User login
Children and COVID: New cases rise to winter levels
Weekly cases of COVID-19 in children topped 100,000 for the first time since early February, according to the American Academy of Pediatrics and the Children’s Hospital Association.
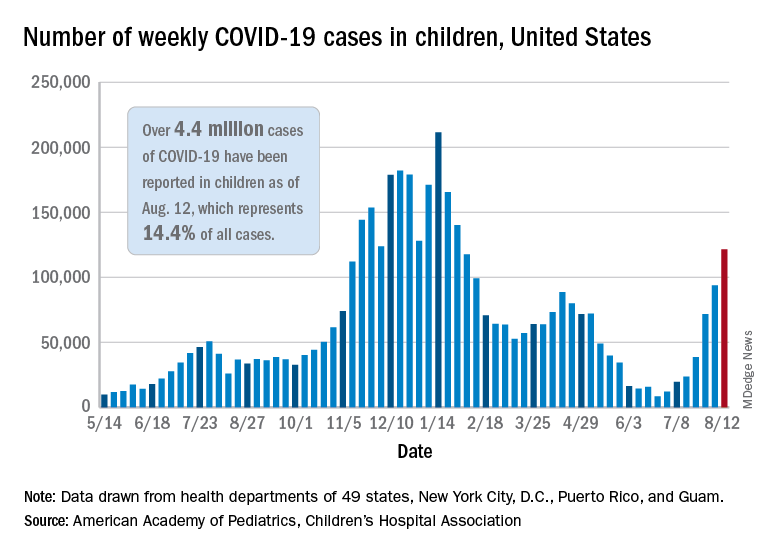
the AAP and CHA said in their weekly COVD-19 report. The recent surge in child COVID has also brought a record high in hospitalizations and shortages of pediatric ICU beds in some areas.
The 121,000 new cases represent an increase of almost 1,400% since June 18-24, when the weekly tally was just 8,447 and at its lowest point in over a year, the AAP/CHA data show.
On the vaccination front in the last week (Aug. 10-16), vaccine initiation for 12- to 17-year-olds was fairly robust but still down slightly, compared with the previous week. Just over 402,000 children aged 12-15 years received a first vaccination, which was down slightly from 411,000 the week before but still higher than any of the 6 weeks from June 22 to Aug. 2, based on data from the Centers for Disease Control and Prevention. Vaccinations were down by a similar margin for 15- to-17-year-olds.
Over 10.9 million children aged 12-17 have had at least one dose of COVID-19 vaccine administered, of whom 8.1 million are fully vaccinated. Among those aged 12-15 years, 44.5% have gotten at least one dose and 31.8% are fully vaccinated, with corresponding figures of 53.9% and 42.5% for 16- and 17-year-olds, according to the CDC’s COVID Data Tracker.
The number of COVID-19 cases reported in children since the start of the pandemic is up to 4.4 million, which makes up 14.4% of all cases in the United States, the AAP and CHA said. Other cumulative figures through Aug. 12 include almost 18,000 hospitalizations – reported by 23 states and New York City – and 378 deaths – reported by 43 states, New York City, Puerto Rico, and Guam.
In the latest edition of their ongoing report, compiled using state data since the summer of 2020, the two groups noted that, “in the summer of 2021, some states have revised cases counts previously reported, begun reporting less frequently, or dropped metrics previously reported.” Among those states are Nebraska, which shut down its online COVID dashboard in late June, and Alabama, which stopped reporting cumulative cases and deaths after July 29.
Weekly cases of COVID-19 in children topped 100,000 for the first time since early February, according to the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and CHA said in their weekly COVD-19 report. The recent surge in child COVID has also brought a record high in hospitalizations and shortages of pediatric ICU beds in some areas.
The 121,000 new cases represent an increase of almost 1,400% since June 18-24, when the weekly tally was just 8,447 and at its lowest point in over a year, the AAP/CHA data show.
On the vaccination front in the last week (Aug. 10-16), vaccine initiation for 12- to 17-year-olds was fairly robust but still down slightly, compared with the previous week. Just over 402,000 children aged 12-15 years received a first vaccination, which was down slightly from 411,000 the week before but still higher than any of the 6 weeks from June 22 to Aug. 2, based on data from the Centers for Disease Control and Prevention. Vaccinations were down by a similar margin for 15- to-17-year-olds.
Over 10.9 million children aged 12-17 have had at least one dose of COVID-19 vaccine administered, of whom 8.1 million are fully vaccinated. Among those aged 12-15 years, 44.5% have gotten at least one dose and 31.8% are fully vaccinated, with corresponding figures of 53.9% and 42.5% for 16- and 17-year-olds, according to the CDC’s COVID Data Tracker.
The number of COVID-19 cases reported in children since the start of the pandemic is up to 4.4 million, which makes up 14.4% of all cases in the United States, the AAP and CHA said. Other cumulative figures through Aug. 12 include almost 18,000 hospitalizations – reported by 23 states and New York City – and 378 deaths – reported by 43 states, New York City, Puerto Rico, and Guam.
In the latest edition of their ongoing report, compiled using state data since the summer of 2020, the two groups noted that, “in the summer of 2021, some states have revised cases counts previously reported, begun reporting less frequently, or dropped metrics previously reported.” Among those states are Nebraska, which shut down its online COVID dashboard in late June, and Alabama, which stopped reporting cumulative cases and deaths after July 29.
Weekly cases of COVID-19 in children topped 100,000 for the first time since early February, according to the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and CHA said in their weekly COVD-19 report. The recent surge in child COVID has also brought a record high in hospitalizations and shortages of pediatric ICU beds in some areas.
The 121,000 new cases represent an increase of almost 1,400% since June 18-24, when the weekly tally was just 8,447 and at its lowest point in over a year, the AAP/CHA data show.
On the vaccination front in the last week (Aug. 10-16), vaccine initiation for 12- to 17-year-olds was fairly robust but still down slightly, compared with the previous week. Just over 402,000 children aged 12-15 years received a first vaccination, which was down slightly from 411,000 the week before but still higher than any of the 6 weeks from June 22 to Aug. 2, based on data from the Centers for Disease Control and Prevention. Vaccinations were down by a similar margin for 15- to-17-year-olds.
Over 10.9 million children aged 12-17 have had at least one dose of COVID-19 vaccine administered, of whom 8.1 million are fully vaccinated. Among those aged 12-15 years, 44.5% have gotten at least one dose and 31.8% are fully vaccinated, with corresponding figures of 53.9% and 42.5% for 16- and 17-year-olds, according to the CDC’s COVID Data Tracker.
The number of COVID-19 cases reported in children since the start of the pandemic is up to 4.4 million, which makes up 14.4% of all cases in the United States, the AAP and CHA said. Other cumulative figures through Aug. 12 include almost 18,000 hospitalizations – reported by 23 states and New York City – and 378 deaths – reported by 43 states, New York City, Puerto Rico, and Guam.
In the latest edition of their ongoing report, compiled using state data since the summer of 2020, the two groups noted that, “in the summer of 2021, some states have revised cases counts previously reported, begun reporting less frequently, or dropped metrics previously reported.” Among those states are Nebraska, which shut down its online COVID dashboard in late June, and Alabama, which stopped reporting cumulative cases and deaths after July 29.
COVID-19 hospitalizations for 30- to 39-year-olds hit record high
Hospitals are reporting record numbers of COVID-19 patients in their 30s, largely because of the contagious Delta variant, according to The Wall Street Journal.
The rate of new hospitalizations for ages 30-39 reached 2.5 per 100,000 people last week, according to the latest CDC data, which is up from the previous peak of 2 per 100,000 people in January.
What’s more, new hospital admissions for patients in their 30s reached an average of 1,113 a day during the last week, which was up from 908 the week before.
“It means Delta is really bad,” James Lawler, MD, an infectious disease doctor and codirector of the Global Center for Health Security at the University of Nebraska Medical Center, told the newspaper.
People in the age group mostly avoided hospitalization throughout the pandemic because of their relatively good health and young age, the newspaper reported. But in recent weeks, those between ages 30 and 39 are contracting the coronavirus because of their active lifestyle – for many in their 30s, these are prime years for working, parenting, and socializing.
Hospitalizations are mostly among unvaccinated adults, according to the Wall Street Journal. Nationally, less than half of those ages 25-39 are fully vaccinated, compared with 61% of all adults, according to CDC data updated Sunday.
“It loves social mobility,” James Fiorica, MD, chief medical officer of Sarasota Memorial Health Care System in Florida, told the newspaper.
“An unvaccinated 30-year-old can be a perfect carrier,” he said.
On top of that, COVID-19 patients in their 30s are arriving at hospitals with more severe disease than in earlier waves, the Journal reported. At the University of Arkansas for Medical Sciences hospital, for instance, doctors are now monitoring younger patients daily with a scoring system for possible organ failure. That wasn’t necessary earlier in the pandemic for people in their 30s.
“This age group pretty much went unscathed,” Nikhil Meena, MD, director of the hospital’s Medical Intensive Care Unit, told the newspaper.
Now, he said, “they’re all out there doing their thing and getting infected and getting sick enough to be in this hospital.”
A version of this article first appeared on WebMD.com.
Hospitals are reporting record numbers of COVID-19 patients in their 30s, largely because of the contagious Delta variant, according to The Wall Street Journal.
The rate of new hospitalizations for ages 30-39 reached 2.5 per 100,000 people last week, according to the latest CDC data, which is up from the previous peak of 2 per 100,000 people in January.
What’s more, new hospital admissions for patients in their 30s reached an average of 1,113 a day during the last week, which was up from 908 the week before.
“It means Delta is really bad,” James Lawler, MD, an infectious disease doctor and codirector of the Global Center for Health Security at the University of Nebraska Medical Center, told the newspaper.
People in the age group mostly avoided hospitalization throughout the pandemic because of their relatively good health and young age, the newspaper reported. But in recent weeks, those between ages 30 and 39 are contracting the coronavirus because of their active lifestyle – for many in their 30s, these are prime years for working, parenting, and socializing.
Hospitalizations are mostly among unvaccinated adults, according to the Wall Street Journal. Nationally, less than half of those ages 25-39 are fully vaccinated, compared with 61% of all adults, according to CDC data updated Sunday.
“It loves social mobility,” James Fiorica, MD, chief medical officer of Sarasota Memorial Health Care System in Florida, told the newspaper.
“An unvaccinated 30-year-old can be a perfect carrier,” he said.
On top of that, COVID-19 patients in their 30s are arriving at hospitals with more severe disease than in earlier waves, the Journal reported. At the University of Arkansas for Medical Sciences hospital, for instance, doctors are now monitoring younger patients daily with a scoring system for possible organ failure. That wasn’t necessary earlier in the pandemic for people in their 30s.
“This age group pretty much went unscathed,” Nikhil Meena, MD, director of the hospital’s Medical Intensive Care Unit, told the newspaper.
Now, he said, “they’re all out there doing their thing and getting infected and getting sick enough to be in this hospital.”
A version of this article first appeared on WebMD.com.
Hospitals are reporting record numbers of COVID-19 patients in their 30s, largely because of the contagious Delta variant, according to The Wall Street Journal.
The rate of new hospitalizations for ages 30-39 reached 2.5 per 100,000 people last week, according to the latest CDC data, which is up from the previous peak of 2 per 100,000 people in January.
What’s more, new hospital admissions for patients in their 30s reached an average of 1,113 a day during the last week, which was up from 908 the week before.
“It means Delta is really bad,” James Lawler, MD, an infectious disease doctor and codirector of the Global Center for Health Security at the University of Nebraska Medical Center, told the newspaper.
People in the age group mostly avoided hospitalization throughout the pandemic because of their relatively good health and young age, the newspaper reported. But in recent weeks, those between ages 30 and 39 are contracting the coronavirus because of their active lifestyle – for many in their 30s, these are prime years for working, parenting, and socializing.
Hospitalizations are mostly among unvaccinated adults, according to the Wall Street Journal. Nationally, less than half of those ages 25-39 are fully vaccinated, compared with 61% of all adults, according to CDC data updated Sunday.
“It loves social mobility,” James Fiorica, MD, chief medical officer of Sarasota Memorial Health Care System in Florida, told the newspaper.
“An unvaccinated 30-year-old can be a perfect carrier,” he said.
On top of that, COVID-19 patients in their 30s are arriving at hospitals with more severe disease than in earlier waves, the Journal reported. At the University of Arkansas for Medical Sciences hospital, for instance, doctors are now monitoring younger patients daily with a scoring system for possible organ failure. That wasn’t necessary earlier in the pandemic for people in their 30s.
“This age group pretty much went unscathed,” Nikhil Meena, MD, director of the hospital’s Medical Intensive Care Unit, told the newspaper.
Now, he said, “they’re all out there doing their thing and getting infected and getting sick enough to be in this hospital.”
A version of this article first appeared on WebMD.com.
Youngest children more likely to spread SARS-CoV-2 to family: Study
Young children are more likely than are their older siblings to transmit SARS-CoV-2 in their households, according to an analysis of public health records in Ontario, Canada – a finding that upends the common belief that children play a minimal role in COVID-19 spread.
The study by researchers from Public Health Ontario, published online in JAMA Pediatrics, found that teenagers (14- to 17-year-olds) were more likely than were their younger siblings to bring the virus into the household, while infants and toddlers (up to age 3) were about 43% more likely than were the older teens to spread it to others in the home.
Children or teens were the source of SARS-CoV-2 in about 1 in 13 Ontario households between June and December 2020, the study shows. The researchers analyzed health records from 6,280 households with a pediatric COVID-19 case and a subset of 1,717 households in which a child up to age 17 was the source of transmission in a household.
When analyzing the data, the researchers controlled for gender differences, month of disease onset, testing delay, and mean family size.
The role of young children in transmission seemed logical to some experts who have been tracking the evolution of the pandemic. “I think what was more surprising was how long the narrative persisted that children weren’t transmitting SARS-CoV-2,” said Samuel Scarpino, PhD, managing director of pathogen surveillance at the Rockefeller Foundation.
Meanwhile, less mask-wearing, the return to school and activities, and the onslaught of the Delta variant have changed the dynamics of spread, said Andrew Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah.
“Adolescents and high-school-aged kids have had much, much higher rates of infection in the past,” he said. “Now when we look at the rates of school-aged kids, they are the same as high-school-aged kids, and we’re seeing more and more in the preschool age groups.”
Cases may be underestimated
If anything, the study may underestimate the role young children play in spreading COVID-19 in families, since it included only symptomatic cases as the initial source and young children are more likely to be asymptomatic, Dr. Pavia said.
The Delta variant heightens the concern; it is more than twice as infectious as previous strains and has spurred a rise in pediatric cases, including some coinfection with other circulating respiratory diseases, such as respiratory syncytial virus (RSV).
The Ontario study covers a period before vaccination and the spread of the Delta variant. “As the number of pediatric cases increases worldwide, the role of children in household transmission will continue to grow,” the authors concluded.
Following recommended respiratory hygiene is clearly more difficult with very young children. For example, parents, caregivers, and older siblings aren’t going to stay 6 feet away from a sick baby or toddler, Susan Coffin, MD, MPH, a pediatric infectious disease physician, and David Rubin, MD, a pediatrician and director of PolicyLab at Children’s Hospital of Philadelphia, noted in an accompanying commentary.
“Cuddling and touching are part and parcel of taking care of a sick young child, and that will obviously come with an increased risk of transmission to parents as well as to older siblings who may be helping to care for their sick brother or sister,” they wrote.
While parents may wash their hands more frequently when caring for a sick child, they aren’t likely to wear a mask, said William Schaffner, MD, an infectious disease specialist at Vanderbilt University, Nashville, Tenn.
“I imagine some moms even take a sick child into bed with them,” he said. “It’s probably just the extensive contact one has with a sick, very small child that augments their capacity to transmit this infection.”
What can be done
What can be done, then, to reduce the household spread of COVID-19? “The obvious solution to protect a household with a sick young infant or toddler is to make sure that all eligible members of the household are vaccinated,” Dr. Coffin and Dr. Rubin stated in their commentary.
The American Academy of Pediatrics recently wrote to Janet Woodcock, MD, acting commissioner of the Food and Drug Administration, asking for the agency to authorize use of SARS-CoV-2 vaccines for children under age 12 “as soon as possible,” noting that “the Delta variant has created a new and pressing risk to children and adolescents across this country, as it has also done for unvaccinated adults.”
The FDA reportedly asked vaccine makers Pfizer and Moderna to expand the clinical trials of children, which may delay authorization for younger age groups. Pfizer has said it plans to submit a request for emergency use authorization of its vaccine for 5- to 11-year-olds in September or October.
As with adult vaccination, hesitancy is likely to be a barrier. Less than half of parents said they are very or somewhat likely to have their children get a COVID-19 vaccine, according to a national survey conducted by researchers at the University of California, Los Angeles.
The Ontario study provides valuable evidence to support taking steps to protect children from transmission in schools, including mask requirements, frequent testing, and improved ventilation, said Dr. Scarpino.
“We’re not going to be able to control COVID without vaccinating younger individuals,” he said.
Dr. Pavia has consulted for GlaxoSmithKline on non–COVID-19–related issues. Sarah Buchan, PhD, study author and scientist at Public Health Ontario, reported grants from the Canadian Institutes of Health Research for research on influenza, RSV, and COVID-19, and grants from the Canadian Immunity Task Force for COVID-19 outside the submitted work. Dr. Coffin reported grants as a Centers for Disease Control and Prevention coinvestigator at a Vaccine and Treatment Evaluation Unit site conducting COVID-19 vaccine trials in children. Dr. Scarpino holds unexercised options in ILiAD Biotechnologies, which is focused on the prevention and treatment of pertussis. Dr. Schaffner is a consultant for VBI Vaccines.
A version of this article first appeared on Medscape.com.
Young children are more likely than are their older siblings to transmit SARS-CoV-2 in their households, according to an analysis of public health records in Ontario, Canada – a finding that upends the common belief that children play a minimal role in COVID-19 spread.
The study by researchers from Public Health Ontario, published online in JAMA Pediatrics, found that teenagers (14- to 17-year-olds) were more likely than were their younger siblings to bring the virus into the household, while infants and toddlers (up to age 3) were about 43% more likely than were the older teens to spread it to others in the home.
Children or teens were the source of SARS-CoV-2 in about 1 in 13 Ontario households between June and December 2020, the study shows. The researchers analyzed health records from 6,280 households with a pediatric COVID-19 case and a subset of 1,717 households in which a child up to age 17 was the source of transmission in a household.
When analyzing the data, the researchers controlled for gender differences, month of disease onset, testing delay, and mean family size.
The role of young children in transmission seemed logical to some experts who have been tracking the evolution of the pandemic. “I think what was more surprising was how long the narrative persisted that children weren’t transmitting SARS-CoV-2,” said Samuel Scarpino, PhD, managing director of pathogen surveillance at the Rockefeller Foundation.
Meanwhile, less mask-wearing, the return to school and activities, and the onslaught of the Delta variant have changed the dynamics of spread, said Andrew Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah.
“Adolescents and high-school-aged kids have had much, much higher rates of infection in the past,” he said. “Now when we look at the rates of school-aged kids, they are the same as high-school-aged kids, and we’re seeing more and more in the preschool age groups.”
Cases may be underestimated
If anything, the study may underestimate the role young children play in spreading COVID-19 in families, since it included only symptomatic cases as the initial source and young children are more likely to be asymptomatic, Dr. Pavia said.
The Delta variant heightens the concern; it is more than twice as infectious as previous strains and has spurred a rise in pediatric cases, including some coinfection with other circulating respiratory diseases, such as respiratory syncytial virus (RSV).
The Ontario study covers a period before vaccination and the spread of the Delta variant. “As the number of pediatric cases increases worldwide, the role of children in household transmission will continue to grow,” the authors concluded.
Following recommended respiratory hygiene is clearly more difficult with very young children. For example, parents, caregivers, and older siblings aren’t going to stay 6 feet away from a sick baby or toddler, Susan Coffin, MD, MPH, a pediatric infectious disease physician, and David Rubin, MD, a pediatrician and director of PolicyLab at Children’s Hospital of Philadelphia, noted in an accompanying commentary.
“Cuddling and touching are part and parcel of taking care of a sick young child, and that will obviously come with an increased risk of transmission to parents as well as to older siblings who may be helping to care for their sick brother or sister,” they wrote.
While parents may wash their hands more frequently when caring for a sick child, they aren’t likely to wear a mask, said William Schaffner, MD, an infectious disease specialist at Vanderbilt University, Nashville, Tenn.
“I imagine some moms even take a sick child into bed with them,” he said. “It’s probably just the extensive contact one has with a sick, very small child that augments their capacity to transmit this infection.”
What can be done
What can be done, then, to reduce the household spread of COVID-19? “The obvious solution to protect a household with a sick young infant or toddler is to make sure that all eligible members of the household are vaccinated,” Dr. Coffin and Dr. Rubin stated in their commentary.
The American Academy of Pediatrics recently wrote to Janet Woodcock, MD, acting commissioner of the Food and Drug Administration, asking for the agency to authorize use of SARS-CoV-2 vaccines for children under age 12 “as soon as possible,” noting that “the Delta variant has created a new and pressing risk to children and adolescents across this country, as it has also done for unvaccinated adults.”
The FDA reportedly asked vaccine makers Pfizer and Moderna to expand the clinical trials of children, which may delay authorization for younger age groups. Pfizer has said it plans to submit a request for emergency use authorization of its vaccine for 5- to 11-year-olds in September or October.
As with adult vaccination, hesitancy is likely to be a barrier. Less than half of parents said they are very or somewhat likely to have their children get a COVID-19 vaccine, according to a national survey conducted by researchers at the University of California, Los Angeles.
The Ontario study provides valuable evidence to support taking steps to protect children from transmission in schools, including mask requirements, frequent testing, and improved ventilation, said Dr. Scarpino.
“We’re not going to be able to control COVID without vaccinating younger individuals,” he said.
Dr. Pavia has consulted for GlaxoSmithKline on non–COVID-19–related issues. Sarah Buchan, PhD, study author and scientist at Public Health Ontario, reported grants from the Canadian Institutes of Health Research for research on influenza, RSV, and COVID-19, and grants from the Canadian Immunity Task Force for COVID-19 outside the submitted work. Dr. Coffin reported grants as a Centers for Disease Control and Prevention coinvestigator at a Vaccine and Treatment Evaluation Unit site conducting COVID-19 vaccine trials in children. Dr. Scarpino holds unexercised options in ILiAD Biotechnologies, which is focused on the prevention and treatment of pertussis. Dr. Schaffner is a consultant for VBI Vaccines.
A version of this article first appeared on Medscape.com.
Young children are more likely than are their older siblings to transmit SARS-CoV-2 in their households, according to an analysis of public health records in Ontario, Canada – a finding that upends the common belief that children play a minimal role in COVID-19 spread.
The study by researchers from Public Health Ontario, published online in JAMA Pediatrics, found that teenagers (14- to 17-year-olds) were more likely than were their younger siblings to bring the virus into the household, while infants and toddlers (up to age 3) were about 43% more likely than were the older teens to spread it to others in the home.
Children or teens were the source of SARS-CoV-2 in about 1 in 13 Ontario households between June and December 2020, the study shows. The researchers analyzed health records from 6,280 households with a pediatric COVID-19 case and a subset of 1,717 households in which a child up to age 17 was the source of transmission in a household.
When analyzing the data, the researchers controlled for gender differences, month of disease onset, testing delay, and mean family size.
The role of young children in transmission seemed logical to some experts who have been tracking the evolution of the pandemic. “I think what was more surprising was how long the narrative persisted that children weren’t transmitting SARS-CoV-2,” said Samuel Scarpino, PhD, managing director of pathogen surveillance at the Rockefeller Foundation.
Meanwhile, less mask-wearing, the return to school and activities, and the onslaught of the Delta variant have changed the dynamics of spread, said Andrew Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah.
“Adolescents and high-school-aged kids have had much, much higher rates of infection in the past,” he said. “Now when we look at the rates of school-aged kids, they are the same as high-school-aged kids, and we’re seeing more and more in the preschool age groups.”
Cases may be underestimated
If anything, the study may underestimate the role young children play in spreading COVID-19 in families, since it included only symptomatic cases as the initial source and young children are more likely to be asymptomatic, Dr. Pavia said.
The Delta variant heightens the concern; it is more than twice as infectious as previous strains and has spurred a rise in pediatric cases, including some coinfection with other circulating respiratory diseases, such as respiratory syncytial virus (RSV).
The Ontario study covers a period before vaccination and the spread of the Delta variant. “As the number of pediatric cases increases worldwide, the role of children in household transmission will continue to grow,” the authors concluded.
Following recommended respiratory hygiene is clearly more difficult with very young children. For example, parents, caregivers, and older siblings aren’t going to stay 6 feet away from a sick baby or toddler, Susan Coffin, MD, MPH, a pediatric infectious disease physician, and David Rubin, MD, a pediatrician and director of PolicyLab at Children’s Hospital of Philadelphia, noted in an accompanying commentary.
“Cuddling and touching are part and parcel of taking care of a sick young child, and that will obviously come with an increased risk of transmission to parents as well as to older siblings who may be helping to care for their sick brother or sister,” they wrote.
While parents may wash their hands more frequently when caring for a sick child, they aren’t likely to wear a mask, said William Schaffner, MD, an infectious disease specialist at Vanderbilt University, Nashville, Tenn.
“I imagine some moms even take a sick child into bed with them,” he said. “It’s probably just the extensive contact one has with a sick, very small child that augments their capacity to transmit this infection.”
What can be done
What can be done, then, to reduce the household spread of COVID-19? “The obvious solution to protect a household with a sick young infant or toddler is to make sure that all eligible members of the household are vaccinated,” Dr. Coffin and Dr. Rubin stated in their commentary.
The American Academy of Pediatrics recently wrote to Janet Woodcock, MD, acting commissioner of the Food and Drug Administration, asking for the agency to authorize use of SARS-CoV-2 vaccines for children under age 12 “as soon as possible,” noting that “the Delta variant has created a new and pressing risk to children and adolescents across this country, as it has also done for unvaccinated adults.”
The FDA reportedly asked vaccine makers Pfizer and Moderna to expand the clinical trials of children, which may delay authorization for younger age groups. Pfizer has said it plans to submit a request for emergency use authorization of its vaccine for 5- to 11-year-olds in September or October.
As with adult vaccination, hesitancy is likely to be a barrier. Less than half of parents said they are very or somewhat likely to have their children get a COVID-19 vaccine, according to a national survey conducted by researchers at the University of California, Los Angeles.
The Ontario study provides valuable evidence to support taking steps to protect children from transmission in schools, including mask requirements, frequent testing, and improved ventilation, said Dr. Scarpino.
“We’re not going to be able to control COVID without vaccinating younger individuals,” he said.
Dr. Pavia has consulted for GlaxoSmithKline on non–COVID-19–related issues. Sarah Buchan, PhD, study author and scientist at Public Health Ontario, reported grants from the Canadian Institutes of Health Research for research on influenza, RSV, and COVID-19, and grants from the Canadian Immunity Task Force for COVID-19 outside the submitted work. Dr. Coffin reported grants as a Centers for Disease Control and Prevention coinvestigator at a Vaccine and Treatment Evaluation Unit site conducting COVID-19 vaccine trials in children. Dr. Scarpino holds unexercised options in ILiAD Biotechnologies, which is focused on the prevention and treatment of pertussis. Dr. Schaffner is a consultant for VBI Vaccines.
A version of this article first appeared on Medscape.com.
U.S. reports record COVID-19 hospitalizations of children
The number of children hospitalized with COVID-19 in the U.S. hit a record high on Aug. 14, with more than 1,900 in hospitals.
Hospitals across the South are running out of beds as the contagious Delta variant spreads, mostly among unvaccinated people. Children make up about 2.4% of the country’s COVID-19 hospitalizations, and those under 12 are particularly vulnerable since they’re not eligible to receive a vaccine.
“This is not last year’s COVID,” Sally Goza, MD, former president of the American Academy of Pediatrics, told CNN on Aug. 14.
“This one is worse, and our children are the ones that are going to be affected by it the most,” she said.
The number of newly hospitalized COVID-19 patients for ages 18-49 also hit record highs during the week of Aug. 9. A fifth of the nation’s hospitalizations are in Florida, where the number of COVID-19 patients hit a record high of 16,100 on Aug. 14. More than 90% of the state’s intensive care unit beds are filled.
More than 90% of the ICU beds in Texas are full as well. On Aug. 13, there were no pediatric ICU beds available in Dallas or the 19 surrounding counties, which means that young patients would be transported father away for care – even Oklahoma City.
“That means if your child’s in a car wreck, if your child has a congenital heart defect or something and needs an ICU bed, or more likely, if they have COVID and need an ICU bed, we don’t have one,” Clay Jenkins, a Dallas County judge, said on Aug. 13.
“Your child will wait for another child to die,” he said.
As children return to classes, educators are talking about the possibility of vaccine mandates. The National Education Association announced its support of mandatory vaccination for its members.
“Our students under 12 can’t get vaccinated,” Becky Pringle, president of the association, told CNN.
“It’s our responsibility to keep them safe,” she said. “Keeping them safe means that everyone who can be vaccinated should be vaccinated.”
The U.S. now has an average of about 129,000 new COVID-19 cases per day, Reuters reported, which has doubled in about 2 weeks. The number of hospitalized patients is at a 6-month high, and about 600 people are dying each day.
Arkansas, Florida, Louisiana, Mississippi, and Oregon have reported record numbers of COVID-19 hospitalizations.
In addition, eight states make up half of all the COVID-19 hospitalizations in the U.S. but only 24% of the nation’s population – Alabama, Arkansas, Florida, Georgia, Louisiana, Mississippi, Nevada, and Texas. These states have vaccination rates lower than the national average, and their COVID-19 patients account for at least 15% of their overall hospitalizations.
To address the surge in hospitalizations, Oregon Gov. Kate Brown has ordered the deployment of up to 1,500 Oregon National Guard members to help health care workers.
“I know this is not the summer many of us envisioned,” Gov. Brown said Aug. 13. “The harsh and frustrating reality is that the Delta variant has changed everything. Delta is highly contagious, and we must take action now.”
A version of this article first appeared on WebMD.com.
The number of children hospitalized with COVID-19 in the U.S. hit a record high on Aug. 14, with more than 1,900 in hospitals.
Hospitals across the South are running out of beds as the contagious Delta variant spreads, mostly among unvaccinated people. Children make up about 2.4% of the country’s COVID-19 hospitalizations, and those under 12 are particularly vulnerable since they’re not eligible to receive a vaccine.
“This is not last year’s COVID,” Sally Goza, MD, former president of the American Academy of Pediatrics, told CNN on Aug. 14.
“This one is worse, and our children are the ones that are going to be affected by it the most,” she said.
The number of newly hospitalized COVID-19 patients for ages 18-49 also hit record highs during the week of Aug. 9. A fifth of the nation’s hospitalizations are in Florida, where the number of COVID-19 patients hit a record high of 16,100 on Aug. 14. More than 90% of the state’s intensive care unit beds are filled.
More than 90% of the ICU beds in Texas are full as well. On Aug. 13, there were no pediatric ICU beds available in Dallas or the 19 surrounding counties, which means that young patients would be transported father away for care – even Oklahoma City.
“That means if your child’s in a car wreck, if your child has a congenital heart defect or something and needs an ICU bed, or more likely, if they have COVID and need an ICU bed, we don’t have one,” Clay Jenkins, a Dallas County judge, said on Aug. 13.
“Your child will wait for another child to die,” he said.
As children return to classes, educators are talking about the possibility of vaccine mandates. The National Education Association announced its support of mandatory vaccination for its members.
“Our students under 12 can’t get vaccinated,” Becky Pringle, president of the association, told CNN.
“It’s our responsibility to keep them safe,” she said. “Keeping them safe means that everyone who can be vaccinated should be vaccinated.”
The U.S. now has an average of about 129,000 new COVID-19 cases per day, Reuters reported, which has doubled in about 2 weeks. The number of hospitalized patients is at a 6-month high, and about 600 people are dying each day.
Arkansas, Florida, Louisiana, Mississippi, and Oregon have reported record numbers of COVID-19 hospitalizations.
In addition, eight states make up half of all the COVID-19 hospitalizations in the U.S. but only 24% of the nation’s population – Alabama, Arkansas, Florida, Georgia, Louisiana, Mississippi, Nevada, and Texas. These states have vaccination rates lower than the national average, and their COVID-19 patients account for at least 15% of their overall hospitalizations.
To address the surge in hospitalizations, Oregon Gov. Kate Brown has ordered the deployment of up to 1,500 Oregon National Guard members to help health care workers.
“I know this is not the summer many of us envisioned,” Gov. Brown said Aug. 13. “The harsh and frustrating reality is that the Delta variant has changed everything. Delta is highly contagious, and we must take action now.”
A version of this article first appeared on WebMD.com.
The number of children hospitalized with COVID-19 in the U.S. hit a record high on Aug. 14, with more than 1,900 in hospitals.
Hospitals across the South are running out of beds as the contagious Delta variant spreads, mostly among unvaccinated people. Children make up about 2.4% of the country’s COVID-19 hospitalizations, and those under 12 are particularly vulnerable since they’re not eligible to receive a vaccine.
“This is not last year’s COVID,” Sally Goza, MD, former president of the American Academy of Pediatrics, told CNN on Aug. 14.
“This one is worse, and our children are the ones that are going to be affected by it the most,” she said.
The number of newly hospitalized COVID-19 patients for ages 18-49 also hit record highs during the week of Aug. 9. A fifth of the nation’s hospitalizations are in Florida, where the number of COVID-19 patients hit a record high of 16,100 on Aug. 14. More than 90% of the state’s intensive care unit beds are filled.
More than 90% of the ICU beds in Texas are full as well. On Aug. 13, there were no pediatric ICU beds available in Dallas or the 19 surrounding counties, which means that young patients would be transported father away for care – even Oklahoma City.
“That means if your child’s in a car wreck, if your child has a congenital heart defect or something and needs an ICU bed, or more likely, if they have COVID and need an ICU bed, we don’t have one,” Clay Jenkins, a Dallas County judge, said on Aug. 13.
“Your child will wait for another child to die,” he said.
As children return to classes, educators are talking about the possibility of vaccine mandates. The National Education Association announced its support of mandatory vaccination for its members.
“Our students under 12 can’t get vaccinated,” Becky Pringle, president of the association, told CNN.
“It’s our responsibility to keep them safe,” she said. “Keeping them safe means that everyone who can be vaccinated should be vaccinated.”
The U.S. now has an average of about 129,000 new COVID-19 cases per day, Reuters reported, which has doubled in about 2 weeks. The number of hospitalized patients is at a 6-month high, and about 600 people are dying each day.
Arkansas, Florida, Louisiana, Mississippi, and Oregon have reported record numbers of COVID-19 hospitalizations.
In addition, eight states make up half of all the COVID-19 hospitalizations in the U.S. but only 24% of the nation’s population – Alabama, Arkansas, Florida, Georgia, Louisiana, Mississippi, Nevada, and Texas. These states have vaccination rates lower than the national average, and their COVID-19 patients account for at least 15% of their overall hospitalizations.
To address the surge in hospitalizations, Oregon Gov. Kate Brown has ordered the deployment of up to 1,500 Oregon National Guard members to help health care workers.
“I know this is not the summer many of us envisioned,” Gov. Brown said Aug. 13. “The harsh and frustrating reality is that the Delta variant has changed everything. Delta is highly contagious, and we must take action now.”
A version of this article first appeared on WebMD.com.
CDC officially endorses third dose of mRNA vaccines for immunocompromised
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, has officially signed off on a recommendation by an independent panel of 11 experts to allow people with weakened immune function to get a third dose of certain COVID-19 vaccines.
The decision follows a unanimous vote by the CDC’s Advisory Committee on Immunization Practices (ACIP), which in turn came hours after the U.S. Food and Drug Administration updated its Emergency Use Authorization (EUA) for the Pfizer and Moderna mRNA vaccines.
About 7 million adults in the United States have moderately to severely impaired immune function because of a medical condition they live with or a medication they take to manage a health condition.
People who fall into this category are at higher risk of being hospitalized or dying if they get COVID-19. They are also more likely to transmit the infection. About 40% of vaccinated patients who are hospitalized with breakthrough cases are immunocompromised.
Recent studies have shown that between one-third and one-half of immunocompromised people who didn’t develop antibodies after two doses of a vaccine do get some level of protection after a third dose.
Even then, however, the protection immunocompromised people get from vaccines is not as robust as someone who has healthy immune function, and some panel members were concerned that a third dose might come with a false sense of security.
“My only concern with adding a third dose for the immunocompromised is the impression that our immunocompromised population [will] then be safe,” said ACIP member Helen Talbot, MD, MPH, an associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn.
“I think the reality is they’ll be safer but still at incredibly high risk for severe disease and death,” she said.
In updating its EUA, the FDA stressed that, even after a third dose, people who are immunocompromised will still need to wear a mask indoors, socially distance, and avoid large crowds. In addition, family members and other close contacts should be fully vaccinated to protect these vulnerable individuals.
Johnson & Johnson not in the mix
The boosters will be available to children as young as 12 years of age who’ve had a Pfizer vaccine or those ages 18 and older who’ve gotten the Moderna vaccine.
For now, people who’ve had the one-dose Johnson & Johnson vaccine have not been cleared to get a second dose of any vaccine.
FDA experts acknowledged the gap but said that people who had received the Johnson & Johnson vaccine represented a small slice of vaccinated Americans, and said they couldn’t act before the FDA had updated its authorization for that vaccine, which the agency is actively exploring.
“We had to do what we’re doing based on the data we have in hand,” said Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the FDA, the division of the agency that regulates vaccines.
“We think at least there is a solution here for the very large majority of immunocompromised individuals, and we believe we will probably have a solution for the remainder in the not-too-distant future,” Dr. Marks said.
In its updated EUA, the FDA said that the third shots were intended for people who had undergone solid organ transplants or have an “equivalent level of immunocompromise.”
The details
Clinical experts on the CDC panel spent a good deal of time trying to suss out exactly what conditions might fall under the FDA’s umbrella for a third dose.
In a presentation to the committee, Neela Goswami, MD, PhD, an assistant professor of infectious diseases at Emory University School of Medicine and of epidemiology at the Emory Rollins School of Public Health, Atlanta, stressed that the shots are intended for patients who are moderately or severely immunocompromised, in close consultation with their doctors, but that people who should qualify would include those:
- Receiving treatment for solid tumors or blood cancers
- Taking immunosuppressing medications after a solid organ transplant
- Within 2 years of receiving CAR-T therapy or a stem cell transplant
- Who have primary immunodeficiencies – rare genetic disorders that prevent the immune system from working properly
- With advanced or untreated
- Taking high-dose corticosteroids (more than 20 milligrams of or its equivalent daily), alkylating agents, antimetabolites, chemotherapy, TNF blockers, or other immunomodulating or immunosuppressing biologics
- With certain chronic medical conditions, such as or asplenia – living without a spleen
- Receiving dialysis
In discussion, CDC experts clarified that these third doses were not intended for people whose immune function had waned with age, such as elderly residents of long-term care facilities or people with chronic diseases like diabetes.
The idea is to try to get a third dose of the vaccine they’ve already had – Moderna or Pfizer – but if that’s not feasible, it’s fine for the third dose to be different from what someone has had before. The third dose should be given at least 28 days after a second dose, and, ideally, before the initiation of immunosuppressive therapy.
Participants in the meeting said that the CDC would post updated materials on its website to help guide physicians on exactly who should receive third doses.
Ultimately, however, the extra doses will be given on an honor system; no prescriptions or other kinds of clinical documentation will be required for people to get a third dose of these shots.
Tests to measure neutralizing antibodies are also not recommended before the shots are given because of differences in the types of tests used to measure these antibodies and the difficulty in interpreting them. It’s unclear right now what level of neutralizing antibodies is needed for protection.
‘Peace of mind’
In public testimony, Heather Braaten, a 44-year-old being treated for ovarian cancer, said she was grateful to have gotten two shots of the Pfizer vaccine last winter, in between rounds of chemotherapy, but she knew she was probably not well protected. She said she’d become obsessive over the past few months reading medical studies and trying to understand her risk.
“I have felt distraught over the situation. My prognosis is poor. I most likely have about two to three years left to live, so everything counts,” Ms. Braaten said.
She said her life ambitions were humble. She wants to visit with friends and family and not have to worry that she’ll be a breakthrough case. She wants to go grocery shopping again and “not panic and leave the store after five minutes.” She’d love to feel free to travel, she said.
“While I understand I still need to be cautious, I am hopeful for the peace of mind and greater freedom a third shot can provide,” Ms. Braaten said.
More boosters on the way?
In the second half of the meeting, the CDC also signaled that it was considering the use of boosters for people whose immunity might have waned in the months since they had completed their vaccine series, particularly seniors. About 75% of people hospitalized with vaccine breakthrough cases are over age 65, according to CDC data.
Those considerations are becoming more urgent as the Delta variant continues to pummel less vaccinated states and counties.
In its presentation to the ACIP, Heather Scobie, PhD, MPH, a member of the CDC’s COVID Response Team, highlighted data from Canada, Israel, Qatar, and the United Kingdom showing that, while the Pfizer vaccine was still highly effective at preventing hospitalizations and death, it’s far less likely when faced with Delta to prevent an infection that causes symptoms.
In Israel, Pfizer’s vaccine prevented symptoms an average of 41% of the time. In Qatar, which is also using the Moderna vaccine, Pfizer’s prevented symptomatic infections with Delta about 54% of the time compared with 85% with Moderna’s.
Dr. Scobie noted that Pfizer’s waning efficacy may have something to do with the fact that it uses a lower dosage than Moderna’s. Pfizer’s recommended dosing interval is also shorter – 3 weeks compared with 4 weeks for Moderna’s. Stretching the time between shots has been shown to boost vaccine effectiveness, she said.
New data from the Mayo clinic, published ahead of peer review, also suggest that Pfizer’s protection may be fading more quickly than Moderna’s.
In February, both shots were nearly 100% effective at preventing the SARS-CoV-2 infection, but by July, against Delta, Pfizer’s efficacy had dropped to somewhere between 13% and 62%, while Moderna’s was still effective at preventing infection between 58% and 87% of the time.
In July, Pfizer’s was between 24% and 94% effective at preventing hospitalization with a COVID-19 infection and Moderna’s was between 33% and 96% effective at preventing hospitalization.
While that may sound like cause for concern, Dr. Scobie noted that, as of August 2, severe COVD-19 outcomes after vaccination are still very rare. Among 164 million fully vaccinated people in the United States there have been about 7,000 hospitalizations and 1,500 deaths; nearly three out of four of these have been in people over the age of 65.
The ACIP will next meet on August 24 to focus solely on the COVID-19 vaccines.
A version of this article first appeared on Medscape.com.
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, has officially signed off on a recommendation by an independent panel of 11 experts to allow people with weakened immune function to get a third dose of certain COVID-19 vaccines.
The decision follows a unanimous vote by the CDC’s Advisory Committee on Immunization Practices (ACIP), which in turn came hours after the U.S. Food and Drug Administration updated its Emergency Use Authorization (EUA) for the Pfizer and Moderna mRNA vaccines.
About 7 million adults in the United States have moderately to severely impaired immune function because of a medical condition they live with or a medication they take to manage a health condition.
People who fall into this category are at higher risk of being hospitalized or dying if they get COVID-19. They are also more likely to transmit the infection. About 40% of vaccinated patients who are hospitalized with breakthrough cases are immunocompromised.
Recent studies have shown that between one-third and one-half of immunocompromised people who didn’t develop antibodies after two doses of a vaccine do get some level of protection after a third dose.
Even then, however, the protection immunocompromised people get from vaccines is not as robust as someone who has healthy immune function, and some panel members were concerned that a third dose might come with a false sense of security.
“My only concern with adding a third dose for the immunocompromised is the impression that our immunocompromised population [will] then be safe,” said ACIP member Helen Talbot, MD, MPH, an associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn.
“I think the reality is they’ll be safer but still at incredibly high risk for severe disease and death,” she said.
In updating its EUA, the FDA stressed that, even after a third dose, people who are immunocompromised will still need to wear a mask indoors, socially distance, and avoid large crowds. In addition, family members and other close contacts should be fully vaccinated to protect these vulnerable individuals.
Johnson & Johnson not in the mix
The boosters will be available to children as young as 12 years of age who’ve had a Pfizer vaccine or those ages 18 and older who’ve gotten the Moderna vaccine.
For now, people who’ve had the one-dose Johnson & Johnson vaccine have not been cleared to get a second dose of any vaccine.
FDA experts acknowledged the gap but said that people who had received the Johnson & Johnson vaccine represented a small slice of vaccinated Americans, and said they couldn’t act before the FDA had updated its authorization for that vaccine, which the agency is actively exploring.
“We had to do what we’re doing based on the data we have in hand,” said Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the FDA, the division of the agency that regulates vaccines.
“We think at least there is a solution here for the very large majority of immunocompromised individuals, and we believe we will probably have a solution for the remainder in the not-too-distant future,” Dr. Marks said.
In its updated EUA, the FDA said that the third shots were intended for people who had undergone solid organ transplants or have an “equivalent level of immunocompromise.”
The details
Clinical experts on the CDC panel spent a good deal of time trying to suss out exactly what conditions might fall under the FDA’s umbrella for a third dose.
In a presentation to the committee, Neela Goswami, MD, PhD, an assistant professor of infectious diseases at Emory University School of Medicine and of epidemiology at the Emory Rollins School of Public Health, Atlanta, stressed that the shots are intended for patients who are moderately or severely immunocompromised, in close consultation with their doctors, but that people who should qualify would include those:
- Receiving treatment for solid tumors or blood cancers
- Taking immunosuppressing medications after a solid organ transplant
- Within 2 years of receiving CAR-T therapy or a stem cell transplant
- Who have primary immunodeficiencies – rare genetic disorders that prevent the immune system from working properly
- With advanced or untreated
- Taking high-dose corticosteroids (more than 20 milligrams of or its equivalent daily), alkylating agents, antimetabolites, chemotherapy, TNF blockers, or other immunomodulating or immunosuppressing biologics
- With certain chronic medical conditions, such as or asplenia – living without a spleen
- Receiving dialysis
In discussion, CDC experts clarified that these third doses were not intended for people whose immune function had waned with age, such as elderly residents of long-term care facilities or people with chronic diseases like diabetes.
The idea is to try to get a third dose of the vaccine they’ve already had – Moderna or Pfizer – but if that’s not feasible, it’s fine for the third dose to be different from what someone has had before. The third dose should be given at least 28 days after a second dose, and, ideally, before the initiation of immunosuppressive therapy.
Participants in the meeting said that the CDC would post updated materials on its website to help guide physicians on exactly who should receive third doses.
Ultimately, however, the extra doses will be given on an honor system; no prescriptions or other kinds of clinical documentation will be required for people to get a third dose of these shots.
Tests to measure neutralizing antibodies are also not recommended before the shots are given because of differences in the types of tests used to measure these antibodies and the difficulty in interpreting them. It’s unclear right now what level of neutralizing antibodies is needed for protection.
‘Peace of mind’
In public testimony, Heather Braaten, a 44-year-old being treated for ovarian cancer, said she was grateful to have gotten two shots of the Pfizer vaccine last winter, in between rounds of chemotherapy, but she knew she was probably not well protected. She said she’d become obsessive over the past few months reading medical studies and trying to understand her risk.
“I have felt distraught over the situation. My prognosis is poor. I most likely have about two to three years left to live, so everything counts,” Ms. Braaten said.
She said her life ambitions were humble. She wants to visit with friends and family and not have to worry that she’ll be a breakthrough case. She wants to go grocery shopping again and “not panic and leave the store after five minutes.” She’d love to feel free to travel, she said.
“While I understand I still need to be cautious, I am hopeful for the peace of mind and greater freedom a third shot can provide,” Ms. Braaten said.
More boosters on the way?
In the second half of the meeting, the CDC also signaled that it was considering the use of boosters for people whose immunity might have waned in the months since they had completed their vaccine series, particularly seniors. About 75% of people hospitalized with vaccine breakthrough cases are over age 65, according to CDC data.
Those considerations are becoming more urgent as the Delta variant continues to pummel less vaccinated states and counties.
In its presentation to the ACIP, Heather Scobie, PhD, MPH, a member of the CDC’s COVID Response Team, highlighted data from Canada, Israel, Qatar, and the United Kingdom showing that, while the Pfizer vaccine was still highly effective at preventing hospitalizations and death, it’s far less likely when faced with Delta to prevent an infection that causes symptoms.
In Israel, Pfizer’s vaccine prevented symptoms an average of 41% of the time. In Qatar, which is also using the Moderna vaccine, Pfizer’s prevented symptomatic infections with Delta about 54% of the time compared with 85% with Moderna’s.
Dr. Scobie noted that Pfizer’s waning efficacy may have something to do with the fact that it uses a lower dosage than Moderna’s. Pfizer’s recommended dosing interval is also shorter – 3 weeks compared with 4 weeks for Moderna’s. Stretching the time between shots has been shown to boost vaccine effectiveness, she said.
New data from the Mayo clinic, published ahead of peer review, also suggest that Pfizer’s protection may be fading more quickly than Moderna’s.
In February, both shots were nearly 100% effective at preventing the SARS-CoV-2 infection, but by July, against Delta, Pfizer’s efficacy had dropped to somewhere between 13% and 62%, while Moderna’s was still effective at preventing infection between 58% and 87% of the time.
In July, Pfizer’s was between 24% and 94% effective at preventing hospitalization with a COVID-19 infection and Moderna’s was between 33% and 96% effective at preventing hospitalization.
While that may sound like cause for concern, Dr. Scobie noted that, as of August 2, severe COVD-19 outcomes after vaccination are still very rare. Among 164 million fully vaccinated people in the United States there have been about 7,000 hospitalizations and 1,500 deaths; nearly three out of four of these have been in people over the age of 65.
The ACIP will next meet on August 24 to focus solely on the COVID-19 vaccines.
A version of this article first appeared on Medscape.com.
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, has officially signed off on a recommendation by an independent panel of 11 experts to allow people with weakened immune function to get a third dose of certain COVID-19 vaccines.
The decision follows a unanimous vote by the CDC’s Advisory Committee on Immunization Practices (ACIP), which in turn came hours after the U.S. Food and Drug Administration updated its Emergency Use Authorization (EUA) for the Pfizer and Moderna mRNA vaccines.
About 7 million adults in the United States have moderately to severely impaired immune function because of a medical condition they live with or a medication they take to manage a health condition.
People who fall into this category are at higher risk of being hospitalized or dying if they get COVID-19. They are also more likely to transmit the infection. About 40% of vaccinated patients who are hospitalized with breakthrough cases are immunocompromised.
Recent studies have shown that between one-third and one-half of immunocompromised people who didn’t develop antibodies after two doses of a vaccine do get some level of protection after a third dose.
Even then, however, the protection immunocompromised people get from vaccines is not as robust as someone who has healthy immune function, and some panel members were concerned that a third dose might come with a false sense of security.
“My only concern with adding a third dose for the immunocompromised is the impression that our immunocompromised population [will] then be safe,” said ACIP member Helen Talbot, MD, MPH, an associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn.
“I think the reality is they’ll be safer but still at incredibly high risk for severe disease and death,” she said.
In updating its EUA, the FDA stressed that, even after a third dose, people who are immunocompromised will still need to wear a mask indoors, socially distance, and avoid large crowds. In addition, family members and other close contacts should be fully vaccinated to protect these vulnerable individuals.
Johnson & Johnson not in the mix
The boosters will be available to children as young as 12 years of age who’ve had a Pfizer vaccine or those ages 18 and older who’ve gotten the Moderna vaccine.
For now, people who’ve had the one-dose Johnson & Johnson vaccine have not been cleared to get a second dose of any vaccine.
FDA experts acknowledged the gap but said that people who had received the Johnson & Johnson vaccine represented a small slice of vaccinated Americans, and said they couldn’t act before the FDA had updated its authorization for that vaccine, which the agency is actively exploring.
“We had to do what we’re doing based on the data we have in hand,” said Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the FDA, the division of the agency that regulates vaccines.
“We think at least there is a solution here for the very large majority of immunocompromised individuals, and we believe we will probably have a solution for the remainder in the not-too-distant future,” Dr. Marks said.
In its updated EUA, the FDA said that the third shots were intended for people who had undergone solid organ transplants or have an “equivalent level of immunocompromise.”
The details
Clinical experts on the CDC panel spent a good deal of time trying to suss out exactly what conditions might fall under the FDA’s umbrella for a third dose.
In a presentation to the committee, Neela Goswami, MD, PhD, an assistant professor of infectious diseases at Emory University School of Medicine and of epidemiology at the Emory Rollins School of Public Health, Atlanta, stressed that the shots are intended for patients who are moderately or severely immunocompromised, in close consultation with their doctors, but that people who should qualify would include those:
- Receiving treatment for solid tumors or blood cancers
- Taking immunosuppressing medications after a solid organ transplant
- Within 2 years of receiving CAR-T therapy or a stem cell transplant
- Who have primary immunodeficiencies – rare genetic disorders that prevent the immune system from working properly
- With advanced or untreated
- Taking high-dose corticosteroids (more than 20 milligrams of or its equivalent daily), alkylating agents, antimetabolites, chemotherapy, TNF blockers, or other immunomodulating or immunosuppressing biologics
- With certain chronic medical conditions, such as or asplenia – living without a spleen
- Receiving dialysis
In discussion, CDC experts clarified that these third doses were not intended for people whose immune function had waned with age, such as elderly residents of long-term care facilities or people with chronic diseases like diabetes.
The idea is to try to get a third dose of the vaccine they’ve already had – Moderna or Pfizer – but if that’s not feasible, it’s fine for the third dose to be different from what someone has had before. The third dose should be given at least 28 days after a second dose, and, ideally, before the initiation of immunosuppressive therapy.
Participants in the meeting said that the CDC would post updated materials on its website to help guide physicians on exactly who should receive third doses.
Ultimately, however, the extra doses will be given on an honor system; no prescriptions or other kinds of clinical documentation will be required for people to get a third dose of these shots.
Tests to measure neutralizing antibodies are also not recommended before the shots are given because of differences in the types of tests used to measure these antibodies and the difficulty in interpreting them. It’s unclear right now what level of neutralizing antibodies is needed for protection.
‘Peace of mind’
In public testimony, Heather Braaten, a 44-year-old being treated for ovarian cancer, said she was grateful to have gotten two shots of the Pfizer vaccine last winter, in between rounds of chemotherapy, but she knew she was probably not well protected. She said she’d become obsessive over the past few months reading medical studies and trying to understand her risk.
“I have felt distraught over the situation. My prognosis is poor. I most likely have about two to three years left to live, so everything counts,” Ms. Braaten said.
She said her life ambitions were humble. She wants to visit with friends and family and not have to worry that she’ll be a breakthrough case. She wants to go grocery shopping again and “not panic and leave the store after five minutes.” She’d love to feel free to travel, she said.
“While I understand I still need to be cautious, I am hopeful for the peace of mind and greater freedom a third shot can provide,” Ms. Braaten said.
More boosters on the way?
In the second half of the meeting, the CDC also signaled that it was considering the use of boosters for people whose immunity might have waned in the months since they had completed their vaccine series, particularly seniors. About 75% of people hospitalized with vaccine breakthrough cases are over age 65, according to CDC data.
Those considerations are becoming more urgent as the Delta variant continues to pummel less vaccinated states and counties.
In its presentation to the ACIP, Heather Scobie, PhD, MPH, a member of the CDC’s COVID Response Team, highlighted data from Canada, Israel, Qatar, and the United Kingdom showing that, while the Pfizer vaccine was still highly effective at preventing hospitalizations and death, it’s far less likely when faced with Delta to prevent an infection that causes symptoms.
In Israel, Pfizer’s vaccine prevented symptoms an average of 41% of the time. In Qatar, which is also using the Moderna vaccine, Pfizer’s prevented symptomatic infections with Delta about 54% of the time compared with 85% with Moderna’s.
Dr. Scobie noted that Pfizer’s waning efficacy may have something to do with the fact that it uses a lower dosage than Moderna’s. Pfizer’s recommended dosing interval is also shorter – 3 weeks compared with 4 weeks for Moderna’s. Stretching the time between shots has been shown to boost vaccine effectiveness, she said.
New data from the Mayo clinic, published ahead of peer review, also suggest that Pfizer’s protection may be fading more quickly than Moderna’s.
In February, both shots were nearly 100% effective at preventing the SARS-CoV-2 infection, but by July, against Delta, Pfizer’s efficacy had dropped to somewhere between 13% and 62%, while Moderna’s was still effective at preventing infection between 58% and 87% of the time.
In July, Pfizer’s was between 24% and 94% effective at preventing hospitalization with a COVID-19 infection and Moderna’s was between 33% and 96% effective at preventing hospitalization.
While that may sound like cause for concern, Dr. Scobie noted that, as of August 2, severe COVD-19 outcomes after vaccination are still very rare. Among 164 million fully vaccinated people in the United States there have been about 7,000 hospitalizations and 1,500 deaths; nearly three out of four of these have been in people over the age of 65.
The ACIP will next meet on August 24 to focus solely on the COVID-19 vaccines.
A version of this article first appeared on Medscape.com.
Heparin’s COVID-19 benefit greatest in moderately ill patients
Critically ill derive no benefit
Therapeutic levels of heparin can have widely varying effects on COVID-19 patients depending on the severity of their disease, according to a multiplatform clinical trial that analyzed patient data from three international trials.
COVID-19 patients in the ICU, or at least receiving ICU-level care, derived no benefit from anticoagulation with heparin, while non–critically ill COVID-19 patients – those who were hospitalized but not receiving ICU-level care – on the same anticoagulation were less likely to progress to need respiratory or cardiovascular organ support despite a slightly heightened risk of bleeding events.
Reporting in two articles published online in the New England Journal of Medicine, authors of three international trials combined their data into one multiplatform trial that makes a strong case for prescribing therapeutic levels of heparin in hospitalized patients not receiving ICU-level care were non–critically ill and critically ill.
“I think this is going to be a game changer,” said Jeffrey S. Berger, MD, ACTIV-4a co–principal investigator and co–first author of the study of non–critically ill patients. “I think that using therapeutic-dose anticoagulation should improve outcomes in the tens of thousands of patients worldwide. I hope our data can have a global impact.”
Outcomes based on disease severity
The multiplatform trial analyzed data from the Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC); A Multicenter, Adaptive, Randomized Controlled Platform Trial of the Safety and Efficacy of Antithrombotic Strategies in Hospitalized Adults with COVID-19 (ACTIV-4a); and Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP).
The trial evaluated 2,219 non–critically ill hospitalized patients, 1,181 of whom were randomized to therapeutic-dose anticoagulation; and 1,098 critically ill patients, 534 of whom were prescribed therapeutic levels of heparin.
In the critically ill patients, those on heparin were no more likely to get discharged or spend fewer days on respiratory or CV organ support – oxygen, mechanical ventilation, life support, vasopressors or inotropes – than were those on usual-care thromboprophylaxis. The investigators stopped the trial in both patient populations: in critically ill patients when it became obvious therapeutic-dose anticoagulation was having no impact; and in moderately ill patients when the trial met the prespecified criteria for the superiority of therapeutic-dose anticoagulation.
ICU patients on therapeutic-level heparin spent an average of 1 day free of organ support vs. 4 for patients on usual-care prophylactic antithrombotic drugs. The percentage of patients who survived to hospital discharge was similar in the therapeutic-level and usual-care critically ill patients: 62.7% and 64.5%, respectively. Major bleeding occurred in 3.8% and 2.8%, respectively. Demographic and clinical characteristics were similar between both patient groups.
However, in non–critically ill patients, therapeutic levels of heparin resulted in a marked improvement in outcomes. The researchers estimated that, for every 1,000 hospitalized patients with what they labeled moderate disease, an initial treatment with therapeutic-dose heparin resulted in 40 additional patients surviving compared to usual-care thromboprophylaxis.
The percentages of patients not needing organ support before hospital discharge was 80.2% on therapeutic-dose heparin and 76.4% on usual-care therapy. In terms of adjusted odds ratio, the anticoagulation group had a 27% improved chance of not needing daily organ support.
Those improvements came with an additional seven major bleeding events per 1,000 patients. That broke down to a rate of 1.9% in the therapeutic-dose and 0.9% in the usual-care patients.
As the Delta variant of COVID-19 spreads, Patrick R. Lawler, MD, MPH, principal investigator of the ATTACC trial, said there’s no reason these findings shouldn’t apply for all variants of the disease.
Dr. Lawler, a physician-scientist at Peter Munk Cardiac Centre at Toronto General Hospital, noted that the multiplatform study did not account for disease variant. “Ongoing clinical trials are tracking the variant patients have or the variants that are most prevalent in an area at that time,” he said. “It may be easier in future trials to look at that question.”
Explaining heparin’s varying effects
The study did not specifically sort out why moderately ill patients fared better on heparin than their critically ill counterparts, but Dr. Lawler speculated on possible reasons. “One might be that the extent of illness severity is too extreme in the ICU-level population for heparin to have a beneficial extent,” he said.
He acknowledged that higher rates of macrovascular thrombosis, such as venous thromboembolism, in ICU patients would suggest that heparin would have a greater beneficial effect, but, he added, “it may also suggest how advanced that process is, and perhaps heparin is not adequate to reverse the course at that point given relatively extensive thrombosis and associate organ failure.”
As clinicians have gained experience dealing with COVID-19, they’ve learned that infected patients carry a high burden of macro- and microthrombosis, Dr. Berger said, which may explain why critically ill patients didn’t respond as well to therapeutic levels of heparin. “I think the cat is out of the bag; patients who are severe are too ill to benefit,” he said. “I would think there’s too much microthrombosis that is already in their bodies.”
However, this doesn’t completely rule out therapeutic levels of heparin in critically ill COVID-19 patients. There are some scenarios where it’s needed, said Dr. Berger, associate professor of medicine and surgery and director of the Center for the Prevention of Cardiovascular Disease at New York University Langone Health. “Anyone who has a known clot already, like a known macrothrombosis in their leg or lung, needs to be on full-dose heparin,” he said.
That rationale can help reconcile the different outcomes in the critically and non–critically ill COVID-19 patients, wrote Hugo ten Cate, MD, PhD, of Maastricht University in the Netherlands, wrote in an accompanying editorial. But differences in the study populations may also explain the divergent outcomes, Dr. ten Cate noted.
The studies suggest that critically ill patients may need hon-heparin antithrombotic approaches “or even profibrinolytic strategies,” Dr. Cate wrote, and that the safety and effectiveness of thromboprophylaxis “remains an important question.” Nonetheless, he added, treating physicians must deal with the bleeding risk when using heparin or low-molecular-weight heparin in moderately ill COVID-19 patients.
Deepak L. Bhatt MD, MPH, of Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that reconciling the two studies was “a bit challenging,” because effective therapies tend to have a greater impact in sicker patients.
“Of course, with antithrombotic therapies, bleeding side effects can sometimes overwhelm benefits in patients who are at high risk of both bleeding and ischemic complications, though that does not seem to be the explanation here,” Dr. Bhatt said. “I do think we need more data to clarify exactly which COVID patients benefit from various antithrombotic regimens, and fortunately, there are other ongoing studies, some of which will report relatively soon.”
He concurred with Dr. Berger that patients who need anticoagulation should receive it “apart from their COVID status,” Dr. Bhatt said. “Sick, hospitalized patients with or without COVID should receive appropriate prophylactic doses of anticoagulation.” However, he added, “Whether we should routinely go beyond that in COVID-positive inpatients, I think we need more data.”
The ATTACC platform received grants from the Canadian Institutes of Health Research and several other research foundations. The ACTIV-4a platform received funding from the National Heart, Lung, and Blood Institute. REMAP-CAP received funding from the European Union and several international research foundations, as well as Amgen and Eisai.
Dr. Lawler had no relationships to disclose. Dr. Berger disclosed receiving grants from the NHLBI, and financial relationships with AstraZeneca, Janssen, and Amgen outside the submitted work. Dr. ten Cate reported relationships with Alveron, Coagulation Profile, Portola/Alexion, Bayer, Pfizer, Stago, Leo Pharma, Daiichi, and Gilead/Galapagos. Dr. Bhatt is chair of the data safety and monitoring board of the FREEDOM COVID anticoagulation clinical trial.
Critically ill derive no benefit
Critically ill derive no benefit
Therapeutic levels of heparin can have widely varying effects on COVID-19 patients depending on the severity of their disease, according to a multiplatform clinical trial that analyzed patient data from three international trials.
COVID-19 patients in the ICU, or at least receiving ICU-level care, derived no benefit from anticoagulation with heparin, while non–critically ill COVID-19 patients – those who were hospitalized but not receiving ICU-level care – on the same anticoagulation were less likely to progress to need respiratory or cardiovascular organ support despite a slightly heightened risk of bleeding events.
Reporting in two articles published online in the New England Journal of Medicine, authors of three international trials combined their data into one multiplatform trial that makes a strong case for prescribing therapeutic levels of heparin in hospitalized patients not receiving ICU-level care were non–critically ill and critically ill.
“I think this is going to be a game changer,” said Jeffrey S. Berger, MD, ACTIV-4a co–principal investigator and co–first author of the study of non–critically ill patients. “I think that using therapeutic-dose anticoagulation should improve outcomes in the tens of thousands of patients worldwide. I hope our data can have a global impact.”
Outcomes based on disease severity
The multiplatform trial analyzed data from the Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC); A Multicenter, Adaptive, Randomized Controlled Platform Trial of the Safety and Efficacy of Antithrombotic Strategies in Hospitalized Adults with COVID-19 (ACTIV-4a); and Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP).
The trial evaluated 2,219 non–critically ill hospitalized patients, 1,181 of whom were randomized to therapeutic-dose anticoagulation; and 1,098 critically ill patients, 534 of whom were prescribed therapeutic levels of heparin.
In the critically ill patients, those on heparin were no more likely to get discharged or spend fewer days on respiratory or CV organ support – oxygen, mechanical ventilation, life support, vasopressors or inotropes – than were those on usual-care thromboprophylaxis. The investigators stopped the trial in both patient populations: in critically ill patients when it became obvious therapeutic-dose anticoagulation was having no impact; and in moderately ill patients when the trial met the prespecified criteria for the superiority of therapeutic-dose anticoagulation.
ICU patients on therapeutic-level heparin spent an average of 1 day free of organ support vs. 4 for patients on usual-care prophylactic antithrombotic drugs. The percentage of patients who survived to hospital discharge was similar in the therapeutic-level and usual-care critically ill patients: 62.7% and 64.5%, respectively. Major bleeding occurred in 3.8% and 2.8%, respectively. Demographic and clinical characteristics were similar between both patient groups.
However, in non–critically ill patients, therapeutic levels of heparin resulted in a marked improvement in outcomes. The researchers estimated that, for every 1,000 hospitalized patients with what they labeled moderate disease, an initial treatment with therapeutic-dose heparin resulted in 40 additional patients surviving compared to usual-care thromboprophylaxis.
The percentages of patients not needing organ support before hospital discharge was 80.2% on therapeutic-dose heparin and 76.4% on usual-care therapy. In terms of adjusted odds ratio, the anticoagulation group had a 27% improved chance of not needing daily organ support.
Those improvements came with an additional seven major bleeding events per 1,000 patients. That broke down to a rate of 1.9% in the therapeutic-dose and 0.9% in the usual-care patients.
As the Delta variant of COVID-19 spreads, Patrick R. Lawler, MD, MPH, principal investigator of the ATTACC trial, said there’s no reason these findings shouldn’t apply for all variants of the disease.
Dr. Lawler, a physician-scientist at Peter Munk Cardiac Centre at Toronto General Hospital, noted that the multiplatform study did not account for disease variant. “Ongoing clinical trials are tracking the variant patients have or the variants that are most prevalent in an area at that time,” he said. “It may be easier in future trials to look at that question.”
Explaining heparin’s varying effects
The study did not specifically sort out why moderately ill patients fared better on heparin than their critically ill counterparts, but Dr. Lawler speculated on possible reasons. “One might be that the extent of illness severity is too extreme in the ICU-level population for heparin to have a beneficial extent,” he said.
He acknowledged that higher rates of macrovascular thrombosis, such as venous thromboembolism, in ICU patients would suggest that heparin would have a greater beneficial effect, but, he added, “it may also suggest how advanced that process is, and perhaps heparin is not adequate to reverse the course at that point given relatively extensive thrombosis and associate organ failure.”
As clinicians have gained experience dealing with COVID-19, they’ve learned that infected patients carry a high burden of macro- and microthrombosis, Dr. Berger said, which may explain why critically ill patients didn’t respond as well to therapeutic levels of heparin. “I think the cat is out of the bag; patients who are severe are too ill to benefit,” he said. “I would think there’s too much microthrombosis that is already in their bodies.”
However, this doesn’t completely rule out therapeutic levels of heparin in critically ill COVID-19 patients. There are some scenarios where it’s needed, said Dr. Berger, associate professor of medicine and surgery and director of the Center for the Prevention of Cardiovascular Disease at New York University Langone Health. “Anyone who has a known clot already, like a known macrothrombosis in their leg or lung, needs to be on full-dose heparin,” he said.
That rationale can help reconcile the different outcomes in the critically and non–critically ill COVID-19 patients, wrote Hugo ten Cate, MD, PhD, of Maastricht University in the Netherlands, wrote in an accompanying editorial. But differences in the study populations may also explain the divergent outcomes, Dr. ten Cate noted.
The studies suggest that critically ill patients may need hon-heparin antithrombotic approaches “or even profibrinolytic strategies,” Dr. Cate wrote, and that the safety and effectiveness of thromboprophylaxis “remains an important question.” Nonetheless, he added, treating physicians must deal with the bleeding risk when using heparin or low-molecular-weight heparin in moderately ill COVID-19 patients.
Deepak L. Bhatt MD, MPH, of Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that reconciling the two studies was “a bit challenging,” because effective therapies tend to have a greater impact in sicker patients.
“Of course, with antithrombotic therapies, bleeding side effects can sometimes overwhelm benefits in patients who are at high risk of both bleeding and ischemic complications, though that does not seem to be the explanation here,” Dr. Bhatt said. “I do think we need more data to clarify exactly which COVID patients benefit from various antithrombotic regimens, and fortunately, there are other ongoing studies, some of which will report relatively soon.”
He concurred with Dr. Berger that patients who need anticoagulation should receive it “apart from their COVID status,” Dr. Bhatt said. “Sick, hospitalized patients with or without COVID should receive appropriate prophylactic doses of anticoagulation.” However, he added, “Whether we should routinely go beyond that in COVID-positive inpatients, I think we need more data.”
The ATTACC platform received grants from the Canadian Institutes of Health Research and several other research foundations. The ACTIV-4a platform received funding from the National Heart, Lung, and Blood Institute. REMAP-CAP received funding from the European Union and several international research foundations, as well as Amgen and Eisai.
Dr. Lawler had no relationships to disclose. Dr. Berger disclosed receiving grants from the NHLBI, and financial relationships with AstraZeneca, Janssen, and Amgen outside the submitted work. Dr. ten Cate reported relationships with Alveron, Coagulation Profile, Portola/Alexion, Bayer, Pfizer, Stago, Leo Pharma, Daiichi, and Gilead/Galapagos. Dr. Bhatt is chair of the data safety and monitoring board of the FREEDOM COVID anticoagulation clinical trial.
Therapeutic levels of heparin can have widely varying effects on COVID-19 patients depending on the severity of their disease, according to a multiplatform clinical trial that analyzed patient data from three international trials.
COVID-19 patients in the ICU, or at least receiving ICU-level care, derived no benefit from anticoagulation with heparin, while non–critically ill COVID-19 patients – those who were hospitalized but not receiving ICU-level care – on the same anticoagulation were less likely to progress to need respiratory or cardiovascular organ support despite a slightly heightened risk of bleeding events.
Reporting in two articles published online in the New England Journal of Medicine, authors of three international trials combined their data into one multiplatform trial that makes a strong case for prescribing therapeutic levels of heparin in hospitalized patients not receiving ICU-level care were non–critically ill and critically ill.
“I think this is going to be a game changer,” said Jeffrey S. Berger, MD, ACTIV-4a co–principal investigator and co–first author of the study of non–critically ill patients. “I think that using therapeutic-dose anticoagulation should improve outcomes in the tens of thousands of patients worldwide. I hope our data can have a global impact.”
Outcomes based on disease severity
The multiplatform trial analyzed data from the Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC); A Multicenter, Adaptive, Randomized Controlled Platform Trial of the Safety and Efficacy of Antithrombotic Strategies in Hospitalized Adults with COVID-19 (ACTIV-4a); and Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP).
The trial evaluated 2,219 non–critically ill hospitalized patients, 1,181 of whom were randomized to therapeutic-dose anticoagulation; and 1,098 critically ill patients, 534 of whom were prescribed therapeutic levels of heparin.
In the critically ill patients, those on heparin were no more likely to get discharged or spend fewer days on respiratory or CV organ support – oxygen, mechanical ventilation, life support, vasopressors or inotropes – than were those on usual-care thromboprophylaxis. The investigators stopped the trial in both patient populations: in critically ill patients when it became obvious therapeutic-dose anticoagulation was having no impact; and in moderately ill patients when the trial met the prespecified criteria for the superiority of therapeutic-dose anticoagulation.
ICU patients on therapeutic-level heparin spent an average of 1 day free of organ support vs. 4 for patients on usual-care prophylactic antithrombotic drugs. The percentage of patients who survived to hospital discharge was similar in the therapeutic-level and usual-care critically ill patients: 62.7% and 64.5%, respectively. Major bleeding occurred in 3.8% and 2.8%, respectively. Demographic and clinical characteristics were similar between both patient groups.
However, in non–critically ill patients, therapeutic levels of heparin resulted in a marked improvement in outcomes. The researchers estimated that, for every 1,000 hospitalized patients with what they labeled moderate disease, an initial treatment with therapeutic-dose heparin resulted in 40 additional patients surviving compared to usual-care thromboprophylaxis.
The percentages of patients not needing organ support before hospital discharge was 80.2% on therapeutic-dose heparin and 76.4% on usual-care therapy. In terms of adjusted odds ratio, the anticoagulation group had a 27% improved chance of not needing daily organ support.
Those improvements came with an additional seven major bleeding events per 1,000 patients. That broke down to a rate of 1.9% in the therapeutic-dose and 0.9% in the usual-care patients.
As the Delta variant of COVID-19 spreads, Patrick R. Lawler, MD, MPH, principal investigator of the ATTACC trial, said there’s no reason these findings shouldn’t apply for all variants of the disease.
Dr. Lawler, a physician-scientist at Peter Munk Cardiac Centre at Toronto General Hospital, noted that the multiplatform study did not account for disease variant. “Ongoing clinical trials are tracking the variant patients have or the variants that are most prevalent in an area at that time,” he said. “It may be easier in future trials to look at that question.”
Explaining heparin’s varying effects
The study did not specifically sort out why moderately ill patients fared better on heparin than their critically ill counterparts, but Dr. Lawler speculated on possible reasons. “One might be that the extent of illness severity is too extreme in the ICU-level population for heparin to have a beneficial extent,” he said.
He acknowledged that higher rates of macrovascular thrombosis, such as venous thromboembolism, in ICU patients would suggest that heparin would have a greater beneficial effect, but, he added, “it may also suggest how advanced that process is, and perhaps heparin is not adequate to reverse the course at that point given relatively extensive thrombosis and associate organ failure.”
As clinicians have gained experience dealing with COVID-19, they’ve learned that infected patients carry a high burden of macro- and microthrombosis, Dr. Berger said, which may explain why critically ill patients didn’t respond as well to therapeutic levels of heparin. “I think the cat is out of the bag; patients who are severe are too ill to benefit,” he said. “I would think there’s too much microthrombosis that is already in their bodies.”
However, this doesn’t completely rule out therapeutic levels of heparin in critically ill COVID-19 patients. There are some scenarios where it’s needed, said Dr. Berger, associate professor of medicine and surgery and director of the Center for the Prevention of Cardiovascular Disease at New York University Langone Health. “Anyone who has a known clot already, like a known macrothrombosis in their leg or lung, needs to be on full-dose heparin,” he said.
That rationale can help reconcile the different outcomes in the critically and non–critically ill COVID-19 patients, wrote Hugo ten Cate, MD, PhD, of Maastricht University in the Netherlands, wrote in an accompanying editorial. But differences in the study populations may also explain the divergent outcomes, Dr. ten Cate noted.
The studies suggest that critically ill patients may need hon-heparin antithrombotic approaches “or even profibrinolytic strategies,” Dr. Cate wrote, and that the safety and effectiveness of thromboprophylaxis “remains an important question.” Nonetheless, he added, treating physicians must deal with the bleeding risk when using heparin or low-molecular-weight heparin in moderately ill COVID-19 patients.
Deepak L. Bhatt MD, MPH, of Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that reconciling the two studies was “a bit challenging,” because effective therapies tend to have a greater impact in sicker patients.
“Of course, with antithrombotic therapies, bleeding side effects can sometimes overwhelm benefits in patients who are at high risk of both bleeding and ischemic complications, though that does not seem to be the explanation here,” Dr. Bhatt said. “I do think we need more data to clarify exactly which COVID patients benefit from various antithrombotic regimens, and fortunately, there are other ongoing studies, some of which will report relatively soon.”
He concurred with Dr. Berger that patients who need anticoagulation should receive it “apart from their COVID status,” Dr. Bhatt said. “Sick, hospitalized patients with or without COVID should receive appropriate prophylactic doses of anticoagulation.” However, he added, “Whether we should routinely go beyond that in COVID-positive inpatients, I think we need more data.”
The ATTACC platform received grants from the Canadian Institutes of Health Research and several other research foundations. The ACTIV-4a platform received funding from the National Heart, Lung, and Blood Institute. REMAP-CAP received funding from the European Union and several international research foundations, as well as Amgen and Eisai.
Dr. Lawler had no relationships to disclose. Dr. Berger disclosed receiving grants from the NHLBI, and financial relationships with AstraZeneca, Janssen, and Amgen outside the submitted work. Dr. ten Cate reported relationships with Alveron, Coagulation Profile, Portola/Alexion, Bayer, Pfizer, Stago, Leo Pharma, Daiichi, and Gilead/Galapagos. Dr. Bhatt is chair of the data safety and monitoring board of the FREEDOM COVID anticoagulation clinical trial.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Tachycardia syndrome may be distinct marker for long COVID
Tachycardia is commonly reported in patients with post-acute COVID-19 syndrome (PACS), also known as long COVID, authors report in a new article. The researchers say tachycardia syndrome should be considered a distinct phenotype.
The study by Marcus Ståhlberg, MD, PhD, of Karolinska University Hospital, Stockholm, and colleagues was published online August 11 in The American Journal of Medicine.
Dr. Ståhlberg told this news organization that although much attention has been paid to cases of clotting and perimyocarditis in patients after COVID, relatively little attention has been paid to tachycardia, despite case reports that show that palpitations are a common complaint.
“We have diagnosed a large number of patients with postural orthostatic tachycardia syndrome [POTS] and other forms of COVID-related tachycardia at our post-COVID outpatient clinic at Karolinska University Hospital and wanted to highlight this phenomenon,” he said.
Between 25% and 50% of patients at the clinic report tachycardia and/or palpitations that last 12 weeks or longer, the authors report.
“Systematic investigations suggest that 9% of Post-acute COVID-19 syndrome patients report palpitations at six months,” the authors write.
The findings also shed light on potential tests and treatments, he said.
“Physicians should be liberal in performing a basic cardiological workup, including an ECG [electrocardiogram], echocardiography, and Holter ECG monitoring in patients complaining of palpitations and/or chest pain,” Dr. Ståhlberg said.
“If orthostatic intolerance is also reported – such as vertigo, nausea, dyspnea – suspicion of POTS should be raised and a head-up tilt test or at least an active standing test should be performed,” he said.
If POTS is confirmed, he said, patients should be offered a heart rate–lowering drug, such as low-dose propranolol or ivabradine. Compression garments, increased fluid intake, and a structured rehabilitation program also help.
“According to our clinical experience, ivabradine can also reduce symptoms in patients with inappropriate sinus tachycardia and post-COVID,” Dr. Ståhlberg said. “Another finding on Holter-ECG to look out for is frequent premature extrasystoles, which could indicate myocarditis and should warrant a cardiac MRI.”
Dr. Ståhlberg said the researchers think the mechanism underlying the tachycardia is autoimmune and that primary SARS-CoV-2 infections trigger an autoimmune response with formation of autoantibodies that can activate receptors regulating blood pressure and heart rate.
Long-lasting symptoms from COVID are prevalent, the authors note, especially in patients who experienced severe forms of the disease.
In the longest follow-up study to date of patients hospitalized with COVID, more than 60% experienced fatigue or muscle weakness 6 months after hospitalization.
PACS should not be considered a single syndrome; the term denotes an array of subsyndromes and phenotypes, the authors write. Typical symptoms include headache, fatigue, dyspnea, and mental fog but can involve multiple organs and systems.
Tachycardia can also be used as a marker to help gauge the severity of long COVID, the authors write.
“[T]achycardia can be considered a universal and easily obtainable quantitative marker of Post-acute COVID-19 syndrome and its severity rather than patient-reported symptoms, blood testing, and thoracic CT-scans,” they write.
An underrecognized complication
Erin D. Michos, MD, MHS, director of women’s cardiovascular health and associate director of preventive cardiology at Johns Hopkins University, Baltimore, said in an interview that she has seen many similar symptoms in the long-COVID patients referred to her practice.
Dr. Michos, who is also an associate professor of medicine and epidemiology, said she’s been receiving a “huge number” of referrals of long-COVID patients with postural tachycardia, inappropriate sinus tachycardia, and POTS.
“I think this is all in the spectrum of autonomic dysfunction that has been recognized a lot since COVID. POTS has been thought to have [a potentially] viral cause that triggers an autoimmune response. Even before COVID, many patients had POTS triggered by a viral infection. The question is whether COVID-related POTS for long COVID is different from other kinds of POTS.”
She says she treats long-COVID patients who complain of elevated heart rates with many of the cardiac workup procedures the authors list and that she treats them in a way similar to the way she treats patients with POTS.
She recommends checking resting oxygen levels and having patients walk the halls and measure their oxygen levels after walking, because their elevated heart rate may be related to ongoing lung injury from COVID.
Eric Adler, MD, a cardiologist with University of San Diego Health, told this news organization that the findings by Dr. Ståhlberg and colleagues are consistent with what he’s seeing in his clinical practice.
Dr. Adler agrees with the authors that tachycardia is an underrecognized complication of long COVID.
He said the article represents further proof that though people may survive COVID, the threat of long-term symptoms, such as heart palpitations, is real and supports the case for vaccinations.
The authors, Dr. Michos, and Dr. Adler have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tachycardia is commonly reported in patients with post-acute COVID-19 syndrome (PACS), also known as long COVID, authors report in a new article. The researchers say tachycardia syndrome should be considered a distinct phenotype.
The study by Marcus Ståhlberg, MD, PhD, of Karolinska University Hospital, Stockholm, and colleagues was published online August 11 in The American Journal of Medicine.
Dr. Ståhlberg told this news organization that although much attention has been paid to cases of clotting and perimyocarditis in patients after COVID, relatively little attention has been paid to tachycardia, despite case reports that show that palpitations are a common complaint.
“We have diagnosed a large number of patients with postural orthostatic tachycardia syndrome [POTS] and other forms of COVID-related tachycardia at our post-COVID outpatient clinic at Karolinska University Hospital and wanted to highlight this phenomenon,” he said.
Between 25% and 50% of patients at the clinic report tachycardia and/or palpitations that last 12 weeks or longer, the authors report.
“Systematic investigations suggest that 9% of Post-acute COVID-19 syndrome patients report palpitations at six months,” the authors write.
The findings also shed light on potential tests and treatments, he said.
“Physicians should be liberal in performing a basic cardiological workup, including an ECG [electrocardiogram], echocardiography, and Holter ECG monitoring in patients complaining of palpitations and/or chest pain,” Dr. Ståhlberg said.
“If orthostatic intolerance is also reported – such as vertigo, nausea, dyspnea – suspicion of POTS should be raised and a head-up tilt test or at least an active standing test should be performed,” he said.
If POTS is confirmed, he said, patients should be offered a heart rate–lowering drug, such as low-dose propranolol or ivabradine. Compression garments, increased fluid intake, and a structured rehabilitation program also help.
“According to our clinical experience, ivabradine can also reduce symptoms in patients with inappropriate sinus tachycardia and post-COVID,” Dr. Ståhlberg said. “Another finding on Holter-ECG to look out for is frequent premature extrasystoles, which could indicate myocarditis and should warrant a cardiac MRI.”
Dr. Ståhlberg said the researchers think the mechanism underlying the tachycardia is autoimmune and that primary SARS-CoV-2 infections trigger an autoimmune response with formation of autoantibodies that can activate receptors regulating blood pressure and heart rate.
Long-lasting symptoms from COVID are prevalent, the authors note, especially in patients who experienced severe forms of the disease.
In the longest follow-up study to date of patients hospitalized with COVID, more than 60% experienced fatigue or muscle weakness 6 months after hospitalization.
PACS should not be considered a single syndrome; the term denotes an array of subsyndromes and phenotypes, the authors write. Typical symptoms include headache, fatigue, dyspnea, and mental fog but can involve multiple organs and systems.
Tachycardia can also be used as a marker to help gauge the severity of long COVID, the authors write.
“[T]achycardia can be considered a universal and easily obtainable quantitative marker of Post-acute COVID-19 syndrome and its severity rather than patient-reported symptoms, blood testing, and thoracic CT-scans,” they write.
An underrecognized complication
Erin D. Michos, MD, MHS, director of women’s cardiovascular health and associate director of preventive cardiology at Johns Hopkins University, Baltimore, said in an interview that she has seen many similar symptoms in the long-COVID patients referred to her practice.
Dr. Michos, who is also an associate professor of medicine and epidemiology, said she’s been receiving a “huge number” of referrals of long-COVID patients with postural tachycardia, inappropriate sinus tachycardia, and POTS.
“I think this is all in the spectrum of autonomic dysfunction that has been recognized a lot since COVID. POTS has been thought to have [a potentially] viral cause that triggers an autoimmune response. Even before COVID, many patients had POTS triggered by a viral infection. The question is whether COVID-related POTS for long COVID is different from other kinds of POTS.”
She says she treats long-COVID patients who complain of elevated heart rates with many of the cardiac workup procedures the authors list and that she treats them in a way similar to the way she treats patients with POTS.
She recommends checking resting oxygen levels and having patients walk the halls and measure their oxygen levels after walking, because their elevated heart rate may be related to ongoing lung injury from COVID.
Eric Adler, MD, a cardiologist with University of San Diego Health, told this news organization that the findings by Dr. Ståhlberg and colleagues are consistent with what he’s seeing in his clinical practice.
Dr. Adler agrees with the authors that tachycardia is an underrecognized complication of long COVID.
He said the article represents further proof that though people may survive COVID, the threat of long-term symptoms, such as heart palpitations, is real and supports the case for vaccinations.
The authors, Dr. Michos, and Dr. Adler have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tachycardia is commonly reported in patients with post-acute COVID-19 syndrome (PACS), also known as long COVID, authors report in a new article. The researchers say tachycardia syndrome should be considered a distinct phenotype.
The study by Marcus Ståhlberg, MD, PhD, of Karolinska University Hospital, Stockholm, and colleagues was published online August 11 in The American Journal of Medicine.
Dr. Ståhlberg told this news organization that although much attention has been paid to cases of clotting and perimyocarditis in patients after COVID, relatively little attention has been paid to tachycardia, despite case reports that show that palpitations are a common complaint.
“We have diagnosed a large number of patients with postural orthostatic tachycardia syndrome [POTS] and other forms of COVID-related tachycardia at our post-COVID outpatient clinic at Karolinska University Hospital and wanted to highlight this phenomenon,” he said.
Between 25% and 50% of patients at the clinic report tachycardia and/or palpitations that last 12 weeks or longer, the authors report.
“Systematic investigations suggest that 9% of Post-acute COVID-19 syndrome patients report palpitations at six months,” the authors write.
The findings also shed light on potential tests and treatments, he said.
“Physicians should be liberal in performing a basic cardiological workup, including an ECG [electrocardiogram], echocardiography, and Holter ECG monitoring in patients complaining of palpitations and/or chest pain,” Dr. Ståhlberg said.
“If orthostatic intolerance is also reported – such as vertigo, nausea, dyspnea – suspicion of POTS should be raised and a head-up tilt test or at least an active standing test should be performed,” he said.
If POTS is confirmed, he said, patients should be offered a heart rate–lowering drug, such as low-dose propranolol or ivabradine. Compression garments, increased fluid intake, and a structured rehabilitation program also help.
“According to our clinical experience, ivabradine can also reduce symptoms in patients with inappropriate sinus tachycardia and post-COVID,” Dr. Ståhlberg said. “Another finding on Holter-ECG to look out for is frequent premature extrasystoles, which could indicate myocarditis and should warrant a cardiac MRI.”
Dr. Ståhlberg said the researchers think the mechanism underlying the tachycardia is autoimmune and that primary SARS-CoV-2 infections trigger an autoimmune response with formation of autoantibodies that can activate receptors regulating blood pressure and heart rate.
Long-lasting symptoms from COVID are prevalent, the authors note, especially in patients who experienced severe forms of the disease.
In the longest follow-up study to date of patients hospitalized with COVID, more than 60% experienced fatigue or muscle weakness 6 months after hospitalization.
PACS should not be considered a single syndrome; the term denotes an array of subsyndromes and phenotypes, the authors write. Typical symptoms include headache, fatigue, dyspnea, and mental fog but can involve multiple organs and systems.
Tachycardia can also be used as a marker to help gauge the severity of long COVID, the authors write.
“[T]achycardia can be considered a universal and easily obtainable quantitative marker of Post-acute COVID-19 syndrome and its severity rather than patient-reported symptoms, blood testing, and thoracic CT-scans,” they write.
An underrecognized complication
Erin D. Michos, MD, MHS, director of women’s cardiovascular health and associate director of preventive cardiology at Johns Hopkins University, Baltimore, said in an interview that she has seen many similar symptoms in the long-COVID patients referred to her practice.
Dr. Michos, who is also an associate professor of medicine and epidemiology, said she’s been receiving a “huge number” of referrals of long-COVID patients with postural tachycardia, inappropriate sinus tachycardia, and POTS.
“I think this is all in the spectrum of autonomic dysfunction that has been recognized a lot since COVID. POTS has been thought to have [a potentially] viral cause that triggers an autoimmune response. Even before COVID, many patients had POTS triggered by a viral infection. The question is whether COVID-related POTS for long COVID is different from other kinds of POTS.”
She says she treats long-COVID patients who complain of elevated heart rates with many of the cardiac workup procedures the authors list and that she treats them in a way similar to the way she treats patients with POTS.
She recommends checking resting oxygen levels and having patients walk the halls and measure their oxygen levels after walking, because their elevated heart rate may be related to ongoing lung injury from COVID.
Eric Adler, MD, a cardiologist with University of San Diego Health, told this news organization that the findings by Dr. Ståhlberg and colleagues are consistent with what he’s seeing in his clinical practice.
Dr. Adler agrees with the authors that tachycardia is an underrecognized complication of long COVID.
He said the article represents further proof that though people may survive COVID, the threat of long-term symptoms, such as heart palpitations, is real and supports the case for vaccinations.
The authors, Dr. Michos, and Dr. Adler have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
German nurse suspected of giving saline instead of COVID-19 vaccine
Those who may be affected are being informed about their possible vulnerability to the coronavirus and will be offered COVID-19 shots, according to CBS News.
“I’m totally shocked by the incident,” Sven Ambrosy, a district administrator of Friesland, wrote in a Facebook post on Aug. 10.
“The district of Friesland will do everything possible to ensure that the affected people receive their vaccination protection as soon as possible,” he said.
In late April, a former Red Cross employee who worked at the Roffhausen Vaccination Center in Friesland, a district in Germany’s northern state of Lower Saxony, told a colleague that she filled six syringes with saline instead of the Pfizer vaccine, according to police reports. The nurse said she dropped a vial containing the vaccine while preparing syringes and tried to cover it up.
The nurse was immediately fired, and local authorities conducted antibody tests on more than 100 people who visited the vaccination center on April 21. Since it was impossible to trace who received the saline shots, everyone who visited the center that day was invited to receive a follow-up shot.
But during a police investigation, authorities found evidence that more people were affected. The case now involves 8,557 vaccinations given between March 5 and April 20 at specific times.
Now, authorities are contacting those who were affected by phone or email to schedule new vaccination appointments. They’ve established a dedicated information phone line as well, according to NPR.
Saline solution is harmless, but most people who received shots in Germany during that time were older adults, who are more likely to have severe COVID-19 if infected, according to Reuters.
The nurse has remained silent about the allegations of her giving saline rather than a vaccine to thousands of people, CBS News reported. And it’s unclear whether there have been any arrests or charges related to the case, according to Reuters.
The nurse hasn’t been named publicly, and the motive hasn’t been shared, NPR reported, though the nurse had purportedly expressed skepticism about COVID-19 vaccines in social media posts.
A version of this article first appeared on WebMD.com.
Those who may be affected are being informed about their possible vulnerability to the coronavirus and will be offered COVID-19 shots, according to CBS News.
“I’m totally shocked by the incident,” Sven Ambrosy, a district administrator of Friesland, wrote in a Facebook post on Aug. 10.
“The district of Friesland will do everything possible to ensure that the affected people receive their vaccination protection as soon as possible,” he said.
In late April, a former Red Cross employee who worked at the Roffhausen Vaccination Center in Friesland, a district in Germany’s northern state of Lower Saxony, told a colleague that she filled six syringes with saline instead of the Pfizer vaccine, according to police reports. The nurse said she dropped a vial containing the vaccine while preparing syringes and tried to cover it up.
The nurse was immediately fired, and local authorities conducted antibody tests on more than 100 people who visited the vaccination center on April 21. Since it was impossible to trace who received the saline shots, everyone who visited the center that day was invited to receive a follow-up shot.
But during a police investigation, authorities found evidence that more people were affected. The case now involves 8,557 vaccinations given between March 5 and April 20 at specific times.
Now, authorities are contacting those who were affected by phone or email to schedule new vaccination appointments. They’ve established a dedicated information phone line as well, according to NPR.
Saline solution is harmless, but most people who received shots in Germany during that time were older adults, who are more likely to have severe COVID-19 if infected, according to Reuters.
The nurse has remained silent about the allegations of her giving saline rather than a vaccine to thousands of people, CBS News reported. And it’s unclear whether there have been any arrests or charges related to the case, according to Reuters.
The nurse hasn’t been named publicly, and the motive hasn’t been shared, NPR reported, though the nurse had purportedly expressed skepticism about COVID-19 vaccines in social media posts.
A version of this article first appeared on WebMD.com.
Those who may be affected are being informed about their possible vulnerability to the coronavirus and will be offered COVID-19 shots, according to CBS News.
“I’m totally shocked by the incident,” Sven Ambrosy, a district administrator of Friesland, wrote in a Facebook post on Aug. 10.
“The district of Friesland will do everything possible to ensure that the affected people receive their vaccination protection as soon as possible,” he said.
In late April, a former Red Cross employee who worked at the Roffhausen Vaccination Center in Friesland, a district in Germany’s northern state of Lower Saxony, told a colleague that she filled six syringes with saline instead of the Pfizer vaccine, according to police reports. The nurse said she dropped a vial containing the vaccine while preparing syringes and tried to cover it up.
The nurse was immediately fired, and local authorities conducted antibody tests on more than 100 people who visited the vaccination center on April 21. Since it was impossible to trace who received the saline shots, everyone who visited the center that day was invited to receive a follow-up shot.
But during a police investigation, authorities found evidence that more people were affected. The case now involves 8,557 vaccinations given between March 5 and April 20 at specific times.
Now, authorities are contacting those who were affected by phone or email to schedule new vaccination appointments. They’ve established a dedicated information phone line as well, according to NPR.
Saline solution is harmless, but most people who received shots in Germany during that time were older adults, who are more likely to have severe COVID-19 if infected, according to Reuters.
The nurse has remained silent about the allegations of her giving saline rather than a vaccine to thousands of people, CBS News reported. And it’s unclear whether there have been any arrests or charges related to the case, according to Reuters.
The nurse hasn’t been named publicly, and the motive hasn’t been shared, NPR reported, though the nurse had purportedly expressed skepticism about COVID-19 vaccines in social media posts.
A version of this article first appeared on WebMD.com.
FDA may okay COVID booster for vulnerable adults before weekend: Media
according to multiple media reports.
The agency, along with the Centers for Disease Control and Prevention (CDC) and the National Institutes of Health, is working through the details of how booster doses for this population would work, and could authorize a third dose of both the Pfizer and Moderna vaccines as early as Aug. 12, Politico reports.
About 2.7% of adults in the United States are immunocompromised, according to the CDC. This group includes people who have cancer, have received solid organ or stem cell transplants, have genetic conditions that weaken the immune function, have HIV, or are people with health conditions that require treatment with medications that turn down immune function, such as rheumatoid arthritis.
Immune function also wanes with age, so the FDA could consider boosters for the elderly.
New research shows that between one-third and one-half of immunocompromised patients who didn’t develop detectable levels of virus-fighting antibodies after two doses of a COVID vaccine will respond to a third dose.
A committee of independent experts that advises the CDC on the use of vaccines in the United States had previously signaled its support for giving boosters to those who are immunocompromised, but noted that it couldn’t officially recommend the strategy until the FDA had updated its emergency-use authorization for the shots or granted them a full biologics license, or “full approval.”
It’s unclear which mechanism the FDA might use, or exactly who will be eligible for the shots.
The United States would follow other nations such as Israel, France, the United Kingdom, and Germany in planning for or authorizing boosters for some vulnerable individuals.
The World Health Organization (WHO) has voiced strong opposition to the use of boosters in wealthy countries while much of the world still doesn’t have access to these lifesaving therapies. The WHO has asked wealthy nations to hold off on giving boosters until at least the end of September to give more people the opportunity to get a first dose.
The CDC’s Advisory Committee on Immunization Practices (ACIP) meets again on Aug. 13 and is expected to discuss booster doses for this population of patients. The ACIP officially makes recommendations on the use of vaccines to the nation’s doctors.
The committee’s recommendation ensures that a vaccine will be covered by public and private insurers. Statutory vaccination requirements are also made based on the ACIP’s recommendations.
A version of this article first appeared on Medscape.com.
according to multiple media reports.
The agency, along with the Centers for Disease Control and Prevention (CDC) and the National Institutes of Health, is working through the details of how booster doses for this population would work, and could authorize a third dose of both the Pfizer and Moderna vaccines as early as Aug. 12, Politico reports.
About 2.7% of adults in the United States are immunocompromised, according to the CDC. This group includes people who have cancer, have received solid organ or stem cell transplants, have genetic conditions that weaken the immune function, have HIV, or are people with health conditions that require treatment with medications that turn down immune function, such as rheumatoid arthritis.
Immune function also wanes with age, so the FDA could consider boosters for the elderly.
New research shows that between one-third and one-half of immunocompromised patients who didn’t develop detectable levels of virus-fighting antibodies after two doses of a COVID vaccine will respond to a third dose.
A committee of independent experts that advises the CDC on the use of vaccines in the United States had previously signaled its support for giving boosters to those who are immunocompromised, but noted that it couldn’t officially recommend the strategy until the FDA had updated its emergency-use authorization for the shots or granted them a full biologics license, or “full approval.”
It’s unclear which mechanism the FDA might use, or exactly who will be eligible for the shots.
The United States would follow other nations such as Israel, France, the United Kingdom, and Germany in planning for or authorizing boosters for some vulnerable individuals.
The World Health Organization (WHO) has voiced strong opposition to the use of boosters in wealthy countries while much of the world still doesn’t have access to these lifesaving therapies. The WHO has asked wealthy nations to hold off on giving boosters until at least the end of September to give more people the opportunity to get a first dose.
The CDC’s Advisory Committee on Immunization Practices (ACIP) meets again on Aug. 13 and is expected to discuss booster doses for this population of patients. The ACIP officially makes recommendations on the use of vaccines to the nation’s doctors.
The committee’s recommendation ensures that a vaccine will be covered by public and private insurers. Statutory vaccination requirements are also made based on the ACIP’s recommendations.
A version of this article first appeared on Medscape.com.
according to multiple media reports.
The agency, along with the Centers for Disease Control and Prevention (CDC) and the National Institutes of Health, is working through the details of how booster doses for this population would work, and could authorize a third dose of both the Pfizer and Moderna vaccines as early as Aug. 12, Politico reports.
About 2.7% of adults in the United States are immunocompromised, according to the CDC. This group includes people who have cancer, have received solid organ or stem cell transplants, have genetic conditions that weaken the immune function, have HIV, or are people with health conditions that require treatment with medications that turn down immune function, such as rheumatoid arthritis.
Immune function also wanes with age, so the FDA could consider boosters for the elderly.
New research shows that between one-third and one-half of immunocompromised patients who didn’t develop detectable levels of virus-fighting antibodies after two doses of a COVID vaccine will respond to a third dose.
A committee of independent experts that advises the CDC on the use of vaccines in the United States had previously signaled its support for giving boosters to those who are immunocompromised, but noted that it couldn’t officially recommend the strategy until the FDA had updated its emergency-use authorization for the shots or granted them a full biologics license, or “full approval.”
It’s unclear which mechanism the FDA might use, or exactly who will be eligible for the shots.
The United States would follow other nations such as Israel, France, the United Kingdom, and Germany in planning for or authorizing boosters for some vulnerable individuals.
The World Health Organization (WHO) has voiced strong opposition to the use of boosters in wealthy countries while much of the world still doesn’t have access to these lifesaving therapies. The WHO has asked wealthy nations to hold off on giving boosters until at least the end of September to give more people the opportunity to get a first dose.
The CDC’s Advisory Committee on Immunization Practices (ACIP) meets again on Aug. 13 and is expected to discuss booster doses for this population of patients. The ACIP officially makes recommendations on the use of vaccines to the nation’s doctors.
The committee’s recommendation ensures that a vaccine will be covered by public and private insurers. Statutory vaccination requirements are also made based on the ACIP’s recommendations.
A version of this article first appeared on Medscape.com.
Masking in school: A battle of the op-eds
Traditionally, as the ides of August descend upon us we expect to be bombarded with advertisements encouraging parents and students to finish up their back-to-school shopping. But, this year the question on every parent and school administrator’s mind is not which color back pack will be the most popular this year but whether a mask should be a required part of the back-to-school ensemble.
The American Academy of Pediatrics has recommended that “All students older than 2 years and all school staff should wear a mask at school” (“American Academy of Pediatrics Updates Recommendations for Opening Schools in Fall 2021.” 2021 Jul 19). The academy’s statement includes a generous list of common sense caveats but it does not include a statement that masks have been shown to be protective for children in school environments. The Centers for Disease Control and Prevention “recommends” universal indoor masking along with keeping a 3-foot separation but again fails to include any references to support the effectiveness of masks (“Guidance for COVID-19 Prevention in K-12 Schools.” 2021 Aug 5).
Not surprisingly, into this void have stepped two pairs of experts – one group purporting to have evidence that masking is effective in school environments and the other warning that masks may not only be ineffective but that they also carry some significant downsides. And, where can you find these opposing positions? Not in The Lancet. Not in the New England Journal of Medicine. We don’t have time for any of that peer-reviewed monkey business. No, this is pandemic-era science where we have an abundance of opinions and paucity of facts. You will find these opposing articles on the op-ed pages of two of this country’s major newspapers.
In the Aug. 10, 2021, edition of the New York Times you will find an article (“We Studied One Million Students. This Is What We Learned About Masking”) by two pediatricians, Kanecia Zimmerman, MD, and Danny Benjamin Jr., MD, who have “studied” a million students in North Carolina school systems and tell us universal masking is “one of the most effective and efficient strategies for preventing SARS-CoV-2 transmission in schools. These investigators write that they “believe” the low rate of in school transmission they observed in North Carolina was “because of the mask-on-mask school environment.”
However, in the next paragraph the authors admit, “Because North Carolina had a mask mandate for all K-12 schools, we could not compare masked schools with unmasked schools.” They lean instead on studies from three other states with mask mandates that also had low transmission rates and a single report of an outbreak in Israel that employed neither masking nor safe distancing.
On the other side of the divide is an article in the Wall Street Journal titled “The Case Against Masks for Children” by Marty Makary, MD, and H. Cody Meissner, MD, (2021 Aug 9). The authors, one a pediatric infectious disease specialist, argue that there is “no science behind mask mandates for children.” And, observe that, of the $46 billion spent on research grants by the National Institutes of Health, “not a single grant was dedicated to studying masking in children.”
Dr. Makary and Dr. Meissner present a variety of concerns about the effects of masking including those on the development and communication skills of young children. None of their theoretical concerns of course are supported by controlled studies. They also observe that in previous studies children seem to be less likely to transmit COVID-19 than adults. Although we all know the landscape is changing with the emergence of the delta strain. In their strongest statement the authors claim, “It is abusive to force kids who struggle with them [masks] to sacrifice for the sake of unvaccinated adults.”
So there you have it. It is a situation we have come to expect over the last 2 years – plenty of opinions and too few facts supported by controlled studies. Both pairs of authors, however, agree on two things: Vaccination should continue to be considered our primary tool in prevention and control of COVID-19. and children need to be in school. Based on nothing more than a hunch and 7 decades of hunching, I tend to side with Dr. Makary and Dr. Meissner. Depending on the situation, I suggest masking but wouldn’t mandate it for children in school.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Traditionally, as the ides of August descend upon us we expect to be bombarded with advertisements encouraging parents and students to finish up their back-to-school shopping. But, this year the question on every parent and school administrator’s mind is not which color back pack will be the most popular this year but whether a mask should be a required part of the back-to-school ensemble.
The American Academy of Pediatrics has recommended that “All students older than 2 years and all school staff should wear a mask at school” (“American Academy of Pediatrics Updates Recommendations for Opening Schools in Fall 2021.” 2021 Jul 19). The academy’s statement includes a generous list of common sense caveats but it does not include a statement that masks have been shown to be protective for children in school environments. The Centers for Disease Control and Prevention “recommends” universal indoor masking along with keeping a 3-foot separation but again fails to include any references to support the effectiveness of masks (“Guidance for COVID-19 Prevention in K-12 Schools.” 2021 Aug 5).
Not surprisingly, into this void have stepped two pairs of experts – one group purporting to have evidence that masking is effective in school environments and the other warning that masks may not only be ineffective but that they also carry some significant downsides. And, where can you find these opposing positions? Not in The Lancet. Not in the New England Journal of Medicine. We don’t have time for any of that peer-reviewed monkey business. No, this is pandemic-era science where we have an abundance of opinions and paucity of facts. You will find these opposing articles on the op-ed pages of two of this country’s major newspapers.
In the Aug. 10, 2021, edition of the New York Times you will find an article (“We Studied One Million Students. This Is What We Learned About Masking”) by two pediatricians, Kanecia Zimmerman, MD, and Danny Benjamin Jr., MD, who have “studied” a million students in North Carolina school systems and tell us universal masking is “one of the most effective and efficient strategies for preventing SARS-CoV-2 transmission in schools. These investigators write that they “believe” the low rate of in school transmission they observed in North Carolina was “because of the mask-on-mask school environment.”
However, in the next paragraph the authors admit, “Because North Carolina had a mask mandate for all K-12 schools, we could not compare masked schools with unmasked schools.” They lean instead on studies from three other states with mask mandates that also had low transmission rates and a single report of an outbreak in Israel that employed neither masking nor safe distancing.
On the other side of the divide is an article in the Wall Street Journal titled “The Case Against Masks for Children” by Marty Makary, MD, and H. Cody Meissner, MD, (2021 Aug 9). The authors, one a pediatric infectious disease specialist, argue that there is “no science behind mask mandates for children.” And, observe that, of the $46 billion spent on research grants by the National Institutes of Health, “not a single grant was dedicated to studying masking in children.”
Dr. Makary and Dr. Meissner present a variety of concerns about the effects of masking including those on the development and communication skills of young children. None of their theoretical concerns of course are supported by controlled studies. They also observe that in previous studies children seem to be less likely to transmit COVID-19 than adults. Although we all know the landscape is changing with the emergence of the delta strain. In their strongest statement the authors claim, “It is abusive to force kids who struggle with them [masks] to sacrifice for the sake of unvaccinated adults.”
So there you have it. It is a situation we have come to expect over the last 2 years – plenty of opinions and too few facts supported by controlled studies. Both pairs of authors, however, agree on two things: Vaccination should continue to be considered our primary tool in prevention and control of COVID-19. and children need to be in school. Based on nothing more than a hunch and 7 decades of hunching, I tend to side with Dr. Makary and Dr. Meissner. Depending on the situation, I suggest masking but wouldn’t mandate it for children in school.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Traditionally, as the ides of August descend upon us we expect to be bombarded with advertisements encouraging parents and students to finish up their back-to-school shopping. But, this year the question on every parent and school administrator’s mind is not which color back pack will be the most popular this year but whether a mask should be a required part of the back-to-school ensemble.
The American Academy of Pediatrics has recommended that “All students older than 2 years and all school staff should wear a mask at school” (“American Academy of Pediatrics Updates Recommendations for Opening Schools in Fall 2021.” 2021 Jul 19). The academy’s statement includes a generous list of common sense caveats but it does not include a statement that masks have been shown to be protective for children in school environments. The Centers for Disease Control and Prevention “recommends” universal indoor masking along with keeping a 3-foot separation but again fails to include any references to support the effectiveness of masks (“Guidance for COVID-19 Prevention in K-12 Schools.” 2021 Aug 5).
Not surprisingly, into this void have stepped two pairs of experts – one group purporting to have evidence that masking is effective in school environments and the other warning that masks may not only be ineffective but that they also carry some significant downsides. And, where can you find these opposing positions? Not in The Lancet. Not in the New England Journal of Medicine. We don’t have time for any of that peer-reviewed monkey business. No, this is pandemic-era science where we have an abundance of opinions and paucity of facts. You will find these opposing articles on the op-ed pages of two of this country’s major newspapers.
In the Aug. 10, 2021, edition of the New York Times you will find an article (“We Studied One Million Students. This Is What We Learned About Masking”) by two pediatricians, Kanecia Zimmerman, MD, and Danny Benjamin Jr., MD, who have “studied” a million students in North Carolina school systems and tell us universal masking is “one of the most effective and efficient strategies for preventing SARS-CoV-2 transmission in schools. These investigators write that they “believe” the low rate of in school transmission they observed in North Carolina was “because of the mask-on-mask school environment.”
However, in the next paragraph the authors admit, “Because North Carolina had a mask mandate for all K-12 schools, we could not compare masked schools with unmasked schools.” They lean instead on studies from three other states with mask mandates that also had low transmission rates and a single report of an outbreak in Israel that employed neither masking nor safe distancing.
On the other side of the divide is an article in the Wall Street Journal titled “The Case Against Masks for Children” by Marty Makary, MD, and H. Cody Meissner, MD, (2021 Aug 9). The authors, one a pediatric infectious disease specialist, argue that there is “no science behind mask mandates for children.” And, observe that, of the $46 billion spent on research grants by the National Institutes of Health, “not a single grant was dedicated to studying masking in children.”
Dr. Makary and Dr. Meissner present a variety of concerns about the effects of masking including those on the development and communication skills of young children. None of their theoretical concerns of course are supported by controlled studies. They also observe that in previous studies children seem to be less likely to transmit COVID-19 than adults. Although we all know the landscape is changing with the emergence of the delta strain. In their strongest statement the authors claim, “It is abusive to force kids who struggle with them [masks] to sacrifice for the sake of unvaccinated adults.”
So there you have it. It is a situation we have come to expect over the last 2 years – plenty of opinions and too few facts supported by controlled studies. Both pairs of authors, however, agree on two things: Vaccination should continue to be considered our primary tool in prevention and control of COVID-19. and children need to be in school. Based on nothing more than a hunch and 7 decades of hunching, I tend to side with Dr. Makary and Dr. Meissner. Depending on the situation, I suggest masking but wouldn’t mandate it for children in school.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].





