User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Aerobic exercise augments PTSD therapy
Investigators randomly assigned individuals with PTSD to receive either exposure therapy with aerobic exercise or exposure therapy with passive stretching for 9 weeks. At 6 months post intervention, participants in the aerobic exercise group showed greater reductions in PTSD severity, compared with those in the stretching group.
“There is a critical need to improve outcomes for treating people with PTSD, and this finding points to one potentially cheap and ready-to-use strategy that all clinicians could employ with most patients,” lead author Richard Bryant, MPsych, PhD, DSc, director of the Traumatic Stress Clinic and Scientia Professor of Psychology at the University of New South Wales, Sydney, told this news organization.
The study was published online in The Lancet Psychiatry.
Promoting BDNF
“Trauma-focused psychotherapy is the recommended treatment for PTSD, but up to half of patients do not respond to this treatment,” Dr. Bryant said.
“We know that brain-derived neurotrophic factors [BDNF] are critical for synaptic plasticity, which underpins the learning that occurs in therapy so that reminders of trauma are no longer fear-provoking,” he continued. “Preclinical animal and human research inform us that brief aerobic exercise can promote BDNF and new learning that inhibits fear responses.”
The researchers “hypothesized that brief exercise after exposure therapy to trauma memories – which is the key ingredient of trauma-focused psychotherapy – would lead to greater reductions in PTSD, relative to standard trauma-focused therapy,” he said.
To investigate the question, the researchers randomly assigned 130 adults with PTSD (mean age, 39 years; 61% female; 76% White) to receive nine 90-minute sessions of exposure therapy with either aerobic exercise or passive stretching (n = 65 in each group).
There were no differences at baseline in sociodemographic characteristics or psychopathology measures, although the mean age of the stretching group was slightly older than that of the aerobic group (40 years vs. 37 years, respectively), and there was a slightly higher proportion of women in the stretching group (68% vs. 54%).
Participants did not differ on weekly exercise either at baseline, immediately post treatment, or at 6-week follow-up.
PTSD severity (the primary outcome) was measured using the clinician-administered PTSD scale CAPS-2, with assessments conducted at baseline, 1 week post treatment, and 6 months post treatment.
The aerobic exercise regimen was tailored to each participant, based on an assessment of his/her aerobic target zone.
The exposure therapy sessions were identical for both groups. Following the exposure sessions, participants engaged in their respective exercises: Those in the passive stretching group engaged in 20 minutes of exercise, while those in the aerobic group participated in a total of 20 minutes of exercise, with 10 conducted at their personal aerobic target heart rate.
“This level of exercise was chosen because BDNF concentration in the serum is increased by two 3-minute bouts of aerobic exercise, and 10 minutes of aerobic exercise can facilitate extinction learning,” the authors explained.
The aerobic activity consisted of running on a stepper exercise platform while having cardiac activity recorded. A small portion (10%) of the therapy sessions were recorded and rated for treatment fidelity.
Change in PTSD was the primary outcome, with secondary outcomes consisting of changes in depression, anxiety, alcohol use disorder, and posttraumatic cognitions.
Few barriers
The researchers found no significant differences in PTSD severity, as measured by CAPS-2 score, between treatment groups at 10 weeks – that is, immediately post treatment (mean difference, 7.0; 95% confidence interval, –2.3 to 16.4; P = .14).
However, significantly greater reductions in PTSD severity were found in the aerobic versus the stretching group at 6-month follow-up (mean difference, 12.1;95% CI, 2.4-21.8; P = .023), pointing to a “moderate effect size” (d = 0.6; 95% CI, 0.1-1.1]).
Although there were no differences found at 6-month assessment between rates of PTSD diagnosis (25% of the aerobic vs 27% of the stretching group), more participants in the aerobic group reached a “minimal clinically important difference,” compared to those in the stretching group (96% vs. 84%, respectively, x2 = 4.4; P = .036).
There were also superior benefits found in the aerobic versus the stretching group on depression severity at 6 months (a secondary outcome), with a mean difference in Beck Depression Inventory-2 score of 5.7 (95% CI, 0.5-10.9; P = .022), yielding a “moderate effect size” (d = 0.5; 95% CI, 0.1-1.0]).
There were no adverse events associated with the intervention, and almost all the sessions (88%) complied with the treatment protocol.
The researchers noted several limitations. For example, they did not obtain plasma to measure BDNF concentrations, so they could not “infer whether the mechanism of change involved BDNF.”
In addition, they did not perform sex-specific analyses. “Future studies could increase the sample size to investigate sex differences because females display less BDNF change following exercise than do males,” they wrote.
Nevertheless, the study “provides initial evidence of a simple and accessible strategy that clinicians could readily apply in combination with exposure therapy,” they stated. “Whereas many pharmacologic interventions pose barriers, including cost, requirement for prescriptions, and patient resistance to drugs, exercise offers clinicians a strategy that can be implemented with few barriers.”
Dr. Bryant emphasized that one study “does not represent a body of evidence, and so it is essential that this finding be replicated in other trials before it can be recommended for clinical use.” He noted that other trials are “currently underway.”
Easy augmentation
In a comment, Barbara Rothbaum, PhD, professor in psychiatry and director of the Trauma and Anxiety Recovery Program at Emory University, Atlanta, called it a “well-controlled trial augmenting exposure therapy for PTSD with brief aerobic exercise and finding some benefits of the augmented condition at 6 months posttreatment but not immediately posttreatment.”
The study’s methodology – that is, using independent standard assessment of PTSD and rating audio recordings of therapy sessions for treatment fidelity and quality – can lead us to “be confident in their [the researchers’] conclusions,” she said.
Dr. Rothbaum, who was not associated with this study, described research into methods to augment exposure therapy for PTSD as “timely and clinically relevant.”
Exercise “would be an easy augmentation for many clinicians if it is helpful,” she noted.
The study was funded by the Australian National Health and Medical Research Council. The authors and Dr. Rothbaum reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators randomly assigned individuals with PTSD to receive either exposure therapy with aerobic exercise or exposure therapy with passive stretching for 9 weeks. At 6 months post intervention, participants in the aerobic exercise group showed greater reductions in PTSD severity, compared with those in the stretching group.
“There is a critical need to improve outcomes for treating people with PTSD, and this finding points to one potentially cheap and ready-to-use strategy that all clinicians could employ with most patients,” lead author Richard Bryant, MPsych, PhD, DSc, director of the Traumatic Stress Clinic and Scientia Professor of Psychology at the University of New South Wales, Sydney, told this news organization.
The study was published online in The Lancet Psychiatry.
Promoting BDNF
“Trauma-focused psychotherapy is the recommended treatment for PTSD, but up to half of patients do not respond to this treatment,” Dr. Bryant said.
“We know that brain-derived neurotrophic factors [BDNF] are critical for synaptic plasticity, which underpins the learning that occurs in therapy so that reminders of trauma are no longer fear-provoking,” he continued. “Preclinical animal and human research inform us that brief aerobic exercise can promote BDNF and new learning that inhibits fear responses.”
The researchers “hypothesized that brief exercise after exposure therapy to trauma memories – which is the key ingredient of trauma-focused psychotherapy – would lead to greater reductions in PTSD, relative to standard trauma-focused therapy,” he said.
To investigate the question, the researchers randomly assigned 130 adults with PTSD (mean age, 39 years; 61% female; 76% White) to receive nine 90-minute sessions of exposure therapy with either aerobic exercise or passive stretching (n = 65 in each group).
There were no differences at baseline in sociodemographic characteristics or psychopathology measures, although the mean age of the stretching group was slightly older than that of the aerobic group (40 years vs. 37 years, respectively), and there was a slightly higher proportion of women in the stretching group (68% vs. 54%).
Participants did not differ on weekly exercise either at baseline, immediately post treatment, or at 6-week follow-up.
PTSD severity (the primary outcome) was measured using the clinician-administered PTSD scale CAPS-2, with assessments conducted at baseline, 1 week post treatment, and 6 months post treatment.
The aerobic exercise regimen was tailored to each participant, based on an assessment of his/her aerobic target zone.
The exposure therapy sessions were identical for both groups. Following the exposure sessions, participants engaged in their respective exercises: Those in the passive stretching group engaged in 20 minutes of exercise, while those in the aerobic group participated in a total of 20 minutes of exercise, with 10 conducted at their personal aerobic target heart rate.
“This level of exercise was chosen because BDNF concentration in the serum is increased by two 3-minute bouts of aerobic exercise, and 10 minutes of aerobic exercise can facilitate extinction learning,” the authors explained.
The aerobic activity consisted of running on a stepper exercise platform while having cardiac activity recorded. A small portion (10%) of the therapy sessions were recorded and rated for treatment fidelity.
Change in PTSD was the primary outcome, with secondary outcomes consisting of changes in depression, anxiety, alcohol use disorder, and posttraumatic cognitions.
Few barriers
The researchers found no significant differences in PTSD severity, as measured by CAPS-2 score, between treatment groups at 10 weeks – that is, immediately post treatment (mean difference, 7.0; 95% confidence interval, –2.3 to 16.4; P = .14).
However, significantly greater reductions in PTSD severity were found in the aerobic versus the stretching group at 6-month follow-up (mean difference, 12.1;95% CI, 2.4-21.8; P = .023), pointing to a “moderate effect size” (d = 0.6; 95% CI, 0.1-1.1]).
Although there were no differences found at 6-month assessment between rates of PTSD diagnosis (25% of the aerobic vs 27% of the stretching group), more participants in the aerobic group reached a “minimal clinically important difference,” compared to those in the stretching group (96% vs. 84%, respectively, x2 = 4.4; P = .036).
There were also superior benefits found in the aerobic versus the stretching group on depression severity at 6 months (a secondary outcome), with a mean difference in Beck Depression Inventory-2 score of 5.7 (95% CI, 0.5-10.9; P = .022), yielding a “moderate effect size” (d = 0.5; 95% CI, 0.1-1.0]).
There were no adverse events associated with the intervention, and almost all the sessions (88%) complied with the treatment protocol.
The researchers noted several limitations. For example, they did not obtain plasma to measure BDNF concentrations, so they could not “infer whether the mechanism of change involved BDNF.”
In addition, they did not perform sex-specific analyses. “Future studies could increase the sample size to investigate sex differences because females display less BDNF change following exercise than do males,” they wrote.
Nevertheless, the study “provides initial evidence of a simple and accessible strategy that clinicians could readily apply in combination with exposure therapy,” they stated. “Whereas many pharmacologic interventions pose barriers, including cost, requirement for prescriptions, and patient resistance to drugs, exercise offers clinicians a strategy that can be implemented with few barriers.”
Dr. Bryant emphasized that one study “does not represent a body of evidence, and so it is essential that this finding be replicated in other trials before it can be recommended for clinical use.” He noted that other trials are “currently underway.”
Easy augmentation
In a comment, Barbara Rothbaum, PhD, professor in psychiatry and director of the Trauma and Anxiety Recovery Program at Emory University, Atlanta, called it a “well-controlled trial augmenting exposure therapy for PTSD with brief aerobic exercise and finding some benefits of the augmented condition at 6 months posttreatment but not immediately posttreatment.”
The study’s methodology – that is, using independent standard assessment of PTSD and rating audio recordings of therapy sessions for treatment fidelity and quality – can lead us to “be confident in their [the researchers’] conclusions,” she said.
Dr. Rothbaum, who was not associated with this study, described research into methods to augment exposure therapy for PTSD as “timely and clinically relevant.”
Exercise “would be an easy augmentation for many clinicians if it is helpful,” she noted.
The study was funded by the Australian National Health and Medical Research Council. The authors and Dr. Rothbaum reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators randomly assigned individuals with PTSD to receive either exposure therapy with aerobic exercise or exposure therapy with passive stretching for 9 weeks. At 6 months post intervention, participants in the aerobic exercise group showed greater reductions in PTSD severity, compared with those in the stretching group.
“There is a critical need to improve outcomes for treating people with PTSD, and this finding points to one potentially cheap and ready-to-use strategy that all clinicians could employ with most patients,” lead author Richard Bryant, MPsych, PhD, DSc, director of the Traumatic Stress Clinic and Scientia Professor of Psychology at the University of New South Wales, Sydney, told this news organization.
The study was published online in The Lancet Psychiatry.
Promoting BDNF
“Trauma-focused psychotherapy is the recommended treatment for PTSD, but up to half of patients do not respond to this treatment,” Dr. Bryant said.
“We know that brain-derived neurotrophic factors [BDNF] are critical for synaptic plasticity, which underpins the learning that occurs in therapy so that reminders of trauma are no longer fear-provoking,” he continued. “Preclinical animal and human research inform us that brief aerobic exercise can promote BDNF and new learning that inhibits fear responses.”
The researchers “hypothesized that brief exercise after exposure therapy to trauma memories – which is the key ingredient of trauma-focused psychotherapy – would lead to greater reductions in PTSD, relative to standard trauma-focused therapy,” he said.
To investigate the question, the researchers randomly assigned 130 adults with PTSD (mean age, 39 years; 61% female; 76% White) to receive nine 90-minute sessions of exposure therapy with either aerobic exercise or passive stretching (n = 65 in each group).
There were no differences at baseline in sociodemographic characteristics or psychopathology measures, although the mean age of the stretching group was slightly older than that of the aerobic group (40 years vs. 37 years, respectively), and there was a slightly higher proportion of women in the stretching group (68% vs. 54%).
Participants did not differ on weekly exercise either at baseline, immediately post treatment, or at 6-week follow-up.
PTSD severity (the primary outcome) was measured using the clinician-administered PTSD scale CAPS-2, with assessments conducted at baseline, 1 week post treatment, and 6 months post treatment.
The aerobic exercise regimen was tailored to each participant, based on an assessment of his/her aerobic target zone.
The exposure therapy sessions were identical for both groups. Following the exposure sessions, participants engaged in their respective exercises: Those in the passive stretching group engaged in 20 minutes of exercise, while those in the aerobic group participated in a total of 20 minutes of exercise, with 10 conducted at their personal aerobic target heart rate.
“This level of exercise was chosen because BDNF concentration in the serum is increased by two 3-minute bouts of aerobic exercise, and 10 minutes of aerobic exercise can facilitate extinction learning,” the authors explained.
The aerobic activity consisted of running on a stepper exercise platform while having cardiac activity recorded. A small portion (10%) of the therapy sessions were recorded and rated for treatment fidelity.
Change in PTSD was the primary outcome, with secondary outcomes consisting of changes in depression, anxiety, alcohol use disorder, and posttraumatic cognitions.
Few barriers
The researchers found no significant differences in PTSD severity, as measured by CAPS-2 score, between treatment groups at 10 weeks – that is, immediately post treatment (mean difference, 7.0; 95% confidence interval, –2.3 to 16.4; P = .14).
However, significantly greater reductions in PTSD severity were found in the aerobic versus the stretching group at 6-month follow-up (mean difference, 12.1;95% CI, 2.4-21.8; P = .023), pointing to a “moderate effect size” (d = 0.6; 95% CI, 0.1-1.1]).
Although there were no differences found at 6-month assessment between rates of PTSD diagnosis (25% of the aerobic vs 27% of the stretching group), more participants in the aerobic group reached a “minimal clinically important difference,” compared to those in the stretching group (96% vs. 84%, respectively, x2 = 4.4; P = .036).
There were also superior benefits found in the aerobic versus the stretching group on depression severity at 6 months (a secondary outcome), with a mean difference in Beck Depression Inventory-2 score of 5.7 (95% CI, 0.5-10.9; P = .022), yielding a “moderate effect size” (d = 0.5; 95% CI, 0.1-1.0]).
There were no adverse events associated with the intervention, and almost all the sessions (88%) complied with the treatment protocol.
The researchers noted several limitations. For example, they did not obtain plasma to measure BDNF concentrations, so they could not “infer whether the mechanism of change involved BDNF.”
In addition, they did not perform sex-specific analyses. “Future studies could increase the sample size to investigate sex differences because females display less BDNF change following exercise than do males,” they wrote.
Nevertheless, the study “provides initial evidence of a simple and accessible strategy that clinicians could readily apply in combination with exposure therapy,” they stated. “Whereas many pharmacologic interventions pose barriers, including cost, requirement for prescriptions, and patient resistance to drugs, exercise offers clinicians a strategy that can be implemented with few barriers.”
Dr. Bryant emphasized that one study “does not represent a body of evidence, and so it is essential that this finding be replicated in other trials before it can be recommended for clinical use.” He noted that other trials are “currently underway.”
Easy augmentation
In a comment, Barbara Rothbaum, PhD, professor in psychiatry and director of the Trauma and Anxiety Recovery Program at Emory University, Atlanta, called it a “well-controlled trial augmenting exposure therapy for PTSD with brief aerobic exercise and finding some benefits of the augmented condition at 6 months posttreatment but not immediately posttreatment.”
The study’s methodology – that is, using independent standard assessment of PTSD and rating audio recordings of therapy sessions for treatment fidelity and quality – can lead us to “be confident in their [the researchers’] conclusions,” she said.
Dr. Rothbaum, who was not associated with this study, described research into methods to augment exposure therapy for PTSD as “timely and clinically relevant.”
Exercise “would be an easy augmentation for many clinicians if it is helpful,” she noted.
The study was funded by the Australian National Health and Medical Research Council. The authors and Dr. Rothbaum reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET PSYCHIATRY
Antipsychotic shows benefit for Alzheimer’s agitation
SAN FRANCISCO – In a widely anticipated report,
Members of a panel of dementia specialists here at the 15th Clinical Trials on Alzheimer’s Disease (CTAD) conference said that the results were encouraging. But they also noted that the available data make it difficult to understand the impact of the drug on the day-to-day life on patients.
“I’d like to be able to translate that into something else to understand the risk benefit calculus,” said neurologist and neuroscientist Alireza Atri, MD, PhD, of Banner Sun Health Research Institute in Phoenix. “How does it affect the patients themselves, their quality of life, and the family members and their burden?”
Currently, there’s no Food and Drug Administration–approved treatment for agitation in AD.
In 2015, the FDA approved brexpiprazole, an oral medication, as a treatment for schizophrenia and an adjunctive treatment for major depressive disorder (MDD). It is an expensive drug with an average retail price per GoodRx of $1,582 per month, and no generic is available.
Researchers released the results of a trio of phase 3 clinical trials at CTAD that examined various doses of brexpiprazole. The results of the first two trials had been released earlier in 2018.
Three trials
All trials were multicenter, 12-week, randomized, double-blind and placebo-controlled.
Study participants were aged 55-90 years, had probable AD diagnoses, and had agitation per various scales. The average age in the groups was 74 years, 56.0%-61.7% were women, and 94.3%-98.1% were White.
The first trial examined two fixed doses (1 mg/d, n = 137; and 2 mg/d, n = 140) or placebo (n = 136). “The study initially included a 0.5 mg/day arm,” the researchers reported, “which was removed in a protocol amendment, and patients randomized to that arm were not included in efficacy analyses.”
The second trial looked at a flexible dose (0.5-2 mg/d, n = 133) or placebo (n = 137).
In a CTAD presentation, Nanco Hefting of Lundbeck, a codeveloper of the drug, said that the researchers learned from the first two trials that 2 mg/d might be an appropriate dose, and the FDA recommended they also examine 3 mg/day. As a result, the third trial examined two fixed doses (2 mg/d, n = 75; 3 mg/d, n = 153; or placebo, n = 117).
In the third trial, both the placebo and drug groups improved per a measurement of agitation; those in the drug group improved somewhat more.
The mean change in baseline on the Cohen-Mansfield Agitation Inventory scale – the primary endpoint – was –5.32 for the 2-mg/d and 3-mg/d groups vs. placebo (P = .0026); the score in the placebo group fell by about 18 and by about 22 in the drug group.
The key secondary endpoint was an improvement from baseline to week 12 in the Clinical Global Impression–Severity (CGI-S) score related to agitation. Compared with the placebo group, this score was –0.27 in the drug group (P = .0078). Both scores hovered around –1.0.
Safety data show the percentage of treatment-emergent events ranged from 45.9% in the placebo group to 49.0%-56.8% for brexpiprazole in the three trials. The percentage of these events leading to discontinuation was 6.3% among those receiving the drug and 3.4% in the placebo group.
University of Exeter dementia researcher Clive Ballard, MD, MB ChB, one of the panelists who discussed the research after the CTAD presentation, praised the trials as “well-conducted” and said that he was pleased that subjects in institutions were included. “It’s not an easy environment to do trials in. They should be really commended for doing for doing that.”
But he echoed fellow panelist Dr. Atri by noting that more data are needed to understand how well the drug works. “I would like to see the effect sizes and a little bit more detail to understand the clinical meaningfulness of that level of benefit.”
What’s next? A spokeswoman for Otsuka, a codeveloper of brexpiprazole, said that it hopes to hear in 2023 about a supplemental new drug application that was filed in November 2022.
Otsuka and Lundbeck funded the research. Mr. Hefting is an employee of Lundbeck, and several other authors work for Lundbeck or Otsuka. The single non-employee author reports various disclosures. Disclosures for Dr. Atri and Dr. Ballard were not provided.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – In a widely anticipated report,
Members of a panel of dementia specialists here at the 15th Clinical Trials on Alzheimer’s Disease (CTAD) conference said that the results were encouraging. But they also noted that the available data make it difficult to understand the impact of the drug on the day-to-day life on patients.
“I’d like to be able to translate that into something else to understand the risk benefit calculus,” said neurologist and neuroscientist Alireza Atri, MD, PhD, of Banner Sun Health Research Institute in Phoenix. “How does it affect the patients themselves, their quality of life, and the family members and their burden?”
Currently, there’s no Food and Drug Administration–approved treatment for agitation in AD.
In 2015, the FDA approved brexpiprazole, an oral medication, as a treatment for schizophrenia and an adjunctive treatment for major depressive disorder (MDD). It is an expensive drug with an average retail price per GoodRx of $1,582 per month, and no generic is available.
Researchers released the results of a trio of phase 3 clinical trials at CTAD that examined various doses of brexpiprazole. The results of the first two trials had been released earlier in 2018.
Three trials
All trials were multicenter, 12-week, randomized, double-blind and placebo-controlled.
Study participants were aged 55-90 years, had probable AD diagnoses, and had agitation per various scales. The average age in the groups was 74 years, 56.0%-61.7% were women, and 94.3%-98.1% were White.
The first trial examined two fixed doses (1 mg/d, n = 137; and 2 mg/d, n = 140) or placebo (n = 136). “The study initially included a 0.5 mg/day arm,” the researchers reported, “which was removed in a protocol amendment, and patients randomized to that arm were not included in efficacy analyses.”
The second trial looked at a flexible dose (0.5-2 mg/d, n = 133) or placebo (n = 137).
In a CTAD presentation, Nanco Hefting of Lundbeck, a codeveloper of the drug, said that the researchers learned from the first two trials that 2 mg/d might be an appropriate dose, and the FDA recommended they also examine 3 mg/day. As a result, the third trial examined two fixed doses (2 mg/d, n = 75; 3 mg/d, n = 153; or placebo, n = 117).
In the third trial, both the placebo and drug groups improved per a measurement of agitation; those in the drug group improved somewhat more.
The mean change in baseline on the Cohen-Mansfield Agitation Inventory scale – the primary endpoint – was –5.32 for the 2-mg/d and 3-mg/d groups vs. placebo (P = .0026); the score in the placebo group fell by about 18 and by about 22 in the drug group.
The key secondary endpoint was an improvement from baseline to week 12 in the Clinical Global Impression–Severity (CGI-S) score related to agitation. Compared with the placebo group, this score was –0.27 in the drug group (P = .0078). Both scores hovered around –1.0.
Safety data show the percentage of treatment-emergent events ranged from 45.9% in the placebo group to 49.0%-56.8% for brexpiprazole in the three trials. The percentage of these events leading to discontinuation was 6.3% among those receiving the drug and 3.4% in the placebo group.
University of Exeter dementia researcher Clive Ballard, MD, MB ChB, one of the panelists who discussed the research after the CTAD presentation, praised the trials as “well-conducted” and said that he was pleased that subjects in institutions were included. “It’s not an easy environment to do trials in. They should be really commended for doing for doing that.”
But he echoed fellow panelist Dr. Atri by noting that more data are needed to understand how well the drug works. “I would like to see the effect sizes and a little bit more detail to understand the clinical meaningfulness of that level of benefit.”
What’s next? A spokeswoman for Otsuka, a codeveloper of brexpiprazole, said that it hopes to hear in 2023 about a supplemental new drug application that was filed in November 2022.
Otsuka and Lundbeck funded the research. Mr. Hefting is an employee of Lundbeck, and several other authors work for Lundbeck or Otsuka. The single non-employee author reports various disclosures. Disclosures for Dr. Atri and Dr. Ballard were not provided.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – In a widely anticipated report,
Members of a panel of dementia specialists here at the 15th Clinical Trials on Alzheimer’s Disease (CTAD) conference said that the results were encouraging. But they also noted that the available data make it difficult to understand the impact of the drug on the day-to-day life on patients.
“I’d like to be able to translate that into something else to understand the risk benefit calculus,” said neurologist and neuroscientist Alireza Atri, MD, PhD, of Banner Sun Health Research Institute in Phoenix. “How does it affect the patients themselves, their quality of life, and the family members and their burden?”
Currently, there’s no Food and Drug Administration–approved treatment for agitation in AD.
In 2015, the FDA approved brexpiprazole, an oral medication, as a treatment for schizophrenia and an adjunctive treatment for major depressive disorder (MDD). It is an expensive drug with an average retail price per GoodRx of $1,582 per month, and no generic is available.
Researchers released the results of a trio of phase 3 clinical trials at CTAD that examined various doses of brexpiprazole. The results of the first two trials had been released earlier in 2018.
Three trials
All trials were multicenter, 12-week, randomized, double-blind and placebo-controlled.
Study participants were aged 55-90 years, had probable AD diagnoses, and had agitation per various scales. The average age in the groups was 74 years, 56.0%-61.7% were women, and 94.3%-98.1% were White.
The first trial examined two fixed doses (1 mg/d, n = 137; and 2 mg/d, n = 140) or placebo (n = 136). “The study initially included a 0.5 mg/day arm,” the researchers reported, “which was removed in a protocol amendment, and patients randomized to that arm were not included in efficacy analyses.”
The second trial looked at a flexible dose (0.5-2 mg/d, n = 133) or placebo (n = 137).
In a CTAD presentation, Nanco Hefting of Lundbeck, a codeveloper of the drug, said that the researchers learned from the first two trials that 2 mg/d might be an appropriate dose, and the FDA recommended they also examine 3 mg/day. As a result, the third trial examined two fixed doses (2 mg/d, n = 75; 3 mg/d, n = 153; or placebo, n = 117).
In the third trial, both the placebo and drug groups improved per a measurement of agitation; those in the drug group improved somewhat more.
The mean change in baseline on the Cohen-Mansfield Agitation Inventory scale – the primary endpoint – was –5.32 for the 2-mg/d and 3-mg/d groups vs. placebo (P = .0026); the score in the placebo group fell by about 18 and by about 22 in the drug group.
The key secondary endpoint was an improvement from baseline to week 12 in the Clinical Global Impression–Severity (CGI-S) score related to agitation. Compared with the placebo group, this score was –0.27 in the drug group (P = .0078). Both scores hovered around –1.0.
Safety data show the percentage of treatment-emergent events ranged from 45.9% in the placebo group to 49.0%-56.8% for brexpiprazole in the three trials. The percentage of these events leading to discontinuation was 6.3% among those receiving the drug and 3.4% in the placebo group.
University of Exeter dementia researcher Clive Ballard, MD, MB ChB, one of the panelists who discussed the research after the CTAD presentation, praised the trials as “well-conducted” and said that he was pleased that subjects in institutions were included. “It’s not an easy environment to do trials in. They should be really commended for doing for doing that.”
But he echoed fellow panelist Dr. Atri by noting that more data are needed to understand how well the drug works. “I would like to see the effect sizes and a little bit more detail to understand the clinical meaningfulness of that level of benefit.”
What’s next? A spokeswoman for Otsuka, a codeveloper of brexpiprazole, said that it hopes to hear in 2023 about a supplemental new drug application that was filed in November 2022.
Otsuka and Lundbeck funded the research. Mr. Hefting is an employee of Lundbeck, and several other authors work for Lundbeck or Otsuka. The single non-employee author reports various disclosures. Disclosures for Dr. Atri and Dr. Ballard were not provided.
A version of this article first appeared on Medscape.com.
AT CTAD 2022
Mindfulness, exercise strike out in memory trial
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
We are coming to the end of the year, which always makes me think about getting older. Much like the search for the fountain of youth, many promising leads have ultimately led to dead ends. And yet, I had high hopes for a trial that focused on two cornerstones of wellness – exercise and mindfulness – to address the subjective loss of memory that comes with aging. Alas, meditation and exercise do not appear to be the fountain of youth.
I’m talking about this study, appearing in JAMA, known as the MEDEX trial.
It’s a clever design: a 2 x 2 factorial randomized trial where participants could be randomized to a mindfulness intervention, an exercise intervention, both, or neither.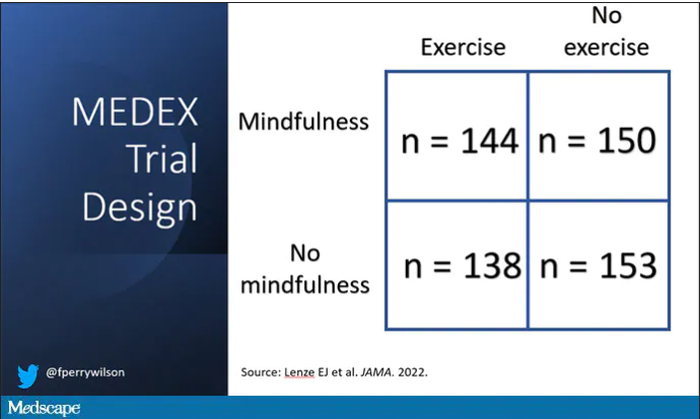
In this manner, you can test multiple hypotheses exploiting a shared control group. Or as a mentor of mine used to say, you get two trials for the price of one and a half.
The participants were older adults, aged 65-84, living in the community. They had to be relatively sedentary at baseline and not engaging in mindfulness practices. They had to subjectively report some memory or concentration issues but had to be cognitively intact, based on a standard dementia screening test. In other words, these are your average older people who are worried that they aren’t as sharp as they used to be.
The interventions themselves were fairly intense. The exercise group had instructor-led sessions for 90 minutes twice a week for the first 6 months of the study, once a week thereafter. And participants were encouraged to exercise at home such that they had a total of 300 minutes of weekly exercise.
The mindfulness program was characterized by eight weekly classes of 2.5 hours each as well as a half-day retreat to teach the tenets of mindfulness and meditation, with monthly refreshers thereafter. Participants were instructed to meditate for 60 minutes a day in addition to the classes.
For the 144 people who were randomized to both meditation and exercise, this trial amounted to something of a part-time job. So you might think that adherence to the interventions was low, but apparently that’s not the case. Attendance to the mindfulness classes was over 90%, and over 80% for the exercise classes. And diary-based reporting of home efforts was also pretty good.
The control group wasn’t left to their own devices. Recognizing that the community aspect of exercise or mindfulness classes might convey a benefit independent of the actual exercise or mindfulness, the control group met on a similar schedule to discuss health education, but no mention of exercise or mindfulness occurred in that setting.
The primary outcome was change in memory and executive function scores across a battery of neuropsychologic testing, but the story is told in just a few pictures.
Memory scores improved in all three groups – mindfulness, exercise, and health education – over time. Cognitive composite score improved in all three groups similarly. There was no synergistic effect of mindfulness and exercise either. Basically, everyone got a bit better.
But the study did way more than look at scores on tests. Researchers used MRI to measure brain anatomic outcomes as well. And the surprising thing is that virtually none of these outcomes were different between the groups either.
Hippocampal volume decreased a bit in all the groups. Dorsolateral prefrontal cortex volume was flat. There was no change in scores measuring tasks of daily living.
When you see negative results like this, right away you worry that the intervention wasn’t properly delivered. Were these people really exercising and meditating? Well, the authors showed that individuals randomized to exercise, at least, had less sleep latency, greater aerobic fitness, and greater strength. So we know something was happening.
They then asked, would the people in the exercise group with the greatest changes in those physiologic parameters show some improvement in cognitive parameters? In other words, we know you were exercising because you got stronger and are sleeping better; is your memory better? The answer? Surprisingly, still no. Even in that honestly somewhat cherry-picked group, the interventions had no effect.
Could it be that the control was inappropriate, that the “health education” intervention was actually so helpful that it obscured the benefits of exercise and meditation? After all, cognitive scores did improve in all groups. The authors doubt it. They say they think the improvement in cognitive scores reflects the fact that patients had learned a bit about how to take the tests. This is pretty common in the neuropsychiatric literature.
So here we are and I just want to say, well, shoot. This is not the result I wanted. And I think the reason I’m so disappointed is because aging and the loss of cognitive faculties that comes with aging are just sort of scary. We are all looking for some control over that fear, and how nice it would be to be able to tell ourselves not to worry – that we won’t have those problems as we get older because we exercise, or meditate, or drink red wine, or don’t drink wine, or whatever. And while I have no doubt that staying healthier physically will keep you healthier mentally, it may take more than one simple thing to move the needle.
Dr. Wilson is associate professor, department of medicine, and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
We are coming to the end of the year, which always makes me think about getting older. Much like the search for the fountain of youth, many promising leads have ultimately led to dead ends. And yet, I had high hopes for a trial that focused on two cornerstones of wellness – exercise and mindfulness – to address the subjective loss of memory that comes with aging. Alas, meditation and exercise do not appear to be the fountain of youth.
I’m talking about this study, appearing in JAMA, known as the MEDEX trial.
It’s a clever design: a 2 x 2 factorial randomized trial where participants could be randomized to a mindfulness intervention, an exercise intervention, both, or neither.
In this manner, you can test multiple hypotheses exploiting a shared control group. Or as a mentor of mine used to say, you get two trials for the price of one and a half.
The participants were older adults, aged 65-84, living in the community. They had to be relatively sedentary at baseline and not engaging in mindfulness practices. They had to subjectively report some memory or concentration issues but had to be cognitively intact, based on a standard dementia screening test. In other words, these are your average older people who are worried that they aren’t as sharp as they used to be.
The interventions themselves were fairly intense. The exercise group had instructor-led sessions for 90 minutes twice a week for the first 6 months of the study, once a week thereafter. And participants were encouraged to exercise at home such that they had a total of 300 minutes of weekly exercise.
The mindfulness program was characterized by eight weekly classes of 2.5 hours each as well as a half-day retreat to teach the tenets of mindfulness and meditation, with monthly refreshers thereafter. Participants were instructed to meditate for 60 minutes a day in addition to the classes.
For the 144 people who were randomized to both meditation and exercise, this trial amounted to something of a part-time job. So you might think that adherence to the interventions was low, but apparently that’s not the case. Attendance to the mindfulness classes was over 90%, and over 80% for the exercise classes. And diary-based reporting of home efforts was also pretty good.
The control group wasn’t left to their own devices. Recognizing that the community aspect of exercise or mindfulness classes might convey a benefit independent of the actual exercise or mindfulness, the control group met on a similar schedule to discuss health education, but no mention of exercise or mindfulness occurred in that setting.
The primary outcome was change in memory and executive function scores across a battery of neuropsychologic testing, but the story is told in just a few pictures.
Memory scores improved in all three groups – mindfulness, exercise, and health education – over time. Cognitive composite score improved in all three groups similarly. There was no synergistic effect of mindfulness and exercise either. Basically, everyone got a bit better.
But the study did way more than look at scores on tests. Researchers used MRI to measure brain anatomic outcomes as well. And the surprising thing is that virtually none of these outcomes were different between the groups either.
Hippocampal volume decreased a bit in all the groups. Dorsolateral prefrontal cortex volume was flat. There was no change in scores measuring tasks of daily living.
When you see negative results like this, right away you worry that the intervention wasn’t properly delivered. Were these people really exercising and meditating? Well, the authors showed that individuals randomized to exercise, at least, had less sleep latency, greater aerobic fitness, and greater strength. So we know something was happening.
They then asked, would the people in the exercise group with the greatest changes in those physiologic parameters show some improvement in cognitive parameters? In other words, we know you were exercising because you got stronger and are sleeping better; is your memory better? The answer? Surprisingly, still no. Even in that honestly somewhat cherry-picked group, the interventions had no effect.
Could it be that the control was inappropriate, that the “health education” intervention was actually so helpful that it obscured the benefits of exercise and meditation? After all, cognitive scores did improve in all groups. The authors doubt it. They say they think the improvement in cognitive scores reflects the fact that patients had learned a bit about how to take the tests. This is pretty common in the neuropsychiatric literature.
So here we are and I just want to say, well, shoot. This is not the result I wanted. And I think the reason I’m so disappointed is because aging and the loss of cognitive faculties that comes with aging are just sort of scary. We are all looking for some control over that fear, and how nice it would be to be able to tell ourselves not to worry – that we won’t have those problems as we get older because we exercise, or meditate, or drink red wine, or don’t drink wine, or whatever. And while I have no doubt that staying healthier physically will keep you healthier mentally, it may take more than one simple thing to move the needle.
Dr. Wilson is associate professor, department of medicine, and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
We are coming to the end of the year, which always makes me think about getting older. Much like the search for the fountain of youth, many promising leads have ultimately led to dead ends. And yet, I had high hopes for a trial that focused on two cornerstones of wellness – exercise and mindfulness – to address the subjective loss of memory that comes with aging. Alas, meditation and exercise do not appear to be the fountain of youth.
I’m talking about this study, appearing in JAMA, known as the MEDEX trial.
It’s a clever design: a 2 x 2 factorial randomized trial where participants could be randomized to a mindfulness intervention, an exercise intervention, both, or neither.
In this manner, you can test multiple hypotheses exploiting a shared control group. Or as a mentor of mine used to say, you get two trials for the price of one and a half.
The participants were older adults, aged 65-84, living in the community. They had to be relatively sedentary at baseline and not engaging in mindfulness practices. They had to subjectively report some memory or concentration issues but had to be cognitively intact, based on a standard dementia screening test. In other words, these are your average older people who are worried that they aren’t as sharp as they used to be.
The interventions themselves were fairly intense. The exercise group had instructor-led sessions for 90 minutes twice a week for the first 6 months of the study, once a week thereafter. And participants were encouraged to exercise at home such that they had a total of 300 minutes of weekly exercise.
The mindfulness program was characterized by eight weekly classes of 2.5 hours each as well as a half-day retreat to teach the tenets of mindfulness and meditation, with monthly refreshers thereafter. Participants were instructed to meditate for 60 minutes a day in addition to the classes.
For the 144 people who were randomized to both meditation and exercise, this trial amounted to something of a part-time job. So you might think that adherence to the interventions was low, but apparently that’s not the case. Attendance to the mindfulness classes was over 90%, and over 80% for the exercise classes. And diary-based reporting of home efforts was also pretty good.
The control group wasn’t left to their own devices. Recognizing that the community aspect of exercise or mindfulness classes might convey a benefit independent of the actual exercise or mindfulness, the control group met on a similar schedule to discuss health education, but no mention of exercise or mindfulness occurred in that setting.
The primary outcome was change in memory and executive function scores across a battery of neuropsychologic testing, but the story is told in just a few pictures.
Memory scores improved in all three groups – mindfulness, exercise, and health education – over time. Cognitive composite score improved in all three groups similarly. There was no synergistic effect of mindfulness and exercise either. Basically, everyone got a bit better.
But the study did way more than look at scores on tests. Researchers used MRI to measure brain anatomic outcomes as well. And the surprising thing is that virtually none of these outcomes were different between the groups either.
Hippocampal volume decreased a bit in all the groups. Dorsolateral prefrontal cortex volume was flat. There was no change in scores measuring tasks of daily living.
When you see negative results like this, right away you worry that the intervention wasn’t properly delivered. Were these people really exercising and meditating? Well, the authors showed that individuals randomized to exercise, at least, had less sleep latency, greater aerobic fitness, and greater strength. So we know something was happening.
They then asked, would the people in the exercise group with the greatest changes in those physiologic parameters show some improvement in cognitive parameters? In other words, we know you were exercising because you got stronger and are sleeping better; is your memory better? The answer? Surprisingly, still no. Even in that honestly somewhat cherry-picked group, the interventions had no effect.
Could it be that the control was inappropriate, that the “health education” intervention was actually so helpful that it obscured the benefits of exercise and meditation? After all, cognitive scores did improve in all groups. The authors doubt it. They say they think the improvement in cognitive scores reflects the fact that patients had learned a bit about how to take the tests. This is pretty common in the neuropsychiatric literature.
So here we are and I just want to say, well, shoot. This is not the result I wanted. And I think the reason I’m so disappointed is because aging and the loss of cognitive faculties that comes with aging are just sort of scary. We are all looking for some control over that fear, and how nice it would be to be able to tell ourselves not to worry – that we won’t have those problems as we get older because we exercise, or meditate, or drink red wine, or don’t drink wine, or whatever. And while I have no doubt that staying healthier physically will keep you healthier mentally, it may take more than one simple thing to move the needle.
Dr. Wilson is associate professor, department of medicine, and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Have you heard the one about the cow in the doctor’s office?
Maybe the cow was late for its appointment
It’s been a long day running the front desk at your doctor’s office. People calling in prescriptions, a million appointments, you’ve been running yourself ragged keeping things together. Finally, it’s almost closing time. The last patient of the day has just checked out and you turn back to the waiting room, expecting to see it blessedly empty.
Instead, a 650-pound cow is staring at you.
“I’m sorry, sir or madam, we’re about to close.”
Moo.
“I understand it’s important, but seriously, the doctor’s about to …”
Moo.
“Fine, I’ll see what we can do for you. What’s your insurance?”
Moo Cross Moo Shield.
“Sorry, we don’t take that. You’ll have to go someplace else.”
This is probably not how things went down recently at Orange (Va.) Family Physicians, when they had a cow break into the office. Cows don’t have health insurance.
The intrepid bovine was being transferred to a new home when it jumped off the trailer and wandered an eighth of a mile to Orange Family Physicians, where the cow wranglers found it hanging around outside. Unfortunately, this was a smart cow, and it bolted as it saw the wranglers, crashing through the glass doors into the doctor’s office. Though neither man had ever wrangled a cow from inside a building, they ultimately secured a rope around the cow’s neck and escorted it back outside, tying it to a nearby pole to keep it from further adventures.
One of the wranglers summed up the situation quite nicely on his Facebook page: “You ain’t no cowboy if you don’t rope a calf out of a [doctor’s] office.”
We can see that decision in your eyes
The cliché that eyes are the windows to the soul doesn’t tell the whole story about how telling eyes really are. It’s all about how they move. In a recent study, researchers determined that a type of eye movement known as a saccade reveals your choice before you even decide.
Saccades involve the eyes jumping from one fixation point to another, senior author Alaa Ahmed of the University of Colorado, Boulder, explained in a statement from the university. Saccade vigor was the key in how aligned the type of decisions were made by the 22 study participants.
In the study, subjects walked on a treadmill at varied inclines for a period of time. Then they sat in front of a monitor and a high-speed camera that tracked their eye movements as the monitor presented them with a series of exercise options. The participants had only 4 seconds to choose between them.
After they made their choices, participants went back on the treadmill to perform the exercises they had chosen. The researchers found that participants’ eyes jumped between the options slowly then faster to the option they eventually picked. The more impulsive decision-makers also tended to move their eyes even more rapidly before slowing down after a decision was made, making it pretty conclusive that the eyes were revealing their choices.
The way your eyes shift gives you away without saying a thing. Might be wise, then, to wear sunglasses to your next poker tournament.
Let them eat soap
Okay, we admit it: LOTME spends a lot of time in the bathroom. Today, though, we’re interested in the sinks. Specifically, the P-traps under the sinks. You know, the curvy bit that keeps sewer gas from wafting back into the room?
Well, researchers from the University of Reading (England) recently found some fungi while examining a bunch of sinks on the university’s Whiteknights campus. “It isn’t a big surprise to find fungi in a warm, wet environment. But sinks and P-traps have thus far been overlooked as potential reservoirs of these microorganisms,” they said in a written statement.
Samples collected from 289 P-traps contained “a very similar community of yeasts and molds, showing that sinks in use in public environments share a role as reservoirs of fungal organisms,” they noted.
The fungi living in the traps survived conditions with high temperatures, low pH, and little in the way of nutrients. So what were they eating? Some varieties, they said, “use detergents, found in soap, as a source of carbon-rich food.” We’ll repeat that last part: They used the soap as food.
WARNING: Rant Ahead.
There are a lot of cleaning products for sale that say they will make your home safe by killing 99.9% of germs and bacteria. Not fungi, exactly, but we’re still talking microorganisms. Molds, bacteria, and viruses are all stuff that can infect humans and make them sick.
So you kill 99.9% of them. Great, but that leaves 0.1% that you just made angry. And what do they do next? They learn to eat soap. Then University of Reading investigators find out that all the extra hand washing going on during the COVID-19 pandemic was “clogging up sinks with nasty disease-causing bacteria.”
These are microorganisms we’re talking about people. They’ve been at this for a billion years! Rats can’t beat them, cockroaches won’t stop them – Earth’s ultimate survivors are powerless against the invisible horde.
We’re doomed.
Maybe the cow was late for its appointment
It’s been a long day running the front desk at your doctor’s office. People calling in prescriptions, a million appointments, you’ve been running yourself ragged keeping things together. Finally, it’s almost closing time. The last patient of the day has just checked out and you turn back to the waiting room, expecting to see it blessedly empty.
Instead, a 650-pound cow is staring at you.
“I’m sorry, sir or madam, we’re about to close.”
Moo.
“I understand it’s important, but seriously, the doctor’s about to …”
Moo.
“Fine, I’ll see what we can do for you. What’s your insurance?”
Moo Cross Moo Shield.
“Sorry, we don’t take that. You’ll have to go someplace else.”
This is probably not how things went down recently at Orange (Va.) Family Physicians, when they had a cow break into the office. Cows don’t have health insurance.
The intrepid bovine was being transferred to a new home when it jumped off the trailer and wandered an eighth of a mile to Orange Family Physicians, where the cow wranglers found it hanging around outside. Unfortunately, this was a smart cow, and it bolted as it saw the wranglers, crashing through the glass doors into the doctor’s office. Though neither man had ever wrangled a cow from inside a building, they ultimately secured a rope around the cow’s neck and escorted it back outside, tying it to a nearby pole to keep it from further adventures.
One of the wranglers summed up the situation quite nicely on his Facebook page: “You ain’t no cowboy if you don’t rope a calf out of a [doctor’s] office.”
We can see that decision in your eyes
The cliché that eyes are the windows to the soul doesn’t tell the whole story about how telling eyes really are. It’s all about how they move. In a recent study, researchers determined that a type of eye movement known as a saccade reveals your choice before you even decide.
Saccades involve the eyes jumping from one fixation point to another, senior author Alaa Ahmed of the University of Colorado, Boulder, explained in a statement from the university. Saccade vigor was the key in how aligned the type of decisions were made by the 22 study participants.
In the study, subjects walked on a treadmill at varied inclines for a period of time. Then they sat in front of a monitor and a high-speed camera that tracked their eye movements as the monitor presented them with a series of exercise options. The participants had only 4 seconds to choose between them.
After they made their choices, participants went back on the treadmill to perform the exercises they had chosen. The researchers found that participants’ eyes jumped between the options slowly then faster to the option they eventually picked. The more impulsive decision-makers also tended to move their eyes even more rapidly before slowing down after a decision was made, making it pretty conclusive that the eyes were revealing their choices.
The way your eyes shift gives you away without saying a thing. Might be wise, then, to wear sunglasses to your next poker tournament.
Let them eat soap
Okay, we admit it: LOTME spends a lot of time in the bathroom. Today, though, we’re interested in the sinks. Specifically, the P-traps under the sinks. You know, the curvy bit that keeps sewer gas from wafting back into the room?
Well, researchers from the University of Reading (England) recently found some fungi while examining a bunch of sinks on the university’s Whiteknights campus. “It isn’t a big surprise to find fungi in a warm, wet environment. But sinks and P-traps have thus far been overlooked as potential reservoirs of these microorganisms,” they said in a written statement.
Samples collected from 289 P-traps contained “a very similar community of yeasts and molds, showing that sinks in use in public environments share a role as reservoirs of fungal organisms,” they noted.
The fungi living in the traps survived conditions with high temperatures, low pH, and little in the way of nutrients. So what were they eating? Some varieties, they said, “use detergents, found in soap, as a source of carbon-rich food.” We’ll repeat that last part: They used the soap as food.
WARNING: Rant Ahead.
There are a lot of cleaning products for sale that say they will make your home safe by killing 99.9% of germs and bacteria. Not fungi, exactly, but we’re still talking microorganisms. Molds, bacteria, and viruses are all stuff that can infect humans and make them sick.
So you kill 99.9% of them. Great, but that leaves 0.1% that you just made angry. And what do they do next? They learn to eat soap. Then University of Reading investigators find out that all the extra hand washing going on during the COVID-19 pandemic was “clogging up sinks with nasty disease-causing bacteria.”
These are microorganisms we’re talking about people. They’ve been at this for a billion years! Rats can’t beat them, cockroaches won’t stop them – Earth’s ultimate survivors are powerless against the invisible horde.
We’re doomed.
Maybe the cow was late for its appointment
It’s been a long day running the front desk at your doctor’s office. People calling in prescriptions, a million appointments, you’ve been running yourself ragged keeping things together. Finally, it’s almost closing time. The last patient of the day has just checked out and you turn back to the waiting room, expecting to see it blessedly empty.
Instead, a 650-pound cow is staring at you.
“I’m sorry, sir or madam, we’re about to close.”
Moo.
“I understand it’s important, but seriously, the doctor’s about to …”
Moo.
“Fine, I’ll see what we can do for you. What’s your insurance?”
Moo Cross Moo Shield.
“Sorry, we don’t take that. You’ll have to go someplace else.”
This is probably not how things went down recently at Orange (Va.) Family Physicians, when they had a cow break into the office. Cows don’t have health insurance.
The intrepid bovine was being transferred to a new home when it jumped off the trailer and wandered an eighth of a mile to Orange Family Physicians, where the cow wranglers found it hanging around outside. Unfortunately, this was a smart cow, and it bolted as it saw the wranglers, crashing through the glass doors into the doctor’s office. Though neither man had ever wrangled a cow from inside a building, they ultimately secured a rope around the cow’s neck and escorted it back outside, tying it to a nearby pole to keep it from further adventures.
One of the wranglers summed up the situation quite nicely on his Facebook page: “You ain’t no cowboy if you don’t rope a calf out of a [doctor’s] office.”
We can see that decision in your eyes
The cliché that eyes are the windows to the soul doesn’t tell the whole story about how telling eyes really are. It’s all about how they move. In a recent study, researchers determined that a type of eye movement known as a saccade reveals your choice before you even decide.
Saccades involve the eyes jumping from one fixation point to another, senior author Alaa Ahmed of the University of Colorado, Boulder, explained in a statement from the university. Saccade vigor was the key in how aligned the type of decisions were made by the 22 study participants.
In the study, subjects walked on a treadmill at varied inclines for a period of time. Then they sat in front of a monitor and a high-speed camera that tracked their eye movements as the monitor presented them with a series of exercise options. The participants had only 4 seconds to choose between them.
After they made their choices, participants went back on the treadmill to perform the exercises they had chosen. The researchers found that participants’ eyes jumped between the options slowly then faster to the option they eventually picked. The more impulsive decision-makers also tended to move their eyes even more rapidly before slowing down after a decision was made, making it pretty conclusive that the eyes were revealing their choices.
The way your eyes shift gives you away without saying a thing. Might be wise, then, to wear sunglasses to your next poker tournament.
Let them eat soap
Okay, we admit it: LOTME spends a lot of time in the bathroom. Today, though, we’re interested in the sinks. Specifically, the P-traps under the sinks. You know, the curvy bit that keeps sewer gas from wafting back into the room?
Well, researchers from the University of Reading (England) recently found some fungi while examining a bunch of sinks on the university’s Whiteknights campus. “It isn’t a big surprise to find fungi in a warm, wet environment. But sinks and P-traps have thus far been overlooked as potential reservoirs of these microorganisms,” they said in a written statement.
Samples collected from 289 P-traps contained “a very similar community of yeasts and molds, showing that sinks in use in public environments share a role as reservoirs of fungal organisms,” they noted.
The fungi living in the traps survived conditions with high temperatures, low pH, and little in the way of nutrients. So what were they eating? Some varieties, they said, “use detergents, found in soap, as a source of carbon-rich food.” We’ll repeat that last part: They used the soap as food.
WARNING: Rant Ahead.
There are a lot of cleaning products for sale that say they will make your home safe by killing 99.9% of germs and bacteria. Not fungi, exactly, but we’re still talking microorganisms. Molds, bacteria, and viruses are all stuff that can infect humans and make them sick.
So you kill 99.9% of them. Great, but that leaves 0.1% that you just made angry. And what do they do next? They learn to eat soap. Then University of Reading investigators find out that all the extra hand washing going on during the COVID-19 pandemic was “clogging up sinks with nasty disease-causing bacteria.”
These are microorganisms we’re talking about people. They’ve been at this for a billion years! Rats can’t beat them, cockroaches won’t stop them – Earth’s ultimate survivors are powerless against the invisible horde.
We’re doomed.
Can a Mediterranean diet ease depression in young men?
This transcript has been edited for clarity.
Drew Ramsey, MD: Welcome back, everyone. I’m Dr. Drew Ramsey. I’m on the editorial board with Medscape Psychiatry and I’m an assistant clinical professor of psychiatry at Columbia University. We have a special guest today.
I’m here with nutritionist Jessica Bayes, who’s at the University of Technology Sydney, and she’s the lead author of the AMMEND trial. [Editor’s note: Since completing her PhD, Bayes is now at Southern Cross University.]
Jessica, welcome to Medscape.
Jessica Bayes, PhD: Thank you for having me.
The AMMEND Trial
Dr. Ramsey: Thank you for coming on board and helping all of us as clinicians understand some of your research and some of what is suggested by your research – that young men can change their diet and it helped their depression. Tell us a little bit about the AMMEND trial.
Dr. Bayes: The AMMEND trial was a 12-week randomized controlled trial in young men, 18-25 years old, who had diagnosed moderate to severe clinical depression. They had a poor baseline diet and we got them to eat a healthy Mediterranean diet, which improved their symptoms of depression.
Dr. Ramsey: It was a remarkable trial. Jessica, if I recall, you helped individuals improve the Mediterranean dietary pattern score by 8 points on a 14-point scale. That led to a 20-point reduction in their Beck Depression Inventory. Tell us what that looked like on the ground.
Dr. Bayes: It’s a huge improvement. Obviously, they were feeling much better in the end in terms of their depressive symptoms, but we also measured their energy, sleep, and quality of life. Many of them at the end were at a score cutoff that suggests no depression or in remission.
Dr. Ramsey: There were 72 people in your total trial, so 36% in your intervention arm went into full remission.
Dr. Bayes: Which is just amazing.
Dr. Ramsey: It also follows up the SMILES trial, which was a little bit of a different trial. You had two nutritional counseling sessions and the SMILES trial had seven, but in the SMILES trial, 32.3% of the patients went into full remission when they adopted a Mediterranean-style diet.
Jessica, what is the secret that you and your team know? I think many clinicians, especially clinicians who are parents and have teens, are kind of shaking their heads in disbelief. They’ve been telling their kids to eat healthy. What do you guys know about how to help young men change their diet?
How to Aid Adherence to Mediterranean Diet
Dr. Bayes: Prior to starting this, when I would say this idea to people, everyone would say, “Great idea. There’s no way you’re going to get depressed young men to change their diet. Not going to happen.” We went to them and we asked them. We said, “We’re going to do this study. What do you want from us? What resources would you need? How many appointments would you like? What’s too little or too many?”
We really got their feedback on board when we designed the study, and that obviously paid off. We had a personalized approach and we met them where they were at. We gave them the skills, resources, recipes, meal ideas – all those things – so we could really set them up to succeed.
Dr. Ramsey: You were telling me earlier about a few of the dietary changes that you felt made a big difference for these young men. What were those?
Dr. Bayes: Increasing the vegetables, olive oil, and legumes are probably the big ones that most of them were really not doing beforehand. They were really able to take that on board and make significant improvements in those areas.
Dr. Ramsey: These are really some of the top food categories in nutritional psychiatry as we think about how we help our efforts to improve mental health by thinking about nutrition, nutritional quality, and nutritional density. Certainly, those food categories – nuts and legumes, plants, and olive oil – are really what help get us there.
You also gave the students a food hamper. If you were going to be in charge of mental health in Australia and America and you got to give every college freshman a little box with a note, what would be in that box?
Dr. Bayes: I’d want to put everything in that box! It would be full of brightly colored fruits and vegetables, different nuts and seeds, and legumes. It would be full of recipes and ideas of how to cook things and how to prepare really delicious things. It would be full of different herbs and spices and all of those things to get people really excited about food.
Dr. Ramsey: Did the young men pick up on your enthusiasm and excitement around food? Did they begin to adopt some of that, shifting their view of how they saw the food and how they saw that it is related to their depression?
Dr. Bayes: Hopefully. I do think energy is infectious. I’m sure that played a role somewhat, but trying to get them excited about food can be really quite daunting, thinking, I’ve got to change my entire diet and I’ve got to learn to cook and go out and buy groceries. I don’t even know what to do with a piece of salmon. Trying to get them curious, interested, and just reminding them that it’s not all-or-nothing. Make small changes, give it a go, and have fun.
Dr. Ramsey: You also have a unique aspect of your research that you’re interested in male mental health, and that’s not something that’s been widely researched. Can you tell us a little bit about what these men were like in terms of coming into your trial as depressed young men?
Dr. Bayes: In the context of the COVID-19 pandemic, mental health was at the forefront of many people’s minds. They joined the study saying, “I’ve never seen anything like this before. I’ve never seen myself represented in research. I wanted to contribute. I want to add to that conversation because I feel like we are overlooked.”
Dr. Ramsey: I love hearing this notion that maybe young men aren’t quite who we think they are. They are wanting to be seen around their mental health. They can learn to use olive oil and to cook, and they can engage in mental health interventions that work. We just need to ask, give them some food, encourage them, and it makes a big difference.
Jessica Bayes, thank you so much for joining us and sharing some of your research. Everyone, it’s the AMMEND trial. We will drop a link to the trial below so you can take a peek and tell us what you think.
Please, in the comments, let us know what you think about this notion of helping young men with depression through nutritional interventions. Take a peek at the great work that Jessica and Professor Sibbritt from the University of Technology Sydney have published and put out into the scientific literature for us all.
Thanks so much, Jessica. I look forward to seeing you soon.
Dr. Bayes: Thank you.
Dr. Ramsey is assistant clinical professor, department of psychiatry, Columbia University, New York. He has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for InterContinental Hotels Group; National Kale Day 501(c)3. Received income in an amount equal to or greater than $250 from: Sharecare. Dr. Bayes is a postdoctoral research fellow; clinical nutritionist, Southern Cross University, National Center for Naturopathic Medicine, Lismore, New South Wales, Australia. She has disclosed the following relevant financial relationships: Received research grant from Endeavour College. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Drew Ramsey, MD: Welcome back, everyone. I’m Dr. Drew Ramsey. I’m on the editorial board with Medscape Psychiatry and I’m an assistant clinical professor of psychiatry at Columbia University. We have a special guest today.
I’m here with nutritionist Jessica Bayes, who’s at the University of Technology Sydney, and she’s the lead author of the AMMEND trial. [Editor’s note: Since completing her PhD, Bayes is now at Southern Cross University.]
Jessica, welcome to Medscape.
Jessica Bayes, PhD: Thank you for having me.
The AMMEND Trial
Dr. Ramsey: Thank you for coming on board and helping all of us as clinicians understand some of your research and some of what is suggested by your research – that young men can change their diet and it helped their depression. Tell us a little bit about the AMMEND trial.
Dr. Bayes: The AMMEND trial was a 12-week randomized controlled trial in young men, 18-25 years old, who had diagnosed moderate to severe clinical depression. They had a poor baseline diet and we got them to eat a healthy Mediterranean diet, which improved their symptoms of depression.
Dr. Ramsey: It was a remarkable trial. Jessica, if I recall, you helped individuals improve the Mediterranean dietary pattern score by 8 points on a 14-point scale. That led to a 20-point reduction in their Beck Depression Inventory. Tell us what that looked like on the ground.
Dr. Bayes: It’s a huge improvement. Obviously, they were feeling much better in the end in terms of their depressive symptoms, but we also measured their energy, sleep, and quality of life. Many of them at the end were at a score cutoff that suggests no depression or in remission.
Dr. Ramsey: There were 72 people in your total trial, so 36% in your intervention arm went into full remission.
Dr. Bayes: Which is just amazing.
Dr. Ramsey: It also follows up the SMILES trial, which was a little bit of a different trial. You had two nutritional counseling sessions and the SMILES trial had seven, but in the SMILES trial, 32.3% of the patients went into full remission when they adopted a Mediterranean-style diet.
Jessica, what is the secret that you and your team know? I think many clinicians, especially clinicians who are parents and have teens, are kind of shaking their heads in disbelief. They’ve been telling their kids to eat healthy. What do you guys know about how to help young men change their diet?
How to Aid Adherence to Mediterranean Diet
Dr. Bayes: Prior to starting this, when I would say this idea to people, everyone would say, “Great idea. There’s no way you’re going to get depressed young men to change their diet. Not going to happen.” We went to them and we asked them. We said, “We’re going to do this study. What do you want from us? What resources would you need? How many appointments would you like? What’s too little or too many?”
We really got their feedback on board when we designed the study, and that obviously paid off. We had a personalized approach and we met them where they were at. We gave them the skills, resources, recipes, meal ideas – all those things – so we could really set them up to succeed.
Dr. Ramsey: You were telling me earlier about a few of the dietary changes that you felt made a big difference for these young men. What were those?
Dr. Bayes: Increasing the vegetables, olive oil, and legumes are probably the big ones that most of them were really not doing beforehand. They were really able to take that on board and make significant improvements in those areas.
Dr. Ramsey: These are really some of the top food categories in nutritional psychiatry as we think about how we help our efforts to improve mental health by thinking about nutrition, nutritional quality, and nutritional density. Certainly, those food categories – nuts and legumes, plants, and olive oil – are really what help get us there.
You also gave the students a food hamper. If you were going to be in charge of mental health in Australia and America and you got to give every college freshman a little box with a note, what would be in that box?
Dr. Bayes: I’d want to put everything in that box! It would be full of brightly colored fruits and vegetables, different nuts and seeds, and legumes. It would be full of recipes and ideas of how to cook things and how to prepare really delicious things. It would be full of different herbs and spices and all of those things to get people really excited about food.
Dr. Ramsey: Did the young men pick up on your enthusiasm and excitement around food? Did they begin to adopt some of that, shifting their view of how they saw the food and how they saw that it is related to their depression?
Dr. Bayes: Hopefully. I do think energy is infectious. I’m sure that played a role somewhat, but trying to get them excited about food can be really quite daunting, thinking, I’ve got to change my entire diet and I’ve got to learn to cook and go out and buy groceries. I don’t even know what to do with a piece of salmon. Trying to get them curious, interested, and just reminding them that it’s not all-or-nothing. Make small changes, give it a go, and have fun.
Dr. Ramsey: You also have a unique aspect of your research that you’re interested in male mental health, and that’s not something that’s been widely researched. Can you tell us a little bit about what these men were like in terms of coming into your trial as depressed young men?
Dr. Bayes: In the context of the COVID-19 pandemic, mental health was at the forefront of many people’s minds. They joined the study saying, “I’ve never seen anything like this before. I’ve never seen myself represented in research. I wanted to contribute. I want to add to that conversation because I feel like we are overlooked.”
Dr. Ramsey: I love hearing this notion that maybe young men aren’t quite who we think they are. They are wanting to be seen around their mental health. They can learn to use olive oil and to cook, and they can engage in mental health interventions that work. We just need to ask, give them some food, encourage them, and it makes a big difference.
Jessica Bayes, thank you so much for joining us and sharing some of your research. Everyone, it’s the AMMEND trial. We will drop a link to the trial below so you can take a peek and tell us what you think.
Please, in the comments, let us know what you think about this notion of helping young men with depression through nutritional interventions. Take a peek at the great work that Jessica and Professor Sibbritt from the University of Technology Sydney have published and put out into the scientific literature for us all.
Thanks so much, Jessica. I look forward to seeing you soon.
Dr. Bayes: Thank you.
Dr. Ramsey is assistant clinical professor, department of psychiatry, Columbia University, New York. He has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for InterContinental Hotels Group; National Kale Day 501(c)3. Received income in an amount equal to or greater than $250 from: Sharecare. Dr. Bayes is a postdoctoral research fellow; clinical nutritionist, Southern Cross University, National Center for Naturopathic Medicine, Lismore, New South Wales, Australia. She has disclosed the following relevant financial relationships: Received research grant from Endeavour College. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Drew Ramsey, MD: Welcome back, everyone. I’m Dr. Drew Ramsey. I’m on the editorial board with Medscape Psychiatry and I’m an assistant clinical professor of psychiatry at Columbia University. We have a special guest today.
I’m here with nutritionist Jessica Bayes, who’s at the University of Technology Sydney, and she’s the lead author of the AMMEND trial. [Editor’s note: Since completing her PhD, Bayes is now at Southern Cross University.]
Jessica, welcome to Medscape.
Jessica Bayes, PhD: Thank you for having me.
The AMMEND Trial
Dr. Ramsey: Thank you for coming on board and helping all of us as clinicians understand some of your research and some of what is suggested by your research – that young men can change their diet and it helped their depression. Tell us a little bit about the AMMEND trial.
Dr. Bayes: The AMMEND trial was a 12-week randomized controlled trial in young men, 18-25 years old, who had diagnosed moderate to severe clinical depression. They had a poor baseline diet and we got them to eat a healthy Mediterranean diet, which improved their symptoms of depression.
Dr. Ramsey: It was a remarkable trial. Jessica, if I recall, you helped individuals improve the Mediterranean dietary pattern score by 8 points on a 14-point scale. That led to a 20-point reduction in their Beck Depression Inventory. Tell us what that looked like on the ground.
Dr. Bayes: It’s a huge improvement. Obviously, they were feeling much better in the end in terms of their depressive symptoms, but we also measured their energy, sleep, and quality of life. Many of them at the end were at a score cutoff that suggests no depression or in remission.
Dr. Ramsey: There were 72 people in your total trial, so 36% in your intervention arm went into full remission.
Dr. Bayes: Which is just amazing.
Dr. Ramsey: It also follows up the SMILES trial, which was a little bit of a different trial. You had two nutritional counseling sessions and the SMILES trial had seven, but in the SMILES trial, 32.3% of the patients went into full remission when they adopted a Mediterranean-style diet.
Jessica, what is the secret that you and your team know? I think many clinicians, especially clinicians who are parents and have teens, are kind of shaking their heads in disbelief. They’ve been telling their kids to eat healthy. What do you guys know about how to help young men change their diet?
How to Aid Adherence to Mediterranean Diet
Dr. Bayes: Prior to starting this, when I would say this idea to people, everyone would say, “Great idea. There’s no way you’re going to get depressed young men to change their diet. Not going to happen.” We went to them and we asked them. We said, “We’re going to do this study. What do you want from us? What resources would you need? How many appointments would you like? What’s too little or too many?”
We really got their feedback on board when we designed the study, and that obviously paid off. We had a personalized approach and we met them where they were at. We gave them the skills, resources, recipes, meal ideas – all those things – so we could really set them up to succeed.
Dr. Ramsey: You were telling me earlier about a few of the dietary changes that you felt made a big difference for these young men. What were those?
Dr. Bayes: Increasing the vegetables, olive oil, and legumes are probably the big ones that most of them were really not doing beforehand. They were really able to take that on board and make significant improvements in those areas.
Dr. Ramsey: These are really some of the top food categories in nutritional psychiatry as we think about how we help our efforts to improve mental health by thinking about nutrition, nutritional quality, and nutritional density. Certainly, those food categories – nuts and legumes, plants, and olive oil – are really what help get us there.
You also gave the students a food hamper. If you were going to be in charge of mental health in Australia and America and you got to give every college freshman a little box with a note, what would be in that box?
Dr. Bayes: I’d want to put everything in that box! It would be full of brightly colored fruits and vegetables, different nuts and seeds, and legumes. It would be full of recipes and ideas of how to cook things and how to prepare really delicious things. It would be full of different herbs and spices and all of those things to get people really excited about food.
Dr. Ramsey: Did the young men pick up on your enthusiasm and excitement around food? Did they begin to adopt some of that, shifting their view of how they saw the food and how they saw that it is related to their depression?
Dr. Bayes: Hopefully. I do think energy is infectious. I’m sure that played a role somewhat, but trying to get them excited about food can be really quite daunting, thinking, I’ve got to change my entire diet and I’ve got to learn to cook and go out and buy groceries. I don’t even know what to do with a piece of salmon. Trying to get them curious, interested, and just reminding them that it’s not all-or-nothing. Make small changes, give it a go, and have fun.
Dr. Ramsey: You also have a unique aspect of your research that you’re interested in male mental health, and that’s not something that’s been widely researched. Can you tell us a little bit about what these men were like in terms of coming into your trial as depressed young men?
Dr. Bayes: In the context of the COVID-19 pandemic, mental health was at the forefront of many people’s minds. They joined the study saying, “I’ve never seen anything like this before. I’ve never seen myself represented in research. I wanted to contribute. I want to add to that conversation because I feel like we are overlooked.”
Dr. Ramsey: I love hearing this notion that maybe young men aren’t quite who we think they are. They are wanting to be seen around their mental health. They can learn to use olive oil and to cook, and they can engage in mental health interventions that work. We just need to ask, give them some food, encourage them, and it makes a big difference.
Jessica Bayes, thank you so much for joining us and sharing some of your research. Everyone, it’s the AMMEND trial. We will drop a link to the trial below so you can take a peek and tell us what you think.
Please, in the comments, let us know what you think about this notion of helping young men with depression through nutritional interventions. Take a peek at the great work that Jessica and Professor Sibbritt from the University of Technology Sydney have published and put out into the scientific literature for us all.
Thanks so much, Jessica. I look forward to seeing you soon.
Dr. Bayes: Thank you.
Dr. Ramsey is assistant clinical professor, department of psychiatry, Columbia University, New York. He has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for InterContinental Hotels Group; National Kale Day 501(c)3. Received income in an amount equal to or greater than $250 from: Sharecare. Dr. Bayes is a postdoctoral research fellow; clinical nutritionist, Southern Cross University, National Center for Naturopathic Medicine, Lismore, New South Wales, Australia. She has disclosed the following relevant financial relationships: Received research grant from Endeavour College. A version of this article first appeared on Medscape.com.
Noninvasive laser therapy tied to improved short-term memory
Investigators compared the effect of 1,064 nm of tPBM delivered over a 12-minute session to the right PFC vs. three other treatment arms: delivery of the same intervention to the left PFC, delivery of the intervention at a lower frequency, and a sham intervention.
All participants were shown a series of items prior to the intervention and asked to recall them after the intervention. Those who received tPBM 1,064 nm to the right PFC showed a superior performance of up to 25% in the memory tasks compared with the other groups.
Patients with attention-related conditions, such as attention deficit hyperactivity disorder, “could benefit from this type of treatment, which is safe, simple, and noninvasive, with no side effects,” coinvestigator Dongwei Li, a visiting PhD student at the Centre for Human Brain Health, University of Birmingham, England, said in a news release.
The findings were published online in Science Advances.
Differing wavelengths
The researchers note that “in the past decades,” noninvasive brain stimulation technology using transcranial application of direct or alternating electrical or magnetic fields “has been proven to be useful” in the improvement of working memory (WM).
When applied to the right PFC, tPBM has been shown to improve accuracy and speed of reaction time in WM tasks and improvements in “high-order cognitive functions,” such as sustained attention, emotion, and executive functions.
The investigators wanted to assess the impact of tPBM applied to different parts of the brain and at different wavelengths. They conducted four double-blind, sham-controlled experiments encompassing 90 neurotypical college students (mean age, 22 years). Each student participated in only one of the four experiments.
All completed two different tPBM sessions, separated by a week, in which sham and active tPBM were compared. Two different types of change-detection memory tasks were given: one requiring participants to remember the orientation of a series of items before and after the intervention and one other requiring them to remember the color of the items (experiments 1 and 2).
A series of follow-up experiments focused on comparing different wavelengths (1,064 nm vs. 852 nm) and different stimulation sites (right vs. left PFC; experiments 3 and 4).
EEG recordings were obtained during the intervention and the memory tasks.
Each experiment consisted of one active tPBM session and one sham tPBM session, with sessions consisting of 12 minutes of laser light (or sham) intervention. These sessions were conducted on the first and the seventh day; then, on the eighth day, participants were asked to report (or guess) which session was the active tPBM session.
Stimulating astrocytes
Results showed that, compared with sham tPBM, there was an improvement in WM capacity and scores by the 1,064 nm intervention in the orientation as well as the color task.
Participants who received the targeted treatment were able to remember between four and five test objects, whereas those with the treatment variations were only able to remember between three and four objects.
“These results support the hypothesis that 1,064 nm tPBM on the right PFC enhances WM capacity,” the investigators wrote.
They also found improvements in WM in participants receiving tPBM vs. sham regardless of whether their performance in the WM task was at a low or high level. This finding held true in both the orientation and the color tasks.
“Therefore, participants with good and poor WM capacity improved after 1,064 nm tPBM,” the researchers noted.
In addition, participants were unable to guess or report whether they had received sham or active tPBM.
EEG monitoring showed changes in brain activity that predicted the improvements in memory performance. In particular, 1,064 tPBM applied to the right PFC increased occipitoparietal contralateral delay activity (CDA), with CDA mediating the WM improvement.
This is “consistent with previous research that CDA is indicative of the number of maintained objects in visual working memory,” the investigators wrote.
Pearson correlation analyses showed that the differences in CDA set-size effects between active and sham session “correlated positively” with the behavioral differences between these sessions. For the orientation task, the r was 0.446 (P < .04); and for the color task, the r was .563 (P < .02).
No similar improvements were found with the 852 nm tPBM.
“We need further research to understand exactly why the tPBM is having this positive effect,” coinvestigator Ole Jensen, PhD, professor in translational neuroscience and codirector of the Centre for Human Brain Health, said in the release.
“It’s possible that the light is stimulating the astrocytes – the powerplants – in the nerve cells within the PFC, and this has a positive effect on the cells’ efficiency,” he noted.
Dr. Jensen added that his team “will also be investigating how long the effects might last. Clearly, if these experiments are to lead to a clinical intervention, we will need to see long-lasting benefits.”
Beneficial cognitive, emotional effects
Commenting for this news organization, Francisco Gonzalez-Lima, PhD, professor in the department of psychology, University of Texas at Austin, called the study “well done.”
Dr. Gonzalez-Lima was one of the first researchers to demonstrate that 1,064 nm transcranial infrared laser stimulation “produces beneficial cognitive and emotional effects in humans, including improving visual working memory,” he said.
The current study “reported an additional brain effect linked to the improved visual working memory that consists of an EEG-derived response, which is a new finding,” noted Dr. Gonzales-Lima, who was not involved with the new research.
He added that the same laser method “has been found by the Gonzalez-Lima lab to be effective at improving cognition in older adults and depressed and bipolar patients.”
The study was supported by the National Natural Science Foundation of China, the Ministry of Science and Technology of the People’s Republic of China, and the National Defence Basic Scientific Research Program of China. The investigators and Dr. Gonzalez-Lima report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared the effect of 1,064 nm of tPBM delivered over a 12-minute session to the right PFC vs. three other treatment arms: delivery of the same intervention to the left PFC, delivery of the intervention at a lower frequency, and a sham intervention.
All participants were shown a series of items prior to the intervention and asked to recall them after the intervention. Those who received tPBM 1,064 nm to the right PFC showed a superior performance of up to 25% in the memory tasks compared with the other groups.
Patients with attention-related conditions, such as attention deficit hyperactivity disorder, “could benefit from this type of treatment, which is safe, simple, and noninvasive, with no side effects,” coinvestigator Dongwei Li, a visiting PhD student at the Centre for Human Brain Health, University of Birmingham, England, said in a news release.
The findings were published online in Science Advances.
Differing wavelengths
The researchers note that “in the past decades,” noninvasive brain stimulation technology using transcranial application of direct or alternating electrical or magnetic fields “has been proven to be useful” in the improvement of working memory (WM).
When applied to the right PFC, tPBM has been shown to improve accuracy and speed of reaction time in WM tasks and improvements in “high-order cognitive functions,” such as sustained attention, emotion, and executive functions.
The investigators wanted to assess the impact of tPBM applied to different parts of the brain and at different wavelengths. They conducted four double-blind, sham-controlled experiments encompassing 90 neurotypical college students (mean age, 22 years). Each student participated in only one of the four experiments.
All completed two different tPBM sessions, separated by a week, in which sham and active tPBM were compared. Two different types of change-detection memory tasks were given: one requiring participants to remember the orientation of a series of items before and after the intervention and one other requiring them to remember the color of the items (experiments 1 and 2).
A series of follow-up experiments focused on comparing different wavelengths (1,064 nm vs. 852 nm) and different stimulation sites (right vs. left PFC; experiments 3 and 4).
EEG recordings were obtained during the intervention and the memory tasks.
Each experiment consisted of one active tPBM session and one sham tPBM session, with sessions consisting of 12 minutes of laser light (or sham) intervention. These sessions were conducted on the first and the seventh day; then, on the eighth day, participants were asked to report (or guess) which session was the active tPBM session.
Stimulating astrocytes
Results showed that, compared with sham tPBM, there was an improvement in WM capacity and scores by the 1,064 nm intervention in the orientation as well as the color task.
Participants who received the targeted treatment were able to remember between four and five test objects, whereas those with the treatment variations were only able to remember between three and four objects.
“These results support the hypothesis that 1,064 nm tPBM on the right PFC enhances WM capacity,” the investigators wrote.
They also found improvements in WM in participants receiving tPBM vs. sham regardless of whether their performance in the WM task was at a low or high level. This finding held true in both the orientation and the color tasks.
“Therefore, participants with good and poor WM capacity improved after 1,064 nm tPBM,” the researchers noted.
In addition, participants were unable to guess or report whether they had received sham or active tPBM.
EEG monitoring showed changes in brain activity that predicted the improvements in memory performance. In particular, 1,064 tPBM applied to the right PFC increased occipitoparietal contralateral delay activity (CDA), with CDA mediating the WM improvement.
This is “consistent with previous research that CDA is indicative of the number of maintained objects in visual working memory,” the investigators wrote.
Pearson correlation analyses showed that the differences in CDA set-size effects between active and sham session “correlated positively” with the behavioral differences between these sessions. For the orientation task, the r was 0.446 (P < .04); and for the color task, the r was .563 (P < .02).
No similar improvements were found with the 852 nm tPBM.
“We need further research to understand exactly why the tPBM is having this positive effect,” coinvestigator Ole Jensen, PhD, professor in translational neuroscience and codirector of the Centre for Human Brain Health, said in the release.
“It’s possible that the light is stimulating the astrocytes – the powerplants – in the nerve cells within the PFC, and this has a positive effect on the cells’ efficiency,” he noted.
Dr. Jensen added that his team “will also be investigating how long the effects might last. Clearly, if these experiments are to lead to a clinical intervention, we will need to see long-lasting benefits.”
Beneficial cognitive, emotional effects
Commenting for this news organization, Francisco Gonzalez-Lima, PhD, professor in the department of psychology, University of Texas at Austin, called the study “well done.”
Dr. Gonzalez-Lima was one of the first researchers to demonstrate that 1,064 nm transcranial infrared laser stimulation “produces beneficial cognitive and emotional effects in humans, including improving visual working memory,” he said.
The current study “reported an additional brain effect linked to the improved visual working memory that consists of an EEG-derived response, which is a new finding,” noted Dr. Gonzales-Lima, who was not involved with the new research.
He added that the same laser method “has been found by the Gonzalez-Lima lab to be effective at improving cognition in older adults and depressed and bipolar patients.”
The study was supported by the National Natural Science Foundation of China, the Ministry of Science and Technology of the People’s Republic of China, and the National Defence Basic Scientific Research Program of China. The investigators and Dr. Gonzalez-Lima report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared the effect of 1,064 nm of tPBM delivered over a 12-minute session to the right PFC vs. three other treatment arms: delivery of the same intervention to the left PFC, delivery of the intervention at a lower frequency, and a sham intervention.
All participants were shown a series of items prior to the intervention and asked to recall them after the intervention. Those who received tPBM 1,064 nm to the right PFC showed a superior performance of up to 25% in the memory tasks compared with the other groups.
Patients with attention-related conditions, such as attention deficit hyperactivity disorder, “could benefit from this type of treatment, which is safe, simple, and noninvasive, with no side effects,” coinvestigator Dongwei Li, a visiting PhD student at the Centre for Human Brain Health, University of Birmingham, England, said in a news release.
The findings were published online in Science Advances.
Differing wavelengths
The researchers note that “in the past decades,” noninvasive brain stimulation technology using transcranial application of direct or alternating electrical or magnetic fields “has been proven to be useful” in the improvement of working memory (WM).
When applied to the right PFC, tPBM has been shown to improve accuracy and speed of reaction time in WM tasks and improvements in “high-order cognitive functions,” such as sustained attention, emotion, and executive functions.
The investigators wanted to assess the impact of tPBM applied to different parts of the brain and at different wavelengths. They conducted four double-blind, sham-controlled experiments encompassing 90 neurotypical college students (mean age, 22 years). Each student participated in only one of the four experiments.
All completed two different tPBM sessions, separated by a week, in which sham and active tPBM were compared. Two different types of change-detection memory tasks were given: one requiring participants to remember the orientation of a series of items before and after the intervention and one other requiring them to remember the color of the items (experiments 1 and 2).
A series of follow-up experiments focused on comparing different wavelengths (1,064 nm vs. 852 nm) and different stimulation sites (right vs. left PFC; experiments 3 and 4).
EEG recordings were obtained during the intervention and the memory tasks.
Each experiment consisted of one active tPBM session and one sham tPBM session, with sessions consisting of 12 minutes of laser light (or sham) intervention. These sessions were conducted on the first and the seventh day; then, on the eighth day, participants were asked to report (or guess) which session was the active tPBM session.
Stimulating astrocytes
Results showed that, compared with sham tPBM, there was an improvement in WM capacity and scores by the 1,064 nm intervention in the orientation as well as the color task.
Participants who received the targeted treatment were able to remember between four and five test objects, whereas those with the treatment variations were only able to remember between three and four objects.
“These results support the hypothesis that 1,064 nm tPBM on the right PFC enhances WM capacity,” the investigators wrote.
They also found improvements in WM in participants receiving tPBM vs. sham regardless of whether their performance in the WM task was at a low or high level. This finding held true in both the orientation and the color tasks.
“Therefore, participants with good and poor WM capacity improved after 1,064 nm tPBM,” the researchers noted.
In addition, participants were unable to guess or report whether they had received sham or active tPBM.
EEG monitoring showed changes in brain activity that predicted the improvements in memory performance. In particular, 1,064 tPBM applied to the right PFC increased occipitoparietal contralateral delay activity (CDA), with CDA mediating the WM improvement.
This is “consistent with previous research that CDA is indicative of the number of maintained objects in visual working memory,” the investigators wrote.
Pearson correlation analyses showed that the differences in CDA set-size effects between active and sham session “correlated positively” with the behavioral differences between these sessions. For the orientation task, the r was 0.446 (P < .04); and for the color task, the r was .563 (P < .02).
No similar improvements were found with the 852 nm tPBM.
“We need further research to understand exactly why the tPBM is having this positive effect,” coinvestigator Ole Jensen, PhD, professor in translational neuroscience and codirector of the Centre for Human Brain Health, said in the release.
“It’s possible that the light is stimulating the astrocytes – the powerplants – in the nerve cells within the PFC, and this has a positive effect on the cells’ efficiency,” he noted.
Dr. Jensen added that his team “will also be investigating how long the effects might last. Clearly, if these experiments are to lead to a clinical intervention, we will need to see long-lasting benefits.”
Beneficial cognitive, emotional effects
Commenting for this news organization, Francisco Gonzalez-Lima, PhD, professor in the department of psychology, University of Texas at Austin, called the study “well done.”
Dr. Gonzalez-Lima was one of the first researchers to demonstrate that 1,064 nm transcranial infrared laser stimulation “produces beneficial cognitive and emotional effects in humans, including improving visual working memory,” he said.
The current study “reported an additional brain effect linked to the improved visual working memory that consists of an EEG-derived response, which is a new finding,” noted Dr. Gonzales-Lima, who was not involved with the new research.
He added that the same laser method “has been found by the Gonzalez-Lima lab to be effective at improving cognition in older adults and depressed and bipolar patients.”
The study was supported by the National Natural Science Foundation of China, the Ministry of Science and Technology of the People’s Republic of China, and the National Defence Basic Scientific Research Program of China. The investigators and Dr. Gonzalez-Lima report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SCIENCE ADVANCES
Terminally ill cancer patients struggle to access psilocybin
In March 2020, when the world was struck by the news of the COVID-19 pandemic, Erinn Baldeschwiler received her own gut punch. She was diagnosed with stage IV metastatic breast cancer and was given about 2 years to live.
Then 48, the mother of two teenagers had just started a new chapter in her life. She’d gotten divorced, moved to a new home, and left a small business she had spent 18 years cultivating. The prospect that her life story might soon be ending, that she wouldn’t see her children grow up, was a twist of fate almost too devastating to bear.
“Are you kidding me that this is happening?” she thought.
But she also wanted to keep learning and growing in her remaining years, to devote them to creating meaningful memories, contemplating her mortality, and trying to find inner peace.
“The last 2 years have kind of been this dance with Lady Death,” she said.
They have also been a dance with Lady Justice.
In March 2021, Ms. Baldeschwiler, along with Michal Bloom, who also has terminal cancer, and their palliative care physician, Sunil Aggarwal, MD, PhD, decided to sue the Drug Enforcement Administration (DEA) for the right to access psilocybin, the psychoactive ingredient in “magic” mushrooms.
Psilocybin-assisted therapy has been shown to help terminally ill people overcome their fear, anxiety, and despair about death and to experience the kind of peace Ms. Baldeschwiler is seeking.
Psilocybin is illegal in the United States, but the plaintiffs argue they should be able to take the substance through the Right to Try Act. The 2018 federal law says that people with life-threatening conditions who have exhausted all approved treatment options can access drugs that have not yet been approved by the Food and Drug Administration but have passed phase 1 clinical trials.
This case marks the first time patients have fought to use a Schedule I drug under the Right to Try Act.
The push to expand access to psilocybin is picking up steam in the United States. In 2023, facilitated use of psilocybin will become legal in Oregon and Colorado. Recent proposals from the Biden administration and members of Congress could make psilocybin more widely accessible in the next few years.
It is also gaining momentum outside the United States. In Canada, patients are suing the government to help patients obtain psilocybin-assisted therapy for medical purposes.
“I think what we have here is a confluence of events that are driving toward the mandatory opening of a path to access psilocybin for therapeutic use sooner rather than later,” said Kathryn Tucker, lead counsel in the case against the DEA.
Reverberations of Right to Try
The story of Right to Try began with Abigail Burroughs, who was diagnosed with head and neck cancer at age 19.
After conventional therapies failed, Ms. Burroughs’ oncologist recommended cetuximab, a drug targeting EGFR that was experimental at the time. Because the drug was available only through colon cancer trials, she was denied access.
She died in 2001 at age 21.
Ms. Burroughs’ father, Frank Burroughs, formed an organization that in 2003 sued the FDA to provide terminally ill patients access to unapproved drugs. In 2005, they lost, and subsequent attempts to appeal the decision failed.
Still, the case sparked a Right to Try movement.
“Right to Try laws swept the U.S. in a firestorm,” Ms. Tucker said.
Along with the federal law, which passed in 2018, 41 states have enacted Right to Try laws.
The movement intrigued Dr. Aggarwal, codirector of the Advanced Integrative Medical Science (AIMS) Institute in Seattle. Dr. Aggarwal had been treating patients with cannabis, and after taking psilocybin himself and finding it therapeutic, he thought Ms. Baldeschwiler could benefit.
“I always knew that the powerful medicines within Schedule I had a significant role to play in healing,” he said. “That was baked into my decision to become a doctor, to research, and to innovate.”
He applied for the right to cultivate psilocybin mushrooms, but the fungus doesn’t meet Right to Try requirements. He then found a manufacturer willing to supply synthesized psilocybin, but because it’s a Schedule I drug, the manufacturer needed an okay from the DEA.
Dr. Aggarwal joined forces with Ms. Tucker, who has spent 35 years protecting the rights of terminally ill patients. In January 2021, Ms. Tucker contacted the DEA about allowing dying patients, including Ms. Baldeschwiler and Mr. Bloom, to access psilocybin-assisted therapy.
The response, she said, was predictable.
“The DEA’s knee always jerks in the direction of no access,” Ms. Tucker said. “So it said ‘no access.’ “
The reason: In a letter dated February 2021, the DEA said it “has no authority to waive” any requirements of the Controlled Substances Act under Right to Try laws.
Suing the DEA
Dr. Aggarwal and Ms. Tucker did not accept the DEA’s “no access” answer.
They decided to sue.
Dr. Aggarwal and Ms. Tucker took the matter to the Ninth Circuit Court in March 2021. In January 2022, the court dismissed the case after the DEA claimed its initial denial was not final.
The following month, the plaintiffs petitioned the DEA to deliver a concrete answer.
In May, while waiting for a response, demonstrators gathered at the DEA’s headquarters to call for legal access to psilocybin. One of the protesters was Ms. Baldeschwiler, who choked back tears as she told the crowd she was likely missing her last Mother’s Day with her children to attend the event. She was arrested, along with 16 other people.
In late June, the DEA provided its final answer: No access.
In a letter addressed to Ms. Tucker, Thomas W. Prevoznik, the DEA’s deputy assistant administrator, said it “finds no basis” to reconsider its initial denial in February 2021 “because the legal and factual considerations remain unchanged.”
In an appeal, Ms. Tucker wrote: “In denying Petitioners’ requested accommodation in the Final Agency Action, DEA hides behind a smokescreen, neglecting its duty to implement the federal [Right to Try Act] and violating the state [Right to Try law].”
The government’s response is due in January 2023.
Ms. Tucker and her legal team also petitioned the DEA on behalf of Dr. Aggarwal to reschedule psilocybin from Schedule I to Schedule II.
The DEA defines Schedule I substances as “drugs with no currently accepted medical use and a high potential for abuse.” But the FDA has designated psilocybin as a breakthrough therapy for depression, which, Ms. Tucker noted, “reflects that there is a currently accepted medical use.”
Nevertheless, in September, the DEA denied Ms. Tucker’s petition to reschedule psilocybin, and her team is now petitioning the Ninth Circuit Court for a review of that decision.
Despite the setbacks, actions from the Biden administration and members of Congress could help improve access.
In July, Senators Cory Booker and Rand Paul introduced the Right to Try Clarification Act to clarify that the federal law includes Schedule I substances. If passed, Ms. Tucker said, it would negate the DEA’s “no access” argument.
And earlier this year, the Biden administration announced plans to establish a federal task force to address the “myriad of complex issues” associated with the anticipated FDA approval of psilocybin to treat depression. The task force will explore “the potential of psychedelic-assisted therapies” to tackle the mental health crisis as well as any “risks to public health” that “may require harm reduction, risk mitigation, and safety monitoring.”
The fight north of the border
In 2016, Canadian resident Thomas Hartle, then 48, awoke from surgery for a bowel obstruction to learn he had stage IV colon cancer.
After another surgery, his doctors believed the tumors were gone. But in 2019, the cancer came back, along with extreme anxiety and distress over his impending death and how his two special-needs children would cope.
Mr. Hartle wanted to try magic mushroom–assisted psychotherapy. The Saskatoon resident sought help from TheraPsil, a Canadian nonprofit organization that advocates for therapeutic psilocybin. They applied for access under Section 56, which allows health officials to exempt patients from certain provisions of drug law.
In 2020, Hartle became the first Canadian to legally obtain psilocybin-assisted therapy.
“It has been nothing short of life changing for me,” Mr. Hartle said at a palliative care conference in Saskatoon this past June. “I am now no longer actively dying. I feel like I am genuinely actively living.”
TheraPsil has obtained Section 56 exemptions for around 60 patients to access psilocybin-assisted therapy as well as 19 health care professionals who are training to become psilocybin-assisted therapists.
But then an election ushered in new health ministers, and in early 2022, the exemptions evaporated. Thousands of patients and health care practitioners on TheraPsil’s waiting list were left in limbo.
Health Canada told CBC News that the rule change came about because “while psilocybin has shown promise in clinical trials for the treatment of some indications, further research is still needed to determine its safety and efficacy.”
As an alternative, TheraPsil began applying for access under Canada’s Special Access Program, which is similar to Right to Try laws in the United States. But Canada’s program doesn’t apply to therapists in training, and the petition process is so slow that many patients die before requests can be approved.
“People like to pretend that the Special Access Program is not political, but it is very political,” said TheraPsil’s CEO, Spencer Hawkswell. “It means a patient and a doctor are asking a politician for access to their medicine, which is absolutely unacceptable.”
Now, TheraPsil is helping patients take the Canadian government to court. In July, Mr. Hartle and seven others with conditions ranging from cancer to chronic pain filed a lawsuit against Canada’s health ministry that challenges the limited legal pathways to the use of psilocybin. The lawsuit argues that patients have a “constitutional right to access psilocybin for medicinal purposes,” and it advocates for access to regulated psilocybin products from licensed dealers, much like Canada’s medical marijuana program already does.
In the filing, TheraPsil said that as of February 2022, it has a wait-list of more than 800 patients who are requesting help in obtaining psilocybin-assisted psychotherapy.
An uncertain future
Despite the groundswell of support, many unknowns remain about the safety of expanding access to psilocybin-assisted therapy.
When Oregon and Colorado launch their psilocybin programs in 2023, the licensed centers will provide testing grounds for the safety and efficacy of broader access to psilocybin for people with depression or terminal cancer as well as those looking to grow spiritually.
Although in clinical trials psilocybin has been found to ease symptoms of depression and end-of-life demoralization for people with life-threatening conditions, it has not been adequately tested in people with a range of mental health problems, traumas, and racial backgrounds.
That uncertainty has given some people pause. In recent months, some researchers and journalists have pushed back against the frenzy over the promise of psychedelics.
In September, David Yaden, PhD, a psychedelics researcher at Johns Hopkins, spoke at the Interdisciplinary Conference on Psychedelic Research in the Netherlands. He encouraged people to pay more attention to potential adverse effects of psychedelics, which could include anything from headaches to lingering dysphoria.
“Oftentimes, we hear only the positive anecdotes,” Dr. Yaden said. “We don’t hear ... neutral or negative ones. So, I think all of those anecdotes need to be part of the picture.”
A recent piece in Wired noted that mentioning the potential harms of psychedelics amid its renaissance has been “taboo,” but the authors cautioned that as clinical trials involving psychedelics grow larger and the drugs become commercialized, “more negative outcomes are likely to transpire.”
But Ms. Baldeschwiler remains steadfast in her pursuit. While it’s important to approach broader access to psychedelics with caution, “end-of-life patients don’t have time to wait,” she said.
A version of this article first appeared on Medscape.com.
In March 2020, when the world was struck by the news of the COVID-19 pandemic, Erinn Baldeschwiler received her own gut punch. She was diagnosed with stage IV metastatic breast cancer and was given about 2 years to live.
Then 48, the mother of two teenagers had just started a new chapter in her life. She’d gotten divorced, moved to a new home, and left a small business she had spent 18 years cultivating. The prospect that her life story might soon be ending, that she wouldn’t see her children grow up, was a twist of fate almost too devastating to bear.
“Are you kidding me that this is happening?” she thought.
But she also wanted to keep learning and growing in her remaining years, to devote them to creating meaningful memories, contemplating her mortality, and trying to find inner peace.
“The last 2 years have kind of been this dance with Lady Death,” she said.
They have also been a dance with Lady Justice.
In March 2021, Ms. Baldeschwiler, along with Michal Bloom, who also has terminal cancer, and their palliative care physician, Sunil Aggarwal, MD, PhD, decided to sue the Drug Enforcement Administration (DEA) for the right to access psilocybin, the psychoactive ingredient in “magic” mushrooms.
Psilocybin-assisted therapy has been shown to help terminally ill people overcome their fear, anxiety, and despair about death and to experience the kind of peace Ms. Baldeschwiler is seeking.
Psilocybin is illegal in the United States, but the plaintiffs argue they should be able to take the substance through the Right to Try Act. The 2018 federal law says that people with life-threatening conditions who have exhausted all approved treatment options can access drugs that have not yet been approved by the Food and Drug Administration but have passed phase 1 clinical trials.
This case marks the first time patients have fought to use a Schedule I drug under the Right to Try Act.
The push to expand access to psilocybin is picking up steam in the United States. In 2023, facilitated use of psilocybin will become legal in Oregon and Colorado. Recent proposals from the Biden administration and members of Congress could make psilocybin more widely accessible in the next few years.
It is also gaining momentum outside the United States. In Canada, patients are suing the government to help patients obtain psilocybin-assisted therapy for medical purposes.
“I think what we have here is a confluence of events that are driving toward the mandatory opening of a path to access psilocybin for therapeutic use sooner rather than later,” said Kathryn Tucker, lead counsel in the case against the DEA.
Reverberations of Right to Try
The story of Right to Try began with Abigail Burroughs, who was diagnosed with head and neck cancer at age 19.
After conventional therapies failed, Ms. Burroughs’ oncologist recommended cetuximab, a drug targeting EGFR that was experimental at the time. Because the drug was available only through colon cancer trials, she was denied access.
She died in 2001 at age 21.
Ms. Burroughs’ father, Frank Burroughs, formed an organization that in 2003 sued the FDA to provide terminally ill patients access to unapproved drugs. In 2005, they lost, and subsequent attempts to appeal the decision failed.
Still, the case sparked a Right to Try movement.
“Right to Try laws swept the U.S. in a firestorm,” Ms. Tucker said.
Along with the federal law, which passed in 2018, 41 states have enacted Right to Try laws.
The movement intrigued Dr. Aggarwal, codirector of the Advanced Integrative Medical Science (AIMS) Institute in Seattle. Dr. Aggarwal had been treating patients with cannabis, and after taking psilocybin himself and finding it therapeutic, he thought Ms. Baldeschwiler could benefit.
“I always knew that the powerful medicines within Schedule I had a significant role to play in healing,” he said. “That was baked into my decision to become a doctor, to research, and to innovate.”
He applied for the right to cultivate psilocybin mushrooms, but the fungus doesn’t meet Right to Try requirements. He then found a manufacturer willing to supply synthesized psilocybin, but because it’s a Schedule I drug, the manufacturer needed an okay from the DEA.
Dr. Aggarwal joined forces with Ms. Tucker, who has spent 35 years protecting the rights of terminally ill patients. In January 2021, Ms. Tucker contacted the DEA about allowing dying patients, including Ms. Baldeschwiler and Mr. Bloom, to access psilocybin-assisted therapy.
The response, she said, was predictable.
“The DEA’s knee always jerks in the direction of no access,” Ms. Tucker said. “So it said ‘no access.’ “
The reason: In a letter dated February 2021, the DEA said it “has no authority to waive” any requirements of the Controlled Substances Act under Right to Try laws.
Suing the DEA
Dr. Aggarwal and Ms. Tucker did not accept the DEA’s “no access” answer.
They decided to sue.
Dr. Aggarwal and Ms. Tucker took the matter to the Ninth Circuit Court in March 2021. In January 2022, the court dismissed the case after the DEA claimed its initial denial was not final.
The following month, the plaintiffs petitioned the DEA to deliver a concrete answer.
In May, while waiting for a response, demonstrators gathered at the DEA’s headquarters to call for legal access to psilocybin. One of the protesters was Ms. Baldeschwiler, who choked back tears as she told the crowd she was likely missing her last Mother’s Day with her children to attend the event. She was arrested, along with 16 other people.
In late June, the DEA provided its final answer: No access.
In a letter addressed to Ms. Tucker, Thomas W. Prevoznik, the DEA’s deputy assistant administrator, said it “finds no basis” to reconsider its initial denial in February 2021 “because the legal and factual considerations remain unchanged.”
In an appeal, Ms. Tucker wrote: “In denying Petitioners’ requested accommodation in the Final Agency Action, DEA hides behind a smokescreen, neglecting its duty to implement the federal [Right to Try Act] and violating the state [Right to Try law].”
The government’s response is due in January 2023.
Ms. Tucker and her legal team also petitioned the DEA on behalf of Dr. Aggarwal to reschedule psilocybin from Schedule I to Schedule II.
The DEA defines Schedule I substances as “drugs with no currently accepted medical use and a high potential for abuse.” But the FDA has designated psilocybin as a breakthrough therapy for depression, which, Ms. Tucker noted, “reflects that there is a currently accepted medical use.”
Nevertheless, in September, the DEA denied Ms. Tucker’s petition to reschedule psilocybin, and her team is now petitioning the Ninth Circuit Court for a review of that decision.
Despite the setbacks, actions from the Biden administration and members of Congress could help improve access.
In July, Senators Cory Booker and Rand Paul introduced the Right to Try Clarification Act to clarify that the federal law includes Schedule I substances. If passed, Ms. Tucker said, it would negate the DEA’s “no access” argument.
And earlier this year, the Biden administration announced plans to establish a federal task force to address the “myriad of complex issues” associated with the anticipated FDA approval of psilocybin to treat depression. The task force will explore “the potential of psychedelic-assisted therapies” to tackle the mental health crisis as well as any “risks to public health” that “may require harm reduction, risk mitigation, and safety monitoring.”
The fight north of the border
In 2016, Canadian resident Thomas Hartle, then 48, awoke from surgery for a bowel obstruction to learn he had stage IV colon cancer.
After another surgery, his doctors believed the tumors were gone. But in 2019, the cancer came back, along with extreme anxiety and distress over his impending death and how his two special-needs children would cope.
Mr. Hartle wanted to try magic mushroom–assisted psychotherapy. The Saskatoon resident sought help from TheraPsil, a Canadian nonprofit organization that advocates for therapeutic psilocybin. They applied for access under Section 56, which allows health officials to exempt patients from certain provisions of drug law.
In 2020, Hartle became the first Canadian to legally obtain psilocybin-assisted therapy.
“It has been nothing short of life changing for me,” Mr. Hartle said at a palliative care conference in Saskatoon this past June. “I am now no longer actively dying. I feel like I am genuinely actively living.”
TheraPsil has obtained Section 56 exemptions for around 60 patients to access psilocybin-assisted therapy as well as 19 health care professionals who are training to become psilocybin-assisted therapists.
But then an election ushered in new health ministers, and in early 2022, the exemptions evaporated. Thousands of patients and health care practitioners on TheraPsil’s waiting list were left in limbo.
Health Canada told CBC News that the rule change came about because “while psilocybin has shown promise in clinical trials for the treatment of some indications, further research is still needed to determine its safety and efficacy.”
As an alternative, TheraPsil began applying for access under Canada’s Special Access Program, which is similar to Right to Try laws in the United States. But Canada’s program doesn’t apply to therapists in training, and the petition process is so slow that many patients die before requests can be approved.
“People like to pretend that the Special Access Program is not political, but it is very political,” said TheraPsil’s CEO, Spencer Hawkswell. “It means a patient and a doctor are asking a politician for access to their medicine, which is absolutely unacceptable.”
Now, TheraPsil is helping patients take the Canadian government to court. In July, Mr. Hartle and seven others with conditions ranging from cancer to chronic pain filed a lawsuit against Canada’s health ministry that challenges the limited legal pathways to the use of psilocybin. The lawsuit argues that patients have a “constitutional right to access psilocybin for medicinal purposes,” and it advocates for access to regulated psilocybin products from licensed dealers, much like Canada’s medical marijuana program already does.
In the filing, TheraPsil said that as of February 2022, it has a wait-list of more than 800 patients who are requesting help in obtaining psilocybin-assisted psychotherapy.
An uncertain future
Despite the groundswell of support, many unknowns remain about the safety of expanding access to psilocybin-assisted therapy.
When Oregon and Colorado launch their psilocybin programs in 2023, the licensed centers will provide testing grounds for the safety and efficacy of broader access to psilocybin for people with depression or terminal cancer as well as those looking to grow spiritually.
Although in clinical trials psilocybin has been found to ease symptoms of depression and end-of-life demoralization for people with life-threatening conditions, it has not been adequately tested in people with a range of mental health problems, traumas, and racial backgrounds.
That uncertainty has given some people pause. In recent months, some researchers and journalists have pushed back against the frenzy over the promise of psychedelics.
In September, David Yaden, PhD, a psychedelics researcher at Johns Hopkins, spoke at the Interdisciplinary Conference on Psychedelic Research in the Netherlands. He encouraged people to pay more attention to potential adverse effects of psychedelics, which could include anything from headaches to lingering dysphoria.
“Oftentimes, we hear only the positive anecdotes,” Dr. Yaden said. “We don’t hear ... neutral or negative ones. So, I think all of those anecdotes need to be part of the picture.”
A recent piece in Wired noted that mentioning the potential harms of psychedelics amid its renaissance has been “taboo,” but the authors cautioned that as clinical trials involving psychedelics grow larger and the drugs become commercialized, “more negative outcomes are likely to transpire.”
But Ms. Baldeschwiler remains steadfast in her pursuit. While it’s important to approach broader access to psychedelics with caution, “end-of-life patients don’t have time to wait,” she said.
A version of this article first appeared on Medscape.com.
In March 2020, when the world was struck by the news of the COVID-19 pandemic, Erinn Baldeschwiler received her own gut punch. She was diagnosed with stage IV metastatic breast cancer and was given about 2 years to live.
Then 48, the mother of two teenagers had just started a new chapter in her life. She’d gotten divorced, moved to a new home, and left a small business she had spent 18 years cultivating. The prospect that her life story might soon be ending, that she wouldn’t see her children grow up, was a twist of fate almost too devastating to bear.
“Are you kidding me that this is happening?” she thought.
But she also wanted to keep learning and growing in her remaining years, to devote them to creating meaningful memories, contemplating her mortality, and trying to find inner peace.
“The last 2 years have kind of been this dance with Lady Death,” she said.
They have also been a dance with Lady Justice.
In March 2021, Ms. Baldeschwiler, along with Michal Bloom, who also has terminal cancer, and their palliative care physician, Sunil Aggarwal, MD, PhD, decided to sue the Drug Enforcement Administration (DEA) for the right to access psilocybin, the psychoactive ingredient in “magic” mushrooms.
Psilocybin-assisted therapy has been shown to help terminally ill people overcome their fear, anxiety, and despair about death and to experience the kind of peace Ms. Baldeschwiler is seeking.
Psilocybin is illegal in the United States, but the plaintiffs argue they should be able to take the substance through the Right to Try Act. The 2018 federal law says that people with life-threatening conditions who have exhausted all approved treatment options can access drugs that have not yet been approved by the Food and Drug Administration but have passed phase 1 clinical trials.
This case marks the first time patients have fought to use a Schedule I drug under the Right to Try Act.
The push to expand access to psilocybin is picking up steam in the United States. In 2023, facilitated use of psilocybin will become legal in Oregon and Colorado. Recent proposals from the Biden administration and members of Congress could make psilocybin more widely accessible in the next few years.
It is also gaining momentum outside the United States. In Canada, patients are suing the government to help patients obtain psilocybin-assisted therapy for medical purposes.
“I think what we have here is a confluence of events that are driving toward the mandatory opening of a path to access psilocybin for therapeutic use sooner rather than later,” said Kathryn Tucker, lead counsel in the case against the DEA.
Reverberations of Right to Try
The story of Right to Try began with Abigail Burroughs, who was diagnosed with head and neck cancer at age 19.
After conventional therapies failed, Ms. Burroughs’ oncologist recommended cetuximab, a drug targeting EGFR that was experimental at the time. Because the drug was available only through colon cancer trials, she was denied access.
She died in 2001 at age 21.
Ms. Burroughs’ father, Frank Burroughs, formed an organization that in 2003 sued the FDA to provide terminally ill patients access to unapproved drugs. In 2005, they lost, and subsequent attempts to appeal the decision failed.
Still, the case sparked a Right to Try movement.
“Right to Try laws swept the U.S. in a firestorm,” Ms. Tucker said.
Along with the federal law, which passed in 2018, 41 states have enacted Right to Try laws.
The movement intrigued Dr. Aggarwal, codirector of the Advanced Integrative Medical Science (AIMS) Institute in Seattle. Dr. Aggarwal had been treating patients with cannabis, and after taking psilocybin himself and finding it therapeutic, he thought Ms. Baldeschwiler could benefit.
“I always knew that the powerful medicines within Schedule I had a significant role to play in healing,” he said. “That was baked into my decision to become a doctor, to research, and to innovate.”
He applied for the right to cultivate psilocybin mushrooms, but the fungus doesn’t meet Right to Try requirements. He then found a manufacturer willing to supply synthesized psilocybin, but because it’s a Schedule I drug, the manufacturer needed an okay from the DEA.
Dr. Aggarwal joined forces with Ms. Tucker, who has spent 35 years protecting the rights of terminally ill patients. In January 2021, Ms. Tucker contacted the DEA about allowing dying patients, including Ms. Baldeschwiler and Mr. Bloom, to access psilocybin-assisted therapy.
The response, she said, was predictable.
“The DEA’s knee always jerks in the direction of no access,” Ms. Tucker said. “So it said ‘no access.’ “
The reason: In a letter dated February 2021, the DEA said it “has no authority to waive” any requirements of the Controlled Substances Act under Right to Try laws.
Suing the DEA
Dr. Aggarwal and Ms. Tucker did not accept the DEA’s “no access” answer.
They decided to sue.
Dr. Aggarwal and Ms. Tucker took the matter to the Ninth Circuit Court in March 2021. In January 2022, the court dismissed the case after the DEA claimed its initial denial was not final.
The following month, the plaintiffs petitioned the DEA to deliver a concrete answer.
In May, while waiting for a response, demonstrators gathered at the DEA’s headquarters to call for legal access to psilocybin. One of the protesters was Ms. Baldeschwiler, who choked back tears as she told the crowd she was likely missing her last Mother’s Day with her children to attend the event. She was arrested, along with 16 other people.
In late June, the DEA provided its final answer: No access.
In a letter addressed to Ms. Tucker, Thomas W. Prevoznik, the DEA’s deputy assistant administrator, said it “finds no basis” to reconsider its initial denial in February 2021 “because the legal and factual considerations remain unchanged.”
In an appeal, Ms. Tucker wrote: “In denying Petitioners’ requested accommodation in the Final Agency Action, DEA hides behind a smokescreen, neglecting its duty to implement the federal [Right to Try Act] and violating the state [Right to Try law].”
The government’s response is due in January 2023.
Ms. Tucker and her legal team also petitioned the DEA on behalf of Dr. Aggarwal to reschedule psilocybin from Schedule I to Schedule II.
The DEA defines Schedule I substances as “drugs with no currently accepted medical use and a high potential for abuse.” But the FDA has designated psilocybin as a breakthrough therapy for depression, which, Ms. Tucker noted, “reflects that there is a currently accepted medical use.”
Nevertheless, in September, the DEA denied Ms. Tucker’s petition to reschedule psilocybin, and her team is now petitioning the Ninth Circuit Court for a review of that decision.
Despite the setbacks, actions from the Biden administration and members of Congress could help improve access.
In July, Senators Cory Booker and Rand Paul introduced the Right to Try Clarification Act to clarify that the federal law includes Schedule I substances. If passed, Ms. Tucker said, it would negate the DEA’s “no access” argument.
And earlier this year, the Biden administration announced plans to establish a federal task force to address the “myriad of complex issues” associated with the anticipated FDA approval of psilocybin to treat depression. The task force will explore “the potential of psychedelic-assisted therapies” to tackle the mental health crisis as well as any “risks to public health” that “may require harm reduction, risk mitigation, and safety monitoring.”
The fight north of the border
In 2016, Canadian resident Thomas Hartle, then 48, awoke from surgery for a bowel obstruction to learn he had stage IV colon cancer.
After another surgery, his doctors believed the tumors were gone. But in 2019, the cancer came back, along with extreme anxiety and distress over his impending death and how his two special-needs children would cope.
Mr. Hartle wanted to try magic mushroom–assisted psychotherapy. The Saskatoon resident sought help from TheraPsil, a Canadian nonprofit organization that advocates for therapeutic psilocybin. They applied for access under Section 56, which allows health officials to exempt patients from certain provisions of drug law.
In 2020, Hartle became the first Canadian to legally obtain psilocybin-assisted therapy.
“It has been nothing short of life changing for me,” Mr. Hartle said at a palliative care conference in Saskatoon this past June. “I am now no longer actively dying. I feel like I am genuinely actively living.”
TheraPsil has obtained Section 56 exemptions for around 60 patients to access psilocybin-assisted therapy as well as 19 health care professionals who are training to become psilocybin-assisted therapists.
But then an election ushered in new health ministers, and in early 2022, the exemptions evaporated. Thousands of patients and health care practitioners on TheraPsil’s waiting list were left in limbo.
Health Canada told CBC News that the rule change came about because “while psilocybin has shown promise in clinical trials for the treatment of some indications, further research is still needed to determine its safety and efficacy.”
As an alternative, TheraPsil began applying for access under Canada’s Special Access Program, which is similar to Right to Try laws in the United States. But Canada’s program doesn’t apply to therapists in training, and the petition process is so slow that many patients die before requests can be approved.
“People like to pretend that the Special Access Program is not political, but it is very political,” said TheraPsil’s CEO, Spencer Hawkswell. “It means a patient and a doctor are asking a politician for access to their medicine, which is absolutely unacceptable.”
Now, TheraPsil is helping patients take the Canadian government to court. In July, Mr. Hartle and seven others with conditions ranging from cancer to chronic pain filed a lawsuit against Canada’s health ministry that challenges the limited legal pathways to the use of psilocybin. The lawsuit argues that patients have a “constitutional right to access psilocybin for medicinal purposes,” and it advocates for access to regulated psilocybin products from licensed dealers, much like Canada’s medical marijuana program already does.
In the filing, TheraPsil said that as of February 2022, it has a wait-list of more than 800 patients who are requesting help in obtaining psilocybin-assisted psychotherapy.
An uncertain future
Despite the groundswell of support, many unknowns remain about the safety of expanding access to psilocybin-assisted therapy.
When Oregon and Colorado launch their psilocybin programs in 2023, the licensed centers will provide testing grounds for the safety and efficacy of broader access to psilocybin for people with depression or terminal cancer as well as those looking to grow spiritually.
Although in clinical trials psilocybin has been found to ease symptoms of depression and end-of-life demoralization for people with life-threatening conditions, it has not been adequately tested in people with a range of mental health problems, traumas, and racial backgrounds.
That uncertainty has given some people pause. In recent months, some researchers and journalists have pushed back against the frenzy over the promise of psychedelics.
In September, David Yaden, PhD, a psychedelics researcher at Johns Hopkins, spoke at the Interdisciplinary Conference on Psychedelic Research in the Netherlands. He encouraged people to pay more attention to potential adverse effects of psychedelics, which could include anything from headaches to lingering dysphoria.
“Oftentimes, we hear only the positive anecdotes,” Dr. Yaden said. “We don’t hear ... neutral or negative ones. So, I think all of those anecdotes need to be part of the picture.”
A recent piece in Wired noted that mentioning the potential harms of psychedelics amid its renaissance has been “taboo,” but the authors cautioned that as clinical trials involving psychedelics grow larger and the drugs become commercialized, “more negative outcomes are likely to transpire.”
But Ms. Baldeschwiler remains steadfast in her pursuit. While it’s important to approach broader access to psychedelics with caution, “end-of-life patients don’t have time to wait,” she said.
A version of this article first appeared on Medscape.com.
Should you quit employment to open a practice? These docs share how they did it
“Everyone said private practice is dying,” said Omar Maniya, MD, an emergency physician who left his hospital job for family practice in New Jersey. “But I think it could be one of the best models we have to advance our health care system and prevent burnout – and bring joy back to the practice of medicine.”
But employment doesn’t necessarily mean happiness. In the Medscape “Employed Physicians: Loving the Focus, Hating the Bureaucracy” ” report, more than 1,350 U.S. physicians employed by a health care organization, hospital, large group practice, or other medical group were surveyedabout their work. As the subtitle suggests, many are torn.
In the survey, employed doctors cited three main downsides to the lifestyle: They have less autonomy, more corporate rules than they’d like, and lower earning potential. Nearly one-third say they’re unhappy about their work-life balance, too, which raises the risk for burnout.
Some physicians find that employment has more cons than pros and turn to private practice instead.
A system skewed toward employment
In the mid-1990s, when James Milford, MD, completed his residency, going straight into private practice was the norm. The family physician bucked that trend by joining a large regional medical center in Wisconsin. He spent the next 20+ years working to establish a network of medical clinics.
“It was very satisfying,” Dr. Milford said. “When I started, I had a lot of input, a lot of control.”
Since then, the pendulum has been swinging toward employment. Brieanna Seefeldt, DO, a family physician outside Denver, completed her residency in 2012.
“I told the recruiter I wanted my own practice,” Dr. Seefeldt said, “They said if you’re not independently wealthy, there’s no way.”
Sonal G. Patel, MD, a pediatric neurologist in Bethesda, finished her residency the same year as Dr. Seefeldt. Dr. Patel never even considered private practice.
“I always thought I would have a certain amount of clinic time where I have my regular patients,” she said, “but I’d also be doing hospital rounds and reading EEG studies at the hospital.”
For Dr. Maniya, who completed his residency in 2021, the choice was simple. Growing up, he watched his immigrant parents, both doctors in private practice, struggle to keep up.
“I opted for a big, sophisticated health system,” he said. “I thought we’d be pushing the envelope of what was possible in medicine.”
Becoming disillusioned with employment
All four of these physicians are now in private practice and are much happier.
Within a few years of starting her job, Dr. Seefeldt was one of the top producers in her area but felt tremendous pressure to see more and more patients. The last straw came after an unpaid maternity leave.
“They told me I owed them for my maternity leave, for lack of productivity,” she said. “I was in practice for only 4 years, but already feeling the effects of burnout.”
Dr. Patel only lasted 2 years before realizing employment didn’t suit her.
“There was an excessive amount of hospital calls,” she said. “And there were bureaucratic issues that made it very difficult to practice the way I thought my practice would be.”
It took just 18 months for Dr. Maniya’s light-bulb moment. He was working at a hospital when COVID-19 hit.
“At my big health care system, it took 9 months to come up with a way to get COVID swabs for free,” he said. “At the same time, I was helping out the family business, a private practice. It took me two calls and 48 hours to get free swabs for not just the practice, not just our patients, but the entire city of Hamilton, New Jersey.”
Milford lasted the longest as an employee – nearly 25 years. The end came after a healthcare company with hospitals in 30 states bought out the medical center.
“My control gradually eroded,” he said. “It got to the point where I had no input regarding things like employees or processes we wanted to improve.”
Making the leap to private practice
Private practice can take different forms.
Dr. Seefeldt opted for direct primary care, a model in which her patients pay a set monthly fee for care whenever needed. Her practice doesn’t take any insurance besides Medicaid.
“Direct primary care is about working directly with the patient and cost-conscious, transparent care,” she said. “And I don’t have to deal with insurance.”
For Dr. Patel, working with an accountable care organization made the transition easier. She owns her practice solo but works with a company called Privia for administrative needs. Privia sent a consultant to set up her office in the company’s electronic medical record. Things were up and running within the first week.
Dr. Maniya joined his mother’s practice, easing his way in over 18 months.
And then there’s what Milford did, building a private practice from the ground up.
“We did a lot of Googling, a lot of meeting with accountants, meeting with small business development from the state of Wisconsin,” he said. “We asked people that were in business, ‘What are the things businesses fail on? Not medical practices, but businesses.’” All that research helped him launch successfully.
Making the dollars and cents add up
Moving from employment into private practice takes time, effort, and of course, money. How much of each varies depending on where you live, your specialty, whether you choose to rent or buy office space, staffing needs, and other factors.
Dr. Seefeldt, Dr. Patel, Dr. Milford, and Dr. Maniya illustrate the range.
- Dr. Seefeldt got a home equity loan of $50,000 to cover startup costs – and paid it back within 6 months.
- Purchasing EEG equipment added to Dr. Patel’s budget; she spent $130,000 of her own money to launch her practice in a temporary office and took out a $150,000 loan to finance the buildout of her final space. It took her 3 years to pay it back.
- When Dr. Milford left employment, he borrowed the buildout and startup costs for his practice from his father, a retired surgeon, to the tune of $500,000.
- Dr. Maniya assumed the largest risk. When he took over the family practice, he borrowed $1.5 million to modernize and build a new office. The practice has now quintupled in size. “It’s going great,” he said. “One of our questions is, should we pay back the loan at a faster pace rather than make the minimum payments?”
Several years in, Dr. Patel reports she’s easily making three to four times as much as she would have at a hospital. However, Dr. Maniya’s guaranteed compensation is 10% less than his old job.
“But as a partner in a private practice, if it succeeds, it could be 100%-150% more in a good year,” he said. On the flip side, if the practice runs into financial trouble, so does he. “Does the risk keep me up at night, give me heartburn? You betcha.”
Dr. Milford and Dr. Seefeldt have both chosen to take less compensation than they could, opting to reinvest in and nurture their practices.
“I love it,” said Dr. Milford. “I joke that I have half as much in my pocketbook, twice as much in my heart. But it’s not really half as much, 5 years in. If I weren’t growing the business, I’d be making more than before.”
Private practice is not without challenges
Being the big cheese does have drawbacks. In the current climate, staffing is a persistent issue for doctors in private practice – both maintaining a full staff and managing their employees.
And without the backing of a large corporation, doctors are sometimes called on to do less than pleasant tasks.
“If the toilet gets clogged and the plumber can’t come for a few hours, the patients still need a bathroom,” Dr. Maniya said. “I’ll go in with my $400 shoes and snake the toilet.”
Dr. Milford pointed out that when the buck stops with you, small mistakes can have enormous ramifications. “But with the bad comes the great potential for good. You have the ability to positively affect patients and healthcare, and to make a difference for people. It creates great personal satisfaction.”
Is running your own practice all it’s cracked up to be?
If it’s not yet apparent, all four doctors highly recommend moving from employment to private practice when possible. The autonomy and the improved work-life balance have helped them find the satisfaction they’d been missing while making burnout less likely.
“When you don’t have to spend 30% of your day apologizing to patients for how bad the health care system is, it reignites your passion for why you went into medicine in the first place,” said Dr. Maniya. In his practice, he’s made a conscious decision to pursue a mix of demographics. “Thirty percent of our patients are Medicaid. The vast majority are middle to low income.”
For physicians who are also parents, the ability to set their own schedules is life-changing.
“My son got an award ... and the teacher invited me to the assembly. In a corporate-based world, I’d struggle to be able to go,” said Dr. Seefeldt. As her own boss, she didn’t have to forgo this special event. Instead, she coordinated directly with her scheduled patient to make time for it.
In Medscape’s report, 61% of employed physicians indicated that they don’t have a say on key management decisions. However, doctors who launch private practices embrace the chance to set their own standards.
“We make sure from the minute someone calls they know they’re in good hands, we’re responsive, we address concerns right away. That’s the difference with private practice – the one-on-one connection is huge,” said Dr. Patel.
“This is exactly what I always wanted. It brings me joy knowing we’ve made a difference in these children’s lives, in their parents’ lives,” she concluded.
A version of this article first appeared on Medscape.com.
“Everyone said private practice is dying,” said Omar Maniya, MD, an emergency physician who left his hospital job for family practice in New Jersey. “But I think it could be one of the best models we have to advance our health care system and prevent burnout – and bring joy back to the practice of medicine.”
But employment doesn’t necessarily mean happiness. In the Medscape “Employed Physicians: Loving the Focus, Hating the Bureaucracy” ” report, more than 1,350 U.S. physicians employed by a health care organization, hospital, large group practice, or other medical group were surveyedabout their work. As the subtitle suggests, many are torn.
In the survey, employed doctors cited three main downsides to the lifestyle: They have less autonomy, more corporate rules than they’d like, and lower earning potential. Nearly one-third say they’re unhappy about their work-life balance, too, which raises the risk for burnout.
Some physicians find that employment has more cons than pros and turn to private practice instead.
A system skewed toward employment
In the mid-1990s, when James Milford, MD, completed his residency, going straight into private practice was the norm. The family physician bucked that trend by joining a large regional medical center in Wisconsin. He spent the next 20+ years working to establish a network of medical clinics.
“It was very satisfying,” Dr. Milford said. “When I started, I had a lot of input, a lot of control.”
Since then, the pendulum has been swinging toward employment. Brieanna Seefeldt, DO, a family physician outside Denver, completed her residency in 2012.
“I told the recruiter I wanted my own practice,” Dr. Seefeldt said, “They said if you’re not independently wealthy, there’s no way.”
Sonal G. Patel, MD, a pediatric neurologist in Bethesda, finished her residency the same year as Dr. Seefeldt. Dr. Patel never even considered private practice.
“I always thought I would have a certain amount of clinic time where I have my regular patients,” she said, “but I’d also be doing hospital rounds and reading EEG studies at the hospital.”
For Dr. Maniya, who completed his residency in 2021, the choice was simple. Growing up, he watched his immigrant parents, both doctors in private practice, struggle to keep up.
“I opted for a big, sophisticated health system,” he said. “I thought we’d be pushing the envelope of what was possible in medicine.”
Becoming disillusioned with employment
All four of these physicians are now in private practice and are much happier.
Within a few years of starting her job, Dr. Seefeldt was one of the top producers in her area but felt tremendous pressure to see more and more patients. The last straw came after an unpaid maternity leave.
“They told me I owed them for my maternity leave, for lack of productivity,” she said. “I was in practice for only 4 years, but already feeling the effects of burnout.”
Dr. Patel only lasted 2 years before realizing employment didn’t suit her.
“There was an excessive amount of hospital calls,” she said. “And there were bureaucratic issues that made it very difficult to practice the way I thought my practice would be.”
It took just 18 months for Dr. Maniya’s light-bulb moment. He was working at a hospital when COVID-19 hit.
“At my big health care system, it took 9 months to come up with a way to get COVID swabs for free,” he said. “At the same time, I was helping out the family business, a private practice. It took me two calls and 48 hours to get free swabs for not just the practice, not just our patients, but the entire city of Hamilton, New Jersey.”
Milford lasted the longest as an employee – nearly 25 years. The end came after a healthcare company with hospitals in 30 states bought out the medical center.
“My control gradually eroded,” he said. “It got to the point where I had no input regarding things like employees or processes we wanted to improve.”
Making the leap to private practice
Private practice can take different forms.
Dr. Seefeldt opted for direct primary care, a model in which her patients pay a set monthly fee for care whenever needed. Her practice doesn’t take any insurance besides Medicaid.
“Direct primary care is about working directly with the patient and cost-conscious, transparent care,” she said. “And I don’t have to deal with insurance.”
For Dr. Patel, working with an accountable care organization made the transition easier. She owns her practice solo but works with a company called Privia for administrative needs. Privia sent a consultant to set up her office in the company’s electronic medical record. Things were up and running within the first week.
Dr. Maniya joined his mother’s practice, easing his way in over 18 months.
And then there’s what Milford did, building a private practice from the ground up.
“We did a lot of Googling, a lot of meeting with accountants, meeting with small business development from the state of Wisconsin,” he said. “We asked people that were in business, ‘What are the things businesses fail on? Not medical practices, but businesses.’” All that research helped him launch successfully.
Making the dollars and cents add up
Moving from employment into private practice takes time, effort, and of course, money. How much of each varies depending on where you live, your specialty, whether you choose to rent or buy office space, staffing needs, and other factors.
Dr. Seefeldt, Dr. Patel, Dr. Milford, and Dr. Maniya illustrate the range.
- Dr. Seefeldt got a home equity loan of $50,000 to cover startup costs – and paid it back within 6 months.
- Purchasing EEG equipment added to Dr. Patel’s budget; she spent $130,000 of her own money to launch her practice in a temporary office and took out a $150,000 loan to finance the buildout of her final space. It took her 3 years to pay it back.
- When Dr. Milford left employment, he borrowed the buildout and startup costs for his practice from his father, a retired surgeon, to the tune of $500,000.
- Dr. Maniya assumed the largest risk. When he took over the family practice, he borrowed $1.5 million to modernize and build a new office. The practice has now quintupled in size. “It’s going great,” he said. “One of our questions is, should we pay back the loan at a faster pace rather than make the minimum payments?”
Several years in, Dr. Patel reports she’s easily making three to four times as much as she would have at a hospital. However, Dr. Maniya’s guaranteed compensation is 10% less than his old job.
“But as a partner in a private practice, if it succeeds, it could be 100%-150% more in a good year,” he said. On the flip side, if the practice runs into financial trouble, so does he. “Does the risk keep me up at night, give me heartburn? You betcha.”
Dr. Milford and Dr. Seefeldt have both chosen to take less compensation than they could, opting to reinvest in and nurture their practices.
“I love it,” said Dr. Milford. “I joke that I have half as much in my pocketbook, twice as much in my heart. But it’s not really half as much, 5 years in. If I weren’t growing the business, I’d be making more than before.”
Private practice is not without challenges
Being the big cheese does have drawbacks. In the current climate, staffing is a persistent issue for doctors in private practice – both maintaining a full staff and managing their employees.
And without the backing of a large corporation, doctors are sometimes called on to do less than pleasant tasks.
“If the toilet gets clogged and the plumber can’t come for a few hours, the patients still need a bathroom,” Dr. Maniya said. “I’ll go in with my $400 shoes and snake the toilet.”
Dr. Milford pointed out that when the buck stops with you, small mistakes can have enormous ramifications. “But with the bad comes the great potential for good. You have the ability to positively affect patients and healthcare, and to make a difference for people. It creates great personal satisfaction.”
Is running your own practice all it’s cracked up to be?
If it’s not yet apparent, all four doctors highly recommend moving from employment to private practice when possible. The autonomy and the improved work-life balance have helped them find the satisfaction they’d been missing while making burnout less likely.
“When you don’t have to spend 30% of your day apologizing to patients for how bad the health care system is, it reignites your passion for why you went into medicine in the first place,” said Dr. Maniya. In his practice, he’s made a conscious decision to pursue a mix of demographics. “Thirty percent of our patients are Medicaid. The vast majority are middle to low income.”
For physicians who are also parents, the ability to set their own schedules is life-changing.
“My son got an award ... and the teacher invited me to the assembly. In a corporate-based world, I’d struggle to be able to go,” said Dr. Seefeldt. As her own boss, she didn’t have to forgo this special event. Instead, she coordinated directly with her scheduled patient to make time for it.
In Medscape’s report, 61% of employed physicians indicated that they don’t have a say on key management decisions. However, doctors who launch private practices embrace the chance to set their own standards.
“We make sure from the minute someone calls they know they’re in good hands, we’re responsive, we address concerns right away. That’s the difference with private practice – the one-on-one connection is huge,” said Dr. Patel.
“This is exactly what I always wanted. It brings me joy knowing we’ve made a difference in these children’s lives, in their parents’ lives,” she concluded.
A version of this article first appeared on Medscape.com.
“Everyone said private practice is dying,” said Omar Maniya, MD, an emergency physician who left his hospital job for family practice in New Jersey. “But I think it could be one of the best models we have to advance our health care system and prevent burnout – and bring joy back to the practice of medicine.”
But employment doesn’t necessarily mean happiness. In the Medscape “Employed Physicians: Loving the Focus, Hating the Bureaucracy” ” report, more than 1,350 U.S. physicians employed by a health care organization, hospital, large group practice, or other medical group were surveyedabout their work. As the subtitle suggests, many are torn.
In the survey, employed doctors cited three main downsides to the lifestyle: They have less autonomy, more corporate rules than they’d like, and lower earning potential. Nearly one-third say they’re unhappy about their work-life balance, too, which raises the risk for burnout.
Some physicians find that employment has more cons than pros and turn to private practice instead.
A system skewed toward employment
In the mid-1990s, when James Milford, MD, completed his residency, going straight into private practice was the norm. The family physician bucked that trend by joining a large regional medical center in Wisconsin. He spent the next 20+ years working to establish a network of medical clinics.
“It was very satisfying,” Dr. Milford said. “When I started, I had a lot of input, a lot of control.”
Since then, the pendulum has been swinging toward employment. Brieanna Seefeldt, DO, a family physician outside Denver, completed her residency in 2012.
“I told the recruiter I wanted my own practice,” Dr. Seefeldt said, “They said if you’re not independently wealthy, there’s no way.”
Sonal G. Patel, MD, a pediatric neurologist in Bethesda, finished her residency the same year as Dr. Seefeldt. Dr. Patel never even considered private practice.
“I always thought I would have a certain amount of clinic time where I have my regular patients,” she said, “but I’d also be doing hospital rounds and reading EEG studies at the hospital.”
For Dr. Maniya, who completed his residency in 2021, the choice was simple. Growing up, he watched his immigrant parents, both doctors in private practice, struggle to keep up.
“I opted for a big, sophisticated health system,” he said. “I thought we’d be pushing the envelope of what was possible in medicine.”
Becoming disillusioned with employment
All four of these physicians are now in private practice and are much happier.
Within a few years of starting her job, Dr. Seefeldt was one of the top producers in her area but felt tremendous pressure to see more and more patients. The last straw came after an unpaid maternity leave.
“They told me I owed them for my maternity leave, for lack of productivity,” she said. “I was in practice for only 4 years, but already feeling the effects of burnout.”
Dr. Patel only lasted 2 years before realizing employment didn’t suit her.
“There was an excessive amount of hospital calls,” she said. “And there were bureaucratic issues that made it very difficult to practice the way I thought my practice would be.”
It took just 18 months for Dr. Maniya’s light-bulb moment. He was working at a hospital when COVID-19 hit.
“At my big health care system, it took 9 months to come up with a way to get COVID swabs for free,” he said. “At the same time, I was helping out the family business, a private practice. It took me two calls and 48 hours to get free swabs for not just the practice, not just our patients, but the entire city of Hamilton, New Jersey.”
Milford lasted the longest as an employee – nearly 25 years. The end came after a healthcare company with hospitals in 30 states bought out the medical center.
“My control gradually eroded,” he said. “It got to the point where I had no input regarding things like employees or processes we wanted to improve.”
Making the leap to private practice
Private practice can take different forms.
Dr. Seefeldt opted for direct primary care, a model in which her patients pay a set monthly fee for care whenever needed. Her practice doesn’t take any insurance besides Medicaid.
“Direct primary care is about working directly with the patient and cost-conscious, transparent care,” she said. “And I don’t have to deal with insurance.”
For Dr. Patel, working with an accountable care organization made the transition easier. She owns her practice solo but works with a company called Privia for administrative needs. Privia sent a consultant to set up her office in the company’s electronic medical record. Things were up and running within the first week.
Dr. Maniya joined his mother’s practice, easing his way in over 18 months.
And then there’s what Milford did, building a private practice from the ground up.
“We did a lot of Googling, a lot of meeting with accountants, meeting with small business development from the state of Wisconsin,” he said. “We asked people that were in business, ‘What are the things businesses fail on? Not medical practices, but businesses.’” All that research helped him launch successfully.
Making the dollars and cents add up
Moving from employment into private practice takes time, effort, and of course, money. How much of each varies depending on where you live, your specialty, whether you choose to rent or buy office space, staffing needs, and other factors.
Dr. Seefeldt, Dr. Patel, Dr. Milford, and Dr. Maniya illustrate the range.
- Dr. Seefeldt got a home equity loan of $50,000 to cover startup costs – and paid it back within 6 months.
- Purchasing EEG equipment added to Dr. Patel’s budget; she spent $130,000 of her own money to launch her practice in a temporary office and took out a $150,000 loan to finance the buildout of her final space. It took her 3 years to pay it back.
- When Dr. Milford left employment, he borrowed the buildout and startup costs for his practice from his father, a retired surgeon, to the tune of $500,000.
- Dr. Maniya assumed the largest risk. When he took over the family practice, he borrowed $1.5 million to modernize and build a new office. The practice has now quintupled in size. “It’s going great,” he said. “One of our questions is, should we pay back the loan at a faster pace rather than make the minimum payments?”
Several years in, Dr. Patel reports she’s easily making three to four times as much as she would have at a hospital. However, Dr. Maniya’s guaranteed compensation is 10% less than his old job.
“But as a partner in a private practice, if it succeeds, it could be 100%-150% more in a good year,” he said. On the flip side, if the practice runs into financial trouble, so does he. “Does the risk keep me up at night, give me heartburn? You betcha.”
Dr. Milford and Dr. Seefeldt have both chosen to take less compensation than they could, opting to reinvest in and nurture their practices.
“I love it,” said Dr. Milford. “I joke that I have half as much in my pocketbook, twice as much in my heart. But it’s not really half as much, 5 years in. If I weren’t growing the business, I’d be making more than before.”
Private practice is not without challenges
Being the big cheese does have drawbacks. In the current climate, staffing is a persistent issue for doctors in private practice – both maintaining a full staff and managing their employees.
And without the backing of a large corporation, doctors are sometimes called on to do less than pleasant tasks.
“If the toilet gets clogged and the plumber can’t come for a few hours, the patients still need a bathroom,” Dr. Maniya said. “I’ll go in with my $400 shoes and snake the toilet.”
Dr. Milford pointed out that when the buck stops with you, small mistakes can have enormous ramifications. “But with the bad comes the great potential for good. You have the ability to positively affect patients and healthcare, and to make a difference for people. It creates great personal satisfaction.”
Is running your own practice all it’s cracked up to be?
If it’s not yet apparent, all four doctors highly recommend moving from employment to private practice when possible. The autonomy and the improved work-life balance have helped them find the satisfaction they’d been missing while making burnout less likely.
“When you don’t have to spend 30% of your day apologizing to patients for how bad the health care system is, it reignites your passion for why you went into medicine in the first place,” said Dr. Maniya. In his practice, he’s made a conscious decision to pursue a mix of demographics. “Thirty percent of our patients are Medicaid. The vast majority are middle to low income.”
For physicians who are also parents, the ability to set their own schedules is life-changing.
“My son got an award ... and the teacher invited me to the assembly. In a corporate-based world, I’d struggle to be able to go,” said Dr. Seefeldt. As her own boss, she didn’t have to forgo this special event. Instead, she coordinated directly with her scheduled patient to make time for it.
In Medscape’s report, 61% of employed physicians indicated that they don’t have a say on key management decisions. However, doctors who launch private practices embrace the chance to set their own standards.
“We make sure from the minute someone calls they know they’re in good hands, we’re responsive, we address concerns right away. That’s the difference with private practice – the one-on-one connection is huge,” said Dr. Patel.
“This is exactly what I always wanted. It brings me joy knowing we’ve made a difference in these children’s lives, in their parents’ lives,” she concluded.
A version of this article first appeared on Medscape.com.
The kids may not be alright, but psychiatry can help
When I was growing up, I can remember experiencing “duck and cover” drills at school. If a flash appeared in our peripheral vision, we were told we should not look at it but crawl under our desks. My classmates and I were being taught how to protect ourselves in case of a nuclear attack.
Clearly, had there been such an attack, ducking under our desks would not have saved us. Thankfully, such a conflict never occurred – and hopefully never will. Still, the warning did penetrate our psyches. In those days, families and children in schools were worried, and some were scared.
The situation is quite different today. Our children and grandchildren are being taught to protect themselves not from actions overseas – that never happened – but from what someone living in their community might do that has been occurring in real time. According to my daughter-in-law, her young children are taught during “lockdowns” to hide in their classrooms’ closets. During these drills, some children are directed to line up against a wall that would be out of sight of a shooter, and to stay as still as possible.
Since 2017, the number of intentional shootings in U.S. kindergarten through grade 12 schools increased precipitously (Prev Med. 2022 Dec. doi: 10.1016/j.ypmed.2022.107280). Imagine the psychological impact that the vigilance required to deal with such impending threats must be having on our children, as they learn to fear injury and possible death every day they go to school. I’ve talked with numerous parents about this, including my own adult children, and this is clearly a new dimension of life that is on everyone’s minds. Schools, once bastions of safety, are no longer that safe.
For many years, I’ve written about the need to destigmatize mental illness so that it is treated on a par with physical illness. As we look at the challenges faced by young people, reframing mental illness is more important now than ever. This means finding ways to increase the funding of studies that help us understand young people with mental health issues. It also means encouraging patients to pursue treatment from psychiatrists, psychologists, or mental health counselors who specialize in short-term therapy.
The emphasis here on short-term therapy is not to discourage longer-term care when needed, but clearly short-term care strategies, such as cognitive-behavioral therapies, not only work for problem resolution, they also help in the destigmatization of mental health care – as the circumscribed treatment with a clear beginning, middle, and end is consistent with CBT and consistent with much of medical care for physical disorders.
Furthermore, as we aim to destigmatize mental health care, it’s important to equate it with physical care. For example, taking a day or two from school or work for a sprained ankle, seeing a dentist, or an eye exam, plus a myriad of physical issues is quite acceptable. Why is it not also acceptable for a mental health issue and evaluation, such as for anxiety or PTSD, plus being able to talk about it without stigma? Seeing the “shrink” needs to be removed as a negative but viewed as a very positive move toward care for oneself.
In addition, children and adolescents are battling countless other health challenges that could have implications for mental health professionals, for example:
- During the height of the coronavirus pandemic, pediatric endocrinologists reportedly saw a surge of referrals for girls experiencing early puberty. Puberty should never be medicalized, but early maturation has been linked to numerous psychiatric disorders such as depression, anxiety, and eating disorders (J Pediatr Adolec Gynecol. 2022 Oct. doi: 10.1016/j.jpag.2022.05.005).
- A global epidemiologic study of children estimates that nearly 8 million youth lost a parent or caregiver because of a pandemic-related cause between Jan. 1, 2020, and May 1, 2022. An additional 2.5 million children were affected by the loss of secondary caregivers such as grandparents (JAMA Pediatr. 2022 Sept. doi: 10.1001/jamapediatrics.2022.3157).
- The inpatient and outpatient volume of adolescents and young adults receiving care for eating disorders skyrocketed before and after the pandemic, according to the results of case study series (JAMA Pediatrics. 2022 Nov 7. doi: 10.1001/jamapediatrics.2022.4346).
- Children and adolescents who developed COVID-19 suffered tremendously during the height of the pandemic. A nationwide analysis shows that COVID-19 nearly tripled children’s risks of developing new mental health illnesses, such as attention-deficit/hyperactivity disorder, anxiety, trauma, or stress disorder (Psychiatric Services. 2022 Jun 2. doi: 10.1176/appi.ps.202100646).
In addition to those challenges, young children are facing an increase in respiratory syncytial virus (RSV) infection. We were told the “flu” would be quite bad this year and to beware of monkeypox. However, very little mention is made of the equally distressing “epidemic” of mental health issues, PTSD, anxiety, and depression as we are still in the midst of the COVID pandemic in the United States with almost 400 deaths a day – a very unacceptable number.
Interestingly, we seem to have abandoned the use of masks as protection against COVID and other respiratory diseases, despite their effectiveness. A study in Boston that looked at children in two school districts that did not lift mask mandates demonstrated that mask wearing does indeed lead to significant reductions in the number of pediatric COVID cases. In addition to societal violence and school shootings – which certainly exacerbate anxiety – the fear of dying or the death of a loved one, tied to COVID, may lead to epidemic proportions of PTSD in children. As an article in WebMD noted, “pediatricians are imploring the federal government to declare a national emergency as cases of pediatric respiratory illnesses continue to soar.”
In light of the acknowledged mental health crisis in children, which appears epidemic, I would hope the psychiatric and psychological associations would publicly sound an alarm so that resources could be brought to bear to address this critical issue. I believe doing so would also aid in destigmatizing mental disorders, and increase education and treatment.
Layered on top of those issues are natural disasters, such as the fallout from Tropical Storm Nicole when it recently caused devastation across western Florida. The mental health trauma caused by recent tropical storms seems all but forgotten – except for those who are still suffering. All of this adds up to a society-wide mental health crisis, which seems far more expansive than monkeypox, for example. Yet monkeypox, which did lead to thousands of cases and approximately 29 deaths in the United States, was declared a national public health emergency.
Additionally, RSV killed 100-500 U.S. children under age 5 each year before the pandemic, according to the Centers for Disease Control and Prevention, and currently it appears even worse. Yet despite the seriousness of RSV, it nowhere matches the emotional toll COVID has taken on children globally.
Let’s make it standard practice for children – and of course, adults – to be taught that anxiety is a normal response at times. We should teach that, in some cases, feeling “down” or in despair and even experiencing symptoms of PTSD based on what’s going on personally and within our environment (i.e., COVID, school shootings, etc.) are triggers and responses that can be addressed and often quickly treated by talking with a mental health professional.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
When I was growing up, I can remember experiencing “duck and cover” drills at school. If a flash appeared in our peripheral vision, we were told we should not look at it but crawl under our desks. My classmates and I were being taught how to protect ourselves in case of a nuclear attack.
Clearly, had there been such an attack, ducking under our desks would not have saved us. Thankfully, such a conflict never occurred – and hopefully never will. Still, the warning did penetrate our psyches. In those days, families and children in schools were worried, and some were scared.
The situation is quite different today. Our children and grandchildren are being taught to protect themselves not from actions overseas – that never happened – but from what someone living in their community might do that has been occurring in real time. According to my daughter-in-law, her young children are taught during “lockdowns” to hide in their classrooms’ closets. During these drills, some children are directed to line up against a wall that would be out of sight of a shooter, and to stay as still as possible.
Since 2017, the number of intentional shootings in U.S. kindergarten through grade 12 schools increased precipitously (Prev Med. 2022 Dec. doi: 10.1016/j.ypmed.2022.107280). Imagine the psychological impact that the vigilance required to deal with such impending threats must be having on our children, as they learn to fear injury and possible death every day they go to school. I’ve talked with numerous parents about this, including my own adult children, and this is clearly a new dimension of life that is on everyone’s minds. Schools, once bastions of safety, are no longer that safe.
For many years, I’ve written about the need to destigmatize mental illness so that it is treated on a par with physical illness. As we look at the challenges faced by young people, reframing mental illness is more important now than ever. This means finding ways to increase the funding of studies that help us understand young people with mental health issues. It also means encouraging patients to pursue treatment from psychiatrists, psychologists, or mental health counselors who specialize in short-term therapy.
The emphasis here on short-term therapy is not to discourage longer-term care when needed, but clearly short-term care strategies, such as cognitive-behavioral therapies, not only work for problem resolution, they also help in the destigmatization of mental health care – as the circumscribed treatment with a clear beginning, middle, and end is consistent with CBT and consistent with much of medical care for physical disorders.
Furthermore, as we aim to destigmatize mental health care, it’s important to equate it with physical care. For example, taking a day or two from school or work for a sprained ankle, seeing a dentist, or an eye exam, plus a myriad of physical issues is quite acceptable. Why is it not also acceptable for a mental health issue and evaluation, such as for anxiety or PTSD, plus being able to talk about it without stigma? Seeing the “shrink” needs to be removed as a negative but viewed as a very positive move toward care for oneself.
In addition, children and adolescents are battling countless other health challenges that could have implications for mental health professionals, for example:
- During the height of the coronavirus pandemic, pediatric endocrinologists reportedly saw a surge of referrals for girls experiencing early puberty. Puberty should never be medicalized, but early maturation has been linked to numerous psychiatric disorders such as depression, anxiety, and eating disorders (J Pediatr Adolec Gynecol. 2022 Oct. doi: 10.1016/j.jpag.2022.05.005).
- A global epidemiologic study of children estimates that nearly 8 million youth lost a parent or caregiver because of a pandemic-related cause between Jan. 1, 2020, and May 1, 2022. An additional 2.5 million children were affected by the loss of secondary caregivers such as grandparents (JAMA Pediatr. 2022 Sept. doi: 10.1001/jamapediatrics.2022.3157).
- The inpatient and outpatient volume of adolescents and young adults receiving care for eating disorders skyrocketed before and after the pandemic, according to the results of case study series (JAMA Pediatrics. 2022 Nov 7. doi: 10.1001/jamapediatrics.2022.4346).
- Children and adolescents who developed COVID-19 suffered tremendously during the height of the pandemic. A nationwide analysis shows that COVID-19 nearly tripled children’s risks of developing new mental health illnesses, such as attention-deficit/hyperactivity disorder, anxiety, trauma, or stress disorder (Psychiatric Services. 2022 Jun 2. doi: 10.1176/appi.ps.202100646).
In addition to those challenges, young children are facing an increase in respiratory syncytial virus (RSV) infection. We were told the “flu” would be quite bad this year and to beware of monkeypox. However, very little mention is made of the equally distressing “epidemic” of mental health issues, PTSD, anxiety, and depression as we are still in the midst of the COVID pandemic in the United States with almost 400 deaths a day – a very unacceptable number.
Interestingly, we seem to have abandoned the use of masks as protection against COVID and other respiratory diseases, despite their effectiveness. A study in Boston that looked at children in two school districts that did not lift mask mandates demonstrated that mask wearing does indeed lead to significant reductions in the number of pediatric COVID cases. In addition to societal violence and school shootings – which certainly exacerbate anxiety – the fear of dying or the death of a loved one, tied to COVID, may lead to epidemic proportions of PTSD in children. As an article in WebMD noted, “pediatricians are imploring the federal government to declare a national emergency as cases of pediatric respiratory illnesses continue to soar.”
In light of the acknowledged mental health crisis in children, which appears epidemic, I would hope the psychiatric and psychological associations would publicly sound an alarm so that resources could be brought to bear to address this critical issue. I believe doing so would also aid in destigmatizing mental disorders, and increase education and treatment.
Layered on top of those issues are natural disasters, such as the fallout from Tropical Storm Nicole when it recently caused devastation across western Florida. The mental health trauma caused by recent tropical storms seems all but forgotten – except for those who are still suffering. All of this adds up to a society-wide mental health crisis, which seems far more expansive than monkeypox, for example. Yet monkeypox, which did lead to thousands of cases and approximately 29 deaths in the United States, was declared a national public health emergency.
Additionally, RSV killed 100-500 U.S. children under age 5 each year before the pandemic, according to the Centers for Disease Control and Prevention, and currently it appears even worse. Yet despite the seriousness of RSV, it nowhere matches the emotional toll COVID has taken on children globally.
Let’s make it standard practice for children – and of course, adults – to be taught that anxiety is a normal response at times. We should teach that, in some cases, feeling “down” or in despair and even experiencing symptoms of PTSD based on what’s going on personally and within our environment (i.e., COVID, school shootings, etc.) are triggers and responses that can be addressed and often quickly treated by talking with a mental health professional.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
When I was growing up, I can remember experiencing “duck and cover” drills at school. If a flash appeared in our peripheral vision, we were told we should not look at it but crawl under our desks. My classmates and I were being taught how to protect ourselves in case of a nuclear attack.
Clearly, had there been such an attack, ducking under our desks would not have saved us. Thankfully, such a conflict never occurred – and hopefully never will. Still, the warning did penetrate our psyches. In those days, families and children in schools were worried, and some were scared.
The situation is quite different today. Our children and grandchildren are being taught to protect themselves not from actions overseas – that never happened – but from what someone living in their community might do that has been occurring in real time. According to my daughter-in-law, her young children are taught during “lockdowns” to hide in their classrooms’ closets. During these drills, some children are directed to line up against a wall that would be out of sight of a shooter, and to stay as still as possible.
Since 2017, the number of intentional shootings in U.S. kindergarten through grade 12 schools increased precipitously (Prev Med. 2022 Dec. doi: 10.1016/j.ypmed.2022.107280). Imagine the psychological impact that the vigilance required to deal with such impending threats must be having on our children, as they learn to fear injury and possible death every day they go to school. I’ve talked with numerous parents about this, including my own adult children, and this is clearly a new dimension of life that is on everyone’s minds. Schools, once bastions of safety, are no longer that safe.
For many years, I’ve written about the need to destigmatize mental illness so that it is treated on a par with physical illness. As we look at the challenges faced by young people, reframing mental illness is more important now than ever. This means finding ways to increase the funding of studies that help us understand young people with mental health issues. It also means encouraging patients to pursue treatment from psychiatrists, psychologists, or mental health counselors who specialize in short-term therapy.
The emphasis here on short-term therapy is not to discourage longer-term care when needed, but clearly short-term care strategies, such as cognitive-behavioral therapies, not only work for problem resolution, they also help in the destigmatization of mental health care – as the circumscribed treatment with a clear beginning, middle, and end is consistent with CBT and consistent with much of medical care for physical disorders.
Furthermore, as we aim to destigmatize mental health care, it’s important to equate it with physical care. For example, taking a day or two from school or work for a sprained ankle, seeing a dentist, or an eye exam, plus a myriad of physical issues is quite acceptable. Why is it not also acceptable for a mental health issue and evaluation, such as for anxiety or PTSD, plus being able to talk about it without stigma? Seeing the “shrink” needs to be removed as a negative but viewed as a very positive move toward care for oneself.
In addition, children and adolescents are battling countless other health challenges that could have implications for mental health professionals, for example:
- During the height of the coronavirus pandemic, pediatric endocrinologists reportedly saw a surge of referrals for girls experiencing early puberty. Puberty should never be medicalized, but early maturation has been linked to numerous psychiatric disorders such as depression, anxiety, and eating disorders (J Pediatr Adolec Gynecol. 2022 Oct. doi: 10.1016/j.jpag.2022.05.005).
- A global epidemiologic study of children estimates that nearly 8 million youth lost a parent or caregiver because of a pandemic-related cause between Jan. 1, 2020, and May 1, 2022. An additional 2.5 million children were affected by the loss of secondary caregivers such as grandparents (JAMA Pediatr. 2022 Sept. doi: 10.1001/jamapediatrics.2022.3157).
- The inpatient and outpatient volume of adolescents and young adults receiving care for eating disorders skyrocketed before and after the pandemic, according to the results of case study series (JAMA Pediatrics. 2022 Nov 7. doi: 10.1001/jamapediatrics.2022.4346).
- Children and adolescents who developed COVID-19 suffered tremendously during the height of the pandemic. A nationwide analysis shows that COVID-19 nearly tripled children’s risks of developing new mental health illnesses, such as attention-deficit/hyperactivity disorder, anxiety, trauma, or stress disorder (Psychiatric Services. 2022 Jun 2. doi: 10.1176/appi.ps.202100646).
In addition to those challenges, young children are facing an increase in respiratory syncytial virus (RSV) infection. We were told the “flu” would be quite bad this year and to beware of monkeypox. However, very little mention is made of the equally distressing “epidemic” of mental health issues, PTSD, anxiety, and depression as we are still in the midst of the COVID pandemic in the United States with almost 400 deaths a day – a very unacceptable number.
Interestingly, we seem to have abandoned the use of masks as protection against COVID and other respiratory diseases, despite their effectiveness. A study in Boston that looked at children in two school districts that did not lift mask mandates demonstrated that mask wearing does indeed lead to significant reductions in the number of pediatric COVID cases. In addition to societal violence and school shootings – which certainly exacerbate anxiety – the fear of dying or the death of a loved one, tied to COVID, may lead to epidemic proportions of PTSD in children. As an article in WebMD noted, “pediatricians are imploring the federal government to declare a national emergency as cases of pediatric respiratory illnesses continue to soar.”
In light of the acknowledged mental health crisis in children, which appears epidemic, I would hope the psychiatric and psychological associations would publicly sound an alarm so that resources could be brought to bear to address this critical issue. I believe doing so would also aid in destigmatizing mental disorders, and increase education and treatment.
Layered on top of those issues are natural disasters, such as the fallout from Tropical Storm Nicole when it recently caused devastation across western Florida. The mental health trauma caused by recent tropical storms seems all but forgotten – except for those who are still suffering. All of this adds up to a society-wide mental health crisis, which seems far more expansive than monkeypox, for example. Yet monkeypox, which did lead to thousands of cases and approximately 29 deaths in the United States, was declared a national public health emergency.
Additionally, RSV killed 100-500 U.S. children under age 5 each year before the pandemic, according to the Centers for Disease Control and Prevention, and currently it appears even worse. Yet despite the seriousness of RSV, it nowhere matches the emotional toll COVID has taken on children globally.
Let’s make it standard practice for children – and of course, adults – to be taught that anxiety is a normal response at times. We should teach that, in some cases, feeling “down” or in despair and even experiencing symptoms of PTSD based on what’s going on personally and within our environment (i.e., COVID, school shootings, etc.) are triggers and responses that can be addressed and often quickly treated by talking with a mental health professional.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
Seizures in dementia hasten decline and death
NASHVILLE, TENN. – , according to a multicenter study presented at the 2022 annual meeting of the American Epilepsy Society.
“When we compared patients with seizures with those who did not have seizures, we found that patients with seizures were more likely to have more severe cognitive impairment; they were more likely to have physical dependence and so worse functional outcomes; and they also had higher mortality rates at a younger age,” lead study author Ifrah Zawar, MD, an assistant professor of neurology at the University of Virginia, Charlottesville, said in an interview.
“The average age of mortality for seizure patients was around 72 years and the average age of mortality for nonseizure patients was around 79 years, so there was a 7- to 8-year difference in mortality,” she said.
Seizures make matters worse
The study analyzed data on 26,425 patients with dementia, 374 (1.4%) of whom had seizures, collected from 2005 to 2021 at 39 Alzheimer’s disease centers in the United States. Patients who had seizures were significantly younger when cognitive decline began (ages 62.9 vs. 68.4 years, P < .001) and died younger (72.99 vs. 79.72 years, P < .001).
The study also found a number of factors associated with active seizures, including a history of dominant Alzheimer’s disease mutation (odds ratio, 5.55; P < .001), stroke (OR, 3.17; P < .001), transient ischemic attack (OR, 1.72; P = .003), traumatic brain injury (OR, 1.92; P < .001), Parkinson’s disease (OR, 1.79; P = .025), active depression (OR, 1.61; P < .001) and lower education (OR, 0.97; P =.043).
After the study made adjustments for sex and other associated factors, it found that patients with seizures were still at a 76% higher risk of dying younger (hazard ratio, 1.76; P < .001).
The study also determined that patients with seizures had worse functional assessment scores and were more likely to be physically dependent on others (OR, 2.52; P < .001). Seizure patients also performed worse on Mini-Mental Status Examination (18.50 vs. 22.88; P < .001) and Clinical Dementia Rating-Sum of boxes (7.95 vs. 4.28; P < .001) after adjusting for age and duration of cognitive decline.
A tip for caregivers
Dr. Zawar acknowledged that differentiating seizures from transient bouts of confusion in people with dementia can be difficult for family members and caregivers, but she offered advice to help them do so. “If they notice any unusual confusion or any altered mentation which is episodic in nature,” she said, “they should bring it to the neurologist’s attention as early as possible, because there are studies that have shown the diagnosis of seizures is delayed, and if they are treated in time they can be well-controlled.” Electroencephalography can also confirm the presence of seizures, she added.
Double whammy
One limitation of this study is the lack of details on the types of seizures the participants had along with the inconsistency of EEGs performed on the study population. “In future studies, I would like to have more EEG data on the types of seizures and the frequency of seizures to assess these factors further,” Dr. Zawar said.
Having more detailed information on the seizures would make the findings more valuable, Andrew J. Cole, MD, director of the epilepsy service at Massachusetts General Hospital in Boston said in an interview. “We know a lot about clinically apparent seizures, as witnessed by this paper, but we still don’t know a whole lot about clinically silent or cryptic or nighttime-only seizures that maybe no one would really recognize as such unless they were specifically looking for them, and this paper doesn’t address that issue,” he said.
While the finding that patients with other neurologic diseases have more seizures even if they also have Alzheimer’s disease isn’t “a huge surprise,” Dr. Cole added. “On the other hand, the paper is important because it shows us that in the course of having Alzheimer’s disease, having seizures also makes your outcome worse, the speed of progression faster, and it complicates the management and living with this disease, and they make that point quite clear.”
Dr. Zawar and Dr. Cole have no relevant disclosures.
NASHVILLE, TENN. – , according to a multicenter study presented at the 2022 annual meeting of the American Epilepsy Society.
“When we compared patients with seizures with those who did not have seizures, we found that patients with seizures were more likely to have more severe cognitive impairment; they were more likely to have physical dependence and so worse functional outcomes; and they also had higher mortality rates at a younger age,” lead study author Ifrah Zawar, MD, an assistant professor of neurology at the University of Virginia, Charlottesville, said in an interview.
“The average age of mortality for seizure patients was around 72 years and the average age of mortality for nonseizure patients was around 79 years, so there was a 7- to 8-year difference in mortality,” she said.
Seizures make matters worse
The study analyzed data on 26,425 patients with dementia, 374 (1.4%) of whom had seizures, collected from 2005 to 2021 at 39 Alzheimer’s disease centers in the United States. Patients who had seizures were significantly younger when cognitive decline began (ages 62.9 vs. 68.4 years, P < .001) and died younger (72.99 vs. 79.72 years, P < .001).
The study also found a number of factors associated with active seizures, including a history of dominant Alzheimer’s disease mutation (odds ratio, 5.55; P < .001), stroke (OR, 3.17; P < .001), transient ischemic attack (OR, 1.72; P = .003), traumatic brain injury (OR, 1.92; P < .001), Parkinson’s disease (OR, 1.79; P = .025), active depression (OR, 1.61; P < .001) and lower education (OR, 0.97; P =.043).
After the study made adjustments for sex and other associated factors, it found that patients with seizures were still at a 76% higher risk of dying younger (hazard ratio, 1.76; P < .001).
The study also determined that patients with seizures had worse functional assessment scores and were more likely to be physically dependent on others (OR, 2.52; P < .001). Seizure patients also performed worse on Mini-Mental Status Examination (18.50 vs. 22.88; P < .001) and Clinical Dementia Rating-Sum of boxes (7.95 vs. 4.28; P < .001) after adjusting for age and duration of cognitive decline.
A tip for caregivers
Dr. Zawar acknowledged that differentiating seizures from transient bouts of confusion in people with dementia can be difficult for family members and caregivers, but she offered advice to help them do so. “If they notice any unusual confusion or any altered mentation which is episodic in nature,” she said, “they should bring it to the neurologist’s attention as early as possible, because there are studies that have shown the diagnosis of seizures is delayed, and if they are treated in time they can be well-controlled.” Electroencephalography can also confirm the presence of seizures, she added.
Double whammy
One limitation of this study is the lack of details on the types of seizures the participants had along with the inconsistency of EEGs performed on the study population. “In future studies, I would like to have more EEG data on the types of seizures and the frequency of seizures to assess these factors further,” Dr. Zawar said.
Having more detailed information on the seizures would make the findings more valuable, Andrew J. Cole, MD, director of the epilepsy service at Massachusetts General Hospital in Boston said in an interview. “We know a lot about clinically apparent seizures, as witnessed by this paper, but we still don’t know a whole lot about clinically silent or cryptic or nighttime-only seizures that maybe no one would really recognize as such unless they were specifically looking for them, and this paper doesn’t address that issue,” he said.
While the finding that patients with other neurologic diseases have more seizures even if they also have Alzheimer’s disease isn’t “a huge surprise,” Dr. Cole added. “On the other hand, the paper is important because it shows us that in the course of having Alzheimer’s disease, having seizures also makes your outcome worse, the speed of progression faster, and it complicates the management and living with this disease, and they make that point quite clear.”
Dr. Zawar and Dr. Cole have no relevant disclosures.
NASHVILLE, TENN. – , according to a multicenter study presented at the 2022 annual meeting of the American Epilepsy Society.
“When we compared patients with seizures with those who did not have seizures, we found that patients with seizures were more likely to have more severe cognitive impairment; they were more likely to have physical dependence and so worse functional outcomes; and they also had higher mortality rates at a younger age,” lead study author Ifrah Zawar, MD, an assistant professor of neurology at the University of Virginia, Charlottesville, said in an interview.
“The average age of mortality for seizure patients was around 72 years and the average age of mortality for nonseizure patients was around 79 years, so there was a 7- to 8-year difference in mortality,” she said.
Seizures make matters worse
The study analyzed data on 26,425 patients with dementia, 374 (1.4%) of whom had seizures, collected from 2005 to 2021 at 39 Alzheimer’s disease centers in the United States. Patients who had seizures were significantly younger when cognitive decline began (ages 62.9 vs. 68.4 years, P < .001) and died younger (72.99 vs. 79.72 years, P < .001).
The study also found a number of factors associated with active seizures, including a history of dominant Alzheimer’s disease mutation (odds ratio, 5.55; P < .001), stroke (OR, 3.17; P < .001), transient ischemic attack (OR, 1.72; P = .003), traumatic brain injury (OR, 1.92; P < .001), Parkinson’s disease (OR, 1.79; P = .025), active depression (OR, 1.61; P < .001) and lower education (OR, 0.97; P =.043).
After the study made adjustments for sex and other associated factors, it found that patients with seizures were still at a 76% higher risk of dying younger (hazard ratio, 1.76; P < .001).
The study also determined that patients with seizures had worse functional assessment scores and were more likely to be physically dependent on others (OR, 2.52; P < .001). Seizure patients also performed worse on Mini-Mental Status Examination (18.50 vs. 22.88; P < .001) and Clinical Dementia Rating-Sum of boxes (7.95 vs. 4.28; P < .001) after adjusting for age and duration of cognitive decline.
A tip for caregivers
Dr. Zawar acknowledged that differentiating seizures from transient bouts of confusion in people with dementia can be difficult for family members and caregivers, but she offered advice to help them do so. “If they notice any unusual confusion or any altered mentation which is episodic in nature,” she said, “they should bring it to the neurologist’s attention as early as possible, because there are studies that have shown the diagnosis of seizures is delayed, and if they are treated in time they can be well-controlled.” Electroencephalography can also confirm the presence of seizures, she added.
Double whammy
One limitation of this study is the lack of details on the types of seizures the participants had along with the inconsistency of EEGs performed on the study population. “In future studies, I would like to have more EEG data on the types of seizures and the frequency of seizures to assess these factors further,” Dr. Zawar said.
Having more detailed information on the seizures would make the findings more valuable, Andrew J. Cole, MD, director of the epilepsy service at Massachusetts General Hospital in Boston said in an interview. “We know a lot about clinically apparent seizures, as witnessed by this paper, but we still don’t know a whole lot about clinically silent or cryptic or nighttime-only seizures that maybe no one would really recognize as such unless they were specifically looking for them, and this paper doesn’t address that issue,” he said.
While the finding that patients with other neurologic diseases have more seizures even if they also have Alzheimer’s disease isn’t “a huge surprise,” Dr. Cole added. “On the other hand, the paper is important because it shows us that in the course of having Alzheimer’s disease, having seizures also makes your outcome worse, the speed of progression faster, and it complicates the management and living with this disease, and they make that point quite clear.”
Dr. Zawar and Dr. Cole have no relevant disclosures.
AT AES 2022





