User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'medstat-accordion-set article-series')]
Oral penicillin advised for high-risk rheumatic heart disease
Some patients with rheumatic heart disease who are thought to have an allergic response to injectable penicillin may actually be experiencing a cardiac reaction to the injection, new information suggests.
This has resulted in new advice from the American Heart Association suggesting that oral penicillin may be a safer option for people with rheumatic heart disease who are at high risk of a cardiac reaction.
Those at high risk of a cardiac reaction include those with rheumatic heart disease and severe valvular heart disease with or without reduced ventricular function, those with aortic insufficiency or decreased left ventricular systolic function, and those who have active symptoms of rheumatic heart disease.
This new guidance is the subject of an AHA “presidential advisory” published online in the Journal of the American Heart Association on Jan. 20, 2022.
The advisory notes that more than 39 million people worldwide have rheumatic heart disease, a condition in which the heart’s valves are permanently damaged by rheumatic fever, which can occur if a strep throat infection or scarlet fever is untreated or inadequately treated.
Most cases of rheumatic heart disease occur in people living in low- and middle-income countries, where the condition is often diagnosed after severe valvular heart disease or other cardiovascular complications have already developed, leading to higher rates of death and lower life expectancy.
The recommended treatment for rheumatic heart disease is an intramuscular injection of benzathine penicillin G (BPG) given every 3-4 weeks for many years or even lifelong. Treatment with BPG for rheumatic heart disease has been limited in part because of patients’ and clinicians’ fears of anaphylaxis.
However, a growing number of reports of BPG-related deaths have not shown the features of classic anaphylaxis and instead point to a cardiovascular reaction, specifically, a vasovagal episode, the advisory states.
Signs of a vasovagal episode often occur immediately after administration of BPG, sometimes even during injection, and include low blood pressure, which can improve if patients are put into a supine position, slow heart rate, and fainting, all of which may lead to low blood flow to the heart, irregular heart rhythm, and sudden cardiac death.
On the other hand, signs of anaphylaxis after BPG injection are usually slightly delayed after the injection, even up to an hour later, and include coughing, respiratory distress, rapid heart rate, low blood pressure that doesn’t respond to position change, fainting, itching and redness at the injection site, the document notes.
The risks of a cardiovascular reaction to BPG are highest among individuals with severe mitral stenosis, aortic stenosis, aortic insufficiency or decreased left ventricular systolic function (ejection fraction <50%), and those who have active symptoms of rheumatic heart disease. For these patients, treatment with oral penicillin should be strongly considered.
People with rheumatic heart disease who are at low risk of this cardiovascular reaction and who do not have a history of being allergic to penicillin or anaphylaxis can still be prescribed BPG for treatment and prevention of rheumatic heart disease, which has been proven to be the best treatment for prevention of recurrent rheumatic fever.
The advisory recommended the following standard practices for all patients receiving BPG for rheumatic heart disease:
- Reducing injection pain and patient anxiety, both of which are known risk factors for injection-related fainting. Methods for pain reduction include applying firm pressure to the site for 10 seconds or application of an ice pack or the use of analgesics (such as acetaminophen, ibuprofen, or other NSAIDs).
- Patients should be well hydrated prior to injection and should drink at least 500 mL of water before injection to prevent reflexive fainting.
- Eating a small amount of solid food within the hour before injection.
- Receiving the injection while lying down, which may reduce the risk of blood pooling in the extremities.
- Providers who administer BPG should be taught how to recognize and quickly treat symptoms such as low blood pressure, low heart rate, or fainting.
A version of this article first appeared on Medscape.com.
Some patients with rheumatic heart disease who are thought to have an allergic response to injectable penicillin may actually be experiencing a cardiac reaction to the injection, new information suggests.
This has resulted in new advice from the American Heart Association suggesting that oral penicillin may be a safer option for people with rheumatic heart disease who are at high risk of a cardiac reaction.
Those at high risk of a cardiac reaction include those with rheumatic heart disease and severe valvular heart disease with or without reduced ventricular function, those with aortic insufficiency or decreased left ventricular systolic function, and those who have active symptoms of rheumatic heart disease.
This new guidance is the subject of an AHA “presidential advisory” published online in the Journal of the American Heart Association on Jan. 20, 2022.
The advisory notes that more than 39 million people worldwide have rheumatic heart disease, a condition in which the heart’s valves are permanently damaged by rheumatic fever, which can occur if a strep throat infection or scarlet fever is untreated or inadequately treated.
Most cases of rheumatic heart disease occur in people living in low- and middle-income countries, where the condition is often diagnosed after severe valvular heart disease or other cardiovascular complications have already developed, leading to higher rates of death and lower life expectancy.
The recommended treatment for rheumatic heart disease is an intramuscular injection of benzathine penicillin G (BPG) given every 3-4 weeks for many years or even lifelong. Treatment with BPG for rheumatic heart disease has been limited in part because of patients’ and clinicians’ fears of anaphylaxis.
However, a growing number of reports of BPG-related deaths have not shown the features of classic anaphylaxis and instead point to a cardiovascular reaction, specifically, a vasovagal episode, the advisory states.
Signs of a vasovagal episode often occur immediately after administration of BPG, sometimes even during injection, and include low blood pressure, which can improve if patients are put into a supine position, slow heart rate, and fainting, all of which may lead to low blood flow to the heart, irregular heart rhythm, and sudden cardiac death.
On the other hand, signs of anaphylaxis after BPG injection are usually slightly delayed after the injection, even up to an hour later, and include coughing, respiratory distress, rapid heart rate, low blood pressure that doesn’t respond to position change, fainting, itching and redness at the injection site, the document notes.
The risks of a cardiovascular reaction to BPG are highest among individuals with severe mitral stenosis, aortic stenosis, aortic insufficiency or decreased left ventricular systolic function (ejection fraction <50%), and those who have active symptoms of rheumatic heart disease. For these patients, treatment with oral penicillin should be strongly considered.
People with rheumatic heart disease who are at low risk of this cardiovascular reaction and who do not have a history of being allergic to penicillin or anaphylaxis can still be prescribed BPG for treatment and prevention of rheumatic heart disease, which has been proven to be the best treatment for prevention of recurrent rheumatic fever.
The advisory recommended the following standard practices for all patients receiving BPG for rheumatic heart disease:
- Reducing injection pain and patient anxiety, both of which are known risk factors for injection-related fainting. Methods for pain reduction include applying firm pressure to the site for 10 seconds or application of an ice pack or the use of analgesics (such as acetaminophen, ibuprofen, or other NSAIDs).
- Patients should be well hydrated prior to injection and should drink at least 500 mL of water before injection to prevent reflexive fainting.
- Eating a small amount of solid food within the hour before injection.
- Receiving the injection while lying down, which may reduce the risk of blood pooling in the extremities.
- Providers who administer BPG should be taught how to recognize and quickly treat symptoms such as low blood pressure, low heart rate, or fainting.
A version of this article first appeared on Medscape.com.
Some patients with rheumatic heart disease who are thought to have an allergic response to injectable penicillin may actually be experiencing a cardiac reaction to the injection, new information suggests.
This has resulted in new advice from the American Heart Association suggesting that oral penicillin may be a safer option for people with rheumatic heart disease who are at high risk of a cardiac reaction.
Those at high risk of a cardiac reaction include those with rheumatic heart disease and severe valvular heart disease with or without reduced ventricular function, those with aortic insufficiency or decreased left ventricular systolic function, and those who have active symptoms of rheumatic heart disease.
This new guidance is the subject of an AHA “presidential advisory” published online in the Journal of the American Heart Association on Jan. 20, 2022.
The advisory notes that more than 39 million people worldwide have rheumatic heart disease, a condition in which the heart’s valves are permanently damaged by rheumatic fever, which can occur if a strep throat infection or scarlet fever is untreated or inadequately treated.
Most cases of rheumatic heart disease occur in people living in low- and middle-income countries, where the condition is often diagnosed after severe valvular heart disease or other cardiovascular complications have already developed, leading to higher rates of death and lower life expectancy.
The recommended treatment for rheumatic heart disease is an intramuscular injection of benzathine penicillin G (BPG) given every 3-4 weeks for many years or even lifelong. Treatment with BPG for rheumatic heart disease has been limited in part because of patients’ and clinicians’ fears of anaphylaxis.
However, a growing number of reports of BPG-related deaths have not shown the features of classic anaphylaxis and instead point to a cardiovascular reaction, specifically, a vasovagal episode, the advisory states.
Signs of a vasovagal episode often occur immediately after administration of BPG, sometimes even during injection, and include low blood pressure, which can improve if patients are put into a supine position, slow heart rate, and fainting, all of which may lead to low blood flow to the heart, irregular heart rhythm, and sudden cardiac death.
On the other hand, signs of anaphylaxis after BPG injection are usually slightly delayed after the injection, even up to an hour later, and include coughing, respiratory distress, rapid heart rate, low blood pressure that doesn’t respond to position change, fainting, itching and redness at the injection site, the document notes.
The risks of a cardiovascular reaction to BPG are highest among individuals with severe mitral stenosis, aortic stenosis, aortic insufficiency or decreased left ventricular systolic function (ejection fraction <50%), and those who have active symptoms of rheumatic heart disease. For these patients, treatment with oral penicillin should be strongly considered.
People with rheumatic heart disease who are at low risk of this cardiovascular reaction and who do not have a history of being allergic to penicillin or anaphylaxis can still be prescribed BPG for treatment and prevention of rheumatic heart disease, which has been proven to be the best treatment for prevention of recurrent rheumatic fever.
The advisory recommended the following standard practices for all patients receiving BPG for rheumatic heart disease:
- Reducing injection pain and patient anxiety, both of which are known risk factors for injection-related fainting. Methods for pain reduction include applying firm pressure to the site for 10 seconds or application of an ice pack or the use of analgesics (such as acetaminophen, ibuprofen, or other NSAIDs).
- Patients should be well hydrated prior to injection and should drink at least 500 mL of water before injection to prevent reflexive fainting.
- Eating a small amount of solid food within the hour before injection.
- Receiving the injection while lying down, which may reduce the risk of blood pooling in the extremities.
- Providers who administer BPG should be taught how to recognize and quickly treat symptoms such as low blood pressure, low heart rate, or fainting.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Physician burnout, depression compounded by COVID: Survey
In 2020, it was hard to imagine that the situation could get worse for doctors.
But 2021 presented a new set of challenges. As quarantines lifted and physicians tried to get back to work, they were forced to deal with reduced staff, continuing COVID stress, and pandemic-related anxieties about family and loved ones.
Medscape’s National Burnout and Depression Report 2022 asked more than 13,000 physicians from 29 specialties to share details about their lives and struggles with burnout and depression in 2021. The results paint a picture of physicians trying to fulfill their mission to care for patients, but struggling to maintain their own well-being amid a global pandemic.
Burnout bump
In 2021’s report, 42% of physicians said they were burned out. In 2022, that number increased to 47%. Perhaps not surprisingly, burnout among emergency physicians took the biggest leap, increasing from 43% to 60%. Critical care (56%), ob.gyn. (53%), and infectious disease and family medicine (both at 51%) rounded out the top five specialties with doctors experiencing burnout in 2021.
Burnout has typically been a greater problem for women than men physicians, and the pandemic hasn’t changed that. “There’s no question that women have reported far more role strain during the pandemic than men,” says Carol A. Bernstein, MD, psychiatrist at Montefiore Health System and professor and vice chair for faculty development and well-being at the Albert Einstein College of Medicine, both in New York. And indeed, 56% of women and 41% of men reported burnout in the 2022 survey.
The causes, however, weren’t especially pandemic related – or at least not directly. As in previous surveys, the major contributing factor to burnout was too much paperwork (60%), such as charting and other bureaucratic tasks. Treating COVID-19 patients was cited as the major source of stress by 10% of respondents. About 34% said too many hours at work was the biggest contributing factor to burnout.
The nature of the beast
What is burnout like for these doctors? One described the conditions that lead to burnout like this: “I barely spend enough time with most patients, just running from one to the next; and then after work, I spend hours documenting, charting, dealing with reports. I feel like an overpaid clerk.” Another said: “Where’s the relationships with patients that used to make this worthwhile?” Others fingered staffing shortages at work or an overwhelming home life: “Staff calls in sick; we’re all running around trying to find things and get things done. It never ends.”
Of those who do experience burnout, the problem reaches beyond the workplace, with 54% saying that their burnout has a strong/severe impact on life and 68% reporting that burnout affects their relationships. One respondent said: “I’m always tired; I have trouble concentrating, no time for the children, more arguments with my hubby.” Another put it this way: “Home is just as busy and chaotic as work. I can never relax.”
It doesn’t help matters that physicians are likely to think they’re the only professionals experiencing job burnout. For example, only 36% of respondents believe teachers experience comparable burnout, yet more than 41% of teachers leave the profession within 5 years of starting – often because of burnout.
When it comes to methods for coping with burnout, exercise is the clear favorite, with 63% of respondents saying exercise helps maintain their mental health. About 41% talk with family members or close friends. However, less healthy coping mechanisms were cited as well, such as isolating themselves from others (45%), sleeping (41%), and eating junk food (35%) or drinking alcohol (24%).
When it comes to trying to alleviate burnout, 29% have tried meditation or similar stress-reduction techniques, while others have reduced their work hours (29%) or changed their work settings (19%).
‘Now I feel like there’s no hope’
About a fifth of physicians (21%) said they suffered from clinical depression, and 64% reported feeling “blue, down, or sad.” One physician characterized their depression this way: “I used to think my life would be great. Now I feel like there’s no hope, this will never get better, I’ll never be happy.”
Of doctors reporting depression, 53% said their illness did not affect their interactions with patients, while 34% said depression caused them to be more easily exasperated by patients.
When asked about seeking help for depression, about half (49%) said they believed they could deal with emotional stress on their own. Unfortunately, fear of medical boards finding out keeps 43% of physicians from reaching out for help, according to the survey.
A version of this article first appeared on Medscape.com.
In 2020, it was hard to imagine that the situation could get worse for doctors.
But 2021 presented a new set of challenges. As quarantines lifted and physicians tried to get back to work, they were forced to deal with reduced staff, continuing COVID stress, and pandemic-related anxieties about family and loved ones.
Medscape’s National Burnout and Depression Report 2022 asked more than 13,000 physicians from 29 specialties to share details about their lives and struggles with burnout and depression in 2021. The results paint a picture of physicians trying to fulfill their mission to care for patients, but struggling to maintain their own well-being amid a global pandemic.
Burnout bump
In 2021’s report, 42% of physicians said they were burned out. In 2022, that number increased to 47%. Perhaps not surprisingly, burnout among emergency physicians took the biggest leap, increasing from 43% to 60%. Critical care (56%), ob.gyn. (53%), and infectious disease and family medicine (both at 51%) rounded out the top five specialties with doctors experiencing burnout in 2021.
Burnout has typically been a greater problem for women than men physicians, and the pandemic hasn’t changed that. “There’s no question that women have reported far more role strain during the pandemic than men,” says Carol A. Bernstein, MD, psychiatrist at Montefiore Health System and professor and vice chair for faculty development and well-being at the Albert Einstein College of Medicine, both in New York. And indeed, 56% of women and 41% of men reported burnout in the 2022 survey.
The causes, however, weren’t especially pandemic related – or at least not directly. As in previous surveys, the major contributing factor to burnout was too much paperwork (60%), such as charting and other bureaucratic tasks. Treating COVID-19 patients was cited as the major source of stress by 10% of respondents. About 34% said too many hours at work was the biggest contributing factor to burnout.
The nature of the beast
What is burnout like for these doctors? One described the conditions that lead to burnout like this: “I barely spend enough time with most patients, just running from one to the next; and then after work, I spend hours documenting, charting, dealing with reports. I feel like an overpaid clerk.” Another said: “Where’s the relationships with patients that used to make this worthwhile?” Others fingered staffing shortages at work or an overwhelming home life: “Staff calls in sick; we’re all running around trying to find things and get things done. It never ends.”
Of those who do experience burnout, the problem reaches beyond the workplace, with 54% saying that their burnout has a strong/severe impact on life and 68% reporting that burnout affects their relationships. One respondent said: “I’m always tired; I have trouble concentrating, no time for the children, more arguments with my hubby.” Another put it this way: “Home is just as busy and chaotic as work. I can never relax.”
It doesn’t help matters that physicians are likely to think they’re the only professionals experiencing job burnout. For example, only 36% of respondents believe teachers experience comparable burnout, yet more than 41% of teachers leave the profession within 5 years of starting – often because of burnout.
When it comes to methods for coping with burnout, exercise is the clear favorite, with 63% of respondents saying exercise helps maintain their mental health. About 41% talk with family members or close friends. However, less healthy coping mechanisms were cited as well, such as isolating themselves from others (45%), sleeping (41%), and eating junk food (35%) or drinking alcohol (24%).
When it comes to trying to alleviate burnout, 29% have tried meditation or similar stress-reduction techniques, while others have reduced their work hours (29%) or changed their work settings (19%).
‘Now I feel like there’s no hope’
About a fifth of physicians (21%) said they suffered from clinical depression, and 64% reported feeling “blue, down, or sad.” One physician characterized their depression this way: “I used to think my life would be great. Now I feel like there’s no hope, this will never get better, I’ll never be happy.”
Of doctors reporting depression, 53% said their illness did not affect their interactions with patients, while 34% said depression caused them to be more easily exasperated by patients.
When asked about seeking help for depression, about half (49%) said they believed they could deal with emotional stress on their own. Unfortunately, fear of medical boards finding out keeps 43% of physicians from reaching out for help, according to the survey.
A version of this article first appeared on Medscape.com.
In 2020, it was hard to imagine that the situation could get worse for doctors.
But 2021 presented a new set of challenges. As quarantines lifted and physicians tried to get back to work, they were forced to deal with reduced staff, continuing COVID stress, and pandemic-related anxieties about family and loved ones.
Medscape’s National Burnout and Depression Report 2022 asked more than 13,000 physicians from 29 specialties to share details about their lives and struggles with burnout and depression in 2021. The results paint a picture of physicians trying to fulfill their mission to care for patients, but struggling to maintain their own well-being amid a global pandemic.
Burnout bump
In 2021’s report, 42% of physicians said they were burned out. In 2022, that number increased to 47%. Perhaps not surprisingly, burnout among emergency physicians took the biggest leap, increasing from 43% to 60%. Critical care (56%), ob.gyn. (53%), and infectious disease and family medicine (both at 51%) rounded out the top five specialties with doctors experiencing burnout in 2021.
Burnout has typically been a greater problem for women than men physicians, and the pandemic hasn’t changed that. “There’s no question that women have reported far more role strain during the pandemic than men,” says Carol A. Bernstein, MD, psychiatrist at Montefiore Health System and professor and vice chair for faculty development and well-being at the Albert Einstein College of Medicine, both in New York. And indeed, 56% of women and 41% of men reported burnout in the 2022 survey.
The causes, however, weren’t especially pandemic related – or at least not directly. As in previous surveys, the major contributing factor to burnout was too much paperwork (60%), such as charting and other bureaucratic tasks. Treating COVID-19 patients was cited as the major source of stress by 10% of respondents. About 34% said too many hours at work was the biggest contributing factor to burnout.
The nature of the beast
What is burnout like for these doctors? One described the conditions that lead to burnout like this: “I barely spend enough time with most patients, just running from one to the next; and then after work, I spend hours documenting, charting, dealing with reports. I feel like an overpaid clerk.” Another said: “Where’s the relationships with patients that used to make this worthwhile?” Others fingered staffing shortages at work or an overwhelming home life: “Staff calls in sick; we’re all running around trying to find things and get things done. It never ends.”
Of those who do experience burnout, the problem reaches beyond the workplace, with 54% saying that their burnout has a strong/severe impact on life and 68% reporting that burnout affects their relationships. One respondent said: “I’m always tired; I have trouble concentrating, no time for the children, more arguments with my hubby.” Another put it this way: “Home is just as busy and chaotic as work. I can never relax.”
It doesn’t help matters that physicians are likely to think they’re the only professionals experiencing job burnout. For example, only 36% of respondents believe teachers experience comparable burnout, yet more than 41% of teachers leave the profession within 5 years of starting – often because of burnout.
When it comes to methods for coping with burnout, exercise is the clear favorite, with 63% of respondents saying exercise helps maintain their mental health. About 41% talk with family members or close friends. However, less healthy coping mechanisms were cited as well, such as isolating themselves from others (45%), sleeping (41%), and eating junk food (35%) or drinking alcohol (24%).
When it comes to trying to alleviate burnout, 29% have tried meditation or similar stress-reduction techniques, while others have reduced their work hours (29%) or changed their work settings (19%).
‘Now I feel like there’s no hope’
About a fifth of physicians (21%) said they suffered from clinical depression, and 64% reported feeling “blue, down, or sad.” One physician characterized their depression this way: “I used to think my life would be great. Now I feel like there’s no hope, this will never get better, I’ll never be happy.”
Of doctors reporting depression, 53% said their illness did not affect their interactions with patients, while 34% said depression caused them to be more easily exasperated by patients.
When asked about seeking help for depression, about half (49%) said they believed they could deal with emotional stress on their own. Unfortunately, fear of medical boards finding out keeps 43% of physicians from reaching out for help, according to the survey.
A version of this article first appeared on Medscape.com.
Rituximab and COVID-19 vaccines: Studies begin to answer key questions
Rituximab has presented something of a conundrum for patients taking the monoclonal antibody during the COVID-19 pandemic.
Used to manage a variety of autoimmune diseases and cancers, rituximab acts against CD20 proteins expressed on the surface of B cells, causing B-cell depletion. However, it is this B-cell depletion that may put these patients at greater risk of COVID-19 development, progression to more severe disease, and in-hospital mortality. Evidence for this appears to be mixed, with studies showing both that patients using rituximab to manage various diseases are and are not at increased risk for SARS-CoV-2 infection, COVID-19 progression, and mortality.
As COVID-19 vaccine rollouts take place across the world, more questions have been raised about the relationship between B-cell depletion from anti-CD20 therapies and COVID-19 vaccines. Do rituximab and other anti-CD20 therapies affect a patient’s response to COVID-19 vaccines? If this is the case, does the timing of anti-CD20 treatment matter to maximize B-cell levels and improve the vaccine’s effectiveness? And how do COVID-19 vaccine booster doses factor into the equation?
Humoral and cell-mediated responses following COVID-19 vaccination
First, the bad news: The vaccine is unquestionably safe to administer in patients taking rituximab, but one thing that has been well established is that antibody response to COVID-19 vaccination in these individuals does is reduced. This isn’t entirely unprecedented, as previous studies have shown a weakened immune response to pneumococcal polysaccharide and keyhole limpet hemocyanin vaccines among patients taking rituximab.
“Compromised immunogenicity to the SARS-CoV-2 vaccines has been demonstrated in rituximab-treated patients, which is of particular concern given the observation that B-cell–depleting therapies may be associated with worse COVID outcomes,” Robert F. Spiera, MD, director of the Scleroderma, Vasculitis, and Myositis Center at the Hospital for Special Surgery in New York, said in an interview.
For example, in a recent study from the Medical University of Vienna, 29 (39%) of 74 patients receiving rituximab (43% as monotherapy, 57% with conventional-synthetic disease-modifying antirheumatic drugs) who were vaccinated with either the Comirnaty (Pfizer-BioNTech) or Spikevax (Moderna) COVID-19 vaccine achieved seroconversion, compared with 100% of patients in a healthy control group, and all but 1 patient without detectable CD19+ peripheral B cells did not develop anti–SARS-CoV-2 receptor-binding domain antibodies.
“There is an increasing number of studies in this field, and they confirm that patients treated with rituximab and other anti-CD20 agents have severely reduced serological responses to COVID-19 vaccines,” Ingrid Jyssum, MD, of the division of rheumatology and research at Diakonhjemmet Hospital in Oslo, said in an interview.
One silver lining is that patients treated with anti-CD20 therapies appear to have a cell-mediated response following vaccination even if they don’t develop SARS-CoV-2 antibodies. “Studies that also investigate T-cell responses are starting to emerge, and so far, they show that, even if the patients do not have antibodies, they may have T-cell responses,” Dr. Jyssum said.
One study of 24 patients with autoimmune diseases taking rituximab that evaluated humoral and T-cell responses following vaccination with the Comirnaty vaccine found that none had a humoral response to the vaccine, but the T-cell response from that group did not significantly differ from 35 patients receiving other immunosuppressants and 26 patients in a healthy control group. In another study of rituximab- or ocrelizumab-treated patients who received mRNA-based COVID-19 vaccines, 69.4% developed SARS-CoV-2–specific antibodies, compared with a control group, but 96.2% of patients taking ocrelizumab and 81.8% of patients taking rituximab mounted a spike-specific CD8+ T-cell response, compared with 66.7% in the control group, and there were comparable rates (85%-90%) of spike-specific CD4+ T cells in all groups. In the study from the Medical University of Vienna, T-cell response was detected in rituximab-treated patients who both did and did not mount an antibody response.
The clinical relevance of how a blunted humoral immune response but a respectable T-cell response to COVID-19 vaccines affects patients treated with anti-CD20 therapies isn’t currently known, Dr. Jyssum said.
While these data are reassuring, they’re also incomplete, Dr. Spiera noted. “The ultimate outcome of relevance to assess vaccine efficacy is protection from COVID and from severe outcomes of COVID infection (i.e., hospitalization, mechanical ventilation, death). That data will require assessment of very large numbers of rituximab-treated vaccinated patients to be compared with rituximab-treated unvaccinated patients, and is unlikely to be forthcoming in the very near future.
“In the meantime, however, achieving serologic positivity, meaning having evidence of serologic as well as cellular immunity following vaccination, is a desired outcome, and likely implies more robust immunity.”
Does treatment timing impact COVID-19 vaccine response?
Given enough time, B-cell reconstitution will occur in patients taking rituximab. With that in mind, is it beneficial to wait a certain amount of time after a patient has stopped rituximab therapy or time since their last dose before giving them a COVID-19 vaccine? In their guidance on COVID-19 vaccines for patients with rheumatic and musculoskeletal diseases, the American College of Rheumatology said there is moderate evidence to consider “optimal timing of dosing and vaccination with the rheumatology provider before proceeding.”
“Guidelines and preliminary studies of serologic response to COVID vaccine in rituximab-treated patients have suggested that longer time from last rituximab exposure is associated with a greater likelihood of a serologic response,” Dr. Spiera said.
In a brief report published in Arthritis & Rheumatology, Dr. Spiera and colleagues performed a retrospective chart review of 56 patients with varying levels of last exposure to rituximab who received a COVID-19 vaccine. Their results showed that, when patients were vaccinated 6-12 months after the last rituximab dose, 55% were seronegative, and when this was more than 12 months, only 13% were seronegative, compared with seronegativity in 86% who were vaccinated less than 6 months after their last rituximab dose.
The RituxiVac trial, conducted by researchers in Switzerland, also examined vaccine responses of 96 rituximab-treated patients who received Comirnaty or Spikevax; results recently published in The Lancet Rheumatology showed findings similar to other studies, with reduced humoral and cell-mediated responses. In the RituxiVac trial, the median time to last anti-CD20 treatment was 1.07 years.
“The typical interval between rituximab doses [for treatment of rheumatoid arthritis, as well as for remission maintenance in antineutrophil cytoplasmic antibody–associated vasculitis] is typically 6 months, and this has become widely used as the interval from last rituximab to time of COVID vaccination, with a recommendation to wait 4 weeks (if possible) from time of vaccination until the next rituximab administration,” Dr. Spiera explained. However, this window seems to vary depending on the study.
Recent research published in Arthritis & Rheumatology indicates B-cell levels could be a relevant indicator for humoral and cell-mediated response in patients with rheumatic diseases treated with rituximab, with a level of 10 B cells/mcL (0.4% of lymphocytes) identified as one potential marker for likely seroconversion following COVID-19 vaccination.
“In some smaller case series, it has been further recognized that rituximab-treated patients who were beginning to reconstitute peripheral B cells were most likely to respond serologically. Our present study confirmed those findings, demonstrating that the presence of detectable B cells was strongly associated with vaccine responsiveness, and affords complementary information to time from last [rituximab dose] in informing the likelihood of a vaccine response,” Dr. Spiera said.
However, the literature is limited in this area, and an exact cutoff for B-cell counts in these patients isn’t currently known, Dr. Jyssum said. A better metric is time away from anti-CD20 therapies, with CD19 cell count being highly correlated with last infusion.
Dr. Spiera agreed that there is no consistent B-cell percentage that works as a cutoff. “In our study, we looked at it as a binary variable, although we did find that a higher percentage of B cells in the peripheral lymphocyte population was associated with a higher likelihood of seroconversion. We did not, however, identify a ‘threshold’ for vaccine serologic responsiveness.”
Should clinicians measure antibodies?
The Food and Drug Administration and the Centers for Disease Control and Prevention have recommended that health care providers and the public not use COVID-19 antibody tests as a way to gauge immunity after exposure to SARS-CoV-2 and after receiving a COVID-19 vaccination. The ACR’s guidance on COVID-19 vaccination for patients with rheumatic and musculoskeletal diseases strongly recommends against ordering antibody tests for patients with autoimmune inflammatory rheumatic diseases as a way to measure immunity.
“Generally, such measurements are not recommended as the clinical correlate of various antibody levels are not known,” Dr. Jyssum said. “With regular infusions of rituximab or other anti-CD20 agents, one cannot expect that these patients will develop significant levels of antibodies.”
However, she said there might be situations where it’s useful to know whether a patient has developed antibodies at all. “Assessing the significance of specific antibody levels is difficult, and the subject of scientific studies. Patients lacking a humoral vaccine response are left to rely on their T-cell responses and on infectious control measures to prevent disease.”
Dr. Spiera said he disagreed with guidelines recommending against checking antibody levels after vaccination, “particularly in patients treated with immunosuppressive medications that might be expected to blunt their serologic response to the vaccines.
“Although we cannot be sure what level of measurable antibodies offer what level of protection, most clinicians would agree that patients who demonstrate no detectable antibodies (which is a common finding in rituximab-treated patients) should be considered at higher risk,” he said. “Indeed, recommendations regarding booster vaccine administration in general was initially based on the observation of declining antibody levels with longer time from vaccination.”
Do COVID-19 vaccine boosters help patients on anti-CD20 therapy?
As of January 2022, the FDA and CDC have recommended a third primary series shot of COVID-19 vaccines for some moderately to severely immunocompromised patients as young as 5 years old (for Comirnaty vaccine) or a booster shot of either Comirnaty or Spikevax for everyone aged 12 years and older, including immunocompromised people, while the ACR goes into more detail and recommends clinicians time a patient’s booster shot with temporary treatment interruption.
In The Lancet Rheumatology, Dr. Jyssum and colleagues recently published results from the prospective Nor-vaC study examining the humoral and cell-mediated immune responses of 87 patients with RA being treated with rituximab who received the Comirnaty, Spikevax, or Vaxzevria (AstraZeneca) COVID-19 vaccines; of these, 49 patients received a booster dose at a median of 70 days after completing their primary series. The results showed 19 patients (28.1%) had a serologic response after their primary series, while 8 of 49 patients (16.3%) who received their booster dose had a serologic response.
All patients who received a third dose in the study had a T-cell response, Dr. Jyssum said. “This is reassuring for patients and clinicians. T cells have been found to be important in countering COVID-19 disease, but whether we can rely on the T-cell response alone in the absence of antibodies to protect patients from infection or from serious COVID disease is still not determined,” she said.
When asked if she would recommend COVID-19 vaccine booster doses for patients on rituximab, Dr. Jyssum replied: “Absolutely.”
Another study, recently published in Annals of the Rheumatic Diseases, examined heterologous and homologous booster doses for 60 patients receiving rituximab without seroconversion after their COVID-19 vaccine primary series. The results showed no significant difference in new seroconversion at 4 weeks based on whether the patient received a vector or mRNA vaccine (22% vs. 32%), but all patients who received a booster dose with a vector vaccine had specific T-cell responses, compared with 81% of patients who received an mRNA vaccine booster. There was a new humoral and/or cellular response in 9 of 11 patients (82%), and most patients with peripheral B cells (12 of 18 patients; 67%) achieved seroconversion.
“Our data show that a cellular and/or humoral immune response can be achieved on a third COVID-19 vaccination in most of the patients who initially developed neither a humoral nor a cellular immune response,” the researchers concluded. “The efficacy data together with the safety data seen in our trial provide a favorable risk/benefit ratio and support the implementation of a third vaccination for nonseroconverted high-risk autoimmune disease patients treated with B-cell–depleting agents.”
Dr. Spiera said booster doses are an important part of the equation, and “it is important to consider factors that would be associated with a greater likelihood of achieving a serologic response, particularly in those patients who did not demonstrate a serologic response to the initial vaccines series.
“Preliminary data shows that the beginnings of B-cell reconstitution is also associated with a positive serologic response following a booster of the COVID-19 vaccine,” he said.
The authors of the cited studies reported numerous relevant financial disclosures. Dr. Spiera and Dr. Jyssum reported no relevant financial disclosures.
Rituximab has presented something of a conundrum for patients taking the monoclonal antibody during the COVID-19 pandemic.
Used to manage a variety of autoimmune diseases and cancers, rituximab acts against CD20 proteins expressed on the surface of B cells, causing B-cell depletion. However, it is this B-cell depletion that may put these patients at greater risk of COVID-19 development, progression to more severe disease, and in-hospital mortality. Evidence for this appears to be mixed, with studies showing both that patients using rituximab to manage various diseases are and are not at increased risk for SARS-CoV-2 infection, COVID-19 progression, and mortality.
As COVID-19 vaccine rollouts take place across the world, more questions have been raised about the relationship between B-cell depletion from anti-CD20 therapies and COVID-19 vaccines. Do rituximab and other anti-CD20 therapies affect a patient’s response to COVID-19 vaccines? If this is the case, does the timing of anti-CD20 treatment matter to maximize B-cell levels and improve the vaccine’s effectiveness? And how do COVID-19 vaccine booster doses factor into the equation?
Humoral and cell-mediated responses following COVID-19 vaccination
First, the bad news: The vaccine is unquestionably safe to administer in patients taking rituximab, but one thing that has been well established is that antibody response to COVID-19 vaccination in these individuals does is reduced. This isn’t entirely unprecedented, as previous studies have shown a weakened immune response to pneumococcal polysaccharide and keyhole limpet hemocyanin vaccines among patients taking rituximab.
“Compromised immunogenicity to the SARS-CoV-2 vaccines has been demonstrated in rituximab-treated patients, which is of particular concern given the observation that B-cell–depleting therapies may be associated with worse COVID outcomes,” Robert F. Spiera, MD, director of the Scleroderma, Vasculitis, and Myositis Center at the Hospital for Special Surgery in New York, said in an interview.
For example, in a recent study from the Medical University of Vienna, 29 (39%) of 74 patients receiving rituximab (43% as monotherapy, 57% with conventional-synthetic disease-modifying antirheumatic drugs) who were vaccinated with either the Comirnaty (Pfizer-BioNTech) or Spikevax (Moderna) COVID-19 vaccine achieved seroconversion, compared with 100% of patients in a healthy control group, and all but 1 patient without detectable CD19+ peripheral B cells did not develop anti–SARS-CoV-2 receptor-binding domain antibodies.
“There is an increasing number of studies in this field, and they confirm that patients treated with rituximab and other anti-CD20 agents have severely reduced serological responses to COVID-19 vaccines,” Ingrid Jyssum, MD, of the division of rheumatology and research at Diakonhjemmet Hospital in Oslo, said in an interview.
One silver lining is that patients treated with anti-CD20 therapies appear to have a cell-mediated response following vaccination even if they don’t develop SARS-CoV-2 antibodies. “Studies that also investigate T-cell responses are starting to emerge, and so far, they show that, even if the patients do not have antibodies, they may have T-cell responses,” Dr. Jyssum said.
One study of 24 patients with autoimmune diseases taking rituximab that evaluated humoral and T-cell responses following vaccination with the Comirnaty vaccine found that none had a humoral response to the vaccine, but the T-cell response from that group did not significantly differ from 35 patients receiving other immunosuppressants and 26 patients in a healthy control group. In another study of rituximab- or ocrelizumab-treated patients who received mRNA-based COVID-19 vaccines, 69.4% developed SARS-CoV-2–specific antibodies, compared with a control group, but 96.2% of patients taking ocrelizumab and 81.8% of patients taking rituximab mounted a spike-specific CD8+ T-cell response, compared with 66.7% in the control group, and there were comparable rates (85%-90%) of spike-specific CD4+ T cells in all groups. In the study from the Medical University of Vienna, T-cell response was detected in rituximab-treated patients who both did and did not mount an antibody response.
The clinical relevance of how a blunted humoral immune response but a respectable T-cell response to COVID-19 vaccines affects patients treated with anti-CD20 therapies isn’t currently known, Dr. Jyssum said.
While these data are reassuring, they’re also incomplete, Dr. Spiera noted. “The ultimate outcome of relevance to assess vaccine efficacy is protection from COVID and from severe outcomes of COVID infection (i.e., hospitalization, mechanical ventilation, death). That data will require assessment of very large numbers of rituximab-treated vaccinated patients to be compared with rituximab-treated unvaccinated patients, and is unlikely to be forthcoming in the very near future.
“In the meantime, however, achieving serologic positivity, meaning having evidence of serologic as well as cellular immunity following vaccination, is a desired outcome, and likely implies more robust immunity.”
Does treatment timing impact COVID-19 vaccine response?
Given enough time, B-cell reconstitution will occur in patients taking rituximab. With that in mind, is it beneficial to wait a certain amount of time after a patient has stopped rituximab therapy or time since their last dose before giving them a COVID-19 vaccine? In their guidance on COVID-19 vaccines for patients with rheumatic and musculoskeletal diseases, the American College of Rheumatology said there is moderate evidence to consider “optimal timing of dosing and vaccination with the rheumatology provider before proceeding.”
“Guidelines and preliminary studies of serologic response to COVID vaccine in rituximab-treated patients have suggested that longer time from last rituximab exposure is associated with a greater likelihood of a serologic response,” Dr. Spiera said.
In a brief report published in Arthritis & Rheumatology, Dr. Spiera and colleagues performed a retrospective chart review of 56 patients with varying levels of last exposure to rituximab who received a COVID-19 vaccine. Their results showed that, when patients were vaccinated 6-12 months after the last rituximab dose, 55% were seronegative, and when this was more than 12 months, only 13% were seronegative, compared with seronegativity in 86% who were vaccinated less than 6 months after their last rituximab dose.
The RituxiVac trial, conducted by researchers in Switzerland, also examined vaccine responses of 96 rituximab-treated patients who received Comirnaty or Spikevax; results recently published in The Lancet Rheumatology showed findings similar to other studies, with reduced humoral and cell-mediated responses. In the RituxiVac trial, the median time to last anti-CD20 treatment was 1.07 years.
“The typical interval between rituximab doses [for treatment of rheumatoid arthritis, as well as for remission maintenance in antineutrophil cytoplasmic antibody–associated vasculitis] is typically 6 months, and this has become widely used as the interval from last rituximab to time of COVID vaccination, with a recommendation to wait 4 weeks (if possible) from time of vaccination until the next rituximab administration,” Dr. Spiera explained. However, this window seems to vary depending on the study.
Recent research published in Arthritis & Rheumatology indicates B-cell levels could be a relevant indicator for humoral and cell-mediated response in patients with rheumatic diseases treated with rituximab, with a level of 10 B cells/mcL (0.4% of lymphocytes) identified as one potential marker for likely seroconversion following COVID-19 vaccination.
“In some smaller case series, it has been further recognized that rituximab-treated patients who were beginning to reconstitute peripheral B cells were most likely to respond serologically. Our present study confirmed those findings, demonstrating that the presence of detectable B cells was strongly associated with vaccine responsiveness, and affords complementary information to time from last [rituximab dose] in informing the likelihood of a vaccine response,” Dr. Spiera said.
However, the literature is limited in this area, and an exact cutoff for B-cell counts in these patients isn’t currently known, Dr. Jyssum said. A better metric is time away from anti-CD20 therapies, with CD19 cell count being highly correlated with last infusion.
Dr. Spiera agreed that there is no consistent B-cell percentage that works as a cutoff. “In our study, we looked at it as a binary variable, although we did find that a higher percentage of B cells in the peripheral lymphocyte population was associated with a higher likelihood of seroconversion. We did not, however, identify a ‘threshold’ for vaccine serologic responsiveness.”
Should clinicians measure antibodies?
The Food and Drug Administration and the Centers for Disease Control and Prevention have recommended that health care providers and the public not use COVID-19 antibody tests as a way to gauge immunity after exposure to SARS-CoV-2 and after receiving a COVID-19 vaccination. The ACR’s guidance on COVID-19 vaccination for patients with rheumatic and musculoskeletal diseases strongly recommends against ordering antibody tests for patients with autoimmune inflammatory rheumatic diseases as a way to measure immunity.
“Generally, such measurements are not recommended as the clinical correlate of various antibody levels are not known,” Dr. Jyssum said. “With regular infusions of rituximab or other anti-CD20 agents, one cannot expect that these patients will develop significant levels of antibodies.”
However, she said there might be situations where it’s useful to know whether a patient has developed antibodies at all. “Assessing the significance of specific antibody levels is difficult, and the subject of scientific studies. Patients lacking a humoral vaccine response are left to rely on their T-cell responses and on infectious control measures to prevent disease.”
Dr. Spiera said he disagreed with guidelines recommending against checking antibody levels after vaccination, “particularly in patients treated with immunosuppressive medications that might be expected to blunt their serologic response to the vaccines.
“Although we cannot be sure what level of measurable antibodies offer what level of protection, most clinicians would agree that patients who demonstrate no detectable antibodies (which is a common finding in rituximab-treated patients) should be considered at higher risk,” he said. “Indeed, recommendations regarding booster vaccine administration in general was initially based on the observation of declining antibody levels with longer time from vaccination.”
Do COVID-19 vaccine boosters help patients on anti-CD20 therapy?
As of January 2022, the FDA and CDC have recommended a third primary series shot of COVID-19 vaccines for some moderately to severely immunocompromised patients as young as 5 years old (for Comirnaty vaccine) or a booster shot of either Comirnaty or Spikevax for everyone aged 12 years and older, including immunocompromised people, while the ACR goes into more detail and recommends clinicians time a patient’s booster shot with temporary treatment interruption.
In The Lancet Rheumatology, Dr. Jyssum and colleagues recently published results from the prospective Nor-vaC study examining the humoral and cell-mediated immune responses of 87 patients with RA being treated with rituximab who received the Comirnaty, Spikevax, or Vaxzevria (AstraZeneca) COVID-19 vaccines; of these, 49 patients received a booster dose at a median of 70 days after completing their primary series. The results showed 19 patients (28.1%) had a serologic response after their primary series, while 8 of 49 patients (16.3%) who received their booster dose had a serologic response.
All patients who received a third dose in the study had a T-cell response, Dr. Jyssum said. “This is reassuring for patients and clinicians. T cells have been found to be important in countering COVID-19 disease, but whether we can rely on the T-cell response alone in the absence of antibodies to protect patients from infection or from serious COVID disease is still not determined,” she said.
When asked if she would recommend COVID-19 vaccine booster doses for patients on rituximab, Dr. Jyssum replied: “Absolutely.”
Another study, recently published in Annals of the Rheumatic Diseases, examined heterologous and homologous booster doses for 60 patients receiving rituximab without seroconversion after their COVID-19 vaccine primary series. The results showed no significant difference in new seroconversion at 4 weeks based on whether the patient received a vector or mRNA vaccine (22% vs. 32%), but all patients who received a booster dose with a vector vaccine had specific T-cell responses, compared with 81% of patients who received an mRNA vaccine booster. There was a new humoral and/or cellular response in 9 of 11 patients (82%), and most patients with peripheral B cells (12 of 18 patients; 67%) achieved seroconversion.
“Our data show that a cellular and/or humoral immune response can be achieved on a third COVID-19 vaccination in most of the patients who initially developed neither a humoral nor a cellular immune response,” the researchers concluded. “The efficacy data together with the safety data seen in our trial provide a favorable risk/benefit ratio and support the implementation of a third vaccination for nonseroconverted high-risk autoimmune disease patients treated with B-cell–depleting agents.”
Dr. Spiera said booster doses are an important part of the equation, and “it is important to consider factors that would be associated with a greater likelihood of achieving a serologic response, particularly in those patients who did not demonstrate a serologic response to the initial vaccines series.
“Preliminary data shows that the beginnings of B-cell reconstitution is also associated with a positive serologic response following a booster of the COVID-19 vaccine,” he said.
The authors of the cited studies reported numerous relevant financial disclosures. Dr. Spiera and Dr. Jyssum reported no relevant financial disclosures.
Rituximab has presented something of a conundrum for patients taking the monoclonal antibody during the COVID-19 pandemic.
Used to manage a variety of autoimmune diseases and cancers, rituximab acts against CD20 proteins expressed on the surface of B cells, causing B-cell depletion. However, it is this B-cell depletion that may put these patients at greater risk of COVID-19 development, progression to more severe disease, and in-hospital mortality. Evidence for this appears to be mixed, with studies showing both that patients using rituximab to manage various diseases are and are not at increased risk for SARS-CoV-2 infection, COVID-19 progression, and mortality.
As COVID-19 vaccine rollouts take place across the world, more questions have been raised about the relationship between B-cell depletion from anti-CD20 therapies and COVID-19 vaccines. Do rituximab and other anti-CD20 therapies affect a patient’s response to COVID-19 vaccines? If this is the case, does the timing of anti-CD20 treatment matter to maximize B-cell levels and improve the vaccine’s effectiveness? And how do COVID-19 vaccine booster doses factor into the equation?
Humoral and cell-mediated responses following COVID-19 vaccination
First, the bad news: The vaccine is unquestionably safe to administer in patients taking rituximab, but one thing that has been well established is that antibody response to COVID-19 vaccination in these individuals does is reduced. This isn’t entirely unprecedented, as previous studies have shown a weakened immune response to pneumococcal polysaccharide and keyhole limpet hemocyanin vaccines among patients taking rituximab.
“Compromised immunogenicity to the SARS-CoV-2 vaccines has been demonstrated in rituximab-treated patients, which is of particular concern given the observation that B-cell–depleting therapies may be associated with worse COVID outcomes,” Robert F. Spiera, MD, director of the Scleroderma, Vasculitis, and Myositis Center at the Hospital for Special Surgery in New York, said in an interview.
For example, in a recent study from the Medical University of Vienna, 29 (39%) of 74 patients receiving rituximab (43% as monotherapy, 57% with conventional-synthetic disease-modifying antirheumatic drugs) who were vaccinated with either the Comirnaty (Pfizer-BioNTech) or Spikevax (Moderna) COVID-19 vaccine achieved seroconversion, compared with 100% of patients in a healthy control group, and all but 1 patient without detectable CD19+ peripheral B cells did not develop anti–SARS-CoV-2 receptor-binding domain antibodies.
“There is an increasing number of studies in this field, and they confirm that patients treated with rituximab and other anti-CD20 agents have severely reduced serological responses to COVID-19 vaccines,” Ingrid Jyssum, MD, of the division of rheumatology and research at Diakonhjemmet Hospital in Oslo, said in an interview.
One silver lining is that patients treated with anti-CD20 therapies appear to have a cell-mediated response following vaccination even if they don’t develop SARS-CoV-2 antibodies. “Studies that also investigate T-cell responses are starting to emerge, and so far, they show that, even if the patients do not have antibodies, they may have T-cell responses,” Dr. Jyssum said.
One study of 24 patients with autoimmune diseases taking rituximab that evaluated humoral and T-cell responses following vaccination with the Comirnaty vaccine found that none had a humoral response to the vaccine, but the T-cell response from that group did not significantly differ from 35 patients receiving other immunosuppressants and 26 patients in a healthy control group. In another study of rituximab- or ocrelizumab-treated patients who received mRNA-based COVID-19 vaccines, 69.4% developed SARS-CoV-2–specific antibodies, compared with a control group, but 96.2% of patients taking ocrelizumab and 81.8% of patients taking rituximab mounted a spike-specific CD8+ T-cell response, compared with 66.7% in the control group, and there were comparable rates (85%-90%) of spike-specific CD4+ T cells in all groups. In the study from the Medical University of Vienna, T-cell response was detected in rituximab-treated patients who both did and did not mount an antibody response.
The clinical relevance of how a blunted humoral immune response but a respectable T-cell response to COVID-19 vaccines affects patients treated with anti-CD20 therapies isn’t currently known, Dr. Jyssum said.
While these data are reassuring, they’re also incomplete, Dr. Spiera noted. “The ultimate outcome of relevance to assess vaccine efficacy is protection from COVID and from severe outcomes of COVID infection (i.e., hospitalization, mechanical ventilation, death). That data will require assessment of very large numbers of rituximab-treated vaccinated patients to be compared with rituximab-treated unvaccinated patients, and is unlikely to be forthcoming in the very near future.
“In the meantime, however, achieving serologic positivity, meaning having evidence of serologic as well as cellular immunity following vaccination, is a desired outcome, and likely implies more robust immunity.”
Does treatment timing impact COVID-19 vaccine response?
Given enough time, B-cell reconstitution will occur in patients taking rituximab. With that in mind, is it beneficial to wait a certain amount of time after a patient has stopped rituximab therapy or time since their last dose before giving them a COVID-19 vaccine? In their guidance on COVID-19 vaccines for patients with rheumatic and musculoskeletal diseases, the American College of Rheumatology said there is moderate evidence to consider “optimal timing of dosing and vaccination with the rheumatology provider before proceeding.”
“Guidelines and preliminary studies of serologic response to COVID vaccine in rituximab-treated patients have suggested that longer time from last rituximab exposure is associated with a greater likelihood of a serologic response,” Dr. Spiera said.
In a brief report published in Arthritis & Rheumatology, Dr. Spiera and colleagues performed a retrospective chart review of 56 patients with varying levels of last exposure to rituximab who received a COVID-19 vaccine. Their results showed that, when patients were vaccinated 6-12 months after the last rituximab dose, 55% were seronegative, and when this was more than 12 months, only 13% were seronegative, compared with seronegativity in 86% who were vaccinated less than 6 months after their last rituximab dose.
The RituxiVac trial, conducted by researchers in Switzerland, also examined vaccine responses of 96 rituximab-treated patients who received Comirnaty or Spikevax; results recently published in The Lancet Rheumatology showed findings similar to other studies, with reduced humoral and cell-mediated responses. In the RituxiVac trial, the median time to last anti-CD20 treatment was 1.07 years.
“The typical interval between rituximab doses [for treatment of rheumatoid arthritis, as well as for remission maintenance in antineutrophil cytoplasmic antibody–associated vasculitis] is typically 6 months, and this has become widely used as the interval from last rituximab to time of COVID vaccination, with a recommendation to wait 4 weeks (if possible) from time of vaccination until the next rituximab administration,” Dr. Spiera explained. However, this window seems to vary depending on the study.
Recent research published in Arthritis & Rheumatology indicates B-cell levels could be a relevant indicator for humoral and cell-mediated response in patients with rheumatic diseases treated with rituximab, with a level of 10 B cells/mcL (0.4% of lymphocytes) identified as one potential marker for likely seroconversion following COVID-19 vaccination.
“In some smaller case series, it has been further recognized that rituximab-treated patients who were beginning to reconstitute peripheral B cells were most likely to respond serologically. Our present study confirmed those findings, demonstrating that the presence of detectable B cells was strongly associated with vaccine responsiveness, and affords complementary information to time from last [rituximab dose] in informing the likelihood of a vaccine response,” Dr. Spiera said.
However, the literature is limited in this area, and an exact cutoff for B-cell counts in these patients isn’t currently known, Dr. Jyssum said. A better metric is time away from anti-CD20 therapies, with CD19 cell count being highly correlated with last infusion.
Dr. Spiera agreed that there is no consistent B-cell percentage that works as a cutoff. “In our study, we looked at it as a binary variable, although we did find that a higher percentage of B cells in the peripheral lymphocyte population was associated with a higher likelihood of seroconversion. We did not, however, identify a ‘threshold’ for vaccine serologic responsiveness.”
Should clinicians measure antibodies?
The Food and Drug Administration and the Centers for Disease Control and Prevention have recommended that health care providers and the public not use COVID-19 antibody tests as a way to gauge immunity after exposure to SARS-CoV-2 and after receiving a COVID-19 vaccination. The ACR’s guidance on COVID-19 vaccination for patients with rheumatic and musculoskeletal diseases strongly recommends against ordering antibody tests for patients with autoimmune inflammatory rheumatic diseases as a way to measure immunity.
“Generally, such measurements are not recommended as the clinical correlate of various antibody levels are not known,” Dr. Jyssum said. “With regular infusions of rituximab or other anti-CD20 agents, one cannot expect that these patients will develop significant levels of antibodies.”
However, she said there might be situations where it’s useful to know whether a patient has developed antibodies at all. “Assessing the significance of specific antibody levels is difficult, and the subject of scientific studies. Patients lacking a humoral vaccine response are left to rely on their T-cell responses and on infectious control measures to prevent disease.”
Dr. Spiera said he disagreed with guidelines recommending against checking antibody levels after vaccination, “particularly in patients treated with immunosuppressive medications that might be expected to blunt their serologic response to the vaccines.
“Although we cannot be sure what level of measurable antibodies offer what level of protection, most clinicians would agree that patients who demonstrate no detectable antibodies (which is a common finding in rituximab-treated patients) should be considered at higher risk,” he said. “Indeed, recommendations regarding booster vaccine administration in general was initially based on the observation of declining antibody levels with longer time from vaccination.”
Do COVID-19 vaccine boosters help patients on anti-CD20 therapy?
As of January 2022, the FDA and CDC have recommended a third primary series shot of COVID-19 vaccines for some moderately to severely immunocompromised patients as young as 5 years old (for Comirnaty vaccine) or a booster shot of either Comirnaty or Spikevax for everyone aged 12 years and older, including immunocompromised people, while the ACR goes into more detail and recommends clinicians time a patient’s booster shot with temporary treatment interruption.
In The Lancet Rheumatology, Dr. Jyssum and colleagues recently published results from the prospective Nor-vaC study examining the humoral and cell-mediated immune responses of 87 patients with RA being treated with rituximab who received the Comirnaty, Spikevax, or Vaxzevria (AstraZeneca) COVID-19 vaccines; of these, 49 patients received a booster dose at a median of 70 days after completing their primary series. The results showed 19 patients (28.1%) had a serologic response after their primary series, while 8 of 49 patients (16.3%) who received their booster dose had a serologic response.
All patients who received a third dose in the study had a T-cell response, Dr. Jyssum said. “This is reassuring for patients and clinicians. T cells have been found to be important in countering COVID-19 disease, but whether we can rely on the T-cell response alone in the absence of antibodies to protect patients from infection or from serious COVID disease is still not determined,” she said.
When asked if she would recommend COVID-19 vaccine booster doses for patients on rituximab, Dr. Jyssum replied: “Absolutely.”
Another study, recently published in Annals of the Rheumatic Diseases, examined heterologous and homologous booster doses for 60 patients receiving rituximab without seroconversion after their COVID-19 vaccine primary series. The results showed no significant difference in new seroconversion at 4 weeks based on whether the patient received a vector or mRNA vaccine (22% vs. 32%), but all patients who received a booster dose with a vector vaccine had specific T-cell responses, compared with 81% of patients who received an mRNA vaccine booster. There was a new humoral and/or cellular response in 9 of 11 patients (82%), and most patients with peripheral B cells (12 of 18 patients; 67%) achieved seroconversion.
“Our data show that a cellular and/or humoral immune response can be achieved on a third COVID-19 vaccination in most of the patients who initially developed neither a humoral nor a cellular immune response,” the researchers concluded. “The efficacy data together with the safety data seen in our trial provide a favorable risk/benefit ratio and support the implementation of a third vaccination for nonseroconverted high-risk autoimmune disease patients treated with B-cell–depleting agents.”
Dr. Spiera said booster doses are an important part of the equation, and “it is important to consider factors that would be associated with a greater likelihood of achieving a serologic response, particularly in those patients who did not demonstrate a serologic response to the initial vaccines series.
“Preliminary data shows that the beginnings of B-cell reconstitution is also associated with a positive serologic response following a booster of the COVID-19 vaccine,” he said.
The authors of the cited studies reported numerous relevant financial disclosures. Dr. Spiera and Dr. Jyssum reported no relevant financial disclosures.
Five things you should know about ‘free’ at-home COVID tests
Americans keep hearing that it is important to test frequently for COVID-19 at home. But just try to find an “at-home” rapid COVID test in a store and at a price that makes frequent tests affordable.
Testing, as well as mask-wearing, is an important measure if the country ever hopes to beat COVID, restore normal routines and get the economy running efficiently. To get Americans cheaper tests, the federal government now plans to have insurance companies pay for them.
You can either get one without any out-of-pocket expense from retail pharmacies that are part of an insurance company’s network or buy it at any store and get reimbursed by the insurer.
Congress said private insurers must cover all COVID testing and any associated medical services when it passed the Families First Coronavirus Response Act and the Coronavirus Aid, Relief and Economic Security, or CARES, Act. The have-insurance-pay-for-it solution has been used frequently through the pandemic. Insurance companies have been told to pay for polymerase chain reaction tests, COVID treatments and the administration of vaccines. (Taxpayers are paying for the cost of the vaccines themselves.) It appears to be an elegant solution for a politician because it looks free and isn’t using taxpayer money.
1. Are the tests really free?
Well, no. As many an economist will tell you, there ain’t no such thing as a free lunch. Someone has to pick up the tab. Initially, the insurance companies bear the cost. Cynthia Cox, a vice president at KFF who studies the Affordable Care Act and private insurers, said the total bill could amount to billions of dollars. Exactly how much depends on “how easy it is to get them, and how many will be reimbursed,” she said.
2. Will the insurance company just swallow those imposed costs?
If companies draw from the time-tested insurance giants’ playbook, they’ll pass along those costs to customers. “This will put upward pressure on premiums,” said Emily Gee, vice president and coordinator for health policy at the Center for American Progress.
Major insurance companies like Cigna, Anthem, UnitedHealthcare, and Aetna did not respond to requests to discuss this issue.
3. If that’s the case, why haven’t I been hit with higher premiums already?
Insurance companies had the chance last year to raise premiums but, mostly, they did not.
Why? Perhaps because insurers have so far made so much money during the pandemic they didn’t need to. For example, the industry’s profits in 2020 increased 41% to $31 billion from $22 billion, according to the National Association of Insurance Commissioners. The NAIC said the industry has continued its “tremendous growth trend” that started before COVID emerged. Companies will be reporting 2021 results soon.
The reason behind these profits is clear. You were paying premiums based on projections your insurance company made about how much health care consumers would use that year. Because people stayed home, had fewer accidents, postponed surgeries and often avoided going to visit the doctor or the hospital, insurers paid out less. They rebated some of their earnings back to customers, but they pocketed a lot more.
As the companies’ actuaries work on predicting 2023 expenditures, premiums could go up if they foresee more claims and expenses. Paying for millions of rapid tests is something they would include in their calculations.
4. Regardless of my premiums, will the tests cost me money directly?
It’s quite possible. If your insurance company doesn’t have an arrangement with a retailer where you can simply pick up your allotted tests, you’ll have to pay for them – at whatever price the store sets. If that’s the case, you’ll need to fill out a form to request a reimbursement from the insurance company. How many times have you lost receipts or just plain neglected to mail in for rebates on something you bought? A lot, right?
Here’s another thing: The reimbursement is set at $12 per test. If you pay $30 for a test – and that is not unheard of – your insurer is only on the hook for $12. You eat the $18.
And by the way, people on Medicare will have to pay for their tests themselves. People who get their health care covered by Medicaid can obtain free test kits at community centers.
A few free tests are supposed to arrive at every American home via the U.S. Postal Service. And the Biden administration has activated a website where Americans can order free tests from a cache of a billion the federal government ordered.
5. Will this help bring down the costs of at-home tests and make them easier to find?
The free COVID tests are unlikely to have much immediate impact on general cost and availability. You will still need to search for them. The federal measures likely will stimulate the demand for tests, which in the short term may make them harder to find.
But the demand, and some government guarantees to manufacturers, may induce test makers to make more of them faster. The increased competition and supply theoretically could bring down the price. There is certainly room for prices to decline since the wholesale cost of the test is between $5 and $7, analysts estimate. “It’s a big step in the right direction,” Ms. Gee said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Americans keep hearing that it is important to test frequently for COVID-19 at home. But just try to find an “at-home” rapid COVID test in a store and at a price that makes frequent tests affordable.
Testing, as well as mask-wearing, is an important measure if the country ever hopes to beat COVID, restore normal routines and get the economy running efficiently. To get Americans cheaper tests, the federal government now plans to have insurance companies pay for them.
You can either get one without any out-of-pocket expense from retail pharmacies that are part of an insurance company’s network or buy it at any store and get reimbursed by the insurer.
Congress said private insurers must cover all COVID testing and any associated medical services when it passed the Families First Coronavirus Response Act and the Coronavirus Aid, Relief and Economic Security, or CARES, Act. The have-insurance-pay-for-it solution has been used frequently through the pandemic. Insurance companies have been told to pay for polymerase chain reaction tests, COVID treatments and the administration of vaccines. (Taxpayers are paying for the cost of the vaccines themselves.) It appears to be an elegant solution for a politician because it looks free and isn’t using taxpayer money.
1. Are the tests really free?
Well, no. As many an economist will tell you, there ain’t no such thing as a free lunch. Someone has to pick up the tab. Initially, the insurance companies bear the cost. Cynthia Cox, a vice president at KFF who studies the Affordable Care Act and private insurers, said the total bill could amount to billions of dollars. Exactly how much depends on “how easy it is to get them, and how many will be reimbursed,” she said.
2. Will the insurance company just swallow those imposed costs?
If companies draw from the time-tested insurance giants’ playbook, they’ll pass along those costs to customers. “This will put upward pressure on premiums,” said Emily Gee, vice president and coordinator for health policy at the Center for American Progress.
Major insurance companies like Cigna, Anthem, UnitedHealthcare, and Aetna did not respond to requests to discuss this issue.
3. If that’s the case, why haven’t I been hit with higher premiums already?
Insurance companies had the chance last year to raise premiums but, mostly, they did not.
Why? Perhaps because insurers have so far made so much money during the pandemic they didn’t need to. For example, the industry’s profits in 2020 increased 41% to $31 billion from $22 billion, according to the National Association of Insurance Commissioners. The NAIC said the industry has continued its “tremendous growth trend” that started before COVID emerged. Companies will be reporting 2021 results soon.
The reason behind these profits is clear. You were paying premiums based on projections your insurance company made about how much health care consumers would use that year. Because people stayed home, had fewer accidents, postponed surgeries and often avoided going to visit the doctor or the hospital, insurers paid out less. They rebated some of their earnings back to customers, but they pocketed a lot more.
As the companies’ actuaries work on predicting 2023 expenditures, premiums could go up if they foresee more claims and expenses. Paying for millions of rapid tests is something they would include in their calculations.
4. Regardless of my premiums, will the tests cost me money directly?
It’s quite possible. If your insurance company doesn’t have an arrangement with a retailer where you can simply pick up your allotted tests, you’ll have to pay for them – at whatever price the store sets. If that’s the case, you’ll need to fill out a form to request a reimbursement from the insurance company. How many times have you lost receipts or just plain neglected to mail in for rebates on something you bought? A lot, right?
Here’s another thing: The reimbursement is set at $12 per test. If you pay $30 for a test – and that is not unheard of – your insurer is only on the hook for $12. You eat the $18.
And by the way, people on Medicare will have to pay for their tests themselves. People who get their health care covered by Medicaid can obtain free test kits at community centers.
A few free tests are supposed to arrive at every American home via the U.S. Postal Service. And the Biden administration has activated a website where Americans can order free tests from a cache of a billion the federal government ordered.
5. Will this help bring down the costs of at-home tests and make them easier to find?
The free COVID tests are unlikely to have much immediate impact on general cost and availability. You will still need to search for them. The federal measures likely will stimulate the demand for tests, which in the short term may make them harder to find.
But the demand, and some government guarantees to manufacturers, may induce test makers to make more of them faster. The increased competition and supply theoretically could bring down the price. There is certainly room for prices to decline since the wholesale cost of the test is between $5 and $7, analysts estimate. “It’s a big step in the right direction,” Ms. Gee said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Americans keep hearing that it is important to test frequently for COVID-19 at home. But just try to find an “at-home” rapid COVID test in a store and at a price that makes frequent tests affordable.
Testing, as well as mask-wearing, is an important measure if the country ever hopes to beat COVID, restore normal routines and get the economy running efficiently. To get Americans cheaper tests, the federal government now plans to have insurance companies pay for them.
You can either get one without any out-of-pocket expense from retail pharmacies that are part of an insurance company’s network or buy it at any store and get reimbursed by the insurer.
Congress said private insurers must cover all COVID testing and any associated medical services when it passed the Families First Coronavirus Response Act and the Coronavirus Aid, Relief and Economic Security, or CARES, Act. The have-insurance-pay-for-it solution has been used frequently through the pandemic. Insurance companies have been told to pay for polymerase chain reaction tests, COVID treatments and the administration of vaccines. (Taxpayers are paying for the cost of the vaccines themselves.) It appears to be an elegant solution for a politician because it looks free and isn’t using taxpayer money.
1. Are the tests really free?
Well, no. As many an economist will tell you, there ain’t no such thing as a free lunch. Someone has to pick up the tab. Initially, the insurance companies bear the cost. Cynthia Cox, a vice president at KFF who studies the Affordable Care Act and private insurers, said the total bill could amount to billions of dollars. Exactly how much depends on “how easy it is to get them, and how many will be reimbursed,” she said.
2. Will the insurance company just swallow those imposed costs?
If companies draw from the time-tested insurance giants’ playbook, they’ll pass along those costs to customers. “This will put upward pressure on premiums,” said Emily Gee, vice president and coordinator for health policy at the Center for American Progress.
Major insurance companies like Cigna, Anthem, UnitedHealthcare, and Aetna did not respond to requests to discuss this issue.
3. If that’s the case, why haven’t I been hit with higher premiums already?
Insurance companies had the chance last year to raise premiums but, mostly, they did not.
Why? Perhaps because insurers have so far made so much money during the pandemic they didn’t need to. For example, the industry’s profits in 2020 increased 41% to $31 billion from $22 billion, according to the National Association of Insurance Commissioners. The NAIC said the industry has continued its “tremendous growth trend” that started before COVID emerged. Companies will be reporting 2021 results soon.
The reason behind these profits is clear. You were paying premiums based on projections your insurance company made about how much health care consumers would use that year. Because people stayed home, had fewer accidents, postponed surgeries and often avoided going to visit the doctor or the hospital, insurers paid out less. They rebated some of their earnings back to customers, but they pocketed a lot more.
As the companies’ actuaries work on predicting 2023 expenditures, premiums could go up if they foresee more claims and expenses. Paying for millions of rapid tests is something they would include in their calculations.
4. Regardless of my premiums, will the tests cost me money directly?
It’s quite possible. If your insurance company doesn’t have an arrangement with a retailer where you can simply pick up your allotted tests, you’ll have to pay for them – at whatever price the store sets. If that’s the case, you’ll need to fill out a form to request a reimbursement from the insurance company. How many times have you lost receipts or just plain neglected to mail in for rebates on something you bought? A lot, right?
Here’s another thing: The reimbursement is set at $12 per test. If you pay $30 for a test – and that is not unheard of – your insurer is only on the hook for $12. You eat the $18.
And by the way, people on Medicare will have to pay for their tests themselves. People who get their health care covered by Medicaid can obtain free test kits at community centers.
A few free tests are supposed to arrive at every American home via the U.S. Postal Service. And the Biden administration has activated a website where Americans can order free tests from a cache of a billion the federal government ordered.
5. Will this help bring down the costs of at-home tests and make them easier to find?
The free COVID tests are unlikely to have much immediate impact on general cost and availability. You will still need to search for them. The federal measures likely will stimulate the demand for tests, which in the short term may make them harder to find.
But the demand, and some government guarantees to manufacturers, may induce test makers to make more of them faster. The increased competition and supply theoretically could bring down the price. There is certainly room for prices to decline since the wholesale cost of the test is between $5 and $7, analysts estimate. “It’s a big step in the right direction,” Ms. Gee said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
COVID at 2 years: Preparing for a different ‘normal’
Two years into the COVID-19 pandemic, the United States is still breaking records in hospital overcrowding and new cases.
The United States is logging nearly 800,000 cases a day, hospitals are starting to fray, and deaths have topped 850,000. Schools oscillate from remote to in-person learning, polarizing communities.
The vaccines are lifesaving for many, yet frustration mounts as the numbers of unvaccinated people in this country stays relatively stagnant (63% in the United States are fully vaccinated) and other parts of the world have seen hardly a single dose. Africa has the slowest vaccination rate among continents, with only 14% of the population receiving one shot, according to the New York Times tracker.
Yet
Effective vaccines and treatments that can keep people out of the hospital were developed at an astounding pace, and advances in tracking and testing – in both access and effectiveness – are starting to pay off.
Some experts say it’s possible that the raging Omicron surge will slow by late spring, providing some relief and maybe shifting the pandemic to a slower-burning endemic.
But other experts caution to keep our guard up, saying it’s time to settle into a “new normal” and upend the strategy for fighting COVID-19.
Time to change COVID thinking
Three former members of the Biden-Harris Transition COVID-19 Advisory Board wrote recently in JAMA that COVID-19 has now become one of the many viral respiratory diseases that health care providers and patients will manage each year.
The group of experts from the University of Pennsylvania, University of Minnesota, and New York University write that “many of the measures to reduce transmission of SARS-CoV-2 (for example, ventilation) will also reduce transmission of other respiratory viruses. Thus, policy makers should retire previous public health categorizations, including deaths from pneumonia and influenza or pneumonia, influenza, and COVID-19, and focus on a new category: the aggregate risk of all respiratory virus infections.”
Other experts, including Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore, have said it’s been clear since the early days of SARS-CoV-2 that we must learn to live with the virus because it “will be ever present for the remaining history of our species.”
But that doesn’t mean the virus will always have the upper hand. Although the United States has been reaching record numbers of hospitalizations in January, these hospitalizations differ from those of last year – marked by fewer extreme lifesaving measures, fewer deaths, and shorter hospital stays – caused in part by medical and therapeutic advances and in part to the nature of the Omicron variant itself.
One sign of progress, Dr. Adalja said, will be the widespread decoupling of cases from hospitalizations, something that has already happened in countries such as the United Kingdom.
“That’s a reflection of how well they have vaccinated their high-risk population and how poorly we have vaccinated our high-risk population,” he said.
Omicron will bump up natural immunity
Dr. Adalja said though the numbers of unvaccinated in the United States appear to be stuck, Omicron’s sweep will make the difference, leaving behind more natural immunity in the population.
Currently, hospitals are struggling with staffing concerns as a “direct result” of too many unvaccinated people, he said.
Andrew Badley, MD, an infectious diseases specialist at Mayo Clinic in Rochester, Minn., and director of the clinic’s COVID-19 Task Force, said the good news with Omicron is that nearly all people it infects will recover.
Over time, when the body sees foreign antigens repeatedly, the quantity and quality of the antibodies the immune system produces increase and the body becomes better at fighting disease.
So “a large amount of the population will have recovered and have a degree of immunity,” Dr. Badley said.
His optimism is tempered by his belief that “it’s going to get worse before it gets better.”
But Dr. Badley still predicts a turnaround. “We’ll see a downturn in COVID in late spring or early summer,” and well into the second quarter of 2022, “we’ll see a reemergence of control.”
Right now, with Omicron, one infected person is infecting three to five others, he said. The hope is that it will eventually reach one-to-one endemic levels.
As for the threat of new variants, Badley said, “it’s not predictable whether they will be stronger or weaker.”
Masks may be around for years
Many experts predict that masks will continue to be part of the national wardrobe for the foreseeable future.
“We will continue to see new cases for years and years to come. Some will respond to that with masks in public places for a very long time. I personally will do so,” Dr. Badley said.
Two mindsets: Inside/outside the hospital
Emily Landon, MD, an infectious disease doctor and the executive medical director of infection prevention and control at University of Chicago Medicine, told this news organization she views the pandemic from two different vantage points.
As a health care provider, she sees her hospital, like others worldwide, overwhelmed. Supplies of a major weapon to help prevent hospitalization, the monoclonal antibody sotrovimab, are running out. Dr. Landon said she has been calling other hospitals to see if they have supplies and, if so, whether Omicron patients can transfer there.
Bottom line: The things they relied on a month ago to keep people out of the hospital are no longer there, she said.
Meanwhile, “We have more COVID patients than we have ever had,” Dr. Landon said.
Last year, UChicago hit a high of 170 people hospitalized with COVID. This year, so far, the peak was 270.
Dr. Landon said she is frustrated when she leaves that overburdened world inside the hospital for the outside world, where people wear no masks or ineffective face coverings and gather unsafely. Although some of that behavior reflects an intention to flout the advice of medical experts, some is caused in part, she said, by the lack of a clear national health strategy and garbled communication from those in charge of public safety.
Americans are deciding for themselves, on an a la carte basis, whether to wear a mask or get tested or travel, and school districts decide individually when it’s time to go virtual.
“People are exhausted from having to do a risk-benefit analysis for every single activity they, their friends, their kids want to participate in,” she said.
U.S. behind in several areas
Despite our self-image as the global leader in science and medicine, the United States stumbled badly in its response to the pandemic, with grave consequences both at home and abroad, experts say.
In a recent commentary in JAMA, Lawrence Gostin, JD, from Georgetown University, Washington, and Jennifer Nuzzo, DrPH, at Johns Hopkins University, Baltimore, pointed to several critical shortfalls in the nation’s efforts to control the disease.
One such shortfall is public trust.
This news organization reported in June 2021 that a poll of its readers found that 44% said their trust in the CDC had waned during the pandemic, and 33% said their trust in the FDA had eroded as well.
Health care providers who responded to the poll lost trust as well. About half of the doctors and nurses who responded said they disagreed with the FDA’s decision-making during the pandemic. Nearly 60% of doctors and 65% of nurses said they disagreed with the CDC’s overall pandemic guidance.
Lack of trust can make people resist vaccines and efforts to fight the virus, the authors wrote.
“This will become really relevant when we have ample supply of Pfizer’s antiviral medication,” Mr. Gostin, who directs the O’Neill Institute for National and Global Health Law at Georgetown, told this news organization. “The next phase of the pandemic is not to link testing to contact tracing, because we’re way past that, but to link testing to treatment.”
Lack of regional manufacturing of products is also thwarting global progress.
“It is extraordinarily important that our pharmaceutical industry transfer technology in a pandemic,” Mr. Gostin said. “The most glaring failure to do that is the mRNA vaccines. We’ve got this enormously effective vaccine and the two manufacturers – Pfizer and Moderna – are refusing to share the technology with producers in other countries. That keeps coming back to haunt us.”
Another problem: When the vaccines are shared with other countries, they are being delivered close to the date they expire or arriving at a shipyards without warning, so even some of the doses that get delivered are going to waste, Mr. Gostin said.
“It’s one of the greatest moral failures of my lifetime,” he said.
Also a failure is the “jaw-dropping” state of testing 2 years into the pandemic, he said, as people continue to pay high prices for tests or endure long lines.
The U.S. government updated its calculations and ordered 1 billion tests for the general public. The COVIDtests.gov website to order the free tests is now live.
It’s a step in the right direction. Mr. Gostin and Dr. Nuzzo wrote that there is every reason to expect future epidemics that are as serious or more serious than COVID.
“Failure to address clearly observed weaknesses in the COVID-19 response will have preventable adverse health, social, and economic consequences when the next novel outbreak occurs,” they wrote.
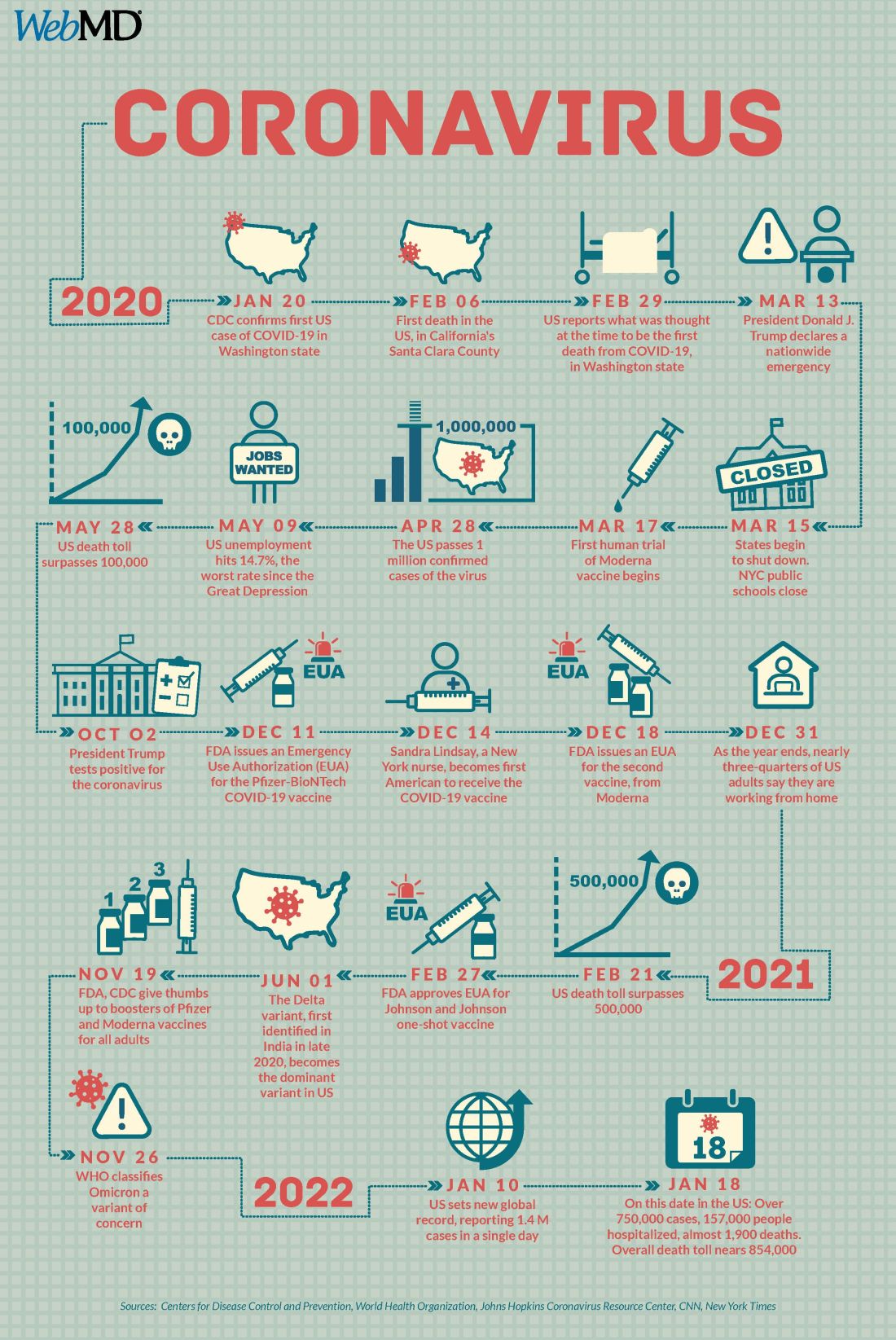
A version of this article first appeared on WebMD.com.
Two years into the COVID-19 pandemic, the United States is still breaking records in hospital overcrowding and new cases.
The United States is logging nearly 800,000 cases a day, hospitals are starting to fray, and deaths have topped 850,000. Schools oscillate from remote to in-person learning, polarizing communities.
The vaccines are lifesaving for many, yet frustration mounts as the numbers of unvaccinated people in this country stays relatively stagnant (63% in the United States are fully vaccinated) and other parts of the world have seen hardly a single dose. Africa has the slowest vaccination rate among continents, with only 14% of the population receiving one shot, according to the New York Times tracker.
Yet
Effective vaccines and treatments that can keep people out of the hospital were developed at an astounding pace, and advances in tracking and testing – in both access and effectiveness – are starting to pay off.
Some experts say it’s possible that the raging Omicron surge will slow by late spring, providing some relief and maybe shifting the pandemic to a slower-burning endemic.
But other experts caution to keep our guard up, saying it’s time to settle into a “new normal” and upend the strategy for fighting COVID-19.
Time to change COVID thinking
Three former members of the Biden-Harris Transition COVID-19 Advisory Board wrote recently in JAMA that COVID-19 has now become one of the many viral respiratory diseases that health care providers and patients will manage each year.
The group of experts from the University of Pennsylvania, University of Minnesota, and New York University write that “many of the measures to reduce transmission of SARS-CoV-2 (for example, ventilation) will also reduce transmission of other respiratory viruses. Thus, policy makers should retire previous public health categorizations, including deaths from pneumonia and influenza or pneumonia, influenza, and COVID-19, and focus on a new category: the aggregate risk of all respiratory virus infections.”
Other experts, including Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore, have said it’s been clear since the early days of SARS-CoV-2 that we must learn to live with the virus because it “will be ever present for the remaining history of our species.”
But that doesn’t mean the virus will always have the upper hand. Although the United States has been reaching record numbers of hospitalizations in January, these hospitalizations differ from those of last year – marked by fewer extreme lifesaving measures, fewer deaths, and shorter hospital stays – caused in part by medical and therapeutic advances and in part to the nature of the Omicron variant itself.
One sign of progress, Dr. Adalja said, will be the widespread decoupling of cases from hospitalizations, something that has already happened in countries such as the United Kingdom.
“That’s a reflection of how well they have vaccinated their high-risk population and how poorly we have vaccinated our high-risk population,” he said.
Omicron will bump up natural immunity
Dr. Adalja said though the numbers of unvaccinated in the United States appear to be stuck, Omicron’s sweep will make the difference, leaving behind more natural immunity in the population.
Currently, hospitals are struggling with staffing concerns as a “direct result” of too many unvaccinated people, he said.
Andrew Badley, MD, an infectious diseases specialist at Mayo Clinic in Rochester, Minn., and director of the clinic’s COVID-19 Task Force, said the good news with Omicron is that nearly all people it infects will recover.
Over time, when the body sees foreign antigens repeatedly, the quantity and quality of the antibodies the immune system produces increase and the body becomes better at fighting disease.
So “a large amount of the population will have recovered and have a degree of immunity,” Dr. Badley said.
His optimism is tempered by his belief that “it’s going to get worse before it gets better.”
But Dr. Badley still predicts a turnaround. “We’ll see a downturn in COVID in late spring or early summer,” and well into the second quarter of 2022, “we’ll see a reemergence of control.”
Right now, with Omicron, one infected person is infecting three to five others, he said. The hope is that it will eventually reach one-to-one endemic levels.
As for the threat of new variants, Badley said, “it’s not predictable whether they will be stronger or weaker.”
Masks may be around for years
Many experts predict that masks will continue to be part of the national wardrobe for the foreseeable future.
“We will continue to see new cases for years and years to come. Some will respond to that with masks in public places for a very long time. I personally will do so,” Dr. Badley said.
Two mindsets: Inside/outside the hospital
Emily Landon, MD, an infectious disease doctor and the executive medical director of infection prevention and control at University of Chicago Medicine, told this news organization she views the pandemic from two different vantage points.
As a health care provider, she sees her hospital, like others worldwide, overwhelmed. Supplies of a major weapon to help prevent hospitalization, the monoclonal antibody sotrovimab, are running out. Dr. Landon said she has been calling other hospitals to see if they have supplies and, if so, whether Omicron patients can transfer there.
Bottom line: The things they relied on a month ago to keep people out of the hospital are no longer there, she said.
Meanwhile, “We have more COVID patients than we have ever had,” Dr. Landon said.
Last year, UChicago hit a high of 170 people hospitalized with COVID. This year, so far, the peak was 270.
Dr. Landon said she is frustrated when she leaves that overburdened world inside the hospital for the outside world, where people wear no masks or ineffective face coverings and gather unsafely. Although some of that behavior reflects an intention to flout the advice of medical experts, some is caused in part, she said, by the lack of a clear national health strategy and garbled communication from those in charge of public safety.
Americans are deciding for themselves, on an a la carte basis, whether to wear a mask or get tested or travel, and school districts decide individually when it’s time to go virtual.
“People are exhausted from having to do a risk-benefit analysis for every single activity they, their friends, their kids want to participate in,” she said.
U.S. behind in several areas
Despite our self-image as the global leader in science and medicine, the United States stumbled badly in its response to the pandemic, with grave consequences both at home and abroad, experts say.
In a recent commentary in JAMA, Lawrence Gostin, JD, from Georgetown University, Washington, and Jennifer Nuzzo, DrPH, at Johns Hopkins University, Baltimore, pointed to several critical shortfalls in the nation’s efforts to control the disease.
One such shortfall is public trust.
This news organization reported in June 2021 that a poll of its readers found that 44% said their trust in the CDC had waned during the pandemic, and 33% said their trust in the FDA had eroded as well.
Health care providers who responded to the poll lost trust as well. About half of the doctors and nurses who responded said they disagreed with the FDA’s decision-making during the pandemic. Nearly 60% of doctors and 65% of nurses said they disagreed with the CDC’s overall pandemic guidance.
Lack of trust can make people resist vaccines and efforts to fight the virus, the authors wrote.
“This will become really relevant when we have ample supply of Pfizer’s antiviral medication,” Mr. Gostin, who directs the O’Neill Institute for National and Global Health Law at Georgetown, told this news organization. “The next phase of the pandemic is not to link testing to contact tracing, because we’re way past that, but to link testing to treatment.”
Lack of regional manufacturing of products is also thwarting global progress.
“It is extraordinarily important that our pharmaceutical industry transfer technology in a pandemic,” Mr. Gostin said. “The most glaring failure to do that is the mRNA vaccines. We’ve got this enormously effective vaccine and the two manufacturers – Pfizer and Moderna – are refusing to share the technology with producers in other countries. That keeps coming back to haunt us.”
Another problem: When the vaccines are shared with other countries, they are being delivered close to the date they expire or arriving at a shipyards without warning, so even some of the doses that get delivered are going to waste, Mr. Gostin said.
“It’s one of the greatest moral failures of my lifetime,” he said.
Also a failure is the “jaw-dropping” state of testing 2 years into the pandemic, he said, as people continue to pay high prices for tests or endure long lines.
The U.S. government updated its calculations and ordered 1 billion tests for the general public. The COVIDtests.gov website to order the free tests is now live.
It’s a step in the right direction. Mr. Gostin and Dr. Nuzzo wrote that there is every reason to expect future epidemics that are as serious or more serious than COVID.
“Failure to address clearly observed weaknesses in the COVID-19 response will have preventable adverse health, social, and economic consequences when the next novel outbreak occurs,” they wrote.

A version of this article first appeared on WebMD.com.
Two years into the COVID-19 pandemic, the United States is still breaking records in hospital overcrowding and new cases.
The United States is logging nearly 800,000 cases a day, hospitals are starting to fray, and deaths have topped 850,000. Schools oscillate from remote to in-person learning, polarizing communities.
The vaccines are lifesaving for many, yet frustration mounts as the numbers of unvaccinated people in this country stays relatively stagnant (63% in the United States are fully vaccinated) and other parts of the world have seen hardly a single dose. Africa has the slowest vaccination rate among continents, with only 14% of the population receiving one shot, according to the New York Times tracker.
Yet
Effective vaccines and treatments that can keep people out of the hospital were developed at an astounding pace, and advances in tracking and testing – in both access and effectiveness – are starting to pay off.
Some experts say it’s possible that the raging Omicron surge will slow by late spring, providing some relief and maybe shifting the pandemic to a slower-burning endemic.
But other experts caution to keep our guard up, saying it’s time to settle into a “new normal” and upend the strategy for fighting COVID-19.
Time to change COVID thinking
Three former members of the Biden-Harris Transition COVID-19 Advisory Board wrote recently in JAMA that COVID-19 has now become one of the many viral respiratory diseases that health care providers and patients will manage each year.
The group of experts from the University of Pennsylvania, University of Minnesota, and New York University write that “many of the measures to reduce transmission of SARS-CoV-2 (for example, ventilation) will also reduce transmission of other respiratory viruses. Thus, policy makers should retire previous public health categorizations, including deaths from pneumonia and influenza or pneumonia, influenza, and COVID-19, and focus on a new category: the aggregate risk of all respiratory virus infections.”
Other experts, including Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore, have said it’s been clear since the early days of SARS-CoV-2 that we must learn to live with the virus because it “will be ever present for the remaining history of our species.”
But that doesn’t mean the virus will always have the upper hand. Although the United States has been reaching record numbers of hospitalizations in January, these hospitalizations differ from those of last year – marked by fewer extreme lifesaving measures, fewer deaths, and shorter hospital stays – caused in part by medical and therapeutic advances and in part to the nature of the Omicron variant itself.
One sign of progress, Dr. Adalja said, will be the widespread decoupling of cases from hospitalizations, something that has already happened in countries such as the United Kingdom.
“That’s a reflection of how well they have vaccinated their high-risk population and how poorly we have vaccinated our high-risk population,” he said.
Omicron will bump up natural immunity
Dr. Adalja said though the numbers of unvaccinated in the United States appear to be stuck, Omicron’s sweep will make the difference, leaving behind more natural immunity in the population.
Currently, hospitals are struggling with staffing concerns as a “direct result” of too many unvaccinated people, he said.
Andrew Badley, MD, an infectious diseases specialist at Mayo Clinic in Rochester, Minn., and director of the clinic’s COVID-19 Task Force, said the good news with Omicron is that nearly all people it infects will recover.
Over time, when the body sees foreign antigens repeatedly, the quantity and quality of the antibodies the immune system produces increase and the body becomes better at fighting disease.
So “a large amount of the population will have recovered and have a degree of immunity,” Dr. Badley said.
His optimism is tempered by his belief that “it’s going to get worse before it gets better.”
But Dr. Badley still predicts a turnaround. “We’ll see a downturn in COVID in late spring or early summer,” and well into the second quarter of 2022, “we’ll see a reemergence of control.”
Right now, with Omicron, one infected person is infecting three to five others, he said. The hope is that it will eventually reach one-to-one endemic levels.
As for the threat of new variants, Badley said, “it’s not predictable whether they will be stronger or weaker.”
Masks may be around for years
Many experts predict that masks will continue to be part of the national wardrobe for the foreseeable future.
“We will continue to see new cases for years and years to come. Some will respond to that with masks in public places for a very long time. I personally will do so,” Dr. Badley said.
Two mindsets: Inside/outside the hospital
Emily Landon, MD, an infectious disease doctor and the executive medical director of infection prevention and control at University of Chicago Medicine, told this news organization she views the pandemic from two different vantage points.
As a health care provider, she sees her hospital, like others worldwide, overwhelmed. Supplies of a major weapon to help prevent hospitalization, the monoclonal antibody sotrovimab, are running out. Dr. Landon said she has been calling other hospitals to see if they have supplies and, if so, whether Omicron patients can transfer there.
Bottom line: The things they relied on a month ago to keep people out of the hospital are no longer there, she said.
Meanwhile, “We have more COVID patients than we have ever had,” Dr. Landon said.
Last year, UChicago hit a high of 170 people hospitalized with COVID. This year, so far, the peak was 270.
Dr. Landon said she is frustrated when she leaves that overburdened world inside the hospital for the outside world, where people wear no masks or ineffective face coverings and gather unsafely. Although some of that behavior reflects an intention to flout the advice of medical experts, some is caused in part, she said, by the lack of a clear national health strategy and garbled communication from those in charge of public safety.
Americans are deciding for themselves, on an a la carte basis, whether to wear a mask or get tested or travel, and school districts decide individually when it’s time to go virtual.
“People are exhausted from having to do a risk-benefit analysis for every single activity they, their friends, their kids want to participate in,” she said.
U.S. behind in several areas
Despite our self-image as the global leader in science and medicine, the United States stumbled badly in its response to the pandemic, with grave consequences both at home and abroad, experts say.
In a recent commentary in JAMA, Lawrence Gostin, JD, from Georgetown University, Washington, and Jennifer Nuzzo, DrPH, at Johns Hopkins University, Baltimore, pointed to several critical shortfalls in the nation’s efforts to control the disease.
One such shortfall is public trust.
This news organization reported in June 2021 that a poll of its readers found that 44% said their trust in the CDC had waned during the pandemic, and 33% said their trust in the FDA had eroded as well.
Health care providers who responded to the poll lost trust as well. About half of the doctors and nurses who responded said they disagreed with the FDA’s decision-making during the pandemic. Nearly 60% of doctors and 65% of nurses said they disagreed with the CDC’s overall pandemic guidance.
Lack of trust can make people resist vaccines and efforts to fight the virus, the authors wrote.
“This will become really relevant when we have ample supply of Pfizer’s antiviral medication,” Mr. Gostin, who directs the O’Neill Institute for National and Global Health Law at Georgetown, told this news organization. “The next phase of the pandemic is not to link testing to contact tracing, because we’re way past that, but to link testing to treatment.”
Lack of regional manufacturing of products is also thwarting global progress.
“It is extraordinarily important that our pharmaceutical industry transfer technology in a pandemic,” Mr. Gostin said. “The most glaring failure to do that is the mRNA vaccines. We’ve got this enormously effective vaccine and the two manufacturers – Pfizer and Moderna – are refusing to share the technology with producers in other countries. That keeps coming back to haunt us.”
Another problem: When the vaccines are shared with other countries, they are being delivered close to the date they expire or arriving at a shipyards without warning, so even some of the doses that get delivered are going to waste, Mr. Gostin said.
“It’s one of the greatest moral failures of my lifetime,” he said.
Also a failure is the “jaw-dropping” state of testing 2 years into the pandemic, he said, as people continue to pay high prices for tests or endure long lines.
The U.S. government updated its calculations and ordered 1 billion tests for the general public. The COVIDtests.gov website to order the free tests is now live.
It’s a step in the right direction. Mr. Gostin and Dr. Nuzzo wrote that there is every reason to expect future epidemics that are as serious or more serious than COVID.
“Failure to address clearly observed weaknesses in the COVID-19 response will have preventable adverse health, social, and economic consequences when the next novel outbreak occurs,” they wrote.

A version of this article first appeared on WebMD.com.
Make America beautiful: Support mask mandates
In space, no one can hear your red blood cells scream
There are many reasons why space is the final frontier, not least of which are the major health issues space travel places on the human body. So until a shady billionaire finds an alien protomolecule on a Saturnian moon and starts splicing it with human DNA so we can hang out in space all day without a spacesuit, we’re stuck with things like space anemia, a condition many astronauts develop after extended time in space.
Space anemia has been known for many years, but it was assumed that it developed as a reaction to microgravity and was a short-term phenomenon only – a temporary compensation as fluids and blood volume adjusted themselves. But as new research shows, that assumption seems to be wrong.
For the study, published in Nature Medicine, 13 astronauts who were in space for at least 120 days – long enough for all their red blood cells to have been produced in space – had their blood tested consistently. Before their flights, the astronauts created and destroyed 2 million red blood cells per second, but while they were in space, they destroyed 3 million cells per second. Notably, this process continued for the entire duration of the space flight. So, not a temporary reaction.
Consequently, 5 of the 13 astronauts developed anemia when they returned to Earth. (Interesting space fact: Having fewer blood cells isn’t a problem while you’re in space; the effects of anemia only manifest when the body returns to full gravity.) The anemia disappeared after a few months, but the astronauts were still destroying 30% more red blood cells a year after their spaceflight than they were before leaving Earth.
You may be thinking: Well, if they were destroying 50% more red blood cells while in space, how come they didn’t all develop severe anemia? The researchers theorized that production was boosted as well, which sounds like a good thing. The body is compensating, as it should. Unfortunately, that increased production stresses bone marrow function and the demand for energy spikes. That’s not such a good thing. And of course, many of the astronauts got anemia anyway.
To tackle the issue, the researchers emphasized the importance of feeding astronauts a proper diet, plus potential supplements before spaceflight. So don’t worry, Captain Kirk will be able to arm wrestle Klingons and romance suspiciously human-looking aliens without fear of keeling over from anemia-induced fatigue. Earth will stay safe.
Tell me with your eyes
Communication can be hard, even under the best of circumstances, but for many nonverbal patients in the intensive care unit who can’t move, getting a point across to the health care team can be a huge struggle in itself.
Health care professionals have been making do with eye-blinking or head-nodding, but what if that’s just not enough? New research shows that it’s not, and there’s a more effective way for patients to say what they mean just by looking.
In a study published in the Journal of Trauma and Acute Care Surgery, researchers looked into using eye-tracking systems for nonverbal ICU patients to communicate. Eye-tracking isn’t anything new, but using it as a form of communication among nonverbal patients with critical illness hasn’t been looked at before.
How does it work? The eye-tracking system is set up in the patient’s line of sight and its various algorithms and software collect data to calculate where exactly the patient is looking. Established scores and scales assess the patient’s mood, quality of life, pain, and self-esteem.
The researchers found that participating patients were actually experiencing more negative moods, pain, and feelings of frustration than was once believed. Making this tool even more valuable for treatment adjustment and meeting patients’ needs.
In this case, it means that health care providers are getting an eyeful … of communication.
Make America grave again
Here we go again. Somebody just found something else that the United States is not the best at. To go along with math and science education, infrastructure investment, quality of life …
That’s going to go on for a while, so let’s get to the new stuff. An international group of researchers surveyed end-of-life care in 81 countries and ranked them based on the assessment of 181 experts in those countries. They looked at 13 different factors, including proper management of pain and comfort, having a clean and safe space, being treated kindly, lack of cost barriers to appropriate care, and treatments that address quality of life and don’t just extend life.
… press freedom, industrial production, racial equality, Internet connectivity …
Their report card, published in the Journal of Pain and Symptom Management, gave six countries an A, with Great Britain at the top. The other five were Ireland, Taiwan, Australia, South Korea, and Costa Rica. The lowest grade went to Paraguay in 81st place, with Lebanon, Brazil, Senegal, and Haiti just ahead.
… environmental stewardship, body-mass index, social mobility, COVID safeness …
The United States, getting a firm grasp on mediocrity, ranked 43rd. Here are some countries that did better: North Macedonia (7th), Sri Lanka (16th), Uganda (31st), and Uruguay 33rd). In the United States, “we spend so much money trying to get people to live longer, but we don’t spend enough money in helping people die better,” lead author Eric A. Finkelstein, PhD, said in a written statement.
… economic stability, and soccer; we’re also not the best at dying. Wait, did we already say that?
The face mask that launched a thousand ships
Face masks, clearly, have been a source of social strife during the pandemic. People may not agree on mandates, but a mask can be a pretty-low-maintenance face shield if you don’t feel like putting on make-up or want to cover up some blemishes.
Before the pandemic, people thought that those wearing face masks were less attractive because the masks represented illness or disease, according to Dr. Michael Lewis of Cardiff (Wales) University. Back then, no one really wore masks besides doctors and nurses, so if you saw someone wearing one on the street, you probably wondered what they were trying to hide.
Now, though, the subject of face mask attractiveness has been revisited by Dr. Lewis and his associate, Oliver Hies, who found that face masks now make people more attractive.
“Our study suggests faces are considered most attractive when covered by medical face masks. … At a time when we feel vulnerable, we may find the wearing of medical masks reassuring and so feel more positive towards the wearer,” Dr. Lewis told the Guardian.
He suggested that we’re no longer looking at people wearing a mask as disease riddled, but rather doing their part to protect society. Or maybe we focus more on someone’s eyes when that’s all there is to look at. Or, maybe we wind up making up what the rest of someone’s face looks like to meet our attractiveness criteria.
However you feel about masks, they’re cheaper than plastic surgery. And you can go out wearing a new face every day.
In space, no one can hear your red blood cells scream
There are many reasons why space is the final frontier, not least of which are the major health issues space travel places on the human body. So until a shady billionaire finds an alien protomolecule on a Saturnian moon and starts splicing it with human DNA so we can hang out in space all day without a spacesuit, we’re stuck with things like space anemia, a condition many astronauts develop after extended time in space.
Space anemia has been known for many years, but it was assumed that it developed as a reaction to microgravity and was a short-term phenomenon only – a temporary compensation as fluids and blood volume adjusted themselves. But as new research shows, that assumption seems to be wrong.
For the study, published in Nature Medicine, 13 astronauts who were in space for at least 120 days – long enough for all their red blood cells to have been produced in space – had their blood tested consistently. Before their flights, the astronauts created and destroyed 2 million red blood cells per second, but while they were in space, they destroyed 3 million cells per second. Notably, this process continued for the entire duration of the space flight. So, not a temporary reaction.
Consequently, 5 of the 13 astronauts developed anemia when they returned to Earth. (Interesting space fact: Having fewer blood cells isn’t a problem while you’re in space; the effects of anemia only manifest when the body returns to full gravity.) The anemia disappeared after a few months, but the astronauts were still destroying 30% more red blood cells a year after their spaceflight than they were before leaving Earth.
You may be thinking: Well, if they were destroying 50% more red blood cells while in space, how come they didn’t all develop severe anemia? The researchers theorized that production was boosted as well, which sounds like a good thing. The body is compensating, as it should. Unfortunately, that increased production stresses bone marrow function and the demand for energy spikes. That’s not such a good thing. And of course, many of the astronauts got anemia anyway.
To tackle the issue, the researchers emphasized the importance of feeding astronauts a proper diet, plus potential supplements before spaceflight. So don’t worry, Captain Kirk will be able to arm wrestle Klingons and romance suspiciously human-looking aliens without fear of keeling over from anemia-induced fatigue. Earth will stay safe.
Tell me with your eyes
Communication can be hard, even under the best of circumstances, but for many nonverbal patients in the intensive care unit who can’t move, getting a point across to the health care team can be a huge struggle in itself.
Health care professionals have been making do with eye-blinking or head-nodding, but what if that’s just not enough? New research shows that it’s not, and there’s a more effective way for patients to say what they mean just by looking.
In a study published in the Journal of Trauma and Acute Care Surgery, researchers looked into using eye-tracking systems for nonverbal ICU patients to communicate. Eye-tracking isn’t anything new, but using it as a form of communication among nonverbal patients with critical illness hasn’t been looked at before.
How does it work? The eye-tracking system is set up in the patient’s line of sight and its various algorithms and software collect data to calculate where exactly the patient is looking. Established scores and scales assess the patient’s mood, quality of life, pain, and self-esteem.
The researchers found that participating patients were actually experiencing more negative moods, pain, and feelings of frustration than was once believed. Making this tool even more valuable for treatment adjustment and meeting patients’ needs.
In this case, it means that health care providers are getting an eyeful … of communication.
Make America grave again
Here we go again. Somebody just found something else that the United States is not the best at. To go along with math and science education, infrastructure investment, quality of life …
That’s going to go on for a while, so let’s get to the new stuff. An international group of researchers surveyed end-of-life care in 81 countries and ranked them based on the assessment of 181 experts in those countries. They looked at 13 different factors, including proper management of pain and comfort, having a clean and safe space, being treated kindly, lack of cost barriers to appropriate care, and treatments that address quality of life and don’t just extend life.
… press freedom, industrial production, racial equality, Internet connectivity …
Their report card, published in the Journal of Pain and Symptom Management, gave six countries an A, with Great Britain at the top. The other five were Ireland, Taiwan, Australia, South Korea, and Costa Rica. The lowest grade went to Paraguay in 81st place, with Lebanon, Brazil, Senegal, and Haiti just ahead.
… environmental stewardship, body-mass index, social mobility, COVID safeness …
The United States, getting a firm grasp on mediocrity, ranked 43rd. Here are some countries that did better: North Macedonia (7th), Sri Lanka (16th), Uganda (31st), and Uruguay 33rd). In the United States, “we spend so much money trying to get people to live longer, but we don’t spend enough money in helping people die better,” lead author Eric A. Finkelstein, PhD, said in a written statement.
… economic stability, and soccer; we’re also not the best at dying. Wait, did we already say that?
The face mask that launched a thousand ships
Face masks, clearly, have been a source of social strife during the pandemic. People may not agree on mandates, but a mask can be a pretty-low-maintenance face shield if you don’t feel like putting on make-up or want to cover up some blemishes.
Before the pandemic, people thought that those wearing face masks were less attractive because the masks represented illness or disease, according to Dr. Michael Lewis of Cardiff (Wales) University. Back then, no one really wore masks besides doctors and nurses, so if you saw someone wearing one on the street, you probably wondered what they were trying to hide.
Now, though, the subject of face mask attractiveness has been revisited by Dr. Lewis and his associate, Oliver Hies, who found that face masks now make people more attractive.
“Our study suggests faces are considered most attractive when covered by medical face masks. … At a time when we feel vulnerable, we may find the wearing of medical masks reassuring and so feel more positive towards the wearer,” Dr. Lewis told the Guardian.
He suggested that we’re no longer looking at people wearing a mask as disease riddled, but rather doing their part to protect society. Or maybe we focus more on someone’s eyes when that’s all there is to look at. Or, maybe we wind up making up what the rest of someone’s face looks like to meet our attractiveness criteria.
However you feel about masks, they’re cheaper than plastic surgery. And you can go out wearing a new face every day.
In space, no one can hear your red blood cells scream
There are many reasons why space is the final frontier, not least of which are the major health issues space travel places on the human body. So until a shady billionaire finds an alien protomolecule on a Saturnian moon and starts splicing it with human DNA so we can hang out in space all day without a spacesuit, we’re stuck with things like space anemia, a condition many astronauts develop after extended time in space.
Space anemia has been known for many years, but it was assumed that it developed as a reaction to microgravity and was a short-term phenomenon only – a temporary compensation as fluids and blood volume adjusted themselves. But as new research shows, that assumption seems to be wrong.
For the study, published in Nature Medicine, 13 astronauts who were in space for at least 120 days – long enough for all their red blood cells to have been produced in space – had their blood tested consistently. Before their flights, the astronauts created and destroyed 2 million red blood cells per second, but while they were in space, they destroyed 3 million cells per second. Notably, this process continued for the entire duration of the space flight. So, not a temporary reaction.
Consequently, 5 of the 13 astronauts developed anemia when they returned to Earth. (Interesting space fact: Having fewer blood cells isn’t a problem while you’re in space; the effects of anemia only manifest when the body returns to full gravity.) The anemia disappeared after a few months, but the astronauts were still destroying 30% more red blood cells a year after their spaceflight than they were before leaving Earth.
You may be thinking: Well, if they were destroying 50% more red blood cells while in space, how come they didn’t all develop severe anemia? The researchers theorized that production was boosted as well, which sounds like a good thing. The body is compensating, as it should. Unfortunately, that increased production stresses bone marrow function and the demand for energy spikes. That’s not such a good thing. And of course, many of the astronauts got anemia anyway.
To tackle the issue, the researchers emphasized the importance of feeding astronauts a proper diet, plus potential supplements before spaceflight. So don’t worry, Captain Kirk will be able to arm wrestle Klingons and romance suspiciously human-looking aliens without fear of keeling over from anemia-induced fatigue. Earth will stay safe.
Tell me with your eyes
Communication can be hard, even under the best of circumstances, but for many nonverbal patients in the intensive care unit who can’t move, getting a point across to the health care team can be a huge struggle in itself.
Health care professionals have been making do with eye-blinking or head-nodding, but what if that’s just not enough? New research shows that it’s not, and there’s a more effective way for patients to say what they mean just by looking.
In a study published in the Journal of Trauma and Acute Care Surgery, researchers looked into using eye-tracking systems for nonverbal ICU patients to communicate. Eye-tracking isn’t anything new, but using it as a form of communication among nonverbal patients with critical illness hasn’t been looked at before.
How does it work? The eye-tracking system is set up in the patient’s line of sight and its various algorithms and software collect data to calculate where exactly the patient is looking. Established scores and scales assess the patient’s mood, quality of life, pain, and self-esteem.
The researchers found that participating patients were actually experiencing more negative moods, pain, and feelings of frustration than was once believed. Making this tool even more valuable for treatment adjustment and meeting patients’ needs.
In this case, it means that health care providers are getting an eyeful … of communication.
Make America grave again
Here we go again. Somebody just found something else that the United States is not the best at. To go along with math and science education, infrastructure investment, quality of life …
That’s going to go on for a while, so let’s get to the new stuff. An international group of researchers surveyed end-of-life care in 81 countries and ranked them based on the assessment of 181 experts in those countries. They looked at 13 different factors, including proper management of pain and comfort, having a clean and safe space, being treated kindly, lack of cost barriers to appropriate care, and treatments that address quality of life and don’t just extend life.
… press freedom, industrial production, racial equality, Internet connectivity …
Their report card, published in the Journal of Pain and Symptom Management, gave six countries an A, with Great Britain at the top. The other five were Ireland, Taiwan, Australia, South Korea, and Costa Rica. The lowest grade went to Paraguay in 81st place, with Lebanon, Brazil, Senegal, and Haiti just ahead.
… environmental stewardship, body-mass index, social mobility, COVID safeness …
The United States, getting a firm grasp on mediocrity, ranked 43rd. Here are some countries that did better: North Macedonia (7th), Sri Lanka (16th), Uganda (31st), and Uruguay 33rd). In the United States, “we spend so much money trying to get people to live longer, but we don’t spend enough money in helping people die better,” lead author Eric A. Finkelstein, PhD, said in a written statement.
… economic stability, and soccer; we’re also not the best at dying. Wait, did we already say that?
The face mask that launched a thousand ships
Face masks, clearly, have been a source of social strife during the pandemic. People may not agree on mandates, but a mask can be a pretty-low-maintenance face shield if you don’t feel like putting on make-up or want to cover up some blemishes.
Before the pandemic, people thought that those wearing face masks were less attractive because the masks represented illness or disease, according to Dr. Michael Lewis of Cardiff (Wales) University. Back then, no one really wore masks besides doctors and nurses, so if you saw someone wearing one on the street, you probably wondered what they were trying to hide.
Now, though, the subject of face mask attractiveness has been revisited by Dr. Lewis and his associate, Oliver Hies, who found that face masks now make people more attractive.
“Our study suggests faces are considered most attractive when covered by medical face masks. … At a time when we feel vulnerable, we may find the wearing of medical masks reassuring and so feel more positive towards the wearer,” Dr. Lewis told the Guardian.
He suggested that we’re no longer looking at people wearing a mask as disease riddled, but rather doing their part to protect society. Or maybe we focus more on someone’s eyes when that’s all there is to look at. Or, maybe we wind up making up what the rest of someone’s face looks like to meet our attractiveness criteria.
However you feel about masks, they’re cheaper than plastic surgery. And you can go out wearing a new face every day.
Pandemic weighing on physicians’ happiness outside of work: survey
One of the unexpected consequences of the pandemic is that many people are rethinking their priorities and lifestyles, and physicians are no exception.
Pets, prayer, and partners
The pandemic has taken a toll on physicians outside of work as well as on the job. Eight in 10 physicians (82% of men and 80% of women) said they were “somewhat” or “very” happy outside of work before the pandemic. This is almost exactly the same result as in last year’s survey.
However, when asked how happy they are outside of work currently, only 6 in 10 (59%) reported being “somewhat” or “very” happy. While the pandemic has made life difficult for everyone, health care professionals face particular stresses even outside of work. Wayne M. Sotile, PhD, founder of the Center for Physician Resilience, says he has counseled doctors who witnessed COVID-related suffering and death at work, then came home to a partner who didn’t believe that the pandemic was real.
Still, physicians reported that spending time with people they love and engaging in favorite activities helps them stay happy. “Spending time with pets” and “religious practice/prayer” were frequent “other” responses to the question, “What do you do to maintain happiness and mental health?” Seven in 10 physicians reported having some kind of religious or spiritual beliefs.
The majority of physicians (83%) are either married or living with a partner, with male physicians edging out their female peers (89% vs. 75%). Among married physicians, 8 in 10 physicians reported that their union is “good” or “very good.” The pandemic may have helped in this respect. Dr. Sotile says he’s heard physicians say that they’ve connected more with their families in the past 18 months. Specialists with the highest rates of happy marriages were otolaryngologists and immunologists (both 91%), followed closely by dermatologists, rheumatologists, and nephrologists (all 90%).
Among physicians balancing a medical career and parenthood, female physicians reported feeling conflicted more often than males (48% vs. 29%). Nicole A. Sparks, MD, an ob.gyn. and a health and lifestyle blogger, cites not being there for her kids as a source of stress. She notes that her two young children notice when she’s not there to help with homework, read bedtime stories, or make their dinner. “Mom guilt can definitely set in if I have to miss important events,” she says.
Work-life balance is an important, if elusive, goal for physicians, and not just females. Sixty percent of female doctors and 53% of male doctors said they would be willing to take a cut in pay if it meant more free time and a better work-life balance. Many doctors do manage to get away from work occasionally, with one-fifth of all physicians taking 5 or more weeks of vacation each year.
Seeking a ‘balanced life’
Alexis Polles, MD, medical director for the Professionals Resource Network, points out the importance of taking time for personal health and wellness. “When we work with professionals who have problems with mental health or substance abuse, they often don’t have a balanced life,” she says. “They are usually in a workaholic mindset and disregard their own needs.”
Few physicians seem to prioritize self-care, with a third indicating they “always” or “most of the time” spend enough time on their own health and wellness. But of those who do, males (38%) are more likely than females (27%) to spend enough time on their own health and wellness. Dr. Polles adds that exercising after a shift can help physicians better make the transition from professional to personal life. Though they did not report when they exercised, about a third of physicians reported doing so four or more times per week. Controlling weight is an issue as well, with 49% of male and 55% of female physicians saying they are currently trying to lose weight.
Of physicians who drink alcohol, about a third have three or more drinks per week. (The CDC defines “heavy drinking” as consuming 15 drinks or more per week for men and eight drinks or more per week for women.)
Of those surveyed, 92% say they do not regularly use cannabidiol or cannabis, and a mere 4% of respondents said they would use at least one of these substances if they were to become legal in their state.
A version of this article first appeared on Medscape.com.
One of the unexpected consequences of the pandemic is that many people are rethinking their priorities and lifestyles, and physicians are no exception.
Pets, prayer, and partners
The pandemic has taken a toll on physicians outside of work as well as on the job. Eight in 10 physicians (82% of men and 80% of women) said they were “somewhat” or “very” happy outside of work before the pandemic. This is almost exactly the same result as in last year’s survey.
However, when asked how happy they are outside of work currently, only 6 in 10 (59%) reported being “somewhat” or “very” happy. While the pandemic has made life difficult for everyone, health care professionals face particular stresses even outside of work. Wayne M. Sotile, PhD, founder of the Center for Physician Resilience, says he has counseled doctors who witnessed COVID-related suffering and death at work, then came home to a partner who didn’t believe that the pandemic was real.
Still, physicians reported that spending time with people they love and engaging in favorite activities helps them stay happy. “Spending time with pets” and “religious practice/prayer” were frequent “other” responses to the question, “What do you do to maintain happiness and mental health?” Seven in 10 physicians reported having some kind of religious or spiritual beliefs.
The majority of physicians (83%) are either married or living with a partner, with male physicians edging out their female peers (89% vs. 75%). Among married physicians, 8 in 10 physicians reported that their union is “good” or “very good.” The pandemic may have helped in this respect. Dr. Sotile says he’s heard physicians say that they’ve connected more with their families in the past 18 months. Specialists with the highest rates of happy marriages were otolaryngologists and immunologists (both 91%), followed closely by dermatologists, rheumatologists, and nephrologists (all 90%).
Among physicians balancing a medical career and parenthood, female physicians reported feeling conflicted more often than males (48% vs. 29%). Nicole A. Sparks, MD, an ob.gyn. and a health and lifestyle blogger, cites not being there for her kids as a source of stress. She notes that her two young children notice when she’s not there to help with homework, read bedtime stories, or make their dinner. “Mom guilt can definitely set in if I have to miss important events,” she says.
Work-life balance is an important, if elusive, goal for physicians, and not just females. Sixty percent of female doctors and 53% of male doctors said they would be willing to take a cut in pay if it meant more free time and a better work-life balance. Many doctors do manage to get away from work occasionally, with one-fifth of all physicians taking 5 or more weeks of vacation each year.
Seeking a ‘balanced life’
Alexis Polles, MD, medical director for the Professionals Resource Network, points out the importance of taking time for personal health and wellness. “When we work with professionals who have problems with mental health or substance abuse, they often don’t have a balanced life,” she says. “They are usually in a workaholic mindset and disregard their own needs.”
Few physicians seem to prioritize self-care, with a third indicating they “always” or “most of the time” spend enough time on their own health and wellness. But of those who do, males (38%) are more likely than females (27%) to spend enough time on their own health and wellness. Dr. Polles adds that exercising after a shift can help physicians better make the transition from professional to personal life. Though they did not report when they exercised, about a third of physicians reported doing so four or more times per week. Controlling weight is an issue as well, with 49% of male and 55% of female physicians saying they are currently trying to lose weight.
Of physicians who drink alcohol, about a third have three or more drinks per week. (The CDC defines “heavy drinking” as consuming 15 drinks or more per week for men and eight drinks or more per week for women.)
Of those surveyed, 92% say they do not regularly use cannabidiol or cannabis, and a mere 4% of respondents said they would use at least one of these substances if they were to become legal in their state.
A version of this article first appeared on Medscape.com.
One of the unexpected consequences of the pandemic is that many people are rethinking their priorities and lifestyles, and physicians are no exception.
Pets, prayer, and partners
The pandemic has taken a toll on physicians outside of work as well as on the job. Eight in 10 physicians (82% of men and 80% of women) said they were “somewhat” or “very” happy outside of work before the pandemic. This is almost exactly the same result as in last year’s survey.
However, when asked how happy they are outside of work currently, only 6 in 10 (59%) reported being “somewhat” or “very” happy. While the pandemic has made life difficult for everyone, health care professionals face particular stresses even outside of work. Wayne M. Sotile, PhD, founder of the Center for Physician Resilience, says he has counseled doctors who witnessed COVID-related suffering and death at work, then came home to a partner who didn’t believe that the pandemic was real.
Still, physicians reported that spending time with people they love and engaging in favorite activities helps them stay happy. “Spending time with pets” and “religious practice/prayer” were frequent “other” responses to the question, “What do you do to maintain happiness and mental health?” Seven in 10 physicians reported having some kind of religious or spiritual beliefs.
The majority of physicians (83%) are either married or living with a partner, with male physicians edging out their female peers (89% vs. 75%). Among married physicians, 8 in 10 physicians reported that their union is “good” or “very good.” The pandemic may have helped in this respect. Dr. Sotile says he’s heard physicians say that they’ve connected more with their families in the past 18 months. Specialists with the highest rates of happy marriages were otolaryngologists and immunologists (both 91%), followed closely by dermatologists, rheumatologists, and nephrologists (all 90%).
Among physicians balancing a medical career and parenthood, female physicians reported feeling conflicted more often than males (48% vs. 29%). Nicole A. Sparks, MD, an ob.gyn. and a health and lifestyle blogger, cites not being there for her kids as a source of stress. She notes that her two young children notice when she’s not there to help with homework, read bedtime stories, or make their dinner. “Mom guilt can definitely set in if I have to miss important events,” she says.
Work-life balance is an important, if elusive, goal for physicians, and not just females. Sixty percent of female doctors and 53% of male doctors said they would be willing to take a cut in pay if it meant more free time and a better work-life balance. Many doctors do manage to get away from work occasionally, with one-fifth of all physicians taking 5 or more weeks of vacation each year.
Seeking a ‘balanced life’
Alexis Polles, MD, medical director for the Professionals Resource Network, points out the importance of taking time for personal health and wellness. “When we work with professionals who have problems with mental health or substance abuse, they often don’t have a balanced life,” she says. “They are usually in a workaholic mindset and disregard their own needs.”
Few physicians seem to prioritize self-care, with a third indicating they “always” or “most of the time” spend enough time on their own health and wellness. But of those who do, males (38%) are more likely than females (27%) to spend enough time on their own health and wellness. Dr. Polles adds that exercising after a shift can help physicians better make the transition from professional to personal life. Though they did not report when they exercised, about a third of physicians reported doing so four or more times per week. Controlling weight is an issue as well, with 49% of male and 55% of female physicians saying they are currently trying to lose weight.
Of physicians who drink alcohol, about a third have three or more drinks per week. (The CDC defines “heavy drinking” as consuming 15 drinks or more per week for men and eight drinks or more per week for women.)
Of those surveyed, 92% say they do not regularly use cannabidiol or cannabis, and a mere 4% of respondents said they would use at least one of these substances if they were to become legal in their state.
A version of this article first appeared on Medscape.com.
Could stopping ‘thousand cuts’ by insurers and PBMs help rheumatology’s workforce shortage?
I am hearing more and more often from colleagues about the number of rheumatologists taking early retirement because of the frustration of having doctor-patient shared decision-making taken out of their hands and given to the insurance companies and their pharmacy benefit managers (PBMs). Often the right medication for the patient is not available on the formulary, causing unnecessary administrative barriers to providing care. When you put that together with the decreased reimbursement and the many obstacles to the “buy-and-bill” model, many rheumatologists have just had enough and called it quits earlier than they thought they would. This is a significant contributor to the growing workforce problem in rheumatology.
Many of the issues affecting the availability of medications happen throughout the development and distribution of a drug treatment – regulatory approval and obstacles to commercial launch, such as patent thickets, “pay for delay,” and other anticompetitive tactics by the manufacturers. And once a medication is launched on to the marketplace, rheumatologists are at the mercy of the health plans and PBMs as to whether, when, and even where a medication can be used. Here is where much of the frustration begins, amplified by the knowledge that profit for the PBM is the driving force behind formulary construction.
To support rheumatologists in addressing these challenges, the Coalition of State Rheumatology Organizations started a “Reporting Insurance/Payer Issues” page. Here, rheumatologists can describe issues or complaints they have with payers regarding patient care. The responses we’ve received so far always have a sense of urgency and frustration in the description of whatever obstacle to care is being thrown up by an insurance company or PBM.
One of the recent issues that has arisen via the CSRO’s reporting form involves a new policy for an insurance plan that removes the availability of the intravenous formulation of a medication if it has a subcutaneous (sub Q) formulation. It is a commercial version of the Medicare self-administered drug list, but worse. At least Medicare takes the time to look at the actual usage of a formulation before moving it from Part B to Part D. This new policy flatly states that no patients will have access to the IV formulation until the sub Q formulation has been tried. This includes switching all stable IV patients over to the sub Q formulation. Because the IV formulation is weight based, switching patients from IV to sub Q can reduce their dosage by more than 50%. It appears that loss of disease control is a small price to pay for increased PBM profits (called “savings” by the PBM). Notably, IV medications through physician “buy and bill” offer no revenue to the insurance company, while sub Q medications increase profits through rebates, fees, and other price concessions.
The CSRO outlined these concerns in its Jan. 18, 2022, response to the insurance company’s reply to the coalition’s original letter, urging them to value patients over profits. In this response, the CSRO addressed nonmedical switching, site of care cost, outcome documentation, and grandfathering stable patients, and finished with a discussion on ERISA (Employee Retirement Income Security Act of 1974) protections.
While our Reporting Insurance/Payer Issues form cannot handle reimbursement issues, there needs to be a word about money and profit when it comes to physicians. Physicians whose specialties have few to no procedures, including rheumatologists, rely on office visits and ancillary services such as infusion suites for income. That income sustains their practice and maintains all their attendant expenses. Many of the recent policies put forth by health plans not only intrude on the doctor-patient relationship in treatment decisions, but also reduce reimbursements and place obstacles to “buy and bill,” shifting revenue from the physician to the insurance company.
All these insurance/payer issues boil down to a version of “death by a thousand cuts.” These cuts harm patients and impede rheumatologists’ ability to sustain their practices. They are a type of moral injury (among the many we see in health care providers) that are causing rheumatologists to retire early. Clearly, these issues ultimately affect the workforce. We need advocacy on many levels if we have any hope of dulling the knives that are delivering these “cuts.”
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is President of the CSRO, past chair of the Alliance for Safe Biologic Medicines, and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
I am hearing more and more often from colleagues about the number of rheumatologists taking early retirement because of the frustration of having doctor-patient shared decision-making taken out of their hands and given to the insurance companies and their pharmacy benefit managers (PBMs). Often the right medication for the patient is not available on the formulary, causing unnecessary administrative barriers to providing care. When you put that together with the decreased reimbursement and the many obstacles to the “buy-and-bill” model, many rheumatologists have just had enough and called it quits earlier than they thought they would. This is a significant contributor to the growing workforce problem in rheumatology.
Many of the issues affecting the availability of medications happen throughout the development and distribution of a drug treatment – regulatory approval and obstacles to commercial launch, such as patent thickets, “pay for delay,” and other anticompetitive tactics by the manufacturers. And once a medication is launched on to the marketplace, rheumatologists are at the mercy of the health plans and PBMs as to whether, when, and even where a medication can be used. Here is where much of the frustration begins, amplified by the knowledge that profit for the PBM is the driving force behind formulary construction.
To support rheumatologists in addressing these challenges, the Coalition of State Rheumatology Organizations started a “Reporting Insurance/Payer Issues” page. Here, rheumatologists can describe issues or complaints they have with payers regarding patient care. The responses we’ve received so far always have a sense of urgency and frustration in the description of whatever obstacle to care is being thrown up by an insurance company or PBM.
One of the recent issues that has arisen via the CSRO’s reporting form involves a new policy for an insurance plan that removes the availability of the intravenous formulation of a medication if it has a subcutaneous (sub Q) formulation. It is a commercial version of the Medicare self-administered drug list, but worse. At least Medicare takes the time to look at the actual usage of a formulation before moving it from Part B to Part D. This new policy flatly states that no patients will have access to the IV formulation until the sub Q formulation has been tried. This includes switching all stable IV patients over to the sub Q formulation. Because the IV formulation is weight based, switching patients from IV to sub Q can reduce their dosage by more than 50%. It appears that loss of disease control is a small price to pay for increased PBM profits (called “savings” by the PBM). Notably, IV medications through physician “buy and bill” offer no revenue to the insurance company, while sub Q medications increase profits through rebates, fees, and other price concessions.
The CSRO outlined these concerns in its Jan. 18, 2022, response to the insurance company’s reply to the coalition’s original letter, urging them to value patients over profits. In this response, the CSRO addressed nonmedical switching, site of care cost, outcome documentation, and grandfathering stable patients, and finished with a discussion on ERISA (Employee Retirement Income Security Act of 1974) protections.
While our Reporting Insurance/Payer Issues form cannot handle reimbursement issues, there needs to be a word about money and profit when it comes to physicians. Physicians whose specialties have few to no procedures, including rheumatologists, rely on office visits and ancillary services such as infusion suites for income. That income sustains their practice and maintains all their attendant expenses. Many of the recent policies put forth by health plans not only intrude on the doctor-patient relationship in treatment decisions, but also reduce reimbursements and place obstacles to “buy and bill,” shifting revenue from the physician to the insurance company.
All these insurance/payer issues boil down to a version of “death by a thousand cuts.” These cuts harm patients and impede rheumatologists’ ability to sustain their practices. They are a type of moral injury (among the many we see in health care providers) that are causing rheumatologists to retire early. Clearly, these issues ultimately affect the workforce. We need advocacy on many levels if we have any hope of dulling the knives that are delivering these “cuts.”
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is President of the CSRO, past chair of the Alliance for Safe Biologic Medicines, and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
I am hearing more and more often from colleagues about the number of rheumatologists taking early retirement because of the frustration of having doctor-patient shared decision-making taken out of their hands and given to the insurance companies and their pharmacy benefit managers (PBMs). Often the right medication for the patient is not available on the formulary, causing unnecessary administrative barriers to providing care. When you put that together with the decreased reimbursement and the many obstacles to the “buy-and-bill” model, many rheumatologists have just had enough and called it quits earlier than they thought they would. This is a significant contributor to the growing workforce problem in rheumatology.
Many of the issues affecting the availability of medications happen throughout the development and distribution of a drug treatment – regulatory approval and obstacles to commercial launch, such as patent thickets, “pay for delay,” and other anticompetitive tactics by the manufacturers. And once a medication is launched on to the marketplace, rheumatologists are at the mercy of the health plans and PBMs as to whether, when, and even where a medication can be used. Here is where much of the frustration begins, amplified by the knowledge that profit for the PBM is the driving force behind formulary construction.
To support rheumatologists in addressing these challenges, the Coalition of State Rheumatology Organizations started a “Reporting Insurance/Payer Issues” page. Here, rheumatologists can describe issues or complaints they have with payers regarding patient care. The responses we’ve received so far always have a sense of urgency and frustration in the description of whatever obstacle to care is being thrown up by an insurance company or PBM.
One of the recent issues that has arisen via the CSRO’s reporting form involves a new policy for an insurance plan that removes the availability of the intravenous formulation of a medication if it has a subcutaneous (sub Q) formulation. It is a commercial version of the Medicare self-administered drug list, but worse. At least Medicare takes the time to look at the actual usage of a formulation before moving it from Part B to Part D. This new policy flatly states that no patients will have access to the IV formulation until the sub Q formulation has been tried. This includes switching all stable IV patients over to the sub Q formulation. Because the IV formulation is weight based, switching patients from IV to sub Q can reduce their dosage by more than 50%. It appears that loss of disease control is a small price to pay for increased PBM profits (called “savings” by the PBM). Notably, IV medications through physician “buy and bill” offer no revenue to the insurance company, while sub Q medications increase profits through rebates, fees, and other price concessions.
The CSRO outlined these concerns in its Jan. 18, 2022, response to the insurance company’s reply to the coalition’s original letter, urging them to value patients over profits. In this response, the CSRO addressed nonmedical switching, site of care cost, outcome documentation, and grandfathering stable patients, and finished with a discussion on ERISA (Employee Retirement Income Security Act of 1974) protections.
While our Reporting Insurance/Payer Issues form cannot handle reimbursement issues, there needs to be a word about money and profit when it comes to physicians. Physicians whose specialties have few to no procedures, including rheumatologists, rely on office visits and ancillary services such as infusion suites for income. That income sustains their practice and maintains all their attendant expenses. Many of the recent policies put forth by health plans not only intrude on the doctor-patient relationship in treatment decisions, but also reduce reimbursements and place obstacles to “buy and bill,” shifting revenue from the physician to the insurance company.
All these insurance/payer issues boil down to a version of “death by a thousand cuts.” These cuts harm patients and impede rheumatologists’ ability to sustain their practices. They are a type of moral injury (among the many we see in health care providers) that are causing rheumatologists to retire early. Clearly, these issues ultimately affect the workforce. We need advocacy on many levels if we have any hope of dulling the knives that are delivering these “cuts.”
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is President of the CSRO, past chair of the Alliance for Safe Biologic Medicines, and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
When the patient wants to speak to a manager
A patient swore at me the other day. Not as in “she used a curse word.” As in she spewed fury, spitting out a vulgar, adverbial word before “... terrible doctor” while jabbing her finger toward me. In my 15 years of practice, I’d never had that happen before. Equally surprising, I was not surprised by her outburst. The level of incivility from patients is at an all-time high.
Her anger was misdirected. She wanted me to write a letter to her employer excusing her from getting a vaccine. It was neither indicated nor ethical for me to do so. I did my best to redirect her, but without success. As our chief of service, I often help with service concerns and am happy to see patients who want another opinion or want to speak with the department head (aka, “the manager”). Usually I can help. Lately, it’s become harder.
Not only are such rude incidents more frequent, but they are also more dramatic and inappropriate. For example, I cannot imagine writing a complaint against a doctor stating that she must be a foreign medical grad (as it happens, she’s Ivy League-trained) or demanding money back when a biopsy result turned out to be benign, or threatening to report a doctor to the medical board because he failed to schedule a follow-up appointment (that doctor had been retired for months). Patients have hung up on our staff mid-sentence and slammed a clinic door when they left in a huff. Why are so many previously sensible people throwing childlike tantrums?
It’s the same phenomenon happening to our fellow service agents across all industries. The Federal Aviation Administration’s graph of unruly passenger incidents is a flat line from 1995 to 2019, then it goes straight vertical. A recent survey showed that Americans’ sense of civility is low and worse, that people’s expectations that civility will improve is going down. It’s palpable. Last month, I witnessed a man and woman screaming at each other over Christmas lights in a busy store. An army of aproned walkie-talkie staff surrounded them and escorted them out – their coordination and efficiency clearly indicated they’d done this before. Customers everywhere are mad, frustrated, disenfranchised. Lately, a lot of things just are not working out for them. Supplies are out. Kids are sent home from school. No elective surgery appointments are available. The insta-gratification they’ve grown accustomed to from Amazon and DoorDash is colliding with the reality that not everything works that way.
The word “patient’’ you’ll recall comes from the Latin “patior,” meaning to suffer or bear. With virus variants raging, inflation growing, and call center wait times approaching infinity, many of our patients, it seems, cannot bear any more. I’m confident this situation will improve and our patients will be more reasonable in their expectations, but I am afraid that, in the end, we’ll have lost some decorum and dignity that we may never find again in medicine.
For my potty-mouthed patient, I made an excuse to leave the room to get my dermatoscope and walked out. It gave her time to calm down. I returned in a few minutes to do a skin exam. As I was wrapping up, I advised her that she cannot raise her voice or use offensive language and that she should know that I and everyone in our office cares about her and wants to help. She did apologize for her behavior, but then had to add that, if I really cared, I’d write the letter for her.
I guess the customer is not always right.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
A patient swore at me the other day. Not as in “she used a curse word.” As in she spewed fury, spitting out a vulgar, adverbial word before “... terrible doctor” while jabbing her finger toward me. In my 15 years of practice, I’d never had that happen before. Equally surprising, I was not surprised by her outburst. The level of incivility from patients is at an all-time high.
Her anger was misdirected. She wanted me to write a letter to her employer excusing her from getting a vaccine. It was neither indicated nor ethical for me to do so. I did my best to redirect her, but without success. As our chief of service, I often help with service concerns and am happy to see patients who want another opinion or want to speak with the department head (aka, “the manager”). Usually I can help. Lately, it’s become harder.
Not only are such rude incidents more frequent, but they are also more dramatic and inappropriate. For example, I cannot imagine writing a complaint against a doctor stating that she must be a foreign medical grad (as it happens, she’s Ivy League-trained) or demanding money back when a biopsy result turned out to be benign, or threatening to report a doctor to the medical board because he failed to schedule a follow-up appointment (that doctor had been retired for months). Patients have hung up on our staff mid-sentence and slammed a clinic door when they left in a huff. Why are so many previously sensible people throwing childlike tantrums?
It’s the same phenomenon happening to our fellow service agents across all industries. The Federal Aviation Administration’s graph of unruly passenger incidents is a flat line from 1995 to 2019, then it goes straight vertical. A recent survey showed that Americans’ sense of civility is low and worse, that people’s expectations that civility will improve is going down. It’s palpable. Last month, I witnessed a man and woman screaming at each other over Christmas lights in a busy store. An army of aproned walkie-talkie staff surrounded them and escorted them out – their coordination and efficiency clearly indicated they’d done this before. Customers everywhere are mad, frustrated, disenfranchised. Lately, a lot of things just are not working out for them. Supplies are out. Kids are sent home from school. No elective surgery appointments are available. The insta-gratification they’ve grown accustomed to from Amazon and DoorDash is colliding with the reality that not everything works that way.
The word “patient’’ you’ll recall comes from the Latin “patior,” meaning to suffer or bear. With virus variants raging, inflation growing, and call center wait times approaching infinity, many of our patients, it seems, cannot bear any more. I’m confident this situation will improve and our patients will be more reasonable in their expectations, but I am afraid that, in the end, we’ll have lost some decorum and dignity that we may never find again in medicine.
For my potty-mouthed patient, I made an excuse to leave the room to get my dermatoscope and walked out. It gave her time to calm down. I returned in a few minutes to do a skin exam. As I was wrapping up, I advised her that she cannot raise her voice or use offensive language and that she should know that I and everyone in our office cares about her and wants to help. She did apologize for her behavior, but then had to add that, if I really cared, I’d write the letter for her.
I guess the customer is not always right.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
A patient swore at me the other day. Not as in “she used a curse word.” As in she spewed fury, spitting out a vulgar, adverbial word before “... terrible doctor” while jabbing her finger toward me. In my 15 years of practice, I’d never had that happen before. Equally surprising, I was not surprised by her outburst. The level of incivility from patients is at an all-time high.
Her anger was misdirected. She wanted me to write a letter to her employer excusing her from getting a vaccine. It was neither indicated nor ethical for me to do so. I did my best to redirect her, but without success. As our chief of service, I often help with service concerns and am happy to see patients who want another opinion or want to speak with the department head (aka, “the manager”). Usually I can help. Lately, it’s become harder.
Not only are such rude incidents more frequent, but they are also more dramatic and inappropriate. For example, I cannot imagine writing a complaint against a doctor stating that she must be a foreign medical grad (as it happens, she’s Ivy League-trained) or demanding money back when a biopsy result turned out to be benign, or threatening to report a doctor to the medical board because he failed to schedule a follow-up appointment (that doctor had been retired for months). Patients have hung up on our staff mid-sentence and slammed a clinic door when they left in a huff. Why are so many previously sensible people throwing childlike tantrums?
It’s the same phenomenon happening to our fellow service agents across all industries. The Federal Aviation Administration’s graph of unruly passenger incidents is a flat line from 1995 to 2019, then it goes straight vertical. A recent survey showed that Americans’ sense of civility is low and worse, that people’s expectations that civility will improve is going down. It’s palpable. Last month, I witnessed a man and woman screaming at each other over Christmas lights in a busy store. An army of aproned walkie-talkie staff surrounded them and escorted them out – their coordination and efficiency clearly indicated they’d done this before. Customers everywhere are mad, frustrated, disenfranchised. Lately, a lot of things just are not working out for them. Supplies are out. Kids are sent home from school. No elective surgery appointments are available. The insta-gratification they’ve grown accustomed to from Amazon and DoorDash is colliding with the reality that not everything works that way.
The word “patient’’ you’ll recall comes from the Latin “patior,” meaning to suffer or bear. With virus variants raging, inflation growing, and call center wait times approaching infinity, many of our patients, it seems, cannot bear any more. I’m confident this situation will improve and our patients will be more reasonable in their expectations, but I am afraid that, in the end, we’ll have lost some decorum and dignity that we may never find again in medicine.
For my potty-mouthed patient, I made an excuse to leave the room to get my dermatoscope and walked out. It gave her time to calm down. I returned in a few minutes to do a skin exam. As I was wrapping up, I advised her that she cannot raise her voice or use offensive language and that she should know that I and everyone in our office cares about her and wants to help. She did apologize for her behavior, but then had to add that, if I really cared, I’d write the letter for her.
I guess the customer is not always right.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Study finds genetic factor for COVID smell and taste loss
, according to a new study published in the journal Nature Genetics
The finding could eventually help the 1.6 million people in the United States who still can’t smell or have had a change in their ability to smell more than 6 months after getting the coronavirus. The exact cause related to COVID-19 is still unknown, but researchers believe it could be because of damage in a part of the nose called the olfactory epithelium.
“How we get from infection to smell loss remains unclear,” Justin Turner, MD, an associate professor of otolaryngology at Vanderbilt University, Nashville, Tenn., told NBC News. Dr. Turner was not part of the research team.
“Early data suggest that supporting cells of the olfactory epithelium are the ones mostly being infected by the virus, and presumably this leads to the death of the neurons themselves,” he said. “But we don’t really, really know why and when that happens, and why it seems to preferentially happen in certain individuals.”
Researchers at 23andMe, a genomics and biotechnology company, did the study as part of a larger COVID-19 project, which includes people in the United States and the United Kingdom. They analyzed data from nearly 70,000 people who took online surveys after receiving a positive coronavirus test. Among those, 68% reported a loss of smell or taste as a symptom.
The study team compared the genetic differences between those who lost their sense of smell and taste and those who didn’t. They found that a location near two olfactory genes – UGT2A1 and UGT2A2 – is associated with COVID-19 loss of smell and taste. The genetic risk factor makes it 11% more likely for a person with COVID-19 to lose their sense of smell or taste.
The research team also found that women were 11% more likely than men to report a loss of smell and taste. About 73% of those who reported a loss of smell and taste were ages 26-35.
The researchers aren’t sure how the genes are involved, though they suspect that infected cells could lead to smell loss. Typically, the genes are expressed in tissue inside the nose involved with smell and play a role in processing things that have an odor. To use the findings, researchers need to learn more about the genes, how they are expressed, and what their functions are, NBC News reported.
The findings could help lead to treatments. Other research has shown that the loss of taste and smell is related to a “failure to protect the sensory cells of the nose and tongue from viral infection,” Danielle Reed, PhD, associate director of the Monell Chemical Senses Center in Philadelphia, told NBC News. She was not part of the research team but studies person-to-person differences in the loss of these senses because of COVID-19.
“This study suggests a different direction,” she said. “The pathways that break down the chemicals that cause taste and smell in the first place might be over or underactive, reducing or distorting the ability to taste and smell.”
A version of this article first appeared on WebMD.com.
, according to a new study published in the journal Nature Genetics
The finding could eventually help the 1.6 million people in the United States who still can’t smell or have had a change in their ability to smell more than 6 months after getting the coronavirus. The exact cause related to COVID-19 is still unknown, but researchers believe it could be because of damage in a part of the nose called the olfactory epithelium.
“How we get from infection to smell loss remains unclear,” Justin Turner, MD, an associate professor of otolaryngology at Vanderbilt University, Nashville, Tenn., told NBC News. Dr. Turner was not part of the research team.
“Early data suggest that supporting cells of the olfactory epithelium are the ones mostly being infected by the virus, and presumably this leads to the death of the neurons themselves,” he said. “But we don’t really, really know why and when that happens, and why it seems to preferentially happen in certain individuals.”
Researchers at 23andMe, a genomics and biotechnology company, did the study as part of a larger COVID-19 project, which includes people in the United States and the United Kingdom. They analyzed data from nearly 70,000 people who took online surveys after receiving a positive coronavirus test. Among those, 68% reported a loss of smell or taste as a symptom.
The study team compared the genetic differences between those who lost their sense of smell and taste and those who didn’t. They found that a location near two olfactory genes – UGT2A1 and UGT2A2 – is associated with COVID-19 loss of smell and taste. The genetic risk factor makes it 11% more likely for a person with COVID-19 to lose their sense of smell or taste.
The research team also found that women were 11% more likely than men to report a loss of smell and taste. About 73% of those who reported a loss of smell and taste were ages 26-35.
The researchers aren’t sure how the genes are involved, though they suspect that infected cells could lead to smell loss. Typically, the genes are expressed in tissue inside the nose involved with smell and play a role in processing things that have an odor. To use the findings, researchers need to learn more about the genes, how they are expressed, and what their functions are, NBC News reported.
The findings could help lead to treatments. Other research has shown that the loss of taste and smell is related to a “failure to protect the sensory cells of the nose and tongue from viral infection,” Danielle Reed, PhD, associate director of the Monell Chemical Senses Center in Philadelphia, told NBC News. She was not part of the research team but studies person-to-person differences in the loss of these senses because of COVID-19.
“This study suggests a different direction,” she said. “The pathways that break down the chemicals that cause taste and smell in the first place might be over or underactive, reducing or distorting the ability to taste and smell.”
A version of this article first appeared on WebMD.com.
, according to a new study published in the journal Nature Genetics
The finding could eventually help the 1.6 million people in the United States who still can’t smell or have had a change in their ability to smell more than 6 months after getting the coronavirus. The exact cause related to COVID-19 is still unknown, but researchers believe it could be because of damage in a part of the nose called the olfactory epithelium.
“How we get from infection to smell loss remains unclear,” Justin Turner, MD, an associate professor of otolaryngology at Vanderbilt University, Nashville, Tenn., told NBC News. Dr. Turner was not part of the research team.
“Early data suggest that supporting cells of the olfactory epithelium are the ones mostly being infected by the virus, and presumably this leads to the death of the neurons themselves,” he said. “But we don’t really, really know why and when that happens, and why it seems to preferentially happen in certain individuals.”
Researchers at 23andMe, a genomics and biotechnology company, did the study as part of a larger COVID-19 project, which includes people in the United States and the United Kingdom. They analyzed data from nearly 70,000 people who took online surveys after receiving a positive coronavirus test. Among those, 68% reported a loss of smell or taste as a symptom.
The study team compared the genetic differences between those who lost their sense of smell and taste and those who didn’t. They found that a location near two olfactory genes – UGT2A1 and UGT2A2 – is associated with COVID-19 loss of smell and taste. The genetic risk factor makes it 11% more likely for a person with COVID-19 to lose their sense of smell or taste.
The research team also found that women were 11% more likely than men to report a loss of smell and taste. About 73% of those who reported a loss of smell and taste were ages 26-35.
The researchers aren’t sure how the genes are involved, though they suspect that infected cells could lead to smell loss. Typically, the genes are expressed in tissue inside the nose involved with smell and play a role in processing things that have an odor. To use the findings, researchers need to learn more about the genes, how they are expressed, and what their functions are, NBC News reported.
The findings could help lead to treatments. Other research has shown that the loss of taste and smell is related to a “failure to protect the sensory cells of the nose and tongue from viral infection,” Danielle Reed, PhD, associate director of the Monell Chemical Senses Center in Philadelphia, told NBC News. She was not part of the research team but studies person-to-person differences in the loss of these senses because of COVID-19.
“This study suggests a different direction,” she said. “The pathways that break down the chemicals that cause taste and smell in the first place might be over or underactive, reducing or distorting the ability to taste and smell.”
A version of this article first appeared on WebMD.com.
FROM NATURE GENETICS













