User login
Diagnostic challenges in primary care: Identifying and avoiding cognitive bias
Medical errors in all settings contributed to as many as 250,000 deaths per year in the United States between 2000 and 2008, according to a 2016 study.1 Diagnostic error, in particular, remains a leading cause of morbidity and mortality in the United States and worldwide. In 2017, 12 million patients (roughly 5% of all US adults) who sought outpatient care experienced missed, delayed, or incorrect diagnosis at least once.2
In his classic work, How Doctors Think, Jerome Groopman, MD, explored the diagnostic process with a focus on the role of cognitive bias in clinical decision-making. Groopman examined how physicians can become sidetracked in their thinking and “blinded” to potential alternative diagnoses.3 Medical error is not necessarily because of a deficiency in medical knowledge; rather, physicians become susceptible to medical error when defective and faulty reasoning distort their diagnostic ability.4
Cognitive bias in the diagnostic process has been extensively studied, and a full review is beyond the scope of this article.5 However, here we will examine pathways leading to diagnostic errors in the primary care setting, specifically the role of cognitive bias in the work-up of polymyalgia rheumatica (PMR), ovarian cancer (OC), Lewy body dementia (LBD), and fibromyalgia (FM). As these 4 disease states are seen with low-to-moderate frequency in primary care, cognitive bias can complicate accurate diagnosis. But first, a word about how to understand clinical reasoning.
There are 2 types of reasoning (and 1 is more prone to error)
Physician clinical reasoning can be divided into 2 different cognitive approaches.
Type 1 reasoning employs intuition and heuristics; this type is automatic, reflexive, and quick.5 While the use of mental shortcuts in type 1 increases the speed with which decisions are made, it also makes this form of reasoning more prone to error.
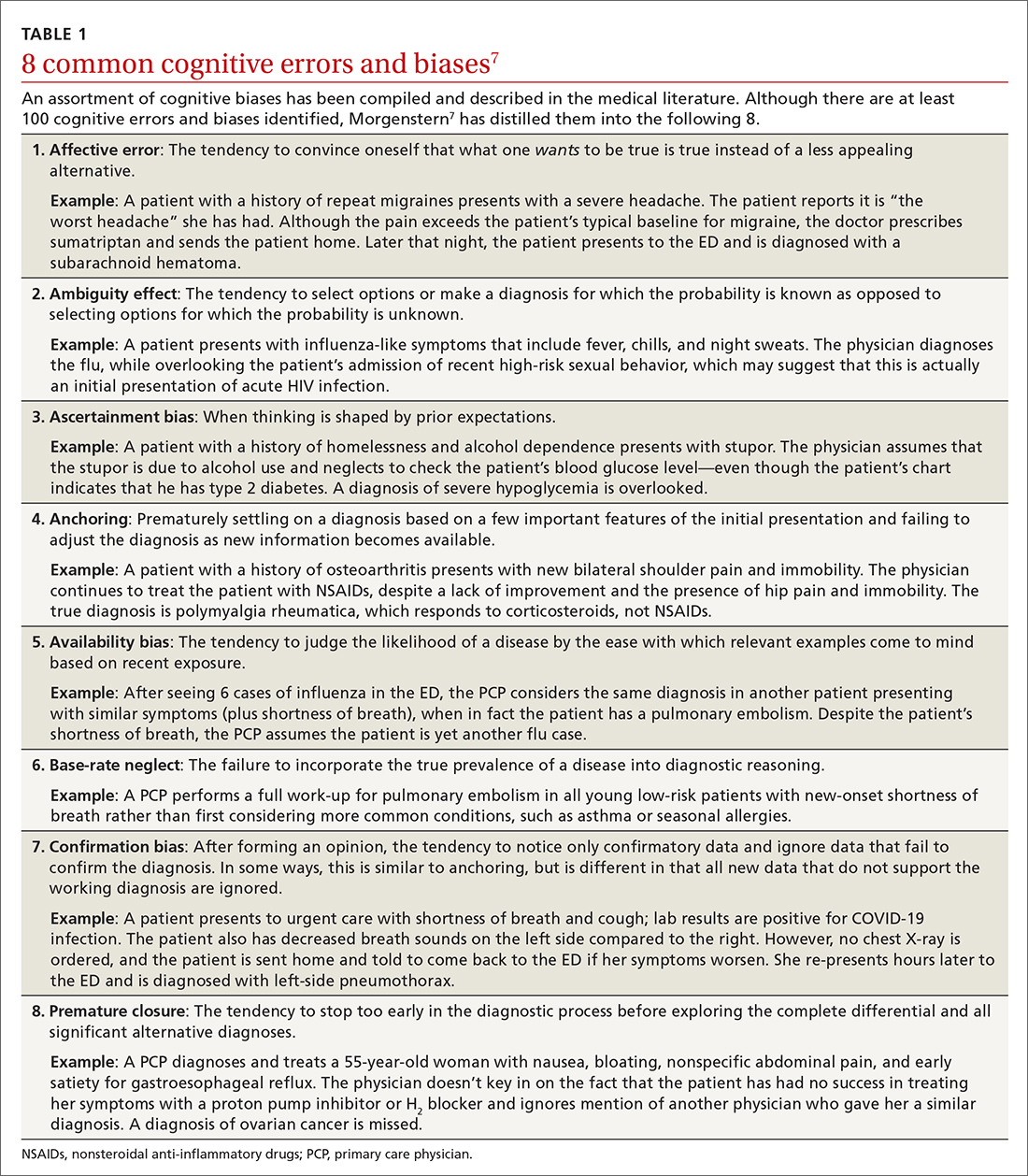
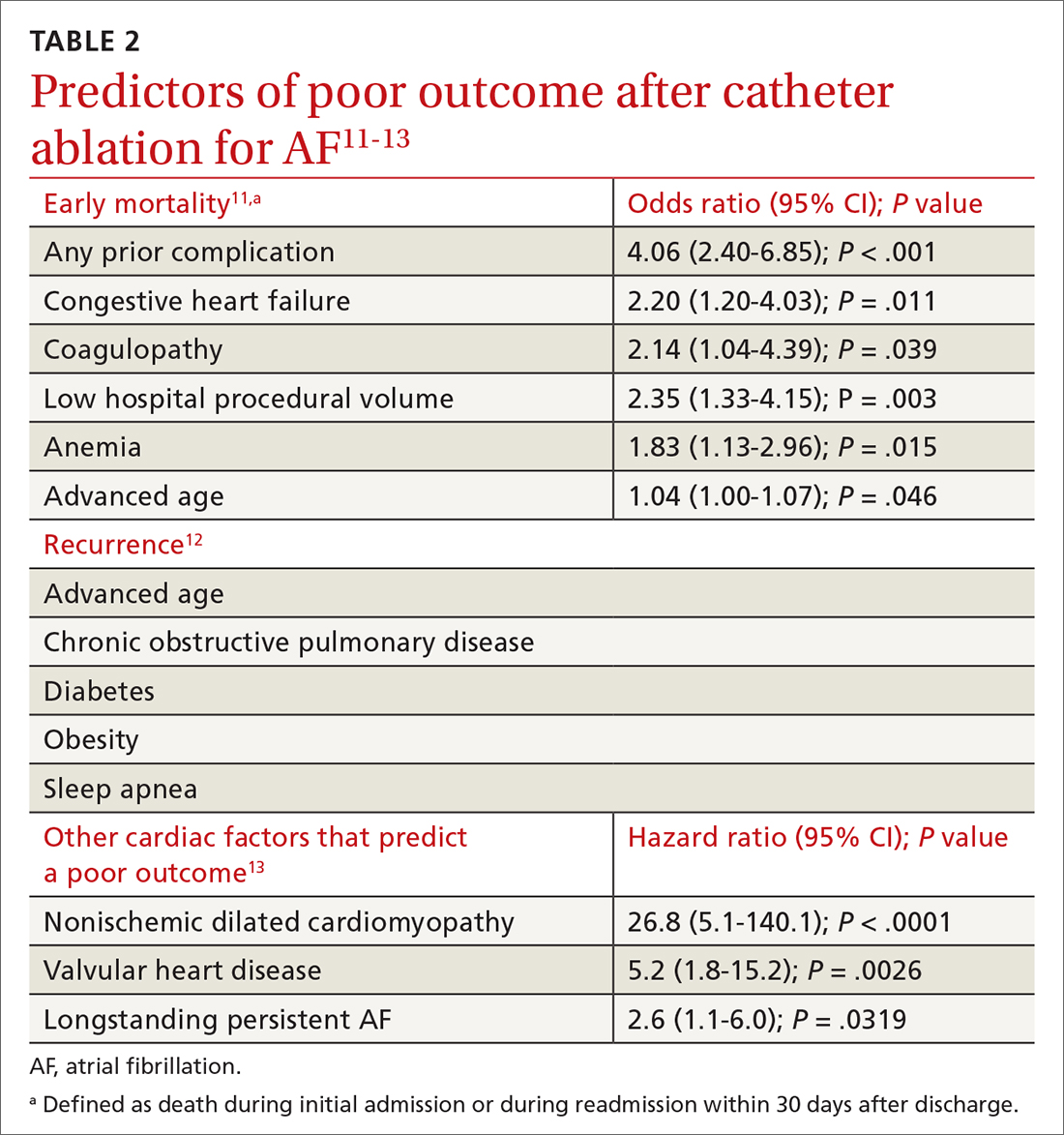
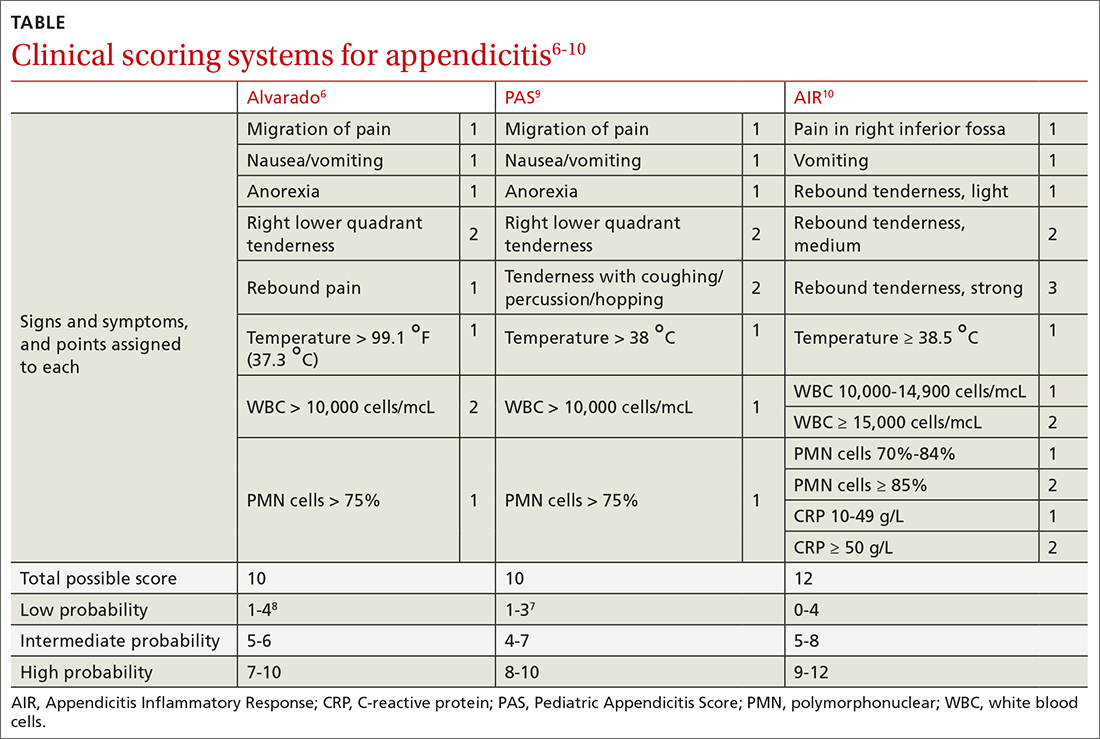
Type 2 reasoning requires conscious effort. It is goal directed and rigorous and therefore slower than type 1 reasoning. Extrapolated to the clinical context, clinicians transition from type 2 to type 1 reasoning as they gain experience and training throughout their careers and develop their own conscious and subconscious heuristics. Deviations from accurate decision-making occur in a systematic manner due to cognitive biases and result in medical error.6table 17 lists common types of cognitive bias.
An important question to ask. Physicians tend to fall into a pattern of quick, type 1 reasoning. However, it’s important to strive to maintain a broad differential diagnosis and avoid premature closure of the diagnostic process. It’s critical that we consider alternative diagnoses (ie, consciously move from type 1 to type 2 thinking) and continue to ask ourselves, “What else?” while working through differential diagnoses. This can be a powerful debiasing technique.
Continue to: The discussion...
The discussion of the following 4 disease states demonstrates how cognitive bias can lead to diagnostic error.
The patient is barely able to ambulate and appears to be in considerable pain. She is relying heavily on her walker and is assisted by her granddaughter. The primary care physician (PCP) obtains a detailed history that includes chronic shoulder and hip pain. Given that the patient has not responded to NSAID treatment over the previous 6 months, the PCP takes a moment to reconsider the diagnosis of OA and considers other options.
In light of the high prevalence of PMR in older women, the physician pursues a more specific physical examination tailored to ferret out PMR. He had learned this diagnostic shortcut as a resident, remembered it, and adeptly applied it whenever circumstances warranted. He asks the patient to raise her arms above her head (goalpost sign). She is unable to perform this task and experiences severe bilateral shoulder pain on trial. The PCP then places the patient on the examining table and attempts to assist her in rolling toward him. The patient is also unable to perform this maneuver and experiences significant bilateral hip pain on trial.
Based primarily on the patient’s history and physical exam findings, the PCP makes a presumptive diagnosis of PMR vs OA vs combined PMR with OA, orders an erythrocyte sedimentation rate (ESR) and basic rheumatologic
PMR can be mistaken for OA
PMR is the most common inflammatory rheumatic disease in older patients.8 It is a debilitating illness with simple, effective treatment but has devastating consequences if missed or left untreated.9 PMR typically manifests in patients older than age 50, with a peak incidence at 80 years of age. It is also far more common in women.10
Approximately 80% of patients with PMR initially present to their PCP, often posing a diagnostic challenge to many clinicians.11 Due to overlap in symptoms, the condition is often misdiagnosed as OA, a more common condition seen by PCPs. Also, there are no specific diagnostic tests for PMR. An elevated ESR can help confirm the diagnosis, but one-third of patients with PMR have a normal ESR.12 Therefore, the diagnostic conundrum the physician faces is OA vs rheumatoid arthritis (RA), PMR, or another condition.
Continue to: The consequences...
The consequences of a missed and delayed PMR diagnosis range from seriously impaired quality of life to significantly increased risk of vascular events (eg, blindness, stroke) due to temporal arteritis.13 Early diagnosis is even more critical as the risk of a vascular event and death is highest during initial phases of the disease course.14
FPs often miss this Dx. A timely diagnosis relies almost exclusively on an accurate, thorough history and physical exam. However, PCPs often struggle to correctly diagnose PMR. According to a study by Bahlas and colleagues,15 the accuracy rate for correctly diagnosing PMR was 24% among a cohort of family physicians.
The differential diagnosis for PMR is broad and includes seronegative spondyloarthropathies, malignancy, Lyme disease, hypothyroidism, and both RA and OA.16
PCPs are extremely adept at correctly diagnosing RA, but not PMR. A study by Blaauw and colleagues17 comparing PCPs and rheumatologists found PCPs correctly identified 92% of RA cases but only 55% of PMR cases. When rheumatologists reviewed these same cases, they correctly identified PMR and RA almost 100% of the time.17 The difference in diagnostic accuracy between rheumatologists and PCPs suggests limited experience and gaps in fund of knowledge.
Making the diagnosis. The diagnosis of PMR is often made on empiric response to corticosteroid treatment, but doing so based solely on a patient’s response is controversial.18 There are rare instances in which patients with PMR fail to respond to treatment. On the other hand, some inflammatory conditions that mimic or share symptoms with PMR also respond to corticosteroids, potentially resulting in erroneous confirmation bias.
Some classification criteria use rapid response to low-dose prednisone/prednisolone (≤ 20 mg) to confirm the diagnosis,19 while other more recent guidelines no longer include this approach.20 If PMR continues to be suspected after a trial of steroids is unsuccessful, the PCP can try another course of higher dose steroids or consult with Rheumatology.
Continue to: A full history...
A full history and physical exam revealed a myriad of gastrointestinal (GI) complaints, such as diarrhea. But the PCP recalled a recent roundtable discussion on debiasing techniques specifically related to gynecologic disorders, including OC. Therefore, he decided to include OC in the differential diagnosis—something he would not routinely have done given the preponderance of GI symptoms. Despite the patient’s reluctance and time constraints, the PCP ordered a transvaginal ultrasound. Findings from the ultrasound study revealed stage II OC, which carries a good prognosis. The patient is currently undergoing treatment and was last reported as doing well.
Early signs of ovarian cancer can be chalked up to a “GI issue”
OC is the second most common gynecologic cancer21 and the fifth leading cause of cancer-related death22 in US women. Compared to other cancers, the prognosis for localized early-stage OC is surprisingly good, with a 5-year survival rate approaching 93%.23 However, most disease is detected in later stages, and the 5-year survival rate drops to a low of 29%.24
There remains no established screening protocol for OC. Fewer than a quarter of all cases are diagnosed in stage I, and detection of OC relies heavily on the physician’s ability to decipher vague symptomatology that overlaps with other, more common maladies. This poses an obvious diagnostic challenge and, not surprisingly, a high level of susceptibility to cognitive bias.
More than 90% of patients with OC present with some combination of the following symptoms prior to diagnosis: abdominal (77%), GI (70%), pain (58%), constitutional (50%), urinary (34%), and pelvic (26%).25 The 3 most common isolated symptoms in patients with OC are abdominal bloating, decrease in appetite, and frank abdominal pain.26 Patients with biopsy-confirmed OC experience these symptoms an average of 6 months prior to actual diagnosis.27
Knowledge gaps play a role. Studies assessing the ability of health care providers to identify presenting symptoms of OC reveal specific knowledge gaps. For instance, in a survey by Gajjar and colleagues,28 most PCPs correctly identified bloating as a key symptom of OC; however, they weren’t as good at identifying less common symptoms, such as inability to finish a meal and early satiety. Moreover, survey participants misinterpreted or missed GI symptoms as an important manifestation of early OC disease.28 These specific knowledge gaps combine with physician errors in thinking, further obscuring and extending the diagnostic process. The point prevalence for OC is relatively low, and many PCPs only encounter a few cases during their entire career.29 This low pre-test probability may also fuel the delay in diagnosis.
Watch for these forms of bias. Since nonspecific symptoms of early-stage OC resemble those of other more benign conditions, a form of anchoring error known as multiple alternatives bias can arise. In this scenario, clinicians investigate only 1 potential plausible diagnosis and remain focused on that single, often faulty, conclusion. This persists despite other equally plausible alternatives that arise as the investigation proceeds.28
Affective error may also play a role in missed or delayed diagnosis. For example, a physician would prefer to diagnose and treat a common GI illness than consider OC. Another distortion involves outcome bias wherein the physician gives more significance to benign conditions such as irritable bowel syndrome because they have a more favorable outcome and clear treatment path. Physicians also favor these benign conditions because they encounter them more frequently than OC in the clinic setting. (This is known as availability bias.) Outcome bias and multiple alternatives bias can result in noninvestigation of symptoms and inefficient or improper management, leading to a delay in arriving at the correct diagnosis or anchoring on a plausible but incorrect diagnosis.
Continue to: An incorrect initial diagnostic...
An incorrect initial diagnostic path often triggers a cascade of subsequent errors. The physician orders additional unhelpful and expensive tests in an effort to characterize the suspected GI pathology. This then leads the physician to prematurely terminate the work-up and accept the most favored diagnosis. Lastly, sunk-cost fallacy comes into play: The physician has “invested” time and energy investigating a particular diagnosis and rather than abandon the presumed diagnosis, continues to put more time and effort in going down an incorrect diagnostic path.
A series of failures. These biases and miscues have been observed in several studies. For example, a survey of 1725 women by Goff and colleagues30 sought to identify factors related to delayed OC diagnosis. The authors found that the following factors were significantly associated with a delayed diagnosis: omission of a pelvic exam at initial presentation, a separate exploration of a multitude of collateral symptoms, a failure to order ultrasound/computed tomography/CA-125 test, and a failure to consider age as a factor (especially if the patient was outside the norm).
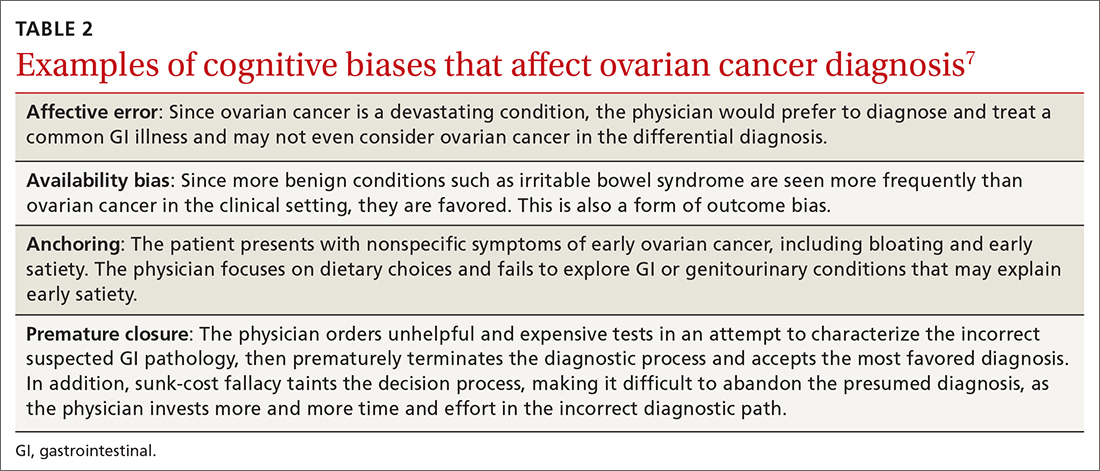
Responses from the survey also revealed that physicians initially ordered work-ups related to GI etiology and only later considered a pelvic work-up. This suggests that well-known presenting signs and symptoms or a constellation of typical and atypical symptoms of OC often failed to trigger physician recognition. Understandably, patients presenting with menorrhagia or gynecologic complaints are more likely to have OC detected at an earlier stage than patients who present with GI or abdominal signs alone.31 table 27 summarizes some of the cognitive biases seen in the diagnostic path of OC.
While in the hospital, he becomes acutely upset by the hallucinations and is given haloperidol and lorazepam by house staff. In the morning, the patient exhibits severe signs of Parkinson disease that include rigidity and masked facies.
Given the patient’s poor response to haloperidol and continued confusion, the team consulted Neurology and Psychiatry. Gathering a more detailed history from the patient and family, the patient is given a diagnosis of classic LBD. The antipsychotic medications are stopped. The patient and his family receive education about LBD treatment and management, and the patient is discharged to outpatient care.
Psychiatric symptoms can be an early “misdirect” in cases of Lewy body disease
LBD, the second leading neurodegenerative dementia after Alzheimer disease (AD), affects 1.5 million Americans,32 representing about 10% of all dementia cases. LBD and AD overlap in 25% of dementia cases.33 In patients older than 85 years, the prevalence jumps to 5% of the general population and 22% of all cases of dementia.33 Despite its prevalence, a recent study showed that only 6% of PCPs correctly identified LBD as the primary diagnosis when presented with typical case examples.32
Continue to: 3 stages of presentation
3 stages of presentation. Unlike other forms of dementia, LBD typically presents first with psychiatric symptoms, then with cognitive impairment, and last with parkinsonian symptoms. Additionally, rapid eye movement sleep behavior disorder and often subtle elements of nonmemory cognitive impairment distinguish LBD from both AD and vascular dementia.32 The primary cognitive deficit in LBD is not in memory but in attention, executive functioning, and visuospatial ability.34 Only in the later stages of the disease do patients exhibit gradual and progressive memory loss.
Mistaken for many things. When evaluating patients exhibiting signs of dementia, it’s important to include LBD in the differential, with increased suspicion for patients experiencing episodes of psychosis or delirium. The uniqueness of LBD lies in its psychotic symptomatology, particularly during earlier stages of the disease. This feature helps distinguish LBD from both AD and vascular dementia. As seen in the case, LBD can also be confused with acute delirium.
Older adult patients presenting to the ED or clinic with visual hallucinations, delirium, and mental confusion may receive a false diagnosis of schizophrenia, medication- or substance-induced psychosis, Parkinson disease, or delirium of unknown etiology.35 Unfortunately, LBD is often overlooked and not considered in the differential diagnosis. Due to underrecognition, patients may receive treatment with typical antipsychotics. The addition of a neuroleptic to help control the psychotic symptoms causes patients with LBD to develop severe extrapyramidal symptoms and worsening mental status,36 leading to severe parkinsonian signs, which further muddies the diagnostic process. In addition, treatment for suspected Parkinson disease, including carbidopa-levodopa, has no benefit for patients with LBD and may increase psychotic symptoms.37
First-line treatment for LBD includes psychoeducation for the patient and family, cholinesterase inhibitors (eg, rivastigmine), and avoidance of high-potency antipsychotics, such as haloperidol. Although persistent hallucinations and psychosis remain difficult to treat in LBD, low-dose quetiapine is 1 option. Incorrectly diagnosing and prescribing treatment for another condition exacerbates symptoms in this patient population.
The patient has been experiencing chronic pain for the past few years after a motor vehicle accident. She has seen a physiatrist and another provider, both of whom found no “objective” causes of her chronic pain. They started the patient on sertraline for depression and an analgesic, both of which were ineffective.
The patient likes to exercise at a gym twice a week by doing light cardio (treadmill) exercise and light weightlifting. Lately, however, she has been unable to exercise due to the pain. At this visit, she mentions having low energy, poor sleep, frequent fatigue, and generalized soreness and pain in multiple areas of her body. The PCP recognizes the patient’s presenting symptoms as significant for FM and starts her on pregabalin and hydrotherapy, with positive results.
Continue to: Fibromyalgia skepticism may lead to a Dx of depression
Fibromyalgia skepticism may lead to a Dx of depression
FM, the second most common disorder seen in rheumatologic practice after OA, is estimated to affect approximately 1 in 20 patients (approximately 5 million Americans) in the primary care setting.38,39 The condition has a high female-to-male preponderance (3.4% vs 0.5%).40 While the primary symptom of FM is chronic pain, patients commonly present with fatigue and sleep disturbance.41 Comorbid conditions include headaches, irritable bowel syndrome, and mood disturbances (most commonly anxiety and depression).
Several studies have explored reasons for the misdiagnosis and underdiagnosis of FM. One important factor is ongoing skepticism among some physicians and the public, in general, as to whether FM is a real disease. This issue was addressed by a study by White and colleagues,42 who estimated and compared the point prevalence of FM and related disorders in Amish vs non-Amish adults. The authors hypothesized that if litigation and/or compensation availability have a major impact on FM prevalence, then there would be a near zero prevalence of FM in the Amish community. And yet, researchers found an overall age- and sex-adjusted FM prevalence of 7.3% (95% CI; 5.3%-9.7%); this was both statistically greater than zero (P < .0001) and greater than 2 control populations of non-Amish adults (both P < .05).
Many physicians consider FM fundamentally an emotional disturbance, and the high preponderance of FM in female patients may contribute to this misconception as reports of pain and emotional distress by women are often dismissed as hysteria.43 Physicians often explore the emotional aspects of FM, incorrectly diagnosing patients with depression and subsequently treating them with a psychotropic drug.39 Alternatively, they may focus on the musculoskeletal presentations of FM and prescribe analgesics or physical therapy, both of which do little to alleviate FM.
To make the correct diagnosis of FM, the American College of Rheumatology created a specific set of criteria in 1990, which was updated in 2010.44 For a diagnosis of FM, a patient must have at least a 3-month history of bilateral pain above and below the waist and along the axial skeletal spine. Although not included in the updated 2010 criteria, many clinicians continue to check for tender points, following the 1990 criteria requiring the presence of 11 of 18 points to make the diagnosis.
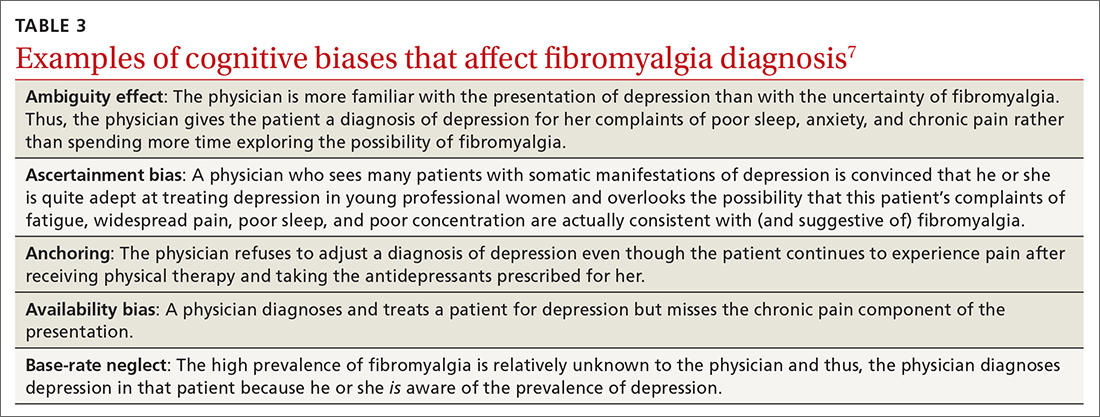
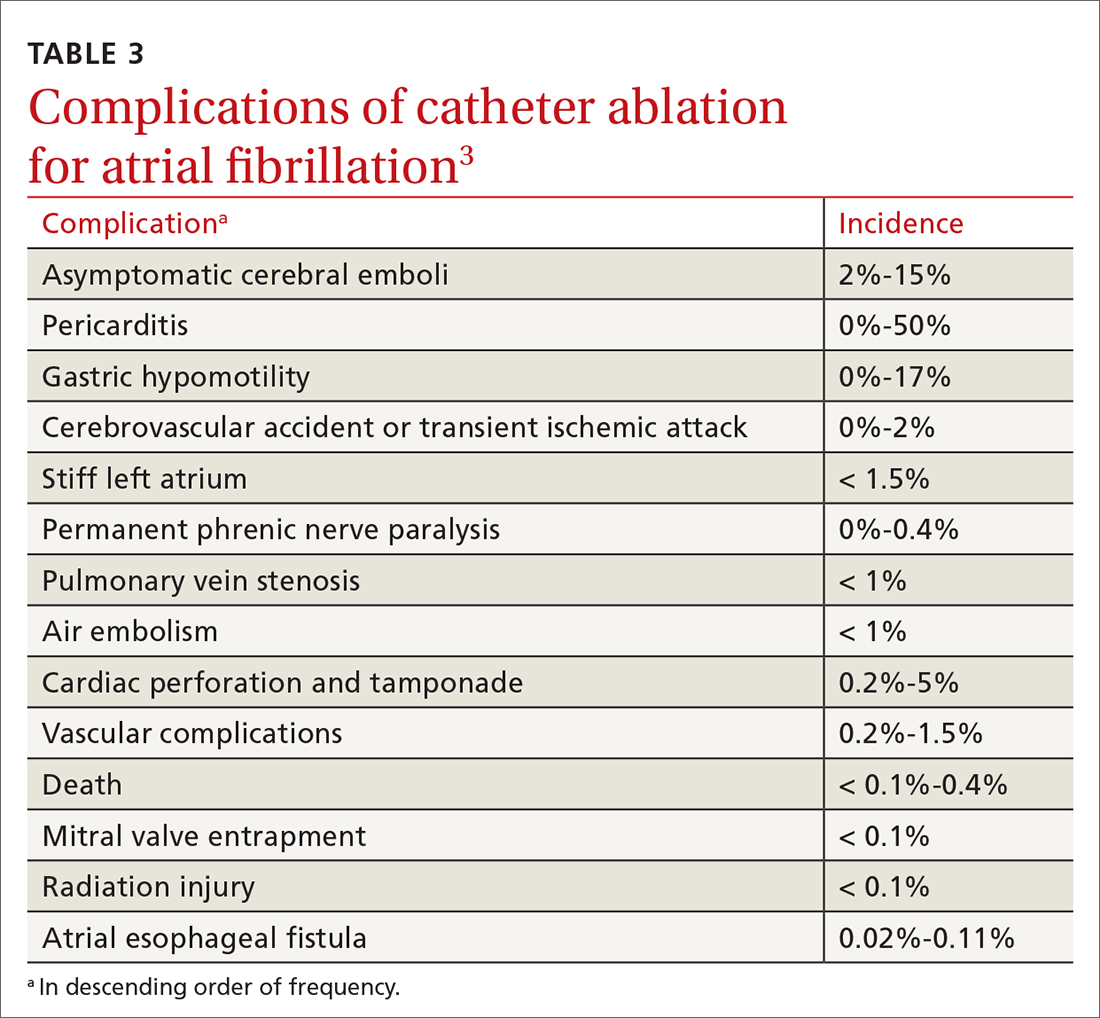
At least 3 cognitive biases relating to FM apply: anchoring, availability, and fundamental attribution error (see table 3).7 Anchoring occurs when the PCP settles on a psychiatric diagnosis of exaggerated pain syndrome, muscle overuse, or OA and fails to explore alternative etiology. Availability bias may obscure the true diagnosis of FM. Since PCPs see many patients with RA or OA, they may overlook or dismiss the possibility of FM. Attribution error happens when physicians dismiss the complaints of patients with FM as merely due to psychological distress, hysteria, or acting out.43
Patients with FM, who are often otherwise healthy, often present multiple times to the same PCP with a chief complaint of chronic pain. These repeat presentations can result in compassion fatigue and impact care. As Aloush and colleagues40 noted in their study, “FM patients were perceived as more difficult than RA patients, with a high level of concern and emotional response. A high proportion of physicians were reluctant to accept them because they feel emotional/psychological difficulties meeting and coping with these patients.”In response, patients with undiagnosed FM or inadequately treated FM may visit other PCPs, which may or may not result in a correct diagnosis and treatment.
We can do better
Primary care physicians face the daunting task of diagnosing and treating a wide range of common conditions while also trying to recognize less-common conditions with atypical presentations—all during a busy clinic workday. Nonetheless, we should strive to overcome internal (eg, cognitive bias and fund-of-knowledge deficits) and external (eg, time constraints, limited resources) pressures to improve diagnostic accuracy and care.
Each of the 4 disease states we’ve discussed have high rates of missed and/or delayed diagnosis. Each presents a unique set of confounders: PMR with its overlapping symptoms of many other rheumatologic diseases; OC with its often vague and misleading GI symptomatology; LBD with overlapping features of AD and Parkinson disease; and FM with skepticism. As gatekeepers to health care, it falls on PCPs to sort out these diagnostic dilemmas to avoid medical errors. Fundamental knowledge of each disease, its unique pathophysiology and symptoms, and varying presentations can be learned, internalized, and subsequently put into clinical practice to improve patient outcomes.
CORRESPONDENCE
Paul D. Rosen MD, Brooklyn Hospital Center, Department of Family Medicine, 121 Dekalb Avenue, Brooklyn, New York 11201; [email protected]
1. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139. doi: 10.1136/bmj.i2139
2. Van Such M, Lohr R, Beckman T, et al. Extent of diagnostic agreement among medical referrals. J Eval Clin Pract. 2017;23:870-874. doi: 10.1111/jep.12747
3. Groopman JE. How Doctors Think. Houghton Mifflin; 2007.
4. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185:1124-1131. doi: 10.1126/science.185.4157.1124
5. Norman GR, Monteiro SD, Sherbino J, et al. The causes of errors in clinical reasoning: Cognitive biases, knowledge deficits, and dual process thinking. Acad Med. 2017;92:23-30. doi: 10.1097/ACM.0000000000001421
6. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780. doi: 10.1097/00001888-200308000-00003
7. Morgenstern J. Cognitive errors in medicine: The common errors. First10EM blog. September 15, 2015. Updated September 22, 2019. Accessed February 8, 2022. https://first10em.com/cognitive-errors/
8. Gazitt T, Zisman D, Gardner G. Polymyalgia rheumatica: a common disease in seniors. Curr Rheumatol Rep. 2020;22:40. doi: 10.1007/s11926-020-00919-2
9. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35. doi: 10.1002/art.23176
10. Doran MF, Crowson CS, O’Fallon WM, et al. Trends in the incidence of polymyalgia rheumatica over a 30 year period in Olmsted County, Minnesota, USA. J Rheumatol. 2002;29:1694-1697.
11. Barraclough K, Liddell WG, du Toit J, et al. Polymyalgia rheumatica in primary care: a cohort study of the diagnostic criteria and outcome. Fam Pract. 2008;25:328-33. doi: 10.1093/fampra/cmn044
12. Manzo C. Polymyalgia rheumatica (PMR) with normal values of both erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) concentration at the time of diagnosis in a centenarian man: a case report. Diseases. 2018;6:84. doi: 10.3390/diseases6040084
13. Crowson CS, Matteson EL. Contemporary prevalence estimates for giant cell arteritis and polymyalgia rheumatica, 2015. Semin Arthritis Rheum. 2017;47:253-256. doi: 10.1016/j.semarthrit.2017.04.001
14. Nordborg E, Bengtsson BA. Death rates and causes of death in 284 consecutive patients with giant cell arteritis confirmed by biopsy. BMJ. 1989;299:549-550. doi: 10.1136/bmj.299.6698.549
15. Bahlas S, Ramos-Remus C, Davis P. Utilisation and costs of investigations, and accuracy of diagnosis of polymyalgia rheumatica by family physicians. Clin Rheumatol. 2000;19:278-280. doi: 10.1007/s100670070045
16. Brooks RC, McGee SR. Diagnostic dilemmas in polymyalgia rheumatica. Arch Intern Med. 1997;157:162-168.
17. Blaauw AA, Schuwirth LW, van der Vleuten CP, et al. Assessing clinical competence: recognition of case descriptions of rheumatic diseases by general practitioners. Br J Rheumatol. 1995;34:375-379. doi:10.1093/rheumatology/34.4.375
18. Mager DR. Polymylagia rheumatica: common disease, elusive diagnosis. Home Healthc Now. 2015;33:132-138. doi:10.1097/NHH.0000000000000199
19. Kermani TA, Warrington KJ. Polymyalgia rheumatica. Lancet. 381;63-72. doi: 10.1016/S0140-6736(12)60680-1. Published correction appears in Lancet. 20135;381:28.
20. Liew DF, Owen CE, Buchanan RR. Prescribing for polymyalgia rheumatica. Aust Prescr. 2018;41:14-19. doi: 10.18773/austprescr.2018.001
21. Ovarian cancer statistics. Centers for Disease Control and Prevention. Reviewed June 8, 2021. Accessed February 22, 2022. www.cdc.gov/cancer/ovarian/statistics/index.htm
22. Key statistics for ovarian cancer. American Cancer Society. Revised January 12, 2022. Accessed February 22, 2022. www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html
23. Survival rates for ovarian cancer. American Cancer Society. Revised January 25, 2021. Accessed February 22, 2022. www.cancer.org/cancer/ovarian-cancer/detection-diagnosis-staging/survival-rates.html
24. Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med. 2017;14:9-32. doi: 10.20892/j.issn.2095-3941.2016.0084
25. Goff BA, Mandel LS, Melancon CH, et al. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291:2705-2712. doi: 10.1001/jama.291.22.2705
26. Olson SH, Mignone L, Nakraseive C, et al. Symptoms of ovarian cancer. Obstet Gynecol. 2001;98:212-217. doi: 10.1016/s0029-7844(01)01457-0
27. Allgar VL, Neal RD. Delays in the diagnosis of six cancers: analysis of data from the National Survey of NHS patients: Cancer. Br J Cancer. 2005;92:1959-1970. doi: 10.1038/sj.bjc.6602587
28. Gajjar K, Ogden G, Mujahid MI, et al. Symptoms and risk factors of ovarian cancer: a survey in primary care. Obstet Gynecol. 2012:754197. doi: 10.5402/2012/754197
29. Austoker J. Diagnosis of ovarian cancer in primary care. BMJ. 2009;339:b3286. doi: 10.1136/bmj.b3286
30. Goff BA, Mandel L, Muntz HG, et al. Ovarian carcinoma diagnosis: results of a national ovarian cancer survey. Cancer. 2000;89:2068-2075. doi: 10.1002/1097-0142(20001115)89:10<2068::aid-cncr6>3.0.co;2-z
31. Smith EM, Anderson B. The effects of symptoms and delay in seeking diagnosis on stage of disease at diagnosis among women with cancers of the ovary. Cancer. 1985;56:2727-2732. doi: 10.1002/1097-0142(19851201)56:11<2727::aid-cncr2820561138>3.0.co;2-8
32. Galvin JE, Duda JE, Kaufer DI, et al. Lewy body dementia: the caregiver experience of clinical care. Parkinsonism Relat Disord. 2010;16:388-392. doi: 10.1016/j.parkreldis.2010.03.007
33. McKeith I. Dementia with Lewy bodies. Dialogues Clin Neurosci. 2004;6:333-341. doi: 10.31887/DCNS.2004.6.3/imckeith
34. Mrak RE, Griffin WS. Dementia with Lewy bodies: definitions, diagnosis, and pathogenic relationship to Alzheimer’s disease. Neuropsychiatr Dis Treat. 2007;3:619-625.
35. Khotianov N, Singh R, Singh S. Lewy body dementia: case report and discussion. J Am Board Fam Pract. 2002;15:50-54.
36. Stinton C, McKeith I, Taylor JP, et al. Pharmacological management of Lewy body dementia: a systematic review and meta-analysis. Am J Psychiatry. 2015;172:731-742. doi: 10.1176/appi.ajp.2015.14121582
37. Velayudhan L, Ffytche D, Ballard C, et al. New therapeutic strategies of Lewy body dementias. Curr Neurol Neurosci Rep. 2017;17:68. doi: 10.1007/s11910-017-0778-2
38. Arnold LM, Clauw DJ, McCarberg BH; FibroCollaborative. Improving the recognition and diagnosis of fibromyalgia. Mayo Clin Proc. 2011;86:457-464. doi: 10.4065/mcp.2010.0738
39. Arnold LM, Gebke KB, Choy EHS. Fibromyalgia: management strategies for primary care providers. Int J Clin Pract. 2016;70:99-112. doi: 10.1111/ijcp.12757
40. Aloush V, Niv D, Ablin JN, et al. Good pain, bad pain: illness perception and physician attitudes towards rheumatoid arthritis and fibromyalgia patients. Clin Exp Rheumatol. 2021;39(suppl 130):54-60.
41. Vincent A, Lahr BD, Wolfe F, et al. Prevalence of fibromyalgia: a population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis Care Res (Hoboken). 2013;65:786-792. doi: 10.1002/acr.21896
42. White KP, Thompson J. Fibromyalgia syndrome in an Amish community: a controlled study to determine disease and symptom prevalence. J Rheumatol. 2003;30:1835-1840.
43. Lobo CP, Pfalzgraf AR, Giannetti V, et al. Impact of invalidation and trust in physicians on health outcomes in fibromyalgia patients. Prim Care Companion CNS Disord. 2014;16:10.4088/PCC.14m01664. doi: 10.4088/PCC.14m01664
44. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken). 2010;62:600-610. doi:10.1002/acr.20140
Medical errors in all settings contributed to as many as 250,000 deaths per year in the United States between 2000 and 2008, according to a 2016 study.1 Diagnostic error, in particular, remains a leading cause of morbidity and mortality in the United States and worldwide. In 2017, 12 million patients (roughly 5% of all US adults) who sought outpatient care experienced missed, delayed, or incorrect diagnosis at least once.2
In his classic work, How Doctors Think, Jerome Groopman, MD, explored the diagnostic process with a focus on the role of cognitive bias in clinical decision-making. Groopman examined how physicians can become sidetracked in their thinking and “blinded” to potential alternative diagnoses.3 Medical error is not necessarily because of a deficiency in medical knowledge; rather, physicians become susceptible to medical error when defective and faulty reasoning distort their diagnostic ability.4
Cognitive bias in the diagnostic process has been extensively studied, and a full review is beyond the scope of this article.5 However, here we will examine pathways leading to diagnostic errors in the primary care setting, specifically the role of cognitive bias in the work-up of polymyalgia rheumatica (PMR), ovarian cancer (OC), Lewy body dementia (LBD), and fibromyalgia (FM). As these 4 disease states are seen with low-to-moderate frequency in primary care, cognitive bias can complicate accurate diagnosis. But first, a word about how to understand clinical reasoning.
There are 2 types of reasoning (and 1 is more prone to error)
Physician clinical reasoning can be divided into 2 different cognitive approaches.
Type 1 reasoning employs intuition and heuristics; this type is automatic, reflexive, and quick.5 While the use of mental shortcuts in type 1 increases the speed with which decisions are made, it also makes this form of reasoning more prone to error.
Type 2 reasoning requires conscious effort. It is goal directed and rigorous and therefore slower than type 1 reasoning. Extrapolated to the clinical context, clinicians transition from type 2 to type 1 reasoning as they gain experience and training throughout their careers and develop their own conscious and subconscious heuristics. Deviations from accurate decision-making occur in a systematic manner due to cognitive biases and result in medical error.6table 17 lists common types of cognitive bias.
An important question to ask. Physicians tend to fall into a pattern of quick, type 1 reasoning. However, it’s important to strive to maintain a broad differential diagnosis and avoid premature closure of the diagnostic process. It’s critical that we consider alternative diagnoses (ie, consciously move from type 1 to type 2 thinking) and continue to ask ourselves, “What else?” while working through differential diagnoses. This can be a powerful debiasing technique.
Continue to: The discussion...
The discussion of the following 4 disease states demonstrates how cognitive bias can lead to diagnostic error.
The patient is barely able to ambulate and appears to be in considerable pain. She is relying heavily on her walker and is assisted by her granddaughter. The primary care physician (PCP) obtains a detailed history that includes chronic shoulder and hip pain. Given that the patient has not responded to NSAID treatment over the previous 6 months, the PCP takes a moment to reconsider the diagnosis of OA and considers other options.
In light of the high prevalence of PMR in older women, the physician pursues a more specific physical examination tailored to ferret out PMR. He had learned this diagnostic shortcut as a resident, remembered it, and adeptly applied it whenever circumstances warranted. He asks the patient to raise her arms above her head (goalpost sign). She is unable to perform this task and experiences severe bilateral shoulder pain on trial. The PCP then places the patient on the examining table and attempts to assist her in rolling toward him. The patient is also unable to perform this maneuver and experiences significant bilateral hip pain on trial.
Based primarily on the patient’s history and physical exam findings, the PCP makes a presumptive diagnosis of PMR vs OA vs combined PMR with OA, orders an erythrocyte sedimentation rate (ESR) and basic rheumatologic
PMR can be mistaken for OA
PMR is the most common inflammatory rheumatic disease in older patients.8 It is a debilitating illness with simple, effective treatment but has devastating consequences if missed or left untreated.9 PMR typically manifests in patients older than age 50, with a peak incidence at 80 years of age. It is also far more common in women.10
Approximately 80% of patients with PMR initially present to their PCP, often posing a diagnostic challenge to many clinicians.11 Due to overlap in symptoms, the condition is often misdiagnosed as OA, a more common condition seen by PCPs. Also, there are no specific diagnostic tests for PMR. An elevated ESR can help confirm the diagnosis, but one-third of patients with PMR have a normal ESR.12 Therefore, the diagnostic conundrum the physician faces is OA vs rheumatoid arthritis (RA), PMR, or another condition.
Continue to: The consequences...
The consequences of a missed and delayed PMR diagnosis range from seriously impaired quality of life to significantly increased risk of vascular events (eg, blindness, stroke) due to temporal arteritis.13 Early diagnosis is even more critical as the risk of a vascular event and death is highest during initial phases of the disease course.14
FPs often miss this Dx. A timely diagnosis relies almost exclusively on an accurate, thorough history and physical exam. However, PCPs often struggle to correctly diagnose PMR. According to a study by Bahlas and colleagues,15 the accuracy rate for correctly diagnosing PMR was 24% among a cohort of family physicians.
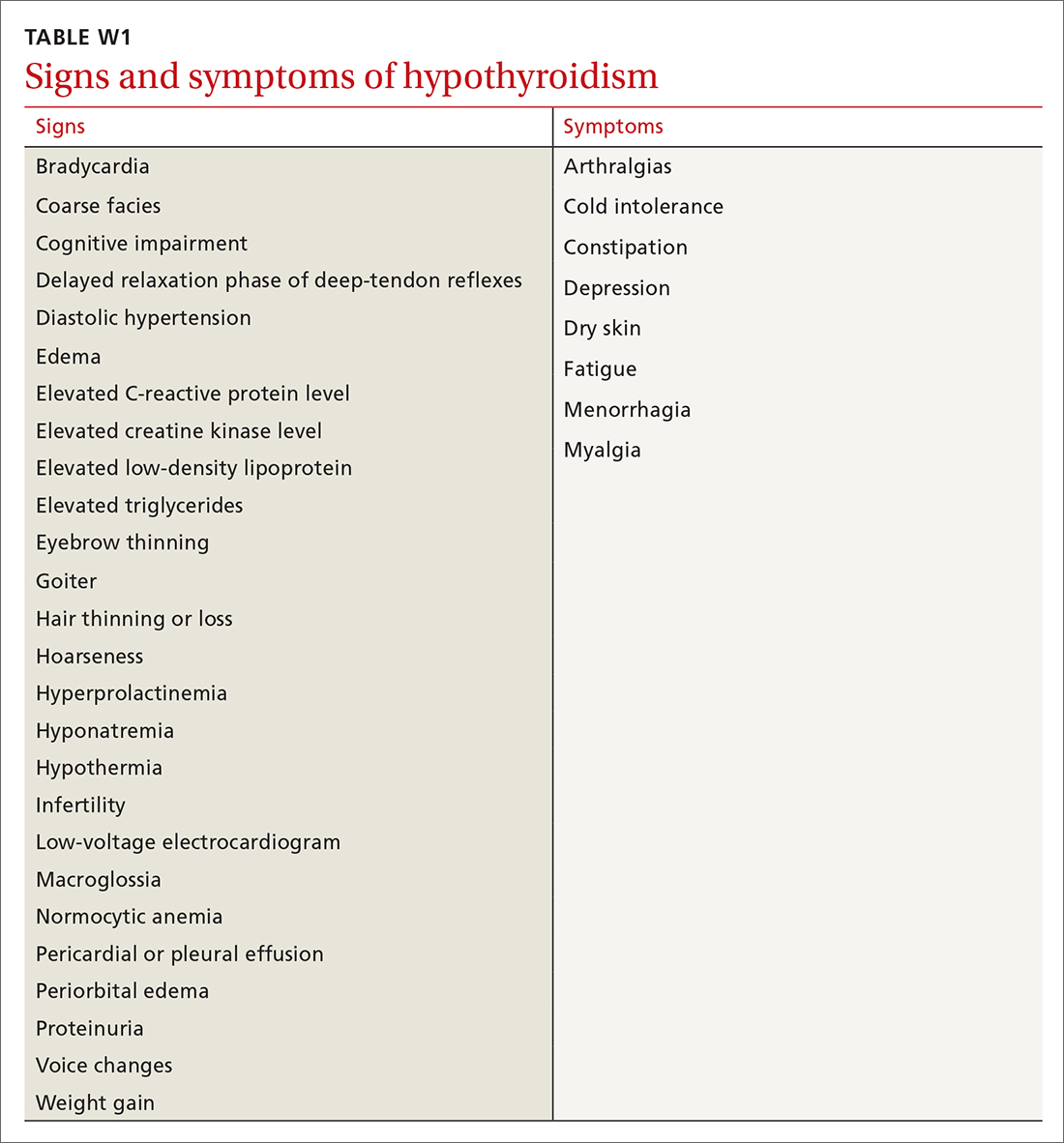
The differential diagnosis for PMR is broad and includes seronegative spondyloarthropathies, malignancy, Lyme disease, hypothyroidism, and both RA and OA.16
PCPs are extremely adept at correctly diagnosing RA, but not PMR. A study by Blaauw and colleagues17 comparing PCPs and rheumatologists found PCPs correctly identified 92% of RA cases but only 55% of PMR cases. When rheumatologists reviewed these same cases, they correctly identified PMR and RA almost 100% of the time.17 The difference in diagnostic accuracy between rheumatologists and PCPs suggests limited experience and gaps in fund of knowledge.
Making the diagnosis. The diagnosis of PMR is often made on empiric response to corticosteroid treatment, but doing so based solely on a patient’s response is controversial.18 There are rare instances in which patients with PMR fail to respond to treatment. On the other hand, some inflammatory conditions that mimic or share symptoms with PMR also respond to corticosteroids, potentially resulting in erroneous confirmation bias.
Some classification criteria use rapid response to low-dose prednisone/prednisolone (≤ 20 mg) to confirm the diagnosis,19 while other more recent guidelines no longer include this approach.20 If PMR continues to be suspected after a trial of steroids is unsuccessful, the PCP can try another course of higher dose steroids or consult with Rheumatology.
Continue to: A full history...
A full history and physical exam revealed a myriad of gastrointestinal (GI) complaints, such as diarrhea. But the PCP recalled a recent roundtable discussion on debiasing techniques specifically related to gynecologic disorders, including OC. Therefore, he decided to include OC in the differential diagnosis—something he would not routinely have done given the preponderance of GI symptoms. Despite the patient’s reluctance and time constraints, the PCP ordered a transvaginal ultrasound. Findings from the ultrasound study revealed stage II OC, which carries a good prognosis. The patient is currently undergoing treatment and was last reported as doing well.
Early signs of ovarian cancer can be chalked up to a “GI issue”
OC is the second most common gynecologic cancer21 and the fifth leading cause of cancer-related death22 in US women. Compared to other cancers, the prognosis for localized early-stage OC is surprisingly good, with a 5-year survival rate approaching 93%.23 However, most disease is detected in later stages, and the 5-year survival rate drops to a low of 29%.24
There remains no established screening protocol for OC. Fewer than a quarter of all cases are diagnosed in stage I, and detection of OC relies heavily on the physician’s ability to decipher vague symptomatology that overlaps with other, more common maladies. This poses an obvious diagnostic challenge and, not surprisingly, a high level of susceptibility to cognitive bias.
More than 90% of patients with OC present with some combination of the following symptoms prior to diagnosis: abdominal (77%), GI (70%), pain (58%), constitutional (50%), urinary (34%), and pelvic (26%).25 The 3 most common isolated symptoms in patients with OC are abdominal bloating, decrease in appetite, and frank abdominal pain.26 Patients with biopsy-confirmed OC experience these symptoms an average of 6 months prior to actual diagnosis.27
Knowledge gaps play a role. Studies assessing the ability of health care providers to identify presenting symptoms of OC reveal specific knowledge gaps. For instance, in a survey by Gajjar and colleagues,28 most PCPs correctly identified bloating as a key symptom of OC; however, they weren’t as good at identifying less common symptoms, such as inability to finish a meal and early satiety. Moreover, survey participants misinterpreted or missed GI symptoms as an important manifestation of early OC disease.28 These specific knowledge gaps combine with physician errors in thinking, further obscuring and extending the diagnostic process. The point prevalence for OC is relatively low, and many PCPs only encounter a few cases during their entire career.29 This low pre-test probability may also fuel the delay in diagnosis.
Watch for these forms of bias. Since nonspecific symptoms of early-stage OC resemble those of other more benign conditions, a form of anchoring error known as multiple alternatives bias can arise. In this scenario, clinicians investigate only 1 potential plausible diagnosis and remain focused on that single, often faulty, conclusion. This persists despite other equally plausible alternatives that arise as the investigation proceeds.28
Affective error may also play a role in missed or delayed diagnosis. For example, a physician would prefer to diagnose and treat a common GI illness than consider OC. Another distortion involves outcome bias wherein the physician gives more significance to benign conditions such as irritable bowel syndrome because they have a more favorable outcome and clear treatment path. Physicians also favor these benign conditions because they encounter them more frequently than OC in the clinic setting. (This is known as availability bias.) Outcome bias and multiple alternatives bias can result in noninvestigation of symptoms and inefficient or improper management, leading to a delay in arriving at the correct diagnosis or anchoring on a plausible but incorrect diagnosis.
Continue to: An incorrect initial diagnostic...
An incorrect initial diagnostic path often triggers a cascade of subsequent errors. The physician orders additional unhelpful and expensive tests in an effort to characterize the suspected GI pathology. This then leads the physician to prematurely terminate the work-up and accept the most favored diagnosis. Lastly, sunk-cost fallacy comes into play: The physician has “invested” time and energy investigating a particular diagnosis and rather than abandon the presumed diagnosis, continues to put more time and effort in going down an incorrect diagnostic path.
A series of failures. These biases and miscues have been observed in several studies. For example, a survey of 1725 women by Goff and colleagues30 sought to identify factors related to delayed OC diagnosis. The authors found that the following factors were significantly associated with a delayed diagnosis: omission of a pelvic exam at initial presentation, a separate exploration of a multitude of collateral symptoms, a failure to order ultrasound/computed tomography/CA-125 test, and a failure to consider age as a factor (especially if the patient was outside the norm).
Responses from the survey also revealed that physicians initially ordered work-ups related to GI etiology and only later considered a pelvic work-up. This suggests that well-known presenting signs and symptoms or a constellation of typical and atypical symptoms of OC often failed to trigger physician recognition. Understandably, patients presenting with menorrhagia or gynecologic complaints are more likely to have OC detected at an earlier stage than patients who present with GI or abdominal signs alone.31 table 27 summarizes some of the cognitive biases seen in the diagnostic path of OC.
While in the hospital, he becomes acutely upset by the hallucinations and is given haloperidol and lorazepam by house staff. In the morning, the patient exhibits severe signs of Parkinson disease that include rigidity and masked facies.
Given the patient’s poor response to haloperidol and continued confusion, the team consulted Neurology and Psychiatry. Gathering a more detailed history from the patient and family, the patient is given a diagnosis of classic LBD. The antipsychotic medications are stopped. The patient and his family receive education about LBD treatment and management, and the patient is discharged to outpatient care.
Psychiatric symptoms can be an early “misdirect” in cases of Lewy body disease
LBD, the second leading neurodegenerative dementia after Alzheimer disease (AD), affects 1.5 million Americans,32 representing about 10% of all dementia cases. LBD and AD overlap in 25% of dementia cases.33 In patients older than 85 years, the prevalence jumps to 5% of the general population and 22% of all cases of dementia.33 Despite its prevalence, a recent study showed that only 6% of PCPs correctly identified LBD as the primary diagnosis when presented with typical case examples.32
Continue to: 3 stages of presentation
3 stages of presentation. Unlike other forms of dementia, LBD typically presents first with psychiatric symptoms, then with cognitive impairment, and last with parkinsonian symptoms. Additionally, rapid eye movement sleep behavior disorder and often subtle elements of nonmemory cognitive impairment distinguish LBD from both AD and vascular dementia.32 The primary cognitive deficit in LBD is not in memory but in attention, executive functioning, and visuospatial ability.34 Only in the later stages of the disease do patients exhibit gradual and progressive memory loss.
Mistaken for many things. When evaluating patients exhibiting signs of dementia, it’s important to include LBD in the differential, with increased suspicion for patients experiencing episodes of psychosis or delirium. The uniqueness of LBD lies in its psychotic symptomatology, particularly during earlier stages of the disease. This feature helps distinguish LBD from both AD and vascular dementia. As seen in the case, LBD can also be confused with acute delirium.
Older adult patients presenting to the ED or clinic with visual hallucinations, delirium, and mental confusion may receive a false diagnosis of schizophrenia, medication- or substance-induced psychosis, Parkinson disease, or delirium of unknown etiology.35 Unfortunately, LBD is often overlooked and not considered in the differential diagnosis. Due to underrecognition, patients may receive treatment with typical antipsychotics. The addition of a neuroleptic to help control the psychotic symptoms causes patients with LBD to develop severe extrapyramidal symptoms and worsening mental status,36 leading to severe parkinsonian signs, which further muddies the diagnostic process. In addition, treatment for suspected Parkinson disease, including carbidopa-levodopa, has no benefit for patients with LBD and may increase psychotic symptoms.37
First-line treatment for LBD includes psychoeducation for the patient and family, cholinesterase inhibitors (eg, rivastigmine), and avoidance of high-potency antipsychotics, such as haloperidol. Although persistent hallucinations and psychosis remain difficult to treat in LBD, low-dose quetiapine is 1 option. Incorrectly diagnosing and prescribing treatment for another condition exacerbates symptoms in this patient population.
The patient has been experiencing chronic pain for the past few years after a motor vehicle accident. She has seen a physiatrist and another provider, both of whom found no “objective” causes of her chronic pain. They started the patient on sertraline for depression and an analgesic, both of which were ineffective.
The patient likes to exercise at a gym twice a week by doing light cardio (treadmill) exercise and light weightlifting. Lately, however, she has been unable to exercise due to the pain. At this visit, she mentions having low energy, poor sleep, frequent fatigue, and generalized soreness and pain in multiple areas of her body. The PCP recognizes the patient’s presenting symptoms as significant for FM and starts her on pregabalin and hydrotherapy, with positive results.
Continue to: Fibromyalgia skepticism may lead to a Dx of depression
Fibromyalgia skepticism may lead to a Dx of depression
FM, the second most common disorder seen in rheumatologic practice after OA, is estimated to affect approximately 1 in 20 patients (approximately 5 million Americans) in the primary care setting.38,39 The condition has a high female-to-male preponderance (3.4% vs 0.5%).40 While the primary symptom of FM is chronic pain, patients commonly present with fatigue and sleep disturbance.41 Comorbid conditions include headaches, irritable bowel syndrome, and mood disturbances (most commonly anxiety and depression).
Several studies have explored reasons for the misdiagnosis and underdiagnosis of FM. One important factor is ongoing skepticism among some physicians and the public, in general, as to whether FM is a real disease. This issue was addressed by a study by White and colleagues,42 who estimated and compared the point prevalence of FM and related disorders in Amish vs non-Amish adults. The authors hypothesized that if litigation and/or compensation availability have a major impact on FM prevalence, then there would be a near zero prevalence of FM in the Amish community. And yet, researchers found an overall age- and sex-adjusted FM prevalence of 7.3% (95% CI; 5.3%-9.7%); this was both statistically greater than zero (P < .0001) and greater than 2 control populations of non-Amish adults (both P < .05).
Many physicians consider FM fundamentally an emotional disturbance, and the high preponderance of FM in female patients may contribute to this misconception as reports of pain and emotional distress by women are often dismissed as hysteria.43 Physicians often explore the emotional aspects of FM, incorrectly diagnosing patients with depression and subsequently treating them with a psychotropic drug.39 Alternatively, they may focus on the musculoskeletal presentations of FM and prescribe analgesics or physical therapy, both of which do little to alleviate FM.
To make the correct diagnosis of FM, the American College of Rheumatology created a specific set of criteria in 1990, which was updated in 2010.44 For a diagnosis of FM, a patient must have at least a 3-month history of bilateral pain above and below the waist and along the axial skeletal spine. Although not included in the updated 2010 criteria, many clinicians continue to check for tender points, following the 1990 criteria requiring the presence of 11 of 18 points to make the diagnosis.
At least 3 cognitive biases relating to FM apply: anchoring, availability, and fundamental attribution error (see table 3).7 Anchoring occurs when the PCP settles on a psychiatric diagnosis of exaggerated pain syndrome, muscle overuse, or OA and fails to explore alternative etiology. Availability bias may obscure the true diagnosis of FM. Since PCPs see many patients with RA or OA, they may overlook or dismiss the possibility of FM. Attribution error happens when physicians dismiss the complaints of patients with FM as merely due to psychological distress, hysteria, or acting out.43
Patients with FM, who are often otherwise healthy, often present multiple times to the same PCP with a chief complaint of chronic pain. These repeat presentations can result in compassion fatigue and impact care. As Aloush and colleagues40 noted in their study, “FM patients were perceived as more difficult than RA patients, with a high level of concern and emotional response. A high proportion of physicians were reluctant to accept them because they feel emotional/psychological difficulties meeting and coping with these patients.”In response, patients with undiagnosed FM or inadequately treated FM may visit other PCPs, which may or may not result in a correct diagnosis and treatment.
We can do better
Primary care physicians face the daunting task of diagnosing and treating a wide range of common conditions while also trying to recognize less-common conditions with atypical presentations—all during a busy clinic workday. Nonetheless, we should strive to overcome internal (eg, cognitive bias and fund-of-knowledge deficits) and external (eg, time constraints, limited resources) pressures to improve diagnostic accuracy and care.
Each of the 4 disease states we’ve discussed have high rates of missed and/or delayed diagnosis. Each presents a unique set of confounders: PMR with its overlapping symptoms of many other rheumatologic diseases; OC with its often vague and misleading GI symptomatology; LBD with overlapping features of AD and Parkinson disease; and FM with skepticism. As gatekeepers to health care, it falls on PCPs to sort out these diagnostic dilemmas to avoid medical errors. Fundamental knowledge of each disease, its unique pathophysiology and symptoms, and varying presentations can be learned, internalized, and subsequently put into clinical practice to improve patient outcomes.
CORRESPONDENCE
Paul D. Rosen MD, Brooklyn Hospital Center, Department of Family Medicine, 121 Dekalb Avenue, Brooklyn, New York 11201; [email protected]
Medical errors in all settings contributed to as many as 250,000 deaths per year in the United States between 2000 and 2008, according to a 2016 study.1 Diagnostic error, in particular, remains a leading cause of morbidity and mortality in the United States and worldwide. In 2017, 12 million patients (roughly 5% of all US adults) who sought outpatient care experienced missed, delayed, or incorrect diagnosis at least once.2
In his classic work, How Doctors Think, Jerome Groopman, MD, explored the diagnostic process with a focus on the role of cognitive bias in clinical decision-making. Groopman examined how physicians can become sidetracked in their thinking and “blinded” to potential alternative diagnoses.3 Medical error is not necessarily because of a deficiency in medical knowledge; rather, physicians become susceptible to medical error when defective and faulty reasoning distort their diagnostic ability.4
Cognitive bias in the diagnostic process has been extensively studied, and a full review is beyond the scope of this article.5 However, here we will examine pathways leading to diagnostic errors in the primary care setting, specifically the role of cognitive bias in the work-up of polymyalgia rheumatica (PMR), ovarian cancer (OC), Lewy body dementia (LBD), and fibromyalgia (FM). As these 4 disease states are seen with low-to-moderate frequency in primary care, cognitive bias can complicate accurate diagnosis. But first, a word about how to understand clinical reasoning.
There are 2 types of reasoning (and 1 is more prone to error)
Physician clinical reasoning can be divided into 2 different cognitive approaches.
Type 1 reasoning employs intuition and heuristics; this type is automatic, reflexive, and quick.5 While the use of mental shortcuts in type 1 increases the speed with which decisions are made, it also makes this form of reasoning more prone to error.
Type 2 reasoning requires conscious effort. It is goal directed and rigorous and therefore slower than type 1 reasoning. Extrapolated to the clinical context, clinicians transition from type 2 to type 1 reasoning as they gain experience and training throughout their careers and develop their own conscious and subconscious heuristics. Deviations from accurate decision-making occur in a systematic manner due to cognitive biases and result in medical error.6table 17 lists common types of cognitive bias.
An important question to ask. Physicians tend to fall into a pattern of quick, type 1 reasoning. However, it’s important to strive to maintain a broad differential diagnosis and avoid premature closure of the diagnostic process. It’s critical that we consider alternative diagnoses (ie, consciously move from type 1 to type 2 thinking) and continue to ask ourselves, “What else?” while working through differential diagnoses. This can be a powerful debiasing technique.
Continue to: The discussion...
The discussion of the following 4 disease states demonstrates how cognitive bias can lead to diagnostic error.
The patient is barely able to ambulate and appears to be in considerable pain. She is relying heavily on her walker and is assisted by her granddaughter. The primary care physician (PCP) obtains a detailed history that includes chronic shoulder and hip pain. Given that the patient has not responded to NSAID treatment over the previous 6 months, the PCP takes a moment to reconsider the diagnosis of OA and considers other options.
In light of the high prevalence of PMR in older women, the physician pursues a more specific physical examination tailored to ferret out PMR. He had learned this diagnostic shortcut as a resident, remembered it, and adeptly applied it whenever circumstances warranted. He asks the patient to raise her arms above her head (goalpost sign). She is unable to perform this task and experiences severe bilateral shoulder pain on trial. The PCP then places the patient on the examining table and attempts to assist her in rolling toward him. The patient is also unable to perform this maneuver and experiences significant bilateral hip pain on trial.
Based primarily on the patient’s history and physical exam findings, the PCP makes a presumptive diagnosis of PMR vs OA vs combined PMR with OA, orders an erythrocyte sedimentation rate (ESR) and basic rheumatologic
PMR can be mistaken for OA
PMR is the most common inflammatory rheumatic disease in older patients.8 It is a debilitating illness with simple, effective treatment but has devastating consequences if missed or left untreated.9 PMR typically manifests in patients older than age 50, with a peak incidence at 80 years of age. It is also far more common in women.10
Approximately 80% of patients with PMR initially present to their PCP, often posing a diagnostic challenge to many clinicians.11 Due to overlap in symptoms, the condition is often misdiagnosed as OA, a more common condition seen by PCPs. Also, there are no specific diagnostic tests for PMR. An elevated ESR can help confirm the diagnosis, but one-third of patients with PMR have a normal ESR.12 Therefore, the diagnostic conundrum the physician faces is OA vs rheumatoid arthritis (RA), PMR, or another condition.
Continue to: The consequences...
The consequences of a missed and delayed PMR diagnosis range from seriously impaired quality of life to significantly increased risk of vascular events (eg, blindness, stroke) due to temporal arteritis.13 Early diagnosis is even more critical as the risk of a vascular event and death is highest during initial phases of the disease course.14
FPs often miss this Dx. A timely diagnosis relies almost exclusively on an accurate, thorough history and physical exam. However, PCPs often struggle to correctly diagnose PMR. According to a study by Bahlas and colleagues,15 the accuracy rate for correctly diagnosing PMR was 24% among a cohort of family physicians.
The differential diagnosis for PMR is broad and includes seronegative spondyloarthropathies, malignancy, Lyme disease, hypothyroidism, and both RA and OA.16
PCPs are extremely adept at correctly diagnosing RA, but not PMR. A study by Blaauw and colleagues17 comparing PCPs and rheumatologists found PCPs correctly identified 92% of RA cases but only 55% of PMR cases. When rheumatologists reviewed these same cases, they correctly identified PMR and RA almost 100% of the time.17 The difference in diagnostic accuracy between rheumatologists and PCPs suggests limited experience and gaps in fund of knowledge.
Making the diagnosis. The diagnosis of PMR is often made on empiric response to corticosteroid treatment, but doing so based solely on a patient’s response is controversial.18 There are rare instances in which patients with PMR fail to respond to treatment. On the other hand, some inflammatory conditions that mimic or share symptoms with PMR also respond to corticosteroids, potentially resulting in erroneous confirmation bias.
Some classification criteria use rapid response to low-dose prednisone/prednisolone (≤ 20 mg) to confirm the diagnosis,19 while other more recent guidelines no longer include this approach.20 If PMR continues to be suspected after a trial of steroids is unsuccessful, the PCP can try another course of higher dose steroids or consult with Rheumatology.
Continue to: A full history...
A full history and physical exam revealed a myriad of gastrointestinal (GI) complaints, such as diarrhea. But the PCP recalled a recent roundtable discussion on debiasing techniques specifically related to gynecologic disorders, including OC. Therefore, he decided to include OC in the differential diagnosis—something he would not routinely have done given the preponderance of GI symptoms. Despite the patient’s reluctance and time constraints, the PCP ordered a transvaginal ultrasound. Findings from the ultrasound study revealed stage II OC, which carries a good prognosis. The patient is currently undergoing treatment and was last reported as doing well.
Early signs of ovarian cancer can be chalked up to a “GI issue”
OC is the second most common gynecologic cancer21 and the fifth leading cause of cancer-related death22 in US women. Compared to other cancers, the prognosis for localized early-stage OC is surprisingly good, with a 5-year survival rate approaching 93%.23 However, most disease is detected in later stages, and the 5-year survival rate drops to a low of 29%.24
There remains no established screening protocol for OC. Fewer than a quarter of all cases are diagnosed in stage I, and detection of OC relies heavily on the physician’s ability to decipher vague symptomatology that overlaps with other, more common maladies. This poses an obvious diagnostic challenge and, not surprisingly, a high level of susceptibility to cognitive bias.
More than 90% of patients with OC present with some combination of the following symptoms prior to diagnosis: abdominal (77%), GI (70%), pain (58%), constitutional (50%), urinary (34%), and pelvic (26%).25 The 3 most common isolated symptoms in patients with OC are abdominal bloating, decrease in appetite, and frank abdominal pain.26 Patients with biopsy-confirmed OC experience these symptoms an average of 6 months prior to actual diagnosis.27
Knowledge gaps play a role. Studies assessing the ability of health care providers to identify presenting symptoms of OC reveal specific knowledge gaps. For instance, in a survey by Gajjar and colleagues,28 most PCPs correctly identified bloating as a key symptom of OC; however, they weren’t as good at identifying less common symptoms, such as inability to finish a meal and early satiety. Moreover, survey participants misinterpreted or missed GI symptoms as an important manifestation of early OC disease.28 These specific knowledge gaps combine with physician errors in thinking, further obscuring and extending the diagnostic process. The point prevalence for OC is relatively low, and many PCPs only encounter a few cases during their entire career.29 This low pre-test probability may also fuel the delay in diagnosis.
Watch for these forms of bias. Since nonspecific symptoms of early-stage OC resemble those of other more benign conditions, a form of anchoring error known as multiple alternatives bias can arise. In this scenario, clinicians investigate only 1 potential plausible diagnosis and remain focused on that single, often faulty, conclusion. This persists despite other equally plausible alternatives that arise as the investigation proceeds.28
Affective error may also play a role in missed or delayed diagnosis. For example, a physician would prefer to diagnose and treat a common GI illness than consider OC. Another distortion involves outcome bias wherein the physician gives more significance to benign conditions such as irritable bowel syndrome because they have a more favorable outcome and clear treatment path. Physicians also favor these benign conditions because they encounter them more frequently than OC in the clinic setting. (This is known as availability bias.) Outcome bias and multiple alternatives bias can result in noninvestigation of symptoms and inefficient or improper management, leading to a delay in arriving at the correct diagnosis or anchoring on a plausible but incorrect diagnosis.
Continue to: An incorrect initial diagnostic...
An incorrect initial diagnostic path often triggers a cascade of subsequent errors. The physician orders additional unhelpful and expensive tests in an effort to characterize the suspected GI pathology. This then leads the physician to prematurely terminate the work-up and accept the most favored diagnosis. Lastly, sunk-cost fallacy comes into play: The physician has “invested” time and energy investigating a particular diagnosis and rather than abandon the presumed diagnosis, continues to put more time and effort in going down an incorrect diagnostic path.
A series of failures. These biases and miscues have been observed in several studies. For example, a survey of 1725 women by Goff and colleagues30 sought to identify factors related to delayed OC diagnosis. The authors found that the following factors were significantly associated with a delayed diagnosis: omission of a pelvic exam at initial presentation, a separate exploration of a multitude of collateral symptoms, a failure to order ultrasound/computed tomography/CA-125 test, and a failure to consider age as a factor (especially if the patient was outside the norm).
Responses from the survey also revealed that physicians initially ordered work-ups related to GI etiology and only later considered a pelvic work-up. This suggests that well-known presenting signs and symptoms or a constellation of typical and atypical symptoms of OC often failed to trigger physician recognition. Understandably, patients presenting with menorrhagia or gynecologic complaints are more likely to have OC detected at an earlier stage than patients who present with GI or abdominal signs alone.31 table 27 summarizes some of the cognitive biases seen in the diagnostic path of OC.
While in the hospital, he becomes acutely upset by the hallucinations and is given haloperidol and lorazepam by house staff. In the morning, the patient exhibits severe signs of Parkinson disease that include rigidity and masked facies.
Given the patient’s poor response to haloperidol and continued confusion, the team consulted Neurology and Psychiatry. Gathering a more detailed history from the patient and family, the patient is given a diagnosis of classic LBD. The antipsychotic medications are stopped. The patient and his family receive education about LBD treatment and management, and the patient is discharged to outpatient care.
Psychiatric symptoms can be an early “misdirect” in cases of Lewy body disease
LBD, the second leading neurodegenerative dementia after Alzheimer disease (AD), affects 1.5 million Americans,32 representing about 10% of all dementia cases. LBD and AD overlap in 25% of dementia cases.33 In patients older than 85 years, the prevalence jumps to 5% of the general population and 22% of all cases of dementia.33 Despite its prevalence, a recent study showed that only 6% of PCPs correctly identified LBD as the primary diagnosis when presented with typical case examples.32
Continue to: 3 stages of presentation
3 stages of presentation. Unlike other forms of dementia, LBD typically presents first with psychiatric symptoms, then with cognitive impairment, and last with parkinsonian symptoms. Additionally, rapid eye movement sleep behavior disorder and often subtle elements of nonmemory cognitive impairment distinguish LBD from both AD and vascular dementia.32 The primary cognitive deficit in LBD is not in memory but in attention, executive functioning, and visuospatial ability.34 Only in the later stages of the disease do patients exhibit gradual and progressive memory loss.
Mistaken for many things. When evaluating patients exhibiting signs of dementia, it’s important to include LBD in the differential, with increased suspicion for patients experiencing episodes of psychosis or delirium. The uniqueness of LBD lies in its psychotic symptomatology, particularly during earlier stages of the disease. This feature helps distinguish LBD from both AD and vascular dementia. As seen in the case, LBD can also be confused with acute delirium.
Older adult patients presenting to the ED or clinic with visual hallucinations, delirium, and mental confusion may receive a false diagnosis of schizophrenia, medication- or substance-induced psychosis, Parkinson disease, or delirium of unknown etiology.35 Unfortunately, LBD is often overlooked and not considered in the differential diagnosis. Due to underrecognition, patients may receive treatment with typical antipsychotics. The addition of a neuroleptic to help control the psychotic symptoms causes patients with LBD to develop severe extrapyramidal symptoms and worsening mental status,36 leading to severe parkinsonian signs, which further muddies the diagnostic process. In addition, treatment for suspected Parkinson disease, including carbidopa-levodopa, has no benefit for patients with LBD and may increase psychotic symptoms.37
First-line treatment for LBD includes psychoeducation for the patient and family, cholinesterase inhibitors (eg, rivastigmine), and avoidance of high-potency antipsychotics, such as haloperidol. Although persistent hallucinations and psychosis remain difficult to treat in LBD, low-dose quetiapine is 1 option. Incorrectly diagnosing and prescribing treatment for another condition exacerbates symptoms in this patient population.
The patient has been experiencing chronic pain for the past few years after a motor vehicle accident. She has seen a physiatrist and another provider, both of whom found no “objective” causes of her chronic pain. They started the patient on sertraline for depression and an analgesic, both of which were ineffective.
The patient likes to exercise at a gym twice a week by doing light cardio (treadmill) exercise and light weightlifting. Lately, however, she has been unable to exercise due to the pain. At this visit, she mentions having low energy, poor sleep, frequent fatigue, and generalized soreness and pain in multiple areas of her body. The PCP recognizes the patient’s presenting symptoms as significant for FM and starts her on pregabalin and hydrotherapy, with positive results.
Continue to: Fibromyalgia skepticism may lead to a Dx of depression
Fibromyalgia skepticism may lead to a Dx of depression
FM, the second most common disorder seen in rheumatologic practice after OA, is estimated to affect approximately 1 in 20 patients (approximately 5 million Americans) in the primary care setting.38,39 The condition has a high female-to-male preponderance (3.4% vs 0.5%).40 While the primary symptom of FM is chronic pain, patients commonly present with fatigue and sleep disturbance.41 Comorbid conditions include headaches, irritable bowel syndrome, and mood disturbances (most commonly anxiety and depression).
Several studies have explored reasons for the misdiagnosis and underdiagnosis of FM. One important factor is ongoing skepticism among some physicians and the public, in general, as to whether FM is a real disease. This issue was addressed by a study by White and colleagues,42 who estimated and compared the point prevalence of FM and related disorders in Amish vs non-Amish adults. The authors hypothesized that if litigation and/or compensation availability have a major impact on FM prevalence, then there would be a near zero prevalence of FM in the Amish community. And yet, researchers found an overall age- and sex-adjusted FM prevalence of 7.3% (95% CI; 5.3%-9.7%); this was both statistically greater than zero (P < .0001) and greater than 2 control populations of non-Amish adults (both P < .05).
Many physicians consider FM fundamentally an emotional disturbance, and the high preponderance of FM in female patients may contribute to this misconception as reports of pain and emotional distress by women are often dismissed as hysteria.43 Physicians often explore the emotional aspects of FM, incorrectly diagnosing patients with depression and subsequently treating them with a psychotropic drug.39 Alternatively, they may focus on the musculoskeletal presentations of FM and prescribe analgesics or physical therapy, both of which do little to alleviate FM.
To make the correct diagnosis of FM, the American College of Rheumatology created a specific set of criteria in 1990, which was updated in 2010.44 For a diagnosis of FM, a patient must have at least a 3-month history of bilateral pain above and below the waist and along the axial skeletal spine. Although not included in the updated 2010 criteria, many clinicians continue to check for tender points, following the 1990 criteria requiring the presence of 11 of 18 points to make the diagnosis.
At least 3 cognitive biases relating to FM apply: anchoring, availability, and fundamental attribution error (see table 3).7 Anchoring occurs when the PCP settles on a psychiatric diagnosis of exaggerated pain syndrome, muscle overuse, or OA and fails to explore alternative etiology. Availability bias may obscure the true diagnosis of FM. Since PCPs see many patients with RA or OA, they may overlook or dismiss the possibility of FM. Attribution error happens when physicians dismiss the complaints of patients with FM as merely due to psychological distress, hysteria, or acting out.43
Patients with FM, who are often otherwise healthy, often present multiple times to the same PCP with a chief complaint of chronic pain. These repeat presentations can result in compassion fatigue and impact care. As Aloush and colleagues40 noted in their study, “FM patients were perceived as more difficult than RA patients, with a high level of concern and emotional response. A high proportion of physicians were reluctant to accept them because they feel emotional/psychological difficulties meeting and coping with these patients.”In response, patients with undiagnosed FM or inadequately treated FM may visit other PCPs, which may or may not result in a correct diagnosis and treatment.
We can do better
Primary care physicians face the daunting task of diagnosing and treating a wide range of common conditions while also trying to recognize less-common conditions with atypical presentations—all during a busy clinic workday. Nonetheless, we should strive to overcome internal (eg, cognitive bias and fund-of-knowledge deficits) and external (eg, time constraints, limited resources) pressures to improve diagnostic accuracy and care.
Each of the 4 disease states we’ve discussed have high rates of missed and/or delayed diagnosis. Each presents a unique set of confounders: PMR with its overlapping symptoms of many other rheumatologic diseases; OC with its often vague and misleading GI symptomatology; LBD with overlapping features of AD and Parkinson disease; and FM with skepticism. As gatekeepers to health care, it falls on PCPs to sort out these diagnostic dilemmas to avoid medical errors. Fundamental knowledge of each disease, its unique pathophysiology and symptoms, and varying presentations can be learned, internalized, and subsequently put into clinical practice to improve patient outcomes.
CORRESPONDENCE
Paul D. Rosen MD, Brooklyn Hospital Center, Department of Family Medicine, 121 Dekalb Avenue, Brooklyn, New York 11201; [email protected]
1. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139. doi: 10.1136/bmj.i2139
2. Van Such M, Lohr R, Beckman T, et al. Extent of diagnostic agreement among medical referrals. J Eval Clin Pract. 2017;23:870-874. doi: 10.1111/jep.12747
3. Groopman JE. How Doctors Think. Houghton Mifflin; 2007.
4. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185:1124-1131. doi: 10.1126/science.185.4157.1124
5. Norman GR, Monteiro SD, Sherbino J, et al. The causes of errors in clinical reasoning: Cognitive biases, knowledge deficits, and dual process thinking. Acad Med. 2017;92:23-30. doi: 10.1097/ACM.0000000000001421
6. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780. doi: 10.1097/00001888-200308000-00003
7. Morgenstern J. Cognitive errors in medicine: The common errors. First10EM blog. September 15, 2015. Updated September 22, 2019. Accessed February 8, 2022. https://first10em.com/cognitive-errors/
8. Gazitt T, Zisman D, Gardner G. Polymyalgia rheumatica: a common disease in seniors. Curr Rheumatol Rep. 2020;22:40. doi: 10.1007/s11926-020-00919-2
9. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35. doi: 10.1002/art.23176
10. Doran MF, Crowson CS, O’Fallon WM, et al. Trends in the incidence of polymyalgia rheumatica over a 30 year period in Olmsted County, Minnesota, USA. J Rheumatol. 2002;29:1694-1697.
11. Barraclough K, Liddell WG, du Toit J, et al. Polymyalgia rheumatica in primary care: a cohort study of the diagnostic criteria and outcome. Fam Pract. 2008;25:328-33. doi: 10.1093/fampra/cmn044
12. Manzo C. Polymyalgia rheumatica (PMR) with normal values of both erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) concentration at the time of diagnosis in a centenarian man: a case report. Diseases. 2018;6:84. doi: 10.3390/diseases6040084
13. Crowson CS, Matteson EL. Contemporary prevalence estimates for giant cell arteritis and polymyalgia rheumatica, 2015. Semin Arthritis Rheum. 2017;47:253-256. doi: 10.1016/j.semarthrit.2017.04.001
14. Nordborg E, Bengtsson BA. Death rates and causes of death in 284 consecutive patients with giant cell arteritis confirmed by biopsy. BMJ. 1989;299:549-550. doi: 10.1136/bmj.299.6698.549
15. Bahlas S, Ramos-Remus C, Davis P. Utilisation and costs of investigations, and accuracy of diagnosis of polymyalgia rheumatica by family physicians. Clin Rheumatol. 2000;19:278-280. doi: 10.1007/s100670070045
16. Brooks RC, McGee SR. Diagnostic dilemmas in polymyalgia rheumatica. Arch Intern Med. 1997;157:162-168.
17. Blaauw AA, Schuwirth LW, van der Vleuten CP, et al. Assessing clinical competence: recognition of case descriptions of rheumatic diseases by general practitioners. Br J Rheumatol. 1995;34:375-379. doi:10.1093/rheumatology/34.4.375
18. Mager DR. Polymylagia rheumatica: common disease, elusive diagnosis. Home Healthc Now. 2015;33:132-138. doi:10.1097/NHH.0000000000000199
19. Kermani TA, Warrington KJ. Polymyalgia rheumatica. Lancet. 381;63-72. doi: 10.1016/S0140-6736(12)60680-1. Published correction appears in Lancet. 20135;381:28.
20. Liew DF, Owen CE, Buchanan RR. Prescribing for polymyalgia rheumatica. Aust Prescr. 2018;41:14-19. doi: 10.18773/austprescr.2018.001
21. Ovarian cancer statistics. Centers for Disease Control and Prevention. Reviewed June 8, 2021. Accessed February 22, 2022. www.cdc.gov/cancer/ovarian/statistics/index.htm
22. Key statistics for ovarian cancer. American Cancer Society. Revised January 12, 2022. Accessed February 22, 2022. www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html
23. Survival rates for ovarian cancer. American Cancer Society. Revised January 25, 2021. Accessed February 22, 2022. www.cancer.org/cancer/ovarian-cancer/detection-diagnosis-staging/survival-rates.html
24. Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med. 2017;14:9-32. doi: 10.20892/j.issn.2095-3941.2016.0084
25. Goff BA, Mandel LS, Melancon CH, et al. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291:2705-2712. doi: 10.1001/jama.291.22.2705
26. Olson SH, Mignone L, Nakraseive C, et al. Symptoms of ovarian cancer. Obstet Gynecol. 2001;98:212-217. doi: 10.1016/s0029-7844(01)01457-0
27. Allgar VL, Neal RD. Delays in the diagnosis of six cancers: analysis of data from the National Survey of NHS patients: Cancer. Br J Cancer. 2005;92:1959-1970. doi: 10.1038/sj.bjc.6602587
28. Gajjar K, Ogden G, Mujahid MI, et al. Symptoms and risk factors of ovarian cancer: a survey in primary care. Obstet Gynecol. 2012:754197. doi: 10.5402/2012/754197
29. Austoker J. Diagnosis of ovarian cancer in primary care. BMJ. 2009;339:b3286. doi: 10.1136/bmj.b3286
30. Goff BA, Mandel L, Muntz HG, et al. Ovarian carcinoma diagnosis: results of a national ovarian cancer survey. Cancer. 2000;89:2068-2075. doi: 10.1002/1097-0142(20001115)89:10<2068::aid-cncr6>3.0.co;2-z
31. Smith EM, Anderson B. The effects of symptoms and delay in seeking diagnosis on stage of disease at diagnosis among women with cancers of the ovary. Cancer. 1985;56:2727-2732. doi: 10.1002/1097-0142(19851201)56:11<2727::aid-cncr2820561138>3.0.co;2-8
32. Galvin JE, Duda JE, Kaufer DI, et al. Lewy body dementia: the caregiver experience of clinical care. Parkinsonism Relat Disord. 2010;16:388-392. doi: 10.1016/j.parkreldis.2010.03.007
33. McKeith I. Dementia with Lewy bodies. Dialogues Clin Neurosci. 2004;6:333-341. doi: 10.31887/DCNS.2004.6.3/imckeith
34. Mrak RE, Griffin WS. Dementia with Lewy bodies: definitions, diagnosis, and pathogenic relationship to Alzheimer’s disease. Neuropsychiatr Dis Treat. 2007;3:619-625.
35. Khotianov N, Singh R, Singh S. Lewy body dementia: case report and discussion. J Am Board Fam Pract. 2002;15:50-54.
36. Stinton C, McKeith I, Taylor JP, et al. Pharmacological management of Lewy body dementia: a systematic review and meta-analysis. Am J Psychiatry. 2015;172:731-742. doi: 10.1176/appi.ajp.2015.14121582
37. Velayudhan L, Ffytche D, Ballard C, et al. New therapeutic strategies of Lewy body dementias. Curr Neurol Neurosci Rep. 2017;17:68. doi: 10.1007/s11910-017-0778-2
38. Arnold LM, Clauw DJ, McCarberg BH; FibroCollaborative. Improving the recognition and diagnosis of fibromyalgia. Mayo Clin Proc. 2011;86:457-464. doi: 10.4065/mcp.2010.0738
39. Arnold LM, Gebke KB, Choy EHS. Fibromyalgia: management strategies for primary care providers. Int J Clin Pract. 2016;70:99-112. doi: 10.1111/ijcp.12757
40. Aloush V, Niv D, Ablin JN, et al. Good pain, bad pain: illness perception and physician attitudes towards rheumatoid arthritis and fibromyalgia patients. Clin Exp Rheumatol. 2021;39(suppl 130):54-60.
41. Vincent A, Lahr BD, Wolfe F, et al. Prevalence of fibromyalgia: a population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis Care Res (Hoboken). 2013;65:786-792. doi: 10.1002/acr.21896
42. White KP, Thompson J. Fibromyalgia syndrome in an Amish community: a controlled study to determine disease and symptom prevalence. J Rheumatol. 2003;30:1835-1840.
43. Lobo CP, Pfalzgraf AR, Giannetti V, et al. Impact of invalidation and trust in physicians on health outcomes in fibromyalgia patients. Prim Care Companion CNS Disord. 2014;16:10.4088/PCC.14m01664. doi: 10.4088/PCC.14m01664
44. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken). 2010;62:600-610. doi:10.1002/acr.20140
1. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139. doi: 10.1136/bmj.i2139
2. Van Such M, Lohr R, Beckman T, et al. Extent of diagnostic agreement among medical referrals. J Eval Clin Pract. 2017;23:870-874. doi: 10.1111/jep.12747
3. Groopman JE. How Doctors Think. Houghton Mifflin; 2007.
4. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185:1124-1131. doi: 10.1126/science.185.4157.1124
5. Norman GR, Monteiro SD, Sherbino J, et al. The causes of errors in clinical reasoning: Cognitive biases, knowledge deficits, and dual process thinking. Acad Med. 2017;92:23-30. doi: 10.1097/ACM.0000000000001421
6. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780. doi: 10.1097/00001888-200308000-00003
7. Morgenstern J. Cognitive errors in medicine: The common errors. First10EM blog. September 15, 2015. Updated September 22, 2019. Accessed February 8, 2022. https://first10em.com/cognitive-errors/
8. Gazitt T, Zisman D, Gardner G. Polymyalgia rheumatica: a common disease in seniors. Curr Rheumatol Rep. 2020;22:40. doi: 10.1007/s11926-020-00919-2
9. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35. doi: 10.1002/art.23176
10. Doran MF, Crowson CS, O’Fallon WM, et al. Trends in the incidence of polymyalgia rheumatica over a 30 year period in Olmsted County, Minnesota, USA. J Rheumatol. 2002;29:1694-1697.
11. Barraclough K, Liddell WG, du Toit J, et al. Polymyalgia rheumatica in primary care: a cohort study of the diagnostic criteria and outcome. Fam Pract. 2008;25:328-33. doi: 10.1093/fampra/cmn044
12. Manzo C. Polymyalgia rheumatica (PMR) with normal values of both erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) concentration at the time of diagnosis in a centenarian man: a case report. Diseases. 2018;6:84. doi: 10.3390/diseases6040084
13. Crowson CS, Matteson EL. Contemporary prevalence estimates for giant cell arteritis and polymyalgia rheumatica, 2015. Semin Arthritis Rheum. 2017;47:253-256. doi: 10.1016/j.semarthrit.2017.04.001
14. Nordborg E, Bengtsson BA. Death rates and causes of death in 284 consecutive patients with giant cell arteritis confirmed by biopsy. BMJ. 1989;299:549-550. doi: 10.1136/bmj.299.6698.549
15. Bahlas S, Ramos-Remus C, Davis P. Utilisation and costs of investigations, and accuracy of diagnosis of polymyalgia rheumatica by family physicians. Clin Rheumatol. 2000;19:278-280. doi: 10.1007/s100670070045
16. Brooks RC, McGee SR. Diagnostic dilemmas in polymyalgia rheumatica. Arch Intern Med. 1997;157:162-168.
17. Blaauw AA, Schuwirth LW, van der Vleuten CP, et al. Assessing clinical competence: recognition of case descriptions of rheumatic diseases by general practitioners. Br J Rheumatol. 1995;34:375-379. doi:10.1093/rheumatology/34.4.375
18. Mager DR. Polymylagia rheumatica: common disease, elusive diagnosis. Home Healthc Now. 2015;33:132-138. doi:10.1097/NHH.0000000000000199
19. Kermani TA, Warrington KJ. Polymyalgia rheumatica. Lancet. 381;63-72. doi: 10.1016/S0140-6736(12)60680-1. Published correction appears in Lancet. 20135;381:28.
20. Liew DF, Owen CE, Buchanan RR. Prescribing for polymyalgia rheumatica. Aust Prescr. 2018;41:14-19. doi: 10.18773/austprescr.2018.001
21. Ovarian cancer statistics. Centers for Disease Control and Prevention. Reviewed June 8, 2021. Accessed February 22, 2022. www.cdc.gov/cancer/ovarian/statistics/index.htm
22. Key statistics for ovarian cancer. American Cancer Society. Revised January 12, 2022. Accessed February 22, 2022. www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html
23. Survival rates for ovarian cancer. American Cancer Society. Revised January 25, 2021. Accessed February 22, 2022. www.cancer.org/cancer/ovarian-cancer/detection-diagnosis-staging/survival-rates.html
24. Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med. 2017;14:9-32. doi: 10.20892/j.issn.2095-3941.2016.0084
25. Goff BA, Mandel LS, Melancon CH, et al. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291:2705-2712. doi: 10.1001/jama.291.22.2705
26. Olson SH, Mignone L, Nakraseive C, et al. Symptoms of ovarian cancer. Obstet Gynecol. 2001;98:212-217. doi: 10.1016/s0029-7844(01)01457-0
27. Allgar VL, Neal RD. Delays in the diagnosis of six cancers: analysis of data from the National Survey of NHS patients: Cancer. Br J Cancer. 2005;92:1959-1970. doi: 10.1038/sj.bjc.6602587
28. Gajjar K, Ogden G, Mujahid MI, et al. Symptoms and risk factors of ovarian cancer: a survey in primary care. Obstet Gynecol. 2012:754197. doi: 10.5402/2012/754197
29. Austoker J. Diagnosis of ovarian cancer in primary care. BMJ. 2009;339:b3286. doi: 10.1136/bmj.b3286
30. Goff BA, Mandel L, Muntz HG, et al. Ovarian carcinoma diagnosis: results of a national ovarian cancer survey. Cancer. 2000;89:2068-2075. doi: 10.1002/1097-0142(20001115)89:10<2068::aid-cncr6>3.0.co;2-z
31. Smith EM, Anderson B. The effects of symptoms and delay in seeking diagnosis on stage of disease at diagnosis among women with cancers of the ovary. Cancer. 1985;56:2727-2732. doi: 10.1002/1097-0142(19851201)56:11<2727::aid-cncr2820561138>3.0.co;2-8
32. Galvin JE, Duda JE, Kaufer DI, et al. Lewy body dementia: the caregiver experience of clinical care. Parkinsonism Relat Disord. 2010;16:388-392. doi: 10.1016/j.parkreldis.2010.03.007
33. McKeith I. Dementia with Lewy bodies. Dialogues Clin Neurosci. 2004;6:333-341. doi: 10.31887/DCNS.2004.6.3/imckeith
34. Mrak RE, Griffin WS. Dementia with Lewy bodies: definitions, diagnosis, and pathogenic relationship to Alzheimer’s disease. Neuropsychiatr Dis Treat. 2007;3:619-625.
35. Khotianov N, Singh R, Singh S. Lewy body dementia: case report and discussion. J Am Board Fam Pract. 2002;15:50-54.
36. Stinton C, McKeith I, Taylor JP, et al. Pharmacological management of Lewy body dementia: a systematic review and meta-analysis. Am J Psychiatry. 2015;172:731-742. doi: 10.1176/appi.ajp.2015.14121582
37. Velayudhan L, Ffytche D, Ballard C, et al. New therapeutic strategies of Lewy body dementias. Curr Neurol Neurosci Rep. 2017;17:68. doi: 10.1007/s11910-017-0778-2
38. Arnold LM, Clauw DJ, McCarberg BH; FibroCollaborative. Improving the recognition and diagnosis of fibromyalgia. Mayo Clin Proc. 2011;86:457-464. doi: 10.4065/mcp.2010.0738
39. Arnold LM, Gebke KB, Choy EHS. Fibromyalgia: management strategies for primary care providers. Int J Clin Pract. 2016;70:99-112. doi: 10.1111/ijcp.12757
40. Aloush V, Niv D, Ablin JN, et al. Good pain, bad pain: illness perception and physician attitudes towards rheumatoid arthritis and fibromyalgia patients. Clin Exp Rheumatol. 2021;39(suppl 130):54-60.
41. Vincent A, Lahr BD, Wolfe F, et al. Prevalence of fibromyalgia: a population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis Care Res (Hoboken). 2013;65:786-792. doi: 10.1002/acr.21896
42. White KP, Thompson J. Fibromyalgia syndrome in an Amish community: a controlled study to determine disease and symptom prevalence. J Rheumatol. 2003;30:1835-1840.
43. Lobo CP, Pfalzgraf AR, Giannetti V, et al. Impact of invalidation and trust in physicians on health outcomes in fibromyalgia patients. Prim Care Companion CNS Disord. 2014;16:10.4088/PCC.14m01664. doi: 10.4088/PCC.14m01664
44. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken). 2010;62:600-610. doi:10.1002/acr.20140
Strategies for improved management of hypothyroidism
The hormones thyroxine (T4) and triiodothyronine (T3), produced by the thyroid gland, are crucial for maintaining metabolism. A deficit of thyroid hormone production—hypothyroidism—is a common endocrine disorder seen in primary care.
Although the diagnosis and management of hypothyroidism are considered straightforward, many patients with hypothyroidism do not achieve optimal treatment goals or see an improvement in their quality of life. In this article, we address the questionable utility of screening; outline the diagnostic approach, including the central role of laboratory testing; and explain why treatment requires a precise approach to address the range of patient types.
Epidemiology and classification
Estimates are that almost 5% of Americans 12 years or older have hypothyroidism; older people and women are more likely to develop the condition. 1 In the US National Health and Nutrition Examination Survey (NHANES III) of 13,344 people without known thyroid disease or a family history, hypothyroidism was found in 4.6% (overt [clinical] in 0.3% and subclinical in 4.3%); 11% had a high serum thyroid peroxidase antibody level, which increases their risk of hypothyroidism, and is treated the same as hypothyroidism of other causes; and, overall, lower serum thyroid-stimulating hormone (TSH) levels were seen in Blacks, compared to Whites and Mexican Americans.1
Primary hypothyroidism accounts for > 95% of cases of hypothyroidism, representing a failure of the thyroid gland to produce sufficient hormone. It has been shown that, in iodine-replete countries such as the United States, the prevalence of spontaneous hypothyroidism is 1% to 2%, and it is 10 times more common in women.2,3
Central hypothyroidism is caused by insufficient stimulation of the thyroid gland by TSH, due to pituitary (secondary hypothyroidism) or hypothalamic (tertiary hypothyroidism) disease and is estimated to occur in 1 in every 20,000 to 80,000 people in the general population.4
How does hypothyroidism manifest?
Signs and symptoms. Manifestations of hypothyroidism range from life-threatening to minimal or no clinical signs and symptoms (TABLE W1). Signs and symptoms of low thyroid function vary by the degree of hypothyroidism at presentation.
Common signs and symptoms of low thyroid function include fatigue, weight gain, dry skin, brittle hair, hair loss, morning stiffness, muscle aches, joint pain, cold intolerance, diffuse headache, constipation, difficulty concentrating, low libido, depression, and menstrual irregularities. On physical examination, a patient might present with bradycardia, hypotension, hypothermia with slow speech or movement, coarse facial appearance, goiter, diffuse hair loss, cold hands and feet, and a prolonged Achilles tendon reflex.5 Skin findings, such as keratosis pilaris, palmoplantar keratoderma (thickening of the skin), and pityriasis rubra pilar, can be associated with autoimmune hypothyroidism.6,7
Continue to: Carpal tunnel syndrome...
Carpal tunnel syndrome, plantar fasciitis, infertility or miscarriage, dyspepsia, and small intestinal bacterial overgrowth can be associated with hypothyroidism; thyroid function should therefore be assessed in patients who have any of these conditions, along with other signs and symptoms of low thyroid function.8,9 A patient with severe hypothyroidism might present with hemodynamic instability, pericardial or pleural effusion, and myxedema coma.10
Clues in the history and from the lab. A history of radiation to the head, neck, or chest area and a history or family history of autoimmune disorders are risk factors for autoimmune thyroid disease.11,12 Laboratory findings can include markers of oxidative stress, such as elevated levels of low-density lipoprotein cholesterol and serum malondialdehyde.13-15
Screening and diagnosis
Screening. The US Preventive Services Task Force has asserted that evidence is insufficient by which to evaluate the benefits and risks of routine screening for thyroid dysfunction in nonpregnant, asymptomatic adults.16 According to the American Thyroid Association and the American Association of Clinical Endocrinologists, screening should be considered in high-risk patients, including those who take medication that affects thyroid function or the results of thyroid hormone assays (TABLE W2, available at mdedge.com/familymedicine).17-20
Screening inpatients is challenging and usually not recommended unless thyroid disease is strongly suspected. This is because changes in the levels of thyroid hormones, binding proteins, and the TSH concentration can occur in severe nonthyroidal illness; in addition, assay interference by antibodies and other substances can affect thyroid hormone measurement.21
Testing strategy. Generally, screening and diagnosis of hypothyroidism are based primarily on laboratory testing, because signs and symptoms are nonspecific (FIGURE 15). A serum TSH level is usually the initial test when screening for thyroid dysfunction. A normal serum TSH value ranges from 0.5-5.0 mIU/L.
When an abnormal serum TSH level is found, further tests can be performed to investigate, including a serum free thyroxine (FT4) test. (Our preference is to order TSH and FT4 assays simultaneously to facilitate and confirm the diagnosis.) An FT4 test measures the amount of unattached, or free, thyroxine in blood by immunoassay. A normal FT4 value usually ranges from 0.7-1.9 ng/dL.
The combination of a high TSH level and a low FT4 level could be an indication of an underactive thyroid gland (ie, clinical or overt hypothyroidism). Milder, subclinical hypothyroidism is characterized by a higher-than-normal TSH level but a normal FT4 level.22 Central (secondary) hypothyroidism is characterized by a low serum FT4 level and a serum TSH level that can be below the reference range, low normal, or even slightly high.4
Continue to: These measurements...
These measurements must be interpreted within the context of the laboratory-specific normal range for each test. Third-generation serum TSH assays are more sensitive and specific than serum FT4 measurements for hypothyroidism. FT4 is usually measured by automated analogue immunoassay, which generally provides reliable results; abnormal binding proteins or other interferences occur in some patients, however, resulting in reporting of a falsely high, or falsely low, FT4 level. In such cases, FT4 by direct dialysis, or total T4, can be measured for further evaluation. In primary care, you are most likely to encounter primary hypothyroidism; secondary (central) hypothyroidism is much rarer (< 5% of cases).4
The ins and outs of treatment
For most patients, hypothyroidism is a permanent disorder requiring lifelong thyroid hormone replacement therapy—unless the disease is transient (ie, painless or subacute thyroiditis); reversible, because it is caused by medication; or responsive to medical intervention that addresses the underlying autoimmune condition.19 Goals of treatment (Figure 25,23) are to:
- normalize the TSH level to 0.5-5.0 mIU/L (the main goal), with an age-related shift toward a higher TSH goal in older patients (and an upper limit of normal of 7.5 mIU/L in patients who are ≥ 80 years of age)20
- restore the euthyroid state
- relieve symptoms
- reduce any goiter
- avoid overtreatment (iatrogenic thyrotoxicosis).
Desiccated thyroid extract (DTE), developed in the late 1880s and made from the dried thyroid gland of pigs, sheep, or cows, was the earliest treatment for hypothyroidism. The use of DTE has declined since the introduction of synthetic thyroxine (T4, or levothyroxine [here, referred to as LT4]), which is now the standard treatment.20-22 LT4 is deiodinated in peripheral tissues to form T3, the active thyroid hormone; this process accounts for 80% of total T3 production daily.24
LT4 formulations. LT4 is commercially available in tablet, soft-gel, and liquid preparations. Most patients are treated with the tablet; the soft-gel capsule or liquid is an option for patients who absorb the tablet poorly (because of atrophic gastritis, celiac disease, or gluten sensitivity or because they are post bariatric surgery). Increasing the dosage of the tablet form of LT4, with ongoing TSH monitoring, is more cost effective than moving to an alternative preparation.
If a switch of LT4 formulation is made (ie, from one manufacturer to another), test the serum TSH level to ensure that the therapeutic goal is being reached. Also, in our experience, it is best to prescribe a brand-name preparation of levothyroxine, not a generic, whenever possible, due to the variability in generic formulations and the potential presence of other (inert) ingredients.25
Dosing (TABLE 320,23). The average full replacement dosage of LT4 for a young, healthy adult is approximately 1.6 mcg/kg/d. Older patients (> 65 years) or those with coronary artery disease (CAD) should be started on a lower dosage (25-50 mcg/d) and titrated to goal accordingly.
LT4 (tablets, soft-gel capsules, or liquid) should be administered on an empty stomach, with water only, 30 to 60 minutes before breakfast. Medications that interfere with LT4 absorption (eg, bile acid resins, calcium carbonate, ferrous sulfate) should be taken several hours after LT4. For patients who cannot take LT4 in the morning, taking it at bedtime (≥ 2-3 hours after the last meal) is acceptable.
Continue to: Monitoring and titrating
Monitoring and titrating. Hypothyroid symptoms usually improve after 2 or 3 weeks of LT4 treatment; in severe hypothyroidism, complete recovery might take months. Approximately 6 weeks after LT4 therapy is initiated, serum TSH should be measured. After assessing whether administration of LT4 at the starting dosage is appropriate, that dosage can be increased, or decreased, every 4 to 6 weeks until the TSH goal is reached. Once the patient is maintained at a given dosage, measure serum TSH once a year—more often if there is an abnormal result or a change in the patient’s health status.23
Adverse effects of LT4 therapy are rare, unless over-replacement occurs. Rarely, patients have an allergy to the dye or an excipient (filler) in the tablet.26-28 The white, 50-mcg tablets can be given safely to patients with dye sensitivity. For those who have an allergy to an excipient (except gelatin) or gluten intolerance, the LT4 soft-gel capsule or liquid preparation (Tirosint) can be prescribed.
Pure LT4, in a capsule made from vegetable sources, can be ordered through a compounding pharmacy for patients who are allergic to animal products.
Anemia, especially iron-deficiency anemia, can cause intolerance to LT4 therapy; in such patients, lowering the starting dosage and treating anemia are indicated.29
Persistent symptoms (despite a normal TSH level). Because many hypothyroid symptoms are nonspecific, patients might come to think that their LT4 dosage is inadequate if they feel tired or gain weight. Persistent hypothyroid symptoms despite a normal serum TSH level might be due to (1) the inability of LT4 therapy to restore tissue thyroid hormone levels to normal or (2) other variables unrelated to hypothyroidism, including disorders associated with inflammation or autoimmune disease, certain medications, diet, lifestyle, and environmental toxins.
These patients might benefit from a detailed history to identify other causes and a switch to either LT4 + liothyronine (LT3; synthetic T3) combination therapy or DTE26,30-33 (TABLE 434), although a beneficial effect of LT4 + LT3 therapy was not seen in several studies.35,36 Over-replacement with LT4 should be discouraged, due to concerns about thyrotoxicosis and its complications (eg, atrial fibrillation, accelerated bone loss).
DTE and LT4 + LT3. Use of DTE has decreased since the 1970s, when LT4 became the therapy of choice. Subsequently, anecdotal evidence emerged that some patients did not feel well on LT4 and preferred to return to DTE.32,33
Continue to: Several clinical trials...
Several clinical trials addressed the question of whether residual symptoms could be resolved through LT4 + LT3 combination therapy31-39 (TABLE 434), but evidence of any consistent superiority of combination therapy was not demonstrated.35-39 In selected cases, patients might prefer the combination approach.31,33,39 The quality of life of hypothyroid patients was found to be similarly improved with LT4 or DTE, but the latter was associated with modest weight loss (approximately 4 lbs); nearly 50% of study patients preferred treatment with DTE over LT4.33 A follow-up study did not confirm weight loss with DTE, however.34
When LT4 monotherapy and LT4 + LT3 combination therapy were compared, results were mixed31-39; responsiveness to therapy containing LT3 might therefore depend on multiple variables, including genetic background, nutritional and lifestyle factors, stress, presence of comorbidities and autoimmune disorders, and other unidentified or poorly defined variables.40-48
Although combination therapy and DTE are not generally recommended over LT4 monotherapy, they might offer better options for patients who are still symptomatic when being treated with LT4 only: In a randomized, double-blind, crossover study that compared LT4 with DTE and with LT4 + LT3, one-third of the most highly symptomatic patients who had low scores on mood, cognitive, and quality-of-life assessments improved significantly after they were switched to combination therapy or DTE.34
The 2014 American Thyroid Association guidelines24 do not support routine use of LT4 + LT3 in hypothyroid patients who have residual symptoms after LT4 monotherapy; however, a therapeutic trial of LT4 + LT3, while maintaining a normal serum TSH, is reasonable in selected patients. Candidates for DTE or LT4 + LT3 might include patients who do not feel well on LT4 monotherapy, are post thyroidectomy or post radioiodine therapy, or have a low serum T3 level. DTE and combination therapy are discouraged in older patients, patients who have underlying CAD, and pregnant patients.
Special treatment circumstances
A number of patient variables have the potential to alter management strategies for hypothyroidism.18,20,23,40,49-53
Age, comorbidity. Older patients (> 65 years) and patients with cardiopulmonary disease or CAD should be treated with LT4, 25 to 50 mcg/d, initially; that dosage can be titrated upward by 12.5 to 25 mcg/d every 4 to 6 weeks until the TSH goal is reached—preferably, in the range of 4 to 8 mIU/L. An increase in the dosage of LT4 might be required in the presence of malabsorption (eg, gastrointestinal disorders, celiac disease) and in nephrotic syndrome.18,20,23
Body weight. A decrease in the dosage of LT4 might be indicated in the setting of significant weight loss (> 10% body weight).23
Continue to: Co-pharmacy
Co-pharmacy. An increase in the dosage of LT4 might be required when other drugs (eg, phenytoin, phenobarbital, rifampin, and carbamazepine) have led to an increased rate of thyroid hormone metabolism. A decrease in the dosage of LT4 might be necessary after initiation of androgen therapy.23
Pregnancy. Women with pre-existing hypothyroidism require an increase of 25% to 50% in their LT4 dosage during pregnancy to maintain a TSH level in the recommended pregnancy reference range. Thyroid function should be monitored every 4 to 6 weeks to ensure that the TSH target for each trimester is reached (first trimester, 0.1-4 mIU/L; second trimester, 0.2-4 mIU/L; third trimester, 0.3-4 mIU/L). Postpartum, LT4 can be reduced to the prepartum dosage; TSH should be checked every 4 to 6 weeks to maintain the TSH goal.23
Estrogen therapy. Hypothyroid women who are receiving estrogen therapy might require an increase in their LT4 dosage because serum thyroxine-binding globulin levels are increased by estrogens or through other mechanisms that have not been identified.23
Surgical candidacy. Observational studies show few adverse outcomes in surgical patients with mild (subclinical) hypothyroidism or moderate hypothyroidism; however, the risk of adverse surgical outcome might be increased in patients with severe hypothyroidism. For patients in whom surgery is planned and who have:
- subclinical hypothyroidism (elevated TSH and normal FT4), we recommend that surgery—urgent or elective—not be posptoned but proceed.
- moderate (overt) hypothyroidism who require urgent surgery, we recommend not postponing surgery, even though minor perioperative complications might develop. Such patients should be treated with LT4 as soon as the diagnosis for which surgery is required has been made. Alternatively, when moderate hypothyroidism is discovered in a patient who is being evaluated for elective surgery, we recommend postponing surgery until the euthyroid state is restored.
- severe hypothyroidism (myxedema coma [discussed in a bit]; severe clinical symptoms of chronic hypothyroidism, such as altered mental status, pericardial effusion, or heart failure; or a very low level of T4), surgery should be delayed until hypothyroidism has been treated. When emergency surgery is required for a severely hypothyroid patient, they should be treated with LT4 as soon as the diagnosis for which surgery is indicated has been made. When emergency surgery must be performed in a patient with myxedema coma, we recommend treatment with LT4 + LT3, rather than LT4 alone, often administered intravenously because LT4 is poorly absorbed in these patients.
Nonadherence. For patients who do not take LT4 regularly or do not respond to efforts to improve adherence, LT4 can be given weekly, instead of daily, although this interval is not ideal. Weekly dosing should not be used in older patients with CAD.23
Thyroid cancer. Patients who are post total thyroidectomy for thyroid cancer need to take LT4 to treat hypothyroidism and to prevent recurrence of thyroid cancer. The goal TSH level should be based on the cancer stage and risk of recurrence and should be monitored by an endocrinologist.
Myxedema coma. This medical emergency has high mortality. Myxedema coma occurs when severe hypothyroidism leads to any, or a combination, of the following: diminished mentation; hypothermia; bradycardia; hyponatremia; hypotension; cardiovascular, respiratory, and gastrointestinal dysfunction; and renal insufficiency. LT4, LT3, and glucocorticoids should be administered intravenously and the patient monitored closely—preferably in consultation with an endocrinologist.
Continue to: When to seek consultation
When to seek consultation
A patient with hypothyroidism should be referred to Endocrinology if they are < 18 years of age, pregnant, unresponsive to therapy, or have cardiac disease, coexisting endocrine disease, suspected myxedema coma, goiter or thyroid nodules, or a structural thyroid abnormality.
What we know about nutrition and hypothyroidism
Although it is commonly recognized that iodine is essential for production of thyroid hormone, other nutritional factors might contribute to proper production of thyroid hormones, including:
- adequate intake of iron, tyrosine, selenium, zinc, and vitamins E, B2, B3, B6, C, and D44,45
- selenium and zinc, which increase conversion of T4 to T3 and might be important in the management of hypothyroid patients40,46
- vitamin A, zinc, and regular exercise, which have been shown to improve cellular sensitivity to thyroid hormones.
Low iron stores can contribute to persistent symptoms and poor quality of life in patients with hypothyroidism, despite their being treated according to guidelines.29,47
Despite what is known about these nutritional connections, there is insufficient evidence that improving nutrition can reverse hypothyroidism.
Prevention
Prevention of hypothyroidism should take into account variables that affect or inhibit thyroid function, such as stress, infection (eg, Epstein-Barr virus), excessive fluoride intake, toxins (eg, pesticides, solvents, mercury, cadmium, and lead), autoimmune disease (eg, celiac disease), and food sensitivity.54,55 Oxidative stress can also cause thyroid impairment.40-48,54-58
Otherwise, there are, at present, no effective strategies for preventing thyroid disorders.
Subclinical hypothyroidism: Elusive management target
Subclinical hypothyroidism is defined as a normal serum FT4 level in the presence of an elevated serum TSH level. The prevalence of subclinical hypothyroidism varies from 3% to 15%, depending on the population studied; a higher incidence has been noted in women and older people.59 In the NHANES III,1 which excluded people with previously diagnosed thyroid disease, the incidence of subclinical hypothyroidism was 4.3%.
Continue to: Causes of subclinical...
Causes of subclinical hypothyroidism are the same as those of overt hypothyroidism, and include Hashimoto disease. The combination of an elevated TSH level and a normal FT4 level is associated with disorders characterized by protein-binding variations (eg, pregnancy, genetic disorders, drugs), TSH-secreting pituitary adenoma, class II and III obesity (respectively, body mass index, ≥ 35 but < 40 and ≥ 40), and assay variability.49,51
Lab diagnosis: Fraught with difficulty
The serum TSH level and either the total T4 level or the FT4 level should be measured to make a diagnosis of subclinical hypothyroidism. Most laboratories use a 1-step analogue immunoassay to determine free thyroid hormones; protein-binding variations can thus affect measurement of FT4.
Several scenarios that can result in inaccurate measurement of FT4 by radioimmunoassay include genetic disorders that affect binding proteins; pregnancy; use of certain drugs, including heparin, furosemide, antiepileptic agents, salicylate, ferrous sulfate, and cholesterol-binding resins; and some medical conditions, including cardiac surgery, critical illness, and renal failure. Variables that inhibit proper production of thyroid hormones—stress, infection, fluoride (an iodine antagonist), toxins (pesticides, mercury, cadmium, lead) and autoimmune conditions, such as celiac disease—should be considered when attempting to determine the cause of subclinical hypothyroidism.
Liquid chromatography–mass spectrometry measurement of thyroid hormones might be more accurate than immunoassay.53 Measuring serum total T4 and FT4 by dialysis, free from interfering proteins, might also be useful when measurement of FT4 by immunoassay is affected by binding-protein variations.
Features of subclinical hypothyroidism
Most patients who have subclinical hypothyroidism and a serum TSH level < 10 mIU/L are asymptomatic. Some might have nonspecific symptoms of hypothyroidism, however, such as reduced quality of life, poor cognitive function, and poor memory—symptoms that do not typically correlate with the serum TSH level.
It has been suggested that some elderly people normally have a higher level of serum TSH, and that they might have even a prolonged lifespan.51 Additionally, it has been shown that, in nonpregnant adult patients with subclinical hypothyroidism and a serum TSH level of 4.5 to 10 mIU/L, treatment with LT4 was not associated with improvement in thyroid-related symptoms or general quality of life.52
Treat, or don't treat, subclinical hypothyroidism?
It is well accepted that the goal of therapy in hypothyroid patients is to normalize the serum TSH level; however, the American Thyroid Association and the American Association of Clinical Endocrinology recommend starting LT4 in patients with a serum TSH level ≥ 10 mIU/L (TABLE 5).59-62 The principal reason for not treating subclinical hypothyroidism is the lack of benefit in reducing the risk of cardiovascular morbidity and mortality when the TSH level is between 7.5 and 10 mIU/L.62
Continue to: Routine treatment
Routine treatment of patients with a serum TSH level of 4.5 to 10 mIU/L remains controversial. When TSH is 7.0 to 9.9 mIU/L, treatment is recommended for (1) patients < 65 years and (2) for older patients (> 65 years) only when there are convincing hypothyroid symptoms because of concern about unintended overtreatment.
When the TSH level is anywhere above the upper limit of normal to 6.9 mIU/L, treatment is recommended for patients < 65 years old, patients who have a high titer of thyroid peroxidase antibodies, and patients with goiter—but not for patients > 65 years (and, especially, not for octogenarians) because their upper limit of normal could be as high as 6 to 8 mIU/L, especially if they are otherwise healthy.
Treatment should be considered for women with subclinical hypothyroidism who are trying to conceive or experiencing an infertility problem.
For patients with subclinical hypothyroidism who are not being treated, monitor thyroid function every 6 to 12 months by testing TSH and FT4.
CORRESPONDENCE
Thanh D. Hoang, DO, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
1. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499. doi: 10.1210/jcem.87.2.8182
2. Vanderpump MPJ. The epidemiology of thyroid disease. Br Med Bull. 2011;99:39-51. doi: 10.1093/bmb/ldr030
3. Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526-534. doi: 10.1001/archinte.160.4.526
4. Persani L. Central hypothyroidism: pathogenic, diagnostic, and therapeutic challenges. J Clin Endocrinol Metab. 2012;97:3068-3078. doi: 10.1210/jc.2012-1616
5. Almandoz JP, Gharib H. Hypothyroidism: etiology, diagnosis, and management. Med Clin North Am. 2012;96:203-221. doi: 10.1016/j.mcna.2012.01.005
6. Ai J, Leonhardt JM, Heymann WR. Autoimmune thyroid diseases: etiology, pathogenesis and dermatologic manifestations. J Am Acad Dermatol. 2003;48:641-659. doi: 10.1067/mjd.2003.257
7. Franzotti AM, Avelar JCD, Cardoso TA, et al. Pityriasis rubra pilar and hypothyroidism. An Bras Dermatol. 2014;89:497-500. doi: 10.1590/abd1806-4841.20142994
8. Yaylali O, Kirac S, Yilmaz M, et al. Does hypothyroidism affect gastrointestinal motility? Gastroenterol Res Pract. 2009;2009:529802. doi: 10.1155/2009/529802
9. Patil AD. Link between hypothyroidism and small intestinal bacterial overgrowth. Indian J Endocrinol Metab. 2014;18:307-309.
10. Ono Y, Ono S, Yasunaga H, et al. Clinical characteristics and outcomes of myxedema coma: analysis of a national inpatient database in Japan. J Epidemiol. 2017;27:117-122. doi: 10.1016/j.je.2016.04.002
11. Boomsma MJ, Bijl HP, Langendijk JA. Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol. 2011;99:1-5. doi: 10.1016/j.radonc.2011.03.002
12. Boelaert K, Newby PR, Simmonds MJ, et al. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med. 2010;123:183.e1-e9. doi: 10.1016/j.amjmed.2009.06.030
13. Cheserek MJ, Wu G-R, Ntazinda A, et al. Association between thyroid hormones, lipids and oxidative stress markers in subclinical hypothyroidism. J Med Biochem. 2015;34:323-331. doi: 10.2478/jomb-2014-0044
14. Zha K, Zuo C, Wang A, et al. LDL in patients with subclinical hypothyroidism shows increased lipid peroxidation. Lipids Health Dis. 2015;14:95. doi: 10.1186/s12944-015-0092-4
15. Tejovathi B, Suchitra MM, Suresh V, et al. Association of lipid oxidation with endothelial dysfunction in patients with overt hypothyroidism. Exp Clin Endocrinol Diabetes. 2013;121:306-309. doi: 10.1055/s-0032-1333298
16. LeFevre ML; U.S. Preventive Services Task Force. Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162:641-650. doi: 10.7326/M15-0483
17. Chaker L, Bianco AC, Jonklaas J, et al. Hypothyroidism. Lancet. 2017;390:1550-1562. doi: 10.1016/S0140-6736(17)30703-1
18. Vaidya B, Pearce SHS. Management of hypothyroidism in adults. BMJ. 2008;337:a801. doi: 10.1136/bmj.a801
19. Iyer PC, Cabanillas ME, Waguespack SG, et al. Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid. 2018;28:1243-1251. doi: 10.1089/thy.2018.0116
20. Garber JR, Cobin RH, Gharib H, et al; American Association Of Clinical Endocrinologists And American Thyroid Association Taskforce On Hypothyroidism In Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22:1200-1235. doi: 10.1089/thy.2012.0205
21. Maiden MJ, Torpy DJ. Thyroid hormones in critical illness. Crit Care Clin. 2019;35:375-388. doi: 10.1016/j.ccc.2018.11.012
22. Peeters RP. Subclinical hypothyroidism. N Engl J Med. 2017;376:2556-2565. doi: 10.1056/NEJMcp1611144
23. Benvenga S, Carlé A. Levothyroxine formulations: pharmacological and clinical implications of generic substitution. Adv Ther. 2019;36(suppl 2):59-71. doi: 10.1007/s12325-019-01079-1
24. Jonklaas J, Bianco AC, Bauer AJ, et al; . Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid. 2014;24:1670-1751. doi: 10.1089/thy.2014.0028
25. Engler D, Burger AG. The deiodination of the iodothyronines and of their derivatives in man. Endocr Rev. 1984;5:151-184. doi: 10.1210/edrv-5-2-151
26. Ettleson MD, Bianco AC. Individualized therapy for hypothyroidism: is T4 enough for everyone? J Clin Endocrinol Metab. 2020;105:e3090-e3104. doi: 10.1210/clinem/dgaa430
27. Slayden TA, Shakir MKM, Hoang TD. A bull in a pill shop: alpha-gal allergy complicating treatment options for postprocedural hypothyroidism. AACE Clin Case Rep. 2020;6:e101-e104. doi: 10.4158/ACCR-2019-0495
28. Chamorro-Pareja N, Carrillo-Martin I, Haehn DA, et al. Self-reported allergy to thyroid replacement therapy: a multicenter retrospective chart review. Endocr Pract. 2020;26:761-767. doi: 10.4158/EP-2019-0488
29. Shakir MKM, Turton D, Aprill BS, et al. Anemia: a cause of intolerance to thyroxine sodium. Mayo Clin Proc. 2000;75:189-192.
30. Jonklaas J, Bianco AC, Cappola AR, et al. Evidence-based use of levothyroxine/liothyronine combinations in treating hypothyroidism: a consensus document. Thyroid. 2021;31:156-182. doi: 10.1089/thy.2020.0720
31. Appelhof BC, Fliers E, Wekking EM, et al. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. J Clin Endocrinol Metab. 2005;90:2666-2674. doi: 10.1210/jc.2004-2111
32. Escobar-Morreale HF, Botella-Carretero JI, M, et al. Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing L-thyroxine plus liothyronine with L-thyroxine alone. Ann Intern Med. 2005;142:412-424. doi: 10.7326/0003-4819-142-6-200503150-00007
33. Hoang TD, Olsen CH, Mai VQ, et al. Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab. 2013;98:1982-1990. doi: 10.1210/jc.2012-4107
34. Shakir MKM, Brooks DI, McAninch EA, et al. Comparative effectiveness of levothyroxine, desiccated thyroid extract, and levothyroxine+liothyronine in hypothyroidism. J Clin Endocrinol Metab. 2021;106:e4400-e4413. doi: 10.1210/clinem/dgab478
35. Valizadeh M, Seyyed-Majidi MR, Hajibeigloo H, et al. Efficacy of combined levothyroxine and liothyronine as compared with levothyroxine monotherapy in primary hypothyroidism: a randomized controlled trial. Endocr Res. 2009;34:80-89. doi: 10.1080/07435800903156340
36. Walsh JP, Shiels L, Lim EM, et al. Combined thyroxine/liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. J Clin Endocrinol Metab. 2003;88:4543-4550. doi: 10.1210/jc.2003-030249
37. Rodriguez T, Lavis VR, Meininger JC, et al. Substitution of liothyronine at a 1:5 ratio for a portion of levothyroxine: effect on fatigue, symptoms of depression, and working memory versus treatment with levothyroxine alone. Endocr Pract. 2005;11:223-233. doi: 10.4158/EP.11.4.223
38. Sawka AM, Gerstein HC, Marriott MJ, et al. Does a combination regimen of thyroxine (T4) and 3,5,3’-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? Results of a double-blind, randomized, controlled trial. J Clin Endocrinol Metab. 2003;88:4551-4555. doi: 10.1210/jc.2003-030139
39. Clyde PW, Harari AE, Getka EJ, et al. Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism: a randomized controlled trial. JAMA. 2003;290:2952-2958. doi: 10.1001/jama.290.22.2952
40. Duntas LH. Selenium and the thyroid: a close-knit connection. J Clin Endocrinol Metab. 2010;95:5180-5188. doi: 10.1210/jc.2010-0191
41. Winther KH, Wichman JEM, Bonnema SJ, et al. Insufficient documentation for clinical efficacy of selenium supplementation in chronic autoimmune thyroiditis, based on a systematic review and meta-analysis. Endocrine. 2017;55:376-385. doi: 10.1007/s12020-016-1098-z
42. Parva NR, Tadepalli S, Singh P, et al. Prevalence of vitamin D deficiency and associated risk factors in the US population (2011-2012). Cureus. 2018;10:e2741. doi: 10.7759/cureus.2741
43. Wang J, Lv S, Chen G, et al. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients. 2015,7:2485-2498. doi: 10.3390/nu7042485
44. Wilson MM, Reedy J, Krebs-Smith SM. American diet quality: where it is, where it is heading, and what it could be. J Acad Nutr Diet. 2016;116:302-310.e1. doi: 10.1016/j.jand.2015.09.020
45. Babiker A, Alawi A, Al Atawi M, et al. The role of micronutrients in thyroid dysfunction. Sudan J Paediatr. 2020;20:13-19. doi: 10.24911/SJP.106-1587138942
46. Knezevic J, Starchl C, Tmava Berisha A, et al. Thyroid-gut-axis: How does the microbiota influence thyroid function? Nutrients. 2020;12:1769. doi: 10.3390/nu12061769
47. Rayman MP. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc Nutr Soc. 2019;78:34-44. doi: 10.1017/S0029665118001192
48. Chakrabarti SK, Ghosh S, Banerjee S, et al. Oxidative stress in hypothyroid patients and the role of antioxidant supplementation. Indian J Endocrinol Metab. 2016;20:674-678. doi: 10.4103/2230-8210.190555
49. Tseng F-Y, Lin W-Y, Lin C-C, et al. Subclinical hypothyroidism is associated with increased risk for all-cause and cardiovascular mortality in adults. J Am Coll Cardiol. 2012;60:730-737. doi: 10.1016/j.jacc.2012.03.047
50. Roberts LM, Pattison H, Roalfe A, et al. Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Intern Med. 2006;145:573-581. doi: 10.7326/0003-4819-145-8-200610170-00006
51. Gussekloo J, van Exel E, de Craen AJM, et al. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292:2591-2599. doi: 10.1001/jama.292.21.2591
52. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA. 2018;320:1349-1359. doi: 10.1001/jama.2018.13770
53. Monzani F, Dardano A, Caraccio N. Does treating subclinical hypothyroidism improve markers of cardiovascular risk? Treat Endocrinol. 2006;5:65-81. doi: 10.2165/00024677-200605020-00001
54. Duntas LH. Does celiac disease trigger autoimmune thyroiditis? Nat Rev Endocrinol. 2009;5:190-191. doi: 10.1038/nrendo.2009.46
55. Lerner A, Jeremias P, Matthias T. Gut-thyroid axis and celiac disease. Endocr Connect. 2017;6:R52-R58. doi: 10.1530/EC-17-0021
56. Janegova A, Janega P, Rychly B, et al. The role of Epstein-Barr virus infection in the development of autoimmune thyroid diseases. Endokrynol Pol. 2015;66:132-136. doi: 10.5603/EP.2015.0020
57. Brent GA. Environmental exposures and autoimmune thyroid disease. Thyroid. 2010;20:755-761. doi: 10.1089/thy.2010.1636
58. Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44-84. doi: 10.1016/j.biocel.2006.07.001
59. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028. doi: 10.4158/EP12280.GL
60. Welsh KJ, Soldin SJ. Diagnosis of endocrine disease: How reliable are free thyroid and total T3 hormone assays? Eur J Endocrinol. 2016;175:R255-R263. doi: 10.1530/EJE-16-0193
61. Grossman A, Feldhamer I, Meyerovitch J. Treatment with levothyroxin in subclinical hypothyroidism is associated with increased mortality in the elderly. Eur J Intern Med. 2018;50:65-68. doi: 10.1016/j.ejim.2017.11.010
62. Pearce SHS, Brabant G, Duntas LH, et al. 2013 ETA Guideline: management of subclinical hypothyroidism. Eur Thyroid J. 2013;2:215-228. doi: 10.1159/000356507
The hormones thyroxine (T4) and triiodothyronine (T3), produced by the thyroid gland, are crucial for maintaining metabolism. A deficit of thyroid hormone production—hypothyroidism—is a common endocrine disorder seen in primary care.
Although the diagnosis and management of hypothyroidism are considered straightforward, many patients with hypothyroidism do not achieve optimal treatment goals or see an improvement in their quality of life. In this article, we address the questionable utility of screening; outline the diagnostic approach, including the central role of laboratory testing; and explain why treatment requires a precise approach to address the range of patient types.
Epidemiology and classification
Estimates are that almost 5% of Americans 12 years or older have hypothyroidism; older people and women are more likely to develop the condition. 1 In the US National Health and Nutrition Examination Survey (NHANES III) of 13,344 people without known thyroid disease or a family history, hypothyroidism was found in 4.6% (overt [clinical] in 0.3% and subclinical in 4.3%); 11% had a high serum thyroid peroxidase antibody level, which increases their risk of hypothyroidism, and is treated the same as hypothyroidism of other causes; and, overall, lower serum thyroid-stimulating hormone (TSH) levels were seen in Blacks, compared to Whites and Mexican Americans.1
Primary hypothyroidism accounts for > 95% of cases of hypothyroidism, representing a failure of the thyroid gland to produce sufficient hormone. It has been shown that, in iodine-replete countries such as the United States, the prevalence of spontaneous hypothyroidism is 1% to 2%, and it is 10 times more common in women.2,3
Central hypothyroidism is caused by insufficient stimulation of the thyroid gland by TSH, due to pituitary (secondary hypothyroidism) or hypothalamic (tertiary hypothyroidism) disease and is estimated to occur in 1 in every 20,000 to 80,000 people in the general population.4
How does hypothyroidism manifest?
Signs and symptoms. Manifestations of hypothyroidism range from life-threatening to minimal or no clinical signs and symptoms (TABLE W1). Signs and symptoms of low thyroid function vary by the degree of hypothyroidism at presentation.
Common signs and symptoms of low thyroid function include fatigue, weight gain, dry skin, brittle hair, hair loss, morning stiffness, muscle aches, joint pain, cold intolerance, diffuse headache, constipation, difficulty concentrating, low libido, depression, and menstrual irregularities. On physical examination, a patient might present with bradycardia, hypotension, hypothermia with slow speech or movement, coarse facial appearance, goiter, diffuse hair loss, cold hands and feet, and a prolonged Achilles tendon reflex.5 Skin findings, such as keratosis pilaris, palmoplantar keratoderma (thickening of the skin), and pityriasis rubra pilar, can be associated with autoimmune hypothyroidism.6,7
Continue to: Carpal tunnel syndrome...
Carpal tunnel syndrome, plantar fasciitis, infertility or miscarriage, dyspepsia, and small intestinal bacterial overgrowth can be associated with hypothyroidism; thyroid function should therefore be assessed in patients who have any of these conditions, along with other signs and symptoms of low thyroid function.8,9 A patient with severe hypothyroidism might present with hemodynamic instability, pericardial or pleural effusion, and myxedema coma.10
Clues in the history and from the lab. A history of radiation to the head, neck, or chest area and a history or family history of autoimmune disorders are risk factors for autoimmune thyroid disease.11,12 Laboratory findings can include markers of oxidative stress, such as elevated levels of low-density lipoprotein cholesterol and serum malondialdehyde.13-15
Screening and diagnosis
Screening. The US Preventive Services Task Force has asserted that evidence is insufficient by which to evaluate the benefits and risks of routine screening for thyroid dysfunction in nonpregnant, asymptomatic adults.16 According to the American Thyroid Association and the American Association of Clinical Endocrinologists, screening should be considered in high-risk patients, including those who take medication that affects thyroid function or the results of thyroid hormone assays (TABLE W2, available at mdedge.com/familymedicine).17-20
Screening inpatients is challenging and usually not recommended unless thyroid disease is strongly suspected. This is because changes in the levels of thyroid hormones, binding proteins, and the TSH concentration can occur in severe nonthyroidal illness; in addition, assay interference by antibodies and other substances can affect thyroid hormone measurement.21
Testing strategy. Generally, screening and diagnosis of hypothyroidism are based primarily on laboratory testing, because signs and symptoms are nonspecific (FIGURE 15). A serum TSH level is usually the initial test when screening for thyroid dysfunction. A normal serum TSH value ranges from 0.5-5.0 mIU/L.
When an abnormal serum TSH level is found, further tests can be performed to investigate, including a serum free thyroxine (FT4) test. (Our preference is to order TSH and FT4 assays simultaneously to facilitate and confirm the diagnosis.) An FT4 test measures the amount of unattached, or free, thyroxine in blood by immunoassay. A normal FT4 value usually ranges from 0.7-1.9 ng/dL.
The combination of a high TSH level and a low FT4 level could be an indication of an underactive thyroid gland (ie, clinical or overt hypothyroidism). Milder, subclinical hypothyroidism is characterized by a higher-than-normal TSH level but a normal FT4 level.22 Central (secondary) hypothyroidism is characterized by a low serum FT4 level and a serum TSH level that can be below the reference range, low normal, or even slightly high.4
Continue to: These measurements...
These measurements must be interpreted within the context of the laboratory-specific normal range for each test. Third-generation serum TSH assays are more sensitive and specific than serum FT4 measurements for hypothyroidism. FT4 is usually measured by automated analogue immunoassay, which generally provides reliable results; abnormal binding proteins or other interferences occur in some patients, however, resulting in reporting of a falsely high, or falsely low, FT4 level. In such cases, FT4 by direct dialysis, or total T4, can be measured for further evaluation. In primary care, you are most likely to encounter primary hypothyroidism; secondary (central) hypothyroidism is much rarer (< 5% of cases).4
The ins and outs of treatment
For most patients, hypothyroidism is a permanent disorder requiring lifelong thyroid hormone replacement therapy—unless the disease is transient (ie, painless or subacute thyroiditis); reversible, because it is caused by medication; or responsive to medical intervention that addresses the underlying autoimmune condition.19 Goals of treatment (Figure 25,23) are to:
- normalize the TSH level to 0.5-5.0 mIU/L (the main goal), with an age-related shift toward a higher TSH goal in older patients (and an upper limit of normal of 7.5 mIU/L in patients who are ≥ 80 years of age)20
- restore the euthyroid state
- relieve symptoms
- reduce any goiter
- avoid overtreatment (iatrogenic thyrotoxicosis).
Desiccated thyroid extract (DTE), developed in the late 1880s and made from the dried thyroid gland of pigs, sheep, or cows, was the earliest treatment for hypothyroidism. The use of DTE has declined since the introduction of synthetic thyroxine (T4, or levothyroxine [here, referred to as LT4]), which is now the standard treatment.20-22 LT4 is deiodinated in peripheral tissues to form T3, the active thyroid hormone; this process accounts for 80% of total T3 production daily.24
LT4 formulations. LT4 is commercially available in tablet, soft-gel, and liquid preparations. Most patients are treated with the tablet; the soft-gel capsule or liquid is an option for patients who absorb the tablet poorly (because of atrophic gastritis, celiac disease, or gluten sensitivity or because they are post bariatric surgery). Increasing the dosage of the tablet form of LT4, with ongoing TSH monitoring, is more cost effective than moving to an alternative preparation.
If a switch of LT4 formulation is made (ie, from one manufacturer to another), test the serum TSH level to ensure that the therapeutic goal is being reached. Also, in our experience, it is best to prescribe a brand-name preparation of levothyroxine, not a generic, whenever possible, due to the variability in generic formulations and the potential presence of other (inert) ingredients.25
Dosing (TABLE 320,23). The average full replacement dosage of LT4 for a young, healthy adult is approximately 1.6 mcg/kg/d. Older patients (> 65 years) or those with coronary artery disease (CAD) should be started on a lower dosage (25-50 mcg/d) and titrated to goal accordingly.
LT4 (tablets, soft-gel capsules, or liquid) should be administered on an empty stomach, with water only, 30 to 60 minutes before breakfast. Medications that interfere with LT4 absorption (eg, bile acid resins, calcium carbonate, ferrous sulfate) should be taken several hours after LT4. For patients who cannot take LT4 in the morning, taking it at bedtime (≥ 2-3 hours after the last meal) is acceptable.
Continue to: Monitoring and titrating
Monitoring and titrating. Hypothyroid symptoms usually improve after 2 or 3 weeks of LT4 treatment; in severe hypothyroidism, complete recovery might take months. Approximately 6 weeks after LT4 therapy is initiated, serum TSH should be measured. After assessing whether administration of LT4 at the starting dosage is appropriate, that dosage can be increased, or decreased, every 4 to 6 weeks until the TSH goal is reached. Once the patient is maintained at a given dosage, measure serum TSH once a year—more often if there is an abnormal result or a change in the patient’s health status.23
Adverse effects of LT4 therapy are rare, unless over-replacement occurs. Rarely, patients have an allergy to the dye or an excipient (filler) in the tablet.26-28 The white, 50-mcg tablets can be given safely to patients with dye sensitivity. For those who have an allergy to an excipient (except gelatin) or gluten intolerance, the LT4 soft-gel capsule or liquid preparation (Tirosint) can be prescribed.
Pure LT4, in a capsule made from vegetable sources, can be ordered through a compounding pharmacy for patients who are allergic to animal products.
Anemia, especially iron-deficiency anemia, can cause intolerance to LT4 therapy; in such patients, lowering the starting dosage and treating anemia are indicated.29
Persistent symptoms (despite a normal TSH level). Because many hypothyroid symptoms are nonspecific, patients might come to think that their LT4 dosage is inadequate if they feel tired or gain weight. Persistent hypothyroid symptoms despite a normal serum TSH level might be due to (1) the inability of LT4 therapy to restore tissue thyroid hormone levels to normal or (2) other variables unrelated to hypothyroidism, including disorders associated with inflammation or autoimmune disease, certain medications, diet, lifestyle, and environmental toxins.
These patients might benefit from a detailed history to identify other causes and a switch to either LT4 + liothyronine (LT3; synthetic T3) combination therapy or DTE26,30-33 (TABLE 434), although a beneficial effect of LT4 + LT3 therapy was not seen in several studies.35,36 Over-replacement with LT4 should be discouraged, due to concerns about thyrotoxicosis and its complications (eg, atrial fibrillation, accelerated bone loss).
DTE and LT4 + LT3. Use of DTE has decreased since the 1970s, when LT4 became the therapy of choice. Subsequently, anecdotal evidence emerged that some patients did not feel well on LT4 and preferred to return to DTE.32,33
Continue to: Several clinical trials...
Several clinical trials addressed the question of whether residual symptoms could be resolved through LT4 + LT3 combination therapy31-39 (TABLE 434), but evidence of any consistent superiority of combination therapy was not demonstrated.35-39 In selected cases, patients might prefer the combination approach.31,33,39 The quality of life of hypothyroid patients was found to be similarly improved with LT4 or DTE, but the latter was associated with modest weight loss (approximately 4 lbs); nearly 50% of study patients preferred treatment with DTE over LT4.33 A follow-up study did not confirm weight loss with DTE, however.34
When LT4 monotherapy and LT4 + LT3 combination therapy were compared, results were mixed31-39; responsiveness to therapy containing LT3 might therefore depend on multiple variables, including genetic background, nutritional and lifestyle factors, stress, presence of comorbidities and autoimmune disorders, and other unidentified or poorly defined variables.40-48
Although combination therapy and DTE are not generally recommended over LT4 monotherapy, they might offer better options for patients who are still symptomatic when being treated with LT4 only: In a randomized, double-blind, crossover study that compared LT4 with DTE and with LT4 + LT3, one-third of the most highly symptomatic patients who had low scores on mood, cognitive, and quality-of-life assessments improved significantly after they were switched to combination therapy or DTE.34
The 2014 American Thyroid Association guidelines24 do not support routine use of LT4 + LT3 in hypothyroid patients who have residual symptoms after LT4 monotherapy; however, a therapeutic trial of LT4 + LT3, while maintaining a normal serum TSH, is reasonable in selected patients. Candidates for DTE or LT4 + LT3 might include patients who do not feel well on LT4 monotherapy, are post thyroidectomy or post radioiodine therapy, or have a low serum T3 level. DTE and combination therapy are discouraged in older patients, patients who have underlying CAD, and pregnant patients.
Special treatment circumstances
A number of patient variables have the potential to alter management strategies for hypothyroidism.18,20,23,40,49-53
Age, comorbidity. Older patients (> 65 years) and patients with cardiopulmonary disease or CAD should be treated with LT4, 25 to 50 mcg/d, initially; that dosage can be titrated upward by 12.5 to 25 mcg/d every 4 to 6 weeks until the TSH goal is reached—preferably, in the range of 4 to 8 mIU/L. An increase in the dosage of LT4 might be required in the presence of malabsorption (eg, gastrointestinal disorders, celiac disease) and in nephrotic syndrome.18,20,23
Body weight. A decrease in the dosage of LT4 might be indicated in the setting of significant weight loss (> 10% body weight).23
Continue to: Co-pharmacy
Co-pharmacy. An increase in the dosage of LT4 might be required when other drugs (eg, phenytoin, phenobarbital, rifampin, and carbamazepine) have led to an increased rate of thyroid hormone metabolism. A decrease in the dosage of LT4 might be necessary after initiation of androgen therapy.23
Pregnancy. Women with pre-existing hypothyroidism require an increase of 25% to 50% in their LT4 dosage during pregnancy to maintain a TSH level in the recommended pregnancy reference range. Thyroid function should be monitored every 4 to 6 weeks to ensure that the TSH target for each trimester is reached (first trimester, 0.1-4 mIU/L; second trimester, 0.2-4 mIU/L; third trimester, 0.3-4 mIU/L). Postpartum, LT4 can be reduced to the prepartum dosage; TSH should be checked every 4 to 6 weeks to maintain the TSH goal.23
Estrogen therapy. Hypothyroid women who are receiving estrogen therapy might require an increase in their LT4 dosage because serum thyroxine-binding globulin levels are increased by estrogens or through other mechanisms that have not been identified.23
Surgical candidacy. Observational studies show few adverse outcomes in surgical patients with mild (subclinical) hypothyroidism or moderate hypothyroidism; however, the risk of adverse surgical outcome might be increased in patients with severe hypothyroidism. For patients in whom surgery is planned and who have:
- subclinical hypothyroidism (elevated TSH and normal FT4), we recommend that surgery—urgent or elective—not be posptoned but proceed.
- moderate (overt) hypothyroidism who require urgent surgery, we recommend not postponing surgery, even though minor perioperative complications might develop. Such patients should be treated with LT4 as soon as the diagnosis for which surgery is required has been made. Alternatively, when moderate hypothyroidism is discovered in a patient who is being evaluated for elective surgery, we recommend postponing surgery until the euthyroid state is restored.
- severe hypothyroidism (myxedema coma [discussed in a bit]; severe clinical symptoms of chronic hypothyroidism, such as altered mental status, pericardial effusion, or heart failure; or a very low level of T4), surgery should be delayed until hypothyroidism has been treated. When emergency surgery is required for a severely hypothyroid patient, they should be treated with LT4 as soon as the diagnosis for which surgery is indicated has been made. When emergency surgery must be performed in a patient with myxedema coma, we recommend treatment with LT4 + LT3, rather than LT4 alone, often administered intravenously because LT4 is poorly absorbed in these patients.
Nonadherence. For patients who do not take LT4 regularly or do not respond to efforts to improve adherence, LT4 can be given weekly, instead of daily, although this interval is not ideal. Weekly dosing should not be used in older patients with CAD.23
Thyroid cancer. Patients who are post total thyroidectomy for thyroid cancer need to take LT4 to treat hypothyroidism and to prevent recurrence of thyroid cancer. The goal TSH level should be based on the cancer stage and risk of recurrence and should be monitored by an endocrinologist.
Myxedema coma. This medical emergency has high mortality. Myxedema coma occurs when severe hypothyroidism leads to any, or a combination, of the following: diminished mentation; hypothermia; bradycardia; hyponatremia; hypotension; cardiovascular, respiratory, and gastrointestinal dysfunction; and renal insufficiency. LT4, LT3, and glucocorticoids should be administered intravenously and the patient monitored closely—preferably in consultation with an endocrinologist.
Continue to: When to seek consultation
When to seek consultation
A patient with hypothyroidism should be referred to Endocrinology if they are < 18 years of age, pregnant, unresponsive to therapy, or have cardiac disease, coexisting endocrine disease, suspected myxedema coma, goiter or thyroid nodules, or a structural thyroid abnormality.
What we know about nutrition and hypothyroidism
Although it is commonly recognized that iodine is essential for production of thyroid hormone, other nutritional factors might contribute to proper production of thyroid hormones, including:
- adequate intake of iron, tyrosine, selenium, zinc, and vitamins E, B2, B3, B6, C, and D44,45
- selenium and zinc, which increase conversion of T4 to T3 and might be important in the management of hypothyroid patients40,46
- vitamin A, zinc, and regular exercise, which have been shown to improve cellular sensitivity to thyroid hormones.
Low iron stores can contribute to persistent symptoms and poor quality of life in patients with hypothyroidism, despite their being treated according to guidelines.29,47
Despite what is known about these nutritional connections, there is insufficient evidence that improving nutrition can reverse hypothyroidism.
Prevention
Prevention of hypothyroidism should take into account variables that affect or inhibit thyroid function, such as stress, infection (eg, Epstein-Barr virus), excessive fluoride intake, toxins (eg, pesticides, solvents, mercury, cadmium, and lead), autoimmune disease (eg, celiac disease), and food sensitivity.54,55 Oxidative stress can also cause thyroid impairment.40-48,54-58
Otherwise, there are, at present, no effective strategies for preventing thyroid disorders.
Subclinical hypothyroidism: Elusive management target
Subclinical hypothyroidism is defined as a normal serum FT4 level in the presence of an elevated serum TSH level. The prevalence of subclinical hypothyroidism varies from 3% to 15%, depending on the population studied; a higher incidence has been noted in women and older people.59 In the NHANES III,1 which excluded people with previously diagnosed thyroid disease, the incidence of subclinical hypothyroidism was 4.3%.
Continue to: Causes of subclinical...
Causes of subclinical hypothyroidism are the same as those of overt hypothyroidism, and include Hashimoto disease. The combination of an elevated TSH level and a normal FT4 level is associated with disorders characterized by protein-binding variations (eg, pregnancy, genetic disorders, drugs), TSH-secreting pituitary adenoma, class II and III obesity (respectively, body mass index, ≥ 35 but < 40 and ≥ 40), and assay variability.49,51
Lab diagnosis: Fraught with difficulty
The serum TSH level and either the total T4 level or the FT4 level should be measured to make a diagnosis of subclinical hypothyroidism. Most laboratories use a 1-step analogue immunoassay to determine free thyroid hormones; protein-binding variations can thus affect measurement of FT4.
Several scenarios that can result in inaccurate measurement of FT4 by radioimmunoassay include genetic disorders that affect binding proteins; pregnancy; use of certain drugs, including heparin, furosemide, antiepileptic agents, salicylate, ferrous sulfate, and cholesterol-binding resins; and some medical conditions, including cardiac surgery, critical illness, and renal failure. Variables that inhibit proper production of thyroid hormones—stress, infection, fluoride (an iodine antagonist), toxins (pesticides, mercury, cadmium, lead) and autoimmune conditions, such as celiac disease—should be considered when attempting to determine the cause of subclinical hypothyroidism.
Liquid chromatography–mass spectrometry measurement of thyroid hormones might be more accurate than immunoassay.53 Measuring serum total T4 and FT4 by dialysis, free from interfering proteins, might also be useful when measurement of FT4 by immunoassay is affected by binding-protein variations.
Features of subclinical hypothyroidism
Most patients who have subclinical hypothyroidism and a serum TSH level < 10 mIU/L are asymptomatic. Some might have nonspecific symptoms of hypothyroidism, however, such as reduced quality of life, poor cognitive function, and poor memory—symptoms that do not typically correlate with the serum TSH level.
It has been suggested that some elderly people normally have a higher level of serum TSH, and that they might have even a prolonged lifespan.51 Additionally, it has been shown that, in nonpregnant adult patients with subclinical hypothyroidism and a serum TSH level of 4.5 to 10 mIU/L, treatment with LT4 was not associated with improvement in thyroid-related symptoms or general quality of life.52
Treat, or don't treat, subclinical hypothyroidism?
It is well accepted that the goal of therapy in hypothyroid patients is to normalize the serum TSH level; however, the American Thyroid Association and the American Association of Clinical Endocrinology recommend starting LT4 in patients with a serum TSH level ≥ 10 mIU/L (TABLE 5).59-62 The principal reason for not treating subclinical hypothyroidism is the lack of benefit in reducing the risk of cardiovascular morbidity and mortality when the TSH level is between 7.5 and 10 mIU/L.62
Continue to: Routine treatment
Routine treatment of patients with a serum TSH level of 4.5 to 10 mIU/L remains controversial. When TSH is 7.0 to 9.9 mIU/L, treatment is recommended for (1) patients < 65 years and (2) for older patients (> 65 years) only when there are convincing hypothyroid symptoms because of concern about unintended overtreatment.
When the TSH level is anywhere above the upper limit of normal to 6.9 mIU/L, treatment is recommended for patients < 65 years old, patients who have a high titer of thyroid peroxidase antibodies, and patients with goiter—but not for patients > 65 years (and, especially, not for octogenarians) because their upper limit of normal could be as high as 6 to 8 mIU/L, especially if they are otherwise healthy.
Treatment should be considered for women with subclinical hypothyroidism who are trying to conceive or experiencing an infertility problem.
For patients with subclinical hypothyroidism who are not being treated, monitor thyroid function every 6 to 12 months by testing TSH and FT4.
CORRESPONDENCE
Thanh D. Hoang, DO, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
The hormones thyroxine (T4) and triiodothyronine (T3), produced by the thyroid gland, are crucial for maintaining metabolism. A deficit of thyroid hormone production—hypothyroidism—is a common endocrine disorder seen in primary care.
Although the diagnosis and management of hypothyroidism are considered straightforward, many patients with hypothyroidism do not achieve optimal treatment goals or see an improvement in their quality of life. In this article, we address the questionable utility of screening; outline the diagnostic approach, including the central role of laboratory testing; and explain why treatment requires a precise approach to address the range of patient types.
Epidemiology and classification
Estimates are that almost 5% of Americans 12 years or older have hypothyroidism; older people and women are more likely to develop the condition. 1 In the US National Health and Nutrition Examination Survey (NHANES III) of 13,344 people without known thyroid disease or a family history, hypothyroidism was found in 4.6% (overt [clinical] in 0.3% and subclinical in 4.3%); 11% had a high serum thyroid peroxidase antibody level, which increases their risk of hypothyroidism, and is treated the same as hypothyroidism of other causes; and, overall, lower serum thyroid-stimulating hormone (TSH) levels were seen in Blacks, compared to Whites and Mexican Americans.1
Primary hypothyroidism accounts for > 95% of cases of hypothyroidism, representing a failure of the thyroid gland to produce sufficient hormone. It has been shown that, in iodine-replete countries such as the United States, the prevalence of spontaneous hypothyroidism is 1% to 2%, and it is 10 times more common in women.2,3
Central hypothyroidism is caused by insufficient stimulation of the thyroid gland by TSH, due to pituitary (secondary hypothyroidism) or hypothalamic (tertiary hypothyroidism) disease and is estimated to occur in 1 in every 20,000 to 80,000 people in the general population.4
How does hypothyroidism manifest?
Signs and symptoms. Manifestations of hypothyroidism range from life-threatening to minimal or no clinical signs and symptoms (TABLE W1). Signs and symptoms of low thyroid function vary by the degree of hypothyroidism at presentation.
Common signs and symptoms of low thyroid function include fatigue, weight gain, dry skin, brittle hair, hair loss, morning stiffness, muscle aches, joint pain, cold intolerance, diffuse headache, constipation, difficulty concentrating, low libido, depression, and menstrual irregularities. On physical examination, a patient might present with bradycardia, hypotension, hypothermia with slow speech or movement, coarse facial appearance, goiter, diffuse hair loss, cold hands and feet, and a prolonged Achilles tendon reflex.5 Skin findings, such as keratosis pilaris, palmoplantar keratoderma (thickening of the skin), and pityriasis rubra pilar, can be associated with autoimmune hypothyroidism.6,7
Continue to: Carpal tunnel syndrome...
Carpal tunnel syndrome, plantar fasciitis, infertility or miscarriage, dyspepsia, and small intestinal bacterial overgrowth can be associated with hypothyroidism; thyroid function should therefore be assessed in patients who have any of these conditions, along with other signs and symptoms of low thyroid function.8,9 A patient with severe hypothyroidism might present with hemodynamic instability, pericardial or pleural effusion, and myxedema coma.10
Clues in the history and from the lab. A history of radiation to the head, neck, or chest area and a history or family history of autoimmune disorders are risk factors for autoimmune thyroid disease.11,12 Laboratory findings can include markers of oxidative stress, such as elevated levels of low-density lipoprotein cholesterol and serum malondialdehyde.13-15
Screening and diagnosis
Screening. The US Preventive Services Task Force has asserted that evidence is insufficient by which to evaluate the benefits and risks of routine screening for thyroid dysfunction in nonpregnant, asymptomatic adults.16 According to the American Thyroid Association and the American Association of Clinical Endocrinologists, screening should be considered in high-risk patients, including those who take medication that affects thyroid function or the results of thyroid hormone assays (TABLE W2, available at mdedge.com/familymedicine).17-20
Screening inpatients is challenging and usually not recommended unless thyroid disease is strongly suspected. This is because changes in the levels of thyroid hormones, binding proteins, and the TSH concentration can occur in severe nonthyroidal illness; in addition, assay interference by antibodies and other substances can affect thyroid hormone measurement.21
Testing strategy. Generally, screening and diagnosis of hypothyroidism are based primarily on laboratory testing, because signs and symptoms are nonspecific (FIGURE 15). A serum TSH level is usually the initial test when screening for thyroid dysfunction. A normal serum TSH value ranges from 0.5-5.0 mIU/L.
When an abnormal serum TSH level is found, further tests can be performed to investigate, including a serum free thyroxine (FT4) test. (Our preference is to order TSH and FT4 assays simultaneously to facilitate and confirm the diagnosis.) An FT4 test measures the amount of unattached, or free, thyroxine in blood by immunoassay. A normal FT4 value usually ranges from 0.7-1.9 ng/dL.
The combination of a high TSH level and a low FT4 level could be an indication of an underactive thyroid gland (ie, clinical or overt hypothyroidism). Milder, subclinical hypothyroidism is characterized by a higher-than-normal TSH level but a normal FT4 level.22 Central (secondary) hypothyroidism is characterized by a low serum FT4 level and a serum TSH level that can be below the reference range, low normal, or even slightly high.4
Continue to: These measurements...
These measurements must be interpreted within the context of the laboratory-specific normal range for each test. Third-generation serum TSH assays are more sensitive and specific than serum FT4 measurements for hypothyroidism. FT4 is usually measured by automated analogue immunoassay, which generally provides reliable results; abnormal binding proteins or other interferences occur in some patients, however, resulting in reporting of a falsely high, or falsely low, FT4 level. In such cases, FT4 by direct dialysis, or total T4, can be measured for further evaluation. In primary care, you are most likely to encounter primary hypothyroidism; secondary (central) hypothyroidism is much rarer (< 5% of cases).4
The ins and outs of treatment
For most patients, hypothyroidism is a permanent disorder requiring lifelong thyroid hormone replacement therapy—unless the disease is transient (ie, painless or subacute thyroiditis); reversible, because it is caused by medication; or responsive to medical intervention that addresses the underlying autoimmune condition.19 Goals of treatment (Figure 25,23) are to:
- normalize the TSH level to 0.5-5.0 mIU/L (the main goal), with an age-related shift toward a higher TSH goal in older patients (and an upper limit of normal of 7.5 mIU/L in patients who are ≥ 80 years of age)20
- restore the euthyroid state
- relieve symptoms
- reduce any goiter
- avoid overtreatment (iatrogenic thyrotoxicosis).
Desiccated thyroid extract (DTE), developed in the late 1880s and made from the dried thyroid gland of pigs, sheep, or cows, was the earliest treatment for hypothyroidism. The use of DTE has declined since the introduction of synthetic thyroxine (T4, or levothyroxine [here, referred to as LT4]), which is now the standard treatment.20-22 LT4 is deiodinated in peripheral tissues to form T3, the active thyroid hormone; this process accounts for 80% of total T3 production daily.24
LT4 formulations. LT4 is commercially available in tablet, soft-gel, and liquid preparations. Most patients are treated with the tablet; the soft-gel capsule or liquid is an option for patients who absorb the tablet poorly (because of atrophic gastritis, celiac disease, or gluten sensitivity or because they are post bariatric surgery). Increasing the dosage of the tablet form of LT4, with ongoing TSH monitoring, is more cost effective than moving to an alternative preparation.
If a switch of LT4 formulation is made (ie, from one manufacturer to another), test the serum TSH level to ensure that the therapeutic goal is being reached. Also, in our experience, it is best to prescribe a brand-name preparation of levothyroxine, not a generic, whenever possible, due to the variability in generic formulations and the potential presence of other (inert) ingredients.25
Dosing (TABLE 320,23). The average full replacement dosage of LT4 for a young, healthy adult is approximately 1.6 mcg/kg/d. Older patients (> 65 years) or those with coronary artery disease (CAD) should be started on a lower dosage (25-50 mcg/d) and titrated to goal accordingly.
LT4 (tablets, soft-gel capsules, or liquid) should be administered on an empty stomach, with water only, 30 to 60 minutes before breakfast. Medications that interfere with LT4 absorption (eg, bile acid resins, calcium carbonate, ferrous sulfate) should be taken several hours after LT4. For patients who cannot take LT4 in the morning, taking it at bedtime (≥ 2-3 hours after the last meal) is acceptable.
Continue to: Monitoring and titrating
Monitoring and titrating. Hypothyroid symptoms usually improve after 2 or 3 weeks of LT4 treatment; in severe hypothyroidism, complete recovery might take months. Approximately 6 weeks after LT4 therapy is initiated, serum TSH should be measured. After assessing whether administration of LT4 at the starting dosage is appropriate, that dosage can be increased, or decreased, every 4 to 6 weeks until the TSH goal is reached. Once the patient is maintained at a given dosage, measure serum TSH once a year—more often if there is an abnormal result or a change in the patient’s health status.23
Adverse effects of LT4 therapy are rare, unless over-replacement occurs. Rarely, patients have an allergy to the dye or an excipient (filler) in the tablet.26-28 The white, 50-mcg tablets can be given safely to patients with dye sensitivity. For those who have an allergy to an excipient (except gelatin) or gluten intolerance, the LT4 soft-gel capsule or liquid preparation (Tirosint) can be prescribed.
Pure LT4, in a capsule made from vegetable sources, can be ordered through a compounding pharmacy for patients who are allergic to animal products.
Anemia, especially iron-deficiency anemia, can cause intolerance to LT4 therapy; in such patients, lowering the starting dosage and treating anemia are indicated.29
Persistent symptoms (despite a normal TSH level). Because many hypothyroid symptoms are nonspecific, patients might come to think that their LT4 dosage is inadequate if they feel tired or gain weight. Persistent hypothyroid symptoms despite a normal serum TSH level might be due to (1) the inability of LT4 therapy to restore tissue thyroid hormone levels to normal or (2) other variables unrelated to hypothyroidism, including disorders associated with inflammation or autoimmune disease, certain medications, diet, lifestyle, and environmental toxins.
These patients might benefit from a detailed history to identify other causes and a switch to either LT4 + liothyronine (LT3; synthetic T3) combination therapy or DTE26,30-33 (TABLE 434), although a beneficial effect of LT4 + LT3 therapy was not seen in several studies.35,36 Over-replacement with LT4 should be discouraged, due to concerns about thyrotoxicosis and its complications (eg, atrial fibrillation, accelerated bone loss).
DTE and LT4 + LT3. Use of DTE has decreased since the 1970s, when LT4 became the therapy of choice. Subsequently, anecdotal evidence emerged that some patients did not feel well on LT4 and preferred to return to DTE.32,33
Continue to: Several clinical trials...
Several clinical trials addressed the question of whether residual symptoms could be resolved through LT4 + LT3 combination therapy31-39 (TABLE 434), but evidence of any consistent superiority of combination therapy was not demonstrated.35-39 In selected cases, patients might prefer the combination approach.31,33,39 The quality of life of hypothyroid patients was found to be similarly improved with LT4 or DTE, but the latter was associated with modest weight loss (approximately 4 lbs); nearly 50% of study patients preferred treatment with DTE over LT4.33 A follow-up study did not confirm weight loss with DTE, however.34
When LT4 monotherapy and LT4 + LT3 combination therapy were compared, results were mixed31-39; responsiveness to therapy containing LT3 might therefore depend on multiple variables, including genetic background, nutritional and lifestyle factors, stress, presence of comorbidities and autoimmune disorders, and other unidentified or poorly defined variables.40-48
Although combination therapy and DTE are not generally recommended over LT4 monotherapy, they might offer better options for patients who are still symptomatic when being treated with LT4 only: In a randomized, double-blind, crossover study that compared LT4 with DTE and with LT4 + LT3, one-third of the most highly symptomatic patients who had low scores on mood, cognitive, and quality-of-life assessments improved significantly after they were switched to combination therapy or DTE.34
The 2014 American Thyroid Association guidelines24 do not support routine use of LT4 + LT3 in hypothyroid patients who have residual symptoms after LT4 monotherapy; however, a therapeutic trial of LT4 + LT3, while maintaining a normal serum TSH, is reasonable in selected patients. Candidates for DTE or LT4 + LT3 might include patients who do not feel well on LT4 monotherapy, are post thyroidectomy or post radioiodine therapy, or have a low serum T3 level. DTE and combination therapy are discouraged in older patients, patients who have underlying CAD, and pregnant patients.
Special treatment circumstances
A number of patient variables have the potential to alter management strategies for hypothyroidism.18,20,23,40,49-53
Age, comorbidity. Older patients (> 65 years) and patients with cardiopulmonary disease or CAD should be treated with LT4, 25 to 50 mcg/d, initially; that dosage can be titrated upward by 12.5 to 25 mcg/d every 4 to 6 weeks until the TSH goal is reached—preferably, in the range of 4 to 8 mIU/L. An increase in the dosage of LT4 might be required in the presence of malabsorption (eg, gastrointestinal disorders, celiac disease) and in nephrotic syndrome.18,20,23
Body weight. A decrease in the dosage of LT4 might be indicated in the setting of significant weight loss (> 10% body weight).23
Continue to: Co-pharmacy
Co-pharmacy. An increase in the dosage of LT4 might be required when other drugs (eg, phenytoin, phenobarbital, rifampin, and carbamazepine) have led to an increased rate of thyroid hormone metabolism. A decrease in the dosage of LT4 might be necessary after initiation of androgen therapy.23
Pregnancy. Women with pre-existing hypothyroidism require an increase of 25% to 50% in their LT4 dosage during pregnancy to maintain a TSH level in the recommended pregnancy reference range. Thyroid function should be monitored every 4 to 6 weeks to ensure that the TSH target for each trimester is reached (first trimester, 0.1-4 mIU/L; second trimester, 0.2-4 mIU/L; third trimester, 0.3-4 mIU/L). Postpartum, LT4 can be reduced to the prepartum dosage; TSH should be checked every 4 to 6 weeks to maintain the TSH goal.23
Estrogen therapy. Hypothyroid women who are receiving estrogen therapy might require an increase in their LT4 dosage because serum thyroxine-binding globulin levels are increased by estrogens or through other mechanisms that have not been identified.23
Surgical candidacy. Observational studies show few adverse outcomes in surgical patients with mild (subclinical) hypothyroidism or moderate hypothyroidism; however, the risk of adverse surgical outcome might be increased in patients with severe hypothyroidism. For patients in whom surgery is planned and who have:
- subclinical hypothyroidism (elevated TSH and normal FT4), we recommend that surgery—urgent or elective—not be posptoned but proceed.
- moderate (overt) hypothyroidism who require urgent surgery, we recommend not postponing surgery, even though minor perioperative complications might develop. Such patients should be treated with LT4 as soon as the diagnosis for which surgery is required has been made. Alternatively, when moderate hypothyroidism is discovered in a patient who is being evaluated for elective surgery, we recommend postponing surgery until the euthyroid state is restored.
- severe hypothyroidism (myxedema coma [discussed in a bit]; severe clinical symptoms of chronic hypothyroidism, such as altered mental status, pericardial effusion, or heart failure; or a very low level of T4), surgery should be delayed until hypothyroidism has been treated. When emergency surgery is required for a severely hypothyroid patient, they should be treated with LT4 as soon as the diagnosis for which surgery is indicated has been made. When emergency surgery must be performed in a patient with myxedema coma, we recommend treatment with LT4 + LT3, rather than LT4 alone, often administered intravenously because LT4 is poorly absorbed in these patients.
Nonadherence. For patients who do not take LT4 regularly or do not respond to efforts to improve adherence, LT4 can be given weekly, instead of daily, although this interval is not ideal. Weekly dosing should not be used in older patients with CAD.23
Thyroid cancer. Patients who are post total thyroidectomy for thyroid cancer need to take LT4 to treat hypothyroidism and to prevent recurrence of thyroid cancer. The goal TSH level should be based on the cancer stage and risk of recurrence and should be monitored by an endocrinologist.
Myxedema coma. This medical emergency has high mortality. Myxedema coma occurs when severe hypothyroidism leads to any, or a combination, of the following: diminished mentation; hypothermia; bradycardia; hyponatremia; hypotension; cardiovascular, respiratory, and gastrointestinal dysfunction; and renal insufficiency. LT4, LT3, and glucocorticoids should be administered intravenously and the patient monitored closely—preferably in consultation with an endocrinologist.
Continue to: When to seek consultation
When to seek consultation
A patient with hypothyroidism should be referred to Endocrinology if they are < 18 years of age, pregnant, unresponsive to therapy, or have cardiac disease, coexisting endocrine disease, suspected myxedema coma, goiter or thyroid nodules, or a structural thyroid abnormality.
What we know about nutrition and hypothyroidism
Although it is commonly recognized that iodine is essential for production of thyroid hormone, other nutritional factors might contribute to proper production of thyroid hormones, including:
- adequate intake of iron, tyrosine, selenium, zinc, and vitamins E, B2, B3, B6, C, and D44,45
- selenium and zinc, which increase conversion of T4 to T3 and might be important in the management of hypothyroid patients40,46
- vitamin A, zinc, and regular exercise, which have been shown to improve cellular sensitivity to thyroid hormones.
Low iron stores can contribute to persistent symptoms and poor quality of life in patients with hypothyroidism, despite their being treated according to guidelines.29,47
Despite what is known about these nutritional connections, there is insufficient evidence that improving nutrition can reverse hypothyroidism.
Prevention
Prevention of hypothyroidism should take into account variables that affect or inhibit thyroid function, such as stress, infection (eg, Epstein-Barr virus), excessive fluoride intake, toxins (eg, pesticides, solvents, mercury, cadmium, and lead), autoimmune disease (eg, celiac disease), and food sensitivity.54,55 Oxidative stress can also cause thyroid impairment.40-48,54-58
Otherwise, there are, at present, no effective strategies for preventing thyroid disorders.
Subclinical hypothyroidism: Elusive management target
Subclinical hypothyroidism is defined as a normal serum FT4 level in the presence of an elevated serum TSH level. The prevalence of subclinical hypothyroidism varies from 3% to 15%, depending on the population studied; a higher incidence has been noted in women and older people.59 In the NHANES III,1 which excluded people with previously diagnosed thyroid disease, the incidence of subclinical hypothyroidism was 4.3%.
Continue to: Causes of subclinical...
Causes of subclinical hypothyroidism are the same as those of overt hypothyroidism, and include Hashimoto disease. The combination of an elevated TSH level and a normal FT4 level is associated with disorders characterized by protein-binding variations (eg, pregnancy, genetic disorders, drugs), TSH-secreting pituitary adenoma, class II and III obesity (respectively, body mass index, ≥ 35 but < 40 and ≥ 40), and assay variability.49,51
Lab diagnosis: Fraught with difficulty
The serum TSH level and either the total T4 level or the FT4 level should be measured to make a diagnosis of subclinical hypothyroidism. Most laboratories use a 1-step analogue immunoassay to determine free thyroid hormones; protein-binding variations can thus affect measurement of FT4.
Several scenarios that can result in inaccurate measurement of FT4 by radioimmunoassay include genetic disorders that affect binding proteins; pregnancy; use of certain drugs, including heparin, furosemide, antiepileptic agents, salicylate, ferrous sulfate, and cholesterol-binding resins; and some medical conditions, including cardiac surgery, critical illness, and renal failure. Variables that inhibit proper production of thyroid hormones—stress, infection, fluoride (an iodine antagonist), toxins (pesticides, mercury, cadmium, lead) and autoimmune conditions, such as celiac disease—should be considered when attempting to determine the cause of subclinical hypothyroidism.
Liquid chromatography–mass spectrometry measurement of thyroid hormones might be more accurate than immunoassay.53 Measuring serum total T4 and FT4 by dialysis, free from interfering proteins, might also be useful when measurement of FT4 by immunoassay is affected by binding-protein variations.
Features of subclinical hypothyroidism
Most patients who have subclinical hypothyroidism and a serum TSH level < 10 mIU/L are asymptomatic. Some might have nonspecific symptoms of hypothyroidism, however, such as reduced quality of life, poor cognitive function, and poor memory—symptoms that do not typically correlate with the serum TSH level.
It has been suggested that some elderly people normally have a higher level of serum TSH, and that they might have even a prolonged lifespan.51 Additionally, it has been shown that, in nonpregnant adult patients with subclinical hypothyroidism and a serum TSH level of 4.5 to 10 mIU/L, treatment with LT4 was not associated with improvement in thyroid-related symptoms or general quality of life.52
Treat, or don't treat, subclinical hypothyroidism?
It is well accepted that the goal of therapy in hypothyroid patients is to normalize the serum TSH level; however, the American Thyroid Association and the American Association of Clinical Endocrinology recommend starting LT4 in patients with a serum TSH level ≥ 10 mIU/L (TABLE 5).59-62 The principal reason for not treating subclinical hypothyroidism is the lack of benefit in reducing the risk of cardiovascular morbidity and mortality when the TSH level is between 7.5 and 10 mIU/L.62
Continue to: Routine treatment
Routine treatment of patients with a serum TSH level of 4.5 to 10 mIU/L remains controversial. When TSH is 7.0 to 9.9 mIU/L, treatment is recommended for (1) patients < 65 years and (2) for older patients (> 65 years) only when there are convincing hypothyroid symptoms because of concern about unintended overtreatment.
When the TSH level is anywhere above the upper limit of normal to 6.9 mIU/L, treatment is recommended for patients < 65 years old, patients who have a high titer of thyroid peroxidase antibodies, and patients with goiter—but not for patients > 65 years (and, especially, not for octogenarians) because their upper limit of normal could be as high as 6 to 8 mIU/L, especially if they are otherwise healthy.
Treatment should be considered for women with subclinical hypothyroidism who are trying to conceive or experiencing an infertility problem.
For patients with subclinical hypothyroidism who are not being treated, monitor thyroid function every 6 to 12 months by testing TSH and FT4.
CORRESPONDENCE
Thanh D. Hoang, DO, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
1. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499. doi: 10.1210/jcem.87.2.8182
2. Vanderpump MPJ. The epidemiology of thyroid disease. Br Med Bull. 2011;99:39-51. doi: 10.1093/bmb/ldr030
3. Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526-534. doi: 10.1001/archinte.160.4.526
4. Persani L. Central hypothyroidism: pathogenic, diagnostic, and therapeutic challenges. J Clin Endocrinol Metab. 2012;97:3068-3078. doi: 10.1210/jc.2012-1616
5. Almandoz JP, Gharib H. Hypothyroidism: etiology, diagnosis, and management. Med Clin North Am. 2012;96:203-221. doi: 10.1016/j.mcna.2012.01.005
6. Ai J, Leonhardt JM, Heymann WR. Autoimmune thyroid diseases: etiology, pathogenesis and dermatologic manifestations. J Am Acad Dermatol. 2003;48:641-659. doi: 10.1067/mjd.2003.257
7. Franzotti AM, Avelar JCD, Cardoso TA, et al. Pityriasis rubra pilar and hypothyroidism. An Bras Dermatol. 2014;89:497-500. doi: 10.1590/abd1806-4841.20142994
8. Yaylali O, Kirac S, Yilmaz M, et al. Does hypothyroidism affect gastrointestinal motility? Gastroenterol Res Pract. 2009;2009:529802. doi: 10.1155/2009/529802
9. Patil AD. Link between hypothyroidism and small intestinal bacterial overgrowth. Indian J Endocrinol Metab. 2014;18:307-309.
10. Ono Y, Ono S, Yasunaga H, et al. Clinical characteristics and outcomes of myxedema coma: analysis of a national inpatient database in Japan. J Epidemiol. 2017;27:117-122. doi: 10.1016/j.je.2016.04.002
11. Boomsma MJ, Bijl HP, Langendijk JA. Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol. 2011;99:1-5. doi: 10.1016/j.radonc.2011.03.002
12. Boelaert K, Newby PR, Simmonds MJ, et al. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med. 2010;123:183.e1-e9. doi: 10.1016/j.amjmed.2009.06.030
13. Cheserek MJ, Wu G-R, Ntazinda A, et al. Association between thyroid hormones, lipids and oxidative stress markers in subclinical hypothyroidism. J Med Biochem. 2015;34:323-331. doi: 10.2478/jomb-2014-0044
14. Zha K, Zuo C, Wang A, et al. LDL in patients with subclinical hypothyroidism shows increased lipid peroxidation. Lipids Health Dis. 2015;14:95. doi: 10.1186/s12944-015-0092-4
15. Tejovathi B, Suchitra MM, Suresh V, et al. Association of lipid oxidation with endothelial dysfunction in patients with overt hypothyroidism. Exp Clin Endocrinol Diabetes. 2013;121:306-309. doi: 10.1055/s-0032-1333298
16. LeFevre ML; U.S. Preventive Services Task Force. Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162:641-650. doi: 10.7326/M15-0483
17. Chaker L, Bianco AC, Jonklaas J, et al. Hypothyroidism. Lancet. 2017;390:1550-1562. doi: 10.1016/S0140-6736(17)30703-1
18. Vaidya B, Pearce SHS. Management of hypothyroidism in adults. BMJ. 2008;337:a801. doi: 10.1136/bmj.a801
19. Iyer PC, Cabanillas ME, Waguespack SG, et al. Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid. 2018;28:1243-1251. doi: 10.1089/thy.2018.0116
20. Garber JR, Cobin RH, Gharib H, et al; American Association Of Clinical Endocrinologists And American Thyroid Association Taskforce On Hypothyroidism In Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22:1200-1235. doi: 10.1089/thy.2012.0205
21. Maiden MJ, Torpy DJ. Thyroid hormones in critical illness. Crit Care Clin. 2019;35:375-388. doi: 10.1016/j.ccc.2018.11.012
22. Peeters RP. Subclinical hypothyroidism. N Engl J Med. 2017;376:2556-2565. doi: 10.1056/NEJMcp1611144
23. Benvenga S, Carlé A. Levothyroxine formulations: pharmacological and clinical implications of generic substitution. Adv Ther. 2019;36(suppl 2):59-71. doi: 10.1007/s12325-019-01079-1
24. Jonklaas J, Bianco AC, Bauer AJ, et al; . Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid. 2014;24:1670-1751. doi: 10.1089/thy.2014.0028
25. Engler D, Burger AG. The deiodination of the iodothyronines and of their derivatives in man. Endocr Rev. 1984;5:151-184. doi: 10.1210/edrv-5-2-151
26. Ettleson MD, Bianco AC. Individualized therapy for hypothyroidism: is T4 enough for everyone? J Clin Endocrinol Metab. 2020;105:e3090-e3104. doi: 10.1210/clinem/dgaa430
27. Slayden TA, Shakir MKM, Hoang TD. A bull in a pill shop: alpha-gal allergy complicating treatment options for postprocedural hypothyroidism. AACE Clin Case Rep. 2020;6:e101-e104. doi: 10.4158/ACCR-2019-0495
28. Chamorro-Pareja N, Carrillo-Martin I, Haehn DA, et al. Self-reported allergy to thyroid replacement therapy: a multicenter retrospective chart review. Endocr Pract. 2020;26:761-767. doi: 10.4158/EP-2019-0488
29. Shakir MKM, Turton D, Aprill BS, et al. Anemia: a cause of intolerance to thyroxine sodium. Mayo Clin Proc. 2000;75:189-192.
30. Jonklaas J, Bianco AC, Cappola AR, et al. Evidence-based use of levothyroxine/liothyronine combinations in treating hypothyroidism: a consensus document. Thyroid. 2021;31:156-182. doi: 10.1089/thy.2020.0720
31. Appelhof BC, Fliers E, Wekking EM, et al. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. J Clin Endocrinol Metab. 2005;90:2666-2674. doi: 10.1210/jc.2004-2111
32. Escobar-Morreale HF, Botella-Carretero JI, M, et al. Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing L-thyroxine plus liothyronine with L-thyroxine alone. Ann Intern Med. 2005;142:412-424. doi: 10.7326/0003-4819-142-6-200503150-00007
33. Hoang TD, Olsen CH, Mai VQ, et al. Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab. 2013;98:1982-1990. doi: 10.1210/jc.2012-4107
34. Shakir MKM, Brooks DI, McAninch EA, et al. Comparative effectiveness of levothyroxine, desiccated thyroid extract, and levothyroxine+liothyronine in hypothyroidism. J Clin Endocrinol Metab. 2021;106:e4400-e4413. doi: 10.1210/clinem/dgab478
35. Valizadeh M, Seyyed-Majidi MR, Hajibeigloo H, et al. Efficacy of combined levothyroxine and liothyronine as compared with levothyroxine monotherapy in primary hypothyroidism: a randomized controlled trial. Endocr Res. 2009;34:80-89. doi: 10.1080/07435800903156340
36. Walsh JP, Shiels L, Lim EM, et al. Combined thyroxine/liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. J Clin Endocrinol Metab. 2003;88:4543-4550. doi: 10.1210/jc.2003-030249
37. Rodriguez T, Lavis VR, Meininger JC, et al. Substitution of liothyronine at a 1:5 ratio for a portion of levothyroxine: effect on fatigue, symptoms of depression, and working memory versus treatment with levothyroxine alone. Endocr Pract. 2005;11:223-233. doi: 10.4158/EP.11.4.223
38. Sawka AM, Gerstein HC, Marriott MJ, et al. Does a combination regimen of thyroxine (T4) and 3,5,3’-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? Results of a double-blind, randomized, controlled trial. J Clin Endocrinol Metab. 2003;88:4551-4555. doi: 10.1210/jc.2003-030139
39. Clyde PW, Harari AE, Getka EJ, et al. Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism: a randomized controlled trial. JAMA. 2003;290:2952-2958. doi: 10.1001/jama.290.22.2952
40. Duntas LH. Selenium and the thyroid: a close-knit connection. J Clin Endocrinol Metab. 2010;95:5180-5188. doi: 10.1210/jc.2010-0191
41. Winther KH, Wichman JEM, Bonnema SJ, et al. Insufficient documentation for clinical efficacy of selenium supplementation in chronic autoimmune thyroiditis, based on a systematic review and meta-analysis. Endocrine. 2017;55:376-385. doi: 10.1007/s12020-016-1098-z
42. Parva NR, Tadepalli S, Singh P, et al. Prevalence of vitamin D deficiency and associated risk factors in the US population (2011-2012). Cureus. 2018;10:e2741. doi: 10.7759/cureus.2741
43. Wang J, Lv S, Chen G, et al. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients. 2015,7:2485-2498. doi: 10.3390/nu7042485
44. Wilson MM, Reedy J, Krebs-Smith SM. American diet quality: where it is, where it is heading, and what it could be. J Acad Nutr Diet. 2016;116:302-310.e1. doi: 10.1016/j.jand.2015.09.020
45. Babiker A, Alawi A, Al Atawi M, et al. The role of micronutrients in thyroid dysfunction. Sudan J Paediatr. 2020;20:13-19. doi: 10.24911/SJP.106-1587138942
46. Knezevic J, Starchl C, Tmava Berisha A, et al. Thyroid-gut-axis: How does the microbiota influence thyroid function? Nutrients. 2020;12:1769. doi: 10.3390/nu12061769
47. Rayman MP. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc Nutr Soc. 2019;78:34-44. doi: 10.1017/S0029665118001192
48. Chakrabarti SK, Ghosh S, Banerjee S, et al. Oxidative stress in hypothyroid patients and the role of antioxidant supplementation. Indian J Endocrinol Metab. 2016;20:674-678. doi: 10.4103/2230-8210.190555
49. Tseng F-Y, Lin W-Y, Lin C-C, et al. Subclinical hypothyroidism is associated with increased risk for all-cause and cardiovascular mortality in adults. J Am Coll Cardiol. 2012;60:730-737. doi: 10.1016/j.jacc.2012.03.047
50. Roberts LM, Pattison H, Roalfe A, et al. Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Intern Med. 2006;145:573-581. doi: 10.7326/0003-4819-145-8-200610170-00006
51. Gussekloo J, van Exel E, de Craen AJM, et al. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292:2591-2599. doi: 10.1001/jama.292.21.2591
52. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA. 2018;320:1349-1359. doi: 10.1001/jama.2018.13770
53. Monzani F, Dardano A, Caraccio N. Does treating subclinical hypothyroidism improve markers of cardiovascular risk? Treat Endocrinol. 2006;5:65-81. doi: 10.2165/00024677-200605020-00001
54. Duntas LH. Does celiac disease trigger autoimmune thyroiditis? Nat Rev Endocrinol. 2009;5:190-191. doi: 10.1038/nrendo.2009.46
55. Lerner A, Jeremias P, Matthias T. Gut-thyroid axis and celiac disease. Endocr Connect. 2017;6:R52-R58. doi: 10.1530/EC-17-0021
56. Janegova A, Janega P, Rychly B, et al. The role of Epstein-Barr virus infection in the development of autoimmune thyroid diseases. Endokrynol Pol. 2015;66:132-136. doi: 10.5603/EP.2015.0020
57. Brent GA. Environmental exposures and autoimmune thyroid disease. Thyroid. 2010;20:755-761. doi: 10.1089/thy.2010.1636
58. Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44-84. doi: 10.1016/j.biocel.2006.07.001
59. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028. doi: 10.4158/EP12280.GL
60. Welsh KJ, Soldin SJ. Diagnosis of endocrine disease: How reliable are free thyroid and total T3 hormone assays? Eur J Endocrinol. 2016;175:R255-R263. doi: 10.1530/EJE-16-0193
61. Grossman A, Feldhamer I, Meyerovitch J. Treatment with levothyroxin in subclinical hypothyroidism is associated with increased mortality in the elderly. Eur J Intern Med. 2018;50:65-68. doi: 10.1016/j.ejim.2017.11.010
62. Pearce SHS, Brabant G, Duntas LH, et al. 2013 ETA Guideline: management of subclinical hypothyroidism. Eur Thyroid J. 2013;2:215-228. doi: 10.1159/000356507
1. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499. doi: 10.1210/jcem.87.2.8182
2. Vanderpump MPJ. The epidemiology of thyroid disease. Br Med Bull. 2011;99:39-51. doi: 10.1093/bmb/ldr030
3. Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526-534. doi: 10.1001/archinte.160.4.526
4. Persani L. Central hypothyroidism: pathogenic, diagnostic, and therapeutic challenges. J Clin Endocrinol Metab. 2012;97:3068-3078. doi: 10.1210/jc.2012-1616
5. Almandoz JP, Gharib H. Hypothyroidism: etiology, diagnosis, and management. Med Clin North Am. 2012;96:203-221. doi: 10.1016/j.mcna.2012.01.005
6. Ai J, Leonhardt JM, Heymann WR. Autoimmune thyroid diseases: etiology, pathogenesis and dermatologic manifestations. J Am Acad Dermatol. 2003;48:641-659. doi: 10.1067/mjd.2003.257
7. Franzotti AM, Avelar JCD, Cardoso TA, et al. Pityriasis rubra pilar and hypothyroidism. An Bras Dermatol. 2014;89:497-500. doi: 10.1590/abd1806-4841.20142994
8. Yaylali O, Kirac S, Yilmaz M, et al. Does hypothyroidism affect gastrointestinal motility? Gastroenterol Res Pract. 2009;2009:529802. doi: 10.1155/2009/529802
9. Patil AD. Link between hypothyroidism and small intestinal bacterial overgrowth. Indian J Endocrinol Metab. 2014;18:307-309.
10. Ono Y, Ono S, Yasunaga H, et al. Clinical characteristics and outcomes of myxedema coma: analysis of a national inpatient database in Japan. J Epidemiol. 2017;27:117-122. doi: 10.1016/j.je.2016.04.002
11. Boomsma MJ, Bijl HP, Langendijk JA. Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol. 2011;99:1-5. doi: 10.1016/j.radonc.2011.03.002
12. Boelaert K, Newby PR, Simmonds MJ, et al. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med. 2010;123:183.e1-e9. doi: 10.1016/j.amjmed.2009.06.030
13. Cheserek MJ, Wu G-R, Ntazinda A, et al. Association between thyroid hormones, lipids and oxidative stress markers in subclinical hypothyroidism. J Med Biochem. 2015;34:323-331. doi: 10.2478/jomb-2014-0044
14. Zha K, Zuo C, Wang A, et al. LDL in patients with subclinical hypothyroidism shows increased lipid peroxidation. Lipids Health Dis. 2015;14:95. doi: 10.1186/s12944-015-0092-4
15. Tejovathi B, Suchitra MM, Suresh V, et al. Association of lipid oxidation with endothelial dysfunction in patients with overt hypothyroidism. Exp Clin Endocrinol Diabetes. 2013;121:306-309. doi: 10.1055/s-0032-1333298
16. LeFevre ML; U.S. Preventive Services Task Force. Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162:641-650. doi: 10.7326/M15-0483
17. Chaker L, Bianco AC, Jonklaas J, et al. Hypothyroidism. Lancet. 2017;390:1550-1562. doi: 10.1016/S0140-6736(17)30703-1
18. Vaidya B, Pearce SHS. Management of hypothyroidism in adults. BMJ. 2008;337:a801. doi: 10.1136/bmj.a801
19. Iyer PC, Cabanillas ME, Waguespack SG, et al. Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid. 2018;28:1243-1251. doi: 10.1089/thy.2018.0116
20. Garber JR, Cobin RH, Gharib H, et al; American Association Of Clinical Endocrinologists And American Thyroid Association Taskforce On Hypothyroidism In Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22:1200-1235. doi: 10.1089/thy.2012.0205
21. Maiden MJ, Torpy DJ. Thyroid hormones in critical illness. Crit Care Clin. 2019;35:375-388. doi: 10.1016/j.ccc.2018.11.012
22. Peeters RP. Subclinical hypothyroidism. N Engl J Med. 2017;376:2556-2565. doi: 10.1056/NEJMcp1611144
23. Benvenga S, Carlé A. Levothyroxine formulations: pharmacological and clinical implications of generic substitution. Adv Ther. 2019;36(suppl 2):59-71. doi: 10.1007/s12325-019-01079-1
24. Jonklaas J, Bianco AC, Bauer AJ, et al; . Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid. 2014;24:1670-1751. doi: 10.1089/thy.2014.0028
25. Engler D, Burger AG. The deiodination of the iodothyronines and of their derivatives in man. Endocr Rev. 1984;5:151-184. doi: 10.1210/edrv-5-2-151
26. Ettleson MD, Bianco AC. Individualized therapy for hypothyroidism: is T4 enough for everyone? J Clin Endocrinol Metab. 2020;105:e3090-e3104. doi: 10.1210/clinem/dgaa430
27. Slayden TA, Shakir MKM, Hoang TD. A bull in a pill shop: alpha-gal allergy complicating treatment options for postprocedural hypothyroidism. AACE Clin Case Rep. 2020;6:e101-e104. doi: 10.4158/ACCR-2019-0495
28. Chamorro-Pareja N, Carrillo-Martin I, Haehn DA, et al. Self-reported allergy to thyroid replacement therapy: a multicenter retrospective chart review. Endocr Pract. 2020;26:761-767. doi: 10.4158/EP-2019-0488
29. Shakir MKM, Turton D, Aprill BS, et al. Anemia: a cause of intolerance to thyroxine sodium. Mayo Clin Proc. 2000;75:189-192.
30. Jonklaas J, Bianco AC, Cappola AR, et al. Evidence-based use of levothyroxine/liothyronine combinations in treating hypothyroidism: a consensus document. Thyroid. 2021;31:156-182. doi: 10.1089/thy.2020.0720
31. Appelhof BC, Fliers E, Wekking EM, et al. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. J Clin Endocrinol Metab. 2005;90:2666-2674. doi: 10.1210/jc.2004-2111
32. Escobar-Morreale HF, Botella-Carretero JI, M, et al. Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing L-thyroxine plus liothyronine with L-thyroxine alone. Ann Intern Med. 2005;142:412-424. doi: 10.7326/0003-4819-142-6-200503150-00007
33. Hoang TD, Olsen CH, Mai VQ, et al. Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab. 2013;98:1982-1990. doi: 10.1210/jc.2012-4107
34. Shakir MKM, Brooks DI, McAninch EA, et al. Comparative effectiveness of levothyroxine, desiccated thyroid extract, and levothyroxine+liothyronine in hypothyroidism. J Clin Endocrinol Metab. 2021;106:e4400-e4413. doi: 10.1210/clinem/dgab478
35. Valizadeh M, Seyyed-Majidi MR, Hajibeigloo H, et al. Efficacy of combined levothyroxine and liothyronine as compared with levothyroxine monotherapy in primary hypothyroidism: a randomized controlled trial. Endocr Res. 2009;34:80-89. doi: 10.1080/07435800903156340
36. Walsh JP, Shiels L, Lim EM, et al. Combined thyroxine/liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. J Clin Endocrinol Metab. 2003;88:4543-4550. doi: 10.1210/jc.2003-030249
37. Rodriguez T, Lavis VR, Meininger JC, et al. Substitution of liothyronine at a 1:5 ratio for a portion of levothyroxine: effect on fatigue, symptoms of depression, and working memory versus treatment with levothyroxine alone. Endocr Pract. 2005;11:223-233. doi: 10.4158/EP.11.4.223
38. Sawka AM, Gerstein HC, Marriott MJ, et al. Does a combination regimen of thyroxine (T4) and 3,5,3’-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? Results of a double-blind, randomized, controlled trial. J Clin Endocrinol Metab. 2003;88:4551-4555. doi: 10.1210/jc.2003-030139
39. Clyde PW, Harari AE, Getka EJ, et al. Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism: a randomized controlled trial. JAMA. 2003;290:2952-2958. doi: 10.1001/jama.290.22.2952
40. Duntas LH. Selenium and the thyroid: a close-knit connection. J Clin Endocrinol Metab. 2010;95:5180-5188. doi: 10.1210/jc.2010-0191
41. Winther KH, Wichman JEM, Bonnema SJ, et al. Insufficient documentation for clinical efficacy of selenium supplementation in chronic autoimmune thyroiditis, based on a systematic review and meta-analysis. Endocrine. 2017;55:376-385. doi: 10.1007/s12020-016-1098-z
42. Parva NR, Tadepalli S, Singh P, et al. Prevalence of vitamin D deficiency and associated risk factors in the US population (2011-2012). Cureus. 2018;10:e2741. doi: 10.7759/cureus.2741
43. Wang J, Lv S, Chen G, et al. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients. 2015,7:2485-2498. doi: 10.3390/nu7042485
44. Wilson MM, Reedy J, Krebs-Smith SM. American diet quality: where it is, where it is heading, and what it could be. J Acad Nutr Diet. 2016;116:302-310.e1. doi: 10.1016/j.jand.2015.09.020
45. Babiker A, Alawi A, Al Atawi M, et al. The role of micronutrients in thyroid dysfunction. Sudan J Paediatr. 2020;20:13-19. doi: 10.24911/SJP.106-1587138942
46. Knezevic J, Starchl C, Tmava Berisha A, et al. Thyroid-gut-axis: How does the microbiota influence thyroid function? Nutrients. 2020;12:1769. doi: 10.3390/nu12061769
47. Rayman MP. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc Nutr Soc. 2019;78:34-44. doi: 10.1017/S0029665118001192
48. Chakrabarti SK, Ghosh S, Banerjee S, et al. Oxidative stress in hypothyroid patients and the role of antioxidant supplementation. Indian J Endocrinol Metab. 2016;20:674-678. doi: 10.4103/2230-8210.190555
49. Tseng F-Y, Lin W-Y, Lin C-C, et al. Subclinical hypothyroidism is associated with increased risk for all-cause and cardiovascular mortality in adults. J Am Coll Cardiol. 2012;60:730-737. doi: 10.1016/j.jacc.2012.03.047
50. Roberts LM, Pattison H, Roalfe A, et al. Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Intern Med. 2006;145:573-581. doi: 10.7326/0003-4819-145-8-200610170-00006
51. Gussekloo J, van Exel E, de Craen AJM, et al. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292:2591-2599. doi: 10.1001/jama.292.21.2591
52. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA. 2018;320:1349-1359. doi: 10.1001/jama.2018.13770
53. Monzani F, Dardano A, Caraccio N. Does treating subclinical hypothyroidism improve markers of cardiovascular risk? Treat Endocrinol. 2006;5:65-81. doi: 10.2165/00024677-200605020-00001
54. Duntas LH. Does celiac disease trigger autoimmune thyroiditis? Nat Rev Endocrinol. 2009;5:190-191. doi: 10.1038/nrendo.2009.46
55. Lerner A, Jeremias P, Matthias T. Gut-thyroid axis and celiac disease. Endocr Connect. 2017;6:R52-R58. doi: 10.1530/EC-17-0021
56. Janegova A, Janega P, Rychly B, et al. The role of Epstein-Barr virus infection in the development of autoimmune thyroid diseases. Endokrynol Pol. 2015;66:132-136. doi: 10.5603/EP.2015.0020
57. Brent GA. Environmental exposures and autoimmune thyroid disease. Thyroid. 2010;20:755-761. doi: 10.1089/thy.2010.1636
58. Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44-84. doi: 10.1016/j.biocel.2006.07.001
59. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028. doi: 10.4158/EP12280.GL
60. Welsh KJ, Soldin SJ. Diagnosis of endocrine disease: How reliable are free thyroid and total T3 hormone assays? Eur J Endocrinol. 2016;175:R255-R263. doi: 10.1530/EJE-16-0193
61. Grossman A, Feldhamer I, Meyerovitch J. Treatment with levothyroxin in subclinical hypothyroidism is associated with increased mortality in the elderly. Eur J Intern Med. 2018;50:65-68. doi: 10.1016/j.ejim.2017.11.010
62. Pearce SHS, Brabant G, Duntas LH, et al. 2013 ETA Guideline: management of subclinical hypothyroidism. Eur Thyroid J. 2013;2:215-228. doi: 10.1159/000356507
PRACTICE RECOMMENDATIONS
› Prescribe levothyroxine (LT4) to maintain thyroid-stimulating hormone (TSH) at 4 to 7 mIU/L in select patients with primary hypothyroidism for whom that range of the serum TSH level can be considered appropriate (ie, those older than 65 years and those who have underlying coronary artery disease or another debilitating chronic disorder). A
› Counsel all women of childbearing age with primary hypothyroidism that they need to have their dosage of LT4 increased as soon as pregnancy is suspected. A
› Keep in mind that treating hypothyroidism is not always necessary in older patients who have subclinical disease and a serum TSH level < 10 mIU/L. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Dyspareunia: Keys to biopsychosocial evaluation and treatment planning
Dyspareunia is persistent or recurrent pain before, during, or after sexual contact and is not limited to cisgender individuals or vaginal intercourse.1-3 With a prevalence as high as 45% in the United States,2-5 it is one of the most common complaints in gynecologic practices.5,6
Causes and contributing factors
There are many possible causes of dyspareunia.2,4,6 While some patients have a single cause, most cases are complex, with multiple overlapping causes and maintaining factors.4,6 Identifying each contributing factor can help you appropriately address all components.
Physical conditions. The range of physical contributors to dyspareunia includes inflammatory processes, structural abnormalities, musculoskeletal dysfunctions, pelvic organ disorders, injuries, iatrogenic effects, infections, allergic reactions, sensitization, hormonal changes, medication effects, adhesions, autoimmune disorders, and other pain syndromes (TABLE 12-4,6-11).

Inadequate arousal. One of the primary causes of pain during vaginal penetration is inadequate arousal and lubrication.1,2,9-11 Arousal is the phase of the sexual response cycle that leads to genital tumescence and prepares the genitals for sexual contact through penile/clitoral erection, vaginal engorgement, and lubrication, which prevents pain and enhances pleasurable sensation.9-11
While some physical conditions can lead to an inability to lubricate, the most common causes of inadequate lubrication are psychosocial-behavioral, wherein patients have the same physical ability to lubricate as patients without genital pain but do not progress through the arousal phase.9-11 Behavioral factors such as inadequate or ineffective foreplay can fail to produce engorgement and lubrication, while psychosocial factors such as low attraction to partner, relationship stressors, anxiety, or low self-esteem can have an inhibitory effect on sexual arousal.1,2,9-11 Psychosocial and behavioral factors may also be maintaining factors or consequences of dyspareunia, and need to be assessed and treated.1,2,9-11
Psychological trauma. Exposure to psychological traumas and the development of posttraumatic stress disorder (PTSD) have been linked with the development of pain disorders in general and dyspareunia specifically. Most patients seeking treatment for chronic pain disorders have a history of physical or sexual abuse.12 Changes in physiologic processes (eg, neurochemical, endocrine) that occur with PTSD interfere with the sexual response cycle, and sexual traumas specifically have been linked with pelvic floor dysfunction.13,14 Additionally, when PTSD is caused by a sexual trauma, even consensual sexual encounters can trigger flashbacks, intrusive memories, hyperarousal, and muscle tension that interfere with the sexual response cycle and contribute to genital pain.13
Vaginismus is both a physiologic and psychological contributor to dyspareunia.1,2,4 Patients experiencing pain can develop anxiety about repeated pain and involuntarily contract their pelvic muscles, thereby creating more pain, increasing anxiety, decreasing lubrication, and causing pelvic floor dysfunction.1-4,6 Consequently, all patients with dyspareunia should be assessed and continually monitored for symptoms of vaginismus.
Continue to: Anxiety
Anxiety. As with other pain disorders, anxiety develops around pain triggers.10,15 When expecting sexual activity, patients can experience extreme worry and panic attacks.10,15,16 The distress of sexual encounters can interfere with physiologic arousal and sexual desire, impacting all phases of the sexual response cycle.1,2
Relationship issues. Difficulty engaging in or avoidance of sexual activity can interfere with romantic relationships.2,10,16 Severe pain or vaginismus contractions can prevent penetration, leading to unconsummated marriages and an inability to conceive through intercourse.10 The distress surrounding sexual encounters can precipitate erectile dysfunction in male partners, or partners may continue to demand sexual encounters despite the patient’s pain, further impacting the relationship and heightening sexual distress.10 These stressors have led to relationships ending, patients reluctantly agreeing to nonmonogamy to appease their partners, and patients avoiding relationships altogether.10,16
Devalued self-image. Difficulties with sexuality and relationships impact the self-image of patients with dyspareunia. Diminished self-image may include feeling “inadequate” as a woman and as a sexual partner, or feeling like a “failure.”16 Women with dyspareunia often have more distress related to their body image, physical appearance, and genital self-image than do women without genital pain.17 Feeling resentment toward their body, or feeling “ugly,” embarrassed, shamed, “broken,” and “useless” also contribute to increased depressive symptoms found in patients with dyspareunia.16,18
Making the diagnosis
Most patients do not report symptoms unless directly asked2,7; therefore, it is recommended that all patients be screened as a part of an initial intake and before any genital exam (TABLE 22-4,6,7,9,11,19,20).4,7,21 If this screen is positive, a separate appointment may be needed for a thorough evaluation and before any attempt is made at a genital exam.4,7
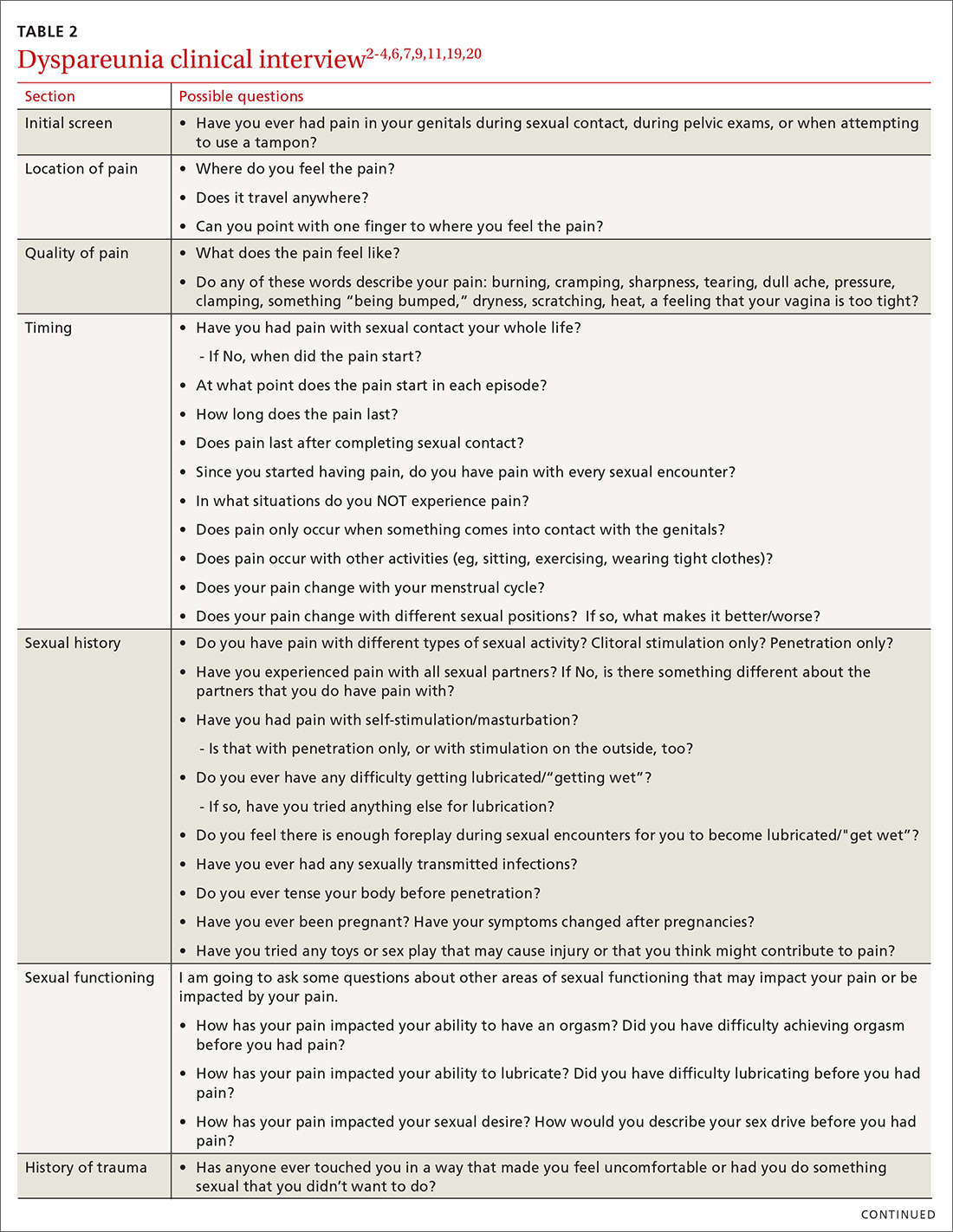
Items to include in the clinical interview
Given the range of possible causes of dyspareunia and its contributing factors and symptoms, a thorough clinical interview is essential. Begin with a review of the patient’s complete medical and surgical history to identify possible known contributors to genital pain.4 Pregnancy history is of particular importance as the prevalence of postpartum dyspareunia is 35%, with risk being greater for patients who experienced dyspareunia symptoms before pregnancy.22

Knowing the location and quality of pain is important for differentiating between possible diagnoses, as is specifying dyspareunia as lifelong or acquired, superficial or deep, and primary or secondary.1-4,6 Confirm the specific location(s) of pain—eg, at the introitus, in the vestibule, on the labia, in the perineum, or near the clitoris.2,4,6 A diagram or model may be needed to help patients to localize pain.4
To help narrow the differential, include the following elements in your assessment: pain quality, timing (eg, initial onset, episode onset, episode duration, situational triggers), alleviating factors, symptoms in surrounding structures (eg, bladder, bowel, muscles, bones), sexual history, other areas of sexual functioning, history of psychological trauma, relationship effects, and mental health (TABLE 22-4,6,7,9,11,19,20 and Table 323-28). Screening for a history of sexual trauma is particularly important, as a recent systematic review and meta-analysis found that women with a history of sexual assault had a 42% higher risk of gynecologic problems overall, a 74% higher risk of dyspareunia, and a 71% higher risk of vaginismus than women without a history of sexual assault.29 Using measures such as the Female Sexual Function Index or the McGill Pain Questionnaire can help patients more thoroughly describe their symptoms (TABLE 323-28).3
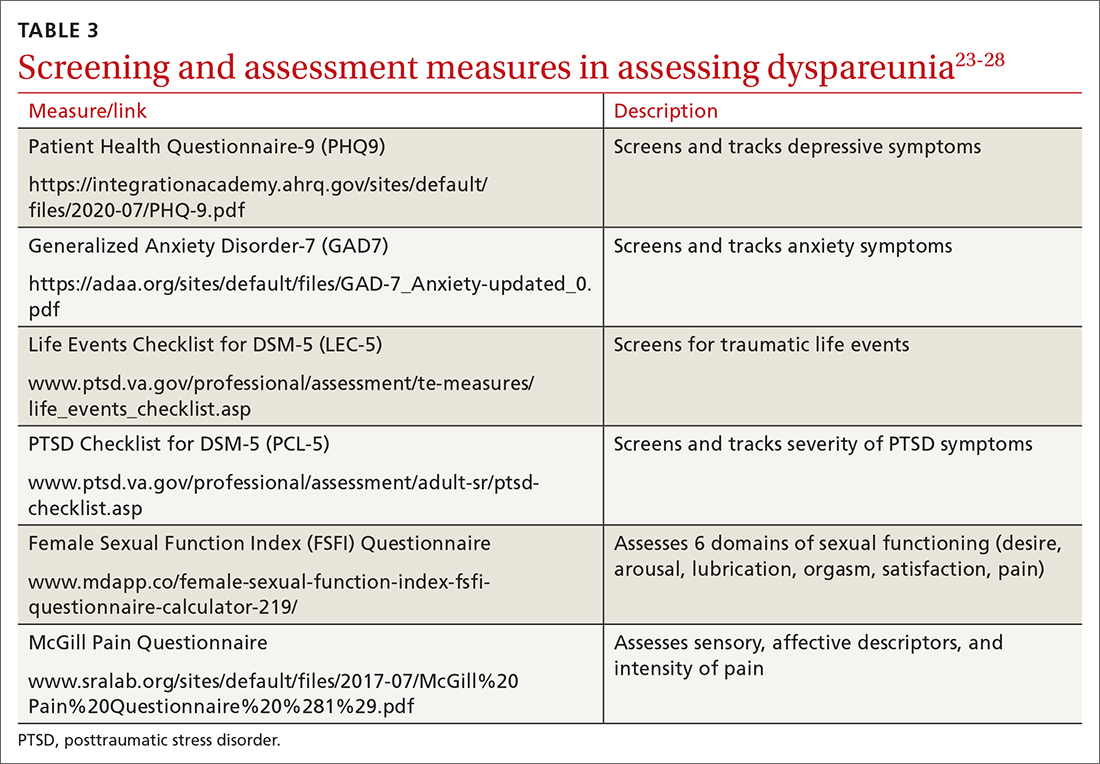
Continue to: Guidelines for the physical exam
Guidelines for the physical exam
Before the exam, ensure the patient has not used any topical genital treatment in the past 2 weeks that may interfere with sensitivity to the exam.4 To decrease patients’ anxiety about the exam, remind them that they can stop the exam at any time.7 Also consider offering the use of a mirror to better pinpoint the location of pain, and to possibly help the patient learn more about her anatomy.2,7
Begin the exam by palpating surrounding areas that may be involved in pain, including the abdomen and musculoskeletal features.3,6,19 Next visually inspect the external genitalia for lesions, abrasions, discoloration, erythema, or other abnormal findings.2,3,6 Ask the patient for permission before contacting the genitals. Because the labia may be a site of pain, apply gentle pressure in retracting it to fully examine the vestibule.6,7 Contraction of the pelvic floor muscles during approach or initial palpation could signal possible vaginismus.4
After visual inspection of external genitalia, use a cotton swab to map the vulva and vestibule in a clockwise fashion to precisely identify any painful locations.2-4,6 If the patient’s history of pain has been intermittent, it’s possible that the cotton swab will not elicit pain on the day of the initial exam, but it may on other days.4
Begin the internal exam by inserting a single finger into the first inch of the vagina and have the patient squeeze and release to assess tenderness, muscle tightness, and control.2,6 Advance the finger further into the vagina and palpate clockwise, examining the levator muscles, obturator muscles, rectum, urethra, and bladder for abnormal tightness or reproduction of pain.2,4,6 Complete a bimanual exam to evaluate the pelvic organs and adnexa.2,4 If indicated, a more thorough evaluation of pelvic floor musculature can be performed by a physical therapist or gynecologist who specializes in pelvic pain.2-4
If the patient consents to further evaluation, consider using a small speculum, advanced slowly, for further internal examination, noting any lesions, abrasions, discharge, ectropion, or tenderness.2-4,7 A rectal exam may also be needed in cases of deep dyspareunia.6 Initial work-up may include a potassium hydroxide wet prep, sexually transmitted infection testing, and pelvic ultrasound.2,4 In some cases, laparoscopy or biopsy may be needed.2,4
Treatments for common causes
Treatment often begins with education about anatomy, to help patients communicate about symptoms and engage more fully in their care.3 Additional education may be needed on genital functioning and the necessity of adequate stimulation and lubrication prior to penetration.1,2,9-11 A discussion of treatments for the wide range of possible causes of dyspareunia is outside the scope of this article. However, some basic behavioral changes may help patients address some of the more common contributing factors.
For example, if vaginal infection is suspected, advise patients to discontinue the use of harsh soaps, known vaginal irritants (eg, perfumed products, bath additives), and douches.3 Recommend using only preservative- and alcohol-free lubricants for sexual contact, and avoiding lubricants with added functions (eg, warming).3 It’s worth noting that avoidance of tight clothing and thong underwear due to possible risk for infections may not be necessary. A recent study found that women who frequently wore thong underwear (more than half of the time) were no more likely to develop urinary tract infections, yeast vaginitis, or bacterial vaginosis than those who avoid such items.30 However, noncotton underwear fabric, rather than tightness, was associated with yeast vaginitis30; therefore, patients may want to consider using only breathable underwear.3
Continue to: Medication
Medication. Medication may be used to treat the underlying contributing conditions or the symptom of pain directly. Some common options are particularly important for patients whose dyspareunia does not have an identifiable cause. These medications include anti-inflammatory agents, topical anesthetics, tricyclic antidepressants, and hormonal treatments.2-4 Since effectiveness varies based on subtypes of pain, select a medication according to the location, timing, and hypothesized mechanism of pain.3,31,32
Medication for deep pain. A meta-analysis and systematic review found that patients with some types of chronic pelvic pain with pain deep in the vagina or pelvis experienced greater than 50% reduction in pain using medroxyprogesterone acetate compared with placebo.33 Other treatments for deep pain depend on physical exam findings.
Medication for superficial pain. Many remedies have been tried, with at least 26 different treatments for vulvodynia pain alone.16 Only some of these treatments have supporting evidence. For patients with vulvar pain, an intent-to-treat RCT found that patients using a topical steroid experienced a 23% reduction in pain from pre-treatment to 6-month follow-up.32
Surgery is also effective for vulvar pain.34,35 For provoked vestibulodynia (in which pain is localized to the vestibule and triggered by contact with the vulva), or vulvar vestibulitis, RCTs have found that vestibulectomy has stronger effects on pain than other treatments,31,35 with a 53% reduction in pain during intercourse and a 70% reduction in vestibular pain overall.35 However, while vestibulectomy is effective for provoked vestibulodynia, it is not recommended for generalized vulvodynia, in which pain is diffuse across the vulva and occurs without vulvar contact.34
Unsupported treatments. A number of other treatments have not yet been found effective. Although lidocaine for vulvar pain is often used, RCTs have not found any significant reduction in symptoms, and a double-blind RCT found that lidocaine ointment actually performed worse than placebo.31,34 Similarly, oral tricyclics have not been found to decrease vulvar pain more than placebo in double-blind studies.31,34 Furthermore, a meta-analysis of RCTs comparing treatments with placebo for vestibular pain found no significant decrease in dyspareunia for topical conjugated estrogen, topical lidocaine, oral desipramine, oral desipramine with topical lidocaine, laser therapy, or transcranial direct current.32
Tx risks to consider. Risks and benefits of dyspareunia treatment options should be thoroughly weighed and discussed with the patient.2-4 Vestibulectomy, despite reducing pain for many patients, has led to increased pain for 9% of patients who underwent the procedure.35 Topical treatments may lead to allergic reactions, inflammation, and worsening of symptoms,4 and hormonal treatments have been found to increase the risk of weight gain and bloating and are not appropriate for patients trying to conceive.33
Coordinate care with other providers
While medications and surgery can reduce pain, they have not been shown to improve other aspects of sexual functioning such as sexual satisfaction, frequency of sexual intercourse, or overall sense of sexual functioning.35 Additionally, pain reduction does not address muscle tension, anxiety, self-esteem, and relationship problems. As a result, a multidisciplinary approach is generally needed.3,4,32,33
Continue to: Physical therapists
Physical therapists. Pelvic floor physical therapists are often members of the dyspareunia treatment team and can provide a thorough evaluation and treatment of pelvic floor disorders.2-4 An RCT with intent-to-treat analysis found that pain was reduced by 71% following pelvic floor physical therapy.36 Another RCT found that 90% of patients reported a clinically meaningful decrease in pain with pelvic floor physical therapy.37 In addition to addressing pain, pelvic floor physical therapy has also been found to improve sexual functioning, sexual satisfaction, distress, and patient perception of improvement.34,36,37
Behavioral health specialists. Psychotherapists, especially those trained in sex therapy, couples therapy, or cognitive behavioral therapy (CBT), are also typically on the treatment team. Multiple RCTs have found evidence of CBT’s effectiveness in the direct treatment of dyspareunia pain. Bergeron et al35 found a 37.5% reduction in vulvar vestibulitis pain intensity during intercourse after patients completed group CBT. Another intent-to-treat RCT found that patients receiving CBT experienced more pain reduction (~ 30%) than patients who were treated with a topical steroid.38
In addition to having a direct impact on pain, CBT has also been found to have a clinically and statistically significant positive impact on other aspects of sexual experience, such as overall sexuality, self-efficacy, overall sexual functioning, frequency of intercourse, and catastrophizing.34,38 A recent meta-analysis of RCTs found that about 80% of vaginismus patients were able to achieve penetrative intercourse after treatment with behavioral sex therapy or CBT.39 This success rate was not exceeded by physical or surgical treatments.39
When PTSD is thought to be a contributing factor, trauma therapy will likely be needed in addition to treatments for dyspareunia. First-line treatments for PTSD include cognitive processing therapy, prolonged exposure, trauma-focused CBT, and cognitive therapy.40
Psychotherapists can also help patients reduce anxiety, reintroduce sexual contact without triggering pain or anxiety, address emotional and self-esteem effects of dyspareunia, address relationship issues, and refocus sexual encounters on pleasure rather than pain avoidance.2-4 Despite patient reports of high treatment satisfaction following therapy,38 many patients may initially lack confidence in psychotherapy as a treatment for pain35 and may need to be educated on its effectiveness and multidimensional benefits.
Gynecologists. Often a gynecologist with specialization in pelvic pain is an essential member of the team for diagnostic clarification, recommendation of treatment options, and performance of more advanced treatments.2,3 If pain has become chronic, the patient may also benefit from a pain management team and support groups.2,3
Follow-up steps
Patients who screen negative for dyspareunia should be re-screened periodically. Continue to assess patients diagnosed with dyspareunia for vaginismus symptoms (if they are not initially present) to ensure that the treatment plan is appropriately adjusted. Once treatment has begun, ask about adverse effects and confidence in the treatment plan to minimize negative impacts on treatment adherence and to anticipate a need for a change in the treatment approach.31,35 In addition to tracking treatment effects on pain, continue to assess for patient-centered outcomes such as emotional functioning, self-esteem, and sexual and relationship satisfaction.34 The Female Sexual Function Index can be a useful tool to track symptoms.27,34
Finally, patients who do not experience sufficient improvement in symptoms and functioning with initial treatment may need continued support and encouragement. Given the broad range of contributing factors and the high number of potential treatments, patients may find hope in learning that multiple other treatment options may be available.
CORRESPONDENCE
Adrienne A. Williams, PhD, Department of Family and Community Medicine, University of Illinois at Chicago College of Medicine, 1919 W Taylor Street, MC 663, Chicago, IL 60612; [email protected]
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Ed. American Psychiatric Publishing; 2013.
2. Seehusen DA, Baird DC, Bode DV. Dyspareunia in women. Am Fam Phys. 2014;90:465-470.
3. Sorensen J, Bautista KE, Lamvu G, et al. Evaluation and treatment of female sexual pain: a clinical review. Cureus. 2018;10:e2379.
4. MacNeill C. Dyspareunia. Obstet Gynecol Clin North Am. 2006;33:565-77.
5. Latthe P, Latthe M, Say L, et al. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health. 2006;6:177.
6. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009;113:1124-1136.
7. Williams AA, Williams M. A guide to performing pelvic speculum exams: a patient-centered approach to reducing iatrogenic effects. Teach Learn Med. 2013;25:383-391.
8. Ünlü Z, Yentur A, Çakil N. Pudendal nerve neuropathy: An unknown-rare cause of pelvic pain. Arch Rheumatol. 2016;31:102-103.
9. Dewitte M, Borg C, Lowenstein L. A psychosocial approach to female genital pain. Nat Rev Urol. 2018;15:25-41.
10. Masters WH, Johnson VE. Human Sexual Inadequacy. 1st ed. Little, Brown; 1970.
11. Rathus SA, Nevid JS, Fichner-Rathus L. Human Sexuality in a World of Diversity. 5th ed. Allyn and Bacon; 2002.
12. Bailey BE, Freedenfeld RN, Kiser RS, et al. Lifetime physical and sexual abuse in chronic pain patients: psychosocial correlates and treatment outcomes. Disabil Rehabil. 2003;25:331-342.
13. Yehuda R, Lehrner A, Rosenbaum TY. PTSD and sexual dysfunction in men and women. J Sex Med. 2015;12:1107-1119.
14. Postma R, Bicanic I, van der Vaart H, et al. Pelvic floor muscle problems mediate sexual problems in young adult rape victims. J Sex Med. 2013;10:1978-1987.
15. Binik YM, Bergeron S, Khalifé S. Dyspareunia and vaginismus: so-called sexual pain. In: Leiblum SR, ed. 4th ed. Principles and Practice of Sex Therapy. The Guilford Press; 2007:124-156.
16. Ayling K, Ussher JM. “If sex hurts, am I still a woman?” The subjective experience of vulvodynia in hetero-sexual women. Arch Sex Behav. 2008;37:294-304.
17. Pazmany E, Bergeron S, Van Oudenhove L, et al. Body image and genital self-image in pre-menopausal women with dyspareunia. Arch Sex Behav. 2013;42:999-1010.
18. Maillé DL, Bergeron S, Lambert B. Body image in women with primary and secondary provoked vestibulodynia: a controlled study. J Sex Med. 2015;12:505-515.
19. Ryan L, Hawton K. Female dyspareunia. BMJ. 2004;328:1357.
20. Waldura JF, Arora I, Randall AM, et al. Fifty shades of stigma: exploring the health care experiences of kink-oriented patients. J Sex Med. 2016;13:1918-1929.
21. Hinchliff S, Gott M. Seeking medical help for sexual concerns in mid- and later life: a review of the literature. J Sex Res. 2011;48:106-117.
22. Banaei M, Kariman N, Ozgoli G, et al. Prevalence of postpartum dyspareunia: a systematic review and meta-analysis. Int J Gynaecol Obstet. 2021;153:14-24.
23. Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509-515.
24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
25. U.S. Department of Veterans Affairs. PTSD: National Center for PTSD. Life events checklist for DSM-5 (LEC-5). Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp
26. Weathers FW, Litz BT, Keane TM, et al. The PTSD checklist for DSM-5 (PCL-5). 2013. Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp
27. Rosen R, Brown C, Heiman J, et al. The female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191-208.
28. Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191-197.
29. Hassam T, Kelso E, Chowdary P, et al. Sexual assault as a risk factor for gynaecological morbidity: an exploratory systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;255:222-230.
30. Hamlin AA, Sheeder J, Muffly TM. Brief versus thong hygiene in obstetrics and gynecology (B-THONG): a survey study. J Obstet Gynaecol Res. 2019;45:1190-1196.
31. Foster DC, Kotok MB, Huang LS, et al. Oral desipramine and topical lidocaine for vulvodynia: a randomized controlled trial. Obstet Gynecol. 2010;116:583-593.
32. Pérez-López FR, Bueno-Notivol J, Hernandez AV, et al. Systematic review and meta-analysis of the effects of treatment modalities for vestibulodynia in women. Eur J Contracept Reprod Health Care. 2019;24:337-346.
33. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;(3):CD008797.
34. Goldstein AT, Pukall CF, Brown C, et al. Vulvodynia: assessment and treatment. J Sex Med. 2016;13:572-590.
35. Bergeron S, Binik YM, Khalifé S, et al. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91:297-306.
36. Schvartzman R, Schvartzman L, Ferreira CF, et al. Physical therapy intervention for women with dyspareunia: a randomized clinical trial. J Sex Marital Ther. 2019;45:378-394.
37. Morin M, Dumoulin C, Bergeron S, et al. Multimodal physical therapy versus topical lidocaine for provoked vestibulodynia: a multicenter, randomized trial. Am J Obstet Gynecol. 2021;224:189.e1-189.e12.
38. Bergeron S, Khalifé S, Dupuis M-J, et al. A randomized clinical trial comparing group cognitive-behavioral therapy and a topical steroid for women with dyspareunia. J Consult Clin Psychol. 2016;84:259-268.
39. Maseroli E, Scavello I, Rastrelli G, et al. Outcome of medical and psychosexual interventions for vaginismus: a systematic review and meta-analysis. J Sex Med. 2018;15:1752-1764.
40. American Psychological Association. Clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. 2017. Accessed February 3, 2022. www.apa.org/ptsd-guideline/ptsd.pdf
Dyspareunia is persistent or recurrent pain before, during, or after sexual contact and is not limited to cisgender individuals or vaginal intercourse.1-3 With a prevalence as high as 45% in the United States,2-5 it is one of the most common complaints in gynecologic practices.5,6
Causes and contributing factors
There are many possible causes of dyspareunia.2,4,6 While some patients have a single cause, most cases are complex, with multiple overlapping causes and maintaining factors.4,6 Identifying each contributing factor can help you appropriately address all components.
Physical conditions. The range of physical contributors to dyspareunia includes inflammatory processes, structural abnormalities, musculoskeletal dysfunctions, pelvic organ disorders, injuries, iatrogenic effects, infections, allergic reactions, sensitization, hormonal changes, medication effects, adhesions, autoimmune disorders, and other pain syndromes (TABLE 12-4,6-11).

Inadequate arousal. One of the primary causes of pain during vaginal penetration is inadequate arousal and lubrication.1,2,9-11 Arousal is the phase of the sexual response cycle that leads to genital tumescence and prepares the genitals for sexual contact through penile/clitoral erection, vaginal engorgement, and lubrication, which prevents pain and enhances pleasurable sensation.9-11
While some physical conditions can lead to an inability to lubricate, the most common causes of inadequate lubrication are psychosocial-behavioral, wherein patients have the same physical ability to lubricate as patients without genital pain but do not progress through the arousal phase.9-11 Behavioral factors such as inadequate or ineffective foreplay can fail to produce engorgement and lubrication, while psychosocial factors such as low attraction to partner, relationship stressors, anxiety, or low self-esteem can have an inhibitory effect on sexual arousal.1,2,9-11 Psychosocial and behavioral factors may also be maintaining factors or consequences of dyspareunia, and need to be assessed and treated.1,2,9-11
Psychological trauma. Exposure to psychological traumas and the development of posttraumatic stress disorder (PTSD) have been linked with the development of pain disorders in general and dyspareunia specifically. Most patients seeking treatment for chronic pain disorders have a history of physical or sexual abuse.12 Changes in physiologic processes (eg, neurochemical, endocrine) that occur with PTSD interfere with the sexual response cycle, and sexual traumas specifically have been linked with pelvic floor dysfunction.13,14 Additionally, when PTSD is caused by a sexual trauma, even consensual sexual encounters can trigger flashbacks, intrusive memories, hyperarousal, and muscle tension that interfere with the sexual response cycle and contribute to genital pain.13
Vaginismus is both a physiologic and psychological contributor to dyspareunia.1,2,4 Patients experiencing pain can develop anxiety about repeated pain and involuntarily contract their pelvic muscles, thereby creating more pain, increasing anxiety, decreasing lubrication, and causing pelvic floor dysfunction.1-4,6 Consequently, all patients with dyspareunia should be assessed and continually monitored for symptoms of vaginismus.
Continue to: Anxiety
Anxiety. As with other pain disorders, anxiety develops around pain triggers.10,15 When expecting sexual activity, patients can experience extreme worry and panic attacks.10,15,16 The distress of sexual encounters can interfere with physiologic arousal and sexual desire, impacting all phases of the sexual response cycle.1,2
Relationship issues. Difficulty engaging in or avoidance of sexual activity can interfere with romantic relationships.2,10,16 Severe pain or vaginismus contractions can prevent penetration, leading to unconsummated marriages and an inability to conceive through intercourse.10 The distress surrounding sexual encounters can precipitate erectile dysfunction in male partners, or partners may continue to demand sexual encounters despite the patient’s pain, further impacting the relationship and heightening sexual distress.10 These stressors have led to relationships ending, patients reluctantly agreeing to nonmonogamy to appease their partners, and patients avoiding relationships altogether.10,16
Devalued self-image. Difficulties with sexuality and relationships impact the self-image of patients with dyspareunia. Diminished self-image may include feeling “inadequate” as a woman and as a sexual partner, or feeling like a “failure.”16 Women with dyspareunia often have more distress related to their body image, physical appearance, and genital self-image than do women without genital pain.17 Feeling resentment toward their body, or feeling “ugly,” embarrassed, shamed, “broken,” and “useless” also contribute to increased depressive symptoms found in patients with dyspareunia.16,18
Making the diagnosis
Most patients do not report symptoms unless directly asked2,7; therefore, it is recommended that all patients be screened as a part of an initial intake and before any genital exam (TABLE 22-4,6,7,9,11,19,20).4,7,21 If this screen is positive, a separate appointment may be needed for a thorough evaluation and before any attempt is made at a genital exam.4,7

Items to include in the clinical interview
Given the range of possible causes of dyspareunia and its contributing factors and symptoms, a thorough clinical interview is essential. Begin with a review of the patient’s complete medical and surgical history to identify possible known contributors to genital pain.4 Pregnancy history is of particular importance as the prevalence of postpartum dyspareunia is 35%, with risk being greater for patients who experienced dyspareunia symptoms before pregnancy.22

Knowing the location and quality of pain is important for differentiating between possible diagnoses, as is specifying dyspareunia as lifelong or acquired, superficial or deep, and primary or secondary.1-4,6 Confirm the specific location(s) of pain—eg, at the introitus, in the vestibule, on the labia, in the perineum, or near the clitoris.2,4,6 A diagram or model may be needed to help patients to localize pain.4
To help narrow the differential, include the following elements in your assessment: pain quality, timing (eg, initial onset, episode onset, episode duration, situational triggers), alleviating factors, symptoms in surrounding structures (eg, bladder, bowel, muscles, bones), sexual history, other areas of sexual functioning, history of psychological trauma, relationship effects, and mental health (TABLE 22-4,6,7,9,11,19,20 and Table 323-28). Screening for a history of sexual trauma is particularly important, as a recent systematic review and meta-analysis found that women with a history of sexual assault had a 42% higher risk of gynecologic problems overall, a 74% higher risk of dyspareunia, and a 71% higher risk of vaginismus than women without a history of sexual assault.29 Using measures such as the Female Sexual Function Index or the McGill Pain Questionnaire can help patients more thoroughly describe their symptoms (TABLE 323-28).3

Continue to: Guidelines for the physical exam
Guidelines for the physical exam
Before the exam, ensure the patient has not used any topical genital treatment in the past 2 weeks that may interfere with sensitivity to the exam.4 To decrease patients’ anxiety about the exam, remind them that they can stop the exam at any time.7 Also consider offering the use of a mirror to better pinpoint the location of pain, and to possibly help the patient learn more about her anatomy.2,7
Begin the exam by palpating surrounding areas that may be involved in pain, including the abdomen and musculoskeletal features.3,6,19 Next visually inspect the external genitalia for lesions, abrasions, discoloration, erythema, or other abnormal findings.2,3,6 Ask the patient for permission before contacting the genitals. Because the labia may be a site of pain, apply gentle pressure in retracting it to fully examine the vestibule.6,7 Contraction of the pelvic floor muscles during approach or initial palpation could signal possible vaginismus.4
After visual inspection of external genitalia, use a cotton swab to map the vulva and vestibule in a clockwise fashion to precisely identify any painful locations.2-4,6 If the patient’s history of pain has been intermittent, it’s possible that the cotton swab will not elicit pain on the day of the initial exam, but it may on other days.4
Begin the internal exam by inserting a single finger into the first inch of the vagina and have the patient squeeze and release to assess tenderness, muscle tightness, and control.2,6 Advance the finger further into the vagina and palpate clockwise, examining the levator muscles, obturator muscles, rectum, urethra, and bladder for abnormal tightness or reproduction of pain.2,4,6 Complete a bimanual exam to evaluate the pelvic organs and adnexa.2,4 If indicated, a more thorough evaluation of pelvic floor musculature can be performed by a physical therapist or gynecologist who specializes in pelvic pain.2-4
If the patient consents to further evaluation, consider using a small speculum, advanced slowly, for further internal examination, noting any lesions, abrasions, discharge, ectropion, or tenderness.2-4,7 A rectal exam may also be needed in cases of deep dyspareunia.6 Initial work-up may include a potassium hydroxide wet prep, sexually transmitted infection testing, and pelvic ultrasound.2,4 In some cases, laparoscopy or biopsy may be needed.2,4
Treatments for common causes
Treatment often begins with education about anatomy, to help patients communicate about symptoms and engage more fully in their care.3 Additional education may be needed on genital functioning and the necessity of adequate stimulation and lubrication prior to penetration.1,2,9-11 A discussion of treatments for the wide range of possible causes of dyspareunia is outside the scope of this article. However, some basic behavioral changes may help patients address some of the more common contributing factors.
For example, if vaginal infection is suspected, advise patients to discontinue the use of harsh soaps, known vaginal irritants (eg, perfumed products, bath additives), and douches.3 Recommend using only preservative- and alcohol-free lubricants for sexual contact, and avoiding lubricants with added functions (eg, warming).3 It’s worth noting that avoidance of tight clothing and thong underwear due to possible risk for infections may not be necessary. A recent study found that women who frequently wore thong underwear (more than half of the time) were no more likely to develop urinary tract infections, yeast vaginitis, or bacterial vaginosis than those who avoid such items.30 However, noncotton underwear fabric, rather than tightness, was associated with yeast vaginitis30; therefore, patients may want to consider using only breathable underwear.3
Continue to: Medication
Medication. Medication may be used to treat the underlying contributing conditions or the symptom of pain directly. Some common options are particularly important for patients whose dyspareunia does not have an identifiable cause. These medications include anti-inflammatory agents, topical anesthetics, tricyclic antidepressants, and hormonal treatments.2-4 Since effectiveness varies based on subtypes of pain, select a medication according to the location, timing, and hypothesized mechanism of pain.3,31,32
Medication for deep pain. A meta-analysis and systematic review found that patients with some types of chronic pelvic pain with pain deep in the vagina or pelvis experienced greater than 50% reduction in pain using medroxyprogesterone acetate compared with placebo.33 Other treatments for deep pain depend on physical exam findings.
Medication for superficial pain. Many remedies have been tried, with at least 26 different treatments for vulvodynia pain alone.16 Only some of these treatments have supporting evidence. For patients with vulvar pain, an intent-to-treat RCT found that patients using a topical steroid experienced a 23% reduction in pain from pre-treatment to 6-month follow-up.32
Surgery is also effective for vulvar pain.34,35 For provoked vestibulodynia (in which pain is localized to the vestibule and triggered by contact with the vulva), or vulvar vestibulitis, RCTs have found that vestibulectomy has stronger effects on pain than other treatments,31,35 with a 53% reduction in pain during intercourse and a 70% reduction in vestibular pain overall.35 However, while vestibulectomy is effective for provoked vestibulodynia, it is not recommended for generalized vulvodynia, in which pain is diffuse across the vulva and occurs without vulvar contact.34
Unsupported treatments. A number of other treatments have not yet been found effective. Although lidocaine for vulvar pain is often used, RCTs have not found any significant reduction in symptoms, and a double-blind RCT found that lidocaine ointment actually performed worse than placebo.31,34 Similarly, oral tricyclics have not been found to decrease vulvar pain more than placebo in double-blind studies.31,34 Furthermore, a meta-analysis of RCTs comparing treatments with placebo for vestibular pain found no significant decrease in dyspareunia for topical conjugated estrogen, topical lidocaine, oral desipramine, oral desipramine with topical lidocaine, laser therapy, or transcranial direct current.32
Tx risks to consider. Risks and benefits of dyspareunia treatment options should be thoroughly weighed and discussed with the patient.2-4 Vestibulectomy, despite reducing pain for many patients, has led to increased pain for 9% of patients who underwent the procedure.35 Topical treatments may lead to allergic reactions, inflammation, and worsening of symptoms,4 and hormonal treatments have been found to increase the risk of weight gain and bloating and are not appropriate for patients trying to conceive.33
Coordinate care with other providers
While medications and surgery can reduce pain, they have not been shown to improve other aspects of sexual functioning such as sexual satisfaction, frequency of sexual intercourse, or overall sense of sexual functioning.35 Additionally, pain reduction does not address muscle tension, anxiety, self-esteem, and relationship problems. As a result, a multidisciplinary approach is generally needed.3,4,32,33
Continue to: Physical therapists
Physical therapists. Pelvic floor physical therapists are often members of the dyspareunia treatment team and can provide a thorough evaluation and treatment of pelvic floor disorders.2-4 An RCT with intent-to-treat analysis found that pain was reduced by 71% following pelvic floor physical therapy.36 Another RCT found that 90% of patients reported a clinically meaningful decrease in pain with pelvic floor physical therapy.37 In addition to addressing pain, pelvic floor physical therapy has also been found to improve sexual functioning, sexual satisfaction, distress, and patient perception of improvement.34,36,37
Behavioral health specialists. Psychotherapists, especially those trained in sex therapy, couples therapy, or cognitive behavioral therapy (CBT), are also typically on the treatment team. Multiple RCTs have found evidence of CBT’s effectiveness in the direct treatment of dyspareunia pain. Bergeron et al35 found a 37.5% reduction in vulvar vestibulitis pain intensity during intercourse after patients completed group CBT. Another intent-to-treat RCT found that patients receiving CBT experienced more pain reduction (~ 30%) than patients who were treated with a topical steroid.38
In addition to having a direct impact on pain, CBT has also been found to have a clinically and statistically significant positive impact on other aspects of sexual experience, such as overall sexuality, self-efficacy, overall sexual functioning, frequency of intercourse, and catastrophizing.34,38 A recent meta-analysis of RCTs found that about 80% of vaginismus patients were able to achieve penetrative intercourse after treatment with behavioral sex therapy or CBT.39 This success rate was not exceeded by physical or surgical treatments.39
When PTSD is thought to be a contributing factor, trauma therapy will likely be needed in addition to treatments for dyspareunia. First-line treatments for PTSD include cognitive processing therapy, prolonged exposure, trauma-focused CBT, and cognitive therapy.40
Psychotherapists can also help patients reduce anxiety, reintroduce sexual contact without triggering pain or anxiety, address emotional and self-esteem effects of dyspareunia, address relationship issues, and refocus sexual encounters on pleasure rather than pain avoidance.2-4 Despite patient reports of high treatment satisfaction following therapy,38 many patients may initially lack confidence in psychotherapy as a treatment for pain35 and may need to be educated on its effectiveness and multidimensional benefits.
Gynecologists. Often a gynecologist with specialization in pelvic pain is an essential member of the team for diagnostic clarification, recommendation of treatment options, and performance of more advanced treatments.2,3 If pain has become chronic, the patient may also benefit from a pain management team and support groups.2,3
Follow-up steps
Patients who screen negative for dyspareunia should be re-screened periodically. Continue to assess patients diagnosed with dyspareunia for vaginismus symptoms (if they are not initially present) to ensure that the treatment plan is appropriately adjusted. Once treatment has begun, ask about adverse effects and confidence in the treatment plan to minimize negative impacts on treatment adherence and to anticipate a need for a change in the treatment approach.31,35 In addition to tracking treatment effects on pain, continue to assess for patient-centered outcomes such as emotional functioning, self-esteem, and sexual and relationship satisfaction.34 The Female Sexual Function Index can be a useful tool to track symptoms.27,34
Finally, patients who do not experience sufficient improvement in symptoms and functioning with initial treatment may need continued support and encouragement. Given the broad range of contributing factors and the high number of potential treatments, patients may find hope in learning that multiple other treatment options may be available.
CORRESPONDENCE
Adrienne A. Williams, PhD, Department of Family and Community Medicine, University of Illinois at Chicago College of Medicine, 1919 W Taylor Street, MC 663, Chicago, IL 60612; [email protected]
Dyspareunia is persistent or recurrent pain before, during, or after sexual contact and is not limited to cisgender individuals or vaginal intercourse.1-3 With a prevalence as high as 45% in the United States,2-5 it is one of the most common complaints in gynecologic practices.5,6
Causes and contributing factors
There are many possible causes of dyspareunia.2,4,6 While some patients have a single cause, most cases are complex, with multiple overlapping causes and maintaining factors.4,6 Identifying each contributing factor can help you appropriately address all components.
Physical conditions. The range of physical contributors to dyspareunia includes inflammatory processes, structural abnormalities, musculoskeletal dysfunctions, pelvic organ disorders, injuries, iatrogenic effects, infections, allergic reactions, sensitization, hormonal changes, medication effects, adhesions, autoimmune disorders, and other pain syndromes (TABLE 12-4,6-11).

Inadequate arousal. One of the primary causes of pain during vaginal penetration is inadequate arousal and lubrication.1,2,9-11 Arousal is the phase of the sexual response cycle that leads to genital tumescence and prepares the genitals for sexual contact through penile/clitoral erection, vaginal engorgement, and lubrication, which prevents pain and enhances pleasurable sensation.9-11
While some physical conditions can lead to an inability to lubricate, the most common causes of inadequate lubrication are psychosocial-behavioral, wherein patients have the same physical ability to lubricate as patients without genital pain but do not progress through the arousal phase.9-11 Behavioral factors such as inadequate or ineffective foreplay can fail to produce engorgement and lubrication, while psychosocial factors such as low attraction to partner, relationship stressors, anxiety, or low self-esteem can have an inhibitory effect on sexual arousal.1,2,9-11 Psychosocial and behavioral factors may also be maintaining factors or consequences of dyspareunia, and need to be assessed and treated.1,2,9-11
Psychological trauma. Exposure to psychological traumas and the development of posttraumatic stress disorder (PTSD) have been linked with the development of pain disorders in general and dyspareunia specifically. Most patients seeking treatment for chronic pain disorders have a history of physical or sexual abuse.12 Changes in physiologic processes (eg, neurochemical, endocrine) that occur with PTSD interfere with the sexual response cycle, and sexual traumas specifically have been linked with pelvic floor dysfunction.13,14 Additionally, when PTSD is caused by a sexual trauma, even consensual sexual encounters can trigger flashbacks, intrusive memories, hyperarousal, and muscle tension that interfere with the sexual response cycle and contribute to genital pain.13
Vaginismus is both a physiologic and psychological contributor to dyspareunia.1,2,4 Patients experiencing pain can develop anxiety about repeated pain and involuntarily contract their pelvic muscles, thereby creating more pain, increasing anxiety, decreasing lubrication, and causing pelvic floor dysfunction.1-4,6 Consequently, all patients with dyspareunia should be assessed and continually monitored for symptoms of vaginismus.
Continue to: Anxiety
Anxiety. As with other pain disorders, anxiety develops around pain triggers.10,15 When expecting sexual activity, patients can experience extreme worry and panic attacks.10,15,16 The distress of sexual encounters can interfere with physiologic arousal and sexual desire, impacting all phases of the sexual response cycle.1,2
Relationship issues. Difficulty engaging in or avoidance of sexual activity can interfere with romantic relationships.2,10,16 Severe pain or vaginismus contractions can prevent penetration, leading to unconsummated marriages and an inability to conceive through intercourse.10 The distress surrounding sexual encounters can precipitate erectile dysfunction in male partners, or partners may continue to demand sexual encounters despite the patient’s pain, further impacting the relationship and heightening sexual distress.10 These stressors have led to relationships ending, patients reluctantly agreeing to nonmonogamy to appease their partners, and patients avoiding relationships altogether.10,16
Devalued self-image. Difficulties with sexuality and relationships impact the self-image of patients with dyspareunia. Diminished self-image may include feeling “inadequate” as a woman and as a sexual partner, or feeling like a “failure.”16 Women with dyspareunia often have more distress related to their body image, physical appearance, and genital self-image than do women without genital pain.17 Feeling resentment toward their body, or feeling “ugly,” embarrassed, shamed, “broken,” and “useless” also contribute to increased depressive symptoms found in patients with dyspareunia.16,18
Making the diagnosis
Most patients do not report symptoms unless directly asked2,7; therefore, it is recommended that all patients be screened as a part of an initial intake and before any genital exam (TABLE 22-4,6,7,9,11,19,20).4,7,21 If this screen is positive, a separate appointment may be needed for a thorough evaluation and before any attempt is made at a genital exam.4,7

Items to include in the clinical interview
Given the range of possible causes of dyspareunia and its contributing factors and symptoms, a thorough clinical interview is essential. Begin with a review of the patient’s complete medical and surgical history to identify possible known contributors to genital pain.4 Pregnancy history is of particular importance as the prevalence of postpartum dyspareunia is 35%, with risk being greater for patients who experienced dyspareunia symptoms before pregnancy.22

Knowing the location and quality of pain is important for differentiating between possible diagnoses, as is specifying dyspareunia as lifelong or acquired, superficial or deep, and primary or secondary.1-4,6 Confirm the specific location(s) of pain—eg, at the introitus, in the vestibule, on the labia, in the perineum, or near the clitoris.2,4,6 A diagram or model may be needed to help patients to localize pain.4
To help narrow the differential, include the following elements in your assessment: pain quality, timing (eg, initial onset, episode onset, episode duration, situational triggers), alleviating factors, symptoms in surrounding structures (eg, bladder, bowel, muscles, bones), sexual history, other areas of sexual functioning, history of psychological trauma, relationship effects, and mental health (TABLE 22-4,6,7,9,11,19,20 and Table 323-28). Screening for a history of sexual trauma is particularly important, as a recent systematic review and meta-analysis found that women with a history of sexual assault had a 42% higher risk of gynecologic problems overall, a 74% higher risk of dyspareunia, and a 71% higher risk of vaginismus than women without a history of sexual assault.29 Using measures such as the Female Sexual Function Index or the McGill Pain Questionnaire can help patients more thoroughly describe their symptoms (TABLE 323-28).3

Continue to: Guidelines for the physical exam
Guidelines for the physical exam
Before the exam, ensure the patient has not used any topical genital treatment in the past 2 weeks that may interfere with sensitivity to the exam.4 To decrease patients’ anxiety about the exam, remind them that they can stop the exam at any time.7 Also consider offering the use of a mirror to better pinpoint the location of pain, and to possibly help the patient learn more about her anatomy.2,7
Begin the exam by palpating surrounding areas that may be involved in pain, including the abdomen and musculoskeletal features.3,6,19 Next visually inspect the external genitalia for lesions, abrasions, discoloration, erythema, or other abnormal findings.2,3,6 Ask the patient for permission before contacting the genitals. Because the labia may be a site of pain, apply gentle pressure in retracting it to fully examine the vestibule.6,7 Contraction of the pelvic floor muscles during approach or initial palpation could signal possible vaginismus.4
After visual inspection of external genitalia, use a cotton swab to map the vulva and vestibule in a clockwise fashion to precisely identify any painful locations.2-4,6 If the patient’s history of pain has been intermittent, it’s possible that the cotton swab will not elicit pain on the day of the initial exam, but it may on other days.4
Begin the internal exam by inserting a single finger into the first inch of the vagina and have the patient squeeze and release to assess tenderness, muscle tightness, and control.2,6 Advance the finger further into the vagina and palpate clockwise, examining the levator muscles, obturator muscles, rectum, urethra, and bladder for abnormal tightness or reproduction of pain.2,4,6 Complete a bimanual exam to evaluate the pelvic organs and adnexa.2,4 If indicated, a more thorough evaluation of pelvic floor musculature can be performed by a physical therapist or gynecologist who specializes in pelvic pain.2-4
If the patient consents to further evaluation, consider using a small speculum, advanced slowly, for further internal examination, noting any lesions, abrasions, discharge, ectropion, or tenderness.2-4,7 A rectal exam may also be needed in cases of deep dyspareunia.6 Initial work-up may include a potassium hydroxide wet prep, sexually transmitted infection testing, and pelvic ultrasound.2,4 In some cases, laparoscopy or biopsy may be needed.2,4
Treatments for common causes
Treatment often begins with education about anatomy, to help patients communicate about symptoms and engage more fully in their care.3 Additional education may be needed on genital functioning and the necessity of adequate stimulation and lubrication prior to penetration.1,2,9-11 A discussion of treatments for the wide range of possible causes of dyspareunia is outside the scope of this article. However, some basic behavioral changes may help patients address some of the more common contributing factors.
For example, if vaginal infection is suspected, advise patients to discontinue the use of harsh soaps, known vaginal irritants (eg, perfumed products, bath additives), and douches.3 Recommend using only preservative- and alcohol-free lubricants for sexual contact, and avoiding lubricants with added functions (eg, warming).3 It’s worth noting that avoidance of tight clothing and thong underwear due to possible risk for infections may not be necessary. A recent study found that women who frequently wore thong underwear (more than half of the time) were no more likely to develop urinary tract infections, yeast vaginitis, or bacterial vaginosis than those who avoid such items.30 However, noncotton underwear fabric, rather than tightness, was associated with yeast vaginitis30; therefore, patients may want to consider using only breathable underwear.3
Continue to: Medication
Medication. Medication may be used to treat the underlying contributing conditions or the symptom of pain directly. Some common options are particularly important for patients whose dyspareunia does not have an identifiable cause. These medications include anti-inflammatory agents, topical anesthetics, tricyclic antidepressants, and hormonal treatments.2-4 Since effectiveness varies based on subtypes of pain, select a medication according to the location, timing, and hypothesized mechanism of pain.3,31,32
Medication for deep pain. A meta-analysis and systematic review found that patients with some types of chronic pelvic pain with pain deep in the vagina or pelvis experienced greater than 50% reduction in pain using medroxyprogesterone acetate compared with placebo.33 Other treatments for deep pain depend on physical exam findings.
Medication for superficial pain. Many remedies have been tried, with at least 26 different treatments for vulvodynia pain alone.16 Only some of these treatments have supporting evidence. For patients with vulvar pain, an intent-to-treat RCT found that patients using a topical steroid experienced a 23% reduction in pain from pre-treatment to 6-month follow-up.32
Surgery is also effective for vulvar pain.34,35 For provoked vestibulodynia (in which pain is localized to the vestibule and triggered by contact with the vulva), or vulvar vestibulitis, RCTs have found that vestibulectomy has stronger effects on pain than other treatments,31,35 with a 53% reduction in pain during intercourse and a 70% reduction in vestibular pain overall.35 However, while vestibulectomy is effective for provoked vestibulodynia, it is not recommended for generalized vulvodynia, in which pain is diffuse across the vulva and occurs without vulvar contact.34
Unsupported treatments. A number of other treatments have not yet been found effective. Although lidocaine for vulvar pain is often used, RCTs have not found any significant reduction in symptoms, and a double-blind RCT found that lidocaine ointment actually performed worse than placebo.31,34 Similarly, oral tricyclics have not been found to decrease vulvar pain more than placebo in double-blind studies.31,34 Furthermore, a meta-analysis of RCTs comparing treatments with placebo for vestibular pain found no significant decrease in dyspareunia for topical conjugated estrogen, topical lidocaine, oral desipramine, oral desipramine with topical lidocaine, laser therapy, or transcranial direct current.32
Tx risks to consider. Risks and benefits of dyspareunia treatment options should be thoroughly weighed and discussed with the patient.2-4 Vestibulectomy, despite reducing pain for many patients, has led to increased pain for 9% of patients who underwent the procedure.35 Topical treatments may lead to allergic reactions, inflammation, and worsening of symptoms,4 and hormonal treatments have been found to increase the risk of weight gain and bloating and are not appropriate for patients trying to conceive.33
Coordinate care with other providers
While medications and surgery can reduce pain, they have not been shown to improve other aspects of sexual functioning such as sexual satisfaction, frequency of sexual intercourse, or overall sense of sexual functioning.35 Additionally, pain reduction does not address muscle tension, anxiety, self-esteem, and relationship problems. As a result, a multidisciplinary approach is generally needed.3,4,32,33
Continue to: Physical therapists
Physical therapists. Pelvic floor physical therapists are often members of the dyspareunia treatment team and can provide a thorough evaluation and treatment of pelvic floor disorders.2-4 An RCT with intent-to-treat analysis found that pain was reduced by 71% following pelvic floor physical therapy.36 Another RCT found that 90% of patients reported a clinically meaningful decrease in pain with pelvic floor physical therapy.37 In addition to addressing pain, pelvic floor physical therapy has also been found to improve sexual functioning, sexual satisfaction, distress, and patient perception of improvement.34,36,37
Behavioral health specialists. Psychotherapists, especially those trained in sex therapy, couples therapy, or cognitive behavioral therapy (CBT), are also typically on the treatment team. Multiple RCTs have found evidence of CBT’s effectiveness in the direct treatment of dyspareunia pain. Bergeron et al35 found a 37.5% reduction in vulvar vestibulitis pain intensity during intercourse after patients completed group CBT. Another intent-to-treat RCT found that patients receiving CBT experienced more pain reduction (~ 30%) than patients who were treated with a topical steroid.38
In addition to having a direct impact on pain, CBT has also been found to have a clinically and statistically significant positive impact on other aspects of sexual experience, such as overall sexuality, self-efficacy, overall sexual functioning, frequency of intercourse, and catastrophizing.34,38 A recent meta-analysis of RCTs found that about 80% of vaginismus patients were able to achieve penetrative intercourse after treatment with behavioral sex therapy or CBT.39 This success rate was not exceeded by physical or surgical treatments.39
When PTSD is thought to be a contributing factor, trauma therapy will likely be needed in addition to treatments for dyspareunia. First-line treatments for PTSD include cognitive processing therapy, prolonged exposure, trauma-focused CBT, and cognitive therapy.40
Psychotherapists can also help patients reduce anxiety, reintroduce sexual contact without triggering pain or anxiety, address emotional and self-esteem effects of dyspareunia, address relationship issues, and refocus sexual encounters on pleasure rather than pain avoidance.2-4 Despite patient reports of high treatment satisfaction following therapy,38 many patients may initially lack confidence in psychotherapy as a treatment for pain35 and may need to be educated on its effectiveness and multidimensional benefits.
Gynecologists. Often a gynecologist with specialization in pelvic pain is an essential member of the team for diagnostic clarification, recommendation of treatment options, and performance of more advanced treatments.2,3 If pain has become chronic, the patient may also benefit from a pain management team and support groups.2,3
Follow-up steps
Patients who screen negative for dyspareunia should be re-screened periodically. Continue to assess patients diagnosed with dyspareunia for vaginismus symptoms (if they are not initially present) to ensure that the treatment plan is appropriately adjusted. Once treatment has begun, ask about adverse effects and confidence in the treatment plan to minimize negative impacts on treatment adherence and to anticipate a need for a change in the treatment approach.31,35 In addition to tracking treatment effects on pain, continue to assess for patient-centered outcomes such as emotional functioning, self-esteem, and sexual and relationship satisfaction.34 The Female Sexual Function Index can be a useful tool to track symptoms.27,34
Finally, patients who do not experience sufficient improvement in symptoms and functioning with initial treatment may need continued support and encouragement. Given the broad range of contributing factors and the high number of potential treatments, patients may find hope in learning that multiple other treatment options may be available.
CORRESPONDENCE
Adrienne A. Williams, PhD, Department of Family and Community Medicine, University of Illinois at Chicago College of Medicine, 1919 W Taylor Street, MC 663, Chicago, IL 60612; [email protected]
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Ed. American Psychiatric Publishing; 2013.
2. Seehusen DA, Baird DC, Bode DV. Dyspareunia in women. Am Fam Phys. 2014;90:465-470.
3. Sorensen J, Bautista KE, Lamvu G, et al. Evaluation and treatment of female sexual pain: a clinical review. Cureus. 2018;10:e2379.
4. MacNeill C. Dyspareunia. Obstet Gynecol Clin North Am. 2006;33:565-77.
5. Latthe P, Latthe M, Say L, et al. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health. 2006;6:177.
6. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009;113:1124-1136.
7. Williams AA, Williams M. A guide to performing pelvic speculum exams: a patient-centered approach to reducing iatrogenic effects. Teach Learn Med. 2013;25:383-391.
8. Ünlü Z, Yentur A, Çakil N. Pudendal nerve neuropathy: An unknown-rare cause of pelvic pain. Arch Rheumatol. 2016;31:102-103.
9. Dewitte M, Borg C, Lowenstein L. A psychosocial approach to female genital pain. Nat Rev Urol. 2018;15:25-41.
10. Masters WH, Johnson VE. Human Sexual Inadequacy. 1st ed. Little, Brown; 1970.
11. Rathus SA, Nevid JS, Fichner-Rathus L. Human Sexuality in a World of Diversity. 5th ed. Allyn and Bacon; 2002.
12. Bailey BE, Freedenfeld RN, Kiser RS, et al. Lifetime physical and sexual abuse in chronic pain patients: psychosocial correlates and treatment outcomes. Disabil Rehabil. 2003;25:331-342.
13. Yehuda R, Lehrner A, Rosenbaum TY. PTSD and sexual dysfunction in men and women. J Sex Med. 2015;12:1107-1119.
14. Postma R, Bicanic I, van der Vaart H, et al. Pelvic floor muscle problems mediate sexual problems in young adult rape victims. J Sex Med. 2013;10:1978-1987.
15. Binik YM, Bergeron S, Khalifé S. Dyspareunia and vaginismus: so-called sexual pain. In: Leiblum SR, ed. 4th ed. Principles and Practice of Sex Therapy. The Guilford Press; 2007:124-156.
16. Ayling K, Ussher JM. “If sex hurts, am I still a woman?” The subjective experience of vulvodynia in hetero-sexual women. Arch Sex Behav. 2008;37:294-304.
17. Pazmany E, Bergeron S, Van Oudenhove L, et al. Body image and genital self-image in pre-menopausal women with dyspareunia. Arch Sex Behav. 2013;42:999-1010.
18. Maillé DL, Bergeron S, Lambert B. Body image in women with primary and secondary provoked vestibulodynia: a controlled study. J Sex Med. 2015;12:505-515.
19. Ryan L, Hawton K. Female dyspareunia. BMJ. 2004;328:1357.
20. Waldura JF, Arora I, Randall AM, et al. Fifty shades of stigma: exploring the health care experiences of kink-oriented patients. J Sex Med. 2016;13:1918-1929.
21. Hinchliff S, Gott M. Seeking medical help for sexual concerns in mid- and later life: a review of the literature. J Sex Res. 2011;48:106-117.
22. Banaei M, Kariman N, Ozgoli G, et al. Prevalence of postpartum dyspareunia: a systematic review and meta-analysis. Int J Gynaecol Obstet. 2021;153:14-24.
23. Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509-515.
24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
25. U.S. Department of Veterans Affairs. PTSD: National Center for PTSD. Life events checklist for DSM-5 (LEC-5). Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp
26. Weathers FW, Litz BT, Keane TM, et al. The PTSD checklist for DSM-5 (PCL-5). 2013. Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp
27. Rosen R, Brown C, Heiman J, et al. The female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191-208.
28. Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191-197.
29. Hassam T, Kelso E, Chowdary P, et al. Sexual assault as a risk factor for gynaecological morbidity: an exploratory systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;255:222-230.
30. Hamlin AA, Sheeder J, Muffly TM. Brief versus thong hygiene in obstetrics and gynecology (B-THONG): a survey study. J Obstet Gynaecol Res. 2019;45:1190-1196.
31. Foster DC, Kotok MB, Huang LS, et al. Oral desipramine and topical lidocaine for vulvodynia: a randomized controlled trial. Obstet Gynecol. 2010;116:583-593.
32. Pérez-López FR, Bueno-Notivol J, Hernandez AV, et al. Systematic review and meta-analysis of the effects of treatment modalities for vestibulodynia in women. Eur J Contracept Reprod Health Care. 2019;24:337-346.
33. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;(3):CD008797.
34. Goldstein AT, Pukall CF, Brown C, et al. Vulvodynia: assessment and treatment. J Sex Med. 2016;13:572-590.
35. Bergeron S, Binik YM, Khalifé S, et al. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91:297-306.
36. Schvartzman R, Schvartzman L, Ferreira CF, et al. Physical therapy intervention for women with dyspareunia: a randomized clinical trial. J Sex Marital Ther. 2019;45:378-394.
37. Morin M, Dumoulin C, Bergeron S, et al. Multimodal physical therapy versus topical lidocaine for provoked vestibulodynia: a multicenter, randomized trial. Am J Obstet Gynecol. 2021;224:189.e1-189.e12.
38. Bergeron S, Khalifé S, Dupuis M-J, et al. A randomized clinical trial comparing group cognitive-behavioral therapy and a topical steroid for women with dyspareunia. J Consult Clin Psychol. 2016;84:259-268.
39. Maseroli E, Scavello I, Rastrelli G, et al. Outcome of medical and psychosexual interventions for vaginismus: a systematic review and meta-analysis. J Sex Med. 2018;15:1752-1764.
40. American Psychological Association. Clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. 2017. Accessed February 3, 2022. www.apa.org/ptsd-guideline/ptsd.pdf
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Ed. American Psychiatric Publishing; 2013.
2. Seehusen DA, Baird DC, Bode DV. Dyspareunia in women. Am Fam Phys. 2014;90:465-470.
3. Sorensen J, Bautista KE, Lamvu G, et al. Evaluation and treatment of female sexual pain: a clinical review. Cureus. 2018;10:e2379.
4. MacNeill C. Dyspareunia. Obstet Gynecol Clin North Am. 2006;33:565-77.
5. Latthe P, Latthe M, Say L, et al. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health. 2006;6:177.
6. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009;113:1124-1136.
7. Williams AA, Williams M. A guide to performing pelvic speculum exams: a patient-centered approach to reducing iatrogenic effects. Teach Learn Med. 2013;25:383-391.
8. Ünlü Z, Yentur A, Çakil N. Pudendal nerve neuropathy: An unknown-rare cause of pelvic pain. Arch Rheumatol. 2016;31:102-103.
9. Dewitte M, Borg C, Lowenstein L. A psychosocial approach to female genital pain. Nat Rev Urol. 2018;15:25-41.
10. Masters WH, Johnson VE. Human Sexual Inadequacy. 1st ed. Little, Brown; 1970.
11. Rathus SA, Nevid JS, Fichner-Rathus L. Human Sexuality in a World of Diversity. 5th ed. Allyn and Bacon; 2002.
12. Bailey BE, Freedenfeld RN, Kiser RS, et al. Lifetime physical and sexual abuse in chronic pain patients: psychosocial correlates and treatment outcomes. Disabil Rehabil. 2003;25:331-342.
13. Yehuda R, Lehrner A, Rosenbaum TY. PTSD and sexual dysfunction in men and women. J Sex Med. 2015;12:1107-1119.
14. Postma R, Bicanic I, van der Vaart H, et al. Pelvic floor muscle problems mediate sexual problems in young adult rape victims. J Sex Med. 2013;10:1978-1987.
15. Binik YM, Bergeron S, Khalifé S. Dyspareunia and vaginismus: so-called sexual pain. In: Leiblum SR, ed. 4th ed. Principles and Practice of Sex Therapy. The Guilford Press; 2007:124-156.
16. Ayling K, Ussher JM. “If sex hurts, am I still a woman?” The subjective experience of vulvodynia in hetero-sexual women. Arch Sex Behav. 2008;37:294-304.
17. Pazmany E, Bergeron S, Van Oudenhove L, et al. Body image and genital self-image in pre-menopausal women with dyspareunia. Arch Sex Behav. 2013;42:999-1010.
18. Maillé DL, Bergeron S, Lambert B. Body image in women with primary and secondary provoked vestibulodynia: a controlled study. J Sex Med. 2015;12:505-515.
19. Ryan L, Hawton K. Female dyspareunia. BMJ. 2004;328:1357.
20. Waldura JF, Arora I, Randall AM, et al. Fifty shades of stigma: exploring the health care experiences of kink-oriented patients. J Sex Med. 2016;13:1918-1929.
21. Hinchliff S, Gott M. Seeking medical help for sexual concerns in mid- and later life: a review of the literature. J Sex Res. 2011;48:106-117.
22. Banaei M, Kariman N, Ozgoli G, et al. Prevalence of postpartum dyspareunia: a systematic review and meta-analysis. Int J Gynaecol Obstet. 2021;153:14-24.
23. Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509-515.
24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
25. U.S. Department of Veterans Affairs. PTSD: National Center for PTSD. Life events checklist for DSM-5 (LEC-5). Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp
26. Weathers FW, Litz BT, Keane TM, et al. The PTSD checklist for DSM-5 (PCL-5). 2013. Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp
27. Rosen R, Brown C, Heiman J, et al. The female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191-208.
28. Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191-197.
29. Hassam T, Kelso E, Chowdary P, et al. Sexual assault as a risk factor for gynaecological morbidity: an exploratory systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;255:222-230.
30. Hamlin AA, Sheeder J, Muffly TM. Brief versus thong hygiene in obstetrics and gynecology (B-THONG): a survey study. J Obstet Gynaecol Res. 2019;45:1190-1196.
31. Foster DC, Kotok MB, Huang LS, et al. Oral desipramine and topical lidocaine for vulvodynia: a randomized controlled trial. Obstet Gynecol. 2010;116:583-593.
32. Pérez-López FR, Bueno-Notivol J, Hernandez AV, et al. Systematic review and meta-analysis of the effects of treatment modalities for vestibulodynia in women. Eur J Contracept Reprod Health Care. 2019;24:337-346.
33. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;(3):CD008797.
34. Goldstein AT, Pukall CF, Brown C, et al. Vulvodynia: assessment and treatment. J Sex Med. 2016;13:572-590.
35. Bergeron S, Binik YM, Khalifé S, et al. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91:297-306.
36. Schvartzman R, Schvartzman L, Ferreira CF, et al. Physical therapy intervention for women with dyspareunia: a randomized clinical trial. J Sex Marital Ther. 2019;45:378-394.
37. Morin M, Dumoulin C, Bergeron S, et al. Multimodal physical therapy versus topical lidocaine for provoked vestibulodynia: a multicenter, randomized trial. Am J Obstet Gynecol. 2021;224:189.e1-189.e12.
38. Bergeron S, Khalifé S, Dupuis M-J, et al. A randomized clinical trial comparing group cognitive-behavioral therapy and a topical steroid for women with dyspareunia. J Consult Clin Psychol. 2016;84:259-268.
39. Maseroli E, Scavello I, Rastrelli G, et al. Outcome of medical and psychosexual interventions for vaginismus: a systematic review and meta-analysis. J Sex Med. 2018;15:1752-1764.
40. American Psychological Association. Clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. 2017. Accessed February 3, 2022. www.apa.org/ptsd-guideline/ptsd.pdf
PRACTICE RECOMMENDATIONS
› Screen all patients for sexual dysfunctions, as patients often do not report symptoms on their own. B
› Refer patients with dyspareunia for psychotherapy to address both pain and psychosocial causes and sequela of dyspareunia. A
› Refer patients with dyspareunia for pelvic floor physical therapy to address pain and sexual functioning. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
When is catheter ablation a sound option for your patient with A-fib?
CASE
Jack Z, a 75-year-old man with well-controlled hypertension, diabetes controlled by diet, and atrial fibrillation (AF) presents to the family medicine clinic to establish care with you after moving to the community from out of town.
The patient describes a 1-year history of AF. He provides you with an echocardiography report from 6 months ago that shows no evidence of structural heart disease. He takes lisinopril, to control blood pressure; an anticoagulant; a beta-blocker; and amiodarone for rhythm control. Initially, he took flecainide, which was ineffective for rhythm control, before being switched to amiodarone. He had 2 cardioversion procedures, each time after episodes of symptoms. He does not smoke or drink alcohol.
Mr. Z describes worsening palpitations and shortness of breath over the past 9 months. Symptoms now include episodes of exertional fatigue, even when he is not having palpitations. Prior to the episodes of worsening symptoms, he tells you that he lived a “fairly active” life, golfing twice a week.
The patient’s previous primary care physician had encouraged him to talk to his cardiologist about “other options” for managing AF, because levels of his liver enzymes had started to rise (a known adverse effect of amiodarone1) when measured 3 months ago. He did not undertake that conversation, but asks you now about other treatments for AF.
Atrial fibrillation is the most common sustained cardiac arrhythmia, characterized by discordant electrical activation of the atria due to structural or electrophysiological abnormalities, or both. The disorder is associated with an increased rate of stroke and heart failure and is independently associated with a 1.5- to 2-fold risk of all-cause mortality.2
In this article, we review the pathophysiology of AF; management, including the role of, and indications for, catheter ablation; and patient- and disease-related factors associated with ablation (including odds of success, complications, risk of recurrence, and continuing need for thromboprophylaxis) that family physicians should consider when contemplating referral to a cardiologist or electrophysiologist for catheter ablation for AF.
What provokes AF?
AF is thought to occur as a result of an interaction among 3 phenomena:
- enhanced automaticity of abnormal atrial tissue
- triggered activity of ectopic foci within 1 or more pulmonary veins, lying within the left atrium
- re-entry, in which there is propagation of electrical impulses from an ectopic beat through another pathway.
Continue to: In patients who progress...
In patients who progress from paroxysmal to persistent AF (see “Subtypes,” below), 2 distinct pathways, facilitated by the presence of abnormal tissue, continuously activate one another, thus maintaining the arrhythmia. Myocardial tissue in the pulmonary veins is responsible for most ectopic electrical impulses in patients with drug-refractory AF (see “Rhythm control”).
Subtypes. For the purpose of planning treatment, AF is classified as:
- Paroxysmal. Terminates spontaneously or with intervention ≤ 7 days after onset.
- Persistent. Continuous and sustained for > 7 days.
- Longstanding persistent. Continuous for > 12 months.
- Permanent. The patient and physician accept that there will be no further attempt to restore or maintain sinus rhythm.
Goals of treatment
Primary management goals in patients with AF are 2-fold: control of symptoms and prevention of thromboembolism. A patient with new-onset AF who presents acutely with inadequate rate control and hemodynamic compromise requires urgent assessment to determine the cause of the arrhythmia and need for cardioversion.3 A symptomatic patient with AF who does not have high-risk features (eg, valvular heart disease, mechanical valves) might be a candidate for rhythm control in addition to rate control.3,4
Rate control. After evaluation in the hospital, a patient who has a rapid ventricular response but remains hemodynamically stable, without evidence of heart failure, should be initiated on a rate-controlling medication, such as a beta-blocker or nondihydropyridine calcium-channel blocker. A resting heart rate goal of < 80 beats per minute (bpm) is recommended for a symptomatic patient with AF. The heart rate goal can be relaxed, to < 110 bpm, in an asymptomatic patient with preserved left ventricular function.5,6
Rhythm control, indicated in patients who remain symptomatic on rate-controlling medication, can be achieved either with an antiarrhythmic drug (AAD) or by catheter ablation.4,5 In stable patients, rhythm control should be considered only after a thorough work-up for a reversible cause of AF, and can be achieved with an oral AAD or, in select patients, through catheter ablation (TABLE 13,6). Other indications for chronic rhythm control include treatment of patients with tachycardia-induced cardiomyopathy.5
A major study that documented the benefit of early rhythm control evaluated long-term outcomes in 2789 patients with AF who were undergoing catheter ablation.7 Patients were randomized to early rhythm control (catheter ablation or AAD) or “usual care”—ie, in this study, rhythm control limited to symptomatic patients. Primary outcomes were death from cardiovascular causes, stroke, and hospitalization with worsening heart failure or acute coronary syndrome. A first primary outcome event occurred in 249 patients (3.9/100 person-years) assigned to early rhythm control, compared to 316 (5.0 per 100 person-years) in the group assigned to usual care.
The study was terminated early (after 5.1 years) because of overwhelming evidence of efficacy (number need to treat = 7). Although early rhythm control was obtained through both catheter ablation and AAD (hazard ratio [HR] = 0.79; 96% CI, 0.66-0.94; P = .005), success was attributed to the use of catheter ablation for a rhythm-control strategy and its use among patients whose AF was present for < 1 year. Most patients in both treatment groups continued to receive anticoagulation, rate control, and optimization of cardiovascular risk.7
Continue to: Notably, direct studies...
Notably, direct studies comparing ablation and AAD have not confirmed the benefit of ablation over AAD in outcomes of all-cause mortality, bleeding, stroke, or cardiac arrest over a 5-year period.8
Adverse effects and mortality outcomes with AAD. Concern over using AAD for rhythm control is based mostly on adverse effects and long-term (1-year) mortality outcomes. Long-term AAD therapy has been shown to decrease the recurrence of AF—but without evidence to suggest other mortality benefits.
A meta-analysis of 59 randomized controlled trials reviewed 20,981 patients receiving AAD (including quinidine, disopyramide, propafenone, flecainide, metoprolol, amiodarone, dofetilide, dronedarone, and sotalol) for long-term effects on death, stroke, adverse reactions, and recurrence of AF.9 Findings at 10 months suggest that:
- Compared to placebo, amiodarone and sotalol increased the risk of all-cause mortality during the study period.
- There was minimal difference in mortality among patients taking dofetilide or dronedarone, compared to placebo.
- There were insufficient data to draw conclusions about the effect of disopyramide, flecainide, and propafenone on mortality.
Before starting a patient on AAD, the risk of arrhythmias and the potential for these agents to cause toxicity and adverse events should always be discussed.
CASE
You tell Mr. Z that you need to know the status of his comorbidities to make a recommendation about “other” management options, and proceed to take a detailed history.
Recent history. Mr. Z reveals that “today is a good day”: He has had “only 1” episode of palpitations, which resolved on its own. The previous episode, he explains, was 3 days ago, when palpitations were associated with lightheadedness and shortness of breath. He denies chest pains or swelling of the legs.
Physical exam. The patient appears spry, comfortable, and in no acute distress. Vital signs are within normal limits. A body mass index of 28.4 puts him in the “overweight” category. His blood pressure is 118/75 mm Hg.
Continue to: Cardiac examination...
Cardiac examination is significant for an irregular rhythm without murmurs, rubs, or gallops. His lungs are clear bilaterally; his abdomen is soft and nondistended. His extremities show no edema.
Testing. You obtain an electrocardiogram, which demonstrates a controlled ventricular rate of 88 bpm and AF. You order a complete blood count, comprehensive metabolic panel, tests of hemoglobin A1C and thyroid-stimulating hormone, lipid panel, echocardiogram, and a chest radiograph.
Results. The chest radiograph is negative for an acute cardiopulmonary process; cardiac size is normal. Aspartate aminotransferase and alanine aminotransferase levels are higher than twice the normal limit. The echocardiogram reveals an estimated left ventricular ejection fraction of 55% to 60%; no structural abnormalities are noted.
In which AF patients is catheter ablation indicated?
Ablation is recommended for select patients (TABLE 13,6) with symptomatic paroxysmal AF that is refractory to AAD or who are intolerant of AAD.3,6 It is a reasonable first-line therapy for high-performing athletes in whom AAD would affect athletic performance.3,10 It is also a reasonable option in select patients > 75 years and as an alternative to AAD therapy.3 Finally, catheter ablation should be considered in symptomatic patients with longstanding persistent AF and congestive heart failure, with or without reduced left ventricular ejection fraction.3
CASE
You inform Mr. Z that his symptoms are likely a result of symptomatic paroxysmal AF, which was refractory to flecainide and amiodarone, and that his abnormal liver function test results preclude continued use of amiodarone. You propose Holter monitoring to correlate timing of symptoms with the arrhythmia, but he reports this has been done, and the correlation confirmed, by his previous physician.
You explain that, because the diagnosis of symptomatic paroxysmal AF refractory to AADs has been confirmed, he is categorized as a patient who might benefit from catheter ablation, based on:
- the type of AF (ie, paroxysmal AF is associated with better ablation outcomes)
- persistent symptoms that are refractory to AADs
- his intolerance of AAD
- the length of time since onset of symptoms.
Mr. Z agrees to consider your recommendation.
Continue to: What are the benefits of catheter ablation?
What are the benefits of catheter ablation?
Ablation can be achieved through radiofrequency (RF) ablation, cryoablation, or newer, laser-based balloon ablation. Primary outcomes used to determine the success of any options for performing ablation include mortality, stroke, and hospitalization. Other endpoints include maintenance of sinus rhythm, freedom from AF, reduction in AF burden (estimated through patients’ report of symptoms, recurrence rate, need for a second ablation procedure, and serial long-term monitoring through an implantable cardiac monitoring device), quality of life, and prevention of AF progression.3
Patient and disease variables (TABLE 211-13). The success rate of catheter ablation, defined as freedom from either symptomatic or asymptomatic episodes of AF, is dependent on several factors,3,14 including:
- type of AF (paroxysmal or persistent)
- duration and degree of symptoms
- age
- sex
- comorbidities, including heart failure and structural heart or lung disease.
Overall, in patients with paroxysmal AF, an estimated 75% are symptom free 1 year after ablation.15 Patients with persistent and longstanding persistent AF experience a lower success rate.
RF catheter ablation has demonstrated superiority to AAD in reducing the need for cardioversion (relative risk [RR] = 0.62; 95% CI, 0.47-0.82) and cardiac-related hospitalization (RR = 0.27; 95% CI, 0.10-0.72;) at 12 months in patients with nonparoxysmal AF (persistent or longstanding persistent).16
Effect on mortality. Among patients with heart failure with reduced ejection fraction, long-term studies of cardiovascular outcomes 5 years post ablation concluded that ablation is associated with a decrease in all-cause mortality (RR = 0.69; 95% CI, 0.54-0.88; P = .003) and a reduction in hospitalization (RR = 0.62; 95% CI, 0.47-0.82; P = .0006); younger (< 65 years) and male patients derive greater benefit.6,17 Indications for ablation in patients with heart failure are similar to those in patients without heart failure; ablation can therefore be considered for select heart failure patients who remain symptomatic or for whom AAD has failed.3
Older patients. Ablation can be considered for patients > 75 years with symptomatic paroxysmal AF refractory to AAD or who are intolerant of AAD.3 A study that assessed the benefit of catheter ablation reviewed 587 older (> 75 years) patients with AF, of whom 324 were eligible for ablation. Endpoints were maintenance of sinus rhythm, stroke, death, and major bleeding. Return to normal sinus rhythm was an independent factor, associated with a decrease in the risk of mortality among all patient groups that underwent ablation (HR = 0.36; 95% CI, 0.2-0.63; P = .0005). Age > 75 years (HR = 1.09; 95% CI, 1.01-1.16; P > .02) and depressed ejection fraction < 40% (HR = 2.38; 95% CI, 1.28-4.4; P = .006) were determined to be unfavorable parameters for survival.18
Complications and risks
Complications of catheter ablation for AF, although infrequent, can be severe (TABLE 33). Early mortality, defined as death during initial admission or 30-day readmission, occurs in approximately 0.5% of cases; half of deaths take place during readmission.11
Continue to: Complications vary...
Complications vary, based on the type and site of ablation.19,20 Cardiac tamponade or perforation, the most life-threatening complications, taken together occur in an estimated 1.9% of patients (odds ratio [OR] = 2.98; 95% CI, 1.36-6.56; P = .007).11 Other in-hospital complications independently predictive of death include any cardiac complications (OR = 12.8; 95% CI, 6.86 to 23.8; P < .001) and neurologic complications (cerebrovascular accident and transient ischemic attack) (OR = 8.72; 95% CI, 2.71-28.1; P < .001).
Other complications that do not cause death but might prolong the hospital stay include pericarditis without effusion, anesthesia-related complications, and vascular-access complications. Patients whose ablation is performed at an institution where the volume of ablations is low are also at higher risk of early mortality (OR = 2.35; 95% CI, 1.33-4.15; P = .003).16
Recurrence is common (TABLE 211-13). Risk of recurrence following ablation is significant; early (within 3 months after ablation) recurrence is seen in 50% of patients.21,22 However, this is a so-called "blanking period"—ie, a temporary period of inflammatory and proarrhythmic changes that are not predictors of later recurrence. The 5-year post-ablation recurrence rate is approximately 25.5%; longstanding persistent and persistent AF and the presence of comorbidities are major risk factors for recurrence.13,23
Recurrence is also associated with the type of procedure; pulmonary vein isolation, alone or in combination with another type of procedure, results in higher long-term success.21,23
Other variables affect outcome (TABLE 211-13). Following AF ablation, patients with nonparoxysmal AF at baseline, advanced age, sleep apnea and obesity, left atrial enlargement, and any structural heart disease tend to have a poorer long-term (5-year) outcome (ie, freedom from extended episodes of AF).3,13,23,24
Patients who undergo repeat procedures have higher arrhythmia-free survival; the highest ablation success rate is for patients with paroxysmal AF.13,23
Exposure to ionizing radiation. Fluoroscopy is required for multiple components of atrial mapping and ablation during RF ablation, including navigation, visualization, and monitoring of catheter placement. Patients undergoing this particular procedure therefore receive significant exposure to ionizing radiation. A reduction in, even complete elimination of, fluoroscopy has been achieved with:
- nonfluoroscopic 3-dimensional mapping systems25
- intracardiac echocardiography, which utilizes ultrasonographic imaging as the primary visual mode for tracking and manipulating the catheter
- robotic guided navigation.26-28
Continue to: CASE
CASE
At his return visit, Mr. Z says that he is concerned about, first, undergoing catheter ablation at his age and, second, the risks associated with the procedure. You explain that it is true that ablation is ideal in younger patients who have minimal comorbidities and that the risk of complications increases with age—but that there is no cutoff or absolute age contraindication to ablation.
You tell Mr. Z that you will work with him on risk-factor modification in anticipation of ablation. You also assure him that the decision whether to ablate must be a joint one—between him and a cardiologist experienced both in electrophysiology and in performing this highly technical procedure. And you explain that a highly practiced specialist can identify Mr. Z’s risk factors that might make ablation more difficult to perform and affect the long-term outcome.
With Mr. Z’s agreement, you screen for sleep apnea and start him on a lifestyle modification plan to achieve a more ideal weight, explaining that the risk of recurrence of AF after catheter ablation is increased by obesity and sleep apnea, in addition to age. You explain that, based on his CHA2DS2–VASc (congestive heart failure; hypertension; age, ≥ 75 years; diabetes; prior stroke, transient ischemic attack, or thromboembolism; vascular disease; age, 65 to 74 years; sex category) score of 3, he will remain on anticoagulation whether or not he has the ablation.
You refer the patient to the nearest high-volume cardiac ablation center.
Last, you caution Mr. Z that, based on his lipid levels, his 10-year risk of heart disease or stroke is elevated. You recommend treatment with a statin agent while he continues his other medications.
Delivering energy to myocardium
Myocardial tissue in pulmonary veins is responsible for most ectopic electrical impulses in patients with drug-refractory AF. The goal of catheter ablation in AF is destruction (scarring) of tissue that is the source of abnormal vein potentials.15
How RF ablation works. Ablation is most commonly performed using RF energy, a high-frequency form of electrical energy. Electrophysiology studies are carried out at the time of ablation by percutaneous, fluoroscopically guided insertion of 2 to 5 catheters, usually through the femoral or internal jugular vein, which are then positioned within several areas of the heart—usually, the right atrium, bundle of His, right ventricle, and coronary sinus.
Continue to: Electrical current...
Electrical current is applied through the catheters from an external generator to stimulate the myocardium and thus determine its electrophysiologic properties. The anatomic and electrical activity of the left atrium and pulmonary veins is then identified, a technique known as electro-anatomical mapping (FIGURE). After arrhythmogenic myocardial tissue is mapped, ablation is carried out with RF energy through the catheter to the pathogenic myocardium from which arrhythmias are initiated or conducted. The result is thermal destruction of tissue and creation of small, shallow lesions that vary in size with the type of catheter and the force of contact pressure applied.3,29
Other energy sources used in catheter ablation include cryothermal energy, which utilizes liquid nitrous oxide under pressure through a cryocatheter or cryoballoon catheter. Application of cryothermal energy freezes tissue and disrupts cell membranes and any electrical activity. Cryoballoon ablation has been shown to be similarly safe and efficacious as RF ablation in patients with paroxysmal AF.30,31
Newer laser-based balloon ablations are performed under ultrasonographic guidance and utilize arcs of laser energy delivered to the pathogenic myocardium.3
Thromboembolism prophylaxis
Oral anticoagulation to decrease the risk of stroke is initiated in all patients with AF, based on a thromboembolic risk profile determined by their CHA2DS2–VASc score, with anticoagulation recommended when the score is ≥ 2 in men and ≥ 3 in women. Options for anticoagulation include warfarin and one of the novel oral anticoagulants dabigatran, apixaban, rivaroxaban, and edoxaban.4 Recommendations are as follows3:
- For patients with a CHA2DS2–VASc score of ≥ 2 (men) or ≥ 3 (women), anticoagulation should be continued indefinitely, regardless of how successful the ablation procedure is.
- When patients choose to discontinue anticoagulation, they should be counseled in detail about the risk of doing so. The continued need for frequent arrhythmia monitoring should be emphasized.
The route from primary careto catheter ablation
Perform a thorough evaluation. Patients who present to you with palpitations should first undergo a routine workup for AF, followed by confirmation of the diagnosis. Exclude structural heart disease with echocardiography. Undertake monitoring, which is essential to determine whether symptoms are a reflection of the arrhythmia, using noncontinuous or continuous electrocardiographic (EKG) monitoring. Noncontinuous detection devices include:
- scheduled or symptom-initiated EKG
- a Holter monitor, worn for at least 24 hours and as long as 7 days
- trans-telephonic recordings and patient- or automatically activated devices
- an external loop recorder.32
Continuous EKG monitoring is more permanent (≥ 12 months). This is usually achieved through an implantable loop powered by a battery that lasts as long as 3 years.3
Ablation: Yes or no? Ablation is not recommended to avoid anticoagulation or when anticoagulation is contraindicated.5 With regard to specific patient criteria, the ideal patient:
- is symptomatic
- has failed AAD therapy
- does not have pulmonary disease
- has a normal or mildly dilated left atrium or normal or mildly reduced left ventricular ejection fraction.5
Continue to: There is no absolute age...
There is no absolute age or comorbidity contraindication to ablation. The patient should be referred to a cardiologist who has received appropriate training in electrophysiology, to identify comorbidities that (1) increase the technical difficulty of the procedure and baseline risk and (2) affect long-term outcome,12 and who performs the procedure in a center that has considerable experience with catheter ablation.33
Once the decision is made to perform ablation, you can provide strategies that optimize the outcome (freedom from AF episodes). Those tactics include weight loss and screening evaluation and, if indicated, treatment for sleep apnea.3
Protocol. Prior to the procedure, the patient fasts overnight; they might be asked to taper or discontinue cardiac medications that have electrophysiologic effects. Studies suggest a low risk of bleeding associated with catheter ablation; anticoagulation should therefore continue uninterrupted for patients undergoing catheter ablation for AF3,4,34,35; however, this practice varies with the cardiologist or electrophysiologist performing ablation.
Because of the length and complexity of the procedure, electro-anatomical mapping and ablation are conducted with the patient under general anesthesia.3 The patient is kept supine, and remains so for 2 to 4 hours afterward to allow for hemostasis at puncture sites.3
Patients might be monitored overnight, although same-day catheter ablation has been shown to be safe and cost-effective in select patients.36,37 Post ablation, patients follow up with the cardiologist and electrophysiologist. Long-term arrhythmia monitoring is required.3 Anticoagulation is continued for at least 2 months, and is discontinued based on the patient’s risk for stroke, utilizing their CHA2DS2–VASc score.3,4
CASE
At Mr. Z’s 6-month primary care follow-up, he confirms what has been reported to you as the referring physician: He had a successful catheter ablation and continues to have regular follow-up monitoring with the cardiologist. He is no longer taking amiodarone.
At this visit, he reports no recurrence of AF-associated symptoms or detectable AF on cardiac monitoring. He has lost 8 lbs. You counsel to him to continue to maintain a healthy lifestyle.
CORRESPONDENCE
Amimi S. Osayande MD, FAAFP, Northside-Gwinnett Family Medicine Residency Program, Strickland Family Medicine Center, 665 Duluth Highway, Suite 501, Lawrenceville, GA 30046; [email protected]
1. Amiodarone hydrochloride (marketed as Cordarone and Pacerone) information. Silver Spring, Md.: US Food & Drug Administration. Reviewed March 23, 2015. Accessed January 16, 2022. www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/amiodarone-hydrochloride-marketed-cordarone-and-pacerone-information
2. Gómez-Outes A, Suárez-Gea ML,García-Pinilla JM. Causes of death in atrial fibrillation: challenges and opportunities. Trends Cardiovasc Med. 2017;27:494-503. doi: 10.1016/j.tcm.2017.05.002
3. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/ APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Arrhythm. 2017;33:369-409. doi: 10.1016/j.joa.2017.08.001
4. Camm AJ, Lip GYH, De Caterina R, et al; ESC Committee for Practice Guidelines-CPG; Document Reviewers. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation—developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14:1385-1413. doi: 10.1093/europace/eus305
5. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071-2104. doi: 10.1161/CIR.0000000000000040
6. January CT, Wann LS, Calkins H, et al; Writing Group Members. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/ HRS guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019;16:e66-e93. doi: 10.1016/j.hrthm.2019.01.024
7. Kirchhof P, Camm AJ, Goette A, et al; EAST-AFNET 4 Trial Investigators. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305-1316. doi: 10.1056/ NEJMoa2019422
8. Packer DL, Mark DB, Robb RA, et al; CABANA Investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261-1274. doi: 10.1001/jama.2019.0693
9. Valembois L, Audureau E, Takeda A, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2019;9:CD005049. doi: 10.1002/14651858.CD005049
10. Koopman P, Nuyens D, Garweg C, et al. Efficacy of radiofrequency catheter ablation in athletes with atrial fibrillation. Europace. 2011;13:1386-1393. doi: 10.1093/europace/eur142
11. Hakalahti A, Biancari F, Nielsen JC, et al. Radiofrequency ablation vs. antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: systematic review and meta-analysis. Europace. 2015;17:370-378. doi: 10.1093/europace/euu376
12. Nyong J, Amit G, Adler AJ, et al. Efficacy and safety of ablation for people with non-paroxysmal atrial fibrillation. Cochrane Database Syst Rev. 2016;11:CD012088. doi: 10.1002/14651858. CD012088.pub2
13. Andrade JG, Champagne J, Dubuc M, et al; CIRCA-DOSE Study Investigators. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. 2019;140:1779-1788. doi: 10.1161/ CIRCULATIONAHA.119.042622
14. Asad ZUA, Yousif A, Khan MS, et al. Catheter ablation versus medical therapy for atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. Circ Arrhythm Electrophysiol. 2019;12:e007414. doi: 10.1161/ CIRCEP.119.007414
15. Nademanee K, Amnueypol M, Lee F, et al. Benefits and risks of catheter ablation in elderly patients with atrial fibrillation. Heart Rhythm. 2015;12:44-51. doi: 10.1016/j.hrthm.2014.09.049
16. Cheng EP, Liu CF, Yeo I, et al. Risk of mortality following catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2019;74: 2254-2264. doi: 10.1016/j.jacc.2019.08.1036
17. Brugada J, Katritsis DG, Arbelo E, et al; ESC Scientific Document Group. 2019 ESC Guidelines for the management of patients with supraventricular tachycardia. The Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Developed in collaboration with the Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2020;41:655-720. doi: 10.1093/eurheartj/ehz467
18. Hosseini SM, Rozen G, Saleh A, et al. Catheter ablation for cardiac arrhythmias: utilization and in-hospital complications, 2000 to 2013. JACC Clin Electrophysiol. 2017;3:1240-1248. doi: 10.1016/j.jacep.2017.05.005
19. Andrade JG, Macle L, Khairy P, et al. Incidence and significance of early recurrences associated with different ablation strategies for AF: a STAR-AF substudy. J Cardiovasc Electrophysiol. 2012;23:1295-1301. doi: 10.1111/j.1540-8167.2012.02399.x
20. Joshi S, Choi AD, Kamath GS, et al. Prevalence, predictors, and prognosis of atrial fibrillation early after pulmonary vein isolation: findings from 3 months of continuous automatic ECG loop recordings. J Cardiovasc Electrophysiol. 2009;20:1089-1094. doi: 10.1111/j.1540-8167.2009.01506.x
21. Weerasooriya R, Khairy P, Litalien J, et al. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160-166. doi: 10.1016/j.jacc.2010.05.061
22. Ouyang F, Tilz R, Chun J, et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation. 2010;122:2368-2377. doi: 10.1161/ CIRCULATIONAHA.110.946806
23. Tilz RR, Rillig A, Thum A-M, et al. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J Am Coll Cardiol. 2012;60: 1921-1929. doi: 10.1016/j.jacc.2012.04.060
24. Forkmann M, Schwab C, Busch S. [Catheter ablation of supraventricular tachycardia]. Herzschrittmacherther Elektrophysiol. 2019;30:336-342. doi: 10.1007/s00399-019-00654-x
25. Bulava A, Hanis J, Eisenberger M. Catheter ablation of atrial fibrillation using zero-fluoroscopy technique: a randomized trial. Pacing Clin Electrophysiol. 2015;38:797-806. doi: 10.1111/pace.12634
26. Haegeli LM, Stutz L, Mohsen M, et al. Feasibility of zero or near zero fluoroscopy during catheter ablation procedures. Cardiol J. 2019;26:226-232. doi: 10.5603/CJ.a2018.0029
27. Steven D, Servatius H, Rostock T, et al. Reduced fluoroscopy during atrial fibrillation ablation: benefits of robotic guided navigation. J Cardiovasc Electrophysiol. 2010;21:6-12. doi: 10.1111/j.1540-8167.2009.01592.x
28. General therapy for cardiac arrhythmias. In: Zipes DP, Libby P, Bonow RO, et al. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 11th ed. Elsevier; 2019.
29. Kuck K-H, Brugada J, Albenque J-P. Cryoballoon or radiofrequency ablation for atrial fibrillation. N Engl J Med. 2016;375: 1100-1101. doi: 10.1056/NEJMc1609160
30. Chen Y-H, Lu Z-Y, Xiang Y, et al. Cryoablation vs. radiofrequency ablation for treatment of paroxysmal atrial fibrillation: a systematic review and meta-analysis. Europace. 2017;19:784-794. doi: 10.1093/europace/euw330
31. Locati ET, Vecchi AM, Vargiu S, et al. Role of extended external loop recorders for the diagnosis of unexplained syncope, presyncope, and sustained palpitations. Europace. 2014;16:914-922. doi: 10.1093/europace/eut337
32. Calkins H, Kuck KH, Cappato R, et al; Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2012;9:632-696.e21. doi: 10.1016/j.hrthm.2011.12.016
33. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609-1678. doi: 10.1093/ europace/euw295
34. Nairooz R, Sardar P, Payne J, et al. Meta-analysis of major bleeding with uninterrupted warfarin compared to interrupted warfarin and heparin bridging in ablation of atrial fibrillation. Int J Cardiol. 2015;187:426-429. doi: 10.1016/j.ijcard.2015.03.376
35. Romero J, Cerrud-Rodriguez RC, Diaz JC, et al. Uninterrupted direct oral anticoagulants vs. uninterrupted vitamin K antagonists during catheter ablation of non-valvular atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. Europace. 2018;20:1612-1620. doi: 10.1093/europace/euy133
36. Deyell MW, Leather RA, Macle L, et al. Efficacy and safety of same-day discharge for atrial fibrillation ablation. JACC Clin Electrophysiol. 2020;6:609-619. doi: 10.1016/j.jacep.2020.02.009
37. Theodoreson MD, Chohan BC, McAloon CJ, et al. Same-day cardiac catheter ablation is safe and cost-effective: experience from a UK tertiary center. Heart Rhythm. 2015;12:1756-1761. doi: 10.1016/j.hrthm.2015.05.006
CASE
Jack Z, a 75-year-old man with well-controlled hypertension, diabetes controlled by diet, and atrial fibrillation (AF) presents to the family medicine clinic to establish care with you after moving to the community from out of town.
The patient describes a 1-year history of AF. He provides you with an echocardiography report from 6 months ago that shows no evidence of structural heart disease. He takes lisinopril, to control blood pressure; an anticoagulant; a beta-blocker; and amiodarone for rhythm control. Initially, he took flecainide, which was ineffective for rhythm control, before being switched to amiodarone. He had 2 cardioversion procedures, each time after episodes of symptoms. He does not smoke or drink alcohol.
Mr. Z describes worsening palpitations and shortness of breath over the past 9 months. Symptoms now include episodes of exertional fatigue, even when he is not having palpitations. Prior to the episodes of worsening symptoms, he tells you that he lived a “fairly active” life, golfing twice a week.
The patient’s previous primary care physician had encouraged him to talk to his cardiologist about “other options” for managing AF, because levels of his liver enzymes had started to rise (a known adverse effect of amiodarone1) when measured 3 months ago. He did not undertake that conversation, but asks you now about other treatments for AF.
Atrial fibrillation is the most common sustained cardiac arrhythmia, characterized by discordant electrical activation of the atria due to structural or electrophysiological abnormalities, or both. The disorder is associated with an increased rate of stroke and heart failure and is independently associated with a 1.5- to 2-fold risk of all-cause mortality.2
In this article, we review the pathophysiology of AF; management, including the role of, and indications for, catheter ablation; and patient- and disease-related factors associated with ablation (including odds of success, complications, risk of recurrence, and continuing need for thromboprophylaxis) that family physicians should consider when contemplating referral to a cardiologist or electrophysiologist for catheter ablation for AF.
What provokes AF?
AF is thought to occur as a result of an interaction among 3 phenomena:
- enhanced automaticity of abnormal atrial tissue
- triggered activity of ectopic foci within 1 or more pulmonary veins, lying within the left atrium
- re-entry, in which there is propagation of electrical impulses from an ectopic beat through another pathway.
Continue to: In patients who progress...
In patients who progress from paroxysmal to persistent AF (see “Subtypes,” below), 2 distinct pathways, facilitated by the presence of abnormal tissue, continuously activate one another, thus maintaining the arrhythmia. Myocardial tissue in the pulmonary veins is responsible for most ectopic electrical impulses in patients with drug-refractory AF (see “Rhythm control”).
Subtypes. For the purpose of planning treatment, AF is classified as:
- Paroxysmal. Terminates spontaneously or with intervention ≤ 7 days after onset.
- Persistent. Continuous and sustained for > 7 days.
- Longstanding persistent. Continuous for > 12 months.
- Permanent. The patient and physician accept that there will be no further attempt to restore or maintain sinus rhythm.
Goals of treatment
Primary management goals in patients with AF are 2-fold: control of symptoms and prevention of thromboembolism. A patient with new-onset AF who presents acutely with inadequate rate control and hemodynamic compromise requires urgent assessment to determine the cause of the arrhythmia and need for cardioversion.3 A symptomatic patient with AF who does not have high-risk features (eg, valvular heart disease, mechanical valves) might be a candidate for rhythm control in addition to rate control.3,4
Rate control. After evaluation in the hospital, a patient who has a rapid ventricular response but remains hemodynamically stable, without evidence of heart failure, should be initiated on a rate-controlling medication, such as a beta-blocker or nondihydropyridine calcium-channel blocker. A resting heart rate goal of < 80 beats per minute (bpm) is recommended for a symptomatic patient with AF. The heart rate goal can be relaxed, to < 110 bpm, in an asymptomatic patient with preserved left ventricular function.5,6
Rhythm control, indicated in patients who remain symptomatic on rate-controlling medication, can be achieved either with an antiarrhythmic drug (AAD) or by catheter ablation.4,5 In stable patients, rhythm control should be considered only after a thorough work-up for a reversible cause of AF, and can be achieved with an oral AAD or, in select patients, through catheter ablation (TABLE 13,6). Other indications for chronic rhythm control include treatment of patients with tachycardia-induced cardiomyopathy.5
A major study that documented the benefit of early rhythm control evaluated long-term outcomes in 2789 patients with AF who were undergoing catheter ablation.7 Patients were randomized to early rhythm control (catheter ablation or AAD) or “usual care”—ie, in this study, rhythm control limited to symptomatic patients. Primary outcomes were death from cardiovascular causes, stroke, and hospitalization with worsening heart failure or acute coronary syndrome. A first primary outcome event occurred in 249 patients (3.9/100 person-years) assigned to early rhythm control, compared to 316 (5.0 per 100 person-years) in the group assigned to usual care.
The study was terminated early (after 5.1 years) because of overwhelming evidence of efficacy (number need to treat = 7). Although early rhythm control was obtained through both catheter ablation and AAD (hazard ratio [HR] = 0.79; 96% CI, 0.66-0.94; P = .005), success was attributed to the use of catheter ablation for a rhythm-control strategy and its use among patients whose AF was present for < 1 year. Most patients in both treatment groups continued to receive anticoagulation, rate control, and optimization of cardiovascular risk.7
Continue to: Notably, direct studies...
Notably, direct studies comparing ablation and AAD have not confirmed the benefit of ablation over AAD in outcomes of all-cause mortality, bleeding, stroke, or cardiac arrest over a 5-year period.8
Adverse effects and mortality outcomes with AAD. Concern over using AAD for rhythm control is based mostly on adverse effects and long-term (1-year) mortality outcomes. Long-term AAD therapy has been shown to decrease the recurrence of AF—but without evidence to suggest other mortality benefits.
A meta-analysis of 59 randomized controlled trials reviewed 20,981 patients receiving AAD (including quinidine, disopyramide, propafenone, flecainide, metoprolol, amiodarone, dofetilide, dronedarone, and sotalol) for long-term effects on death, stroke, adverse reactions, and recurrence of AF.9 Findings at 10 months suggest that:
- Compared to placebo, amiodarone and sotalol increased the risk of all-cause mortality during the study period.
- There was minimal difference in mortality among patients taking dofetilide or dronedarone, compared to placebo.
- There were insufficient data to draw conclusions about the effect of disopyramide, flecainide, and propafenone on mortality.
Before starting a patient on AAD, the risk of arrhythmias and the potential for these agents to cause toxicity and adverse events should always be discussed.
CASE
You tell Mr. Z that you need to know the status of his comorbidities to make a recommendation about “other” management options, and proceed to take a detailed history.
Recent history. Mr. Z reveals that “today is a good day”: He has had “only 1” episode of palpitations, which resolved on its own. The previous episode, he explains, was 3 days ago, when palpitations were associated with lightheadedness and shortness of breath. He denies chest pains or swelling of the legs.
Physical exam. The patient appears spry, comfortable, and in no acute distress. Vital signs are within normal limits. A body mass index of 28.4 puts him in the “overweight” category. His blood pressure is 118/75 mm Hg.
Continue to: Cardiac examination...
Cardiac examination is significant for an irregular rhythm without murmurs, rubs, or gallops. His lungs are clear bilaterally; his abdomen is soft and nondistended. His extremities show no edema.
Testing. You obtain an electrocardiogram, which demonstrates a controlled ventricular rate of 88 bpm and AF. You order a complete blood count, comprehensive metabolic panel, tests of hemoglobin A1C and thyroid-stimulating hormone, lipid panel, echocardiogram, and a chest radiograph.
Results. The chest radiograph is negative for an acute cardiopulmonary process; cardiac size is normal. Aspartate aminotransferase and alanine aminotransferase levels are higher than twice the normal limit. The echocardiogram reveals an estimated left ventricular ejection fraction of 55% to 60%; no structural abnormalities are noted.
In which AF patients is catheter ablation indicated?
Ablation is recommended for select patients (TABLE 13,6) with symptomatic paroxysmal AF that is refractory to AAD or who are intolerant of AAD.3,6 It is a reasonable first-line therapy for high-performing athletes in whom AAD would affect athletic performance.3,10 It is also a reasonable option in select patients > 75 years and as an alternative to AAD therapy.3 Finally, catheter ablation should be considered in symptomatic patients with longstanding persistent AF and congestive heart failure, with or without reduced left ventricular ejection fraction.3
CASE
You inform Mr. Z that his symptoms are likely a result of symptomatic paroxysmal AF, which was refractory to flecainide and amiodarone, and that his abnormal liver function test results preclude continued use of amiodarone. You propose Holter monitoring to correlate timing of symptoms with the arrhythmia, but he reports this has been done, and the correlation confirmed, by his previous physician.
You explain that, because the diagnosis of symptomatic paroxysmal AF refractory to AADs has been confirmed, he is categorized as a patient who might benefit from catheter ablation, based on:
- the type of AF (ie, paroxysmal AF is associated with better ablation outcomes)
- persistent symptoms that are refractory to AADs
- his intolerance of AAD
- the length of time since onset of symptoms.
Mr. Z agrees to consider your recommendation.
Continue to: What are the benefits of catheter ablation?
What are the benefits of catheter ablation?
Ablation can be achieved through radiofrequency (RF) ablation, cryoablation, or newer, laser-based balloon ablation. Primary outcomes used to determine the success of any options for performing ablation include mortality, stroke, and hospitalization. Other endpoints include maintenance of sinus rhythm, freedom from AF, reduction in AF burden (estimated through patients’ report of symptoms, recurrence rate, need for a second ablation procedure, and serial long-term monitoring through an implantable cardiac monitoring device), quality of life, and prevention of AF progression.3
Patient and disease variables (TABLE 211-13). The success rate of catheter ablation, defined as freedom from either symptomatic or asymptomatic episodes of AF, is dependent on several factors,3,14 including:
- type of AF (paroxysmal or persistent)
- duration and degree of symptoms
- age
- sex
- comorbidities, including heart failure and structural heart or lung disease.
Overall, in patients with paroxysmal AF, an estimated 75% are symptom free 1 year after ablation.15 Patients with persistent and longstanding persistent AF experience a lower success rate.
RF catheter ablation has demonstrated superiority to AAD in reducing the need for cardioversion (relative risk [RR] = 0.62; 95% CI, 0.47-0.82) and cardiac-related hospitalization (RR = 0.27; 95% CI, 0.10-0.72;) at 12 months in patients with nonparoxysmal AF (persistent or longstanding persistent).16
Effect on mortality. Among patients with heart failure with reduced ejection fraction, long-term studies of cardiovascular outcomes 5 years post ablation concluded that ablation is associated with a decrease in all-cause mortality (RR = 0.69; 95% CI, 0.54-0.88; P = .003) and a reduction in hospitalization (RR = 0.62; 95% CI, 0.47-0.82; P = .0006); younger (< 65 years) and male patients derive greater benefit.6,17 Indications for ablation in patients with heart failure are similar to those in patients without heart failure; ablation can therefore be considered for select heart failure patients who remain symptomatic or for whom AAD has failed.3
Older patients. Ablation can be considered for patients > 75 years with symptomatic paroxysmal AF refractory to AAD or who are intolerant of AAD.3 A study that assessed the benefit of catheter ablation reviewed 587 older (> 75 years) patients with AF, of whom 324 were eligible for ablation. Endpoints were maintenance of sinus rhythm, stroke, death, and major bleeding. Return to normal sinus rhythm was an independent factor, associated with a decrease in the risk of mortality among all patient groups that underwent ablation (HR = 0.36; 95% CI, 0.2-0.63; P = .0005). Age > 75 years (HR = 1.09; 95% CI, 1.01-1.16; P > .02) and depressed ejection fraction < 40% (HR = 2.38; 95% CI, 1.28-4.4; P = .006) were determined to be unfavorable parameters for survival.18
Complications and risks
Complications of catheter ablation for AF, although infrequent, can be severe (TABLE 33). Early mortality, defined as death during initial admission or 30-day readmission, occurs in approximately 0.5% of cases; half of deaths take place during readmission.11
Continue to: Complications vary...
Complications vary, based on the type and site of ablation.19,20 Cardiac tamponade or perforation, the most life-threatening complications, taken together occur in an estimated 1.9% of patients (odds ratio [OR] = 2.98; 95% CI, 1.36-6.56; P = .007).11 Other in-hospital complications independently predictive of death include any cardiac complications (OR = 12.8; 95% CI, 6.86 to 23.8; P < .001) and neurologic complications (cerebrovascular accident and transient ischemic attack) (OR = 8.72; 95% CI, 2.71-28.1; P < .001).
Other complications that do not cause death but might prolong the hospital stay include pericarditis without effusion, anesthesia-related complications, and vascular-access complications. Patients whose ablation is performed at an institution where the volume of ablations is low are also at higher risk of early mortality (OR = 2.35; 95% CI, 1.33-4.15; P = .003).16
Recurrence is common (TABLE 211-13). Risk of recurrence following ablation is significant; early (within 3 months after ablation) recurrence is seen in 50% of patients.21,22 However, this is a so-called "blanking period"—ie, a temporary period of inflammatory and proarrhythmic changes that are not predictors of later recurrence. The 5-year post-ablation recurrence rate is approximately 25.5%; longstanding persistent and persistent AF and the presence of comorbidities are major risk factors for recurrence.13,23
Recurrence is also associated with the type of procedure; pulmonary vein isolation, alone or in combination with another type of procedure, results in higher long-term success.21,23
Other variables affect outcome (TABLE 211-13). Following AF ablation, patients with nonparoxysmal AF at baseline, advanced age, sleep apnea and obesity, left atrial enlargement, and any structural heart disease tend to have a poorer long-term (5-year) outcome (ie, freedom from extended episodes of AF).3,13,23,24
Patients who undergo repeat procedures have higher arrhythmia-free survival; the highest ablation success rate is for patients with paroxysmal AF.13,23
Exposure to ionizing radiation. Fluoroscopy is required for multiple components of atrial mapping and ablation during RF ablation, including navigation, visualization, and monitoring of catheter placement. Patients undergoing this particular procedure therefore receive significant exposure to ionizing radiation. A reduction in, even complete elimination of, fluoroscopy has been achieved with:
- nonfluoroscopic 3-dimensional mapping systems25
- intracardiac echocardiography, which utilizes ultrasonographic imaging as the primary visual mode for tracking and manipulating the catheter
- robotic guided navigation.26-28
Continue to: CASE
CASE
At his return visit, Mr. Z says that he is concerned about, first, undergoing catheter ablation at his age and, second, the risks associated with the procedure. You explain that it is true that ablation is ideal in younger patients who have minimal comorbidities and that the risk of complications increases with age—but that there is no cutoff or absolute age contraindication to ablation.
You tell Mr. Z that you will work with him on risk-factor modification in anticipation of ablation. You also assure him that the decision whether to ablate must be a joint one—between him and a cardiologist experienced both in electrophysiology and in performing this highly technical procedure. And you explain that a highly practiced specialist can identify Mr. Z’s risk factors that might make ablation more difficult to perform and affect the long-term outcome.
With Mr. Z’s agreement, you screen for sleep apnea and start him on a lifestyle modification plan to achieve a more ideal weight, explaining that the risk of recurrence of AF after catheter ablation is increased by obesity and sleep apnea, in addition to age. You explain that, based on his CHA2DS2–VASc (congestive heart failure; hypertension; age, ≥ 75 years; diabetes; prior stroke, transient ischemic attack, or thromboembolism; vascular disease; age, 65 to 74 years; sex category) score of 3, he will remain on anticoagulation whether or not he has the ablation.
You refer the patient to the nearest high-volume cardiac ablation center.
Last, you caution Mr. Z that, based on his lipid levels, his 10-year risk of heart disease or stroke is elevated. You recommend treatment with a statin agent while he continues his other medications.
Delivering energy to myocardium
Myocardial tissue in pulmonary veins is responsible for most ectopic electrical impulses in patients with drug-refractory AF. The goal of catheter ablation in AF is destruction (scarring) of tissue that is the source of abnormal vein potentials.15
How RF ablation works. Ablation is most commonly performed using RF energy, a high-frequency form of electrical energy. Electrophysiology studies are carried out at the time of ablation by percutaneous, fluoroscopically guided insertion of 2 to 5 catheters, usually through the femoral or internal jugular vein, which are then positioned within several areas of the heart—usually, the right atrium, bundle of His, right ventricle, and coronary sinus.
Continue to: Electrical current...
Electrical current is applied through the catheters from an external generator to stimulate the myocardium and thus determine its electrophysiologic properties. The anatomic and electrical activity of the left atrium and pulmonary veins is then identified, a technique known as electro-anatomical mapping (FIGURE). After arrhythmogenic myocardial tissue is mapped, ablation is carried out with RF energy through the catheter to the pathogenic myocardium from which arrhythmias are initiated or conducted. The result is thermal destruction of tissue and creation of small, shallow lesions that vary in size with the type of catheter and the force of contact pressure applied.3,29
Other energy sources used in catheter ablation include cryothermal energy, which utilizes liquid nitrous oxide under pressure through a cryocatheter or cryoballoon catheter. Application of cryothermal energy freezes tissue and disrupts cell membranes and any electrical activity. Cryoballoon ablation has been shown to be similarly safe and efficacious as RF ablation in patients with paroxysmal AF.30,31
Newer laser-based balloon ablations are performed under ultrasonographic guidance and utilize arcs of laser energy delivered to the pathogenic myocardium.3
Thromboembolism prophylaxis
Oral anticoagulation to decrease the risk of stroke is initiated in all patients with AF, based on a thromboembolic risk profile determined by their CHA2DS2–VASc score, with anticoagulation recommended when the score is ≥ 2 in men and ≥ 3 in women. Options for anticoagulation include warfarin and one of the novel oral anticoagulants dabigatran, apixaban, rivaroxaban, and edoxaban.4 Recommendations are as follows3:
- For patients with a CHA2DS2–VASc score of ≥ 2 (men) or ≥ 3 (women), anticoagulation should be continued indefinitely, regardless of how successful the ablation procedure is.
- When patients choose to discontinue anticoagulation, they should be counseled in detail about the risk of doing so. The continued need for frequent arrhythmia monitoring should be emphasized.
The route from primary careto catheter ablation
Perform a thorough evaluation. Patients who present to you with palpitations should first undergo a routine workup for AF, followed by confirmation of the diagnosis. Exclude structural heart disease with echocardiography. Undertake monitoring, which is essential to determine whether symptoms are a reflection of the arrhythmia, using noncontinuous or continuous electrocardiographic (EKG) monitoring. Noncontinuous detection devices include:
- scheduled or symptom-initiated EKG
- a Holter monitor, worn for at least 24 hours and as long as 7 days
- trans-telephonic recordings and patient- or automatically activated devices
- an external loop recorder.32
Continuous EKG monitoring is more permanent (≥ 12 months). This is usually achieved through an implantable loop powered by a battery that lasts as long as 3 years.3
Ablation: Yes or no? Ablation is not recommended to avoid anticoagulation or when anticoagulation is contraindicated.5 With regard to specific patient criteria, the ideal patient:
- is symptomatic
- has failed AAD therapy
- does not have pulmonary disease
- has a normal or mildly dilated left atrium or normal or mildly reduced left ventricular ejection fraction.5
Continue to: There is no absolute age...
There is no absolute age or comorbidity contraindication to ablation. The patient should be referred to a cardiologist who has received appropriate training in electrophysiology, to identify comorbidities that (1) increase the technical difficulty of the procedure and baseline risk and (2) affect long-term outcome,12 and who performs the procedure in a center that has considerable experience with catheter ablation.33
Once the decision is made to perform ablation, you can provide strategies that optimize the outcome (freedom from AF episodes). Those tactics include weight loss and screening evaluation and, if indicated, treatment for sleep apnea.3
Protocol. Prior to the procedure, the patient fasts overnight; they might be asked to taper or discontinue cardiac medications that have electrophysiologic effects. Studies suggest a low risk of bleeding associated with catheter ablation; anticoagulation should therefore continue uninterrupted for patients undergoing catheter ablation for AF3,4,34,35; however, this practice varies with the cardiologist or electrophysiologist performing ablation.
Because of the length and complexity of the procedure, electro-anatomical mapping and ablation are conducted with the patient under general anesthesia.3 The patient is kept supine, and remains so for 2 to 4 hours afterward to allow for hemostasis at puncture sites.3
Patients might be monitored overnight, although same-day catheter ablation has been shown to be safe and cost-effective in select patients.36,37 Post ablation, patients follow up with the cardiologist and electrophysiologist. Long-term arrhythmia monitoring is required.3 Anticoagulation is continued for at least 2 months, and is discontinued based on the patient’s risk for stroke, utilizing their CHA2DS2–VASc score.3,4
CASE
At Mr. Z’s 6-month primary care follow-up, he confirms what has been reported to you as the referring physician: He had a successful catheter ablation and continues to have regular follow-up monitoring with the cardiologist. He is no longer taking amiodarone.
At this visit, he reports no recurrence of AF-associated symptoms or detectable AF on cardiac monitoring. He has lost 8 lbs. You counsel to him to continue to maintain a healthy lifestyle.
CORRESPONDENCE
Amimi S. Osayande MD, FAAFP, Northside-Gwinnett Family Medicine Residency Program, Strickland Family Medicine Center, 665 Duluth Highway, Suite 501, Lawrenceville, GA 30046; [email protected]
CASE
Jack Z, a 75-year-old man with well-controlled hypertension, diabetes controlled by diet, and atrial fibrillation (AF) presents to the family medicine clinic to establish care with you after moving to the community from out of town.
The patient describes a 1-year history of AF. He provides you with an echocardiography report from 6 months ago that shows no evidence of structural heart disease. He takes lisinopril, to control blood pressure; an anticoagulant; a beta-blocker; and amiodarone for rhythm control. Initially, he took flecainide, which was ineffective for rhythm control, before being switched to amiodarone. He had 2 cardioversion procedures, each time after episodes of symptoms. He does not smoke or drink alcohol.
Mr. Z describes worsening palpitations and shortness of breath over the past 9 months. Symptoms now include episodes of exertional fatigue, even when he is not having palpitations. Prior to the episodes of worsening symptoms, he tells you that he lived a “fairly active” life, golfing twice a week.
The patient’s previous primary care physician had encouraged him to talk to his cardiologist about “other options” for managing AF, because levels of his liver enzymes had started to rise (a known adverse effect of amiodarone1) when measured 3 months ago. He did not undertake that conversation, but asks you now about other treatments for AF.
Atrial fibrillation is the most common sustained cardiac arrhythmia, characterized by discordant electrical activation of the atria due to structural or electrophysiological abnormalities, or both. The disorder is associated with an increased rate of stroke and heart failure and is independently associated with a 1.5- to 2-fold risk of all-cause mortality.2
In this article, we review the pathophysiology of AF; management, including the role of, and indications for, catheter ablation; and patient- and disease-related factors associated with ablation (including odds of success, complications, risk of recurrence, and continuing need for thromboprophylaxis) that family physicians should consider when contemplating referral to a cardiologist or electrophysiologist for catheter ablation for AF.
What provokes AF?
AF is thought to occur as a result of an interaction among 3 phenomena:
- enhanced automaticity of abnormal atrial tissue
- triggered activity of ectopic foci within 1 or more pulmonary veins, lying within the left atrium
- re-entry, in which there is propagation of electrical impulses from an ectopic beat through another pathway.
Continue to: In patients who progress...
In patients who progress from paroxysmal to persistent AF (see “Subtypes,” below), 2 distinct pathways, facilitated by the presence of abnormal tissue, continuously activate one another, thus maintaining the arrhythmia. Myocardial tissue in the pulmonary veins is responsible for most ectopic electrical impulses in patients with drug-refractory AF (see “Rhythm control”).
Subtypes. For the purpose of planning treatment, AF is classified as:
- Paroxysmal. Terminates spontaneously or with intervention ≤ 7 days after onset.
- Persistent. Continuous and sustained for > 7 days.
- Longstanding persistent. Continuous for > 12 months.
- Permanent. The patient and physician accept that there will be no further attempt to restore or maintain sinus rhythm.
Goals of treatment
Primary management goals in patients with AF are 2-fold: control of symptoms and prevention of thromboembolism. A patient with new-onset AF who presents acutely with inadequate rate control and hemodynamic compromise requires urgent assessment to determine the cause of the arrhythmia and need for cardioversion.3 A symptomatic patient with AF who does not have high-risk features (eg, valvular heart disease, mechanical valves) might be a candidate for rhythm control in addition to rate control.3,4
Rate control. After evaluation in the hospital, a patient who has a rapid ventricular response but remains hemodynamically stable, without evidence of heart failure, should be initiated on a rate-controlling medication, such as a beta-blocker or nondihydropyridine calcium-channel blocker. A resting heart rate goal of < 80 beats per minute (bpm) is recommended for a symptomatic patient with AF. The heart rate goal can be relaxed, to < 110 bpm, in an asymptomatic patient with preserved left ventricular function.5,6
Rhythm control, indicated in patients who remain symptomatic on rate-controlling medication, can be achieved either with an antiarrhythmic drug (AAD) or by catheter ablation.4,5 In stable patients, rhythm control should be considered only after a thorough work-up for a reversible cause of AF, and can be achieved with an oral AAD or, in select patients, through catheter ablation (TABLE 13,6). Other indications for chronic rhythm control include treatment of patients with tachycardia-induced cardiomyopathy.5
A major study that documented the benefit of early rhythm control evaluated long-term outcomes in 2789 patients with AF who were undergoing catheter ablation.7 Patients were randomized to early rhythm control (catheter ablation or AAD) or “usual care”—ie, in this study, rhythm control limited to symptomatic patients. Primary outcomes were death from cardiovascular causes, stroke, and hospitalization with worsening heart failure or acute coronary syndrome. A first primary outcome event occurred in 249 patients (3.9/100 person-years) assigned to early rhythm control, compared to 316 (5.0 per 100 person-years) in the group assigned to usual care.
The study was terminated early (after 5.1 years) because of overwhelming evidence of efficacy (number need to treat = 7). Although early rhythm control was obtained through both catheter ablation and AAD (hazard ratio [HR] = 0.79; 96% CI, 0.66-0.94; P = .005), success was attributed to the use of catheter ablation for a rhythm-control strategy and its use among patients whose AF was present for < 1 year. Most patients in both treatment groups continued to receive anticoagulation, rate control, and optimization of cardiovascular risk.7
Continue to: Notably, direct studies...
Notably, direct studies comparing ablation and AAD have not confirmed the benefit of ablation over AAD in outcomes of all-cause mortality, bleeding, stroke, or cardiac arrest over a 5-year period.8
Adverse effects and mortality outcomes with AAD. Concern over using AAD for rhythm control is based mostly on adverse effects and long-term (1-year) mortality outcomes. Long-term AAD therapy has been shown to decrease the recurrence of AF—but without evidence to suggest other mortality benefits.
A meta-analysis of 59 randomized controlled trials reviewed 20,981 patients receiving AAD (including quinidine, disopyramide, propafenone, flecainide, metoprolol, amiodarone, dofetilide, dronedarone, and sotalol) for long-term effects on death, stroke, adverse reactions, and recurrence of AF.9 Findings at 10 months suggest that:
- Compared to placebo, amiodarone and sotalol increased the risk of all-cause mortality during the study period.
- There was minimal difference in mortality among patients taking dofetilide or dronedarone, compared to placebo.
- There were insufficient data to draw conclusions about the effect of disopyramide, flecainide, and propafenone on mortality.
Before starting a patient on AAD, the risk of arrhythmias and the potential for these agents to cause toxicity and adverse events should always be discussed.
CASE
You tell Mr. Z that you need to know the status of his comorbidities to make a recommendation about “other” management options, and proceed to take a detailed history.
Recent history. Mr. Z reveals that “today is a good day”: He has had “only 1” episode of palpitations, which resolved on its own. The previous episode, he explains, was 3 days ago, when palpitations were associated with lightheadedness and shortness of breath. He denies chest pains or swelling of the legs.
Physical exam. The patient appears spry, comfortable, and in no acute distress. Vital signs are within normal limits. A body mass index of 28.4 puts him in the “overweight” category. His blood pressure is 118/75 mm Hg.
Continue to: Cardiac examination...
Cardiac examination is significant for an irregular rhythm without murmurs, rubs, or gallops. His lungs are clear bilaterally; his abdomen is soft and nondistended. His extremities show no edema.
Testing. You obtain an electrocardiogram, which demonstrates a controlled ventricular rate of 88 bpm and AF. You order a complete blood count, comprehensive metabolic panel, tests of hemoglobin A1C and thyroid-stimulating hormone, lipid panel, echocardiogram, and a chest radiograph.
Results. The chest radiograph is negative for an acute cardiopulmonary process; cardiac size is normal. Aspartate aminotransferase and alanine aminotransferase levels are higher than twice the normal limit. The echocardiogram reveals an estimated left ventricular ejection fraction of 55% to 60%; no structural abnormalities are noted.
In which AF patients is catheter ablation indicated?
Ablation is recommended for select patients (TABLE 13,6) with symptomatic paroxysmal AF that is refractory to AAD or who are intolerant of AAD.3,6 It is a reasonable first-line therapy for high-performing athletes in whom AAD would affect athletic performance.3,10 It is also a reasonable option in select patients > 75 years and as an alternative to AAD therapy.3 Finally, catheter ablation should be considered in symptomatic patients with longstanding persistent AF and congestive heart failure, with or without reduced left ventricular ejection fraction.3
CASE
You inform Mr. Z that his symptoms are likely a result of symptomatic paroxysmal AF, which was refractory to flecainide and amiodarone, and that his abnormal liver function test results preclude continued use of amiodarone. You propose Holter monitoring to correlate timing of symptoms with the arrhythmia, but he reports this has been done, and the correlation confirmed, by his previous physician.
You explain that, because the diagnosis of symptomatic paroxysmal AF refractory to AADs has been confirmed, he is categorized as a patient who might benefit from catheter ablation, based on:
- the type of AF (ie, paroxysmal AF is associated with better ablation outcomes)
- persistent symptoms that are refractory to AADs
- his intolerance of AAD
- the length of time since onset of symptoms.
Mr. Z agrees to consider your recommendation.
Continue to: What are the benefits of catheter ablation?
What are the benefits of catheter ablation?
Ablation can be achieved through radiofrequency (RF) ablation, cryoablation, or newer, laser-based balloon ablation. Primary outcomes used to determine the success of any options for performing ablation include mortality, stroke, and hospitalization. Other endpoints include maintenance of sinus rhythm, freedom from AF, reduction in AF burden (estimated through patients’ report of symptoms, recurrence rate, need for a second ablation procedure, and serial long-term monitoring through an implantable cardiac monitoring device), quality of life, and prevention of AF progression.3
Patient and disease variables (TABLE 211-13). The success rate of catheter ablation, defined as freedom from either symptomatic or asymptomatic episodes of AF, is dependent on several factors,3,14 including:
- type of AF (paroxysmal or persistent)
- duration and degree of symptoms
- age
- sex
- comorbidities, including heart failure and structural heart or lung disease.
Overall, in patients with paroxysmal AF, an estimated 75% are symptom free 1 year after ablation.15 Patients with persistent and longstanding persistent AF experience a lower success rate.
RF catheter ablation has demonstrated superiority to AAD in reducing the need for cardioversion (relative risk [RR] = 0.62; 95% CI, 0.47-0.82) and cardiac-related hospitalization (RR = 0.27; 95% CI, 0.10-0.72;) at 12 months in patients with nonparoxysmal AF (persistent or longstanding persistent).16
Effect on mortality. Among patients with heart failure with reduced ejection fraction, long-term studies of cardiovascular outcomes 5 years post ablation concluded that ablation is associated with a decrease in all-cause mortality (RR = 0.69; 95% CI, 0.54-0.88; P = .003) and a reduction in hospitalization (RR = 0.62; 95% CI, 0.47-0.82; P = .0006); younger (< 65 years) and male patients derive greater benefit.6,17 Indications for ablation in patients with heart failure are similar to those in patients without heart failure; ablation can therefore be considered for select heart failure patients who remain symptomatic or for whom AAD has failed.3
Older patients. Ablation can be considered for patients > 75 years with symptomatic paroxysmal AF refractory to AAD or who are intolerant of AAD.3 A study that assessed the benefit of catheter ablation reviewed 587 older (> 75 years) patients with AF, of whom 324 were eligible for ablation. Endpoints were maintenance of sinus rhythm, stroke, death, and major bleeding. Return to normal sinus rhythm was an independent factor, associated with a decrease in the risk of mortality among all patient groups that underwent ablation (HR = 0.36; 95% CI, 0.2-0.63; P = .0005). Age > 75 years (HR = 1.09; 95% CI, 1.01-1.16; P > .02) and depressed ejection fraction < 40% (HR = 2.38; 95% CI, 1.28-4.4; P = .006) were determined to be unfavorable parameters for survival.18
Complications and risks
Complications of catheter ablation for AF, although infrequent, can be severe (TABLE 33). Early mortality, defined as death during initial admission or 30-day readmission, occurs in approximately 0.5% of cases; half of deaths take place during readmission.11
Continue to: Complications vary...
Complications vary, based on the type and site of ablation.19,20 Cardiac tamponade or perforation, the most life-threatening complications, taken together occur in an estimated 1.9% of patients (odds ratio [OR] = 2.98; 95% CI, 1.36-6.56; P = .007).11 Other in-hospital complications independently predictive of death include any cardiac complications (OR = 12.8; 95% CI, 6.86 to 23.8; P < .001) and neurologic complications (cerebrovascular accident and transient ischemic attack) (OR = 8.72; 95% CI, 2.71-28.1; P < .001).
Other complications that do not cause death but might prolong the hospital stay include pericarditis without effusion, anesthesia-related complications, and vascular-access complications. Patients whose ablation is performed at an institution where the volume of ablations is low are also at higher risk of early mortality (OR = 2.35; 95% CI, 1.33-4.15; P = .003).16
Recurrence is common (TABLE 211-13). Risk of recurrence following ablation is significant; early (within 3 months after ablation) recurrence is seen in 50% of patients.21,22 However, this is a so-called "blanking period"—ie, a temporary period of inflammatory and proarrhythmic changes that are not predictors of later recurrence. The 5-year post-ablation recurrence rate is approximately 25.5%; longstanding persistent and persistent AF and the presence of comorbidities are major risk factors for recurrence.13,23
Recurrence is also associated with the type of procedure; pulmonary vein isolation, alone or in combination with another type of procedure, results in higher long-term success.21,23
Other variables affect outcome (TABLE 211-13). Following AF ablation, patients with nonparoxysmal AF at baseline, advanced age, sleep apnea and obesity, left atrial enlargement, and any structural heart disease tend to have a poorer long-term (5-year) outcome (ie, freedom from extended episodes of AF).3,13,23,24
Patients who undergo repeat procedures have higher arrhythmia-free survival; the highest ablation success rate is for patients with paroxysmal AF.13,23
Exposure to ionizing radiation. Fluoroscopy is required for multiple components of atrial mapping and ablation during RF ablation, including navigation, visualization, and monitoring of catheter placement. Patients undergoing this particular procedure therefore receive significant exposure to ionizing radiation. A reduction in, even complete elimination of, fluoroscopy has been achieved with:
- nonfluoroscopic 3-dimensional mapping systems25
- intracardiac echocardiography, which utilizes ultrasonographic imaging as the primary visual mode for tracking and manipulating the catheter
- robotic guided navigation.26-28
Continue to: CASE
CASE
At his return visit, Mr. Z says that he is concerned about, first, undergoing catheter ablation at his age and, second, the risks associated with the procedure. You explain that it is true that ablation is ideal in younger patients who have minimal comorbidities and that the risk of complications increases with age—but that there is no cutoff or absolute age contraindication to ablation.
You tell Mr. Z that you will work with him on risk-factor modification in anticipation of ablation. You also assure him that the decision whether to ablate must be a joint one—between him and a cardiologist experienced both in electrophysiology and in performing this highly technical procedure. And you explain that a highly practiced specialist can identify Mr. Z’s risk factors that might make ablation more difficult to perform and affect the long-term outcome.
With Mr. Z’s agreement, you screen for sleep apnea and start him on a lifestyle modification plan to achieve a more ideal weight, explaining that the risk of recurrence of AF after catheter ablation is increased by obesity and sleep apnea, in addition to age. You explain that, based on his CHA2DS2–VASc (congestive heart failure; hypertension; age, ≥ 75 years; diabetes; prior stroke, transient ischemic attack, or thromboembolism; vascular disease; age, 65 to 74 years; sex category) score of 3, he will remain on anticoagulation whether or not he has the ablation.
You refer the patient to the nearest high-volume cardiac ablation center.
Last, you caution Mr. Z that, based on his lipid levels, his 10-year risk of heart disease or stroke is elevated. You recommend treatment with a statin agent while he continues his other medications.
Delivering energy to myocardium
Myocardial tissue in pulmonary veins is responsible for most ectopic electrical impulses in patients with drug-refractory AF. The goal of catheter ablation in AF is destruction (scarring) of tissue that is the source of abnormal vein potentials.15
How RF ablation works. Ablation is most commonly performed using RF energy, a high-frequency form of electrical energy. Electrophysiology studies are carried out at the time of ablation by percutaneous, fluoroscopically guided insertion of 2 to 5 catheters, usually through the femoral or internal jugular vein, which are then positioned within several areas of the heart—usually, the right atrium, bundle of His, right ventricle, and coronary sinus.
Continue to: Electrical current...
Electrical current is applied through the catheters from an external generator to stimulate the myocardium and thus determine its electrophysiologic properties. The anatomic and electrical activity of the left atrium and pulmonary veins is then identified, a technique known as electro-anatomical mapping (FIGURE). After arrhythmogenic myocardial tissue is mapped, ablation is carried out with RF energy through the catheter to the pathogenic myocardium from which arrhythmias are initiated or conducted. The result is thermal destruction of tissue and creation of small, shallow lesions that vary in size with the type of catheter and the force of contact pressure applied.3,29
Other energy sources used in catheter ablation include cryothermal energy, which utilizes liquid nitrous oxide under pressure through a cryocatheter or cryoballoon catheter. Application of cryothermal energy freezes tissue and disrupts cell membranes and any electrical activity. Cryoballoon ablation has been shown to be similarly safe and efficacious as RF ablation in patients with paroxysmal AF.30,31
Newer laser-based balloon ablations are performed under ultrasonographic guidance and utilize arcs of laser energy delivered to the pathogenic myocardium.3
Thromboembolism prophylaxis
Oral anticoagulation to decrease the risk of stroke is initiated in all patients with AF, based on a thromboembolic risk profile determined by their CHA2DS2–VASc score, with anticoagulation recommended when the score is ≥ 2 in men and ≥ 3 in women. Options for anticoagulation include warfarin and one of the novel oral anticoagulants dabigatran, apixaban, rivaroxaban, and edoxaban.4 Recommendations are as follows3:
- For patients with a CHA2DS2–VASc score of ≥ 2 (men) or ≥ 3 (women), anticoagulation should be continued indefinitely, regardless of how successful the ablation procedure is.
- When patients choose to discontinue anticoagulation, they should be counseled in detail about the risk of doing so. The continued need for frequent arrhythmia monitoring should be emphasized.
The route from primary careto catheter ablation
Perform a thorough evaluation. Patients who present to you with palpitations should first undergo a routine workup for AF, followed by confirmation of the diagnosis. Exclude structural heart disease with echocardiography. Undertake monitoring, which is essential to determine whether symptoms are a reflection of the arrhythmia, using noncontinuous or continuous electrocardiographic (EKG) monitoring. Noncontinuous detection devices include:
- scheduled or symptom-initiated EKG
- a Holter monitor, worn for at least 24 hours and as long as 7 days
- trans-telephonic recordings and patient- or automatically activated devices
- an external loop recorder.32
Continuous EKG monitoring is more permanent (≥ 12 months). This is usually achieved through an implantable loop powered by a battery that lasts as long as 3 years.3
Ablation: Yes or no? Ablation is not recommended to avoid anticoagulation or when anticoagulation is contraindicated.5 With regard to specific patient criteria, the ideal patient:
- is symptomatic
- has failed AAD therapy
- does not have pulmonary disease
- has a normal or mildly dilated left atrium or normal or mildly reduced left ventricular ejection fraction.5
Continue to: There is no absolute age...
There is no absolute age or comorbidity contraindication to ablation. The patient should be referred to a cardiologist who has received appropriate training in electrophysiology, to identify comorbidities that (1) increase the technical difficulty of the procedure and baseline risk and (2) affect long-term outcome,12 and who performs the procedure in a center that has considerable experience with catheter ablation.33
Once the decision is made to perform ablation, you can provide strategies that optimize the outcome (freedom from AF episodes). Those tactics include weight loss and screening evaluation and, if indicated, treatment for sleep apnea.3
Protocol. Prior to the procedure, the patient fasts overnight; they might be asked to taper or discontinue cardiac medications that have electrophysiologic effects. Studies suggest a low risk of bleeding associated with catheter ablation; anticoagulation should therefore continue uninterrupted for patients undergoing catheter ablation for AF3,4,34,35; however, this practice varies with the cardiologist or electrophysiologist performing ablation.
Because of the length and complexity of the procedure, electro-anatomical mapping and ablation are conducted with the patient under general anesthesia.3 The patient is kept supine, and remains so for 2 to 4 hours afterward to allow for hemostasis at puncture sites.3
Patients might be monitored overnight, although same-day catheter ablation has been shown to be safe and cost-effective in select patients.36,37 Post ablation, patients follow up with the cardiologist and electrophysiologist. Long-term arrhythmia monitoring is required.3 Anticoagulation is continued for at least 2 months, and is discontinued based on the patient’s risk for stroke, utilizing their CHA2DS2–VASc score.3,4
CASE
At Mr. Z’s 6-month primary care follow-up, he confirms what has been reported to you as the referring physician: He had a successful catheter ablation and continues to have regular follow-up monitoring with the cardiologist. He is no longer taking amiodarone.
At this visit, he reports no recurrence of AF-associated symptoms or detectable AF on cardiac monitoring. He has lost 8 lbs. You counsel to him to continue to maintain a healthy lifestyle.
CORRESPONDENCE
Amimi S. Osayande MD, FAAFP, Northside-Gwinnett Family Medicine Residency Program, Strickland Family Medicine Center, 665 Duluth Highway, Suite 501, Lawrenceville, GA 30046; [email protected]
1. Amiodarone hydrochloride (marketed as Cordarone and Pacerone) information. Silver Spring, Md.: US Food & Drug Administration. Reviewed March 23, 2015. Accessed January 16, 2022. www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/amiodarone-hydrochloride-marketed-cordarone-and-pacerone-information
2. Gómez-Outes A, Suárez-Gea ML,García-Pinilla JM. Causes of death in atrial fibrillation: challenges and opportunities. Trends Cardiovasc Med. 2017;27:494-503. doi: 10.1016/j.tcm.2017.05.002
3. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/ APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Arrhythm. 2017;33:369-409. doi: 10.1016/j.joa.2017.08.001
4. Camm AJ, Lip GYH, De Caterina R, et al; ESC Committee for Practice Guidelines-CPG; Document Reviewers. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation—developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14:1385-1413. doi: 10.1093/europace/eus305
5. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071-2104. doi: 10.1161/CIR.0000000000000040
6. January CT, Wann LS, Calkins H, et al; Writing Group Members. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/ HRS guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019;16:e66-e93. doi: 10.1016/j.hrthm.2019.01.024
7. Kirchhof P, Camm AJ, Goette A, et al; EAST-AFNET 4 Trial Investigators. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305-1316. doi: 10.1056/ NEJMoa2019422
8. Packer DL, Mark DB, Robb RA, et al; CABANA Investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261-1274. doi: 10.1001/jama.2019.0693
9. Valembois L, Audureau E, Takeda A, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2019;9:CD005049. doi: 10.1002/14651858.CD005049
10. Koopman P, Nuyens D, Garweg C, et al. Efficacy of radiofrequency catheter ablation in athletes with atrial fibrillation. Europace. 2011;13:1386-1393. doi: 10.1093/europace/eur142
11. Hakalahti A, Biancari F, Nielsen JC, et al. Radiofrequency ablation vs. antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: systematic review and meta-analysis. Europace. 2015;17:370-378. doi: 10.1093/europace/euu376
12. Nyong J, Amit G, Adler AJ, et al. Efficacy and safety of ablation for people with non-paroxysmal atrial fibrillation. Cochrane Database Syst Rev. 2016;11:CD012088. doi: 10.1002/14651858. CD012088.pub2
13. Andrade JG, Champagne J, Dubuc M, et al; CIRCA-DOSE Study Investigators. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. 2019;140:1779-1788. doi: 10.1161/ CIRCULATIONAHA.119.042622
14. Asad ZUA, Yousif A, Khan MS, et al. Catheter ablation versus medical therapy for atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. Circ Arrhythm Electrophysiol. 2019;12:e007414. doi: 10.1161/ CIRCEP.119.007414
15. Nademanee K, Amnueypol M, Lee F, et al. Benefits and risks of catheter ablation in elderly patients with atrial fibrillation. Heart Rhythm. 2015;12:44-51. doi: 10.1016/j.hrthm.2014.09.049
16. Cheng EP, Liu CF, Yeo I, et al. Risk of mortality following catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2019;74: 2254-2264. doi: 10.1016/j.jacc.2019.08.1036
17. Brugada J, Katritsis DG, Arbelo E, et al; ESC Scientific Document Group. 2019 ESC Guidelines for the management of patients with supraventricular tachycardia. The Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Developed in collaboration with the Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2020;41:655-720. doi: 10.1093/eurheartj/ehz467
18. Hosseini SM, Rozen G, Saleh A, et al. Catheter ablation for cardiac arrhythmias: utilization and in-hospital complications, 2000 to 2013. JACC Clin Electrophysiol. 2017;3:1240-1248. doi: 10.1016/j.jacep.2017.05.005
19. Andrade JG, Macle L, Khairy P, et al. Incidence and significance of early recurrences associated with different ablation strategies for AF: a STAR-AF substudy. J Cardiovasc Electrophysiol. 2012;23:1295-1301. doi: 10.1111/j.1540-8167.2012.02399.x
20. Joshi S, Choi AD, Kamath GS, et al. Prevalence, predictors, and prognosis of atrial fibrillation early after pulmonary vein isolation: findings from 3 months of continuous automatic ECG loop recordings. J Cardiovasc Electrophysiol. 2009;20:1089-1094. doi: 10.1111/j.1540-8167.2009.01506.x
21. Weerasooriya R, Khairy P, Litalien J, et al. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160-166. doi: 10.1016/j.jacc.2010.05.061
22. Ouyang F, Tilz R, Chun J, et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation. 2010;122:2368-2377. doi: 10.1161/ CIRCULATIONAHA.110.946806
23. Tilz RR, Rillig A, Thum A-M, et al. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J Am Coll Cardiol. 2012;60: 1921-1929. doi: 10.1016/j.jacc.2012.04.060
24. Forkmann M, Schwab C, Busch S. [Catheter ablation of supraventricular tachycardia]. Herzschrittmacherther Elektrophysiol. 2019;30:336-342. doi: 10.1007/s00399-019-00654-x
25. Bulava A, Hanis J, Eisenberger M. Catheter ablation of atrial fibrillation using zero-fluoroscopy technique: a randomized trial. Pacing Clin Electrophysiol. 2015;38:797-806. doi: 10.1111/pace.12634
26. Haegeli LM, Stutz L, Mohsen M, et al. Feasibility of zero or near zero fluoroscopy during catheter ablation procedures. Cardiol J. 2019;26:226-232. doi: 10.5603/CJ.a2018.0029
27. Steven D, Servatius H, Rostock T, et al. Reduced fluoroscopy during atrial fibrillation ablation: benefits of robotic guided navigation. J Cardiovasc Electrophysiol. 2010;21:6-12. doi: 10.1111/j.1540-8167.2009.01592.x
28. General therapy for cardiac arrhythmias. In: Zipes DP, Libby P, Bonow RO, et al. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 11th ed. Elsevier; 2019.
29. Kuck K-H, Brugada J, Albenque J-P. Cryoballoon or radiofrequency ablation for atrial fibrillation. N Engl J Med. 2016;375: 1100-1101. doi: 10.1056/NEJMc1609160
30. Chen Y-H, Lu Z-Y, Xiang Y, et al. Cryoablation vs. radiofrequency ablation for treatment of paroxysmal atrial fibrillation: a systematic review and meta-analysis. Europace. 2017;19:784-794. doi: 10.1093/europace/euw330
31. Locati ET, Vecchi AM, Vargiu S, et al. Role of extended external loop recorders for the diagnosis of unexplained syncope, presyncope, and sustained palpitations. Europace. 2014;16:914-922. doi: 10.1093/europace/eut337
32. Calkins H, Kuck KH, Cappato R, et al; Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2012;9:632-696.e21. doi: 10.1016/j.hrthm.2011.12.016
33. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609-1678. doi: 10.1093/ europace/euw295
34. Nairooz R, Sardar P, Payne J, et al. Meta-analysis of major bleeding with uninterrupted warfarin compared to interrupted warfarin and heparin bridging in ablation of atrial fibrillation. Int J Cardiol. 2015;187:426-429. doi: 10.1016/j.ijcard.2015.03.376
35. Romero J, Cerrud-Rodriguez RC, Diaz JC, et al. Uninterrupted direct oral anticoagulants vs. uninterrupted vitamin K antagonists during catheter ablation of non-valvular atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. Europace. 2018;20:1612-1620. doi: 10.1093/europace/euy133
36. Deyell MW, Leather RA, Macle L, et al. Efficacy and safety of same-day discharge for atrial fibrillation ablation. JACC Clin Electrophysiol. 2020;6:609-619. doi: 10.1016/j.jacep.2020.02.009
37. Theodoreson MD, Chohan BC, McAloon CJ, et al. Same-day cardiac catheter ablation is safe and cost-effective: experience from a UK tertiary center. Heart Rhythm. 2015;12:1756-1761. doi: 10.1016/j.hrthm.2015.05.006
1. Amiodarone hydrochloride (marketed as Cordarone and Pacerone) information. Silver Spring, Md.: US Food & Drug Administration. Reviewed March 23, 2015. Accessed January 16, 2022. www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/amiodarone-hydrochloride-marketed-cordarone-and-pacerone-information
2. Gómez-Outes A, Suárez-Gea ML,García-Pinilla JM. Causes of death in atrial fibrillation: challenges and opportunities. Trends Cardiovasc Med. 2017;27:494-503. doi: 10.1016/j.tcm.2017.05.002
3. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/ APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Arrhythm. 2017;33:369-409. doi: 10.1016/j.joa.2017.08.001
4. Camm AJ, Lip GYH, De Caterina R, et al; ESC Committee for Practice Guidelines-CPG; Document Reviewers. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation—developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14:1385-1413. doi: 10.1093/europace/eus305
5. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071-2104. doi: 10.1161/CIR.0000000000000040
6. January CT, Wann LS, Calkins H, et al; Writing Group Members. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/ HRS guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019;16:e66-e93. doi: 10.1016/j.hrthm.2019.01.024
7. Kirchhof P, Camm AJ, Goette A, et al; EAST-AFNET 4 Trial Investigators. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305-1316. doi: 10.1056/ NEJMoa2019422
8. Packer DL, Mark DB, Robb RA, et al; CABANA Investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261-1274. doi: 10.1001/jama.2019.0693
9. Valembois L, Audureau E, Takeda A, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2019;9:CD005049. doi: 10.1002/14651858.CD005049
10. Koopman P, Nuyens D, Garweg C, et al. Efficacy of radiofrequency catheter ablation in athletes with atrial fibrillation. Europace. 2011;13:1386-1393. doi: 10.1093/europace/eur142
11. Hakalahti A, Biancari F, Nielsen JC, et al. Radiofrequency ablation vs. antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: systematic review and meta-analysis. Europace. 2015;17:370-378. doi: 10.1093/europace/euu376
12. Nyong J, Amit G, Adler AJ, et al. Efficacy and safety of ablation for people with non-paroxysmal atrial fibrillation. Cochrane Database Syst Rev. 2016;11:CD012088. doi: 10.1002/14651858. CD012088.pub2
13. Andrade JG, Champagne J, Dubuc M, et al; CIRCA-DOSE Study Investigators. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. 2019;140:1779-1788. doi: 10.1161/ CIRCULATIONAHA.119.042622
14. Asad ZUA, Yousif A, Khan MS, et al. Catheter ablation versus medical therapy for atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. Circ Arrhythm Electrophysiol. 2019;12:e007414. doi: 10.1161/ CIRCEP.119.007414
15. Nademanee K, Amnueypol M, Lee F, et al. Benefits and risks of catheter ablation in elderly patients with atrial fibrillation. Heart Rhythm. 2015;12:44-51. doi: 10.1016/j.hrthm.2014.09.049
16. Cheng EP, Liu CF, Yeo I, et al. Risk of mortality following catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2019;74: 2254-2264. doi: 10.1016/j.jacc.2019.08.1036
17. Brugada J, Katritsis DG, Arbelo E, et al; ESC Scientific Document Group. 2019 ESC Guidelines for the management of patients with supraventricular tachycardia. The Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Developed in collaboration with the Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2020;41:655-720. doi: 10.1093/eurheartj/ehz467
18. Hosseini SM, Rozen G, Saleh A, et al. Catheter ablation for cardiac arrhythmias: utilization and in-hospital complications, 2000 to 2013. JACC Clin Electrophysiol. 2017;3:1240-1248. doi: 10.1016/j.jacep.2017.05.005
19. Andrade JG, Macle L, Khairy P, et al. Incidence and significance of early recurrences associated with different ablation strategies for AF: a STAR-AF substudy. J Cardiovasc Electrophysiol. 2012;23:1295-1301. doi: 10.1111/j.1540-8167.2012.02399.x
20. Joshi S, Choi AD, Kamath GS, et al. Prevalence, predictors, and prognosis of atrial fibrillation early after pulmonary vein isolation: findings from 3 months of continuous automatic ECG loop recordings. J Cardiovasc Electrophysiol. 2009;20:1089-1094. doi: 10.1111/j.1540-8167.2009.01506.x
21. Weerasooriya R, Khairy P, Litalien J, et al. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160-166. doi: 10.1016/j.jacc.2010.05.061
22. Ouyang F, Tilz R, Chun J, et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation. 2010;122:2368-2377. doi: 10.1161/ CIRCULATIONAHA.110.946806
23. Tilz RR, Rillig A, Thum A-M, et al. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J Am Coll Cardiol. 2012;60: 1921-1929. doi: 10.1016/j.jacc.2012.04.060
24. Forkmann M, Schwab C, Busch S. [Catheter ablation of supraventricular tachycardia]. Herzschrittmacherther Elektrophysiol. 2019;30:336-342. doi: 10.1007/s00399-019-00654-x
25. Bulava A, Hanis J, Eisenberger M. Catheter ablation of atrial fibrillation using zero-fluoroscopy technique: a randomized trial. Pacing Clin Electrophysiol. 2015;38:797-806. doi: 10.1111/pace.12634
26. Haegeli LM, Stutz L, Mohsen M, et al. Feasibility of zero or near zero fluoroscopy during catheter ablation procedures. Cardiol J. 2019;26:226-232. doi: 10.5603/CJ.a2018.0029
27. Steven D, Servatius H, Rostock T, et al. Reduced fluoroscopy during atrial fibrillation ablation: benefits of robotic guided navigation. J Cardiovasc Electrophysiol. 2010;21:6-12. doi: 10.1111/j.1540-8167.2009.01592.x
28. General therapy for cardiac arrhythmias. In: Zipes DP, Libby P, Bonow RO, et al. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 11th ed. Elsevier; 2019.
29. Kuck K-H, Brugada J, Albenque J-P. Cryoballoon or radiofrequency ablation for atrial fibrillation. N Engl J Med. 2016;375: 1100-1101. doi: 10.1056/NEJMc1609160
30. Chen Y-H, Lu Z-Y, Xiang Y, et al. Cryoablation vs. radiofrequency ablation for treatment of paroxysmal atrial fibrillation: a systematic review and meta-analysis. Europace. 2017;19:784-794. doi: 10.1093/europace/euw330
31. Locati ET, Vecchi AM, Vargiu S, et al. Role of extended external loop recorders for the diagnosis of unexplained syncope, presyncope, and sustained palpitations. Europace. 2014;16:914-922. doi: 10.1093/europace/eut337
32. Calkins H, Kuck KH, Cappato R, et al; Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2012;9:632-696.e21. doi: 10.1016/j.hrthm.2011.12.016
33. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609-1678. doi: 10.1093/ europace/euw295
34. Nairooz R, Sardar P, Payne J, et al. Meta-analysis of major bleeding with uninterrupted warfarin compared to interrupted warfarin and heparin bridging in ablation of atrial fibrillation. Int J Cardiol. 2015;187:426-429. doi: 10.1016/j.ijcard.2015.03.376
35. Romero J, Cerrud-Rodriguez RC, Diaz JC, et al. Uninterrupted direct oral anticoagulants vs. uninterrupted vitamin K antagonists during catheter ablation of non-valvular atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. Europace. 2018;20:1612-1620. doi: 10.1093/europace/euy133
36. Deyell MW, Leather RA, Macle L, et al. Efficacy and safety of same-day discharge for atrial fibrillation ablation. JACC Clin Electrophysiol. 2020;6:609-619. doi: 10.1016/j.jacep.2020.02.009
37. Theodoreson MD, Chohan BC, McAloon CJ, et al. Same-day cardiac catheter ablation is safe and cost-effective: experience from a UK tertiary center. Heart Rhythm. 2015;12:1756-1761. doi: 10.1016/j.hrthm.2015.05.006
PRACTICE RECOMMENDATIONS
› Refer patients with atrial fibrillation (AF) to Cardiology for consideration of catheter ablation, a recommended treatment in select cases of (1) symptomatic paroxysmal AF in the setting of intolerance of antiarrhythmic drug therapy and (2) persistence of symptoms despite antiarrhythmic drug therapy. A
› Continue long-term oral anticoagulation therapy post ablation in patients with paroxysmal AF who have undergone catheter ablation if their CHA2DS2–VASc score is ≥ 2 (men) or ≥ 3 (women). C
› Regard catheter ablation as a reasonable alternative to antiarrhythmic drug therapy in select older patients with AF, and refer to a cardiologist as appropriate. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Right place, right time: Facilitating end-of-life conversations
As the geriatric population continues to grow and treatment advances blur the lines between improving the length of life vs improving its quality, end-of-life (EOL) conversations are becoming increasingly important. These discussions are a crucial part of the advance care planning (ACP) process, in which patients discuss their treatment preferences and values with their caregiver/surrogate decision maker and health care provider to ultimately improve EOL decision-making and care. 1,2
EOL conversations are most helpful when incorporated in the outpatient setting as part of the patient’s ongoing health care plan or when initiating treatment for a chronic or life-threatening disease. Because family physicians promote general wellness, understand the patient’s health status and medical history, and have an ongoing—and often longstanding—relationship with patients and their families, we are ideally positioned to engage patients in EOL discussions. However, these conversations can be challenging in the outpatient setting, and often clinicians struggle not only to find ways to raise the subject, but also to find the time to have these supportive, meaningful conversations.3
In this article, we will address the importance of having EOL discussions in the outpatient setting, specifically about advance directives (ADs), and the reasons why patients and physicians might avoid these discussions. The role of palliative care in EOL care, along with its benefits and methods for overcoming patient and physician barriers to its successful use, are reviewed. Finally, we examine specific challenges associated with discussing EOL care with patients with decreased mental capacity, such as those with dementia, and provide strategies to successfully facilitate EOL discussions in these populations.
Moving patients toward completion of advance directives
Although many older patients express a desire to document their wishes before EOL situations arise, they may not fully understand the benefits of an AD or how to complete one. 4 Often the family physician is best equipped to address the patient’s concerns and discuss their goals for EOL care, as well as the potential situations that might arise.
Managing an aging population. Projections suggest that primary care physicians will encounter increasing numbers of geriatric patients in the next 2 decades. Thus it is essential for those in primary care to receive proper training during their residency for the care of this group of patients. According to a group of academic educators and geriatricians from internal medicine and family medicine whose goal was to define a set of minimal and essential competencies in the care of older adults, this includes training on how to discuss and document “advance care planning and goals of care with all patients with chronic or complex illness,” as well as how to differentiate among “types of code status, health care proxies, and advanced directives” within the state in which training occurs. 5
Educate patients and ease fears. Patients often avoid EOL conversations or wait for their family physician to start the conversation. They may not understand how ADs can help guide care or they may believe they are “too healthy” to have these conversations at this time. 4 Simply asking about existing ADs or providing forms to patients during an outpatient visit can open the door to more in-depth discussions. Some examples of opening phrases include:
- Do you have a living will or durable power of attorney for health care?
- Have you ever discussed your health care wishes with your loved ones?
- Who would you want to speak for you regarding your health care if you could not speak for yourself? Have you discussed your health care wishes with that person?
By normalizing the conversation as a routine part of comprehensive, patient-centered care, the family physician can allay patient fears, foster open and honest conversations, and encourage ongoing discussions with loved ones as situations arise.6
Continue to: When ADs are executed...
When ADs are executed, patients often fail to have meaningful conversations with their surrogates about specific treatment wishes or EOL scenarios. As a result, the surrogate may not feel prepared to serve as a proxy decision maker or may find the role extremely stressful.7 Physicians should encourage open conversations between patients and their surrogates about potential EOL scenarios when possible. When possible and appropriate, it is also important to encourage the patient to include the surrogate in future outpatient visits so that the surrogate can understand the patient’s health status and potential decisions they may need to make.
Don’t overlook clinician barriers. Family physicians also might avoid AD discussions because they do not understand laws that govern ADs, which vary from state to state. Various online resources for patients and physicians exist that clarify state-specific regulations and provide state-specific forms (TABLE).
Time constraints present another challenge for family physicians. This can be addressed by establishing workflows that include EOL elements. Also, the Centers for Medicare and Medicaid Services (CMS) has provided separate billing codes for AD discussion based on time spent explaining and discussing how to complete forms.8 CPT codes 99497 and 99498 are time-based codes that cover the first 30 minutes and each additional 30 minutes, respectively, of time spent explaining and discussing how to complete standard forms in a face-to-face setting (TABLE).9 CMS also includes discussion of AD documents as an optional element of the annual Medicare wellness visit.8
Improve quality of life for patients with any serious illness
Unlike hospice, which focuses on providing comfort rather than cure in the final months of a patient’s life, palliative care strives to prevent and relieve the patient’s suffering from a serious illness that is not immediately life-threatening. Palliative care focuses on the early identification, careful assessment, and treatment of the physical, psychosocial, and spiritual symptoms associated with a patient’s condition(s).10,11 It has been well established that palliative care has a positive effect on many clinical outcomes including symptom burden, quality of life, satisfaction with care, and survival.12-14 Patients who receive palliative care consultation also tend to perceive a higher quality of care.15
Conversations lead to better outcomes. Palliative care consultation is being increasingly used in the outpatient setting and can be introduced early in a disease process. Doing so provides an additional opportunity for the family physician to introduce an EOL discussion. A comparison of outcomes between patients who had initial inpatient palliative care consultation vs outpatient palliative care referral found that outpatient referral improved quality EOL care and was associated with significantly fewer emergency department visits (68% vs 48%; P < .001) and hospital admissions (86% vs 52%; P < .001), as well as shorter hospital stays in the last 30 days of life (3-11 vs 5-14 days; P = .01).14 Despite these benefits, 60% to 90% of patients with a serious illness report never having discussed EOL care issues with their clinician.16,17
Continue to: Early EOL discussions...
Early EOL discussions have also been shown to have a positive impact on families. In a US study, family members stated that timely EOL care discussions allowed them to make use of hospice and palliative care services sooner and to make the most of their time with the patient.18
Timing and communication are key
Logistically it can be difficult to gather the right people (patient, family, etc) in the right place and at the right time. For physicians, the most often cited barriers include inadequate time to conduct an EOL discussion, 19 a perceived lack of competence in EOL conversations, 1,20 difficulty navigating patient readiness, 21 and a fear of destroying hope due to prognostic uncertainty. 19,20
A prospective, observational study used the Quality of Communication (QOC) questionnaire to assess life-sustaining treatment preferences, ACP, and the quality of EOL care communication in Dutch outpatients with clinically stable but severe chronic obstructive pulmonary disease (n = 105) or congestive heart failure (n = 80). The QOC questionnaire is a validated instrument that asks patients to rate their physician on several communication skills from 0 (“the very worst” or “My doctor didn’t do this”) to 10 (“the very best”). In this study, quality communication was identified by patients as one of the most important skills for physicians to provide adequate EOL care. 22 While QOC ratings were high for general communication skills (median, 8.0 points), quality EOL care communication was rated very low (median, 0.0 points). Researchers say that this was primarily because most EOL topics were not discussed—especially spirituality, prognosis, and what dying might be like. 22 In a secondary analysis that evaluated quality of EOL care communication during 1-year follow-up of patients with advanced chronic organ failure (n = 265) with the QOC questionnaire, patient ratings improved to moderate to good (medians, 6-8 points) when these topics were addressed. 23
Pick a strategy and prepare. As the older population continues to grow, the demands of palliative care management cannot be met by specialists alone and the responsibility of discussing EOL care with patients and their families will increasingly fall to family physicians as well. 24 Several strategies and approaches have evolved to assist family physicians with acquiring the skills to conduct productive EOL discussions. These include widely referenced resources, such as VitalTalk 25 and the ABCDE Plan. 26 VitalTalk teaches skills to help clinicians navigate difficult conversations, 25 and the “ABCDE” method provides a pneumonic for recommendations for how to deliver bad news ( A dvance preparation; B uild a therapeutic environment/relationship; C ommunicate well; D eal with patient and family reactions; E ncourage and validate emotions). 26
Other strategies include familiarizing oneself with the patient’s medical history and present situation (eg, What are the patient’s symptoms? What do other involved clinicians think and recommend? What therapies have been attempted? What are the relevant social and emotional dynamics?); asking the patient who they want present for the EOL conversation; scheduling the conversation for when you can set aside an appropriate amount of time and in a private place where there will be no interruptions; and going into the meeting with your goal in mind, whether it is to deliver bad news, clarify the prognosis, establish goals of care, or communicate the patient’s goals and wishes for the EOL to those in attendance. 27 It can be very helpful to begin the conversation by clarifying what the patient and their family/surrogate understand about the current diagnosis and prognosis. From there, the family physician can present a “headline” that prepares them for the current conversation (eg, “I have your latest test results, and I need to share some serious news”). This can facilitate a more detailed discussion of the patient’s and surrogate’s goals of care. Using these strategies, family physicians can lead a productive EOL discussion that benefits everyone.
Continue to: How to navigate EOL discussions with patients with dementia
How to navigate EOL discussions with patients with dementia
EOL discussions with patients with dementia become even more complex and warrant specific discussion because one must consider the timing of such discussions, 2,28,29 the trajectory of the disease and how that affects the patient’s capacity for EOL conversations, and the critical importance of engaging caregivers/surrogate decision makers in these discussions. 2 ACP provides an opportunity for the physician, patient, and caregiver/surrogate to jointly explore the patient’s values, beliefs, and preferences for care through the EOL as the disease progresses and the patient’s decisional capacity declines.
Ensure meaningful participation with timing. EOL discussions should occur while the patient has the cognitive capacity to actively participate in the planning process. A National Institutes of Health stage I behavioral intervention development trial evaluated a structured psychoeducational intervention, known as SPIRIT (Sharing Patient’s Illness Representation to Increase Trust), that aimed to promote cognitive and emotional preparation for EOL decisions for patients and their surrogates.28 It was found to be effective in patients, including those with end-stage renal disease and advanced heart failure, and their surrogates.28 Preliminary results from the trial confirmed that people with mild-to-moderate dementia (recent Montreal Cognitive Assessment score ≥ 13) are able to participate meaningfully in EOL discussions and ACP.28
Song et al29 adapted SPIRIT for use with patients with dementia and conducted a feasibility study with 23 patient-surrogate dyads.The mixed-methods study involved an expert panel review of the adapted SPIRIT, followed by a randomized trial with qualitative interviews. All 23 patients with dementia, including 14 with moderate dementia, were able to articulate their values and EOL preferences somewhat or very coherently (91.3% inter-rater reliability).29 In addition, dyad care goal congruence (agreement between patient’s EOL preferences and surrogate’s understanding of those preferences) and surrogate decision-making confidence (comfort in performing as a surrogate) were high and patient decisional conflict (patient difficulty in weighing the benefits and burdens of life-sustaining treatments and decision-making) was low, both at baseline as well as post intervention.29 Although preparedness for EOL decision-making outcome measures did not change, people with dementia and their surrogates perceived SPIRIT to be beneficial, particularly in helping them be on the same page.29
The randomized trial portion of the study (phase 2) continues to recruit 120 patient-surrogate dyads. Patient and surrogate self-reported preparedness for EOL decision-making are the primary outcomes, measured at baseline and 2 to 3 days post intervention. The estimated study completion date is May 31, 2022.30
Evidence-based clinical guidance can improve the process. Following the Belgian Centre for Evidence-Based Medicine’s procedures as a sample methodology, Piers et al2 developed evidence-based clinical recommendations for providers to use in the practical application of ACP in their care of patients with dementia.The researchers searched the literature; developed recommendations based on the evidence obtained, as well as their collective expert opinion; and performed validation using expert and end-user feedback and peer review. The study resulted in 32 recommendations focused on 8 domains that ranged from the beginning of the process (preconditions for optimal implementation of ACP) to later stages (ACP when it is difficult/no longer possible to communicate).2Specific guidance for ACP in dementia care include the following:
- ACP initiation. Begin conversations around the time of diagnosis, continue them throughout ongoing care, and revisit them when changes occur in the patient’s health, financial, or residential status.
- ACP conversations. Use conversations to identify significant others in the patient’s life (potential caregivers and/or surrogate decision makers) and explore the patient’s awareness of the disease and its trajectory. Discuss the patient’s values and beliefs, as well as their fears about, and preferences for, future care and the EOL.
- Role of significant others in the ACP process. Involve a patient’s significant others early in the ACP process, educate them about the surrogate decision-maker role, assess their disease awareness, and inform them about the disease trajectory and anticipated EOL decisions. 2
Continue to: Incorporate and document patients' values and preferences with LEAD
Incorporate and document patients’ values and preferences with LEAD. Dassel et al31 developed the Life-planning in Early Alzheimer’s and Dementia (LEAD) tool, which is a validated dementia-focused EOL planning tool that can be used to promote discussion and document a patient’s care preferences and values within the context of their changing cognitive ability.Dassel et al31 used a 4-phase mixed-method design that included (1) focus groups of patients with early-stage dementia and family caregivers, (2) clinical utility evaluation by content experts, (3) instrument completion sampling to evaluate its psychometric properties, and (4) additional focus groups to inform how the instrument should be used by families and in clinical practice.Six scales with high internal consistency and high test-retest reliability were identified: 3 scales represented patient values (concern about being a burden, the importance of quality [vs length] of life, and the preference for autonomy in decision-making) and 3 scales represented patient preferences (use of life-prolonging measures, controlling the timing of death, and the location of EOL care).31
The LEAD Guide can be used as a self-assessment tool that is completed individually and then shared with the surrogate decision maker and health care provider.32 It also can be used to guide conversations with the surrogate and physician, as well as with trusted family and friends. Using this framework, family physicians can facilitate EOL planning with the patient and their surrogate that is based on the patient’s values and preferences for EOL care prior to, and in anticipation of, the patient’s loss of decisional capacity.31
Facilitate discussions that improve outcomes
Conversations about EOL care are taking on increased importance as the population ages and treatments advance. Understanding the concerns of patients and their surrogate decision makers, as well as the resources available to guide these difficult discussions ( TABLE ), will help family physicians conduct effective conversations that enhance care, reduce the burden on surrogate decision makers, and have a positive impact on many clinical outcomes.
CORRESPONDENCE
Shirley Bodi, MD, 3000 Arlington Avenue, Department of Family Medicine, Dowling Hall, Suite 2200, University of Toledo College of Medicine and Life Sciences, Toledo, OH 43614; [email protected]
1. Bergenholtz Heidi, Timm HU, Missel M. Talking about end of life in general palliative care – what’s going on? A qualitative study on end-of-life conversations in an acute care hospital in Denmark. BMC Palliat Care. 2019;18:62. doi: 10.1186/s12904-019-0448-z
2. Piers R, Albers G, Gilissen J, et al. Advance care planning in dementia: recommendations for healthcare professionals. BMC Palliat Care. 2018;17:88. doi: 10.1186/s12904-018-0332-2
3. Tunzi M, Ventres W. A reflective case study in family medicine advance care planning conversations. J Am Board Fam Med. 2019;32:108-114. doi: 10.3122/jabfm.2019.01.180198
4. Schickedanz AD, Schillinger D, Landefeld CS, et al. A clinical framework for improving the advance care planning process: start with patients’ self-identified barriers. J Am Geriatr Soc. 2009;57:31-39. doi: 10.1111/j.1532-5415.2008.02093.x
5. Williams BC, Warshaw G, Fabiny AR, et al. Medicine in the 21st century: recommended essential geriatrics competencies for internal medicine and family medicine residents. J Grad Med Ed. 2010;2:373-383. doi: 10.4300/JGME-D-10-00065.1
6. Alano G, Pekmezaris R, Tai J, et al. Factors influencing older adults to complete advance directives. Palliat Support Care. 2010;8:267-275. doi: 10.1017/S1478951510000064
7. Wendler D, Rid A. Systematic review: the effect on surrogates of making treatment decisions for others. Ann Intern Med. 2011;154:336-346. doi: 10.7326/0003-4819-154-5-201103010-00008
8. Edelberg C. Advance care planning with and without an annual wellness visit. Ed Management website. June 1, 2016. Accessed November 16, 2021. ww.reliasmedia.com/articles/137829-advanced-care-planning-with-and-without-an-annual-wellness-visit
9. Centers for Medicare and Medicaid Services. Frequently asked questions about billing the physician fee schedule for advance care planning services. July 14, 2016. Accessed December 20, 2021. www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/FAQ-Advance-Care-Planning.pdf
10. World Health Organization. Palliative care fact sheet. August 5, 2020. Accessed November 16, 2021. www.who.int/news-room/fact-sheets/detail/palliative-care
11. National Institute on Aging. What are palliative care and hospice care? Reviewed May 14, 2021. Accessed December 20, 2021. www.nia.nih.gov/health/what-are-palliative-care-and-hospice-care#palliative-vs-hospice
12. Rabow MW, Dibble SL, Pantilat, SZ, et al. The comprehensive care team: a controlled trial of outpatient palliative medicine consultation. Arch Intern Med. 2004;164:83-91. doi: 10.1001/archinte.164.1.83
13. Muir JC, Daley F, Davis MS, et al. Integrating palliative care into the outpatient, private practice oncology setting. J Pain Symptom Manage. 2010;40:126-135. doi: 10.1016/j.jpainsymman.2009.12.017
14. Hui D, Kim SH, Roquemore J, et al. Impact of timing and setting of palliative care referral on quality of end-of-life care in cancer patients. Cancer. 2014;120:1743-1749. doi: 10.1002/cncr.28628
15. Leung JM, Udris EM, Uman J, e al. The effect of end-of-life discussions on perceived quality of care and health status among patients with COPD. Chest. 2012;142:128-133. doi: 10.1378/chest.11-2222
16. Davison SN. End-of-life care preferences and needs: perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:195-204. doi: 10.2215/CJN.05960809
17. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patients mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665-1673. doi: 10.1001/jama.300.14.1665
18. Park E, Check DK, Yopp JM, et al. An exploratory study of end-of-life prognostic communication needs as reported by widowed fathers due to cancer. Psychooncology. 2015;24:1471-1476. doi: 10.1002/pon.3757
19. Tavares N, Jarrett N, Hunt K, et al. Palliative and end-of-life care conversations in COPD: a systematic literature review. ERJ Open Res. 2017;3:00068-2016. doi: 10.1183/23120541.00068-2016
20. Hancock K, Clayton JM, Parker SM, et al. Truth-telling in discussing prognosis in advanced life-limiting illnesses: a systematic review. Palliat Med. 2007;21:507-517. doi: 10.1177/0269216307080823
21. Parker SM, Clayton JM, Hancock K, et al. A systematic review of prognostic/end-of-life communication with adults in the advanced stages of a life-limiting illness: patient/caregiver preferences for the content, style, and timing of information. J Pain Symptom Manage. 2007;34:81-93. doi: 10.1016/j.jpainsymman.2006.09.035
22. Janssen DJA, Spruit MA, Schols JMGA, et al. A call for high-quality advance care planning in outpatients with severe COPD or chronic heart failure. Chest. 2011;139:1081-1088. doi: 10.1378/chest.10-1753
23. Houben CHM, Spruit MA, Schols JM, et al. Patient-clinician communication about end-of-life care on patients with advanced chronic organ failure during one year. J Pain Symptom Manage. 2015;49:1109-1115. doi: 10.1016/j.jpainsymman.2014.12.008
24. Brighton LJ, Bristowe K. Communication in palliative care: talking about the end of life, before the end of life. Postgrad Med J. 2016;92:466-470. doi: 10.1136/postgradmedj-2015-133368
25. VitalTalk website. Accessed December 20, 2021. vitaltalk.org
26. Rabow MQ, McPhee SJ. Beyond breaking bad news: how to help patients who suffer. Wes J Med. 1999;171:260-263. www.ncbi.nlm.nih.gov/pmc/articles/PMC1305864
27. Pfeifer M, Head B. Which critical communication skills are essential for interdisciplinary end-of-life discussions? AMA J Ethics. 2018;8:E724-E731. doi: 10.1001/amajethics.2018.724
28. Song M-K, Ward SE, Hepburn K, et al. SPIRIT advance care planning intervention in early stage dementias: an NIH stage I behavioral intervention development trial. Contemp Clin Trials. 2018;71:55-62. doi: 10.1016/j.cct.2018.06.005
29. Song M-K, Ward SE, Hepburn K, et al. Can persons with dementia meaningfully participate in advance care planning discussions? A mixed-methods study of SPIRIT. J Palliat Med. 2019;22:1410-1416. doi: 10.1089/jpm.2019.0088
30. Two-phased study of SPIRIT in mild dementia. ClinicalTrials.gov Identifier: NCT03311711. Updated August 23, 2021. Accessed December 20, 2021. clinicaltrials.gov/ct2/show/NCT03311711
31. Dassel K, Utz R, Supiano K, et al. Development of a dementia-focused end-of-life planning tool: the LEAD Guide (Life-planning in Early Alzheimer’s and Dementia). Innov Aging. 2019;3:igz024. doi: 10.1093/geroni/igz024
32. Dassel K, Supiano K, Utz R, et al. The LEAD Guide. Life-planning in Early Alzheimer’s and Dementia. 2019. Accessed December 20, 2021. utahgwep.org/resources/search-all-resources/send/10-dementia/27-the-lead-guide#:~:text=The%20LEAD%20Guide%20(Life%2DPlanning,your%20decisions%20about%20your%20care
As the geriatric population continues to grow and treatment advances blur the lines between improving the length of life vs improving its quality, end-of-life (EOL) conversations are becoming increasingly important. These discussions are a crucial part of the advance care planning (ACP) process, in which patients discuss their treatment preferences and values with their caregiver/surrogate decision maker and health care provider to ultimately improve EOL decision-making and care. 1,2
EOL conversations are most helpful when incorporated in the outpatient setting as part of the patient’s ongoing health care plan or when initiating treatment for a chronic or life-threatening disease. Because family physicians promote general wellness, understand the patient’s health status and medical history, and have an ongoing—and often longstanding—relationship with patients and their families, we are ideally positioned to engage patients in EOL discussions. However, these conversations can be challenging in the outpatient setting, and often clinicians struggle not only to find ways to raise the subject, but also to find the time to have these supportive, meaningful conversations.3
In this article, we will address the importance of having EOL discussions in the outpatient setting, specifically about advance directives (ADs), and the reasons why patients and physicians might avoid these discussions. The role of palliative care in EOL care, along with its benefits and methods for overcoming patient and physician barriers to its successful use, are reviewed. Finally, we examine specific challenges associated with discussing EOL care with patients with decreased mental capacity, such as those with dementia, and provide strategies to successfully facilitate EOL discussions in these populations.
Moving patients toward completion of advance directives
Although many older patients express a desire to document their wishes before EOL situations arise, they may not fully understand the benefits of an AD or how to complete one. 4 Often the family physician is best equipped to address the patient’s concerns and discuss their goals for EOL care, as well as the potential situations that might arise.
Managing an aging population. Projections suggest that primary care physicians will encounter increasing numbers of geriatric patients in the next 2 decades. Thus it is essential for those in primary care to receive proper training during their residency for the care of this group of patients. According to a group of academic educators and geriatricians from internal medicine and family medicine whose goal was to define a set of minimal and essential competencies in the care of older adults, this includes training on how to discuss and document “advance care planning and goals of care with all patients with chronic or complex illness,” as well as how to differentiate among “types of code status, health care proxies, and advanced directives” within the state in which training occurs. 5
Educate patients and ease fears. Patients often avoid EOL conversations or wait for their family physician to start the conversation. They may not understand how ADs can help guide care or they may believe they are “too healthy” to have these conversations at this time. 4 Simply asking about existing ADs or providing forms to patients during an outpatient visit can open the door to more in-depth discussions. Some examples of opening phrases include:
- Do you have a living will or durable power of attorney for health care?
- Have you ever discussed your health care wishes with your loved ones?
- Who would you want to speak for you regarding your health care if you could not speak for yourself? Have you discussed your health care wishes with that person?
By normalizing the conversation as a routine part of comprehensive, patient-centered care, the family physician can allay patient fears, foster open and honest conversations, and encourage ongoing discussions with loved ones as situations arise.6
Continue to: When ADs are executed...
When ADs are executed, patients often fail to have meaningful conversations with their surrogates about specific treatment wishes or EOL scenarios. As a result, the surrogate may not feel prepared to serve as a proxy decision maker or may find the role extremely stressful.7 Physicians should encourage open conversations between patients and their surrogates about potential EOL scenarios when possible. When possible and appropriate, it is also important to encourage the patient to include the surrogate in future outpatient visits so that the surrogate can understand the patient’s health status and potential decisions they may need to make.
Don’t overlook clinician barriers. Family physicians also might avoid AD discussions because they do not understand laws that govern ADs, which vary from state to state. Various online resources for patients and physicians exist that clarify state-specific regulations and provide state-specific forms (TABLE).
Time constraints present another challenge for family physicians. This can be addressed by establishing workflows that include EOL elements. Also, the Centers for Medicare and Medicaid Services (CMS) has provided separate billing codes for AD discussion based on time spent explaining and discussing how to complete forms.8 CPT codes 99497 and 99498 are time-based codes that cover the first 30 minutes and each additional 30 minutes, respectively, of time spent explaining and discussing how to complete standard forms in a face-to-face setting (TABLE).9 CMS also includes discussion of AD documents as an optional element of the annual Medicare wellness visit.8
Improve quality of life for patients with any serious illness
Unlike hospice, which focuses on providing comfort rather than cure in the final months of a patient’s life, palliative care strives to prevent and relieve the patient’s suffering from a serious illness that is not immediately life-threatening. Palliative care focuses on the early identification, careful assessment, and treatment of the physical, psychosocial, and spiritual symptoms associated with a patient’s condition(s).10,11 It has been well established that palliative care has a positive effect on many clinical outcomes including symptom burden, quality of life, satisfaction with care, and survival.12-14 Patients who receive palliative care consultation also tend to perceive a higher quality of care.15
Conversations lead to better outcomes. Palliative care consultation is being increasingly used in the outpatient setting and can be introduced early in a disease process. Doing so provides an additional opportunity for the family physician to introduce an EOL discussion. A comparison of outcomes between patients who had initial inpatient palliative care consultation vs outpatient palliative care referral found that outpatient referral improved quality EOL care and was associated with significantly fewer emergency department visits (68% vs 48%; P < .001) and hospital admissions (86% vs 52%; P < .001), as well as shorter hospital stays in the last 30 days of life (3-11 vs 5-14 days; P = .01).14 Despite these benefits, 60% to 90% of patients with a serious illness report never having discussed EOL care issues with their clinician.16,17
Continue to: Early EOL discussions...
Early EOL discussions have also been shown to have a positive impact on families. In a US study, family members stated that timely EOL care discussions allowed them to make use of hospice and palliative care services sooner and to make the most of their time with the patient.18
Timing and communication are key
Logistically it can be difficult to gather the right people (patient, family, etc) in the right place and at the right time. For physicians, the most often cited barriers include inadequate time to conduct an EOL discussion, 19 a perceived lack of competence in EOL conversations, 1,20 difficulty navigating patient readiness, 21 and a fear of destroying hope due to prognostic uncertainty. 19,20
A prospective, observational study used the Quality of Communication (QOC) questionnaire to assess life-sustaining treatment preferences, ACP, and the quality of EOL care communication in Dutch outpatients with clinically stable but severe chronic obstructive pulmonary disease (n = 105) or congestive heart failure (n = 80). The QOC questionnaire is a validated instrument that asks patients to rate their physician on several communication skills from 0 (“the very worst” or “My doctor didn’t do this”) to 10 (“the very best”). In this study, quality communication was identified by patients as one of the most important skills for physicians to provide adequate EOL care. 22 While QOC ratings were high for general communication skills (median, 8.0 points), quality EOL care communication was rated very low (median, 0.0 points). Researchers say that this was primarily because most EOL topics were not discussed—especially spirituality, prognosis, and what dying might be like. 22 In a secondary analysis that evaluated quality of EOL care communication during 1-year follow-up of patients with advanced chronic organ failure (n = 265) with the QOC questionnaire, patient ratings improved to moderate to good (medians, 6-8 points) when these topics were addressed. 23
Pick a strategy and prepare. As the older population continues to grow, the demands of palliative care management cannot be met by specialists alone and the responsibility of discussing EOL care with patients and their families will increasingly fall to family physicians as well. 24 Several strategies and approaches have evolved to assist family physicians with acquiring the skills to conduct productive EOL discussions. These include widely referenced resources, such as VitalTalk 25 and the ABCDE Plan. 26 VitalTalk teaches skills to help clinicians navigate difficult conversations, 25 and the “ABCDE” method provides a pneumonic for recommendations for how to deliver bad news ( A dvance preparation; B uild a therapeutic environment/relationship; C ommunicate well; D eal with patient and family reactions; E ncourage and validate emotions). 26
Other strategies include familiarizing oneself with the patient’s medical history and present situation (eg, What are the patient’s symptoms? What do other involved clinicians think and recommend? What therapies have been attempted? What are the relevant social and emotional dynamics?); asking the patient who they want present for the EOL conversation; scheduling the conversation for when you can set aside an appropriate amount of time and in a private place where there will be no interruptions; and going into the meeting with your goal in mind, whether it is to deliver bad news, clarify the prognosis, establish goals of care, or communicate the patient’s goals and wishes for the EOL to those in attendance. 27 It can be very helpful to begin the conversation by clarifying what the patient and their family/surrogate understand about the current diagnosis and prognosis. From there, the family physician can present a “headline” that prepares them for the current conversation (eg, “I have your latest test results, and I need to share some serious news”). This can facilitate a more detailed discussion of the patient’s and surrogate’s goals of care. Using these strategies, family physicians can lead a productive EOL discussion that benefits everyone.
Continue to: How to navigate EOL discussions with patients with dementia
How to navigate EOL discussions with patients with dementia
EOL discussions with patients with dementia become even more complex and warrant specific discussion because one must consider the timing of such discussions, 2,28,29 the trajectory of the disease and how that affects the patient’s capacity for EOL conversations, and the critical importance of engaging caregivers/surrogate decision makers in these discussions. 2 ACP provides an opportunity for the physician, patient, and caregiver/surrogate to jointly explore the patient’s values, beliefs, and preferences for care through the EOL as the disease progresses and the patient’s decisional capacity declines.
Ensure meaningful participation with timing. EOL discussions should occur while the patient has the cognitive capacity to actively participate in the planning process. A National Institutes of Health stage I behavioral intervention development trial evaluated a structured psychoeducational intervention, known as SPIRIT (Sharing Patient’s Illness Representation to Increase Trust), that aimed to promote cognitive and emotional preparation for EOL decisions for patients and their surrogates.28 It was found to be effective in patients, including those with end-stage renal disease and advanced heart failure, and their surrogates.28 Preliminary results from the trial confirmed that people with mild-to-moderate dementia (recent Montreal Cognitive Assessment score ≥ 13) are able to participate meaningfully in EOL discussions and ACP.28
Song et al29 adapted SPIRIT for use with patients with dementia and conducted a feasibility study with 23 patient-surrogate dyads.The mixed-methods study involved an expert panel review of the adapted SPIRIT, followed by a randomized trial with qualitative interviews. All 23 patients with dementia, including 14 with moderate dementia, were able to articulate their values and EOL preferences somewhat or very coherently (91.3% inter-rater reliability).29 In addition, dyad care goal congruence (agreement between patient’s EOL preferences and surrogate’s understanding of those preferences) and surrogate decision-making confidence (comfort in performing as a surrogate) were high and patient decisional conflict (patient difficulty in weighing the benefits and burdens of life-sustaining treatments and decision-making) was low, both at baseline as well as post intervention.29 Although preparedness for EOL decision-making outcome measures did not change, people with dementia and their surrogates perceived SPIRIT to be beneficial, particularly in helping them be on the same page.29
The randomized trial portion of the study (phase 2) continues to recruit 120 patient-surrogate dyads. Patient and surrogate self-reported preparedness for EOL decision-making are the primary outcomes, measured at baseline and 2 to 3 days post intervention. The estimated study completion date is May 31, 2022.30
Evidence-based clinical guidance can improve the process. Following the Belgian Centre for Evidence-Based Medicine’s procedures as a sample methodology, Piers et al2 developed evidence-based clinical recommendations for providers to use in the practical application of ACP in their care of patients with dementia.The researchers searched the literature; developed recommendations based on the evidence obtained, as well as their collective expert opinion; and performed validation using expert and end-user feedback and peer review. The study resulted in 32 recommendations focused on 8 domains that ranged from the beginning of the process (preconditions for optimal implementation of ACP) to later stages (ACP when it is difficult/no longer possible to communicate).2Specific guidance for ACP in dementia care include the following:
- ACP initiation. Begin conversations around the time of diagnosis, continue them throughout ongoing care, and revisit them when changes occur in the patient’s health, financial, or residential status.
- ACP conversations. Use conversations to identify significant others in the patient’s life (potential caregivers and/or surrogate decision makers) and explore the patient’s awareness of the disease and its trajectory. Discuss the patient’s values and beliefs, as well as their fears about, and preferences for, future care and the EOL.
- Role of significant others in the ACP process. Involve a patient’s significant others early in the ACP process, educate them about the surrogate decision-maker role, assess their disease awareness, and inform them about the disease trajectory and anticipated EOL decisions. 2
Continue to: Incorporate and document patients' values and preferences with LEAD
Incorporate and document patients’ values and preferences with LEAD. Dassel et al31 developed the Life-planning in Early Alzheimer’s and Dementia (LEAD) tool, which is a validated dementia-focused EOL planning tool that can be used to promote discussion and document a patient’s care preferences and values within the context of their changing cognitive ability.Dassel et al31 used a 4-phase mixed-method design that included (1) focus groups of patients with early-stage dementia and family caregivers, (2) clinical utility evaluation by content experts, (3) instrument completion sampling to evaluate its psychometric properties, and (4) additional focus groups to inform how the instrument should be used by families and in clinical practice.Six scales with high internal consistency and high test-retest reliability were identified: 3 scales represented patient values (concern about being a burden, the importance of quality [vs length] of life, and the preference for autonomy in decision-making) and 3 scales represented patient preferences (use of life-prolonging measures, controlling the timing of death, and the location of EOL care).31
The LEAD Guide can be used as a self-assessment tool that is completed individually and then shared with the surrogate decision maker and health care provider.32 It also can be used to guide conversations with the surrogate and physician, as well as with trusted family and friends. Using this framework, family physicians can facilitate EOL planning with the patient and their surrogate that is based on the patient’s values and preferences for EOL care prior to, and in anticipation of, the patient’s loss of decisional capacity.31
Facilitate discussions that improve outcomes
Conversations about EOL care are taking on increased importance as the population ages and treatments advance. Understanding the concerns of patients and their surrogate decision makers, as well as the resources available to guide these difficult discussions ( TABLE ), will help family physicians conduct effective conversations that enhance care, reduce the burden on surrogate decision makers, and have a positive impact on many clinical outcomes.
CORRESPONDENCE
Shirley Bodi, MD, 3000 Arlington Avenue, Department of Family Medicine, Dowling Hall, Suite 2200, University of Toledo College of Medicine and Life Sciences, Toledo, OH 43614; [email protected]
As the geriatric population continues to grow and treatment advances blur the lines between improving the length of life vs improving its quality, end-of-life (EOL) conversations are becoming increasingly important. These discussions are a crucial part of the advance care planning (ACP) process, in which patients discuss their treatment preferences and values with their caregiver/surrogate decision maker and health care provider to ultimately improve EOL decision-making and care. 1,2
EOL conversations are most helpful when incorporated in the outpatient setting as part of the patient’s ongoing health care plan or when initiating treatment for a chronic or life-threatening disease. Because family physicians promote general wellness, understand the patient’s health status and medical history, and have an ongoing—and often longstanding—relationship with patients and their families, we are ideally positioned to engage patients in EOL discussions. However, these conversations can be challenging in the outpatient setting, and often clinicians struggle not only to find ways to raise the subject, but also to find the time to have these supportive, meaningful conversations.3
In this article, we will address the importance of having EOL discussions in the outpatient setting, specifically about advance directives (ADs), and the reasons why patients and physicians might avoid these discussions. The role of palliative care in EOL care, along with its benefits and methods for overcoming patient and physician barriers to its successful use, are reviewed. Finally, we examine specific challenges associated with discussing EOL care with patients with decreased mental capacity, such as those with dementia, and provide strategies to successfully facilitate EOL discussions in these populations.
Moving patients toward completion of advance directives
Although many older patients express a desire to document their wishes before EOL situations arise, they may not fully understand the benefits of an AD or how to complete one. 4 Often the family physician is best equipped to address the patient’s concerns and discuss their goals for EOL care, as well as the potential situations that might arise.
Managing an aging population. Projections suggest that primary care physicians will encounter increasing numbers of geriatric patients in the next 2 decades. Thus it is essential for those in primary care to receive proper training during their residency for the care of this group of patients. According to a group of academic educators and geriatricians from internal medicine and family medicine whose goal was to define a set of minimal and essential competencies in the care of older adults, this includes training on how to discuss and document “advance care planning and goals of care with all patients with chronic or complex illness,” as well as how to differentiate among “types of code status, health care proxies, and advanced directives” within the state in which training occurs. 5
Educate patients and ease fears. Patients often avoid EOL conversations or wait for their family physician to start the conversation. They may not understand how ADs can help guide care or they may believe they are “too healthy” to have these conversations at this time. 4 Simply asking about existing ADs or providing forms to patients during an outpatient visit can open the door to more in-depth discussions. Some examples of opening phrases include:
- Do you have a living will or durable power of attorney for health care?
- Have you ever discussed your health care wishes with your loved ones?
- Who would you want to speak for you regarding your health care if you could not speak for yourself? Have you discussed your health care wishes with that person?
By normalizing the conversation as a routine part of comprehensive, patient-centered care, the family physician can allay patient fears, foster open and honest conversations, and encourage ongoing discussions with loved ones as situations arise.6
Continue to: When ADs are executed...
When ADs are executed, patients often fail to have meaningful conversations with their surrogates about specific treatment wishes or EOL scenarios. As a result, the surrogate may not feel prepared to serve as a proxy decision maker or may find the role extremely stressful.7 Physicians should encourage open conversations between patients and their surrogates about potential EOL scenarios when possible. When possible and appropriate, it is also important to encourage the patient to include the surrogate in future outpatient visits so that the surrogate can understand the patient’s health status and potential decisions they may need to make.
Don’t overlook clinician barriers. Family physicians also might avoid AD discussions because they do not understand laws that govern ADs, which vary from state to state. Various online resources for patients and physicians exist that clarify state-specific regulations and provide state-specific forms (TABLE).
Time constraints present another challenge for family physicians. This can be addressed by establishing workflows that include EOL elements. Also, the Centers for Medicare and Medicaid Services (CMS) has provided separate billing codes for AD discussion based on time spent explaining and discussing how to complete forms.8 CPT codes 99497 and 99498 are time-based codes that cover the first 30 minutes and each additional 30 minutes, respectively, of time spent explaining and discussing how to complete standard forms in a face-to-face setting (TABLE).9 CMS also includes discussion of AD documents as an optional element of the annual Medicare wellness visit.8
Improve quality of life for patients with any serious illness
Unlike hospice, which focuses on providing comfort rather than cure in the final months of a patient’s life, palliative care strives to prevent and relieve the patient’s suffering from a serious illness that is not immediately life-threatening. Palliative care focuses on the early identification, careful assessment, and treatment of the physical, psychosocial, and spiritual symptoms associated with a patient’s condition(s).10,11 It has been well established that palliative care has a positive effect on many clinical outcomes including symptom burden, quality of life, satisfaction with care, and survival.12-14 Patients who receive palliative care consultation also tend to perceive a higher quality of care.15
Conversations lead to better outcomes. Palliative care consultation is being increasingly used in the outpatient setting and can be introduced early in a disease process. Doing so provides an additional opportunity for the family physician to introduce an EOL discussion. A comparison of outcomes between patients who had initial inpatient palliative care consultation vs outpatient palliative care referral found that outpatient referral improved quality EOL care and was associated with significantly fewer emergency department visits (68% vs 48%; P < .001) and hospital admissions (86% vs 52%; P < .001), as well as shorter hospital stays in the last 30 days of life (3-11 vs 5-14 days; P = .01).14 Despite these benefits, 60% to 90% of patients with a serious illness report never having discussed EOL care issues with their clinician.16,17
Continue to: Early EOL discussions...
Early EOL discussions have also been shown to have a positive impact on families. In a US study, family members stated that timely EOL care discussions allowed them to make use of hospice and palliative care services sooner and to make the most of their time with the patient.18
Timing and communication are key
Logistically it can be difficult to gather the right people (patient, family, etc) in the right place and at the right time. For physicians, the most often cited barriers include inadequate time to conduct an EOL discussion, 19 a perceived lack of competence in EOL conversations, 1,20 difficulty navigating patient readiness, 21 and a fear of destroying hope due to prognostic uncertainty. 19,20
A prospective, observational study used the Quality of Communication (QOC) questionnaire to assess life-sustaining treatment preferences, ACP, and the quality of EOL care communication in Dutch outpatients with clinically stable but severe chronic obstructive pulmonary disease (n = 105) or congestive heart failure (n = 80). The QOC questionnaire is a validated instrument that asks patients to rate their physician on several communication skills from 0 (“the very worst” or “My doctor didn’t do this”) to 10 (“the very best”). In this study, quality communication was identified by patients as one of the most important skills for physicians to provide adequate EOL care. 22 While QOC ratings were high for general communication skills (median, 8.0 points), quality EOL care communication was rated very low (median, 0.0 points). Researchers say that this was primarily because most EOL topics were not discussed—especially spirituality, prognosis, and what dying might be like. 22 In a secondary analysis that evaluated quality of EOL care communication during 1-year follow-up of patients with advanced chronic organ failure (n = 265) with the QOC questionnaire, patient ratings improved to moderate to good (medians, 6-8 points) when these topics were addressed. 23
Pick a strategy and prepare. As the older population continues to grow, the demands of palliative care management cannot be met by specialists alone and the responsibility of discussing EOL care with patients and their families will increasingly fall to family physicians as well. 24 Several strategies and approaches have evolved to assist family physicians with acquiring the skills to conduct productive EOL discussions. These include widely referenced resources, such as VitalTalk 25 and the ABCDE Plan. 26 VitalTalk teaches skills to help clinicians navigate difficult conversations, 25 and the “ABCDE” method provides a pneumonic for recommendations for how to deliver bad news ( A dvance preparation; B uild a therapeutic environment/relationship; C ommunicate well; D eal with patient and family reactions; E ncourage and validate emotions). 26
Other strategies include familiarizing oneself with the patient’s medical history and present situation (eg, What are the patient’s symptoms? What do other involved clinicians think and recommend? What therapies have been attempted? What are the relevant social and emotional dynamics?); asking the patient who they want present for the EOL conversation; scheduling the conversation for when you can set aside an appropriate amount of time and in a private place where there will be no interruptions; and going into the meeting with your goal in mind, whether it is to deliver bad news, clarify the prognosis, establish goals of care, or communicate the patient’s goals and wishes for the EOL to those in attendance. 27 It can be very helpful to begin the conversation by clarifying what the patient and their family/surrogate understand about the current diagnosis and prognosis. From there, the family physician can present a “headline” that prepares them for the current conversation (eg, “I have your latest test results, and I need to share some serious news”). This can facilitate a more detailed discussion of the patient’s and surrogate’s goals of care. Using these strategies, family physicians can lead a productive EOL discussion that benefits everyone.
Continue to: How to navigate EOL discussions with patients with dementia
How to navigate EOL discussions with patients with dementia
EOL discussions with patients with dementia become even more complex and warrant specific discussion because one must consider the timing of such discussions, 2,28,29 the trajectory of the disease and how that affects the patient’s capacity for EOL conversations, and the critical importance of engaging caregivers/surrogate decision makers in these discussions. 2 ACP provides an opportunity for the physician, patient, and caregiver/surrogate to jointly explore the patient’s values, beliefs, and preferences for care through the EOL as the disease progresses and the patient’s decisional capacity declines.
Ensure meaningful participation with timing. EOL discussions should occur while the patient has the cognitive capacity to actively participate in the planning process. A National Institutes of Health stage I behavioral intervention development trial evaluated a structured psychoeducational intervention, known as SPIRIT (Sharing Patient’s Illness Representation to Increase Trust), that aimed to promote cognitive and emotional preparation for EOL decisions for patients and their surrogates.28 It was found to be effective in patients, including those with end-stage renal disease and advanced heart failure, and their surrogates.28 Preliminary results from the trial confirmed that people with mild-to-moderate dementia (recent Montreal Cognitive Assessment score ≥ 13) are able to participate meaningfully in EOL discussions and ACP.28
Song et al29 adapted SPIRIT for use with patients with dementia and conducted a feasibility study with 23 patient-surrogate dyads.The mixed-methods study involved an expert panel review of the adapted SPIRIT, followed by a randomized trial with qualitative interviews. All 23 patients with dementia, including 14 with moderate dementia, were able to articulate their values and EOL preferences somewhat or very coherently (91.3% inter-rater reliability).29 In addition, dyad care goal congruence (agreement between patient’s EOL preferences and surrogate’s understanding of those preferences) and surrogate decision-making confidence (comfort in performing as a surrogate) were high and patient decisional conflict (patient difficulty in weighing the benefits and burdens of life-sustaining treatments and decision-making) was low, both at baseline as well as post intervention.29 Although preparedness for EOL decision-making outcome measures did not change, people with dementia and their surrogates perceived SPIRIT to be beneficial, particularly in helping them be on the same page.29
The randomized trial portion of the study (phase 2) continues to recruit 120 patient-surrogate dyads. Patient and surrogate self-reported preparedness for EOL decision-making are the primary outcomes, measured at baseline and 2 to 3 days post intervention. The estimated study completion date is May 31, 2022.30
Evidence-based clinical guidance can improve the process. Following the Belgian Centre for Evidence-Based Medicine’s procedures as a sample methodology, Piers et al2 developed evidence-based clinical recommendations for providers to use in the practical application of ACP in their care of patients with dementia.The researchers searched the literature; developed recommendations based on the evidence obtained, as well as their collective expert opinion; and performed validation using expert and end-user feedback and peer review. The study resulted in 32 recommendations focused on 8 domains that ranged from the beginning of the process (preconditions for optimal implementation of ACP) to later stages (ACP when it is difficult/no longer possible to communicate).2Specific guidance for ACP in dementia care include the following:
- ACP initiation. Begin conversations around the time of diagnosis, continue them throughout ongoing care, and revisit them when changes occur in the patient’s health, financial, or residential status.
- ACP conversations. Use conversations to identify significant others in the patient’s life (potential caregivers and/or surrogate decision makers) and explore the patient’s awareness of the disease and its trajectory. Discuss the patient’s values and beliefs, as well as their fears about, and preferences for, future care and the EOL.
- Role of significant others in the ACP process. Involve a patient’s significant others early in the ACP process, educate them about the surrogate decision-maker role, assess their disease awareness, and inform them about the disease trajectory and anticipated EOL decisions. 2
Continue to: Incorporate and document patients' values and preferences with LEAD
Incorporate and document patients’ values and preferences with LEAD. Dassel et al31 developed the Life-planning in Early Alzheimer’s and Dementia (LEAD) tool, which is a validated dementia-focused EOL planning tool that can be used to promote discussion and document a patient’s care preferences and values within the context of their changing cognitive ability.Dassel et al31 used a 4-phase mixed-method design that included (1) focus groups of patients with early-stage dementia and family caregivers, (2) clinical utility evaluation by content experts, (3) instrument completion sampling to evaluate its psychometric properties, and (4) additional focus groups to inform how the instrument should be used by families and in clinical practice.Six scales with high internal consistency and high test-retest reliability were identified: 3 scales represented patient values (concern about being a burden, the importance of quality [vs length] of life, and the preference for autonomy in decision-making) and 3 scales represented patient preferences (use of life-prolonging measures, controlling the timing of death, and the location of EOL care).31
The LEAD Guide can be used as a self-assessment tool that is completed individually and then shared with the surrogate decision maker and health care provider.32 It also can be used to guide conversations with the surrogate and physician, as well as with trusted family and friends. Using this framework, family physicians can facilitate EOL planning with the patient and their surrogate that is based on the patient’s values and preferences for EOL care prior to, and in anticipation of, the patient’s loss of decisional capacity.31
Facilitate discussions that improve outcomes
Conversations about EOL care are taking on increased importance as the population ages and treatments advance. Understanding the concerns of patients and their surrogate decision makers, as well as the resources available to guide these difficult discussions ( TABLE ), will help family physicians conduct effective conversations that enhance care, reduce the burden on surrogate decision makers, and have a positive impact on many clinical outcomes.
CORRESPONDENCE
Shirley Bodi, MD, 3000 Arlington Avenue, Department of Family Medicine, Dowling Hall, Suite 2200, University of Toledo College of Medicine and Life Sciences, Toledo, OH 43614; [email protected]
1. Bergenholtz Heidi, Timm HU, Missel M. Talking about end of life in general palliative care – what’s going on? A qualitative study on end-of-life conversations in an acute care hospital in Denmark. BMC Palliat Care. 2019;18:62. doi: 10.1186/s12904-019-0448-z
2. Piers R, Albers G, Gilissen J, et al. Advance care planning in dementia: recommendations for healthcare professionals. BMC Palliat Care. 2018;17:88. doi: 10.1186/s12904-018-0332-2
3. Tunzi M, Ventres W. A reflective case study in family medicine advance care planning conversations. J Am Board Fam Med. 2019;32:108-114. doi: 10.3122/jabfm.2019.01.180198
4. Schickedanz AD, Schillinger D, Landefeld CS, et al. A clinical framework for improving the advance care planning process: start with patients’ self-identified barriers. J Am Geriatr Soc. 2009;57:31-39. doi: 10.1111/j.1532-5415.2008.02093.x
5. Williams BC, Warshaw G, Fabiny AR, et al. Medicine in the 21st century: recommended essential geriatrics competencies for internal medicine and family medicine residents. J Grad Med Ed. 2010;2:373-383. doi: 10.4300/JGME-D-10-00065.1
6. Alano G, Pekmezaris R, Tai J, et al. Factors influencing older adults to complete advance directives. Palliat Support Care. 2010;8:267-275. doi: 10.1017/S1478951510000064
7. Wendler D, Rid A. Systematic review: the effect on surrogates of making treatment decisions for others. Ann Intern Med. 2011;154:336-346. doi: 10.7326/0003-4819-154-5-201103010-00008
8. Edelberg C. Advance care planning with and without an annual wellness visit. Ed Management website. June 1, 2016. Accessed November 16, 2021. ww.reliasmedia.com/articles/137829-advanced-care-planning-with-and-without-an-annual-wellness-visit
9. Centers for Medicare and Medicaid Services. Frequently asked questions about billing the physician fee schedule for advance care planning services. July 14, 2016. Accessed December 20, 2021. www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/FAQ-Advance-Care-Planning.pdf
10. World Health Organization. Palliative care fact sheet. August 5, 2020. Accessed November 16, 2021. www.who.int/news-room/fact-sheets/detail/palliative-care
11. National Institute on Aging. What are palliative care and hospice care? Reviewed May 14, 2021. Accessed December 20, 2021. www.nia.nih.gov/health/what-are-palliative-care-and-hospice-care#palliative-vs-hospice
12. Rabow MW, Dibble SL, Pantilat, SZ, et al. The comprehensive care team: a controlled trial of outpatient palliative medicine consultation. Arch Intern Med. 2004;164:83-91. doi: 10.1001/archinte.164.1.83
13. Muir JC, Daley F, Davis MS, et al. Integrating palliative care into the outpatient, private practice oncology setting. J Pain Symptom Manage. 2010;40:126-135. doi: 10.1016/j.jpainsymman.2009.12.017
14. Hui D, Kim SH, Roquemore J, et al. Impact of timing and setting of palliative care referral on quality of end-of-life care in cancer patients. Cancer. 2014;120:1743-1749. doi: 10.1002/cncr.28628
15. Leung JM, Udris EM, Uman J, e al. The effect of end-of-life discussions on perceived quality of care and health status among patients with COPD. Chest. 2012;142:128-133. doi: 10.1378/chest.11-2222
16. Davison SN. End-of-life care preferences and needs: perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:195-204. doi: 10.2215/CJN.05960809
17. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patients mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665-1673. doi: 10.1001/jama.300.14.1665
18. Park E, Check DK, Yopp JM, et al. An exploratory study of end-of-life prognostic communication needs as reported by widowed fathers due to cancer. Psychooncology. 2015;24:1471-1476. doi: 10.1002/pon.3757
19. Tavares N, Jarrett N, Hunt K, et al. Palliative and end-of-life care conversations in COPD: a systematic literature review. ERJ Open Res. 2017;3:00068-2016. doi: 10.1183/23120541.00068-2016
20. Hancock K, Clayton JM, Parker SM, et al. Truth-telling in discussing prognosis in advanced life-limiting illnesses: a systematic review. Palliat Med. 2007;21:507-517. doi: 10.1177/0269216307080823
21. Parker SM, Clayton JM, Hancock K, et al. A systematic review of prognostic/end-of-life communication with adults in the advanced stages of a life-limiting illness: patient/caregiver preferences for the content, style, and timing of information. J Pain Symptom Manage. 2007;34:81-93. doi: 10.1016/j.jpainsymman.2006.09.035
22. Janssen DJA, Spruit MA, Schols JMGA, et al. A call for high-quality advance care planning in outpatients with severe COPD or chronic heart failure. Chest. 2011;139:1081-1088. doi: 10.1378/chest.10-1753
23. Houben CHM, Spruit MA, Schols JM, et al. Patient-clinician communication about end-of-life care on patients with advanced chronic organ failure during one year. J Pain Symptom Manage. 2015;49:1109-1115. doi: 10.1016/j.jpainsymman.2014.12.008
24. Brighton LJ, Bristowe K. Communication in palliative care: talking about the end of life, before the end of life. Postgrad Med J. 2016;92:466-470. doi: 10.1136/postgradmedj-2015-133368
25. VitalTalk website. Accessed December 20, 2021. vitaltalk.org
26. Rabow MQ, McPhee SJ. Beyond breaking bad news: how to help patients who suffer. Wes J Med. 1999;171:260-263. www.ncbi.nlm.nih.gov/pmc/articles/PMC1305864
27. Pfeifer M, Head B. Which critical communication skills are essential for interdisciplinary end-of-life discussions? AMA J Ethics. 2018;8:E724-E731. doi: 10.1001/amajethics.2018.724
28. Song M-K, Ward SE, Hepburn K, et al. SPIRIT advance care planning intervention in early stage dementias: an NIH stage I behavioral intervention development trial. Contemp Clin Trials. 2018;71:55-62. doi: 10.1016/j.cct.2018.06.005
29. Song M-K, Ward SE, Hepburn K, et al. Can persons with dementia meaningfully participate in advance care planning discussions? A mixed-methods study of SPIRIT. J Palliat Med. 2019;22:1410-1416. doi: 10.1089/jpm.2019.0088
30. Two-phased study of SPIRIT in mild dementia. ClinicalTrials.gov Identifier: NCT03311711. Updated August 23, 2021. Accessed December 20, 2021. clinicaltrials.gov/ct2/show/NCT03311711
31. Dassel K, Utz R, Supiano K, et al. Development of a dementia-focused end-of-life planning tool: the LEAD Guide (Life-planning in Early Alzheimer’s and Dementia). Innov Aging. 2019;3:igz024. doi: 10.1093/geroni/igz024
32. Dassel K, Supiano K, Utz R, et al. The LEAD Guide. Life-planning in Early Alzheimer’s and Dementia. 2019. Accessed December 20, 2021. utahgwep.org/resources/search-all-resources/send/10-dementia/27-the-lead-guide#:~:text=The%20LEAD%20Guide%20(Life%2DPlanning,your%20decisions%20about%20your%20care
1. Bergenholtz Heidi, Timm HU, Missel M. Talking about end of life in general palliative care – what’s going on? A qualitative study on end-of-life conversations in an acute care hospital in Denmark. BMC Palliat Care. 2019;18:62. doi: 10.1186/s12904-019-0448-z
2. Piers R, Albers G, Gilissen J, et al. Advance care planning in dementia: recommendations for healthcare professionals. BMC Palliat Care. 2018;17:88. doi: 10.1186/s12904-018-0332-2
3. Tunzi M, Ventres W. A reflective case study in family medicine advance care planning conversations. J Am Board Fam Med. 2019;32:108-114. doi: 10.3122/jabfm.2019.01.180198
4. Schickedanz AD, Schillinger D, Landefeld CS, et al. A clinical framework for improving the advance care planning process: start with patients’ self-identified barriers. J Am Geriatr Soc. 2009;57:31-39. doi: 10.1111/j.1532-5415.2008.02093.x
5. Williams BC, Warshaw G, Fabiny AR, et al. Medicine in the 21st century: recommended essential geriatrics competencies for internal medicine and family medicine residents. J Grad Med Ed. 2010;2:373-383. doi: 10.4300/JGME-D-10-00065.1
6. Alano G, Pekmezaris R, Tai J, et al. Factors influencing older adults to complete advance directives. Palliat Support Care. 2010;8:267-275. doi: 10.1017/S1478951510000064
7. Wendler D, Rid A. Systematic review: the effect on surrogates of making treatment decisions for others. Ann Intern Med. 2011;154:336-346. doi: 10.7326/0003-4819-154-5-201103010-00008
8. Edelberg C. Advance care planning with and without an annual wellness visit. Ed Management website. June 1, 2016. Accessed November 16, 2021. ww.reliasmedia.com/articles/137829-advanced-care-planning-with-and-without-an-annual-wellness-visit
9. Centers for Medicare and Medicaid Services. Frequently asked questions about billing the physician fee schedule for advance care planning services. July 14, 2016. Accessed December 20, 2021. www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/FAQ-Advance-Care-Planning.pdf
10. World Health Organization. Palliative care fact sheet. August 5, 2020. Accessed November 16, 2021. www.who.int/news-room/fact-sheets/detail/palliative-care
11. National Institute on Aging. What are palliative care and hospice care? Reviewed May 14, 2021. Accessed December 20, 2021. www.nia.nih.gov/health/what-are-palliative-care-and-hospice-care#palliative-vs-hospice
12. Rabow MW, Dibble SL, Pantilat, SZ, et al. The comprehensive care team: a controlled trial of outpatient palliative medicine consultation. Arch Intern Med. 2004;164:83-91. doi: 10.1001/archinte.164.1.83
13. Muir JC, Daley F, Davis MS, et al. Integrating palliative care into the outpatient, private practice oncology setting. J Pain Symptom Manage. 2010;40:126-135. doi: 10.1016/j.jpainsymman.2009.12.017
14. Hui D, Kim SH, Roquemore J, et al. Impact of timing and setting of palliative care referral on quality of end-of-life care in cancer patients. Cancer. 2014;120:1743-1749. doi: 10.1002/cncr.28628
15. Leung JM, Udris EM, Uman J, e al. The effect of end-of-life discussions on perceived quality of care and health status among patients with COPD. Chest. 2012;142:128-133. doi: 10.1378/chest.11-2222
16. Davison SN. End-of-life care preferences and needs: perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:195-204. doi: 10.2215/CJN.05960809
17. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patients mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665-1673. doi: 10.1001/jama.300.14.1665
18. Park E, Check DK, Yopp JM, et al. An exploratory study of end-of-life prognostic communication needs as reported by widowed fathers due to cancer. Psychooncology. 2015;24:1471-1476. doi: 10.1002/pon.3757
19. Tavares N, Jarrett N, Hunt K, et al. Palliative and end-of-life care conversations in COPD: a systematic literature review. ERJ Open Res. 2017;3:00068-2016. doi: 10.1183/23120541.00068-2016
20. Hancock K, Clayton JM, Parker SM, et al. Truth-telling in discussing prognosis in advanced life-limiting illnesses: a systematic review. Palliat Med. 2007;21:507-517. doi: 10.1177/0269216307080823
21. Parker SM, Clayton JM, Hancock K, et al. A systematic review of prognostic/end-of-life communication with adults in the advanced stages of a life-limiting illness: patient/caregiver preferences for the content, style, and timing of information. J Pain Symptom Manage. 2007;34:81-93. doi: 10.1016/j.jpainsymman.2006.09.035
22. Janssen DJA, Spruit MA, Schols JMGA, et al. A call for high-quality advance care planning in outpatients with severe COPD or chronic heart failure. Chest. 2011;139:1081-1088. doi: 10.1378/chest.10-1753
23. Houben CHM, Spruit MA, Schols JM, et al. Patient-clinician communication about end-of-life care on patients with advanced chronic organ failure during one year. J Pain Symptom Manage. 2015;49:1109-1115. doi: 10.1016/j.jpainsymman.2014.12.008
24. Brighton LJ, Bristowe K. Communication in palliative care: talking about the end of life, before the end of life. Postgrad Med J. 2016;92:466-470. doi: 10.1136/postgradmedj-2015-133368
25. VitalTalk website. Accessed December 20, 2021. vitaltalk.org
26. Rabow MQ, McPhee SJ. Beyond breaking bad news: how to help patients who suffer. Wes J Med. 1999;171:260-263. www.ncbi.nlm.nih.gov/pmc/articles/PMC1305864
27. Pfeifer M, Head B. Which critical communication skills are essential for interdisciplinary end-of-life discussions? AMA J Ethics. 2018;8:E724-E731. doi: 10.1001/amajethics.2018.724
28. Song M-K, Ward SE, Hepburn K, et al. SPIRIT advance care planning intervention in early stage dementias: an NIH stage I behavioral intervention development trial. Contemp Clin Trials. 2018;71:55-62. doi: 10.1016/j.cct.2018.06.005
29. Song M-K, Ward SE, Hepburn K, et al. Can persons with dementia meaningfully participate in advance care planning discussions? A mixed-methods study of SPIRIT. J Palliat Med. 2019;22:1410-1416. doi: 10.1089/jpm.2019.0088
30. Two-phased study of SPIRIT in mild dementia. ClinicalTrials.gov Identifier: NCT03311711. Updated August 23, 2021. Accessed December 20, 2021. clinicaltrials.gov/ct2/show/NCT03311711
31. Dassel K, Utz R, Supiano K, et al. Development of a dementia-focused end-of-life planning tool: the LEAD Guide (Life-planning in Early Alzheimer’s and Dementia). Innov Aging. 2019;3:igz024. doi: 10.1093/geroni/igz024
32. Dassel K, Supiano K, Utz R, et al. The LEAD Guide. Life-planning in Early Alzheimer’s and Dementia. 2019. Accessed December 20, 2021. utahgwep.org/resources/search-all-resources/send/10-dementia/27-the-lead-guide#:~:text=The%20LEAD%20Guide%20(Life%2DPlanning,your%20decisions%20about%20your%20care
PRACTICE RECOMMENDATIONS
› Improve patients’ quality of life and satisfaction with care through the successful implementation of palliative care. C
› Initiate end-of-life (EOL) discussions with patients with dementia at diagnosis, while the patient is cognizant and able to actively express their values and preferences for EOL care. C
› Engage surrogate decision makers in conversations about dementia, its trajectory, and their role in EOL care early in the process. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Gestational diabetes: Optimizing Dx and management in primary care
Gestational diabetes mellitus (GDM), defined as new-onset hyperglycemia detected in a pregnant woman after 24 weeks of gestation, affects 4% to 10% of pregnancies in the United States annually1 and is a major challenge for health care professionals.2 During pregnancy, the body’s physiologic responses are altered to support the growing fetus. One of these changes is an increase in insulin resistance, which suggests that pregnancy alone increases the patient’s risk for type 2 diabetes (T2D). However, several other factors also increase this risk, including maternal age, social barriers to care, obesity, poor weight control, and family history.
If not controlled, GDM results in poor health outcomes for the mother, such as preeclampsia, preterm labor, and maternal T2D.3-5 For the infant, intrauterine exposure to persistent hyperglycemia is correlated with neonatal macrosomia, hypoglycemia, perinatal complications (eg, preterm delivery, fetal demise), and obesity and insulin resistance later in life.4
Primary care physicians (PCPs) are the patient’s main point of contact prior to pregnancy. This relationship makes PCPs a resource for the patient and specialists during and after pregnancy. In this article, we discuss risk factors and how to screen for GDM, provide an update on practice recommendations for treatment and management of GDM in primary care, and describe the effects of uncontrolled GDM.
Know the key risk factors
Prevention begins with identifying the major risk factors that contribute to the development of GDM. These include maternal age, social barriers to care, family history of prediabetes, and obesity and poor weight control.
Older age. A meta-analysis of 24 studies noted strong positive correlation between GDM risk and maternal age.6 One of the population-based cohort studies in the meta-analysis examined relationships between maternal age and pregnancy outcomes in women living in British Columbia, Canada (n = 203,414). Data suggested that the relative risk of GDM increased linearly with maternal age to 3.2, 4.2, and 4.4 among women ages ≥ 35, ≥ 40, and ≥ 45 years, respectively.7
Social barriers to care. Although the prevalence of GDM has increased over the past few decades,1 from 2011 to 2019 the increase in GDM in individuals at first live birth was significantly higher in non-Hispanic Asian and Hispanic/Latina women than in non-Hispanic White women.8 Data from the Centers for Disease Control and Prevention further suggest that diabetes was more prevalent among individuals with a lower socioeconomic status as indicated by their level of education.9 Ogunwole et al10 suggest that racism is the root cause of these disparities and leads to long-term barriers to care (eg, socioeconomic deprivation, lack of health insurance, limited access to care, and poor health literacy), which ultimately contribute to the development of GDM and progression of diabetes. It is important for PCPs and all health professionals to be aware of these barriers so that they may practice mindfulness and deliver culturally sensitive care to patients from marginalized communities.
Family history of prediabetes. In a population-based cohort study (n = 7020), women with prediabetes (A1C, 5.7%-6.4%) were 2.8 times more likely to develop GDM compared with women with normal A1C (< 5.7%).11 Similar results were seen in a retrospective cohort study (n = 2812), in which women with prediabetes were more likely than women with a normal first trimester A1C to have GDM (29.1% vs 13.7%, respectively; adjusted relative risk = 1.48; 95% CI, 1.15-1.89).12 In both studies, prediabetes was not associated with a higher risk for adverse maternal or neonatal outcomes.11,12
Continue to: While there are no current...
While there are no current guidelines for treating prediabetes in pregnancy, women diagnosed with prediabetes in 1 study were found to have significantly less weight gain during pregnancy compared with patients with normal A1C,12 suggesting there may be a benefit in early identification and intervention, although further research is needed.11 In a separate case-control study (n = 345 women with GDM; n = 800 control), high rates of gestational weight gain (> 0.41 kg/wk) were associated with an increased risk of GDM (odds ratio [OR] = 1.74; 95% CI, 1.16-2.60) compared with women with the lowest rate of gestational weight gain (0.27-0.4 kg/wk [OR = 1.43; 95% CI, 0.96-2.14]).13 Thus, it is helpful to have proactive conversations about family planning and adequate weight and glycemic control with high-risk patients to prepare for a healthy pregnancy.
Obesity and weight management. Patients who are overweight (body mass index [BMI], 25-29.9) or obese (BMI > 30) have a substantially increased risk of GDM (adjusted OR = 1.44; 95% CI, 1.04-1.81), as seen in a retrospective cohort study of 1951 pregnant Malaysian women.14 Several factors have been found to contribute to successful weight control, including calorie prescription, a structured meal plan, high physical activity goals (60-90 min/d), daily weighing and monitoring of food intake, behavior therapy, and continued patient–provider contact.15
The safety, efficacy, and sustainability of weight loss with various dietary plans have been studied in individuals who are overweight and obese.16 Ultimately, energy expenditure must be greater than energy intake to promote weight loss. Conventional diets with continuous energy restriction (ie, low-fat, low-carbohydrate, and high-protein diets) have proven to be effective for short-term weight loss but data on long-term weight maintenance are limited.16 The Mediterranean diet, which is comprised mostly of vegetables, fruits, legumes, fish, and grains—with a lower intake of meat and dairy—may reduce gestational weight gain and risk of GDM as suggested by a randomized controlled trial (RCT; n = 1252).17 Although the choice of diet is up to the patient, it is important to be aware of different diets or refer the patient to a registered dietician who can help the patient if needed.
Reduce risk with adequate weight and glycemic control
Prevention of GDM during pregnancy should focus on weight maintenance and optimal glycemic control. Two systematic reviews, one with 8 RCTs (n = 1792) and another with 5 studies (n = 539), assessed the efficacy and safety of energy-restricted dietary intervention on GDM prevention.18 The first review found a significant reduction in gestational weight gain and improved glycemic control without increased risk of adverse maternal and fetal outcomes.18 The second review showed no clear difference between energy-restricted and non–energy-restricted diets on outcomes such as preeclampsia, gestational weight gain, large for gestational age, and macrosomia.18 These data suggest that while energy-restricted dietary interventions made no difference on maternal and fetal complications, they may still be safely used in pregnancy to reduce gestational weight gain and improve glycemic control.18
Once a woman is pregnant, it becomes difficult to lose weight because additional calories are needed to support a growing fetus. It is recommended that patients with healthy pregestational BMI consume an extra 200 to 300 calories/d after the first trimester. However, extra caloric intake in a woman with obesity who is pregnant leads to metabolic impairment and increased risk of diabetes for both the mother and fetus.19 Therefore, it is recommended that patients with obese pregestational BMI not consume additional calories because excess maternal fat is sufficient to support the energy needs of the growing fetus.19
Continue to: Ultimately, earlier intervention...
Ultimately, earlier intervention—prior to conception—helps patients prepare for a healthier pregnancy, resulting in better long-term outcomes. It is helpful to be familiar with the advantages and disadvantages of common approaches to weight management and to be able to refer patients to nutritionists for optimal planning. When establishing a dietary plan, consider patient-specific factors, such as cultural diets, financial and time constraints, and the patient’s readiness to make and maintain these changes. Consistent follow-up and behavioral therapy are necessary to maintain successful weight control.
There are many screening tools, but 1 is preferred in pregnancy
There are several ways to diagnose diabetes in patients who are not pregnant, including A1C, a fasting glucose test, an oral glucose tolerance test (OGTT), or random glucose testing (plus symptoms). However, the preferred method for diagnosing GDM is OGTT because it has a higher sensitivity.20 A1C, while a good measure of hyperglycemic stability, does not register hyperglycemia early enough to diagnose GDM and fasting glucose testing is less sensitive because for most women with GDM, that abnormal postprandial glucose level is the first glycemic abnormality.21
When to screen. Blood glucose levels should be checked in all pregnant women as part of their metabolic panel at the first prenatal visit. A reflex A1C for high glucose levels can be ordered based on the physician’s preference. This may help you to identify patients with prediabetes who are at risk for GDM and implement early behavioral and lifestyle changes. However, further research is needed to determine if intervention early in pregnancy can truly reduce the risk of GDM.11
Screening for GDM should be completed at 24 to 28 weeks of gestation20 because it is likely that this is when the hormonal effects of the placenta that contribute to insulin resistance set the woman up for postprandial hyperglycemia. Currently, there are no evidence-based guidelines for the use of continuous glucose monitoring prior to 24 weeks of gestation to identify GDM.20 If persistent hyperglycemia is present before 24 weeks of gestation, it is considered evidence of a pre-existing metabolic abnormality and is diagnosed as “pregestational diabetes.” Treatment should follow guidelines established for women who had diabetes prior to pregnancy.
How to screen? There is ongoing discussion about what is the optimal screening method for GDM: a 1-step strategy with a fasting 75-g OGTT only, or a 2-step strategy with a 50-g non-fasting glucose load test followed by a fasting 100-g OGTT in women who do not meet the plasma glucose cutoff (TABLE 1).22-24 Hillier et al25 compared the effectiveness of these strategies in diagnosing GDM and identifying pregnancy complications for the mother and infant. They found that while the 1-step strategy resulted in a 2-fold increase in the diagnosis of GDM, it did not lead to better outcomes for mothers and infants when compared with the 2-step method.25 Currently, the majority of obstetricians (95%) prefer to use the 2-step method.24
Continue to: Manage lifestyle, monitor glucose
Manage lifestyle, monitor glucose
Management of GDM in most women starts with diabetes self-management education and support for therapeutic lifestyle changes, such as nutritional interventions that reduce hyperglycemia and contribute to healthy weight gain during pregnancy.20 This may include medical nutrition therapy that focuses on adequate nutrition for the mother and fetus. Currently, the recommended dietary intake for women who are pregnant (regardless of diabetes) includes a minimum of 175 g of carbohydrates, 71 g of daily protein, and at least 28 g of fiber. Further refinement of dietary intake, including carbohydrate restriction, should be done with guidance from a registered dietitian.20 If the obstetrics team does not include a registered dietitian, a referral to one may be necessary. Regular physical activity should be continued throughout pregnancy as tolerated. Social support, stress reduction, and good sleep hygiene should be encouraged as much as possible.
For successful outcomes, therapeutic lifestyle changes should be coupled with glucose monitoring. The Fifth International Workshop-Conference on Gestational Diabetes Mellitus recommends that women with GDM monitor fasting blood glucose and typically 1-hour postprandial glucose. The glucose goals in GDM are as follows26:
- Fasting glucose < 95 mg/dL (5.3 mmol/L), and either
- 1-hour postprandial glucose < 140 mg/dL (7.8 mmol/L), or
- 2-hour postprandial glucose < 120 mg/dL (6.7 mmol/L).
Importantly, in the second and third trimester, the A1C goal for women with GDM is 6.0%. This is lower than the more traditional A1C goal for 2 reasons: (1) increases in A1C, even within the normal range, increase adverse outcomes; and (2) pregnant women will have an increased red blood cell count turnover, which can lower the A1C.27 In a historical cohort study (n = 27,213), Abell et al28 found that women who have an A1C < 6.0% in the second and third trimester have the lowest risk of giving birth to large-for-gestational-age infants and for having preeclampsia.
Add insulin if glucose targets are not met
Most women who engage in therapeutic lifestyle change (70%-85%) can achieve an A1C < 6% and will not need to take medication to manage GDM.29 If pharmacotherapy is needed to manage glucose, insulin is the preferred treatment for all women with GDM.20 Treatment should be individualized based on the glucose trends the woman is experiencing. Common treatments include bedtime NPH if fasting hyperglycemia is most prominent and analogue insulin at mealtimes for women with prominent postprandial hyperglycemia.
Noninsulin agents such as metformin and sulfonylureas are not currently recommended by the American College of Obstetricians and Gynecologists or the American Diabetes Association for use in GDM.20,24 Despite being used for years in women with pregestational diabetes, metabolic syndrome, and polycystic ovary syndrome, there is evidence that metformin crosses the placenta and fetal safety has not yet been established in RCTs. The Metformin in Gestational Diabetes: The Offspring Follow-Up (MiG TOFU) study was a longitudinal follow-up study that evaluated body composition and metabolic outcomes in children (ages 7-9 years) of women with GDM who had received metformin or insulin while pregnant.30 At age 9 years, children who were exposed to metformin weighed more and had a higher waist-to-height ratio and waist circumference than those exposed to insulin.30
Continue to: Sulfonylureas are no longer recommended...
Sulfonylureas are no longer recommended because of the risk of maternal and fetal hypoglycemia and concerns about this medication crossing the placenta.24,31,32 Specifically, in a 2015 meta-analysis and systematic review of 15 articles (n = 2509), glyburide had a higher risk of neonatal hypoglycemia and macrosomia than insulin or metformin.33 For women who cannot manage their glucose with therapeutic lifestyle changes and cannot take insulin, oral therapies may be considered if the risk-benefit ratio is balanced for that person.34
Watch for effects of poor glycemic control on mother, infant
Preeclampsia is defined as new-onset hypertension and proteinuria after 20 weeks of gestation. The correlation between GDM and preeclampsia has partly been explained by their shared overlapping risk factors, including maternal obesity, excessive gestational weight gain, and persistent hyperglycemia.35 On a biochemical level, these risk factors contribute to oxidative stress and systemic vascular dysfunction, which have been hypothesized as the underlying pathophysiology for the development of preeclampsia.35
Neonatal macrosomia, defined as a birth weight ≥ 4000 g, is a common complication that develops in 15% to 45% of infants of mothers with GDM.36 Placental transfer of glucose in mothers with hyperglycemia stimulates the secretion of neonatal insulin and the ultimate storage of the excess glucose as body fat. After delivery, the abrupt discontinuation of placental transfer of glucose to an infant who is actively secreting insulin leads to neonatal hypoglycemia, which if not detected or managed, can lead to long-term neurologic deficits, including recurrent seizures and developmental delays.37 Therefore, it is essential to screen for neonatal hypoglycemia immediately after birth and serially up to 12 hours.38
Postpartum T2D. Poor glycemic control increases the risk of increasing insulin resistance developing into T2D postpartum for mothers.39 It also increases the risk of obesity and insulin resistance later in life for the infant.40 A retrospective cohort study (n = 461) found a positive correlation between exposure to maternal GDM and elevated BMI in children ages 6 to 13 years.41 Kamana et al36 further discussed this correlation and suggested that exposure to maternal hyperglycemia in utero contributes to fetal programming of later adipose deposition. Children may develop without a notable increase in BMI until after puberty.42
Partner with specialists to improve outcomes
Although most women with GDM are managed by specialists (obstetricians, endocrinologists, and maternal-fetal medicine specialists),43 these patients are still seeking care from their family physicians for other complaints. These visits provide key touchpoints during pregnancy and are opportunities for PCPs to identify a pregnancy-related complication or provide additional education or referral to the obstetrician.
Continue to: Also, if you work in an area...
Also, if you work in an area where specialists are less accessible, you may be the clinician providing the majority of care to a patient with GDM. If this is the case, you’ll want to watch for the following risk factors, which should prompt a referral to specialty care:
- a previous pregnancy with GDM20
- a previous birth of an infant weighing > 4000 g44
- baseline history of hypertension45
- evidence of insulin resistance or polycystic ovary syndrome46,47
- a history of cardiovascular disease20
- a need to treat GDM with pharmacotherapy.48
Ensuring a smooth transition after the birth
Optimal communication and hand-offs throughout pregnancy and after delivery will benefit everyone. When the pregnant patient’s care has been managed by an obstetrician, it is important to address the following issues during the hand-off:
- baseline medical problems
- medical screenings and treatments in pregnancy (retinopathy and nephropathy screening)
- aspirin initiation, if indicated
- management of thyroid abnormalities
- management of mental health conditions
- postpartum glucose management and T2D screening postpartum
- management of complications identified during pregnancy (retinopathy and nephropathy).
Timing and other elements of postpartum care. The first postpartum screen should occur at 4 to 12 weeks postpartum. OGTT is recommended instead of A1C at this time because A1C may still be lowered by the increased red blood cell turnover related to pregnancy and blood loss at delivery. Because women with GDM have a 50% to 75% lifetime risk of T2D,20 patients with normal test results should be re-tested every 1 to 3 years using any of the standard screening methods (A1C, fasting glucose, or OGTT).20
After delivery it may be difficult for women to follow-up with their own personal health care because they are focused on the care of their baby. The increased use of telehealth may make postpartum follow-up visits easier to attend.
Visits present opportunities. Postpartum visits present another opportunity for PCPs to screen for diabetes and other postpartum complications, including depression and thyroid abnormalities. Visits are also an opportunity to discuss timely contraception so as to prevent an early, unplanned pregnancy. Other important aspects of postpartum care are outlined in TABLE 2.20,49
CORRESPONDENCE
Connie L. Ha, BS, OMS IV, Department of Primary Care, 1310 Club Drive, Touro University California, Vallejo, CA 94592; [email protected]
1. Sheiner E. Gestational diabetes mellitus: long-term consequences for the mother and child grand challenge: how to move on towards secondary prevention? Front Clin Diabetes Healthc. 2020. doi: 10.3389/fcdhc.2020.546256
2. Angueira AR, Ludvik AE, Reddy TE, et al. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327-334. doi: 10.2337/db14-0877
3. Shou C, Wei Y-M, Wang C, et al. Updates in long-term maternal and fetal adverse effects of gestational diabetes mellitus. Maternal-Fetal Med. 2019;1:91-94. doi: 10.1097/FM9.0000000000000019
4. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342. doi: 10.3390/ijms19113342
5. Kulshrestha V, Agarwal N. Maternal complications in pregnancy with diabetes. J Pak Med Assoc. 2016;66(9 suppl 1):S74-S77.
6. Li Y, Ren X, He L, et al. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. doi: 10.1016/j.diabres.2020.108044
7. Schummers L, Hutcheon JA, Hacker MR, et al. Absolute risks of obstetric outcomes by maternal age at first birth: a population-based cohort. Epidemiology. 2018;29:379-387. doi: 10.1097/EDE.0000000000000818
8. Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326:660-669. doi: 10.1001/jama.2021.7217
9. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. Accessed February 2, 2022. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
10. Ogunwole SM, Golden SH. Social determinants of health and structural inequities—root causes of diabetes disparities. Diabetes Care. 2021;44:11-13. doi: 10.2337/dci20-0060
11. Chen L, Pocobelli G, Yu O, et al. Early pregnancy hemoglobin A1C and pregnancy outcomes: a population-based study. Am J Perinatol. 2019;36:1045-1053. doi: 10.1055/s-0038-1675619
12. Osmundson S, Zhao BS, Kunz L, et al. First trimester hemoglobin A1C prediction of gestational diabetes. Am J Perinatol. 2016;33:977-982. doi: 10.1055/s-0036-1581055
13. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus [published correction appears in Obstet Gynecol. 2010;115:1092]. Obstet Gynecol. 2010;115:597-604. doi: 10.1097/AOG.0b013e3181cfce4f
14. Yong HY, Mohd Shariff Z, Mohd Yusof BN, et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep. 2020;10:8486. doi: 10.1038/s41598-020-65251-2
15. Phelan S. Windows of opportunity for lifestyle interventions to prevent gestational diabetes mellitus. Am J Perinatol. 2016;33:1291-1299. doi: 10.1055/s-0036-1586504
16. Koliaki C, Spinos T, Spinou M, et al. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel). 2018;6:73. doi: 10.3390/healthcare6030073
17. Al Wattar BH, Dodds J, Placzek A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLOS Med. 2019;16:e1002857. doi: 10.1371/journal.pmed.1002857
18. Zarogiannis S. Are novel lifestyle approaches to management of type 2 diabetes applicable to prevention and treatment of women with gestational diabetes mellitus? Global Diabetes Open Access J. 2019;1:1-14.
19. Most J, Amant MS, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690. doi: 10.1172/JCI130341
20. American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S200-S210. doi: 10.2337/dc21-S014
21. McIntyre HD, Sacks DA, Barbour LA, et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care. 2016;39:53-54. doi: 10.2337/dc15-1887
22. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
23. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
24. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49-e64. doi: 10.1097/AOG.0000000000002501
25. Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384:895-904. doi: 10.1056/NEJMoa2026028
26. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260. doi: 10.2337/dc07-s225
27. Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200-1201. doi: 10.2337/diacare.27.5.1200
28. Abell SK, Boyle JA, de Courten B, et al. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2017;57:308-314. doi: 10.1111/ajo.12521
29. Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014;37:3345-3355. doi: 10.2337/dc14-1530
30. Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018;6:e000456. doi: 10.1136/bmjdrc-2017-000456
31. Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85:607-614. doi: 10.1038/clpt.2009.5
32. Malek R, Davis SN. Pharmacokinetics, efficacy and safety of glyburide for treatment of gestational diabetes mellitus. Expert Opin Drug Metab Toxicol. 2016;12:691-699. doi: 10.1080/17425255.2016.1187131
33. Balsells M, García-Patterson A, Solà I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. doi: 10.1136/bmj.h102
34. Kavitha N, De S, Kanagasabai S. Oral hypoglycemic agents in pregnancy: an update. J Obstet Gynaecol India. 2013;63:82-87. doi: 10.1007/s13224-012-0312-z
35. Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15:9. doi: 10.1007/s11892-015-0579-4
36. Kamana KC, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(suppl 2):14-20. doi: 10.1159/000371628
37. Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734-743. doi: 10.4239/wjd.v6.i5.734
38. Stanescu A, Stoicescu SM. Neonatal hypoglycemia screening in newborns from diabetic mothers—arguments and controversies. J Med Life. 2014;7(spec iss 3):51-52.
39. Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med. 2014;31:292-301. doi: 10.1111/dme.12382
40. Stewart A, Malhotra A. Gestational diabetes and the neonate: challenges and solutions. Res Rep Neonatol. 2015;5:31-39. doi: 10.2147/RRN.S30971
41. Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87-92. doi: 10.1007/s00125-010-1925-3
42. Crume TL, Ogden L, Daniels S, et al. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 2011;158:941-946. doi: 10.1016/j.jpeds.2010.12.007
43. Levels of maternal care. Obstetric Care Consensus No. 9. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2019;134:e41-e55. doi: 10.1097/AOG.0000000000003383
44. Caughey AB, Cheng YW, Stotland NE, et al. Maternal and paternal race/ethnicity are both associated with gestational diabetes. Am J Obstet Gynecol. 2010;202:616.e1-e5. doi: 10.1016/j.ajog.2010.01.082
45. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191:1655-1660. doi: 10.1016/j.ajog.2004.03.074
46. Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;5:CD011970. doi: 10.1002/14651858.CD011970.pub2
47. Ceysens G, Rouiller D, Boulvain M. Exercise for the diabetic pregnant woman. Cochrane Database Syst Rev. 2006;3:CD004225. doi: 10.1002/14651858.CD004225.pub2
48. Chawla R, Mukherjee JJ, Chawla M, et al. Expert group recommendations on the effective use of bolus insulin in the management of type 2 diabetes mellitus. Med Sci (Basel). 2021;9:38. doi: 10.3390/medsci9020038
49. American Diabetes Association. Introduction: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
Gestational diabetes mellitus (GDM), defined as new-onset hyperglycemia detected in a pregnant woman after 24 weeks of gestation, affects 4% to 10% of pregnancies in the United States annually1 and is a major challenge for health care professionals.2 During pregnancy, the body’s physiologic responses are altered to support the growing fetus. One of these changes is an increase in insulin resistance, which suggests that pregnancy alone increases the patient’s risk for type 2 diabetes (T2D). However, several other factors also increase this risk, including maternal age, social barriers to care, obesity, poor weight control, and family history.
If not controlled, GDM results in poor health outcomes for the mother, such as preeclampsia, preterm labor, and maternal T2D.3-5 For the infant, intrauterine exposure to persistent hyperglycemia is correlated with neonatal macrosomia, hypoglycemia, perinatal complications (eg, preterm delivery, fetal demise), and obesity and insulin resistance later in life.4
Primary care physicians (PCPs) are the patient’s main point of contact prior to pregnancy. This relationship makes PCPs a resource for the patient and specialists during and after pregnancy. In this article, we discuss risk factors and how to screen for GDM, provide an update on practice recommendations for treatment and management of GDM in primary care, and describe the effects of uncontrolled GDM.
Know the key risk factors
Prevention begins with identifying the major risk factors that contribute to the development of GDM. These include maternal age, social barriers to care, family history of prediabetes, and obesity and poor weight control.
Older age. A meta-analysis of 24 studies noted strong positive correlation between GDM risk and maternal age.6 One of the population-based cohort studies in the meta-analysis examined relationships between maternal age and pregnancy outcomes in women living in British Columbia, Canada (n = 203,414). Data suggested that the relative risk of GDM increased linearly with maternal age to 3.2, 4.2, and 4.4 among women ages ≥ 35, ≥ 40, and ≥ 45 years, respectively.7
Social barriers to care. Although the prevalence of GDM has increased over the past few decades,1 from 2011 to 2019 the increase in GDM in individuals at first live birth was significantly higher in non-Hispanic Asian and Hispanic/Latina women than in non-Hispanic White women.8 Data from the Centers for Disease Control and Prevention further suggest that diabetes was more prevalent among individuals with a lower socioeconomic status as indicated by their level of education.9 Ogunwole et al10 suggest that racism is the root cause of these disparities and leads to long-term barriers to care (eg, socioeconomic deprivation, lack of health insurance, limited access to care, and poor health literacy), which ultimately contribute to the development of GDM and progression of diabetes. It is important for PCPs and all health professionals to be aware of these barriers so that they may practice mindfulness and deliver culturally sensitive care to patients from marginalized communities.
Family history of prediabetes. In a population-based cohort study (n = 7020), women with prediabetes (A1C, 5.7%-6.4%) were 2.8 times more likely to develop GDM compared with women with normal A1C (< 5.7%).11 Similar results were seen in a retrospective cohort study (n = 2812), in which women with prediabetes were more likely than women with a normal first trimester A1C to have GDM (29.1% vs 13.7%, respectively; adjusted relative risk = 1.48; 95% CI, 1.15-1.89).12 In both studies, prediabetes was not associated with a higher risk for adverse maternal or neonatal outcomes.11,12
Continue to: While there are no current...
While there are no current guidelines for treating prediabetes in pregnancy, women diagnosed with prediabetes in 1 study were found to have significantly less weight gain during pregnancy compared with patients with normal A1C,12 suggesting there may be a benefit in early identification and intervention, although further research is needed.11 In a separate case-control study (n = 345 women with GDM; n = 800 control), high rates of gestational weight gain (> 0.41 kg/wk) were associated with an increased risk of GDM (odds ratio [OR] = 1.74; 95% CI, 1.16-2.60) compared with women with the lowest rate of gestational weight gain (0.27-0.4 kg/wk [OR = 1.43; 95% CI, 0.96-2.14]).13 Thus, it is helpful to have proactive conversations about family planning and adequate weight and glycemic control with high-risk patients to prepare for a healthy pregnancy.
Obesity and weight management. Patients who are overweight (body mass index [BMI], 25-29.9) or obese (BMI > 30) have a substantially increased risk of GDM (adjusted OR = 1.44; 95% CI, 1.04-1.81), as seen in a retrospective cohort study of 1951 pregnant Malaysian women.14 Several factors have been found to contribute to successful weight control, including calorie prescription, a structured meal plan, high physical activity goals (60-90 min/d), daily weighing and monitoring of food intake, behavior therapy, and continued patient–provider contact.15
The safety, efficacy, and sustainability of weight loss with various dietary plans have been studied in individuals who are overweight and obese.16 Ultimately, energy expenditure must be greater than energy intake to promote weight loss. Conventional diets with continuous energy restriction (ie, low-fat, low-carbohydrate, and high-protein diets) have proven to be effective for short-term weight loss but data on long-term weight maintenance are limited.16 The Mediterranean diet, which is comprised mostly of vegetables, fruits, legumes, fish, and grains—with a lower intake of meat and dairy—may reduce gestational weight gain and risk of GDM as suggested by a randomized controlled trial (RCT; n = 1252).17 Although the choice of diet is up to the patient, it is important to be aware of different diets or refer the patient to a registered dietician who can help the patient if needed.
Reduce risk with adequate weight and glycemic control
Prevention of GDM during pregnancy should focus on weight maintenance and optimal glycemic control. Two systematic reviews, one with 8 RCTs (n = 1792) and another with 5 studies (n = 539), assessed the efficacy and safety of energy-restricted dietary intervention on GDM prevention.18 The first review found a significant reduction in gestational weight gain and improved glycemic control without increased risk of adverse maternal and fetal outcomes.18 The second review showed no clear difference between energy-restricted and non–energy-restricted diets on outcomes such as preeclampsia, gestational weight gain, large for gestational age, and macrosomia.18 These data suggest that while energy-restricted dietary interventions made no difference on maternal and fetal complications, they may still be safely used in pregnancy to reduce gestational weight gain and improve glycemic control.18
Once a woman is pregnant, it becomes difficult to lose weight because additional calories are needed to support a growing fetus. It is recommended that patients with healthy pregestational BMI consume an extra 200 to 300 calories/d after the first trimester. However, extra caloric intake in a woman with obesity who is pregnant leads to metabolic impairment and increased risk of diabetes for both the mother and fetus.19 Therefore, it is recommended that patients with obese pregestational BMI not consume additional calories because excess maternal fat is sufficient to support the energy needs of the growing fetus.19
Continue to: Ultimately, earlier intervention...
Ultimately, earlier intervention—prior to conception—helps patients prepare for a healthier pregnancy, resulting in better long-term outcomes. It is helpful to be familiar with the advantages and disadvantages of common approaches to weight management and to be able to refer patients to nutritionists for optimal planning. When establishing a dietary plan, consider patient-specific factors, such as cultural diets, financial and time constraints, and the patient’s readiness to make and maintain these changes. Consistent follow-up and behavioral therapy are necessary to maintain successful weight control.
There are many screening tools, but 1 is preferred in pregnancy
There are several ways to diagnose diabetes in patients who are not pregnant, including A1C, a fasting glucose test, an oral glucose tolerance test (OGTT), or random glucose testing (plus symptoms). However, the preferred method for diagnosing GDM is OGTT because it has a higher sensitivity.20 A1C, while a good measure of hyperglycemic stability, does not register hyperglycemia early enough to diagnose GDM and fasting glucose testing is less sensitive because for most women with GDM, that abnormal postprandial glucose level is the first glycemic abnormality.21
When to screen. Blood glucose levels should be checked in all pregnant women as part of their metabolic panel at the first prenatal visit. A reflex A1C for high glucose levels can be ordered based on the physician’s preference. This may help you to identify patients with prediabetes who are at risk for GDM and implement early behavioral and lifestyle changes. However, further research is needed to determine if intervention early in pregnancy can truly reduce the risk of GDM.11
Screening for GDM should be completed at 24 to 28 weeks of gestation20 because it is likely that this is when the hormonal effects of the placenta that contribute to insulin resistance set the woman up for postprandial hyperglycemia. Currently, there are no evidence-based guidelines for the use of continuous glucose monitoring prior to 24 weeks of gestation to identify GDM.20 If persistent hyperglycemia is present before 24 weeks of gestation, it is considered evidence of a pre-existing metabolic abnormality and is diagnosed as “pregestational diabetes.” Treatment should follow guidelines established for women who had diabetes prior to pregnancy.
How to screen? There is ongoing discussion about what is the optimal screening method for GDM: a 1-step strategy with a fasting 75-g OGTT only, or a 2-step strategy with a 50-g non-fasting glucose load test followed by a fasting 100-g OGTT in women who do not meet the plasma glucose cutoff (TABLE 1).22-24 Hillier et al25 compared the effectiveness of these strategies in diagnosing GDM and identifying pregnancy complications for the mother and infant. They found that while the 1-step strategy resulted in a 2-fold increase in the diagnosis of GDM, it did not lead to better outcomes for mothers and infants when compared with the 2-step method.25 Currently, the majority of obstetricians (95%) prefer to use the 2-step method.24
Continue to: Manage lifestyle, monitor glucose
Manage lifestyle, monitor glucose
Management of GDM in most women starts with diabetes self-management education and support for therapeutic lifestyle changes, such as nutritional interventions that reduce hyperglycemia and contribute to healthy weight gain during pregnancy.20 This may include medical nutrition therapy that focuses on adequate nutrition for the mother and fetus. Currently, the recommended dietary intake for women who are pregnant (regardless of diabetes) includes a minimum of 175 g of carbohydrates, 71 g of daily protein, and at least 28 g of fiber. Further refinement of dietary intake, including carbohydrate restriction, should be done with guidance from a registered dietitian.20 If the obstetrics team does not include a registered dietitian, a referral to one may be necessary. Regular physical activity should be continued throughout pregnancy as tolerated. Social support, stress reduction, and good sleep hygiene should be encouraged as much as possible.
For successful outcomes, therapeutic lifestyle changes should be coupled with glucose monitoring. The Fifth International Workshop-Conference on Gestational Diabetes Mellitus recommends that women with GDM monitor fasting blood glucose and typically 1-hour postprandial glucose. The glucose goals in GDM are as follows26:
- Fasting glucose < 95 mg/dL (5.3 mmol/L), and either
- 1-hour postprandial glucose < 140 mg/dL (7.8 mmol/L), or
- 2-hour postprandial glucose < 120 mg/dL (6.7 mmol/L).
Importantly, in the second and third trimester, the A1C goal for women with GDM is 6.0%. This is lower than the more traditional A1C goal for 2 reasons: (1) increases in A1C, even within the normal range, increase adverse outcomes; and (2) pregnant women will have an increased red blood cell count turnover, which can lower the A1C.27 In a historical cohort study (n = 27,213), Abell et al28 found that women who have an A1C < 6.0% in the second and third trimester have the lowest risk of giving birth to large-for-gestational-age infants and for having preeclampsia.
Add insulin if glucose targets are not met
Most women who engage in therapeutic lifestyle change (70%-85%) can achieve an A1C < 6% and will not need to take medication to manage GDM.29 If pharmacotherapy is needed to manage glucose, insulin is the preferred treatment for all women with GDM.20 Treatment should be individualized based on the glucose trends the woman is experiencing. Common treatments include bedtime NPH if fasting hyperglycemia is most prominent and analogue insulin at mealtimes for women with prominent postprandial hyperglycemia.
Noninsulin agents such as metformin and sulfonylureas are not currently recommended by the American College of Obstetricians and Gynecologists or the American Diabetes Association for use in GDM.20,24 Despite being used for years in women with pregestational diabetes, metabolic syndrome, and polycystic ovary syndrome, there is evidence that metformin crosses the placenta and fetal safety has not yet been established in RCTs. The Metformin in Gestational Diabetes: The Offspring Follow-Up (MiG TOFU) study was a longitudinal follow-up study that evaluated body composition and metabolic outcomes in children (ages 7-9 years) of women with GDM who had received metformin or insulin while pregnant.30 At age 9 years, children who were exposed to metformin weighed more and had a higher waist-to-height ratio and waist circumference than those exposed to insulin.30
Continue to: Sulfonylureas are no longer recommended...
Sulfonylureas are no longer recommended because of the risk of maternal and fetal hypoglycemia and concerns about this medication crossing the placenta.24,31,32 Specifically, in a 2015 meta-analysis and systematic review of 15 articles (n = 2509), glyburide had a higher risk of neonatal hypoglycemia and macrosomia than insulin or metformin.33 For women who cannot manage their glucose with therapeutic lifestyle changes and cannot take insulin, oral therapies may be considered if the risk-benefit ratio is balanced for that person.34
Watch for effects of poor glycemic control on mother, infant
Preeclampsia is defined as new-onset hypertension and proteinuria after 20 weeks of gestation. The correlation between GDM and preeclampsia has partly been explained by their shared overlapping risk factors, including maternal obesity, excessive gestational weight gain, and persistent hyperglycemia.35 On a biochemical level, these risk factors contribute to oxidative stress and systemic vascular dysfunction, which have been hypothesized as the underlying pathophysiology for the development of preeclampsia.35
Neonatal macrosomia, defined as a birth weight ≥ 4000 g, is a common complication that develops in 15% to 45% of infants of mothers with GDM.36 Placental transfer of glucose in mothers with hyperglycemia stimulates the secretion of neonatal insulin and the ultimate storage of the excess glucose as body fat. After delivery, the abrupt discontinuation of placental transfer of glucose to an infant who is actively secreting insulin leads to neonatal hypoglycemia, which if not detected or managed, can lead to long-term neurologic deficits, including recurrent seizures and developmental delays.37 Therefore, it is essential to screen for neonatal hypoglycemia immediately after birth and serially up to 12 hours.38
Postpartum T2D. Poor glycemic control increases the risk of increasing insulin resistance developing into T2D postpartum for mothers.39 It also increases the risk of obesity and insulin resistance later in life for the infant.40 A retrospective cohort study (n = 461) found a positive correlation between exposure to maternal GDM and elevated BMI in children ages 6 to 13 years.41 Kamana et al36 further discussed this correlation and suggested that exposure to maternal hyperglycemia in utero contributes to fetal programming of later adipose deposition. Children may develop without a notable increase in BMI until after puberty.42
Partner with specialists to improve outcomes
Although most women with GDM are managed by specialists (obstetricians, endocrinologists, and maternal-fetal medicine specialists),43 these patients are still seeking care from their family physicians for other complaints. These visits provide key touchpoints during pregnancy and are opportunities for PCPs to identify a pregnancy-related complication or provide additional education or referral to the obstetrician.
Continue to: Also, if you work in an area...
Also, if you work in an area where specialists are less accessible, you may be the clinician providing the majority of care to a patient with GDM. If this is the case, you’ll want to watch for the following risk factors, which should prompt a referral to specialty care:
- a previous pregnancy with GDM20
- a previous birth of an infant weighing > 4000 g44
- baseline history of hypertension45
- evidence of insulin resistance or polycystic ovary syndrome46,47
- a history of cardiovascular disease20
- a need to treat GDM with pharmacotherapy.48
Ensuring a smooth transition after the birth
Optimal communication and hand-offs throughout pregnancy and after delivery will benefit everyone. When the pregnant patient’s care has been managed by an obstetrician, it is important to address the following issues during the hand-off:
- baseline medical problems
- medical screenings and treatments in pregnancy (retinopathy and nephropathy screening)
- aspirin initiation, if indicated
- management of thyroid abnormalities
- management of mental health conditions
- postpartum glucose management and T2D screening postpartum
- management of complications identified during pregnancy (retinopathy and nephropathy).
Timing and other elements of postpartum care. The first postpartum screen should occur at 4 to 12 weeks postpartum. OGTT is recommended instead of A1C at this time because A1C may still be lowered by the increased red blood cell turnover related to pregnancy and blood loss at delivery. Because women with GDM have a 50% to 75% lifetime risk of T2D,20 patients with normal test results should be re-tested every 1 to 3 years using any of the standard screening methods (A1C, fasting glucose, or OGTT).20
After delivery it may be difficult for women to follow-up with their own personal health care because they are focused on the care of their baby. The increased use of telehealth may make postpartum follow-up visits easier to attend.
Visits present opportunities. Postpartum visits present another opportunity for PCPs to screen for diabetes and other postpartum complications, including depression and thyroid abnormalities. Visits are also an opportunity to discuss timely contraception so as to prevent an early, unplanned pregnancy. Other important aspects of postpartum care are outlined in TABLE 2.20,49
CORRESPONDENCE
Connie L. Ha, BS, OMS IV, Department of Primary Care, 1310 Club Drive, Touro University California, Vallejo, CA 94592; [email protected]
Gestational diabetes mellitus (GDM), defined as new-onset hyperglycemia detected in a pregnant woman after 24 weeks of gestation, affects 4% to 10% of pregnancies in the United States annually1 and is a major challenge for health care professionals.2 During pregnancy, the body’s physiologic responses are altered to support the growing fetus. One of these changes is an increase in insulin resistance, which suggests that pregnancy alone increases the patient’s risk for type 2 diabetes (T2D). However, several other factors also increase this risk, including maternal age, social barriers to care, obesity, poor weight control, and family history.
If not controlled, GDM results in poor health outcomes for the mother, such as preeclampsia, preterm labor, and maternal T2D.3-5 For the infant, intrauterine exposure to persistent hyperglycemia is correlated with neonatal macrosomia, hypoglycemia, perinatal complications (eg, preterm delivery, fetal demise), and obesity and insulin resistance later in life.4
Primary care physicians (PCPs) are the patient’s main point of contact prior to pregnancy. This relationship makes PCPs a resource for the patient and specialists during and after pregnancy. In this article, we discuss risk factors and how to screen for GDM, provide an update on practice recommendations for treatment and management of GDM in primary care, and describe the effects of uncontrolled GDM.
Know the key risk factors
Prevention begins with identifying the major risk factors that contribute to the development of GDM. These include maternal age, social barriers to care, family history of prediabetes, and obesity and poor weight control.
Older age. A meta-analysis of 24 studies noted strong positive correlation between GDM risk and maternal age.6 One of the population-based cohort studies in the meta-analysis examined relationships between maternal age and pregnancy outcomes in women living in British Columbia, Canada (n = 203,414). Data suggested that the relative risk of GDM increased linearly with maternal age to 3.2, 4.2, and 4.4 among women ages ≥ 35, ≥ 40, and ≥ 45 years, respectively.7
Social barriers to care. Although the prevalence of GDM has increased over the past few decades,1 from 2011 to 2019 the increase in GDM in individuals at first live birth was significantly higher in non-Hispanic Asian and Hispanic/Latina women than in non-Hispanic White women.8 Data from the Centers for Disease Control and Prevention further suggest that diabetes was more prevalent among individuals with a lower socioeconomic status as indicated by their level of education.9 Ogunwole et al10 suggest that racism is the root cause of these disparities and leads to long-term barriers to care (eg, socioeconomic deprivation, lack of health insurance, limited access to care, and poor health literacy), which ultimately contribute to the development of GDM and progression of diabetes. It is important for PCPs and all health professionals to be aware of these barriers so that they may practice mindfulness and deliver culturally sensitive care to patients from marginalized communities.
Family history of prediabetes. In a population-based cohort study (n = 7020), women with prediabetes (A1C, 5.7%-6.4%) were 2.8 times more likely to develop GDM compared with women with normal A1C (< 5.7%).11 Similar results were seen in a retrospective cohort study (n = 2812), in which women with prediabetes were more likely than women with a normal first trimester A1C to have GDM (29.1% vs 13.7%, respectively; adjusted relative risk = 1.48; 95% CI, 1.15-1.89).12 In both studies, prediabetes was not associated with a higher risk for adverse maternal or neonatal outcomes.11,12
Continue to: While there are no current...
While there are no current guidelines for treating prediabetes in pregnancy, women diagnosed with prediabetes in 1 study were found to have significantly less weight gain during pregnancy compared with patients with normal A1C,12 suggesting there may be a benefit in early identification and intervention, although further research is needed.11 In a separate case-control study (n = 345 women with GDM; n = 800 control), high rates of gestational weight gain (> 0.41 kg/wk) were associated with an increased risk of GDM (odds ratio [OR] = 1.74; 95% CI, 1.16-2.60) compared with women with the lowest rate of gestational weight gain (0.27-0.4 kg/wk [OR = 1.43; 95% CI, 0.96-2.14]).13 Thus, it is helpful to have proactive conversations about family planning and adequate weight and glycemic control with high-risk patients to prepare for a healthy pregnancy.
Obesity and weight management. Patients who are overweight (body mass index [BMI], 25-29.9) or obese (BMI > 30) have a substantially increased risk of GDM (adjusted OR = 1.44; 95% CI, 1.04-1.81), as seen in a retrospective cohort study of 1951 pregnant Malaysian women.14 Several factors have been found to contribute to successful weight control, including calorie prescription, a structured meal plan, high physical activity goals (60-90 min/d), daily weighing and monitoring of food intake, behavior therapy, and continued patient–provider contact.15
The safety, efficacy, and sustainability of weight loss with various dietary plans have been studied in individuals who are overweight and obese.16 Ultimately, energy expenditure must be greater than energy intake to promote weight loss. Conventional diets with continuous energy restriction (ie, low-fat, low-carbohydrate, and high-protein diets) have proven to be effective for short-term weight loss but data on long-term weight maintenance are limited.16 The Mediterranean diet, which is comprised mostly of vegetables, fruits, legumes, fish, and grains—with a lower intake of meat and dairy—may reduce gestational weight gain and risk of GDM as suggested by a randomized controlled trial (RCT; n = 1252).17 Although the choice of diet is up to the patient, it is important to be aware of different diets or refer the patient to a registered dietician who can help the patient if needed.
Reduce risk with adequate weight and glycemic control
Prevention of GDM during pregnancy should focus on weight maintenance and optimal glycemic control. Two systematic reviews, one with 8 RCTs (n = 1792) and another with 5 studies (n = 539), assessed the efficacy and safety of energy-restricted dietary intervention on GDM prevention.18 The first review found a significant reduction in gestational weight gain and improved glycemic control without increased risk of adverse maternal and fetal outcomes.18 The second review showed no clear difference between energy-restricted and non–energy-restricted diets on outcomes such as preeclampsia, gestational weight gain, large for gestational age, and macrosomia.18 These data suggest that while energy-restricted dietary interventions made no difference on maternal and fetal complications, they may still be safely used in pregnancy to reduce gestational weight gain and improve glycemic control.18
Once a woman is pregnant, it becomes difficult to lose weight because additional calories are needed to support a growing fetus. It is recommended that patients with healthy pregestational BMI consume an extra 200 to 300 calories/d after the first trimester. However, extra caloric intake in a woman with obesity who is pregnant leads to metabolic impairment and increased risk of diabetes for both the mother and fetus.19 Therefore, it is recommended that patients with obese pregestational BMI not consume additional calories because excess maternal fat is sufficient to support the energy needs of the growing fetus.19
Continue to: Ultimately, earlier intervention...
Ultimately, earlier intervention—prior to conception—helps patients prepare for a healthier pregnancy, resulting in better long-term outcomes. It is helpful to be familiar with the advantages and disadvantages of common approaches to weight management and to be able to refer patients to nutritionists for optimal planning. When establishing a dietary plan, consider patient-specific factors, such as cultural diets, financial and time constraints, and the patient’s readiness to make and maintain these changes. Consistent follow-up and behavioral therapy are necessary to maintain successful weight control.
There are many screening tools, but 1 is preferred in pregnancy
There are several ways to diagnose diabetes in patients who are not pregnant, including A1C, a fasting glucose test, an oral glucose tolerance test (OGTT), or random glucose testing (plus symptoms). However, the preferred method for diagnosing GDM is OGTT because it has a higher sensitivity.20 A1C, while a good measure of hyperglycemic stability, does not register hyperglycemia early enough to diagnose GDM and fasting glucose testing is less sensitive because for most women with GDM, that abnormal postprandial glucose level is the first glycemic abnormality.21
When to screen. Blood glucose levels should be checked in all pregnant women as part of their metabolic panel at the first prenatal visit. A reflex A1C for high glucose levels can be ordered based on the physician’s preference. This may help you to identify patients with prediabetes who are at risk for GDM and implement early behavioral and lifestyle changes. However, further research is needed to determine if intervention early in pregnancy can truly reduce the risk of GDM.11
Screening for GDM should be completed at 24 to 28 weeks of gestation20 because it is likely that this is when the hormonal effects of the placenta that contribute to insulin resistance set the woman up for postprandial hyperglycemia. Currently, there are no evidence-based guidelines for the use of continuous glucose monitoring prior to 24 weeks of gestation to identify GDM.20 If persistent hyperglycemia is present before 24 weeks of gestation, it is considered evidence of a pre-existing metabolic abnormality and is diagnosed as “pregestational diabetes.” Treatment should follow guidelines established for women who had diabetes prior to pregnancy.
How to screen? There is ongoing discussion about what is the optimal screening method for GDM: a 1-step strategy with a fasting 75-g OGTT only, or a 2-step strategy with a 50-g non-fasting glucose load test followed by a fasting 100-g OGTT in women who do not meet the plasma glucose cutoff (TABLE 1).22-24 Hillier et al25 compared the effectiveness of these strategies in diagnosing GDM and identifying pregnancy complications for the mother and infant. They found that while the 1-step strategy resulted in a 2-fold increase in the diagnosis of GDM, it did not lead to better outcomes for mothers and infants when compared with the 2-step method.25 Currently, the majority of obstetricians (95%) prefer to use the 2-step method.24
Continue to: Manage lifestyle, monitor glucose
Manage lifestyle, monitor glucose
Management of GDM in most women starts with diabetes self-management education and support for therapeutic lifestyle changes, such as nutritional interventions that reduce hyperglycemia and contribute to healthy weight gain during pregnancy.20 This may include medical nutrition therapy that focuses on adequate nutrition for the mother and fetus. Currently, the recommended dietary intake for women who are pregnant (regardless of diabetes) includes a minimum of 175 g of carbohydrates, 71 g of daily protein, and at least 28 g of fiber. Further refinement of dietary intake, including carbohydrate restriction, should be done with guidance from a registered dietitian.20 If the obstetrics team does not include a registered dietitian, a referral to one may be necessary. Regular physical activity should be continued throughout pregnancy as tolerated. Social support, stress reduction, and good sleep hygiene should be encouraged as much as possible.
For successful outcomes, therapeutic lifestyle changes should be coupled with glucose monitoring. The Fifth International Workshop-Conference on Gestational Diabetes Mellitus recommends that women with GDM monitor fasting blood glucose and typically 1-hour postprandial glucose. The glucose goals in GDM are as follows26:
- Fasting glucose < 95 mg/dL (5.3 mmol/L), and either
- 1-hour postprandial glucose < 140 mg/dL (7.8 mmol/L), or
- 2-hour postprandial glucose < 120 mg/dL (6.7 mmol/L).
Importantly, in the second and third trimester, the A1C goal for women with GDM is 6.0%. This is lower than the more traditional A1C goal for 2 reasons: (1) increases in A1C, even within the normal range, increase adverse outcomes; and (2) pregnant women will have an increased red blood cell count turnover, which can lower the A1C.27 In a historical cohort study (n = 27,213), Abell et al28 found that women who have an A1C < 6.0% in the second and third trimester have the lowest risk of giving birth to large-for-gestational-age infants and for having preeclampsia.
Add insulin if glucose targets are not met
Most women who engage in therapeutic lifestyle change (70%-85%) can achieve an A1C < 6% and will not need to take medication to manage GDM.29 If pharmacotherapy is needed to manage glucose, insulin is the preferred treatment for all women with GDM.20 Treatment should be individualized based on the glucose trends the woman is experiencing. Common treatments include bedtime NPH if fasting hyperglycemia is most prominent and analogue insulin at mealtimes for women with prominent postprandial hyperglycemia.
Noninsulin agents such as metformin and sulfonylureas are not currently recommended by the American College of Obstetricians and Gynecologists or the American Diabetes Association for use in GDM.20,24 Despite being used for years in women with pregestational diabetes, metabolic syndrome, and polycystic ovary syndrome, there is evidence that metformin crosses the placenta and fetal safety has not yet been established in RCTs. The Metformin in Gestational Diabetes: The Offspring Follow-Up (MiG TOFU) study was a longitudinal follow-up study that evaluated body composition and metabolic outcomes in children (ages 7-9 years) of women with GDM who had received metformin or insulin while pregnant.30 At age 9 years, children who were exposed to metformin weighed more and had a higher waist-to-height ratio and waist circumference than those exposed to insulin.30
Continue to: Sulfonylureas are no longer recommended...
Sulfonylureas are no longer recommended because of the risk of maternal and fetal hypoglycemia and concerns about this medication crossing the placenta.24,31,32 Specifically, in a 2015 meta-analysis and systematic review of 15 articles (n = 2509), glyburide had a higher risk of neonatal hypoglycemia and macrosomia than insulin or metformin.33 For women who cannot manage their glucose with therapeutic lifestyle changes and cannot take insulin, oral therapies may be considered if the risk-benefit ratio is balanced for that person.34
Watch for effects of poor glycemic control on mother, infant
Preeclampsia is defined as new-onset hypertension and proteinuria after 20 weeks of gestation. The correlation between GDM and preeclampsia has partly been explained by their shared overlapping risk factors, including maternal obesity, excessive gestational weight gain, and persistent hyperglycemia.35 On a biochemical level, these risk factors contribute to oxidative stress and systemic vascular dysfunction, which have been hypothesized as the underlying pathophysiology for the development of preeclampsia.35
Neonatal macrosomia, defined as a birth weight ≥ 4000 g, is a common complication that develops in 15% to 45% of infants of mothers with GDM.36 Placental transfer of glucose in mothers with hyperglycemia stimulates the secretion of neonatal insulin and the ultimate storage of the excess glucose as body fat. After delivery, the abrupt discontinuation of placental transfer of glucose to an infant who is actively secreting insulin leads to neonatal hypoglycemia, which if not detected or managed, can lead to long-term neurologic deficits, including recurrent seizures and developmental delays.37 Therefore, it is essential to screen for neonatal hypoglycemia immediately after birth and serially up to 12 hours.38
Postpartum T2D. Poor glycemic control increases the risk of increasing insulin resistance developing into T2D postpartum for mothers.39 It also increases the risk of obesity and insulin resistance later in life for the infant.40 A retrospective cohort study (n = 461) found a positive correlation between exposure to maternal GDM and elevated BMI in children ages 6 to 13 years.41 Kamana et al36 further discussed this correlation and suggested that exposure to maternal hyperglycemia in utero contributes to fetal programming of later adipose deposition. Children may develop without a notable increase in BMI until after puberty.42
Partner with specialists to improve outcomes
Although most women with GDM are managed by specialists (obstetricians, endocrinologists, and maternal-fetal medicine specialists),43 these patients are still seeking care from their family physicians for other complaints. These visits provide key touchpoints during pregnancy and are opportunities for PCPs to identify a pregnancy-related complication or provide additional education or referral to the obstetrician.
Continue to: Also, if you work in an area...
Also, if you work in an area where specialists are less accessible, you may be the clinician providing the majority of care to a patient with GDM. If this is the case, you’ll want to watch for the following risk factors, which should prompt a referral to specialty care:
- a previous pregnancy with GDM20
- a previous birth of an infant weighing > 4000 g44
- baseline history of hypertension45
- evidence of insulin resistance or polycystic ovary syndrome46,47
- a history of cardiovascular disease20
- a need to treat GDM with pharmacotherapy.48
Ensuring a smooth transition after the birth
Optimal communication and hand-offs throughout pregnancy and after delivery will benefit everyone. When the pregnant patient’s care has been managed by an obstetrician, it is important to address the following issues during the hand-off:
- baseline medical problems
- medical screenings and treatments in pregnancy (retinopathy and nephropathy screening)
- aspirin initiation, if indicated
- management of thyroid abnormalities
- management of mental health conditions
- postpartum glucose management and T2D screening postpartum
- management of complications identified during pregnancy (retinopathy and nephropathy).
Timing and other elements of postpartum care. The first postpartum screen should occur at 4 to 12 weeks postpartum. OGTT is recommended instead of A1C at this time because A1C may still be lowered by the increased red blood cell turnover related to pregnancy and blood loss at delivery. Because women with GDM have a 50% to 75% lifetime risk of T2D,20 patients with normal test results should be re-tested every 1 to 3 years using any of the standard screening methods (A1C, fasting glucose, or OGTT).20
After delivery it may be difficult for women to follow-up with their own personal health care because they are focused on the care of their baby. The increased use of telehealth may make postpartum follow-up visits easier to attend.
Visits present opportunities. Postpartum visits present another opportunity for PCPs to screen for diabetes and other postpartum complications, including depression and thyroid abnormalities. Visits are also an opportunity to discuss timely contraception so as to prevent an early, unplanned pregnancy. Other important aspects of postpartum care are outlined in TABLE 2.20,49
CORRESPONDENCE
Connie L. Ha, BS, OMS IV, Department of Primary Care, 1310 Club Drive, Touro University California, Vallejo, CA 94592; [email protected]
1. Sheiner E. Gestational diabetes mellitus: long-term consequences for the mother and child grand challenge: how to move on towards secondary prevention? Front Clin Diabetes Healthc. 2020. doi: 10.3389/fcdhc.2020.546256
2. Angueira AR, Ludvik AE, Reddy TE, et al. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327-334. doi: 10.2337/db14-0877
3. Shou C, Wei Y-M, Wang C, et al. Updates in long-term maternal and fetal adverse effects of gestational diabetes mellitus. Maternal-Fetal Med. 2019;1:91-94. doi: 10.1097/FM9.0000000000000019
4. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342. doi: 10.3390/ijms19113342
5. Kulshrestha V, Agarwal N. Maternal complications in pregnancy with diabetes. J Pak Med Assoc. 2016;66(9 suppl 1):S74-S77.
6. Li Y, Ren X, He L, et al. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. doi: 10.1016/j.diabres.2020.108044
7. Schummers L, Hutcheon JA, Hacker MR, et al. Absolute risks of obstetric outcomes by maternal age at first birth: a population-based cohort. Epidemiology. 2018;29:379-387. doi: 10.1097/EDE.0000000000000818
8. Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326:660-669. doi: 10.1001/jama.2021.7217
9. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. Accessed February 2, 2022. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
10. Ogunwole SM, Golden SH. Social determinants of health and structural inequities—root causes of diabetes disparities. Diabetes Care. 2021;44:11-13. doi: 10.2337/dci20-0060
11. Chen L, Pocobelli G, Yu O, et al. Early pregnancy hemoglobin A1C and pregnancy outcomes: a population-based study. Am J Perinatol. 2019;36:1045-1053. doi: 10.1055/s-0038-1675619
12. Osmundson S, Zhao BS, Kunz L, et al. First trimester hemoglobin A1C prediction of gestational diabetes. Am J Perinatol. 2016;33:977-982. doi: 10.1055/s-0036-1581055
13. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus [published correction appears in Obstet Gynecol. 2010;115:1092]. Obstet Gynecol. 2010;115:597-604. doi: 10.1097/AOG.0b013e3181cfce4f
14. Yong HY, Mohd Shariff Z, Mohd Yusof BN, et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep. 2020;10:8486. doi: 10.1038/s41598-020-65251-2
15. Phelan S. Windows of opportunity for lifestyle interventions to prevent gestational diabetes mellitus. Am J Perinatol. 2016;33:1291-1299. doi: 10.1055/s-0036-1586504
16. Koliaki C, Spinos T, Spinou M, et al. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel). 2018;6:73. doi: 10.3390/healthcare6030073
17. Al Wattar BH, Dodds J, Placzek A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLOS Med. 2019;16:e1002857. doi: 10.1371/journal.pmed.1002857
18. Zarogiannis S. Are novel lifestyle approaches to management of type 2 diabetes applicable to prevention and treatment of women with gestational diabetes mellitus? Global Diabetes Open Access J. 2019;1:1-14.
19. Most J, Amant MS, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690. doi: 10.1172/JCI130341
20. American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S200-S210. doi: 10.2337/dc21-S014
21. McIntyre HD, Sacks DA, Barbour LA, et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care. 2016;39:53-54. doi: 10.2337/dc15-1887
22. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
23. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
24. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49-e64. doi: 10.1097/AOG.0000000000002501
25. Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384:895-904. doi: 10.1056/NEJMoa2026028
26. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260. doi: 10.2337/dc07-s225
27. Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200-1201. doi: 10.2337/diacare.27.5.1200
28. Abell SK, Boyle JA, de Courten B, et al. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2017;57:308-314. doi: 10.1111/ajo.12521
29. Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014;37:3345-3355. doi: 10.2337/dc14-1530
30. Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018;6:e000456. doi: 10.1136/bmjdrc-2017-000456
31. Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85:607-614. doi: 10.1038/clpt.2009.5
32. Malek R, Davis SN. Pharmacokinetics, efficacy and safety of glyburide for treatment of gestational diabetes mellitus. Expert Opin Drug Metab Toxicol. 2016;12:691-699. doi: 10.1080/17425255.2016.1187131
33. Balsells M, García-Patterson A, Solà I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. doi: 10.1136/bmj.h102
34. Kavitha N, De S, Kanagasabai S. Oral hypoglycemic agents in pregnancy: an update. J Obstet Gynaecol India. 2013;63:82-87. doi: 10.1007/s13224-012-0312-z
35. Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15:9. doi: 10.1007/s11892-015-0579-4
36. Kamana KC, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(suppl 2):14-20. doi: 10.1159/000371628
37. Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734-743. doi: 10.4239/wjd.v6.i5.734
38. Stanescu A, Stoicescu SM. Neonatal hypoglycemia screening in newborns from diabetic mothers—arguments and controversies. J Med Life. 2014;7(spec iss 3):51-52.
39. Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med. 2014;31:292-301. doi: 10.1111/dme.12382
40. Stewart A, Malhotra A. Gestational diabetes and the neonate: challenges and solutions. Res Rep Neonatol. 2015;5:31-39. doi: 10.2147/RRN.S30971
41. Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87-92. doi: 10.1007/s00125-010-1925-3
42. Crume TL, Ogden L, Daniels S, et al. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 2011;158:941-946. doi: 10.1016/j.jpeds.2010.12.007
43. Levels of maternal care. Obstetric Care Consensus No. 9. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2019;134:e41-e55. doi: 10.1097/AOG.0000000000003383
44. Caughey AB, Cheng YW, Stotland NE, et al. Maternal and paternal race/ethnicity are both associated with gestational diabetes. Am J Obstet Gynecol. 2010;202:616.e1-e5. doi: 10.1016/j.ajog.2010.01.082
45. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191:1655-1660. doi: 10.1016/j.ajog.2004.03.074
46. Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;5:CD011970. doi: 10.1002/14651858.CD011970.pub2
47. Ceysens G, Rouiller D, Boulvain M. Exercise for the diabetic pregnant woman. Cochrane Database Syst Rev. 2006;3:CD004225. doi: 10.1002/14651858.CD004225.pub2
48. Chawla R, Mukherjee JJ, Chawla M, et al. Expert group recommendations on the effective use of bolus insulin in the management of type 2 diabetes mellitus. Med Sci (Basel). 2021;9:38. doi: 10.3390/medsci9020038
49. American Diabetes Association. Introduction: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
1. Sheiner E. Gestational diabetes mellitus: long-term consequences for the mother and child grand challenge: how to move on towards secondary prevention? Front Clin Diabetes Healthc. 2020. doi: 10.3389/fcdhc.2020.546256
2. Angueira AR, Ludvik AE, Reddy TE, et al. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327-334. doi: 10.2337/db14-0877
3. Shou C, Wei Y-M, Wang C, et al. Updates in long-term maternal and fetal adverse effects of gestational diabetes mellitus. Maternal-Fetal Med. 2019;1:91-94. doi: 10.1097/FM9.0000000000000019
4. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342. doi: 10.3390/ijms19113342
5. Kulshrestha V, Agarwal N. Maternal complications in pregnancy with diabetes. J Pak Med Assoc. 2016;66(9 suppl 1):S74-S77.
6. Li Y, Ren X, He L, et al. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. doi: 10.1016/j.diabres.2020.108044
7. Schummers L, Hutcheon JA, Hacker MR, et al. Absolute risks of obstetric outcomes by maternal age at first birth: a population-based cohort. Epidemiology. 2018;29:379-387. doi: 10.1097/EDE.0000000000000818
8. Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326:660-669. doi: 10.1001/jama.2021.7217
9. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. Accessed February 2, 2022. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
10. Ogunwole SM, Golden SH. Social determinants of health and structural inequities—root causes of diabetes disparities. Diabetes Care. 2021;44:11-13. doi: 10.2337/dci20-0060
11. Chen L, Pocobelli G, Yu O, et al. Early pregnancy hemoglobin A1C and pregnancy outcomes: a population-based study. Am J Perinatol. 2019;36:1045-1053. doi: 10.1055/s-0038-1675619
12. Osmundson S, Zhao BS, Kunz L, et al. First trimester hemoglobin A1C prediction of gestational diabetes. Am J Perinatol. 2016;33:977-982. doi: 10.1055/s-0036-1581055
13. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus [published correction appears in Obstet Gynecol. 2010;115:1092]. Obstet Gynecol. 2010;115:597-604. doi: 10.1097/AOG.0b013e3181cfce4f
14. Yong HY, Mohd Shariff Z, Mohd Yusof BN, et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep. 2020;10:8486. doi: 10.1038/s41598-020-65251-2
15. Phelan S. Windows of opportunity for lifestyle interventions to prevent gestational diabetes mellitus. Am J Perinatol. 2016;33:1291-1299. doi: 10.1055/s-0036-1586504
16. Koliaki C, Spinos T, Spinou M, et al. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel). 2018;6:73. doi: 10.3390/healthcare6030073
17. Al Wattar BH, Dodds J, Placzek A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLOS Med. 2019;16:e1002857. doi: 10.1371/journal.pmed.1002857
18. Zarogiannis S. Are novel lifestyle approaches to management of type 2 diabetes applicable to prevention and treatment of women with gestational diabetes mellitus? Global Diabetes Open Access J. 2019;1:1-14.
19. Most J, Amant MS, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690. doi: 10.1172/JCI130341
20. American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S200-S210. doi: 10.2337/dc21-S014
21. McIntyre HD, Sacks DA, Barbour LA, et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care. 2016;39:53-54. doi: 10.2337/dc15-1887
22. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
23. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
24. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49-e64. doi: 10.1097/AOG.0000000000002501
25. Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384:895-904. doi: 10.1056/NEJMoa2026028
26. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260. doi: 10.2337/dc07-s225
27. Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200-1201. doi: 10.2337/diacare.27.5.1200
28. Abell SK, Boyle JA, de Courten B, et al. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2017;57:308-314. doi: 10.1111/ajo.12521
29. Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014;37:3345-3355. doi: 10.2337/dc14-1530
30. Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018;6:e000456. doi: 10.1136/bmjdrc-2017-000456
31. Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85:607-614. doi: 10.1038/clpt.2009.5
32. Malek R, Davis SN. Pharmacokinetics, efficacy and safety of glyburide for treatment of gestational diabetes mellitus. Expert Opin Drug Metab Toxicol. 2016;12:691-699. doi: 10.1080/17425255.2016.1187131
33. Balsells M, García-Patterson A, Solà I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. doi: 10.1136/bmj.h102
34. Kavitha N, De S, Kanagasabai S. Oral hypoglycemic agents in pregnancy: an update. J Obstet Gynaecol India. 2013;63:82-87. doi: 10.1007/s13224-012-0312-z
35. Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15:9. doi: 10.1007/s11892-015-0579-4
36. Kamana KC, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(suppl 2):14-20. doi: 10.1159/000371628
37. Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734-743. doi: 10.4239/wjd.v6.i5.734
38. Stanescu A, Stoicescu SM. Neonatal hypoglycemia screening in newborns from diabetic mothers—arguments and controversies. J Med Life. 2014;7(spec iss 3):51-52.
39. Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med. 2014;31:292-301. doi: 10.1111/dme.12382
40. Stewart A, Malhotra A. Gestational diabetes and the neonate: challenges and solutions. Res Rep Neonatol. 2015;5:31-39. doi: 10.2147/RRN.S30971
41. Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87-92. doi: 10.1007/s00125-010-1925-3
42. Crume TL, Ogden L, Daniels S, et al. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 2011;158:941-946. doi: 10.1016/j.jpeds.2010.12.007
43. Levels of maternal care. Obstetric Care Consensus No. 9. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2019;134:e41-e55. doi: 10.1097/AOG.0000000000003383
44. Caughey AB, Cheng YW, Stotland NE, et al. Maternal and paternal race/ethnicity are both associated with gestational diabetes. Am J Obstet Gynecol. 2010;202:616.e1-e5. doi: 10.1016/j.ajog.2010.01.082
45. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191:1655-1660. doi: 10.1016/j.ajog.2004.03.074
46. Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;5:CD011970. doi: 10.1002/14651858.CD011970.pub2
47. Ceysens G, Rouiller D, Boulvain M. Exercise for the diabetic pregnant woman. Cochrane Database Syst Rev. 2006;3:CD004225. doi: 10.1002/14651858.CD004225.pub2
48. Chawla R, Mukherjee JJ, Chawla M, et al. Expert group recommendations on the effective use of bolus insulin in the management of type 2 diabetes mellitus. Med Sci (Basel). 2021;9:38. doi: 10.3390/medsci9020038
49. American Diabetes Association. Introduction: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
PRACTICE RECOMMENDATIONS
› Manage gestational diabetes mellitus (GDM) with lifestyle behavior changes first and add insulin as a secondary treatment only if glycemic targets are not being met. A
› Treat hyperglycemia in GDM with insulin, not metformin or glyburide; these agents cross the placenta to the fetus. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Easing dementia caregiver burden, addressing interpersonal violence
The number of people with dementia globally is expected to reach 74.7 million by 2030 and 131.5 million by 2050.1 Because dementia is progressive, many patients will exhibit severe symptoms termed behavioral crises. Deteriorating interpersonal conduct and escalating antisocial acts result in an acquired sociopathy.2 Increasing cognitive impairment causes these patients to misunderstand intimate care and perceive it as a threat, often resulting in outbursts of violence against their caregivers.3
Available studies (TABLE4-17) make evident the incidence of interpersonal violence experienced by caregivers secondary to aggressive acts by patients with dementia. This violence ranges from verbal abuse, including racial slurs, to physical abuse—sometimes resulting in significant physical injury. Aggressive behavior by patients with dementia, resulting in violence towards their caregivers or partners, stems from progressive cognitive decline, which can make optimal care difficult. Such episodes may also impair the psychological and physical well-being of caregivers, increasing their risk of depression, anxiety, and even post-traumatic stress disorder (PTSD).18 The extent of the impact is also determined by the interpretation of the abuse by the caregivers themselves. One study suggested that the perception of aggressive or violent behavior as “normal” by a caregiver reduced the overall negative effect of the interactions.7Our review emphasizes the unintended burden that can fall to caregivers of patients with dementia. We also address the role of primary care providers (PCPs) in identifying these instances of violence and intervening appropriately by providing safety strategies, education, resources, and support.
CASE
A 67-year-old man with a medical history of PTSD with depression, type 2 diabetes, alcohol use disorder/dependence, hypertension, and obstructive sleep apnea was brought to his PCP by his wife. She said he had recently been unable to keep appointment times, pay bills, or take his usual medications, venlafaxine and bupropion. She also said his PTSD symptoms had worsened. He was sleeping 12 to 14 hours per day and was increasingly irritable. The patient denied any concerns or changes in his behavior.
The PCP administered a Saint Louis University Mental Status (SLUMS) examination to screen for cognitive impairment.19 The patient scored 14/30 (less than 20 is indicative of dementia). He was unable to complete a simple math problem, recall any items from a list of 5, count in reverse, draw a clock correctly, or recall a full story. Throughout the exam, the patient demonstrated minimal effort and was often only able to complete a task after further prompting by the examiner.
A computed tomography scan of the head revealed no signs of hemorrhage or damage. Thyroid-stimulating hormone levels and vitamin B12 levels were normal. A rapid plasma reagin test result was negative. The patient was given a diagnosis of Alzheimer disease. Donepezil was added to the patient’s medications, starting at 5 mg and then increased to 10 mg. His wife began to assist him with his tasks of daily living. His mood improved, and his wife noted he began to remember his appointments and take his medications with assistance.
However, the patient’s irritability continued to escalate. He grew paranoid and accused his wife of mismanaging their money. This pattern steadily worsened over the course of 6 months. The situation escalated until one day the patient’s wife called a mental health hotline reporting that her husband was holding her hostage and threatening to kill her with a gun. He told her, “I can do something to you, and they won’t even find a fingernail. It doesn’t have to be with a gun either.” She was counseled to try to stay calm to avoid aggravating the situation and to go to a safe place and stay there until help arrived.
His memory had worsened to the point that he could not recall any events from the previous 2 years. He was paranoid about anyone entering his home and would not allow his deteriorating roof to be repaired or his yard to be maintained. He did not shower for weeks at a time. He slept holding a rifle and accused his wife of embezzlement.
Continue to: The patient was evaluated...
The patient was evaluated by another specialist, who assessed his SLUMS score to be 18/30. He increased the patient’s donepezil dose, initiated a bupropion taper, and added sertraline to the regimen. The PCP spoke to the patient’s wife regarding options for her safety including leaving the home, hiding firearms, and calling the police in cases of interpersonal violence. The wife said she did not want to pursue these options. She expressed worry that he might be harmed if he was uncooperative with the police and said there was no one except her to take care of him.
Caregivers struggle to care for their loved ones
Instances of personal violence lead to shock, astonishment, heartbreak, and fear. Anticipation of a recurrence of violence causes many partners and caregivers to feel exhausted, because there is minimal hope for any chance of improvement. There are a few exceptions, however, as our case will show. In addition to emotional exhaustion, there is also a never-ending sense of self-doubt, leading many caregivers to question their ability to handle their family member.20,21 Over time, this leads to caregiver burnout, leaving them unable to understand their family member’s aggression. The sudden loss of caregiver control in dealing with the patient may also result in the family member exhibiting behavioral changes reflecting emotional trauma. For caregivers who do not live with the patient, they may choose to make fewer or shorter visits—or not visit at all—because they fear being abused.7,22
Caregivers of patients with dementia often feel helpless and powerless once abrupt and drastic changes in personality lead to some form of interpersonal violence. Additionally, caregivers with a poor health status are more likely to have lower physical function and experience greater caregiving stress overall.23 Other factors increasing stress are longer years of caregiving and the severity of a patient’s dementia and functional impairment.23
Interventions to reduce caregiver burden
Many studies have assessed the role of different interventions to reduce caregiver burden, such as teaching them problem-solving skills, increasing their knowledge of dementia, recommending social resources, providing emotional support, changing caregiver perceptions of the care situation, introducing coping strategies, relying on strengths and experiences in caregiving, help-seeking, and engaging in activity programs.24-28 For Hispanic caregivers, a structured and self-paced online telenovela format has been effective in improving care and relieving caregiver stress.29 Online positive emotion regulators helped in significantly improving quality of life and physical health in the caregivers.30 In this last intervention, caregivers had 6 online sessions with a facilitator who taught them emotional regulation skills that included: noticing positive events, capitalizing on them, and feeling gratitude; practicing mindfulness; doing a positive reappraisal; acknowledging personal strengths and setting attainable goals; and performing acts of kindness. Empowerment programs have also shown significant improvement in the well-being of caregivers.31
Caregivers may reject support.
Continue to: These practical tips can help
These practical tips can help
Based on our review of the literature, we recommend offering the following supports to caregivers:
- Counsel caregivers early on in a patient’s dementia that behavior changes are likely and may be unpredictable. Explain that dementia can involve changes to personality and behavior as well as memory difficulties.33,34
- Describe resources for support, such as day programs for senior adults, insurance coverage for caregiver respite programs, and the Alzheimer’s Association (www.alz.org/). Encourage caregivers to seek general medical and mental health care for themselves. Caregivers should have opportunities and support to discuss their experiences and to be appropriately trained for the challenge of caring for a family member with dementia.35
- Encourage disclosure about abrupt changes in the patient’s behavior. This invites families to discuss issues with you and may make them more comfortable with such conversations.
- Involve ancillary services (eg, social worker) to plan for a higher level of care well in advance of it becoming necessary.
- Discuss safety strategies for the caregiver, including when it is appropriate to alter a patient’s set routines such as bedtimes and mealtimes.33,34
- Discuss when and how to involve law enforcement, if necessary.33,34 Emphasize the importance of removing firearms from the home as a safety measure. Although federal laws do not explicitly prohibit possession of arms by patients with neurologic damage, a few states mention “organic brain syndrome” or “dementia” as conditions prohibiting use or possession of firearms.36
- Suggest, as feasible, nonpharmacologic aids for the patient such as massage therapy, animal-assisted therapy, personalized interventions, music therapy, and light therapy.37 Prescribe medications to the patient to aid in behavior modification when appropriate.
- Screen caregivers and family members for signs of interpersonal violence. Take notice of changes in caregiver behavior or irregularity in attending follow-up appointments.
CASE
Over the next month, the patient’s symptoms further deteriorated. His PCP recommended hospitalization, but the patient and his wife declined. Magnetic resonance imaging of the patient’s brain revealed severe confluent and patchy regions of white matter and T2 signal hyperintensity, consistent with chronic microvascular ischemic disease. An old, small, left parietal lobe infarct was also noted.
One month later, the patient presented to the emergency department. His symptoms were largely unchanged, but his wife indicated that she could no longer live at home due to burnout. The patient’s medications were adjusted, but he was not admitted for inpatient care. His wife said they needed help at home, but the patient opposed the idea any time that it was mentioned.
A few weeks later, the patient presented for outpatient follow-up. He was delusional, believing that the government was compelling citizens to take sertraline in order to harm their mental health. He had also begun viewing online pornography in front of his wife and attempting to remove all of his money from the bank. He was prescribed aripiprazole 15 mg, and his symptoms began to improve. Soon after, however, he threatened to kill his grandson, then took all his Lasix pills (a 7-day supply) simultaneously. The patient denied that this was a suicide attempt.
Over the course of the next month, the patient began to report hearing voices. A neuropsychological evaluation confirmed a diagnosis of dementia with psychiatric symptoms due to neurologic injury. The patient was referred to a geriatric psychiatrist and continued to be managed medically. He was assigned a multidisciplinary team comprising palliative care, social work, and care management to assist in his care and provide support to the family. His behavior improved.
Continue to: At the time of this publication...
At the time of this publication, the patient’s irritability and paranoia had subsided and he had made no further threats to his family. He has allowed a home health aide into the house and has agreed to have his roof repaired. His wife still lives with him and assists him with activities of daily living.
Interprofessional teams are key
Caregiver burnout increases the risk of patient neglect or abuse, as individuals who have been the targets of aggressive behavior are more likely to leave demented patients unattended.8,16,23 Although tools are available to screen caregivers for depression and burnout, an important step forward would be to develop an interprofessional team to aid in identifying and closely following high-risk patient–caregiver groups. This continual and varied assessment of psychosocial stressors could help prevent the development of violent interactions. These teams would allow integration with the primary health care system by frequent and effective shared communication of knowledge, development of goals, and shared decision-making.38 Setting expectations, providing support, and discussing safety strategies can improve the health and welfare of caregivers and patients with dementia alike.
CORRESPONDENCE
Abu Baker Sheikh, MD, MSC 10-5550, 1 University of New Mexico, Albuquerque, NM 87131; [email protected].
1. Wu YT, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time - current evidence. Nat Rev Neurol. 2017;13:327-339.
2. Cipriani G, Borin G, Vedovello M, et al. Sociopathic behavior and dementia. Acta Neurol Belg. 2013;113:111-115.
3. Cipriani G, Lucetti C, Danti S, et al. Violent and criminal manifestations in dementia patients. Geriatr Gerontol Int. 2016;16:541-549.
4. Skovdahl K, Kihlgren AL, Kihlgren M. Different attitudes when handling aggressive behaviour in dementia—narratives from two caregiver groups. Aging Ment Health. 2003;7:277-286.
5. Kristiansen L, Hellzén O, Asplund K. Swedish assistant nurses’ experiences of job satisfaction when caring for persons suffering from dementia and behavioural disturbances. An interview study. Int J Qualitat Stud Health Well-being. 2006;1:245-256.
6. Wharton TC, Ford BK. What is known about dementia care recipient violence and aggression against caregivers? J Gerontol Soc Work. 2014;57:460-477.
7. Ostaszkiewicz J, Lakhan P, O’Connell B, et al. Ongoing challenges responding to behavioural and psychological symptoms of dementia. Int Nurs Rev. 2015;62:506-516.
8. Kim J, De Bellis AM, Xiao LD. The experience of paid family-care workers of people with dementia in South Korea. Asian Nurs Res (Korean Soc Nurs Sci). 2018;12:34-41.
9. Band-Winterstein T, Avieli H. Women coping with a partner’s dementia-related violence: a qualitative study. J Nurs Scholarsh. 2019; 51:368-379.
10. Munkejord MC, Stefansdottir OA, Sveinbjarnardottir EK. Who cares for the carer? The suffering, struggles and unmet needs of older women caring for husbands living with cognitive decline. Int Pract Devel J. 2020;10:1-11.
11. Seidel D, Thyrian JR. Burden of caring for people with dementia - comparing family caregivers and professional caregivers. A descriptive study. J Multidiscip Healthc. 2019;12:655-663.
12. Tang W, Friedman DB, Kannaley K, et al. Experiences of caregivers by care recipient’s health condition: a study of caregivers for Alzheimer’s disease and related dementias versus other chronic conditions. Geriatr Nurs. 2019;40:181-184.
13. Benbow SM, Bhattacharyya S, Kingston P. Older adults and violence: an analysis of domestic homicide reviews in England involving adults over 60 years of age. Ageing Soc. 2018;39:1097-1121.
14. Herron RV, Wrathall MA. Putting responsive behaviours in place: examining how formal and informal carers understand the actions of people with dementia. Soc Sci Med. 2018;204:9-15.
15. Herron RV, Rosenberg MW. Responding to aggression and reactive behaviours in the home. Dementia (London). 2019;18:1328-1340.
16. Spencer D, Funk LM, Herron RV, et al. Fear, defensive strategies and caring for cognitively impaired family members. J Gerontol Soc Work. 2019;62:67-85.
17. Skovdahl K, Kihlgren AL, Kihlgren M. Dementia and aggressiveness: stimulated recall interviews with caregivers after video-recorded interactions. J Clin Nurs. 2004;13:515-525.
18. Needham I, Abderhalden C, Halfens RJ, et al. Non-somatic effects of patient aggression on nurses: a systematic review. J Adv Nurs. 2005;49:283-296.
19. Tariq SH, Tumosa N, Chibnall JT, et al. The Saint Louis University Mental Status (SLUMS) Examination for detecting mild cognitive impairment and dementia is more sensitive than the Mini-Mental Status Examination (MMSE) - a pilot study. Am J Geriatr Psych. 2006;14:900-910.
20. Janzen S, Zecevic AA, Kloseck M, et al. Managing agitation using nonpharmacological interventions for seniors with dementia. Am J Alzheimers Dis Other Demen. 2013;28:524-532.
21. Zeller A, Dassen T, Kok G, et al. Nursing home caregivers’ explanations for and coping strategies with residents’ aggression: a qualitative study. J Clin Nurs. 2011;20:2469-2478.
22. Alzheimer’s Society. Fix dementia care: homecare. Accessed December 28, 2021. https://www.alzheimers.org.uk/sites/default/files/migrate/downloads/fix_dementia_care_homecare_report.pdf
23. von Känel R, Mausbach BT, Dimsdale JE, et al. Refining caregiver vulnerability for clinical practice: determinants of self-rated health in spousal dementia caregivers. BMC Geriatr. 2019;19:18.
24. Chen HM, Huang MF, Yeh YC, et al. Effectiveness of coping strategies intervention on caregiver burden among caregivers of elderly patients with dementia. Psychogeriatrics. 2015; 15:20-25.
25. Wawrziczny E, Larochette C, Papo D, et al. A customized intervention for dementia caregivers: a quasi-experimental design. J Aging Health. 2019;31:1172-1195.
26. Gitlin LN, Piersol CV, Hodgson N, et al. Reducing neuropsychiatric symptoms in persons with dementia and associated burden in family caregivers using tailored activities: Design and methods of a randomized clinical trial. Contemp Clin Trials. 2016;49:92-102.
27. de Oliveira AM, Radanovic M, Homem de Mello PC, et al. An intervention to reduce neuropsychiatric symptoms and caregiver burden in dementia: preliminary results from a randomized trial of the tailored activity program-outpatient version. Int J Geriatr Psychiatry. 2019;34:1301-1307.
28. Livingston G, Barber J, Rapaport P, et al. Clinical effectiveness of a manual based coping strategy programme (START, STrAtegies for RelaTives) in promoting the mental health of carers of family members with dementia: pragmatic randomised controlled trial. BMJ. 2013;347:f6276.
29. Kajiyama B, Fernandez G, Carter EA, et al. Helping Hispanic dementia caregivers cope with stress using technology-based resources. Clin Gerontol. 2018;41:209-216.
30. Moskowitz JT, Cheung EO, Snowberg KE, et al. Randomized controlled trial of a facilitated online positive emotion regulation intervention for dementia caregivers. Health Psychol. 2019;38:391-402.
31. Yoon HK, Kim GS. An empowerment program for family caregivers of people with dementia. Public Health Nurs. 2020;37:222-233.
32. Zwingmann I, Dreier-Wolfgramm A, Esser A, et al. Why do family dementia caregivers reject caregiver support services? Analyzing types of rejection and associated health-impairments in a cluster-randomized controlled intervention trial. BMC Health Serv Res. 2020;20:121.
33. Nybakken S, Strandås M, Bondas T. Caregivers’ perceptions of aggressive behaviour in nursing home residents living with dementia: A meta-ethnography. J Adv Nurs. 2018;74:2713-2726.
34. Nakaishi L, Moss H, Weinstein M, et al. Exploring workplace violence among home care workers in a consumer-driven home health care program. Workplace Health Saf. 2013;61:441-450.
35. Medical Advisory Secretariat. Caregiver- and patient-directed interventions for dementia: an evidence-based analysis. Ont Health Technol Assess Ser. 2008;8:1-98.
36. Betz ME, McCourt AD, Vernick JS, et al. Firearms and dementia: clinical considerations. Ann Intern Med. 2018;169:47-49.
37. Leng M, Zhao Y, Wang Z. Comparative efficacy of non-pharmacological interventions on agitation in people with dementia: a systematic review and Bayesian network meta-analysis. Int J Nurs Stud. 2020;102:103489.
38. Morgan S, Pullon S, McKinlay E. Observation of interprofessional collaborative practice in primary care teams: an integrative literature review. Int J Nurs Stud. 2015;52:1217-1230.
The number of people with dementia globally is expected to reach 74.7 million by 2030 and 131.5 million by 2050.1 Because dementia is progressive, many patients will exhibit severe symptoms termed behavioral crises. Deteriorating interpersonal conduct and escalating antisocial acts result in an acquired sociopathy.2 Increasing cognitive impairment causes these patients to misunderstand intimate care and perceive it as a threat, often resulting in outbursts of violence against their caregivers.3
Available studies (TABLE4-17) make evident the incidence of interpersonal violence experienced by caregivers secondary to aggressive acts by patients with dementia. This violence ranges from verbal abuse, including racial slurs, to physical abuse—sometimes resulting in significant physical injury. Aggressive behavior by patients with dementia, resulting in violence towards their caregivers or partners, stems from progressive cognitive decline, which can make optimal care difficult. Such episodes may also impair the psychological and physical well-being of caregivers, increasing their risk of depression, anxiety, and even post-traumatic stress disorder (PTSD).18 The extent of the impact is also determined by the interpretation of the abuse by the caregivers themselves. One study suggested that the perception of aggressive or violent behavior as “normal” by a caregiver reduced the overall negative effect of the interactions.7Our review emphasizes the unintended burden that can fall to caregivers of patients with dementia. We also address the role of primary care providers (PCPs) in identifying these instances of violence and intervening appropriately by providing safety strategies, education, resources, and support.
CASE
A 67-year-old man with a medical history of PTSD with depression, type 2 diabetes, alcohol use disorder/dependence, hypertension, and obstructive sleep apnea was brought to his PCP by his wife. She said he had recently been unable to keep appointment times, pay bills, or take his usual medications, venlafaxine and bupropion. She also said his PTSD symptoms had worsened. He was sleeping 12 to 14 hours per day and was increasingly irritable. The patient denied any concerns or changes in his behavior.
The PCP administered a Saint Louis University Mental Status (SLUMS) examination to screen for cognitive impairment.19 The patient scored 14/30 (less than 20 is indicative of dementia). He was unable to complete a simple math problem, recall any items from a list of 5, count in reverse, draw a clock correctly, or recall a full story. Throughout the exam, the patient demonstrated minimal effort and was often only able to complete a task after further prompting by the examiner.
A computed tomography scan of the head revealed no signs of hemorrhage or damage. Thyroid-stimulating hormone levels and vitamin B12 levels were normal. A rapid plasma reagin test result was negative. The patient was given a diagnosis of Alzheimer disease. Donepezil was added to the patient’s medications, starting at 5 mg and then increased to 10 mg. His wife began to assist him with his tasks of daily living. His mood improved, and his wife noted he began to remember his appointments and take his medications with assistance.
However, the patient’s irritability continued to escalate. He grew paranoid and accused his wife of mismanaging their money. This pattern steadily worsened over the course of 6 months. The situation escalated until one day the patient’s wife called a mental health hotline reporting that her husband was holding her hostage and threatening to kill her with a gun. He told her, “I can do something to you, and they won’t even find a fingernail. It doesn’t have to be with a gun either.” She was counseled to try to stay calm to avoid aggravating the situation and to go to a safe place and stay there until help arrived.
His memory had worsened to the point that he could not recall any events from the previous 2 years. He was paranoid about anyone entering his home and would not allow his deteriorating roof to be repaired or his yard to be maintained. He did not shower for weeks at a time. He slept holding a rifle and accused his wife of embezzlement.
Continue to: The patient was evaluated...
The patient was evaluated by another specialist, who assessed his SLUMS score to be 18/30. He increased the patient’s donepezil dose, initiated a bupropion taper, and added sertraline to the regimen. The PCP spoke to the patient’s wife regarding options for her safety including leaving the home, hiding firearms, and calling the police in cases of interpersonal violence. The wife said she did not want to pursue these options. She expressed worry that he might be harmed if he was uncooperative with the police and said there was no one except her to take care of him.
Caregivers struggle to care for their loved ones
Instances of personal violence lead to shock, astonishment, heartbreak, and fear. Anticipation of a recurrence of violence causes many partners and caregivers to feel exhausted, because there is minimal hope for any chance of improvement. There are a few exceptions, however, as our case will show. In addition to emotional exhaustion, there is also a never-ending sense of self-doubt, leading many caregivers to question their ability to handle their family member.20,21 Over time, this leads to caregiver burnout, leaving them unable to understand their family member’s aggression. The sudden loss of caregiver control in dealing with the patient may also result in the family member exhibiting behavioral changes reflecting emotional trauma. For caregivers who do not live with the patient, they may choose to make fewer or shorter visits—or not visit at all—because they fear being abused.7,22
Caregivers of patients with dementia often feel helpless and powerless once abrupt and drastic changes in personality lead to some form of interpersonal violence. Additionally, caregivers with a poor health status are more likely to have lower physical function and experience greater caregiving stress overall.23 Other factors increasing stress are longer years of caregiving and the severity of a patient’s dementia and functional impairment.23
Interventions to reduce caregiver burden
Many studies have assessed the role of different interventions to reduce caregiver burden, such as teaching them problem-solving skills, increasing their knowledge of dementia, recommending social resources, providing emotional support, changing caregiver perceptions of the care situation, introducing coping strategies, relying on strengths and experiences in caregiving, help-seeking, and engaging in activity programs.24-28 For Hispanic caregivers, a structured and self-paced online telenovela format has been effective in improving care and relieving caregiver stress.29 Online positive emotion regulators helped in significantly improving quality of life and physical health in the caregivers.30 In this last intervention, caregivers had 6 online sessions with a facilitator who taught them emotional regulation skills that included: noticing positive events, capitalizing on them, and feeling gratitude; practicing mindfulness; doing a positive reappraisal; acknowledging personal strengths and setting attainable goals; and performing acts of kindness. Empowerment programs have also shown significant improvement in the well-being of caregivers.31
Caregivers may reject support.
Continue to: These practical tips can help
These practical tips can help
Based on our review of the literature, we recommend offering the following supports to caregivers:
- Counsel caregivers early on in a patient’s dementia that behavior changes are likely and may be unpredictable. Explain that dementia can involve changes to personality and behavior as well as memory difficulties.33,34
- Describe resources for support, such as day programs for senior adults, insurance coverage for caregiver respite programs, and the Alzheimer’s Association (www.alz.org/). Encourage caregivers to seek general medical and mental health care for themselves. Caregivers should have opportunities and support to discuss their experiences and to be appropriately trained for the challenge of caring for a family member with dementia.35
- Encourage disclosure about abrupt changes in the patient’s behavior. This invites families to discuss issues with you and may make them more comfortable with such conversations.
- Involve ancillary services (eg, social worker) to plan for a higher level of care well in advance of it becoming necessary.
- Discuss safety strategies for the caregiver, including when it is appropriate to alter a patient’s set routines such as bedtimes and mealtimes.33,34
- Discuss when and how to involve law enforcement, if necessary.33,34 Emphasize the importance of removing firearms from the home as a safety measure. Although federal laws do not explicitly prohibit possession of arms by patients with neurologic damage, a few states mention “organic brain syndrome” or “dementia” as conditions prohibiting use or possession of firearms.36
- Suggest, as feasible, nonpharmacologic aids for the patient such as massage therapy, animal-assisted therapy, personalized interventions, music therapy, and light therapy.37 Prescribe medications to the patient to aid in behavior modification when appropriate.
- Screen caregivers and family members for signs of interpersonal violence. Take notice of changes in caregiver behavior or irregularity in attending follow-up appointments.
CASE
Over the next month, the patient’s symptoms further deteriorated. His PCP recommended hospitalization, but the patient and his wife declined. Magnetic resonance imaging of the patient’s brain revealed severe confluent and patchy regions of white matter and T2 signal hyperintensity, consistent with chronic microvascular ischemic disease. An old, small, left parietal lobe infarct was also noted.
One month later, the patient presented to the emergency department. His symptoms were largely unchanged, but his wife indicated that she could no longer live at home due to burnout. The patient’s medications were adjusted, but he was not admitted for inpatient care. His wife said they needed help at home, but the patient opposed the idea any time that it was mentioned.
A few weeks later, the patient presented for outpatient follow-up. He was delusional, believing that the government was compelling citizens to take sertraline in order to harm their mental health. He had also begun viewing online pornography in front of his wife and attempting to remove all of his money from the bank. He was prescribed aripiprazole 15 mg, and his symptoms began to improve. Soon after, however, he threatened to kill his grandson, then took all his Lasix pills (a 7-day supply) simultaneously. The patient denied that this was a suicide attempt.
Over the course of the next month, the patient began to report hearing voices. A neuropsychological evaluation confirmed a diagnosis of dementia with psychiatric symptoms due to neurologic injury. The patient was referred to a geriatric psychiatrist and continued to be managed medically. He was assigned a multidisciplinary team comprising palliative care, social work, and care management to assist in his care and provide support to the family. His behavior improved.
Continue to: At the time of this publication...
At the time of this publication, the patient’s irritability and paranoia had subsided and he had made no further threats to his family. He has allowed a home health aide into the house and has agreed to have his roof repaired. His wife still lives with him and assists him with activities of daily living.
Interprofessional teams are key
Caregiver burnout increases the risk of patient neglect or abuse, as individuals who have been the targets of aggressive behavior are more likely to leave demented patients unattended.8,16,23 Although tools are available to screen caregivers for depression and burnout, an important step forward would be to develop an interprofessional team to aid in identifying and closely following high-risk patient–caregiver groups. This continual and varied assessment of psychosocial stressors could help prevent the development of violent interactions. These teams would allow integration with the primary health care system by frequent and effective shared communication of knowledge, development of goals, and shared decision-making.38 Setting expectations, providing support, and discussing safety strategies can improve the health and welfare of caregivers and patients with dementia alike.
CORRESPONDENCE
Abu Baker Sheikh, MD, MSC 10-5550, 1 University of New Mexico, Albuquerque, NM 87131; [email protected].
The number of people with dementia globally is expected to reach 74.7 million by 2030 and 131.5 million by 2050.1 Because dementia is progressive, many patients will exhibit severe symptoms termed behavioral crises. Deteriorating interpersonal conduct and escalating antisocial acts result in an acquired sociopathy.2 Increasing cognitive impairment causes these patients to misunderstand intimate care and perceive it as a threat, often resulting in outbursts of violence against their caregivers.3
Available studies (TABLE4-17) make evident the incidence of interpersonal violence experienced by caregivers secondary to aggressive acts by patients with dementia. This violence ranges from verbal abuse, including racial slurs, to physical abuse—sometimes resulting in significant physical injury. Aggressive behavior by patients with dementia, resulting in violence towards their caregivers or partners, stems from progressive cognitive decline, which can make optimal care difficult. Such episodes may also impair the psychological and physical well-being of caregivers, increasing their risk of depression, anxiety, and even post-traumatic stress disorder (PTSD).18 The extent of the impact is also determined by the interpretation of the abuse by the caregivers themselves. One study suggested that the perception of aggressive or violent behavior as “normal” by a caregiver reduced the overall negative effect of the interactions.7Our review emphasizes the unintended burden that can fall to caregivers of patients with dementia. We also address the role of primary care providers (PCPs) in identifying these instances of violence and intervening appropriately by providing safety strategies, education, resources, and support.
CASE
A 67-year-old man with a medical history of PTSD with depression, type 2 diabetes, alcohol use disorder/dependence, hypertension, and obstructive sleep apnea was brought to his PCP by his wife. She said he had recently been unable to keep appointment times, pay bills, or take his usual medications, venlafaxine and bupropion. She also said his PTSD symptoms had worsened. He was sleeping 12 to 14 hours per day and was increasingly irritable. The patient denied any concerns or changes in his behavior.
The PCP administered a Saint Louis University Mental Status (SLUMS) examination to screen for cognitive impairment.19 The patient scored 14/30 (less than 20 is indicative of dementia). He was unable to complete a simple math problem, recall any items from a list of 5, count in reverse, draw a clock correctly, or recall a full story. Throughout the exam, the patient demonstrated minimal effort and was often only able to complete a task after further prompting by the examiner.
A computed tomography scan of the head revealed no signs of hemorrhage or damage. Thyroid-stimulating hormone levels and vitamin B12 levels were normal. A rapid plasma reagin test result was negative. The patient was given a diagnosis of Alzheimer disease. Donepezil was added to the patient’s medications, starting at 5 mg and then increased to 10 mg. His wife began to assist him with his tasks of daily living. His mood improved, and his wife noted he began to remember his appointments and take his medications with assistance.
However, the patient’s irritability continued to escalate. He grew paranoid and accused his wife of mismanaging their money. This pattern steadily worsened over the course of 6 months. The situation escalated until one day the patient’s wife called a mental health hotline reporting that her husband was holding her hostage and threatening to kill her with a gun. He told her, “I can do something to you, and they won’t even find a fingernail. It doesn’t have to be with a gun either.” She was counseled to try to stay calm to avoid aggravating the situation and to go to a safe place and stay there until help arrived.
His memory had worsened to the point that he could not recall any events from the previous 2 years. He was paranoid about anyone entering his home and would not allow his deteriorating roof to be repaired or his yard to be maintained. He did not shower for weeks at a time. He slept holding a rifle and accused his wife of embezzlement.
Continue to: The patient was evaluated...
The patient was evaluated by another specialist, who assessed his SLUMS score to be 18/30. He increased the patient’s donepezil dose, initiated a bupropion taper, and added sertraline to the regimen. The PCP spoke to the patient’s wife regarding options for her safety including leaving the home, hiding firearms, and calling the police in cases of interpersonal violence. The wife said she did not want to pursue these options. She expressed worry that he might be harmed if he was uncooperative with the police and said there was no one except her to take care of him.
Caregivers struggle to care for their loved ones
Instances of personal violence lead to shock, astonishment, heartbreak, and fear. Anticipation of a recurrence of violence causes many partners and caregivers to feel exhausted, because there is minimal hope for any chance of improvement. There are a few exceptions, however, as our case will show. In addition to emotional exhaustion, there is also a never-ending sense of self-doubt, leading many caregivers to question their ability to handle their family member.20,21 Over time, this leads to caregiver burnout, leaving them unable to understand their family member’s aggression. The sudden loss of caregiver control in dealing with the patient may also result in the family member exhibiting behavioral changes reflecting emotional trauma. For caregivers who do not live with the patient, they may choose to make fewer or shorter visits—or not visit at all—because they fear being abused.7,22
Caregivers of patients with dementia often feel helpless and powerless once abrupt and drastic changes in personality lead to some form of interpersonal violence. Additionally, caregivers with a poor health status are more likely to have lower physical function and experience greater caregiving stress overall.23 Other factors increasing stress are longer years of caregiving and the severity of a patient’s dementia and functional impairment.23
Interventions to reduce caregiver burden
Many studies have assessed the role of different interventions to reduce caregiver burden, such as teaching them problem-solving skills, increasing their knowledge of dementia, recommending social resources, providing emotional support, changing caregiver perceptions of the care situation, introducing coping strategies, relying on strengths and experiences in caregiving, help-seeking, and engaging in activity programs.24-28 For Hispanic caregivers, a structured and self-paced online telenovela format has been effective in improving care and relieving caregiver stress.29 Online positive emotion regulators helped in significantly improving quality of life and physical health in the caregivers.30 In this last intervention, caregivers had 6 online sessions with a facilitator who taught them emotional regulation skills that included: noticing positive events, capitalizing on them, and feeling gratitude; practicing mindfulness; doing a positive reappraisal; acknowledging personal strengths and setting attainable goals; and performing acts of kindness. Empowerment programs have also shown significant improvement in the well-being of caregivers.31
Caregivers may reject support.
Continue to: These practical tips can help
These practical tips can help
Based on our review of the literature, we recommend offering the following supports to caregivers:
- Counsel caregivers early on in a patient’s dementia that behavior changes are likely and may be unpredictable. Explain that dementia can involve changes to personality and behavior as well as memory difficulties.33,34
- Describe resources for support, such as day programs for senior adults, insurance coverage for caregiver respite programs, and the Alzheimer’s Association (www.alz.org/). Encourage caregivers to seek general medical and mental health care for themselves. Caregivers should have opportunities and support to discuss their experiences and to be appropriately trained for the challenge of caring for a family member with dementia.35
- Encourage disclosure about abrupt changes in the patient’s behavior. This invites families to discuss issues with you and may make them more comfortable with such conversations.
- Involve ancillary services (eg, social worker) to plan for a higher level of care well in advance of it becoming necessary.
- Discuss safety strategies for the caregiver, including when it is appropriate to alter a patient’s set routines such as bedtimes and mealtimes.33,34
- Discuss when and how to involve law enforcement, if necessary.33,34 Emphasize the importance of removing firearms from the home as a safety measure. Although federal laws do not explicitly prohibit possession of arms by patients with neurologic damage, a few states mention “organic brain syndrome” or “dementia” as conditions prohibiting use or possession of firearms.36
- Suggest, as feasible, nonpharmacologic aids for the patient such as massage therapy, animal-assisted therapy, personalized interventions, music therapy, and light therapy.37 Prescribe medications to the patient to aid in behavior modification when appropriate.
- Screen caregivers and family members for signs of interpersonal violence. Take notice of changes in caregiver behavior or irregularity in attending follow-up appointments.
CASE
Over the next month, the patient’s symptoms further deteriorated. His PCP recommended hospitalization, but the patient and his wife declined. Magnetic resonance imaging of the patient’s brain revealed severe confluent and patchy regions of white matter and T2 signal hyperintensity, consistent with chronic microvascular ischemic disease. An old, small, left parietal lobe infarct was also noted.
One month later, the patient presented to the emergency department. His symptoms were largely unchanged, but his wife indicated that she could no longer live at home due to burnout. The patient’s medications were adjusted, but he was not admitted for inpatient care. His wife said they needed help at home, but the patient opposed the idea any time that it was mentioned.
A few weeks later, the patient presented for outpatient follow-up. He was delusional, believing that the government was compelling citizens to take sertraline in order to harm their mental health. He had also begun viewing online pornography in front of his wife and attempting to remove all of his money from the bank. He was prescribed aripiprazole 15 mg, and his symptoms began to improve. Soon after, however, he threatened to kill his grandson, then took all his Lasix pills (a 7-day supply) simultaneously. The patient denied that this was a suicide attempt.
Over the course of the next month, the patient began to report hearing voices. A neuropsychological evaluation confirmed a diagnosis of dementia with psychiatric symptoms due to neurologic injury. The patient was referred to a geriatric psychiatrist and continued to be managed medically. He was assigned a multidisciplinary team comprising palliative care, social work, and care management to assist in his care and provide support to the family. His behavior improved.
Continue to: At the time of this publication...
At the time of this publication, the patient’s irritability and paranoia had subsided and he had made no further threats to his family. He has allowed a home health aide into the house and has agreed to have his roof repaired. His wife still lives with him and assists him with activities of daily living.
Interprofessional teams are key
Caregiver burnout increases the risk of patient neglect or abuse, as individuals who have been the targets of aggressive behavior are more likely to leave demented patients unattended.8,16,23 Although tools are available to screen caregivers for depression and burnout, an important step forward would be to develop an interprofessional team to aid in identifying and closely following high-risk patient–caregiver groups. This continual and varied assessment of psychosocial stressors could help prevent the development of violent interactions. These teams would allow integration with the primary health care system by frequent and effective shared communication of knowledge, development of goals, and shared decision-making.38 Setting expectations, providing support, and discussing safety strategies can improve the health and welfare of caregivers and patients with dementia alike.
CORRESPONDENCE
Abu Baker Sheikh, MD, MSC 10-5550, 1 University of New Mexico, Albuquerque, NM 87131; [email protected].
1. Wu YT, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time - current evidence. Nat Rev Neurol. 2017;13:327-339.
2. Cipriani G, Borin G, Vedovello M, et al. Sociopathic behavior and dementia. Acta Neurol Belg. 2013;113:111-115.
3. Cipriani G, Lucetti C, Danti S, et al. Violent and criminal manifestations in dementia patients. Geriatr Gerontol Int. 2016;16:541-549.
4. Skovdahl K, Kihlgren AL, Kihlgren M. Different attitudes when handling aggressive behaviour in dementia—narratives from two caregiver groups. Aging Ment Health. 2003;7:277-286.
5. Kristiansen L, Hellzén O, Asplund K. Swedish assistant nurses’ experiences of job satisfaction when caring for persons suffering from dementia and behavioural disturbances. An interview study. Int J Qualitat Stud Health Well-being. 2006;1:245-256.
6. Wharton TC, Ford BK. What is known about dementia care recipient violence and aggression against caregivers? J Gerontol Soc Work. 2014;57:460-477.
7. Ostaszkiewicz J, Lakhan P, O’Connell B, et al. Ongoing challenges responding to behavioural and psychological symptoms of dementia. Int Nurs Rev. 2015;62:506-516.
8. Kim J, De Bellis AM, Xiao LD. The experience of paid family-care workers of people with dementia in South Korea. Asian Nurs Res (Korean Soc Nurs Sci). 2018;12:34-41.
9. Band-Winterstein T, Avieli H. Women coping with a partner’s dementia-related violence: a qualitative study. J Nurs Scholarsh. 2019; 51:368-379.
10. Munkejord MC, Stefansdottir OA, Sveinbjarnardottir EK. Who cares for the carer? The suffering, struggles and unmet needs of older women caring for husbands living with cognitive decline. Int Pract Devel J. 2020;10:1-11.
11. Seidel D, Thyrian JR. Burden of caring for people with dementia - comparing family caregivers and professional caregivers. A descriptive study. J Multidiscip Healthc. 2019;12:655-663.
12. Tang W, Friedman DB, Kannaley K, et al. Experiences of caregivers by care recipient’s health condition: a study of caregivers for Alzheimer’s disease and related dementias versus other chronic conditions. Geriatr Nurs. 2019;40:181-184.
13. Benbow SM, Bhattacharyya S, Kingston P. Older adults and violence: an analysis of domestic homicide reviews in England involving adults over 60 years of age. Ageing Soc. 2018;39:1097-1121.
14. Herron RV, Wrathall MA. Putting responsive behaviours in place: examining how formal and informal carers understand the actions of people with dementia. Soc Sci Med. 2018;204:9-15.
15. Herron RV, Rosenberg MW. Responding to aggression and reactive behaviours in the home. Dementia (London). 2019;18:1328-1340.
16. Spencer D, Funk LM, Herron RV, et al. Fear, defensive strategies and caring for cognitively impaired family members. J Gerontol Soc Work. 2019;62:67-85.
17. Skovdahl K, Kihlgren AL, Kihlgren M. Dementia and aggressiveness: stimulated recall interviews with caregivers after video-recorded interactions. J Clin Nurs. 2004;13:515-525.
18. Needham I, Abderhalden C, Halfens RJ, et al. Non-somatic effects of patient aggression on nurses: a systematic review. J Adv Nurs. 2005;49:283-296.
19. Tariq SH, Tumosa N, Chibnall JT, et al. The Saint Louis University Mental Status (SLUMS) Examination for detecting mild cognitive impairment and dementia is more sensitive than the Mini-Mental Status Examination (MMSE) - a pilot study. Am J Geriatr Psych. 2006;14:900-910.
20. Janzen S, Zecevic AA, Kloseck M, et al. Managing agitation using nonpharmacological interventions for seniors with dementia. Am J Alzheimers Dis Other Demen. 2013;28:524-532.
21. Zeller A, Dassen T, Kok G, et al. Nursing home caregivers’ explanations for and coping strategies with residents’ aggression: a qualitative study. J Clin Nurs. 2011;20:2469-2478.
22. Alzheimer’s Society. Fix dementia care: homecare. Accessed December 28, 2021. https://www.alzheimers.org.uk/sites/default/files/migrate/downloads/fix_dementia_care_homecare_report.pdf
23. von Känel R, Mausbach BT, Dimsdale JE, et al. Refining caregiver vulnerability for clinical practice: determinants of self-rated health in spousal dementia caregivers. BMC Geriatr. 2019;19:18.
24. Chen HM, Huang MF, Yeh YC, et al. Effectiveness of coping strategies intervention on caregiver burden among caregivers of elderly patients with dementia. Psychogeriatrics. 2015; 15:20-25.
25. Wawrziczny E, Larochette C, Papo D, et al. A customized intervention for dementia caregivers: a quasi-experimental design. J Aging Health. 2019;31:1172-1195.
26. Gitlin LN, Piersol CV, Hodgson N, et al. Reducing neuropsychiatric symptoms in persons with dementia and associated burden in family caregivers using tailored activities: Design and methods of a randomized clinical trial. Contemp Clin Trials. 2016;49:92-102.
27. de Oliveira AM, Radanovic M, Homem de Mello PC, et al. An intervention to reduce neuropsychiatric symptoms and caregiver burden in dementia: preliminary results from a randomized trial of the tailored activity program-outpatient version. Int J Geriatr Psychiatry. 2019;34:1301-1307.
28. Livingston G, Barber J, Rapaport P, et al. Clinical effectiveness of a manual based coping strategy programme (START, STrAtegies for RelaTives) in promoting the mental health of carers of family members with dementia: pragmatic randomised controlled trial. BMJ. 2013;347:f6276.
29. Kajiyama B, Fernandez G, Carter EA, et al. Helping Hispanic dementia caregivers cope with stress using technology-based resources. Clin Gerontol. 2018;41:209-216.
30. Moskowitz JT, Cheung EO, Snowberg KE, et al. Randomized controlled trial of a facilitated online positive emotion regulation intervention for dementia caregivers. Health Psychol. 2019;38:391-402.
31. Yoon HK, Kim GS. An empowerment program for family caregivers of people with dementia. Public Health Nurs. 2020;37:222-233.
32. Zwingmann I, Dreier-Wolfgramm A, Esser A, et al. Why do family dementia caregivers reject caregiver support services? Analyzing types of rejection and associated health-impairments in a cluster-randomized controlled intervention trial. BMC Health Serv Res. 2020;20:121.
33. Nybakken S, Strandås M, Bondas T. Caregivers’ perceptions of aggressive behaviour in nursing home residents living with dementia: A meta-ethnography. J Adv Nurs. 2018;74:2713-2726.
34. Nakaishi L, Moss H, Weinstein M, et al. Exploring workplace violence among home care workers in a consumer-driven home health care program. Workplace Health Saf. 2013;61:441-450.
35. Medical Advisory Secretariat. Caregiver- and patient-directed interventions for dementia: an evidence-based analysis. Ont Health Technol Assess Ser. 2008;8:1-98.
36. Betz ME, McCourt AD, Vernick JS, et al. Firearms and dementia: clinical considerations. Ann Intern Med. 2018;169:47-49.
37. Leng M, Zhao Y, Wang Z. Comparative efficacy of non-pharmacological interventions on agitation in people with dementia: a systematic review and Bayesian network meta-analysis. Int J Nurs Stud. 2020;102:103489.
38. Morgan S, Pullon S, McKinlay E. Observation of interprofessional collaborative practice in primary care teams: an integrative literature review. Int J Nurs Stud. 2015;52:1217-1230.
1. Wu YT, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time - current evidence. Nat Rev Neurol. 2017;13:327-339.
2. Cipriani G, Borin G, Vedovello M, et al. Sociopathic behavior and dementia. Acta Neurol Belg. 2013;113:111-115.
3. Cipriani G, Lucetti C, Danti S, et al. Violent and criminal manifestations in dementia patients. Geriatr Gerontol Int. 2016;16:541-549.
4. Skovdahl K, Kihlgren AL, Kihlgren M. Different attitudes when handling aggressive behaviour in dementia—narratives from two caregiver groups. Aging Ment Health. 2003;7:277-286.
5. Kristiansen L, Hellzén O, Asplund K. Swedish assistant nurses’ experiences of job satisfaction when caring for persons suffering from dementia and behavioural disturbances. An interview study. Int J Qualitat Stud Health Well-being. 2006;1:245-256.
6. Wharton TC, Ford BK. What is known about dementia care recipient violence and aggression against caregivers? J Gerontol Soc Work. 2014;57:460-477.
7. Ostaszkiewicz J, Lakhan P, O’Connell B, et al. Ongoing challenges responding to behavioural and psychological symptoms of dementia. Int Nurs Rev. 2015;62:506-516.
8. Kim J, De Bellis AM, Xiao LD. The experience of paid family-care workers of people with dementia in South Korea. Asian Nurs Res (Korean Soc Nurs Sci). 2018;12:34-41.
9. Band-Winterstein T, Avieli H. Women coping with a partner’s dementia-related violence: a qualitative study. J Nurs Scholarsh. 2019; 51:368-379.
10. Munkejord MC, Stefansdottir OA, Sveinbjarnardottir EK. Who cares for the carer? The suffering, struggles and unmet needs of older women caring for husbands living with cognitive decline. Int Pract Devel J. 2020;10:1-11.
11. Seidel D, Thyrian JR. Burden of caring for people with dementia - comparing family caregivers and professional caregivers. A descriptive study. J Multidiscip Healthc. 2019;12:655-663.
12. Tang W, Friedman DB, Kannaley K, et al. Experiences of caregivers by care recipient’s health condition: a study of caregivers for Alzheimer’s disease and related dementias versus other chronic conditions. Geriatr Nurs. 2019;40:181-184.
13. Benbow SM, Bhattacharyya S, Kingston P. Older adults and violence: an analysis of domestic homicide reviews in England involving adults over 60 years of age. Ageing Soc. 2018;39:1097-1121.
14. Herron RV, Wrathall MA. Putting responsive behaviours in place: examining how formal and informal carers understand the actions of people with dementia. Soc Sci Med. 2018;204:9-15.
15. Herron RV, Rosenberg MW. Responding to aggression and reactive behaviours in the home. Dementia (London). 2019;18:1328-1340.
16. Spencer D, Funk LM, Herron RV, et al. Fear, defensive strategies and caring for cognitively impaired family members. J Gerontol Soc Work. 2019;62:67-85.
17. Skovdahl K, Kihlgren AL, Kihlgren M. Dementia and aggressiveness: stimulated recall interviews with caregivers after video-recorded interactions. J Clin Nurs. 2004;13:515-525.
18. Needham I, Abderhalden C, Halfens RJ, et al. Non-somatic effects of patient aggression on nurses: a systematic review. J Adv Nurs. 2005;49:283-296.
19. Tariq SH, Tumosa N, Chibnall JT, et al. The Saint Louis University Mental Status (SLUMS) Examination for detecting mild cognitive impairment and dementia is more sensitive than the Mini-Mental Status Examination (MMSE) - a pilot study. Am J Geriatr Psych. 2006;14:900-910.
20. Janzen S, Zecevic AA, Kloseck M, et al. Managing agitation using nonpharmacological interventions for seniors with dementia. Am J Alzheimers Dis Other Demen. 2013;28:524-532.
21. Zeller A, Dassen T, Kok G, et al. Nursing home caregivers’ explanations for and coping strategies with residents’ aggression: a qualitative study. J Clin Nurs. 2011;20:2469-2478.
22. Alzheimer’s Society. Fix dementia care: homecare. Accessed December 28, 2021. https://www.alzheimers.org.uk/sites/default/files/migrate/downloads/fix_dementia_care_homecare_report.pdf
23. von Känel R, Mausbach BT, Dimsdale JE, et al. Refining caregiver vulnerability for clinical practice: determinants of self-rated health in spousal dementia caregivers. BMC Geriatr. 2019;19:18.
24. Chen HM, Huang MF, Yeh YC, et al. Effectiveness of coping strategies intervention on caregiver burden among caregivers of elderly patients with dementia. Psychogeriatrics. 2015; 15:20-25.
25. Wawrziczny E, Larochette C, Papo D, et al. A customized intervention for dementia caregivers: a quasi-experimental design. J Aging Health. 2019;31:1172-1195.
26. Gitlin LN, Piersol CV, Hodgson N, et al. Reducing neuropsychiatric symptoms in persons with dementia and associated burden in family caregivers using tailored activities: Design and methods of a randomized clinical trial. Contemp Clin Trials. 2016;49:92-102.
27. de Oliveira AM, Radanovic M, Homem de Mello PC, et al. An intervention to reduce neuropsychiatric symptoms and caregiver burden in dementia: preliminary results from a randomized trial of the tailored activity program-outpatient version. Int J Geriatr Psychiatry. 2019;34:1301-1307.
28. Livingston G, Barber J, Rapaport P, et al. Clinical effectiveness of a manual based coping strategy programme (START, STrAtegies for RelaTives) in promoting the mental health of carers of family members with dementia: pragmatic randomised controlled trial. BMJ. 2013;347:f6276.
29. Kajiyama B, Fernandez G, Carter EA, et al. Helping Hispanic dementia caregivers cope with stress using technology-based resources. Clin Gerontol. 2018;41:209-216.
30. Moskowitz JT, Cheung EO, Snowberg KE, et al. Randomized controlled trial of a facilitated online positive emotion regulation intervention for dementia caregivers. Health Psychol. 2019;38:391-402.
31. Yoon HK, Kim GS. An empowerment program for family caregivers of people with dementia. Public Health Nurs. 2020;37:222-233.
32. Zwingmann I, Dreier-Wolfgramm A, Esser A, et al. Why do family dementia caregivers reject caregiver support services? Analyzing types of rejection and associated health-impairments in a cluster-randomized controlled intervention trial. BMC Health Serv Res. 2020;20:121.
33. Nybakken S, Strandås M, Bondas T. Caregivers’ perceptions of aggressive behaviour in nursing home residents living with dementia: A meta-ethnography. J Adv Nurs. 2018;74:2713-2726.
34. Nakaishi L, Moss H, Weinstein M, et al. Exploring workplace violence among home care workers in a consumer-driven home health care program. Workplace Health Saf. 2013;61:441-450.
35. Medical Advisory Secretariat. Caregiver- and patient-directed interventions for dementia: an evidence-based analysis. Ont Health Technol Assess Ser. 2008;8:1-98.
36. Betz ME, McCourt AD, Vernick JS, et al. Firearms and dementia: clinical considerations. Ann Intern Med. 2018;169:47-49.
37. Leng M, Zhao Y, Wang Z. Comparative efficacy of non-pharmacological interventions on agitation in people with dementia: a systematic review and Bayesian network meta-analysis. Int J Nurs Stud. 2020;102:103489.
38. Morgan S, Pullon S, McKinlay E. Observation of interprofessional collaborative practice in primary care teams: an integrative literature review. Int J Nurs Stud. 2015;52:1217-1230.
PRACTICE RECOMMENDATIONS
› Screen caregivers and family members of patients with dementia for signs of interpersonal violence. C
› Counsel caregivers early on that behavior changes in patients with dementia are likely and may be unpredictable. C
› Discuss safety strategies for the caregiver, including when it is appropriate to alter routines such as bedtimes and meals. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A practical guide to appendicitis evaluation and treatment
CASE
A 35-year-old man with a body mass index of 20 presented to the emergency department after 24 hours of abdominal pain that began in the periumbilical region and then migrated to the right lower quadrant. The pain was exacerbated during ambulation and was intense when the car transporting him to the hospital encountered bumps in the road. After his pain started, he had associated anorexia, followed by nausea and emesis. He reported fever and chills. On examination, his temperature was 100.8 °F (38.2 °C), and palpation of the right and left lower quadrants elicited right lower quadrant pain. Laboratory evaluation revealed a white blood cell (WBC) count of 14,000 cells/mcL with 85% neutrophils, C-reactive protein of 40 mg/L, and a negative urinalysis.
How would you proceed with this patient?
Acute appendicitis is the most common cause of abdominal pain resulting in the need for surgical treatment; lifetime risk of appendicitis is 6% to 7%.1 Appendicitis is caused by intraluminal obstruction in the appendix from enlarged lymphoid tissue or a fecalith. The obstruction leads to elevated intraluminal pressure due to persistent mucus and gas production by bacteria, ultimately leading to ischemia and perforation.1 Additionally, obstruction leads to bacterial overgrowth, most commonly colonic flora such as Escherichia coli, Bacteroides fragilis, Streptococcus viridans, Enterococcus sp., Pseudomonas aeruginosa, and Klebsiella pneumoniaei.1,2
The following review provides a look at how 3 clinical scoring systems compare in the identification of acute appendicitis and details which imaging studies you should order—and when. But first, we’ll quickly detail the relevant physical findings and lab values that point to a diagnosis of acute appendicitis.
Physical findings. The patient typically first experiences vague abdominal pain that then localizes to the right lower quadrant due to peritoneal inflammation. Anorexia and nausea typically follow the abdominal pain. On examination, the patient often appears ill and exhibits abdominal guarding due to peritonitis. Tachycardia and fever are common; however, the absence of either does not exclude appendicitis. Classically, on palpation, the patient will have pain at McBurney’s point (one-third the distance from the anterior iliac spine to the umbilicus). The exact point of maximal tenderness can differ because of the varying anatomy of the appendix (retrocecal, paracolic, pelvic, pre/post ileal, promontoric, or subcecal).1 Right lower quadrant pain, abdominal rigidity, and radiation of periumbilical pain to the right lower quadrant are the most accurate findings in adults to rule in appendicitis.3 For children, physical exam findings have the highest likelihood in predicting appendicitis and include a positive Obturator sign, positive Rovsing sign, or a positive Psoas sign, and absent or decreased bowel sounds.4
Laboratory studies can support a diagnosis of appendicitis but cannot exclude it. Leukocytosis with neutrophil predominance is present in 90% of cases.5 An elevated C-reactive protein level renders the highest diagnostic accuracy.5 Perform a pregnancy test for any woman of child-bearing age, to assist in the diagnosis and guide imaging choices for evaluation. Additional laboratory tests are not needed unless there are concerns about volume depletion.
Clinical scoring systems
Several clinical scoring systems (TABLE6-10) have been validated to aid clinicians in evaluating patients with possible appendicitis, to decrease unnecessary exposure to ionizing radiation from computed tomography (CT) scans, to identify and reassure patients with low likelihoods of appendicitis, and to conduct outpatient follow-up.
Continue to: The Alvarado score
The Alvarado score is the oldest scoring rule, developed in 1986; it entails 8 clinical and laboratory variables.6 Ebell et al altered the proposed cutoff values of the Alvarado score to be low risk (< 4), intermediate risk (4-8), and high risk (≥ 9), effectively improving the sensitivity and specificity rates.7
In a meta-analysis of the Alvarado score that included 42 studies of men, women, and children, the sensitivity for “ruling out” appendicitis with a cutoff of 5 points was 96% for men, 99% for women, and 99% for children.8 The accuracy of a high-risk score (> 7) for “ruling in” appendicitis was less with an overall specificity of 82%.8 The Alvarado score did seem to overestimate appendicitis in women in all score categories.8
The Pediatric Appendicitis Score (PAS) is similar to Alvarado and was prospectively validated in 1170 children in 2002 for more specific guidance in this age group.9 The PAS had excellent specificity in the study; those with a score of ≥ 6 had a high probability of appendicitis. In a study comparing Alvarado with PAS in 311 patients, insignificant differences were noted at a score of ≥ 7 for both tests (sensitivity 86% vs 89%, and specificity 59% vs 50%, respectively).11 No scoring system has been found to be sufficiently accurate for use in children 4 years of age and younger.12
The Appendicitis Inflammatory Response (AIR) Score was prospectively validated in 545 patients representing all age groups.10 Subsequently, in a larger prospective multicenter study of 3878 patients older than 5 years, the original cut points were altered, thereby improving test sensitivity and negative predictive value to 99% for those with low probability (0 to 3), and test specificity to 98% for those with high-probability (9 to 12).13 Compared with the Alvarado Score, the AIR Score has higher specificity for those in the high-probability range, and similar exclusion rates in the low-probability range.14
Caveats with clinical decision scores. These tools are accepted and often used. However, challenges that affect generalizability of study data include differences in patient selection for each study (undifferentiated abdominal pain vs appendicitis), prospective vs retrospective designs, and age and gender variations in the patient populations. Despite the numerous scoring systems developed, none can accurately be used to rule in appendicitis. They are best used to assist in ruling out appendicitis and to aid in deciding for or against imaging.
Continue to: A look at the imaging options
A look at the imaging options
Abdominal CT has sensitivity and specificity rates between 76% and 100% and 83% and 100%, respectively.15,20,21 Ultrasonography has sensitivity and specificity rates of 71% to 94% and 81% to 98%, respectively.15,20,21 Formal US is reliable to confirm appendicitis, but less so to rule out appendicitis. Special considerations for imagining in pregnant patients and children are discussed in a bit.
Timing of surgical consultation
Surgical consultation is paramount once the diagnosis of appendicitis is probable. Imaging is best obtained prior to surgical consultation to streamline evaluation and enhance decision- making. Typically, patients will be categorized as complicated or uncomplicated based on the presence or absence of perforation, a gangrenous appendix, an intra-abdominal abscess (IAA), or purulent peritonitis. Active continuous surgical involvement (co-management or assumption of care) is recommended in all cases of appendicitis, especially if nonoperative management is selected, given that some cases must convert to immediate operative treatment or may be selected for delayed future (interval) appendectomy.22
Management
Uncomplicated appendicitis
Prompt appendectomy has been the gold standard of care for uncomplicated acute appendicitis for 60 years. However, several studies have investigated an antibiotic-based strategy rather than surgical treatment for uncomplicated appendicitis.
Antibiotics vs appendectomy. In 2020, the CODA Collaborative published a randomized trial comparing a 10-day course of antibiotics with appendectomy in patients with uncomplicated appendicitis. In this multicenter study based in the United States, 1552 patients 18 years of age or older were randomized to receive antibiotics or undergo appendectomy (95% performed laparoscopically). The antibiotic treatment consisted of at least 24 hours of IV antibiotics, with or without admission to the hospital. Antibiotic choice was individualized according to guidelines for intra-abdominal infection published by the Infectious Diseases Society of America, with the most common IV medications being ertapenem, cefoxitin, or metronidazole plus one of the following: ceftriaxone, cefazolin, or levofloxacin. For the remaining 10 days, oral metronidazole plus ciprofloxacin or cefdinir were used.22
Continue to: The primary endpoint...
The primary endpoint was the European Quality of Life-5 Dimensions (EQ-5D) questionnaire, with secondary outcomes including appendectomy in the antibiotics group and complications through 90 days. Exclusion criteria included pregnancy, sepsis, peritonitis, recurrent appendicitis, severe phlegmon on imaging, or evidence of neoplasm.22
Antibiotics were noninferior to appendectomy for the 30-day study. However, antibiotics failed in 29%, who then proceeded to appendectomy by 90 days; these patients also accounted for 41% of those with an appendicolith. Overall complications were more common in the antibiotics group than in the appendectomy group (8.1 vs 3.5 per 100 participants; 95% CI, 1.3-3.98). Also more common in the antibiotic group were serious adverse events (4 vs 3 per 100 participants; hazard ratio [HR] = 1.29; 95% CI, 0.67-2.50). The presence of an appendicolith in the antibiotics group increased the conversion risk to appendectomy, as well as adverse events risk.22
The takeaway. Antibiotic treatment is a noninferior method to treat acute uncomplicated appendicitis. However, the informed consent process is important, given the ~30% failure rate. Patient factors such as continued access to care should help inform the decision.
Two main surgical approaches exist for appendectomy: open and minimally invasive. At this time, the minimally invasive options include laparoscopic, single incision laparoscopic surgery (SILS), and robotic appendectomy. A study comparing cost, availability, or complications of these options has not been conducted at this time.
A large Cochrane review of 67 studies examining open vs laparoscopic appendectomy in adults and children completed in 2018 revealed that the laparoscopic approach reduced early postoperative pain intensity and led to a shorter hospital stay, earlier return to work or usual activities, and a decrease in wound infections.23 The odds of IAA occurring with laparoscopic appendectomy increased by 65% compared with an open procedure; however, postoperative bowel obstruction and incisional hernias were less likely to occur.23 Additionally, following laparoscopic surgery, postoperative bowel obstruction and incisional hernias are less likely to occur. The laparoscopic approach is preferred due to overall increased patient satisfaction and a reduction in most, if not all, complications.
Continue to: Complicated appendicitis
Complicated appendicitis
Excluding patients with severe sepsis or purulent peritonitis requiring resuscitation and immediate surgical intervention of intra-abdominal infection, the approach to patients with complicated appendicitis varies between aggressive surgical intervention and nonoperative management.
In a 2007 meta-analysis reviewing nonsurgical treatment of appendiceal abscess/phlegmon, immediate surgery was associated with higher morbidity.24 Within the nonoperative management group 7.2% (CI, 4.0-10.5) required surgical intervention and 19.7% (CI, 11.0-28.3) required abscess drainage. Malignant disease was detected in 1.2% (CI, 0.6-1.7).24 Small subsequent studies concluded different results.25
Ultimately, the 2015 European Association of Endoscopic Surgery guidelines recommend a new systematic review; but with current data, initial nonoperative management is preferred.15 After initial nonoperative treatment, the only benefits from interval appendectomy are identification of an underlying malignancy (6% to 20%) and mitigating the risk of recurrent appendicitis (5% to 44%).15,25-30
Multiple single institutional series found increased neoplasm incidence (9% to 20%) in complicated appendicitis in patients 40 years and older.26-30 Prior to interval appendectomy in patients 45 years and older, ensuring they have an up-to-date screening colonoscopy is important. This is in line with 2021 US Preventive Services Task Force (Grade “B” recommendation), 2018 American Cancer Society (qualified recommendation), and 2021 American College of Gastroenterology (conditional recommendation) guidelines for colorectal cancer screening to start at age 45 in average-risk patients.31 Patients younger than 45 can consider screening through shared decision-making.
Special populations
Pregnant patients
In pregnancy, challenges exist with the presence of traditional signs and symptoms of appendicitis, with the most predictive sign being a WBC count higher than 18,000.32 The American College of Radiology’s (ACR) Appropriateness Criteria recommend US as the imaging modality of choice in pregnancy, with MRI as the best option when US is inconclusive.33 Two meta-analyses demonstrated high sensitivity (91.8%-96.6%) and specificity (95.9%-97.9%) of MRI in diagnosing appendicitis.34,35 CT scan is not the preferred initial imagining modality in pregnancy unless urgent information is needed and other modalities are insufficient or unavailable.36
Continue to: The most common...
The most common nonobstetric surgical intervention during pregnancy is appendectomy, at a rate of 6.3/10,000 person-years, which increases to 9.9/10,000 in the postpartum period.37 Two large population studies demonstrate the rate of appendicitis varies over the course of pregnancy, with the lowest rates in the third trimester,38,39 and a significant rebound lasting for 2 years postpartum.39 Peritonitis, septic shock, pneumonia, postoperative infection, and longer hospital stays occur more frequently in pregnant women than in nonpregnant women with appendicitis.40 Fetal loss is higher in the first trimester.32
In a 14-year review of 63,145 appendicitis cases, an increased risk of fetal loss and maternal death was noted across ages and ethnicities, with the largest risk of maternal death occurring in Hispanics and fetal death in non-Hispanic Blacks.41 In a large study of 1018 adverse events after appendectomy or cholecystectomy, the 3 most common events were preterm delivery (35.4%), preterm labor without preterm delivery (26.4%), and miscarriage (25.7%).42 The surgery itself was not a major risk factor for adverse events. Major risk factors included cervical incompetence (odds ratio [OR] = 24.3), preterm labor in current pregnancy (OR = 18.3), and presence of vulvovaginitis (OR = 5.2).42
Nonoperative management in pregnancy is not recommended; only 1 prospective trial has been done, with 20 patients, showing a 25% failure rate.43 Two meta-analyses published in 2019 highlight the potential increase of fetal loss with laparoscopic approaches to appendectomy.44,45 However, recently published literature demonstrates no significant maternal-fetal morbidity. Current guidelines of the Society of American Gastrointestinal and Endoscopic Surgeons agree that laparoscopy is the operative choice in pregnancy.36
Children
Acute appendicitis is the most common surgical emergency in children.4 Physical exam findings and laboratory results are not classic in this population, obtaining an accurate history can be challenging, and results of clinical scoring systems can be inconclusive.4 Additional serum biomarkers, procalcitonin and calprotectin, are gaining evidence for use in improving scoring systems to refine low-risk groups. Unavailability of timely, reliable biomarker testing in rural practice locations limits definitive recommendations at this time.46 ACR recommends no imaging in a pediatric patient whose risk of having appendicitis is low based on any of several scoring systems.47 For those assessed as having higher risk, US is the recommended initial modality,with CT with IV contrast or MRI without contrast equally recommended if the US is equivocal.47
Despite promising data from trials of nonoperative treatment for adults with appendicitis, no definitive evidence and recommendations are available for children. Two systematic reviews show nonoperative treatment is safe, with an efficacy rate of 76% to 82% at long-term follow-up,48,49 although the success of antibiotic regimens varies. Within the nonoperative treatment group, 16% of patients had appendectomy during the follow-up period, which varied from 8 weeks to 4 years.48 A randomized controlled trial is needed for final guidance.
Continue to: CASE
CASE
The patient had an Alvarado score of 9 (high probability) and an AIR score of 6 (intermediate probability). A CT with IV contrast showed a 9-mm fluid-filled appendix with periappendiceal fluid. During surgical consultation, he was offered laparoscopic appendectomy or nonoperative treatment with antibiotics. He opted for a preoperative dose of piperacillin-tazobactam 3.375 g IV and laparoscopic appendectomy. The patient was discharged home 6 hours after his procedure.
CORRESPONDENCE
Jessica Servey, MD, MHPE, 4301 Jones Bridge Road, Bethesda, MD 20814; [email protected]
1. Prystowsky JB, Pugh CM, Nagle AP. Current problems in surgery. Appendicitis. Curr Probl Surg. 2005;42:688-742.
2. Song DW, Park BK, Suh SW, et al. Bacterial culture and antibiotic susceptibility in patients with acute appendicitis. Int J Colorectal Dis. 2018;33:441-447.
3. Wagner JM, McKinney WP, Carpenter JL. Does this patient have appendicitis? JAMA. 1996;276:1589-1594.
4. Benabbas R, Hanna M, Shah J, et al. Diagnostic accuracy of history, physical examination, laboratory tests, and point-of-care ultrasound for pediatric acute appendicitis in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2017;24:523-551.
5. Andersson RE. Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg. 2004;91:28-37.
6. Alvarado A. A practical score for the early diagnosis of acute appendicitis. Ann Emerg Med. 1986;15:557-564.
7. Ebell MH, Shinholser J. What are the most clinically useful cutoffs for the Alvarado and Pediatric Appendicitis Scores? A systematic review. Ann Emerg Med. 2014;64:365-372.e2.
8. Ohle R, O’Reilly F, O’Brien KK, et al. The Alvarado score for predicting acute appendicitis: a systematic review. BMC Med. 2011;9:139.
9. Samuel M. Pediatric appendicitis score. J Pediatr Surg. 2002;37:877-881.
10. Andersson M, Andersson RE. The appendicitis inflammatory response score: a tool for the diagnosis of acute appendicitis that outperforms the Alvarado score. World J Surg. 2008;32:1843-1849.
11. Pogorelić Z, Rak S, Mrklić I, et al. Prospective validation of Alvarado score and Pediatric Appendicitis Score for the diagnosis of acute appendicitis in children. Pediatr Emerg Care. 2015;31:164-168.
12. Rassi R, Muse F, Sánchez-Martínez J, et al. Diagnostic value of clinical prediction scores for acute appendicitis in children younger than 4 years. Eur J Pediatr Surg. 2021. [Online ahead of print]
13. Andersson M, Kolodziej B, Andersson RE. Validation of the Appendicitis Inflammatory Response (AIR) score. World J Surg. 2021;45:2081-2091.
14. Kollár D, McCartan DP, Bourke M, et al. Predicting acute appendicitis? A comparison of the Alvarado score, the Appendicitis Inflammatory Response Score and clinical assessment. World J Surg. 2015;39:104-109.
15. Gorter RR, Eker HH, Gorter-Stam MA, et al. Diagnosis and management of acute appendicitis. EAES consensus development conference 2015. Surg Endosc. 2016;30:4668-4690.
16. Matthew Fields J, Davis J, Alsup C, et al. Accuracy of point-of-care ultrasonography for diagnosing acute appendicitis: a systematic review and meta-analysis. Acad Emerg Med. 2017;24:1124-1136.
17. Sharif S, Skitch S, Vlahaki D, et al. Point-of-care ultrasound to diagnose appendicitis in a Canadian emergency department. CJEM. 2018;20:732-735.
18. Doniger SJ, Kornblith A. Point-of-care ultrasound integrated into a staged diagnostic algorithm for pediatric appendicitis. Pediatr Emerg Care. 2018;34:109-115.
19. Menon N, Kumar S, Keeler B, et al. A systematic review of point-of-care abdominal ultrasound scans performed by general surgeons. Surgeon. 2021. [Online ahead of print]
20. Doria AS, Moineddin R, Kellenberger CJ, et al. US or CT for diagnosis of appendicitis in children and adults? A meta-analysis. Radiology. 2006;241:83-94.
21. van Randen A, Laméris W, van Es HW, et al. A comparison of the accuracy of ultrasound and computed tomography in common diagnoses causing acute abdominal pain. Eur Radiol. 2011;21:1535-1545.
22. Flum DR, Davidson GH, Monsell SE, et al. A randomized trial comparing antibiotics with appendectomy for appendicitis. N Engl J Med. 2020;383:1907-1919.
23. Jaschinski T, Mosch CG, Eikermann M, et al. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev. 2018;11:CD001546.
24. Andersson RE, Petzold MG. Nonsurgical treatment of appendiceal abscess or phlegmon: a systematic review and meta-analysis. Ann Surg. 2007;246:741-748.
25. Deelder JD, Richir MC, Schoorl T, et al. How to treat an appendiceal inflammatory mass: operatively or nonoperatively? J Gastrointest Surg. 2014;18:641-645.
26. Carpenter SG, Chapital AB, Merritt MV, et al. Increased risk of neoplasm in appendicitis treated with interval appendectomy: single-institution experience and literature review. Am Surg. 2012;78:339-343.
27. Hayes D, Reiter S, Hagen E, et al. Is interval appendectomy really needed? A closer look at neoplasm rates in adult patients undergoing interval appendectomy after complicated appendicitis. Surg Endosc. 2021;35:3855-3860.
28. Peltrini R, Cantoni V, Green R, et al. Risk of appendiceal neoplasm after interval appendectomy for complicated appendicitis: a systematic review and meta-analysis. Surgeon. 2021. [Online ahead of print.]
29. Mällinen J, Rautio T, Grönroos J, et al. Risk of appendiceal neoplasm in periappendicular abscess in patients treated with interval appendectomy vs follow-up with magnetic resonance imaging: 1-year outcomes of the peri-appendicitis acuta randomized clinical trial. JAMA Surg. 2019;154:200-207.
30. Son J, Park YJ, Lee SR, et al. Increased risk of neoplasms in adult patients undergoing interval appendectomy. Ann Coloproctol. 2020;36:311-315.
31. Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977.
32. Theilen LH, Mellnick VM, Shanks AL, et al. Acute appendicitis in pregnancy: predictive clinical factors and pregnancy outcomes. Am J Perinatol. 2017;34:523-528.
33. Garcia EM, Camacho MA, Karolyi DR, et al. ACR Appropriateness Criteria right lower quadrant pain-suspected appendicitis. J Am Coll Radiol. 2018;15:S373-s387.
34. Kave M, Parooie F, Salarzaei M. Pregnancy and appendicitis: a systematic review and meta-analysis on the clinical use of MRI in diagnosis of appendicitis in pregnant women. World J Emerg Surg. 2019;14:37.
35. Repplinger MD, Levy JF, Peethumnongsin E, et al. Systematic review and meta-analysis of the accuracy of MRI to diagnose appendicitis in the general population. J Magn Reson Imaging. 2016;43:1346-1354.
36. Pearl JP, Price RR, Tonkin AE, et al. SAGES guidelines for the use of laparoscopy during pregnancy. Surg Endosc. 2017;31:3767-3782.
37. Zingone F, Sultan AA, Humes DJ, et al. Risk of acute appendicitis in and around pregnancy: a population-based cohort study from England. Ann Surg. 2015;261:332-337.
38. Andersson RE, Lambe M. Incidence of appendicitis during pregnancy. Int J Epidemiol. 2001;30:1281-1285.
39. Moltubak E, Landerholm K, Blomberg M, et al. Major variation in the incidence of appendicitis before, during and after pregnancy: a population-based cohort study. World J Surg. 2020;44:2601-2608.
40. Abbasi N, Patenaude V, Abenhaim HA. Management and outcomes of acute appendicitis in pregnancy-population-based study of over 7000 cases. BJOG. 2014;121:1509-1514.
41. Dongarwar D, Taylor J, Ajewole V, et al. Trends in appendicitis among pregnant women, the risk for cardiac arrest, and maternal-fetal mortality. World J Surg. 2020;44:3999-4005.
42. Sachs A, Guglielminotti J, Miller R, et al. Risk factors and risk stratification for adverse obstetrical outcomes after appendectomy or cholecystectomy during pregnancy. JAMA Surg. 2017;152:436-441.
43. Joo JI, Park HC, Kim MJ, et al. Outcomes of antibiotic therapy for uncomplicated appendicitis in pregnancy. Am J Med. 2017;130:1467-1469.
44. Lee SH, Lee JY, Choi YY, Lee JG. Laparoscopic appendectomy versus open appendectomy for suspected appendicitis during pregnancy: a systematic review and updated meta-analysis. BMC Surg. 2019;19:41.
45. Frountzas M, Nikolaou C, Stergios K, et al. Is the laparoscopic approach a safe choice for the management of acute appendicitis in pregnant women? A meta-analysis of observational studies. Ann R Coll Surg Engl. 2019;101:235-248.
46. Di Saverio S, Podda M, De Simone B, et al. Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J Emerg Surg. 2020;15:27.
47. Koberlein GC, Trout AT, Rigsby CK, et al. ACR Appropriateness Criteria suspected appendicitis-child. J Am Coll Radiol. 2019;16:S252-S263.
48. Maita S, Andersson B, Svensson JF, et al. Nonoperative treatment for nonperforated appendicitis in children: a systematic review and meta-analysis. Pediatr Surg Int. 2020;36:261-269.
49. Georgiou R, Eaton S, Stanton MP, et al. Efficacy and safety of nonoperative treatment for acute appendicitis: a meta-analysis. Pediatrics. 2017;139:e20163003.
CASE
A 35-year-old man with a body mass index of 20 presented to the emergency department after 24 hours of abdominal pain that began in the periumbilical region and then migrated to the right lower quadrant. The pain was exacerbated during ambulation and was intense when the car transporting him to the hospital encountered bumps in the road. After his pain started, he had associated anorexia, followed by nausea and emesis. He reported fever and chills. On examination, his temperature was 100.8 °F (38.2 °C), and palpation of the right and left lower quadrants elicited right lower quadrant pain. Laboratory evaluation revealed a white blood cell (WBC) count of 14,000 cells/mcL with 85% neutrophils, C-reactive protein of 40 mg/L, and a negative urinalysis.
How would you proceed with this patient?
Acute appendicitis is the most common cause of abdominal pain resulting in the need for surgical treatment; lifetime risk of appendicitis is 6% to 7%.1 Appendicitis is caused by intraluminal obstruction in the appendix from enlarged lymphoid tissue or a fecalith. The obstruction leads to elevated intraluminal pressure due to persistent mucus and gas production by bacteria, ultimately leading to ischemia and perforation.1 Additionally, obstruction leads to bacterial overgrowth, most commonly colonic flora such as Escherichia coli, Bacteroides fragilis, Streptococcus viridans, Enterococcus sp., Pseudomonas aeruginosa, and Klebsiella pneumoniaei.1,2
The following review provides a look at how 3 clinical scoring systems compare in the identification of acute appendicitis and details which imaging studies you should order—and when. But first, we’ll quickly detail the relevant physical findings and lab values that point to a diagnosis of acute appendicitis.
Physical findings. The patient typically first experiences vague abdominal pain that then localizes to the right lower quadrant due to peritoneal inflammation. Anorexia and nausea typically follow the abdominal pain. On examination, the patient often appears ill and exhibits abdominal guarding due to peritonitis. Tachycardia and fever are common; however, the absence of either does not exclude appendicitis. Classically, on palpation, the patient will have pain at McBurney’s point (one-third the distance from the anterior iliac spine to the umbilicus). The exact point of maximal tenderness can differ because of the varying anatomy of the appendix (retrocecal, paracolic, pelvic, pre/post ileal, promontoric, or subcecal).1 Right lower quadrant pain, abdominal rigidity, and radiation of periumbilical pain to the right lower quadrant are the most accurate findings in adults to rule in appendicitis.3 For children, physical exam findings have the highest likelihood in predicting appendicitis and include a positive Obturator sign, positive Rovsing sign, or a positive Psoas sign, and absent or decreased bowel sounds.4
Laboratory studies can support a diagnosis of appendicitis but cannot exclude it. Leukocytosis with neutrophil predominance is present in 90% of cases.5 An elevated C-reactive protein level renders the highest diagnostic accuracy.5 Perform a pregnancy test for any woman of child-bearing age, to assist in the diagnosis and guide imaging choices for evaluation. Additional laboratory tests are not needed unless there are concerns about volume depletion.
Clinical scoring systems
Several clinical scoring systems (TABLE6-10) have been validated to aid clinicians in evaluating patients with possible appendicitis, to decrease unnecessary exposure to ionizing radiation from computed tomography (CT) scans, to identify and reassure patients with low likelihoods of appendicitis, and to conduct outpatient follow-up.
Continue to: The Alvarado score
The Alvarado score is the oldest scoring rule, developed in 1986; it entails 8 clinical and laboratory variables.6 Ebell et al altered the proposed cutoff values of the Alvarado score to be low risk (< 4), intermediate risk (4-8), and high risk (≥ 9), effectively improving the sensitivity and specificity rates.7
In a meta-analysis of the Alvarado score that included 42 studies of men, women, and children, the sensitivity for “ruling out” appendicitis with a cutoff of 5 points was 96% for men, 99% for women, and 99% for children.8 The accuracy of a high-risk score (> 7) for “ruling in” appendicitis was less with an overall specificity of 82%.8 The Alvarado score did seem to overestimate appendicitis in women in all score categories.8
The Pediatric Appendicitis Score (PAS) is similar to Alvarado and was prospectively validated in 1170 children in 2002 for more specific guidance in this age group.9 The PAS had excellent specificity in the study; those with a score of ≥ 6 had a high probability of appendicitis. In a study comparing Alvarado with PAS in 311 patients, insignificant differences were noted at a score of ≥ 7 for both tests (sensitivity 86% vs 89%, and specificity 59% vs 50%, respectively).11 No scoring system has been found to be sufficiently accurate for use in children 4 years of age and younger.12
The Appendicitis Inflammatory Response (AIR) Score was prospectively validated in 545 patients representing all age groups.10 Subsequently, in a larger prospective multicenter study of 3878 patients older than 5 years, the original cut points were altered, thereby improving test sensitivity and negative predictive value to 99% for those with low probability (0 to 3), and test specificity to 98% for those with high-probability (9 to 12).13 Compared with the Alvarado Score, the AIR Score has higher specificity for those in the high-probability range, and similar exclusion rates in the low-probability range.14
Caveats with clinical decision scores. These tools are accepted and often used. However, challenges that affect generalizability of study data include differences in patient selection for each study (undifferentiated abdominal pain vs appendicitis), prospective vs retrospective designs, and age and gender variations in the patient populations. Despite the numerous scoring systems developed, none can accurately be used to rule in appendicitis. They are best used to assist in ruling out appendicitis and to aid in deciding for or against imaging.
Continue to: A look at the imaging options
A look at the imaging options
Abdominal CT has sensitivity and specificity rates between 76% and 100% and 83% and 100%, respectively.15,20,21 Ultrasonography has sensitivity and specificity rates of 71% to 94% and 81% to 98%, respectively.15,20,21 Formal US is reliable to confirm appendicitis, but less so to rule out appendicitis. Special considerations for imagining in pregnant patients and children are discussed in a bit.
Timing of surgical consultation
Surgical consultation is paramount once the diagnosis of appendicitis is probable. Imaging is best obtained prior to surgical consultation to streamline evaluation and enhance decision- making. Typically, patients will be categorized as complicated or uncomplicated based on the presence or absence of perforation, a gangrenous appendix, an intra-abdominal abscess (IAA), or purulent peritonitis. Active continuous surgical involvement (co-management or assumption of care) is recommended in all cases of appendicitis, especially if nonoperative management is selected, given that some cases must convert to immediate operative treatment or may be selected for delayed future (interval) appendectomy.22
Management
Uncomplicated appendicitis
Prompt appendectomy has been the gold standard of care for uncomplicated acute appendicitis for 60 years. However, several studies have investigated an antibiotic-based strategy rather than surgical treatment for uncomplicated appendicitis.
Antibiotics vs appendectomy. In 2020, the CODA Collaborative published a randomized trial comparing a 10-day course of antibiotics with appendectomy in patients with uncomplicated appendicitis. In this multicenter study based in the United States, 1552 patients 18 years of age or older were randomized to receive antibiotics or undergo appendectomy (95% performed laparoscopically). The antibiotic treatment consisted of at least 24 hours of IV antibiotics, with or without admission to the hospital. Antibiotic choice was individualized according to guidelines for intra-abdominal infection published by the Infectious Diseases Society of America, with the most common IV medications being ertapenem, cefoxitin, or metronidazole plus one of the following: ceftriaxone, cefazolin, or levofloxacin. For the remaining 10 days, oral metronidazole plus ciprofloxacin or cefdinir were used.22
Continue to: The primary endpoint...
The primary endpoint was the European Quality of Life-5 Dimensions (EQ-5D) questionnaire, with secondary outcomes including appendectomy in the antibiotics group and complications through 90 days. Exclusion criteria included pregnancy, sepsis, peritonitis, recurrent appendicitis, severe phlegmon on imaging, or evidence of neoplasm.22
Antibiotics were noninferior to appendectomy for the 30-day study. However, antibiotics failed in 29%, who then proceeded to appendectomy by 90 days; these patients also accounted for 41% of those with an appendicolith. Overall complications were more common in the antibiotics group than in the appendectomy group (8.1 vs 3.5 per 100 participants; 95% CI, 1.3-3.98). Also more common in the antibiotic group were serious adverse events (4 vs 3 per 100 participants; hazard ratio [HR] = 1.29; 95% CI, 0.67-2.50). The presence of an appendicolith in the antibiotics group increased the conversion risk to appendectomy, as well as adverse events risk.22
The takeaway. Antibiotic treatment is a noninferior method to treat acute uncomplicated appendicitis. However, the informed consent process is important, given the ~30% failure rate. Patient factors such as continued access to care should help inform the decision.
Two main surgical approaches exist for appendectomy: open and minimally invasive. At this time, the minimally invasive options include laparoscopic, single incision laparoscopic surgery (SILS), and robotic appendectomy. A study comparing cost, availability, or complications of these options has not been conducted at this time.
A large Cochrane review of 67 studies examining open vs laparoscopic appendectomy in adults and children completed in 2018 revealed that the laparoscopic approach reduced early postoperative pain intensity and led to a shorter hospital stay, earlier return to work or usual activities, and a decrease in wound infections.23 The odds of IAA occurring with laparoscopic appendectomy increased by 65% compared with an open procedure; however, postoperative bowel obstruction and incisional hernias were less likely to occur.23 Additionally, following laparoscopic surgery, postoperative bowel obstruction and incisional hernias are less likely to occur. The laparoscopic approach is preferred due to overall increased patient satisfaction and a reduction in most, if not all, complications.
Continue to: Complicated appendicitis
Complicated appendicitis
Excluding patients with severe sepsis or purulent peritonitis requiring resuscitation and immediate surgical intervention of intra-abdominal infection, the approach to patients with complicated appendicitis varies between aggressive surgical intervention and nonoperative management.
In a 2007 meta-analysis reviewing nonsurgical treatment of appendiceal abscess/phlegmon, immediate surgery was associated with higher morbidity.24 Within the nonoperative management group 7.2% (CI, 4.0-10.5) required surgical intervention and 19.7% (CI, 11.0-28.3) required abscess drainage. Malignant disease was detected in 1.2% (CI, 0.6-1.7).24 Small subsequent studies concluded different results.25
Ultimately, the 2015 European Association of Endoscopic Surgery guidelines recommend a new systematic review; but with current data, initial nonoperative management is preferred.15 After initial nonoperative treatment, the only benefits from interval appendectomy are identification of an underlying malignancy (6% to 20%) and mitigating the risk of recurrent appendicitis (5% to 44%).15,25-30
Multiple single institutional series found increased neoplasm incidence (9% to 20%) in complicated appendicitis in patients 40 years and older.26-30 Prior to interval appendectomy in patients 45 years and older, ensuring they have an up-to-date screening colonoscopy is important. This is in line with 2021 US Preventive Services Task Force (Grade “B” recommendation), 2018 American Cancer Society (qualified recommendation), and 2021 American College of Gastroenterology (conditional recommendation) guidelines for colorectal cancer screening to start at age 45 in average-risk patients.31 Patients younger than 45 can consider screening through shared decision-making.
Special populations
Pregnant patients
In pregnancy, challenges exist with the presence of traditional signs and symptoms of appendicitis, with the most predictive sign being a WBC count higher than 18,000.32 The American College of Radiology’s (ACR) Appropriateness Criteria recommend US as the imaging modality of choice in pregnancy, with MRI as the best option when US is inconclusive.33 Two meta-analyses demonstrated high sensitivity (91.8%-96.6%) and specificity (95.9%-97.9%) of MRI in diagnosing appendicitis.34,35 CT scan is not the preferred initial imagining modality in pregnancy unless urgent information is needed and other modalities are insufficient or unavailable.36
Continue to: The most common...
The most common nonobstetric surgical intervention during pregnancy is appendectomy, at a rate of 6.3/10,000 person-years, which increases to 9.9/10,000 in the postpartum period.37 Two large population studies demonstrate the rate of appendicitis varies over the course of pregnancy, with the lowest rates in the third trimester,38,39 and a significant rebound lasting for 2 years postpartum.39 Peritonitis, septic shock, pneumonia, postoperative infection, and longer hospital stays occur more frequently in pregnant women than in nonpregnant women with appendicitis.40 Fetal loss is higher in the first trimester.32
In a 14-year review of 63,145 appendicitis cases, an increased risk of fetal loss and maternal death was noted across ages and ethnicities, with the largest risk of maternal death occurring in Hispanics and fetal death in non-Hispanic Blacks.41 In a large study of 1018 adverse events after appendectomy or cholecystectomy, the 3 most common events were preterm delivery (35.4%), preterm labor without preterm delivery (26.4%), and miscarriage (25.7%).42 The surgery itself was not a major risk factor for adverse events. Major risk factors included cervical incompetence (odds ratio [OR] = 24.3), preterm labor in current pregnancy (OR = 18.3), and presence of vulvovaginitis (OR = 5.2).42
Nonoperative management in pregnancy is not recommended; only 1 prospective trial has been done, with 20 patients, showing a 25% failure rate.43 Two meta-analyses published in 2019 highlight the potential increase of fetal loss with laparoscopic approaches to appendectomy.44,45 However, recently published literature demonstrates no significant maternal-fetal morbidity. Current guidelines of the Society of American Gastrointestinal and Endoscopic Surgeons agree that laparoscopy is the operative choice in pregnancy.36
Children
Acute appendicitis is the most common surgical emergency in children.4 Physical exam findings and laboratory results are not classic in this population, obtaining an accurate history can be challenging, and results of clinical scoring systems can be inconclusive.4 Additional serum biomarkers, procalcitonin and calprotectin, are gaining evidence for use in improving scoring systems to refine low-risk groups. Unavailability of timely, reliable biomarker testing in rural practice locations limits definitive recommendations at this time.46 ACR recommends no imaging in a pediatric patient whose risk of having appendicitis is low based on any of several scoring systems.47 For those assessed as having higher risk, US is the recommended initial modality,with CT with IV contrast or MRI without contrast equally recommended if the US is equivocal.47
Despite promising data from trials of nonoperative treatment for adults with appendicitis, no definitive evidence and recommendations are available for children. Two systematic reviews show nonoperative treatment is safe, with an efficacy rate of 76% to 82% at long-term follow-up,48,49 although the success of antibiotic regimens varies. Within the nonoperative treatment group, 16% of patients had appendectomy during the follow-up period, which varied from 8 weeks to 4 years.48 A randomized controlled trial is needed for final guidance.
Continue to: CASE
CASE
The patient had an Alvarado score of 9 (high probability) and an AIR score of 6 (intermediate probability). A CT with IV contrast showed a 9-mm fluid-filled appendix with periappendiceal fluid. During surgical consultation, he was offered laparoscopic appendectomy or nonoperative treatment with antibiotics. He opted for a preoperative dose of piperacillin-tazobactam 3.375 g IV and laparoscopic appendectomy. The patient was discharged home 6 hours after his procedure.
CORRESPONDENCE
Jessica Servey, MD, MHPE, 4301 Jones Bridge Road, Bethesda, MD 20814; [email protected]
CASE
A 35-year-old man with a body mass index of 20 presented to the emergency department after 24 hours of abdominal pain that began in the periumbilical region and then migrated to the right lower quadrant. The pain was exacerbated during ambulation and was intense when the car transporting him to the hospital encountered bumps in the road. After his pain started, he had associated anorexia, followed by nausea and emesis. He reported fever and chills. On examination, his temperature was 100.8 °F (38.2 °C), and palpation of the right and left lower quadrants elicited right lower quadrant pain. Laboratory evaluation revealed a white blood cell (WBC) count of 14,000 cells/mcL with 85% neutrophils, C-reactive protein of 40 mg/L, and a negative urinalysis.
How would you proceed with this patient?
Acute appendicitis is the most common cause of abdominal pain resulting in the need for surgical treatment; lifetime risk of appendicitis is 6% to 7%.1 Appendicitis is caused by intraluminal obstruction in the appendix from enlarged lymphoid tissue or a fecalith. The obstruction leads to elevated intraluminal pressure due to persistent mucus and gas production by bacteria, ultimately leading to ischemia and perforation.1 Additionally, obstruction leads to bacterial overgrowth, most commonly colonic flora such as Escherichia coli, Bacteroides fragilis, Streptococcus viridans, Enterococcus sp., Pseudomonas aeruginosa, and Klebsiella pneumoniaei.1,2
The following review provides a look at how 3 clinical scoring systems compare in the identification of acute appendicitis and details which imaging studies you should order—and when. But first, we’ll quickly detail the relevant physical findings and lab values that point to a diagnosis of acute appendicitis.
Physical findings. The patient typically first experiences vague abdominal pain that then localizes to the right lower quadrant due to peritoneal inflammation. Anorexia and nausea typically follow the abdominal pain. On examination, the patient often appears ill and exhibits abdominal guarding due to peritonitis. Tachycardia and fever are common; however, the absence of either does not exclude appendicitis. Classically, on palpation, the patient will have pain at McBurney’s point (one-third the distance from the anterior iliac spine to the umbilicus). The exact point of maximal tenderness can differ because of the varying anatomy of the appendix (retrocecal, paracolic, pelvic, pre/post ileal, promontoric, or subcecal).1 Right lower quadrant pain, abdominal rigidity, and radiation of periumbilical pain to the right lower quadrant are the most accurate findings in adults to rule in appendicitis.3 For children, physical exam findings have the highest likelihood in predicting appendicitis and include a positive Obturator sign, positive Rovsing sign, or a positive Psoas sign, and absent or decreased bowel sounds.4
Laboratory studies can support a diagnosis of appendicitis but cannot exclude it. Leukocytosis with neutrophil predominance is present in 90% of cases.5 An elevated C-reactive protein level renders the highest diagnostic accuracy.5 Perform a pregnancy test for any woman of child-bearing age, to assist in the diagnosis and guide imaging choices for evaluation. Additional laboratory tests are not needed unless there are concerns about volume depletion.
Clinical scoring systems
Several clinical scoring systems (TABLE6-10) have been validated to aid clinicians in evaluating patients with possible appendicitis, to decrease unnecessary exposure to ionizing radiation from computed tomography (CT) scans, to identify and reassure patients with low likelihoods of appendicitis, and to conduct outpatient follow-up.
Continue to: The Alvarado score
The Alvarado score is the oldest scoring rule, developed in 1986; it entails 8 clinical and laboratory variables.6 Ebell et al altered the proposed cutoff values of the Alvarado score to be low risk (< 4), intermediate risk (4-8), and high risk (≥ 9), effectively improving the sensitivity and specificity rates.7
In a meta-analysis of the Alvarado score that included 42 studies of men, women, and children, the sensitivity for “ruling out” appendicitis with a cutoff of 5 points was 96% for men, 99% for women, and 99% for children.8 The accuracy of a high-risk score (> 7) for “ruling in” appendicitis was less with an overall specificity of 82%.8 The Alvarado score did seem to overestimate appendicitis in women in all score categories.8
The Pediatric Appendicitis Score (PAS) is similar to Alvarado and was prospectively validated in 1170 children in 2002 for more specific guidance in this age group.9 The PAS had excellent specificity in the study; those with a score of ≥ 6 had a high probability of appendicitis. In a study comparing Alvarado with PAS in 311 patients, insignificant differences were noted at a score of ≥ 7 for both tests (sensitivity 86% vs 89%, and specificity 59% vs 50%, respectively).11 No scoring system has been found to be sufficiently accurate for use in children 4 years of age and younger.12
The Appendicitis Inflammatory Response (AIR) Score was prospectively validated in 545 patients representing all age groups.10 Subsequently, in a larger prospective multicenter study of 3878 patients older than 5 years, the original cut points were altered, thereby improving test sensitivity and negative predictive value to 99% for those with low probability (0 to 3), and test specificity to 98% for those with high-probability (9 to 12).13 Compared with the Alvarado Score, the AIR Score has higher specificity for those in the high-probability range, and similar exclusion rates in the low-probability range.14
Caveats with clinical decision scores. These tools are accepted and often used. However, challenges that affect generalizability of study data include differences in patient selection for each study (undifferentiated abdominal pain vs appendicitis), prospective vs retrospective designs, and age and gender variations in the patient populations. Despite the numerous scoring systems developed, none can accurately be used to rule in appendicitis. They are best used to assist in ruling out appendicitis and to aid in deciding for or against imaging.
Continue to: A look at the imaging options
A look at the imaging options
Abdominal CT has sensitivity and specificity rates between 76% and 100% and 83% and 100%, respectively.15,20,21 Ultrasonography has sensitivity and specificity rates of 71% to 94% and 81% to 98%, respectively.15,20,21 Formal US is reliable to confirm appendicitis, but less so to rule out appendicitis. Special considerations for imagining in pregnant patients and children are discussed in a bit.
Timing of surgical consultation
Surgical consultation is paramount once the diagnosis of appendicitis is probable. Imaging is best obtained prior to surgical consultation to streamline evaluation and enhance decision- making. Typically, patients will be categorized as complicated or uncomplicated based on the presence or absence of perforation, a gangrenous appendix, an intra-abdominal abscess (IAA), or purulent peritonitis. Active continuous surgical involvement (co-management or assumption of care) is recommended in all cases of appendicitis, especially if nonoperative management is selected, given that some cases must convert to immediate operative treatment or may be selected for delayed future (interval) appendectomy.22
Management
Uncomplicated appendicitis
Prompt appendectomy has been the gold standard of care for uncomplicated acute appendicitis for 60 years. However, several studies have investigated an antibiotic-based strategy rather than surgical treatment for uncomplicated appendicitis.
Antibiotics vs appendectomy. In 2020, the CODA Collaborative published a randomized trial comparing a 10-day course of antibiotics with appendectomy in patients with uncomplicated appendicitis. In this multicenter study based in the United States, 1552 patients 18 years of age or older were randomized to receive antibiotics or undergo appendectomy (95% performed laparoscopically). The antibiotic treatment consisted of at least 24 hours of IV antibiotics, with or without admission to the hospital. Antibiotic choice was individualized according to guidelines for intra-abdominal infection published by the Infectious Diseases Society of America, with the most common IV medications being ertapenem, cefoxitin, or metronidazole plus one of the following: ceftriaxone, cefazolin, or levofloxacin. For the remaining 10 days, oral metronidazole plus ciprofloxacin or cefdinir were used.22
Continue to: The primary endpoint...
The primary endpoint was the European Quality of Life-5 Dimensions (EQ-5D) questionnaire, with secondary outcomes including appendectomy in the antibiotics group and complications through 90 days. Exclusion criteria included pregnancy, sepsis, peritonitis, recurrent appendicitis, severe phlegmon on imaging, or evidence of neoplasm.22
Antibiotics were noninferior to appendectomy for the 30-day study. However, antibiotics failed in 29%, who then proceeded to appendectomy by 90 days; these patients also accounted for 41% of those with an appendicolith. Overall complications were more common in the antibiotics group than in the appendectomy group (8.1 vs 3.5 per 100 participants; 95% CI, 1.3-3.98). Also more common in the antibiotic group were serious adverse events (4 vs 3 per 100 participants; hazard ratio [HR] = 1.29; 95% CI, 0.67-2.50). The presence of an appendicolith in the antibiotics group increased the conversion risk to appendectomy, as well as adverse events risk.22
The takeaway. Antibiotic treatment is a noninferior method to treat acute uncomplicated appendicitis. However, the informed consent process is important, given the ~30% failure rate. Patient factors such as continued access to care should help inform the decision.
Two main surgical approaches exist for appendectomy: open and minimally invasive. At this time, the minimally invasive options include laparoscopic, single incision laparoscopic surgery (SILS), and robotic appendectomy. A study comparing cost, availability, or complications of these options has not been conducted at this time.
A large Cochrane review of 67 studies examining open vs laparoscopic appendectomy in adults and children completed in 2018 revealed that the laparoscopic approach reduced early postoperative pain intensity and led to a shorter hospital stay, earlier return to work or usual activities, and a decrease in wound infections.23 The odds of IAA occurring with laparoscopic appendectomy increased by 65% compared with an open procedure; however, postoperative bowel obstruction and incisional hernias were less likely to occur.23 Additionally, following laparoscopic surgery, postoperative bowel obstruction and incisional hernias are less likely to occur. The laparoscopic approach is preferred due to overall increased patient satisfaction and a reduction in most, if not all, complications.
Continue to: Complicated appendicitis
Complicated appendicitis
Excluding patients with severe sepsis or purulent peritonitis requiring resuscitation and immediate surgical intervention of intra-abdominal infection, the approach to patients with complicated appendicitis varies between aggressive surgical intervention and nonoperative management.
In a 2007 meta-analysis reviewing nonsurgical treatment of appendiceal abscess/phlegmon, immediate surgery was associated with higher morbidity.24 Within the nonoperative management group 7.2% (CI, 4.0-10.5) required surgical intervention and 19.7% (CI, 11.0-28.3) required abscess drainage. Malignant disease was detected in 1.2% (CI, 0.6-1.7).24 Small subsequent studies concluded different results.25
Ultimately, the 2015 European Association of Endoscopic Surgery guidelines recommend a new systematic review; but with current data, initial nonoperative management is preferred.15 After initial nonoperative treatment, the only benefits from interval appendectomy are identification of an underlying malignancy (6% to 20%) and mitigating the risk of recurrent appendicitis (5% to 44%).15,25-30
Multiple single institutional series found increased neoplasm incidence (9% to 20%) in complicated appendicitis in patients 40 years and older.26-30 Prior to interval appendectomy in patients 45 years and older, ensuring they have an up-to-date screening colonoscopy is important. This is in line with 2021 US Preventive Services Task Force (Grade “B” recommendation), 2018 American Cancer Society (qualified recommendation), and 2021 American College of Gastroenterology (conditional recommendation) guidelines for colorectal cancer screening to start at age 45 in average-risk patients.31 Patients younger than 45 can consider screening through shared decision-making.
Special populations
Pregnant patients
In pregnancy, challenges exist with the presence of traditional signs and symptoms of appendicitis, with the most predictive sign being a WBC count higher than 18,000.32 The American College of Radiology’s (ACR) Appropriateness Criteria recommend US as the imaging modality of choice in pregnancy, with MRI as the best option when US is inconclusive.33 Two meta-analyses demonstrated high sensitivity (91.8%-96.6%) and specificity (95.9%-97.9%) of MRI in diagnosing appendicitis.34,35 CT scan is not the preferred initial imagining modality in pregnancy unless urgent information is needed and other modalities are insufficient or unavailable.36
Continue to: The most common...
The most common nonobstetric surgical intervention during pregnancy is appendectomy, at a rate of 6.3/10,000 person-years, which increases to 9.9/10,000 in the postpartum period.37 Two large population studies demonstrate the rate of appendicitis varies over the course of pregnancy, with the lowest rates in the third trimester,38,39 and a significant rebound lasting for 2 years postpartum.39 Peritonitis, septic shock, pneumonia, postoperative infection, and longer hospital stays occur more frequently in pregnant women than in nonpregnant women with appendicitis.40 Fetal loss is higher in the first trimester.32
In a 14-year review of 63,145 appendicitis cases, an increased risk of fetal loss and maternal death was noted across ages and ethnicities, with the largest risk of maternal death occurring in Hispanics and fetal death in non-Hispanic Blacks.41 In a large study of 1018 adverse events after appendectomy or cholecystectomy, the 3 most common events were preterm delivery (35.4%), preterm labor without preterm delivery (26.4%), and miscarriage (25.7%).42 The surgery itself was not a major risk factor for adverse events. Major risk factors included cervical incompetence (odds ratio [OR] = 24.3), preterm labor in current pregnancy (OR = 18.3), and presence of vulvovaginitis (OR = 5.2).42
Nonoperative management in pregnancy is not recommended; only 1 prospective trial has been done, with 20 patients, showing a 25% failure rate.43 Two meta-analyses published in 2019 highlight the potential increase of fetal loss with laparoscopic approaches to appendectomy.44,45 However, recently published literature demonstrates no significant maternal-fetal morbidity. Current guidelines of the Society of American Gastrointestinal and Endoscopic Surgeons agree that laparoscopy is the operative choice in pregnancy.36
Children
Acute appendicitis is the most common surgical emergency in children.4 Physical exam findings and laboratory results are not classic in this population, obtaining an accurate history can be challenging, and results of clinical scoring systems can be inconclusive.4 Additional serum biomarkers, procalcitonin and calprotectin, are gaining evidence for use in improving scoring systems to refine low-risk groups. Unavailability of timely, reliable biomarker testing in rural practice locations limits definitive recommendations at this time.46 ACR recommends no imaging in a pediatric patient whose risk of having appendicitis is low based on any of several scoring systems.47 For those assessed as having higher risk, US is the recommended initial modality,with CT with IV contrast or MRI without contrast equally recommended if the US is equivocal.47
Despite promising data from trials of nonoperative treatment for adults with appendicitis, no definitive evidence and recommendations are available for children. Two systematic reviews show nonoperative treatment is safe, with an efficacy rate of 76% to 82% at long-term follow-up,48,49 although the success of antibiotic regimens varies. Within the nonoperative treatment group, 16% of patients had appendectomy during the follow-up period, which varied from 8 weeks to 4 years.48 A randomized controlled trial is needed for final guidance.
Continue to: CASE
CASE
The patient had an Alvarado score of 9 (high probability) and an AIR score of 6 (intermediate probability). A CT with IV contrast showed a 9-mm fluid-filled appendix with periappendiceal fluid. During surgical consultation, he was offered laparoscopic appendectomy or nonoperative treatment with antibiotics. He opted for a preoperative dose of piperacillin-tazobactam 3.375 g IV and laparoscopic appendectomy. The patient was discharged home 6 hours after his procedure.
CORRESPONDENCE
Jessica Servey, MD, MHPE, 4301 Jones Bridge Road, Bethesda, MD 20814; [email protected]
1. Prystowsky JB, Pugh CM, Nagle AP. Current problems in surgery. Appendicitis. Curr Probl Surg. 2005;42:688-742.
2. Song DW, Park BK, Suh SW, et al. Bacterial culture and antibiotic susceptibility in patients with acute appendicitis. Int J Colorectal Dis. 2018;33:441-447.
3. Wagner JM, McKinney WP, Carpenter JL. Does this patient have appendicitis? JAMA. 1996;276:1589-1594.
4. Benabbas R, Hanna M, Shah J, et al. Diagnostic accuracy of history, physical examination, laboratory tests, and point-of-care ultrasound for pediatric acute appendicitis in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2017;24:523-551.
5. Andersson RE. Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg. 2004;91:28-37.
6. Alvarado A. A practical score for the early diagnosis of acute appendicitis. Ann Emerg Med. 1986;15:557-564.
7. Ebell MH, Shinholser J. What are the most clinically useful cutoffs for the Alvarado and Pediatric Appendicitis Scores? A systematic review. Ann Emerg Med. 2014;64:365-372.e2.
8. Ohle R, O’Reilly F, O’Brien KK, et al. The Alvarado score for predicting acute appendicitis: a systematic review. BMC Med. 2011;9:139.
9. Samuel M. Pediatric appendicitis score. J Pediatr Surg. 2002;37:877-881.
10. Andersson M, Andersson RE. The appendicitis inflammatory response score: a tool for the diagnosis of acute appendicitis that outperforms the Alvarado score. World J Surg. 2008;32:1843-1849.
11. Pogorelić Z, Rak S, Mrklić I, et al. Prospective validation of Alvarado score and Pediatric Appendicitis Score for the diagnosis of acute appendicitis in children. Pediatr Emerg Care. 2015;31:164-168.
12. Rassi R, Muse F, Sánchez-Martínez J, et al. Diagnostic value of clinical prediction scores for acute appendicitis in children younger than 4 years. Eur J Pediatr Surg. 2021. [Online ahead of print]
13. Andersson M, Kolodziej B, Andersson RE. Validation of the Appendicitis Inflammatory Response (AIR) score. World J Surg. 2021;45:2081-2091.
14. Kollár D, McCartan DP, Bourke M, et al. Predicting acute appendicitis? A comparison of the Alvarado score, the Appendicitis Inflammatory Response Score and clinical assessment. World J Surg. 2015;39:104-109.
15. Gorter RR, Eker HH, Gorter-Stam MA, et al. Diagnosis and management of acute appendicitis. EAES consensus development conference 2015. Surg Endosc. 2016;30:4668-4690.
16. Matthew Fields J, Davis J, Alsup C, et al. Accuracy of point-of-care ultrasonography for diagnosing acute appendicitis: a systematic review and meta-analysis. Acad Emerg Med. 2017;24:1124-1136.
17. Sharif S, Skitch S, Vlahaki D, et al. Point-of-care ultrasound to diagnose appendicitis in a Canadian emergency department. CJEM. 2018;20:732-735.
18. Doniger SJ, Kornblith A. Point-of-care ultrasound integrated into a staged diagnostic algorithm for pediatric appendicitis. Pediatr Emerg Care. 2018;34:109-115.
19. Menon N, Kumar S, Keeler B, et al. A systematic review of point-of-care abdominal ultrasound scans performed by general surgeons. Surgeon. 2021. [Online ahead of print]
20. Doria AS, Moineddin R, Kellenberger CJ, et al. US or CT for diagnosis of appendicitis in children and adults? A meta-analysis. Radiology. 2006;241:83-94.
21. van Randen A, Laméris W, van Es HW, et al. A comparison of the accuracy of ultrasound and computed tomography in common diagnoses causing acute abdominal pain. Eur Radiol. 2011;21:1535-1545.
22. Flum DR, Davidson GH, Monsell SE, et al. A randomized trial comparing antibiotics with appendectomy for appendicitis. N Engl J Med. 2020;383:1907-1919.
23. Jaschinski T, Mosch CG, Eikermann M, et al. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev. 2018;11:CD001546.
24. Andersson RE, Petzold MG. Nonsurgical treatment of appendiceal abscess or phlegmon: a systematic review and meta-analysis. Ann Surg. 2007;246:741-748.
25. Deelder JD, Richir MC, Schoorl T, et al. How to treat an appendiceal inflammatory mass: operatively or nonoperatively? J Gastrointest Surg. 2014;18:641-645.
26. Carpenter SG, Chapital AB, Merritt MV, et al. Increased risk of neoplasm in appendicitis treated with interval appendectomy: single-institution experience and literature review. Am Surg. 2012;78:339-343.
27. Hayes D, Reiter S, Hagen E, et al. Is interval appendectomy really needed? A closer look at neoplasm rates in adult patients undergoing interval appendectomy after complicated appendicitis. Surg Endosc. 2021;35:3855-3860.
28. Peltrini R, Cantoni V, Green R, et al. Risk of appendiceal neoplasm after interval appendectomy for complicated appendicitis: a systematic review and meta-analysis. Surgeon. 2021. [Online ahead of print.]
29. Mällinen J, Rautio T, Grönroos J, et al. Risk of appendiceal neoplasm in periappendicular abscess in patients treated with interval appendectomy vs follow-up with magnetic resonance imaging: 1-year outcomes of the peri-appendicitis acuta randomized clinical trial. JAMA Surg. 2019;154:200-207.
30. Son J, Park YJ, Lee SR, et al. Increased risk of neoplasms in adult patients undergoing interval appendectomy. Ann Coloproctol. 2020;36:311-315.
31. Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977.
32. Theilen LH, Mellnick VM, Shanks AL, et al. Acute appendicitis in pregnancy: predictive clinical factors and pregnancy outcomes. Am J Perinatol. 2017;34:523-528.
33. Garcia EM, Camacho MA, Karolyi DR, et al. ACR Appropriateness Criteria right lower quadrant pain-suspected appendicitis. J Am Coll Radiol. 2018;15:S373-s387.
34. Kave M, Parooie F, Salarzaei M. Pregnancy and appendicitis: a systematic review and meta-analysis on the clinical use of MRI in diagnosis of appendicitis in pregnant women. World J Emerg Surg. 2019;14:37.
35. Repplinger MD, Levy JF, Peethumnongsin E, et al. Systematic review and meta-analysis of the accuracy of MRI to diagnose appendicitis in the general population. J Magn Reson Imaging. 2016;43:1346-1354.
36. Pearl JP, Price RR, Tonkin AE, et al. SAGES guidelines for the use of laparoscopy during pregnancy. Surg Endosc. 2017;31:3767-3782.
37. Zingone F, Sultan AA, Humes DJ, et al. Risk of acute appendicitis in and around pregnancy: a population-based cohort study from England. Ann Surg. 2015;261:332-337.
38. Andersson RE, Lambe M. Incidence of appendicitis during pregnancy. Int J Epidemiol. 2001;30:1281-1285.
39. Moltubak E, Landerholm K, Blomberg M, et al. Major variation in the incidence of appendicitis before, during and after pregnancy: a population-based cohort study. World J Surg. 2020;44:2601-2608.
40. Abbasi N, Patenaude V, Abenhaim HA. Management and outcomes of acute appendicitis in pregnancy-population-based study of over 7000 cases. BJOG. 2014;121:1509-1514.
41. Dongarwar D, Taylor J, Ajewole V, et al. Trends in appendicitis among pregnant women, the risk for cardiac arrest, and maternal-fetal mortality. World J Surg. 2020;44:3999-4005.
42. Sachs A, Guglielminotti J, Miller R, et al. Risk factors and risk stratification for adverse obstetrical outcomes after appendectomy or cholecystectomy during pregnancy. JAMA Surg. 2017;152:436-441.
43. Joo JI, Park HC, Kim MJ, et al. Outcomes of antibiotic therapy for uncomplicated appendicitis in pregnancy. Am J Med. 2017;130:1467-1469.
44. Lee SH, Lee JY, Choi YY, Lee JG. Laparoscopic appendectomy versus open appendectomy for suspected appendicitis during pregnancy: a systematic review and updated meta-analysis. BMC Surg. 2019;19:41.
45. Frountzas M, Nikolaou C, Stergios K, et al. Is the laparoscopic approach a safe choice for the management of acute appendicitis in pregnant women? A meta-analysis of observational studies. Ann R Coll Surg Engl. 2019;101:235-248.
46. Di Saverio S, Podda M, De Simone B, et al. Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J Emerg Surg. 2020;15:27.
47. Koberlein GC, Trout AT, Rigsby CK, et al. ACR Appropriateness Criteria suspected appendicitis-child. J Am Coll Radiol. 2019;16:S252-S263.
48. Maita S, Andersson B, Svensson JF, et al. Nonoperative treatment for nonperforated appendicitis in children: a systematic review and meta-analysis. Pediatr Surg Int. 2020;36:261-269.
49. Georgiou R, Eaton S, Stanton MP, et al. Efficacy and safety of nonoperative treatment for acute appendicitis: a meta-analysis. Pediatrics. 2017;139:e20163003.
1. Prystowsky JB, Pugh CM, Nagle AP. Current problems in surgery. Appendicitis. Curr Probl Surg. 2005;42:688-742.
2. Song DW, Park BK, Suh SW, et al. Bacterial culture and antibiotic susceptibility in patients with acute appendicitis. Int J Colorectal Dis. 2018;33:441-447.
3. Wagner JM, McKinney WP, Carpenter JL. Does this patient have appendicitis? JAMA. 1996;276:1589-1594.
4. Benabbas R, Hanna M, Shah J, et al. Diagnostic accuracy of history, physical examination, laboratory tests, and point-of-care ultrasound for pediatric acute appendicitis in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2017;24:523-551.
5. Andersson RE. Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg. 2004;91:28-37.
6. Alvarado A. A practical score for the early diagnosis of acute appendicitis. Ann Emerg Med. 1986;15:557-564.
7. Ebell MH, Shinholser J. What are the most clinically useful cutoffs for the Alvarado and Pediatric Appendicitis Scores? A systematic review. Ann Emerg Med. 2014;64:365-372.e2.
8. Ohle R, O’Reilly F, O’Brien KK, et al. The Alvarado score for predicting acute appendicitis: a systematic review. BMC Med. 2011;9:139.
9. Samuel M. Pediatric appendicitis score. J Pediatr Surg. 2002;37:877-881.
10. Andersson M, Andersson RE. The appendicitis inflammatory response score: a tool for the diagnosis of acute appendicitis that outperforms the Alvarado score. World J Surg. 2008;32:1843-1849.
11. Pogorelić Z, Rak S, Mrklić I, et al. Prospective validation of Alvarado score and Pediatric Appendicitis Score for the diagnosis of acute appendicitis in children. Pediatr Emerg Care. 2015;31:164-168.
12. Rassi R, Muse F, Sánchez-Martínez J, et al. Diagnostic value of clinical prediction scores for acute appendicitis in children younger than 4 years. Eur J Pediatr Surg. 2021. [Online ahead of print]
13. Andersson M, Kolodziej B, Andersson RE. Validation of the Appendicitis Inflammatory Response (AIR) score. World J Surg. 2021;45:2081-2091.
14. Kollár D, McCartan DP, Bourke M, et al. Predicting acute appendicitis? A comparison of the Alvarado score, the Appendicitis Inflammatory Response Score and clinical assessment. World J Surg. 2015;39:104-109.
15. Gorter RR, Eker HH, Gorter-Stam MA, et al. Diagnosis and management of acute appendicitis. EAES consensus development conference 2015. Surg Endosc. 2016;30:4668-4690.
16. Matthew Fields J, Davis J, Alsup C, et al. Accuracy of point-of-care ultrasonography for diagnosing acute appendicitis: a systematic review and meta-analysis. Acad Emerg Med. 2017;24:1124-1136.
17. Sharif S, Skitch S, Vlahaki D, et al. Point-of-care ultrasound to diagnose appendicitis in a Canadian emergency department. CJEM. 2018;20:732-735.
18. Doniger SJ, Kornblith A. Point-of-care ultrasound integrated into a staged diagnostic algorithm for pediatric appendicitis. Pediatr Emerg Care. 2018;34:109-115.
19. Menon N, Kumar S, Keeler B, et al. A systematic review of point-of-care abdominal ultrasound scans performed by general surgeons. Surgeon. 2021. [Online ahead of print]
20. Doria AS, Moineddin R, Kellenberger CJ, et al. US or CT for diagnosis of appendicitis in children and adults? A meta-analysis. Radiology. 2006;241:83-94.
21. van Randen A, Laméris W, van Es HW, et al. A comparison of the accuracy of ultrasound and computed tomography in common diagnoses causing acute abdominal pain. Eur Radiol. 2011;21:1535-1545.
22. Flum DR, Davidson GH, Monsell SE, et al. A randomized trial comparing antibiotics with appendectomy for appendicitis. N Engl J Med. 2020;383:1907-1919.
23. Jaschinski T, Mosch CG, Eikermann M, et al. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev. 2018;11:CD001546.
24. Andersson RE, Petzold MG. Nonsurgical treatment of appendiceal abscess or phlegmon: a systematic review and meta-analysis. Ann Surg. 2007;246:741-748.
25. Deelder JD, Richir MC, Schoorl T, et al. How to treat an appendiceal inflammatory mass: operatively or nonoperatively? J Gastrointest Surg. 2014;18:641-645.
26. Carpenter SG, Chapital AB, Merritt MV, et al. Increased risk of neoplasm in appendicitis treated with interval appendectomy: single-institution experience and literature review. Am Surg. 2012;78:339-343.
27. Hayes D, Reiter S, Hagen E, et al. Is interval appendectomy really needed? A closer look at neoplasm rates in adult patients undergoing interval appendectomy after complicated appendicitis. Surg Endosc. 2021;35:3855-3860.
28. Peltrini R, Cantoni V, Green R, et al. Risk of appendiceal neoplasm after interval appendectomy for complicated appendicitis: a systematic review and meta-analysis. Surgeon. 2021. [Online ahead of print.]
29. Mällinen J, Rautio T, Grönroos J, et al. Risk of appendiceal neoplasm in periappendicular abscess in patients treated with interval appendectomy vs follow-up with magnetic resonance imaging: 1-year outcomes of the peri-appendicitis acuta randomized clinical trial. JAMA Surg. 2019;154:200-207.
30. Son J, Park YJ, Lee SR, et al. Increased risk of neoplasms in adult patients undergoing interval appendectomy. Ann Coloproctol. 2020;36:311-315.
31. Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977.
32. Theilen LH, Mellnick VM, Shanks AL, et al. Acute appendicitis in pregnancy: predictive clinical factors and pregnancy outcomes. Am J Perinatol. 2017;34:523-528.
33. Garcia EM, Camacho MA, Karolyi DR, et al. ACR Appropriateness Criteria right lower quadrant pain-suspected appendicitis. J Am Coll Radiol. 2018;15:S373-s387.
34. Kave M, Parooie F, Salarzaei M. Pregnancy and appendicitis: a systematic review and meta-analysis on the clinical use of MRI in diagnosis of appendicitis in pregnant women. World J Emerg Surg. 2019;14:37.
35. Repplinger MD, Levy JF, Peethumnongsin E, et al. Systematic review and meta-analysis of the accuracy of MRI to diagnose appendicitis in the general population. J Magn Reson Imaging. 2016;43:1346-1354.
36. Pearl JP, Price RR, Tonkin AE, et al. SAGES guidelines for the use of laparoscopy during pregnancy. Surg Endosc. 2017;31:3767-3782.
37. Zingone F, Sultan AA, Humes DJ, et al. Risk of acute appendicitis in and around pregnancy: a population-based cohort study from England. Ann Surg. 2015;261:332-337.
38. Andersson RE, Lambe M. Incidence of appendicitis during pregnancy. Int J Epidemiol. 2001;30:1281-1285.
39. Moltubak E, Landerholm K, Blomberg M, et al. Major variation in the incidence of appendicitis before, during and after pregnancy: a population-based cohort study. World J Surg. 2020;44:2601-2608.
40. Abbasi N, Patenaude V, Abenhaim HA. Management and outcomes of acute appendicitis in pregnancy-population-based study of over 7000 cases. BJOG. 2014;121:1509-1514.
41. Dongarwar D, Taylor J, Ajewole V, et al. Trends in appendicitis among pregnant women, the risk for cardiac arrest, and maternal-fetal mortality. World J Surg. 2020;44:3999-4005.
42. Sachs A, Guglielminotti J, Miller R, et al. Risk factors and risk stratification for adverse obstetrical outcomes after appendectomy or cholecystectomy during pregnancy. JAMA Surg. 2017;152:436-441.
43. Joo JI, Park HC, Kim MJ, et al. Outcomes of antibiotic therapy for uncomplicated appendicitis in pregnancy. Am J Med. 2017;130:1467-1469.
44. Lee SH, Lee JY, Choi YY, Lee JG. Laparoscopic appendectomy versus open appendectomy for suspected appendicitis during pregnancy: a systematic review and updated meta-analysis. BMC Surg. 2019;19:41.
45. Frountzas M, Nikolaou C, Stergios K, et al. Is the laparoscopic approach a safe choice for the management of acute appendicitis in pregnant women? A meta-analysis of observational studies. Ann R Coll Surg Engl. 2019;101:235-248.
46. Di Saverio S, Podda M, De Simone B, et al. Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J Emerg Surg. 2020;15:27.
47. Koberlein GC, Trout AT, Rigsby CK, et al. ACR Appropriateness Criteria suspected appendicitis-child. J Am Coll Radiol. 2019;16:S252-S263.
48. Maita S, Andersson B, Svensson JF, et al. Nonoperative treatment for nonperforated appendicitis in children: a systematic review and meta-analysis. Pediatr Surg Int. 2020;36:261-269.
49. Georgiou R, Eaton S, Stanton MP, et al. Efficacy and safety of nonoperative treatment for acute appendicitis: a meta-analysis. Pediatrics. 2017;139:e20163003.
PRACTICE RECOMMENDATIONS
› Use the Alvarado Score, Pediatric Appendicitis Score, or Appendicitis Inflammatory Response Score to help rule out appendicitis and thereby reduce unnecessary imaging. A
› Choose ultrasound first as the imaging procedure for children and pregnant women, followed by magnetic resonance imaging if needed, to reduce ionizing radiation in these populations. B
› Consider an antibiotic-based strategy under the care of a surgeon in lieu of immediate surgery for uncomplicated appendicitis. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series