User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Mild and moderate OSAS often resolves in children
Mild to moderate obstructive sleep apnea syndrome (OSAS) resolves spontaneously in many children in as few as 7 months, based on polysomnography results from the control arm of the Childhood Adenotonsillectomy Trial (CHAT).
Symptomatic improvement in snoring, however, was less common. Nonetheless, “watchful waiting may be a reasonable option in children with low OSAS symptom burden and, especially, little snoring, who also have low AHIs [apnea/hypopnea indexes] and do not have central obesity,” markers that were most likely to be associated with resolution, wrote Dr. Ronald D. Chervin of the University of Michigan, Ann Arbor, and his colleagues. “Without surgery, habitual snoring resolves in one-half to two-thirds of affected children within 1-3 years.”
The study enrolled 453 children, aged 5-9, with an AHI of at least 2 events per hour of sleep, or an obstructive apnea index (OHI) of at least 1. All children were recruited from pediatric sleep clinics and otolaryngology practices. The study did not include children with severe OSAS, which was defined as having an apnea/hypopnea index of greater than 30, an obstructive apnea index greater than 20, or oxygen saturation less than 90% for at least 2% of total sleep time. None of the study participants had recurrent tonsillitis, had a BMI z-score of at least 3, or were taking medication for attention-deficit/hyperactivity disorder, the investigators reported (Chest. 2015;148[5]:1204-13).
Among 453 children randomized in CHAT, 194 in the control arm had complete follow-up, remained untreated surgically, and provided data for the current analyses. Mean AHI at baseline was 6.7 (range, 1.1-29.3), mean oxygen saturation at baseline was 88.8% (range, 59%-97%), and mean score on the Pediatric Sleep Questionnaire Sleep-Related Breathing Disorder (PSQ-SRBD) scale at baseline was 0.48 (range, 0.05-0.90).
Primary endpoints based on polysomnography results at 7-month follow-up were reaching an AHI of less than 2 and an obstructive apnea index of less than 1. In addition, researchers defined “substantive resolution” of symptoms related to OSAS as a total PSQ-SRBD score of 0.33 or more at baseline that declined below 0.33 and was at least 25% below the baseline value at 7-month follow-up.
At 7 months, OSAS had spontaneously resolved by polysomnography measures in 82 of the 194 children based on achieving an AHI less than 2 and an OAI less than 1. The results did not differ by sex.
However, symptomatic improvement was less common. Of 167 children with PSQ-SRBD scores of at least 0.33 at baseline, only 25 children (15%) had scores less than 0.33 and at least a 25% reduction in PSQ-SRBD score at 7 months. Of the 25, 17 were girls and 8 were boys, indicating a higher rate of spontaneous resolution in girls than in boys (P = .033). In addition, only 20 children (12%) showed both polysomnographic and symptomatic resolution of symptoms at 7 months.
“Symptoms often matter more to patients and families than do laboratory results,” the authors wrote. “In our cohort, 34 of 147 habitual snorers (23%) were no longer habitual snorers at follow-up.”
The CHAT Study was supported by the National Institutes of Health. Dr. Chervin disclosed that he is named in or has developed, patented, and copyrighted materials owned by the University of Michigan and designed to assist with assessment or treatment of sleep disorders, including the Pediatric Sleep Questionnaire Sleep-Related Breathing Disorder scale used in this study. He also has received support for research and education from Philips Respironics and Fisher & Paykel Healthcare, and has consulted for MC3 and Zansors.
Because polysomnography is expensive, time consuming, and often unavailable, otolaryngologists will often perform an adenotonsillectomy based on a strong clinical history and parental observation in a child with snoring and chronically enlarged adenoids and tonsils. The findings of this study challenge that approach. Surgery may not be the treatment of choice for children who have a low symptom burden, little snoring, low apnea/hypopnea indexes, and no central obesity. For these children, mild OSAS resolves as they grow and a period of watchful waiting makes sense.
Admittedly, adenotonsillectomy is relatively safe, but even relatively safe surgical procedures can have complications. Further, the financial impact associated with professional fees, facility fees, medications, and parental lost time at work is substantial.
We need to better understand who requires surgery and who does not. Encouraging empirical evidence suggests anti-inflammatory agents may be effective in treating mild to moderate OSAS. As we learn more about who is affected by this disorder, new treatments will likely emerge. In the meantime, we can feel comfortable that watchful waiting can be an appropriate strategy for many children.
Dr. Ian Nathanson is a pediatric pulmonologist in Maitland, Fla. He made his comments in an editorial that accompanied the article (Chest. 2015;148[5]:1129-1130).
Because polysomnography is expensive, time consuming, and often unavailable, otolaryngologists will often perform an adenotonsillectomy based on a strong clinical history and parental observation in a child with snoring and chronically enlarged adenoids and tonsils. The findings of this study challenge that approach. Surgery may not be the treatment of choice for children who have a low symptom burden, little snoring, low apnea/hypopnea indexes, and no central obesity. For these children, mild OSAS resolves as they grow and a period of watchful waiting makes sense.
Admittedly, adenotonsillectomy is relatively safe, but even relatively safe surgical procedures can have complications. Further, the financial impact associated with professional fees, facility fees, medications, and parental lost time at work is substantial.
We need to better understand who requires surgery and who does not. Encouraging empirical evidence suggests anti-inflammatory agents may be effective in treating mild to moderate OSAS. As we learn more about who is affected by this disorder, new treatments will likely emerge. In the meantime, we can feel comfortable that watchful waiting can be an appropriate strategy for many children.
Dr. Ian Nathanson is a pediatric pulmonologist in Maitland, Fla. He made his comments in an editorial that accompanied the article (Chest. 2015;148[5]:1129-1130).
Because polysomnography is expensive, time consuming, and often unavailable, otolaryngologists will often perform an adenotonsillectomy based on a strong clinical history and parental observation in a child with snoring and chronically enlarged adenoids and tonsils. The findings of this study challenge that approach. Surgery may not be the treatment of choice for children who have a low symptom burden, little snoring, low apnea/hypopnea indexes, and no central obesity. For these children, mild OSAS resolves as they grow and a period of watchful waiting makes sense.
Admittedly, adenotonsillectomy is relatively safe, but even relatively safe surgical procedures can have complications. Further, the financial impact associated with professional fees, facility fees, medications, and parental lost time at work is substantial.
We need to better understand who requires surgery and who does not. Encouraging empirical evidence suggests anti-inflammatory agents may be effective in treating mild to moderate OSAS. As we learn more about who is affected by this disorder, new treatments will likely emerge. In the meantime, we can feel comfortable that watchful waiting can be an appropriate strategy for many children.
Dr. Ian Nathanson is a pediatric pulmonologist in Maitland, Fla. He made his comments in an editorial that accompanied the article (Chest. 2015;148[5]:1129-1130).
Mild to moderate obstructive sleep apnea syndrome (OSAS) resolves spontaneously in many children in as few as 7 months, based on polysomnography results from the control arm of the Childhood Adenotonsillectomy Trial (CHAT).
Symptomatic improvement in snoring, however, was less common. Nonetheless, “watchful waiting may be a reasonable option in children with low OSAS symptom burden and, especially, little snoring, who also have low AHIs [apnea/hypopnea indexes] and do not have central obesity,” markers that were most likely to be associated with resolution, wrote Dr. Ronald D. Chervin of the University of Michigan, Ann Arbor, and his colleagues. “Without surgery, habitual snoring resolves in one-half to two-thirds of affected children within 1-3 years.”
The study enrolled 453 children, aged 5-9, with an AHI of at least 2 events per hour of sleep, or an obstructive apnea index (OHI) of at least 1. All children were recruited from pediatric sleep clinics and otolaryngology practices. The study did not include children with severe OSAS, which was defined as having an apnea/hypopnea index of greater than 30, an obstructive apnea index greater than 20, or oxygen saturation less than 90% for at least 2% of total sleep time. None of the study participants had recurrent tonsillitis, had a BMI z-score of at least 3, or were taking medication for attention-deficit/hyperactivity disorder, the investigators reported (Chest. 2015;148[5]:1204-13).
Among 453 children randomized in CHAT, 194 in the control arm had complete follow-up, remained untreated surgically, and provided data for the current analyses. Mean AHI at baseline was 6.7 (range, 1.1-29.3), mean oxygen saturation at baseline was 88.8% (range, 59%-97%), and mean score on the Pediatric Sleep Questionnaire Sleep-Related Breathing Disorder (PSQ-SRBD) scale at baseline was 0.48 (range, 0.05-0.90).
Primary endpoints based on polysomnography results at 7-month follow-up were reaching an AHI of less than 2 and an obstructive apnea index of less than 1. In addition, researchers defined “substantive resolution” of symptoms related to OSAS as a total PSQ-SRBD score of 0.33 or more at baseline that declined below 0.33 and was at least 25% below the baseline value at 7-month follow-up.
At 7 months, OSAS had spontaneously resolved by polysomnography measures in 82 of the 194 children based on achieving an AHI less than 2 and an OAI less than 1. The results did not differ by sex.
However, symptomatic improvement was less common. Of 167 children with PSQ-SRBD scores of at least 0.33 at baseline, only 25 children (15%) had scores less than 0.33 and at least a 25% reduction in PSQ-SRBD score at 7 months. Of the 25, 17 were girls and 8 were boys, indicating a higher rate of spontaneous resolution in girls than in boys (P = .033). In addition, only 20 children (12%) showed both polysomnographic and symptomatic resolution of symptoms at 7 months.
“Symptoms often matter more to patients and families than do laboratory results,” the authors wrote. “In our cohort, 34 of 147 habitual snorers (23%) were no longer habitual snorers at follow-up.”
The CHAT Study was supported by the National Institutes of Health. Dr. Chervin disclosed that he is named in or has developed, patented, and copyrighted materials owned by the University of Michigan and designed to assist with assessment or treatment of sleep disorders, including the Pediatric Sleep Questionnaire Sleep-Related Breathing Disorder scale used in this study. He also has received support for research and education from Philips Respironics and Fisher & Paykel Healthcare, and has consulted for MC3 and Zansors.
Mild to moderate obstructive sleep apnea syndrome (OSAS) resolves spontaneously in many children in as few as 7 months, based on polysomnography results from the control arm of the Childhood Adenotonsillectomy Trial (CHAT).
Symptomatic improvement in snoring, however, was less common. Nonetheless, “watchful waiting may be a reasonable option in children with low OSAS symptom burden and, especially, little snoring, who also have low AHIs [apnea/hypopnea indexes] and do not have central obesity,” markers that were most likely to be associated with resolution, wrote Dr. Ronald D. Chervin of the University of Michigan, Ann Arbor, and his colleagues. “Without surgery, habitual snoring resolves in one-half to two-thirds of affected children within 1-3 years.”
The study enrolled 453 children, aged 5-9, with an AHI of at least 2 events per hour of sleep, or an obstructive apnea index (OHI) of at least 1. All children were recruited from pediatric sleep clinics and otolaryngology practices. The study did not include children with severe OSAS, which was defined as having an apnea/hypopnea index of greater than 30, an obstructive apnea index greater than 20, or oxygen saturation less than 90% for at least 2% of total sleep time. None of the study participants had recurrent tonsillitis, had a BMI z-score of at least 3, or were taking medication for attention-deficit/hyperactivity disorder, the investigators reported (Chest. 2015;148[5]:1204-13).
Among 453 children randomized in CHAT, 194 in the control arm had complete follow-up, remained untreated surgically, and provided data for the current analyses. Mean AHI at baseline was 6.7 (range, 1.1-29.3), mean oxygen saturation at baseline was 88.8% (range, 59%-97%), and mean score on the Pediatric Sleep Questionnaire Sleep-Related Breathing Disorder (PSQ-SRBD) scale at baseline was 0.48 (range, 0.05-0.90).
Primary endpoints based on polysomnography results at 7-month follow-up were reaching an AHI of less than 2 and an obstructive apnea index of less than 1. In addition, researchers defined “substantive resolution” of symptoms related to OSAS as a total PSQ-SRBD score of 0.33 or more at baseline that declined below 0.33 and was at least 25% below the baseline value at 7-month follow-up.
At 7 months, OSAS had spontaneously resolved by polysomnography measures in 82 of the 194 children based on achieving an AHI less than 2 and an OAI less than 1. The results did not differ by sex.
However, symptomatic improvement was less common. Of 167 children with PSQ-SRBD scores of at least 0.33 at baseline, only 25 children (15%) had scores less than 0.33 and at least a 25% reduction in PSQ-SRBD score at 7 months. Of the 25, 17 were girls and 8 were boys, indicating a higher rate of spontaneous resolution in girls than in boys (P = .033). In addition, only 20 children (12%) showed both polysomnographic and symptomatic resolution of symptoms at 7 months.
“Symptoms often matter more to patients and families than do laboratory results,” the authors wrote. “In our cohort, 34 of 147 habitual snorers (23%) were no longer habitual snorers at follow-up.”
The CHAT Study was supported by the National Institutes of Health. Dr. Chervin disclosed that he is named in or has developed, patented, and copyrighted materials owned by the University of Michigan and designed to assist with assessment or treatment of sleep disorders, including the Pediatric Sleep Questionnaire Sleep-Related Breathing Disorder scale used in this study. He also has received support for research and education from Philips Respironics and Fisher & Paykel Healthcare, and has consulted for MC3 and Zansors.
FROM CHEST
Key clinical point: Advise parents that moderate obstructive sleep apnea syndrome can spontaneously resolve in children with a low baseline apnea/hypopnea index and a normal waist circumference, or if they have a low Pediatric Sleep Questionnaire and snoring score.
Major finding: After 7 months, 82 of 194 children no longer met polysomnographic criteria for OSAS; 25 of 167 children with baseline PSQ scores of at least 0.33 still had symptoms after 7 months.
Data source: A prospective cohort study of 194 children, aged 5-9 years.
Disclosures: The study was supported by the National Institutes of Health; Dr. Chervin disclosed that he is named in or has developed, patented, and copyrighted materials owned by the University of Michigan and designed to assist with assessment or treatment of sleep disorders, including the Pediatric Sleep Questionnaire Sleep-Related Breathing Disorder scale used in this study.
Riociguat deemed suitable for PAH in connective tissue disease
SAN FRANCISCO – Phase III clinical trial experience in using riociguat indicates that the drug is just as safe and effective in treating pulmonary arterial hypertension associated with connective tissue disease (CTD), particularly systemic sclerosis, as it is for the overall group of patients with PAH caused by various conditions.
“Riociguat [Adempas] slowed deterioration of exercise capacity in patients with scleroderma, with clinical effects sustained over the 2-year follow-up. Long-term safety and tolerability of riociguat in patients with PAH-CTD was quite acceptable and was similar to the overall PATENT population, with no new safety signals,” lead investigator Dr. Christopher P. Denton said at the annual meeting of the American College of Rheumatology.
The phase III, randomized, placebo-controlled PATENT-1 trial demonstrated riociguat’s benefits over 12 weeks, principally the increased exercise capacity in terms of 6-minute walk distance. The open-label extension PATENT-2 trial confirmed the long-term nature of the improvements out to 2 years. The trials were pivotal in securing approval of the novel soluble guanylate cyclase stimulator for treatment of PAH.
PAH-CTD cases comprised 25% of the total PATENT population, second only to patients with idiopathic disease. About half of the PAH-CTD cases were patients with systemic sclerosis (SSc), who typically have an especially poor prognosis. This offered the opportunity to fine-tune the analysis of riociguat’s efficacy and safety to this subgroup.
All PAH-CTD patients had appreciable signs of pulmonary hypertension on physical exertion. Most were already receiving PAH therapy, so any improvements would be on top of already existing treatment-related gains. At week 12 of the PATENT-1 trial, more PAH-CTD patients in the placebo group had died, worsened clinically, or withdrawn than in the riociguat group (24% vs. 8%). Clinical worsening had occurred in 12% of those receiving placebo, compared with none in the riociguat group. The benefits of riociguat in terms of 6-minute walk distance performance and improved World Health Organization functional class were especially pronounced for SSc patients. Analysis of PATENT-2 data demonstrated the durability of the improvements over the 2-year follow-up.
The types and frequencies of adverse events and serious adverse events were similar in PAH-CTD patients and the overall population. “Very reassuringly,” the 2-year survival rate exceeded 90% overall and was 93% in PAH-CTD patients and 94% in PAH patients with SSc, said Dr. Denton of University College London.
“The results are consistent with what is emerging from long-term trials and registry analyses, with an improvement in the longer-term outcome of patients with CTD- and SSc-associated PAH, which is likely a reflection of the more intense approach we are taking in the utility of drugs used in combination to target different pathogenic pathways,” Dr. Denton said.
Dr. Denton reported financial disclosures involving research funding with Actelion, Novartis, Roche, CSL Behring, and Bayer Healthcare. Bayer markets riociguat and sponsored the trials.
SAN FRANCISCO – Phase III clinical trial experience in using riociguat indicates that the drug is just as safe and effective in treating pulmonary arterial hypertension associated with connective tissue disease (CTD), particularly systemic sclerosis, as it is for the overall group of patients with PAH caused by various conditions.
“Riociguat [Adempas] slowed deterioration of exercise capacity in patients with scleroderma, with clinical effects sustained over the 2-year follow-up. Long-term safety and tolerability of riociguat in patients with PAH-CTD was quite acceptable and was similar to the overall PATENT population, with no new safety signals,” lead investigator Dr. Christopher P. Denton said at the annual meeting of the American College of Rheumatology.
The phase III, randomized, placebo-controlled PATENT-1 trial demonstrated riociguat’s benefits over 12 weeks, principally the increased exercise capacity in terms of 6-minute walk distance. The open-label extension PATENT-2 trial confirmed the long-term nature of the improvements out to 2 years. The trials were pivotal in securing approval of the novel soluble guanylate cyclase stimulator for treatment of PAH.
PAH-CTD cases comprised 25% of the total PATENT population, second only to patients with idiopathic disease. About half of the PAH-CTD cases were patients with systemic sclerosis (SSc), who typically have an especially poor prognosis. This offered the opportunity to fine-tune the analysis of riociguat’s efficacy and safety to this subgroup.
All PAH-CTD patients had appreciable signs of pulmonary hypertension on physical exertion. Most were already receiving PAH therapy, so any improvements would be on top of already existing treatment-related gains. At week 12 of the PATENT-1 trial, more PAH-CTD patients in the placebo group had died, worsened clinically, or withdrawn than in the riociguat group (24% vs. 8%). Clinical worsening had occurred in 12% of those receiving placebo, compared with none in the riociguat group. The benefits of riociguat in terms of 6-minute walk distance performance and improved World Health Organization functional class were especially pronounced for SSc patients. Analysis of PATENT-2 data demonstrated the durability of the improvements over the 2-year follow-up.
The types and frequencies of adverse events and serious adverse events were similar in PAH-CTD patients and the overall population. “Very reassuringly,” the 2-year survival rate exceeded 90% overall and was 93% in PAH-CTD patients and 94% in PAH patients with SSc, said Dr. Denton of University College London.
“The results are consistent with what is emerging from long-term trials and registry analyses, with an improvement in the longer-term outcome of patients with CTD- and SSc-associated PAH, which is likely a reflection of the more intense approach we are taking in the utility of drugs used in combination to target different pathogenic pathways,” Dr. Denton said.
Dr. Denton reported financial disclosures involving research funding with Actelion, Novartis, Roche, CSL Behring, and Bayer Healthcare. Bayer markets riociguat and sponsored the trials.
SAN FRANCISCO – Phase III clinical trial experience in using riociguat indicates that the drug is just as safe and effective in treating pulmonary arterial hypertension associated with connective tissue disease (CTD), particularly systemic sclerosis, as it is for the overall group of patients with PAH caused by various conditions.
“Riociguat [Adempas] slowed deterioration of exercise capacity in patients with scleroderma, with clinical effects sustained over the 2-year follow-up. Long-term safety and tolerability of riociguat in patients with PAH-CTD was quite acceptable and was similar to the overall PATENT population, with no new safety signals,” lead investigator Dr. Christopher P. Denton said at the annual meeting of the American College of Rheumatology.
The phase III, randomized, placebo-controlled PATENT-1 trial demonstrated riociguat’s benefits over 12 weeks, principally the increased exercise capacity in terms of 6-minute walk distance. The open-label extension PATENT-2 trial confirmed the long-term nature of the improvements out to 2 years. The trials were pivotal in securing approval of the novel soluble guanylate cyclase stimulator for treatment of PAH.
PAH-CTD cases comprised 25% of the total PATENT population, second only to patients with idiopathic disease. About half of the PAH-CTD cases were patients with systemic sclerosis (SSc), who typically have an especially poor prognosis. This offered the opportunity to fine-tune the analysis of riociguat’s efficacy and safety to this subgroup.
All PAH-CTD patients had appreciable signs of pulmonary hypertension on physical exertion. Most were already receiving PAH therapy, so any improvements would be on top of already existing treatment-related gains. At week 12 of the PATENT-1 trial, more PAH-CTD patients in the placebo group had died, worsened clinically, or withdrawn than in the riociguat group (24% vs. 8%). Clinical worsening had occurred in 12% of those receiving placebo, compared with none in the riociguat group. The benefits of riociguat in terms of 6-minute walk distance performance and improved World Health Organization functional class were especially pronounced for SSc patients. Analysis of PATENT-2 data demonstrated the durability of the improvements over the 2-year follow-up.
The types and frequencies of adverse events and serious adverse events were similar in PAH-CTD patients and the overall population. “Very reassuringly,” the 2-year survival rate exceeded 90% overall and was 93% in PAH-CTD patients and 94% in PAH patients with SSc, said Dr. Denton of University College London.
“The results are consistent with what is emerging from long-term trials and registry analyses, with an improvement in the longer-term outcome of patients with CTD- and SSc-associated PAH, which is likely a reflection of the more intense approach we are taking in the utility of drugs used in combination to target different pathogenic pathways,” Dr. Denton said.
Dr. Denton reported financial disclosures involving research funding with Actelion, Novartis, Roche, CSL Behring, and Bayer Healthcare. Bayer markets riociguat and sponsored the trials.
AT THE ACR ANNUAL MEETING
Key clinical point: Patients with pulmonary arterial hypertension and connective tissue disease can benefit from riociguat treatment.
Major finding: At week 12, more PAH-CTD patients in the placebo group had died, worsened clinically, or withdrawn than in the riociguat group (24% vs. 8%).
Data source: Subgroup analysis of PATENT-1 randomized, phase III, placebo-controlled, single-blinded trial and PATENT-2 open-label extension phase.
Disclosures: Dr. Denton reported financial disclosures involving research funding with Actelion, Novartis, Roche, CSL Behring, and Bayer Healthcare. Bayer markets riociguat and sponsored the trials.
A good night’s sleep with placebo
Is it just me? I don’t think that I have visited with a single patient this week who’s not struggling with sleep. Our heavily caffeinated, media-obsessed, melatonin-killing, computer-screen-staring, tragic work-life imbalances explain away a lot of it. Knowing that, though, patients in front of me seek immediate relief.
A prescription takes substantially less effort than does training patients on sleep hygiene or sleep restriction. The problem with “z” drugs is, of course, that tolerance can develop within 4 weeks with daily use. These drugs have been associated with mood and cognitive changes and increased mortality. Many of my patients cannot use them sparingly, because relief of insomnia is something of which they cannot get enough. Then we are either back to square one or playing the dose-escalation game.
I have always wondered how much of this is the “placebo effect” and how we could use this to our clinical advantage. This is certainly what I hear from my cynical colleagues when I recommend melatonin – which, by the way, I personally believe is an extremely effective and underutilized intervention. We should remind ourselves to be cognizant of the “if I can’t prescribe it, it can’t work that well” mentality.
Alexander Winkler and Winfried Rief of the University of Marburg, Germany, conducted a brilliant systematic review examining the effect of placebo conditions in randomized trials including polysomnography addressing primary insomnia (Sleep. 2015 Jun 1;38[6]:925-31).
The investigators identified 32 studies with almost 4,000 patients in 82 treatment conditions: The studies were published between 1992 and 2012. Of those 82 treatment conditions, 17 included hypnotic drugs.
Results suggest that 64% of drug response to medications for primary insomnia is achieved in the placebo group.
So what does this mean clinically? I think the first thing it means is that we should consider (re)launching frank discourse about the ethics surrounding the use of placebo in clinical medicine. Are we all too horrified to think that patients may get better despite us rather than because of us to dig too deeply here?
The second thing it means is that patients should be instructed to use the medications only when needed. If a minority of the drug effect for insomnia is from the drug itself, we should minimize the buildup of tolerance by using it sparingly. Studies that have alternated a hypnotic with a placebo show that this works just as well as using a hypnotic every night.
Patients should be encouraged to alternate therapy, such as trying melatonin first and resorting to a “z” drug only if sleep remains elusive. Alternating drug therapy may also enhance the efficacy of the medication.
Always try to combine pharmacotherapy with good sleep hygiene to maximize benefits.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article. Follow him on Twitter @jonebbert.
Is it just me? I don’t think that I have visited with a single patient this week who’s not struggling with sleep. Our heavily caffeinated, media-obsessed, melatonin-killing, computer-screen-staring, tragic work-life imbalances explain away a lot of it. Knowing that, though, patients in front of me seek immediate relief.
A prescription takes substantially less effort than does training patients on sleep hygiene or sleep restriction. The problem with “z” drugs is, of course, that tolerance can develop within 4 weeks with daily use. These drugs have been associated with mood and cognitive changes and increased mortality. Many of my patients cannot use them sparingly, because relief of insomnia is something of which they cannot get enough. Then we are either back to square one or playing the dose-escalation game.
I have always wondered how much of this is the “placebo effect” and how we could use this to our clinical advantage. This is certainly what I hear from my cynical colleagues when I recommend melatonin – which, by the way, I personally believe is an extremely effective and underutilized intervention. We should remind ourselves to be cognizant of the “if I can’t prescribe it, it can’t work that well” mentality.
Alexander Winkler and Winfried Rief of the University of Marburg, Germany, conducted a brilliant systematic review examining the effect of placebo conditions in randomized trials including polysomnography addressing primary insomnia (Sleep. 2015 Jun 1;38[6]:925-31).
The investigators identified 32 studies with almost 4,000 patients in 82 treatment conditions: The studies were published between 1992 and 2012. Of those 82 treatment conditions, 17 included hypnotic drugs.
Results suggest that 64% of drug response to medications for primary insomnia is achieved in the placebo group.
So what does this mean clinically? I think the first thing it means is that we should consider (re)launching frank discourse about the ethics surrounding the use of placebo in clinical medicine. Are we all too horrified to think that patients may get better despite us rather than because of us to dig too deeply here?
The second thing it means is that patients should be instructed to use the medications only when needed. If a minority of the drug effect for insomnia is from the drug itself, we should minimize the buildup of tolerance by using it sparingly. Studies that have alternated a hypnotic with a placebo show that this works just as well as using a hypnotic every night.
Patients should be encouraged to alternate therapy, such as trying melatonin first and resorting to a “z” drug only if sleep remains elusive. Alternating drug therapy may also enhance the efficacy of the medication.
Always try to combine pharmacotherapy with good sleep hygiene to maximize benefits.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article. Follow him on Twitter @jonebbert.
Is it just me? I don’t think that I have visited with a single patient this week who’s not struggling with sleep. Our heavily caffeinated, media-obsessed, melatonin-killing, computer-screen-staring, tragic work-life imbalances explain away a lot of it. Knowing that, though, patients in front of me seek immediate relief.
A prescription takes substantially less effort than does training patients on sleep hygiene or sleep restriction. The problem with “z” drugs is, of course, that tolerance can develop within 4 weeks with daily use. These drugs have been associated with mood and cognitive changes and increased mortality. Many of my patients cannot use them sparingly, because relief of insomnia is something of which they cannot get enough. Then we are either back to square one or playing the dose-escalation game.
I have always wondered how much of this is the “placebo effect” and how we could use this to our clinical advantage. This is certainly what I hear from my cynical colleagues when I recommend melatonin – which, by the way, I personally believe is an extremely effective and underutilized intervention. We should remind ourselves to be cognizant of the “if I can’t prescribe it, it can’t work that well” mentality.
Alexander Winkler and Winfried Rief of the University of Marburg, Germany, conducted a brilliant systematic review examining the effect of placebo conditions in randomized trials including polysomnography addressing primary insomnia (Sleep. 2015 Jun 1;38[6]:925-31).
The investigators identified 32 studies with almost 4,000 patients in 82 treatment conditions: The studies were published between 1992 and 2012. Of those 82 treatment conditions, 17 included hypnotic drugs.
Results suggest that 64% of drug response to medications for primary insomnia is achieved in the placebo group.
So what does this mean clinically? I think the first thing it means is that we should consider (re)launching frank discourse about the ethics surrounding the use of placebo in clinical medicine. Are we all too horrified to think that patients may get better despite us rather than because of us to dig too deeply here?
The second thing it means is that patients should be instructed to use the medications only when needed. If a minority of the drug effect for insomnia is from the drug itself, we should minimize the buildup of tolerance by using it sparingly. Studies that have alternated a hypnotic with a placebo show that this works just as well as using a hypnotic every night.
Patients should be encouraged to alternate therapy, such as trying melatonin first and resorting to a “z” drug only if sleep remains elusive. Alternating drug therapy may also enhance the efficacy of the medication.
Always try to combine pharmacotherapy with good sleep hygiene to maximize benefits.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article. Follow him on Twitter @jonebbert.
Careful with that cough…
September, October, and November are well known as the “coughing months” in any general pediatrician’s office. We all can relate to the frustration we feel when that handful of patients returns for the unrelenting cough after steroids and albuterol have failed. Codeine has been known to be a good cough suppressant, and when coupled with promethazine (antihistamine), you have a very effective cough syrup that been used as such for decades.
Unfortunately, over the last few decades, this cough syrup has gained notoriety for use other than cough, and now is under great scrutiny. This common syrup is now the main ingredient of a poplar drink among teens known as “Sizzurp” or the “Purple Drank.” Well known artists tweet about it, post pictures of it on Instagram, sing about it in their songs, and glamorize it in their videos.
The mixture is simple; promethazine with codeine, lemon-lime sodas, and hard candies has all the makings of a party drink. It is fizzy, colorful, and sweet, with no horrible aftertaste, so gulping is easy. Most teens don’t limit their drinking to just the purple drink, so now we have a mixture of codeine with alcohol and or marijuana. All of which result in respiratory depression and potentially death.
The abuse of this cough syrup has become so great that Actavis was reported to pull it from production. Already the pint-size bottle sells on the street for $800, and limited access will only skyrocket its value.
As clinicians, we must be aware of the misuse and abuse of common prescription medications because teens prey on busy practices with false or exaggerated symptoms to try to obtain a prescription. Despite the American Academy of Pediatrics’ warning against codeine’s use as an antitussive in children (Pediatrics. 1997 Jun;99[6]:918-20.), there has not been a significant decline in its use (Pediatrics 2014 May;133[5]:e1139-47). Pediatricians need to use extreme caution, and be vigilant to identify frequent flyers or teens known to be at risk for drug abuse. Prescribe nonnarcotic-containing products first, and only prescribe small amounts promethazine/codeine products to prevent leftovers from being kept around the house for unsupervised use.
Most importantly, educate parents and families about the danger of overdose with these products so they can monitor its use.
Dr. Pearce is a pediatrician in Frankfort, Ill.
September, October, and November are well known as the “coughing months” in any general pediatrician’s office. We all can relate to the frustration we feel when that handful of patients returns for the unrelenting cough after steroids and albuterol have failed. Codeine has been known to be a good cough suppressant, and when coupled with promethazine (antihistamine), you have a very effective cough syrup that been used as such for decades.
Unfortunately, over the last few decades, this cough syrup has gained notoriety for use other than cough, and now is under great scrutiny. This common syrup is now the main ingredient of a poplar drink among teens known as “Sizzurp” or the “Purple Drank.” Well known artists tweet about it, post pictures of it on Instagram, sing about it in their songs, and glamorize it in their videos.
The mixture is simple; promethazine with codeine, lemon-lime sodas, and hard candies has all the makings of a party drink. It is fizzy, colorful, and sweet, with no horrible aftertaste, so gulping is easy. Most teens don’t limit their drinking to just the purple drink, so now we have a mixture of codeine with alcohol and or marijuana. All of which result in respiratory depression and potentially death.
The abuse of this cough syrup has become so great that Actavis was reported to pull it from production. Already the pint-size bottle sells on the street for $800, and limited access will only skyrocket its value.
As clinicians, we must be aware of the misuse and abuse of common prescription medications because teens prey on busy practices with false or exaggerated symptoms to try to obtain a prescription. Despite the American Academy of Pediatrics’ warning against codeine’s use as an antitussive in children (Pediatrics. 1997 Jun;99[6]:918-20.), there has not been a significant decline in its use (Pediatrics 2014 May;133[5]:e1139-47). Pediatricians need to use extreme caution, and be vigilant to identify frequent flyers or teens known to be at risk for drug abuse. Prescribe nonnarcotic-containing products first, and only prescribe small amounts promethazine/codeine products to prevent leftovers from being kept around the house for unsupervised use.
Most importantly, educate parents and families about the danger of overdose with these products so they can monitor its use.
Dr. Pearce is a pediatrician in Frankfort, Ill.
September, October, and November are well known as the “coughing months” in any general pediatrician’s office. We all can relate to the frustration we feel when that handful of patients returns for the unrelenting cough after steroids and albuterol have failed. Codeine has been known to be a good cough suppressant, and when coupled with promethazine (antihistamine), you have a very effective cough syrup that been used as such for decades.
Unfortunately, over the last few decades, this cough syrup has gained notoriety for use other than cough, and now is under great scrutiny. This common syrup is now the main ingredient of a poplar drink among teens known as “Sizzurp” or the “Purple Drank.” Well known artists tweet about it, post pictures of it on Instagram, sing about it in their songs, and glamorize it in their videos.
The mixture is simple; promethazine with codeine, lemon-lime sodas, and hard candies has all the makings of a party drink. It is fizzy, colorful, and sweet, with no horrible aftertaste, so gulping is easy. Most teens don’t limit their drinking to just the purple drink, so now we have a mixture of codeine with alcohol and or marijuana. All of which result in respiratory depression and potentially death.
The abuse of this cough syrup has become so great that Actavis was reported to pull it from production. Already the pint-size bottle sells on the street for $800, and limited access will only skyrocket its value.
As clinicians, we must be aware of the misuse and abuse of common prescription medications because teens prey on busy practices with false or exaggerated symptoms to try to obtain a prescription. Despite the American Academy of Pediatrics’ warning against codeine’s use as an antitussive in children (Pediatrics. 1997 Jun;99[6]:918-20.), there has not been a significant decline in its use (Pediatrics 2014 May;133[5]:e1139-47). Pediatricians need to use extreme caution, and be vigilant to identify frequent flyers or teens known to be at risk for drug abuse. Prescribe nonnarcotic-containing products first, and only prescribe small amounts promethazine/codeine products to prevent leftovers from being kept around the house for unsupervised use.
Most importantly, educate parents and families about the danger of overdose with these products so they can monitor its use.
Dr. Pearce is a pediatrician in Frankfort, Ill.
Heroin smoking linked to emphysema
Inhalation or smoking of heroin can lead to early onset chronic obstructive pulmonary disease (COPD), according to Dr. Paul P. Walker and his colleagues from the University Hospital Aintree and the University of Liverpool, England.
“We believe that we have accumulated sufficient evidence of both physiologic impairment and structural damage to identify a discrete form of early onset COPD, commonly involving emphysema, which can be attributed to inhaled opiate use,” the researchers wrote. “The widespread use of opiates as recreational drugs in some communities means that we are likely to see more obstructive lung disease in the future.”
Recreational use of opiates has been linked to asthma, but there has not been a definitive link to airway disease. “Little is known about the association between heroin inhalation and COPD beyond a study by Buster et al. [and] no previous study has examined measures of emphysema, such as detailed lung function testing or CT scan,” the researchers wrote (Chest. 2015 Nov;148[5]:1156-1163).
The researchers studied 73 individuals who were aged 40 years or younger when they developed symptoms, were diagnosed with COPD, and smoked heroin regularly within the last 2 years. The mean history of smoking heroin was 14 years. The study participants additionally were regular smokers for at least 5 years, most were heavy smokers, and did not have a primary clinical diagnosis of asthma. All had completed spirometry on at least one occasion when clinically stable.
Data was collected during 2005-2013, via lung function testing done when subjects were both clinically stable and a minimum of 4 weeks postexacerbation. Lung function testing was done in 12 subjects via spirometry, either prebronchodilator or postbronchodilator. High-resolution CT scans (slice thickness was no greater than 2 mm) were performed in 32 subjects each analyzed by two thoracic radiologists. Emphysema was scored on a scale of 1-5 based on guidelines produced by Sakai et al., which requires examination of a cranial level taken 1 cm above the superior margin of the aortic arch, a middle level taken 1 cm below the carina, and a caudal level taken about 3 cm above the top of the diaphragm.
Data were available from 44 of the initial 73 subjects. In the 32 who had high-resolution CT scans, their mean score – taking into account the scans of the upper, middle, and lower lung – was 2.3, indicating a 5%-25% chance of emphysema; 15 of 32 individuals had a score greater than 3, indicating a 25%-50% likelihood of emphysema, in the upper lung alone.
In the 12 subjects who underwent lung function testing, the range of the diffusing capacity of the lung for carbon monoxide was 35.5-63.0, with a median of 48.0 and a mean of 50.7. Eleven of these subjects (92%) had score that qualified as “abnormal.”
As a result of “lifestyle and varying motivation” not all subjects completed the planned sequence of the investigation or returned for follow-up spirometric measurement, the researchers wrote. “Taking a history of inhaled drug use is important in patients with early-onset COPD, as is the provision of appropriate education about this new hazard of opiate use among drug users and their caretakers. In some areas and populations there may be a role for case finding using spirometry.”
Dr. Walker and his coauthors did not report any relevant financial disclosures.
This case series of heroin smokers who developed early-onset emphysema may offer insights into the development of COPD and emphysema in cigarette smokers who don’t smoke opiates.
How might narcotic use contribute to the development of COPD and emphysema? There are several possible explanations. Smokers of heroin and other illicit substances typically take a deep inhalation, combined with a Valsalva maneuver to enhance absorption of the drug into the body. This behavior has been described previously in heroin users and users of other smoked substances. In addition, these agents often burn at a very high temperature, with the potential to cause damage deep within the lung.
The depth of inhalation, dynamic hyperinflation, and barotrauma may be important factors in some patients who develop emphysema related to cigarette smoking or other factors, as well. Physicians should be aware of this problem, and the public must be educated about the dangers associated with the inhalation of these and other burned substances.
Dr. David M. Mannino is the chair of preventative medicine and environmental health in the department of epidemiology at the University of Kentucky in Lexington. He disclosed having served as a consultant for Boehringer Ingelheim GmbH, GlaxoSmithKline, AstraZeneca, Novartis AG, Merck, and Forest Pharmaceuticals, and has received research grants from GSK, Novartis, Boehringer Ingelheim, Forest Pharmaceuticals, and Pfizer. He is also compensated by Up-to-Date, has served as an expert in tobacco-related cases, and is on the board of the COPD Foundation. He made his remarks in an editorial published with the study.
This case series of heroin smokers who developed early-onset emphysema may offer insights into the development of COPD and emphysema in cigarette smokers who don’t smoke opiates.
How might narcotic use contribute to the development of COPD and emphysema? There are several possible explanations. Smokers of heroin and other illicit substances typically take a deep inhalation, combined with a Valsalva maneuver to enhance absorption of the drug into the body. This behavior has been described previously in heroin users and users of other smoked substances. In addition, these agents often burn at a very high temperature, with the potential to cause damage deep within the lung.
The depth of inhalation, dynamic hyperinflation, and barotrauma may be important factors in some patients who develop emphysema related to cigarette smoking or other factors, as well. Physicians should be aware of this problem, and the public must be educated about the dangers associated with the inhalation of these and other burned substances.
Dr. David M. Mannino is the chair of preventative medicine and environmental health in the department of epidemiology at the University of Kentucky in Lexington. He disclosed having served as a consultant for Boehringer Ingelheim GmbH, GlaxoSmithKline, AstraZeneca, Novartis AG, Merck, and Forest Pharmaceuticals, and has received research grants from GSK, Novartis, Boehringer Ingelheim, Forest Pharmaceuticals, and Pfizer. He is also compensated by Up-to-Date, has served as an expert in tobacco-related cases, and is on the board of the COPD Foundation. He made his remarks in an editorial published with the study.
This case series of heroin smokers who developed early-onset emphysema may offer insights into the development of COPD and emphysema in cigarette smokers who don’t smoke opiates.
How might narcotic use contribute to the development of COPD and emphysema? There are several possible explanations. Smokers of heroin and other illicit substances typically take a deep inhalation, combined with a Valsalva maneuver to enhance absorption of the drug into the body. This behavior has been described previously in heroin users and users of other smoked substances. In addition, these agents often burn at a very high temperature, with the potential to cause damage deep within the lung.
The depth of inhalation, dynamic hyperinflation, and barotrauma may be important factors in some patients who develop emphysema related to cigarette smoking or other factors, as well. Physicians should be aware of this problem, and the public must be educated about the dangers associated with the inhalation of these and other burned substances.
Dr. David M. Mannino is the chair of preventative medicine and environmental health in the department of epidemiology at the University of Kentucky in Lexington. He disclosed having served as a consultant for Boehringer Ingelheim GmbH, GlaxoSmithKline, AstraZeneca, Novartis AG, Merck, and Forest Pharmaceuticals, and has received research grants from GSK, Novartis, Boehringer Ingelheim, Forest Pharmaceuticals, and Pfizer. He is also compensated by Up-to-Date, has served as an expert in tobacco-related cases, and is on the board of the COPD Foundation. He made his remarks in an editorial published with the study.
Inhalation or smoking of heroin can lead to early onset chronic obstructive pulmonary disease (COPD), according to Dr. Paul P. Walker and his colleagues from the University Hospital Aintree and the University of Liverpool, England.
“We believe that we have accumulated sufficient evidence of both physiologic impairment and structural damage to identify a discrete form of early onset COPD, commonly involving emphysema, which can be attributed to inhaled opiate use,” the researchers wrote. “The widespread use of opiates as recreational drugs in some communities means that we are likely to see more obstructive lung disease in the future.”
Recreational use of opiates has been linked to asthma, but there has not been a definitive link to airway disease. “Little is known about the association between heroin inhalation and COPD beyond a study by Buster et al. [and] no previous study has examined measures of emphysema, such as detailed lung function testing or CT scan,” the researchers wrote (Chest. 2015 Nov;148[5]:1156-1163).
The researchers studied 73 individuals who were aged 40 years or younger when they developed symptoms, were diagnosed with COPD, and smoked heroin regularly within the last 2 years. The mean history of smoking heroin was 14 years. The study participants additionally were regular smokers for at least 5 years, most were heavy smokers, and did not have a primary clinical diagnosis of asthma. All had completed spirometry on at least one occasion when clinically stable.
Data was collected during 2005-2013, via lung function testing done when subjects were both clinically stable and a minimum of 4 weeks postexacerbation. Lung function testing was done in 12 subjects via spirometry, either prebronchodilator or postbronchodilator. High-resolution CT scans (slice thickness was no greater than 2 mm) were performed in 32 subjects each analyzed by two thoracic radiologists. Emphysema was scored on a scale of 1-5 based on guidelines produced by Sakai et al., which requires examination of a cranial level taken 1 cm above the superior margin of the aortic arch, a middle level taken 1 cm below the carina, and a caudal level taken about 3 cm above the top of the diaphragm.
Data were available from 44 of the initial 73 subjects. In the 32 who had high-resolution CT scans, their mean score – taking into account the scans of the upper, middle, and lower lung – was 2.3, indicating a 5%-25% chance of emphysema; 15 of 32 individuals had a score greater than 3, indicating a 25%-50% likelihood of emphysema, in the upper lung alone.
In the 12 subjects who underwent lung function testing, the range of the diffusing capacity of the lung for carbon monoxide was 35.5-63.0, with a median of 48.0 and a mean of 50.7. Eleven of these subjects (92%) had score that qualified as “abnormal.”
As a result of “lifestyle and varying motivation” not all subjects completed the planned sequence of the investigation or returned for follow-up spirometric measurement, the researchers wrote. “Taking a history of inhaled drug use is important in patients with early-onset COPD, as is the provision of appropriate education about this new hazard of opiate use among drug users and their caretakers. In some areas and populations there may be a role for case finding using spirometry.”
Dr. Walker and his coauthors did not report any relevant financial disclosures.
Inhalation or smoking of heroin can lead to early onset chronic obstructive pulmonary disease (COPD), according to Dr. Paul P. Walker and his colleagues from the University Hospital Aintree and the University of Liverpool, England.
“We believe that we have accumulated sufficient evidence of both physiologic impairment and structural damage to identify a discrete form of early onset COPD, commonly involving emphysema, which can be attributed to inhaled opiate use,” the researchers wrote. “The widespread use of opiates as recreational drugs in some communities means that we are likely to see more obstructive lung disease in the future.”
Recreational use of opiates has been linked to asthma, but there has not been a definitive link to airway disease. “Little is known about the association between heroin inhalation and COPD beyond a study by Buster et al. [and] no previous study has examined measures of emphysema, such as detailed lung function testing or CT scan,” the researchers wrote (Chest. 2015 Nov;148[5]:1156-1163).
The researchers studied 73 individuals who were aged 40 years or younger when they developed symptoms, were diagnosed with COPD, and smoked heroin regularly within the last 2 years. The mean history of smoking heroin was 14 years. The study participants additionally were regular smokers for at least 5 years, most were heavy smokers, and did not have a primary clinical diagnosis of asthma. All had completed spirometry on at least one occasion when clinically stable.
Data was collected during 2005-2013, via lung function testing done when subjects were both clinically stable and a minimum of 4 weeks postexacerbation. Lung function testing was done in 12 subjects via spirometry, either prebronchodilator or postbronchodilator. High-resolution CT scans (slice thickness was no greater than 2 mm) were performed in 32 subjects each analyzed by two thoracic radiologists. Emphysema was scored on a scale of 1-5 based on guidelines produced by Sakai et al., which requires examination of a cranial level taken 1 cm above the superior margin of the aortic arch, a middle level taken 1 cm below the carina, and a caudal level taken about 3 cm above the top of the diaphragm.
Data were available from 44 of the initial 73 subjects. In the 32 who had high-resolution CT scans, their mean score – taking into account the scans of the upper, middle, and lower lung – was 2.3, indicating a 5%-25% chance of emphysema; 15 of 32 individuals had a score greater than 3, indicating a 25%-50% likelihood of emphysema, in the upper lung alone.
In the 12 subjects who underwent lung function testing, the range of the diffusing capacity of the lung for carbon monoxide was 35.5-63.0, with a median of 48.0 and a mean of 50.7. Eleven of these subjects (92%) had score that qualified as “abnormal.”
As a result of “lifestyle and varying motivation” not all subjects completed the planned sequence of the investigation or returned for follow-up spirometric measurement, the researchers wrote. “Taking a history of inhaled drug use is important in patients with early-onset COPD, as is the provision of appropriate education about this new hazard of opiate use among drug users and their caretakers. In some areas and populations there may be a role for case finding using spirometry.”
Dr. Walker and his coauthors did not report any relevant financial disclosures.
FROM CHEST
Key clinical point: Recreational smoking of heroin is associated with a higher risk of developing early-onset emphysema.
Major finding: Overall high-resolution CT scan emphysema score averaged across the upper, middle, and lower part of the lung was 2.3 (5%-25% emphysema), with 47% subjects having an upper lobe emphysema score 3 (25%-50% emphysema).
Data source: Cohort study of 73 heroin smokers with clinician-diagnosed and spirometrically confirmed COPD, whose symptoms developed before age 40 years.
Disclosures: The authors did not report any relevant financial disclosures.
Prophylactic Antibiotics Don’t Prevent Poststroke Pneumonia
Prophylactic antibiotics don’t prevent poststroke pneumonia or reduce mortality, even in patients who have stroke-induced dysphagia and are at high risk of aspiration, according to a report published in the Lancet.
In a prospective open-label cluster-randomized clinical trial, researchers randomly assigned 37 stroke units in the United Kingdom to give new patients either prophylactic antibiotics for 7 days plus standard stroke care (564 patients) or standard stroke care alone (524 patients). All study participants were considered “unsafe to swallow” because they had impaired consciousness, they failed a bedside swallow test, or they had a nasogastric tube, said Lalit Kalra, Ph.D., of the department of basic and clinical neurosciences and the Institute of Psychiatry, Psychology, and Neuroscience at King’s College, London, and his associates.
Each hospital was allowed to choose which prophylactic antibiotics to use according to their local guidelines, as well as which dosage and route of administration. The primary outcome was the incidence of post-stroke pneumonia within 2 weeks of hospitalization, which was assessed by two separate methods: a statistician masked to treatment assignment diagnosed pneumonia according to a criteria-based hierarchical algorithm, and a local treating physician diagnosed pneumonia according to clinical findings.
According to the algorithm, poststroke pneumonia developed in 13% of patients given prophylactic antibiotics and 10% of the control group, for an OR of 1.21. According to the clinical findings, poststroke pneumonia developed in 16% of the intervention group and 15% of the control group, for an OR of 1.01. By either definition, prophylactic antibiotics failed to reduce the incidence of poststroke pneumonia, the investigators said (Lancet 2015;386:1835-44).
In addition, all-cause mortality at 14 days (10%) and at 90 days (39%) was not significantly different between the two study groups. And there was no significant difference in the percentage of patients with good functional outcomes. Prophylactic antibiotics were associated with longer hospital stays than standard treatment.
On the positive side, prophylactic antibiotics did reduce the number of nonpneumonia infections, especially urosepsis.
Adverse effects, including cases of Clostridium difficile-positive diarrhea and MRSA colonization, were rare and occurred in equal numbers across the two study groups.
The findings indicate that routine use of antibiotics to prevent poststroke pneumonia “cannot be recommended and should be used judiciously ... in patients after stroke who are managed on stroke units, even if they are at high risk of aspiration,” Dr. Kalra and associates said.
The most likely explanation for this study’s negative findings is that prophylactic antibiotics “do not add to existing preventive measures such as positioning, regular suction, swallowing techniques, modified diets, and early initiation of antibiotics” if patients are suspected of developing pneumonia. It also is possible that poststroke pneumonia is not just a straightforward infection but a complex respiratory syndrome stemming from multiple bacterial, chemical, and immunologic causes that might not respond to antibiotics alone, they added.
This study was funded by the U.K. National Institute for Health Research. Dr. Kalra and associates reported having no relevant financial disclosures.
Prophylactic antibiotics don’t prevent poststroke pneumonia or reduce mortality, even in patients who have stroke-induced dysphagia and are at high risk of aspiration, according to a report published in the Lancet.
In a prospective open-label cluster-randomized clinical trial, researchers randomly assigned 37 stroke units in the United Kingdom to give new patients either prophylactic antibiotics for 7 days plus standard stroke care (564 patients) or standard stroke care alone (524 patients). All study participants were considered “unsafe to swallow” because they had impaired consciousness, they failed a bedside swallow test, or they had a nasogastric tube, said Lalit Kalra, Ph.D., of the department of basic and clinical neurosciences and the Institute of Psychiatry, Psychology, and Neuroscience at King’s College, London, and his associates.
Each hospital was allowed to choose which prophylactic antibiotics to use according to their local guidelines, as well as which dosage and route of administration. The primary outcome was the incidence of post-stroke pneumonia within 2 weeks of hospitalization, which was assessed by two separate methods: a statistician masked to treatment assignment diagnosed pneumonia according to a criteria-based hierarchical algorithm, and a local treating physician diagnosed pneumonia according to clinical findings.
According to the algorithm, poststroke pneumonia developed in 13% of patients given prophylactic antibiotics and 10% of the control group, for an OR of 1.21. According to the clinical findings, poststroke pneumonia developed in 16% of the intervention group and 15% of the control group, for an OR of 1.01. By either definition, prophylactic antibiotics failed to reduce the incidence of poststroke pneumonia, the investigators said (Lancet 2015;386:1835-44).
In addition, all-cause mortality at 14 days (10%) and at 90 days (39%) was not significantly different between the two study groups. And there was no significant difference in the percentage of patients with good functional outcomes. Prophylactic antibiotics were associated with longer hospital stays than standard treatment.
On the positive side, prophylactic antibiotics did reduce the number of nonpneumonia infections, especially urosepsis.
Adverse effects, including cases of Clostridium difficile-positive diarrhea and MRSA colonization, were rare and occurred in equal numbers across the two study groups.
The findings indicate that routine use of antibiotics to prevent poststroke pneumonia “cannot be recommended and should be used judiciously ... in patients after stroke who are managed on stroke units, even if they are at high risk of aspiration,” Dr. Kalra and associates said.
The most likely explanation for this study’s negative findings is that prophylactic antibiotics “do not add to existing preventive measures such as positioning, regular suction, swallowing techniques, modified diets, and early initiation of antibiotics” if patients are suspected of developing pneumonia. It also is possible that poststroke pneumonia is not just a straightforward infection but a complex respiratory syndrome stemming from multiple bacterial, chemical, and immunologic causes that might not respond to antibiotics alone, they added.
This study was funded by the U.K. National Institute for Health Research. Dr. Kalra and associates reported having no relevant financial disclosures.
Prophylactic antibiotics don’t prevent poststroke pneumonia or reduce mortality, even in patients who have stroke-induced dysphagia and are at high risk of aspiration, according to a report published in the Lancet.
In a prospective open-label cluster-randomized clinical trial, researchers randomly assigned 37 stroke units in the United Kingdom to give new patients either prophylactic antibiotics for 7 days plus standard stroke care (564 patients) or standard stroke care alone (524 patients). All study participants were considered “unsafe to swallow” because they had impaired consciousness, they failed a bedside swallow test, or they had a nasogastric tube, said Lalit Kalra, Ph.D., of the department of basic and clinical neurosciences and the Institute of Psychiatry, Psychology, and Neuroscience at King’s College, London, and his associates.
Each hospital was allowed to choose which prophylactic antibiotics to use according to their local guidelines, as well as which dosage and route of administration. The primary outcome was the incidence of post-stroke pneumonia within 2 weeks of hospitalization, which was assessed by two separate methods: a statistician masked to treatment assignment diagnosed pneumonia according to a criteria-based hierarchical algorithm, and a local treating physician diagnosed pneumonia according to clinical findings.
According to the algorithm, poststroke pneumonia developed in 13% of patients given prophylactic antibiotics and 10% of the control group, for an OR of 1.21. According to the clinical findings, poststroke pneumonia developed in 16% of the intervention group and 15% of the control group, for an OR of 1.01. By either definition, prophylactic antibiotics failed to reduce the incidence of poststroke pneumonia, the investigators said (Lancet 2015;386:1835-44).
In addition, all-cause mortality at 14 days (10%) and at 90 days (39%) was not significantly different between the two study groups. And there was no significant difference in the percentage of patients with good functional outcomes. Prophylactic antibiotics were associated with longer hospital stays than standard treatment.
On the positive side, prophylactic antibiotics did reduce the number of nonpneumonia infections, especially urosepsis.
Adverse effects, including cases of Clostridium difficile-positive diarrhea and MRSA colonization, were rare and occurred in equal numbers across the two study groups.
The findings indicate that routine use of antibiotics to prevent poststroke pneumonia “cannot be recommended and should be used judiciously ... in patients after stroke who are managed on stroke units, even if they are at high risk of aspiration,” Dr. Kalra and associates said.
The most likely explanation for this study’s negative findings is that prophylactic antibiotics “do not add to existing preventive measures such as positioning, regular suction, swallowing techniques, modified diets, and early initiation of antibiotics” if patients are suspected of developing pneumonia. It also is possible that poststroke pneumonia is not just a straightforward infection but a complex respiratory syndrome stemming from multiple bacterial, chemical, and immunologic causes that might not respond to antibiotics alone, they added.
This study was funded by the U.K. National Institute for Health Research. Dr. Kalra and associates reported having no relevant financial disclosures.
FROM THE LANCET
Prophylactic antibiotics don’t prevent poststroke pneumonia
Prophylactic antibiotics don’t prevent poststroke pneumonia or reduce mortality, even in patients who have stroke-induced dysphagia and are at high risk of aspiration, according to a report published in the Lancet.
In a prospective open-label cluster-randomized clinical trial, researchers randomly assigned 37 stroke units in the United Kingdom to give new patients either prophylactic antibiotics for 7 days plus standard stroke care (564 patients) or standard stroke care alone (524 patients). All study participants were considered “unsafe to swallow” because they had impaired consciousness, they failed a bedside swallow test, or they had a nasogastric tube, said Lalit Kalra, Ph.D., of the department of basic and clinical neurosciences and the Institute of Psychiatry, Psychology, and Neuroscience at King’s College, London, and his associates.
Each hospital was allowed to choose which prophylactic antibiotics to use according to their local guidelines, as well as which dosage and route of administration. The primary outcome was the incidence of post-stroke pneumonia within 2 weeks of hospitalization, which was assessed by two separate methods: a statistician masked to treatment assignment diagnosed pneumonia according to a criteria-based hierarchical algorithm, and a local treating physician diagnosed pneumonia according to clinical findings.
According to the algorithm, poststroke pneumonia developed in 13% of patients given prophylactic antibiotics and 10% of the control group, for an OR of 1.21. According to the clinical findings, poststroke pneumonia developed in 16% of the intervention group and 15% of the control group, for an OR of 1.01. By either definition, prophylactic antibiotics failed to reduce the incidence of poststroke pneumonia, the investigators said (Lancet 2015;386:1835-44).
In addition, all-cause mortality at 14 days (10%) and at 90 days (39%) was not significantly different between the two study groups. And there was no significant difference in the percentage of patients with good functional outcomes. Prophylactic antibiotics were associated with longer hospital stays than standard treatment.
On the positive side, prophylactic antibiotics did reduce the number of nonpneumonia infections, especially urosepsis.
Adverse effects, including cases of Clostridium difficile-positive diarrhea and MRSA colonization, were rare and occurred in equal numbers across the two study groups.
The findings indicate that routine use of antibiotics to prevent poststroke pneumonia “cannot be recommended and should be used judiciously ... in patients after stroke who are managed on stroke units, even if they are at high risk of aspiration,” Dr. Kalra and associates said.
The most likely explanation for this study’s negative findings is that prophylactic antibiotics “do not add to existing preventive measures such as positioning, regular suction, swallowing techniques, modified diets, and early initiation of antibiotics” if patients are suspected of developing pneumonia. It also is possible that poststroke pneumonia is not just a straightforward infection but a complex respiratory syndrome stemming from multiple bacterial, chemical, and immunologic causes that might not respond to antibiotics alone, they added.
This study was funded by the U.K. National Institute for Health Research. Dr. Kalra and associates reported having no relevant financial disclosures.
Prophylactic antibiotics don’t prevent poststroke pneumonia or reduce mortality, even in patients who have stroke-induced dysphagia and are at high risk of aspiration, according to a report published in the Lancet.
In a prospective open-label cluster-randomized clinical trial, researchers randomly assigned 37 stroke units in the United Kingdom to give new patients either prophylactic antibiotics for 7 days plus standard stroke care (564 patients) or standard stroke care alone (524 patients). All study participants were considered “unsafe to swallow” because they had impaired consciousness, they failed a bedside swallow test, or they had a nasogastric tube, said Lalit Kalra, Ph.D., of the department of basic and clinical neurosciences and the Institute of Psychiatry, Psychology, and Neuroscience at King’s College, London, and his associates.
Each hospital was allowed to choose which prophylactic antibiotics to use according to their local guidelines, as well as which dosage and route of administration. The primary outcome was the incidence of post-stroke pneumonia within 2 weeks of hospitalization, which was assessed by two separate methods: a statistician masked to treatment assignment diagnosed pneumonia according to a criteria-based hierarchical algorithm, and a local treating physician diagnosed pneumonia according to clinical findings.
According to the algorithm, poststroke pneumonia developed in 13% of patients given prophylactic antibiotics and 10% of the control group, for an OR of 1.21. According to the clinical findings, poststroke pneumonia developed in 16% of the intervention group and 15% of the control group, for an OR of 1.01. By either definition, prophylactic antibiotics failed to reduce the incidence of poststroke pneumonia, the investigators said (Lancet 2015;386:1835-44).
In addition, all-cause mortality at 14 days (10%) and at 90 days (39%) was not significantly different between the two study groups. And there was no significant difference in the percentage of patients with good functional outcomes. Prophylactic antibiotics were associated with longer hospital stays than standard treatment.
On the positive side, prophylactic antibiotics did reduce the number of nonpneumonia infections, especially urosepsis.
Adverse effects, including cases of Clostridium difficile-positive diarrhea and MRSA colonization, were rare and occurred in equal numbers across the two study groups.
The findings indicate that routine use of antibiotics to prevent poststroke pneumonia “cannot be recommended and should be used judiciously ... in patients after stroke who are managed on stroke units, even if they are at high risk of aspiration,” Dr. Kalra and associates said.
The most likely explanation for this study’s negative findings is that prophylactic antibiotics “do not add to existing preventive measures such as positioning, regular suction, swallowing techniques, modified diets, and early initiation of antibiotics” if patients are suspected of developing pneumonia. It also is possible that poststroke pneumonia is not just a straightforward infection but a complex respiratory syndrome stemming from multiple bacterial, chemical, and immunologic causes that might not respond to antibiotics alone, they added.
This study was funded by the U.K. National Institute for Health Research. Dr. Kalra and associates reported having no relevant financial disclosures.
Prophylactic antibiotics don’t prevent poststroke pneumonia or reduce mortality, even in patients who have stroke-induced dysphagia and are at high risk of aspiration, according to a report published in the Lancet.
In a prospective open-label cluster-randomized clinical trial, researchers randomly assigned 37 stroke units in the United Kingdom to give new patients either prophylactic antibiotics for 7 days plus standard stroke care (564 patients) or standard stroke care alone (524 patients). All study participants were considered “unsafe to swallow” because they had impaired consciousness, they failed a bedside swallow test, or they had a nasogastric tube, said Lalit Kalra, Ph.D., of the department of basic and clinical neurosciences and the Institute of Psychiatry, Psychology, and Neuroscience at King’s College, London, and his associates.
Each hospital was allowed to choose which prophylactic antibiotics to use according to their local guidelines, as well as which dosage and route of administration. The primary outcome was the incidence of post-stroke pneumonia within 2 weeks of hospitalization, which was assessed by two separate methods: a statistician masked to treatment assignment diagnosed pneumonia according to a criteria-based hierarchical algorithm, and a local treating physician diagnosed pneumonia according to clinical findings.
According to the algorithm, poststroke pneumonia developed in 13% of patients given prophylactic antibiotics and 10% of the control group, for an OR of 1.21. According to the clinical findings, poststroke pneumonia developed in 16% of the intervention group and 15% of the control group, for an OR of 1.01. By either definition, prophylactic antibiotics failed to reduce the incidence of poststroke pneumonia, the investigators said (Lancet 2015;386:1835-44).
In addition, all-cause mortality at 14 days (10%) and at 90 days (39%) was not significantly different between the two study groups. And there was no significant difference in the percentage of patients with good functional outcomes. Prophylactic antibiotics were associated with longer hospital stays than standard treatment.
On the positive side, prophylactic antibiotics did reduce the number of nonpneumonia infections, especially urosepsis.
Adverse effects, including cases of Clostridium difficile-positive diarrhea and MRSA colonization, were rare and occurred in equal numbers across the two study groups.
The findings indicate that routine use of antibiotics to prevent poststroke pneumonia “cannot be recommended and should be used judiciously ... in patients after stroke who are managed on stroke units, even if they are at high risk of aspiration,” Dr. Kalra and associates said.
The most likely explanation for this study’s negative findings is that prophylactic antibiotics “do not add to existing preventive measures such as positioning, regular suction, swallowing techniques, modified diets, and early initiation of antibiotics” if patients are suspected of developing pneumonia. It also is possible that poststroke pneumonia is not just a straightforward infection but a complex respiratory syndrome stemming from multiple bacterial, chemical, and immunologic causes that might not respond to antibiotics alone, they added.
This study was funded by the U.K. National Institute for Health Research. Dr. Kalra and associates reported having no relevant financial disclosures.
FROM THE LANCET
Key clinical point: Prophylactic antibiotics don’t prevent poststroke pneumonia in patients with dysphagia.
Major finding: According to the algorithm, poststroke pneumonia developed in 13% of patients given prophylactic antibiotics and 10% of the control group (OR 1.21), while according to the clinical findings, poststroke pneumonia developed in 16% of the intervention group and 15% of the control group, for an OR of 1.01.
Data source: A prospective multicenter cluster-randomized open-label clinical trial involving 1,088 stroke patients followed for 90 days.
Disclosures: This study was funded by the U.K. National Institute for Health Research. Dr. Kalra and associates reported having no relevant financial disclosures.
Judicious antibiotic use key in ambulatory settings
I was recently asked to evaluate a young child with a urinary tract infection caused by an extended spectrum beta-lactamase (ESBL)–producing Escherichia coli.
I’d just broken the bad news to the mother: There was no oral medication available to treat the baby, so she’d have to stay in the hospital for a full intravenous course.
“Has your child been treated with antibiotics recently?” I asked the mother, wondering how the baby had come to have such a resistant infection.
“She had a couple days of runny nose and a low-grade fever a couple of weeks ago,” she told me. “Her doctor treated her for a sinus infection.”
In 2011, doctors in outpatient settings across the United States wrote 262.5 million prescriptions for antibiotics – 73.7 million for children – and according to the Centers for Disease Control and Prevention, about 50% of these were completely unnecessary because they were prescribed for viral respiratory tract infections (Clin Infect Dis. 2015 May 1;60[9]:1308-16).
Prescribing practices varied by region, with the highest rates in the South. Don’t think I’m judging. I live in Kentucky, the state with the highest rate of antibiotic prescribing at 1,281 prescriptions per 1,000 persons. Is it any wonder that we’re seeing kids with very resistant infections?
The CDC estimates that at least two million people in the United States are infected annually with antibiotic-resistant bacteria and at least 23,000 of them die as a result of these infections. It is estimated that prevention strategies that include better antibiotic prescribing could prevent as many as 619,000 infections and 37,000 deaths over 5 years. Fortunately, my little patient recovered fully, but it has made me think about antimicrobial stewardship, especially its role in the outpatient setting.
According the American Academy of Pediatrics, the goal of antimicrobial stewardship is “to optimize antimicrobial use, with the aim of decreasing inappropriate use that leads to unwarranted toxicity and to selection and spread of resistant organisms.”
Antimicrobial stewardship programs (ASPs) are increasingly common in inpatient settings and have been shown to reduce antibiotic use. These programs can take many forms. The hospital where I work relies primarily on clinical guidelines emphasizing appropriate empiric therapy for a variety of common conditions. Other hospitals employ prospective audit and feedback, as well as a restricted formulary. Medicare and Medicaid Conditions of Participation will soon require hospitals that receive funds from the Centers for Medicare and Medicaid Services have an ASP.
Comparatively little has been published about ASPs in the outpatient setting. The American Academy of Pediatrics suggests that effective strategies include patient education, provider education, provider audit and feedback, and clinical decision support. We have at least some data that these work, at least in a research setting.
From 2000 to 2003, a controlled, cluster-randomized trial in 16 Massachusetts communities demonstrated that a 3-year, multifaceted, community-level intervention was “modestly successful” in reducing antibiotic use (Pediatrics. 2008 Jan;121[1]:e15-23). As a part of this intervention, parents received education via direct mail and in primary care settings, pharmacies, and child care centers while physicians received small-group education, frequent updates and educational materials, and prescribing feedback. Antibiotic prescribing was measured via health insurance claims data from all children who were 6 years of age or younger and resided in study communities, and were insured by one of four participating health plans. Coincident with the intervention, there was 4.2% decrease in antibiotic prescribing among children aged 24 to <48 months and a 6.7% decrease among those aged 48-72 months. The effect was greatest among Medicaid-insured children.
More recently, 18 primary care practices in Pennsylvania and New Jersey were randomized to an intervention that consisted of a 1-hour, on-site education session followed by 1 year of personalized, quarterly audit and feedback of prescribing for bacterial and viral acute respiratory tract infections (ARTIs), or usual practice (JAMA. 2013 Jun 12;309[22]:2345-52). The prescribing practices of 162 clinicians were included in the analysis.
Broad spectrum–antibiotic prescribing decreased in intervention practices, compared with controls (26.8% to 14.3% among intervention practices vs. 28.4% to 22.6% in controls), as did “off-guideline” prescribing for pneumonia and acute sinusitis. Antibiotic prescribing for viral infections was relatively low at baseline and did not change. The authors concluded that “extending antimicrobial stewardship to the ambulatory setting, where such programs have generally not been implemented, may have important health benefits.” Unfortunately, the positive effect in these practices was not sustained after the audit and feedback stopped (JAMA. 2014 Dec 17;312[23]:2569-70).
Not all antimicrobial stewardship interventions need to be time- and resource-intensive. Investigators in California found that providers who publicly pledged to reducing inappropriate antibiotic use for ARTIs by signing and posting a commitment letter in exam rooms actually prescribed fewer inappropriate antibiotic courses for their adult patients (JAMA Intern Med. 2014 Mar;174[3]:425-31).
“When you have a cough, sore throat, or other illness, your doctor will help you select the best possible treatments. If an antibiotic would do more harm than good, your doctor will explain this to you, and may offer other treatments that are better for you,” the letter read in part. There was a 19.7 absolute percentage reduction in inappropriate antibiotic prescribing for ARTIs among clinicians randomized to the commitment letter invention relative to controls.
Can antimicrobial strategies work in the “real” world, in a busy pediatrician’s office? According to Dr. Patricia Purcell, a physician with East Louisville Pediatrics in Louisville, Ky., the answer is “yes.”
“We actually start with education in the newborn period,” Dr. Purcell said. “We let parents know that we are not going to call in antibiotics over the phone, and we’re not going to prescribe them for an upper respiratory tract infection.”
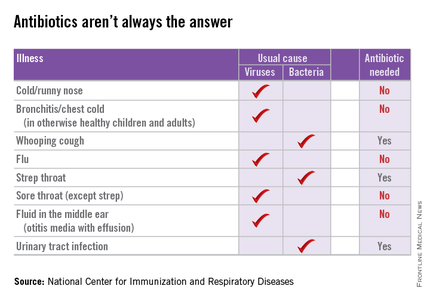
Dr. Purcell and her partners have committed to following evidence-based guidelines for antibiotic practices, such as the AAP’s guidelines for otitis media and sinusitis. She also noted that at least one major insurance company is starting to provide the group feedback about their antibiotic-prescribing practices. “They want to make sure we are not prescribing antibiotics for viruses,” she said.
Still, the message that antibiotics are not always the answer can be a bitter pill for some parents to swallow. A pediatrician friend in Alabama notes: “I have these conversations every day, and a lot of parents are mad at me for not prescribing antibiotics for their child’s ‘terrible cold.’” Another friend notes that watchful waiting can be a burden for parents who have high copays or difficulties with transportation.
Still, many parents would welcome a frank discussion about the risks and benefits of antibiotics. After I shared some of the CDC information for parents with a nursing colleague, she told me that her daughter recently had a febrile illness and was diagnosed with otitis media. “I don’t like giving my kids meds they don’t need,” she told me. “However, if the doc says they need antibiotics and they prescribe them, I give them. I never say, ‘Do we really need antibiotics for that?’”
Now she is rethinking that approach. “Was 10 days of amoxicillin necessary for a ‘red’ eardrum?! I’m just a mom. ... I don’t know the answer to that! Was her ear red because she had been crying or because of her fever? Did she get ‘treatment’ she did not need? Did the doctor give me antibiotics without education because she assumed that is why I brought her in?”
This year’s “Get Smart About Antibiotics Week” was Nov. 16-22. This annual 1-week observance is intended to raise awareness of the threat of antibiotic resistance and the importance of appropriate prescribing and use. Kudos if you celebrated this in your office. If you missed it, it’s not too late to check out some of the activities suggested by the CDC, and try one or two in your own practice. Email me with your ideas about stewardship in the outpatient setting, and I’ll try to feature at least some of them in a future column.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. Dr. Bryant disclosed that she has been an investigator for clinical trials funded by Pfizer for the past 2 years. Email her at [email protected].
I was recently asked to evaluate a young child with a urinary tract infection caused by an extended spectrum beta-lactamase (ESBL)–producing Escherichia coli.
I’d just broken the bad news to the mother: There was no oral medication available to treat the baby, so she’d have to stay in the hospital for a full intravenous course.
“Has your child been treated with antibiotics recently?” I asked the mother, wondering how the baby had come to have such a resistant infection.
“She had a couple days of runny nose and a low-grade fever a couple of weeks ago,” she told me. “Her doctor treated her for a sinus infection.”
In 2011, doctors in outpatient settings across the United States wrote 262.5 million prescriptions for antibiotics – 73.7 million for children – and according to the Centers for Disease Control and Prevention, about 50% of these were completely unnecessary because they were prescribed for viral respiratory tract infections (Clin Infect Dis. 2015 May 1;60[9]:1308-16).
Prescribing practices varied by region, with the highest rates in the South. Don’t think I’m judging. I live in Kentucky, the state with the highest rate of antibiotic prescribing at 1,281 prescriptions per 1,000 persons. Is it any wonder that we’re seeing kids with very resistant infections?
The CDC estimates that at least two million people in the United States are infected annually with antibiotic-resistant bacteria and at least 23,000 of them die as a result of these infections. It is estimated that prevention strategies that include better antibiotic prescribing could prevent as many as 619,000 infections and 37,000 deaths over 5 years. Fortunately, my little patient recovered fully, but it has made me think about antimicrobial stewardship, especially its role in the outpatient setting.
According the American Academy of Pediatrics, the goal of antimicrobial stewardship is “to optimize antimicrobial use, with the aim of decreasing inappropriate use that leads to unwarranted toxicity and to selection and spread of resistant organisms.”
Antimicrobial stewardship programs (ASPs) are increasingly common in inpatient settings and have been shown to reduce antibiotic use. These programs can take many forms. The hospital where I work relies primarily on clinical guidelines emphasizing appropriate empiric therapy for a variety of common conditions. Other hospitals employ prospective audit and feedback, as well as a restricted formulary. Medicare and Medicaid Conditions of Participation will soon require hospitals that receive funds from the Centers for Medicare and Medicaid Services have an ASP.
Comparatively little has been published about ASPs in the outpatient setting. The American Academy of Pediatrics suggests that effective strategies include patient education, provider education, provider audit and feedback, and clinical decision support. We have at least some data that these work, at least in a research setting.
From 2000 to 2003, a controlled, cluster-randomized trial in 16 Massachusetts communities demonstrated that a 3-year, multifaceted, community-level intervention was “modestly successful” in reducing antibiotic use (Pediatrics. 2008 Jan;121[1]:e15-23). As a part of this intervention, parents received education via direct mail and in primary care settings, pharmacies, and child care centers while physicians received small-group education, frequent updates and educational materials, and prescribing feedback. Antibiotic prescribing was measured via health insurance claims data from all children who were 6 years of age or younger and resided in study communities, and were insured by one of four participating health plans. Coincident with the intervention, there was 4.2% decrease in antibiotic prescribing among children aged 24 to <48 months and a 6.7% decrease among those aged 48-72 months. The effect was greatest among Medicaid-insured children.
More recently, 18 primary care practices in Pennsylvania and New Jersey were randomized to an intervention that consisted of a 1-hour, on-site education session followed by 1 year of personalized, quarterly audit and feedback of prescribing for bacterial and viral acute respiratory tract infections (ARTIs), or usual practice (JAMA. 2013 Jun 12;309[22]:2345-52). The prescribing practices of 162 clinicians were included in the analysis.
Broad spectrum–antibiotic prescribing decreased in intervention practices, compared with controls (26.8% to 14.3% among intervention practices vs. 28.4% to 22.6% in controls), as did “off-guideline” prescribing for pneumonia and acute sinusitis. Antibiotic prescribing for viral infections was relatively low at baseline and did not change. The authors concluded that “extending antimicrobial stewardship to the ambulatory setting, where such programs have generally not been implemented, may have important health benefits.” Unfortunately, the positive effect in these practices was not sustained after the audit and feedback stopped (JAMA. 2014 Dec 17;312[23]:2569-70).
Not all antimicrobial stewardship interventions need to be time- and resource-intensive. Investigators in California found that providers who publicly pledged to reducing inappropriate antibiotic use for ARTIs by signing and posting a commitment letter in exam rooms actually prescribed fewer inappropriate antibiotic courses for their adult patients (JAMA Intern Med. 2014 Mar;174[3]:425-31).
“When you have a cough, sore throat, or other illness, your doctor will help you select the best possible treatments. If an antibiotic would do more harm than good, your doctor will explain this to you, and may offer other treatments that are better for you,” the letter read in part. There was a 19.7 absolute percentage reduction in inappropriate antibiotic prescribing for ARTIs among clinicians randomized to the commitment letter invention relative to controls.
Can antimicrobial strategies work in the “real” world, in a busy pediatrician’s office? According to Dr. Patricia Purcell, a physician with East Louisville Pediatrics in Louisville, Ky., the answer is “yes.”
“We actually start with education in the newborn period,” Dr. Purcell said. “We let parents know that we are not going to call in antibiotics over the phone, and we’re not going to prescribe them for an upper respiratory tract infection.”

Dr. Purcell and her partners have committed to following evidence-based guidelines for antibiotic practices, such as the AAP’s guidelines for otitis media and sinusitis. She also noted that at least one major insurance company is starting to provide the group feedback about their antibiotic-prescribing practices. “They want to make sure we are not prescribing antibiotics for viruses,” she said.
Still, the message that antibiotics are not always the answer can be a bitter pill for some parents to swallow. A pediatrician friend in Alabama notes: “I have these conversations every day, and a lot of parents are mad at me for not prescribing antibiotics for their child’s ‘terrible cold.’” Another friend notes that watchful waiting can be a burden for parents who have high copays or difficulties with transportation.
Still, many parents would welcome a frank discussion about the risks and benefits of antibiotics. After I shared some of the CDC information for parents with a nursing colleague, she told me that her daughter recently had a febrile illness and was diagnosed with otitis media. “I don’t like giving my kids meds they don’t need,” she told me. “However, if the doc says they need antibiotics and they prescribe them, I give them. I never say, ‘Do we really need antibiotics for that?’”
Now she is rethinking that approach. “Was 10 days of amoxicillin necessary for a ‘red’ eardrum?! I’m just a mom. ... I don’t know the answer to that! Was her ear red because she had been crying or because of her fever? Did she get ‘treatment’ she did not need? Did the doctor give me antibiotics without education because she assumed that is why I brought her in?”
This year’s “Get Smart About Antibiotics Week” was Nov. 16-22. This annual 1-week observance is intended to raise awareness of the threat of antibiotic resistance and the importance of appropriate prescribing and use. Kudos if you celebrated this in your office. If you missed it, it’s not too late to check out some of the activities suggested by the CDC, and try one or two in your own practice. Email me with your ideas about stewardship in the outpatient setting, and I’ll try to feature at least some of them in a future column.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. Dr. Bryant disclosed that she has been an investigator for clinical trials funded by Pfizer for the past 2 years. Email her at [email protected].
I was recently asked to evaluate a young child with a urinary tract infection caused by an extended spectrum beta-lactamase (ESBL)–producing Escherichia coli.
I’d just broken the bad news to the mother: There was no oral medication available to treat the baby, so she’d have to stay in the hospital for a full intravenous course.
“Has your child been treated with antibiotics recently?” I asked the mother, wondering how the baby had come to have such a resistant infection.
“She had a couple days of runny nose and a low-grade fever a couple of weeks ago,” she told me. “Her doctor treated her for a sinus infection.”
In 2011, doctors in outpatient settings across the United States wrote 262.5 million prescriptions for antibiotics – 73.7 million for children – and according to the Centers for Disease Control and Prevention, about 50% of these were completely unnecessary because they were prescribed for viral respiratory tract infections (Clin Infect Dis. 2015 May 1;60[9]:1308-16).
Prescribing practices varied by region, with the highest rates in the South. Don’t think I’m judging. I live in Kentucky, the state with the highest rate of antibiotic prescribing at 1,281 prescriptions per 1,000 persons. Is it any wonder that we’re seeing kids with very resistant infections?
The CDC estimates that at least two million people in the United States are infected annually with antibiotic-resistant bacteria and at least 23,000 of them die as a result of these infections. It is estimated that prevention strategies that include better antibiotic prescribing could prevent as many as 619,000 infections and 37,000 deaths over 5 years. Fortunately, my little patient recovered fully, but it has made me think about antimicrobial stewardship, especially its role in the outpatient setting.
According the American Academy of Pediatrics, the goal of antimicrobial stewardship is “to optimize antimicrobial use, with the aim of decreasing inappropriate use that leads to unwarranted toxicity and to selection and spread of resistant organisms.”
Antimicrobial stewardship programs (ASPs) are increasingly common in inpatient settings and have been shown to reduce antibiotic use. These programs can take many forms. The hospital where I work relies primarily on clinical guidelines emphasizing appropriate empiric therapy for a variety of common conditions. Other hospitals employ prospective audit and feedback, as well as a restricted formulary. Medicare and Medicaid Conditions of Participation will soon require hospitals that receive funds from the Centers for Medicare and Medicaid Services have an ASP.
Comparatively little has been published about ASPs in the outpatient setting. The American Academy of Pediatrics suggests that effective strategies include patient education, provider education, provider audit and feedback, and clinical decision support. We have at least some data that these work, at least in a research setting.
From 2000 to 2003, a controlled, cluster-randomized trial in 16 Massachusetts communities demonstrated that a 3-year, multifaceted, community-level intervention was “modestly successful” in reducing antibiotic use (Pediatrics. 2008 Jan;121[1]:e15-23). As a part of this intervention, parents received education via direct mail and in primary care settings, pharmacies, and child care centers while physicians received small-group education, frequent updates and educational materials, and prescribing feedback. Antibiotic prescribing was measured via health insurance claims data from all children who were 6 years of age or younger and resided in study communities, and were insured by one of four participating health plans. Coincident with the intervention, there was 4.2% decrease in antibiotic prescribing among children aged 24 to <48 months and a 6.7% decrease among those aged 48-72 months. The effect was greatest among Medicaid-insured children.
More recently, 18 primary care practices in Pennsylvania and New Jersey were randomized to an intervention that consisted of a 1-hour, on-site education session followed by 1 year of personalized, quarterly audit and feedback of prescribing for bacterial and viral acute respiratory tract infections (ARTIs), or usual practice (JAMA. 2013 Jun 12;309[22]:2345-52). The prescribing practices of 162 clinicians were included in the analysis.
Broad spectrum–antibiotic prescribing decreased in intervention practices, compared with controls (26.8% to 14.3% among intervention practices vs. 28.4% to 22.6% in controls), as did “off-guideline” prescribing for pneumonia and acute sinusitis. Antibiotic prescribing for viral infections was relatively low at baseline and did not change. The authors concluded that “extending antimicrobial stewardship to the ambulatory setting, where such programs have generally not been implemented, may have important health benefits.” Unfortunately, the positive effect in these practices was not sustained after the audit and feedback stopped (JAMA. 2014 Dec 17;312[23]:2569-70).
Not all antimicrobial stewardship interventions need to be time- and resource-intensive. Investigators in California found that providers who publicly pledged to reducing inappropriate antibiotic use for ARTIs by signing and posting a commitment letter in exam rooms actually prescribed fewer inappropriate antibiotic courses for their adult patients (JAMA Intern Med. 2014 Mar;174[3]:425-31).
“When you have a cough, sore throat, or other illness, your doctor will help you select the best possible treatments. If an antibiotic would do more harm than good, your doctor will explain this to you, and may offer other treatments that are better for you,” the letter read in part. There was a 19.7 absolute percentage reduction in inappropriate antibiotic prescribing for ARTIs among clinicians randomized to the commitment letter invention relative to controls.
Can antimicrobial strategies work in the “real” world, in a busy pediatrician’s office? According to Dr. Patricia Purcell, a physician with East Louisville Pediatrics in Louisville, Ky., the answer is “yes.”
“We actually start with education in the newborn period,” Dr. Purcell said. “We let parents know that we are not going to call in antibiotics over the phone, and we’re not going to prescribe them for an upper respiratory tract infection.”

Dr. Purcell and her partners have committed to following evidence-based guidelines for antibiotic practices, such as the AAP’s guidelines for otitis media and sinusitis. She also noted that at least one major insurance company is starting to provide the group feedback about their antibiotic-prescribing practices. “They want to make sure we are not prescribing antibiotics for viruses,” she said.
Still, the message that antibiotics are not always the answer can be a bitter pill for some parents to swallow. A pediatrician friend in Alabama notes: “I have these conversations every day, and a lot of parents are mad at me for not prescribing antibiotics for their child’s ‘terrible cold.’” Another friend notes that watchful waiting can be a burden for parents who have high copays or difficulties with transportation.
Still, many parents would welcome a frank discussion about the risks and benefits of antibiotics. After I shared some of the CDC information for parents with a nursing colleague, she told me that her daughter recently had a febrile illness and was diagnosed with otitis media. “I don’t like giving my kids meds they don’t need,” she told me. “However, if the doc says they need antibiotics and they prescribe them, I give them. I never say, ‘Do we really need antibiotics for that?’”
Now she is rethinking that approach. “Was 10 days of amoxicillin necessary for a ‘red’ eardrum?! I’m just a mom. ... I don’t know the answer to that! Was her ear red because she had been crying or because of her fever? Did she get ‘treatment’ she did not need? Did the doctor give me antibiotics without education because she assumed that is why I brought her in?”
This year’s “Get Smart About Antibiotics Week” was Nov. 16-22. This annual 1-week observance is intended to raise awareness of the threat of antibiotic resistance and the importance of appropriate prescribing and use. Kudos if you celebrated this in your office. If you missed it, it’s not too late to check out some of the activities suggested by the CDC, and try one or two in your own practice. Email me with your ideas about stewardship in the outpatient setting, and I’ll try to feature at least some of them in a future column.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. Dr. Bryant disclosed that she has been an investigator for clinical trials funded by Pfizer for the past 2 years. Email her at [email protected].
Shared decision making reduces antibiotic use
Shared decision making between doctors and patients for the treatment of acute respiratory infections can achieve significant short-term reductions in antibiotic use, according to a Cochrane review published Nov. 11.
“Shared decision making is … a set of communication and evidence-based practice skills that elicits patients’ expectations, clarifies any misperceptions, and discusses the best available evidence for benefits and harms of treatment,” wrote Peter Coxeter of the Centre for Research in Evidence-Based Practice at Bond University, Australia.
Dr. Coxeter and his coauthors analyzed 10 published reports from nine randomized controlled trials involving more than 1,100 physicians and 492,000 patients, and found that shared decision making interventions were associated with a 39% overall reduction in antibiotic use (95% confidence interval, 0.55-0.68) within 6 weeks of the consultation, with a trend suggesting those reductions were maintained in the longer term.
The analysis also showed that this reduction did not lead to an increase in patient-initiated reconsultations or a decrease in patient satisfaction, although there were not enough data to determine the impact of these interventions on longer-term outcomes such as hospital admissions, pneumonia, or mortality (Cochrane Database Syst Rev. 2015 Nov 11. doi: 10.1002/14651858.CD010907.pub2).
“Further research should also aim to determine which aspects of these interventions provide the greatest benefit to adapt program implementation and uptake in diverse clinical settings,” the authors wrote.
The review was supported by the National Health and Medical Research Council (Australia). No conflicts of interest were declared.
Shared decision making between doctors and patients for the treatment of acute respiratory infections can achieve significant short-term reductions in antibiotic use, according to a Cochrane review published Nov. 11.
“Shared decision making is … a set of communication and evidence-based practice skills that elicits patients’ expectations, clarifies any misperceptions, and discusses the best available evidence for benefits and harms of treatment,” wrote Peter Coxeter of the Centre for Research in Evidence-Based Practice at Bond University, Australia.
Dr. Coxeter and his coauthors analyzed 10 published reports from nine randomized controlled trials involving more than 1,100 physicians and 492,000 patients, and found that shared decision making interventions were associated with a 39% overall reduction in antibiotic use (95% confidence interval, 0.55-0.68) within 6 weeks of the consultation, with a trend suggesting those reductions were maintained in the longer term.
The analysis also showed that this reduction did not lead to an increase in patient-initiated reconsultations or a decrease in patient satisfaction, although there were not enough data to determine the impact of these interventions on longer-term outcomes such as hospital admissions, pneumonia, or mortality (Cochrane Database Syst Rev. 2015 Nov 11. doi: 10.1002/14651858.CD010907.pub2).
“Further research should also aim to determine which aspects of these interventions provide the greatest benefit to adapt program implementation and uptake in diverse clinical settings,” the authors wrote.
The review was supported by the National Health and Medical Research Council (Australia). No conflicts of interest were declared.
Shared decision making between doctors and patients for the treatment of acute respiratory infections can achieve significant short-term reductions in antibiotic use, according to a Cochrane review published Nov. 11.
“Shared decision making is … a set of communication and evidence-based practice skills that elicits patients’ expectations, clarifies any misperceptions, and discusses the best available evidence for benefits and harms of treatment,” wrote Peter Coxeter of the Centre for Research in Evidence-Based Practice at Bond University, Australia.
Dr. Coxeter and his coauthors analyzed 10 published reports from nine randomized controlled trials involving more than 1,100 physicians and 492,000 patients, and found that shared decision making interventions were associated with a 39% overall reduction in antibiotic use (95% confidence interval, 0.55-0.68) within 6 weeks of the consultation, with a trend suggesting those reductions were maintained in the longer term.
The analysis also showed that this reduction did not lead to an increase in patient-initiated reconsultations or a decrease in patient satisfaction, although there were not enough data to determine the impact of these interventions on longer-term outcomes such as hospital admissions, pneumonia, or mortality (Cochrane Database Syst Rev. 2015 Nov 11. doi: 10.1002/14651858.CD010907.pub2).
“Further research should also aim to determine which aspects of these interventions provide the greatest benefit to adapt program implementation and uptake in diverse clinical settings,” the authors wrote.
The review was supported by the National Health and Medical Research Council (Australia). No conflicts of interest were declared.
FROM THE COCHRANE DATABASE OF SYSTEMATIC REVIEWS
Key clinical point:Shared decision making between doctors and patients for the treatment of acute respiratory infections can achieve significant short-term reductions in antibiotic use.
Major finding: Shared decision making interventions achieved an overall 39% reduction in antibiotic use in the 6 weeks after the consultation.
Data source: A review of nine randomized controlled trials involving more than 1,100 physicians and 492,000 patients.
Disclosures: The review was supported by the National Health and Medical Research Council (Australia). No conflicts of interest were declared.
Starting ACS patients on varenicline in the hospital boosted smoking quit rates
ORLANDO – Starting varenicline in smokers while hospitalized for an acute coronary syndrome resulted in substantially higher smoking abstinence rates than with placebo at all time points through 6 months of follow-up in the double-blind, randomized EVITA trial.
“The ACS population is typically older, they’re long-term smokers, and they come into the hospital with a life-threatening condition. Their family is all around them. They’ve had angioplasty or CABG [coronary artery bypass graft] surgery. So they have pressure on them to stop smoking. This is a teachable moment, a window of opportunity. The public health benefit for smoking cessation in this population is huge. You can cut their risk of death and significant morbidity in half if you can get them to stop,” Dr. Mark J. Eisenberg said in presenting the EVITA results at the American Heart Association scientific sessions.
“To our knowledge, this is the highest-risk population that’s been exposed to varenicline,” said Dr. Eisenberg, professor of medicine at McGill University and director of the cardiovascular health services research program at Jewish General Hospital in Montreal.
The primary study endpoint was continuous self-reported abstinence since baseline backed by biochemical confirmation in the form of an exhaled carbon monoxide level of 10 ppm or less at week 24 as well as at all the earlier follow-up visits. The rate was 47.3% in the varenicline group, compared with 32.5% in placebo-treated controls. That placebo response rate is in line with numerous prior studies that have shown that less than one-third of smokers with ACS remain abstinent after leaving the hospital.
“Most cardiologists would say, ‘All my patients stop smoking.’ But in the clinic if you look in the patients’ pockets, you find a pack of cigarettes. They stop smoking while in hospital, but as soon as they’re discharged, the relapse rate is almost immediate. Most patients are smoking the day they get out of hospital,” according to Dr. Eisenberg.
In EVITA, the number-needed-to-treat with varenicline for 12 weeks in order to produce 1 extra nonsmoker at 6 months was 6.8 patients.
The secondary endpoint of at least a 50% reduction in the number of cigarettes per day from baseline to 6 months was met by 67.4% of the varenicline group and 55.6% of controls, with a number-needed-to-treat of 8.5, he continued.
No safety issues emerged in the study, although as Dr. Eisenberg noted, EVITA wasn’t sufficiently powered to look at safety. The only side effect more common in varenicline-treated patients was abnormal dreams, with a 12-week incidence of 15%, threefold higher than in controls, a phenomenon seen in other, larger varenicline studies as well.
The EVITA investigators plan to follow participants out to 12 months. “If we see someone who at 1 year post MI is still smoking, maybe it’s time to go after them again, perhaps with another medication or behavioral therapy,” he said.
There are no randomized clinical trials demonstrating that starting nicotine patches or bupropion in the hospital is effective for smoking cessation in the ACS population, according to the cardiologist.
Dr. Eisenberg predicted this study will change clinical practice. In much the same way physicians now routinely start ACS patients on a statin, beta-blocker, and aspirin before they leave the hospital, physicians will capitalize on this opportunity to help ACS patients quit smoking as well, he said.
“The use of varenicline in ACS patients before they leave the hospital is a very important step forward. Cardiologists are increasingly comfortable with the idea of starting secondary prevention medications in the hospital, and there’s very little more important for a person with heart disease who smokes cigarettes than to help them quit smoking. It’s probably the No. 1 priority. So evidence that we can start a medication in the hospital and get more people who smoke cigarettes to quit smoking is definitely game changing, I think,” said Dr. Goff, professor of epidemiology and dean of the Colorado School of Public Health in Aurora.
Dr. Eisenberg reported receiving funding from Pfizer to perform the EVITA trial. He has also gotten honoraria from the company for providing continuing medical education talks on smoking cessation.
ORLANDO – Starting varenicline in smokers while hospitalized for an acute coronary syndrome resulted in substantially higher smoking abstinence rates than with placebo at all time points through 6 months of follow-up in the double-blind, randomized EVITA trial.
“The ACS population is typically older, they’re long-term smokers, and they come into the hospital with a life-threatening condition. Their family is all around them. They’ve had angioplasty or CABG [coronary artery bypass graft] surgery. So they have pressure on them to stop smoking. This is a teachable moment, a window of opportunity. The public health benefit for smoking cessation in this population is huge. You can cut their risk of death and significant morbidity in half if you can get them to stop,” Dr. Mark J. Eisenberg said in presenting the EVITA results at the American Heart Association scientific sessions.
“To our knowledge, this is the highest-risk population that’s been exposed to varenicline,” said Dr. Eisenberg, professor of medicine at McGill University and director of the cardiovascular health services research program at Jewish General Hospital in Montreal.
The primary study endpoint was continuous self-reported abstinence since baseline backed by biochemical confirmation in the form of an exhaled carbon monoxide level of 10 ppm or less at week 24 as well as at all the earlier follow-up visits. The rate was 47.3% in the varenicline group, compared with 32.5% in placebo-treated controls. That placebo response rate is in line with numerous prior studies that have shown that less than one-third of smokers with ACS remain abstinent after leaving the hospital.
“Most cardiologists would say, ‘All my patients stop smoking.’ But in the clinic if you look in the patients’ pockets, you find a pack of cigarettes. They stop smoking while in hospital, but as soon as they’re discharged, the relapse rate is almost immediate. Most patients are smoking the day they get out of hospital,” according to Dr. Eisenberg.
In EVITA, the number-needed-to-treat with varenicline for 12 weeks in order to produce 1 extra nonsmoker at 6 months was 6.8 patients.
The secondary endpoint of at least a 50% reduction in the number of cigarettes per day from baseline to 6 months was met by 67.4% of the varenicline group and 55.6% of controls, with a number-needed-to-treat of 8.5, he continued.
No safety issues emerged in the study, although as Dr. Eisenberg noted, EVITA wasn’t sufficiently powered to look at safety. The only side effect more common in varenicline-treated patients was abnormal dreams, with a 12-week incidence of 15%, threefold higher than in controls, a phenomenon seen in other, larger varenicline studies as well.
The EVITA investigators plan to follow participants out to 12 months. “If we see someone who at 1 year post MI is still smoking, maybe it’s time to go after them again, perhaps with another medication or behavioral therapy,” he said.
There are no randomized clinical trials demonstrating that starting nicotine patches or bupropion in the hospital is effective for smoking cessation in the ACS population, according to the cardiologist.
Dr. Eisenberg predicted this study will change clinical practice. In much the same way physicians now routinely start ACS patients on a statin, beta-blocker, and aspirin before they leave the hospital, physicians will capitalize on this opportunity to help ACS patients quit smoking as well, he said.
“The use of varenicline in ACS patients before they leave the hospital is a very important step forward. Cardiologists are increasingly comfortable with the idea of starting secondary prevention medications in the hospital, and there’s very little more important for a person with heart disease who smokes cigarettes than to help them quit smoking. It’s probably the No. 1 priority. So evidence that we can start a medication in the hospital and get more people who smoke cigarettes to quit smoking is definitely game changing, I think,” said Dr. Goff, professor of epidemiology and dean of the Colorado School of Public Health in Aurora.
Dr. Eisenberg reported receiving funding from Pfizer to perform the EVITA trial. He has also gotten honoraria from the company for providing continuing medical education talks on smoking cessation.
ORLANDO – Starting varenicline in smokers while hospitalized for an acute coronary syndrome resulted in substantially higher smoking abstinence rates than with placebo at all time points through 6 months of follow-up in the double-blind, randomized EVITA trial.
“The ACS population is typically older, they’re long-term smokers, and they come into the hospital with a life-threatening condition. Their family is all around them. They’ve had angioplasty or CABG [coronary artery bypass graft] surgery. So they have pressure on them to stop smoking. This is a teachable moment, a window of opportunity. The public health benefit for smoking cessation in this population is huge. You can cut their risk of death and significant morbidity in half if you can get them to stop,” Dr. Mark J. Eisenberg said in presenting the EVITA results at the American Heart Association scientific sessions.
“To our knowledge, this is the highest-risk population that’s been exposed to varenicline,” said Dr. Eisenberg, professor of medicine at McGill University and director of the cardiovascular health services research program at Jewish General Hospital in Montreal.
The primary study endpoint was continuous self-reported abstinence since baseline backed by biochemical confirmation in the form of an exhaled carbon monoxide level of 10 ppm or less at week 24 as well as at all the earlier follow-up visits. The rate was 47.3% in the varenicline group, compared with 32.5% in placebo-treated controls. That placebo response rate is in line with numerous prior studies that have shown that less than one-third of smokers with ACS remain abstinent after leaving the hospital.
“Most cardiologists would say, ‘All my patients stop smoking.’ But in the clinic if you look in the patients’ pockets, you find a pack of cigarettes. They stop smoking while in hospital, but as soon as they’re discharged, the relapse rate is almost immediate. Most patients are smoking the day they get out of hospital,” according to Dr. Eisenberg.
In EVITA, the number-needed-to-treat with varenicline for 12 weeks in order to produce 1 extra nonsmoker at 6 months was 6.8 patients.
The secondary endpoint of at least a 50% reduction in the number of cigarettes per day from baseline to 6 months was met by 67.4% of the varenicline group and 55.6% of controls, with a number-needed-to-treat of 8.5, he continued.
No safety issues emerged in the study, although as Dr. Eisenberg noted, EVITA wasn’t sufficiently powered to look at safety. The only side effect more common in varenicline-treated patients was abnormal dreams, with a 12-week incidence of 15%, threefold higher than in controls, a phenomenon seen in other, larger varenicline studies as well.
The EVITA investigators plan to follow participants out to 12 months. “If we see someone who at 1 year post MI is still smoking, maybe it’s time to go after them again, perhaps with another medication or behavioral therapy,” he said.
There are no randomized clinical trials demonstrating that starting nicotine patches or bupropion in the hospital is effective for smoking cessation in the ACS population, according to the cardiologist.
Dr. Eisenberg predicted this study will change clinical practice. In much the same way physicians now routinely start ACS patients on a statin, beta-blocker, and aspirin before they leave the hospital, physicians will capitalize on this opportunity to help ACS patients quit smoking as well, he said.
“The use of varenicline in ACS patients before they leave the hospital is a very important step forward. Cardiologists are increasingly comfortable with the idea of starting secondary prevention medications in the hospital, and there’s very little more important for a person with heart disease who smokes cigarettes than to help them quit smoking. It’s probably the No. 1 priority. So evidence that we can start a medication in the hospital and get more people who smoke cigarettes to quit smoking is definitely game changing, I think,” said Dr. Goff, professor of epidemiology and dean of the Colorado School of Public Health in Aurora.
Dr. Eisenberg reported receiving funding from Pfizer to perform the EVITA trial. He has also gotten honoraria from the company for providing continuing medical education talks on smoking cessation.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: The number of smokers who need to be started on varenicline while hospitalized for an acute coronary syndrome in order to produce one extra nonsmoker at 6 months is 6.8.
Data source: EVITA, a double-blind, randomized multicenter trial in 302 smokers hospitalized for ACS who were prospectively followed for 6 months.
Disclosures: The EVITA study was funded by Pfizer. The presenter reported receiving a grant from the company to conduct the trial, as well as honoraria for giving continuing medical education talks on smoking cessation.