User login
A guide to GERD, H pylori infection, and Barrett esophagus
Three conditions seen in primary care—gastroesophageal reflux disease (GERD), Helicobacter pylori (H pylori) infection, and Barrett esophagus (BE)—evolve in a gastric acid environment and are treated in part through gastric acid suppression. While GERD is a risk factor for the development of BE, H pylori is not associated with BE.1 Patients with H pylori are actually less likely to have GERD symptoms.2,3 In this article, we describe similarities and differences in patient presentations, diagnostic testing, and management, and review screening recommendations.
Gastroesophageal reflux disease
GERD is a clinical diagnosis based on symptoms of regurgitation and heartburn or the presence of one of its known complications (esophagitis, peptic strictures, or BE).2,4 Chest pain is also common. Atypical symptoms are dysphagia, bleeding, chronic cough, asthma, chronic laryngitis, hoarseness, wheezing, teeth erosions, belching, and bloating.2,5-7
The worldwide prevalence of gastroesophageal reflux symptoms in adults is 14.8%.8 When using a stringent definition of GERD—weekly symptoms occurring for at least 3 months—prevalence drops to 9.4%.9 GERD symptoms vary markedly by geographic location; the highest rates are in Central America (19.6%) and the lowest rates are in Southeast Asia (7.4%).8TABLE 12,9-11 lists risk factors for GERD.
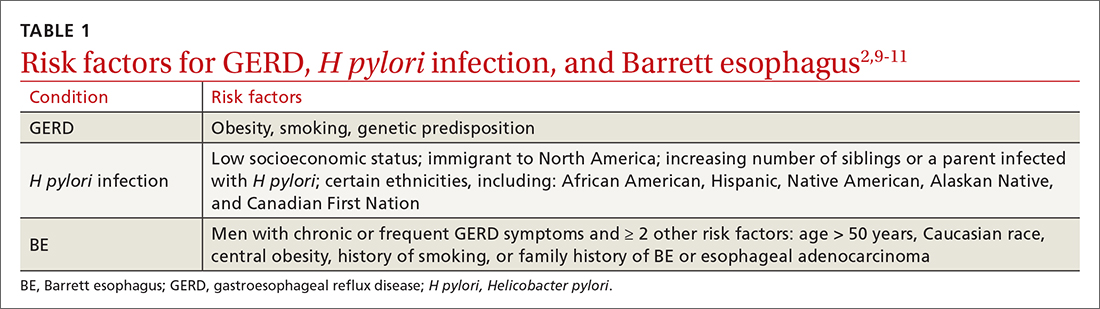
GERD results from dysfunction of the esophagogastric junction that permits regurgitation of acidic gastric contents into the esophagus. Normally, the lower esophageal sphincter (LES) relaxes temporarily with gastric distention; when this relaxation is frequent and prolonged, it causes GERD.2,12 Several medications, particularly those with anticholinergic effects (eg, tricyclic antidepressants) can decrease LES tone and contribute to symptoms. Nonsteroidal anti-inflammatory drugs (NSAIDs) are often linked to dyspepsia and gastritis and should be avoided in patients who have symptoms of GERD. Pathologic reflux can also occur in conditions that increase intra-abdominal pressure, such as obesity and pregnancy, and with esophageal dysmotility, hiatal hernia, and delayed gastric emptying.5 When gastric contents travel proximally, this contributes to extraesophageal symptoms, such as chronic cough, asthma, laryngitis, dyspepsia, bloating, and belching.2,4
Treatment
Proton pump inhibitors (PPIs) are the most effective treatment for GERD, but lifestyle modifications are also recommended for all patients.2,6,13-16 Consider selective elimination of beverages and foods that are commonly associated with heartburn (eg, alcohol, caffeine, chocolate, citrus, and spicy foods) if patients note a correlation to symptoms.5,6,13 Also, advise weight loss and smoking cessation, as appropriate, and suggest that the patient elevate the head of their bed when sleeping.
All PPIs are equally effective in suppressing acid when given at equivalent doses (TABLE 217), so they can be used interchangeably.17 Treat uncomplicated GERD with a once-daily PPI 30 to 60 minutes prior to a meal for 4 to 8 weeks. If treatment is effective, you’ll want to try to reduce or stop the medication after the 4- to 8-week period. (It’s worth noting that the benefits of treatment for those with extraesophageal GERD are less predictable than for those with heartburn or esophagitis symptoms.5)
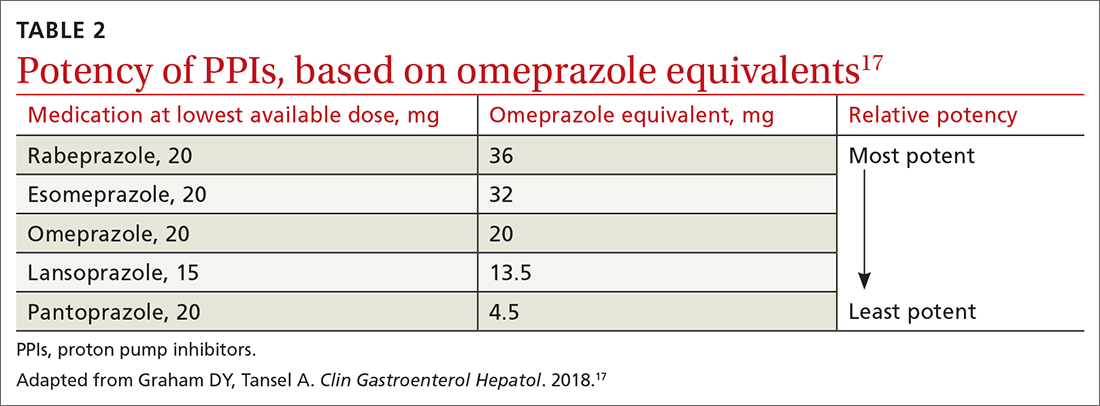
If GERD symptoms reemerge after the PPI is stopped, the medication can be restarted but should be limited to the least potent effective dose, no matter if it is taken daily or only as needed.2,6,17 In patients with esophagitis, you may need to continue PPI treatment indefinitely at the lowest possible dose given the increased risk of recurrent esophagitis.2,13,16
Continue to: Keep in mind...
Keep in mind that the safety of long-term PPI use has not been fully established. While observational studies have shown that long-term PPI use may be associated with adverse events, including kidney damage, Clostridioides difficile infection, osteoporosis, and gastric cancer, subsequent prospective studies have not shown any significant risks with long-term PPI use.2,13,14,16,18,19 If a decision is made to discontinue PPIs after long-term use, the patient should be advised that rebound acid hypersecretion may occur, although this possibility can be mitigated by gradually tapering the PPI dose.
Another maintenance therapy option. Histamine-2 receptor antagonists (H2RAs) are a reasonable alternative to PPIs as maintenance therapy, but they are ineffective in healing esophagitis6,13 and may be best used as adjunctive therapy at bedtime for breakthrough symptoms while a patient is on maintenance PPIs.6,19 Antacids (eg, calcium carbonate, aluminum hydroxide, or magnesium hydroxide) and alginate may provide some symptomatic relief, as well.
When PPIs don’t work. If initial lifestyle changes and PPI treatment do not provide adequate relief, consider the possibility of nonadherence with medication or lifestyle directives. If nonadherence does not appear to be an issue, twice-daily PPI dosing is also an option. Recognize, though, that PPI treatment failure occurs in as many as 40% of patients and is much more common in those with atypical symptoms.6
Consider upper gastrointestinal (GI) endoscopy—and perhaps esophageal manometry or pH testing—if a patient does not respond to empiric treatment with a PPI for 4 to 8 weeks at a standard, once-daily dose.2,4,13 (Alternative diagnoses may also need to be considered.) Upper endoscopy is also appropriate for patients who have symptoms concerning for malignancy (progressive dysphagia, unintentional weight loss, or bleeding).
Esophagitis detected on endoscopy confirms GERD, although it is seen in only 18% to 25% of patients with GERD symptoms.2,4 (The absence of esophagitis only indicates a lack of mucosal injury and not the absence of GERD.4) Acid exposure can cause fibrotic scarring and, in turn, strictures visible on endoscopy.2 BE, the precursor to esophageal adenocarcinoma, is also a complication of GERD and is defined by columnar metaplasia replacing the normal squamous cell esophageal epithelium; it is detected on pathology review of biopsies.2
Continue to: GERD confirmed but PPIs aren't working?
GERD confirmed but PPIs aren’t working? Laparoscopic fundoplication is an effective treatment for GERD. However, due to its adverse effects (dysphagia, bloating, flatulence) and risk of treatment failure or breakdown within 5 to 10 years, it should be reserved for those poorly managed with PPIs.2,13,19
Considerations in pregnancy. GERD is reported by 40% to 85% of pregnant women,20,21 and its clinical presentation, diagnosis, and treatment are similar to that of nonpregnant adults.21 If lifestyle modification is not effective, pharmacologic therapy may be considered. Often, lifestyle modifications and antacids followed by the addition of sucralfate will be used first given the lack of systemic effects. H2RAs can be used next based on long-term historical use and reported safety.21 As with nonpregnant patients, PPIs are more effective than other medical therapies. If PPIs are used, dexlansoprazole, lansoprazole, pantoprazole, and rabeprazole are preferred. Omeprazole and esomeprazole are typically avoided due to findings of embryonic and fetal mortality in early animal studies, although subsequent human studies have noted no teratogenicity.2,20,21
Considerations in children. As with adults, findings in the history and exam are sufficient to diagnose and initiate treatment of GERD in children, provided there are no warning signs (eg, bilious vomiting, GI bleeding, consistent forceful vomiting, fever, lethargy, hepatosplenomegaly, bulging fontanelle, macro- or microcephaly, seizures, abdominal tenderness/distention, or genetic/metabolic syndromes). Lifestyle changes are first-line treatment, followed by medication. Acid suppressants are preferred, with PPIs showing superior efficacy compared with H2RAs.15 Some PPIs (omeprazole, lansoprazole, and esomeprazole) have US Food and Drug Administration (FDA) approval beginning at age 1 year, while rabeprazole has FDA approval beginning at age 12.22 As in adults, if PPIs are ineffective, consider alternative diagnoses.15,22
Helicobacter pylori infection
H pylori is a gram-negative spiral-shaped bacterium found in the stomach of humans and other mammals. It survives the acidic environment by metabolizing urea into alkaline ammonia and carbon dioxide. H pylori infection increases the risk of peptic ulcer disease, gastric cancer, iron deficiency anemia, and immune thrombocytopenia. It may be associated with dyspepsia, increased ulcer risk with use of an NSAID, and chronic gastritis.9 Infection with H pylori can decrease the risk of GERD.2 The bacterial infection causes atrophic gastritis and subsequent hypochlorhydria, which then diminishes the acidity of the reflux contents.19 There is no link between H pylori infection and BE.1
TABLE 12,9-11 shows those at highest risk of H pylori. The estimated prevalence of infection is 40% to 48%23 worldwide but lower in North America, at 32% to 42%.24H pylori is often acquired in childhood, and risk of infection is more likely if the parents (particularly mothers) are infected.9
Continue to: Whom to test, and how
Whom to test, and how
Test for H pylori in those with active peptic ulcer disease or a history of peptic ulcer disease that was not investigated for H pylori. Also test individuals who have gastric mucosa-associated lymphoid tissue lymphoma, have a history of gastric cancer or family history of gastric cancer, are scheduled for endoscopic evaluation for dyspepsia, or are starting chronic NSAID therapy. Patients with typical GERD symptoms do not need to be tested for H pylori.9,25
Means of testing for H pylori include the urea breath test, stool antigen studies, endoscopically obtained biopsies, or serum antibody tests. Antibody testing is discouraged because it has a lower diagnostic utility and cannot determine if the patient’s infection is current or past. Before undergoing urea breath tests, stool antigen tests, or biopsies for H pylori identification, patients should have abstained from taking the following agents for the time periods indicated: PPIs, 1 to 2 weeks; H2RAs, at least 1 day and preferably 2 weeks; and antibiotics, 4 weeks.9
The urea breath test and endoscopically obtained biopsies have the greatest diagnostic utility and, where available, should be the first-line tests. Stool antigen studies are useful for ruling out H pylori infection (very low negative likelihood ratio), but a positive test result is not as useful for confirming an infection, as false-positives do occur (moderate positive likelihood ratio).9,26,27 Stool antigen testing is less expensive and, in many cases, more convenient and readily available for patients than urea breath testing and endoscopic biopsies.
Treatment
Offer treatment to all patients who test positive for H pylori. Eradication rates range from 70% to 91% using first-line treatment options.9 Treatment regimens consist of acid suppression and 2 to 3 antibiotics in combination (TABLE 39,28). The single greatest predictive factor for treatment failure is antibiotic resistance, so a detailed antibiotic history is essential. In particular, ask about macrolide antibiotic usage and penicillin allergies.
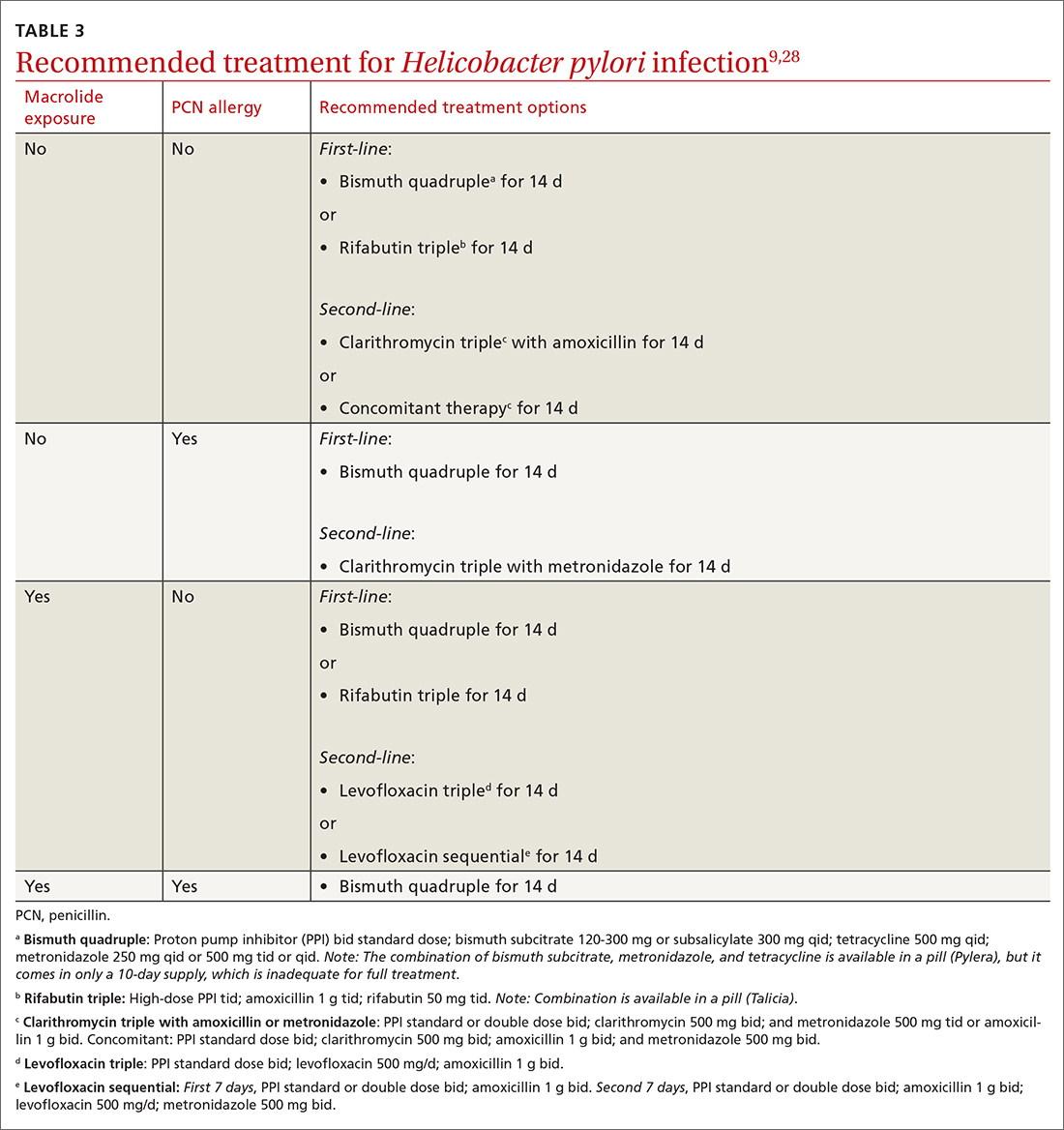
People living in areas with population macrolide resistance ≥ 15% should avoid clarithromycin-based regimens unless bacterial sensitivity testing has been done and shows sensitivity to these agents.9,28,29 For cases that do not resolve with a first-line treatment program, choose an alternative regimen with different antibiotics.9,29
Continue to: Additionally, adequate...
Additionally, adequate acid suppression is directly related to successful eradication. Thus, the likelihood of treatment success can be improved by using higher doses of PPIs and avoiding ones that are more likely to be metabolized quickly in some patients (lansoprazole, omeprazole). Patient adherence to the treatment regimen is an important determinant of effectiveness.9,29 Adding vitamin C 400 to 1000 mg/d, vitamin E 100 to 400 mg/d, and probiotics may improve the effectiveness of treatment.9,30
Duration of treatment is directly related to treatment effectiveness. Whenever possible, opt for 14 days of treatment instead of just 7.9
Test of cure. Patients treated for H pylori should be re-tested no sooner than 4 weeks after completion of therapy. Urea breath testing, stool antigen testing, and endoscopic biopsies (if endoscopy is indicated for some other reason) can all be used post treatment for test of cure.9
Barrett esophagus
Whom to screen
The American College of Gastroenterology recommends consideration of screening with upper endoscopy for men with chronic GERD (> 5 years) or frequent GERD symptoms (once weekly or more often), plus 2
Continue to: Not everyone with BE...
Not everyone with BE experiences GERD symptoms; sometimes BE may be diagnosed incidentally on upper endoscopy performed for unrelated symptoms.11 GERD patients who are currently asymptomatic and had a normal prior upper endoscopy do not require surveillance.
Diagnosis and management
BE is diagnosed based on specific endoscopic and histologic findings. The presence of dysplasia (either low grade or high grade) or its absence has important treatment implications. When histology is indefinite for dysplasia, treat reflux and, following acid suppression with PPIs for 3 to 6 months, repeat endoscopy (since reactive changes with reflux may obscure results).11
Nondysplastic BE has a risk of progressing to cancer in only 0.2% to 0.5% of affected patients per year.11 Guidelines for BE without dysplasia advise repeating surveillance endoscopy every 3 to 5 years after appropriate counseling regarding overall low risk of cancer progression.11,31 Surveillance endoscopy recommendations exist despite the lack of prospective randomized trials that demonstrate benefit. The rationale for surveillance is that survival in EAC is stage dependent and often EAC metastasizes prior to the development of symptoms from the tumor. Observational cohort studies in BE have demonstrated that surveillance endoscopy programs find EAC at earlier stages with improved survival; however, lead and length time bias may attenuate or eliminate these surveillance benefits.11,32
Risk for neoplastic progression increases with degree of dysplasia. BE with low-grade dysplasia and high-grade dysplasia have a risk of cancer progression of 0.7% per year and 7% per year, respectively.11
Historically, esophagectomy was the preferred treatment for BE with dysplasia. Now, endoscopic eradication therapies, including radiofrequency ablation and endoscopic mucosal resection for nodular BE, are the usual treatment for either low- or high-grade dysplasia.11
Chemoprophylaxis with PPIs. Most patients with BE have symptoms of GERD or reflux esophagitis, so treatment with a PPI is indicated for symptom control. In patients with BE without GERD, PPI use may still be indicated, although this is controversial. Current guidelines recommend once-daily PPI therapy for BE (twice daily only if needed for symptom control) to reduce reflux-associated inflammation and recommend against routine prescription of aspirin or NSAIDs for BE.11 In vitro and observational studies support PPI use to prevent progression to EAC11,33; however, data from randomized controlled trials to support their use are limited.34,35
CORRESPONDENCE
Megan Everson, MD, Medical College of Wisconsin, 229 South Morrison Street, Appleton WI, 54911; [email protected]
1. Wang C, Yuan Y, Hunt RH. Helicobacter pylori infection and Barrett’s esophagus: a systematic review and meta-analysis. Am J Gastroenterol. 2009;104:492-500. doi: 10.1038/ajg.2008.37
2. Maret-Ouda J, Markar SR, Lagergren J. Gastroesophageal reflux disease: a review. JAMA. 2020;324:2536-2547. doi: 10.1001/jama.2020.21360
3. Scida S, Russo M, Miraglia C, et al. Relationship between Helicobacter pylori infection and GERD. Acta Biomed. 2018;89:40-43. doi: 10.23750/abm.v89i8-S.7918
4. Vakil N, Van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-1920. doi: 10.1111/j.1572-0241.2006.00630.x
5. Mikami DJ, Murayama KM. Physiology and pathogenesis of gastroesophageal reflux disease. Surg Clin North Am. 2015;95:515-525. doi: 10.1016/j.suc.2015.02.006
6. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308-328. doi: 10.1038/ajg.2012.444
7. Sidhwa F, Moore A, Alligood E, et al. Diagnosis and treatment of the extraesophageal manifestations of gastroesophageal reflux disease. Ann Surg. 2017;265:63-67. doi: 10.1097/SLA.0000000000001907
8. Eusebi LH, Ratnakumaran R, Yuan Y, et al. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. 2018;67:430-440. doi: 10.1136/gutjnl-2016-313589
9. Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212-239. doi: 10.1038/ajg.2016.563
10. Eusebi LH, Cirota GG, Zagari RM, et al. Global prevalence of Barrett’s oesophagus and oesophageal cancer in individuals with gastro-oesophageal reflux: a systematic review and meta-analysis. Gut. 2021;70:456-463. doi: 10.1136/gutjnl-2020-321365
11. Shaheen NJ, Falk GW, Iyer PG, et al; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol. 2016;111:30-50. doi: 10.1038/ajg.2015.322
12. Savarino E, Bredenoord AJ, Fox M, et al; International Working Group for Disorders of Gastrointestinal Motility and Function. Expert consensus document: advances in the physiological assessment and diagnosis of GERD. Nat Rev Gastroenterol Hepatol. 2017;14:665-676. doi: 10.1038/nrgastro.2017.130
13. Kahrilas PJ. Clinical practice. Gastroesophageal reflux disease. N Engl J Med. 2008;359:1700-1707. doi: 10.1056/NEJMcp0804684
14. Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152:706-715. doi: 10.1053/j.gastro.2017.01.031
15. Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:516-554. doi: 10.1097/MPG.0000000000001889
16. Kahrilas PJ, Shaheen NJ, Vaezi MF, et al. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383-1391. doi: 10.1053/j.gastro.2008.08.045
17. Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol. 2018;16:800-808. doi: 10.1016/j.cgh.2017.09.033
18. Moayyedi P, Eikelboom JW, Bosch J, et al. Safety of proton pump inhibitors based on a large, multi-year, randomized trial of patients receiving rivaroxaban or aspirin. Gastroenterology. 2019;157:682-691. doi: 10.1053/j.gastro.2019.05.056
19. Katzka DA, Kahrilas PJ. Advances in the diagnosis and management of gastroesophageal reflux disease. BMJ. 2020;371:m3786. doi: 10.1136/bmj.m3786
20. Ali RA, Egan LJ. Gastroesophageal reflux disease in pregnancy. Best Pract Res Clin Gastroenterol. 2007;21:793-806. doi: 10.1016/j.bpg.2007.05.006
21. Body C, Christie JA. Gastrointestinal diseases in pregnancy: nausea, vomiting, hyperemesis gravidarum, gastroesophageal reflux disease, constipation, and diarrhea. Gastroenterol Clin North Am. 2016;45:267-283. doi: 10.1016/j.gtc.2016.02.005
22. Lightdale JR, Gremse DA, et al. Gastroesophageal reflux: management guidance for the pediatrician. Pediatrics. 2013;131;e1684-e1695. doi: 10.1542/peds.2013-0421
23. Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153:420-429. doi: 10.1053/j.gastro.2017.04.022
24. Zamani M, Ebrahimtabar F, Zamani V, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47:868-876. doi: 10.1111/apt.14561
25. Choi IJ, Kim CG, Lee JY, et al. Family history of gastric cancer and Helicobacter pylori treatment. N Engl J Med. 2020;382:427-436. doi: 10.1056/NEJMoa1909666
26. Gisbert JP, Abraira V. Accuracy of Helicobacter pylori diagnostic tests in patients with bleeding peptic ulcer: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101:848-863. doi: 10.1111/j.1572-0241.2006.00528.x
27. Best LM, Takwoingi Y, Siddique S, et al. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst Rev. 2018;3:CD012080. doi: 10.1002/14651858.CD012080.pub2
28. Howden CW, Graham DY. Recent developments pertaining to H. pylori infection. Am J Gastroenterol. 2021;116:1-3. doi: 10.14309/ajg.0000000000001031
29. Shah SC, Iyer PG, Moss SF. AGA Clinical practice update on the management of refractory Helicobacter pylori infection: expert review. Gastroenterology. 2021;160:1831-1841. doi: 10.1053/j.gastro.2020.11.059
30. Yang-Ou YB, Hu Y, Zhu Y, et al. The effect of antioxidants on Helicobacter pylori eradication: a systematic review with meta-analysis. Helicobacter. 2018;23:e12535. doi: 10.1111/hel.12535
31. Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:1084-1091. doi: 10.1053/j.gastro.2011.01.030
32. Codipilly DC, Chandar AK, Singh S, et al. The effect of endoscopic surveillance in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gastroenterology. 2018;154:2068-2086. doi: 10.1053/j.gastro.2018.02.022
33. Singh S, Garg SK, Singh PP, et al. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta-analysis. Gut. 2014;63:1229-1237. doi: 10.1136/gutjnl-2013-305997
34. Jankowski JAZ, de Caestecker J, Love SB, et al. Esomeprazole and aspirin in Barrett’s oesophagus (AspECT): a randomised factorial trial. Lancet. 2018;392:400-408. doi: 10.1016/S0140-6736(18)31388-6
35. Hu Q, Sun TT, Hong J, et al. Proton pump inhibitors do not reduce the risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a systematic review and meta-analysis. PLoS One. 2017;12:e0169691. doi: 10.1371/journal.pone.0169691
Three conditions seen in primary care—gastroesophageal reflux disease (GERD), Helicobacter pylori (H pylori) infection, and Barrett esophagus (BE)—evolve in a gastric acid environment and are treated in part through gastric acid suppression. While GERD is a risk factor for the development of BE, H pylori is not associated with BE.1 Patients with H pylori are actually less likely to have GERD symptoms.2,3 In this article, we describe similarities and differences in patient presentations, diagnostic testing, and management, and review screening recommendations.
Gastroesophageal reflux disease
GERD is a clinical diagnosis based on symptoms of regurgitation and heartburn or the presence of one of its known complications (esophagitis, peptic strictures, or BE).2,4 Chest pain is also common. Atypical symptoms are dysphagia, bleeding, chronic cough, asthma, chronic laryngitis, hoarseness, wheezing, teeth erosions, belching, and bloating.2,5-7
The worldwide prevalence of gastroesophageal reflux symptoms in adults is 14.8%.8 When using a stringent definition of GERD—weekly symptoms occurring for at least 3 months—prevalence drops to 9.4%.9 GERD symptoms vary markedly by geographic location; the highest rates are in Central America (19.6%) and the lowest rates are in Southeast Asia (7.4%).8TABLE 12,9-11 lists risk factors for GERD.

GERD results from dysfunction of the esophagogastric junction that permits regurgitation of acidic gastric contents into the esophagus. Normally, the lower esophageal sphincter (LES) relaxes temporarily with gastric distention; when this relaxation is frequent and prolonged, it causes GERD.2,12 Several medications, particularly those with anticholinergic effects (eg, tricyclic antidepressants) can decrease LES tone and contribute to symptoms. Nonsteroidal anti-inflammatory drugs (NSAIDs) are often linked to dyspepsia and gastritis and should be avoided in patients who have symptoms of GERD. Pathologic reflux can also occur in conditions that increase intra-abdominal pressure, such as obesity and pregnancy, and with esophageal dysmotility, hiatal hernia, and delayed gastric emptying.5 When gastric contents travel proximally, this contributes to extraesophageal symptoms, such as chronic cough, asthma, laryngitis, dyspepsia, bloating, and belching.2,4
Treatment
Proton pump inhibitors (PPIs) are the most effective treatment for GERD, but lifestyle modifications are also recommended for all patients.2,6,13-16 Consider selective elimination of beverages and foods that are commonly associated with heartburn (eg, alcohol, caffeine, chocolate, citrus, and spicy foods) if patients note a correlation to symptoms.5,6,13 Also, advise weight loss and smoking cessation, as appropriate, and suggest that the patient elevate the head of their bed when sleeping.
All PPIs are equally effective in suppressing acid when given at equivalent doses (TABLE 217), so they can be used interchangeably.17 Treat uncomplicated GERD with a once-daily PPI 30 to 60 minutes prior to a meal for 4 to 8 weeks. If treatment is effective, you’ll want to try to reduce or stop the medication after the 4- to 8-week period. (It’s worth noting that the benefits of treatment for those with extraesophageal GERD are less predictable than for those with heartburn or esophagitis symptoms.5)

If GERD symptoms reemerge after the PPI is stopped, the medication can be restarted but should be limited to the least potent effective dose, no matter if it is taken daily or only as needed.2,6,17 In patients with esophagitis, you may need to continue PPI treatment indefinitely at the lowest possible dose given the increased risk of recurrent esophagitis.2,13,16
Continue to: Keep in mind...
Keep in mind that the safety of long-term PPI use has not been fully established. While observational studies have shown that long-term PPI use may be associated with adverse events, including kidney damage, Clostridioides difficile infection, osteoporosis, and gastric cancer, subsequent prospective studies have not shown any significant risks with long-term PPI use.2,13,14,16,18,19 If a decision is made to discontinue PPIs after long-term use, the patient should be advised that rebound acid hypersecretion may occur, although this possibility can be mitigated by gradually tapering the PPI dose.
Another maintenance therapy option. Histamine-2 receptor antagonists (H2RAs) are a reasonable alternative to PPIs as maintenance therapy, but they are ineffective in healing esophagitis6,13 and may be best used as adjunctive therapy at bedtime for breakthrough symptoms while a patient is on maintenance PPIs.6,19 Antacids (eg, calcium carbonate, aluminum hydroxide, or magnesium hydroxide) and alginate may provide some symptomatic relief, as well.
When PPIs don’t work. If initial lifestyle changes and PPI treatment do not provide adequate relief, consider the possibility of nonadherence with medication or lifestyle directives. If nonadherence does not appear to be an issue, twice-daily PPI dosing is also an option. Recognize, though, that PPI treatment failure occurs in as many as 40% of patients and is much more common in those with atypical symptoms.6
Consider upper gastrointestinal (GI) endoscopy—and perhaps esophageal manometry or pH testing—if a patient does not respond to empiric treatment with a PPI for 4 to 8 weeks at a standard, once-daily dose.2,4,13 (Alternative diagnoses may also need to be considered.) Upper endoscopy is also appropriate for patients who have symptoms concerning for malignancy (progressive dysphagia, unintentional weight loss, or bleeding).
Esophagitis detected on endoscopy confirms GERD, although it is seen in only 18% to 25% of patients with GERD symptoms.2,4 (The absence of esophagitis only indicates a lack of mucosal injury and not the absence of GERD.4) Acid exposure can cause fibrotic scarring and, in turn, strictures visible on endoscopy.2 BE, the precursor to esophageal adenocarcinoma, is also a complication of GERD and is defined by columnar metaplasia replacing the normal squamous cell esophageal epithelium; it is detected on pathology review of biopsies.2
Continue to: GERD confirmed but PPIs aren't working?
GERD confirmed but PPIs aren’t working? Laparoscopic fundoplication is an effective treatment for GERD. However, due to its adverse effects (dysphagia, bloating, flatulence) and risk of treatment failure or breakdown within 5 to 10 years, it should be reserved for those poorly managed with PPIs.2,13,19
Considerations in pregnancy. GERD is reported by 40% to 85% of pregnant women,20,21 and its clinical presentation, diagnosis, and treatment are similar to that of nonpregnant adults.21 If lifestyle modification is not effective, pharmacologic therapy may be considered. Often, lifestyle modifications and antacids followed by the addition of sucralfate will be used first given the lack of systemic effects. H2RAs can be used next based on long-term historical use and reported safety.21 As with nonpregnant patients, PPIs are more effective than other medical therapies. If PPIs are used, dexlansoprazole, lansoprazole, pantoprazole, and rabeprazole are preferred. Omeprazole and esomeprazole are typically avoided due to findings of embryonic and fetal mortality in early animal studies, although subsequent human studies have noted no teratogenicity.2,20,21
Considerations in children. As with adults, findings in the history and exam are sufficient to diagnose and initiate treatment of GERD in children, provided there are no warning signs (eg, bilious vomiting, GI bleeding, consistent forceful vomiting, fever, lethargy, hepatosplenomegaly, bulging fontanelle, macro- or microcephaly, seizures, abdominal tenderness/distention, or genetic/metabolic syndromes). Lifestyle changes are first-line treatment, followed by medication. Acid suppressants are preferred, with PPIs showing superior efficacy compared with H2RAs.15 Some PPIs (omeprazole, lansoprazole, and esomeprazole) have US Food and Drug Administration (FDA) approval beginning at age 1 year, while rabeprazole has FDA approval beginning at age 12.22 As in adults, if PPIs are ineffective, consider alternative diagnoses.15,22
Helicobacter pylori infection
H pylori is a gram-negative spiral-shaped bacterium found in the stomach of humans and other mammals. It survives the acidic environment by metabolizing urea into alkaline ammonia and carbon dioxide. H pylori infection increases the risk of peptic ulcer disease, gastric cancer, iron deficiency anemia, and immune thrombocytopenia. It may be associated with dyspepsia, increased ulcer risk with use of an NSAID, and chronic gastritis.9 Infection with H pylori can decrease the risk of GERD.2 The bacterial infection causes atrophic gastritis and subsequent hypochlorhydria, which then diminishes the acidity of the reflux contents.19 There is no link between H pylori infection and BE.1
TABLE 12,9-11 shows those at highest risk of H pylori. The estimated prevalence of infection is 40% to 48%23 worldwide but lower in North America, at 32% to 42%.24H pylori is often acquired in childhood, and risk of infection is more likely if the parents (particularly mothers) are infected.9
Continue to: Whom to test, and how
Whom to test, and how
Test for H pylori in those with active peptic ulcer disease or a history of peptic ulcer disease that was not investigated for H pylori. Also test individuals who have gastric mucosa-associated lymphoid tissue lymphoma, have a history of gastric cancer or family history of gastric cancer, are scheduled for endoscopic evaluation for dyspepsia, or are starting chronic NSAID therapy. Patients with typical GERD symptoms do not need to be tested for H pylori.9,25
Means of testing for H pylori include the urea breath test, stool antigen studies, endoscopically obtained biopsies, or serum antibody tests. Antibody testing is discouraged because it has a lower diagnostic utility and cannot determine if the patient’s infection is current or past. Before undergoing urea breath tests, stool antigen tests, or biopsies for H pylori identification, patients should have abstained from taking the following agents for the time periods indicated: PPIs, 1 to 2 weeks; H2RAs, at least 1 day and preferably 2 weeks; and antibiotics, 4 weeks.9
The urea breath test and endoscopically obtained biopsies have the greatest diagnostic utility and, where available, should be the first-line tests. Stool antigen studies are useful for ruling out H pylori infection (very low negative likelihood ratio), but a positive test result is not as useful for confirming an infection, as false-positives do occur (moderate positive likelihood ratio).9,26,27 Stool antigen testing is less expensive and, in many cases, more convenient and readily available for patients than urea breath testing and endoscopic biopsies.
Treatment
Offer treatment to all patients who test positive for H pylori. Eradication rates range from 70% to 91% using first-line treatment options.9 Treatment regimens consist of acid suppression and 2 to 3 antibiotics in combination (TABLE 39,28). The single greatest predictive factor for treatment failure is antibiotic resistance, so a detailed antibiotic history is essential. In particular, ask about macrolide antibiotic usage and penicillin allergies.

People living in areas with population macrolide resistance ≥ 15% should avoid clarithromycin-based regimens unless bacterial sensitivity testing has been done and shows sensitivity to these agents.9,28,29 For cases that do not resolve with a first-line treatment program, choose an alternative regimen with different antibiotics.9,29
Continue to: Additionally, adequate...
Additionally, adequate acid suppression is directly related to successful eradication. Thus, the likelihood of treatment success can be improved by using higher doses of PPIs and avoiding ones that are more likely to be metabolized quickly in some patients (lansoprazole, omeprazole). Patient adherence to the treatment regimen is an important determinant of effectiveness.9,29 Adding vitamin C 400 to 1000 mg/d, vitamin E 100 to 400 mg/d, and probiotics may improve the effectiveness of treatment.9,30
Duration of treatment is directly related to treatment effectiveness. Whenever possible, opt for 14 days of treatment instead of just 7.9
Test of cure. Patients treated for H pylori should be re-tested no sooner than 4 weeks after completion of therapy. Urea breath testing, stool antigen testing, and endoscopic biopsies (if endoscopy is indicated for some other reason) can all be used post treatment for test of cure.9
Barrett esophagus
Whom to screen
The American College of Gastroenterology recommends consideration of screening with upper endoscopy for men with chronic GERD (> 5 years) or frequent GERD symptoms (once weekly or more often), plus 2
Continue to: Not everyone with BE...
Not everyone with BE experiences GERD symptoms; sometimes BE may be diagnosed incidentally on upper endoscopy performed for unrelated symptoms.11 GERD patients who are currently asymptomatic and had a normal prior upper endoscopy do not require surveillance.
Diagnosis and management
BE is diagnosed based on specific endoscopic and histologic findings. The presence of dysplasia (either low grade or high grade) or its absence has important treatment implications. When histology is indefinite for dysplasia, treat reflux and, following acid suppression with PPIs for 3 to 6 months, repeat endoscopy (since reactive changes with reflux may obscure results).11
Nondysplastic BE has a risk of progressing to cancer in only 0.2% to 0.5% of affected patients per year.11 Guidelines for BE without dysplasia advise repeating surveillance endoscopy every 3 to 5 years after appropriate counseling regarding overall low risk of cancer progression.11,31 Surveillance endoscopy recommendations exist despite the lack of prospective randomized trials that demonstrate benefit. The rationale for surveillance is that survival in EAC is stage dependent and often EAC metastasizes prior to the development of symptoms from the tumor. Observational cohort studies in BE have demonstrated that surveillance endoscopy programs find EAC at earlier stages with improved survival; however, lead and length time bias may attenuate or eliminate these surveillance benefits.11,32
Risk for neoplastic progression increases with degree of dysplasia. BE with low-grade dysplasia and high-grade dysplasia have a risk of cancer progression of 0.7% per year and 7% per year, respectively.11
Historically, esophagectomy was the preferred treatment for BE with dysplasia. Now, endoscopic eradication therapies, including radiofrequency ablation and endoscopic mucosal resection for nodular BE, are the usual treatment for either low- or high-grade dysplasia.11
Chemoprophylaxis with PPIs. Most patients with BE have symptoms of GERD or reflux esophagitis, so treatment with a PPI is indicated for symptom control. In patients with BE without GERD, PPI use may still be indicated, although this is controversial. Current guidelines recommend once-daily PPI therapy for BE (twice daily only if needed for symptom control) to reduce reflux-associated inflammation and recommend against routine prescription of aspirin or NSAIDs for BE.11 In vitro and observational studies support PPI use to prevent progression to EAC11,33; however, data from randomized controlled trials to support their use are limited.34,35
CORRESPONDENCE
Megan Everson, MD, Medical College of Wisconsin, 229 South Morrison Street, Appleton WI, 54911; [email protected]
Three conditions seen in primary care—gastroesophageal reflux disease (GERD), Helicobacter pylori (H pylori) infection, and Barrett esophagus (BE)—evolve in a gastric acid environment and are treated in part through gastric acid suppression. While GERD is a risk factor for the development of BE, H pylori is not associated with BE.1 Patients with H pylori are actually less likely to have GERD symptoms.2,3 In this article, we describe similarities and differences in patient presentations, diagnostic testing, and management, and review screening recommendations.
Gastroesophageal reflux disease
GERD is a clinical diagnosis based on symptoms of regurgitation and heartburn or the presence of one of its known complications (esophagitis, peptic strictures, or BE).2,4 Chest pain is also common. Atypical symptoms are dysphagia, bleeding, chronic cough, asthma, chronic laryngitis, hoarseness, wheezing, teeth erosions, belching, and bloating.2,5-7
The worldwide prevalence of gastroesophageal reflux symptoms in adults is 14.8%.8 When using a stringent definition of GERD—weekly symptoms occurring for at least 3 months—prevalence drops to 9.4%.9 GERD symptoms vary markedly by geographic location; the highest rates are in Central America (19.6%) and the lowest rates are in Southeast Asia (7.4%).8TABLE 12,9-11 lists risk factors for GERD.

GERD results from dysfunction of the esophagogastric junction that permits regurgitation of acidic gastric contents into the esophagus. Normally, the lower esophageal sphincter (LES) relaxes temporarily with gastric distention; when this relaxation is frequent and prolonged, it causes GERD.2,12 Several medications, particularly those with anticholinergic effects (eg, tricyclic antidepressants) can decrease LES tone and contribute to symptoms. Nonsteroidal anti-inflammatory drugs (NSAIDs) are often linked to dyspepsia and gastritis and should be avoided in patients who have symptoms of GERD. Pathologic reflux can also occur in conditions that increase intra-abdominal pressure, such as obesity and pregnancy, and with esophageal dysmotility, hiatal hernia, and delayed gastric emptying.5 When gastric contents travel proximally, this contributes to extraesophageal symptoms, such as chronic cough, asthma, laryngitis, dyspepsia, bloating, and belching.2,4
Treatment
Proton pump inhibitors (PPIs) are the most effective treatment for GERD, but lifestyle modifications are also recommended for all patients.2,6,13-16 Consider selective elimination of beverages and foods that are commonly associated with heartburn (eg, alcohol, caffeine, chocolate, citrus, and spicy foods) if patients note a correlation to symptoms.5,6,13 Also, advise weight loss and smoking cessation, as appropriate, and suggest that the patient elevate the head of their bed when sleeping.
All PPIs are equally effective in suppressing acid when given at equivalent doses (TABLE 217), so they can be used interchangeably.17 Treat uncomplicated GERD with a once-daily PPI 30 to 60 minutes prior to a meal for 4 to 8 weeks. If treatment is effective, you’ll want to try to reduce or stop the medication after the 4- to 8-week period. (It’s worth noting that the benefits of treatment for those with extraesophageal GERD are less predictable than for those with heartburn or esophagitis symptoms.5)

If GERD symptoms reemerge after the PPI is stopped, the medication can be restarted but should be limited to the least potent effective dose, no matter if it is taken daily or only as needed.2,6,17 In patients with esophagitis, you may need to continue PPI treatment indefinitely at the lowest possible dose given the increased risk of recurrent esophagitis.2,13,16
Continue to: Keep in mind...
Keep in mind that the safety of long-term PPI use has not been fully established. While observational studies have shown that long-term PPI use may be associated with adverse events, including kidney damage, Clostridioides difficile infection, osteoporosis, and gastric cancer, subsequent prospective studies have not shown any significant risks with long-term PPI use.2,13,14,16,18,19 If a decision is made to discontinue PPIs after long-term use, the patient should be advised that rebound acid hypersecretion may occur, although this possibility can be mitigated by gradually tapering the PPI dose.
Another maintenance therapy option. Histamine-2 receptor antagonists (H2RAs) are a reasonable alternative to PPIs as maintenance therapy, but they are ineffective in healing esophagitis6,13 and may be best used as adjunctive therapy at bedtime for breakthrough symptoms while a patient is on maintenance PPIs.6,19 Antacids (eg, calcium carbonate, aluminum hydroxide, or magnesium hydroxide) and alginate may provide some symptomatic relief, as well.
When PPIs don’t work. If initial lifestyle changes and PPI treatment do not provide adequate relief, consider the possibility of nonadherence with medication or lifestyle directives. If nonadherence does not appear to be an issue, twice-daily PPI dosing is also an option. Recognize, though, that PPI treatment failure occurs in as many as 40% of patients and is much more common in those with atypical symptoms.6
Consider upper gastrointestinal (GI) endoscopy—and perhaps esophageal manometry or pH testing—if a patient does not respond to empiric treatment with a PPI for 4 to 8 weeks at a standard, once-daily dose.2,4,13 (Alternative diagnoses may also need to be considered.) Upper endoscopy is also appropriate for patients who have symptoms concerning for malignancy (progressive dysphagia, unintentional weight loss, or bleeding).
Esophagitis detected on endoscopy confirms GERD, although it is seen in only 18% to 25% of patients with GERD symptoms.2,4 (The absence of esophagitis only indicates a lack of mucosal injury and not the absence of GERD.4) Acid exposure can cause fibrotic scarring and, in turn, strictures visible on endoscopy.2 BE, the precursor to esophageal adenocarcinoma, is also a complication of GERD and is defined by columnar metaplasia replacing the normal squamous cell esophageal epithelium; it is detected on pathology review of biopsies.2
Continue to: GERD confirmed but PPIs aren't working?
GERD confirmed but PPIs aren’t working? Laparoscopic fundoplication is an effective treatment for GERD. However, due to its adverse effects (dysphagia, bloating, flatulence) and risk of treatment failure or breakdown within 5 to 10 years, it should be reserved for those poorly managed with PPIs.2,13,19
Considerations in pregnancy. GERD is reported by 40% to 85% of pregnant women,20,21 and its clinical presentation, diagnosis, and treatment are similar to that of nonpregnant adults.21 If lifestyle modification is not effective, pharmacologic therapy may be considered. Often, lifestyle modifications and antacids followed by the addition of sucralfate will be used first given the lack of systemic effects. H2RAs can be used next based on long-term historical use and reported safety.21 As with nonpregnant patients, PPIs are more effective than other medical therapies. If PPIs are used, dexlansoprazole, lansoprazole, pantoprazole, and rabeprazole are preferred. Omeprazole and esomeprazole are typically avoided due to findings of embryonic and fetal mortality in early animal studies, although subsequent human studies have noted no teratogenicity.2,20,21
Considerations in children. As with adults, findings in the history and exam are sufficient to diagnose and initiate treatment of GERD in children, provided there are no warning signs (eg, bilious vomiting, GI bleeding, consistent forceful vomiting, fever, lethargy, hepatosplenomegaly, bulging fontanelle, macro- or microcephaly, seizures, abdominal tenderness/distention, or genetic/metabolic syndromes). Lifestyle changes are first-line treatment, followed by medication. Acid suppressants are preferred, with PPIs showing superior efficacy compared with H2RAs.15 Some PPIs (omeprazole, lansoprazole, and esomeprazole) have US Food and Drug Administration (FDA) approval beginning at age 1 year, while rabeprazole has FDA approval beginning at age 12.22 As in adults, if PPIs are ineffective, consider alternative diagnoses.15,22
Helicobacter pylori infection
H pylori is a gram-negative spiral-shaped bacterium found in the stomach of humans and other mammals. It survives the acidic environment by metabolizing urea into alkaline ammonia and carbon dioxide. H pylori infection increases the risk of peptic ulcer disease, gastric cancer, iron deficiency anemia, and immune thrombocytopenia. It may be associated with dyspepsia, increased ulcer risk with use of an NSAID, and chronic gastritis.9 Infection with H pylori can decrease the risk of GERD.2 The bacterial infection causes atrophic gastritis and subsequent hypochlorhydria, which then diminishes the acidity of the reflux contents.19 There is no link between H pylori infection and BE.1
TABLE 12,9-11 shows those at highest risk of H pylori. The estimated prevalence of infection is 40% to 48%23 worldwide but lower in North America, at 32% to 42%.24H pylori is often acquired in childhood, and risk of infection is more likely if the parents (particularly mothers) are infected.9
Continue to: Whom to test, and how
Whom to test, and how
Test for H pylori in those with active peptic ulcer disease or a history of peptic ulcer disease that was not investigated for H pylori. Also test individuals who have gastric mucosa-associated lymphoid tissue lymphoma, have a history of gastric cancer or family history of gastric cancer, are scheduled for endoscopic evaluation for dyspepsia, or are starting chronic NSAID therapy. Patients with typical GERD symptoms do not need to be tested for H pylori.9,25
Means of testing for H pylori include the urea breath test, stool antigen studies, endoscopically obtained biopsies, or serum antibody tests. Antibody testing is discouraged because it has a lower diagnostic utility and cannot determine if the patient’s infection is current or past. Before undergoing urea breath tests, stool antigen tests, or biopsies for H pylori identification, patients should have abstained from taking the following agents for the time periods indicated: PPIs, 1 to 2 weeks; H2RAs, at least 1 day and preferably 2 weeks; and antibiotics, 4 weeks.9
The urea breath test and endoscopically obtained biopsies have the greatest diagnostic utility and, where available, should be the first-line tests. Stool antigen studies are useful for ruling out H pylori infection (very low negative likelihood ratio), but a positive test result is not as useful for confirming an infection, as false-positives do occur (moderate positive likelihood ratio).9,26,27 Stool antigen testing is less expensive and, in many cases, more convenient and readily available for patients than urea breath testing and endoscopic biopsies.
Treatment
Offer treatment to all patients who test positive for H pylori. Eradication rates range from 70% to 91% using first-line treatment options.9 Treatment regimens consist of acid suppression and 2 to 3 antibiotics in combination (TABLE 39,28). The single greatest predictive factor for treatment failure is antibiotic resistance, so a detailed antibiotic history is essential. In particular, ask about macrolide antibiotic usage and penicillin allergies.

People living in areas with population macrolide resistance ≥ 15% should avoid clarithromycin-based regimens unless bacterial sensitivity testing has been done and shows sensitivity to these agents.9,28,29 For cases that do not resolve with a first-line treatment program, choose an alternative regimen with different antibiotics.9,29
Continue to: Additionally, adequate...
Additionally, adequate acid suppression is directly related to successful eradication. Thus, the likelihood of treatment success can be improved by using higher doses of PPIs and avoiding ones that are more likely to be metabolized quickly in some patients (lansoprazole, omeprazole). Patient adherence to the treatment regimen is an important determinant of effectiveness.9,29 Adding vitamin C 400 to 1000 mg/d, vitamin E 100 to 400 mg/d, and probiotics may improve the effectiveness of treatment.9,30
Duration of treatment is directly related to treatment effectiveness. Whenever possible, opt for 14 days of treatment instead of just 7.9
Test of cure. Patients treated for H pylori should be re-tested no sooner than 4 weeks after completion of therapy. Urea breath testing, stool antigen testing, and endoscopic biopsies (if endoscopy is indicated for some other reason) can all be used post treatment for test of cure.9
Barrett esophagus
Whom to screen
The American College of Gastroenterology recommends consideration of screening with upper endoscopy for men with chronic GERD (> 5 years) or frequent GERD symptoms (once weekly or more often), plus 2
Continue to: Not everyone with BE...
Not everyone with BE experiences GERD symptoms; sometimes BE may be diagnosed incidentally on upper endoscopy performed for unrelated symptoms.11 GERD patients who are currently asymptomatic and had a normal prior upper endoscopy do not require surveillance.
Diagnosis and management
BE is diagnosed based on specific endoscopic and histologic findings. The presence of dysplasia (either low grade or high grade) or its absence has important treatment implications. When histology is indefinite for dysplasia, treat reflux and, following acid suppression with PPIs for 3 to 6 months, repeat endoscopy (since reactive changes with reflux may obscure results).11
Nondysplastic BE has a risk of progressing to cancer in only 0.2% to 0.5% of affected patients per year.11 Guidelines for BE without dysplasia advise repeating surveillance endoscopy every 3 to 5 years after appropriate counseling regarding overall low risk of cancer progression.11,31 Surveillance endoscopy recommendations exist despite the lack of prospective randomized trials that demonstrate benefit. The rationale for surveillance is that survival in EAC is stage dependent and often EAC metastasizes prior to the development of symptoms from the tumor. Observational cohort studies in BE have demonstrated that surveillance endoscopy programs find EAC at earlier stages with improved survival; however, lead and length time bias may attenuate or eliminate these surveillance benefits.11,32
Risk for neoplastic progression increases with degree of dysplasia. BE with low-grade dysplasia and high-grade dysplasia have a risk of cancer progression of 0.7% per year and 7% per year, respectively.11
Historically, esophagectomy was the preferred treatment for BE with dysplasia. Now, endoscopic eradication therapies, including radiofrequency ablation and endoscopic mucosal resection for nodular BE, are the usual treatment for either low- or high-grade dysplasia.11
Chemoprophylaxis with PPIs. Most patients with BE have symptoms of GERD or reflux esophagitis, so treatment with a PPI is indicated for symptom control. In patients with BE without GERD, PPI use may still be indicated, although this is controversial. Current guidelines recommend once-daily PPI therapy for BE (twice daily only if needed for symptom control) to reduce reflux-associated inflammation and recommend against routine prescription of aspirin or NSAIDs for BE.11 In vitro and observational studies support PPI use to prevent progression to EAC11,33; however, data from randomized controlled trials to support their use are limited.34,35
CORRESPONDENCE
Megan Everson, MD, Medical College of Wisconsin, 229 South Morrison Street, Appleton WI, 54911; [email protected]
1. Wang C, Yuan Y, Hunt RH. Helicobacter pylori infection and Barrett’s esophagus: a systematic review and meta-analysis. Am J Gastroenterol. 2009;104:492-500. doi: 10.1038/ajg.2008.37
2. Maret-Ouda J, Markar SR, Lagergren J. Gastroesophageal reflux disease: a review. JAMA. 2020;324:2536-2547. doi: 10.1001/jama.2020.21360
3. Scida S, Russo M, Miraglia C, et al. Relationship between Helicobacter pylori infection and GERD. Acta Biomed. 2018;89:40-43. doi: 10.23750/abm.v89i8-S.7918
4. Vakil N, Van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-1920. doi: 10.1111/j.1572-0241.2006.00630.x
5. Mikami DJ, Murayama KM. Physiology and pathogenesis of gastroesophageal reflux disease. Surg Clin North Am. 2015;95:515-525. doi: 10.1016/j.suc.2015.02.006
6. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308-328. doi: 10.1038/ajg.2012.444
7. Sidhwa F, Moore A, Alligood E, et al. Diagnosis and treatment of the extraesophageal manifestations of gastroesophageal reflux disease. Ann Surg. 2017;265:63-67. doi: 10.1097/SLA.0000000000001907
8. Eusebi LH, Ratnakumaran R, Yuan Y, et al. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. 2018;67:430-440. doi: 10.1136/gutjnl-2016-313589
9. Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212-239. doi: 10.1038/ajg.2016.563
10. Eusebi LH, Cirota GG, Zagari RM, et al. Global prevalence of Barrett’s oesophagus and oesophageal cancer in individuals with gastro-oesophageal reflux: a systematic review and meta-analysis. Gut. 2021;70:456-463. doi: 10.1136/gutjnl-2020-321365
11. Shaheen NJ, Falk GW, Iyer PG, et al; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol. 2016;111:30-50. doi: 10.1038/ajg.2015.322
12. Savarino E, Bredenoord AJ, Fox M, et al; International Working Group for Disorders of Gastrointestinal Motility and Function. Expert consensus document: advances in the physiological assessment and diagnosis of GERD. Nat Rev Gastroenterol Hepatol. 2017;14:665-676. doi: 10.1038/nrgastro.2017.130
13. Kahrilas PJ. Clinical practice. Gastroesophageal reflux disease. N Engl J Med. 2008;359:1700-1707. doi: 10.1056/NEJMcp0804684
14. Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152:706-715. doi: 10.1053/j.gastro.2017.01.031
15. Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:516-554. doi: 10.1097/MPG.0000000000001889
16. Kahrilas PJ, Shaheen NJ, Vaezi MF, et al. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383-1391. doi: 10.1053/j.gastro.2008.08.045
17. Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol. 2018;16:800-808. doi: 10.1016/j.cgh.2017.09.033
18. Moayyedi P, Eikelboom JW, Bosch J, et al. Safety of proton pump inhibitors based on a large, multi-year, randomized trial of patients receiving rivaroxaban or aspirin. Gastroenterology. 2019;157:682-691. doi: 10.1053/j.gastro.2019.05.056
19. Katzka DA, Kahrilas PJ. Advances in the diagnosis and management of gastroesophageal reflux disease. BMJ. 2020;371:m3786. doi: 10.1136/bmj.m3786
20. Ali RA, Egan LJ. Gastroesophageal reflux disease in pregnancy. Best Pract Res Clin Gastroenterol. 2007;21:793-806. doi: 10.1016/j.bpg.2007.05.006
21. Body C, Christie JA. Gastrointestinal diseases in pregnancy: nausea, vomiting, hyperemesis gravidarum, gastroesophageal reflux disease, constipation, and diarrhea. Gastroenterol Clin North Am. 2016;45:267-283. doi: 10.1016/j.gtc.2016.02.005
22. Lightdale JR, Gremse DA, et al. Gastroesophageal reflux: management guidance for the pediatrician. Pediatrics. 2013;131;e1684-e1695. doi: 10.1542/peds.2013-0421
23. Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153:420-429. doi: 10.1053/j.gastro.2017.04.022
24. Zamani M, Ebrahimtabar F, Zamani V, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47:868-876. doi: 10.1111/apt.14561
25. Choi IJ, Kim CG, Lee JY, et al. Family history of gastric cancer and Helicobacter pylori treatment. N Engl J Med. 2020;382:427-436. doi: 10.1056/NEJMoa1909666
26. Gisbert JP, Abraira V. Accuracy of Helicobacter pylori diagnostic tests in patients with bleeding peptic ulcer: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101:848-863. doi: 10.1111/j.1572-0241.2006.00528.x
27. Best LM, Takwoingi Y, Siddique S, et al. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst Rev. 2018;3:CD012080. doi: 10.1002/14651858.CD012080.pub2
28. Howden CW, Graham DY. Recent developments pertaining to H. pylori infection. Am J Gastroenterol. 2021;116:1-3. doi: 10.14309/ajg.0000000000001031
29. Shah SC, Iyer PG, Moss SF. AGA Clinical practice update on the management of refractory Helicobacter pylori infection: expert review. Gastroenterology. 2021;160:1831-1841. doi: 10.1053/j.gastro.2020.11.059
30. Yang-Ou YB, Hu Y, Zhu Y, et al. The effect of antioxidants on Helicobacter pylori eradication: a systematic review with meta-analysis. Helicobacter. 2018;23:e12535. doi: 10.1111/hel.12535
31. Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:1084-1091. doi: 10.1053/j.gastro.2011.01.030
32. Codipilly DC, Chandar AK, Singh S, et al. The effect of endoscopic surveillance in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gastroenterology. 2018;154:2068-2086. doi: 10.1053/j.gastro.2018.02.022
33. Singh S, Garg SK, Singh PP, et al. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta-analysis. Gut. 2014;63:1229-1237. doi: 10.1136/gutjnl-2013-305997
34. Jankowski JAZ, de Caestecker J, Love SB, et al. Esomeprazole and aspirin in Barrett’s oesophagus (AspECT): a randomised factorial trial. Lancet. 2018;392:400-408. doi: 10.1016/S0140-6736(18)31388-6
35. Hu Q, Sun TT, Hong J, et al. Proton pump inhibitors do not reduce the risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a systematic review and meta-analysis. PLoS One. 2017;12:e0169691. doi: 10.1371/journal.pone.0169691
1. Wang C, Yuan Y, Hunt RH. Helicobacter pylori infection and Barrett’s esophagus: a systematic review and meta-analysis. Am J Gastroenterol. 2009;104:492-500. doi: 10.1038/ajg.2008.37
2. Maret-Ouda J, Markar SR, Lagergren J. Gastroesophageal reflux disease: a review. JAMA. 2020;324:2536-2547. doi: 10.1001/jama.2020.21360
3. Scida S, Russo M, Miraglia C, et al. Relationship between Helicobacter pylori infection and GERD. Acta Biomed. 2018;89:40-43. doi: 10.23750/abm.v89i8-S.7918
4. Vakil N, Van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-1920. doi: 10.1111/j.1572-0241.2006.00630.x
5. Mikami DJ, Murayama KM. Physiology and pathogenesis of gastroesophageal reflux disease. Surg Clin North Am. 2015;95:515-525. doi: 10.1016/j.suc.2015.02.006
6. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308-328. doi: 10.1038/ajg.2012.444
7. Sidhwa F, Moore A, Alligood E, et al. Diagnosis and treatment of the extraesophageal manifestations of gastroesophageal reflux disease. Ann Surg. 2017;265:63-67. doi: 10.1097/SLA.0000000000001907
8. Eusebi LH, Ratnakumaran R, Yuan Y, et al. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. 2018;67:430-440. doi: 10.1136/gutjnl-2016-313589
9. Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212-239. doi: 10.1038/ajg.2016.563
10. Eusebi LH, Cirota GG, Zagari RM, et al. Global prevalence of Barrett’s oesophagus and oesophageal cancer in individuals with gastro-oesophageal reflux: a systematic review and meta-analysis. Gut. 2021;70:456-463. doi: 10.1136/gutjnl-2020-321365
11. Shaheen NJ, Falk GW, Iyer PG, et al; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol. 2016;111:30-50. doi: 10.1038/ajg.2015.322
12. Savarino E, Bredenoord AJ, Fox M, et al; International Working Group for Disorders of Gastrointestinal Motility and Function. Expert consensus document: advances in the physiological assessment and diagnosis of GERD. Nat Rev Gastroenterol Hepatol. 2017;14:665-676. doi: 10.1038/nrgastro.2017.130
13. Kahrilas PJ. Clinical practice. Gastroesophageal reflux disease. N Engl J Med. 2008;359:1700-1707. doi: 10.1056/NEJMcp0804684
14. Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152:706-715. doi: 10.1053/j.gastro.2017.01.031
15. Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:516-554. doi: 10.1097/MPG.0000000000001889
16. Kahrilas PJ, Shaheen NJ, Vaezi MF, et al. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383-1391. doi: 10.1053/j.gastro.2008.08.045
17. Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol. 2018;16:800-808. doi: 10.1016/j.cgh.2017.09.033
18. Moayyedi P, Eikelboom JW, Bosch J, et al. Safety of proton pump inhibitors based on a large, multi-year, randomized trial of patients receiving rivaroxaban or aspirin. Gastroenterology. 2019;157:682-691. doi: 10.1053/j.gastro.2019.05.056
19. Katzka DA, Kahrilas PJ. Advances in the diagnosis and management of gastroesophageal reflux disease. BMJ. 2020;371:m3786. doi: 10.1136/bmj.m3786
20. Ali RA, Egan LJ. Gastroesophageal reflux disease in pregnancy. Best Pract Res Clin Gastroenterol. 2007;21:793-806. doi: 10.1016/j.bpg.2007.05.006
21. Body C, Christie JA. Gastrointestinal diseases in pregnancy: nausea, vomiting, hyperemesis gravidarum, gastroesophageal reflux disease, constipation, and diarrhea. Gastroenterol Clin North Am. 2016;45:267-283. doi: 10.1016/j.gtc.2016.02.005
22. Lightdale JR, Gremse DA, et al. Gastroesophageal reflux: management guidance for the pediatrician. Pediatrics. 2013;131;e1684-e1695. doi: 10.1542/peds.2013-0421
23. Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153:420-429. doi: 10.1053/j.gastro.2017.04.022
24. Zamani M, Ebrahimtabar F, Zamani V, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47:868-876. doi: 10.1111/apt.14561
25. Choi IJ, Kim CG, Lee JY, et al. Family history of gastric cancer and Helicobacter pylori treatment. N Engl J Med. 2020;382:427-436. doi: 10.1056/NEJMoa1909666
26. Gisbert JP, Abraira V. Accuracy of Helicobacter pylori diagnostic tests in patients with bleeding peptic ulcer: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101:848-863. doi: 10.1111/j.1572-0241.2006.00528.x
27. Best LM, Takwoingi Y, Siddique S, et al. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst Rev. 2018;3:CD012080. doi: 10.1002/14651858.CD012080.pub2
28. Howden CW, Graham DY. Recent developments pertaining to H. pylori infection. Am J Gastroenterol. 2021;116:1-3. doi: 10.14309/ajg.0000000000001031
29. Shah SC, Iyer PG, Moss SF. AGA Clinical practice update on the management of refractory Helicobacter pylori infection: expert review. Gastroenterology. 2021;160:1831-1841. doi: 10.1053/j.gastro.2020.11.059
30. Yang-Ou YB, Hu Y, Zhu Y, et al. The effect of antioxidants on Helicobacter pylori eradication: a systematic review with meta-analysis. Helicobacter. 2018;23:e12535. doi: 10.1111/hel.12535
31. Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:1084-1091. doi: 10.1053/j.gastro.2011.01.030
32. Codipilly DC, Chandar AK, Singh S, et al. The effect of endoscopic surveillance in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gastroenterology. 2018;154:2068-2086. doi: 10.1053/j.gastro.2018.02.022
33. Singh S, Garg SK, Singh PP, et al. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta-analysis. Gut. 2014;63:1229-1237. doi: 10.1136/gutjnl-2013-305997
34. Jankowski JAZ, de Caestecker J, Love SB, et al. Esomeprazole and aspirin in Barrett’s oesophagus (AspECT): a randomised factorial trial. Lancet. 2018;392:400-408. doi: 10.1016/S0140-6736(18)31388-6
35. Hu Q, Sun TT, Hong J, et al. Proton pump inhibitors do not reduce the risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a systematic review and meta-analysis. PLoS One. 2017;12:e0169691. doi: 10.1371/journal.pone.0169691
PRACTICE RECOMMENDATIONS
› Recommend endoscopy for patients with gastroesophageal reflux disease (GERD) and red flag symptoms: dysphagia, unintentional weight loss, or bleeding. B
› Recommend long-term use of a proton pump inhibitor at the lowest tolerated dose in patients with esophagitis or Barrett esophagus. C
› Test for Helicobacter pylori in patients with peptic ulcer disease, in those with past ulcers not investigated for H pylori, and in those starting chronic nonsteroidal anti-inflammatory drug therapy. A
› Use a urea breath test, stool antigen study, or endoscopically obtained biopsy to test for H pylori. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
