User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'medstat-accordion-set article-series')]
Clinical Edge Commentary: RA July 2021
Several recent studies have evaluated risks of therapy in rheumatoid arthritis (RA). One question regarding treatment of early RA is whether different initial treatment strategies confer different risks. In a systematic review with network meta-analysis, Adas et al reviewed differences between methotrexate, biologic disease-modifying antirheumatic drug (bDMARD), and steroid use in early RA. Overall, risk of serious adverse events was higher with bDMARD monotherapy than methotrexate monotherapy. Of note, while generally long-term steroid use is disfavored due to adverse effects, serious adverse events were not increased in patients treated with methotrexate and steroids together. The size of differences in risk was small and study heterogeneity, including the class of bDMARDs, limits generalizability of this information; thus, variations in the studies themselves may account for these differences.
Pazmino et al also looked at treatment strategies in early RA in a post hoc analysis of participants at “low-risk” for poor prognosis in the CareRA trial, in which patients were randomized to step-up methotrexate without glucocorticoids or step-down with glucocorticoids. While pain scores and disease activity scores were similar among the two groups, analgesic use (including non-steroidal anti-inflammatory drugs [NSAIDs] and opioids) was significantly lower among those randomized to the glucocorticoid-bridging arm. Though this information is reassuring as to the utility of glucocorticoids, it is not clear that this correlation is broadly applicable, for example, among the “higher-risk” patients who might otherwise be more likely to receive glucocorticoids.
A recent analysis of the COVID-19 global rheumatology alliance physician registry by Sparks et al of cases of COVID-19 in patients with rheumatic disease looked more specifically at COVID-19 outcomes in patients with RA on biologic therapy. These are of interest due both to the risk of immunosuppression overall as well as the use of immunosuppressive medications in COVID-19-associated hyperinflammation. The study evaluated outcomes of hospitalization (including respiratory support and mortality). While hospitalization is difficult to evaluate as an outcome without knowing the background rate of COVID in the different areas, of the hospitalized patients, patients who used Janus kinase inhibitors (JAKi) and rituximab received oxygen or ventilator support and had higher mortality than those who were on abatacept, IL-6 inhibitors, or TNF inhibitors. Wider interpretation is difficult due to lack of knowledge of when medications were given (including rituximab dosing), but the results suggest that concern is warranted in improving outcomes for patients with RA on these therapies.
Finally, regarding the well-known cardiovascular risk associated with RA, several observational studies have suggested that methotrexate is associated with reduction in risk of cardiovascular events. This cohort study of the Veterans Affairs RA registry followed over 2000 patients for a mean of about 5 years; a reduction in incidence of cardiovascular events was associated with methotrexate use, independent of age, body mass index (BMI), cardiovascular risk factors, RA disease activity, and other RA therapies. It may be that methotrexate use is associated with an unknown mediator of cardiovascular disease not evaluated in this study, such as reduced glucocorticoid or NSAID use, but this area deserves further investigation.
Several recent studies have evaluated risks of therapy in rheumatoid arthritis (RA). One question regarding treatment of early RA is whether different initial treatment strategies confer different risks. In a systematic review with network meta-analysis, Adas et al reviewed differences between methotrexate, biologic disease-modifying antirheumatic drug (bDMARD), and steroid use in early RA. Overall, risk of serious adverse events was higher with bDMARD monotherapy than methotrexate monotherapy. Of note, while generally long-term steroid use is disfavored due to adverse effects, serious adverse events were not increased in patients treated with methotrexate and steroids together. The size of differences in risk was small and study heterogeneity, including the class of bDMARDs, limits generalizability of this information; thus, variations in the studies themselves may account for these differences.
Pazmino et al also looked at treatment strategies in early RA in a post hoc analysis of participants at “low-risk” for poor prognosis in the CareRA trial, in which patients were randomized to step-up methotrexate without glucocorticoids or step-down with glucocorticoids. While pain scores and disease activity scores were similar among the two groups, analgesic use (including non-steroidal anti-inflammatory drugs [NSAIDs] and opioids) was significantly lower among those randomized to the glucocorticoid-bridging arm. Though this information is reassuring as to the utility of glucocorticoids, it is not clear that this correlation is broadly applicable, for example, among the “higher-risk” patients who might otherwise be more likely to receive glucocorticoids.
A recent analysis of the COVID-19 global rheumatology alliance physician registry by Sparks et al of cases of COVID-19 in patients with rheumatic disease looked more specifically at COVID-19 outcomes in patients with RA on biologic therapy. These are of interest due both to the risk of immunosuppression overall as well as the use of immunosuppressive medications in COVID-19-associated hyperinflammation. The study evaluated outcomes of hospitalization (including respiratory support and mortality). While hospitalization is difficult to evaluate as an outcome without knowing the background rate of COVID in the different areas, of the hospitalized patients, patients who used Janus kinase inhibitors (JAKi) and rituximab received oxygen or ventilator support and had higher mortality than those who were on abatacept, IL-6 inhibitors, or TNF inhibitors. Wider interpretation is difficult due to lack of knowledge of when medications were given (including rituximab dosing), but the results suggest that concern is warranted in improving outcomes for patients with RA on these therapies.
Finally, regarding the well-known cardiovascular risk associated with RA, several observational studies have suggested that methotrexate is associated with reduction in risk of cardiovascular events. This cohort study of the Veterans Affairs RA registry followed over 2000 patients for a mean of about 5 years; a reduction in incidence of cardiovascular events was associated with methotrexate use, independent of age, body mass index (BMI), cardiovascular risk factors, RA disease activity, and other RA therapies. It may be that methotrexate use is associated with an unknown mediator of cardiovascular disease not evaluated in this study, such as reduced glucocorticoid or NSAID use, but this area deserves further investigation.
Several recent studies have evaluated risks of therapy in rheumatoid arthritis (RA). One question regarding treatment of early RA is whether different initial treatment strategies confer different risks. In a systematic review with network meta-analysis, Adas et al reviewed differences between methotrexate, biologic disease-modifying antirheumatic drug (bDMARD), and steroid use in early RA. Overall, risk of serious adverse events was higher with bDMARD monotherapy than methotrexate monotherapy. Of note, while generally long-term steroid use is disfavored due to adverse effects, serious adverse events were not increased in patients treated with methotrexate and steroids together. The size of differences in risk was small and study heterogeneity, including the class of bDMARDs, limits generalizability of this information; thus, variations in the studies themselves may account for these differences.
Pazmino et al also looked at treatment strategies in early RA in a post hoc analysis of participants at “low-risk” for poor prognosis in the CareRA trial, in which patients were randomized to step-up methotrexate without glucocorticoids or step-down with glucocorticoids. While pain scores and disease activity scores were similar among the two groups, analgesic use (including non-steroidal anti-inflammatory drugs [NSAIDs] and opioids) was significantly lower among those randomized to the glucocorticoid-bridging arm. Though this information is reassuring as to the utility of glucocorticoids, it is not clear that this correlation is broadly applicable, for example, among the “higher-risk” patients who might otherwise be more likely to receive glucocorticoids.
A recent analysis of the COVID-19 global rheumatology alliance physician registry by Sparks et al of cases of COVID-19 in patients with rheumatic disease looked more specifically at COVID-19 outcomes in patients with RA on biologic therapy. These are of interest due both to the risk of immunosuppression overall as well as the use of immunosuppressive medications in COVID-19-associated hyperinflammation. The study evaluated outcomes of hospitalization (including respiratory support and mortality). While hospitalization is difficult to evaluate as an outcome without knowing the background rate of COVID in the different areas, of the hospitalized patients, patients who used Janus kinase inhibitors (JAKi) and rituximab received oxygen or ventilator support and had higher mortality than those who were on abatacept, IL-6 inhibitors, or TNF inhibitors. Wider interpretation is difficult due to lack of knowledge of when medications were given (including rituximab dosing), but the results suggest that concern is warranted in improving outcomes for patients with RA on these therapies.
Finally, regarding the well-known cardiovascular risk associated with RA, several observational studies have suggested that methotrexate is associated with reduction in risk of cardiovascular events. This cohort study of the Veterans Affairs RA registry followed over 2000 patients for a mean of about 5 years; a reduction in incidence of cardiovascular events was associated with methotrexate use, independent of age, body mass index (BMI), cardiovascular risk factors, RA disease activity, and other RA therapies. It may be that methotrexate use is associated with an unknown mediator of cardiovascular disease not evaluated in this study, such as reduced glucocorticoid or NSAID use, but this area deserves further investigation.
Female doctors of color say they feel pressure to change their look
It started when a Latina doctor tweeted that she lost points on a practical exam in medical school because of her hoop earrings, with the evaluator writing “earrings, unprofessional.”
That led other female doctors to cite their own experiences, reported The Lily, a Washington Post publication aimed at millennial women. Many women posted photos of themselves wearing hoops, which have long been associated with Latina and African American women, the outlet said.
“There’s a big movement to police women of color and how they present themselves in medical spaces,” said Briana Christophers, an MD-PhD student at the Tri-Institutional MD-PhD Program in New York. “I think in part it’s a way of trying to make people who don’t usually fit the mold, fit the mold.”
Ms. Christophers, who identifies as Latina, said she was urged to wear a black or navy suit when interviewing for doctorate programs. She wore a black suit with a lavender blouse and received comments about that – some positive, some not, she said.
“Sometimes you don’t know how to interpret those sorts of comments,” Ms. Christophers said. “Do you remember because you like the shirt, or because you don’t think I should have done that?”
Doctors of color still stand out in American medicine. The Lily cited the Association of American Medical Colleges as saying that in 2018, Hispanics made up 5.8% of active American doctors and African Americans made up 5%.
Studies show that medical professionals of color often don’t receive the same respect as their White counterparts, with some people questioning whether they’re actually doctors.
“At work, wearing my white coat that has my name pretty big on it with a badge that says doctor on it, I still get asked if I’m the environmental services staff,” Alexandra Sims, MD, a pediatrician in Cincinnati, told The Lily. “I think it just demonstrates how deeply ingrained bias, racism, and sexism are in society and that we have a lot of work to do to disrupt that.”
Dr. Sims said the tweet about hoop earrings led her to wonder about daily decisions she makes about dress.
“Am I too much? Is this too much? Is this earring too big? Is this nail polish color too loud? And how will that be received at work?” she said, noting that she may opt not to wear hoops in certain situations, such as when she’s dealing with a grabby baby.
Monica Verduzco-Gutierrez, MD, professor and chair of the department of rehabilitation medicine at University of Texas Health, San Antonio, said doctors should be judged on the care they provide, not their appearance.
“Judging someone based on their earrings or their jumpsuit or whatever else that they’re noticing about the student is not an appropriate way to judge the student’s ability to take care of a patient,” Dr. Verduzco-Gutierrez said, noting that she was not speaking on behalf of the school.
A version of this article was first published on WebMD.com .
It started when a Latina doctor tweeted that she lost points on a practical exam in medical school because of her hoop earrings, with the evaluator writing “earrings, unprofessional.”
That led other female doctors to cite their own experiences, reported The Lily, a Washington Post publication aimed at millennial women. Many women posted photos of themselves wearing hoops, which have long been associated with Latina and African American women, the outlet said.
“There’s a big movement to police women of color and how they present themselves in medical spaces,” said Briana Christophers, an MD-PhD student at the Tri-Institutional MD-PhD Program in New York. “I think in part it’s a way of trying to make people who don’t usually fit the mold, fit the mold.”
Ms. Christophers, who identifies as Latina, said she was urged to wear a black or navy suit when interviewing for doctorate programs. She wore a black suit with a lavender blouse and received comments about that – some positive, some not, she said.
“Sometimes you don’t know how to interpret those sorts of comments,” Ms. Christophers said. “Do you remember because you like the shirt, or because you don’t think I should have done that?”
Doctors of color still stand out in American medicine. The Lily cited the Association of American Medical Colleges as saying that in 2018, Hispanics made up 5.8% of active American doctors and African Americans made up 5%.
Studies show that medical professionals of color often don’t receive the same respect as their White counterparts, with some people questioning whether they’re actually doctors.
“At work, wearing my white coat that has my name pretty big on it with a badge that says doctor on it, I still get asked if I’m the environmental services staff,” Alexandra Sims, MD, a pediatrician in Cincinnati, told The Lily. “I think it just demonstrates how deeply ingrained bias, racism, and sexism are in society and that we have a lot of work to do to disrupt that.”
Dr. Sims said the tweet about hoop earrings led her to wonder about daily decisions she makes about dress.
“Am I too much? Is this too much? Is this earring too big? Is this nail polish color too loud? And how will that be received at work?” she said, noting that she may opt not to wear hoops in certain situations, such as when she’s dealing with a grabby baby.
Monica Verduzco-Gutierrez, MD, professor and chair of the department of rehabilitation medicine at University of Texas Health, San Antonio, said doctors should be judged on the care they provide, not their appearance.
“Judging someone based on their earrings or their jumpsuit or whatever else that they’re noticing about the student is not an appropriate way to judge the student’s ability to take care of a patient,” Dr. Verduzco-Gutierrez said, noting that she was not speaking on behalf of the school.
A version of this article was first published on WebMD.com .
It started when a Latina doctor tweeted that she lost points on a practical exam in medical school because of her hoop earrings, with the evaluator writing “earrings, unprofessional.”
That led other female doctors to cite their own experiences, reported The Lily, a Washington Post publication aimed at millennial women. Many women posted photos of themselves wearing hoops, which have long been associated with Latina and African American women, the outlet said.
“There’s a big movement to police women of color and how they present themselves in medical spaces,” said Briana Christophers, an MD-PhD student at the Tri-Institutional MD-PhD Program in New York. “I think in part it’s a way of trying to make people who don’t usually fit the mold, fit the mold.”
Ms. Christophers, who identifies as Latina, said she was urged to wear a black or navy suit when interviewing for doctorate programs. She wore a black suit with a lavender blouse and received comments about that – some positive, some not, she said.
“Sometimes you don’t know how to interpret those sorts of comments,” Ms. Christophers said. “Do you remember because you like the shirt, or because you don’t think I should have done that?”
Doctors of color still stand out in American medicine. The Lily cited the Association of American Medical Colleges as saying that in 2018, Hispanics made up 5.8% of active American doctors and African Americans made up 5%.
Studies show that medical professionals of color often don’t receive the same respect as their White counterparts, with some people questioning whether they’re actually doctors.
“At work, wearing my white coat that has my name pretty big on it with a badge that says doctor on it, I still get asked if I’m the environmental services staff,” Alexandra Sims, MD, a pediatrician in Cincinnati, told The Lily. “I think it just demonstrates how deeply ingrained bias, racism, and sexism are in society and that we have a lot of work to do to disrupt that.”
Dr. Sims said the tweet about hoop earrings led her to wonder about daily decisions she makes about dress.
“Am I too much? Is this too much? Is this earring too big? Is this nail polish color too loud? And how will that be received at work?” she said, noting that she may opt not to wear hoops in certain situations, such as when she’s dealing with a grabby baby.
Monica Verduzco-Gutierrez, MD, professor and chair of the department of rehabilitation medicine at University of Texas Health, San Antonio, said doctors should be judged on the care they provide, not their appearance.
“Judging someone based on their earrings or their jumpsuit or whatever else that they’re noticing about the student is not an appropriate way to judge the student’s ability to take care of a patient,” Dr. Verduzco-Gutierrez said, noting that she was not speaking on behalf of the school.
A version of this article was first published on WebMD.com .
New analysis puts U.S. psoriasis prevalence at 3%
, according to an analysis of national survey data from 2011 to 2014.
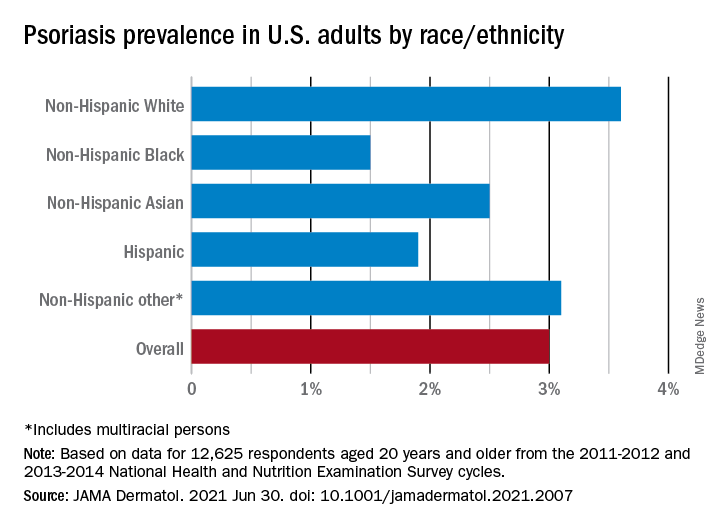
“The adult prevalence rate of 3.0% continues to place psoriasis as one of the most common immune-mediated diseases affecting adults” in the United States, April W. Armstrong, MD, MPH, and associates said in a report published in JAMA Dermatology. At that rate, approximately 7,560,000 Americans aged 20 years or older have psoriasis.
That overall rate among adults aged 20 years and older, based on data from the 2011-2012 and 2013-2014 cycles of the National Health and Nutrition Examination Survey (NHANES), did not change significantly when compared with the 2003-2004 NHANES, when it was 3.15% among those aged 20-59, said Dr. Armstrong, professor of dermatology, University of Southern California, Los Angeles, and associates.
For the 2011-2014 period, psoriasis prevalence was similar between women (3.2%) and men (2.8%) but was significantly associated with older age and White/non-White status. Those aged 50-59 years had the highest prevalence of any age group at 4.3% and those aged 70 and older had a rate of 3.9%, while those aged 20-29 were the lowest at 1.6%, the investigators reported.
The prevalence in non-Hispanic Whites in the United States was 3.6% over the study period, and their odds ratio for having psoriasis was 1.92, compared with non-White individuals. Asian respondents had a prevalence of 2.5%, with the Hispanic population at 1.9%, non-Hispanic Black respondents at 1.5%, and those identifying as other (including multiracial persons) at 3.1%, they said.
The NHANES sample consisted of 12,638 people who had participated in the question that asked if they had ever been diagnosed with psoriasis by a physician or other health care professional, of whom 12,625 gave a definitive yes or no answer, the investigators noted.
A much smaller number, 329, also answered a question about the severity of their disease: Fifty-six percent had little or no psoriasis, almost 22% reported 1-2 palms of involvement, 16% had 3-10 palms of involvement, and 5.5% said the coverage was more than 10 palms. Since the survey did not distinguish between treated and untreated patients, however, some “of those reporting low body surface area involvement may be receiving treatments that are controlling their otherwise more extensive disease,” they wrote.
Dr. Armstrong and another investigator said that they have received grants, personal fees, and honoraria from a number of pharmaceutical companies; two other investigators are employees of the National Psoriasis Foundation.
, according to an analysis of national survey data from 2011 to 2014.

“The adult prevalence rate of 3.0% continues to place psoriasis as one of the most common immune-mediated diseases affecting adults” in the United States, April W. Armstrong, MD, MPH, and associates said in a report published in JAMA Dermatology. At that rate, approximately 7,560,000 Americans aged 20 years or older have psoriasis.
That overall rate among adults aged 20 years and older, based on data from the 2011-2012 and 2013-2014 cycles of the National Health and Nutrition Examination Survey (NHANES), did not change significantly when compared with the 2003-2004 NHANES, when it was 3.15% among those aged 20-59, said Dr. Armstrong, professor of dermatology, University of Southern California, Los Angeles, and associates.
For the 2011-2014 period, psoriasis prevalence was similar between women (3.2%) and men (2.8%) but was significantly associated with older age and White/non-White status. Those aged 50-59 years had the highest prevalence of any age group at 4.3% and those aged 70 and older had a rate of 3.9%, while those aged 20-29 were the lowest at 1.6%, the investigators reported.
The prevalence in non-Hispanic Whites in the United States was 3.6% over the study period, and their odds ratio for having psoriasis was 1.92, compared with non-White individuals. Asian respondents had a prevalence of 2.5%, with the Hispanic population at 1.9%, non-Hispanic Black respondents at 1.5%, and those identifying as other (including multiracial persons) at 3.1%, they said.
The NHANES sample consisted of 12,638 people who had participated in the question that asked if they had ever been diagnosed with psoriasis by a physician or other health care professional, of whom 12,625 gave a definitive yes or no answer, the investigators noted.
A much smaller number, 329, also answered a question about the severity of their disease: Fifty-six percent had little or no psoriasis, almost 22% reported 1-2 palms of involvement, 16% had 3-10 palms of involvement, and 5.5% said the coverage was more than 10 palms. Since the survey did not distinguish between treated and untreated patients, however, some “of those reporting low body surface area involvement may be receiving treatments that are controlling their otherwise more extensive disease,” they wrote.
Dr. Armstrong and another investigator said that they have received grants, personal fees, and honoraria from a number of pharmaceutical companies; two other investigators are employees of the National Psoriasis Foundation.
, according to an analysis of national survey data from 2011 to 2014.

“The adult prevalence rate of 3.0% continues to place psoriasis as one of the most common immune-mediated diseases affecting adults” in the United States, April W. Armstrong, MD, MPH, and associates said in a report published in JAMA Dermatology. At that rate, approximately 7,560,000 Americans aged 20 years or older have psoriasis.
That overall rate among adults aged 20 years and older, based on data from the 2011-2012 and 2013-2014 cycles of the National Health and Nutrition Examination Survey (NHANES), did not change significantly when compared with the 2003-2004 NHANES, when it was 3.15% among those aged 20-59, said Dr. Armstrong, professor of dermatology, University of Southern California, Los Angeles, and associates.
For the 2011-2014 period, psoriasis prevalence was similar between women (3.2%) and men (2.8%) but was significantly associated with older age and White/non-White status. Those aged 50-59 years had the highest prevalence of any age group at 4.3% and those aged 70 and older had a rate of 3.9%, while those aged 20-29 were the lowest at 1.6%, the investigators reported.
The prevalence in non-Hispanic Whites in the United States was 3.6% over the study period, and their odds ratio for having psoriasis was 1.92, compared with non-White individuals. Asian respondents had a prevalence of 2.5%, with the Hispanic population at 1.9%, non-Hispanic Black respondents at 1.5%, and those identifying as other (including multiracial persons) at 3.1%, they said.
The NHANES sample consisted of 12,638 people who had participated in the question that asked if they had ever been diagnosed with psoriasis by a physician or other health care professional, of whom 12,625 gave a definitive yes or no answer, the investigators noted.
A much smaller number, 329, also answered a question about the severity of their disease: Fifty-six percent had little or no psoriasis, almost 22% reported 1-2 palms of involvement, 16% had 3-10 palms of involvement, and 5.5% said the coverage was more than 10 palms. Since the survey did not distinguish between treated and untreated patients, however, some “of those reporting low body surface area involvement may be receiving treatments that are controlling their otherwise more extensive disease,” they wrote.
Dr. Armstrong and another investigator said that they have received grants, personal fees, and honoraria from a number of pharmaceutical companies; two other investigators are employees of the National Psoriasis Foundation.
FROM JAMA DERMATOLOGY
New details of myocarditis linked to COVID vaccines
Further details from multiple cases of myocarditis linked to the Pfizer and Moderna mRNA COVID vaccines have been described in recent papers in the medical literature.
The cases appear to occur almost exclusively in males and most often in younger age groups. While symptoms and signs of myocarditis mostly resolved with a few days of supportive care, long-term effects are unknown at present.
The authors of all the reports and of two accompanying editorials in JAMA Cardiology are unanimous in their opinion that the benefits of vaccination still outweigh the risks.
The Centers for Disease Control and Prevention’s but committee members delivered a strong endorsement for continuing to vaccinate young people with the mRNA vaccines.
The current case reports are published in two papers in JAMA Cardiology and in three in Circulation.
U.S. military reports 23 cases
In one report in JAMA Cardiology, authors led by Jay Montgomery, MD, from Walter Reed National Military Medical Center in Bethesda, Md., described 23 cases from the U.S. Military Health System of individuals with acute myocarditis who presented within 4 days after mRNA-based COVID-19 vaccination (7 Pfizer and 16 Moderna).
All patients were male, 22 of 23 were on active duty, and the median age was 25 years (range, 20-51); 20 of the 23 cases occurred after receipt of a second dose of an mRNA COVID-19 vaccine.
The patients all presented with acute onset of marked chest pain. All patients had significantly elevated cardiac troponin levels. Among eight patients who underwent cardiac MRI (cMRI), all had findings consistent with the clinical diagnosis of myocarditis.
Additional testing did not identify other possible causes of myocarditis. All patients received brief supportive care and were recovered or recovering.
The authors reported that the military administered more than 2.8 million doses of mRNA COVID-19 vaccine in this period, and while the observed number of myocarditis cases was small, the number was “substantially higher” than expected among male military members after a second vaccine dose.
They noted that, based on historical data, among the 544,000 second doses to military members there may have been 0-10 expected myocarditis cases, but they observed 19 cases.
“All patients in this series reflect substantial similarities in demographic characteristics, proximate vaccine dose, onset interval, and character of vaccine-associated myocarditis. The consistent pattern of clinical presentation, rapid recovery, and absence of evidence of other causes support the diagnosis of hypersensitivity myocarditis,” they stated.
They added that presentation after a second vaccine dose or, in three patients, when vaccination followed SARS-CoV-2 infection, suggests that prior exposure was relevant in the hypersensitivity response.
“The spectrum of clinical presentation and reliance on patients seeking health care and on health care professionals recognizing a rare vaccine-associated adverse event limits determination of the true incidence of this condition,” the authors wrote.
They stressed that recognition of vaccine-associated myocarditis is clinically important because diagnosis impacts management, recommendations for exercise, and monitoring for cardiomyopathy.
But the authors also acknowledged that it is important to frame concerns about potential vaccine-associated myocarditis within the context of the current pandemic.
“Infection with SARS-CoV-2 is a clear cause of serious cardiac injury in many patients. ... Prevalence of cardiac injury may be as high as 60% in seriously ill patients. Notably, nearly 1% of highly fit athletes with mild COVID-19 infection have evidence of myocarditis on cMRI,” they wrote.
“Given that COVID-19 vaccines are remarkably effective at preventing infection, any risk of rare adverse events following immunization must be carefully weighed against the very substantial benefit of vaccination,” they concluded.
Four cases at Duke
In the second paper in JAMA Cardiology, a group led by Han W. Kim, MD, reported four patients with acute myocarditis occurring within days of mRNA COVID-19 vaccination (two Pfizer and two Moderna) in patients treated at Duke University Medical Center, Durham, N.C. The hospital courses of the four patients with myocarditis following COVID-19 vaccination were uneventful, and they were discharged within 2-4 days.
The authors said that, although a causal relationship cannot be established, none of the patients had a viral prodrome or had coincident testing that revealed an alternative explanation.
They stated that these four patients represent the majority of patients with acute myocarditis identified in the past 3 months at their institution, and this led to the highest total number of patients with acute myocarditis, compared with the same 3-month period for the past 5 years.
“Additionally, we identified only those patients with severe unremitting chest pain who sought medical attention. Those with mild or moderate chest pain might not seek medical attention, and it is possible that subclinical myocarditis may occur and could be detected by active surveillance, as has been described with smallpox vaccination,” they wrote.
Further case reports
In one of the papers in Circulation, a group led by Kathryn F. Larson, MD, from the Mayo Clinic in Rochester, Minn., described eight patients hospitalized with chest pain who were diagnosed with myocarditis within 2-4 days of receiving either the Pfizer or Moderna vaccine.
Two of the patients had previously been infected by SARS-CoV-2 without need for hospitalization. All individuals were otherwise healthy males between the ages of 21 and 56 years. All but one patient developed symptoms after their second dose, and the one patient who developed myocarditis after the first vaccine dose had previously been infected with SARS-CoV-2.
Systemic symptoms began within 24 hours after vaccine administration in five of eight patients, with chest pain presenting between 48 and 96 hours later. Troponin values were elevated in all individuals and appeared to peak the day after admission, whereas none had eosinophilia.
Cardiac MRI revealed findings consistent with myocarditis in all patients. All patients had resolution of their chest pain and were discharged from the hospital in stable condition.
“The patients presented here demonstrated typical signs, symptoms, and diagnostic features of acute myocarditis. The temporal association between receiving an mRNA-based COVID-19 vaccine and the development of myocarditis is notable,” the authors said.
They added that they would consider the use of corticosteroids in these patients but cautioned that this could reduce the specific immune response against SARS-COV-2 triggered by the vaccine. “Thus, the duration of corticosteroid administration should be limited to the resolution of the symptoms or ventricular arrhythmias or the recovery of the left ventricular ejection fraction.”
Pending publication of long-term outcome data after SARS-CoV-2 vaccine–related myocarditis, they suggest adherence to the current consensus recommendation to abstain from competitive sports for a period of 3-6 months with reevaluation prior to sports participation.
In another of the Circulation papers, a group led by Carolyn M. Rosner, MSN, presented a case series of seven patients hospitalized for acute myocarditis-like illness following COVID-19 vaccination, from two U.S. medical centers, in Falls Church, Va., and Dallas. All patients were males below the age of 40 years and of White or Hispanic race/ethnicity. Only one patient reported prior history of COVID-19 infection. Six patients received mRNA (Moderna or Pfizer) and one received the adenovirus (Johnson & Johnson) vaccine. All patients presented 3-7 days post vaccination with acute onset chest pain and biochemical evidence of myocardial injury.
Hospital length of stay was 3 days, and all patients’ symptoms resolved by hospital discharge.
And finally, the third paper in Circulation reported a detailed description of one patient – a 52-year-old, previously healthy male who presented with acute myocarditis 3 days after the administration of the second dose of Moderna’s COVID-19 vaccine. The symptoms resolved, and there was a gradual improvement in cMRI findings. Ischemic injury and other potential causes of acute myocardial injury were excluded, as were other potential infectious causes of myocarditis, and there was no evidence of systemic autoimmune disease.
“Clinicians should be aware that myocarditis may be present in patients exhibiting cardiac signs and symptoms 2-4 days after COVID-19 vaccination,” the authors said.
They added that additional surveillance of such adverse events post–COVID-19 vaccination will help identify subgroups at higher risk for this vaccine-related effect, and whether additional precautions are necessary.
‘Benefits outweigh risk’
In an accompanying editorial in JAMA Cardiology, three doctors from the CDC cite several other reports of myocarditis after mRNA COVID vaccination. These include a case report published in Pediatrics of seven male adolescents aged 14-19 years who presented with myocarditis or myopericarditis within 4 days after receipt of a second dose of the Pfizer vaccine.
But the editorialists noted that the most comprehensive data about the risk for myocarditis following immunization with mRNA vaccines comes from Israel.
The Israeli Ministry of Health recently posted data describing 121 myocarditis cases occurring within 30 days of a second dose of mRNA vaccine among 5,049,424 persons, suggesting a crude incidence rate of approximately 24 cases per million.
On the current case reports, the CDC doctors wrote: “The striking clinical similarities in the presentations of these patients, their recent vaccination with an mRNA-based COVID-19 vaccine, and the lack of any alternative etiologies for acute myocarditis suggest an association with immunization.”
They said that acute onset of chest pain 3-5 days after vaccine administration, usually after a second dose, is a typical feature of reported cases and suggests an immune-mediated mechanism.
But SARS-CoV-2 infection also causes cardiac injury which may result in severe outcomes, and based on currently available data, myocarditis following immunization with current mRNA-based vaccines is rare.
“At present, the benefits of immunization in preventing severe morbidity favors continued COVID-19 vaccination, particularly considering the increasing COVID-19 hospitalization rates among adolescents reported during spring 2021,” the editorialists stated.
But they added that many questions remain. These include whether modifications are needed to the vaccine schedule among persons with a history of possible or confirmed myocarditis after COVID vaccine, how should postvaccine myocarditis be managed, how often should follow-up assessments be performed, how might follow-up assessments affect recommendations to avoid vigorous physical activity following the diagnosis of myocarditis, and do all likely cases of acute myocarditis that appear to be uncomplicated require cardiac MRI for more definitive diagnosis?
“While the data needed to answer such questions are being collected, there is an opportunity for researchers with expertise in myocarditis to develop a comprehensive, national assessment of the natural history, pathogenesis, and treatment of acute myocarditis associated with receipt of mRNA-based COVID-19 vaccines,” they concluded.
In a second editorial in JAMA Cardiology, a group of editors from the journal acknowledged that publication of the current case reports may contribute to additional public concern regarding immunization. But they added that clinicians discussing immunization with patients should recognize that these case series suggest that the symptomatic events consistent with myocarditis are still very rare and appear to be self-limiting.
“Given the risks of COVID-19, including the risk of myocarditis from COVID-19 infection, the editors do not believe these case reports are sufficient to interrupt the march toward maximal vaccination against SARS-CoV-2 as expeditiously as possible,” they said.
A version of this article first appeared on Medscape.com.
Further details from multiple cases of myocarditis linked to the Pfizer and Moderna mRNA COVID vaccines have been described in recent papers in the medical literature.
The cases appear to occur almost exclusively in males and most often in younger age groups. While symptoms and signs of myocarditis mostly resolved with a few days of supportive care, long-term effects are unknown at present.
The authors of all the reports and of two accompanying editorials in JAMA Cardiology are unanimous in their opinion that the benefits of vaccination still outweigh the risks.
The Centers for Disease Control and Prevention’s but committee members delivered a strong endorsement for continuing to vaccinate young people with the mRNA vaccines.
The current case reports are published in two papers in JAMA Cardiology and in three in Circulation.
U.S. military reports 23 cases
In one report in JAMA Cardiology, authors led by Jay Montgomery, MD, from Walter Reed National Military Medical Center in Bethesda, Md., described 23 cases from the U.S. Military Health System of individuals with acute myocarditis who presented within 4 days after mRNA-based COVID-19 vaccination (7 Pfizer and 16 Moderna).
All patients were male, 22 of 23 were on active duty, and the median age was 25 years (range, 20-51); 20 of the 23 cases occurred after receipt of a second dose of an mRNA COVID-19 vaccine.
The patients all presented with acute onset of marked chest pain. All patients had significantly elevated cardiac troponin levels. Among eight patients who underwent cardiac MRI (cMRI), all had findings consistent with the clinical diagnosis of myocarditis.
Additional testing did not identify other possible causes of myocarditis. All patients received brief supportive care and were recovered or recovering.
The authors reported that the military administered more than 2.8 million doses of mRNA COVID-19 vaccine in this period, and while the observed number of myocarditis cases was small, the number was “substantially higher” than expected among male military members after a second vaccine dose.
They noted that, based on historical data, among the 544,000 second doses to military members there may have been 0-10 expected myocarditis cases, but they observed 19 cases.
“All patients in this series reflect substantial similarities in demographic characteristics, proximate vaccine dose, onset interval, and character of vaccine-associated myocarditis. The consistent pattern of clinical presentation, rapid recovery, and absence of evidence of other causes support the diagnosis of hypersensitivity myocarditis,” they stated.
They added that presentation after a second vaccine dose or, in three patients, when vaccination followed SARS-CoV-2 infection, suggests that prior exposure was relevant in the hypersensitivity response.
“The spectrum of clinical presentation and reliance on patients seeking health care and on health care professionals recognizing a rare vaccine-associated adverse event limits determination of the true incidence of this condition,” the authors wrote.
They stressed that recognition of vaccine-associated myocarditis is clinically important because diagnosis impacts management, recommendations for exercise, and monitoring for cardiomyopathy.
But the authors also acknowledged that it is important to frame concerns about potential vaccine-associated myocarditis within the context of the current pandemic.
“Infection with SARS-CoV-2 is a clear cause of serious cardiac injury in many patients. ... Prevalence of cardiac injury may be as high as 60% in seriously ill patients. Notably, nearly 1% of highly fit athletes with mild COVID-19 infection have evidence of myocarditis on cMRI,” they wrote.
“Given that COVID-19 vaccines are remarkably effective at preventing infection, any risk of rare adverse events following immunization must be carefully weighed against the very substantial benefit of vaccination,” they concluded.
Four cases at Duke
In the second paper in JAMA Cardiology, a group led by Han W. Kim, MD, reported four patients with acute myocarditis occurring within days of mRNA COVID-19 vaccination (two Pfizer and two Moderna) in patients treated at Duke University Medical Center, Durham, N.C. The hospital courses of the four patients with myocarditis following COVID-19 vaccination were uneventful, and they were discharged within 2-4 days.
The authors said that, although a causal relationship cannot be established, none of the patients had a viral prodrome or had coincident testing that revealed an alternative explanation.
They stated that these four patients represent the majority of patients with acute myocarditis identified in the past 3 months at their institution, and this led to the highest total number of patients with acute myocarditis, compared with the same 3-month period for the past 5 years.
“Additionally, we identified only those patients with severe unremitting chest pain who sought medical attention. Those with mild or moderate chest pain might not seek medical attention, and it is possible that subclinical myocarditis may occur and could be detected by active surveillance, as has been described with smallpox vaccination,” they wrote.
Further case reports
In one of the papers in Circulation, a group led by Kathryn F. Larson, MD, from the Mayo Clinic in Rochester, Minn., described eight patients hospitalized with chest pain who were diagnosed with myocarditis within 2-4 days of receiving either the Pfizer or Moderna vaccine.
Two of the patients had previously been infected by SARS-CoV-2 without need for hospitalization. All individuals were otherwise healthy males between the ages of 21 and 56 years. All but one patient developed symptoms after their second dose, and the one patient who developed myocarditis after the first vaccine dose had previously been infected with SARS-CoV-2.
Systemic symptoms began within 24 hours after vaccine administration in five of eight patients, with chest pain presenting between 48 and 96 hours later. Troponin values were elevated in all individuals and appeared to peak the day after admission, whereas none had eosinophilia.
Cardiac MRI revealed findings consistent with myocarditis in all patients. All patients had resolution of their chest pain and were discharged from the hospital in stable condition.
“The patients presented here demonstrated typical signs, symptoms, and diagnostic features of acute myocarditis. The temporal association between receiving an mRNA-based COVID-19 vaccine and the development of myocarditis is notable,” the authors said.
They added that they would consider the use of corticosteroids in these patients but cautioned that this could reduce the specific immune response against SARS-COV-2 triggered by the vaccine. “Thus, the duration of corticosteroid administration should be limited to the resolution of the symptoms or ventricular arrhythmias or the recovery of the left ventricular ejection fraction.”
Pending publication of long-term outcome data after SARS-CoV-2 vaccine–related myocarditis, they suggest adherence to the current consensus recommendation to abstain from competitive sports for a period of 3-6 months with reevaluation prior to sports participation.
In another of the Circulation papers, a group led by Carolyn M. Rosner, MSN, presented a case series of seven patients hospitalized for acute myocarditis-like illness following COVID-19 vaccination, from two U.S. medical centers, in Falls Church, Va., and Dallas. All patients were males below the age of 40 years and of White or Hispanic race/ethnicity. Only one patient reported prior history of COVID-19 infection. Six patients received mRNA (Moderna or Pfizer) and one received the adenovirus (Johnson & Johnson) vaccine. All patients presented 3-7 days post vaccination with acute onset chest pain and biochemical evidence of myocardial injury.
Hospital length of stay was 3 days, and all patients’ symptoms resolved by hospital discharge.
And finally, the third paper in Circulation reported a detailed description of one patient – a 52-year-old, previously healthy male who presented with acute myocarditis 3 days after the administration of the second dose of Moderna’s COVID-19 vaccine. The symptoms resolved, and there was a gradual improvement in cMRI findings. Ischemic injury and other potential causes of acute myocardial injury were excluded, as were other potential infectious causes of myocarditis, and there was no evidence of systemic autoimmune disease.
“Clinicians should be aware that myocarditis may be present in patients exhibiting cardiac signs and symptoms 2-4 days after COVID-19 vaccination,” the authors said.
They added that additional surveillance of such adverse events post–COVID-19 vaccination will help identify subgroups at higher risk for this vaccine-related effect, and whether additional precautions are necessary.
‘Benefits outweigh risk’
In an accompanying editorial in JAMA Cardiology, three doctors from the CDC cite several other reports of myocarditis after mRNA COVID vaccination. These include a case report published in Pediatrics of seven male adolescents aged 14-19 years who presented with myocarditis or myopericarditis within 4 days after receipt of a second dose of the Pfizer vaccine.
But the editorialists noted that the most comprehensive data about the risk for myocarditis following immunization with mRNA vaccines comes from Israel.
The Israeli Ministry of Health recently posted data describing 121 myocarditis cases occurring within 30 days of a second dose of mRNA vaccine among 5,049,424 persons, suggesting a crude incidence rate of approximately 24 cases per million.
On the current case reports, the CDC doctors wrote: “The striking clinical similarities in the presentations of these patients, their recent vaccination with an mRNA-based COVID-19 vaccine, and the lack of any alternative etiologies for acute myocarditis suggest an association with immunization.”
They said that acute onset of chest pain 3-5 days after vaccine administration, usually after a second dose, is a typical feature of reported cases and suggests an immune-mediated mechanism.
But SARS-CoV-2 infection also causes cardiac injury which may result in severe outcomes, and based on currently available data, myocarditis following immunization with current mRNA-based vaccines is rare.
“At present, the benefits of immunization in preventing severe morbidity favors continued COVID-19 vaccination, particularly considering the increasing COVID-19 hospitalization rates among adolescents reported during spring 2021,” the editorialists stated.
But they added that many questions remain. These include whether modifications are needed to the vaccine schedule among persons with a history of possible or confirmed myocarditis after COVID vaccine, how should postvaccine myocarditis be managed, how often should follow-up assessments be performed, how might follow-up assessments affect recommendations to avoid vigorous physical activity following the diagnosis of myocarditis, and do all likely cases of acute myocarditis that appear to be uncomplicated require cardiac MRI for more definitive diagnosis?
“While the data needed to answer such questions are being collected, there is an opportunity for researchers with expertise in myocarditis to develop a comprehensive, national assessment of the natural history, pathogenesis, and treatment of acute myocarditis associated with receipt of mRNA-based COVID-19 vaccines,” they concluded.
In a second editorial in JAMA Cardiology, a group of editors from the journal acknowledged that publication of the current case reports may contribute to additional public concern regarding immunization. But they added that clinicians discussing immunization with patients should recognize that these case series suggest that the symptomatic events consistent with myocarditis are still very rare and appear to be self-limiting.
“Given the risks of COVID-19, including the risk of myocarditis from COVID-19 infection, the editors do not believe these case reports are sufficient to interrupt the march toward maximal vaccination against SARS-CoV-2 as expeditiously as possible,” they said.
A version of this article first appeared on Medscape.com.
Further details from multiple cases of myocarditis linked to the Pfizer and Moderna mRNA COVID vaccines have been described in recent papers in the medical literature.
The cases appear to occur almost exclusively in males and most often in younger age groups. While symptoms and signs of myocarditis mostly resolved with a few days of supportive care, long-term effects are unknown at present.
The authors of all the reports and of two accompanying editorials in JAMA Cardiology are unanimous in their opinion that the benefits of vaccination still outweigh the risks.
The Centers for Disease Control and Prevention’s but committee members delivered a strong endorsement for continuing to vaccinate young people with the mRNA vaccines.
The current case reports are published in two papers in JAMA Cardiology and in three in Circulation.
U.S. military reports 23 cases
In one report in JAMA Cardiology, authors led by Jay Montgomery, MD, from Walter Reed National Military Medical Center in Bethesda, Md., described 23 cases from the U.S. Military Health System of individuals with acute myocarditis who presented within 4 days after mRNA-based COVID-19 vaccination (7 Pfizer and 16 Moderna).
All patients were male, 22 of 23 were on active duty, and the median age was 25 years (range, 20-51); 20 of the 23 cases occurred after receipt of a second dose of an mRNA COVID-19 vaccine.
The patients all presented with acute onset of marked chest pain. All patients had significantly elevated cardiac troponin levels. Among eight patients who underwent cardiac MRI (cMRI), all had findings consistent with the clinical diagnosis of myocarditis.
Additional testing did not identify other possible causes of myocarditis. All patients received brief supportive care and were recovered or recovering.
The authors reported that the military administered more than 2.8 million doses of mRNA COVID-19 vaccine in this period, and while the observed number of myocarditis cases was small, the number was “substantially higher” than expected among male military members after a second vaccine dose.
They noted that, based on historical data, among the 544,000 second doses to military members there may have been 0-10 expected myocarditis cases, but they observed 19 cases.
“All patients in this series reflect substantial similarities in demographic characteristics, proximate vaccine dose, onset interval, and character of vaccine-associated myocarditis. The consistent pattern of clinical presentation, rapid recovery, and absence of evidence of other causes support the diagnosis of hypersensitivity myocarditis,” they stated.
They added that presentation after a second vaccine dose or, in three patients, when vaccination followed SARS-CoV-2 infection, suggests that prior exposure was relevant in the hypersensitivity response.
“The spectrum of clinical presentation and reliance on patients seeking health care and on health care professionals recognizing a rare vaccine-associated adverse event limits determination of the true incidence of this condition,” the authors wrote.
They stressed that recognition of vaccine-associated myocarditis is clinically important because diagnosis impacts management, recommendations for exercise, and monitoring for cardiomyopathy.
But the authors also acknowledged that it is important to frame concerns about potential vaccine-associated myocarditis within the context of the current pandemic.
“Infection with SARS-CoV-2 is a clear cause of serious cardiac injury in many patients. ... Prevalence of cardiac injury may be as high as 60% in seriously ill patients. Notably, nearly 1% of highly fit athletes with mild COVID-19 infection have evidence of myocarditis on cMRI,” they wrote.
“Given that COVID-19 vaccines are remarkably effective at preventing infection, any risk of rare adverse events following immunization must be carefully weighed against the very substantial benefit of vaccination,” they concluded.
Four cases at Duke
In the second paper in JAMA Cardiology, a group led by Han W. Kim, MD, reported four patients with acute myocarditis occurring within days of mRNA COVID-19 vaccination (two Pfizer and two Moderna) in patients treated at Duke University Medical Center, Durham, N.C. The hospital courses of the four patients with myocarditis following COVID-19 vaccination were uneventful, and they were discharged within 2-4 days.
The authors said that, although a causal relationship cannot be established, none of the patients had a viral prodrome or had coincident testing that revealed an alternative explanation.
They stated that these four patients represent the majority of patients with acute myocarditis identified in the past 3 months at their institution, and this led to the highest total number of patients with acute myocarditis, compared with the same 3-month period for the past 5 years.
“Additionally, we identified only those patients with severe unremitting chest pain who sought medical attention. Those with mild or moderate chest pain might not seek medical attention, and it is possible that subclinical myocarditis may occur and could be detected by active surveillance, as has been described with smallpox vaccination,” they wrote.
Further case reports
In one of the papers in Circulation, a group led by Kathryn F. Larson, MD, from the Mayo Clinic in Rochester, Minn., described eight patients hospitalized with chest pain who were diagnosed with myocarditis within 2-4 days of receiving either the Pfizer or Moderna vaccine.
Two of the patients had previously been infected by SARS-CoV-2 without need for hospitalization. All individuals were otherwise healthy males between the ages of 21 and 56 years. All but one patient developed symptoms after their second dose, and the one patient who developed myocarditis after the first vaccine dose had previously been infected with SARS-CoV-2.
Systemic symptoms began within 24 hours after vaccine administration in five of eight patients, with chest pain presenting between 48 and 96 hours later. Troponin values were elevated in all individuals and appeared to peak the day after admission, whereas none had eosinophilia.
Cardiac MRI revealed findings consistent with myocarditis in all patients. All patients had resolution of their chest pain and were discharged from the hospital in stable condition.
“The patients presented here demonstrated typical signs, symptoms, and diagnostic features of acute myocarditis. The temporal association between receiving an mRNA-based COVID-19 vaccine and the development of myocarditis is notable,” the authors said.
They added that they would consider the use of corticosteroids in these patients but cautioned that this could reduce the specific immune response against SARS-COV-2 triggered by the vaccine. “Thus, the duration of corticosteroid administration should be limited to the resolution of the symptoms or ventricular arrhythmias or the recovery of the left ventricular ejection fraction.”
Pending publication of long-term outcome data after SARS-CoV-2 vaccine–related myocarditis, they suggest adherence to the current consensus recommendation to abstain from competitive sports for a period of 3-6 months with reevaluation prior to sports participation.
In another of the Circulation papers, a group led by Carolyn M. Rosner, MSN, presented a case series of seven patients hospitalized for acute myocarditis-like illness following COVID-19 vaccination, from two U.S. medical centers, in Falls Church, Va., and Dallas. All patients were males below the age of 40 years and of White or Hispanic race/ethnicity. Only one patient reported prior history of COVID-19 infection. Six patients received mRNA (Moderna or Pfizer) and one received the adenovirus (Johnson & Johnson) vaccine. All patients presented 3-7 days post vaccination with acute onset chest pain and biochemical evidence of myocardial injury.
Hospital length of stay was 3 days, and all patients’ symptoms resolved by hospital discharge.
And finally, the third paper in Circulation reported a detailed description of one patient – a 52-year-old, previously healthy male who presented with acute myocarditis 3 days after the administration of the second dose of Moderna’s COVID-19 vaccine. The symptoms resolved, and there was a gradual improvement in cMRI findings. Ischemic injury and other potential causes of acute myocardial injury were excluded, as were other potential infectious causes of myocarditis, and there was no evidence of systemic autoimmune disease.
“Clinicians should be aware that myocarditis may be present in patients exhibiting cardiac signs and symptoms 2-4 days after COVID-19 vaccination,” the authors said.
They added that additional surveillance of such adverse events post–COVID-19 vaccination will help identify subgroups at higher risk for this vaccine-related effect, and whether additional precautions are necessary.
‘Benefits outweigh risk’
In an accompanying editorial in JAMA Cardiology, three doctors from the CDC cite several other reports of myocarditis after mRNA COVID vaccination. These include a case report published in Pediatrics of seven male adolescents aged 14-19 years who presented with myocarditis or myopericarditis within 4 days after receipt of a second dose of the Pfizer vaccine.
But the editorialists noted that the most comprehensive data about the risk for myocarditis following immunization with mRNA vaccines comes from Israel.
The Israeli Ministry of Health recently posted data describing 121 myocarditis cases occurring within 30 days of a second dose of mRNA vaccine among 5,049,424 persons, suggesting a crude incidence rate of approximately 24 cases per million.
On the current case reports, the CDC doctors wrote: “The striking clinical similarities in the presentations of these patients, their recent vaccination with an mRNA-based COVID-19 vaccine, and the lack of any alternative etiologies for acute myocarditis suggest an association with immunization.”
They said that acute onset of chest pain 3-5 days after vaccine administration, usually after a second dose, is a typical feature of reported cases and suggests an immune-mediated mechanism.
But SARS-CoV-2 infection also causes cardiac injury which may result in severe outcomes, and based on currently available data, myocarditis following immunization with current mRNA-based vaccines is rare.
“At present, the benefits of immunization in preventing severe morbidity favors continued COVID-19 vaccination, particularly considering the increasing COVID-19 hospitalization rates among adolescents reported during spring 2021,” the editorialists stated.
But they added that many questions remain. These include whether modifications are needed to the vaccine schedule among persons with a history of possible or confirmed myocarditis after COVID vaccine, how should postvaccine myocarditis be managed, how often should follow-up assessments be performed, how might follow-up assessments affect recommendations to avoid vigorous physical activity following the diagnosis of myocarditis, and do all likely cases of acute myocarditis that appear to be uncomplicated require cardiac MRI for more definitive diagnosis?
“While the data needed to answer such questions are being collected, there is an opportunity for researchers with expertise in myocarditis to develop a comprehensive, national assessment of the natural history, pathogenesis, and treatment of acute myocarditis associated with receipt of mRNA-based COVID-19 vaccines,” they concluded.
In a second editorial in JAMA Cardiology, a group of editors from the journal acknowledged that publication of the current case reports may contribute to additional public concern regarding immunization. But they added that clinicians discussing immunization with patients should recognize that these case series suggest that the symptomatic events consistent with myocarditis are still very rare and appear to be self-limiting.
“Given the risks of COVID-19, including the risk of myocarditis from COVID-19 infection, the editors do not believe these case reports are sufficient to interrupt the march toward maximal vaccination against SARS-CoV-2 as expeditiously as possible,” they said.
A version of this article first appeared on Medscape.com.
Post–COVID-19 lung injury: What we know so far
With vaccination rates increasing and new infections declining, we all hope the worst of the COVID-19 pandemic is over (fingers crossed really tight). Regardless, the post–COVID-19 syndrome pandemic has already begun. What is post–COVID-19 syndrome (or long-haulers or long-COVID)? Is it standard postviral fatigue? Prolonged deconditioning following debilitating illness? Permanent lung or vascular injury? Common sense and past experience say it’s all of these.
In theory, the burden of actual lung injury post COVID-19 should be the easiest to quantify, so let’s discuss what we think we know. I’ve heard experts break post–COVID-19 lung injury into three broad categories:
- Preexisting lung disease that is exacerbated by acute COVID-19 infection.
- Acute COVID-19 infection that causes acute respiratory distress syndrome (ARDS) or other acute lung injury (ALI).
- Non–critically ill acute COVID-19 with residual lung damage and abnormal repair.
These categories are necessarily imprecise, making it challenging to fit some patients neatly into a single definition.
For patients in the first category, management will be dictated largely by the nature of the preexisting lung disease. For those in category two, we already know a lot about what their recovery from ARDS will look like. There’s no longer reason to believe that COVID-19–related ARDS is particularly unique, and all things being equal, lung recovery should mimic that seen with non–COVID-19 ARDS.
It’s going to take patience and time, and beyond targeted rehabilitation it’s not clear that we have anything available to expedite the process.
The third category of patients is the most intriguing. Is there a group of patients who have residual lung injury but didn’t have evident ARDS/ALI during their acute COVID-19 infection? Anecdotally we think so, but we know little about prevalence and less about management. A recent study published in Annals of the American Thoracic Society addresses both issues. In an observational report on patients recovering after being hospitalized with COVID-19 infection, the authors found that 3.6% of patients had residual lung injury that improved with 3 weeks of corticosteroid treatment.
The report is timely and helpful but hardly definitive. It’s observational, and patients required extensive screening and identification by a multidisciplinary committee of experts in interstitial lung disease. Patients were diagnosed as having organizing pneumonia (OP) as their “lung injury” if certain radiographic criteria were met. There were no biopsies. Last, there was no control group. Still, this report is critically important. It tells us that at 6 weeks post discharge, about 3.6% of patients who were hospitalized for COVID-19 will have persistent symptoms, radiographic abnormalities, and a plateau in their recovery.
Beyond that, it tells us little. Did these patients really have OP? It’s impossible to know. The CT findings used to establish the diagnosis are nonspecific. Response to steroids is consistent with OP, but the treatment course was quite short. If truly OP, one would expect a high relapse rate after steroid withdrawal. Patients weren’t followed long enough to monitor recurrence rates. Also, as appropriately discussed in the accompanying editorial, there’s no control group so we can’t know whether the patients treated with steroids would have recovered without treatment. There was objective improvement in lung function for the two to three patients they followed who did not receive steroids. However, it was of lesser magnitude than in the steroid group.
Post–COVID-19 symptoms will remain a challenge for the foreseeable future. More than 30 million patients have been diagnosed with COVID-19 in the United States and close to half will experience persistent dyspnea. Putting the numbers together, I conclude that the vast majority will not have identifiable lung injury that will benefit from steroids. I wish I could prescribe patience to both physicians and patients.
Dr. Holley is associate professor of medicine at Uniformed Services University and program director of pulmonary and critical care medicine at Walter Reed National Military Medical Center. He covers a wide range of topics in pulmonary, critical care, and sleep medicine.
A version of this article first appeared on Medscape.com.
With vaccination rates increasing and new infections declining, we all hope the worst of the COVID-19 pandemic is over (fingers crossed really tight). Regardless, the post–COVID-19 syndrome pandemic has already begun. What is post–COVID-19 syndrome (or long-haulers or long-COVID)? Is it standard postviral fatigue? Prolonged deconditioning following debilitating illness? Permanent lung or vascular injury? Common sense and past experience say it’s all of these.
In theory, the burden of actual lung injury post COVID-19 should be the easiest to quantify, so let’s discuss what we think we know. I’ve heard experts break post–COVID-19 lung injury into three broad categories:
- Preexisting lung disease that is exacerbated by acute COVID-19 infection.
- Acute COVID-19 infection that causes acute respiratory distress syndrome (ARDS) or other acute lung injury (ALI).
- Non–critically ill acute COVID-19 with residual lung damage and abnormal repair.
These categories are necessarily imprecise, making it challenging to fit some patients neatly into a single definition.
For patients in the first category, management will be dictated largely by the nature of the preexisting lung disease. For those in category two, we already know a lot about what their recovery from ARDS will look like. There’s no longer reason to believe that COVID-19–related ARDS is particularly unique, and all things being equal, lung recovery should mimic that seen with non–COVID-19 ARDS.
It’s going to take patience and time, and beyond targeted rehabilitation it’s not clear that we have anything available to expedite the process.
The third category of patients is the most intriguing. Is there a group of patients who have residual lung injury but didn’t have evident ARDS/ALI during their acute COVID-19 infection? Anecdotally we think so, but we know little about prevalence and less about management. A recent study published in Annals of the American Thoracic Society addresses both issues. In an observational report on patients recovering after being hospitalized with COVID-19 infection, the authors found that 3.6% of patients had residual lung injury that improved with 3 weeks of corticosteroid treatment.
The report is timely and helpful but hardly definitive. It’s observational, and patients required extensive screening and identification by a multidisciplinary committee of experts in interstitial lung disease. Patients were diagnosed as having organizing pneumonia (OP) as their “lung injury” if certain radiographic criteria were met. There were no biopsies. Last, there was no control group. Still, this report is critically important. It tells us that at 6 weeks post discharge, about 3.6% of patients who were hospitalized for COVID-19 will have persistent symptoms, radiographic abnormalities, and a plateau in their recovery.
Beyond that, it tells us little. Did these patients really have OP? It’s impossible to know. The CT findings used to establish the diagnosis are nonspecific. Response to steroids is consistent with OP, but the treatment course was quite short. If truly OP, one would expect a high relapse rate after steroid withdrawal. Patients weren’t followed long enough to monitor recurrence rates. Also, as appropriately discussed in the accompanying editorial, there’s no control group so we can’t know whether the patients treated with steroids would have recovered without treatment. There was objective improvement in lung function for the two to three patients they followed who did not receive steroids. However, it was of lesser magnitude than in the steroid group.
Post–COVID-19 symptoms will remain a challenge for the foreseeable future. More than 30 million patients have been diagnosed with COVID-19 in the United States and close to half will experience persistent dyspnea. Putting the numbers together, I conclude that the vast majority will not have identifiable lung injury that will benefit from steroids. I wish I could prescribe patience to both physicians and patients.
Dr. Holley is associate professor of medicine at Uniformed Services University and program director of pulmonary and critical care medicine at Walter Reed National Military Medical Center. He covers a wide range of topics in pulmonary, critical care, and sleep medicine.
A version of this article first appeared on Medscape.com.
With vaccination rates increasing and new infections declining, we all hope the worst of the COVID-19 pandemic is over (fingers crossed really tight). Regardless, the post–COVID-19 syndrome pandemic has already begun. What is post–COVID-19 syndrome (or long-haulers or long-COVID)? Is it standard postviral fatigue? Prolonged deconditioning following debilitating illness? Permanent lung or vascular injury? Common sense and past experience say it’s all of these.
In theory, the burden of actual lung injury post COVID-19 should be the easiest to quantify, so let’s discuss what we think we know. I’ve heard experts break post–COVID-19 lung injury into three broad categories:
- Preexisting lung disease that is exacerbated by acute COVID-19 infection.
- Acute COVID-19 infection that causes acute respiratory distress syndrome (ARDS) or other acute lung injury (ALI).
- Non–critically ill acute COVID-19 with residual lung damage and abnormal repair.
These categories are necessarily imprecise, making it challenging to fit some patients neatly into a single definition.
For patients in the first category, management will be dictated largely by the nature of the preexisting lung disease. For those in category two, we already know a lot about what their recovery from ARDS will look like. There’s no longer reason to believe that COVID-19–related ARDS is particularly unique, and all things being equal, lung recovery should mimic that seen with non–COVID-19 ARDS.
It’s going to take patience and time, and beyond targeted rehabilitation it’s not clear that we have anything available to expedite the process.
The third category of patients is the most intriguing. Is there a group of patients who have residual lung injury but didn’t have evident ARDS/ALI during their acute COVID-19 infection? Anecdotally we think so, but we know little about prevalence and less about management. A recent study published in Annals of the American Thoracic Society addresses both issues. In an observational report on patients recovering after being hospitalized with COVID-19 infection, the authors found that 3.6% of patients had residual lung injury that improved with 3 weeks of corticosteroid treatment.
The report is timely and helpful but hardly definitive. It’s observational, and patients required extensive screening and identification by a multidisciplinary committee of experts in interstitial lung disease. Patients were diagnosed as having organizing pneumonia (OP) as their “lung injury” if certain radiographic criteria were met. There were no biopsies. Last, there was no control group. Still, this report is critically important. It tells us that at 6 weeks post discharge, about 3.6% of patients who were hospitalized for COVID-19 will have persistent symptoms, radiographic abnormalities, and a plateau in their recovery.
Beyond that, it tells us little. Did these patients really have OP? It’s impossible to know. The CT findings used to establish the diagnosis are nonspecific. Response to steroids is consistent with OP, but the treatment course was quite short. If truly OP, one would expect a high relapse rate after steroid withdrawal. Patients weren’t followed long enough to monitor recurrence rates. Also, as appropriately discussed in the accompanying editorial, there’s no control group so we can’t know whether the patients treated with steroids would have recovered without treatment. There was objective improvement in lung function for the two to three patients they followed who did not receive steroids. However, it was of lesser magnitude than in the steroid group.
Post–COVID-19 symptoms will remain a challenge for the foreseeable future. More than 30 million patients have been diagnosed with COVID-19 in the United States and close to half will experience persistent dyspnea. Putting the numbers together, I conclude that the vast majority will not have identifiable lung injury that will benefit from steroids. I wish I could prescribe patience to both physicians and patients.
Dr. Holley is associate professor of medicine at Uniformed Services University and program director of pulmonary and critical care medicine at Walter Reed National Military Medical Center. He covers a wide range of topics in pulmonary, critical care, and sleep medicine.
A version of this article first appeared on Medscape.com.
Malignancy risk: Secukinumab shows long-term safety for psoriasis, PsA, ankylosing spondylitis
that included 49 clinical trials.
Secukinumab (Cosentyx), an interleukin-17A antagonist, is approved for several conditions: moderate to severe psoriasis in children and adults, PsA, ankylosing spondylitis (AS), and nonradiographic axial spondyloarthritis.
Although secukinumab has demonstrated safety and tolerability, data on long-term malignancy rates are limited, wrote Mark Lebwohl, MD, professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and coauthors.
In a study published in the British Journal of Dermatology, they analyzed the combined safety data from clinical trials and postmarketing surveillance. The study population included 10,685 patients with psoriasis, 2,523 patients with PsA, and 1,311 patients with ankylosing spondylitis who received at least one approved dose of secukinumab (300 mg or 150 mg). The maximum follow-up was 5 years. The exposure-adjusted incidence rate was defined as the incidence rates per 100 patient treatment-years (PTY). The cumulative exposure for patients with psoriasis, PsA, and AS was 16,482, 4,944, and 2,668 PTY, respectively, with average follow-up times of 1.54, 1.96, and 2.03 years, respectively.
The observed and the expected number of malignancies were comparable, with a standardized incidence ratio (SIR) for malignancy of 0.99 across all treatment indications, the researchers said. In further analysis of malignancy by indication, the SIR was 0.87, 1.16, and 1.61 for psoriasis, PsA, and AS, respectively.
Data from postmarketing surveillance showed similar results: The estimated crude cumulative incidence reporting rate per 100 PTY was 0.27 for malignancy across all indications. The cumulative exposure was 285,811 PTY.
The study findings were limited by several factors including the post hoc design, differences in clinical trial methodologies, and lack of controlling for confounding variables, such as smoking status and previous exposure to systemic and biologic treatments, the researchers noted. In addition, the analysis did not include postexposure follow-up data, or data on patients who discontinued clinical trials, they said.
Overall, the analysis is the largest to date and supports the low risk of malignancy in patients with psoriasis, PsA, and AS treated with secukinumab, the researchers noted.
However, “while this assessment provides a broader understanding of the safety of secukinumab and supports its long-term use in these chronic systemic inflammatory conditions, registry data are further warranted to fully understand the real-world effect of biologics on malignancy risk,” they concluded.
“Secukinumab is a relatively newer biologic, approved in 2015, and there is currently a lack of longer-term data on the incidence of malignancy in secukinumab-treated patients, so it’s important to look at the data we have so far on this topic so we can better understand the long-term risks and counsel our psoriasis and psoriatic arthritis patients,” Flavia Fedeles, MD, of the department of dermatology at Massachusetts General Hospital, Boston, said in an interview.
Dr. Fedeles, who was not involved with the study, said that she was not surprised by the study results. “Data reported in the past from phase 3 clinical trials of secukinumab compared with placebo did not show an increase in risk of malignancy, though at that time no long-term safety data or data from patients with history of malignancy was available,” she said. “This study is reassuring in that there wasn’t a signal of increased malignancy events up to 5 years of secukinumab treatment,” said Dr. Fedeles.
However, she noted that the study has a number of limitations, including the use of clinical trials data, which have stringent inclusion/exclusion criteria that can lead to selection bias, the use of postmarketing surveillance data, the post hoc nature of the analysis, and the fact that the sponsor of the trial was the manufacturer of secukinumab, which “potentially can lead to bias to this study.”
She added that “registry data are needed to fully understand the real-world long-term effect of secukinumab on malignancy risk.”
The study was funded by Novartis. Lead author Dr. Lebwohl disclosed participating in advisory boards and/or as an investigator and/or speaker and receiving grants and/or honoraria from multiple companies including Novartis. Several study coauthors are employees of Novartis.
Dr. Fedeles had no financial conflicts to disclose.
that included 49 clinical trials.
Secukinumab (Cosentyx), an interleukin-17A antagonist, is approved for several conditions: moderate to severe psoriasis in children and adults, PsA, ankylosing spondylitis (AS), and nonradiographic axial spondyloarthritis.
Although secukinumab has demonstrated safety and tolerability, data on long-term malignancy rates are limited, wrote Mark Lebwohl, MD, professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and coauthors.
In a study published in the British Journal of Dermatology, they analyzed the combined safety data from clinical trials and postmarketing surveillance. The study population included 10,685 patients with psoriasis, 2,523 patients with PsA, and 1,311 patients with ankylosing spondylitis who received at least one approved dose of secukinumab (300 mg or 150 mg). The maximum follow-up was 5 years. The exposure-adjusted incidence rate was defined as the incidence rates per 100 patient treatment-years (PTY). The cumulative exposure for patients with psoriasis, PsA, and AS was 16,482, 4,944, and 2,668 PTY, respectively, with average follow-up times of 1.54, 1.96, and 2.03 years, respectively.
The observed and the expected number of malignancies were comparable, with a standardized incidence ratio (SIR) for malignancy of 0.99 across all treatment indications, the researchers said. In further analysis of malignancy by indication, the SIR was 0.87, 1.16, and 1.61 for psoriasis, PsA, and AS, respectively.
Data from postmarketing surveillance showed similar results: The estimated crude cumulative incidence reporting rate per 100 PTY was 0.27 for malignancy across all indications. The cumulative exposure was 285,811 PTY.
The study findings were limited by several factors including the post hoc design, differences in clinical trial methodologies, and lack of controlling for confounding variables, such as smoking status and previous exposure to systemic and biologic treatments, the researchers noted. In addition, the analysis did not include postexposure follow-up data, or data on patients who discontinued clinical trials, they said.
Overall, the analysis is the largest to date and supports the low risk of malignancy in patients with psoriasis, PsA, and AS treated with secukinumab, the researchers noted.
However, “while this assessment provides a broader understanding of the safety of secukinumab and supports its long-term use in these chronic systemic inflammatory conditions, registry data are further warranted to fully understand the real-world effect of biologics on malignancy risk,” they concluded.
“Secukinumab is a relatively newer biologic, approved in 2015, and there is currently a lack of longer-term data on the incidence of malignancy in secukinumab-treated patients, so it’s important to look at the data we have so far on this topic so we can better understand the long-term risks and counsel our psoriasis and psoriatic arthritis patients,” Flavia Fedeles, MD, of the department of dermatology at Massachusetts General Hospital, Boston, said in an interview.
Dr. Fedeles, who was not involved with the study, said that she was not surprised by the study results. “Data reported in the past from phase 3 clinical trials of secukinumab compared with placebo did not show an increase in risk of malignancy, though at that time no long-term safety data or data from patients with history of malignancy was available,” she said. “This study is reassuring in that there wasn’t a signal of increased malignancy events up to 5 years of secukinumab treatment,” said Dr. Fedeles.
However, she noted that the study has a number of limitations, including the use of clinical trials data, which have stringent inclusion/exclusion criteria that can lead to selection bias, the use of postmarketing surveillance data, the post hoc nature of the analysis, and the fact that the sponsor of the trial was the manufacturer of secukinumab, which “potentially can lead to bias to this study.”
She added that “registry data are needed to fully understand the real-world long-term effect of secukinumab on malignancy risk.”
The study was funded by Novartis. Lead author Dr. Lebwohl disclosed participating in advisory boards and/or as an investigator and/or speaker and receiving grants and/or honoraria from multiple companies including Novartis. Several study coauthors are employees of Novartis.
Dr. Fedeles had no financial conflicts to disclose.
that included 49 clinical trials.
Secukinumab (Cosentyx), an interleukin-17A antagonist, is approved for several conditions: moderate to severe psoriasis in children and adults, PsA, ankylosing spondylitis (AS), and nonradiographic axial spondyloarthritis.
Although secukinumab has demonstrated safety and tolerability, data on long-term malignancy rates are limited, wrote Mark Lebwohl, MD, professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and coauthors.
In a study published in the British Journal of Dermatology, they analyzed the combined safety data from clinical trials and postmarketing surveillance. The study population included 10,685 patients with psoriasis, 2,523 patients with PsA, and 1,311 patients with ankylosing spondylitis who received at least one approved dose of secukinumab (300 mg or 150 mg). The maximum follow-up was 5 years. The exposure-adjusted incidence rate was defined as the incidence rates per 100 patient treatment-years (PTY). The cumulative exposure for patients with psoriasis, PsA, and AS was 16,482, 4,944, and 2,668 PTY, respectively, with average follow-up times of 1.54, 1.96, and 2.03 years, respectively.
The observed and the expected number of malignancies were comparable, with a standardized incidence ratio (SIR) for malignancy of 0.99 across all treatment indications, the researchers said. In further analysis of malignancy by indication, the SIR was 0.87, 1.16, and 1.61 for psoriasis, PsA, and AS, respectively.
Data from postmarketing surveillance showed similar results: The estimated crude cumulative incidence reporting rate per 100 PTY was 0.27 for malignancy across all indications. The cumulative exposure was 285,811 PTY.
The study findings were limited by several factors including the post hoc design, differences in clinical trial methodologies, and lack of controlling for confounding variables, such as smoking status and previous exposure to systemic and biologic treatments, the researchers noted. In addition, the analysis did not include postexposure follow-up data, or data on patients who discontinued clinical trials, they said.
Overall, the analysis is the largest to date and supports the low risk of malignancy in patients with psoriasis, PsA, and AS treated with secukinumab, the researchers noted.
However, “while this assessment provides a broader understanding of the safety of secukinumab and supports its long-term use in these chronic systemic inflammatory conditions, registry data are further warranted to fully understand the real-world effect of biologics on malignancy risk,” they concluded.
“Secukinumab is a relatively newer biologic, approved in 2015, and there is currently a lack of longer-term data on the incidence of malignancy in secukinumab-treated patients, so it’s important to look at the data we have so far on this topic so we can better understand the long-term risks and counsel our psoriasis and psoriatic arthritis patients,” Flavia Fedeles, MD, of the department of dermatology at Massachusetts General Hospital, Boston, said in an interview.
Dr. Fedeles, who was not involved with the study, said that she was not surprised by the study results. “Data reported in the past from phase 3 clinical trials of secukinumab compared with placebo did not show an increase in risk of malignancy, though at that time no long-term safety data or data from patients with history of malignancy was available,” she said. “This study is reassuring in that there wasn’t a signal of increased malignancy events up to 5 years of secukinumab treatment,” said Dr. Fedeles.
However, she noted that the study has a number of limitations, including the use of clinical trials data, which have stringent inclusion/exclusion criteria that can lead to selection bias, the use of postmarketing surveillance data, the post hoc nature of the analysis, and the fact that the sponsor of the trial was the manufacturer of secukinumab, which “potentially can lead to bias to this study.”
She added that “registry data are needed to fully understand the real-world long-term effect of secukinumab on malignancy risk.”
The study was funded by Novartis. Lead author Dr. Lebwohl disclosed participating in advisory boards and/or as an investigator and/or speaker and receiving grants and/or honoraria from multiple companies including Novartis. Several study coauthors are employees of Novartis.
Dr. Fedeles had no financial conflicts to disclose.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
A pacemaker that 'just disappears' and a magnetic diet device
Ignore this pacemaker and it will go away
At some point – and now seems to be that point – we have to say enough is enough. The throwaway culture that produces phones, TVs, and computers that get tossed in the trash because they can’t be repaired has gone too far. That’s right, we’re looking at you, medical science!
This time, it’s a pacemaker that just disappears when it’s no longer needed. Some lazy heart surgeon decided that it was way too much trouble to do another surgery to remove the leads when a temporary pacemaker was no longer needed. You know the type: “It sure would be nice if the pacemaker components were biocompatible and were naturally absorbed by the body over the course of a few weeks and wouldn’t need to be surgically extracted.” Slacker.
Well, get a load of this. Researchers at Northwestern and George Washington universities say that they have come up with a transient pacemaker that “harvests energy from an external, remote antenna using near-field communication protocols – the same technology used in smartphones for electronic payments and in RFID tags.”
That means no batteries and no wires that have to be removed and can cause infections. Because the infectious disease docs also are too lazy to do their jobs, apparently.
The lack of onboard infrastructure means that the device can be very small – it weighs less than half a gram and is only 250 microns thick. And yes, it is bioresorbable and completely harmless. It fully degrades and disappears in 5-7 weeks through the body’s natural biologic processes, “thereby avoiding the need for physical removal of the pacemaker electrodes. This is potentially a major victory for postoperative patients,” said Dr. Rishi Arora, one of the investigators.
A victory for patients, he says. Not a word about the time and effort saved by the surgeons. Typical.
It’s a mask! No, it’s a COVID-19 test!
Mask wearing has gotten more lax as people get vaccinated for COVID-19, but as wearing masks for virus prevention is becoming more normalized in western society, some saw an opportunity to make them work for diagnosis.
Researchers from the Massachusetts Institute of Technology and the Wyss Institute for Biologically Inspired Engineering at Harvard University have found a way to do just that with their wearable freeze-dried cell-free (wFDCF) technology. A single push of a button releases water from a reservoir in the mask that sequentially activates three different freeze-dried biological reactions, which detect the SARS-CoV-2 virus in the wearer’s breath.
Initially meant as a tool for the Zika outbreak in 2015, the team made a quick pivot in May 2020. But this isn’t just some run-of-the-mill, at-home test. The data prove that the wFDCF mask is comparable to polymerase chain reactions tests, the standard in COVID-19 detection. Plus there aren’t any extra factors to deal with, like room or instrument temperature to ensure accuracy. In just 90 minutes, the mask gives results on a readout in a way similar to that of a pregnancy test. Voilà! To have COVID-19 or not to have COVID-19 is an easily answered question.
At LOTME, we think this is a big improvement from having dogs, or even three-foot rats, sniffing out coronavirus.
But wait, there’s more. “In addition to face masks, our programmable biosensors can be integrated into other garments to provide on-the-go detection of dangerous substances including viruses, bacteria, toxins, and chemical agents,” said Peter Nguyen, PhD, study coauthor and research scientist at the Wyss Institute. The technology can be used on lab coats, scrubs, military uniforms, and uniforms of first responders who may come in contact with hazardous pathogens and toxins. Think of all the lives saved and possible avoidances.
If only it could diagnose bad breath.
Finally, an excuse for the all-beer diet
Weight loss is hard work. Extremely hard work, and, as evidenced by the constant inundation and advertisement of quick fixes, crash diets, and expensive gym memberships, there’s not really a solid, 100% solution to the issue. Until now, thanks to a team of doctors from New Zealand, who’ve decided that the best way to combat obesity is to leave you in constant agony.
The DentalSlim Diet Control device is certainly a radical yet comically logical attempt to combat obesity. The creators say that the biggest problem with dieting is compliance, and, well, it’s difficult to eat too much if you can’t actually open your mouth. The metal contraption is mounted onto your teeth and uses magnetic locks to prevent the user from opening their mouths more than 2 mm. That’s less than a tenth of an inch. Which is not a lot. So not a lot that essentially all you can consume is liquid.
Oh, and they’ve got results to back up their madness. In a small study, seven otherwise healthy obese women lost an average of 5.1% of their body weight after using the DentalSlim for 2 weeks, though they did complain that the device was difficult to use, caused discomfort and difficulty speaking, made them more tense, and in general made life “less satisfying.” And one participant was able to cheat the system and consume nonhealthy food like chocolate by melting it.
So, there you are, if you want a weight-loss solution that tortures you and has far bigger holes than the one it leaves for your mouth, try the DentalSlim. Or, you know, don’t eat that eighth slice of pizza and maybe go for a walk later. Your choice.
Ignore this pacemaker and it will go away
At some point – and now seems to be that point – we have to say enough is enough. The throwaway culture that produces phones, TVs, and computers that get tossed in the trash because they can’t be repaired has gone too far. That’s right, we’re looking at you, medical science!
This time, it’s a pacemaker that just disappears when it’s no longer needed. Some lazy heart surgeon decided that it was way too much trouble to do another surgery to remove the leads when a temporary pacemaker was no longer needed. You know the type: “It sure would be nice if the pacemaker components were biocompatible and were naturally absorbed by the body over the course of a few weeks and wouldn’t need to be surgically extracted.” Slacker.
Well, get a load of this. Researchers at Northwestern and George Washington universities say that they have come up with a transient pacemaker that “harvests energy from an external, remote antenna using near-field communication protocols – the same technology used in smartphones for electronic payments and in RFID tags.”
That means no batteries and no wires that have to be removed and can cause infections. Because the infectious disease docs also are too lazy to do their jobs, apparently.
The lack of onboard infrastructure means that the device can be very small – it weighs less than half a gram and is only 250 microns thick. And yes, it is bioresorbable and completely harmless. It fully degrades and disappears in 5-7 weeks through the body’s natural biologic processes, “thereby avoiding the need for physical removal of the pacemaker electrodes. This is potentially a major victory for postoperative patients,” said Dr. Rishi Arora, one of the investigators.
A victory for patients, he says. Not a word about the time and effort saved by the surgeons. Typical.
It’s a mask! No, it’s a COVID-19 test!
Mask wearing has gotten more lax as people get vaccinated for COVID-19, but as wearing masks for virus prevention is becoming more normalized in western society, some saw an opportunity to make them work for diagnosis.
Researchers from the Massachusetts Institute of Technology and the Wyss Institute for Biologically Inspired Engineering at Harvard University have found a way to do just that with their wearable freeze-dried cell-free (wFDCF) technology. A single push of a button releases water from a reservoir in the mask that sequentially activates three different freeze-dried biological reactions, which detect the SARS-CoV-2 virus in the wearer’s breath.
Initially meant as a tool for the Zika outbreak in 2015, the team made a quick pivot in May 2020. But this isn’t just some run-of-the-mill, at-home test. The data prove that the wFDCF mask is comparable to polymerase chain reactions tests, the standard in COVID-19 detection. Plus there aren’t any extra factors to deal with, like room or instrument temperature to ensure accuracy. In just 90 minutes, the mask gives results on a readout in a way similar to that of a pregnancy test. Voilà! To have COVID-19 or not to have COVID-19 is an easily answered question.
At LOTME, we think this is a big improvement from having dogs, or even three-foot rats, sniffing out coronavirus.
But wait, there’s more. “In addition to face masks, our programmable biosensors can be integrated into other garments to provide on-the-go detection of dangerous substances including viruses, bacteria, toxins, and chemical agents,” said Peter Nguyen, PhD, study coauthor and research scientist at the Wyss Institute. The technology can be used on lab coats, scrubs, military uniforms, and uniforms of first responders who may come in contact with hazardous pathogens and toxins. Think of all the lives saved and possible avoidances.
If only it could diagnose bad breath.
Finally, an excuse for the all-beer diet
Weight loss is hard work. Extremely hard work, and, as evidenced by the constant inundation and advertisement of quick fixes, crash diets, and expensive gym memberships, there’s not really a solid, 100% solution to the issue. Until now, thanks to a team of doctors from New Zealand, who’ve decided that the best way to combat obesity is to leave you in constant agony.
The DentalSlim Diet Control device is certainly a radical yet comically logical attempt to combat obesity. The creators say that the biggest problem with dieting is compliance, and, well, it’s difficult to eat too much if you can’t actually open your mouth. The metal contraption is mounted onto your teeth and uses magnetic locks to prevent the user from opening their mouths more than 2 mm. That’s less than a tenth of an inch. Which is not a lot. So not a lot that essentially all you can consume is liquid.
Oh, and they’ve got results to back up their madness. In a small study, seven otherwise healthy obese women lost an average of 5.1% of their body weight after using the DentalSlim for 2 weeks, though they did complain that the device was difficult to use, caused discomfort and difficulty speaking, made them more tense, and in general made life “less satisfying.” And one participant was able to cheat the system and consume nonhealthy food like chocolate by melting it.
So, there you are, if you want a weight-loss solution that tortures you and has far bigger holes than the one it leaves for your mouth, try the DentalSlim. Or, you know, don’t eat that eighth slice of pizza and maybe go for a walk later. Your choice.
Ignore this pacemaker and it will go away
At some point – and now seems to be that point – we have to say enough is enough. The throwaway culture that produces phones, TVs, and computers that get tossed in the trash because they can’t be repaired has gone too far. That’s right, we’re looking at you, medical science!
This time, it’s a pacemaker that just disappears when it’s no longer needed. Some lazy heart surgeon decided that it was way too much trouble to do another surgery to remove the leads when a temporary pacemaker was no longer needed. You know the type: “It sure would be nice if the pacemaker components were biocompatible and were naturally absorbed by the body over the course of a few weeks and wouldn’t need to be surgically extracted.” Slacker.
Well, get a load of this. Researchers at Northwestern and George Washington universities say that they have come up with a transient pacemaker that “harvests energy from an external, remote antenna using near-field communication protocols – the same technology used in smartphones for electronic payments and in RFID tags.”
That means no batteries and no wires that have to be removed and can cause infections. Because the infectious disease docs also are too lazy to do their jobs, apparently.
The lack of onboard infrastructure means that the device can be very small – it weighs less than half a gram and is only 250 microns thick. And yes, it is bioresorbable and completely harmless. It fully degrades and disappears in 5-7 weeks through the body’s natural biologic processes, “thereby avoiding the need for physical removal of the pacemaker electrodes. This is potentially a major victory for postoperative patients,” said Dr. Rishi Arora, one of the investigators.
A victory for patients, he says. Not a word about the time and effort saved by the surgeons. Typical.
It’s a mask! No, it’s a COVID-19 test!
Mask wearing has gotten more lax as people get vaccinated for COVID-19, but as wearing masks for virus prevention is becoming more normalized in western society, some saw an opportunity to make them work for diagnosis.
Researchers from the Massachusetts Institute of Technology and the Wyss Institute for Biologically Inspired Engineering at Harvard University have found a way to do just that with their wearable freeze-dried cell-free (wFDCF) technology. A single push of a button releases water from a reservoir in the mask that sequentially activates three different freeze-dried biological reactions, which detect the SARS-CoV-2 virus in the wearer’s breath.
Initially meant as a tool for the Zika outbreak in 2015, the team made a quick pivot in May 2020. But this isn’t just some run-of-the-mill, at-home test. The data prove that the wFDCF mask is comparable to polymerase chain reactions tests, the standard in COVID-19 detection. Plus there aren’t any extra factors to deal with, like room or instrument temperature to ensure accuracy. In just 90 minutes, the mask gives results on a readout in a way similar to that of a pregnancy test. Voilà! To have COVID-19 or not to have COVID-19 is an easily answered question.
At LOTME, we think this is a big improvement from having dogs, or even three-foot rats, sniffing out coronavirus.
But wait, there’s more. “In addition to face masks, our programmable biosensors can be integrated into other garments to provide on-the-go detection of dangerous substances including viruses, bacteria, toxins, and chemical agents,” said Peter Nguyen, PhD, study coauthor and research scientist at the Wyss Institute. The technology can be used on lab coats, scrubs, military uniforms, and uniforms of first responders who may come in contact with hazardous pathogens and toxins. Think of all the lives saved and possible avoidances.
If only it could diagnose bad breath.
Finally, an excuse for the all-beer diet
Weight loss is hard work. Extremely hard work, and, as evidenced by the constant inundation and advertisement of quick fixes, crash diets, and expensive gym memberships, there’s not really a solid, 100% solution to the issue. Until now, thanks to a team of doctors from New Zealand, who’ve decided that the best way to combat obesity is to leave you in constant agony.
The DentalSlim Diet Control device is certainly a radical yet comically logical attempt to combat obesity. The creators say that the biggest problem with dieting is compliance, and, well, it’s difficult to eat too much if you can’t actually open your mouth. The metal contraption is mounted onto your teeth and uses magnetic locks to prevent the user from opening their mouths more than 2 mm. That’s less than a tenth of an inch. Which is not a lot. So not a lot that essentially all you can consume is liquid.
Oh, and they’ve got results to back up their madness. In a small study, seven otherwise healthy obese women lost an average of 5.1% of their body weight after using the DentalSlim for 2 weeks, though they did complain that the device was difficult to use, caused discomfort and difficulty speaking, made them more tense, and in general made life “less satisfying.” And one participant was able to cheat the system and consume nonhealthy food like chocolate by melting it.
So, there you are, if you want a weight-loss solution that tortures you and has far bigger holes than the one it leaves for your mouth, try the DentalSlim. Or, you know, don’t eat that eighth slice of pizza and maybe go for a walk later. Your choice.
Almost all U.S. COVID-19 deaths now in the unvaccinated
If you, a friend, or a loved one remain unvaccinated against COVID-19 at this point – for whatever reason – you are at higher risk of dying if you become infected.
That’s the conclusion of a new report released by the Associated Press looking at COVID-19 deaths during May 2021.
Of more than 18,000 people who died from COVID-19, for example, only about 150 were fully vaccinated. That’s less than 1%.
“Recently, I was working in the emergency room [and] I saw a 21-year-old African American who came in with shortness of breath,” said Vino K. Palli, MD, MPH, a physician specializing in emergency medicine, internal medicine, and urgent care.
The patient rapidly deteriorated and required intubation and ventilation. She was transferred to a specialized hospital for possible extracorporeal membrane oxygenation (ECMO) treatment.
“This patient was unvaccinated, along with her entire family. This would have been easily preventable,” added Dr. Palli, who is also founder and CEO of MiDoctor Urgent Care in New York City.
“Vaccine misinformation, compounded with vaccine inertia and vaccine access, have contributed to this,” he added. “Even though we have a surplus amount of vaccines at this time, we are only seeing 50% to 55% of completely vaccinated patients.”
Authors of the Associated Press report also acknowledge that some people who are fully vaccinated can get a breakthrough infection. These occurred in fewer than 1,200 of more than 853,000 people hospitalized for COVID-19 in May, or about 0.1%.
The Associated Press came up with these numbers using data from the Centers for Disease Control and Prevention. The CDC tracks the numbers of cases, hospitalizations, and deaths but does not breakdown rates by vaccination status.
Stronger argument for vaccination?
“The fact that only 0.8% of COVID-19 deaths are in the fully vaccinated should persuade those people still hesitant about vaccination,” said Hugh Cassiere, MD, medical director of Respiratory Therapy Services at North Shore University Hospital in Manhasset, New York.
Stuart C. Ray, MD, professor of medicine and oncology in the Division of Infectious Diseases at Johns Hopkins University, Baltimore, agreed. “It seems compelling, even for skeptics, that unvaccinated people represent 99% of those now dying from COVID-19 when they represent less than 50% of the adult population in the United States.”
The findings from the study could be more persuasive than previous arguments made in favor of immunization, Dr. Ray said. “These recent findings of striking reductions in risk of death in the vaccinated are more directly attributable and harder to ignore or dismiss.”
Brian Labus, PhD, MPH, of the University of Nevada Las Vegas (UNLV) is less convinced. “While this might change some peoples’ minds, it probably won’t make a major difference. People have many different reasons for not getting vaccinated, and this is only one of the things they consider.”
The study adds information that was not available before, said Dr. Labus, assistant professor in the Department of Epidemiology and Biostatistics at the UNLV School of Public Health. “We study the vaccine under tightly controlled, ideal conditions. This is the evidence that it works as well in the real world as it did in the trials, and that is what is most important in implementing a vaccination program,” added Dr. Labus.
“The scientific data has honed in on one thing: Vaccines are effective in preventing hospitalizations, ICU admissions, ventilations, and deaths,” agreed Dr. Palli.
“We now know that almost all deaths occurred in patients who were not vaccinated. We also know that all vaccines are effective against various strains that are in circulation right now, including the Delta variant, which is rapidly spreading,” Dr. Palli said.
Dr. Cassiere pointed out that the unvaccinated are not only at higher risk of developing COVID-19 but also of spreading, being hospitalized for, and dying from the infection. Avoiding “long hauler” symptoms is another argument in favor of immunization, he added.
As of June 28, the CDC reports that 63% of Americans 12 years and older have received at least one dose of a COVID-19 vaccine, and 54% are fully vaccinated.
Worldwide worry?
Although overall rates of U.S. COVID-19 hospitalizations and deaths are down, the outlook may not remain as encouraging. “I hope I’m wrong about this, but I anticipate that the coming fall and winter will bring increasingly localized versions of similar findings – severe disease and death due to SARS-CoV-2 infection in regions or groups with lower vaccination rates,” Dr. Ray said.
There could be a silver lining, he added: “If this unfortunate surge occurs, the health and economic consequences seem likely to erode much of the remaining hesitancy regarding vaccination.”
The rise of more infectious SARS-CoV-2 variants, such as the Delta variant, could also throw a wrench in controlling COVID-19. “This isn’t just a domestic issue,” Dr. Ray said. “We have learned that the world is a small place in pandemic times.”
The Associated Press investigators state that their findings support the high efficacy of the vaccine. Also, given the current widespread availability of COVID-19 vaccines in the United States, they believe many of the COVID-19 deaths now occurring are preventable.
Public health measures should have continued longer to protect unvaccinated individuals, especially Black Americans, Hispanic Americans, and other minorities, Dr. Palli said. “Only time will tell if re-opening and abandoning all public health measures by the CDC was premature.”
A version of this article first appeared on Medscape.com.
If you, a friend, or a loved one remain unvaccinated against COVID-19 at this point – for whatever reason – you are at higher risk of dying if you become infected.
That’s the conclusion of a new report released by the Associated Press looking at COVID-19 deaths during May 2021.
Of more than 18,000 people who died from COVID-19, for example, only about 150 were fully vaccinated. That’s less than 1%.
“Recently, I was working in the emergency room [and] I saw a 21-year-old African American who came in with shortness of breath,” said Vino K. Palli, MD, MPH, a physician specializing in emergency medicine, internal medicine, and urgent care.
The patient rapidly deteriorated and required intubation and ventilation. She was transferred to a specialized hospital for possible extracorporeal membrane oxygenation (ECMO) treatment.
“This patient was unvaccinated, along with her entire family. This would have been easily preventable,” added Dr. Palli, who is also founder and CEO of MiDoctor Urgent Care in New York City.
“Vaccine misinformation, compounded with vaccine inertia and vaccine access, have contributed to this,” he added. “Even though we have a surplus amount of vaccines at this time, we are only seeing 50% to 55% of completely vaccinated patients.”
Authors of the Associated Press report also acknowledge that some people who are fully vaccinated can get a breakthrough infection. These occurred in fewer than 1,200 of more than 853,000 people hospitalized for COVID-19 in May, or about 0.1%.
The Associated Press came up with these numbers using data from the Centers for Disease Control and Prevention. The CDC tracks the numbers of cases, hospitalizations, and deaths but does not breakdown rates by vaccination status.
Stronger argument for vaccination?
“The fact that only 0.8% of COVID-19 deaths are in the fully vaccinated should persuade those people still hesitant about vaccination,” said Hugh Cassiere, MD, medical director of Respiratory Therapy Services at North Shore University Hospital in Manhasset, New York.
Stuart C. Ray, MD, professor of medicine and oncology in the Division of Infectious Diseases at Johns Hopkins University, Baltimore, agreed. “It seems compelling, even for skeptics, that unvaccinated people represent 99% of those now dying from COVID-19 when they represent less than 50% of the adult population in the United States.”
The findings from the study could be more persuasive than previous arguments made in favor of immunization, Dr. Ray said. “These recent findings of striking reductions in risk of death in the vaccinated are more directly attributable and harder to ignore or dismiss.”
Brian Labus, PhD, MPH, of the University of Nevada Las Vegas (UNLV) is less convinced. “While this might change some peoples’ minds, it probably won’t make a major difference. People have many different reasons for not getting vaccinated, and this is only one of the things they consider.”
The study adds information that was not available before, said Dr. Labus, assistant professor in the Department of Epidemiology and Biostatistics at the UNLV School of Public Health. “We study the vaccine under tightly controlled, ideal conditions. This is the evidence that it works as well in the real world as it did in the trials, and that is what is most important in implementing a vaccination program,” added Dr. Labus.
“The scientific data has honed in on one thing: Vaccines are effective in preventing hospitalizations, ICU admissions, ventilations, and deaths,” agreed Dr. Palli.
“We now know that almost all deaths occurred in patients who were not vaccinated. We also know that all vaccines are effective against various strains that are in circulation right now, including the Delta variant, which is rapidly spreading,” Dr. Palli said.
Dr. Cassiere pointed out that the unvaccinated are not only at higher risk of developing COVID-19 but also of spreading, being hospitalized for, and dying from the infection. Avoiding “long hauler” symptoms is another argument in favor of immunization, he added.
As of June 28, the CDC reports that 63% of Americans 12 years and older have received at least one dose of a COVID-19 vaccine, and 54% are fully vaccinated.
Worldwide worry?
Although overall rates of U.S. COVID-19 hospitalizations and deaths are down, the outlook may not remain as encouraging. “I hope I’m wrong about this, but I anticipate that the coming fall and winter will bring increasingly localized versions of similar findings – severe disease and death due to SARS-CoV-2 infection in regions or groups with lower vaccination rates,” Dr. Ray said.
There could be a silver lining, he added: “If this unfortunate surge occurs, the health and economic consequences seem likely to erode much of the remaining hesitancy regarding vaccination.”
The rise of more infectious SARS-CoV-2 variants, such as the Delta variant, could also throw a wrench in controlling COVID-19. “This isn’t just a domestic issue,” Dr. Ray said. “We have learned that the world is a small place in pandemic times.”
The Associated Press investigators state that their findings support the high efficacy of the vaccine. Also, given the current widespread availability of COVID-19 vaccines in the United States, they believe many of the COVID-19 deaths now occurring are preventable.
Public health measures should have continued longer to protect unvaccinated individuals, especially Black Americans, Hispanic Americans, and other minorities, Dr. Palli said. “Only time will tell if re-opening and abandoning all public health measures by the CDC was premature.”
A version of this article first appeared on Medscape.com.
If you, a friend, or a loved one remain unvaccinated against COVID-19 at this point – for whatever reason – you are at higher risk of dying if you become infected.
That’s the conclusion of a new report released by the Associated Press looking at COVID-19 deaths during May 2021.
Of more than 18,000 people who died from COVID-19, for example, only about 150 were fully vaccinated. That’s less than 1%.
“Recently, I was working in the emergency room [and] I saw a 21-year-old African American who came in with shortness of breath,” said Vino K. Palli, MD, MPH, a physician specializing in emergency medicine, internal medicine, and urgent care.
The patient rapidly deteriorated and required intubation and ventilation. She was transferred to a specialized hospital for possible extracorporeal membrane oxygenation (ECMO) treatment.
“This patient was unvaccinated, along with her entire family. This would have been easily preventable,” added Dr. Palli, who is also founder and CEO of MiDoctor Urgent Care in New York City.
“Vaccine misinformation, compounded with vaccine inertia and vaccine access, have contributed to this,” he added. “Even though we have a surplus amount of vaccines at this time, we are only seeing 50% to 55% of completely vaccinated patients.”
Authors of the Associated Press report also acknowledge that some people who are fully vaccinated can get a breakthrough infection. These occurred in fewer than 1,200 of more than 853,000 people hospitalized for COVID-19 in May, or about 0.1%.
The Associated Press came up with these numbers using data from the Centers for Disease Control and Prevention. The CDC tracks the numbers of cases, hospitalizations, and deaths but does not breakdown rates by vaccination status.
Stronger argument for vaccination?
“The fact that only 0.8% of COVID-19 deaths are in the fully vaccinated should persuade those people still hesitant about vaccination,” said Hugh Cassiere, MD, medical director of Respiratory Therapy Services at North Shore University Hospital in Manhasset, New York.
Stuart C. Ray, MD, professor of medicine and oncology in the Division of Infectious Diseases at Johns Hopkins University, Baltimore, agreed. “It seems compelling, even for skeptics, that unvaccinated people represent 99% of those now dying from COVID-19 when they represent less than 50% of the adult population in the United States.”
The findings from the study could be more persuasive than previous arguments made in favor of immunization, Dr. Ray said. “These recent findings of striking reductions in risk of death in the vaccinated are more directly attributable and harder to ignore or dismiss.”
Brian Labus, PhD, MPH, of the University of Nevada Las Vegas (UNLV) is less convinced. “While this might change some peoples’ minds, it probably won’t make a major difference. People have many different reasons for not getting vaccinated, and this is only one of the things they consider.”
The study adds information that was not available before, said Dr. Labus, assistant professor in the Department of Epidemiology and Biostatistics at the UNLV School of Public Health. “We study the vaccine under tightly controlled, ideal conditions. This is the evidence that it works as well in the real world as it did in the trials, and that is what is most important in implementing a vaccination program,” added Dr. Labus.
“The scientific data has honed in on one thing: Vaccines are effective in preventing hospitalizations, ICU admissions, ventilations, and deaths,” agreed Dr. Palli.
“We now know that almost all deaths occurred in patients who were not vaccinated. We also know that all vaccines are effective against various strains that are in circulation right now, including the Delta variant, which is rapidly spreading,” Dr. Palli said.
Dr. Cassiere pointed out that the unvaccinated are not only at higher risk of developing COVID-19 but also of spreading, being hospitalized for, and dying from the infection. Avoiding “long hauler” symptoms is another argument in favor of immunization, he added.
As of June 28, the CDC reports that 63% of Americans 12 years and older have received at least one dose of a COVID-19 vaccine, and 54% are fully vaccinated.
Worldwide worry?
Although overall rates of U.S. COVID-19 hospitalizations and deaths are down, the outlook may not remain as encouraging. “I hope I’m wrong about this, but I anticipate that the coming fall and winter will bring increasingly localized versions of similar findings – severe disease and death due to SARS-CoV-2 infection in regions or groups with lower vaccination rates,” Dr. Ray said.
There could be a silver lining, he added: “If this unfortunate surge occurs, the health and economic consequences seem likely to erode much of the remaining hesitancy regarding vaccination.”
The rise of more infectious SARS-CoV-2 variants, such as the Delta variant, could also throw a wrench in controlling COVID-19. “This isn’t just a domestic issue,” Dr. Ray said. “We have learned that the world is a small place in pandemic times.”
The Associated Press investigators state that their findings support the high efficacy of the vaccine. Also, given the current widespread availability of COVID-19 vaccines in the United States, they believe many of the COVID-19 deaths now occurring are preventable.
Public health measures should have continued longer to protect unvaccinated individuals, especially Black Americans, Hispanic Americans, and other minorities, Dr. Palli said. “Only time will tell if re-opening and abandoning all public health measures by the CDC was premature.”
A version of this article first appeared on Medscape.com.
Physician fired after slurs, including ‘cannibalism,’ against Israel
Fidaa Wishah, MD, a pediatric radiologist at Phoenix Children’s Hospital in Arizona, has been fired after the hospital reviewed evidence that included her anti-Israel comments on social media, according to the hospital’s statement.
On May 26, Dr. Wishah posted, “We will uncover your thirst to kill our Palestinian children. … We sense your fear. The fear of your collapse. A state based on atrocity, inhumanity, racism and cannibalism never last long! Hey #israel … your end is coming sooner than you think.”
Phoenix Children’s Hospital did not respond to this news organization’s request for comment but said in a statement to the Jewish News Syndicate : “After a thorough review of the facts related to this matter, this individual is no longer providing care at Phoenix Children’s. All children in the care of Phoenix Children’s receive hope, healing and the best possible health care, regardless of race, color, disability, religion, gender, gender identity, sexual orientation or national origin.”
Dr. Wishah’s profile has been removed from the hospital website. Her LinkedIn profile indicates she had been a pediatric radiology fellow at Stanford (Calif.) University, specializing in advanced magnetic resonance imaging and fetal imaging and had been a senior staff pediatric radiologist at Henry Ford Health System in Detroit.
It wasn’t the first time antisemitic comments have led to the firing of a physician. Last year, this news organization wrote about Lara Kollab, DO, a first-year resident fired for her antisemitic tweets. She was subsequently barred from medicine.
In the same post from May 26, Dr. Wishah also wrote: “We will not be #censored anymore! Bomb our media buildings and we have the phones[.] Bribe the mainstream media and we have our small #socialmedia platforms[.] From our windows ... from our streets ... next the rubble we will expose you to the world[.] We will expose the #massacre and #genocide you #zionists are proud of[.]”
Today, CAIR-AZ, a group whose mission is to “enhance understanding of Islam, protect civil rights, promote justice, and empower American Muslims,” according to its website, announced that it, along with three private law firms, will represent Dr. Wishah in what they referred to as “her wrongful termination case against Phoenix Children’s Hospital.”
The announcement, which mentions that Dr. Wishah was born and raised in Gaza, said, “Dr. Wishah has been a medical doctor since 2010 and has spent the vast majority of her career as a pediatric physician. Despite caring for thousands of children, many of whom are Jewish, she has never been accused of discriminating against any of her patients or colleagues.”
The statement added, “PCH’s decision to terminate Dr. Wishah is shameful and an attack on freedom of speech.”
A version of this article first appeared on Medscape.com.
Fidaa Wishah, MD, a pediatric radiologist at Phoenix Children’s Hospital in Arizona, has been fired after the hospital reviewed evidence that included her anti-Israel comments on social media, according to the hospital’s statement.
On May 26, Dr. Wishah posted, “We will uncover your thirst to kill our Palestinian children. … We sense your fear. The fear of your collapse. A state based on atrocity, inhumanity, racism and cannibalism never last long! Hey #israel … your end is coming sooner than you think.”
Phoenix Children’s Hospital did not respond to this news organization’s request for comment but said in a statement to the Jewish News Syndicate : “After a thorough review of the facts related to this matter, this individual is no longer providing care at Phoenix Children’s. All children in the care of Phoenix Children’s receive hope, healing and the best possible health care, regardless of race, color, disability, religion, gender, gender identity, sexual orientation or national origin.”
Dr. Wishah’s profile has been removed from the hospital website. Her LinkedIn profile indicates she had been a pediatric radiology fellow at Stanford (Calif.) University, specializing in advanced magnetic resonance imaging and fetal imaging and had been a senior staff pediatric radiologist at Henry Ford Health System in Detroit.
It wasn’t the first time antisemitic comments have led to the firing of a physician. Last year, this news organization wrote about Lara Kollab, DO, a first-year resident fired for her antisemitic tweets. She was subsequently barred from medicine.
In the same post from May 26, Dr. Wishah also wrote: “We will not be #censored anymore! Bomb our media buildings and we have the phones[.] Bribe the mainstream media and we have our small #socialmedia platforms[.] From our windows ... from our streets ... next the rubble we will expose you to the world[.] We will expose the #massacre and #genocide you #zionists are proud of[.]”
Today, CAIR-AZ, a group whose mission is to “enhance understanding of Islam, protect civil rights, promote justice, and empower American Muslims,” according to its website, announced that it, along with three private law firms, will represent Dr. Wishah in what they referred to as “her wrongful termination case against Phoenix Children’s Hospital.”
The announcement, which mentions that Dr. Wishah was born and raised in Gaza, said, “Dr. Wishah has been a medical doctor since 2010 and has spent the vast majority of her career as a pediatric physician. Despite caring for thousands of children, many of whom are Jewish, she has never been accused of discriminating against any of her patients or colleagues.”
The statement added, “PCH’s decision to terminate Dr. Wishah is shameful and an attack on freedom of speech.”
A version of this article first appeared on Medscape.com.
Fidaa Wishah, MD, a pediatric radiologist at Phoenix Children’s Hospital in Arizona, has been fired after the hospital reviewed evidence that included her anti-Israel comments on social media, according to the hospital’s statement.
On May 26, Dr. Wishah posted, “We will uncover your thirst to kill our Palestinian children. … We sense your fear. The fear of your collapse. A state based on atrocity, inhumanity, racism and cannibalism never last long! Hey #israel … your end is coming sooner than you think.”
Phoenix Children’s Hospital did not respond to this news organization’s request for comment but said in a statement to the Jewish News Syndicate : “After a thorough review of the facts related to this matter, this individual is no longer providing care at Phoenix Children’s. All children in the care of Phoenix Children’s receive hope, healing and the best possible health care, regardless of race, color, disability, religion, gender, gender identity, sexual orientation or national origin.”
Dr. Wishah’s profile has been removed from the hospital website. Her LinkedIn profile indicates she had been a pediatric radiology fellow at Stanford (Calif.) University, specializing in advanced magnetic resonance imaging and fetal imaging and had been a senior staff pediatric radiologist at Henry Ford Health System in Detroit.
It wasn’t the first time antisemitic comments have led to the firing of a physician. Last year, this news organization wrote about Lara Kollab, DO, a first-year resident fired for her antisemitic tweets. She was subsequently barred from medicine.
In the same post from May 26, Dr. Wishah also wrote: “We will not be #censored anymore! Bomb our media buildings and we have the phones[.] Bribe the mainstream media and we have our small #socialmedia platforms[.] From our windows ... from our streets ... next the rubble we will expose you to the world[.] We will expose the #massacre and #genocide you #zionists are proud of[.]”
Today, CAIR-AZ, a group whose mission is to “enhance understanding of Islam, protect civil rights, promote justice, and empower American Muslims,” according to its website, announced that it, along with three private law firms, will represent Dr. Wishah in what they referred to as “her wrongful termination case against Phoenix Children’s Hospital.”
The announcement, which mentions that Dr. Wishah was born and raised in Gaza, said, “Dr. Wishah has been a medical doctor since 2010 and has spent the vast majority of her career as a pediatric physician. Despite caring for thousands of children, many of whom are Jewish, she has never been accused of discriminating against any of her patients or colleagues.”
The statement added, “PCH’s decision to terminate Dr. Wishah is shameful and an attack on freedom of speech.”
A version of this article first appeared on Medscape.com.
Wrong-site surgery doc says he can’t be sued
A neurosurgeon who operated on the wrong side of his patient’s spine claims he can’t be sued because of a federal law that protects health care professionals during a public health emergency, according to a report by KSDK, an NBC-affiliated television station in St. Louis.
Natalie Avilez, who lives in Missouri with her husband and five children, had been suffering from intense back pain. At some point in the recent past (the story doesn’t identify precisely when), she was referred to Fangxiang Chen, MD, a neurosurgeon affiliated with Mercy Hospital and Mercy Hospital South, in St. Louis. Ms. Avilez reportedly claims that Dr. Chen told her that an “easy” surgery – a hemilaminectomy – could relieve her back pain.
Something went wrong during the procedure, however. Dr. Chen ended up operating on the left side of Avilez’s spine instead of the right side, where he had initially diagnosed disk-related pressure. Dr. Chen realized his mistake while his patient was under anesthesia but couldn’t remedy it.
As the patient awakened, Dr. Chen asked her to authorize an immediate right-side surgery, but, as Ms. Avilez told the TV station, her “charge nurse would not let him get authorization because I wasn’t fully awake.” In the recovery room afterward, Dr. Chen explained what had happened to his patient, who permitted him to redo the surgery the following day.
But the redo didn’t remedy Ms. Avilez’s pain; in fact, the second surgery made things worse. “I’m always in constant pain,” she said. “I kind of feel like I would have been better off not even doing it at all.”
In January of this year, Ms. Avilez filed a medical malpractice suit against Dr. Chen and Mercy. But the neurosurgeon made a surprising claim:
Initially passed in 2005, PREP was intended to shield doctors and other licensed health care professionals from liability during a public health emergency except in cases of willful misconduct. On March 17, 2020, then–Health and Human Services Secretary Alex Azar invoked the PREP Act “for activities related to medical countermeasures against COVID-19.”
But could this declaration – which has since been amended multiple times – shield a physician from a claim of wrong-site surgery?
Ms. Avilez’s attorney, Morgan Murphy, doesn’t think so. “Obviously, we are not claiming that COVID had anything to do with the fact that Dr. Chen operated on the incorrect side of Natalie’s spine. It is a fairly straightforward situation. A doctor should never perform the incorrect surgery, period.”
Other observers are less certain that the Chen defense won’t hold. It’s true the PREP Act doesn’t protect doctors against claims of willful or intentional misconduct, says Deidre Gilbert, who leads a national medical malpractice patient-advocacy group. But such claims are, she quickly adds, very difficult to prove, never more so than during a pandemic.
Several states, including Missouri, have passed or are considering additional measures to protect health care professionals against the expected wave of COVID-related claims. (One estimate places the number of those claims at almost 6,000 as of February 2021.) “We want to make sure that there is a heightened standard for holding somebody liable in ... COVID transmission cases,” said the sponsor of the proposed Show-Me State legislation.
As for Ms. Avilez, she feels lucky that she’s not even worse off than she is now. She worries, though, about other patients who are less fortunate and who are told that the pandemic protects their health care professionals from liability. “That’s just not fair,” she says.
Hidden beliefs about people of color raise liability risks
Clinicians’ “implicit bias” can exacerbate medical disparities and also malpractice claims, a story in the Dayton Daily News reports.
The story’s authors cite La Fleur Small, PhD, a medical sociologist at Wayne State University, in Detroit, who sees “implicit bias” as a set of “unconscious associations and judgments” that affect social behavior, causing people to act in ways that are often contrary to their perceived value system. In the medical profession, such thinking can have unintended consequences, especially for people of color.
Implicit bias can erode the physician-patient relationship, which in turn can make a malpractice suit more likely should an adverse event occur. Studies reported in recent years in the AMA Journal of Ethics, for instance, found that poor communication was a factor in almost three-quarters of closed claims. Other studies have revealed that, of patients seeking legal advice following a medical mishap, more than half cited a poor doctor-patient relationship as a contributing factor in their decision.
To remedy things, it would be helpful to boost the number of doctors of color, at least to the point that it more closely reflects the percentage in the general population, say experts. Currently, although Black and Hispanic persons constitute 13.4% and 18.5%, respectively, of the overall U.S. population, they make up only 5.0% and 5.8% of active physicians. (As of 2018, 56.2% of all physicians were White and 17.2% were Asian, according to data from the Association of American Medical Colleges.)
Father of impaired baby seeks mega damages
An Oregon man whose son sustained permanent neurologic injuries during childbirth has sued the hospital where the 2017 delivery took place, as reported in The Astorian.
In the suit on behalf of his son, Wesley Humphries claims that Columbia Memorial Hospital in Astoria, Oregon, failed to monitor the baby’s heart rate and other aspects of the labor and delivery. As a consequence, the baby needed to be transferred to Oregon Health and Science University Hospital in Portland, approximately 100 miles away, for emergency treatment. Doctors there diagnosed the child as having hypoxic ischemic encephalopathy, which his lawyers say resulted in cerebral palsy, among other neurologic conditions.
Because of his son’s permanent impairment, Mr. Humphries is seeking significant damages: more than $45 million in medical, custodial, and life-care expenses and $65 million in noneconomic damages. Should his claim prove successful, the payout would mark one of the largest awards – if not the largest award – in Oregon State history. The hospital has declined to comment.
At press time, a trial date hadn’t been set.
A version of this article first appeared on Medscape.com.
A neurosurgeon who operated on the wrong side of his patient’s spine claims he can’t be sued because of a federal law that protects health care professionals during a public health emergency, according to a report by KSDK, an NBC-affiliated television station in St. Louis.
Natalie Avilez, who lives in Missouri with her husband and five children, had been suffering from intense back pain. At some point in the recent past (the story doesn’t identify precisely when), she was referred to Fangxiang Chen, MD, a neurosurgeon affiliated with Mercy Hospital and Mercy Hospital South, in St. Louis. Ms. Avilez reportedly claims that Dr. Chen told her that an “easy” surgery – a hemilaminectomy – could relieve her back pain.
Something went wrong during the procedure, however. Dr. Chen ended up operating on the left side of Avilez’s spine instead of the right side, where he had initially diagnosed disk-related pressure. Dr. Chen realized his mistake while his patient was under anesthesia but couldn’t remedy it.
As the patient awakened, Dr. Chen asked her to authorize an immediate right-side surgery, but, as Ms. Avilez told the TV station, her “charge nurse would not let him get authorization because I wasn’t fully awake.” In the recovery room afterward, Dr. Chen explained what had happened to his patient, who permitted him to redo the surgery the following day.
But the redo didn’t remedy Ms. Avilez’s pain; in fact, the second surgery made things worse. “I’m always in constant pain,” she said. “I kind of feel like I would have been better off not even doing it at all.”
In January of this year, Ms. Avilez filed a medical malpractice suit against Dr. Chen and Mercy. But the neurosurgeon made a surprising claim:
Initially passed in 2005, PREP was intended to shield doctors and other licensed health care professionals from liability during a public health emergency except in cases of willful misconduct. On March 17, 2020, then–Health and Human Services Secretary Alex Azar invoked the PREP Act “for activities related to medical countermeasures against COVID-19.”
But could this declaration – which has since been amended multiple times – shield a physician from a claim of wrong-site surgery?
Ms. Avilez’s attorney, Morgan Murphy, doesn’t think so. “Obviously, we are not claiming that COVID had anything to do with the fact that Dr. Chen operated on the incorrect side of Natalie’s spine. It is a fairly straightforward situation. A doctor should never perform the incorrect surgery, period.”
Other observers are less certain that the Chen defense won’t hold. It’s true the PREP Act doesn’t protect doctors against claims of willful or intentional misconduct, says Deidre Gilbert, who leads a national medical malpractice patient-advocacy group. But such claims are, she quickly adds, very difficult to prove, never more so than during a pandemic.
Several states, including Missouri, have passed or are considering additional measures to protect health care professionals against the expected wave of COVID-related claims. (One estimate places the number of those claims at almost 6,000 as of February 2021.) “We want to make sure that there is a heightened standard for holding somebody liable in ... COVID transmission cases,” said the sponsor of the proposed Show-Me State legislation.
As for Ms. Avilez, she feels lucky that she’s not even worse off than she is now. She worries, though, about other patients who are less fortunate and who are told that the pandemic protects their health care professionals from liability. “That’s just not fair,” she says.
Hidden beliefs about people of color raise liability risks
Clinicians’ “implicit bias” can exacerbate medical disparities and also malpractice claims, a story in the Dayton Daily News reports.
The story’s authors cite La Fleur Small, PhD, a medical sociologist at Wayne State University, in Detroit, who sees “implicit bias” as a set of “unconscious associations and judgments” that affect social behavior, causing people to act in ways that are often contrary to their perceived value system. In the medical profession, such thinking can have unintended consequences, especially for people of color.
Implicit bias can erode the physician-patient relationship, which in turn can make a malpractice suit more likely should an adverse event occur. Studies reported in recent years in the AMA Journal of Ethics, for instance, found that poor communication was a factor in almost three-quarters of closed claims. Other studies have revealed that, of patients seeking legal advice following a medical mishap, more than half cited a poor doctor-patient relationship as a contributing factor in their decision.
To remedy things, it would be helpful to boost the number of doctors of color, at least to the point that it more closely reflects the percentage in the general population, say experts. Currently, although Black and Hispanic persons constitute 13.4% and 18.5%, respectively, of the overall U.S. population, they make up only 5.0% and 5.8% of active physicians. (As of 2018, 56.2% of all physicians were White and 17.2% were Asian, according to data from the Association of American Medical Colleges.)
Father of impaired baby seeks mega damages
An Oregon man whose son sustained permanent neurologic injuries during childbirth has sued the hospital where the 2017 delivery took place, as reported in The Astorian.
In the suit on behalf of his son, Wesley Humphries claims that Columbia Memorial Hospital in Astoria, Oregon, failed to monitor the baby’s heart rate and other aspects of the labor and delivery. As a consequence, the baby needed to be transferred to Oregon Health and Science University Hospital in Portland, approximately 100 miles away, for emergency treatment. Doctors there diagnosed the child as having hypoxic ischemic encephalopathy, which his lawyers say resulted in cerebral palsy, among other neurologic conditions.
Because of his son’s permanent impairment, Mr. Humphries is seeking significant damages: more than $45 million in medical, custodial, and life-care expenses and $65 million in noneconomic damages. Should his claim prove successful, the payout would mark one of the largest awards – if not the largest award – in Oregon State history. The hospital has declined to comment.
At press time, a trial date hadn’t been set.
A version of this article first appeared on Medscape.com.
A neurosurgeon who operated on the wrong side of his patient’s spine claims he can’t be sued because of a federal law that protects health care professionals during a public health emergency, according to a report by KSDK, an NBC-affiliated television station in St. Louis.
Natalie Avilez, who lives in Missouri with her husband and five children, had been suffering from intense back pain. At some point in the recent past (the story doesn’t identify precisely when), she was referred to Fangxiang Chen, MD, a neurosurgeon affiliated with Mercy Hospital and Mercy Hospital South, in St. Louis. Ms. Avilez reportedly claims that Dr. Chen told her that an “easy” surgery – a hemilaminectomy – could relieve her back pain.
Something went wrong during the procedure, however. Dr. Chen ended up operating on the left side of Avilez’s spine instead of the right side, where he had initially diagnosed disk-related pressure. Dr. Chen realized his mistake while his patient was under anesthesia but couldn’t remedy it.
As the patient awakened, Dr. Chen asked her to authorize an immediate right-side surgery, but, as Ms. Avilez told the TV station, her “charge nurse would not let him get authorization because I wasn’t fully awake.” In the recovery room afterward, Dr. Chen explained what had happened to his patient, who permitted him to redo the surgery the following day.
But the redo didn’t remedy Ms. Avilez’s pain; in fact, the second surgery made things worse. “I’m always in constant pain,” she said. “I kind of feel like I would have been better off not even doing it at all.”
In January of this year, Ms. Avilez filed a medical malpractice suit against Dr. Chen and Mercy. But the neurosurgeon made a surprising claim:
Initially passed in 2005, PREP was intended to shield doctors and other licensed health care professionals from liability during a public health emergency except in cases of willful misconduct. On March 17, 2020, then–Health and Human Services Secretary Alex Azar invoked the PREP Act “for activities related to medical countermeasures against COVID-19.”
But could this declaration – which has since been amended multiple times – shield a physician from a claim of wrong-site surgery?
Ms. Avilez’s attorney, Morgan Murphy, doesn’t think so. “Obviously, we are not claiming that COVID had anything to do with the fact that Dr. Chen operated on the incorrect side of Natalie’s spine. It is a fairly straightforward situation. A doctor should never perform the incorrect surgery, period.”
Other observers are less certain that the Chen defense won’t hold. It’s true the PREP Act doesn’t protect doctors against claims of willful or intentional misconduct, says Deidre Gilbert, who leads a national medical malpractice patient-advocacy group. But such claims are, she quickly adds, very difficult to prove, never more so than during a pandemic.
Several states, including Missouri, have passed or are considering additional measures to protect health care professionals against the expected wave of COVID-related claims. (One estimate places the number of those claims at almost 6,000 as of February 2021.) “We want to make sure that there is a heightened standard for holding somebody liable in ... COVID transmission cases,” said the sponsor of the proposed Show-Me State legislation.
As for Ms. Avilez, she feels lucky that she’s not even worse off than she is now. She worries, though, about other patients who are less fortunate and who are told that the pandemic protects their health care professionals from liability. “That’s just not fair,” she says.
Hidden beliefs about people of color raise liability risks
Clinicians’ “implicit bias” can exacerbate medical disparities and also malpractice claims, a story in the Dayton Daily News reports.
The story’s authors cite La Fleur Small, PhD, a medical sociologist at Wayne State University, in Detroit, who sees “implicit bias” as a set of “unconscious associations and judgments” that affect social behavior, causing people to act in ways that are often contrary to their perceived value system. In the medical profession, such thinking can have unintended consequences, especially for people of color.
Implicit bias can erode the physician-patient relationship, which in turn can make a malpractice suit more likely should an adverse event occur. Studies reported in recent years in the AMA Journal of Ethics, for instance, found that poor communication was a factor in almost three-quarters of closed claims. Other studies have revealed that, of patients seeking legal advice following a medical mishap, more than half cited a poor doctor-patient relationship as a contributing factor in their decision.
To remedy things, it would be helpful to boost the number of doctors of color, at least to the point that it more closely reflects the percentage in the general population, say experts. Currently, although Black and Hispanic persons constitute 13.4% and 18.5%, respectively, of the overall U.S. population, they make up only 5.0% and 5.8% of active physicians. (As of 2018, 56.2% of all physicians were White and 17.2% were Asian, according to data from the Association of American Medical Colleges.)
Father of impaired baby seeks mega damages
An Oregon man whose son sustained permanent neurologic injuries during childbirth has sued the hospital where the 2017 delivery took place, as reported in The Astorian.
In the suit on behalf of his son, Wesley Humphries claims that Columbia Memorial Hospital in Astoria, Oregon, failed to monitor the baby’s heart rate and other aspects of the labor and delivery. As a consequence, the baby needed to be transferred to Oregon Health and Science University Hospital in Portland, approximately 100 miles away, for emergency treatment. Doctors there diagnosed the child as having hypoxic ischemic encephalopathy, which his lawyers say resulted in cerebral palsy, among other neurologic conditions.
Because of his son’s permanent impairment, Mr. Humphries is seeking significant damages: more than $45 million in medical, custodial, and life-care expenses and $65 million in noneconomic damages. Should his claim prove successful, the payout would mark one of the largest awards – if not the largest award – in Oregon State history. The hospital has declined to comment.
At press time, a trial date hadn’t been set.
A version of this article first appeared on Medscape.com.