User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
It’s time for moonshot thinking in psychiatry
“I believe that this nation should commit itself to achieving the goal, before the decade is out, of landing a man on the Moon and returning him safely to Earth.”
President John F. Kennedy, May 25, 1961
Despite significant progress, there remain many unmet needs in psychiatry. These include a granular understanding of the neurobiology of various psychopathologies, an objective and valid diagnostic schema, and disease-modifying treatments for chronic and disabling psychiatric disorders. Several moonshots are needed to address those festering needs.
A “moonshot” is an extremely ambitious, dramatic, imaginative, and inspiring goal. Landing on the Moon was generally believed to be impossible when President Kennedy boldly set that as a goal for the United States in 1961. Yet, 8 short years later, on July 20, 1969, Neil Armstrong stepped off the lunar module ladder onto the Moon’s surface, a feat that captured the imagination of the nation and the world. I distinctly remember watching it on television with amazement as a young boy. It was a surreal experience. That’s what achieving a moonshot feels like.
Successful organizations should always have 1 or more moonshots (American Psychiatric Association and National Institute of Mental Health [NIMH], are you listening?). Setting lofty goals that require monumental determination and effort to accomplish will have a transformative, long-lasting impact. The construction of the Panama Canal to connect 2 oceans and the Manhattan Project to develop the first nuclear bomb, which ended World War II, are examples of moonshots that continue to reverberate. A more recent moonshot is the driverless car, which in the past was a laughable idea but is now a reality that will change society and the world in many ways. Innovative billionaire moguls now speak loudly about colonizing Mars, which sounds improbable and highly risky, but it’s a moonshot that may be achieved within a few years. Establishing world peace is a moonshot that requires collective Kennedy-esque vision and motivation among world leaders, which currently is sadly lacking.
So, for contemporary psychiatry, what is the equivalent of landing on the Moon? Here is the list that pops in my brain’s mind (let us know which of these would be your top 3 moonshots by taking our survey at https://bit.ly/3qkKqTa):
- A cure for schizophrenia (across positive, negative, and cognitive symptom domains)
- A cure for mood disorders, unipolar and bipolar (including suicide)
- A cure for anxiety disorders
- A cure for obsessive-compulsive disorder
- A cure for posttraumatic stress disorder
- A cure for alcoholism/addiction
- A cure for autism
- A cure for Alzheimer’s disease and other dementias
- A cure for personality disorders, especially antisocial and borderline
- A cure for the visceral hatred across political parties that permeates our society (obviously not a psychiatric category, but perhaps it should be added to DSM because it is so destructive).
Those moonshots may be regarded as absurd, and totally unachievable, but so was landing on the Moon, until it was accomplished. Psychiatry must stop thinking small and being content with tiny advances (which is like changing the chairs to more comfortable sofas on the deck of the Titanic and calling it “progress…”). Psychiatry needs to be unified under the flag of “moonshot thinking” by several visionary and transformative leaders to start believing in a miraculously better future for our patients. But to pave the way for moonshots in psychiatry, the leading organizations must collaborate closely to open the door for unprecedented scientific and medical breakthroughs of a moonshot by:
- Lobbying effectively to secure massive funding for research from federal, state, corporate, and foundation sources (perhaps convincing the Gates Foundation that schizophrenia is as devastating worldwide as malaria may bring a few badly needed billions into psychiatric brain research).
- Reminding members of Congress that in the United States, costs associated with psychiatric brain disorders total an estimated $700 billion annually,1 and that this must be addressed by boosting the meager NIMH budget by at least an order of magnitude. The NIMH should disproportionately invest its resources on severe brain disorders such as schizophrenia because breakthrough advances in its neurobiology will provide unprecedented insights to the pathophysiology of other severe psychiatric brain disorders.
- Partnering intimately with the pharmaceutical industry in a powerful public-private coalition to exploit the extensive research infrastructure of this industry.
- Creating the necessary army of researchers (physician-scientists) by providing huge incentives to medical students and psychiatric residents to pursue careers in neuroscience research. Incentives can include paying for an individual’s entire medical education and research training, and providing generous salaries that match or exceed the income of a very successful clinical practice.
- Convincing all psychiatric clinicians to support research by referring patients to research projects. Clinical psychiatrists are badly needed to care for the population, but they must be reminded that every treatment they are using today was a research project in the past, and that the research of today will evolve into the treatments (or cures) of tomorrow.
Pursuing lofty moonshots via innovative research is very likely to enhance serendipity and lead to unexpected discoveries along the way. As Louis Pasteur said, “chance only favors the prepared mind.”2 Moonshot thinking in psychiatry today is more feasible than ever before because of the many advances in research methods (neuroimaging, pluripotent cells, optogenetics, CRISPR, etc) and complex data management technologies (big data, machine learning, artificial intelligence), each of which qualifies as a preparatory moonshot in its own right.
Given the tragic consequences of psychiatric brain disorders, it is imperative that we “think big.” Humanity expects us to do that. We must envision the future of psychiatry as dramatically different from the present. Moonshot thinking is the indispensable vehicle to take us there.
1. Discovery Mood and Anxiety Program. The rising cost of mental health and substance abuse in the United States. Accessed January 13, 2022. https://discoverymood.com/blog/cost-of-mental-health-increase/
2. Wikiquote. Louis Pasteur. Accessed January 10, 2022. https://en.wikiquote.org/wiki/Louis_Pasteur
“I believe that this nation should commit itself to achieving the goal, before the decade is out, of landing a man on the Moon and returning him safely to Earth.”
President John F. Kennedy, May 25, 1961
Despite significant progress, there remain many unmet needs in psychiatry. These include a granular understanding of the neurobiology of various psychopathologies, an objective and valid diagnostic schema, and disease-modifying treatments for chronic and disabling psychiatric disorders. Several moonshots are needed to address those festering needs.
A “moonshot” is an extremely ambitious, dramatic, imaginative, and inspiring goal. Landing on the Moon was generally believed to be impossible when President Kennedy boldly set that as a goal for the United States in 1961. Yet, 8 short years later, on July 20, 1969, Neil Armstrong stepped off the lunar module ladder onto the Moon’s surface, a feat that captured the imagination of the nation and the world. I distinctly remember watching it on television with amazement as a young boy. It was a surreal experience. That’s what achieving a moonshot feels like.
Successful organizations should always have 1 or more moonshots (American Psychiatric Association and National Institute of Mental Health [NIMH], are you listening?). Setting lofty goals that require monumental determination and effort to accomplish will have a transformative, long-lasting impact. The construction of the Panama Canal to connect 2 oceans and the Manhattan Project to develop the first nuclear bomb, which ended World War II, are examples of moonshots that continue to reverberate. A more recent moonshot is the driverless car, which in the past was a laughable idea but is now a reality that will change society and the world in many ways. Innovative billionaire moguls now speak loudly about colonizing Mars, which sounds improbable and highly risky, but it’s a moonshot that may be achieved within a few years. Establishing world peace is a moonshot that requires collective Kennedy-esque vision and motivation among world leaders, which currently is sadly lacking.
So, for contemporary psychiatry, what is the equivalent of landing on the Moon? Here is the list that pops in my brain’s mind (let us know which of these would be your top 3 moonshots by taking our survey at https://bit.ly/3qkKqTa):
- A cure for schizophrenia (across positive, negative, and cognitive symptom domains)
- A cure for mood disorders, unipolar and bipolar (including suicide)
- A cure for anxiety disorders
- A cure for obsessive-compulsive disorder
- A cure for posttraumatic stress disorder
- A cure for alcoholism/addiction
- A cure for autism
- A cure for Alzheimer’s disease and other dementias
- A cure for personality disorders, especially antisocial and borderline
- A cure for the visceral hatred across political parties that permeates our society (obviously not a psychiatric category, but perhaps it should be added to DSM because it is so destructive).
Those moonshots may be regarded as absurd, and totally unachievable, but so was landing on the Moon, until it was accomplished. Psychiatry must stop thinking small and being content with tiny advances (which is like changing the chairs to more comfortable sofas on the deck of the Titanic and calling it “progress…”). Psychiatry needs to be unified under the flag of “moonshot thinking” by several visionary and transformative leaders to start believing in a miraculously better future for our patients. But to pave the way for moonshots in psychiatry, the leading organizations must collaborate closely to open the door for unprecedented scientific and medical breakthroughs of a moonshot by:
- Lobbying effectively to secure massive funding for research from federal, state, corporate, and foundation sources (perhaps convincing the Gates Foundation that schizophrenia is as devastating worldwide as malaria may bring a few badly needed billions into psychiatric brain research).
- Reminding members of Congress that in the United States, costs associated with psychiatric brain disorders total an estimated $700 billion annually,1 and that this must be addressed by boosting the meager NIMH budget by at least an order of magnitude. The NIMH should disproportionately invest its resources on severe brain disorders such as schizophrenia because breakthrough advances in its neurobiology will provide unprecedented insights to the pathophysiology of other severe psychiatric brain disorders.
- Partnering intimately with the pharmaceutical industry in a powerful public-private coalition to exploit the extensive research infrastructure of this industry.
- Creating the necessary army of researchers (physician-scientists) by providing huge incentives to medical students and psychiatric residents to pursue careers in neuroscience research. Incentives can include paying for an individual’s entire medical education and research training, and providing generous salaries that match or exceed the income of a very successful clinical practice.
- Convincing all psychiatric clinicians to support research by referring patients to research projects. Clinical psychiatrists are badly needed to care for the population, but they must be reminded that every treatment they are using today was a research project in the past, and that the research of today will evolve into the treatments (or cures) of tomorrow.
Pursuing lofty moonshots via innovative research is very likely to enhance serendipity and lead to unexpected discoveries along the way. As Louis Pasteur said, “chance only favors the prepared mind.”2 Moonshot thinking in psychiatry today is more feasible than ever before because of the many advances in research methods (neuroimaging, pluripotent cells, optogenetics, CRISPR, etc) and complex data management technologies (big data, machine learning, artificial intelligence), each of which qualifies as a preparatory moonshot in its own right.
Given the tragic consequences of psychiatric brain disorders, it is imperative that we “think big.” Humanity expects us to do that. We must envision the future of psychiatry as dramatically different from the present. Moonshot thinking is the indispensable vehicle to take us there.
“I believe that this nation should commit itself to achieving the goal, before the decade is out, of landing a man on the Moon and returning him safely to Earth.”
President John F. Kennedy, May 25, 1961
Despite significant progress, there remain many unmet needs in psychiatry. These include a granular understanding of the neurobiology of various psychopathologies, an objective and valid diagnostic schema, and disease-modifying treatments for chronic and disabling psychiatric disorders. Several moonshots are needed to address those festering needs.
A “moonshot” is an extremely ambitious, dramatic, imaginative, and inspiring goal. Landing on the Moon was generally believed to be impossible when President Kennedy boldly set that as a goal for the United States in 1961. Yet, 8 short years later, on July 20, 1969, Neil Armstrong stepped off the lunar module ladder onto the Moon’s surface, a feat that captured the imagination of the nation and the world. I distinctly remember watching it on television with amazement as a young boy. It was a surreal experience. That’s what achieving a moonshot feels like.
Successful organizations should always have 1 or more moonshots (American Psychiatric Association and National Institute of Mental Health [NIMH], are you listening?). Setting lofty goals that require monumental determination and effort to accomplish will have a transformative, long-lasting impact. The construction of the Panama Canal to connect 2 oceans and the Manhattan Project to develop the first nuclear bomb, which ended World War II, are examples of moonshots that continue to reverberate. A more recent moonshot is the driverless car, which in the past was a laughable idea but is now a reality that will change society and the world in many ways. Innovative billionaire moguls now speak loudly about colonizing Mars, which sounds improbable and highly risky, but it’s a moonshot that may be achieved within a few years. Establishing world peace is a moonshot that requires collective Kennedy-esque vision and motivation among world leaders, which currently is sadly lacking.
So, for contemporary psychiatry, what is the equivalent of landing on the Moon? Here is the list that pops in my brain’s mind (let us know which of these would be your top 3 moonshots by taking our survey at https://bit.ly/3qkKqTa):
- A cure for schizophrenia (across positive, negative, and cognitive symptom domains)
- A cure for mood disorders, unipolar and bipolar (including suicide)
- A cure for anxiety disorders
- A cure for obsessive-compulsive disorder
- A cure for posttraumatic stress disorder
- A cure for alcoholism/addiction
- A cure for autism
- A cure for Alzheimer’s disease and other dementias
- A cure for personality disorders, especially antisocial and borderline
- A cure for the visceral hatred across political parties that permeates our society (obviously not a psychiatric category, but perhaps it should be added to DSM because it is so destructive).
Those moonshots may be regarded as absurd, and totally unachievable, but so was landing on the Moon, until it was accomplished. Psychiatry must stop thinking small and being content with tiny advances (which is like changing the chairs to more comfortable sofas on the deck of the Titanic and calling it “progress…”). Psychiatry needs to be unified under the flag of “moonshot thinking” by several visionary and transformative leaders to start believing in a miraculously better future for our patients. But to pave the way for moonshots in psychiatry, the leading organizations must collaborate closely to open the door for unprecedented scientific and medical breakthroughs of a moonshot by:
- Lobbying effectively to secure massive funding for research from federal, state, corporate, and foundation sources (perhaps convincing the Gates Foundation that schizophrenia is as devastating worldwide as malaria may bring a few badly needed billions into psychiatric brain research).
- Reminding members of Congress that in the United States, costs associated with psychiatric brain disorders total an estimated $700 billion annually,1 and that this must be addressed by boosting the meager NIMH budget by at least an order of magnitude. The NIMH should disproportionately invest its resources on severe brain disorders such as schizophrenia because breakthrough advances in its neurobiology will provide unprecedented insights to the pathophysiology of other severe psychiatric brain disorders.
- Partnering intimately with the pharmaceutical industry in a powerful public-private coalition to exploit the extensive research infrastructure of this industry.
- Creating the necessary army of researchers (physician-scientists) by providing huge incentives to medical students and psychiatric residents to pursue careers in neuroscience research. Incentives can include paying for an individual’s entire medical education and research training, and providing generous salaries that match or exceed the income of a very successful clinical practice.
- Convincing all psychiatric clinicians to support research by referring patients to research projects. Clinical psychiatrists are badly needed to care for the population, but they must be reminded that every treatment they are using today was a research project in the past, and that the research of today will evolve into the treatments (or cures) of tomorrow.
Pursuing lofty moonshots via innovative research is very likely to enhance serendipity and lead to unexpected discoveries along the way. As Louis Pasteur said, “chance only favors the prepared mind.”2 Moonshot thinking in psychiatry today is more feasible than ever before because of the many advances in research methods (neuroimaging, pluripotent cells, optogenetics, CRISPR, etc) and complex data management technologies (big data, machine learning, artificial intelligence), each of which qualifies as a preparatory moonshot in its own right.
Given the tragic consequences of psychiatric brain disorders, it is imperative that we “think big.” Humanity expects us to do that. We must envision the future of psychiatry as dramatically different from the present. Moonshot thinking is the indispensable vehicle to take us there.
1. Discovery Mood and Anxiety Program. The rising cost of mental health and substance abuse in the United States. Accessed January 13, 2022. https://discoverymood.com/blog/cost-of-mental-health-increase/
2. Wikiquote. Louis Pasteur. Accessed January 10, 2022. https://en.wikiquote.org/wiki/Louis_Pasteur
1. Discovery Mood and Anxiety Program. The rising cost of mental health and substance abuse in the United States. Accessed January 13, 2022. https://discoverymood.com/blog/cost-of-mental-health-increase/
2. Wikiquote. Louis Pasteur. Accessed January 10, 2022. https://en.wikiquote.org/wiki/Louis_Pasteur
Honor thy parents? Understanding parricide and associated spree killings
Mr. B, age 37, presents to a community mental health center for an appointment following a recent emergency department visit. He is diagnosed with schizophrenia, and has been treated for approximately 1 year. Six months ago, Mr. B stopped taking his antipsychotic due to its adverse effects. Despite compliance with another agent, he has become increasingly disorganized and paranoid.
He now believes that his mother, with whom he has lived all his life and who serves as his guardian, is poisoning his food and trying to kill him. She is an employee at a local grocery store, and Mr. B has expressed concern that her coworkers are assisting her in the plot to kill him.
Following a home visit, Mr. B’s case manager indicates that the patient showed them the collection of weapons he is amassing to “defend” himself. This leads to a concern for the safety of the patient, his mother, and others.
Although parricide—killing one’s parent—is a relatively rare event, its sensationalistic nature has long captured the attention of headline writers and the general public. This article discusses the diagnostic and demographic factors that may be seen among individuals who kill their parents, with an emphasis on those who commit matricide (murder of one’s mother) and associated spree killings, where an individual kills multiple people within a single brief but contiguous time period. Understanding these characteristics can help clinicians identify and more safely manage patients who may be at risk of harming their parents in addition to others.
Characteristics of perpetrators of parricide
Worldwide, approximately 2% to 4% of homicides involve parricide, or killing one’s parent.1,2 Most offenders are men in early adulthood, though a proportion are adolescents and some are women.1,3 They are often single, unemployed, and live with the parent prior to the killing.1 Patricide occurs more frequently than matricide.4 In the United States, approximately 150 fathers and 100 mothers are killed by their child each year.5
In a study of all homicides in England and Wales between 1997 and 2014, two-thirds of parricide offenders had previously been diagnosed with a mental disorder.1 One-third were diagnosed with schizophrenia.1 In a Canadian study focusing on 43 adult perpetrators found not criminally responsible,6 most were experiencing psychotic symptoms at the time of parricide; symptoms of a personality disorder were the second-most prevalent symptoms. Similarly, Bourget et al4 studied Canadian coroner records for 64 parents killed by their children. Of the children involved in those parricides, 15% attempted suicide after the killing. Two-thirds of the male offenders evidenced delusional thinking, and/or excessive violence (overkill) was common. Some cases (16%) followed an argument, and some of those perpetrators were intoxicated or psychotic. From our clinical experience, when there are identifiable nonpsychotic triggers, they often can be small things such as an argument over food, smoking, or video games. Often, the perpetrator was financially dependent on their parents and were “trapped in a difficult/hostile/dependence/love relationship” with that parent.6 Adolescent males who kill their parents may not have psychosis7; however, they may be victims of longstanding serious abuse at the hands of their parents. These perpetrators often express relief rather than remorse after committing murder.
Three categories to classify the perpetrators of parricide have been proposed: severely abused, severely mentally ill, and “dangerously antisocial.”3 While severe mental illness was most common in adult defendants, severe abuse was most common in adolescent offenders. There may be significant commonalities between adolescent and adult perpetrators. A more recent latent class analysis by Bojanic et al1 indicated 3 unique types of parricide offenders (Table 1).
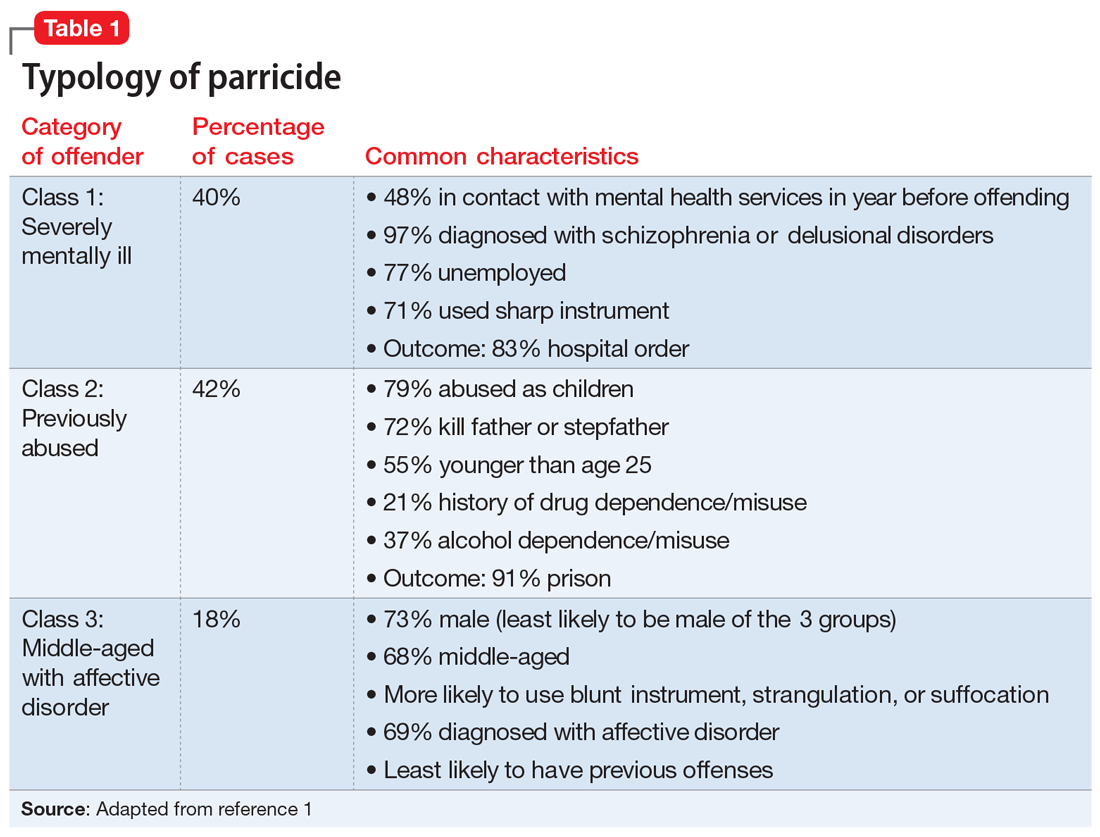
Continue to: Matricide: A closer look...
Matricide: A closer look
Though multiple studies have found a higher rate of psychosis among perpetrators of matricide, it is important to note that most people with psychotic disorders would never kill their mother. These events, however, tend to grab headlines and may be highly focused upon by the general population. In addition, matricide may be part of a larger crime that draws additional attention. For example, the 1966 University of Texas Bell Tower shooter and the 2012 Sandy Hook Elementary school shooter both killed their mothers before engaging in mass homicide. Often in cases of matricide, a longer-term dysfunctional relationship existed between the mother and the child. The mother is frequently described as controlling and intrusive and the (often adult) child as overly dependent, while the father may be absent or ineffectual. Hostility and mutual dependence are usual hallmarks of these relationships.8
However, in some cases where an individual with a psychotic disorder kills their mother, there may have been a traditional nurturing relationship.8 Alternative motivations unrelated to psychosis must be considered, including crimes motivated by money/inheritance or those perpetrated out of nonpsychotic anger. Green8 described motive categories for matricide that include paranoid and persecutory, altruistic, and other. In the “paranoid and persecutory” group, delusional beliefs about the mother occur; for example, the perpetrator may believe their mother was the devil. Sexual elements are found in some cases.8 Alternatively, the “altruistic” group demonstrated rather selfless reasons for killing, such as altruistic infanticide cases, or killing out of love.9 The altruistic matricide perpetrator may believe their mother is unwell, which may be a delusion or actually true. Finally, the “other” category contains cases related to jealousy, rage, and impulsivity.
In a study of 15 matricidal men in New York conducted by Campion et al,10 individuals seen by forensic psychiatric services for the crime of matricide included those with schizophrenia, substance-induced psychosis, and impulse control disorders. The authors noted there was often “a serious chronic derangement in the relationships of most matricidal men and their mothers.” Psychometric testing in these cases indicated feelings of dependency, weakness, and difficulty accepting an adult role separate from their mother. Some had conceptualized their mothers as a threat to their masculinity, while others had become enraged at their mothers.
Prevention requires addressing underlying issues
As described above, several factors are common among individuals who commit parricide, and these can be used to develop prevention strategies that focus on addressing underlying issues (Table 2). It is important to consider the relationship dynamics between the potential victim and perpetrator, as well as the motive, rather than focusing solely on mental illness or substance misuse.2
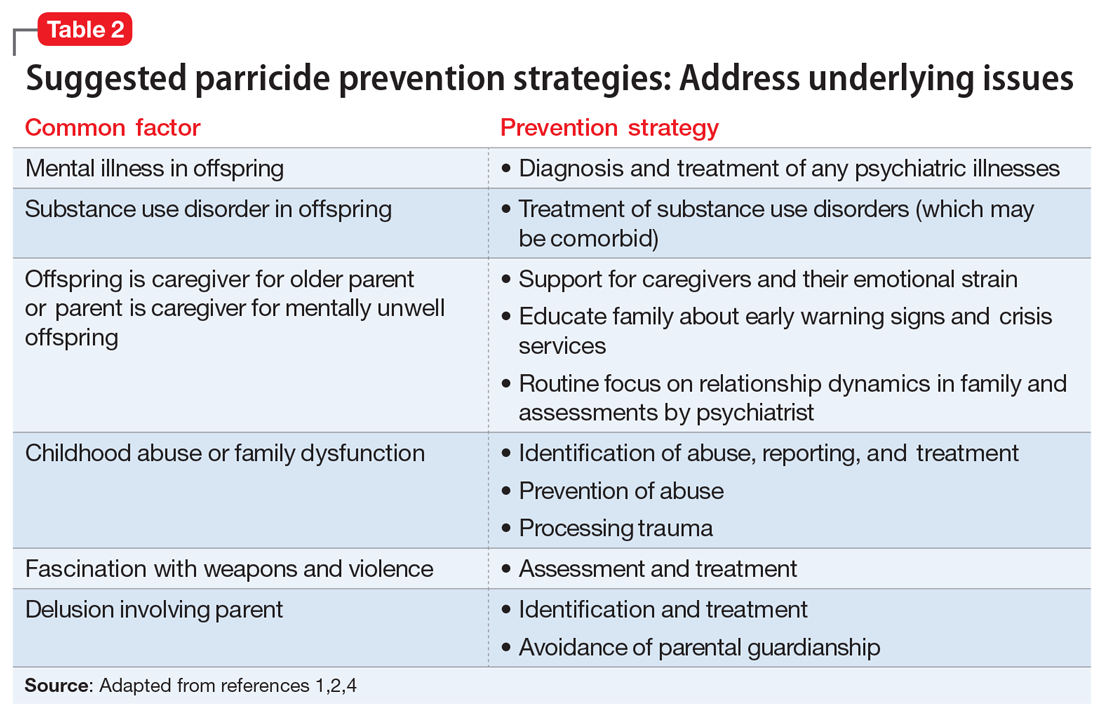
Spree killings that start as parricide
Although spree killing is a relatively rare event, a subset of spree killings involve parricide. One infamous recent event occurred in 2012 at Sandy Hook Elementary School, where the gunman killed his mother before going to the school and killing 26 additional people, many of whom were children.11,12 Because such events are rare, and because in these cases there is a high likelihood that the perpetrator is deceased (eg, died by suicide or killed by the police), much remains unknown about specific motivations and causative factors.
Information is often pieced together from postmortem reviews, which can be hampered by hindsight/recall bias and lack of contemporaneous documentation. Even worse, when these events occur, they may lead to a bias that all parricides or mass murders follow the pattern of the most recent case. This can result in overgeneralization of an individual’s history as being actionable risk violence factors for all potential parricide cases both by the public (eg, “My sister’s son has autism, and the Sandy Hook shooter was reported to have autism—should I be worried for my sister?”) and professionals (eg, “Will I be blamed for the next Sandy Hook by not taking more aggressive action even though I am not sure it is clinically warranted?”).
To identify trends for individuals committing parricide who engage in mass murder events (such as spree killing), we reviewed the 2000-2019 FBI active shooter list.12 Of the 333 events identified, 46 could be classified as domestic violence situations (eg, the perpetrator was in a romantic or familial relationship/former relationship and engaged in an active shooting incident involving at least 1 person from that relationship). We classified 11 of those 46 cases as parricide. Ten of those 11 parricides involved a child killing a parent (Table 3), and the other involved a grandchild killing a grandfather who served as their primary caregiver. Of the 11 incidents, mothers were involved (killed or wounded) in 4, and father figures (including the grandfather serving as a father and a stepfather) were killed in 9, with 2 incidents involving both parents. In 4 of the 11 parricides, other family members were killed in addition to the parent (including siblings, grandparents, or extended family). When considering spree shooters who committed parricide, 4 alleged perpetrators died by suicide, 1 was killed at the scene, and the rest were apprehended. The most common active shooting site endangering the public was an educational location (5), followed by commerce locations (4), with 2 involving open spaces. Eight of the 11 parricides occurred before the event was designated as an active shooting. The mean age for a parricide plus spree shooter was 23, once the oldest (age 61) and youngest (age 14) were removed from the calculation. The majority of the cases fell into the age range of 16 to 25 (n = 6), followed by 3 individuals who were age 26 to 31 (n = 3). All suspected individuals were male.
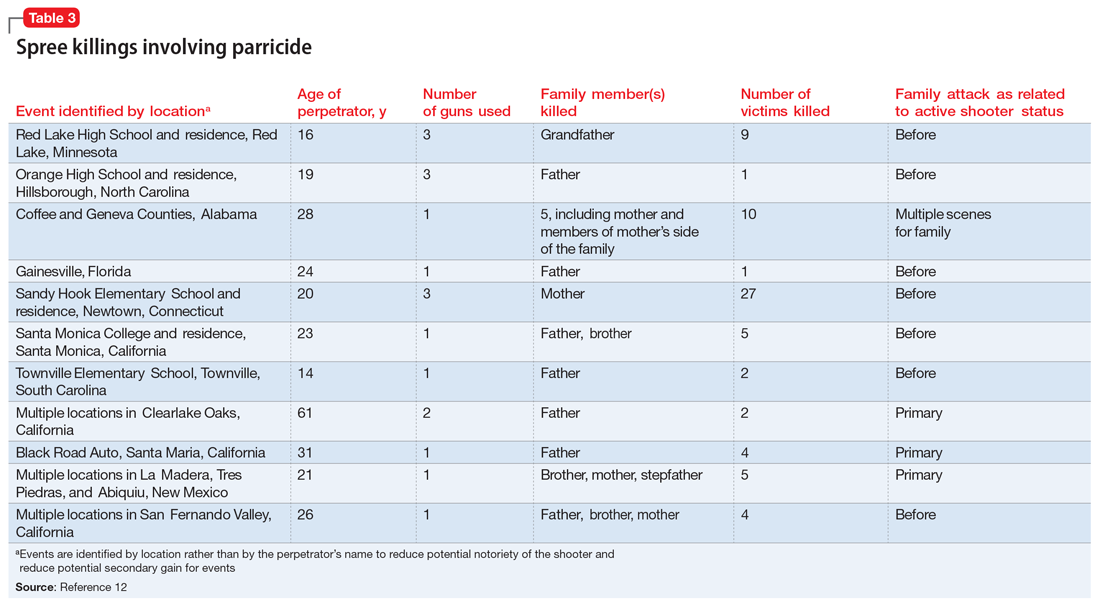
It is difficult to ascertain the existence of prior mental health care because perpetrators’ medical records may be confidential, juvenile court records may be sealed, and there may not even be a trial due to death or suicide (leading to limited forensic psychiatry examination or public testimony). Among those apprehended, many individuals raise some form of mental health defense, but the validity of their diagnosis may be undermined by concerns of possible malingering, especially in cases where the individual did not have a history of psychiatric treatment prior to the event.11 In summary, based on FBI data,12 spree shooters who committed parricide were usually male, in their late adolescence or early 20s, and more frequently targeted father figures. They often committed the parricide first and at a different location from later “active” shootings. Police were usually not aware of the parricide until after the spree is over.
Continue to: Parricide and society...
Parricide and society
For centuries, mothers and fathers have been honored and revered. Therefore, it is not surprising that killing of one’s mother or father attracts a great deal of macabre interest. Examples of parricide are present throughout popular culture, in mythology, comic books, movies, and television. As all psychiatrists know, Oedipus killed his father and married his mother. Other popular culture examples include: Grant Morrison’s Arkham Asylum: A Serious House on Serious Earth, Alfred Hitchcock’s Psycho, Oliver Stone’s Natural Born Killers, Peter Jackson’s Heavenly Creatures, The Affair drama series, and Star Wars: The Force Awakens.13,14
CASE CONTINUED
In Mr. B’s case, it is imperative for the treatment team to inquire about his history of violence, paying particular attention to prior violent acts towards his mother. His clinicians should consider hospitalization with the guardian’s consent if the danger appears imminent, especially considering the presence of weapons at home. They should attempt to stabilize Mr. B on effective, tolerable medications to ameliorate his psychosis, and to refer him for long-term psychotherapy to address difficult dynamic issues in the family relationship and encourage compliance with treatment. These steps may help avert a tragedy.
Bottom Line
Individuals who commit parricide often have a history of psychosis, a mood disorder, childhood abuse, and/or difficult relationship dynamics with the parent they kill. Some go on a spree killing in the community. Through careful consideration of individual risk factors, psychiatrists may help prevent some cases of parent murder by a child and possibly more tragedy in the community.
1. Bojanié L, Flynn S, Gianatsi M, et al. The typology of parricide and the role of mental illness: data-driven approach. Aggress Behav. 2020;46(6):516-522.
2. Pinals DS. Parricide. In Friedman SH, ed. Family Murder: Pathologies of Love and Hate. American Psychiatric Publishing; 2019:113-138.
3. Heide KM. Matricide and stepmatricide victims and offenders: an empirical analysis of US arrest data. Behav Sci Law. 2013;31(2):203-214.
4. Bourget D, Gagné P, Labelle ME. Parricide: a comparative study of matricide versus patricide. J Am Acad Psychiatry Law. 2007;35(3):306-312.
5. Heide KM, Frei A. Matricide: a critique of the literature. Trauma Violence Abuse. 2010;11(1):3-17.
6. Marleau JD, Auclair N, Millaud F. Comparison of factors associated with parricide in adults and adolescents. J Fam Viol. 2006;21:321-325.
7. West SG, Feldsher M. Parricide: characteristics of sons and daughters who kill their parents. Current Psychiatry. 2010;9(11):20-38.
8. Green CM. Matricide by sons. Med Sci Law. 1981;21(3):207-214.
9. Friedman SH. Conclusions. In Friedman SH, ed. Family Murder: Pathologies of Love and Hate. American Psychiatric Publishing; 2019:161-164.
10. Campion J, Cravens JM, Rotholc A, et al. A study of 15 matricidal men. Am J Psychiatry. 1985;142(3):312-317.
11. Hall RCW, Friedman SH, Sorrentino R, et al. The myth of school shooters and psychotropic medications. Behav Sci Law. 2019;37(5):540-558.
12. Department of Justice Federal Bureau of Investigation. Active Shooter Incidents: 20-Year Review, 2000-2019. June 1, 2021. Accessed October 12, 2021. https://www.fbi.gov/file-repository/active-shooter-incidents-20-year-review-2000-2019-060121.pdf/view
13. Friedman SH, Hall RCW. Star Wars: The Force Awakens, forensic teaching about matricide. J Am Acad Psychiatry Law. 2017;45(1):128-130.
14. Friedman SH, Hall RCW. Deadly and dysfunctional family dynamics: when fiction mirrors fact. In: Packer S, Fredrick DR, eds. Welcome to Arkham Asylum: Essays on Psychiatry and the Gotham City Institution. McFarland; 2019:65-75.
Mr. B, age 37, presents to a community mental health center for an appointment following a recent emergency department visit. He is diagnosed with schizophrenia, and has been treated for approximately 1 year. Six months ago, Mr. B stopped taking his antipsychotic due to its adverse effects. Despite compliance with another agent, he has become increasingly disorganized and paranoid.
He now believes that his mother, with whom he has lived all his life and who serves as his guardian, is poisoning his food and trying to kill him. She is an employee at a local grocery store, and Mr. B has expressed concern that her coworkers are assisting her in the plot to kill him.
Following a home visit, Mr. B’s case manager indicates that the patient showed them the collection of weapons he is amassing to “defend” himself. This leads to a concern for the safety of the patient, his mother, and others.
Although parricide—killing one’s parent—is a relatively rare event, its sensationalistic nature has long captured the attention of headline writers and the general public. This article discusses the diagnostic and demographic factors that may be seen among individuals who kill their parents, with an emphasis on those who commit matricide (murder of one’s mother) and associated spree killings, where an individual kills multiple people within a single brief but contiguous time period. Understanding these characteristics can help clinicians identify and more safely manage patients who may be at risk of harming their parents in addition to others.
Characteristics of perpetrators of parricide
Worldwide, approximately 2% to 4% of homicides involve parricide, or killing one’s parent.1,2 Most offenders are men in early adulthood, though a proportion are adolescents and some are women.1,3 They are often single, unemployed, and live with the parent prior to the killing.1 Patricide occurs more frequently than matricide.4 In the United States, approximately 150 fathers and 100 mothers are killed by their child each year.5
In a study of all homicides in England and Wales between 1997 and 2014, two-thirds of parricide offenders had previously been diagnosed with a mental disorder.1 One-third were diagnosed with schizophrenia.1 In a Canadian study focusing on 43 adult perpetrators found not criminally responsible,6 most were experiencing psychotic symptoms at the time of parricide; symptoms of a personality disorder were the second-most prevalent symptoms. Similarly, Bourget et al4 studied Canadian coroner records for 64 parents killed by their children. Of the children involved in those parricides, 15% attempted suicide after the killing. Two-thirds of the male offenders evidenced delusional thinking, and/or excessive violence (overkill) was common. Some cases (16%) followed an argument, and some of those perpetrators were intoxicated or psychotic. From our clinical experience, when there are identifiable nonpsychotic triggers, they often can be small things such as an argument over food, smoking, or video games. Often, the perpetrator was financially dependent on their parents and were “trapped in a difficult/hostile/dependence/love relationship” with that parent.6 Adolescent males who kill their parents may not have psychosis7; however, they may be victims of longstanding serious abuse at the hands of their parents. These perpetrators often express relief rather than remorse after committing murder.
Three categories to classify the perpetrators of parricide have been proposed: severely abused, severely mentally ill, and “dangerously antisocial.”3 While severe mental illness was most common in adult defendants, severe abuse was most common in adolescent offenders. There may be significant commonalities between adolescent and adult perpetrators. A more recent latent class analysis by Bojanic et al1 indicated 3 unique types of parricide offenders (Table 1).

Continue to: Matricide: A closer look...
Matricide: A closer look
Though multiple studies have found a higher rate of psychosis among perpetrators of matricide, it is important to note that most people with psychotic disorders would never kill their mother. These events, however, tend to grab headlines and may be highly focused upon by the general population. In addition, matricide may be part of a larger crime that draws additional attention. For example, the 1966 University of Texas Bell Tower shooter and the 2012 Sandy Hook Elementary school shooter both killed their mothers before engaging in mass homicide. Often in cases of matricide, a longer-term dysfunctional relationship existed between the mother and the child. The mother is frequently described as controlling and intrusive and the (often adult) child as overly dependent, while the father may be absent or ineffectual. Hostility and mutual dependence are usual hallmarks of these relationships.8
However, in some cases where an individual with a psychotic disorder kills their mother, there may have been a traditional nurturing relationship.8 Alternative motivations unrelated to psychosis must be considered, including crimes motivated by money/inheritance or those perpetrated out of nonpsychotic anger. Green8 described motive categories for matricide that include paranoid and persecutory, altruistic, and other. In the “paranoid and persecutory” group, delusional beliefs about the mother occur; for example, the perpetrator may believe their mother was the devil. Sexual elements are found in some cases.8 Alternatively, the “altruistic” group demonstrated rather selfless reasons for killing, such as altruistic infanticide cases, or killing out of love.9 The altruistic matricide perpetrator may believe their mother is unwell, which may be a delusion or actually true. Finally, the “other” category contains cases related to jealousy, rage, and impulsivity.
In a study of 15 matricidal men in New York conducted by Campion et al,10 individuals seen by forensic psychiatric services for the crime of matricide included those with schizophrenia, substance-induced psychosis, and impulse control disorders. The authors noted there was often “a serious chronic derangement in the relationships of most matricidal men and their mothers.” Psychometric testing in these cases indicated feelings of dependency, weakness, and difficulty accepting an adult role separate from their mother. Some had conceptualized their mothers as a threat to their masculinity, while others had become enraged at their mothers.
Prevention requires addressing underlying issues
As described above, several factors are common among individuals who commit parricide, and these can be used to develop prevention strategies that focus on addressing underlying issues (Table 2). It is important to consider the relationship dynamics between the potential victim and perpetrator, as well as the motive, rather than focusing solely on mental illness or substance misuse.2

Spree killings that start as parricide
Although spree killing is a relatively rare event, a subset of spree killings involve parricide. One infamous recent event occurred in 2012 at Sandy Hook Elementary School, where the gunman killed his mother before going to the school and killing 26 additional people, many of whom were children.11,12 Because such events are rare, and because in these cases there is a high likelihood that the perpetrator is deceased (eg, died by suicide or killed by the police), much remains unknown about specific motivations and causative factors.
Information is often pieced together from postmortem reviews, which can be hampered by hindsight/recall bias and lack of contemporaneous documentation. Even worse, when these events occur, they may lead to a bias that all parricides or mass murders follow the pattern of the most recent case. This can result in overgeneralization of an individual’s history as being actionable risk violence factors for all potential parricide cases both by the public (eg, “My sister’s son has autism, and the Sandy Hook shooter was reported to have autism—should I be worried for my sister?”) and professionals (eg, “Will I be blamed for the next Sandy Hook by not taking more aggressive action even though I am not sure it is clinically warranted?”).
To identify trends for individuals committing parricide who engage in mass murder events (such as spree killing), we reviewed the 2000-2019 FBI active shooter list.12 Of the 333 events identified, 46 could be classified as domestic violence situations (eg, the perpetrator was in a romantic or familial relationship/former relationship and engaged in an active shooting incident involving at least 1 person from that relationship). We classified 11 of those 46 cases as parricide. Ten of those 11 parricides involved a child killing a parent (Table 3), and the other involved a grandchild killing a grandfather who served as their primary caregiver. Of the 11 incidents, mothers were involved (killed or wounded) in 4, and father figures (including the grandfather serving as a father and a stepfather) were killed in 9, with 2 incidents involving both parents. In 4 of the 11 parricides, other family members were killed in addition to the parent (including siblings, grandparents, or extended family). When considering spree shooters who committed parricide, 4 alleged perpetrators died by suicide, 1 was killed at the scene, and the rest were apprehended. The most common active shooting site endangering the public was an educational location (5), followed by commerce locations (4), with 2 involving open spaces. Eight of the 11 parricides occurred before the event was designated as an active shooting. The mean age for a parricide plus spree shooter was 23, once the oldest (age 61) and youngest (age 14) were removed from the calculation. The majority of the cases fell into the age range of 16 to 25 (n = 6), followed by 3 individuals who were age 26 to 31 (n = 3). All suspected individuals were male.

It is difficult to ascertain the existence of prior mental health care because perpetrators’ medical records may be confidential, juvenile court records may be sealed, and there may not even be a trial due to death or suicide (leading to limited forensic psychiatry examination or public testimony). Among those apprehended, many individuals raise some form of mental health defense, but the validity of their diagnosis may be undermined by concerns of possible malingering, especially in cases where the individual did not have a history of psychiatric treatment prior to the event.11 In summary, based on FBI data,12 spree shooters who committed parricide were usually male, in their late adolescence or early 20s, and more frequently targeted father figures. They often committed the parricide first and at a different location from later “active” shootings. Police were usually not aware of the parricide until after the spree is over.
Continue to: Parricide and society...
Parricide and society
For centuries, mothers and fathers have been honored and revered. Therefore, it is not surprising that killing of one’s mother or father attracts a great deal of macabre interest. Examples of parricide are present throughout popular culture, in mythology, comic books, movies, and television. As all psychiatrists know, Oedipus killed his father and married his mother. Other popular culture examples include: Grant Morrison’s Arkham Asylum: A Serious House on Serious Earth, Alfred Hitchcock’s Psycho, Oliver Stone’s Natural Born Killers, Peter Jackson’s Heavenly Creatures, The Affair drama series, and Star Wars: The Force Awakens.13,14
CASE CONTINUED
In Mr. B’s case, it is imperative for the treatment team to inquire about his history of violence, paying particular attention to prior violent acts towards his mother. His clinicians should consider hospitalization with the guardian’s consent if the danger appears imminent, especially considering the presence of weapons at home. They should attempt to stabilize Mr. B on effective, tolerable medications to ameliorate his psychosis, and to refer him for long-term psychotherapy to address difficult dynamic issues in the family relationship and encourage compliance with treatment. These steps may help avert a tragedy.
Bottom Line
Individuals who commit parricide often have a history of psychosis, a mood disorder, childhood abuse, and/or difficult relationship dynamics with the parent they kill. Some go on a spree killing in the community. Through careful consideration of individual risk factors, psychiatrists may help prevent some cases of parent murder by a child and possibly more tragedy in the community.
Mr. B, age 37, presents to a community mental health center for an appointment following a recent emergency department visit. He is diagnosed with schizophrenia, and has been treated for approximately 1 year. Six months ago, Mr. B stopped taking his antipsychotic due to its adverse effects. Despite compliance with another agent, he has become increasingly disorganized and paranoid.
He now believes that his mother, with whom he has lived all his life and who serves as his guardian, is poisoning his food and trying to kill him. She is an employee at a local grocery store, and Mr. B has expressed concern that her coworkers are assisting her in the plot to kill him.
Following a home visit, Mr. B’s case manager indicates that the patient showed them the collection of weapons he is amassing to “defend” himself. This leads to a concern for the safety of the patient, his mother, and others.
Although parricide—killing one’s parent—is a relatively rare event, its sensationalistic nature has long captured the attention of headline writers and the general public. This article discusses the diagnostic and demographic factors that may be seen among individuals who kill their parents, with an emphasis on those who commit matricide (murder of one’s mother) and associated spree killings, where an individual kills multiple people within a single brief but contiguous time period. Understanding these characteristics can help clinicians identify and more safely manage patients who may be at risk of harming their parents in addition to others.
Characteristics of perpetrators of parricide
Worldwide, approximately 2% to 4% of homicides involve parricide, or killing one’s parent.1,2 Most offenders are men in early adulthood, though a proportion are adolescents and some are women.1,3 They are often single, unemployed, and live with the parent prior to the killing.1 Patricide occurs more frequently than matricide.4 In the United States, approximately 150 fathers and 100 mothers are killed by their child each year.5
In a study of all homicides in England and Wales between 1997 and 2014, two-thirds of parricide offenders had previously been diagnosed with a mental disorder.1 One-third were diagnosed with schizophrenia.1 In a Canadian study focusing on 43 adult perpetrators found not criminally responsible,6 most were experiencing psychotic symptoms at the time of parricide; symptoms of a personality disorder were the second-most prevalent symptoms. Similarly, Bourget et al4 studied Canadian coroner records for 64 parents killed by their children. Of the children involved in those parricides, 15% attempted suicide after the killing. Two-thirds of the male offenders evidenced delusional thinking, and/or excessive violence (overkill) was common. Some cases (16%) followed an argument, and some of those perpetrators were intoxicated or psychotic. From our clinical experience, when there are identifiable nonpsychotic triggers, they often can be small things such as an argument over food, smoking, or video games. Often, the perpetrator was financially dependent on their parents and were “trapped in a difficult/hostile/dependence/love relationship” with that parent.6 Adolescent males who kill their parents may not have psychosis7; however, they may be victims of longstanding serious abuse at the hands of their parents. These perpetrators often express relief rather than remorse after committing murder.
Three categories to classify the perpetrators of parricide have been proposed: severely abused, severely mentally ill, and “dangerously antisocial.”3 While severe mental illness was most common in adult defendants, severe abuse was most common in adolescent offenders. There may be significant commonalities between adolescent and adult perpetrators. A more recent latent class analysis by Bojanic et al1 indicated 3 unique types of parricide offenders (Table 1).

Continue to: Matricide: A closer look...
Matricide: A closer look
Though multiple studies have found a higher rate of psychosis among perpetrators of matricide, it is important to note that most people with psychotic disorders would never kill their mother. These events, however, tend to grab headlines and may be highly focused upon by the general population. In addition, matricide may be part of a larger crime that draws additional attention. For example, the 1966 University of Texas Bell Tower shooter and the 2012 Sandy Hook Elementary school shooter both killed their mothers before engaging in mass homicide. Often in cases of matricide, a longer-term dysfunctional relationship existed between the mother and the child. The mother is frequently described as controlling and intrusive and the (often adult) child as overly dependent, while the father may be absent or ineffectual. Hostility and mutual dependence are usual hallmarks of these relationships.8
However, in some cases where an individual with a psychotic disorder kills their mother, there may have been a traditional nurturing relationship.8 Alternative motivations unrelated to psychosis must be considered, including crimes motivated by money/inheritance or those perpetrated out of nonpsychotic anger. Green8 described motive categories for matricide that include paranoid and persecutory, altruistic, and other. In the “paranoid and persecutory” group, delusional beliefs about the mother occur; for example, the perpetrator may believe their mother was the devil. Sexual elements are found in some cases.8 Alternatively, the “altruistic” group demonstrated rather selfless reasons for killing, such as altruistic infanticide cases, or killing out of love.9 The altruistic matricide perpetrator may believe their mother is unwell, which may be a delusion or actually true. Finally, the “other” category contains cases related to jealousy, rage, and impulsivity.
In a study of 15 matricidal men in New York conducted by Campion et al,10 individuals seen by forensic psychiatric services for the crime of matricide included those with schizophrenia, substance-induced psychosis, and impulse control disorders. The authors noted there was often “a serious chronic derangement in the relationships of most matricidal men and their mothers.” Psychometric testing in these cases indicated feelings of dependency, weakness, and difficulty accepting an adult role separate from their mother. Some had conceptualized their mothers as a threat to their masculinity, while others had become enraged at their mothers.
Prevention requires addressing underlying issues
As described above, several factors are common among individuals who commit parricide, and these can be used to develop prevention strategies that focus on addressing underlying issues (Table 2). It is important to consider the relationship dynamics between the potential victim and perpetrator, as well as the motive, rather than focusing solely on mental illness or substance misuse.2

Spree killings that start as parricide
Although spree killing is a relatively rare event, a subset of spree killings involve parricide. One infamous recent event occurred in 2012 at Sandy Hook Elementary School, where the gunman killed his mother before going to the school and killing 26 additional people, many of whom were children.11,12 Because such events are rare, and because in these cases there is a high likelihood that the perpetrator is deceased (eg, died by suicide or killed by the police), much remains unknown about specific motivations and causative factors.
Information is often pieced together from postmortem reviews, which can be hampered by hindsight/recall bias and lack of contemporaneous documentation. Even worse, when these events occur, they may lead to a bias that all parricides or mass murders follow the pattern of the most recent case. This can result in overgeneralization of an individual’s history as being actionable risk violence factors for all potential parricide cases both by the public (eg, “My sister’s son has autism, and the Sandy Hook shooter was reported to have autism—should I be worried for my sister?”) and professionals (eg, “Will I be blamed for the next Sandy Hook by not taking more aggressive action even though I am not sure it is clinically warranted?”).
To identify trends for individuals committing parricide who engage in mass murder events (such as spree killing), we reviewed the 2000-2019 FBI active shooter list.12 Of the 333 events identified, 46 could be classified as domestic violence situations (eg, the perpetrator was in a romantic or familial relationship/former relationship and engaged in an active shooting incident involving at least 1 person from that relationship). We classified 11 of those 46 cases as parricide. Ten of those 11 parricides involved a child killing a parent (Table 3), and the other involved a grandchild killing a grandfather who served as their primary caregiver. Of the 11 incidents, mothers were involved (killed or wounded) in 4, and father figures (including the grandfather serving as a father and a stepfather) were killed in 9, with 2 incidents involving both parents. In 4 of the 11 parricides, other family members were killed in addition to the parent (including siblings, grandparents, or extended family). When considering spree shooters who committed parricide, 4 alleged perpetrators died by suicide, 1 was killed at the scene, and the rest were apprehended. The most common active shooting site endangering the public was an educational location (5), followed by commerce locations (4), with 2 involving open spaces. Eight of the 11 parricides occurred before the event was designated as an active shooting. The mean age for a parricide plus spree shooter was 23, once the oldest (age 61) and youngest (age 14) were removed from the calculation. The majority of the cases fell into the age range of 16 to 25 (n = 6), followed by 3 individuals who were age 26 to 31 (n = 3). All suspected individuals were male.

It is difficult to ascertain the existence of prior mental health care because perpetrators’ medical records may be confidential, juvenile court records may be sealed, and there may not even be a trial due to death or suicide (leading to limited forensic psychiatry examination or public testimony). Among those apprehended, many individuals raise some form of mental health defense, but the validity of their diagnosis may be undermined by concerns of possible malingering, especially in cases where the individual did not have a history of psychiatric treatment prior to the event.11 In summary, based on FBI data,12 spree shooters who committed parricide were usually male, in their late adolescence or early 20s, and more frequently targeted father figures. They often committed the parricide first and at a different location from later “active” shootings. Police were usually not aware of the parricide until after the spree is over.
Continue to: Parricide and society...
Parricide and society
For centuries, mothers and fathers have been honored and revered. Therefore, it is not surprising that killing of one’s mother or father attracts a great deal of macabre interest. Examples of parricide are present throughout popular culture, in mythology, comic books, movies, and television. As all psychiatrists know, Oedipus killed his father and married his mother. Other popular culture examples include: Grant Morrison’s Arkham Asylum: A Serious House on Serious Earth, Alfred Hitchcock’s Psycho, Oliver Stone’s Natural Born Killers, Peter Jackson’s Heavenly Creatures, The Affair drama series, and Star Wars: The Force Awakens.13,14
CASE CONTINUED
In Mr. B’s case, it is imperative for the treatment team to inquire about his history of violence, paying particular attention to prior violent acts towards his mother. His clinicians should consider hospitalization with the guardian’s consent if the danger appears imminent, especially considering the presence of weapons at home. They should attempt to stabilize Mr. B on effective, tolerable medications to ameliorate his psychosis, and to refer him for long-term psychotherapy to address difficult dynamic issues in the family relationship and encourage compliance with treatment. These steps may help avert a tragedy.
Bottom Line
Individuals who commit parricide often have a history of psychosis, a mood disorder, childhood abuse, and/or difficult relationship dynamics with the parent they kill. Some go on a spree killing in the community. Through careful consideration of individual risk factors, psychiatrists may help prevent some cases of parent murder by a child and possibly more tragedy in the community.
1. Bojanié L, Flynn S, Gianatsi M, et al. The typology of parricide and the role of mental illness: data-driven approach. Aggress Behav. 2020;46(6):516-522.
2. Pinals DS. Parricide. In Friedman SH, ed. Family Murder: Pathologies of Love and Hate. American Psychiatric Publishing; 2019:113-138.
3. Heide KM. Matricide and stepmatricide victims and offenders: an empirical analysis of US arrest data. Behav Sci Law. 2013;31(2):203-214.
4. Bourget D, Gagné P, Labelle ME. Parricide: a comparative study of matricide versus patricide. J Am Acad Psychiatry Law. 2007;35(3):306-312.
5. Heide KM, Frei A. Matricide: a critique of the literature. Trauma Violence Abuse. 2010;11(1):3-17.
6. Marleau JD, Auclair N, Millaud F. Comparison of factors associated with parricide in adults and adolescents. J Fam Viol. 2006;21:321-325.
7. West SG, Feldsher M. Parricide: characteristics of sons and daughters who kill their parents. Current Psychiatry. 2010;9(11):20-38.
8. Green CM. Matricide by sons. Med Sci Law. 1981;21(3):207-214.
9. Friedman SH. Conclusions. In Friedman SH, ed. Family Murder: Pathologies of Love and Hate. American Psychiatric Publishing; 2019:161-164.
10. Campion J, Cravens JM, Rotholc A, et al. A study of 15 matricidal men. Am J Psychiatry. 1985;142(3):312-317.
11. Hall RCW, Friedman SH, Sorrentino R, et al. The myth of school shooters and psychotropic medications. Behav Sci Law. 2019;37(5):540-558.
12. Department of Justice Federal Bureau of Investigation. Active Shooter Incidents: 20-Year Review, 2000-2019. June 1, 2021. Accessed October 12, 2021. https://www.fbi.gov/file-repository/active-shooter-incidents-20-year-review-2000-2019-060121.pdf/view
13. Friedman SH, Hall RCW. Star Wars: The Force Awakens, forensic teaching about matricide. J Am Acad Psychiatry Law. 2017;45(1):128-130.
14. Friedman SH, Hall RCW. Deadly and dysfunctional family dynamics: when fiction mirrors fact. In: Packer S, Fredrick DR, eds. Welcome to Arkham Asylum: Essays on Psychiatry and the Gotham City Institution. McFarland; 2019:65-75.
1. Bojanié L, Flynn S, Gianatsi M, et al. The typology of parricide and the role of mental illness: data-driven approach. Aggress Behav. 2020;46(6):516-522.
2. Pinals DS. Parricide. In Friedman SH, ed. Family Murder: Pathologies of Love and Hate. American Psychiatric Publishing; 2019:113-138.
3. Heide KM. Matricide and stepmatricide victims and offenders: an empirical analysis of US arrest data. Behav Sci Law. 2013;31(2):203-214.
4. Bourget D, Gagné P, Labelle ME. Parricide: a comparative study of matricide versus patricide. J Am Acad Psychiatry Law. 2007;35(3):306-312.
5. Heide KM, Frei A. Matricide: a critique of the literature. Trauma Violence Abuse. 2010;11(1):3-17.
6. Marleau JD, Auclair N, Millaud F. Comparison of factors associated with parricide in adults and adolescents. J Fam Viol. 2006;21:321-325.
7. West SG, Feldsher M. Parricide: characteristics of sons and daughters who kill their parents. Current Psychiatry. 2010;9(11):20-38.
8. Green CM. Matricide by sons. Med Sci Law. 1981;21(3):207-214.
9. Friedman SH. Conclusions. In Friedman SH, ed. Family Murder: Pathologies of Love and Hate. American Psychiatric Publishing; 2019:161-164.
10. Campion J, Cravens JM, Rotholc A, et al. A study of 15 matricidal men. Am J Psychiatry. 1985;142(3):312-317.
11. Hall RCW, Friedman SH, Sorrentino R, et al. The myth of school shooters and psychotropic medications. Behav Sci Law. 2019;37(5):540-558.
12. Department of Justice Federal Bureau of Investigation. Active Shooter Incidents: 20-Year Review, 2000-2019. June 1, 2021. Accessed October 12, 2021. https://www.fbi.gov/file-repository/active-shooter-incidents-20-year-review-2000-2019-060121.pdf/view
13. Friedman SH, Hall RCW. Star Wars: The Force Awakens, forensic teaching about matricide. J Am Acad Psychiatry Law. 2017;45(1):128-130.
14. Friedman SH, Hall RCW. Deadly and dysfunctional family dynamics: when fiction mirrors fact. In: Packer S, Fredrick DR, eds. Welcome to Arkham Asylum: Essays on Psychiatry and the Gotham City Institution. McFarland; 2019:65-75.
Antipsychotic-induced priapism: Mitigating the risk
Mr. J, age 35, is brought to the hospital from prison due to priapism that does not improve with treatment. He says he has had priapism 5 times previously, with the first incidence occurring “years ago” due to trazodone.
Recently, he has been receiving risperidone, which the treatment team believes is the cause of his current priapism. His medical history includes asthma, schizophrenia, hypertension, seizures, and sickle cell trait. Mr. J is experiencing auditory hallucinations, which he describes as “continuous, neutral voices that are annoying.” He would like relief from his auditory hallucinations and is willing to change his antipsychotic, but does not want additional treatment for his priapism. His present medications include risperidone, 1 mg twice a day, escitalopram, 10 mg/d, benztropine, 1 mg twice a day, and phenytoin, 500 mg/d at bedtime.
Priapism is a prolonged, persistent, and often painful erection that occurs without sexual stimulation. Although relatively rare, it can result in potentially serious long-term complications, including impotence and gangrene, and requires immediate evaluation and management.
There are 2 types of priapism: nonischemic, or “high-flow,” priapism, and ischemic, or “low-flow,” priapism (Table 1). While nonischemic priapism is typically caused by penile or perineal trauma, ischemic priapism can occur as a result of medications, including antipsychotics, antidepressants, anxiolytics, and antihypertensives, or hematological conditions such as sickle cell disease.1 Other risk factors associated with priapism include substance abuse, hyperprolactinemia, diabetes, and liver disease.4
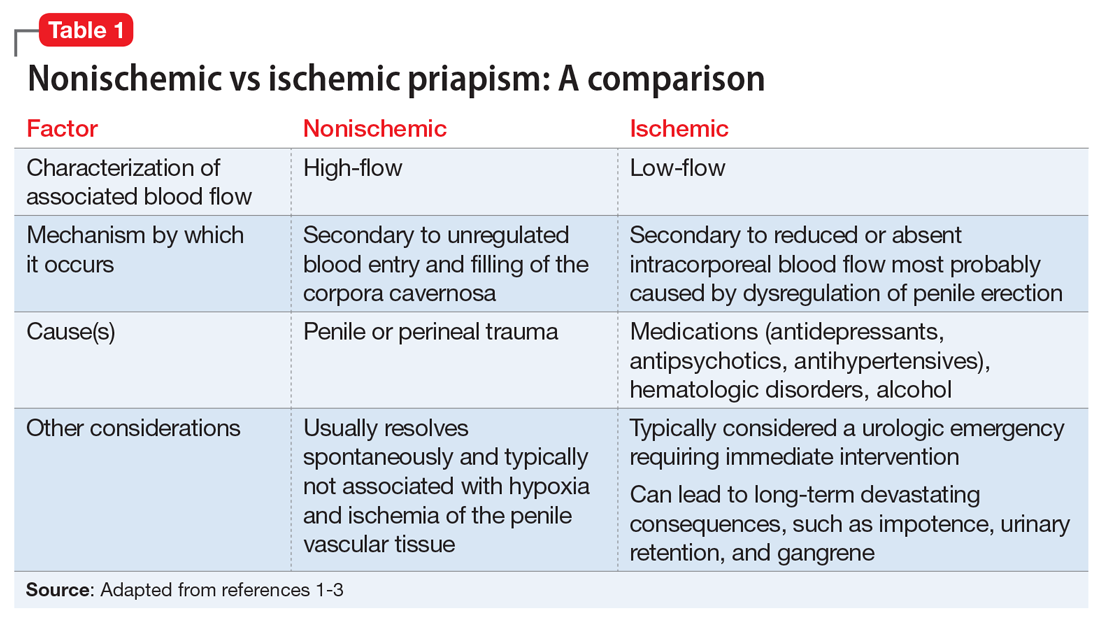
Antipsychotic-induced priapism
Medication-induced priapism is a rare adverse drug reaction (ADR). Of the medication classes associated with priapism, antipsychotics have the highest incidence and account for approximately 20% of all cases.1
The mechanism of priapism associated with antipsychotics is thought to be related to alpha-1 blockade in the corpora cavernosa of the penis. Although antipsychotics within each class share common characteristics, each agent has a unique profile of receptor affinities. As such, antipsychotics have varying affinities for the alpha-adrenergic receptor (Table 2). Agents such as ziprasidone, chlorpromazine, and risperidone—which have the highest affinity for the alpha-1 adrenoceptors—may be more likely to cause priapism compared with agents with lower affinity, such as olanzapine. Priapism may occur at any time during antipsychotic treatment, and does not appear to be dose-related.
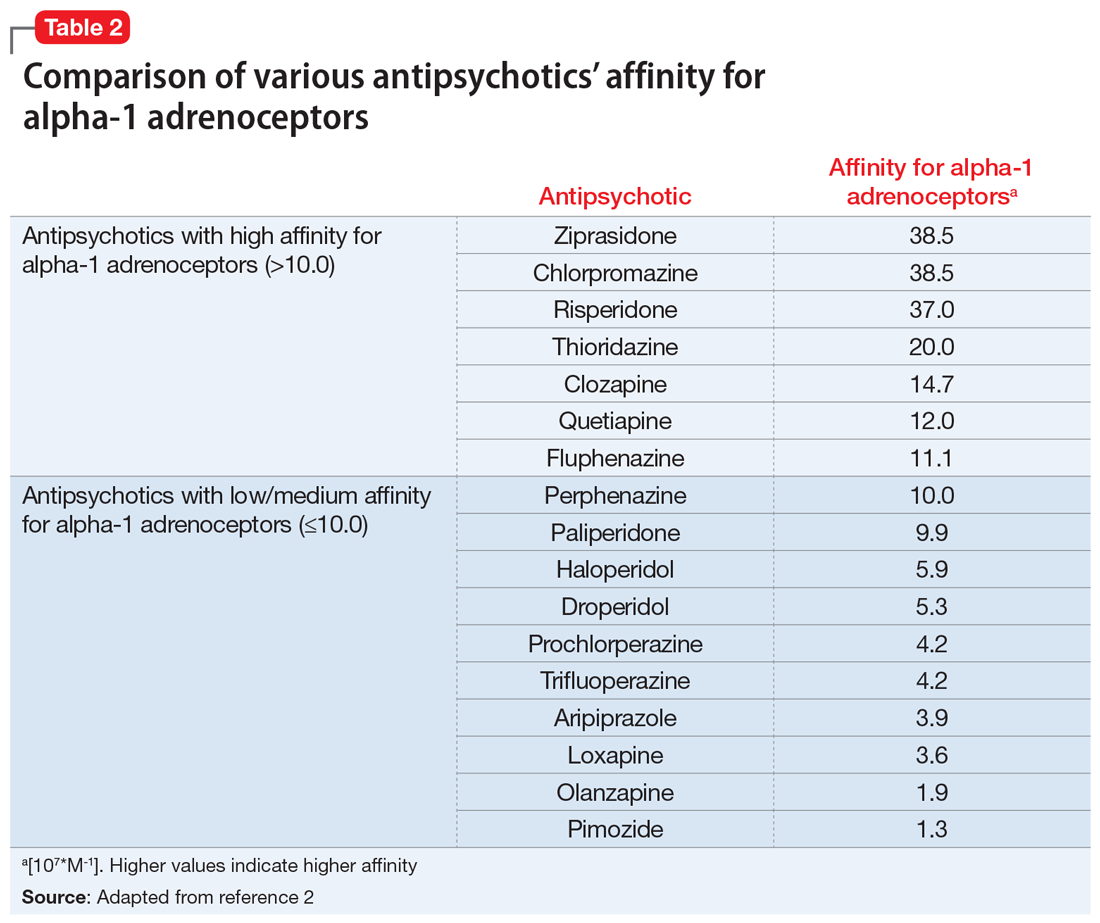
Continue to: Antipsychotic drug interactions and priapism...
Antipsychotic drug interactions and priapism
Patients who are receiving multiple medications as treatment for chronic medical or psychiatric conditions have an increased likelihood of experiencing drug-drug interactions (DDIs) that lead to adverse effects.
Various case reports have described priapism as a result of DDIs related to antipsychotic agents combined with other psychotropic or nonpsychotropic medications.3 Most of these DDIs have been attributed to the cytochrome P450 (CYP) family of enzymes, including CYP2D6, CYP1A2, and CYP3A4/5, which are major enzymes implicated in the metabolism of antipsychotics (Table 3).
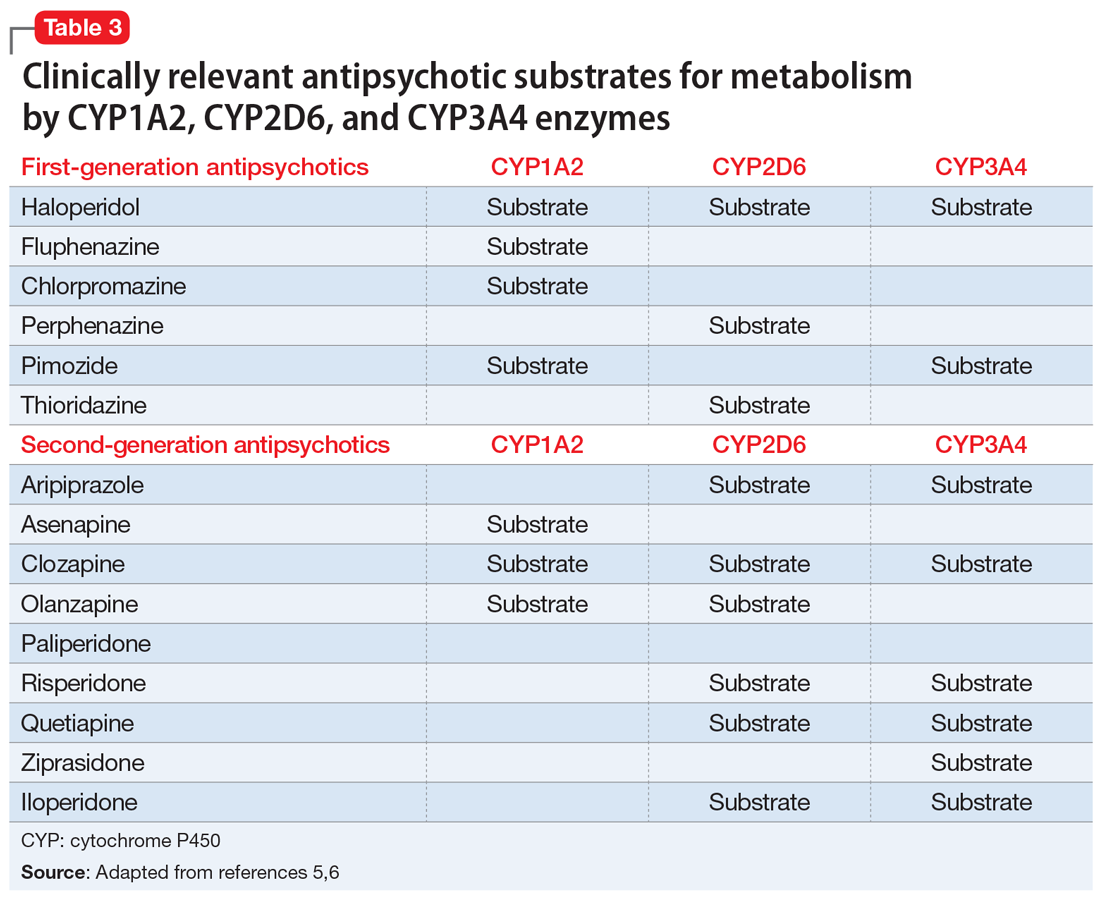
It is imperative to be vigilant during the concomitant administration of antipsychotics with other medications that may be substrates, inducers, or inhibitors of CYP enzymes, as this could alter the metabolism and kinetics of the antipsychotic and result in ADRs such as priapism. For example, drug interactions exist between strong CYP2D6 inhibitors—such as the antidepressants paroxetine, fluoxetine, and bupropion—and antipsychotics that are substrates of CYP2D6, such as risperidone, aripiprazole, haloperidol, and perphenazine. This interaction can lead to higher levels of the antipsychotic, which would increase the patient’s risk of experiencing ADRs. Because psychotic illnesses and depression/anxiety often coexist, it is not uncommon for individuals with these conditions to be receiving both an antipsychotic and an antidepressant.
Because there is a high incidence of comorbidities such as HIV and cardiovascular disease among individuals with mental illnesses, clinicians must also be cognizant of any nonpsychotropic medications the patient may be taking. For instance, clinically relevant DDIs exist between protease inhibitors, such as ritonavir, a strong CYP3A4 inhibitor, and antipsychotics that are substrates of CYP3A4, such as pimozide, aripiprazole, and quetiapine.
Mitigating the risk of priapism
Although there are associated risk factors for priapism, there are no concrete indicators to predict the onset or development of the condition. The best predictor may be a history of prolonged and painless erections.3
As such, when choosing an antipsychotic, it is critical to screen the patient for the previously mentioned risk factors, including the presence of medications with strong alpha-1 receptor affinity and CYP interactions, especially to minimize the risk of recurrence of priapism in those with prior or similar episodes. Management of patients with priapism due to antipsychotics has involved reducing the dose of the offending agent and/or changing the medication to one with a lower alpha-adrenergic affinity (Table 22).
Similar to most situations, management is patient-specific and depends on several factors, including the severity of the patient’s psychiatric disease, history/severity of priapism and treatment, concurrent medication list, etc. For example, although clozapine is considered to have relatively high affinity for the alpha-1 receptor, it is also the agent of choice for treatment-refractory schizophrenia. Risks and benefits must be weighed on a individualized basis. Case reports have described symptom improvement via lowering the dose of clozapine and adding on or switching to an antipsychotic agent with minimal alpha-1 receptor affinity.4
After considering Mr. J’s history, risk factors, and preferences, the treatment team discontinues risperidone and initiates haloperidol, 5 mg twice a day. Soon after, Mr. J no longer experiences priapism.
1. Weiner DM, Lowe FC. Psychotropic drug-induced priapism. Mol Diag Ther 9. 1998;371-379. doi:10.2165/00023210-199809050-00004
2. Andersohn F, Schmedt N, Weinmann S, et al. Priapism associated with antipsychotics: role of alpha1 adrenoceptor affinity. J Clin Psychopharmacol. 2010;30(1):68-71. doi:10.1097/JCP.0b013e3181c8273d
3. Sood S, James W, Bailon MJ. Priapism associated with atypical antipsychotic medications: a review. Int Clin Psychopharmacol. 2008;23(1):9-17.
4. Sinkeviciute I, Kroken RA, Johnsen E. Priapism in antipsychotic drug use: a rare but important side effect. Case Rep Psychiatry. 2012;2012:496364. doi:10.1155/2012/496364
5. Mora F, Martín JDD, Zubillaga E, et al. CYP450 and its implications in the clinical use of antipsychotic drugs. Clin Exp Pharmacol. 2015;5(176):1-10. doi:10.4172/2161-1459.1000176
6. Puangpetch A, Vanwong N, Nuntamool N, et al. CYP2D6 polymorphisms and their influence on risperidone treatment. Pharmgenomics Pers Med. 2016;9:131-147. doi:10.2147/PGPM.S107772
Mr. J, age 35, is brought to the hospital from prison due to priapism that does not improve with treatment. He says he has had priapism 5 times previously, with the first incidence occurring “years ago” due to trazodone.
Recently, he has been receiving risperidone, which the treatment team believes is the cause of his current priapism. His medical history includes asthma, schizophrenia, hypertension, seizures, and sickle cell trait. Mr. J is experiencing auditory hallucinations, which he describes as “continuous, neutral voices that are annoying.” He would like relief from his auditory hallucinations and is willing to change his antipsychotic, but does not want additional treatment for his priapism. His present medications include risperidone, 1 mg twice a day, escitalopram, 10 mg/d, benztropine, 1 mg twice a day, and phenytoin, 500 mg/d at bedtime.
Priapism is a prolonged, persistent, and often painful erection that occurs without sexual stimulation. Although relatively rare, it can result in potentially serious long-term complications, including impotence and gangrene, and requires immediate evaluation and management.
There are 2 types of priapism: nonischemic, or “high-flow,” priapism, and ischemic, or “low-flow,” priapism (Table 1). While nonischemic priapism is typically caused by penile or perineal trauma, ischemic priapism can occur as a result of medications, including antipsychotics, antidepressants, anxiolytics, and antihypertensives, or hematological conditions such as sickle cell disease.1 Other risk factors associated with priapism include substance abuse, hyperprolactinemia, diabetes, and liver disease.4

Antipsychotic-induced priapism
Medication-induced priapism is a rare adverse drug reaction (ADR). Of the medication classes associated with priapism, antipsychotics have the highest incidence and account for approximately 20% of all cases.1
The mechanism of priapism associated with antipsychotics is thought to be related to alpha-1 blockade in the corpora cavernosa of the penis. Although antipsychotics within each class share common characteristics, each agent has a unique profile of receptor affinities. As such, antipsychotics have varying affinities for the alpha-adrenergic receptor (Table 2). Agents such as ziprasidone, chlorpromazine, and risperidone—which have the highest affinity for the alpha-1 adrenoceptors—may be more likely to cause priapism compared with agents with lower affinity, such as olanzapine. Priapism may occur at any time during antipsychotic treatment, and does not appear to be dose-related.

Continue to: Antipsychotic drug interactions and priapism...
Antipsychotic drug interactions and priapism
Patients who are receiving multiple medications as treatment for chronic medical or psychiatric conditions have an increased likelihood of experiencing drug-drug interactions (DDIs) that lead to adverse effects.
Various case reports have described priapism as a result of DDIs related to antipsychotic agents combined with other psychotropic or nonpsychotropic medications.3 Most of these DDIs have been attributed to the cytochrome P450 (CYP) family of enzymes, including CYP2D6, CYP1A2, and CYP3A4/5, which are major enzymes implicated in the metabolism of antipsychotics (Table 3).

It is imperative to be vigilant during the concomitant administration of antipsychotics with other medications that may be substrates, inducers, or inhibitors of CYP enzymes, as this could alter the metabolism and kinetics of the antipsychotic and result in ADRs such as priapism. For example, drug interactions exist between strong CYP2D6 inhibitors—such as the antidepressants paroxetine, fluoxetine, and bupropion—and antipsychotics that are substrates of CYP2D6, such as risperidone, aripiprazole, haloperidol, and perphenazine. This interaction can lead to higher levels of the antipsychotic, which would increase the patient’s risk of experiencing ADRs. Because psychotic illnesses and depression/anxiety often coexist, it is not uncommon for individuals with these conditions to be receiving both an antipsychotic and an antidepressant.
Because there is a high incidence of comorbidities such as HIV and cardiovascular disease among individuals with mental illnesses, clinicians must also be cognizant of any nonpsychotropic medications the patient may be taking. For instance, clinically relevant DDIs exist between protease inhibitors, such as ritonavir, a strong CYP3A4 inhibitor, and antipsychotics that are substrates of CYP3A4, such as pimozide, aripiprazole, and quetiapine.
Mitigating the risk of priapism
Although there are associated risk factors for priapism, there are no concrete indicators to predict the onset or development of the condition. The best predictor may be a history of prolonged and painless erections.3
As such, when choosing an antipsychotic, it is critical to screen the patient for the previously mentioned risk factors, including the presence of medications with strong alpha-1 receptor affinity and CYP interactions, especially to minimize the risk of recurrence of priapism in those with prior or similar episodes. Management of patients with priapism due to antipsychotics has involved reducing the dose of the offending agent and/or changing the medication to one with a lower alpha-adrenergic affinity (Table 22).
Similar to most situations, management is patient-specific and depends on several factors, including the severity of the patient’s psychiatric disease, history/severity of priapism and treatment, concurrent medication list, etc. For example, although clozapine is considered to have relatively high affinity for the alpha-1 receptor, it is also the agent of choice for treatment-refractory schizophrenia. Risks and benefits must be weighed on a individualized basis. Case reports have described symptom improvement via lowering the dose of clozapine and adding on or switching to an antipsychotic agent with minimal alpha-1 receptor affinity.4
After considering Mr. J’s history, risk factors, and preferences, the treatment team discontinues risperidone and initiates haloperidol, 5 mg twice a day. Soon after, Mr. J no longer experiences priapism.
Mr. J, age 35, is brought to the hospital from prison due to priapism that does not improve with treatment. He says he has had priapism 5 times previously, with the first incidence occurring “years ago” due to trazodone.
Recently, he has been receiving risperidone, which the treatment team believes is the cause of his current priapism. His medical history includes asthma, schizophrenia, hypertension, seizures, and sickle cell trait. Mr. J is experiencing auditory hallucinations, which he describes as “continuous, neutral voices that are annoying.” He would like relief from his auditory hallucinations and is willing to change his antipsychotic, but does not want additional treatment for his priapism. His present medications include risperidone, 1 mg twice a day, escitalopram, 10 mg/d, benztropine, 1 mg twice a day, and phenytoin, 500 mg/d at bedtime.
Priapism is a prolonged, persistent, and often painful erection that occurs without sexual stimulation. Although relatively rare, it can result in potentially serious long-term complications, including impotence and gangrene, and requires immediate evaluation and management.
There are 2 types of priapism: nonischemic, or “high-flow,” priapism, and ischemic, or “low-flow,” priapism (Table 1). While nonischemic priapism is typically caused by penile or perineal trauma, ischemic priapism can occur as a result of medications, including antipsychotics, antidepressants, anxiolytics, and antihypertensives, or hematological conditions such as sickle cell disease.1 Other risk factors associated with priapism include substance abuse, hyperprolactinemia, diabetes, and liver disease.4

Antipsychotic-induced priapism
Medication-induced priapism is a rare adverse drug reaction (ADR). Of the medication classes associated with priapism, antipsychotics have the highest incidence and account for approximately 20% of all cases.1
The mechanism of priapism associated with antipsychotics is thought to be related to alpha-1 blockade in the corpora cavernosa of the penis. Although antipsychotics within each class share common characteristics, each agent has a unique profile of receptor affinities. As such, antipsychotics have varying affinities for the alpha-adrenergic receptor (Table 2). Agents such as ziprasidone, chlorpromazine, and risperidone—which have the highest affinity for the alpha-1 adrenoceptors—may be more likely to cause priapism compared with agents with lower affinity, such as olanzapine. Priapism may occur at any time during antipsychotic treatment, and does not appear to be dose-related.

Continue to: Antipsychotic drug interactions and priapism...
Antipsychotic drug interactions and priapism
Patients who are receiving multiple medications as treatment for chronic medical or psychiatric conditions have an increased likelihood of experiencing drug-drug interactions (DDIs) that lead to adverse effects.
Various case reports have described priapism as a result of DDIs related to antipsychotic agents combined with other psychotropic or nonpsychotropic medications.3 Most of these DDIs have been attributed to the cytochrome P450 (CYP) family of enzymes, including CYP2D6, CYP1A2, and CYP3A4/5, which are major enzymes implicated in the metabolism of antipsychotics (Table 3).

It is imperative to be vigilant during the concomitant administration of antipsychotics with other medications that may be substrates, inducers, or inhibitors of CYP enzymes, as this could alter the metabolism and kinetics of the antipsychotic and result in ADRs such as priapism. For example, drug interactions exist between strong CYP2D6 inhibitors—such as the antidepressants paroxetine, fluoxetine, and bupropion—and antipsychotics that are substrates of CYP2D6, such as risperidone, aripiprazole, haloperidol, and perphenazine. This interaction can lead to higher levels of the antipsychotic, which would increase the patient’s risk of experiencing ADRs. Because psychotic illnesses and depression/anxiety often coexist, it is not uncommon for individuals with these conditions to be receiving both an antipsychotic and an antidepressant.
Because there is a high incidence of comorbidities such as HIV and cardiovascular disease among individuals with mental illnesses, clinicians must also be cognizant of any nonpsychotropic medications the patient may be taking. For instance, clinically relevant DDIs exist between protease inhibitors, such as ritonavir, a strong CYP3A4 inhibitor, and antipsychotics that are substrates of CYP3A4, such as pimozide, aripiprazole, and quetiapine.
Mitigating the risk of priapism
Although there are associated risk factors for priapism, there are no concrete indicators to predict the onset or development of the condition. The best predictor may be a history of prolonged and painless erections.3
As such, when choosing an antipsychotic, it is critical to screen the patient for the previously mentioned risk factors, including the presence of medications with strong alpha-1 receptor affinity and CYP interactions, especially to minimize the risk of recurrence of priapism in those with prior or similar episodes. Management of patients with priapism due to antipsychotics has involved reducing the dose of the offending agent and/or changing the medication to one with a lower alpha-adrenergic affinity (Table 22).
Similar to most situations, management is patient-specific and depends on several factors, including the severity of the patient’s psychiatric disease, history/severity of priapism and treatment, concurrent medication list, etc. For example, although clozapine is considered to have relatively high affinity for the alpha-1 receptor, it is also the agent of choice for treatment-refractory schizophrenia. Risks and benefits must be weighed on a individualized basis. Case reports have described symptom improvement via lowering the dose of clozapine and adding on or switching to an antipsychotic agent with minimal alpha-1 receptor affinity.4
After considering Mr. J’s history, risk factors, and preferences, the treatment team discontinues risperidone and initiates haloperidol, 5 mg twice a day. Soon after, Mr. J no longer experiences priapism.
1. Weiner DM, Lowe FC. Psychotropic drug-induced priapism. Mol Diag Ther 9. 1998;371-379. doi:10.2165/00023210-199809050-00004
2. Andersohn F, Schmedt N, Weinmann S, et al. Priapism associated with antipsychotics: role of alpha1 adrenoceptor affinity. J Clin Psychopharmacol. 2010;30(1):68-71. doi:10.1097/JCP.0b013e3181c8273d
3. Sood S, James W, Bailon MJ. Priapism associated with atypical antipsychotic medications: a review. Int Clin Psychopharmacol. 2008;23(1):9-17.
4. Sinkeviciute I, Kroken RA, Johnsen E. Priapism in antipsychotic drug use: a rare but important side effect. Case Rep Psychiatry. 2012;2012:496364. doi:10.1155/2012/496364
5. Mora F, Martín JDD, Zubillaga E, et al. CYP450 and its implications in the clinical use of antipsychotic drugs. Clin Exp Pharmacol. 2015;5(176):1-10. doi:10.4172/2161-1459.1000176
6. Puangpetch A, Vanwong N, Nuntamool N, et al. CYP2D6 polymorphisms and their influence on risperidone treatment. Pharmgenomics Pers Med. 2016;9:131-147. doi:10.2147/PGPM.S107772
1. Weiner DM, Lowe FC. Psychotropic drug-induced priapism. Mol Diag Ther 9. 1998;371-379. doi:10.2165/00023210-199809050-00004
2. Andersohn F, Schmedt N, Weinmann S, et al. Priapism associated with antipsychotics: role of alpha1 adrenoceptor affinity. J Clin Psychopharmacol. 2010;30(1):68-71. doi:10.1097/JCP.0b013e3181c8273d
3. Sood S, James W, Bailon MJ. Priapism associated with atypical antipsychotic medications: a review. Int Clin Psychopharmacol. 2008;23(1):9-17.
4. Sinkeviciute I, Kroken RA, Johnsen E. Priapism in antipsychotic drug use: a rare but important side effect. Case Rep Psychiatry. 2012;2012:496364. doi:10.1155/2012/496364
5. Mora F, Martín JDD, Zubillaga E, et al. CYP450 and its implications in the clinical use of antipsychotic drugs. Clin Exp Pharmacol. 2015;5(176):1-10. doi:10.4172/2161-1459.1000176
6. Puangpetch A, Vanwong N, Nuntamool N, et al. CYP2D6 polymorphisms and their influence on risperidone treatment. Pharmgenomics Pers Med. 2016;9:131-147. doi:10.2147/PGPM.S107772
Depressed and awkward: Is it more than that?
CASE Treatment-resistant MDD
Ms. P, age 21, presents to the outpatient clinic. She has diagnoses of treatment-resistant major depressive disorder (MDD) and schizoid personality disorder (SPD). Ms. P was diagnosed with MDD 3 years ago after reporting symptoms of prevailing sadness for approximately 8 years, described as feelings of worthlessness, anhedonia, social withdrawal, and decreased hygiene and self-care behaviors, as well as suicidal ideation and self-harm. SPD was diagnosed 1 year earlier based on her “odd” behaviors and disheveled appearance following observation and in collateral with her family. Her odd behaviors are described as spending most of her time alone, preferring solitary activities, and having little contact with people other than her parents.
Ms. P reports that she was previously treated with citalopram, 20 mg/d, bupropion, 150 mg/d, aripiprazole, 3.75 mg/d, topiramate, 100 mg twice daily, and melatonin, 9 mg/d at bedtime, but discontinued follow-up appointments and medications after no significant improvement in symptoms.
[polldaddy:11027942]
The authors’ observations
The term “schizoid” first made its debut in the medical community to describe the prodromal social withdrawal and isolation observed in schizophrenia.1 The use of schizoid to describe a personality type first occurred in DSM-III in 1980.2 SPD is a Cluster A personality disorder that groups personalities characterized by common traits that are “odd” or “eccentric” and may resemble the positive and/or negative symptoms of schizophrenia.3,4 Relatively uncommon in clinical settings, SPD includes individuals who do not desire or enjoy close relationships. Those afflicted with SPD will be described as isolated, aloof, and detached from social relationships with others, even immediate family members. Individuals with SPD may appear indifferent to criticism and praise, and may take pleasure in only a few activities. They may exhibit a general absence of affective range, which contributes to their characterization as flat, blunted, or emotionally vacant. SPD is more commonly diagnosed in males and may be present in childhood and adolescence. These children are typified by solitariness, poor peer relationships, and underachievement in school. SPD impacts 3.1% to 4.9% of the United States population and approximately 1% of community populations.5,6
EVALUATION Persistent depressive symptoms
Ms. P is accompanied by her parents for the examination. She reports a chronic, persistent sad mood, hopelessness, anergia, insomnia, anhedonia, and decreased concentration and appetite. She says she experiences episodes of intense worry, along with tension, restlessness, feelings of being on the edge, irritability, and difficulty relaxing. Socially, she is withdrawn, preferring to stay alone in her room most of the day watching YouTube or trying to write stories. She has 2 friends with whom she does not interact with in person, but rather through digital means. Ms. P has never enjoyed attending school and feels “nervous” when she is around people. She has difficulty expressing her thoughts and often looks to her parents for help. Her parents add that getting Ms. P to attend school was a struggle, which resulted in periods of home schooling throughout high school.
The treating team prescribes citalopram, 10 mg/d, and aripiprazole, 2 mg/d. On subsequent follow-up visits, Ms. P’s depression improves with an increase in citalopram to 40 mg/d. Psychotherapy is added to her treatment plan to help address the persistent social deficits, odd behavior, and anxieties.
Continue to: Evaluation Psychological assessment...
EVALUATION Psychological assessment
At her psychotherapy intake appointment with the clinical neuropsychologist, Ms. P is dressed in purple from head to toe and sits clutching her purse and looking at the ground. She is overweight with clean, fitting clothing. Ms. P takes a secondary role during most of the interview, allowing her parents to answer most questions. When asked why she is starting therapy, Ms. P replies, “Well, I’ve been using the bathroom a lot.” She describes a feeling of comfort and calmness while in the restroom. Suddenly, she asks her parents to exit the exam room for a moment. Once they leave, she leans in and whispers, “Have you ever heard of self-sabotage? I think that’s what I’m doing.”
Her mood is euthymic, with a blunted affect. She scores 2 on the Patient Health Questionnaire-9 (PHQ-9) and 10 on the Generalized Anxiety Disorder 7-item scale (GAD-7), which indicates the positive impact of medication on her depressive symptoms but continuing moderate anxious distress. She endorses fear of the night, insomnia, and suicidal ideation. She reports an unusual “constant itching sensation,” resulting in hours of repetitive excoriation. Physical examination reveals several significant scars and scabs covering her bilateral upper and lower extremities. Her vocational history is brief; she had held 2 entry-level customer service positions that lasted <1 year. She was fired due to excessive bathroom use.
As the interview progresses, the intake clinician’s background in neuropsychological assessment facilitates screening for possible developmental disorders. Given the nature of the referral and psychotherapy intake, a full neuropsychological assessment is not conducted. The clinician emphasizes verbal abstraction and theory of mind. Ms. P’s IQ was estimated to be average by Wide Range Achievement Test 4 word reading and interview questions about her academic history. Questions are abstracted from the Autism Diagnostic Observation Schedule, Module 4, to assess for conversation ability, emotional insight, awareness and expression, relationships, and areas of functioning in daily living. Developmental history questions, such as those found on the Adaptive Behavior Assessment System, 3rd edition, help guide developmental information provided by parents in the areas of communication, emotion and eye-gaze, gestures, sensory function, language, social functioning, hygiene behavior, and specific interests.
Ms. P’s mother describes a normal pregnancy and delivery; however, she states that Ms. P was “born with problems,” including difficulty with rooting and sucking, and required gastrointestinal intubation until age 3. Cyclical vomiting followed normal food consumption. Ambulation, language acquisition, toilet training, and hygiene behavior were delayed. Ms. P experienced improvements with early intervention in intensive physical and occupational therapy.
Ms. P’s hygiene is well below average, and she requires cueing from her parents. She attended general education until she reached high school, when she began special education. She was sensitive to sensory stimulation from infancy, with sensory sensitivity to textures. Ms. P continues to report sensory sensitivity and lapses in hygiene.
She has difficulty establishing and maintaining relationships with her peers, and prefers solitary activities. Ms. P has no history of romantic relationships, although she does desire one. When asked about her understanding of various relationships, Ms. P’s responses are stereotyped, such as “I know someone is my friend because they are nice to me” and “People get married because they love each other.” She struggles to offer greater insight into the nuances that form lasting relationships and bonds. Ms. P struggles to imitate and describe the physical and internal cues of several basic emotions (eg, fear, joy, anger).
Her conversational and social skills are assessed by asking her to engage in a conversation with the examiner as if meeting for the first time. Her speech is reciprocal, aprosodic, and delayed. The conversation is one-sided, and the examiner fills in several awkward pauses. Ms. P’s gaze at times is intense and prolonged, especially when responding to questions. She tends to use descriptive statements (eg, “I like your purple pen, I like your shirt”) to engage in conversation, rather than gathering more information through reflective statements, questions, or expressing a shared interest.
Ms. P’s verbal abstraction is screened using questions from the Wechsler Adult Intelligence Scale, 4th edition Similarities subtest, to which she provides several responses within normal limits. Her understanding of colloquial speech is assessed by asking her the meaning of common phrases (eg, “Get knocked down 9 times, get up 10,” “Jack and Jill are 2 peas in a pod”). On many occasions, she is able to limit her response to 1 word, (eg, “resiliency”), demonstrating intact ability to decipher idioms.
[polldaddy:11027971]
The authors’ observations
Upon reflection of Ms. P’s clinical presentation and history of developmental delays, social deficits, sensory sensitivity since infancy, and repetitive behaviors (all which continue to impact her), the clinical team concluded that the diagnosis of autism spectrum disorder (ASD) helps explain the patient’s “odd” behaviors, more so than SPD.
ASD is a heterogenous, complex neuropsychiatric disorder characterized by a persistent deficit in social reciprocity, verbal, and nonverbal communication, and includes a pattern of restricted, repetitive and/or stereotyped behaviors and/or interests.5 The term “autismus” is Greek meaning “self,” and was first used to classify the qualities of “morbid self-admiration” observed in prodromal schizophrenia.7

To properly distinguish these disorders, keep in mind that patients with ASD have repetitive and restricted patterns of behaviors or interests that are not found in SPD, and experience deficits in forming, maintaining, and understanding relationships since they lack those skills, while patients with SPD are more prone to desire solitary activities and limited relationships.5,9
There has been an increased interest in determining why for some patients the diagnosis of ASD is delayed until they reach adulthood. Limited or no access to the patient’s childhood caregiver to obtain a developmental history, as well as generational differences on what constitutes typical childhood behavior, could contribute to a delayed diagnosis of ASD until adulthood. Some patients develop camouflaging strategies that allow them to navigate social expectations to a limited degree, such as learning stock phrases, imitating gestures, and telling anecdotes. Another factor to consider is that co-occurring psychiatric disorders may take center stage when patients present for mental health services.10 Fusar-Poli et al11 investigated the characteristics of patients who received a diagnosis of ASD in adulthood. They found that the median time from the initial clinical evaluation to diagnosis of ASD in adulthood was 11 years. In adults identified with ASD, their cognitive abilities ranged from average to above average, and they required less support. Additionally, they also had higher rates of being previously diagnosed with psychotic disorders and personality disorders.11
It is important to keep in mind that the wide spectrum of autism as currently defined by DSM-5 and its overlap of symptoms with other psychiatric disorders can make the diagnosis challenging for both child and adolescent psychiatrists and adult psychiatrists and might help explain why severe cases of ASD are more readily identified earlier than milder cases of ASD.10
Ms. P’s case is also an example of how women are more likely than men to be overlooked when evaluated for ASD. According to DSM-5, the estimated gender ratio for ASD is believed to be 4:1 (male:female).5 However, upon systematic review and meta-analysis, Loomes et al12 found that the gender ratio may be closer to 3:1 (male:female). These authors suggested that diagnostic bias and a failure of passive case ascertainment to estimate gender ratios as stated by DSM-5 in identifying ASD might explain the lower gender ratio.12 A growing body of evidence suggests that ASD is different in males and females. A 2019 qualitative study by Milner et al13 found that female participants reported using masking and camouflaging strategies to appear neurotypical. Compensatory behaviors were found to be linked to a delay in diagnosis and support for ASD.13
Cognitive ability as measured by IQ has also been found to be a factor in receiving a diagnosis of ASD. In a 2010 secondary analysis of a population-based study of the prevalence of ASD, Giarelli et al14found that girls with cognitive impairments as measured by IQ were less likely to be diagnosed with ASD than boys with cognitive impairment, despite meeting the criteria for ASD. Females tend to exhibit fewer repetitive behaviors than males, and tend to be more likely to show accompanying intellectual disability, which suggests that females with ASD may go unrecognized when they exhibit average intelligence with less impairment of behavior and subtler manifestation of social and communication deficits.15 Consequently, females tend to receive this diagnosis later than males.
Continue to: Treatment...
TREATMENT Adding CBT
At an interdisciplinary session several weeks later that includes Ms. P and her parents, the treatment team discusses the revised diagnoses of ASD and MDD, a treatment recommendation for cognitive-behavioral therapy (CBT), and continued use of medication. At this session, Ms. P discloses that she has not been consistent with her medication regimen since her last appointment, which helps explain the increase in her PHQ-9 score from 2 to 14 and GAD-7 score
[polldaddy:11027990]
The authors’ observations
CBT can be helpful in improving medication adherence, developing coping skills, and modifying maladaptive behaviors.

OUTCOME Improvement with psychotherapy
Ms. P and family agree with the team’s recommendations. The aims of Ms. P’s psychotherapy are to maintain medication compliance; implement behavioral modification, vocational rehabilitation, and community engagement; develop social skills; increase functional independence; and develop coping skills for depression and anxiety.
Bottom Line
The prevalence of schizoid personality disorder (SPD) is low, and its symptoms overlap with those of autism spectrum disorder. Therefore, before diagnosing SPD in an adult patient, it is important to obtain a detailed developmental history and include an interdisciplinary team to assess for autism spectrum disorder.
1. Fariba K, Gupta V. Schizoid personality disorder. StatPearls Publishing. Updated June 9, 2021. Accessed January 6, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559234/
2. Diagnostic and Statistical Manual of Mental Disorders: DSM-III. 3rd ed rev. American Psychiatric Association; 1987.
3. Esterberg ML, Goulding SM, Walker EF. Cluster A personality disorders: schizotypal, schizoid and paranoid personality disorders in childhood and adolescence. J Psychopathol Behav Assess. 2010;32(4):515-528. doi:10.1007/s10862-010-9183-8
4. Kalus O, Bernstein DP, Siever LJ. Schizoid personality disorder: a review of current status and implications for DSM-IV. Journal of Personality Disorders. 1993;7(1), 43-52.
5. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; 2013.
6. Eaton NR, Greene AL. Personality disorders: community prevalence and socio-demographic correlates. Curr Opin Psychol. 2018;21:28-32. doi:10.1016/j.copsyc.2017.09.001
7. Vatano
8. Ritsner MS. Handbook of Schizophrenia Spectrum Disorders, Volume I: Conceptual Issues and Neurobiological Advances. Springer; 2011.
9. Cook ML, Zhang Y, Constantino JN. On the continuity between autistic and schizoid personality disorder trait burden: a prospective study in adolescence. J Nerv Ment Dis. 2020;208(2):94-100. doi:10.1097/NMD.0000000000001105
10. Lai MC, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry. 2015;2(11):1013-1027. doi:10.1016/S2215-0366(15)00277-1
11. Fusar-Poli L, Brondino N, Politi P, et al. Missed diagnoses and misdiagnoses of adults with autism spectrum disorder. Eur Arch Psychiatry Clin Neurosci. 2020;10.1007/s00406-020-01189-2. doi:10.1007/s00406-020-01189-w
12. Loomes R, Hull L, Mandy WPL. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2017;56(6):466-474. doi:10.1016/j.jaac.2017.03.013
13. Milner V, McIntosh H, Colvert E, et al. A qualitative exploration of the female experience of autism spectrum disorder (ASD). J Autism Dev Disord. 2019;49(6):2389-2402. doi:10.1007/s10803-019-03906-4
14. Giarelli E, Wiggins LD, Rice CE, et al. Sex differences in the evaluation and diagnosis of autism spectrum disorders among children. Disabil Health J. 2010;3(2):107-116. doi:10.1016/j.dhjo.2009.07.001
15. Frazier TW, Georgiades S, Bishop SL, et al. Behavioral and cognitive characteristics of females and males with autism in the Simons Simplex Collection. J Am Acad Child Adolesc Psychiatry. 2014;53(3):329-40.e403. doi:10.1016/j.jaac.2013.12.004
16. Julius RJ, Novitsky MA Jr, et al. Medication adherence: a review of the literature and implications for clinical practice. J Psychiatr Pract. 2009;15(1):34-44. doi:10.1097/01.pra.0000344917.43780.77
17. Spain D, Sin J, Chalder T, et al. Cognitive behaviour therapy for adults with autism spectrum disorders and psychiatric co-morbidity: a review. Research in Autism Spectrum Disorders. 2015;9, 151-162. https://doi.org/10.1016/j.rasd.2014.10.019
18. Bishop-Fitzpatrick L, Minshew NJ, Eack SM. A systematic review of psychosocial interventions for adults with autism spectrum disorders. J Autism Dev Disord. 2013;43(3):687-694. doi:10.1007/s10803-012-1615-8
CASE Treatment-resistant MDD
Ms. P, age 21, presents to the outpatient clinic. She has diagnoses of treatment-resistant major depressive disorder (MDD) and schizoid personality disorder (SPD). Ms. P was diagnosed with MDD 3 years ago after reporting symptoms of prevailing sadness for approximately 8 years, described as feelings of worthlessness, anhedonia, social withdrawal, and decreased hygiene and self-care behaviors, as well as suicidal ideation and self-harm. SPD was diagnosed 1 year earlier based on her “odd” behaviors and disheveled appearance following observation and in collateral with her family. Her odd behaviors are described as spending most of her time alone, preferring solitary activities, and having little contact with people other than her parents.
Ms. P reports that she was previously treated with citalopram, 20 mg/d, bupropion, 150 mg/d, aripiprazole, 3.75 mg/d, topiramate, 100 mg twice daily, and melatonin, 9 mg/d at bedtime, but discontinued follow-up appointments and medications after no significant improvement in symptoms.
[polldaddy:11027942]
The authors’ observations
The term “schizoid” first made its debut in the medical community to describe the prodromal social withdrawal and isolation observed in schizophrenia.1 The use of schizoid to describe a personality type first occurred in DSM-III in 1980.2 SPD is a Cluster A personality disorder that groups personalities characterized by common traits that are “odd” or “eccentric” and may resemble the positive and/or negative symptoms of schizophrenia.3,4 Relatively uncommon in clinical settings, SPD includes individuals who do not desire or enjoy close relationships. Those afflicted with SPD will be described as isolated, aloof, and detached from social relationships with others, even immediate family members. Individuals with SPD may appear indifferent to criticism and praise, and may take pleasure in only a few activities. They may exhibit a general absence of affective range, which contributes to their characterization as flat, blunted, or emotionally vacant. SPD is more commonly diagnosed in males and may be present in childhood and adolescence. These children are typified by solitariness, poor peer relationships, and underachievement in school. SPD impacts 3.1% to 4.9% of the United States population and approximately 1% of community populations.5,6
EVALUATION Persistent depressive symptoms
Ms. P is accompanied by her parents for the examination. She reports a chronic, persistent sad mood, hopelessness, anergia, insomnia, anhedonia, and decreased concentration and appetite. She says she experiences episodes of intense worry, along with tension, restlessness, feelings of being on the edge, irritability, and difficulty relaxing. Socially, she is withdrawn, preferring to stay alone in her room most of the day watching YouTube or trying to write stories. She has 2 friends with whom she does not interact with in person, but rather through digital means. Ms. P has never enjoyed attending school and feels “nervous” when she is around people. She has difficulty expressing her thoughts and often looks to her parents for help. Her parents add that getting Ms. P to attend school was a struggle, which resulted in periods of home schooling throughout high school.
The treating team prescribes citalopram, 10 mg/d, and aripiprazole, 2 mg/d. On subsequent follow-up visits, Ms. P’s depression improves with an increase in citalopram to 40 mg/d. Psychotherapy is added to her treatment plan to help address the persistent social deficits, odd behavior, and anxieties.
Continue to: Evaluation Psychological assessment...
EVALUATION Psychological assessment
At her psychotherapy intake appointment with the clinical neuropsychologist, Ms. P is dressed in purple from head to toe and sits clutching her purse and looking at the ground. She is overweight with clean, fitting clothing. Ms. P takes a secondary role during most of the interview, allowing her parents to answer most questions. When asked why she is starting therapy, Ms. P replies, “Well, I’ve been using the bathroom a lot.” She describes a feeling of comfort and calmness while in the restroom. Suddenly, she asks her parents to exit the exam room for a moment. Once they leave, she leans in and whispers, “Have you ever heard of self-sabotage? I think that’s what I’m doing.”
Her mood is euthymic, with a blunted affect. She scores 2 on the Patient Health Questionnaire-9 (PHQ-9) and 10 on the Generalized Anxiety Disorder 7-item scale (GAD-7), which indicates the positive impact of medication on her depressive symptoms but continuing moderate anxious distress. She endorses fear of the night, insomnia, and suicidal ideation. She reports an unusual “constant itching sensation,” resulting in hours of repetitive excoriation. Physical examination reveals several significant scars and scabs covering her bilateral upper and lower extremities. Her vocational history is brief; she had held 2 entry-level customer service positions that lasted <1 year. She was fired due to excessive bathroom use.
As the interview progresses, the intake clinician’s background in neuropsychological assessment facilitates screening for possible developmental disorders. Given the nature of the referral and psychotherapy intake, a full neuropsychological assessment is not conducted. The clinician emphasizes verbal abstraction and theory of mind. Ms. P’s IQ was estimated to be average by Wide Range Achievement Test 4 word reading and interview questions about her academic history. Questions are abstracted from the Autism Diagnostic Observation Schedule, Module 4, to assess for conversation ability, emotional insight, awareness and expression, relationships, and areas of functioning in daily living. Developmental history questions, such as those found on the Adaptive Behavior Assessment System, 3rd edition, help guide developmental information provided by parents in the areas of communication, emotion and eye-gaze, gestures, sensory function, language, social functioning, hygiene behavior, and specific interests.
Ms. P’s mother describes a normal pregnancy and delivery; however, she states that Ms. P was “born with problems,” including difficulty with rooting and sucking, and required gastrointestinal intubation until age 3. Cyclical vomiting followed normal food consumption. Ambulation, language acquisition, toilet training, and hygiene behavior were delayed. Ms. P experienced improvements with early intervention in intensive physical and occupational therapy.
Ms. P’s hygiene is well below average, and she requires cueing from her parents. She attended general education until she reached high school, when she began special education. She was sensitive to sensory stimulation from infancy, with sensory sensitivity to textures. Ms. P continues to report sensory sensitivity and lapses in hygiene.
She has difficulty establishing and maintaining relationships with her peers, and prefers solitary activities. Ms. P has no history of romantic relationships, although she does desire one. When asked about her understanding of various relationships, Ms. P’s responses are stereotyped, such as “I know someone is my friend because they are nice to me” and “People get married because they love each other.” She struggles to offer greater insight into the nuances that form lasting relationships and bonds. Ms. P struggles to imitate and describe the physical and internal cues of several basic emotions (eg, fear, joy, anger).
Her conversational and social skills are assessed by asking her to engage in a conversation with the examiner as if meeting for the first time. Her speech is reciprocal, aprosodic, and delayed. The conversation is one-sided, and the examiner fills in several awkward pauses. Ms. P’s gaze at times is intense and prolonged, especially when responding to questions. She tends to use descriptive statements (eg, “I like your purple pen, I like your shirt”) to engage in conversation, rather than gathering more information through reflective statements, questions, or expressing a shared interest.
Ms. P’s verbal abstraction is screened using questions from the Wechsler Adult Intelligence Scale, 4th edition Similarities subtest, to which she provides several responses within normal limits. Her understanding of colloquial speech is assessed by asking her the meaning of common phrases (eg, “Get knocked down 9 times, get up 10,” “Jack and Jill are 2 peas in a pod”). On many occasions, she is able to limit her response to 1 word, (eg, “resiliency”), demonstrating intact ability to decipher idioms.
[polldaddy:11027971]
The authors’ observations
Upon reflection of Ms. P’s clinical presentation and history of developmental delays, social deficits, sensory sensitivity since infancy, and repetitive behaviors (all which continue to impact her), the clinical team concluded that the diagnosis of autism spectrum disorder (ASD) helps explain the patient’s “odd” behaviors, more so than SPD.
ASD is a heterogenous, complex neuropsychiatric disorder characterized by a persistent deficit in social reciprocity, verbal, and nonverbal communication, and includes a pattern of restricted, repetitive and/or stereotyped behaviors and/or interests.5 The term “autismus” is Greek meaning “self,” and was first used to classify the qualities of “morbid self-admiration” observed in prodromal schizophrenia.7

To properly distinguish these disorders, keep in mind that patients with ASD have repetitive and restricted patterns of behaviors or interests that are not found in SPD, and experience deficits in forming, maintaining, and understanding relationships since they lack those skills, while patients with SPD are more prone to desire solitary activities and limited relationships.5,9
There has been an increased interest in determining why for some patients the diagnosis of ASD is delayed until they reach adulthood. Limited or no access to the patient’s childhood caregiver to obtain a developmental history, as well as generational differences on what constitutes typical childhood behavior, could contribute to a delayed diagnosis of ASD until adulthood. Some patients develop camouflaging strategies that allow them to navigate social expectations to a limited degree, such as learning stock phrases, imitating gestures, and telling anecdotes. Another factor to consider is that co-occurring psychiatric disorders may take center stage when patients present for mental health services.10 Fusar-Poli et al11 investigated the characteristics of patients who received a diagnosis of ASD in adulthood. They found that the median time from the initial clinical evaluation to diagnosis of ASD in adulthood was 11 years. In adults identified with ASD, their cognitive abilities ranged from average to above average, and they required less support. Additionally, they also had higher rates of being previously diagnosed with psychotic disorders and personality disorders.11
It is important to keep in mind that the wide spectrum of autism as currently defined by DSM-5 and its overlap of symptoms with other psychiatric disorders can make the diagnosis challenging for both child and adolescent psychiatrists and adult psychiatrists and might help explain why severe cases of ASD are more readily identified earlier than milder cases of ASD.10
Ms. P’s case is also an example of how women are more likely than men to be overlooked when evaluated for ASD. According to DSM-5, the estimated gender ratio for ASD is believed to be 4:1 (male:female).5 However, upon systematic review and meta-analysis, Loomes et al12 found that the gender ratio may be closer to 3:1 (male:female). These authors suggested that diagnostic bias and a failure of passive case ascertainment to estimate gender ratios as stated by DSM-5 in identifying ASD might explain the lower gender ratio.12 A growing body of evidence suggests that ASD is different in males and females. A 2019 qualitative study by Milner et al13 found that female participants reported using masking and camouflaging strategies to appear neurotypical. Compensatory behaviors were found to be linked to a delay in diagnosis and support for ASD.13
Cognitive ability as measured by IQ has also been found to be a factor in receiving a diagnosis of ASD. In a 2010 secondary analysis of a population-based study of the prevalence of ASD, Giarelli et al14found that girls with cognitive impairments as measured by IQ were less likely to be diagnosed with ASD than boys with cognitive impairment, despite meeting the criteria for ASD. Females tend to exhibit fewer repetitive behaviors than males, and tend to be more likely to show accompanying intellectual disability, which suggests that females with ASD may go unrecognized when they exhibit average intelligence with less impairment of behavior and subtler manifestation of social and communication deficits.15 Consequently, females tend to receive this diagnosis later than males.
Continue to: Treatment...
TREATMENT Adding CBT
At an interdisciplinary session several weeks later that includes Ms. P and her parents, the treatment team discusses the revised diagnoses of ASD and MDD, a treatment recommendation for cognitive-behavioral therapy (CBT), and continued use of medication. At this session, Ms. P discloses that she has not been consistent with her medication regimen since her last appointment, which helps explain the increase in her PHQ-9 score from 2 to 14 and GAD-7 score
[polldaddy:11027990]
The authors’ observations
CBT can be helpful in improving medication adherence, developing coping skills, and modifying maladaptive behaviors.

OUTCOME Improvement with psychotherapy
Ms. P and family agree with the team’s recommendations. The aims of Ms. P’s psychotherapy are to maintain medication compliance; implement behavioral modification, vocational rehabilitation, and community engagement; develop social skills; increase functional independence; and develop coping skills for depression and anxiety.
Bottom Line
The prevalence of schizoid personality disorder (SPD) is low, and its symptoms overlap with those of autism spectrum disorder. Therefore, before diagnosing SPD in an adult patient, it is important to obtain a detailed developmental history and include an interdisciplinary team to assess for autism spectrum disorder.
CASE Treatment-resistant MDD
Ms. P, age 21, presents to the outpatient clinic. She has diagnoses of treatment-resistant major depressive disorder (MDD) and schizoid personality disorder (SPD). Ms. P was diagnosed with MDD 3 years ago after reporting symptoms of prevailing sadness for approximately 8 years, described as feelings of worthlessness, anhedonia, social withdrawal, and decreased hygiene and self-care behaviors, as well as suicidal ideation and self-harm. SPD was diagnosed 1 year earlier based on her “odd” behaviors and disheveled appearance following observation and in collateral with her family. Her odd behaviors are described as spending most of her time alone, preferring solitary activities, and having little contact with people other than her parents.
Ms. P reports that she was previously treated with citalopram, 20 mg/d, bupropion, 150 mg/d, aripiprazole, 3.75 mg/d, topiramate, 100 mg twice daily, and melatonin, 9 mg/d at bedtime, but discontinued follow-up appointments and medications after no significant improvement in symptoms.
[polldaddy:11027942]
The authors’ observations
The term “schizoid” first made its debut in the medical community to describe the prodromal social withdrawal and isolation observed in schizophrenia.1 The use of schizoid to describe a personality type first occurred in DSM-III in 1980.2 SPD is a Cluster A personality disorder that groups personalities characterized by common traits that are “odd” or “eccentric” and may resemble the positive and/or negative symptoms of schizophrenia.3,4 Relatively uncommon in clinical settings, SPD includes individuals who do not desire or enjoy close relationships. Those afflicted with SPD will be described as isolated, aloof, and detached from social relationships with others, even immediate family members. Individuals with SPD may appear indifferent to criticism and praise, and may take pleasure in only a few activities. They may exhibit a general absence of affective range, which contributes to their characterization as flat, blunted, or emotionally vacant. SPD is more commonly diagnosed in males and may be present in childhood and adolescence. These children are typified by solitariness, poor peer relationships, and underachievement in school. SPD impacts 3.1% to 4.9% of the United States population and approximately 1% of community populations.5,6
EVALUATION Persistent depressive symptoms
Ms. P is accompanied by her parents for the examination. She reports a chronic, persistent sad mood, hopelessness, anergia, insomnia, anhedonia, and decreased concentration and appetite. She says she experiences episodes of intense worry, along with tension, restlessness, feelings of being on the edge, irritability, and difficulty relaxing. Socially, she is withdrawn, preferring to stay alone in her room most of the day watching YouTube or trying to write stories. She has 2 friends with whom she does not interact with in person, but rather through digital means. Ms. P has never enjoyed attending school and feels “nervous” when she is around people. She has difficulty expressing her thoughts and often looks to her parents for help. Her parents add that getting Ms. P to attend school was a struggle, which resulted in periods of home schooling throughout high school.
The treating team prescribes citalopram, 10 mg/d, and aripiprazole, 2 mg/d. On subsequent follow-up visits, Ms. P’s depression improves with an increase in citalopram to 40 mg/d. Psychotherapy is added to her treatment plan to help address the persistent social deficits, odd behavior, and anxieties.
Continue to: Evaluation Psychological assessment...
EVALUATION Psychological assessment
At her psychotherapy intake appointment with the clinical neuropsychologist, Ms. P is dressed in purple from head to toe and sits clutching her purse and looking at the ground. She is overweight with clean, fitting clothing. Ms. P takes a secondary role during most of the interview, allowing her parents to answer most questions. When asked why she is starting therapy, Ms. P replies, “Well, I’ve been using the bathroom a lot.” She describes a feeling of comfort and calmness while in the restroom. Suddenly, she asks her parents to exit the exam room for a moment. Once they leave, she leans in and whispers, “Have you ever heard of self-sabotage? I think that’s what I’m doing.”
Her mood is euthymic, with a blunted affect. She scores 2 on the Patient Health Questionnaire-9 (PHQ-9) and 10 on the Generalized Anxiety Disorder 7-item scale (GAD-7), which indicates the positive impact of medication on her depressive symptoms but continuing moderate anxious distress. She endorses fear of the night, insomnia, and suicidal ideation. She reports an unusual “constant itching sensation,” resulting in hours of repetitive excoriation. Physical examination reveals several significant scars and scabs covering her bilateral upper and lower extremities. Her vocational history is brief; she had held 2 entry-level customer service positions that lasted <1 year. She was fired due to excessive bathroom use.
As the interview progresses, the intake clinician’s background in neuropsychological assessment facilitates screening for possible developmental disorders. Given the nature of the referral and psychotherapy intake, a full neuropsychological assessment is not conducted. The clinician emphasizes verbal abstraction and theory of mind. Ms. P’s IQ was estimated to be average by Wide Range Achievement Test 4 word reading and interview questions about her academic history. Questions are abstracted from the Autism Diagnostic Observation Schedule, Module 4, to assess for conversation ability, emotional insight, awareness and expression, relationships, and areas of functioning in daily living. Developmental history questions, such as those found on the Adaptive Behavior Assessment System, 3rd edition, help guide developmental information provided by parents in the areas of communication, emotion and eye-gaze, gestures, sensory function, language, social functioning, hygiene behavior, and specific interests.
Ms. P’s mother describes a normal pregnancy and delivery; however, she states that Ms. P was “born with problems,” including difficulty with rooting and sucking, and required gastrointestinal intubation until age 3. Cyclical vomiting followed normal food consumption. Ambulation, language acquisition, toilet training, and hygiene behavior were delayed. Ms. P experienced improvements with early intervention in intensive physical and occupational therapy.
Ms. P’s hygiene is well below average, and she requires cueing from her parents. She attended general education until she reached high school, when she began special education. She was sensitive to sensory stimulation from infancy, with sensory sensitivity to textures. Ms. P continues to report sensory sensitivity and lapses in hygiene.
She has difficulty establishing and maintaining relationships with her peers, and prefers solitary activities. Ms. P has no history of romantic relationships, although she does desire one. When asked about her understanding of various relationships, Ms. P’s responses are stereotyped, such as “I know someone is my friend because they are nice to me” and “People get married because they love each other.” She struggles to offer greater insight into the nuances that form lasting relationships and bonds. Ms. P struggles to imitate and describe the physical and internal cues of several basic emotions (eg, fear, joy, anger).
Her conversational and social skills are assessed by asking her to engage in a conversation with the examiner as if meeting for the first time. Her speech is reciprocal, aprosodic, and delayed. The conversation is one-sided, and the examiner fills in several awkward pauses. Ms. P’s gaze at times is intense and prolonged, especially when responding to questions. She tends to use descriptive statements (eg, “I like your purple pen, I like your shirt”) to engage in conversation, rather than gathering more information through reflective statements, questions, or expressing a shared interest.
Ms. P’s verbal abstraction is screened using questions from the Wechsler Adult Intelligence Scale, 4th edition Similarities subtest, to which she provides several responses within normal limits. Her understanding of colloquial speech is assessed by asking her the meaning of common phrases (eg, “Get knocked down 9 times, get up 10,” “Jack and Jill are 2 peas in a pod”). On many occasions, she is able to limit her response to 1 word, (eg, “resiliency”), demonstrating intact ability to decipher idioms.
[polldaddy:11027971]
The authors’ observations
Upon reflection of Ms. P’s clinical presentation and history of developmental delays, social deficits, sensory sensitivity since infancy, and repetitive behaviors (all which continue to impact her), the clinical team concluded that the diagnosis of autism spectrum disorder (ASD) helps explain the patient’s “odd” behaviors, more so than SPD.
ASD is a heterogenous, complex neuropsychiatric disorder characterized by a persistent deficit in social reciprocity, verbal, and nonverbal communication, and includes a pattern of restricted, repetitive and/or stereotyped behaviors and/or interests.5 The term “autismus” is Greek meaning “self,” and was first used to classify the qualities of “morbid self-admiration” observed in prodromal schizophrenia.7

To properly distinguish these disorders, keep in mind that patients with ASD have repetitive and restricted patterns of behaviors or interests that are not found in SPD, and experience deficits in forming, maintaining, and understanding relationships since they lack those skills, while patients with SPD are more prone to desire solitary activities and limited relationships.5,9
There has been an increased interest in determining why for some patients the diagnosis of ASD is delayed until they reach adulthood. Limited or no access to the patient’s childhood caregiver to obtain a developmental history, as well as generational differences on what constitutes typical childhood behavior, could contribute to a delayed diagnosis of ASD until adulthood. Some patients develop camouflaging strategies that allow them to navigate social expectations to a limited degree, such as learning stock phrases, imitating gestures, and telling anecdotes. Another factor to consider is that co-occurring psychiatric disorders may take center stage when patients present for mental health services.10 Fusar-Poli et al11 investigated the characteristics of patients who received a diagnosis of ASD in adulthood. They found that the median time from the initial clinical evaluation to diagnosis of ASD in adulthood was 11 years. In adults identified with ASD, their cognitive abilities ranged from average to above average, and they required less support. Additionally, they also had higher rates of being previously diagnosed with psychotic disorders and personality disorders.11
It is important to keep in mind that the wide spectrum of autism as currently defined by DSM-5 and its overlap of symptoms with other psychiatric disorders can make the diagnosis challenging for both child and adolescent psychiatrists and adult psychiatrists and might help explain why severe cases of ASD are more readily identified earlier than milder cases of ASD.10
Ms. P’s case is also an example of how women are more likely than men to be overlooked when evaluated for ASD. According to DSM-5, the estimated gender ratio for ASD is believed to be 4:1 (male:female).5 However, upon systematic review and meta-analysis, Loomes et al12 found that the gender ratio may be closer to 3:1 (male:female). These authors suggested that diagnostic bias and a failure of passive case ascertainment to estimate gender ratios as stated by DSM-5 in identifying ASD might explain the lower gender ratio.12 A growing body of evidence suggests that ASD is different in males and females. A 2019 qualitative study by Milner et al13 found that female participants reported using masking and camouflaging strategies to appear neurotypical. Compensatory behaviors were found to be linked to a delay in diagnosis and support for ASD.13
Cognitive ability as measured by IQ has also been found to be a factor in receiving a diagnosis of ASD. In a 2010 secondary analysis of a population-based study of the prevalence of ASD, Giarelli et al14found that girls with cognitive impairments as measured by IQ were less likely to be diagnosed with ASD than boys with cognitive impairment, despite meeting the criteria for ASD. Females tend to exhibit fewer repetitive behaviors than males, and tend to be more likely to show accompanying intellectual disability, which suggests that females with ASD may go unrecognized when they exhibit average intelligence with less impairment of behavior and subtler manifestation of social and communication deficits.15 Consequently, females tend to receive this diagnosis later than males.
Continue to: Treatment...
TREATMENT Adding CBT
At an interdisciplinary session several weeks later that includes Ms. P and her parents, the treatment team discusses the revised diagnoses of ASD and MDD, a treatment recommendation for cognitive-behavioral therapy (CBT), and continued use of medication. At this session, Ms. P discloses that she has not been consistent with her medication regimen since her last appointment, which helps explain the increase in her PHQ-9 score from 2 to 14 and GAD-7 score
[polldaddy:11027990]
The authors’ observations
CBT can be helpful in improving medication adherence, developing coping skills, and modifying maladaptive behaviors.

OUTCOME Improvement with psychotherapy
Ms. P and family agree with the team’s recommendations. The aims of Ms. P’s psychotherapy are to maintain medication compliance; implement behavioral modification, vocational rehabilitation, and community engagement; develop social skills; increase functional independence; and develop coping skills for depression and anxiety.
Bottom Line
The prevalence of schizoid personality disorder (SPD) is low, and its symptoms overlap with those of autism spectrum disorder. Therefore, before diagnosing SPD in an adult patient, it is important to obtain a detailed developmental history and include an interdisciplinary team to assess for autism spectrum disorder.
1. Fariba K, Gupta V. Schizoid personality disorder. StatPearls Publishing. Updated June 9, 2021. Accessed January 6, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559234/
2. Diagnostic and Statistical Manual of Mental Disorders: DSM-III. 3rd ed rev. American Psychiatric Association; 1987.
3. Esterberg ML, Goulding SM, Walker EF. Cluster A personality disorders: schizotypal, schizoid and paranoid personality disorders in childhood and adolescence. J Psychopathol Behav Assess. 2010;32(4):515-528. doi:10.1007/s10862-010-9183-8
4. Kalus O, Bernstein DP, Siever LJ. Schizoid personality disorder: a review of current status and implications for DSM-IV. Journal of Personality Disorders. 1993;7(1), 43-52.
5. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; 2013.
6. Eaton NR, Greene AL. Personality disorders: community prevalence and socio-demographic correlates. Curr Opin Psychol. 2018;21:28-32. doi:10.1016/j.copsyc.2017.09.001
7. Vatano
8. Ritsner MS. Handbook of Schizophrenia Spectrum Disorders, Volume I: Conceptual Issues and Neurobiological Advances. Springer; 2011.
9. Cook ML, Zhang Y, Constantino JN. On the continuity between autistic and schizoid personality disorder trait burden: a prospective study in adolescence. J Nerv Ment Dis. 2020;208(2):94-100. doi:10.1097/NMD.0000000000001105
10. Lai MC, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry. 2015;2(11):1013-1027. doi:10.1016/S2215-0366(15)00277-1
11. Fusar-Poli L, Brondino N, Politi P, et al. Missed diagnoses and misdiagnoses of adults with autism spectrum disorder. Eur Arch Psychiatry Clin Neurosci. 2020;10.1007/s00406-020-01189-2. doi:10.1007/s00406-020-01189-w
12. Loomes R, Hull L, Mandy WPL. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2017;56(6):466-474. doi:10.1016/j.jaac.2017.03.013
13. Milner V, McIntosh H, Colvert E, et al. A qualitative exploration of the female experience of autism spectrum disorder (ASD). J Autism Dev Disord. 2019;49(6):2389-2402. doi:10.1007/s10803-019-03906-4
14. Giarelli E, Wiggins LD, Rice CE, et al. Sex differences in the evaluation and diagnosis of autism spectrum disorders among children. Disabil Health J. 2010;3(2):107-116. doi:10.1016/j.dhjo.2009.07.001
15. Frazier TW, Georgiades S, Bishop SL, et al. Behavioral and cognitive characteristics of females and males with autism in the Simons Simplex Collection. J Am Acad Child Adolesc Psychiatry. 2014;53(3):329-40.e403. doi:10.1016/j.jaac.2013.12.004
16. Julius RJ, Novitsky MA Jr, et al. Medication adherence: a review of the literature and implications for clinical practice. J Psychiatr Pract. 2009;15(1):34-44. doi:10.1097/01.pra.0000344917.43780.77
17. Spain D, Sin J, Chalder T, et al. Cognitive behaviour therapy for adults with autism spectrum disorders and psychiatric co-morbidity: a review. Research in Autism Spectrum Disorders. 2015;9, 151-162. https://doi.org/10.1016/j.rasd.2014.10.019
18. Bishop-Fitzpatrick L, Minshew NJ, Eack SM. A systematic review of psychosocial interventions for adults with autism spectrum disorders. J Autism Dev Disord. 2013;43(3):687-694. doi:10.1007/s10803-012-1615-8
1. Fariba K, Gupta V. Schizoid personality disorder. StatPearls Publishing. Updated June 9, 2021. Accessed January 6, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559234/
2. Diagnostic and Statistical Manual of Mental Disorders: DSM-III. 3rd ed rev. American Psychiatric Association; 1987.
3. Esterberg ML, Goulding SM, Walker EF. Cluster A personality disorders: schizotypal, schizoid and paranoid personality disorders in childhood and adolescence. J Psychopathol Behav Assess. 2010;32(4):515-528. doi:10.1007/s10862-010-9183-8
4. Kalus O, Bernstein DP, Siever LJ. Schizoid personality disorder: a review of current status and implications for DSM-IV. Journal of Personality Disorders. 1993;7(1), 43-52.
5. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; 2013.
6. Eaton NR, Greene AL. Personality disorders: community prevalence and socio-demographic correlates. Curr Opin Psychol. 2018;21:28-32. doi:10.1016/j.copsyc.2017.09.001
7. Vatano
8. Ritsner MS. Handbook of Schizophrenia Spectrum Disorders, Volume I: Conceptual Issues and Neurobiological Advances. Springer; 2011.
9. Cook ML, Zhang Y, Constantino JN. On the continuity between autistic and schizoid personality disorder trait burden: a prospective study in adolescence. J Nerv Ment Dis. 2020;208(2):94-100. doi:10.1097/NMD.0000000000001105
10. Lai MC, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry. 2015;2(11):1013-1027. doi:10.1016/S2215-0366(15)00277-1
11. Fusar-Poli L, Brondino N, Politi P, et al. Missed diagnoses and misdiagnoses of adults with autism spectrum disorder. Eur Arch Psychiatry Clin Neurosci. 2020;10.1007/s00406-020-01189-2. doi:10.1007/s00406-020-01189-w
12. Loomes R, Hull L, Mandy WPL. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2017;56(6):466-474. doi:10.1016/j.jaac.2017.03.013
13. Milner V, McIntosh H, Colvert E, et al. A qualitative exploration of the female experience of autism spectrum disorder (ASD). J Autism Dev Disord. 2019;49(6):2389-2402. doi:10.1007/s10803-019-03906-4
14. Giarelli E, Wiggins LD, Rice CE, et al. Sex differences in the evaluation and diagnosis of autism spectrum disorders among children. Disabil Health J. 2010;3(2):107-116. doi:10.1016/j.dhjo.2009.07.001
15. Frazier TW, Georgiades S, Bishop SL, et al. Behavioral and cognitive characteristics of females and males with autism in the Simons Simplex Collection. J Am Acad Child Adolesc Psychiatry. 2014;53(3):329-40.e403. doi:10.1016/j.jaac.2013.12.004
16. Julius RJ, Novitsky MA Jr, et al. Medication adherence: a review of the literature and implications for clinical practice. J Psychiatr Pract. 2009;15(1):34-44. doi:10.1097/01.pra.0000344917.43780.77
17. Spain D, Sin J, Chalder T, et al. Cognitive behaviour therapy for adults with autism spectrum disorders and psychiatric co-morbidity: a review. Research in Autism Spectrum Disorders. 2015;9, 151-162. https://doi.org/10.1016/j.rasd.2014.10.019
18. Bishop-Fitzpatrick L, Minshew NJ, Eack SM. A systematic review of psychosocial interventions for adults with autism spectrum disorders. J Autism Dev Disord. 2013;43(3):687-694. doi:10.1007/s10803-012-1615-8
The skill of administering IM medications: 3 questions to consider
The intramuscular (IM) route is commonly used to administer medication in various clinical settings. Even when an IM medication is administered appropriately, patient factors such as high subcutaneous tissue, greater body mass index, and gender can lower the success rate of injections.1 A key but infrequently discussed issue is the skill of the individual administering the IM medication. Incorrectly administering an IM medication can lead to complications, such as abscesses, nerve injury, and skeletal muscle fibrosis.2 Poor IM injection technique can impact patient care and safety.1 For example, a poorly administered antipsychotic medication might lead to the patient receiving a subtherapeutic dose, and could prompt a clinician to ask, “Does this agitated patient need more emergent medication because the medication being given is not effective, or because the medication is not being administered properly?”
This article offers 3 questions to ask when clinicians are evaluating how IM medications are being administered in their clinical setting.
1. Who is administering the medication?
Is the person a registered nurse, licensed psychiatric technician, certified nursing assistant, licensed vocational nurse, or medical assistant? What a specific clinician is permitted to do in one state may not be permitted in another state. For example, in the state of Washington, under certain conditions a medical assistant is allowed to administer an IM medication.3
2. What is the individual’s training in administering IM medications?
Has the person been trained in the proper technique, depending on the body location? Is the injection being properly prepared? Is the correct needle gauge being used?
3. What is the individual’s comfort level with administering IM medications?
Is the person comfortable administering medication only when a patient is calm? Or are they comfortable administering medication when a patient is agitated and being physically held or in 4-point restraints, such as in inpatient psychiatric units or emergency departments?
1. Soliman E, Ranjan S, Xu T, et al. A narrative review of the success of intramuscular gluteal injections and its impact in psychiatry. Biodes Manuf. 2018;1(3):161-170.
2. Nicoll LH, Hesby A. Intramuscular injection: an integrative research review and guideline for evidence-based practice. Appl Nurs Res. 2002;15(3):149-162.
3. Washington State Legislature. WAC 246-827-0240. Medical assistant-certified—Administering medications and injections. Accessed January 10, 2022. https://apps.leg.wa.gov/wac/default.aspx?cite=246-827-0240
The intramuscular (IM) route is commonly used to administer medication in various clinical settings. Even when an IM medication is administered appropriately, patient factors such as high subcutaneous tissue, greater body mass index, and gender can lower the success rate of injections.1 A key but infrequently discussed issue is the skill of the individual administering the IM medication. Incorrectly administering an IM medication can lead to complications, such as abscesses, nerve injury, and skeletal muscle fibrosis.2 Poor IM injection technique can impact patient care and safety.1 For example, a poorly administered antipsychotic medication might lead to the patient receiving a subtherapeutic dose, and could prompt a clinician to ask, “Does this agitated patient need more emergent medication because the medication being given is not effective, or because the medication is not being administered properly?”
This article offers 3 questions to ask when clinicians are evaluating how IM medications are being administered in their clinical setting.
1. Who is administering the medication?
Is the person a registered nurse, licensed psychiatric technician, certified nursing assistant, licensed vocational nurse, or medical assistant? What a specific clinician is permitted to do in one state may not be permitted in another state. For example, in the state of Washington, under certain conditions a medical assistant is allowed to administer an IM medication.3
2. What is the individual’s training in administering IM medications?
Has the person been trained in the proper technique, depending on the body location? Is the injection being properly prepared? Is the correct needle gauge being used?
3. What is the individual’s comfort level with administering IM medications?
Is the person comfortable administering medication only when a patient is calm? Or are they comfortable administering medication when a patient is agitated and being physically held or in 4-point restraints, such as in inpatient psychiatric units or emergency departments?
The intramuscular (IM) route is commonly used to administer medication in various clinical settings. Even when an IM medication is administered appropriately, patient factors such as high subcutaneous tissue, greater body mass index, and gender can lower the success rate of injections.1 A key but infrequently discussed issue is the skill of the individual administering the IM medication. Incorrectly administering an IM medication can lead to complications, such as abscesses, nerve injury, and skeletal muscle fibrosis.2 Poor IM injection technique can impact patient care and safety.1 For example, a poorly administered antipsychotic medication might lead to the patient receiving a subtherapeutic dose, and could prompt a clinician to ask, “Does this agitated patient need more emergent medication because the medication being given is not effective, or because the medication is not being administered properly?”
This article offers 3 questions to ask when clinicians are evaluating how IM medications are being administered in their clinical setting.
1. Who is administering the medication?
Is the person a registered nurse, licensed psychiatric technician, certified nursing assistant, licensed vocational nurse, or medical assistant? What a specific clinician is permitted to do in one state may not be permitted in another state. For example, in the state of Washington, under certain conditions a medical assistant is allowed to administer an IM medication.3
2. What is the individual’s training in administering IM medications?
Has the person been trained in the proper technique, depending on the body location? Is the injection being properly prepared? Is the correct needle gauge being used?
3. What is the individual’s comfort level with administering IM medications?
Is the person comfortable administering medication only when a patient is calm? Or are they comfortable administering medication when a patient is agitated and being physically held or in 4-point restraints, such as in inpatient psychiatric units or emergency departments?
1. Soliman E, Ranjan S, Xu T, et al. A narrative review of the success of intramuscular gluteal injections and its impact in psychiatry. Biodes Manuf. 2018;1(3):161-170.
2. Nicoll LH, Hesby A. Intramuscular injection: an integrative research review and guideline for evidence-based practice. Appl Nurs Res. 2002;15(3):149-162.
3. Washington State Legislature. WAC 246-827-0240. Medical assistant-certified—Administering medications and injections. Accessed January 10, 2022. https://apps.leg.wa.gov/wac/default.aspx?cite=246-827-0240
1. Soliman E, Ranjan S, Xu T, et al. A narrative review of the success of intramuscular gluteal injections and its impact in psychiatry. Biodes Manuf. 2018;1(3):161-170.
2. Nicoll LH, Hesby A. Intramuscular injection: an integrative research review and guideline for evidence-based practice. Appl Nurs Res. 2002;15(3):149-162.
3. Washington State Legislature. WAC 246-827-0240. Medical assistant-certified—Administering medications and injections. Accessed January 10, 2022. https://apps.leg.wa.gov/wac/default.aspx?cite=246-827-0240
Intermittent fasting: What to tell patients
Intermittent fasting is the purposeful, restricted intake of food (and sometimes water), usually for health or religious reasons. Common forms are alternative-day fasting or time-restricted fasting, with variable ratios of days or hours for fasting and eating/drinking.1 For example, fasting during Ramadan, the ninth month of the Islamic calendar, occurs from dawn to sunset, for a variable duration due to latitude and seasonal shifts.2 Clinicians are likely to care for a patient who occasionally fasts. While there are potential benefits of fasting, clinicians need to consider the implications for patients who fast, particularly those receiving psychotropic medications.
Potential benefits for weight loss, mood
Some research suggests fasting is popular and may have benefits for an individual’s physical and mental health. In a 2020 online poll (N = 1,241), 24% of respondents said they had tried intermittent fasting, and 87% said the practice was very effective (50%) or somewhat effective (37%) in helping them lose weight.3 While more randomized control trials are needed to examine the practice’s effectiveness in promoting and maintaining weight loss, fasting has been linked to better glucose control in both humans and animals, and patients may have better adherence with fasting compared to caloric restriction alone.1 Improved mood, alertness, tranquility, and sometimes euphoria have been documented among individuals who fast, but these benefits may not be sustained.4 A prospective study of 462 participants who fasted during Ramadan found the practice reduced depression in patients with diabetes, possibly due to mindfulness, decreased inflammation from improved insulin sensitivity, and/or social cohesion.5
Be aware of the potential risks
Fasting may either improve or destabilize mood in people with bipolar disorder by disrupting circadian rhythm and sleep.2 Fasting might exacerbate underlying eating disorders.2 Increased dehydration escalates the risk for orthostatic hypotension, which might require discontinuing clozapine.6 Hypotension and toxicity might arise during lithium pharmacotherapy. The Table4 summarizes things to consider when caring for a patient who fasts while receiving pharmacotherapy.

Provide patients with guidance
Advise patients not to fast if you believe it might exacerbate their mental illness, and encourage them to discuss with their primary care physicians any potential worsening of physical illnesses.2 When caring for a patient who fasts for religious reasons, consider consulting with the patient’s religious leaders.2 If patients choose to fast, monitor them for mood destabilization and/or medication adverse effects. If possible, avoid altering drug treatment regimens during fasting, and carefully monitor whenever a pharmaceutical change is necessary. When appropriate, the use of long-acting injectable medications may minimize adverse effects while maintaining mood stability. Encourage patients who fast to ensure they remain hydrated and practice sleep hygiene while they fast.7
1. Dong TA, Sandesara PB, Dhindsa DS, et al. Intermittent fasting: a heart healthy dietary pattern? Am J Med. 2020;133(8):901-907.
2. Fond G, Macgregor A, Leboyer M, et al. Fasting in mood disorders: neurobiology and effectiveness. A review of the literature. Psychiatry Res. 2013;209(3):253-258.
3. Ballard J. Americans say this popular diet is effective and inexpensive. YouGov. February 24, 2020. Accessed January 6, 2022. https://today.yougov.com/topics/food/articles-reports/2020/02/24/most-effective-diet-intermittent-fasting-poll
4. Furqan Z, Awaad R, Kurdyak P, et al. Considerations for clinicians treating Muslim patients with psychiatric disorders during Ramadan. Lancet Psychiatry. 2019;6(7):556-557.
5. Al-Ozairi E, AlAwadhi MM, Al-Ozairi A, et al. A prospective study of the effect of fasting during the month of Ramadan on depression and diabetes distress in people with type 2 diabetes. Diabet Res Clin Pract. 2019;153:145-149.
6. Chehovich C, Demler TL, Leppien E. Impact of Ramadan fasting on medical and psychiatric health. Int Clin Psychopharmacol. 2019;34(6):317-322.
7. Farooq S, Nazar Z, Akhtar J, et al. Effect of fasting during Ramadan on serum lithium level and mental state in bipolar affective disorder. Int Clin Psychopharmacol. 2010;25(6):323-327.
Intermittent fasting is the purposeful, restricted intake of food (and sometimes water), usually for health or religious reasons. Common forms are alternative-day fasting or time-restricted fasting, with variable ratios of days or hours for fasting and eating/drinking.1 For example, fasting during Ramadan, the ninth month of the Islamic calendar, occurs from dawn to sunset, for a variable duration due to latitude and seasonal shifts.2 Clinicians are likely to care for a patient who occasionally fasts. While there are potential benefits of fasting, clinicians need to consider the implications for patients who fast, particularly those receiving psychotropic medications.
Potential benefits for weight loss, mood
Some research suggests fasting is popular and may have benefits for an individual’s physical and mental health. In a 2020 online poll (N = 1,241), 24% of respondents said they had tried intermittent fasting, and 87% said the practice was very effective (50%) or somewhat effective (37%) in helping them lose weight.3 While more randomized control trials are needed to examine the practice’s effectiveness in promoting and maintaining weight loss, fasting has been linked to better glucose control in both humans and animals, and patients may have better adherence with fasting compared to caloric restriction alone.1 Improved mood, alertness, tranquility, and sometimes euphoria have been documented among individuals who fast, but these benefits may not be sustained.4 A prospective study of 462 participants who fasted during Ramadan found the practice reduced depression in patients with diabetes, possibly due to mindfulness, decreased inflammation from improved insulin sensitivity, and/or social cohesion.5
Be aware of the potential risks
Fasting may either improve or destabilize mood in people with bipolar disorder by disrupting circadian rhythm and sleep.2 Fasting might exacerbate underlying eating disorders.2 Increased dehydration escalates the risk for orthostatic hypotension, which might require discontinuing clozapine.6 Hypotension and toxicity might arise during lithium pharmacotherapy. The Table4 summarizes things to consider when caring for a patient who fasts while receiving pharmacotherapy.

Provide patients with guidance
Advise patients not to fast if you believe it might exacerbate their mental illness, and encourage them to discuss with their primary care physicians any potential worsening of physical illnesses.2 When caring for a patient who fasts for religious reasons, consider consulting with the patient’s religious leaders.2 If patients choose to fast, monitor them for mood destabilization and/or medication adverse effects. If possible, avoid altering drug treatment regimens during fasting, and carefully monitor whenever a pharmaceutical change is necessary. When appropriate, the use of long-acting injectable medications may minimize adverse effects while maintaining mood stability. Encourage patients who fast to ensure they remain hydrated and practice sleep hygiene while they fast.7
Intermittent fasting is the purposeful, restricted intake of food (and sometimes water), usually for health or religious reasons. Common forms are alternative-day fasting or time-restricted fasting, with variable ratios of days or hours for fasting and eating/drinking.1 For example, fasting during Ramadan, the ninth month of the Islamic calendar, occurs from dawn to sunset, for a variable duration due to latitude and seasonal shifts.2 Clinicians are likely to care for a patient who occasionally fasts. While there are potential benefits of fasting, clinicians need to consider the implications for patients who fast, particularly those receiving psychotropic medications.
Potential benefits for weight loss, mood
Some research suggests fasting is popular and may have benefits for an individual’s physical and mental health. In a 2020 online poll (N = 1,241), 24% of respondents said they had tried intermittent fasting, and 87% said the practice was very effective (50%) or somewhat effective (37%) in helping them lose weight.3 While more randomized control trials are needed to examine the practice’s effectiveness in promoting and maintaining weight loss, fasting has been linked to better glucose control in both humans and animals, and patients may have better adherence with fasting compared to caloric restriction alone.1 Improved mood, alertness, tranquility, and sometimes euphoria have been documented among individuals who fast, but these benefits may not be sustained.4 A prospective study of 462 participants who fasted during Ramadan found the practice reduced depression in patients with diabetes, possibly due to mindfulness, decreased inflammation from improved insulin sensitivity, and/or social cohesion.5
Be aware of the potential risks
Fasting may either improve or destabilize mood in people with bipolar disorder by disrupting circadian rhythm and sleep.2 Fasting might exacerbate underlying eating disorders.2 Increased dehydration escalates the risk for orthostatic hypotension, which might require discontinuing clozapine.6 Hypotension and toxicity might arise during lithium pharmacotherapy. The Table4 summarizes things to consider when caring for a patient who fasts while receiving pharmacotherapy.

Provide patients with guidance
Advise patients not to fast if you believe it might exacerbate their mental illness, and encourage them to discuss with their primary care physicians any potential worsening of physical illnesses.2 When caring for a patient who fasts for religious reasons, consider consulting with the patient’s religious leaders.2 If patients choose to fast, monitor them for mood destabilization and/or medication adverse effects. If possible, avoid altering drug treatment regimens during fasting, and carefully monitor whenever a pharmaceutical change is necessary. When appropriate, the use of long-acting injectable medications may minimize adverse effects while maintaining mood stability. Encourage patients who fast to ensure they remain hydrated and practice sleep hygiene while they fast.7
1. Dong TA, Sandesara PB, Dhindsa DS, et al. Intermittent fasting: a heart healthy dietary pattern? Am J Med. 2020;133(8):901-907.
2. Fond G, Macgregor A, Leboyer M, et al. Fasting in mood disorders: neurobiology and effectiveness. A review of the literature. Psychiatry Res. 2013;209(3):253-258.
3. Ballard J. Americans say this popular diet is effective and inexpensive. YouGov. February 24, 2020. Accessed January 6, 2022. https://today.yougov.com/topics/food/articles-reports/2020/02/24/most-effective-diet-intermittent-fasting-poll
4. Furqan Z, Awaad R, Kurdyak P, et al. Considerations for clinicians treating Muslim patients with psychiatric disorders during Ramadan. Lancet Psychiatry. 2019;6(7):556-557.
5. Al-Ozairi E, AlAwadhi MM, Al-Ozairi A, et al. A prospective study of the effect of fasting during the month of Ramadan on depression and diabetes distress in people with type 2 diabetes. Diabet Res Clin Pract. 2019;153:145-149.
6. Chehovich C, Demler TL, Leppien E. Impact of Ramadan fasting on medical and psychiatric health. Int Clin Psychopharmacol. 2019;34(6):317-322.
7. Farooq S, Nazar Z, Akhtar J, et al. Effect of fasting during Ramadan on serum lithium level and mental state in bipolar affective disorder. Int Clin Psychopharmacol. 2010;25(6):323-327.
1. Dong TA, Sandesara PB, Dhindsa DS, et al. Intermittent fasting: a heart healthy dietary pattern? Am J Med. 2020;133(8):901-907.
2. Fond G, Macgregor A, Leboyer M, et al. Fasting in mood disorders: neurobiology and effectiveness. A review of the literature. Psychiatry Res. 2013;209(3):253-258.
3. Ballard J. Americans say this popular diet is effective and inexpensive. YouGov. February 24, 2020. Accessed January 6, 2022. https://today.yougov.com/topics/food/articles-reports/2020/02/24/most-effective-diet-intermittent-fasting-poll
4. Furqan Z, Awaad R, Kurdyak P, et al. Considerations for clinicians treating Muslim patients with psychiatric disorders during Ramadan. Lancet Psychiatry. 2019;6(7):556-557.
5. Al-Ozairi E, AlAwadhi MM, Al-Ozairi A, et al. A prospective study of the effect of fasting during the month of Ramadan on depression and diabetes distress in people with type 2 diabetes. Diabet Res Clin Pract. 2019;153:145-149.
6. Chehovich C, Demler TL, Leppien E. Impact of Ramadan fasting on medical and psychiatric health. Int Clin Psychopharmacol. 2019;34(6):317-322.
7. Farooq S, Nazar Z, Akhtar J, et al. Effect of fasting during Ramadan on serum lithium level and mental state in bipolar affective disorder. Int Clin Psychopharmacol. 2010;25(6):323-327.
Buprenorphine may curb opioid-induced respiratory depression
High plasma concentrations of buprenorphine may reduce fentanyl-induced respiratory depression, new research suggests.
The primary endpoint measure in a small “proof of principal” pharmacology study was effect of escalating fentanyl dosing on respiratory depression by way of decreased isohypercapnic minute ventilation (VE) – or volume of gas inhaled or exhaled per minute from the lungs.
Results showed the maximum decrease in highest-dose fentanyl-induced VE was almost 50% less for opioid-tolerant patients receiving a 2.0 ng/mL concentration of steady-state plasma buprenorphine than when receiving matching placebo.
Risk for apnea requiring stimulation after fentanyl dosing was also significantly lower with buprenorphine.
“Even though the study is small, a lot of data were collected which will allow us to very accurately predict which plasma concentrations, and therefore drug doses, are needed to protect people adequately in practice,” study coinvestigator Geert Jan Groeneveld, MD, PhD, neurologist and clinical pharmacologist at the Centre for Human Drug Research, Leiden, the Netherlands, and professor of clinical neuropharmacology at Leiden University Medical Center, told this news organization.
He added the “beautiful results” were in line with what the researchers expected and although further research is needed, the study provides a lot of useful information for clinicians.
“I think this is an approach that works, and this study makes that clear,” Dr. Groeneveld added.
The findings were published online Jan. 27, 2022, in PLoS One.
High death rate from synthetic opioids
A recent report from the Centers for Disease Control and Prevention noted that, between June 2020 and June 2021, there were more than 100,000 drug overdose deaths in the United States. Of these, more than 73,000 were attributed to opioids and more than 60,000 to synthetic opioids such as fentanyl.
Most opioid-related overdose deaths in the United States are attributable to synthetic opioids “that can unexpectedly cause respiratory depression by being ingested as a substitute for heroin or with [other] drugs,” Indivior noted in a press release.
Buprenorphine is a partial agonist that “binds with high affinity to mu-opioid receptors but displays partial respiratory depression effects,” the investigators wrote.
As reported by this news organization, the Food and Drug Administration approved buprenorphine extended release (Sublocade, Indivior) in 2017 as the first once-monthly injection for the treatment of opioid use disorder.
In the current study, which was conducted in Leiden, the Netherlands, the investigators used continuous intravenous buprenorphine in order to “mimic” the sustained plasma concentrations of the drug that can be delivered with the long-acting injectable, noted Christian Heidbreder, PhD, chief scientific officer at Indivior.
“This was an experimental medicine study, whereby we used intravenous buprenorphine to really understand the interaction with escalating doses of fentanyl” on respiratory depression, he told this news organization.
Two-part, two-period study
In part A, period one of the two-period crossover study, 14 healthy volunteers were randomly assigned to receive for 360 minutes continuous infusion of 0.02 or 0.05 mg/70 kg per hour of buprenorphine to target plasma concentrations of 0.2 or 0.5 ng/mL, respectively, or matching placebo. In the second period, participants received the alternative infusion – either placebo or the active drug.
In part B, eight opioid-tolerant patients who had used high-dose opioids for at least 3 months prior received a higher infusion rate of 0.1, 0.2, or 0.5 mg/70 kg per hour to target plasma concentrations of 1, 2, or 5 ng/mL, respectively.
The 2 ng/mL “is a very important threshold for us” and the result from several previous experiments, Dr. Heidbreder noted. So the investigators targeted that concentration as well as one below and one “much higher” in the current study.
“Because tolerance to opioid effects is poorly characterized in patients receiving long-term opioids, opioid-tolerant participants in part B had a fixed treatment sequence, receiving placebo infusion plus fentanyl challenges in period 1 to optimize the fentanyl dose escalation before buprenorphine and fentanyl were coadministered in period 2,” the investigators reported.
All participants received up to four escalating doses of intravenous fentanyl after reaching target buprenorphine plasma concentrations.
For healthy volunteers, the planned fentanyl doses were 0.075, 0.15, 0.25, and 0.35 mg/70 kg. For the opioid-tolerant patients, the doses were 0.25, 0.35, 0.5, and 0.7 mg/70 kg.
The infusions began after baseline VE had stabilized at 20 plus or minus 2 L/min, which is about four times above normal resting VE.
First clinical evidence?
Results showed fentanyl-induced adverse changes in VE were less at higher concentrations of buprenorphine plasma.
Opioid-tolerant patients receiving the 2.0 ng/mL concentration of buprenorphine had a 33.7% decrease in highest dose fentanyl-induced VE versus an 82.3% decrease when receiving placebo.
In addition, fentanyl reduced VE up to 49% (95% confidence interval, 21%-76%) in opioid-tolerant patients in all buprenorphine concentration groups combined versus reducing VE up to 100% (95% CI, 68%-132%) during placebo infusion (P = .006).
In addition, buprenorphine was associated with a lower risk versus placebo for apnea requiring verbal stimulation after fentanyl dosing (odds ratio, 0.07; P = .001).
For the healthy volunteers, the first fentanyl bolus reduced VE by 26% for those at target buprenorphine concentration of 0.5 ng/mL versus 51% when receiving placebo (P = .001). The second bolus reduced VE by 47% versus 79%, respectively (P < .001).
“Discontinuations for apnea limited treatment comparisons beyond the second fentanyl injection,” the investigators reported.
Overall, the findings “provide the first clinical evidence that high sustained plasma concentrations of buprenorphine may protect against respiratory depression induced by potent opioids,” they added.
Additional research is now “warranted to assess the competitive interaction of buprenorphine and fentanyl (as well as other illicitly manufactured fentanyl analogs) as we continue to deepen our understanding of buprenorphine as an evidence-based treatment for patients struggling with opioid use disorder,” Dr. Heidbreder said in a press release.
It’s unclear whether the study’s findings are generalizable to other populations, said Dr. Heidbreder.
“So and for that we’ll be using [the injectable] Sublocade as the medication of choice,” said Dr. Heidbreder.
“Conceptually, we feel confident about these data, but now we need to demonstrate what is happening in the real world,” he added.
The study was funded by Indivior. Dr. Groeneveld has reported no relevant financial relationships. Dr. Heidbreder is an employee of Indivior.
A version of this article first appeared on Medscape.com.
High plasma concentrations of buprenorphine may reduce fentanyl-induced respiratory depression, new research suggests.
The primary endpoint measure in a small “proof of principal” pharmacology study was effect of escalating fentanyl dosing on respiratory depression by way of decreased isohypercapnic minute ventilation (VE) – or volume of gas inhaled or exhaled per minute from the lungs.
Results showed the maximum decrease in highest-dose fentanyl-induced VE was almost 50% less for opioid-tolerant patients receiving a 2.0 ng/mL concentration of steady-state plasma buprenorphine than when receiving matching placebo.
Risk for apnea requiring stimulation after fentanyl dosing was also significantly lower with buprenorphine.
“Even though the study is small, a lot of data were collected which will allow us to very accurately predict which plasma concentrations, and therefore drug doses, are needed to protect people adequately in practice,” study coinvestigator Geert Jan Groeneveld, MD, PhD, neurologist and clinical pharmacologist at the Centre for Human Drug Research, Leiden, the Netherlands, and professor of clinical neuropharmacology at Leiden University Medical Center, told this news organization.
He added the “beautiful results” were in line with what the researchers expected and although further research is needed, the study provides a lot of useful information for clinicians.
“I think this is an approach that works, and this study makes that clear,” Dr. Groeneveld added.
The findings were published online Jan. 27, 2022, in PLoS One.
High death rate from synthetic opioids
A recent report from the Centers for Disease Control and Prevention noted that, between June 2020 and June 2021, there were more than 100,000 drug overdose deaths in the United States. Of these, more than 73,000 were attributed to opioids and more than 60,000 to synthetic opioids such as fentanyl.
Most opioid-related overdose deaths in the United States are attributable to synthetic opioids “that can unexpectedly cause respiratory depression by being ingested as a substitute for heroin or with [other] drugs,” Indivior noted in a press release.
Buprenorphine is a partial agonist that “binds with high affinity to mu-opioid receptors but displays partial respiratory depression effects,” the investigators wrote.
As reported by this news organization, the Food and Drug Administration approved buprenorphine extended release (Sublocade, Indivior) in 2017 as the first once-monthly injection for the treatment of opioid use disorder.
In the current study, which was conducted in Leiden, the Netherlands, the investigators used continuous intravenous buprenorphine in order to “mimic” the sustained plasma concentrations of the drug that can be delivered with the long-acting injectable, noted Christian Heidbreder, PhD, chief scientific officer at Indivior.
“This was an experimental medicine study, whereby we used intravenous buprenorphine to really understand the interaction with escalating doses of fentanyl” on respiratory depression, he told this news organization.
Two-part, two-period study
In part A, period one of the two-period crossover study, 14 healthy volunteers were randomly assigned to receive for 360 minutes continuous infusion of 0.02 or 0.05 mg/70 kg per hour of buprenorphine to target plasma concentrations of 0.2 or 0.5 ng/mL, respectively, or matching placebo. In the second period, participants received the alternative infusion – either placebo or the active drug.
In part B, eight opioid-tolerant patients who had used high-dose opioids for at least 3 months prior received a higher infusion rate of 0.1, 0.2, or 0.5 mg/70 kg per hour to target plasma concentrations of 1, 2, or 5 ng/mL, respectively.
The 2 ng/mL “is a very important threshold for us” and the result from several previous experiments, Dr. Heidbreder noted. So the investigators targeted that concentration as well as one below and one “much higher” in the current study.
“Because tolerance to opioid effects is poorly characterized in patients receiving long-term opioids, opioid-tolerant participants in part B had a fixed treatment sequence, receiving placebo infusion plus fentanyl challenges in period 1 to optimize the fentanyl dose escalation before buprenorphine and fentanyl were coadministered in period 2,” the investigators reported.
All participants received up to four escalating doses of intravenous fentanyl after reaching target buprenorphine plasma concentrations.
For healthy volunteers, the planned fentanyl doses were 0.075, 0.15, 0.25, and 0.35 mg/70 kg. For the opioid-tolerant patients, the doses were 0.25, 0.35, 0.5, and 0.7 mg/70 kg.
The infusions began after baseline VE had stabilized at 20 plus or minus 2 L/min, which is about four times above normal resting VE.
First clinical evidence?
Results showed fentanyl-induced adverse changes in VE were less at higher concentrations of buprenorphine plasma.
Opioid-tolerant patients receiving the 2.0 ng/mL concentration of buprenorphine had a 33.7% decrease in highest dose fentanyl-induced VE versus an 82.3% decrease when receiving placebo.
In addition, fentanyl reduced VE up to 49% (95% confidence interval, 21%-76%) in opioid-tolerant patients in all buprenorphine concentration groups combined versus reducing VE up to 100% (95% CI, 68%-132%) during placebo infusion (P = .006).
In addition, buprenorphine was associated with a lower risk versus placebo for apnea requiring verbal stimulation after fentanyl dosing (odds ratio, 0.07; P = .001).
For the healthy volunteers, the first fentanyl bolus reduced VE by 26% for those at target buprenorphine concentration of 0.5 ng/mL versus 51% when receiving placebo (P = .001). The second bolus reduced VE by 47% versus 79%, respectively (P < .001).
“Discontinuations for apnea limited treatment comparisons beyond the second fentanyl injection,” the investigators reported.
Overall, the findings “provide the first clinical evidence that high sustained plasma concentrations of buprenorphine may protect against respiratory depression induced by potent opioids,” they added.
Additional research is now “warranted to assess the competitive interaction of buprenorphine and fentanyl (as well as other illicitly manufactured fentanyl analogs) as we continue to deepen our understanding of buprenorphine as an evidence-based treatment for patients struggling with opioid use disorder,” Dr. Heidbreder said in a press release.
It’s unclear whether the study’s findings are generalizable to other populations, said Dr. Heidbreder.
“So and for that we’ll be using [the injectable] Sublocade as the medication of choice,” said Dr. Heidbreder.
“Conceptually, we feel confident about these data, but now we need to demonstrate what is happening in the real world,” he added.
The study was funded by Indivior. Dr. Groeneveld has reported no relevant financial relationships. Dr. Heidbreder is an employee of Indivior.
A version of this article first appeared on Medscape.com.
High plasma concentrations of buprenorphine may reduce fentanyl-induced respiratory depression, new research suggests.
The primary endpoint measure in a small “proof of principal” pharmacology study was effect of escalating fentanyl dosing on respiratory depression by way of decreased isohypercapnic minute ventilation (VE) – or volume of gas inhaled or exhaled per minute from the lungs.
Results showed the maximum decrease in highest-dose fentanyl-induced VE was almost 50% less for opioid-tolerant patients receiving a 2.0 ng/mL concentration of steady-state plasma buprenorphine than when receiving matching placebo.
Risk for apnea requiring stimulation after fentanyl dosing was also significantly lower with buprenorphine.
“Even though the study is small, a lot of data were collected which will allow us to very accurately predict which plasma concentrations, and therefore drug doses, are needed to protect people adequately in practice,” study coinvestigator Geert Jan Groeneveld, MD, PhD, neurologist and clinical pharmacologist at the Centre for Human Drug Research, Leiden, the Netherlands, and professor of clinical neuropharmacology at Leiden University Medical Center, told this news organization.
He added the “beautiful results” were in line with what the researchers expected and although further research is needed, the study provides a lot of useful information for clinicians.
“I think this is an approach that works, and this study makes that clear,” Dr. Groeneveld added.
The findings were published online Jan. 27, 2022, in PLoS One.
High death rate from synthetic opioids
A recent report from the Centers for Disease Control and Prevention noted that, between June 2020 and June 2021, there were more than 100,000 drug overdose deaths in the United States. Of these, more than 73,000 were attributed to opioids and more than 60,000 to synthetic opioids such as fentanyl.
Most opioid-related overdose deaths in the United States are attributable to synthetic opioids “that can unexpectedly cause respiratory depression by being ingested as a substitute for heroin or with [other] drugs,” Indivior noted in a press release.
Buprenorphine is a partial agonist that “binds with high affinity to mu-opioid receptors but displays partial respiratory depression effects,” the investigators wrote.
As reported by this news organization, the Food and Drug Administration approved buprenorphine extended release (Sublocade, Indivior) in 2017 as the first once-monthly injection for the treatment of opioid use disorder.
In the current study, which was conducted in Leiden, the Netherlands, the investigators used continuous intravenous buprenorphine in order to “mimic” the sustained plasma concentrations of the drug that can be delivered with the long-acting injectable, noted Christian Heidbreder, PhD, chief scientific officer at Indivior.
“This was an experimental medicine study, whereby we used intravenous buprenorphine to really understand the interaction with escalating doses of fentanyl” on respiratory depression, he told this news organization.
Two-part, two-period study
In part A, period one of the two-period crossover study, 14 healthy volunteers were randomly assigned to receive for 360 minutes continuous infusion of 0.02 or 0.05 mg/70 kg per hour of buprenorphine to target plasma concentrations of 0.2 or 0.5 ng/mL, respectively, or matching placebo. In the second period, participants received the alternative infusion – either placebo or the active drug.
In part B, eight opioid-tolerant patients who had used high-dose opioids for at least 3 months prior received a higher infusion rate of 0.1, 0.2, or 0.5 mg/70 kg per hour to target plasma concentrations of 1, 2, or 5 ng/mL, respectively.
The 2 ng/mL “is a very important threshold for us” and the result from several previous experiments, Dr. Heidbreder noted. So the investigators targeted that concentration as well as one below and one “much higher” in the current study.
“Because tolerance to opioid effects is poorly characterized in patients receiving long-term opioids, opioid-tolerant participants in part B had a fixed treatment sequence, receiving placebo infusion plus fentanyl challenges in period 1 to optimize the fentanyl dose escalation before buprenorphine and fentanyl were coadministered in period 2,” the investigators reported.
All participants received up to four escalating doses of intravenous fentanyl after reaching target buprenorphine plasma concentrations.
For healthy volunteers, the planned fentanyl doses were 0.075, 0.15, 0.25, and 0.35 mg/70 kg. For the opioid-tolerant patients, the doses were 0.25, 0.35, 0.5, and 0.7 mg/70 kg.
The infusions began after baseline VE had stabilized at 20 plus or minus 2 L/min, which is about four times above normal resting VE.
First clinical evidence?
Results showed fentanyl-induced adverse changes in VE were less at higher concentrations of buprenorphine plasma.
Opioid-tolerant patients receiving the 2.0 ng/mL concentration of buprenorphine had a 33.7% decrease in highest dose fentanyl-induced VE versus an 82.3% decrease when receiving placebo.
In addition, fentanyl reduced VE up to 49% (95% confidence interval, 21%-76%) in opioid-tolerant patients in all buprenorphine concentration groups combined versus reducing VE up to 100% (95% CI, 68%-132%) during placebo infusion (P = .006).
In addition, buprenorphine was associated with a lower risk versus placebo for apnea requiring verbal stimulation after fentanyl dosing (odds ratio, 0.07; P = .001).
For the healthy volunteers, the first fentanyl bolus reduced VE by 26% for those at target buprenorphine concentration of 0.5 ng/mL versus 51% when receiving placebo (P = .001). The second bolus reduced VE by 47% versus 79%, respectively (P < .001).
“Discontinuations for apnea limited treatment comparisons beyond the second fentanyl injection,” the investigators reported.
Overall, the findings “provide the first clinical evidence that high sustained plasma concentrations of buprenorphine may protect against respiratory depression induced by potent opioids,” they added.
Additional research is now “warranted to assess the competitive interaction of buprenorphine and fentanyl (as well as other illicitly manufactured fentanyl analogs) as we continue to deepen our understanding of buprenorphine as an evidence-based treatment for patients struggling with opioid use disorder,” Dr. Heidbreder said in a press release.
It’s unclear whether the study’s findings are generalizable to other populations, said Dr. Heidbreder.
“So and for that we’ll be using [the injectable] Sublocade as the medication of choice,” said Dr. Heidbreder.
“Conceptually, we feel confident about these data, but now we need to demonstrate what is happening in the real world,” he added.
The study was funded by Indivior. Dr. Groeneveld has reported no relevant financial relationships. Dr. Heidbreder is an employee of Indivior.
A version of this article first appeared on Medscape.com.
FROM PLOS ONE
FDA grants full approval to Moderna COVID-19 vaccine
Moderna announced today that its mRNA COVID-19 vaccine has received full Food and Drug Administration approval for adults 18 years and older.
The move lifts an FDA emergency use authorization for the vaccine, which started Dec. 18, 2020.
The Moderna vaccine also now has a new trade name: Spikevax.
The FDA approval comes a little more than 5 months after the agency granted full approval to the Pfizer/BioNTech COVID-19 vaccine on Aug. 23. At the time, the Pfizer vaccine received the trade name Comirnaty.
The FDA approved the Moderna vaccine based on how well it works and its safety for 6 months after a second dose, including follow-up data from a phase 3 study, Moderna announced this morning through a news release. The FDA also announced the news.
Spikevax is the first Moderna product to be fully licensed in the United States.
The United States joins more than 70 other countries where regulators have approved the vaccine. A total of 807 million doses of Moderna’s COVID-19 vaccine were shipped worldwide in 2021, the company reported.
“The full licensure of Spikevax in the U.S. now joins that in Canada, Japan, the European Union, the U.K., Israel, and other countries, where the adolescent indication is also approved,” Stéphane Bancel, Moderna chief executive officer, said in the release.
A version of this article first appeared on WebMD.com.
Moderna announced today that its mRNA COVID-19 vaccine has received full Food and Drug Administration approval for adults 18 years and older.
The move lifts an FDA emergency use authorization for the vaccine, which started Dec. 18, 2020.
The Moderna vaccine also now has a new trade name: Spikevax.
The FDA approval comes a little more than 5 months after the agency granted full approval to the Pfizer/BioNTech COVID-19 vaccine on Aug. 23. At the time, the Pfizer vaccine received the trade name Comirnaty.
The FDA approved the Moderna vaccine based on how well it works and its safety for 6 months after a second dose, including follow-up data from a phase 3 study, Moderna announced this morning through a news release. The FDA also announced the news.
Spikevax is the first Moderna product to be fully licensed in the United States.
The United States joins more than 70 other countries where regulators have approved the vaccine. A total of 807 million doses of Moderna’s COVID-19 vaccine were shipped worldwide in 2021, the company reported.
“The full licensure of Spikevax in the U.S. now joins that in Canada, Japan, the European Union, the U.K., Israel, and other countries, where the adolescent indication is also approved,” Stéphane Bancel, Moderna chief executive officer, said in the release.
A version of this article first appeared on WebMD.com.
Moderna announced today that its mRNA COVID-19 vaccine has received full Food and Drug Administration approval for adults 18 years and older.
The move lifts an FDA emergency use authorization for the vaccine, which started Dec. 18, 2020.
The Moderna vaccine also now has a new trade name: Spikevax.
The FDA approval comes a little more than 5 months after the agency granted full approval to the Pfizer/BioNTech COVID-19 vaccine on Aug. 23. At the time, the Pfizer vaccine received the trade name Comirnaty.
The FDA approved the Moderna vaccine based on how well it works and its safety for 6 months after a second dose, including follow-up data from a phase 3 study, Moderna announced this morning through a news release. The FDA also announced the news.
Spikevax is the first Moderna product to be fully licensed in the United States.
The United States joins more than 70 other countries where regulators have approved the vaccine. A total of 807 million doses of Moderna’s COVID-19 vaccine were shipped worldwide in 2021, the company reported.
“The full licensure of Spikevax in the U.S. now joins that in Canada, Japan, the European Union, the U.K., Israel, and other countries, where the adolescent indication is also approved,” Stéphane Bancel, Moderna chief executive officer, said in the release.
A version of this article first appeared on WebMD.com.
Will I really feel better if I eat fermented foods?
I’m in a crowded commercial kitchen, and everywhere I look I see bottles of colorful drinks and jars holding faded vegetables suspended in brine. The smell of fermented cabbage permeates the room. I open a mason jar, which lets out a loud hiss. I’d spent months researching the gut-brain axis during my PhD, hoping to understand the role that fermented food may play in our mental health. So I enrolled in a class on how to make fermented foods.
The teacher is praising these ancient foods as a magical cure for every ailment you can imagine. I’m uncomfortable – not because of the smell, but because I’ve never found a scientific article that definitively supported this idea. I’m subconsciously applying a fact filter and wondering what the other unsuspecting students must think. I let this slide, since I’m here to learn the art of fermentation. I bravely take a spoonful of sauerkraut. The salty brine overwhelms my senses. Gulp!
If you’ve ever eaten sauerkraut, kimchi, tempeh, kombucha, or kefir, then you’ve had a fermented food (or drink). The first time I gave them a proper go (with a mind open to enjoying them), I noticed the sour, vinegar-like taste and the noticeable absence of sugar. It didn’t take me long to get used to the taste. After a while of drinking my bubbly kombucha, I noticed that my palate had adapted and sweet flavors felt overpowering.
Fermentation is a natural process of curdling or culturing that has been used for thousands of years to preserve foods. Fermented foods and drinks are made through “desired microbial growth and enzymatic conversions of food components” (as opposed to undesirable microbial growth, which happens when your food spoils). Fermented foods are made either by the bacteria and yeast already present in the environment/food material or by introducing bacteria or yeast to help start the fermentation process.
For example, when I made sauerkraut, I shredded the cabbage, added salt, then pummeled and squeezed the cabbage until it released its own juices, which also allowed the “probiotic” lactic acid bacteria in the cabbage to kickstart the fermentation process. Probiotic bacteria like Lactobacillus and Bifidobacterium are considered probiotic good bugs, and are also present in many yogurts and cheeses.
We can’t necessarily call our sauerkraut a “probiotic food” because we don’t know the exact probiotic strains that are in our sauerkraut and whether they are present in the correct “probiotic” dose. It’s also worth noting that foods and drinks that are produced by fermentation don’t necessarily need to have live bacteria in them when you eat them to still be considered a fermented food. For example, sourdough is born from a bubbly live starter culture that contains yeast and bacteria, but once cooked it might no longer have any live bacteria in it.
So, what about the health claims?
Microbial fermentation may interact with health through multiple different biological pathways. It can enhance the nutritional composition of the final food, create bioactive compounds, and change the composition of the gut microbiota (potentially outcompeting harmful pathogens). The lactic acid bacteria in fermented food might also help to influence your immune system and strengthen your intestinal barrier. Some fermented foods, like tempeh, also contain prebiotics; these are fibers that escape your digestion and are broken down by your gut bacteria, including your lactic acid bacteria, which feed off prebiotic fiber to help grow their colonies. In a recent diet experiment, a high-fiber diet was compared with a diet high in fermented foods (eg, yogurt, fermented vegetables, kefir, fermented cheese); those who ate higher fermented food had lower markers of inflammation and an increased diversity of gut microbiota (which is thought to be a good thing in adults). So, in theory, fermented foods sound good.
Still wanting to understand more, and dispel a few myths, a team of researchers and I investigated what’s known about the link between fermented foods and mental health. We looked at the pathways by which fermented foods might affect mental health, such as by reducing inflammation and strengthening the intestinal barrier. These pathways are relevant because they might reduce your brain’s exposure to certain inflammatory molecules that can impact brain function and mental health.
Fermented foods also contain neurotransmitters that are important to mental health. Research about fermented food and mental health is still in its early infancy. Animal studies provide experimental evidence that fermented foods can help with symptoms of depression and anxiety – but that’s in animals. The problem is in knowing how the animal findings relate to our human experience.
We found eight studies in humans that experimented with fermented foods (for example, fermented milk products) to measure their impact on depression, anxiety, and stress in adults, but the studies were all so different that we were unable to make firm conclusions. It is still difficult to know what the active ingredient in fermented foods is. Is it the microbes? Is it the byproducts? Is it the nutrition? And how much of each is needed, and what are safe levels of each? We really need more studies, with detailed descriptions of exactly what is in each food being tested. At this stage, there is not enough human evidence to make firm clinical recommendations for eating fermented food to improve mental health symptoms.
I’ve since moved on from sauerkraut to making sourdough bread as a COVID lockdown project (as this involves a fermented starter culture). When my delicious fresh bread comes out of the oven, my world is paused for a few minutes, and my family mill around to enjoy the warm, fresh bread. While it may be too soon to tell whether fermented foods help our mental health, my sourdough itself has sure helped us.
Dr. Dawson is a nutritionist and bioinformatician research fellow at the Food & Mood Centre at Deakin University, Geelong, Australia. She disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
I’m in a crowded commercial kitchen, and everywhere I look I see bottles of colorful drinks and jars holding faded vegetables suspended in brine. The smell of fermented cabbage permeates the room. I open a mason jar, which lets out a loud hiss. I’d spent months researching the gut-brain axis during my PhD, hoping to understand the role that fermented food may play in our mental health. So I enrolled in a class on how to make fermented foods.
The teacher is praising these ancient foods as a magical cure for every ailment you can imagine. I’m uncomfortable – not because of the smell, but because I’ve never found a scientific article that definitively supported this idea. I’m subconsciously applying a fact filter and wondering what the other unsuspecting students must think. I let this slide, since I’m here to learn the art of fermentation. I bravely take a spoonful of sauerkraut. The salty brine overwhelms my senses. Gulp!
If you’ve ever eaten sauerkraut, kimchi, tempeh, kombucha, or kefir, then you’ve had a fermented food (or drink). The first time I gave them a proper go (with a mind open to enjoying them), I noticed the sour, vinegar-like taste and the noticeable absence of sugar. It didn’t take me long to get used to the taste. After a while of drinking my bubbly kombucha, I noticed that my palate had adapted and sweet flavors felt overpowering.
Fermentation is a natural process of curdling or culturing that has been used for thousands of years to preserve foods. Fermented foods and drinks are made through “desired microbial growth and enzymatic conversions of food components” (as opposed to undesirable microbial growth, which happens when your food spoils). Fermented foods are made either by the bacteria and yeast already present in the environment/food material or by introducing bacteria or yeast to help start the fermentation process.
For example, when I made sauerkraut, I shredded the cabbage, added salt, then pummeled and squeezed the cabbage until it released its own juices, which also allowed the “probiotic” lactic acid bacteria in the cabbage to kickstart the fermentation process. Probiotic bacteria like Lactobacillus and Bifidobacterium are considered probiotic good bugs, and are also present in many yogurts and cheeses.
We can’t necessarily call our sauerkraut a “probiotic food” because we don’t know the exact probiotic strains that are in our sauerkraut and whether they are present in the correct “probiotic” dose. It’s also worth noting that foods and drinks that are produced by fermentation don’t necessarily need to have live bacteria in them when you eat them to still be considered a fermented food. For example, sourdough is born from a bubbly live starter culture that contains yeast and bacteria, but once cooked it might no longer have any live bacteria in it.
So, what about the health claims?
Microbial fermentation may interact with health through multiple different biological pathways. It can enhance the nutritional composition of the final food, create bioactive compounds, and change the composition of the gut microbiota (potentially outcompeting harmful pathogens). The lactic acid bacteria in fermented food might also help to influence your immune system and strengthen your intestinal barrier. Some fermented foods, like tempeh, also contain prebiotics; these are fibers that escape your digestion and are broken down by your gut bacteria, including your lactic acid bacteria, which feed off prebiotic fiber to help grow their colonies. In a recent diet experiment, a high-fiber diet was compared with a diet high in fermented foods (eg, yogurt, fermented vegetables, kefir, fermented cheese); those who ate higher fermented food had lower markers of inflammation and an increased diversity of gut microbiota (which is thought to be a good thing in adults). So, in theory, fermented foods sound good.
Still wanting to understand more, and dispel a few myths, a team of researchers and I investigated what’s known about the link between fermented foods and mental health. We looked at the pathways by which fermented foods might affect mental health, such as by reducing inflammation and strengthening the intestinal barrier. These pathways are relevant because they might reduce your brain’s exposure to certain inflammatory molecules that can impact brain function and mental health.
Fermented foods also contain neurotransmitters that are important to mental health. Research about fermented food and mental health is still in its early infancy. Animal studies provide experimental evidence that fermented foods can help with symptoms of depression and anxiety – but that’s in animals. The problem is in knowing how the animal findings relate to our human experience.
We found eight studies in humans that experimented with fermented foods (for example, fermented milk products) to measure their impact on depression, anxiety, and stress in adults, but the studies were all so different that we were unable to make firm conclusions. It is still difficult to know what the active ingredient in fermented foods is. Is it the microbes? Is it the byproducts? Is it the nutrition? And how much of each is needed, and what are safe levels of each? We really need more studies, with detailed descriptions of exactly what is in each food being tested. At this stage, there is not enough human evidence to make firm clinical recommendations for eating fermented food to improve mental health symptoms.
I’ve since moved on from sauerkraut to making sourdough bread as a COVID lockdown project (as this involves a fermented starter culture). When my delicious fresh bread comes out of the oven, my world is paused for a few minutes, and my family mill around to enjoy the warm, fresh bread. While it may be too soon to tell whether fermented foods help our mental health, my sourdough itself has sure helped us.
Dr. Dawson is a nutritionist and bioinformatician research fellow at the Food & Mood Centre at Deakin University, Geelong, Australia. She disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
I’m in a crowded commercial kitchen, and everywhere I look I see bottles of colorful drinks and jars holding faded vegetables suspended in brine. The smell of fermented cabbage permeates the room. I open a mason jar, which lets out a loud hiss. I’d spent months researching the gut-brain axis during my PhD, hoping to understand the role that fermented food may play in our mental health. So I enrolled in a class on how to make fermented foods.
The teacher is praising these ancient foods as a magical cure for every ailment you can imagine. I’m uncomfortable – not because of the smell, but because I’ve never found a scientific article that definitively supported this idea. I’m subconsciously applying a fact filter and wondering what the other unsuspecting students must think. I let this slide, since I’m here to learn the art of fermentation. I bravely take a spoonful of sauerkraut. The salty brine overwhelms my senses. Gulp!
If you’ve ever eaten sauerkraut, kimchi, tempeh, kombucha, or kefir, then you’ve had a fermented food (or drink). The first time I gave them a proper go (with a mind open to enjoying them), I noticed the sour, vinegar-like taste and the noticeable absence of sugar. It didn’t take me long to get used to the taste. After a while of drinking my bubbly kombucha, I noticed that my palate had adapted and sweet flavors felt overpowering.
Fermentation is a natural process of curdling or culturing that has been used for thousands of years to preserve foods. Fermented foods and drinks are made through “desired microbial growth and enzymatic conversions of food components” (as opposed to undesirable microbial growth, which happens when your food spoils). Fermented foods are made either by the bacteria and yeast already present in the environment/food material or by introducing bacteria or yeast to help start the fermentation process.
For example, when I made sauerkraut, I shredded the cabbage, added salt, then pummeled and squeezed the cabbage until it released its own juices, which also allowed the “probiotic” lactic acid bacteria in the cabbage to kickstart the fermentation process. Probiotic bacteria like Lactobacillus and Bifidobacterium are considered probiotic good bugs, and are also present in many yogurts and cheeses.
We can’t necessarily call our sauerkraut a “probiotic food” because we don’t know the exact probiotic strains that are in our sauerkraut and whether they are present in the correct “probiotic” dose. It’s also worth noting that foods and drinks that are produced by fermentation don’t necessarily need to have live bacteria in them when you eat them to still be considered a fermented food. For example, sourdough is born from a bubbly live starter culture that contains yeast and bacteria, but once cooked it might no longer have any live bacteria in it.
So, what about the health claims?
Microbial fermentation may interact with health through multiple different biological pathways. It can enhance the nutritional composition of the final food, create bioactive compounds, and change the composition of the gut microbiota (potentially outcompeting harmful pathogens). The lactic acid bacteria in fermented food might also help to influence your immune system and strengthen your intestinal barrier. Some fermented foods, like tempeh, also contain prebiotics; these are fibers that escape your digestion and are broken down by your gut bacteria, including your lactic acid bacteria, which feed off prebiotic fiber to help grow their colonies. In a recent diet experiment, a high-fiber diet was compared with a diet high in fermented foods (eg, yogurt, fermented vegetables, kefir, fermented cheese); those who ate higher fermented food had lower markers of inflammation and an increased diversity of gut microbiota (which is thought to be a good thing in adults). So, in theory, fermented foods sound good.
Still wanting to understand more, and dispel a few myths, a team of researchers and I investigated what’s known about the link between fermented foods and mental health. We looked at the pathways by which fermented foods might affect mental health, such as by reducing inflammation and strengthening the intestinal barrier. These pathways are relevant because they might reduce your brain’s exposure to certain inflammatory molecules that can impact brain function and mental health.
Fermented foods also contain neurotransmitters that are important to mental health. Research about fermented food and mental health is still in its early infancy. Animal studies provide experimental evidence that fermented foods can help with symptoms of depression and anxiety – but that’s in animals. The problem is in knowing how the animal findings relate to our human experience.
We found eight studies in humans that experimented with fermented foods (for example, fermented milk products) to measure their impact on depression, anxiety, and stress in adults, but the studies were all so different that we were unable to make firm conclusions. It is still difficult to know what the active ingredient in fermented foods is. Is it the microbes? Is it the byproducts? Is it the nutrition? And how much of each is needed, and what are safe levels of each? We really need more studies, with detailed descriptions of exactly what is in each food being tested. At this stage, there is not enough human evidence to make firm clinical recommendations for eating fermented food to improve mental health symptoms.
I’ve since moved on from sauerkraut to making sourdough bread as a COVID lockdown project (as this involves a fermented starter culture). When my delicious fresh bread comes out of the oven, my world is paused for a few minutes, and my family mill around to enjoy the warm, fresh bread. While it may be too soon to tell whether fermented foods help our mental health, my sourdough itself has sure helped us.
Dr. Dawson is a nutritionist and bioinformatician research fellow at the Food & Mood Centre at Deakin University, Geelong, Australia. She disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Billionaire Mark Cuban launches online pharmacy for generics
The Mark Cuban Cost Plus Drugs Company (MCCPDC) plans to offer the leukemia therapy imatinib for $47 per month, for example, compared with $120 or more with a common voucher and a retail price of $9,657 per month.
Other examples of lower-priced generics include the ulcerative colitis treatment mesalamine, which goes for $32.40 per month on the new online pharmacy versus $940 per month retail. In addition, the MCCPDC will offer the gout treatment colchicine at a lower price, charging $8.70, compared with $182 per month retail.
Likely in part because of claims of significant cost savings and in part because of Mr. Cuban’s celebrity status, the new venture is getting widespread media attention. Forbes, NPR, and TMZ have shared the news since the new digital pharmacy was announced earlier this month.
The new venture plans to charge consumers 15% above the manufacturing cost for the generic medications, plus a $3 fee for pharmacists and $5 for shipping. People will still require a prescription from their doctor to get the medications.
Generic pricing and social benefit
The top 100 generic products account for about half of generic sales, and there is enough competition for these high-demand medications that “the prices have come down close to zero,” said William Comanor, PhD, a health economist and professor of health policy and management at the University of California, Los Angeles. The remaining generic agents have lower-volume demand.
One prominent example is Daraprim, a decades-old treatment for the life-threatening parasitic infection toxoplasmosis. The drug jumped into the spotlight in 2015 when Martin Shkreli and his company Vyera Pharmaceuticals bought the rights to make the generic drug and raised the price overnight from $13.50 to $750. In January 2022, a U.S. judge banned Mr. Shkreli from the pharmaceutical industry and ordered him to pay an almost $65 million fine.
Dr. Comanor agreed the price should have been raised – $13.50 “was not economically viable” – but not as steep as $750.
“Say Mark Cuban says he will cut the price from $750 to $300. He will still make money. There is a market for these low-volume products,” he said. “There would also be a social benefit.”
A direct-to-consumer digital pharmacy
MCCPDC is “cutting out the middleman” in two ways. The business model calls for charging consumers out of pocket, so insurance companies are not involved. Also, the company created its own pharmacy business manager firm in October 2021, allowing it to negotiate prices with drugmakers in house.
The company also announced plans to complete construction of a 22,000-square-foot pharmaceutical factory in Dallas by the end of 2022.
Reactions on social media ranged from celebratory to people disappointed their generic medication would not cost significantly less or is not provided by the digital pharmacy.
When weighted by the number of prescriptions, prices for generics have declined in the United States.
“Overall, U.S. generic prices are the lowest in the world,” Dr. Comanor said. “People say U.S. drug prices are the highest in the world. That’s true for branded, but it’s not true for generics.
“So if someone asks if U.S. drug prices are the highest or lowest in the world, the answer is both,” he said.
“Maybe there is a role to play for this new pharmacy,” Dr. Comanor said when asked if the initiative seems like a positive development.
The state of California also announced plans to provide its own generic drugs, he said.
“But you won’t see a lot of entrepreneurs getting into this because the volumes are so low. If Cuban called me, I would tell him to provide Daraprim and similar, low-volume products,” Dr. Comanor said of the billionaire. “He’s a rich guy; maybe he can do it.”
A version of this article first appeared on WebMD.com.
The Mark Cuban Cost Plus Drugs Company (MCCPDC) plans to offer the leukemia therapy imatinib for $47 per month, for example, compared with $120 or more with a common voucher and a retail price of $9,657 per month.
Other examples of lower-priced generics include the ulcerative colitis treatment mesalamine, which goes for $32.40 per month on the new online pharmacy versus $940 per month retail. In addition, the MCCPDC will offer the gout treatment colchicine at a lower price, charging $8.70, compared with $182 per month retail.
Likely in part because of claims of significant cost savings and in part because of Mr. Cuban’s celebrity status, the new venture is getting widespread media attention. Forbes, NPR, and TMZ have shared the news since the new digital pharmacy was announced earlier this month.
The new venture plans to charge consumers 15% above the manufacturing cost for the generic medications, plus a $3 fee for pharmacists and $5 for shipping. People will still require a prescription from their doctor to get the medications.
Generic pricing and social benefit
The top 100 generic products account for about half of generic sales, and there is enough competition for these high-demand medications that “the prices have come down close to zero,” said William Comanor, PhD, a health economist and professor of health policy and management at the University of California, Los Angeles. The remaining generic agents have lower-volume demand.
One prominent example is Daraprim, a decades-old treatment for the life-threatening parasitic infection toxoplasmosis. The drug jumped into the spotlight in 2015 when Martin Shkreli and his company Vyera Pharmaceuticals bought the rights to make the generic drug and raised the price overnight from $13.50 to $750. In January 2022, a U.S. judge banned Mr. Shkreli from the pharmaceutical industry and ordered him to pay an almost $65 million fine.
Dr. Comanor agreed the price should have been raised – $13.50 “was not economically viable” – but not as steep as $750.
“Say Mark Cuban says he will cut the price from $750 to $300. He will still make money. There is a market for these low-volume products,” he said. “There would also be a social benefit.”
A direct-to-consumer digital pharmacy
MCCPDC is “cutting out the middleman” in two ways. The business model calls for charging consumers out of pocket, so insurance companies are not involved. Also, the company created its own pharmacy business manager firm in October 2021, allowing it to negotiate prices with drugmakers in house.
The company also announced plans to complete construction of a 22,000-square-foot pharmaceutical factory in Dallas by the end of 2022.
Reactions on social media ranged from celebratory to people disappointed their generic medication would not cost significantly less or is not provided by the digital pharmacy.
When weighted by the number of prescriptions, prices for generics have declined in the United States.
“Overall, U.S. generic prices are the lowest in the world,” Dr. Comanor said. “People say U.S. drug prices are the highest in the world. That’s true for branded, but it’s not true for generics.
“So if someone asks if U.S. drug prices are the highest or lowest in the world, the answer is both,” he said.
“Maybe there is a role to play for this new pharmacy,” Dr. Comanor said when asked if the initiative seems like a positive development.
The state of California also announced plans to provide its own generic drugs, he said.
“But you won’t see a lot of entrepreneurs getting into this because the volumes are so low. If Cuban called me, I would tell him to provide Daraprim and similar, low-volume products,” Dr. Comanor said of the billionaire. “He’s a rich guy; maybe he can do it.”
A version of this article first appeared on WebMD.com.
The Mark Cuban Cost Plus Drugs Company (MCCPDC) plans to offer the leukemia therapy imatinib for $47 per month, for example, compared with $120 or more with a common voucher and a retail price of $9,657 per month.
Other examples of lower-priced generics include the ulcerative colitis treatment mesalamine, which goes for $32.40 per month on the new online pharmacy versus $940 per month retail. In addition, the MCCPDC will offer the gout treatment colchicine at a lower price, charging $8.70, compared with $182 per month retail.
Likely in part because of claims of significant cost savings and in part because of Mr. Cuban’s celebrity status, the new venture is getting widespread media attention. Forbes, NPR, and TMZ have shared the news since the new digital pharmacy was announced earlier this month.
The new venture plans to charge consumers 15% above the manufacturing cost for the generic medications, plus a $3 fee for pharmacists and $5 for shipping. People will still require a prescription from their doctor to get the medications.
Generic pricing and social benefit
The top 100 generic products account for about half of generic sales, and there is enough competition for these high-demand medications that “the prices have come down close to zero,” said William Comanor, PhD, a health economist and professor of health policy and management at the University of California, Los Angeles. The remaining generic agents have lower-volume demand.
One prominent example is Daraprim, a decades-old treatment for the life-threatening parasitic infection toxoplasmosis. The drug jumped into the spotlight in 2015 when Martin Shkreli and his company Vyera Pharmaceuticals bought the rights to make the generic drug and raised the price overnight from $13.50 to $750. In January 2022, a U.S. judge banned Mr. Shkreli from the pharmaceutical industry and ordered him to pay an almost $65 million fine.
Dr. Comanor agreed the price should have been raised – $13.50 “was not economically viable” – but not as steep as $750.
“Say Mark Cuban says he will cut the price from $750 to $300. He will still make money. There is a market for these low-volume products,” he said. “There would also be a social benefit.”
A direct-to-consumer digital pharmacy
MCCPDC is “cutting out the middleman” in two ways. The business model calls for charging consumers out of pocket, so insurance companies are not involved. Also, the company created its own pharmacy business manager firm in October 2021, allowing it to negotiate prices with drugmakers in house.
The company also announced plans to complete construction of a 22,000-square-foot pharmaceutical factory in Dallas by the end of 2022.
Reactions on social media ranged from celebratory to people disappointed their generic medication would not cost significantly less or is not provided by the digital pharmacy.
When weighted by the number of prescriptions, prices for generics have declined in the United States.
“Overall, U.S. generic prices are the lowest in the world,” Dr. Comanor said. “People say U.S. drug prices are the highest in the world. That’s true for branded, but it’s not true for generics.
“So if someone asks if U.S. drug prices are the highest or lowest in the world, the answer is both,” he said.
“Maybe there is a role to play for this new pharmacy,” Dr. Comanor said when asked if the initiative seems like a positive development.
The state of California also announced plans to provide its own generic drugs, he said.
“But you won’t see a lot of entrepreneurs getting into this because the volumes are so low. If Cuban called me, I would tell him to provide Daraprim and similar, low-volume products,” Dr. Comanor said of the billionaire. “He’s a rich guy; maybe he can do it.”
A version of this article first appeared on WebMD.com.


