User login
How to better identify and manage women with elevated breast cancer risk
Breast cancer is the most common invasive cancer in women in the United States; it is estimated that there will be 287,850 new cases of breast cancer in the United States during 2022 with 43,250 deaths.1 Lives are extended and saved every day because of a robust arsenal of treatments and interventions available to those who have been given a diagnosis of breast cancer. And, of course, lives are also extended and saved when we identify women at risk and provide early interventions. But in busy offices where time is short and there are competing demands on our time, proper assessment of a woman’s risk of breast cancer does not always happen. As a result, women with a higher risk of breast cancer may not be getting appropriate management.2,3
Familiarizing yourself with several risk-assessment tools and knowing when genetic testing is needed can make a big difference. Knowing the timing of mammograms and magnetic resonance imaging (MRI) for women deemed to be at high risk is also key. The following review employs a case-based approach (with an accompanying ALGORITHM) to illustrate how best to identify women who are at heightened risk of breast cancer and maximize their care. We also discuss the chemoprophylaxis regimens that may be used for those at increased risk.
CASE
Rachel P, age 37, presents to establish care. She has an Ashkenazi Jewish background and wonders if she should start doing breast cancer screening before age 40. She has 2 children, ages 4 years and 2 years. Her maternal aunt had unilateral breast cancer at age 54, and her maternal grandmother died of ovarian cancer at age 65.
Risk assessment
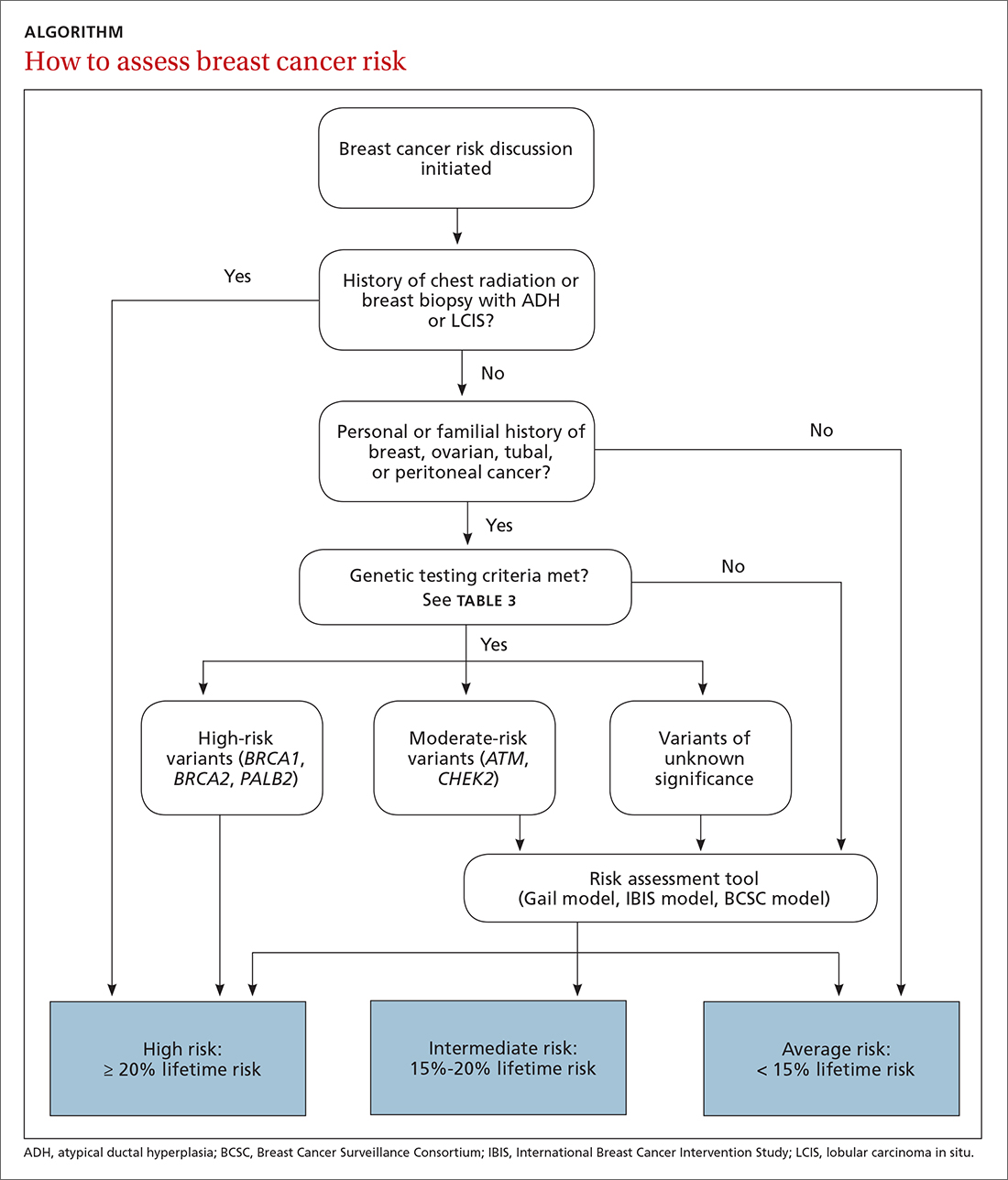
The risk assessment process (see ALGORITHM) must start with either the clinician or the patient initiating the discussion about breast cancer risk. The clinician may initiate the discussion with a new patient or at an annual physical examination. The patient may start the discussion because they are experiencing new breast symptoms, have anxiety about developing breast cancer, or have a family member with a new cancer diagnosis.
Risk factors. There are single factors that convey enough risk to automatically designate the patient as high risk (see TABLE 14-9). These factors include having a history of chest radiation between the ages of 10 and 30, a history of breast biopsy with either lobular carcinoma in situ (LCIS) or atypical ductal hyperplasia (ADH), past breast and/or ovarian cancer, and either a family or personal history of a high penetrant genetic variant for breast cancer.4-9
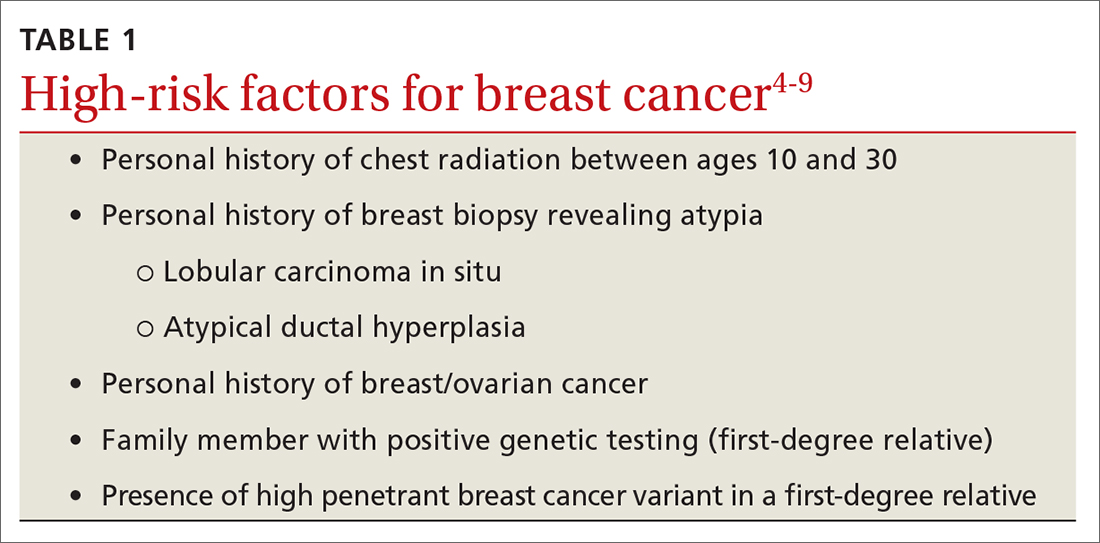
In women with previous chest radiation, breast cancer risk correlates with the total dose of radiation.5 For women with a personal history of breast cancer, the younger the age at diagnosis, the higher the risk of contralateral breast cancer.5 Precancerous changes such as ADH, LCIS, and ductal carcinoma in situ (DCIS) also confer moderate increases in risk. Women with these diagnoses will commonly have follow-up with specialists.
Risk assessment tools. There are several models available to assess a woman’s breast cancer risk (see TABLE 210-12). The Gail model (https://bcrisktool.cancer.gov/) is the oldest, quickest, and most widely known. However, the Gail model only accounts for first-degree relatives diagnosed with breast cancer, may underpredict risk in women with a more extensive family history, and has not been studied in women younger than 35. The International Breast Cancer Intervention Study (IBIS) Risk Evaluation Tool (https://ibis-risk-calculator.magview.com/), commonly referred to as the Tyrer-Cuzick model, incorporates second-degree relatives into the prediction model—although women may not know their full family history. Both the IBIS and the Breast Cancer Surveillance Consortium (BCSC) model (https://tools.bcsc-scc.org/BC5yearRisk/intro.htm) include breast density in the prediction algorithm. The choice of tool depends on clinician comfort and individual patient risk factors. There is no evidence that one model is better than another.10-12

Continue to: CASE
CASE
Ms. P’s clinician starts with an assessment using the Gail model. However, when the result comes back with average risk, the clinician decides to follow up with the Tyrer-Cuzick model in order to incorporate Ms. P’s multiple second-degree relatives with breast and ovarian cancer. (The BCSC model was not used because it only includes first-degree relatives.)
Genetic testing
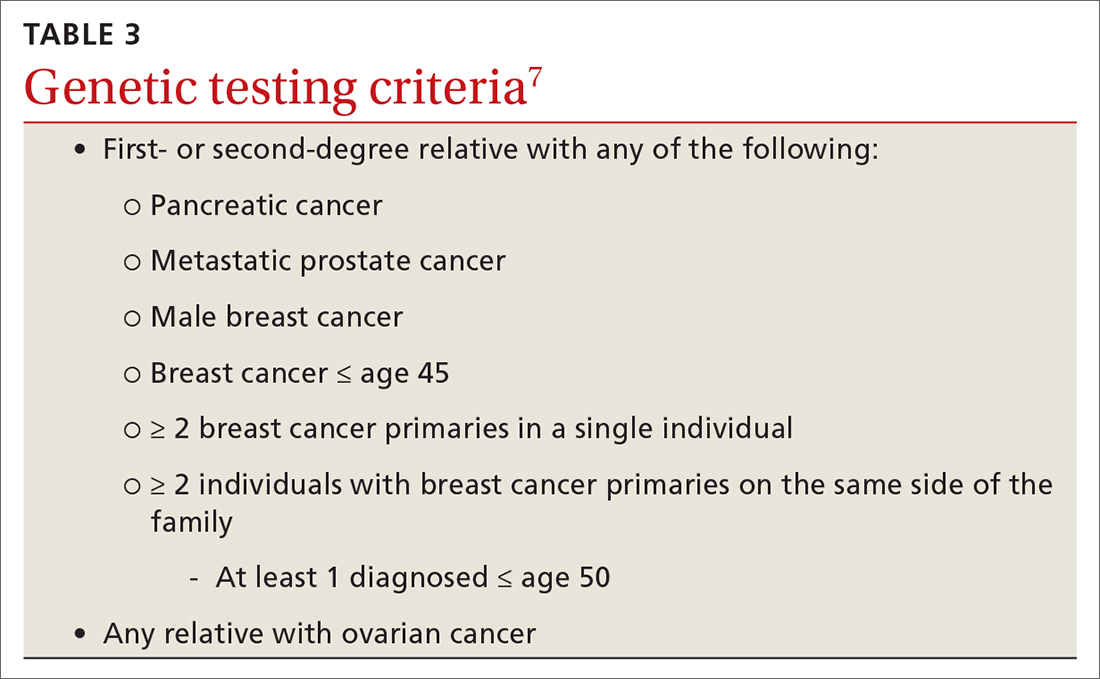
The National Comprehensive Cancer Network (NCCN) guidelines recommend genetic testing if a woman has a first- or second-degree relative with pancreatic cancer, metastatic prostate cancer, male breast cancer, breast cancer at age 45 or younger, 2 or more breast cancers in a single person, 2 or more people on the same side of the family with at least 1 diagnosed at age 50 or younger, or any relative with ovarian cancer (see TABLE 3).7 Before ordering genetic testing, it is useful to refer the patient to a genetic counselor for a thorough discussion of options.
Results of genetic testing may include high-risk variants, moderate-risk variants, and variants of unknown significance (VUS), or be negative for any variants. High-risk variants for breast cancer include BRCA1, BRCA2, PALB2, and cancer syndrome variants such as TP53, PTEN, STK11, and CDH1.5,6,9,13-15 These high-risk variants confer sufficient risk that women with these mutations are automatically categorized in the high-risk group. It is estimated that high-risk variants account for only 25% of the genetic risk for breast cancer.16
BRCA1/2 and PTEN mutations confer greater than 80% lifetime risk, while other high-risk variants such as TP53, CDH1, and STK11 confer risks between 25% and 40%. These variants are also associated with cancers of other organs, depending on the mutation.17
Moderate-risk variants—ATM and CHEK2—do not confer sufficient risk to elevate women into the high-risk group. However, they do qualify these intermediate-risk women to participate in a specialized management strategy.5,9,13,18
VUS are those for which the associated risk is unclear, but more research may be done to categorize the risk.9 The clinical management of women with VUS usually entails close monitoring.
In an effort to better characterize breast cancer risk using a combination of pathogenic variants found in broad multi-gene cancer predisposition panels, researchers have developed a method to combine risks in a “polygenic risk score” (PRS) that can be used to counsel women (see “What is a polygenic risk score for breast cancer?” on page 203).19-21PRS predicts an additional 18% of genetic risk in women of European descent.21
SIDEBAR
What is a polygenic risk score for breast cancer?
- A polygenic risk score (PRS) is a mathematical method to combine results from a variety of different single nucleotide polymorphisms (SNPs; ie, single base pair variants) into a prediction tool that can estimate a woman’s lifetime risk of breast cancer.
- A PRS may be most accurate in determining risk for women with intermediate pathogenic variants, such as ATM and CHEK2. 19,20
- PRS has not been studied in non-White women.21
Continue to: CASE
CASE
Using the assessment results, the clinician talks to Ms. P about her lifetime risk for breast cancer. The Gail model indicates her lifetime risk is 13.3%, just slightly higher than the average (12.5%), and her 5-year risk is 0.5% (average, 0.4%). The IBIS or Tyrer-Cuzick model, which takes into account her second-degree relatives with breast and ovarian cancer and her Ashkenazi ethnicity (which confers increased risk due to elevated risk of BRCA mutations), predicts her lifetime risk of breast cancer to be 20.4%. This categorizes Ms. P as high risk.
Enhanced screening recommendations for women at high risk
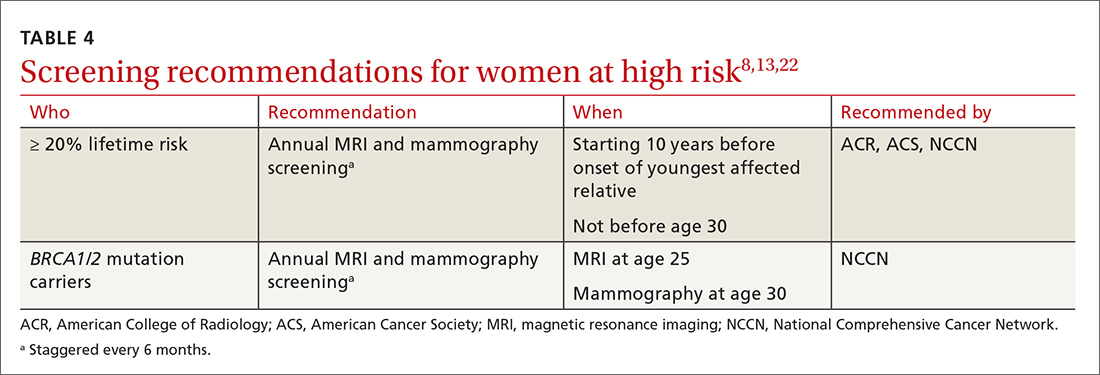
TABLE 48,13,22 summarizes screening recommendations for women deemed to be at high risk for breast cancer. The American Cancer Society (ACS), NCCN, and the American College of Radiology (ACR) recommend that women with at least a 20% lifetime risk have yearly magnetic resonance imaging (MRI) and mammography (staggered so that the patient has 1 test every 6 months) starting 10 years before the age of onset for the youngest affected relative but not before age 30.8 For carriers of high-risk (as well as intermediate-risk) genes, NCCN recommends annual MRI screening starting at age 40.13BRCA1/2 screening includes annual MRI starting at age 25 and annual mammography every 6 months starting at age 30.22 Clinicians should counsel women with moderate risk factors (elevated breast density; personal history of ADH, LCIS, or DCIS) about the potential risks and benefits of enhanced screening and chemoprophylaxis.
Risk-reduction strategies
Chemoprophylaxis
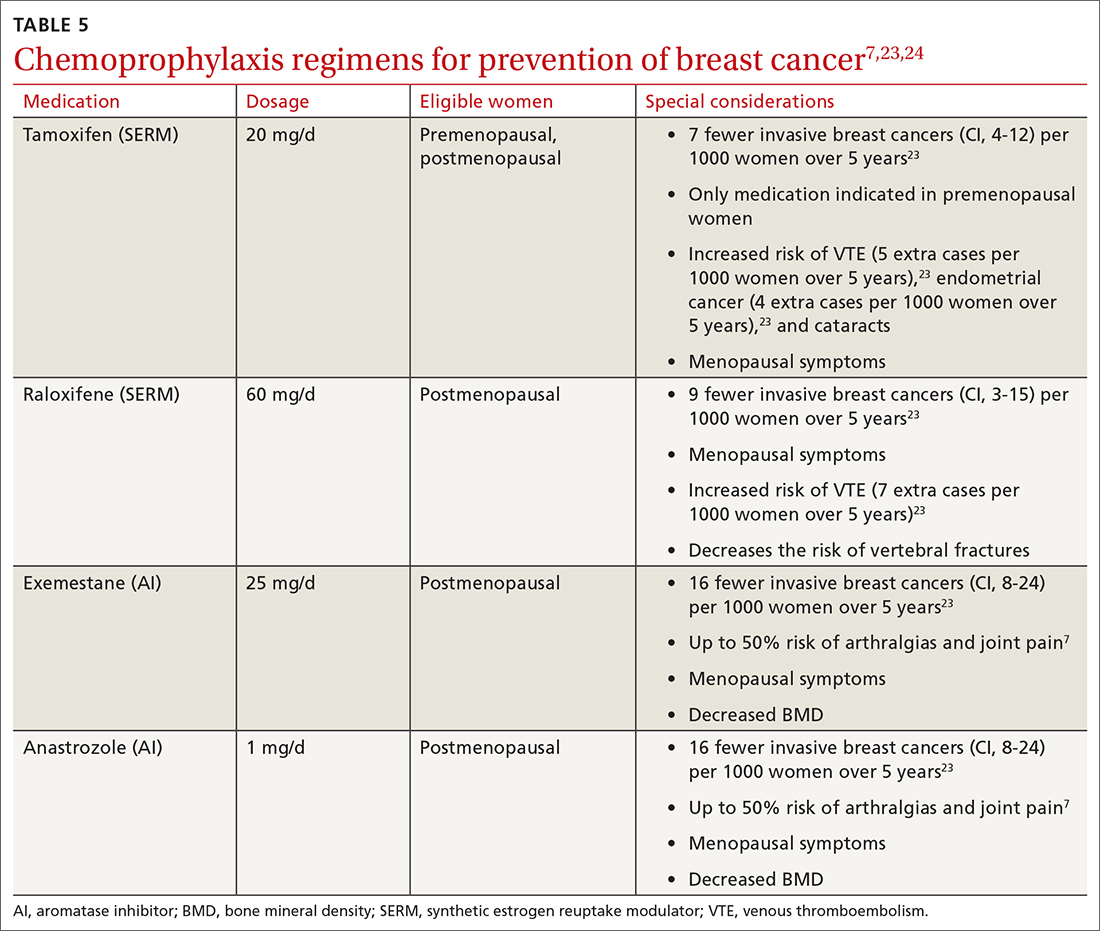
The US Preventive Services Task Force (USPSTF) recommends that all women at increased risk for breast cancer consider chemoprophylaxis (B recommendation)23 based on convincing evidence that 5 years of treatment with either a synthetic estrogen reuptake modulator (SERM) or an aromatase inhibitor (AI) decreases the incidence of estrogen receptor positive breast cancers. (See TABLE 57,23,24 for absolute risk reduction.) There is no benefit for chemoprophylaxis in women at average risk (D recommendation).23 It is unclear whether chemoprophylaxis is indicated in women with moderate increased risk (ie, who do not meet the 20% lifetime risk criteria). Chemoprophylaxis may not be effective in women with BRCA1 mutations, as they often develop triple-negative breast cancers.
Accurate risk assessment and shared decision-making enable the clinician and patient to discuss the potential risks and benefits of chemoprophylaxis.7,24 The USPSTF did not find that any 1 risk prediction tool was better than another to identify women who should be counseled about chemoprophylaxis. Clinicians should counsel all women taking AIs about optimizing bone health with adequate calcium and vitamin D intake and routine bone density tests.
Surgical risk reduction
The NCCN guidelines state that risk-reducing bilateral mastectomy is reserved for individuals with high-risk gene variants and individuals with prior chest radiation between ages 10 and 30.25 NCCN also recommends discussing risk-reducing mastectomy with all women with BRCA mutations.22
Bilateral mastectomy is the most effective method to reduce breast cancer risk and should be discussed after age 25 in women with BRCA mutations and at least 8 years after chest radiation is completed.26 There is a reduction in breast cancer incidence of 90%.25 Breast imaging for screening (mammography or MRI) is not indicated after risk-reducing mastectomy. However, clinical breast examinations of the surgical site are important, because there is a small risk of developing breast cancer in that area.26
Risk-reducing oophorectomy is the standard of care for women with BRCA mutations to reduce the risk of ovarian cancer. It can also reduce the risk of breast cancer in women with BRCA mutations.27
Continue to: CASE
CASE
Based on her risk assessment results, family history, and genetic heritage, Ms. P qualifies for referral to a genetic counselor for discussion of BRCA testing. The clinician discusses adding annual MRI to Ms. P’s breast cancer screening regimen, based on ACS, NCCN, and ACR recommendations, due to her 20.4% lifetime risk. Discussion of whether and when to start chemoprophylaxis is typically based on breast cancer risk, projected benefit, and the potential impact of medication adverse effects. A high-risk woman is eligible for 5 years of chemoprophylaxis (tamoxifen if premenopausal) based on her lifetime risk. The clinician discusses timing with Ms. P, and even though she is finished with childbearing, she would like to wait until she is age 45, which is before the age at which her aunt was given a diagnosis of breast cancer.
Conclusion
Primary care clinicians are well positioned to identify women with an elevated risk of breast cancer and refer them for enhanced screening and chemoprophylaxis (see ALGORITHM). Shared decision-making with the inclusion of patient decision aids (https://decisionaid.ohri.ca/AZsearch.php?criteria=breast+cancer) about genetic testing, chemoprophylaxis, and prophylactic mastectomy or oophorectomy may help women at intermediate or high risk of breast cancer feel empowered to make decisions about their breast—and overall—health.
CORRESPONDENCE
Sarina Schrager, MD, MS, Professor, Department of Family Medicine and Community Health, University of Wisconsin, 1100 Delaplaine Court, Madison, WI 53715; [email protected]
1. National Cancer Institute. Cancer stat facts: female breast cancer. Accessed May 13, 2022. https://seer.cancer.gov/statfacts/html/breast.html
2. Guerra CE, Sherman M, Armstrong K. Diffusion of breast cancer risk assessment in primary care. J Am Board Fam Med. 2009;22:272-279. doi:10.3122/jabfm.2009.03.080153
3. Hamilton JG, Abdiwahab E, Edwards HM, et al. Primary care providers’ cancer genetic testing-related knowledge, attitudes, and communication behaviors: a systematic review and research agenda. J Gen Intern Med. 2017;32:315-324. doi:10.1007/s11606-016-3943-4
4. Eden KB, Ivlev I, Bensching KL, et al. Use of an online breast cancer risk assessment and patient decision aid in primary care practices. J Womens Health (Larchmt). 2020;29:763-769. doi: 10.1089/jwh.2019.8143
5. Kleibl Z, Kristensen VN. Women at high risk of breast cancer: molecular characteristics, clinical presentation and management. Breast. 2016;28:136-44. doi: 10.1016/j.breast.2016.05.006
6. Sciaraffa T, Guido B, Khan SA, et al. Breast cancer risk assessment and management programs: a practical guide. Breast J. 2020;26:1556-1564. doi: 10.1111/tbj.13967
7. Farkas A, Vanderberg R, Merriam S, et al. Breast cancer chemoprevention: a practical guide for the primary care provider. J Womens Health (Larchmt). 2020;29:46-56. doi: 10.1089/jwh.2018.7643
8. McClintock AH, Golob AL, Laya MB. Breast cancer risk assessment: a step-wise approach for primary care providers on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275. doi: 10.1016/j.mayocp.2020.04.017
9. Catana A, Apostu AP, Antemie RG. Multi gene panel testing for hereditary breast cancer - is it ready to be used? Med Pharm Rep. 2019;92:220-225. doi: 10.15386/mpr-1083
10. Barke LD, Freivogel ME. Breast cancer risk assessment models and high-risk screening. Radiol Clin North Am. 2017;55:457-474. doi: 10.1016/j.rcl.2016.12.013
11. Amir E, Freedman OC, Seruga B, et al. Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010;102:680-91. doi: 10.1093/jnci/djq088
12. Kim G, Bahl M. Assessing risk of breast cancer: a review of risk prediction models. J Breast Imaging. 2021;3:144-155. doi: 10.1093/jbi/wbab001
13. Narod SA. Which genes for hereditary breast cancer? N Engl J Med. 2021;384:471-473. doi: 10.1056/NEJMe2035083
14. Couch FJ, Shimelis H, Hu C, et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017;3:1190-1196. doi: 10.1001/jamaoncol.2017.0424
15. Obeid EI, Hall MJ, Daly MB. Multigene panel testing and breast cancer risk: is it time to scale down? JAMA Oncol. 2017;3:1176-1177. doi: 10.1001/jamaoncol.2017.0342
16. Michailidou K, Lindström S, Dennis J, et al. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551:92-94. doi: 10.1038/nature24284
17. Shiovitz S, Korde LA. Genetics of breast cancer: a topic in evolution. Ann Oncol. 2015;26:1291-1299. doi: 10.1093/annonc/mdv022
18. Hu C, Hart SN, Gnanaolivu R, et al. A population-based study of genes previously implicated in breast cancer. N Engl J Med. 2021;384:440-451. doi: 10.1056/NEJMoa2005936
19. Gao C, Polley EC, Hart SN, et al. Risk of breast cancer among carriers of pathogenic variants in breast cancer predisposition genes varies by polygenic risk score. J Clin Oncol. 2021;39:2564-2573. doi: 10.1200/JCO.20.01992
20. Gallagher S, Hughes E, Wagner S, et al. Association of a polygenic risk score with breast cancer among women carriers of high- and moderate-risk breast cancer genes. JAMA Netw Open. 2020;3:e208501. doi: 10.1001/jamanetworkopen.2020.8501
21. Yanes T, Young MA, Meiser B, et al. Clinical applications of polygenic breast cancer risk: a critical review and perspectives of an emerging field. Breast Cancer Res. 2020;22:21. doi: 10.1186/s13058-020-01260-3
22. Schrager S, Torell E, Ledford K, et al. Managing a woman with BRCA mutations? Shared decision-making is key. J Fam Pract. 2020;69:237-243
23. US Preventive Services Task Force; Owens DK, Davidson KW, Krist AH, et al. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:857-867. doi: 10.1001/jama.2019.11885
24. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol 2015;22:3230-3235. doi: 10.1245/s10434-015-4715-9
25. Britt KL, Cuzick J, Phillips KA. Key steps for effective breast cancer prevention. Nat Rev Cancer. 2020;20:417-436. doi: 10.1038/s41568-020-0266-x
26. Jatoi I, Kemp Z. Risk-reducing mastectomy. JAMA. 2021;325:1781-1782. doi: 10.1001/jama.2020.22414
27. Choi Y, Terry MB, Daly MB, et al. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7:585-592. doi:10.1001/jamaoncol.2020.7995
Breast cancer is the most common invasive cancer in women in the United States; it is estimated that there will be 287,850 new cases of breast cancer in the United States during 2022 with 43,250 deaths.1 Lives are extended and saved every day because of a robust arsenal of treatments and interventions available to those who have been given a diagnosis of breast cancer. And, of course, lives are also extended and saved when we identify women at risk and provide early interventions. But in busy offices where time is short and there are competing demands on our time, proper assessment of a woman’s risk of breast cancer does not always happen. As a result, women with a higher risk of breast cancer may not be getting appropriate management.2,3
Familiarizing yourself with several risk-assessment tools and knowing when genetic testing is needed can make a big difference. Knowing the timing of mammograms and magnetic resonance imaging (MRI) for women deemed to be at high risk is also key. The following review employs a case-based approach (with an accompanying ALGORITHM) to illustrate how best to identify women who are at heightened risk of breast cancer and maximize their care. We also discuss the chemoprophylaxis regimens that may be used for those at increased risk.

CASE
Rachel P, age 37, presents to establish care. She has an Ashkenazi Jewish background and wonders if she should start doing breast cancer screening before age 40. She has 2 children, ages 4 years and 2 years. Her maternal aunt had unilateral breast cancer at age 54, and her maternal grandmother died of ovarian cancer at age 65.
Risk assessment
The risk assessment process (see ALGORITHM) must start with either the clinician or the patient initiating the discussion about breast cancer risk. The clinician may initiate the discussion with a new patient or at an annual physical examination. The patient may start the discussion because they are experiencing new breast symptoms, have anxiety about developing breast cancer, or have a family member with a new cancer diagnosis.
Risk factors. There are single factors that convey enough risk to automatically designate the patient as high risk (see TABLE 14-9). These factors include having a history of chest radiation between the ages of 10 and 30, a history of breast biopsy with either lobular carcinoma in situ (LCIS) or atypical ductal hyperplasia (ADH), past breast and/or ovarian cancer, and either a family or personal history of a high penetrant genetic variant for breast cancer.4-9

In women with previous chest radiation, breast cancer risk correlates with the total dose of radiation.5 For women with a personal history of breast cancer, the younger the age at diagnosis, the higher the risk of contralateral breast cancer.5 Precancerous changes such as ADH, LCIS, and ductal carcinoma in situ (DCIS) also confer moderate increases in risk. Women with these diagnoses will commonly have follow-up with specialists.
Risk assessment tools. There are several models available to assess a woman’s breast cancer risk (see TABLE 210-12). The Gail model (https://bcrisktool.cancer.gov/) is the oldest, quickest, and most widely known. However, the Gail model only accounts for first-degree relatives diagnosed with breast cancer, may underpredict risk in women with a more extensive family history, and has not been studied in women younger than 35. The International Breast Cancer Intervention Study (IBIS) Risk Evaluation Tool (https://ibis-risk-calculator.magview.com/), commonly referred to as the Tyrer-Cuzick model, incorporates second-degree relatives into the prediction model—although women may not know their full family history. Both the IBIS and the Breast Cancer Surveillance Consortium (BCSC) model (https://tools.bcsc-scc.org/BC5yearRisk/intro.htm) include breast density in the prediction algorithm. The choice of tool depends on clinician comfort and individual patient risk factors. There is no evidence that one model is better than another.10-12

Continue to: CASE
CASE
Ms. P’s clinician starts with an assessment using the Gail model. However, when the result comes back with average risk, the clinician decides to follow up with the Tyrer-Cuzick model in order to incorporate Ms. P’s multiple second-degree relatives with breast and ovarian cancer. (The BCSC model was not used because it only includes first-degree relatives.)
Genetic testing
The National Comprehensive Cancer Network (NCCN) guidelines recommend genetic testing if a woman has a first- or second-degree relative with pancreatic cancer, metastatic prostate cancer, male breast cancer, breast cancer at age 45 or younger, 2 or more breast cancers in a single person, 2 or more people on the same side of the family with at least 1 diagnosed at age 50 or younger, or any relative with ovarian cancer (see TABLE 3).7 Before ordering genetic testing, it is useful to refer the patient to a genetic counselor for a thorough discussion of options.

Results of genetic testing may include high-risk variants, moderate-risk variants, and variants of unknown significance (VUS), or be negative for any variants. High-risk variants for breast cancer include BRCA1, BRCA2, PALB2, and cancer syndrome variants such as TP53, PTEN, STK11, and CDH1.5,6,9,13-15 These high-risk variants confer sufficient risk that women with these mutations are automatically categorized in the high-risk group. It is estimated that high-risk variants account for only 25% of the genetic risk for breast cancer.16
BRCA1/2 and PTEN mutations confer greater than 80% lifetime risk, while other high-risk variants such as TP53, CDH1, and STK11 confer risks between 25% and 40%. These variants are also associated with cancers of other organs, depending on the mutation.17
Moderate-risk variants—ATM and CHEK2—do not confer sufficient risk to elevate women into the high-risk group. However, they do qualify these intermediate-risk women to participate in a specialized management strategy.5,9,13,18
VUS are those for which the associated risk is unclear, but more research may be done to categorize the risk.9 The clinical management of women with VUS usually entails close monitoring.
In an effort to better characterize breast cancer risk using a combination of pathogenic variants found in broad multi-gene cancer predisposition panels, researchers have developed a method to combine risks in a “polygenic risk score” (PRS) that can be used to counsel women (see “What is a polygenic risk score for breast cancer?” on page 203).19-21PRS predicts an additional 18% of genetic risk in women of European descent.21
SIDEBAR
What is a polygenic risk score for breast cancer?
- A polygenic risk score (PRS) is a mathematical method to combine results from a variety of different single nucleotide polymorphisms (SNPs; ie, single base pair variants) into a prediction tool that can estimate a woman’s lifetime risk of breast cancer.
- A PRS may be most accurate in determining risk for women with intermediate pathogenic variants, such as ATM and CHEK2. 19,20
- PRS has not been studied in non-White women.21
Continue to: CASE
CASE
Using the assessment results, the clinician talks to Ms. P about her lifetime risk for breast cancer. The Gail model indicates her lifetime risk is 13.3%, just slightly higher than the average (12.5%), and her 5-year risk is 0.5% (average, 0.4%). The IBIS or Tyrer-Cuzick model, which takes into account her second-degree relatives with breast and ovarian cancer and her Ashkenazi ethnicity (which confers increased risk due to elevated risk of BRCA mutations), predicts her lifetime risk of breast cancer to be 20.4%. This categorizes Ms. P as high risk.
Enhanced screening recommendations for women at high risk
TABLE 48,13,22 summarizes screening recommendations for women deemed to be at high risk for breast cancer. The American Cancer Society (ACS), NCCN, and the American College of Radiology (ACR) recommend that women with at least a 20% lifetime risk have yearly magnetic resonance imaging (MRI) and mammography (staggered so that the patient has 1 test every 6 months) starting 10 years before the age of onset for the youngest affected relative but not before age 30.8 For carriers of high-risk (as well as intermediate-risk) genes, NCCN recommends annual MRI screening starting at age 40.13BRCA1/2 screening includes annual MRI starting at age 25 and annual mammography every 6 months starting at age 30.22 Clinicians should counsel women with moderate risk factors (elevated breast density; personal history of ADH, LCIS, or DCIS) about the potential risks and benefits of enhanced screening and chemoprophylaxis.

Risk-reduction strategies
Chemoprophylaxis
The US Preventive Services Task Force (USPSTF) recommends that all women at increased risk for breast cancer consider chemoprophylaxis (B recommendation)23 based on convincing evidence that 5 years of treatment with either a synthetic estrogen reuptake modulator (SERM) or an aromatase inhibitor (AI) decreases the incidence of estrogen receptor positive breast cancers. (See TABLE 57,23,24 for absolute risk reduction.) There is no benefit for chemoprophylaxis in women at average risk (D recommendation).23 It is unclear whether chemoprophylaxis is indicated in women with moderate increased risk (ie, who do not meet the 20% lifetime risk criteria). Chemoprophylaxis may not be effective in women with BRCA1 mutations, as they often develop triple-negative breast cancers.

Accurate risk assessment and shared decision-making enable the clinician and patient to discuss the potential risks and benefits of chemoprophylaxis.7,24 The USPSTF did not find that any 1 risk prediction tool was better than another to identify women who should be counseled about chemoprophylaxis. Clinicians should counsel all women taking AIs about optimizing bone health with adequate calcium and vitamin D intake and routine bone density tests.
Surgical risk reduction
The NCCN guidelines state that risk-reducing bilateral mastectomy is reserved for individuals with high-risk gene variants and individuals with prior chest radiation between ages 10 and 30.25 NCCN also recommends discussing risk-reducing mastectomy with all women with BRCA mutations.22
Bilateral mastectomy is the most effective method to reduce breast cancer risk and should be discussed after age 25 in women with BRCA mutations and at least 8 years after chest radiation is completed.26 There is a reduction in breast cancer incidence of 90%.25 Breast imaging for screening (mammography or MRI) is not indicated after risk-reducing mastectomy. However, clinical breast examinations of the surgical site are important, because there is a small risk of developing breast cancer in that area.26
Risk-reducing oophorectomy is the standard of care for women with BRCA mutations to reduce the risk of ovarian cancer. It can also reduce the risk of breast cancer in women with BRCA mutations.27
Continue to: CASE
CASE
Based on her risk assessment results, family history, and genetic heritage, Ms. P qualifies for referral to a genetic counselor for discussion of BRCA testing. The clinician discusses adding annual MRI to Ms. P’s breast cancer screening regimen, based on ACS, NCCN, and ACR recommendations, due to her 20.4% lifetime risk. Discussion of whether and when to start chemoprophylaxis is typically based on breast cancer risk, projected benefit, and the potential impact of medication adverse effects. A high-risk woman is eligible for 5 years of chemoprophylaxis (tamoxifen if premenopausal) based on her lifetime risk. The clinician discusses timing with Ms. P, and even though she is finished with childbearing, she would like to wait until she is age 45, which is before the age at which her aunt was given a diagnosis of breast cancer.
Conclusion
Primary care clinicians are well positioned to identify women with an elevated risk of breast cancer and refer them for enhanced screening and chemoprophylaxis (see ALGORITHM). Shared decision-making with the inclusion of patient decision aids (https://decisionaid.ohri.ca/AZsearch.php?criteria=breast+cancer) about genetic testing, chemoprophylaxis, and prophylactic mastectomy or oophorectomy may help women at intermediate or high risk of breast cancer feel empowered to make decisions about their breast—and overall—health.
CORRESPONDENCE
Sarina Schrager, MD, MS, Professor, Department of Family Medicine and Community Health, University of Wisconsin, 1100 Delaplaine Court, Madison, WI 53715; [email protected]
Breast cancer is the most common invasive cancer in women in the United States; it is estimated that there will be 287,850 new cases of breast cancer in the United States during 2022 with 43,250 deaths.1 Lives are extended and saved every day because of a robust arsenal of treatments and interventions available to those who have been given a diagnosis of breast cancer. And, of course, lives are also extended and saved when we identify women at risk and provide early interventions. But in busy offices where time is short and there are competing demands on our time, proper assessment of a woman’s risk of breast cancer does not always happen. As a result, women with a higher risk of breast cancer may not be getting appropriate management.2,3
Familiarizing yourself with several risk-assessment tools and knowing when genetic testing is needed can make a big difference. Knowing the timing of mammograms and magnetic resonance imaging (MRI) for women deemed to be at high risk is also key. The following review employs a case-based approach (with an accompanying ALGORITHM) to illustrate how best to identify women who are at heightened risk of breast cancer and maximize their care. We also discuss the chemoprophylaxis regimens that may be used for those at increased risk.

CASE
Rachel P, age 37, presents to establish care. She has an Ashkenazi Jewish background and wonders if she should start doing breast cancer screening before age 40. She has 2 children, ages 4 years and 2 years. Her maternal aunt had unilateral breast cancer at age 54, and her maternal grandmother died of ovarian cancer at age 65.
Risk assessment
The risk assessment process (see ALGORITHM) must start with either the clinician or the patient initiating the discussion about breast cancer risk. The clinician may initiate the discussion with a new patient or at an annual physical examination. The patient may start the discussion because they are experiencing new breast symptoms, have anxiety about developing breast cancer, or have a family member with a new cancer diagnosis.
Risk factors. There are single factors that convey enough risk to automatically designate the patient as high risk (see TABLE 14-9). These factors include having a history of chest radiation between the ages of 10 and 30, a history of breast biopsy with either lobular carcinoma in situ (LCIS) or atypical ductal hyperplasia (ADH), past breast and/or ovarian cancer, and either a family or personal history of a high penetrant genetic variant for breast cancer.4-9

In women with previous chest radiation, breast cancer risk correlates with the total dose of radiation.5 For women with a personal history of breast cancer, the younger the age at diagnosis, the higher the risk of contralateral breast cancer.5 Precancerous changes such as ADH, LCIS, and ductal carcinoma in situ (DCIS) also confer moderate increases in risk. Women with these diagnoses will commonly have follow-up with specialists.
Risk assessment tools. There are several models available to assess a woman’s breast cancer risk (see TABLE 210-12). The Gail model (https://bcrisktool.cancer.gov/) is the oldest, quickest, and most widely known. However, the Gail model only accounts for first-degree relatives diagnosed with breast cancer, may underpredict risk in women with a more extensive family history, and has not been studied in women younger than 35. The International Breast Cancer Intervention Study (IBIS) Risk Evaluation Tool (https://ibis-risk-calculator.magview.com/), commonly referred to as the Tyrer-Cuzick model, incorporates second-degree relatives into the prediction model—although women may not know their full family history. Both the IBIS and the Breast Cancer Surveillance Consortium (BCSC) model (https://tools.bcsc-scc.org/BC5yearRisk/intro.htm) include breast density in the prediction algorithm. The choice of tool depends on clinician comfort and individual patient risk factors. There is no evidence that one model is better than another.10-12

Continue to: CASE
CASE
Ms. P’s clinician starts with an assessment using the Gail model. However, when the result comes back with average risk, the clinician decides to follow up with the Tyrer-Cuzick model in order to incorporate Ms. P’s multiple second-degree relatives with breast and ovarian cancer. (The BCSC model was not used because it only includes first-degree relatives.)
Genetic testing
The National Comprehensive Cancer Network (NCCN) guidelines recommend genetic testing if a woman has a first- or second-degree relative with pancreatic cancer, metastatic prostate cancer, male breast cancer, breast cancer at age 45 or younger, 2 or more breast cancers in a single person, 2 or more people on the same side of the family with at least 1 diagnosed at age 50 or younger, or any relative with ovarian cancer (see TABLE 3).7 Before ordering genetic testing, it is useful to refer the patient to a genetic counselor for a thorough discussion of options.

Results of genetic testing may include high-risk variants, moderate-risk variants, and variants of unknown significance (VUS), or be negative for any variants. High-risk variants for breast cancer include BRCA1, BRCA2, PALB2, and cancer syndrome variants such as TP53, PTEN, STK11, and CDH1.5,6,9,13-15 These high-risk variants confer sufficient risk that women with these mutations are automatically categorized in the high-risk group. It is estimated that high-risk variants account for only 25% of the genetic risk for breast cancer.16
BRCA1/2 and PTEN mutations confer greater than 80% lifetime risk, while other high-risk variants such as TP53, CDH1, and STK11 confer risks between 25% and 40%. These variants are also associated with cancers of other organs, depending on the mutation.17
Moderate-risk variants—ATM and CHEK2—do not confer sufficient risk to elevate women into the high-risk group. However, they do qualify these intermediate-risk women to participate in a specialized management strategy.5,9,13,18
VUS are those for which the associated risk is unclear, but more research may be done to categorize the risk.9 The clinical management of women with VUS usually entails close monitoring.
In an effort to better characterize breast cancer risk using a combination of pathogenic variants found in broad multi-gene cancer predisposition panels, researchers have developed a method to combine risks in a “polygenic risk score” (PRS) that can be used to counsel women (see “What is a polygenic risk score for breast cancer?” on page 203).19-21PRS predicts an additional 18% of genetic risk in women of European descent.21
SIDEBAR
What is a polygenic risk score for breast cancer?
- A polygenic risk score (PRS) is a mathematical method to combine results from a variety of different single nucleotide polymorphisms (SNPs; ie, single base pair variants) into a prediction tool that can estimate a woman’s lifetime risk of breast cancer.
- A PRS may be most accurate in determining risk for women with intermediate pathogenic variants, such as ATM and CHEK2. 19,20
- PRS has not been studied in non-White women.21
Continue to: CASE
CASE
Using the assessment results, the clinician talks to Ms. P about her lifetime risk for breast cancer. The Gail model indicates her lifetime risk is 13.3%, just slightly higher than the average (12.5%), and her 5-year risk is 0.5% (average, 0.4%). The IBIS or Tyrer-Cuzick model, which takes into account her second-degree relatives with breast and ovarian cancer and her Ashkenazi ethnicity (which confers increased risk due to elevated risk of BRCA mutations), predicts her lifetime risk of breast cancer to be 20.4%. This categorizes Ms. P as high risk.
Enhanced screening recommendations for women at high risk
TABLE 48,13,22 summarizes screening recommendations for women deemed to be at high risk for breast cancer. The American Cancer Society (ACS), NCCN, and the American College of Radiology (ACR) recommend that women with at least a 20% lifetime risk have yearly magnetic resonance imaging (MRI) and mammography (staggered so that the patient has 1 test every 6 months) starting 10 years before the age of onset for the youngest affected relative but not before age 30.8 For carriers of high-risk (as well as intermediate-risk) genes, NCCN recommends annual MRI screening starting at age 40.13BRCA1/2 screening includes annual MRI starting at age 25 and annual mammography every 6 months starting at age 30.22 Clinicians should counsel women with moderate risk factors (elevated breast density; personal history of ADH, LCIS, or DCIS) about the potential risks and benefits of enhanced screening and chemoprophylaxis.

Risk-reduction strategies
Chemoprophylaxis
The US Preventive Services Task Force (USPSTF) recommends that all women at increased risk for breast cancer consider chemoprophylaxis (B recommendation)23 based on convincing evidence that 5 years of treatment with either a synthetic estrogen reuptake modulator (SERM) or an aromatase inhibitor (AI) decreases the incidence of estrogen receptor positive breast cancers. (See TABLE 57,23,24 for absolute risk reduction.) There is no benefit for chemoprophylaxis in women at average risk (D recommendation).23 It is unclear whether chemoprophylaxis is indicated in women with moderate increased risk (ie, who do not meet the 20% lifetime risk criteria). Chemoprophylaxis may not be effective in women with BRCA1 mutations, as they often develop triple-negative breast cancers.

Accurate risk assessment and shared decision-making enable the clinician and patient to discuss the potential risks and benefits of chemoprophylaxis.7,24 The USPSTF did not find that any 1 risk prediction tool was better than another to identify women who should be counseled about chemoprophylaxis. Clinicians should counsel all women taking AIs about optimizing bone health with adequate calcium and vitamin D intake and routine bone density tests.
Surgical risk reduction
The NCCN guidelines state that risk-reducing bilateral mastectomy is reserved for individuals with high-risk gene variants and individuals with prior chest radiation between ages 10 and 30.25 NCCN also recommends discussing risk-reducing mastectomy with all women with BRCA mutations.22
Bilateral mastectomy is the most effective method to reduce breast cancer risk and should be discussed after age 25 in women with BRCA mutations and at least 8 years after chest radiation is completed.26 There is a reduction in breast cancer incidence of 90%.25 Breast imaging for screening (mammography or MRI) is not indicated after risk-reducing mastectomy. However, clinical breast examinations of the surgical site are important, because there is a small risk of developing breast cancer in that area.26
Risk-reducing oophorectomy is the standard of care for women with BRCA mutations to reduce the risk of ovarian cancer. It can also reduce the risk of breast cancer in women with BRCA mutations.27
Continue to: CASE
CASE
Based on her risk assessment results, family history, and genetic heritage, Ms. P qualifies for referral to a genetic counselor for discussion of BRCA testing. The clinician discusses adding annual MRI to Ms. P’s breast cancer screening regimen, based on ACS, NCCN, and ACR recommendations, due to her 20.4% lifetime risk. Discussion of whether and when to start chemoprophylaxis is typically based on breast cancer risk, projected benefit, and the potential impact of medication adverse effects. A high-risk woman is eligible for 5 years of chemoprophylaxis (tamoxifen if premenopausal) based on her lifetime risk. The clinician discusses timing with Ms. P, and even though she is finished with childbearing, she would like to wait until she is age 45, which is before the age at which her aunt was given a diagnosis of breast cancer.
Conclusion
Primary care clinicians are well positioned to identify women with an elevated risk of breast cancer and refer them for enhanced screening and chemoprophylaxis (see ALGORITHM). Shared decision-making with the inclusion of patient decision aids (https://decisionaid.ohri.ca/AZsearch.php?criteria=breast+cancer) about genetic testing, chemoprophylaxis, and prophylactic mastectomy or oophorectomy may help women at intermediate or high risk of breast cancer feel empowered to make decisions about their breast—and overall—health.
CORRESPONDENCE
Sarina Schrager, MD, MS, Professor, Department of Family Medicine and Community Health, University of Wisconsin, 1100 Delaplaine Court, Madison, WI 53715; [email protected]
1. National Cancer Institute. Cancer stat facts: female breast cancer. Accessed May 13, 2022. https://seer.cancer.gov/statfacts/html/breast.html
2. Guerra CE, Sherman M, Armstrong K. Diffusion of breast cancer risk assessment in primary care. J Am Board Fam Med. 2009;22:272-279. doi:10.3122/jabfm.2009.03.080153
3. Hamilton JG, Abdiwahab E, Edwards HM, et al. Primary care providers’ cancer genetic testing-related knowledge, attitudes, and communication behaviors: a systematic review and research agenda. J Gen Intern Med. 2017;32:315-324. doi:10.1007/s11606-016-3943-4
4. Eden KB, Ivlev I, Bensching KL, et al. Use of an online breast cancer risk assessment and patient decision aid in primary care practices. J Womens Health (Larchmt). 2020;29:763-769. doi: 10.1089/jwh.2019.8143
5. Kleibl Z, Kristensen VN. Women at high risk of breast cancer: molecular characteristics, clinical presentation and management. Breast. 2016;28:136-44. doi: 10.1016/j.breast.2016.05.006
6. Sciaraffa T, Guido B, Khan SA, et al. Breast cancer risk assessment and management programs: a practical guide. Breast J. 2020;26:1556-1564. doi: 10.1111/tbj.13967
7. Farkas A, Vanderberg R, Merriam S, et al. Breast cancer chemoprevention: a practical guide for the primary care provider. J Womens Health (Larchmt). 2020;29:46-56. doi: 10.1089/jwh.2018.7643
8. McClintock AH, Golob AL, Laya MB. Breast cancer risk assessment: a step-wise approach for primary care providers on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275. doi: 10.1016/j.mayocp.2020.04.017
9. Catana A, Apostu AP, Antemie RG. Multi gene panel testing for hereditary breast cancer - is it ready to be used? Med Pharm Rep. 2019;92:220-225. doi: 10.15386/mpr-1083
10. Barke LD, Freivogel ME. Breast cancer risk assessment models and high-risk screening. Radiol Clin North Am. 2017;55:457-474. doi: 10.1016/j.rcl.2016.12.013
11. Amir E, Freedman OC, Seruga B, et al. Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010;102:680-91. doi: 10.1093/jnci/djq088
12. Kim G, Bahl M. Assessing risk of breast cancer: a review of risk prediction models. J Breast Imaging. 2021;3:144-155. doi: 10.1093/jbi/wbab001
13. Narod SA. Which genes for hereditary breast cancer? N Engl J Med. 2021;384:471-473. doi: 10.1056/NEJMe2035083
14. Couch FJ, Shimelis H, Hu C, et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017;3:1190-1196. doi: 10.1001/jamaoncol.2017.0424
15. Obeid EI, Hall MJ, Daly MB. Multigene panel testing and breast cancer risk: is it time to scale down? JAMA Oncol. 2017;3:1176-1177. doi: 10.1001/jamaoncol.2017.0342
16. Michailidou K, Lindström S, Dennis J, et al. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551:92-94. doi: 10.1038/nature24284
17. Shiovitz S, Korde LA. Genetics of breast cancer: a topic in evolution. Ann Oncol. 2015;26:1291-1299. doi: 10.1093/annonc/mdv022
18. Hu C, Hart SN, Gnanaolivu R, et al. A population-based study of genes previously implicated in breast cancer. N Engl J Med. 2021;384:440-451. doi: 10.1056/NEJMoa2005936
19. Gao C, Polley EC, Hart SN, et al. Risk of breast cancer among carriers of pathogenic variants in breast cancer predisposition genes varies by polygenic risk score. J Clin Oncol. 2021;39:2564-2573. doi: 10.1200/JCO.20.01992
20. Gallagher S, Hughes E, Wagner S, et al. Association of a polygenic risk score with breast cancer among women carriers of high- and moderate-risk breast cancer genes. JAMA Netw Open. 2020;3:e208501. doi: 10.1001/jamanetworkopen.2020.8501
21. Yanes T, Young MA, Meiser B, et al. Clinical applications of polygenic breast cancer risk: a critical review and perspectives of an emerging field. Breast Cancer Res. 2020;22:21. doi: 10.1186/s13058-020-01260-3
22. Schrager S, Torell E, Ledford K, et al. Managing a woman with BRCA mutations? Shared decision-making is key. J Fam Pract. 2020;69:237-243
23. US Preventive Services Task Force; Owens DK, Davidson KW, Krist AH, et al. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:857-867. doi: 10.1001/jama.2019.11885
24. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol 2015;22:3230-3235. doi: 10.1245/s10434-015-4715-9
25. Britt KL, Cuzick J, Phillips KA. Key steps for effective breast cancer prevention. Nat Rev Cancer. 2020;20:417-436. doi: 10.1038/s41568-020-0266-x
26. Jatoi I, Kemp Z. Risk-reducing mastectomy. JAMA. 2021;325:1781-1782. doi: 10.1001/jama.2020.22414
27. Choi Y, Terry MB, Daly MB, et al. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7:585-592. doi:10.1001/jamaoncol.2020.7995
1. National Cancer Institute. Cancer stat facts: female breast cancer. Accessed May 13, 2022. https://seer.cancer.gov/statfacts/html/breast.html
2. Guerra CE, Sherman M, Armstrong K. Diffusion of breast cancer risk assessment in primary care. J Am Board Fam Med. 2009;22:272-279. doi:10.3122/jabfm.2009.03.080153
3. Hamilton JG, Abdiwahab E, Edwards HM, et al. Primary care providers’ cancer genetic testing-related knowledge, attitudes, and communication behaviors: a systematic review and research agenda. J Gen Intern Med. 2017;32:315-324. doi:10.1007/s11606-016-3943-4
4. Eden KB, Ivlev I, Bensching KL, et al. Use of an online breast cancer risk assessment and patient decision aid in primary care practices. J Womens Health (Larchmt). 2020;29:763-769. doi: 10.1089/jwh.2019.8143
5. Kleibl Z, Kristensen VN. Women at high risk of breast cancer: molecular characteristics, clinical presentation and management. Breast. 2016;28:136-44. doi: 10.1016/j.breast.2016.05.006
6. Sciaraffa T, Guido B, Khan SA, et al. Breast cancer risk assessment and management programs: a practical guide. Breast J. 2020;26:1556-1564. doi: 10.1111/tbj.13967
7. Farkas A, Vanderberg R, Merriam S, et al. Breast cancer chemoprevention: a practical guide for the primary care provider. J Womens Health (Larchmt). 2020;29:46-56. doi: 10.1089/jwh.2018.7643
8. McClintock AH, Golob AL, Laya MB. Breast cancer risk assessment: a step-wise approach for primary care providers on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275. doi: 10.1016/j.mayocp.2020.04.017
9. Catana A, Apostu AP, Antemie RG. Multi gene panel testing for hereditary breast cancer - is it ready to be used? Med Pharm Rep. 2019;92:220-225. doi: 10.15386/mpr-1083
10. Barke LD, Freivogel ME. Breast cancer risk assessment models and high-risk screening. Radiol Clin North Am. 2017;55:457-474. doi: 10.1016/j.rcl.2016.12.013
11. Amir E, Freedman OC, Seruga B, et al. Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010;102:680-91. doi: 10.1093/jnci/djq088
12. Kim G, Bahl M. Assessing risk of breast cancer: a review of risk prediction models. J Breast Imaging. 2021;3:144-155. doi: 10.1093/jbi/wbab001
13. Narod SA. Which genes for hereditary breast cancer? N Engl J Med. 2021;384:471-473. doi: 10.1056/NEJMe2035083
14. Couch FJ, Shimelis H, Hu C, et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017;3:1190-1196. doi: 10.1001/jamaoncol.2017.0424
15. Obeid EI, Hall MJ, Daly MB. Multigene panel testing and breast cancer risk: is it time to scale down? JAMA Oncol. 2017;3:1176-1177. doi: 10.1001/jamaoncol.2017.0342
16. Michailidou K, Lindström S, Dennis J, et al. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551:92-94. doi: 10.1038/nature24284
17. Shiovitz S, Korde LA. Genetics of breast cancer: a topic in evolution. Ann Oncol. 2015;26:1291-1299. doi: 10.1093/annonc/mdv022
18. Hu C, Hart SN, Gnanaolivu R, et al. A population-based study of genes previously implicated in breast cancer. N Engl J Med. 2021;384:440-451. doi: 10.1056/NEJMoa2005936
19. Gao C, Polley EC, Hart SN, et al. Risk of breast cancer among carriers of pathogenic variants in breast cancer predisposition genes varies by polygenic risk score. J Clin Oncol. 2021;39:2564-2573. doi: 10.1200/JCO.20.01992
20. Gallagher S, Hughes E, Wagner S, et al. Association of a polygenic risk score with breast cancer among women carriers of high- and moderate-risk breast cancer genes. JAMA Netw Open. 2020;3:e208501. doi: 10.1001/jamanetworkopen.2020.8501
21. Yanes T, Young MA, Meiser B, et al. Clinical applications of polygenic breast cancer risk: a critical review and perspectives of an emerging field. Breast Cancer Res. 2020;22:21. doi: 10.1186/s13058-020-01260-3
22. Schrager S, Torell E, Ledford K, et al. Managing a woman with BRCA mutations? Shared decision-making is key. J Fam Pract. 2020;69:237-243
23. US Preventive Services Task Force; Owens DK, Davidson KW, Krist AH, et al. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:857-867. doi: 10.1001/jama.2019.11885
24. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol 2015;22:3230-3235. doi: 10.1245/s10434-015-4715-9
25. Britt KL, Cuzick J, Phillips KA. Key steps for effective breast cancer prevention. Nat Rev Cancer. 2020;20:417-436. doi: 10.1038/s41568-020-0266-x
26. Jatoi I, Kemp Z. Risk-reducing mastectomy. JAMA. 2021;325:1781-1782. doi: 10.1001/jama.2020.22414
27. Choi Y, Terry MB, Daly MB, et al. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7:585-592. doi:10.1001/jamaoncol.2020.7995
PRACTICE RECOMMENDATIONS
› Assess breast cancer risk in all women starting at age 35. C
› Perform enhanced screening in all women with a lifetime risk of breast cancer > 20%. A
› Discuss chemoprevention for all women at elevated risk for breast cancer. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A guide to GERD, H pylori infection, and Barrett esophagus
Three conditions seen in primary care—gastroesophageal reflux disease (GERD), Helicobacter pylori (H pylori) infection, and Barrett esophagus (BE)—evolve in a gastric acid environment and are treated in part through gastric acid suppression. While GERD is a risk factor for the development of BE, H pylori is not associated with BE.1 Patients with H pylori are actually less likely to have GERD symptoms.2,3 In this article, we describe similarities and differences in patient presentations, diagnostic testing, and management, and review screening recommendations.
Gastroesophageal reflux disease
GERD is a clinical diagnosis based on symptoms of regurgitation and heartburn or the presence of one of its known complications (esophagitis, peptic strictures, or BE).2,4 Chest pain is also common. Atypical symptoms are dysphagia, bleeding, chronic cough, asthma, chronic laryngitis, hoarseness, wheezing, teeth erosions, belching, and bloating.2,5-7
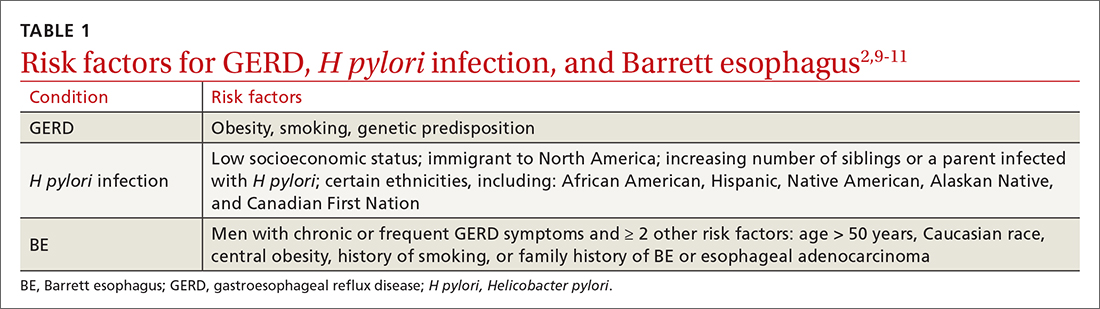
The worldwide prevalence of gastroesophageal reflux symptoms in adults is 14.8%.8 When using a stringent definition of GERD—weekly symptoms occurring for at least 3 months—prevalence drops to 9.4%.9 GERD symptoms vary markedly by geographic location; the highest rates are in Central America (19.6%) and the lowest rates are in Southeast Asia (7.4%).8TABLE 12,9-11 lists risk factors for GERD.
GERD results from dysfunction of the esophagogastric junction that permits regurgitation of acidic gastric contents into the esophagus. Normally, the lower esophageal sphincter (LES) relaxes temporarily with gastric distention; when this relaxation is frequent and prolonged, it causes GERD.2,12 Several medications, particularly those with anticholinergic effects (eg, tricyclic antidepressants) can decrease LES tone and contribute to symptoms. Nonsteroidal anti-inflammatory drugs (NSAIDs) are often linked to dyspepsia and gastritis and should be avoided in patients who have symptoms of GERD. Pathologic reflux can also occur in conditions that increase intra-abdominal pressure, such as obesity and pregnancy, and with esophageal dysmotility, hiatal hernia, and delayed gastric emptying.5 When gastric contents travel proximally, this contributes to extraesophageal symptoms, such as chronic cough, asthma, laryngitis, dyspepsia, bloating, and belching.2,4
Treatment
Proton pump inhibitors (PPIs) are the most effective treatment for GERD, but lifestyle modifications are also recommended for all patients.2,6,13-16 Consider selective elimination of beverages and foods that are commonly associated with heartburn (eg, alcohol, caffeine, chocolate, citrus, and spicy foods) if patients note a correlation to symptoms.5,6,13 Also, advise weight loss and smoking cessation, as appropriate, and suggest that the patient elevate the head of their bed when sleeping.
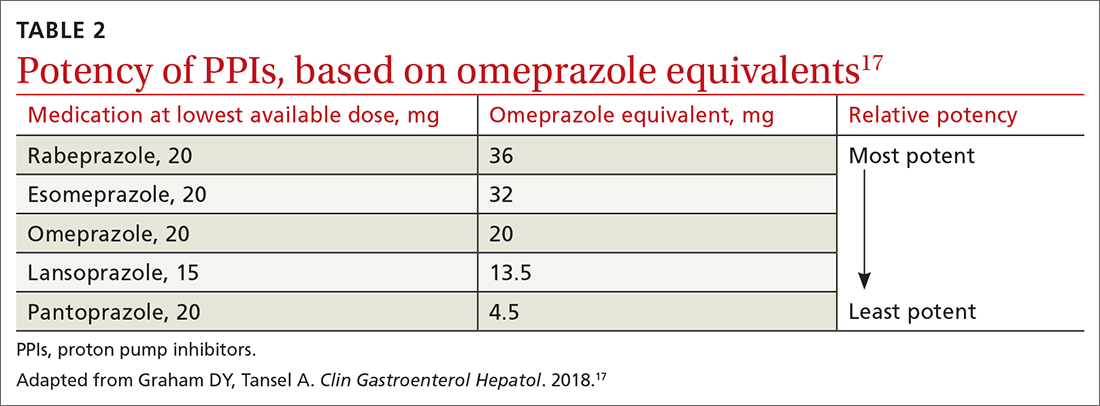
All PPIs are equally effective in suppressing acid when given at equivalent doses (TABLE 217), so they can be used interchangeably.17 Treat uncomplicated GERD with a once-daily PPI 30 to 60 minutes prior to a meal for 4 to 8 weeks. If treatment is effective, you’ll want to try to reduce or stop the medication after the 4- to 8-week period. (It’s worth noting that the benefits of treatment for those with extraesophageal GERD are less predictable than for those with heartburn or esophagitis symptoms.5)
If GERD symptoms reemerge after the PPI is stopped, the medication can be restarted but should be limited to the least potent effective dose, no matter if it is taken daily or only as needed.2,6,17 In patients with esophagitis, you may need to continue PPI treatment indefinitely at the lowest possible dose given the increased risk of recurrent esophagitis.2,13,16
Continue to: Keep in mind...
Keep in mind that the safety of long-term PPI use has not been fully established. While observational studies have shown that long-term PPI use may be associated with adverse events, including kidney damage, Clostridioides difficile infection, osteoporosis, and gastric cancer, subsequent prospective studies have not shown any significant risks with long-term PPI use.2,13,14,16,18,19 If a decision is made to discontinue PPIs after long-term use, the patient should be advised that rebound acid hypersecretion may occur, although this possibility can be mitigated by gradually tapering the PPI dose.
Another maintenance therapy option. Histamine-2 receptor antagonists (H2RAs) are a reasonable alternative to PPIs as maintenance therapy, but they are ineffective in healing esophagitis6,13 and may be best used as adjunctive therapy at bedtime for breakthrough symptoms while a patient is on maintenance PPIs.6,19 Antacids (eg, calcium carbonate, aluminum hydroxide, or magnesium hydroxide) and alginate may provide some symptomatic relief, as well.
When PPIs don’t work. If initial lifestyle changes and PPI treatment do not provide adequate relief, consider the possibility of nonadherence with medication or lifestyle directives. If nonadherence does not appear to be an issue, twice-daily PPI dosing is also an option. Recognize, though, that PPI treatment failure occurs in as many as 40% of patients and is much more common in those with atypical symptoms.6
Consider upper gastrointestinal (GI) endoscopy—and perhaps esophageal manometry or pH testing—if a patient does not respond to empiric treatment with a PPI for 4 to 8 weeks at a standard, once-daily dose.2,4,13 (Alternative diagnoses may also need to be considered.) Upper endoscopy is also appropriate for patients who have symptoms concerning for malignancy (progressive dysphagia, unintentional weight loss, or bleeding).
Esophagitis detected on endoscopy confirms GERD, although it is seen in only 18% to 25% of patients with GERD symptoms.2,4 (The absence of esophagitis only indicates a lack of mucosal injury and not the absence of GERD.4) Acid exposure can cause fibrotic scarring and, in turn, strictures visible on endoscopy.2 BE, the precursor to esophageal adenocarcinoma, is also a complication of GERD and is defined by columnar metaplasia replacing the normal squamous cell esophageal epithelium; it is detected on pathology review of biopsies.2
Continue to: GERD confirmed but PPIs aren't working?
GERD confirmed but PPIs aren’t working? Laparoscopic fundoplication is an effective treatment for GERD. However, due to its adverse effects (dysphagia, bloating, flatulence) and risk of treatment failure or breakdown within 5 to 10 years, it should be reserved for those poorly managed with PPIs.2,13,19
Considerations in pregnancy. GERD is reported by 40% to 85% of pregnant women,20,21 and its clinical presentation, diagnosis, and treatment are similar to that of nonpregnant adults.21 If lifestyle modification is not effective, pharmacologic therapy may be considered. Often, lifestyle modifications and antacids followed by the addition of sucralfate will be used first given the lack of systemic effects. H2RAs can be used next based on long-term historical use and reported safety.21 As with nonpregnant patients, PPIs are more effective than other medical therapies. If PPIs are used, dexlansoprazole, lansoprazole, pantoprazole, and rabeprazole are preferred. Omeprazole and esomeprazole are typically avoided due to findings of embryonic and fetal mortality in early animal studies, although subsequent human studies have noted no teratogenicity.2,20,21
Considerations in children. As with adults, findings in the history and exam are sufficient to diagnose and initiate treatment of GERD in children, provided there are no warning signs (eg, bilious vomiting, GI bleeding, consistent forceful vomiting, fever, lethargy, hepatosplenomegaly, bulging fontanelle, macro- or microcephaly, seizures, abdominal tenderness/distention, or genetic/metabolic syndromes). Lifestyle changes are first-line treatment, followed by medication. Acid suppressants are preferred, with PPIs showing superior efficacy compared with H2RAs.15 Some PPIs (omeprazole, lansoprazole, and esomeprazole) have US Food and Drug Administration (FDA) approval beginning at age 1 year, while rabeprazole has FDA approval beginning at age 12.22 As in adults, if PPIs are ineffective, consider alternative diagnoses.15,22
Helicobacter pylori infection
H pylori is a gram-negative spiral-shaped bacterium found in the stomach of humans and other mammals. It survives the acidic environment by metabolizing urea into alkaline ammonia and carbon dioxide. H pylori infection increases the risk of peptic ulcer disease, gastric cancer, iron deficiency anemia, and immune thrombocytopenia. It may be associated with dyspepsia, increased ulcer risk with use of an NSAID, and chronic gastritis.9 Infection with H pylori can decrease the risk of GERD.2 The bacterial infection causes atrophic gastritis and subsequent hypochlorhydria, which then diminishes the acidity of the reflux contents.19 There is no link between H pylori infection and BE.1
TABLE 12,9-11 shows those at highest risk of H pylori. The estimated prevalence of infection is 40% to 48%23 worldwide but lower in North America, at 32% to 42%.24H pylori is often acquired in childhood, and risk of infection is more likely if the parents (particularly mothers) are infected.9
Continue to: Whom to test, and how
Whom to test, and how
Test for H pylori in those with active peptic ulcer disease or a history of peptic ulcer disease that was not investigated for H pylori. Also test individuals who have gastric mucosa-associated lymphoid tissue lymphoma, have a history of gastric cancer or family history of gastric cancer, are scheduled for endoscopic evaluation for dyspepsia, or are starting chronic NSAID therapy. Patients with typical GERD symptoms do not need to be tested for H pylori.9,25
Means of testing for H pylori include the urea breath test, stool antigen studies, endoscopically obtained biopsies, or serum antibody tests. Antibody testing is discouraged because it has a lower diagnostic utility and cannot determine if the patient’s infection is current or past. Before undergoing urea breath tests, stool antigen tests, or biopsies for H pylori identification, patients should have abstained from taking the following agents for the time periods indicated: PPIs, 1 to 2 weeks; H2RAs, at least 1 day and preferably 2 weeks; and antibiotics, 4 weeks.9
The urea breath test and endoscopically obtained biopsies have the greatest diagnostic utility and, where available, should be the first-line tests. Stool antigen studies are useful for ruling out H pylori infection (very low negative likelihood ratio), but a positive test result is not as useful for confirming an infection, as false-positives do occur (moderate positive likelihood ratio).9,26,27 Stool antigen testing is less expensive and, in many cases, more convenient and readily available for patients than urea breath testing and endoscopic biopsies.
Treatment
Offer treatment to all patients who test positive for H pylori. Eradication rates range from 70% to 91% using first-line treatment options.9 Treatment regimens consist of acid suppression and 2 to 3 antibiotics in combination (TABLE 39,28). The single greatest predictive factor for treatment failure is antibiotic resistance, so a detailed antibiotic history is essential. In particular, ask about macrolide antibiotic usage and penicillin allergies.
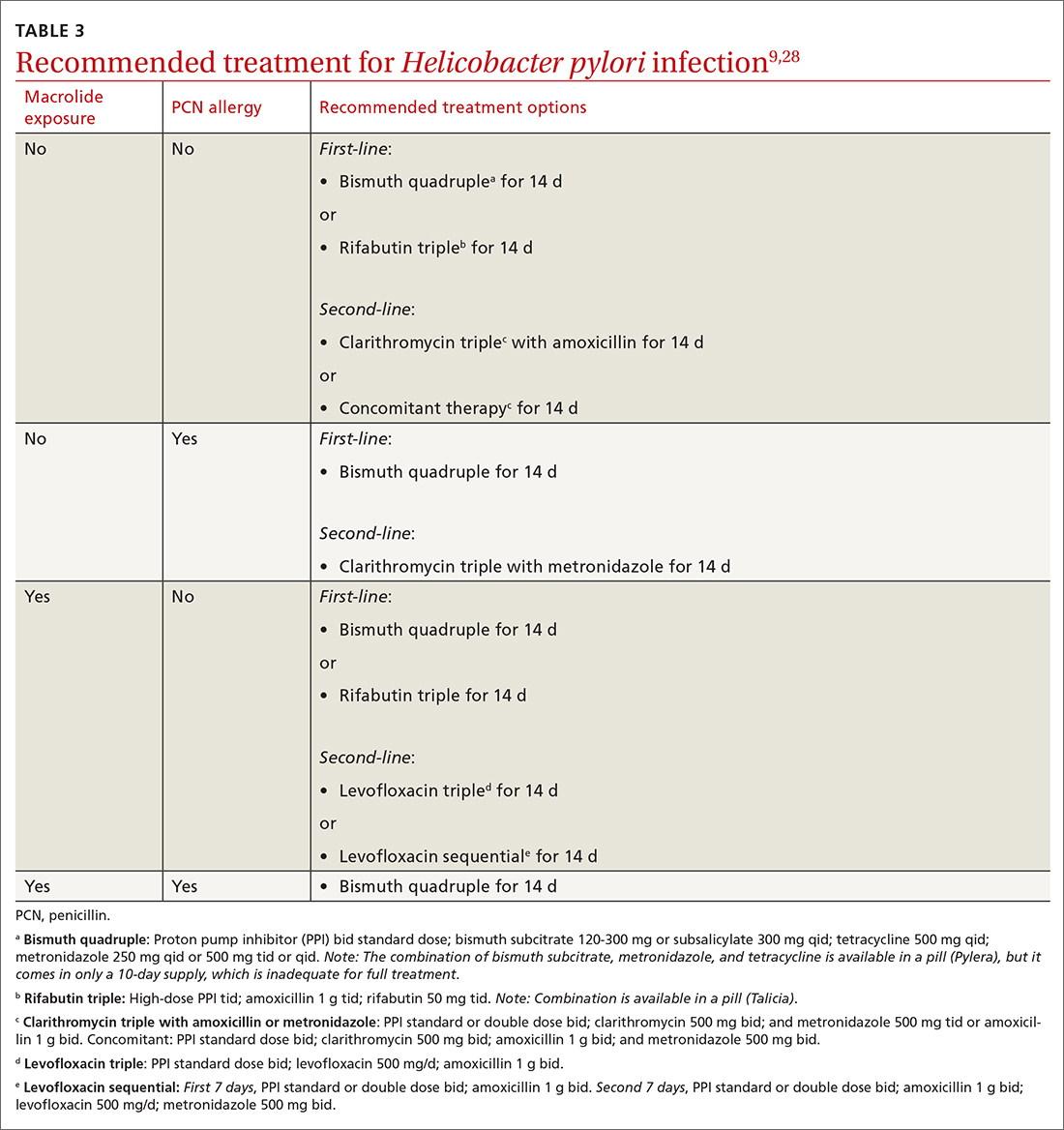
People living in areas with population macrolide resistance ≥ 15% should avoid clarithromycin-based regimens unless bacterial sensitivity testing has been done and shows sensitivity to these agents.9,28,29 For cases that do not resolve with a first-line treatment program, choose an alternative regimen with different antibiotics.9,29
Continue to: Additionally, adequate...
Additionally, adequate acid suppression is directly related to successful eradication. Thus, the likelihood of treatment success can be improved by using higher doses of PPIs and avoiding ones that are more likely to be metabolized quickly in some patients (lansoprazole, omeprazole). Patient adherence to the treatment regimen is an important determinant of effectiveness.9,29 Adding vitamin C 400 to 1000 mg/d, vitamin E 100 to 400 mg/d, and probiotics may improve the effectiveness of treatment.9,30
Duration of treatment is directly related to treatment effectiveness. Whenever possible, opt for 14 days of treatment instead of just 7.9
Test of cure. Patients treated for H pylori should be re-tested no sooner than 4 weeks after completion of therapy. Urea breath testing, stool antigen testing, and endoscopic biopsies (if endoscopy is indicated for some other reason) can all be used post treatment for test of cure.9
Barrett esophagus
Whom to screen
The American College of Gastroenterology recommends consideration of screening with upper endoscopy for men with chronic GERD (> 5 years) or frequent GERD symptoms (once weekly or more often), plus 2
Continue to: Not everyone with BE...
Not everyone with BE experiences GERD symptoms; sometimes BE may be diagnosed incidentally on upper endoscopy performed for unrelated symptoms.11 GERD patients who are currently asymptomatic and had a normal prior upper endoscopy do not require surveillance.
Diagnosis and management
BE is diagnosed based on specific endoscopic and histologic findings. The presence of dysplasia (either low grade or high grade) or its absence has important treatment implications. When histology is indefinite for dysplasia, treat reflux and, following acid suppression with PPIs for 3 to 6 months, repeat endoscopy (since reactive changes with reflux may obscure results).11
Nondysplastic BE has a risk of progressing to cancer in only 0.2% to 0.5% of affected patients per year.11 Guidelines for BE without dysplasia advise repeating surveillance endoscopy every 3 to 5 years after appropriate counseling regarding overall low risk of cancer progression.11,31 Surveillance endoscopy recommendations exist despite the lack of prospective randomized trials that demonstrate benefit. The rationale for surveillance is that survival in EAC is stage dependent and often EAC metastasizes prior to the development of symptoms from the tumor. Observational cohort studies in BE have demonstrated that surveillance endoscopy programs find EAC at earlier stages with improved survival; however, lead and length time bias may attenuate or eliminate these surveillance benefits.11,32
Risk for neoplastic progression increases with degree of dysplasia. BE with low-grade dysplasia and high-grade dysplasia have a risk of cancer progression of 0.7% per year and 7% per year, respectively.11
Historically, esophagectomy was the preferred treatment for BE with dysplasia. Now, endoscopic eradication therapies, including radiofrequency ablation and endoscopic mucosal resection for nodular BE, are the usual treatment for either low- or high-grade dysplasia.11
Chemoprophylaxis with PPIs. Most patients with BE have symptoms of GERD or reflux esophagitis, so treatment with a PPI is indicated for symptom control. In patients with BE without GERD, PPI use may still be indicated, although this is controversial. Current guidelines recommend once-daily PPI therapy for BE (twice daily only if needed for symptom control) to reduce reflux-associated inflammation and recommend against routine prescription of aspirin or NSAIDs for BE.11 In vitro and observational studies support PPI use to prevent progression to EAC11,33; however, data from randomized controlled trials to support their use are limited.34,35
CORRESPONDENCE
Megan Everson, MD, Medical College of Wisconsin, 229 South Morrison Street, Appleton WI, 54911; [email protected]
1. Wang C, Yuan Y, Hunt RH. Helicobacter pylori infection and Barrett’s esophagus: a systematic review and meta-analysis. Am J Gastroenterol. 2009;104:492-500. doi: 10.1038/ajg.2008.37
2. Maret-Ouda J, Markar SR, Lagergren J. Gastroesophageal reflux disease: a review. JAMA. 2020;324:2536-2547. doi: 10.1001/jama.2020.21360
3. Scida S, Russo M, Miraglia C, et al. Relationship between Helicobacter pylori infection and GERD. Acta Biomed. 2018;89:40-43. doi: 10.23750/abm.v89i8-S.7918
4. Vakil N, Van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-1920. doi: 10.1111/j.1572-0241.2006.00630.x
5. Mikami DJ, Murayama KM. Physiology and pathogenesis of gastroesophageal reflux disease. Surg Clin North Am. 2015;95:515-525. doi: 10.1016/j.suc.2015.02.006
6. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308-328. doi: 10.1038/ajg.2012.444
7. Sidhwa F, Moore A, Alligood E, et al. Diagnosis and treatment of the extraesophageal manifestations of gastroesophageal reflux disease. Ann Surg. 2017;265:63-67. doi: 10.1097/SLA.0000000000001907
8. Eusebi LH, Ratnakumaran R, Yuan Y, et al. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. 2018;67:430-440. doi: 10.1136/gutjnl-2016-313589
9. Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212-239. doi: 10.1038/ajg.2016.563
10. Eusebi LH, Cirota GG, Zagari RM, et al. Global prevalence of Barrett’s oesophagus and oesophageal cancer in individuals with gastro-oesophageal reflux: a systematic review and meta-analysis. Gut. 2021;70:456-463. doi: 10.1136/gutjnl-2020-321365
11. Shaheen NJ, Falk GW, Iyer PG, et al; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol. 2016;111:30-50. doi: 10.1038/ajg.2015.322
12. Savarino E, Bredenoord AJ, Fox M, et al; International Working Group for Disorders of Gastrointestinal Motility and Function. Expert consensus document: advances in the physiological assessment and diagnosis of GERD. Nat Rev Gastroenterol Hepatol. 2017;14:665-676. doi: 10.1038/nrgastro.2017.130
13. Kahrilas PJ. Clinical practice. Gastroesophageal reflux disease. N Engl J Med. 2008;359:1700-1707. doi: 10.1056/NEJMcp0804684
14. Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152:706-715. doi: 10.1053/j.gastro.2017.01.031
15. Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:516-554. doi: 10.1097/MPG.0000000000001889
16. Kahrilas PJ, Shaheen NJ, Vaezi MF, et al. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383-1391. doi: 10.1053/j.gastro.2008.08.045
17. Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol. 2018;16:800-808. doi: 10.1016/j.cgh.2017.09.033
18. Moayyedi P, Eikelboom JW, Bosch J, et al. Safety of proton pump inhibitors based on a large, multi-year, randomized trial of patients receiving rivaroxaban or aspirin. Gastroenterology. 2019;157:682-691. doi: 10.1053/j.gastro.2019.05.056
19. Katzka DA, Kahrilas PJ. Advances in the diagnosis and management of gastroesophageal reflux disease. BMJ. 2020;371:m3786. doi: 10.1136/bmj.m3786
20. Ali RA, Egan LJ. Gastroesophageal reflux disease in pregnancy. Best Pract Res Clin Gastroenterol. 2007;21:793-806. doi: 10.1016/j.bpg.2007.05.006
21. Body C, Christie JA. Gastrointestinal diseases in pregnancy: nausea, vomiting, hyperemesis gravidarum, gastroesophageal reflux disease, constipation, and diarrhea. Gastroenterol Clin North Am. 2016;45:267-283. doi: 10.1016/j.gtc.2016.02.005
22. Lightdale JR, Gremse DA, et al. Gastroesophageal reflux: management guidance for the pediatrician. Pediatrics. 2013;131;e1684-e1695. doi: 10.1542/peds.2013-0421
23. Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153:420-429. doi: 10.1053/j.gastro.2017.04.022
24. Zamani M, Ebrahimtabar F, Zamani V, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47:868-876. doi: 10.1111/apt.14561
25. Choi IJ, Kim CG, Lee JY, et al. Family history of gastric cancer and Helicobacter pylori treatment. N Engl J Med. 2020;382:427-436. doi: 10.1056/NEJMoa1909666
26. Gisbert JP, Abraira V. Accuracy of Helicobacter pylori diagnostic tests in patients with bleeding peptic ulcer: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101:848-863. doi: 10.1111/j.1572-0241.2006.00528.x
27. Best LM, Takwoingi Y, Siddique S, et al. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst Rev. 2018;3:CD012080. doi: 10.1002/14651858.CD012080.pub2
28. Howden CW, Graham DY. Recent developments pertaining to H. pylori infection. Am J Gastroenterol. 2021;116:1-3. doi: 10.14309/ajg.0000000000001031
29. Shah SC, Iyer PG, Moss SF. AGA Clinical practice update on the management of refractory Helicobacter pylori infection: expert review. Gastroenterology. 2021;160:1831-1841. doi: 10.1053/j.gastro.2020.11.059
30. Yang-Ou YB, Hu Y, Zhu Y, et al. The effect of antioxidants on Helicobacter pylori eradication: a systematic review with meta-analysis. Helicobacter. 2018;23:e12535. doi: 10.1111/hel.12535
31. Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:1084-1091. doi: 10.1053/j.gastro.2011.01.030
32. Codipilly DC, Chandar AK, Singh S, et al. The effect of endoscopic surveillance in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gastroenterology. 2018;154:2068-2086. doi: 10.1053/j.gastro.2018.02.022
33. Singh S, Garg SK, Singh PP, et al. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta-analysis. Gut. 2014;63:1229-1237. doi: 10.1136/gutjnl-2013-305997
34. Jankowski JAZ, de Caestecker J, Love SB, et al. Esomeprazole and aspirin in Barrett’s oesophagus (AspECT): a randomised factorial trial. Lancet. 2018;392:400-408. doi: 10.1016/S0140-6736(18)31388-6
35. Hu Q, Sun TT, Hong J, et al. Proton pump inhibitors do not reduce the risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a systematic review and meta-analysis. PLoS One. 2017;12:e0169691. doi: 10.1371/journal.pone.0169691
Three conditions seen in primary care—gastroesophageal reflux disease (GERD), Helicobacter pylori (H pylori) infection, and Barrett esophagus (BE)—evolve in a gastric acid environment and are treated in part through gastric acid suppression. While GERD is a risk factor for the development of BE, H pylori is not associated with BE.1 Patients with H pylori are actually less likely to have GERD symptoms.2,3 In this article, we describe similarities and differences in patient presentations, diagnostic testing, and management, and review screening recommendations.
Gastroesophageal reflux disease
GERD is a clinical diagnosis based on symptoms of regurgitation and heartburn or the presence of one of its known complications (esophagitis, peptic strictures, or BE).2,4 Chest pain is also common. Atypical symptoms are dysphagia, bleeding, chronic cough, asthma, chronic laryngitis, hoarseness, wheezing, teeth erosions, belching, and bloating.2,5-7
The worldwide prevalence of gastroesophageal reflux symptoms in adults is 14.8%.8 When using a stringent definition of GERD—weekly symptoms occurring for at least 3 months—prevalence drops to 9.4%.9 GERD symptoms vary markedly by geographic location; the highest rates are in Central America (19.6%) and the lowest rates are in Southeast Asia (7.4%).8TABLE 12,9-11 lists risk factors for GERD.

GERD results from dysfunction of the esophagogastric junction that permits regurgitation of acidic gastric contents into the esophagus. Normally, the lower esophageal sphincter (LES) relaxes temporarily with gastric distention; when this relaxation is frequent and prolonged, it causes GERD.2,12 Several medications, particularly those with anticholinergic effects (eg, tricyclic antidepressants) can decrease LES tone and contribute to symptoms. Nonsteroidal anti-inflammatory drugs (NSAIDs) are often linked to dyspepsia and gastritis and should be avoided in patients who have symptoms of GERD. Pathologic reflux can also occur in conditions that increase intra-abdominal pressure, such as obesity and pregnancy, and with esophageal dysmotility, hiatal hernia, and delayed gastric emptying.5 When gastric contents travel proximally, this contributes to extraesophageal symptoms, such as chronic cough, asthma, laryngitis, dyspepsia, bloating, and belching.2,4
Treatment
Proton pump inhibitors (PPIs) are the most effective treatment for GERD, but lifestyle modifications are also recommended for all patients.2,6,13-16 Consider selective elimination of beverages and foods that are commonly associated with heartburn (eg, alcohol, caffeine, chocolate, citrus, and spicy foods) if patients note a correlation to symptoms.5,6,13 Also, advise weight loss and smoking cessation, as appropriate, and suggest that the patient elevate the head of their bed when sleeping.
All PPIs are equally effective in suppressing acid when given at equivalent doses (TABLE 217), so they can be used interchangeably.17 Treat uncomplicated GERD with a once-daily PPI 30 to 60 minutes prior to a meal for 4 to 8 weeks. If treatment is effective, you’ll want to try to reduce or stop the medication after the 4- to 8-week period. (It’s worth noting that the benefits of treatment for those with extraesophageal GERD are less predictable than for those with heartburn or esophagitis symptoms.5)

If GERD symptoms reemerge after the PPI is stopped, the medication can be restarted but should be limited to the least potent effective dose, no matter if it is taken daily or only as needed.2,6,17 In patients with esophagitis, you may need to continue PPI treatment indefinitely at the lowest possible dose given the increased risk of recurrent esophagitis.2,13,16
Continue to: Keep in mind...
Keep in mind that the safety of long-term PPI use has not been fully established. While observational studies have shown that long-term PPI use may be associated with adverse events, including kidney damage, Clostridioides difficile infection, osteoporosis, and gastric cancer, subsequent prospective studies have not shown any significant risks with long-term PPI use.2,13,14,16,18,19 If a decision is made to discontinue PPIs after long-term use, the patient should be advised that rebound acid hypersecretion may occur, although this possibility can be mitigated by gradually tapering the PPI dose.
Another maintenance therapy option. Histamine-2 receptor antagonists (H2RAs) are a reasonable alternative to PPIs as maintenance therapy, but they are ineffective in healing esophagitis6,13 and may be best used as adjunctive therapy at bedtime for breakthrough symptoms while a patient is on maintenance PPIs.6,19 Antacids (eg, calcium carbonate, aluminum hydroxide, or magnesium hydroxide) and alginate may provide some symptomatic relief, as well.
When PPIs don’t work. If initial lifestyle changes and PPI treatment do not provide adequate relief, consider the possibility of nonadherence with medication or lifestyle directives. If nonadherence does not appear to be an issue, twice-daily PPI dosing is also an option. Recognize, though, that PPI treatment failure occurs in as many as 40% of patients and is much more common in those with atypical symptoms.6
Consider upper gastrointestinal (GI) endoscopy—and perhaps esophageal manometry or pH testing—if a patient does not respond to empiric treatment with a PPI for 4 to 8 weeks at a standard, once-daily dose.2,4,13 (Alternative diagnoses may also need to be considered.) Upper endoscopy is also appropriate for patients who have symptoms concerning for malignancy (progressive dysphagia, unintentional weight loss, or bleeding).
Esophagitis detected on endoscopy confirms GERD, although it is seen in only 18% to 25% of patients with GERD symptoms.2,4 (The absence of esophagitis only indicates a lack of mucosal injury and not the absence of GERD.4) Acid exposure can cause fibrotic scarring and, in turn, strictures visible on endoscopy.2 BE, the precursor to esophageal adenocarcinoma, is also a complication of GERD and is defined by columnar metaplasia replacing the normal squamous cell esophageal epithelium; it is detected on pathology review of biopsies.2
Continue to: GERD confirmed but PPIs aren't working?
GERD confirmed but PPIs aren’t working? Laparoscopic fundoplication is an effective treatment for GERD. However, due to its adverse effects (dysphagia, bloating, flatulence) and risk of treatment failure or breakdown within 5 to 10 years, it should be reserved for those poorly managed with PPIs.2,13,19
Considerations in pregnancy. GERD is reported by 40% to 85% of pregnant women,20,21 and its clinical presentation, diagnosis, and treatment are similar to that of nonpregnant adults.21 If lifestyle modification is not effective, pharmacologic therapy may be considered. Often, lifestyle modifications and antacids followed by the addition of sucralfate will be used first given the lack of systemic effects. H2RAs can be used next based on long-term historical use and reported safety.21 As with nonpregnant patients, PPIs are more effective than other medical therapies. If PPIs are used, dexlansoprazole, lansoprazole, pantoprazole, and rabeprazole are preferred. Omeprazole and esomeprazole are typically avoided due to findings of embryonic and fetal mortality in early animal studies, although subsequent human studies have noted no teratogenicity.2,20,21
Considerations in children. As with adults, findings in the history and exam are sufficient to diagnose and initiate treatment of GERD in children, provided there are no warning signs (eg, bilious vomiting, GI bleeding, consistent forceful vomiting, fever, lethargy, hepatosplenomegaly, bulging fontanelle, macro- or microcephaly, seizures, abdominal tenderness/distention, or genetic/metabolic syndromes). Lifestyle changes are first-line treatment, followed by medication. Acid suppressants are preferred, with PPIs showing superior efficacy compared with H2RAs.15 Some PPIs (omeprazole, lansoprazole, and esomeprazole) have US Food and Drug Administration (FDA) approval beginning at age 1 year, while rabeprazole has FDA approval beginning at age 12.22 As in adults, if PPIs are ineffective, consider alternative diagnoses.15,22
Helicobacter pylori infection
H pylori is a gram-negative spiral-shaped bacterium found in the stomach of humans and other mammals. It survives the acidic environment by metabolizing urea into alkaline ammonia and carbon dioxide. H pylori infection increases the risk of peptic ulcer disease, gastric cancer, iron deficiency anemia, and immune thrombocytopenia. It may be associated with dyspepsia, increased ulcer risk with use of an NSAID, and chronic gastritis.9 Infection with H pylori can decrease the risk of GERD.2 The bacterial infection causes atrophic gastritis and subsequent hypochlorhydria, which then diminishes the acidity of the reflux contents.19 There is no link between H pylori infection and BE.1
TABLE 12,9-11 shows those at highest risk of H pylori. The estimated prevalence of infection is 40% to 48%23 worldwide but lower in North America, at 32% to 42%.24H pylori is often acquired in childhood, and risk of infection is more likely if the parents (particularly mothers) are infected.9
Continue to: Whom to test, and how
Whom to test, and how
Test for H pylori in those with active peptic ulcer disease or a history of peptic ulcer disease that was not investigated for H pylori. Also test individuals who have gastric mucosa-associated lymphoid tissue lymphoma, have a history of gastric cancer or family history of gastric cancer, are scheduled for endoscopic evaluation for dyspepsia, or are starting chronic NSAID therapy. Patients with typical GERD symptoms do not need to be tested for H pylori.9,25
Means of testing for H pylori include the urea breath test, stool antigen studies, endoscopically obtained biopsies, or serum antibody tests. Antibody testing is discouraged because it has a lower diagnostic utility and cannot determine if the patient’s infection is current or past. Before undergoing urea breath tests, stool antigen tests, or biopsies for H pylori identification, patients should have abstained from taking the following agents for the time periods indicated: PPIs, 1 to 2 weeks; H2RAs, at least 1 day and preferably 2 weeks; and antibiotics, 4 weeks.9
The urea breath test and endoscopically obtained biopsies have the greatest diagnostic utility and, where available, should be the first-line tests. Stool antigen studies are useful for ruling out H pylori infection (very low negative likelihood ratio), but a positive test result is not as useful for confirming an infection, as false-positives do occur (moderate positive likelihood ratio).9,26,27 Stool antigen testing is less expensive and, in many cases, more convenient and readily available for patients than urea breath testing and endoscopic biopsies.
Treatment
Offer treatment to all patients who test positive for H pylori. Eradication rates range from 70% to 91% using first-line treatment options.9 Treatment regimens consist of acid suppression and 2 to 3 antibiotics in combination (TABLE 39,28). The single greatest predictive factor for treatment failure is antibiotic resistance, so a detailed antibiotic history is essential. In particular, ask about macrolide antibiotic usage and penicillin allergies.

People living in areas with population macrolide resistance ≥ 15% should avoid clarithromycin-based regimens unless bacterial sensitivity testing has been done and shows sensitivity to these agents.9,28,29 For cases that do not resolve with a first-line treatment program, choose an alternative regimen with different antibiotics.9,29
Continue to: Additionally, adequate...
Additionally, adequate acid suppression is directly related to successful eradication. Thus, the likelihood of treatment success can be improved by using higher doses of PPIs and avoiding ones that are more likely to be metabolized quickly in some patients (lansoprazole, omeprazole). Patient adherence to the treatment regimen is an important determinant of effectiveness.9,29 Adding vitamin C 400 to 1000 mg/d, vitamin E 100 to 400 mg/d, and probiotics may improve the effectiveness of treatment.9,30
Duration of treatment is directly related to treatment effectiveness. Whenever possible, opt for 14 days of treatment instead of just 7.9
Test of cure. Patients treated for H pylori should be re-tested no sooner than 4 weeks after completion of therapy. Urea breath testing, stool antigen testing, and endoscopic biopsies (if endoscopy is indicated for some other reason) can all be used post treatment for test of cure.9
Barrett esophagus
Whom to screen
The American College of Gastroenterology recommends consideration of screening with upper endoscopy for men with chronic GERD (> 5 years) or frequent GERD symptoms (once weekly or more often), plus 2
Continue to: Not everyone with BE...
Not everyone with BE experiences GERD symptoms; sometimes BE may be diagnosed incidentally on upper endoscopy performed for unrelated symptoms.11 GERD patients who are currently asymptomatic and had a normal prior upper endoscopy do not require surveillance.
Diagnosis and management
BE is diagnosed based on specific endoscopic and histologic findings. The presence of dysplasia (either low grade or high grade) or its absence has important treatment implications. When histology is indefinite for dysplasia, treat reflux and, following acid suppression with PPIs for 3 to 6 months, repeat endoscopy (since reactive changes with reflux may obscure results).11
Nondysplastic BE has a risk of progressing to cancer in only 0.2% to 0.5% of affected patients per year.11 Guidelines for BE without dysplasia advise repeating surveillance endoscopy every 3 to 5 years after appropriate counseling regarding overall low risk of cancer progression.11,31 Surveillance endoscopy recommendations exist despite the lack of prospective randomized trials that demonstrate benefit. The rationale for surveillance is that survival in EAC is stage dependent and often EAC metastasizes prior to the development of symptoms from the tumor. Observational cohort studies in BE have demonstrated that surveillance endoscopy programs find EAC at earlier stages with improved survival; however, lead and length time bias may attenuate or eliminate these surveillance benefits.11,32
Risk for neoplastic progression increases with degree of dysplasia. BE with low-grade dysplasia and high-grade dysplasia have a risk of cancer progression of 0.7% per year and 7% per year, respectively.11
Historically, esophagectomy was the preferred treatment for BE with dysplasia. Now, endoscopic eradication therapies, including radiofrequency ablation and endoscopic mucosal resection for nodular BE, are the usual treatment for either low- or high-grade dysplasia.11
Chemoprophylaxis with PPIs. Most patients with BE have symptoms of GERD or reflux esophagitis, so treatment with a PPI is indicated for symptom control. In patients with BE without GERD, PPI use may still be indicated, although this is controversial. Current guidelines recommend once-daily PPI therapy for BE (twice daily only if needed for symptom control) to reduce reflux-associated inflammation and recommend against routine prescription of aspirin or NSAIDs for BE.11 In vitro and observational studies support PPI use to prevent progression to EAC11,33; however, data from randomized controlled trials to support their use are limited.34,35
CORRESPONDENCE
Megan Everson, MD, Medical College of Wisconsin, 229 South Morrison Street, Appleton WI, 54911; [email protected]
Three conditions seen in primary care—gastroesophageal reflux disease (GERD), Helicobacter pylori (H pylori) infection, and Barrett esophagus (BE)—evolve in a gastric acid environment and are treated in part through gastric acid suppression. While GERD is a risk factor for the development of BE, H pylori is not associated with BE.1 Patients with H pylori are actually less likely to have GERD symptoms.2,3 In this article, we describe similarities and differences in patient presentations, diagnostic testing, and management, and review screening recommendations.
Gastroesophageal reflux disease
GERD is a clinical diagnosis based on symptoms of regurgitation and heartburn or the presence of one of its known complications (esophagitis, peptic strictures, or BE).2,4 Chest pain is also common. Atypical symptoms are dysphagia, bleeding, chronic cough, asthma, chronic laryngitis, hoarseness, wheezing, teeth erosions, belching, and bloating.2,5-7
The worldwide prevalence of gastroesophageal reflux symptoms in adults is 14.8%.8 When using a stringent definition of GERD—weekly symptoms occurring for at least 3 months—prevalence drops to 9.4%.9 GERD symptoms vary markedly by geographic location; the highest rates are in Central America (19.6%) and the lowest rates are in Southeast Asia (7.4%).8TABLE 12,9-11 lists risk factors for GERD.

GERD results from dysfunction of the esophagogastric junction that permits regurgitation of acidic gastric contents into the esophagus. Normally, the lower esophageal sphincter (LES) relaxes temporarily with gastric distention; when this relaxation is frequent and prolonged, it causes GERD.2,12 Several medications, particularly those with anticholinergic effects (eg, tricyclic antidepressants) can decrease LES tone and contribute to symptoms. Nonsteroidal anti-inflammatory drugs (NSAIDs) are often linked to dyspepsia and gastritis and should be avoided in patients who have symptoms of GERD. Pathologic reflux can also occur in conditions that increase intra-abdominal pressure, such as obesity and pregnancy, and with esophageal dysmotility, hiatal hernia, and delayed gastric emptying.5 When gastric contents travel proximally, this contributes to extraesophageal symptoms, such as chronic cough, asthma, laryngitis, dyspepsia, bloating, and belching.2,4
Treatment
Proton pump inhibitors (PPIs) are the most effective treatment for GERD, but lifestyle modifications are also recommended for all patients.2,6,13-16 Consider selective elimination of beverages and foods that are commonly associated with heartburn (eg, alcohol, caffeine, chocolate, citrus, and spicy foods) if patients note a correlation to symptoms.5,6,13 Also, advise weight loss and smoking cessation, as appropriate, and suggest that the patient elevate the head of their bed when sleeping.
All PPIs are equally effective in suppressing acid when given at equivalent doses (TABLE 217), so they can be used interchangeably.17 Treat uncomplicated GERD with a once-daily PPI 30 to 60 minutes prior to a meal for 4 to 8 weeks. If treatment is effective, you’ll want to try to reduce or stop the medication after the 4- to 8-week period. (It’s worth noting that the benefits of treatment for those with extraesophageal GERD are less predictable than for those with heartburn or esophagitis symptoms.5)

If GERD symptoms reemerge after the PPI is stopped, the medication can be restarted but should be limited to the least potent effective dose, no matter if it is taken daily or only as needed.2,6,17 In patients with esophagitis, you may need to continue PPI treatment indefinitely at the lowest possible dose given the increased risk of recurrent esophagitis.2,13,16
Continue to: Keep in mind...
Keep in mind that the safety of long-term PPI use has not been fully established. While observational studies have shown that long-term PPI use may be associated with adverse events, including kidney damage, Clostridioides difficile infection, osteoporosis, and gastric cancer, subsequent prospective studies have not shown any significant risks with long-term PPI use.2,13,14,16,18,19 If a decision is made to discontinue PPIs after long-term use, the patient should be advised that rebound acid hypersecretion may occur, although this possibility can be mitigated by gradually tapering the PPI dose.
Another maintenance therapy option. Histamine-2 receptor antagonists (H2RAs) are a reasonable alternative to PPIs as maintenance therapy, but they are ineffective in healing esophagitis6,13 and may be best used as adjunctive therapy at bedtime for breakthrough symptoms while a patient is on maintenance PPIs.6,19 Antacids (eg, calcium carbonate, aluminum hydroxide, or magnesium hydroxide) and alginate may provide some symptomatic relief, as well.
When PPIs don’t work. If initial lifestyle changes and PPI treatment do not provide adequate relief, consider the possibility of nonadherence with medication or lifestyle directives. If nonadherence does not appear to be an issue, twice-daily PPI dosing is also an option. Recognize, though, that PPI treatment failure occurs in as many as 40% of patients and is much more common in those with atypical symptoms.6
Consider upper gastrointestinal (GI) endoscopy—and perhaps esophageal manometry or pH testing—if a patient does not respond to empiric treatment with a PPI for 4 to 8 weeks at a standard, once-daily dose.2,4,13 (Alternative diagnoses may also need to be considered.) Upper endoscopy is also appropriate for patients who have symptoms concerning for malignancy (progressive dysphagia, unintentional weight loss, or bleeding).
Esophagitis detected on endoscopy confirms GERD, although it is seen in only 18% to 25% of patients with GERD symptoms.2,4 (The absence of esophagitis only indicates a lack of mucosal injury and not the absence of GERD.4) Acid exposure can cause fibrotic scarring and, in turn, strictures visible on endoscopy.2 BE, the precursor to esophageal adenocarcinoma, is also a complication of GERD and is defined by columnar metaplasia replacing the normal squamous cell esophageal epithelium; it is detected on pathology review of biopsies.2
Continue to: GERD confirmed but PPIs aren't working?
GERD confirmed but PPIs aren’t working? Laparoscopic fundoplication is an effective treatment for GERD. However, due to its adverse effects (dysphagia, bloating, flatulence) and risk of treatment failure or breakdown within 5 to 10 years, it should be reserved for those poorly managed with PPIs.2,13,19
Considerations in pregnancy. GERD is reported by 40% to 85% of pregnant women,20,21 and its clinical presentation, diagnosis, and treatment are similar to that of nonpregnant adults.21 If lifestyle modification is not effective, pharmacologic therapy may be considered. Often, lifestyle modifications and antacids followed by the addition of sucralfate will be used first given the lack of systemic effects. H2RAs can be used next based on long-term historical use and reported safety.21 As with nonpregnant patients, PPIs are more effective than other medical therapies. If PPIs are used, dexlansoprazole, lansoprazole, pantoprazole, and rabeprazole are preferred. Omeprazole and esomeprazole are typically avoided due to findings of embryonic and fetal mortality in early animal studies, although subsequent human studies have noted no teratogenicity.2,20,21
Considerations in children. As with adults, findings in the history and exam are sufficient to diagnose and initiate treatment of GERD in children, provided there are no warning signs (eg, bilious vomiting, GI bleeding, consistent forceful vomiting, fever, lethargy, hepatosplenomegaly, bulging fontanelle, macro- or microcephaly, seizures, abdominal tenderness/distention, or genetic/metabolic syndromes). Lifestyle changes are first-line treatment, followed by medication. Acid suppressants are preferred, with PPIs showing superior efficacy compared with H2RAs.15 Some PPIs (omeprazole, lansoprazole, and esomeprazole) have US Food and Drug Administration (FDA) approval beginning at age 1 year, while rabeprazole has FDA approval beginning at age 12.22 As in adults, if PPIs are ineffective, consider alternative diagnoses.15,22
Helicobacter pylori infection
H pylori is a gram-negative spiral-shaped bacterium found in the stomach of humans and other mammals. It survives the acidic environment by metabolizing urea into alkaline ammonia and carbon dioxide. H pylori infection increases the risk of peptic ulcer disease, gastric cancer, iron deficiency anemia, and immune thrombocytopenia. It may be associated with dyspepsia, increased ulcer risk with use of an NSAID, and chronic gastritis.9 Infection with H pylori can decrease the risk of GERD.2 The bacterial infection causes atrophic gastritis and subsequent hypochlorhydria, which then diminishes the acidity of the reflux contents.19 There is no link between H pylori infection and BE.1
TABLE 12,9-11 shows those at highest risk of H pylori. The estimated prevalence of infection is 40% to 48%23 worldwide but lower in North America, at 32% to 42%.24H pylori is often acquired in childhood, and risk of infection is more likely if the parents (particularly mothers) are infected.9
Continue to: Whom to test, and how
Whom to test, and how
Test for H pylori in those with active peptic ulcer disease or a history of peptic ulcer disease that was not investigated for H pylori. Also test individuals who have gastric mucosa-associated lymphoid tissue lymphoma, have a history of gastric cancer or family history of gastric cancer, are scheduled for endoscopic evaluation for dyspepsia, or are starting chronic NSAID therapy. Patients with typical GERD symptoms do not need to be tested for H pylori.9,25
Means of testing for H pylori include the urea breath test, stool antigen studies, endoscopically obtained biopsies, or serum antibody tests. Antibody testing is discouraged because it has a lower diagnostic utility and cannot determine if the patient’s infection is current or past. Before undergoing urea breath tests, stool antigen tests, or biopsies for H pylori identification, patients should have abstained from taking the following agents for the time periods indicated: PPIs, 1 to 2 weeks; H2RAs, at least 1 day and preferably 2 weeks; and antibiotics, 4 weeks.9
The urea breath test and endoscopically obtained biopsies have the greatest diagnostic utility and, where available, should be the first-line tests. Stool antigen studies are useful for ruling out H pylori infection (very low negative likelihood ratio), but a positive test result is not as useful for confirming an infection, as false-positives do occur (moderate positive likelihood ratio).9,26,27 Stool antigen testing is less expensive and, in many cases, more convenient and readily available for patients than urea breath testing and endoscopic biopsies.
Treatment
Offer treatment to all patients who test positive for H pylori. Eradication rates range from 70% to 91% using first-line treatment options.9 Treatment regimens consist of acid suppression and 2 to 3 antibiotics in combination (TABLE 39,28). The single greatest predictive factor for treatment failure is antibiotic resistance, so a detailed antibiotic history is essential. In particular, ask about macrolide antibiotic usage and penicillin allergies.

People living in areas with population macrolide resistance ≥ 15% should avoid clarithromycin-based regimens unless bacterial sensitivity testing has been done and shows sensitivity to these agents.9,28,29 For cases that do not resolve with a first-line treatment program, choose an alternative regimen with different antibiotics.9,29
Continue to: Additionally, adequate...
Additionally, adequate acid suppression is directly related to successful eradication. Thus, the likelihood of treatment success can be improved by using higher doses of PPIs and avoiding ones that are more likely to be metabolized quickly in some patients (lansoprazole, omeprazole). Patient adherence to the treatment regimen is an important determinant of effectiveness.9,29 Adding vitamin C 400 to 1000 mg/d, vitamin E 100 to 400 mg/d, and probiotics may improve the effectiveness of treatment.9,30
Duration of treatment is directly related to treatment effectiveness. Whenever possible, opt for 14 days of treatment instead of just 7.9
Test of cure. Patients treated for H pylori should be re-tested no sooner than 4 weeks after completion of therapy. Urea breath testing, stool antigen testing, and endoscopic biopsies (if endoscopy is indicated for some other reason) can all be used post treatment for test of cure.9
Barrett esophagus
Whom to screen
The American College of Gastroenterology recommends consideration of screening with upper endoscopy for men with chronic GERD (> 5 years) or frequent GERD symptoms (once weekly or more often), plus 2
Continue to: Not everyone with BE...
Not everyone with BE experiences GERD symptoms; sometimes BE may be diagnosed incidentally on upper endoscopy performed for unrelated symptoms.11 GERD patients who are currently asymptomatic and had a normal prior upper endoscopy do not require surveillance.
Diagnosis and management
BE is diagnosed based on specific endoscopic and histologic findings. The presence of dysplasia (either low grade or high grade) or its absence has important treatment implications. When histology is indefinite for dysplasia, treat reflux and, following acid suppression with PPIs for 3 to 6 months, repeat endoscopy (since reactive changes with reflux may obscure results).11
Nondysplastic BE has a risk of progressing to cancer in only 0.2% to 0.5% of affected patients per year.11 Guidelines for BE without dysplasia advise repeating surveillance endoscopy every 3 to 5 years after appropriate counseling regarding overall low risk of cancer progression.11,31 Surveillance endoscopy recommendations exist despite the lack of prospective randomized trials that demonstrate benefit. The rationale for surveillance is that survival in EAC is stage dependent and often EAC metastasizes prior to the development of symptoms from the tumor. Observational cohort studies in BE have demonstrated that surveillance endoscopy programs find EAC at earlier stages with improved survival; however, lead and length time bias may attenuate or eliminate these surveillance benefits.11,32
Risk for neoplastic progression increases with degree of dysplasia. BE with low-grade dysplasia and high-grade dysplasia have a risk of cancer progression of 0.7% per year and 7% per year, respectively.11
Historically, esophagectomy was the preferred treatment for BE with dysplasia. Now, endoscopic eradication therapies, including radiofrequency ablation and endoscopic mucosal resection for nodular BE, are the usual treatment for either low- or high-grade dysplasia.11
Chemoprophylaxis with PPIs. Most patients with BE have symptoms of GERD or reflux esophagitis, so treatment with a PPI is indicated for symptom control. In patients with BE without GERD, PPI use may still be indicated, although this is controversial. Current guidelines recommend once-daily PPI therapy for BE (twice daily only if needed for symptom control) to reduce reflux-associated inflammation and recommend against routine prescription of aspirin or NSAIDs for BE.11 In vitro and observational studies support PPI use to prevent progression to EAC11,33; however, data from randomized controlled trials to support their use are limited.34,35
CORRESPONDENCE
Megan Everson, MD, Medical College of Wisconsin, 229 South Morrison Street, Appleton WI, 54911; [email protected]
1. Wang C, Yuan Y, Hunt RH. Helicobacter pylori infection and Barrett’s esophagus: a systematic review and meta-analysis. Am J Gastroenterol. 2009;104:492-500. doi: 10.1038/ajg.2008.37
2. Maret-Ouda J, Markar SR, Lagergren J. Gastroesophageal reflux disease: a review. JAMA. 2020;324:2536-2547. doi: 10.1001/jama.2020.21360
3. Scida S, Russo M, Miraglia C, et al. Relationship between Helicobacter pylori infection and GERD. Acta Biomed. 2018;89:40-43. doi: 10.23750/abm.v89i8-S.7918
4. Vakil N, Van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-1920. doi: 10.1111/j.1572-0241.2006.00630.x
5. Mikami DJ, Murayama KM. Physiology and pathogenesis of gastroesophageal reflux disease. Surg Clin North Am. 2015;95:515-525. doi: 10.1016/j.suc.2015.02.006
6. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308-328. doi: 10.1038/ajg.2012.444
7. Sidhwa F, Moore A, Alligood E, et al. Diagnosis and treatment of the extraesophageal manifestations of gastroesophageal reflux disease. Ann Surg. 2017;265:63-67. doi: 10.1097/SLA.0000000000001907
8. Eusebi LH, Ratnakumaran R, Yuan Y, et al. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. 2018;67:430-440. doi: 10.1136/gutjnl-2016-313589
9. Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212-239. doi: 10.1038/ajg.2016.563
10. Eusebi LH, Cirota GG, Zagari RM, et al. Global prevalence of Barrett’s oesophagus and oesophageal cancer in individuals with gastro-oesophageal reflux: a systematic review and meta-analysis. Gut. 2021;70:456-463. doi: 10.1136/gutjnl-2020-321365
11. Shaheen NJ, Falk GW, Iyer PG, et al; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol. 2016;111:30-50. doi: 10.1038/ajg.2015.322
12. Savarino E, Bredenoord AJ, Fox M, et al; International Working Group for Disorders of Gastrointestinal Motility and Function. Expert consensus document: advances in the physiological assessment and diagnosis of GERD. Nat Rev Gastroenterol Hepatol. 2017;14:665-676. doi: 10.1038/nrgastro.2017.130
13. Kahrilas PJ. Clinical practice. Gastroesophageal reflux disease. N Engl J Med. 2008;359:1700-1707. doi: 10.1056/NEJMcp0804684
14. Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152:706-715. doi: 10.1053/j.gastro.2017.01.031
15. Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:516-554. doi: 10.1097/MPG.0000000000001889
16. Kahrilas PJ, Shaheen NJ, Vaezi MF, et al. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383-1391. doi: 10.1053/j.gastro.2008.08.045
17. Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol. 2018;16:800-808. doi: 10.1016/j.cgh.2017.09.033
18. Moayyedi P, Eikelboom JW, Bosch J, et al. Safety of proton pump inhibitors based on a large, multi-year, randomized trial of patients receiving rivaroxaban or aspirin. Gastroenterology. 2019;157:682-691. doi: 10.1053/j.gastro.2019.05.056
19. Katzka DA, Kahrilas PJ. Advances in the diagnosis and management of gastroesophageal reflux disease. BMJ. 2020;371:m3786. doi: 10.1136/bmj.m3786
20. Ali RA, Egan LJ. Gastroesophageal reflux disease in pregnancy. Best Pract Res Clin Gastroenterol. 2007;21:793-806. doi: 10.1016/j.bpg.2007.05.006
21. Body C, Christie JA. Gastrointestinal diseases in pregnancy: nausea, vomiting, hyperemesis gravidarum, gastroesophageal reflux disease, constipation, and diarrhea. Gastroenterol Clin North Am. 2016;45:267-283. doi: 10.1016/j.gtc.2016.02.005
22. Lightdale JR, Gremse DA, et al. Gastroesophageal reflux: management guidance for the pediatrician. Pediatrics. 2013;131;e1684-e1695. doi: 10.1542/peds.2013-0421
23. Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153:420-429. doi: 10.1053/j.gastro.2017.04.022
24. Zamani M, Ebrahimtabar F, Zamani V, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47:868-876. doi: 10.1111/apt.14561
25. Choi IJ, Kim CG, Lee JY, et al. Family history of gastric cancer and Helicobacter pylori treatment. N Engl J Med. 2020;382:427-436. doi: 10.1056/NEJMoa1909666
26. Gisbert JP, Abraira V. Accuracy of Helicobacter pylori diagnostic tests in patients with bleeding peptic ulcer: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101:848-863. doi: 10.1111/j.1572-0241.2006.00528.x
27. Best LM, Takwoingi Y, Siddique S, et al. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst Rev. 2018;3:CD012080. doi: 10.1002/14651858.CD012080.pub2
28. Howden CW, Graham DY. Recent developments pertaining to H. pylori infection. Am J Gastroenterol. 2021;116:1-3. doi: 10.14309/ajg.0000000000001031
29. Shah SC, Iyer PG, Moss SF. AGA Clinical practice update on the management of refractory Helicobacter pylori infection: expert review. Gastroenterology. 2021;160:1831-1841. doi: 10.1053/j.gastro.2020.11.059
30. Yang-Ou YB, Hu Y, Zhu Y, et al. The effect of antioxidants on Helicobacter pylori eradication: a systematic review with meta-analysis. Helicobacter. 2018;23:e12535. doi: 10.1111/hel.12535
31. Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:1084-1091. doi: 10.1053/j.gastro.2011.01.030
32. Codipilly DC, Chandar AK, Singh S, et al. The effect of endoscopic surveillance in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gastroenterology. 2018;154:2068-2086. doi: 10.1053/j.gastro.2018.02.022
33. Singh S, Garg SK, Singh PP, et al. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta-analysis. Gut. 2014;63:1229-1237. doi: 10.1136/gutjnl-2013-305997
34. Jankowski JAZ, de Caestecker J, Love SB, et al. Esomeprazole and aspirin in Barrett’s oesophagus (AspECT): a randomised factorial trial. Lancet. 2018;392:400-408. doi: 10.1016/S0140-6736(18)31388-6
35. Hu Q, Sun TT, Hong J, et al. Proton pump inhibitors do not reduce the risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a systematic review and meta-analysis. PLoS One. 2017;12:e0169691. doi: 10.1371/journal.pone.0169691
1. Wang C, Yuan Y, Hunt RH. Helicobacter pylori infection and Barrett’s esophagus: a systematic review and meta-analysis. Am J Gastroenterol. 2009;104:492-500. doi: 10.1038/ajg.2008.37
2. Maret-Ouda J, Markar SR, Lagergren J. Gastroesophageal reflux disease: a review. JAMA. 2020;324:2536-2547. doi: 10.1001/jama.2020.21360
3. Scida S, Russo M, Miraglia C, et al. Relationship between Helicobacter pylori infection and GERD. Acta Biomed. 2018;89:40-43. doi: 10.23750/abm.v89i8-S.7918
4. Vakil N, Van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-1920. doi: 10.1111/j.1572-0241.2006.00630.x
5. Mikami DJ, Murayama KM. Physiology and pathogenesis of gastroesophageal reflux disease. Surg Clin North Am. 2015;95:515-525. doi: 10.1016/j.suc.2015.02.006
6. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308-328. doi: 10.1038/ajg.2012.444
7. Sidhwa F, Moore A, Alligood E, et al. Diagnosis and treatment of the extraesophageal manifestations of gastroesophageal reflux disease. Ann Surg. 2017;265:63-67. doi: 10.1097/SLA.0000000000001907
8. Eusebi LH, Ratnakumaran R, Yuan Y, et al. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. 2018;67:430-440. doi: 10.1136/gutjnl-2016-313589
9. Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212-239. doi: 10.1038/ajg.2016.563
10. Eusebi LH, Cirota GG, Zagari RM, et al. Global prevalence of Barrett’s oesophagus and oesophageal cancer in individuals with gastro-oesophageal reflux: a systematic review and meta-analysis. Gut. 2021;70:456-463. doi: 10.1136/gutjnl-2020-321365
11. Shaheen NJ, Falk GW, Iyer PG, et al; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol. 2016;111:30-50. doi: 10.1038/ajg.2015.322
12. Savarino E, Bredenoord AJ, Fox M, et al; International Working Group for Disorders of Gastrointestinal Motility and Function. Expert consensus document: advances in the physiological assessment and diagnosis of GERD. Nat Rev Gastroenterol Hepatol. 2017;14:665-676. doi: 10.1038/nrgastro.2017.130
13. Kahrilas PJ. Clinical practice. Gastroesophageal reflux disease. N Engl J Med. 2008;359:1700-1707. doi: 10.1056/NEJMcp0804684
14. Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152:706-715. doi: 10.1053/j.gastro.2017.01.031
15. Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:516-554. doi: 10.1097/MPG.0000000000001889
16. Kahrilas PJ, Shaheen NJ, Vaezi MF, et al. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383-1391. doi: 10.1053/j.gastro.2008.08.045
17. Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol. 2018;16:800-808. doi: 10.1016/j.cgh.2017.09.033
18. Moayyedi P, Eikelboom JW, Bosch J, et al. Safety of proton pump inhibitors based on a large, multi-year, randomized trial of patients receiving rivaroxaban or aspirin. Gastroenterology. 2019;157:682-691. doi: 10.1053/j.gastro.2019.05.056
19. Katzka DA, Kahrilas PJ. Advances in the diagnosis and management of gastroesophageal reflux disease. BMJ. 2020;371:m3786. doi: 10.1136/bmj.m3786
20. Ali RA, Egan LJ. Gastroesophageal reflux disease in pregnancy. Best Pract Res Clin Gastroenterol. 2007;21:793-806. doi: 10.1016/j.bpg.2007.05.006
21. Body C, Christie JA. Gastrointestinal diseases in pregnancy: nausea, vomiting, hyperemesis gravidarum, gastroesophageal reflux disease, constipation, and diarrhea. Gastroenterol Clin North Am. 2016;45:267-283. doi: 10.1016/j.gtc.2016.02.005
22. Lightdale JR, Gremse DA, et al. Gastroesophageal reflux: management guidance for the pediatrician. Pediatrics. 2013;131;e1684-e1695. doi: 10.1542/peds.2013-0421
23. Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153:420-429. doi: 10.1053/j.gastro.2017.04.022
24. Zamani M, Ebrahimtabar F, Zamani V, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47:868-876. doi: 10.1111/apt.14561
25. Choi IJ, Kim CG, Lee JY, et al. Family history of gastric cancer and Helicobacter pylori treatment. N Engl J Med. 2020;382:427-436. doi: 10.1056/NEJMoa1909666
26. Gisbert JP, Abraira V. Accuracy of Helicobacter pylori diagnostic tests in patients with bleeding peptic ulcer: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101:848-863. doi: 10.1111/j.1572-0241.2006.00528.x
27. Best LM, Takwoingi Y, Siddique S, et al. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst Rev. 2018;3:CD012080. doi: 10.1002/14651858.CD012080.pub2
28. Howden CW, Graham DY. Recent developments pertaining to H. pylori infection. Am J Gastroenterol. 2021;116:1-3. doi: 10.14309/ajg.0000000000001031
29. Shah SC, Iyer PG, Moss SF. AGA Clinical practice update on the management of refractory Helicobacter pylori infection: expert review. Gastroenterology. 2021;160:1831-1841. doi: 10.1053/j.gastro.2020.11.059
30. Yang-Ou YB, Hu Y, Zhu Y, et al. The effect of antioxidants on Helicobacter pylori eradication: a systematic review with meta-analysis. Helicobacter. 2018;23:e12535. doi: 10.1111/hel.12535
31. Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:1084-1091. doi: 10.1053/j.gastro.2011.01.030
32. Codipilly DC, Chandar AK, Singh S, et al. The effect of endoscopic surveillance in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gastroenterology. 2018;154:2068-2086. doi: 10.1053/j.gastro.2018.02.022
33. Singh S, Garg SK, Singh PP, et al. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta-analysis. Gut. 2014;63:1229-1237. doi: 10.1136/gutjnl-2013-305997
34. Jankowski JAZ, de Caestecker J, Love SB, et al. Esomeprazole and aspirin in Barrett’s oesophagus (AspECT): a randomised factorial trial. Lancet. 2018;392:400-408. doi: 10.1016/S0140-6736(18)31388-6
35. Hu Q, Sun TT, Hong J, et al. Proton pump inhibitors do not reduce the risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a systematic review and meta-analysis. PLoS One. 2017;12:e0169691. doi: 10.1371/journal.pone.0169691
PRACTICE RECOMMENDATIONS
› Recommend endoscopy for patients with gastroesophageal reflux disease (GERD) and red flag symptoms: dysphagia, unintentional weight loss, or bleeding. B
› Recommend long-term use of a proton pump inhibitor at the lowest tolerated dose in patients with esophagitis or Barrett esophagus. C
› Test for Helicobacter pylori in patients with peptic ulcer disease, in those with past ulcers not investigated for H pylori, and in those starting chronic nonsteroidal anti-inflammatory drug therapy. A
› Use a urea breath test, stool antigen study, or endoscopically obtained biopsy to test for H pylori. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Tips for managing 4 common soft-tissue finger and thumb injuries
Finger injuries are often seen in the primary care physician’s office. The evidence—and our experience in sports medicine—indicates that many of these injuries can be managed conservatively with bracing or injection; a subset, however, requires surgical referral. In this article, we provide a refresher on finger anatomy (see “A guide to the anatomic structures of the digits of the hand”1,2) and review the diagnosis and management of 4 common soft-tissue finger and thumb injuries in adults: trigger finger, jersey finger, mallet finger, and skier’s thumb (TABLE2-18).
Trigger finger
Also called stenosing flexor tenosynovitis, trigger finger is caused by abnormal flexor tendon movement that results from impingement at the level of the A1 pulley.
Causes and incidence. Impingement usually occurs because of thickening of the A1 pulley but can also be caused by inflammation or a nodule on the flexor tendon.3,4 The A1 pulley at the metacarpal head is the most proximal part of the retinacular sheath and therefore experiences the greatest force upon finger flexion, making it the most common site of inflammation and constriction.4

Trigger finger occurs in 2% to 3% of the general population and in as many as 10% of people with diabetes.5 The condition typically affects the long and ring fingers of the dominant hand; most cases occur in women in the sixth and seventh decades.3-5
Multiple systemic conditions predispose to trigger finger, including endocrine disorders (eg, diabetes, hypothyroidism), inflammatory arthropathies (gout, pseudogout), and autoimmune disorders (rheumatoid arthritis, sarcoidosis).3,5 Diabetes commonly causes bilateral hand and multiple digit involvement, as well as more severe disease.3,5 Occupation is also a risk factor for trigger finger because repetitive movements and manual work can exacerbate triggering.4
Presentation and exam. Patients report pain at the metacarpal head or metacarpophalangeal (MCP) joint, difficulty grasping objects, and, possibly, clicking and catching of the digit and locking of the digit in flexion.3,5
On exam, there might be tenderness at the level of the A1 pulley over the volar MCP joint or a palpable nodule. In severe cases, the proximal interphalangeal (PIP) joint or entire finger can be fixed in flexion.5 Repeated compound finger flexion (eg, closing and opening a fist) or holding a fist for as long as 1 minute and then slowly opening it might provoke triggering.
More than 60% of patients with trigger finger also have carpal tunnel syndrome.5 This makes it important to assess for (1) sensory changes in the distribution of the median nerve and (2) nerve compression, by eliciting Phalen and Tinel signs.4,5
Continue to: Imaging
Imaging. Trigger finger is a clinical diagnosis. Imaging is therefore unnecessary for diagnosis or treatment.5
Treatment. Trigger finger resolves spontaneously in 52% of cases.3 Most patients experience relief in 8 to 12 months.3
First-line treatment is injection of a corticosteroid into the flexor tendon sheath, which often alleviates symptoms.4,5 Injection is performed at the level of the A1 pulley on the palmar surface, just proximal to the MCP joint at the level of the distal palmar crease6 (FIGURE 1). The needle is inserted at an oblique angle until there is an increase in resistance. The needle is then slightly withdrawn to reposition it in the tendon sheath; 0.5 to 1 mL of 50% corticosteroid and 50% local anesthetic without epinephrine is then injected.6
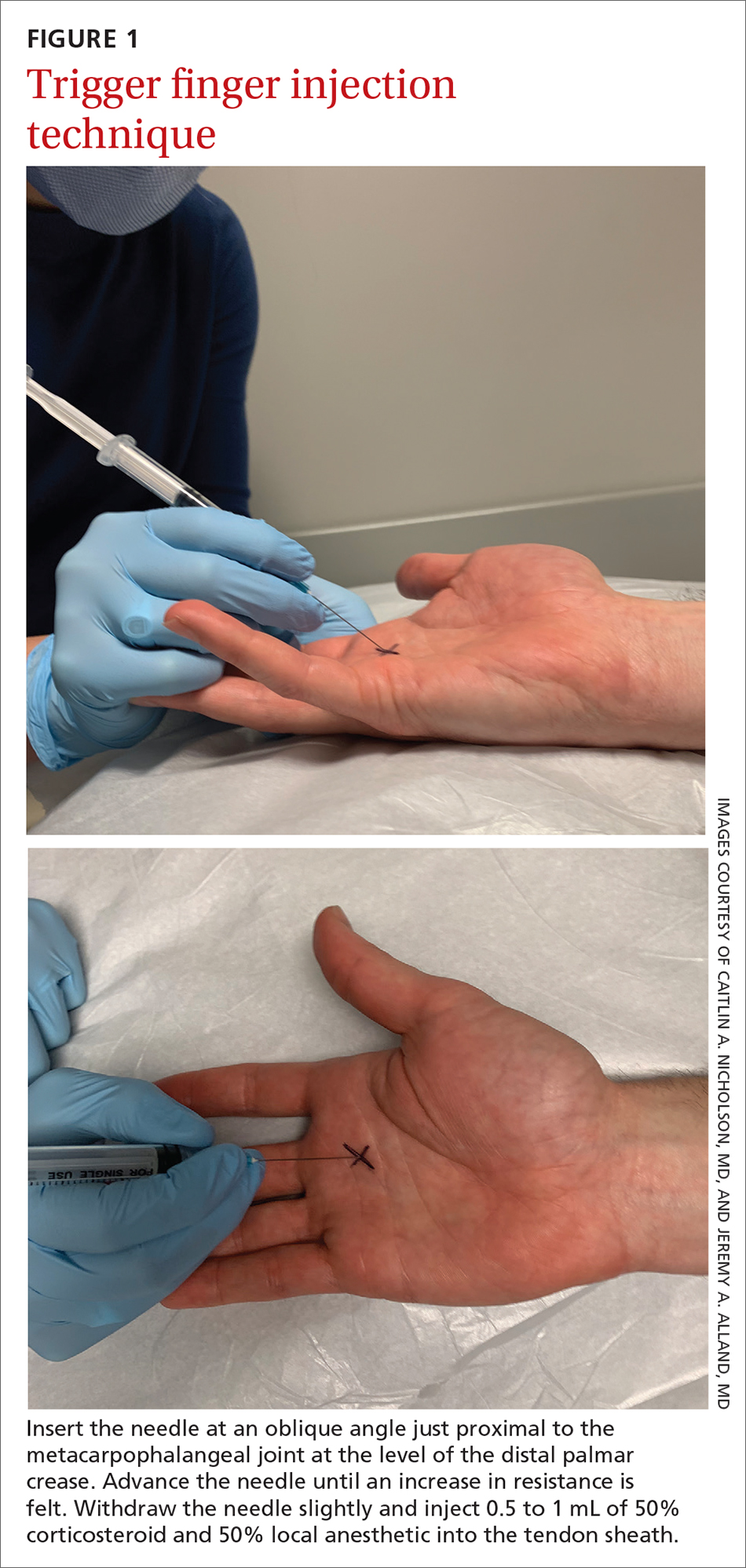
The cure rate of trigger finger is 57% to 70% with 1 injection and 82% to 86% after 2 injections.3,4,19
Many patients experience symptom relief in 1 to 4 weeks after a corticosteroid injection; however, as many as 56% experience repeat triggering within 6 months—often making multiple injections (maximum, 3 per digit) necessary.19,20 Patients who have a longer duration of symptoms, more severe symptoms, and multiple trigger fingers are less likely to experience relief with injections.3,5
Continue to: Splinting is an effective treatment...
Splinting is an effective treatment for patients who cannot undergo corticosteroid injection or surgery. The MCP or PIP joint is immobilized in extension while movement of the distal interphalangeal (DIP) joint is maintained. Instruct the patient that the splint must be worn day and night; splinting is continued for ≥ 6 weeks.21 Splinting relieves symptoms in 47% to 70% of cases and is most effective in patients whose symptoms have been present for < 6 months.3,7
Patients whose trigger finger is locked in flexion and those who have not experienced improvement after 2 or 3 corticosteroid injections should be referred for surgery.4 The surgical cure rate is nearly 100%; only 6% of patients experience repeat triggering 6 to 12 months postoperatively.4,7,22
Jersey finger
Causes and incidence. Jersey finger is caused by avulsion injury to the flexor digitorum profundus (FDP) tendon at its insertion on the distal phalanx.8,9 It occurs when a flexed finger is forced into extension, such as when a football or rugby player grabs another player’s jersey during a tackle.9,10 This action causes the FDP tendon to detach from the distal phalanx, sometimes with a bony fragment.9,11 Once detached, the tendon might retract proximally within the finger or to the palm, with consequent loss of its blood supply.9
Although jersey finger is not as common as the other conditions discussed in this article,9 it is important not to miss this diagnosis because of the risk of chronic disability when it is not treated promptly. Seventy-five percent of cases occur in the ring finger, which is more susceptible to injury because it extends past the other digits in a power grip.8,9
Presentation and exam. On exam, the affected finger lies in slight extension compared to the other digits; the patient is unable to actively flex the DIP joint.8,9 There may be tenderness to palpation over the volar distal phalanx. The retracted FDP tendon might be palpable more proximally in the digit.
Continue to: Imaging
Imaging. Anteroposterior (AP), oblique, and lateral radiographs, although unnecessary for diagnosis, are recommended to assess for an avulsion fragment, associated fracture, or dislocation.9,11 Ultrasonography or magnetic resonance imaging is useful in chronic cases to quantify the degree of tendon retraction.9
Treatment. Refer acute cases of jersey finger for surgical management urgently because most cases require flexor tendon repair within 1 or 2 weeks for a successful outcome.9 Chronic jersey finger, in which injury occurred > 6 weeks before presentation, also requires surgical repair, although not as urgently.9
Complications of jersey finger include flexion contracture at the DIP joint and the so-called quadriga effect, in which the patient is unable to fully flex the fingers adjacent to the injured digit.8 These complications can cause chronic disability in the affected hand, making early diagnosis and referral key to successful treatment.9
Mallet finger
Also called drop finger, mallet finger is a result of loss of active extension at the DIP joint.12,13
Causes and incidence. Mallet finger is a relatively common injury that typically affects the long, ring, or small finger of the dominant hand in young to middle-aged men and older women.12,14,23 The condition is the result of forced flexion or hyperextension injury, which disrupts the extensor tendon.6,14
Continue to: Sudden forced flexion...
Sudden forced flexion of an extended DIP joint during work or sports (eg, catching a ball) is the most common mechanism of injury.12,15 This action causes stretching or tearing of the extensor tendon as well as a possible avulsion fracture of the distal phalanx.13 Mallet finger can also result from a laceration or crush injury of the extensor tendon (open mallet finger) or hyperextension of the DIP joint, causing a fracture at the dorsal base of the distal phalanx.12
Presentation. Through any of the aforementioned mechanisms, the delicate balance between the flexor and extensor tendons is disrupted, causing the patient to present with a flexed DIP joint that can be passively, but not actively, extended.6,12 The DIP joint might also be painful and swollen. Patients whose injury occurred > 4 weeks prior to presentation (chronic mallet finger) might also have a so-called swan-neck deformity, with hyperextension of the PIP joint in the affected finger.12
Imaging. AP, oblique, and lateral radiographs are recommended to assess for bony injury.
Treatment. Splinting is the first-line treatment for almost all mallet finger injuries that are not the result of a laceration or crush injury. Immobilize the DIP joint in extension for 6 to 8 weeks, with an additional 2 to 4 weeks of splinting at night.6,12 The splint must be worn continuously in the initial 6 to 8 weeks, and the DIP joint should remain in extension—even when the patient is performing daily hygiene.12 It is imperative that patients comply with that period of continuous immobilization; if the DIP joint is allowed to flex, the course of treatment must be restarted.13
Many different types of splints exist; functional outcomes are equivalent across all of them.24,25 In our practice, we manage mallet finger with a volar-based splint (FIGURE 2), which is associated with fewer dermatologic complications and has provided the most success for our patients.23
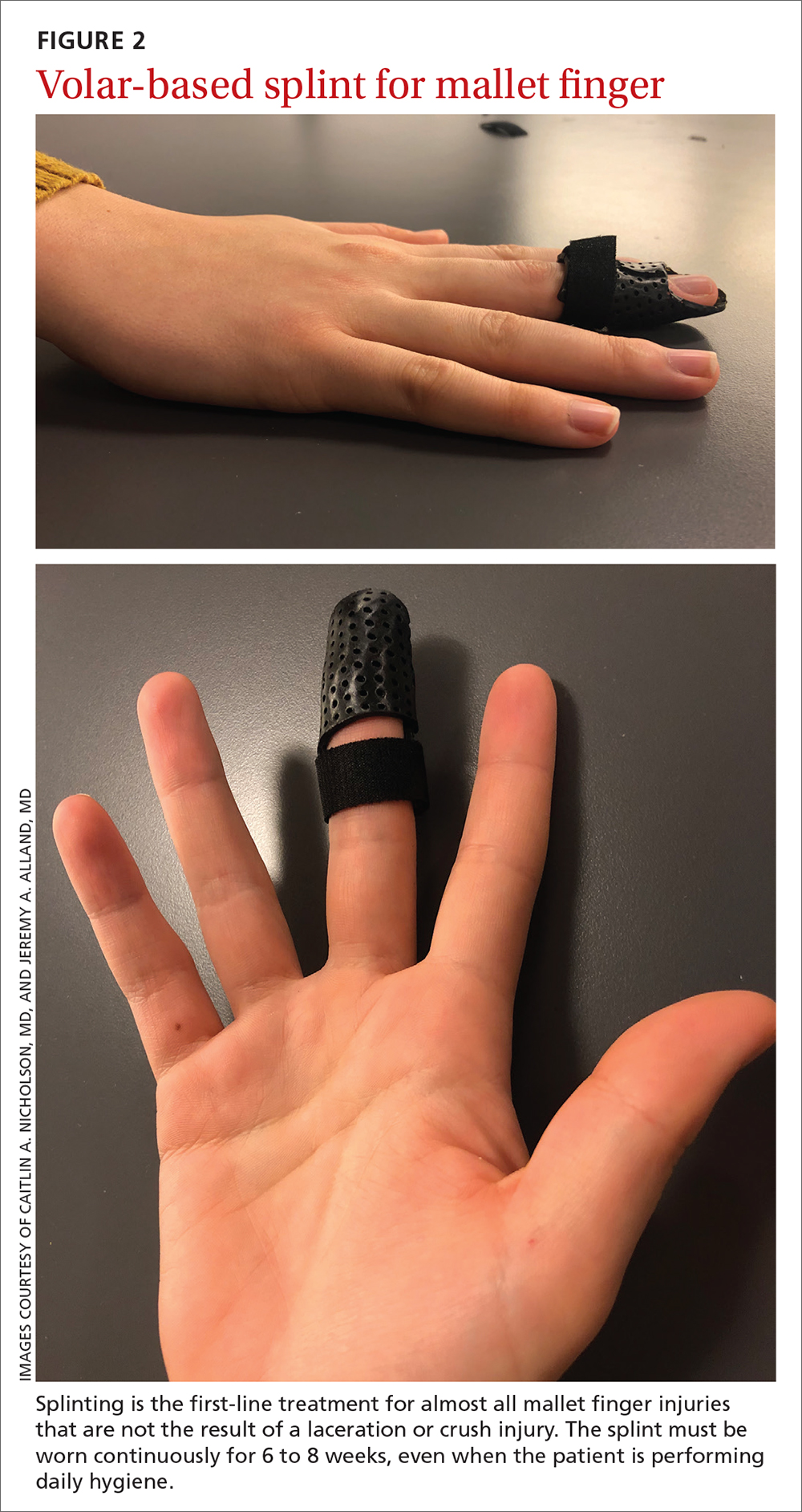
Continue to: Surgical repair of mallet finger injury...
Surgical repair of mallet finger injury is indicated in any of these situations12,14:
- injury is caused by laceration
- there is volar subluxation of the DIP joint
- more than one-third of the articular surface is involved in an avulsion fracture.
Patients who cannot comply with wearing a splint 24 hours per day or whose occupation precludes wearing a splint at all (eg, surgeons, dentists, musicians) are also surgical candidates.12
Surgical and conservative treatments have similar clinical and functional outcomes, including loss of approximately 5° to 7° of active extension and an increased risk of DIP joint osteoarthritis.12,14,24 Patients with chronic mallet finger can be managed with 6 weeks of splinting initially but will likely require surgery.6,12,13
Skier’s thumb
This relatively common injury is a tear of the ulnar collateral ligament (UCL) at the MCP joint of the thumb.16
Causes and incidence. Skier’s thumb occurs when a valgus force hyperabducts the thumb,16 and is so named because the injury is often seen in recreational skiers who fall while holding a ski pole.15-17 It can also occur in racquet sports when a ball or racquet strikes the ulnar side of thumb.16
Continue to: In chronic cases...
In chronic cases, the UCL can be injured by occupational demands and is termed gamekeeper’s thumb because it was first described in this population, who killed game by breaking the animal's neck between the thumb and index finger against the ground.16,18 A UCL tear causes instability at the thumb MCP joint, which affects a person’s ability to grip and pinch.2,16,18
Presentation. On exam, the affected thumb is swollen and, possibly, bruised. There might be radial deviation and volar subluxation of the proximal phalanx. The ulnar side of the MCP joint is tender to palpation.16 If the distal UCL is torn completely, it can displace proximally and present as a palpable mass over the ulnar side of the MCP joint, known as a Stener lesion.16
Stress testing of the MCP joint is the most important part of the physical exam for skier’s thumb. Stabilize the metacarpal neck and apply a valgus stress on the proximal phalanx at both 0° and 30° of MCP flexion (FIGURE 3), which allows for assessment of both the proper and accessory bands of the UCL.2,16 (A common pitfall during stress testing is to allow the MCP joint to rotate, which can mimic instability.2) Intra-articular local anesthesia might be necessary for this exam because it can be painful.16,18,26 A stress exam should assess for laxity and a soft or firm endpoint; the result should be compared to that of a stress exam on the contralateral side.16,17
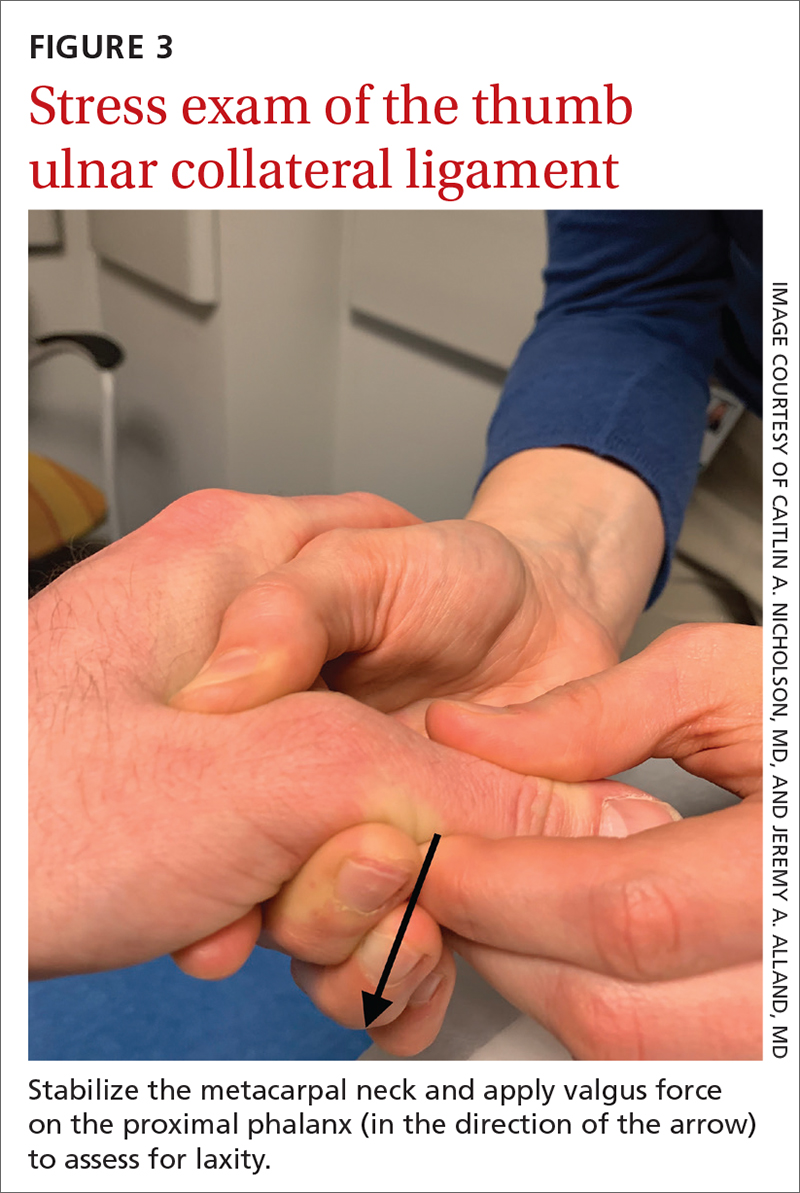
Imaging. AP, oblique, and lateral radiographs of the thumb should be obtained to assess for instability, avulsion injury, and associated fracture. Subluxation (volar or radial) or supination of the proximal phalanx relative to the metacarpal on imaging suggests MCP instability of the MCP joint.16,17
If the stress exam is equivocal, magnetic resonance imaging is recommended for further assessment.2,18
Continue to: Stress radiographs...
Stress radiographs (ie, radiographs of the thumb with valgus stress applied at the MCP joint) can aid in diagnosis but are controversial. Some experts think that these stress views can further damage the UCL; others recommend against them because they carry a false-negative rate ≥ 25%.15,16 If you choose to perform stress views, order standard radiographs beforehand to rule out bony injury.17
Treatment. UCL tears are classified as 3 tiers to guide treatment.
- Grade 1 injury (a partial tear) is characterized by pain upon palpation but no instability on the stress exam.
- Grade 2 injury (also a partial tear) is marked by laxity on the stress exam with a firm endpoint.
- Grade 3 injury (complete tear) shows laxity and a soft endpoint on a stress exam16,17; Stener lesions are seen only in grade 3 tears.16,17
Grades 1 and 2 UCL tears without fracture or with a nondisplaced avulsion fracture can be managed nonoperatively by immobilizing the thumb in a spica splint or cast for 4 to 6 weeks.16,18 The MCP joint is immobilized and the interphalangeal joint is allowed to move freely.2,16,17
Grade 3 injuries should be referred to a hand specialist for surgical repair.16 Patients presenting > 12 weeks after acute injury or with a chronic UCL tear should also be referred for surgical repair.16
CORRESPONDENCE
Caitlin A. Nicholson, MD, 1611 West Harrison Street, Suite 300, Chicago, IL 60612; [email protected]
1. Hirt B, Seyhan H, Wagner M, et al. Hand and Wrist Anatomy and Biomechanics: A Comprehensive Guide. Thieme; 2017:57,58,71,72,75-80.
2. Daley D, Geary M, Gaston RG. Thumb metacarpophalangeal ulnar and radial collateral ligament injuries. Clin Sports Med. 2020;39:443-455. doi: 10.1016/j.csm.2019.12.003
3. Gil JA, Hresko AM, Weiss AC. Current concepts in the management of trigger finger in adults. J Am Acad Orthop Surg. 2020;28:e642-e650. doi: 10.5435/JAAOS-D-19-00614
4. Henton J, Jain A, Medhurst C, et al. Adult trigger finger. BMJ. 2012;345:e5743. doi: 10.1136/bmj.e5743
5. Bates T, Dunn J. Trigger finger. Orthobullets [Internet]. Updated December 8, 2021. Accessed April 14, 2022. www.orthobullets.com/hand/6027/trigger-finger
6. Chhabra AB, Deal ND. Soft tissue injuries of the wrist and hand. In: O’Connor FG, Casa DJ, Davis BA, et al. ACSM’s Sports Medicine: A Comprehensive Review. Lippincott Williams & Wilkins; 2012:370-373.
7. Ballard TNS, Kozlow JH. Trigger finger in adults. CMAJ. 2016;188:61. doi: 10.1503/cmaj.150225
8. Vitale M. Jersey finger. Orthobullets [Internet]. Updated May 22, 2021. 2019. Accessed April 15, 2022. www.orthobullets.com/hand/6015/jersey-finger
9. Shapiro LM, Kamal RN. Evaluation and treatment of flexor tendon and pulley injuries in athletes. Clin Sports Med. 2020;39:279-297. doi: 10.1016/j.csm.2019.12.004
10. Goodson A, Morgan M, Rajeswaran G, et al. Current management of Jersey finger in rugby players: case series and literature review. Hand Surg. 2010;15:103-107. doi: 10.1142/S0218810410004710
11. Lapegue F, Andre A, Brun C, et al. Traumatic flexor tendon injuries. Diagn Interv Imaging. 2015;96:1279-1292. doi: 10.1016/j.diii.2015.09.010
12. Bendre AA, Hartigan BJ, Kalainov DM. Mallet finger. J Am Acad Orthop Surg. 2005;13:336-344. doi: 10.5435/00124635-200509000-00007
13. Lamaris GA, Matthew MK. The diagnosis and management of mallet finger injuries. Hand (N Y). 2017;12:223-228. doi: 10.1177/1558944716642763
14. Sheth U. Mallet finger. Orthobullets [Internet]. Updated August 5, 2021. Accessed April 15, 2022. www.orthobullets.com/hand/6014/mallet-finger
15. Weintraub MD, Hansford BG, Stilwill SE, et al. Avulsion injuries of the hand and wrist. Radiographics. 2020;40:163-180. doi: 10.1148/rg.2020190085
16. Avery III DM, Inkellis ER, Carlson MG. Thumb collateral ligament injuries in the athlete. Curr Rev Musculoskelet Med. 2017;10:28-37. doi: 10.1007/s12178-017-9381-z
17. Steffes MJ. Thumb collateral ligament injury. Orthobullets [Internet]. Updated February 18, 2022. Accessed April 15, 2022. www.orthobullets.com/hand/6040/thumb-collateral-ligament-injury
18. Madan SS, Pai DR, Kaur A, et al. Injury to ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7. doi: 10.1111/os.12084
19. Dardas AZ, VandenBerg J, Shen T, et al. Long-term effectiveness of repeat corticosteroid injections for trigger finger. J Hand Surg Am. 2017;42:227-235. doi: 10.1016/j.jhsa.2017.02.001
20. Huisstede BM, Gladdines S, Randsdorp MS, et al. Effectiveness of conservative, surgical, and postsurgical interventions for trigger finger, Dupuytren disease, and de Quervain disease: a systematic review. Arch Phys Med Rehabil. 2018;99:1635-1649.e21. doi: 10.1016/j.apmr.2017.07.014
21. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
22. Fiorini HJ, Tamaoki MJ, Lenza M, et al. Surgery for trigger finger. Cochrane Database Syst Rev. 2018;2:CD009860. doi: 10.1002/14651858.CD009860.pub2
23. Salazar Botero S, Hidalgo Diaz JJ, Benaïda A, et al. Review of acute traumatic closed mallet finger injuries in adults. Arch Plast Surg. 2016;43:134-144. doi: 10.5999/aps.2016.43.2.134
24. Lin JS, Samora JB. Surgical and nonsurgical management of mallet finger: a systematic review. J Hand Surg Am. 2018;43:146-163.e2. doi: 10.1016/j.jhsa.2017.10.004
25. Handoll H, Vaghela MV. Interventions for treating mallet finger injuries. Cochrane Database Syst Rev. 2004;(3):CD004574. doi: 10.1002/14651858.CD004574.pub2
26. Pulos N, Shin AY. Treatment of ulnar collateral ligament injuries of the thumb: a critical analysis review. JBJS Rev. 2017;5:e3. doi: 10.2106/JBJS.RVW.16.00051
Finger injuries are often seen in the primary care physician’s office. The evidence—and our experience in sports medicine—indicates that many of these injuries can be managed conservatively with bracing or injection; a subset, however, requires surgical referral. In this article, we provide a refresher on finger anatomy (see “A guide to the anatomic structures of the digits of the hand”1,2) and review the diagnosis and management of 4 common soft-tissue finger and thumb injuries in adults: trigger finger, jersey finger, mallet finger, and skier’s thumb (TABLE2-18).


Trigger finger
Also called stenosing flexor tenosynovitis, trigger finger is caused by abnormal flexor tendon movement that results from impingement at the level of the A1 pulley.
Causes and incidence. Impingement usually occurs because of thickening of the A1 pulley but can also be caused by inflammation or a nodule on the flexor tendon.3,4 The A1 pulley at the metacarpal head is the most proximal part of the retinacular sheath and therefore experiences the greatest force upon finger flexion, making it the most common site of inflammation and constriction.4

Trigger finger occurs in 2% to 3% of the general population and in as many as 10% of people with diabetes.5 The condition typically affects the long and ring fingers of the dominant hand; most cases occur in women in the sixth and seventh decades.3-5
Multiple systemic conditions predispose to trigger finger, including endocrine disorders (eg, diabetes, hypothyroidism), inflammatory arthropathies (gout, pseudogout), and autoimmune disorders (rheumatoid arthritis, sarcoidosis).3,5 Diabetes commonly causes bilateral hand and multiple digit involvement, as well as more severe disease.3,5 Occupation is also a risk factor for trigger finger because repetitive movements and manual work can exacerbate triggering.4
Presentation and exam. Patients report pain at the metacarpal head or metacarpophalangeal (MCP) joint, difficulty grasping objects, and, possibly, clicking and catching of the digit and locking of the digit in flexion.3,5
On exam, there might be tenderness at the level of the A1 pulley over the volar MCP joint or a palpable nodule. In severe cases, the proximal interphalangeal (PIP) joint or entire finger can be fixed in flexion.5 Repeated compound finger flexion (eg, closing and opening a fist) or holding a fist for as long as 1 minute and then slowly opening it might provoke triggering.
More than 60% of patients with trigger finger also have carpal tunnel syndrome.5 This makes it important to assess for (1) sensory changes in the distribution of the median nerve and (2) nerve compression, by eliciting Phalen and Tinel signs.4,5
Continue to: Imaging
Imaging. Trigger finger is a clinical diagnosis. Imaging is therefore unnecessary for diagnosis or treatment.5
Treatment. Trigger finger resolves spontaneously in 52% of cases.3 Most patients experience relief in 8 to 12 months.3
First-line treatment is injection of a corticosteroid into the flexor tendon sheath, which often alleviates symptoms.4,5 Injection is performed at the level of the A1 pulley on the palmar surface, just proximal to the MCP joint at the level of the distal palmar crease6 (FIGURE 1). The needle is inserted at an oblique angle until there is an increase in resistance. The needle is then slightly withdrawn to reposition it in the tendon sheath; 0.5 to 1 mL of 50% corticosteroid and 50% local anesthetic without epinephrine is then injected.6

The cure rate of trigger finger is 57% to 70% with 1 injection and 82% to 86% after 2 injections.3,4,19
Many patients experience symptom relief in 1 to 4 weeks after a corticosteroid injection; however, as many as 56% experience repeat triggering within 6 months—often making multiple injections (maximum, 3 per digit) necessary.19,20 Patients who have a longer duration of symptoms, more severe symptoms, and multiple trigger fingers are less likely to experience relief with injections.3,5
Continue to: Splinting is an effective treatment...
Splinting is an effective treatment for patients who cannot undergo corticosteroid injection or surgery. The MCP or PIP joint is immobilized in extension while movement of the distal interphalangeal (DIP) joint is maintained. Instruct the patient that the splint must be worn day and night; splinting is continued for ≥ 6 weeks.21 Splinting relieves symptoms in 47% to 70% of cases and is most effective in patients whose symptoms have been present for < 6 months.3,7
Patients whose trigger finger is locked in flexion and those who have not experienced improvement after 2 or 3 corticosteroid injections should be referred for surgery.4 The surgical cure rate is nearly 100%; only 6% of patients experience repeat triggering 6 to 12 months postoperatively.4,7,22
Jersey finger
Causes and incidence. Jersey finger is caused by avulsion injury to the flexor digitorum profundus (FDP) tendon at its insertion on the distal phalanx.8,9 It occurs when a flexed finger is forced into extension, such as when a football or rugby player grabs another player’s jersey during a tackle.9,10 This action causes the FDP tendon to detach from the distal phalanx, sometimes with a bony fragment.9,11 Once detached, the tendon might retract proximally within the finger or to the palm, with consequent loss of its blood supply.9
Although jersey finger is not as common as the other conditions discussed in this article,9 it is important not to miss this diagnosis because of the risk of chronic disability when it is not treated promptly. Seventy-five percent of cases occur in the ring finger, which is more susceptible to injury because it extends past the other digits in a power grip.8,9
Presentation and exam. On exam, the affected finger lies in slight extension compared to the other digits; the patient is unable to actively flex the DIP joint.8,9 There may be tenderness to palpation over the volar distal phalanx. The retracted FDP tendon might be palpable more proximally in the digit.
Continue to: Imaging
Imaging. Anteroposterior (AP), oblique, and lateral radiographs, although unnecessary for diagnosis, are recommended to assess for an avulsion fragment, associated fracture, or dislocation.9,11 Ultrasonography or magnetic resonance imaging is useful in chronic cases to quantify the degree of tendon retraction.9
Treatment. Refer acute cases of jersey finger for surgical management urgently because most cases require flexor tendon repair within 1 or 2 weeks for a successful outcome.9 Chronic jersey finger, in which injury occurred > 6 weeks before presentation, also requires surgical repair, although not as urgently.9
Complications of jersey finger include flexion contracture at the DIP joint and the so-called quadriga effect, in which the patient is unable to fully flex the fingers adjacent to the injured digit.8 These complications can cause chronic disability in the affected hand, making early diagnosis and referral key to successful treatment.9
Mallet finger
Also called drop finger, mallet finger is a result of loss of active extension at the DIP joint.12,13
Causes and incidence. Mallet finger is a relatively common injury that typically affects the long, ring, or small finger of the dominant hand in young to middle-aged men and older women.12,14,23 The condition is the result of forced flexion or hyperextension injury, which disrupts the extensor tendon.6,14
Continue to: Sudden forced flexion...
Sudden forced flexion of an extended DIP joint during work or sports (eg, catching a ball) is the most common mechanism of injury.12,15 This action causes stretching or tearing of the extensor tendon as well as a possible avulsion fracture of the distal phalanx.13 Mallet finger can also result from a laceration or crush injury of the extensor tendon (open mallet finger) or hyperextension of the DIP joint, causing a fracture at the dorsal base of the distal phalanx.12
Presentation. Through any of the aforementioned mechanisms, the delicate balance between the flexor and extensor tendons is disrupted, causing the patient to present with a flexed DIP joint that can be passively, but not actively, extended.6,12 The DIP joint might also be painful and swollen. Patients whose injury occurred > 4 weeks prior to presentation (chronic mallet finger) might also have a so-called swan-neck deformity, with hyperextension of the PIP joint in the affected finger.12
Imaging. AP, oblique, and lateral radiographs are recommended to assess for bony injury.
Treatment. Splinting is the first-line treatment for almost all mallet finger injuries that are not the result of a laceration or crush injury. Immobilize the DIP joint in extension for 6 to 8 weeks, with an additional 2 to 4 weeks of splinting at night.6,12 The splint must be worn continuously in the initial 6 to 8 weeks, and the DIP joint should remain in extension—even when the patient is performing daily hygiene.12 It is imperative that patients comply with that period of continuous immobilization; if the DIP joint is allowed to flex, the course of treatment must be restarted.13
Many different types of splints exist; functional outcomes are equivalent across all of them.24,25 In our practice, we manage mallet finger with a volar-based splint (FIGURE 2), which is associated with fewer dermatologic complications and has provided the most success for our patients.23

Continue to: Surgical repair of mallet finger injury...
Surgical repair of mallet finger injury is indicated in any of these situations12,14:
- injury is caused by laceration
- there is volar subluxation of the DIP joint
- more than one-third of the articular surface is involved in an avulsion fracture.
Patients who cannot comply with wearing a splint 24 hours per day or whose occupation precludes wearing a splint at all (eg, surgeons, dentists, musicians) are also surgical candidates.12
Surgical and conservative treatments have similar clinical and functional outcomes, including loss of approximately 5° to 7° of active extension and an increased risk of DIP joint osteoarthritis.12,14,24 Patients with chronic mallet finger can be managed with 6 weeks of splinting initially but will likely require surgery.6,12,13
Skier’s thumb
This relatively common injury is a tear of the ulnar collateral ligament (UCL) at the MCP joint of the thumb.16
Causes and incidence. Skier’s thumb occurs when a valgus force hyperabducts the thumb,16 and is so named because the injury is often seen in recreational skiers who fall while holding a ski pole.15-17 It can also occur in racquet sports when a ball or racquet strikes the ulnar side of thumb.16
Continue to: In chronic cases...
In chronic cases, the UCL can be injured by occupational demands and is termed gamekeeper’s thumb because it was first described in this population, who killed game by breaking the animal's neck between the thumb and index finger against the ground.16,18 A UCL tear causes instability at the thumb MCP joint, which affects a person’s ability to grip and pinch.2,16,18
Presentation. On exam, the affected thumb is swollen and, possibly, bruised. There might be radial deviation and volar subluxation of the proximal phalanx. The ulnar side of the MCP joint is tender to palpation.16 If the distal UCL is torn completely, it can displace proximally and present as a palpable mass over the ulnar side of the MCP joint, known as a Stener lesion.16
Stress testing of the MCP joint is the most important part of the physical exam for skier’s thumb. Stabilize the metacarpal neck and apply a valgus stress on the proximal phalanx at both 0° and 30° of MCP flexion (FIGURE 3), which allows for assessment of both the proper and accessory bands of the UCL.2,16 (A common pitfall during stress testing is to allow the MCP joint to rotate, which can mimic instability.2) Intra-articular local anesthesia might be necessary for this exam because it can be painful.16,18,26 A stress exam should assess for laxity and a soft or firm endpoint; the result should be compared to that of a stress exam on the contralateral side.16,17

Imaging. AP, oblique, and lateral radiographs of the thumb should be obtained to assess for instability, avulsion injury, and associated fracture. Subluxation (volar or radial) or supination of the proximal phalanx relative to the metacarpal on imaging suggests MCP instability of the MCP joint.16,17
If the stress exam is equivocal, magnetic resonance imaging is recommended for further assessment.2,18
Continue to: Stress radiographs...
Stress radiographs (ie, radiographs of the thumb with valgus stress applied at the MCP joint) can aid in diagnosis but are controversial. Some experts think that these stress views can further damage the UCL; others recommend against them because they carry a false-negative rate ≥ 25%.15,16 If you choose to perform stress views, order standard radiographs beforehand to rule out bony injury.17
Treatment. UCL tears are classified as 3 tiers to guide treatment.
- Grade 1 injury (a partial tear) is characterized by pain upon palpation but no instability on the stress exam.
- Grade 2 injury (also a partial tear) is marked by laxity on the stress exam with a firm endpoint.
- Grade 3 injury (complete tear) shows laxity and a soft endpoint on a stress exam16,17; Stener lesions are seen only in grade 3 tears.16,17
Grades 1 and 2 UCL tears without fracture or with a nondisplaced avulsion fracture can be managed nonoperatively by immobilizing the thumb in a spica splint or cast for 4 to 6 weeks.16,18 The MCP joint is immobilized and the interphalangeal joint is allowed to move freely.2,16,17
Grade 3 injuries should be referred to a hand specialist for surgical repair.16 Patients presenting > 12 weeks after acute injury or with a chronic UCL tear should also be referred for surgical repair.16
CORRESPONDENCE
Caitlin A. Nicholson, MD, 1611 West Harrison Street, Suite 300, Chicago, IL 60612; [email protected]
Finger injuries are often seen in the primary care physician’s office. The evidence—and our experience in sports medicine—indicates that many of these injuries can be managed conservatively with bracing or injection; a subset, however, requires surgical referral. In this article, we provide a refresher on finger anatomy (see “A guide to the anatomic structures of the digits of the hand”1,2) and review the diagnosis and management of 4 common soft-tissue finger and thumb injuries in adults: trigger finger, jersey finger, mallet finger, and skier’s thumb (TABLE2-18).


Trigger finger
Also called stenosing flexor tenosynovitis, trigger finger is caused by abnormal flexor tendon movement that results from impingement at the level of the A1 pulley.
Causes and incidence. Impingement usually occurs because of thickening of the A1 pulley but can also be caused by inflammation or a nodule on the flexor tendon.3,4 The A1 pulley at the metacarpal head is the most proximal part of the retinacular sheath and therefore experiences the greatest force upon finger flexion, making it the most common site of inflammation and constriction.4

Trigger finger occurs in 2% to 3% of the general population and in as many as 10% of people with diabetes.5 The condition typically affects the long and ring fingers of the dominant hand; most cases occur in women in the sixth and seventh decades.3-5
Multiple systemic conditions predispose to trigger finger, including endocrine disorders (eg, diabetes, hypothyroidism), inflammatory arthropathies (gout, pseudogout), and autoimmune disorders (rheumatoid arthritis, sarcoidosis).3,5 Diabetes commonly causes bilateral hand and multiple digit involvement, as well as more severe disease.3,5 Occupation is also a risk factor for trigger finger because repetitive movements and manual work can exacerbate triggering.4
Presentation and exam. Patients report pain at the metacarpal head or metacarpophalangeal (MCP) joint, difficulty grasping objects, and, possibly, clicking and catching of the digit and locking of the digit in flexion.3,5
On exam, there might be tenderness at the level of the A1 pulley over the volar MCP joint or a palpable nodule. In severe cases, the proximal interphalangeal (PIP) joint or entire finger can be fixed in flexion.5 Repeated compound finger flexion (eg, closing and opening a fist) or holding a fist for as long as 1 minute and then slowly opening it might provoke triggering.
More than 60% of patients with trigger finger also have carpal tunnel syndrome.5 This makes it important to assess for (1) sensory changes in the distribution of the median nerve and (2) nerve compression, by eliciting Phalen and Tinel signs.4,5
Continue to: Imaging
Imaging. Trigger finger is a clinical diagnosis. Imaging is therefore unnecessary for diagnosis or treatment.5
Treatment. Trigger finger resolves spontaneously in 52% of cases.3 Most patients experience relief in 8 to 12 months.3
First-line treatment is injection of a corticosteroid into the flexor tendon sheath, which often alleviates symptoms.4,5 Injection is performed at the level of the A1 pulley on the palmar surface, just proximal to the MCP joint at the level of the distal palmar crease6 (FIGURE 1). The needle is inserted at an oblique angle until there is an increase in resistance. The needle is then slightly withdrawn to reposition it in the tendon sheath; 0.5 to 1 mL of 50% corticosteroid and 50% local anesthetic without epinephrine is then injected.6

The cure rate of trigger finger is 57% to 70% with 1 injection and 82% to 86% after 2 injections.3,4,19
Many patients experience symptom relief in 1 to 4 weeks after a corticosteroid injection; however, as many as 56% experience repeat triggering within 6 months—often making multiple injections (maximum, 3 per digit) necessary.19,20 Patients who have a longer duration of symptoms, more severe symptoms, and multiple trigger fingers are less likely to experience relief with injections.3,5
Continue to: Splinting is an effective treatment...
Splinting is an effective treatment for patients who cannot undergo corticosteroid injection or surgery. The MCP or PIP joint is immobilized in extension while movement of the distal interphalangeal (DIP) joint is maintained. Instruct the patient that the splint must be worn day and night; splinting is continued for ≥ 6 weeks.21 Splinting relieves symptoms in 47% to 70% of cases and is most effective in patients whose symptoms have been present for < 6 months.3,7
Patients whose trigger finger is locked in flexion and those who have not experienced improvement after 2 or 3 corticosteroid injections should be referred for surgery.4 The surgical cure rate is nearly 100%; only 6% of patients experience repeat triggering 6 to 12 months postoperatively.4,7,22
Jersey finger
Causes and incidence. Jersey finger is caused by avulsion injury to the flexor digitorum profundus (FDP) tendon at its insertion on the distal phalanx.8,9 It occurs when a flexed finger is forced into extension, such as when a football or rugby player grabs another player’s jersey during a tackle.9,10 This action causes the FDP tendon to detach from the distal phalanx, sometimes with a bony fragment.9,11 Once detached, the tendon might retract proximally within the finger or to the palm, with consequent loss of its blood supply.9
Although jersey finger is not as common as the other conditions discussed in this article,9 it is important not to miss this diagnosis because of the risk of chronic disability when it is not treated promptly. Seventy-five percent of cases occur in the ring finger, which is more susceptible to injury because it extends past the other digits in a power grip.8,9
Presentation and exam. On exam, the affected finger lies in slight extension compared to the other digits; the patient is unable to actively flex the DIP joint.8,9 There may be tenderness to palpation over the volar distal phalanx. The retracted FDP tendon might be palpable more proximally in the digit.
Continue to: Imaging
Imaging. Anteroposterior (AP), oblique, and lateral radiographs, although unnecessary for diagnosis, are recommended to assess for an avulsion fragment, associated fracture, or dislocation.9,11 Ultrasonography or magnetic resonance imaging is useful in chronic cases to quantify the degree of tendon retraction.9
Treatment. Refer acute cases of jersey finger for surgical management urgently because most cases require flexor tendon repair within 1 or 2 weeks for a successful outcome.9 Chronic jersey finger, in which injury occurred > 6 weeks before presentation, also requires surgical repair, although not as urgently.9
Complications of jersey finger include flexion contracture at the DIP joint and the so-called quadriga effect, in which the patient is unable to fully flex the fingers adjacent to the injured digit.8 These complications can cause chronic disability in the affected hand, making early diagnosis and referral key to successful treatment.9
Mallet finger
Also called drop finger, mallet finger is a result of loss of active extension at the DIP joint.12,13
Causes and incidence. Mallet finger is a relatively common injury that typically affects the long, ring, or small finger of the dominant hand in young to middle-aged men and older women.12,14,23 The condition is the result of forced flexion or hyperextension injury, which disrupts the extensor tendon.6,14
Continue to: Sudden forced flexion...
Sudden forced flexion of an extended DIP joint during work or sports (eg, catching a ball) is the most common mechanism of injury.12,15 This action causes stretching or tearing of the extensor tendon as well as a possible avulsion fracture of the distal phalanx.13 Mallet finger can also result from a laceration or crush injury of the extensor tendon (open mallet finger) or hyperextension of the DIP joint, causing a fracture at the dorsal base of the distal phalanx.12
Presentation. Through any of the aforementioned mechanisms, the delicate balance between the flexor and extensor tendons is disrupted, causing the patient to present with a flexed DIP joint that can be passively, but not actively, extended.6,12 The DIP joint might also be painful and swollen. Patients whose injury occurred > 4 weeks prior to presentation (chronic mallet finger) might also have a so-called swan-neck deformity, with hyperextension of the PIP joint in the affected finger.12
Imaging. AP, oblique, and lateral radiographs are recommended to assess for bony injury.
Treatment. Splinting is the first-line treatment for almost all mallet finger injuries that are not the result of a laceration or crush injury. Immobilize the DIP joint in extension for 6 to 8 weeks, with an additional 2 to 4 weeks of splinting at night.6,12 The splint must be worn continuously in the initial 6 to 8 weeks, and the DIP joint should remain in extension—even when the patient is performing daily hygiene.12 It is imperative that patients comply with that period of continuous immobilization; if the DIP joint is allowed to flex, the course of treatment must be restarted.13
Many different types of splints exist; functional outcomes are equivalent across all of them.24,25 In our practice, we manage mallet finger with a volar-based splint (FIGURE 2), which is associated with fewer dermatologic complications and has provided the most success for our patients.23

Continue to: Surgical repair of mallet finger injury...
Surgical repair of mallet finger injury is indicated in any of these situations12,14:
- injury is caused by laceration
- there is volar subluxation of the DIP joint
- more than one-third of the articular surface is involved in an avulsion fracture.
Patients who cannot comply with wearing a splint 24 hours per day or whose occupation precludes wearing a splint at all (eg, surgeons, dentists, musicians) are also surgical candidates.12
Surgical and conservative treatments have similar clinical and functional outcomes, including loss of approximately 5° to 7° of active extension and an increased risk of DIP joint osteoarthritis.12,14,24 Patients with chronic mallet finger can be managed with 6 weeks of splinting initially but will likely require surgery.6,12,13
Skier’s thumb
This relatively common injury is a tear of the ulnar collateral ligament (UCL) at the MCP joint of the thumb.16
Causes and incidence. Skier’s thumb occurs when a valgus force hyperabducts the thumb,16 and is so named because the injury is often seen in recreational skiers who fall while holding a ski pole.15-17 It can also occur in racquet sports when a ball or racquet strikes the ulnar side of thumb.16
Continue to: In chronic cases...
In chronic cases, the UCL can be injured by occupational demands and is termed gamekeeper’s thumb because it was first described in this population, who killed game by breaking the animal's neck between the thumb and index finger against the ground.16,18 A UCL tear causes instability at the thumb MCP joint, which affects a person’s ability to grip and pinch.2,16,18
Presentation. On exam, the affected thumb is swollen and, possibly, bruised. There might be radial deviation and volar subluxation of the proximal phalanx. The ulnar side of the MCP joint is tender to palpation.16 If the distal UCL is torn completely, it can displace proximally and present as a palpable mass over the ulnar side of the MCP joint, known as a Stener lesion.16
Stress testing of the MCP joint is the most important part of the physical exam for skier’s thumb. Stabilize the metacarpal neck and apply a valgus stress on the proximal phalanx at both 0° and 30° of MCP flexion (FIGURE 3), which allows for assessment of both the proper and accessory bands of the UCL.2,16 (A common pitfall during stress testing is to allow the MCP joint to rotate, which can mimic instability.2) Intra-articular local anesthesia might be necessary for this exam because it can be painful.16,18,26 A stress exam should assess for laxity and a soft or firm endpoint; the result should be compared to that of a stress exam on the contralateral side.16,17

Imaging. AP, oblique, and lateral radiographs of the thumb should be obtained to assess for instability, avulsion injury, and associated fracture. Subluxation (volar or radial) or supination of the proximal phalanx relative to the metacarpal on imaging suggests MCP instability of the MCP joint.16,17
If the stress exam is equivocal, magnetic resonance imaging is recommended for further assessment.2,18
Continue to: Stress radiographs...
Stress radiographs (ie, radiographs of the thumb with valgus stress applied at the MCP joint) can aid in diagnosis but are controversial. Some experts think that these stress views can further damage the UCL; others recommend against them because they carry a false-negative rate ≥ 25%.15,16 If you choose to perform stress views, order standard radiographs beforehand to rule out bony injury.17
Treatment. UCL tears are classified as 3 tiers to guide treatment.
- Grade 1 injury (a partial tear) is characterized by pain upon palpation but no instability on the stress exam.
- Grade 2 injury (also a partial tear) is marked by laxity on the stress exam with a firm endpoint.
- Grade 3 injury (complete tear) shows laxity and a soft endpoint on a stress exam16,17; Stener lesions are seen only in grade 3 tears.16,17
Grades 1 and 2 UCL tears without fracture or with a nondisplaced avulsion fracture can be managed nonoperatively by immobilizing the thumb in a spica splint or cast for 4 to 6 weeks.16,18 The MCP joint is immobilized and the interphalangeal joint is allowed to move freely.2,16,17
Grade 3 injuries should be referred to a hand specialist for surgical repair.16 Patients presenting > 12 weeks after acute injury or with a chronic UCL tear should also be referred for surgical repair.16
CORRESPONDENCE
Caitlin A. Nicholson, MD, 1611 West Harrison Street, Suite 300, Chicago, IL 60612; [email protected]
1. Hirt B, Seyhan H, Wagner M, et al. Hand and Wrist Anatomy and Biomechanics: A Comprehensive Guide. Thieme; 2017:57,58,71,72,75-80.
2. Daley D, Geary M, Gaston RG. Thumb metacarpophalangeal ulnar and radial collateral ligament injuries. Clin Sports Med. 2020;39:443-455. doi: 10.1016/j.csm.2019.12.003
3. Gil JA, Hresko AM, Weiss AC. Current concepts in the management of trigger finger in adults. J Am Acad Orthop Surg. 2020;28:e642-e650. doi: 10.5435/JAAOS-D-19-00614
4. Henton J, Jain A, Medhurst C, et al. Adult trigger finger. BMJ. 2012;345:e5743. doi: 10.1136/bmj.e5743
5. Bates T, Dunn J. Trigger finger. Orthobullets [Internet]. Updated December 8, 2021. Accessed April 14, 2022. www.orthobullets.com/hand/6027/trigger-finger
6. Chhabra AB, Deal ND. Soft tissue injuries of the wrist and hand. In: O’Connor FG, Casa DJ, Davis BA, et al. ACSM’s Sports Medicine: A Comprehensive Review. Lippincott Williams & Wilkins; 2012:370-373.
7. Ballard TNS, Kozlow JH. Trigger finger in adults. CMAJ. 2016;188:61. doi: 10.1503/cmaj.150225
8. Vitale M. Jersey finger. Orthobullets [Internet]. Updated May 22, 2021. 2019. Accessed April 15, 2022. www.orthobullets.com/hand/6015/jersey-finger
9. Shapiro LM, Kamal RN. Evaluation and treatment of flexor tendon and pulley injuries in athletes. Clin Sports Med. 2020;39:279-297. doi: 10.1016/j.csm.2019.12.004
10. Goodson A, Morgan M, Rajeswaran G, et al. Current management of Jersey finger in rugby players: case series and literature review. Hand Surg. 2010;15:103-107. doi: 10.1142/S0218810410004710
11. Lapegue F, Andre A, Brun C, et al. Traumatic flexor tendon injuries. Diagn Interv Imaging. 2015;96:1279-1292. doi: 10.1016/j.diii.2015.09.010
12. Bendre AA, Hartigan BJ, Kalainov DM. Mallet finger. J Am Acad Orthop Surg. 2005;13:336-344. doi: 10.5435/00124635-200509000-00007
13. Lamaris GA, Matthew MK. The diagnosis and management of mallet finger injuries. Hand (N Y). 2017;12:223-228. doi: 10.1177/1558944716642763
14. Sheth U. Mallet finger. Orthobullets [Internet]. Updated August 5, 2021. Accessed April 15, 2022. www.orthobullets.com/hand/6014/mallet-finger
15. Weintraub MD, Hansford BG, Stilwill SE, et al. Avulsion injuries of the hand and wrist. Radiographics. 2020;40:163-180. doi: 10.1148/rg.2020190085
16. Avery III DM, Inkellis ER, Carlson MG. Thumb collateral ligament injuries in the athlete. Curr Rev Musculoskelet Med. 2017;10:28-37. doi: 10.1007/s12178-017-9381-z
17. Steffes MJ. Thumb collateral ligament injury. Orthobullets [Internet]. Updated February 18, 2022. Accessed April 15, 2022. www.orthobullets.com/hand/6040/thumb-collateral-ligament-injury
18. Madan SS, Pai DR, Kaur A, et al. Injury to ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7. doi: 10.1111/os.12084
19. Dardas AZ, VandenBerg J, Shen T, et al. Long-term effectiveness of repeat corticosteroid injections for trigger finger. J Hand Surg Am. 2017;42:227-235. doi: 10.1016/j.jhsa.2017.02.001
20. Huisstede BM, Gladdines S, Randsdorp MS, et al. Effectiveness of conservative, surgical, and postsurgical interventions for trigger finger, Dupuytren disease, and de Quervain disease: a systematic review. Arch Phys Med Rehabil. 2018;99:1635-1649.e21. doi: 10.1016/j.apmr.2017.07.014
21. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
22. Fiorini HJ, Tamaoki MJ, Lenza M, et al. Surgery for trigger finger. Cochrane Database Syst Rev. 2018;2:CD009860. doi: 10.1002/14651858.CD009860.pub2
23. Salazar Botero S, Hidalgo Diaz JJ, Benaïda A, et al. Review of acute traumatic closed mallet finger injuries in adults. Arch Plast Surg. 2016;43:134-144. doi: 10.5999/aps.2016.43.2.134
24. Lin JS, Samora JB. Surgical and nonsurgical management of mallet finger: a systematic review. J Hand Surg Am. 2018;43:146-163.e2. doi: 10.1016/j.jhsa.2017.10.004
25. Handoll H, Vaghela MV. Interventions for treating mallet finger injuries. Cochrane Database Syst Rev. 2004;(3):CD004574. doi: 10.1002/14651858.CD004574.pub2
26. Pulos N, Shin AY. Treatment of ulnar collateral ligament injuries of the thumb: a critical analysis review. JBJS Rev. 2017;5:e3. doi: 10.2106/JBJS.RVW.16.00051
1. Hirt B, Seyhan H, Wagner M, et al. Hand and Wrist Anatomy and Biomechanics: A Comprehensive Guide. Thieme; 2017:57,58,71,72,75-80.
2. Daley D, Geary M, Gaston RG. Thumb metacarpophalangeal ulnar and radial collateral ligament injuries. Clin Sports Med. 2020;39:443-455. doi: 10.1016/j.csm.2019.12.003
3. Gil JA, Hresko AM, Weiss AC. Current concepts in the management of trigger finger in adults. J Am Acad Orthop Surg. 2020;28:e642-e650. doi: 10.5435/JAAOS-D-19-00614
4. Henton J, Jain A, Medhurst C, et al. Adult trigger finger. BMJ. 2012;345:e5743. doi: 10.1136/bmj.e5743
5. Bates T, Dunn J. Trigger finger. Orthobullets [Internet]. Updated December 8, 2021. Accessed April 14, 2022. www.orthobullets.com/hand/6027/trigger-finger
6. Chhabra AB, Deal ND. Soft tissue injuries of the wrist and hand. In: O’Connor FG, Casa DJ, Davis BA, et al. ACSM’s Sports Medicine: A Comprehensive Review. Lippincott Williams & Wilkins; 2012:370-373.
7. Ballard TNS, Kozlow JH. Trigger finger in adults. CMAJ. 2016;188:61. doi: 10.1503/cmaj.150225
8. Vitale M. Jersey finger. Orthobullets [Internet]. Updated May 22, 2021. 2019. Accessed April 15, 2022. www.orthobullets.com/hand/6015/jersey-finger
9. Shapiro LM, Kamal RN. Evaluation and treatment of flexor tendon and pulley injuries in athletes. Clin Sports Med. 2020;39:279-297. doi: 10.1016/j.csm.2019.12.004
10. Goodson A, Morgan M, Rajeswaran G, et al. Current management of Jersey finger in rugby players: case series and literature review. Hand Surg. 2010;15:103-107. doi: 10.1142/S0218810410004710
11. Lapegue F, Andre A, Brun C, et al. Traumatic flexor tendon injuries. Diagn Interv Imaging. 2015;96:1279-1292. doi: 10.1016/j.diii.2015.09.010
12. Bendre AA, Hartigan BJ, Kalainov DM. Mallet finger. J Am Acad Orthop Surg. 2005;13:336-344. doi: 10.5435/00124635-200509000-00007
13. Lamaris GA, Matthew MK. The diagnosis and management of mallet finger injuries. Hand (N Y). 2017;12:223-228. doi: 10.1177/1558944716642763
14. Sheth U. Mallet finger. Orthobullets [Internet]. Updated August 5, 2021. Accessed April 15, 2022. www.orthobullets.com/hand/6014/mallet-finger
15. Weintraub MD, Hansford BG, Stilwill SE, et al. Avulsion injuries of the hand and wrist. Radiographics. 2020;40:163-180. doi: 10.1148/rg.2020190085
16. Avery III DM, Inkellis ER, Carlson MG. Thumb collateral ligament injuries in the athlete. Curr Rev Musculoskelet Med. 2017;10:28-37. doi: 10.1007/s12178-017-9381-z
17. Steffes MJ. Thumb collateral ligament injury. Orthobullets [Internet]. Updated February 18, 2022. Accessed April 15, 2022. www.orthobullets.com/hand/6040/thumb-collateral-ligament-injury
18. Madan SS, Pai DR, Kaur A, et al. Injury to ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7. doi: 10.1111/os.12084
19. Dardas AZ, VandenBerg J, Shen T, et al. Long-term effectiveness of repeat corticosteroid injections for trigger finger. J Hand Surg Am. 2017;42:227-235. doi: 10.1016/j.jhsa.2017.02.001
20. Huisstede BM, Gladdines S, Randsdorp MS, et al. Effectiveness of conservative, surgical, and postsurgical interventions for trigger finger, Dupuytren disease, and de Quervain disease: a systematic review. Arch Phys Med Rehabil. 2018;99:1635-1649.e21. doi: 10.1016/j.apmr.2017.07.014
21. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
22. Fiorini HJ, Tamaoki MJ, Lenza M, et al. Surgery for trigger finger. Cochrane Database Syst Rev. 2018;2:CD009860. doi: 10.1002/14651858.CD009860.pub2
23. Salazar Botero S, Hidalgo Diaz JJ, Benaïda A, et al. Review of acute traumatic closed mallet finger injuries in adults. Arch Plast Surg. 2016;43:134-144. doi: 10.5999/aps.2016.43.2.134
24. Lin JS, Samora JB. Surgical and nonsurgical management of mallet finger: a systematic review. J Hand Surg Am. 2018;43:146-163.e2. doi: 10.1016/j.jhsa.2017.10.004
25. Handoll H, Vaghela MV. Interventions for treating mallet finger injuries. Cochrane Database Syst Rev. 2004;(3):CD004574. doi: 10.1002/14651858.CD004574.pub2
26. Pulos N, Shin AY. Treatment of ulnar collateral ligament injuries of the thumb: a critical analysis review. JBJS Rev. 2017;5:e3. doi: 10.2106/JBJS.RVW.16.00051
PRACTICE RECOMMENDATIONS
› Treat trigger finger with a corticosteroid injection into the flexor tendon sheath. A
› Refer a case of jersey finger to a hand surgeon within 1 week after injury for flexor tendon repair. C
› Treat mallet finger with strict distal interphalangeal joint immobilization for 6 to 8 weeks. A
› Treat Grades 1 and 2 skier’s thumb with immobilization in a thumb spica splint or a cast for 4 to 6 weeks. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Patients asking about APOE gene test results? Here’s what to tell them
Advances in Alzheimer disease (AD) genes and biomarkers now allow older adults to undergo testing and learn about their risk for AD.1 Current routes for doing so include testing in cardiology, screening for enrollment in secondary prevention trials (which use these tests to determine trial eligibility),2 and direct-to-consumer (DTC) services that provide these results as part of large panels.3 Patients may also obtain apolipoprotein (APOE) genotype information as part of an assessment of the risks and benefits of treatment with aducanumab (Aduhelm) or other anti-amyloid therapies that have been developed to stop or slow the progression of AD pathologies.
Expanded access to testing, in combination with limited guidance from DTC companies, suggests more older adults may consult their primary care physicians about this testing. In this narrative review, we use a vignette-driven approach to summarize the current scientific knowledge of the topic and to offer guidance on provider-patient discussions and follow-up.
First, a look at APOE genotyping
In cognitively unimpaired older adults, the APOE gene is a known risk factor for mild cognitive impairment (MCI) or AD.3 A person has 2 alleles of the APOE gene, which has 3 variants: ε2, ε3, and ε4. The combination of alleles conveys varying levels of risk for developing clinical symptoms (TABLE 14), with ε4 increasing risk and ε2 decreasing risk compared to the more common ε3; thus the ε4/ε4 genotype conveys the most risk and the ε2/ε2 the least.
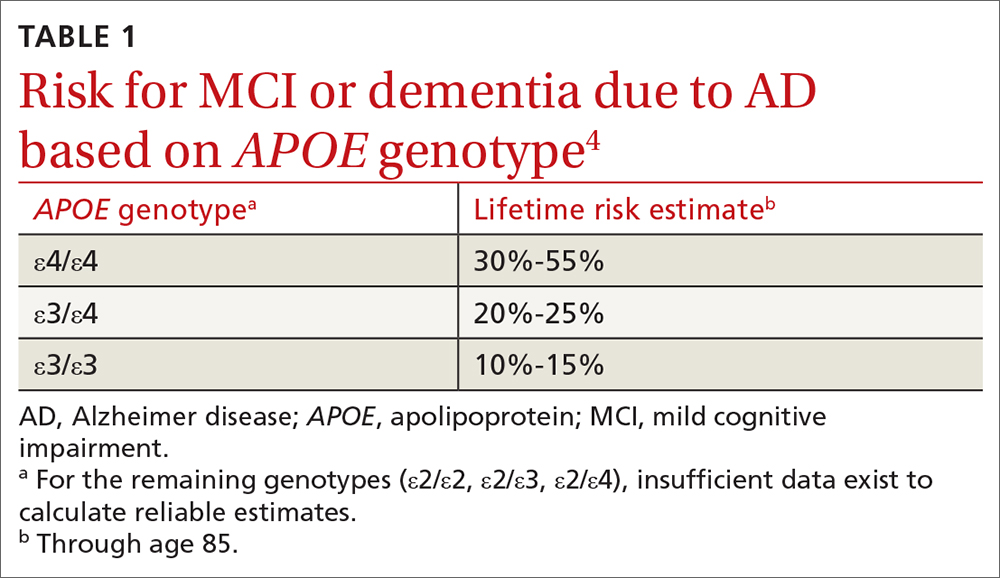
The APOE gene differs from other genes that have been identified in early-onset familial AD. These other genes, which include APP, PSEN1, and PSEN2, are deterministic genes that are fully penetrant. The APOE gene is not deterministic, meaning there is no combination of APOE alleles that are necessary or sufficient to cause late-onset AD dementia.
In clinical trials of amyloid-modifying therapies, the APOE gene has been shown to convey a risk of amyloid-related imaging abnormalities (ARIA).5 That is, in addition to conveying a risk for AD, the gene also conveys a risk for adverse effects of emerging treatments that can result in serious injury or death. This includes the drug aducanumab that was recently approved by the US Food and Drug Administration (FDA).6 In this review, we focus primarily on common clinical scenarios related to APOE. However, in light of the recent controversy over aducanumab and whether the drug should be offered to patients,7-9 we also describe how a patient’s APOE genotype may factor into drug candidacy decisions.
Testing, in clinic and “at home.” To date, practice guidelines have consistently recommended against APOE genetic testing in routine clinical practice. This is primarily due to low clinical prognostic utility and the lack of actionable results. Furthermore, no lifestyle or pharmaceutical interventions based on APOE genotype currently exist (although trials are underway10).
In 2017, the FDA approved marketing of DTC testing for the APOE gene.11 While DTC companies tend to issue standardized test result reports, the content and quality can vary widely. In fact, some provide risk estimates that are too high and too definitive and may not reflect the most recent science.12
Continue to: 7 clinical scenarios and how to approach them
7 clinical scenarios and how to approach them
Six of the following vignettes describe common clinical scenarios in which patients seek medical advice regarding APOE test results. The seventh vignette describes a patient whose APOE genotype may play a role in possible disease-modifying treatments down the road. Each vignette is designed to guide your approach to patient discussions and follow-up. Recommendations and considerations are also summarized in TABLE 213-16.
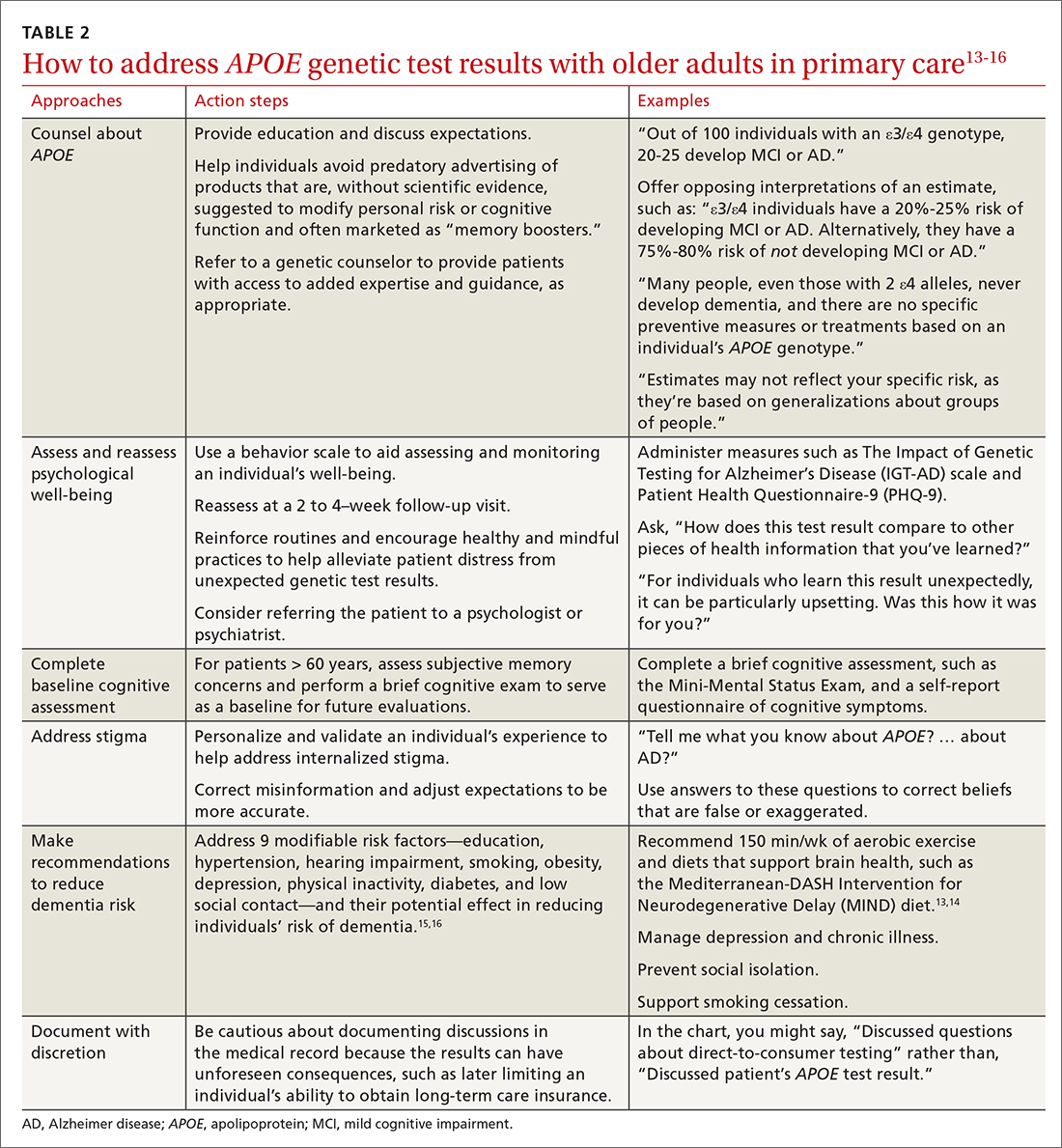
Vignette 1
Janet W, age 65, comes to the clinic for a new patient visit. She has no concerns about her memory but recently purchased DTC genetic testing to learn about her genetic health risks. Her results showed an APOE ε4/ε4 genotype. She is now concerned about developing AD. Her mother was diagnosed with AD in her 70s.
Several important pieces of information can be conveyed by the primary care physician. First, patients such as Ms. W should be told that the APOE gene is not deterministic; many people, even those with 2 ε4 alleles, never develop dementia. Second, no specific preventive measures or treatments exist based on an individual’s APOE genotype (see Vignette 5 for additional discussion).
In this scenario, patients may ask for numeric quantification of their risk for dementia (see TABLE 14 for estimates). When conveying probabilistic risk, consider using simple percentages or pictographs (eg, out of 100 individuals with an ε4/ε4 genotype, 30 to 55 develop MCI or AD). Additionally, because people tend to exhibit confirmatory bias in thinking about probabilistic risk, providing opposing interpretations of an estimate may help them to consider alternative possibilities.17 For example
There are important caveats to the interpretation of APOE risk estimates. Because APOE risk estimates are probabilistic and averaged across a broader spectrum of people in large population cohorts,4 estimates may not accurately reflect a given individual’s risk. The ranges reflect the uncertainty in the estimates. The uncertainty arises from relatively small samples, the rareness of some genotypes (notably ε4/ε4) even in large samples, and variations in methods and sampling that can lead to differences in estimates beyond statistical variation.
Vignette 2
Eric J, age 85, presents for a new patient visit accompanied by his daughter. He lives independently, volunteers at a senior center several times a week, and exercises regularly, and neither he nor his daughter has any concerns about his memory. As a gift, he recently underwent DTC genetic testing and unexpectedly learned his APOE result, which is ε4/ε4. He wants to know about his chances of developing AD.
Risk conveyed by APOE genotype can be modified by a patient’s age. At age 85, Mr. J is healthy, highly functional, and cognitively unimpaired. Given his age, Mr. J has likely “outlived” much of the risk for dementia attributable to the ε4/ε4 genotype. His risk for dementia remains high, but this risk is likely driven more by age than by his APOE genotype. Data for individuals older than age 80 are limited, and thus risk estimates lack precision. Given Mr. J’s good health and functional status, his physician may want to perform a brief cognitive screening test to serve as a baseline for future evaluations.
Continue to: Vignette 3
Vignette 3
Audrey S is a 60-year-old African American woman who comes to the clinic for her annual visit. Because her father had AD, she recently purchased DTC genetic testing to learn about her APOE genotype and risk for AD. Her results are ε3/ε4. She is wondering what this may mean for her future.
Lack of diversity in research cohorts often limits the generalizability of estimates. For example, both the frequency and impact of APOE ε4 differ across racial groups.18 But most of the data on APOE lifetime risk estimates are from largely White patient samples. While APOE ε4 seems to confer increased risk for AD across sociocultural groups, these effects may be attenuated in African American and Hispanic populations.19,20 If Ms. S is interested in numeric risk estimates, the physician can provide the estimate for ε3/ε4 (20%-25% lifetime risk), with the important caveat that this estimate may not be reflective of her individual risk.
It may be prudent to determine whether Ms. S, at age 60, has subjective memory concerns and if she does, to perform a brief cognitive exam to serve as a baseline for future evaluations. Additionally, while the Genetic Information Nondiscrimination Act (GINA, 2008) prohibits health insurers and employers from discriminating based on genetic testing results, no legal provisions exist regarding long-term care, disability, or life insurance. Documented conversations about APOE test results in the medical record may become part of patients’ applications for these insurance products, and physicians should be cautious before documenting such discussions in the medical record.
Vignette 4
Tina L, age 60, comes to the clinic for a routine wellness visit. She recently developed an interest in genealogy and purchased a DNA testing kit to learn more about her family tree. As part of this testing, she unexpectedly learned that she has an APOE ε4/ε4 genotype. She describes feeling distraught and anxious about what the result means for her future.
Ms. L’s reaction to receiving unexpected genetic results highlights a concern of DTC APOE testing. Her experience is quite different from individuals undergoing medically recommended genetic testing or those who are participating in research studies. They receive comprehensive pre-test counseling by licensed genetic counselors. The counseling includes psychological assessment, education, and discussion of expectations.2
In Ms. L’s case, it may be helpful to explain the limits of APOE lifetime risk estimates (see Vignettes 1-3). But it’s also important to address her concerns. There are behavior scales that can aid the assessment and monitoring of an individual’s well-being. The Impact of Genetic Testing for Alzheimer’s Disease (IGT-AD) scale is a tool that assesses psychological impact. It can help physicians to identify, monitor, and address concerns.21 Other useful tools include the Patient Health Questionnaire-9 (PHQ-9) and the Geriatric Depression Scale (GDS) for depression, and a suicide or self-harm assessment.2,22,23 Finally, a follow-up visit at 2 to 4 weeks may be useful to reassess psychological well-being.
Vignette 4 (cont’d)
Ms. L returns to the clinic 2 weeks later, reporting continued anxiety about her APOE test result and feelings of hopelessness and despair.
Continue to: Some patients struggle...
Some patients struggle with knowing their APOE test result. Test result–related distress is often a combination of depression (as with Ms. L), anger, confusion, and grief.24 Cognitions often include worries about uncertainty, stereotyped threat, and internalized stigma.25,26 These issues can spill over to patient concerns about sharing an APOE test result with others.27
Intolerance of uncertainty is a transdiagnostic risk factor that can influence psychological suffering.28 Brief cognitive behavioral interventions that reinforce routines and encourage healthy and mindful practices may help alleviate patient distress from unexpected genetic test results.29 Interventions that personalize and validate an individual’s experience can help address internalized stigma.30 Referral to a psychologist or psychiatrist could be warranted. Additionally, referral to a genetic counselor may help provide patients with access to added expertise and guidance; useful web-based resources for identifying an appropriate referral include https://medlineplus.gov/genetics/understanding/consult/findingprofessional/ and https://findageneticcounselor.nsgc.org/.
Vignette 5
Bob K, age 65, comes to the clinic for his annual exam. He is a current smoker and says he’s hoping to be more physically active now that he is retired. He says that his mother and grandmother both had AD. He recently purchased DTC genetic testing to learn more about his risk for AD. His learned his APOE genotype is ε3/ε4 and is wondering what he can do to decrease his chances of developing AD.
Mr. K likely would have benefited from pre-test counseling regarding the lack of current therapies to modify one’s genetic risk for AD. A pre-test counseling session often includes education about APOE testing and a brief evaluation to assess psychological readiness to undergo testing. Posttest educational information may help Mr. K avoid predatory advertising of products claiming—without scientific evidence—to modify risk for cognitive decline or to improve cognitive function.
There are several important pieces of information that should be communicated to Mr. K. Emerging evidence from randomized controlled trials suggests that healthy lifestyle modifications may benefit cognition in individuals with APOE ε4 alleles.31 It would be prudent to address proper blood pressure control32 and counsel Mr. K on how he may be able to avoid diabetes through exercise and weight maintenance. Lifestyle recommendations for Mr. K could include: smoking cessation, regular aerobic exercise (eg, 150 min/wk), and a brain-healthy diet (eg, the Mediterranean-DASH Intervention for Neurodegenerative Delay [MIND] diet).13,14 Moreover, dementia prevention also includes appropriately managing depression and chronic illnesses and preventing social isolation and hearing loss.15,16 This information should be thoughtfully conveyed, as these interventions can improve overall (especially cardiovascular) health, as well as mitigating one’s personal risk for AD.
Vignette 6
Juan L, age 45, comes in for his annual physical exam. He has a strong family history of heart disease. His cardiologist recently ordered lipid disorder genetic testing for familial hypercholesterolemia. This panel included APOE testing and showed Mr. L’s genotype is ε2/ε4. He read that the APOE gene can be associated with an increased AD risk and asks for information about his genotype.
Mr. L received genetic testing results that were ordered by a physician for another health purpose. Current recommendations for genetic testing in cardiology advise pre-test genetic counseling.33 But this counseling may not include discussion of the relationship of APOE and risk for MCI or AD. This additional information may be unexpected for Mr. L. Moreover, its significance in the context of his present concerns about cardiovascular disease may influence his reaction.
Continue to: The ε2/ε4 genotype...
The ε2/ε4 genotype is rare. One study showed that in healthy adults, the frequency was 7 in 210 (0.02 [0.01-0.04]).34 Given the rarity of the ε2/ε4 genotype, data about it are sparse. However, since the ε4 allele increases risk but the ε2 allele decreases risk, it is likely that any increase in risk is more modest than with ε3/ε4. In addition, it would help Mr. L to know that AD occurs infrequently before age 60.35 Given his relatively young age, he is unlikely to develop AD any time in the near future. In addition, particularly if he starts early, he might be able to mitigate any increased risk through some of the advice provided to Mr. K in Vignette 5.
Vignette 7
Joe J, age 65, comes to the clinic for a new patient visit. He has no concerns about his memory but has a family history of dementia and recently purchased DTC genetic testing to learn about his genetic health risks. His results showed an APOE ε4/ε4 genotype. He is concerned about developing AD. He heard on the news that there is a drug that can treat AD and wants to know if he is a candidate for this treatment.
Mr. J would benefit from the education provided to Ms. W in Vignette 1. Patients such as Mr. J should be advised that while an APOE ε4/ε4 genotype conveys an increased risk for AD, it is not deterministic of the disease. While there are no specific preventive measures or treatments based on APOE genotype, careful medical care and lifestyle factors can offset some of the risk (see Vignette 5 for discussion).
Recently (and controversially), the FDA approved aducanumab, a drug that targets amyloid.6,36 Of note, brain amyloid is more common in individuals with the APOE ε4/ε4 genotype, such as Mr. J. However, there would be no point in testing Mr. J for brain amyloid because at present the drug is only indicated in symptomatic individuals—and, even in this setting, it is controversial. One reason for the controversy is that aducanumab has potentially severe adverse effects. Patients with the ε4/ε4 genotype should know that this genotype carries increased risk for the most serious adverse event, ARIA—which can include brain edema and microhemorrhages.
What lies ahead?
More research is needed to explore the impact that greater AD gene and biomarker testing will have on the health system and workforce development. In addition, graduate schools and training programs will need to prepare clinicians to address probabilistic risk estimates for common diseases, such as AD. Finally, health systems and medical groups that employ clinicians may want to offer simulated training—similar to the vignettes in this article—as a practice requirement or as continuing medical education. This may also allow health systems or medical groups to put in place frameworks that support clinicians in proactively answering questions for patients and families about APOE and other emerging markers of disease risk.
CORRESPONDENCE
Shana Stites, University of Pennsylvania, 3615 Chestnut Street, Philadelphia, PA 19104; [email protected]
1. Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2018;14:535-562. doi: 10.1016/j.jalz.2018.02.018 PMCID:PMC5958625
2. Langlois CM, Bradbury A, Wood EM, et al. Alzheimer’s Prevention Initiative Generation Program: development of an APOE genetic counseling and disclosure process in the context of clinical trials. Alzheimers Dement Transl Res Clin Interv. 2019;5:705-716. doi: 10.1016/j.trci.2019.09.013
3. Frank L, Wesson Ashford J, Bayley PJ, et al. Genetic risk of Alzheimer’s disease: three wishes now that the genie is out of the bottle. J Alzheimers Dis. 2018;66:421-423. doi: 10.3233/JAD-180629
4. Qian J, Wolters FJ, Beiser A, et al. APOE-related risk of mild cognitive impairment and dementia for prevention trials: an analysis of four cohorts. PLOS Med. 2017;14:e1002254. doi: 10.1371/journal.pmed.1002254
5. Sperling RA, Jack CR, Black SE, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7:367-385. doi: 10.1016/j.jalz.2011.05.2351
6. FDA. November 6, 2020: Meeting of the Peripheral and Central Nervous System Drugs Advisory Committee Meeting Announcement. Published November 12, 2020. Accessed January 14, 2021. www.fda.gov/advisory-committees/advisory-committee-calendar/november-6-2020-meeting-peripheral-and-central-nervous-system-drugs-advisory-committee-meeting
7. Cummings J. Why aducanumab is important. Nat Med. 2021;27:1498-1498. doi: 10.1038/s41591-021-01478-4
8. Alexander GC, Karlawish J. The problem of aducanumab for the treatment of Alzheimer disease. Ann Intern Med. 2021;174:1303-1304. doi: 10.7326/M21-2603
9. Mullard A. More Alzheimer’s drugs head for FDA review: what scientists are watching. Nature. 2021;599:544-545. doi: 10.1038/d41586-021-03410-9
10. Rosenberg A, Mangialasche F, Ngandu T, et al. Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: from finger to world-wide fingers. J Prev Alzheimers Dis. 2019:1-8. doi: 10.14283/jpad.2019.41
11. FDA. Commissioner of the FDA allows marketing of first direct-to-consumer tests that provide genetic risk information for certain conditions. Published March 24, 2020. Accessed November 7, 2020. www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-direct-consumer-tests-provide-genetic-risk-information-certain-conditions
12. Blell M, Hunter MA. Direct-to-consumer genetic testing’s red herring: “genetic ancestry” and personalized medicine. Front Med. 2019;6:48. doi: 10.3389/fmed.2019.00048
13. Ekstrand B, Scheers N, Rasmussen MK, et al. Brain foods - the role of diet in brain performance and health. Nutr Rev. 2021;79:693-708. doi: 10.1093/nutrit/nuaa091
14. Cherian L, Wang Y, Fakuda K, et al. Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) diet slows cognitive decline after stroke. J Prev Alzheimers Dis. 2019;6:267-273. doi: 10.14283/jpad.2019.28
15. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396:413-446. doi: 10.1016/S0140-6736(20)30367-6
16. Livingston PG, Sommerlad A, Orgeta V, et al. The Lancet International Commission on Dementia Prevention and Care. 2017. Accessed March 30, 2022. https://discovery.ucl.ac.uk/id/eprint/1567635/1/Livingston_Dementia_prevention_intervention_care.pdf
17. Peters U. What is the function of confirmation bias? Erkenntnis. April 2020. doi: 10.1007/s10670-020-00252-1
18. Barnes LL, Bennett DA. Cognitive resilience in APOE*ε4 carriers—is race important? Nat Rev Neurol. 2015;11:190-191. doi: 10.1038/nrneurol.2015.38
19. Farrer LA. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278:1349. doi: 10.1001/jama.1997.03550160069041
20. Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60:185. doi: 10.1001/archneur.60.2.185
21. Chung WW, Chen CA, Cupples LA, et al. A new scale measuring psychologic impact of genetic susceptibility testing for Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:50-56. doi: 10.1097/WAD.0b013e318188429e
22. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613. doi: 10.1046/j.1525-1497.2001.016009606.x
23. Yesavage JA, Sheikh JI. 9/Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165-173. doi: 10.1300/J018v05n01_09
24. Green RC, Roberts JS, Cupples LA, et al. Disclosure of APOE genotype for risk of Alzheimer’s disease. N Engl J Med. 2009;361:245-254. doi: 10.1056/NEJMoa0809578
25. Lineweaver TT, Bondi MW, Galasko D, et al. Effect of knowledge of APOE genotype on subjective and objective memory performance in healthy older adults. Am J Psychiatry. 2014;171:201-208. doi: 10.1176/appi.ajp.2013.12121590
26. Karlawish J. Understanding the impact of learning an amyloid PET scan result: preliminary findings from the SOKRATES study. Alzheimers Dement J Alzheimers Assoc. 2016;12:P325. doi: 10.1016/j.jalz.2016.06.594
27. Stites SD. Cognitively healthy individuals want to know their risk for Alzheimer’s disease: what should we do? J Alzheimers Dis. 2018;62:499-502. doi: 10.3233/JAD-171089
28. Milne S, Lomax C, Freeston MH. A review of the relationship between intolerance of uncertainty and threat appraisal in anxiety. Cogn Behav Ther. 2019;12:e38. doi: 10.1017/S1754470X19000230
29. Hebert EA, Dugas MJ. Behavioral experiments for intolerance of uncertainty: challenging the unknown in the treatment of generalized anxiety disorder. Cogn Behav Pract. 2019;26:421-436. doi: 10.1016/j.cbpra.2018.07.007
30. Stites SD, Karlawish, J. Stigma of Alzheimer’s disease dementia: considerations for practice. Pract Neurol. Published June 2018. Accessed January 31, 2019. http://practicalneurology.com/2018/06/stigma-of-alzheimers-disease-dementia/
31. Solomon A, Turunen H, Ngandu T, et al. Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 2018;75:462. doi: 10.1001/jamaneurol.2017.4365
32. Peters R, Warwick J, Anstey KJ, et al. Blood pressure and dementia: what the SPRINT-MIND trial adds and what we still need to know. Neurology. 2019;92:1017-1018. doi: 10.1212/WNL.0000000000007543
33. Musunuru K, Hershberger RE, Day SM, et al. Genetic testing for inherited cardiovascular diseases: a Scientific Statement from the American Heart Association. Circ Genom Precis Med. 2020;13: e000067. doi: 10.1161/HCG.0000000000000067
34. Margaglione M, Seripa D, Gravina C, et al. Prevalence of apolipoprotein E alleles in healthy subjects and survivors of ischemic stroke. Stroke. 1998;29:399-403. doi: 10.1161/01.STR.29.2.399
35. National Institute on Aging. Alzheimer’s disease genetics fact sheet. Reviewed December 24, 2019. Accessed April 10, 2022. www.nia.nih.gov/health/alzheimers-disease-genetics-fact-sheet
36. Belluck P, Kaplan S, Robbins R. How Aduhelm, an unproven Alzheimer’s drug, got approved. The New York Times. Published July 19, 2021. Updated Oct. 20, 2021. Accessed December 1, 2021. www.nytimes.com/2021/07/19/health/alzheimers-drug-aduhelm-fda.html
Advances in Alzheimer disease (AD) genes and biomarkers now allow older adults to undergo testing and learn about their risk for AD.1 Current routes for doing so include testing in cardiology, screening for enrollment in secondary prevention trials (which use these tests to determine trial eligibility),2 and direct-to-consumer (DTC) services that provide these results as part of large panels.3 Patients may also obtain apolipoprotein (APOE) genotype information as part of an assessment of the risks and benefits of treatment with aducanumab (Aduhelm) or other anti-amyloid therapies that have been developed to stop or slow the progression of AD pathologies.
Expanded access to testing, in combination with limited guidance from DTC companies, suggests more older adults may consult their primary care physicians about this testing. In this narrative review, we use a vignette-driven approach to summarize the current scientific knowledge of the topic and to offer guidance on provider-patient discussions and follow-up.
First, a look at APOE genotyping
In cognitively unimpaired older adults, the APOE gene is a known risk factor for mild cognitive impairment (MCI) or AD.3 A person has 2 alleles of the APOE gene, which has 3 variants: ε2, ε3, and ε4. The combination of alleles conveys varying levels of risk for developing clinical symptoms (TABLE 14), with ε4 increasing risk and ε2 decreasing risk compared to the more common ε3; thus the ε4/ε4 genotype conveys the most risk and the ε2/ε2 the least.

The APOE gene differs from other genes that have been identified in early-onset familial AD. These other genes, which include APP, PSEN1, and PSEN2, are deterministic genes that are fully penetrant. The APOE gene is not deterministic, meaning there is no combination of APOE alleles that are necessary or sufficient to cause late-onset AD dementia.
In clinical trials of amyloid-modifying therapies, the APOE gene has been shown to convey a risk of amyloid-related imaging abnormalities (ARIA).5 That is, in addition to conveying a risk for AD, the gene also conveys a risk for adverse effects of emerging treatments that can result in serious injury or death. This includes the drug aducanumab that was recently approved by the US Food and Drug Administration (FDA).6 In this review, we focus primarily on common clinical scenarios related to APOE. However, in light of the recent controversy over aducanumab and whether the drug should be offered to patients,7-9 we also describe how a patient’s APOE genotype may factor into drug candidacy decisions.
Testing, in clinic and “at home.” To date, practice guidelines have consistently recommended against APOE genetic testing in routine clinical practice. This is primarily due to low clinical prognostic utility and the lack of actionable results. Furthermore, no lifestyle or pharmaceutical interventions based on APOE genotype currently exist (although trials are underway10).
In 2017, the FDA approved marketing of DTC testing for the APOE gene.11 While DTC companies tend to issue standardized test result reports, the content and quality can vary widely. In fact, some provide risk estimates that are too high and too definitive and may not reflect the most recent science.12
Continue to: 7 clinical scenarios and how to approach them
7 clinical scenarios and how to approach them
Six of the following vignettes describe common clinical scenarios in which patients seek medical advice regarding APOE test results. The seventh vignette describes a patient whose APOE genotype may play a role in possible disease-modifying treatments down the road. Each vignette is designed to guide your approach to patient discussions and follow-up. Recommendations and considerations are also summarized in TABLE 213-16.

Vignette 1
Janet W, age 65, comes to the clinic for a new patient visit. She has no concerns about her memory but recently purchased DTC genetic testing to learn about her genetic health risks. Her results showed an APOE ε4/ε4 genotype. She is now concerned about developing AD. Her mother was diagnosed with AD in her 70s.
Several important pieces of information can be conveyed by the primary care physician. First, patients such as Ms. W should be told that the APOE gene is not deterministic; many people, even those with 2 ε4 alleles, never develop dementia. Second, no specific preventive measures or treatments exist based on an individual’s APOE genotype (see Vignette 5 for additional discussion).
In this scenario, patients may ask for numeric quantification of their risk for dementia (see TABLE 14 for estimates). When conveying probabilistic risk, consider using simple percentages or pictographs (eg, out of 100 individuals with an ε4/ε4 genotype, 30 to 55 develop MCI or AD). Additionally, because people tend to exhibit confirmatory bias in thinking about probabilistic risk, providing opposing interpretations of an estimate may help them to consider alternative possibilities.17 For example
There are important caveats to the interpretation of APOE risk estimates. Because APOE risk estimates are probabilistic and averaged across a broader spectrum of people in large population cohorts,4 estimates may not accurately reflect a given individual’s risk. The ranges reflect the uncertainty in the estimates. The uncertainty arises from relatively small samples, the rareness of some genotypes (notably ε4/ε4) even in large samples, and variations in methods and sampling that can lead to differences in estimates beyond statistical variation.
Vignette 2
Eric J, age 85, presents for a new patient visit accompanied by his daughter. He lives independently, volunteers at a senior center several times a week, and exercises regularly, and neither he nor his daughter has any concerns about his memory. As a gift, he recently underwent DTC genetic testing and unexpectedly learned his APOE result, which is ε4/ε4. He wants to know about his chances of developing AD.
Risk conveyed by APOE genotype can be modified by a patient’s age. At age 85, Mr. J is healthy, highly functional, and cognitively unimpaired. Given his age, Mr. J has likely “outlived” much of the risk for dementia attributable to the ε4/ε4 genotype. His risk for dementia remains high, but this risk is likely driven more by age than by his APOE genotype. Data for individuals older than age 80 are limited, and thus risk estimates lack precision. Given Mr. J’s good health and functional status, his physician may want to perform a brief cognitive screening test to serve as a baseline for future evaluations.
Continue to: Vignette 3
Vignette 3
Audrey S is a 60-year-old African American woman who comes to the clinic for her annual visit. Because her father had AD, she recently purchased DTC genetic testing to learn about her APOE genotype and risk for AD. Her results are ε3/ε4. She is wondering what this may mean for her future.
Lack of diversity in research cohorts often limits the generalizability of estimates. For example, both the frequency and impact of APOE ε4 differ across racial groups.18 But most of the data on APOE lifetime risk estimates are from largely White patient samples. While APOE ε4 seems to confer increased risk for AD across sociocultural groups, these effects may be attenuated in African American and Hispanic populations.19,20 If Ms. S is interested in numeric risk estimates, the physician can provide the estimate for ε3/ε4 (20%-25% lifetime risk), with the important caveat that this estimate may not be reflective of her individual risk.
It may be prudent to determine whether Ms. S, at age 60, has subjective memory concerns and if she does, to perform a brief cognitive exam to serve as a baseline for future evaluations. Additionally, while the Genetic Information Nondiscrimination Act (GINA, 2008) prohibits health insurers and employers from discriminating based on genetic testing results, no legal provisions exist regarding long-term care, disability, or life insurance. Documented conversations about APOE test results in the medical record may become part of patients’ applications for these insurance products, and physicians should be cautious before documenting such discussions in the medical record.
Vignette 4
Tina L, age 60, comes to the clinic for a routine wellness visit. She recently developed an interest in genealogy and purchased a DNA testing kit to learn more about her family tree. As part of this testing, she unexpectedly learned that she has an APOE ε4/ε4 genotype. She describes feeling distraught and anxious about what the result means for her future.
Ms. L’s reaction to receiving unexpected genetic results highlights a concern of DTC APOE testing. Her experience is quite different from individuals undergoing medically recommended genetic testing or those who are participating in research studies. They receive comprehensive pre-test counseling by licensed genetic counselors. The counseling includes psychological assessment, education, and discussion of expectations.2
In Ms. L’s case, it may be helpful to explain the limits of APOE lifetime risk estimates (see Vignettes 1-3). But it’s also important to address her concerns. There are behavior scales that can aid the assessment and monitoring of an individual’s well-being. The Impact of Genetic Testing for Alzheimer’s Disease (IGT-AD) scale is a tool that assesses psychological impact. It can help physicians to identify, monitor, and address concerns.21 Other useful tools include the Patient Health Questionnaire-9 (PHQ-9) and the Geriatric Depression Scale (GDS) for depression, and a suicide or self-harm assessment.2,22,23 Finally, a follow-up visit at 2 to 4 weeks may be useful to reassess psychological well-being.
Vignette 4 (cont’d)
Ms. L returns to the clinic 2 weeks later, reporting continued anxiety about her APOE test result and feelings of hopelessness and despair.
Continue to: Some patients struggle...
Some patients struggle with knowing their APOE test result. Test result–related distress is often a combination of depression (as with Ms. L), anger, confusion, and grief.24 Cognitions often include worries about uncertainty, stereotyped threat, and internalized stigma.25,26 These issues can spill over to patient concerns about sharing an APOE test result with others.27
Intolerance of uncertainty is a transdiagnostic risk factor that can influence psychological suffering.28 Brief cognitive behavioral interventions that reinforce routines and encourage healthy and mindful practices may help alleviate patient distress from unexpected genetic test results.29 Interventions that personalize and validate an individual’s experience can help address internalized stigma.30 Referral to a psychologist or psychiatrist could be warranted. Additionally, referral to a genetic counselor may help provide patients with access to added expertise and guidance; useful web-based resources for identifying an appropriate referral include https://medlineplus.gov/genetics/understanding/consult/findingprofessional/ and https://findageneticcounselor.nsgc.org/.
Vignette 5
Bob K, age 65, comes to the clinic for his annual exam. He is a current smoker and says he’s hoping to be more physically active now that he is retired. He says that his mother and grandmother both had AD. He recently purchased DTC genetic testing to learn more about his risk for AD. His learned his APOE genotype is ε3/ε4 and is wondering what he can do to decrease his chances of developing AD.
Mr. K likely would have benefited from pre-test counseling regarding the lack of current therapies to modify one’s genetic risk for AD. A pre-test counseling session often includes education about APOE testing and a brief evaluation to assess psychological readiness to undergo testing. Posttest educational information may help Mr. K avoid predatory advertising of products claiming—without scientific evidence—to modify risk for cognitive decline or to improve cognitive function.
There are several important pieces of information that should be communicated to Mr. K. Emerging evidence from randomized controlled trials suggests that healthy lifestyle modifications may benefit cognition in individuals with APOE ε4 alleles.31 It would be prudent to address proper blood pressure control32 and counsel Mr. K on how he may be able to avoid diabetes through exercise and weight maintenance. Lifestyle recommendations for Mr. K could include: smoking cessation, regular aerobic exercise (eg, 150 min/wk), and a brain-healthy diet (eg, the Mediterranean-DASH Intervention for Neurodegenerative Delay [MIND] diet).13,14 Moreover, dementia prevention also includes appropriately managing depression and chronic illnesses and preventing social isolation and hearing loss.15,16 This information should be thoughtfully conveyed, as these interventions can improve overall (especially cardiovascular) health, as well as mitigating one’s personal risk for AD.
Vignette 6
Juan L, age 45, comes in for his annual physical exam. He has a strong family history of heart disease. His cardiologist recently ordered lipid disorder genetic testing for familial hypercholesterolemia. This panel included APOE testing and showed Mr. L’s genotype is ε2/ε4. He read that the APOE gene can be associated with an increased AD risk and asks for information about his genotype.
Mr. L received genetic testing results that were ordered by a physician for another health purpose. Current recommendations for genetic testing in cardiology advise pre-test genetic counseling.33 But this counseling may not include discussion of the relationship of APOE and risk for MCI or AD. This additional information may be unexpected for Mr. L. Moreover, its significance in the context of his present concerns about cardiovascular disease may influence his reaction.
Continue to: The ε2/ε4 genotype...
The ε2/ε4 genotype is rare. One study showed that in healthy adults, the frequency was 7 in 210 (0.02 [0.01-0.04]).34 Given the rarity of the ε2/ε4 genotype, data about it are sparse. However, since the ε4 allele increases risk but the ε2 allele decreases risk, it is likely that any increase in risk is more modest than with ε3/ε4. In addition, it would help Mr. L to know that AD occurs infrequently before age 60.35 Given his relatively young age, he is unlikely to develop AD any time in the near future. In addition, particularly if he starts early, he might be able to mitigate any increased risk through some of the advice provided to Mr. K in Vignette 5.
Vignette 7
Joe J, age 65, comes to the clinic for a new patient visit. He has no concerns about his memory but has a family history of dementia and recently purchased DTC genetic testing to learn about his genetic health risks. His results showed an APOE ε4/ε4 genotype. He is concerned about developing AD. He heard on the news that there is a drug that can treat AD and wants to know if he is a candidate for this treatment.
Mr. J would benefit from the education provided to Ms. W in Vignette 1. Patients such as Mr. J should be advised that while an APOE ε4/ε4 genotype conveys an increased risk for AD, it is not deterministic of the disease. While there are no specific preventive measures or treatments based on APOE genotype, careful medical care and lifestyle factors can offset some of the risk (see Vignette 5 for discussion).
Recently (and controversially), the FDA approved aducanumab, a drug that targets amyloid.6,36 Of note, brain amyloid is more common in individuals with the APOE ε4/ε4 genotype, such as Mr. J. However, there would be no point in testing Mr. J for brain amyloid because at present the drug is only indicated in symptomatic individuals—and, even in this setting, it is controversial. One reason for the controversy is that aducanumab has potentially severe adverse effects. Patients with the ε4/ε4 genotype should know that this genotype carries increased risk for the most serious adverse event, ARIA—which can include brain edema and microhemorrhages.
What lies ahead?
More research is needed to explore the impact that greater AD gene and biomarker testing will have on the health system and workforce development. In addition, graduate schools and training programs will need to prepare clinicians to address probabilistic risk estimates for common diseases, such as AD. Finally, health systems and medical groups that employ clinicians may want to offer simulated training—similar to the vignettes in this article—as a practice requirement or as continuing medical education. This may also allow health systems or medical groups to put in place frameworks that support clinicians in proactively answering questions for patients and families about APOE and other emerging markers of disease risk.
CORRESPONDENCE
Shana Stites, University of Pennsylvania, 3615 Chestnut Street, Philadelphia, PA 19104; [email protected]
Advances in Alzheimer disease (AD) genes and biomarkers now allow older adults to undergo testing and learn about their risk for AD.1 Current routes for doing so include testing in cardiology, screening for enrollment in secondary prevention trials (which use these tests to determine trial eligibility),2 and direct-to-consumer (DTC) services that provide these results as part of large panels.3 Patients may also obtain apolipoprotein (APOE) genotype information as part of an assessment of the risks and benefits of treatment with aducanumab (Aduhelm) or other anti-amyloid therapies that have been developed to stop or slow the progression of AD pathologies.
Expanded access to testing, in combination with limited guidance from DTC companies, suggests more older adults may consult their primary care physicians about this testing. In this narrative review, we use a vignette-driven approach to summarize the current scientific knowledge of the topic and to offer guidance on provider-patient discussions and follow-up.
First, a look at APOE genotyping
In cognitively unimpaired older adults, the APOE gene is a known risk factor for mild cognitive impairment (MCI) or AD.3 A person has 2 alleles of the APOE gene, which has 3 variants: ε2, ε3, and ε4. The combination of alleles conveys varying levels of risk for developing clinical symptoms (TABLE 14), with ε4 increasing risk and ε2 decreasing risk compared to the more common ε3; thus the ε4/ε4 genotype conveys the most risk and the ε2/ε2 the least.

The APOE gene differs from other genes that have been identified in early-onset familial AD. These other genes, which include APP, PSEN1, and PSEN2, are deterministic genes that are fully penetrant. The APOE gene is not deterministic, meaning there is no combination of APOE alleles that are necessary or sufficient to cause late-onset AD dementia.
In clinical trials of amyloid-modifying therapies, the APOE gene has been shown to convey a risk of amyloid-related imaging abnormalities (ARIA).5 That is, in addition to conveying a risk for AD, the gene also conveys a risk for adverse effects of emerging treatments that can result in serious injury or death. This includes the drug aducanumab that was recently approved by the US Food and Drug Administration (FDA).6 In this review, we focus primarily on common clinical scenarios related to APOE. However, in light of the recent controversy over aducanumab and whether the drug should be offered to patients,7-9 we also describe how a patient’s APOE genotype may factor into drug candidacy decisions.
Testing, in clinic and “at home.” To date, practice guidelines have consistently recommended against APOE genetic testing in routine clinical practice. This is primarily due to low clinical prognostic utility and the lack of actionable results. Furthermore, no lifestyle or pharmaceutical interventions based on APOE genotype currently exist (although trials are underway10).
In 2017, the FDA approved marketing of DTC testing for the APOE gene.11 While DTC companies tend to issue standardized test result reports, the content and quality can vary widely. In fact, some provide risk estimates that are too high and too definitive and may not reflect the most recent science.12
Continue to: 7 clinical scenarios and how to approach them
7 clinical scenarios and how to approach them
Six of the following vignettes describe common clinical scenarios in which patients seek medical advice regarding APOE test results. The seventh vignette describes a patient whose APOE genotype may play a role in possible disease-modifying treatments down the road. Each vignette is designed to guide your approach to patient discussions and follow-up. Recommendations and considerations are also summarized in TABLE 213-16.

Vignette 1
Janet W, age 65, comes to the clinic for a new patient visit. She has no concerns about her memory but recently purchased DTC genetic testing to learn about her genetic health risks. Her results showed an APOE ε4/ε4 genotype. She is now concerned about developing AD. Her mother was diagnosed with AD in her 70s.
Several important pieces of information can be conveyed by the primary care physician. First, patients such as Ms. W should be told that the APOE gene is not deterministic; many people, even those with 2 ε4 alleles, never develop dementia. Second, no specific preventive measures or treatments exist based on an individual’s APOE genotype (see Vignette 5 for additional discussion).
In this scenario, patients may ask for numeric quantification of their risk for dementia (see TABLE 14 for estimates). When conveying probabilistic risk, consider using simple percentages or pictographs (eg, out of 100 individuals with an ε4/ε4 genotype, 30 to 55 develop MCI or AD). Additionally, because people tend to exhibit confirmatory bias in thinking about probabilistic risk, providing opposing interpretations of an estimate may help them to consider alternative possibilities.17 For example
There are important caveats to the interpretation of APOE risk estimates. Because APOE risk estimates are probabilistic and averaged across a broader spectrum of people in large population cohorts,4 estimates may not accurately reflect a given individual’s risk. The ranges reflect the uncertainty in the estimates. The uncertainty arises from relatively small samples, the rareness of some genotypes (notably ε4/ε4) even in large samples, and variations in methods and sampling that can lead to differences in estimates beyond statistical variation.
Vignette 2
Eric J, age 85, presents for a new patient visit accompanied by his daughter. He lives independently, volunteers at a senior center several times a week, and exercises regularly, and neither he nor his daughter has any concerns about his memory. As a gift, he recently underwent DTC genetic testing and unexpectedly learned his APOE result, which is ε4/ε4. He wants to know about his chances of developing AD.
Risk conveyed by APOE genotype can be modified by a patient’s age. At age 85, Mr. J is healthy, highly functional, and cognitively unimpaired. Given his age, Mr. J has likely “outlived” much of the risk for dementia attributable to the ε4/ε4 genotype. His risk for dementia remains high, but this risk is likely driven more by age than by his APOE genotype. Data for individuals older than age 80 are limited, and thus risk estimates lack precision. Given Mr. J’s good health and functional status, his physician may want to perform a brief cognitive screening test to serve as a baseline for future evaluations.
Continue to: Vignette 3
Vignette 3
Audrey S is a 60-year-old African American woman who comes to the clinic for her annual visit. Because her father had AD, she recently purchased DTC genetic testing to learn about her APOE genotype and risk for AD. Her results are ε3/ε4. She is wondering what this may mean for her future.
Lack of diversity in research cohorts often limits the generalizability of estimates. For example, both the frequency and impact of APOE ε4 differ across racial groups.18 But most of the data on APOE lifetime risk estimates are from largely White patient samples. While APOE ε4 seems to confer increased risk for AD across sociocultural groups, these effects may be attenuated in African American and Hispanic populations.19,20 If Ms. S is interested in numeric risk estimates, the physician can provide the estimate for ε3/ε4 (20%-25% lifetime risk), with the important caveat that this estimate may not be reflective of her individual risk.
It may be prudent to determine whether Ms. S, at age 60, has subjective memory concerns and if she does, to perform a brief cognitive exam to serve as a baseline for future evaluations. Additionally, while the Genetic Information Nondiscrimination Act (GINA, 2008) prohibits health insurers and employers from discriminating based on genetic testing results, no legal provisions exist regarding long-term care, disability, or life insurance. Documented conversations about APOE test results in the medical record may become part of patients’ applications for these insurance products, and physicians should be cautious before documenting such discussions in the medical record.
Vignette 4
Tina L, age 60, comes to the clinic for a routine wellness visit. She recently developed an interest in genealogy and purchased a DNA testing kit to learn more about her family tree. As part of this testing, she unexpectedly learned that she has an APOE ε4/ε4 genotype. She describes feeling distraught and anxious about what the result means for her future.
Ms. L’s reaction to receiving unexpected genetic results highlights a concern of DTC APOE testing. Her experience is quite different from individuals undergoing medically recommended genetic testing or those who are participating in research studies. They receive comprehensive pre-test counseling by licensed genetic counselors. The counseling includes psychological assessment, education, and discussion of expectations.2
In Ms. L’s case, it may be helpful to explain the limits of APOE lifetime risk estimates (see Vignettes 1-3). But it’s also important to address her concerns. There are behavior scales that can aid the assessment and monitoring of an individual’s well-being. The Impact of Genetic Testing for Alzheimer’s Disease (IGT-AD) scale is a tool that assesses psychological impact. It can help physicians to identify, monitor, and address concerns.21 Other useful tools include the Patient Health Questionnaire-9 (PHQ-9) and the Geriatric Depression Scale (GDS) for depression, and a suicide or self-harm assessment.2,22,23 Finally, a follow-up visit at 2 to 4 weeks may be useful to reassess psychological well-being.
Vignette 4 (cont’d)
Ms. L returns to the clinic 2 weeks later, reporting continued anxiety about her APOE test result and feelings of hopelessness and despair.
Continue to: Some patients struggle...
Some patients struggle with knowing their APOE test result. Test result–related distress is often a combination of depression (as with Ms. L), anger, confusion, and grief.24 Cognitions often include worries about uncertainty, stereotyped threat, and internalized stigma.25,26 These issues can spill over to patient concerns about sharing an APOE test result with others.27
Intolerance of uncertainty is a transdiagnostic risk factor that can influence psychological suffering.28 Brief cognitive behavioral interventions that reinforce routines and encourage healthy and mindful practices may help alleviate patient distress from unexpected genetic test results.29 Interventions that personalize and validate an individual’s experience can help address internalized stigma.30 Referral to a psychologist or psychiatrist could be warranted. Additionally, referral to a genetic counselor may help provide patients with access to added expertise and guidance; useful web-based resources for identifying an appropriate referral include https://medlineplus.gov/genetics/understanding/consult/findingprofessional/ and https://findageneticcounselor.nsgc.org/.
Vignette 5
Bob K, age 65, comes to the clinic for his annual exam. He is a current smoker and says he’s hoping to be more physically active now that he is retired. He says that his mother and grandmother both had AD. He recently purchased DTC genetic testing to learn more about his risk for AD. His learned his APOE genotype is ε3/ε4 and is wondering what he can do to decrease his chances of developing AD.
Mr. K likely would have benefited from pre-test counseling regarding the lack of current therapies to modify one’s genetic risk for AD. A pre-test counseling session often includes education about APOE testing and a brief evaluation to assess psychological readiness to undergo testing. Posttest educational information may help Mr. K avoid predatory advertising of products claiming—without scientific evidence—to modify risk for cognitive decline or to improve cognitive function.
There are several important pieces of information that should be communicated to Mr. K. Emerging evidence from randomized controlled trials suggests that healthy lifestyle modifications may benefit cognition in individuals with APOE ε4 alleles.31 It would be prudent to address proper blood pressure control32 and counsel Mr. K on how he may be able to avoid diabetes through exercise and weight maintenance. Lifestyle recommendations for Mr. K could include: smoking cessation, regular aerobic exercise (eg, 150 min/wk), and a brain-healthy diet (eg, the Mediterranean-DASH Intervention for Neurodegenerative Delay [MIND] diet).13,14 Moreover, dementia prevention also includes appropriately managing depression and chronic illnesses and preventing social isolation and hearing loss.15,16 This information should be thoughtfully conveyed, as these interventions can improve overall (especially cardiovascular) health, as well as mitigating one’s personal risk for AD.
Vignette 6
Juan L, age 45, comes in for his annual physical exam. He has a strong family history of heart disease. His cardiologist recently ordered lipid disorder genetic testing for familial hypercholesterolemia. This panel included APOE testing and showed Mr. L’s genotype is ε2/ε4. He read that the APOE gene can be associated with an increased AD risk and asks for information about his genotype.
Mr. L received genetic testing results that were ordered by a physician for another health purpose. Current recommendations for genetic testing in cardiology advise pre-test genetic counseling.33 But this counseling may not include discussion of the relationship of APOE and risk for MCI or AD. This additional information may be unexpected for Mr. L. Moreover, its significance in the context of his present concerns about cardiovascular disease may influence his reaction.
Continue to: The ε2/ε4 genotype...
The ε2/ε4 genotype is rare. One study showed that in healthy adults, the frequency was 7 in 210 (0.02 [0.01-0.04]).34 Given the rarity of the ε2/ε4 genotype, data about it are sparse. However, since the ε4 allele increases risk but the ε2 allele decreases risk, it is likely that any increase in risk is more modest than with ε3/ε4. In addition, it would help Mr. L to know that AD occurs infrequently before age 60.35 Given his relatively young age, he is unlikely to develop AD any time in the near future. In addition, particularly if he starts early, he might be able to mitigate any increased risk through some of the advice provided to Mr. K in Vignette 5.
Vignette 7
Joe J, age 65, comes to the clinic for a new patient visit. He has no concerns about his memory but has a family history of dementia and recently purchased DTC genetic testing to learn about his genetic health risks. His results showed an APOE ε4/ε4 genotype. He is concerned about developing AD. He heard on the news that there is a drug that can treat AD and wants to know if he is a candidate for this treatment.
Mr. J would benefit from the education provided to Ms. W in Vignette 1. Patients such as Mr. J should be advised that while an APOE ε4/ε4 genotype conveys an increased risk for AD, it is not deterministic of the disease. While there are no specific preventive measures or treatments based on APOE genotype, careful medical care and lifestyle factors can offset some of the risk (see Vignette 5 for discussion).
Recently (and controversially), the FDA approved aducanumab, a drug that targets amyloid.6,36 Of note, brain amyloid is more common in individuals with the APOE ε4/ε4 genotype, such as Mr. J. However, there would be no point in testing Mr. J for brain amyloid because at present the drug is only indicated in symptomatic individuals—and, even in this setting, it is controversial. One reason for the controversy is that aducanumab has potentially severe adverse effects. Patients with the ε4/ε4 genotype should know that this genotype carries increased risk for the most serious adverse event, ARIA—which can include brain edema and microhemorrhages.
What lies ahead?
More research is needed to explore the impact that greater AD gene and biomarker testing will have on the health system and workforce development. In addition, graduate schools and training programs will need to prepare clinicians to address probabilistic risk estimates for common diseases, such as AD. Finally, health systems and medical groups that employ clinicians may want to offer simulated training—similar to the vignettes in this article—as a practice requirement or as continuing medical education. This may also allow health systems or medical groups to put in place frameworks that support clinicians in proactively answering questions for patients and families about APOE and other emerging markers of disease risk.
CORRESPONDENCE
Shana Stites, University of Pennsylvania, 3615 Chestnut Street, Philadelphia, PA 19104; [email protected]
1. Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2018;14:535-562. doi: 10.1016/j.jalz.2018.02.018 PMCID:PMC5958625
2. Langlois CM, Bradbury A, Wood EM, et al. Alzheimer’s Prevention Initiative Generation Program: development of an APOE genetic counseling and disclosure process in the context of clinical trials. Alzheimers Dement Transl Res Clin Interv. 2019;5:705-716. doi: 10.1016/j.trci.2019.09.013
3. Frank L, Wesson Ashford J, Bayley PJ, et al. Genetic risk of Alzheimer’s disease: three wishes now that the genie is out of the bottle. J Alzheimers Dis. 2018;66:421-423. doi: 10.3233/JAD-180629
4. Qian J, Wolters FJ, Beiser A, et al. APOE-related risk of mild cognitive impairment and dementia for prevention trials: an analysis of four cohorts. PLOS Med. 2017;14:e1002254. doi: 10.1371/journal.pmed.1002254
5. Sperling RA, Jack CR, Black SE, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7:367-385. doi: 10.1016/j.jalz.2011.05.2351
6. FDA. November 6, 2020: Meeting of the Peripheral and Central Nervous System Drugs Advisory Committee Meeting Announcement. Published November 12, 2020. Accessed January 14, 2021. www.fda.gov/advisory-committees/advisory-committee-calendar/november-6-2020-meeting-peripheral-and-central-nervous-system-drugs-advisory-committee-meeting
7. Cummings J. Why aducanumab is important. Nat Med. 2021;27:1498-1498. doi: 10.1038/s41591-021-01478-4
8. Alexander GC, Karlawish J. The problem of aducanumab for the treatment of Alzheimer disease. Ann Intern Med. 2021;174:1303-1304. doi: 10.7326/M21-2603
9. Mullard A. More Alzheimer’s drugs head for FDA review: what scientists are watching. Nature. 2021;599:544-545. doi: 10.1038/d41586-021-03410-9
10. Rosenberg A, Mangialasche F, Ngandu T, et al. Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: from finger to world-wide fingers. J Prev Alzheimers Dis. 2019:1-8. doi: 10.14283/jpad.2019.41
11. FDA. Commissioner of the FDA allows marketing of first direct-to-consumer tests that provide genetic risk information for certain conditions. Published March 24, 2020. Accessed November 7, 2020. www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-direct-consumer-tests-provide-genetic-risk-information-certain-conditions
12. Blell M, Hunter MA. Direct-to-consumer genetic testing’s red herring: “genetic ancestry” and personalized medicine. Front Med. 2019;6:48. doi: 10.3389/fmed.2019.00048
13. Ekstrand B, Scheers N, Rasmussen MK, et al. Brain foods - the role of diet in brain performance and health. Nutr Rev. 2021;79:693-708. doi: 10.1093/nutrit/nuaa091
14. Cherian L, Wang Y, Fakuda K, et al. Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) diet slows cognitive decline after stroke. J Prev Alzheimers Dis. 2019;6:267-273. doi: 10.14283/jpad.2019.28
15. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396:413-446. doi: 10.1016/S0140-6736(20)30367-6
16. Livingston PG, Sommerlad A, Orgeta V, et al. The Lancet International Commission on Dementia Prevention and Care. 2017. Accessed March 30, 2022. https://discovery.ucl.ac.uk/id/eprint/1567635/1/Livingston_Dementia_prevention_intervention_care.pdf
17. Peters U. What is the function of confirmation bias? Erkenntnis. April 2020. doi: 10.1007/s10670-020-00252-1
18. Barnes LL, Bennett DA. Cognitive resilience in APOE*ε4 carriers—is race important? Nat Rev Neurol. 2015;11:190-191. doi: 10.1038/nrneurol.2015.38
19. Farrer LA. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278:1349. doi: 10.1001/jama.1997.03550160069041
20. Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60:185. doi: 10.1001/archneur.60.2.185
21. Chung WW, Chen CA, Cupples LA, et al. A new scale measuring psychologic impact of genetic susceptibility testing for Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:50-56. doi: 10.1097/WAD.0b013e318188429e
22. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613. doi: 10.1046/j.1525-1497.2001.016009606.x
23. Yesavage JA, Sheikh JI. 9/Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165-173. doi: 10.1300/J018v05n01_09
24. Green RC, Roberts JS, Cupples LA, et al. Disclosure of APOE genotype for risk of Alzheimer’s disease. N Engl J Med. 2009;361:245-254. doi: 10.1056/NEJMoa0809578
25. Lineweaver TT, Bondi MW, Galasko D, et al. Effect of knowledge of APOE genotype on subjective and objective memory performance in healthy older adults. Am J Psychiatry. 2014;171:201-208. doi: 10.1176/appi.ajp.2013.12121590
26. Karlawish J. Understanding the impact of learning an amyloid PET scan result: preliminary findings from the SOKRATES study. Alzheimers Dement J Alzheimers Assoc. 2016;12:P325. doi: 10.1016/j.jalz.2016.06.594
27. Stites SD. Cognitively healthy individuals want to know their risk for Alzheimer’s disease: what should we do? J Alzheimers Dis. 2018;62:499-502. doi: 10.3233/JAD-171089
28. Milne S, Lomax C, Freeston MH. A review of the relationship between intolerance of uncertainty and threat appraisal in anxiety. Cogn Behav Ther. 2019;12:e38. doi: 10.1017/S1754470X19000230
29. Hebert EA, Dugas MJ. Behavioral experiments for intolerance of uncertainty: challenging the unknown in the treatment of generalized anxiety disorder. Cogn Behav Pract. 2019;26:421-436. doi: 10.1016/j.cbpra.2018.07.007
30. Stites SD, Karlawish, J. Stigma of Alzheimer’s disease dementia: considerations for practice. Pract Neurol. Published June 2018. Accessed January 31, 2019. http://practicalneurology.com/2018/06/stigma-of-alzheimers-disease-dementia/
31. Solomon A, Turunen H, Ngandu T, et al. Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 2018;75:462. doi: 10.1001/jamaneurol.2017.4365
32. Peters R, Warwick J, Anstey KJ, et al. Blood pressure and dementia: what the SPRINT-MIND trial adds and what we still need to know. Neurology. 2019;92:1017-1018. doi: 10.1212/WNL.0000000000007543
33. Musunuru K, Hershberger RE, Day SM, et al. Genetic testing for inherited cardiovascular diseases: a Scientific Statement from the American Heart Association. Circ Genom Precis Med. 2020;13: e000067. doi: 10.1161/HCG.0000000000000067
34. Margaglione M, Seripa D, Gravina C, et al. Prevalence of apolipoprotein E alleles in healthy subjects and survivors of ischemic stroke. Stroke. 1998;29:399-403. doi: 10.1161/01.STR.29.2.399
35. National Institute on Aging. Alzheimer’s disease genetics fact sheet. Reviewed December 24, 2019. Accessed April 10, 2022. www.nia.nih.gov/health/alzheimers-disease-genetics-fact-sheet
36. Belluck P, Kaplan S, Robbins R. How Aduhelm, an unproven Alzheimer’s drug, got approved. The New York Times. Published July 19, 2021. Updated Oct. 20, 2021. Accessed December 1, 2021. www.nytimes.com/2021/07/19/health/alzheimers-drug-aduhelm-fda.html
1. Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2018;14:535-562. doi: 10.1016/j.jalz.2018.02.018 PMCID:PMC5958625
2. Langlois CM, Bradbury A, Wood EM, et al. Alzheimer’s Prevention Initiative Generation Program: development of an APOE genetic counseling and disclosure process in the context of clinical trials. Alzheimers Dement Transl Res Clin Interv. 2019;5:705-716. doi: 10.1016/j.trci.2019.09.013
3. Frank L, Wesson Ashford J, Bayley PJ, et al. Genetic risk of Alzheimer’s disease: three wishes now that the genie is out of the bottle. J Alzheimers Dis. 2018;66:421-423. doi: 10.3233/JAD-180629
4. Qian J, Wolters FJ, Beiser A, et al. APOE-related risk of mild cognitive impairment and dementia for prevention trials: an analysis of four cohorts. PLOS Med. 2017;14:e1002254. doi: 10.1371/journal.pmed.1002254
5. Sperling RA, Jack CR, Black SE, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7:367-385. doi: 10.1016/j.jalz.2011.05.2351
6. FDA. November 6, 2020: Meeting of the Peripheral and Central Nervous System Drugs Advisory Committee Meeting Announcement. Published November 12, 2020. Accessed January 14, 2021. www.fda.gov/advisory-committees/advisory-committee-calendar/november-6-2020-meeting-peripheral-and-central-nervous-system-drugs-advisory-committee-meeting
7. Cummings J. Why aducanumab is important. Nat Med. 2021;27:1498-1498. doi: 10.1038/s41591-021-01478-4
8. Alexander GC, Karlawish J. The problem of aducanumab for the treatment of Alzheimer disease. Ann Intern Med. 2021;174:1303-1304. doi: 10.7326/M21-2603
9. Mullard A. More Alzheimer’s drugs head for FDA review: what scientists are watching. Nature. 2021;599:544-545. doi: 10.1038/d41586-021-03410-9
10. Rosenberg A, Mangialasche F, Ngandu T, et al. Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: from finger to world-wide fingers. J Prev Alzheimers Dis. 2019:1-8. doi: 10.14283/jpad.2019.41
11. FDA. Commissioner of the FDA allows marketing of first direct-to-consumer tests that provide genetic risk information for certain conditions. Published March 24, 2020. Accessed November 7, 2020. www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-direct-consumer-tests-provide-genetic-risk-information-certain-conditions
12. Blell M, Hunter MA. Direct-to-consumer genetic testing’s red herring: “genetic ancestry” and personalized medicine. Front Med. 2019;6:48. doi: 10.3389/fmed.2019.00048
13. Ekstrand B, Scheers N, Rasmussen MK, et al. Brain foods - the role of diet in brain performance and health. Nutr Rev. 2021;79:693-708. doi: 10.1093/nutrit/nuaa091
14. Cherian L, Wang Y, Fakuda K, et al. Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) diet slows cognitive decline after stroke. J Prev Alzheimers Dis. 2019;6:267-273. doi: 10.14283/jpad.2019.28
15. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396:413-446. doi: 10.1016/S0140-6736(20)30367-6
16. Livingston PG, Sommerlad A, Orgeta V, et al. The Lancet International Commission on Dementia Prevention and Care. 2017. Accessed March 30, 2022. https://discovery.ucl.ac.uk/id/eprint/1567635/1/Livingston_Dementia_prevention_intervention_care.pdf
17. Peters U. What is the function of confirmation bias? Erkenntnis. April 2020. doi: 10.1007/s10670-020-00252-1
18. Barnes LL, Bennett DA. Cognitive resilience in APOE*ε4 carriers—is race important? Nat Rev Neurol. 2015;11:190-191. doi: 10.1038/nrneurol.2015.38
19. Farrer LA. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278:1349. doi: 10.1001/jama.1997.03550160069041
20. Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60:185. doi: 10.1001/archneur.60.2.185
21. Chung WW, Chen CA, Cupples LA, et al. A new scale measuring psychologic impact of genetic susceptibility testing for Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:50-56. doi: 10.1097/WAD.0b013e318188429e
22. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613. doi: 10.1046/j.1525-1497.2001.016009606.x
23. Yesavage JA, Sheikh JI. 9/Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165-173. doi: 10.1300/J018v05n01_09
24. Green RC, Roberts JS, Cupples LA, et al. Disclosure of APOE genotype for risk of Alzheimer’s disease. N Engl J Med. 2009;361:245-254. doi: 10.1056/NEJMoa0809578
25. Lineweaver TT, Bondi MW, Galasko D, et al. Effect of knowledge of APOE genotype on subjective and objective memory performance in healthy older adults. Am J Psychiatry. 2014;171:201-208. doi: 10.1176/appi.ajp.2013.12121590
26. Karlawish J. Understanding the impact of learning an amyloid PET scan result: preliminary findings from the SOKRATES study. Alzheimers Dement J Alzheimers Assoc. 2016;12:P325. doi: 10.1016/j.jalz.2016.06.594
27. Stites SD. Cognitively healthy individuals want to know their risk for Alzheimer’s disease: what should we do? J Alzheimers Dis. 2018;62:499-502. doi: 10.3233/JAD-171089
28. Milne S, Lomax C, Freeston MH. A review of the relationship between intolerance of uncertainty and threat appraisal in anxiety. Cogn Behav Ther. 2019;12:e38. doi: 10.1017/S1754470X19000230
29. Hebert EA, Dugas MJ. Behavioral experiments for intolerance of uncertainty: challenging the unknown in the treatment of generalized anxiety disorder. Cogn Behav Pract. 2019;26:421-436. doi: 10.1016/j.cbpra.2018.07.007
30. Stites SD, Karlawish, J. Stigma of Alzheimer’s disease dementia: considerations for practice. Pract Neurol. Published June 2018. Accessed January 31, 2019. http://practicalneurology.com/2018/06/stigma-of-alzheimers-disease-dementia/
31. Solomon A, Turunen H, Ngandu T, et al. Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 2018;75:462. doi: 10.1001/jamaneurol.2017.4365
32. Peters R, Warwick J, Anstey KJ, et al. Blood pressure and dementia: what the SPRINT-MIND trial adds and what we still need to know. Neurology. 2019;92:1017-1018. doi: 10.1212/WNL.0000000000007543
33. Musunuru K, Hershberger RE, Day SM, et al. Genetic testing for inherited cardiovascular diseases: a Scientific Statement from the American Heart Association. Circ Genom Precis Med. 2020;13: e000067. doi: 10.1161/HCG.0000000000000067
34. Margaglione M, Seripa D, Gravina C, et al. Prevalence of apolipoprotein E alleles in healthy subjects and survivors of ischemic stroke. Stroke. 1998;29:399-403. doi: 10.1161/01.STR.29.2.399
35. National Institute on Aging. Alzheimer’s disease genetics fact sheet. Reviewed December 24, 2019. Accessed April 10, 2022. www.nia.nih.gov/health/alzheimers-disease-genetics-fact-sheet
36. Belluck P, Kaplan S, Robbins R. How Aduhelm, an unproven Alzheimer’s drug, got approved. The New York Times. Published July 19, 2021. Updated Oct. 20, 2021. Accessed December 1, 2021. www.nytimes.com/2021/07/19/health/alzheimers-drug-aduhelm-fda.html
Hypertension—or not? Looking beyond office BP readings
Normal blood pressure (BP) is defined as systolic BP (SBP) < 120 mm Hg and diastolic BP (DBP) < 80 mm Hg.1 The thresholds for hypertension (HTN) are shown in TABLE 1.1 These thresholds must be met on at least 2 separate occasions to merit a diagnosis of HTN.1
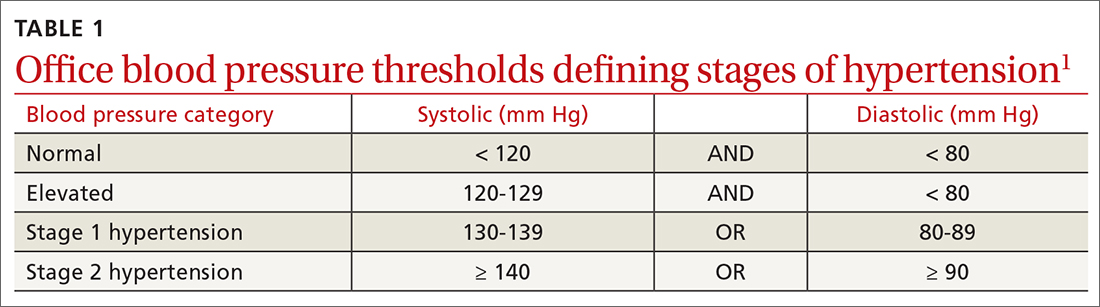
Given the high prevalence of HTN and its associated comorbidities, the US Preventive Services Task Force (USPSTF) recently reaffirmed its recommendation that every adult be screened for HTN, regardless of risk factors.2 Patients 40 years of age and older and those with risk factors (obesity, family history of HTN, diabetes) should have their BP checked at least annually. Individuals ages 18 to 39 years without risk factors who are initially normotensive should be rescreened within 3 to 5 years.2
Patients are most commonly screened for HTN in the outpatient setting. However, office BP measurements may be inaccurate and are of limited diagnostic utility when taken as a single reading.1,3,4 As will be described later, office BP measurements are subject to multiple sources of error that can result in a mean underestimation of 24 mm Hg to a mean overestimation of 33 mm Hg for SBP, and a mean underestimation of 14 mm Hg to a mean overestimation of 23 mm Hg for DBP.4
Differences to this degree between true BP and measured BP can have important implications for the diagnosis, surveillance, and management of HTN. To diminish this potential for error, the American Heart Association HTN guideline and USPSTF recommendation advise clinicians to obtain out-of-office BP measurements to confirm a diagnosis of HTN before initiating treatment.1,2 The preferred methods for out-of-office BP assessment are home BP monitoring (HBPM) and 24-hour ambulatory BP monitoring (ABPM).
Limitations of office BP measurement
Multiple sources of error can lead to wide variability in the measurement of office BP, whether taken via the traditional sphygmomanometer auscultatory approach or with an oscillometric monitor.1,4 Measurement error can be patient related (eg, talking during the reading, or eating or using tobacco prior to measurement), device related (eg, device has not been calibrated or validated), or procedure related (eg, miscuffing, improper patient positioning).
Although use of validated oscillometric monitors eliminates some sources of error such as terminal digit bias, rapid cuff deflation, and missed Korotkoff sounds, their use does not eliminate other sources of error. For example, a patient’s use of tobacco 30 to 60 minutes prior to measurement can raise SBP by 2.8 to 25 mm Hg and DBP 2 to 18 mm Hg.4 Having a full bladder can elevate SBP by 4.2 to 33 mm Hg and DBP by 2.8 to 18.5 mm Hg.4 If the patient is talking during measurement, is crossing one leg over the opposite knee, or has an unsupported arm below the level of the heart, SBP and DBP can rise, respectively, by an estimated mean 2 to 23 mm Hg and 2 to 14 mm Hg.4
Although many sources of BP measurement error can be reduced or eliminated through standardization of technique across office staff, some sources of inaccuracy will persist. Even if all variables are optimized, relying solely on office BP monitoring will still misclassify BP phenotypes, which require out-of-office BP assessments.1,3FIGURE 1 reviews key tips for maximizing the accuracy of BP measurement, regardless of where the measurement is done.
Continue to: Automated office BP
Automated office BP (AOBP) lessens some of the limitations inherent with the traditional sphygmomanometer auscultatory and single-measurement oscillometric devices. AOBP combines oscillometric technology with the capacity to record multiple BP readings within a single activation, thereby providing an average of these readings.1 The total time required for AOBP is 4 to 6 minutes, including a brief rest period before the measurement starts. Studies have reported comparable readings between staff-attended and unattended AOBP, which is an encouraging way to eliminate some measurement error (eg, talking with the patient) and to improve efficiency.5,6
Waiting several minutes per patient to record BP may not be practical in a busy office setting and may require an alteration of workflow. There is a paucity of literature evaluating practice realities, which makes it difficult to know how many patients are getting their BP checked in this manner. Several studies have shown that BP measured with AOBP is closer to awake out-of-office BP as measured with ABPM (discussed in a bit),5-8 largely through mitigation of white-coat effect. Canada now recommends AOBP as the preferred method for diagnosing HTN and monitoring BP.9
Home blood pressure monitoring
HBPM refers to individuals measuring their own BP at home. It is important to remember this definition,
There is strong evidence that HBPM adds value over and above office measurements in predicting end-organ damage and cardiovascular disease (CVD) outcomes, and it has a stronger relationship with CVD risk than office BP.1 Compared with office BP measurement, HBPM is a better predictor of echocardiographic left ventricular mass index, urinary albumin-to-creatinine ratio, proteinuria, silent cerebrovascular disease, nonfatal cardiovascular outcomes, cardiovascular mortality, and all-cause mortality.15,16 There is no strong evidence demonstrating the superiority of HBPM over ABPM, or vice versa, for predicting CVD events or mortality.17 Both ABPM and HBPM have important roles in out-of-office monitoring (FIGURE 23).
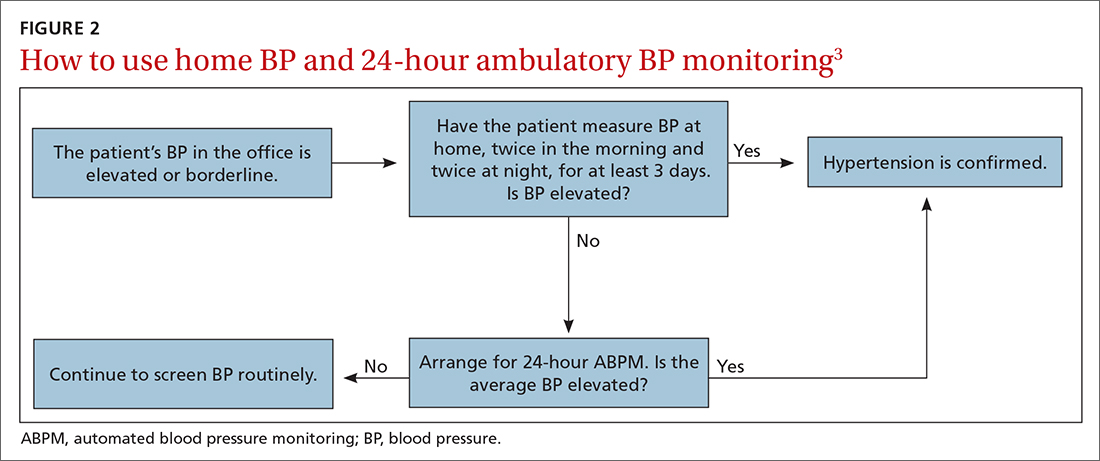
Clinical indications for HBPM
HBPM can facilitate diagnosis of white-coat HTN or effect (if already on BP-lowering medication) as well as masked uncontrolled HTN and masked HTN. Importantly, masked HTN is associated with nearly the same risk of target organ damage and cardiovascular events as sustained HTN. In one meta-analysis the overall adjusted hazard ratio for CVD events was 2.00 (95% CI, 1.58-2.52) for masked HTN and 2.28 (95% CI, 1.87-2.78) for sustained HTN, compared with normotensive individuals.18 Other studies support these results, demonstrating that masked HTN confers risk similar to sustained HTN.19,20
Even treated subjects with masked uncontrolled HTN (normal office and high home BP) have higher CVD risk, likely due to undertreatment given lower BP in the office setting. Among 1451 treated patients in a large cohort study who were followed for a median of 8.3 years, CVD was higher in those with masked uncontrolled HTN (adjusted hazard ratio = 1.76; 95% CI, 1.23-2.53) compared to treated controlled patients (normal office and home BP).21
HBPM also can be used to monitor BP levels over time, to increase patient involvement in chronic disease management, and to improve adherence with medications. Since 2008, several meta-analyses have been published showing improved BP control when HBPM is combined with other interventions and patient education.22-25 Particularly relevant in the age of increased telehealth, several meta-analyses demonstrate improvement in BP control when HBPM is combined with web- or phone-based support, systematic medication titration, patient education, and provider counseling.22-25 A comprehensive systematic review found HBPM with this kind of ongoing support (compared with usual care) led to clinic SBP reductions of 3.2 mm Hg (95% CI, 1.6-4.9) at 12 months.22
Continue to: HBPM nuts and bolts
HBPM nuts and bolts
When using HBPM to obtain a BP average either for confirming a diagnosis or assessing HTN control, patients should be instructed to record their BP measurements twice in the morning and twice at night for a minimum of 3 days (ie, 12 readings).26,27 For each monitoring period, both SBP and DBP readings should be recorded, although protocols differ as to whether to discard the initial reading of each day, or the entire first day of readings.26-29 Consecutive days of monitoring are preferred, although nonconsecutive days also are likely to provide valid data. Once BP stabilizes, monitoring 1 to 3 days a week is likely sufficient.
Most guidelines cite a mean BP of ≥ 135/85 mm Hg as the indication of high BP on HBPM.1,28,29 This value corresponds to an office BP average of 140/90 mm Hg. TABLE 21 shows the comparison of home, ambulatory, and office BP thresholds.
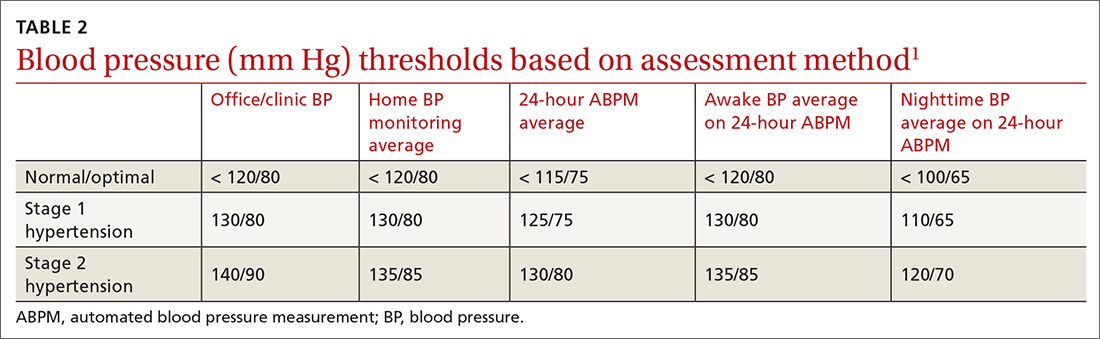
Device selection and validation
As with any BP device, validation and proper technique are important. Recommend only upper-arm cuff devices that have passed validation protocols.30 To eliminate the burden on patients to accurately record and store their BP readings, and to eliminate this step as a source of bias, additionally recommend devices with built-in memory. Although easy-to-use wrist and finger monitors have become popular, there are important limitations in terms of accurate positioning and a lack of validated protocols.31,32
The brachial artery is still the recommended measurement location, unless otherwise precluded due to arm size (the largest size for most validated upper-arm cuffs is 42 cm), patient discomfort, medical contraindication (eg, lymphedema), or immobility (eg, due to injury). Arm size limitation is particularly important as obesity rates continue to rise. Data from the National Health and Nutrition Examination Survey indicate that 52% of men and 38% of women with HTN need a different cuff size than the US standard.33 If the brachial artery is not an option, there are no definitive data to recommend finger over wrist devices, as both are limited by lack of validated protocols.
The website www.stridebp.org maintains a current list of validated and preferred BP devices, and is supported by the European Society of Hypertension, the International Society of Hypertension, and the World Hypertension League. There are more than 4000 devices on the global market, but only 8% have been validated according to StrideBP.
Advances in HBPM that offset previous limitations
The usefulness of HBPM depends on patient factors such as a commitment to monitoring, applying standardized technique, and accurately recording measurements. Discuss these matters with patients before recommending HBPM. Until recently, HBPM devices could not measure BP during sleep. However, a device that assesses BP during sleep has now come on the US market, with preliminary data suggesting the BP measurements are similar to those obtained with ABPM.34 Advances in device memory and data storage and increased availability of electronic health record connection continue to improve the standardization and reliability of HBPM. In fact, there is a growing list of electronic health portals that can be synced with apps for direct transfer of HBPM data.
Ambulatory blood pressure monitoring
ABPM involves wearing a small device connected to an arm BP cuff that measures BP at pre-programmed intervals over a 24-hour period, during sleep and wakefulness. ABPM is the standard against which HBPM and office BP are compared.1-3
Continue to: Clinical indications for ABPM
Clinical indications for ABPM
Compared with office-based BP measurements, ABPM has a stronger positive correlation with clinical CVD outcomes and HTN-related organ damage.1 ABPM has the advantage of being able to provide a large number of measurements over the course of a patient’s daily activities, including sleep. It is useful to evaluate for a wide spectrum of hypertensive or hypotensive patterns, including nocturnal, postprandial, and drug-related patterns. ABPM also is used to assess for white-coat HTN and masked HTN.1
Among these BP phenotypes, an estimated 15% to 30% of adults in the United States exhibit white-coat HTN.1 Most evidence suggests that white-coat HTN confers similar cardiovascular risk as normotension, and it therefore does not require treatment.35 Confirming this diagnosis saves the individual and the health care system the cost of unnecessary diagnosis and treatment.
One cost-effectiveness study using ABPM for annual screening with subsequent treatment for those confirmed to be hypertensive found that ABPM reduced treatment-years by correctly identifying white-coat HTN, and also delayed treatment for those who would eventually develop HTN with advancing age.36 The estimates in savings were 3% to 14% for total cost of care for hypertension and 10% to 23% reduction in treatment days.36 An Australian study showed similar cost reductions.37 A more recent analysis demonstrated that compared with clinic BP measurement alone, incorporation of ABPM is associated with lifetime cost-savings ranging from $77 to $5013, depending on the age and sex of the patients modeled.38
ABPM can also be used to rule out white-coat effect in patients being evaluated for resistant HTN. Several studies demonstrate that among patients with apparent resistant HTN, approximately one-third have controlled BP when assessed by ABPM.39-41 Thus, it is recommended to conduct an out-of-office BP assessment in patients with apparent resistant HTN prior to adding another medication.41Twelve percent of US adults have masked HTN.42 As described earlier, these patients, unrecognized without out-of-office BP assessment, are twice as likely to experience a CVD event compared with normotensive patients.1,42,43
ABPM nuts and bolts
ABPM devices are typically worn for 24 hours and with little interruption to daily routines. Prior to BP capture, the device will alert the patient to ensure the patient’s arm can be held still while the BP measurement is being captured.44 At the completion of 24 hours, specific software uses the stored data to calculate the BP and heart rate averages, as well as minimums and maximums throughout the monitoring period. Clinical decision-making should be driven by the average BP measurements during times of sleep and wakefulness.1,14,44FIGURE 3 is an example of output from an ABPM session. TABLE 31,44 offers a comparison of HBPM and ABPM.
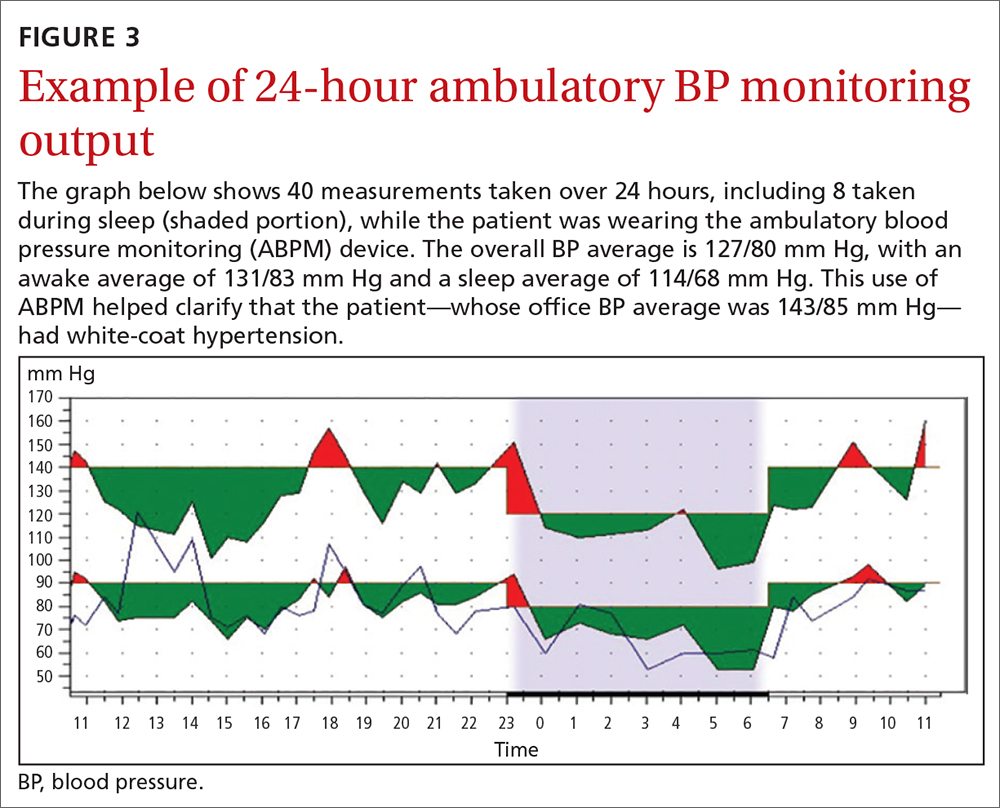
Limitations of ABPM
While ABPM has been designed to be almost effortless to use, some may find it inconvenient to wear. The repeated cuff inflations can cause discomfort or bruising, and the device can interfere with sleep.45 Inconsistent or incorrect wear of ABPM can diminish the quality of BP measurements, which can potentially affect interpretation and subsequent clinical decision-making. Therefore, consider the likelihood of correct and complete usage before ordering ABPM for your patient. Such deliberation is particularly relevant when there is concern for BP phenotypes such as nocturnal nondipping (failure of BP to fall appropriately during sleep) and postprandial HTN and hypotension.
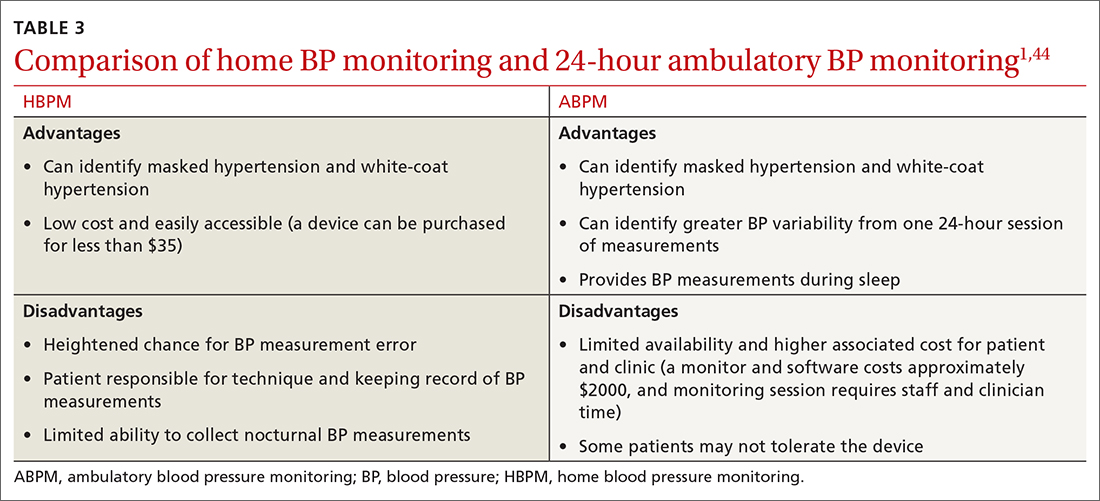
Trained personnel are needed to oversee coordination of the ABPM service within the clinic and to educate patients about proper wear. Additionally, ABPM has not been widely used in US clinical practices to date, in part because this diagnostic strategy is not favorably reimbursed. Based on geographic region, Medicare currently pays between $56 and $122 per 24-hour ABPM session, and only for suspected white-coat HTN.38 Discrepancies remain between commercial and Medicaid/Medicare coverage.44
Continue to: Other modes of monitoring BP
Other modes of monitoring BP
The COVID pandemic has changed health care in many ways, including the frequency of in-person visits. As clinics come to rely more on virtual visits and telehealth, accurate monitoring of out-of-office BP has become more important. Kiosks and smart technology offer the opportunity to supplement traditional in-office BP readings. Kiosks are commonly found in pharmacies and grocery stores. These stations facilitate BP monitoring, as long as the device is appropriately validated and calibrated. Unfortunately, most kiosks have only one cuff size that is too small for many US adults, and some do not have a back support.46,47 Additionally, despite US Food and Drug Administration clearance, many kiosks do not have validated protocols, and the reproducibility of kiosk-measured BP is questionable.46,47
Mobile health technology is increasingly being examined as an effective means of providing health information, support, and management in chronic disease. Smartphone technology, wearable sensors, and cuffless BP monitors offer promise for providing BP data in more convenient ways. However, as with kiosk devices, very few of these have been validated, and several have been shown to have poor accuracy compared with oscillometric devices.48-50 For these reasons, kiosk and smart technology for BP monitoring are not recommended at this time, unless no alternatives are available to the patient.
CORRESPONDENCE
Anthony J. Viera, MD, Department of Family Medicine and Community Health, Duke University School of Medicine, 2200 West Main Street, Suite 400, Durham, NC 27705; [email protected]
1. Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35-e66. doi: 10.1161/HYP.0000000000000087
2. Krist AH, Davidson KW, Mangione CM, et al; U.S. Preventive Services Task Force. Screening for hypertension in adults: U.S. Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2021;325:1650-1656. doi: 10.1001/jama.2021.4987
3. Viera AJ, Yano Y, Lin FC, et al. Does this adult patient have hypertension?: the Rational Clinical Examination systematic review. JAMA. 2021;326:339-347. doi: 10.1001/jama.2021.4533
4. Kallioinen N, Hill A, Horswill MS, et al. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017; 35:421-441. doi: 10.1097/HJH.0000000000001197
5. Armstrong D, Matangi M, Brouillard D, et al. Automated office blood pressure: being alone and not location is what matters most. Blood Press Monit. 2015;20:204-208. doi: 10.1097/MBP.0000000000000133
6. Myers MG, Valdivieso M, Kiss A. Consistent relationship between automated office blood pressure recorded in different settings. Blood Press Monit. 2009;14:108-111. doi: 10.1097/MBP.0b013e32832c5167
7. Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomized parallel design controlled trial. BMJ. 2011;342:d286. doi: 10.1136/bmj.d286
8. Ringrose JS, Cena J, Ip S, et al. Comparability of automated office blood pressure to daytime 24-hour ambulatory blood pressure. Can J Cardiol. 2018;34:61-65. doi: 10.1016/j.cjca.2017.09.022
9. Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33:557-576. doi: 10.1016/j.cjca.2017.03.005
10. Sakuma M, Imai Y, Nagai K, et al. Reproducibility of home blood pressure measurements over a 1-year period. Am J Hypertens. 1997;10:798-803. doi: 10.1016/s0895-7061(97)00117-9
11. Brody S, Veit R, Rau H. Four-year test-retest reliability of self-measured blood pressure. Arch Intern Med. 1999;159:1007-1008. doi: 10.1001/archinte.159.9.1007
12. Calvo-Vargas C, Padilla Rios V, Troyo-Sanromán R, et al. Reproducibility and cost of blood pressure self-measurement using the ‘Loaned Self-measurement Equipment Model.’ Blood Press Monit. 2001;6:225-232. doi: 10.1097/00126097-200110000-00001
13. Scisney-Matlock M, Grand A, Steigerwalt SP, et al. Reliability and reproducibility of clinic and home blood pressure measurements in hypertensive women according to age and ethnicity. Blood Press Monit. 2009;14:49-57. doi: 10.1097/MBP.0b013e3283263064
14. Shimbo D, Abdalla M, Falzon L, et al. Role of ambulatory and home blood pressure monitoring in clinical practice: a narrative review. Ann Intern Med. 2015;163:691-700. doi: 10.7326/M15-1270
15. Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30:1289-1299. doi: 10.1097/HJH.0b013e3283531eaf
16. Fuchs SC, Mello RG, Fuchs FC. Home blood pressure monitoring is better predictor of cardiovascular disease and target organ damage than office blood pressure: a systematic review and meta-analysis. Curr Cardiol Rep.2013;15:413. doi: 10.1007/s11886-013-0413-z
17. Shimbo D, Abdalla M, Falzon L, et al. Studies comparing ambulatory blood pressure and home blood pressure on cardiovascular disease and mortality outcomes: a systematic review. J Am Soc Hypertens. 2016;10:224-234. doi: 10.1016/j.jash.2015.12.013
18. Fagard RH, Cornelessen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25:2193-2198. doi: 10.1097/HJH.0b013e3282ef6185
19. Pierdomenico SD, Cuccurullo F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta-analysis. Am J Hypertens. 2011;24:52-58. doi: 10.1038/ajh.2010.203
20. Ohkubo T, Kikuya M, Metoki H, et al. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol. 2005;46:508-515. doi: 10.1016/j.jacc.2005.03.070
21. Stergiou GS, Asayama K, Thijs L, et al; on behalf of the International Database on Home blood pressure in relation to Cardiovascular Outcome (IDHOCO) Investigators. Prognosis of white-coat and masked hypertension: International Database of HOme blood pressure in relation to Cardiovascular Outcome. Hypertension. 2014;63:675-682. doi: 10.1161/HYPERTENSIONAHA.113.02741
22. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389. doi: 10.1371/journal.pmed.1002389
23. Bray EP, Holder R, Mant J, et al. Does self-monitoring reduce blood pressure? Meta-analysis with meta-regression of randomized controlled trials. Ann Med. 2010;42:371-386. doi: 10.3109/07853890.2010.489567
24. Glynn LG, Murphy AW, Smith SM, et al. Self-monitoring and other non-pharmacological interventions to improve the management of hypertension in primary care: a systematic review. Br J Gen Pract. 2010;60:e476-e488. doi: 10.3399/bjgp10X544113
25. Agarwal R, Bills JE, Hecht TJ, et al. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57:29-38. doi: 10.1161/HYPERTENSIONAHA.110.160911
26. Stergiou GS, Skeva II, Zourbaki AS, et al. Self-monitoring of blood pressure at home: how many measurements are needed? J Hypertens. 1998;16:725-773. doi: 10.1097/00004872-199816060-00002
27. Stergiou GS, Nasothimiou EG, Kalogeropoulos PG, et al. The optimal home blood pressure monitoring schedule based on the Didima outcome study. J Hum Hypertens. 2010;24:158-164. doi: 10.1038/jhh.2009.54
28. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785. doi: 10.1038/jhh.2010.54
29. Imai Y, Kario K, Shimada K, et al; Japanese Society of Hypertension Committee for Guidelines for Self-monitoring of Blood Pressure at Home. The Japanese Society of Hypertension guidelines for self-monitoring of blood pressure at home (second edition). Hypertens Res.2012;35:777-795. doi: 10.1038/hr.2012.56
30. O’Brien E, Atkins N, Stergiou G, et al; Working Group on Blood Pressure Monitoring of the European Society of Hypertension. European Society of Hypertension international protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010; 15:23-38. doi: 10.1097/MBP.0b013e3283360e98
31. Casiglia E, Tikhonoff V, Albertini F, et al. Poor reliability of wrist blood pressure self-measurement at home: a population-based study. Hypertension. 2016;68:896-903. doi: 10.1161/HYPERTENSIONAHA.116.07961
32. Harju J, Vehkaoja A, Kumpulainen P, et al. Comparison of non-invasive blood pressure monitoring using modified arterial applanation tonometry with intra-arterial measurement. J Clin Monit Comput. 2018;32:13-22. doi: 10.1007/s10877-017-9984-3
33. Ostchega Y, Hughes JP, Zhang G, et al. Mean mid-arm circumference and blood pressure cuff sizes for U.S. adults: National Health and Nutrition Examination Survey, 1999-2010. Blood Press Monit. 2013;18:138-143. doi: 10.1097/MBP.0b013e3283617606
34. White WB, Barber V. Ambulatory monitoring of blood pressure: an overview of devices, analyses, and clinical utility. In: White WB, ed. Blood Pressure Monitoring in Cardiovascular Medicine and Therapeutics. Springer International Publishing; 2016:55-76.
35. Franklin SS, Thijs L, Asayama K, et al; IDACO Investigators. The cardiovascular risk of white-coat hypertension. J Am Coll Cardiol. 2016;68:2033-2043. doi: 10.1016/j.jacc.2016.08.035
36. Krakoff LR. Cost-effectiveness of ambulatory blood pressure: a reanalysis. Hypertension. 2006;47:29-34. doi: 10.1161/01.HYP.0000197195.84725.66
37. Ewald B, Pekarsky B. Cost analysis of ambulatory blood pressure monitoring in initiating antihypertensive drug treatment in Australian general practice. Med J Aust. 2002;176:580-583. doi: 10.5694/j.1326-5377.2002.tb04588.x
38. Beyhaghi H, Viera AJ. Comparative cost-effectiveness of clinic, home, or ambulatory blood pressure measurement for hypertension diagnosis in US adults. Hypertension. 2019;73:121-131. doi: 10.1161/HYPERTENSIONAHA.118.11715
39. De la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898-902. doi: 10.1161/HYPERTENSIONAHA.110.168948
40. Brown MA, Buddle ML, Martin A. Is resistant hypertension really resistant? Am J Hypertens. 2001;14:1263-1269. doi: 10.1016/s0895-7061(01)02193-8
41. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
42. Wang YC, Shimbo D, Muntner P, et al. Prevalence of masked hypertension among US adults with non-elevated clinic blood pressure. Am J Epidemiol. 2017;185:194-202. doi: 10.1093/aje/kww237
43. Thakkar HV, Pope A, Anpalahan M. Masked hypertension: a systematic review. Heart Lung Circ. 2020;29:102-111. doi: 10.1016/j.hlc.2019.08.006
44. Kronish IM, Hughes C, Quispe K, et al. Implementing ambulatory blood pressure monitoring in primary care practice. Fam Pract Manag. 2020;27:19-25.
45. Viera AJ, Lingley K, Hinderliter AL. Tolerability of the Oscar 2 ambulatory blood pressure monitor among research participants: a cross-sectional repeated measures study. BMC Med Res Methodol. 2011;11:59. doi: 10.1186/1471-2288-11-59
46. Alpert BS, Dart RA, Sica DA. Public-use blood pressure measurement: the kiosk quandary. J Am Soc Hypertens. 2014;8:739-742. doi: 10.1016/j.jash.2014.07.034
47. Al Hamarneh YN, Houle SK, Chatterley P, et al. The validity of blood pressure kiosk validation studies: a systematic review. Blood Press Monit. 2013;18:167-172. doi: 10.1097/MBP.0b013e328360fb85
48. Kumar N, Khunger M, Gupta A, et al. A content analysis of smartphone-based applications for hypertension management. J Am Soc Hypertens. 2015;9:130-136. doi: 10.1016/j.jash.2014.12.001
49. Bruining N, Caiani E, Chronaki C, et al. Acquisition and analysis of cardiovascular signals on smartphones: potential, pitfalls and perspectives: by the Task Force of the e-Cardiology Working Group of European Society of Cardiology. Eur J Prev Cardiol. 2014;21(suppl 2):4-13. doi: 10.1177/2047487314552604
50. Chandrasekaran V, Dantu R, Jonnada S, et al. Cuffless differential blood pressure estimation using smart phones. IEEE Trans Biomed Eng. 2013;60:1080-1089. doi: 10.1109/TBME.2012.2211078
Normal blood pressure (BP) is defined as systolic BP (SBP) < 120 mm Hg and diastolic BP (DBP) < 80 mm Hg.1 The thresholds for hypertension (HTN) are shown in TABLE 1.1 These thresholds must be met on at least 2 separate occasions to merit a diagnosis of HTN.1

Given the high prevalence of HTN and its associated comorbidities, the US Preventive Services Task Force (USPSTF) recently reaffirmed its recommendation that every adult be screened for HTN, regardless of risk factors.2 Patients 40 years of age and older and those with risk factors (obesity, family history of HTN, diabetes) should have their BP checked at least annually. Individuals ages 18 to 39 years without risk factors who are initially normotensive should be rescreened within 3 to 5 years.2
Patients are most commonly screened for HTN in the outpatient setting. However, office BP measurements may be inaccurate and are of limited diagnostic utility when taken as a single reading.1,3,4 As will be described later, office BP measurements are subject to multiple sources of error that can result in a mean underestimation of 24 mm Hg to a mean overestimation of 33 mm Hg for SBP, and a mean underestimation of 14 mm Hg to a mean overestimation of 23 mm Hg for DBP.4
Differences to this degree between true BP and measured BP can have important implications for the diagnosis, surveillance, and management of HTN. To diminish this potential for error, the American Heart Association HTN guideline and USPSTF recommendation advise clinicians to obtain out-of-office BP measurements to confirm a diagnosis of HTN before initiating treatment.1,2 The preferred methods for out-of-office BP assessment are home BP monitoring (HBPM) and 24-hour ambulatory BP monitoring (ABPM).
Limitations of office BP measurement
Multiple sources of error can lead to wide variability in the measurement of office BP, whether taken via the traditional sphygmomanometer auscultatory approach or with an oscillometric monitor.1,4 Measurement error can be patient related (eg, talking during the reading, or eating or using tobacco prior to measurement), device related (eg, device has not been calibrated or validated), or procedure related (eg, miscuffing, improper patient positioning).
Although use of validated oscillometric monitors eliminates some sources of error such as terminal digit bias, rapid cuff deflation, and missed Korotkoff sounds, their use does not eliminate other sources of error. For example, a patient’s use of tobacco 30 to 60 minutes prior to measurement can raise SBP by 2.8 to 25 mm Hg and DBP 2 to 18 mm Hg.4 Having a full bladder can elevate SBP by 4.2 to 33 mm Hg and DBP by 2.8 to 18.5 mm Hg.4 If the patient is talking during measurement, is crossing one leg over the opposite knee, or has an unsupported arm below the level of the heart, SBP and DBP can rise, respectively, by an estimated mean 2 to 23 mm Hg and 2 to 14 mm Hg.4
Although many sources of BP measurement error can be reduced or eliminated through standardization of technique across office staff, some sources of inaccuracy will persist. Even if all variables are optimized, relying solely on office BP monitoring will still misclassify BP phenotypes, which require out-of-office BP assessments.1,3FIGURE 1 reviews key tips for maximizing the accuracy of BP measurement, regardless of where the measurement is done.

Continue to: Automated office BP
Automated office BP (AOBP) lessens some of the limitations inherent with the traditional sphygmomanometer auscultatory and single-measurement oscillometric devices. AOBP combines oscillometric technology with the capacity to record multiple BP readings within a single activation, thereby providing an average of these readings.1 The total time required for AOBP is 4 to 6 minutes, including a brief rest period before the measurement starts. Studies have reported comparable readings between staff-attended and unattended AOBP, which is an encouraging way to eliminate some measurement error (eg, talking with the patient) and to improve efficiency.5,6
Waiting several minutes per patient to record BP may not be practical in a busy office setting and may require an alteration of workflow. There is a paucity of literature evaluating practice realities, which makes it difficult to know how many patients are getting their BP checked in this manner. Several studies have shown that BP measured with AOBP is closer to awake out-of-office BP as measured with ABPM (discussed in a bit),5-8 largely through mitigation of white-coat effect. Canada now recommends AOBP as the preferred method for diagnosing HTN and monitoring BP.9
Home blood pressure monitoring
HBPM refers to individuals measuring their own BP at home. It is important to remember this definition,
There is strong evidence that HBPM adds value over and above office measurements in predicting end-organ damage and cardiovascular disease (CVD) outcomes, and it has a stronger relationship with CVD risk than office BP.1 Compared with office BP measurement, HBPM is a better predictor of echocardiographic left ventricular mass index, urinary albumin-to-creatinine ratio, proteinuria, silent cerebrovascular disease, nonfatal cardiovascular outcomes, cardiovascular mortality, and all-cause mortality.15,16 There is no strong evidence demonstrating the superiority of HBPM over ABPM, or vice versa, for predicting CVD events or mortality.17 Both ABPM and HBPM have important roles in out-of-office monitoring (FIGURE 23).

Clinical indications for HBPM
HBPM can facilitate diagnosis of white-coat HTN or effect (if already on BP-lowering medication) as well as masked uncontrolled HTN and masked HTN. Importantly, masked HTN is associated with nearly the same risk of target organ damage and cardiovascular events as sustained HTN. In one meta-analysis the overall adjusted hazard ratio for CVD events was 2.00 (95% CI, 1.58-2.52) for masked HTN and 2.28 (95% CI, 1.87-2.78) for sustained HTN, compared with normotensive individuals.18 Other studies support these results, demonstrating that masked HTN confers risk similar to sustained HTN.19,20
Even treated subjects with masked uncontrolled HTN (normal office and high home BP) have higher CVD risk, likely due to undertreatment given lower BP in the office setting. Among 1451 treated patients in a large cohort study who were followed for a median of 8.3 years, CVD was higher in those with masked uncontrolled HTN (adjusted hazard ratio = 1.76; 95% CI, 1.23-2.53) compared to treated controlled patients (normal office and home BP).21
HBPM also can be used to monitor BP levels over time, to increase patient involvement in chronic disease management, and to improve adherence with medications. Since 2008, several meta-analyses have been published showing improved BP control when HBPM is combined with other interventions and patient education.22-25 Particularly relevant in the age of increased telehealth, several meta-analyses demonstrate improvement in BP control when HBPM is combined with web- or phone-based support, systematic medication titration, patient education, and provider counseling.22-25 A comprehensive systematic review found HBPM with this kind of ongoing support (compared with usual care) led to clinic SBP reductions of 3.2 mm Hg (95% CI, 1.6-4.9) at 12 months.22
Continue to: HBPM nuts and bolts
HBPM nuts and bolts
When using HBPM to obtain a BP average either for confirming a diagnosis or assessing HTN control, patients should be instructed to record their BP measurements twice in the morning and twice at night for a minimum of 3 days (ie, 12 readings).26,27 For each monitoring period, both SBP and DBP readings should be recorded, although protocols differ as to whether to discard the initial reading of each day, or the entire first day of readings.26-29 Consecutive days of monitoring are preferred, although nonconsecutive days also are likely to provide valid data. Once BP stabilizes, monitoring 1 to 3 days a week is likely sufficient.
Most guidelines cite a mean BP of ≥ 135/85 mm Hg as the indication of high BP on HBPM.1,28,29 This value corresponds to an office BP average of 140/90 mm Hg. TABLE 21 shows the comparison of home, ambulatory, and office BP thresholds.

Device selection and validation
As with any BP device, validation and proper technique are important. Recommend only upper-arm cuff devices that have passed validation protocols.30 To eliminate the burden on patients to accurately record and store their BP readings, and to eliminate this step as a source of bias, additionally recommend devices with built-in memory. Although easy-to-use wrist and finger monitors have become popular, there are important limitations in terms of accurate positioning and a lack of validated protocols.31,32
The brachial artery is still the recommended measurement location, unless otherwise precluded due to arm size (the largest size for most validated upper-arm cuffs is 42 cm), patient discomfort, medical contraindication (eg, lymphedema), or immobility (eg, due to injury). Arm size limitation is particularly important as obesity rates continue to rise. Data from the National Health and Nutrition Examination Survey indicate that 52% of men and 38% of women with HTN need a different cuff size than the US standard.33 If the brachial artery is not an option, there are no definitive data to recommend finger over wrist devices, as both are limited by lack of validated protocols.
The website www.stridebp.org maintains a current list of validated and preferred BP devices, and is supported by the European Society of Hypertension, the International Society of Hypertension, and the World Hypertension League. There are more than 4000 devices on the global market, but only 8% have been validated according to StrideBP.
Advances in HBPM that offset previous limitations
The usefulness of HBPM depends on patient factors such as a commitment to monitoring, applying standardized technique, and accurately recording measurements. Discuss these matters with patients before recommending HBPM. Until recently, HBPM devices could not measure BP during sleep. However, a device that assesses BP during sleep has now come on the US market, with preliminary data suggesting the BP measurements are similar to those obtained with ABPM.34 Advances in device memory and data storage and increased availability of electronic health record connection continue to improve the standardization and reliability of HBPM. In fact, there is a growing list of electronic health portals that can be synced with apps for direct transfer of HBPM data.
Ambulatory blood pressure monitoring
ABPM involves wearing a small device connected to an arm BP cuff that measures BP at pre-programmed intervals over a 24-hour period, during sleep and wakefulness. ABPM is the standard against which HBPM and office BP are compared.1-3
Continue to: Clinical indications for ABPM
Clinical indications for ABPM
Compared with office-based BP measurements, ABPM has a stronger positive correlation with clinical CVD outcomes and HTN-related organ damage.1 ABPM has the advantage of being able to provide a large number of measurements over the course of a patient’s daily activities, including sleep. It is useful to evaluate for a wide spectrum of hypertensive or hypotensive patterns, including nocturnal, postprandial, and drug-related patterns. ABPM also is used to assess for white-coat HTN and masked HTN.1
Among these BP phenotypes, an estimated 15% to 30% of adults in the United States exhibit white-coat HTN.1 Most evidence suggests that white-coat HTN confers similar cardiovascular risk as normotension, and it therefore does not require treatment.35 Confirming this diagnosis saves the individual and the health care system the cost of unnecessary diagnosis and treatment.
One cost-effectiveness study using ABPM for annual screening with subsequent treatment for those confirmed to be hypertensive found that ABPM reduced treatment-years by correctly identifying white-coat HTN, and also delayed treatment for those who would eventually develop HTN with advancing age.36 The estimates in savings were 3% to 14% for total cost of care for hypertension and 10% to 23% reduction in treatment days.36 An Australian study showed similar cost reductions.37 A more recent analysis demonstrated that compared with clinic BP measurement alone, incorporation of ABPM is associated with lifetime cost-savings ranging from $77 to $5013, depending on the age and sex of the patients modeled.38
ABPM can also be used to rule out white-coat effect in patients being evaluated for resistant HTN. Several studies demonstrate that among patients with apparent resistant HTN, approximately one-third have controlled BP when assessed by ABPM.39-41 Thus, it is recommended to conduct an out-of-office BP assessment in patients with apparent resistant HTN prior to adding another medication.41Twelve percent of US adults have masked HTN.42 As described earlier, these patients, unrecognized without out-of-office BP assessment, are twice as likely to experience a CVD event compared with normotensive patients.1,42,43
ABPM nuts and bolts
ABPM devices are typically worn for 24 hours and with little interruption to daily routines. Prior to BP capture, the device will alert the patient to ensure the patient’s arm can be held still while the BP measurement is being captured.44 At the completion of 24 hours, specific software uses the stored data to calculate the BP and heart rate averages, as well as minimums and maximums throughout the monitoring period. Clinical decision-making should be driven by the average BP measurements during times of sleep and wakefulness.1,14,44FIGURE 3 is an example of output from an ABPM session. TABLE 31,44 offers a comparison of HBPM and ABPM.

Limitations of ABPM
While ABPM has been designed to be almost effortless to use, some may find it inconvenient to wear. The repeated cuff inflations can cause discomfort or bruising, and the device can interfere with sleep.45 Inconsistent or incorrect wear of ABPM can diminish the quality of BP measurements, which can potentially affect interpretation and subsequent clinical decision-making. Therefore, consider the likelihood of correct and complete usage before ordering ABPM for your patient. Such deliberation is particularly relevant when there is concern for BP phenotypes such as nocturnal nondipping (failure of BP to fall appropriately during sleep) and postprandial HTN and hypotension.

Trained personnel are needed to oversee coordination of the ABPM service within the clinic and to educate patients about proper wear. Additionally, ABPM has not been widely used in US clinical practices to date, in part because this diagnostic strategy is not favorably reimbursed. Based on geographic region, Medicare currently pays between $56 and $122 per 24-hour ABPM session, and only for suspected white-coat HTN.38 Discrepancies remain between commercial and Medicaid/Medicare coverage.44
Continue to: Other modes of monitoring BP
Other modes of monitoring BP
The COVID pandemic has changed health care in many ways, including the frequency of in-person visits. As clinics come to rely more on virtual visits and telehealth, accurate monitoring of out-of-office BP has become more important. Kiosks and smart technology offer the opportunity to supplement traditional in-office BP readings. Kiosks are commonly found in pharmacies and grocery stores. These stations facilitate BP monitoring, as long as the device is appropriately validated and calibrated. Unfortunately, most kiosks have only one cuff size that is too small for many US adults, and some do not have a back support.46,47 Additionally, despite US Food and Drug Administration clearance, many kiosks do not have validated protocols, and the reproducibility of kiosk-measured BP is questionable.46,47
Mobile health technology is increasingly being examined as an effective means of providing health information, support, and management in chronic disease. Smartphone technology, wearable sensors, and cuffless BP monitors offer promise for providing BP data in more convenient ways. However, as with kiosk devices, very few of these have been validated, and several have been shown to have poor accuracy compared with oscillometric devices.48-50 For these reasons, kiosk and smart technology for BP monitoring are not recommended at this time, unless no alternatives are available to the patient.
CORRESPONDENCE
Anthony J. Viera, MD, Department of Family Medicine and Community Health, Duke University School of Medicine, 2200 West Main Street, Suite 400, Durham, NC 27705; [email protected]
Normal blood pressure (BP) is defined as systolic BP (SBP) < 120 mm Hg and diastolic BP (DBP) < 80 mm Hg.1 The thresholds for hypertension (HTN) are shown in TABLE 1.1 These thresholds must be met on at least 2 separate occasions to merit a diagnosis of HTN.1

Given the high prevalence of HTN and its associated comorbidities, the US Preventive Services Task Force (USPSTF) recently reaffirmed its recommendation that every adult be screened for HTN, regardless of risk factors.2 Patients 40 years of age and older and those with risk factors (obesity, family history of HTN, diabetes) should have their BP checked at least annually. Individuals ages 18 to 39 years without risk factors who are initially normotensive should be rescreened within 3 to 5 years.2
Patients are most commonly screened for HTN in the outpatient setting. However, office BP measurements may be inaccurate and are of limited diagnostic utility when taken as a single reading.1,3,4 As will be described later, office BP measurements are subject to multiple sources of error that can result in a mean underestimation of 24 mm Hg to a mean overestimation of 33 mm Hg for SBP, and a mean underestimation of 14 mm Hg to a mean overestimation of 23 mm Hg for DBP.4
Differences to this degree between true BP and measured BP can have important implications for the diagnosis, surveillance, and management of HTN. To diminish this potential for error, the American Heart Association HTN guideline and USPSTF recommendation advise clinicians to obtain out-of-office BP measurements to confirm a diagnosis of HTN before initiating treatment.1,2 The preferred methods for out-of-office BP assessment are home BP monitoring (HBPM) and 24-hour ambulatory BP monitoring (ABPM).
Limitations of office BP measurement
Multiple sources of error can lead to wide variability in the measurement of office BP, whether taken via the traditional sphygmomanometer auscultatory approach or with an oscillometric monitor.1,4 Measurement error can be patient related (eg, talking during the reading, or eating or using tobacco prior to measurement), device related (eg, device has not been calibrated or validated), or procedure related (eg, miscuffing, improper patient positioning).
Although use of validated oscillometric monitors eliminates some sources of error such as terminal digit bias, rapid cuff deflation, and missed Korotkoff sounds, their use does not eliminate other sources of error. For example, a patient’s use of tobacco 30 to 60 minutes prior to measurement can raise SBP by 2.8 to 25 mm Hg and DBP 2 to 18 mm Hg.4 Having a full bladder can elevate SBP by 4.2 to 33 mm Hg and DBP by 2.8 to 18.5 mm Hg.4 If the patient is talking during measurement, is crossing one leg over the opposite knee, or has an unsupported arm below the level of the heart, SBP and DBP can rise, respectively, by an estimated mean 2 to 23 mm Hg and 2 to 14 mm Hg.4
Although many sources of BP measurement error can be reduced or eliminated through standardization of technique across office staff, some sources of inaccuracy will persist. Even if all variables are optimized, relying solely on office BP monitoring will still misclassify BP phenotypes, which require out-of-office BP assessments.1,3FIGURE 1 reviews key tips for maximizing the accuracy of BP measurement, regardless of where the measurement is done.

Continue to: Automated office BP
Automated office BP (AOBP) lessens some of the limitations inherent with the traditional sphygmomanometer auscultatory and single-measurement oscillometric devices. AOBP combines oscillometric technology with the capacity to record multiple BP readings within a single activation, thereby providing an average of these readings.1 The total time required for AOBP is 4 to 6 minutes, including a brief rest period before the measurement starts. Studies have reported comparable readings between staff-attended and unattended AOBP, which is an encouraging way to eliminate some measurement error (eg, talking with the patient) and to improve efficiency.5,6
Waiting several minutes per patient to record BP may not be practical in a busy office setting and may require an alteration of workflow. There is a paucity of literature evaluating practice realities, which makes it difficult to know how many patients are getting their BP checked in this manner. Several studies have shown that BP measured with AOBP is closer to awake out-of-office BP as measured with ABPM (discussed in a bit),5-8 largely through mitigation of white-coat effect. Canada now recommends AOBP as the preferred method for diagnosing HTN and monitoring BP.9
Home blood pressure monitoring
HBPM refers to individuals measuring their own BP at home. It is important to remember this definition,
There is strong evidence that HBPM adds value over and above office measurements in predicting end-organ damage and cardiovascular disease (CVD) outcomes, and it has a stronger relationship with CVD risk than office BP.1 Compared with office BP measurement, HBPM is a better predictor of echocardiographic left ventricular mass index, urinary albumin-to-creatinine ratio, proteinuria, silent cerebrovascular disease, nonfatal cardiovascular outcomes, cardiovascular mortality, and all-cause mortality.15,16 There is no strong evidence demonstrating the superiority of HBPM over ABPM, or vice versa, for predicting CVD events or mortality.17 Both ABPM and HBPM have important roles in out-of-office monitoring (FIGURE 23).

Clinical indications for HBPM
HBPM can facilitate diagnosis of white-coat HTN or effect (if already on BP-lowering medication) as well as masked uncontrolled HTN and masked HTN. Importantly, masked HTN is associated with nearly the same risk of target organ damage and cardiovascular events as sustained HTN. In one meta-analysis the overall adjusted hazard ratio for CVD events was 2.00 (95% CI, 1.58-2.52) for masked HTN and 2.28 (95% CI, 1.87-2.78) for sustained HTN, compared with normotensive individuals.18 Other studies support these results, demonstrating that masked HTN confers risk similar to sustained HTN.19,20
Even treated subjects with masked uncontrolled HTN (normal office and high home BP) have higher CVD risk, likely due to undertreatment given lower BP in the office setting. Among 1451 treated patients in a large cohort study who were followed for a median of 8.3 years, CVD was higher in those with masked uncontrolled HTN (adjusted hazard ratio = 1.76; 95% CI, 1.23-2.53) compared to treated controlled patients (normal office and home BP).21
HBPM also can be used to monitor BP levels over time, to increase patient involvement in chronic disease management, and to improve adherence with medications. Since 2008, several meta-analyses have been published showing improved BP control when HBPM is combined with other interventions and patient education.22-25 Particularly relevant in the age of increased telehealth, several meta-analyses demonstrate improvement in BP control when HBPM is combined with web- or phone-based support, systematic medication titration, patient education, and provider counseling.22-25 A comprehensive systematic review found HBPM with this kind of ongoing support (compared with usual care) led to clinic SBP reductions of 3.2 mm Hg (95% CI, 1.6-4.9) at 12 months.22
Continue to: HBPM nuts and bolts
HBPM nuts and bolts
When using HBPM to obtain a BP average either for confirming a diagnosis or assessing HTN control, patients should be instructed to record their BP measurements twice in the morning and twice at night for a minimum of 3 days (ie, 12 readings).26,27 For each monitoring period, both SBP and DBP readings should be recorded, although protocols differ as to whether to discard the initial reading of each day, or the entire first day of readings.26-29 Consecutive days of monitoring are preferred, although nonconsecutive days also are likely to provide valid data. Once BP stabilizes, monitoring 1 to 3 days a week is likely sufficient.
Most guidelines cite a mean BP of ≥ 135/85 mm Hg as the indication of high BP on HBPM.1,28,29 This value corresponds to an office BP average of 140/90 mm Hg. TABLE 21 shows the comparison of home, ambulatory, and office BP thresholds.

Device selection and validation
As with any BP device, validation and proper technique are important. Recommend only upper-arm cuff devices that have passed validation protocols.30 To eliminate the burden on patients to accurately record and store their BP readings, and to eliminate this step as a source of bias, additionally recommend devices with built-in memory. Although easy-to-use wrist and finger monitors have become popular, there are important limitations in terms of accurate positioning and a lack of validated protocols.31,32
The brachial artery is still the recommended measurement location, unless otherwise precluded due to arm size (the largest size for most validated upper-arm cuffs is 42 cm), patient discomfort, medical contraindication (eg, lymphedema), or immobility (eg, due to injury). Arm size limitation is particularly important as obesity rates continue to rise. Data from the National Health and Nutrition Examination Survey indicate that 52% of men and 38% of women with HTN need a different cuff size than the US standard.33 If the brachial artery is not an option, there are no definitive data to recommend finger over wrist devices, as both are limited by lack of validated protocols.
The website www.stridebp.org maintains a current list of validated and preferred BP devices, and is supported by the European Society of Hypertension, the International Society of Hypertension, and the World Hypertension League. There are more than 4000 devices on the global market, but only 8% have been validated according to StrideBP.
Advances in HBPM that offset previous limitations
The usefulness of HBPM depends on patient factors such as a commitment to monitoring, applying standardized technique, and accurately recording measurements. Discuss these matters with patients before recommending HBPM. Until recently, HBPM devices could not measure BP during sleep. However, a device that assesses BP during sleep has now come on the US market, with preliminary data suggesting the BP measurements are similar to those obtained with ABPM.34 Advances in device memory and data storage and increased availability of electronic health record connection continue to improve the standardization and reliability of HBPM. In fact, there is a growing list of electronic health portals that can be synced with apps for direct transfer of HBPM data.
Ambulatory blood pressure monitoring
ABPM involves wearing a small device connected to an arm BP cuff that measures BP at pre-programmed intervals over a 24-hour period, during sleep and wakefulness. ABPM is the standard against which HBPM and office BP are compared.1-3
Continue to: Clinical indications for ABPM
Clinical indications for ABPM
Compared with office-based BP measurements, ABPM has a stronger positive correlation with clinical CVD outcomes and HTN-related organ damage.1 ABPM has the advantage of being able to provide a large number of measurements over the course of a patient’s daily activities, including sleep. It is useful to evaluate for a wide spectrum of hypertensive or hypotensive patterns, including nocturnal, postprandial, and drug-related patterns. ABPM also is used to assess for white-coat HTN and masked HTN.1
Among these BP phenotypes, an estimated 15% to 30% of adults in the United States exhibit white-coat HTN.1 Most evidence suggests that white-coat HTN confers similar cardiovascular risk as normotension, and it therefore does not require treatment.35 Confirming this diagnosis saves the individual and the health care system the cost of unnecessary diagnosis and treatment.
One cost-effectiveness study using ABPM for annual screening with subsequent treatment for those confirmed to be hypertensive found that ABPM reduced treatment-years by correctly identifying white-coat HTN, and also delayed treatment for those who would eventually develop HTN with advancing age.36 The estimates in savings were 3% to 14% for total cost of care for hypertension and 10% to 23% reduction in treatment days.36 An Australian study showed similar cost reductions.37 A more recent analysis demonstrated that compared with clinic BP measurement alone, incorporation of ABPM is associated with lifetime cost-savings ranging from $77 to $5013, depending on the age and sex of the patients modeled.38
ABPM can also be used to rule out white-coat effect in patients being evaluated for resistant HTN. Several studies demonstrate that among patients with apparent resistant HTN, approximately one-third have controlled BP when assessed by ABPM.39-41 Thus, it is recommended to conduct an out-of-office BP assessment in patients with apparent resistant HTN prior to adding another medication.41Twelve percent of US adults have masked HTN.42 As described earlier, these patients, unrecognized without out-of-office BP assessment, are twice as likely to experience a CVD event compared with normotensive patients.1,42,43
ABPM nuts and bolts
ABPM devices are typically worn for 24 hours and with little interruption to daily routines. Prior to BP capture, the device will alert the patient to ensure the patient’s arm can be held still while the BP measurement is being captured.44 At the completion of 24 hours, specific software uses the stored data to calculate the BP and heart rate averages, as well as minimums and maximums throughout the monitoring period. Clinical decision-making should be driven by the average BP measurements during times of sleep and wakefulness.1,14,44FIGURE 3 is an example of output from an ABPM session. TABLE 31,44 offers a comparison of HBPM and ABPM.

Limitations of ABPM
While ABPM has been designed to be almost effortless to use, some may find it inconvenient to wear. The repeated cuff inflations can cause discomfort or bruising, and the device can interfere with sleep.45 Inconsistent or incorrect wear of ABPM can diminish the quality of BP measurements, which can potentially affect interpretation and subsequent clinical decision-making. Therefore, consider the likelihood of correct and complete usage before ordering ABPM for your patient. Such deliberation is particularly relevant when there is concern for BP phenotypes such as nocturnal nondipping (failure of BP to fall appropriately during sleep) and postprandial HTN and hypotension.

Trained personnel are needed to oversee coordination of the ABPM service within the clinic and to educate patients about proper wear. Additionally, ABPM has not been widely used in US clinical practices to date, in part because this diagnostic strategy is not favorably reimbursed. Based on geographic region, Medicare currently pays between $56 and $122 per 24-hour ABPM session, and only for suspected white-coat HTN.38 Discrepancies remain between commercial and Medicaid/Medicare coverage.44
Continue to: Other modes of monitoring BP
Other modes of monitoring BP
The COVID pandemic has changed health care in many ways, including the frequency of in-person visits. As clinics come to rely more on virtual visits and telehealth, accurate monitoring of out-of-office BP has become more important. Kiosks and smart technology offer the opportunity to supplement traditional in-office BP readings. Kiosks are commonly found in pharmacies and grocery stores. These stations facilitate BP monitoring, as long as the device is appropriately validated and calibrated. Unfortunately, most kiosks have only one cuff size that is too small for many US adults, and some do not have a back support.46,47 Additionally, despite US Food and Drug Administration clearance, many kiosks do not have validated protocols, and the reproducibility of kiosk-measured BP is questionable.46,47
Mobile health technology is increasingly being examined as an effective means of providing health information, support, and management in chronic disease. Smartphone technology, wearable sensors, and cuffless BP monitors offer promise for providing BP data in more convenient ways. However, as with kiosk devices, very few of these have been validated, and several have been shown to have poor accuracy compared with oscillometric devices.48-50 For these reasons, kiosk and smart technology for BP monitoring are not recommended at this time, unless no alternatives are available to the patient.
CORRESPONDENCE
Anthony J. Viera, MD, Department of Family Medicine and Community Health, Duke University School of Medicine, 2200 West Main Street, Suite 400, Durham, NC 27705; [email protected]
1. Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35-e66. doi: 10.1161/HYP.0000000000000087
2. Krist AH, Davidson KW, Mangione CM, et al; U.S. Preventive Services Task Force. Screening for hypertension in adults: U.S. Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2021;325:1650-1656. doi: 10.1001/jama.2021.4987
3. Viera AJ, Yano Y, Lin FC, et al. Does this adult patient have hypertension?: the Rational Clinical Examination systematic review. JAMA. 2021;326:339-347. doi: 10.1001/jama.2021.4533
4. Kallioinen N, Hill A, Horswill MS, et al. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017; 35:421-441. doi: 10.1097/HJH.0000000000001197
5. Armstrong D, Matangi M, Brouillard D, et al. Automated office blood pressure: being alone and not location is what matters most. Blood Press Monit. 2015;20:204-208. doi: 10.1097/MBP.0000000000000133
6. Myers MG, Valdivieso M, Kiss A. Consistent relationship between automated office blood pressure recorded in different settings. Blood Press Monit. 2009;14:108-111. doi: 10.1097/MBP.0b013e32832c5167
7. Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomized parallel design controlled trial. BMJ. 2011;342:d286. doi: 10.1136/bmj.d286
8. Ringrose JS, Cena J, Ip S, et al. Comparability of automated office blood pressure to daytime 24-hour ambulatory blood pressure. Can J Cardiol. 2018;34:61-65. doi: 10.1016/j.cjca.2017.09.022
9. Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33:557-576. doi: 10.1016/j.cjca.2017.03.005
10. Sakuma M, Imai Y, Nagai K, et al. Reproducibility of home blood pressure measurements over a 1-year period. Am J Hypertens. 1997;10:798-803. doi: 10.1016/s0895-7061(97)00117-9
11. Brody S, Veit R, Rau H. Four-year test-retest reliability of self-measured blood pressure. Arch Intern Med. 1999;159:1007-1008. doi: 10.1001/archinte.159.9.1007
12. Calvo-Vargas C, Padilla Rios V, Troyo-Sanromán R, et al. Reproducibility and cost of blood pressure self-measurement using the ‘Loaned Self-measurement Equipment Model.’ Blood Press Monit. 2001;6:225-232. doi: 10.1097/00126097-200110000-00001
13. Scisney-Matlock M, Grand A, Steigerwalt SP, et al. Reliability and reproducibility of clinic and home blood pressure measurements in hypertensive women according to age and ethnicity. Blood Press Monit. 2009;14:49-57. doi: 10.1097/MBP.0b013e3283263064
14. Shimbo D, Abdalla M, Falzon L, et al. Role of ambulatory and home blood pressure monitoring in clinical practice: a narrative review. Ann Intern Med. 2015;163:691-700. doi: 10.7326/M15-1270
15. Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30:1289-1299. doi: 10.1097/HJH.0b013e3283531eaf
16. Fuchs SC, Mello RG, Fuchs FC. Home blood pressure monitoring is better predictor of cardiovascular disease and target organ damage than office blood pressure: a systematic review and meta-analysis. Curr Cardiol Rep.2013;15:413. doi: 10.1007/s11886-013-0413-z
17. Shimbo D, Abdalla M, Falzon L, et al. Studies comparing ambulatory blood pressure and home blood pressure on cardiovascular disease and mortality outcomes: a systematic review. J Am Soc Hypertens. 2016;10:224-234. doi: 10.1016/j.jash.2015.12.013
18. Fagard RH, Cornelessen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25:2193-2198. doi: 10.1097/HJH.0b013e3282ef6185
19. Pierdomenico SD, Cuccurullo F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta-analysis. Am J Hypertens. 2011;24:52-58. doi: 10.1038/ajh.2010.203
20. Ohkubo T, Kikuya M, Metoki H, et al. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol. 2005;46:508-515. doi: 10.1016/j.jacc.2005.03.070
21. Stergiou GS, Asayama K, Thijs L, et al; on behalf of the International Database on Home blood pressure in relation to Cardiovascular Outcome (IDHOCO) Investigators. Prognosis of white-coat and masked hypertension: International Database of HOme blood pressure in relation to Cardiovascular Outcome. Hypertension. 2014;63:675-682. doi: 10.1161/HYPERTENSIONAHA.113.02741
22. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389. doi: 10.1371/journal.pmed.1002389
23. Bray EP, Holder R, Mant J, et al. Does self-monitoring reduce blood pressure? Meta-analysis with meta-regression of randomized controlled trials. Ann Med. 2010;42:371-386. doi: 10.3109/07853890.2010.489567
24. Glynn LG, Murphy AW, Smith SM, et al. Self-monitoring and other non-pharmacological interventions to improve the management of hypertension in primary care: a systematic review. Br J Gen Pract. 2010;60:e476-e488. doi: 10.3399/bjgp10X544113
25. Agarwal R, Bills JE, Hecht TJ, et al. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57:29-38. doi: 10.1161/HYPERTENSIONAHA.110.160911
26. Stergiou GS, Skeva II, Zourbaki AS, et al. Self-monitoring of blood pressure at home: how many measurements are needed? J Hypertens. 1998;16:725-773. doi: 10.1097/00004872-199816060-00002
27. Stergiou GS, Nasothimiou EG, Kalogeropoulos PG, et al. The optimal home blood pressure monitoring schedule based on the Didima outcome study. J Hum Hypertens. 2010;24:158-164. doi: 10.1038/jhh.2009.54
28. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785. doi: 10.1038/jhh.2010.54
29. Imai Y, Kario K, Shimada K, et al; Japanese Society of Hypertension Committee for Guidelines for Self-monitoring of Blood Pressure at Home. The Japanese Society of Hypertension guidelines for self-monitoring of blood pressure at home (second edition). Hypertens Res.2012;35:777-795. doi: 10.1038/hr.2012.56
30. O’Brien E, Atkins N, Stergiou G, et al; Working Group on Blood Pressure Monitoring of the European Society of Hypertension. European Society of Hypertension international protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010; 15:23-38. doi: 10.1097/MBP.0b013e3283360e98
31. Casiglia E, Tikhonoff V, Albertini F, et al. Poor reliability of wrist blood pressure self-measurement at home: a population-based study. Hypertension. 2016;68:896-903. doi: 10.1161/HYPERTENSIONAHA.116.07961
32. Harju J, Vehkaoja A, Kumpulainen P, et al. Comparison of non-invasive blood pressure monitoring using modified arterial applanation tonometry with intra-arterial measurement. J Clin Monit Comput. 2018;32:13-22. doi: 10.1007/s10877-017-9984-3
33. Ostchega Y, Hughes JP, Zhang G, et al. Mean mid-arm circumference and blood pressure cuff sizes for U.S. adults: National Health and Nutrition Examination Survey, 1999-2010. Blood Press Monit. 2013;18:138-143. doi: 10.1097/MBP.0b013e3283617606
34. White WB, Barber V. Ambulatory monitoring of blood pressure: an overview of devices, analyses, and clinical utility. In: White WB, ed. Blood Pressure Monitoring in Cardiovascular Medicine and Therapeutics. Springer International Publishing; 2016:55-76.
35. Franklin SS, Thijs L, Asayama K, et al; IDACO Investigators. The cardiovascular risk of white-coat hypertension. J Am Coll Cardiol. 2016;68:2033-2043. doi: 10.1016/j.jacc.2016.08.035
36. Krakoff LR. Cost-effectiveness of ambulatory blood pressure: a reanalysis. Hypertension. 2006;47:29-34. doi: 10.1161/01.HYP.0000197195.84725.66
37. Ewald B, Pekarsky B. Cost analysis of ambulatory blood pressure monitoring in initiating antihypertensive drug treatment in Australian general practice. Med J Aust. 2002;176:580-583. doi: 10.5694/j.1326-5377.2002.tb04588.x
38. Beyhaghi H, Viera AJ. Comparative cost-effectiveness of clinic, home, or ambulatory blood pressure measurement for hypertension diagnosis in US adults. Hypertension. 2019;73:121-131. doi: 10.1161/HYPERTENSIONAHA.118.11715
39. De la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898-902. doi: 10.1161/HYPERTENSIONAHA.110.168948
40. Brown MA, Buddle ML, Martin A. Is resistant hypertension really resistant? Am J Hypertens. 2001;14:1263-1269. doi: 10.1016/s0895-7061(01)02193-8
41. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
42. Wang YC, Shimbo D, Muntner P, et al. Prevalence of masked hypertension among US adults with non-elevated clinic blood pressure. Am J Epidemiol. 2017;185:194-202. doi: 10.1093/aje/kww237
43. Thakkar HV, Pope A, Anpalahan M. Masked hypertension: a systematic review. Heart Lung Circ. 2020;29:102-111. doi: 10.1016/j.hlc.2019.08.006
44. Kronish IM, Hughes C, Quispe K, et al. Implementing ambulatory blood pressure monitoring in primary care practice. Fam Pract Manag. 2020;27:19-25.
45. Viera AJ, Lingley K, Hinderliter AL. Tolerability of the Oscar 2 ambulatory blood pressure monitor among research participants: a cross-sectional repeated measures study. BMC Med Res Methodol. 2011;11:59. doi: 10.1186/1471-2288-11-59
46. Alpert BS, Dart RA, Sica DA. Public-use blood pressure measurement: the kiosk quandary. J Am Soc Hypertens. 2014;8:739-742. doi: 10.1016/j.jash.2014.07.034
47. Al Hamarneh YN, Houle SK, Chatterley P, et al. The validity of blood pressure kiosk validation studies: a systematic review. Blood Press Monit. 2013;18:167-172. doi: 10.1097/MBP.0b013e328360fb85
48. Kumar N, Khunger M, Gupta A, et al. A content analysis of smartphone-based applications for hypertension management. J Am Soc Hypertens. 2015;9:130-136. doi: 10.1016/j.jash.2014.12.001
49. Bruining N, Caiani E, Chronaki C, et al. Acquisition and analysis of cardiovascular signals on smartphones: potential, pitfalls and perspectives: by the Task Force of the e-Cardiology Working Group of European Society of Cardiology. Eur J Prev Cardiol. 2014;21(suppl 2):4-13. doi: 10.1177/2047487314552604
50. Chandrasekaran V, Dantu R, Jonnada S, et al. Cuffless differential blood pressure estimation using smart phones. IEEE Trans Biomed Eng. 2013;60:1080-1089. doi: 10.1109/TBME.2012.2211078
1. Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35-e66. doi: 10.1161/HYP.0000000000000087
2. Krist AH, Davidson KW, Mangione CM, et al; U.S. Preventive Services Task Force. Screening for hypertension in adults: U.S. Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2021;325:1650-1656. doi: 10.1001/jama.2021.4987
3. Viera AJ, Yano Y, Lin FC, et al. Does this adult patient have hypertension?: the Rational Clinical Examination systematic review. JAMA. 2021;326:339-347. doi: 10.1001/jama.2021.4533
4. Kallioinen N, Hill A, Horswill MS, et al. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017; 35:421-441. doi: 10.1097/HJH.0000000000001197
5. Armstrong D, Matangi M, Brouillard D, et al. Automated office blood pressure: being alone and not location is what matters most. Blood Press Monit. 2015;20:204-208. doi: 10.1097/MBP.0000000000000133
6. Myers MG, Valdivieso M, Kiss A. Consistent relationship between automated office blood pressure recorded in different settings. Blood Press Monit. 2009;14:108-111. doi: 10.1097/MBP.0b013e32832c5167
7. Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomized parallel design controlled trial. BMJ. 2011;342:d286. doi: 10.1136/bmj.d286
8. Ringrose JS, Cena J, Ip S, et al. Comparability of automated office blood pressure to daytime 24-hour ambulatory blood pressure. Can J Cardiol. 2018;34:61-65. doi: 10.1016/j.cjca.2017.09.022
9. Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33:557-576. doi: 10.1016/j.cjca.2017.03.005
10. Sakuma M, Imai Y, Nagai K, et al. Reproducibility of home blood pressure measurements over a 1-year period. Am J Hypertens. 1997;10:798-803. doi: 10.1016/s0895-7061(97)00117-9
11. Brody S, Veit R, Rau H. Four-year test-retest reliability of self-measured blood pressure. Arch Intern Med. 1999;159:1007-1008. doi: 10.1001/archinte.159.9.1007
12. Calvo-Vargas C, Padilla Rios V, Troyo-Sanromán R, et al. Reproducibility and cost of blood pressure self-measurement using the ‘Loaned Self-measurement Equipment Model.’ Blood Press Monit. 2001;6:225-232. doi: 10.1097/00126097-200110000-00001
13. Scisney-Matlock M, Grand A, Steigerwalt SP, et al. Reliability and reproducibility of clinic and home blood pressure measurements in hypertensive women according to age and ethnicity. Blood Press Monit. 2009;14:49-57. doi: 10.1097/MBP.0b013e3283263064
14. Shimbo D, Abdalla M, Falzon L, et al. Role of ambulatory and home blood pressure monitoring in clinical practice: a narrative review. Ann Intern Med. 2015;163:691-700. doi: 10.7326/M15-1270
15. Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30:1289-1299. doi: 10.1097/HJH.0b013e3283531eaf
16. Fuchs SC, Mello RG, Fuchs FC. Home blood pressure monitoring is better predictor of cardiovascular disease and target organ damage than office blood pressure: a systematic review and meta-analysis. Curr Cardiol Rep.2013;15:413. doi: 10.1007/s11886-013-0413-z
17. Shimbo D, Abdalla M, Falzon L, et al. Studies comparing ambulatory blood pressure and home blood pressure on cardiovascular disease and mortality outcomes: a systematic review. J Am Soc Hypertens. 2016;10:224-234. doi: 10.1016/j.jash.2015.12.013
18. Fagard RH, Cornelessen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25:2193-2198. doi: 10.1097/HJH.0b013e3282ef6185
19. Pierdomenico SD, Cuccurullo F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta-analysis. Am J Hypertens. 2011;24:52-58. doi: 10.1038/ajh.2010.203
20. Ohkubo T, Kikuya M, Metoki H, et al. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol. 2005;46:508-515. doi: 10.1016/j.jacc.2005.03.070
21. Stergiou GS, Asayama K, Thijs L, et al; on behalf of the International Database on Home blood pressure in relation to Cardiovascular Outcome (IDHOCO) Investigators. Prognosis of white-coat and masked hypertension: International Database of HOme blood pressure in relation to Cardiovascular Outcome. Hypertension. 2014;63:675-682. doi: 10.1161/HYPERTENSIONAHA.113.02741
22. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389. doi: 10.1371/journal.pmed.1002389
23. Bray EP, Holder R, Mant J, et al. Does self-monitoring reduce blood pressure? Meta-analysis with meta-regression of randomized controlled trials. Ann Med. 2010;42:371-386. doi: 10.3109/07853890.2010.489567
24. Glynn LG, Murphy AW, Smith SM, et al. Self-monitoring and other non-pharmacological interventions to improve the management of hypertension in primary care: a systematic review. Br J Gen Pract. 2010;60:e476-e488. doi: 10.3399/bjgp10X544113
25. Agarwal R, Bills JE, Hecht TJ, et al. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57:29-38. doi: 10.1161/HYPERTENSIONAHA.110.160911
26. Stergiou GS, Skeva II, Zourbaki AS, et al. Self-monitoring of blood pressure at home: how many measurements are needed? J Hypertens. 1998;16:725-773. doi: 10.1097/00004872-199816060-00002
27. Stergiou GS, Nasothimiou EG, Kalogeropoulos PG, et al. The optimal home blood pressure monitoring schedule based on the Didima outcome study. J Hum Hypertens. 2010;24:158-164. doi: 10.1038/jhh.2009.54
28. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785. doi: 10.1038/jhh.2010.54
29. Imai Y, Kario K, Shimada K, et al; Japanese Society of Hypertension Committee for Guidelines for Self-monitoring of Blood Pressure at Home. The Japanese Society of Hypertension guidelines for self-monitoring of blood pressure at home (second edition). Hypertens Res.2012;35:777-795. doi: 10.1038/hr.2012.56
30. O’Brien E, Atkins N, Stergiou G, et al; Working Group on Blood Pressure Monitoring of the European Society of Hypertension. European Society of Hypertension international protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010; 15:23-38. doi: 10.1097/MBP.0b013e3283360e98
31. Casiglia E, Tikhonoff V, Albertini F, et al. Poor reliability of wrist blood pressure self-measurement at home: a population-based study. Hypertension. 2016;68:896-903. doi: 10.1161/HYPERTENSIONAHA.116.07961
32. Harju J, Vehkaoja A, Kumpulainen P, et al. Comparison of non-invasive blood pressure monitoring using modified arterial applanation tonometry with intra-arterial measurement. J Clin Monit Comput. 2018;32:13-22. doi: 10.1007/s10877-017-9984-3
33. Ostchega Y, Hughes JP, Zhang G, et al. Mean mid-arm circumference and blood pressure cuff sizes for U.S. adults: National Health and Nutrition Examination Survey, 1999-2010. Blood Press Monit. 2013;18:138-143. doi: 10.1097/MBP.0b013e3283617606
34. White WB, Barber V. Ambulatory monitoring of blood pressure: an overview of devices, analyses, and clinical utility. In: White WB, ed. Blood Pressure Monitoring in Cardiovascular Medicine and Therapeutics. Springer International Publishing; 2016:55-76.
35. Franklin SS, Thijs L, Asayama K, et al; IDACO Investigators. The cardiovascular risk of white-coat hypertension. J Am Coll Cardiol. 2016;68:2033-2043. doi: 10.1016/j.jacc.2016.08.035
36. Krakoff LR. Cost-effectiveness of ambulatory blood pressure: a reanalysis. Hypertension. 2006;47:29-34. doi: 10.1161/01.HYP.0000197195.84725.66
37. Ewald B, Pekarsky B. Cost analysis of ambulatory blood pressure monitoring in initiating antihypertensive drug treatment in Australian general practice. Med J Aust. 2002;176:580-583. doi: 10.5694/j.1326-5377.2002.tb04588.x
38. Beyhaghi H, Viera AJ. Comparative cost-effectiveness of clinic, home, or ambulatory blood pressure measurement for hypertension diagnosis in US adults. Hypertension. 2019;73:121-131. doi: 10.1161/HYPERTENSIONAHA.118.11715
39. De la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898-902. doi: 10.1161/HYPERTENSIONAHA.110.168948
40. Brown MA, Buddle ML, Martin A. Is resistant hypertension really resistant? Am J Hypertens. 2001;14:1263-1269. doi: 10.1016/s0895-7061(01)02193-8
41. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
42. Wang YC, Shimbo D, Muntner P, et al. Prevalence of masked hypertension among US adults with non-elevated clinic blood pressure. Am J Epidemiol. 2017;185:194-202. doi: 10.1093/aje/kww237
43. Thakkar HV, Pope A, Anpalahan M. Masked hypertension: a systematic review. Heart Lung Circ. 2020;29:102-111. doi: 10.1016/j.hlc.2019.08.006
44. Kronish IM, Hughes C, Quispe K, et al. Implementing ambulatory blood pressure monitoring in primary care practice. Fam Pract Manag. 2020;27:19-25.
45. Viera AJ, Lingley K, Hinderliter AL. Tolerability of the Oscar 2 ambulatory blood pressure monitor among research participants: a cross-sectional repeated measures study. BMC Med Res Methodol. 2011;11:59. doi: 10.1186/1471-2288-11-59
46. Alpert BS, Dart RA, Sica DA. Public-use blood pressure measurement: the kiosk quandary. J Am Soc Hypertens. 2014;8:739-742. doi: 10.1016/j.jash.2014.07.034
47. Al Hamarneh YN, Houle SK, Chatterley P, et al. The validity of blood pressure kiosk validation studies: a systematic review. Blood Press Monit. 2013;18:167-172. doi: 10.1097/MBP.0b013e328360fb85
48. Kumar N, Khunger M, Gupta A, et al. A content analysis of smartphone-based applications for hypertension management. J Am Soc Hypertens. 2015;9:130-136. doi: 10.1016/j.jash.2014.12.001
49. Bruining N, Caiani E, Chronaki C, et al. Acquisition and analysis of cardiovascular signals on smartphones: potential, pitfalls and perspectives: by the Task Force of the e-Cardiology Working Group of European Society of Cardiology. Eur J Prev Cardiol. 2014;21(suppl 2):4-13. doi: 10.1177/2047487314552604
50. Chandrasekaran V, Dantu R, Jonnada S, et al. Cuffless differential blood pressure estimation using smart phones. IEEE Trans Biomed Eng. 2013;60:1080-1089. doi: 10.1109/TBME.2012.2211078
PRACTICE RECOMMENDATIONS
› Use home blood pressure measurement (HBPM) for initial out-of-office evaluation to confirm hypertension. A
› Use 24-hour ambulatory measurement only when the results between office and HBPM are discordant. A
› Instruct patients to record their home BP measurements twice in the morning and twice at night for a minimum of 3 days. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Managing TIA: Early action and essential risk-reduction steps
As many as 240,000 people per year in the United States experience a transient ischemic attack (TIA),1,2 which is now defined by the American Heart Association and American Stroke Association as a “transient episode of neurological dysfunction caused by focal brain, spinal cord, or retinal ischemia, without acute infarction.”3 An older definition of TIA was based on the duration of the event (ie, resolution of symptoms at 24 hours); in the updated (2009) definition, the diagnostic criterion is the extent of focal tissue damage.3 Using the 2009 definition might mean a decrease in the number of patients who have a diagnosis of a TIA and an increase in the number who are determined to have had a stroke because an infarction is found on initial imaging.
Guided by the 2009 revised definition of a TIA, we review here the work-up and treatment of TIA, emphasizing immediacy of management to (1) prevent further tissue damage and (2) decrease the risk of a second event.
CASE
Martin L, 69 years old, retired, a nonsmoker, and with a history of peripheral arterial disease and hypercholesterolemia, presents to the emergency department (ED) of a rural hospital complaining of slurred speech and left-side facial numbness. He had an episode of facial numbness that lasted 30 minutes, then resolved, each of the 2 previous evenings; he did not seek care at those times. Now, in the ED, Mr. L is normotensive.
The patient’s medication history includes a selective serotonin reuptake inhibitor and melatonin to improve sleep. He reports having discontinued a statin because he could not tolerate its adverse effects.
What immediate steps are recommended for Mr. L’s care?
Common event callsfor quick action
A TIA is the strongest predictor of subsequent stroke and stroke-related death; the highest period of risk of these devastating outcomes is immediately following a TIA.1,2,4,5 It is essential, therefore, for the physician who sees a patient with a current complaint or recent history of suspected focal neurologic deficits to direct that patient to an ED for an accurate diagnosis and, as appropriate, early treatment for the best possible outcome.
Imaging—preferably, diffusion-weighted magnetic resonance imaging (DW-MRI), the gold standard for diagnosing stroke (see “Diagnosis includes ruling out mimics”)2,3—should be performed as soon as the patient with a suspected TIA arrives in the ED. Imaging should not be held while waiting for a stroke to declare itself—ie, by allowing symptoms to persist for longer than 24 hours. 6
Continue to: Late presentation
Late presentation. Some patients present ≥ 48 hours after onset of early symptoms of a TIA; for them, the work-up is the same as for prompt presentation but can be completed in the outpatient clinic—as long as the patient is stable clinically and imaging is accessible there. DW-MRI should be completed within 48 hours after late presentation. In such cases, the patient should be cautioned regarding risks and any recurrence of symptoms.7,8
Diagnosis includes ruling out mimics
All patients in whom a stroke is suspected should be evaluated on an emergency basis with brain imaging upon arrival at the hospital, before any therapy is initiated. As noted, DW-MRI is the preferred modality; noncontrast computed tomography (CT) or CT angiography can be used if MRI is unavailable.2,3
Mimics. Stroke has many mimics; quickly eliminating them from the differential diagnosis is important so that appropriate therapy can be initiated. Mimics usually have a prolonged presentation of symptoms, whereas the presentation of a TIA is usually abrupt. The 3 more common diagnoses that mimic a TIA are migraine with aura, seizure, and syncope.9,10 Symptoms that generally are not associated with a TIA are chest pain, generalized weakness, and confusion.11 A complete history and physical exam provide the path to the imaging, laboratory, and cardiac testing that is needed to differentiate these diagnoses from a TIA.
A thorough history is best obtained from the patient and a witness, if available, and should include identification of any focal neurologic deficits and the duration and time to resolution of symptoms. Obtain a history of risk factors for ischemia—tobacco use, diabetes, obesity, dyslipidemia, hypertension, previous TIA or stroke, atrial fibrillation, and any coagulopathy. Ask questions about a family history of TIA, stroke, and coagulopathy.11
A comprehensive physical exam, including vital signs, cardiac exam, a check for carotid bruits, and complete neurologic exam, should be performed. Most patients present with concerns for unilateral weakness and changes in speech, which are usually associated with infarction on DW-MRI.12 The most common findings on physical exam include cranial nerve abnormalities, such as diplopia, hemianopia, monocular blindness, disconjugate gaze, facial drooping, lateral tongue movement, dysphagia, and vestibular dysfunction. Cerebellar abnormalities are also often noted, including past pointing, dystaxia, ataxia, nystagmus, and motor abnormalities (eg, spasticity, clonus, or unilateral weakness in the face or extremities).11
Electrocardiography at the bedside can confirm atrial fibrillation or another arrhythmia quickly.
Essential laboratory testing includes measurement of blood glucose and serum electrolytes to determine if these particular imbalances are the cause of symptoms. The presence of a hypercoaguable state is determined by a complete blood count and coagulation studies.3,13 Urine toxicology should also be obtained to rule out other causes of symptoms. A lipid profile is beneficial for making long-term treatment decisions.
Continue to: ABCD2 score
ABCD2 score. Patients who have had a TIA and present within 72 hours after symptoms have resolved should be hospitalized if they have an ABCD2 (Age, Blood pressure [BP], Clinical presentation, Diabetes mellitus [type 1 or 2], Duration of symptoms) prediction system score > 3.14 ABCD2 criteria can be used to help identify patients who are at higher risk of stroke or need further therapy (TABLE 1).14,15
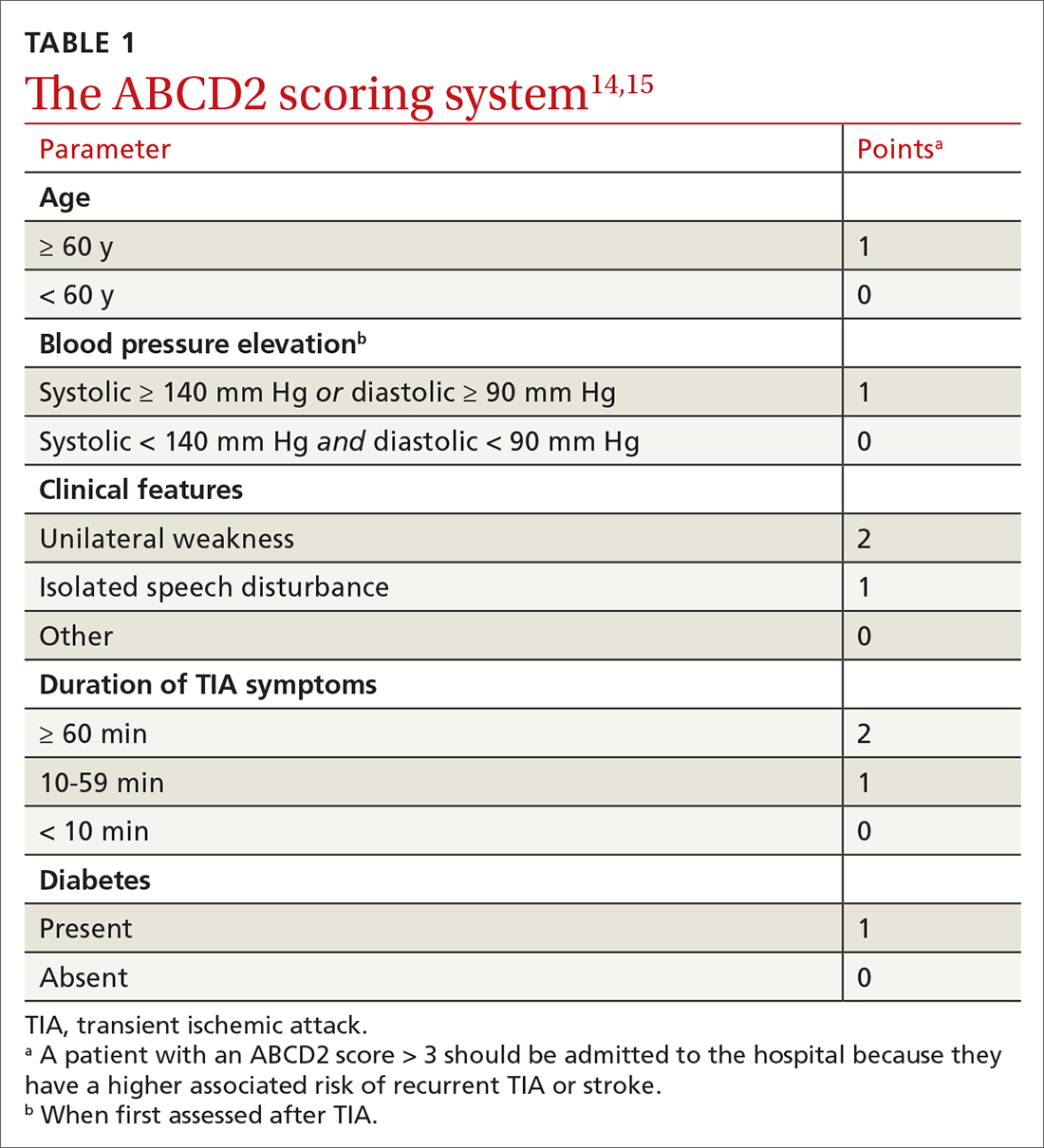
The ABCD2 score is also used to determine whether a patient needs dual antiplatelet therapy. Patients who score at the higher end of the ABCD2 system usually have an increased risk of stroke, longer hospitalization, and greater disability.
CASE
In the ED, Mr. L is immediately assessed and airlifted to a larger regional medical center, where MRI confirms a stroke.
Management
Initial management of a TIA is aimed at reducing the risk of recurrent TIA or stroke. Early medical and possibly surgical treatment are key for preventing stroke and improving outcomes. The first 48 hours after a TIA are the most critical because the incidence of recurrent TIA or stroke is highest during this period.16-18
What is the accepted strategy for early treatment?
Initial treatment must include antiplatelet therapy, BP management, anticoagulation, statin therapy, and carotid endarterectomy as indicated.2,19,20 Control of hypertension and anticoagulation decrease the risk of recurrent stroke by the largest margin20; both are “A”-level Strength of Recommendation Taxonomy interventions.2,3
Step 1: Antiplatelet therapy. After initial imaging is complete and if there are no contraindications, antiplatelet agents are recommended for patients who have had a noncardioembolic TIA. The American Heart Association and American Stroke Association recommend either aspirin, clopidogrel, dipyridamole + aspirin (available in a single capsule [Aggrenox]), or clopidogrel + aspirin as first-line therapy.2,20 The choice of agent needs to be individualized, based on tolerability and adverse effects (TABLE 22,20,21).
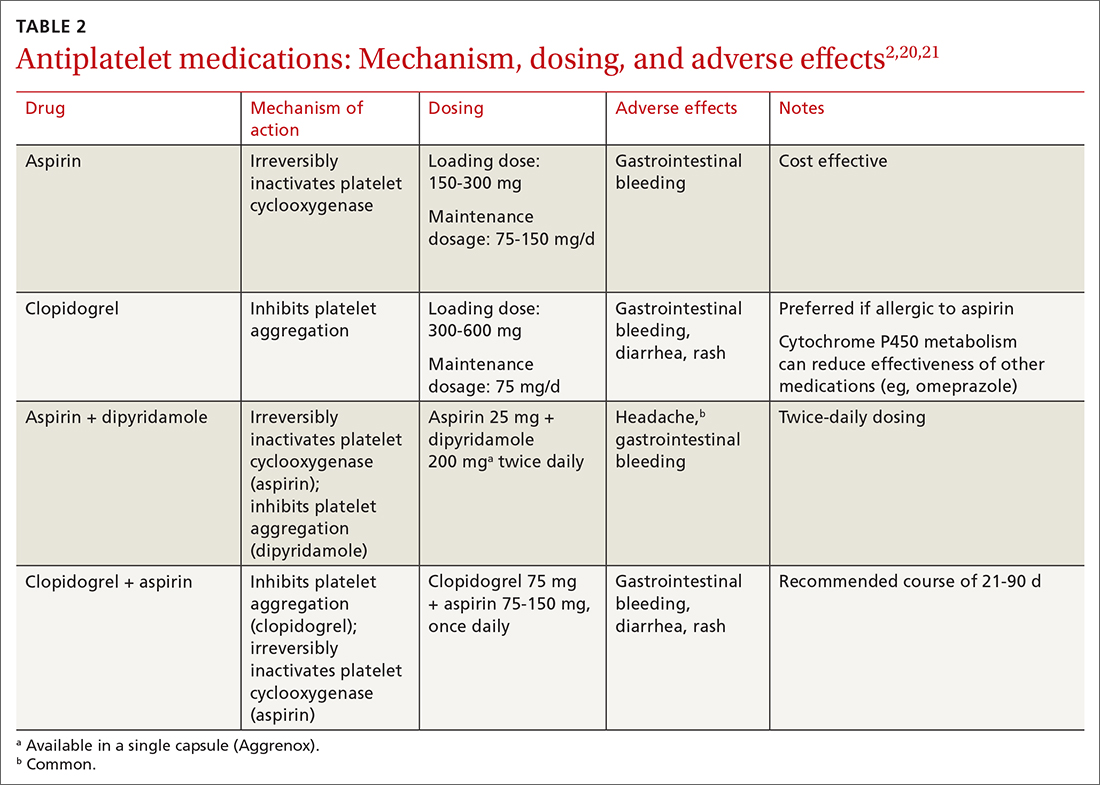
A meta-analysis of antiplatelet therapy reviewed the optimum dosing of each medication.21,22 Reduction of the risk of ischemic stroke with aspirin is 21% to 22% at the optimal dosing of 75 to 150 mg/d, which also reduces the risk of gastrointestinal bleeding.
Continue to: For a patient who has...
For a patient who has an ABCD2 score ≥ 4, has had a prior TIA, or has large-vessel disease, dual antiplatelet therapy is recommended for the first 21 days, with a subsequent return to monotherapy. Dual antiplatelet therapy of clopidogrel + aspirin increases the risk of adverse reactions and has not been shown to have greater long-term benefit23-25 (TABLE 22,20,21).
Step 2: BP management. This is the next immediate step. As many as 80% of patients who present with a TIA have elevated BP upon admission. BP needs to be treated and carefully monitored during this early treatment phase. The recommendation is for a systolic BP < 185 mm Hg and a diastolic BP < 110 mm Hg.24
Step 3: Anticoagulation. Treatment with warfarin or a direct oral anticoagulant (DOAC) is recommended for patients who have the potential for forming emboli—eg, in the setting of atrial fibrillation, ventricular thrombus, mechanical heart valve, or venous thromboembolism.
Step 4. High-intensity statin. A statin agent is recommended as part of immediate and long-term medical management, regardless of the low-density lipoprotein cholesterol (LDL-C) level, to reduce the risk of stroke.2,24
Carotid artery management. Surgical intervention is not always considered a component of immediate medical management. However, guidelines recommend that carotid endarterectomy or stenting be considered in patients who have stenosis > 70%.2
CASE
Mr. L is admitted to the hospital and undergoes neurosurgical intervention. Medical management is instituted.
Long-term management and secondary prevention
The main risk factors for stroke can be divided into modifiable, vascular, and unmodifiable. Addressing both modifiable and vascular risks is important for secondary prevention.
Continue to: Modifiable and vascular risk factors
Modifiable and vascular risk factors
Modifiable risk factors for stroke include hypertension, diabetes, dyslipidemia, smoking, and physical activity; the most important of these, for preventing subsequent stroke after an initial TIA, is hypertension.26
The 2 more significant vascular risk factors for stroke are carotid artery stenosis and atrial fibrillation.
Hypertension. Improving control of hypertension can improve secondary risk reduction for recurrent stroke. Control of both systolic and diastolic BP is important in this regard, with larger systolic BP reductions having a greater impact on decreasing the risk of recurrent stroke.24 Evidence supports lowering BP to improve secondary risk reduction in people with and without diagnosed hypertension: The goal is to lower systolic BP by ≥ 10 mm Hg and diastolic BP by 5 mm Hg.24 No particular class of antihypertensive is recommended in the first line, although preliminary evidence shows that a diuretic, with or without an angiotensin-converting enzyme inhibitor, might be more beneficial than other options.24
Diabetes. The risk of cardiovascular disease, including stroke, is higher in people with diabetes. Evidence shows that various (but not all) agents in 2 pharmaceutical classes—glucagon-like peptide-1 (GLP-1) receptor agonists and the sodium glucose-2 cotransporter (SGLT2) inhibitors—reduce the risk of major cardiovascular events and improve secondary prevention of recurrent stroke:
- EMPA-REG OUTCOME (ClinicalTrials.gov Identifier: NCT01131676) was the first trial to show cardiovascular benefit from an SGLT2 inhibitor (empagliflozin); subsequent studies confirmed the cardiovascular benefits found in EMPA-REG OUTCOME.27,28
- The ELIXA trial (ClinicalTrials.gov Identifier: NCT01147250) was the first to show cardiovascular benefit from a GLP-1 receptor agonist (lixisenatide); subsequent studies supported this finding.29,30
Appropriate agents in these 2 classes should be considered as first-line or adjunctive in patients with both diabetes and known cardiovascular disease, as long as there are no contraindications.27,28
Pioglitazone, a thiazolidinedione-class antidiabetic agent, was once considered a potential option to improve secondary prevention of stroke. However, the thiazolidinediones are generally no longer considered; instead, the SGLT2 inhibitors and GLP-1 receptor agonists are favored.31
Evidence demonstrates the effect of hyperglycemia on cardiovascular events; however, it is important to note that hypoglycemia can result in symptoms and focal changes that mimic a stroke. In addition, some evidence suggests that hypoglycemia can increase cardiovascular risk—thereby supporting the importance of strict control of diabetes and maintenance of euglycemia in reducing overall cardiovascular risk.32
Continue to: Lipids
Lipids. The SPARCL trial (ClinicalTrials.gov Identifier: NCT00147602) was the first study to demonstrate the benefit of high-intensity statin therapy—specifically, atorvastatin 80 mg/d—for secondary prevention for recurrent stroke.33 The recommendation is to use high-intensity statin therapy to decrease the risk of recurrent stroke by reducing the level of LDL-C—by ≥ 50% or to < 70 mg/dL, for maximum risk reduction.24,34
The IMPROVE-IT trial (ClinicalTrials.gov Identifier: NCT00202878) demonstrated the benefit of adding ezetimibe, 10 mg/d, to a moderate-to-high-intensity statin (simvastatin, 40-80 mg/d) to reduce the risk of recurrent stroke.35
Results of recent studies support the use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors for regulating levels of LDL-C, as an additional option to consider—if needed to further reduce the LDL-C level or if statins are contraindicated in a particular patient.34
Smoking cessation. Cigarette smoking is known to increase the risk of ischemic stroke; newer evidence shows that second-hand exposure to smoke also increases the risk of ischemic stroke.36,37 Although these studies focused on primary prevention of ischemic stroke, the data can reasonably be applied to secondary prevention.38 The recommendation for secondary prevention is to quit smoking and avoid secondhand smoke.24
Alcohol. Evidence demonstrates that heavy alcohol consumption and alcoholism increase the risk of stroke; similar to what is known about smoking, most available data relate to primary prevention.38 The recommendation for providing secondary stroke prevention is to stop or decrease alcohol intake.24
Weight reduction. Obesity (body mass index > 30) increases the risk of ischemic stroke. However, there is, as yet, no evidence that weight loss diminishes the risk of subsequent stroke for secondary prevention.24
Physical activity. Aerobic exercise and strength-training programs after a stroke improve cardiovascular health and mobility. There is no evidence that exercise leads to a reduction in the risk of subsequent stroke.24
Continue to: Nutrition
Nutrition. No current randomized controlled trials are focused on the relationship between diet and recurrent stroke for purposes of prevention; however, evidence for both BP and lipid control incorporate dietary guidance. Recommendations include reducing intake of saturated fats and of sodium (the latter, to < 2.3 g/d) and increasing intake of fruits and vegetables, both of which are beneficial for controlling BP and lipid levels and promoting overall cardiovascular health.38
Carotid artery stenosis. Several randomized controlled trials have demonstrated benefit from treating carotid stenosis (> 70% stenosis but not < 50%) with carotid endarterectomy to reduce the risk of recurrent stroke after TIA.2 The ideal timing of carotid endarterectomy is still being studied; however, available evidence supports intervention within 2 to 6 weeks after TIA or stroke.25 Studies are ongoing that compare carotid angioplasty and stenting against carotid endarterectomy. Medical therapy, with antiplatelet agents and statins, is recommended after carotid endarterectomy.25
Atrial fibrillation increases the risk of recurrent stroke after a TIA, and is the most important indication for secondary stroke prevention with anticoagulation therapy:
- Warfarin. Several studies have shown that warfarin provides a 68% relative risk reduction and a 1.4% absolute risk reduction in the annual stroke rate.24 To achieve this reduction in risk, the optimal international normalized ratio is 2.5 (range, 2-3).24
- Aspirin provides a 13% relative risk reduction for recurrent stroke, although there is evidence that long-term anticoagulation provides more benefit than aspirin after a TIA.39-41 Optimal dosing of aspirin ranges from 75-100 mg/d; greatest benefit is likely in the 12 weeks after stroke, when the risk of recurrent stroke is highest.31,41,42
- DOACs have similar efficacy to warfarin but more rapid onset, lower risk of bleeding, fewer drug interactions, and no requirement for monitoring—often making them a more tolerable long-term choice. Options are rivaroxaban 20 mg/d, dabigatran 150 mg twice daily, apixaban 5 mg twice daily, and edoxaban 60 mg/d.39
When to start anticoagulation and the choice of agent should be weighed against a risk of bleeding, which is highest after the initial stroke. Cost is also a consideration: DOACs are more expensive than warfarin.
CASE
Mr. L is discharged 3 days after carotid endarterectomy and free of residual deficits. He is started on dual antiplatelet therapy (aspirin + clopidogrel) for 21 days, to be followed by a return to monotherapy. He is restarted on a high-intensity statin. He is instructed to resume taking the selective serotonin reuptake inhibitor and melatonin for sleep, as needed. Last, he is told to schedule follow-up with his primary care physician in 7 to 10 days to begin post-stroke care.
Final thoughts
Primary care physicians are often the first point of contact for patients with current or remote TIA symptoms. Based on that provider–patient relationship, evidence supports several recommendations for diagnosing and treating a TIA and for reducing the risk of recurrent stroke after TIA. Addressing each of these areas, in this order, is imperative to reduce the risk of recurrent stroke and improve overall cardiovascular outcomes:
- Obtain an accurate diagnosis of a TIA, using DW-MRI or comparable brain imaging, to allow for prompt intervention.
- Initiate BP management promptly in the acute setting and establish optimal BP control over the long term.
- Begin appropriate antiplatelet therapy.
- When indicated (eg, atrial fibrillation), begin anticoagulation therapy with a DOAC or warfarin.
- Begin high-intensity statin therapy.
- Consider treating patients with diabetes using an SGLT2 inhibitor or GLP-1 receptor agonist.
- Encourage smoking cessation, prescribe quit-smoking medications, or refer a smoker for behavioral support.
Education. Last, it is important to educate patients—especially those who have risk factors for a TIA or stroke—about the presentation of events, so that they know to seek immediate medical attention.
CORRESPONDENCE
Kristen Rundell, MD, Department of Family and Community Medicine, University of Arizona College of Medicine, 655 North Alvernon Way, Suite 228, Tucson, AZ 85711; [email protected]
1. Kleindorfer D, Panagos P, Pancioli A, et al. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke. 2005;36:720-723. doi: 10.1161/01.STR.0000158917.59233.b7
2. Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2021;52:e364-e467. doi: 10.1161/STR.0000000000000375
3. Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276-2293. doi: 10.1161/STROKEAHA.108.192218
4. Thacker EL, Wiggins KL, Rice KM, et al. Short-term and long-term risk of incident ischemic stroke after transient ischemic attack. Stroke. 2010;41:239-243. doi: 10.1161/STROKEAHA.109.569707
5. Hill MD, Yiannakoulias N, Jeerakathil T, et al. The high risk of stroke immediately after transient ischemic attack: a population-based study. Neurology. 2004;62:2015-2020. doi: 10.1212/01.wnl.0000129482.70315.2f
6. Giles MF, Albers GW, Amarenco P, et al. Early stroke risk and ABCD2 score performance in tissue- vs time-defined TIA: a multicenter study. Neurology. 2011;77:1222-1228. doi: 10.1212/WNL.0b013e3182309f91
7. Cucchiara BL, Kasner SE. All patients should be admitted to the hospital after a transient ischemic attack. Stroke. 2012;43:1446-1447. doi: 10.1161/STROKEAHA.111.636746
8. Amarenco P. Not all patients should be admitted to the hospital for observation after a transient ischemic attack. Stroke. 2012;43:1448-1449. doi: 10.1161/STROKEAHA.111.636753
9. Amort M, Fluri F, Schäfer J, et al. Transient ischemic attack versus transient ischemic attack mimics: frequency, clinical characteristics and outcome. Cerebrovasc Dis. 2011;32:57-64. doi: 10.1159/000327034
10. Hand PJ, Kwan J, Lindley RI, et al. Distinguishing between stroke and mimic at the bedside: The Brain Attack Study. Stroke. 2006;37:769-775. doi: 10.1161/01.STR.0000204041.13466.4c
11. Shah KH, Edlow JA. Transient ischemic attack: review for the emergency physician. Ann Emerg Med. 2004;43:592-604. doi: 10.1016/S0196064404000058
12. Crisostomo RA, Garcia MM, Tong DC. Detection of diffusion-weighted MRI abnormalities in patients with transient ischemic attack: correlation with clinical characteristics. Stroke. 2003;34:932-937. doi: 10.1161/01.STR.0000061496.00669.5E
13. Adams HP Jr, del Zoppo G, Alberts MJ, et al; ; ; ; ; . Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655-1711. doi: 10.1161/STROKEAHA.107.181486
14. Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369:283-292. doi: 10.1016/S0140-6736(07)60150-0
15. Cucchiara BL, Messe SR, Taylor RA, et al. Is the ABCD score useful for risk stratification of patients with acute transient ischemic attack? Stroke. 2006;37:1710-1714. doi: 10.1161/01.STR.0000227195.46336.93
16. Amarenco P, Lavallée PC, Labreuche J, et al; . One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542. doi: 10.1056/NEJMoa1412981
17. Wu CM, McLaughlin K, Lorenzetti DL, et al. Early risk of stroke after transient ischemic attack: a systematic review and meta-analysis. Arch Intern Med. 2007;167:2417-2422. doi: 10.1001/archinte.167.22.2417
18. Rothwell PM, Warlow CP. Timing of TIAs preceding stroke: time window for prevention is very short. Neurology. 2005;64:817-820. doi: 10.1212/01.WNL.0000152985.32732.EE
19. Kernan WN, Ovbiagele B, Black HR, et al; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236. doi: 10.1161/STR.0000000000000024
20. Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370:1432-1442. doi: 10.1016/S0140-6736(07)61448-2
21. Hackam DG, Spence JD. Antiplatelet therapy in ischemic stroke and transient ischemic attack: an overview of major trials and meta-analyses. Stroke. 2019;50:773-778. doi: c10.1161/STROKEAHA.118.023954
22. Bhatia K, Jain V, Aggarwal D, et al. Dual antiplatelet therapy versus aspirin in patients with stroke or transient ischemic attack: meta-analysis of randomized controlled trials. Stroke. 2021;52:e217-e223. doi: 10.1161/STROKEAHA.120.033033
23. Wang Y, Pan Y, Zhao X, et al; CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack (CHANCE) trial: one-year outcomes. Circulation. 2015;132:40-46. doi: 10.1161/CIRCULATIONAHA.114.014791
24. Furie KL, Kasner SE, Adams RJ, et al; . Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227-276. doi: 10.1161/STR.0b013e3181f7d043
25. Powers WJ, Rabinstein AA, Ackerson T, et al; American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110. doi: 10.1161/STR.0000000000000158
26. O’Donnell MJ, Chin SL, Rangarajan S, et al; INTERSTROKE Investigators. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388:761-775. doi: 10.1016/S0140-6736(16)30506-2
27. Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776-785. doi:10.1016/S2213-8587(19)30249-9
28. Bertoccini L, Baroni MG. GLP-1 receptor agonists and SGLT2 inhibitors for the treatment of type 2 diabetes: new insights and opportunities for cardiovascular protection. Adv Exp Med Biol. 2021;1307:193-212. doi:10.1007/5584_2020_494
29. Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome N Engl J Med. 2015;373:2247-2257. doi: 10.1056/NEJMoa1509225
30. Sheahan KH, Wahlberg EA, Gilbert MP. An overview of GLP-1 agonists and recent cardiovascular outcomes trials. Postgrad Med J. 2020;96:156-161. doi:10.1136/postgradmedj-2019-137186
31. Kim AS. Medical management for secondary stroke prevention. Continuum (Minneap Minn). 2020;26:435-456. doi:10.1212/CON.0000000000000849
32. Smith L, Chakraborty D, Bhattacharya P, et al. Exposure to hypoglycemia and risk of stroke. Ann N Y Acad Sci. 2018;1431:25-34. doi:10.1111/nyas.13872
33. Amarenco P, Bogousslavsky J, Callahan A 3rd, et al; . High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559. doi:10.1056/NEJMoa061894
34. Castilla-Guerra, L, Fernandez-Moreno M, Leon-Jimenez D, et al. Statins in ischemic stroke prevention: what have we learned in the post-SPARCL (The Stroke Prevention by Aggressive Reduction in Cholesterol Levels) decade? Curr Treat Options Neurol. 2019;21:22. doi: 10.1007/s11940-019-0563-4
35. Bohula EA, Wiviott SD, Giugliano RP, et al. Prevention of stroke with the addition of ezetimibe to statin therapy in patients with acute coronary syndrome in IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Circulation. 2017;136:2440-2450. doi:10.1161/CIRCULATIONAHA.117.029095
36. Moritsugu KP. The 2006 report of the Surgeon General: the health consequences of involuntary exposure to tobacco smoke. Am J Prev Med. 20067;32:542-543. doi: 10.1016/j.amepre.2007.02.026
37. Wolf PA, D’Agostino RB, Kannel WB, et al. Cigarette smoking as a risk factor for stroke: the Framingham Study. JAMA. 1988;259:1025-1029.
38. Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:1583-1633. doi: 10.1161/01.STR.0000223048.70103.F1
39. Klijn CJ, Paciaroni M, Berge E, et al. Antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation: A European Stroke Organisation guideline. Eur Stroke J. 2019;4:198-223. doi:10.1177/2396987319841187
40. Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860. doi:10.1016/S0140-6736(09)60503-1
41. Singer DE, Albers GW, Dalen JE, et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):546S–592S. doi: 10.1378/chest.08-0678
42. Rothwell PM, Algra A, Chen Z, et al. Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: time-course analysis of randomised trials. Lancet. 2016;388:365-375. doi:10.1016/S0140-6736(16)30468-8
As many as 240,000 people per year in the United States experience a transient ischemic attack (TIA),1,2 which is now defined by the American Heart Association and American Stroke Association as a “transient episode of neurological dysfunction caused by focal brain, spinal cord, or retinal ischemia, without acute infarction.”3 An older definition of TIA was based on the duration of the event (ie, resolution of symptoms at 24 hours); in the updated (2009) definition, the diagnostic criterion is the extent of focal tissue damage.3 Using the 2009 definition might mean a decrease in the number of patients who have a diagnosis of a TIA and an increase in the number who are determined to have had a stroke because an infarction is found on initial imaging.
Guided by the 2009 revised definition of a TIA, we review here the work-up and treatment of TIA, emphasizing immediacy of management to (1) prevent further tissue damage and (2) decrease the risk of a second event.

CASE
Martin L, 69 years old, retired, a nonsmoker, and with a history of peripheral arterial disease and hypercholesterolemia, presents to the emergency department (ED) of a rural hospital complaining of slurred speech and left-side facial numbness. He had an episode of facial numbness that lasted 30 minutes, then resolved, each of the 2 previous evenings; he did not seek care at those times. Now, in the ED, Mr. L is normotensive.
The patient’s medication history includes a selective serotonin reuptake inhibitor and melatonin to improve sleep. He reports having discontinued a statin because he could not tolerate its adverse effects.
What immediate steps are recommended for Mr. L’s care?
Common event callsfor quick action
A TIA is the strongest predictor of subsequent stroke and stroke-related death; the highest period of risk of these devastating outcomes is immediately following a TIA.1,2,4,5 It is essential, therefore, for the physician who sees a patient with a current complaint or recent history of suspected focal neurologic deficits to direct that patient to an ED for an accurate diagnosis and, as appropriate, early treatment for the best possible outcome.
Imaging—preferably, diffusion-weighted magnetic resonance imaging (DW-MRI), the gold standard for diagnosing stroke (see “Diagnosis includes ruling out mimics”)2,3—should be performed as soon as the patient with a suspected TIA arrives in the ED. Imaging should not be held while waiting for a stroke to declare itself—ie, by allowing symptoms to persist for longer than 24 hours. 6
Continue to: Late presentation
Late presentation. Some patients present ≥ 48 hours after onset of early symptoms of a TIA; for them, the work-up is the same as for prompt presentation but can be completed in the outpatient clinic—as long as the patient is stable clinically and imaging is accessible there. DW-MRI should be completed within 48 hours after late presentation. In such cases, the patient should be cautioned regarding risks and any recurrence of symptoms.7,8
Diagnosis includes ruling out mimics
All patients in whom a stroke is suspected should be evaluated on an emergency basis with brain imaging upon arrival at the hospital, before any therapy is initiated. As noted, DW-MRI is the preferred modality; noncontrast computed tomography (CT) or CT angiography can be used if MRI is unavailable.2,3
Mimics. Stroke has many mimics; quickly eliminating them from the differential diagnosis is important so that appropriate therapy can be initiated. Mimics usually have a prolonged presentation of symptoms, whereas the presentation of a TIA is usually abrupt. The 3 more common diagnoses that mimic a TIA are migraine with aura, seizure, and syncope.9,10 Symptoms that generally are not associated with a TIA are chest pain, generalized weakness, and confusion.11 A complete history and physical exam provide the path to the imaging, laboratory, and cardiac testing that is needed to differentiate these diagnoses from a TIA.
A thorough history is best obtained from the patient and a witness, if available, and should include identification of any focal neurologic deficits and the duration and time to resolution of symptoms. Obtain a history of risk factors for ischemia—tobacco use, diabetes, obesity, dyslipidemia, hypertension, previous TIA or stroke, atrial fibrillation, and any coagulopathy. Ask questions about a family history of TIA, stroke, and coagulopathy.11
A comprehensive physical exam, including vital signs, cardiac exam, a check for carotid bruits, and complete neurologic exam, should be performed. Most patients present with concerns for unilateral weakness and changes in speech, which are usually associated with infarction on DW-MRI.12 The most common findings on physical exam include cranial nerve abnormalities, such as diplopia, hemianopia, monocular blindness, disconjugate gaze, facial drooping, lateral tongue movement, dysphagia, and vestibular dysfunction. Cerebellar abnormalities are also often noted, including past pointing, dystaxia, ataxia, nystagmus, and motor abnormalities (eg, spasticity, clonus, or unilateral weakness in the face or extremities).11
Electrocardiography at the bedside can confirm atrial fibrillation or another arrhythmia quickly.
Essential laboratory testing includes measurement of blood glucose and serum electrolytes to determine if these particular imbalances are the cause of symptoms. The presence of a hypercoaguable state is determined by a complete blood count and coagulation studies.3,13 Urine toxicology should also be obtained to rule out other causes of symptoms. A lipid profile is beneficial for making long-term treatment decisions.
Continue to: ABCD2 score
ABCD2 score. Patients who have had a TIA and present within 72 hours after symptoms have resolved should be hospitalized if they have an ABCD2 (Age, Blood pressure [BP], Clinical presentation, Diabetes mellitus [type 1 or 2], Duration of symptoms) prediction system score > 3.14 ABCD2 criteria can be used to help identify patients who are at higher risk of stroke or need further therapy (TABLE 1).14,15

The ABCD2 score is also used to determine whether a patient needs dual antiplatelet therapy. Patients who score at the higher end of the ABCD2 system usually have an increased risk of stroke, longer hospitalization, and greater disability.
CASE
In the ED, Mr. L is immediately assessed and airlifted to a larger regional medical center, where MRI confirms a stroke.
Management
Initial management of a TIA is aimed at reducing the risk of recurrent TIA or stroke. Early medical and possibly surgical treatment are key for preventing stroke and improving outcomes. The first 48 hours after a TIA are the most critical because the incidence of recurrent TIA or stroke is highest during this period.16-18
What is the accepted strategy for early treatment?
Initial treatment must include antiplatelet therapy, BP management, anticoagulation, statin therapy, and carotid endarterectomy as indicated.2,19,20 Control of hypertension and anticoagulation decrease the risk of recurrent stroke by the largest margin20; both are “A”-level Strength of Recommendation Taxonomy interventions.2,3
Step 1: Antiplatelet therapy. After initial imaging is complete and if there are no contraindications, antiplatelet agents are recommended for patients who have had a noncardioembolic TIA. The American Heart Association and American Stroke Association recommend either aspirin, clopidogrel, dipyridamole + aspirin (available in a single capsule [Aggrenox]), or clopidogrel + aspirin as first-line therapy.2,20 The choice of agent needs to be individualized, based on tolerability and adverse effects (TABLE 22,20,21).

A meta-analysis of antiplatelet therapy reviewed the optimum dosing of each medication.21,22 Reduction of the risk of ischemic stroke with aspirin is 21% to 22% at the optimal dosing of 75 to 150 mg/d, which also reduces the risk of gastrointestinal bleeding.
Continue to: For a patient who has...
For a patient who has an ABCD2 score ≥ 4, has had a prior TIA, or has large-vessel disease, dual antiplatelet therapy is recommended for the first 21 days, with a subsequent return to monotherapy. Dual antiplatelet therapy of clopidogrel + aspirin increases the risk of adverse reactions and has not been shown to have greater long-term benefit23-25 (TABLE 22,20,21).
Step 2: BP management. This is the next immediate step. As many as 80% of patients who present with a TIA have elevated BP upon admission. BP needs to be treated and carefully monitored during this early treatment phase. The recommendation is for a systolic BP < 185 mm Hg and a diastolic BP < 110 mm Hg.24
Step 3: Anticoagulation. Treatment with warfarin or a direct oral anticoagulant (DOAC) is recommended for patients who have the potential for forming emboli—eg, in the setting of atrial fibrillation, ventricular thrombus, mechanical heart valve, or venous thromboembolism.
Step 4. High-intensity statin. A statin agent is recommended as part of immediate and long-term medical management, regardless of the low-density lipoprotein cholesterol (LDL-C) level, to reduce the risk of stroke.2,24
Carotid artery management. Surgical intervention is not always considered a component of immediate medical management. However, guidelines recommend that carotid endarterectomy or stenting be considered in patients who have stenosis > 70%.2
CASE
Mr. L is admitted to the hospital and undergoes neurosurgical intervention. Medical management is instituted.
Long-term management and secondary prevention
The main risk factors for stroke can be divided into modifiable, vascular, and unmodifiable. Addressing both modifiable and vascular risks is important for secondary prevention.
Continue to: Modifiable and vascular risk factors
Modifiable and vascular risk factors
Modifiable risk factors for stroke include hypertension, diabetes, dyslipidemia, smoking, and physical activity; the most important of these, for preventing subsequent stroke after an initial TIA, is hypertension.26
The 2 more significant vascular risk factors for stroke are carotid artery stenosis and atrial fibrillation.
Hypertension. Improving control of hypertension can improve secondary risk reduction for recurrent stroke. Control of both systolic and diastolic BP is important in this regard, with larger systolic BP reductions having a greater impact on decreasing the risk of recurrent stroke.24 Evidence supports lowering BP to improve secondary risk reduction in people with and without diagnosed hypertension: The goal is to lower systolic BP by ≥ 10 mm Hg and diastolic BP by 5 mm Hg.24 No particular class of antihypertensive is recommended in the first line, although preliminary evidence shows that a diuretic, with or without an angiotensin-converting enzyme inhibitor, might be more beneficial than other options.24
Diabetes. The risk of cardiovascular disease, including stroke, is higher in people with diabetes. Evidence shows that various (but not all) agents in 2 pharmaceutical classes—glucagon-like peptide-1 (GLP-1) receptor agonists and the sodium glucose-2 cotransporter (SGLT2) inhibitors—reduce the risk of major cardiovascular events and improve secondary prevention of recurrent stroke:
- EMPA-REG OUTCOME (ClinicalTrials.gov Identifier: NCT01131676) was the first trial to show cardiovascular benefit from an SGLT2 inhibitor (empagliflozin); subsequent studies confirmed the cardiovascular benefits found in EMPA-REG OUTCOME.27,28
- The ELIXA trial (ClinicalTrials.gov Identifier: NCT01147250) was the first to show cardiovascular benefit from a GLP-1 receptor agonist (lixisenatide); subsequent studies supported this finding.29,30
Appropriate agents in these 2 classes should be considered as first-line or adjunctive in patients with both diabetes and known cardiovascular disease, as long as there are no contraindications.27,28
Pioglitazone, a thiazolidinedione-class antidiabetic agent, was once considered a potential option to improve secondary prevention of stroke. However, the thiazolidinediones are generally no longer considered; instead, the SGLT2 inhibitors and GLP-1 receptor agonists are favored.31
Evidence demonstrates the effect of hyperglycemia on cardiovascular events; however, it is important to note that hypoglycemia can result in symptoms and focal changes that mimic a stroke. In addition, some evidence suggests that hypoglycemia can increase cardiovascular risk—thereby supporting the importance of strict control of diabetes and maintenance of euglycemia in reducing overall cardiovascular risk.32
Continue to: Lipids
Lipids. The SPARCL trial (ClinicalTrials.gov Identifier: NCT00147602) was the first study to demonstrate the benefit of high-intensity statin therapy—specifically, atorvastatin 80 mg/d—for secondary prevention for recurrent stroke.33 The recommendation is to use high-intensity statin therapy to decrease the risk of recurrent stroke by reducing the level of LDL-C—by ≥ 50% or to < 70 mg/dL, for maximum risk reduction.24,34
The IMPROVE-IT trial (ClinicalTrials.gov Identifier: NCT00202878) demonstrated the benefit of adding ezetimibe, 10 mg/d, to a moderate-to-high-intensity statin (simvastatin, 40-80 mg/d) to reduce the risk of recurrent stroke.35
Results of recent studies support the use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors for regulating levels of LDL-C, as an additional option to consider—if needed to further reduce the LDL-C level or if statins are contraindicated in a particular patient.34
Smoking cessation. Cigarette smoking is known to increase the risk of ischemic stroke; newer evidence shows that second-hand exposure to smoke also increases the risk of ischemic stroke.36,37 Although these studies focused on primary prevention of ischemic stroke, the data can reasonably be applied to secondary prevention.38 The recommendation for secondary prevention is to quit smoking and avoid secondhand smoke.24
Alcohol. Evidence demonstrates that heavy alcohol consumption and alcoholism increase the risk of stroke; similar to what is known about smoking, most available data relate to primary prevention.38 The recommendation for providing secondary stroke prevention is to stop or decrease alcohol intake.24
Weight reduction. Obesity (body mass index > 30) increases the risk of ischemic stroke. However, there is, as yet, no evidence that weight loss diminishes the risk of subsequent stroke for secondary prevention.24
Physical activity. Aerobic exercise and strength-training programs after a stroke improve cardiovascular health and mobility. There is no evidence that exercise leads to a reduction in the risk of subsequent stroke.24
Continue to: Nutrition
Nutrition. No current randomized controlled trials are focused on the relationship between diet and recurrent stroke for purposes of prevention; however, evidence for both BP and lipid control incorporate dietary guidance. Recommendations include reducing intake of saturated fats and of sodium (the latter, to < 2.3 g/d) and increasing intake of fruits and vegetables, both of which are beneficial for controlling BP and lipid levels and promoting overall cardiovascular health.38
Carotid artery stenosis. Several randomized controlled trials have demonstrated benefit from treating carotid stenosis (> 70% stenosis but not < 50%) with carotid endarterectomy to reduce the risk of recurrent stroke after TIA.2 The ideal timing of carotid endarterectomy is still being studied; however, available evidence supports intervention within 2 to 6 weeks after TIA or stroke.25 Studies are ongoing that compare carotid angioplasty and stenting against carotid endarterectomy. Medical therapy, with antiplatelet agents and statins, is recommended after carotid endarterectomy.25
Atrial fibrillation increases the risk of recurrent stroke after a TIA, and is the most important indication for secondary stroke prevention with anticoagulation therapy:
- Warfarin. Several studies have shown that warfarin provides a 68% relative risk reduction and a 1.4% absolute risk reduction in the annual stroke rate.24 To achieve this reduction in risk, the optimal international normalized ratio is 2.5 (range, 2-3).24
- Aspirin provides a 13% relative risk reduction for recurrent stroke, although there is evidence that long-term anticoagulation provides more benefit than aspirin after a TIA.39-41 Optimal dosing of aspirin ranges from 75-100 mg/d; greatest benefit is likely in the 12 weeks after stroke, when the risk of recurrent stroke is highest.31,41,42
- DOACs have similar efficacy to warfarin but more rapid onset, lower risk of bleeding, fewer drug interactions, and no requirement for monitoring—often making them a more tolerable long-term choice. Options are rivaroxaban 20 mg/d, dabigatran 150 mg twice daily, apixaban 5 mg twice daily, and edoxaban 60 mg/d.39
When to start anticoagulation and the choice of agent should be weighed against a risk of bleeding, which is highest after the initial stroke. Cost is also a consideration: DOACs are more expensive than warfarin.
CASE
Mr. L is discharged 3 days after carotid endarterectomy and free of residual deficits. He is started on dual antiplatelet therapy (aspirin + clopidogrel) for 21 days, to be followed by a return to monotherapy. He is restarted on a high-intensity statin. He is instructed to resume taking the selective serotonin reuptake inhibitor and melatonin for sleep, as needed. Last, he is told to schedule follow-up with his primary care physician in 7 to 10 days to begin post-stroke care.
Final thoughts
Primary care physicians are often the first point of contact for patients with current or remote TIA symptoms. Based on that provider–patient relationship, evidence supports several recommendations for diagnosing and treating a TIA and for reducing the risk of recurrent stroke after TIA. Addressing each of these areas, in this order, is imperative to reduce the risk of recurrent stroke and improve overall cardiovascular outcomes:
- Obtain an accurate diagnosis of a TIA, using DW-MRI or comparable brain imaging, to allow for prompt intervention.
- Initiate BP management promptly in the acute setting and establish optimal BP control over the long term.
- Begin appropriate antiplatelet therapy.
- When indicated (eg, atrial fibrillation), begin anticoagulation therapy with a DOAC or warfarin.
- Begin high-intensity statin therapy.
- Consider treating patients with diabetes using an SGLT2 inhibitor or GLP-1 receptor agonist.
- Encourage smoking cessation, prescribe quit-smoking medications, or refer a smoker for behavioral support.
Education. Last, it is important to educate patients—especially those who have risk factors for a TIA or stroke—about the presentation of events, so that they know to seek immediate medical attention.
CORRESPONDENCE
Kristen Rundell, MD, Department of Family and Community Medicine, University of Arizona College of Medicine, 655 North Alvernon Way, Suite 228, Tucson, AZ 85711; [email protected]
As many as 240,000 people per year in the United States experience a transient ischemic attack (TIA),1,2 which is now defined by the American Heart Association and American Stroke Association as a “transient episode of neurological dysfunction caused by focal brain, spinal cord, or retinal ischemia, without acute infarction.”3 An older definition of TIA was based on the duration of the event (ie, resolution of symptoms at 24 hours); in the updated (2009) definition, the diagnostic criterion is the extent of focal tissue damage.3 Using the 2009 definition might mean a decrease in the number of patients who have a diagnosis of a TIA and an increase in the number who are determined to have had a stroke because an infarction is found on initial imaging.
Guided by the 2009 revised definition of a TIA, we review here the work-up and treatment of TIA, emphasizing immediacy of management to (1) prevent further tissue damage and (2) decrease the risk of a second event.

CASE
Martin L, 69 years old, retired, a nonsmoker, and with a history of peripheral arterial disease and hypercholesterolemia, presents to the emergency department (ED) of a rural hospital complaining of slurred speech and left-side facial numbness. He had an episode of facial numbness that lasted 30 minutes, then resolved, each of the 2 previous evenings; he did not seek care at those times. Now, in the ED, Mr. L is normotensive.
The patient’s medication history includes a selective serotonin reuptake inhibitor and melatonin to improve sleep. He reports having discontinued a statin because he could not tolerate its adverse effects.
What immediate steps are recommended for Mr. L’s care?
Common event callsfor quick action
A TIA is the strongest predictor of subsequent stroke and stroke-related death; the highest period of risk of these devastating outcomes is immediately following a TIA.1,2,4,5 It is essential, therefore, for the physician who sees a patient with a current complaint or recent history of suspected focal neurologic deficits to direct that patient to an ED for an accurate diagnosis and, as appropriate, early treatment for the best possible outcome.
Imaging—preferably, diffusion-weighted magnetic resonance imaging (DW-MRI), the gold standard for diagnosing stroke (see “Diagnosis includes ruling out mimics”)2,3—should be performed as soon as the patient with a suspected TIA arrives in the ED. Imaging should not be held while waiting for a stroke to declare itself—ie, by allowing symptoms to persist for longer than 24 hours. 6
Continue to: Late presentation
Late presentation. Some patients present ≥ 48 hours after onset of early symptoms of a TIA; for them, the work-up is the same as for prompt presentation but can be completed in the outpatient clinic—as long as the patient is stable clinically and imaging is accessible there. DW-MRI should be completed within 48 hours after late presentation. In such cases, the patient should be cautioned regarding risks and any recurrence of symptoms.7,8
Diagnosis includes ruling out mimics
All patients in whom a stroke is suspected should be evaluated on an emergency basis with brain imaging upon arrival at the hospital, before any therapy is initiated. As noted, DW-MRI is the preferred modality; noncontrast computed tomography (CT) or CT angiography can be used if MRI is unavailable.2,3
Mimics. Stroke has many mimics; quickly eliminating them from the differential diagnosis is important so that appropriate therapy can be initiated. Mimics usually have a prolonged presentation of symptoms, whereas the presentation of a TIA is usually abrupt. The 3 more common diagnoses that mimic a TIA are migraine with aura, seizure, and syncope.9,10 Symptoms that generally are not associated with a TIA are chest pain, generalized weakness, and confusion.11 A complete history and physical exam provide the path to the imaging, laboratory, and cardiac testing that is needed to differentiate these diagnoses from a TIA.
A thorough history is best obtained from the patient and a witness, if available, and should include identification of any focal neurologic deficits and the duration and time to resolution of symptoms. Obtain a history of risk factors for ischemia—tobacco use, diabetes, obesity, dyslipidemia, hypertension, previous TIA or stroke, atrial fibrillation, and any coagulopathy. Ask questions about a family history of TIA, stroke, and coagulopathy.11
A comprehensive physical exam, including vital signs, cardiac exam, a check for carotid bruits, and complete neurologic exam, should be performed. Most patients present with concerns for unilateral weakness and changes in speech, which are usually associated with infarction on DW-MRI.12 The most common findings on physical exam include cranial nerve abnormalities, such as diplopia, hemianopia, monocular blindness, disconjugate gaze, facial drooping, lateral tongue movement, dysphagia, and vestibular dysfunction. Cerebellar abnormalities are also often noted, including past pointing, dystaxia, ataxia, nystagmus, and motor abnormalities (eg, spasticity, clonus, or unilateral weakness in the face or extremities).11
Electrocardiography at the bedside can confirm atrial fibrillation or another arrhythmia quickly.
Essential laboratory testing includes measurement of blood glucose and serum electrolytes to determine if these particular imbalances are the cause of symptoms. The presence of a hypercoaguable state is determined by a complete blood count and coagulation studies.3,13 Urine toxicology should also be obtained to rule out other causes of symptoms. A lipid profile is beneficial for making long-term treatment decisions.
Continue to: ABCD2 score
ABCD2 score. Patients who have had a TIA and present within 72 hours after symptoms have resolved should be hospitalized if they have an ABCD2 (Age, Blood pressure [BP], Clinical presentation, Diabetes mellitus [type 1 or 2], Duration of symptoms) prediction system score > 3.14 ABCD2 criteria can be used to help identify patients who are at higher risk of stroke or need further therapy (TABLE 1).14,15

The ABCD2 score is also used to determine whether a patient needs dual antiplatelet therapy. Patients who score at the higher end of the ABCD2 system usually have an increased risk of stroke, longer hospitalization, and greater disability.
CASE
In the ED, Mr. L is immediately assessed and airlifted to a larger regional medical center, where MRI confirms a stroke.
Management
Initial management of a TIA is aimed at reducing the risk of recurrent TIA or stroke. Early medical and possibly surgical treatment are key for preventing stroke and improving outcomes. The first 48 hours after a TIA are the most critical because the incidence of recurrent TIA or stroke is highest during this period.16-18
What is the accepted strategy for early treatment?
Initial treatment must include antiplatelet therapy, BP management, anticoagulation, statin therapy, and carotid endarterectomy as indicated.2,19,20 Control of hypertension and anticoagulation decrease the risk of recurrent stroke by the largest margin20; both are “A”-level Strength of Recommendation Taxonomy interventions.2,3
Step 1: Antiplatelet therapy. After initial imaging is complete and if there are no contraindications, antiplatelet agents are recommended for patients who have had a noncardioembolic TIA. The American Heart Association and American Stroke Association recommend either aspirin, clopidogrel, dipyridamole + aspirin (available in a single capsule [Aggrenox]), or clopidogrel + aspirin as first-line therapy.2,20 The choice of agent needs to be individualized, based on tolerability and adverse effects (TABLE 22,20,21).

A meta-analysis of antiplatelet therapy reviewed the optimum dosing of each medication.21,22 Reduction of the risk of ischemic stroke with aspirin is 21% to 22% at the optimal dosing of 75 to 150 mg/d, which also reduces the risk of gastrointestinal bleeding.
Continue to: For a patient who has...
For a patient who has an ABCD2 score ≥ 4, has had a prior TIA, or has large-vessel disease, dual antiplatelet therapy is recommended for the first 21 days, with a subsequent return to monotherapy. Dual antiplatelet therapy of clopidogrel + aspirin increases the risk of adverse reactions and has not been shown to have greater long-term benefit23-25 (TABLE 22,20,21).
Step 2: BP management. This is the next immediate step. As many as 80% of patients who present with a TIA have elevated BP upon admission. BP needs to be treated and carefully monitored during this early treatment phase. The recommendation is for a systolic BP < 185 mm Hg and a diastolic BP < 110 mm Hg.24
Step 3: Anticoagulation. Treatment with warfarin or a direct oral anticoagulant (DOAC) is recommended for patients who have the potential for forming emboli—eg, in the setting of atrial fibrillation, ventricular thrombus, mechanical heart valve, or venous thromboembolism.
Step 4. High-intensity statin. A statin agent is recommended as part of immediate and long-term medical management, regardless of the low-density lipoprotein cholesterol (LDL-C) level, to reduce the risk of stroke.2,24
Carotid artery management. Surgical intervention is not always considered a component of immediate medical management. However, guidelines recommend that carotid endarterectomy or stenting be considered in patients who have stenosis > 70%.2
CASE
Mr. L is admitted to the hospital and undergoes neurosurgical intervention. Medical management is instituted.
Long-term management and secondary prevention
The main risk factors for stroke can be divided into modifiable, vascular, and unmodifiable. Addressing both modifiable and vascular risks is important for secondary prevention.
Continue to: Modifiable and vascular risk factors
Modifiable and vascular risk factors
Modifiable risk factors for stroke include hypertension, diabetes, dyslipidemia, smoking, and physical activity; the most important of these, for preventing subsequent stroke after an initial TIA, is hypertension.26
The 2 more significant vascular risk factors for stroke are carotid artery stenosis and atrial fibrillation.
Hypertension. Improving control of hypertension can improve secondary risk reduction for recurrent stroke. Control of both systolic and diastolic BP is important in this regard, with larger systolic BP reductions having a greater impact on decreasing the risk of recurrent stroke.24 Evidence supports lowering BP to improve secondary risk reduction in people with and without diagnosed hypertension: The goal is to lower systolic BP by ≥ 10 mm Hg and diastolic BP by 5 mm Hg.24 No particular class of antihypertensive is recommended in the first line, although preliminary evidence shows that a diuretic, with or without an angiotensin-converting enzyme inhibitor, might be more beneficial than other options.24
Diabetes. The risk of cardiovascular disease, including stroke, is higher in people with diabetes. Evidence shows that various (but not all) agents in 2 pharmaceutical classes—glucagon-like peptide-1 (GLP-1) receptor agonists and the sodium glucose-2 cotransporter (SGLT2) inhibitors—reduce the risk of major cardiovascular events and improve secondary prevention of recurrent stroke:
- EMPA-REG OUTCOME (ClinicalTrials.gov Identifier: NCT01131676) was the first trial to show cardiovascular benefit from an SGLT2 inhibitor (empagliflozin); subsequent studies confirmed the cardiovascular benefits found in EMPA-REG OUTCOME.27,28
- The ELIXA trial (ClinicalTrials.gov Identifier: NCT01147250) was the first to show cardiovascular benefit from a GLP-1 receptor agonist (lixisenatide); subsequent studies supported this finding.29,30
Appropriate agents in these 2 classes should be considered as first-line or adjunctive in patients with both diabetes and known cardiovascular disease, as long as there are no contraindications.27,28
Pioglitazone, a thiazolidinedione-class antidiabetic agent, was once considered a potential option to improve secondary prevention of stroke. However, the thiazolidinediones are generally no longer considered; instead, the SGLT2 inhibitors and GLP-1 receptor agonists are favored.31
Evidence demonstrates the effect of hyperglycemia on cardiovascular events; however, it is important to note that hypoglycemia can result in symptoms and focal changes that mimic a stroke. In addition, some evidence suggests that hypoglycemia can increase cardiovascular risk—thereby supporting the importance of strict control of diabetes and maintenance of euglycemia in reducing overall cardiovascular risk.32
Continue to: Lipids
Lipids. The SPARCL trial (ClinicalTrials.gov Identifier: NCT00147602) was the first study to demonstrate the benefit of high-intensity statin therapy—specifically, atorvastatin 80 mg/d—for secondary prevention for recurrent stroke.33 The recommendation is to use high-intensity statin therapy to decrease the risk of recurrent stroke by reducing the level of LDL-C—by ≥ 50% or to < 70 mg/dL, for maximum risk reduction.24,34
The IMPROVE-IT trial (ClinicalTrials.gov Identifier: NCT00202878) demonstrated the benefit of adding ezetimibe, 10 mg/d, to a moderate-to-high-intensity statin (simvastatin, 40-80 mg/d) to reduce the risk of recurrent stroke.35
Results of recent studies support the use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors for regulating levels of LDL-C, as an additional option to consider—if needed to further reduce the LDL-C level or if statins are contraindicated in a particular patient.34
Smoking cessation. Cigarette smoking is known to increase the risk of ischemic stroke; newer evidence shows that second-hand exposure to smoke also increases the risk of ischemic stroke.36,37 Although these studies focused on primary prevention of ischemic stroke, the data can reasonably be applied to secondary prevention.38 The recommendation for secondary prevention is to quit smoking and avoid secondhand smoke.24
Alcohol. Evidence demonstrates that heavy alcohol consumption and alcoholism increase the risk of stroke; similar to what is known about smoking, most available data relate to primary prevention.38 The recommendation for providing secondary stroke prevention is to stop or decrease alcohol intake.24
Weight reduction. Obesity (body mass index > 30) increases the risk of ischemic stroke. However, there is, as yet, no evidence that weight loss diminishes the risk of subsequent stroke for secondary prevention.24
Physical activity. Aerobic exercise and strength-training programs after a stroke improve cardiovascular health and mobility. There is no evidence that exercise leads to a reduction in the risk of subsequent stroke.24
Continue to: Nutrition
Nutrition. No current randomized controlled trials are focused on the relationship between diet and recurrent stroke for purposes of prevention; however, evidence for both BP and lipid control incorporate dietary guidance. Recommendations include reducing intake of saturated fats and of sodium (the latter, to < 2.3 g/d) and increasing intake of fruits and vegetables, both of which are beneficial for controlling BP and lipid levels and promoting overall cardiovascular health.38
Carotid artery stenosis. Several randomized controlled trials have demonstrated benefit from treating carotid stenosis (> 70% stenosis but not < 50%) with carotid endarterectomy to reduce the risk of recurrent stroke after TIA.2 The ideal timing of carotid endarterectomy is still being studied; however, available evidence supports intervention within 2 to 6 weeks after TIA or stroke.25 Studies are ongoing that compare carotid angioplasty and stenting against carotid endarterectomy. Medical therapy, with antiplatelet agents and statins, is recommended after carotid endarterectomy.25
Atrial fibrillation increases the risk of recurrent stroke after a TIA, and is the most important indication for secondary stroke prevention with anticoagulation therapy:
- Warfarin. Several studies have shown that warfarin provides a 68% relative risk reduction and a 1.4% absolute risk reduction in the annual stroke rate.24 To achieve this reduction in risk, the optimal international normalized ratio is 2.5 (range, 2-3).24
- Aspirin provides a 13% relative risk reduction for recurrent stroke, although there is evidence that long-term anticoagulation provides more benefit than aspirin after a TIA.39-41 Optimal dosing of aspirin ranges from 75-100 mg/d; greatest benefit is likely in the 12 weeks after stroke, when the risk of recurrent stroke is highest.31,41,42
- DOACs have similar efficacy to warfarin but more rapid onset, lower risk of bleeding, fewer drug interactions, and no requirement for monitoring—often making them a more tolerable long-term choice. Options are rivaroxaban 20 mg/d, dabigatran 150 mg twice daily, apixaban 5 mg twice daily, and edoxaban 60 mg/d.39
When to start anticoagulation and the choice of agent should be weighed against a risk of bleeding, which is highest after the initial stroke. Cost is also a consideration: DOACs are more expensive than warfarin.
CASE
Mr. L is discharged 3 days after carotid endarterectomy and free of residual deficits. He is started on dual antiplatelet therapy (aspirin + clopidogrel) for 21 days, to be followed by a return to monotherapy. He is restarted on a high-intensity statin. He is instructed to resume taking the selective serotonin reuptake inhibitor and melatonin for sleep, as needed. Last, he is told to schedule follow-up with his primary care physician in 7 to 10 days to begin post-stroke care.
Final thoughts
Primary care physicians are often the first point of contact for patients with current or remote TIA symptoms. Based on that provider–patient relationship, evidence supports several recommendations for diagnosing and treating a TIA and for reducing the risk of recurrent stroke after TIA. Addressing each of these areas, in this order, is imperative to reduce the risk of recurrent stroke and improve overall cardiovascular outcomes:
- Obtain an accurate diagnosis of a TIA, using DW-MRI or comparable brain imaging, to allow for prompt intervention.
- Initiate BP management promptly in the acute setting and establish optimal BP control over the long term.
- Begin appropriate antiplatelet therapy.
- When indicated (eg, atrial fibrillation), begin anticoagulation therapy with a DOAC or warfarin.
- Begin high-intensity statin therapy.
- Consider treating patients with diabetes using an SGLT2 inhibitor or GLP-1 receptor agonist.
- Encourage smoking cessation, prescribe quit-smoking medications, or refer a smoker for behavioral support.
Education. Last, it is important to educate patients—especially those who have risk factors for a TIA or stroke—about the presentation of events, so that they know to seek immediate medical attention.
CORRESPONDENCE
Kristen Rundell, MD, Department of Family and Community Medicine, University of Arizona College of Medicine, 655 North Alvernon Way, Suite 228, Tucson, AZ 85711; [email protected]
1. Kleindorfer D, Panagos P, Pancioli A, et al. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke. 2005;36:720-723. doi: 10.1161/01.STR.0000158917.59233.b7
2. Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2021;52:e364-e467. doi: 10.1161/STR.0000000000000375
3. Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276-2293. doi: 10.1161/STROKEAHA.108.192218
4. Thacker EL, Wiggins KL, Rice KM, et al. Short-term and long-term risk of incident ischemic stroke after transient ischemic attack. Stroke. 2010;41:239-243. doi: 10.1161/STROKEAHA.109.569707
5. Hill MD, Yiannakoulias N, Jeerakathil T, et al. The high risk of stroke immediately after transient ischemic attack: a population-based study. Neurology. 2004;62:2015-2020. doi: 10.1212/01.wnl.0000129482.70315.2f
6. Giles MF, Albers GW, Amarenco P, et al. Early stroke risk and ABCD2 score performance in tissue- vs time-defined TIA: a multicenter study. Neurology. 2011;77:1222-1228. doi: 10.1212/WNL.0b013e3182309f91
7. Cucchiara BL, Kasner SE. All patients should be admitted to the hospital after a transient ischemic attack. Stroke. 2012;43:1446-1447. doi: 10.1161/STROKEAHA.111.636746
8. Amarenco P. Not all patients should be admitted to the hospital for observation after a transient ischemic attack. Stroke. 2012;43:1448-1449. doi: 10.1161/STROKEAHA.111.636753
9. Amort M, Fluri F, Schäfer J, et al. Transient ischemic attack versus transient ischemic attack mimics: frequency, clinical characteristics and outcome. Cerebrovasc Dis. 2011;32:57-64. doi: 10.1159/000327034
10. Hand PJ, Kwan J, Lindley RI, et al. Distinguishing between stroke and mimic at the bedside: The Brain Attack Study. Stroke. 2006;37:769-775. doi: 10.1161/01.STR.0000204041.13466.4c
11. Shah KH, Edlow JA. Transient ischemic attack: review for the emergency physician. Ann Emerg Med. 2004;43:592-604. doi: 10.1016/S0196064404000058
12. Crisostomo RA, Garcia MM, Tong DC. Detection of diffusion-weighted MRI abnormalities in patients with transient ischemic attack: correlation with clinical characteristics. Stroke. 2003;34:932-937. doi: 10.1161/01.STR.0000061496.00669.5E
13. Adams HP Jr, del Zoppo G, Alberts MJ, et al; ; ; ; ; . Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655-1711. doi: 10.1161/STROKEAHA.107.181486
14. Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369:283-292. doi: 10.1016/S0140-6736(07)60150-0
15. Cucchiara BL, Messe SR, Taylor RA, et al. Is the ABCD score useful for risk stratification of patients with acute transient ischemic attack? Stroke. 2006;37:1710-1714. doi: 10.1161/01.STR.0000227195.46336.93
16. Amarenco P, Lavallée PC, Labreuche J, et al; . One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542. doi: 10.1056/NEJMoa1412981
17. Wu CM, McLaughlin K, Lorenzetti DL, et al. Early risk of stroke after transient ischemic attack: a systematic review and meta-analysis. Arch Intern Med. 2007;167:2417-2422. doi: 10.1001/archinte.167.22.2417
18. Rothwell PM, Warlow CP. Timing of TIAs preceding stroke: time window for prevention is very short. Neurology. 2005;64:817-820. doi: 10.1212/01.WNL.0000152985.32732.EE
19. Kernan WN, Ovbiagele B, Black HR, et al; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236. doi: 10.1161/STR.0000000000000024
20. Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370:1432-1442. doi: 10.1016/S0140-6736(07)61448-2
21. Hackam DG, Spence JD. Antiplatelet therapy in ischemic stroke and transient ischemic attack: an overview of major trials and meta-analyses. Stroke. 2019;50:773-778. doi: c10.1161/STROKEAHA.118.023954
22. Bhatia K, Jain V, Aggarwal D, et al. Dual antiplatelet therapy versus aspirin in patients with stroke or transient ischemic attack: meta-analysis of randomized controlled trials. Stroke. 2021;52:e217-e223. doi: 10.1161/STROKEAHA.120.033033
23. Wang Y, Pan Y, Zhao X, et al; CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack (CHANCE) trial: one-year outcomes. Circulation. 2015;132:40-46. doi: 10.1161/CIRCULATIONAHA.114.014791
24. Furie KL, Kasner SE, Adams RJ, et al; . Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227-276. doi: 10.1161/STR.0b013e3181f7d043
25. Powers WJ, Rabinstein AA, Ackerson T, et al; American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110. doi: 10.1161/STR.0000000000000158
26. O’Donnell MJ, Chin SL, Rangarajan S, et al; INTERSTROKE Investigators. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388:761-775. doi: 10.1016/S0140-6736(16)30506-2
27. Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776-785. doi:10.1016/S2213-8587(19)30249-9
28. Bertoccini L, Baroni MG. GLP-1 receptor agonists and SGLT2 inhibitors for the treatment of type 2 diabetes: new insights and opportunities for cardiovascular protection. Adv Exp Med Biol. 2021;1307:193-212. doi:10.1007/5584_2020_494
29. Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome N Engl J Med. 2015;373:2247-2257. doi: 10.1056/NEJMoa1509225
30. Sheahan KH, Wahlberg EA, Gilbert MP. An overview of GLP-1 agonists and recent cardiovascular outcomes trials. Postgrad Med J. 2020;96:156-161. doi:10.1136/postgradmedj-2019-137186
31. Kim AS. Medical management for secondary stroke prevention. Continuum (Minneap Minn). 2020;26:435-456. doi:10.1212/CON.0000000000000849
32. Smith L, Chakraborty D, Bhattacharya P, et al. Exposure to hypoglycemia and risk of stroke. Ann N Y Acad Sci. 2018;1431:25-34. doi:10.1111/nyas.13872
33. Amarenco P, Bogousslavsky J, Callahan A 3rd, et al; . High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559. doi:10.1056/NEJMoa061894
34. Castilla-Guerra, L, Fernandez-Moreno M, Leon-Jimenez D, et al. Statins in ischemic stroke prevention: what have we learned in the post-SPARCL (The Stroke Prevention by Aggressive Reduction in Cholesterol Levels) decade? Curr Treat Options Neurol. 2019;21:22. doi: 10.1007/s11940-019-0563-4
35. Bohula EA, Wiviott SD, Giugliano RP, et al. Prevention of stroke with the addition of ezetimibe to statin therapy in patients with acute coronary syndrome in IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Circulation. 2017;136:2440-2450. doi:10.1161/CIRCULATIONAHA.117.029095
36. Moritsugu KP. The 2006 report of the Surgeon General: the health consequences of involuntary exposure to tobacco smoke. Am J Prev Med. 20067;32:542-543. doi: 10.1016/j.amepre.2007.02.026
37. Wolf PA, D’Agostino RB, Kannel WB, et al. Cigarette smoking as a risk factor for stroke: the Framingham Study. JAMA. 1988;259:1025-1029.
38. Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:1583-1633. doi: 10.1161/01.STR.0000223048.70103.F1
39. Klijn CJ, Paciaroni M, Berge E, et al. Antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation: A European Stroke Organisation guideline. Eur Stroke J. 2019;4:198-223. doi:10.1177/2396987319841187
40. Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860. doi:10.1016/S0140-6736(09)60503-1
41. Singer DE, Albers GW, Dalen JE, et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):546S–592S. doi: 10.1378/chest.08-0678
42. Rothwell PM, Algra A, Chen Z, et al. Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: time-course analysis of randomised trials. Lancet. 2016;388:365-375. doi:10.1016/S0140-6736(16)30468-8
1. Kleindorfer D, Panagos P, Pancioli A, et al. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke. 2005;36:720-723. doi: 10.1161/01.STR.0000158917.59233.b7
2. Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2021;52:e364-e467. doi: 10.1161/STR.0000000000000375
3. Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276-2293. doi: 10.1161/STROKEAHA.108.192218
4. Thacker EL, Wiggins KL, Rice KM, et al. Short-term and long-term risk of incident ischemic stroke after transient ischemic attack. Stroke. 2010;41:239-243. doi: 10.1161/STROKEAHA.109.569707
5. Hill MD, Yiannakoulias N, Jeerakathil T, et al. The high risk of stroke immediately after transient ischemic attack: a population-based study. Neurology. 2004;62:2015-2020. doi: 10.1212/01.wnl.0000129482.70315.2f
6. Giles MF, Albers GW, Amarenco P, et al. Early stroke risk and ABCD2 score performance in tissue- vs time-defined TIA: a multicenter study. Neurology. 2011;77:1222-1228. doi: 10.1212/WNL.0b013e3182309f91
7. Cucchiara BL, Kasner SE. All patients should be admitted to the hospital after a transient ischemic attack. Stroke. 2012;43:1446-1447. doi: 10.1161/STROKEAHA.111.636746
8. Amarenco P. Not all patients should be admitted to the hospital for observation after a transient ischemic attack. Stroke. 2012;43:1448-1449. doi: 10.1161/STROKEAHA.111.636753
9. Amort M, Fluri F, Schäfer J, et al. Transient ischemic attack versus transient ischemic attack mimics: frequency, clinical characteristics and outcome. Cerebrovasc Dis. 2011;32:57-64. doi: 10.1159/000327034
10. Hand PJ, Kwan J, Lindley RI, et al. Distinguishing between stroke and mimic at the bedside: The Brain Attack Study. Stroke. 2006;37:769-775. doi: 10.1161/01.STR.0000204041.13466.4c
11. Shah KH, Edlow JA. Transient ischemic attack: review for the emergency physician. Ann Emerg Med. 2004;43:592-604. doi: 10.1016/S0196064404000058
12. Crisostomo RA, Garcia MM, Tong DC. Detection of diffusion-weighted MRI abnormalities in patients with transient ischemic attack: correlation with clinical characteristics. Stroke. 2003;34:932-937. doi: 10.1161/01.STR.0000061496.00669.5E
13. Adams HP Jr, del Zoppo G, Alberts MJ, et al; ; ; ; ; . Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655-1711. doi: 10.1161/STROKEAHA.107.181486
14. Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369:283-292. doi: 10.1016/S0140-6736(07)60150-0
15. Cucchiara BL, Messe SR, Taylor RA, et al. Is the ABCD score useful for risk stratification of patients with acute transient ischemic attack? Stroke. 2006;37:1710-1714. doi: 10.1161/01.STR.0000227195.46336.93
16. Amarenco P, Lavallée PC, Labreuche J, et al; . One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542. doi: 10.1056/NEJMoa1412981
17. Wu CM, McLaughlin K, Lorenzetti DL, et al. Early risk of stroke after transient ischemic attack: a systematic review and meta-analysis. Arch Intern Med. 2007;167:2417-2422. doi: 10.1001/archinte.167.22.2417
18. Rothwell PM, Warlow CP. Timing of TIAs preceding stroke: time window for prevention is very short. Neurology. 2005;64:817-820. doi: 10.1212/01.WNL.0000152985.32732.EE
19. Kernan WN, Ovbiagele B, Black HR, et al; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236. doi: 10.1161/STR.0000000000000024
20. Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370:1432-1442. doi: 10.1016/S0140-6736(07)61448-2
21. Hackam DG, Spence JD. Antiplatelet therapy in ischemic stroke and transient ischemic attack: an overview of major trials and meta-analyses. Stroke. 2019;50:773-778. doi: c10.1161/STROKEAHA.118.023954
22. Bhatia K, Jain V, Aggarwal D, et al. Dual antiplatelet therapy versus aspirin in patients with stroke or transient ischemic attack: meta-analysis of randomized controlled trials. Stroke. 2021;52:e217-e223. doi: 10.1161/STROKEAHA.120.033033
23. Wang Y, Pan Y, Zhao X, et al; CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack (CHANCE) trial: one-year outcomes. Circulation. 2015;132:40-46. doi: 10.1161/CIRCULATIONAHA.114.014791
24. Furie KL, Kasner SE, Adams RJ, et al; . Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227-276. doi: 10.1161/STR.0b013e3181f7d043
25. Powers WJ, Rabinstein AA, Ackerson T, et al; American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110. doi: 10.1161/STR.0000000000000158
26. O’Donnell MJ, Chin SL, Rangarajan S, et al; INTERSTROKE Investigators. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388:761-775. doi: 10.1016/S0140-6736(16)30506-2
27. Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776-785. doi:10.1016/S2213-8587(19)30249-9
28. Bertoccini L, Baroni MG. GLP-1 receptor agonists and SGLT2 inhibitors for the treatment of type 2 diabetes: new insights and opportunities for cardiovascular protection. Adv Exp Med Biol. 2021;1307:193-212. doi:10.1007/5584_2020_494
29. Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome N Engl J Med. 2015;373:2247-2257. doi: 10.1056/NEJMoa1509225
30. Sheahan KH, Wahlberg EA, Gilbert MP. An overview of GLP-1 agonists and recent cardiovascular outcomes trials. Postgrad Med J. 2020;96:156-161. doi:10.1136/postgradmedj-2019-137186
31. Kim AS. Medical management for secondary stroke prevention. Continuum (Minneap Minn). 2020;26:435-456. doi:10.1212/CON.0000000000000849
32. Smith L, Chakraborty D, Bhattacharya P, et al. Exposure to hypoglycemia and risk of stroke. Ann N Y Acad Sci. 2018;1431:25-34. doi:10.1111/nyas.13872
33. Amarenco P, Bogousslavsky J, Callahan A 3rd, et al; . High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559. doi:10.1056/NEJMoa061894
34. Castilla-Guerra, L, Fernandez-Moreno M, Leon-Jimenez D, et al. Statins in ischemic stroke prevention: what have we learned in the post-SPARCL (The Stroke Prevention by Aggressive Reduction in Cholesterol Levels) decade? Curr Treat Options Neurol. 2019;21:22. doi: 10.1007/s11940-019-0563-4
35. Bohula EA, Wiviott SD, Giugliano RP, et al. Prevention of stroke with the addition of ezetimibe to statin therapy in patients with acute coronary syndrome in IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Circulation. 2017;136:2440-2450. doi:10.1161/CIRCULATIONAHA.117.029095
36. Moritsugu KP. The 2006 report of the Surgeon General: the health consequences of involuntary exposure to tobacco smoke. Am J Prev Med. 20067;32:542-543. doi: 10.1016/j.amepre.2007.02.026
37. Wolf PA, D’Agostino RB, Kannel WB, et al. Cigarette smoking as a risk factor for stroke: the Framingham Study. JAMA. 1988;259:1025-1029.
38. Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:1583-1633. doi: 10.1161/01.STR.0000223048.70103.F1
39. Klijn CJ, Paciaroni M, Berge E, et al. Antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation: A European Stroke Organisation guideline. Eur Stroke J. 2019;4:198-223. doi:10.1177/2396987319841187
40. Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860. doi:10.1016/S0140-6736(09)60503-1
41. Singer DE, Albers GW, Dalen JE, et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):546S–592S. doi: 10.1378/chest.08-0678
42. Rothwell PM, Algra A, Chen Z, et al. Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: time-course analysis of randomised trials. Lancet. 2016;388:365-375. doi:10.1016/S0140-6736(16)30468-8
PRACTICE RECOMMENDATIONS
In the hospital, the treating physician should:
› Immediately initiate brain imaging with diffusion-weighted magnetic resonance imaging when TIA is suspected, upon the patient’s arrival at the hospital. A
› Control blood pressure when a TIA is confirmed, to decrease the risk of recurrent stroke. A
› Initiate antiplatelet therapy, to decrease the risk of recurrent stroke. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Benzodiazepine and Z-hypnotic stewardship
Benzodiazepines (BZDs) and Z-hypnotics have been available for decades, yet uncertainties about their use remain. They are prescribed and overprescribed most often for anxiety and insomnia, for which they have value but also the potential for significant adverse consequences, notably physiologic dependence. Use of these agents should be limited, and planned deprescribing is a fundamental aspect of prescribing.
A brief history. BZDs are a subset of benzodiazepine receptor agonists (BZRAs), which enhance the inhibitory effect of centrally acting γ-amino butyric acid (GABA) at the GABAA receptor through allosteric modulation. In 1960, the first BZD, chlordiazepoxide, was marketed for clinical use, and as other agents in the class became available, BZDs supplanted the more toxic barbiturates, another BZRA subset (TABLE 1). By the late 1970s, BZDs had risen to the top of most prescribed medications, with one agent in particular—diazepam (Valium)—earning a reputation as “mother’s little helper,” a phrase derived from a Rolling Stones' song with that title produced in 1966.1
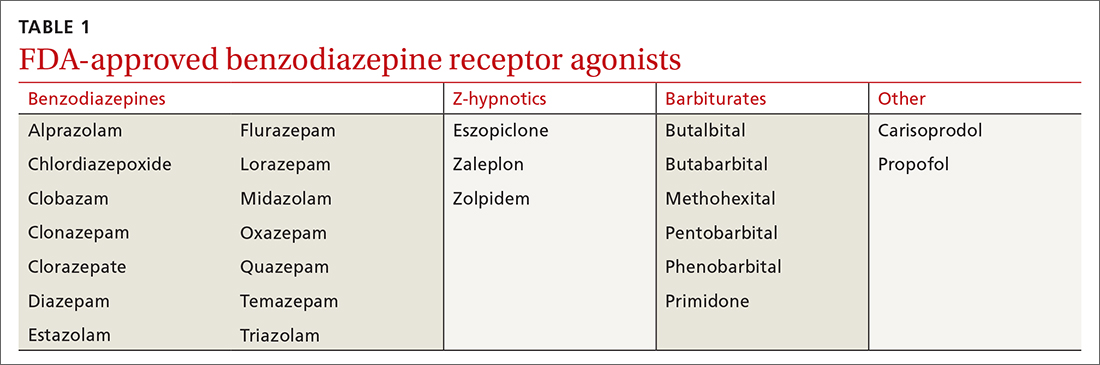
With recognition of the problems associated with BZDs, their popularity diminished somewhat but remained high. BZDs were listed under Schedule IV by the Drug Enforcement Administration in 1975 due to the risk for addiction, and on the American Geriatrics Society Beers Criteria list in 1991 because of significant adverse consequences in the elderly. Researchers began to question their use as early as the 1970s, and the landmark Ashton Manual, guidance for patients and clinicians alike, was published in 2002.2
Currently, there are 14 BZDs approved by the Food and Drug Administration (FDA) as well as 3 Z-hypnotics, termed such as they include the letter “z” in their generic names (TABLE 1). In recent years, BZD prescribing has risen; a 2019 study found that 1 of 8 American adults reported using a BZD in the previous year.3
Limited benefits of benzodiazepine receptor agonists
BZRAs can be of benefit in a limited range of medical conditions, including some for which they are first-line considerations. (See TABLE 2 for a list of indications for BZDs.) They are most often prescribed for anxiety and insomnia, although they are not first-line treatments for these conditions and should be prescribed only when symptoms limit a patient’s daily functioning.

BZRAs are not intended for long-term use. In recent decades, the percentage of patients prescribed BZRAs has doubled, and more than 80% of these patients indicate usage for more than 6 months.4 Evidence, however, does not support long-term daily use.
Observation periods in most studies are far shorter than the number of years over which BZDs are actually prescribed, and flawed research methodology has introduced the risk of bias. Specifically, the generalizability of reported outcomes must be qualified, since efficacy trials performed under ideal study conditions (eg, exclusion criteria to minimize confounders) differ from circumstances seen in clinical practice. Conclusions are also limited by the inherent bias of pharmaceutical industry sponsorship and unavailability of unpublished trials that may have demonstrated unfavorable results.
Continue to: Insomnia
Insomnia, a current (past 30 days) complaint in more than 40% of US adults, is associated with a variety of symptoms.5 About 20% of adults have an insomnia disorder, defined as a predominant problem for at least 1 month involving sleep initiation, maintenance, or nonrestorative sleep along with daytime function-limiting fatigue.5 Meta-analyses indicate BZRAs can reduce sleep latency (BZDs, by 4 minutes; Z-hypnotics, 22 minutes) and may increase sleep duration (BZDs, 62 minutes per limited data; Z-hypnotics, data insufficient).6,7 Definitive evidence for long-term (> 2-4 weeks) BZD benefit is lacking, and cognitive behavioral therapy for insomnia (CBT-I) is well established as first-line treatment yielding improvements that may last at least 18 months after completion of therapy. 8,9
Although CBT-I is generally provided by behavioral health specialists, elements of CBT-I and sleep hygiene measures can be effectively used by primary care clinicians.10 Data indicate other nonpharmacologic interventions are also effective,11 including acceptance and commitment therapy,12 meditation,13 and acupuncture.14
Episodic fear and anxiety are universal and essential for survival. Fear is an alarm warning of an immediate hazard. Anxiety (the emotion) paired with worry (the thought) relate to a perceived future threat. Transient (state) anxiety should not be suppressed altogether if self-management can curb its intensity and thereby allow effective problem engagement. However, when individuals are incapacitated by crisis anxiety or sporadic specific phobias such as flight anxiety, episodic BZDs do have a role.
Ongoing anxiety is a more complex treatment situation. Obsessive-compulsive disorder and posttraumatic stress disorder are no longer categorized as anxiety disorders, but they often involve anxiety. Here, BZDs have no indication aside from exceptional acute crisis presentations. Anxiety disorders are defined by a core persistent (trait) anxiety disproportionate to the actual threat, limited daily functioning, and more than 6 months’ duration. One of 3 Americans older than 13 years meet the criteria for anxiety in their lifetime; 1 of 5 meet the criteria in any single year.15
BZDs are effective in treating anxiety disorders in the short term (2-4 weeks)2,16,17; however, benefit may fade over time.18-21 For some individuals, data suggest BZDs themselves might actually generate anxiety, as evidenced by reduced symptom intensity following discontinuation.22,23 Recommended first-line medications for anxiety disorders include certain antidepressants and pregabalin, which exhibit efficacy similar to that of BZDs.24 Mindfulness and various psychotherapies have value, as well.16 Among the latter, CBT is considered first line with benefit comparable to BZDs in the short term; yet unlike BZDs, CBT gains can last 12 months or longer after the conclusion of therapy. 25,26 Because there may be a delay between the start of CBT and the onset of benefit, BZDs, which work quickly, may be used to bridge functionally impaired patients in the short term.
Continue to: Risks with benzodiazepine receptor agonists
Risks with benzodiazepine receptor agonists
Harms from BZRA use are common, tempering their utility. Sedation, dyscognition, and psychomotor impairments are often seen upon initiation of BZRA use. These adverse effects can—although not always—improve with continuous BZRA exposure, an effect known as tolerance, which is due to neuropharmacologic adaptation.
Cognitive issues include problems with memory, judgment, and decision making. These may be unrecognized or, if noted, attributed to other issues such as aging, and may become clear only when BZRAs are discontinued. Anterograde amnesia and parasomnias occur less often.
Psychomotor impairment can result in falls, fractures, and other injuries, especially in the elderly. Decrements in mood, including emergent depression and paradoxical anxiety, can occur. Some individuals experience disinhibition that is expressed through irritability, agitation, aggression, and violence.
Misuse of BZRAs is not unusual and can be related to dosing errors or attempts to ease intrusive symptoms. Nonmedical use almost always occurs in the context of an underlying use disorder, whereby BZRAs serve to amplify euphoria or ameliorate withdrawal from opioids or alcohol. Addiction per se, which entails BZRA craving and compulsive use leading to adverse consequences, is unusual.
BZRAs are associated with increased mortality, including all-cause mortality and suicide. They are respiratory depressants, although when taken alone in excess rarely result in death. They are, however, strongly implicated in opioid-related overdose fatalities, as their presence has been identified in 1 of 3 such decedents.27
Continue to: Physiologic dependence with BZRAs
Physiologic dependence with BZRAs
Among the more important adverse outcomes with ongoing BZRA exposure is physiologic dependence. This occurs primarily because of neuroadaptation of GABAA and glutaminergic receptors, but dependence probably also involves changes in the adenosine A2A, serotonergic, and peripheral benzodiazepine receptors, the latter being present on mitochondrial membranes. The hypothalamic-pituitary-adrenal axis also appears to be involved.
Physiologic dependence is expressed through BZRA tolerance and characteristic physical and psychological symptoms upon withdrawal. Tolerance refers to a reduced effect with continued substance exposure or the need for an increased dose to get the same effect. Drug withdrawal can result in manifestations distinctive to addiction-prone substances, as well as to some medications without addiction liability, such as corticosteroids and antidepressants. Tolerance and withdrawal are not applicable criteria in the diagnosis of sedative-hypnotic use disorder when BZRAs are prescribed.28
Withdrawal. Reported prevalence of BZRA physiologic dependence differs according to populations studied, criteria used, and the deprescribing process employed. Some researchers have found rates of one-third and others exceeding one-half among individuals using BZRAs for longer than a month.23,29
Deprescribing BZRAs
Because benefits are limited and adverse outcomes including physiologic dependence are common, it is recommended that clinicians urge deprescribing of BZRAs for any patient taking them consistently for more than 1 month. Published deprescribing investigations and guidance are insufficient, heterogenous, and confusing. Still, some approaches can work well, and success rates as high as 80% have been achieved among the elderly, for example.35 Brief interventions such as providing individualized advice, support, and management are effective.36,37 Abrupt discontinuation is inappropriate and can be life threatening.38 Forced cessation is also inappropriate unless significant respiratory depression is identified.
The Ashton Manual is a useful guide, readable by patients. Proceed with tapering slowly at a rate led by the patient’s response.2,39 Avoid discrediting patients’ reports of unusual withdrawal symptoms, as this can lead to misdiagnosis (eg, somatic symptom disorder) or ineffective treatment (eg, addiction recovery approaches). Adding CBT to tapering improves outcomes, and adjunctive medications may be helpful, although not without their own problems.29 Consistent support of patients by others involved in treatment (prescriber, pharmacist, behavioral health specialists, peer coach, significant others) is essential. Complex challenges generally resolve through authentic listening and response but may require referral to others with necessary skills and experience. Complete cessation may take 12 to 18 months (or longer). Even if complete cessation is not possible, the least dose necessary can be achieved.
CORRESPONDENCE
Steven Wright, MD, 1975 Ashland Mine Road, Ashland, OR 97520; [email protected]
- Chandler A, Whittaker A, Williams N, et al. Mother’s little helper? Contrasting accounts of benzodiazepine and methadone use among drug-dependent parents in the UK. Drugs (Abingdon Engl). 2014;21:470-475. doi: 10.3109/09687637.2014.930814Ash
- ton CH. Benzodiazepines: How They Work & How to Withdraw (the Ashton Manual). 2002. Accessed March 17, 2022. www.benzoinfo.com/ashtonmanual/
- Maust DT, Lin LA, Blow FC. Benzodiazepine use and misuse among adults in the United States. Psychiatr Serv. 2019;70:97-106. doi: 10.1176/appi.ps.201800321
- Kaufmann CN, Spira AP, Depp CA, et al. Long-term use of benzodiazepines and non-benzodiazepine hypnotics from 1999 to 2014: results from the National Health and Nutrition Examination Survey. Psychiatr Serv. 2018;69:235-238. doi: 10.1176/appi.ps.201700095
- Walsh JK, Coulouvrat C, Hajak G, et al. Nighttime insomnia symptoms and perceived health in the America Insomnia Survey (AIS). Sleep. 2011;34:997-1011. doi: 10.5665/SLEEP.1150
- Holbrook AM, Crowther R, Lotter A, et al. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162:225-233.
- Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343
- Curran HV, Collins R, Fletcher S, et al. Older adults and withdrawal from benzodiazepine hypnotics in general practice: effects on cognitive function, sleep, mood and quality of life. Psychol Med. 2003;33:1223-1237. doi: 10.1017/s0033291703008213
- Geiger-Brown JM, Rogers VE, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54-67. doi: 10.1016/j.smrv.2014.11.007
- Sorscher AJ. Insomnia: getting to the cause, facilitating relief. J Fam Pract. 2017;66:216-225
- Laura Hrehová L, Mezian K. Non-pharmacologic treatment of insomnia in primary care settings. Int J Clin Pract. 2021;75:e14084. doi: 10.1111/ijcp.14084.
- Daly-Eichenhardt A, Scott W, Howard-Jones M, et al. Changes in sleep problems and psychological flexibility following interdisciplinary acceptance and commitment therapy for chronic pain: an observational cohort study. Front Psychol. 2016;7:1326. doi: 10.3389/fpsyg.2016.01326
- Rusch HL, Rosario M, Levison LM, et al. The effect of mindfulness meditation on sleep quality: a systematic review and meta-analysis of randomized controlled trials. Ann N Y Acad Sci. 2019;1445:5-16. doi: 10.1111/nyas.13996
- Cao H, Pan X, Li H, et al. Acupuncture for treatment of insomnia: a systematic review of randomized controlled trials. J Altern Complement Med. 2009;15:1171-1186. doi: 10.1089/acm.2009.0041
- Kessler RC, Petukhova M, Sampson NA, et al. Twelve‐month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169-184. doi: 10.1002/mpr.1359
- Bandelow B, Reitt M, Röver C, et al. Efficacy of treatments for anxiety disorders: a meta-analysis. Int Clin Psychopharmacol. 2015;30:183-192. doi: 10.1097/YIC.0000000000000078
- Lader M. Benzodiazepines revisited—will we ever learn? Addiction. 2011;106:2086-2109. doi: 10.1111/j.1360-0443.2011.03563.x
- Fava GA. Fading of therapeutic effects of alprazolam in agoraphobia. Case reports. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:109-112. doi: 10.1016/0278-5846(88)90066-8
- Fava GA, Grandi S, Belluardo P, et al. Benzodiazepines and anxiety sensitivity in panic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:1163-1168. doi: 10.1016/0278-5846(94)90118-x
- Pélissolo A, Maniere F, Boutges B, et al. Anxiety and depressive disorders in 4,425 long term benzodiazepine users in general practice. Encephale. 2007;33:32-38. doi: 10.1016/s0013-7006(07)91556-0
- Gale C, Glue P, Guaiana G, et al. Influence of covariates on heterogeneity in Hamilton Anxiety Scale ratings in placebo-controlled trials of benzodiazepines in generalized anxiety disorder: systematic review and meta-analysis. J Psychopharmacol. 2019;33:543-547. doi: 10.1177/0269881118822146
- Ashton CH. Benzodiazepine withdrawal: outcome in 50 patients. Br J Addict. 1987;82:655-671. Accessed February 22, 2022. www.benzo.org.uk/ashbzoc.htm
- Rickels K, Schweizer E, Case WG, et al. Long-term therapeutic use of benzodiazepines. I. Effects of abrupt discontinuation. Arch Gen Psychiatry. 1990;47:899-907. doi: 10.1001/archpsyc.1990.01810220015002
- Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16:77-84. Accessed March 17, 2022. www.wfsbp.org/fileadmin/user_upload/Treatment_Guidelines/Bandelow_et_al_01.pdf
- Imai H, Tajika A, Chen P, et al. Psychological therapies versus pharmacological interventions for panic disorder with or without agoraphobia in adults. Cochrane Database Syst Rev. 2016;10:CD011170. doi: 10.1002/14651858.CD011170.pub2
- van Dis EAM, van Veen SC, Hagenaars MA, et al. Long-term outcomes of cognitive behavioral therapy for anxiety-related disorders. A systematic review and meta-analysis. JAMA Psychiatry. 2020;77:265-273. doi:10.1001/jamapsychiatry.2019.3986
- Chen LH, Hedegaard H, Warner M. Drug-poisoning deaths Involving opioid analgesics: United States, 1999-2011. NCHS Data Brief. 2014;(166):1-8. Accessed March 17, 2022. www.cdc.gov/nchs/data/databriefs/db166.pdf
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013:550-555.
- Marriott S, Tyrer P. Benzodiazepine dependence: avoidance and withdrawal. Drug Safety. 1993;9:93-103. doi: 10.2165/00002018-199309020-00003
- Griffiths RR, Evans SM, Guarino JJ, et al. Intravenous flumazenil following acute and repeated exposure to lorazepam in healthy volunteers: antagonism and precipitated withdrawal. J Pharmacol Exp Ther. 1993;265:1163-1174.
- Ashton H. Benzodiazepine withdrawal: an unfinished story. Br Med J. 1984;288:1135-1140. Accessed March 17, 2022. www.ncbi.nlm.nih.gov/pmc/articles/PMC1441411/pdf/bmjcred00496-0031.pdf
- Lugoboni F, Quaglio G. Exploring the dark side of the moon: the treatment of benzodiazepine tolerance. Br J Clin Pharmacol. 2014;77:239-41. doi: 10.1111/bcp.12148
- Ashton CH. Protracted withdrawal from benzodiazepines: the post-withdrawal syndrome. Psychiatr Ann. 1995;25:174-179. Accessed March 17, 2022. https://benzo.org.uk/pha-1.htm
- Fixsen AM, Ridge D. Stories of hell and healing: internet users’ construction of benzodiazepine distress and withdrawal. Qual Health Res. 2017;27:2030-2041. doi: 10.1177/1049732317728053
- Ng BJ, Le Couteur DG, Hilmer SN. Deprescribing benzodiazepines in older patients: impact of interventions targeting physicians, pharmacists, and patients. Drugs Aging. 2018;35:493-521. doi: 10.1007/s40266-018-0544-4
- Lynch T, Ryan C, Hughes CM, et al. Brief interventions targeting long-term benzodiazepine and Z-drug use in primary care: a systematic review and meta-analysis. Addiction. 2020;115:1618-1639. doi: 10.1111/add.14981
- Darker CD, Sweeney BP, Barry JM, et al. Psychosocial interventions for benzodiazepine harmful use, abuse or dependence. Cochrane Database Syst Rev. 2015;(5):CD009652. doi: 10.1002/14651858.CD009652.pub2
- Hu X. Benzodiazepine withdrawal seizures and management. J Okla State Med Assoc. 2011;104:62-65.
- Wright SL. Benzodiazepine withdrawal: clinical aspects. In Peppin J, Raffa R, Pergolizzi J, Wright SL, eds. The Benzodiazepines Crisis: The Ramifications of an Overused Drug Class. Oxford University Press. 2020:117-148.
Benzodiazepines (BZDs) and Z-hypnotics have been available for decades, yet uncertainties about their use remain. They are prescribed and overprescribed most often for anxiety and insomnia, for which they have value but also the potential for significant adverse consequences, notably physiologic dependence. Use of these agents should be limited, and planned deprescribing is a fundamental aspect of prescribing.
A brief history. BZDs are a subset of benzodiazepine receptor agonists (BZRAs), which enhance the inhibitory effect of centrally acting γ-amino butyric acid (GABA) at the GABAA receptor through allosteric modulation. In 1960, the first BZD, chlordiazepoxide, was marketed for clinical use, and as other agents in the class became available, BZDs supplanted the more toxic barbiturates, another BZRA subset (TABLE 1). By the late 1970s, BZDs had risen to the top of most prescribed medications, with one agent in particular—diazepam (Valium)—earning a reputation as “mother’s little helper,” a phrase derived from a Rolling Stones' song with that title produced in 1966.1

With recognition of the problems associated with BZDs, their popularity diminished somewhat but remained high. BZDs were listed under Schedule IV by the Drug Enforcement Administration in 1975 due to the risk for addiction, and on the American Geriatrics Society Beers Criteria list in 1991 because of significant adverse consequences in the elderly. Researchers began to question their use as early as the 1970s, and the landmark Ashton Manual, guidance for patients and clinicians alike, was published in 2002.2
Currently, there are 14 BZDs approved by the Food and Drug Administration (FDA) as well as 3 Z-hypnotics, termed such as they include the letter “z” in their generic names (TABLE 1). In recent years, BZD prescribing has risen; a 2019 study found that 1 of 8 American adults reported using a BZD in the previous year.3
Limited benefits of benzodiazepine receptor agonists
BZRAs can be of benefit in a limited range of medical conditions, including some for which they are first-line considerations. (See TABLE 2 for a list of indications for BZDs.) They are most often prescribed for anxiety and insomnia, although they are not first-line treatments for these conditions and should be prescribed only when symptoms limit a patient’s daily functioning.

BZRAs are not intended for long-term use. In recent decades, the percentage of patients prescribed BZRAs has doubled, and more than 80% of these patients indicate usage for more than 6 months.4 Evidence, however, does not support long-term daily use.
Observation periods in most studies are far shorter than the number of years over which BZDs are actually prescribed, and flawed research methodology has introduced the risk of bias. Specifically, the generalizability of reported outcomes must be qualified, since efficacy trials performed under ideal study conditions (eg, exclusion criteria to minimize confounders) differ from circumstances seen in clinical practice. Conclusions are also limited by the inherent bias of pharmaceutical industry sponsorship and unavailability of unpublished trials that may have demonstrated unfavorable results.
Continue to: Insomnia
Insomnia, a current (past 30 days) complaint in more than 40% of US adults, is associated with a variety of symptoms.5 About 20% of adults have an insomnia disorder, defined as a predominant problem for at least 1 month involving sleep initiation, maintenance, or nonrestorative sleep along with daytime function-limiting fatigue.5 Meta-analyses indicate BZRAs can reduce sleep latency (BZDs, by 4 minutes; Z-hypnotics, 22 minutes) and may increase sleep duration (BZDs, 62 minutes per limited data; Z-hypnotics, data insufficient).6,7 Definitive evidence for long-term (> 2-4 weeks) BZD benefit is lacking, and cognitive behavioral therapy for insomnia (CBT-I) is well established as first-line treatment yielding improvements that may last at least 18 months after completion of therapy. 8,9
Although CBT-I is generally provided by behavioral health specialists, elements of CBT-I and sleep hygiene measures can be effectively used by primary care clinicians.10 Data indicate other nonpharmacologic interventions are also effective,11 including acceptance and commitment therapy,12 meditation,13 and acupuncture.14
Episodic fear and anxiety are universal and essential for survival. Fear is an alarm warning of an immediate hazard. Anxiety (the emotion) paired with worry (the thought) relate to a perceived future threat. Transient (state) anxiety should not be suppressed altogether if self-management can curb its intensity and thereby allow effective problem engagement. However, when individuals are incapacitated by crisis anxiety or sporadic specific phobias such as flight anxiety, episodic BZDs do have a role.
Ongoing anxiety is a more complex treatment situation. Obsessive-compulsive disorder and posttraumatic stress disorder are no longer categorized as anxiety disorders, but they often involve anxiety. Here, BZDs have no indication aside from exceptional acute crisis presentations. Anxiety disorders are defined by a core persistent (trait) anxiety disproportionate to the actual threat, limited daily functioning, and more than 6 months’ duration. One of 3 Americans older than 13 years meet the criteria for anxiety in their lifetime; 1 of 5 meet the criteria in any single year.15
BZDs are effective in treating anxiety disorders in the short term (2-4 weeks)2,16,17; however, benefit may fade over time.18-21 For some individuals, data suggest BZDs themselves might actually generate anxiety, as evidenced by reduced symptom intensity following discontinuation.22,23 Recommended first-line medications for anxiety disorders include certain antidepressants and pregabalin, which exhibit efficacy similar to that of BZDs.24 Mindfulness and various psychotherapies have value, as well.16 Among the latter, CBT is considered first line with benefit comparable to BZDs in the short term; yet unlike BZDs, CBT gains can last 12 months or longer after the conclusion of therapy. 25,26 Because there may be a delay between the start of CBT and the onset of benefit, BZDs, which work quickly, may be used to bridge functionally impaired patients in the short term.
Continue to: Risks with benzodiazepine receptor agonists
Risks with benzodiazepine receptor agonists
Harms from BZRA use are common, tempering their utility. Sedation, dyscognition, and psychomotor impairments are often seen upon initiation of BZRA use. These adverse effects can—although not always—improve with continuous BZRA exposure, an effect known as tolerance, which is due to neuropharmacologic adaptation.
Cognitive issues include problems with memory, judgment, and decision making. These may be unrecognized or, if noted, attributed to other issues such as aging, and may become clear only when BZRAs are discontinued. Anterograde amnesia and parasomnias occur less often.
Psychomotor impairment can result in falls, fractures, and other injuries, especially in the elderly. Decrements in mood, including emergent depression and paradoxical anxiety, can occur. Some individuals experience disinhibition that is expressed through irritability, agitation, aggression, and violence.
Misuse of BZRAs is not unusual and can be related to dosing errors or attempts to ease intrusive symptoms. Nonmedical use almost always occurs in the context of an underlying use disorder, whereby BZRAs serve to amplify euphoria or ameliorate withdrawal from opioids or alcohol. Addiction per se, which entails BZRA craving and compulsive use leading to adverse consequences, is unusual.
BZRAs are associated with increased mortality, including all-cause mortality and suicide. They are respiratory depressants, although when taken alone in excess rarely result in death. They are, however, strongly implicated in opioid-related overdose fatalities, as their presence has been identified in 1 of 3 such decedents.27
Continue to: Physiologic dependence with BZRAs
Physiologic dependence with BZRAs
Among the more important adverse outcomes with ongoing BZRA exposure is physiologic dependence. This occurs primarily because of neuroadaptation of GABAA and glutaminergic receptors, but dependence probably also involves changes in the adenosine A2A, serotonergic, and peripheral benzodiazepine receptors, the latter being present on mitochondrial membranes. The hypothalamic-pituitary-adrenal axis also appears to be involved.
Physiologic dependence is expressed through BZRA tolerance and characteristic physical and psychological symptoms upon withdrawal. Tolerance refers to a reduced effect with continued substance exposure or the need for an increased dose to get the same effect. Drug withdrawal can result in manifestations distinctive to addiction-prone substances, as well as to some medications without addiction liability, such as corticosteroids and antidepressants. Tolerance and withdrawal are not applicable criteria in the diagnosis of sedative-hypnotic use disorder when BZRAs are prescribed.28
Withdrawal. Reported prevalence of BZRA physiologic dependence differs according to populations studied, criteria used, and the deprescribing process employed. Some researchers have found rates of one-third and others exceeding one-half among individuals using BZRAs for longer than a month.23,29
Deprescribing BZRAs
Because benefits are limited and adverse outcomes including physiologic dependence are common, it is recommended that clinicians urge deprescribing of BZRAs for any patient taking them consistently for more than 1 month. Published deprescribing investigations and guidance are insufficient, heterogenous, and confusing. Still, some approaches can work well, and success rates as high as 80% have been achieved among the elderly, for example.35 Brief interventions such as providing individualized advice, support, and management are effective.36,37 Abrupt discontinuation is inappropriate and can be life threatening.38 Forced cessation is also inappropriate unless significant respiratory depression is identified.
The Ashton Manual is a useful guide, readable by patients. Proceed with tapering slowly at a rate led by the patient’s response.2,39 Avoid discrediting patients’ reports of unusual withdrawal symptoms, as this can lead to misdiagnosis (eg, somatic symptom disorder) or ineffective treatment (eg, addiction recovery approaches). Adding CBT to tapering improves outcomes, and adjunctive medications may be helpful, although not without their own problems.29 Consistent support of patients by others involved in treatment (prescriber, pharmacist, behavioral health specialists, peer coach, significant others) is essential. Complex challenges generally resolve through authentic listening and response but may require referral to others with necessary skills and experience. Complete cessation may take 12 to 18 months (or longer). Even if complete cessation is not possible, the least dose necessary can be achieved.
CORRESPONDENCE
Steven Wright, MD, 1975 Ashland Mine Road, Ashland, OR 97520; [email protected]
Benzodiazepines (BZDs) and Z-hypnotics have been available for decades, yet uncertainties about their use remain. They are prescribed and overprescribed most often for anxiety and insomnia, for which they have value but also the potential for significant adverse consequences, notably physiologic dependence. Use of these agents should be limited, and planned deprescribing is a fundamental aspect of prescribing.
A brief history. BZDs are a subset of benzodiazepine receptor agonists (BZRAs), which enhance the inhibitory effect of centrally acting γ-amino butyric acid (GABA) at the GABAA receptor through allosteric modulation. In 1960, the first BZD, chlordiazepoxide, was marketed for clinical use, and as other agents in the class became available, BZDs supplanted the more toxic barbiturates, another BZRA subset (TABLE 1). By the late 1970s, BZDs had risen to the top of most prescribed medications, with one agent in particular—diazepam (Valium)—earning a reputation as “mother’s little helper,” a phrase derived from a Rolling Stones' song with that title produced in 1966.1

With recognition of the problems associated with BZDs, their popularity diminished somewhat but remained high. BZDs were listed under Schedule IV by the Drug Enforcement Administration in 1975 due to the risk for addiction, and on the American Geriatrics Society Beers Criteria list in 1991 because of significant adverse consequences in the elderly. Researchers began to question their use as early as the 1970s, and the landmark Ashton Manual, guidance for patients and clinicians alike, was published in 2002.2
Currently, there are 14 BZDs approved by the Food and Drug Administration (FDA) as well as 3 Z-hypnotics, termed such as they include the letter “z” in their generic names (TABLE 1). In recent years, BZD prescribing has risen; a 2019 study found that 1 of 8 American adults reported using a BZD in the previous year.3
Limited benefits of benzodiazepine receptor agonists
BZRAs can be of benefit in a limited range of medical conditions, including some for which they are first-line considerations. (See TABLE 2 for a list of indications for BZDs.) They are most often prescribed for anxiety and insomnia, although they are not first-line treatments for these conditions and should be prescribed only when symptoms limit a patient’s daily functioning.

BZRAs are not intended for long-term use. In recent decades, the percentage of patients prescribed BZRAs has doubled, and more than 80% of these patients indicate usage for more than 6 months.4 Evidence, however, does not support long-term daily use.
Observation periods in most studies are far shorter than the number of years over which BZDs are actually prescribed, and flawed research methodology has introduced the risk of bias. Specifically, the generalizability of reported outcomes must be qualified, since efficacy trials performed under ideal study conditions (eg, exclusion criteria to minimize confounders) differ from circumstances seen in clinical practice. Conclusions are also limited by the inherent bias of pharmaceutical industry sponsorship and unavailability of unpublished trials that may have demonstrated unfavorable results.
Continue to: Insomnia
Insomnia, a current (past 30 days) complaint in more than 40% of US adults, is associated with a variety of symptoms.5 About 20% of adults have an insomnia disorder, defined as a predominant problem for at least 1 month involving sleep initiation, maintenance, or nonrestorative sleep along with daytime function-limiting fatigue.5 Meta-analyses indicate BZRAs can reduce sleep latency (BZDs, by 4 minutes; Z-hypnotics, 22 minutes) and may increase sleep duration (BZDs, 62 minutes per limited data; Z-hypnotics, data insufficient).6,7 Definitive evidence for long-term (> 2-4 weeks) BZD benefit is lacking, and cognitive behavioral therapy for insomnia (CBT-I) is well established as first-line treatment yielding improvements that may last at least 18 months after completion of therapy. 8,9
Although CBT-I is generally provided by behavioral health specialists, elements of CBT-I and sleep hygiene measures can be effectively used by primary care clinicians.10 Data indicate other nonpharmacologic interventions are also effective,11 including acceptance and commitment therapy,12 meditation,13 and acupuncture.14
Episodic fear and anxiety are universal and essential for survival. Fear is an alarm warning of an immediate hazard. Anxiety (the emotion) paired with worry (the thought) relate to a perceived future threat. Transient (state) anxiety should not be suppressed altogether if self-management can curb its intensity and thereby allow effective problem engagement. However, when individuals are incapacitated by crisis anxiety or sporadic specific phobias such as flight anxiety, episodic BZDs do have a role.
Ongoing anxiety is a more complex treatment situation. Obsessive-compulsive disorder and posttraumatic stress disorder are no longer categorized as anxiety disorders, but they often involve anxiety. Here, BZDs have no indication aside from exceptional acute crisis presentations. Anxiety disorders are defined by a core persistent (trait) anxiety disproportionate to the actual threat, limited daily functioning, and more than 6 months’ duration. One of 3 Americans older than 13 years meet the criteria for anxiety in their lifetime; 1 of 5 meet the criteria in any single year.15
BZDs are effective in treating anxiety disorders in the short term (2-4 weeks)2,16,17; however, benefit may fade over time.18-21 For some individuals, data suggest BZDs themselves might actually generate anxiety, as evidenced by reduced symptom intensity following discontinuation.22,23 Recommended first-line medications for anxiety disorders include certain antidepressants and pregabalin, which exhibit efficacy similar to that of BZDs.24 Mindfulness and various psychotherapies have value, as well.16 Among the latter, CBT is considered first line with benefit comparable to BZDs in the short term; yet unlike BZDs, CBT gains can last 12 months or longer after the conclusion of therapy. 25,26 Because there may be a delay between the start of CBT and the onset of benefit, BZDs, which work quickly, may be used to bridge functionally impaired patients in the short term.
Continue to: Risks with benzodiazepine receptor agonists
Risks with benzodiazepine receptor agonists
Harms from BZRA use are common, tempering their utility. Sedation, dyscognition, and psychomotor impairments are often seen upon initiation of BZRA use. These adverse effects can—although not always—improve with continuous BZRA exposure, an effect known as tolerance, which is due to neuropharmacologic adaptation.
Cognitive issues include problems with memory, judgment, and decision making. These may be unrecognized or, if noted, attributed to other issues such as aging, and may become clear only when BZRAs are discontinued. Anterograde amnesia and parasomnias occur less often.
Psychomotor impairment can result in falls, fractures, and other injuries, especially in the elderly. Decrements in mood, including emergent depression and paradoxical anxiety, can occur. Some individuals experience disinhibition that is expressed through irritability, agitation, aggression, and violence.
Misuse of BZRAs is not unusual and can be related to dosing errors or attempts to ease intrusive symptoms. Nonmedical use almost always occurs in the context of an underlying use disorder, whereby BZRAs serve to amplify euphoria or ameliorate withdrawal from opioids or alcohol. Addiction per se, which entails BZRA craving and compulsive use leading to adverse consequences, is unusual.
BZRAs are associated with increased mortality, including all-cause mortality and suicide. They are respiratory depressants, although when taken alone in excess rarely result in death. They are, however, strongly implicated in opioid-related overdose fatalities, as their presence has been identified in 1 of 3 such decedents.27
Continue to: Physiologic dependence with BZRAs
Physiologic dependence with BZRAs
Among the more important adverse outcomes with ongoing BZRA exposure is physiologic dependence. This occurs primarily because of neuroadaptation of GABAA and glutaminergic receptors, but dependence probably also involves changes in the adenosine A2A, serotonergic, and peripheral benzodiazepine receptors, the latter being present on mitochondrial membranes. The hypothalamic-pituitary-adrenal axis also appears to be involved.
Physiologic dependence is expressed through BZRA tolerance and characteristic physical and psychological symptoms upon withdrawal. Tolerance refers to a reduced effect with continued substance exposure or the need for an increased dose to get the same effect. Drug withdrawal can result in manifestations distinctive to addiction-prone substances, as well as to some medications without addiction liability, such as corticosteroids and antidepressants. Tolerance and withdrawal are not applicable criteria in the diagnosis of sedative-hypnotic use disorder when BZRAs are prescribed.28
Withdrawal. Reported prevalence of BZRA physiologic dependence differs according to populations studied, criteria used, and the deprescribing process employed. Some researchers have found rates of one-third and others exceeding one-half among individuals using BZRAs for longer than a month.23,29
Deprescribing BZRAs
Because benefits are limited and adverse outcomes including physiologic dependence are common, it is recommended that clinicians urge deprescribing of BZRAs for any patient taking them consistently for more than 1 month. Published deprescribing investigations and guidance are insufficient, heterogenous, and confusing. Still, some approaches can work well, and success rates as high as 80% have been achieved among the elderly, for example.35 Brief interventions such as providing individualized advice, support, and management are effective.36,37 Abrupt discontinuation is inappropriate and can be life threatening.38 Forced cessation is also inappropriate unless significant respiratory depression is identified.
The Ashton Manual is a useful guide, readable by patients. Proceed with tapering slowly at a rate led by the patient’s response.2,39 Avoid discrediting patients’ reports of unusual withdrawal symptoms, as this can lead to misdiagnosis (eg, somatic symptom disorder) or ineffective treatment (eg, addiction recovery approaches). Adding CBT to tapering improves outcomes, and adjunctive medications may be helpful, although not without their own problems.29 Consistent support of patients by others involved in treatment (prescriber, pharmacist, behavioral health specialists, peer coach, significant others) is essential. Complex challenges generally resolve through authentic listening and response but may require referral to others with necessary skills and experience. Complete cessation may take 12 to 18 months (or longer). Even if complete cessation is not possible, the least dose necessary can be achieved.
CORRESPONDENCE
Steven Wright, MD, 1975 Ashland Mine Road, Ashland, OR 97520; [email protected]
- Chandler A, Whittaker A, Williams N, et al. Mother’s little helper? Contrasting accounts of benzodiazepine and methadone use among drug-dependent parents in the UK. Drugs (Abingdon Engl). 2014;21:470-475. doi: 10.3109/09687637.2014.930814Ash
- ton CH. Benzodiazepines: How They Work & How to Withdraw (the Ashton Manual). 2002. Accessed March 17, 2022. www.benzoinfo.com/ashtonmanual/
- Maust DT, Lin LA, Blow FC. Benzodiazepine use and misuse among adults in the United States. Psychiatr Serv. 2019;70:97-106. doi: 10.1176/appi.ps.201800321
- Kaufmann CN, Spira AP, Depp CA, et al. Long-term use of benzodiazepines and non-benzodiazepine hypnotics from 1999 to 2014: results from the National Health and Nutrition Examination Survey. Psychiatr Serv. 2018;69:235-238. doi: 10.1176/appi.ps.201700095
- Walsh JK, Coulouvrat C, Hajak G, et al. Nighttime insomnia symptoms and perceived health in the America Insomnia Survey (AIS). Sleep. 2011;34:997-1011. doi: 10.5665/SLEEP.1150
- Holbrook AM, Crowther R, Lotter A, et al. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162:225-233.
- Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343
- Curran HV, Collins R, Fletcher S, et al. Older adults and withdrawal from benzodiazepine hypnotics in general practice: effects on cognitive function, sleep, mood and quality of life. Psychol Med. 2003;33:1223-1237. doi: 10.1017/s0033291703008213
- Geiger-Brown JM, Rogers VE, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54-67. doi: 10.1016/j.smrv.2014.11.007
- Sorscher AJ. Insomnia: getting to the cause, facilitating relief. J Fam Pract. 2017;66:216-225
- Laura Hrehová L, Mezian K. Non-pharmacologic treatment of insomnia in primary care settings. Int J Clin Pract. 2021;75:e14084. doi: 10.1111/ijcp.14084.
- Daly-Eichenhardt A, Scott W, Howard-Jones M, et al. Changes in sleep problems and psychological flexibility following interdisciplinary acceptance and commitment therapy for chronic pain: an observational cohort study. Front Psychol. 2016;7:1326. doi: 10.3389/fpsyg.2016.01326
- Rusch HL, Rosario M, Levison LM, et al. The effect of mindfulness meditation on sleep quality: a systematic review and meta-analysis of randomized controlled trials. Ann N Y Acad Sci. 2019;1445:5-16. doi: 10.1111/nyas.13996
- Cao H, Pan X, Li H, et al. Acupuncture for treatment of insomnia: a systematic review of randomized controlled trials. J Altern Complement Med. 2009;15:1171-1186. doi: 10.1089/acm.2009.0041
- Kessler RC, Petukhova M, Sampson NA, et al. Twelve‐month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169-184. doi: 10.1002/mpr.1359
- Bandelow B, Reitt M, Röver C, et al. Efficacy of treatments for anxiety disorders: a meta-analysis. Int Clin Psychopharmacol. 2015;30:183-192. doi: 10.1097/YIC.0000000000000078
- Lader M. Benzodiazepines revisited—will we ever learn? Addiction. 2011;106:2086-2109. doi: 10.1111/j.1360-0443.2011.03563.x
- Fava GA. Fading of therapeutic effects of alprazolam in agoraphobia. Case reports. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:109-112. doi: 10.1016/0278-5846(88)90066-8
- Fava GA, Grandi S, Belluardo P, et al. Benzodiazepines and anxiety sensitivity in panic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:1163-1168. doi: 10.1016/0278-5846(94)90118-x
- Pélissolo A, Maniere F, Boutges B, et al. Anxiety and depressive disorders in 4,425 long term benzodiazepine users in general practice. Encephale. 2007;33:32-38. doi: 10.1016/s0013-7006(07)91556-0
- Gale C, Glue P, Guaiana G, et al. Influence of covariates on heterogeneity in Hamilton Anxiety Scale ratings in placebo-controlled trials of benzodiazepines in generalized anxiety disorder: systematic review and meta-analysis. J Psychopharmacol. 2019;33:543-547. doi: 10.1177/0269881118822146
- Ashton CH. Benzodiazepine withdrawal: outcome in 50 patients. Br J Addict. 1987;82:655-671. Accessed February 22, 2022. www.benzo.org.uk/ashbzoc.htm
- Rickels K, Schweizer E, Case WG, et al. Long-term therapeutic use of benzodiazepines. I. Effects of abrupt discontinuation. Arch Gen Psychiatry. 1990;47:899-907. doi: 10.1001/archpsyc.1990.01810220015002
- Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16:77-84. Accessed March 17, 2022. www.wfsbp.org/fileadmin/user_upload/Treatment_Guidelines/Bandelow_et_al_01.pdf
- Imai H, Tajika A, Chen P, et al. Psychological therapies versus pharmacological interventions for panic disorder with or without agoraphobia in adults. Cochrane Database Syst Rev. 2016;10:CD011170. doi: 10.1002/14651858.CD011170.pub2
- van Dis EAM, van Veen SC, Hagenaars MA, et al. Long-term outcomes of cognitive behavioral therapy for anxiety-related disorders. A systematic review and meta-analysis. JAMA Psychiatry. 2020;77:265-273. doi:10.1001/jamapsychiatry.2019.3986
- Chen LH, Hedegaard H, Warner M. Drug-poisoning deaths Involving opioid analgesics: United States, 1999-2011. NCHS Data Brief. 2014;(166):1-8. Accessed March 17, 2022. www.cdc.gov/nchs/data/databriefs/db166.pdf
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013:550-555.
- Marriott S, Tyrer P. Benzodiazepine dependence: avoidance and withdrawal. Drug Safety. 1993;9:93-103. doi: 10.2165/00002018-199309020-00003
- Griffiths RR, Evans SM, Guarino JJ, et al. Intravenous flumazenil following acute and repeated exposure to lorazepam in healthy volunteers: antagonism and precipitated withdrawal. J Pharmacol Exp Ther. 1993;265:1163-1174.
- Ashton H. Benzodiazepine withdrawal: an unfinished story. Br Med J. 1984;288:1135-1140. Accessed March 17, 2022. www.ncbi.nlm.nih.gov/pmc/articles/PMC1441411/pdf/bmjcred00496-0031.pdf
- Lugoboni F, Quaglio G. Exploring the dark side of the moon: the treatment of benzodiazepine tolerance. Br J Clin Pharmacol. 2014;77:239-41. doi: 10.1111/bcp.12148
- Ashton CH. Protracted withdrawal from benzodiazepines: the post-withdrawal syndrome. Psychiatr Ann. 1995;25:174-179. Accessed March 17, 2022. https://benzo.org.uk/pha-1.htm
- Fixsen AM, Ridge D. Stories of hell and healing: internet users’ construction of benzodiazepine distress and withdrawal. Qual Health Res. 2017;27:2030-2041. doi: 10.1177/1049732317728053
- Ng BJ, Le Couteur DG, Hilmer SN. Deprescribing benzodiazepines in older patients: impact of interventions targeting physicians, pharmacists, and patients. Drugs Aging. 2018;35:493-521. doi: 10.1007/s40266-018-0544-4
- Lynch T, Ryan C, Hughes CM, et al. Brief interventions targeting long-term benzodiazepine and Z-drug use in primary care: a systematic review and meta-analysis. Addiction. 2020;115:1618-1639. doi: 10.1111/add.14981
- Darker CD, Sweeney BP, Barry JM, et al. Psychosocial interventions for benzodiazepine harmful use, abuse or dependence. Cochrane Database Syst Rev. 2015;(5):CD009652. doi: 10.1002/14651858.CD009652.pub2
- Hu X. Benzodiazepine withdrawal seizures and management. J Okla State Med Assoc. 2011;104:62-65.
- Wright SL. Benzodiazepine withdrawal: clinical aspects. In Peppin J, Raffa R, Pergolizzi J, Wright SL, eds. The Benzodiazepines Crisis: The Ramifications of an Overused Drug Class. Oxford University Press. 2020:117-148.
- Chandler A, Whittaker A, Williams N, et al. Mother’s little helper? Contrasting accounts of benzodiazepine and methadone use among drug-dependent parents in the UK. Drugs (Abingdon Engl). 2014;21:470-475. doi: 10.3109/09687637.2014.930814Ash
- ton CH. Benzodiazepines: How They Work & How to Withdraw (the Ashton Manual). 2002. Accessed March 17, 2022. www.benzoinfo.com/ashtonmanual/
- Maust DT, Lin LA, Blow FC. Benzodiazepine use and misuse among adults in the United States. Psychiatr Serv. 2019;70:97-106. doi: 10.1176/appi.ps.201800321
- Kaufmann CN, Spira AP, Depp CA, et al. Long-term use of benzodiazepines and non-benzodiazepine hypnotics from 1999 to 2014: results from the National Health and Nutrition Examination Survey. Psychiatr Serv. 2018;69:235-238. doi: 10.1176/appi.ps.201700095
- Walsh JK, Coulouvrat C, Hajak G, et al. Nighttime insomnia symptoms and perceived health in the America Insomnia Survey (AIS). Sleep. 2011;34:997-1011. doi: 10.5665/SLEEP.1150
- Holbrook AM, Crowther R, Lotter A, et al. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162:225-233.
- Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343
- Curran HV, Collins R, Fletcher S, et al. Older adults and withdrawal from benzodiazepine hypnotics in general practice: effects on cognitive function, sleep, mood and quality of life. Psychol Med. 2003;33:1223-1237. doi: 10.1017/s0033291703008213
- Geiger-Brown JM, Rogers VE, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54-67. doi: 10.1016/j.smrv.2014.11.007
- Sorscher AJ. Insomnia: getting to the cause, facilitating relief. J Fam Pract. 2017;66:216-225
- Laura Hrehová L, Mezian K. Non-pharmacologic treatment of insomnia in primary care settings. Int J Clin Pract. 2021;75:e14084. doi: 10.1111/ijcp.14084.
- Daly-Eichenhardt A, Scott W, Howard-Jones M, et al. Changes in sleep problems and psychological flexibility following interdisciplinary acceptance and commitment therapy for chronic pain: an observational cohort study. Front Psychol. 2016;7:1326. doi: 10.3389/fpsyg.2016.01326
- Rusch HL, Rosario M, Levison LM, et al. The effect of mindfulness meditation on sleep quality: a systematic review and meta-analysis of randomized controlled trials. Ann N Y Acad Sci. 2019;1445:5-16. doi: 10.1111/nyas.13996
- Cao H, Pan X, Li H, et al. Acupuncture for treatment of insomnia: a systematic review of randomized controlled trials. J Altern Complement Med. 2009;15:1171-1186. doi: 10.1089/acm.2009.0041
- Kessler RC, Petukhova M, Sampson NA, et al. Twelve‐month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169-184. doi: 10.1002/mpr.1359
- Bandelow B, Reitt M, Röver C, et al. Efficacy of treatments for anxiety disorders: a meta-analysis. Int Clin Psychopharmacol. 2015;30:183-192. doi: 10.1097/YIC.0000000000000078
- Lader M. Benzodiazepines revisited—will we ever learn? Addiction. 2011;106:2086-2109. doi: 10.1111/j.1360-0443.2011.03563.x
- Fava GA. Fading of therapeutic effects of alprazolam in agoraphobia. Case reports. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:109-112. doi: 10.1016/0278-5846(88)90066-8
- Fava GA, Grandi S, Belluardo P, et al. Benzodiazepines and anxiety sensitivity in panic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:1163-1168. doi: 10.1016/0278-5846(94)90118-x
- Pélissolo A, Maniere F, Boutges B, et al. Anxiety and depressive disorders in 4,425 long term benzodiazepine users in general practice. Encephale. 2007;33:32-38. doi: 10.1016/s0013-7006(07)91556-0
- Gale C, Glue P, Guaiana G, et al. Influence of covariates on heterogeneity in Hamilton Anxiety Scale ratings in placebo-controlled trials of benzodiazepines in generalized anxiety disorder: systematic review and meta-analysis. J Psychopharmacol. 2019;33:543-547. doi: 10.1177/0269881118822146
- Ashton CH. Benzodiazepine withdrawal: outcome in 50 patients. Br J Addict. 1987;82:655-671. Accessed February 22, 2022. www.benzo.org.uk/ashbzoc.htm
- Rickels K, Schweizer E, Case WG, et al. Long-term therapeutic use of benzodiazepines. I. Effects of abrupt discontinuation. Arch Gen Psychiatry. 1990;47:899-907. doi: 10.1001/archpsyc.1990.01810220015002
- Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16:77-84. Accessed March 17, 2022. www.wfsbp.org/fileadmin/user_upload/Treatment_Guidelines/Bandelow_et_al_01.pdf
- Imai H, Tajika A, Chen P, et al. Psychological therapies versus pharmacological interventions for panic disorder with or without agoraphobia in adults. Cochrane Database Syst Rev. 2016;10:CD011170. doi: 10.1002/14651858.CD011170.pub2
- van Dis EAM, van Veen SC, Hagenaars MA, et al. Long-term outcomes of cognitive behavioral therapy for anxiety-related disorders. A systematic review and meta-analysis. JAMA Psychiatry. 2020;77:265-273. doi:10.1001/jamapsychiatry.2019.3986
- Chen LH, Hedegaard H, Warner M. Drug-poisoning deaths Involving opioid analgesics: United States, 1999-2011. NCHS Data Brief. 2014;(166):1-8. Accessed March 17, 2022. www.cdc.gov/nchs/data/databriefs/db166.pdf
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013:550-555.
- Marriott S, Tyrer P. Benzodiazepine dependence: avoidance and withdrawal. Drug Safety. 1993;9:93-103. doi: 10.2165/00002018-199309020-00003
- Griffiths RR, Evans SM, Guarino JJ, et al. Intravenous flumazenil following acute and repeated exposure to lorazepam in healthy volunteers: antagonism and precipitated withdrawal. J Pharmacol Exp Ther. 1993;265:1163-1174.
- Ashton H. Benzodiazepine withdrawal: an unfinished story. Br Med J. 1984;288:1135-1140. Accessed March 17, 2022. www.ncbi.nlm.nih.gov/pmc/articles/PMC1441411/pdf/bmjcred00496-0031.pdf
- Lugoboni F, Quaglio G. Exploring the dark side of the moon: the treatment of benzodiazepine tolerance. Br J Clin Pharmacol. 2014;77:239-41. doi: 10.1111/bcp.12148
- Ashton CH. Protracted withdrawal from benzodiazepines: the post-withdrawal syndrome. Psychiatr Ann. 1995;25:174-179. Accessed March 17, 2022. https://benzo.org.uk/pha-1.htm
- Fixsen AM, Ridge D. Stories of hell and healing: internet users’ construction of benzodiazepine distress and withdrawal. Qual Health Res. 2017;27:2030-2041. doi: 10.1177/1049732317728053
- Ng BJ, Le Couteur DG, Hilmer SN. Deprescribing benzodiazepines in older patients: impact of interventions targeting physicians, pharmacists, and patients. Drugs Aging. 2018;35:493-521. doi: 10.1007/s40266-018-0544-4
- Lynch T, Ryan C, Hughes CM, et al. Brief interventions targeting long-term benzodiazepine and Z-drug use in primary care: a systematic review and meta-analysis. Addiction. 2020;115:1618-1639. doi: 10.1111/add.14981
- Darker CD, Sweeney BP, Barry JM, et al. Psychosocial interventions for benzodiazepine harmful use, abuse or dependence. Cochrane Database Syst Rev. 2015;(5):CD009652. doi: 10.1002/14651858.CD009652.pub2
- Hu X. Benzodiazepine withdrawal seizures and management. J Okla State Med Assoc. 2011;104:62-65.
- Wright SL. Benzodiazepine withdrawal: clinical aspects. In Peppin J, Raffa R, Pergolizzi J, Wright SL, eds. The Benzodiazepines Crisis: The Ramifications of an Overused Drug Class. Oxford University Press. 2020:117-148.
PRACTICE RECOMMENDATIONS
› Recommend cognitive behavioral therapy as first-line treatment for anxiety and insomnia. A
› Limit benzodiazepine prescribing to ≤ 2 to 4 weeks for anxiety and insomnia. B
› Taper benzodiazepines slowly and flexibly. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series