User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'medstat-accordion-set article-series')]
Dining restrictions, mask mandates tied to less illness, death, CDC reaffirms
The numbers are in to back up two policies designed to restrict the spread of the COVID-19 pandemic.
Researchers at the Centers for Disease Control and Prevention) found that when states lifted restrictions on dining on premises at restaurants, rates of daily COVID-19 cases jumped 41-100 days later. COVID-19-related deaths also increased significantly after 60 days.
On the other hand, the same report demonstrates that state mask mandates slowed the spread of SARS-CoV-2 within a few weeks.
The study was published online March 5 in the CDC Morbidity and Mortality Weekly Report.
The investigators did not distinguish between outdoor and indoor restaurant dining. But they did compare COVID-19 case and death rates before and after most states banned restaurants from serving patrons on-premises in March and April 2020.
They found, for example, that COVID-19 daily cases increased by 0.9% at 41-60 days after on-premise dining was permitted. Similarly, rates jumped by 1.2% at 61-80 days, and 1.1% at 81-100 days after the restaurant restrictions were lifted.
The differences were statistically significant, with P values of .02, <.01, and .04, respectively.
COVID-19–related death rates did not increase significantly at first – but did jump 2.2% between 61 and 80 days after the return of on-premises dining, for example. Deaths also increased by 3% at 81-100 days.
Both these differences were statistically significant (P < .01).
This is not the first report where the CDC announced reservations about in-person dining. In September 2020, CDC investigators implicated the inability to wear a mask while eating and drinking as likely contributing to the heightened risk.
Masks make a difference
The CDC report also provided more evidence to back mask-wearing policies for public spaces. Between March 1 and Dec. 31, 2020, 74% of U.S. counties issued mask mandates.
Investigators found that these policies had a more immediate effect, reducing daily COVID-19 cases by 0.5% in the first 20 days. Mask mandates likewise were linked to daily cases dropping 1.1% between 21 and 40 days, 1.5% between 41 and 60 days, 1.7% between 61 and 80 days, and 1.8% between 81 and 100 days.
These decreases in daily COVID-19 cases were statistically significant (P < .01) compared with a reference period before March 1, 2020.
The CDC also linked mask mandates to lower mortality. For example, these state policies were associated with 0.7% fewer deaths at 1-20 days post implementation. The effect increased thereafter – 1.0% drop at 21-40 days, 1.4% decrease at 41-60 days, 1.6% drop between 61 and 80 days, and 1.9% fewer deaths between 81 and 100 days.
The decrease in deaths was statistically significant at 1-20 days after the mask mandate (P = .03), as well as during the other periods (each P < .01) compared with the reference period.
CDC Director Rochelle Walensky, MD, reacted to the new findings at a White House press briefing. She cited how increases in COVID-19 cases and death rates “slowed significantly within 20 days of putting mask mandates into place. This is why I’m asking you to double down on prevention measures.
“We have seen this movie before,” Dr. Walensky added. “When prevention measures like mask-wearing mandates are lifted, cases go up.”
Recently, multiple states have announced plans to roll back restrictions related to the pandemic, including mask mandates, which prompted warnings from some public health officials.
These are not the first CDC data to show that mask mandates make a difference.
In February 2021, for example, the agency pointed out that state-wide mask mandates reduced COVID-19 hospitalizations by 5.5% among adults 18-64 years old within 3 weeks of implementation.
Restrictions regarding on-premises restaurant dining and implementation of state-wide mask mandates are two tactics within a more comprehensive CDC strategy to reduce the spread of SARS-CoV-2. The researchers note that “such efforts are increasingly important given the emergence of highly transmissible SARS-CoV-2 variants in the United States.”
The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The numbers are in to back up two policies designed to restrict the spread of the COVID-19 pandemic.
Researchers at the Centers for Disease Control and Prevention) found that when states lifted restrictions on dining on premises at restaurants, rates of daily COVID-19 cases jumped 41-100 days later. COVID-19-related deaths also increased significantly after 60 days.
On the other hand, the same report demonstrates that state mask mandates slowed the spread of SARS-CoV-2 within a few weeks.
The study was published online March 5 in the CDC Morbidity and Mortality Weekly Report.
The investigators did not distinguish between outdoor and indoor restaurant dining. But they did compare COVID-19 case and death rates before and after most states banned restaurants from serving patrons on-premises in March and April 2020.
They found, for example, that COVID-19 daily cases increased by 0.9% at 41-60 days after on-premise dining was permitted. Similarly, rates jumped by 1.2% at 61-80 days, and 1.1% at 81-100 days after the restaurant restrictions were lifted.
The differences were statistically significant, with P values of .02, <.01, and .04, respectively.
COVID-19–related death rates did not increase significantly at first – but did jump 2.2% between 61 and 80 days after the return of on-premises dining, for example. Deaths also increased by 3% at 81-100 days.
Both these differences were statistically significant (P < .01).
This is not the first report where the CDC announced reservations about in-person dining. In September 2020, CDC investigators implicated the inability to wear a mask while eating and drinking as likely contributing to the heightened risk.
Masks make a difference
The CDC report also provided more evidence to back mask-wearing policies for public spaces. Between March 1 and Dec. 31, 2020, 74% of U.S. counties issued mask mandates.
Investigators found that these policies had a more immediate effect, reducing daily COVID-19 cases by 0.5% in the first 20 days. Mask mandates likewise were linked to daily cases dropping 1.1% between 21 and 40 days, 1.5% between 41 and 60 days, 1.7% between 61 and 80 days, and 1.8% between 81 and 100 days.
These decreases in daily COVID-19 cases were statistically significant (P < .01) compared with a reference period before March 1, 2020.
The CDC also linked mask mandates to lower mortality. For example, these state policies were associated with 0.7% fewer deaths at 1-20 days post implementation. The effect increased thereafter – 1.0% drop at 21-40 days, 1.4% decrease at 41-60 days, 1.6% drop between 61 and 80 days, and 1.9% fewer deaths between 81 and 100 days.
The decrease in deaths was statistically significant at 1-20 days after the mask mandate (P = .03), as well as during the other periods (each P < .01) compared with the reference period.
CDC Director Rochelle Walensky, MD, reacted to the new findings at a White House press briefing. She cited how increases in COVID-19 cases and death rates “slowed significantly within 20 days of putting mask mandates into place. This is why I’m asking you to double down on prevention measures.
“We have seen this movie before,” Dr. Walensky added. “When prevention measures like mask-wearing mandates are lifted, cases go up.”
Recently, multiple states have announced plans to roll back restrictions related to the pandemic, including mask mandates, which prompted warnings from some public health officials.
These are not the first CDC data to show that mask mandates make a difference.
In February 2021, for example, the agency pointed out that state-wide mask mandates reduced COVID-19 hospitalizations by 5.5% among adults 18-64 years old within 3 weeks of implementation.
Restrictions regarding on-premises restaurant dining and implementation of state-wide mask mandates are two tactics within a more comprehensive CDC strategy to reduce the spread of SARS-CoV-2. The researchers note that “such efforts are increasingly important given the emergence of highly transmissible SARS-CoV-2 variants in the United States.”
The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The numbers are in to back up two policies designed to restrict the spread of the COVID-19 pandemic.
Researchers at the Centers for Disease Control and Prevention) found that when states lifted restrictions on dining on premises at restaurants, rates of daily COVID-19 cases jumped 41-100 days later. COVID-19-related deaths also increased significantly after 60 days.
On the other hand, the same report demonstrates that state mask mandates slowed the spread of SARS-CoV-2 within a few weeks.
The study was published online March 5 in the CDC Morbidity and Mortality Weekly Report.
The investigators did not distinguish between outdoor and indoor restaurant dining. But they did compare COVID-19 case and death rates before and after most states banned restaurants from serving patrons on-premises in March and April 2020.
They found, for example, that COVID-19 daily cases increased by 0.9% at 41-60 days after on-premise dining was permitted. Similarly, rates jumped by 1.2% at 61-80 days, and 1.1% at 81-100 days after the restaurant restrictions were lifted.
The differences were statistically significant, with P values of .02, <.01, and .04, respectively.
COVID-19–related death rates did not increase significantly at first – but did jump 2.2% between 61 and 80 days after the return of on-premises dining, for example. Deaths also increased by 3% at 81-100 days.
Both these differences were statistically significant (P < .01).
This is not the first report where the CDC announced reservations about in-person dining. In September 2020, CDC investigators implicated the inability to wear a mask while eating and drinking as likely contributing to the heightened risk.
Masks make a difference
The CDC report also provided more evidence to back mask-wearing policies for public spaces. Between March 1 and Dec. 31, 2020, 74% of U.S. counties issued mask mandates.
Investigators found that these policies had a more immediate effect, reducing daily COVID-19 cases by 0.5% in the first 20 days. Mask mandates likewise were linked to daily cases dropping 1.1% between 21 and 40 days, 1.5% between 41 and 60 days, 1.7% between 61 and 80 days, and 1.8% between 81 and 100 days.
These decreases in daily COVID-19 cases were statistically significant (P < .01) compared with a reference period before March 1, 2020.
The CDC also linked mask mandates to lower mortality. For example, these state policies were associated with 0.7% fewer deaths at 1-20 days post implementation. The effect increased thereafter – 1.0% drop at 21-40 days, 1.4% decrease at 41-60 days, 1.6% drop between 61 and 80 days, and 1.9% fewer deaths between 81 and 100 days.
The decrease in deaths was statistically significant at 1-20 days after the mask mandate (P = .03), as well as during the other periods (each P < .01) compared with the reference period.
CDC Director Rochelle Walensky, MD, reacted to the new findings at a White House press briefing. She cited how increases in COVID-19 cases and death rates “slowed significantly within 20 days of putting mask mandates into place. This is why I’m asking you to double down on prevention measures.
“We have seen this movie before,” Dr. Walensky added. “When prevention measures like mask-wearing mandates are lifted, cases go up.”
Recently, multiple states have announced plans to roll back restrictions related to the pandemic, including mask mandates, which prompted warnings from some public health officials.
These are not the first CDC data to show that mask mandates make a difference.
In February 2021, for example, the agency pointed out that state-wide mask mandates reduced COVID-19 hospitalizations by 5.5% among adults 18-64 years old within 3 weeks of implementation.
Restrictions regarding on-premises restaurant dining and implementation of state-wide mask mandates are two tactics within a more comprehensive CDC strategy to reduce the spread of SARS-CoV-2. The researchers note that “such efforts are increasingly important given the emergence of highly transmissible SARS-CoV-2 variants in the United States.”
The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tocilizumab (Actemra) scores FDA approval for systemic sclerosis–associated interstitial lung disease
The Food and Drug Administration has approved subcutaneously-injected tocilizumab (Actemra) to reduce the rate of pulmonary function decline in systemic sclerosis–associated interstitial lung disease (SSc-ILD) patients, according to a press release from manufacturer Genentech.
Tocilizumab is the first biologic to be approved by the agency for adults with SSc-ILD, a rare and potentially life-threatening condition that may affect up to 80% of SSc patients and lead to lung inflammation and scarring.
The approval was based primarily on data from a phase 3 randomized, double-blind, placebo-controlled clinical trial (the focuSSced trial) that included 212 adults with SSc. Although that study failed to meet its primary endpoint of change from baseline to 48 weeks in the modified Rodnan Skin Score, the researchers observed a significantly reduced lung function decline as measured by forced vital capacity (FVC) and percent predicted forced vital capacity (ppFVC) among tocilizumab-treated patients, compared with those who received placebo. A total of 68 patients (65%) in the tocilizumab group and 68 patients (64%) in the placebo group had SSc-ILD at baseline.
In a subgroup analysis, patients taking tocilizumab had a smaller decline in mean ppFVC, compared with placebo patients (0.07% vs. –6.4%; mean difference, 6.47%), and a smaller decline in FVC (mean change –14 mL vs. –255 mL with placebo; mean difference, 241 mL).
The mean change from baseline to week 48 in modified Rodnan Skin Score was –5.88 for patients on tocilizumab and –3.77 with placebo.
Safety data were similar between tocilizumab and placebo groups through 48 weeks, and similar for patients with and without SSc-ILD. In general, tocilizumab side effects include increased susceptibility to infections, and serious side effects may include stomach tears, hepatotoxicity, and increased risk of cancer and hepatitis B, according to the prescribing information. However, the most common side effects are upper respiratory tract infections, headache, hypertension, and injection-site reactions.
Tocilizumab, an interleukin-6 receptor antagonist, is already approved for the treatment of adult patients with moderately to severely active rheumatoid arthritis, as well as for adult patients with giant cell arteritis; patients aged 2 years and older with active polyarticular juvenile idiopathic arthritis or active systemic juvenile idiopathic arthritis; and adults and pediatric patients 2 years of age and older with chimeric antigen receptor T-cell–induced severe or life-threatening cytokine release syndrome.
Prescribing information is available here.
The Food and Drug Administration has approved subcutaneously-injected tocilizumab (Actemra) to reduce the rate of pulmonary function decline in systemic sclerosis–associated interstitial lung disease (SSc-ILD) patients, according to a press release from manufacturer Genentech.
Tocilizumab is the first biologic to be approved by the agency for adults with SSc-ILD, a rare and potentially life-threatening condition that may affect up to 80% of SSc patients and lead to lung inflammation and scarring.
The approval was based primarily on data from a phase 3 randomized, double-blind, placebo-controlled clinical trial (the focuSSced trial) that included 212 adults with SSc. Although that study failed to meet its primary endpoint of change from baseline to 48 weeks in the modified Rodnan Skin Score, the researchers observed a significantly reduced lung function decline as measured by forced vital capacity (FVC) and percent predicted forced vital capacity (ppFVC) among tocilizumab-treated patients, compared with those who received placebo. A total of 68 patients (65%) in the tocilizumab group and 68 patients (64%) in the placebo group had SSc-ILD at baseline.
In a subgroup analysis, patients taking tocilizumab had a smaller decline in mean ppFVC, compared with placebo patients (0.07% vs. –6.4%; mean difference, 6.47%), and a smaller decline in FVC (mean change –14 mL vs. –255 mL with placebo; mean difference, 241 mL).
The mean change from baseline to week 48 in modified Rodnan Skin Score was –5.88 for patients on tocilizumab and –3.77 with placebo.
Safety data were similar between tocilizumab and placebo groups through 48 weeks, and similar for patients with and without SSc-ILD. In general, tocilizumab side effects include increased susceptibility to infections, and serious side effects may include stomach tears, hepatotoxicity, and increased risk of cancer and hepatitis B, according to the prescribing information. However, the most common side effects are upper respiratory tract infections, headache, hypertension, and injection-site reactions.
Tocilizumab, an interleukin-6 receptor antagonist, is already approved for the treatment of adult patients with moderately to severely active rheumatoid arthritis, as well as for adult patients with giant cell arteritis; patients aged 2 years and older with active polyarticular juvenile idiopathic arthritis or active systemic juvenile idiopathic arthritis; and adults and pediatric patients 2 years of age and older with chimeric antigen receptor T-cell–induced severe or life-threatening cytokine release syndrome.
Prescribing information is available here.
The Food and Drug Administration has approved subcutaneously-injected tocilizumab (Actemra) to reduce the rate of pulmonary function decline in systemic sclerosis–associated interstitial lung disease (SSc-ILD) patients, according to a press release from manufacturer Genentech.
Tocilizumab is the first biologic to be approved by the agency for adults with SSc-ILD, a rare and potentially life-threatening condition that may affect up to 80% of SSc patients and lead to lung inflammation and scarring.
The approval was based primarily on data from a phase 3 randomized, double-blind, placebo-controlled clinical trial (the focuSSced trial) that included 212 adults with SSc. Although that study failed to meet its primary endpoint of change from baseline to 48 weeks in the modified Rodnan Skin Score, the researchers observed a significantly reduced lung function decline as measured by forced vital capacity (FVC) and percent predicted forced vital capacity (ppFVC) among tocilizumab-treated patients, compared with those who received placebo. A total of 68 patients (65%) in the tocilizumab group and 68 patients (64%) in the placebo group had SSc-ILD at baseline.
In a subgroup analysis, patients taking tocilizumab had a smaller decline in mean ppFVC, compared with placebo patients (0.07% vs. –6.4%; mean difference, 6.47%), and a smaller decline in FVC (mean change –14 mL vs. –255 mL with placebo; mean difference, 241 mL).
The mean change from baseline to week 48 in modified Rodnan Skin Score was –5.88 for patients on tocilizumab and –3.77 with placebo.
Safety data were similar between tocilizumab and placebo groups through 48 weeks, and similar for patients with and without SSc-ILD. In general, tocilizumab side effects include increased susceptibility to infections, and serious side effects may include stomach tears, hepatotoxicity, and increased risk of cancer and hepatitis B, according to the prescribing information. However, the most common side effects are upper respiratory tract infections, headache, hypertension, and injection-site reactions.
Tocilizumab, an interleukin-6 receptor antagonist, is already approved for the treatment of adult patients with moderately to severely active rheumatoid arthritis, as well as for adult patients with giant cell arteritis; patients aged 2 years and older with active polyarticular juvenile idiopathic arthritis or active systemic juvenile idiopathic arthritis; and adults and pediatric patients 2 years of age and older with chimeric antigen receptor T-cell–induced severe or life-threatening cytokine release syndrome.
Prescribing information is available here.
Routine vaccinations missed by older adults during pandemic
Physicians are going to have to play catch-up when it comes to getting older patients their routine, but important, vaccinations missed during the pandemic.
and have recovered only partially and gradually, according to a report by Kai Hong, PhD, and colleagues at the Centers for Disease Control and Prevention, published in the Morbidity and Mortality Weekly Report. “As the pandemic continues,” the investigators stated, “vaccination providers should continue efforts to resolve disruptions in routine adult vaccination.”
The CDC issued guidance recommending postponement of routine adult vaccination in response to the March 13, 2020, COVID-19 national emergency declaration by the U.S. government and also to state and local shelter-in-place orders. Health care facility operations were restricted because of safety concerns around exposure to the SARS-CoV-2 virus. The result was a significant drop in routine medical care including adult vaccinations.
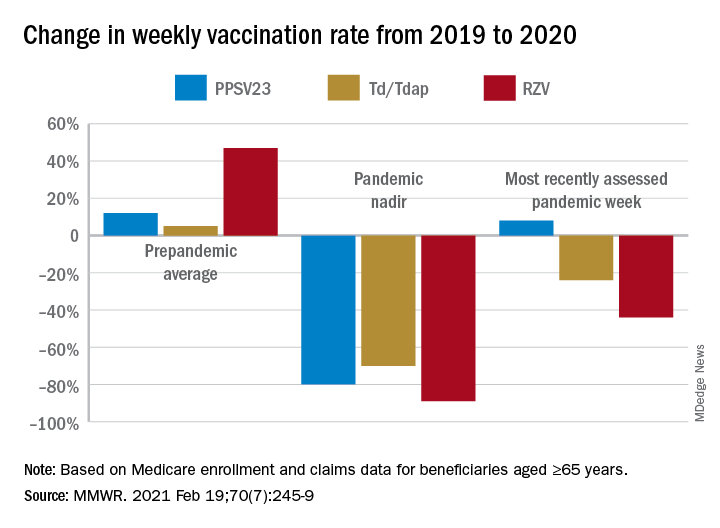
The investigators examined Medicare enrollment and claims data to assess the change in weekly receipt of four routine adult vaccines by Medicare beneficiaries aged ≥65 during the pandemic: (13-valent pneumococcal conjugate vaccine [PCV13], 23-valent pneumococcal polysaccharide vaccine [PPSV23], tetanus-diphtheria or tetanus-diphtheria-acellular pertussis vaccine [Td/Tdap], and recombinant zoster vaccine [RZV]). The comparison periods were Jan. 6–July 20, 2019, and Jan. 5–July 18, 2020.
Of the Medicare enrollees in the study sample, 85% were White, 7% Black, 2% Asian, 2% Hispanic, and 4% other racial and ethnic groups. For each of the four vaccines overall, weekly rates of vaccination declined sharply after the emergency declaration, compared with corresponding weeks in 2019. In the period prior to the emergency declaration (Jan. 5–March 14, 2020), weekly percentages of Medicare beneficiaries vaccinated with PPSV23, Td/Tdap, and RZV were consistently higher than rates during the same period in 2019.
After the March 13 declaration, while weekly vaccination rates plummeted 25% for PPSV23 and 62% for RZV in the first week, the greatest weekly declines were during April 5-11, 2020, for PCV13, PPSV23, and Td/Tdap, and during April 12-18, 2020, for RZV. The pandemic weekly vaccination rate nadirs revealed declines of 88% for PCV13, 80% for PPSV23, 70% for Td/Tdap, and 89% for RZV.
Routine vaccinations increased midyear
Vaccination rates recovered gradually. For the most recently assessed pandemic week (July 12-18, 2020), the rate for PPSV23 was 8% higher than in the corresponding period in 2019. Weekly corresponding rates for other examined vaccines, however, remained much lower than in 2019: 44% lower for RZV, 24% lower for Td/Tdap and 43% lower for PCV13. The CDC Advisory Committee on Immunization Practices voted in June 2019 to stop recommending PCV13 for adults aged ≥65 years and so vaccination with PCV13 among this population declined in 2020, compared with that in 2019.
Another significant drop in the rates of adult vaccinations may have occurred because of the surge in COVID-19 infections in the fall of 2020 and subsequent closures and renewal of lockdown in many localities.
Disparities in routine vaccination trends
Dr. Hong and colleagues noted that their findings are consistent with prior reports of declines in pediatric vaccine ordering, administration, and coverage during the pandemic. While the reductions were similar across all racial and ethnic groups, the magnitudes of recovery varied, with vaccination rates lower among racial and ethnic minority adults than among White adults.
In view of the disproportionate COVID-19 pandemic effects among some racial and ethnic minorities, the investigators recommended monitoring and subsequent early intervention to mitigate similar indirect pandemic effects, such as reduced utilization of other preventive services. “Many members of racial and ethnic minority groups face barriers to routine medical care, which means they have fewer opportunities to receive preventive interventions such as vaccination,” Dr. Hong said in an interview. “When clinicians are following up with patients who have missed vaccinations, it is important for them to remember that patients may face new barriers to vaccination such as loss of income or health insurance, and to work with them to remove those barriers,” he added.
“If vaccination is deferred, older adults and adults with underlying medical conditions who subsequently become infected with a vaccine-preventable disease are at increased risk for complications,” Dr. Hong said. “The most important thing clinicians can do is identify patients who are due for or who have missed vaccinations, and contact them to schedule visits. Immunization Information Systems and electronic health records may be able to support this work. In addition, the vaccination status of all patients should be assessed at every health care visit to reduce missed opportunities for vaccination.”
Physicians are going to have to play catch-up when it comes to getting older patients their routine, but important, vaccinations missed during the pandemic.
and have recovered only partially and gradually, according to a report by Kai Hong, PhD, and colleagues at the Centers for Disease Control and Prevention, published in the Morbidity and Mortality Weekly Report. “As the pandemic continues,” the investigators stated, “vaccination providers should continue efforts to resolve disruptions in routine adult vaccination.”
The CDC issued guidance recommending postponement of routine adult vaccination in response to the March 13, 2020, COVID-19 national emergency declaration by the U.S. government and also to state and local shelter-in-place orders. Health care facility operations were restricted because of safety concerns around exposure to the SARS-CoV-2 virus. The result was a significant drop in routine medical care including adult vaccinations.
The investigators examined Medicare enrollment and claims data to assess the change in weekly receipt of four routine adult vaccines by Medicare beneficiaries aged ≥65 during the pandemic: (13-valent pneumococcal conjugate vaccine [PCV13], 23-valent pneumococcal polysaccharide vaccine [PPSV23], tetanus-diphtheria or tetanus-diphtheria-acellular pertussis vaccine [Td/Tdap], and recombinant zoster vaccine [RZV]). The comparison periods were Jan. 6–July 20, 2019, and Jan. 5–July 18, 2020.
Of the Medicare enrollees in the study sample, 85% were White, 7% Black, 2% Asian, 2% Hispanic, and 4% other racial and ethnic groups. For each of the four vaccines overall, weekly rates of vaccination declined sharply after the emergency declaration, compared with corresponding weeks in 2019. In the period prior to the emergency declaration (Jan. 5–March 14, 2020), weekly percentages of Medicare beneficiaries vaccinated with PPSV23, Td/Tdap, and RZV were consistently higher than rates during the same period in 2019.
After the March 13 declaration, while weekly vaccination rates plummeted 25% for PPSV23 and 62% for RZV in the first week, the greatest weekly declines were during April 5-11, 2020, for PCV13, PPSV23, and Td/Tdap, and during April 12-18, 2020, for RZV. The pandemic weekly vaccination rate nadirs revealed declines of 88% for PCV13, 80% for PPSV23, 70% for Td/Tdap, and 89% for RZV.
Routine vaccinations increased midyear
Vaccination rates recovered gradually. For the most recently assessed pandemic week (July 12-18, 2020), the rate for PPSV23 was 8% higher than in the corresponding period in 2019. Weekly corresponding rates for other examined vaccines, however, remained much lower than in 2019: 44% lower for RZV, 24% lower for Td/Tdap and 43% lower for PCV13. The CDC Advisory Committee on Immunization Practices voted in June 2019 to stop recommending PCV13 for adults aged ≥65 years and so vaccination with PCV13 among this population declined in 2020, compared with that in 2019.
Another significant drop in the rates of adult vaccinations may have occurred because of the surge in COVID-19 infections in the fall of 2020 and subsequent closures and renewal of lockdown in many localities.
Disparities in routine vaccination trends
Dr. Hong and colleagues noted that their findings are consistent with prior reports of declines in pediatric vaccine ordering, administration, and coverage during the pandemic. While the reductions were similar across all racial and ethnic groups, the magnitudes of recovery varied, with vaccination rates lower among racial and ethnic minority adults than among White adults.

In view of the disproportionate COVID-19 pandemic effects among some racial and ethnic minorities, the investigators recommended monitoring and subsequent early intervention to mitigate similar indirect pandemic effects, such as reduced utilization of other preventive services. “Many members of racial and ethnic minority groups face barriers to routine medical care, which means they have fewer opportunities to receive preventive interventions such as vaccination,” Dr. Hong said in an interview. “When clinicians are following up with patients who have missed vaccinations, it is important for them to remember that patients may face new barriers to vaccination such as loss of income or health insurance, and to work with them to remove those barriers,” he added.
“If vaccination is deferred, older adults and adults with underlying medical conditions who subsequently become infected with a vaccine-preventable disease are at increased risk for complications,” Dr. Hong said. “The most important thing clinicians can do is identify patients who are due for or who have missed vaccinations, and contact them to schedule visits. Immunization Information Systems and electronic health records may be able to support this work. In addition, the vaccination status of all patients should be assessed at every health care visit to reduce missed opportunities for vaccination.”
Physicians are going to have to play catch-up when it comes to getting older patients their routine, but important, vaccinations missed during the pandemic.
and have recovered only partially and gradually, according to a report by Kai Hong, PhD, and colleagues at the Centers for Disease Control and Prevention, published in the Morbidity and Mortality Weekly Report. “As the pandemic continues,” the investigators stated, “vaccination providers should continue efforts to resolve disruptions in routine adult vaccination.”
The CDC issued guidance recommending postponement of routine adult vaccination in response to the March 13, 2020, COVID-19 national emergency declaration by the U.S. government and also to state and local shelter-in-place orders. Health care facility operations were restricted because of safety concerns around exposure to the SARS-CoV-2 virus. The result was a significant drop in routine medical care including adult vaccinations.
The investigators examined Medicare enrollment and claims data to assess the change in weekly receipt of four routine adult vaccines by Medicare beneficiaries aged ≥65 during the pandemic: (13-valent pneumococcal conjugate vaccine [PCV13], 23-valent pneumococcal polysaccharide vaccine [PPSV23], tetanus-diphtheria or tetanus-diphtheria-acellular pertussis vaccine [Td/Tdap], and recombinant zoster vaccine [RZV]). The comparison periods were Jan. 6–July 20, 2019, and Jan. 5–July 18, 2020.
Of the Medicare enrollees in the study sample, 85% were White, 7% Black, 2% Asian, 2% Hispanic, and 4% other racial and ethnic groups. For each of the four vaccines overall, weekly rates of vaccination declined sharply after the emergency declaration, compared with corresponding weeks in 2019. In the period prior to the emergency declaration (Jan. 5–March 14, 2020), weekly percentages of Medicare beneficiaries vaccinated with PPSV23, Td/Tdap, and RZV were consistently higher than rates during the same period in 2019.
After the March 13 declaration, while weekly vaccination rates plummeted 25% for PPSV23 and 62% for RZV in the first week, the greatest weekly declines were during April 5-11, 2020, for PCV13, PPSV23, and Td/Tdap, and during April 12-18, 2020, for RZV. The pandemic weekly vaccination rate nadirs revealed declines of 88% for PCV13, 80% for PPSV23, 70% for Td/Tdap, and 89% for RZV.
Routine vaccinations increased midyear
Vaccination rates recovered gradually. For the most recently assessed pandemic week (July 12-18, 2020), the rate for PPSV23 was 8% higher than in the corresponding period in 2019. Weekly corresponding rates for other examined vaccines, however, remained much lower than in 2019: 44% lower for RZV, 24% lower for Td/Tdap and 43% lower for PCV13. The CDC Advisory Committee on Immunization Practices voted in June 2019 to stop recommending PCV13 for adults aged ≥65 years and so vaccination with PCV13 among this population declined in 2020, compared with that in 2019.
Another significant drop in the rates of adult vaccinations may have occurred because of the surge in COVID-19 infections in the fall of 2020 and subsequent closures and renewal of lockdown in many localities.
Disparities in routine vaccination trends
Dr. Hong and colleagues noted that their findings are consistent with prior reports of declines in pediatric vaccine ordering, administration, and coverage during the pandemic. While the reductions were similar across all racial and ethnic groups, the magnitudes of recovery varied, with vaccination rates lower among racial and ethnic minority adults than among White adults.

In view of the disproportionate COVID-19 pandemic effects among some racial and ethnic minorities, the investigators recommended monitoring and subsequent early intervention to mitigate similar indirect pandemic effects, such as reduced utilization of other preventive services. “Many members of racial and ethnic minority groups face barriers to routine medical care, which means they have fewer opportunities to receive preventive interventions such as vaccination,” Dr. Hong said in an interview. “When clinicians are following up with patients who have missed vaccinations, it is important for them to remember that patients may face new barriers to vaccination such as loss of income or health insurance, and to work with them to remove those barriers,” he added.
“If vaccination is deferred, older adults and adults with underlying medical conditions who subsequently become infected with a vaccine-preventable disease are at increased risk for complications,” Dr. Hong said. “The most important thing clinicians can do is identify patients who are due for or who have missed vaccinations, and contact them to schedule visits. Immunization Information Systems and electronic health records may be able to support this work. In addition, the vaccination status of all patients should be assessed at every health care visit to reduce missed opportunities for vaccination.”
FROM MMWR
BMI, age, and sex affect COVID-19 vaccine antibody response
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
JAMA podcast on racism in medicine faces backlash
Published on Feb. 23, the episode is hosted on JAMA’s learning platform for doctors and is available for continuing medical education credits.
“No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast,” JAMA wrote in a Twitter post to promote the episode. That tweet has since been deleted.

The episode features host Ed Livingston, MD, deputy editor for clinical reviews and education at JAMA, and guest Mitchell Katz, MD, president and CEO for NYC Health + Hospitals and deputy editor for JAMA Internal Medicine. Dr. Livingston approaches the episode as “structural racism for skeptics,” and Dr. Katz tries to explain how structural racism deepens health disparities and what health systems can do about it.
“Many physicians are skeptical of structural racism, the idea that economic, educational, and other societal systems preferentially disadvantage Black Americans and other communities of color,” the episode description says.
In the podcast, Dr. Livingston and Dr. Katz speak about health care disparities and racial inequality. Dr. Livingston, who says he “didn’t understand the concept” going into the episode, suggests that racism was made illegal in the 1960s and that the discussion of “structural racism” should shift away from the term “racism” and focus on socioeconomic status instead.
“What you’re talking about isn’t so much racism ... it isn’t their race, it isn’t their color, it’s their socioeconomic status,” Dr. Livingston says. “Is that a fair statement?”
But Dr. Katz says that “acknowledging structural racism can be helpful to us. Structural racism refers to a system in which policies or practices or how we look at people perpetuates racial inequality.”
Dr. Katz points to the creation of a hospital in San Francisco in the 1880s to treat patients of Chinese ethnicity separately. Outside of health care, he talks about environmental racism between neighborhoods with inequalities in hospitals, schools, and social services.
“All of those things have an impact on that minority person,” Dr. Katz says. “The big thing we can all do is move away from trying to interrogate each other’s opinions and move to a place where we are looking at the policies of our institutions and making sure that they promote equality.”
Dr. Livingston concludes the episode by reemphasizing that “racism” should be taken out of the conversation and it should instead focus on the “structural” aspect of socioeconomics.
“Minorities ... aren’t [in those neighborhoods] because they’re not allowed to buy houses or they can’t get a job because they’re Black or Hispanic. That would be illegal,” Dr. Livingston says. “But disproportionality does exist.”
Efforts to reach Dr. Livingston were unsuccessful. Dr. Katz distanced himself from Dr. Livingston in a statement released on March 4.
“Systemic and interpersonal racism both still exist in our country — they must be rooted out. I do not share the JAMA host’s belief of doing away with the word ‘racism’ will help us be more successful in ending inequities that exists across racial and ethnic lines,” Dr. Katz said. “Further, I believe that we will only produce an equitable society when social and political structures do not continue to produce and perpetuate disparate results based on social race and ethnicity.”
Dr. Katz reiterated that both interpersonal and structural racism continue to exist in the United States, “and it is woefully naive to say that no physician is a racist just because the Civil Rights Act of 1964 forbade it.”
He also recommended JAMA use this controversy “as a learning opportunity for continued dialogue and create another podcast series as an open conversation that invites diverse experts in the field to have an open discussion about structural racism in healthcare.”
The podcast and JAMA’s tweet promoting it were widely criticized on Twitter. In interviews with WebMD, many doctors expressed disbelief that such a respected journal would lend its name to this podcast episode.
B. Bobby Chiong, MD, a radiologist in New York, said although JAMA’s effort to engage with its audience about racism is laudable, it missed the mark.
“I think the backlash comes from how they tried to make a podcast about the subject and somehow made themselves an example of unconscious bias and unfamiliarity with just how embedded in our system is structural racism,” he said.
Perhaps the podcast’s worst offense was its failure to address the painful history of racial bias in this country that still permeates the medical community, says Tamara Saint-Surin, MD, assistant professor at the University of North Carolina at Chapel Hill.
“For physicians in leadership to have the belief that structural racism does not exist in medicine, they don’t really appreciate what affects their patients and what their patients were dealing with,” Dr. Saint-Surin said in an interview. “It was a very harmful podcast and goes to show we still have so much work to do.”
Along with a flawed premise, she says, the podcast was not nearly long enough to address such a nuanced issue. And Dr. Livingston focused on interpersonal racism rather than structural racism, she said, failing to address widespread problems such as higher rates of asthma among Black populations living in areas with poor air quality.
The number of Black doctors remains low and the lack of representation adds to an environment already rife with racism, according to many medical professionals.
Shirlene Obuobi, MD, an internal medicine doctor in Chicago, said JAMA failed to live up to its own standards by publishing material that lacked research and expertise.
“I can’t submit a clinical trial to JAMA without them combing through methods with a fine-tooth comb,” Dr. Obuobi said. “They didn’t uphold the standards they normally apply to anyone else.”
Both the editor of JAMA and the head of the American Medical Association issued statements criticizing the episode and the tweet that promoted it.
JAMA Editor-in-Chief Howard Bauchner, MD, said, “The language of the tweet, and some portions of the podcast, do not reflect my commitment as editorial leader of JAMA and JAMA Network to call out and discuss the adverse effects of injustice, inequity, and racism in society and medicine as JAMA has done for many years.” He said JAMA will schedule a future podcast to address the concerns raised about the recent episode.
AMA CEO James L. Madara, MD, said, “The AMA’s House of Delegates passed policy stating that racism is structural, systemic, cultural, and interpersonal, and we are deeply disturbed – and angered – by a recent JAMA podcast that questioned the existence of structural racism and the affiliated tweet that promoted the podcast and stated ‘no physician is racist, so how can there be structural racism in health care?’ ”
He continued: “JAMA has editorial independence from AMA, but this tweet and podcast are inconsistent with the policies and views of AMA, and I’m concerned about and acknowledge the harms they have caused. Structural racism in health care and our society exists, and it is incumbent on all of us to fix it.”
This article was updated 3/5/21.
A version of this article first appeared on WebMD.com.
Published on Feb. 23, the episode is hosted on JAMA’s learning platform for doctors and is available for continuing medical education credits.
“No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast,” JAMA wrote in a Twitter post to promote the episode. That tweet has since been deleted.

The episode features host Ed Livingston, MD, deputy editor for clinical reviews and education at JAMA, and guest Mitchell Katz, MD, president and CEO for NYC Health + Hospitals and deputy editor for JAMA Internal Medicine. Dr. Livingston approaches the episode as “structural racism for skeptics,” and Dr. Katz tries to explain how structural racism deepens health disparities and what health systems can do about it.
“Many physicians are skeptical of structural racism, the idea that economic, educational, and other societal systems preferentially disadvantage Black Americans and other communities of color,” the episode description says.
In the podcast, Dr. Livingston and Dr. Katz speak about health care disparities and racial inequality. Dr. Livingston, who says he “didn’t understand the concept” going into the episode, suggests that racism was made illegal in the 1960s and that the discussion of “structural racism” should shift away from the term “racism” and focus on socioeconomic status instead.
“What you’re talking about isn’t so much racism ... it isn’t their race, it isn’t their color, it’s their socioeconomic status,” Dr. Livingston says. “Is that a fair statement?”
But Dr. Katz says that “acknowledging structural racism can be helpful to us. Structural racism refers to a system in which policies or practices or how we look at people perpetuates racial inequality.”
Dr. Katz points to the creation of a hospital in San Francisco in the 1880s to treat patients of Chinese ethnicity separately. Outside of health care, he talks about environmental racism between neighborhoods with inequalities in hospitals, schools, and social services.
“All of those things have an impact on that minority person,” Dr. Katz says. “The big thing we can all do is move away from trying to interrogate each other’s opinions and move to a place where we are looking at the policies of our institutions and making sure that they promote equality.”
Dr. Livingston concludes the episode by reemphasizing that “racism” should be taken out of the conversation and it should instead focus on the “structural” aspect of socioeconomics.
“Minorities ... aren’t [in those neighborhoods] because they’re not allowed to buy houses or they can’t get a job because they’re Black or Hispanic. That would be illegal,” Dr. Livingston says. “But disproportionality does exist.”
Efforts to reach Dr. Livingston were unsuccessful. Dr. Katz distanced himself from Dr. Livingston in a statement released on March 4.
“Systemic and interpersonal racism both still exist in our country — they must be rooted out. I do not share the JAMA host’s belief of doing away with the word ‘racism’ will help us be more successful in ending inequities that exists across racial and ethnic lines,” Dr. Katz said. “Further, I believe that we will only produce an equitable society when social and political structures do not continue to produce and perpetuate disparate results based on social race and ethnicity.”
Dr. Katz reiterated that both interpersonal and structural racism continue to exist in the United States, “and it is woefully naive to say that no physician is a racist just because the Civil Rights Act of 1964 forbade it.”
He also recommended JAMA use this controversy “as a learning opportunity for continued dialogue and create another podcast series as an open conversation that invites diverse experts in the field to have an open discussion about structural racism in healthcare.”
The podcast and JAMA’s tweet promoting it were widely criticized on Twitter. In interviews with WebMD, many doctors expressed disbelief that such a respected journal would lend its name to this podcast episode.
B. Bobby Chiong, MD, a radiologist in New York, said although JAMA’s effort to engage with its audience about racism is laudable, it missed the mark.
“I think the backlash comes from how they tried to make a podcast about the subject and somehow made themselves an example of unconscious bias and unfamiliarity with just how embedded in our system is structural racism,” he said.
Perhaps the podcast’s worst offense was its failure to address the painful history of racial bias in this country that still permeates the medical community, says Tamara Saint-Surin, MD, assistant professor at the University of North Carolina at Chapel Hill.
“For physicians in leadership to have the belief that structural racism does not exist in medicine, they don’t really appreciate what affects their patients and what their patients were dealing with,” Dr. Saint-Surin said in an interview. “It was a very harmful podcast and goes to show we still have so much work to do.”
Along with a flawed premise, she says, the podcast was not nearly long enough to address such a nuanced issue. And Dr. Livingston focused on interpersonal racism rather than structural racism, she said, failing to address widespread problems such as higher rates of asthma among Black populations living in areas with poor air quality.
The number of Black doctors remains low and the lack of representation adds to an environment already rife with racism, according to many medical professionals.
Shirlene Obuobi, MD, an internal medicine doctor in Chicago, said JAMA failed to live up to its own standards by publishing material that lacked research and expertise.
“I can’t submit a clinical trial to JAMA without them combing through methods with a fine-tooth comb,” Dr. Obuobi said. “They didn’t uphold the standards they normally apply to anyone else.”
Both the editor of JAMA and the head of the American Medical Association issued statements criticizing the episode and the tweet that promoted it.
JAMA Editor-in-Chief Howard Bauchner, MD, said, “The language of the tweet, and some portions of the podcast, do not reflect my commitment as editorial leader of JAMA and JAMA Network to call out and discuss the adverse effects of injustice, inequity, and racism in society and medicine as JAMA has done for many years.” He said JAMA will schedule a future podcast to address the concerns raised about the recent episode.
AMA CEO James L. Madara, MD, said, “The AMA’s House of Delegates passed policy stating that racism is structural, systemic, cultural, and interpersonal, and we are deeply disturbed – and angered – by a recent JAMA podcast that questioned the existence of structural racism and the affiliated tweet that promoted the podcast and stated ‘no physician is racist, so how can there be structural racism in health care?’ ”
He continued: “JAMA has editorial independence from AMA, but this tweet and podcast are inconsistent with the policies and views of AMA, and I’m concerned about and acknowledge the harms they have caused. Structural racism in health care and our society exists, and it is incumbent on all of us to fix it.”
This article was updated 3/5/21.
A version of this article first appeared on WebMD.com.
Published on Feb. 23, the episode is hosted on JAMA’s learning platform for doctors and is available for continuing medical education credits.
“No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast,” JAMA wrote in a Twitter post to promote the episode. That tweet has since been deleted.

The episode features host Ed Livingston, MD, deputy editor for clinical reviews and education at JAMA, and guest Mitchell Katz, MD, president and CEO for NYC Health + Hospitals and deputy editor for JAMA Internal Medicine. Dr. Livingston approaches the episode as “structural racism for skeptics,” and Dr. Katz tries to explain how structural racism deepens health disparities and what health systems can do about it.
“Many physicians are skeptical of structural racism, the idea that economic, educational, and other societal systems preferentially disadvantage Black Americans and other communities of color,” the episode description says.
In the podcast, Dr. Livingston and Dr. Katz speak about health care disparities and racial inequality. Dr. Livingston, who says he “didn’t understand the concept” going into the episode, suggests that racism was made illegal in the 1960s and that the discussion of “structural racism” should shift away from the term “racism” and focus on socioeconomic status instead.
“What you’re talking about isn’t so much racism ... it isn’t their race, it isn’t their color, it’s their socioeconomic status,” Dr. Livingston says. “Is that a fair statement?”
But Dr. Katz says that “acknowledging structural racism can be helpful to us. Structural racism refers to a system in which policies or practices or how we look at people perpetuates racial inequality.”
Dr. Katz points to the creation of a hospital in San Francisco in the 1880s to treat patients of Chinese ethnicity separately. Outside of health care, he talks about environmental racism between neighborhoods with inequalities in hospitals, schools, and social services.
“All of those things have an impact on that minority person,” Dr. Katz says. “The big thing we can all do is move away from trying to interrogate each other’s opinions and move to a place where we are looking at the policies of our institutions and making sure that they promote equality.”
Dr. Livingston concludes the episode by reemphasizing that “racism” should be taken out of the conversation and it should instead focus on the “structural” aspect of socioeconomics.
“Minorities ... aren’t [in those neighborhoods] because they’re not allowed to buy houses or they can’t get a job because they’re Black or Hispanic. That would be illegal,” Dr. Livingston says. “But disproportionality does exist.”
Efforts to reach Dr. Livingston were unsuccessful. Dr. Katz distanced himself from Dr. Livingston in a statement released on March 4.
“Systemic and interpersonal racism both still exist in our country — they must be rooted out. I do not share the JAMA host’s belief of doing away with the word ‘racism’ will help us be more successful in ending inequities that exists across racial and ethnic lines,” Dr. Katz said. “Further, I believe that we will only produce an equitable society when social and political structures do not continue to produce and perpetuate disparate results based on social race and ethnicity.”
Dr. Katz reiterated that both interpersonal and structural racism continue to exist in the United States, “and it is woefully naive to say that no physician is a racist just because the Civil Rights Act of 1964 forbade it.”
He also recommended JAMA use this controversy “as a learning opportunity for continued dialogue and create another podcast series as an open conversation that invites diverse experts in the field to have an open discussion about structural racism in healthcare.”
The podcast and JAMA’s tweet promoting it were widely criticized on Twitter. In interviews with WebMD, many doctors expressed disbelief that such a respected journal would lend its name to this podcast episode.
B. Bobby Chiong, MD, a radiologist in New York, said although JAMA’s effort to engage with its audience about racism is laudable, it missed the mark.
“I think the backlash comes from how they tried to make a podcast about the subject and somehow made themselves an example of unconscious bias and unfamiliarity with just how embedded in our system is structural racism,” he said.
Perhaps the podcast’s worst offense was its failure to address the painful history of racial bias in this country that still permeates the medical community, says Tamara Saint-Surin, MD, assistant professor at the University of North Carolina at Chapel Hill.
“For physicians in leadership to have the belief that structural racism does not exist in medicine, they don’t really appreciate what affects their patients and what their patients were dealing with,” Dr. Saint-Surin said in an interview. “It was a very harmful podcast and goes to show we still have so much work to do.”
Along with a flawed premise, she says, the podcast was not nearly long enough to address such a nuanced issue. And Dr. Livingston focused on interpersonal racism rather than structural racism, she said, failing to address widespread problems such as higher rates of asthma among Black populations living in areas with poor air quality.
The number of Black doctors remains low and the lack of representation adds to an environment already rife with racism, according to many medical professionals.
Shirlene Obuobi, MD, an internal medicine doctor in Chicago, said JAMA failed to live up to its own standards by publishing material that lacked research and expertise.
“I can’t submit a clinical trial to JAMA without them combing through methods with a fine-tooth comb,” Dr. Obuobi said. “They didn’t uphold the standards they normally apply to anyone else.”
Both the editor of JAMA and the head of the American Medical Association issued statements criticizing the episode and the tweet that promoted it.
JAMA Editor-in-Chief Howard Bauchner, MD, said, “The language of the tweet, and some portions of the podcast, do not reflect my commitment as editorial leader of JAMA and JAMA Network to call out and discuss the adverse effects of injustice, inequity, and racism in society and medicine as JAMA has done for many years.” He said JAMA will schedule a future podcast to address the concerns raised about the recent episode.
AMA CEO James L. Madara, MD, said, “The AMA’s House of Delegates passed policy stating that racism is structural, systemic, cultural, and interpersonal, and we are deeply disturbed – and angered – by a recent JAMA podcast that questioned the existence of structural racism and the affiliated tweet that promoted the podcast and stated ‘no physician is racist, so how can there be structural racism in health care?’ ”
He continued: “JAMA has editorial independence from AMA, but this tweet and podcast are inconsistent with the policies and views of AMA, and I’m concerned about and acknowledge the harms they have caused. Structural racism in health care and our society exists, and it is incumbent on all of us to fix it.”
This article was updated 3/5/21.
A version of this article first appeared on WebMD.com.
Docs become dog groomers and warehouse workers after COVID-19 work loss
One of the biggest conundrums of the COVID-19 pandemic has been the simultaneous panic-hiring of medical professionals in hot spots and significant downsizing of staff across the country. From huge hospital systems to private practices, the stoppage of breast reductions and knee replacements, not to mention the drops in motor vehicle accidents and bar fights, have quieted operating rooms and emergency departments and put doctors’ jobs on the chopping block. A widely cited survey suggests that 21% of doctors have had a work reduction due to COVID-19.
For many American doctors, this is their first extended period of unemployment. Unlike engineers or those with MBAs who might see their fortunes rise and fall with the whims of recessions and boom times, physicians are not exactly accustomed to being laid off. However, doctors were already smarting for years due to falling salaries and decreased autonomy, punctuated by endless clicks on electronic medical records software.
Stephanie Eschenbach Morgan, MD, a breast radiologist in North Carolina, trained for 10 years after college before earning a true physician’s salary.
“Being furloughed was awful. Initially, it was only going to be 2 weeks, and then it turned into 2 months with no pay,” she reflected.
Dr. Eschenbach Morgan and her surgeon husband, who lost a full quarter’s salary, had to ask for grace periods on their credit card and mortgage payments because they had paid a large tax bill right before the pandemic began. “We couldn’t get any stimulus help, so that added insult to injury,” she said.
With her time spent waiting in a holding pattern, Dr. Eschenbach Morgan homeschooled her two young children and started putting a home gym together. She went on a home organizing spree, started a garden, and, perhaps most impressively, caught up with 5 years of photo albums.
A bonus she noted: “I didn’t set an alarm for 2 months.”
Shella Farooki, MD, a radiologist in California, was also focused on homeschooling, itself a demanding job, and veered toward retirement. When one of her work contracts furloughed her (“at one point, I made $30K a month for [their business]”), she started saving money at home, teaching the kids, and applied for a Paycheck Protection Program loan. Her husband, a hospitalist, had had his shifts cut. Dr. Farooki tried a radiology artificial intelligence firm but backed out when she was asked to read 9,200 studies for them for $2,000 per month.
Now, she thinks about leaving medicine “every day.”
Some doctors are questioning whether they should be in medicine in the first place. Family medicine physician Jonathan Polak, MD, faced with his own pink slip, turned to pink T-shirts instead. His girlfriend manages an outlet of the teen fashion retailer Justice. Dr. Polak, who finished his residency just 2 years ago, didn’t hesitate to take a $10-an-hour gig as a stock doc, once even finding himself delivering a shelving unit from the shuttering store to a physician fleeing the city for rural New Hampshire to “escape.”
There’s no escape for him – yet. Saddled with “astronomical” student loans, he had considered grocery store work as well. Dr. Polak knows he can’t work part time or go into teaching long term, as he might like.
Even so, he’s doing everything he can to not be in patient care for the long haul – it’s just not what he thought it would be.
“The culture of medicine, bureaucracy, endless paperwork and charting, and threat of litigation sucks a lot of the joy out of it to the point that I don’t see myself doing it forever when imagining myself 5-10 years into it.”
Still, he recently took an 18-month hospital contract that will force him to move to Florida, but he’s also been turning himself into a veritable Renaissance man; composing music, training for an ultramarathon, studying the latest medical findings, roadtripping, and launching a podcast about dog grooming with a master groomer. “We found parallels between medicine and dog grooming,” he says, somewhat convincingly.
Also working the ruff life is Jen Tserng, MD, a former forensic pathologist who landed on news websites in recent years for becoming a professional dogwalker and housesitter without a permanent home. Dr. Tserng knows doctors were restless and unhappy before COVID-19, their thoughts wandering where the grass might be greener.
As her profile grew, she found her inbox gathering messages from disaffected medical minions: students with a fear of failing or staring down residency application season and employed doctors sick of the constant grind. As she recounted those de facto life coach conversations (“What do you really enjoy?” “Do you really like dogs?”) by phone from New York, she said matter-of-factly, “They don’t call because of COVID. They call because they hate their lives.”
Michelle Mudge-Riley, MD, a physician in Texas, has been seeing this shift for some time as well. She recently held a virtual version of her Physicians Helping Physicians conference, where doctors hear from their peers working successfully in fields like pharmaceuticals and real estate investing.
When COVID-19 hit, Dr. Mudge-Riley quickly pivoted to a virtual platform, where the MDs and DOs huddled in breakout rooms having honest chats about their fears and tentative hopes about their new careers.
“There has been increased interest in nonclinical exploration into full- and part-time careers, as well as side hustles, since COVID began,” she said. “Many physicians have had their hours or pay cut, and some have been laid off. Others are furloughed. Some just want out of an environment where they don’t feel safe.”
An ear, nose, and throat surgeon, Maansi Doshi, MD, from central California, didn’t feel safe – so she left. She had returned from India sick with a mystery virus right as the pandemic began (she said her COVID-19 tests were all negative) and was waiting to get well enough to go back to her private practice job. However, she said she clashed with Trump-supporting colleagues she feared might not be taking the pandemic seriously enough.
Finally getting over a relapse of her mystery virus, Dr. Doshi emailed her resignation in May. Her husband, family practice doctor Mark Mangiapane, MD, gave his job notice weeks later in solidarity because he worked in the same building. Together, they have embraced gardening, a Peloton splurge, and learning business skills to open private practices – solo primary care for him; ENT with a focus on her favorite surgery, rhinoplasty, for her.
Dr. Mangiapane had considered editing medical brochures and also tried to apply for a job as a county public health officer in rural California, but he received his own shock when he learned the county intended to open schools in the midst of the pandemic despite advisement to the contrary by the former health officer.
He retreated from job listings altogether after hearing his would-be peers were getting death threats – targeting their children.
Both doctors felt COVID-19 pushed them beyond their comfort zones. “If COVID hadn’t happened, I would be working. ... Be ‘owned.’ In a weird way, COVID made me more independent and take a risk with my career.”
Obstetrician Kwandaa Roberts, MD, certainly did; she took a budding interest in decorating dollhouses straight to Instagram and national news fame, and she is now a TV-show expert on “Sell This House.”
Like Dr. Doshi and Dr. Mangiapane, Dr. Polak wants to be more in control of his future – even if selling T-shirts at a mall means a certain loss of status along the way.
“Aside from my passion to learn and to have that connection with people, I went into medicine ... because of the job security I thought existed,” he said. “I would say that my getting furloughed has changed my view of the United States in a dramatic way. I do not feel as confident in the U.S. economy and general way of life as I did a year ago. And I am taking a number of steps to put myself in a more fluid, adaptable position in case another crisis like this occurs or if the current state of things worsens.”
A version of this article first appeared on Medscape.com.
One of the biggest conundrums of the COVID-19 pandemic has been the simultaneous panic-hiring of medical professionals in hot spots and significant downsizing of staff across the country. From huge hospital systems to private practices, the stoppage of breast reductions and knee replacements, not to mention the drops in motor vehicle accidents and bar fights, have quieted operating rooms and emergency departments and put doctors’ jobs on the chopping block. A widely cited survey suggests that 21% of doctors have had a work reduction due to COVID-19.
For many American doctors, this is their first extended period of unemployment. Unlike engineers or those with MBAs who might see their fortunes rise and fall with the whims of recessions and boom times, physicians are not exactly accustomed to being laid off. However, doctors were already smarting for years due to falling salaries and decreased autonomy, punctuated by endless clicks on electronic medical records software.
Stephanie Eschenbach Morgan, MD, a breast radiologist in North Carolina, trained for 10 years after college before earning a true physician’s salary.
“Being furloughed was awful. Initially, it was only going to be 2 weeks, and then it turned into 2 months with no pay,” she reflected.
Dr. Eschenbach Morgan and her surgeon husband, who lost a full quarter’s salary, had to ask for grace periods on their credit card and mortgage payments because they had paid a large tax bill right before the pandemic began. “We couldn’t get any stimulus help, so that added insult to injury,” she said.
With her time spent waiting in a holding pattern, Dr. Eschenbach Morgan homeschooled her two young children and started putting a home gym together. She went on a home organizing spree, started a garden, and, perhaps most impressively, caught up with 5 years of photo albums.
A bonus she noted: “I didn’t set an alarm for 2 months.”
Shella Farooki, MD, a radiologist in California, was also focused on homeschooling, itself a demanding job, and veered toward retirement. When one of her work contracts furloughed her (“at one point, I made $30K a month for [their business]”), she started saving money at home, teaching the kids, and applied for a Paycheck Protection Program loan. Her husband, a hospitalist, had had his shifts cut. Dr. Farooki tried a radiology artificial intelligence firm but backed out when she was asked to read 9,200 studies for them for $2,000 per month.
Now, she thinks about leaving medicine “every day.”
Some doctors are questioning whether they should be in medicine in the first place. Family medicine physician Jonathan Polak, MD, faced with his own pink slip, turned to pink T-shirts instead. His girlfriend manages an outlet of the teen fashion retailer Justice. Dr. Polak, who finished his residency just 2 years ago, didn’t hesitate to take a $10-an-hour gig as a stock doc, once even finding himself delivering a shelving unit from the shuttering store to a physician fleeing the city for rural New Hampshire to “escape.”
There’s no escape for him – yet. Saddled with “astronomical” student loans, he had considered grocery store work as well. Dr. Polak knows he can’t work part time or go into teaching long term, as he might like.
Even so, he’s doing everything he can to not be in patient care for the long haul – it’s just not what he thought it would be.
“The culture of medicine, bureaucracy, endless paperwork and charting, and threat of litigation sucks a lot of the joy out of it to the point that I don’t see myself doing it forever when imagining myself 5-10 years into it.”
Still, he recently took an 18-month hospital contract that will force him to move to Florida, but he’s also been turning himself into a veritable Renaissance man; composing music, training for an ultramarathon, studying the latest medical findings, roadtripping, and launching a podcast about dog grooming with a master groomer. “We found parallels between medicine and dog grooming,” he says, somewhat convincingly.
Also working the ruff life is Jen Tserng, MD, a former forensic pathologist who landed on news websites in recent years for becoming a professional dogwalker and housesitter without a permanent home. Dr. Tserng knows doctors were restless and unhappy before COVID-19, their thoughts wandering where the grass might be greener.
As her profile grew, she found her inbox gathering messages from disaffected medical minions: students with a fear of failing or staring down residency application season and employed doctors sick of the constant grind. As she recounted those de facto life coach conversations (“What do you really enjoy?” “Do you really like dogs?”) by phone from New York, she said matter-of-factly, “They don’t call because of COVID. They call because they hate their lives.”
Michelle Mudge-Riley, MD, a physician in Texas, has been seeing this shift for some time as well. She recently held a virtual version of her Physicians Helping Physicians conference, where doctors hear from their peers working successfully in fields like pharmaceuticals and real estate investing.
When COVID-19 hit, Dr. Mudge-Riley quickly pivoted to a virtual platform, where the MDs and DOs huddled in breakout rooms having honest chats about their fears and tentative hopes about their new careers.
“There has been increased interest in nonclinical exploration into full- and part-time careers, as well as side hustles, since COVID began,” she said. “Many physicians have had their hours or pay cut, and some have been laid off. Others are furloughed. Some just want out of an environment where they don’t feel safe.”
An ear, nose, and throat surgeon, Maansi Doshi, MD, from central California, didn’t feel safe – so she left. She had returned from India sick with a mystery virus right as the pandemic began (she said her COVID-19 tests were all negative) and was waiting to get well enough to go back to her private practice job. However, she said she clashed with Trump-supporting colleagues she feared might not be taking the pandemic seriously enough.
Finally getting over a relapse of her mystery virus, Dr. Doshi emailed her resignation in May. Her husband, family practice doctor Mark Mangiapane, MD, gave his job notice weeks later in solidarity because he worked in the same building. Together, they have embraced gardening, a Peloton splurge, and learning business skills to open private practices – solo primary care for him; ENT with a focus on her favorite surgery, rhinoplasty, for her.
Dr. Mangiapane had considered editing medical brochures and also tried to apply for a job as a county public health officer in rural California, but he received his own shock when he learned the county intended to open schools in the midst of the pandemic despite advisement to the contrary by the former health officer.
He retreated from job listings altogether after hearing his would-be peers were getting death threats – targeting their children.
Both doctors felt COVID-19 pushed them beyond their comfort zones. “If COVID hadn’t happened, I would be working. ... Be ‘owned.’ In a weird way, COVID made me more independent and take a risk with my career.”
Obstetrician Kwandaa Roberts, MD, certainly did; she took a budding interest in decorating dollhouses straight to Instagram and national news fame, and she is now a TV-show expert on “Sell This House.”
Like Dr. Doshi and Dr. Mangiapane, Dr. Polak wants to be more in control of his future – even if selling T-shirts at a mall means a certain loss of status along the way.
“Aside from my passion to learn and to have that connection with people, I went into medicine ... because of the job security I thought existed,” he said. “I would say that my getting furloughed has changed my view of the United States in a dramatic way. I do not feel as confident in the U.S. economy and general way of life as I did a year ago. And I am taking a number of steps to put myself in a more fluid, adaptable position in case another crisis like this occurs or if the current state of things worsens.”
A version of this article first appeared on Medscape.com.
One of the biggest conundrums of the COVID-19 pandemic has been the simultaneous panic-hiring of medical professionals in hot spots and significant downsizing of staff across the country. From huge hospital systems to private practices, the stoppage of breast reductions and knee replacements, not to mention the drops in motor vehicle accidents and bar fights, have quieted operating rooms and emergency departments and put doctors’ jobs on the chopping block. A widely cited survey suggests that 21% of doctors have had a work reduction due to COVID-19.
For many American doctors, this is their first extended period of unemployment. Unlike engineers or those with MBAs who might see their fortunes rise and fall with the whims of recessions and boom times, physicians are not exactly accustomed to being laid off. However, doctors were already smarting for years due to falling salaries and decreased autonomy, punctuated by endless clicks on electronic medical records software.
Stephanie Eschenbach Morgan, MD, a breast radiologist in North Carolina, trained for 10 years after college before earning a true physician’s salary.
“Being furloughed was awful. Initially, it was only going to be 2 weeks, and then it turned into 2 months with no pay,” she reflected.
Dr. Eschenbach Morgan and her surgeon husband, who lost a full quarter’s salary, had to ask for grace periods on their credit card and mortgage payments because they had paid a large tax bill right before the pandemic began. “We couldn’t get any stimulus help, so that added insult to injury,” she said.
With her time spent waiting in a holding pattern, Dr. Eschenbach Morgan homeschooled her two young children and started putting a home gym together. She went on a home organizing spree, started a garden, and, perhaps most impressively, caught up with 5 years of photo albums.
A bonus she noted: “I didn’t set an alarm for 2 months.”
Shella Farooki, MD, a radiologist in California, was also focused on homeschooling, itself a demanding job, and veered toward retirement. When one of her work contracts furloughed her (“at one point, I made $30K a month for [their business]”), she started saving money at home, teaching the kids, and applied for a Paycheck Protection Program loan. Her husband, a hospitalist, had had his shifts cut. Dr. Farooki tried a radiology artificial intelligence firm but backed out when she was asked to read 9,200 studies for them for $2,000 per month.
Now, she thinks about leaving medicine “every day.”
Some doctors are questioning whether they should be in medicine in the first place. Family medicine physician Jonathan Polak, MD, faced with his own pink slip, turned to pink T-shirts instead. His girlfriend manages an outlet of the teen fashion retailer Justice. Dr. Polak, who finished his residency just 2 years ago, didn’t hesitate to take a $10-an-hour gig as a stock doc, once even finding himself delivering a shelving unit from the shuttering store to a physician fleeing the city for rural New Hampshire to “escape.”
There’s no escape for him – yet. Saddled with “astronomical” student loans, he had considered grocery store work as well. Dr. Polak knows he can’t work part time or go into teaching long term, as he might like.
Even so, he’s doing everything he can to not be in patient care for the long haul – it’s just not what he thought it would be.
“The culture of medicine, bureaucracy, endless paperwork and charting, and threat of litigation sucks a lot of the joy out of it to the point that I don’t see myself doing it forever when imagining myself 5-10 years into it.”
Still, he recently took an 18-month hospital contract that will force him to move to Florida, but he’s also been turning himself into a veritable Renaissance man; composing music, training for an ultramarathon, studying the latest medical findings, roadtripping, and launching a podcast about dog grooming with a master groomer. “We found parallels between medicine and dog grooming,” he says, somewhat convincingly.
Also working the ruff life is Jen Tserng, MD, a former forensic pathologist who landed on news websites in recent years for becoming a professional dogwalker and housesitter without a permanent home. Dr. Tserng knows doctors were restless and unhappy before COVID-19, their thoughts wandering where the grass might be greener.
As her profile grew, she found her inbox gathering messages from disaffected medical minions: students with a fear of failing or staring down residency application season and employed doctors sick of the constant grind. As she recounted those de facto life coach conversations (“What do you really enjoy?” “Do you really like dogs?”) by phone from New York, she said matter-of-factly, “They don’t call because of COVID. They call because they hate their lives.”
Michelle Mudge-Riley, MD, a physician in Texas, has been seeing this shift for some time as well. She recently held a virtual version of her Physicians Helping Physicians conference, where doctors hear from their peers working successfully in fields like pharmaceuticals and real estate investing.
When COVID-19 hit, Dr. Mudge-Riley quickly pivoted to a virtual platform, where the MDs and DOs huddled in breakout rooms having honest chats about their fears and tentative hopes about their new careers.
“There has been increased interest in nonclinical exploration into full- and part-time careers, as well as side hustles, since COVID began,” she said. “Many physicians have had their hours or pay cut, and some have been laid off. Others are furloughed. Some just want out of an environment where they don’t feel safe.”
An ear, nose, and throat surgeon, Maansi Doshi, MD, from central California, didn’t feel safe – so she left. She had returned from India sick with a mystery virus right as the pandemic began (she said her COVID-19 tests were all negative) and was waiting to get well enough to go back to her private practice job. However, she said she clashed with Trump-supporting colleagues she feared might not be taking the pandemic seriously enough.
Finally getting over a relapse of her mystery virus, Dr. Doshi emailed her resignation in May. Her husband, family practice doctor Mark Mangiapane, MD, gave his job notice weeks later in solidarity because he worked in the same building. Together, they have embraced gardening, a Peloton splurge, and learning business skills to open private practices – solo primary care for him; ENT with a focus on her favorite surgery, rhinoplasty, for her.
Dr. Mangiapane had considered editing medical brochures and also tried to apply for a job as a county public health officer in rural California, but he received his own shock when he learned the county intended to open schools in the midst of the pandemic despite advisement to the contrary by the former health officer.
He retreated from job listings altogether after hearing his would-be peers were getting death threats – targeting their children.
Both doctors felt COVID-19 pushed them beyond their comfort zones. “If COVID hadn’t happened, I would be working. ... Be ‘owned.’ In a weird way, COVID made me more independent and take a risk with my career.”
Obstetrician Kwandaa Roberts, MD, certainly did; she took a budding interest in decorating dollhouses straight to Instagram and national news fame, and she is now a TV-show expert on “Sell This House.”
Like Dr. Doshi and Dr. Mangiapane, Dr. Polak wants to be more in control of his future – even if selling T-shirts at a mall means a certain loss of status along the way.
“Aside from my passion to learn and to have that connection with people, I went into medicine ... because of the job security I thought existed,” he said. “I would say that my getting furloughed has changed my view of the United States in a dramatic way. I do not feel as confident in the U.S. economy and general way of life as I did a year ago. And I am taking a number of steps to put myself in a more fluid, adaptable position in case another crisis like this occurs or if the current state of things worsens.”
A version of this article first appeared on Medscape.com.
Recent psoriasis pathophysiology insights carry treatment implications
at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually.
Among these unexpected developments was the startling finding that skin inflammation in mild psoriasis is at least as great as in severe disease; evidence that psoriasis may actually be an autoimmune disease rather than a nonspecific immune-mediated disease; and the newly appreciated importance of interleukin-19 (IL-19) in keratinocyte proliferation, according to Dr. Gordon, professor and chair of the department of dermatology at the Medical College of Wisconsin, Milwaukee.
“Our understanding of the pathophysiology of psoriasis is still a work in progress,” the dermatologist observed.
Immunoregulatory deficits in mild vs. severe psoriasis
Conventional wisdom has held that mild psoriasis as defined by limited affected body surface area involves less skin inflammation than more extensive severe psoriasis, so less-potent topical therapies are appropriate. Not so, according to Dr. Gordon, who highlighted work by James G. Krueger, MD, PhD, head of the laboratory of investigative dermatology at Rockefeller University, New York, and coinvestigators. They demonstrated that overall skin inflammation expressed as the sum of T-cell activation and IL-19-mediated epidermal responses didn’t differ in lesions of mild as compared with severe psoriasis. Indeed, mild skin lesions featured a greater number of T-cells, stronger expression of proinflammatory cytokine IL-17A, and greater expression of the central psoriasis transcriptome. The big difference between skin lesions of mild versus severe psoriasis was that severe psoriasis was characterized by strikingly weaker expression of immunoregulatory genes, including programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA4), than that of mild lesions.
The implication is that IL-17-targeted therapies may be of benefit in a much larger segment of the psoriatic population: namely, those with mild disease, who comprise the majority of psoriasis patients by a wide margin, according to the investigators.
Dr. Gordon concurs.
“The primary problem in psoriasis is not so much the inflammatory activity, but the ability to turn off the activity,” he explained. “That implies that if a patient wants to get clear or have significant improvement in disease, you can’t use a less effective medication just because they have less amount of disease. You’re going to need to treat it just as aggressively because the great majority of our medications block the proinflammatory pathways.”
The deficit in immunoregulatory action identified by Dr. Krueger and colleagues in patients with severe disease could provide a novel therapeutic target. If the deficient immunoregulation could be boosted, it might achieve disease control without need for continuous anti-inflammatory therapy.
Autoimmunity in psoriasis
“When I started work in psoriasis, we always thought there would be a common antigen for the immune process in the disease. We never found it. So for that reason, we sort of put it aside and called psoriasis a nonspecific immune-mediated disease,” Dr. Gordon recalled.
That view is being reexamined. “While we’re not completely certain, there is now some evidence that there might be autoimmunity in psoriasis,” he said.
He cited work by an international team of investigators who identified the cathelicidin antimicrobial peptide LL37 as being overexpressed in psoriatic skin, where it appears to serve as a T-cell autoantigen. LL37-specific CD4+ and CD8+ T-cells are skin homing: They can infiltrate lesional skin, where they produce interferon-gamma and proinflammatory Th17 cytokines. The investigators reported that levels of circulating LL37-specific T cells correlated with disease activity such that they were found in three-quarters of patients with moderate to severe plaque psoriasis.
“As LL37 is able to activate innate immune cells and break innate tolerance to self-nucleic acids, it represents an even more appealing target to treat psoriasis. Therapeutic targeting of LL37-specific T cells may provide new avenues to prevent or treat psoriasis without inducing indiscriminate immunosuppression,” the investigators concluded.
Similarly, German investigators have identified ADAMTS-like protein 5 (ADAMTSL5) as an autoantigen specific for melanocytes in psoriasis patients who possess the central psoriasis risk gene, known as HLA-C*06:02, which is present in two-thirds of patients with psoriasis. They proposed that their newly recognized autoimmune pathway may explain how HLA-C*06.02 predisposes to psoriasis.
Growing clinical relevance of IL-19
It’s now well-established that IL-17 is the pivotal force driving the changes in keratinocytes that define the visible expressions of psoriasis, including plaque scale and thickness, which are due to abnormal keratinocyte maturation and proliferation, respectively. Less well appreciated is the fact that IL-17-activated keratinocytes produce IL-19, which feeds back and further stimulates keratinocyte proliferation.
In light of mounting evidence that IL-19 plays an important role in the pathogenesis of psoriasis and that naked eye assessment of visible psoriasis may not reflect the true extent of inflammation, Brian J. Nickoloff, MD, PhD, and coworkers at Lilly Research Laboratories have developed a novel serum IL-19 immunoassay that appears to provide a much-needed objective biomarker of disease activity in psoriasis patients. They demonstrated that serum IL-19 levels correlated with Psoriasis Area and Severity Index scores, and that treatment with the anti-IL-17A biologic ixekizumab (Taltz) led to rapid reduction of IL-19 down to a normal level.
Moreover, following withdrawal of ixekizumab, IL-19 levels rose prior to clinical relapse, then dropped again in response to retreatment. The hope is that this assay will serve as an accurate tool for assessment of response to therapy.
Dr. Gordon reported receiving research funding and/or honoraria from more than a dozen pharmaceutical companies involved in psoriasis therapy.
MedscapeLive and this news organization are owned by the same parent company.
at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually.
Among these unexpected developments was the startling finding that skin inflammation in mild psoriasis is at least as great as in severe disease; evidence that psoriasis may actually be an autoimmune disease rather than a nonspecific immune-mediated disease; and the newly appreciated importance of interleukin-19 (IL-19) in keratinocyte proliferation, according to Dr. Gordon, professor and chair of the department of dermatology at the Medical College of Wisconsin, Milwaukee.
“Our understanding of the pathophysiology of psoriasis is still a work in progress,” the dermatologist observed.
Immunoregulatory deficits in mild vs. severe psoriasis
Conventional wisdom has held that mild psoriasis as defined by limited affected body surface area involves less skin inflammation than more extensive severe psoriasis, so less-potent topical therapies are appropriate. Not so, according to Dr. Gordon, who highlighted work by James G. Krueger, MD, PhD, head of the laboratory of investigative dermatology at Rockefeller University, New York, and coinvestigators. They demonstrated that overall skin inflammation expressed as the sum of T-cell activation and IL-19-mediated epidermal responses didn’t differ in lesions of mild as compared with severe psoriasis. Indeed, mild skin lesions featured a greater number of T-cells, stronger expression of proinflammatory cytokine IL-17A, and greater expression of the central psoriasis transcriptome. The big difference between skin lesions of mild versus severe psoriasis was that severe psoriasis was characterized by strikingly weaker expression of immunoregulatory genes, including programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA4), than that of mild lesions.
The implication is that IL-17-targeted therapies may be of benefit in a much larger segment of the psoriatic population: namely, those with mild disease, who comprise the majority of psoriasis patients by a wide margin, according to the investigators.
Dr. Gordon concurs.
“The primary problem in psoriasis is not so much the inflammatory activity, but the ability to turn off the activity,” he explained. “That implies that if a patient wants to get clear or have significant improvement in disease, you can’t use a less effective medication just because they have less amount of disease. You’re going to need to treat it just as aggressively because the great majority of our medications block the proinflammatory pathways.”
The deficit in immunoregulatory action identified by Dr. Krueger and colleagues in patients with severe disease could provide a novel therapeutic target. If the deficient immunoregulation could be boosted, it might achieve disease control without need for continuous anti-inflammatory therapy.
Autoimmunity in psoriasis
“When I started work in psoriasis, we always thought there would be a common antigen for the immune process in the disease. We never found it. So for that reason, we sort of put it aside and called psoriasis a nonspecific immune-mediated disease,” Dr. Gordon recalled.
That view is being reexamined. “While we’re not completely certain, there is now some evidence that there might be autoimmunity in psoriasis,” he said.
He cited work by an international team of investigators who identified the cathelicidin antimicrobial peptide LL37 as being overexpressed in psoriatic skin, where it appears to serve as a T-cell autoantigen. LL37-specific CD4+ and CD8+ T-cells are skin homing: They can infiltrate lesional skin, where they produce interferon-gamma and proinflammatory Th17 cytokines. The investigators reported that levels of circulating LL37-specific T cells correlated with disease activity such that they were found in three-quarters of patients with moderate to severe plaque psoriasis.
“As LL37 is able to activate innate immune cells and break innate tolerance to self-nucleic acids, it represents an even more appealing target to treat psoriasis. Therapeutic targeting of LL37-specific T cells may provide new avenues to prevent or treat psoriasis without inducing indiscriminate immunosuppression,” the investigators concluded.
Similarly, German investigators have identified ADAMTS-like protein 5 (ADAMTSL5) as an autoantigen specific for melanocytes in psoriasis patients who possess the central psoriasis risk gene, known as HLA-C*06:02, which is present in two-thirds of patients with psoriasis. They proposed that their newly recognized autoimmune pathway may explain how HLA-C*06.02 predisposes to psoriasis.
Growing clinical relevance of IL-19
It’s now well-established that IL-17 is the pivotal force driving the changes in keratinocytes that define the visible expressions of psoriasis, including plaque scale and thickness, which are due to abnormal keratinocyte maturation and proliferation, respectively. Less well appreciated is the fact that IL-17-activated keratinocytes produce IL-19, which feeds back and further stimulates keratinocyte proliferation.
In light of mounting evidence that IL-19 plays an important role in the pathogenesis of psoriasis and that naked eye assessment of visible psoriasis may not reflect the true extent of inflammation, Brian J. Nickoloff, MD, PhD, and coworkers at Lilly Research Laboratories have developed a novel serum IL-19 immunoassay that appears to provide a much-needed objective biomarker of disease activity in psoriasis patients. They demonstrated that serum IL-19 levels correlated with Psoriasis Area and Severity Index scores, and that treatment with the anti-IL-17A biologic ixekizumab (Taltz) led to rapid reduction of IL-19 down to a normal level.
Moreover, following withdrawal of ixekizumab, IL-19 levels rose prior to clinical relapse, then dropped again in response to retreatment. The hope is that this assay will serve as an accurate tool for assessment of response to therapy.
Dr. Gordon reported receiving research funding and/or honoraria from more than a dozen pharmaceutical companies involved in psoriasis therapy.
MedscapeLive and this news organization are owned by the same parent company.
at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually.
Among these unexpected developments was the startling finding that skin inflammation in mild psoriasis is at least as great as in severe disease; evidence that psoriasis may actually be an autoimmune disease rather than a nonspecific immune-mediated disease; and the newly appreciated importance of interleukin-19 (IL-19) in keratinocyte proliferation, according to Dr. Gordon, professor and chair of the department of dermatology at the Medical College of Wisconsin, Milwaukee.
“Our understanding of the pathophysiology of psoriasis is still a work in progress,” the dermatologist observed.
Immunoregulatory deficits in mild vs. severe psoriasis
Conventional wisdom has held that mild psoriasis as defined by limited affected body surface area involves less skin inflammation than more extensive severe psoriasis, so less-potent topical therapies are appropriate. Not so, according to Dr. Gordon, who highlighted work by James G. Krueger, MD, PhD, head of the laboratory of investigative dermatology at Rockefeller University, New York, and coinvestigators. They demonstrated that overall skin inflammation expressed as the sum of T-cell activation and IL-19-mediated epidermal responses didn’t differ in lesions of mild as compared with severe psoriasis. Indeed, mild skin lesions featured a greater number of T-cells, stronger expression of proinflammatory cytokine IL-17A, and greater expression of the central psoriasis transcriptome. The big difference between skin lesions of mild versus severe psoriasis was that severe psoriasis was characterized by strikingly weaker expression of immunoregulatory genes, including programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA4), than that of mild lesions.
The implication is that IL-17-targeted therapies may be of benefit in a much larger segment of the psoriatic population: namely, those with mild disease, who comprise the majority of psoriasis patients by a wide margin, according to the investigators.
Dr. Gordon concurs.
“The primary problem in psoriasis is not so much the inflammatory activity, but the ability to turn off the activity,” he explained. “That implies that if a patient wants to get clear or have significant improvement in disease, you can’t use a less effective medication just because they have less amount of disease. You’re going to need to treat it just as aggressively because the great majority of our medications block the proinflammatory pathways.”
The deficit in immunoregulatory action identified by Dr. Krueger and colleagues in patients with severe disease could provide a novel therapeutic target. If the deficient immunoregulation could be boosted, it might achieve disease control without need for continuous anti-inflammatory therapy.
Autoimmunity in psoriasis
“When I started work in psoriasis, we always thought there would be a common antigen for the immune process in the disease. We never found it. So for that reason, we sort of put it aside and called psoriasis a nonspecific immune-mediated disease,” Dr. Gordon recalled.
That view is being reexamined. “While we’re not completely certain, there is now some evidence that there might be autoimmunity in psoriasis,” he said.
He cited work by an international team of investigators who identified the cathelicidin antimicrobial peptide LL37 as being overexpressed in psoriatic skin, where it appears to serve as a T-cell autoantigen. LL37-specific CD4+ and CD8+ T-cells are skin homing: They can infiltrate lesional skin, where they produce interferon-gamma and proinflammatory Th17 cytokines. The investigators reported that levels of circulating LL37-specific T cells correlated with disease activity such that they were found in three-quarters of patients with moderate to severe plaque psoriasis.
“As LL37 is able to activate innate immune cells and break innate tolerance to self-nucleic acids, it represents an even more appealing target to treat psoriasis. Therapeutic targeting of LL37-specific T cells may provide new avenues to prevent or treat psoriasis without inducing indiscriminate immunosuppression,” the investigators concluded.
Similarly, German investigators have identified ADAMTS-like protein 5 (ADAMTSL5) as an autoantigen specific for melanocytes in psoriasis patients who possess the central psoriasis risk gene, known as HLA-C*06:02, which is present in two-thirds of patients with psoriasis. They proposed that their newly recognized autoimmune pathway may explain how HLA-C*06.02 predisposes to psoriasis.
Growing clinical relevance of IL-19
It’s now well-established that IL-17 is the pivotal force driving the changes in keratinocytes that define the visible expressions of psoriasis, including plaque scale and thickness, which are due to abnormal keratinocyte maturation and proliferation, respectively. Less well appreciated is the fact that IL-17-activated keratinocytes produce IL-19, which feeds back and further stimulates keratinocyte proliferation.
In light of mounting evidence that IL-19 plays an important role in the pathogenesis of psoriasis and that naked eye assessment of visible psoriasis may not reflect the true extent of inflammation, Brian J. Nickoloff, MD, PhD, and coworkers at Lilly Research Laboratories have developed a novel serum IL-19 immunoassay that appears to provide a much-needed objective biomarker of disease activity in psoriasis patients. They demonstrated that serum IL-19 levels correlated with Psoriasis Area and Severity Index scores, and that treatment with the anti-IL-17A biologic ixekizumab (Taltz) led to rapid reduction of IL-19 down to a normal level.
Moreover, following withdrawal of ixekizumab, IL-19 levels rose prior to clinical relapse, then dropped again in response to retreatment. The hope is that this assay will serve as an accurate tool for assessment of response to therapy.
Dr. Gordon reported receiving research funding and/or honoraria from more than a dozen pharmaceutical companies involved in psoriasis therapy.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
RA experts highlight key developments over the past year
A massive Swedish cohort study suggesting that biologic agents reduce the known excess risk of lymphoma in patients with rheumatoid arthritis was hailed as one of the high points of the last year by speakers at the 2021 Rheumatology Winter Clinical Symposium.
The speakers gave additional shout-outs regarding other key developments in RA during the pandemic year, including two huge studies showing that even low-dose corticosteroids at 5 mg/day or less carry the price of increased risk of cardiovascular disease and serious infections; Swedish data showing that RA disease activity can be used for venous thromboembolism (VTE) risk stratification; evidence from the venerable BeST study showing at 17 years of follow-up that treat-to-target strategies didn’t prevent an increased mortality risk of RA compared to the general population; and a signal that artificial intelligence using machine learning will become useful in clinical practice sooner than many realize.
Also, the speakers offered kudos to the investigators in the SEAM-RA trial, whose results they expect to be practice changing. In addition, they praised the SELECT-CHOICE trial for providing insight into the relative safety and efficacy of upadacitinib (Rinvoq) and abatacept (Orencia) as second- or third-line therapy in RA. And they warned their colleagues of rocky upcoming months for tofacitinib (Xeljanz) as the Food and Drug Administration reviews concerning new data from a long-term postmarketing safety trial.
Swedes show biologics may reduce excess lymphoma risk in RA
Decades ago, Swedish investigators harnessed their nation’s comprehensive linked health registries to demonstrate that patients with RA are inherently at greater risk for developing lymphoma. Now the Swedes have used the registries to identify a strong signal that this excess risk of lymphoma is reduced in RA patients treated with biologic drugs.
The study included 16,392 RA patients who initiated treatment with a biologic during the study period, 55,253 who were biologic-naive, and 229,047 age- and sex-matched controls from the general population. During the years 2006-2016, there was an adjusted 31% reduction in the risk of incident lymphoma in RA patients who started a first biologic agent, compared with those who did not, and a 54% reduction in lymphoma in biologic-treated patients, compared with those who switched from one conventional synthetic disease-modifying antirheumatic drug (DMARD) to another.
Moreover, while RA patients with 0-2 years of accumulated time on biologics were at a statistically significant 3.42-fold increased risk of lymphoma, compared with the matched general population, that risk shrunk with longer exposure to these medications, dropping to a nonsignificant 1.53-fold increase with 3-4 years of biologic exposure and a 1.04-fold risk with 5 years or more. The lymphoma risks didn’t vary significantly among the TNF inhibitors, nor with the use of TNF inhibitors versus other biologics.
“I think this is really quite encouraging,” said John J. Cush, MD, professor of internal medicine at the University of Texas, Dallas. “If the lymphoma risk was related to inflammation, I think you’d see results like this. It really does speak to the fact that control of inflammation over time does lessen one’s risk of cancer.”
Arthur Kavanaugh, MD, observed that patients with RA continue to ask their rheumatologists if going on a TNF inhibitor will increase their risk of lymphoma, so these Swedish data are going to be useful in conversations in the clinic.
“I thought this study was very exciting,” said Dr. Kavanaugh, professor of medicine at the University of California, San Diego, and director of RWCS. “Maybe we can make the case now that the longer you’re on biologic therapies and the more recently you’re being treated, the better you do in terms of lymphoma risk.”
Beware underappreciated hazards of very-low-dose glucocorticoids
The increased risk of osteoporotic fractures associated with corticosteroid therapy gets tons of attention. But the past year brought two cautionary studies that newly identified significantly increased risks of cardiovascular events and serious infection as well, even at doses of less than 5 mg/day, which have traditionally been believed safe long term.
Investigators at the University of Leeds (England) conducted a population-based cohort study in which they used the U.K. Clinical Practice Research Datalink to analyze the oral corticosteroid dose-dependent cardiovascular risk in 25,324 patients with RA and 62,470 with five other immune-mediated inflammatory diseases: inflammatory bowel disease, giant cell arteritis, polymyalgia rheumatica, systemic lupus erythematosus, and vasculitis. None had known cardiovascular disease at baseline. The analysis accounted for changes in oral steroid dose over time and for the use of potentially confounding medications.
Over a median 5 years of follow-up, a strong steroid dose-dependent risk of acute MI, heart failure, atrial fibrillation, cerebrovascular disease, and abdominal aortic aneurysm was evident in patients with any of the six of the immune-mediated inflammatory diseases. The 1-year adjusted cumulative risk of major adverse cardiovascular events jumped from 1.4% during periods of nonuse to 8.9% at a daily dose of 25 mg or more. Notably, when patients were on oral steroids at doses less than 5 mg/day, their risk of one or more of these major adverse cardiovascular events was 74% greater than when off steroids.
A separate red flag regarding low-dose glucocorticoids was raised by a retrospective cohort study of the 1-year risk of hospitalization for serious infection in 172,041 RA patients in the Medicare claims database for the years 2005-2015. After 6 months of stable use of DMARDs, 47.1% of those patients were on corticosteroids. Their 1-year cumulative incidence of severe infection resulting in hospitalization was 11% if their dose was 5 mg/day or less, 14.4% at >5 to 10 mg/day, and 17.7% at >10 mg/day, all significantly greater than the 8.6% rate in patients not on steroids. The same pattern held true when the investigators separately scrutinized 44,118 RA patients in the Optum Clinformatics Data Mart database for the years 2006-2015.
“This is a really important paper telling us what you’ve always known, which is that there’s a dose-related increase in serious infections in people on steroids. Everybody knows that, but usually people think of 10 mg/day and above,” Dr. Cush said. “This is a little bit sobering for those of you who think, ‘Well, I’m doing OK using 2.5 or 4 mg/day.’ In this study, even at doses of 5 mg/day or less, there’s a significant increase in serious hospitalizable infections.”
Taken together, the message of these two huge studies is clear, he added: “The idea is we probably shouldn’t be using steroids.”
Given the large array of therapies now available to address various aspects of the inflammatory and immune responses in RA, Dr. Kavanaugh asked, why do so many rheumatologists still maintain their patients on low-dose steroids?
”Because when we find something that works, we stick to it,” Dr. Cush replied.
RA disease activity predicts VTE risk
The risk of VTE climbs with RA disease activity such that patients with high disease activity – defined using the erythrocyte sedimentation rate-based Disease Activity Score in 28 joints (DAS28-ESR) – have double the VTE risk of those in disease remission, according to a Swedish nationwide cohort study using data from the Swedish Rheumatology Quality Register. The investigators concluded that RA disease activity can be used in clinical practice as an additional tool for VTE risk stratification, alongside such established risk factors as immobilization, age, surgery, and comorbid conditions.
The study included 46,316 patients with RA who collectively experienced 2,241 VTE events within 1 year after one of their collective 322,601 visits to their rheumatologist. The overall cumulative 1-year incidence of VTE in the RA population was 0.71%, versus 0.36% in a control group composed of 215,843 randomly selected age- and sex-matched individuals drawn from the general Swedish population. That translates to an adjusted 1.88-fold increased risk in the RA group.
The VTE incidence was 0.52% in the year following a rheumatologist visit at which RA patients were found to be in DAS28-ESR remission. The adjusted risk for VTE climbed significantly in stepwise fashion with increasing disease activity: 12% greater risk with DAS28-ESR low disease activity, 48% greater risk with moderate disease activity, and 2.03-fold greater risk with high disease activity.
SEAM-RA shows which drug to withdraw to maintain remission gained on combo therapy
The SEAM-RA trial presented at the 2020 annual meeting of the American College of Rheumatology (ACR) and published shortly afterward in Arthritis & Rheumatology provides guidance on how to best implement a drug tapering strategy in patients who have achieved sustained remission on combination therapy with etanercept (Enbrel) and methotrexate. The purpose of such a strategy is to reduce patients’ medication burden and exposure to safety concerns inherent in continuing therapy. SEAM-RA included 371 RA patients in sustained disease remission during 24 weeks of open-label treatment with etanercept plus methotrexate. At that point, they were randomized 2:2:1 to 48 weeks of double-blind methotrexate monotherapy with etanercept withdrawal, etanercept monotherapy, or continued combination therapy. Those who experienced disease worsening on double-blind monotherapy were eligible for combination rescue therapy with both drugs.
The primary endpoint was maintenance of remission without disease worsening at week 48. This was accomplished in 52.9% of the combination therapy group, with a similar 49.5% success rate in patients on etanercept monotherapy, both significantly better than the 28.7% rate with methotrexate monotherapy. The inference is that biologic monotherapy might be an advantageous strategy for maintenance after achieving prolonged remission on combination therapy.
“This study could impact guidelines,” Dr. Cush predicted.
Among the key study findings, in his view, was that disease worsening occurred early following the switch to methotrexate monotherapy: typically within the first 2-4 weeks. And combination rescue therapy worked: Almost 75% of patients were able to recapture disease remission regardless of which drug they’d been on as monotherapy, and almost 90% of patients on rescue therapy achieved a low-disease-activity state.
Still, just over half of patients were able to maintain long-term remission after downshifting to etanercept monotherapy, Dr. Kavanaugh noted. “The bedeviling thing is we don’t know which patients can do this,” he said.
This was made abundantly clear in PREDICTRA, a phase 4, double-blind study in which 122 RA patients in sustained remission on 40 mg of adalimumab (Humira) every 2 weeks were randomized 5:1 to adalimumab taper or withdrawal for 36 weeks. Overall, 36% of the taper group and 45% of those who halted the biologic flared within 36 weeks. Nothing on an extensive list of candidate baseline predictors predicted flare in either group.
”I think the interesting thing here is that nothing predicted it, not drug levels, antidrug antibodies, not even MRI evidence of inflammation while in remission. The lack of predictors of who’s going to do well off therapy makes us crazy,” Dr. Kavanaugh said. Also noteworthy was that when full-dose, open-label adalimumab was reinstituted as rescue therapy in patients who flared, only half of them regained remission during the following 4 months, he observed.
Dr. Cush didn’t mince words regarding his view of the concept underlying PREDICTRA.
“Why are we even talking about this? We spend our whole lives trying to get these patients into remission, we’re heavily invested in combination therapy, we’re very proud of our great successes, and now we want to find data to condone, ‘Let’s get off drug therapy?’ ” he argued.
“There are untold consequences to this,” the rheumatologist continued. “There are cardiovascular consequences, and we’ve seen the x-ray studies that show that patients may be doing clinically better but still have x-ray worsening because you’ve withdrawn the TNF inhibitor. I think this is all nonsense, and I’m totally against it.”
Making a SELECT-CHOICE in refractory RA
In patients whose RA has proved refractory to one or more biologics, opting for the oral Janus kinase inhibitor upadacitinib as next-line therapy is significantly more effective than turning to the T-cell costimulation modulator abatacept, but at the cost of a doubled risk of severe adverse events, according to the findings of the phase 3, double-blind, 24-week, randomized SELECT-CHOICE trial.
In this 612-patient study, the week-12 clinical remission rate as defined by a C-reactive protein (CRP)-based DAS28 below 2.6 was 30% in the upadacitinib arm, compared to 13.3% with abatacept. At week 24, the remission rates were 46% and 31%, respectively. However, the severe adverse event rate was 6.3% in the upadacitinib group, including one death, one stroke, and two cases of VTE, compared to 3.2% with abatacept.
“Maybe this is a win for upadacitinib, but abatacept is a win when it comes to safety,” Dr. Cush said. “So the question is, when you’re making your second or third change in a biologic DMARD, are you going for safety or are you going for efficacy?”
Make way for machine learning
Studies of machine learning and artificial intelligence aimed at developing a more individualized treatment approach are popping up with increasing frequency at the major rheumatology meetings.
“This is really, I think, the future because for most of us, in most of our choices, as great as all of us are in managing RA, it is a crystal ball and a coin toss as to whether our next therapy is going to work,” Dr. Cush said.
He highlighted a study presented at the 2020 ACR annual meeting as an example of how machine learning can generate better evidence for selecting therapies. An international team of investigators used artificial intelligence to develop a simple rule to predict an RA patient’s likely response to the interleukin-6 receptor inhibitor sarilumab (Kevzara). The researchers developed a list of 42 candidate predictors of an ACR20 response, including demographics, biomarkers, and disease activity scores, then applied machine learning to data from the 1,197-patient, phase 3 MOBILITY trial of sarilumab versus placebo in order to generate a predictive rule of better outcomes.
Out of the 42 candidate predictive values, the best predictive rule turned out to be the combination of the presence of anti-citrullinated protein antibodies and a baseline CRP level greater than 12.3 mg/L. This rule was then put to the test using data from four phase 3 clinical trials in which 34%-51% of RA patients randomized to sarilumab were rule-positive. The rule-positive patients had worse baseline prognostic factors and more severe RA. Yet rule-positive patients had a better outcome than that of those who were rule-negative. For example, a week-24 ACR70 response was achieved in 34% of rule-positive patients, nearly fivefold greater than the 7% rate in the rule-negative group.
Treat-to-target strategies don’t tame excess mortality in RA
At a mean of 17 years of follow-up in the Dutch BeST study – a pioneering treat-to-target study in RA – patients with early RA who were assigned to one of four treat-to-target strategies for 10 years showed excess mortality compared to the general age- and sex-matched Dutch population, Johanna M. Maassen, MD, reported at the 2020 annual meeting of the European Alliance of Associations for Rheumatology (EULAR). Indeed, mortality was 37% higher in the RA patients than in the comparator population, with no significant differences in survival curves evident between the four tight-control strategies.
“This raises the question of whether survival in patients not strictly treated to target would have been even lower. Are we not doing as well as we think we’re doing?” Dr. Kavanaugh said.
“This is very sobering. It tells us we really do need to be better at managing RA. And I would say based on this data we need to be better at managing RA really early to get better outcomes really late,” said Dr. Cush. “The damage that led to cardiovascular risk could have been incurred earlier on and then carried forward when you look out to 15-20 years.”
More trouble for tofacitinib
Tofacitinib faces an uncertain future following Pfizer’s late-January press release announcing that the JAK inhibitor failed to meet noninferiority compared to anti-TNF therapy in terms of adjudicated major adverse cardiovascular events (MACE) and adjudicated malignancies in an FDA-mandated postmarketing safety study. The study, known as ORAL Surveillance, or Study A3921133 – ‘1133’ for short – randomized 4,362 RA patients aged 50 years or older with at least one additional cardiovascular risk factor to tofacitinib at 5 or 10 mg twice daily or to a TNF inhibitor.
The Pfizer press release was soon followed by an FDA safety warning and announcement that the agency is reviewing the full study data before making any final recommendations. The FDA already requires black box warnings for tofacitinib regarding increased risks of blood clots and death, but only at the 10-mg twice-daily dose. In the ORAL Surveillance study, however, the primary endpoint was noninferiority of the combined tofacitinib doses, and there was no evidence of a difference in endpoints between the 5- and 10-mg twice-daily doses.
Only the topline results released in the Pfizer press release are publicly available so far. The combined tofacitinib doses were associated with a 1.33-fold greater risk of adjudicated MACE and a 1.48-fold increase in adjudicated malignancies other than nonmelanoma skin cancer, compared to adalimumab or etanercept.
The jury is still out pending release of the full study data, in Dr. Kavanaugh’s view.
“When I get to look at the data, I want to see who are the people who had the events, their characteristics,” he said.
Alexis R. Ogdie-Beatty, MD, said the study’s topline results “make me a little nervous.
“The effect size is pretty large. It’s in the 1.4 range, which means there might be something there for real,” said Dr. Ogdie-Beatty, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia.
In contrast, Eric M. Ruderman, MD, said he’s not concerned “just yet” about the results.
“Frankly, the FDA was just plain wrong in their press release because the confidence intervals for the MACE events crossed 1.0 meaningfully [0.91, 1.94], so there’s nothing there. And the confidence intervals hit 1.0 for the malignancies,” observed Dr. Ruderman, professor of medicine at Northwestern University, Chicago.
This was a nearly 4,400-patient study, and given that it couldn’t convincingly show a safety risk, such risk – if it even exists – would seem to be tiny, he added.
“It’s probably not going to change the way we prescribe these drugs. So I don’t see that trial taking away from the big run for JAK inhibitors we’re going to see in the next year,” the rheumatologist said.
The speakers reported having financial relationships with numerous pharmaceutical companies.
A massive Swedish cohort study suggesting that biologic agents reduce the known excess risk of lymphoma in patients with rheumatoid arthritis was hailed as one of the high points of the last year by speakers at the 2021 Rheumatology Winter Clinical Symposium.
The speakers gave additional shout-outs regarding other key developments in RA during the pandemic year, including two huge studies showing that even low-dose corticosteroids at 5 mg/day or less carry the price of increased risk of cardiovascular disease and serious infections; Swedish data showing that RA disease activity can be used for venous thromboembolism (VTE) risk stratification; evidence from the venerable BeST study showing at 17 years of follow-up that treat-to-target strategies didn’t prevent an increased mortality risk of RA compared to the general population; and a signal that artificial intelligence using machine learning will become useful in clinical practice sooner than many realize.
Also, the speakers offered kudos to the investigators in the SEAM-RA trial, whose results they expect to be practice changing. In addition, they praised the SELECT-CHOICE trial for providing insight into the relative safety and efficacy of upadacitinib (Rinvoq) and abatacept (Orencia) as second- or third-line therapy in RA. And they warned their colleagues of rocky upcoming months for tofacitinib (Xeljanz) as the Food and Drug Administration reviews concerning new data from a long-term postmarketing safety trial.
Swedes show biologics may reduce excess lymphoma risk in RA
Decades ago, Swedish investigators harnessed their nation’s comprehensive linked health registries to demonstrate that patients with RA are inherently at greater risk for developing lymphoma. Now the Swedes have used the registries to identify a strong signal that this excess risk of lymphoma is reduced in RA patients treated with biologic drugs.
The study included 16,392 RA patients who initiated treatment with a biologic during the study period, 55,253 who were biologic-naive, and 229,047 age- and sex-matched controls from the general population. During the years 2006-2016, there was an adjusted 31% reduction in the risk of incident lymphoma in RA patients who started a first biologic agent, compared with those who did not, and a 54% reduction in lymphoma in biologic-treated patients, compared with those who switched from one conventional synthetic disease-modifying antirheumatic drug (DMARD) to another.
Moreover, while RA patients with 0-2 years of accumulated time on biologics were at a statistically significant 3.42-fold increased risk of lymphoma, compared with the matched general population, that risk shrunk with longer exposure to these medications, dropping to a nonsignificant 1.53-fold increase with 3-4 years of biologic exposure and a 1.04-fold risk with 5 years or more. The lymphoma risks didn’t vary significantly among the TNF inhibitors, nor with the use of TNF inhibitors versus other biologics.
“I think this is really quite encouraging,” said John J. Cush, MD, professor of internal medicine at the University of Texas, Dallas. “If the lymphoma risk was related to inflammation, I think you’d see results like this. It really does speak to the fact that control of inflammation over time does lessen one’s risk of cancer.”
Arthur Kavanaugh, MD, observed that patients with RA continue to ask their rheumatologists if going on a TNF inhibitor will increase their risk of lymphoma, so these Swedish data are going to be useful in conversations in the clinic.
“I thought this study was very exciting,” said Dr. Kavanaugh, professor of medicine at the University of California, San Diego, and director of RWCS. “Maybe we can make the case now that the longer you’re on biologic therapies and the more recently you’re being treated, the better you do in terms of lymphoma risk.”
Beware underappreciated hazards of very-low-dose glucocorticoids
The increased risk of osteoporotic fractures associated with corticosteroid therapy gets tons of attention. But the past year brought two cautionary studies that newly identified significantly increased risks of cardiovascular events and serious infection as well, even at doses of less than 5 mg/day, which have traditionally been believed safe long term.
Investigators at the University of Leeds (England) conducted a population-based cohort study in which they used the U.K. Clinical Practice Research Datalink to analyze the oral corticosteroid dose-dependent cardiovascular risk in 25,324 patients with RA and 62,470 with five other immune-mediated inflammatory diseases: inflammatory bowel disease, giant cell arteritis, polymyalgia rheumatica, systemic lupus erythematosus, and vasculitis. None had known cardiovascular disease at baseline. The analysis accounted for changes in oral steroid dose over time and for the use of potentially confounding medications.
Over a median 5 years of follow-up, a strong steroid dose-dependent risk of acute MI, heart failure, atrial fibrillation, cerebrovascular disease, and abdominal aortic aneurysm was evident in patients with any of the six of the immune-mediated inflammatory diseases. The 1-year adjusted cumulative risk of major adverse cardiovascular events jumped from 1.4% during periods of nonuse to 8.9% at a daily dose of 25 mg or more. Notably, when patients were on oral steroids at doses less than 5 mg/day, their risk of one or more of these major adverse cardiovascular events was 74% greater than when off steroids.
A separate red flag regarding low-dose glucocorticoids was raised by a retrospective cohort study of the 1-year risk of hospitalization for serious infection in 172,041 RA patients in the Medicare claims database for the years 2005-2015. After 6 months of stable use of DMARDs, 47.1% of those patients were on corticosteroids. Their 1-year cumulative incidence of severe infection resulting in hospitalization was 11% if their dose was 5 mg/day or less, 14.4% at >5 to 10 mg/day, and 17.7% at >10 mg/day, all significantly greater than the 8.6% rate in patients not on steroids. The same pattern held true when the investigators separately scrutinized 44,118 RA patients in the Optum Clinformatics Data Mart database for the years 2006-2015.
“This is a really important paper telling us what you’ve always known, which is that there’s a dose-related increase in serious infections in people on steroids. Everybody knows that, but usually people think of 10 mg/day and above,” Dr. Cush said. “This is a little bit sobering for those of you who think, ‘Well, I’m doing OK using 2.5 or 4 mg/day.’ In this study, even at doses of 5 mg/day or less, there’s a significant increase in serious hospitalizable infections.”
Taken together, the message of these two huge studies is clear, he added: “The idea is we probably shouldn’t be using steroids.”
Given the large array of therapies now available to address various aspects of the inflammatory and immune responses in RA, Dr. Kavanaugh asked, why do so many rheumatologists still maintain their patients on low-dose steroids?
”Because when we find something that works, we stick to it,” Dr. Cush replied.
RA disease activity predicts VTE risk
The risk of VTE climbs with RA disease activity such that patients with high disease activity – defined using the erythrocyte sedimentation rate-based Disease Activity Score in 28 joints (DAS28-ESR) – have double the VTE risk of those in disease remission, according to a Swedish nationwide cohort study using data from the Swedish Rheumatology Quality Register. The investigators concluded that RA disease activity can be used in clinical practice as an additional tool for VTE risk stratification, alongside such established risk factors as immobilization, age, surgery, and comorbid conditions.
The study included 46,316 patients with RA who collectively experienced 2,241 VTE events within 1 year after one of their collective 322,601 visits to their rheumatologist. The overall cumulative 1-year incidence of VTE in the RA population was 0.71%, versus 0.36% in a control group composed of 215,843 randomly selected age- and sex-matched individuals drawn from the general Swedish population. That translates to an adjusted 1.88-fold increased risk in the RA group.
The VTE incidence was 0.52% in the year following a rheumatologist visit at which RA patients were found to be in DAS28-ESR remission. The adjusted risk for VTE climbed significantly in stepwise fashion with increasing disease activity: 12% greater risk with DAS28-ESR low disease activity, 48% greater risk with moderate disease activity, and 2.03-fold greater risk with high disease activity.
SEAM-RA shows which drug to withdraw to maintain remission gained on combo therapy
The SEAM-RA trial presented at the 2020 annual meeting of the American College of Rheumatology (ACR) and published shortly afterward in Arthritis & Rheumatology provides guidance on how to best implement a drug tapering strategy in patients who have achieved sustained remission on combination therapy with etanercept (Enbrel) and methotrexate. The purpose of such a strategy is to reduce patients’ medication burden and exposure to safety concerns inherent in continuing therapy. SEAM-RA included 371 RA patients in sustained disease remission during 24 weeks of open-label treatment with etanercept plus methotrexate. At that point, they were randomized 2:2:1 to 48 weeks of double-blind methotrexate monotherapy with etanercept withdrawal, etanercept monotherapy, or continued combination therapy. Those who experienced disease worsening on double-blind monotherapy were eligible for combination rescue therapy with both drugs.
The primary endpoint was maintenance of remission without disease worsening at week 48. This was accomplished in 52.9% of the combination therapy group, with a similar 49.5% success rate in patients on etanercept monotherapy, both significantly better than the 28.7% rate with methotrexate monotherapy. The inference is that biologic monotherapy might be an advantageous strategy for maintenance after achieving prolonged remission on combination therapy.
“This study could impact guidelines,” Dr. Cush predicted.
Among the key study findings, in his view, was that disease worsening occurred early following the switch to methotrexate monotherapy: typically within the first 2-4 weeks. And combination rescue therapy worked: Almost 75% of patients were able to recapture disease remission regardless of which drug they’d been on as monotherapy, and almost 90% of patients on rescue therapy achieved a low-disease-activity state.
Still, just over half of patients were able to maintain long-term remission after downshifting to etanercept monotherapy, Dr. Kavanaugh noted. “The bedeviling thing is we don’t know which patients can do this,” he said.
This was made abundantly clear in PREDICTRA, a phase 4, double-blind study in which 122 RA patients in sustained remission on 40 mg of adalimumab (Humira) every 2 weeks were randomized 5:1 to adalimumab taper or withdrawal for 36 weeks. Overall, 36% of the taper group and 45% of those who halted the biologic flared within 36 weeks. Nothing on an extensive list of candidate baseline predictors predicted flare in either group.
”I think the interesting thing here is that nothing predicted it, not drug levels, antidrug antibodies, not even MRI evidence of inflammation while in remission. The lack of predictors of who’s going to do well off therapy makes us crazy,” Dr. Kavanaugh said. Also noteworthy was that when full-dose, open-label adalimumab was reinstituted as rescue therapy in patients who flared, only half of them regained remission during the following 4 months, he observed.
Dr. Cush didn’t mince words regarding his view of the concept underlying PREDICTRA.
“Why are we even talking about this? We spend our whole lives trying to get these patients into remission, we’re heavily invested in combination therapy, we’re very proud of our great successes, and now we want to find data to condone, ‘Let’s get off drug therapy?’ ” he argued.
“There are untold consequences to this,” the rheumatologist continued. “There are cardiovascular consequences, and we’ve seen the x-ray studies that show that patients may be doing clinically better but still have x-ray worsening because you’ve withdrawn the TNF inhibitor. I think this is all nonsense, and I’m totally against it.”
Making a SELECT-CHOICE in refractory RA
In patients whose RA has proved refractory to one or more biologics, opting for the oral Janus kinase inhibitor upadacitinib as next-line therapy is significantly more effective than turning to the T-cell costimulation modulator abatacept, but at the cost of a doubled risk of severe adverse events, according to the findings of the phase 3, double-blind, 24-week, randomized SELECT-CHOICE trial.
In this 612-patient study, the week-12 clinical remission rate as defined by a C-reactive protein (CRP)-based DAS28 below 2.6 was 30% in the upadacitinib arm, compared to 13.3% with abatacept. At week 24, the remission rates were 46% and 31%, respectively. However, the severe adverse event rate was 6.3% in the upadacitinib group, including one death, one stroke, and two cases of VTE, compared to 3.2% with abatacept.
“Maybe this is a win for upadacitinib, but abatacept is a win when it comes to safety,” Dr. Cush said. “So the question is, when you’re making your second or third change in a biologic DMARD, are you going for safety or are you going for efficacy?”
Make way for machine learning
Studies of machine learning and artificial intelligence aimed at developing a more individualized treatment approach are popping up with increasing frequency at the major rheumatology meetings.
“This is really, I think, the future because for most of us, in most of our choices, as great as all of us are in managing RA, it is a crystal ball and a coin toss as to whether our next therapy is going to work,” Dr. Cush said.
He highlighted a study presented at the 2020 ACR annual meeting as an example of how machine learning can generate better evidence for selecting therapies. An international team of investigators used artificial intelligence to develop a simple rule to predict an RA patient’s likely response to the interleukin-6 receptor inhibitor sarilumab (Kevzara). The researchers developed a list of 42 candidate predictors of an ACR20 response, including demographics, biomarkers, and disease activity scores, then applied machine learning to data from the 1,197-patient, phase 3 MOBILITY trial of sarilumab versus placebo in order to generate a predictive rule of better outcomes.
Out of the 42 candidate predictive values, the best predictive rule turned out to be the combination of the presence of anti-citrullinated protein antibodies and a baseline CRP level greater than 12.3 mg/L. This rule was then put to the test using data from four phase 3 clinical trials in which 34%-51% of RA patients randomized to sarilumab were rule-positive. The rule-positive patients had worse baseline prognostic factors and more severe RA. Yet rule-positive patients had a better outcome than that of those who were rule-negative. For example, a week-24 ACR70 response was achieved in 34% of rule-positive patients, nearly fivefold greater than the 7% rate in the rule-negative group.
Treat-to-target strategies don’t tame excess mortality in RA
At a mean of 17 years of follow-up in the Dutch BeST study – a pioneering treat-to-target study in RA – patients with early RA who were assigned to one of four treat-to-target strategies for 10 years showed excess mortality compared to the general age- and sex-matched Dutch population, Johanna M. Maassen, MD, reported at the 2020 annual meeting of the European Alliance of Associations for Rheumatology (EULAR). Indeed, mortality was 37% higher in the RA patients than in the comparator population, with no significant differences in survival curves evident between the four tight-control strategies.
“This raises the question of whether survival in patients not strictly treated to target would have been even lower. Are we not doing as well as we think we’re doing?” Dr. Kavanaugh said.
“This is very sobering. It tells us we really do need to be better at managing RA. And I would say based on this data we need to be better at managing RA really early to get better outcomes really late,” said Dr. Cush. “The damage that led to cardiovascular risk could have been incurred earlier on and then carried forward when you look out to 15-20 years.”
More trouble for tofacitinib
Tofacitinib faces an uncertain future following Pfizer’s late-January press release announcing that the JAK inhibitor failed to meet noninferiority compared to anti-TNF therapy in terms of adjudicated major adverse cardiovascular events (MACE) and adjudicated malignancies in an FDA-mandated postmarketing safety study. The study, known as ORAL Surveillance, or Study A3921133 – ‘1133’ for short – randomized 4,362 RA patients aged 50 years or older with at least one additional cardiovascular risk factor to tofacitinib at 5 or 10 mg twice daily or to a TNF inhibitor.
The Pfizer press release was soon followed by an FDA safety warning and announcement that the agency is reviewing the full study data before making any final recommendations. The FDA already requires black box warnings for tofacitinib regarding increased risks of blood clots and death, but only at the 10-mg twice-daily dose. In the ORAL Surveillance study, however, the primary endpoint was noninferiority of the combined tofacitinib doses, and there was no evidence of a difference in endpoints between the 5- and 10-mg twice-daily doses.
Only the topline results released in the Pfizer press release are publicly available so far. The combined tofacitinib doses were associated with a 1.33-fold greater risk of adjudicated MACE and a 1.48-fold increase in adjudicated malignancies other than nonmelanoma skin cancer, compared to adalimumab or etanercept.
The jury is still out pending release of the full study data, in Dr. Kavanaugh’s view.
“When I get to look at the data, I want to see who are the people who had the events, their characteristics,” he said.
Alexis R. Ogdie-Beatty, MD, said the study’s topline results “make me a little nervous.
“The effect size is pretty large. It’s in the 1.4 range, which means there might be something there for real,” said Dr. Ogdie-Beatty, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia.
In contrast, Eric M. Ruderman, MD, said he’s not concerned “just yet” about the results.
“Frankly, the FDA was just plain wrong in their press release because the confidence intervals for the MACE events crossed 1.0 meaningfully [0.91, 1.94], so there’s nothing there. And the confidence intervals hit 1.0 for the malignancies,” observed Dr. Ruderman, professor of medicine at Northwestern University, Chicago.
This was a nearly 4,400-patient study, and given that it couldn’t convincingly show a safety risk, such risk – if it even exists – would seem to be tiny, he added.
“It’s probably not going to change the way we prescribe these drugs. So I don’t see that trial taking away from the big run for JAK inhibitors we’re going to see in the next year,” the rheumatologist said.
The speakers reported having financial relationships with numerous pharmaceutical companies.
A massive Swedish cohort study suggesting that biologic agents reduce the known excess risk of lymphoma in patients with rheumatoid arthritis was hailed as one of the high points of the last year by speakers at the 2021 Rheumatology Winter Clinical Symposium.
The speakers gave additional shout-outs regarding other key developments in RA during the pandemic year, including two huge studies showing that even low-dose corticosteroids at 5 mg/day or less carry the price of increased risk of cardiovascular disease and serious infections; Swedish data showing that RA disease activity can be used for venous thromboembolism (VTE) risk stratification; evidence from the venerable BeST study showing at 17 years of follow-up that treat-to-target strategies didn’t prevent an increased mortality risk of RA compared to the general population; and a signal that artificial intelligence using machine learning will become useful in clinical practice sooner than many realize.
Also, the speakers offered kudos to the investigators in the SEAM-RA trial, whose results they expect to be practice changing. In addition, they praised the SELECT-CHOICE trial for providing insight into the relative safety and efficacy of upadacitinib (Rinvoq) and abatacept (Orencia) as second- or third-line therapy in RA. And they warned their colleagues of rocky upcoming months for tofacitinib (Xeljanz) as the Food and Drug Administration reviews concerning new data from a long-term postmarketing safety trial.
Swedes show biologics may reduce excess lymphoma risk in RA
Decades ago, Swedish investigators harnessed their nation’s comprehensive linked health registries to demonstrate that patients with RA are inherently at greater risk for developing lymphoma. Now the Swedes have used the registries to identify a strong signal that this excess risk of lymphoma is reduced in RA patients treated with biologic drugs.
The study included 16,392 RA patients who initiated treatment with a biologic during the study period, 55,253 who were biologic-naive, and 229,047 age- and sex-matched controls from the general population. During the years 2006-2016, there was an adjusted 31% reduction in the risk of incident lymphoma in RA patients who started a first biologic agent, compared with those who did not, and a 54% reduction in lymphoma in biologic-treated patients, compared with those who switched from one conventional synthetic disease-modifying antirheumatic drug (DMARD) to another.
Moreover, while RA patients with 0-2 years of accumulated time on biologics were at a statistically significant 3.42-fold increased risk of lymphoma, compared with the matched general population, that risk shrunk with longer exposure to these medications, dropping to a nonsignificant 1.53-fold increase with 3-4 years of biologic exposure and a 1.04-fold risk with 5 years or more. The lymphoma risks didn’t vary significantly among the TNF inhibitors, nor with the use of TNF inhibitors versus other biologics.
“I think this is really quite encouraging,” said John J. Cush, MD, professor of internal medicine at the University of Texas, Dallas. “If the lymphoma risk was related to inflammation, I think you’d see results like this. It really does speak to the fact that control of inflammation over time does lessen one’s risk of cancer.”
Arthur Kavanaugh, MD, observed that patients with RA continue to ask their rheumatologists if going on a TNF inhibitor will increase their risk of lymphoma, so these Swedish data are going to be useful in conversations in the clinic.
“I thought this study was very exciting,” said Dr. Kavanaugh, professor of medicine at the University of California, San Diego, and director of RWCS. “Maybe we can make the case now that the longer you’re on biologic therapies and the more recently you’re being treated, the better you do in terms of lymphoma risk.”
Beware underappreciated hazards of very-low-dose glucocorticoids
The increased risk of osteoporotic fractures associated with corticosteroid therapy gets tons of attention. But the past year brought two cautionary studies that newly identified significantly increased risks of cardiovascular events and serious infection as well, even at doses of less than 5 mg/day, which have traditionally been believed safe long term.
Investigators at the University of Leeds (England) conducted a population-based cohort study in which they used the U.K. Clinical Practice Research Datalink to analyze the oral corticosteroid dose-dependent cardiovascular risk in 25,324 patients with RA and 62,470 with five other immune-mediated inflammatory diseases: inflammatory bowel disease, giant cell arteritis, polymyalgia rheumatica, systemic lupus erythematosus, and vasculitis. None had known cardiovascular disease at baseline. The analysis accounted for changes in oral steroid dose over time and for the use of potentially confounding medications.
Over a median 5 years of follow-up, a strong steroid dose-dependent risk of acute MI, heart failure, atrial fibrillation, cerebrovascular disease, and abdominal aortic aneurysm was evident in patients with any of the six of the immune-mediated inflammatory diseases. The 1-year adjusted cumulative risk of major adverse cardiovascular events jumped from 1.4% during periods of nonuse to 8.9% at a daily dose of 25 mg or more. Notably, when patients were on oral steroids at doses less than 5 mg/day, their risk of one or more of these major adverse cardiovascular events was 74% greater than when off steroids.
A separate red flag regarding low-dose glucocorticoids was raised by a retrospective cohort study of the 1-year risk of hospitalization for serious infection in 172,041 RA patients in the Medicare claims database for the years 2005-2015. After 6 months of stable use of DMARDs, 47.1% of those patients were on corticosteroids. Their 1-year cumulative incidence of severe infection resulting in hospitalization was 11% if their dose was 5 mg/day or less, 14.4% at >5 to 10 mg/day, and 17.7% at >10 mg/day, all significantly greater than the 8.6% rate in patients not on steroids. The same pattern held true when the investigators separately scrutinized 44,118 RA patients in the Optum Clinformatics Data Mart database for the years 2006-2015.
“This is a really important paper telling us what you’ve always known, which is that there’s a dose-related increase in serious infections in people on steroids. Everybody knows that, but usually people think of 10 mg/day and above,” Dr. Cush said. “This is a little bit sobering for those of you who think, ‘Well, I’m doing OK using 2.5 or 4 mg/day.’ In this study, even at doses of 5 mg/day or less, there’s a significant increase in serious hospitalizable infections.”
Taken together, the message of these two huge studies is clear, he added: “The idea is we probably shouldn’t be using steroids.”
Given the large array of therapies now available to address various aspects of the inflammatory and immune responses in RA, Dr. Kavanaugh asked, why do so many rheumatologists still maintain their patients on low-dose steroids?
”Because when we find something that works, we stick to it,” Dr. Cush replied.
RA disease activity predicts VTE risk
The risk of VTE climbs with RA disease activity such that patients with high disease activity – defined using the erythrocyte sedimentation rate-based Disease Activity Score in 28 joints (DAS28-ESR) – have double the VTE risk of those in disease remission, according to a Swedish nationwide cohort study using data from the Swedish Rheumatology Quality Register. The investigators concluded that RA disease activity can be used in clinical practice as an additional tool for VTE risk stratification, alongside such established risk factors as immobilization, age, surgery, and comorbid conditions.
The study included 46,316 patients with RA who collectively experienced 2,241 VTE events within 1 year after one of their collective 322,601 visits to their rheumatologist. The overall cumulative 1-year incidence of VTE in the RA population was 0.71%, versus 0.36% in a control group composed of 215,843 randomly selected age- and sex-matched individuals drawn from the general Swedish population. That translates to an adjusted 1.88-fold increased risk in the RA group.
The VTE incidence was 0.52% in the year following a rheumatologist visit at which RA patients were found to be in DAS28-ESR remission. The adjusted risk for VTE climbed significantly in stepwise fashion with increasing disease activity: 12% greater risk with DAS28-ESR low disease activity, 48% greater risk with moderate disease activity, and 2.03-fold greater risk with high disease activity.
SEAM-RA shows which drug to withdraw to maintain remission gained on combo therapy
The SEAM-RA trial presented at the 2020 annual meeting of the American College of Rheumatology (ACR) and published shortly afterward in Arthritis & Rheumatology provides guidance on how to best implement a drug tapering strategy in patients who have achieved sustained remission on combination therapy with etanercept (Enbrel) and methotrexate. The purpose of such a strategy is to reduce patients’ medication burden and exposure to safety concerns inherent in continuing therapy. SEAM-RA included 371 RA patients in sustained disease remission during 24 weeks of open-label treatment with etanercept plus methotrexate. At that point, they were randomized 2:2:1 to 48 weeks of double-blind methotrexate monotherapy with etanercept withdrawal, etanercept monotherapy, or continued combination therapy. Those who experienced disease worsening on double-blind monotherapy were eligible for combination rescue therapy with both drugs.
The primary endpoint was maintenance of remission without disease worsening at week 48. This was accomplished in 52.9% of the combination therapy group, with a similar 49.5% success rate in patients on etanercept monotherapy, both significantly better than the 28.7% rate with methotrexate monotherapy. The inference is that biologic monotherapy might be an advantageous strategy for maintenance after achieving prolonged remission on combination therapy.
“This study could impact guidelines,” Dr. Cush predicted.
Among the key study findings, in his view, was that disease worsening occurred early following the switch to methotrexate monotherapy: typically within the first 2-4 weeks. And combination rescue therapy worked: Almost 75% of patients were able to recapture disease remission regardless of which drug they’d been on as monotherapy, and almost 90% of patients on rescue therapy achieved a low-disease-activity state.
Still, just over half of patients were able to maintain long-term remission after downshifting to etanercept monotherapy, Dr. Kavanaugh noted. “The bedeviling thing is we don’t know which patients can do this,” he said.
This was made abundantly clear in PREDICTRA, a phase 4, double-blind study in which 122 RA patients in sustained remission on 40 mg of adalimumab (Humira) every 2 weeks were randomized 5:1 to adalimumab taper or withdrawal for 36 weeks. Overall, 36% of the taper group and 45% of those who halted the biologic flared within 36 weeks. Nothing on an extensive list of candidate baseline predictors predicted flare in either group.
”I think the interesting thing here is that nothing predicted it, not drug levels, antidrug antibodies, not even MRI evidence of inflammation while in remission. The lack of predictors of who’s going to do well off therapy makes us crazy,” Dr. Kavanaugh said. Also noteworthy was that when full-dose, open-label adalimumab was reinstituted as rescue therapy in patients who flared, only half of them regained remission during the following 4 months, he observed.
Dr. Cush didn’t mince words regarding his view of the concept underlying PREDICTRA.
“Why are we even talking about this? We spend our whole lives trying to get these patients into remission, we’re heavily invested in combination therapy, we’re very proud of our great successes, and now we want to find data to condone, ‘Let’s get off drug therapy?’ ” he argued.
“There are untold consequences to this,” the rheumatologist continued. “There are cardiovascular consequences, and we’ve seen the x-ray studies that show that patients may be doing clinically better but still have x-ray worsening because you’ve withdrawn the TNF inhibitor. I think this is all nonsense, and I’m totally against it.”
Making a SELECT-CHOICE in refractory RA
In patients whose RA has proved refractory to one or more biologics, opting for the oral Janus kinase inhibitor upadacitinib as next-line therapy is significantly more effective than turning to the T-cell costimulation modulator abatacept, but at the cost of a doubled risk of severe adverse events, according to the findings of the phase 3, double-blind, 24-week, randomized SELECT-CHOICE trial.
In this 612-patient study, the week-12 clinical remission rate as defined by a C-reactive protein (CRP)-based DAS28 below 2.6 was 30% in the upadacitinib arm, compared to 13.3% with abatacept. At week 24, the remission rates were 46% and 31%, respectively. However, the severe adverse event rate was 6.3% in the upadacitinib group, including one death, one stroke, and two cases of VTE, compared to 3.2% with abatacept.
“Maybe this is a win for upadacitinib, but abatacept is a win when it comes to safety,” Dr. Cush said. “So the question is, when you’re making your second or third change in a biologic DMARD, are you going for safety or are you going for efficacy?”
Make way for machine learning
Studies of machine learning and artificial intelligence aimed at developing a more individualized treatment approach are popping up with increasing frequency at the major rheumatology meetings.
“This is really, I think, the future because for most of us, in most of our choices, as great as all of us are in managing RA, it is a crystal ball and a coin toss as to whether our next therapy is going to work,” Dr. Cush said.
He highlighted a study presented at the 2020 ACR annual meeting as an example of how machine learning can generate better evidence for selecting therapies. An international team of investigators used artificial intelligence to develop a simple rule to predict an RA patient’s likely response to the interleukin-6 receptor inhibitor sarilumab (Kevzara). The researchers developed a list of 42 candidate predictors of an ACR20 response, including demographics, biomarkers, and disease activity scores, then applied machine learning to data from the 1,197-patient, phase 3 MOBILITY trial of sarilumab versus placebo in order to generate a predictive rule of better outcomes.
Out of the 42 candidate predictive values, the best predictive rule turned out to be the combination of the presence of anti-citrullinated protein antibodies and a baseline CRP level greater than 12.3 mg/L. This rule was then put to the test using data from four phase 3 clinical trials in which 34%-51% of RA patients randomized to sarilumab were rule-positive. The rule-positive patients had worse baseline prognostic factors and more severe RA. Yet rule-positive patients had a better outcome than that of those who were rule-negative. For example, a week-24 ACR70 response was achieved in 34% of rule-positive patients, nearly fivefold greater than the 7% rate in the rule-negative group.
Treat-to-target strategies don’t tame excess mortality in RA
At a mean of 17 years of follow-up in the Dutch BeST study – a pioneering treat-to-target study in RA – patients with early RA who were assigned to one of four treat-to-target strategies for 10 years showed excess mortality compared to the general age- and sex-matched Dutch population, Johanna M. Maassen, MD, reported at the 2020 annual meeting of the European Alliance of Associations for Rheumatology (EULAR). Indeed, mortality was 37% higher in the RA patients than in the comparator population, with no significant differences in survival curves evident between the four tight-control strategies.
“This raises the question of whether survival in patients not strictly treated to target would have been even lower. Are we not doing as well as we think we’re doing?” Dr. Kavanaugh said.
“This is very sobering. It tells us we really do need to be better at managing RA. And I would say based on this data we need to be better at managing RA really early to get better outcomes really late,” said Dr. Cush. “The damage that led to cardiovascular risk could have been incurred earlier on and then carried forward when you look out to 15-20 years.”
More trouble for tofacitinib
Tofacitinib faces an uncertain future following Pfizer’s late-January press release announcing that the JAK inhibitor failed to meet noninferiority compared to anti-TNF therapy in terms of adjudicated major adverse cardiovascular events (MACE) and adjudicated malignancies in an FDA-mandated postmarketing safety study. The study, known as ORAL Surveillance, or Study A3921133 – ‘1133’ for short – randomized 4,362 RA patients aged 50 years or older with at least one additional cardiovascular risk factor to tofacitinib at 5 or 10 mg twice daily or to a TNF inhibitor.
The Pfizer press release was soon followed by an FDA safety warning and announcement that the agency is reviewing the full study data before making any final recommendations. The FDA already requires black box warnings for tofacitinib regarding increased risks of blood clots and death, but only at the 10-mg twice-daily dose. In the ORAL Surveillance study, however, the primary endpoint was noninferiority of the combined tofacitinib doses, and there was no evidence of a difference in endpoints between the 5- and 10-mg twice-daily doses.
Only the topline results released in the Pfizer press release are publicly available so far. The combined tofacitinib doses were associated with a 1.33-fold greater risk of adjudicated MACE and a 1.48-fold increase in adjudicated malignancies other than nonmelanoma skin cancer, compared to adalimumab or etanercept.
The jury is still out pending release of the full study data, in Dr. Kavanaugh’s view.
“When I get to look at the data, I want to see who are the people who had the events, their characteristics,” he said.
Alexis R. Ogdie-Beatty, MD, said the study’s topline results “make me a little nervous.
“The effect size is pretty large. It’s in the 1.4 range, which means there might be something there for real,” said Dr. Ogdie-Beatty, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia.
In contrast, Eric M. Ruderman, MD, said he’s not concerned “just yet” about the results.
“Frankly, the FDA was just plain wrong in their press release because the confidence intervals for the MACE events crossed 1.0 meaningfully [0.91, 1.94], so there’s nothing there. And the confidence intervals hit 1.0 for the malignancies,” observed Dr. Ruderman, professor of medicine at Northwestern University, Chicago.
This was a nearly 4,400-patient study, and given that it couldn’t convincingly show a safety risk, such risk – if it even exists – would seem to be tiny, he added.
“It’s probably not going to change the way we prescribe these drugs. So I don’t see that trial taking away from the big run for JAK inhibitors we’re going to see in the next year,” the rheumatologist said.
The speakers reported having financial relationships with numerous pharmaceutical companies.
FROM RWCS 2021
Study clarifies who gets post–COVID-19 interstitial lung disease
A study of post–COVID-19 patients in the United Kingdom who developed severe lung inflammation after they left the hospital may provide greater clarity on which patients are most likely to have persistent lung dysfunction.
In addition to pinpointing those most at risk, the findings showed that conventional corticosteroid treatment is highly effective in improving lung function and reducing symptoms.
Researchers from Guy’s and St. Thomas’ National Health Foundation Trust in London reported that a small percentage of patients – 4.8%, or 35 of 837 patients in the study – had severe persistent interstitial lung disease (ILD), mostly organizing pneumonia, 4 weeks after discharge. Of these patients, 30 received steroid treatment, all of whom showed improvement in lung function.
Lead author Katherine Jane Myall, MRCP, and colleagues wrote that the most common radiologic finding in acute COVID-19 is bilateral ground-glass opacification, and findings of organizing pneumonia are common. However, no reports exist of the role of inflammatory infiltrates during recovery from COVID-19 or of the effectiveness of treatments for persistent ILD. “The long-term respiratory morbidity remains unclear,” Dr. Myall and colleagues wrote.
The study findings are significant because they quantify the degree of lung disease that patients have after COVID-19, said Sachin Gupta, MD, FCCP, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif. He added that the disease course and presentation followed the pattern of organizing pneumonia in some patients, and traditional corticosteroid therapy seemed to resolve symptoms and improve lung function.
“This is a really important piece to get out there because it describes what a lot of us are worried about in patients with post-COVID lung disease and about what type of lung disease they have. It offers a potential treatment,” he said.
Dr. Myall and colleagues noted that even a “relatively small proportion” of patients with persistent, severe ILD – as reported in this study – pose “a significant disease burden.” They added: “Prompt therapy may avoid potentially permanent fibrosis and functional impairment.”
The single-center, prospective, observational study followed discharged patients with telephone calls 4 weeks after discharge to determine their status. At that point, 39% of the study cohort (n = 325) reported ongoing symptoms.
The patients had outpatient examinations at 6 weeks post discharge, at which time 42.9% (n = 138) had no signs or symptoms of persistent disease; 33.8% (n = 110) had symptoms but no radiologic findings and received referrals to other departments; and 24% (n = 77) were referred to the post-COVID lung disease multidisciplinary team. A total of 59 were diagnosed with persistent post-COVID interstitial change, 35 of whom had organizing pneumonia, hence the rationale for using steroids in this group, Dr. Myall and colleagues stated.
The 30 patients treated with corticosteroids received a maximum initial dose of 0.5 mg/kg prednisolone, which was rapidly weaned over 3 weeks. Some patients received lower doses depending on their comorbidities.
Treatment resulted in an average relative increase in transfer factor of 31.6% (P < .001) and forced vital capacity of 9.6% (P = .014), along with significant improvement in symptoms and x-ray signs.
The study identified some key characteristics of the patients who had persistent post–COVID-19 inflammatory ILD. They were mostly male (71.5%) and overweight with an average body mass index of 28.3, but only 26% were obese. Most had at least one comorbidity, with the most common being diabetes and asthma (22.9%). Their average hospital stay was 16.9 days, 82.9% required oxygen, 55% were in the ICU, and 46% needed invasive mechanical ventilation.
The patients most vulnerable to ILD and organizing pneumonia were the “sicker” of the whole cohort, Dr. Gupta said. “In one sense, it’s reassuring that this is not just happening in anyone; this is happening in patients who had the worst course and were hospitalized in the ICU for the most part.”
The study shows that identifying these patients early on and initiating steroid therapy could avoid persistent lung injury and scarring, Dr. Gupta said.
The London researchers noted that theirs wasn’t a radiologic study, so CT scans weren’t formally scored before and after treatment. They also acknowledged vagueness about imaging and clinical findings representing “nothing other than slow ongoing recovery.”
Patients with post–COVID-19 ILD will require ongoing follow-up to better understand the disease course, Dr. Myall and colleagues stated, although they predicted organizing pneumonia is unlikely to recur once it resolves.
Dr. Myall and coauthors had no relevant relationships to disclose. Dr. Gupta disclosed he is also an employee and shareholder at Genentech.
A study of post–COVID-19 patients in the United Kingdom who developed severe lung inflammation after they left the hospital may provide greater clarity on which patients are most likely to have persistent lung dysfunction.
In addition to pinpointing those most at risk, the findings showed that conventional corticosteroid treatment is highly effective in improving lung function and reducing symptoms.
Researchers from Guy’s and St. Thomas’ National Health Foundation Trust in London reported that a small percentage of patients – 4.8%, or 35 of 837 patients in the study – had severe persistent interstitial lung disease (ILD), mostly organizing pneumonia, 4 weeks after discharge. Of these patients, 30 received steroid treatment, all of whom showed improvement in lung function.
Lead author Katherine Jane Myall, MRCP, and colleagues wrote that the most common radiologic finding in acute COVID-19 is bilateral ground-glass opacification, and findings of organizing pneumonia are common. However, no reports exist of the role of inflammatory infiltrates during recovery from COVID-19 or of the effectiveness of treatments for persistent ILD. “The long-term respiratory morbidity remains unclear,” Dr. Myall and colleagues wrote.
The study findings are significant because they quantify the degree of lung disease that patients have after COVID-19, said Sachin Gupta, MD, FCCP, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif. He added that the disease course and presentation followed the pattern of organizing pneumonia in some patients, and traditional corticosteroid therapy seemed to resolve symptoms and improve lung function.
“This is a really important piece to get out there because it describes what a lot of us are worried about in patients with post-COVID lung disease and about what type of lung disease they have. It offers a potential treatment,” he said.
Dr. Myall and colleagues noted that even a “relatively small proportion” of patients with persistent, severe ILD – as reported in this study – pose “a significant disease burden.” They added: “Prompt therapy may avoid potentially permanent fibrosis and functional impairment.”
The single-center, prospective, observational study followed discharged patients with telephone calls 4 weeks after discharge to determine their status. At that point, 39% of the study cohort (n = 325) reported ongoing symptoms.
The patients had outpatient examinations at 6 weeks post discharge, at which time 42.9% (n = 138) had no signs or symptoms of persistent disease; 33.8% (n = 110) had symptoms but no radiologic findings and received referrals to other departments; and 24% (n = 77) were referred to the post-COVID lung disease multidisciplinary team. A total of 59 were diagnosed with persistent post-COVID interstitial change, 35 of whom had organizing pneumonia, hence the rationale for using steroids in this group, Dr. Myall and colleagues stated.
The 30 patients treated with corticosteroids received a maximum initial dose of 0.5 mg/kg prednisolone, which was rapidly weaned over 3 weeks. Some patients received lower doses depending on their comorbidities.
Treatment resulted in an average relative increase in transfer factor of 31.6% (P < .001) and forced vital capacity of 9.6% (P = .014), along with significant improvement in symptoms and x-ray signs.
The study identified some key characteristics of the patients who had persistent post–COVID-19 inflammatory ILD. They were mostly male (71.5%) and overweight with an average body mass index of 28.3, but only 26% were obese. Most had at least one comorbidity, with the most common being diabetes and asthma (22.9%). Their average hospital stay was 16.9 days, 82.9% required oxygen, 55% were in the ICU, and 46% needed invasive mechanical ventilation.
The patients most vulnerable to ILD and organizing pneumonia were the “sicker” of the whole cohort, Dr. Gupta said. “In one sense, it’s reassuring that this is not just happening in anyone; this is happening in patients who had the worst course and were hospitalized in the ICU for the most part.”
The study shows that identifying these patients early on and initiating steroid therapy could avoid persistent lung injury and scarring, Dr. Gupta said.
The London researchers noted that theirs wasn’t a radiologic study, so CT scans weren’t formally scored before and after treatment. They also acknowledged vagueness about imaging and clinical findings representing “nothing other than slow ongoing recovery.”
Patients with post–COVID-19 ILD will require ongoing follow-up to better understand the disease course, Dr. Myall and colleagues stated, although they predicted organizing pneumonia is unlikely to recur once it resolves.
Dr. Myall and coauthors had no relevant relationships to disclose. Dr. Gupta disclosed he is also an employee and shareholder at Genentech.
A study of post–COVID-19 patients in the United Kingdom who developed severe lung inflammation after they left the hospital may provide greater clarity on which patients are most likely to have persistent lung dysfunction.
In addition to pinpointing those most at risk, the findings showed that conventional corticosteroid treatment is highly effective in improving lung function and reducing symptoms.
Researchers from Guy’s and St. Thomas’ National Health Foundation Trust in London reported that a small percentage of patients – 4.8%, or 35 of 837 patients in the study – had severe persistent interstitial lung disease (ILD), mostly organizing pneumonia, 4 weeks after discharge. Of these patients, 30 received steroid treatment, all of whom showed improvement in lung function.
Lead author Katherine Jane Myall, MRCP, and colleagues wrote that the most common radiologic finding in acute COVID-19 is bilateral ground-glass opacification, and findings of organizing pneumonia are common. However, no reports exist of the role of inflammatory infiltrates during recovery from COVID-19 or of the effectiveness of treatments for persistent ILD. “The long-term respiratory morbidity remains unclear,” Dr. Myall and colleagues wrote.
The study findings are significant because they quantify the degree of lung disease that patients have after COVID-19, said Sachin Gupta, MD, FCCP, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif. He added that the disease course and presentation followed the pattern of organizing pneumonia in some patients, and traditional corticosteroid therapy seemed to resolve symptoms and improve lung function.
“This is a really important piece to get out there because it describes what a lot of us are worried about in patients with post-COVID lung disease and about what type of lung disease they have. It offers a potential treatment,” he said.
Dr. Myall and colleagues noted that even a “relatively small proportion” of patients with persistent, severe ILD – as reported in this study – pose “a significant disease burden.” They added: “Prompt therapy may avoid potentially permanent fibrosis and functional impairment.”
The single-center, prospective, observational study followed discharged patients with telephone calls 4 weeks after discharge to determine their status. At that point, 39% of the study cohort (n = 325) reported ongoing symptoms.
The patients had outpatient examinations at 6 weeks post discharge, at which time 42.9% (n = 138) had no signs or symptoms of persistent disease; 33.8% (n = 110) had symptoms but no radiologic findings and received referrals to other departments; and 24% (n = 77) were referred to the post-COVID lung disease multidisciplinary team. A total of 59 were diagnosed with persistent post-COVID interstitial change, 35 of whom had organizing pneumonia, hence the rationale for using steroids in this group, Dr. Myall and colleagues stated.
The 30 patients treated with corticosteroids received a maximum initial dose of 0.5 mg/kg prednisolone, which was rapidly weaned over 3 weeks. Some patients received lower doses depending on their comorbidities.
Treatment resulted in an average relative increase in transfer factor of 31.6% (P < .001) and forced vital capacity of 9.6% (P = .014), along with significant improvement in symptoms and x-ray signs.
The study identified some key characteristics of the patients who had persistent post–COVID-19 inflammatory ILD. They were mostly male (71.5%) and overweight with an average body mass index of 28.3, but only 26% were obese. Most had at least one comorbidity, with the most common being diabetes and asthma (22.9%). Their average hospital stay was 16.9 days, 82.9% required oxygen, 55% were in the ICU, and 46% needed invasive mechanical ventilation.
The patients most vulnerable to ILD and organizing pneumonia were the “sicker” of the whole cohort, Dr. Gupta said. “In one sense, it’s reassuring that this is not just happening in anyone; this is happening in patients who had the worst course and were hospitalized in the ICU for the most part.”
The study shows that identifying these patients early on and initiating steroid therapy could avoid persistent lung injury and scarring, Dr. Gupta said.
The London researchers noted that theirs wasn’t a radiologic study, so CT scans weren’t formally scored before and after treatment. They also acknowledged vagueness about imaging and clinical findings representing “nothing other than slow ongoing recovery.”
Patients with post–COVID-19 ILD will require ongoing follow-up to better understand the disease course, Dr. Myall and colleagues stated, although they predicted organizing pneumonia is unlikely to recur once it resolves.
Dr. Myall and coauthors had no relevant relationships to disclose. Dr. Gupta disclosed he is also an employee and shareholder at Genentech.
FROM ANNALS OF THE AMERICAN THORACIC SOCIETY
Fired for good judgment a sign of physicians’ lost respect
What happened to Hasan Gokal, MD, should stick painfully in the craws of all physicians. It should serve as a call to action, because Dr. Gokal is sitting at home today without a job and under threat of further legal action while we continue about our day.
Dr. Gokal’s “crime” is that he vaccinated 10 strangers and acquaintances with soon-to-expire doses of the Moderna COVID-19 vaccine. He drove to the homes of some in the dark of night and injected others on his Sugar Land, Texas, lawn. He spent hours in a frantic search for willing recipients to beat the expiration clock. With minutes to spare, he gave the last dose to his at-risk wife, who has symptomatic pulmonary sarcoidosis, but whose age meant she did not fall into a vaccine priority tier.
According to the New York Times, Dr. Gokal’s wife was hesitant, afraid he might get into trouble. But why would she be hesitant? He wasn’t doing anything immoral. Perhaps she knew how far physicians have fallen and how bitterly they both could suffer.
In Barren County, Ky., where I live, a state of emergency was declared by our judge executive because of inclement weather. This directive allows our emergency management to “waive procedures and formalities otherwise required by the law.” It’s too bad that the same courtesy was not afforded to Dr. Gokal in Texas. It’s a shame that ice and snow didn’t drive his actions. Perhaps that would have protected him against the harsh criticism. Rather, it was his oath to patients and dedication to his fellow humans that motivated him, and for that, he was made to suffer.
Dr. Gokal was right to think that pouring the last 10 vaccine doses down the toilet would be an egregious act. But he was wrong in thinking his decision to find takers for the vaccine would be viewed as expedient. Instead, he was accused of graft and even nepotism. And there is the rub. That he was fired and charged with the theft of $137 worth of vaccines says everything about how physicians are treated in the year 2021. Dr. Gokal’s lawyer says the charge carried a maximum penalty of 1 year in prison and a fine of nearly $4,000.
Thank God a sage judge threw out the case and “rebuked” the office of District Attorney Kim Ogg. That hasn’t stopped her from threatening to bring the case to a grand jury. That threat invites anyone faced with the same scenario to flush the extra vaccine doses into the septic system. It encourages us to choose the toilet handle to avoid a mug shot.
And we can’t ignore the racial slant to this story. The Times reported that Dr. Gokal asked the officials, “Are you suggesting that there were too many Indian names in this group?”
“Exactly” was the answer. Let that sink in.
None of this would have happened 20 years ago. Back then, no one would have questioned the wisdom a physician gains from all our years of training and residency. In an age when anyone who conducts an office visit is now called “doctor,” respect for the letters “MD” has been leveled. We physicians have lost our autonomy and been cowed into submission.
But whatever his profession, Hasan Gokal was fired for being a good human. Today, the sun rose on 10 individuals who now enjoy better protection against a deadly pandemic. They include a bed-bound nonagenarian. A woman in her 80s with dementia. A mother with a child who uses a ventilator. All now have antibodies against SARS-CoV2 because of the tireless actions of Dr. Gokal.
Yet Dr. Gokal’s future is uncertain. Will we help him, or will we leave him to the wolves? In an email exchange with his lawyer’s office, I learned that Dr. Gokal has received offers of employment but is unable to entertain them because the actions by the Harris County District Attorney triggered an automatic review by the Texas Medical Board. A GoFundMe page was launched, but an appreciative Dr. Gokal stated publicly that he’d rather the money go to a needy charity.
In the last paragraph of the Times article, Dr. Gokal asks, “How can I take it back?” referencing stories about “the Pakistani doctor in Houston who stole all those vaccines.”
Let’s help him take back his story. In helping him, perhaps we can take back a little control. We could start with letters of support that could be mailed to his lawyer, Paul Doyle, Esq., of Houston, or tweet, respectfully of course, to the district attorney @Kimoggforda.
We can also let the Harris County Public Health Department in Houston know what we think of their actions.
On Martin Luther King Day, Kim Ogg, the district attorney who charged Dr. Gokal, tweeted MLK’s famous quote: “Injustice anywhere is a threat to justice everywhere.”
Let that motivate us to action.
Melissa Walton-Shirley, MD, is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. In addition to opinion writing, she enjoys spending time with her husband, daughters and parents, and sidelines as a backing vocalist for local rock bands. A version of this article first appeared on Medscape.com.
What happened to Hasan Gokal, MD, should stick painfully in the craws of all physicians. It should serve as a call to action, because Dr. Gokal is sitting at home today without a job and under threat of further legal action while we continue about our day.
Dr. Gokal’s “crime” is that he vaccinated 10 strangers and acquaintances with soon-to-expire doses of the Moderna COVID-19 vaccine. He drove to the homes of some in the dark of night and injected others on his Sugar Land, Texas, lawn. He spent hours in a frantic search for willing recipients to beat the expiration clock. With minutes to spare, he gave the last dose to his at-risk wife, who has symptomatic pulmonary sarcoidosis, but whose age meant she did not fall into a vaccine priority tier.
According to the New York Times, Dr. Gokal’s wife was hesitant, afraid he might get into trouble. But why would she be hesitant? He wasn’t doing anything immoral. Perhaps she knew how far physicians have fallen and how bitterly they both could suffer.
In Barren County, Ky., where I live, a state of emergency was declared by our judge executive because of inclement weather. This directive allows our emergency management to “waive procedures and formalities otherwise required by the law.” It’s too bad that the same courtesy was not afforded to Dr. Gokal in Texas. It’s a shame that ice and snow didn’t drive his actions. Perhaps that would have protected him against the harsh criticism. Rather, it was his oath to patients and dedication to his fellow humans that motivated him, and for that, he was made to suffer.
Dr. Gokal was right to think that pouring the last 10 vaccine doses down the toilet would be an egregious act. But he was wrong in thinking his decision to find takers for the vaccine would be viewed as expedient. Instead, he was accused of graft and even nepotism. And there is the rub. That he was fired and charged with the theft of $137 worth of vaccines says everything about how physicians are treated in the year 2021. Dr. Gokal’s lawyer says the charge carried a maximum penalty of 1 year in prison and a fine of nearly $4,000.
Thank God a sage judge threw out the case and “rebuked” the office of District Attorney Kim Ogg. That hasn’t stopped her from threatening to bring the case to a grand jury. That threat invites anyone faced with the same scenario to flush the extra vaccine doses into the septic system. It encourages us to choose the toilet handle to avoid a mug shot.
And we can’t ignore the racial slant to this story. The Times reported that Dr. Gokal asked the officials, “Are you suggesting that there were too many Indian names in this group?”
“Exactly” was the answer. Let that sink in.
None of this would have happened 20 years ago. Back then, no one would have questioned the wisdom a physician gains from all our years of training and residency. In an age when anyone who conducts an office visit is now called “doctor,” respect for the letters “MD” has been leveled. We physicians have lost our autonomy and been cowed into submission.
But whatever his profession, Hasan Gokal was fired for being a good human. Today, the sun rose on 10 individuals who now enjoy better protection against a deadly pandemic. They include a bed-bound nonagenarian. A woman in her 80s with dementia. A mother with a child who uses a ventilator. All now have antibodies against SARS-CoV2 because of the tireless actions of Dr. Gokal.
Yet Dr. Gokal’s future is uncertain. Will we help him, or will we leave him to the wolves? In an email exchange with his lawyer’s office, I learned that Dr. Gokal has received offers of employment but is unable to entertain them because the actions by the Harris County District Attorney triggered an automatic review by the Texas Medical Board. A GoFundMe page was launched, but an appreciative Dr. Gokal stated publicly that he’d rather the money go to a needy charity.
In the last paragraph of the Times article, Dr. Gokal asks, “How can I take it back?” referencing stories about “the Pakistani doctor in Houston who stole all those vaccines.”
Let’s help him take back his story. In helping him, perhaps we can take back a little control. We could start with letters of support that could be mailed to his lawyer, Paul Doyle, Esq., of Houston, or tweet, respectfully of course, to the district attorney @Kimoggforda.
We can also let the Harris County Public Health Department in Houston know what we think of their actions.
On Martin Luther King Day, Kim Ogg, the district attorney who charged Dr. Gokal, tweeted MLK’s famous quote: “Injustice anywhere is a threat to justice everywhere.”
Let that motivate us to action.
Melissa Walton-Shirley, MD, is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. In addition to opinion writing, she enjoys spending time with her husband, daughters and parents, and sidelines as a backing vocalist for local rock bands. A version of this article first appeared on Medscape.com.
What happened to Hasan Gokal, MD, should stick painfully in the craws of all physicians. It should serve as a call to action, because Dr. Gokal is sitting at home today without a job and under threat of further legal action while we continue about our day.
Dr. Gokal’s “crime” is that he vaccinated 10 strangers and acquaintances with soon-to-expire doses of the Moderna COVID-19 vaccine. He drove to the homes of some in the dark of night and injected others on his Sugar Land, Texas, lawn. He spent hours in a frantic search for willing recipients to beat the expiration clock. With minutes to spare, he gave the last dose to his at-risk wife, who has symptomatic pulmonary sarcoidosis, but whose age meant she did not fall into a vaccine priority tier.
According to the New York Times, Dr. Gokal’s wife was hesitant, afraid he might get into trouble. But why would she be hesitant? He wasn’t doing anything immoral. Perhaps she knew how far physicians have fallen and how bitterly they both could suffer.
In Barren County, Ky., where I live, a state of emergency was declared by our judge executive because of inclement weather. This directive allows our emergency management to “waive procedures and formalities otherwise required by the law.” It’s too bad that the same courtesy was not afforded to Dr. Gokal in Texas. It’s a shame that ice and snow didn’t drive his actions. Perhaps that would have protected him against the harsh criticism. Rather, it was his oath to patients and dedication to his fellow humans that motivated him, and for that, he was made to suffer.
Dr. Gokal was right to think that pouring the last 10 vaccine doses down the toilet would be an egregious act. But he was wrong in thinking his decision to find takers for the vaccine would be viewed as expedient. Instead, he was accused of graft and even nepotism. And there is the rub. That he was fired and charged with the theft of $137 worth of vaccines says everything about how physicians are treated in the year 2021. Dr. Gokal’s lawyer says the charge carried a maximum penalty of 1 year in prison and a fine of nearly $4,000.
Thank God a sage judge threw out the case and “rebuked” the office of District Attorney Kim Ogg. That hasn’t stopped her from threatening to bring the case to a grand jury. That threat invites anyone faced with the same scenario to flush the extra vaccine doses into the septic system. It encourages us to choose the toilet handle to avoid a mug shot.
And we can’t ignore the racial slant to this story. The Times reported that Dr. Gokal asked the officials, “Are you suggesting that there were too many Indian names in this group?”
“Exactly” was the answer. Let that sink in.
None of this would have happened 20 years ago. Back then, no one would have questioned the wisdom a physician gains from all our years of training and residency. In an age when anyone who conducts an office visit is now called “doctor,” respect for the letters “MD” has been leveled. We physicians have lost our autonomy and been cowed into submission.
But whatever his profession, Hasan Gokal was fired for being a good human. Today, the sun rose on 10 individuals who now enjoy better protection against a deadly pandemic. They include a bed-bound nonagenarian. A woman in her 80s with dementia. A mother with a child who uses a ventilator. All now have antibodies against SARS-CoV2 because of the tireless actions of Dr. Gokal.
Yet Dr. Gokal’s future is uncertain. Will we help him, or will we leave him to the wolves? In an email exchange with his lawyer’s office, I learned that Dr. Gokal has received offers of employment but is unable to entertain them because the actions by the Harris County District Attorney triggered an automatic review by the Texas Medical Board. A GoFundMe page was launched, but an appreciative Dr. Gokal stated publicly that he’d rather the money go to a needy charity.
In the last paragraph of the Times article, Dr. Gokal asks, “How can I take it back?” referencing stories about “the Pakistani doctor in Houston who stole all those vaccines.”
Let’s help him take back his story. In helping him, perhaps we can take back a little control. We could start with letters of support that could be mailed to his lawyer, Paul Doyle, Esq., of Houston, or tweet, respectfully of course, to the district attorney @Kimoggforda.
We can also let the Harris County Public Health Department in Houston know what we think of their actions.
On Martin Luther King Day, Kim Ogg, the district attorney who charged Dr. Gokal, tweeted MLK’s famous quote: “Injustice anywhere is a threat to justice everywhere.”
Let that motivate us to action.
Melissa Walton-Shirley, MD, is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. In addition to opinion writing, she enjoys spending time with her husband, daughters and parents, and sidelines as a backing vocalist for local rock bands. A version of this article first appeared on Medscape.com.