User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Don’t delay treatment for patients with TB and HIV
Clinicians should treat patients diagnosed with HIV and tuberculosis for both conditions immediately, according to new guidelines on the treatment of drug-susceptible tuberculosis.
The clinical practice guidelines were issued collectively by three organizations: the American Thoracic Society (ATS), the U.S. Centers for Disease Control and Prevention (CDC), and the Infectious Diseases Society of America (IDSA), and published online in Clinical Infectious Diseases.
The guidelines recommend starting TB treatment for all patients as soon as an infection is suspected, rather than waiting for test results, and focusing on daily therapy to reduce the risk of relapse. In addition, all TB patients should receive comprehensive care, including direct observed therapy (DOT) when appropriate (Clin Infect Dis. 2016 Aug 10. doi: 10.1093/cid/ciw376).
“Treatment of tuberculosis is focused on both curing the individual patient and minimizing the transmission,” wrote Payam Nahid, MD, professor of medicine at the University of California, San Francisco, and his colleagues on the guidelines committee.
The guidelines’ section on treatment of tuberculosis in special situations addresses management of TB in patients with conditions including HIV, extrapulmonary TB, culture-negative pulmonary TB, pregnancy, renal disease, and hepatic disease, as well as treatment of children and the elderly.
With regard to HIV, the guidelines recommend the standard 6-month daily TB treatment for HIV patients on antiretroviral therapy. This treatment includes 2 months of isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), and ethambutol (EMB), followed by a continuation phase of 4 months of INH and RIF.
“Patients with HIV infection and tuberculosis are at an increased risk of developing paradoxical worsening of symptoms, signs, or clinical manifestations of tuberculosis after beginning antituberculosis and antiretroviral treatments,” according to the guidelines. These responses are defined as Immune Reconstitution Inflammatory Syndrome (IRIS). However, IRIS does not appear to impact the simultaneous treatment of TB and HIV, and the condition can be managed symptomatically if it occurs, the researchers noted.
The guidelines identified several areas in need of further study, including new TB drugs and treatment plans; the effects of biomarkers to help design individual therapy; TB in special populations including HIV patients, pregnant women, and children; and treatment delivery strategies.
The guidelines also are endorsed by the European Respiratory Society (ERS) and the U.S. National Tuberculosis Controllers Association (NCTA).
The American Thoracic Society, the Infections Diseases Society of America, and the Centers for Disease Control and Prevention provided financial support. Lead author Dr. Nahid had no financial conflicts to disclose.
Clinicians should treat patients diagnosed with HIV and tuberculosis for both conditions immediately, according to new guidelines on the treatment of drug-susceptible tuberculosis.
The clinical practice guidelines were issued collectively by three organizations: the American Thoracic Society (ATS), the U.S. Centers for Disease Control and Prevention (CDC), and the Infectious Diseases Society of America (IDSA), and published online in Clinical Infectious Diseases.
The guidelines recommend starting TB treatment for all patients as soon as an infection is suspected, rather than waiting for test results, and focusing on daily therapy to reduce the risk of relapse. In addition, all TB patients should receive comprehensive care, including direct observed therapy (DOT) when appropriate (Clin Infect Dis. 2016 Aug 10. doi: 10.1093/cid/ciw376).
“Treatment of tuberculosis is focused on both curing the individual patient and minimizing the transmission,” wrote Payam Nahid, MD, professor of medicine at the University of California, San Francisco, and his colleagues on the guidelines committee.
The guidelines’ section on treatment of tuberculosis in special situations addresses management of TB in patients with conditions including HIV, extrapulmonary TB, culture-negative pulmonary TB, pregnancy, renal disease, and hepatic disease, as well as treatment of children and the elderly.
With regard to HIV, the guidelines recommend the standard 6-month daily TB treatment for HIV patients on antiretroviral therapy. This treatment includes 2 months of isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), and ethambutol (EMB), followed by a continuation phase of 4 months of INH and RIF.
“Patients with HIV infection and tuberculosis are at an increased risk of developing paradoxical worsening of symptoms, signs, or clinical manifestations of tuberculosis after beginning antituberculosis and antiretroviral treatments,” according to the guidelines. These responses are defined as Immune Reconstitution Inflammatory Syndrome (IRIS). However, IRIS does not appear to impact the simultaneous treatment of TB and HIV, and the condition can be managed symptomatically if it occurs, the researchers noted.
The guidelines identified several areas in need of further study, including new TB drugs and treatment plans; the effects of biomarkers to help design individual therapy; TB in special populations including HIV patients, pregnant women, and children; and treatment delivery strategies.
The guidelines also are endorsed by the European Respiratory Society (ERS) and the U.S. National Tuberculosis Controllers Association (NCTA).
The American Thoracic Society, the Infections Diseases Society of America, and the Centers for Disease Control and Prevention provided financial support. Lead author Dr. Nahid had no financial conflicts to disclose.
Clinicians should treat patients diagnosed with HIV and tuberculosis for both conditions immediately, according to new guidelines on the treatment of drug-susceptible tuberculosis.
The clinical practice guidelines were issued collectively by three organizations: the American Thoracic Society (ATS), the U.S. Centers for Disease Control and Prevention (CDC), and the Infectious Diseases Society of America (IDSA), and published online in Clinical Infectious Diseases.
The guidelines recommend starting TB treatment for all patients as soon as an infection is suspected, rather than waiting for test results, and focusing on daily therapy to reduce the risk of relapse. In addition, all TB patients should receive comprehensive care, including direct observed therapy (DOT) when appropriate (Clin Infect Dis. 2016 Aug 10. doi: 10.1093/cid/ciw376).
“Treatment of tuberculosis is focused on both curing the individual patient and minimizing the transmission,” wrote Payam Nahid, MD, professor of medicine at the University of California, San Francisco, and his colleagues on the guidelines committee.
The guidelines’ section on treatment of tuberculosis in special situations addresses management of TB in patients with conditions including HIV, extrapulmonary TB, culture-negative pulmonary TB, pregnancy, renal disease, and hepatic disease, as well as treatment of children and the elderly.
With regard to HIV, the guidelines recommend the standard 6-month daily TB treatment for HIV patients on antiretroviral therapy. This treatment includes 2 months of isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), and ethambutol (EMB), followed by a continuation phase of 4 months of INH and RIF.
“Patients with HIV infection and tuberculosis are at an increased risk of developing paradoxical worsening of symptoms, signs, or clinical manifestations of tuberculosis after beginning antituberculosis and antiretroviral treatments,” according to the guidelines. These responses are defined as Immune Reconstitution Inflammatory Syndrome (IRIS). However, IRIS does not appear to impact the simultaneous treatment of TB and HIV, and the condition can be managed symptomatically if it occurs, the researchers noted.
The guidelines identified several areas in need of further study, including new TB drugs and treatment plans; the effects of biomarkers to help design individual therapy; TB in special populations including HIV patients, pregnant women, and children; and treatment delivery strategies.
The guidelines also are endorsed by the European Respiratory Society (ERS) and the U.S. National Tuberculosis Controllers Association (NCTA).
The American Thoracic Society, the Infections Diseases Society of America, and the Centers for Disease Control and Prevention provided financial support. Lead author Dr. Nahid had no financial conflicts to disclose.
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point: Clinicians should treat patients diagnosed with HIV and tuberculosis for both conditions immediately.
Major finding: A four-drug regimen of INH, RIF, PZA, and EMB remains the preferred initial treatment for drug-susceptible pulmonary tuberculosis. Treatment should be initiated promptly even before diagnostic test results are known in patients with high likelihood of having tuberculosis.
Data source: Nine PICO (population, intervention, comparators, outcomes) questions and associated recommendations for the treatment of patients diagnosed with both HIV and TB, developed based on the evidence appraised using GRADE (Grading of Recommendations Assessment, Development, and Evaluation) methodology.
Disclosures: The American Thoracic Society, the Infections Diseases Society of America, and the Centers for Disease Control and Prevention provided financial support. Lead author Dr. Nahid had no financial conflicts to disclose.
CMV viremia not culprit in high mortality of TB/HIV coinfection
DURBAN, SOUTH AFRICA – Cytomegalovirus viremia is common among patients hospitalized for HIV-associated tuberculosis, but it appears to be a bystander rather than a contributor to the high mortality seen in this population, Amy Ward, MD, said at the 21st International AIDS Conference.
“CMV [cytomegalovirus] viremia is likely a marker of more severe immunodeficiency rather than a direct contributor to mortality,” she concluded based upon the findings of her prospective cohort study. The finding means therapies for CMV viremia will not open up a new avenue of potentially life-saving treatments for these patients.
In other severe immunodeficiency conditions, such as after organ transplant, CMV viremia is directly related to increased mortality, and ganciclovir therapy can prevent progression to clinical disease and death, explained Dr. Ward of the University of Cape Town, South Africa.
She presented a prospective cohort study of 256 HIV-infected South African adults, median age 36 years, who were hospitalized with a new diagnosis of TB. At enrollment, their median CD4 count was 64 cells/mm3. Only 35% were on antiretroviral therapy (ART); 44% had previously been on ART, 21% were ART-naive, and 41% had a positive TB blood culture.
CMV viremia was present in 31%, and CMV viral load was 1,000 copies/mL or more in half of them. None had CMV retinitis, based on panoptic fundoscopy at enrollment. HIV-related retinal pathologies at enrollment included disseminated cryptococcal disease, ocular TB granules, and HIV retinitis.
The primary endpoint of the study was mortality at 12 weeks on anti-TB therapy. The mortality rate was 38% in the CMV viremic group, significantly higher than the 17.8% mortality rate seen in the CMV-negative patients.
In a univariate Cox proportional hazards regression analysis, CMV viremia was associated with a 2.1-fold increased risk for 12-week mortality. But advancing age, a low CD4 count, and decreasing serum albumin were also risk factors.
When these variables were incorporated in a multivariate regression analysis along with HIV viral load, tuberculosis blood culture results, and gender, CMV viremia was no longer a significant risk factor for 12-week mortality. Age was the sole significant predictor of death. Patients who were at least 36 years old had a 32.8% mortality rate, compared with a 14.1% rate in those who were younger. The CD4 count didn’t differ significantly by age; however, the prevalence of CMV viremia was 38% in the older group and 26.3% in patients under age 36.
“Those patients who were 36 years old and above had a higher mortality and were more likely to have CMV viremia, both findings perhaps reflecting premature aging of the immune system,” Dr. Ward said.
Also, no dose-response was seen between CMV viral load and mortality risk. The 12-week mortality rate was 33.3% in patients with a CMV viral load below 1,000 copies/mL and similar at 34.1% in those with a viral load above that cutpoint, she noted.
The study was funded by the Wellcome Trust and the South African Medical Research Council. Dr. Ward reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – Cytomegalovirus viremia is common among patients hospitalized for HIV-associated tuberculosis, but it appears to be a bystander rather than a contributor to the high mortality seen in this population, Amy Ward, MD, said at the 21st International AIDS Conference.
“CMV [cytomegalovirus] viremia is likely a marker of more severe immunodeficiency rather than a direct contributor to mortality,” she concluded based upon the findings of her prospective cohort study. The finding means therapies for CMV viremia will not open up a new avenue of potentially life-saving treatments for these patients.
In other severe immunodeficiency conditions, such as after organ transplant, CMV viremia is directly related to increased mortality, and ganciclovir therapy can prevent progression to clinical disease and death, explained Dr. Ward of the University of Cape Town, South Africa.
She presented a prospective cohort study of 256 HIV-infected South African adults, median age 36 years, who were hospitalized with a new diagnosis of TB. At enrollment, their median CD4 count was 64 cells/mm3. Only 35% were on antiretroviral therapy (ART); 44% had previously been on ART, 21% were ART-naive, and 41% had a positive TB blood culture.
CMV viremia was present in 31%, and CMV viral load was 1,000 copies/mL or more in half of them. None had CMV retinitis, based on panoptic fundoscopy at enrollment. HIV-related retinal pathologies at enrollment included disseminated cryptococcal disease, ocular TB granules, and HIV retinitis.
The primary endpoint of the study was mortality at 12 weeks on anti-TB therapy. The mortality rate was 38% in the CMV viremic group, significantly higher than the 17.8% mortality rate seen in the CMV-negative patients.
In a univariate Cox proportional hazards regression analysis, CMV viremia was associated with a 2.1-fold increased risk for 12-week mortality. But advancing age, a low CD4 count, and decreasing serum albumin were also risk factors.
When these variables were incorporated in a multivariate regression analysis along with HIV viral load, tuberculosis blood culture results, and gender, CMV viremia was no longer a significant risk factor for 12-week mortality. Age was the sole significant predictor of death. Patients who were at least 36 years old had a 32.8% mortality rate, compared with a 14.1% rate in those who were younger. The CD4 count didn’t differ significantly by age; however, the prevalence of CMV viremia was 38% in the older group and 26.3% in patients under age 36.
“Those patients who were 36 years old and above had a higher mortality and were more likely to have CMV viremia, both findings perhaps reflecting premature aging of the immune system,” Dr. Ward said.
Also, no dose-response was seen between CMV viral load and mortality risk. The 12-week mortality rate was 33.3% in patients with a CMV viral load below 1,000 copies/mL and similar at 34.1% in those with a viral load above that cutpoint, she noted.
The study was funded by the Wellcome Trust and the South African Medical Research Council. Dr. Ward reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – Cytomegalovirus viremia is common among patients hospitalized for HIV-associated tuberculosis, but it appears to be a bystander rather than a contributor to the high mortality seen in this population, Amy Ward, MD, said at the 21st International AIDS Conference.
“CMV [cytomegalovirus] viremia is likely a marker of more severe immunodeficiency rather than a direct contributor to mortality,” she concluded based upon the findings of her prospective cohort study. The finding means therapies for CMV viremia will not open up a new avenue of potentially life-saving treatments for these patients.
In other severe immunodeficiency conditions, such as after organ transplant, CMV viremia is directly related to increased mortality, and ganciclovir therapy can prevent progression to clinical disease and death, explained Dr. Ward of the University of Cape Town, South Africa.
She presented a prospective cohort study of 256 HIV-infected South African adults, median age 36 years, who were hospitalized with a new diagnosis of TB. At enrollment, their median CD4 count was 64 cells/mm3. Only 35% were on antiretroviral therapy (ART); 44% had previously been on ART, 21% were ART-naive, and 41% had a positive TB blood culture.
CMV viremia was present in 31%, and CMV viral load was 1,000 copies/mL or more in half of them. None had CMV retinitis, based on panoptic fundoscopy at enrollment. HIV-related retinal pathologies at enrollment included disseminated cryptococcal disease, ocular TB granules, and HIV retinitis.
The primary endpoint of the study was mortality at 12 weeks on anti-TB therapy. The mortality rate was 38% in the CMV viremic group, significantly higher than the 17.8% mortality rate seen in the CMV-negative patients.
In a univariate Cox proportional hazards regression analysis, CMV viremia was associated with a 2.1-fold increased risk for 12-week mortality. But advancing age, a low CD4 count, and decreasing serum albumin were also risk factors.
When these variables were incorporated in a multivariate regression analysis along with HIV viral load, tuberculosis blood culture results, and gender, CMV viremia was no longer a significant risk factor for 12-week mortality. Age was the sole significant predictor of death. Patients who were at least 36 years old had a 32.8% mortality rate, compared with a 14.1% rate in those who were younger. The CD4 count didn’t differ significantly by age; however, the prevalence of CMV viremia was 38% in the older group and 26.3% in patients under age 36.
“Those patients who were 36 years old and above had a higher mortality and were more likely to have CMV viremia, both findings perhaps reflecting premature aging of the immune system,” Dr. Ward said.
Also, no dose-response was seen between CMV viral load and mortality risk. The 12-week mortality rate was 33.3% in patients with a CMV viral load below 1,000 copies/mL and similar at 34.1% in those with a viral load above that cutpoint, she noted.
The study was funded by the Wellcome Trust and the South African Medical Research Council. Dr. Ward reported having no financial conflicts of interest.
AT AIDS 2016
Key clinical point: Cytomegalovirus viremia is common in patients hospitalized for HIV-associated tuberculosis, but treating the CMV infection is unlikely to reduce the coinfected group’s high mortality rate.
Major finding: Cytomegalovirus viremia was present in nearly one-third of a group of hospitalized patients with HIV infection and tuberculosis, but was not an independent risk factor for their 23% mortality rate at 12 weeks.
Data source: This was a prospective cohort study including 256 hospitalized patients coinfected with HIV and newly diagnosed tuberculosis.
Disclosures: The study was funded by the Wellcome Trust and the South African Medical Research Council. The presenter reported having no financial conflicts of interest.
FDA approves generic version of Tamiflu
The Food and Drug Administration has approved the first generic version of Tamiflu (oseltamivir phosphate), a medication for the treatment of influenza A and B.
The announcement was made Aug. 3, 2016, on the Drugs@FDA website and in an email from the FDA’s Division of Drug Information (DDI). Tamiflu was first approved in 1999.
Oseltamivir phosphate is intended for use in patients 2 weeks of age and older who have had flu symptoms for no more than 48 hours, and for prevention of influenza in patients 1 year of age and older. According to the FDA, the drug does not treat or prevent illness caused by viral infections other than the influenza virus, and does not prevent bacterial infections that may happen with the flu.
Products in the FDA generic approval application submitted by Natco Pharma Ltd., an India-based drug company, include the oral capsule form of the drug, in 30-, 45-, and 75-mg strengths.
The FDA acknowledged in its approval that it does not know if oseltamivir phosphate is effective in patients who start treatment after 2 days of developing symptoms, or have weakened immune systems. The most common side effects reported by patients using oseltamivir phosphate in clinical trials included nausea and vomiting.
For more information on oseltamivir phosphate, see the Tamiflu drug label.
On Twitter @richpizzi
The Food and Drug Administration has approved the first generic version of Tamiflu (oseltamivir phosphate), a medication for the treatment of influenza A and B.
The announcement was made Aug. 3, 2016, on the Drugs@FDA website and in an email from the FDA’s Division of Drug Information (DDI). Tamiflu was first approved in 1999.
Oseltamivir phosphate is intended for use in patients 2 weeks of age and older who have had flu symptoms for no more than 48 hours, and for prevention of influenza in patients 1 year of age and older. According to the FDA, the drug does not treat or prevent illness caused by viral infections other than the influenza virus, and does not prevent bacterial infections that may happen with the flu.
Products in the FDA generic approval application submitted by Natco Pharma Ltd., an India-based drug company, include the oral capsule form of the drug, in 30-, 45-, and 75-mg strengths.
The FDA acknowledged in its approval that it does not know if oseltamivir phosphate is effective in patients who start treatment after 2 days of developing symptoms, or have weakened immune systems. The most common side effects reported by patients using oseltamivir phosphate in clinical trials included nausea and vomiting.
For more information on oseltamivir phosphate, see the Tamiflu drug label.
On Twitter @richpizzi
The Food and Drug Administration has approved the first generic version of Tamiflu (oseltamivir phosphate), a medication for the treatment of influenza A and B.
The announcement was made Aug. 3, 2016, on the Drugs@FDA website and in an email from the FDA’s Division of Drug Information (DDI). Tamiflu was first approved in 1999.
Oseltamivir phosphate is intended for use in patients 2 weeks of age and older who have had flu symptoms for no more than 48 hours, and for prevention of influenza in patients 1 year of age and older. According to the FDA, the drug does not treat or prevent illness caused by viral infections other than the influenza virus, and does not prevent bacterial infections that may happen with the flu.
Products in the FDA generic approval application submitted by Natco Pharma Ltd., an India-based drug company, include the oral capsule form of the drug, in 30-, 45-, and 75-mg strengths.
The FDA acknowledged in its approval that it does not know if oseltamivir phosphate is effective in patients who start treatment after 2 days of developing symptoms, or have weakened immune systems. The most common side effects reported by patients using oseltamivir phosphate in clinical trials included nausea and vomiting.
For more information on oseltamivir phosphate, see the Tamiflu drug label.
On Twitter @richpizzi
Hypoxia of obstructive sleep apnea aggravates NAFLD, NASH in adolescents
A new study has found that a strong association exists in adolescents who have obstructive sleep apnea, and their risks of developing more highly progressed forms of nonalcoholic fatty liver disease (NAFLD) or nonalcoholic steatohepatitis (NASH).
“Substantial evidence suggests oxidative stress is a central mediator in NAFLD pathogenesis and progression, although the specific trigger for reactive oxygen species (ROS) generation has not been clearly delineated,” wrote the authors, led by Shikha S. Sundaram, MD of the University of Colorado at Denver, Aurora, adding that “Emerging evidence demonstrates that obesity-related obstructive sleep apnea (OSA) and intermittent nocturnal hypoxia are associated with NAFLD progression.”
Dr. Sundaram and her coinvestigators looked at patients admitted to the Children’s Hospital Colorado Pediatric Liver Center from June 2009 through January 2014. Subjects included were children ages 8-18 years, male and female, who were classified as Tanner stage 2-4 with liver biopsy evidence of NAFLD.
“In our center, a clinical liver biopsy for suspected NAFLD is performed in overweight or obese children (body mass index greater than 85% for age and gender) with chronically elevated aminotransferases in whom a diagnosis is unclear based on serologic testing,” Dr. Sundaram and her coauthors clarified regarding the screening process.
Additionally, age-matched “lean” children, that is, those with a body mass index lower than 85%, were also enrolled as controls; these subjects were included if they had no evidence of hepatomegaly or liver disease – translated to AST and ALT levels of 640 IU/L – and were also Tanner stage 2-4. The authors explained that this Tanner stage range was chosen in order to “minimize variations in insulin sensitivity that may confound the interpretation of potential associations between OSA/hypoxia and NAFLD.”
Ultimately, 36 NAFLD adolescent subjects and 14 controls completed the study. A total of 25 of the 36 NAFLD subjects (69.4%) had OSA and/or nocturnal hypoxia; of these, 15 were classified as having isolated OSA, 9 had both OSA and hypoxia, and 1 had isolated hypoxia. Polysomnograms found that all NAFLD subjects spent more than 12% of their total time asleep in REM sleep, which was deemed adequate enough to consider the findings valid.
Based on liver histology scoring, laboratory testing, urine F2-isoprostanes, and 4-hydroxynonenal liver immunohistochemistry tests that were conducted on all subjects, Dr. Sundaram and her coinvestigators found that subjects with OSA or hypoxia had more severe fibrosis than did those without. While the latter cohort were 100% stage 0-2, only 64% of those with OSA/hypoxia were stage 0-2, while the remaining 36% were stage 3 (P = .03). Additionally, higher F2-isoprostanes – used to measure lipid peroxidation – correlated with apnea/hypoxia index (r = 0.39; P = .03), and the most severe OSA/hypoxia occurred in subjects that had the greatest 4-hydroxynonenal staining (P = .03). Furthermore, an increase in both F2-isoprostanes and 4-hydroxynonenal hepatic staining was shown to lead to a higher risk of worse steatosis: r = 0.32 and r = 0.47, respectively (P = .04 and P = .007).
“These data support sleep disordered breathing as an important trigger of oxidative stress that promotes progression of pediatric NAFLD to NASH,” the authors concluded, adding that “this study confirms that OSA/hypoxia is common in pediatric NAFLD and that more severe OSA/hypoxia is associated with elevated aminotransferases, hepatic steatosis, inflammation, NAS [NAFLD activity score], and fibrosis.”
Dr. Sundaram and her coauthors call for further research to examine if “prevention or reversal of NASH following effective therapy of OSA and nocturnal hypoxia in obese patients” is viable.
This study was supported by funding from the National Institutes of Health. Dr. Sundaram and her coinvestigators did not report any relevant financial disclosures.
A new study has found that a strong association exists in adolescents who have obstructive sleep apnea, and their risks of developing more highly progressed forms of nonalcoholic fatty liver disease (NAFLD) or nonalcoholic steatohepatitis (NASH).
“Substantial evidence suggests oxidative stress is a central mediator in NAFLD pathogenesis and progression, although the specific trigger for reactive oxygen species (ROS) generation has not been clearly delineated,” wrote the authors, led by Shikha S. Sundaram, MD of the University of Colorado at Denver, Aurora, adding that “Emerging evidence demonstrates that obesity-related obstructive sleep apnea (OSA) and intermittent nocturnal hypoxia are associated with NAFLD progression.”
Dr. Sundaram and her coinvestigators looked at patients admitted to the Children’s Hospital Colorado Pediatric Liver Center from June 2009 through January 2014. Subjects included were children ages 8-18 years, male and female, who were classified as Tanner stage 2-4 with liver biopsy evidence of NAFLD.
“In our center, a clinical liver biopsy for suspected NAFLD is performed in overweight or obese children (body mass index greater than 85% for age and gender) with chronically elevated aminotransferases in whom a diagnosis is unclear based on serologic testing,” Dr. Sundaram and her coauthors clarified regarding the screening process.
Additionally, age-matched “lean” children, that is, those with a body mass index lower than 85%, were also enrolled as controls; these subjects were included if they had no evidence of hepatomegaly or liver disease – translated to AST and ALT levels of 640 IU/L – and were also Tanner stage 2-4. The authors explained that this Tanner stage range was chosen in order to “minimize variations in insulin sensitivity that may confound the interpretation of potential associations between OSA/hypoxia and NAFLD.”
Ultimately, 36 NAFLD adolescent subjects and 14 controls completed the study. A total of 25 of the 36 NAFLD subjects (69.4%) had OSA and/or nocturnal hypoxia; of these, 15 were classified as having isolated OSA, 9 had both OSA and hypoxia, and 1 had isolated hypoxia. Polysomnograms found that all NAFLD subjects spent more than 12% of their total time asleep in REM sleep, which was deemed adequate enough to consider the findings valid.
Based on liver histology scoring, laboratory testing, urine F2-isoprostanes, and 4-hydroxynonenal liver immunohistochemistry tests that were conducted on all subjects, Dr. Sundaram and her coinvestigators found that subjects with OSA or hypoxia had more severe fibrosis than did those without. While the latter cohort were 100% stage 0-2, only 64% of those with OSA/hypoxia were stage 0-2, while the remaining 36% were stage 3 (P = .03). Additionally, higher F2-isoprostanes – used to measure lipid peroxidation – correlated with apnea/hypoxia index (r = 0.39; P = .03), and the most severe OSA/hypoxia occurred in subjects that had the greatest 4-hydroxynonenal staining (P = .03). Furthermore, an increase in both F2-isoprostanes and 4-hydroxynonenal hepatic staining was shown to lead to a higher risk of worse steatosis: r = 0.32 and r = 0.47, respectively (P = .04 and P = .007).
“These data support sleep disordered breathing as an important trigger of oxidative stress that promotes progression of pediatric NAFLD to NASH,” the authors concluded, adding that “this study confirms that OSA/hypoxia is common in pediatric NAFLD and that more severe OSA/hypoxia is associated with elevated aminotransferases, hepatic steatosis, inflammation, NAS [NAFLD activity score], and fibrosis.”
Dr. Sundaram and her coauthors call for further research to examine if “prevention or reversal of NASH following effective therapy of OSA and nocturnal hypoxia in obese patients” is viable.
This study was supported by funding from the National Institutes of Health. Dr. Sundaram and her coinvestigators did not report any relevant financial disclosures.
A new study has found that a strong association exists in adolescents who have obstructive sleep apnea, and their risks of developing more highly progressed forms of nonalcoholic fatty liver disease (NAFLD) or nonalcoholic steatohepatitis (NASH).
“Substantial evidence suggests oxidative stress is a central mediator in NAFLD pathogenesis and progression, although the specific trigger for reactive oxygen species (ROS) generation has not been clearly delineated,” wrote the authors, led by Shikha S. Sundaram, MD of the University of Colorado at Denver, Aurora, adding that “Emerging evidence demonstrates that obesity-related obstructive sleep apnea (OSA) and intermittent nocturnal hypoxia are associated with NAFLD progression.”
Dr. Sundaram and her coinvestigators looked at patients admitted to the Children’s Hospital Colorado Pediatric Liver Center from June 2009 through January 2014. Subjects included were children ages 8-18 years, male and female, who were classified as Tanner stage 2-4 with liver biopsy evidence of NAFLD.
“In our center, a clinical liver biopsy for suspected NAFLD is performed in overweight or obese children (body mass index greater than 85% for age and gender) with chronically elevated aminotransferases in whom a diagnosis is unclear based on serologic testing,” Dr. Sundaram and her coauthors clarified regarding the screening process.
Additionally, age-matched “lean” children, that is, those with a body mass index lower than 85%, were also enrolled as controls; these subjects were included if they had no evidence of hepatomegaly or liver disease – translated to AST and ALT levels of 640 IU/L – and were also Tanner stage 2-4. The authors explained that this Tanner stage range was chosen in order to “minimize variations in insulin sensitivity that may confound the interpretation of potential associations between OSA/hypoxia and NAFLD.”
Ultimately, 36 NAFLD adolescent subjects and 14 controls completed the study. A total of 25 of the 36 NAFLD subjects (69.4%) had OSA and/or nocturnal hypoxia; of these, 15 were classified as having isolated OSA, 9 had both OSA and hypoxia, and 1 had isolated hypoxia. Polysomnograms found that all NAFLD subjects spent more than 12% of their total time asleep in REM sleep, which was deemed adequate enough to consider the findings valid.
Based on liver histology scoring, laboratory testing, urine F2-isoprostanes, and 4-hydroxynonenal liver immunohistochemistry tests that were conducted on all subjects, Dr. Sundaram and her coinvestigators found that subjects with OSA or hypoxia had more severe fibrosis than did those without. While the latter cohort were 100% stage 0-2, only 64% of those with OSA/hypoxia were stage 0-2, while the remaining 36% were stage 3 (P = .03). Additionally, higher F2-isoprostanes – used to measure lipid peroxidation – correlated with apnea/hypoxia index (r = 0.39; P = .03), and the most severe OSA/hypoxia occurred in subjects that had the greatest 4-hydroxynonenal staining (P = .03). Furthermore, an increase in both F2-isoprostanes and 4-hydroxynonenal hepatic staining was shown to lead to a higher risk of worse steatosis: r = 0.32 and r = 0.47, respectively (P = .04 and P = .007).
“These data support sleep disordered breathing as an important trigger of oxidative stress that promotes progression of pediatric NAFLD to NASH,” the authors concluded, adding that “this study confirms that OSA/hypoxia is common in pediatric NAFLD and that more severe OSA/hypoxia is associated with elevated aminotransferases, hepatic steatosis, inflammation, NAS [NAFLD activity score], and fibrosis.”
Dr. Sundaram and her coauthors call for further research to examine if “prevention or reversal of NASH following effective therapy of OSA and nocturnal hypoxia in obese patients” is viable.
This study was supported by funding from the National Institutes of Health. Dr. Sundaram and her coinvestigators did not report any relevant financial disclosures.
FROM THE JOURNAL OF HEPATOLOGY
Key clinical point: Adolescents with obstructive sleep apnea have a higher risk for nonalcoholic fatty liver disease, because of liver tissue scarring.
Major finding: The cohort of subjects with OSA had more severe fibrosis (64%, stages 0-2; 36% stage 3) than those without OSA (100%, stages 0-2) (P = .03).
Data source: Prospective cohort study of 36 adolescents with NAFLD and 14 lean controls.
Disclosures: Funding provided by the NIH. Authors reported no relevant financial disclosures.
Extended pneumococcal vaccination schedule boosts early immunity for preemies
A randomized clinical trial evaluating three dosing strategies for 13-valent pneumococcal vaccine (PCV13) in preterm infants found that more widely spaced priming vaccinations resulted in higher immunoglobulin G (IgG) during the first 12 months of life, but reduced the immune response seen after the 12-month booster was given.
After the primary schedule, the percent of infants lacking seroprotection for more than one half of the serotypes in the PCV13 formulation was 25% on a reduced two-dose schedule, 12% on an accelerated schedule, and 3% on an extended schedule (P less than .001).
Conversely, “A reduced priming schedule of PCV13 resulted in higher post-booster IgG concentrations but lower post-primary concentrations,” wrote Alison Kent, MBChB, and her coinvestigators in the PUNS (Prems Under New Schedule) Study Group (Pediatrics. 2016;138[3]:e20153945).
“Infants who received the extended schedule had lower fold increases in concentrations after booster vaccination than the other groups,” wrote Dr. Kent of the Pediatric Infectious Diseases Research Group and Vaccine Institute, St. George’s, University of London, and her collaborators. Participants receiving the extended schedule had lower geometric mean concentrations (GMCs) of antibodies than did those on the reduced schedule for nine serotypes and those on the accelerated schedule for four serotypes.
The study enrolled 210 premature infants in a phase IV, controlled, open-label trial at 12 sites in the United Kingdom. Infants of less than 35 weeks gestation, and between 7 and 12 weeks of age, were randomly assigned to receive PCV13 on one of three schedules. The reduced schedule gave two priming doses at 2 and 4 months of age; the accelerated schedule gave the doses at 2, 3, and 4 months of age; and the extended schedule gave doses at 2, 4, and 6 months of age. All infants received a booster vaccination at 12 or 13 months of age, and all received a standard suite of childhood immunizations for other diseases. The entire study was completed by 194 patients.
Serotype-specific IgG concentrations were obtained pre-vaccination, 1 month after the primary vaccination, and before and 1 month after the booster vaccination was given. IgG levels were reported for each PCV serotype; “there was considerable variation between serotypes,” ranging from 0.16 ng/mL for serotype 6b on the reduced schedule to 8.49 ng/mL for serotype 14 on the extended schedule, the investigators said.
Dr. Kent and her collaborators also used logistic regression analysis to explore how the vaccine’s effectiveness was affected by a number of factors. These included gestational length, the receipt of blood transfusions or pre- or post-natal steroids, BCG vaccination, early postvaccination acetaminophen, and the presence of chronic lung disease.
Later gestation was associated with increased seroprotection for four serotypes at 2 months of age, and with an increase in post-primary vaccination IgG concentrations for three others (P-values ranging from P less than .001 to P = .021).
No other factors were associated with protective IgG levels at any point, except that receipt of prenatal steroids had a negative association with seroprotection for several serotypes. “At no time points were antenatal steroids associated with higher antibody concentrations,” wrote the investigators.
Most studies of immunogenicity of infant vaccination schedules have been completed using term infants, with limited knowledge about efficacy in preterm infants. Previous work had shown that preterm infants had lower IgG concentrations after the primary and booster vaccinations for eight serotypes of PCV, compared with term infants. “The lower immunogenicity ... is concerning because premature infants are also less likely to benefit from the protective maternal antibodies transferred during late pregnancy,” Dr. Kent and her coauthors wrote.
The lower booster immunogenicity after the extended schedule is an effect that has been previously observed with other vaccinations and may be related to the formation of immune complexes with previously existing antibodies with the vaccine antigen, said Dr. Kent and her coauthors. The variation in immunogenicity timing for the various priming schedules, they said, will be helpful for those caring for preterm infants, enabling them “to consider this finding in the context of their own immunization programs and epidemiologic situations.”
The study was funded by Pfizer as an investigator-led study, without Pfizer’s input on the conduct of the trial, analysis of data, interpretation of results, or the preparation of this manuscript. Pfizer manufactures Prevnar 13.
On Twitter @karioakes
The needs of varying populations, the prevalence of various serotypes, and other local epidemiologic and economic factors all influence vaccination schedules. For PCV, the present study showed how widely seroprotection varied between serotypes and between different priming schedules.
Invasive pneumococcal disease (IPD) can be devastating in the vulnerable preterm population, as can pneumococcal pneumonia. Though the current vaccination schedule recommendations in the United States takes into account age-related changes in the immune system, truly optimized vaccine delivery for all populations, including this vulnerable one, is still more a goal than a reality.
However, each of the schedules examined in this study have been studied in areas where they are in clinical use, and all are generally protective of IPD. The timing of other vaccinations, as well as economic and logistic realities, will also affect vaccination schedules, and must be taken into account.
The findings of this study show that no one schedule is best for all populations, and also highlight why those making vaccine policy around the globe will continue to arrive at varying answers when considering the needs of their populations.
Mark H. Sawyer, MD, is a professor of pediatrics at the University of California, San Diego. Mobeen Rathore, MD, is director of the University of Florida Center for HIV/AIDS Research, Education and Service. They had no conflicts of interest to declare. Their remarks are drawn from a companion commentary in Pediatrics (Pediatrics. 2016;138[3]:e20160975).
The needs of varying populations, the prevalence of various serotypes, and other local epidemiologic and economic factors all influence vaccination schedules. For PCV, the present study showed how widely seroprotection varied between serotypes and between different priming schedules.
Invasive pneumococcal disease (IPD) can be devastating in the vulnerable preterm population, as can pneumococcal pneumonia. Though the current vaccination schedule recommendations in the United States takes into account age-related changes in the immune system, truly optimized vaccine delivery for all populations, including this vulnerable one, is still more a goal than a reality.
However, each of the schedules examined in this study have been studied in areas where they are in clinical use, and all are generally protective of IPD. The timing of other vaccinations, as well as economic and logistic realities, will also affect vaccination schedules, and must be taken into account.
The findings of this study show that no one schedule is best for all populations, and also highlight why those making vaccine policy around the globe will continue to arrive at varying answers when considering the needs of their populations.
Mark H. Sawyer, MD, is a professor of pediatrics at the University of California, San Diego. Mobeen Rathore, MD, is director of the University of Florida Center for HIV/AIDS Research, Education and Service. They had no conflicts of interest to declare. Their remarks are drawn from a companion commentary in Pediatrics (Pediatrics. 2016;138[3]:e20160975).
The needs of varying populations, the prevalence of various serotypes, and other local epidemiologic and economic factors all influence vaccination schedules. For PCV, the present study showed how widely seroprotection varied between serotypes and between different priming schedules.
Invasive pneumococcal disease (IPD) can be devastating in the vulnerable preterm population, as can pneumococcal pneumonia. Though the current vaccination schedule recommendations in the United States takes into account age-related changes in the immune system, truly optimized vaccine delivery for all populations, including this vulnerable one, is still more a goal than a reality.
However, each of the schedules examined in this study have been studied in areas where they are in clinical use, and all are generally protective of IPD. The timing of other vaccinations, as well as economic and logistic realities, will also affect vaccination schedules, and must be taken into account.
The findings of this study show that no one schedule is best for all populations, and also highlight why those making vaccine policy around the globe will continue to arrive at varying answers when considering the needs of their populations.
Mark H. Sawyer, MD, is a professor of pediatrics at the University of California, San Diego. Mobeen Rathore, MD, is director of the University of Florida Center for HIV/AIDS Research, Education and Service. They had no conflicts of interest to declare. Their remarks are drawn from a companion commentary in Pediatrics (Pediatrics. 2016;138[3]:e20160975).
A randomized clinical trial evaluating three dosing strategies for 13-valent pneumococcal vaccine (PCV13) in preterm infants found that more widely spaced priming vaccinations resulted in higher immunoglobulin G (IgG) during the first 12 months of life, but reduced the immune response seen after the 12-month booster was given.
After the primary schedule, the percent of infants lacking seroprotection for more than one half of the serotypes in the PCV13 formulation was 25% on a reduced two-dose schedule, 12% on an accelerated schedule, and 3% on an extended schedule (P less than .001).
Conversely, “A reduced priming schedule of PCV13 resulted in higher post-booster IgG concentrations but lower post-primary concentrations,” wrote Alison Kent, MBChB, and her coinvestigators in the PUNS (Prems Under New Schedule) Study Group (Pediatrics. 2016;138[3]:e20153945).
“Infants who received the extended schedule had lower fold increases in concentrations after booster vaccination than the other groups,” wrote Dr. Kent of the Pediatric Infectious Diseases Research Group and Vaccine Institute, St. George’s, University of London, and her collaborators. Participants receiving the extended schedule had lower geometric mean concentrations (GMCs) of antibodies than did those on the reduced schedule for nine serotypes and those on the accelerated schedule for four serotypes.
The study enrolled 210 premature infants in a phase IV, controlled, open-label trial at 12 sites in the United Kingdom. Infants of less than 35 weeks gestation, and between 7 and 12 weeks of age, were randomly assigned to receive PCV13 on one of three schedules. The reduced schedule gave two priming doses at 2 and 4 months of age; the accelerated schedule gave the doses at 2, 3, and 4 months of age; and the extended schedule gave doses at 2, 4, and 6 months of age. All infants received a booster vaccination at 12 or 13 months of age, and all received a standard suite of childhood immunizations for other diseases. The entire study was completed by 194 patients.
Serotype-specific IgG concentrations were obtained pre-vaccination, 1 month after the primary vaccination, and before and 1 month after the booster vaccination was given. IgG levels were reported for each PCV serotype; “there was considerable variation between serotypes,” ranging from 0.16 ng/mL for serotype 6b on the reduced schedule to 8.49 ng/mL for serotype 14 on the extended schedule, the investigators said.
Dr. Kent and her collaborators also used logistic regression analysis to explore how the vaccine’s effectiveness was affected by a number of factors. These included gestational length, the receipt of blood transfusions or pre- or post-natal steroids, BCG vaccination, early postvaccination acetaminophen, and the presence of chronic lung disease.
Later gestation was associated with increased seroprotection for four serotypes at 2 months of age, and with an increase in post-primary vaccination IgG concentrations for three others (P-values ranging from P less than .001 to P = .021).
No other factors were associated with protective IgG levels at any point, except that receipt of prenatal steroids had a negative association with seroprotection for several serotypes. “At no time points were antenatal steroids associated with higher antibody concentrations,” wrote the investigators.
Most studies of immunogenicity of infant vaccination schedules have been completed using term infants, with limited knowledge about efficacy in preterm infants. Previous work had shown that preterm infants had lower IgG concentrations after the primary and booster vaccinations for eight serotypes of PCV, compared with term infants. “The lower immunogenicity ... is concerning because premature infants are also less likely to benefit from the protective maternal antibodies transferred during late pregnancy,” Dr. Kent and her coauthors wrote.
The lower booster immunogenicity after the extended schedule is an effect that has been previously observed with other vaccinations and may be related to the formation of immune complexes with previously existing antibodies with the vaccine antigen, said Dr. Kent and her coauthors. The variation in immunogenicity timing for the various priming schedules, they said, will be helpful for those caring for preterm infants, enabling them “to consider this finding in the context of their own immunization programs and epidemiologic situations.”
The study was funded by Pfizer as an investigator-led study, without Pfizer’s input on the conduct of the trial, analysis of data, interpretation of results, or the preparation of this manuscript. Pfizer manufactures Prevnar 13.
On Twitter @karioakes
A randomized clinical trial evaluating three dosing strategies for 13-valent pneumococcal vaccine (PCV13) in preterm infants found that more widely spaced priming vaccinations resulted in higher immunoglobulin G (IgG) during the first 12 months of life, but reduced the immune response seen after the 12-month booster was given.
After the primary schedule, the percent of infants lacking seroprotection for more than one half of the serotypes in the PCV13 formulation was 25% on a reduced two-dose schedule, 12% on an accelerated schedule, and 3% on an extended schedule (P less than .001).
Conversely, “A reduced priming schedule of PCV13 resulted in higher post-booster IgG concentrations but lower post-primary concentrations,” wrote Alison Kent, MBChB, and her coinvestigators in the PUNS (Prems Under New Schedule) Study Group (Pediatrics. 2016;138[3]:e20153945).
“Infants who received the extended schedule had lower fold increases in concentrations after booster vaccination than the other groups,” wrote Dr. Kent of the Pediatric Infectious Diseases Research Group and Vaccine Institute, St. George’s, University of London, and her collaborators. Participants receiving the extended schedule had lower geometric mean concentrations (GMCs) of antibodies than did those on the reduced schedule for nine serotypes and those on the accelerated schedule for four serotypes.
The study enrolled 210 premature infants in a phase IV, controlled, open-label trial at 12 sites in the United Kingdom. Infants of less than 35 weeks gestation, and between 7 and 12 weeks of age, were randomly assigned to receive PCV13 on one of three schedules. The reduced schedule gave two priming doses at 2 and 4 months of age; the accelerated schedule gave the doses at 2, 3, and 4 months of age; and the extended schedule gave doses at 2, 4, and 6 months of age. All infants received a booster vaccination at 12 or 13 months of age, and all received a standard suite of childhood immunizations for other diseases. The entire study was completed by 194 patients.
Serotype-specific IgG concentrations were obtained pre-vaccination, 1 month after the primary vaccination, and before and 1 month after the booster vaccination was given. IgG levels were reported for each PCV serotype; “there was considerable variation between serotypes,” ranging from 0.16 ng/mL for serotype 6b on the reduced schedule to 8.49 ng/mL for serotype 14 on the extended schedule, the investigators said.
Dr. Kent and her collaborators also used logistic regression analysis to explore how the vaccine’s effectiveness was affected by a number of factors. These included gestational length, the receipt of blood transfusions or pre- or post-natal steroids, BCG vaccination, early postvaccination acetaminophen, and the presence of chronic lung disease.
Later gestation was associated with increased seroprotection for four serotypes at 2 months of age, and with an increase in post-primary vaccination IgG concentrations for three others (P-values ranging from P less than .001 to P = .021).
No other factors were associated with protective IgG levels at any point, except that receipt of prenatal steroids had a negative association with seroprotection for several serotypes. “At no time points were antenatal steroids associated with higher antibody concentrations,” wrote the investigators.
Most studies of immunogenicity of infant vaccination schedules have been completed using term infants, with limited knowledge about efficacy in preterm infants. Previous work had shown that preterm infants had lower IgG concentrations after the primary and booster vaccinations for eight serotypes of PCV, compared with term infants. “The lower immunogenicity ... is concerning because premature infants are also less likely to benefit from the protective maternal antibodies transferred during late pregnancy,” Dr. Kent and her coauthors wrote.
The lower booster immunogenicity after the extended schedule is an effect that has been previously observed with other vaccinations and may be related to the formation of immune complexes with previously existing antibodies with the vaccine antigen, said Dr. Kent and her coauthors. The variation in immunogenicity timing for the various priming schedules, they said, will be helpful for those caring for preterm infants, enabling them “to consider this finding in the context of their own immunization programs and epidemiologic situations.”
The study was funded by Pfizer as an investigator-led study, without Pfizer’s input on the conduct of the trial, analysis of data, interpretation of results, or the preparation of this manuscript. Pfizer manufactures Prevnar 13.
On Twitter @karioakes
FROM PEDIATRICS
Key clinical point: More widely-spaced pneumococcal vaccinations boosted early immunity but reduced the effectiveness of a 12-month booster in preterm infants.
Major finding: Of preterm infants on an extended pneumococcal conjugate vaccine (PCV13) schedule, just 3% lacked seroprotection for over half of the serotypes.
Data source: Randomized, placebo-controlled, open-label study of 210 preterm infants receiving PCV13vaccination on one of three dosing schedules.
Disclosures: The study was funded by Pfizer as an investigator-led study, without Pfizer’s input on the conduct of the trial, analysis of data, interpretation of results, or the preparation of this manuscript. Pfizer manufactures Prevnar 13.
Blood viral RNA may indicate severity of MERS coronavirus clinical course
The presence of blood viral RNA in patients presenting with possible Middle East respiratory syndrome coronavirus (MERS-CoV) may be a very reliable indicator of the severity of the infection’s clinical course, according to a new study published in Emerging Infectious Diseases.
“Our study aimed to evaluate the diagnostic utility of blood specimens for MERS-CoV infection by using large numbers of patients with a single viral origin and to determine the relationship between blood viral detection and clinical characteristics,” wrote the authors, led by So Yeon Kim, MD, of the National Medical Center in Seoul, South Korea.
The investigators recruited 21 MERS-CoV patients within South Korea, all of whom had been previously diagnosed by the Korea Centers for Disease Control and Prevention via respiratory samples and were of “a single viral origin.” All subjects contributed ethylenediaminetetraacetic acid (EDTA)-treated whole blood and serum specimens, from which viral RNA was extracted (Emerg Infect Dis. 2016 Oct 15;22[10]. doi: 10.3201/eid2210.160218).
Viral RNA was detected in 6 of 21 whole blood samples and 6 of 21 serum samples at hospital admission. However, because two patients were viral positive in either specimen subtype of EDTA-treated whole blood or serum, the overall detection rate for the population was 7 of 21 (33%). Being positive for blood viral RNA at admission was found to be associated with a fever of higher than 37.5 °C (99.5 °F) on the date of sample collection (P = .007), being placed on mechanical ventilation at some point during the clinical course (P = .003), extracorporeal membrane oxygenation (P = .025), and death (P = .025).
However, “between the blood viral RNA-positive and -negative groups, we found no differences in age, duration from symptom onset to diagnosis of MERS-CoV infection, or an invasive procedure before the specimens were obtained,” the investigators noted.
The takeaway, the authors underscore, is that although early blood viral RNA presence may not be a useful diagnostic tool, it “might be a good prognostic indicator of severe outcome” due to its high association with worse clinical course.
The research was funded by the National Medical Center Research Institute.
The presence of blood viral RNA in patients presenting with possible Middle East respiratory syndrome coronavirus (MERS-CoV) may be a very reliable indicator of the severity of the infection’s clinical course, according to a new study published in Emerging Infectious Diseases.
“Our study aimed to evaluate the diagnostic utility of blood specimens for MERS-CoV infection by using large numbers of patients with a single viral origin and to determine the relationship between blood viral detection and clinical characteristics,” wrote the authors, led by So Yeon Kim, MD, of the National Medical Center in Seoul, South Korea.
The investigators recruited 21 MERS-CoV patients within South Korea, all of whom had been previously diagnosed by the Korea Centers for Disease Control and Prevention via respiratory samples and were of “a single viral origin.” All subjects contributed ethylenediaminetetraacetic acid (EDTA)-treated whole blood and serum specimens, from which viral RNA was extracted (Emerg Infect Dis. 2016 Oct 15;22[10]. doi: 10.3201/eid2210.160218).
Viral RNA was detected in 6 of 21 whole blood samples and 6 of 21 serum samples at hospital admission. However, because two patients were viral positive in either specimen subtype of EDTA-treated whole blood or serum, the overall detection rate for the population was 7 of 21 (33%). Being positive for blood viral RNA at admission was found to be associated with a fever of higher than 37.5 °C (99.5 °F) on the date of sample collection (P = .007), being placed on mechanical ventilation at some point during the clinical course (P = .003), extracorporeal membrane oxygenation (P = .025), and death (P = .025).
However, “between the blood viral RNA-positive and -negative groups, we found no differences in age, duration from symptom onset to diagnosis of MERS-CoV infection, or an invasive procedure before the specimens were obtained,” the investigators noted.
The takeaway, the authors underscore, is that although early blood viral RNA presence may not be a useful diagnostic tool, it “might be a good prognostic indicator of severe outcome” due to its high association with worse clinical course.
The research was funded by the National Medical Center Research Institute.
The presence of blood viral RNA in patients presenting with possible Middle East respiratory syndrome coronavirus (MERS-CoV) may be a very reliable indicator of the severity of the infection’s clinical course, according to a new study published in Emerging Infectious Diseases.
“Our study aimed to evaluate the diagnostic utility of blood specimens for MERS-CoV infection by using large numbers of patients with a single viral origin and to determine the relationship between blood viral detection and clinical characteristics,” wrote the authors, led by So Yeon Kim, MD, of the National Medical Center in Seoul, South Korea.
The investigators recruited 21 MERS-CoV patients within South Korea, all of whom had been previously diagnosed by the Korea Centers for Disease Control and Prevention via respiratory samples and were of “a single viral origin.” All subjects contributed ethylenediaminetetraacetic acid (EDTA)-treated whole blood and serum specimens, from which viral RNA was extracted (Emerg Infect Dis. 2016 Oct 15;22[10]. doi: 10.3201/eid2210.160218).
Viral RNA was detected in 6 of 21 whole blood samples and 6 of 21 serum samples at hospital admission. However, because two patients were viral positive in either specimen subtype of EDTA-treated whole blood or serum, the overall detection rate for the population was 7 of 21 (33%). Being positive for blood viral RNA at admission was found to be associated with a fever of higher than 37.5 °C (99.5 °F) on the date of sample collection (P = .007), being placed on mechanical ventilation at some point during the clinical course (P = .003), extracorporeal membrane oxygenation (P = .025), and death (P = .025).
However, “between the blood viral RNA-positive and -negative groups, we found no differences in age, duration from symptom onset to diagnosis of MERS-CoV infection, or an invasive procedure before the specimens were obtained,” the investigators noted.
The takeaway, the authors underscore, is that although early blood viral RNA presence may not be a useful diagnostic tool, it “might be a good prognostic indicator of severe outcome” due to its high association with worse clinical course.
The research was funded by the National Medical Center Research Institute.
FROM EMERGING INFECTIOUS DISEASES
Key clinical point: Checking for blood viral RNA at hospital admission may be a reliable indicator of the severity of MERS coronavirus infection clinical course.
Major finding: Blood viral RNA positivity at admission was associated with fever higher than 37.5 °C on the sampling date (P = .007), requirement for mechanical ventilation during the following clinical course (P = .003), extracorporeal membrane oxygenation (P = .025), and patient death (P = .025).
Data source: Prospective analysis of 21 patients with Middle East respiratory syndrome coronavirus (MERS-CoV).
Disclosures: The research was funded by the National Medical Center Research Institute.
LABA achieves better asthma control when combined with FDC inhaler
Long-acting beta-2 agonists achieve better asthma control when added to inhaled corticosteroids in a fixed-dose combination, compared with use of a LABA as a separate inhaler, according to Steve Turner, MD, and his associates.
At baseline, 35% of children in the FDC ICS (fixed-dose combination inhaled corticosteroids)/LABA cohort and in the separate ICS+LABA cohort had achieved overall asthma control. After 2 years, 43% of children in the FDC ICS/LABA cohort had achieved overall asthma control, compared with 37% of children in the separate ICS+LABA cohort. The adjusted odds ratio for overall asthma control in the separate ICS+LABA cohort was 0.77.
The adjusted relative risk of acute respiratory events for the separate ICS+LABA cohort was 1.21, compared with the FDC ICS/LABA cohort, and the aRR for severe exacerbations was 1.31 for the separate ICS+LABA cohort. More children in the separate ICS+LABA cohort were treated with antibiotics; however, the incidence of thrush was higher in the FDC ICS/LABA cohort.
“This small effect may be partly explained by improvement in all outcomes in both groups as the children became older. An additional factor may be that adherence was relatively poor for all participants (22%-33%), and poor adherence is associated with poor control. This may have led to the decision to step up and also to a relatively disappointing response to treatment,” the investigators wrote.
Find the full study in the Journal of Allergy and Clinical Immunology (doi:10.1016/j.jaip.2016.06.009).
Long-acting beta-2 agonists achieve better asthma control when added to inhaled corticosteroids in a fixed-dose combination, compared with use of a LABA as a separate inhaler, according to Steve Turner, MD, and his associates.
At baseline, 35% of children in the FDC ICS (fixed-dose combination inhaled corticosteroids)/LABA cohort and in the separate ICS+LABA cohort had achieved overall asthma control. After 2 years, 43% of children in the FDC ICS/LABA cohort had achieved overall asthma control, compared with 37% of children in the separate ICS+LABA cohort. The adjusted odds ratio for overall asthma control in the separate ICS+LABA cohort was 0.77.
The adjusted relative risk of acute respiratory events for the separate ICS+LABA cohort was 1.21, compared with the FDC ICS/LABA cohort, and the aRR for severe exacerbations was 1.31 for the separate ICS+LABA cohort. More children in the separate ICS+LABA cohort were treated with antibiotics; however, the incidence of thrush was higher in the FDC ICS/LABA cohort.
“This small effect may be partly explained by improvement in all outcomes in both groups as the children became older. An additional factor may be that adherence was relatively poor for all participants (22%-33%), and poor adherence is associated with poor control. This may have led to the decision to step up and also to a relatively disappointing response to treatment,” the investigators wrote.
Find the full study in the Journal of Allergy and Clinical Immunology (doi:10.1016/j.jaip.2016.06.009).
Long-acting beta-2 agonists achieve better asthma control when added to inhaled corticosteroids in a fixed-dose combination, compared with use of a LABA as a separate inhaler, according to Steve Turner, MD, and his associates.
At baseline, 35% of children in the FDC ICS (fixed-dose combination inhaled corticosteroids)/LABA cohort and in the separate ICS+LABA cohort had achieved overall asthma control. After 2 years, 43% of children in the FDC ICS/LABA cohort had achieved overall asthma control, compared with 37% of children in the separate ICS+LABA cohort. The adjusted odds ratio for overall asthma control in the separate ICS+LABA cohort was 0.77.
The adjusted relative risk of acute respiratory events for the separate ICS+LABA cohort was 1.21, compared with the FDC ICS/LABA cohort, and the aRR for severe exacerbations was 1.31 for the separate ICS+LABA cohort. More children in the separate ICS+LABA cohort were treated with antibiotics; however, the incidence of thrush was higher in the FDC ICS/LABA cohort.
“This small effect may be partly explained by improvement in all outcomes in both groups as the children became older. An additional factor may be that adherence was relatively poor for all participants (22%-33%), and poor adherence is associated with poor control. This may have led to the decision to step up and also to a relatively disappointing response to treatment,” the investigators wrote.
Find the full study in the Journal of Allergy and Clinical Immunology (doi:10.1016/j.jaip.2016.06.009).
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY
Serum vitamin D levels, atopy not significantly linked
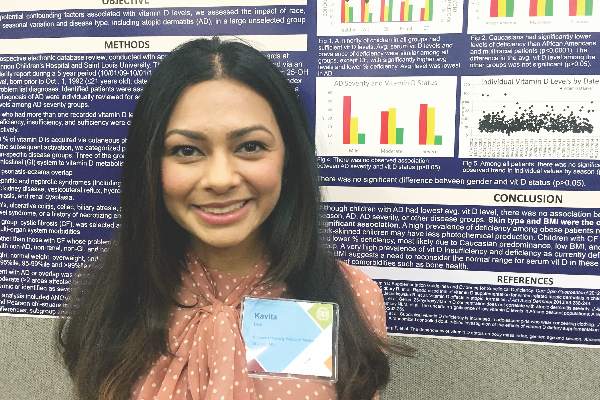
SCOTTSDALE, ARIZ. – Serum vitamin D level was not significantly associated with atopic dermatitis or disease severity in a single-center study of more than 600 children and adolescents.
However, “we did observe a strong correlation between average serum vitamin D levels and skin type, as well as body mass index,” said Kavita Darji, a medical student at Saint Louis (Mo.) University, who presented the findings in a poster at the annual meeting of the Society for Investigative Dermatology. Those findings challenge the logic of following universal definitions of vitamin D deficiency, especially given the phenotypic heterogeneity of patients in the United States, she added in an interview.
Serum vitamin D testing is one of most common laboratory assays in this country, but clinicians still debate the risks and benefits of supplementing children and adolescents who test below the Endocrine Society’s threshold for sufficiency (30.0 ng/mL).
To identify factors affecting vitamin D levels, Ms. Darji and her associates reviewed electronic medical charts for patients under age 22 years at Saint Louis University medical centers between 2009 and 2014. The cohort of 655 patients was primarily white (64%) or black (29%), and was nearly equally balanced by gender; their average age was 10 years. The researchers analyzed only the first vitamin D serum measurement for each patient, and defined deficiency as a level under 20 ng/mL, insufficiency as a level between 20 and 29.9 ng/mL, and sufficiency as a level of at least 30 ng/mL.
Serum vitamin D levels were slightly lower among atopic patients, compared with those without atopy, but the difference did not reach statistical significance (about 25 ng/mL vs. about 38 ng/mL; P greater than .05). “We also did not find an association between AD severity and vitamin D level,” Ms. Darji reported. Instead, race and body mass index were the most significant predictors of vitamin D deficiency, probably because these factors directly affect cutaneous photo-induced vitamin D synthesis and the sequestration of fat-soluble vitamins in adipose tissue, she said.
Using the standard definitions, more than 50% of black patients were vitamin D deficient, while less than 30% had sufficient vitamin D levels. In contrast, about 25% of white patients were vitamin D deficient, while nearly 40% had sufficient vitamin D levels (P less than .0001 for proportions of deficiency by race). Furthermore, only about 10% of obese children (those who exceeded the 99th percentile of BMI for age) had sufficient vitamin D levels, compared with more than 40% of underweight children and about 30% of normal-weight children (P less than .00001).
Since vitamin D deficiency was more common among black and obese patients, “maybe they could benefit from a different cut-off value than the standard 30 ng per mL that we used,” Ms. Darji said. “The question is, do they really require these supplements? It may be beneficial to look at the unique characteristics of each patient before supplementing, because the risks of supplementation are considerable in terms of bone health and cardiovascular disease.”
Vitamin D levels did not vary significantly by gender or by month or season measured, Ms. Darji noted. She reported no funding sources and had no disclosures.
SCOTTSDALE, ARIZ. – Serum vitamin D level was not significantly associated with atopic dermatitis or disease severity in a single-center study of more than 600 children and adolescents.
However, “we did observe a strong correlation between average serum vitamin D levels and skin type, as well as body mass index,” said Kavita Darji, a medical student at Saint Louis (Mo.) University, who presented the findings in a poster at the annual meeting of the Society for Investigative Dermatology. Those findings challenge the logic of following universal definitions of vitamin D deficiency, especially given the phenotypic heterogeneity of patients in the United States, she added in an interview.
Serum vitamin D testing is one of most common laboratory assays in this country, but clinicians still debate the risks and benefits of supplementing children and adolescents who test below the Endocrine Society’s threshold for sufficiency (30.0 ng/mL).
To identify factors affecting vitamin D levels, Ms. Darji and her associates reviewed electronic medical charts for patients under age 22 years at Saint Louis University medical centers between 2009 and 2014. The cohort of 655 patients was primarily white (64%) or black (29%), and was nearly equally balanced by gender; their average age was 10 years. The researchers analyzed only the first vitamin D serum measurement for each patient, and defined deficiency as a level under 20 ng/mL, insufficiency as a level between 20 and 29.9 ng/mL, and sufficiency as a level of at least 30 ng/mL.
Serum vitamin D levels were slightly lower among atopic patients, compared with those without atopy, but the difference did not reach statistical significance (about 25 ng/mL vs. about 38 ng/mL; P greater than .05). “We also did not find an association between AD severity and vitamin D level,” Ms. Darji reported. Instead, race and body mass index were the most significant predictors of vitamin D deficiency, probably because these factors directly affect cutaneous photo-induced vitamin D synthesis and the sequestration of fat-soluble vitamins in adipose tissue, she said.
Using the standard definitions, more than 50% of black patients were vitamin D deficient, while less than 30% had sufficient vitamin D levels. In contrast, about 25% of white patients were vitamin D deficient, while nearly 40% had sufficient vitamin D levels (P less than .0001 for proportions of deficiency by race). Furthermore, only about 10% of obese children (those who exceeded the 99th percentile of BMI for age) had sufficient vitamin D levels, compared with more than 40% of underweight children and about 30% of normal-weight children (P less than .00001).
Since vitamin D deficiency was more common among black and obese patients, “maybe they could benefit from a different cut-off value than the standard 30 ng per mL that we used,” Ms. Darji said. “The question is, do they really require these supplements? It may be beneficial to look at the unique characteristics of each patient before supplementing, because the risks of supplementation are considerable in terms of bone health and cardiovascular disease.”
Vitamin D levels did not vary significantly by gender or by month or season measured, Ms. Darji noted. She reported no funding sources and had no disclosures.
SCOTTSDALE, ARIZ. – Serum vitamin D level was not significantly associated with atopic dermatitis or disease severity in a single-center study of more than 600 children and adolescents.
However, “we did observe a strong correlation between average serum vitamin D levels and skin type, as well as body mass index,” said Kavita Darji, a medical student at Saint Louis (Mo.) University, who presented the findings in a poster at the annual meeting of the Society for Investigative Dermatology. Those findings challenge the logic of following universal definitions of vitamin D deficiency, especially given the phenotypic heterogeneity of patients in the United States, she added in an interview.
Serum vitamin D testing is one of most common laboratory assays in this country, but clinicians still debate the risks and benefits of supplementing children and adolescents who test below the Endocrine Society’s threshold for sufficiency (30.0 ng/mL).
To identify factors affecting vitamin D levels, Ms. Darji and her associates reviewed electronic medical charts for patients under age 22 years at Saint Louis University medical centers between 2009 and 2014. The cohort of 655 patients was primarily white (64%) or black (29%), and was nearly equally balanced by gender; their average age was 10 years. The researchers analyzed only the first vitamin D serum measurement for each patient, and defined deficiency as a level under 20 ng/mL, insufficiency as a level between 20 and 29.9 ng/mL, and sufficiency as a level of at least 30 ng/mL.
Serum vitamin D levels were slightly lower among atopic patients, compared with those without atopy, but the difference did not reach statistical significance (about 25 ng/mL vs. about 38 ng/mL; P greater than .05). “We also did not find an association between AD severity and vitamin D level,” Ms. Darji reported. Instead, race and body mass index were the most significant predictors of vitamin D deficiency, probably because these factors directly affect cutaneous photo-induced vitamin D synthesis and the sequestration of fat-soluble vitamins in adipose tissue, she said.
Using the standard definitions, more than 50% of black patients were vitamin D deficient, while less than 30% had sufficient vitamin D levels. In contrast, about 25% of white patients were vitamin D deficient, while nearly 40% had sufficient vitamin D levels (P less than .0001 for proportions of deficiency by race). Furthermore, only about 10% of obese children (those who exceeded the 99th percentile of BMI for age) had sufficient vitamin D levels, compared with more than 40% of underweight children and about 30% of normal-weight children (P less than .00001).
Since vitamin D deficiency was more common among black and obese patients, “maybe they could benefit from a different cut-off value than the standard 30 ng per mL that we used,” Ms. Darji said. “The question is, do they really require these supplements? It may be beneficial to look at the unique characteristics of each patient before supplementing, because the risks of supplementation are considerable in terms of bone health and cardiovascular disease.”
Vitamin D levels did not vary significantly by gender or by month or season measured, Ms. Darji noted. She reported no funding sources and had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Serum vitamin D was not a significant marker for pediatric atopic dermatitis or disease severity.
Major finding: The average serum vitamin D level was lower among patients with atopic dermatitis than healthy children, but the difference did not reach statistical significance.
Data source: A single-center retrospective review of electronic medical records from 655 patients aged 21 years and younger (average age, 10 years).
Disclosures: Ms. Darji reported no funding sources and had no disclosures.
Influenza: A vaccine we love to hate
The Centers for Disease Control and Prevention, American Academy of Pediatrics, and American Academy of Family Physicians recommend that everyone 6 months of age and older get a seasonal flu vaccine. Emphasizing influenza vaccination in children recognizes the high burden of morbidity and significant mortality associated with influenza in young children as well as their role in transmission in the community.
In 2015-2016, the CDC reported 83 influenza deaths in children, and estimated the rate of hospitalization for children younger than 4 years of age to be 42/100,000 (at press time). In 2015-2016, the H1N1 strain was dominant in the community overall, with influenza B being most prevalent late in the season. The CDC estimates that nearly 75% of children less than 24 months and 68% between 2 and 4 years of age were immunized this year. Overall vaccine efficacy in children 6 months through 8 years was reported at 47% last season from a CDC study using a study design that compares vaccination odds among influenza reverse transcription polymerase chain reaction (RT-PCR)–positive cases and RT-PCR–negative controls.
Influenza virus vaccines are unique in that they are updated, often annually, to include the most current hemagglutinin (HA) antigens based on estimates from circulating strains. In the United States, influenza vaccine manufacturers submit a supplement to their license and obtain Food and Drug Administration approval. These applications require only a limited study of safety in approximately 300 adults, essentially to verify attenuation (Influenza Other Respir Viruses. 2016. doi: 10.111/irv.1283). They do not require clinical proof of efficacy or even a threshold of immunogenicity.
At the June 2016 CDC’s Advisory Committee on Immunization Practices (ACIP) meeting, data were presented comparing the efficacy of this season’s live attenuated influenza vaccine (LAIV) with inactivated influenza vaccine (IIV) by age and specific influenza type and subtype. Data from the U.S. Flu Vaccine Effectiveness (VE) Network, a consortium of five CDC-funded sites that conducts annual studies of influenza vaccine effectiveness, failed to demonstrate efficacy for LAIV in children aged 2-8 years. There was an absence of efficacy against the primary circulating strain, A(H1N1). This contrasted with the 62% efficacy report for IIV against A(H1N1).
The concern for efficacy for LAIV was not limited to 2015-2016; efficacy was poor in 2013-2014 during a year in which A(H1N1) was the dominant virus as well, and in 2014-2015 when the prevalent strain was a drifted A(H3N2). The lack of efficacy in 2015-2016 and 2013-2014 when A(H1N1) was the prevalent strain was especially enigmatic given its high efficacy against A(H1N1) between 2009 and 2011. Studies of LAIV from Astra Zeneca and the U.S. Department of Defense were consistent with those from the U.S. Flu VE Network; however, there were discordant data from Finland where vaccine efficacy was present. As a result of these studies, the ACIP voted that LAIV should not be used during the 2016-2017 flu season. This vote reinforces the importance of monitoring the effectiveness of annual flu vaccination and other public health interventions.
ACIP recommendations for 2016-2017
• Children younger than 2 years of age and those with chronic health problems such as asthma, diabetes, and disorders of the brain or nervous system are at especially high risk of developing serious flu complications.
• Annual influenza immunization, with either the IIV or recombinant influenza vaccine (RIV), for everyone 6 months and older, remains the only effective strategy for decreasing influenza disease in the community.
• LAIV should not be used during the 2016-2017 flu season.
ACIP recommendations must be reviewed and approved by the CDC’s director before becoming CDC policy. The final annual recommendations on the prevention and control of influenza with vaccines will be published in CDC Morbidity and Mortality Weekly Report (MMWR) Recommendations and Reports in late summer or early fall.
Flu vaccines available for children for 2016-2017
• The trivalent flu vaccine protects against three flu viruses; two influenza A viruses and an influenza B virus. Standard dose trivalent shots are manufactured with viruses grown in eggs. These are approved for children aged 6 months and older. There are different brands of this type of vaccine; each specific formulation has different age-based approvals.

• The quadrivalent flu vaccine protects against four flu viruses; two influenza A viruses and two influenza B viruses. A standard dose quadrivalent formulation is available for children; one brand is approved for children 6 months and older while others are approved for those 3 years and older.
• A cell-based vaccine, developed through a manufacturing process different from the traditional egg-based manufacturing process, was approved as a quadrivalent formulation for use in children 4 years of age and older.
Unanswered questions for the 2016-2017 influenza season
• Children 6 months to 8 years who are getting vaccinated for the first time need two doses. How should we consider influenza-naive children who received two doses of LAIV last year? The reason for the LAIV’s loss of efficacy in the years 2014 through 2016 is unknown, although it has been hypothesized that reduced immunogenicity is one possible cause for the lack of protection. Rather than speculate, we need to wait for ACIP to gather more data and then publish recommendations as to whether to consider such children vaccine naive (and therefore requiring two doses this season) or previously immunized (and therefore in need of only a single dose).
• Will supply be adequate this year? LAIV represents about 8% of the 171-176 million doses that were projected to be available during the 2016-2017 season; however, it represents nearly one-third of doses given to children. Thus, the potential for shortages in pediatric offices is real, and pediatricians and vaccine manufacturers need to work together to make sure sufficient pediatric formulation is available. The CDC is working with manufacturers to ensure there is sufficient supply to meet the demand.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He has received honoraria from Sanofi Pasteur and Seqirus for participation in vaccine advisory boards in the prior 12 months. Email him at [email protected].
The Centers for Disease Control and Prevention, American Academy of Pediatrics, and American Academy of Family Physicians recommend that everyone 6 months of age and older get a seasonal flu vaccine. Emphasizing influenza vaccination in children recognizes the high burden of morbidity and significant mortality associated with influenza in young children as well as their role in transmission in the community.
In 2015-2016, the CDC reported 83 influenza deaths in children, and estimated the rate of hospitalization for children younger than 4 years of age to be 42/100,000 (at press time). In 2015-2016, the H1N1 strain was dominant in the community overall, with influenza B being most prevalent late in the season. The CDC estimates that nearly 75% of children less than 24 months and 68% between 2 and 4 years of age were immunized this year. Overall vaccine efficacy in children 6 months through 8 years was reported at 47% last season from a CDC study using a study design that compares vaccination odds among influenza reverse transcription polymerase chain reaction (RT-PCR)–positive cases and RT-PCR–negative controls.
Influenza virus vaccines are unique in that they are updated, often annually, to include the most current hemagglutinin (HA) antigens based on estimates from circulating strains. In the United States, influenza vaccine manufacturers submit a supplement to their license and obtain Food and Drug Administration approval. These applications require only a limited study of safety in approximately 300 adults, essentially to verify attenuation (Influenza Other Respir Viruses. 2016. doi: 10.111/irv.1283). They do not require clinical proof of efficacy or even a threshold of immunogenicity.
At the June 2016 CDC’s Advisory Committee on Immunization Practices (ACIP) meeting, data were presented comparing the efficacy of this season’s live attenuated influenza vaccine (LAIV) with inactivated influenza vaccine (IIV) by age and specific influenza type and subtype. Data from the U.S. Flu Vaccine Effectiveness (VE) Network, a consortium of five CDC-funded sites that conducts annual studies of influenza vaccine effectiveness, failed to demonstrate efficacy for LAIV in children aged 2-8 years. There was an absence of efficacy against the primary circulating strain, A(H1N1). This contrasted with the 62% efficacy report for IIV against A(H1N1).
The concern for efficacy for LAIV was not limited to 2015-2016; efficacy was poor in 2013-2014 during a year in which A(H1N1) was the dominant virus as well, and in 2014-2015 when the prevalent strain was a drifted A(H3N2). The lack of efficacy in 2015-2016 and 2013-2014 when A(H1N1) was the prevalent strain was especially enigmatic given its high efficacy against A(H1N1) between 2009 and 2011. Studies of LAIV from Astra Zeneca and the U.S. Department of Defense were consistent with those from the U.S. Flu VE Network; however, there were discordant data from Finland where vaccine efficacy was present. As a result of these studies, the ACIP voted that LAIV should not be used during the 2016-2017 flu season. This vote reinforces the importance of monitoring the effectiveness of annual flu vaccination and other public health interventions.

ACIP recommendations for 2016-2017
• Children younger than 2 years of age and those with chronic health problems such as asthma, diabetes, and disorders of the brain or nervous system are at especially high risk of developing serious flu complications.
• Annual influenza immunization, with either the IIV or recombinant influenza vaccine (RIV), for everyone 6 months and older, remains the only effective strategy for decreasing influenza disease in the community.
• LAIV should not be used during the 2016-2017 flu season.
ACIP recommendations must be reviewed and approved by the CDC’s director before becoming CDC policy. The final annual recommendations on the prevention and control of influenza with vaccines will be published in CDC Morbidity and Mortality Weekly Report (MMWR) Recommendations and Reports in late summer or early fall.
Flu vaccines available for children for 2016-2017
• The trivalent flu vaccine protects against three flu viruses; two influenza A viruses and an influenza B virus. Standard dose trivalent shots are manufactured with viruses grown in eggs. These are approved for children aged 6 months and older. There are different brands of this type of vaccine; each specific formulation has different age-based approvals.

• The quadrivalent flu vaccine protects against four flu viruses; two influenza A viruses and two influenza B viruses. A standard dose quadrivalent formulation is available for children; one brand is approved for children 6 months and older while others are approved for those 3 years and older.
• A cell-based vaccine, developed through a manufacturing process different from the traditional egg-based manufacturing process, was approved as a quadrivalent formulation for use in children 4 years of age and older.
Unanswered questions for the 2016-2017 influenza season
• Children 6 months to 8 years who are getting vaccinated for the first time need two doses. How should we consider influenza-naive children who received two doses of LAIV last year? The reason for the LAIV’s loss of efficacy in the years 2014 through 2016 is unknown, although it has been hypothesized that reduced immunogenicity is one possible cause for the lack of protection. Rather than speculate, we need to wait for ACIP to gather more data and then publish recommendations as to whether to consider such children vaccine naive (and therefore requiring two doses this season) or previously immunized (and therefore in need of only a single dose).
• Will supply be adequate this year? LAIV represents about 8% of the 171-176 million doses that were projected to be available during the 2016-2017 season; however, it represents nearly one-third of doses given to children. Thus, the potential for shortages in pediatric offices is real, and pediatricians and vaccine manufacturers need to work together to make sure sufficient pediatric formulation is available. The CDC is working with manufacturers to ensure there is sufficient supply to meet the demand.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He has received honoraria from Sanofi Pasteur and Seqirus for participation in vaccine advisory boards in the prior 12 months. Email him at [email protected].
The Centers for Disease Control and Prevention, American Academy of Pediatrics, and American Academy of Family Physicians recommend that everyone 6 months of age and older get a seasonal flu vaccine. Emphasizing influenza vaccination in children recognizes the high burden of morbidity and significant mortality associated with influenza in young children as well as their role in transmission in the community.
In 2015-2016, the CDC reported 83 influenza deaths in children, and estimated the rate of hospitalization for children younger than 4 years of age to be 42/100,000 (at press time). In 2015-2016, the H1N1 strain was dominant in the community overall, with influenza B being most prevalent late in the season. The CDC estimates that nearly 75% of children less than 24 months and 68% between 2 and 4 years of age were immunized this year. Overall vaccine efficacy in children 6 months through 8 years was reported at 47% last season from a CDC study using a study design that compares vaccination odds among influenza reverse transcription polymerase chain reaction (RT-PCR)–positive cases and RT-PCR–negative controls.
Influenza virus vaccines are unique in that they are updated, often annually, to include the most current hemagglutinin (HA) antigens based on estimates from circulating strains. In the United States, influenza vaccine manufacturers submit a supplement to their license and obtain Food and Drug Administration approval. These applications require only a limited study of safety in approximately 300 adults, essentially to verify attenuation (Influenza Other Respir Viruses. 2016. doi: 10.111/irv.1283). They do not require clinical proof of efficacy or even a threshold of immunogenicity.
At the June 2016 CDC’s Advisory Committee on Immunization Practices (ACIP) meeting, data were presented comparing the efficacy of this season’s live attenuated influenza vaccine (LAIV) with inactivated influenza vaccine (IIV) by age and specific influenza type and subtype. Data from the U.S. Flu Vaccine Effectiveness (VE) Network, a consortium of five CDC-funded sites that conducts annual studies of influenza vaccine effectiveness, failed to demonstrate efficacy for LAIV in children aged 2-8 years. There was an absence of efficacy against the primary circulating strain, A(H1N1). This contrasted with the 62% efficacy report for IIV against A(H1N1).
The concern for efficacy for LAIV was not limited to 2015-2016; efficacy was poor in 2013-2014 during a year in which A(H1N1) was the dominant virus as well, and in 2014-2015 when the prevalent strain was a drifted A(H3N2). The lack of efficacy in 2015-2016 and 2013-2014 when A(H1N1) was the prevalent strain was especially enigmatic given its high efficacy against A(H1N1) between 2009 and 2011. Studies of LAIV from Astra Zeneca and the U.S. Department of Defense were consistent with those from the U.S. Flu VE Network; however, there were discordant data from Finland where vaccine efficacy was present. As a result of these studies, the ACIP voted that LAIV should not be used during the 2016-2017 flu season. This vote reinforces the importance of monitoring the effectiveness of annual flu vaccination and other public health interventions.

ACIP recommendations for 2016-2017
• Children younger than 2 years of age and those with chronic health problems such as asthma, diabetes, and disorders of the brain or nervous system are at especially high risk of developing serious flu complications.
• Annual influenza immunization, with either the IIV or recombinant influenza vaccine (RIV), for everyone 6 months and older, remains the only effective strategy for decreasing influenza disease in the community.
• LAIV should not be used during the 2016-2017 flu season.
ACIP recommendations must be reviewed and approved by the CDC’s director before becoming CDC policy. The final annual recommendations on the prevention and control of influenza with vaccines will be published in CDC Morbidity and Mortality Weekly Report (MMWR) Recommendations and Reports in late summer or early fall.
Flu vaccines available for children for 2016-2017
• The trivalent flu vaccine protects against three flu viruses; two influenza A viruses and an influenza B virus. Standard dose trivalent shots are manufactured with viruses grown in eggs. These are approved for children aged 6 months and older. There are different brands of this type of vaccine; each specific formulation has different age-based approvals.

• The quadrivalent flu vaccine protects against four flu viruses; two influenza A viruses and two influenza B viruses. A standard dose quadrivalent formulation is available for children; one brand is approved for children 6 months and older while others are approved for those 3 years and older.
• A cell-based vaccine, developed through a manufacturing process different from the traditional egg-based manufacturing process, was approved as a quadrivalent formulation for use in children 4 years of age and older.
Unanswered questions for the 2016-2017 influenza season
• Children 6 months to 8 years who are getting vaccinated for the first time need two doses. How should we consider influenza-naive children who received two doses of LAIV last year? The reason for the LAIV’s loss of efficacy in the years 2014 through 2016 is unknown, although it has been hypothesized that reduced immunogenicity is one possible cause for the lack of protection. Rather than speculate, we need to wait for ACIP to gather more data and then publish recommendations as to whether to consider such children vaccine naive (and therefore requiring two doses this season) or previously immunized (and therefore in need of only a single dose).
• Will supply be adequate this year? LAIV represents about 8% of the 171-176 million doses that were projected to be available during the 2016-2017 season; however, it represents nearly one-third of doses given to children. Thus, the potential for shortages in pediatric offices is real, and pediatricians and vaccine manufacturers need to work together to make sure sufficient pediatric formulation is available. The CDC is working with manufacturers to ensure there is sufficient supply to meet the demand.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He has received honoraria from Sanofi Pasteur and Seqirus for participation in vaccine advisory boards in the prior 12 months. Email him at [email protected].
Did Somebody Say “Precepting”?
But First, a Word About Vaping …
As advocates for tobacco control, my colleagues and I took great interest in Randy D. Danielsen’s editorial, “Vaping: Are Its ‘Benefits’ a Lot of Hot Air?” (Clinician Reviews. 2016;26[6]:15-16). Our practice offers evidence-based cessation treatment for individuals with nicotine addiction through counseling, pharmacotherapy, and the use of nicotine replacement products.
At our center, we often interact with clients who have had multiple quit attempts. Many of our clients state that they have been unsuccessful using an e-cigarette as a smoking cessation strategy. More often than not, they report smoking a cigarette “here and there” along with “vaping,” until they eventually relapse to their usual smoking pattern. Some report that they smoke even more than before they tried to quit. We have concerns about how vaping may renormalize the behaviors associated with smoking. Our clients say that when they vape, it reminds them of the “social” aspects of smoking— “being part of a group” and participating in an activity that keeps their hands busy.
Recent literature suggests that curiosity is the primary reason adolescents engage in e-cigarette use. While the newly implemented FDA regulations on e-cigarettes may keep these products out of the hands of some adolescents by prohibiting sales to those younger than 18, there is much more to consider. Along with exposure to nicotine, these devices offer a variety of kid-friendly flavorings that make these products attractive to middle and high school youth. Flavorings will not be regulated at this point in time.
According to researchers, this is a major concern. Findings from studies report that when inhaled, certain flavors are more harmful than others. For example, very high—even toxic—levels of benzaldehyde are inhaled by the user when cherry-flavored e-liquid is heated at high temperatures. The chemical diacetyl, a respiratory irritant known to be associated with bronchiolitis obliterans (popcorn lung), is produced by the aerosol vapors from buttered popcorn and certain fruit-flavored e-cigarette liquids.
As public health advocates, we must provide research to the FDA about the health hazards of the flavoring added to e-cigarettes and continue to fight for this regulation. We must support evidence-based tobacco control interventions, such as hard-hitting media campaigns and tobacco excise taxes, and promote access to cessation treatment, smoke-free policies, and statewide funding. Elimination of tobacco products will reduce the public health burden of tobacco-related illness.
Andrea Spatarella, DNP, RN, FNP-BC, Christine Fardellone, DNP, RN, Raisa Abramova, FNP-BC, RN
Great Neck, NY
Continue for Precepting & E-Quality of Care >>
Precepting & E-Quality of Care
As a woman of the baby-boomer generation, I was raised in an era when feminism was a focus for many. There was a great deal being written and discussed to encourage women to attain equal pay for equal work. Because nursing was (and still is) a profession dominated by women, this was a frequent topic in the classroom. We were repeatedly told, “Don’t give away your knowledge for free” and “You deserve to be paid what you’re worth, don’t discount yourself.”
I find it very telling that the same female-dominated academic programs that encouraged me to seek proper payment are now taking advantage of my free labor. I am somewhat offended by this attitude and consider it a step backward. Each time NPs are guilted or browbeaten into teaching without proper compensation, the profession is devalued. To continue to participate is to enable a problematic, if not broken, system.
NP education is in need of major reform. The precepting issue is the weak link in becoming a qualified professional who is able to meet the demands and responsibilities that academics and politicos are pushing harder and harder for. Our physician and PA colleagues can rightly argue that their clinical education is superior to ours—and I cannot fault our colleagues for expressing concern about quality of care. If nursing really wants an equal place at the table, this weakness must be improved, or the naysayers will have plenty of evidence that they were correct in the years to come.
Rebecca Shively, MSN, RN, FNP-BC
San Marcos, TX
Continue for NP Schools & Their Rigid Rules >>
NP Schools & Their Rigid Rules
I have been a preceptor for at least a dozen NP students and have yet to be offered compensation. Preceptors take the place of a paid instructor, giving away free advice and experiences. I don’t mind doing this, but at times it can be a struggle. Some students, for example, have never done a pelvic exam. Letting an inexperienced NP student practice a pelvic exam on a patient who made an appointment to see an experienced provider is unjust and unfair to the patient—I won’t do it. These schools need to provide practice sessions on paid patients so their students can learn these skills.
I have my beef with the institutes of higher learning, not the students. It feels like a one-way street. You fill out the forms they require in order to precept, which takes up valuable work time. You equip their students with the skills they need to practice safely and correctly, and then try to fill out their evaluation sheets on things that students are not licensed to do.
Schools present their contracts and won’t adapt them to match what your employer wants. We are doing them a service, yet they dictate how we do it. My practice no longer takes students from certain schools, simply because we do not agree with their contracts. These poor students are thrown out without a life raft to find their preceptors! Aren’t their schools getting paid to do something?
Carol Glascock, WHNP-BC
Columbia, MO
Continue for Teaching & Precepting: Two Sides of the Same Coin >>
Teaching & Precepting: Two Sides of the Same Coin
I am a 64-year-old NP who has been precepting in Montana for the past four years. The students I precept are responsible for finding their own preceptors, just as I was 20+ years ago. However, preceptors are hard to find here, as the population is widely scattered; this places an emotional burden on students. They cannot be picky in choosing where they go. Thus, students may not be familiar with the preceptor’s practice or ability to teach.
The students I precept are in doctorate programs. My experience has shown that these students have very little understanding of practical application and instead have an overabundance of theoretical knowledge that does not always apply to seeing and treating patients. I believe that this, and the suggested “lack of preparedness,” is the fault of the program—not of the student.
Regardless of program faults, students are looking to learn from our experience. Teaching is part of being a preceptor; if you do not want to teach, being a preceptor is not for you. If you want to share your experience and knowledge with those following you (mindful that they may treat you in the future), precepting is an enjoyable experience. But—a good practitioner does not always make a good teacher.
Before becoming a preceptor, you must consider your time constraints, as well as your staff’s. You also must consider how your patients will react to seeing a student in your place.
Preceptors need to have a relationship with the student’s university apart from signing a paper saying they, the NP, will be the student’s preceptor. The university needs to be more proactive, as medical schools are, when finding preceptors willing to take students.
Compensation is another consideration that is rarely mentioned or discussed. Compensation would eliminate some of the negative reactions and might get more preceptors to sign on.
Harold W. Bruce, MSN, FNP-BC
Butte, MT
Continue for Collision of Causes for Precepting Hurdles >>
Collision of Causes for Precepting Hurdles
I am a family NP practicing in a large internal medicine practice owned by a university-based health care system. I precept NP students because I feel an obligation to my profession. However, the stress and additional workload that precepting places on me will probably lead me to stop sooner than I would like.
The inability to locate enough quality preceptors is a multifaceted issue. Too many students in too many programs, as mentioned in the editorial, is one contributing problem. I have been told by nursing professors that universities profit from their NP programs. They have an incentive to admit a large quantity of students and push them through. We could learn from our MD colleagues, who recognize the value of limiting student numbers.
The rise in NP students has led to a high number of poorly prepared students who enter their programs with no experience as RNs. Preceptors should not teach the basics, and professors should not expect preceptors to do so. Likewise, professors should not expect employers to fill in the gaps for new NPs they hire.
Many NP students have no “real-life” clinical experience to supplement their knowledge and skills. A strong foundation that combines nursing and medical knowledge, clinical experiences, basic assessment skills, and an understanding of human nature and human responses is crucial to being a successful NP. The latter is only developed through experience with patients. Students cannot develop these skills when their professors push them to immediately enroll in NP or DNP programs upon graduation from their BSN or basic non-NP MSN programs.
Our programs would do well to provide all the didactic classroom hours prior to the start of clinical rotations. Thus, the limited clinical hours can be used to hone clinical skills, instead of the current practice of students learning basics while also trying to incorporate knowledge with practice. It is a disservice to our NP students not to have completed classroom learning before starting their limited clinical rotations.
Preceptor overload and “burnout” occurs when very busy NPs are expected to fit precepting into their usual clinical sessions. There are strict mandates that dictate the number of residents a physician can precept. Those rules also allot physicians time reserved just for precepting. Why are NPs expected to precept during their already overworked day? Why haven’t our Boards of Nursing and nursing educators demanded this?
Precepting puts us behind during our clinical sessions. In some cases, it can impact our relative value units or patient numbers and salaries. We are teaching on our own time, with no incentives or monetary gain, yet we are expected to devote time and resources to our students.
Most of us do not receive merit-based financial rewards for the extra work. When did it become wrong to expect to be paid for our work? No other profession has this sense of guilt or self-recrimination when asking to be paid for services.
Preceptor training is another issue. Unlike physicians, we are not acculturated in the “see one, do one, teach one” manner. In nursing, we are trained that we must be taught, observed, and tested before being allowed to do anything new. We have a need to be taught everything, including how to precept. That being said, precepting is both an art and a science that involves grasping the basic tenets of learning and mentoring. These are skills that should be taught through observation or in classes so that we can pass on our knowledge. If our NP programs were longer and more step-by-step—in terms of first acquiring knowledge, then incorporating clinical skills with practice—we might learn the skills of teaching and mentoring without feeling we need additional “education” in precepting.
I have been in nursing for more than 40 years and love my profession. There are challenges ahead of us that we can only meet if we are brave enough to look clearly at the way we teach younger nurses, create improved ways of teaching those who will replace us, and actually recognize the value and efforts of those we ask to precept the next generation.
Theresa Dippolito, MSN, NP-C, CRNP, APN, CCM
Levittown, PA
Continue for Raising the Bar >>
Raising the Bar
I no longer want to be involved in precepting. I, too, find the students to be poorly prepared, and I was flabbergasted when I read a recent post on Facebook—a student offered to pay her preceptor to sign off on her clinicals!
I graduated from an FNP program in 1998 and also felt unprepared at first. My class thought like nurses, in that we expected things to be presented to us. Very few of us were aware that we should prepare ourselves, and the program I went through did nothing to inform us of this. It was a rude awakening.
NP programs should have improved since then, but they certainly have not. I have precepted multiple students who did not know how to do a proper physical exam, despite having passed their related courses. I have also precepted students who thought they knew everything and felt I should let them practice solo. Sadly, the majority were simultaneously in both groups.
There is still the stigma that we should remain within a nursing philosophy when we practice, when the reality is that we practice side by side with the doctors. We need to think critically, as they do, and have our programs teach such thinking via competent instructors.
My suggestions include a competency exam for NP instructors so that we can assure a higher, more standardized level of teaching. There should also be a prep course for potential NP students on how to think, including an explanation that it will be their responsibility to go after knowledge as well. Finally, we need to stray from the nursing philosophy-type teaching in NP programs and instead focus on stronger clinical knowledge and competence.
Nikki Knight, MSN, FNP-C
San Francisco, CA
But First, a Word About Vaping …
As advocates for tobacco control, my colleagues and I took great interest in Randy D. Danielsen’s editorial, “Vaping: Are Its ‘Benefits’ a Lot of Hot Air?” (Clinician Reviews. 2016;26[6]:15-16). Our practice offers evidence-based cessation treatment for individuals with nicotine addiction through counseling, pharmacotherapy, and the use of nicotine replacement products.
At our center, we often interact with clients who have had multiple quit attempts. Many of our clients state that they have been unsuccessful using an e-cigarette as a smoking cessation strategy. More often than not, they report smoking a cigarette “here and there” along with “vaping,” until they eventually relapse to their usual smoking pattern. Some report that they smoke even more than before they tried to quit. We have concerns about how vaping may renormalize the behaviors associated with smoking. Our clients say that when they vape, it reminds them of the “social” aspects of smoking— “being part of a group” and participating in an activity that keeps their hands busy.
Recent literature suggests that curiosity is the primary reason adolescents engage in e-cigarette use. While the newly implemented FDA regulations on e-cigarettes may keep these products out of the hands of some adolescents by prohibiting sales to those younger than 18, there is much more to consider. Along with exposure to nicotine, these devices offer a variety of kid-friendly flavorings that make these products attractive to middle and high school youth. Flavorings will not be regulated at this point in time.
According to researchers, this is a major concern. Findings from studies report that when inhaled, certain flavors are more harmful than others. For example, very high—even toxic—levels of benzaldehyde are inhaled by the user when cherry-flavored e-liquid is heated at high temperatures. The chemical diacetyl, a respiratory irritant known to be associated with bronchiolitis obliterans (popcorn lung), is produced by the aerosol vapors from buttered popcorn and certain fruit-flavored e-cigarette liquids.
As public health advocates, we must provide research to the FDA about the health hazards of the flavoring added to e-cigarettes and continue to fight for this regulation. We must support evidence-based tobacco control interventions, such as hard-hitting media campaigns and tobacco excise taxes, and promote access to cessation treatment, smoke-free policies, and statewide funding. Elimination of tobacco products will reduce the public health burden of tobacco-related illness.
Andrea Spatarella, DNP, RN, FNP-BC, Christine Fardellone, DNP, RN, Raisa Abramova, FNP-BC, RN
Great Neck, NY
Continue for Precepting & E-Quality of Care >>
Precepting & E-Quality of Care
As a woman of the baby-boomer generation, I was raised in an era when feminism was a focus for many. There was a great deal being written and discussed to encourage women to attain equal pay for equal work. Because nursing was (and still is) a profession dominated by women, this was a frequent topic in the classroom. We were repeatedly told, “Don’t give away your knowledge for free” and “You deserve to be paid what you’re worth, don’t discount yourself.”
I find it very telling that the same female-dominated academic programs that encouraged me to seek proper payment are now taking advantage of my free labor. I am somewhat offended by this attitude and consider it a step backward. Each time NPs are guilted or browbeaten into teaching without proper compensation, the profession is devalued. To continue to participate is to enable a problematic, if not broken, system.
NP education is in need of major reform. The precepting issue is the weak link in becoming a qualified professional who is able to meet the demands and responsibilities that academics and politicos are pushing harder and harder for. Our physician and PA colleagues can rightly argue that their clinical education is superior to ours—and I cannot fault our colleagues for expressing concern about quality of care. If nursing really wants an equal place at the table, this weakness must be improved, or the naysayers will have plenty of evidence that they were correct in the years to come.
Rebecca Shively, MSN, RN, FNP-BC
San Marcos, TX
Continue for NP Schools & Their Rigid Rules >>
NP Schools & Their Rigid Rules
I have been a preceptor for at least a dozen NP students and have yet to be offered compensation. Preceptors take the place of a paid instructor, giving away free advice and experiences. I don’t mind doing this, but at times it can be a struggle. Some students, for example, have never done a pelvic exam. Letting an inexperienced NP student practice a pelvic exam on a patient who made an appointment to see an experienced provider is unjust and unfair to the patient—I won’t do it. These schools need to provide practice sessions on paid patients so their students can learn these skills.
I have my beef with the institutes of higher learning, not the students. It feels like a one-way street. You fill out the forms they require in order to precept, which takes up valuable work time. You equip their students with the skills they need to practice safely and correctly, and then try to fill out their evaluation sheets on things that students are not licensed to do.
Schools present their contracts and won’t adapt them to match what your employer wants. We are doing them a service, yet they dictate how we do it. My practice no longer takes students from certain schools, simply because we do not agree with their contracts. These poor students are thrown out without a life raft to find their preceptors! Aren’t their schools getting paid to do something?
Carol Glascock, WHNP-BC
Columbia, MO
Continue for Teaching & Precepting: Two Sides of the Same Coin >>
Teaching & Precepting: Two Sides of the Same Coin
I am a 64-year-old NP who has been precepting in Montana for the past four years. The students I precept are responsible for finding their own preceptors, just as I was 20+ years ago. However, preceptors are hard to find here, as the population is widely scattered; this places an emotional burden on students. They cannot be picky in choosing where they go. Thus, students may not be familiar with the preceptor’s practice or ability to teach.
The students I precept are in doctorate programs. My experience has shown that these students have very little understanding of practical application and instead have an overabundance of theoretical knowledge that does not always apply to seeing and treating patients. I believe that this, and the suggested “lack of preparedness,” is the fault of the program—not of the student.
Regardless of program faults, students are looking to learn from our experience. Teaching is part of being a preceptor; if you do not want to teach, being a preceptor is not for you. If you want to share your experience and knowledge with those following you (mindful that they may treat you in the future), precepting is an enjoyable experience. But—a good practitioner does not always make a good teacher.
Before becoming a preceptor, you must consider your time constraints, as well as your staff’s. You also must consider how your patients will react to seeing a student in your place.
Preceptors need to have a relationship with the student’s university apart from signing a paper saying they, the NP, will be the student’s preceptor. The university needs to be more proactive, as medical schools are, when finding preceptors willing to take students.
Compensation is another consideration that is rarely mentioned or discussed. Compensation would eliminate some of the negative reactions and might get more preceptors to sign on.
Harold W. Bruce, MSN, FNP-BC
Butte, MT
Continue for Collision of Causes for Precepting Hurdles >>
Collision of Causes for Precepting Hurdles
I am a family NP practicing in a large internal medicine practice owned by a university-based health care system. I precept NP students because I feel an obligation to my profession. However, the stress and additional workload that precepting places on me will probably lead me to stop sooner than I would like.
The inability to locate enough quality preceptors is a multifaceted issue. Too many students in too many programs, as mentioned in the editorial, is one contributing problem. I have been told by nursing professors that universities profit from their NP programs. They have an incentive to admit a large quantity of students and push them through. We could learn from our MD colleagues, who recognize the value of limiting student numbers.
The rise in NP students has led to a high number of poorly prepared students who enter their programs with no experience as RNs. Preceptors should not teach the basics, and professors should not expect preceptors to do so. Likewise, professors should not expect employers to fill in the gaps for new NPs they hire.
Many NP students have no “real-life” clinical experience to supplement their knowledge and skills. A strong foundation that combines nursing and medical knowledge, clinical experiences, basic assessment skills, and an understanding of human nature and human responses is crucial to being a successful NP. The latter is only developed through experience with patients. Students cannot develop these skills when their professors push them to immediately enroll in NP or DNP programs upon graduation from their BSN or basic non-NP MSN programs.
Our programs would do well to provide all the didactic classroom hours prior to the start of clinical rotations. Thus, the limited clinical hours can be used to hone clinical skills, instead of the current practice of students learning basics while also trying to incorporate knowledge with practice. It is a disservice to our NP students not to have completed classroom learning before starting their limited clinical rotations.
Preceptor overload and “burnout” occurs when very busy NPs are expected to fit precepting into their usual clinical sessions. There are strict mandates that dictate the number of residents a physician can precept. Those rules also allot physicians time reserved just for precepting. Why are NPs expected to precept during their already overworked day? Why haven’t our Boards of Nursing and nursing educators demanded this?
Precepting puts us behind during our clinical sessions. In some cases, it can impact our relative value units or patient numbers and salaries. We are teaching on our own time, with no incentives or monetary gain, yet we are expected to devote time and resources to our students.
Most of us do not receive merit-based financial rewards for the extra work. When did it become wrong to expect to be paid for our work? No other profession has this sense of guilt or self-recrimination when asking to be paid for services.
Preceptor training is another issue. Unlike physicians, we are not acculturated in the “see one, do one, teach one” manner. In nursing, we are trained that we must be taught, observed, and tested before being allowed to do anything new. We have a need to be taught everything, including how to precept. That being said, precepting is both an art and a science that involves grasping the basic tenets of learning and mentoring. These are skills that should be taught through observation or in classes so that we can pass on our knowledge. If our NP programs were longer and more step-by-step—in terms of first acquiring knowledge, then incorporating clinical skills with practice—we might learn the skills of teaching and mentoring without feeling we need additional “education” in precepting.
I have been in nursing for more than 40 years and love my profession. There are challenges ahead of us that we can only meet if we are brave enough to look clearly at the way we teach younger nurses, create improved ways of teaching those who will replace us, and actually recognize the value and efforts of those we ask to precept the next generation.
Theresa Dippolito, MSN, NP-C, CRNP, APN, CCM
Levittown, PA
Continue for Raising the Bar >>
Raising the Bar
I no longer want to be involved in precepting. I, too, find the students to be poorly prepared, and I was flabbergasted when I read a recent post on Facebook—a student offered to pay her preceptor to sign off on her clinicals!
I graduated from an FNP program in 1998 and also felt unprepared at first. My class thought like nurses, in that we expected things to be presented to us. Very few of us were aware that we should prepare ourselves, and the program I went through did nothing to inform us of this. It was a rude awakening.
NP programs should have improved since then, but they certainly have not. I have precepted multiple students who did not know how to do a proper physical exam, despite having passed their related courses. I have also precepted students who thought they knew everything and felt I should let them practice solo. Sadly, the majority were simultaneously in both groups.
There is still the stigma that we should remain within a nursing philosophy when we practice, when the reality is that we practice side by side with the doctors. We need to think critically, as they do, and have our programs teach such thinking via competent instructors.
My suggestions include a competency exam for NP instructors so that we can assure a higher, more standardized level of teaching. There should also be a prep course for potential NP students on how to think, including an explanation that it will be their responsibility to go after knowledge as well. Finally, we need to stray from the nursing philosophy-type teaching in NP programs and instead focus on stronger clinical knowledge and competence.
Nikki Knight, MSN, FNP-C
San Francisco, CA
But First, a Word About Vaping …
As advocates for tobacco control, my colleagues and I took great interest in Randy D. Danielsen’s editorial, “Vaping: Are Its ‘Benefits’ a Lot of Hot Air?” (Clinician Reviews. 2016;26[6]:15-16). Our practice offers evidence-based cessation treatment for individuals with nicotine addiction through counseling, pharmacotherapy, and the use of nicotine replacement products.
At our center, we often interact with clients who have had multiple quit attempts. Many of our clients state that they have been unsuccessful using an e-cigarette as a smoking cessation strategy. More often than not, they report smoking a cigarette “here and there” along with “vaping,” until they eventually relapse to their usual smoking pattern. Some report that they smoke even more than before they tried to quit. We have concerns about how vaping may renormalize the behaviors associated with smoking. Our clients say that when they vape, it reminds them of the “social” aspects of smoking— “being part of a group” and participating in an activity that keeps their hands busy.
Recent literature suggests that curiosity is the primary reason adolescents engage in e-cigarette use. While the newly implemented FDA regulations on e-cigarettes may keep these products out of the hands of some adolescents by prohibiting sales to those younger than 18, there is much more to consider. Along with exposure to nicotine, these devices offer a variety of kid-friendly flavorings that make these products attractive to middle and high school youth. Flavorings will not be regulated at this point in time.
According to researchers, this is a major concern. Findings from studies report that when inhaled, certain flavors are more harmful than others. For example, very high—even toxic—levels of benzaldehyde are inhaled by the user when cherry-flavored e-liquid is heated at high temperatures. The chemical diacetyl, a respiratory irritant known to be associated with bronchiolitis obliterans (popcorn lung), is produced by the aerosol vapors from buttered popcorn and certain fruit-flavored e-cigarette liquids.
As public health advocates, we must provide research to the FDA about the health hazards of the flavoring added to e-cigarettes and continue to fight for this regulation. We must support evidence-based tobacco control interventions, such as hard-hitting media campaigns and tobacco excise taxes, and promote access to cessation treatment, smoke-free policies, and statewide funding. Elimination of tobacco products will reduce the public health burden of tobacco-related illness.
Andrea Spatarella, DNP, RN, FNP-BC, Christine Fardellone, DNP, RN, Raisa Abramova, FNP-BC, RN
Great Neck, NY
Continue for Precepting & E-Quality of Care >>
Precepting & E-Quality of Care
As a woman of the baby-boomer generation, I was raised in an era when feminism was a focus for many. There was a great deal being written and discussed to encourage women to attain equal pay for equal work. Because nursing was (and still is) a profession dominated by women, this was a frequent topic in the classroom. We were repeatedly told, “Don’t give away your knowledge for free” and “You deserve to be paid what you’re worth, don’t discount yourself.”
I find it very telling that the same female-dominated academic programs that encouraged me to seek proper payment are now taking advantage of my free labor. I am somewhat offended by this attitude and consider it a step backward. Each time NPs are guilted or browbeaten into teaching without proper compensation, the profession is devalued. To continue to participate is to enable a problematic, if not broken, system.
NP education is in need of major reform. The precepting issue is the weak link in becoming a qualified professional who is able to meet the demands and responsibilities that academics and politicos are pushing harder and harder for. Our physician and PA colleagues can rightly argue that their clinical education is superior to ours—and I cannot fault our colleagues for expressing concern about quality of care. If nursing really wants an equal place at the table, this weakness must be improved, or the naysayers will have plenty of evidence that they were correct in the years to come.
Rebecca Shively, MSN, RN, FNP-BC
San Marcos, TX
Continue for NP Schools & Their Rigid Rules >>
NP Schools & Their Rigid Rules
I have been a preceptor for at least a dozen NP students and have yet to be offered compensation. Preceptors take the place of a paid instructor, giving away free advice and experiences. I don’t mind doing this, but at times it can be a struggle. Some students, for example, have never done a pelvic exam. Letting an inexperienced NP student practice a pelvic exam on a patient who made an appointment to see an experienced provider is unjust and unfair to the patient—I won’t do it. These schools need to provide practice sessions on paid patients so their students can learn these skills.
I have my beef with the institutes of higher learning, not the students. It feels like a one-way street. You fill out the forms they require in order to precept, which takes up valuable work time. You equip their students with the skills they need to practice safely and correctly, and then try to fill out their evaluation sheets on things that students are not licensed to do.
Schools present their contracts and won’t adapt them to match what your employer wants. We are doing them a service, yet they dictate how we do it. My practice no longer takes students from certain schools, simply because we do not agree with their contracts. These poor students are thrown out without a life raft to find their preceptors! Aren’t their schools getting paid to do something?
Carol Glascock, WHNP-BC
Columbia, MO
Continue for Teaching & Precepting: Two Sides of the Same Coin >>
Teaching & Precepting: Two Sides of the Same Coin
I am a 64-year-old NP who has been precepting in Montana for the past four years. The students I precept are responsible for finding their own preceptors, just as I was 20+ years ago. However, preceptors are hard to find here, as the population is widely scattered; this places an emotional burden on students. They cannot be picky in choosing where they go. Thus, students may not be familiar with the preceptor’s practice or ability to teach.
The students I precept are in doctorate programs. My experience has shown that these students have very little understanding of practical application and instead have an overabundance of theoretical knowledge that does not always apply to seeing and treating patients. I believe that this, and the suggested “lack of preparedness,” is the fault of the program—not of the student.
Regardless of program faults, students are looking to learn from our experience. Teaching is part of being a preceptor; if you do not want to teach, being a preceptor is not for you. If you want to share your experience and knowledge with those following you (mindful that they may treat you in the future), precepting is an enjoyable experience. But—a good practitioner does not always make a good teacher.
Before becoming a preceptor, you must consider your time constraints, as well as your staff’s. You also must consider how your patients will react to seeing a student in your place.
Preceptors need to have a relationship with the student’s university apart from signing a paper saying they, the NP, will be the student’s preceptor. The university needs to be more proactive, as medical schools are, when finding preceptors willing to take students.
Compensation is another consideration that is rarely mentioned or discussed. Compensation would eliminate some of the negative reactions and might get more preceptors to sign on.
Harold W. Bruce, MSN, FNP-BC
Butte, MT
Continue for Collision of Causes for Precepting Hurdles >>
Collision of Causes for Precepting Hurdles
I am a family NP practicing in a large internal medicine practice owned by a university-based health care system. I precept NP students because I feel an obligation to my profession. However, the stress and additional workload that precepting places on me will probably lead me to stop sooner than I would like.
The inability to locate enough quality preceptors is a multifaceted issue. Too many students in too many programs, as mentioned in the editorial, is one contributing problem. I have been told by nursing professors that universities profit from their NP programs. They have an incentive to admit a large quantity of students and push them through. We could learn from our MD colleagues, who recognize the value of limiting student numbers.
The rise in NP students has led to a high number of poorly prepared students who enter their programs with no experience as RNs. Preceptors should not teach the basics, and professors should not expect preceptors to do so. Likewise, professors should not expect employers to fill in the gaps for new NPs they hire.
Many NP students have no “real-life” clinical experience to supplement their knowledge and skills. A strong foundation that combines nursing and medical knowledge, clinical experiences, basic assessment skills, and an understanding of human nature and human responses is crucial to being a successful NP. The latter is only developed through experience with patients. Students cannot develop these skills when their professors push them to immediately enroll in NP or DNP programs upon graduation from their BSN or basic non-NP MSN programs.
Our programs would do well to provide all the didactic classroom hours prior to the start of clinical rotations. Thus, the limited clinical hours can be used to hone clinical skills, instead of the current practice of students learning basics while also trying to incorporate knowledge with practice. It is a disservice to our NP students not to have completed classroom learning before starting their limited clinical rotations.
Preceptor overload and “burnout” occurs when very busy NPs are expected to fit precepting into their usual clinical sessions. There are strict mandates that dictate the number of residents a physician can precept. Those rules also allot physicians time reserved just for precepting. Why are NPs expected to precept during their already overworked day? Why haven’t our Boards of Nursing and nursing educators demanded this?
Precepting puts us behind during our clinical sessions. In some cases, it can impact our relative value units or patient numbers and salaries. We are teaching on our own time, with no incentives or monetary gain, yet we are expected to devote time and resources to our students.
Most of us do not receive merit-based financial rewards for the extra work. When did it become wrong to expect to be paid for our work? No other profession has this sense of guilt or self-recrimination when asking to be paid for services.
Preceptor training is another issue. Unlike physicians, we are not acculturated in the “see one, do one, teach one” manner. In nursing, we are trained that we must be taught, observed, and tested before being allowed to do anything new. We have a need to be taught everything, including how to precept. That being said, precepting is both an art and a science that involves grasping the basic tenets of learning and mentoring. These are skills that should be taught through observation or in classes so that we can pass on our knowledge. If our NP programs were longer and more step-by-step—in terms of first acquiring knowledge, then incorporating clinical skills with practice—we might learn the skills of teaching and mentoring without feeling we need additional “education” in precepting.
I have been in nursing for more than 40 years and love my profession. There are challenges ahead of us that we can only meet if we are brave enough to look clearly at the way we teach younger nurses, create improved ways of teaching those who will replace us, and actually recognize the value and efforts of those we ask to precept the next generation.
Theresa Dippolito, MSN, NP-C, CRNP, APN, CCM
Levittown, PA
Continue for Raising the Bar >>
Raising the Bar
I no longer want to be involved in precepting. I, too, find the students to be poorly prepared, and I was flabbergasted when I read a recent post on Facebook—a student offered to pay her preceptor to sign off on her clinicals!
I graduated from an FNP program in 1998 and also felt unprepared at first. My class thought like nurses, in that we expected things to be presented to us. Very few of us were aware that we should prepare ourselves, and the program I went through did nothing to inform us of this. It was a rude awakening.
NP programs should have improved since then, but they certainly have not. I have precepted multiple students who did not know how to do a proper physical exam, despite having passed their related courses. I have also precepted students who thought they knew everything and felt I should let them practice solo. Sadly, the majority were simultaneously in both groups.
There is still the stigma that we should remain within a nursing philosophy when we practice, when the reality is that we practice side by side with the doctors. We need to think critically, as they do, and have our programs teach such thinking via competent instructors.
My suggestions include a competency exam for NP instructors so that we can assure a higher, more standardized level of teaching. There should also be a prep course for potential NP students on how to think, including an explanation that it will be their responsibility to go after knowledge as well. Finally, we need to stray from the nursing philosophy-type teaching in NP programs and instead focus on stronger clinical knowledge and competence.
Nikki Knight, MSN, FNP-C
San Francisco, CA