User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'medstat-accordion-set article-series')]
Omicron may require fourth vaccine dose, Pfizer says
, Pfizer officials said on Dec. 8.
The standard two doses may be less effective against the variant, the company announced earlier in the day, and a booster dose increases neutralizing antibodies.
But the timeline might need to be moved up for a fourth dose. Previously, Pfizer CEO Albert Bourla, PhD, said another dose might be needed about a year after a third shot. Now the company’s scientists believe that a fourth shot, which targets the Omicron variant, could be required sooner.
“With Omicron, we need to wait and see because we have very little information. We may need it faster,” Dr. Bourla said on CNBC’s Squawk Box.
“But for right now, the most important thing is that we have winter in front of us,” he said. “From a healthcare perspective, it is important to understand that we need to be well-protected to go through the winter.”
A third dose should provide protection throughout the winter, Dr. Bourla said. That may buy time until the early spring to develop new shots that target Omicron, which Pfizer could have ready by March, according to Bloomberg News.
As of the afternoon of Dec. 8, 43 people in 19 states had tested positive for the Omicron variant, according to The Associated Press. More than 75% had been vaccinated, and a third had had booster shots. About a third had traveled internationally.
Nearly all of them have had mild symptoms so far, the AP reported, with the most common symptoms being a cough, congestion, and fatigue. One person has been hospitalized, but no deaths have been reported so far.
The CDC is still trying to determine how the Omicron variant may affect the course of the pandemic and whether the strain is more contagious or causes more severe disease.
“What we generally know is the more mutations a variant has, the higher level you need your immunity to be,” Rochelle Walensky, MD, director of the CDC, told the AP.
“We want to make sure we bolster everybody’s immunity,” she said. “And that’s really what motivated the decision to expand our guidance [on boosters for all adults].”
The Omicron variant has been reported in 57 countries so far, World Health Organization officials reported Dec. 8, and they expect that number to continue growing.
“Certain features of Omicron, including its global spread and large number of mutations, suggest it could have a major impact on the course of the pandemic. Exactly what that impact will be is still difficult to know,” Tedros Adhanom Ghebreyesus, PhD, the World Health Organization’s director-general, said during a media briefing.
Several studies suggest that Omicron leads to a rapid increase in transmission, he said, though scientists are still trying to understand whether it can “outcompete Delta.” Data from South Africa also suggests a higher risk of reinfection with Omicron, though it appears to cause milder disease than Delta, he noted.
“Even though we still need answers to some crucial questions, we are not defenseless against Omicron or Delta,” he said. “The steps countries take today, and in the coming days and weeks, will determine how Omicron unfolds.”
A version of this article first appeared on WebMD.com.
, Pfizer officials said on Dec. 8.
The standard two doses may be less effective against the variant, the company announced earlier in the day, and a booster dose increases neutralizing antibodies.
But the timeline might need to be moved up for a fourth dose. Previously, Pfizer CEO Albert Bourla, PhD, said another dose might be needed about a year after a third shot. Now the company’s scientists believe that a fourth shot, which targets the Omicron variant, could be required sooner.
“With Omicron, we need to wait and see because we have very little information. We may need it faster,” Dr. Bourla said on CNBC’s Squawk Box.
“But for right now, the most important thing is that we have winter in front of us,” he said. “From a healthcare perspective, it is important to understand that we need to be well-protected to go through the winter.”
A third dose should provide protection throughout the winter, Dr. Bourla said. That may buy time until the early spring to develop new shots that target Omicron, which Pfizer could have ready by March, according to Bloomberg News.
As of the afternoon of Dec. 8, 43 people in 19 states had tested positive for the Omicron variant, according to The Associated Press. More than 75% had been vaccinated, and a third had had booster shots. About a third had traveled internationally.
Nearly all of them have had mild symptoms so far, the AP reported, with the most common symptoms being a cough, congestion, and fatigue. One person has been hospitalized, but no deaths have been reported so far.
The CDC is still trying to determine how the Omicron variant may affect the course of the pandemic and whether the strain is more contagious or causes more severe disease.
“What we generally know is the more mutations a variant has, the higher level you need your immunity to be,” Rochelle Walensky, MD, director of the CDC, told the AP.
“We want to make sure we bolster everybody’s immunity,” she said. “And that’s really what motivated the decision to expand our guidance [on boosters for all adults].”
The Omicron variant has been reported in 57 countries so far, World Health Organization officials reported Dec. 8, and they expect that number to continue growing.
“Certain features of Omicron, including its global spread and large number of mutations, suggest it could have a major impact on the course of the pandemic. Exactly what that impact will be is still difficult to know,” Tedros Adhanom Ghebreyesus, PhD, the World Health Organization’s director-general, said during a media briefing.
Several studies suggest that Omicron leads to a rapid increase in transmission, he said, though scientists are still trying to understand whether it can “outcompete Delta.” Data from South Africa also suggests a higher risk of reinfection with Omicron, though it appears to cause milder disease than Delta, he noted.
“Even though we still need answers to some crucial questions, we are not defenseless against Omicron or Delta,” he said. “The steps countries take today, and in the coming days and weeks, will determine how Omicron unfolds.”
A version of this article first appeared on WebMD.com.
, Pfizer officials said on Dec. 8.
The standard two doses may be less effective against the variant, the company announced earlier in the day, and a booster dose increases neutralizing antibodies.
But the timeline might need to be moved up for a fourth dose. Previously, Pfizer CEO Albert Bourla, PhD, said another dose might be needed about a year after a third shot. Now the company’s scientists believe that a fourth shot, which targets the Omicron variant, could be required sooner.
“With Omicron, we need to wait and see because we have very little information. We may need it faster,” Dr. Bourla said on CNBC’s Squawk Box.
“But for right now, the most important thing is that we have winter in front of us,” he said. “From a healthcare perspective, it is important to understand that we need to be well-protected to go through the winter.”
A third dose should provide protection throughout the winter, Dr. Bourla said. That may buy time until the early spring to develop new shots that target Omicron, which Pfizer could have ready by March, according to Bloomberg News.
As of the afternoon of Dec. 8, 43 people in 19 states had tested positive for the Omicron variant, according to The Associated Press. More than 75% had been vaccinated, and a third had had booster shots. About a third had traveled internationally.
Nearly all of them have had mild symptoms so far, the AP reported, with the most common symptoms being a cough, congestion, and fatigue. One person has been hospitalized, but no deaths have been reported so far.
The CDC is still trying to determine how the Omicron variant may affect the course of the pandemic and whether the strain is more contagious or causes more severe disease.
“What we generally know is the more mutations a variant has, the higher level you need your immunity to be,” Rochelle Walensky, MD, director of the CDC, told the AP.
“We want to make sure we bolster everybody’s immunity,” she said. “And that’s really what motivated the decision to expand our guidance [on boosters for all adults].”
The Omicron variant has been reported in 57 countries so far, World Health Organization officials reported Dec. 8, and they expect that number to continue growing.
“Certain features of Omicron, including its global spread and large number of mutations, suggest it could have a major impact on the course of the pandemic. Exactly what that impact will be is still difficult to know,” Tedros Adhanom Ghebreyesus, PhD, the World Health Organization’s director-general, said during a media briefing.
Several studies suggest that Omicron leads to a rapid increase in transmission, he said, though scientists are still trying to understand whether it can “outcompete Delta.” Data from South Africa also suggests a higher risk of reinfection with Omicron, though it appears to cause milder disease than Delta, he noted.
“Even though we still need answers to some crucial questions, we are not defenseless against Omicron or Delta,” he said. “The steps countries take today, and in the coming days and weeks, will determine how Omicron unfolds.”
A version of this article first appeared on WebMD.com.
Medical board stops warning docs against giving false COVID information
Under pressure from Republican state lawmakers, t
The board’s 7-3 vote on December 7 to delete the statement followed repeated threats by a powerful state House Republican to dissolve the board and appoint all new members if it did not immediately take it down.
The Tennessee board’s statement was a verbatim restatement of a warning to physicians issued by the Federation of State Medical Boards in July. The federation cited a “dramatic increase” in dissemination of misinformation and disinformation about the COVID-19 vaccine by physicians. It said that’s dangerous because physicians enjoy a high degree of public credibility.
Across the country, state medical licensing boards and state and national medical associations and specialty boards are struggling with how to respond to scientifically baseless public statements about COVID-19 by some physicians, which they say are increasing public confusion, political conflict, and preventable illnesses and deaths.
There have been only a small number of disciplinary actions by medical boards against physicians for spreading false COVID-19 information. Critics say the boards have been weak in responding to these dangerous violations of medical standards. As an example, they cite the State Medical Board of Ohio’s September renewal of the medical license of Sherri Tenpenny, DO, who had previously testified before Ohio lawmakers that COVID-19 vaccines magnetize their recipients and “interface” with cell phone towers.
“I’m not satisfied with what medical boards have done, and we are ramping up our efforts to press the boards to hold these physicians accountable,” said Nick Sawyer, MD, an emergency physician in Sacramento, Calif., who heads a group of healthcare professionals called No License for Disinformation.
Still, Tennessee board members insisted that the board’s policy of disciplining physicians who disseminate false information about COVID-19 vaccinations remains in effect, because state law empowers the board to take action against doctors whose unprofessional behavior endangers the public.
“COVID misinformation and disinformation has caused undue loss of life and jobs and other incalculable loss in our society,” said Melanie Blake, MD, MBA, a Chattanooga internist who’s president of the board. “Physicians have a responsibility to uphold their oath and put forward consensus-driven medical principles.”
But state Rep. John Ragan, the Republican co-chairman of the Joint Government Operations Committee, told the Tennessean newspaper that deleting the statement from the board’s website was equivalent to rescinding the policy. Ragan, who identifies himself as a business consultant and retired Air Force pilot, did not respond to a request for comment for this article.
Blake acknowledged that removing the statement from the board’s website has the potential to confuse Tennessee physicians. And the pressure from GOP lawmakers, who overwhelmingly control the Tennessee legislature, could discourage investigations and disciplinary actions against physicians who allegedly spread COVID-19 misinformation, she added. “It’s hard for me to answer whether this puts a chill on us,” she said.
In September, the Tennessee board, besides approving the general statement that physicians who spread COVID-19 disinformation could face licensure action, also directed the State Department of Health to prioritize investigations of physicians who spread outrageous claims. The board cited statements such as the vaccines are poisonous, cause infertility, contain microchips, or magnetize the body.
In response, the Tennessee General Assembly passed a bill in late October prohibiting the board from implementing any disciplinary process regarding the prescribing of “medication for COVID-19” without review and approval by Ragan’s committee. It’s not clear whether that language covers vaccines.
Last summer, in a similar move, Ragan threatened to dissolve the State Department of Health because its top vaccination official wrote a letter to medical providers explaining that state law allowed them to give COVID-19 vaccinations to minors older than 14 without parental consent. That official, Michelle Fiscus, MD, was fired in July.
Republican Sen. Richard Briggs, MD, a cardiothoracic surgeon who voted against the October legislation affecting COVID-related disciplinary actions, criticized his GOP colleagues’ interference in the medical board’s licensure decisions. “The mission of the board is to protect the health and safety of Tennessee citizens, and this was in complete conflict with that mission,” he said.
The Federation of State Medical Boards similarly condemned the Tennessee lawmakers’ moves. “The FSMB strongly opposes restricting a board’s authority to evaluate the standard of care and assess potential risk for patient harm,” a spokesman said. “Any interference, politically motivated or otherwise, is unhelpful and dangerous.”
But Arthur Caplan, PhD, a professor of bioethics at NYU School of Medicine, doubts that state medical boards are up to the task of policing disinformation spread by physicians. That’s because they ultimately are under the control of elected state officials, who may force the boards to base policy on ideology rather than science.
He said medical board members in Florida and another GOP-controlled state have told him they do not want to pursue disciplinary actions against physicians for COVID-19 misinformation for fear of political backlash.
Michele Heisler, MD, medical director of Physicians for Human Rights, agreed that the Tennessee situation highlights the looming political threat to the independence of state medical boards. She urged other medical organizations, particularly medical specialty boards, to step in.
“As a profession, we need to take a stance against this,” said Heisler, who’s a professor of internal medicine and public health at the University of Michigan. “Our credibility as physicians is at stake.”
A version of this article first appeared on Medscape.com.
Under pressure from Republican state lawmakers, t
The board’s 7-3 vote on December 7 to delete the statement followed repeated threats by a powerful state House Republican to dissolve the board and appoint all new members if it did not immediately take it down.
The Tennessee board’s statement was a verbatim restatement of a warning to physicians issued by the Federation of State Medical Boards in July. The federation cited a “dramatic increase” in dissemination of misinformation and disinformation about the COVID-19 vaccine by physicians. It said that’s dangerous because physicians enjoy a high degree of public credibility.
Across the country, state medical licensing boards and state and national medical associations and specialty boards are struggling with how to respond to scientifically baseless public statements about COVID-19 by some physicians, which they say are increasing public confusion, political conflict, and preventable illnesses and deaths.
There have been only a small number of disciplinary actions by medical boards against physicians for spreading false COVID-19 information. Critics say the boards have been weak in responding to these dangerous violations of medical standards. As an example, they cite the State Medical Board of Ohio’s September renewal of the medical license of Sherri Tenpenny, DO, who had previously testified before Ohio lawmakers that COVID-19 vaccines magnetize their recipients and “interface” with cell phone towers.
“I’m not satisfied with what medical boards have done, and we are ramping up our efforts to press the boards to hold these physicians accountable,” said Nick Sawyer, MD, an emergency physician in Sacramento, Calif., who heads a group of healthcare professionals called No License for Disinformation.
Still, Tennessee board members insisted that the board’s policy of disciplining physicians who disseminate false information about COVID-19 vaccinations remains in effect, because state law empowers the board to take action against doctors whose unprofessional behavior endangers the public.
“COVID misinformation and disinformation has caused undue loss of life and jobs and other incalculable loss in our society,” said Melanie Blake, MD, MBA, a Chattanooga internist who’s president of the board. “Physicians have a responsibility to uphold their oath and put forward consensus-driven medical principles.”
But state Rep. John Ragan, the Republican co-chairman of the Joint Government Operations Committee, told the Tennessean newspaper that deleting the statement from the board’s website was equivalent to rescinding the policy. Ragan, who identifies himself as a business consultant and retired Air Force pilot, did not respond to a request for comment for this article.
Blake acknowledged that removing the statement from the board’s website has the potential to confuse Tennessee physicians. And the pressure from GOP lawmakers, who overwhelmingly control the Tennessee legislature, could discourage investigations and disciplinary actions against physicians who allegedly spread COVID-19 misinformation, she added. “It’s hard for me to answer whether this puts a chill on us,” she said.
In September, the Tennessee board, besides approving the general statement that physicians who spread COVID-19 disinformation could face licensure action, also directed the State Department of Health to prioritize investigations of physicians who spread outrageous claims. The board cited statements such as the vaccines are poisonous, cause infertility, contain microchips, or magnetize the body.
In response, the Tennessee General Assembly passed a bill in late October prohibiting the board from implementing any disciplinary process regarding the prescribing of “medication for COVID-19” without review and approval by Ragan’s committee. It’s not clear whether that language covers vaccines.
Last summer, in a similar move, Ragan threatened to dissolve the State Department of Health because its top vaccination official wrote a letter to medical providers explaining that state law allowed them to give COVID-19 vaccinations to minors older than 14 without parental consent. That official, Michelle Fiscus, MD, was fired in July.
Republican Sen. Richard Briggs, MD, a cardiothoracic surgeon who voted against the October legislation affecting COVID-related disciplinary actions, criticized his GOP colleagues’ interference in the medical board’s licensure decisions. “The mission of the board is to protect the health and safety of Tennessee citizens, and this was in complete conflict with that mission,” he said.
The Federation of State Medical Boards similarly condemned the Tennessee lawmakers’ moves. “The FSMB strongly opposes restricting a board’s authority to evaluate the standard of care and assess potential risk for patient harm,” a spokesman said. “Any interference, politically motivated or otherwise, is unhelpful and dangerous.”
But Arthur Caplan, PhD, a professor of bioethics at NYU School of Medicine, doubts that state medical boards are up to the task of policing disinformation spread by physicians. That’s because they ultimately are under the control of elected state officials, who may force the boards to base policy on ideology rather than science.
He said medical board members in Florida and another GOP-controlled state have told him they do not want to pursue disciplinary actions against physicians for COVID-19 misinformation for fear of political backlash.
Michele Heisler, MD, medical director of Physicians for Human Rights, agreed that the Tennessee situation highlights the looming political threat to the independence of state medical boards. She urged other medical organizations, particularly medical specialty boards, to step in.
“As a profession, we need to take a stance against this,” said Heisler, who’s a professor of internal medicine and public health at the University of Michigan. “Our credibility as physicians is at stake.”
A version of this article first appeared on Medscape.com.
Under pressure from Republican state lawmakers, t
The board’s 7-3 vote on December 7 to delete the statement followed repeated threats by a powerful state House Republican to dissolve the board and appoint all new members if it did not immediately take it down.
The Tennessee board’s statement was a verbatim restatement of a warning to physicians issued by the Federation of State Medical Boards in July. The federation cited a “dramatic increase” in dissemination of misinformation and disinformation about the COVID-19 vaccine by physicians. It said that’s dangerous because physicians enjoy a high degree of public credibility.
Across the country, state medical licensing boards and state and national medical associations and specialty boards are struggling with how to respond to scientifically baseless public statements about COVID-19 by some physicians, which they say are increasing public confusion, political conflict, and preventable illnesses and deaths.
There have been only a small number of disciplinary actions by medical boards against physicians for spreading false COVID-19 information. Critics say the boards have been weak in responding to these dangerous violations of medical standards. As an example, they cite the State Medical Board of Ohio’s September renewal of the medical license of Sherri Tenpenny, DO, who had previously testified before Ohio lawmakers that COVID-19 vaccines magnetize their recipients and “interface” with cell phone towers.
“I’m not satisfied with what medical boards have done, and we are ramping up our efforts to press the boards to hold these physicians accountable,” said Nick Sawyer, MD, an emergency physician in Sacramento, Calif., who heads a group of healthcare professionals called No License for Disinformation.
Still, Tennessee board members insisted that the board’s policy of disciplining physicians who disseminate false information about COVID-19 vaccinations remains in effect, because state law empowers the board to take action against doctors whose unprofessional behavior endangers the public.
“COVID misinformation and disinformation has caused undue loss of life and jobs and other incalculable loss in our society,” said Melanie Blake, MD, MBA, a Chattanooga internist who’s president of the board. “Physicians have a responsibility to uphold their oath and put forward consensus-driven medical principles.”
But state Rep. John Ragan, the Republican co-chairman of the Joint Government Operations Committee, told the Tennessean newspaper that deleting the statement from the board’s website was equivalent to rescinding the policy. Ragan, who identifies himself as a business consultant and retired Air Force pilot, did not respond to a request for comment for this article.
Blake acknowledged that removing the statement from the board’s website has the potential to confuse Tennessee physicians. And the pressure from GOP lawmakers, who overwhelmingly control the Tennessee legislature, could discourage investigations and disciplinary actions against physicians who allegedly spread COVID-19 misinformation, she added. “It’s hard for me to answer whether this puts a chill on us,” she said.
In September, the Tennessee board, besides approving the general statement that physicians who spread COVID-19 disinformation could face licensure action, also directed the State Department of Health to prioritize investigations of physicians who spread outrageous claims. The board cited statements such as the vaccines are poisonous, cause infertility, contain microchips, or magnetize the body.
In response, the Tennessee General Assembly passed a bill in late October prohibiting the board from implementing any disciplinary process regarding the prescribing of “medication for COVID-19” without review and approval by Ragan’s committee. It’s not clear whether that language covers vaccines.
Last summer, in a similar move, Ragan threatened to dissolve the State Department of Health because its top vaccination official wrote a letter to medical providers explaining that state law allowed them to give COVID-19 vaccinations to minors older than 14 without parental consent. That official, Michelle Fiscus, MD, was fired in July.
Republican Sen. Richard Briggs, MD, a cardiothoracic surgeon who voted against the October legislation affecting COVID-related disciplinary actions, criticized his GOP colleagues’ interference in the medical board’s licensure decisions. “The mission of the board is to protect the health and safety of Tennessee citizens, and this was in complete conflict with that mission,” he said.
The Federation of State Medical Boards similarly condemned the Tennessee lawmakers’ moves. “The FSMB strongly opposes restricting a board’s authority to evaluate the standard of care and assess potential risk for patient harm,” a spokesman said. “Any interference, politically motivated or otherwise, is unhelpful and dangerous.”
But Arthur Caplan, PhD, a professor of bioethics at NYU School of Medicine, doubts that state medical boards are up to the task of policing disinformation spread by physicians. That’s because they ultimately are under the control of elected state officials, who may force the boards to base policy on ideology rather than science.
He said medical board members in Florida and another GOP-controlled state have told him they do not want to pursue disciplinary actions against physicians for COVID-19 misinformation for fear of political backlash.
Michele Heisler, MD, medical director of Physicians for Human Rights, agreed that the Tennessee situation highlights the looming political threat to the independence of state medical boards. She urged other medical organizations, particularly medical specialty boards, to step in.
“As a profession, we need to take a stance against this,” said Heisler, who’s a professor of internal medicine and public health at the University of Michigan. “Our credibility as physicians is at stake.”
A version of this article first appeared on Medscape.com.
AMA, hospital group sue federal government over surprise billing law
which tilts toward using prevailing rates paid for services.
The American Hospital Association and American Medical Association said they will ask the U.S. District Court for the District of Columbia to try to prevent implementation of certain provisions of new federal rules on surprise bills. This court is often a venue for fights over federal rules. Also joining the suit are Nevada-based Renown Health, UMass Memorial Health, and two physicians based in North Carolina, AHA and AMA said.
Federal agencies, including the Department of Health & Human Services, in September had unveiled the rule on surprise medical bills that will take effect Jan. 1.
Under this rule, a key benchmark for payment disputes would be the qualifying payment amount (QPA), which is pegged to median contracted rates. In the dispute-resolution process outlined in the rule, there is a presumption that the QPA is the appropriate out-of-network rate.
The rule allows for exceptions in which the independent mediating organization handling the payment dispute resolution has “credible information” as to why the QPA is materially different from the appropriate out-of-network rate.
In the view of the federal agencies that issued the rule, this approach “encourages predictable outcomes,” which likely would reduce the number of disputes that go through the resolution process while also “providing equitable and clear standards” for cases to appropriately deviate from QPA. HHS was joined in issuing the rule by the Treasury and Labor Departments and the Office of Personnel Management.
AMA and AHA disagree with their view, seeing this approach as a boon for insurers at the expense of physicians and hospitals.
In a press release, they said the rule’s approach to surprise billing would “all but ensure that hospitals, physicians, and other providers will routinely be undercompensated by commercial insurers, and patients will have fewer choices for access to in-network services.”
The rule is part of the implementation of a federal law passed in December 2020, known as the No Surprises Act. In their statement, AHA and AMA said their legal challenge would not prevent “core patient protections’’ of that law from moving forward.
“No patient should fear receiving a surprise medical bill,” Rick Pollack, AHA president and chief executive, said in the statement. “That is why hospitals and health systems supported the No Surprises Act to protect patients and keep them out of the middle of disputes between providers and insurers. Congress carefully crafted the law with a balanced, patient-friendly approach and it should be implemented as intended.”
AMA President Gerald E. Harmon, MD, added the approach used in the rule on surprise billing could create “an unsustainable situation for physicians.”
“Our legal challenge urges regulators to ensure there is a fair and meaningful process to resolve disputes between health care providers and insurance companies,” Dr. Harmon said.
AHA and AMA included with their statement a link to a November letter from more than 150 members of Congress, who also objected to the approach taken in designing the independent dispute-resolution (IDR) process.
“This directive establishes a de facto benchmark rate, making the median in-network rate the default factor considered in the IDR process. This approach is contrary to statute and could incentivize insurance companies to set artificially low payment rates, which would narrow provider networks and jeopardize patient access to care – the exact opposite of the goal of the law,” wrote the members of Congress, including Rep. Raul Ruiz, MD, a California Democrat, and Rep. Larry Bucshon, MD, an Indiana Republican.
A version of this article first appeared on Medscape.com.
which tilts toward using prevailing rates paid for services.
The American Hospital Association and American Medical Association said they will ask the U.S. District Court for the District of Columbia to try to prevent implementation of certain provisions of new federal rules on surprise bills. This court is often a venue for fights over federal rules. Also joining the suit are Nevada-based Renown Health, UMass Memorial Health, and two physicians based in North Carolina, AHA and AMA said.
Federal agencies, including the Department of Health & Human Services, in September had unveiled the rule on surprise medical bills that will take effect Jan. 1.
Under this rule, a key benchmark for payment disputes would be the qualifying payment amount (QPA), which is pegged to median contracted rates. In the dispute-resolution process outlined in the rule, there is a presumption that the QPA is the appropriate out-of-network rate.
The rule allows for exceptions in which the independent mediating organization handling the payment dispute resolution has “credible information” as to why the QPA is materially different from the appropriate out-of-network rate.
In the view of the federal agencies that issued the rule, this approach “encourages predictable outcomes,” which likely would reduce the number of disputes that go through the resolution process while also “providing equitable and clear standards” for cases to appropriately deviate from QPA. HHS was joined in issuing the rule by the Treasury and Labor Departments and the Office of Personnel Management.
AMA and AHA disagree with their view, seeing this approach as a boon for insurers at the expense of physicians and hospitals.
In a press release, they said the rule’s approach to surprise billing would “all but ensure that hospitals, physicians, and other providers will routinely be undercompensated by commercial insurers, and patients will have fewer choices for access to in-network services.”
The rule is part of the implementation of a federal law passed in December 2020, known as the No Surprises Act. In their statement, AHA and AMA said their legal challenge would not prevent “core patient protections’’ of that law from moving forward.
“No patient should fear receiving a surprise medical bill,” Rick Pollack, AHA president and chief executive, said in the statement. “That is why hospitals and health systems supported the No Surprises Act to protect patients and keep them out of the middle of disputes between providers and insurers. Congress carefully crafted the law with a balanced, patient-friendly approach and it should be implemented as intended.”
AMA President Gerald E. Harmon, MD, added the approach used in the rule on surprise billing could create “an unsustainable situation for physicians.”
“Our legal challenge urges regulators to ensure there is a fair and meaningful process to resolve disputes between health care providers and insurance companies,” Dr. Harmon said.
AHA and AMA included with their statement a link to a November letter from more than 150 members of Congress, who also objected to the approach taken in designing the independent dispute-resolution (IDR) process.
“This directive establishes a de facto benchmark rate, making the median in-network rate the default factor considered in the IDR process. This approach is contrary to statute and could incentivize insurance companies to set artificially low payment rates, which would narrow provider networks and jeopardize patient access to care – the exact opposite of the goal of the law,” wrote the members of Congress, including Rep. Raul Ruiz, MD, a California Democrat, and Rep. Larry Bucshon, MD, an Indiana Republican.
A version of this article first appeared on Medscape.com.
which tilts toward using prevailing rates paid for services.
The American Hospital Association and American Medical Association said they will ask the U.S. District Court for the District of Columbia to try to prevent implementation of certain provisions of new federal rules on surprise bills. This court is often a venue for fights over federal rules. Also joining the suit are Nevada-based Renown Health, UMass Memorial Health, and two physicians based in North Carolina, AHA and AMA said.
Federal agencies, including the Department of Health & Human Services, in September had unveiled the rule on surprise medical bills that will take effect Jan. 1.
Under this rule, a key benchmark for payment disputes would be the qualifying payment amount (QPA), which is pegged to median contracted rates. In the dispute-resolution process outlined in the rule, there is a presumption that the QPA is the appropriate out-of-network rate.
The rule allows for exceptions in which the independent mediating organization handling the payment dispute resolution has “credible information” as to why the QPA is materially different from the appropriate out-of-network rate.
In the view of the federal agencies that issued the rule, this approach “encourages predictable outcomes,” which likely would reduce the number of disputes that go through the resolution process while also “providing equitable and clear standards” for cases to appropriately deviate from QPA. HHS was joined in issuing the rule by the Treasury and Labor Departments and the Office of Personnel Management.
AMA and AHA disagree with their view, seeing this approach as a boon for insurers at the expense of physicians and hospitals.
In a press release, they said the rule’s approach to surprise billing would “all but ensure that hospitals, physicians, and other providers will routinely be undercompensated by commercial insurers, and patients will have fewer choices for access to in-network services.”
The rule is part of the implementation of a federal law passed in December 2020, known as the No Surprises Act. In their statement, AHA and AMA said their legal challenge would not prevent “core patient protections’’ of that law from moving forward.
“No patient should fear receiving a surprise medical bill,” Rick Pollack, AHA president and chief executive, said in the statement. “That is why hospitals and health systems supported the No Surprises Act to protect patients and keep them out of the middle of disputes between providers and insurers. Congress carefully crafted the law with a balanced, patient-friendly approach and it should be implemented as intended.”
AMA President Gerald E. Harmon, MD, added the approach used in the rule on surprise billing could create “an unsustainable situation for physicians.”
“Our legal challenge urges regulators to ensure there is a fair and meaningful process to resolve disputes between health care providers and insurance companies,” Dr. Harmon said.
AHA and AMA included with their statement a link to a November letter from more than 150 members of Congress, who also objected to the approach taken in designing the independent dispute-resolution (IDR) process.
“This directive establishes a de facto benchmark rate, making the median in-network rate the default factor considered in the IDR process. This approach is contrary to statute and could incentivize insurance companies to set artificially low payment rates, which would narrow provider networks and jeopardize patient access to care – the exact opposite of the goal of the law,” wrote the members of Congress, including Rep. Raul Ruiz, MD, a California Democrat, and Rep. Larry Bucshon, MD, an Indiana Republican.
A version of this article first appeared on Medscape.com.
Risk for severe COVID-19 and death plummets with Pfizer booster
Both studies were completed before the advent of the Omicron variant.
In one study that included data on more than 4 million patients, led by Yinon M. Bar-On, MSc, of the Weizmann Institute of Science in Rehovot, Israel, the rate of confirmed SARS-CoV-2 infection was lower in the booster group than in the nonbooster group by a factor of about 10.
This was true across all five age groups studied (range among the groups [starting with age 16], 9.0-17.2).
The risk for severe COVID-19 in the primary analysis decreased in the booster group by a factor of 17.9 (95% confidence interval, 15.1-21.2), among those aged 60 years or older. Risk for severe illness in those ages 40-59 was lower by a factor of 21.7 (95% CI, 10.6-44.2).
Among the 60 and older age group, risk for death was also reduced by a factor of 14.7 (95% CI, 10.0-21.4).
Researchers analyzed data for the period from July 30 to Oct. 10, 2021, from the Israel Ministry of Health database on 4.69 million people at least 16 years old who had received two Pfizer doses at least 5 months earlier.
In the main analysis, the researchers compared the rates of confirmed COVID-19, severe disease, and death among those who had gotten a booster at least 12 days earlier with the rates in a nonbooster group.
The authors wrote: “Booster vaccination programs may provide a way to control transmission without costly social-distancing measures and quarantines. Our findings provide evidence for the short-term effectiveness of the booster dose against the currently dominant Delta variant in persons 16 years of age or older.”
Death risk down by 90%
A second study, led by Ronen Arbel, PhD, with the community medical services division, Clalit Health Services (CHS), Tel Aviv, which included more than 800,000 participants, also found mortality risk was greatly reduced among those who received the booster compared with those who didn’t get the booster.
Participants aged 50 years or older who received a booster at least 5 months after a second Pfizer dose had 90% lower mortality risk because of COVID-19 than participants who did not get the booster.
The adjusted hazard ratio for death as a result of COVID-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% CI, 0.07-0.14; P < .001). Of the 843,208 eligible participants, 758,118 (90%) received the booster during the 54-day study period.
The study included all CHS members who were aged 50 years or older on the study start date and had received two Pfizer doses at least 5 months earlier. CHS covers about 52% of the Israeli population and is the largest of four health care organizations in Israel that provide mandatory health care.
The authors noted that, although the study period was only 54 days (Aug. 6–Sept. 29), during that time “the incidence of COVID-19 in Israel was one of the highest in the world.”
The authors of both original articles pointed out that the studies are limited by short time periods and that longer-term studies are needed to see how the booster shots stand up to known and future variants, such as Omicron.
None of the authors involved in both studies reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both studies were completed before the advent of the Omicron variant.
In one study that included data on more than 4 million patients, led by Yinon M. Bar-On, MSc, of the Weizmann Institute of Science in Rehovot, Israel, the rate of confirmed SARS-CoV-2 infection was lower in the booster group than in the nonbooster group by a factor of about 10.
This was true across all five age groups studied (range among the groups [starting with age 16], 9.0-17.2).
The risk for severe COVID-19 in the primary analysis decreased in the booster group by a factor of 17.9 (95% confidence interval, 15.1-21.2), among those aged 60 years or older. Risk for severe illness in those ages 40-59 was lower by a factor of 21.7 (95% CI, 10.6-44.2).
Among the 60 and older age group, risk for death was also reduced by a factor of 14.7 (95% CI, 10.0-21.4).
Researchers analyzed data for the period from July 30 to Oct. 10, 2021, from the Israel Ministry of Health database on 4.69 million people at least 16 years old who had received two Pfizer doses at least 5 months earlier.
In the main analysis, the researchers compared the rates of confirmed COVID-19, severe disease, and death among those who had gotten a booster at least 12 days earlier with the rates in a nonbooster group.
The authors wrote: “Booster vaccination programs may provide a way to control transmission without costly social-distancing measures and quarantines. Our findings provide evidence for the short-term effectiveness of the booster dose against the currently dominant Delta variant in persons 16 years of age or older.”
Death risk down by 90%
A second study, led by Ronen Arbel, PhD, with the community medical services division, Clalit Health Services (CHS), Tel Aviv, which included more than 800,000 participants, also found mortality risk was greatly reduced among those who received the booster compared with those who didn’t get the booster.
Participants aged 50 years or older who received a booster at least 5 months after a second Pfizer dose had 90% lower mortality risk because of COVID-19 than participants who did not get the booster.
The adjusted hazard ratio for death as a result of COVID-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% CI, 0.07-0.14; P < .001). Of the 843,208 eligible participants, 758,118 (90%) received the booster during the 54-day study period.
The study included all CHS members who were aged 50 years or older on the study start date and had received two Pfizer doses at least 5 months earlier. CHS covers about 52% of the Israeli population and is the largest of four health care organizations in Israel that provide mandatory health care.
The authors noted that, although the study period was only 54 days (Aug. 6–Sept. 29), during that time “the incidence of COVID-19 in Israel was one of the highest in the world.”
The authors of both original articles pointed out that the studies are limited by short time periods and that longer-term studies are needed to see how the booster shots stand up to known and future variants, such as Omicron.
None of the authors involved in both studies reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both studies were completed before the advent of the Omicron variant.
In one study that included data on more than 4 million patients, led by Yinon M. Bar-On, MSc, of the Weizmann Institute of Science in Rehovot, Israel, the rate of confirmed SARS-CoV-2 infection was lower in the booster group than in the nonbooster group by a factor of about 10.
This was true across all five age groups studied (range among the groups [starting with age 16], 9.0-17.2).
The risk for severe COVID-19 in the primary analysis decreased in the booster group by a factor of 17.9 (95% confidence interval, 15.1-21.2), among those aged 60 years or older. Risk for severe illness in those ages 40-59 was lower by a factor of 21.7 (95% CI, 10.6-44.2).
Among the 60 and older age group, risk for death was also reduced by a factor of 14.7 (95% CI, 10.0-21.4).
Researchers analyzed data for the period from July 30 to Oct. 10, 2021, from the Israel Ministry of Health database on 4.69 million people at least 16 years old who had received two Pfizer doses at least 5 months earlier.
In the main analysis, the researchers compared the rates of confirmed COVID-19, severe disease, and death among those who had gotten a booster at least 12 days earlier with the rates in a nonbooster group.
The authors wrote: “Booster vaccination programs may provide a way to control transmission without costly social-distancing measures and quarantines. Our findings provide evidence for the short-term effectiveness of the booster dose against the currently dominant Delta variant in persons 16 years of age or older.”
Death risk down by 90%
A second study, led by Ronen Arbel, PhD, with the community medical services division, Clalit Health Services (CHS), Tel Aviv, which included more than 800,000 participants, also found mortality risk was greatly reduced among those who received the booster compared with those who didn’t get the booster.
Participants aged 50 years or older who received a booster at least 5 months after a second Pfizer dose had 90% lower mortality risk because of COVID-19 than participants who did not get the booster.
The adjusted hazard ratio for death as a result of COVID-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% CI, 0.07-0.14; P < .001). Of the 843,208 eligible participants, 758,118 (90%) received the booster during the 54-day study period.
The study included all CHS members who were aged 50 years or older on the study start date and had received two Pfizer doses at least 5 months earlier. CHS covers about 52% of the Israeli population and is the largest of four health care organizations in Israel that provide mandatory health care.
The authors noted that, although the study period was only 54 days (Aug. 6–Sept. 29), during that time “the incidence of COVID-19 in Israel was one of the highest in the world.”
The authors of both original articles pointed out that the studies are limited by short time periods and that longer-term studies are needed to see how the booster shots stand up to known and future variants, such as Omicron.
None of the authors involved in both studies reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Spam filter failure: Selling physician emails equals big $$
Despite the best efforts of my institution’s spam filter, I’ve realized that I spend at least 4 minutes every day of the week removing junk email from my in basket: EMR vendors, predatory journals trying to lure me into paying their outrageous publication fees, people who want to help me with my billing software (evidently that .edu extension hasn’t clicked for them yet), headhunters trying to fill specialty positions in other states, market researchers offering a gift card for 40 minutes filling out a survey.
If you do the math, 4 minutes daily is 1,460 minutes per year. That’s an entire day of my life lost each year to this useless nonsense, which I never agreed to receive in the first place. Now multiply that by the 22 million health care workers in the United States, or even just by the 985,000 licensed physicians in this country. Then factor in the $638 per hour in gross revenue generated by the average primary care physician, as a conservative, well-documented value.
By my reckoning, these bozos owe the United States alone over $15 billion in lost GDP each year.
So why don’t we shut it down!? The CAN-SPAM Act of 2003 attempted to at least mitigate the problem. It applies only to commercial entities (I know, I’d love to report some political groups, too). To avoid violating the law and risking fines of up to $16,000 per individual email, senders must:
- Not use misleading header info (including domain name and email address)
- Not use deceptive subject lines
- Clearly label the email as an ad
- Give an actual physical address of the sender
- Tell recipients how to opt out of future emails
- Honor opt-out requests within 10 business days
- Monitor the activities of any subcontractor sending email on their behalf
I can say with certainty that much of the trash in my inbox violates at least one of these. But that doesn’t matter if there is not an efficient way to report the violators and ensure that they’ll be tracked down. Hard enough if they live here, impossible if the email is routed from overseas, as much of it clearly is.
If you receive email in violation of the act, experts recommend that you write down the email address and the business name of the sender, fill out a complaint form on the Federal Trade Commission website, or send an email to [email protected], then send an email to your Internet service provider’s abuse desk. If you’re not working within a big institution like mine that has hot and cold running IT personnel that operate their own abuse prevention office, the address you’ll need is likely abuse@domain_name or postmaster@domain_name. Just hitting the spam button at the top of your browser/email software may do the trick. There’s more good advice at the FTC’s consumer spam page.
The answer came, ironically, to my email inbox in the form of one of those emails that did indeed violate the law.
I rolled my eyes and started into my reporting subroutine but then stopped cold. Just 1 second. If this person is selling lists of email addresses of conference attendees, somebody within the conference structure must be providing them. How is that legal? I have never agreed, in registering for a medical conference, to allow them to share my email address with anyone. To think that they are making money from that is extremely galling.
Vermont, at least, has enacted a law requiring companies that traffic in such email lists to register with the state. Although it has been in effect for 2 years, the jury is out regarding its efficacy. Our European counterparts are protected by the General Data Protection Regulation, which specifies that commercial email can be sent only to individuals who have explicitly opted into such mailings, and that purchased email lists are not compliant with the requirement.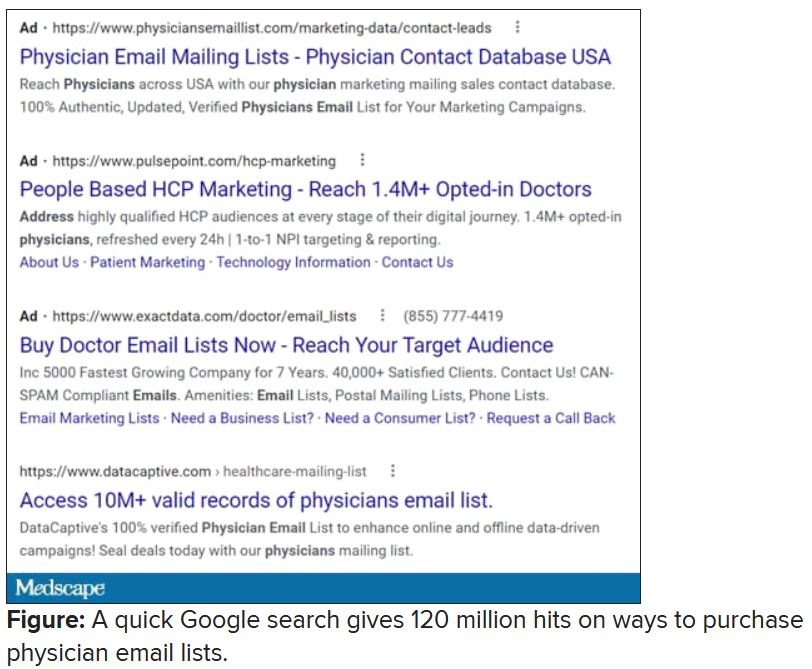
Anybody have the inside scoop on this? Can we demand that our professional societies safeguard their attendee databases so this won’t happen? If they won’t, why am I paying big money to attend their conferences, only for them to make even more money at my expense?
Dr. Hitchcock is assistant professor, department of radiation oncology, at the University of Florida, Gainesville. She reported receiving research grant money from Merck. A version of this article first appeared on Medscape.com.
Despite the best efforts of my institution’s spam filter, I’ve realized that I spend at least 4 minutes every day of the week removing junk email from my in basket: EMR vendors, predatory journals trying to lure me into paying their outrageous publication fees, people who want to help me with my billing software (evidently that .edu extension hasn’t clicked for them yet), headhunters trying to fill specialty positions in other states, market researchers offering a gift card for 40 minutes filling out a survey.
If you do the math, 4 minutes daily is 1,460 minutes per year. That’s an entire day of my life lost each year to this useless nonsense, which I never agreed to receive in the first place. Now multiply that by the 22 million health care workers in the United States, or even just by the 985,000 licensed physicians in this country. Then factor in the $638 per hour in gross revenue generated by the average primary care physician, as a conservative, well-documented value.
By my reckoning, these bozos owe the United States alone over $15 billion in lost GDP each year.
So why don’t we shut it down!? The CAN-SPAM Act of 2003 attempted to at least mitigate the problem. It applies only to commercial entities (I know, I’d love to report some political groups, too). To avoid violating the law and risking fines of up to $16,000 per individual email, senders must:
- Not use misleading header info (including domain name and email address)
- Not use deceptive subject lines
- Clearly label the email as an ad
- Give an actual physical address of the sender
- Tell recipients how to opt out of future emails
- Honor opt-out requests within 10 business days
- Monitor the activities of any subcontractor sending email on their behalf
I can say with certainty that much of the trash in my inbox violates at least one of these. But that doesn’t matter if there is not an efficient way to report the violators and ensure that they’ll be tracked down. Hard enough if they live here, impossible if the email is routed from overseas, as much of it clearly is.
If you receive email in violation of the act, experts recommend that you write down the email address and the business name of the sender, fill out a complaint form on the Federal Trade Commission website, or send an email to [email protected], then send an email to your Internet service provider’s abuse desk. If you’re not working within a big institution like mine that has hot and cold running IT personnel that operate their own abuse prevention office, the address you’ll need is likely abuse@domain_name or postmaster@domain_name. Just hitting the spam button at the top of your browser/email software may do the trick. There’s more good advice at the FTC’s consumer spam page.
The answer came, ironically, to my email inbox in the form of one of those emails that did indeed violate the law.
I rolled my eyes and started into my reporting subroutine but then stopped cold. Just 1 second. If this person is selling lists of email addresses of conference attendees, somebody within the conference structure must be providing them. How is that legal? I have never agreed, in registering for a medical conference, to allow them to share my email address with anyone. To think that they are making money from that is extremely galling.
Vermont, at least, has enacted a law requiring companies that traffic in such email lists to register with the state. Although it has been in effect for 2 years, the jury is out regarding its efficacy. Our European counterparts are protected by the General Data Protection Regulation, which specifies that commercial email can be sent only to individuals who have explicitly opted into such mailings, and that purchased email lists are not compliant with the requirement.
Anybody have the inside scoop on this? Can we demand that our professional societies safeguard their attendee databases so this won’t happen? If they won’t, why am I paying big money to attend their conferences, only for them to make even more money at my expense?
Dr. Hitchcock is assistant professor, department of radiation oncology, at the University of Florida, Gainesville. She reported receiving research grant money from Merck. A version of this article first appeared on Medscape.com.
Despite the best efforts of my institution’s spam filter, I’ve realized that I spend at least 4 minutes every day of the week removing junk email from my in basket: EMR vendors, predatory journals trying to lure me into paying their outrageous publication fees, people who want to help me with my billing software (evidently that .edu extension hasn’t clicked for them yet), headhunters trying to fill specialty positions in other states, market researchers offering a gift card for 40 minutes filling out a survey.
If you do the math, 4 minutes daily is 1,460 minutes per year. That’s an entire day of my life lost each year to this useless nonsense, which I never agreed to receive in the first place. Now multiply that by the 22 million health care workers in the United States, or even just by the 985,000 licensed physicians in this country. Then factor in the $638 per hour in gross revenue generated by the average primary care physician, as a conservative, well-documented value.
By my reckoning, these bozos owe the United States alone over $15 billion in lost GDP each year.
So why don’t we shut it down!? The CAN-SPAM Act of 2003 attempted to at least mitigate the problem. It applies only to commercial entities (I know, I’d love to report some political groups, too). To avoid violating the law and risking fines of up to $16,000 per individual email, senders must:
- Not use misleading header info (including domain name and email address)
- Not use deceptive subject lines
- Clearly label the email as an ad
- Give an actual physical address of the sender
- Tell recipients how to opt out of future emails
- Honor opt-out requests within 10 business days
- Monitor the activities of any subcontractor sending email on their behalf
I can say with certainty that much of the trash in my inbox violates at least one of these. But that doesn’t matter if there is not an efficient way to report the violators and ensure that they’ll be tracked down. Hard enough if they live here, impossible if the email is routed from overseas, as much of it clearly is.
If you receive email in violation of the act, experts recommend that you write down the email address and the business name of the sender, fill out a complaint form on the Federal Trade Commission website, or send an email to [email protected], then send an email to your Internet service provider’s abuse desk. If you’re not working within a big institution like mine that has hot and cold running IT personnel that operate their own abuse prevention office, the address you’ll need is likely abuse@domain_name or postmaster@domain_name. Just hitting the spam button at the top of your browser/email software may do the trick. There’s more good advice at the FTC’s consumer spam page.
The answer came, ironically, to my email inbox in the form of one of those emails that did indeed violate the law.
I rolled my eyes and started into my reporting subroutine but then stopped cold. Just 1 second. If this person is selling lists of email addresses of conference attendees, somebody within the conference structure must be providing them. How is that legal? I have never agreed, in registering for a medical conference, to allow them to share my email address with anyone. To think that they are making money from that is extremely galling.
Vermont, at least, has enacted a law requiring companies that traffic in such email lists to register with the state. Although it has been in effect for 2 years, the jury is out regarding its efficacy. Our European counterparts are protected by the General Data Protection Regulation, which specifies that commercial email can be sent only to individuals who have explicitly opted into such mailings, and that purchased email lists are not compliant with the requirement.
Anybody have the inside scoop on this? Can we demand that our professional societies safeguard their attendee databases so this won’t happen? If they won’t, why am I paying big money to attend their conferences, only for them to make even more money at my expense?
Dr. Hitchcock is assistant professor, department of radiation oncology, at the University of Florida, Gainesville. She reported receiving research grant money from Merck. A version of this article first appeared on Medscape.com.
Closing your practice
“I might have to close my office,” a colleague wrote me recently. “I can’t find reliable medical assistants; no one good applies. Sad, but oh, well.”
A paucity of good employees is just one of many reasons given by physicians who have decided to close up shop. (See my recent column, “Finding Employees During a Pandemic”).
to address in order to ensure a smooth exit.
First, this cannot (and should not) be a hasty process. You will need at least a year to do it correctly, because there is a lot to do.
Once you have settled on a closing date, inform your attorney. If the firm you are using does not have experience in medical practice sales or closures, ask them to recommend one that does. You will need expert legal guidance during many of the steps that follow.
Next, review all of your contracts and leases. Most of them cannot be terminated at the drop of a hat. Facility and equipment leases may require a year’s notice, or even longer. Contracts with managed care, maintenance, cleaning, and hazardous waste disposal companies, and others such as answering services and website managers, should be reviewed to determine what sort of advance notice you will need to give.
Another step to take well in advance is to contact your malpractice insurance carrier. Most carriers have specific guidelines for when to notify your patients – and that notification will vary from carrier to carrier, state to state, and situation to situation. If you have a claims-made policy, you also need to inquire about the necessity of purchasing “tail” coverage, which will protect you in the event of a lawsuit after your practice has closed. Many carriers include tail coverage at no charge if you are retiring completely, but if you expect to do part-time, locum tenens, or volunteer medical work, you will need to pay for it.
Once you have the basics nailed down, notify your employees. You will want them to hear the news from you, not through the grapevine, and certainly not from your patients. You may be worried that some will quit, but keeping them in the dark will not prevent that, as they will find out soon enough. Besides, if you help them by assisting in finding them new employment, they will most likely help you by staying to the end.
At this point, you should also begin thinking about disposition of your patients’ records. You can’t just shred them, much as you might be tempted. Your attorney and malpractice carrier will guide you in how long they must be retained; 7-10 years is typical in many states, but it could be longer in yours. Unless you are selling part or all of your practice to another physician, you will have to designate someone else to be the legal custodian of the records and obtain a written custodial agreement from that person or organization.
Once that is arranged, you can notify your patients. Send them a letter or e-mail (or both) informing them of the date that you intend to close the practice. Let them know where their records will be kept, who to contact for a copy, and that their written consent will be required to obtain it. Some states also require that a notice be placed in the local newspaper or online, including the date of closure and how to request records.
This is also the time to inform all your third-party payers, including Medicare and Medicaid if applicable, any hospitals where you have privileges, and referring physicians. Notify any business concerns not notified already, such as utilities and other ancillary services. Your state medical board and the Drug Enforcement Agency will need to know as well. Contact a liquidator or used equipment dealer to arrange for disposal of any office equipment that has resale value. It is also a good time to decide how you will handle patient collections that trickle in after closing, and where mail should be forwarded.
As the closing date approaches, determine how to properly dispose of any medications you have on-hand. Your state may have requirements for disposal of controlled substances, and possibly for noncontrolled pharmaceuticals as well. Check your state’s controlled substances reporting system and other applicable regulators. Once the office is closed, don’t forget to shred any blank prescription pads and dissolve your corporation, if you have one.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
“I might have to close my office,” a colleague wrote me recently. “I can’t find reliable medical assistants; no one good applies. Sad, but oh, well.”
A paucity of good employees is just one of many reasons given by physicians who have decided to close up shop. (See my recent column, “Finding Employees During a Pandemic”).
to address in order to ensure a smooth exit.
First, this cannot (and should not) be a hasty process. You will need at least a year to do it correctly, because there is a lot to do.
Once you have settled on a closing date, inform your attorney. If the firm you are using does not have experience in medical practice sales or closures, ask them to recommend one that does. You will need expert legal guidance during many of the steps that follow.
Next, review all of your contracts and leases. Most of them cannot be terminated at the drop of a hat. Facility and equipment leases may require a year’s notice, or even longer. Contracts with managed care, maintenance, cleaning, and hazardous waste disposal companies, and others such as answering services and website managers, should be reviewed to determine what sort of advance notice you will need to give.
Another step to take well in advance is to contact your malpractice insurance carrier. Most carriers have specific guidelines for when to notify your patients – and that notification will vary from carrier to carrier, state to state, and situation to situation. If you have a claims-made policy, you also need to inquire about the necessity of purchasing “tail” coverage, which will protect you in the event of a lawsuit after your practice has closed. Many carriers include tail coverage at no charge if you are retiring completely, but if you expect to do part-time, locum tenens, or volunteer medical work, you will need to pay for it.
Once you have the basics nailed down, notify your employees. You will want them to hear the news from you, not through the grapevine, and certainly not from your patients. You may be worried that some will quit, but keeping them in the dark will not prevent that, as they will find out soon enough. Besides, if you help them by assisting in finding them new employment, they will most likely help you by staying to the end.
At this point, you should also begin thinking about disposition of your patients’ records. You can’t just shred them, much as you might be tempted. Your attorney and malpractice carrier will guide you in how long they must be retained; 7-10 years is typical in many states, but it could be longer in yours. Unless you are selling part or all of your practice to another physician, you will have to designate someone else to be the legal custodian of the records and obtain a written custodial agreement from that person or organization.
Once that is arranged, you can notify your patients. Send them a letter or e-mail (or both) informing them of the date that you intend to close the practice. Let them know where their records will be kept, who to contact for a copy, and that their written consent will be required to obtain it. Some states also require that a notice be placed in the local newspaper or online, including the date of closure and how to request records.
This is also the time to inform all your third-party payers, including Medicare and Medicaid if applicable, any hospitals where you have privileges, and referring physicians. Notify any business concerns not notified already, such as utilities and other ancillary services. Your state medical board and the Drug Enforcement Agency will need to know as well. Contact a liquidator or used equipment dealer to arrange for disposal of any office equipment that has resale value. It is also a good time to decide how you will handle patient collections that trickle in after closing, and where mail should be forwarded.
As the closing date approaches, determine how to properly dispose of any medications you have on-hand. Your state may have requirements for disposal of controlled substances, and possibly for noncontrolled pharmaceuticals as well. Check your state’s controlled substances reporting system and other applicable regulators. Once the office is closed, don’t forget to shred any blank prescription pads and dissolve your corporation, if you have one.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
“I might have to close my office,” a colleague wrote me recently. “I can’t find reliable medical assistants; no one good applies. Sad, but oh, well.”
A paucity of good employees is just one of many reasons given by physicians who have decided to close up shop. (See my recent column, “Finding Employees During a Pandemic”).
to address in order to ensure a smooth exit.
First, this cannot (and should not) be a hasty process. You will need at least a year to do it correctly, because there is a lot to do.
Once you have settled on a closing date, inform your attorney. If the firm you are using does not have experience in medical practice sales or closures, ask them to recommend one that does. You will need expert legal guidance during many of the steps that follow.
Next, review all of your contracts and leases. Most of them cannot be terminated at the drop of a hat. Facility and equipment leases may require a year’s notice, or even longer. Contracts with managed care, maintenance, cleaning, and hazardous waste disposal companies, and others such as answering services and website managers, should be reviewed to determine what sort of advance notice you will need to give.
Another step to take well in advance is to contact your malpractice insurance carrier. Most carriers have specific guidelines for when to notify your patients – and that notification will vary from carrier to carrier, state to state, and situation to situation. If you have a claims-made policy, you also need to inquire about the necessity of purchasing “tail” coverage, which will protect you in the event of a lawsuit after your practice has closed. Many carriers include tail coverage at no charge if you are retiring completely, but if you expect to do part-time, locum tenens, or volunteer medical work, you will need to pay for it.
Once you have the basics nailed down, notify your employees. You will want them to hear the news from you, not through the grapevine, and certainly not from your patients. You may be worried that some will quit, but keeping them in the dark will not prevent that, as they will find out soon enough. Besides, if you help them by assisting in finding them new employment, they will most likely help you by staying to the end.
At this point, you should also begin thinking about disposition of your patients’ records. You can’t just shred them, much as you might be tempted. Your attorney and malpractice carrier will guide you in how long they must be retained; 7-10 years is typical in many states, but it could be longer in yours. Unless you are selling part or all of your practice to another physician, you will have to designate someone else to be the legal custodian of the records and obtain a written custodial agreement from that person or organization.
Once that is arranged, you can notify your patients. Send them a letter or e-mail (or both) informing them of the date that you intend to close the practice. Let them know where their records will be kept, who to contact for a copy, and that their written consent will be required to obtain it. Some states also require that a notice be placed in the local newspaper or online, including the date of closure and how to request records.
This is also the time to inform all your third-party payers, including Medicare and Medicaid if applicable, any hospitals where you have privileges, and referring physicians. Notify any business concerns not notified already, such as utilities and other ancillary services. Your state medical board and the Drug Enforcement Agency will need to know as well. Contact a liquidator or used equipment dealer to arrange for disposal of any office equipment that has resale value. It is also a good time to decide how you will handle patient collections that trickle in after closing, and where mail should be forwarded.
As the closing date approaches, determine how to properly dispose of any medications you have on-hand. Your state may have requirements for disposal of controlled substances, and possibly for noncontrolled pharmaceuticals as well. Check your state’s controlled substances reporting system and other applicable regulators. Once the office is closed, don’t forget to shred any blank prescription pads and dissolve your corporation, if you have one.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
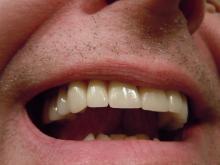
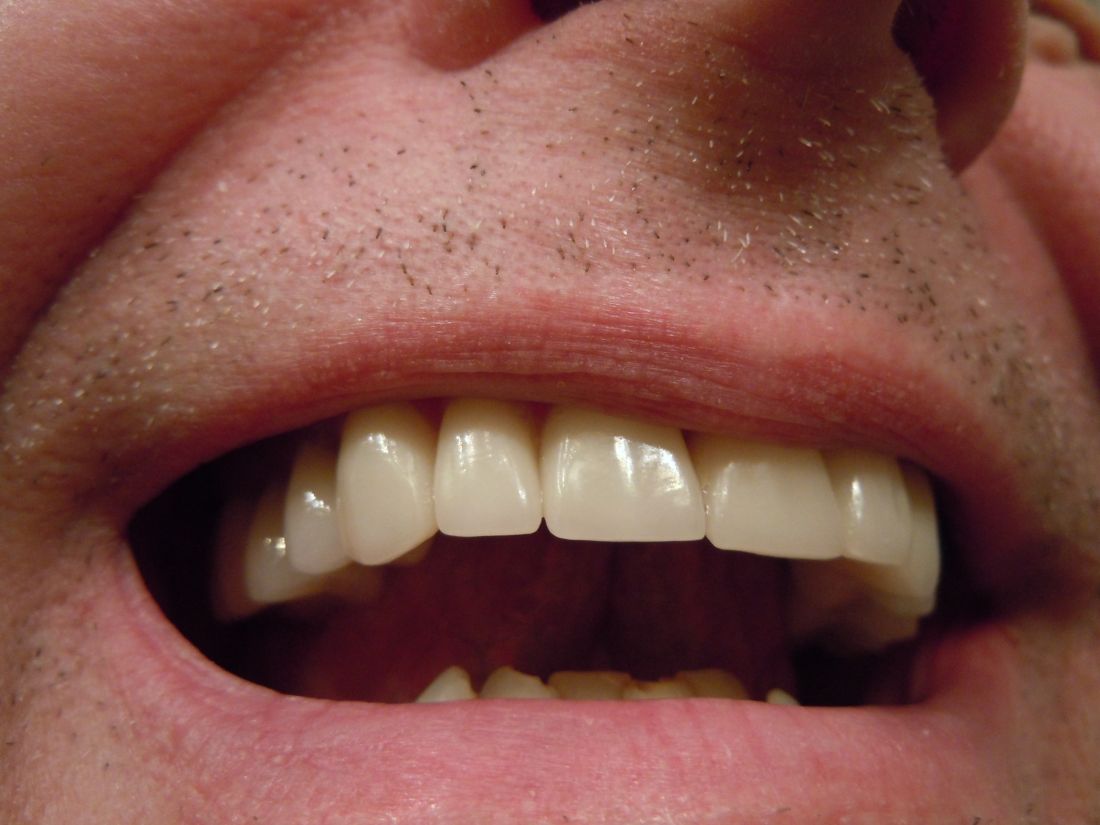
A very strange place to find a tooth
A nose for the tooth
Have you ever had a stuffy nose that just wouldn’t go away? Those irritating head colds have nothing on the stuffy nose a man in New York recently had to go through. A stuffy nose to top all stuffy noses. One stuffy nose to rule them all, as it were.
This man went to a Mount Sinai clinic with difficulty breathing through his right nostril, a problem that had been going on for years. Let us repeat that: A stuffy nose that lasted for years. The exam revealed a white mass jutting through the back of the septum and a CT scan confirmed the diagnosis. Perhaps you’ve already guessed, since the headline does give things away. Yes, this man had a tooth growing into his nose.
The problem was a half-inch-long ectopic tooth. Ectopic teeth are rare, occurring in less than 1% of people, but an ectopic tooth growing backward into the nasal cavity? Well, that’s so uncommon that this man got a case report in the New England Journal of Medicine.
This story does have a happy ending. Not all ectopic teeth need to be treated, but this one really did have to go. The offending tooth was surgically removed and, at a 3-month follow-up, the stuffy nose issue was completely resolved. So our friend gets the best of both worlds: His issue gets cured and he gets a case report in a major medical publication. If that’s not living the dream, we don’t know what is, and that’s the tooth.
Lettuce recommend you a sleep aid
Lettuce is great for many things. The star in a salad? Of course. The fresh element in a BLT? Yep. A sleep aid? According to a TikTok hack with almost 5 million views, the pinch hitter in a sandwich is switching leagues to be used like a tea for faster sleep. But, does it really work? Researchers say yes and no, according to a recent report at Tyla.com.
Studies conducted in 2013 and 2017 pointed toward a compound called lactucin, which is found in the plant’s n-butanol fraction. In the 2013 study, mice that received n-butanol fraction fell asleep faster and stayed asleep longer. In 2017, researchers found that lettuce made mice sleep longer and helped protect against cell inflammation and damage.
OK, so it works on mice. But what about humans? In the TikTok video, user Shapla Hoque pours hot water on a few lettuce leaves in a mug with a peppermint tea bag (for flavor). After 10 minutes, when the leaves are soaked and soggy, she removes them and drinks the lettuce tea. By the end of the video she’s visibly drowsy and ready to crash. Does this hold water?
Here’s the no. Dr. Charlotte Norton of the Slimming Clinic told Tyla.com that yeah, there are some properties in lettuce that will help you fall asleep, such as lactucarium, which is prominent in romaine. But you would need a massive amount of lettuce to get any effect. The TikTok video, she said, is an example of the placebo effect.
Brains get a rise out of Viagra
A lot of medications are used off label. Antidepressants for COVID have taken the cake recently, but here’s a new one: Viagra for Alzheimer’s disease.
Although there’s no definite link yet between the two, neuron models derived from induced pluripotent stem cells from patients with Alzheimer’s suggest that sildenafil increases neurite growth and decreases phospho-tau expression, Jiansong Fang, PhD, of the Cleveland Clinic, and associates said in Nature Aging.
Their research is an attempt to find untapped sources of new treatments among existing drugs. They began the search with 1,600 approved drugs and focused on those that target the buildup of beta amyloid and tau proteins in the brain, according to the Daily Beast.
Since sildenafil is obviously for men, more research will need to be done on how this drug affects women. Don’t start stocking up just yet.
Omicron is not a social-distancing robot
COVID, safe to say, has not been your typical, run-of-the-mill pandemic. People have protested social distancing. People have protested lockdowns. People have protested mask mandates. People have protested vaccine mandates. People have protested people protesting vaccine mandates.
Someone used a fake arm to get a COVID vaccine card. People have tried to reverse their COVID vaccinations. People had COVID contamination parties.
The common denominator? People. Humans. Maybe what we need is a nonhuman intervention. To fight COVID, we need a hero. A robotic hero.
And where can we find such a hero? The University of Maryland, of course, where computer scientists and engineers are working on an autonomous mobile robot to enforce indoor social-distancing rules.
Their robot can detect lapses in social distancing using cameras, both thermal and visual, along with a LiDAR (Light Detection and Ranging) sensor. It then sorts the offenders into various groups depending on whether they are standing still or moving and predicts their future movement using a state-of-the-art hybrid collision avoidance method known as Frozone, Adarsh Jagan Sathyamoorthy and associates explained in PLOS One.
“Once it reaches the breach, the robot encourages people to move apart via text that appears on a mounted display,” ScienceDaily said.
Maybe you were expecting a Terminator-type robot coming to enforce social distancing requirements rather than a simple text message. Let’s just hope that all COVID guidelines are followed, including social distancing, so the pandemic will finally end and won’t “be back.”
A nose for the tooth
Have you ever had a stuffy nose that just wouldn’t go away? Those irritating head colds have nothing on the stuffy nose a man in New York recently had to go through. A stuffy nose to top all stuffy noses. One stuffy nose to rule them all, as it were.
This man went to a Mount Sinai clinic with difficulty breathing through his right nostril, a problem that had been going on for years. Let us repeat that: A stuffy nose that lasted for years. The exam revealed a white mass jutting through the back of the septum and a CT scan confirmed the diagnosis. Perhaps you’ve already guessed, since the headline does give things away. Yes, this man had a tooth growing into his nose.
The problem was a half-inch-long ectopic tooth. Ectopic teeth are rare, occurring in less than 1% of people, but an ectopic tooth growing backward into the nasal cavity? Well, that’s so uncommon that this man got a case report in the New England Journal of Medicine.
This story does have a happy ending. Not all ectopic teeth need to be treated, but this one really did have to go. The offending tooth was surgically removed and, at a 3-month follow-up, the stuffy nose issue was completely resolved. So our friend gets the best of both worlds: His issue gets cured and he gets a case report in a major medical publication. If that’s not living the dream, we don’t know what is, and that’s the tooth.
Lettuce recommend you a sleep aid
Lettuce is great for many things. The star in a salad? Of course. The fresh element in a BLT? Yep. A sleep aid? According to a TikTok hack with almost 5 million views, the pinch hitter in a sandwich is switching leagues to be used like a tea for faster sleep. But, does it really work? Researchers say yes and no, according to a recent report at Tyla.com.
Studies conducted in 2013 and 2017 pointed toward a compound called lactucin, which is found in the plant’s n-butanol fraction. In the 2013 study, mice that received n-butanol fraction fell asleep faster and stayed asleep longer. In 2017, researchers found that lettuce made mice sleep longer and helped protect against cell inflammation and damage.
OK, so it works on mice. But what about humans? In the TikTok video, user Shapla Hoque pours hot water on a few lettuce leaves in a mug with a peppermint tea bag (for flavor). After 10 minutes, when the leaves are soaked and soggy, she removes them and drinks the lettuce tea. By the end of the video she’s visibly drowsy and ready to crash. Does this hold water?
Here’s the no. Dr. Charlotte Norton of the Slimming Clinic told Tyla.com that yeah, there are some properties in lettuce that will help you fall asleep, such as lactucarium, which is prominent in romaine. But you would need a massive amount of lettuce to get any effect. The TikTok video, she said, is an example of the placebo effect.
Brains get a rise out of Viagra
A lot of medications are used off label. Antidepressants for COVID have taken the cake recently, but here’s a new one: Viagra for Alzheimer’s disease.
Although there’s no definite link yet between the two, neuron models derived from induced pluripotent stem cells from patients with Alzheimer’s suggest that sildenafil increases neurite growth and decreases phospho-tau expression, Jiansong Fang, PhD, of the Cleveland Clinic, and associates said in Nature Aging.
Their research is an attempt to find untapped sources of new treatments among existing drugs. They began the search with 1,600 approved drugs and focused on those that target the buildup of beta amyloid and tau proteins in the brain, according to the Daily Beast.
Since sildenafil is obviously for men, more research will need to be done on how this drug affects women. Don’t start stocking up just yet.
Omicron is not a social-distancing robot
COVID, safe to say, has not been your typical, run-of-the-mill pandemic. People have protested social distancing. People have protested lockdowns. People have protested mask mandates. People have protested vaccine mandates. People have protested people protesting vaccine mandates.
Someone used a fake arm to get a COVID vaccine card. People have tried to reverse their COVID vaccinations. People had COVID contamination parties.
The common denominator? People. Humans. Maybe what we need is a nonhuman intervention. To fight COVID, we need a hero. A robotic hero.
And where can we find such a hero? The University of Maryland, of course, where computer scientists and engineers are working on an autonomous mobile robot to enforce indoor social-distancing rules.
Their robot can detect lapses in social distancing using cameras, both thermal and visual, along with a LiDAR (Light Detection and Ranging) sensor. It then sorts the offenders into various groups depending on whether they are standing still or moving and predicts their future movement using a state-of-the-art hybrid collision avoidance method known as Frozone, Adarsh Jagan Sathyamoorthy and associates explained in PLOS One.
“Once it reaches the breach, the robot encourages people to move apart via text that appears on a mounted display,” ScienceDaily said.
Maybe you were expecting a Terminator-type robot coming to enforce social distancing requirements rather than a simple text message. Let’s just hope that all COVID guidelines are followed, including social distancing, so the pandemic will finally end and won’t “be back.”
A nose for the tooth
Have you ever had a stuffy nose that just wouldn’t go away? Those irritating head colds have nothing on the stuffy nose a man in New York recently had to go through. A stuffy nose to top all stuffy noses. One stuffy nose to rule them all, as it were.
This man went to a Mount Sinai clinic with difficulty breathing through his right nostril, a problem that had been going on for years. Let us repeat that: A stuffy nose that lasted for years. The exam revealed a white mass jutting through the back of the septum and a CT scan confirmed the diagnosis. Perhaps you’ve already guessed, since the headline does give things away. Yes, this man had a tooth growing into his nose.
The problem was a half-inch-long ectopic tooth. Ectopic teeth are rare, occurring in less than 1% of people, but an ectopic tooth growing backward into the nasal cavity? Well, that’s so uncommon that this man got a case report in the New England Journal of Medicine.
This story does have a happy ending. Not all ectopic teeth need to be treated, but this one really did have to go. The offending tooth was surgically removed and, at a 3-month follow-up, the stuffy nose issue was completely resolved. So our friend gets the best of both worlds: His issue gets cured and he gets a case report in a major medical publication. If that’s not living the dream, we don’t know what is, and that’s the tooth.
Lettuce recommend you a sleep aid
Lettuce is great for many things. The star in a salad? Of course. The fresh element in a BLT? Yep. A sleep aid? According to a TikTok hack with almost 5 million views, the pinch hitter in a sandwich is switching leagues to be used like a tea for faster sleep. But, does it really work? Researchers say yes and no, according to a recent report at Tyla.com.
Studies conducted in 2013 and 2017 pointed toward a compound called lactucin, which is found in the plant’s n-butanol fraction. In the 2013 study, mice that received n-butanol fraction fell asleep faster and stayed asleep longer. In 2017, researchers found that lettuce made mice sleep longer and helped protect against cell inflammation and damage.
OK, so it works on mice. But what about humans? In the TikTok video, user Shapla Hoque pours hot water on a few lettuce leaves in a mug with a peppermint tea bag (for flavor). After 10 minutes, when the leaves are soaked and soggy, she removes them and drinks the lettuce tea. By the end of the video she’s visibly drowsy and ready to crash. Does this hold water?
Here’s the no. Dr. Charlotte Norton of the Slimming Clinic told Tyla.com that yeah, there are some properties in lettuce that will help you fall asleep, such as lactucarium, which is prominent in romaine. But you would need a massive amount of lettuce to get any effect. The TikTok video, she said, is an example of the placebo effect.
Brains get a rise out of Viagra
A lot of medications are used off label. Antidepressants for COVID have taken the cake recently, but here’s a new one: Viagra for Alzheimer’s disease.
Although there’s no definite link yet between the two, neuron models derived from induced pluripotent stem cells from patients with Alzheimer’s suggest that sildenafil increases neurite growth and decreases phospho-tau expression, Jiansong Fang, PhD, of the Cleveland Clinic, and associates said in Nature Aging.
Their research is an attempt to find untapped sources of new treatments among existing drugs. They began the search with 1,600 approved drugs and focused on those that target the buildup of beta amyloid and tau proteins in the brain, according to the Daily Beast.
Since sildenafil is obviously for men, more research will need to be done on how this drug affects women. Don’t start stocking up just yet.
Omicron is not a social-distancing robot
COVID, safe to say, has not been your typical, run-of-the-mill pandemic. People have protested social distancing. People have protested lockdowns. People have protested mask mandates. People have protested vaccine mandates. People have protested people protesting vaccine mandates.
Someone used a fake arm to get a COVID vaccine card. People have tried to reverse their COVID vaccinations. People had COVID contamination parties.
The common denominator? People. Humans. Maybe what we need is a nonhuman intervention. To fight COVID, we need a hero. A robotic hero.
And where can we find such a hero? The University of Maryland, of course, where computer scientists and engineers are working on an autonomous mobile robot to enforce indoor social-distancing rules.
Their robot can detect lapses in social distancing using cameras, both thermal and visual, along with a LiDAR (Light Detection and Ranging) sensor. It then sorts the offenders into various groups depending on whether they are standing still or moving and predicts their future movement using a state-of-the-art hybrid collision avoidance method known as Frozone, Adarsh Jagan Sathyamoorthy and associates explained in PLOS One.
“Once it reaches the breach, the robot encourages people to move apart via text that appears on a mounted display,” ScienceDaily said.
Maybe you were expecting a Terminator-type robot coming to enforce social distancing requirements rather than a simple text message. Let’s just hope that all COVID guidelines are followed, including social distancing, so the pandemic will finally end and won’t “be back.”
Vaccine protection drops against Omicron, making boosters crucial
A raft of new
The new studies, from teams of researchers in Germany, South Africa, Sweden, and the drug company Pfizer, showed 25 to 40-fold drops in the ability of antibodies created by two doses of the Pfizer-BioNTech vaccine to neutralize the virus.
But there seemed to be a bright spot in the studies too. The virus didn’t completely escape the immunity from the vaccines, and giving a third, booster dose appeared to restore antibodies to a level that’s been associated with protection against variants in the past.
“One of the silver linings of this pandemic so far is that mRNA vaccines manufactured based on the ancestral SARS-CoV-2 continue to work in the laboratory and, importantly, in real life against variant strains,” said Hana El Sahly, MD, professor of molecular virology and microbiology at Baylor College of Medicine in Houston. “The strains so far vary by their degree of being neutralized by the antibodies from these vaccines, but they are being neutralized nonetheless.”
Dr. El Sahly points out that the Beta variant was associated with a 10-fold drop in antibodies, but two doses of the vaccines still protected against it.
President Biden hailed the study results as good news.
“That Pfizer lab report came back saying that the expectation is that the existing vaccines protect against Omicron. But if you get the booster, you’re really in good shape. And so that’s very encouraging,” he said in a press briefing Dec. 8.
More research needed
Other scientists, however, stressed that these studies are from lab tests, and don’t necessarily reflect what will happen with Omicron in the real world. They cautioned about a worldwide push for boosters with so many countries still struggling to give first doses of vaccines.
Soumya Swaminathan, MD, chief scientist for the World Health Organization, stressed in a press briefing Dec. 8 that the results from the four studies varied widely, showing dips in neutralizing activity with Omicron that ranged from 5-fold to 40-fold.
The types of lab tests that were run were different, too, and involved small numbers of blood samples from patients.
She stressed that immunity depends not just on neutralizing antibodies, which act as a first line of defense when a virus invades, but also on B cells and T cells, and so far, tests show that these crucial components — which are important for preventing severe disease and death — had been less impacted than antibodies.
“So, I think it’s premature to conclude that this reduction in neutralizing activity would result in a significant reduction in vaccine effectiveness,” she said.
Whether or not these first-generation vaccines will be enough to stop Omicron, though, remains to be seen. A study of the Pfizer, Moderna, and AstraZeneca vaccines, led by German physician Sandra Ciesek, MD, who directs the Institute of Medical Virology at the University of Frankfurt, shows a booster didn’t appear to hold up well over time.
Dr. Ciesek and her team exposed Omicron viruses to the antibodies of volunteers who had been boosted with the Pfizer vaccine 3 months prior.
She also compared the results to what happened to those same 3-month antibody levels against Delta variant viruses. She found only a 25% neutralization of Omicron compared with a 95% neutralization of Delta. That represented about a 37-fold reduction in the ability of the antibodies to neutralize Omicron vs Delta.
“The data confirm that developing a vaccine adapted for Omicron makes sense,” she tweeted as part of a long thread she posted on her results.
Retool the vaccines?
Both Pfizer and Moderna are retooling their vaccines to better match them to the changes in the Omicron variant. In a press release, Pfizer said it could start deliveries of that updated vaccine by March, pending U.S. Food and Drug Administration authorization.
“What the booster really does in neutralizing Omicron right now, they don’t know, they have no idea,” said Peter Palese, PhD, chair of the department of microbiology at the Mount Sinai School of Medicine in New York City.
Dr. Palese said he was definitely concerned about a possible Omicron wave.
“There are four major sites on the spike protein targeted by antibodies from the vaccines, and all four sites have mutations,” he said. “All these important antigenic sites are changed.
“If Omicron becomes the new Delta, and the old vaccines really aren’t good enough, then we have to make new Omicron vaccines. Then we have to revaccinate everybody twice,” he said, and the costs could be staggering. “I am worried.”
Tedros Adhanom Ghebreyesus, PhD, director general of the WHO, urged countries to move quickly.
“Don’t wait. Act now,” he said, even before all the science is in hand. “All of us, every government, every individual should use all the tools we have right now,” to drive down transmission, increase testing and surveillance, and share scientific findings.
“We can prevent Omicron [from] becoming a global crisis right now,” he said.
A version of this article first appeared on Medscape.com.
A raft of new
The new studies, from teams of researchers in Germany, South Africa, Sweden, and the drug company Pfizer, showed 25 to 40-fold drops in the ability of antibodies created by two doses of the Pfizer-BioNTech vaccine to neutralize the virus.
But there seemed to be a bright spot in the studies too. The virus didn’t completely escape the immunity from the vaccines, and giving a third, booster dose appeared to restore antibodies to a level that’s been associated with protection against variants in the past.
“One of the silver linings of this pandemic so far is that mRNA vaccines manufactured based on the ancestral SARS-CoV-2 continue to work in the laboratory and, importantly, in real life against variant strains,” said Hana El Sahly, MD, professor of molecular virology and microbiology at Baylor College of Medicine in Houston. “The strains so far vary by their degree of being neutralized by the antibodies from these vaccines, but they are being neutralized nonetheless.”
Dr. El Sahly points out that the Beta variant was associated with a 10-fold drop in antibodies, but two doses of the vaccines still protected against it.
President Biden hailed the study results as good news.
“That Pfizer lab report came back saying that the expectation is that the existing vaccines protect against Omicron. But if you get the booster, you’re really in good shape. And so that’s very encouraging,” he said in a press briefing Dec. 8.
More research needed
Other scientists, however, stressed that these studies are from lab tests, and don’t necessarily reflect what will happen with Omicron in the real world. They cautioned about a worldwide push for boosters with so many countries still struggling to give first doses of vaccines.
Soumya Swaminathan, MD, chief scientist for the World Health Organization, stressed in a press briefing Dec. 8 that the results from the four studies varied widely, showing dips in neutralizing activity with Omicron that ranged from 5-fold to 40-fold.
The types of lab tests that were run were different, too, and involved small numbers of blood samples from patients.
She stressed that immunity depends not just on neutralizing antibodies, which act as a first line of defense when a virus invades, but also on B cells and T cells, and so far, tests show that these crucial components — which are important for preventing severe disease and death — had been less impacted than antibodies.
“So, I think it’s premature to conclude that this reduction in neutralizing activity would result in a significant reduction in vaccine effectiveness,” she said.
Whether or not these first-generation vaccines will be enough to stop Omicron, though, remains to be seen. A study of the Pfizer, Moderna, and AstraZeneca vaccines, led by German physician Sandra Ciesek, MD, who directs the Institute of Medical Virology at the University of Frankfurt, shows a booster didn’t appear to hold up well over time.
Dr. Ciesek and her team exposed Omicron viruses to the antibodies of volunteers who had been boosted with the Pfizer vaccine 3 months prior.
She also compared the results to what happened to those same 3-month antibody levels against Delta variant viruses. She found only a 25% neutralization of Omicron compared with a 95% neutralization of Delta. That represented about a 37-fold reduction in the ability of the antibodies to neutralize Omicron vs Delta.
“The data confirm that developing a vaccine adapted for Omicron makes sense,” she tweeted as part of a long thread she posted on her results.
Retool the vaccines?
Both Pfizer and Moderna are retooling their vaccines to better match them to the changes in the Omicron variant. In a press release, Pfizer said it could start deliveries of that updated vaccine by March, pending U.S. Food and Drug Administration authorization.
“What the booster really does in neutralizing Omicron right now, they don’t know, they have no idea,” said Peter Palese, PhD, chair of the department of microbiology at the Mount Sinai School of Medicine in New York City.
Dr. Palese said he was definitely concerned about a possible Omicron wave.
“There are four major sites on the spike protein targeted by antibodies from the vaccines, and all four sites have mutations,” he said. “All these important antigenic sites are changed.
“If Omicron becomes the new Delta, and the old vaccines really aren’t good enough, then we have to make new Omicron vaccines. Then we have to revaccinate everybody twice,” he said, and the costs could be staggering. “I am worried.”
Tedros Adhanom Ghebreyesus, PhD, director general of the WHO, urged countries to move quickly.
“Don’t wait. Act now,” he said, even before all the science is in hand. “All of us, every government, every individual should use all the tools we have right now,” to drive down transmission, increase testing and surveillance, and share scientific findings.
“We can prevent Omicron [from] becoming a global crisis right now,” he said.
A version of this article first appeared on Medscape.com.
A raft of new
The new studies, from teams of researchers in Germany, South Africa, Sweden, and the drug company Pfizer, showed 25 to 40-fold drops in the ability of antibodies created by two doses of the Pfizer-BioNTech vaccine to neutralize the virus.
But there seemed to be a bright spot in the studies too. The virus didn’t completely escape the immunity from the vaccines, and giving a third, booster dose appeared to restore antibodies to a level that’s been associated with protection against variants in the past.
“One of the silver linings of this pandemic so far is that mRNA vaccines manufactured based on the ancestral SARS-CoV-2 continue to work in the laboratory and, importantly, in real life against variant strains,” said Hana El Sahly, MD, professor of molecular virology and microbiology at Baylor College of Medicine in Houston. “The strains so far vary by their degree of being neutralized by the antibodies from these vaccines, but they are being neutralized nonetheless.”
Dr. El Sahly points out that the Beta variant was associated with a 10-fold drop in antibodies, but two doses of the vaccines still protected against it.
President Biden hailed the study results as good news.
“That Pfizer lab report came back saying that the expectation is that the existing vaccines protect against Omicron. But if you get the booster, you’re really in good shape. And so that’s very encouraging,” he said in a press briefing Dec. 8.
More research needed
Other scientists, however, stressed that these studies are from lab tests, and don’t necessarily reflect what will happen with Omicron in the real world. They cautioned about a worldwide push for boosters with so many countries still struggling to give first doses of vaccines.
Soumya Swaminathan, MD, chief scientist for the World Health Organization, stressed in a press briefing Dec. 8 that the results from the four studies varied widely, showing dips in neutralizing activity with Omicron that ranged from 5-fold to 40-fold.
The types of lab tests that were run were different, too, and involved small numbers of blood samples from patients.
She stressed that immunity depends not just on neutralizing antibodies, which act as a first line of defense when a virus invades, but also on B cells and T cells, and so far, tests show that these crucial components — which are important for preventing severe disease and death — had been less impacted than antibodies.
“So, I think it’s premature to conclude that this reduction in neutralizing activity would result in a significant reduction in vaccine effectiveness,” she said.
Whether or not these first-generation vaccines will be enough to stop Omicron, though, remains to be seen. A study of the Pfizer, Moderna, and AstraZeneca vaccines, led by German physician Sandra Ciesek, MD, who directs the Institute of Medical Virology at the University of Frankfurt, shows a booster didn’t appear to hold up well over time.
Dr. Ciesek and her team exposed Omicron viruses to the antibodies of volunteers who had been boosted with the Pfizer vaccine 3 months prior.
She also compared the results to what happened to those same 3-month antibody levels against Delta variant viruses. She found only a 25% neutralization of Omicron compared with a 95% neutralization of Delta. That represented about a 37-fold reduction in the ability of the antibodies to neutralize Omicron vs Delta.
“The data confirm that developing a vaccine adapted for Omicron makes sense,” she tweeted as part of a long thread she posted on her results.
Retool the vaccines?
Both Pfizer and Moderna are retooling their vaccines to better match them to the changes in the Omicron variant. In a press release, Pfizer said it could start deliveries of that updated vaccine by March, pending U.S. Food and Drug Administration authorization.
“What the booster really does in neutralizing Omicron right now, they don’t know, they have no idea,” said Peter Palese, PhD, chair of the department of microbiology at the Mount Sinai School of Medicine in New York City.
Dr. Palese said he was definitely concerned about a possible Omicron wave.
“There are four major sites on the spike protein targeted by antibodies from the vaccines, and all four sites have mutations,” he said. “All these important antigenic sites are changed.
“If Omicron becomes the new Delta, and the old vaccines really aren’t good enough, then we have to make new Omicron vaccines. Then we have to revaccinate everybody twice,” he said, and the costs could be staggering. “I am worried.”
Tedros Adhanom Ghebreyesus, PhD, director general of the WHO, urged countries to move quickly.
“Don’t wait. Act now,” he said, even before all the science is in hand. “All of us, every government, every individual should use all the tools we have right now,” to drive down transmission, increase testing and surveillance, and share scientific findings.
“We can prevent Omicron [from] becoming a global crisis right now,” he said.
A version of this article first appeared on Medscape.com.
New data on rare myocarditis after COVID-19 vaccination
Adolescents and adults younger than age 21 who develop myocarditis after mRNA COVID-19 vaccination frequently have abnormal findings on cardiac MRI (cMRI) but most have a mild clinical course with rapid resolution of symptoms, a new study concludes.
“This study supports what we’ve been seeing. People identified and treated early and appropriately for the rare complication of COVID-19 vaccine-related myocarditis typically experienced only mild cases and short recovery times,” American Heart Association President Donald M. Lloyd-Jones, MD, said in a podcast.
“Overwhelmingly, the data continue to indicate [that] the benefits of COVID-19 vaccine far outweigh any very rare risks of adverse events from the vaccine, including myocarditis,” Dr. Lloyd-Jones added.
The study was published online Dec. 6 in Circulation.
Using data from 26 pediatric medical centers across the United States and Canada, the researchers reviewed the medical records of 139 patients younger than 21 with suspected myocarditis within 1 month of receiving a COVID-19 vaccination.
They made the following key observations:
- Most patients were male (90.6%), White (66.2%) and with a median age of 15.8 years.
- Suspected myocarditis occurred in 136 patients (97.8%) following mRNA vaccine, with 131 (94.2%) following the Pfizer-BioNTech vaccine; 128 cases (91.4%) occurred after the second dose.
- Symptoms started a median of 2 days (range 0 to 22 days) following vaccination administration.
- Chest pain was the most common symptom (99.3%), with fever present in 30.9% of patients and shortness of breath in 27.3%.
- Patients were treated with nonsteroidal anti-inflammatory drugs (81.3%), intravenous immunoglobulin (21.6%), glucocorticoids (21.6%), colchicine (7.9%) or no anti-inflammatory therapies (8.6%).
- Twenty-six patients (18.7%) were admitted to the intensive care unit; 2 received inotropic/vasoactive support; none required extracorporeal membrane oxygenation or died.
- Median time spent in the hospital was 2 days.
- A total of 111 patients had elevated troponin I (8.12 ng/mL) and 28 had elevated troponin T (0.61 ng/mL).
- More than two-thirds (69.8%) had abnormal electrocardiograms and/or arrhythmias (7 with nonsustained ventricular tachycardia).
- Twenty-six patients (18.7%) had left ventricular ejection fraction (LVEF) less than 55% on echocardiogram; LVEF had returned to normal in the 25 who returned for follow-up.
- 75 of 97 patients (77.3%) who underwent cMRI at a median of 5 days from symptom onset had abnormal findings; 74 (76.3%) had late gadolinium enhancement, 54 (55.7%) had myocardial edema, and 49 (50.5%) met Lake Louise criteria for myocarditis.
“These data suggest that most cases of suspected COVID-19 vaccine–related myocarditis in people younger than 21 are mild and resolve quickly,” corresponding author Dongngan Truong, MD, Division of Pediatric Cardiology, University of Utah and Primary Children’s Hospital, Salt Lake City, said in a statement.
“We were very happy to see that type of recovery. However, we are awaiting further studies to better understand the long-term outcomes of patients who have had COVID-19 vaccination-related myocarditis. We also need to study the risk factors and mechanisms for this rare complication,” Dr. Truong added.
Dr. Lloyd-Jones said these findings support the AHA’s position that COVID-19 vaccines are “safe, highly effective, and fundamental to saving lives, protecting our families and communities against COVID-19, and ending the pandemic.”
The study received no funding. Dr. Truong consults for Pfizer on vaccine-associated myocarditis. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
Adolescents and adults younger than age 21 who develop myocarditis after mRNA COVID-19 vaccination frequently have abnormal findings on cardiac MRI (cMRI) but most have a mild clinical course with rapid resolution of symptoms, a new study concludes.
“This study supports what we’ve been seeing. People identified and treated early and appropriately for the rare complication of COVID-19 vaccine-related myocarditis typically experienced only mild cases and short recovery times,” American Heart Association President Donald M. Lloyd-Jones, MD, said in a podcast.
“Overwhelmingly, the data continue to indicate [that] the benefits of COVID-19 vaccine far outweigh any very rare risks of adverse events from the vaccine, including myocarditis,” Dr. Lloyd-Jones added.
The study was published online Dec. 6 in Circulation.
Using data from 26 pediatric medical centers across the United States and Canada, the researchers reviewed the medical records of 139 patients younger than 21 with suspected myocarditis within 1 month of receiving a COVID-19 vaccination.
They made the following key observations:
- Most patients were male (90.6%), White (66.2%) and with a median age of 15.8 years.
- Suspected myocarditis occurred in 136 patients (97.8%) following mRNA vaccine, with 131 (94.2%) following the Pfizer-BioNTech vaccine; 128 cases (91.4%) occurred after the second dose.
- Symptoms started a median of 2 days (range 0 to 22 days) following vaccination administration.
- Chest pain was the most common symptom (99.3%), with fever present in 30.9% of patients and shortness of breath in 27.3%.
- Patients were treated with nonsteroidal anti-inflammatory drugs (81.3%), intravenous immunoglobulin (21.6%), glucocorticoids (21.6%), colchicine (7.9%) or no anti-inflammatory therapies (8.6%).
- Twenty-six patients (18.7%) were admitted to the intensive care unit; 2 received inotropic/vasoactive support; none required extracorporeal membrane oxygenation or died.
- Median time spent in the hospital was 2 days.
- A total of 111 patients had elevated troponin I (8.12 ng/mL) and 28 had elevated troponin T (0.61 ng/mL).
- More than two-thirds (69.8%) had abnormal electrocardiograms and/or arrhythmias (7 with nonsustained ventricular tachycardia).
- Twenty-six patients (18.7%) had left ventricular ejection fraction (LVEF) less than 55% on echocardiogram; LVEF had returned to normal in the 25 who returned for follow-up.
- 75 of 97 patients (77.3%) who underwent cMRI at a median of 5 days from symptom onset had abnormal findings; 74 (76.3%) had late gadolinium enhancement, 54 (55.7%) had myocardial edema, and 49 (50.5%) met Lake Louise criteria for myocarditis.
“These data suggest that most cases of suspected COVID-19 vaccine–related myocarditis in people younger than 21 are mild and resolve quickly,” corresponding author Dongngan Truong, MD, Division of Pediatric Cardiology, University of Utah and Primary Children’s Hospital, Salt Lake City, said in a statement.
“We were very happy to see that type of recovery. However, we are awaiting further studies to better understand the long-term outcomes of patients who have had COVID-19 vaccination-related myocarditis. We also need to study the risk factors and mechanisms for this rare complication,” Dr. Truong added.
Dr. Lloyd-Jones said these findings support the AHA’s position that COVID-19 vaccines are “safe, highly effective, and fundamental to saving lives, protecting our families and communities against COVID-19, and ending the pandemic.”
The study received no funding. Dr. Truong consults for Pfizer on vaccine-associated myocarditis. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
Adolescents and adults younger than age 21 who develop myocarditis after mRNA COVID-19 vaccination frequently have abnormal findings on cardiac MRI (cMRI) but most have a mild clinical course with rapid resolution of symptoms, a new study concludes.
“This study supports what we’ve been seeing. People identified and treated early and appropriately for the rare complication of COVID-19 vaccine-related myocarditis typically experienced only mild cases and short recovery times,” American Heart Association President Donald M. Lloyd-Jones, MD, said in a podcast.
“Overwhelmingly, the data continue to indicate [that] the benefits of COVID-19 vaccine far outweigh any very rare risks of adverse events from the vaccine, including myocarditis,” Dr. Lloyd-Jones added.
The study was published online Dec. 6 in Circulation.
Using data from 26 pediatric medical centers across the United States and Canada, the researchers reviewed the medical records of 139 patients younger than 21 with suspected myocarditis within 1 month of receiving a COVID-19 vaccination.
They made the following key observations:
- Most patients were male (90.6%), White (66.2%) and with a median age of 15.8 years.
- Suspected myocarditis occurred in 136 patients (97.8%) following mRNA vaccine, with 131 (94.2%) following the Pfizer-BioNTech vaccine; 128 cases (91.4%) occurred after the second dose.
- Symptoms started a median of 2 days (range 0 to 22 days) following vaccination administration.
- Chest pain was the most common symptom (99.3%), with fever present in 30.9% of patients and shortness of breath in 27.3%.
- Patients were treated with nonsteroidal anti-inflammatory drugs (81.3%), intravenous immunoglobulin (21.6%), glucocorticoids (21.6%), colchicine (7.9%) or no anti-inflammatory therapies (8.6%).
- Twenty-six patients (18.7%) were admitted to the intensive care unit; 2 received inotropic/vasoactive support; none required extracorporeal membrane oxygenation or died.
- Median time spent in the hospital was 2 days.
- A total of 111 patients had elevated troponin I (8.12 ng/mL) and 28 had elevated troponin T (0.61 ng/mL).
- More than two-thirds (69.8%) had abnormal electrocardiograms and/or arrhythmias (7 with nonsustained ventricular tachycardia).
- Twenty-six patients (18.7%) had left ventricular ejection fraction (LVEF) less than 55% on echocardiogram; LVEF had returned to normal in the 25 who returned for follow-up.
- 75 of 97 patients (77.3%) who underwent cMRI at a median of 5 days from symptom onset had abnormal findings; 74 (76.3%) had late gadolinium enhancement, 54 (55.7%) had myocardial edema, and 49 (50.5%) met Lake Louise criteria for myocarditis.
“These data suggest that most cases of suspected COVID-19 vaccine–related myocarditis in people younger than 21 are mild and resolve quickly,” corresponding author Dongngan Truong, MD, Division of Pediatric Cardiology, University of Utah and Primary Children’s Hospital, Salt Lake City, said in a statement.
“We were very happy to see that type of recovery. However, we are awaiting further studies to better understand the long-term outcomes of patients who have had COVID-19 vaccination-related myocarditis. We also need to study the risk factors and mechanisms for this rare complication,” Dr. Truong added.
Dr. Lloyd-Jones said these findings support the AHA’s position that COVID-19 vaccines are “safe, highly effective, and fundamental to saving lives, protecting our families and communities against COVID-19, and ending the pandemic.”
The study received no funding. Dr. Truong consults for Pfizer on vaccine-associated myocarditis. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
AHA challenges diet doctor’s study alleging COVID vax risks
An abstract and poster presentation questioning the safety of mRNA-based COVID-19 vaccines, embraced by some and lambasted by others, has drawn an “expression of concern” from the American Heart Association, along with a bid for correction.
The abstract in question concludes that COVID vaccines “dramatically increase” levels of certain inflammatory biomarkers, and therefore, the 5-year risk of acute coronary syndromes (ACS), based on pre- and post-vaccination results of an obscure blood panel called the PULS Cardiac Test (GD Biosciences). The findings were presented at the AHA’s 2021 Scientific Sessionsas, an uncontrolled observational study of 566 patients in a preventive cardiology practice.
Some on social media have seized on the abstract as evidence of serious potential harm from the two available mRNA-based SARS-CoV-2 vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). But others contend that the study’s described design and findings are specious and its conclusions overstated.
They also point to the notoriety of its one listed author, Steven R. Gundry, MD, who promotes his diet books and supplements as well as fringe, highly criticized theories about diet and disease on several websites, including drgundry.com. Dr. Gundry has not responded to requests for an interview.
Dr. Gundry’s abstract from the AHA Scientific Sessions 2021, available on the meeting’s program planner, was marked with an “expression of concern” by the AHA that is to stand “until a suitable correction is published, to indicate that the abstract in its current version may not be reliable.”
The expression of concern statement, also published online Nov. 24 in Circulation, says “potential errors in the abstract” were brought to the attention of the meeting planners. “Specifically, there are several typographical errors, there is no data in the abstract regarding myocardial T-cell infiltration, there are no statistical analyses for significance provided, and the author is not clear that only anecdotal data was used.”
The biomarker elevations on which the abstract’s conclusions are based included hepatocyte growth factor, “which serves as a marker for chemotaxis of T-cells into epithelium and cardiac tissue,” it states.
“The expression of concern about the abstract will remain in place until a correction is accepted and published” in Circulation, AHA spokesperson Suzanne Grant told this news organization by email.
“The specific data needed will be up to the abstract author to determine and supply,” she said, noting that Dr. Gundry “has been in communication with the journal throughout this process.”
Submitting researchers “must always attest to the validity of the abstract,” Ms. Grant said. “Abstracts are then curated by independent review panels, blinded to the identities of the abstract authors, and are considered based on the potential to add to the diversity of scientific issues and views discussed at the meeting.”
Regarding the AHA’s system for vetting abstracts vying for acceptance to the scientific sessions, she said it is not primarily intended to “evaluate scientific validity” and that the organization is “currently reviewing its existing abstract submission processes.”
A recent Reuters report reviews the controversy and provides links to criticisms of the study on social media.
A version of this article first appeared on Medscape.com.
An abstract and poster presentation questioning the safety of mRNA-based COVID-19 vaccines, embraced by some and lambasted by others, has drawn an “expression of concern” from the American Heart Association, along with a bid for correction.
The abstract in question concludes that COVID vaccines “dramatically increase” levels of certain inflammatory biomarkers, and therefore, the 5-year risk of acute coronary syndromes (ACS), based on pre- and post-vaccination results of an obscure blood panel called the PULS Cardiac Test (GD Biosciences). The findings were presented at the AHA’s 2021 Scientific Sessionsas, an uncontrolled observational study of 566 patients in a preventive cardiology practice.
Some on social media have seized on the abstract as evidence of serious potential harm from the two available mRNA-based SARS-CoV-2 vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). But others contend that the study’s described design and findings are specious and its conclusions overstated.
They also point to the notoriety of its one listed author, Steven R. Gundry, MD, who promotes his diet books and supplements as well as fringe, highly criticized theories about diet and disease on several websites, including drgundry.com. Dr. Gundry has not responded to requests for an interview.
Dr. Gundry’s abstract from the AHA Scientific Sessions 2021, available on the meeting’s program planner, was marked with an “expression of concern” by the AHA that is to stand “until a suitable correction is published, to indicate that the abstract in its current version may not be reliable.”
The expression of concern statement, also published online Nov. 24 in Circulation, says “potential errors in the abstract” were brought to the attention of the meeting planners. “Specifically, there are several typographical errors, there is no data in the abstract regarding myocardial T-cell infiltration, there are no statistical analyses for significance provided, and the author is not clear that only anecdotal data was used.”
The biomarker elevations on which the abstract’s conclusions are based included hepatocyte growth factor, “which serves as a marker for chemotaxis of T-cells into epithelium and cardiac tissue,” it states.
“The expression of concern about the abstract will remain in place until a correction is accepted and published” in Circulation, AHA spokesperson Suzanne Grant told this news organization by email.
“The specific data needed will be up to the abstract author to determine and supply,” she said, noting that Dr. Gundry “has been in communication with the journal throughout this process.”
Submitting researchers “must always attest to the validity of the abstract,” Ms. Grant said. “Abstracts are then curated by independent review panels, blinded to the identities of the abstract authors, and are considered based on the potential to add to the diversity of scientific issues and views discussed at the meeting.”
Regarding the AHA’s system for vetting abstracts vying for acceptance to the scientific sessions, she said it is not primarily intended to “evaluate scientific validity” and that the organization is “currently reviewing its existing abstract submission processes.”
A recent Reuters report reviews the controversy and provides links to criticisms of the study on social media.
A version of this article first appeared on Medscape.com.
An abstract and poster presentation questioning the safety of mRNA-based COVID-19 vaccines, embraced by some and lambasted by others, has drawn an “expression of concern” from the American Heart Association, along with a bid for correction.
The abstract in question concludes that COVID vaccines “dramatically increase” levels of certain inflammatory biomarkers, and therefore, the 5-year risk of acute coronary syndromes (ACS), based on pre- and post-vaccination results of an obscure blood panel called the PULS Cardiac Test (GD Biosciences). The findings were presented at the AHA’s 2021 Scientific Sessionsas, an uncontrolled observational study of 566 patients in a preventive cardiology practice.
Some on social media have seized on the abstract as evidence of serious potential harm from the two available mRNA-based SARS-CoV-2 vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). But others contend that the study’s described design and findings are specious and its conclusions overstated.
They also point to the notoriety of its one listed author, Steven R. Gundry, MD, who promotes his diet books and supplements as well as fringe, highly criticized theories about diet and disease on several websites, including drgundry.com. Dr. Gundry has not responded to requests for an interview.
Dr. Gundry’s abstract from the AHA Scientific Sessions 2021, available on the meeting’s program planner, was marked with an “expression of concern” by the AHA that is to stand “until a suitable correction is published, to indicate that the abstract in its current version may not be reliable.”
The expression of concern statement, also published online Nov. 24 in Circulation, says “potential errors in the abstract” were brought to the attention of the meeting planners. “Specifically, there are several typographical errors, there is no data in the abstract regarding myocardial T-cell infiltration, there are no statistical analyses for significance provided, and the author is not clear that only anecdotal data was used.”
The biomarker elevations on which the abstract’s conclusions are based included hepatocyte growth factor, “which serves as a marker for chemotaxis of T-cells into epithelium and cardiac tissue,” it states.
“The expression of concern about the abstract will remain in place until a correction is accepted and published” in Circulation, AHA spokesperson Suzanne Grant told this news organization by email.
“The specific data needed will be up to the abstract author to determine and supply,” she said, noting that Dr. Gundry “has been in communication with the journal throughout this process.”
Submitting researchers “must always attest to the validity of the abstract,” Ms. Grant said. “Abstracts are then curated by independent review panels, blinded to the identities of the abstract authors, and are considered based on the potential to add to the diversity of scientific issues and views discussed at the meeting.”
Regarding the AHA’s system for vetting abstracts vying for acceptance to the scientific sessions, she said it is not primarily intended to “evaluate scientific validity” and that the organization is “currently reviewing its existing abstract submission processes.”
A recent Reuters report reviews the controversy and provides links to criticisms of the study on social media.
A version of this article first appeared on Medscape.com.