User login
How do you manage common inpatient oncologic emergencies?
Three routinely encountered emergencies in the inpatient setting
In 2016, there were an estimated 15,338,988 people living with cancer in the United States.1 As such, it is important that hospitalists be proficient in managing oncologic emergencies that can arise during the natural history of cancer or from its treatment. This article will review three emergencies that are routinely encountered in the inpatient setting: malignant spinal cord compression (MSCC), hypercalcemia of malignancy (HCM), and febrile neutropenia (FN).
Case
Mr. Williams is a 56-year-old man with newly diagnosed metastatic prostate cancer, diabetes mellitus, peptic ulcer disease, and hypertension. He is admitted with back pain and lower extremity weakness worsening over 2 weeks. He denies loss of sensation or bowel and bladder incontinence and can walk. MRI confirms cord compression at T10. What initial and subsequent steroid doses would be of most benefit to administer?
Malignant spinal cord compression
Treatment of MSCC usually aims to preserve function rather than reverse established deficits. MSCC from epidural tumor metastasis develops in 5%-14% of all cancer cases,2 with back pain as the most common symptom. Nearly 60%-85% of patients have weakness at the time of diagnosis,3 and unfortunately, nearly two-thirds of patients will be nonambulatory at presentation.
While timely steroid administration in addition to definitive treatment may maintain ambulatory capacity at 1 year after therapy,4 there is no consensus on the optimal loading and maintenance dose and duration of steroids.
Overview of the data
Although there are no formal guidelines on optimal steroid dosing for MSCC, it is common practice for dexamethasone to be initially dosed at 10 mg followed by 4 mg every 4-6 hours.5 The use of higher doses of dexamethasone may result in improvement in neurologic deficits, but has higher risks for toxicity and is not universally supported in the literature.
A study conducted by Vecht and colleagues demonstrated few differences between initial high-dose and low-dose dexamethasone.6 Intravenous administration of either 10 mg or 100 mg dexamethasone, both followed by total 16 mg of dexamethasone orally per day, showed no significant difference in mobility or survival between the groups.
In a prospective study by Heimdal and colleagues that evaluated the relationship between dexamethasone dose and toxicity, higher doses of steroids had no meaningful impact on neurological symptoms and resulted in more severe side effects.7 Patients were either given 96-mg IV loading dose, gradually tapered over 2 weeks, or enrolled in the low-dose group in which they received 4-mg IV dexamethasone four times per day with a taper over 2 weeks. The high-dose group experienced side effects in 28.6% of patients, with 14.3% experiencing serious side effects. Meanwhile, 7.9% of the low-dose group exhibited some side effects, with none experiencing serious adverse effects.The high-dose group did not experience a significant increase in mobility (57.1 vs. 57.9%).
Key takeaways
Dexamthasone 10-mg oral or IV followed by 4 mg every 4-6 hours until definitive treatment is started is associated with improved neurologic outcomes and minimal adverse side effects. Higher doses of steroids are unlikely to offer more benefit. In patients with paraplegia or autonomic dysfunction, the ability to restore neurologic function is reduced and the burdens of steroid treatment may outweigh its benefits.5
Case continued
Mr. Williams completed treatment for MSCC but was still complaining of extreme lethargy and noticed an increase in thirst and no bowel movement in 5 days. His serum calcium was 14 mg/dL.
Hypercalcemia of malignancy
HCM is the most common paraneoplastic syndrome, observed in nearly 30% of patients with advanced cancer. It is a poor prognostic indicator, and approximately half of all patients with HCM will die within 30 days.8 Cancer is the most common reason for hypercalcemia in the inpatient setting9 and is most often associated with multiple myeloma, non–small cell lung cancer, breast cancer, renal cell carcinoma, non-Hodgkins lymphoma, and leukemia.
Hypercalcemia most often presents with cognitive changes and lethargy, anorexia, nausea, constipation, polyuria and polydipsia, and renal failure. Bradycardia and shortened QT interval are seen more with severe hypercalcemia.
Management of hypercalcemia of malignancy
Management of HCM depends on corrected calcium or ionized calcium levels, chronicity, degree of symptoms, and presence of renal failure. In general, mild asymptomatic hypercalcemia can be managed with outpatient care. Serum calcium greater than 14 mg/dL should be treated regardless of symptoms (Table 1).
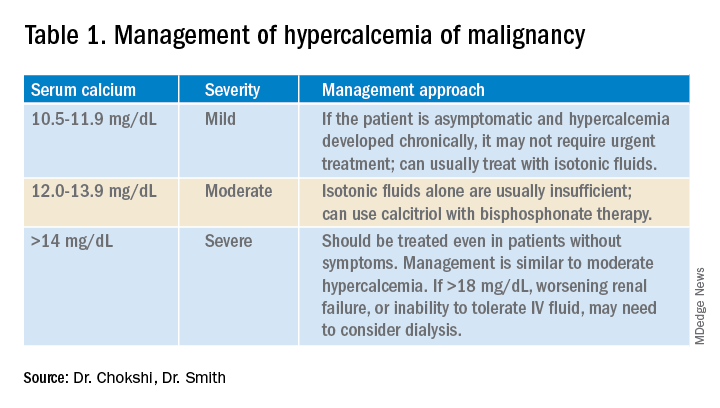
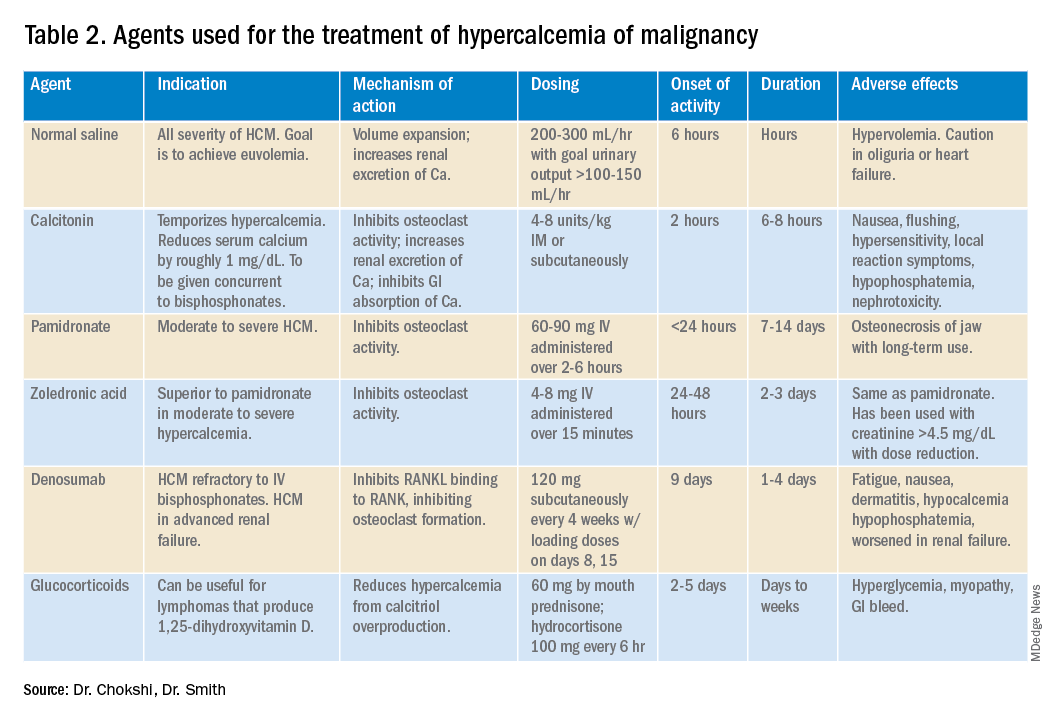
For mild to moderate HCM, management involves saline administration to achieve euvolemia and calcitonin, which has temporizing effects. Early administration of IV bisphosphonates for moderate to severe HCM is beneficial because onset of action is 24-48 hours. Furosemide for management of HCM has fallen out of favor unless the patient develops hypervolemia. Denosumab has been Food and Drug Administration–approved for HCM refractory to bisphosphonate therapy and can manage HCM in 64% of patients who did not respond adequately to bisphosphonate therapy.10 Because it can be used in advanced renal failure without dose adjustment, it is first-line therapy in this population, although the risk for hypocalcemia is increased in renal failure. For patients with serum calcium greater than 18 mg/dL, worsening renal failure, or inability to tolerate IV fluids, dialysis with a low-calcium bath should be considered (Table 2).
Zoledronic acid versus pamidronate
A single dose of zoledronic acid normalizes the serum calcium concentration in 88% of patients, compared with 70% of those who received pamidronate, in a pooled analysis of two phase 3 trials.11 The median duration of normocalcemia was longer for those receiving zoledronic acid (32-43 days vs. 18 days). The efficacy of the 4-mg and 8-mg zoledronic acid doses were similar, but the 4-mg dose was recommended because of renal toxicity and increased mortality associated with the higher dose.Despite this data, many specialists maintain that pamidronate, which is less expensive, is of similar clinical efficacy to ZA.12
Key takeaways
Management of HCM should be determined by the severity of the calcium level. The mainstay of treatment includes hydration with normal saline, calcitonin ,and bisphosphonate therapy; zoledronic acid is preferred over pamidronate. For patients refractory to bisphosphonates or patients with renal insufficiency, denosumab should be used.
Case continued: Febrile neutropenia
Febrile neutropenia is defined as a single oral temperature of 100.9° F or a temperature of 100.4° F sustained over a 1-hour period in a patient with absolute neutrophil count (ANC) less than 1,000 cells/mL or ANC expected to decrease to less than 500 cells/mL within a 48-hour period.13 Up to 30% of patients with solid tumors develop febrile neutropenia after chemotherapy, and nearly 80% of patients with hematologic malignancy or after hematopoietic stem cell therapy (HSCT) experience it.
Even though an infectious etiology is identified in only 30%-40% of cases, all patients with febrile neutropenia should initially receive at least empiric gram-negative coverage. The mortality rate is nearly 70% in neutropenic patients who do not receive empiric antibiotics and is reduced to 4%-20% with antibiotics.14
Risk stratification for febrile neutropenia and early discharge
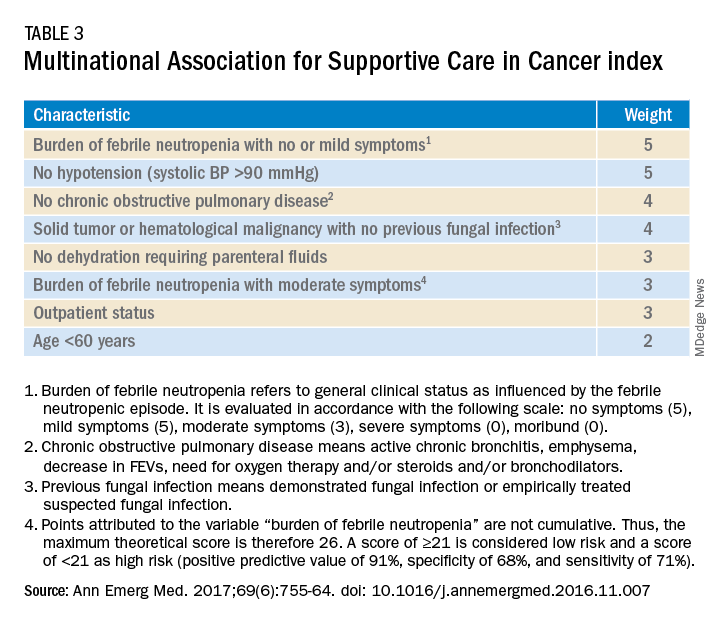
Talcott’s Rules, the Multinational Association for Supportive Care in Cancer (MASCC) score, and the Clinical Index of Stable Febrile Neutropenia (CISNE) are validated tools to determine low-risk febrile neutropenia patients (Tables 3 and 4). The Infectious Diseases Society of America guidelines validated the use of MASCC in 2002 but found that CISNE had better performance than other tools. Coyne and colleagues conducted a retrospective cohort study to assess these two risk stratification tools in the ED and found that the CISNE was 98.3% specific for identifying adverse outcomes, whereas the MASCC was 54.2% specific.15
A study by Talcott and colleagues used Talcott’s Rules to identify low-risk febrile neutropenia patients, who were randomized to early discharge with home intravenous antibiotics versus continued inpatient management. There were no significant differences in the primary outcomes, defined as any change in clinical status requiring medical evaluation.16 Another study suggested that discharge after 24 hours based on clinical stability with outpatient oral antibiotics were noninferior to standard inpatient and intravenous antibiotic therapy.17 A Cochrane review in 2013 of 22 randomized controlled trials determined that oral antibiotics were an acceptable treatment for low-risk patients.18
Key takeaways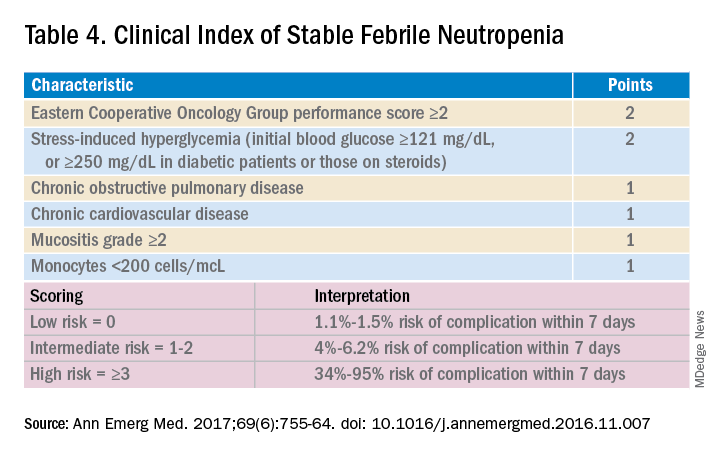
Though the MASCC is highly sensitive in identifying low-risk febrile neutropenia patients, it should be used with clinical caution because up to 11% of patients characterized as low risk developed severe complications.19 If a low-risk patient with solid tumor malignancy has adequate home support, lives within an hour of the hospital, and has access to follow-up within 72 hours, oral antibiotics and early discharge can be considered.
Dr. Chokshi is assistant professor in the division of hospital medicine at Mount Sinai Hospital, New York. Dr. Smith is associate professor in the division of hematology/oncology at Mount Sinai Hospital.
QUIZ
Mrs. Smith is a 64-year-old woman with endometrial cancer with temperature of 100.4° F at home. She takes no antibiotics, has no other medical history, and was sent in from clinic and admitted for further management. She feels well, and preliminary infectious workup is negative. She has been afebrile for more than 24 hours, and her ANC is 600 cells/mL.
Her son’s soccer game is tomorrow, and she would like to be present. Her family is involved in her care. Under what conditions can she be discharged?
A. She should not be discharged until full course of empiric intravenous antibiotics is completed.
B. Consider discharge in another 24 hours if she remains afebrile.
C. Discharge if low risk by MASCC or CISNE, with oral doses of levofloxacin or moxifloxacin or oral ciprofloxacin and amoxicillin/clavulanic acid.
Answer: C. The patient has a solid tumor malignancy, is low risk by both MASCC and CISNE, and can most likely be discharged if she is clinically stable or improved. A 7-day course of antibiotics is recommended with close follow-up.
References
1. SEER. Cancer of Any Site - Cancer Stat Facts. https://seer.cancer.gov/statfacts/html/all.html. Accessed 2019 Jul 17.
2. Kwok Y et al. Clinical Approach to Metastatic Epidural Spinal Cord Compression. Hematol Oncol Clin North Am. 2006;20(6):1297-305.
3. Helweg-Larsen S et al. Prognostic factors in metastatic spinal cord compression: a prospective study using multivariate analysis of variables influencing survival and gait function in 153 patients. Int J Radiat Oncol Biol Phys. 2000;46(5):1163-9.
4. Sørensen P et al. Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: A randomised trial. Eur J Cancer. 1994;30(1):22-7.
5. Skeoch G et al. Corticosteroid treatment for metastatic spinal cord compression: A review. Global Spine J. 2017;7(3):272-9.
6. Vecht C et al. Initial bolus of conventional versus high-dose dexamethasone in metastatic spinal cord compression. Neurology. 1989;39(9):1255-7.
7. Heimdal K et al. High incidence of serious side effects of high-dose dexamethasone treatment in patients with epidural spinal cord compression. J Neurooncol. 1992;12(2):141-4.
8. Ralston S et al. Cancer-associated hypercalcemia: Morbidity and mortality. Clinical experience in 126 treated patients. Ann Intern Med. 1990;112(7):499-504.
9. Lindner G et al. Hypercalcemia in the ED: Prevalence, etiology, and outcome. Am J Emerg Med. 2013;31(4):657-60.
10. Hu M et al. Denosumab for patients with persistent or relapsed hypercalcemia of malignancy despite recent bisphosphonate treatment. J Natl Cancer Inst. 2013;105(18):1417-20.
11. Major P et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: A pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19(2):558-67.
12. Stewart A. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005;352(4):373-9.
13. Freifeld A et al. Executive summary: Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):427-31.
14. Baden L et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(7):882-913.
15. Coyne C et al. Application of the MASCC and CISNE risk-stratification scores to identify low-risk febrile neutropenic patients in the emergency department. Ann Emerg Med. 2017;69(6):755-64.
16. Talcott J et al. Safety of early discharge for low-risk patients with febrile neutropenia: a multicenter randomized controlled trial. J Clin Oncol. 2011;29(30):3977-83.
17. Innes H et al. Oral antibiotics with early hospital discharge compared with in-patient intravenous antibiotics for low-risk febrile neutropenia in patients with cancer: A prospective randomised controlled single centre study. Br J Cancer. 2003;89(1):43-9.
18. Vidal L, et al. Oral versus intravenous antibiotic treatment for febrile neutropenia in cancer patients. Cochrane Database Syst. Rev. 2013.
19. Taplitz RA et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America clinical practice guideline update. J Clin Oncol. 2018;36(14):1443-53.
Three routinely encountered emergencies in the inpatient setting
Three routinely encountered emergencies in the inpatient setting
In 2016, there were an estimated 15,338,988 people living with cancer in the United States.1 As such, it is important that hospitalists be proficient in managing oncologic emergencies that can arise during the natural history of cancer or from its treatment. This article will review three emergencies that are routinely encountered in the inpatient setting: malignant spinal cord compression (MSCC), hypercalcemia of malignancy (HCM), and febrile neutropenia (FN).
Case
Mr. Williams is a 56-year-old man with newly diagnosed metastatic prostate cancer, diabetes mellitus, peptic ulcer disease, and hypertension. He is admitted with back pain and lower extremity weakness worsening over 2 weeks. He denies loss of sensation or bowel and bladder incontinence and can walk. MRI confirms cord compression at T10. What initial and subsequent steroid doses would be of most benefit to administer?
Malignant spinal cord compression
Treatment of MSCC usually aims to preserve function rather than reverse established deficits. MSCC from epidural tumor metastasis develops in 5%-14% of all cancer cases,2 with back pain as the most common symptom. Nearly 60%-85% of patients have weakness at the time of diagnosis,3 and unfortunately, nearly two-thirds of patients will be nonambulatory at presentation.
While timely steroid administration in addition to definitive treatment may maintain ambulatory capacity at 1 year after therapy,4 there is no consensus on the optimal loading and maintenance dose and duration of steroids.
Overview of the data
Although there are no formal guidelines on optimal steroid dosing for MSCC, it is common practice for dexamethasone to be initially dosed at 10 mg followed by 4 mg every 4-6 hours.5 The use of higher doses of dexamethasone may result in improvement in neurologic deficits, but has higher risks for toxicity and is not universally supported in the literature.
A study conducted by Vecht and colleagues demonstrated few differences between initial high-dose and low-dose dexamethasone.6 Intravenous administration of either 10 mg or 100 mg dexamethasone, both followed by total 16 mg of dexamethasone orally per day, showed no significant difference in mobility or survival between the groups.
In a prospective study by Heimdal and colleagues that evaluated the relationship between dexamethasone dose and toxicity, higher doses of steroids had no meaningful impact on neurological symptoms and resulted in more severe side effects.7 Patients were either given 96-mg IV loading dose, gradually tapered over 2 weeks, or enrolled in the low-dose group in which they received 4-mg IV dexamethasone four times per day with a taper over 2 weeks. The high-dose group experienced side effects in 28.6% of patients, with 14.3% experiencing serious side effects. Meanwhile, 7.9% of the low-dose group exhibited some side effects, with none experiencing serious adverse effects.The high-dose group did not experience a significant increase in mobility (57.1 vs. 57.9%).
Key takeaways
Dexamthasone 10-mg oral or IV followed by 4 mg every 4-6 hours until definitive treatment is started is associated with improved neurologic outcomes and minimal adverse side effects. Higher doses of steroids are unlikely to offer more benefit. In patients with paraplegia or autonomic dysfunction, the ability to restore neurologic function is reduced and the burdens of steroid treatment may outweigh its benefits.5
Case continued
Mr. Williams completed treatment for MSCC but was still complaining of extreme lethargy and noticed an increase in thirst and no bowel movement in 5 days. His serum calcium was 14 mg/dL.
Hypercalcemia of malignancy
HCM is the most common paraneoplastic syndrome, observed in nearly 30% of patients with advanced cancer. It is a poor prognostic indicator, and approximately half of all patients with HCM will die within 30 days.8 Cancer is the most common reason for hypercalcemia in the inpatient setting9 and is most often associated with multiple myeloma, non–small cell lung cancer, breast cancer, renal cell carcinoma, non-Hodgkins lymphoma, and leukemia.
Hypercalcemia most often presents with cognitive changes and lethargy, anorexia, nausea, constipation, polyuria and polydipsia, and renal failure. Bradycardia and shortened QT interval are seen more with severe hypercalcemia.
Management of hypercalcemia of malignancy
Management of HCM depends on corrected calcium or ionized calcium levels, chronicity, degree of symptoms, and presence of renal failure. In general, mild asymptomatic hypercalcemia can be managed with outpatient care. Serum calcium greater than 14 mg/dL should be treated regardless of symptoms (Table 1).

For mild to moderate HCM, management involves saline administration to achieve euvolemia and calcitonin, which has temporizing effects. Early administration of IV bisphosphonates for moderate to severe HCM is beneficial because onset of action is 24-48 hours. Furosemide for management of HCM has fallen out of favor unless the patient develops hypervolemia. Denosumab has been Food and Drug Administration–approved for HCM refractory to bisphosphonate therapy and can manage HCM in 64% of patients who did not respond adequately to bisphosphonate therapy.10 Because it can be used in advanced renal failure without dose adjustment, it is first-line therapy in this population, although the risk for hypocalcemia is increased in renal failure. For patients with serum calcium greater than 18 mg/dL, worsening renal failure, or inability to tolerate IV fluids, dialysis with a low-calcium bath should be considered (Table 2).
Zoledronic acid versus pamidronate
A single dose of zoledronic acid normalizes the serum calcium concentration in 88% of patients, compared with 70% of those who received pamidronate, in a pooled analysis of two phase 3 trials.11 The median duration of normocalcemia was longer for those receiving zoledronic acid (32-43 days vs. 18 days). The efficacy of the 4-mg and 8-mg zoledronic acid doses were similar, but the 4-mg dose was recommended because of renal toxicity and increased mortality associated with the higher dose.Despite this data, many specialists maintain that pamidronate, which is less expensive, is of similar clinical efficacy to ZA.12

Key takeaways
Management of HCM should be determined by the severity of the calcium level. The mainstay of treatment includes hydration with normal saline, calcitonin ,and bisphosphonate therapy; zoledronic acid is preferred over pamidronate. For patients refractory to bisphosphonates or patients with renal insufficiency, denosumab should be used.
Case continued: Febrile neutropenia
Febrile neutropenia is defined as a single oral temperature of 100.9° F or a temperature of 100.4° F sustained over a 1-hour period in a patient with absolute neutrophil count (ANC) less than 1,000 cells/mL or ANC expected to decrease to less than 500 cells/mL within a 48-hour period.13 Up to 30% of patients with solid tumors develop febrile neutropenia after chemotherapy, and nearly 80% of patients with hematologic malignancy or after hematopoietic stem cell therapy (HSCT) experience it.
Even though an infectious etiology is identified in only 30%-40% of cases, all patients with febrile neutropenia should initially receive at least empiric gram-negative coverage. The mortality rate is nearly 70% in neutropenic patients who do not receive empiric antibiotics and is reduced to 4%-20% with antibiotics.14
Risk stratification for febrile neutropenia and early discharge

Talcott’s Rules, the Multinational Association for Supportive Care in Cancer (MASCC) score, and the Clinical Index of Stable Febrile Neutropenia (CISNE) are validated tools to determine low-risk febrile neutropenia patients (Tables 3 and 4). The Infectious Diseases Society of America guidelines validated the use of MASCC in 2002 but found that CISNE had better performance than other tools. Coyne and colleagues conducted a retrospective cohort study to assess these two risk stratification tools in the ED and found that the CISNE was 98.3% specific for identifying adverse outcomes, whereas the MASCC was 54.2% specific.15
A study by Talcott and colleagues used Talcott’s Rules to identify low-risk febrile neutropenia patients, who were randomized to early discharge with home intravenous antibiotics versus continued inpatient management. There were no significant differences in the primary outcomes, defined as any change in clinical status requiring medical evaluation.16 Another study suggested that discharge after 24 hours based on clinical stability with outpatient oral antibiotics were noninferior to standard inpatient and intravenous antibiotic therapy.17 A Cochrane review in 2013 of 22 randomized controlled trials determined that oral antibiotics were an acceptable treatment for low-risk patients.18
Key takeaways
Though the MASCC is highly sensitive in identifying low-risk febrile neutropenia patients, it should be used with clinical caution because up to 11% of patients characterized as low risk developed severe complications.19 If a low-risk patient with solid tumor malignancy has adequate home support, lives within an hour of the hospital, and has access to follow-up within 72 hours, oral antibiotics and early discharge can be considered.
Dr. Chokshi is assistant professor in the division of hospital medicine at Mount Sinai Hospital, New York. Dr. Smith is associate professor in the division of hematology/oncology at Mount Sinai Hospital.
QUIZ
Mrs. Smith is a 64-year-old woman with endometrial cancer with temperature of 100.4° F at home. She takes no antibiotics, has no other medical history, and was sent in from clinic and admitted for further management. She feels well, and preliminary infectious workup is negative. She has been afebrile for more than 24 hours, and her ANC is 600 cells/mL.
Her son’s soccer game is tomorrow, and she would like to be present. Her family is involved in her care. Under what conditions can she be discharged?
A. She should not be discharged until full course of empiric intravenous antibiotics is completed.
B. Consider discharge in another 24 hours if she remains afebrile.
C. Discharge if low risk by MASCC or CISNE, with oral doses of levofloxacin or moxifloxacin or oral ciprofloxacin and amoxicillin/clavulanic acid.
Answer: C. The patient has a solid tumor malignancy, is low risk by both MASCC and CISNE, and can most likely be discharged if she is clinically stable or improved. A 7-day course of antibiotics is recommended with close follow-up.
References
1. SEER. Cancer of Any Site - Cancer Stat Facts. https://seer.cancer.gov/statfacts/html/all.html. Accessed 2019 Jul 17.
2. Kwok Y et al. Clinical Approach to Metastatic Epidural Spinal Cord Compression. Hematol Oncol Clin North Am. 2006;20(6):1297-305.
3. Helweg-Larsen S et al. Prognostic factors in metastatic spinal cord compression: a prospective study using multivariate analysis of variables influencing survival and gait function in 153 patients. Int J Radiat Oncol Biol Phys. 2000;46(5):1163-9.
4. Sørensen P et al. Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: A randomised trial. Eur J Cancer. 1994;30(1):22-7.
5. Skeoch G et al. Corticosteroid treatment for metastatic spinal cord compression: A review. Global Spine J. 2017;7(3):272-9.
6. Vecht C et al. Initial bolus of conventional versus high-dose dexamethasone in metastatic spinal cord compression. Neurology. 1989;39(9):1255-7.
7. Heimdal K et al. High incidence of serious side effects of high-dose dexamethasone treatment in patients with epidural spinal cord compression. J Neurooncol. 1992;12(2):141-4.
8. Ralston S et al. Cancer-associated hypercalcemia: Morbidity and mortality. Clinical experience in 126 treated patients. Ann Intern Med. 1990;112(7):499-504.
9. Lindner G et al. Hypercalcemia in the ED: Prevalence, etiology, and outcome. Am J Emerg Med. 2013;31(4):657-60.
10. Hu M et al. Denosumab for patients with persistent or relapsed hypercalcemia of malignancy despite recent bisphosphonate treatment. J Natl Cancer Inst. 2013;105(18):1417-20.
11. Major P et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: A pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19(2):558-67.
12. Stewart A. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005;352(4):373-9.
13. Freifeld A et al. Executive summary: Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):427-31.
14. Baden L et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(7):882-913.
15. Coyne C et al. Application of the MASCC and CISNE risk-stratification scores to identify low-risk febrile neutropenic patients in the emergency department. Ann Emerg Med. 2017;69(6):755-64.
16. Talcott J et al. Safety of early discharge for low-risk patients with febrile neutropenia: a multicenter randomized controlled trial. J Clin Oncol. 2011;29(30):3977-83.
17. Innes H et al. Oral antibiotics with early hospital discharge compared with in-patient intravenous antibiotics for low-risk febrile neutropenia in patients with cancer: A prospective randomised controlled single centre study. Br J Cancer. 2003;89(1):43-9.
18. Vidal L, et al. Oral versus intravenous antibiotic treatment for febrile neutropenia in cancer patients. Cochrane Database Syst. Rev. 2013.
19. Taplitz RA et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America clinical practice guideline update. J Clin Oncol. 2018;36(14):1443-53.
In 2016, there were an estimated 15,338,988 people living with cancer in the United States.1 As such, it is important that hospitalists be proficient in managing oncologic emergencies that can arise during the natural history of cancer or from its treatment. This article will review three emergencies that are routinely encountered in the inpatient setting: malignant spinal cord compression (MSCC), hypercalcemia of malignancy (HCM), and febrile neutropenia (FN).
Case
Mr. Williams is a 56-year-old man with newly diagnosed metastatic prostate cancer, diabetes mellitus, peptic ulcer disease, and hypertension. He is admitted with back pain and lower extremity weakness worsening over 2 weeks. He denies loss of sensation or bowel and bladder incontinence and can walk. MRI confirms cord compression at T10. What initial and subsequent steroid doses would be of most benefit to administer?
Malignant spinal cord compression
Treatment of MSCC usually aims to preserve function rather than reverse established deficits. MSCC from epidural tumor metastasis develops in 5%-14% of all cancer cases,2 with back pain as the most common symptom. Nearly 60%-85% of patients have weakness at the time of diagnosis,3 and unfortunately, nearly two-thirds of patients will be nonambulatory at presentation.
While timely steroid administration in addition to definitive treatment may maintain ambulatory capacity at 1 year after therapy,4 there is no consensus on the optimal loading and maintenance dose and duration of steroids.
Overview of the data
Although there are no formal guidelines on optimal steroid dosing for MSCC, it is common practice for dexamethasone to be initially dosed at 10 mg followed by 4 mg every 4-6 hours.5 The use of higher doses of dexamethasone may result in improvement in neurologic deficits, but has higher risks for toxicity and is not universally supported in the literature.
A study conducted by Vecht and colleagues demonstrated few differences between initial high-dose and low-dose dexamethasone.6 Intravenous administration of either 10 mg or 100 mg dexamethasone, both followed by total 16 mg of dexamethasone orally per day, showed no significant difference in mobility or survival between the groups.
In a prospective study by Heimdal and colleagues that evaluated the relationship between dexamethasone dose and toxicity, higher doses of steroids had no meaningful impact on neurological symptoms and resulted in more severe side effects.7 Patients were either given 96-mg IV loading dose, gradually tapered over 2 weeks, or enrolled in the low-dose group in which they received 4-mg IV dexamethasone four times per day with a taper over 2 weeks. The high-dose group experienced side effects in 28.6% of patients, with 14.3% experiencing serious side effects. Meanwhile, 7.9% of the low-dose group exhibited some side effects, with none experiencing serious adverse effects.The high-dose group did not experience a significant increase in mobility (57.1 vs. 57.9%).
Key takeaways
Dexamthasone 10-mg oral or IV followed by 4 mg every 4-6 hours until definitive treatment is started is associated with improved neurologic outcomes and minimal adverse side effects. Higher doses of steroids are unlikely to offer more benefit. In patients with paraplegia or autonomic dysfunction, the ability to restore neurologic function is reduced and the burdens of steroid treatment may outweigh its benefits.5
Case continued
Mr. Williams completed treatment for MSCC but was still complaining of extreme lethargy and noticed an increase in thirst and no bowel movement in 5 days. His serum calcium was 14 mg/dL.
Hypercalcemia of malignancy
HCM is the most common paraneoplastic syndrome, observed in nearly 30% of patients with advanced cancer. It is a poor prognostic indicator, and approximately half of all patients with HCM will die within 30 days.8 Cancer is the most common reason for hypercalcemia in the inpatient setting9 and is most often associated with multiple myeloma, non–small cell lung cancer, breast cancer, renal cell carcinoma, non-Hodgkins lymphoma, and leukemia.
Hypercalcemia most often presents with cognitive changes and lethargy, anorexia, nausea, constipation, polyuria and polydipsia, and renal failure. Bradycardia and shortened QT interval are seen more with severe hypercalcemia.
Management of hypercalcemia of malignancy
Management of HCM depends on corrected calcium or ionized calcium levels, chronicity, degree of symptoms, and presence of renal failure. In general, mild asymptomatic hypercalcemia can be managed with outpatient care. Serum calcium greater than 14 mg/dL should be treated regardless of symptoms (Table 1).

For mild to moderate HCM, management involves saline administration to achieve euvolemia and calcitonin, which has temporizing effects. Early administration of IV bisphosphonates for moderate to severe HCM is beneficial because onset of action is 24-48 hours. Furosemide for management of HCM has fallen out of favor unless the patient develops hypervolemia. Denosumab has been Food and Drug Administration–approved for HCM refractory to bisphosphonate therapy and can manage HCM in 64% of patients who did not respond adequately to bisphosphonate therapy.10 Because it can be used in advanced renal failure without dose adjustment, it is first-line therapy in this population, although the risk for hypocalcemia is increased in renal failure. For patients with serum calcium greater than 18 mg/dL, worsening renal failure, or inability to tolerate IV fluids, dialysis with a low-calcium bath should be considered (Table 2).
Zoledronic acid versus pamidronate
A single dose of zoledronic acid normalizes the serum calcium concentration in 88% of patients, compared with 70% of those who received pamidronate, in a pooled analysis of two phase 3 trials.11 The median duration of normocalcemia was longer for those receiving zoledronic acid (32-43 days vs. 18 days). The efficacy of the 4-mg and 8-mg zoledronic acid doses were similar, but the 4-mg dose was recommended because of renal toxicity and increased mortality associated with the higher dose.Despite this data, many specialists maintain that pamidronate, which is less expensive, is of similar clinical efficacy to ZA.12

Key takeaways
Management of HCM should be determined by the severity of the calcium level. The mainstay of treatment includes hydration with normal saline, calcitonin ,and bisphosphonate therapy; zoledronic acid is preferred over pamidronate. For patients refractory to bisphosphonates or patients with renal insufficiency, denosumab should be used.
Case continued: Febrile neutropenia
Febrile neutropenia is defined as a single oral temperature of 100.9° F or a temperature of 100.4° F sustained over a 1-hour period in a patient with absolute neutrophil count (ANC) less than 1,000 cells/mL or ANC expected to decrease to less than 500 cells/mL within a 48-hour period.13 Up to 30% of patients with solid tumors develop febrile neutropenia after chemotherapy, and nearly 80% of patients with hematologic malignancy or after hematopoietic stem cell therapy (HSCT) experience it.
Even though an infectious etiology is identified in only 30%-40% of cases, all patients with febrile neutropenia should initially receive at least empiric gram-negative coverage. The mortality rate is nearly 70% in neutropenic patients who do not receive empiric antibiotics and is reduced to 4%-20% with antibiotics.14
Risk stratification for febrile neutropenia and early discharge

Talcott’s Rules, the Multinational Association for Supportive Care in Cancer (MASCC) score, and the Clinical Index of Stable Febrile Neutropenia (CISNE) are validated tools to determine low-risk febrile neutropenia patients (Tables 3 and 4). The Infectious Diseases Society of America guidelines validated the use of MASCC in 2002 but found that CISNE had better performance than other tools. Coyne and colleagues conducted a retrospective cohort study to assess these two risk stratification tools in the ED and found that the CISNE was 98.3% specific for identifying adverse outcomes, whereas the MASCC was 54.2% specific.15
A study by Talcott and colleagues used Talcott’s Rules to identify low-risk febrile neutropenia patients, who were randomized to early discharge with home intravenous antibiotics versus continued inpatient management. There were no significant differences in the primary outcomes, defined as any change in clinical status requiring medical evaluation.16 Another study suggested that discharge after 24 hours based on clinical stability with outpatient oral antibiotics were noninferior to standard inpatient and intravenous antibiotic therapy.17 A Cochrane review in 2013 of 22 randomized controlled trials determined that oral antibiotics were an acceptable treatment for low-risk patients.18
Key takeaways
Though the MASCC is highly sensitive in identifying low-risk febrile neutropenia patients, it should be used with clinical caution because up to 11% of patients characterized as low risk developed severe complications.19 If a low-risk patient with solid tumor malignancy has adequate home support, lives within an hour of the hospital, and has access to follow-up within 72 hours, oral antibiotics and early discharge can be considered.
Dr. Chokshi is assistant professor in the division of hospital medicine at Mount Sinai Hospital, New York. Dr. Smith is associate professor in the division of hematology/oncology at Mount Sinai Hospital.
QUIZ
Mrs. Smith is a 64-year-old woman with endometrial cancer with temperature of 100.4° F at home. She takes no antibiotics, has no other medical history, and was sent in from clinic and admitted for further management. She feels well, and preliminary infectious workup is negative. She has been afebrile for more than 24 hours, and her ANC is 600 cells/mL.
Her son’s soccer game is tomorrow, and she would like to be present. Her family is involved in her care. Under what conditions can she be discharged?
A. She should not be discharged until full course of empiric intravenous antibiotics is completed.
B. Consider discharge in another 24 hours if she remains afebrile.
C. Discharge if low risk by MASCC or CISNE, with oral doses of levofloxacin or moxifloxacin or oral ciprofloxacin and amoxicillin/clavulanic acid.
Answer: C. The patient has a solid tumor malignancy, is low risk by both MASCC and CISNE, and can most likely be discharged if she is clinically stable or improved. A 7-day course of antibiotics is recommended with close follow-up.
References
1. SEER. Cancer of Any Site - Cancer Stat Facts. https://seer.cancer.gov/statfacts/html/all.html. Accessed 2019 Jul 17.
2. Kwok Y et al. Clinical Approach to Metastatic Epidural Spinal Cord Compression. Hematol Oncol Clin North Am. 2006;20(6):1297-305.
3. Helweg-Larsen S et al. Prognostic factors in metastatic spinal cord compression: a prospective study using multivariate analysis of variables influencing survival and gait function in 153 patients. Int J Radiat Oncol Biol Phys. 2000;46(5):1163-9.
4. Sørensen P et al. Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: A randomised trial. Eur J Cancer. 1994;30(1):22-7.
5. Skeoch G et al. Corticosteroid treatment for metastatic spinal cord compression: A review. Global Spine J. 2017;7(3):272-9.
6. Vecht C et al. Initial bolus of conventional versus high-dose dexamethasone in metastatic spinal cord compression. Neurology. 1989;39(9):1255-7.
7. Heimdal K et al. High incidence of serious side effects of high-dose dexamethasone treatment in patients with epidural spinal cord compression. J Neurooncol. 1992;12(2):141-4.
8. Ralston S et al. Cancer-associated hypercalcemia: Morbidity and mortality. Clinical experience in 126 treated patients. Ann Intern Med. 1990;112(7):499-504.
9. Lindner G et al. Hypercalcemia in the ED: Prevalence, etiology, and outcome. Am J Emerg Med. 2013;31(4):657-60.
10. Hu M et al. Denosumab for patients with persistent or relapsed hypercalcemia of malignancy despite recent bisphosphonate treatment. J Natl Cancer Inst. 2013;105(18):1417-20.
11. Major P et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: A pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19(2):558-67.
12. Stewart A. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005;352(4):373-9.
13. Freifeld A et al. Executive summary: Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):427-31.
14. Baden L et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(7):882-913.
15. Coyne C et al. Application of the MASCC and CISNE risk-stratification scores to identify low-risk febrile neutropenic patients in the emergency department. Ann Emerg Med. 2017;69(6):755-64.
16. Talcott J et al. Safety of early discharge for low-risk patients with febrile neutropenia: a multicenter randomized controlled trial. J Clin Oncol. 2011;29(30):3977-83.
17. Innes H et al. Oral antibiotics with early hospital discharge compared with in-patient intravenous antibiotics for low-risk febrile neutropenia in patients with cancer: A prospective randomised controlled single centre study. Br J Cancer. 2003;89(1):43-9.
18. Vidal L, et al. Oral versus intravenous antibiotic treatment for febrile neutropenia in cancer patients. Cochrane Database Syst. Rev. 2013.
19. Taplitz RA et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America clinical practice guideline update. J Clin Oncol. 2018;36(14):1443-53.
COVID spikes exacerbate health worker shortages in Rocky Mountains, Great Plains
In Montana, pandemic-induced staffing shortages have shuttered a clinic in the state’s capital, led a northwestern regional hospital to ask employees exposed to COVID-19 to continue to work and emptied a health department 400 miles to the east.
“Just one more person out and we wouldn’t be able to keep the surgeries going,” said Dr. Shelly Harkins, MD, chief medical officer of St. Peter’s Health in Helena, a city of roughly 32,000 where cases continue to spread. “When the virus is just all around you, it’s almost impossible to not be deemed a contact at some point. One case can take out a whole team of people in a blink of an eye.”
In North Dakota, where cases per resident are growing faster than any other state, hospitals may once again curtail elective surgeries and possibly seek government aid to hire more nurses if the situation gets worse, North Dakota Hospital Association President Tim Blasl said.
“How long can we run at this rate with the workforce that we have?” Blasl said. “You can have all the licensed beds you want, but if you don’t have anybody to staff those beds, it doesn’t do you any good.”
The northern Rocky Mountains, Great Plains and Upper Midwest are seeing the highest surge of COVID-19 cases in the nation, as some residents have ignored recommendations for curtailing the virus, such as wearing masks and avoiding large gatherings. Montana, Idaho, Utah, Wyoming, North Dakota, South Dakota, Nebraska, Iowa, and Wisconsin have recently ranked among the top 10 U.S. states in confirmed cases per 100,000 residents over a 7-day period, according to an analysis by the New York Times.
Such coronavirus infections – and the quarantines that occur because of them – are exacerbating the health care worker shortage that existed in these states well before the pandemic. Unlike in the nation’s metropolitan hubs, these outbreaks are scattered across hundreds of miles. And even in these states’ biggest cities, the ranks of medical professionals are in short supply. Specialists and registered nurses are sometimes harder to track down than ventilators, N95 masks or hospital beds. Without enough care providers, patients may not be able to get the medical attention they need.
Hospitals have asked staffers to cover extra shifts and learn new skills. They have brought in temporary workers from other parts of the country and transferred some patients to less-crowded hospitals. But, at St. Peter’s Health, if the hospital’s one kidney doctor gets sick or is told to quarantine, Dr. Harkins doesn’t expect to find a backup.
“We make a point to not have excessive staff because we have an obligation to keep the cost of health care down for a community – we just don’t have a lot of slack in our rope,” Dr. Harkins said. “What we don’t account for is a mass exodus of staff for 14 days.”
Some hospitals are already at patient capacity or are nearly there. That’s not just because of the growing number of COVID-19 patients. Elective surgeries have resumed, and medical emergencies don’t pause for a pandemic.
Some Montana hospitals formed agreements with local affiliates early in the pandemic to share staff if one came up short. But now that the disease is spreading fast – and widely – the hope is that their needs don’t peak all at once.
Montana state officials keep a list of primarily in-state volunteer workers ready to travel to towns with shortages of contact tracers, nurses and more. But during a press conference on Oct. 15, Democratic Gov. Steve Bullock said the state had exhausted that database, and its nationwide request for National Guard medical staffing hadn’t brought in new workers.
“If you are a registered nurse, licensed practical nurse, paramedic, EMT, CNA or contact tracer, and are able to join our workforce, please do consider joining our team,” Gov. Bullock said.
This month, Kalispell Regional Medical Center in northwestern Montana even stopped quarantining COVID-exposed staff who remain asymptomatic, a change allowed by Centers for Disease Control and Prevention guidelines for health facilities facing staffing shortages.
“That’s very telling for what staffing is going through right now,” said Andrea Lueck, a registered nurse at the center. “We’re so tight that employees are called off of quarantine.”
Financial pressure early in the pandemic led the hospital to furlough staff, but it had to bring most of them back to work because it needs those bodies more than ever. The regional hub is based in Flathead County, which has recorded the state’s second-highest number of active COVID-19 cases.
Mellody Sharpton, a hospital spokesperson, said hospital workers who are exposed to someone infected with the virus are tested within three to five days and monitored for symptoms. The hospital is also pulling in new workers, with 25 traveling health professionals on hand and another 25 temporary ones on the way.
But Ms. Sharpton said the best way to conserve the hospital’s workforce is to stop the disease surge in the community.
Earlier in the pandemic, Central Montana Medical Center in Lewistown, a town of fewer than 6,000, experienced an exodus of part-time workers or those close to retirement who decided their jobs weren’t worth the risk. The facility recently secured two traveling workers, but both backed out because they couldn’t find housing. And, so far, roughly 40 of the hospital’s 322 employees have missed work for reasons connected to COVID-19.
“We’re at a critical staffing shortage and have been since the beginning of COVID,” said Joanie Slaybaugh, Central Montana Medical Center’s director of human resources. “We’re small enough, everybody feels an obligation to protect themselves and to protect each other. But it doesn’t take much to take out our staff.”
Roosevelt County, where roughly 11,000 live on the northeastern edge of Montana, had one of the nation’s highest rates of new cases as of Oct. 15. But by the end of the month, the county health department will lose half of its registered nurses as one person is about to retire and another was hired through a grant that’s ending. That leaves only one registered nurse aside from its director, Patty Presser. The health department already had to close earlier during the pandemic because of COVID exposure and not enough staffers to cover the gap. Now, if Ms. Presser can’t find nurse replacements in time, she hopes volunteers will step in, though she added they typically stay for only a few weeks.
“I need someone to do immunizations for my community, and you don’t become an immunization nurse in 14 days,” she said. “We don’t have the workforce here to deal with this virus, not even right now, and then I’m going to have my best two people go.”
Back in Helena, Dr. Harkins said St. Peter’s Health had to close a specialty outpatient clinic that treats chronic diseases for two weeks at the end of September because the entire staff had to quarantine.
Now the hospital is considering having doctors take turns spending a week working from home, so that if another wave of quarantines hits in the hospital, at least one untainted person can be brought back to work. But that won’t help for some specialties, like the hospital’s sole kidney doctor.
Every time Dr. Harkins’ phone rings, she said, she takes a breath and hopes it’s not another case that will force a whole division to close.
“Because I think immediately of the hundreds of people that need that service and won’t have it for 14 days,” she said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
In Montana, pandemic-induced staffing shortages have shuttered a clinic in the state’s capital, led a northwestern regional hospital to ask employees exposed to COVID-19 to continue to work and emptied a health department 400 miles to the east.
“Just one more person out and we wouldn’t be able to keep the surgeries going,” said Dr. Shelly Harkins, MD, chief medical officer of St. Peter’s Health in Helena, a city of roughly 32,000 where cases continue to spread. “When the virus is just all around you, it’s almost impossible to not be deemed a contact at some point. One case can take out a whole team of people in a blink of an eye.”
In North Dakota, where cases per resident are growing faster than any other state, hospitals may once again curtail elective surgeries and possibly seek government aid to hire more nurses if the situation gets worse, North Dakota Hospital Association President Tim Blasl said.
“How long can we run at this rate with the workforce that we have?” Blasl said. “You can have all the licensed beds you want, but if you don’t have anybody to staff those beds, it doesn’t do you any good.”
The northern Rocky Mountains, Great Plains and Upper Midwest are seeing the highest surge of COVID-19 cases in the nation, as some residents have ignored recommendations for curtailing the virus, such as wearing masks and avoiding large gatherings. Montana, Idaho, Utah, Wyoming, North Dakota, South Dakota, Nebraska, Iowa, and Wisconsin have recently ranked among the top 10 U.S. states in confirmed cases per 100,000 residents over a 7-day period, according to an analysis by the New York Times.
Such coronavirus infections – and the quarantines that occur because of them – are exacerbating the health care worker shortage that existed in these states well before the pandemic. Unlike in the nation’s metropolitan hubs, these outbreaks are scattered across hundreds of miles. And even in these states’ biggest cities, the ranks of medical professionals are in short supply. Specialists and registered nurses are sometimes harder to track down than ventilators, N95 masks or hospital beds. Without enough care providers, patients may not be able to get the medical attention they need.
Hospitals have asked staffers to cover extra shifts and learn new skills. They have brought in temporary workers from other parts of the country and transferred some patients to less-crowded hospitals. But, at St. Peter’s Health, if the hospital’s one kidney doctor gets sick or is told to quarantine, Dr. Harkins doesn’t expect to find a backup.
“We make a point to not have excessive staff because we have an obligation to keep the cost of health care down for a community – we just don’t have a lot of slack in our rope,” Dr. Harkins said. “What we don’t account for is a mass exodus of staff for 14 days.”
Some hospitals are already at patient capacity or are nearly there. That’s not just because of the growing number of COVID-19 patients. Elective surgeries have resumed, and medical emergencies don’t pause for a pandemic.
Some Montana hospitals formed agreements with local affiliates early in the pandemic to share staff if one came up short. But now that the disease is spreading fast – and widely – the hope is that their needs don’t peak all at once.
Montana state officials keep a list of primarily in-state volunteer workers ready to travel to towns with shortages of contact tracers, nurses and more. But during a press conference on Oct. 15, Democratic Gov. Steve Bullock said the state had exhausted that database, and its nationwide request for National Guard medical staffing hadn’t brought in new workers.
“If you are a registered nurse, licensed practical nurse, paramedic, EMT, CNA or contact tracer, and are able to join our workforce, please do consider joining our team,” Gov. Bullock said.
This month, Kalispell Regional Medical Center in northwestern Montana even stopped quarantining COVID-exposed staff who remain asymptomatic, a change allowed by Centers for Disease Control and Prevention guidelines for health facilities facing staffing shortages.
“That’s very telling for what staffing is going through right now,” said Andrea Lueck, a registered nurse at the center. “We’re so tight that employees are called off of quarantine.”
Financial pressure early in the pandemic led the hospital to furlough staff, but it had to bring most of them back to work because it needs those bodies more than ever. The regional hub is based in Flathead County, which has recorded the state’s second-highest number of active COVID-19 cases.
Mellody Sharpton, a hospital spokesperson, said hospital workers who are exposed to someone infected with the virus are tested within three to five days and monitored for symptoms. The hospital is also pulling in new workers, with 25 traveling health professionals on hand and another 25 temporary ones on the way.
But Ms. Sharpton said the best way to conserve the hospital’s workforce is to stop the disease surge in the community.
Earlier in the pandemic, Central Montana Medical Center in Lewistown, a town of fewer than 6,000, experienced an exodus of part-time workers or those close to retirement who decided their jobs weren’t worth the risk. The facility recently secured two traveling workers, but both backed out because they couldn’t find housing. And, so far, roughly 40 of the hospital’s 322 employees have missed work for reasons connected to COVID-19.
“We’re at a critical staffing shortage and have been since the beginning of COVID,” said Joanie Slaybaugh, Central Montana Medical Center’s director of human resources. “We’re small enough, everybody feels an obligation to protect themselves and to protect each other. But it doesn’t take much to take out our staff.”
Roosevelt County, where roughly 11,000 live on the northeastern edge of Montana, had one of the nation’s highest rates of new cases as of Oct. 15. But by the end of the month, the county health department will lose half of its registered nurses as one person is about to retire and another was hired through a grant that’s ending. That leaves only one registered nurse aside from its director, Patty Presser. The health department already had to close earlier during the pandemic because of COVID exposure and not enough staffers to cover the gap. Now, if Ms. Presser can’t find nurse replacements in time, she hopes volunteers will step in, though she added they typically stay for only a few weeks.
“I need someone to do immunizations for my community, and you don’t become an immunization nurse in 14 days,” she said. “We don’t have the workforce here to deal with this virus, not even right now, and then I’m going to have my best two people go.”
Back in Helena, Dr. Harkins said St. Peter’s Health had to close a specialty outpatient clinic that treats chronic diseases for two weeks at the end of September because the entire staff had to quarantine.
Now the hospital is considering having doctors take turns spending a week working from home, so that if another wave of quarantines hits in the hospital, at least one untainted person can be brought back to work. But that won’t help for some specialties, like the hospital’s sole kidney doctor.
Every time Dr. Harkins’ phone rings, she said, she takes a breath and hopes it’s not another case that will force a whole division to close.
“Because I think immediately of the hundreds of people that need that service and won’t have it for 14 days,” she said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
In Montana, pandemic-induced staffing shortages have shuttered a clinic in the state’s capital, led a northwestern regional hospital to ask employees exposed to COVID-19 to continue to work and emptied a health department 400 miles to the east.
“Just one more person out and we wouldn’t be able to keep the surgeries going,” said Dr. Shelly Harkins, MD, chief medical officer of St. Peter’s Health in Helena, a city of roughly 32,000 where cases continue to spread. “When the virus is just all around you, it’s almost impossible to not be deemed a contact at some point. One case can take out a whole team of people in a blink of an eye.”
In North Dakota, where cases per resident are growing faster than any other state, hospitals may once again curtail elective surgeries and possibly seek government aid to hire more nurses if the situation gets worse, North Dakota Hospital Association President Tim Blasl said.
“How long can we run at this rate with the workforce that we have?” Blasl said. “You can have all the licensed beds you want, but if you don’t have anybody to staff those beds, it doesn’t do you any good.”
The northern Rocky Mountains, Great Plains and Upper Midwest are seeing the highest surge of COVID-19 cases in the nation, as some residents have ignored recommendations for curtailing the virus, such as wearing masks and avoiding large gatherings. Montana, Idaho, Utah, Wyoming, North Dakota, South Dakota, Nebraska, Iowa, and Wisconsin have recently ranked among the top 10 U.S. states in confirmed cases per 100,000 residents over a 7-day period, according to an analysis by the New York Times.
Such coronavirus infections – and the quarantines that occur because of them – are exacerbating the health care worker shortage that existed in these states well before the pandemic. Unlike in the nation’s metropolitan hubs, these outbreaks are scattered across hundreds of miles. And even in these states’ biggest cities, the ranks of medical professionals are in short supply. Specialists and registered nurses are sometimes harder to track down than ventilators, N95 masks or hospital beds. Without enough care providers, patients may not be able to get the medical attention they need.
Hospitals have asked staffers to cover extra shifts and learn new skills. They have brought in temporary workers from other parts of the country and transferred some patients to less-crowded hospitals. But, at St. Peter’s Health, if the hospital’s one kidney doctor gets sick or is told to quarantine, Dr. Harkins doesn’t expect to find a backup.
“We make a point to not have excessive staff because we have an obligation to keep the cost of health care down for a community – we just don’t have a lot of slack in our rope,” Dr. Harkins said. “What we don’t account for is a mass exodus of staff for 14 days.”
Some hospitals are already at patient capacity or are nearly there. That’s not just because of the growing number of COVID-19 patients. Elective surgeries have resumed, and medical emergencies don’t pause for a pandemic.
Some Montana hospitals formed agreements with local affiliates early in the pandemic to share staff if one came up short. But now that the disease is spreading fast – and widely – the hope is that their needs don’t peak all at once.
Montana state officials keep a list of primarily in-state volunteer workers ready to travel to towns with shortages of contact tracers, nurses and more. But during a press conference on Oct. 15, Democratic Gov. Steve Bullock said the state had exhausted that database, and its nationwide request for National Guard medical staffing hadn’t brought in new workers.
“If you are a registered nurse, licensed practical nurse, paramedic, EMT, CNA or contact tracer, and are able to join our workforce, please do consider joining our team,” Gov. Bullock said.
This month, Kalispell Regional Medical Center in northwestern Montana even stopped quarantining COVID-exposed staff who remain asymptomatic, a change allowed by Centers for Disease Control and Prevention guidelines for health facilities facing staffing shortages.
“That’s very telling for what staffing is going through right now,” said Andrea Lueck, a registered nurse at the center. “We’re so tight that employees are called off of quarantine.”
Financial pressure early in the pandemic led the hospital to furlough staff, but it had to bring most of them back to work because it needs those bodies more than ever. The regional hub is based in Flathead County, which has recorded the state’s second-highest number of active COVID-19 cases.
Mellody Sharpton, a hospital spokesperson, said hospital workers who are exposed to someone infected with the virus are tested within three to five days and monitored for symptoms. The hospital is also pulling in new workers, with 25 traveling health professionals on hand and another 25 temporary ones on the way.
But Ms. Sharpton said the best way to conserve the hospital’s workforce is to stop the disease surge in the community.
Earlier in the pandemic, Central Montana Medical Center in Lewistown, a town of fewer than 6,000, experienced an exodus of part-time workers or those close to retirement who decided their jobs weren’t worth the risk. The facility recently secured two traveling workers, but both backed out because they couldn’t find housing. And, so far, roughly 40 of the hospital’s 322 employees have missed work for reasons connected to COVID-19.
“We’re at a critical staffing shortage and have been since the beginning of COVID,” said Joanie Slaybaugh, Central Montana Medical Center’s director of human resources. “We’re small enough, everybody feels an obligation to protect themselves and to protect each other. But it doesn’t take much to take out our staff.”
Roosevelt County, where roughly 11,000 live on the northeastern edge of Montana, had one of the nation’s highest rates of new cases as of Oct. 15. But by the end of the month, the county health department will lose half of its registered nurses as one person is about to retire and another was hired through a grant that’s ending. That leaves only one registered nurse aside from its director, Patty Presser. The health department already had to close earlier during the pandemic because of COVID exposure and not enough staffers to cover the gap. Now, if Ms. Presser can’t find nurse replacements in time, she hopes volunteers will step in, though she added they typically stay for only a few weeks.
“I need someone to do immunizations for my community, and you don’t become an immunization nurse in 14 days,” she said. “We don’t have the workforce here to deal with this virus, not even right now, and then I’m going to have my best two people go.”
Back in Helena, Dr. Harkins said St. Peter’s Health had to close a specialty outpatient clinic that treats chronic diseases for two weeks at the end of September because the entire staff had to quarantine.
Now the hospital is considering having doctors take turns spending a week working from home, so that if another wave of quarantines hits in the hospital, at least one untainted person can be brought back to work. But that won’t help for some specialties, like the hospital’s sole kidney doctor.
Every time Dr. Harkins’ phone rings, she said, she takes a breath and hopes it’s not another case that will force a whole division to close.
“Because I think immediately of the hundreds of people that need that service and won’t have it for 14 days,” she said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Around the world in 24 hours: A snapshot of COVID’s global havoc
Some medical societies feature sessions at their annual meetings that feel like they’re 24 hours long, yet few have the courage to schedule a session that actually runs all day and all night. But the five societies sponsoring the IDWeek conference had that courage. The first 24 hours of the meeting was devoted to the most pressing infectious-disease crisis of the last 100 years: the COVID-19 pandemic. They called it “COVID-19: Chasing the Sun.”
Dr. Fauci predicts a vaccine answer in mid-November
In the first segment, at 10 am Eastern time, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases and the nation’s top infectious-disease expert, began the day by noting that five of the six companies the US invested in to develop a vaccine are conducting phase 3 trials. He said, “we feel confident that we will have an answer likely in mid-November to the beginning of December as to whether we have a safe and effective vaccine”. He added he was “cautiously optimistic” that “we will have a safe and effective vaccine by the end of the year, which we can begin to distribute as we go into 2021.” He highlighted the COVID-19 Prevention Network website for more information on the trials.
Glaring racial health disparities in U.S.
Some of the most glaring health disparities surrounding COVID-19 in the United States were described by Carlos del Rio, MD, professor of medicine at Emory University in Atlanta, Georgia. He pointed out that while white people have about 23 cases per 10,000 population, Blacks have about 62 cases per 10,000, and Latinos have 73 cases per 10,000. While whites don’t see a huge jump in cases until age 80, he said, “among Blacks and Latinos you start seeing that huge increase at a younger age. In fact, starting at age 20, you start seeing a major, major change.”
COVID-19 diagnostics
Audrey Odom John, MD, PhD, chief of pediatric infectious diseases at Children’s Hospital of Philadelphia, is working on a new way of diagnosing COVID-19 infection in children by testing their breath. “We’re really taking advantage of a fundamental biological fact, which is that people stink,” she said. Breath shows the health of the body as a whole, “and it’s easy to see how breath volatiles might arise from a respiratory infection.” Testing breath is easy and inexpensive, which makes it particularly attractive as a potential test globally, she said.
Long-term effects of COVID-19
Post-COVID illness threatens to overwhelm the health system in the United States, even if only 1% of the 8 million people who have been infected have some sort of long-term deficit, “which would be a very conservative estimate,” said John O’Horo, MD, MPH, with the Mayo Clinic in Rochester, Minn. Neurologic dysfunction is going to be a “fairly significant thing to keep an eye on,” he added. Preeti Malani, MD, chief health officer in infectious diseases at the University of Michigan, Ann Arbor, said the emotional aspects of the illness are “striking” and may be the major long-term effect for most patients.
Challenging cases in COVID-19: Through fire and water
In a case presented to panelists during an afternoon session, a Mexican-born woman, 42, presents to urgent care with fever, dyspnea, dry cough, and pleuritic pain, for over a week. Multiple family members have had recent respiratory illness as well. She is obese, on no medications, was not traveling. She’s a nonsmoker and lives in a multigenerational household in the Mission District of San Francisco. Her heart rate is 116, respiratory rate is 36, and her oxygen saturation on room air is 77%. She is admitted to a local hospital and quickly declines, is intubated and started on hydroxychloroquine (HCQ). One day later she is transferred to a hospital for consideration of extracorporeal membrane oxygenation (ECMO).
Panelists were asked a variety of questions about how they would treat this patient. For example, would they continue HCQ? Ravina Kullar, PharmD, MPH, an infectious disease expert from Newport Beach, Calif., answered that she would not continue the HCQ because of lack of evidence and potential harms. Asked whether she would start remdesivir, Dr. Kullar said she would steer her away from that if the patient developed renal failure. Co-moderator Peter Chin-Hong, MD, a medical educator with the University of California, San Francisco, noted that contact tracing will be important as the patient returns to her housing-dense community.
In-hospital infection prevention
The CDC acknowledged aerosol spread of COVID-19 this month, but David Weber, MD, MPH, professor in infectious diseases at the University of North Carolina at Chapel Hill, said, “this does not change anything we need to do in the hospital,” as long as protective pandemic protocols continue to be followed.
There is no evidence, he noted, that SARS-CoV-2 is transmitted far enough that a hospitalized patient could infect people in other rooms or corridors or floors. Opening windows in COVID-19 patients’ rooms is “not an option,” he said, and could be harmful as fungal elements in outside air may introduce new pathogens. The degree to which improved ventilation systems reduce transmission has not been identified and studies are needed to look at that, he said.
Preventing COVID transmission in the community
Mary-Margaret Fill, MD, deputy state epidemiologist in Tennessee, highlighted COVID-19’s spread in prisons. As of mid-October, she said, there are more than 147,000 cases among the U.S. prison population and there have been 1,246 deaths. This translates to a case rate of about 9800 cases per 100,000 people, she said, “double the highest case rate for any state in the country and over three times greater than our national case rate of about 2,500 cases per 100,000 persons.”
Testing varies widely, she noted. For instance, some states test only new prisoners, and some test only when they are symptomatic. One of the strategies to fight this spread is having staff, who go in and out of the community, be assigned to work with only certain groups at a prison. Another is widespread testing of all prisoners. And when prisoners have to leave the prison for care or court dates, a third strategy would be quarantining them upon their return.
COVID-19 vaccines
As the session stretched into the evening in the United States, Mary Marovich, MD, director of vaccine research, AIDS division, with the National Institute of Allergy and Infectious Diseases and the National Institutes of Health, said while each of the government-funded vaccine studies has its own trial, there are standardized objectives for direct comparisons. The studies are being conducted within the same clinical trial networks, and collaborative laboratories apply the same immunoassays and define the infections in the same way. They are all randomized, placebo-controlled trials and all but one have a 30,000-volunteer sample size. She said that while a vaccine is the goal to end the pandemic, monoclonal antibodies, such as those in convalescent plasma, “may serve as a critical bridge.”
The good, the bad, and the ugly during COVID-19 in Latin America
Latin America and the Caribbean are currently the regions hardest hit by COVID-19. Gustavo D. Lopardo, of the Asociacion Panamericana de Infectologia, noted that even before the pandemic Latin America suffered from widespread poverty and inequality. While overcrowding and poverty are determining factors in the spread of the virus, diabetes and obesity – both highly prevalent – are worsening COVID outcomes.
The countries of the region have dealt with asynchronous waves of transmission within their borders by implementing different containment strategies, with dissimilar results. The presenters covered the spectrum of the pandemic, from the “ugly” in Peru, which has the highest mortality rate in the region, to the “good” in Uruguay, where testing is “winning against COVID-19.” Paradoxically, Chile has both the highest cumulative incidence and the lowest case fatality rate of COVID-19 in the region.
In the social and political turmoil imposed by COVID-19, Clóvis Arns da Cunha, MD, president of the Brazilian Society of Infectious Diseases and professor at the Federal University of Paraná, pointed out that “fake news [has become] a public health problem in Brazil” and elsewhere.
Diagnostics and therapeutics in Latin America
Eleven of the 15 countries with the highest death rate in the world are located in Latin America or the Caribbean. Dr. Arns de Cunha pointed out that tests are hard to come by and inadequate diagnostic testing is a major problem. Latin American countries have not been able to compete with the United States and Europe in purchasing polymerase chain reaction test kits from China and South Korea. The test is the best diagnostic tool in the first week of symptoms, but its scale-up has proved to be a challenge in Latin America.
Furthermore, the most sensitive serological markers, CLIA and ECLIA, which perform best after 2 weeks of symptom onset, are not widely available in Latin America where many patients do not have access to the public health system. The detection of silent hypoxemia in symptomatic patients with COVID-19 can save lives; hence, Arns da Cunha praised the program that distributed 100,000 digital oximeters to hundreds of cities in Brazil, targeting vulnerable populations.
The COVID-19 experience in Japan
Takuya Yamagishi, MD, PhD, chief of the Antimicrobial Resistance Research Center at the National Institute of Infectious Diseases in Japan, played an instrumental role in the epidemiological investigation that took place on the Diamond Princess Cruise Ship in February 2020. That COVID-19 outbreak is the largest disease outbreak involving a cruise ship to date, with 712 confirmed COVID-19 cases and 13 deaths.
The ship-based quarantine prompted a massive public health response with unique challenges. In those early days, investigators uncovered important facts about COVID-19 epidemiology, generating hot debates regarding the public health strategy at the time. Notably, the majority of asymptomatically infected persons remained asymptomatic throughout the course of the infection, transmission from asymptomatic cases was almost as likely as transmission from symptomatic cases, and isolation of passengers in their cabins prevented inter-cabin transmission but not intra-cabin transmission.
Swift response in Asia Pacific region
Infectious-disease experts from Taiwan, Singapore, and Australia, who have been at the forefront of clinical care, research, and policy-making, spoke about their experiences.
Taiwan was one of the first countries to adopt a swift response to COVID-19, shortly after they recognized an outbreak of pneumonia of unknown etiology in China and long before the WHO declared a public health emergency, said Ping-Ing Lee, MD, PhD, from the National Taiwan University Children’s Hospital.
The country began onboard health checks on flights from Wuhan as early as Dec. 31, 2019. Dr. Lee attributed Taiwan’s success in prevention and control of COVID-19 to the rigorous use of face masks and environmental disinfection procedures. Regarding the country’s antilockdown stance, he said, “Lockdown may be effective; however, it is associated with a tremendous economic loss.”
In his presentation on remdesivir vs corticosteroids, David Lye, MBBS, said, “I think remdesivir as an antiviral seems to work well given early, but steroids will need to be studied further in terms of its conflicting evidence in multiple well-designed RCTs as well as [their] potential side effects.” He is director of the Infectious Disease Research and Training Office, National Centre for Infectious Diseases, Singapore.
Allen C. Cheng, MBBS, PhD, of Monash University in Melbourne, noted that “control is possible. We seemed to have controlled this twice at the moment with fairly draconian action, but every day does matter.”
China past the first wave
China has already passed the first wave, explained Lei Zhou, MD, of the Chinese Center for Disease Control and Prevention, but there are still some small-scale resurgences. So far a total of four waves have been identified. She also mentioned that contact tracing is intense and highlighted the case of Xinfadi Market in Beijing, the site of an outbreak in June 2020.
Gui-Qiang Wang, MD, from the Department of Infectious Disease, Peking University First Hospital, emphasized the importance of a chest CT for the diagnosis of COVID-19. “In the early stage of the disease, patients may not show any symptoms; however, on CT scan you can see pneumonia. Also, early intervention of high-risk groups and monitoring of warning indicators for disease progression is extremely important,” he said.
“Early antiviral therapy is expected to stop progression, but still needs evaluation,” he said. “Convalescent plasma is safe and effective, but its source is limited; steroid therapy needs to explore appropriate population and timing; and thymosin α is safe, and its effect on outcomes needs large-sample clinical trial.”
Time to Call for an ‘Arab CDC?’
The eastern Mediterranean is geographically, politically, economically, and religiously a very distinct and sensitive region, and “COVID-19 is an added insult to this already frail region of the world,” said Zaid Haddadin, MD, Vanderbilt University Medical Center, Nashville, Tenn.
Poor healthcare and poor public health services are a consequence of weak and fragile governments and infrastructure, the result of war and regional conflicts in many countries. Millions of war refugees live in camps with high population densities and shared facilities, which makes social distancing and community mitigation very challenging. Moreover, the culture includes frequent large social gatherings. Millions of pilgrims visit holy sites in different cities in these countries. There is also movement due to trade and tourism. Travel restrictions are challenging, and there is limited comprehension of precautionary measures.
Najwa Khuri-Bulos, professor of pediatrics and infectious diseases at the University of Jordan, was part of a task force headed by the country’s Ministry of Health. A lockdown was implemented, which helped flatten the curve, but the loosening of restrictions has led to a recent increase in cases. She said, “No country can succeed in controlling spread without the regional collaboration. Perhaps it is time to adopt the call for an Arab CDC.”
Africa is “not out of the woods yet”
The Africa CDC has three key pillars as the foundation for their COVID-19 strategy: preventing transmission, preventing deaths, and preventing social harm, according to Raji Tajudeen, MBBS, FWACP, MPH, head of the agency’s Public Health Institutes and Research Division. Africa, with 1.5 million cases of COVID-19, accounts for 5% of global cases. With a recovery rate of 83% and a case fatality rate of 2.4%, the African continent has fared much better than the rest of the world. “Significant improvements have been made, but we are not out of the woods yet,” he cautioned.
Richard Lessells, PhD, from the University of KwaZulu-Natal, agreed. “Unfortunately, South Africa has not been spared from the worst effects of this pandemic despite what you might read in the press and scientific coverage.” He added, “Over 50% of cases and up to two thirds of the deaths in the African region are coming from South Africa.” A bigger challenge for South Africa has been maintaining essential health services during the COVID-19 pandemic, especially since it is also at the heart of the HIV pandemic. On the brighter side, HIV itself has not emerged as a risk factor for COVID-19 infection or severe disease in South Africa.
Dimie Ogoina, MBBS, FWACP, president of the Nigerian Infectious Diseases Society, stated that COVID-19 has significantly affected access to healthcare in Nigeria, particularly immunizations and antenatal care. Immunization uptake is likely to have dropped by 50% in the country.
Diagnostic pitfalls in COVID-19
Technical errors associated with the SARS-CoV-2 diagnostic pipeline are a major source of variations in diagnosis, explained Jim Huggett, PhD, senior lecturer, analytical microbiology, University of Surrey, Guildford, England. He believes that PCR assays are currently too biased for a single cutoff to be broadly used, and false-positive signals are most likely because of contamination.
Dana Wolf, MD, Clinical Virology Unit, Hadassah Hebrew University Medical Center in Israel, presented a large-scale data analysis of more than 133,000 pooled samples. Such a pooling strategy appeared to be highly efficient for a wide range of prevalence rates (<1% to 6%). “Our empirical evidence strongly projects on the feasibility and benefits of pooling in the current pandemic setting, to enhance continued surveillance, control, and community reopening,” she said.
Corine Geurts van Kessel, MD, PhD, Department of Virology, Erasmus University Rotterdam (the Netherlands), discussing antibodies testing for SARS-CoV-2, pointed out that disease severity can affect testing accuracy. “Reinfection cases tell us that we cannot rely on immunity acquired by natural infection to confer herd immunity,” she said.
Misinformation in the first digital pandemic
The world is not only facing a devastating pandemic, but also an alarming “infodemic” of misinformation. Between January and March 2020, a new COVID-19–related tweet appeared on Twitter every 45 milliseconds. Müge Çevik, MD, MSc, MRCP, an infectious disease clinician, scientist, and science communicator, said that “the greatest challenge for science communication is reaching the audience.”
People have always been skeptical of science reporting by journalists and would rather have scientists communicate with them directly, she noted. Science communication plays a dual role. “On one hand is the need to promote science to a wide audience in order to inform and educate and inspire the next generation of scientists, and on the other hand there is also a need to engage effectively in public dialogue,” she added. Dr. Çevik and colleagues think that “The responsibility of academics should not end with finding the truth. It should end after communicating it.”
Treatment in the ICU
Matteo Bassetti, MD, with the University of Genoa (Italy), who was asked about when to use remdesivir in the intensive care unit and for how long, said, “In the majority of cases, 5 days is probably enough.” However, if there is high viremia, he said, physicians may choose to continue the regimen beyond 5 days. Data show it is important to prescribe this drug for patients with oxygen support in an early phase, within 10 days of the first symptoms, he added. “In the late phase, there is a very limited role for remdesivir, as we know that we are already out of the viremic phase.” He also emphasized that there is no role for hydroxychloroquine or lopinavir-ritonavir.
Breaking the chains of transmission
During the wrap-up session, former US CDC Director Tom Frieden, MD, said, “We’re not even halfway through it” about the pandemic trajectory. “And we have to be very clear that the risk of explosive spread will not end with a vaccine.” He is now president and CEO of Resolve to Save Lives.
Different parts of the world will have very different experiences, Dr. Frieden said, noting that Africa, where 4% of the population is older than 65, has a very different risk level than Europe and the United States, where 10%-20% of people are in older age groups.
“We need a one-two punch,” he noted, first preventing spread, and when it does happen, boxing it in. Mask wearing is essential. “States in the US that mandated universal mask-wearing experienced much more rapid declines (in cases) for every 5 days the mandate was in place.”
Michael Ryan, MD, executive director for the WHO’s Health Emergencies Programme, added, “We need to collectively recommit to winning this game. We know how to break the chains of transmission. We need recommitment to a scientific, societal, and political strategy, and an alliance – a contract – between those entities to try to move us forward.”
This article first appeared on Medscape.com.
Some medical societies feature sessions at their annual meetings that feel like they’re 24 hours long, yet few have the courage to schedule a session that actually runs all day and all night. But the five societies sponsoring the IDWeek conference had that courage. The first 24 hours of the meeting was devoted to the most pressing infectious-disease crisis of the last 100 years: the COVID-19 pandemic. They called it “COVID-19: Chasing the Sun.”
Dr. Fauci predicts a vaccine answer in mid-November
In the first segment, at 10 am Eastern time, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases and the nation’s top infectious-disease expert, began the day by noting that five of the six companies the US invested in to develop a vaccine are conducting phase 3 trials. He said, “we feel confident that we will have an answer likely in mid-November to the beginning of December as to whether we have a safe and effective vaccine”. He added he was “cautiously optimistic” that “we will have a safe and effective vaccine by the end of the year, which we can begin to distribute as we go into 2021.” He highlighted the COVID-19 Prevention Network website for more information on the trials.
Glaring racial health disparities in U.S.
Some of the most glaring health disparities surrounding COVID-19 in the United States were described by Carlos del Rio, MD, professor of medicine at Emory University in Atlanta, Georgia. He pointed out that while white people have about 23 cases per 10,000 population, Blacks have about 62 cases per 10,000, and Latinos have 73 cases per 10,000. While whites don’t see a huge jump in cases until age 80, he said, “among Blacks and Latinos you start seeing that huge increase at a younger age. In fact, starting at age 20, you start seeing a major, major change.”
COVID-19 diagnostics
Audrey Odom John, MD, PhD, chief of pediatric infectious diseases at Children’s Hospital of Philadelphia, is working on a new way of diagnosing COVID-19 infection in children by testing their breath. “We’re really taking advantage of a fundamental biological fact, which is that people stink,” she said. Breath shows the health of the body as a whole, “and it’s easy to see how breath volatiles might arise from a respiratory infection.” Testing breath is easy and inexpensive, which makes it particularly attractive as a potential test globally, she said.
Long-term effects of COVID-19
Post-COVID illness threatens to overwhelm the health system in the United States, even if only 1% of the 8 million people who have been infected have some sort of long-term deficit, “which would be a very conservative estimate,” said John O’Horo, MD, MPH, with the Mayo Clinic in Rochester, Minn. Neurologic dysfunction is going to be a “fairly significant thing to keep an eye on,” he added. Preeti Malani, MD, chief health officer in infectious diseases at the University of Michigan, Ann Arbor, said the emotional aspects of the illness are “striking” and may be the major long-term effect for most patients.
Challenging cases in COVID-19: Through fire and water
In a case presented to panelists during an afternoon session, a Mexican-born woman, 42, presents to urgent care with fever, dyspnea, dry cough, and pleuritic pain, for over a week. Multiple family members have had recent respiratory illness as well. She is obese, on no medications, was not traveling. She’s a nonsmoker and lives in a multigenerational household in the Mission District of San Francisco. Her heart rate is 116, respiratory rate is 36, and her oxygen saturation on room air is 77%. She is admitted to a local hospital and quickly declines, is intubated and started on hydroxychloroquine (HCQ). One day later she is transferred to a hospital for consideration of extracorporeal membrane oxygenation (ECMO).
Panelists were asked a variety of questions about how they would treat this patient. For example, would they continue HCQ? Ravina Kullar, PharmD, MPH, an infectious disease expert from Newport Beach, Calif., answered that she would not continue the HCQ because of lack of evidence and potential harms. Asked whether she would start remdesivir, Dr. Kullar said she would steer her away from that if the patient developed renal failure. Co-moderator Peter Chin-Hong, MD, a medical educator with the University of California, San Francisco, noted that contact tracing will be important as the patient returns to her housing-dense community.
In-hospital infection prevention
The CDC acknowledged aerosol spread of COVID-19 this month, but David Weber, MD, MPH, professor in infectious diseases at the University of North Carolina at Chapel Hill, said, “this does not change anything we need to do in the hospital,” as long as protective pandemic protocols continue to be followed.
There is no evidence, he noted, that SARS-CoV-2 is transmitted far enough that a hospitalized patient could infect people in other rooms or corridors or floors. Opening windows in COVID-19 patients’ rooms is “not an option,” he said, and could be harmful as fungal elements in outside air may introduce new pathogens. The degree to which improved ventilation systems reduce transmission has not been identified and studies are needed to look at that, he said.
Preventing COVID transmission in the community
Mary-Margaret Fill, MD, deputy state epidemiologist in Tennessee, highlighted COVID-19’s spread in prisons. As of mid-October, she said, there are more than 147,000 cases among the U.S. prison population and there have been 1,246 deaths. This translates to a case rate of about 9800 cases per 100,000 people, she said, “double the highest case rate for any state in the country and over three times greater than our national case rate of about 2,500 cases per 100,000 persons.”
Testing varies widely, she noted. For instance, some states test only new prisoners, and some test only when they are symptomatic. One of the strategies to fight this spread is having staff, who go in and out of the community, be assigned to work with only certain groups at a prison. Another is widespread testing of all prisoners. And when prisoners have to leave the prison for care or court dates, a third strategy would be quarantining them upon their return.
COVID-19 vaccines
As the session stretched into the evening in the United States, Mary Marovich, MD, director of vaccine research, AIDS division, with the National Institute of Allergy and Infectious Diseases and the National Institutes of Health, said while each of the government-funded vaccine studies has its own trial, there are standardized objectives for direct comparisons. The studies are being conducted within the same clinical trial networks, and collaborative laboratories apply the same immunoassays and define the infections in the same way. They are all randomized, placebo-controlled trials and all but one have a 30,000-volunteer sample size. She said that while a vaccine is the goal to end the pandemic, monoclonal antibodies, such as those in convalescent plasma, “may serve as a critical bridge.”
The good, the bad, and the ugly during COVID-19 in Latin America
Latin America and the Caribbean are currently the regions hardest hit by COVID-19. Gustavo D. Lopardo, of the Asociacion Panamericana de Infectologia, noted that even before the pandemic Latin America suffered from widespread poverty and inequality. While overcrowding and poverty are determining factors in the spread of the virus, diabetes and obesity – both highly prevalent – are worsening COVID outcomes.
The countries of the region have dealt with asynchronous waves of transmission within their borders by implementing different containment strategies, with dissimilar results. The presenters covered the spectrum of the pandemic, from the “ugly” in Peru, which has the highest mortality rate in the region, to the “good” in Uruguay, where testing is “winning against COVID-19.” Paradoxically, Chile has both the highest cumulative incidence and the lowest case fatality rate of COVID-19 in the region.
In the social and political turmoil imposed by COVID-19, Clóvis Arns da Cunha, MD, president of the Brazilian Society of Infectious Diseases and professor at the Federal University of Paraná, pointed out that “fake news [has become] a public health problem in Brazil” and elsewhere.
Diagnostics and therapeutics in Latin America
Eleven of the 15 countries with the highest death rate in the world are located in Latin America or the Caribbean. Dr. Arns de Cunha pointed out that tests are hard to come by and inadequate diagnostic testing is a major problem. Latin American countries have not been able to compete with the United States and Europe in purchasing polymerase chain reaction test kits from China and South Korea. The test is the best diagnostic tool in the first week of symptoms, but its scale-up has proved to be a challenge in Latin America.
Furthermore, the most sensitive serological markers, CLIA and ECLIA, which perform best after 2 weeks of symptom onset, are not widely available in Latin America where many patients do not have access to the public health system. The detection of silent hypoxemia in symptomatic patients with COVID-19 can save lives; hence, Arns da Cunha praised the program that distributed 100,000 digital oximeters to hundreds of cities in Brazil, targeting vulnerable populations.
The COVID-19 experience in Japan
Takuya Yamagishi, MD, PhD, chief of the Antimicrobial Resistance Research Center at the National Institute of Infectious Diseases in Japan, played an instrumental role in the epidemiological investigation that took place on the Diamond Princess Cruise Ship in February 2020. That COVID-19 outbreak is the largest disease outbreak involving a cruise ship to date, with 712 confirmed COVID-19 cases and 13 deaths.
The ship-based quarantine prompted a massive public health response with unique challenges. In those early days, investigators uncovered important facts about COVID-19 epidemiology, generating hot debates regarding the public health strategy at the time. Notably, the majority of asymptomatically infected persons remained asymptomatic throughout the course of the infection, transmission from asymptomatic cases was almost as likely as transmission from symptomatic cases, and isolation of passengers in their cabins prevented inter-cabin transmission but not intra-cabin transmission.
Swift response in Asia Pacific region
Infectious-disease experts from Taiwan, Singapore, and Australia, who have been at the forefront of clinical care, research, and policy-making, spoke about their experiences.
Taiwan was one of the first countries to adopt a swift response to COVID-19, shortly after they recognized an outbreak of pneumonia of unknown etiology in China and long before the WHO declared a public health emergency, said Ping-Ing Lee, MD, PhD, from the National Taiwan University Children’s Hospital.
The country began onboard health checks on flights from Wuhan as early as Dec. 31, 2019. Dr. Lee attributed Taiwan’s success in prevention and control of COVID-19 to the rigorous use of face masks and environmental disinfection procedures. Regarding the country’s antilockdown stance, he said, “Lockdown may be effective; however, it is associated with a tremendous economic loss.”
In his presentation on remdesivir vs corticosteroids, David Lye, MBBS, said, “I think remdesivir as an antiviral seems to work well given early, but steroids will need to be studied further in terms of its conflicting evidence in multiple well-designed RCTs as well as [their] potential side effects.” He is director of the Infectious Disease Research and Training Office, National Centre for Infectious Diseases, Singapore.
Allen C. Cheng, MBBS, PhD, of Monash University in Melbourne, noted that “control is possible. We seemed to have controlled this twice at the moment with fairly draconian action, but every day does matter.”
China past the first wave
China has already passed the first wave, explained Lei Zhou, MD, of the Chinese Center for Disease Control and Prevention, but there are still some small-scale resurgences. So far a total of four waves have been identified. She also mentioned that contact tracing is intense and highlighted the case of Xinfadi Market in Beijing, the site of an outbreak in June 2020.
Gui-Qiang Wang, MD, from the Department of Infectious Disease, Peking University First Hospital, emphasized the importance of a chest CT for the diagnosis of COVID-19. “In the early stage of the disease, patients may not show any symptoms; however, on CT scan you can see pneumonia. Also, early intervention of high-risk groups and monitoring of warning indicators for disease progression is extremely important,” he said.
“Early antiviral therapy is expected to stop progression, but still needs evaluation,” he said. “Convalescent plasma is safe and effective, but its source is limited; steroid therapy needs to explore appropriate population and timing; and thymosin α is safe, and its effect on outcomes needs large-sample clinical trial.”
Time to Call for an ‘Arab CDC?’
The eastern Mediterranean is geographically, politically, economically, and religiously a very distinct and sensitive region, and “COVID-19 is an added insult to this already frail region of the world,” said Zaid Haddadin, MD, Vanderbilt University Medical Center, Nashville, Tenn.
Poor healthcare and poor public health services are a consequence of weak and fragile governments and infrastructure, the result of war and regional conflicts in many countries. Millions of war refugees live in camps with high population densities and shared facilities, which makes social distancing and community mitigation very challenging. Moreover, the culture includes frequent large social gatherings. Millions of pilgrims visit holy sites in different cities in these countries. There is also movement due to trade and tourism. Travel restrictions are challenging, and there is limited comprehension of precautionary measures.
Najwa Khuri-Bulos, professor of pediatrics and infectious diseases at the University of Jordan, was part of a task force headed by the country’s Ministry of Health. A lockdown was implemented, which helped flatten the curve, but the loosening of restrictions has led to a recent increase in cases. She said, “No country can succeed in controlling spread without the regional collaboration. Perhaps it is time to adopt the call for an Arab CDC.”
Africa is “not out of the woods yet”
The Africa CDC has three key pillars as the foundation for their COVID-19 strategy: preventing transmission, preventing deaths, and preventing social harm, according to Raji Tajudeen, MBBS, FWACP, MPH, head of the agency’s Public Health Institutes and Research Division. Africa, with 1.5 million cases of COVID-19, accounts for 5% of global cases. With a recovery rate of 83% and a case fatality rate of 2.4%, the African continent has fared much better than the rest of the world. “Significant improvements have been made, but we are not out of the woods yet,” he cautioned.
Richard Lessells, PhD, from the University of KwaZulu-Natal, agreed. “Unfortunately, South Africa has not been spared from the worst effects of this pandemic despite what you might read in the press and scientific coverage.” He added, “Over 50% of cases and up to two thirds of the deaths in the African region are coming from South Africa.” A bigger challenge for South Africa has been maintaining essential health services during the COVID-19 pandemic, especially since it is also at the heart of the HIV pandemic. On the brighter side, HIV itself has not emerged as a risk factor for COVID-19 infection or severe disease in South Africa.
Dimie Ogoina, MBBS, FWACP, president of the Nigerian Infectious Diseases Society, stated that COVID-19 has significantly affected access to healthcare in Nigeria, particularly immunizations and antenatal care. Immunization uptake is likely to have dropped by 50% in the country.
Diagnostic pitfalls in COVID-19
Technical errors associated with the SARS-CoV-2 diagnostic pipeline are a major source of variations in diagnosis, explained Jim Huggett, PhD, senior lecturer, analytical microbiology, University of Surrey, Guildford, England. He believes that PCR assays are currently too biased for a single cutoff to be broadly used, and false-positive signals are most likely because of contamination.
Dana Wolf, MD, Clinical Virology Unit, Hadassah Hebrew University Medical Center in Israel, presented a large-scale data analysis of more than 133,000 pooled samples. Such a pooling strategy appeared to be highly efficient for a wide range of prevalence rates (<1% to 6%). “Our empirical evidence strongly projects on the feasibility and benefits of pooling in the current pandemic setting, to enhance continued surveillance, control, and community reopening,” she said.
Corine Geurts van Kessel, MD, PhD, Department of Virology, Erasmus University Rotterdam (the Netherlands), discussing antibodies testing for SARS-CoV-2, pointed out that disease severity can affect testing accuracy. “Reinfection cases tell us that we cannot rely on immunity acquired by natural infection to confer herd immunity,” she said.
Misinformation in the first digital pandemic
The world is not only facing a devastating pandemic, but also an alarming “infodemic” of misinformation. Between January and March 2020, a new COVID-19–related tweet appeared on Twitter every 45 milliseconds. Müge Çevik, MD, MSc, MRCP, an infectious disease clinician, scientist, and science communicator, said that “the greatest challenge for science communication is reaching the audience.”
People have always been skeptical of science reporting by journalists and would rather have scientists communicate with them directly, she noted. Science communication plays a dual role. “On one hand is the need to promote science to a wide audience in order to inform and educate and inspire the next generation of scientists, and on the other hand there is also a need to engage effectively in public dialogue,” she added. Dr. Çevik and colleagues think that “The responsibility of academics should not end with finding the truth. It should end after communicating it.”
Treatment in the ICU
Matteo Bassetti, MD, with the University of Genoa (Italy), who was asked about when to use remdesivir in the intensive care unit and for how long, said, “In the majority of cases, 5 days is probably enough.” However, if there is high viremia, he said, physicians may choose to continue the regimen beyond 5 days. Data show it is important to prescribe this drug for patients with oxygen support in an early phase, within 10 days of the first symptoms, he added. “In the late phase, there is a very limited role for remdesivir, as we know that we are already out of the viremic phase.” He also emphasized that there is no role for hydroxychloroquine or lopinavir-ritonavir.
Breaking the chains of transmission
During the wrap-up session, former US CDC Director Tom Frieden, MD, said, “We’re not even halfway through it” about the pandemic trajectory. “And we have to be very clear that the risk of explosive spread will not end with a vaccine.” He is now president and CEO of Resolve to Save Lives.
Different parts of the world will have very different experiences, Dr. Frieden said, noting that Africa, where 4% of the population is older than 65, has a very different risk level than Europe and the United States, where 10%-20% of people are in older age groups.
“We need a one-two punch,” he noted, first preventing spread, and when it does happen, boxing it in. Mask wearing is essential. “States in the US that mandated universal mask-wearing experienced much more rapid declines (in cases) for every 5 days the mandate was in place.”
Michael Ryan, MD, executive director for the WHO’s Health Emergencies Programme, added, “We need to collectively recommit to winning this game. We know how to break the chains of transmission. We need recommitment to a scientific, societal, and political strategy, and an alliance – a contract – between those entities to try to move us forward.”
This article first appeared on Medscape.com.
Some medical societies feature sessions at their annual meetings that feel like they’re 24 hours long, yet few have the courage to schedule a session that actually runs all day and all night. But the five societies sponsoring the IDWeek conference had that courage. The first 24 hours of the meeting was devoted to the most pressing infectious-disease crisis of the last 100 years: the COVID-19 pandemic. They called it “COVID-19: Chasing the Sun.”
Dr. Fauci predicts a vaccine answer in mid-November
In the first segment, at 10 am Eastern time, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases and the nation’s top infectious-disease expert, began the day by noting that five of the six companies the US invested in to develop a vaccine are conducting phase 3 trials. He said, “we feel confident that we will have an answer likely in mid-November to the beginning of December as to whether we have a safe and effective vaccine”. He added he was “cautiously optimistic” that “we will have a safe and effective vaccine by the end of the year, which we can begin to distribute as we go into 2021.” He highlighted the COVID-19 Prevention Network website for more information on the trials.
Glaring racial health disparities in U.S.
Some of the most glaring health disparities surrounding COVID-19 in the United States were described by Carlos del Rio, MD, professor of medicine at Emory University in Atlanta, Georgia. He pointed out that while white people have about 23 cases per 10,000 population, Blacks have about 62 cases per 10,000, and Latinos have 73 cases per 10,000. While whites don’t see a huge jump in cases until age 80, he said, “among Blacks and Latinos you start seeing that huge increase at a younger age. In fact, starting at age 20, you start seeing a major, major change.”
COVID-19 diagnostics
Audrey Odom John, MD, PhD, chief of pediatric infectious diseases at Children’s Hospital of Philadelphia, is working on a new way of diagnosing COVID-19 infection in children by testing their breath. “We’re really taking advantage of a fundamental biological fact, which is that people stink,” she said. Breath shows the health of the body as a whole, “and it’s easy to see how breath volatiles might arise from a respiratory infection.” Testing breath is easy and inexpensive, which makes it particularly attractive as a potential test globally, she said.
Long-term effects of COVID-19
Post-COVID illness threatens to overwhelm the health system in the United States, even if only 1% of the 8 million people who have been infected have some sort of long-term deficit, “which would be a very conservative estimate,” said John O’Horo, MD, MPH, with the Mayo Clinic in Rochester, Minn. Neurologic dysfunction is going to be a “fairly significant thing to keep an eye on,” he added. Preeti Malani, MD, chief health officer in infectious diseases at the University of Michigan, Ann Arbor, said the emotional aspects of the illness are “striking” and may be the major long-term effect for most patients.
Challenging cases in COVID-19: Through fire and water
In a case presented to panelists during an afternoon session, a Mexican-born woman, 42, presents to urgent care with fever, dyspnea, dry cough, and pleuritic pain, for over a week. Multiple family members have had recent respiratory illness as well. She is obese, on no medications, was not traveling. She’s a nonsmoker and lives in a multigenerational household in the Mission District of San Francisco. Her heart rate is 116, respiratory rate is 36, and her oxygen saturation on room air is 77%. She is admitted to a local hospital and quickly declines, is intubated and started on hydroxychloroquine (HCQ). One day later she is transferred to a hospital for consideration of extracorporeal membrane oxygenation (ECMO).
Panelists were asked a variety of questions about how they would treat this patient. For example, would they continue HCQ? Ravina Kullar, PharmD, MPH, an infectious disease expert from Newport Beach, Calif., answered that she would not continue the HCQ because of lack of evidence and potential harms. Asked whether she would start remdesivir, Dr. Kullar said she would steer her away from that if the patient developed renal failure. Co-moderator Peter Chin-Hong, MD, a medical educator with the University of California, San Francisco, noted that contact tracing will be important as the patient returns to her housing-dense community.
In-hospital infection prevention
The CDC acknowledged aerosol spread of COVID-19 this month, but David Weber, MD, MPH, professor in infectious diseases at the University of North Carolina at Chapel Hill, said, “this does not change anything we need to do in the hospital,” as long as protective pandemic protocols continue to be followed.
There is no evidence, he noted, that SARS-CoV-2 is transmitted far enough that a hospitalized patient could infect people in other rooms or corridors or floors. Opening windows in COVID-19 patients’ rooms is “not an option,” he said, and could be harmful as fungal elements in outside air may introduce new pathogens. The degree to which improved ventilation systems reduce transmission has not been identified and studies are needed to look at that, he said.
Preventing COVID transmission in the community
Mary-Margaret Fill, MD, deputy state epidemiologist in Tennessee, highlighted COVID-19’s spread in prisons. As of mid-October, she said, there are more than 147,000 cases among the U.S. prison population and there have been 1,246 deaths. This translates to a case rate of about 9800 cases per 100,000 people, she said, “double the highest case rate for any state in the country and over three times greater than our national case rate of about 2,500 cases per 100,000 persons.”
Testing varies widely, she noted. For instance, some states test only new prisoners, and some test only when they are symptomatic. One of the strategies to fight this spread is having staff, who go in and out of the community, be assigned to work with only certain groups at a prison. Another is widespread testing of all prisoners. And when prisoners have to leave the prison for care or court dates, a third strategy would be quarantining them upon their return.
COVID-19 vaccines
As the session stretched into the evening in the United States, Mary Marovich, MD, director of vaccine research, AIDS division, with the National Institute of Allergy and Infectious Diseases and the National Institutes of Health, said while each of the government-funded vaccine studies has its own trial, there are standardized objectives for direct comparisons. The studies are being conducted within the same clinical trial networks, and collaborative laboratories apply the same immunoassays and define the infections in the same way. They are all randomized, placebo-controlled trials and all but one have a 30,000-volunteer sample size. She said that while a vaccine is the goal to end the pandemic, monoclonal antibodies, such as those in convalescent plasma, “may serve as a critical bridge.”
The good, the bad, and the ugly during COVID-19 in Latin America
Latin America and the Caribbean are currently the regions hardest hit by COVID-19. Gustavo D. Lopardo, of the Asociacion Panamericana de Infectologia, noted that even before the pandemic Latin America suffered from widespread poverty and inequality. While overcrowding and poverty are determining factors in the spread of the virus, diabetes and obesity – both highly prevalent – are worsening COVID outcomes.
The countries of the region have dealt with asynchronous waves of transmission within their borders by implementing different containment strategies, with dissimilar results. The presenters covered the spectrum of the pandemic, from the “ugly” in Peru, which has the highest mortality rate in the region, to the “good” in Uruguay, where testing is “winning against COVID-19.” Paradoxically, Chile has both the highest cumulative incidence and the lowest case fatality rate of COVID-19 in the region.
In the social and political turmoil imposed by COVID-19, Clóvis Arns da Cunha, MD, president of the Brazilian Society of Infectious Diseases and professor at the Federal University of Paraná, pointed out that “fake news [has become] a public health problem in Brazil” and elsewhere.
Diagnostics and therapeutics in Latin America
Eleven of the 15 countries with the highest death rate in the world are located in Latin America or the Caribbean. Dr. Arns de Cunha pointed out that tests are hard to come by and inadequate diagnostic testing is a major problem. Latin American countries have not been able to compete with the United States and Europe in purchasing polymerase chain reaction test kits from China and South Korea. The test is the best diagnostic tool in the first week of symptoms, but its scale-up has proved to be a challenge in Latin America.
Furthermore, the most sensitive serological markers, CLIA and ECLIA, which perform best after 2 weeks of symptom onset, are not widely available in Latin America where many patients do not have access to the public health system. The detection of silent hypoxemia in symptomatic patients with COVID-19 can save lives; hence, Arns da Cunha praised the program that distributed 100,000 digital oximeters to hundreds of cities in Brazil, targeting vulnerable populations.
The COVID-19 experience in Japan
Takuya Yamagishi, MD, PhD, chief of the Antimicrobial Resistance Research Center at the National Institute of Infectious Diseases in Japan, played an instrumental role in the epidemiological investigation that took place on the Diamond Princess Cruise Ship in February 2020. That COVID-19 outbreak is the largest disease outbreak involving a cruise ship to date, with 712 confirmed COVID-19 cases and 13 deaths.
The ship-based quarantine prompted a massive public health response with unique challenges. In those early days, investigators uncovered important facts about COVID-19 epidemiology, generating hot debates regarding the public health strategy at the time. Notably, the majority of asymptomatically infected persons remained asymptomatic throughout the course of the infection, transmission from asymptomatic cases was almost as likely as transmission from symptomatic cases, and isolation of passengers in their cabins prevented inter-cabin transmission but not intra-cabin transmission.
Swift response in Asia Pacific region
Infectious-disease experts from Taiwan, Singapore, and Australia, who have been at the forefront of clinical care, research, and policy-making, spoke about their experiences.
Taiwan was one of the first countries to adopt a swift response to COVID-19, shortly after they recognized an outbreak of pneumonia of unknown etiology in China and long before the WHO declared a public health emergency, said Ping-Ing Lee, MD, PhD, from the National Taiwan University Children’s Hospital.
The country began onboard health checks on flights from Wuhan as early as Dec. 31, 2019. Dr. Lee attributed Taiwan’s success in prevention and control of COVID-19 to the rigorous use of face masks and environmental disinfection procedures. Regarding the country’s antilockdown stance, he said, “Lockdown may be effective; however, it is associated with a tremendous economic loss.”
In his presentation on remdesivir vs corticosteroids, David Lye, MBBS, said, “I think remdesivir as an antiviral seems to work well given early, but steroids will need to be studied further in terms of its conflicting evidence in multiple well-designed RCTs as well as [their] potential side effects.” He is director of the Infectious Disease Research and Training Office, National Centre for Infectious Diseases, Singapore.
Allen C. Cheng, MBBS, PhD, of Monash University in Melbourne, noted that “control is possible. We seemed to have controlled this twice at the moment with fairly draconian action, but every day does matter.”
China past the first wave
China has already passed the first wave, explained Lei Zhou, MD, of the Chinese Center for Disease Control and Prevention, but there are still some small-scale resurgences. So far a total of four waves have been identified. She also mentioned that contact tracing is intense and highlighted the case of Xinfadi Market in Beijing, the site of an outbreak in June 2020.
Gui-Qiang Wang, MD, from the Department of Infectious Disease, Peking University First Hospital, emphasized the importance of a chest CT for the diagnosis of COVID-19. “In the early stage of the disease, patients may not show any symptoms; however, on CT scan you can see pneumonia. Also, early intervention of high-risk groups and monitoring of warning indicators for disease progression is extremely important,” he said.
“Early antiviral therapy is expected to stop progression, but still needs evaluation,” he said. “Convalescent plasma is safe and effective, but its source is limited; steroid therapy needs to explore appropriate population and timing; and thymosin α is safe, and its effect on outcomes needs large-sample clinical trial.”
Time to Call for an ‘Arab CDC?’
The eastern Mediterranean is geographically, politically, economically, and religiously a very distinct and sensitive region, and “COVID-19 is an added insult to this already frail region of the world,” said Zaid Haddadin, MD, Vanderbilt University Medical Center, Nashville, Tenn.
Poor healthcare and poor public health services are a consequence of weak and fragile governments and infrastructure, the result of war and regional conflicts in many countries. Millions of war refugees live in camps with high population densities and shared facilities, which makes social distancing and community mitigation very challenging. Moreover, the culture includes frequent large social gatherings. Millions of pilgrims visit holy sites in different cities in these countries. There is also movement due to trade and tourism. Travel restrictions are challenging, and there is limited comprehension of precautionary measures.
Najwa Khuri-Bulos, professor of pediatrics and infectious diseases at the University of Jordan, was part of a task force headed by the country’s Ministry of Health. A lockdown was implemented, which helped flatten the curve, but the loosening of restrictions has led to a recent increase in cases. She said, “No country can succeed in controlling spread without the regional collaboration. Perhaps it is time to adopt the call for an Arab CDC.”
Africa is “not out of the woods yet”
The Africa CDC has three key pillars as the foundation for their COVID-19 strategy: preventing transmission, preventing deaths, and preventing social harm, according to Raji Tajudeen, MBBS, FWACP, MPH, head of the agency’s Public Health Institutes and Research Division. Africa, with 1.5 million cases of COVID-19, accounts for 5% of global cases. With a recovery rate of 83% and a case fatality rate of 2.4%, the African continent has fared much better than the rest of the world. “Significant improvements have been made, but we are not out of the woods yet,” he cautioned.
Richard Lessells, PhD, from the University of KwaZulu-Natal, agreed. “Unfortunately, South Africa has not been spared from the worst effects of this pandemic despite what you might read in the press and scientific coverage.” He added, “Over 50% of cases and up to two thirds of the deaths in the African region are coming from South Africa.” A bigger challenge for South Africa has been maintaining essential health services during the COVID-19 pandemic, especially since it is also at the heart of the HIV pandemic. On the brighter side, HIV itself has not emerged as a risk factor for COVID-19 infection or severe disease in South Africa.
Dimie Ogoina, MBBS, FWACP, president of the Nigerian Infectious Diseases Society, stated that COVID-19 has significantly affected access to healthcare in Nigeria, particularly immunizations and antenatal care. Immunization uptake is likely to have dropped by 50% in the country.
Diagnostic pitfalls in COVID-19
Technical errors associated with the SARS-CoV-2 diagnostic pipeline are a major source of variations in diagnosis, explained Jim Huggett, PhD, senior lecturer, analytical microbiology, University of Surrey, Guildford, England. He believes that PCR assays are currently too biased for a single cutoff to be broadly used, and false-positive signals are most likely because of contamination.
Dana Wolf, MD, Clinical Virology Unit, Hadassah Hebrew University Medical Center in Israel, presented a large-scale data analysis of more than 133,000 pooled samples. Such a pooling strategy appeared to be highly efficient for a wide range of prevalence rates (<1% to 6%). “Our empirical evidence strongly projects on the feasibility and benefits of pooling in the current pandemic setting, to enhance continued surveillance, control, and community reopening,” she said.
Corine Geurts van Kessel, MD, PhD, Department of Virology, Erasmus University Rotterdam (the Netherlands), discussing antibodies testing for SARS-CoV-2, pointed out that disease severity can affect testing accuracy. “Reinfection cases tell us that we cannot rely on immunity acquired by natural infection to confer herd immunity,” she said.
Misinformation in the first digital pandemic
The world is not only facing a devastating pandemic, but also an alarming “infodemic” of misinformation. Between January and March 2020, a new COVID-19–related tweet appeared on Twitter every 45 milliseconds. Müge Çevik, MD, MSc, MRCP, an infectious disease clinician, scientist, and science communicator, said that “the greatest challenge for science communication is reaching the audience.”
People have always been skeptical of science reporting by journalists and would rather have scientists communicate with them directly, she noted. Science communication plays a dual role. “On one hand is the need to promote science to a wide audience in order to inform and educate and inspire the next generation of scientists, and on the other hand there is also a need to engage effectively in public dialogue,” she added. Dr. Çevik and colleagues think that “The responsibility of academics should not end with finding the truth. It should end after communicating it.”
Treatment in the ICU
Matteo Bassetti, MD, with the University of Genoa (Italy), who was asked about when to use remdesivir in the intensive care unit and for how long, said, “In the majority of cases, 5 days is probably enough.” However, if there is high viremia, he said, physicians may choose to continue the regimen beyond 5 days. Data show it is important to prescribe this drug for patients with oxygen support in an early phase, within 10 days of the first symptoms, he added. “In the late phase, there is a very limited role for remdesivir, as we know that we are already out of the viremic phase.” He also emphasized that there is no role for hydroxychloroquine or lopinavir-ritonavir.
Breaking the chains of transmission
During the wrap-up session, former US CDC Director Tom Frieden, MD, said, “We’re not even halfway through it” about the pandemic trajectory. “And we have to be very clear that the risk of explosive spread will not end with a vaccine.” He is now president and CEO of Resolve to Save Lives.
Different parts of the world will have very different experiences, Dr. Frieden said, noting that Africa, where 4% of the population is older than 65, has a very different risk level than Europe and the United States, where 10%-20% of people are in older age groups.
“We need a one-two punch,” he noted, first preventing spread, and when it does happen, boxing it in. Mask wearing is essential. “States in the US that mandated universal mask-wearing experienced much more rapid declines (in cases) for every 5 days the mandate was in place.”
Michael Ryan, MD, executive director for the WHO’s Health Emergencies Programme, added, “We need to collectively recommit to winning this game. We know how to break the chains of transmission. We need recommitment to a scientific, societal, and political strategy, and an alliance – a contract – between those entities to try to move us forward.”
This article first appeared on Medscape.com.
FROM IDWEEK 2020
Unneeded meds at discharge could cause harm
A significant number of patients leave the hospital with inappropriate drugs because of a lack of medication reconciliation at discharge, new research shows.
Proton pump inhibitors – known to have adverse effects, such as fractures, osteoporosis, and progressive kidney disease – make up 30% of inappropriate prescriptions at discharge.
“These medications can have a significant toxic effect, especially in the long term,” said Harsh Patel, MD, from Medical City Healthcare in Fort Worth, Tex.
And “when we interviewed patients, they were unable to recall ever partaking in a pulmonary function test or endoscopy to warrant the medications,” he said in an interview.
For their retrospective chart review, Dr. Patel and colleagues assessed patients admitted to the ICU in 13 hospitals over a 6-month period in northern Texas. Of the 12,930 patients, 2,557 had not previously received but were prescribed during their hospital stay a bronchodilator, a proton pump inhibitor, or an H2 receptor agonist.
Of those 2,557 patients, 26.8% were discharged on a proton pump inhibitor, 8.4% on an H2 receptor agonist, and 5.49% on a bronchodilator.
There were no corresponding diseases or diagnoses to justify continued use, Dr. Patel said during his presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
Button fatigue
The problem stems from a technology disconnect when patients are transferred from the ICU to the general population.
Doctors expect that the medications will be reconciled at discharge, said one of the study investigators, Prashanth Reddy, MD, from Medical City Las Colinas (Tex.).
But in some instances, clinicians unfamiliar with the case click through the electronic health record to get the patient “out of the ICU to the floor,” he explained. “They don’t always know what medications to keep.”
“They may have button fatigue, so they just accept and continue,” Dr. Reddy said in an interviews.
In light of these findings, the team has kick-started a project to improve transition out of the ICU and minimize overprescription at discharge.
“This is the kind of a problem where we thought we could have some influence,” said Dr. Reddy.
One solution would be to put “stop orders” on potentially harmful medications. “But we don’t want to increase button fatigue even more, so we have to find a happy medium,” he said. “It’s going to take a while to formulate the best path on this.”
The inclusion of pharmacy residents in rounds could make a difference. “When we rounded with pharmacy residents, these issues got addressed,” Dr. Patel said. The pharmacy residents often asked: “Can we go over the meds? Does this person really need all this?”
Medication reconciliations not only have a positive effect on a patient’s health, they can also cut costs by eliminating unneeded drugs. And “patients are always happy to hear we’re taking them off a drug,” Dr. Patel added.
He said he remembers one of his mentors telling him that, if he could get his patients down to five medications, “then you’ve achieved success as a physician.”
“I’m still working toward that,” he said. “The end goal should sometimes be, less is more.”
COPD patients overprescribed home oxygen
In addition to medications, home oxygen therapy is often prescribed when patients are discharged from the hospital.
A study of 69 patients who were continued on home oxygen therapy after hospitalization for an exacerbation of chronic obstructive pulmonary disease was presented by Analisa Taylor, MD, from the University of Illinois at Chicago.
Despite guideline recommendations that patients be reassessed within 90 days of discharge, only 38 patients in the cohort were reassessed, and “28 were considered eligible for discontinuation,” she said during her presentation.
However, “of those, only four were ultimately discontinued,” she reported.
The reason for this gap needs to be examined, noted Dr. Taylor, suggesting that “perhaps clinical inertia plays a role in the continuation of previously prescribed therapy despite a lack of ongoing clinical benefit.”
A version of this article originally appeared on Medscape.com.
A significant number of patients leave the hospital with inappropriate drugs because of a lack of medication reconciliation at discharge, new research shows.
Proton pump inhibitors – known to have adverse effects, such as fractures, osteoporosis, and progressive kidney disease – make up 30% of inappropriate prescriptions at discharge.
“These medications can have a significant toxic effect, especially in the long term,” said Harsh Patel, MD, from Medical City Healthcare in Fort Worth, Tex.
And “when we interviewed patients, they were unable to recall ever partaking in a pulmonary function test or endoscopy to warrant the medications,” he said in an interview.
For their retrospective chart review, Dr. Patel and colleagues assessed patients admitted to the ICU in 13 hospitals over a 6-month period in northern Texas. Of the 12,930 patients, 2,557 had not previously received but were prescribed during their hospital stay a bronchodilator, a proton pump inhibitor, or an H2 receptor agonist.
Of those 2,557 patients, 26.8% were discharged on a proton pump inhibitor, 8.4% on an H2 receptor agonist, and 5.49% on a bronchodilator.
There were no corresponding diseases or diagnoses to justify continued use, Dr. Patel said during his presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
Button fatigue
The problem stems from a technology disconnect when patients are transferred from the ICU to the general population.
Doctors expect that the medications will be reconciled at discharge, said one of the study investigators, Prashanth Reddy, MD, from Medical City Las Colinas (Tex.).
But in some instances, clinicians unfamiliar with the case click through the electronic health record to get the patient “out of the ICU to the floor,” he explained. “They don’t always know what medications to keep.”
“They may have button fatigue, so they just accept and continue,” Dr. Reddy said in an interviews.
In light of these findings, the team has kick-started a project to improve transition out of the ICU and minimize overprescription at discharge.
“This is the kind of a problem where we thought we could have some influence,” said Dr. Reddy.
One solution would be to put “stop orders” on potentially harmful medications. “But we don’t want to increase button fatigue even more, so we have to find a happy medium,” he said. “It’s going to take a while to formulate the best path on this.”
The inclusion of pharmacy residents in rounds could make a difference. “When we rounded with pharmacy residents, these issues got addressed,” Dr. Patel said. The pharmacy residents often asked: “Can we go over the meds? Does this person really need all this?”
Medication reconciliations not only have a positive effect on a patient’s health, they can also cut costs by eliminating unneeded drugs. And “patients are always happy to hear we’re taking them off a drug,” Dr. Patel added.
He said he remembers one of his mentors telling him that, if he could get his patients down to five medications, “then you’ve achieved success as a physician.”
“I’m still working toward that,” he said. “The end goal should sometimes be, less is more.”
COPD patients overprescribed home oxygen
In addition to medications, home oxygen therapy is often prescribed when patients are discharged from the hospital.
A study of 69 patients who were continued on home oxygen therapy after hospitalization for an exacerbation of chronic obstructive pulmonary disease was presented by Analisa Taylor, MD, from the University of Illinois at Chicago.
Despite guideline recommendations that patients be reassessed within 90 days of discharge, only 38 patients in the cohort were reassessed, and “28 were considered eligible for discontinuation,” she said during her presentation.
However, “of those, only four were ultimately discontinued,” she reported.
The reason for this gap needs to be examined, noted Dr. Taylor, suggesting that “perhaps clinical inertia plays a role in the continuation of previously prescribed therapy despite a lack of ongoing clinical benefit.”
A version of this article originally appeared on Medscape.com.
A significant number of patients leave the hospital with inappropriate drugs because of a lack of medication reconciliation at discharge, new research shows.
Proton pump inhibitors – known to have adverse effects, such as fractures, osteoporosis, and progressive kidney disease – make up 30% of inappropriate prescriptions at discharge.
“These medications can have a significant toxic effect, especially in the long term,” said Harsh Patel, MD, from Medical City Healthcare in Fort Worth, Tex.
And “when we interviewed patients, they were unable to recall ever partaking in a pulmonary function test or endoscopy to warrant the medications,” he said in an interview.
For their retrospective chart review, Dr. Patel and colleagues assessed patients admitted to the ICU in 13 hospitals over a 6-month period in northern Texas. Of the 12,930 patients, 2,557 had not previously received but were prescribed during their hospital stay a bronchodilator, a proton pump inhibitor, or an H2 receptor agonist.
Of those 2,557 patients, 26.8% were discharged on a proton pump inhibitor, 8.4% on an H2 receptor agonist, and 5.49% on a bronchodilator.
There were no corresponding diseases or diagnoses to justify continued use, Dr. Patel said during his presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
Button fatigue
The problem stems from a technology disconnect when patients are transferred from the ICU to the general population.
Doctors expect that the medications will be reconciled at discharge, said one of the study investigators, Prashanth Reddy, MD, from Medical City Las Colinas (Tex.).
But in some instances, clinicians unfamiliar with the case click through the electronic health record to get the patient “out of the ICU to the floor,” he explained. “They don’t always know what medications to keep.”
“They may have button fatigue, so they just accept and continue,” Dr. Reddy said in an interviews.
In light of these findings, the team has kick-started a project to improve transition out of the ICU and minimize overprescription at discharge.
“This is the kind of a problem where we thought we could have some influence,” said Dr. Reddy.
One solution would be to put “stop orders” on potentially harmful medications. “But we don’t want to increase button fatigue even more, so we have to find a happy medium,” he said. “It’s going to take a while to formulate the best path on this.”
The inclusion of pharmacy residents in rounds could make a difference. “When we rounded with pharmacy residents, these issues got addressed,” Dr. Patel said. The pharmacy residents often asked: “Can we go over the meds? Does this person really need all this?”
Medication reconciliations not only have a positive effect on a patient’s health, they can also cut costs by eliminating unneeded drugs. And “patients are always happy to hear we’re taking them off a drug,” Dr. Patel added.
He said he remembers one of his mentors telling him that, if he could get his patients down to five medications, “then you’ve achieved success as a physician.”
“I’m still working toward that,” he said. “The end goal should sometimes be, less is more.”
COPD patients overprescribed home oxygen
In addition to medications, home oxygen therapy is often prescribed when patients are discharged from the hospital.
A study of 69 patients who were continued on home oxygen therapy after hospitalization for an exacerbation of chronic obstructive pulmonary disease was presented by Analisa Taylor, MD, from the University of Illinois at Chicago.
Despite guideline recommendations that patients be reassessed within 90 days of discharge, only 38 patients in the cohort were reassessed, and “28 were considered eligible for discontinuation,” she said during her presentation.
However, “of those, only four were ultimately discontinued,” she reported.
The reason for this gap needs to be examined, noted Dr. Taylor, suggesting that “perhaps clinical inertia plays a role in the continuation of previously prescribed therapy despite a lack of ongoing clinical benefit.”
A version of this article originally appeared on Medscape.com.
FROM CHEST 2020
Score predicts risk for ventilation in COVID-19 patients
A new scoring system can predict whether COVID-19 patients will require invasive mechanical ventilation, researchers report.
The score uses three variables to predict future risk: heart rate; the ratio of oxygen saturation (SpO2) to fraction of inspired oxygen (FiO2); and a positive troponin I level.
“What excites us is it’s a really benign tool,” said Muhtadi Alnababteh, MD, from the Medstar Washington (D.C.) Hospital Center. “For the first two variables you only need to look at vital signs, no labs or invasive diagnostics.”
“The third part is a simple lab, which is performed universally and can be done in any hospital,” he told this news organization. “We know that even rural hospitals can do this.”
For their retrospective analysis, Dr. Alnababteh and his colleagues assessed 265 adults with confirmed COVID-19 infection who were admitted to a single tertiary care center in March and April. They looked at demographic characteristics, lab results, and clinical and outcome information.
Ultimately, 54 of these patients required invasive mechanical ventilation.
On multiple-regression analysis, the researchers determined that three variables independently predicted the need for invasive mechanical ventilation.
Calibration of the model was good (Hosmer–Lemeshow score, 6.3; P = .39), as was predictive ability (area under the curve, 0.80).
The risk for invasive mechanical ventilation increased as the number of positive variables increased (P < .001), from 15.4% for those with one positive variable, to 29.0% for those with two, to 60.5% for those with three positive variables.
The team established cutoff points for each variable and developed a points-based scoring system to predict risk.
It was an initial surprise that troponin – a cardiac marker – would be a risk factor. “Originally, we thought COVID-19 only affects the lung,” Dr. Alnababteh explained during his presentation at CHEST 2020. Later studies, however, showed it can cause myocarditis symptoms.
The case for looking at cardiac markers was made when a study of young athletes who recovered from COVID-19 after experiencing mild or no symptoms showed that 15% had signs of myocarditis on cardiac MRI.
“If mild COVID disease in young patients caused cardiac injury, you can imagine what it can do to older patients with severe disease,” Alnababteh said.
This tool will help triage patients who are not sick enough for the ICU but are known to be at high risk for ventilation. “It’s one of the biggest decisions you have to make: Where do you send your patient? This score helps determine that,” he said.
The researchers are now working to validate the score and evaluate how it performs, he reported.
Existing scores evaluated for COVID-19 outcome prediction
The MuLBSTA score can also be used to predict outcomes in patients with COVID-19.
A retrospective evaluation of 163 patients was presented at CHEST 2020 by Jurgena Tusha, MD, from Wayne State University in Detroit.
Patients who survived their illness had a mean MuLBSTA score of 8.67, whereas patients who died had a mean score of 13.60.
The score “correlated significantly with mortality, ventilator support, and length of stay, which may be used to provide guidance to screen patients and make further clinical decisions,” Dr. Tusha said in a press release.
“Further studies are required to validate this study in larger patient cohorts,” she added.
The three-variable scoring system is easier to use than the MuLBSTA, and more specific, said Dr. Alnababteh.
“The main difference between our study and the MuLBSTA study is that we came up with a novel score for COVID-19 patients,” he said. “Our study score doesn’t require chest x-rays or blood cultures, and the outcome is need for invasive mechanical ventilation, not mortality.”
A version of this article originally appeared on Medscape.com.
A new scoring system can predict whether COVID-19 patients will require invasive mechanical ventilation, researchers report.
The score uses three variables to predict future risk: heart rate; the ratio of oxygen saturation (SpO2) to fraction of inspired oxygen (FiO2); and a positive troponin I level.
“What excites us is it’s a really benign tool,” said Muhtadi Alnababteh, MD, from the Medstar Washington (D.C.) Hospital Center. “For the first two variables you only need to look at vital signs, no labs or invasive diagnostics.”
“The third part is a simple lab, which is performed universally and can be done in any hospital,” he told this news organization. “We know that even rural hospitals can do this.”
For their retrospective analysis, Dr. Alnababteh and his colleagues assessed 265 adults with confirmed COVID-19 infection who were admitted to a single tertiary care center in March and April. They looked at demographic characteristics, lab results, and clinical and outcome information.
Ultimately, 54 of these patients required invasive mechanical ventilation.
On multiple-regression analysis, the researchers determined that three variables independently predicted the need for invasive mechanical ventilation.
Calibration of the model was good (Hosmer–Lemeshow score, 6.3; P = .39), as was predictive ability (area under the curve, 0.80).
The risk for invasive mechanical ventilation increased as the number of positive variables increased (P < .001), from 15.4% for those with one positive variable, to 29.0% for those with two, to 60.5% for those with three positive variables.
The team established cutoff points for each variable and developed a points-based scoring system to predict risk.
It was an initial surprise that troponin – a cardiac marker – would be a risk factor. “Originally, we thought COVID-19 only affects the lung,” Dr. Alnababteh explained during his presentation at CHEST 2020. Later studies, however, showed it can cause myocarditis symptoms.
The case for looking at cardiac markers was made when a study of young athletes who recovered from COVID-19 after experiencing mild or no symptoms showed that 15% had signs of myocarditis on cardiac MRI.
“If mild COVID disease in young patients caused cardiac injury, you can imagine what it can do to older patients with severe disease,” Alnababteh said.
This tool will help triage patients who are not sick enough for the ICU but are known to be at high risk for ventilation. “It’s one of the biggest decisions you have to make: Where do you send your patient? This score helps determine that,” he said.
The researchers are now working to validate the score and evaluate how it performs, he reported.
Existing scores evaluated for COVID-19 outcome prediction
The MuLBSTA score can also be used to predict outcomes in patients with COVID-19.
A retrospective evaluation of 163 patients was presented at CHEST 2020 by Jurgena Tusha, MD, from Wayne State University in Detroit.
Patients who survived their illness had a mean MuLBSTA score of 8.67, whereas patients who died had a mean score of 13.60.
The score “correlated significantly with mortality, ventilator support, and length of stay, which may be used to provide guidance to screen patients and make further clinical decisions,” Dr. Tusha said in a press release.
“Further studies are required to validate this study in larger patient cohorts,” she added.
The three-variable scoring system is easier to use than the MuLBSTA, and more specific, said Dr. Alnababteh.
“The main difference between our study and the MuLBSTA study is that we came up with a novel score for COVID-19 patients,” he said. “Our study score doesn’t require chest x-rays or blood cultures, and the outcome is need for invasive mechanical ventilation, not mortality.”
A version of this article originally appeared on Medscape.com.
A new scoring system can predict whether COVID-19 patients will require invasive mechanical ventilation, researchers report.
The score uses three variables to predict future risk: heart rate; the ratio of oxygen saturation (SpO2) to fraction of inspired oxygen (FiO2); and a positive troponin I level.
“What excites us is it’s a really benign tool,” said Muhtadi Alnababteh, MD, from the Medstar Washington (D.C.) Hospital Center. “For the first two variables you only need to look at vital signs, no labs or invasive diagnostics.”
“The third part is a simple lab, which is performed universally and can be done in any hospital,” he told this news organization. “We know that even rural hospitals can do this.”
For their retrospective analysis, Dr. Alnababteh and his colleagues assessed 265 adults with confirmed COVID-19 infection who were admitted to a single tertiary care center in March and April. They looked at demographic characteristics, lab results, and clinical and outcome information.
Ultimately, 54 of these patients required invasive mechanical ventilation.
On multiple-regression analysis, the researchers determined that three variables independently predicted the need for invasive mechanical ventilation.
Calibration of the model was good (Hosmer–Lemeshow score, 6.3; P = .39), as was predictive ability (area under the curve, 0.80).
The risk for invasive mechanical ventilation increased as the number of positive variables increased (P < .001), from 15.4% for those with one positive variable, to 29.0% for those with two, to 60.5% for those with three positive variables.
The team established cutoff points for each variable and developed a points-based scoring system to predict risk.
It was an initial surprise that troponin – a cardiac marker – would be a risk factor. “Originally, we thought COVID-19 only affects the lung,” Dr. Alnababteh explained during his presentation at CHEST 2020. Later studies, however, showed it can cause myocarditis symptoms.
The case for looking at cardiac markers was made when a study of young athletes who recovered from COVID-19 after experiencing mild or no symptoms showed that 15% had signs of myocarditis on cardiac MRI.
“If mild COVID disease in young patients caused cardiac injury, you can imagine what it can do to older patients with severe disease,” Alnababteh said.
This tool will help triage patients who are not sick enough for the ICU but are known to be at high risk for ventilation. “It’s one of the biggest decisions you have to make: Where do you send your patient? This score helps determine that,” he said.
The researchers are now working to validate the score and evaluate how it performs, he reported.
Existing scores evaluated for COVID-19 outcome prediction
The MuLBSTA score can also be used to predict outcomes in patients with COVID-19.
A retrospective evaluation of 163 patients was presented at CHEST 2020 by Jurgena Tusha, MD, from Wayne State University in Detroit.
Patients who survived their illness had a mean MuLBSTA score of 8.67, whereas patients who died had a mean score of 13.60.
The score “correlated significantly with mortality, ventilator support, and length of stay, which may be used to provide guidance to screen patients and make further clinical decisions,” Dr. Tusha said in a press release.
“Further studies are required to validate this study in larger patient cohorts,” she added.
The three-variable scoring system is easier to use than the MuLBSTA, and more specific, said Dr. Alnababteh.
“The main difference between our study and the MuLBSTA study is that we came up with a novel score for COVID-19 patients,” he said. “Our study score doesn’t require chest x-rays or blood cultures, and the outcome is need for invasive mechanical ventilation, not mortality.”
A version of this article originally appeared on Medscape.com.
Trump and Biden face off over COVID-19, ACA in final debate
The COVID-19 pandemic figured prominently in the final debate between President Donald Trump and former Vice President Joe Biden when they met on stage for a 90-minute debate in Nashville, Tennessee, Thursday evening.
The adequacy of the COVID-19 response to date, the likely timeline for vaccine availability, and how to reopen businesses while keeping Americans safe were among the points on which the two candidates disagreed. The two candidates also sparred over the value of the Affordable Care Act (ACA) and the future of healthcare in the United States.
Trump and Biden also differed on whether or not the country is facing a “dark winter” because of the pandemic.
Moderator Kristen Welker, NBC News White House correspondent, asked Trump to comment on the fact that 40,000 people are in the hospital on debate night with COVID-19 and that 16,000 have died since the last presidential debate.
Trump said, “2.2 million people modeled out were expected to die.” He said COVID-19 is a worldwide disease that does not only affect the United States.
“The mortality rate is down 85%, and the excess mortality is also down,” he added. He pointed out that previous spikes in Florida, Texas, and Arizona are now gone, and “spikes and surges in other places will soon be gone.
“It will go away, we are rounding the corner,” Trump said. “From personal experience, I was in the hospital, I had it, and they gave me a therapeutic, some would call it a cure...and now they say I’m immune. Whether it’s for a month or lifetime, nobody has been able to say that, but I’m immune.”
Biden countered by saying that “220,000 people are dead. If you hear nothing else I say tonight, hear this: Anyone who’s responsible for that many deaths should not remain president of the United States of America.”
Biden said there are a thousand deaths a day now and that there are over 70K new cases per day. “The expectation is we will have another 200,000 people down before the end of this year. If we just all wore these masks, we could save 100,000.”
“The New England Journal of Medicine said the way the president has handled this is absolutely tragic,” Biden added.
Vaccine timeline
Welker asked Trump if he could guarantee that there will be a COVID-19 vaccine within weeks.
“I can’t guarantee that, but it will be by end of the year. It will be distributed very quickly,” Trump said. He added that three leading vaccine developers, Johnson & Johnson, Moderna, and Pfizer, “are doing very well.”
“We’re about to go into a dark winter and he has no clear plan,” Biden said. “There is no prospect there will be a vaccine for most Americans by middle of next year.”
“It will not be a dark winter,” Trump responded.
Reopening the economy
Trump and Biden disagreed on how aggressively the economy should be reopened in light of the pandemic.
“I want to open the schools. We can’t keep this country closed,” Trump said. “This is a massive country with a massive economy.” He pointed out that rates of depression and suicide have risen because of the economic shutdown. “The cure cannot be worse than the problem.
“His Democrat governors...shut down so tight, and they’re dying,” the president added, gesturing toward Biden. “We are not going to shut down. We are going to open the schools.” As an example of the resiliency of young people, he mentioned that his son Barron tested positive for COVID-19 and recovered.
“I would shut down the virus, not the country,” Biden said. “It’s his ineptitude that caused so many schools and businesses to close in large part. Instead of being in a sand trap playing golf, he should have been negotiating with Nancy Pelosi.”
“He says we’re learning to live with it,” the former vice president said, but instead, “people are learning to die with it.”
Biden added that reopening the economy and minimizing transmission of COVID-19 are not mutually exclusive. “We can walk and chew gum at the same time.”
Divergence over the ACA
The fate of the ACA also garnered considerable attention. The discussion underlined a vast difference of opinion between the two candidates on the US healthcare system.
The moderator asked Trump what he would do for the 20 million Americans who get their healthcare through the ACA if it’s taken away.
“Through the legislature, I terminated the individual mandate, the worst part of Obamacare,” Trump said. “And now it’s in court because Obamacare is no good.
“Preexisting conditions will stay,” Trump added.
“I want to terminate Obamacare, and I want to come up with a beautiful healthcare [plan],” Trump added, turning the discussion toward private health insurance. “One thing that is very important is we have 180 million out there who have great private healthcare. Joe Biden will terminate all of their healthcare.”
Trump described Biden’s plan as “socialized medicine.” He also emphasized that protections for people with preexisting conditions “will stay.”
The Trump administration is supporting a lawsuit to overturn the ACA. The suit was filed by 18 Republican-led states. Arguments before the US Supreme Court on the constitutionality of the ACA are scheduled for November 10.
The moderator asked what Biden plans to do if the ACA is struck down. “I will pass Obamacare with a public option ― that will be ‘Bidencare.’ “ He said his plan will reduce premiums and drug prices. “I support private insurance. No one lost their private insurance under Obamacare.
“There is no way he can protect preexisting conditions,” Biden said. He added that 10 million people have already lost their private healthcare through unemployment during the pandemic.
Muting the mic
Following what many described as a chaotic first debate at the Cleveland Clinic in Ohio on September 29, the Commission on Presidential Debate opted to allow the muting of the microphone during the first 2 minutes of remarks made by each candidate during each debate segment.
The muting of the microphones appeared to prevent crosstalk during the beginning of each segment of the debate. The candidates did manage to talk over and interrupt each other, as well as the moderator, during portions of the debate.
This article first appeared on Medscape.com.
The COVID-19 pandemic figured prominently in the final debate between President Donald Trump and former Vice President Joe Biden when they met on stage for a 90-minute debate in Nashville, Tennessee, Thursday evening.
The adequacy of the COVID-19 response to date, the likely timeline for vaccine availability, and how to reopen businesses while keeping Americans safe were among the points on which the two candidates disagreed. The two candidates also sparred over the value of the Affordable Care Act (ACA) and the future of healthcare in the United States.
Trump and Biden also differed on whether or not the country is facing a “dark winter” because of the pandemic.
Moderator Kristen Welker, NBC News White House correspondent, asked Trump to comment on the fact that 40,000 people are in the hospital on debate night with COVID-19 and that 16,000 have died since the last presidential debate.
Trump said, “2.2 million people modeled out were expected to die.” He said COVID-19 is a worldwide disease that does not only affect the United States.
“The mortality rate is down 85%, and the excess mortality is also down,” he added. He pointed out that previous spikes in Florida, Texas, and Arizona are now gone, and “spikes and surges in other places will soon be gone.
“It will go away, we are rounding the corner,” Trump said. “From personal experience, I was in the hospital, I had it, and they gave me a therapeutic, some would call it a cure...and now they say I’m immune. Whether it’s for a month or lifetime, nobody has been able to say that, but I’m immune.”
Biden countered by saying that “220,000 people are dead. If you hear nothing else I say tonight, hear this: Anyone who’s responsible for that many deaths should not remain president of the United States of America.”
Biden said there are a thousand deaths a day now and that there are over 70K new cases per day. “The expectation is we will have another 200,000 people down before the end of this year. If we just all wore these masks, we could save 100,000.”
“The New England Journal of Medicine said the way the president has handled this is absolutely tragic,” Biden added.
Vaccine timeline
Welker asked Trump if he could guarantee that there will be a COVID-19 vaccine within weeks.
“I can’t guarantee that, but it will be by end of the year. It will be distributed very quickly,” Trump said. He added that three leading vaccine developers, Johnson & Johnson, Moderna, and Pfizer, “are doing very well.”
“We’re about to go into a dark winter and he has no clear plan,” Biden said. “There is no prospect there will be a vaccine for most Americans by middle of next year.”
“It will not be a dark winter,” Trump responded.
Reopening the economy
Trump and Biden disagreed on how aggressively the economy should be reopened in light of the pandemic.
“I want to open the schools. We can’t keep this country closed,” Trump said. “This is a massive country with a massive economy.” He pointed out that rates of depression and suicide have risen because of the economic shutdown. “The cure cannot be worse than the problem.
“His Democrat governors...shut down so tight, and they’re dying,” the president added, gesturing toward Biden. “We are not going to shut down. We are going to open the schools.” As an example of the resiliency of young people, he mentioned that his son Barron tested positive for COVID-19 and recovered.
“I would shut down the virus, not the country,” Biden said. “It’s his ineptitude that caused so many schools and businesses to close in large part. Instead of being in a sand trap playing golf, he should have been negotiating with Nancy Pelosi.”
“He says we’re learning to live with it,” the former vice president said, but instead, “people are learning to die with it.”
Biden added that reopening the economy and minimizing transmission of COVID-19 are not mutually exclusive. “We can walk and chew gum at the same time.”
Divergence over the ACA
The fate of the ACA also garnered considerable attention. The discussion underlined a vast difference of opinion between the two candidates on the US healthcare system.
The moderator asked Trump what he would do for the 20 million Americans who get their healthcare through the ACA if it’s taken away.
“Through the legislature, I terminated the individual mandate, the worst part of Obamacare,” Trump said. “And now it’s in court because Obamacare is no good.
“Preexisting conditions will stay,” Trump added.
“I want to terminate Obamacare, and I want to come up with a beautiful healthcare [plan],” Trump added, turning the discussion toward private health insurance. “One thing that is very important is we have 180 million out there who have great private healthcare. Joe Biden will terminate all of their healthcare.”
Trump described Biden’s plan as “socialized medicine.” He also emphasized that protections for people with preexisting conditions “will stay.”
The Trump administration is supporting a lawsuit to overturn the ACA. The suit was filed by 18 Republican-led states. Arguments before the US Supreme Court on the constitutionality of the ACA are scheduled for November 10.
The moderator asked what Biden plans to do if the ACA is struck down. “I will pass Obamacare with a public option ― that will be ‘Bidencare.’ “ He said his plan will reduce premiums and drug prices. “I support private insurance. No one lost their private insurance under Obamacare.
“There is no way he can protect preexisting conditions,” Biden said. He added that 10 million people have already lost their private healthcare through unemployment during the pandemic.
Muting the mic
Following what many described as a chaotic first debate at the Cleveland Clinic in Ohio on September 29, the Commission on Presidential Debate opted to allow the muting of the microphone during the first 2 minutes of remarks made by each candidate during each debate segment.
The muting of the microphones appeared to prevent crosstalk during the beginning of each segment of the debate. The candidates did manage to talk over and interrupt each other, as well as the moderator, during portions of the debate.
This article first appeared on Medscape.com.
The COVID-19 pandemic figured prominently in the final debate between President Donald Trump and former Vice President Joe Biden when they met on stage for a 90-minute debate in Nashville, Tennessee, Thursday evening.
The adequacy of the COVID-19 response to date, the likely timeline for vaccine availability, and how to reopen businesses while keeping Americans safe were among the points on which the two candidates disagreed. The two candidates also sparred over the value of the Affordable Care Act (ACA) and the future of healthcare in the United States.
Trump and Biden also differed on whether or not the country is facing a “dark winter” because of the pandemic.
Moderator Kristen Welker, NBC News White House correspondent, asked Trump to comment on the fact that 40,000 people are in the hospital on debate night with COVID-19 and that 16,000 have died since the last presidential debate.
Trump said, “2.2 million people modeled out were expected to die.” He said COVID-19 is a worldwide disease that does not only affect the United States.
“The mortality rate is down 85%, and the excess mortality is also down,” he added. He pointed out that previous spikes in Florida, Texas, and Arizona are now gone, and “spikes and surges in other places will soon be gone.
“It will go away, we are rounding the corner,” Trump said. “From personal experience, I was in the hospital, I had it, and they gave me a therapeutic, some would call it a cure...and now they say I’m immune. Whether it’s for a month or lifetime, nobody has been able to say that, but I’m immune.”
Biden countered by saying that “220,000 people are dead. If you hear nothing else I say tonight, hear this: Anyone who’s responsible for that many deaths should not remain president of the United States of America.”
Biden said there are a thousand deaths a day now and that there are over 70K new cases per day. “The expectation is we will have another 200,000 people down before the end of this year. If we just all wore these masks, we could save 100,000.”
“The New England Journal of Medicine said the way the president has handled this is absolutely tragic,” Biden added.
Vaccine timeline
Welker asked Trump if he could guarantee that there will be a COVID-19 vaccine within weeks.
“I can’t guarantee that, but it will be by end of the year. It will be distributed very quickly,” Trump said. He added that three leading vaccine developers, Johnson & Johnson, Moderna, and Pfizer, “are doing very well.”
“We’re about to go into a dark winter and he has no clear plan,” Biden said. “There is no prospect there will be a vaccine for most Americans by middle of next year.”
“It will not be a dark winter,” Trump responded.
Reopening the economy
Trump and Biden disagreed on how aggressively the economy should be reopened in light of the pandemic.
“I want to open the schools. We can’t keep this country closed,” Trump said. “This is a massive country with a massive economy.” He pointed out that rates of depression and suicide have risen because of the economic shutdown. “The cure cannot be worse than the problem.
“His Democrat governors...shut down so tight, and they’re dying,” the president added, gesturing toward Biden. “We are not going to shut down. We are going to open the schools.” As an example of the resiliency of young people, he mentioned that his son Barron tested positive for COVID-19 and recovered.
“I would shut down the virus, not the country,” Biden said. “It’s his ineptitude that caused so many schools and businesses to close in large part. Instead of being in a sand trap playing golf, he should have been negotiating with Nancy Pelosi.”
“He says we’re learning to live with it,” the former vice president said, but instead, “people are learning to die with it.”
Biden added that reopening the economy and minimizing transmission of COVID-19 are not mutually exclusive. “We can walk and chew gum at the same time.”
Divergence over the ACA
The fate of the ACA also garnered considerable attention. The discussion underlined a vast difference of opinion between the two candidates on the US healthcare system.
The moderator asked Trump what he would do for the 20 million Americans who get their healthcare through the ACA if it’s taken away.
“Through the legislature, I terminated the individual mandate, the worst part of Obamacare,” Trump said. “And now it’s in court because Obamacare is no good.
“Preexisting conditions will stay,” Trump added.
“I want to terminate Obamacare, and I want to come up with a beautiful healthcare [plan],” Trump added, turning the discussion toward private health insurance. “One thing that is very important is we have 180 million out there who have great private healthcare. Joe Biden will terminate all of their healthcare.”
Trump described Biden’s plan as “socialized medicine.” He also emphasized that protections for people with preexisting conditions “will stay.”
The Trump administration is supporting a lawsuit to overturn the ACA. The suit was filed by 18 Republican-led states. Arguments before the US Supreme Court on the constitutionality of the ACA are scheduled for November 10.
The moderator asked what Biden plans to do if the ACA is struck down. “I will pass Obamacare with a public option ― that will be ‘Bidencare.’ “ He said his plan will reduce premiums and drug prices. “I support private insurance. No one lost their private insurance under Obamacare.
“There is no way he can protect preexisting conditions,” Biden said. He added that 10 million people have already lost their private healthcare through unemployment during the pandemic.
Muting the mic
Following what many described as a chaotic first debate at the Cleveland Clinic in Ohio on September 29, the Commission on Presidential Debate opted to allow the muting of the microphone during the first 2 minutes of remarks made by each candidate during each debate segment.
The muting of the microphones appeared to prevent crosstalk during the beginning of each segment of the debate. The candidates did manage to talk over and interrupt each other, as well as the moderator, during portions of the debate.
This article first appeared on Medscape.com.
When should students resume sports after a COVID-19 diagnosis?
Many student athletes who test positive for COVID-19 likely can have an uneventful return to their sports after they have rested for 2 weeks in quarantine, doctors suggest.
There are reasons for caution, however, especially when a patient has symptoms that indicate possible cardiac involvement. In these cases, patients should undergo cardiac testing before a physician clears them to return to play, according to guidance from professional associations. Reports of myocarditis in college athletes who tested positive for SARS-CoV-2 but were asymptomatic are among the reasons for concern. Myocarditis may increase the risk of sudden death during exercise.
“The thing that you need to keep in mind is that this is not just a respiratory illness,” David T. Bernhardt, MD, professor of pediatrics, orthopedics, and rehabilitation at the University of Wisconsin in Madison, said in a presentation at the annual meeting of the American Academy of Pediatrics, held virtually this year. High school and college athletes have had cardiac, neurologic, hematologic, and renal problems that “can complicate their recovery and their return to sport.”
Still, children who test positive for COVID-19 tend to have mild illness and often are asymptomatic. “It is more than likely going to be safe for the majority of the student athletes who are in the elementary and middle school age to return to sport,” said Dr. Bernhardt. Given that 18-year-old college freshmen have had cardiac complications, there may be reason for more caution with high school students.
Limited data
The AAP has released interim guidance on returning to sports and recommends that primary care physicians clear all patients with COVID-19 before they resume training. Physicians should screen for cardiac symptoms such as chest pain, shortness of breath, fatigue, palpitations, or syncope.
Those with severe illness should be restricted from exercise and participation for 3-6 months. Primary care physicians, preferably in consultation with pediatric cardiologists, should clear athletes who experience severe illness.
“Most of the recommendations come from the fact that we simply do not know what we do not know with COVID-19,” Susannah Briskin, MD, a coauthor of the interim guidance, said in an interview. “We have to be cautious in returning individuals to play and closely monitor them as we learn more about the disease process and its effect on kids.”
Patients with severe illness could include those who were hospitalized and experienced hypotension or arrhythmias, required intubation or extracorporeal membrane oxygenation (ECMO) support, had kidney or cardiac failure, or developed multisystem inflammatory syndrome in children (MIS-C), said Dr. Briskin, a specialist in pediatric sports medicine at Case Western Reserve University, Cleveland.
“The majority of COVID-19 cases will not present like this in kids. We have no idea how common myocarditis is in kids post infection. We do know that, if anyone has chest pain, shortness of breath, excessive fatigue, syncope [passing out], or arrhythmia [feeling of their heart skipping beats], they should undergo further evaluation for myocarditis,” Dr. Briskin said.
Patients who are asymptomatic or have mild symptoms should rest for 14 days after their positive test. After their infectious period has passed, a doctor should assess for any concerning cardiac symptoms. “Anyone with prolonged fever or moderate symptoms should see their pediatrician and have an EKG performed, at a minimum, prior to return to sports,” Dr. Briskin said. “Anyone with an abnormal EKG or concerning signs or symptoms should be referred on to pediatric cardiology for a further assessment.”
Most patients who Dr. Briskin has seen have been asymptomatic or mildly symptomatic. “They have done well with a gradual return to physical activity,” she said. “We recommend a gradual return so individuals can be monitored for any signs or symptoms concerning for myocarditis. The far majority of individuals likely have an uneventful return to play.”
Mitigating risk
COVID-19 adds elements of uncertainty and complexity to the usual process of mitigating risk in sports, Dr. Bernhardt noted in his lecture. “You are dealing with an infection that we do not know a lot about,” he said. “And we are trying to mitigate risk not only for the individual who may or may not have underlying health problems, but you are also trying to mitigate risk for anybody else involved with the sport, including athletic trainers and team physicians, coaches, spectators, custodial staff, people working at a snack shack, and all the other people that can be involved in a typical sporting type of atmosphere.”
When patients do return to play after an illness, they should gradually increase the training load to avoid injury. In addition, clinicians should screen for depression and anxiety using tools such as the Four-Item Patient Health Questionnaire (PHQ-4) when they see patients. “The pandemic has been quite stressful for everybody, including our high school student athletes,” Dr. Bernhardt said. “Giving everybody a PHQ-4 when they come into clinic right now probably makes sense in terms of the stress levels that all of us are experiencing.”
If a patient screens positive, take additional history and refer for more in-depth mental health evaluation and treatment if warranted. Sharing breathing and relaxation exercises, promoting healthy behaviors, and paying attention to unhealthy strategies also may help, Dr. Bernhardt suggested.
Ultimately, determining when an athlete with COVID-19 can be medically cleared to return to play may be a challenge. There are limited data on epidemiology and clinical presentations that could help identify cardiac injury related to the disease, Dr. Bernhardt said. Guidance from the American College of Cardiology provides a framework for evaluating athletes for return to play, and pediatric cardiologists have discussed how the guidance relates to a pediatric population. Cardiac assessments may include measures of biomarkers such as troponin, B-type natriuretic peptide, and sedimentation rate, along with electrocardiograms, echocardiograms, and cardiac MRI.
Beyond return-to-play decisions, encourage the use of cloth face coverings on the sidelines and away from the playing field, and stress proper quarantining, Dr. Briskin added. Too often, she hears about children not quarantining properly. “Individuals with a known exposure should be quarantined in their house – ideally in a separate room from everyone else. ... When they come out of their room, they should wash their hands well and wear a cloth face covering. They should not be eating with other people.”
Dr. Bernhardt had no relevant disclosures.
Many student athletes who test positive for COVID-19 likely can have an uneventful return to their sports after they have rested for 2 weeks in quarantine, doctors suggest.
There are reasons for caution, however, especially when a patient has symptoms that indicate possible cardiac involvement. In these cases, patients should undergo cardiac testing before a physician clears them to return to play, according to guidance from professional associations. Reports of myocarditis in college athletes who tested positive for SARS-CoV-2 but were asymptomatic are among the reasons for concern. Myocarditis may increase the risk of sudden death during exercise.
“The thing that you need to keep in mind is that this is not just a respiratory illness,” David T. Bernhardt, MD, professor of pediatrics, orthopedics, and rehabilitation at the University of Wisconsin in Madison, said in a presentation at the annual meeting of the American Academy of Pediatrics, held virtually this year. High school and college athletes have had cardiac, neurologic, hematologic, and renal problems that “can complicate their recovery and their return to sport.”
Still, children who test positive for COVID-19 tend to have mild illness and often are asymptomatic. “It is more than likely going to be safe for the majority of the student athletes who are in the elementary and middle school age to return to sport,” said Dr. Bernhardt. Given that 18-year-old college freshmen have had cardiac complications, there may be reason for more caution with high school students.
Limited data
The AAP has released interim guidance on returning to sports and recommends that primary care physicians clear all patients with COVID-19 before they resume training. Physicians should screen for cardiac symptoms such as chest pain, shortness of breath, fatigue, palpitations, or syncope.
Those with severe illness should be restricted from exercise and participation for 3-6 months. Primary care physicians, preferably in consultation with pediatric cardiologists, should clear athletes who experience severe illness.
“Most of the recommendations come from the fact that we simply do not know what we do not know with COVID-19,” Susannah Briskin, MD, a coauthor of the interim guidance, said in an interview. “We have to be cautious in returning individuals to play and closely monitor them as we learn more about the disease process and its effect on kids.”
Patients with severe illness could include those who were hospitalized and experienced hypotension or arrhythmias, required intubation or extracorporeal membrane oxygenation (ECMO) support, had kidney or cardiac failure, or developed multisystem inflammatory syndrome in children (MIS-C), said Dr. Briskin, a specialist in pediatric sports medicine at Case Western Reserve University, Cleveland.
“The majority of COVID-19 cases will not present like this in kids. We have no idea how common myocarditis is in kids post infection. We do know that, if anyone has chest pain, shortness of breath, excessive fatigue, syncope [passing out], or arrhythmia [feeling of their heart skipping beats], they should undergo further evaluation for myocarditis,” Dr. Briskin said.
Patients who are asymptomatic or have mild symptoms should rest for 14 days after their positive test. After their infectious period has passed, a doctor should assess for any concerning cardiac symptoms. “Anyone with prolonged fever or moderate symptoms should see their pediatrician and have an EKG performed, at a minimum, prior to return to sports,” Dr. Briskin said. “Anyone with an abnormal EKG or concerning signs or symptoms should be referred on to pediatric cardiology for a further assessment.”
Most patients who Dr. Briskin has seen have been asymptomatic or mildly symptomatic. “They have done well with a gradual return to physical activity,” she said. “We recommend a gradual return so individuals can be monitored for any signs or symptoms concerning for myocarditis. The far majority of individuals likely have an uneventful return to play.”
Mitigating risk
COVID-19 adds elements of uncertainty and complexity to the usual process of mitigating risk in sports, Dr. Bernhardt noted in his lecture. “You are dealing with an infection that we do not know a lot about,” he said. “And we are trying to mitigate risk not only for the individual who may or may not have underlying health problems, but you are also trying to mitigate risk for anybody else involved with the sport, including athletic trainers and team physicians, coaches, spectators, custodial staff, people working at a snack shack, and all the other people that can be involved in a typical sporting type of atmosphere.”
When patients do return to play after an illness, they should gradually increase the training load to avoid injury. In addition, clinicians should screen for depression and anxiety using tools such as the Four-Item Patient Health Questionnaire (PHQ-4) when they see patients. “The pandemic has been quite stressful for everybody, including our high school student athletes,” Dr. Bernhardt said. “Giving everybody a PHQ-4 when they come into clinic right now probably makes sense in terms of the stress levels that all of us are experiencing.”
If a patient screens positive, take additional history and refer for more in-depth mental health evaluation and treatment if warranted. Sharing breathing and relaxation exercises, promoting healthy behaviors, and paying attention to unhealthy strategies also may help, Dr. Bernhardt suggested.
Ultimately, determining when an athlete with COVID-19 can be medically cleared to return to play may be a challenge. There are limited data on epidemiology and clinical presentations that could help identify cardiac injury related to the disease, Dr. Bernhardt said. Guidance from the American College of Cardiology provides a framework for evaluating athletes for return to play, and pediatric cardiologists have discussed how the guidance relates to a pediatric population. Cardiac assessments may include measures of biomarkers such as troponin, B-type natriuretic peptide, and sedimentation rate, along with electrocardiograms, echocardiograms, and cardiac MRI.
Beyond return-to-play decisions, encourage the use of cloth face coverings on the sidelines and away from the playing field, and stress proper quarantining, Dr. Briskin added. Too often, she hears about children not quarantining properly. “Individuals with a known exposure should be quarantined in their house – ideally in a separate room from everyone else. ... When they come out of their room, they should wash their hands well and wear a cloth face covering. They should not be eating with other people.”
Dr. Bernhardt had no relevant disclosures.
Many student athletes who test positive for COVID-19 likely can have an uneventful return to their sports after they have rested for 2 weeks in quarantine, doctors suggest.
There are reasons for caution, however, especially when a patient has symptoms that indicate possible cardiac involvement. In these cases, patients should undergo cardiac testing before a physician clears them to return to play, according to guidance from professional associations. Reports of myocarditis in college athletes who tested positive for SARS-CoV-2 but were asymptomatic are among the reasons for concern. Myocarditis may increase the risk of sudden death during exercise.
“The thing that you need to keep in mind is that this is not just a respiratory illness,” David T. Bernhardt, MD, professor of pediatrics, orthopedics, and rehabilitation at the University of Wisconsin in Madison, said in a presentation at the annual meeting of the American Academy of Pediatrics, held virtually this year. High school and college athletes have had cardiac, neurologic, hematologic, and renal problems that “can complicate their recovery and their return to sport.”
Still, children who test positive for COVID-19 tend to have mild illness and often are asymptomatic. “It is more than likely going to be safe for the majority of the student athletes who are in the elementary and middle school age to return to sport,” said Dr. Bernhardt. Given that 18-year-old college freshmen have had cardiac complications, there may be reason for more caution with high school students.
Limited data
The AAP has released interim guidance on returning to sports and recommends that primary care physicians clear all patients with COVID-19 before they resume training. Physicians should screen for cardiac symptoms such as chest pain, shortness of breath, fatigue, palpitations, or syncope.
Those with severe illness should be restricted from exercise and participation for 3-6 months. Primary care physicians, preferably in consultation with pediatric cardiologists, should clear athletes who experience severe illness.
“Most of the recommendations come from the fact that we simply do not know what we do not know with COVID-19,” Susannah Briskin, MD, a coauthor of the interim guidance, said in an interview. “We have to be cautious in returning individuals to play and closely monitor them as we learn more about the disease process and its effect on kids.”
Patients with severe illness could include those who were hospitalized and experienced hypotension or arrhythmias, required intubation or extracorporeal membrane oxygenation (ECMO) support, had kidney or cardiac failure, or developed multisystem inflammatory syndrome in children (MIS-C), said Dr. Briskin, a specialist in pediatric sports medicine at Case Western Reserve University, Cleveland.
“The majority of COVID-19 cases will not present like this in kids. We have no idea how common myocarditis is in kids post infection. We do know that, if anyone has chest pain, shortness of breath, excessive fatigue, syncope [passing out], or arrhythmia [feeling of their heart skipping beats], they should undergo further evaluation for myocarditis,” Dr. Briskin said.
Patients who are asymptomatic or have mild symptoms should rest for 14 days after their positive test. After their infectious period has passed, a doctor should assess for any concerning cardiac symptoms. “Anyone with prolonged fever or moderate symptoms should see their pediatrician and have an EKG performed, at a minimum, prior to return to sports,” Dr. Briskin said. “Anyone with an abnormal EKG or concerning signs or symptoms should be referred on to pediatric cardiology for a further assessment.”
Most patients who Dr. Briskin has seen have been asymptomatic or mildly symptomatic. “They have done well with a gradual return to physical activity,” she said. “We recommend a gradual return so individuals can be monitored for any signs or symptoms concerning for myocarditis. The far majority of individuals likely have an uneventful return to play.”
Mitigating risk
COVID-19 adds elements of uncertainty and complexity to the usual process of mitigating risk in sports, Dr. Bernhardt noted in his lecture. “You are dealing with an infection that we do not know a lot about,” he said. “And we are trying to mitigate risk not only for the individual who may or may not have underlying health problems, but you are also trying to mitigate risk for anybody else involved with the sport, including athletic trainers and team physicians, coaches, spectators, custodial staff, people working at a snack shack, and all the other people that can be involved in a typical sporting type of atmosphere.”
When patients do return to play after an illness, they should gradually increase the training load to avoid injury. In addition, clinicians should screen for depression and anxiety using tools such as the Four-Item Patient Health Questionnaire (PHQ-4) when they see patients. “The pandemic has been quite stressful for everybody, including our high school student athletes,” Dr. Bernhardt said. “Giving everybody a PHQ-4 when they come into clinic right now probably makes sense in terms of the stress levels that all of us are experiencing.”
If a patient screens positive, take additional history and refer for more in-depth mental health evaluation and treatment if warranted. Sharing breathing and relaxation exercises, promoting healthy behaviors, and paying attention to unhealthy strategies also may help, Dr. Bernhardt suggested.
Ultimately, determining when an athlete with COVID-19 can be medically cleared to return to play may be a challenge. There are limited data on epidemiology and clinical presentations that could help identify cardiac injury related to the disease, Dr. Bernhardt said. Guidance from the American College of Cardiology provides a framework for evaluating athletes for return to play, and pediatric cardiologists have discussed how the guidance relates to a pediatric population. Cardiac assessments may include measures of biomarkers such as troponin, B-type natriuretic peptide, and sedimentation rate, along with electrocardiograms, echocardiograms, and cardiac MRI.
Beyond return-to-play decisions, encourage the use of cloth face coverings on the sidelines and away from the playing field, and stress proper quarantining, Dr. Briskin added. Too often, she hears about children not quarantining properly. “Individuals with a known exposure should be quarantined in their house – ideally in a separate room from everyone else. ... When they come out of their room, they should wash their hands well and wear a cloth face covering. They should not be eating with other people.”
Dr. Bernhardt had no relevant disclosures.
FROM AAP 2020
No mortality benefit after intensive glucose control once Hb A1c curves equalize
Background: A previous study reported that a median of 5.6 years of intensive versus standard glucose lowering in veterans with type 2 diabetes resulted in significantly reduced risk of major cardiovascular events after 10 years of combined intervention and observational follow-up.
Study design: Prospective cohort.
Setting: Veterans Affairs Healthcare System.
Synopsis: In the original trial, 1,791 veterans were randomly assigned to receive either intensive or standard glucose control therapy. After conclusion of that study, 1,655 participants were followed using central databases, and 1,391 also provided data via surveys and chart review. Initially the difference in the glycated hemoglobin (Hb A1c) curves between the two groups averaged 1.5%, but it declined to 0.2%-0.3% 3 years after the trial ended. The median Hb A1c then stabilized to 8% in both groups.
Over a period of 15 years of combined intervention and posttrial follow-up, the risks of major cardiovascular events or death were not lower in the intensive-therapy group (hazard ratio for composite outcome, 0.91; 95% confidence interval, 0.78-1.06; P = .23; HR for death, 1.02; 95% CI, 0.88-1.18). The risk of major cardiovascular disease outcomes was reduced during the approximately 10-year interval of separation of the Hb A1c curves (HR, 0.83; 95% CI, 0.70-0.99), but it did not persist after equalization of Hb A1c levels (HR, 1.26; 95% CI, 0.90-1.75). Limitations include the observational study design, the study population of mostly older men, and reliance on administrative data for outcomes.
Bottom line: More than 5 years of intensive glucose lowering, compared with standard therapy, did not show significantly lower risks of cardiovascular events or mortality once the glycated hemoglobin curves equalized during follow-up in years 11-15.
Citation: Reaven PD et al. Intensive glucose control in patients with type 2 diabetes – 15-year follow-up. New Engl J Med. 2019 Jun 6;380(23):2215-24.
Dr. Burke is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
Background: A previous study reported that a median of 5.6 years of intensive versus standard glucose lowering in veterans with type 2 diabetes resulted in significantly reduced risk of major cardiovascular events after 10 years of combined intervention and observational follow-up.
Study design: Prospective cohort.
Setting: Veterans Affairs Healthcare System.
Synopsis: In the original trial, 1,791 veterans were randomly assigned to receive either intensive or standard glucose control therapy. After conclusion of that study, 1,655 participants were followed using central databases, and 1,391 also provided data via surveys and chart review. Initially the difference in the glycated hemoglobin (Hb A1c) curves between the two groups averaged 1.5%, but it declined to 0.2%-0.3% 3 years after the trial ended. The median Hb A1c then stabilized to 8% in both groups.
Over a period of 15 years of combined intervention and posttrial follow-up, the risks of major cardiovascular events or death were not lower in the intensive-therapy group (hazard ratio for composite outcome, 0.91; 95% confidence interval, 0.78-1.06; P = .23; HR for death, 1.02; 95% CI, 0.88-1.18). The risk of major cardiovascular disease outcomes was reduced during the approximately 10-year interval of separation of the Hb A1c curves (HR, 0.83; 95% CI, 0.70-0.99), but it did not persist after equalization of Hb A1c levels (HR, 1.26; 95% CI, 0.90-1.75). Limitations include the observational study design, the study population of mostly older men, and reliance on administrative data for outcomes.
Bottom line: More than 5 years of intensive glucose lowering, compared with standard therapy, did not show significantly lower risks of cardiovascular events or mortality once the glycated hemoglobin curves equalized during follow-up in years 11-15.
Citation: Reaven PD et al. Intensive glucose control in patients with type 2 diabetes – 15-year follow-up. New Engl J Med. 2019 Jun 6;380(23):2215-24.
Dr. Burke is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
Background: A previous study reported that a median of 5.6 years of intensive versus standard glucose lowering in veterans with type 2 diabetes resulted in significantly reduced risk of major cardiovascular events after 10 years of combined intervention and observational follow-up.
Study design: Prospective cohort.
Setting: Veterans Affairs Healthcare System.
Synopsis: In the original trial, 1,791 veterans were randomly assigned to receive either intensive or standard glucose control therapy. After conclusion of that study, 1,655 participants were followed using central databases, and 1,391 also provided data via surveys and chart review. Initially the difference in the glycated hemoglobin (Hb A1c) curves between the two groups averaged 1.5%, but it declined to 0.2%-0.3% 3 years after the trial ended. The median Hb A1c then stabilized to 8% in both groups.
Over a period of 15 years of combined intervention and posttrial follow-up, the risks of major cardiovascular events or death were not lower in the intensive-therapy group (hazard ratio for composite outcome, 0.91; 95% confidence interval, 0.78-1.06; P = .23; HR for death, 1.02; 95% CI, 0.88-1.18). The risk of major cardiovascular disease outcomes was reduced during the approximately 10-year interval of separation of the Hb A1c curves (HR, 0.83; 95% CI, 0.70-0.99), but it did not persist after equalization of Hb A1c levels (HR, 1.26; 95% CI, 0.90-1.75). Limitations include the observational study design, the study population of mostly older men, and reliance on administrative data for outcomes.
Bottom line: More than 5 years of intensive glucose lowering, compared with standard therapy, did not show significantly lower risks of cardiovascular events or mortality once the glycated hemoglobin curves equalized during follow-up in years 11-15.
Citation: Reaven PD et al. Intensive glucose control in patients with type 2 diabetes – 15-year follow-up. New Engl J Med. 2019 Jun 6;380(23):2215-24.
Dr. Burke is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
COVID-19 vaccine standards questioned at FDA advisory meeting
The FDA’s Vaccines and Related Biological Products Advisory Committee met for a wide-ranging discussion beginning around 10 am. The FDA did not ask the panel to weigh in on any particular vaccine. Instead, the FDA asked for the panel’s feedback on a series of questions, including considerations for continuing phase 3 trials if a product were to get an interim clearance known as an emergency use authorization (EUA).
Speakers at the hearing made a variety of requests, including asking for data showing COVID-19 vaccines can prevent serious illness and urging transparency about the agency’s deliberations for each product to be considered.
FDA staff are closely tracking the crop of experimental vaccines that have made it into advanced stages of testing, including products from Pfizer Inc, AstraZeneca, Johnson & Johnson, and Moderna.
‘Time for a reset’
Among the speakers at the public hearing was Peter Lurie, MD, who served as an FDA associate commissioner from 2014 to 2017. Now the president of the Center for Science in the Public Interest, Lurie was among the speakers who asked the agency to make its independence clear.
President Donald Trump has for months been making predictions about COVID-19 vaccine approvals that have been overly optimistic. In one example, the president, who is seeking re-election on November 3, last month spoke about being able to begin distributing a vaccine in October.
“Until now the process of developing candidate vaccines has been inappropriately politicized with an eye on the election calendar, rather than the deliberate timeframe science requires,” Lurie told the FDA advisory panel. “Now is the time for a reset. This committee has a unique opportunity to set a new tone for vaccine deliberations going forward.”
Lurie asked the panel to press the FDA to commit to hold an advisory committee meeting on requests by drugmakers for EUAs. He also asked the panel to demand that informed consent forms and minutes from institutional review board (IRB) discussions of COVID-19 vaccines trials be made public.
Also among the speakers at the public hearing was Peter Doshi, PhD, an associate professor at the University of Maryland School of Pharmacy, who argued that the current trials won’t answer the right questions about the COVID-19 vaccines.
“We could end up with approved vaccines that reduce the risk of mild infection, but do not decrease the risk of hospitalization, ICU use, or death — either at all or by a clinically relevant amount,” Doshi told the panel.
In his presentation, he reiterated points he had made previously, including in an October 21 article in the BMJ, for which he is an associate editor. Doshi also raised these concerns in a September opinion article in The New York Times, co-authored with Eric Topol, MD, director of the Scripps Research Translational Institute and editor-in-chief of Medscape.
Risks of a ‘rushed vaccine’
Other complaints about the FDA’s approach included criticism of a 2-month follow-up time after vaccination, which was seen as too short. ECRI, a nonprofit organization that seeks to improve the safety, quality, and cost-effectiveness of medicines, has argued that approving a weak COVID-19 vaccine might worsen the pandemic.
In an October 21 statement, ECRI noted the risk of a partially effective vaccine, which could be welcomed as a means of slowing transmission of the virus. But public response and attitudes over the past 9 months in the United States suggest that people would relax their precautions as soon as a vaccine is available.
“Resulting infections may offset the vaccine’s impact and end up increasing the mortality and morbidity burden,” ECRI said in the brief.
“The risks and consequences of a rushed vaccine could be very severe if the review is anything shy of thorough,” ECRI Chief Executive Officer Marcus Schabacker, MD, PhD, said in a statement prepared for the hearing.
This article first appeared on Medscape.com.
The FDA’s Vaccines and Related Biological Products Advisory Committee met for a wide-ranging discussion beginning around 10 am. The FDA did not ask the panel to weigh in on any particular vaccine. Instead, the FDA asked for the panel’s feedback on a series of questions, including considerations for continuing phase 3 trials if a product were to get an interim clearance known as an emergency use authorization (EUA).
Speakers at the hearing made a variety of requests, including asking for data showing COVID-19 vaccines can prevent serious illness and urging transparency about the agency’s deliberations for each product to be considered.
FDA staff are closely tracking the crop of experimental vaccines that have made it into advanced stages of testing, including products from Pfizer Inc, AstraZeneca, Johnson & Johnson, and Moderna.
‘Time for a reset’
Among the speakers at the public hearing was Peter Lurie, MD, who served as an FDA associate commissioner from 2014 to 2017. Now the president of the Center for Science in the Public Interest, Lurie was among the speakers who asked the agency to make its independence clear.
President Donald Trump has for months been making predictions about COVID-19 vaccine approvals that have been overly optimistic. In one example, the president, who is seeking re-election on November 3, last month spoke about being able to begin distributing a vaccine in October.
“Until now the process of developing candidate vaccines has been inappropriately politicized with an eye on the election calendar, rather than the deliberate timeframe science requires,” Lurie told the FDA advisory panel. “Now is the time for a reset. This committee has a unique opportunity to set a new tone for vaccine deliberations going forward.”
Lurie asked the panel to press the FDA to commit to hold an advisory committee meeting on requests by drugmakers for EUAs. He also asked the panel to demand that informed consent forms and minutes from institutional review board (IRB) discussions of COVID-19 vaccines trials be made public.
Also among the speakers at the public hearing was Peter Doshi, PhD, an associate professor at the University of Maryland School of Pharmacy, who argued that the current trials won’t answer the right questions about the COVID-19 vaccines.
“We could end up with approved vaccines that reduce the risk of mild infection, but do not decrease the risk of hospitalization, ICU use, or death — either at all or by a clinically relevant amount,” Doshi told the panel.
In his presentation, he reiterated points he had made previously, including in an October 21 article in the BMJ, for which he is an associate editor. Doshi also raised these concerns in a September opinion article in The New York Times, co-authored with Eric Topol, MD, director of the Scripps Research Translational Institute and editor-in-chief of Medscape.
Risks of a ‘rushed vaccine’
Other complaints about the FDA’s approach included criticism of a 2-month follow-up time after vaccination, which was seen as too short. ECRI, a nonprofit organization that seeks to improve the safety, quality, and cost-effectiveness of medicines, has argued that approving a weak COVID-19 vaccine might worsen the pandemic.
In an October 21 statement, ECRI noted the risk of a partially effective vaccine, which could be welcomed as a means of slowing transmission of the virus. But public response and attitudes over the past 9 months in the United States suggest that people would relax their precautions as soon as a vaccine is available.
“Resulting infections may offset the vaccine’s impact and end up increasing the mortality and morbidity burden,” ECRI said in the brief.
“The risks and consequences of a rushed vaccine could be very severe if the review is anything shy of thorough,” ECRI Chief Executive Officer Marcus Schabacker, MD, PhD, said in a statement prepared for the hearing.
This article first appeared on Medscape.com.
The FDA’s Vaccines and Related Biological Products Advisory Committee met for a wide-ranging discussion beginning around 10 am. The FDA did not ask the panel to weigh in on any particular vaccine. Instead, the FDA asked for the panel’s feedback on a series of questions, including considerations for continuing phase 3 trials if a product were to get an interim clearance known as an emergency use authorization (EUA).
Speakers at the hearing made a variety of requests, including asking for data showing COVID-19 vaccines can prevent serious illness and urging transparency about the agency’s deliberations for each product to be considered.
FDA staff are closely tracking the crop of experimental vaccines that have made it into advanced stages of testing, including products from Pfizer Inc, AstraZeneca, Johnson & Johnson, and Moderna.
‘Time for a reset’
Among the speakers at the public hearing was Peter Lurie, MD, who served as an FDA associate commissioner from 2014 to 2017. Now the president of the Center for Science in the Public Interest, Lurie was among the speakers who asked the agency to make its independence clear.
President Donald Trump has for months been making predictions about COVID-19 vaccine approvals that have been overly optimistic. In one example, the president, who is seeking re-election on November 3, last month spoke about being able to begin distributing a vaccine in October.
“Until now the process of developing candidate vaccines has been inappropriately politicized with an eye on the election calendar, rather than the deliberate timeframe science requires,” Lurie told the FDA advisory panel. “Now is the time for a reset. This committee has a unique opportunity to set a new tone for vaccine deliberations going forward.”
Lurie asked the panel to press the FDA to commit to hold an advisory committee meeting on requests by drugmakers for EUAs. He also asked the panel to demand that informed consent forms and minutes from institutional review board (IRB) discussions of COVID-19 vaccines trials be made public.
Also among the speakers at the public hearing was Peter Doshi, PhD, an associate professor at the University of Maryland School of Pharmacy, who argued that the current trials won’t answer the right questions about the COVID-19 vaccines.
“We could end up with approved vaccines that reduce the risk of mild infection, but do not decrease the risk of hospitalization, ICU use, or death — either at all or by a clinically relevant amount,” Doshi told the panel.
In his presentation, he reiterated points he had made previously, including in an October 21 article in the BMJ, for which he is an associate editor. Doshi also raised these concerns in a September opinion article in The New York Times, co-authored with Eric Topol, MD, director of the Scripps Research Translational Institute and editor-in-chief of Medscape.
Risks of a ‘rushed vaccine’
Other complaints about the FDA’s approach included criticism of a 2-month follow-up time after vaccination, which was seen as too short. ECRI, a nonprofit organization that seeks to improve the safety, quality, and cost-effectiveness of medicines, has argued that approving a weak COVID-19 vaccine might worsen the pandemic.
In an October 21 statement, ECRI noted the risk of a partially effective vaccine, which could be welcomed as a means of slowing transmission of the virus. But public response and attitudes over the past 9 months in the United States suggest that people would relax their precautions as soon as a vaccine is available.
“Resulting infections may offset the vaccine’s impact and end up increasing the mortality and morbidity burden,” ECRI said in the brief.
“The risks and consequences of a rushed vaccine could be very severe if the review is anything shy of thorough,” ECRI Chief Executive Officer Marcus Schabacker, MD, PhD, said in a statement prepared for the hearing.
This article first appeared on Medscape.com.
CDC expands definition of COVID-19 exposure from ‘close contact’
New data suggest each close encounter – coming within 6 feet of an infected person – can increase the risk for transmission, CDC director Robert Redfield, MD, said during a media briefing.
“As we get more data and understand the science of COVID, we’re going to continue to incorporate that in our recommendations,” Dr. Redfield said in response to a reporter’s question about a recent study.
Previously, the CDC cautioned against spending 15 minutes or longer in close proximity to an infected person, particularly in enclosed indoor spaces.
In a new report published online Oct. 21 in Morbidity and Mortality Weekly Report, however, investigators “determined that an individual who had a series of shorter contacts that over time added up to more than 15 minutes became infected.”
Beware of brief encounters?
On July 28, a 20-year-old male correctional officer in Vermont had multiple brief encounters with six transferred incarcerated or detained people while their SARS-CoV-2 test results were pending. The six were asymptomatic at the time and were housed in a quarantine unit, reported CDC researcher Julia Pringle, PhD, and colleagues.
The following day, all six inmates tested polymerase chain reaction (PCR) positive for COVID-19. The correctional officer did not spend 15 minutes or more within 6 feet of any of the inmates, according to video surveillance footage, and he continued to work.
On Aug. 4, however, he developed symptoms that included loss of smell and taste, myalgia, runny nose, cough, shortness of breath, headache, loss of appetite, and gastrointestinal symptoms. He stayed home starting the next day and tested PCR positive for COVID-19 on Aug. 11.
Further review of the surveillance video showed that the officer had numerous brief encounters of approximately 1 minute each that cumulatively exceeded 15 minutes over a 24-hour period, the researchers reported.
During all the interactions with inmates, the correctional officer wore a cloth mask, gown, and eye protection. The inmates wore masks while in their cells but did not have them on during brief cell doorway interactions or in the recreation room, according to the report.
No interaction is 100% safe
“We know that every activity that involves interacting with others has some degree of risk right now,” said Jay Butler, MD, CDC deputy director for infectious diseases.
“Unfortunately, we’re seeing a distressing trend here in the United States with COVID-19 cases increasing in nearly 75% of the country,” he said. “We’ve confirmed 8.1 million cases and, sadly, over 220,000 deaths since January.
“I know these are numbers, but these are also people,” Dr. Butler added.
“The pandemic is not over,” Dr. Redfield said. “Earlier this week, COVID virus cases reached over 40 million globally. Here in the United States we are approaching a critical phase.”
Four factors associated with higher risk for transmission are the proximity of each encounter, its duration, whether an interaction takes place indoors or outdoors, and the number of people encountered, Dr. Butler said.
Dr. Butler acknowledged widespread fatigue with adherence to personal protection measures, but added that social distancing, mask-wearing, and other measures are more important now than ever. He noted that more Americans will be spending time indoors with the onset of cooler weather and the upcoming holidays.
A note of optimism
Dr. Redfield remains optimistic about the limited availability of a vaccine or vaccines by year’s end but added that “it’s important for all of us to remain diligent in our efforts to defeat this virus.”
“There is hope on the way, in the form of safe and effective vaccines in a matter of weeks or months. To bridge to that next phase, we have to take steps to keep ourselves, our families, and our communities safe,” said Alex Azar, secretary of the Department of Health & Human Services.
“I know it’s been a difficult year for Americans, but we are going to come through this on the other side,” Dr. Redfield said.
New data suggest each close encounter – coming within 6 feet of an infected person – can increase the risk for transmission, CDC director Robert Redfield, MD, said during a media briefing.
“As we get more data and understand the science of COVID, we’re going to continue to incorporate that in our recommendations,” Dr. Redfield said in response to a reporter’s question about a recent study.
Previously, the CDC cautioned against spending 15 minutes or longer in close proximity to an infected person, particularly in enclosed indoor spaces.
In a new report published online Oct. 21 in Morbidity and Mortality Weekly Report, however, investigators “determined that an individual who had a series of shorter contacts that over time added up to more than 15 minutes became infected.”
Beware of brief encounters?
On July 28, a 20-year-old male correctional officer in Vermont had multiple brief encounters with six transferred incarcerated or detained people while their SARS-CoV-2 test results were pending. The six were asymptomatic at the time and were housed in a quarantine unit, reported CDC researcher Julia Pringle, PhD, and colleagues.
The following day, all six inmates tested polymerase chain reaction (PCR) positive for COVID-19. The correctional officer did not spend 15 minutes or more within 6 feet of any of the inmates, according to video surveillance footage, and he continued to work.
On Aug. 4, however, he developed symptoms that included loss of smell and taste, myalgia, runny nose, cough, shortness of breath, headache, loss of appetite, and gastrointestinal symptoms. He stayed home starting the next day and tested PCR positive for COVID-19 on Aug. 11.
Further review of the surveillance video showed that the officer had numerous brief encounters of approximately 1 minute each that cumulatively exceeded 15 minutes over a 24-hour period, the researchers reported.
During all the interactions with inmates, the correctional officer wore a cloth mask, gown, and eye protection. The inmates wore masks while in their cells but did not have them on during brief cell doorway interactions or in the recreation room, according to the report.
No interaction is 100% safe
“We know that every activity that involves interacting with others has some degree of risk right now,” said Jay Butler, MD, CDC deputy director for infectious diseases.
“Unfortunately, we’re seeing a distressing trend here in the United States with COVID-19 cases increasing in nearly 75% of the country,” he said. “We’ve confirmed 8.1 million cases and, sadly, over 220,000 deaths since January.
“I know these are numbers, but these are also people,” Dr. Butler added.
“The pandemic is not over,” Dr. Redfield said. “Earlier this week, COVID virus cases reached over 40 million globally. Here in the United States we are approaching a critical phase.”
Four factors associated with higher risk for transmission are the proximity of each encounter, its duration, whether an interaction takes place indoors or outdoors, and the number of people encountered, Dr. Butler said.
Dr. Butler acknowledged widespread fatigue with adherence to personal protection measures, but added that social distancing, mask-wearing, and other measures are more important now than ever. He noted that more Americans will be spending time indoors with the onset of cooler weather and the upcoming holidays.
A note of optimism
Dr. Redfield remains optimistic about the limited availability of a vaccine or vaccines by year’s end but added that “it’s important for all of us to remain diligent in our efforts to defeat this virus.”
“There is hope on the way, in the form of safe and effective vaccines in a matter of weeks or months. To bridge to that next phase, we have to take steps to keep ourselves, our families, and our communities safe,” said Alex Azar, secretary of the Department of Health & Human Services.
“I know it’s been a difficult year for Americans, but we are going to come through this on the other side,” Dr. Redfield said.
New data suggest each close encounter – coming within 6 feet of an infected person – can increase the risk for transmission, CDC director Robert Redfield, MD, said during a media briefing.
“As we get more data and understand the science of COVID, we’re going to continue to incorporate that in our recommendations,” Dr. Redfield said in response to a reporter’s question about a recent study.
Previously, the CDC cautioned against spending 15 minutes or longer in close proximity to an infected person, particularly in enclosed indoor spaces.
In a new report published online Oct. 21 in Morbidity and Mortality Weekly Report, however, investigators “determined that an individual who had a series of shorter contacts that over time added up to more than 15 minutes became infected.”
Beware of brief encounters?
On July 28, a 20-year-old male correctional officer in Vermont had multiple brief encounters with six transferred incarcerated or detained people while their SARS-CoV-2 test results were pending. The six were asymptomatic at the time and were housed in a quarantine unit, reported CDC researcher Julia Pringle, PhD, and colleagues.
The following day, all six inmates tested polymerase chain reaction (PCR) positive for COVID-19. The correctional officer did not spend 15 minutes or more within 6 feet of any of the inmates, according to video surveillance footage, and he continued to work.
On Aug. 4, however, he developed symptoms that included loss of smell and taste, myalgia, runny nose, cough, shortness of breath, headache, loss of appetite, and gastrointestinal symptoms. He stayed home starting the next day and tested PCR positive for COVID-19 on Aug. 11.
Further review of the surveillance video showed that the officer had numerous brief encounters of approximately 1 minute each that cumulatively exceeded 15 minutes over a 24-hour period, the researchers reported.
During all the interactions with inmates, the correctional officer wore a cloth mask, gown, and eye protection. The inmates wore masks while in their cells but did not have them on during brief cell doorway interactions or in the recreation room, according to the report.
No interaction is 100% safe
“We know that every activity that involves interacting with others has some degree of risk right now,” said Jay Butler, MD, CDC deputy director for infectious diseases.
“Unfortunately, we’re seeing a distressing trend here in the United States with COVID-19 cases increasing in nearly 75% of the country,” he said. “We’ve confirmed 8.1 million cases and, sadly, over 220,000 deaths since January.
“I know these are numbers, but these are also people,” Dr. Butler added.
“The pandemic is not over,” Dr. Redfield said. “Earlier this week, COVID virus cases reached over 40 million globally. Here in the United States we are approaching a critical phase.”
Four factors associated with higher risk for transmission are the proximity of each encounter, its duration, whether an interaction takes place indoors or outdoors, and the number of people encountered, Dr. Butler said.
Dr. Butler acknowledged widespread fatigue with adherence to personal protection measures, but added that social distancing, mask-wearing, and other measures are more important now than ever. He noted that more Americans will be spending time indoors with the onset of cooler weather and the upcoming holidays.
A note of optimism
Dr. Redfield remains optimistic about the limited availability of a vaccine or vaccines by year’s end but added that “it’s important for all of us to remain diligent in our efforts to defeat this virus.”
“There is hope on the way, in the form of safe and effective vaccines in a matter of weeks or months. To bridge to that next phase, we have to take steps to keep ourselves, our families, and our communities safe,” said Alex Azar, secretary of the Department of Health & Human Services.
“I know it’s been a difficult year for Americans, but we are going to come through this on the other side,” Dr. Redfield said.