User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Physical fitness tied to lower risk of Alzheimer’s disease
, new findings suggest. “One exciting finding of this study is that as people’s fitness improved, their risk of Alzheimer’s disease decreased – it was not an all-or-nothing proposition,” study investigator Edward Zamrini, MD, of the Washington DC VA Medical Center, said in a news release.
The findings suggest that people can work toward making incremental changes and improvements in their physical fitness, which may help decrease their risk of dementia, Dr. Zamrini added.
The findings were presented at the 2022 annual meeting of the American Academy of Neurology.
Effective prevention strategy
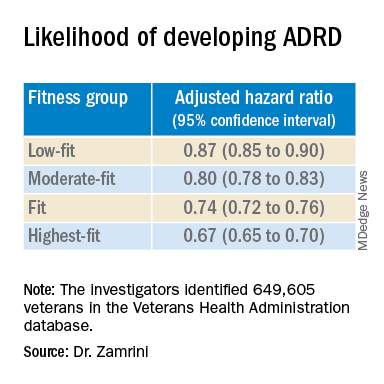
Using the Veterans Health Administration database, Dr. Zamrini and colleagues identified 649,605 veterans (mean age, 61 years) free of Alzheimer’s disease and related disorders (ADRD) when they completed standardized exercise treadmill tests between 2000 and 2017.
They divided participants into five age-specific fitness groups, from least fit to most fit, based on peak metabolic equivalents (METs) achieved during the treadmill test: lowest-fit (METs, ±3.8), low-fit (METs, ±5.8), moderate-fit (METs, ±7.5), fit (METs, ±9.2), and highest-fit (METs, ±11.7).
In unadjusted analysis, veterans with the lowest cardiorespiratory fitness developed ADRD at a rate of 9.5 cases per 1,000 person-years, compared with a rate of 6.4 cases per 1,000 person-years for the most fit group (P < .001).
After adjusting for factors that could affect risk of ADRD, compared with the lowest-fit group, the highest-fit and fit groups were 33% and 26% less likely to develop ADRD, respectively, while the moderate-fit and low-fit groups were 20% and 13% less likely to develop the disease, respectively.
The findings suggest that the association between cardiorespiratory fitness and ADRD risk is “inverse, independent, and graded,” the researchers said in their conference abstract.
“The idea that you can reduce your risk for Alzheimer’s disease by simply increasing your activity is very promising, especially since there are no adequate treatments to prevent or stop the progression of the disease,” Dr. Zamrini added in the news release.
“We hope to develop a simple scale that can be individualized so people can see the benefits that even incremental improvements in fitness can deliver,” he said.
The next vital sign?
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Boston, noted that “for decades and with increasing body of support from studies like this, we have known that preventing dementia is based on healthy behaviors for the brain including a proper diet (NASH and/or Mediterranean), exercise regimen (aerobic/cardio more than anaerobic/weight-lifting), sleep hygiene, and social and intellectual engagements.”
“Frankly, what’s good for the body is good for the brain,” said Dr. Lakhan.
“It should be noted that the measure studied here is cardiorespiratory fitness, which has been associated with heart disease and resulting death, death from any cause, and now brain health,” Dr. Lakhan said.
“This powerful predictor may in fact be the next vital sign, after your heart rate and blood pressure, from which your primary care provider can make a personalized treatment plan,” he added.
“Accelerating this process, the ability to measure cardiorespiratory fitness traditionally from huge stationary machines down to wearables like a watch or ring, or even your iPhone or Android, is just on the horizon,” Dr. Lakhan said.
“Instead of tracking just your weight, shape, and BMI, personal fitness may be tailored to optimizing this indicator and further empowering individuals to take charge of their health,” he said.
The study was supported by the National Institute on Aging, the National Institutes of Health, the U.S. Department of Veterans Affairs, the Washington DC VA Medical Center, and George Washington University. Dr. Zamrini and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new findings suggest. “One exciting finding of this study is that as people’s fitness improved, their risk of Alzheimer’s disease decreased – it was not an all-or-nothing proposition,” study investigator Edward Zamrini, MD, of the Washington DC VA Medical Center, said in a news release.
The findings suggest that people can work toward making incremental changes and improvements in their physical fitness, which may help decrease their risk of dementia, Dr. Zamrini added.
The findings were presented at the 2022 annual meeting of the American Academy of Neurology.
Effective prevention strategy
Using the Veterans Health Administration database, Dr. Zamrini and colleagues identified 649,605 veterans (mean age, 61 years) free of Alzheimer’s disease and related disorders (ADRD) when they completed standardized exercise treadmill tests between 2000 and 2017.
They divided participants into five age-specific fitness groups, from least fit to most fit, based on peak metabolic equivalents (METs) achieved during the treadmill test: lowest-fit (METs, ±3.8), low-fit (METs, ±5.8), moderate-fit (METs, ±7.5), fit (METs, ±9.2), and highest-fit (METs, ±11.7).
In unadjusted analysis, veterans with the lowest cardiorespiratory fitness developed ADRD at a rate of 9.5 cases per 1,000 person-years, compared with a rate of 6.4 cases per 1,000 person-years for the most fit group (P < .001).
After adjusting for factors that could affect risk of ADRD, compared with the lowest-fit group, the highest-fit and fit groups were 33% and 26% less likely to develop ADRD, respectively, while the moderate-fit and low-fit groups were 20% and 13% less likely to develop the disease, respectively.

The findings suggest that the association between cardiorespiratory fitness and ADRD risk is “inverse, independent, and graded,” the researchers said in their conference abstract.
“The idea that you can reduce your risk for Alzheimer’s disease by simply increasing your activity is very promising, especially since there are no adequate treatments to prevent or stop the progression of the disease,” Dr. Zamrini added in the news release.
“We hope to develop a simple scale that can be individualized so people can see the benefits that even incremental improvements in fitness can deliver,” he said.
The next vital sign?
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Boston, noted that “for decades and with increasing body of support from studies like this, we have known that preventing dementia is based on healthy behaviors for the brain including a proper diet (NASH and/or Mediterranean), exercise regimen (aerobic/cardio more than anaerobic/weight-lifting), sleep hygiene, and social and intellectual engagements.”
“Frankly, what’s good for the body is good for the brain,” said Dr. Lakhan.
“It should be noted that the measure studied here is cardiorespiratory fitness, which has been associated with heart disease and resulting death, death from any cause, and now brain health,” Dr. Lakhan said.
“This powerful predictor may in fact be the next vital sign, after your heart rate and blood pressure, from which your primary care provider can make a personalized treatment plan,” he added.
“Accelerating this process, the ability to measure cardiorespiratory fitness traditionally from huge stationary machines down to wearables like a watch or ring, or even your iPhone or Android, is just on the horizon,” Dr. Lakhan said.
“Instead of tracking just your weight, shape, and BMI, personal fitness may be tailored to optimizing this indicator and further empowering individuals to take charge of their health,” he said.
The study was supported by the National Institute on Aging, the National Institutes of Health, the U.S. Department of Veterans Affairs, the Washington DC VA Medical Center, and George Washington University. Dr. Zamrini and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new findings suggest. “One exciting finding of this study is that as people’s fitness improved, their risk of Alzheimer’s disease decreased – it was not an all-or-nothing proposition,” study investigator Edward Zamrini, MD, of the Washington DC VA Medical Center, said in a news release.
The findings suggest that people can work toward making incremental changes and improvements in their physical fitness, which may help decrease their risk of dementia, Dr. Zamrini added.
The findings were presented at the 2022 annual meeting of the American Academy of Neurology.
Effective prevention strategy
Using the Veterans Health Administration database, Dr. Zamrini and colleagues identified 649,605 veterans (mean age, 61 years) free of Alzheimer’s disease and related disorders (ADRD) when they completed standardized exercise treadmill tests between 2000 and 2017.
They divided participants into five age-specific fitness groups, from least fit to most fit, based on peak metabolic equivalents (METs) achieved during the treadmill test: lowest-fit (METs, ±3.8), low-fit (METs, ±5.8), moderate-fit (METs, ±7.5), fit (METs, ±9.2), and highest-fit (METs, ±11.7).
In unadjusted analysis, veterans with the lowest cardiorespiratory fitness developed ADRD at a rate of 9.5 cases per 1,000 person-years, compared with a rate of 6.4 cases per 1,000 person-years for the most fit group (P < .001).
After adjusting for factors that could affect risk of ADRD, compared with the lowest-fit group, the highest-fit and fit groups were 33% and 26% less likely to develop ADRD, respectively, while the moderate-fit and low-fit groups were 20% and 13% less likely to develop the disease, respectively.

The findings suggest that the association between cardiorespiratory fitness and ADRD risk is “inverse, independent, and graded,” the researchers said in their conference abstract.
“The idea that you can reduce your risk for Alzheimer’s disease by simply increasing your activity is very promising, especially since there are no adequate treatments to prevent or stop the progression of the disease,” Dr. Zamrini added in the news release.
“We hope to develop a simple scale that can be individualized so people can see the benefits that even incremental improvements in fitness can deliver,” he said.
The next vital sign?
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Boston, noted that “for decades and with increasing body of support from studies like this, we have known that preventing dementia is based on healthy behaviors for the brain including a proper diet (NASH and/or Mediterranean), exercise regimen (aerobic/cardio more than anaerobic/weight-lifting), sleep hygiene, and social and intellectual engagements.”
“Frankly, what’s good for the body is good for the brain,” said Dr. Lakhan.
“It should be noted that the measure studied here is cardiorespiratory fitness, which has been associated with heart disease and resulting death, death from any cause, and now brain health,” Dr. Lakhan said.
“This powerful predictor may in fact be the next vital sign, after your heart rate and blood pressure, from which your primary care provider can make a personalized treatment plan,” he added.
“Accelerating this process, the ability to measure cardiorespiratory fitness traditionally from huge stationary machines down to wearables like a watch or ring, or even your iPhone or Android, is just on the horizon,” Dr. Lakhan said.
“Instead of tracking just your weight, shape, and BMI, personal fitness may be tailored to optimizing this indicator and further empowering individuals to take charge of their health,” he said.
The study was supported by the National Institute on Aging, the National Institutes of Health, the U.S. Department of Veterans Affairs, the Washington DC VA Medical Center, and George Washington University. Dr. Zamrini and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAN 2022
First comprehensive guidelines for managing anorexia in pregnancy
The first comprehensive guidelines to manage pregnant women with anorexia nervosa (AN) have been released.
Pregnant women with AN are at greater risk of poor outcomes, including stillbirth, underweight infant, or pre-term birth, yet there are no clear guidelines on the management of the condition.
“Anorexia in pregnancy has been an overlooked area of clinical care, as many believed only women in remission become pregnant, and it is clear that is not the case,” lead author Megan Galbally, MBBS, PhD, professor and director, Centre of Women’s and Children’s Mental Health at Monash University School of Clinical Sciences, Melbourne, told this news organization.
“There are great opportunities to support women in their mental health and give them and their babies a healthier start to parenthood and life,” said Dr. Galbally.
“For instance, reducing the likelihood of prematurity or low birth weight at birth that can be associated with anorexia in pregnancy has extraordinary benefits for that child for lifelong health and well-being,” she added.
The guidelines were published online in Lancet Psychiatry.
Spike in cases
Dr. Galbally noted that during her 20 years of working in perinatal mental health within tertiary maternity services, she only ever saw an occasional pregnant woman with current AN.
In contrast, over the last 3 to 4 years, there has been a “steep increase in women presenting in pregnancy with very low body mass index (BMI) and current anorexia nervosa requiring treatment in pregnancy,” Dr. Galbally said.
Despite the complexity of managing AN in pregnancy, few studies are available to guide care. In a systematic literature review, the researchers identified only eight studies that addressed the management of AN in pregnancy. These studies were case studies or case reports examining narrow aspects of management.
Digging deeper, the researchers conducted a state-of-the-art research review in relevant disciplines and areas of expertise for managing anorexia nervosa in pregnancy. They synthesized their findings into “recommendations and principles” for multidisciplinary care of pregnant women with AN.
The researchers note that AN in pregnancy is associated with increased risks of pregnancy complications and poorer outcomes for infants, and measures such as BMI are less accurate in pregnancy for assessing severity or change in anorexia nervosa.
Anorexia affects pregnancy and neonatal outcomes through low calorie intake, nutritional and vitamin deficiencies, stress, fasting, low body mass, and poor placentation and uteroplacental function.
The authors note that managing AN in pregnancy requires multidisciplinary care that considers the substantial physiological changes for women and requirements for monitoring fetal growth and development.
At a minimum, they recommend monitoring the following:
- Sodium, potassium, magnesium, phosphate, and chloride concentration
- Iron status, vitamin D and bone mineral density, blood sugar concentration (fasting or random), and A1c
- Liver function (including bilirubin, aspartate transaminase, alanine aminotransferase, and gamma-glutamyl transferase) and bone marrow function (including full blood examination, white cell count, neutrophil count, platelets, and hemoglobin)
- Inflammatory markers (C-reactive protein and erythrocyte sedimentation rate)
- Cardiac function (electrocardiogram and echocardiogram)
- Blood pressure and heart rate (lying and standing) and body temperature
“There are considerable risks for women and their unborn child in managing moderate to severe AN in pregnancy,” said Dr. Galbally.
“While we have provided some recommendations, it still requires considerable adaptation to individual presentations and circumstances, and this is best done with a maternity service that manages other high-risk pregnancies such as through maternal-fetal medicine teams,” she said.
“While this area of clinical care can be new to high-risk pregnancy teams, it is clearly important that high-risk pregnancy services and mental health work together to improve care for women with anorexia in pregnancy,” Dr. Galbally added.
A nightmare, a dream come true
Reached for comment, Kamryn T. Eddy, PhD, co-director, Eating Disorders Clinical and Research Program, Massachusetts General Hospital, said, “for many with anorexia nervosa, pregnancy realizes their greatest nightmare and dream come true, both at once.”
“The physical demands of pregnancy can be taxing, and for those with anorexia nervosa, closer clinical management makes sense and may help to support patients who are at risk for return to or worsening of symptoms with the increased nutritional needs and weight gain that occur in pregnancy,” Dr. Eddy, associate professor, department of psychiatry, Harvard Medical School, Boston, told this news organization.
“At the same time, the desire to have a child can be a strong motivator for patients to make the changes needed to recover, and for some, the transition to mother can also help in recovery by broadening the range of things that influence their self-worth,” Dr. Eddy added.
This research had no specific funding. Dr. Galbally and Dr. Eddy report no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The first comprehensive guidelines to manage pregnant women with anorexia nervosa (AN) have been released.
Pregnant women with AN are at greater risk of poor outcomes, including stillbirth, underweight infant, or pre-term birth, yet there are no clear guidelines on the management of the condition.
“Anorexia in pregnancy has been an overlooked area of clinical care, as many believed only women in remission become pregnant, and it is clear that is not the case,” lead author Megan Galbally, MBBS, PhD, professor and director, Centre of Women’s and Children’s Mental Health at Monash University School of Clinical Sciences, Melbourne, told this news organization.
“There are great opportunities to support women in their mental health and give them and their babies a healthier start to parenthood and life,” said Dr. Galbally.
“For instance, reducing the likelihood of prematurity or low birth weight at birth that can be associated with anorexia in pregnancy has extraordinary benefits for that child for lifelong health and well-being,” she added.
The guidelines were published online in Lancet Psychiatry.
Spike in cases
Dr. Galbally noted that during her 20 years of working in perinatal mental health within tertiary maternity services, she only ever saw an occasional pregnant woman with current AN.
In contrast, over the last 3 to 4 years, there has been a “steep increase in women presenting in pregnancy with very low body mass index (BMI) and current anorexia nervosa requiring treatment in pregnancy,” Dr. Galbally said.
Despite the complexity of managing AN in pregnancy, few studies are available to guide care. In a systematic literature review, the researchers identified only eight studies that addressed the management of AN in pregnancy. These studies were case studies or case reports examining narrow aspects of management.
Digging deeper, the researchers conducted a state-of-the-art research review in relevant disciplines and areas of expertise for managing anorexia nervosa in pregnancy. They synthesized their findings into “recommendations and principles” for multidisciplinary care of pregnant women with AN.
The researchers note that AN in pregnancy is associated with increased risks of pregnancy complications and poorer outcomes for infants, and measures such as BMI are less accurate in pregnancy for assessing severity or change in anorexia nervosa.
Anorexia affects pregnancy and neonatal outcomes through low calorie intake, nutritional and vitamin deficiencies, stress, fasting, low body mass, and poor placentation and uteroplacental function.
The authors note that managing AN in pregnancy requires multidisciplinary care that considers the substantial physiological changes for women and requirements for monitoring fetal growth and development.
At a minimum, they recommend monitoring the following:
- Sodium, potassium, magnesium, phosphate, and chloride concentration
- Iron status, vitamin D and bone mineral density, blood sugar concentration (fasting or random), and A1c
- Liver function (including bilirubin, aspartate transaminase, alanine aminotransferase, and gamma-glutamyl transferase) and bone marrow function (including full blood examination, white cell count, neutrophil count, platelets, and hemoglobin)
- Inflammatory markers (C-reactive protein and erythrocyte sedimentation rate)
- Cardiac function (electrocardiogram and echocardiogram)
- Blood pressure and heart rate (lying and standing) and body temperature
“There are considerable risks for women and their unborn child in managing moderate to severe AN in pregnancy,” said Dr. Galbally.
“While we have provided some recommendations, it still requires considerable adaptation to individual presentations and circumstances, and this is best done with a maternity service that manages other high-risk pregnancies such as through maternal-fetal medicine teams,” she said.
“While this area of clinical care can be new to high-risk pregnancy teams, it is clearly important that high-risk pregnancy services and mental health work together to improve care for women with anorexia in pregnancy,” Dr. Galbally added.
A nightmare, a dream come true
Reached for comment, Kamryn T. Eddy, PhD, co-director, Eating Disorders Clinical and Research Program, Massachusetts General Hospital, said, “for many with anorexia nervosa, pregnancy realizes their greatest nightmare and dream come true, both at once.”
“The physical demands of pregnancy can be taxing, and for those with anorexia nervosa, closer clinical management makes sense and may help to support patients who are at risk for return to or worsening of symptoms with the increased nutritional needs and weight gain that occur in pregnancy,” Dr. Eddy, associate professor, department of psychiatry, Harvard Medical School, Boston, told this news organization.
“At the same time, the desire to have a child can be a strong motivator for patients to make the changes needed to recover, and for some, the transition to mother can also help in recovery by broadening the range of things that influence their self-worth,” Dr. Eddy added.
This research had no specific funding. Dr. Galbally and Dr. Eddy report no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The first comprehensive guidelines to manage pregnant women with anorexia nervosa (AN) have been released.
Pregnant women with AN are at greater risk of poor outcomes, including stillbirth, underweight infant, or pre-term birth, yet there are no clear guidelines on the management of the condition.
“Anorexia in pregnancy has been an overlooked area of clinical care, as many believed only women in remission become pregnant, and it is clear that is not the case,” lead author Megan Galbally, MBBS, PhD, professor and director, Centre of Women’s and Children’s Mental Health at Monash University School of Clinical Sciences, Melbourne, told this news organization.
“There are great opportunities to support women in their mental health and give them and their babies a healthier start to parenthood and life,” said Dr. Galbally.
“For instance, reducing the likelihood of prematurity or low birth weight at birth that can be associated with anorexia in pregnancy has extraordinary benefits for that child for lifelong health and well-being,” she added.
The guidelines were published online in Lancet Psychiatry.
Spike in cases
Dr. Galbally noted that during her 20 years of working in perinatal mental health within tertiary maternity services, she only ever saw an occasional pregnant woman with current AN.
In contrast, over the last 3 to 4 years, there has been a “steep increase in women presenting in pregnancy with very low body mass index (BMI) and current anorexia nervosa requiring treatment in pregnancy,” Dr. Galbally said.
Despite the complexity of managing AN in pregnancy, few studies are available to guide care. In a systematic literature review, the researchers identified only eight studies that addressed the management of AN in pregnancy. These studies were case studies or case reports examining narrow aspects of management.
Digging deeper, the researchers conducted a state-of-the-art research review in relevant disciplines and areas of expertise for managing anorexia nervosa in pregnancy. They synthesized their findings into “recommendations and principles” for multidisciplinary care of pregnant women with AN.
The researchers note that AN in pregnancy is associated with increased risks of pregnancy complications and poorer outcomes for infants, and measures such as BMI are less accurate in pregnancy for assessing severity or change in anorexia nervosa.
Anorexia affects pregnancy and neonatal outcomes through low calorie intake, nutritional and vitamin deficiencies, stress, fasting, low body mass, and poor placentation and uteroplacental function.
The authors note that managing AN in pregnancy requires multidisciplinary care that considers the substantial physiological changes for women and requirements for monitoring fetal growth and development.
At a minimum, they recommend monitoring the following:
- Sodium, potassium, magnesium, phosphate, and chloride concentration
- Iron status, vitamin D and bone mineral density, blood sugar concentration (fasting or random), and A1c
- Liver function (including bilirubin, aspartate transaminase, alanine aminotransferase, and gamma-glutamyl transferase) and bone marrow function (including full blood examination, white cell count, neutrophil count, platelets, and hemoglobin)
- Inflammatory markers (C-reactive protein and erythrocyte sedimentation rate)
- Cardiac function (electrocardiogram and echocardiogram)
- Blood pressure and heart rate (lying and standing) and body temperature
“There are considerable risks for women and their unborn child in managing moderate to severe AN in pregnancy,” said Dr. Galbally.
“While we have provided some recommendations, it still requires considerable adaptation to individual presentations and circumstances, and this is best done with a maternity service that manages other high-risk pregnancies such as through maternal-fetal medicine teams,” she said.
“While this area of clinical care can be new to high-risk pregnancy teams, it is clearly important that high-risk pregnancy services and mental health work together to improve care for women with anorexia in pregnancy,” Dr. Galbally added.
A nightmare, a dream come true
Reached for comment, Kamryn T. Eddy, PhD, co-director, Eating Disorders Clinical and Research Program, Massachusetts General Hospital, said, “for many with anorexia nervosa, pregnancy realizes their greatest nightmare and dream come true, both at once.”
“The physical demands of pregnancy can be taxing, and for those with anorexia nervosa, closer clinical management makes sense and may help to support patients who are at risk for return to or worsening of symptoms with the increased nutritional needs and weight gain that occur in pregnancy,” Dr. Eddy, associate professor, department of psychiatry, Harvard Medical School, Boston, told this news organization.
“At the same time, the desire to have a child can be a strong motivator for patients to make the changes needed to recover, and for some, the transition to mother can also help in recovery by broadening the range of things that influence their self-worth,” Dr. Eddy added.
This research had no specific funding. Dr. Galbally and Dr. Eddy report no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Ohio bill bans ‘co-pay accumulator’ practice by insurers
The Ohio House of Representatives recently passed a bill that would enable patients to use drug manufacturer coupons and other co-pay assistance as payment toward their annual deductible.
According to the Kaiser Family Foundation, approximately 1 in 4 Americans have difficulty paying for their prescription drugs, while almost half of U.S. adults report difficulty paying out-of-pocket costs not covered by their health insurance.
Supporting the bill that restricts co-pay accumulators are groups such as the Ohio State Medical Association, the Crohn’s and Colitis Foundation, Susan C. Komen, the National Multiple Sclerosis Society, and the American Diabetes Association. The bill faced opposition from health insurers and pharmacy benefit managers, reported The Columbus Dispatch.
“The debate on the management of rising drug costs between manufacturers and insurers unfortunately leaves patients caught in the middle, and practices like co-pay accumulators can have a devastating impact,” Monica Hueckel, senior director of government relations for the Ohio State Medical Association, told this news organization.
“Patients often do not even know about these policies until the coupons are no longer usable. As you can imagine, for patients with expensive medications and/or high deductible health plans, the impact is disastrous,” she said.
Ohio State Representative Susan Manchester, who co-sponsored the bill, told The Columbus Dispatch that the legislation “is needed to assist our constituents who find themselves increasingly subjected to more out-of-pocket costs as part of their insurance coverage.”
Other states blocking health insurers’ co-pay policies
With the passage of the bill, Ohio joins 12 states and Puerto Rico in preventing the use of health insurers’ co-pays to increase patients’ out-of-pocket costs, reported The Columbus Dispatch; 15 states are also considering this type of legislation.
Eighty-three percent of patients are in plans that include a co-pay accumulator, according to consulting firm Avalere, which wrote that, beginning in 2023, the Center for Medicare & Medicaid Services requires patients with Medicaid to receive “the full value of co-pay assistance” on drugs.
According to the National Conference of State Legislatures, co-pay adjustment programs present challenges for patients, with plans that include high cost sharing or co-insurance whereby a patient pays a percentage of the cost instead of a flat amount.
For example, with a co-pay adjustment policy, a patient with a $2,000 deductible plan couldn’t use a $500 coupon toward meeting the deductible, writes the National Conference of State Legislatures. Conversely, a patient in a plan without a co-pay adjustment policy could use the coupon to satisfy their annual deductible.
Patients with complex conditions, such as cancer, rheumatoid arthritis, and diabetes, which often require expensive medications, may have little choice but to fork over the unexpected co-pays, according to the organization that represents state legislatures in the United States.
The bill now moves to the Ohio Senate, reported The Columbus Dispatch.
A version of this article first appeared on Medscape.com.
The Ohio House of Representatives recently passed a bill that would enable patients to use drug manufacturer coupons and other co-pay assistance as payment toward their annual deductible.
According to the Kaiser Family Foundation, approximately 1 in 4 Americans have difficulty paying for their prescription drugs, while almost half of U.S. adults report difficulty paying out-of-pocket costs not covered by their health insurance.
Supporting the bill that restricts co-pay accumulators are groups such as the Ohio State Medical Association, the Crohn’s and Colitis Foundation, Susan C. Komen, the National Multiple Sclerosis Society, and the American Diabetes Association. The bill faced opposition from health insurers and pharmacy benefit managers, reported The Columbus Dispatch.
“The debate on the management of rising drug costs between manufacturers and insurers unfortunately leaves patients caught in the middle, and practices like co-pay accumulators can have a devastating impact,” Monica Hueckel, senior director of government relations for the Ohio State Medical Association, told this news organization.
“Patients often do not even know about these policies until the coupons are no longer usable. As you can imagine, for patients with expensive medications and/or high deductible health plans, the impact is disastrous,” she said.
Ohio State Representative Susan Manchester, who co-sponsored the bill, told The Columbus Dispatch that the legislation “is needed to assist our constituents who find themselves increasingly subjected to more out-of-pocket costs as part of their insurance coverage.”
Other states blocking health insurers’ co-pay policies
With the passage of the bill, Ohio joins 12 states and Puerto Rico in preventing the use of health insurers’ co-pays to increase patients’ out-of-pocket costs, reported The Columbus Dispatch; 15 states are also considering this type of legislation.
Eighty-three percent of patients are in plans that include a co-pay accumulator, according to consulting firm Avalere, which wrote that, beginning in 2023, the Center for Medicare & Medicaid Services requires patients with Medicaid to receive “the full value of co-pay assistance” on drugs.
According to the National Conference of State Legislatures, co-pay adjustment programs present challenges for patients, with plans that include high cost sharing or co-insurance whereby a patient pays a percentage of the cost instead of a flat amount.
For example, with a co-pay adjustment policy, a patient with a $2,000 deductible plan couldn’t use a $500 coupon toward meeting the deductible, writes the National Conference of State Legislatures. Conversely, a patient in a plan without a co-pay adjustment policy could use the coupon to satisfy their annual deductible.
Patients with complex conditions, such as cancer, rheumatoid arthritis, and diabetes, which often require expensive medications, may have little choice but to fork over the unexpected co-pays, according to the organization that represents state legislatures in the United States.
The bill now moves to the Ohio Senate, reported The Columbus Dispatch.
A version of this article first appeared on Medscape.com.
The Ohio House of Representatives recently passed a bill that would enable patients to use drug manufacturer coupons and other co-pay assistance as payment toward their annual deductible.
According to the Kaiser Family Foundation, approximately 1 in 4 Americans have difficulty paying for their prescription drugs, while almost half of U.S. adults report difficulty paying out-of-pocket costs not covered by their health insurance.
Supporting the bill that restricts co-pay accumulators are groups such as the Ohio State Medical Association, the Crohn’s and Colitis Foundation, Susan C. Komen, the National Multiple Sclerosis Society, and the American Diabetes Association. The bill faced opposition from health insurers and pharmacy benefit managers, reported The Columbus Dispatch.
“The debate on the management of rising drug costs between manufacturers and insurers unfortunately leaves patients caught in the middle, and practices like co-pay accumulators can have a devastating impact,” Monica Hueckel, senior director of government relations for the Ohio State Medical Association, told this news organization.
“Patients often do not even know about these policies until the coupons are no longer usable. As you can imagine, for patients with expensive medications and/or high deductible health plans, the impact is disastrous,” she said.
Ohio State Representative Susan Manchester, who co-sponsored the bill, told The Columbus Dispatch that the legislation “is needed to assist our constituents who find themselves increasingly subjected to more out-of-pocket costs as part of their insurance coverage.”
Other states blocking health insurers’ co-pay policies
With the passage of the bill, Ohio joins 12 states and Puerto Rico in preventing the use of health insurers’ co-pays to increase patients’ out-of-pocket costs, reported The Columbus Dispatch; 15 states are also considering this type of legislation.
Eighty-three percent of patients are in plans that include a co-pay accumulator, according to consulting firm Avalere, which wrote that, beginning in 2023, the Center for Medicare & Medicaid Services requires patients with Medicaid to receive “the full value of co-pay assistance” on drugs.
According to the National Conference of State Legislatures, co-pay adjustment programs present challenges for patients, with plans that include high cost sharing or co-insurance whereby a patient pays a percentage of the cost instead of a flat amount.
For example, with a co-pay adjustment policy, a patient with a $2,000 deductible plan couldn’t use a $500 coupon toward meeting the deductible, writes the National Conference of State Legislatures. Conversely, a patient in a plan without a co-pay adjustment policy could use the coupon to satisfy their annual deductible.
Patients with complex conditions, such as cancer, rheumatoid arthritis, and diabetes, which often require expensive medications, may have little choice but to fork over the unexpected co-pays, according to the organization that represents state legislatures in the United States.
The bill now moves to the Ohio Senate, reported The Columbus Dispatch.
A version of this article first appeared on Medscape.com.
Novel medication tied to better quality of life in major depression
DENVER –
In a phase 3 trial that included more than 500 adult patients with MDD, those who received zuranolone for 14 days showed greater improvement at day 15 across numerous QoL outcomes, compared with their counterparts in the placebo group.
In addition, combined analysis of four zuranolone clinical trials showed “mental well-being and functioning improved to near general population norm levels” for the active-treatment group, reported the researchers, led by Anita H. Clayton, MD, chair and professor of psychiatry, University of Virginia, Charlottesville.
“Based on these integrated analyses, the benefit of treatment with zuranolone may extend beyond reduction in depressive symptoms to include potential improvement in quality of life and overall health, as perceived by patients,” they add.
The findings were presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
First oral formulation
Zuranolone represents the second entry in the new class of neuroactive steroid drugs, which modulate GABA-A receptor activity – but it would be the first to have an oral formulation. Brexanolone, which was approved by the Food and Drug Administration in 2019 for postpartum depression, is administered through continuous IV infusion over 60 hours.
As previously reported by this news organization, zuranolone improved depressive symptoms as early as day 3, achieving the primary endpoint of significantly greater reduction in scores on the 17-item Hamilton Rating Scale for Depression from baseline to day 15 versus placebo (P = .014).
In the new analysis, patient-reported measures of functional health and well-being were assessed in the WATERFALL trial. It included 266 patients with MDD who were treated with zuranolone 50 mg daily for 2 weeks and 268 patients with MDD who were treated with placebo.
The study used the Short Form–36 (SF-36v2), which covers a wide range of patient-reported measures, including physical function, bodily pain, general health, vitality, social function, and “role-emotional” symptoms.
Results showed that although the treatment and placebo groups had similar baseline SF-36v2 scores, those receiving zuranolone reported significantly greater improvements at day 15 in almost all of the assessment’s domains, including physical function (treatment difference, 0.8), general health (1.0), vitality (3.1), social functioning (1.1), and role-emotional symptoms (1.5; for all comparisons, P < .05). The only exceptions were in role-physical symptoms and bodily pain.
In measures that included physical function, bodily pain, and general health, the patients achieved improvements at day 15 that were consistent with normal levels, with the improvement in vitality considered clinically meaningful versus placebo.
Integrated data
In further analysis of integrated data from four zuranolone clinical trials in the NEST and LANDSCAPE programs for patients with MDD and postpartum depression, results showed similar improvements at day 15 for zuranolone in QoL and overall health across all of the SF-36v2 functioning and well-being domains (P <.05), with the exceptions of physical measure and bodily pain.
By day 42, all of the domains showed significantly greater improvement with zuranolone versus placebo (all, P <.05).
Among the strongest score improvements in the integrated trials were measures in social functioning, which improved from baseline scores of 29.66 to 42.82 on day 15 and to 43.59 on day 42.
Emotional domain scores improved from 24.43 at baseline to 39.13 on day 15 and to 39.82 on day 42. For mental health, the integrated scores for the zuranolone group improved from 27.13 at baseline to 42.40 on day 15 and 42.62 on day 42.
Of note, the baseline scores for mental health represented just 54.3% of those in the normal population; with the increase at day 15, the level was 84.8% of the normal population.
“Across four completed placebo-controlled NEST and LANDSCAPE clinical trials, patient reports of functional health and well-being as assessed by the SF-36v2 indicated substantial impairment at baseline compared to the population norm,” the researchers reported.
The improvements are especially important in light of the fact that in some patients with MDD, functional improvement is a top priority.
“Patients have often prioritized returning to their usual level of functioning over reduction in depressive symptoms, and functional recovery has been associated with better prognosis of depression,” the investigators wrote.
Zuranolone trials have shown that treatment-emergent adverse events (AEs) occur among about 60% of patients, versus about 44% with placebo. The most common AEs are somnolence, dizziness, headache, sedation, and diarrhea, with no increases in suicidal ideation or withdrawal.
The rates of severe AEs are low, and they are observed in about 3% of patients, versus 1.1% with placebo, the researchers noted.
Further, as opposed to serotonergic antidepressants such as SNRIs and SSRIs, zuranolone does not appear to have the undesirable side effects of decreased libido and sexual dysfunction, they added.
Clinically meaningful?
Andrew J. Cutler, MD, clinical associate professor of psychiatry at State University of New York, Syracuse, said the data are “very significant” for a number of reasons.
“We need more options to treat depression, especially ones with novel mechanisms of action and faster onset of efficacy, such as zuranolone,” said Dr. Cutler, who was not involved in the current study. He has coauthored other studies on zuranolone.
Regarding the study’s QoL outcomes, “while improvement in depressive symptoms is very important, what really matters to patients is improvement in function and quality of life,” Dr. Cutler noted.
Also commenting on the study, Jonathan E. Alpert, MD, PhD, chair of the department of psychiatry and behavioral sciences and professor of psychiatry, neuroscience, and pediatrics at Albert Einstein College of Medicine, New York, said the investigational drug could represent an important addition to the armamentarium for treating depression.
“Zuranolone has good oral bioavailability and would represent the first neuroactive steroid antidepressant available in oral form and, indeed, the first non–monoamine-based antidepressant available in oral form,” he said in an interview.
Dr. Alpert was not involved in the research and has no relationship with the drug’s development.
He noted that although there are modest differences between the patients who received zuranolone and those who received placebo in the trials, “this may have been related to high placebo response rates, which often complicate antidepressant trials.
“Further research is needed to determine whether differences between zuranolone and placebo are clinically meaningful, though the separation between drug and placebo on the primary endpoint, as well as some other measures, such as quality of life measures, is promising,” Dr. Alpert said.
However, he added that comparisons with other active antidepressants in terms of efficacy and tolerability remain to be seen.
“Given the large number of individuals with major depressive disorder who have incomplete response to or do not tolerate monoaminergic antidepressants, the development of agents that leverage novel nonmonoaminergic mechanisms is important,” Dr. Alpert concluded.
The study was funded by Sage Therapeutics and Biogen. Dr. Cutler has been involved in research of zuranolone for Sage Therapeutics. Dr. Alpert has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
DENVER –
In a phase 3 trial that included more than 500 adult patients with MDD, those who received zuranolone for 14 days showed greater improvement at day 15 across numerous QoL outcomes, compared with their counterparts in the placebo group.
In addition, combined analysis of four zuranolone clinical trials showed “mental well-being and functioning improved to near general population norm levels” for the active-treatment group, reported the researchers, led by Anita H. Clayton, MD, chair and professor of psychiatry, University of Virginia, Charlottesville.
“Based on these integrated analyses, the benefit of treatment with zuranolone may extend beyond reduction in depressive symptoms to include potential improvement in quality of life and overall health, as perceived by patients,” they add.
The findings were presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
First oral formulation
Zuranolone represents the second entry in the new class of neuroactive steroid drugs, which modulate GABA-A receptor activity – but it would be the first to have an oral formulation. Brexanolone, which was approved by the Food and Drug Administration in 2019 for postpartum depression, is administered through continuous IV infusion over 60 hours.
As previously reported by this news organization, zuranolone improved depressive symptoms as early as day 3, achieving the primary endpoint of significantly greater reduction in scores on the 17-item Hamilton Rating Scale for Depression from baseline to day 15 versus placebo (P = .014).
In the new analysis, patient-reported measures of functional health and well-being were assessed in the WATERFALL trial. It included 266 patients with MDD who were treated with zuranolone 50 mg daily for 2 weeks and 268 patients with MDD who were treated with placebo.
The study used the Short Form–36 (SF-36v2), which covers a wide range of patient-reported measures, including physical function, bodily pain, general health, vitality, social function, and “role-emotional” symptoms.
Results showed that although the treatment and placebo groups had similar baseline SF-36v2 scores, those receiving zuranolone reported significantly greater improvements at day 15 in almost all of the assessment’s domains, including physical function (treatment difference, 0.8), general health (1.0), vitality (3.1), social functioning (1.1), and role-emotional symptoms (1.5; for all comparisons, P < .05). The only exceptions were in role-physical symptoms and bodily pain.
In measures that included physical function, bodily pain, and general health, the patients achieved improvements at day 15 that were consistent with normal levels, with the improvement in vitality considered clinically meaningful versus placebo.
Integrated data
In further analysis of integrated data from four zuranolone clinical trials in the NEST and LANDSCAPE programs for patients with MDD and postpartum depression, results showed similar improvements at day 15 for zuranolone in QoL and overall health across all of the SF-36v2 functioning and well-being domains (P <.05), with the exceptions of physical measure and bodily pain.
By day 42, all of the domains showed significantly greater improvement with zuranolone versus placebo (all, P <.05).
Among the strongest score improvements in the integrated trials were measures in social functioning, which improved from baseline scores of 29.66 to 42.82 on day 15 and to 43.59 on day 42.
Emotional domain scores improved from 24.43 at baseline to 39.13 on day 15 and to 39.82 on day 42. For mental health, the integrated scores for the zuranolone group improved from 27.13 at baseline to 42.40 on day 15 and 42.62 on day 42.
Of note, the baseline scores for mental health represented just 54.3% of those in the normal population; with the increase at day 15, the level was 84.8% of the normal population.
“Across four completed placebo-controlled NEST and LANDSCAPE clinical trials, patient reports of functional health and well-being as assessed by the SF-36v2 indicated substantial impairment at baseline compared to the population norm,” the researchers reported.
The improvements are especially important in light of the fact that in some patients with MDD, functional improvement is a top priority.
“Patients have often prioritized returning to their usual level of functioning over reduction in depressive symptoms, and functional recovery has been associated with better prognosis of depression,” the investigators wrote.
Zuranolone trials have shown that treatment-emergent adverse events (AEs) occur among about 60% of patients, versus about 44% with placebo. The most common AEs are somnolence, dizziness, headache, sedation, and diarrhea, with no increases in suicidal ideation or withdrawal.
The rates of severe AEs are low, and they are observed in about 3% of patients, versus 1.1% with placebo, the researchers noted.
Further, as opposed to serotonergic antidepressants such as SNRIs and SSRIs, zuranolone does not appear to have the undesirable side effects of decreased libido and sexual dysfunction, they added.
Clinically meaningful?
Andrew J. Cutler, MD, clinical associate professor of psychiatry at State University of New York, Syracuse, said the data are “very significant” for a number of reasons.
“We need more options to treat depression, especially ones with novel mechanisms of action and faster onset of efficacy, such as zuranolone,” said Dr. Cutler, who was not involved in the current study. He has coauthored other studies on zuranolone.
Regarding the study’s QoL outcomes, “while improvement in depressive symptoms is very important, what really matters to patients is improvement in function and quality of life,” Dr. Cutler noted.
Also commenting on the study, Jonathan E. Alpert, MD, PhD, chair of the department of psychiatry and behavioral sciences and professor of psychiatry, neuroscience, and pediatrics at Albert Einstein College of Medicine, New York, said the investigational drug could represent an important addition to the armamentarium for treating depression.
“Zuranolone has good oral bioavailability and would represent the first neuroactive steroid antidepressant available in oral form and, indeed, the first non–monoamine-based antidepressant available in oral form,” he said in an interview.
Dr. Alpert was not involved in the research and has no relationship with the drug’s development.
He noted that although there are modest differences between the patients who received zuranolone and those who received placebo in the trials, “this may have been related to high placebo response rates, which often complicate antidepressant trials.
“Further research is needed to determine whether differences between zuranolone and placebo are clinically meaningful, though the separation between drug and placebo on the primary endpoint, as well as some other measures, such as quality of life measures, is promising,” Dr. Alpert said.
However, he added that comparisons with other active antidepressants in terms of efficacy and tolerability remain to be seen.
“Given the large number of individuals with major depressive disorder who have incomplete response to or do not tolerate monoaminergic antidepressants, the development of agents that leverage novel nonmonoaminergic mechanisms is important,” Dr. Alpert concluded.
The study was funded by Sage Therapeutics and Biogen. Dr. Cutler has been involved in research of zuranolone for Sage Therapeutics. Dr. Alpert has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
DENVER –
In a phase 3 trial that included more than 500 adult patients with MDD, those who received zuranolone for 14 days showed greater improvement at day 15 across numerous QoL outcomes, compared with their counterparts in the placebo group.
In addition, combined analysis of four zuranolone clinical trials showed “mental well-being and functioning improved to near general population norm levels” for the active-treatment group, reported the researchers, led by Anita H. Clayton, MD, chair and professor of psychiatry, University of Virginia, Charlottesville.
“Based on these integrated analyses, the benefit of treatment with zuranolone may extend beyond reduction in depressive symptoms to include potential improvement in quality of life and overall health, as perceived by patients,” they add.
The findings were presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
First oral formulation
Zuranolone represents the second entry in the new class of neuroactive steroid drugs, which modulate GABA-A receptor activity – but it would be the first to have an oral formulation. Brexanolone, which was approved by the Food and Drug Administration in 2019 for postpartum depression, is administered through continuous IV infusion over 60 hours.
As previously reported by this news organization, zuranolone improved depressive symptoms as early as day 3, achieving the primary endpoint of significantly greater reduction in scores on the 17-item Hamilton Rating Scale for Depression from baseline to day 15 versus placebo (P = .014).
In the new analysis, patient-reported measures of functional health and well-being were assessed in the WATERFALL trial. It included 266 patients with MDD who were treated with zuranolone 50 mg daily for 2 weeks and 268 patients with MDD who were treated with placebo.
The study used the Short Form–36 (SF-36v2), which covers a wide range of patient-reported measures, including physical function, bodily pain, general health, vitality, social function, and “role-emotional” symptoms.
Results showed that although the treatment and placebo groups had similar baseline SF-36v2 scores, those receiving zuranolone reported significantly greater improvements at day 15 in almost all of the assessment’s domains, including physical function (treatment difference, 0.8), general health (1.0), vitality (3.1), social functioning (1.1), and role-emotional symptoms (1.5; for all comparisons, P < .05). The only exceptions were in role-physical symptoms and bodily pain.
In measures that included physical function, bodily pain, and general health, the patients achieved improvements at day 15 that were consistent with normal levels, with the improvement in vitality considered clinically meaningful versus placebo.
Integrated data
In further analysis of integrated data from four zuranolone clinical trials in the NEST and LANDSCAPE programs for patients with MDD and postpartum depression, results showed similar improvements at day 15 for zuranolone in QoL and overall health across all of the SF-36v2 functioning and well-being domains (P <.05), with the exceptions of physical measure and bodily pain.
By day 42, all of the domains showed significantly greater improvement with zuranolone versus placebo (all, P <.05).
Among the strongest score improvements in the integrated trials were measures in social functioning, which improved from baseline scores of 29.66 to 42.82 on day 15 and to 43.59 on day 42.
Emotional domain scores improved from 24.43 at baseline to 39.13 on day 15 and to 39.82 on day 42. For mental health, the integrated scores for the zuranolone group improved from 27.13 at baseline to 42.40 on day 15 and 42.62 on day 42.
Of note, the baseline scores for mental health represented just 54.3% of those in the normal population; with the increase at day 15, the level was 84.8% of the normal population.
“Across four completed placebo-controlled NEST and LANDSCAPE clinical trials, patient reports of functional health and well-being as assessed by the SF-36v2 indicated substantial impairment at baseline compared to the population norm,” the researchers reported.
The improvements are especially important in light of the fact that in some patients with MDD, functional improvement is a top priority.
“Patients have often prioritized returning to their usual level of functioning over reduction in depressive symptoms, and functional recovery has been associated with better prognosis of depression,” the investigators wrote.
Zuranolone trials have shown that treatment-emergent adverse events (AEs) occur among about 60% of patients, versus about 44% with placebo. The most common AEs are somnolence, dizziness, headache, sedation, and diarrhea, with no increases in suicidal ideation or withdrawal.
The rates of severe AEs are low, and they are observed in about 3% of patients, versus 1.1% with placebo, the researchers noted.
Further, as opposed to serotonergic antidepressants such as SNRIs and SSRIs, zuranolone does not appear to have the undesirable side effects of decreased libido and sexual dysfunction, they added.
Clinically meaningful?
Andrew J. Cutler, MD, clinical associate professor of psychiatry at State University of New York, Syracuse, said the data are “very significant” for a number of reasons.
“We need more options to treat depression, especially ones with novel mechanisms of action and faster onset of efficacy, such as zuranolone,” said Dr. Cutler, who was not involved in the current study. He has coauthored other studies on zuranolone.
Regarding the study’s QoL outcomes, “while improvement in depressive symptoms is very important, what really matters to patients is improvement in function and quality of life,” Dr. Cutler noted.
Also commenting on the study, Jonathan E. Alpert, MD, PhD, chair of the department of psychiatry and behavioral sciences and professor of psychiatry, neuroscience, and pediatrics at Albert Einstein College of Medicine, New York, said the investigational drug could represent an important addition to the armamentarium for treating depression.
“Zuranolone has good oral bioavailability and would represent the first neuroactive steroid antidepressant available in oral form and, indeed, the first non–monoamine-based antidepressant available in oral form,” he said in an interview.
Dr. Alpert was not involved in the research and has no relationship with the drug’s development.
He noted that although there are modest differences between the patients who received zuranolone and those who received placebo in the trials, “this may have been related to high placebo response rates, which often complicate antidepressant trials.
“Further research is needed to determine whether differences between zuranolone and placebo are clinically meaningful, though the separation between drug and placebo on the primary endpoint, as well as some other measures, such as quality of life measures, is promising,” Dr. Alpert said.
However, he added that comparisons with other active antidepressants in terms of efficacy and tolerability remain to be seen.
“Given the large number of individuals with major depressive disorder who have incomplete response to or do not tolerate monoaminergic antidepressants, the development of agents that leverage novel nonmonoaminergic mechanisms is important,” Dr. Alpert concluded.
The study was funded by Sage Therapeutics and Biogen. Dr. Cutler has been involved in research of zuranolone for Sage Therapeutics. Dr. Alpert has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ADAA 2022
White House announces long-COVID action plan
The National Research Action Plan on Long COVID will gather experts from various agencies, including the Department of Defense and the Department of Veterans Affairs, to expand existing long-COVID clinics and broaden research on symptoms of the virus that persist long after infection.
“We’ll collaborate with academic, industry, state and local partners to better understand long COVID,” Health and Human Services Secretary Xavier Becerra said at a White House briefing April 5. “We need to work as aggressively as we can to make sure no American is left behind.”
The plan will build on the RECOVER Initiative, a $1.15 billion effort announced last year that will study long COVID.
The COVID-19 Response Team also announced that the United States will donate tens of millions of pediatric coronavirus vaccines to other countries. More than 20 countries have asked for the donations, the team said.
The United States has delivered more than 500 million vaccine doses to 114 countries.
Meanwhile, national COVID-19 numbers continue to fall. CDC Director Rochelle Walensky, MD, reported that average daily cases are down 4% this week to 25,000; hospitalizations have dropped 17% to 1,400 per day; and daily deaths are down to 570 a day, which is a decrease of about 17%.
New national estimates show that Omicron’s subvariant BA.2 now accounts for 72% of circulating variants nationally, she said.
Top infectious disease expert Anthony Fauci, MD, reported that recent data supports the need for a second booster among certain people 50 and older – a move authorized by the Food and Drug Administration and Centers for Disease Control and Prevention last week.
“The effectiveness of the first booster dose we know wanes over time, and growing evidence shows a second dose can restore vaccine effectiveness for certain populations,” he said.
Dr. Fauci reported findings from an Israeli study of more than 1 million people 60 and older, which showed that an additional booster dose after 4 months lowered the rate of infection by two times and lowered the rate of severe infection by more than four times.
Another study from Israeli scientists showed that out of half a million people 60 and older, a second booster after 4 months brought a 78% reduction in death, compared to those who received only the first boost.
A version of this article first appeared on WebMD.com.
The National Research Action Plan on Long COVID will gather experts from various agencies, including the Department of Defense and the Department of Veterans Affairs, to expand existing long-COVID clinics and broaden research on symptoms of the virus that persist long after infection.
“We’ll collaborate with academic, industry, state and local partners to better understand long COVID,” Health and Human Services Secretary Xavier Becerra said at a White House briefing April 5. “We need to work as aggressively as we can to make sure no American is left behind.”
The plan will build on the RECOVER Initiative, a $1.15 billion effort announced last year that will study long COVID.
The COVID-19 Response Team also announced that the United States will donate tens of millions of pediatric coronavirus vaccines to other countries. More than 20 countries have asked for the donations, the team said.
The United States has delivered more than 500 million vaccine doses to 114 countries.
Meanwhile, national COVID-19 numbers continue to fall. CDC Director Rochelle Walensky, MD, reported that average daily cases are down 4% this week to 25,000; hospitalizations have dropped 17% to 1,400 per day; and daily deaths are down to 570 a day, which is a decrease of about 17%.
New national estimates show that Omicron’s subvariant BA.2 now accounts for 72% of circulating variants nationally, she said.
Top infectious disease expert Anthony Fauci, MD, reported that recent data supports the need for a second booster among certain people 50 and older – a move authorized by the Food and Drug Administration and Centers for Disease Control and Prevention last week.
“The effectiveness of the first booster dose we know wanes over time, and growing evidence shows a second dose can restore vaccine effectiveness for certain populations,” he said.
Dr. Fauci reported findings from an Israeli study of more than 1 million people 60 and older, which showed that an additional booster dose after 4 months lowered the rate of infection by two times and lowered the rate of severe infection by more than four times.
Another study from Israeli scientists showed that out of half a million people 60 and older, a second booster after 4 months brought a 78% reduction in death, compared to those who received only the first boost.
A version of this article first appeared on WebMD.com.
The National Research Action Plan on Long COVID will gather experts from various agencies, including the Department of Defense and the Department of Veterans Affairs, to expand existing long-COVID clinics and broaden research on symptoms of the virus that persist long after infection.
“We’ll collaborate with academic, industry, state and local partners to better understand long COVID,” Health and Human Services Secretary Xavier Becerra said at a White House briefing April 5. “We need to work as aggressively as we can to make sure no American is left behind.”
The plan will build on the RECOVER Initiative, a $1.15 billion effort announced last year that will study long COVID.
The COVID-19 Response Team also announced that the United States will donate tens of millions of pediatric coronavirus vaccines to other countries. More than 20 countries have asked for the donations, the team said.
The United States has delivered more than 500 million vaccine doses to 114 countries.
Meanwhile, national COVID-19 numbers continue to fall. CDC Director Rochelle Walensky, MD, reported that average daily cases are down 4% this week to 25,000; hospitalizations have dropped 17% to 1,400 per day; and daily deaths are down to 570 a day, which is a decrease of about 17%.
New national estimates show that Omicron’s subvariant BA.2 now accounts for 72% of circulating variants nationally, she said.
Top infectious disease expert Anthony Fauci, MD, reported that recent data supports the need for a second booster among certain people 50 and older – a move authorized by the Food and Drug Administration and Centers for Disease Control and Prevention last week.
“The effectiveness of the first booster dose we know wanes over time, and growing evidence shows a second dose can restore vaccine effectiveness for certain populations,” he said.
Dr. Fauci reported findings from an Israeli study of more than 1 million people 60 and older, which showed that an additional booster dose after 4 months lowered the rate of infection by two times and lowered the rate of severe infection by more than four times.
Another study from Israeli scientists showed that out of half a million people 60 and older, a second booster after 4 months brought a 78% reduction in death, compared to those who received only the first boost.
A version of this article first appeared on WebMD.com.
We all struggle with the unwritten rules of medical culture
There is a two-lane bridge in my town. It is quaint and picturesque, and when we first moved here, I would gaze out at the water as I drove, letting my mind wander along with the seagulls drifting alongside the car. Until one day, crossing back over, I passed a school bus stopped in the other lane, and instead of waving back, the driver gave me such a fierce look of disapproval I felt like I’d been to the principal’s office. What had I done?
I started paying more attention to the pattern of the other cars on the bridge. Although it appeared to be a standard two-lane width, the lanes weren’t quite wide enough if a school bus or large truck needed to cross at the same time as a car coming from the opposite direction. They had to wait until the other lane was clear. It was an unwritten rule of the town that if you saw a school bus on the other side, you stopped your car and yielded the bridge to the bus. It took me weeks to figure this out. When I did, I felt like I finally belonged in the community. Before, I’d been an outsider.
This got me thinking about culture. Every place has its unwritten rules, whether a community or a workplace. But how do we know the culture of a place? It’s pretty much impossible until we experience it for ourselves.
When I did figure out the bridge, I had a little bit of anger, to be honest. How was I supposed to know about the lanes? There weren’t any signs. Geez.
Now, when I approach the bridge, I don’t even think about it. I know what to do if I see a bus coming.
But sometimes I remember that time of confusion before I deciphered the unwritten rule. I still have a twinge of guilt for having done something wrong, even though it hadn’t been my fault.
It reminded me of a memory from medical training. I was an MS4, and my ER rotation was in a busy county hospital with a level I trauma center. To say that the place was chaotic would be an understatement.
On the first morning, I was shown the chart rack (yes, this was back in the day of paper charts). Charts were placed in the order that patients arrived. Med students and residents were to take a chart in chronological order, go triage and assess the patient, and then find an attending. Once finished, you put the chart back on the rack and picked up the next one. This was the extent of my orientation to the ER.
The days and weeks of the rotation flew by. It was a busy and exciting time. By the end of the month, I’d come to feel a part of the team.
Until one day, after finishing discharging a patient, an attending asked me, “Where’s the billing sheet?”
I had no idea what she was talking about. No one had ever shown me a billing sheet. But by this point, as an MS4, I knew well that if an attending asked you something you didn’t know the answer to, you shouldn’t just say that you didn’t know. You should try to figure out if you could at least approximate an answer first.
As I scrambled in my mind to figure out what she was asking me, she took one look at the apprehension in my eyes and asked again, raising her voice, “You haven’t been doing the billing sheets?”
I thought back to the first day of the rotation. The cursory 30-second orientation. Chart rack. Take one. See the patient. Put it back. See the next patient. Nothing about billing sheets.
“No,” I said. “No one ever told me about – ”
But the attending didn’t care that I hadn’t been instructed on the billing sheets. She ripped into me, yelling about how she couldn’t believe I’d been working there the entire month and was not doing the billing sheets. She showed me what they were and where they were supposed to be going and, in front of the whole staff, treated me like not only the biggest idiot she’d ever worked with but that the hospital had ever seen.
As she berated me, I thought about all the patients I’d seen that month. All the billing sheets I hadn’t placed in the pile. All the attendings who hadn’t gotten credit for the patients they’d staffed with me.
But how could I have known? I wanted to ask. How could I have known if nobody showed me or told me?
It was like the bridge. I was in a new environment and somehow expected to know the rules without anyone telling me; and when I didn’t know, people treated me like I’d done it the wrong way on purpose.
I didn’t end up saying anything more to that attending. What could I have said? She had already unleashed a mountain of her pent-up anger at me.
What I did decide in that moment was that I would never be an attending like that.
Like the bridge, this memory years later can still make me feel guilt and shame for doing something wrong. Even though it wasn’t my fault.
I was thinking about this recently with the Match. Thousands of freshly graduated medical students embarking on their new positions as interns in teaching hospitals across the country.
If someone treats you poorly for not knowing something, you are not an idiot. You’ve worked incredibly hard to get where you are, and you deserve to be there.
For attendings and more senior trainees, remember what it was like to be starting in a new place. We all make mistakes, and often it’s simply because of a lack of information.
Trainees shouldn’t have to suffer and be made to feel like outsiders until they figure out the unwritten rules of the place. They belong.
Dr. Lycette is medical director of Providence Oncology and Hematology Care Clinic, Seaside, Ore. She disclosed no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
There is a two-lane bridge in my town. It is quaint and picturesque, and when we first moved here, I would gaze out at the water as I drove, letting my mind wander along with the seagulls drifting alongside the car. Until one day, crossing back over, I passed a school bus stopped in the other lane, and instead of waving back, the driver gave me such a fierce look of disapproval I felt like I’d been to the principal’s office. What had I done?
I started paying more attention to the pattern of the other cars on the bridge. Although it appeared to be a standard two-lane width, the lanes weren’t quite wide enough if a school bus or large truck needed to cross at the same time as a car coming from the opposite direction. They had to wait until the other lane was clear. It was an unwritten rule of the town that if you saw a school bus on the other side, you stopped your car and yielded the bridge to the bus. It took me weeks to figure this out. When I did, I felt like I finally belonged in the community. Before, I’d been an outsider.
This got me thinking about culture. Every place has its unwritten rules, whether a community or a workplace. But how do we know the culture of a place? It’s pretty much impossible until we experience it for ourselves.
When I did figure out the bridge, I had a little bit of anger, to be honest. How was I supposed to know about the lanes? There weren’t any signs. Geez.
Now, when I approach the bridge, I don’t even think about it. I know what to do if I see a bus coming.
But sometimes I remember that time of confusion before I deciphered the unwritten rule. I still have a twinge of guilt for having done something wrong, even though it hadn’t been my fault.
It reminded me of a memory from medical training. I was an MS4, and my ER rotation was in a busy county hospital with a level I trauma center. To say that the place was chaotic would be an understatement.
On the first morning, I was shown the chart rack (yes, this was back in the day of paper charts). Charts were placed in the order that patients arrived. Med students and residents were to take a chart in chronological order, go triage and assess the patient, and then find an attending. Once finished, you put the chart back on the rack and picked up the next one. This was the extent of my orientation to the ER.
The days and weeks of the rotation flew by. It was a busy and exciting time. By the end of the month, I’d come to feel a part of the team.
Until one day, after finishing discharging a patient, an attending asked me, “Where’s the billing sheet?”
I had no idea what she was talking about. No one had ever shown me a billing sheet. But by this point, as an MS4, I knew well that if an attending asked you something you didn’t know the answer to, you shouldn’t just say that you didn’t know. You should try to figure out if you could at least approximate an answer first.
As I scrambled in my mind to figure out what she was asking me, she took one look at the apprehension in my eyes and asked again, raising her voice, “You haven’t been doing the billing sheets?”
I thought back to the first day of the rotation. The cursory 30-second orientation. Chart rack. Take one. See the patient. Put it back. See the next patient. Nothing about billing sheets.
“No,” I said. “No one ever told me about – ”
But the attending didn’t care that I hadn’t been instructed on the billing sheets. She ripped into me, yelling about how she couldn’t believe I’d been working there the entire month and was not doing the billing sheets. She showed me what they were and where they were supposed to be going and, in front of the whole staff, treated me like not only the biggest idiot she’d ever worked with but that the hospital had ever seen.
As she berated me, I thought about all the patients I’d seen that month. All the billing sheets I hadn’t placed in the pile. All the attendings who hadn’t gotten credit for the patients they’d staffed with me.
But how could I have known? I wanted to ask. How could I have known if nobody showed me or told me?
It was like the bridge. I was in a new environment and somehow expected to know the rules without anyone telling me; and when I didn’t know, people treated me like I’d done it the wrong way on purpose.
I didn’t end up saying anything more to that attending. What could I have said? She had already unleashed a mountain of her pent-up anger at me.
What I did decide in that moment was that I would never be an attending like that.
Like the bridge, this memory years later can still make me feel guilt and shame for doing something wrong. Even though it wasn’t my fault.
I was thinking about this recently with the Match. Thousands of freshly graduated medical students embarking on their new positions as interns in teaching hospitals across the country.
If someone treats you poorly for not knowing something, you are not an idiot. You’ve worked incredibly hard to get where you are, and you deserve to be there.
For attendings and more senior trainees, remember what it was like to be starting in a new place. We all make mistakes, and often it’s simply because of a lack of information.
Trainees shouldn’t have to suffer and be made to feel like outsiders until they figure out the unwritten rules of the place. They belong.
Dr. Lycette is medical director of Providence Oncology and Hematology Care Clinic, Seaside, Ore. She disclosed no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
There is a two-lane bridge in my town. It is quaint and picturesque, and when we first moved here, I would gaze out at the water as I drove, letting my mind wander along with the seagulls drifting alongside the car. Until one day, crossing back over, I passed a school bus stopped in the other lane, and instead of waving back, the driver gave me such a fierce look of disapproval I felt like I’d been to the principal’s office. What had I done?
I started paying more attention to the pattern of the other cars on the bridge. Although it appeared to be a standard two-lane width, the lanes weren’t quite wide enough if a school bus or large truck needed to cross at the same time as a car coming from the opposite direction. They had to wait until the other lane was clear. It was an unwritten rule of the town that if you saw a school bus on the other side, you stopped your car and yielded the bridge to the bus. It took me weeks to figure this out. When I did, I felt like I finally belonged in the community. Before, I’d been an outsider.
This got me thinking about culture. Every place has its unwritten rules, whether a community or a workplace. But how do we know the culture of a place? It’s pretty much impossible until we experience it for ourselves.
When I did figure out the bridge, I had a little bit of anger, to be honest. How was I supposed to know about the lanes? There weren’t any signs. Geez.
Now, when I approach the bridge, I don’t even think about it. I know what to do if I see a bus coming.
But sometimes I remember that time of confusion before I deciphered the unwritten rule. I still have a twinge of guilt for having done something wrong, even though it hadn’t been my fault.
It reminded me of a memory from medical training. I was an MS4, and my ER rotation was in a busy county hospital with a level I trauma center. To say that the place was chaotic would be an understatement.
On the first morning, I was shown the chart rack (yes, this was back in the day of paper charts). Charts were placed in the order that patients arrived. Med students and residents were to take a chart in chronological order, go triage and assess the patient, and then find an attending. Once finished, you put the chart back on the rack and picked up the next one. This was the extent of my orientation to the ER.
The days and weeks of the rotation flew by. It was a busy and exciting time. By the end of the month, I’d come to feel a part of the team.
Until one day, after finishing discharging a patient, an attending asked me, “Where’s the billing sheet?”
I had no idea what she was talking about. No one had ever shown me a billing sheet. But by this point, as an MS4, I knew well that if an attending asked you something you didn’t know the answer to, you shouldn’t just say that you didn’t know. You should try to figure out if you could at least approximate an answer first.
As I scrambled in my mind to figure out what she was asking me, she took one look at the apprehension in my eyes and asked again, raising her voice, “You haven’t been doing the billing sheets?”
I thought back to the first day of the rotation. The cursory 30-second orientation. Chart rack. Take one. See the patient. Put it back. See the next patient. Nothing about billing sheets.
“No,” I said. “No one ever told me about – ”
But the attending didn’t care that I hadn’t been instructed on the billing sheets. She ripped into me, yelling about how she couldn’t believe I’d been working there the entire month and was not doing the billing sheets. She showed me what they were and where they were supposed to be going and, in front of the whole staff, treated me like not only the biggest idiot she’d ever worked with but that the hospital had ever seen.
As she berated me, I thought about all the patients I’d seen that month. All the billing sheets I hadn’t placed in the pile. All the attendings who hadn’t gotten credit for the patients they’d staffed with me.
But how could I have known? I wanted to ask. How could I have known if nobody showed me or told me?
It was like the bridge. I was in a new environment and somehow expected to know the rules without anyone telling me; and when I didn’t know, people treated me like I’d done it the wrong way on purpose.
I didn’t end up saying anything more to that attending. What could I have said? She had already unleashed a mountain of her pent-up anger at me.
What I did decide in that moment was that I would never be an attending like that.
Like the bridge, this memory years later can still make me feel guilt and shame for doing something wrong. Even though it wasn’t my fault.
I was thinking about this recently with the Match. Thousands of freshly graduated medical students embarking on their new positions as interns in teaching hospitals across the country.
If someone treats you poorly for not knowing something, you are not an idiot. You’ve worked incredibly hard to get where you are, and you deserve to be there.
For attendings and more senior trainees, remember what it was like to be starting in a new place. We all make mistakes, and often it’s simply because of a lack of information.
Trainees shouldn’t have to suffer and be made to feel like outsiders until they figure out the unwritten rules of the place. They belong.
Dr. Lycette is medical director of Providence Oncology and Hematology Care Clinic, Seaside, Ore. She disclosed no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
The importance of treating insomnia in psychiatric illness
Data suggests this symptom, defined as chronic sleep onset and/or sleep continuity problems associated with impaired daytime functioning, is common in psychiatric illnesses, and can worsen their course.2
The incidence of psychiatric illness in patients with insomnia is estimated at near 50%, with the highest rates found in mood disorders such as depression and bipolar disorder, as well as anxiety disorders.3 In patients with diagnosed major depressive disorder, insomnia rates can approach 90%.4-6
Insomnia has been identified as a risk factor for development of mental illness, including doubling the risk of major depressive disorder and tripling the risk of any depressive or anxiety disorder.7,8 It can also significantly increase the risk of alcohol abuse and psychosis.8
Sleep disturbances can worsen symptoms of diagnosed mental illness, including substance abuse, mood and psychotic disorders.9-10 In one study, nearly 75% of patients with a diagnosis of schizophrenia or bipolar spectrum disorder had at least one type of sleep disturbance (insomnia, hypersomnia, or delayed sleep phase).10 This was almost twice the rate in healthy controls. Importantly, compared with well-rested subjects with mental illness in this study, sleep-disordered participants had higher rates of negative and depressive symptoms on the Positive and Negative Syndrome Scale, as well as significantly lower function via the global assessment of functioning.11,12
Additional data suggests simply being awake during the night (00:00-05:59) elevates risk of suicide. The mean incident rate of completed suicide in one study was a striking four times the rate noted during daytime hours (06:00-23:59 ) (P < .001).13
Although insomnia symptoms can resolve after relief from a particular life stressor, as many as half of patients with more severe symptoms develop a chronic course.14 This then leads to an extended use of many types of sedative-hypnotics designed and studied primarily for short-term use.15 In a survey reviewing national use of prescription drugs for insomnia, as many as 20% of individuals use a medication to target insomnia in a given month.16
Fortunately, despite the many challenges posed by COVID-19, particularly for those with psychiatric illness and limited access to care, telehealth has become more readily available. Additionally, digital versions of evidence-based treatments specifically for sleep problems, such as cognitive-behavioral therapy for insomnia (CBT-I), are regularly being developed.
The benefits of CBT-I have been demonstrated repeatedly and it is recommended as the first line treatment for insomnia by the Clinical Guidelines of the American Academy of Sleep Medicine, the Centers for Disease Control and Prevention, and the National Institutes of Health.17-21 Studies suggest benefits persist long-term, even after completing the therapy sessions, which differ in durability from medication choices.18
One group that may be particularly suited for treatment with CBT-I is women with insomnia during pregnancy or the postpartum period. In these women, options for treatment may be limited by risk of medication during breastfeeding, as well as difficulty traveling to a physician’s or therapist’s office to receive psychotherapy. However, two recent studies evaluated the use of digital CBT-I to treat insomnia during pregnancy and in the postpartum period, respectively.22-23
In both studies,the same group of women with insomnia diagnosed during pregnancy were given six weekly 20-minute sessions of digital CBT-I or standard treatment for insomnia, including medication and psychotherapy per their usual provider.
By study end, the pregnant women receiving the CBT-I intervention not only had significantly improved severity of insomnia, they also experienced improved depression and anxiety symptoms, and a decrease in the use of prescription or over-the-counter sleep aides, compared with the standard treatment group, lowering the fetal exposure to medication during pregnancy.22
In the more recent study, the same group was followed for 6 months post partum.23 Results were again notable, with the women who received CBT-I reporting significantly less insomnia, as well as significantly lower rates of probable major depression at 3 and 6 months (18% vs. 4%, 10% vs. 0%, respectively.) They also exhibited lower rates of moderate to severe anxiety (17% vs. 4%) at 3 months, compared with those receiving standard care. With as many as one in seven women suffering from postpartum depression, these findings represent a substantial public health benefit.
In summary, insomnia is a critical area of focus for any provider diagnosing and treating psychiatric illness. Attempts to optimize sleep, whether through CBT-I or other psychotherapy approaches, or evidence-based medications dosed for appropriate lengths and at safe doses, should be a part of most, if not all, clinical encounters.
Dr. Reid is a board-certified psychiatrist and award-winning medical educator with a private practice in Philadelphia, as well as a clinical faculty role at the University of Pennsylvania, also in Philadelphia. She attended medical school at Columbia University, New York, and completed her psychiatry residency at the University of California, Los Angeles. Dr. Reid is a regular contributor to Psychology Today with her blog, “Think Like a Shrink,” and writes and podcasts as The Reflective Doc.
References
1. Voitsidis P et al. Psychiatry Res. 2020 Jul;289:113076. doi: 10.1016/j.psychres.2020.113076.
2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, Va.: American Psychiatric Publishing, 2013.
3. Ford DE and Kamerow DB. JAMA. 1989;262(11):1479-84. doi: 10.1001/jama.1989.03430110069030.
4. Ohayon MM and Roth T. J Psychiatr Res. Jan-Feb 2003;37(1):9-15. doi: 10.1016/s0022-3956(02)00052-3.
5. Seow LSE et al. J Ment Health. 2016 Dec;25(6):492-9. doi: 10.3109/09638237.2015.1124390.
6. Thase ME. J Clin Psychiatry. 1999;60 Suppl 17:28-31; discussion 46-8.
7. Baglioni C et al. J Affect Disord. 2011 Dec;135(1-3):10-9. doi: 10.1016/j.jad.2011.01.011.
8. Hertenstein E et al. Sleep Med Rev. 2019 Feb;43:96-105. doi: 10.1016/j.smrv.2018.10.006.
9. Brower KJ et al. Medical Hypotheses. 2010;74(5):928-33. doi: 10.1016/j.mehy.2009.10.020.
10. Laskemoen JF et al. Compr Psychiatry. 2019 May;91:6-12. doi: 10.1016/j.comppsych.2019.02.006.
11. Kay SR et al. Schizophr Bull. 1987;13(2):261-76. doi: 10.1093/schbul/13.2.261.
12. Hall R. Psychosomatics. May-Jun 1995;36(3):267-75. doi: 10.1016/S0033-3182(95)71666-8.
13. Perlis ML et al. J Clin Psychiatry. 2016 Jun;77(6):e726-33. doi: 10.4088/JCP.15m10131.
14. Morin CM et al. Arch Intern Med. 2009 Mar 9. doi: 10.1001/archinternmed.2008.610.
15. Cheung J et al. Sleep Med Clin. 2019 Jun;14(2):253-65. doi: 10.1016/j.jsmc.2019.01.006.
16. Bertisch SM et al. Sleep. 2014 Feb 1. doi: 10.5665/sleep.3410.
17. Okajima I et al. Sleep Biol Rhythms. 2010 Nov 28. doi: 10.1111/j.1479-8425.2010.00481.x.
18. Trauer JM et al. Ann Intern Med. 2015 Aug 4. doi: 10.7326/M14-2841.
19. Edinger J et al. J Clin Sleep Med. 2021 Feb 1. doi: 10.5664/jcsm.8986.
20. U.S. Centers for Disease Control and Prevention. https://www.cdc.gov/sleep/for-clinicians.html.
21. National Institutes of Health. Sleep Health. https://www.nhlbi.nih.gov/health-topics/education-and-awareness/sleep-health.
22. Felder JN et al. JAMA Psychiatry. 2020;77(5):484-92. doi:10.1001/jamapsychiatry.2019.4491.
23. Felder JN et al. Sleep. 2022 Feb 14. doi: 10.1093/sleep/zsab280.
Data suggests this symptom, defined as chronic sleep onset and/or sleep continuity problems associated with impaired daytime functioning, is common in psychiatric illnesses, and can worsen their course.2
The incidence of psychiatric illness in patients with insomnia is estimated at near 50%, with the highest rates found in mood disorders such as depression and bipolar disorder, as well as anxiety disorders.3 In patients with diagnosed major depressive disorder, insomnia rates can approach 90%.4-6
Insomnia has been identified as a risk factor for development of mental illness, including doubling the risk of major depressive disorder and tripling the risk of any depressive or anxiety disorder.7,8 It can also significantly increase the risk of alcohol abuse and psychosis.8
Sleep disturbances can worsen symptoms of diagnosed mental illness, including substance abuse, mood and psychotic disorders.9-10 In one study, nearly 75% of patients with a diagnosis of schizophrenia or bipolar spectrum disorder had at least one type of sleep disturbance (insomnia, hypersomnia, or delayed sleep phase).10 This was almost twice the rate in healthy controls. Importantly, compared with well-rested subjects with mental illness in this study, sleep-disordered participants had higher rates of negative and depressive symptoms on the Positive and Negative Syndrome Scale, as well as significantly lower function via the global assessment of functioning.11,12
Additional data suggests simply being awake during the night (00:00-05:59) elevates risk of suicide. The mean incident rate of completed suicide in one study was a striking four times the rate noted during daytime hours (06:00-23:59 ) (P < .001).13
Although insomnia symptoms can resolve after relief from a particular life stressor, as many as half of patients with more severe symptoms develop a chronic course.14 This then leads to an extended use of many types of sedative-hypnotics designed and studied primarily for short-term use.15 In a survey reviewing national use of prescription drugs for insomnia, as many as 20% of individuals use a medication to target insomnia in a given month.16
Fortunately, despite the many challenges posed by COVID-19, particularly for those with psychiatric illness and limited access to care, telehealth has become more readily available. Additionally, digital versions of evidence-based treatments specifically for sleep problems, such as cognitive-behavioral therapy for insomnia (CBT-I), are regularly being developed.
The benefits of CBT-I have been demonstrated repeatedly and it is recommended as the first line treatment for insomnia by the Clinical Guidelines of the American Academy of Sleep Medicine, the Centers for Disease Control and Prevention, and the National Institutes of Health.17-21 Studies suggest benefits persist long-term, even after completing the therapy sessions, which differ in durability from medication choices.18
One group that may be particularly suited for treatment with CBT-I is women with insomnia during pregnancy or the postpartum period. In these women, options for treatment may be limited by risk of medication during breastfeeding, as well as difficulty traveling to a physician’s or therapist’s office to receive psychotherapy. However, two recent studies evaluated the use of digital CBT-I to treat insomnia during pregnancy and in the postpartum period, respectively.22-23
In both studies,the same group of women with insomnia diagnosed during pregnancy were given six weekly 20-minute sessions of digital CBT-I or standard treatment for insomnia, including medication and psychotherapy per their usual provider.
By study end, the pregnant women receiving the CBT-I intervention not only had significantly improved severity of insomnia, they also experienced improved depression and anxiety symptoms, and a decrease in the use of prescription or over-the-counter sleep aides, compared with the standard treatment group, lowering the fetal exposure to medication during pregnancy.22
In the more recent study, the same group was followed for 6 months post partum.23 Results were again notable, with the women who received CBT-I reporting significantly less insomnia, as well as significantly lower rates of probable major depression at 3 and 6 months (18% vs. 4%, 10% vs. 0%, respectively.) They also exhibited lower rates of moderate to severe anxiety (17% vs. 4%) at 3 months, compared with those receiving standard care. With as many as one in seven women suffering from postpartum depression, these findings represent a substantial public health benefit.
In summary, insomnia is a critical area of focus for any provider diagnosing and treating psychiatric illness. Attempts to optimize sleep, whether through CBT-I or other psychotherapy approaches, or evidence-based medications dosed for appropriate lengths and at safe doses, should be a part of most, if not all, clinical encounters.
Dr. Reid is a board-certified psychiatrist and award-winning medical educator with a private practice in Philadelphia, as well as a clinical faculty role at the University of Pennsylvania, also in Philadelphia. She attended medical school at Columbia University, New York, and completed her psychiatry residency at the University of California, Los Angeles. Dr. Reid is a regular contributor to Psychology Today with her blog, “Think Like a Shrink,” and writes and podcasts as The Reflective Doc.
References
1. Voitsidis P et al. Psychiatry Res. 2020 Jul;289:113076. doi: 10.1016/j.psychres.2020.113076.
2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, Va.: American Psychiatric Publishing, 2013.
3. Ford DE and Kamerow DB. JAMA. 1989;262(11):1479-84. doi: 10.1001/jama.1989.03430110069030.
4. Ohayon MM and Roth T. J Psychiatr Res. Jan-Feb 2003;37(1):9-15. doi: 10.1016/s0022-3956(02)00052-3.
5. Seow LSE et al. J Ment Health. 2016 Dec;25(6):492-9. doi: 10.3109/09638237.2015.1124390.
6. Thase ME. J Clin Psychiatry. 1999;60 Suppl 17:28-31; discussion 46-8.
7. Baglioni C et al. J Affect Disord. 2011 Dec;135(1-3):10-9. doi: 10.1016/j.jad.2011.01.011.
8. Hertenstein E et al. Sleep Med Rev. 2019 Feb;43:96-105. doi: 10.1016/j.smrv.2018.10.006.
9. Brower KJ et al. Medical Hypotheses. 2010;74(5):928-33. doi: 10.1016/j.mehy.2009.10.020.
10. Laskemoen JF et al. Compr Psychiatry. 2019 May;91:6-12. doi: 10.1016/j.comppsych.2019.02.006.
11. Kay SR et al. Schizophr Bull. 1987;13(2):261-76. doi: 10.1093/schbul/13.2.261.
12. Hall R. Psychosomatics. May-Jun 1995;36(3):267-75. doi: 10.1016/S0033-3182(95)71666-8.
13. Perlis ML et al. J Clin Psychiatry. 2016 Jun;77(6):e726-33. doi: 10.4088/JCP.15m10131.
14. Morin CM et al. Arch Intern Med. 2009 Mar 9. doi: 10.1001/archinternmed.2008.610.
15. Cheung J et al. Sleep Med Clin. 2019 Jun;14(2):253-65. doi: 10.1016/j.jsmc.2019.01.006.
16. Bertisch SM et al. Sleep. 2014 Feb 1. doi: 10.5665/sleep.3410.
17. Okajima I et al. Sleep Biol Rhythms. 2010 Nov 28. doi: 10.1111/j.1479-8425.2010.00481.x.
18. Trauer JM et al. Ann Intern Med. 2015 Aug 4. doi: 10.7326/M14-2841.
19. Edinger J et al. J Clin Sleep Med. 2021 Feb 1. doi: 10.5664/jcsm.8986.
20. U.S. Centers for Disease Control and Prevention. https://www.cdc.gov/sleep/for-clinicians.html.
21. National Institutes of Health. Sleep Health. https://www.nhlbi.nih.gov/health-topics/education-and-awareness/sleep-health.
22. Felder JN et al. JAMA Psychiatry. 2020;77(5):484-92. doi:10.1001/jamapsychiatry.2019.4491.
23. Felder JN et al. Sleep. 2022 Feb 14. doi: 10.1093/sleep/zsab280.
Data suggests this symptom, defined as chronic sleep onset and/or sleep continuity problems associated with impaired daytime functioning, is common in psychiatric illnesses, and can worsen their course.2
The incidence of psychiatric illness in patients with insomnia is estimated at near 50%, with the highest rates found in mood disorders such as depression and bipolar disorder, as well as anxiety disorders.3 In patients with diagnosed major depressive disorder, insomnia rates can approach 90%.4-6
Insomnia has been identified as a risk factor for development of mental illness, including doubling the risk of major depressive disorder and tripling the risk of any depressive or anxiety disorder.7,8 It can also significantly increase the risk of alcohol abuse and psychosis.8
Sleep disturbances can worsen symptoms of diagnosed mental illness, including substance abuse, mood and psychotic disorders.9-10 In one study, nearly 75% of patients with a diagnosis of schizophrenia or bipolar spectrum disorder had at least one type of sleep disturbance (insomnia, hypersomnia, or delayed sleep phase).10 This was almost twice the rate in healthy controls. Importantly, compared with well-rested subjects with mental illness in this study, sleep-disordered participants had higher rates of negative and depressive symptoms on the Positive and Negative Syndrome Scale, as well as significantly lower function via the global assessment of functioning.11,12
Additional data suggests simply being awake during the night (00:00-05:59) elevates risk of suicide. The mean incident rate of completed suicide in one study was a striking four times the rate noted during daytime hours (06:00-23:59 ) (P < .001).13
Although insomnia symptoms can resolve after relief from a particular life stressor, as many as half of patients with more severe symptoms develop a chronic course.14 This then leads to an extended use of many types of sedative-hypnotics designed and studied primarily for short-term use.15 In a survey reviewing national use of prescription drugs for insomnia, as many as 20% of individuals use a medication to target insomnia in a given month.16
Fortunately, despite the many challenges posed by COVID-19, particularly for those with psychiatric illness and limited access to care, telehealth has become more readily available. Additionally, digital versions of evidence-based treatments specifically for sleep problems, such as cognitive-behavioral therapy for insomnia (CBT-I), are regularly being developed.
The benefits of CBT-I have been demonstrated repeatedly and it is recommended as the first line treatment for insomnia by the Clinical Guidelines of the American Academy of Sleep Medicine, the Centers for Disease Control and Prevention, and the National Institutes of Health.17-21 Studies suggest benefits persist long-term, even after completing the therapy sessions, which differ in durability from medication choices.18
One group that may be particularly suited for treatment with CBT-I is women with insomnia during pregnancy or the postpartum period. In these women, options for treatment may be limited by risk of medication during breastfeeding, as well as difficulty traveling to a physician’s or therapist’s office to receive psychotherapy. However, two recent studies evaluated the use of digital CBT-I to treat insomnia during pregnancy and in the postpartum period, respectively.22-23
In both studies,the same group of women with insomnia diagnosed during pregnancy were given six weekly 20-minute sessions of digital CBT-I or standard treatment for insomnia, including medication and psychotherapy per their usual provider.
By study end, the pregnant women receiving the CBT-I intervention not only had significantly improved severity of insomnia, they also experienced improved depression and anxiety symptoms, and a decrease in the use of prescription or over-the-counter sleep aides, compared with the standard treatment group, lowering the fetal exposure to medication during pregnancy.22
In the more recent study, the same group was followed for 6 months post partum.23 Results were again notable, with the women who received CBT-I reporting significantly less insomnia, as well as significantly lower rates of probable major depression at 3 and 6 months (18% vs. 4%, 10% vs. 0%, respectively.) They also exhibited lower rates of moderate to severe anxiety (17% vs. 4%) at 3 months, compared with those receiving standard care. With as many as one in seven women suffering from postpartum depression, these findings represent a substantial public health benefit.
In summary, insomnia is a critical area of focus for any provider diagnosing and treating psychiatric illness. Attempts to optimize sleep, whether through CBT-I or other psychotherapy approaches, or evidence-based medications dosed for appropriate lengths and at safe doses, should be a part of most, if not all, clinical encounters.
Dr. Reid is a board-certified psychiatrist and award-winning medical educator with a private practice in Philadelphia, as well as a clinical faculty role at the University of Pennsylvania, also in Philadelphia. She attended medical school at Columbia University, New York, and completed her psychiatry residency at the University of California, Los Angeles. Dr. Reid is a regular contributor to Psychology Today with her blog, “Think Like a Shrink,” and writes and podcasts as The Reflective Doc.
References
1. Voitsidis P et al. Psychiatry Res. 2020 Jul;289:113076. doi: 10.1016/j.psychres.2020.113076.
2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, Va.: American Psychiatric Publishing, 2013.
3. Ford DE and Kamerow DB. JAMA. 1989;262(11):1479-84. doi: 10.1001/jama.1989.03430110069030.
4. Ohayon MM and Roth T. J Psychiatr Res. Jan-Feb 2003;37(1):9-15. doi: 10.1016/s0022-3956(02)00052-3.
5. Seow LSE et al. J Ment Health. 2016 Dec;25(6):492-9. doi: 10.3109/09638237.2015.1124390.
6. Thase ME. J Clin Psychiatry. 1999;60 Suppl 17:28-31; discussion 46-8.
7. Baglioni C et al. J Affect Disord. 2011 Dec;135(1-3):10-9. doi: 10.1016/j.jad.2011.01.011.
8. Hertenstein E et al. Sleep Med Rev. 2019 Feb;43:96-105. doi: 10.1016/j.smrv.2018.10.006.
9. Brower KJ et al. Medical Hypotheses. 2010;74(5):928-33. doi: 10.1016/j.mehy.2009.10.020.
10. Laskemoen JF et al. Compr Psychiatry. 2019 May;91:6-12. doi: 10.1016/j.comppsych.2019.02.006.
11. Kay SR et al. Schizophr Bull. 1987;13(2):261-76. doi: 10.1093/schbul/13.2.261.
12. Hall R. Psychosomatics. May-Jun 1995;36(3):267-75. doi: 10.1016/S0033-3182(95)71666-8.
13. Perlis ML et al. J Clin Psychiatry. 2016 Jun;77(6):e726-33. doi: 10.4088/JCP.15m10131.
14. Morin CM et al. Arch Intern Med. 2009 Mar 9. doi: 10.1001/archinternmed.2008.610.
15. Cheung J et al. Sleep Med Clin. 2019 Jun;14(2):253-65. doi: 10.1016/j.jsmc.2019.01.006.
16. Bertisch SM et al. Sleep. 2014 Feb 1. doi: 10.5665/sleep.3410.
17. Okajima I et al. Sleep Biol Rhythms. 2010 Nov 28. doi: 10.1111/j.1479-8425.2010.00481.x.
18. Trauer JM et al. Ann Intern Med. 2015 Aug 4. doi: 10.7326/M14-2841.
19. Edinger J et al. J Clin Sleep Med. 2021 Feb 1. doi: 10.5664/jcsm.8986.
20. U.S. Centers for Disease Control and Prevention. https://www.cdc.gov/sleep/for-clinicians.html.
21. National Institutes of Health. Sleep Health. https://www.nhlbi.nih.gov/health-topics/education-and-awareness/sleep-health.
22. Felder JN et al. JAMA Psychiatry. 2020;77(5):484-92. doi:10.1001/jamapsychiatry.2019.4491.
23. Felder JN et al. Sleep. 2022 Feb 14. doi: 10.1093/sleep/zsab280.
IBD: Patients struggle with presenteeism, mental health
Anxiety and depression are common among individuals with inflammatory bowel disease (IBD) who are in remission, and the conditions are linked to unemployment and presenteeism, according to a prospective study.
Previous studies have found a heightened risk of anxiety and depression in IBD, as well as an association with poor treatment compliance and greater morbidity. Presenteeism, defined as reduced productivity due to a physical or mental condition, is increasingly recognized as an indirect economic cost of chronic conditions that may exact a higher cost than absenteeism.
More than one-third of patients with IBD experienced presenteeism in one study. Other studies have examined exercise in chronic diseases, and most find an association between more exercise and lower rates of anxiety and depression. To date, few studies have examined a combination of physical activity, mental health, and presenteeism in the context of IBD.
The new study, published in the Journal of Crohn’s and Colitis, “is very important, as presenteeism is not commonly discussed in any formal, measurable way in the IBD space,” said Laurie Keefer, PhD, professor of medicine and a gastropsychologist at Icahn School of Medicine, who was asked to comment on the research. “While conducted in Europe and Israel, the study also has relevance in the U.S. since, here, employers typically pay for health care – so absenteeism, presenteeism, and health care cost are all intertwined.”
“The study confirmed high rates of depression and anxiety after a diagnosis of IBD. One high-quality aspect of this study was that the diagnosis of depression and anxiety was confirmed by a medical practitioner,” said Dr. Keefer.
The results suggest that current efforts to screen IBD patients for depression and anxiety may not be enough, according to Stephen Lupe, PsyD, who was also asked to comment on the study. He noted that almost half of the patients had symptoms of anxiety or depression as measured by the Hospital Anxiety and Depression Score (HADS). “We’re missing all kinds of people,” said Dr. Lupe, who is director of behavioral medicine for the department of gastroenterology, hepatology, and nutrition at the Cleveland Clinic. He cited other data that show that, if someone develops depression, they are more likely to have a surgical outcome, and they are more likely to have problems with medication adherence. They likely will have more flares and less time in remission. “We need to be screening for it as part of care,” he said.
The researchers prospectively studied 585 IBD patients who were in clinical remission at 8 centers in Europe and Israel between September 2020 and March 2021. Participants filled out the HADS and The Stanford Presenteeism scale (SPS-6). A total of 62.2% of the participants had CD; 53.0% were male. Participants’ mean age was 39 years. Among the group, 10.8% had a pre existing diagnosis of anxiety or depression at the time of IBD diagnosis, and an additional 14.2% were later diagnosed with anxiety or depression.
Less than half, 46.1%, of IBD patients had a score 8 or higher in the HADS-anxiety (HADS-A) or HADS-depression (HADS-D) subscale, a cutoff that suggests evidence of clinical depression or anxiety. A total of 27.4% had a score of 11 or higher, indicating a mood disorder. High HADS-A score was associated with female gender (odds ratio, 1.91; P < 0.05), long duration of disease (OR, 1.04; P < 0.01), and perianal disease (P < 0.023). The authors speculate that the latter result may be due to a higher burden of symptoms. Three-quarters, 74.5%, of patients were employed; 34.0% experienced presenteeism as defined by SPS-6 score less than or equal to 18.
The researchers found that 23.0% of the patients were sedentary, and this was more common among individuals with HADS-A or HADS-D scores greater than or equal to 8. Among those experiencing presenteeism, 50% were sedentary, 29.4% were active, and 20.6% were moderately active (P < 0.01). Individuals with higher HADS-A or HADS-D scores had a greater likelihood of being sedentary (P < 0.05).
One limitation of the study was that the questionnaires were translated into the respective languages and scores were taken at only one time point.
“Rather than relying on the patient to come forward and seek help,” physicians should familiarize themselves with these validated screening tools “in order to increase the diagnostic rate of such pathologies and enable a better holistic care for the IBD patients,” the authors concluded. “Active involvement of a psychologist and/or a psychiatrist, as part of the IBD team, should be pursued to further improve the patients’ quality of life, which has emerged as one of the top priority outcomes in IBD.”
Dr. Lupe said that the findings regarding presenteeism are consistent with his experience. He pointed out that IBD patients must be more aware of their body and vigilant in managing symptoms, and he speculated that that could detract from concentration at work. He said that the study shows the need for a holistic approach to treatment. “When someone is coping with a chronic disease, like ulcerative colitis or Crohn’s disease, it affects the whole person,” including psychologically, professionally, and personally. “These are bidirectional relationships, so that if someone’s social life starts falling down, it’s more contributory to the development of something like depression and anxiety, and maybe that’s contributory to complications that come up in a disease state like Crohn’s disease or ulcerative colitis.”
The study did not receive funding, but two authors disclosed relations with AbbVie, Janssen, Pfizer, and other companies. Dr. Keefer is a cofounder and has equity ownership In Trellus Health. Dr. Lupe has no relevant financial disclosures.
Anxiety and depression are common among individuals with inflammatory bowel disease (IBD) who are in remission, and the conditions are linked to unemployment and presenteeism, according to a prospective study.
Previous studies have found a heightened risk of anxiety and depression in IBD, as well as an association with poor treatment compliance and greater morbidity. Presenteeism, defined as reduced productivity due to a physical or mental condition, is increasingly recognized as an indirect economic cost of chronic conditions that may exact a higher cost than absenteeism.
More than one-third of patients with IBD experienced presenteeism in one study. Other studies have examined exercise in chronic diseases, and most find an association between more exercise and lower rates of anxiety and depression. To date, few studies have examined a combination of physical activity, mental health, and presenteeism in the context of IBD.
The new study, published in the Journal of Crohn’s and Colitis, “is very important, as presenteeism is not commonly discussed in any formal, measurable way in the IBD space,” said Laurie Keefer, PhD, professor of medicine and a gastropsychologist at Icahn School of Medicine, who was asked to comment on the research. “While conducted in Europe and Israel, the study also has relevance in the U.S. since, here, employers typically pay for health care – so absenteeism, presenteeism, and health care cost are all intertwined.”
“The study confirmed high rates of depression and anxiety after a diagnosis of IBD. One high-quality aspect of this study was that the diagnosis of depression and anxiety was confirmed by a medical practitioner,” said Dr. Keefer.
The results suggest that current efforts to screen IBD patients for depression and anxiety may not be enough, according to Stephen Lupe, PsyD, who was also asked to comment on the study. He noted that almost half of the patients had symptoms of anxiety or depression as measured by the Hospital Anxiety and Depression Score (HADS). “We’re missing all kinds of people,” said Dr. Lupe, who is director of behavioral medicine for the department of gastroenterology, hepatology, and nutrition at the Cleveland Clinic. He cited other data that show that, if someone develops depression, they are more likely to have a surgical outcome, and they are more likely to have problems with medication adherence. They likely will have more flares and less time in remission. “We need to be screening for it as part of care,” he said.
The researchers prospectively studied 585 IBD patients who were in clinical remission at 8 centers in Europe and Israel between September 2020 and March 2021. Participants filled out the HADS and The Stanford Presenteeism scale (SPS-6). A total of 62.2% of the participants had CD; 53.0% were male. Participants’ mean age was 39 years. Among the group, 10.8% had a pre existing diagnosis of anxiety or depression at the time of IBD diagnosis, and an additional 14.2% were later diagnosed with anxiety or depression.
Less than half, 46.1%, of IBD patients had a score 8 or higher in the HADS-anxiety (HADS-A) or HADS-depression (HADS-D) subscale, a cutoff that suggests evidence of clinical depression or anxiety. A total of 27.4% had a score of 11 or higher, indicating a mood disorder. High HADS-A score was associated with female gender (odds ratio, 1.91; P < 0.05), long duration of disease (OR, 1.04; P < 0.01), and perianal disease (P < 0.023). The authors speculate that the latter result may be due to a higher burden of symptoms. Three-quarters, 74.5%, of patients were employed; 34.0% experienced presenteeism as defined by SPS-6 score less than or equal to 18.
The researchers found that 23.0% of the patients were sedentary, and this was more common among individuals with HADS-A or HADS-D scores greater than or equal to 8. Among those experiencing presenteeism, 50% were sedentary, 29.4% were active, and 20.6% were moderately active (P < 0.01). Individuals with higher HADS-A or HADS-D scores had a greater likelihood of being sedentary (P < 0.05).
One limitation of the study was that the questionnaires were translated into the respective languages and scores were taken at only one time point.
“Rather than relying on the patient to come forward and seek help,” physicians should familiarize themselves with these validated screening tools “in order to increase the diagnostic rate of such pathologies and enable a better holistic care for the IBD patients,” the authors concluded. “Active involvement of a psychologist and/or a psychiatrist, as part of the IBD team, should be pursued to further improve the patients’ quality of life, which has emerged as one of the top priority outcomes in IBD.”
Dr. Lupe said that the findings regarding presenteeism are consistent with his experience. He pointed out that IBD patients must be more aware of their body and vigilant in managing symptoms, and he speculated that that could detract from concentration at work. He said that the study shows the need for a holistic approach to treatment. “When someone is coping with a chronic disease, like ulcerative colitis or Crohn’s disease, it affects the whole person,” including psychologically, professionally, and personally. “These are bidirectional relationships, so that if someone’s social life starts falling down, it’s more contributory to the development of something like depression and anxiety, and maybe that’s contributory to complications that come up in a disease state like Crohn’s disease or ulcerative colitis.”
The study did not receive funding, but two authors disclosed relations with AbbVie, Janssen, Pfizer, and other companies. Dr. Keefer is a cofounder and has equity ownership In Trellus Health. Dr. Lupe has no relevant financial disclosures.
Anxiety and depression are common among individuals with inflammatory bowel disease (IBD) who are in remission, and the conditions are linked to unemployment and presenteeism, according to a prospective study.
Previous studies have found a heightened risk of anxiety and depression in IBD, as well as an association with poor treatment compliance and greater morbidity. Presenteeism, defined as reduced productivity due to a physical or mental condition, is increasingly recognized as an indirect economic cost of chronic conditions that may exact a higher cost than absenteeism.
More than one-third of patients with IBD experienced presenteeism in one study. Other studies have examined exercise in chronic diseases, and most find an association between more exercise and lower rates of anxiety and depression. To date, few studies have examined a combination of physical activity, mental health, and presenteeism in the context of IBD.
The new study, published in the Journal of Crohn’s and Colitis, “is very important, as presenteeism is not commonly discussed in any formal, measurable way in the IBD space,” said Laurie Keefer, PhD, professor of medicine and a gastropsychologist at Icahn School of Medicine, who was asked to comment on the research. “While conducted in Europe and Israel, the study also has relevance in the U.S. since, here, employers typically pay for health care – so absenteeism, presenteeism, and health care cost are all intertwined.”
“The study confirmed high rates of depression and anxiety after a diagnosis of IBD. One high-quality aspect of this study was that the diagnosis of depression and anxiety was confirmed by a medical practitioner,” said Dr. Keefer.
The results suggest that current efforts to screen IBD patients for depression and anxiety may not be enough, according to Stephen Lupe, PsyD, who was also asked to comment on the study. He noted that almost half of the patients had symptoms of anxiety or depression as measured by the Hospital Anxiety and Depression Score (HADS). “We’re missing all kinds of people,” said Dr. Lupe, who is director of behavioral medicine for the department of gastroenterology, hepatology, and nutrition at the Cleveland Clinic. He cited other data that show that, if someone develops depression, they are more likely to have a surgical outcome, and they are more likely to have problems with medication adherence. They likely will have more flares and less time in remission. “We need to be screening for it as part of care,” he said.
The researchers prospectively studied 585 IBD patients who were in clinical remission at 8 centers in Europe and Israel between September 2020 and March 2021. Participants filled out the HADS and The Stanford Presenteeism scale (SPS-6). A total of 62.2% of the participants had CD; 53.0% were male. Participants’ mean age was 39 years. Among the group, 10.8% had a pre existing diagnosis of anxiety or depression at the time of IBD diagnosis, and an additional 14.2% were later diagnosed with anxiety or depression.
Less than half, 46.1%, of IBD patients had a score 8 or higher in the HADS-anxiety (HADS-A) or HADS-depression (HADS-D) subscale, a cutoff that suggests evidence of clinical depression or anxiety. A total of 27.4% had a score of 11 or higher, indicating a mood disorder. High HADS-A score was associated with female gender (odds ratio, 1.91; P < 0.05), long duration of disease (OR, 1.04; P < 0.01), and perianal disease (P < 0.023). The authors speculate that the latter result may be due to a higher burden of symptoms. Three-quarters, 74.5%, of patients were employed; 34.0% experienced presenteeism as defined by SPS-6 score less than or equal to 18.
The researchers found that 23.0% of the patients were sedentary, and this was more common among individuals with HADS-A or HADS-D scores greater than or equal to 8. Among those experiencing presenteeism, 50% were sedentary, 29.4% were active, and 20.6% were moderately active (P < 0.01). Individuals with higher HADS-A or HADS-D scores had a greater likelihood of being sedentary (P < 0.05).
One limitation of the study was that the questionnaires were translated into the respective languages and scores were taken at only one time point.
“Rather than relying on the patient to come forward and seek help,” physicians should familiarize themselves with these validated screening tools “in order to increase the diagnostic rate of such pathologies and enable a better holistic care for the IBD patients,” the authors concluded. “Active involvement of a psychologist and/or a psychiatrist, as part of the IBD team, should be pursued to further improve the patients’ quality of life, which has emerged as one of the top priority outcomes in IBD.”
Dr. Lupe said that the findings regarding presenteeism are consistent with his experience. He pointed out that IBD patients must be more aware of their body and vigilant in managing symptoms, and he speculated that that could detract from concentration at work. He said that the study shows the need for a holistic approach to treatment. “When someone is coping with a chronic disease, like ulcerative colitis or Crohn’s disease, it affects the whole person,” including psychologically, professionally, and personally. “These are bidirectional relationships, so that if someone’s social life starts falling down, it’s more contributory to the development of something like depression and anxiety, and maybe that’s contributory to complications that come up in a disease state like Crohn’s disease or ulcerative colitis.”
The study did not receive funding, but two authors disclosed relations with AbbVie, Janssen, Pfizer, and other companies. Dr. Keefer is a cofounder and has equity ownership In Trellus Health. Dr. Lupe has no relevant financial disclosures.
REPORTING FROM JOURNAL OF CROHN'S AND COLITIS
Experimental drug may boost executive function in patients with Alzheimer’s disease
Patients performed better in cognitive testing after just 2 weeks, especially in areas of executive functioning. Clinicians involved in the study also reported improvements in patients’ ability to complete daily activities, especially in complex tasks such as using a computer, carrying out household chores, and managing their medications.
“It’s pretty incredible to see improvement over the course of a week to a week and a half,” said study investigator Aaron Koenig, MD, vice president of Early Clinical Development at Sage Therapeutics in Cambridge, Mass. “Not only are we seeing objective improvement, we’re also seeing a subjective benefit.”
The drug, SAGE-718, is also under study for MCI in patients with Huntington’s disease, the drug’s primary indication, and Parkinson’s disease.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
Improved executive function
SAGE-718 is in a new class of drugs called positive allosteric modulator of N-methyl-D-aspartate (NMDA) receptors, which are thought to improve neuroplasticity.
For the phase 2a open-label LUMINARY trial, researchers enrolled 26 patients ages 50-80 years with Alzheimer’s disease who had MCI. Patients completed a battery of cognitive tests at the study outset, again at the end of treatment, and again after 28 days.
Participants received SAGE-718 daily for 2 weeks and were followed for another 2 weeks.
The study’s primary outcome was safety. Seven patients (26.9%) reported mild or moderate treatment-emergent adverse events (AEs), and there were no serious AEs or deaths.
However, after 14 days, researchers also noted improvements from baseline on multiple tests of executive functioning, learning, and memory. And at 28 days, participants demonstrated significantly better Montreal Cognitive Assessment scores, compared with baseline (+2.3 points; P < .05), suggesting improvement in global cognition.
“We know that in Alzheimer’s and other neurodegenerative conditions there is a change in cognition, the ability to think, the ability to do things,” Dr. Koenig said. “What we’ve seen with SAGE-718 to date, all the way back to our phase 1 studies, is a cognitively beneficial effect, but more specifically an improvement in executive functioning.”
Intentional small study design
Commenting on the findings, Percy Griffin, PhD, MSc, director of scientific engagement for the Alzheimer’s Association, said that because of the small study size, short follow-up, and limited data available in the conference abstract, “we cannot speculate about the efficacy of this investigational therapy.”
“Bigger picture, the real-world clinical meaningfulness of research results that are generated in highly controlled circumstances is an important question that is being discussed right now throughout the Alzheimer’s field,” he added.
However, Dr. Koenig countered that the small study design was intentional. “Over the course of a year, we can get to the answer in different patient populations rather than running these rather large and arduous trials that may pan out to not be positive,” he said.
“The purpose here is to say, directionally, are we seeing improvement that warrants further investigation? If you don’t see an effect in a small number of patients, if you don’t see that effect rather quickly, and if you don’t see an effect that should translate into something meaningful, we at SAGE believe that you may not have a drug there,” he added.
Sage Therapeutics plans to launch a phase 2b placebo-controlled trial later this year to study SAGE-718 in more Alzheimer’s patients over a longer period of time.
The study was funded by SAGE Therapeutics. Dr. Koenig is an employee of SAGE and reports no other conflicts. Dr. Griffin has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients performed better in cognitive testing after just 2 weeks, especially in areas of executive functioning. Clinicians involved in the study also reported improvements in patients’ ability to complete daily activities, especially in complex tasks such as using a computer, carrying out household chores, and managing their medications.
“It’s pretty incredible to see improvement over the course of a week to a week and a half,” said study investigator Aaron Koenig, MD, vice president of Early Clinical Development at Sage Therapeutics in Cambridge, Mass. “Not only are we seeing objective improvement, we’re also seeing a subjective benefit.”
The drug, SAGE-718, is also under study for MCI in patients with Huntington’s disease, the drug’s primary indication, and Parkinson’s disease.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
Improved executive function
SAGE-718 is in a new class of drugs called positive allosteric modulator of N-methyl-D-aspartate (NMDA) receptors, which are thought to improve neuroplasticity.
For the phase 2a open-label LUMINARY trial, researchers enrolled 26 patients ages 50-80 years with Alzheimer’s disease who had MCI. Patients completed a battery of cognitive tests at the study outset, again at the end of treatment, and again after 28 days.
Participants received SAGE-718 daily for 2 weeks and were followed for another 2 weeks.
The study’s primary outcome was safety. Seven patients (26.9%) reported mild or moderate treatment-emergent adverse events (AEs), and there were no serious AEs or deaths.
However, after 14 days, researchers also noted improvements from baseline on multiple tests of executive functioning, learning, and memory. And at 28 days, participants demonstrated significantly better Montreal Cognitive Assessment scores, compared with baseline (+2.3 points; P < .05), suggesting improvement in global cognition.
“We know that in Alzheimer’s and other neurodegenerative conditions there is a change in cognition, the ability to think, the ability to do things,” Dr. Koenig said. “What we’ve seen with SAGE-718 to date, all the way back to our phase 1 studies, is a cognitively beneficial effect, but more specifically an improvement in executive functioning.”
Intentional small study design
Commenting on the findings, Percy Griffin, PhD, MSc, director of scientific engagement for the Alzheimer’s Association, said that because of the small study size, short follow-up, and limited data available in the conference abstract, “we cannot speculate about the efficacy of this investigational therapy.”
“Bigger picture, the real-world clinical meaningfulness of research results that are generated in highly controlled circumstances is an important question that is being discussed right now throughout the Alzheimer’s field,” he added.
However, Dr. Koenig countered that the small study design was intentional. “Over the course of a year, we can get to the answer in different patient populations rather than running these rather large and arduous trials that may pan out to not be positive,” he said.
“The purpose here is to say, directionally, are we seeing improvement that warrants further investigation? If you don’t see an effect in a small number of patients, if you don’t see that effect rather quickly, and if you don’t see an effect that should translate into something meaningful, we at SAGE believe that you may not have a drug there,” he added.
Sage Therapeutics plans to launch a phase 2b placebo-controlled trial later this year to study SAGE-718 in more Alzheimer’s patients over a longer period of time.
The study was funded by SAGE Therapeutics. Dr. Koenig is an employee of SAGE and reports no other conflicts. Dr. Griffin has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients performed better in cognitive testing after just 2 weeks, especially in areas of executive functioning. Clinicians involved in the study also reported improvements in patients’ ability to complete daily activities, especially in complex tasks such as using a computer, carrying out household chores, and managing their medications.
“It’s pretty incredible to see improvement over the course of a week to a week and a half,” said study investigator Aaron Koenig, MD, vice president of Early Clinical Development at Sage Therapeutics in Cambridge, Mass. “Not only are we seeing objective improvement, we’re also seeing a subjective benefit.”
The drug, SAGE-718, is also under study for MCI in patients with Huntington’s disease, the drug’s primary indication, and Parkinson’s disease.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
Improved executive function
SAGE-718 is in a new class of drugs called positive allosteric modulator of N-methyl-D-aspartate (NMDA) receptors, which are thought to improve neuroplasticity.
For the phase 2a open-label LUMINARY trial, researchers enrolled 26 patients ages 50-80 years with Alzheimer’s disease who had MCI. Patients completed a battery of cognitive tests at the study outset, again at the end of treatment, and again after 28 days.
Participants received SAGE-718 daily for 2 weeks and were followed for another 2 weeks.
The study’s primary outcome was safety. Seven patients (26.9%) reported mild or moderate treatment-emergent adverse events (AEs), and there were no serious AEs or deaths.
However, after 14 days, researchers also noted improvements from baseline on multiple tests of executive functioning, learning, and memory. And at 28 days, participants demonstrated significantly better Montreal Cognitive Assessment scores, compared with baseline (+2.3 points; P < .05), suggesting improvement in global cognition.
“We know that in Alzheimer’s and other neurodegenerative conditions there is a change in cognition, the ability to think, the ability to do things,” Dr. Koenig said. “What we’ve seen with SAGE-718 to date, all the way back to our phase 1 studies, is a cognitively beneficial effect, but more specifically an improvement in executive functioning.”
Intentional small study design
Commenting on the findings, Percy Griffin, PhD, MSc, director of scientific engagement for the Alzheimer’s Association, said that because of the small study size, short follow-up, and limited data available in the conference abstract, “we cannot speculate about the efficacy of this investigational therapy.”
“Bigger picture, the real-world clinical meaningfulness of research results that are generated in highly controlled circumstances is an important question that is being discussed right now throughout the Alzheimer’s field,” he added.
However, Dr. Koenig countered that the small study design was intentional. “Over the course of a year, we can get to the answer in different patient populations rather than running these rather large and arduous trials that may pan out to not be positive,” he said.
“The purpose here is to say, directionally, are we seeing improvement that warrants further investigation? If you don’t see an effect in a small number of patients, if you don’t see that effect rather quickly, and if you don’t see an effect that should translate into something meaningful, we at SAGE believe that you may not have a drug there,” he added.
Sage Therapeutics plans to launch a phase 2b placebo-controlled trial later this year to study SAGE-718 in more Alzheimer’s patients over a longer period of time.
The study was funded by SAGE Therapeutics. Dr. Koenig is an employee of SAGE and reports no other conflicts. Dr. Griffin has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAN 2022
Novel drug significantly reduces tics in Tourette syndrome – without side effects
, a new study shows.
Importantly, unlike current medications for the disorder, ecocipam does not lead to weight gain, anxiety, depression, or tardive dyskinesia, compared with placebo – a factor that may lead to better adherence.
For clinicians treating children with Tourette syndrome, the results suggest “help is on the way,” said study investigator Donald Gilbert, MD, professor of pediatrics and neurology, University of Cincinnati Children’s Hospital Medical Center.
“There may be a drug available with a new mechanism of action that is effective to suppress tics without causing weight gain or unwanted neuropsychiatric side effects,” Dr. Gilbert said.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
First-in-class agent
Tourette syndrome is a neurologic condition that causes sudden repetitive involuntary muscle movements and sounds known as tics. These movements typically develop in childhood and worsen during adolescence.
“There’s a risk of injury, such as to the neck, with tics in childhood, so it’s good to have something that makes tics less severe and less socially impairing in junior high,” said Dr. Gilbert.
While tics generally diminish by adulthood, “about 10% of the patients we see as kids persist into adulthood enough to need medical interventions,” said Dr. Gilbert.
Ecopipam is a first-in-class selective D1 receptor antagonist in clinical development for pediatric patients with Tourette syndrome. The compound was previously tested without success in schizophrenia and in obesity, the idea being that because dopamine is linked to pleasure or reward, blocking it might result in weight loss, said Dr. Gilbert.
However, earlier studies in Tourette syndrome suggested that ecopipam reduces tics in children and adults and had low metabolic and movement-related adverse effects.
Drugs currently used to treat tics include haloperidol, pimocide, and aripiprazole. All of these agents block the dopamine-2 (D2) receptor and can cause weight gain and tardive dyskinesia, said Dr. Gilbert.
Placebo-controlled trial
The new study included 149 patients with Tourette syndrome who had a score of at least 20 on the Yale Global Tic Severity Total Tic Score (YGTSS-TTS). The scale measures five aspects of motor and vocal tics: the number, frequency, intensity, complexity, and interference.
With that scale, intensity assesses whether tics cause injury, complexity evaluates the number of muscle group, and interference assesses whether tics interrupt functions, such as speaking or walking.
For each of the five areas, scores range from 0-5, with higher scores indicating greater severity. The motor and vocal parts have a maximum of 25 points each, for a maximum total of 50.
Participants were randomly assigned to receive once-daily oral ecopipam or placebo. A 4-week titration period was followed by an 8-week maintenance period and then a 1-week tapering period.
The primary endpoint was mean change from baseline to week 12 in scores on the YGTSS-TTS.
Results on the YGTSS-TTS showed a significant improvement in the ecopipam group, compared with placebo groups (least square [LS] mean difference: -3.44; 95% confidence interval: -6.09 to -0.79; P = .011).
The analysis indicated a 30% reduction, with an effect size of 0.48, which is “pretty good,” said Dr. Gilbert. “The amount of change is comparable to other drugs that are marketed” to treat tics.
The drug was effective for younger as well as older children. Among those aged 6-11 years, the LS mean difference was -4.95 (95% CI: -9.99 to 0.10; P = .054), and for those aged 12 to 17 years, the LS mean difference was -3.37 (95% CI: -6.51 to -0.24; P = .035).
A key secondary endpoint was the score on the Clinical Global Impression of Tourette Syndrome Severity, which Dr. Gilbert said is a more subjective measure of whether a patient’s life has improved. Here, the mean change at week 12 was significant (P = .001) for the treated group (improvement of 0.91 points), compared with the placebo group (improvement of 0.5 points).
Researchers also assessed safety and tolerability. Treatment-related adverse events (AEs) occurred in 34% of patients taking ecopipam and in 21% of those taking placebo. The most common AEs were headache (9.2%), fatigue (6.6%), somnolence (6.6%), and restlessness (5.3%).
There were no metabolic or movement-related AEs or treatment-related serious AEs.
“This drug doesn’t cause weight gain at all,” said Dr. Gilbert. He noted that there was also no difference in the groups in terms of rates of depression, anxiety, or tardive dyskinesia.
Significant tic reduction
Commenting on the findings, Jessica Frey, MD, a movement disorders fellow at the University of Florida, said the new double-blind, placebo-controlled study “is promising” in that it demonstrates significant tic reduction, compared with placebo without significant side effects.
“Ecopipam could potentially expand pharmacologic treatment options for children and adolescents with Tourette syndrome in the near future,” she said.
Dr. Frey will also be presenting results at the meeting of a study showing a significant correlation between tic severity and social media use among adolescents with Tourette syndrome during the COVID pandemic.
She noted that dopamine is an important neurotransmitter in the underlying pathophysiology of Tourette syndrome. In addition, although D2 receptor blockade can provide significant tic reduction, the “intolerable” side effects often linked to medications with this mechanism “can lead to discontinuation,” said Dr. Frey.
She also noted that ecopipam has previously been evaluated in an open-label study and a follow-up placebo-controlled study that demonstrated safety as well as significant tic reduction.
The study was supported by Emalex Biosciences. Dr. Gilbert and Dr. Frey report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new study shows.
Importantly, unlike current medications for the disorder, ecocipam does not lead to weight gain, anxiety, depression, or tardive dyskinesia, compared with placebo – a factor that may lead to better adherence.
For clinicians treating children with Tourette syndrome, the results suggest “help is on the way,” said study investigator Donald Gilbert, MD, professor of pediatrics and neurology, University of Cincinnati Children’s Hospital Medical Center.
“There may be a drug available with a new mechanism of action that is effective to suppress tics without causing weight gain or unwanted neuropsychiatric side effects,” Dr. Gilbert said.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
First-in-class agent
Tourette syndrome is a neurologic condition that causes sudden repetitive involuntary muscle movements and sounds known as tics. These movements typically develop in childhood and worsen during adolescence.
“There’s a risk of injury, such as to the neck, with tics in childhood, so it’s good to have something that makes tics less severe and less socially impairing in junior high,” said Dr. Gilbert.
While tics generally diminish by adulthood, “about 10% of the patients we see as kids persist into adulthood enough to need medical interventions,” said Dr. Gilbert.
Ecopipam is a first-in-class selective D1 receptor antagonist in clinical development for pediatric patients with Tourette syndrome. The compound was previously tested without success in schizophrenia and in obesity, the idea being that because dopamine is linked to pleasure or reward, blocking it might result in weight loss, said Dr. Gilbert.
However, earlier studies in Tourette syndrome suggested that ecopipam reduces tics in children and adults and had low metabolic and movement-related adverse effects.
Drugs currently used to treat tics include haloperidol, pimocide, and aripiprazole. All of these agents block the dopamine-2 (D2) receptor and can cause weight gain and tardive dyskinesia, said Dr. Gilbert.
Placebo-controlled trial
The new study included 149 patients with Tourette syndrome who had a score of at least 20 on the Yale Global Tic Severity Total Tic Score (YGTSS-TTS). The scale measures five aspects of motor and vocal tics: the number, frequency, intensity, complexity, and interference.
With that scale, intensity assesses whether tics cause injury, complexity evaluates the number of muscle group, and interference assesses whether tics interrupt functions, such as speaking or walking.
For each of the five areas, scores range from 0-5, with higher scores indicating greater severity. The motor and vocal parts have a maximum of 25 points each, for a maximum total of 50.
Participants were randomly assigned to receive once-daily oral ecopipam or placebo. A 4-week titration period was followed by an 8-week maintenance period and then a 1-week tapering period.
The primary endpoint was mean change from baseline to week 12 in scores on the YGTSS-TTS.
Results on the YGTSS-TTS showed a significant improvement in the ecopipam group, compared with placebo groups (least square [LS] mean difference: -3.44; 95% confidence interval: -6.09 to -0.79; P = .011).
The analysis indicated a 30% reduction, with an effect size of 0.48, which is “pretty good,” said Dr. Gilbert. “The amount of change is comparable to other drugs that are marketed” to treat tics.
The drug was effective for younger as well as older children. Among those aged 6-11 years, the LS mean difference was -4.95 (95% CI: -9.99 to 0.10; P = .054), and for those aged 12 to 17 years, the LS mean difference was -3.37 (95% CI: -6.51 to -0.24; P = .035).
A key secondary endpoint was the score on the Clinical Global Impression of Tourette Syndrome Severity, which Dr. Gilbert said is a more subjective measure of whether a patient’s life has improved. Here, the mean change at week 12 was significant (P = .001) for the treated group (improvement of 0.91 points), compared with the placebo group (improvement of 0.5 points).
Researchers also assessed safety and tolerability. Treatment-related adverse events (AEs) occurred in 34% of patients taking ecopipam and in 21% of those taking placebo. The most common AEs were headache (9.2%), fatigue (6.6%), somnolence (6.6%), and restlessness (5.3%).
There were no metabolic or movement-related AEs or treatment-related serious AEs.
“This drug doesn’t cause weight gain at all,” said Dr. Gilbert. He noted that there was also no difference in the groups in terms of rates of depression, anxiety, or tardive dyskinesia.
Significant tic reduction
Commenting on the findings, Jessica Frey, MD, a movement disorders fellow at the University of Florida, said the new double-blind, placebo-controlled study “is promising” in that it demonstrates significant tic reduction, compared with placebo without significant side effects.
“Ecopipam could potentially expand pharmacologic treatment options for children and adolescents with Tourette syndrome in the near future,” she said.
Dr. Frey will also be presenting results at the meeting of a study showing a significant correlation between tic severity and social media use among adolescents with Tourette syndrome during the COVID pandemic.
She noted that dopamine is an important neurotransmitter in the underlying pathophysiology of Tourette syndrome. In addition, although D2 receptor blockade can provide significant tic reduction, the “intolerable” side effects often linked to medications with this mechanism “can lead to discontinuation,” said Dr. Frey.
She also noted that ecopipam has previously been evaluated in an open-label study and a follow-up placebo-controlled study that demonstrated safety as well as significant tic reduction.
The study was supported by Emalex Biosciences. Dr. Gilbert and Dr. Frey report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new study shows.
Importantly, unlike current medications for the disorder, ecocipam does not lead to weight gain, anxiety, depression, or tardive dyskinesia, compared with placebo – a factor that may lead to better adherence.
For clinicians treating children with Tourette syndrome, the results suggest “help is on the way,” said study investigator Donald Gilbert, MD, professor of pediatrics and neurology, University of Cincinnati Children’s Hospital Medical Center.
“There may be a drug available with a new mechanism of action that is effective to suppress tics without causing weight gain or unwanted neuropsychiatric side effects,” Dr. Gilbert said.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
First-in-class agent
Tourette syndrome is a neurologic condition that causes sudden repetitive involuntary muscle movements and sounds known as tics. These movements typically develop in childhood and worsen during adolescence.
“There’s a risk of injury, such as to the neck, with tics in childhood, so it’s good to have something that makes tics less severe and less socially impairing in junior high,” said Dr. Gilbert.
While tics generally diminish by adulthood, “about 10% of the patients we see as kids persist into adulthood enough to need medical interventions,” said Dr. Gilbert.
Ecopipam is a first-in-class selective D1 receptor antagonist in clinical development for pediatric patients with Tourette syndrome. The compound was previously tested without success in schizophrenia and in obesity, the idea being that because dopamine is linked to pleasure or reward, blocking it might result in weight loss, said Dr. Gilbert.
However, earlier studies in Tourette syndrome suggested that ecopipam reduces tics in children and adults and had low metabolic and movement-related adverse effects.
Drugs currently used to treat tics include haloperidol, pimocide, and aripiprazole. All of these agents block the dopamine-2 (D2) receptor and can cause weight gain and tardive dyskinesia, said Dr. Gilbert.
Placebo-controlled trial
The new study included 149 patients with Tourette syndrome who had a score of at least 20 on the Yale Global Tic Severity Total Tic Score (YGTSS-TTS). The scale measures five aspects of motor and vocal tics: the number, frequency, intensity, complexity, and interference.
With that scale, intensity assesses whether tics cause injury, complexity evaluates the number of muscle group, and interference assesses whether tics interrupt functions, such as speaking or walking.
For each of the five areas, scores range from 0-5, with higher scores indicating greater severity. The motor and vocal parts have a maximum of 25 points each, for a maximum total of 50.
Participants were randomly assigned to receive once-daily oral ecopipam or placebo. A 4-week titration period was followed by an 8-week maintenance period and then a 1-week tapering period.
The primary endpoint was mean change from baseline to week 12 in scores on the YGTSS-TTS.
Results on the YGTSS-TTS showed a significant improvement in the ecopipam group, compared with placebo groups (least square [LS] mean difference: -3.44; 95% confidence interval: -6.09 to -0.79; P = .011).
The analysis indicated a 30% reduction, with an effect size of 0.48, which is “pretty good,” said Dr. Gilbert. “The amount of change is comparable to other drugs that are marketed” to treat tics.
The drug was effective for younger as well as older children. Among those aged 6-11 years, the LS mean difference was -4.95 (95% CI: -9.99 to 0.10; P = .054), and for those aged 12 to 17 years, the LS mean difference was -3.37 (95% CI: -6.51 to -0.24; P = .035).
A key secondary endpoint was the score on the Clinical Global Impression of Tourette Syndrome Severity, which Dr. Gilbert said is a more subjective measure of whether a patient’s life has improved. Here, the mean change at week 12 was significant (P = .001) for the treated group (improvement of 0.91 points), compared with the placebo group (improvement of 0.5 points).
Researchers also assessed safety and tolerability. Treatment-related adverse events (AEs) occurred in 34% of patients taking ecopipam and in 21% of those taking placebo. The most common AEs were headache (9.2%), fatigue (6.6%), somnolence (6.6%), and restlessness (5.3%).
There were no metabolic or movement-related AEs or treatment-related serious AEs.
“This drug doesn’t cause weight gain at all,” said Dr. Gilbert. He noted that there was also no difference in the groups in terms of rates of depression, anxiety, or tardive dyskinesia.
Significant tic reduction
Commenting on the findings, Jessica Frey, MD, a movement disorders fellow at the University of Florida, said the new double-blind, placebo-controlled study “is promising” in that it demonstrates significant tic reduction, compared with placebo without significant side effects.
“Ecopipam could potentially expand pharmacologic treatment options for children and adolescents with Tourette syndrome in the near future,” she said.
Dr. Frey will also be presenting results at the meeting of a study showing a significant correlation between tic severity and social media use among adolescents with Tourette syndrome during the COVID pandemic.
She noted that dopamine is an important neurotransmitter in the underlying pathophysiology of Tourette syndrome. In addition, although D2 receptor blockade can provide significant tic reduction, the “intolerable” side effects often linked to medications with this mechanism “can lead to discontinuation,” said Dr. Frey.
She also noted that ecopipam has previously been evaluated in an open-label study and a follow-up placebo-controlled study that demonstrated safety as well as significant tic reduction.
The study was supported by Emalex Biosciences. Dr. Gilbert and Dr. Frey report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAN 2022







