synaptic cleft, while the pathophysiological mechanisms are not clearly known. In some cases, hallucinations emerged after taking the first low dose, which has been thought to be an effect of idiosyncratic mechanism. Stimulants cause an increase of the releasing of catecholamines. Porfirio et al14 argue that high-dose stimulants can deteriorate the response to visual stimuli, causing a different perception of visual stimuli in susceptible children, based on the information that norepinephrine is released in the lateral geniculate nucleus, and it increases the transmission of visual information.
An idiosyncratic drug reaction
Despite the existence of many theories on the pathophysiology of stimulant-induced psychosis (Box15-18), its actual mechanism remains unknown. In R’s case, given the speed with which his symptoms developed, the proposed mechanisms of action may not explain his psychotic symptoms. We must consider an idiosyncratic drug reaction as an explanation. This suggestion is supported by the fact that re-challenging with a stimulant did not re-induce psychosis in 2 out of the 4 cases described in the literature,10,12,13 as well as in R’s case.
Box
Although the subjective effects of methylphenidate and amphetamines are similar, neurochemical effects of the 2 stimulants are distinct, with different mechanisms of action. Methylphenidate targets the dopamine transporter (DAT) and the noradrenaline transporter (NET), inhibiting DA and NA reuptake, and therefore increasing DA and NA levels in the synaptic cleft. Amphetamine targets DAT and NET, inhibiting DA and NA reuptake, and therefore increasing DA and NA levels in the synaptic cleft. It also enters the presynaptic neuron, preventing DA/NA from storing in the vesicles. In addition, it promotes the release of catecholamines from vesicles into the cytosol and ultimately from the cytosol into the synaptic cleft.18
Generally, amphetamines are twice as potent as methylphenidate. As such, lower doses of amphetamine preparations can cause psychotic symptoms when compared with methamphetamine products.17 Griffith15 showed that paranoia manifested itself in all participants who were previously healthy as they underwent repeated administration of 5 to 15 mg of oral dextroamphetamine many times per day for up to 5 days in a row, leading to cumulative doses ranging from 200 to 800 mg.15 At such doses, the effects are similar to those obtained with illicit use of methamphetamine, a drug of abuse for which psychosis-inducing effects are well documented.
Psychosis in reaction to therapeutic doses of methylphenidate may have a mechanism of action that is shared by psychosis in response to chronic use of methamphetamine. Several hypotheses have been suggested to explain the mechanism behind stimulantinduced psychosis in cases of chronic methamphetamine use:
- Young,16 who had one of the first proposed theories in 1981, hypothesized attributing symptoms to dose-related effects at pre- and post-synaptic noradrenergic and dopaminergic receptors.
- Hsieh et al18 hypothesized that methamphetamine use causes an increased flow of dopamine in the striatum, which leads to excessive glutamate release into the cortex. Excess glutamate in the cortex might, over time, cause damage to cortical interneurons. This damage may dysregulate thalamocortical signals, resulting in psychotic symptoms.18
Although the mechanisms by which psychotic symptoms associated with stimulants occur remain unknown, possibilities include10,19:
- genetic predisposition
- changes induced by stimulants at the level of neurotransmitters, synapses, and brain circuits
- an idiosyncratic drug reaction.
Continue to: What to consider before prescribing stimulants
What to consider before prescribing stimulants
While stimulants are clearly beneficial for the vast majority of children with ADHD, there may be a small subgroup of patients for whom stimulants carry increased risk. For example, it is possible that patients with a family history of mood and psychotic disorders may be more vulnerable to stimulant-induced psychotic symptoms that are reversible on discontinuation.20 In our case, R had a first-degree relative (his father) with treatment-refractory schizophrenia.
Attentional dysfunction is a common premorbid presentation for children who later develop schizophrenia or bipolar disorder. Retrospective data from patients with schizophrenia or bipolar disorder document high rates of childhood stimulant use—generally higher even than other groups with attentional dysfunction21 and histories of stimulant-associated adverse behavioral effects.22 In these patients, a history of stimulant use is also associated with an earlier age at onset23 and a more severe course of illness during hospitalization.24 Stimulant exposure in vulnerable individuals may hasten the onset or worsen the course of bipolar or psychotic illnesses.21,25,26
OUTCOME Well-controlled symptoms
R continues to receive short-acting methylphenidate, 5 mg twice a day. His ADHD symptoms remain well-controlled, and he is able to do well academically.
The authors’ observations
Although stimulant-induced psychosis is a rare and unpredictable occurrence, carefully monitoring all patients for any adverse effects of ADHD medication is recommended. When present, psychotic symptoms may quickly remit upon discontinuation of the medication. The question of subsequently reintroducing stimulant medication for a patient with severe ADHD is complicated. One needs to measure the possible risk of a reoccurrence of the psychotic symptoms against the consequences of untreated ADHD. These consequences include increased risk for academic and occupational failure, depression, anxiety, and substance abuse. Psychosocial interventions for ADHD should be implemented, but for optimal results, they often need to be combined with medication. However, if a stimulant medication is to be reintroduced, this should be done with extreme care. Starting dosages need to be low, and increases should be gradual, with frequent monitoring.
Bottom Line
Although stimulant-induced psychosis is a rare occurrence, determine if your pediatric patient with attention-deficit/hyperactivity disorder (ADHD) has a family history of mood or psychotic disorders before initiating stimulants. Carefully monitor all patients for any adverse effects of stimulant medications prescribed for ADHD. If psychotic symptoms occur at therapeutic doses, reduce the dose or discontinue the medication. Once the psychotic or manic symptoms resolve, it may be appropriate to re-challenge with a stimulant.
Related Resource
- Man KK, Coghill D, Chan EW, et al. Methylphenidate and the risk of psychotic disorders and hallucinations in children and adolescents in a large health system. Transl Psychiatry. 2016;6(11):e956. doi: 10.1038/tp.2016.216.
Drug Brand Names
Atomoxetine • Strattera
Dexmethylphenidate • Focalin
Dextroamphetamine/amphetamine • Adderall
Methylphenidate • Metadate, Ritalin
Methylphenidate ER • Concerta
1. Cherland E, Fitzpatrick R. Psychotic side effects of psychostimulants: a 5-year review. Can J Psychiatry. 1999; 44(8):811-813.
2. Ross RG. Psychotic and manic-like symptoms during stimulant treatment of attention deficit hyperactivity disorder. Am. J. Psychiatry. 2006;163(7):1149-1152.
3. Rashid J, Mitelman S. Methylphenidate and somatic hallucinations. J Am Acad Child Adolesc Psychiatry. 2007;46(8):945-946.
4. Rubio JM, Sanjuán J, Flórez-Salamanca L, et al. Examining the course of hallucinatory experiences in children and adolescents: a systematic review. Schizophr Res. 2012;138(2-3):248-254.
5. Iversen L. Neurotransmitter transporters and their impact on the development of psychopharmacology. Br J Pharmacol. 2006;147(Suppl 1):S82-S88.
6. Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69(8):776-786.
7. Bloom AS, Russell LJ, Weisskopf B, et al. Methylphenidate-induced delusional disorder in a child with attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry. 1988;27(1):88-89.
8. Shibib S, Chaloub N. Stimulant induced psychosis. Child Adolesc Ment Health. 2009;14(1):1420-1423.
9. Lucas AR, Weiss M. Methylphenidate hallucinosis. JAMA. 1971;217(8):1079-1081.
10. Kraemer M, Uekermann J, Wiltfang J, et al. Methylphenidate-induced psychosis in adult attention-deficit/hyperactivity disorder: report of 3 new cases and review of the literature. Clin Neuropharmacol. 2010;33(4):204-206.
11. Mosholder AD, Gelperin K, Hammad TA, et al. Hallucinations and other psychotic symptoms associated with the use of attention-deficit/hyperactivity disorder drugs in children. Pediatrics. 2009; 123:611-616.
12. Gross-Tsur V, Joseph A, Shalev RS. Hallucinations during methylphenidate therapy. Neurology. 2004;63(4):753-754.
13. Halevy A, Shuper A. Methylphenidate induction of complex visual hallucinations. J Child Neurol. 2009;24(8):1005-1007.
14. Porfirio MC, Giana G, Giovinazzo S, et al. Methylphenidate-induced visual hallucinations. Neuropediatrics. 2011;42(1):30-31.
15. Griffith J. A study of illicit amphetamine drug traffic in Oklahoma City. Am J Psychiatry. 1966;123(5):560-569.
16. Young JG. Methylphenidate-induced hallucinosis: case histories and possible mechanisms of action. J Dev Behav Pediatr. 1981;2(2):35-38.
17. Stein MA, Sarampote CS, Waldman ID, et al. A dose-response study of OROS methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics. 2003; 112(5):e404. PMID: 14595084.
18. Hsieh JH, Stein DJ, Howells FM. The neurobiology of methamphetamine induced psychosis. Front Hum Neurosci. 2014;8:537. doi:10.3389/fnhum.2014.00537.
19. Shyu YC, Yuan SS, Lee SY, et al. Attention-deficit/hyperactivity disorder, methylphenidate use and the risk of developing schizophrenia spectrum disorders: a nationwide population-based study in Taiwan. Schizophrenia Res. 2015;168(1-2):161-167.
20. MacKenzie LE, Abidi S, Fisher HL, et al. Stimulant medication and psychotic symptoms in offspring of parents with mental illness. Pediatrics. 2016;137(1). doi: 10.1542/peds.2015-2486.
21. Schaeffer J, Ross RG. Childhood-onset schizophrenia: premorbid and prodromal diagnosis and treatment histories. J Am Acad Child Adolesc Psychiatry. 2002;41(5):538-545.
22. Faedda GL, Baldessarini RJ, Blovinsky IP, et al. Treatment-emergent mania in pediatric bipolar disorder: a retrospective case review. J Affect Disord. 2004;82(1):149-158.
23. DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord. 2001;3(2):53-57.
24. Soutullo CA, DelBello MP, Ochsner BS, et al. Severity of bipolarity in hospitalized manic adolescents with history of stimulant or antidepressant treatment. J Affect Disord. 2002;70(3):323-327.
25. Reichart CG, Nolen WA. Earlier onset of bipolar disorder in children by antidepressants or stimulants? An hypothesis. J Affect Disord. 2004;78(1):81-84.
26. Ikeda M, Okahisa Y, Aleksic B, et al. Evidence for shared genetic risk between methamphetamine-induced psychosis and schizophrenia. Neuropsychopharmacology. 2013;38(10):1864-1870.
CASE Disruptive and inattentive
R, age 9, is brought by his mother to our child/adolescent psychiatry clinic, where he has been receiving treatment for attention-deficit/hyperactivity disorder (ADHD), because he is experiencing visual hallucinations and exhibiting aggressive behavior. R had initially been prescribed (and had been taking) short-acting methylphenidate, 5 mg every morning for weeks. During this time, he responded well to the medication; he had reduced hyperactivity, talked less in class, and was able to give increased attention to his academic work. After 2 weeks, because R did not want to take short-acting methylphenidate in school, we switched him to osmotic-controlled release oral delivery system (OROS) methylphenidate, 18 mg every morning.
Two days after starting the OROS methylphenidate formulation, R develops visual hallucinations and aggressive behavior. His visual hallucinations—which occur both at home and at school—involve seeing snakes circling him. When hallucinating, he hits and pushes family members and throws objects at them. He refuses to go to school because he fears the snakes. The hallucinations continue throughout the day and persist for the next 3 to 4 days.
R does not have any comorbid medical or psychiatric illnesses; however, his father has a history of schizophrenia, polysubstance abuse, and multiple prior psychiatric hospitalizations due to medication noncompliance.
R undergoes laboratory workup, which includes a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone level, and urine drug screening. All results are within normal limits.
[polldaddy:10468215]
The authors’ observations
We ruled out delirium by ordering a basic laboratory workup. We considered the possibility of a new mood or psychotic disorder, but began to suspect the OROS methylphenidate might be causing R’s symptoms.
Attention-deficit/hyperactivity disorder is an increasingly prevalent diagnosis in the United States, affecting up to 6.4 million children age 4 to 17. While symptoms of ADHD often first appear in preschool-age children, the average age at which a child receives a diagnosis of ADHD is 7.
Stimulants are a clinically effective treatment for ADHD. In general, their use is safe and well tolerated, especially in pediatric patients. Some common adverse effects of stimulant medications include reduced appetite, headache, and insomnia.1 Psychotic symptoms such as paranoid delusions, visual hallucinations, auditory hallucinations, and tactile hallucinations are rare. In some cases, these psychotic symptoms can be accompanied by increased aggression.2-4
Continue to: Methylphenidate is one of the most...
Methylphenidate is one of the most commonly prescribed stimulants for treating ADHD. Methylphenidate has 2 known mechanisms of action: 1) inhibition of catecholamine reuptake at the presynaptic dopamine reuptake inhibitor, and 2) binding to and blocking intracellular dopamine transporters, inhibiting both dopamine and norepinephrine reuptake.5,6 Because increased levels of synaptic dopamine are implicated in the generation of psychotic symptoms, the pharmacologic mechanism of methylphenidate also implies a potential to induce psychotic symptoms.7
How common is this problem?
On the population level, there is no detectable difference in the event rate (incidence) of psychosis in children treated with stimulants or children not taking stimulants.8 However, there are reports that individual patients can experience psychosis due to treatment with stimulants as an unusual adverse medication reaction. In 1971, Lucas and Weiss9 were among the first to describe 3 cases of methylphenidate-induced psychosis. Since then, many articles in the scientific literature have reported cases of psychosis related to stimulant medications.
A brief review of the literature between 2002 and 2010 revealed 14 cases of stimulant-related psychosis, in patients ranging from age 7 to 45. Six of the patients were children, age 7 to 12; 1 patient was an adolescent, age 15; 4 were young adults, age 18 to 25; and 3 were older adults. Of all 14 individuals, 7 reported visual hallucinations, 4 had tactile hallucinations, 4 had auditory hallucinations, and 3 displayed paranoid delusions.10 With the aim of exploring possible etiologic factors associated with psychotic symptoms, such as type of drug and dosage, it was found that 9 patients received methylphenidate, with total daily doses ranging from 7.5 to 74 mg (3 patients received short-acting methylphenidate; 1 patient received methylphenidate extended release (ER); 1 patient received both; 4 patients received dextroamphetamine, with doses of 30 to 50 mg/d; and 1 patient received amphetamine, 10 mg/d). In terms of family history, 1 patient had a positive family history of schizophrenia; 1 patient had a family history of bipolar disorder; and 6 patients were negative for family history of any psychotic disorder.10
In 2006, due to growing concerns about adverse psychiatric effects of ADHD medications, the FDA Center for Drug Evaluation and Research Office of Surveillance and Epidemiology requested the electronic clinical trial databases of manufacturers of drugs approved for the treatment of ADHD, or those with active clinical development programs for the same indication.11 In that study, Mosholder et al11 analyzed data from 49 randomized, controlled clinical trials that were in pediatric development programs and found that there were psychotic or manic adverse events in 11 individuals in the pooled active drug group. These were observed with methylphenidate, dexmethylphenidate, and atomoxetine. There were no events in the placebo group, which reinforced the causality between the ADHD medication and these symptoms, as participants with untreated ADHD did not develop them.11
It is important to note that ADHD medications taken in excessive doses are much more likely to provoke psychotic adverse effects than when taken at therapeutic doses. However, as seen in our clinical case, patients such as R could develop acute psychosis even with a lower dosage of stimulant medications. An article by Ross2 suggested rates of .25% for this psychiatric adverse effect (1 in 400 children treated with therapeutic doses of stimulants will develop psychosis), which is consistent with the data from the Mosholder et al11 study.
Continue to: TREATMENT Discontinuation and re-challenge
TREATMENT Discontinuation and re-challenge
After 3 days, we discontinue OROS methylphenidate. Five days after discontinuation, R’s visual hallucinations and aggressive behaviors completely resolve. After not receiving stimulants for 2 weeks, R is restarted on short-acting methylphenidate, 5 mg/d, because he had a relatively good clinical response to short-acting methylphenidate previously. After 14 days, the short-acting methylphenidate dosage is increased to 5 mg twice daily without the re-emergence of psychosis or aggressive behaviors.
The authors’ observations
Although stimulant-induced psychosis can be a disturbing adverse effect, severe ADHD greatly affects a person’s functioning at school and at home and can lead to several comorbidities, including depression, anxiety, and substance abuse. For these reasons, most patients with ADHD who experience psychotic symptoms are re-challenged with stimulants.10 Out of the 14 cases discussed above, 4 patients were restarted on the same stimulant or a different ADHD medication; 2 of them had the same psychotic symptoms days after the reintroduction of the drug and the other 2 had no recurrence.10,12,13
Stimulant-induced hallucinations
The emergence of hallucinations with methylphenidate or amphetamines has been attributed to a chronic increase of dopamine levels in the synaptic cleft, while the pathophysiological mechanisms are not clearly known. In some cases, hallucinations emerged after taking the first low dose, which has been thought to be an effect of idiosyncratic mechanism. Stimulants cause an increase of the releasing of catecholamines. Porfirio et al14 argue that high-dose stimulants can deteriorate the response to visual stimuli, causing a different perception of visual stimuli in susceptible children, based on the information that norepinephrine is released in the lateral geniculate nucleus, and it increases the transmission of visual information.
An idiosyncratic drug reaction
Despite the existence of many theories on the pathophysiology of stimulant-induced psychosis (Box15-18), its actual mechanism remains unknown. In R’s case, given the speed with which his symptoms developed, the proposed mechanisms of action may not explain his psychotic symptoms. We must consider an idiosyncratic drug reaction as an explanation. This suggestion is supported by the fact that re-challenging with a stimulant did not re-induce psychosis in 2 out of the 4 cases described in the literature,10,12,13 as well as in R’s case.
Box
Although the subjective effects of methylphenidate and amphetamines are similar, neurochemical effects of the 2 stimulants are distinct, with different mechanisms of action. Methylphenidate targets the dopamine transporter (DAT) and the noradrenaline transporter (NET), inhibiting DA and NA reuptake, and therefore increasing DA and NA levels in the synaptic cleft. Amphetamine targets DAT and NET, inhibiting DA and NA reuptake, and therefore increasing DA and NA levels in the synaptic cleft. It also enters the presynaptic neuron, preventing DA/NA from storing in the vesicles. In addition, it promotes the release of catecholamines from vesicles into the cytosol and ultimately from the cytosol into the synaptic cleft.18
Generally, amphetamines are twice as potent as methylphenidate. As such, lower doses of amphetamine preparations can cause psychotic symptoms when compared with methamphetamine products.17 Griffith15 showed that paranoia manifested itself in all participants who were previously healthy as they underwent repeated administration of 5 to 15 mg of oral dextroamphetamine many times per day for up to 5 days in a row, leading to cumulative doses ranging from 200 to 800 mg.15 At such doses, the effects are similar to those obtained with illicit use of methamphetamine, a drug of abuse for which psychosis-inducing effects are well documented.
Psychosis in reaction to therapeutic doses of methylphenidate may have a mechanism of action that is shared by psychosis in response to chronic use of methamphetamine. Several hypotheses have been suggested to explain the mechanism behind stimulantinduced psychosis in cases of chronic methamphetamine use:
- Young,16 who had one of the first proposed theories in 1981, hypothesized attributing symptoms to dose-related effects at pre- and post-synaptic noradrenergic and dopaminergic receptors.
- Hsieh et al18 hypothesized that methamphetamine use causes an increased flow of dopamine in the striatum, which leads to excessive glutamate release into the cortex. Excess glutamate in the cortex might, over time, cause damage to cortical interneurons. This damage may dysregulate thalamocortical signals, resulting in psychotic symptoms.18
Although the mechanisms by which psychotic symptoms associated with stimulants occur remain unknown, possibilities include10,19:
- genetic predisposition
- changes induced by stimulants at the level of neurotransmitters, synapses, and brain circuits
- an idiosyncratic drug reaction.
Continue to: What to consider before prescribing stimulants
What to consider before prescribing stimulants
While stimulants are clearly beneficial for the vast majority of children with ADHD, there may be a small subgroup of patients for whom stimulants carry increased risk. For example, it is possible that patients with a family history of mood and psychotic disorders may be more vulnerable to stimulant-induced psychotic symptoms that are reversible on discontinuation.20 In our case, R had a first-degree relative (his father) with treatment-refractory schizophrenia.
Attentional dysfunction is a common premorbid presentation for children who later develop schizophrenia or bipolar disorder. Retrospective data from patients with schizophrenia or bipolar disorder document high rates of childhood stimulant use—generally higher even than other groups with attentional dysfunction21 and histories of stimulant-associated adverse behavioral effects.22 In these patients, a history of stimulant use is also associated with an earlier age at onset23 and a more severe course of illness during hospitalization.24 Stimulant exposure in vulnerable individuals may hasten the onset or worsen the course of bipolar or psychotic illnesses.21,25,26
OUTCOME Well-controlled symptoms
R continues to receive short-acting methylphenidate, 5 mg twice a day. His ADHD symptoms remain well-controlled, and he is able to do well academically.
The authors’ observations
Although stimulant-induced psychosis is a rare and unpredictable occurrence, carefully monitoring all patients for any adverse effects of ADHD medication is recommended. When present, psychotic symptoms may quickly remit upon discontinuation of the medication. The question of subsequently reintroducing stimulant medication for a patient with severe ADHD is complicated. One needs to measure the possible risk of a reoccurrence of the psychotic symptoms against the consequences of untreated ADHD. These consequences include increased risk for academic and occupational failure, depression, anxiety, and substance abuse. Psychosocial interventions for ADHD should be implemented, but for optimal results, they often need to be combined with medication. However, if a stimulant medication is to be reintroduced, this should be done with extreme care. Starting dosages need to be low, and increases should be gradual, with frequent monitoring.
Bottom Line
Although stimulant-induced psychosis is a rare occurrence, determine if your pediatric patient with attention-deficit/hyperactivity disorder (ADHD) has a family history of mood or psychotic disorders before initiating stimulants. Carefully monitor all patients for any adverse effects of stimulant medications prescribed for ADHD. If psychotic symptoms occur at therapeutic doses, reduce the dose or discontinue the medication. Once the psychotic or manic symptoms resolve, it may be appropriate to re-challenge with a stimulant.
Related Resource
- Man KK, Coghill D, Chan EW, et al. Methylphenidate and the risk of psychotic disorders and hallucinations in children and adolescents in a large health system. Transl Psychiatry. 2016;6(11):e956. doi: 10.1038/tp.2016.216.
Drug Brand Names
Atomoxetine • Strattera
Dexmethylphenidate • Focalin
Dextroamphetamine/amphetamine • Adderall
Methylphenidate • Metadate, Ritalin
Methylphenidate ER • Concerta
CASE Disruptive and inattentive
R, age 9, is brought by his mother to our child/adolescent psychiatry clinic, where he has been receiving treatment for attention-deficit/hyperactivity disorder (ADHD), because he is experiencing visual hallucinations and exhibiting aggressive behavior. R had initially been prescribed (and had been taking) short-acting methylphenidate, 5 mg every morning for weeks. During this time, he responded well to the medication; he had reduced hyperactivity, talked less in class, and was able to give increased attention to his academic work. After 2 weeks, because R did not want to take short-acting methylphenidate in school, we switched him to osmotic-controlled release oral delivery system (OROS) methylphenidate, 18 mg every morning.
Two days after starting the OROS methylphenidate formulation, R develops visual hallucinations and aggressive behavior. His visual hallucinations—which occur both at home and at school—involve seeing snakes circling him. When hallucinating, he hits and pushes family members and throws objects at them. He refuses to go to school because he fears the snakes. The hallucinations continue throughout the day and persist for the next 3 to 4 days.
R does not have any comorbid medical or psychiatric illnesses; however, his father has a history of schizophrenia, polysubstance abuse, and multiple prior psychiatric hospitalizations due to medication noncompliance.
R undergoes laboratory workup, which includes a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone level, and urine drug screening. All results are within normal limits.
[polldaddy:10468215]
The authors’ observations
We ruled out delirium by ordering a basic laboratory workup. We considered the possibility of a new mood or psychotic disorder, but began to suspect the OROS methylphenidate might be causing R’s symptoms.
Attention-deficit/hyperactivity disorder is an increasingly prevalent diagnosis in the United States, affecting up to 6.4 million children age 4 to 17. While symptoms of ADHD often first appear in preschool-age children, the average age at which a child receives a diagnosis of ADHD is 7.
Stimulants are a clinically effective treatment for ADHD. In general, their use is safe and well tolerated, especially in pediatric patients. Some common adverse effects of stimulant medications include reduced appetite, headache, and insomnia.1 Psychotic symptoms such as paranoid delusions, visual hallucinations, auditory hallucinations, and tactile hallucinations are rare. In some cases, these psychotic symptoms can be accompanied by increased aggression.2-4
Continue to: Methylphenidate is one of the most...
Methylphenidate is one of the most commonly prescribed stimulants for treating ADHD. Methylphenidate has 2 known mechanisms of action: 1) inhibition of catecholamine reuptake at the presynaptic dopamine reuptake inhibitor, and 2) binding to and blocking intracellular dopamine transporters, inhibiting both dopamine and norepinephrine reuptake.5,6 Because increased levels of synaptic dopamine are implicated in the generation of psychotic symptoms, the pharmacologic mechanism of methylphenidate also implies a potential to induce psychotic symptoms.7
How common is this problem?
On the population level, there is no detectable difference in the event rate (incidence) of psychosis in children treated with stimulants or children not taking stimulants.8 However, there are reports that individual patients can experience psychosis due to treatment with stimulants as an unusual adverse medication reaction. In 1971, Lucas and Weiss9 were among the first to describe 3 cases of methylphenidate-induced psychosis. Since then, many articles in the scientific literature have reported cases of psychosis related to stimulant medications.
A brief review of the literature between 2002 and 2010 revealed 14 cases of stimulant-related psychosis, in patients ranging from age 7 to 45. Six of the patients were children, age 7 to 12; 1 patient was an adolescent, age 15; 4 were young adults, age 18 to 25; and 3 were older adults. Of all 14 individuals, 7 reported visual hallucinations, 4 had tactile hallucinations, 4 had auditory hallucinations, and 3 displayed paranoid delusions.10 With the aim of exploring possible etiologic factors associated with psychotic symptoms, such as type of drug and dosage, it was found that 9 patients received methylphenidate, with total daily doses ranging from 7.5 to 74 mg (3 patients received short-acting methylphenidate; 1 patient received methylphenidate extended release (ER); 1 patient received both; 4 patients received dextroamphetamine, with doses of 30 to 50 mg/d; and 1 patient received amphetamine, 10 mg/d). In terms of family history, 1 patient had a positive family history of schizophrenia; 1 patient had a family history of bipolar disorder; and 6 patients were negative for family history of any psychotic disorder.10
In 2006, due to growing concerns about adverse psychiatric effects of ADHD medications, the FDA Center for Drug Evaluation and Research Office of Surveillance and Epidemiology requested the electronic clinical trial databases of manufacturers of drugs approved for the treatment of ADHD, or those with active clinical development programs for the same indication.11 In that study, Mosholder et al11 analyzed data from 49 randomized, controlled clinical trials that were in pediatric development programs and found that there were psychotic or manic adverse events in 11 individuals in the pooled active drug group. These were observed with methylphenidate, dexmethylphenidate, and atomoxetine. There were no events in the placebo group, which reinforced the causality between the ADHD medication and these symptoms, as participants with untreated ADHD did not develop them.11
It is important to note that ADHD medications taken in excessive doses are much more likely to provoke psychotic adverse effects than when taken at therapeutic doses. However, as seen in our clinical case, patients such as R could develop acute psychosis even with a lower dosage of stimulant medications. An article by Ross2 suggested rates of .25% for this psychiatric adverse effect (1 in 400 children treated with therapeutic doses of stimulants will develop psychosis), which is consistent with the data from the Mosholder et al11 study.
Continue to: TREATMENT Discontinuation and re-challenge
TREATMENT Discontinuation and re-challenge
After 3 days, we discontinue OROS methylphenidate. Five days after discontinuation, R’s visual hallucinations and aggressive behaviors completely resolve. After not receiving stimulants for 2 weeks, R is restarted on short-acting methylphenidate, 5 mg/d, because he had a relatively good clinical response to short-acting methylphenidate previously. After 14 days, the short-acting methylphenidate dosage is increased to 5 mg twice daily without the re-emergence of psychosis or aggressive behaviors.
The authors’ observations
Although stimulant-induced psychosis can be a disturbing adverse effect, severe ADHD greatly affects a person’s functioning at school and at home and can lead to several comorbidities, including depression, anxiety, and substance abuse. For these reasons, most patients with ADHD who experience psychotic symptoms are re-challenged with stimulants.10 Out of the 14 cases discussed above, 4 patients were restarted on the same stimulant or a different ADHD medication; 2 of them had the same psychotic symptoms days after the reintroduction of the drug and the other 2 had no recurrence.10,12,13
Stimulant-induced hallucinations
The emergence of hallucinations with methylphenidate or amphetamines has been attributed to a chronic increase of dopamine levels in the synaptic cleft, while the pathophysiological mechanisms are not clearly known. In some cases, hallucinations emerged after taking the first low dose, which has been thought to be an effect of idiosyncratic mechanism. Stimulants cause an increase of the releasing of catecholamines. Porfirio et al14 argue that high-dose stimulants can deteriorate the response to visual stimuli, causing a different perception of visual stimuli in susceptible children, based on the information that norepinephrine is released in the lateral geniculate nucleus, and it increases the transmission of visual information.
An idiosyncratic drug reaction
Despite the existence of many theories on the pathophysiology of stimulant-induced psychosis (Box15-18), its actual mechanism remains unknown. In R’s case, given the speed with which his symptoms developed, the proposed mechanisms of action may not explain his psychotic symptoms. We must consider an idiosyncratic drug reaction as an explanation. This suggestion is supported by the fact that re-challenging with a stimulant did not re-induce psychosis in 2 out of the 4 cases described in the literature,10,12,13 as well as in R’s case.
Box
Although the subjective effects of methylphenidate and amphetamines are similar, neurochemical effects of the 2 stimulants are distinct, with different mechanisms of action. Methylphenidate targets the dopamine transporter (DAT) and the noradrenaline transporter (NET), inhibiting DA and NA reuptake, and therefore increasing DA and NA levels in the synaptic cleft. Amphetamine targets DAT and NET, inhibiting DA and NA reuptake, and therefore increasing DA and NA levels in the synaptic cleft. It also enters the presynaptic neuron, preventing DA/NA from storing in the vesicles. In addition, it promotes the release of catecholamines from vesicles into the cytosol and ultimately from the cytosol into the synaptic cleft.18
Generally, amphetamines are twice as potent as methylphenidate. As such, lower doses of amphetamine preparations can cause psychotic symptoms when compared with methamphetamine products.17 Griffith15 showed that paranoia manifested itself in all participants who were previously healthy as they underwent repeated administration of 5 to 15 mg of oral dextroamphetamine many times per day for up to 5 days in a row, leading to cumulative doses ranging from 200 to 800 mg.15 At such doses, the effects are similar to those obtained with illicit use of methamphetamine, a drug of abuse for which psychosis-inducing effects are well documented.
Psychosis in reaction to therapeutic doses of methylphenidate may have a mechanism of action that is shared by psychosis in response to chronic use of methamphetamine. Several hypotheses have been suggested to explain the mechanism behind stimulantinduced psychosis in cases of chronic methamphetamine use:
- Young,16 who had one of the first proposed theories in 1981, hypothesized attributing symptoms to dose-related effects at pre- and post-synaptic noradrenergic and dopaminergic receptors.
- Hsieh et al18 hypothesized that methamphetamine use causes an increased flow of dopamine in the striatum, which leads to excessive glutamate release into the cortex. Excess glutamate in the cortex might, over time, cause damage to cortical interneurons. This damage may dysregulate thalamocortical signals, resulting in psychotic symptoms.18
Although the mechanisms by which psychotic symptoms associated with stimulants occur remain unknown, possibilities include10,19:
- genetic predisposition
- changes induced by stimulants at the level of neurotransmitters, synapses, and brain circuits
- an idiosyncratic drug reaction.
Continue to: What to consider before prescribing stimulants
What to consider before prescribing stimulants
While stimulants are clearly beneficial for the vast majority of children with ADHD, there may be a small subgroup of patients for whom stimulants carry increased risk. For example, it is possible that patients with a family history of mood and psychotic disorders may be more vulnerable to stimulant-induced psychotic symptoms that are reversible on discontinuation.20 In our case, R had a first-degree relative (his father) with treatment-refractory schizophrenia.
Attentional dysfunction is a common premorbid presentation for children who later develop schizophrenia or bipolar disorder. Retrospective data from patients with schizophrenia or bipolar disorder document high rates of childhood stimulant use—generally higher even than other groups with attentional dysfunction21 and histories of stimulant-associated adverse behavioral effects.22 In these patients, a history of stimulant use is also associated with an earlier age at onset23 and a more severe course of illness during hospitalization.24 Stimulant exposure in vulnerable individuals may hasten the onset or worsen the course of bipolar or psychotic illnesses.21,25,26
OUTCOME Well-controlled symptoms
R continues to receive short-acting methylphenidate, 5 mg twice a day. His ADHD symptoms remain well-controlled, and he is able to do well academically.
The authors’ observations
Although stimulant-induced psychosis is a rare and unpredictable occurrence, carefully monitoring all patients for any adverse effects of ADHD medication is recommended. When present, psychotic symptoms may quickly remit upon discontinuation of the medication. The question of subsequently reintroducing stimulant medication for a patient with severe ADHD is complicated. One needs to measure the possible risk of a reoccurrence of the psychotic symptoms against the consequences of untreated ADHD. These consequences include increased risk for academic and occupational failure, depression, anxiety, and substance abuse. Psychosocial interventions for ADHD should be implemented, but for optimal results, they often need to be combined with medication. However, if a stimulant medication is to be reintroduced, this should be done with extreme care. Starting dosages need to be low, and increases should be gradual, with frequent monitoring.
Bottom Line
Although stimulant-induced psychosis is a rare occurrence, determine if your pediatric patient with attention-deficit/hyperactivity disorder (ADHD) has a family history of mood or psychotic disorders before initiating stimulants. Carefully monitor all patients for any adverse effects of stimulant medications prescribed for ADHD. If psychotic symptoms occur at therapeutic doses, reduce the dose or discontinue the medication. Once the psychotic or manic symptoms resolve, it may be appropriate to re-challenge with a stimulant.
Related Resource
- Man KK, Coghill D, Chan EW, et al. Methylphenidate and the risk of psychotic disorders and hallucinations in children and adolescents in a large health system. Transl Psychiatry. 2016;6(11):e956. doi: 10.1038/tp.2016.216.
Drug Brand Names
Atomoxetine • Strattera
Dexmethylphenidate • Focalin
Dextroamphetamine/amphetamine • Adderall
Methylphenidate • Metadate, Ritalin
Methylphenidate ER • Concerta
1. Cherland E, Fitzpatrick R. Psychotic side effects of psychostimulants: a 5-year review. Can J Psychiatry. 1999; 44(8):811-813.
2. Ross RG. Psychotic and manic-like symptoms during stimulant treatment of attention deficit hyperactivity disorder. Am. J. Psychiatry. 2006;163(7):1149-1152.
3. Rashid J, Mitelman S. Methylphenidate and somatic hallucinations. J Am Acad Child Adolesc Psychiatry. 2007;46(8):945-946.
4. Rubio JM, Sanjuán J, Flórez-Salamanca L, et al. Examining the course of hallucinatory experiences in children and adolescents: a systematic review. Schizophr Res. 2012;138(2-3):248-254.
5. Iversen L. Neurotransmitter transporters and their impact on the development of psychopharmacology. Br J Pharmacol. 2006;147(Suppl 1):S82-S88.
6. Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69(8):776-786.
7. Bloom AS, Russell LJ, Weisskopf B, et al. Methylphenidate-induced delusional disorder in a child with attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry. 1988;27(1):88-89.
8. Shibib S, Chaloub N. Stimulant induced psychosis. Child Adolesc Ment Health. 2009;14(1):1420-1423.
9. Lucas AR, Weiss M. Methylphenidate hallucinosis. JAMA. 1971;217(8):1079-1081.
10. Kraemer M, Uekermann J, Wiltfang J, et al. Methylphenidate-induced psychosis in adult attention-deficit/hyperactivity disorder: report of 3 new cases and review of the literature. Clin Neuropharmacol. 2010;33(4):204-206.
11. Mosholder AD, Gelperin K, Hammad TA, et al. Hallucinations and other psychotic symptoms associated with the use of attention-deficit/hyperactivity disorder drugs in children. Pediatrics. 2009; 123:611-616.
12. Gross-Tsur V, Joseph A, Shalev RS. Hallucinations during methylphenidate therapy. Neurology. 2004;63(4):753-754.
13. Halevy A, Shuper A. Methylphenidate induction of complex visual hallucinations. J Child Neurol. 2009;24(8):1005-1007.
14. Porfirio MC, Giana G, Giovinazzo S, et al. Methylphenidate-induced visual hallucinations. Neuropediatrics. 2011;42(1):30-31.
15. Griffith J. A study of illicit amphetamine drug traffic in Oklahoma City. Am J Psychiatry. 1966;123(5):560-569.
16. Young JG. Methylphenidate-induced hallucinosis: case histories and possible mechanisms of action. J Dev Behav Pediatr. 1981;2(2):35-38.
17. Stein MA, Sarampote CS, Waldman ID, et al. A dose-response study of OROS methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics. 2003; 112(5):e404. PMID: 14595084.
18. Hsieh JH, Stein DJ, Howells FM. The neurobiology of methamphetamine induced psychosis. Front Hum Neurosci. 2014;8:537. doi:10.3389/fnhum.2014.00537.
19. Shyu YC, Yuan SS, Lee SY, et al. Attention-deficit/hyperactivity disorder, methylphenidate use and the risk of developing schizophrenia spectrum disorders: a nationwide population-based study in Taiwan. Schizophrenia Res. 2015;168(1-2):161-167.
20. MacKenzie LE, Abidi S, Fisher HL, et al. Stimulant medication and psychotic symptoms in offspring of parents with mental illness. Pediatrics. 2016;137(1). doi: 10.1542/peds.2015-2486.
21. Schaeffer J, Ross RG. Childhood-onset schizophrenia: premorbid and prodromal diagnosis and treatment histories. J Am Acad Child Adolesc Psychiatry. 2002;41(5):538-545.
22. Faedda GL, Baldessarini RJ, Blovinsky IP, et al. Treatment-emergent mania in pediatric bipolar disorder: a retrospective case review. J Affect Disord. 2004;82(1):149-158.
23. DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord. 2001;3(2):53-57.
24. Soutullo CA, DelBello MP, Ochsner BS, et al. Severity of bipolarity in hospitalized manic adolescents with history of stimulant or antidepressant treatment. J Affect Disord. 2002;70(3):323-327.
25. Reichart CG, Nolen WA. Earlier onset of bipolar disorder in children by antidepressants or stimulants? An hypothesis. J Affect Disord. 2004;78(1):81-84.
26. Ikeda M, Okahisa Y, Aleksic B, et al. Evidence for shared genetic risk between methamphetamine-induced psychosis and schizophrenia. Neuropsychopharmacology. 2013;38(10):1864-1870.
1. Cherland E, Fitzpatrick R. Psychotic side effects of psychostimulants: a 5-year review. Can J Psychiatry. 1999; 44(8):811-813.
2. Ross RG. Psychotic and manic-like symptoms during stimulant treatment of attention deficit hyperactivity disorder. Am. J. Psychiatry. 2006;163(7):1149-1152.
3. Rashid J, Mitelman S. Methylphenidate and somatic hallucinations. J Am Acad Child Adolesc Psychiatry. 2007;46(8):945-946.
4. Rubio JM, Sanjuán J, Flórez-Salamanca L, et al. Examining the course of hallucinatory experiences in children and adolescents: a systematic review. Schizophr Res. 2012;138(2-3):248-254.
5. Iversen L. Neurotransmitter transporters and their impact on the development of psychopharmacology. Br J Pharmacol. 2006;147(Suppl 1):S82-S88.
6. Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69(8):776-786.
7. Bloom AS, Russell LJ, Weisskopf B, et al. Methylphenidate-induced delusional disorder in a child with attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry. 1988;27(1):88-89.
8. Shibib S, Chaloub N. Stimulant induced psychosis. Child Adolesc Ment Health. 2009;14(1):1420-1423.
9. Lucas AR, Weiss M. Methylphenidate hallucinosis. JAMA. 1971;217(8):1079-1081.
10. Kraemer M, Uekermann J, Wiltfang J, et al. Methylphenidate-induced psychosis in adult attention-deficit/hyperactivity disorder: report of 3 new cases and review of the literature. Clin Neuropharmacol. 2010;33(4):204-206.
11. Mosholder AD, Gelperin K, Hammad TA, et al. Hallucinations and other psychotic symptoms associated with the use of attention-deficit/hyperactivity disorder drugs in children. Pediatrics. 2009; 123:611-616.
12. Gross-Tsur V, Joseph A, Shalev RS. Hallucinations during methylphenidate therapy. Neurology. 2004;63(4):753-754.
13. Halevy A, Shuper A. Methylphenidate induction of complex visual hallucinations. J Child Neurol. 2009;24(8):1005-1007.
14. Porfirio MC, Giana G, Giovinazzo S, et al. Methylphenidate-induced visual hallucinations. Neuropediatrics. 2011;42(1):30-31.
15. Griffith J. A study of illicit amphetamine drug traffic in Oklahoma City. Am J Psychiatry. 1966;123(5):560-569.
16. Young JG. Methylphenidate-induced hallucinosis: case histories and possible mechanisms of action. J Dev Behav Pediatr. 1981;2(2):35-38.
17. Stein MA, Sarampote CS, Waldman ID, et al. A dose-response study of OROS methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics. 2003; 112(5):e404. PMID: 14595084.
18. Hsieh JH, Stein DJ, Howells FM. The neurobiology of methamphetamine induced psychosis. Front Hum Neurosci. 2014;8:537. doi:10.3389/fnhum.2014.00537.
19. Shyu YC, Yuan SS, Lee SY, et al. Attention-deficit/hyperactivity disorder, methylphenidate use and the risk of developing schizophrenia spectrum disorders: a nationwide population-based study in Taiwan. Schizophrenia Res. 2015;168(1-2):161-167.
20. MacKenzie LE, Abidi S, Fisher HL, et al. Stimulant medication and psychotic symptoms in offspring of parents with mental illness. Pediatrics. 2016;137(1). doi: 10.1542/peds.2015-2486.
21. Schaeffer J, Ross RG. Childhood-onset schizophrenia: premorbid and prodromal diagnosis and treatment histories. J Am Acad Child Adolesc Psychiatry. 2002;41(5):538-545.
22. Faedda GL, Baldessarini RJ, Blovinsky IP, et al. Treatment-emergent mania in pediatric bipolar disorder: a retrospective case review. J Affect Disord. 2004;82(1):149-158.
23. DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord. 2001;3(2):53-57.
24. Soutullo CA, DelBello MP, Ochsner BS, et al. Severity of bipolarity in hospitalized manic adolescents with history of stimulant or antidepressant treatment. J Affect Disord. 2002;70(3):323-327.
25. Reichart CG, Nolen WA. Earlier onset of bipolar disorder in children by antidepressants or stimulants? An hypothesis. J Affect Disord. 2004;78(1):81-84.
26. Ikeda M, Okahisa Y, Aleksic B, et al. Evidence for shared genetic risk between methamphetamine-induced psychosis and schizophrenia. Neuropsychopharmacology. 2013;38(10):1864-1870.
Suicide screening: How to recognize and treat at-risk adults
THE CASE
Emily T,* a 30-year-old woman, visited her primary care physician as follow-up to reassess her grief over the loss of her father a year earlier. Emily was her father’s primary caretaker and still lived alone in his home. Emily had a history of chronic pain and major depressive disorder and had expressed feelings of worthlessness and hopelessness about her future since her father’s passing. In addition to her continuing grief response, she reported feeling worse on most days. She completed the Patient Health Questionnaire-9, and results indicated anhedonia, depressed mood, psychomotor retardation, hypersomnia, decreased appetite, decreased concentration, and thoughts that she would be better off dead.
- HOW WOULD YOU PROCEED WITH THIS PATIENT?
* The patient’s name has been changed to protect her identity.
In the United States, 1 suicide occurs on average every 12 minutes; lifetime prevalence of suicide attempts ranges from 1.9% to 8.7%.1 Suicide is the 10th overall cause of death in the United States, and it is the second leading cause of death for adults 18 to 34 years of age.2 In one study, nearly half of suicide victims had contact with primary care providers within 1 month of their suicide.3 Unfortunately, additional research suggests that primary care physicians appropriately screen for suicide in fewer than 40% of patient encounters.4,5
Suicide is defined as “death caused by self-directed injurious behavior with any intent to die as a result of the behavior.”6 When screening for suicide, be aware of the many terms related to suicide evaluation (TABLE 16). Be mindful, too, of the differences between suicidal and nonsuicidal ideation (death wish); the continuum of such thoughts ranges from those that lead to suicide to those that do not.
SUICIDE SCREENING RECOMMENDATIONS VARY
Although most health care providers would agree that intervening with a suicidal patient first requires competence in assessing suicide risk, regulating bodies differ on the use of routine screening and on appropriate screening tools for primary care. The Joint Commission recommends assessing suicide risk with all primary care patients,7 while the US Preventive Services Tasks Force (USPSTF) advises against universal suicide screening in primary care8 due to insufficient evidence that its benefit outweighs potential harm (TABLE 27-12). Instead, the USPSTF recommends screening primary care patients with known mental health disorders, recent inpatient psychiatric hospitalization, prior suicide or self-harm attempts, or increased emotional distress.8 USPSTF does support screening for depression with routine mental health measures that include items assessing suicidality.8,13,14 The American Academy of Family Physicians supports the recommendations by USPSTF.13
When screening for suicide, a comprehensive suicide risk assessment is recommended by both the Joint Commission and USPSTF.7,8 A comprehensive suicide risk assessment has 4 components: (1) identification of current suicide risk factors, (2) identification of protective factors, (3) inquiry about suicidal ideation, intent, and plan, and (4) primary care practitioner judgment of risk level and plan for clinical intervention.9-11
Take into account both risks and protective factors
Unfortunately, there is no “typical” description of a patient at risk for suicide and no validated models to predict suicide risk.8,10 A multitude of factors, both individual and societal, can increase or reduce risk of suicide.11,15 Each patient’s unique history includes risk factors for suicide including precipitating events (eg, job loss, termination of a relationship, death of a loved one) and protective factors that may be evaluated to determine overall risk for suicide (TABLE 38,10,11,15). According to the Centers for Disease Control and Prevention (CDC), there are several warning signs for patients who may be at greater risk for suicide: isolation, increased anxiety or anger, obtaining lethal means (eg, guns, knives, ropes), frequent mood swings, sleep changes, feeling trapped or in pain, increased substance use, discussing plans for death or wishes of death, and feeling like a burden.16
CHOOSING FROM AMONG SUICIDE SCREENING TOOLS
Brief mental health screening tools such as the Patient Health Questionnaire-9 (PHQ-9) are commonly used as primary screening tools for suicidal ideation.17 However, to attain a fuller understanding of a patient’s suicidality, select a screening tool that specifically
Continue to: Several screening tools...
Several screening tools are available for exploring a patient’s suicidality. Unfortunately, most of them are supported by limited evidence of effectiveness in identifying suicide risk.8-10 An exception is the well-researched and commonly used Columbia-Suicide Severity Rating Scale (C-SSRS).18,19 In a comparative study conducted at 2 primary care clinics, researchers found that the suicide item included in the PHQ-9 provided poor sensitivity but moderate specificity (60% and 84%, respectively),20 while the C-SSRS showed high sensitivity (100%) and specificity (96%-100%) in accurately identifying various suicidal self-injurious behaviors above and beyond what was identified through a structured clinical interview.20 Free copies of the C-SSRS, training materials, and follow-up assessments in multiple languages can be obtained on The Columbia Lighthouse Project Web site (http://cssrs.columbia.edu/).19
RECOMMENDATIONS FOR INTERVENTION
While there is debate regarding whom to screen for suicide, the importance of intervention when a patient is revealed to be at risk is clear. After completing a
When a patient is at high risk for suicide and reports an imminent plan or intent, ensure their safety through inpatient psychiatric hospitalization and then close follow-up upon hospital discharge. First encourage voluntary hospitalization in a collaborative discussion with the patient; resort to involuntary hospitalization only if the patient resists.
What not to do. When the patient does not require immediate hospitalization, evidence recommends against contracting for patient safety via a written contract or requiring patients to verbally guarantee that they will not commit suicide upon leaving a provider’s office.21 Concerns about such contracts include a lack of evidence supporting their use, decreased vigilance by health care workers when such contracts are in place, and questions regarding informed consent and competence.21 Instead, engage a patient who is at moderate or low risk in safety planning, and meet with the patient frequently to discuss continued safety planning through close follow-up (or with a behavioral health provider if available).10-12,22 With patients previously identified as at high risk for suicide who return from inpatient psychiatric hospitalization, continue to screen them for suicide at subsequent visits and engage them in collaborative safety planning.
Safety planning (TABLE 512), also known as crisis response planning, is considered a best practice and effective suicide prevention intervention by the Suicide Prevention Resource Center and the American Foundation for Suicide Prevention Best Practices Registry for Suicide Prevention.23 Safety planning involves a collaboration between patient and physician to identify risk factors and protective factors along with crisis resources and strategies to reduce
Continue to: THE CASE
THE CASE
Based on the concerning results from the PHQ-9 suicide item, Emily’s physician conducted a comprehensive suicide risk assessment using both clinical interview and the C-SSRS. Emily reported that she was experiencing daily suicidal ideations due to a lack of social support and longing to be with her deceased father. She had not previously attempted suicide and had no imminent intent to commit suicide. Emily did, however, have a plan to overdose on opioid medications she had been collecting for many months. Her physician determined that Emily was at moderate risk for suicide and consulted with the clinic’s behavioral health consultant, a psychologist, to confirm a treatment plan.
Emily and her physician collaboratively developed a safety plan including means reduction. Emily agreed to have her physician contact a friend to assist with safety planning, and she brought her opioid medications to the primary care clinic for disposal. Follow-up appointments were scheduled with the physician for every other week. The psychologist was available at the time of the first biweekly appointment to consult with the physician if needed. This initial appointment was focused on Emily’s suicide risk and her ability to engage in safety planning. In addition, the physician recommended that Emily schedule time with the psychologist so that she could work on her grief and depressive symptoms.
After several weeks of the biweekly appointments with both the primary care provider and the psychologist, Emily was no longer reporting suicidal ideation and she was ready to engage in coping strategies to deal with her grief and depressive symptoms.
CORRESPONDENCE
Meredith L.C. Williamson, PhD, 2900 E. 29th Street, Suite 100, Bryan, TX 77802; [email protected].
1. Nock MK, Borges G, Bromet EJ, et al. Suicide and suicidal behavior. Epidemiol Rev. 2008;30:133-154.
2. National Institute of Mental Health. Suicide. https://www.nimh.nih.gov/health/statistics/suicide.shtml#part_154968. Accessed October 18, 2019.
3. Luoma JB, Martin CE, Pearson JL. Contact with mental health and primary care providers before suicide: a review of the evidence. Am J Psychiatry. 2002;159:909-916.
4. Vannoy SD, Robins LS. Suicide-related discussions with depressed primary care patients in the USA: gender and quality gaps. A mixed methods analysis. BMJ Open. 2011;1:e000198.
5. Feldman MD, Franks P, Duberstein PR, et al. Let’s not talk about it: suicide inquiry in primary care. Ann Fam Med. 2007;5:412-418.
6. U.S. Department of Health and Human Services (HHS) Office of the Surgeon General and National Action Alliance for Suicide Prevention. 2012 National strategy for suicide prevention: goals and objectives for action. https://mnprc.org/wp-content/uploads/2019/01/2012-National-Strategy-for-suicide-prevention-goals-and-objectives-for-action.pdf. Accessed October 18, 2019.
7. The Joint Commission. Detecting and treating suicide ideation in all settings. Sentinel Event Alert. 2016;(56):1-7.
8. LeFevre ML, U.S. Preventive Services Task Force. Screening for suicide risk in adolescents, adults, and older adults in primary care: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:719-726.
9. American Psychiatric Association. Practice guidelines for the assessment and treatment of patients with suicidal behaviors. 2010. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/suicide.pdf. Accessed October 18, 2019.
10. Department of Veterans Affairs & Department of Defense. VA/DoD clinical practice guideline for assessment and management of patients at risk for suicide. 2013. https://www.healthquality.va.gov/guidelines/MH/srb/VADODCP_SuicideRisk_Full.pdf. Accessed October 18, 2019.
11. Western Interstate Commission for Higher Education. Suicide prevention toolkit for primary care practices. 2017. https://www.sprc.org/sites/default/files/Final%20National%20Suicide%20Prevention%20Toolkit%202.15.18%20FINAL.pdf. Accessed October 18, 2019.
12. Stanley B, Brown GK. Safety planning intervention: a brief intervention to mitigate suicide risk. Cogn Behav Pract. 2012;19:256-264.
13. Screening for suicide risk in adolescents, adults, and older adults in primary care: recommendation statement. Am Fam Physician. 2015;91:190F-190I.
14. O’Connor E, Gaynes B, Burda BU, et al. Screening for suicide risk in primary care: a systematic evidence review for the U.S. Preventive Services Task Force. Evidence synthesis no. 103. https://www.ncbi.nlm.nih.gov/books/NBK137737/. Accessed October 25, 2019.
15. Suicide Prevention Resource Center. Risk and protective factors. https://www.sprc.org/about-suicide/risk-protective-factors. Accessed October 18, 2019.
16. CDC. Suicide rising across the US: more than a mental health concern. https://www.cdc.gov/vitalsigns/suicide/index.html. Accessed October 18, 2019.
17. Martin A, Rief W, Klaiberg A, et al. Validity of the Brief Patient Health Questionnaire Mood Scale (PHQ-9) in the general population. Gen Hosp Psychiatry. 2006;28:71-77.
18. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266-1277.
19. The Columbia Lighthouse Project. Identify risk. Prevent suicide. http://cssrs.columbia.edu. Accessed October 25, 2019.
20. Uebelacker LA, German NM, Gaudiano BA, et al. Patient health questionnaire depression scale as a suicide screening instrument in depressed primary care patients: a cross-sectional study. Prim Care Companion CNS Disord. 2011;13:pii: PCC.10m01027.
21. Hoffman RM. Contracting for safety: a misused tool. Pa Patient Saf Advis. 2013;10:82-84.
22. Stanley B, Brown GK, Brenner LA, et al. Comparison of the safety planning intervention with follow-up vs usual care of suicidal patients treated in the emergency department. JAMA Psychiatry. 2018;75:894-900.
23. Suicide Prevention Resource Center. Safety planning in emergency settings. http://www.sprc.org/news/safety-planning-emergency-settings. Accessed October 25, 2019.
THE CASE
Emily T,* a 30-year-old woman, visited her primary care physician as follow-up to reassess her grief over the loss of her father a year earlier. Emily was her father’s primary caretaker and still lived alone in his home. Emily had a history of chronic pain and major depressive disorder and had expressed feelings of worthlessness and hopelessness about her future since her father’s passing. In addition to her continuing grief response, she reported feeling worse on most days. She completed the Patient Health Questionnaire-9, and results indicated anhedonia, depressed mood, psychomotor retardation, hypersomnia, decreased appetite, decreased concentration, and thoughts that she would be better off dead.
- HOW WOULD YOU PROCEED WITH THIS PATIENT?
* The patient’s name has been changed to protect her identity.
In the United States, 1 suicide occurs on average every 12 minutes; lifetime prevalence of suicide attempts ranges from 1.9% to 8.7%.1 Suicide is the 10th overall cause of death in the United States, and it is the second leading cause of death for adults 18 to 34 years of age.2 In one study, nearly half of suicide victims had contact with primary care providers within 1 month of their suicide.3 Unfortunately, additional research suggests that primary care physicians appropriately screen for suicide in fewer than 40% of patient encounters.4,5
Suicide is defined as “death caused by self-directed injurious behavior with any intent to die as a result of the behavior.”6 When screening for suicide, be aware of the many terms related to suicide evaluation (TABLE 16). Be mindful, too, of the differences between suicidal and nonsuicidal ideation (death wish); the continuum of such thoughts ranges from those that lead to suicide to those that do not.
SUICIDE SCREENING RECOMMENDATIONS VARY
Although most health care providers would agree that intervening with a suicidal patient first requires competence in assessing suicide risk, regulating bodies differ on the use of routine screening and on appropriate screening tools for primary care. The Joint Commission recommends assessing suicide risk with all primary care patients,7 while the US Preventive Services Tasks Force (USPSTF) advises against universal suicide screening in primary care8 due to insufficient evidence that its benefit outweighs potential harm (TABLE 27-12). Instead, the USPSTF recommends screening primary care patients with known mental health disorders, recent inpatient psychiatric hospitalization, prior suicide or self-harm attempts, or increased emotional distress.8 USPSTF does support screening for depression with routine mental health measures that include items assessing suicidality.8,13,14 The American Academy of Family Physicians supports the recommendations by USPSTF.13
When screening for suicide, a comprehensive suicide risk assessment is recommended by both the Joint Commission and USPSTF.7,8 A comprehensive suicide risk assessment has 4 components: (1) identification of current suicide risk factors, (2) identification of protective factors, (3) inquiry about suicidal ideation, intent, and plan, and (4) primary care practitioner judgment of risk level and plan for clinical intervention.9-11
Take into account both risks and protective factors
Unfortunately, there is no “typical” description of a patient at risk for suicide and no validated models to predict suicide risk.8,10 A multitude of factors, both individual and societal, can increase or reduce risk of suicide.11,15 Each patient’s unique history includes risk factors for suicide including precipitating events (eg, job loss, termination of a relationship, death of a loved one) and protective factors that may be evaluated to determine overall risk for suicide (TABLE 38,10,11,15). According to the Centers for Disease Control and Prevention (CDC), there are several warning signs for patients who may be at greater risk for suicide: isolation, increased anxiety or anger, obtaining lethal means (eg, guns, knives, ropes), frequent mood swings, sleep changes, feeling trapped or in pain, increased substance use, discussing plans for death or wishes of death, and feeling like a burden.16
CHOOSING FROM AMONG SUICIDE SCREENING TOOLS
Brief mental health screening tools such as the Patient Health Questionnaire-9 (PHQ-9) are commonly used as primary screening tools for suicidal ideation.17 However, to attain a fuller understanding of a patient’s suicidality, select a screening tool that specifically
Continue to: Several screening tools...
Several screening tools are available for exploring a patient’s suicidality. Unfortunately, most of them are supported by limited evidence of effectiveness in identifying suicide risk.8-10 An exception is the well-researched and commonly used Columbia-Suicide Severity Rating Scale (C-SSRS).18,19 In a comparative study conducted at 2 primary care clinics, researchers found that the suicide item included in the PHQ-9 provided poor sensitivity but moderate specificity (60% and 84%, respectively),20 while the C-SSRS showed high sensitivity (100%) and specificity (96%-100%) in accurately identifying various suicidal self-injurious behaviors above and beyond what was identified through a structured clinical interview.20 Free copies of the C-SSRS, training materials, and follow-up assessments in multiple languages can be obtained on The Columbia Lighthouse Project Web site (http://cssrs.columbia.edu/).19
RECOMMENDATIONS FOR INTERVENTION
While there is debate regarding whom to screen for suicide, the importance of intervention when a patient is revealed to be at risk is clear. After completing a
When a patient is at high risk for suicide and reports an imminent plan or intent, ensure their safety through inpatient psychiatric hospitalization and then close follow-up upon hospital discharge. First encourage voluntary hospitalization in a collaborative discussion with the patient; resort to involuntary hospitalization only if the patient resists.
What not to do. When the patient does not require immediate hospitalization, evidence recommends against contracting for patient safety via a written contract or requiring patients to verbally guarantee that they will not commit suicide upon leaving a provider’s office.21 Concerns about such contracts include a lack of evidence supporting their use, decreased vigilance by health care workers when such contracts are in place, and questions regarding informed consent and competence.21 Instead, engage a patient who is at moderate or low risk in safety planning, and meet with the patient frequently to discuss continued safety planning through close follow-up (or with a behavioral health provider if available).10-12,22 With patients previously identified as at high risk for suicide who return from inpatient psychiatric hospitalization, continue to screen them for suicide at subsequent visits and engage them in collaborative safety planning.
Safety planning (TABLE 512), also known as crisis response planning, is considered a best practice and effective suicide prevention intervention by the Suicide Prevention Resource Center and the American Foundation for Suicide Prevention Best Practices Registry for Suicide Prevention.23 Safety planning involves a collaboration between patient and physician to identify risk factors and protective factors along with crisis resources and strategies to reduce
Continue to: THE CASE
THE CASE
Based on the concerning results from the PHQ-9 suicide item, Emily’s physician conducted a comprehensive suicide risk assessment using both clinical interview and the C-SSRS. Emily reported that she was experiencing daily suicidal ideations due to a lack of social support and longing to be with her deceased father. She had not previously attempted suicide and had no imminent intent to commit suicide. Emily did, however, have a plan to overdose on opioid medications she had been collecting for many months. Her physician determined that Emily was at moderate risk for suicide and consulted with the clinic’s behavioral health consultant, a psychologist, to confirm a treatment plan.
Emily and her physician collaboratively developed a safety plan including means reduction. Emily agreed to have her physician contact a friend to assist with safety planning, and she brought her opioid medications to the primary care clinic for disposal. Follow-up appointments were scheduled with the physician for every other week. The psychologist was available at the time of the first biweekly appointment to consult with the physician if needed. This initial appointment was focused on Emily’s suicide risk and her ability to engage in safety planning. In addition, the physician recommended that Emily schedule time with the psychologist so that she could work on her grief and depressive symptoms.
After several weeks of the biweekly appointments with both the primary care provider and the psychologist, Emily was no longer reporting suicidal ideation and she was ready to engage in coping strategies to deal with her grief and depressive symptoms.
CORRESPONDENCE
Meredith L.C. Williamson, PhD, 2900 E. 29th Street, Suite 100, Bryan, TX 77802; [email protected].
THE CASE
Emily T,* a 30-year-old woman, visited her primary care physician as follow-up to reassess her grief over the loss of her father a year earlier. Emily was her father’s primary caretaker and still lived alone in his home. Emily had a history of chronic pain and major depressive disorder and had expressed feelings of worthlessness and hopelessness about her future since her father’s passing. In addition to her continuing grief response, she reported feeling worse on most days. She completed the Patient Health Questionnaire-9, and results indicated anhedonia, depressed mood, psychomotor retardation, hypersomnia, decreased appetite, decreased concentration, and thoughts that she would be better off dead.
- HOW WOULD YOU PROCEED WITH THIS PATIENT?
* The patient’s name has been changed to protect her identity.
In the United States, 1 suicide occurs on average every 12 minutes; lifetime prevalence of suicide attempts ranges from 1.9% to 8.7%.1 Suicide is the 10th overall cause of death in the United States, and it is the second leading cause of death for adults 18 to 34 years of age.2 In one study, nearly half of suicide victims had contact with primary care providers within 1 month of their suicide.3 Unfortunately, additional research suggests that primary care physicians appropriately screen for suicide in fewer than 40% of patient encounters.4,5
Suicide is defined as “death caused by self-directed injurious behavior with any intent to die as a result of the behavior.”6 When screening for suicide, be aware of the many terms related to suicide evaluation (TABLE 16). Be mindful, too, of the differences between suicidal and nonsuicidal ideation (death wish); the continuum of such thoughts ranges from those that lead to suicide to those that do not.
SUICIDE SCREENING RECOMMENDATIONS VARY
Although most health care providers would agree that intervening with a suicidal patient first requires competence in assessing suicide risk, regulating bodies differ on the use of routine screening and on appropriate screening tools for primary care. The Joint Commission recommends assessing suicide risk with all primary care patients,7 while the US Preventive Services Tasks Force (USPSTF) advises against universal suicide screening in primary care8 due to insufficient evidence that its benefit outweighs potential harm (TABLE 27-12). Instead, the USPSTF recommends screening primary care patients with known mental health disorders, recent inpatient psychiatric hospitalization, prior suicide or self-harm attempts, or increased emotional distress.8 USPSTF does support screening for depression with routine mental health measures that include items assessing suicidality.8,13,14 The American Academy of Family Physicians supports the recommendations by USPSTF.13
When screening for suicide, a comprehensive suicide risk assessment is recommended by both the Joint Commission and USPSTF.7,8 A comprehensive suicide risk assessment has 4 components: (1) identification of current suicide risk factors, (2) identification of protective factors, (3) inquiry about suicidal ideation, intent, and plan, and (4) primary care practitioner judgment of risk level and plan for clinical intervention.9-11
Take into account both risks and protective factors
Unfortunately, there is no “typical” description of a patient at risk for suicide and no validated models to predict suicide risk.8,10 A multitude of factors, both individual and societal, can increase or reduce risk of suicide.11,15 Each patient’s unique history includes risk factors for suicide including precipitating events (eg, job loss, termination of a relationship, death of a loved one) and protective factors that may be evaluated to determine overall risk for suicide (TABLE 38,10,11,15). According to the Centers for Disease Control and Prevention (CDC), there are several warning signs for patients who may be at greater risk for suicide: isolation, increased anxiety or anger, obtaining lethal means (eg, guns, knives, ropes), frequent mood swings, sleep changes, feeling trapped or in pain, increased substance use, discussing plans for death or wishes of death, and feeling like a burden.16
CHOOSING FROM AMONG SUICIDE SCREENING TOOLS
Brief mental health screening tools such as the Patient Health Questionnaire-9 (PHQ-9) are commonly used as primary screening tools for suicidal ideation.17 However, to attain a fuller understanding of a patient’s suicidality, select a screening tool that specifically
Continue to: Several screening tools...
Several screening tools are available for exploring a patient’s suicidality. Unfortunately, most of them are supported by limited evidence of effectiveness in identifying suicide risk.8-10 An exception is the well-researched and commonly used Columbia-Suicide Severity Rating Scale (C-SSRS).18,19 In a comparative study conducted at 2 primary care clinics, researchers found that the suicide item included in the PHQ-9 provided poor sensitivity but moderate specificity (60% and 84%, respectively),20 while the C-SSRS showed high sensitivity (100%) and specificity (96%-100%) in accurately identifying various suicidal self-injurious behaviors above and beyond what was identified through a structured clinical interview.20 Free copies of the C-SSRS, training materials, and follow-up assessments in multiple languages can be obtained on The Columbia Lighthouse Project Web site (http://cssrs.columbia.edu/).19
RECOMMENDATIONS FOR INTERVENTION
While there is debate regarding whom to screen for suicide, the importance of intervention when a patient is revealed to be at risk is clear. After completing a
When a patient is at high risk for suicide and reports an imminent plan or intent, ensure their safety through inpatient psychiatric hospitalization and then close follow-up upon hospital discharge. First encourage voluntary hospitalization in a collaborative discussion with the patient; resort to involuntary hospitalization only if the patient resists.
What not to do. When the patient does not require immediate hospitalization, evidence recommends against contracting for patient safety via a written contract or requiring patients to verbally guarantee that they will not commit suicide upon leaving a provider’s office.21 Concerns about such contracts include a lack of evidence supporting their use, decreased vigilance by health care workers when such contracts are in place, and questions regarding informed consent and competence.21 Instead, engage a patient who is at moderate or low risk in safety planning, and meet with the patient frequently to discuss continued safety planning through close follow-up (or with a behavioral health provider if available).10-12,22 With patients previously identified as at high risk for suicide who return from inpatient psychiatric hospitalization, continue to screen them for suicide at subsequent visits and engage them in collaborative safety planning.
Safety planning (TABLE 512), also known as crisis response planning, is considered a best practice and effective suicide prevention intervention by the Suicide Prevention Resource Center and the American Foundation for Suicide Prevention Best Practices Registry for Suicide Prevention.23 Safety planning involves a collaboration between patient and physician to identify risk factors and protective factors along with crisis resources and strategies to reduce
Continue to: THE CASE
THE CASE
Based on the concerning results from the PHQ-9 suicide item, Emily’s physician conducted a comprehensive suicide risk assessment using both clinical interview and the C-SSRS. Emily reported that she was experiencing daily suicidal ideations due to a lack of social support and longing to be with her deceased father. She had not previously attempted suicide and had no imminent intent to commit suicide. Emily did, however, have a plan to overdose on opioid medications she had been collecting for many months. Her physician determined that Emily was at moderate risk for suicide and consulted with the clinic’s behavioral health consultant, a psychologist, to confirm a treatment plan.
Emily and her physician collaboratively developed a safety plan including means reduction. Emily agreed to have her physician contact a friend to assist with safety planning, and she brought her opioid medications to the primary care clinic for disposal. Follow-up appointments were scheduled with the physician for every other week. The psychologist was available at the time of the first biweekly appointment to consult with the physician if needed. This initial appointment was focused on Emily’s suicide risk and her ability to engage in safety planning. In addition, the physician recommended that Emily schedule time with the psychologist so that she could work on her grief and depressive symptoms.
After several weeks of the biweekly appointments with both the primary care provider and the psychologist, Emily was no longer reporting suicidal ideation and she was ready to engage in coping strategies to deal with her grief and depressive symptoms.
CORRESPONDENCE
Meredith L.C. Williamson, PhD, 2900 E. 29th Street, Suite 100, Bryan, TX 77802; [email protected].
1. Nock MK, Borges G, Bromet EJ, et al. Suicide and suicidal behavior. Epidemiol Rev. 2008;30:133-154.
2. National Institute of Mental Health. Suicide. https://www.nimh.nih.gov/health/statistics/suicide.shtml#part_154968. Accessed October 18, 2019.
3. Luoma JB, Martin CE, Pearson JL. Contact with mental health and primary care providers before suicide: a review of the evidence. Am J Psychiatry. 2002;159:909-916.
4. Vannoy SD, Robins LS. Suicide-related discussions with depressed primary care patients in the USA: gender and quality gaps. A mixed methods analysis. BMJ Open. 2011;1:e000198.
5. Feldman MD, Franks P, Duberstein PR, et al. Let’s not talk about it: suicide inquiry in primary care. Ann Fam Med. 2007;5:412-418.
6. U.S. Department of Health and Human Services (HHS) Office of the Surgeon General and National Action Alliance for Suicide Prevention. 2012 National strategy for suicide prevention: goals and objectives for action. https://mnprc.org/wp-content/uploads/2019/01/2012-National-Strategy-for-suicide-prevention-goals-and-objectives-for-action.pdf. Accessed October 18, 2019.
7. The Joint Commission. Detecting and treating suicide ideation in all settings. Sentinel Event Alert. 2016;(56):1-7.
8. LeFevre ML, U.S. Preventive Services Task Force. Screening for suicide risk in adolescents, adults, and older adults in primary care: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:719-726.
9. American Psychiatric Association. Practice guidelines for the assessment and treatment of patients with suicidal behaviors. 2010. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/suicide.pdf. Accessed October 18, 2019.
10. Department of Veterans Affairs & Department of Defense. VA/DoD clinical practice guideline for assessment and management of patients at risk for suicide. 2013. https://www.healthquality.va.gov/guidelines/MH/srb/VADODCP_SuicideRisk_Full.pdf. Accessed October 18, 2019.
11. Western Interstate Commission for Higher Education. Suicide prevention toolkit for primary care practices. 2017. https://www.sprc.org/sites/default/files/Final%20National%20Suicide%20Prevention%20Toolkit%202.15.18%20FINAL.pdf. Accessed October 18, 2019.
12. Stanley B, Brown GK. Safety planning intervention: a brief intervention to mitigate suicide risk. Cogn Behav Pract. 2012;19:256-264.
13. Screening for suicide risk in adolescents, adults, and older adults in primary care: recommendation statement. Am Fam Physician. 2015;91:190F-190I.
14. O’Connor E, Gaynes B, Burda BU, et al. Screening for suicide risk in primary care: a systematic evidence review for the U.S. Preventive Services Task Force. Evidence synthesis no. 103. https://www.ncbi.nlm.nih.gov/books/NBK137737/. Accessed October 25, 2019.
15. Suicide Prevention Resource Center. Risk and protective factors. https://www.sprc.org/about-suicide/risk-protective-factors. Accessed October 18, 2019.
16. CDC. Suicide rising across the US: more than a mental health concern. https://www.cdc.gov/vitalsigns/suicide/index.html. Accessed October 18, 2019.
17. Martin A, Rief W, Klaiberg A, et al. Validity of the Brief Patient Health Questionnaire Mood Scale (PHQ-9) in the general population. Gen Hosp Psychiatry. 2006;28:71-77.
18. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266-1277.
19. The Columbia Lighthouse Project. Identify risk. Prevent suicide. http://cssrs.columbia.edu. Accessed October 25, 2019.
20. Uebelacker LA, German NM, Gaudiano BA, et al. Patient health questionnaire depression scale as a suicide screening instrument in depressed primary care patients: a cross-sectional study. Prim Care Companion CNS Disord. 2011;13:pii: PCC.10m01027.
21. Hoffman RM. Contracting for safety: a misused tool. Pa Patient Saf Advis. 2013;10:82-84.
22. Stanley B, Brown GK, Brenner LA, et al. Comparison of the safety planning intervention with follow-up vs usual care of suicidal patients treated in the emergency department. JAMA Psychiatry. 2018;75:894-900.
23. Suicide Prevention Resource Center. Safety planning in emergency settings. http://www.sprc.org/news/safety-planning-emergency-settings. Accessed October 25, 2019.
1. Nock MK, Borges G, Bromet EJ, et al. Suicide and suicidal behavior. Epidemiol Rev. 2008;30:133-154.
2. National Institute of Mental Health. Suicide. https://www.nimh.nih.gov/health/statistics/suicide.shtml#part_154968. Accessed October 18, 2019.
3. Luoma JB, Martin CE, Pearson JL. Contact with mental health and primary care providers before suicide: a review of the evidence. Am J Psychiatry. 2002;159:909-916.
4. Vannoy SD, Robins LS. Suicide-related discussions with depressed primary care patients in the USA: gender and quality gaps. A mixed methods analysis. BMJ Open. 2011;1:e000198.
5. Feldman MD, Franks P, Duberstein PR, et al. Let’s not talk about it: suicide inquiry in primary care. Ann Fam Med. 2007;5:412-418.
6. U.S. Department of Health and Human Services (HHS) Office of the Surgeon General and National Action Alliance for Suicide Prevention. 2012 National strategy for suicide prevention: goals and objectives for action. https://mnprc.org/wp-content/uploads/2019/01/2012-National-Strategy-for-suicide-prevention-goals-and-objectives-for-action.pdf. Accessed October 18, 2019.
7. The Joint Commission. Detecting and treating suicide ideation in all settings. Sentinel Event Alert. 2016;(56):1-7.
8. LeFevre ML, U.S. Preventive Services Task Force. Screening for suicide risk in adolescents, adults, and older adults in primary care: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:719-726.
9. American Psychiatric Association. Practice guidelines for the assessment and treatment of patients with suicidal behaviors. 2010. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/suicide.pdf. Accessed October 18, 2019.
10. Department of Veterans Affairs & Department of Defense. VA/DoD clinical practice guideline for assessment and management of patients at risk for suicide. 2013. https://www.healthquality.va.gov/guidelines/MH/srb/VADODCP_SuicideRisk_Full.pdf. Accessed October 18, 2019.
11. Western Interstate Commission for Higher Education. Suicide prevention toolkit for primary care practices. 2017. https://www.sprc.org/sites/default/files/Final%20National%20Suicide%20Prevention%20Toolkit%202.15.18%20FINAL.pdf. Accessed October 18, 2019.
12. Stanley B, Brown GK. Safety planning intervention: a brief intervention to mitigate suicide risk. Cogn Behav Pract. 2012;19:256-264.
13. Screening for suicide risk in adolescents, adults, and older adults in primary care: recommendation statement. Am Fam Physician. 2015;91:190F-190I.
14. O’Connor E, Gaynes B, Burda BU, et al. Screening for suicide risk in primary care: a systematic evidence review for the U.S. Preventive Services Task Force. Evidence synthesis no. 103. https://www.ncbi.nlm.nih.gov/books/NBK137737/. Accessed October 25, 2019.
15. Suicide Prevention Resource Center. Risk and protective factors. https://www.sprc.org/about-suicide/risk-protective-factors. Accessed October 18, 2019.
16. CDC. Suicide rising across the US: more than a mental health concern. https://www.cdc.gov/vitalsigns/suicide/index.html. Accessed October 18, 2019.
17. Martin A, Rief W, Klaiberg A, et al. Validity of the Brief Patient Health Questionnaire Mood Scale (PHQ-9) in the general population. Gen Hosp Psychiatry. 2006;28:71-77.
18. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266-1277.
19. The Columbia Lighthouse Project. Identify risk. Prevent suicide. http://cssrs.columbia.edu. Accessed October 25, 2019.
20. Uebelacker LA, German NM, Gaudiano BA, et al. Patient health questionnaire depression scale as a suicide screening instrument in depressed primary care patients: a cross-sectional study. Prim Care Companion CNS Disord. 2011;13:pii: PCC.10m01027.
21. Hoffman RM. Contracting for safety: a misused tool. Pa Patient Saf Advis. 2013;10:82-84.
22. Stanley B, Brown GK, Brenner LA, et al. Comparison of the safety planning intervention with follow-up vs usual care of suicidal patients treated in the emergency department. JAMA Psychiatry. 2018;75:894-900.
23. Suicide Prevention Resource Center. Safety planning in emergency settings. http://www.sprc.org/news/safety-planning-emergency-settings. Accessed October 25, 2019.