User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'medstat-accordion-set article-series')]
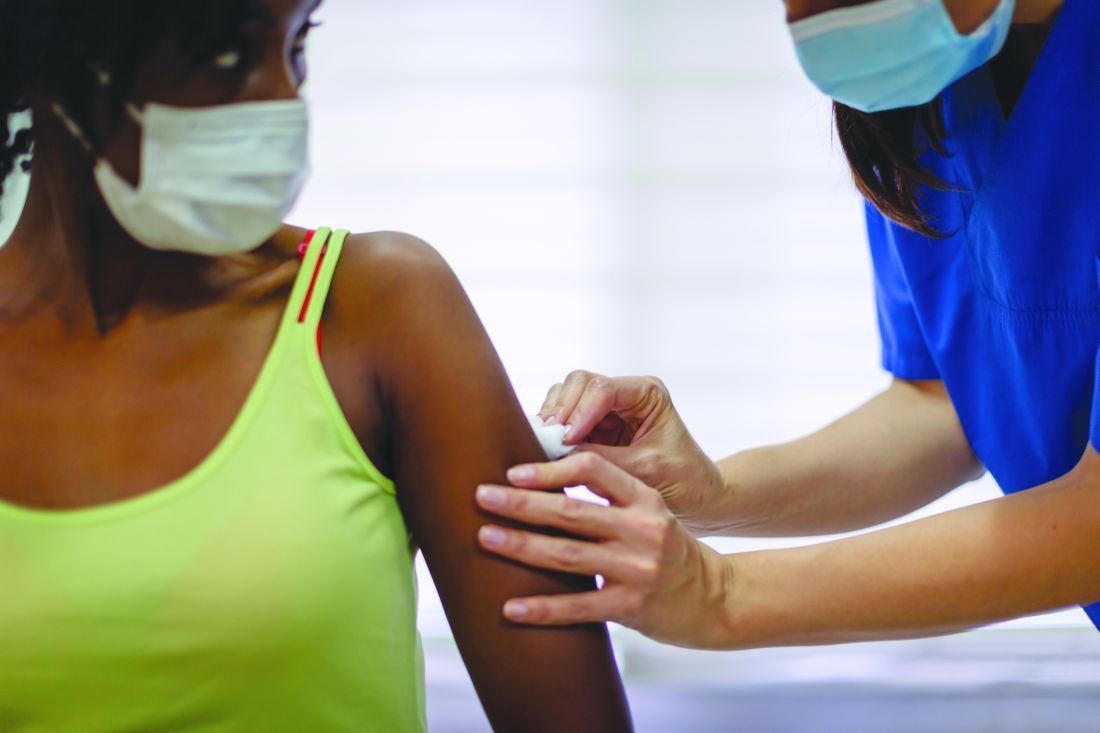
U.S. finally hits its stride with COVID-19 vaccination rollouts
Each afternoon, Cyrus Shahpar, MD, the data guru for the White House COVID-19 Response Team, sends an email to staffers with the daily count of COVID-19 vaccinations delivered in the United States.
The numbers, collected from states ahead of the final figures being posted on the Centers for Disease Control and Prevention website, act as a report card of sorts on the team’s efforts.
On Saturday, April 3, it was a new record: 4.1 million vaccinations delivered in a single day, more than the total population of some states.
While the United States has a long way to go before it is done with COVID-19, there’s finally some good news in the nation’s long and blundering slog through the pandemic.
After a rocky start in December 2020 and January 2021, vaccination is happening faster than nearly anyone thought possible. As more people see their friends and family roll up their sleeves, hesitancy is dropping, too.
In settings where large numbers of people are vaccinated, such as nursing homes, COVID-19 cases and deaths have plunged.
Those gains, however, haven’t been shared equally. According to CDC data, 69% of people who are fully vaccinated are White, while just 8% are Black and about 9% are Hispanic, a group that now represents most new COVID-19 cases.
Officials say that’s partly because the vaccines were rolled out to the elderly first. The average life expectancy for Black people in the United States is now age 72, which means there were fewer people of color represented in the first groups to become eligible. Experts are hopeful that underrepresented groups will start to catch up as more states open up vaccinations to younger people.
Based on overall numbers of daily vaccine doses, the United States ranks third, behind China and India. America ranks fourth – behind Israel, the United Kingdom, and Chile – in the total share of the population that’s been vaccinated, according to the website Our World in Data.
A positive development
It’s a stunning turnaround for a country that failed for months to develop effective tests, and still struggles in some quarters to investigate new cases and quarantine their contacts.
The 7-day rolling average of vaccines administered in the United States is currently more than 3 million a day.
“We knew that we needed to get to 3 million a day at some point, if we were going to get most people vaccinated this year, but I don’t think that most people expected it to happen this early,” said Eric Toner, MD, a senior scholar with the Johns Hopkins Center for Health Security in Baltimore.
Before taking office, President Joe Biden pledged to get 100 million shots in arms within his first 100 days in office. After hitting that goal in late March, he doubled it, to 200 million vaccinations by April 30. After first saying all adults should be eligible to get in line for the vaccine by May 1, on April 6, he bumped up that date to April 19.
Some media reports have seen this repeated moving of the goalposts as calculated – an unstated strategy of underpromising and overdelivering with the aim of rebuilding public trust.
But others pointed out that, even if that’s true, the goals being set aren’t easy, and hitting them has never been a given.
“I think the Biden administration really gets a lot of credit for pushing the companies to get more vaccine out faster than they had planned to,” Dr. Toner said. “And the states have really responded as well as the federal government in terms of getting vaccination sites going. So we’re not only getting the vaccines, we’re getting it into people’s arms faster than expected.”
Others agree.
“We’re doing an amazing job, and I think the U.S. is really beginning to bend the curve,” said Carlos del Rio, MD, an infectious disease specialist and distinguished professor of medicine at Emory University, Atlanta.
“I think overall it’s just that everybody’s putting in a ton of work to get it done,” he said.
On April 3, the day the United States hit its vaccination record, he was volunteering to give vaccinations.
“I mean, of all the bad things we do to people as clinicians, this is one thing that people are very happy about, right?” Dr. del Rio said.
He said he vaccinated a young woman who asked if she could video chat with her mom, who was feeling nervous about getting the shot. He answered her mom’s questions, and later that day, she came down to be vaccinated herself.
‘We view it as a war’
The White House COVID-19 Response Team has worked hard to better coordinate the work of so many people at both the federal and state levels, Andy Slavitt, senior adviser for the team, said in an interview.
“We view it as a war, and in a war, you do everything: You bring experienced personnel; you bring all the resources to bear; you create multiple routes,” Mr. Slavitt said. “You don’t leave anything to chance.”
Among the levers the administration has pulled, using the Defense Production Act has helped vaccine manufacturers get needed supplies, Mr. Slavitt said.
The administration has set up an array of Federal Emergency Management Agency–run community vaccination centers and mobile vaccination sites to complement state-led efforts, and it’s activated a federal health law called the Public Readiness and Emergency Preparedness Act, which provides immunity from liability for retired doctors and nurses, among others, who sign up to help give vaccinations. That’s helped get more people into the field giving shots.
The administration also canceled a plan to allocate vaccines to states based on their pace of administration, which would have punished underperforming states. Instead, doses are allocated based on population.
In a media call on April 7, when asked whether the administration would send additional vaccines to Michigan, a state that’s seeing a surge of COVID-19 cases with more transmissible variants, Mr. Slavitt said they weren’t managing vaccine supply “according to some formula.”
He said they were distributing based on population “because that’s fundamental,” but were also locating vaccines “surgically in places that have had the greatest disease and where people have the greatest exposure.”
He said sites like community health centers and retail pharmacies have the power to order vaccines directly from the federal government, which helps get more supply to harder-hit areas.
Mr. Slavitt said hitting 4.1 million daily vaccinations on April 3 was gratifying.
“I’ve seen photographs ... of people breaking down in tears when they get their vaccine, people who are giving standing ovations to active military for taking care of them,” he said, “and I think about people who have gone for a long time without hope, or who have been very scared.
“It’s incredibly encouraging to think about maybe a few million people taking a step back to normal life again,” he said.
A version of this article first appeared on Medscape.com.
Each afternoon, Cyrus Shahpar, MD, the data guru for the White House COVID-19 Response Team, sends an email to staffers with the daily count of COVID-19 vaccinations delivered in the United States.
The numbers, collected from states ahead of the final figures being posted on the Centers for Disease Control and Prevention website, act as a report card of sorts on the team’s efforts.
On Saturday, April 3, it was a new record: 4.1 million vaccinations delivered in a single day, more than the total population of some states.
While the United States has a long way to go before it is done with COVID-19, there’s finally some good news in the nation’s long and blundering slog through the pandemic.
After a rocky start in December 2020 and January 2021, vaccination is happening faster than nearly anyone thought possible. As more people see their friends and family roll up their sleeves, hesitancy is dropping, too.
In settings where large numbers of people are vaccinated, such as nursing homes, COVID-19 cases and deaths have plunged.
Those gains, however, haven’t been shared equally. According to CDC data, 69% of people who are fully vaccinated are White, while just 8% are Black and about 9% are Hispanic, a group that now represents most new COVID-19 cases.
Officials say that’s partly because the vaccines were rolled out to the elderly first. The average life expectancy for Black people in the United States is now age 72, which means there were fewer people of color represented in the first groups to become eligible. Experts are hopeful that underrepresented groups will start to catch up as more states open up vaccinations to younger people.
Based on overall numbers of daily vaccine doses, the United States ranks third, behind China and India. America ranks fourth – behind Israel, the United Kingdom, and Chile – in the total share of the population that’s been vaccinated, according to the website Our World in Data.
A positive development
It’s a stunning turnaround for a country that failed for months to develop effective tests, and still struggles in some quarters to investigate new cases and quarantine their contacts.
The 7-day rolling average of vaccines administered in the United States is currently more than 3 million a day.
“We knew that we needed to get to 3 million a day at some point, if we were going to get most people vaccinated this year, but I don’t think that most people expected it to happen this early,” said Eric Toner, MD, a senior scholar with the Johns Hopkins Center for Health Security in Baltimore.
Before taking office, President Joe Biden pledged to get 100 million shots in arms within his first 100 days in office. After hitting that goal in late March, he doubled it, to 200 million vaccinations by April 30. After first saying all adults should be eligible to get in line for the vaccine by May 1, on April 6, he bumped up that date to April 19.
Some media reports have seen this repeated moving of the goalposts as calculated – an unstated strategy of underpromising and overdelivering with the aim of rebuilding public trust.
But others pointed out that, even if that’s true, the goals being set aren’t easy, and hitting them has never been a given.
“I think the Biden administration really gets a lot of credit for pushing the companies to get more vaccine out faster than they had planned to,” Dr. Toner said. “And the states have really responded as well as the federal government in terms of getting vaccination sites going. So we’re not only getting the vaccines, we’re getting it into people’s arms faster than expected.”
Others agree.
“We’re doing an amazing job, and I think the U.S. is really beginning to bend the curve,” said Carlos del Rio, MD, an infectious disease specialist and distinguished professor of medicine at Emory University, Atlanta.
“I think overall it’s just that everybody’s putting in a ton of work to get it done,” he said.
On April 3, the day the United States hit its vaccination record, he was volunteering to give vaccinations.
“I mean, of all the bad things we do to people as clinicians, this is one thing that people are very happy about, right?” Dr. del Rio said.
He said he vaccinated a young woman who asked if she could video chat with her mom, who was feeling nervous about getting the shot. He answered her mom’s questions, and later that day, she came down to be vaccinated herself.
‘We view it as a war’
The White House COVID-19 Response Team has worked hard to better coordinate the work of so many people at both the federal and state levels, Andy Slavitt, senior adviser for the team, said in an interview.
“We view it as a war, and in a war, you do everything: You bring experienced personnel; you bring all the resources to bear; you create multiple routes,” Mr. Slavitt said. “You don’t leave anything to chance.”
Among the levers the administration has pulled, using the Defense Production Act has helped vaccine manufacturers get needed supplies, Mr. Slavitt said.
The administration has set up an array of Federal Emergency Management Agency–run community vaccination centers and mobile vaccination sites to complement state-led efforts, and it’s activated a federal health law called the Public Readiness and Emergency Preparedness Act, which provides immunity from liability for retired doctors and nurses, among others, who sign up to help give vaccinations. That’s helped get more people into the field giving shots.
The administration also canceled a plan to allocate vaccines to states based on their pace of administration, which would have punished underperforming states. Instead, doses are allocated based on population.
In a media call on April 7, when asked whether the administration would send additional vaccines to Michigan, a state that’s seeing a surge of COVID-19 cases with more transmissible variants, Mr. Slavitt said they weren’t managing vaccine supply “according to some formula.”
He said they were distributing based on population “because that’s fundamental,” but were also locating vaccines “surgically in places that have had the greatest disease and where people have the greatest exposure.”
He said sites like community health centers and retail pharmacies have the power to order vaccines directly from the federal government, which helps get more supply to harder-hit areas.
Mr. Slavitt said hitting 4.1 million daily vaccinations on April 3 was gratifying.
“I’ve seen photographs ... of people breaking down in tears when they get their vaccine, people who are giving standing ovations to active military for taking care of them,” he said, “and I think about people who have gone for a long time without hope, or who have been very scared.
“It’s incredibly encouraging to think about maybe a few million people taking a step back to normal life again,” he said.
A version of this article first appeared on Medscape.com.
Each afternoon, Cyrus Shahpar, MD, the data guru for the White House COVID-19 Response Team, sends an email to staffers with the daily count of COVID-19 vaccinations delivered in the United States.
The numbers, collected from states ahead of the final figures being posted on the Centers for Disease Control and Prevention website, act as a report card of sorts on the team’s efforts.
On Saturday, April 3, it was a new record: 4.1 million vaccinations delivered in a single day, more than the total population of some states.
While the United States has a long way to go before it is done with COVID-19, there’s finally some good news in the nation’s long and blundering slog through the pandemic.
After a rocky start in December 2020 and January 2021, vaccination is happening faster than nearly anyone thought possible. As more people see their friends and family roll up their sleeves, hesitancy is dropping, too.
In settings where large numbers of people are vaccinated, such as nursing homes, COVID-19 cases and deaths have plunged.
Those gains, however, haven’t been shared equally. According to CDC data, 69% of people who are fully vaccinated are White, while just 8% are Black and about 9% are Hispanic, a group that now represents most new COVID-19 cases.
Officials say that’s partly because the vaccines were rolled out to the elderly first. The average life expectancy for Black people in the United States is now age 72, which means there were fewer people of color represented in the first groups to become eligible. Experts are hopeful that underrepresented groups will start to catch up as more states open up vaccinations to younger people.
Based on overall numbers of daily vaccine doses, the United States ranks third, behind China and India. America ranks fourth – behind Israel, the United Kingdom, and Chile – in the total share of the population that’s been vaccinated, according to the website Our World in Data.
A positive development
It’s a stunning turnaround for a country that failed for months to develop effective tests, and still struggles in some quarters to investigate new cases and quarantine their contacts.
The 7-day rolling average of vaccines administered in the United States is currently more than 3 million a day.
“We knew that we needed to get to 3 million a day at some point, if we were going to get most people vaccinated this year, but I don’t think that most people expected it to happen this early,” said Eric Toner, MD, a senior scholar with the Johns Hopkins Center for Health Security in Baltimore.
Before taking office, President Joe Biden pledged to get 100 million shots in arms within his first 100 days in office. After hitting that goal in late March, he doubled it, to 200 million vaccinations by April 30. After first saying all adults should be eligible to get in line for the vaccine by May 1, on April 6, he bumped up that date to April 19.
Some media reports have seen this repeated moving of the goalposts as calculated – an unstated strategy of underpromising and overdelivering with the aim of rebuilding public trust.
But others pointed out that, even if that’s true, the goals being set aren’t easy, and hitting them has never been a given.
“I think the Biden administration really gets a lot of credit for pushing the companies to get more vaccine out faster than they had planned to,” Dr. Toner said. “And the states have really responded as well as the federal government in terms of getting vaccination sites going. So we’re not only getting the vaccines, we’re getting it into people’s arms faster than expected.”
Others agree.
“We’re doing an amazing job, and I think the U.S. is really beginning to bend the curve,” said Carlos del Rio, MD, an infectious disease specialist and distinguished professor of medicine at Emory University, Atlanta.
“I think overall it’s just that everybody’s putting in a ton of work to get it done,” he said.
On April 3, the day the United States hit its vaccination record, he was volunteering to give vaccinations.
“I mean, of all the bad things we do to people as clinicians, this is one thing that people are very happy about, right?” Dr. del Rio said.
He said he vaccinated a young woman who asked if she could video chat with her mom, who was feeling nervous about getting the shot. He answered her mom’s questions, and later that day, she came down to be vaccinated herself.
‘We view it as a war’
The White House COVID-19 Response Team has worked hard to better coordinate the work of so many people at both the federal and state levels, Andy Slavitt, senior adviser for the team, said in an interview.
“We view it as a war, and in a war, you do everything: You bring experienced personnel; you bring all the resources to bear; you create multiple routes,” Mr. Slavitt said. “You don’t leave anything to chance.”
Among the levers the administration has pulled, using the Defense Production Act has helped vaccine manufacturers get needed supplies, Mr. Slavitt said.
The administration has set up an array of Federal Emergency Management Agency–run community vaccination centers and mobile vaccination sites to complement state-led efforts, and it’s activated a federal health law called the Public Readiness and Emergency Preparedness Act, which provides immunity from liability for retired doctors and nurses, among others, who sign up to help give vaccinations. That’s helped get more people into the field giving shots.
The administration also canceled a plan to allocate vaccines to states based on their pace of administration, which would have punished underperforming states. Instead, doses are allocated based on population.
In a media call on April 7, when asked whether the administration would send additional vaccines to Michigan, a state that’s seeing a surge of COVID-19 cases with more transmissible variants, Mr. Slavitt said they weren’t managing vaccine supply “according to some formula.”
He said they were distributing based on population “because that’s fundamental,” but were also locating vaccines “surgically in places that have had the greatest disease and where people have the greatest exposure.”
He said sites like community health centers and retail pharmacies have the power to order vaccines directly from the federal government, which helps get more supply to harder-hit areas.
Mr. Slavitt said hitting 4.1 million daily vaccinations on April 3 was gratifying.
“I’ve seen photographs ... of people breaking down in tears when they get their vaccine, people who are giving standing ovations to active military for taking care of them,” he said, “and I think about people who have gone for a long time without hope, or who have been very scared.
“It’s incredibly encouraging to think about maybe a few million people taking a step back to normal life again,” he said.
A version of this article first appeared on Medscape.com.
Rheumatology clinics find success with smoking cessation referral program
A new protocol designed to help patients in rheumatology clinics quit smoking proved both efficient and effective in referring willing participants to free tobacco quit lines.
“Rheumatology visits provide a unique opportunity to address smoking as a chronic modifiable risk factor in populations at high risk for cardiovascular disease, pulmonary disease, and rheumatic disease progression,” wrote Christie M. Bartels, MD, chief of the division of rheumatology at the University of Wisconsin, Madison, and colleagues. The study was published in Arthritis Care & Research.
To assess the effectiveness of implementing a smoking cessation protocol for patients with rheumatic diseases, the researchers launched a quasi-experimental cohort study in which their Quit Connect protocol was tested at three rheumatology clinics. Adapting the Ask, Advise, Connect primary care protocol to a new setting, nurses and medical assistants were trained to use electronic health record (EHR) prompts that would check if patients who smoked were ready to quit within 30 days, advise them to do so, and then use electronic referrals to connect them to state-run tobacco quit lines. An extended baseline period – October 2012 to March 2016 – was compared to a 6-month intervention period from April to October 2016.
Across 54,090 pre- and postimplementation rheumatology clinic visits, 4,601 were with current smokers. Demographics were similar across both periods: The mean age of the patients was 51 years, about two-thirds were female, and 85% were White.
Clinicians’ assessment of tobacco use before and after implementation of the program stayed steady at 96% of patient visits, but the percentage of tobacco users’ visits that included checking for readiness to quit within the next 30 days rose from 3% (135 of 4,078) to 80% (421 of 523).
Before the implementation of the program, 0.6% of eligible visits with current smokers included a quit-line referral offer. After implementation, 93 (18%) of the 523 smokers who visited – 122 of whom said they were ready to quit – were offered referrals, a 26-fold increase. Of the 93 offered referrals, 66 (71%) accepted and 16 set a quit date or reported having quit; 11 accepted counseling services and nicotine replacement.
Although clinic staff reported encountering several obstacles, such as the need to craft nonthreatening language for challenging patients, they also contributed their own talking points that were included in the EHR tools and desktop brochures. On average, the protocol took less than 90 seconds to perform.
Rheumatologists can make headway on patients quitting smoking
“While smoking cessation programs require time and resources to implement, this study suggests a role for evidence-based protocols within rheumatology centers,” Medha Barbhaiya, MD, a rheumatologist at the Hospital for Special Surgery in New York, said in an interview. “Given that current smokers are at an increased risk of developing more severe rheumatic disease and cardiovascular disease, and patients often visit their rheumatologist multiple times yearly, rheumatologists may be well-positioned to address smoking cessation with patients.”
In regard to next steps, she noted that “while future large studies in diverse cohorts are needed to confirm these findings, implementing a formal smoking cessation protocol within rheumatology centers may provide a unique opportunity for rheumatologists to directly help patients modify their disease risk, leading to improved health outcomes.”
The authors acknowledged their study’s limitations, including the fact that it was a prepost design and not a randomized trial. They also recognized that many tobacco users require 8-10 attempts before permanently quitting, likely lessening the lasting impact of the short-term study. They did cite expert analysis, however, that says “connecting patients to evidence-based resources makes them more likely to permanently quit.”
The study was supported in part by Pfizer’s office of Independent Grants for Learning and Change and by a grant collaboration from the University of Wisconsin Clinical and Translational Science Award and the University of Wisconsin School of Medicine and Public Health’s Wisconsin Partnership Program, through the NIH National Center for Advancing Translational Sciences.
A new protocol designed to help patients in rheumatology clinics quit smoking proved both efficient and effective in referring willing participants to free tobacco quit lines.
“Rheumatology visits provide a unique opportunity to address smoking as a chronic modifiable risk factor in populations at high risk for cardiovascular disease, pulmonary disease, and rheumatic disease progression,” wrote Christie M. Bartels, MD, chief of the division of rheumatology at the University of Wisconsin, Madison, and colleagues. The study was published in Arthritis Care & Research.
To assess the effectiveness of implementing a smoking cessation protocol for patients with rheumatic diseases, the researchers launched a quasi-experimental cohort study in which their Quit Connect protocol was tested at three rheumatology clinics. Adapting the Ask, Advise, Connect primary care protocol to a new setting, nurses and medical assistants were trained to use electronic health record (EHR) prompts that would check if patients who smoked were ready to quit within 30 days, advise them to do so, and then use electronic referrals to connect them to state-run tobacco quit lines. An extended baseline period – October 2012 to March 2016 – was compared to a 6-month intervention period from April to October 2016.
Across 54,090 pre- and postimplementation rheumatology clinic visits, 4,601 were with current smokers. Demographics were similar across both periods: The mean age of the patients was 51 years, about two-thirds were female, and 85% were White.
Clinicians’ assessment of tobacco use before and after implementation of the program stayed steady at 96% of patient visits, but the percentage of tobacco users’ visits that included checking for readiness to quit within the next 30 days rose from 3% (135 of 4,078) to 80% (421 of 523).
Before the implementation of the program, 0.6% of eligible visits with current smokers included a quit-line referral offer. After implementation, 93 (18%) of the 523 smokers who visited – 122 of whom said they were ready to quit – were offered referrals, a 26-fold increase. Of the 93 offered referrals, 66 (71%) accepted and 16 set a quit date or reported having quit; 11 accepted counseling services and nicotine replacement.
Although clinic staff reported encountering several obstacles, such as the need to craft nonthreatening language for challenging patients, they also contributed their own talking points that were included in the EHR tools and desktop brochures. On average, the protocol took less than 90 seconds to perform.
Rheumatologists can make headway on patients quitting smoking
“While smoking cessation programs require time and resources to implement, this study suggests a role for evidence-based protocols within rheumatology centers,” Medha Barbhaiya, MD, a rheumatologist at the Hospital for Special Surgery in New York, said in an interview. “Given that current smokers are at an increased risk of developing more severe rheumatic disease and cardiovascular disease, and patients often visit their rheumatologist multiple times yearly, rheumatologists may be well-positioned to address smoking cessation with patients.”
In regard to next steps, she noted that “while future large studies in diverse cohorts are needed to confirm these findings, implementing a formal smoking cessation protocol within rheumatology centers may provide a unique opportunity for rheumatologists to directly help patients modify their disease risk, leading to improved health outcomes.”
The authors acknowledged their study’s limitations, including the fact that it was a prepost design and not a randomized trial. They also recognized that many tobacco users require 8-10 attempts before permanently quitting, likely lessening the lasting impact of the short-term study. They did cite expert analysis, however, that says “connecting patients to evidence-based resources makes them more likely to permanently quit.”
The study was supported in part by Pfizer’s office of Independent Grants for Learning and Change and by a grant collaboration from the University of Wisconsin Clinical and Translational Science Award and the University of Wisconsin School of Medicine and Public Health’s Wisconsin Partnership Program, through the NIH National Center for Advancing Translational Sciences.
A new protocol designed to help patients in rheumatology clinics quit smoking proved both efficient and effective in referring willing participants to free tobacco quit lines.
“Rheumatology visits provide a unique opportunity to address smoking as a chronic modifiable risk factor in populations at high risk for cardiovascular disease, pulmonary disease, and rheumatic disease progression,” wrote Christie M. Bartels, MD, chief of the division of rheumatology at the University of Wisconsin, Madison, and colleagues. The study was published in Arthritis Care & Research.
To assess the effectiveness of implementing a smoking cessation protocol for patients with rheumatic diseases, the researchers launched a quasi-experimental cohort study in which their Quit Connect protocol was tested at three rheumatology clinics. Adapting the Ask, Advise, Connect primary care protocol to a new setting, nurses and medical assistants were trained to use electronic health record (EHR) prompts that would check if patients who smoked were ready to quit within 30 days, advise them to do so, and then use electronic referrals to connect them to state-run tobacco quit lines. An extended baseline period – October 2012 to March 2016 – was compared to a 6-month intervention period from April to October 2016.
Across 54,090 pre- and postimplementation rheumatology clinic visits, 4,601 were with current smokers. Demographics were similar across both periods: The mean age of the patients was 51 years, about two-thirds were female, and 85% were White.
Clinicians’ assessment of tobacco use before and after implementation of the program stayed steady at 96% of patient visits, but the percentage of tobacco users’ visits that included checking for readiness to quit within the next 30 days rose from 3% (135 of 4,078) to 80% (421 of 523).
Before the implementation of the program, 0.6% of eligible visits with current smokers included a quit-line referral offer. After implementation, 93 (18%) of the 523 smokers who visited – 122 of whom said they were ready to quit – were offered referrals, a 26-fold increase. Of the 93 offered referrals, 66 (71%) accepted and 16 set a quit date or reported having quit; 11 accepted counseling services and nicotine replacement.
Although clinic staff reported encountering several obstacles, such as the need to craft nonthreatening language for challenging patients, they also contributed their own talking points that were included in the EHR tools and desktop brochures. On average, the protocol took less than 90 seconds to perform.
Rheumatologists can make headway on patients quitting smoking
“While smoking cessation programs require time and resources to implement, this study suggests a role for evidence-based protocols within rheumatology centers,” Medha Barbhaiya, MD, a rheumatologist at the Hospital for Special Surgery in New York, said in an interview. “Given that current smokers are at an increased risk of developing more severe rheumatic disease and cardiovascular disease, and patients often visit their rheumatologist multiple times yearly, rheumatologists may be well-positioned to address smoking cessation with patients.”
In regard to next steps, she noted that “while future large studies in diverse cohorts are needed to confirm these findings, implementing a formal smoking cessation protocol within rheumatology centers may provide a unique opportunity for rheumatologists to directly help patients modify their disease risk, leading to improved health outcomes.”
The authors acknowledged their study’s limitations, including the fact that it was a prepost design and not a randomized trial. They also recognized that many tobacco users require 8-10 attempts before permanently quitting, likely lessening the lasting impact of the short-term study. They did cite expert analysis, however, that says “connecting patients to evidence-based resources makes them more likely to permanently quit.”
The study was supported in part by Pfizer’s office of Independent Grants for Learning and Change and by a grant collaboration from the University of Wisconsin Clinical and Translational Science Award and the University of Wisconsin School of Medicine and Public Health’s Wisconsin Partnership Program, through the NIH National Center for Advancing Translational Sciences.
FROM ARTHRITIS CARE & RESEARCH
Life after death, and the case of the disappearing digit
It’s alive!!!
Calling all “The Walking Dead” fans! Did you know that, after death, certain cells in the brain can stay active and even become colossal?
Researchers evaluated brain tissue to feign the gene expression during autopsy and death. By doing this, they found that these inflammatory cells, called glial cells, can increase gene expression and “grow and sprout long arm-like appendages for many hours after death.”
According to Dr. Jeffrey Loeb, the study’s senior author, the continued growth after death doesn’t come as a shock since these are the cells that do damage control after certain brain injuries, such as stroke.
Maybe those mindless zombies aren’t so mindless after all. We’re not sure if we should be more scared of a zombie that can think, or a zombie that can’t. We’re sensing a spin-off!
Beam me up, Doc!
In the realm of Star Trek, Dr. Leonard “Bones” McCoy isn’t the only physician who seems to find merit in the adventures of the starship Enterprise.
Pediatric cardiologist Victor Grech, it was reported, has been so influenced by the generational hit that the show made special guest appearances in his medical writing.
The alarm was sounded by a student at Oxford University who had suspicions about more than 100 articles published in Early Human Development. Of the articles eventually withdrawn by the journal’s publisher, Elsevier, 26 were on COVID-19 alone.
Just like a Romulan cloaking device, where the stories once stood Elsevier has left a “withdrawn” statement, making the articles vanish out of thin air.
Along with articles on COVID-19, Dr. Grech’s 48-article series with coauthors on how to write a scientific paper rightfully came into question. Elsevier’s statement on the incident says that the journal’s editorial work flow has been redesigned “to ensure that this will not happen again in the future.”
The number of retracted articles boldly puts Dr. Grech in a lane where few men have gone before.
Something’s wrong, but I can’t put my finger on it
Mixed martial arts is not a sport for the faint of heart. However, we doubt fans who were watching the Khetag Pliev/Devin Goodale fight on April 1 were prepared for the announcement that a search was commencing for a missing finger. Not broken, in case you think that was a misprint. Completely 100% removed from the rest of the hand.
One would think that pinpointing the exact moment when the finger, belonging to Mr. Pliev, was severed would be easy, but the video evidence is unclear, with the best guess being that a kick in the first round broke the finger and a grapple in the second severed it completely. Mr. Pliev was not helpful in clearing up the matter; not only did he fail to immediately notice the fact that his finger had broken or severed, he tried to keep the fight going after the second round when the referee noticed some blood where his left ring finger should have been. He thought he was winning. Unfortunately, the doctor on hand, who was clearly a complete drag, felt differently, ending the fight and awarding it to Mr. Goodale in a technical knockout.
Rest assured, there is a happy ending to this gruesome story. After a frantic search, the missing finger was found deep within Mr. Pliev’s glove and was successfully reattached in a Philadelphia emergency room.
The LOTME team commends Mr. Pliev’s commitment to his craft by wanting to continue the fight, but we respectfully disagree with his assertion that he was winning. We’re fairly confident that body part removal is an automatic loss (pun intended), unless you’re the Black Knight from “Monty Python and the Holy Grail.” Then it’s a draw.
Take two cookies and call me in the morning
The placebo effect is a well-known phenomenon. A pharmacologically inactive treatment can help people if they don’t know it’s pharmacologically inactive. But what if they did know? Would it still work?
That’s what researchers at Beth Israel Deaconess Medical Center in Boston wanted to find out. They divided a cohort of patients with irritable bowel syndrome into three groups. One group got pill bottles containing “open-label placebo,” so the subjects knew they were getting a placebo. The second received bottles labeled “double-blind placebo or peppermint oil.” The third got no pills but followed the rest of the study protocol.
Can you see where this is going? Two-thirds of the open-label placebo group had meaningful improvement of their symptoms, there was no difference in improvement between the two placebo groups, and both did significantly better than the no-pill group.
“If the presumption that deception is necessary for placebos to be effective is false, then many theories about the mechanisms that drive placebo effects may need modification,” investigator Ted J. Kaptchuk said in a written statement.
In other words, this changes everything. Who needs real drugs when anything that a doctor gives to a patient will help? Someone who has trouble swallowing pills can get a milkshake instead. Kid doesn’t like the taste of amoxicillin? Prescribe a slice of therapeutic pizza. Vaccine deniers can get a shot of vitamin C … or bourbon. And just imagine all the good that can be done in this crazy, mixed up world with a batch of chocolate chip cookies.
It’s alive!!!
Calling all “The Walking Dead” fans! Did you know that, after death, certain cells in the brain can stay active and even become colossal?
Researchers evaluated brain tissue to feign the gene expression during autopsy and death. By doing this, they found that these inflammatory cells, called glial cells, can increase gene expression and “grow and sprout long arm-like appendages for many hours after death.”
According to Dr. Jeffrey Loeb, the study’s senior author, the continued growth after death doesn’t come as a shock since these are the cells that do damage control after certain brain injuries, such as stroke.
Maybe those mindless zombies aren’t so mindless after all. We’re not sure if we should be more scared of a zombie that can think, or a zombie that can’t. We’re sensing a spin-off!
Beam me up, Doc!
In the realm of Star Trek, Dr. Leonard “Bones” McCoy isn’t the only physician who seems to find merit in the adventures of the starship Enterprise.
Pediatric cardiologist Victor Grech, it was reported, has been so influenced by the generational hit that the show made special guest appearances in his medical writing.
The alarm was sounded by a student at Oxford University who had suspicions about more than 100 articles published in Early Human Development. Of the articles eventually withdrawn by the journal’s publisher, Elsevier, 26 were on COVID-19 alone.
Just like a Romulan cloaking device, where the stories once stood Elsevier has left a “withdrawn” statement, making the articles vanish out of thin air.
Along with articles on COVID-19, Dr. Grech’s 48-article series with coauthors on how to write a scientific paper rightfully came into question. Elsevier’s statement on the incident says that the journal’s editorial work flow has been redesigned “to ensure that this will not happen again in the future.”
The number of retracted articles boldly puts Dr. Grech in a lane where few men have gone before.
Something’s wrong, but I can’t put my finger on it
Mixed martial arts is not a sport for the faint of heart. However, we doubt fans who were watching the Khetag Pliev/Devin Goodale fight on April 1 were prepared for the announcement that a search was commencing for a missing finger. Not broken, in case you think that was a misprint. Completely 100% removed from the rest of the hand.
One would think that pinpointing the exact moment when the finger, belonging to Mr. Pliev, was severed would be easy, but the video evidence is unclear, with the best guess being that a kick in the first round broke the finger and a grapple in the second severed it completely. Mr. Pliev was not helpful in clearing up the matter; not only did he fail to immediately notice the fact that his finger had broken or severed, he tried to keep the fight going after the second round when the referee noticed some blood where his left ring finger should have been. He thought he was winning. Unfortunately, the doctor on hand, who was clearly a complete drag, felt differently, ending the fight and awarding it to Mr. Goodale in a technical knockout.
Rest assured, there is a happy ending to this gruesome story. After a frantic search, the missing finger was found deep within Mr. Pliev’s glove and was successfully reattached in a Philadelphia emergency room.
The LOTME team commends Mr. Pliev’s commitment to his craft by wanting to continue the fight, but we respectfully disagree with his assertion that he was winning. We’re fairly confident that body part removal is an automatic loss (pun intended), unless you’re the Black Knight from “Monty Python and the Holy Grail.” Then it’s a draw.
Take two cookies and call me in the morning
The placebo effect is a well-known phenomenon. A pharmacologically inactive treatment can help people if they don’t know it’s pharmacologically inactive. But what if they did know? Would it still work?
That’s what researchers at Beth Israel Deaconess Medical Center in Boston wanted to find out. They divided a cohort of patients with irritable bowel syndrome into three groups. One group got pill bottles containing “open-label placebo,” so the subjects knew they were getting a placebo. The second received bottles labeled “double-blind placebo or peppermint oil.” The third got no pills but followed the rest of the study protocol.
Can you see where this is going? Two-thirds of the open-label placebo group had meaningful improvement of their symptoms, there was no difference in improvement between the two placebo groups, and both did significantly better than the no-pill group.
“If the presumption that deception is necessary for placebos to be effective is false, then many theories about the mechanisms that drive placebo effects may need modification,” investigator Ted J. Kaptchuk said in a written statement.
In other words, this changes everything. Who needs real drugs when anything that a doctor gives to a patient will help? Someone who has trouble swallowing pills can get a milkshake instead. Kid doesn’t like the taste of amoxicillin? Prescribe a slice of therapeutic pizza. Vaccine deniers can get a shot of vitamin C … or bourbon. And just imagine all the good that can be done in this crazy, mixed up world with a batch of chocolate chip cookies.
It’s alive!!!
Calling all “The Walking Dead” fans! Did you know that, after death, certain cells in the brain can stay active and even become colossal?
Researchers evaluated brain tissue to feign the gene expression during autopsy and death. By doing this, they found that these inflammatory cells, called glial cells, can increase gene expression and “grow and sprout long arm-like appendages for many hours after death.”
According to Dr. Jeffrey Loeb, the study’s senior author, the continued growth after death doesn’t come as a shock since these are the cells that do damage control after certain brain injuries, such as stroke.
Maybe those mindless zombies aren’t so mindless after all. We’re not sure if we should be more scared of a zombie that can think, or a zombie that can’t. We’re sensing a spin-off!
Beam me up, Doc!
In the realm of Star Trek, Dr. Leonard “Bones” McCoy isn’t the only physician who seems to find merit in the adventures of the starship Enterprise.
Pediatric cardiologist Victor Grech, it was reported, has been so influenced by the generational hit that the show made special guest appearances in his medical writing.
The alarm was sounded by a student at Oxford University who had suspicions about more than 100 articles published in Early Human Development. Of the articles eventually withdrawn by the journal’s publisher, Elsevier, 26 were on COVID-19 alone.
Just like a Romulan cloaking device, where the stories once stood Elsevier has left a “withdrawn” statement, making the articles vanish out of thin air.
Along with articles on COVID-19, Dr. Grech’s 48-article series with coauthors on how to write a scientific paper rightfully came into question. Elsevier’s statement on the incident says that the journal’s editorial work flow has been redesigned “to ensure that this will not happen again in the future.”
The number of retracted articles boldly puts Dr. Grech in a lane where few men have gone before.
Something’s wrong, but I can’t put my finger on it
Mixed martial arts is not a sport for the faint of heart. However, we doubt fans who were watching the Khetag Pliev/Devin Goodale fight on April 1 were prepared for the announcement that a search was commencing for a missing finger. Not broken, in case you think that was a misprint. Completely 100% removed from the rest of the hand.
One would think that pinpointing the exact moment when the finger, belonging to Mr. Pliev, was severed would be easy, but the video evidence is unclear, with the best guess being that a kick in the first round broke the finger and a grapple in the second severed it completely. Mr. Pliev was not helpful in clearing up the matter; not only did he fail to immediately notice the fact that his finger had broken or severed, he tried to keep the fight going after the second round when the referee noticed some blood where his left ring finger should have been. He thought he was winning. Unfortunately, the doctor on hand, who was clearly a complete drag, felt differently, ending the fight and awarding it to Mr. Goodale in a technical knockout.
Rest assured, there is a happy ending to this gruesome story. After a frantic search, the missing finger was found deep within Mr. Pliev’s glove and was successfully reattached in a Philadelphia emergency room.
The LOTME team commends Mr. Pliev’s commitment to his craft by wanting to continue the fight, but we respectfully disagree with his assertion that he was winning. We’re fairly confident that body part removal is an automatic loss (pun intended), unless you’re the Black Knight from “Monty Python and the Holy Grail.” Then it’s a draw.
Take two cookies and call me in the morning
The placebo effect is a well-known phenomenon. A pharmacologically inactive treatment can help people if they don’t know it’s pharmacologically inactive. But what if they did know? Would it still work?
That’s what researchers at Beth Israel Deaconess Medical Center in Boston wanted to find out. They divided a cohort of patients with irritable bowel syndrome into three groups. One group got pill bottles containing “open-label placebo,” so the subjects knew they were getting a placebo. The second received bottles labeled “double-blind placebo or peppermint oil.” The third got no pills but followed the rest of the study protocol.
Can you see where this is going? Two-thirds of the open-label placebo group had meaningful improvement of their symptoms, there was no difference in improvement between the two placebo groups, and both did significantly better than the no-pill group.
“If the presumption that deception is necessary for placebos to be effective is false, then many theories about the mechanisms that drive placebo effects may need modification,” investigator Ted J. Kaptchuk said in a written statement.
In other words, this changes everything. Who needs real drugs when anything that a doctor gives to a patient will help? Someone who has trouble swallowing pills can get a milkshake instead. Kid doesn’t like the taste of amoxicillin? Prescribe a slice of therapeutic pizza. Vaccine deniers can get a shot of vitamin C … or bourbon. And just imagine all the good that can be done in this crazy, mixed up world with a batch of chocolate chip cookies.
TAVR feasible, comparable with surgery in rheumatic heart disease
Patients with rheumatic heart disease (RHD) appear to have comparable outcomes, whether undergoing transcatheter or surgical aortic valve replacement (TAVR/SAVR), and when compared with TAVR in patients with nonrheumatic aortic stenosis, a new Medicare study finds.
An analysis of data from 1,159 Medicare beneficiaries with rheumatic aortic stenosis revealed that, over a median follow-up of 19 months, there was no difference in all-cause mortality with TAVR vs. SAVR (11.2 vs. 7.0 per 100 person-years; adjusted hazard ratio, 1.53; P = .2).
Mortality was also similar after a median follow-up of 17 months between TAVR in patients with rheumatic aortic stenosis and 88,554 additional beneficiaries with nonrheumatic aortic stenosis (15.2 vs. 17.7 deaths per 100 person-years; aHR, 0.87; P = .2).
“We need collaboration between industry and society leaders in developed countries to initiate a randomized, controlled trial to address the feasibility of TAVR in rheumatic heart disease in younger populations who aren’t surgical candidates or if there’s a lack of surgical capabilities in countries, but this is an encouraging first sign,” lead author Amgad Mentias, MD, MSc, Cleveland Clinic Foundation, said in an interview.
Although the prevalence of rheumatic heart disease (RHD) has fallen to less than 5% or so in the United States and Europe, it remains a significant problem in developing and low-income countries, with more than 1 million deaths per year, he noted. RHD patients typically present at younger ages, often with concomitant aortic regurgitation and mitral valve disease, but have less calcification than degenerative calcific aortic stenosis.
Commenting on the results, published in the Journal of the American College of Cardiology, David F. Williams, PhD, said in an interview that “it is only now becoming possible to entertain the use of TAVR in such patients, and this paper demonstrates the feasibility of doing so.
“Although the study is based on geriatric patients of an industrialized country, it opens the door to the massive unmet clinical needs in poorer regions as well as emerging economies,” said Dr. Williams, a professor at the Wake Forest Institute for Regenerative Medicine, Winston-Salem, N.C., and coauthor of an accompanying editorial.
The study included Medicare beneficiaries treated from October 2015 to December 2017 for rheumatic aortic stenosis (TAVR, n = 605; SAVR, n = 55) or nonrheumatic aortic stenosis (n = 88,554).
Among those with rheumatic disease, SAVR patients were younger than TAVR patients (73.4 vs. 79.4 years), had a lower prevalence of most comorbidities, and were less frail (median frailty score, 5.3 vs. 11.3).
SAVR was associated with significantly higher weighted risk for in-hospital acute kidney injury (22.3% vs. 11.9%), blood transfusion (19.8% vs. 7.6%), cardiogenic shock (5.7% vs. 1.5%), new-onset atrial fibrillation (21.1% vs. 2.2%), and had longer hospital stays (median, 8 vs. 3 days), whereas new permanent pacemaker implantations trended higher with TAVR (12.5% vs 7.2%).
The TAVR and SAVR groups had comparable rates of adjusted in-hospital mortality (2.4% vs. 3.5%), 30-day mortality (3.6% vs. 3.2%), 30-day stroke (2.4% vs. 2.8%), and 1-year mortality (13.1% vs. 8.9%).
Among the two TAVR cohorts, patients with rheumatic disease were younger than those with nonrheumatic aortic stenosis (79.4 vs. 81.2 years); had a higher prevalence of heart failure, ischemic stroke, atrial fibrillation, and lung disease; and were more frail (median score, 11.3 vs. 6.9).
Still, there was no difference in weighted risk of in-hospital mortality (2.2% vs. 2.6%), 30-day mortality (3.6% vs. 3.7%), 30-day stroke (2.0% vs. 3.3%), or 1-year mortality (16.0% vs. 17.1%) between TAVR patients with and without rheumatic stenosis.
“We didn’t have specific information on echo[cardiography], so we don’t know how that affected our results, but one of the encouraging points is that after a median follow-up of almost 2 years, none of the patients who had TAVR in the rheumatic valve and who survived required redo aortic valve replacement,” Dr. Mentias said. “It’s still short term but it shows that for the short to mid term, the valve is durable.”
Data were not available on paravalvular regurgitation, an Achilles heel for TAVR, but Dr. Mentias said rates of this complication have come down significantly in the past 2 years with modifications to newer-generation TAVR valves.
Dr. Williams and colleagues say one main limitation of the study also highlights the major shortcoming of contemporary TAVRs when treating patients with RHD: “namely, their inadequate suitability for AR [aortic regurgitation], the predominant rheumatic lesion of the aortic valve” in low- to middle-income countries.
They pointed out that patients needing an aortic valve where RHD is rampant are at least 30 years younger than the 79-year-old TAVR recipients in the study.
In a comment, Dr. Williams said there are several unanswered questions about the full impact TAVR could have in the treatment of young RHD patients in underprivileged regions. “These mainly concern the durability of the valves in individuals who could expect greater longevity than the typical heart valve patient in the USA, and the adaptation of transcatheter techniques to provide cost-effective treatment in regions that lack the usual sophisticated clinical infrastructure.”
Dr. Mentias received support from a National Research Service Award institutional grant to the Abboud Cardiovascular Research Center. Dr. Williams and coauthors are directors of Strait Access Technologies.
A version of this article first appeared on Medscape.com.
Patients with rheumatic heart disease (RHD) appear to have comparable outcomes, whether undergoing transcatheter or surgical aortic valve replacement (TAVR/SAVR), and when compared with TAVR in patients with nonrheumatic aortic stenosis, a new Medicare study finds.
An analysis of data from 1,159 Medicare beneficiaries with rheumatic aortic stenosis revealed that, over a median follow-up of 19 months, there was no difference in all-cause mortality with TAVR vs. SAVR (11.2 vs. 7.0 per 100 person-years; adjusted hazard ratio, 1.53; P = .2).
Mortality was also similar after a median follow-up of 17 months between TAVR in patients with rheumatic aortic stenosis and 88,554 additional beneficiaries with nonrheumatic aortic stenosis (15.2 vs. 17.7 deaths per 100 person-years; aHR, 0.87; P = .2).
“We need collaboration between industry and society leaders in developed countries to initiate a randomized, controlled trial to address the feasibility of TAVR in rheumatic heart disease in younger populations who aren’t surgical candidates or if there’s a lack of surgical capabilities in countries, but this is an encouraging first sign,” lead author Amgad Mentias, MD, MSc, Cleveland Clinic Foundation, said in an interview.
Although the prevalence of rheumatic heart disease (RHD) has fallen to less than 5% or so in the United States and Europe, it remains a significant problem in developing and low-income countries, with more than 1 million deaths per year, he noted. RHD patients typically present at younger ages, often with concomitant aortic regurgitation and mitral valve disease, but have less calcification than degenerative calcific aortic stenosis.
Commenting on the results, published in the Journal of the American College of Cardiology, David F. Williams, PhD, said in an interview that “it is only now becoming possible to entertain the use of TAVR in such patients, and this paper demonstrates the feasibility of doing so.
“Although the study is based on geriatric patients of an industrialized country, it opens the door to the massive unmet clinical needs in poorer regions as well as emerging economies,” said Dr. Williams, a professor at the Wake Forest Institute for Regenerative Medicine, Winston-Salem, N.C., and coauthor of an accompanying editorial.
The study included Medicare beneficiaries treated from October 2015 to December 2017 for rheumatic aortic stenosis (TAVR, n = 605; SAVR, n = 55) or nonrheumatic aortic stenosis (n = 88,554).
Among those with rheumatic disease, SAVR patients were younger than TAVR patients (73.4 vs. 79.4 years), had a lower prevalence of most comorbidities, and were less frail (median frailty score, 5.3 vs. 11.3).
SAVR was associated with significantly higher weighted risk for in-hospital acute kidney injury (22.3% vs. 11.9%), blood transfusion (19.8% vs. 7.6%), cardiogenic shock (5.7% vs. 1.5%), new-onset atrial fibrillation (21.1% vs. 2.2%), and had longer hospital stays (median, 8 vs. 3 days), whereas new permanent pacemaker implantations trended higher with TAVR (12.5% vs 7.2%).
The TAVR and SAVR groups had comparable rates of adjusted in-hospital mortality (2.4% vs. 3.5%), 30-day mortality (3.6% vs. 3.2%), 30-day stroke (2.4% vs. 2.8%), and 1-year mortality (13.1% vs. 8.9%).
Among the two TAVR cohorts, patients with rheumatic disease were younger than those with nonrheumatic aortic stenosis (79.4 vs. 81.2 years); had a higher prevalence of heart failure, ischemic stroke, atrial fibrillation, and lung disease; and were more frail (median score, 11.3 vs. 6.9).
Still, there was no difference in weighted risk of in-hospital mortality (2.2% vs. 2.6%), 30-day mortality (3.6% vs. 3.7%), 30-day stroke (2.0% vs. 3.3%), or 1-year mortality (16.0% vs. 17.1%) between TAVR patients with and without rheumatic stenosis.
“We didn’t have specific information on echo[cardiography], so we don’t know how that affected our results, but one of the encouraging points is that after a median follow-up of almost 2 years, none of the patients who had TAVR in the rheumatic valve and who survived required redo aortic valve replacement,” Dr. Mentias said. “It’s still short term but it shows that for the short to mid term, the valve is durable.”
Data were not available on paravalvular regurgitation, an Achilles heel for TAVR, but Dr. Mentias said rates of this complication have come down significantly in the past 2 years with modifications to newer-generation TAVR valves.
Dr. Williams and colleagues say one main limitation of the study also highlights the major shortcoming of contemporary TAVRs when treating patients with RHD: “namely, their inadequate suitability for AR [aortic regurgitation], the predominant rheumatic lesion of the aortic valve” in low- to middle-income countries.
They pointed out that patients needing an aortic valve where RHD is rampant are at least 30 years younger than the 79-year-old TAVR recipients in the study.
In a comment, Dr. Williams said there are several unanswered questions about the full impact TAVR could have in the treatment of young RHD patients in underprivileged regions. “These mainly concern the durability of the valves in individuals who could expect greater longevity than the typical heart valve patient in the USA, and the adaptation of transcatheter techniques to provide cost-effective treatment in regions that lack the usual sophisticated clinical infrastructure.”
Dr. Mentias received support from a National Research Service Award institutional grant to the Abboud Cardiovascular Research Center. Dr. Williams and coauthors are directors of Strait Access Technologies.
A version of this article first appeared on Medscape.com.
Patients with rheumatic heart disease (RHD) appear to have comparable outcomes, whether undergoing transcatheter or surgical aortic valve replacement (TAVR/SAVR), and when compared with TAVR in patients with nonrheumatic aortic stenosis, a new Medicare study finds.
An analysis of data from 1,159 Medicare beneficiaries with rheumatic aortic stenosis revealed that, over a median follow-up of 19 months, there was no difference in all-cause mortality with TAVR vs. SAVR (11.2 vs. 7.0 per 100 person-years; adjusted hazard ratio, 1.53; P = .2).
Mortality was also similar after a median follow-up of 17 months between TAVR in patients with rheumatic aortic stenosis and 88,554 additional beneficiaries with nonrheumatic aortic stenosis (15.2 vs. 17.7 deaths per 100 person-years; aHR, 0.87; P = .2).
“We need collaboration between industry and society leaders in developed countries to initiate a randomized, controlled trial to address the feasibility of TAVR in rheumatic heart disease in younger populations who aren’t surgical candidates or if there’s a lack of surgical capabilities in countries, but this is an encouraging first sign,” lead author Amgad Mentias, MD, MSc, Cleveland Clinic Foundation, said in an interview.
Although the prevalence of rheumatic heart disease (RHD) has fallen to less than 5% or so in the United States and Europe, it remains a significant problem in developing and low-income countries, with more than 1 million deaths per year, he noted. RHD patients typically present at younger ages, often with concomitant aortic regurgitation and mitral valve disease, but have less calcification than degenerative calcific aortic stenosis.
Commenting on the results, published in the Journal of the American College of Cardiology, David F. Williams, PhD, said in an interview that “it is only now becoming possible to entertain the use of TAVR in such patients, and this paper demonstrates the feasibility of doing so.
“Although the study is based on geriatric patients of an industrialized country, it opens the door to the massive unmet clinical needs in poorer regions as well as emerging economies,” said Dr. Williams, a professor at the Wake Forest Institute for Regenerative Medicine, Winston-Salem, N.C., and coauthor of an accompanying editorial.
The study included Medicare beneficiaries treated from October 2015 to December 2017 for rheumatic aortic stenosis (TAVR, n = 605; SAVR, n = 55) or nonrheumatic aortic stenosis (n = 88,554).
Among those with rheumatic disease, SAVR patients were younger than TAVR patients (73.4 vs. 79.4 years), had a lower prevalence of most comorbidities, and were less frail (median frailty score, 5.3 vs. 11.3).
SAVR was associated with significantly higher weighted risk for in-hospital acute kidney injury (22.3% vs. 11.9%), blood transfusion (19.8% vs. 7.6%), cardiogenic shock (5.7% vs. 1.5%), new-onset atrial fibrillation (21.1% vs. 2.2%), and had longer hospital stays (median, 8 vs. 3 days), whereas new permanent pacemaker implantations trended higher with TAVR (12.5% vs 7.2%).
The TAVR and SAVR groups had comparable rates of adjusted in-hospital mortality (2.4% vs. 3.5%), 30-day mortality (3.6% vs. 3.2%), 30-day stroke (2.4% vs. 2.8%), and 1-year mortality (13.1% vs. 8.9%).
Among the two TAVR cohorts, patients with rheumatic disease were younger than those with nonrheumatic aortic stenosis (79.4 vs. 81.2 years); had a higher prevalence of heart failure, ischemic stroke, atrial fibrillation, and lung disease; and were more frail (median score, 11.3 vs. 6.9).
Still, there was no difference in weighted risk of in-hospital mortality (2.2% vs. 2.6%), 30-day mortality (3.6% vs. 3.7%), 30-day stroke (2.0% vs. 3.3%), or 1-year mortality (16.0% vs. 17.1%) between TAVR patients with and without rheumatic stenosis.
“We didn’t have specific information on echo[cardiography], so we don’t know how that affected our results, but one of the encouraging points is that after a median follow-up of almost 2 years, none of the patients who had TAVR in the rheumatic valve and who survived required redo aortic valve replacement,” Dr. Mentias said. “It’s still short term but it shows that for the short to mid term, the valve is durable.”
Data were not available on paravalvular regurgitation, an Achilles heel for TAVR, but Dr. Mentias said rates of this complication have come down significantly in the past 2 years with modifications to newer-generation TAVR valves.
Dr. Williams and colleagues say one main limitation of the study also highlights the major shortcoming of contemporary TAVRs when treating patients with RHD: “namely, their inadequate suitability for AR [aortic regurgitation], the predominant rheumatic lesion of the aortic valve” in low- to middle-income countries.
They pointed out that patients needing an aortic valve where RHD is rampant are at least 30 years younger than the 79-year-old TAVR recipients in the study.
In a comment, Dr. Williams said there are several unanswered questions about the full impact TAVR could have in the treatment of young RHD patients in underprivileged regions. “These mainly concern the durability of the valves in individuals who could expect greater longevity than the typical heart valve patient in the USA, and the adaptation of transcatheter techniques to provide cost-effective treatment in regions that lack the usual sophisticated clinical infrastructure.”
Dr. Mentias received support from a National Research Service Award institutional grant to the Abboud Cardiovascular Research Center. Dr. Williams and coauthors are directors of Strait Access Technologies.
A version of this article first appeared on Medscape.com.
‘Beyond a reasonable doubt’: COVID-19 brain health fallout is real, severe
COVID-19 survivors face a sharply elevated risk of developing psychiatric or neurologic disorders in the 6 months after they contract the virus – a danger that mounts with symptom severity, new research shows.
In what is purported to be the largest study of its kind to date, results showed that among 236,379 COVID-19 patients, one-third were diagnosed with at least 1 of 14 psychiatric or neurologic disorders within a 6-month span.
The rate of illnesses, which ranged from depression to stroke, rose sharply among those with COVID-19 symptoms acute enough to require hospitalization.
“If we look at patients who were hospitalized, that rate increased to 39%, and then increased to about just under 1 in 2 patients who needed ICU admission at the time of the COVID-19 diagnosis,” Maxime Taquet, PhD, University of Oxford (England) department of psychiatry, said at a media briefing.
Incidence jumps to almost two-thirds in patients with encephalopathy at the time of COVID-19 diagnosis, he added.
The study, which examined the brain health of 236,379 survivors of COVID-19 via a U.S. database of 81 million electronic health records, was published online April 6 in The Lancet Psychiatry.
High rate of neurologic, psychiatric disorders
The research team looked at the first-time diagnosis or recurrence of 14 neurologic and psychiatric outcomes in patients with confirmed SARS-CoV-2 infections. They also compared the brain health of this cohort with a control group of those with influenza or with non–COVID-19 respiratory infections over the same period.
All study participants were older than 10 years, diagnosed with COVID-19 on or after Jan. 20, 2020, and still alive as of Dec. 13, 2020.
The psychiatric and neurologic conditions examined included intracranial hemorrhage; ischemic stroke; parkinsonism; Guillain-Barré syndrome; nerve, nerve root and plexus disorders; myoneural junction and muscle disease; encephalitis; dementia; psychotic, mood, and anxiety disorders; substance use disorder; and insomnia.
The investigators used hospitalization, intensive care admissions, and encephalopathy as an indication of the severity of COVID-19 symptoms.
The study benchmarked the primary cohort with four populations of patients diagnosed in the same period with nonrespiratory illnesses, including skin infection, urolithiasis, bone fractures, and pulmonary embolisms.
Results showed that substantially more COVID-19 patients were diagnosed with a neurologic or psychiatric disorder compared with those with other respiratory illnesses.
“On average, in terms of the relative numbers, there was a 44% increased risk of having a neurological or psychiatric diagnosis after COVID-19 than after the flu and a 16% increased risk compared to other respiratory tract infections,” Dr. Taquet told reporters.
Health services should be prepared for an increase in psychiatric and neurologic issues in the months to come, he said, adding that further investigations are needed into why, and how, the coronavirus affects brain health.
Largest study to date
Although previous research suggests a link between the two, this is the largest study of its kind, examines a wider range of neurologic outcomes, and spans the longest time frame to date, said study coinvestigator Paul Harrison, BM BCh, associate head of the University of Oxford department of psychiatry.
There was a lower incidence of mood and anxiety disorders vs. neurologic disorders in patients with severe COVID-19 symptoms, a finding that Dr. Harrison said may indicate pandemic-related psychological stress is driving these disorders vs. biological factors.
“This paper follows up on an earlier study we did where we found much the same association, and our view is that a lot of the mental health consequences of COVID are … to do with the stress of knowing that one has had COVID and all the implications that go with that, rather than its being a direct effect, for example, of the virus on the brain, or of the immune response to the virus on the brain,” he added.
In contrast, neurologic diagnoses were more likely to be “mediated by some direct consequence of the COVID infection,” he added.
Psychosis and dementia, for instance, were less frequent in the overall COVID-19 population but became much more frequent among those with severe symptoms. The research team said these findings, along with those related to the incidence of ischemic stroke, were “concerning.”
“We found that 1 in 50 patients with COVID-19 go on to have an ischemic stroke in the 6 months after the COVID-19 illness,” Dr. Taquet told reporters. “And that rate increased to 1 in 11 patients if we look at patients with encephalopathy at the time of the COVID-19 diagnosis.”
Rates of brain hemorrhages also rose sharply among those with acute symptoms. Just over 1 in 200 total COVID-19 patients were diagnosed with this neurological condition, but that jumped to 1 in 25 of those who experienced encephalopathy at the time of their COVID-19 diagnosis.
Need for replication
Study coauthor Masud Husain, PhD, of University of Oxford’s cognitive neurology department, told reporters that while there is evidence from other neurologic studies that the virus can access the brain, there has been little sign the neurons themselves are affected.
“There isn’t much evidence that the virus itself attacks neurons in the brain, but it can cause inflammation, and it can activate inflammatory cells in the brain,” he said.
“And those effects are probably very important in some of the biological effects on the brain. In addition, of course, we know that the virus can change clotting and the likelihood of thrombosis in the blood, and those effects can also impact upon the brain,” he added.
Dr. Harrison said it would be helpful to replicate the results garnered from the U.S. database in other populations.
“It goes without saying that replication of these results with other electronic health records and in other countries is a priority,” he said, adding that investigations are essential into how and why the virus affects brain health.
Dr. Harrison cited a U.K. Research and Innovation–funded study called COVID CNS that will follow patients with neurologic and/or psychiatric issues during acute COVID-19 in hopes of exploring possible causes.
Beyond a reasonable doubt
Commenting on the findings, Sir Simon Wessely, MD, Regius chair of psychiatry, King’s College London, said in a release: “This is a very important paper. It confirms beyond any reasonable doubt that COVID-19 affects both brain and mind in equal measure.”
Some of these effects, including stroke and anxiety disorders, were already known, but others such as dementia and psychosis were less well known, he added.
“What is very new is the comparisons with all respiratory viruses or influenza, which suggests that these increases are specifically related to COVID-19, and not a general impact of viral infection,” Dr. Wessely said. “In general, the worse the illness, the greater the neurological or psychiatric outcomes, which is perhaps not surprising.
“The worst outcomes were in those with encephalopathy – inflammation of the brain – again, not surprising. The association with dementia was, however, small and might reflect diagnostic issues, whilst so far there doesn’t seem early evidence of a link with parkinsonism, which was a major factor after the great Spanish Flu pandemic, although the authors caution that it is too early to rule this out.”
A version of this article first appeared on Medscape.com.
COVID-19 survivors face a sharply elevated risk of developing psychiatric or neurologic disorders in the 6 months after they contract the virus – a danger that mounts with symptom severity, new research shows.
In what is purported to be the largest study of its kind to date, results showed that among 236,379 COVID-19 patients, one-third were diagnosed with at least 1 of 14 psychiatric or neurologic disorders within a 6-month span.
The rate of illnesses, which ranged from depression to stroke, rose sharply among those with COVID-19 symptoms acute enough to require hospitalization.
“If we look at patients who were hospitalized, that rate increased to 39%, and then increased to about just under 1 in 2 patients who needed ICU admission at the time of the COVID-19 diagnosis,” Maxime Taquet, PhD, University of Oxford (England) department of psychiatry, said at a media briefing.
Incidence jumps to almost two-thirds in patients with encephalopathy at the time of COVID-19 diagnosis, he added.
The study, which examined the brain health of 236,379 survivors of COVID-19 via a U.S. database of 81 million electronic health records, was published online April 6 in The Lancet Psychiatry.
High rate of neurologic, psychiatric disorders
The research team looked at the first-time diagnosis or recurrence of 14 neurologic and psychiatric outcomes in patients with confirmed SARS-CoV-2 infections. They also compared the brain health of this cohort with a control group of those with influenza or with non–COVID-19 respiratory infections over the same period.
All study participants were older than 10 years, diagnosed with COVID-19 on or after Jan. 20, 2020, and still alive as of Dec. 13, 2020.
The psychiatric and neurologic conditions examined included intracranial hemorrhage; ischemic stroke; parkinsonism; Guillain-Barré syndrome; nerve, nerve root and plexus disorders; myoneural junction and muscle disease; encephalitis; dementia; psychotic, mood, and anxiety disorders; substance use disorder; and insomnia.
The investigators used hospitalization, intensive care admissions, and encephalopathy as an indication of the severity of COVID-19 symptoms.
The study benchmarked the primary cohort with four populations of patients diagnosed in the same period with nonrespiratory illnesses, including skin infection, urolithiasis, bone fractures, and pulmonary embolisms.
Results showed that substantially more COVID-19 patients were diagnosed with a neurologic or psychiatric disorder compared with those with other respiratory illnesses.
“On average, in terms of the relative numbers, there was a 44% increased risk of having a neurological or psychiatric diagnosis after COVID-19 than after the flu and a 16% increased risk compared to other respiratory tract infections,” Dr. Taquet told reporters.
Health services should be prepared for an increase in psychiatric and neurologic issues in the months to come, he said, adding that further investigations are needed into why, and how, the coronavirus affects brain health.
Largest study to date
Although previous research suggests a link between the two, this is the largest study of its kind, examines a wider range of neurologic outcomes, and spans the longest time frame to date, said study coinvestigator Paul Harrison, BM BCh, associate head of the University of Oxford department of psychiatry.
There was a lower incidence of mood and anxiety disorders vs. neurologic disorders in patients with severe COVID-19 symptoms, a finding that Dr. Harrison said may indicate pandemic-related psychological stress is driving these disorders vs. biological factors.
“This paper follows up on an earlier study we did where we found much the same association, and our view is that a lot of the mental health consequences of COVID are … to do with the stress of knowing that one has had COVID and all the implications that go with that, rather than its being a direct effect, for example, of the virus on the brain, or of the immune response to the virus on the brain,” he added.
In contrast, neurologic diagnoses were more likely to be “mediated by some direct consequence of the COVID infection,” he added.
Psychosis and dementia, for instance, were less frequent in the overall COVID-19 population but became much more frequent among those with severe symptoms. The research team said these findings, along with those related to the incidence of ischemic stroke, were “concerning.”
“We found that 1 in 50 patients with COVID-19 go on to have an ischemic stroke in the 6 months after the COVID-19 illness,” Dr. Taquet told reporters. “And that rate increased to 1 in 11 patients if we look at patients with encephalopathy at the time of the COVID-19 diagnosis.”
Rates of brain hemorrhages also rose sharply among those with acute symptoms. Just over 1 in 200 total COVID-19 patients were diagnosed with this neurological condition, but that jumped to 1 in 25 of those who experienced encephalopathy at the time of their COVID-19 diagnosis.
Need for replication
Study coauthor Masud Husain, PhD, of University of Oxford’s cognitive neurology department, told reporters that while there is evidence from other neurologic studies that the virus can access the brain, there has been little sign the neurons themselves are affected.
“There isn’t much evidence that the virus itself attacks neurons in the brain, but it can cause inflammation, and it can activate inflammatory cells in the brain,” he said.
“And those effects are probably very important in some of the biological effects on the brain. In addition, of course, we know that the virus can change clotting and the likelihood of thrombosis in the blood, and those effects can also impact upon the brain,” he added.
Dr. Harrison said it would be helpful to replicate the results garnered from the U.S. database in other populations.
“It goes without saying that replication of these results with other electronic health records and in other countries is a priority,” he said, adding that investigations are essential into how and why the virus affects brain health.
Dr. Harrison cited a U.K. Research and Innovation–funded study called COVID CNS that will follow patients with neurologic and/or psychiatric issues during acute COVID-19 in hopes of exploring possible causes.
Beyond a reasonable doubt
Commenting on the findings, Sir Simon Wessely, MD, Regius chair of psychiatry, King’s College London, said in a release: “This is a very important paper. It confirms beyond any reasonable doubt that COVID-19 affects both brain and mind in equal measure.”
Some of these effects, including stroke and anxiety disorders, were already known, but others such as dementia and psychosis were less well known, he added.
“What is very new is the comparisons with all respiratory viruses or influenza, which suggests that these increases are specifically related to COVID-19, and not a general impact of viral infection,” Dr. Wessely said. “In general, the worse the illness, the greater the neurological or psychiatric outcomes, which is perhaps not surprising.
“The worst outcomes were in those with encephalopathy – inflammation of the brain – again, not surprising. The association with dementia was, however, small and might reflect diagnostic issues, whilst so far there doesn’t seem early evidence of a link with parkinsonism, which was a major factor after the great Spanish Flu pandemic, although the authors caution that it is too early to rule this out.”
A version of this article first appeared on Medscape.com.
COVID-19 survivors face a sharply elevated risk of developing psychiatric or neurologic disorders in the 6 months after they contract the virus – a danger that mounts with symptom severity, new research shows.
In what is purported to be the largest study of its kind to date, results showed that among 236,379 COVID-19 patients, one-third were diagnosed with at least 1 of 14 psychiatric or neurologic disorders within a 6-month span.
The rate of illnesses, which ranged from depression to stroke, rose sharply among those with COVID-19 symptoms acute enough to require hospitalization.
“If we look at patients who were hospitalized, that rate increased to 39%, and then increased to about just under 1 in 2 patients who needed ICU admission at the time of the COVID-19 diagnosis,” Maxime Taquet, PhD, University of Oxford (England) department of psychiatry, said at a media briefing.
Incidence jumps to almost two-thirds in patients with encephalopathy at the time of COVID-19 diagnosis, he added.
The study, which examined the brain health of 236,379 survivors of COVID-19 via a U.S. database of 81 million electronic health records, was published online April 6 in The Lancet Psychiatry.
High rate of neurologic, psychiatric disorders
The research team looked at the first-time diagnosis or recurrence of 14 neurologic and psychiatric outcomes in patients with confirmed SARS-CoV-2 infections. They also compared the brain health of this cohort with a control group of those with influenza or with non–COVID-19 respiratory infections over the same period.
All study participants were older than 10 years, diagnosed with COVID-19 on or after Jan. 20, 2020, and still alive as of Dec. 13, 2020.
The psychiatric and neurologic conditions examined included intracranial hemorrhage; ischemic stroke; parkinsonism; Guillain-Barré syndrome; nerve, nerve root and plexus disorders; myoneural junction and muscle disease; encephalitis; dementia; psychotic, mood, and anxiety disorders; substance use disorder; and insomnia.
The investigators used hospitalization, intensive care admissions, and encephalopathy as an indication of the severity of COVID-19 symptoms.
The study benchmarked the primary cohort with four populations of patients diagnosed in the same period with nonrespiratory illnesses, including skin infection, urolithiasis, bone fractures, and pulmonary embolisms.
Results showed that substantially more COVID-19 patients were diagnosed with a neurologic or psychiatric disorder compared with those with other respiratory illnesses.
“On average, in terms of the relative numbers, there was a 44% increased risk of having a neurological or psychiatric diagnosis after COVID-19 than after the flu and a 16% increased risk compared to other respiratory tract infections,” Dr. Taquet told reporters.
Health services should be prepared for an increase in psychiatric and neurologic issues in the months to come, he said, adding that further investigations are needed into why, and how, the coronavirus affects brain health.
Largest study to date
Although previous research suggests a link between the two, this is the largest study of its kind, examines a wider range of neurologic outcomes, and spans the longest time frame to date, said study coinvestigator Paul Harrison, BM BCh, associate head of the University of Oxford department of psychiatry.
There was a lower incidence of mood and anxiety disorders vs. neurologic disorders in patients with severe COVID-19 symptoms, a finding that Dr. Harrison said may indicate pandemic-related psychological stress is driving these disorders vs. biological factors.
“This paper follows up on an earlier study we did where we found much the same association, and our view is that a lot of the mental health consequences of COVID are … to do with the stress of knowing that one has had COVID and all the implications that go with that, rather than its being a direct effect, for example, of the virus on the brain, or of the immune response to the virus on the brain,” he added.
In contrast, neurologic diagnoses were more likely to be “mediated by some direct consequence of the COVID infection,” he added.
Psychosis and dementia, for instance, were less frequent in the overall COVID-19 population but became much more frequent among those with severe symptoms. The research team said these findings, along with those related to the incidence of ischemic stroke, were “concerning.”
“We found that 1 in 50 patients with COVID-19 go on to have an ischemic stroke in the 6 months after the COVID-19 illness,” Dr. Taquet told reporters. “And that rate increased to 1 in 11 patients if we look at patients with encephalopathy at the time of the COVID-19 diagnosis.”
Rates of brain hemorrhages also rose sharply among those with acute symptoms. Just over 1 in 200 total COVID-19 patients were diagnosed with this neurological condition, but that jumped to 1 in 25 of those who experienced encephalopathy at the time of their COVID-19 diagnosis.
Need for replication
Study coauthor Masud Husain, PhD, of University of Oxford’s cognitive neurology department, told reporters that while there is evidence from other neurologic studies that the virus can access the brain, there has been little sign the neurons themselves are affected.
“There isn’t much evidence that the virus itself attacks neurons in the brain, but it can cause inflammation, and it can activate inflammatory cells in the brain,” he said.
“And those effects are probably very important in some of the biological effects on the brain. In addition, of course, we know that the virus can change clotting and the likelihood of thrombosis in the blood, and those effects can also impact upon the brain,” he added.
Dr. Harrison said it would be helpful to replicate the results garnered from the U.S. database in other populations.
“It goes without saying that replication of these results with other electronic health records and in other countries is a priority,” he said, adding that investigations are essential into how and why the virus affects brain health.
Dr. Harrison cited a U.K. Research and Innovation–funded study called COVID CNS that will follow patients with neurologic and/or psychiatric issues during acute COVID-19 in hopes of exploring possible causes.
Beyond a reasonable doubt
Commenting on the findings, Sir Simon Wessely, MD, Regius chair of psychiatry, King’s College London, said in a release: “This is a very important paper. It confirms beyond any reasonable doubt that COVID-19 affects both brain and mind in equal measure.”
Some of these effects, including stroke and anxiety disorders, were already known, but others such as dementia and psychosis were less well known, he added.
“What is very new is the comparisons with all respiratory viruses or influenza, which suggests that these increases are specifically related to COVID-19, and not a general impact of viral infection,” Dr. Wessely said. “In general, the worse the illness, the greater the neurological or psychiatric outcomes, which is perhaps not surprising.
“The worst outcomes were in those with encephalopathy – inflammation of the brain – again, not surprising. The association with dementia was, however, small and might reflect diagnostic issues, whilst so far there doesn’t seem early evidence of a link with parkinsonism, which was a major factor after the great Spanish Flu pandemic, although the authors caution that it is too early to rule this out.”
A version of this article first appeared on Medscape.com.
About one in five clinicians considers quitting because of pandemic
a new survey of more than 5,000 clinicians at an academic medical center illustrates.
About one in five people reported considering leaving the workforce because of the challenges of working during the COVID-19 pandemic. In addition, 30% reported they are considering cutting back work hours.
“There are a substantial number of employees and trainees who are experiencing major stress and work disruptions because of the pandemic,” lead author Rebecca K. Delaney, PhD, said in an interview. “It is particularly alarming that people who have spent 5 or more years in training for their specialty are struggling with their work, so much so that they have even considered leaving the workforce or reducing their hours.”
“Being a caregiver adds another layer of difficulty for faculty, staff, and trainees who are trying to manage work and child care,” added Dr. Delaney, a researcher in the department of population health sciences, University of Utah, Salt Lake City.
The study was published online April 2 in JAMA Network Open.
“This looks like an excellent survey,” Carol A Bernstein, MD, said in an interview when asked to comment. “I do not think it provides particularly new information as these challenges in the workplace, especially for women during COVID, have been well documented in the media and the medical literature to date.”
“That said, to the extent that data helps drive solutions, I would hope that information such as this would be considered as strong further evidence that health care systems must pay close attention to the wellbeing of the workforce,” added Dr. Bernstein, professor and vice chair of faculty development and well-being, departments of psychiatry and behavioral sciences and obstetrics and gynecology and women’s health, Montefiore Medical Center/Albert Einstein College of Medicine, New York.
When the pandemic hits home
A total of 42% of the American workforce rapidly transitioned to working from home at the onset of the COVID-19 pandemic. At the same time, many employees had to provide child care and assistance with schoolwork. This placed a burden on many individuals at academic medical centers, and women in particular.
“Women comprise 74.9% of hospital employees, many of whom are essential clinical workers,” the researchers noted. “The extent of the needs and difficulties for these workers during the pandemic remain largely unknown.”
To learn more, Dr. Delaney, senior author Angie Fagerlin, PhD, and their colleagues emailed a Qualtrics survey to 27,700 faculty, staff, and trainees at University of Utah Health. The survey was conducted Aug. 5-20, 2020 as part of a quality improvement initiative. All responses were anonymous.
Survey questions included if, because of the pandemic, people had considered leaving the workforce, considered reducing their hours, or experienced reduced productivity. The researchers also asked about career impacts and potential solutions in terms of “work culture adaptations.”
Respondents with children aged under 18 years also were asked about child care options. Dr. Delaney and colleagues also inquired about race and ethnicity because they hypothesized that employees from underrepresented groups would likely experience the pandemic differently.
The mean age of the 5,951 (21%) faculty, staff, and trainees who completed the survey was 40 years. A majority of respondents were women, reflecting the higher proportion of women within the health system.
A majority (86%) identified as White or European American. About two-thirds of respondents (66%) were staff, 16% were faculty, and 13% were trainees.
COVID-19 career concerns
Overall, 1,061 respondents (21%) “moderately or very seriously” considered leaving the workforce and 1,505 (30%) considered reducing hours. Respondents who were younger, married, a member of an underrepresented racial/ethnic group, and worked in a clinical setting were more likely to consider leaving the workforce.
The survey showed 27% felt their productivity increased whereas 39% believed their productivity decreased.
Of the 2,412 survey participants with children aged 18 years or younger, 66% reported that they did not have child care fully available.
“Failure to address and provide for child care has long been one of the many significant deficits in U.S. health care systems,” said Dr. Bernstein, lead author of a March 2021 report evaluating staff emotional support at Montefiore Medical Center during the pandemic in The Joint Commission Journal on Quality and Patient Safety.
Furthermore, 47% were “moderately or very seriously worried” about COVID-19 impacting their career development.
Women trainees were significantly more likely than male counterparts to consider leaving the workforce and reducing their work hours. Women in a faculty or trainee role were also more likely to worry about COVID-19’s impact on their career, compared with men, and compared with women in staff positions.
“It was disheartening to have our data support the gender and racial/ethnic disparity that has been highlighted in the media during the pandemic,” Dr. Delaney said. “Women and in some cases racial/ethnic groups that are underrepresented in medicine were most likely to consider leaving the workforce, reducing hours, and were worried about their career development.
“It is critical that we strategically address these important disparities,” she said.
Women also are disproportionately affected by burnout, particularly during the pandemic, according to an analysis of Medscape’s Physician Burnout and Suicide Report.
Furthermore, the COVID-19 pandemic has shifted the medical specialties now considered highest risk for burnout: critical care physicians ranked first in the report, followed by rheumatologists and infectious disease specialists.
Potential solutions
“Given the disproportionate impact COVID-19 has on employees of health systems, institutions must find ways to support their employees, both in terms of workplace cultural adaptations and assistance with familial responsibilities,” the researchers noted.
Telecommuting policies, scheduling flexibility, and expanding employee support programs are potential solutions. Institutional policies also could address the educational and direct care needs of employee children.
Limitations of the study include its generalizability beyond employees of University of Utah Health. Also, respondents included a lower proportion of racial and ethnic groups, compared with national figures, “although this is mostly accounted for by the overall low population of such groups in the state of Utah,” the researchers added.
“Our results suggest that respondents were struggling during the COVID-19 pandemic,” the researchers noted. “As a result, even after investing substantial amounts of time in years of training, many were considering leaving the workforce because of stress and caregiving responsibilities related to the pandemic.”
The Jon M. Huntsman Presidential Endowed Chair supported the work with a financial award to Dr. Fagerlin. Dr. Delaney and Dr. Bernstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new survey of more than 5,000 clinicians at an academic medical center illustrates.
About one in five people reported considering leaving the workforce because of the challenges of working during the COVID-19 pandemic. In addition, 30% reported they are considering cutting back work hours.
“There are a substantial number of employees and trainees who are experiencing major stress and work disruptions because of the pandemic,” lead author Rebecca K. Delaney, PhD, said in an interview. “It is particularly alarming that people who have spent 5 or more years in training for their specialty are struggling with their work, so much so that they have even considered leaving the workforce or reducing their hours.”
“Being a caregiver adds another layer of difficulty for faculty, staff, and trainees who are trying to manage work and child care,” added Dr. Delaney, a researcher in the department of population health sciences, University of Utah, Salt Lake City.
The study was published online April 2 in JAMA Network Open.
“This looks like an excellent survey,” Carol A Bernstein, MD, said in an interview when asked to comment. “I do not think it provides particularly new information as these challenges in the workplace, especially for women during COVID, have been well documented in the media and the medical literature to date.”
“That said, to the extent that data helps drive solutions, I would hope that information such as this would be considered as strong further evidence that health care systems must pay close attention to the wellbeing of the workforce,” added Dr. Bernstein, professor and vice chair of faculty development and well-being, departments of psychiatry and behavioral sciences and obstetrics and gynecology and women’s health, Montefiore Medical Center/Albert Einstein College of Medicine, New York.
When the pandemic hits home
A total of 42% of the American workforce rapidly transitioned to working from home at the onset of the COVID-19 pandemic. At the same time, many employees had to provide child care and assistance with schoolwork. This placed a burden on many individuals at academic medical centers, and women in particular.
“Women comprise 74.9% of hospital employees, many of whom are essential clinical workers,” the researchers noted. “The extent of the needs and difficulties for these workers during the pandemic remain largely unknown.”
To learn more, Dr. Delaney, senior author Angie Fagerlin, PhD, and their colleagues emailed a Qualtrics survey to 27,700 faculty, staff, and trainees at University of Utah Health. The survey was conducted Aug. 5-20, 2020 as part of a quality improvement initiative. All responses were anonymous.
Survey questions included if, because of the pandemic, people had considered leaving the workforce, considered reducing their hours, or experienced reduced productivity. The researchers also asked about career impacts and potential solutions in terms of “work culture adaptations.”
Respondents with children aged under 18 years also were asked about child care options. Dr. Delaney and colleagues also inquired about race and ethnicity because they hypothesized that employees from underrepresented groups would likely experience the pandemic differently.
The mean age of the 5,951 (21%) faculty, staff, and trainees who completed the survey was 40 years. A majority of respondents were women, reflecting the higher proportion of women within the health system.
A majority (86%) identified as White or European American. About two-thirds of respondents (66%) were staff, 16% were faculty, and 13% were trainees.
COVID-19 career concerns
Overall, 1,061 respondents (21%) “moderately or very seriously” considered leaving the workforce and 1,505 (30%) considered reducing hours. Respondents who were younger, married, a member of an underrepresented racial/ethnic group, and worked in a clinical setting were more likely to consider leaving the workforce.
The survey showed 27% felt their productivity increased whereas 39% believed their productivity decreased.
Of the 2,412 survey participants with children aged 18 years or younger, 66% reported that they did not have child care fully available.
“Failure to address and provide for child care has long been one of the many significant deficits in U.S. health care systems,” said Dr. Bernstein, lead author of a March 2021 report evaluating staff emotional support at Montefiore Medical Center during the pandemic in The Joint Commission Journal on Quality and Patient Safety.
Furthermore, 47% were “moderately or very seriously worried” about COVID-19 impacting their career development.
Women trainees were significantly more likely than male counterparts to consider leaving the workforce and reducing their work hours. Women in a faculty or trainee role were also more likely to worry about COVID-19’s impact on their career, compared with men, and compared with women in staff positions.
“It was disheartening to have our data support the gender and racial/ethnic disparity that has been highlighted in the media during the pandemic,” Dr. Delaney said. “Women and in some cases racial/ethnic groups that are underrepresented in medicine were most likely to consider leaving the workforce, reducing hours, and were worried about their career development.
“It is critical that we strategically address these important disparities,” she said.
Women also are disproportionately affected by burnout, particularly during the pandemic, according to an analysis of Medscape’s Physician Burnout and Suicide Report.
Furthermore, the COVID-19 pandemic has shifted the medical specialties now considered highest risk for burnout: critical care physicians ranked first in the report, followed by rheumatologists and infectious disease specialists.
Potential solutions
“Given the disproportionate impact COVID-19 has on employees of health systems, institutions must find ways to support their employees, both in terms of workplace cultural adaptations and assistance with familial responsibilities,” the researchers noted.
Telecommuting policies, scheduling flexibility, and expanding employee support programs are potential solutions. Institutional policies also could address the educational and direct care needs of employee children.
Limitations of the study include its generalizability beyond employees of University of Utah Health. Also, respondents included a lower proportion of racial and ethnic groups, compared with national figures, “although this is mostly accounted for by the overall low population of such groups in the state of Utah,” the researchers added.
“Our results suggest that respondents were struggling during the COVID-19 pandemic,” the researchers noted. “As a result, even after investing substantial amounts of time in years of training, many were considering leaving the workforce because of stress and caregiving responsibilities related to the pandemic.”
The Jon M. Huntsman Presidential Endowed Chair supported the work with a financial award to Dr. Fagerlin. Dr. Delaney and Dr. Bernstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new survey of more than 5,000 clinicians at an academic medical center illustrates.
About one in five people reported considering leaving the workforce because of the challenges of working during the COVID-19 pandemic. In addition, 30% reported they are considering cutting back work hours.
“There are a substantial number of employees and trainees who are experiencing major stress and work disruptions because of the pandemic,” lead author Rebecca K. Delaney, PhD, said in an interview. “It is particularly alarming that people who have spent 5 or more years in training for their specialty are struggling with their work, so much so that they have even considered leaving the workforce or reducing their hours.”
“Being a caregiver adds another layer of difficulty for faculty, staff, and trainees who are trying to manage work and child care,” added Dr. Delaney, a researcher in the department of population health sciences, University of Utah, Salt Lake City.
The study was published online April 2 in JAMA Network Open.
“This looks like an excellent survey,” Carol A Bernstein, MD, said in an interview when asked to comment. “I do not think it provides particularly new information as these challenges in the workplace, especially for women during COVID, have been well documented in the media and the medical literature to date.”
“That said, to the extent that data helps drive solutions, I would hope that information such as this would be considered as strong further evidence that health care systems must pay close attention to the wellbeing of the workforce,” added Dr. Bernstein, professor and vice chair of faculty development and well-being, departments of psychiatry and behavioral sciences and obstetrics and gynecology and women’s health, Montefiore Medical Center/Albert Einstein College of Medicine, New York.
When the pandemic hits home
A total of 42% of the American workforce rapidly transitioned to working from home at the onset of the COVID-19 pandemic. At the same time, many employees had to provide child care and assistance with schoolwork. This placed a burden on many individuals at academic medical centers, and women in particular.
“Women comprise 74.9% of hospital employees, many of whom are essential clinical workers,” the researchers noted. “The extent of the needs and difficulties for these workers during the pandemic remain largely unknown.”
To learn more, Dr. Delaney, senior author Angie Fagerlin, PhD, and their colleagues emailed a Qualtrics survey to 27,700 faculty, staff, and trainees at University of Utah Health. The survey was conducted Aug. 5-20, 2020 as part of a quality improvement initiative. All responses were anonymous.
Survey questions included if, because of the pandemic, people had considered leaving the workforce, considered reducing their hours, or experienced reduced productivity. The researchers also asked about career impacts and potential solutions in terms of “work culture adaptations.”
Respondents with children aged under 18 years also were asked about child care options. Dr. Delaney and colleagues also inquired about race and ethnicity because they hypothesized that employees from underrepresented groups would likely experience the pandemic differently.
The mean age of the 5,951 (21%) faculty, staff, and trainees who completed the survey was 40 years. A majority of respondents were women, reflecting the higher proportion of women within the health system.
A majority (86%) identified as White or European American. About two-thirds of respondents (66%) were staff, 16% were faculty, and 13% were trainees.
COVID-19 career concerns
Overall, 1,061 respondents (21%) “moderately or very seriously” considered leaving the workforce and 1,505 (30%) considered reducing hours. Respondents who were younger, married, a member of an underrepresented racial/ethnic group, and worked in a clinical setting were more likely to consider leaving the workforce.
The survey showed 27% felt their productivity increased whereas 39% believed their productivity decreased.
Of the 2,412 survey participants with children aged 18 years or younger, 66% reported that they did not have child care fully available.
“Failure to address and provide for child care has long been one of the many significant deficits in U.S. health care systems,” said Dr. Bernstein, lead author of a March 2021 report evaluating staff emotional support at Montefiore Medical Center during the pandemic in The Joint Commission Journal on Quality and Patient Safety.
Furthermore, 47% were “moderately or very seriously worried” about COVID-19 impacting their career development.
Women trainees were significantly more likely than male counterparts to consider leaving the workforce and reducing their work hours. Women in a faculty or trainee role were also more likely to worry about COVID-19’s impact on their career, compared with men, and compared with women in staff positions.
“It was disheartening to have our data support the gender and racial/ethnic disparity that has been highlighted in the media during the pandemic,” Dr. Delaney said. “Women and in some cases racial/ethnic groups that are underrepresented in medicine were most likely to consider leaving the workforce, reducing hours, and were worried about their career development.
“It is critical that we strategically address these important disparities,” she said.
Women also are disproportionately affected by burnout, particularly during the pandemic, according to an analysis of Medscape’s Physician Burnout and Suicide Report.
Furthermore, the COVID-19 pandemic has shifted the medical specialties now considered highest risk for burnout: critical care physicians ranked first in the report, followed by rheumatologists and infectious disease specialists.
Potential solutions
“Given the disproportionate impact COVID-19 has on employees of health systems, institutions must find ways to support their employees, both in terms of workplace cultural adaptations and assistance with familial responsibilities,” the researchers noted.
Telecommuting policies, scheduling flexibility, and expanding employee support programs are potential solutions. Institutional policies also could address the educational and direct care needs of employee children.
Limitations of the study include its generalizability beyond employees of University of Utah Health. Also, respondents included a lower proportion of racial and ethnic groups, compared with national figures, “although this is mostly accounted for by the overall low population of such groups in the state of Utah,” the researchers added.
“Our results suggest that respondents were struggling during the COVID-19 pandemic,” the researchers noted. “As a result, even after investing substantial amounts of time in years of training, many were considering leaving the workforce because of stress and caregiving responsibilities related to the pandemic.”
The Jon M. Huntsman Presidential Endowed Chair supported the work with a financial award to Dr. Fagerlin. Dr. Delaney and Dr. Bernstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VEXAS: A novel rheumatologic, hematologic syndrome that’s making waves
Older men with a novel adult-onset, severe autoinflammatory syndrome known by the acronym VEXAS are likely hiding in plain sight in many adult rheumatology, hematology, and dermatology practices. New clinical features are being described to fill out the clinical profile of such patients who may be currently misdiagnosed with other conditions, according to researchers who first described the syndrome in the last quarter of 2020.

VEXAS is often misdiagnosed as treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, or giant cell arteritis. These seemingly unrelated disorders are actually tied together by a single thread recently unraveled by David B. Beck, MD, PhD, a clinical fellow at the National Human Genome Research Institute, and colleagues, including rheumatologist Marcela Ferrada, MD, and others at institutes of the National Institutes of Health, Bethesda, Md. The connection between these disparate clinical presentations lies in somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation. VEXAS appears primarily limited to men because the UBA1 gene lies on the X chromosome, although it may be possible for women to have it because of an acquired loss of X chromosome.
VEXAS is an acronym for:
- Vacuoles in bone marrow cells
- E-1 activating enzyme, which is what UBA1 encodes for
- X-linked
- Autoinflammatory
- Somatic mutation featuring hematologic mosaicism
Dr. Beck said that VEXAS is “probably affecting thousands of Americans,” but it is tough to say this early in the understanding of the disease. He estimated that the prevalence of VEXAS could be 1 per 20,000-30,000 individuals.
A new way of looking for disease
VEXAS has caused a major stir among geneticists because of the novel manner in which Dr. Beck and his coinvestigators made their discovery. Instead of starting out in the traditional path to discovery of a new genetic disease – that is, by looking for clinical similarities among patients with undiagnosed diseases and then conducting a search for a gene or genes that might explain the shared patient symptoms – the investigators took a genotype-first approach. They scanned the mapped genomic sequences of patients in the National Institutes of Health Undiagnosed Diseases Network, which led them to zero in on mutations in UBA1 as their top candidate.
“We targeted the ubiquitin-proteasome pathway, because it has been implicated in many autoinflammatory diseases – for example, HA20 [A20 haploinsufficiency] and CANDLE syndrome [Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature]. Many of these recurrent inflammatory diseases are caused by mutations within this pathway,” Dr. Beck said in an interview.
Next, they analyzed the genomes of patients in other NIH databases and patients from other study populations at the University College London and Leeds Teaching Hospitals NHS Trust in the United Kingdom in a search for UBA1 somatic mutations, eventually identifying 25 men with the shared features they called VEXAS. These 25 formed the basis for their initial report on the syndrome in the New England Journal of Medicine.
Most autoinflammatory diseases appear in childhood because they stem from germline mutations. VEXAS syndrome, because of somatic mutations with mosaicism, appears to manifest later in life: The median age of the initial 25-man cohort was 64 years, ranging from 45 to 80 years. It’s a severe disorder. By the time the investigators were preparing their paper for publication, 10 of the 25 patients, or 40%, had died.
“I think that somatic mutations may account for a significant percentage of severe. adult-onset rheumatologic diseases, and it may change the way we think about treating them based on having a genetic diagnosis,” Dr. Beck said.
“This approach could be expanded to look at other pathways we know are important in inflammation, or alternatively, it could be completely unbiased and look for any shared variation that occurs across undiagnosed patients with inflammatory diseases. I think that one thing that’s important about our study is that previously we had been looking for mutations that really in most cases were the same sort of germline mutations present in [pediatric] patients who have disease at early onset, but now we’re thinking about things differently. There may be a different type of genetics that drives adult-onset rheumatologic disease, and this would be somatic mutations which are not present in every cell of the body, just in the blood, and that’s why there’s just this blood-based disease.”
When to suspect VEXAS syndrome
Consider the possibility of VEXAS in middle-aged or older men in a rheumatology clinic with characteristics suggestive of treatment-refractory relapsing polychondritis, giant cell arteritis, polyarteritis nodosa, or Sweet syndrome. In the original series of 25 men, 15 were diagnosed with relapsing polychondritis, 8 with Sweet syndrome, 3 with polyarteritis nodosa, and 1 with giant cell arteritis.
Men with VEXAS often have periodic fevers, pulmonary infiltrates, a history of unprovoked venous thromboembolic events, neutrophilic dermatoses, and/or hematologic abnormalities such as myelodysplastic syndrome, multiple myeloma, or monoclonal gammopathy of unknown origin.
Bone marrow biopsy will show vacuoles in myeloid and erythroid precursor cells. Inflammatory marker levels are very high: In the NIH series, the median C-reactive protein was 73 mg/L and median erythrocyte sedimentation rate was 97 mm/hr. The diagnosis of VEXAS can be confirmed by genetic testing performed by Dr. Beck and his NIH coworkers ([email protected]).
In interviews, Dr. Beck and Dr. Ferrada emphasized that management of VEXAS requires a multidisciplinary team of clinicians including rheumatologists, hematologists, and dermatologists.
Dr. Ferrada said that rheumatologists could suspect VEXAS in patients who have very high inflammatory markers and do not have a clear diagnosis or do not meet all criteria for other rheumatologic diseases, particularly in older men, but it’s possible in younger men as well. Hematologists could also consider VEXAS in patients with macrocytic anemia or macrocytosis without an explanation and inflammatory features, she said.
Dr. Ferrada, Dr. Beck, and colleagues also published a study in Arthritis & Rheumatology that presents a useful clinical algorithm for deciding whether to order genetic screening for VEXAS in patients with relapsing polychondritis.
First off, Dr. Ferrada and colleagues performed whole-exome sequencing and testing for UBA1 variants in an observational cohort of 92 relapsing polychondritis patients to determine the prevalence of VEXAS, which turned out to be 8%. They added an additional 6 patients with relapsing polychondritis and VEXAS from other cohorts, for a total of 13. The investigators determined that patients with VEXAS were older at disease onset, and more likely to have fever, ear chondritis, DVT, pulmonary infiltrates, skin involvement, and periorbital edema. In contrast, the RP cohort had a significantly higher prevalence of airway chondritis, joint involvement, and vestibular symptoms.
Dr. Ferrada’s algorithm for picking out VEXAS in patients who meet diagnostic criteria for relapsing polychondritis is based upon a few simple factors readily apparent in screening patient charts: male sex; age at onset older than 50 years; macrocytic anemia; and thrombocytopenia. Those four variables, when present, identify VEXAS within an RP cohort with 100% sensitivity and 96% specificity. “As we learn more about [VEXAS] and how it presents earlier, I think we are going to be able to find different manifestations or laboratory data that are going to allow us to diagnose these patients earlier,” she said. “The whole role of that algorithm was to guide clinicians who see patients with relapsing polychondritis to test these patients for the mutation, but I think over time that is going to evolve.”
Researchers are taking similar approaches for other clinical diagnoses to see which should be referred for UBA1 testing, Dr. Beck said.
Myelodysplastic syndrome and hematologic abnormalities
While patients with both myelodysplastic syndrome and relapsing polychondritis have been known in the literature for many years, it’s not until now that researchers are seeing a connection between the two, Dr. Ferrada said.
A majority of the VEXAS patients in the NEJM study had a workup for myelodysplastic syndrome, but only 24% met criteria. However, many were within the spectrum of myelodysplastic disease and some did not meet criteria because their anemia was attributed to a rheumatologic diagnosis and they did not have a known genetic driver of myelodysplastic syndrome, Dr. Beck said. It also fits with this new evidence that UBA1 is probably a driver of myelodysplastic syndrome in and of itself, and that anemia and hematologic involvement are not secondary to the rheumatologic disease; they are linked to the same disease process.
Dr. Beck said that there may be a subset of patients who present with primarily hematologic manifestations, noting the NEJM study could have ascertainment bias because the researchers analyzed mainly patients presenting to their clinic with relapsing polychondritis and severe inflammation. NIH researchers also are still looking in their cohort for any association with hematologic malignancies that preceded clinical manifestations, he said.
More cases reported
As of early April, another 27 cases had been reported in the literature as more researchers have begun to look for patients with UBA1 mutations, some with additional presenting clinical features associated with VEXAS, including chronic progressive inflammatory arthritis, Kikuchi-Fujimoto disease, spondyloarthritis, and bacterial pneumonia.
“Many times with rare diseases, we can’t get enough patients to understand the full spectrum of the disease, but this disease seems to be far more common than we would have expected. We’re actually getting many referrals,” Dr. Beck said.
It appears so far that the range of somatic UBA1 mutations that have been discovered in VEXAS patients does make a difference in the severity of clinical presentation and could potentially be useful in prognosis, Dr. Beck said.
Right now, NIH researchers are asking patients about their natural clinical course, assessing disease activity, and determining which treatments get a response, with the ultimate goal of a treatment trial at the NIH.
Treatment
Developing better treatments for VEXAS syndrome is a priority. In the initial report on VEXAS, the researchers found that the only reliably effective therapy is high-dose corticosteroids. Dr. Ferrada said that NIH investigators have begun thinking about agents that target both the hematologic and inflammatory features of VEXAS. “Most patients get exposed to treatments that are targeted to decrease the inflammatory process, and some of these treatments help partially but not completely to decrease the amount of steroids that patients are taking. For example, one of the medications is tocilizumab. [It was used in] patients who had previous diagnosis of relapsing polychondritis, but they still had to take steroids and their hematologic manifestations keep progressing. We’re in the process of figuring out medications that may help in treating both.” Dr. Ferrada added that because the source of the mutation is in the bone marrow, transplantation may be an effective option.
Laboratory work to identify potential treatments for VEXAS in studies of model organisms could identify treatments outside of the classic anti-inflammatory agents, such as targeting certain cell types in the bone marrow or the ubiquitin-proteasome pathway, Dr. Beck said. “We think that however UBA1 works to initiate inflammation may be important not just in VEXAS but in other diseases. Rare diseases may be informing the mechanisms in common diseases.”
The VEXAS NEJM study was sponsored by the NIH Intramural Research Programs and by an EU Horizon 2020 Research and Innovation Program grant. Dr. Beck reported a patent pending on “Diagnosis and Treatment of VEXAS with Mosaic Missense Mutations in UBA1.”
Older men with a novel adult-onset, severe autoinflammatory syndrome known by the acronym VEXAS are likely hiding in plain sight in many adult rheumatology, hematology, and dermatology practices. New clinical features are being described to fill out the clinical profile of such patients who may be currently misdiagnosed with other conditions, according to researchers who first described the syndrome in the last quarter of 2020.

VEXAS is often misdiagnosed as treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, or giant cell arteritis. These seemingly unrelated disorders are actually tied together by a single thread recently unraveled by David B. Beck, MD, PhD, a clinical fellow at the National Human Genome Research Institute, and colleagues, including rheumatologist Marcela Ferrada, MD, and others at institutes of the National Institutes of Health, Bethesda, Md. The connection between these disparate clinical presentations lies in somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation. VEXAS appears primarily limited to men because the UBA1 gene lies on the X chromosome, although it may be possible for women to have it because of an acquired loss of X chromosome.
VEXAS is an acronym for:
- Vacuoles in bone marrow cells
- E-1 activating enzyme, which is what UBA1 encodes for
- X-linked
- Autoinflammatory
- Somatic mutation featuring hematologic mosaicism
Dr. Beck said that VEXAS is “probably affecting thousands of Americans,” but it is tough to say this early in the understanding of the disease. He estimated that the prevalence of VEXAS could be 1 per 20,000-30,000 individuals.
A new way of looking for disease
VEXAS has caused a major stir among geneticists because of the novel manner in which Dr. Beck and his coinvestigators made their discovery. Instead of starting out in the traditional path to discovery of a new genetic disease – that is, by looking for clinical similarities among patients with undiagnosed diseases and then conducting a search for a gene or genes that might explain the shared patient symptoms – the investigators took a genotype-first approach. They scanned the mapped genomic sequences of patients in the National Institutes of Health Undiagnosed Diseases Network, which led them to zero in on mutations in UBA1 as their top candidate.
“We targeted the ubiquitin-proteasome pathway, because it has been implicated in many autoinflammatory diseases – for example, HA20 [A20 haploinsufficiency] and CANDLE syndrome [Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature]. Many of these recurrent inflammatory diseases are caused by mutations within this pathway,” Dr. Beck said in an interview.
Next, they analyzed the genomes of patients in other NIH databases and patients from other study populations at the University College London and Leeds Teaching Hospitals NHS Trust in the United Kingdom in a search for UBA1 somatic mutations, eventually identifying 25 men with the shared features they called VEXAS. These 25 formed the basis for their initial report on the syndrome in the New England Journal of Medicine.
Most autoinflammatory diseases appear in childhood because they stem from germline mutations. VEXAS syndrome, because of somatic mutations with mosaicism, appears to manifest later in life: The median age of the initial 25-man cohort was 64 years, ranging from 45 to 80 years. It’s a severe disorder. By the time the investigators were preparing their paper for publication, 10 of the 25 patients, or 40%, had died.
“I think that somatic mutations may account for a significant percentage of severe. adult-onset rheumatologic diseases, and it may change the way we think about treating them based on having a genetic diagnosis,” Dr. Beck said.
“This approach could be expanded to look at other pathways we know are important in inflammation, or alternatively, it could be completely unbiased and look for any shared variation that occurs across undiagnosed patients with inflammatory diseases. I think that one thing that’s important about our study is that previously we had been looking for mutations that really in most cases were the same sort of germline mutations present in [pediatric] patients who have disease at early onset, but now we’re thinking about things differently. There may be a different type of genetics that drives adult-onset rheumatologic disease, and this would be somatic mutations which are not present in every cell of the body, just in the blood, and that’s why there’s just this blood-based disease.”
When to suspect VEXAS syndrome
Consider the possibility of VEXAS in middle-aged or older men in a rheumatology clinic with characteristics suggestive of treatment-refractory relapsing polychondritis, giant cell arteritis, polyarteritis nodosa, or Sweet syndrome. In the original series of 25 men, 15 were diagnosed with relapsing polychondritis, 8 with Sweet syndrome, 3 with polyarteritis nodosa, and 1 with giant cell arteritis.
Men with VEXAS often have periodic fevers, pulmonary infiltrates, a history of unprovoked venous thromboembolic events, neutrophilic dermatoses, and/or hematologic abnormalities such as myelodysplastic syndrome, multiple myeloma, or monoclonal gammopathy of unknown origin.
Bone marrow biopsy will show vacuoles in myeloid and erythroid precursor cells. Inflammatory marker levels are very high: In the NIH series, the median C-reactive protein was 73 mg/L and median erythrocyte sedimentation rate was 97 mm/hr. The diagnosis of VEXAS can be confirmed by genetic testing performed by Dr. Beck and his NIH coworkers ([email protected]).
In interviews, Dr. Beck and Dr. Ferrada emphasized that management of VEXAS requires a multidisciplinary team of clinicians including rheumatologists, hematologists, and dermatologists.
Dr. Ferrada said that rheumatologists could suspect VEXAS in patients who have very high inflammatory markers and do not have a clear diagnosis or do not meet all criteria for other rheumatologic diseases, particularly in older men, but it’s possible in younger men as well. Hematologists could also consider VEXAS in patients with macrocytic anemia or macrocytosis without an explanation and inflammatory features, she said.
Dr. Ferrada, Dr. Beck, and colleagues also published a study in Arthritis & Rheumatology that presents a useful clinical algorithm for deciding whether to order genetic screening for VEXAS in patients with relapsing polychondritis.
First off, Dr. Ferrada and colleagues performed whole-exome sequencing and testing for UBA1 variants in an observational cohort of 92 relapsing polychondritis patients to determine the prevalence of VEXAS, which turned out to be 8%. They added an additional 6 patients with relapsing polychondritis and VEXAS from other cohorts, for a total of 13. The investigators determined that patients with VEXAS were older at disease onset, and more likely to have fever, ear chondritis, DVT, pulmonary infiltrates, skin involvement, and periorbital edema. In contrast, the RP cohort had a significantly higher prevalence of airway chondritis, joint involvement, and vestibular symptoms.
Dr. Ferrada’s algorithm for picking out VEXAS in patients who meet diagnostic criteria for relapsing polychondritis is based upon a few simple factors readily apparent in screening patient charts: male sex; age at onset older than 50 years; macrocytic anemia; and thrombocytopenia. Those four variables, when present, identify VEXAS within an RP cohort with 100% sensitivity and 96% specificity. “As we learn more about [VEXAS] and how it presents earlier, I think we are going to be able to find different manifestations or laboratory data that are going to allow us to diagnose these patients earlier,” she said. “The whole role of that algorithm was to guide clinicians who see patients with relapsing polychondritis to test these patients for the mutation, but I think over time that is going to evolve.”
Researchers are taking similar approaches for other clinical diagnoses to see which should be referred for UBA1 testing, Dr. Beck said.
Myelodysplastic syndrome and hematologic abnormalities
While patients with both myelodysplastic syndrome and relapsing polychondritis have been known in the literature for many years, it’s not until now that researchers are seeing a connection between the two, Dr. Ferrada said.
A majority of the VEXAS patients in the NEJM study had a workup for myelodysplastic syndrome, but only 24% met criteria. However, many were within the spectrum of myelodysplastic disease and some did not meet criteria because their anemia was attributed to a rheumatologic diagnosis and they did not have a known genetic driver of myelodysplastic syndrome, Dr. Beck said. It also fits with this new evidence that UBA1 is probably a driver of myelodysplastic syndrome in and of itself, and that anemia and hematologic involvement are not secondary to the rheumatologic disease; they are linked to the same disease process.
Dr. Beck said that there may be a subset of patients who present with primarily hematologic manifestations, noting the NEJM study could have ascertainment bias because the researchers analyzed mainly patients presenting to their clinic with relapsing polychondritis and severe inflammation. NIH researchers also are still looking in their cohort for any association with hematologic malignancies that preceded clinical manifestations, he said.
More cases reported
As of early April, another 27 cases had been reported in the literature as more researchers have begun to look for patients with UBA1 mutations, some with additional presenting clinical features associated with VEXAS, including chronic progressive inflammatory arthritis, Kikuchi-Fujimoto disease, spondyloarthritis, and bacterial pneumonia.
“Many times with rare diseases, we can’t get enough patients to understand the full spectrum of the disease, but this disease seems to be far more common than we would have expected. We’re actually getting many referrals,” Dr. Beck said.
It appears so far that the range of somatic UBA1 mutations that have been discovered in VEXAS patients does make a difference in the severity of clinical presentation and could potentially be useful in prognosis, Dr. Beck said.
Right now, NIH researchers are asking patients about their natural clinical course, assessing disease activity, and determining which treatments get a response, with the ultimate goal of a treatment trial at the NIH.
Treatment
Developing better treatments for VEXAS syndrome is a priority. In the initial report on VEXAS, the researchers found that the only reliably effective therapy is high-dose corticosteroids. Dr. Ferrada said that NIH investigators have begun thinking about agents that target both the hematologic and inflammatory features of VEXAS. “Most patients get exposed to treatments that are targeted to decrease the inflammatory process, and some of these treatments help partially but not completely to decrease the amount of steroids that patients are taking. For example, one of the medications is tocilizumab. [It was used in] patients who had previous diagnosis of relapsing polychondritis, but they still had to take steroids and their hematologic manifestations keep progressing. We’re in the process of figuring out medications that may help in treating both.” Dr. Ferrada added that because the source of the mutation is in the bone marrow, transplantation may be an effective option.
Laboratory work to identify potential treatments for VEXAS in studies of model organisms could identify treatments outside of the classic anti-inflammatory agents, such as targeting certain cell types in the bone marrow or the ubiquitin-proteasome pathway, Dr. Beck said. “We think that however UBA1 works to initiate inflammation may be important not just in VEXAS but in other diseases. Rare diseases may be informing the mechanisms in common diseases.”
The VEXAS NEJM study was sponsored by the NIH Intramural Research Programs and by an EU Horizon 2020 Research and Innovation Program grant. Dr. Beck reported a patent pending on “Diagnosis and Treatment of VEXAS with Mosaic Missense Mutations in UBA1.”
Older men with a novel adult-onset, severe autoinflammatory syndrome known by the acronym VEXAS are likely hiding in plain sight in many adult rheumatology, hematology, and dermatology practices. New clinical features are being described to fill out the clinical profile of such patients who may be currently misdiagnosed with other conditions, according to researchers who first described the syndrome in the last quarter of 2020.

VEXAS is often misdiagnosed as treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, or giant cell arteritis. These seemingly unrelated disorders are actually tied together by a single thread recently unraveled by David B. Beck, MD, PhD, a clinical fellow at the National Human Genome Research Institute, and colleagues, including rheumatologist Marcela Ferrada, MD, and others at institutes of the National Institutes of Health, Bethesda, Md. The connection between these disparate clinical presentations lies in somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation. VEXAS appears primarily limited to men because the UBA1 gene lies on the X chromosome, although it may be possible for women to have it because of an acquired loss of X chromosome.
VEXAS is an acronym for:
- Vacuoles in bone marrow cells
- E-1 activating enzyme, which is what UBA1 encodes for
- X-linked
- Autoinflammatory
- Somatic mutation featuring hematologic mosaicism
Dr. Beck said that VEXAS is “probably affecting thousands of Americans,” but it is tough to say this early in the understanding of the disease. He estimated that the prevalence of VEXAS could be 1 per 20,000-30,000 individuals.
A new way of looking for disease
VEXAS has caused a major stir among geneticists because of the novel manner in which Dr. Beck and his coinvestigators made their discovery. Instead of starting out in the traditional path to discovery of a new genetic disease – that is, by looking for clinical similarities among patients with undiagnosed diseases and then conducting a search for a gene or genes that might explain the shared patient symptoms – the investigators took a genotype-first approach. They scanned the mapped genomic sequences of patients in the National Institutes of Health Undiagnosed Diseases Network, which led them to zero in on mutations in UBA1 as their top candidate.
“We targeted the ubiquitin-proteasome pathway, because it has been implicated in many autoinflammatory diseases – for example, HA20 [A20 haploinsufficiency] and CANDLE syndrome [Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature]. Many of these recurrent inflammatory diseases are caused by mutations within this pathway,” Dr. Beck said in an interview.
Next, they analyzed the genomes of patients in other NIH databases and patients from other study populations at the University College London and Leeds Teaching Hospitals NHS Trust in the United Kingdom in a search for UBA1 somatic mutations, eventually identifying 25 men with the shared features they called VEXAS. These 25 formed the basis for their initial report on the syndrome in the New England Journal of Medicine.
Most autoinflammatory diseases appear in childhood because they stem from germline mutations. VEXAS syndrome, because of somatic mutations with mosaicism, appears to manifest later in life: The median age of the initial 25-man cohort was 64 years, ranging from 45 to 80 years. It’s a severe disorder. By the time the investigators were preparing their paper for publication, 10 of the 25 patients, or 40%, had died.
“I think that somatic mutations may account for a significant percentage of severe. adult-onset rheumatologic diseases, and it may change the way we think about treating them based on having a genetic diagnosis,” Dr. Beck said.
“This approach could be expanded to look at other pathways we know are important in inflammation, or alternatively, it could be completely unbiased and look for any shared variation that occurs across undiagnosed patients with inflammatory diseases. I think that one thing that’s important about our study is that previously we had been looking for mutations that really in most cases were the same sort of germline mutations present in [pediatric] patients who have disease at early onset, but now we’re thinking about things differently. There may be a different type of genetics that drives adult-onset rheumatologic disease, and this would be somatic mutations which are not present in every cell of the body, just in the blood, and that’s why there’s just this blood-based disease.”
When to suspect VEXAS syndrome
Consider the possibility of VEXAS in middle-aged or older men in a rheumatology clinic with characteristics suggestive of treatment-refractory relapsing polychondritis, giant cell arteritis, polyarteritis nodosa, or Sweet syndrome. In the original series of 25 men, 15 were diagnosed with relapsing polychondritis, 8 with Sweet syndrome, 3 with polyarteritis nodosa, and 1 with giant cell arteritis.
Men with VEXAS often have periodic fevers, pulmonary infiltrates, a history of unprovoked venous thromboembolic events, neutrophilic dermatoses, and/or hematologic abnormalities such as myelodysplastic syndrome, multiple myeloma, or monoclonal gammopathy of unknown origin.
Bone marrow biopsy will show vacuoles in myeloid and erythroid precursor cells. Inflammatory marker levels are very high: In the NIH series, the median C-reactive protein was 73 mg/L and median erythrocyte sedimentation rate was 97 mm/hr. The diagnosis of VEXAS can be confirmed by genetic testing performed by Dr. Beck and his NIH coworkers ([email protected]).
In interviews, Dr. Beck and Dr. Ferrada emphasized that management of VEXAS requires a multidisciplinary team of clinicians including rheumatologists, hematologists, and dermatologists.
Dr. Ferrada said that rheumatologists could suspect VEXAS in patients who have very high inflammatory markers and do not have a clear diagnosis or do not meet all criteria for other rheumatologic diseases, particularly in older men, but it’s possible in younger men as well. Hematologists could also consider VEXAS in patients with macrocytic anemia or macrocytosis without an explanation and inflammatory features, she said.
Dr. Ferrada, Dr. Beck, and colleagues also published a study in Arthritis & Rheumatology that presents a useful clinical algorithm for deciding whether to order genetic screening for VEXAS in patients with relapsing polychondritis.
First off, Dr. Ferrada and colleagues performed whole-exome sequencing and testing for UBA1 variants in an observational cohort of 92 relapsing polychondritis patients to determine the prevalence of VEXAS, which turned out to be 8%. They added an additional 6 patients with relapsing polychondritis and VEXAS from other cohorts, for a total of 13. The investigators determined that patients with VEXAS were older at disease onset, and more likely to have fever, ear chondritis, DVT, pulmonary infiltrates, skin involvement, and periorbital edema. In contrast, the RP cohort had a significantly higher prevalence of airway chondritis, joint involvement, and vestibular symptoms.
Dr. Ferrada’s algorithm for picking out VEXAS in patients who meet diagnostic criteria for relapsing polychondritis is based upon a few simple factors readily apparent in screening patient charts: male sex; age at onset older than 50 years; macrocytic anemia; and thrombocytopenia. Those four variables, when present, identify VEXAS within an RP cohort with 100% sensitivity and 96% specificity. “As we learn more about [VEXAS] and how it presents earlier, I think we are going to be able to find different manifestations or laboratory data that are going to allow us to diagnose these patients earlier,” she said. “The whole role of that algorithm was to guide clinicians who see patients with relapsing polychondritis to test these patients for the mutation, but I think over time that is going to evolve.”
Researchers are taking similar approaches for other clinical diagnoses to see which should be referred for UBA1 testing, Dr. Beck said.
Myelodysplastic syndrome and hematologic abnormalities
While patients with both myelodysplastic syndrome and relapsing polychondritis have been known in the literature for many years, it’s not until now that researchers are seeing a connection between the two, Dr. Ferrada said.
A majority of the VEXAS patients in the NEJM study had a workup for myelodysplastic syndrome, but only 24% met criteria. However, many were within the spectrum of myelodysplastic disease and some did not meet criteria because their anemia was attributed to a rheumatologic diagnosis and they did not have a known genetic driver of myelodysplastic syndrome, Dr. Beck said. It also fits with this new evidence that UBA1 is probably a driver of myelodysplastic syndrome in and of itself, and that anemia and hematologic involvement are not secondary to the rheumatologic disease; they are linked to the same disease process.
Dr. Beck said that there may be a subset of patients who present with primarily hematologic manifestations, noting the NEJM study could have ascertainment bias because the researchers analyzed mainly patients presenting to their clinic with relapsing polychondritis and severe inflammation. NIH researchers also are still looking in their cohort for any association with hematologic malignancies that preceded clinical manifestations, he said.
More cases reported
As of early April, another 27 cases had been reported in the literature as more researchers have begun to look for patients with UBA1 mutations, some with additional presenting clinical features associated with VEXAS, including chronic progressive inflammatory arthritis, Kikuchi-Fujimoto disease, spondyloarthritis, and bacterial pneumonia.
“Many times with rare diseases, we can’t get enough patients to understand the full spectrum of the disease, but this disease seems to be far more common than we would have expected. We’re actually getting many referrals,” Dr. Beck said.
It appears so far that the range of somatic UBA1 mutations that have been discovered in VEXAS patients does make a difference in the severity of clinical presentation and could potentially be useful in prognosis, Dr. Beck said.
Right now, NIH researchers are asking patients about their natural clinical course, assessing disease activity, and determining which treatments get a response, with the ultimate goal of a treatment trial at the NIH.
Treatment
Developing better treatments for VEXAS syndrome is a priority. In the initial report on VEXAS, the researchers found that the only reliably effective therapy is high-dose corticosteroids. Dr. Ferrada said that NIH investigators have begun thinking about agents that target both the hematologic and inflammatory features of VEXAS. “Most patients get exposed to treatments that are targeted to decrease the inflammatory process, and some of these treatments help partially but not completely to decrease the amount of steroids that patients are taking. For example, one of the medications is tocilizumab. [It was used in] patients who had previous diagnosis of relapsing polychondritis, but they still had to take steroids and their hematologic manifestations keep progressing. We’re in the process of figuring out medications that may help in treating both.” Dr. Ferrada added that because the source of the mutation is in the bone marrow, transplantation may be an effective option.
Laboratory work to identify potential treatments for VEXAS in studies of model organisms could identify treatments outside of the classic anti-inflammatory agents, such as targeting certain cell types in the bone marrow or the ubiquitin-proteasome pathway, Dr. Beck said. “We think that however UBA1 works to initiate inflammation may be important not just in VEXAS but in other diseases. Rare diseases may be informing the mechanisms in common diseases.”
The VEXAS NEJM study was sponsored by the NIH Intramural Research Programs and by an EU Horizon 2020 Research and Innovation Program grant. Dr. Beck reported a patent pending on “Diagnosis and Treatment of VEXAS with Mosaic Missense Mutations in UBA1.”
New guidelines on antibiotic prescribing focus on shorter courses
An antibiotic course of 5 days is usually just as effective as longer courses but with fewer side effects and decreased overall antibiotic exposure for a number of common bacterial conditions, according to new clinical guidelines published by the American College of Physicians.
The guidelines focus on treatment of uncomplicated cases involving pneumonia, urinary tract infections (UTIs), cellulitis, chronic obstructive pulmonary disease (COPD) exacerbations, and acute bronchitis. The goal of the guidelines is to continue improving antibiotic stewardship given the increasing threat of antibiotic resistance and the adverse effects of antibiotics.
“Any use of antibiotics (including necessary use) has downstream effects outside of treating infection,” Dawn Nolt, MD, MPH, a professor of pediatric infection disease at Oregon Health & Science University, Portland, said in an interview. Dr. Nolt was not involved in developing these guidelines. “Undesirable outcomes include allergic reactions, diarrhea, and antibiotic-resistant bacteria. When we reduce unnecessary antibiotic, we reduce undesirable outcomes,” she said.
According to background information in the paper, 1 in 10 patients receives an antibiotic prescription during visits, yet nearly a third of these (30%) are unnecessary and last too long, especially for sinusitis and bronchitis. Meanwhile, overuse of antibiotics, particularly broad-spectrum ones, leads to resistance and adverse effects in up to 20% of patients.
“Prescribing practices can vary based on the type of provider, the setting where the antibiotic is being prescribed, what geographic area you are looking at, the medical reason for which the antibiotic is being prescribed, the actual germ being targeted, and the type of patient,” Dr. Nolt said. “But this variability can be reduced when prescribing providers are aware and follow best practice standards as through this article.”
The new ACP guidelines are a distillation of recommendations from preexisting infectious disease organizations, Dr. Nolt said, but aimed specifically at those practicing internal medicine.
“We define appropriate antibiotic use as prescribing the right antibiotic at the right dose for the right duration for a specific condition,” Rachael A. Lee, MD, MSPH, of the University of Alabama at Birmingham, and colleagues wrote in the article detailing the new guidelines. “Despite evidence and guidelines supporting shorter durations of antibiotic use, many physicians do not prescribe short-course therapy, frequently defaulting to 10-day courses regardless of the condition.”
The reasons for this default response vary. Though some clinicians prescribe longer courses specifically to prevent antibiotic resistance, no evidence shows that continuing to take antibiotics after symptoms have resolved actually reduces likelihood of resistance, the authors noted.
“In fact, resistance is a documented side effect of prolonged antibiotic use due to natural selection pressure,” they wrote.
Another common reason is habit.
“This was the ‘conventional wisdom’ for so long, just trying to make sure all bacteria causing the infection were completely eradicated, with no stragglers that had been exposed to the antibiotic but were not gone and now could evolve into resistant organisms,” Jacqueline W. Fincher, MD, a primary care physician and president of the ACP, said in an interview. “While antibiotic stewardship has been very important for over a decade, we now have more recent head-to-head studies/data showing that, in these four conditions, shorter courses of treatment are just as efficacious with less side effects and adverse events.”
The researchers reviewed all existing clinical guidelines related to bronchitis with COPD exacerbations, community-acquired pneumonia, UTIs, and cellulitis, as well as any other relevant studies in the literature. Although they did not conduct a formal systematic review, they compiled the guidelines specifically for all internists, family physicians and other clinicians caring for patients with these conditions.
“Although most patients with these infections will be seen in the outpatient setting, these best-practice advice statements also apply to patients who present in the inpatient setting,” the authors wrote. They also note the importance of ensuring the patient has the correct diagnosis and appropriate corresponding antibiotic prescription. “If a patient is not improving with appropriate antibiotics, it is important for the clinician to reassess for other causes of symptoms rather than defaulting to a longer duration of antibiotic therapy,” they wrote, calling a longer course “the exception and not the rule.”
Acute bronchitis with COPD exacerbations
Antibiotic treatment for COPD exacerbations and acute uncomplicated bronchitis with signs of a bacterial infection should last no longer than 5 days. The authors define this condition as an acute respiratory infection with a normal chest x-ray, most often caused by a virus. Although patients with bronchitis do not automatically need antibiotics if there’s no evidence of pneumonia, the authors did advise antibiotics in cases involving COPD and a high likelihood of bacterial infection. Clinicians should base their choice of antibiotics on the most common bacterial etiology: Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis. Ideal candidates for therapy may include aminopenicillin with clavulanic acid, a macrolide, or a tetracycline.
Community-acquired pneumonia
The initial course of antibiotics should be at least 5 days for pneumonia and only extended after considering validated evidence of the patient’s clinical stability, such as resuming normal vital signs, mental activity, and the ability to eat. Multiple randomized, controlled trials have shown no improved benefit from longer courses, though longer courses are linked to increased adverse events and mortality.
Again, antibiotics used should “cover common pathogens, such as S. pneumoniae, H. influenzae, Mycoplasma pneumoniae, and Staphylococcus aureus, and atypical pathogens, such as Legionella species,” the authors wrote. Options include “amoxicillin, doxycycline, or a macrolide for healthy adults or a beta-lactam with a macrolide or a respiratory fluoroquinolone in patients with comorbidities.”
UTIs: Uncomplicated cystitis and pyelonephritis
For women’s bacterial cystitis – 75% of which is caused by Escherichia coli – the guidelines recommend nitrofurantoin for 5 days, trimethoprim-sulfamethoxazole for 3 days, or fosfomycin as a single dose. For uncomplicated pyelonephritis in both men and women, clinicians can consider fluoroquinolones for 5-7 days or trimethoprim-sulfamethoxazole for 14 days, depending on antibiotic susceptibility.
This recommendation does not include UTIs in women who are pregnant or UTIs with other functional abnormalities present, such as obstruction. The authors also intentionally left out acute bacterial prostatitis because of its complexity and how long it can take to treat.
Cellulitis
MRSA, which has been increasing in prevalence, is a leading cause of skin and soft-tissue infections, such as necrotizing infections, cellulitis, and erysipelas. Unless the patient has penetrating trauma, evidence of MRSA infection elsewhere, injection drug use, nasal colonization of MRSA, or systemic inflammatory response syndrome, the guidelines recommend a 5- to 6-day course of cephalosporin, penicillin, or clindamycin, extended only if the infection has not improved in 5 days. Further research can narrow down the most appropriate treatment course.
This guidance does not apply to purulent cellulitis, such as conditions with abscesses, furuncles, or carbuncles that typically require incision and drainage.
Continuing to get the message out
Dr. Fincher emphasized the importance of continuing to disseminate messaging for clinicians about reducing unnecessary antibiotic use.
“In medicine we are constantly bombarded with new information. It is those patients and disease states that we see and treat every day that are especially important for us as physicians and other clinicians to keep our skills and knowledge base up to date when it comes to use of antibiotics,” Dr. Fincher said in an interview. “We just need to continue to educate and push out the data, guidelines, and recommendations.”
Dr. Nolt added that it’s important to emphasize how to translate these national recommendations into local practices since local guidance can also raise awareness and encourage local compliance.
Other strategies for reducing overuse of antibiotics “include restriction on antibiotics available at health care systems (formulary restriction), not allowing use of antibiotics unless there is discussion about the patient’s case (preauthorization), and reviewing cases of patients on antibiotics and advising on next steps (prospective audit and feedback),” she said.
The research was funded by the ACP. Dr. Lee has received personal fees from this news organization and Prime Education. Dr. Fincher owns stock in Johnson & Johnson and Procter and Gamble. Dr. Nolt and the article’s coauthors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An antibiotic course of 5 days is usually just as effective as longer courses but with fewer side effects and decreased overall antibiotic exposure for a number of common bacterial conditions, according to new clinical guidelines published by the American College of Physicians.
The guidelines focus on treatment of uncomplicated cases involving pneumonia, urinary tract infections (UTIs), cellulitis, chronic obstructive pulmonary disease (COPD) exacerbations, and acute bronchitis. The goal of the guidelines is to continue improving antibiotic stewardship given the increasing threat of antibiotic resistance and the adverse effects of antibiotics.
“Any use of antibiotics (including necessary use) has downstream effects outside of treating infection,” Dawn Nolt, MD, MPH, a professor of pediatric infection disease at Oregon Health & Science University, Portland, said in an interview. Dr. Nolt was not involved in developing these guidelines. “Undesirable outcomes include allergic reactions, diarrhea, and antibiotic-resistant bacteria. When we reduce unnecessary antibiotic, we reduce undesirable outcomes,” she said.
According to background information in the paper, 1 in 10 patients receives an antibiotic prescription during visits, yet nearly a third of these (30%) are unnecessary and last too long, especially for sinusitis and bronchitis. Meanwhile, overuse of antibiotics, particularly broad-spectrum ones, leads to resistance and adverse effects in up to 20% of patients.
“Prescribing practices can vary based on the type of provider, the setting where the antibiotic is being prescribed, what geographic area you are looking at, the medical reason for which the antibiotic is being prescribed, the actual germ being targeted, and the type of patient,” Dr. Nolt said. “But this variability can be reduced when prescribing providers are aware and follow best practice standards as through this article.”
The new ACP guidelines are a distillation of recommendations from preexisting infectious disease organizations, Dr. Nolt said, but aimed specifically at those practicing internal medicine.
“We define appropriate antibiotic use as prescribing the right antibiotic at the right dose for the right duration for a specific condition,” Rachael A. Lee, MD, MSPH, of the University of Alabama at Birmingham, and colleagues wrote in the article detailing the new guidelines. “Despite evidence and guidelines supporting shorter durations of antibiotic use, many physicians do not prescribe short-course therapy, frequently defaulting to 10-day courses regardless of the condition.”
The reasons for this default response vary. Though some clinicians prescribe longer courses specifically to prevent antibiotic resistance, no evidence shows that continuing to take antibiotics after symptoms have resolved actually reduces likelihood of resistance, the authors noted.
“In fact, resistance is a documented side effect of prolonged antibiotic use due to natural selection pressure,” they wrote.
Another common reason is habit.
“This was the ‘conventional wisdom’ for so long, just trying to make sure all bacteria causing the infection were completely eradicated, with no stragglers that had been exposed to the antibiotic but were not gone and now could evolve into resistant organisms,” Jacqueline W. Fincher, MD, a primary care physician and president of the ACP, said in an interview. “While antibiotic stewardship has been very important for over a decade, we now have more recent head-to-head studies/data showing that, in these four conditions, shorter courses of treatment are just as efficacious with less side effects and adverse events.”
The researchers reviewed all existing clinical guidelines related to bronchitis with COPD exacerbations, community-acquired pneumonia, UTIs, and cellulitis, as well as any other relevant studies in the literature. Although they did not conduct a formal systematic review, they compiled the guidelines specifically for all internists, family physicians and other clinicians caring for patients with these conditions.
“Although most patients with these infections will be seen in the outpatient setting, these best-practice advice statements also apply to patients who present in the inpatient setting,” the authors wrote. They also note the importance of ensuring the patient has the correct diagnosis and appropriate corresponding antibiotic prescription. “If a patient is not improving with appropriate antibiotics, it is important for the clinician to reassess for other causes of symptoms rather than defaulting to a longer duration of antibiotic therapy,” they wrote, calling a longer course “the exception and not the rule.”
Acute bronchitis with COPD exacerbations
Antibiotic treatment for COPD exacerbations and acute uncomplicated bronchitis with signs of a bacterial infection should last no longer than 5 days. The authors define this condition as an acute respiratory infection with a normal chest x-ray, most often caused by a virus. Although patients with bronchitis do not automatically need antibiotics if there’s no evidence of pneumonia, the authors did advise antibiotics in cases involving COPD and a high likelihood of bacterial infection. Clinicians should base their choice of antibiotics on the most common bacterial etiology: Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis. Ideal candidates for therapy may include aminopenicillin with clavulanic acid, a macrolide, or a tetracycline.
Community-acquired pneumonia
The initial course of antibiotics should be at least 5 days for pneumonia and only extended after considering validated evidence of the patient’s clinical stability, such as resuming normal vital signs, mental activity, and the ability to eat. Multiple randomized, controlled trials have shown no improved benefit from longer courses, though longer courses are linked to increased adverse events and mortality.
Again, antibiotics used should “cover common pathogens, such as S. pneumoniae, H. influenzae, Mycoplasma pneumoniae, and Staphylococcus aureus, and atypical pathogens, such as Legionella species,” the authors wrote. Options include “amoxicillin, doxycycline, or a macrolide for healthy adults or a beta-lactam with a macrolide or a respiratory fluoroquinolone in patients with comorbidities.”
UTIs: Uncomplicated cystitis and pyelonephritis
For women’s bacterial cystitis – 75% of which is caused by Escherichia coli – the guidelines recommend nitrofurantoin for 5 days, trimethoprim-sulfamethoxazole for 3 days, or fosfomycin as a single dose. For uncomplicated pyelonephritis in both men and women, clinicians can consider fluoroquinolones for 5-7 days or trimethoprim-sulfamethoxazole for 14 days, depending on antibiotic susceptibility.
This recommendation does not include UTIs in women who are pregnant or UTIs with other functional abnormalities present, such as obstruction. The authors also intentionally left out acute bacterial prostatitis because of its complexity and how long it can take to treat.
Cellulitis
MRSA, which has been increasing in prevalence, is a leading cause of skin and soft-tissue infections, such as necrotizing infections, cellulitis, and erysipelas. Unless the patient has penetrating trauma, evidence of MRSA infection elsewhere, injection drug use, nasal colonization of MRSA, or systemic inflammatory response syndrome, the guidelines recommend a 5- to 6-day course of cephalosporin, penicillin, or clindamycin, extended only if the infection has not improved in 5 days. Further research can narrow down the most appropriate treatment course.
This guidance does not apply to purulent cellulitis, such as conditions with abscesses, furuncles, or carbuncles that typically require incision and drainage.
Continuing to get the message out
Dr. Fincher emphasized the importance of continuing to disseminate messaging for clinicians about reducing unnecessary antibiotic use.
“In medicine we are constantly bombarded with new information. It is those patients and disease states that we see and treat every day that are especially important for us as physicians and other clinicians to keep our skills and knowledge base up to date when it comes to use of antibiotics,” Dr. Fincher said in an interview. “We just need to continue to educate and push out the data, guidelines, and recommendations.”
Dr. Nolt added that it’s important to emphasize how to translate these national recommendations into local practices since local guidance can also raise awareness and encourage local compliance.
Other strategies for reducing overuse of antibiotics “include restriction on antibiotics available at health care systems (formulary restriction), not allowing use of antibiotics unless there is discussion about the patient’s case (preauthorization), and reviewing cases of patients on antibiotics and advising on next steps (prospective audit and feedback),” she said.
The research was funded by the ACP. Dr. Lee has received personal fees from this news organization and Prime Education. Dr. Fincher owns stock in Johnson & Johnson and Procter and Gamble. Dr. Nolt and the article’s coauthors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An antibiotic course of 5 days is usually just as effective as longer courses but with fewer side effects and decreased overall antibiotic exposure for a number of common bacterial conditions, according to new clinical guidelines published by the American College of Physicians.
The guidelines focus on treatment of uncomplicated cases involving pneumonia, urinary tract infections (UTIs), cellulitis, chronic obstructive pulmonary disease (COPD) exacerbations, and acute bronchitis. The goal of the guidelines is to continue improving antibiotic stewardship given the increasing threat of antibiotic resistance and the adverse effects of antibiotics.
“Any use of antibiotics (including necessary use) has downstream effects outside of treating infection,” Dawn Nolt, MD, MPH, a professor of pediatric infection disease at Oregon Health & Science University, Portland, said in an interview. Dr. Nolt was not involved in developing these guidelines. “Undesirable outcomes include allergic reactions, diarrhea, and antibiotic-resistant bacteria. When we reduce unnecessary antibiotic, we reduce undesirable outcomes,” she said.
According to background information in the paper, 1 in 10 patients receives an antibiotic prescription during visits, yet nearly a third of these (30%) are unnecessary and last too long, especially for sinusitis and bronchitis. Meanwhile, overuse of antibiotics, particularly broad-spectrum ones, leads to resistance and adverse effects in up to 20% of patients.
“Prescribing practices can vary based on the type of provider, the setting where the antibiotic is being prescribed, what geographic area you are looking at, the medical reason for which the antibiotic is being prescribed, the actual germ being targeted, and the type of patient,” Dr. Nolt said. “But this variability can be reduced when prescribing providers are aware and follow best practice standards as through this article.”
The new ACP guidelines are a distillation of recommendations from preexisting infectious disease organizations, Dr. Nolt said, but aimed specifically at those practicing internal medicine.
“We define appropriate antibiotic use as prescribing the right antibiotic at the right dose for the right duration for a specific condition,” Rachael A. Lee, MD, MSPH, of the University of Alabama at Birmingham, and colleagues wrote in the article detailing the new guidelines. “Despite evidence and guidelines supporting shorter durations of antibiotic use, many physicians do not prescribe short-course therapy, frequently defaulting to 10-day courses regardless of the condition.”
The reasons for this default response vary. Though some clinicians prescribe longer courses specifically to prevent antibiotic resistance, no evidence shows that continuing to take antibiotics after symptoms have resolved actually reduces likelihood of resistance, the authors noted.
“In fact, resistance is a documented side effect of prolonged antibiotic use due to natural selection pressure,” they wrote.
Another common reason is habit.
“This was the ‘conventional wisdom’ for so long, just trying to make sure all bacteria causing the infection were completely eradicated, with no stragglers that had been exposed to the antibiotic but were not gone and now could evolve into resistant organisms,” Jacqueline W. Fincher, MD, a primary care physician and president of the ACP, said in an interview. “While antibiotic stewardship has been very important for over a decade, we now have more recent head-to-head studies/data showing that, in these four conditions, shorter courses of treatment are just as efficacious with less side effects and adverse events.”
The researchers reviewed all existing clinical guidelines related to bronchitis with COPD exacerbations, community-acquired pneumonia, UTIs, and cellulitis, as well as any other relevant studies in the literature. Although they did not conduct a formal systematic review, they compiled the guidelines specifically for all internists, family physicians and other clinicians caring for patients with these conditions.
“Although most patients with these infections will be seen in the outpatient setting, these best-practice advice statements also apply to patients who present in the inpatient setting,” the authors wrote. They also note the importance of ensuring the patient has the correct diagnosis and appropriate corresponding antibiotic prescription. “If a patient is not improving with appropriate antibiotics, it is important for the clinician to reassess for other causes of symptoms rather than defaulting to a longer duration of antibiotic therapy,” they wrote, calling a longer course “the exception and not the rule.”
Acute bronchitis with COPD exacerbations
Antibiotic treatment for COPD exacerbations and acute uncomplicated bronchitis with signs of a bacterial infection should last no longer than 5 days. The authors define this condition as an acute respiratory infection with a normal chest x-ray, most often caused by a virus. Although patients with bronchitis do not automatically need antibiotics if there’s no evidence of pneumonia, the authors did advise antibiotics in cases involving COPD and a high likelihood of bacterial infection. Clinicians should base their choice of antibiotics on the most common bacterial etiology: Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis. Ideal candidates for therapy may include aminopenicillin with clavulanic acid, a macrolide, or a tetracycline.
Community-acquired pneumonia
The initial course of antibiotics should be at least 5 days for pneumonia and only extended after considering validated evidence of the patient’s clinical stability, such as resuming normal vital signs, mental activity, and the ability to eat. Multiple randomized, controlled trials have shown no improved benefit from longer courses, though longer courses are linked to increased adverse events and mortality.
Again, antibiotics used should “cover common pathogens, such as S. pneumoniae, H. influenzae, Mycoplasma pneumoniae, and Staphylococcus aureus, and atypical pathogens, such as Legionella species,” the authors wrote. Options include “amoxicillin, doxycycline, or a macrolide for healthy adults or a beta-lactam with a macrolide or a respiratory fluoroquinolone in patients with comorbidities.”
UTIs: Uncomplicated cystitis and pyelonephritis
For women’s bacterial cystitis – 75% of which is caused by Escherichia coli – the guidelines recommend nitrofurantoin for 5 days, trimethoprim-sulfamethoxazole for 3 days, or fosfomycin as a single dose. For uncomplicated pyelonephritis in both men and women, clinicians can consider fluoroquinolones for 5-7 days or trimethoprim-sulfamethoxazole for 14 days, depending on antibiotic susceptibility.
This recommendation does not include UTIs in women who are pregnant or UTIs with other functional abnormalities present, such as obstruction. The authors also intentionally left out acute bacterial prostatitis because of its complexity and how long it can take to treat.
Cellulitis
MRSA, which has been increasing in prevalence, is a leading cause of skin and soft-tissue infections, such as necrotizing infections, cellulitis, and erysipelas. Unless the patient has penetrating trauma, evidence of MRSA infection elsewhere, injection drug use, nasal colonization of MRSA, or systemic inflammatory response syndrome, the guidelines recommend a 5- to 6-day course of cephalosporin, penicillin, or clindamycin, extended only if the infection has not improved in 5 days. Further research can narrow down the most appropriate treatment course.
This guidance does not apply to purulent cellulitis, such as conditions with abscesses, furuncles, or carbuncles that typically require incision and drainage.
Continuing to get the message out
Dr. Fincher emphasized the importance of continuing to disseminate messaging for clinicians about reducing unnecessary antibiotic use.
“In medicine we are constantly bombarded with new information. It is those patients and disease states that we see and treat every day that are especially important for us as physicians and other clinicians to keep our skills and knowledge base up to date when it comes to use of antibiotics,” Dr. Fincher said in an interview. “We just need to continue to educate and push out the data, guidelines, and recommendations.”
Dr. Nolt added that it’s important to emphasize how to translate these national recommendations into local practices since local guidance can also raise awareness and encourage local compliance.
Other strategies for reducing overuse of antibiotics “include restriction on antibiotics available at health care systems (formulary restriction), not allowing use of antibiotics unless there is discussion about the patient’s case (preauthorization), and reviewing cases of patients on antibiotics and advising on next steps (prospective audit and feedback),” she said.
The research was funded by the ACP. Dr. Lee has received personal fees from this news organization and Prime Education. Dr. Fincher owns stock in Johnson & Johnson and Procter and Gamble. Dr. Nolt and the article’s coauthors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers stress importance of second COVID-19 vaccine dose for infliximab users
Patients being treated with infliximab had weakened immune responses to the first dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, compared with patients on vedolizumab (Entyvio), although a very significant number of patients from both groups seroconverted after their second dose, according to a new U.K. study of patients with inflammatory bowel disease (IBD).
“Antibody testing and adapted vaccine schedules should be considered to protect these at-risk patients,” Nicholas A. Kennedy, PhD, MBBS, of the University of Exeter (England) and colleagues wrote in a preprint published March 29 on MedRxiv.
Infliximab is an anti–tumor necrosis factor (anti-TNF) monoclonal antibody that’s approved to treat adult and pediatric Crohn’s disease and ulcerative colitis, as well as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, whereas vedolizumab, a gut selective anti-integrin alpha4beta7 monoclonal antibody that is not associated with impaired systemic immune responses, is approved to treat Crohn’s disease and ulcerative colitis in adults.
A previous study from Kennedy and colleagues revealed that IBD patients on infliximab showed a weakened COVID-19 antibody response compared with patients on vedolizumab. To determine if treatment with anti-TNF drugs impacted the efficacy of the first shot of these two-dose COVID-19 vaccines, the researchers used data from the CLARITY IBD study to assess 865 infliximab- and 428 vedolizumab-treated participants without evidence of prior SARS-CoV-2 infection who had received uninterrupted biologic therapy since being recruited between Sept. 22 and Dec. 23, 2020.
In the 3-10 weeks after initial vaccination, geometric mean concentrations for SARS-CoV-2 anti-spike protein receptor-binding protein antibodies were lower in patients on infliximab, compared with patients on vedolizumab for both the Pfizer (6.0 U/mL [5.9] versus 28.8 U/mL [5.4], P < .0001) and AstraZeneca (4.7 U/mL [4.9] versus 13.8 U/mL [5.9]; P < .0001) vaccines. The researchers’ multivariable models reinforced those findings, with antibody concentrations lower in infliximab-treated patients for both the Pfizer (fold change, 0.29; 95% confidence interval, 0.21-0.40; P < .0001) and AstraZeneca (FC, 0.39; 95% CI, 0.30-0.51; P < .0001) vaccines.
After second doses of the two-dose Pfizer vaccine, 85% of patients on infliximab and 86% of patients on vedolizumab seroconverted (P = .68); similarly high seroconversion rates were seen in patients who had been infected with SARS-CoV-2 prior to receiving either vaccine. Several patient characteristics were associated with lower antibody concentrations regardless of vaccine type: being 60 years or older, use of immunomodulators, having Crohn’s disease, and being a smoker. Alternatively, non-White ethnicity was associated with higher antibody concentrations.
Evidence has ‘unclear clinical significance’
“These data, which require peer review, do not change my opinion on the safety and efficacy of COVID-19 vaccines in patients taking TNF inhibitors such as infliximab as monotherapy for the treatment of psoriatic disease,” Joel M. Gelfand MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, said in an interview.
“First, two peer-reviewed studies found good antibody response in patients on TNF inhibitors receiving COVID-19 vaccines (doi: 10.1136/annrheumdis-2021-220289; 10.1136/annrheumdis-2021-220272). Second, antibody responses were robust in the small cohort that received the second dose of a COVID-19 vaccine. We already know that, for the two messenger RNA-based vaccines available under emergency use authorization in the U.S., a second dose is required for optimal efficacy. Thus, evidence of a reduced antibody response after just one dose is of unclear clinical significance. Third, antibody responses are only a surrogate marker, and a low antibody response doesn’t necessarily mean the patient will not be protected by the vaccine.”
Focus on the second dose of a two-dose regimen
“Tell me about the response in people who got both doses of a vaccine that you’re supposed to get both doses of,” Jeffrey Curtis, MD, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham, said in an interview. “The number of patients in that subset was small [n = 27] but in my opinion that’s the most clinically relevant analysis and the one that patients and clinicians want answered.”
He also emphasized the uncertainty around what ‘protection’ means in these early days of studying COVID-19 vaccine responses. “You can define seroprotection or seroconversion as some absolute level of an antibody response, but if you want to say ‘Mrs. Smith, your antibody level was X,’ on whatever arbitrary scale with whoever’s arbitrary lab test, nobody actually knows that Mrs. Smith is now protected from SARS-CoV-2, or how protected,” he said.
“What is not terribly controversial is: If you can’t detect antibodies, the vaccine didn’t ‘take,’ if you will. But if I tell you that the mean antibody level was X with one drug and then 2X with another drug, does that mean that you’re twice as protected? We don’t know that. I’m fearful that people are looking at these studies and thinking that more is better. It might be, but we don’t know that to be true.”
Debating the cause of weakened immune responses
“The biological plausibility of being on an anti-TNF affecting your immune reaction to a messenger RNA or even a replication-deficient viral vector vaccine doesn’t make sense,” David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the National Scientific Advisory Committee of the Crohn’s and Colitis Foundation, said in an interview.
“I’m sure immunologists may differ with me on this, but given what we have come to appreciate about these vaccine mechanisms, this finding doesn’t make intuitive sense. So we need to make sure that, when this happens, we look to the next studies and try to understand, was there any other confounder that may have resulted in these findings that was not adequately adjusted for or addressed in some other way?
“When you have a study of this size, you argue, ‘Because it’s so large, any effect that was seen must be real,’ ” he added. “Alternatively, to have a study of this size, by its very nature you are limited in being able to control for certain other factors or differences between the groups.”
That said, he commended the authors for their study and acknowledged the potential questions it raises about the single-shot Johnson & Johnson vaccine. “If you only get one and you’re on infliximab, this study implies that maybe that’s not enough,” he said. “Despite the fact that Johnson & Johnson was approved as a single dose, it may be necessary to think about it as the first of two, or maybe it’s not the preferred vaccine in this group of patients.”
The study was supported by the Royal Devon and Exeter and Hull University Hospital Foundation NHS Trusts and unrestricted educational grants from Biogen (Switzerland), Celltrion Healthcare (South Korea), Galapagos NV (Belgium), and F. Hoffmann-La Roche (Switzerland). The authors acknowledged numerous potential conflicts of interest, including receiving grants, personal fees, and nonfinancial support from various pharmaceutical companies.
Patients being treated with infliximab had weakened immune responses to the first dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, compared with patients on vedolizumab (Entyvio), although a very significant number of patients from both groups seroconverted after their second dose, according to a new U.K. study of patients with inflammatory bowel disease (IBD).
“Antibody testing and adapted vaccine schedules should be considered to protect these at-risk patients,” Nicholas A. Kennedy, PhD, MBBS, of the University of Exeter (England) and colleagues wrote in a preprint published March 29 on MedRxiv.
Infliximab is an anti–tumor necrosis factor (anti-TNF) monoclonal antibody that’s approved to treat adult and pediatric Crohn’s disease and ulcerative colitis, as well as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, whereas vedolizumab, a gut selective anti-integrin alpha4beta7 monoclonal antibody that is not associated with impaired systemic immune responses, is approved to treat Crohn’s disease and ulcerative colitis in adults.
A previous study from Kennedy and colleagues revealed that IBD patients on infliximab showed a weakened COVID-19 antibody response compared with patients on vedolizumab. To determine if treatment with anti-TNF drugs impacted the efficacy of the first shot of these two-dose COVID-19 vaccines, the researchers used data from the CLARITY IBD study to assess 865 infliximab- and 428 vedolizumab-treated participants without evidence of prior SARS-CoV-2 infection who had received uninterrupted biologic therapy since being recruited between Sept. 22 and Dec. 23, 2020.
In the 3-10 weeks after initial vaccination, geometric mean concentrations for SARS-CoV-2 anti-spike protein receptor-binding protein antibodies were lower in patients on infliximab, compared with patients on vedolizumab for both the Pfizer (6.0 U/mL [5.9] versus 28.8 U/mL [5.4], P < .0001) and AstraZeneca (4.7 U/mL [4.9] versus 13.8 U/mL [5.9]; P < .0001) vaccines. The researchers’ multivariable models reinforced those findings, with antibody concentrations lower in infliximab-treated patients for both the Pfizer (fold change, 0.29; 95% confidence interval, 0.21-0.40; P < .0001) and AstraZeneca (FC, 0.39; 95% CI, 0.30-0.51; P < .0001) vaccines.
After second doses of the two-dose Pfizer vaccine, 85% of patients on infliximab and 86% of patients on vedolizumab seroconverted (P = .68); similarly high seroconversion rates were seen in patients who had been infected with SARS-CoV-2 prior to receiving either vaccine. Several patient characteristics were associated with lower antibody concentrations regardless of vaccine type: being 60 years or older, use of immunomodulators, having Crohn’s disease, and being a smoker. Alternatively, non-White ethnicity was associated with higher antibody concentrations.
Evidence has ‘unclear clinical significance’
“These data, which require peer review, do not change my opinion on the safety and efficacy of COVID-19 vaccines in patients taking TNF inhibitors such as infliximab as monotherapy for the treatment of psoriatic disease,” Joel M. Gelfand MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, said in an interview.
“First, two peer-reviewed studies found good antibody response in patients on TNF inhibitors receiving COVID-19 vaccines (doi: 10.1136/annrheumdis-2021-220289; 10.1136/annrheumdis-2021-220272). Second, antibody responses were robust in the small cohort that received the second dose of a COVID-19 vaccine. We already know that, for the two messenger RNA-based vaccines available under emergency use authorization in the U.S., a second dose is required for optimal efficacy. Thus, evidence of a reduced antibody response after just one dose is of unclear clinical significance. Third, antibody responses are only a surrogate marker, and a low antibody response doesn’t necessarily mean the patient will not be protected by the vaccine.”
Focus on the second dose of a two-dose regimen
“Tell me about the response in people who got both doses of a vaccine that you’re supposed to get both doses of,” Jeffrey Curtis, MD, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham, said in an interview. “The number of patients in that subset was small [n = 27] but in my opinion that’s the most clinically relevant analysis and the one that patients and clinicians want answered.”
He also emphasized the uncertainty around what ‘protection’ means in these early days of studying COVID-19 vaccine responses. “You can define seroprotection or seroconversion as some absolute level of an antibody response, but if you want to say ‘Mrs. Smith, your antibody level was X,’ on whatever arbitrary scale with whoever’s arbitrary lab test, nobody actually knows that Mrs. Smith is now protected from SARS-CoV-2, or how protected,” he said.
“What is not terribly controversial is: If you can’t detect antibodies, the vaccine didn’t ‘take,’ if you will. But if I tell you that the mean antibody level was X with one drug and then 2X with another drug, does that mean that you’re twice as protected? We don’t know that. I’m fearful that people are looking at these studies and thinking that more is better. It might be, but we don’t know that to be true.”
Debating the cause of weakened immune responses
“The biological plausibility of being on an anti-TNF affecting your immune reaction to a messenger RNA or even a replication-deficient viral vector vaccine doesn’t make sense,” David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the National Scientific Advisory Committee of the Crohn’s and Colitis Foundation, said in an interview.
“I’m sure immunologists may differ with me on this, but given what we have come to appreciate about these vaccine mechanisms, this finding doesn’t make intuitive sense. So we need to make sure that, when this happens, we look to the next studies and try to understand, was there any other confounder that may have resulted in these findings that was not adequately adjusted for or addressed in some other way?
“When you have a study of this size, you argue, ‘Because it’s so large, any effect that was seen must be real,’ ” he added. “Alternatively, to have a study of this size, by its very nature you are limited in being able to control for certain other factors or differences between the groups.”
That said, he commended the authors for their study and acknowledged the potential questions it raises about the single-shot Johnson & Johnson vaccine. “If you only get one and you’re on infliximab, this study implies that maybe that’s not enough,” he said. “Despite the fact that Johnson & Johnson was approved as a single dose, it may be necessary to think about it as the first of two, or maybe it’s not the preferred vaccine in this group of patients.”
The study was supported by the Royal Devon and Exeter and Hull University Hospital Foundation NHS Trusts and unrestricted educational grants from Biogen (Switzerland), Celltrion Healthcare (South Korea), Galapagos NV (Belgium), and F. Hoffmann-La Roche (Switzerland). The authors acknowledged numerous potential conflicts of interest, including receiving grants, personal fees, and nonfinancial support from various pharmaceutical companies.
Patients being treated with infliximab had weakened immune responses to the first dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, compared with patients on vedolizumab (Entyvio), although a very significant number of patients from both groups seroconverted after their second dose, according to a new U.K. study of patients with inflammatory bowel disease (IBD).
“Antibody testing and adapted vaccine schedules should be considered to protect these at-risk patients,” Nicholas A. Kennedy, PhD, MBBS, of the University of Exeter (England) and colleagues wrote in a preprint published March 29 on MedRxiv.
Infliximab is an anti–tumor necrosis factor (anti-TNF) monoclonal antibody that’s approved to treat adult and pediatric Crohn’s disease and ulcerative colitis, as well as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, whereas vedolizumab, a gut selective anti-integrin alpha4beta7 monoclonal antibody that is not associated with impaired systemic immune responses, is approved to treat Crohn’s disease and ulcerative colitis in adults.
A previous study from Kennedy and colleagues revealed that IBD patients on infliximab showed a weakened COVID-19 antibody response compared with patients on vedolizumab. To determine if treatment with anti-TNF drugs impacted the efficacy of the first shot of these two-dose COVID-19 vaccines, the researchers used data from the CLARITY IBD study to assess 865 infliximab- and 428 vedolizumab-treated participants without evidence of prior SARS-CoV-2 infection who had received uninterrupted biologic therapy since being recruited between Sept. 22 and Dec. 23, 2020.
In the 3-10 weeks after initial vaccination, geometric mean concentrations for SARS-CoV-2 anti-spike protein receptor-binding protein antibodies were lower in patients on infliximab, compared with patients on vedolizumab for both the Pfizer (6.0 U/mL [5.9] versus 28.8 U/mL [5.4], P < .0001) and AstraZeneca (4.7 U/mL [4.9] versus 13.8 U/mL [5.9]; P < .0001) vaccines. The researchers’ multivariable models reinforced those findings, with antibody concentrations lower in infliximab-treated patients for both the Pfizer (fold change, 0.29; 95% confidence interval, 0.21-0.40; P < .0001) and AstraZeneca (FC, 0.39; 95% CI, 0.30-0.51; P < .0001) vaccines.
After second doses of the two-dose Pfizer vaccine, 85% of patients on infliximab and 86% of patients on vedolizumab seroconverted (P = .68); similarly high seroconversion rates were seen in patients who had been infected with SARS-CoV-2 prior to receiving either vaccine. Several patient characteristics were associated with lower antibody concentrations regardless of vaccine type: being 60 years or older, use of immunomodulators, having Crohn’s disease, and being a smoker. Alternatively, non-White ethnicity was associated with higher antibody concentrations.
Evidence has ‘unclear clinical significance’
“These data, which require peer review, do not change my opinion on the safety and efficacy of COVID-19 vaccines in patients taking TNF inhibitors such as infliximab as monotherapy for the treatment of psoriatic disease,” Joel M. Gelfand MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, said in an interview.
“First, two peer-reviewed studies found good antibody response in patients on TNF inhibitors receiving COVID-19 vaccines (doi: 10.1136/annrheumdis-2021-220289; 10.1136/annrheumdis-2021-220272). Second, antibody responses were robust in the small cohort that received the second dose of a COVID-19 vaccine. We already know that, for the two messenger RNA-based vaccines available under emergency use authorization in the U.S., a second dose is required for optimal efficacy. Thus, evidence of a reduced antibody response after just one dose is of unclear clinical significance. Third, antibody responses are only a surrogate marker, and a low antibody response doesn’t necessarily mean the patient will not be protected by the vaccine.”
Focus on the second dose of a two-dose regimen
“Tell me about the response in people who got both doses of a vaccine that you’re supposed to get both doses of,” Jeffrey Curtis, MD, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham, said in an interview. “The number of patients in that subset was small [n = 27] but in my opinion that’s the most clinically relevant analysis and the one that patients and clinicians want answered.”
He also emphasized the uncertainty around what ‘protection’ means in these early days of studying COVID-19 vaccine responses. “You can define seroprotection or seroconversion as some absolute level of an antibody response, but if you want to say ‘Mrs. Smith, your antibody level was X,’ on whatever arbitrary scale with whoever’s arbitrary lab test, nobody actually knows that Mrs. Smith is now protected from SARS-CoV-2, or how protected,” he said.
“What is not terribly controversial is: If you can’t detect antibodies, the vaccine didn’t ‘take,’ if you will. But if I tell you that the mean antibody level was X with one drug and then 2X with another drug, does that mean that you’re twice as protected? We don’t know that. I’m fearful that people are looking at these studies and thinking that more is better. It might be, but we don’t know that to be true.”
Debating the cause of weakened immune responses
“The biological plausibility of being on an anti-TNF affecting your immune reaction to a messenger RNA or even a replication-deficient viral vector vaccine doesn’t make sense,” David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the National Scientific Advisory Committee of the Crohn’s and Colitis Foundation, said in an interview.
“I’m sure immunologists may differ with me on this, but given what we have come to appreciate about these vaccine mechanisms, this finding doesn’t make intuitive sense. So we need to make sure that, when this happens, we look to the next studies and try to understand, was there any other confounder that may have resulted in these findings that was not adequately adjusted for or addressed in some other way?
“When you have a study of this size, you argue, ‘Because it’s so large, any effect that was seen must be real,’ ” he added. “Alternatively, to have a study of this size, by its very nature you are limited in being able to control for certain other factors or differences between the groups.”
That said, he commended the authors for their study and acknowledged the potential questions it raises about the single-shot Johnson & Johnson vaccine. “If you only get one and you’re on infliximab, this study implies that maybe that’s not enough,” he said. “Despite the fact that Johnson & Johnson was approved as a single dose, it may be necessary to think about it as the first of two, or maybe it’s not the preferred vaccine in this group of patients.”
The study was supported by the Royal Devon and Exeter and Hull University Hospital Foundation NHS Trusts and unrestricted educational grants from Biogen (Switzerland), Celltrion Healthcare (South Korea), Galapagos NV (Belgium), and F. Hoffmann-La Roche (Switzerland). The authors acknowledged numerous potential conflicts of interest, including receiving grants, personal fees, and nonfinancial support from various pharmaceutical companies.
FROM MEDRXIV