User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Simulating psychoanalysis: A review of Freud’s Bones
While psychiatry has been the subject of many films, video games are not a medium commonly known for examining mental illness.1 There have been PC games over the years with psychiatric themes, such as Sanitarium (1998), Depression Quest (2013), Fran Bow (2015), and Night in the Woods (2017). Now for perhaps the first time a game has been developed with the practice of psychiatry as its primary focus.
Freud’s Bones is a 2022 game developed by independent Italian game studio Fortuna Imperatore. The result of a successful Kickstarter crowdfunding campaign, Freud’s Bones is advertised as “the first point & click narrative-drive game to pay homage to the birth of psychoanalysis and its founder, addressing the themes of sexuality and neuroses filled with existential doubts.”
In Freud’s Bones, you take control of Sigmund Freud and guide him through his daily tasks. Gameplay is of the simple point-and-click variety, modeled after classic LucasArts-style adventure games of the 1990s such as The Secret of Monkey Island or Day of the Tentacle. Prior to seeing your first patient, the game provides several documents the player can peruse to become familiar with basic concepts of psychoanalysis. Although the game was originally written in Italian (and translation gaffes occasionally arise), generally the English wording is easy to read. However, some players may feel intimidated or bored by the sheer quantity of text the game provides. All in-game text, including books and spoken words, are written and there is no recorded voice acting. Audio consists largely of unintrusive background music and occasional sound effects. The graphical style is simple and cartoonish but pleasant.
Freud’s personal life is a major focus of the game. His real life dog Jofi is a constant presence in Freud’s office. At various times the player will witness Freud’s dreams, act as a voice inside his head, and attempt to interpret mystical Egyptian messages he receives. Players are also tasked with managing Freud’s reputation in the scientific community. This is apparently intended as a reflection of in-game clinical acumen, but it was sometimes difficult to tell what direct influence my actions had on Freud’s reputation.
Freud’s energy may flag at various points during the game, and the player may choose to give him a cigar or a dose of cocaine to stimulate him. These options sound interesting on the surface, but I found the effect of these substances on the game’s actual outcome to be minimal. Some tasks are presented in a less than user-friendly manner. For example, on my initial playthrough I could not figure out how to complete several optional errands such as shopping for more tobacco or selecting a cover for Freud’s books. The player is also given the opportunity to make choices that affect Freud’s personal life, such as whether to pursue an extramarital affair. The game does have a few narrative surprises, including appearances from some of Freud’s well-known contemporaries. One particularly vivid sequence late in the game involves navigating Freud through a hallucination with some bizarre, but very Freudian, imagery.
By far the most interesting and enjoyable part of the game is the psychoanalysis sessions. The player guides Freud through multiple sessions with four different patients. Each of them has a unique story and associated symptoms, and the player can choose a variety of responses. For example, will you take a comforting, paternalistic approach to the patient uncomfortable with her first appointment? Or will you take the more stoic, quiet approach of the analyst and allow the patient to speak without prompting? Part of the player’s quest in guiding Freud through these sessions is to help patients bring their unconscious thoughts to conscious awareness. This is depicted graphically as the thought moves vertically through images representing the id, superego, and ego. Skillful questioning can bring these thoughts to the surface, but poor choices can leave valuable insights buried in the unconscious.
These therapy sessions were unique and engaging, and I wish they constituted a larger portion of the gameplay in Freud’s Bones. More patients, more sessions with each patient, and longer sessions would all have been welcome additions. These analytic sessions eventually culminate in an opportunity to offer a diagnosis, and the player’s accuracy in treatment can result in divergent outcomes for each patient. The game is not lengthy, as it can be played in its entirety in roughly 5-6 hours. Selecting different options for Freud’s personal life and the analysis sessions provides some replay value for subsequent playthroughs.
Overall, Freud’s Bones is a worthy effort for being uniquely designed as interactive entertainment simulating psychoanalysis. It provides an experience of interest to psychiatrists but is also accessible to the general public. While the game has flaws in that it can be overly text-heavy and goals are not always clear, it shines in the moments where it allows the player to participate directly in the process of psychoanalysis. Freud’s Bones is available for purchase on Steam (currently priced at $13.99) and can be played on Windows PCs.
Dr. Weber is a psychiatrist at Intermountain Logan Regional Hospital in Logan, Utah. He disclosed no relevant financial relationships.
References
1. See, for example, Gabbard GO, Gabbard K. Psychiatry and the Cinema, 2nd ed. American Psychiatric Press, Inc.; 1999.
While psychiatry has been the subject of many films, video games are not a medium commonly known for examining mental illness.1 There have been PC games over the years with psychiatric themes, such as Sanitarium (1998), Depression Quest (2013), Fran Bow (2015), and Night in the Woods (2017). Now for perhaps the first time a game has been developed with the practice of psychiatry as its primary focus.
Freud’s Bones is a 2022 game developed by independent Italian game studio Fortuna Imperatore. The result of a successful Kickstarter crowdfunding campaign, Freud’s Bones is advertised as “the first point & click narrative-drive game to pay homage to the birth of psychoanalysis and its founder, addressing the themes of sexuality and neuroses filled with existential doubts.”
In Freud’s Bones, you take control of Sigmund Freud and guide him through his daily tasks. Gameplay is of the simple point-and-click variety, modeled after classic LucasArts-style adventure games of the 1990s such as The Secret of Monkey Island or Day of the Tentacle. Prior to seeing your first patient, the game provides several documents the player can peruse to become familiar with basic concepts of psychoanalysis. Although the game was originally written in Italian (and translation gaffes occasionally arise), generally the English wording is easy to read. However, some players may feel intimidated or bored by the sheer quantity of text the game provides. All in-game text, including books and spoken words, are written and there is no recorded voice acting. Audio consists largely of unintrusive background music and occasional sound effects. The graphical style is simple and cartoonish but pleasant.
Freud’s personal life is a major focus of the game. His real life dog Jofi is a constant presence in Freud’s office. At various times the player will witness Freud’s dreams, act as a voice inside his head, and attempt to interpret mystical Egyptian messages he receives. Players are also tasked with managing Freud’s reputation in the scientific community. This is apparently intended as a reflection of in-game clinical acumen, but it was sometimes difficult to tell what direct influence my actions had on Freud’s reputation.
Freud’s energy may flag at various points during the game, and the player may choose to give him a cigar or a dose of cocaine to stimulate him. These options sound interesting on the surface, but I found the effect of these substances on the game’s actual outcome to be minimal. Some tasks are presented in a less than user-friendly manner. For example, on my initial playthrough I could not figure out how to complete several optional errands such as shopping for more tobacco or selecting a cover for Freud’s books. The player is also given the opportunity to make choices that affect Freud’s personal life, such as whether to pursue an extramarital affair. The game does have a few narrative surprises, including appearances from some of Freud’s well-known contemporaries. One particularly vivid sequence late in the game involves navigating Freud through a hallucination with some bizarre, but very Freudian, imagery.
By far the most interesting and enjoyable part of the game is the psychoanalysis sessions. The player guides Freud through multiple sessions with four different patients. Each of them has a unique story and associated symptoms, and the player can choose a variety of responses. For example, will you take a comforting, paternalistic approach to the patient uncomfortable with her first appointment? Or will you take the more stoic, quiet approach of the analyst and allow the patient to speak without prompting? Part of the player’s quest in guiding Freud through these sessions is to help patients bring their unconscious thoughts to conscious awareness. This is depicted graphically as the thought moves vertically through images representing the id, superego, and ego. Skillful questioning can bring these thoughts to the surface, but poor choices can leave valuable insights buried in the unconscious.
These therapy sessions were unique and engaging, and I wish they constituted a larger portion of the gameplay in Freud’s Bones. More patients, more sessions with each patient, and longer sessions would all have been welcome additions. These analytic sessions eventually culminate in an opportunity to offer a diagnosis, and the player’s accuracy in treatment can result in divergent outcomes for each patient. The game is not lengthy, as it can be played in its entirety in roughly 5-6 hours. Selecting different options for Freud’s personal life and the analysis sessions provides some replay value for subsequent playthroughs.
Overall, Freud’s Bones is a worthy effort for being uniquely designed as interactive entertainment simulating psychoanalysis. It provides an experience of interest to psychiatrists but is also accessible to the general public. While the game has flaws in that it can be overly text-heavy and goals are not always clear, it shines in the moments where it allows the player to participate directly in the process of psychoanalysis. Freud’s Bones is available for purchase on Steam (currently priced at $13.99) and can be played on Windows PCs.
Dr. Weber is a psychiatrist at Intermountain Logan Regional Hospital in Logan, Utah. He disclosed no relevant financial relationships.
References
1. See, for example, Gabbard GO, Gabbard K. Psychiatry and the Cinema, 2nd ed. American Psychiatric Press, Inc.; 1999.
While psychiatry has been the subject of many films, video games are not a medium commonly known for examining mental illness.1 There have been PC games over the years with psychiatric themes, such as Sanitarium (1998), Depression Quest (2013), Fran Bow (2015), and Night in the Woods (2017). Now for perhaps the first time a game has been developed with the practice of psychiatry as its primary focus.
Freud’s Bones is a 2022 game developed by independent Italian game studio Fortuna Imperatore. The result of a successful Kickstarter crowdfunding campaign, Freud’s Bones is advertised as “the first point & click narrative-drive game to pay homage to the birth of psychoanalysis and its founder, addressing the themes of sexuality and neuroses filled with existential doubts.”
In Freud’s Bones, you take control of Sigmund Freud and guide him through his daily tasks. Gameplay is of the simple point-and-click variety, modeled after classic LucasArts-style adventure games of the 1990s such as The Secret of Monkey Island or Day of the Tentacle. Prior to seeing your first patient, the game provides several documents the player can peruse to become familiar with basic concepts of psychoanalysis. Although the game was originally written in Italian (and translation gaffes occasionally arise), generally the English wording is easy to read. However, some players may feel intimidated or bored by the sheer quantity of text the game provides. All in-game text, including books and spoken words, are written and there is no recorded voice acting. Audio consists largely of unintrusive background music and occasional sound effects. The graphical style is simple and cartoonish but pleasant.
Freud’s personal life is a major focus of the game. His real life dog Jofi is a constant presence in Freud’s office. At various times the player will witness Freud’s dreams, act as a voice inside his head, and attempt to interpret mystical Egyptian messages he receives. Players are also tasked with managing Freud’s reputation in the scientific community. This is apparently intended as a reflection of in-game clinical acumen, but it was sometimes difficult to tell what direct influence my actions had on Freud’s reputation.
Freud’s energy may flag at various points during the game, and the player may choose to give him a cigar or a dose of cocaine to stimulate him. These options sound interesting on the surface, but I found the effect of these substances on the game’s actual outcome to be minimal. Some tasks are presented in a less than user-friendly manner. For example, on my initial playthrough I could not figure out how to complete several optional errands such as shopping for more tobacco or selecting a cover for Freud’s books. The player is also given the opportunity to make choices that affect Freud’s personal life, such as whether to pursue an extramarital affair. The game does have a few narrative surprises, including appearances from some of Freud’s well-known contemporaries. One particularly vivid sequence late in the game involves navigating Freud through a hallucination with some bizarre, but very Freudian, imagery.
By far the most interesting and enjoyable part of the game is the psychoanalysis sessions. The player guides Freud through multiple sessions with four different patients. Each of them has a unique story and associated symptoms, and the player can choose a variety of responses. For example, will you take a comforting, paternalistic approach to the patient uncomfortable with her first appointment? Or will you take the more stoic, quiet approach of the analyst and allow the patient to speak without prompting? Part of the player’s quest in guiding Freud through these sessions is to help patients bring their unconscious thoughts to conscious awareness. This is depicted graphically as the thought moves vertically through images representing the id, superego, and ego. Skillful questioning can bring these thoughts to the surface, but poor choices can leave valuable insights buried in the unconscious.
These therapy sessions were unique and engaging, and I wish they constituted a larger portion of the gameplay in Freud’s Bones. More patients, more sessions with each patient, and longer sessions would all have been welcome additions. These analytic sessions eventually culminate in an opportunity to offer a diagnosis, and the player’s accuracy in treatment can result in divergent outcomes for each patient. The game is not lengthy, as it can be played in its entirety in roughly 5-6 hours. Selecting different options for Freud’s personal life and the analysis sessions provides some replay value for subsequent playthroughs.
Overall, Freud’s Bones is a worthy effort for being uniquely designed as interactive entertainment simulating psychoanalysis. It provides an experience of interest to psychiatrists but is also accessible to the general public. While the game has flaws in that it can be overly text-heavy and goals are not always clear, it shines in the moments where it allows the player to participate directly in the process of psychoanalysis. Freud’s Bones is available for purchase on Steam (currently priced at $13.99) and can be played on Windows PCs.
Dr. Weber is a psychiatrist at Intermountain Logan Regional Hospital in Logan, Utah. He disclosed no relevant financial relationships.
References
1. See, for example, Gabbard GO, Gabbard K. Psychiatry and the Cinema, 2nd ed. American Psychiatric Press, Inc.; 1999.
Borderline patients have longer time to depression remission
Major depressive episodes (MDEs) occur in major depressive disorder (MDD) and bipolar disorder (BD), John J. Söderholm, MD, of the University of Helsinki and colleagues wrote. Borderline personality disorder (BPD) includes an increased risk for depression, but data on the relationship between BPD symptoms and depressive illness are limited. In particular, they noted “a lack of studies prospectively comparing the presence of (hypo)manic symptoms over time during the recovery process from MDE between MDD, MDE/BD, and MDE/BPD patients.”
In a cohort study published in the Journal of Affective Disorders, the researchers collected data from 39 adult MDE patients with MDD, 33 with BD, and 23 with BPD. The patients were diagnosed with MDE using the SCID-I/P and SCID-II interviews, mixed symptoms were identified using the Mix-MDE scale, and borderline symptoms were identified using the Borderline Personality Disorder Severity Index.
Over a 6-month follow-up period, the participants completed biweekly online assessments. The primary outcomes were time to first full remission of symptoms and duration and nature of mood episodes.
Overall, the mean number of distinct mood states was 5.75, and the median duration was 60.9 days. When identified by subcohorts, the median number of mood state periods for MDD, BD, and BPD was 4.49, 8.05, and 4.67, respectively. The median durations were 69.2 days, 40.30 days, and 75.6 days, respectively.
The rates of remission for depressive symptoms were similar for MDD, MDE/BD, and MDE/BPD patients. However, MDE/BD patients had a significantly shorter time to first remission (hazard ratio, 2.44). Patients in the BPD group had a significantly longer time to first remission (HR, 0.95).
“When the cohort was divided into quintiles according to BPD feature severity, there was an approximately 1-month difference in time to first period of remission between the first and third and between the third and fifth quintiles, with longer times seen in patients with more severe BPD symptoms,” the researchers wrote.
The study findings were limited by several factors including the small sample size and short follow-up period that prevented investigation of depressive recurrence, the researchers noted. Other limitations included the lack of diagnostic blinding and variation in patients’ treatment schedules.
However, the results were strengthened by the representative samples of subjects with various disorders, the prospective and multimodal assessment of affective states, and the comparison of three patient groups in a single study.
As BPD was associated with a longer time to remission from depressive symptoms, the results suggest that BPD severity may be an indicator of more severe disease in patients with MDD in the context of depression, the researchers concluded.
The study was supported by the Finska Lakaresallskapet, the City of Helsinki, the Hospital District of Helsinki and Uusimaa, and the Finnish Psychiatric Association. The researchers had no financial conflicts to disclose.
Major depressive episodes (MDEs) occur in major depressive disorder (MDD) and bipolar disorder (BD), John J. Söderholm, MD, of the University of Helsinki and colleagues wrote. Borderline personality disorder (BPD) includes an increased risk for depression, but data on the relationship between BPD symptoms and depressive illness are limited. In particular, they noted “a lack of studies prospectively comparing the presence of (hypo)manic symptoms over time during the recovery process from MDE between MDD, MDE/BD, and MDE/BPD patients.”
In a cohort study published in the Journal of Affective Disorders, the researchers collected data from 39 adult MDE patients with MDD, 33 with BD, and 23 with BPD. The patients were diagnosed with MDE using the SCID-I/P and SCID-II interviews, mixed symptoms were identified using the Mix-MDE scale, and borderline symptoms were identified using the Borderline Personality Disorder Severity Index.
Over a 6-month follow-up period, the participants completed biweekly online assessments. The primary outcomes were time to first full remission of symptoms and duration and nature of mood episodes.
Overall, the mean number of distinct mood states was 5.75, and the median duration was 60.9 days. When identified by subcohorts, the median number of mood state periods for MDD, BD, and BPD was 4.49, 8.05, and 4.67, respectively. The median durations were 69.2 days, 40.30 days, and 75.6 days, respectively.
The rates of remission for depressive symptoms were similar for MDD, MDE/BD, and MDE/BPD patients. However, MDE/BD patients had a significantly shorter time to first remission (hazard ratio, 2.44). Patients in the BPD group had a significantly longer time to first remission (HR, 0.95).
“When the cohort was divided into quintiles according to BPD feature severity, there was an approximately 1-month difference in time to first period of remission between the first and third and between the third and fifth quintiles, with longer times seen in patients with more severe BPD symptoms,” the researchers wrote.
The study findings were limited by several factors including the small sample size and short follow-up period that prevented investigation of depressive recurrence, the researchers noted. Other limitations included the lack of diagnostic blinding and variation in patients’ treatment schedules.
However, the results were strengthened by the representative samples of subjects with various disorders, the prospective and multimodal assessment of affective states, and the comparison of three patient groups in a single study.
As BPD was associated with a longer time to remission from depressive symptoms, the results suggest that BPD severity may be an indicator of more severe disease in patients with MDD in the context of depression, the researchers concluded.
The study was supported by the Finska Lakaresallskapet, the City of Helsinki, the Hospital District of Helsinki and Uusimaa, and the Finnish Psychiatric Association. The researchers had no financial conflicts to disclose.
Major depressive episodes (MDEs) occur in major depressive disorder (MDD) and bipolar disorder (BD), John J. Söderholm, MD, of the University of Helsinki and colleagues wrote. Borderline personality disorder (BPD) includes an increased risk for depression, but data on the relationship between BPD symptoms and depressive illness are limited. In particular, they noted “a lack of studies prospectively comparing the presence of (hypo)manic symptoms over time during the recovery process from MDE between MDD, MDE/BD, and MDE/BPD patients.”
In a cohort study published in the Journal of Affective Disorders, the researchers collected data from 39 adult MDE patients with MDD, 33 with BD, and 23 with BPD. The patients were diagnosed with MDE using the SCID-I/P and SCID-II interviews, mixed symptoms were identified using the Mix-MDE scale, and borderline symptoms were identified using the Borderline Personality Disorder Severity Index.
Over a 6-month follow-up period, the participants completed biweekly online assessments. The primary outcomes were time to first full remission of symptoms and duration and nature of mood episodes.
Overall, the mean number of distinct mood states was 5.75, and the median duration was 60.9 days. When identified by subcohorts, the median number of mood state periods for MDD, BD, and BPD was 4.49, 8.05, and 4.67, respectively. The median durations were 69.2 days, 40.30 days, and 75.6 days, respectively.
The rates of remission for depressive symptoms were similar for MDD, MDE/BD, and MDE/BPD patients. However, MDE/BD patients had a significantly shorter time to first remission (hazard ratio, 2.44). Patients in the BPD group had a significantly longer time to first remission (HR, 0.95).
“When the cohort was divided into quintiles according to BPD feature severity, there was an approximately 1-month difference in time to first period of remission between the first and third and between the third and fifth quintiles, with longer times seen in patients with more severe BPD symptoms,” the researchers wrote.
The study findings were limited by several factors including the small sample size and short follow-up period that prevented investigation of depressive recurrence, the researchers noted. Other limitations included the lack of diagnostic blinding and variation in patients’ treatment schedules.
However, the results were strengthened by the representative samples of subjects with various disorders, the prospective and multimodal assessment of affective states, and the comparison of three patient groups in a single study.
As BPD was associated with a longer time to remission from depressive symptoms, the results suggest that BPD severity may be an indicator of more severe disease in patients with MDD in the context of depression, the researchers concluded.
The study was supported by the Finska Lakaresallskapet, the City of Helsinki, the Hospital District of Helsinki and Uusimaa, and the Finnish Psychiatric Association. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Can 6 minutes of intense cycling put the brakes on Alzheimer’s?
new research suggests.
In a small study of healthy adults, 6 minutes of high-intensity cycling increased circulating levels of brain-derived neurotrophic factor (BDNF) to a significantly greater extent than prolonged light cycling or fasting.
However, the data do not suggest that 6 minutes of high-intensity exercise “wards off dementia,” cautioned lead investigator Travis Gibbons, MSc, PhD candidate in environmental physiology at the University of Otago (New Zealand), Dunedin, and now postdoctoral fellow at the University of British Columbia – Okanagan, Kelowna.
“Like all science, this is just a small piece that supports a potential mechanistic role for how exercise might improve brain health,” Dr. Gibbons told this news organization.
The findings were published online in the Journal of Physiology.
Targeting BDNF
Both intermittent fasting and exercise have previously been shown to have potent neuroprotective effects; and an acute upregulation of BDNF appears to be a common mechanistic link.
To tease apart the influence of fasting and exercise on BDNF production, Dr. Gibbons and colleagues studied 12 aerobically fit, healthy men (n = 6) and women (n = 6) aged 20-40 years.
In a study that employed a repeated-measures crossover design, they assessed circulating BDNF levels after a 20-hour fast, prolonged (90-min) light cycling, short (6-min) high-intensity cycling, and combined fasting and exercise.
Six minutes of high-intensity exercise appeared to be the most efficient way to increase BDNF.
Fasting for 20 hours led to a ninefold increase in ketone body delivery to the brain but had no effect on any metric of BDNF in peripheral circulation at rest or during exercise.
Six minutes of high-intensity exercise increased every metric of circulating BDNF four to five times more than prolonged low-intensity exercise.
In addition, the increase in plasma-derived BDNF correlated with a sixfold increase in circulating lactate irrespective of feeding or fasting state.
Lactate delivery?
“My leading theory is that, during and following intense exercise, lactate produced by muscles is delivered and consumed by the brain,” Dr. Gibbons noted.
“It takes high-intensity exercise to provoke this ‘cerebral substrate switch’ from glucose to lactate. Critically, this cerebral substrate switch has been shown to contribute to the early processes that upregulate BDNF production in the brain,” he said.
However, “Whether this translates to ‘warding off dementia’ is not clear,” Dr. Gibbons added.
The study also suggests that increases in plasma volume and platelet concentration appear to play a role in concentrating BDNF in the circulation during exercise.
The investigators note that BDNF and other neurotrophic-based pharmaceutical therapies have shown “great promise” in slowing and even arresting neurodegenerative processes in animals, but attempts to harness the protective power of BDNF in human neurodegeneration have thus far failed.
“Whether episodically upregulating BDNF production with intense exercise is an effective strategy to curb age-related cognitive decline in humans is unknown, but animal models indicate that it is and that BDNF plays a primary role,” the researchers write.
Funding for the study was provided by the Healthcare Otago Charitable Trust. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
In a small study of healthy adults, 6 minutes of high-intensity cycling increased circulating levels of brain-derived neurotrophic factor (BDNF) to a significantly greater extent than prolonged light cycling or fasting.
However, the data do not suggest that 6 minutes of high-intensity exercise “wards off dementia,” cautioned lead investigator Travis Gibbons, MSc, PhD candidate in environmental physiology at the University of Otago (New Zealand), Dunedin, and now postdoctoral fellow at the University of British Columbia – Okanagan, Kelowna.
“Like all science, this is just a small piece that supports a potential mechanistic role for how exercise might improve brain health,” Dr. Gibbons told this news organization.
The findings were published online in the Journal of Physiology.
Targeting BDNF
Both intermittent fasting and exercise have previously been shown to have potent neuroprotective effects; and an acute upregulation of BDNF appears to be a common mechanistic link.
To tease apart the influence of fasting and exercise on BDNF production, Dr. Gibbons and colleagues studied 12 aerobically fit, healthy men (n = 6) and women (n = 6) aged 20-40 years.
In a study that employed a repeated-measures crossover design, they assessed circulating BDNF levels after a 20-hour fast, prolonged (90-min) light cycling, short (6-min) high-intensity cycling, and combined fasting and exercise.
Six minutes of high-intensity exercise appeared to be the most efficient way to increase BDNF.
Fasting for 20 hours led to a ninefold increase in ketone body delivery to the brain but had no effect on any metric of BDNF in peripheral circulation at rest or during exercise.
Six minutes of high-intensity exercise increased every metric of circulating BDNF four to five times more than prolonged low-intensity exercise.
In addition, the increase in plasma-derived BDNF correlated with a sixfold increase in circulating lactate irrespective of feeding or fasting state.
Lactate delivery?
“My leading theory is that, during and following intense exercise, lactate produced by muscles is delivered and consumed by the brain,” Dr. Gibbons noted.
“It takes high-intensity exercise to provoke this ‘cerebral substrate switch’ from glucose to lactate. Critically, this cerebral substrate switch has been shown to contribute to the early processes that upregulate BDNF production in the brain,” he said.
However, “Whether this translates to ‘warding off dementia’ is not clear,” Dr. Gibbons added.
The study also suggests that increases in plasma volume and platelet concentration appear to play a role in concentrating BDNF in the circulation during exercise.
The investigators note that BDNF and other neurotrophic-based pharmaceutical therapies have shown “great promise” in slowing and even arresting neurodegenerative processes in animals, but attempts to harness the protective power of BDNF in human neurodegeneration have thus far failed.
“Whether episodically upregulating BDNF production with intense exercise is an effective strategy to curb age-related cognitive decline in humans is unknown, but animal models indicate that it is and that BDNF plays a primary role,” the researchers write.
Funding for the study was provided by the Healthcare Otago Charitable Trust. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
In a small study of healthy adults, 6 minutes of high-intensity cycling increased circulating levels of brain-derived neurotrophic factor (BDNF) to a significantly greater extent than prolonged light cycling or fasting.
However, the data do not suggest that 6 minutes of high-intensity exercise “wards off dementia,” cautioned lead investigator Travis Gibbons, MSc, PhD candidate in environmental physiology at the University of Otago (New Zealand), Dunedin, and now postdoctoral fellow at the University of British Columbia – Okanagan, Kelowna.
“Like all science, this is just a small piece that supports a potential mechanistic role for how exercise might improve brain health,” Dr. Gibbons told this news organization.
The findings were published online in the Journal of Physiology.
Targeting BDNF
Both intermittent fasting and exercise have previously been shown to have potent neuroprotective effects; and an acute upregulation of BDNF appears to be a common mechanistic link.
To tease apart the influence of fasting and exercise on BDNF production, Dr. Gibbons and colleagues studied 12 aerobically fit, healthy men (n = 6) and women (n = 6) aged 20-40 years.
In a study that employed a repeated-measures crossover design, they assessed circulating BDNF levels after a 20-hour fast, prolonged (90-min) light cycling, short (6-min) high-intensity cycling, and combined fasting and exercise.
Six minutes of high-intensity exercise appeared to be the most efficient way to increase BDNF.
Fasting for 20 hours led to a ninefold increase in ketone body delivery to the brain but had no effect on any metric of BDNF in peripheral circulation at rest or during exercise.
Six minutes of high-intensity exercise increased every metric of circulating BDNF four to five times more than prolonged low-intensity exercise.
In addition, the increase in plasma-derived BDNF correlated with a sixfold increase in circulating lactate irrespective of feeding or fasting state.
Lactate delivery?
“My leading theory is that, during and following intense exercise, lactate produced by muscles is delivered and consumed by the brain,” Dr. Gibbons noted.
“It takes high-intensity exercise to provoke this ‘cerebral substrate switch’ from glucose to lactate. Critically, this cerebral substrate switch has been shown to contribute to the early processes that upregulate BDNF production in the brain,” he said.
However, “Whether this translates to ‘warding off dementia’ is not clear,” Dr. Gibbons added.
The study also suggests that increases in plasma volume and platelet concentration appear to play a role in concentrating BDNF in the circulation during exercise.
The investigators note that BDNF and other neurotrophic-based pharmaceutical therapies have shown “great promise” in slowing and even arresting neurodegenerative processes in animals, but attempts to harness the protective power of BDNF in human neurodegeneration have thus far failed.
“Whether episodically upregulating BDNF production with intense exercise is an effective strategy to curb age-related cognitive decline in humans is unknown, but animal models indicate that it is and that BDNF plays a primary role,” the researchers write.
Funding for the study was provided by the Healthcare Otago Charitable Trust. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF PHYSIOLOGY
Low serum LDH shows potential as depression biomarker
The pathogenesis of depression is complex, and recent research has focused on the potential relationship between energy metabolism and depression, wrote Qian Yao, MD, of Wuhan University, Hubei, China, and colleagues.
Previous studies have suggested that serum lactate dehydrogenase (LDH) may be a biomarker for Parkinson’s disease, Huntington’s disease, and post-stroke depression, but the link between lactate metabolism and depression remains unclear, they said.
“We hypothesize that LDH may act as a potential biomarker for MDD, considering it represents a reduced energy metabolic status in depressive patients,” they explained.
In a study published in General Hospital Psychiatry, the researchers examined differences in serum LDH in 232 patients with major depressive disorder (MDD) and 110 healthy controls. They also examined whether LDH was predictive of suicide attempts in the MDD patients. Depression was assessed via the 24-item Hamilton Depression Scale (HAMD-24).
The mean age across both groups was 33 years; other clinical characteristics were similar between the groups.
The serum LDH level of the MDD group was significantly lower than the control group was (177.94 U/L vs. 196.50 U/L; P < .001). Analysis of blood lipid levels showed significantly lower levels of total cholesterol in the MDD group compared with controls, but no significant differences were noted in LDL cholesterol, HDL cholesterol, or triglycerides.
In a further analysis of subgroups of depression, the serum LDH in MDD patients who had attempted suicide was significantly lower compared to those without suicide attempts (169.96 vs. 181.25; P = .002), although the LDH level for the non-suicide MDD patients also was significantly lower than controls (181.25 vs. 196.50; P < .001). No significant correlation was noted between HAMD-24 score and suicide attempts.
Some gender differences also appeared. Both male and female MDD patients had significantly lower LDH levels compared with controls. However, in a regression analysis, a correlation between total cholesterol and LDL cholesterol as potential suicide markers was noted in female MDD patients, but not male MDD patients, which suggests an impact of gender on suicide risk in MDD, the researchers wrote in their discussion.
The findings were limited by several factors including the retrospective design, lack of investigation of changes in LDH isozymes in MDD patients, and lack of assessment of changes in LDH in cerebrospinal fluid, the researchers noted. However, the results “provide clear evidence that the concentration of LDH in serum is associated with early onset and clinical prognosis of depressive symptoms,” in MDD, which may inform diagnosis and guide clinical intervention, including early identification of suicide risk, they concluded.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
The pathogenesis of depression is complex, and recent research has focused on the potential relationship between energy metabolism and depression, wrote Qian Yao, MD, of Wuhan University, Hubei, China, and colleagues.
Previous studies have suggested that serum lactate dehydrogenase (LDH) may be a biomarker for Parkinson’s disease, Huntington’s disease, and post-stroke depression, but the link between lactate metabolism and depression remains unclear, they said.
“We hypothesize that LDH may act as a potential biomarker for MDD, considering it represents a reduced energy metabolic status in depressive patients,” they explained.
In a study published in General Hospital Psychiatry, the researchers examined differences in serum LDH in 232 patients with major depressive disorder (MDD) and 110 healthy controls. They also examined whether LDH was predictive of suicide attempts in the MDD patients. Depression was assessed via the 24-item Hamilton Depression Scale (HAMD-24).
The mean age across both groups was 33 years; other clinical characteristics were similar between the groups.
The serum LDH level of the MDD group was significantly lower than the control group was (177.94 U/L vs. 196.50 U/L; P < .001). Analysis of blood lipid levels showed significantly lower levels of total cholesterol in the MDD group compared with controls, but no significant differences were noted in LDL cholesterol, HDL cholesterol, or triglycerides.
In a further analysis of subgroups of depression, the serum LDH in MDD patients who had attempted suicide was significantly lower compared to those without suicide attempts (169.96 vs. 181.25; P = .002), although the LDH level for the non-suicide MDD patients also was significantly lower than controls (181.25 vs. 196.50; P < .001). No significant correlation was noted between HAMD-24 score and suicide attempts.
Some gender differences also appeared. Both male and female MDD patients had significantly lower LDH levels compared with controls. However, in a regression analysis, a correlation between total cholesterol and LDL cholesterol as potential suicide markers was noted in female MDD patients, but not male MDD patients, which suggests an impact of gender on suicide risk in MDD, the researchers wrote in their discussion.
The findings were limited by several factors including the retrospective design, lack of investigation of changes in LDH isozymes in MDD patients, and lack of assessment of changes in LDH in cerebrospinal fluid, the researchers noted. However, the results “provide clear evidence that the concentration of LDH in serum is associated with early onset and clinical prognosis of depressive symptoms,” in MDD, which may inform diagnosis and guide clinical intervention, including early identification of suicide risk, they concluded.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
The pathogenesis of depression is complex, and recent research has focused on the potential relationship between energy metabolism and depression, wrote Qian Yao, MD, of Wuhan University, Hubei, China, and colleagues.
Previous studies have suggested that serum lactate dehydrogenase (LDH) may be a biomarker for Parkinson’s disease, Huntington’s disease, and post-stroke depression, but the link between lactate metabolism and depression remains unclear, they said.
“We hypothesize that LDH may act as a potential biomarker for MDD, considering it represents a reduced energy metabolic status in depressive patients,” they explained.
In a study published in General Hospital Psychiatry, the researchers examined differences in serum LDH in 232 patients with major depressive disorder (MDD) and 110 healthy controls. They also examined whether LDH was predictive of suicide attempts in the MDD patients. Depression was assessed via the 24-item Hamilton Depression Scale (HAMD-24).
The mean age across both groups was 33 years; other clinical characteristics were similar between the groups.
The serum LDH level of the MDD group was significantly lower than the control group was (177.94 U/L vs. 196.50 U/L; P < .001). Analysis of blood lipid levels showed significantly lower levels of total cholesterol in the MDD group compared with controls, but no significant differences were noted in LDL cholesterol, HDL cholesterol, or triglycerides.
In a further analysis of subgroups of depression, the serum LDH in MDD patients who had attempted suicide was significantly lower compared to those without suicide attempts (169.96 vs. 181.25; P = .002), although the LDH level for the non-suicide MDD patients also was significantly lower than controls (181.25 vs. 196.50; P < .001). No significant correlation was noted between HAMD-24 score and suicide attempts.
Some gender differences also appeared. Both male and female MDD patients had significantly lower LDH levels compared with controls. However, in a regression analysis, a correlation between total cholesterol and LDL cholesterol as potential suicide markers was noted in female MDD patients, but not male MDD patients, which suggests an impact of gender on suicide risk in MDD, the researchers wrote in their discussion.
The findings were limited by several factors including the retrospective design, lack of investigation of changes in LDH isozymes in MDD patients, and lack of assessment of changes in LDH in cerebrospinal fluid, the researchers noted. However, the results “provide clear evidence that the concentration of LDH in serum is associated with early onset and clinical prognosis of depressive symptoms,” in MDD, which may inform diagnosis and guide clinical intervention, including early identification of suicide risk, they concluded.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
FROM GENERAL HOSPITAL PSYCHIATRY
Mood disorder? Assessment in primary care
The assessment and diagnosis of bipolar disorder in youth has a complicated and controversial history. I recall from my child and adolescent fellowship training that there was a thinly veiled faculty argument about the diagnosis itself with strong opinions on each side. To revisit this quandary, I reviewed the most up-to-date literature and outlined a case-based approach to the initial screening assessment. Certainly, the assessment by a child and adolescent psychiatrist would be the standard for diagnosis, but we do know that the pediatrician’s office may be the first setting for a child and parent to present with mood symptoms and concerns about bipolar disorder. What can you do to address this adolescent, Carrie, and her mother’s concerns?
Case
Carrie is a 17-year-old girl who has struggled through her childhood and adolescence with anxious and depressive symptoms which have ebbed and flowed with major life stressors, including her parent’s divorce. She has tried cognitive-behavioral therapy and selective serotonin reuptake inhibitors, but the SSRI seemed to cause feelings of anxiousness and agitation, so she stopped it within weeks.
Her mother presents to you concerned that Carrie has had a more persistently irritable mood toward her, often just wanting to be with her friends or otherwise isolate in her room when home to study.
Most concerning to her mother is that Carrie, as a straight A student, has also developed a pattern of staying up all night to study for tests and then “crashes” and sleeps through the weekend, avoiding her mother and only brightening with her friends.
To complicate matters, Carrie’s biological father had type 1 bipolar disorder and an addiction. Her mother comes to you with an initially nonparticipatory Carrie in tow and says: “My former husband began his manic episodes with a lack of sleep and Carrie is so irritable towards me. I feel like I am walking on eggshells all the time. Could this be bipolar disorder?”
Case discussion
First, it’s always useful to frame a visit stating that you will spend some time with the patient and some time with both the patient and parent. Emphasizing confidentiality about issues such as drug use, which can be comorbid with mood symptoms and go undetected in high-achieving students such as Carrie, is also important. Further emphasizing that information will not be reflexively shared with the parent unless the child presents a danger to herself or others is also paramount to receive an honest report of symptoms.
Second, there are many signs and symptoms of bipolar disorder that naturally overlap with other conditions such as distractibility with attention-deficit/hyperactivity disorder, or irritability in either a unipolar depression or disruptive mood dysregulation disorder.1 You are looking for an episodic (not chronic) course of symptoms with episodes that last over 5 days for hypomania and over the course of weeks for mania all while meeting all the classic criteria for bipolar disorder.
Note that the broadening of diagnostic criteria has been thought to contribute to an inflated sense of prevalence. The actual expert estimate of prevalence is around 0.8%-1.8% in pediatric populations, although there is a large published range depending on whether the criteria are modified or not.2 Use of the unmodified criteria from the DSM-5 is the recommended approach. Bipolar disorder is exceedingly rare in prepubertal children, and it would be more common for prodromal symptoms such as Carrie’s to emerge and escalate over the teenage years, culminating in a clearer diagnosis in the later teens or 20s.3
In my screening questions, I find the idea of an “infatiguable state” is the most pathognomonic one in considering mania in bipolar disorder.4 Carrie’s “crashing” after nights of studying shows that she clearly fatigues. Patients with bipolar disorder within episodes of hypomania or mania have a seismic shift in perceived energy and a matching lack of ability to sleep that can affect their thought processes, speech, and decision-making. At first blush, Carrie’s history does not indicate current symptoms of bipolar disorder.3
Case, continued
When you meet with Carrie alone she shares that she has been experimenting with prescribed stimulants from her older college-aged brother in order to study and ace her tests. She is also experimenting with alcohol and marijuana with her friends. You provide her the CRAFFT tool to deepen your screening of this issue.5
With her mother, you administer the Parent General Behavior Inventory6 and the and the Child Mania Rating Scale7. From these scales, you note that the irritability is more specific to Carrie’s family than pan-present in school and with friends. Her lack of sleep occurs at high-pressure and discreet times.
At this point, you reassure Carrie and her mother that Carrie does not present with symptoms of bipolar disorder but that certainly you will continue screening assessments over time, as they are a good means to track symptoms. You also recommend that Carrie consider mood tracking so she can develop insights into her mood and its relationship to sleep and other events as she prepares for college.8
Case discussion, continued
The strongest risk factor for bipolar disorder in youth is family history (specifically a parent) with bipolar disorder).9 If there is the chance to explore the parent’s illness with open-ended questions, you will want to hear about the parent’s age of symptom onset, course of treatment, any hospitalizations, and stabilizing medications because this has prognostic power for your patient. It is important to ensure that the parent indeed has a diagnosis of bipolar disorder and that it is not just being used colloquially to characterize an adult who has labile moods from hour to hour or day to day. This would give undue anticipatory anxiety to a youth about their risk, which is up to 8- to 10-fold greater with a parent with bipolar disorder.9
Even with a strong family history, we do not often see bipolar disorder emerge in prepubertal children.10,11 There may be still concerning prodromal symptoms in which a diagnosis of unipolar depression with more irritable features and mood lability seems more commonly complicated by substance use, as with Carrie.
Activation with an SSRI, as in Carrie’s case, even if not resulting in full mania or hypomania, can also be a soft sign of the serotonergic sensitivity present in bipolar disorder. However, if there are not additional symptoms of bipolar disorder and you are concerned based on family history alone, you do not want to withhold antidepressant treatment because fear of risk. You would want to consider a “dose low and go slow” titration process with more frequent monitoring.
A diagnostic interview with a child and adolescent psychiatrist and administration of scales such as the Young Mania Rating Scale and the Modified Child Depression Rating Scale are the standard means to assess for bipolar symptoms.12 Considering the dearth of child psychiatrists nationally, it would be useful to improve one’s screening in primary care so as to not inadvertently “refer out” all patients for whom mood dysregulation is a concern.
There is also a more expanded tool that includes several scales integrated with clinical information (parent’s age of mood disorder onset, child’s age) which can culminate in a risk score.13
Lastly, I provide my patients with a handout of the Young Mania Rating Scale to take home as a reference and to complete before our next visit.14
You can repeat scales to monitor for more striking bipolar disorder signs and symptoms that emerge over the course of one’s longitudinal treatment of a pediatric patient. This can be an ongoing, episodic assessment since the emergence of bipolar disorder has been shown to range from the teenage years and beyond into the 20s and sometimes 30s.
Case, continued
Carrie presents to you again while in her first semester of college at the age of 19. She is taking a leave of absence after she began experimenting with cocaine at college and had a manic episode characterized by a lack of sleep without fatigue, persistent unabating energy, rapid and pressured speech, and ultimately, concern from her college friends. She was admitted to a psychiatric unit and stabilized on a second-generation antipsychotic, risperidone, which has solid evidence for mania, but she and you are now concerned about longer-term metabolic effects.15,16
You discuss monitoring her lipid profile and hemoglobin A1c, in addition to weight gain and waist circumference. She has connected with a therapist and psychiatrist through the college counseling center and hopes to return next semester with a fresh start and commitment to sobriety and social rhythms therapy known to be helpful for patients with bipolar disorder.17
While it is challenging to manage a chronic illness at her age, she feels hopeful that she can make better choices for her overall health with your support and the support of her family and mental health team.
Dr. Pawlowski is a child and adolescent consulting psychiatrist. She is a division chief at the University of Vermont Medical Center, Burlington, where she focuses on primary care mental health integration within primary care pediatrics, internal medicine, and family medicine.
References
1. Bipolar Disord. 2016 Jan 9 doi: 10.1111/bdi.12358.
2. Int J Bipolar Disord. 2021 Jun 25. doi: 10.1186/s40345-021-00225-5.
3. Am J Psychiatry. 2018 Dec 11. doi: 10.1176/appi.ajp.2018.18040461.
4. DSM-5 Changes: Implications for Child Serious Emotional Disturbance. Rockville, Md.: Substance Abuse and Mental Health Services Administration, 2016.
5. The CRAFFT tool.
6. General Behavior Inventory. Parent Version (P-GBI) Short Form – H/B (Revised Version, 2008).
7. Child Mania Rating Scale, Parent Version (CMRS-P).
8. https://www.moodtracker.com.
9. J Clin Psychiatry. 2000 Sep. doi: 10.4088/jcp.v61n0906.
10. Int J Bipolar Disord. 2020 Apr 20. doi: 10.1186/s40345-020-00185-2.
11. Int J Bipolar Disord. 2021 Jun 25. doi: 10.1186/s40345-021-00225-5.
12. Bipolar Disord. 2017 Sep 25. doi: 10.1111/bdi.12556.
13. www.cabsresearch.pitt.edu/bpriskcalculator/.
14. Parent Version of the Young Mania Rating Scale (PYMRS).
15. Arch Gen Psychiatry. 2012 Jan 2. doi: 10.1001/archgenpsychiatry.2011.1508.
16. The Carlat Child Psychiatry Report. “Bipolar Disorder” Newburyport, Mass.: Carlat Publishing, 2012.
17. https://www.ipsrt.org/.
The assessment and diagnosis of bipolar disorder in youth has a complicated and controversial history. I recall from my child and adolescent fellowship training that there was a thinly veiled faculty argument about the diagnosis itself with strong opinions on each side. To revisit this quandary, I reviewed the most up-to-date literature and outlined a case-based approach to the initial screening assessment. Certainly, the assessment by a child and adolescent psychiatrist would be the standard for diagnosis, but we do know that the pediatrician’s office may be the first setting for a child and parent to present with mood symptoms and concerns about bipolar disorder. What can you do to address this adolescent, Carrie, and her mother’s concerns?
Case
Carrie is a 17-year-old girl who has struggled through her childhood and adolescence with anxious and depressive symptoms which have ebbed and flowed with major life stressors, including her parent’s divorce. She has tried cognitive-behavioral therapy and selective serotonin reuptake inhibitors, but the SSRI seemed to cause feelings of anxiousness and agitation, so she stopped it within weeks.
Her mother presents to you concerned that Carrie has had a more persistently irritable mood toward her, often just wanting to be with her friends or otherwise isolate in her room when home to study.
Most concerning to her mother is that Carrie, as a straight A student, has also developed a pattern of staying up all night to study for tests and then “crashes” and sleeps through the weekend, avoiding her mother and only brightening with her friends.
To complicate matters, Carrie’s biological father had type 1 bipolar disorder and an addiction. Her mother comes to you with an initially nonparticipatory Carrie in tow and says: “My former husband began his manic episodes with a lack of sleep and Carrie is so irritable towards me. I feel like I am walking on eggshells all the time. Could this be bipolar disorder?”
Case discussion
First, it’s always useful to frame a visit stating that you will spend some time with the patient and some time with both the patient and parent. Emphasizing confidentiality about issues such as drug use, which can be comorbid with mood symptoms and go undetected in high-achieving students such as Carrie, is also important. Further emphasizing that information will not be reflexively shared with the parent unless the child presents a danger to herself or others is also paramount to receive an honest report of symptoms.
Second, there are many signs and symptoms of bipolar disorder that naturally overlap with other conditions such as distractibility with attention-deficit/hyperactivity disorder, or irritability in either a unipolar depression or disruptive mood dysregulation disorder.1 You are looking for an episodic (not chronic) course of symptoms with episodes that last over 5 days for hypomania and over the course of weeks for mania all while meeting all the classic criteria for bipolar disorder.
Note that the broadening of diagnostic criteria has been thought to contribute to an inflated sense of prevalence. The actual expert estimate of prevalence is around 0.8%-1.8% in pediatric populations, although there is a large published range depending on whether the criteria are modified or not.2 Use of the unmodified criteria from the DSM-5 is the recommended approach. Bipolar disorder is exceedingly rare in prepubertal children, and it would be more common for prodromal symptoms such as Carrie’s to emerge and escalate over the teenage years, culminating in a clearer diagnosis in the later teens or 20s.3
In my screening questions, I find the idea of an “infatiguable state” is the most pathognomonic one in considering mania in bipolar disorder.4 Carrie’s “crashing” after nights of studying shows that she clearly fatigues. Patients with bipolar disorder within episodes of hypomania or mania have a seismic shift in perceived energy and a matching lack of ability to sleep that can affect their thought processes, speech, and decision-making. At first blush, Carrie’s history does not indicate current symptoms of bipolar disorder.3
Case, continued
When you meet with Carrie alone she shares that she has been experimenting with prescribed stimulants from her older college-aged brother in order to study and ace her tests. She is also experimenting with alcohol and marijuana with her friends. You provide her the CRAFFT tool to deepen your screening of this issue.5
With her mother, you administer the Parent General Behavior Inventory6 and the and the Child Mania Rating Scale7. From these scales, you note that the irritability is more specific to Carrie’s family than pan-present in school and with friends. Her lack of sleep occurs at high-pressure and discreet times.
At this point, you reassure Carrie and her mother that Carrie does not present with symptoms of bipolar disorder but that certainly you will continue screening assessments over time, as they are a good means to track symptoms. You also recommend that Carrie consider mood tracking so she can develop insights into her mood and its relationship to sleep and other events as she prepares for college.8
Case discussion, continued
The strongest risk factor for bipolar disorder in youth is family history (specifically a parent) with bipolar disorder).9 If there is the chance to explore the parent’s illness with open-ended questions, you will want to hear about the parent’s age of symptom onset, course of treatment, any hospitalizations, and stabilizing medications because this has prognostic power for your patient. It is important to ensure that the parent indeed has a diagnosis of bipolar disorder and that it is not just being used colloquially to characterize an adult who has labile moods from hour to hour or day to day. This would give undue anticipatory anxiety to a youth about their risk, which is up to 8- to 10-fold greater with a parent with bipolar disorder.9
Even with a strong family history, we do not often see bipolar disorder emerge in prepubertal children.10,11 There may be still concerning prodromal symptoms in which a diagnosis of unipolar depression with more irritable features and mood lability seems more commonly complicated by substance use, as with Carrie.
Activation with an SSRI, as in Carrie’s case, even if not resulting in full mania or hypomania, can also be a soft sign of the serotonergic sensitivity present in bipolar disorder. However, if there are not additional symptoms of bipolar disorder and you are concerned based on family history alone, you do not want to withhold antidepressant treatment because fear of risk. You would want to consider a “dose low and go slow” titration process with more frequent monitoring.
A diagnostic interview with a child and adolescent psychiatrist and administration of scales such as the Young Mania Rating Scale and the Modified Child Depression Rating Scale are the standard means to assess for bipolar symptoms.12 Considering the dearth of child psychiatrists nationally, it would be useful to improve one’s screening in primary care so as to not inadvertently “refer out” all patients for whom mood dysregulation is a concern.
There is also a more expanded tool that includes several scales integrated with clinical information (parent’s age of mood disorder onset, child’s age) which can culminate in a risk score.13
Lastly, I provide my patients with a handout of the Young Mania Rating Scale to take home as a reference and to complete before our next visit.14
You can repeat scales to monitor for more striking bipolar disorder signs and symptoms that emerge over the course of one’s longitudinal treatment of a pediatric patient. This can be an ongoing, episodic assessment since the emergence of bipolar disorder has been shown to range from the teenage years and beyond into the 20s and sometimes 30s.
Case, continued
Carrie presents to you again while in her first semester of college at the age of 19. She is taking a leave of absence after she began experimenting with cocaine at college and had a manic episode characterized by a lack of sleep without fatigue, persistent unabating energy, rapid and pressured speech, and ultimately, concern from her college friends. She was admitted to a psychiatric unit and stabilized on a second-generation antipsychotic, risperidone, which has solid evidence for mania, but she and you are now concerned about longer-term metabolic effects.15,16
You discuss monitoring her lipid profile and hemoglobin A1c, in addition to weight gain and waist circumference. She has connected with a therapist and psychiatrist through the college counseling center and hopes to return next semester with a fresh start and commitment to sobriety and social rhythms therapy known to be helpful for patients with bipolar disorder.17
While it is challenging to manage a chronic illness at her age, she feels hopeful that she can make better choices for her overall health with your support and the support of her family and mental health team.
Dr. Pawlowski is a child and adolescent consulting psychiatrist. She is a division chief at the University of Vermont Medical Center, Burlington, where she focuses on primary care mental health integration within primary care pediatrics, internal medicine, and family medicine.
References
1. Bipolar Disord. 2016 Jan 9 doi: 10.1111/bdi.12358.
2. Int J Bipolar Disord. 2021 Jun 25. doi: 10.1186/s40345-021-00225-5.
3. Am J Psychiatry. 2018 Dec 11. doi: 10.1176/appi.ajp.2018.18040461.
4. DSM-5 Changes: Implications for Child Serious Emotional Disturbance. Rockville, Md.: Substance Abuse and Mental Health Services Administration, 2016.
5. The CRAFFT tool.
6. General Behavior Inventory. Parent Version (P-GBI) Short Form – H/B (Revised Version, 2008).
7. Child Mania Rating Scale, Parent Version (CMRS-P).
8. https://www.moodtracker.com.
9. J Clin Psychiatry. 2000 Sep. doi: 10.4088/jcp.v61n0906.
10. Int J Bipolar Disord. 2020 Apr 20. doi: 10.1186/s40345-020-00185-2.
11. Int J Bipolar Disord. 2021 Jun 25. doi: 10.1186/s40345-021-00225-5.
12. Bipolar Disord. 2017 Sep 25. doi: 10.1111/bdi.12556.
13. www.cabsresearch.pitt.edu/bpriskcalculator/.
14. Parent Version of the Young Mania Rating Scale (PYMRS).
15. Arch Gen Psychiatry. 2012 Jan 2. doi: 10.1001/archgenpsychiatry.2011.1508.
16. The Carlat Child Psychiatry Report. “Bipolar Disorder” Newburyport, Mass.: Carlat Publishing, 2012.
17. https://www.ipsrt.org/.
The assessment and diagnosis of bipolar disorder in youth has a complicated and controversial history. I recall from my child and adolescent fellowship training that there was a thinly veiled faculty argument about the diagnosis itself with strong opinions on each side. To revisit this quandary, I reviewed the most up-to-date literature and outlined a case-based approach to the initial screening assessment. Certainly, the assessment by a child and adolescent psychiatrist would be the standard for diagnosis, but we do know that the pediatrician’s office may be the first setting for a child and parent to present with mood symptoms and concerns about bipolar disorder. What can you do to address this adolescent, Carrie, and her mother’s concerns?
Case
Carrie is a 17-year-old girl who has struggled through her childhood and adolescence with anxious and depressive symptoms which have ebbed and flowed with major life stressors, including her parent’s divorce. She has tried cognitive-behavioral therapy and selective serotonin reuptake inhibitors, but the SSRI seemed to cause feelings of anxiousness and agitation, so she stopped it within weeks.
Her mother presents to you concerned that Carrie has had a more persistently irritable mood toward her, often just wanting to be with her friends or otherwise isolate in her room when home to study.
Most concerning to her mother is that Carrie, as a straight A student, has also developed a pattern of staying up all night to study for tests and then “crashes” and sleeps through the weekend, avoiding her mother and only brightening with her friends.
To complicate matters, Carrie’s biological father had type 1 bipolar disorder and an addiction. Her mother comes to you with an initially nonparticipatory Carrie in tow and says: “My former husband began his manic episodes with a lack of sleep and Carrie is so irritable towards me. I feel like I am walking on eggshells all the time. Could this be bipolar disorder?”
Case discussion
First, it’s always useful to frame a visit stating that you will spend some time with the patient and some time with both the patient and parent. Emphasizing confidentiality about issues such as drug use, which can be comorbid with mood symptoms and go undetected in high-achieving students such as Carrie, is also important. Further emphasizing that information will not be reflexively shared with the parent unless the child presents a danger to herself or others is also paramount to receive an honest report of symptoms.
Second, there are many signs and symptoms of bipolar disorder that naturally overlap with other conditions such as distractibility with attention-deficit/hyperactivity disorder, or irritability in either a unipolar depression or disruptive mood dysregulation disorder.1 You are looking for an episodic (not chronic) course of symptoms with episodes that last over 5 days for hypomania and over the course of weeks for mania all while meeting all the classic criteria for bipolar disorder.
Note that the broadening of diagnostic criteria has been thought to contribute to an inflated sense of prevalence. The actual expert estimate of prevalence is around 0.8%-1.8% in pediatric populations, although there is a large published range depending on whether the criteria are modified or not.2 Use of the unmodified criteria from the DSM-5 is the recommended approach. Bipolar disorder is exceedingly rare in prepubertal children, and it would be more common for prodromal symptoms such as Carrie’s to emerge and escalate over the teenage years, culminating in a clearer diagnosis in the later teens or 20s.3
In my screening questions, I find the idea of an “infatiguable state” is the most pathognomonic one in considering mania in bipolar disorder.4 Carrie’s “crashing” after nights of studying shows that she clearly fatigues. Patients with bipolar disorder within episodes of hypomania or mania have a seismic shift in perceived energy and a matching lack of ability to sleep that can affect their thought processes, speech, and decision-making. At first blush, Carrie’s history does not indicate current symptoms of bipolar disorder.3
Case, continued
When you meet with Carrie alone she shares that she has been experimenting with prescribed stimulants from her older college-aged brother in order to study and ace her tests. She is also experimenting with alcohol and marijuana with her friends. You provide her the CRAFFT tool to deepen your screening of this issue.5
With her mother, you administer the Parent General Behavior Inventory6 and the and the Child Mania Rating Scale7. From these scales, you note that the irritability is more specific to Carrie’s family than pan-present in school and with friends. Her lack of sleep occurs at high-pressure and discreet times.
At this point, you reassure Carrie and her mother that Carrie does not present with symptoms of bipolar disorder but that certainly you will continue screening assessments over time, as they are a good means to track symptoms. You also recommend that Carrie consider mood tracking so she can develop insights into her mood and its relationship to sleep and other events as she prepares for college.8
Case discussion, continued
The strongest risk factor for bipolar disorder in youth is family history (specifically a parent) with bipolar disorder).9 If there is the chance to explore the parent’s illness with open-ended questions, you will want to hear about the parent’s age of symptom onset, course of treatment, any hospitalizations, and stabilizing medications because this has prognostic power for your patient. It is important to ensure that the parent indeed has a diagnosis of bipolar disorder and that it is not just being used colloquially to characterize an adult who has labile moods from hour to hour or day to day. This would give undue anticipatory anxiety to a youth about their risk, which is up to 8- to 10-fold greater with a parent with bipolar disorder.9
Even with a strong family history, we do not often see bipolar disorder emerge in prepubertal children.10,11 There may be still concerning prodromal symptoms in which a diagnosis of unipolar depression with more irritable features and mood lability seems more commonly complicated by substance use, as with Carrie.
Activation with an SSRI, as in Carrie’s case, even if not resulting in full mania or hypomania, can also be a soft sign of the serotonergic sensitivity present in bipolar disorder. However, if there are not additional symptoms of bipolar disorder and you are concerned based on family history alone, you do not want to withhold antidepressant treatment because fear of risk. You would want to consider a “dose low and go slow” titration process with more frequent monitoring.
A diagnostic interview with a child and adolescent psychiatrist and administration of scales such as the Young Mania Rating Scale and the Modified Child Depression Rating Scale are the standard means to assess for bipolar symptoms.12 Considering the dearth of child psychiatrists nationally, it would be useful to improve one’s screening in primary care so as to not inadvertently “refer out” all patients for whom mood dysregulation is a concern.
There is also a more expanded tool that includes several scales integrated with clinical information (parent’s age of mood disorder onset, child’s age) which can culminate in a risk score.13
Lastly, I provide my patients with a handout of the Young Mania Rating Scale to take home as a reference and to complete before our next visit.14
You can repeat scales to monitor for more striking bipolar disorder signs and symptoms that emerge over the course of one’s longitudinal treatment of a pediatric patient. This can be an ongoing, episodic assessment since the emergence of bipolar disorder has been shown to range from the teenage years and beyond into the 20s and sometimes 30s.
Case, continued
Carrie presents to you again while in her first semester of college at the age of 19. She is taking a leave of absence after she began experimenting with cocaine at college and had a manic episode characterized by a lack of sleep without fatigue, persistent unabating energy, rapid and pressured speech, and ultimately, concern from her college friends. She was admitted to a psychiatric unit and stabilized on a second-generation antipsychotic, risperidone, which has solid evidence for mania, but she and you are now concerned about longer-term metabolic effects.15,16
You discuss monitoring her lipid profile and hemoglobin A1c, in addition to weight gain and waist circumference. She has connected with a therapist and psychiatrist through the college counseling center and hopes to return next semester with a fresh start and commitment to sobriety and social rhythms therapy known to be helpful for patients with bipolar disorder.17
While it is challenging to manage a chronic illness at her age, she feels hopeful that she can make better choices for her overall health with your support and the support of her family and mental health team.
Dr. Pawlowski is a child and adolescent consulting psychiatrist. She is a division chief at the University of Vermont Medical Center, Burlington, where she focuses on primary care mental health integration within primary care pediatrics, internal medicine, and family medicine.
References
1. Bipolar Disord. 2016 Jan 9 doi: 10.1111/bdi.12358.
2. Int J Bipolar Disord. 2021 Jun 25. doi: 10.1186/s40345-021-00225-5.
3. Am J Psychiatry. 2018 Dec 11. doi: 10.1176/appi.ajp.2018.18040461.
4. DSM-5 Changes: Implications for Child Serious Emotional Disturbance. Rockville, Md.: Substance Abuse and Mental Health Services Administration, 2016.
5. The CRAFFT tool.
6. General Behavior Inventory. Parent Version (P-GBI) Short Form – H/B (Revised Version, 2008).
7. Child Mania Rating Scale, Parent Version (CMRS-P).
8. https://www.moodtracker.com.
9. J Clin Psychiatry. 2000 Sep. doi: 10.4088/jcp.v61n0906.
10. Int J Bipolar Disord. 2020 Apr 20. doi: 10.1186/s40345-020-00185-2.
11. Int J Bipolar Disord. 2021 Jun 25. doi: 10.1186/s40345-021-00225-5.
12. Bipolar Disord. 2017 Sep 25. doi: 10.1111/bdi.12556.
13. www.cabsresearch.pitt.edu/bpriskcalculator/.
14. Parent Version of the Young Mania Rating Scale (PYMRS).
15. Arch Gen Psychiatry. 2012 Jan 2. doi: 10.1001/archgenpsychiatry.2011.1508.
16. The Carlat Child Psychiatry Report. “Bipolar Disorder” Newburyport, Mass.: Carlat Publishing, 2012.
17. https://www.ipsrt.org/.
The pediatrician’s office may be the first setting for a child to present with mood symptoms.
Self-management app may boost quality of life
In a randomized clinical trial of usual care plus the experimental smartphone-based intervention known as LiveWell vs. usual care alone, participants in the smartphone group who were categorized as low-risk or in asymptomatic recovery at baseline also showed reduced manic symptom severity.
The results suggest that “apps for individuals with bipolar disorder will likely be useful for some people in managing medication use, sleep duration, routine, and monitoring for and managing signs and symptoms” of the disorder, coinvestigator Evan H. Goulding, MD, PhD, assistant professor of psychiatry and behavioral sciences, Northwestern University, Chicago, told this news organization.
Use of the app may also “lead to decreased recurrence of mood episodes, impact overall depressive and manic symptom levels, and improve some aspects of quality of life,” Dr. Goulding added.
The findings were published online in JAMA Psychiatry.
Daily check-ins
The researchers randomly assigned 205 patients with BD to receive either usual care (n = 81; 56% women; mean age, 39 years) or usual care plus the smartphone-based self-management intervention LiveWell (n = 124; 65% women; mean age, 43 years) between March 2017 and April 2020. To be included, participants could not be experiencing a current mood episode or suicidal ideation.
The smartphone intervention included a daily check-in to monitor medication adherence, sleep, and wellness levels; coach visits to support adherence to the app; six phone calls over 16 weeks; and support from mental health professionals whenever needed. Participants in this group were asked to engage their mental health providers in the intervention as well.
Each participant in the control group had a visit with a coach who facilitated self-management support.
Investigators assessed all participants every 8 weeks until week 48 to gather information on mood symptoms and severity over the past 2 weeks and on quality of life.
The patients were also stratified into high- and low-risk relapse groups. The low-risk group was in asymptomatic recovery, meaning that they experienced two or fewer moderate symptoms of mania or depression in the previous 8 weeks. In addition, they had no moderate symptoms of mania or depression at study enrollment.
Patients in the high-risk group were recovering from an episode of mania or depression. They also had two or fewer moderate symptoms, but for 8 weeks or less.
Low-risk group fares better
Results showed that the smartphone intervention was significantly associated with a reduction in depressive symptoms vs. usual care (P = .02), as well as improvement in one aspect of the World Health Organization Quality of Life Assessment that measures social relationships (P = .02).
When the investigators stratified participants into risk groups, they found that for those in the low-risk group the smartphone-based intervention was associated with lower episode-relapse rates, lower mean percentage time symptomatic, and decreased manic symptom severity.
Mean estimated relapse rates by 48 weeks for the low-risk group were 12% for those in the intervention group and 37.2% for those in the control group. No differences were noted for the high-risk group.
Low-risk patients in the intervention group also had lower mean percentage-time symptomatic (17.9%) than those in the control group (26.1%) (Cohen d = .31).
“Our results are consistent with literature emphasizing the identification and facilitation of management plans for early warning signs of mood episodes and using these plans as an important self-management technique for avoiding relapse,” Dr. Goulding said.
Study limitations included low engagement by mental health professionals and low data generalizability to other populations, as the sample was mostly White (84% of the app group and 81% of the control group).
“There is a fairly large literature on risk factors, longitudinal trajectories, and stages of diseases that suggest we should already be able to predict relapse risk for individuals,” Dr. Goulding said.
“However, moving from overall risk to individual risk is trickier and will require larger datasets with longer follow-up to better understand what types of help should be delivered when and to whom,” he added.
‘Requires commitment’
John Torous, MD, director of the division of digital psychiatry at Beth Israel Deaconess Medical Center, Boston, noted that mental health apps such as LiveWell require “time and energy devoted by both the patient and their clinician for maximal efficacy, which requires commitment from and training for both parties as well.
“But with such an investment in people, there is good evidence apps can help people with bipolar disorder even during the more severe periods of the illness,” added Dr. Torous, who was not involved with the research.
The study was funded by the National Institute of Mental Health.
Dr. Goulding reports having received honoraria from Otsuka. Dr. Torous has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized clinical trial of usual care plus the experimental smartphone-based intervention known as LiveWell vs. usual care alone, participants in the smartphone group who were categorized as low-risk or in asymptomatic recovery at baseline also showed reduced manic symptom severity.
The results suggest that “apps for individuals with bipolar disorder will likely be useful for some people in managing medication use, sleep duration, routine, and monitoring for and managing signs and symptoms” of the disorder, coinvestigator Evan H. Goulding, MD, PhD, assistant professor of psychiatry and behavioral sciences, Northwestern University, Chicago, told this news organization.
Use of the app may also “lead to decreased recurrence of mood episodes, impact overall depressive and manic symptom levels, and improve some aspects of quality of life,” Dr. Goulding added.
The findings were published online in JAMA Psychiatry.
Daily check-ins
The researchers randomly assigned 205 patients with BD to receive either usual care (n = 81; 56% women; mean age, 39 years) or usual care plus the smartphone-based self-management intervention LiveWell (n = 124; 65% women; mean age, 43 years) between March 2017 and April 2020. To be included, participants could not be experiencing a current mood episode or suicidal ideation.
The smartphone intervention included a daily check-in to monitor medication adherence, sleep, and wellness levels; coach visits to support adherence to the app; six phone calls over 16 weeks; and support from mental health professionals whenever needed. Participants in this group were asked to engage their mental health providers in the intervention as well.
Each participant in the control group had a visit with a coach who facilitated self-management support.
Investigators assessed all participants every 8 weeks until week 48 to gather information on mood symptoms and severity over the past 2 weeks and on quality of life.
The patients were also stratified into high- and low-risk relapse groups. The low-risk group was in asymptomatic recovery, meaning that they experienced two or fewer moderate symptoms of mania or depression in the previous 8 weeks. In addition, they had no moderate symptoms of mania or depression at study enrollment.
Patients in the high-risk group were recovering from an episode of mania or depression. They also had two or fewer moderate symptoms, but for 8 weeks or less.
Low-risk group fares better
Results showed that the smartphone intervention was significantly associated with a reduction in depressive symptoms vs. usual care (P = .02), as well as improvement in one aspect of the World Health Organization Quality of Life Assessment that measures social relationships (P = .02).
When the investigators stratified participants into risk groups, they found that for those in the low-risk group the smartphone-based intervention was associated with lower episode-relapse rates, lower mean percentage time symptomatic, and decreased manic symptom severity.
Mean estimated relapse rates by 48 weeks for the low-risk group were 12% for those in the intervention group and 37.2% for those in the control group. No differences were noted for the high-risk group.
Low-risk patients in the intervention group also had lower mean percentage-time symptomatic (17.9%) than those in the control group (26.1%) (Cohen d = .31).
“Our results are consistent with literature emphasizing the identification and facilitation of management plans for early warning signs of mood episodes and using these plans as an important self-management technique for avoiding relapse,” Dr. Goulding said.
Study limitations included low engagement by mental health professionals and low data generalizability to other populations, as the sample was mostly White (84% of the app group and 81% of the control group).
“There is a fairly large literature on risk factors, longitudinal trajectories, and stages of diseases that suggest we should already be able to predict relapse risk for individuals,” Dr. Goulding said.
“However, moving from overall risk to individual risk is trickier and will require larger datasets with longer follow-up to better understand what types of help should be delivered when and to whom,” he added.
‘Requires commitment’
John Torous, MD, director of the division of digital psychiatry at Beth Israel Deaconess Medical Center, Boston, noted that mental health apps such as LiveWell require “time and energy devoted by both the patient and their clinician for maximal efficacy, which requires commitment from and training for both parties as well.
“But with such an investment in people, there is good evidence apps can help people with bipolar disorder even during the more severe periods of the illness,” added Dr. Torous, who was not involved with the research.
The study was funded by the National Institute of Mental Health.
Dr. Goulding reports having received honoraria from Otsuka. Dr. Torous has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized clinical trial of usual care plus the experimental smartphone-based intervention known as LiveWell vs. usual care alone, participants in the smartphone group who were categorized as low-risk or in asymptomatic recovery at baseline also showed reduced manic symptom severity.
The results suggest that “apps for individuals with bipolar disorder will likely be useful for some people in managing medication use, sleep duration, routine, and monitoring for and managing signs and symptoms” of the disorder, coinvestigator Evan H. Goulding, MD, PhD, assistant professor of psychiatry and behavioral sciences, Northwestern University, Chicago, told this news organization.
Use of the app may also “lead to decreased recurrence of mood episodes, impact overall depressive and manic symptom levels, and improve some aspects of quality of life,” Dr. Goulding added.
The findings were published online in JAMA Psychiatry.
Daily check-ins
The researchers randomly assigned 205 patients with BD to receive either usual care (n = 81; 56% women; mean age, 39 years) or usual care plus the smartphone-based self-management intervention LiveWell (n = 124; 65% women; mean age, 43 years) between March 2017 and April 2020. To be included, participants could not be experiencing a current mood episode or suicidal ideation.
The smartphone intervention included a daily check-in to monitor medication adherence, sleep, and wellness levels; coach visits to support adherence to the app; six phone calls over 16 weeks; and support from mental health professionals whenever needed. Participants in this group were asked to engage their mental health providers in the intervention as well.
Each participant in the control group had a visit with a coach who facilitated self-management support.
Investigators assessed all participants every 8 weeks until week 48 to gather information on mood symptoms and severity over the past 2 weeks and on quality of life.
The patients were also stratified into high- and low-risk relapse groups. The low-risk group was in asymptomatic recovery, meaning that they experienced two or fewer moderate symptoms of mania or depression in the previous 8 weeks. In addition, they had no moderate symptoms of mania or depression at study enrollment.
Patients in the high-risk group were recovering from an episode of mania or depression. They also had two or fewer moderate symptoms, but for 8 weeks or less.
Low-risk group fares better
Results showed that the smartphone intervention was significantly associated with a reduction in depressive symptoms vs. usual care (P = .02), as well as improvement in one aspect of the World Health Organization Quality of Life Assessment that measures social relationships (P = .02).
When the investigators stratified participants into risk groups, they found that for those in the low-risk group the smartphone-based intervention was associated with lower episode-relapse rates, lower mean percentage time symptomatic, and decreased manic symptom severity.
Mean estimated relapse rates by 48 weeks for the low-risk group were 12% for those in the intervention group and 37.2% for those in the control group. No differences were noted for the high-risk group.
Low-risk patients in the intervention group also had lower mean percentage-time symptomatic (17.9%) than those in the control group (26.1%) (Cohen d = .31).
“Our results are consistent with literature emphasizing the identification and facilitation of management plans for early warning signs of mood episodes and using these plans as an important self-management technique for avoiding relapse,” Dr. Goulding said.
Study limitations included low engagement by mental health professionals and low data generalizability to other populations, as the sample was mostly White (84% of the app group and 81% of the control group).
“There is a fairly large literature on risk factors, longitudinal trajectories, and stages of diseases that suggest we should already be able to predict relapse risk for individuals,” Dr. Goulding said.
“However, moving from overall risk to individual risk is trickier and will require larger datasets with longer follow-up to better understand what types of help should be delivered when and to whom,” he added.
‘Requires commitment’
John Torous, MD, director of the division of digital psychiatry at Beth Israel Deaconess Medical Center, Boston, noted that mental health apps such as LiveWell require “time and energy devoted by both the patient and their clinician for maximal efficacy, which requires commitment from and training for both parties as well.
“But with such an investment in people, there is good evidence apps can help people with bipolar disorder even during the more severe periods of the illness,” added Dr. Torous, who was not involved with the research.
The study was funded by the National Institute of Mental Health.
Dr. Goulding reports having received honoraria from Otsuka. Dr. Torous has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Postconcussion symptoms tied to high risk of depression
Results of a large meta-analysis that included 18 studies and more than 9,000 patients showed a fourfold higher risk of developing depressive symptoms in those with PPCS versus those without PPCS.
“In this meta-analysis, experiencing PPCS was associated with a higher risk of experiencing depressive symptoms,” write the investigators, led by Maude Lambert, PhD, of the School of Psychology, University of Ottawa, and Bloorview Research Institute, Toronto.
“There are several important clinical and health policy implications of the findings. Most notably, the development of strategies for effective prevention and earlier intervention to optimize mental health recovery following a concussion should be supported,” they add.
The study was published online in JAMA Network Open.
‘Important minority’
An “important minority” of 15%-30% of those with concussions continue to experience symptoms for months, or even years, following the injury, the investigators note.
Symptoms vary but can include headaches, fatigue, dizziness, cognitive difficulties, and emotional changes, which can “significantly impact an individual’s everyday functioning.”
The association between PPCS and mental health outcomes “has emerged as an area of interest” over the past decade, with multiple studies pointing to bidirectional associations between depressive symptoms and PPCS, the researchers note. Individuals with PPCS are at significantly higher risk of experiencing depressive symptoms, and depressive symptoms, in turn, predict more prolonged postconcussion recovery, they add.
The authors conducted a previous scoping review that showed individuals with PPCS had “greater mental health difficulties than individuals who recovered from concussion or healthy controls.”
But “quantitative summaries evaluating the magnitude and nature of the association between PPCS and mental health outcomes were not conducted,” so they decided to conduct a follow-up meta-analysis to corroborate the hypothesis that PPCS may be associated with depressive symptoms.
The researchers also wanted to “investigate potential moderators of that association and determine whether the association between depressive symptoms and PPCS differed based on age, sex, mental illness, history of concussion, and time since the injury.”
This could have “significant public health implications” as it represents an “important step” toward understanding the association between PPCS and mental health, paving the way for the “development of optimal postconcussion intervention strategies, targeting effective prevention and earlier intervention to enhance recovery trajectories, improve mental health, and promote well-being following concussion.”
To be included in the meta-analysis, a study had to focus on participants who had experienced a concussion, diagnosed by a health care professional, or as classified by diagnostic measures, and who experienced greater than or equal to 1 concussion symptom lasting greater than 4 weeks.
There was no explicit upper limit on duration, and individuals of all ages were eligible.
Depressive symptoms were defined as “an outcome that must be measured by a validated and standardized measure of depression.”
Biopsychosocial model
Of 580 reports assessed for eligibility, 18 were included in the meta-analysis, incorporating a total of 9,101 participants, with a median (range) sample size of 154 (48-4,462) participants and a mean (SD) participant age of 33.7 (14.4) years.
The mean length of time since the concussion was 21.3 (18.7) weeks. Of the participants, a mean of 36.1% (11.1%) had a history of greater than or equal to 2 concussions.
Close to three-quarters of the studies (72%) used a cross-sectional design, with most studies conducted in North America, and the remaining conducted in Europe, China, and New Zealand.
The researchers found a “significant positive association” between PPCS and postinjury depressive symptoms (odds ratio, 4.87; 95% confidence interval, 3.01-7.90; P < .001), “representing a large effect size.”
Funnel plot and Egger test analyses “suggested the presence of a publication bias.” However, even after accounting for publication bias, the effect size “of large magnitude” remained, the authors report (OR, 4.56; 95% CI, 2.82-7.37; P < .001).
No significant moderators were identified, “likely due to the small number of studies included,” they speculate.
They note that the current study “does not allow inference about the causal directionality of the association” between PPCS and postinjury depressive symptoms, so the question remains: Do PPCS induce depressive symptoms, or do depressive symptoms induce PPCS?”
Despite this unanswered question, the findings still have important clinical and public health implications, highlighting “the need for a greater understanding of the mechanisms of development and etiology of depressive symptoms postconcussion” and emphasizing “the necessary emergence for timely and effective treatment interventions for depressive symptoms to optimize the long-term prognosis of concussion,” the authors note.
They add that several research teams “have aimed to gain more insight into the etiology and underlying mechanisms of development and course of mental health difficulties in individuals who experience a concussion” and have arrived at a biopsychosocial framework, in light of “the myriad of contributing physiological, biological, and psychosocial factors.”
They recommend the establishment of “specialized multidisciplinary or interdisciplinary concussion care programs should include health care professionals with strong clinical foundations and training in mental health conditions.”
Speedy multidisciplinary care
Commenting on the research, Charles Tator, MD, PhD, professor of neurosurgery, University of Toronto, Division of Neurosurgery, Toronto Western Hospital, said the researchers “performed a thorough systematic review” showing “emphatically that depression occurs in this population.”
Dr. Tator, the director of the Canadian Concussion Centre, who was not involved with the current study, continued: “Nowadays clinical discoveries are validated through a progression of case reports, single-center retrospective cohort studies like ours, referenced by [Dr.] Lambert et al., and then confirmatory systematic reviews, each adding important layers of evidence.”
“This evaluative process has now endorsed the importance of early treatment of mental health symptoms in patients with persisting symptoms, which can include depression, anxiety, and PTSD,” he said.
He recommended that treatment should start with family physicians and nurse practitioners “but may require escalation to psychologists and social workers and then to psychiatrists who are often more skilled in medication selection.”
He encouraged “speedy multidisciplinary care,” noting that the possibility of suicide is worrisome.
No source of study funding was listed. A study coauthor, Shannon Scratch, PhD, has reported receiving funds from the Holland Bloorview Kids Rehabilitation Hospital Foundation (via the Holland Family Professorship in Acquired Brain Injury) during the conduct of this study. No other disclosures were reported. Dr. Tator has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a large meta-analysis that included 18 studies and more than 9,000 patients showed a fourfold higher risk of developing depressive symptoms in those with PPCS versus those without PPCS.
“In this meta-analysis, experiencing PPCS was associated with a higher risk of experiencing depressive symptoms,” write the investigators, led by Maude Lambert, PhD, of the School of Psychology, University of Ottawa, and Bloorview Research Institute, Toronto.
“There are several important clinical and health policy implications of the findings. Most notably, the development of strategies for effective prevention and earlier intervention to optimize mental health recovery following a concussion should be supported,” they add.
The study was published online in JAMA Network Open.
‘Important minority’
An “important minority” of 15%-30% of those with concussions continue to experience symptoms for months, or even years, following the injury, the investigators note.
Symptoms vary but can include headaches, fatigue, dizziness, cognitive difficulties, and emotional changes, which can “significantly impact an individual’s everyday functioning.”
The association between PPCS and mental health outcomes “has emerged as an area of interest” over the past decade, with multiple studies pointing to bidirectional associations between depressive symptoms and PPCS, the researchers note. Individuals with PPCS are at significantly higher risk of experiencing depressive symptoms, and depressive symptoms, in turn, predict more prolonged postconcussion recovery, they add.
The authors conducted a previous scoping review that showed individuals with PPCS had “greater mental health difficulties than individuals who recovered from concussion or healthy controls.”
But “quantitative summaries evaluating the magnitude and nature of the association between PPCS and mental health outcomes were not conducted,” so they decided to conduct a follow-up meta-analysis to corroborate the hypothesis that PPCS may be associated with depressive symptoms.
The researchers also wanted to “investigate potential moderators of that association and determine whether the association between depressive symptoms and PPCS differed based on age, sex, mental illness, history of concussion, and time since the injury.”
This could have “significant public health implications” as it represents an “important step” toward understanding the association between PPCS and mental health, paving the way for the “development of optimal postconcussion intervention strategies, targeting effective prevention and earlier intervention to enhance recovery trajectories, improve mental health, and promote well-being following concussion.”
To be included in the meta-analysis, a study had to focus on participants who had experienced a concussion, diagnosed by a health care professional, or as classified by diagnostic measures, and who experienced greater than or equal to 1 concussion symptom lasting greater than 4 weeks.
There was no explicit upper limit on duration, and individuals of all ages were eligible.
Depressive symptoms were defined as “an outcome that must be measured by a validated and standardized measure of depression.”
Biopsychosocial model
Of 580 reports assessed for eligibility, 18 were included in the meta-analysis, incorporating a total of 9,101 participants, with a median (range) sample size of 154 (48-4,462) participants and a mean (SD) participant age of 33.7 (14.4) years.
The mean length of time since the concussion was 21.3 (18.7) weeks. Of the participants, a mean of 36.1% (11.1%) had a history of greater than or equal to 2 concussions.
Close to three-quarters of the studies (72%) used a cross-sectional design, with most studies conducted in North America, and the remaining conducted in Europe, China, and New Zealand.
The researchers found a “significant positive association” between PPCS and postinjury depressive symptoms (odds ratio, 4.87; 95% confidence interval, 3.01-7.90; P < .001), “representing a large effect size.”
Funnel plot and Egger test analyses “suggested the presence of a publication bias.” However, even after accounting for publication bias, the effect size “of large magnitude” remained, the authors report (OR, 4.56; 95% CI, 2.82-7.37; P < .001).
No significant moderators were identified, “likely due to the small number of studies included,” they speculate.
They note that the current study “does not allow inference about the causal directionality of the association” between PPCS and postinjury depressive symptoms, so the question remains: Do PPCS induce depressive symptoms, or do depressive symptoms induce PPCS?”
Despite this unanswered question, the findings still have important clinical and public health implications, highlighting “the need for a greater understanding of the mechanisms of development and etiology of depressive symptoms postconcussion” and emphasizing “the necessary emergence for timely and effective treatment interventions for depressive symptoms to optimize the long-term prognosis of concussion,” the authors note.
They add that several research teams “have aimed to gain more insight into the etiology and underlying mechanisms of development and course of mental health difficulties in individuals who experience a concussion” and have arrived at a biopsychosocial framework, in light of “the myriad of contributing physiological, biological, and psychosocial factors.”
They recommend the establishment of “specialized multidisciplinary or interdisciplinary concussion care programs should include health care professionals with strong clinical foundations and training in mental health conditions.”
Speedy multidisciplinary care
Commenting on the research, Charles Tator, MD, PhD, professor of neurosurgery, University of Toronto, Division of Neurosurgery, Toronto Western Hospital, said the researchers “performed a thorough systematic review” showing “emphatically that depression occurs in this population.”
Dr. Tator, the director of the Canadian Concussion Centre, who was not involved with the current study, continued: “Nowadays clinical discoveries are validated through a progression of case reports, single-center retrospective cohort studies like ours, referenced by [Dr.] Lambert et al., and then confirmatory systematic reviews, each adding important layers of evidence.”
“This evaluative process has now endorsed the importance of early treatment of mental health symptoms in patients with persisting symptoms, which can include depression, anxiety, and PTSD,” he said.
He recommended that treatment should start with family physicians and nurse practitioners “but may require escalation to psychologists and social workers and then to psychiatrists who are often more skilled in medication selection.”
He encouraged “speedy multidisciplinary care,” noting that the possibility of suicide is worrisome.
No source of study funding was listed. A study coauthor, Shannon Scratch, PhD, has reported receiving funds from the Holland Bloorview Kids Rehabilitation Hospital Foundation (via the Holland Family Professorship in Acquired Brain Injury) during the conduct of this study. No other disclosures were reported. Dr. Tator has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a large meta-analysis that included 18 studies and more than 9,000 patients showed a fourfold higher risk of developing depressive symptoms in those with PPCS versus those without PPCS.
“In this meta-analysis, experiencing PPCS was associated with a higher risk of experiencing depressive symptoms,” write the investigators, led by Maude Lambert, PhD, of the School of Psychology, University of Ottawa, and Bloorview Research Institute, Toronto.
“There are several important clinical and health policy implications of the findings. Most notably, the development of strategies for effective prevention and earlier intervention to optimize mental health recovery following a concussion should be supported,” they add.
The study was published online in JAMA Network Open.
‘Important minority’
An “important minority” of 15%-30% of those with concussions continue to experience symptoms for months, or even years, following the injury, the investigators note.
Symptoms vary but can include headaches, fatigue, dizziness, cognitive difficulties, and emotional changes, which can “significantly impact an individual’s everyday functioning.”
The association between PPCS and mental health outcomes “has emerged as an area of interest” over the past decade, with multiple studies pointing to bidirectional associations between depressive symptoms and PPCS, the researchers note. Individuals with PPCS are at significantly higher risk of experiencing depressive symptoms, and depressive symptoms, in turn, predict more prolonged postconcussion recovery, they add.
The authors conducted a previous scoping review that showed individuals with PPCS had “greater mental health difficulties than individuals who recovered from concussion or healthy controls.”
But “quantitative summaries evaluating the magnitude and nature of the association between PPCS and mental health outcomes were not conducted,” so they decided to conduct a follow-up meta-analysis to corroborate the hypothesis that PPCS may be associated with depressive symptoms.
The researchers also wanted to “investigate potential moderators of that association and determine whether the association between depressive symptoms and PPCS differed based on age, sex, mental illness, history of concussion, and time since the injury.”
This could have “significant public health implications” as it represents an “important step” toward understanding the association between PPCS and mental health, paving the way for the “development of optimal postconcussion intervention strategies, targeting effective prevention and earlier intervention to enhance recovery trajectories, improve mental health, and promote well-being following concussion.”
To be included in the meta-analysis, a study had to focus on participants who had experienced a concussion, diagnosed by a health care professional, or as classified by diagnostic measures, and who experienced greater than or equal to 1 concussion symptom lasting greater than 4 weeks.
There was no explicit upper limit on duration, and individuals of all ages were eligible.
Depressive symptoms were defined as “an outcome that must be measured by a validated and standardized measure of depression.”
Biopsychosocial model
Of 580 reports assessed for eligibility, 18 were included in the meta-analysis, incorporating a total of 9,101 participants, with a median (range) sample size of 154 (48-4,462) participants and a mean (SD) participant age of 33.7 (14.4) years.
The mean length of time since the concussion was 21.3 (18.7) weeks. Of the participants, a mean of 36.1% (11.1%) had a history of greater than or equal to 2 concussions.
Close to three-quarters of the studies (72%) used a cross-sectional design, with most studies conducted in North America, and the remaining conducted in Europe, China, and New Zealand.
The researchers found a “significant positive association” between PPCS and postinjury depressive symptoms (odds ratio, 4.87; 95% confidence interval, 3.01-7.90; P < .001), “representing a large effect size.”
Funnel plot and Egger test analyses “suggested the presence of a publication bias.” However, even after accounting for publication bias, the effect size “of large magnitude” remained, the authors report (OR, 4.56; 95% CI, 2.82-7.37; P < .001).
No significant moderators were identified, “likely due to the small number of studies included,” they speculate.
They note that the current study “does not allow inference about the causal directionality of the association” between PPCS and postinjury depressive symptoms, so the question remains: Do PPCS induce depressive symptoms, or do depressive symptoms induce PPCS?”
Despite this unanswered question, the findings still have important clinical and public health implications, highlighting “the need for a greater understanding of the mechanisms of development and etiology of depressive symptoms postconcussion” and emphasizing “the necessary emergence for timely and effective treatment interventions for depressive symptoms to optimize the long-term prognosis of concussion,” the authors note.
They add that several research teams “have aimed to gain more insight into the etiology and underlying mechanisms of development and course of mental health difficulties in individuals who experience a concussion” and have arrived at a biopsychosocial framework, in light of “the myriad of contributing physiological, biological, and psychosocial factors.”
They recommend the establishment of “specialized multidisciplinary or interdisciplinary concussion care programs should include health care professionals with strong clinical foundations and training in mental health conditions.”
Speedy multidisciplinary care
Commenting on the research, Charles Tator, MD, PhD, professor of neurosurgery, University of Toronto, Division of Neurosurgery, Toronto Western Hospital, said the researchers “performed a thorough systematic review” showing “emphatically that depression occurs in this population.”
Dr. Tator, the director of the Canadian Concussion Centre, who was not involved with the current study, continued: “Nowadays clinical discoveries are validated through a progression of case reports, single-center retrospective cohort studies like ours, referenced by [Dr.] Lambert et al., and then confirmatory systematic reviews, each adding important layers of evidence.”
“This evaluative process has now endorsed the importance of early treatment of mental health symptoms in patients with persisting symptoms, which can include depression, anxiety, and PTSD,” he said.
He recommended that treatment should start with family physicians and nurse practitioners “but may require escalation to psychologists and social workers and then to psychiatrists who are often more skilled in medication selection.”
He encouraged “speedy multidisciplinary care,” noting that the possibility of suicide is worrisome.
No source of study funding was listed. A study coauthor, Shannon Scratch, PhD, has reported receiving funds from the Holland Bloorview Kids Rehabilitation Hospital Foundation (via the Holland Family Professorship in Acquired Brain Injury) during the conduct of this study. No other disclosures were reported. Dr. Tator has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Sleep complaints in major depression flag risk for other psychiatric disorders
Investigators studied 3-year incidence rates of psychiatric disorders in almost 3,000 patients experiencing an MDE. Results showed that having a history of difficulty falling asleep, early morning awakening, and hypersomnia increased risk for incident psychiatric disorders.
“The findings of this study suggest the potential value of including insomnia and hypersomnia in clinical assessments of all psychiatric disorders,” write the investigators, led by Bénédicte Barbotin, MD, Département de Psychiatrie et d’Addictologie, Assistance Publique-Hôpitaux de Paris, Hôpital Bichat-Claude Bernard, France.
“Insomnia and hypersomnia symptoms may be prodromal transdiagnostic biomarkers and easily modifiable therapeutic targets for the prevention of psychiatric disorders,” they add.
The findings were published online recently in the Journal of Clinical Psychiatry.
Bidirectional association
The researchers note that sleep disturbance is “one of the most common symptoms” associated with major depressive disorder (MDD) and may be “both a consequence and a cause.”
Moreover, improving sleep disturbances for patients with an MDE “tends to improve depressive symptom and outcomes,” they add.
Although the possibility of a bidirectional association between MDEs and sleep disturbances “offers a new perspective that sleep complaints might be a predictive prodromal symptom,” the association of sleep complaints with the subsequent development of other psychiatric disorders in MDEs “remains poorly documented,” the investigators write.
The observation that sleep complaints are associated with psychiatric complications and adverse outcomes, such as suicidality and substance overdose, suggests that longitudinal studies “may help to better understand these relationships.”
To investigate these issues, the researchers examined three sleep complaints among patients with MDE: trouble falling asleep, early morning awakening, and hypersomnia. They adjusted for an array of variables, including antisocial personality disorders, use of sedatives or tranquilizers, sociodemographic characteristics, MDE severity, poverty, obesity, educational level, and stressful life events.
They also used a “bifactor latent variable approach” to “disentangle” a number of effects, including those shared by all psychiatric disorders; those specific to dimensions of psychopathology, such as internalizing dimension; and those specific to individual psychiatric disorders, such as dysthymia.
“To our knowledge, this is the most extensive prospective assessment [ever conducted] of associations between sleep complaints and incident psychiatric disorders,” the investigators write.
They drew on data from Waves 1 and 2 of the National Epidemiological Survey on Alcohol and Related Conditions, a large nationally representative survey conducted in 2001-2002 (Wave 1) and 2004-2005 (Wave 2) by the National Institute on Alcoholism and Alcohol Abuse.
The analysis included 2,864 participants who experienced MDE in the year prior to Wave 1 and who completed interviews at both waves.
Researchers assessed past-year DSM-IV Axis I disorders and baseline sleep complaints at Wave 1, as well as incident DSM-IV Axis I disorders between the two waves – including substance use, mood, and anxiety disorders.
Screening needed?
Results showed a wide range of incidence rates for psychiatric disorders between Wave 1 and Wave 2, ranging from 2.7% for cannabis use to 8.2% for generalized anxiety disorder.
The lifetime prevalence of sleep complaints was higher among participants who developed a psychiatric disorder between the two waves than among those who did not have sleep complaints. The range (from lowest to highest percentage) is shown in the accompanying table.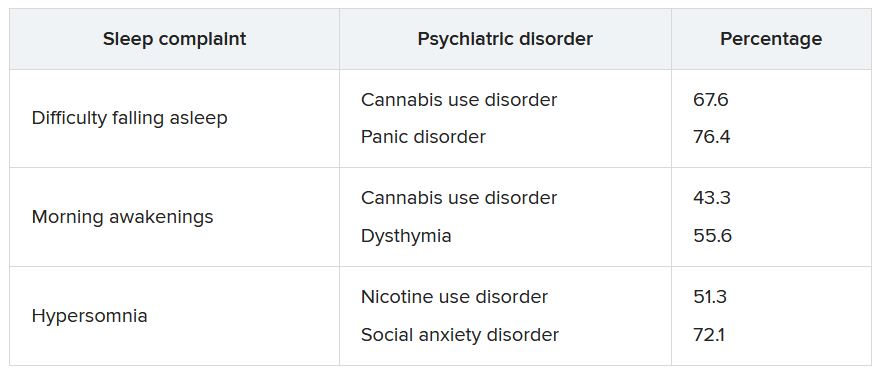
A higher number of sleep complaints was also associated with higher percentages of psychiatric disorders.
Hypersomnia, in particular, significantly increased the odds of having another psychiatric disorder. For patients with MDD who reported hypersomnia, the mean number of sleep disorders was significantly higher than for patients without hypersomnia (2.08 vs. 1.32; P < .001).
“This explains why hypersomnia appears more strongly associated with the incidence of psychiatric disorders,” the investigators write.
After adjusting for sociodemographic and clinical characteristics and antisocial personality disorder, the effects shared across all sleep complaints were “significantly associated with the incident general psychopathology factor, representing mechanisms that may lead to incidence of all psychiatric disorder in the model,” they add.
The researchers note that insomnia and hypersomnia can impair cognitive function, decision-making, problem-solving, and emotion processing networks, thereby increasing the onset of psychiatric disorders in vulnerable individuals.
Shared biological determinants, such as monoamine neurotransmitters that play a major role in depression, anxiety, substance use disorders, and the regulation of sleep stages, may also underlie both sleep disturbances and psychiatric disorders, they speculate.
“These results suggest the importance of systematically assessing insomnia and hypersomnia when evaluating psychiatric disorders and considering these symptoms as nonspecific prodromal or at-risk symptoms, also shared with suicidal behaviors,” the investigators write.
“In addition, since most individuals who developed a psychiatric disorder had at least one sleep complaint, all psychiatric disorders should be carefully screened among individuals with sleep complaints,” they add.
Transdiagnostic phenomenon
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, noted that the study replicates previous observations that a bidirectional relationship exists between sleep disturbances and mental disorders and that there “seems to be a relationship between sleep disturbance and suicidality that is bidirectional.”
He added that he appreciated the fact that the investigators “took this knowledge one step further; and what they are saying is that within the syndrome of depression, it is the sleep disturbance that is predicting future problems.”
Dr. McIntyre, who is also chairman and executive director of the Brain and Cognitive Discover Foundation in Toronto, was not involved with the study.
The data suggest that, “conceptually, sleep disturbance is a transdiagnostic phenomenon that may also be the nexus when multiple comorbid mental disorders occur,” he said.
“If this is the case, clinically, there is an opportunity here to prevent incident mental disorders in persons with depression and sleep disturbance, prioritizing sleep management in any patient with a mood disorder,” Dr. McIntyre added.
He noted that “the testable hypothesis” is how this is occurring mechanistically.
“I would conjecture that it could be inflammation and/or insulin resistance that is part of sleep disturbance that could predispose and portend other mental illnesses – and likely other medical conditions too, such as obesity and diabetes,” he said.
The study received no specific funding from any funding agency, commercial, or not-for-profit sectors. The investigators’ relevant financial relationships are listed in the original article. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China and the Milken Institute; has received speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences; and is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
Investigators studied 3-year incidence rates of psychiatric disorders in almost 3,000 patients experiencing an MDE. Results showed that having a history of difficulty falling asleep, early morning awakening, and hypersomnia increased risk for incident psychiatric disorders.
“The findings of this study suggest the potential value of including insomnia and hypersomnia in clinical assessments of all psychiatric disorders,” write the investigators, led by Bénédicte Barbotin, MD, Département de Psychiatrie et d’Addictologie, Assistance Publique-Hôpitaux de Paris, Hôpital Bichat-Claude Bernard, France.
“Insomnia and hypersomnia symptoms may be prodromal transdiagnostic biomarkers and easily modifiable therapeutic targets for the prevention of psychiatric disorders,” they add.
The findings were published online recently in the Journal of Clinical Psychiatry.
Bidirectional association
The researchers note that sleep disturbance is “one of the most common symptoms” associated with major depressive disorder (MDD) and may be “both a consequence and a cause.”
Moreover, improving sleep disturbances for patients with an MDE “tends to improve depressive symptom and outcomes,” they add.
Although the possibility of a bidirectional association between MDEs and sleep disturbances “offers a new perspective that sleep complaints might be a predictive prodromal symptom,” the association of sleep complaints with the subsequent development of other psychiatric disorders in MDEs “remains poorly documented,” the investigators write.
The observation that sleep complaints are associated with psychiatric complications and adverse outcomes, such as suicidality and substance overdose, suggests that longitudinal studies “may help to better understand these relationships.”
To investigate these issues, the researchers examined three sleep complaints among patients with MDE: trouble falling asleep, early morning awakening, and hypersomnia. They adjusted for an array of variables, including antisocial personality disorders, use of sedatives or tranquilizers, sociodemographic characteristics, MDE severity, poverty, obesity, educational level, and stressful life events.
They also used a “bifactor latent variable approach” to “disentangle” a number of effects, including those shared by all psychiatric disorders; those specific to dimensions of psychopathology, such as internalizing dimension; and those specific to individual psychiatric disorders, such as dysthymia.
“To our knowledge, this is the most extensive prospective assessment [ever conducted] of associations between sleep complaints and incident psychiatric disorders,” the investigators write.
They drew on data from Waves 1 and 2 of the National Epidemiological Survey on Alcohol and Related Conditions, a large nationally representative survey conducted in 2001-2002 (Wave 1) and 2004-2005 (Wave 2) by the National Institute on Alcoholism and Alcohol Abuse.
The analysis included 2,864 participants who experienced MDE in the year prior to Wave 1 and who completed interviews at both waves.
Researchers assessed past-year DSM-IV Axis I disorders and baseline sleep complaints at Wave 1, as well as incident DSM-IV Axis I disorders between the two waves – including substance use, mood, and anxiety disorders.
Screening needed?
Results showed a wide range of incidence rates for psychiatric disorders between Wave 1 and Wave 2, ranging from 2.7% for cannabis use to 8.2% for generalized anxiety disorder.
The lifetime prevalence of sleep complaints was higher among participants who developed a psychiatric disorder between the two waves than among those who did not have sleep complaints. The range (from lowest to highest percentage) is shown in the accompanying table.
A higher number of sleep complaints was also associated with higher percentages of psychiatric disorders.
Hypersomnia, in particular, significantly increased the odds of having another psychiatric disorder. For patients with MDD who reported hypersomnia, the mean number of sleep disorders was significantly higher than for patients without hypersomnia (2.08 vs. 1.32; P < .001).
“This explains why hypersomnia appears more strongly associated with the incidence of psychiatric disorders,” the investigators write.
After adjusting for sociodemographic and clinical characteristics and antisocial personality disorder, the effects shared across all sleep complaints were “significantly associated with the incident general psychopathology factor, representing mechanisms that may lead to incidence of all psychiatric disorder in the model,” they add.
The researchers note that insomnia and hypersomnia can impair cognitive function, decision-making, problem-solving, and emotion processing networks, thereby increasing the onset of psychiatric disorders in vulnerable individuals.
Shared biological determinants, such as monoamine neurotransmitters that play a major role in depression, anxiety, substance use disorders, and the regulation of sleep stages, may also underlie both sleep disturbances and psychiatric disorders, they speculate.
“These results suggest the importance of systematically assessing insomnia and hypersomnia when evaluating psychiatric disorders and considering these symptoms as nonspecific prodromal or at-risk symptoms, also shared with suicidal behaviors,” the investigators write.
“In addition, since most individuals who developed a psychiatric disorder had at least one sleep complaint, all psychiatric disorders should be carefully screened among individuals with sleep complaints,” they add.
Transdiagnostic phenomenon
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, noted that the study replicates previous observations that a bidirectional relationship exists between sleep disturbances and mental disorders and that there “seems to be a relationship between sleep disturbance and suicidality that is bidirectional.”
He added that he appreciated the fact that the investigators “took this knowledge one step further; and what they are saying is that within the syndrome of depression, it is the sleep disturbance that is predicting future problems.”
Dr. McIntyre, who is also chairman and executive director of the Brain and Cognitive Discover Foundation in Toronto, was not involved with the study.
The data suggest that, “conceptually, sleep disturbance is a transdiagnostic phenomenon that may also be the nexus when multiple comorbid mental disorders occur,” he said.
“If this is the case, clinically, there is an opportunity here to prevent incident mental disorders in persons with depression and sleep disturbance, prioritizing sleep management in any patient with a mood disorder,” Dr. McIntyre added.
He noted that “the testable hypothesis” is how this is occurring mechanistically.
“I would conjecture that it could be inflammation and/or insulin resistance that is part of sleep disturbance that could predispose and portend other mental illnesses – and likely other medical conditions too, such as obesity and diabetes,” he said.
The study received no specific funding from any funding agency, commercial, or not-for-profit sectors. The investigators’ relevant financial relationships are listed in the original article. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China and the Milken Institute; has received speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences; and is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
Investigators studied 3-year incidence rates of psychiatric disorders in almost 3,000 patients experiencing an MDE. Results showed that having a history of difficulty falling asleep, early morning awakening, and hypersomnia increased risk for incident psychiatric disorders.
“The findings of this study suggest the potential value of including insomnia and hypersomnia in clinical assessments of all psychiatric disorders,” write the investigators, led by Bénédicte Barbotin, MD, Département de Psychiatrie et d’Addictologie, Assistance Publique-Hôpitaux de Paris, Hôpital Bichat-Claude Bernard, France.
“Insomnia and hypersomnia symptoms may be prodromal transdiagnostic biomarkers and easily modifiable therapeutic targets for the prevention of psychiatric disorders,” they add.
The findings were published online recently in the Journal of Clinical Psychiatry.
Bidirectional association
The researchers note that sleep disturbance is “one of the most common symptoms” associated with major depressive disorder (MDD) and may be “both a consequence and a cause.”
Moreover, improving sleep disturbances for patients with an MDE “tends to improve depressive symptom and outcomes,” they add.
Although the possibility of a bidirectional association between MDEs and sleep disturbances “offers a new perspective that sleep complaints might be a predictive prodromal symptom,” the association of sleep complaints with the subsequent development of other psychiatric disorders in MDEs “remains poorly documented,” the investigators write.
The observation that sleep complaints are associated with psychiatric complications and adverse outcomes, such as suicidality and substance overdose, suggests that longitudinal studies “may help to better understand these relationships.”
To investigate these issues, the researchers examined three sleep complaints among patients with MDE: trouble falling asleep, early morning awakening, and hypersomnia. They adjusted for an array of variables, including antisocial personality disorders, use of sedatives or tranquilizers, sociodemographic characteristics, MDE severity, poverty, obesity, educational level, and stressful life events.
They also used a “bifactor latent variable approach” to “disentangle” a number of effects, including those shared by all psychiatric disorders; those specific to dimensions of psychopathology, such as internalizing dimension; and those specific to individual psychiatric disorders, such as dysthymia.
“To our knowledge, this is the most extensive prospective assessment [ever conducted] of associations between sleep complaints and incident psychiatric disorders,” the investigators write.
They drew on data from Waves 1 and 2 of the National Epidemiological Survey on Alcohol and Related Conditions, a large nationally representative survey conducted in 2001-2002 (Wave 1) and 2004-2005 (Wave 2) by the National Institute on Alcoholism and Alcohol Abuse.
The analysis included 2,864 participants who experienced MDE in the year prior to Wave 1 and who completed interviews at both waves.
Researchers assessed past-year DSM-IV Axis I disorders and baseline sleep complaints at Wave 1, as well as incident DSM-IV Axis I disorders between the two waves – including substance use, mood, and anxiety disorders.
Screening needed?
Results showed a wide range of incidence rates for psychiatric disorders between Wave 1 and Wave 2, ranging from 2.7% for cannabis use to 8.2% for generalized anxiety disorder.
The lifetime prevalence of sleep complaints was higher among participants who developed a psychiatric disorder between the two waves than among those who did not have sleep complaints. The range (from lowest to highest percentage) is shown in the accompanying table.
A higher number of sleep complaints was also associated with higher percentages of psychiatric disorders.
Hypersomnia, in particular, significantly increased the odds of having another psychiatric disorder. For patients with MDD who reported hypersomnia, the mean number of sleep disorders was significantly higher than for patients without hypersomnia (2.08 vs. 1.32; P < .001).
“This explains why hypersomnia appears more strongly associated with the incidence of psychiatric disorders,” the investigators write.
After adjusting for sociodemographic and clinical characteristics and antisocial personality disorder, the effects shared across all sleep complaints were “significantly associated with the incident general psychopathology factor, representing mechanisms that may lead to incidence of all psychiatric disorder in the model,” they add.
The researchers note that insomnia and hypersomnia can impair cognitive function, decision-making, problem-solving, and emotion processing networks, thereby increasing the onset of psychiatric disorders in vulnerable individuals.
Shared biological determinants, such as monoamine neurotransmitters that play a major role in depression, anxiety, substance use disorders, and the regulation of sleep stages, may also underlie both sleep disturbances and psychiatric disorders, they speculate.
“These results suggest the importance of systematically assessing insomnia and hypersomnia when evaluating psychiatric disorders and considering these symptoms as nonspecific prodromal or at-risk symptoms, also shared with suicidal behaviors,” the investigators write.
“In addition, since most individuals who developed a psychiatric disorder had at least one sleep complaint, all psychiatric disorders should be carefully screened among individuals with sleep complaints,” they add.
Transdiagnostic phenomenon
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, noted that the study replicates previous observations that a bidirectional relationship exists between sleep disturbances and mental disorders and that there “seems to be a relationship between sleep disturbance and suicidality that is bidirectional.”
He added that he appreciated the fact that the investigators “took this knowledge one step further; and what they are saying is that within the syndrome of depression, it is the sleep disturbance that is predicting future problems.”
Dr. McIntyre, who is also chairman and executive director of the Brain and Cognitive Discover Foundation in Toronto, was not involved with the study.
The data suggest that, “conceptually, sleep disturbance is a transdiagnostic phenomenon that may also be the nexus when multiple comorbid mental disorders occur,” he said.
“If this is the case, clinically, there is an opportunity here to prevent incident mental disorders in persons with depression and sleep disturbance, prioritizing sleep management in any patient with a mood disorder,” Dr. McIntyre added.
He noted that “the testable hypothesis” is how this is occurring mechanistically.
“I would conjecture that it could be inflammation and/or insulin resistance that is part of sleep disturbance that could predispose and portend other mental illnesses – and likely other medical conditions too, such as obesity and diabetes,” he said.
The study received no specific funding from any funding agency, commercial, or not-for-profit sectors. The investigators’ relevant financial relationships are listed in the original article. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China and the Milken Institute; has received speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences; and is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
A remote mountain bike crash forces a doctor to take knife in hand
It started as a mountain biking excursion with two friends. When we drove into the trailhead parking lot, we saw several emergency vehicles. Then a helicopter passed overhead.
Half a mile down the trail, we encountered another police officer. He asked if we would be willing to go back to get an oxygen tank from the ambulance and carry it out to the scene. The three of us turned around, went back to the parking lot and were able to snag a tank of oxygen. We put it in a backpack and biked out again.
We found the scene about a mile down the trail. An adult male was lying on his back in the dirt after a crash. His eyes were closed and he wasn’t moving except for occasional breaths. Six emergency medical personnel huddled around him, one assisting breaths with a bag mask. I didn’t introduce myself initially. I just listened to hear what was happening.
They were debating the dose of medication to give him in order to intubate. I knew the answer to that question, so I introduced myself. They were happy to have somebody else to assist.
They already had an IV in place and quite a lot of supplies. They administered the meds and the paramedic attempted to intubate through the mouth. Within a few seconds, she pulled the intubating blade out and said, “I’m not going to be able to get this. His tongue is too big.”
I took the blade myself and kneeled at the head of the victim. I made three attempts at intubating, and each time couldn’t view the landmarks. I wasn’t sure if his tongue was too large or if there was some traumatic injury. To make it more difficult, a lot of secretions clogged the airway. The paramedics had a portable suction, which was somewhat functional, but I still couldn’t visualize the landmarks.
I started asking about alternative methods of establishing an airway. They had an i-gel, which is a supraglottic device that goes into the back of the mouth. So, we placed it. But when we attached the bag, air still wasn’t getting into the lungs.
We removed it and put the bag mask back on. Now I was worried. We were having difficulty keeping his oxygen above 90%. I examined the chest and abdomen again. I was wondering if perhaps he was having some gastric distention, which can result from prolonged bagging, but that didn’t seem to be the case.
Bagging became progressively more difficult, and the oxygen slowly trended down through the 80s. Then the 70s. Heart rate dropped below 60 beats per minute. The trajectory was obvious.
That’s when I asked if they had the tools for a surgical airway.
No one thought the question was crazy. In fact, they pulled out a scalpel from an equipment bag.
But now I had to actually do it. I knelt next to the patient, trying to palpate the front of the neck to identify the correct location to cut. I had difficulty finding the appropriate landmarks there as well. Frustrating.
I glanced at the monitor. O2 was now in the 60s. Later the paramedic told me the heart rate was down to 30.
One of the medics looked me in the eye and said, “We’ve got to do something. The time is now.” That helped me snap out of it and act. I made my large vertical incision on the front of the victim’s neck, which of course resulted in quite a bit of bleeding.
My two friends, who were watching, later told me this was the moment the intensity of the scene really increased (it was already pretty intense for me, thanks).
Next, I made the horizontal stab incision. Then I probed with my finger, but it seems the incision hadn’t reached the trachea. I had to make the stab much deeper than I would’ve thought.
And then air bubbled out through the blood. A paramedic was ready with the ET tube in hand and she put it through the incision. We attached the bag. We had air movement into the lungs, and within minutes the oxygen came up.
Not long after, the flight paramedics from the helicopter showed up, having jogged a mile through the woods. They seemed rather surprised to find a patient with a cricothyrotomy. We filled them in on the situation. Now we had to get the patient out of the woods (literally and figuratively).
The emergency responders had a really great transport device: A litter with one big wheel underneath in the middle so we could roll the patient down the mountain bike trail over rocks relatively safely. One person’s job was to hold the tube as we went since we didn’t have suture to hold it in place.
We got back to the parking lot and loaded him into the ambulance, which drove another mile to the helicopter, which then had to take him a hundred miles to the hospital.
To be honest, I thought the prognosis was poor. I suspected he had an intercranial bleed slowly squeezing his brain (that later turned out to not be the case). Even though we had established an airway, it took us so long to get him to the ambulance.
The director of the local EMS called me that evening and said the patient had made it to the hospital. I had never been a part of anything with this intensity. I definitely lost sleep over it. Partly just from the uncertainty of not knowing what the outcome would be. But also second-guessing if I had done everything that I could have.
The story doesn’t quite end there, however.
A week later, a friend of the patient called me. He had recovered well and was going to be discharged from the hospital. He’d chosen to share the story with the media, and the local TV station was going to interview him. They had asked if I would agree to be interviewed.
After the local news story ran, it was kind of a media blitz. In came numerous media requests. But honestly, the portrayal of the story made me feel really weird. It was overly dramatized and not entirely accurate. It really didn’t sit well with me.
Friends all over the country saw the story, and here’s what they got from the coverage:
I was biking behind the patient when he crashed.
I had my own tools. Even the patient himself was told I used my own blade to make the incision.
The true story is what I just told you: A half-dozen emergency medical personnel were already there when I arrived. It was a combination of all of us – together – in the right place at the right time.
A month later, the patient and his family drove to the city where I live to take me out to lunch. It was emotional. There were plenty of tears. His wife and daughter were expressing a lot of gratitude and had some gifts for me. I was able to get his version of the story and learned some details. He had facial trauma in the past with some reconstruction. I realized that perhaps those anatomical changes affected my ability to do the intubation.
I hope to never again have to do this outside of the hospital. But I suppose I’m more prepared than ever now. I’ve reviewed my cricothyrotomy technique many times since then.
I was trained as a family doctor and did clinic and hospital medicine for several years. It was only in 2020 that I transitioned to doing emergency medicine work in a rural hospital. So, 2 years earlier, I’m not sure I would’ve been able to do what I did that day. To me, it was almost symbolic of the transition of my practice to emergency medicine.
I’m still in touch with the patient. We’ve talked about biking together. That hasn’t happened yet, but it may very well happen someday.
Jesse Coenen, MD, is an emergency medicine physician at Hayward Area Memorial Hospital in Hayward, Wisc.
A version of this article first appeared on Medscape.com.
It started as a mountain biking excursion with two friends. When we drove into the trailhead parking lot, we saw several emergency vehicles. Then a helicopter passed overhead.
Half a mile down the trail, we encountered another police officer. He asked if we would be willing to go back to get an oxygen tank from the ambulance and carry it out to the scene. The three of us turned around, went back to the parking lot and were able to snag a tank of oxygen. We put it in a backpack and biked out again.
We found the scene about a mile down the trail. An adult male was lying on his back in the dirt after a crash. His eyes were closed and he wasn’t moving except for occasional breaths. Six emergency medical personnel huddled around him, one assisting breaths with a bag mask. I didn’t introduce myself initially. I just listened to hear what was happening.
They were debating the dose of medication to give him in order to intubate. I knew the answer to that question, so I introduced myself. They were happy to have somebody else to assist.
They already had an IV in place and quite a lot of supplies. They administered the meds and the paramedic attempted to intubate through the mouth. Within a few seconds, she pulled the intubating blade out and said, “I’m not going to be able to get this. His tongue is too big.”
I took the blade myself and kneeled at the head of the victim. I made three attempts at intubating, and each time couldn’t view the landmarks. I wasn’t sure if his tongue was too large or if there was some traumatic injury. To make it more difficult, a lot of secretions clogged the airway. The paramedics had a portable suction, which was somewhat functional, but I still couldn’t visualize the landmarks.
I started asking about alternative methods of establishing an airway. They had an i-gel, which is a supraglottic device that goes into the back of the mouth. So, we placed it. But when we attached the bag, air still wasn’t getting into the lungs.
We removed it and put the bag mask back on. Now I was worried. We were having difficulty keeping his oxygen above 90%. I examined the chest and abdomen again. I was wondering if perhaps he was having some gastric distention, which can result from prolonged bagging, but that didn’t seem to be the case.
Bagging became progressively more difficult, and the oxygen slowly trended down through the 80s. Then the 70s. Heart rate dropped below 60 beats per minute. The trajectory was obvious.
That’s when I asked if they had the tools for a surgical airway.
No one thought the question was crazy. In fact, they pulled out a scalpel from an equipment bag.
But now I had to actually do it. I knelt next to the patient, trying to palpate the front of the neck to identify the correct location to cut. I had difficulty finding the appropriate landmarks there as well. Frustrating.
I glanced at the monitor. O2 was now in the 60s. Later the paramedic told me the heart rate was down to 30.
One of the medics looked me in the eye and said, “We’ve got to do something. The time is now.” That helped me snap out of it and act. I made my large vertical incision on the front of the victim’s neck, which of course resulted in quite a bit of bleeding.
My two friends, who were watching, later told me this was the moment the intensity of the scene really increased (it was already pretty intense for me, thanks).
Next, I made the horizontal stab incision. Then I probed with my finger, but it seems the incision hadn’t reached the trachea. I had to make the stab much deeper than I would’ve thought.
And then air bubbled out through the blood. A paramedic was ready with the ET tube in hand and she put it through the incision. We attached the bag. We had air movement into the lungs, and within minutes the oxygen came up.
Not long after, the flight paramedics from the helicopter showed up, having jogged a mile through the woods. They seemed rather surprised to find a patient with a cricothyrotomy. We filled them in on the situation. Now we had to get the patient out of the woods (literally and figuratively).
The emergency responders had a really great transport device: A litter with one big wheel underneath in the middle so we could roll the patient down the mountain bike trail over rocks relatively safely. One person’s job was to hold the tube as we went since we didn’t have suture to hold it in place.
We got back to the parking lot and loaded him into the ambulance, which drove another mile to the helicopter, which then had to take him a hundred miles to the hospital.
To be honest, I thought the prognosis was poor. I suspected he had an intercranial bleed slowly squeezing his brain (that later turned out to not be the case). Even though we had established an airway, it took us so long to get him to the ambulance.
The director of the local EMS called me that evening and said the patient had made it to the hospital. I had never been a part of anything with this intensity. I definitely lost sleep over it. Partly just from the uncertainty of not knowing what the outcome would be. But also second-guessing if I had done everything that I could have.
The story doesn’t quite end there, however.
A week later, a friend of the patient called me. He had recovered well and was going to be discharged from the hospital. He’d chosen to share the story with the media, and the local TV station was going to interview him. They had asked if I would agree to be interviewed.
After the local news story ran, it was kind of a media blitz. In came numerous media requests. But honestly, the portrayal of the story made me feel really weird. It was overly dramatized and not entirely accurate. It really didn’t sit well with me.
Friends all over the country saw the story, and here’s what they got from the coverage:
I was biking behind the patient when he crashed.
I had my own tools. Even the patient himself was told I used my own blade to make the incision.
The true story is what I just told you: A half-dozen emergency medical personnel were already there when I arrived. It was a combination of all of us – together – in the right place at the right time.
A month later, the patient and his family drove to the city where I live to take me out to lunch. It was emotional. There were plenty of tears. His wife and daughter were expressing a lot of gratitude and had some gifts for me. I was able to get his version of the story and learned some details. He had facial trauma in the past with some reconstruction. I realized that perhaps those anatomical changes affected my ability to do the intubation.
I hope to never again have to do this outside of the hospital. But I suppose I’m more prepared than ever now. I’ve reviewed my cricothyrotomy technique many times since then.
I was trained as a family doctor and did clinic and hospital medicine for several years. It was only in 2020 that I transitioned to doing emergency medicine work in a rural hospital. So, 2 years earlier, I’m not sure I would’ve been able to do what I did that day. To me, it was almost symbolic of the transition of my practice to emergency medicine.
I’m still in touch with the patient. We’ve talked about biking together. That hasn’t happened yet, but it may very well happen someday.
Jesse Coenen, MD, is an emergency medicine physician at Hayward Area Memorial Hospital in Hayward, Wisc.
A version of this article first appeared on Medscape.com.
It started as a mountain biking excursion with two friends. When we drove into the trailhead parking lot, we saw several emergency vehicles. Then a helicopter passed overhead.
Half a mile down the trail, we encountered another police officer. He asked if we would be willing to go back to get an oxygen tank from the ambulance and carry it out to the scene. The three of us turned around, went back to the parking lot and were able to snag a tank of oxygen. We put it in a backpack and biked out again.
We found the scene about a mile down the trail. An adult male was lying on his back in the dirt after a crash. His eyes were closed and he wasn’t moving except for occasional breaths. Six emergency medical personnel huddled around him, one assisting breaths with a bag mask. I didn’t introduce myself initially. I just listened to hear what was happening.
They were debating the dose of medication to give him in order to intubate. I knew the answer to that question, so I introduced myself. They were happy to have somebody else to assist.
They already had an IV in place and quite a lot of supplies. They administered the meds and the paramedic attempted to intubate through the mouth. Within a few seconds, she pulled the intubating blade out and said, “I’m not going to be able to get this. His tongue is too big.”
I took the blade myself and kneeled at the head of the victim. I made three attempts at intubating, and each time couldn’t view the landmarks. I wasn’t sure if his tongue was too large or if there was some traumatic injury. To make it more difficult, a lot of secretions clogged the airway. The paramedics had a portable suction, which was somewhat functional, but I still couldn’t visualize the landmarks.
I started asking about alternative methods of establishing an airway. They had an i-gel, which is a supraglottic device that goes into the back of the mouth. So, we placed it. But when we attached the bag, air still wasn’t getting into the lungs.
We removed it and put the bag mask back on. Now I was worried. We were having difficulty keeping his oxygen above 90%. I examined the chest and abdomen again. I was wondering if perhaps he was having some gastric distention, which can result from prolonged bagging, but that didn’t seem to be the case.
Bagging became progressively more difficult, and the oxygen slowly trended down through the 80s. Then the 70s. Heart rate dropped below 60 beats per minute. The trajectory was obvious.
That’s when I asked if they had the tools for a surgical airway.
No one thought the question was crazy. In fact, they pulled out a scalpel from an equipment bag.
But now I had to actually do it. I knelt next to the patient, trying to palpate the front of the neck to identify the correct location to cut. I had difficulty finding the appropriate landmarks there as well. Frustrating.
I glanced at the monitor. O2 was now in the 60s. Later the paramedic told me the heart rate was down to 30.
One of the medics looked me in the eye and said, “We’ve got to do something. The time is now.” That helped me snap out of it and act. I made my large vertical incision on the front of the victim’s neck, which of course resulted in quite a bit of bleeding.
My two friends, who were watching, later told me this was the moment the intensity of the scene really increased (it was already pretty intense for me, thanks).
Next, I made the horizontal stab incision. Then I probed with my finger, but it seems the incision hadn’t reached the trachea. I had to make the stab much deeper than I would’ve thought.
And then air bubbled out through the blood. A paramedic was ready with the ET tube in hand and she put it through the incision. We attached the bag. We had air movement into the lungs, and within minutes the oxygen came up.
Not long after, the flight paramedics from the helicopter showed up, having jogged a mile through the woods. They seemed rather surprised to find a patient with a cricothyrotomy. We filled them in on the situation. Now we had to get the patient out of the woods (literally and figuratively).
The emergency responders had a really great transport device: A litter with one big wheel underneath in the middle so we could roll the patient down the mountain bike trail over rocks relatively safely. One person’s job was to hold the tube as we went since we didn’t have suture to hold it in place.
We got back to the parking lot and loaded him into the ambulance, which drove another mile to the helicopter, which then had to take him a hundred miles to the hospital.
To be honest, I thought the prognosis was poor. I suspected he had an intercranial bleed slowly squeezing his brain (that later turned out to not be the case). Even though we had established an airway, it took us so long to get him to the ambulance.
The director of the local EMS called me that evening and said the patient had made it to the hospital. I had never been a part of anything with this intensity. I definitely lost sleep over it. Partly just from the uncertainty of not knowing what the outcome would be. But also second-guessing if I had done everything that I could have.
The story doesn’t quite end there, however.
A week later, a friend of the patient called me. He had recovered well and was going to be discharged from the hospital. He’d chosen to share the story with the media, and the local TV station was going to interview him. They had asked if I would agree to be interviewed.
After the local news story ran, it was kind of a media blitz. In came numerous media requests. But honestly, the portrayal of the story made me feel really weird. It was overly dramatized and not entirely accurate. It really didn’t sit well with me.
Friends all over the country saw the story, and here’s what they got from the coverage:
I was biking behind the patient when he crashed.
I had my own tools. Even the patient himself was told I used my own blade to make the incision.
The true story is what I just told you: A half-dozen emergency medical personnel were already there when I arrived. It was a combination of all of us – together – in the right place at the right time.
A month later, the patient and his family drove to the city where I live to take me out to lunch. It was emotional. There were plenty of tears. His wife and daughter were expressing a lot of gratitude and had some gifts for me. I was able to get his version of the story and learned some details. He had facial trauma in the past with some reconstruction. I realized that perhaps those anatomical changes affected my ability to do the intubation.
I hope to never again have to do this outside of the hospital. But I suppose I’m more prepared than ever now. I’ve reviewed my cricothyrotomy technique many times since then.
I was trained as a family doctor and did clinic and hospital medicine for several years. It was only in 2020 that I transitioned to doing emergency medicine work in a rural hospital. So, 2 years earlier, I’m not sure I would’ve been able to do what I did that day. To me, it was almost symbolic of the transition of my practice to emergency medicine.
I’m still in touch with the patient. We’ve talked about biking together. That hasn’t happened yet, but it may very well happen someday.
Jesse Coenen, MD, is an emergency medicine physician at Hayward Area Memorial Hospital in Hayward, Wisc.
A version of this article first appeared on Medscape.com.
Early retirement and the terrible, horrible, no good, very bad cognitive decline
The ‘scheme’ in the name should have been a clue
Retirement. The shiny reward to a lifetime’s worth of working and saving. We’re all literally working to get there, some of us more to get there early, but current research reveals that early retirement isn’t the relaxing finish line we dream about, cognitively speaking.
Researchers at Binghamton (N.Y.) University set out to examine just how retirement plans affect cognitive performance. They started off with China’s New Rural Pension Scheme (scheme probably has a less negative connotation in Chinese), a plan that financially aids the growing rural retirement-age population in the country. Then they looked at data from the Chinese Health and Retirement Longitudinal Survey, which tests cognition with a focus on episodic memory and parts of intact mental status.
What they found was the opposite of what you would expect out of retirees with nothing but time on their hands.
The pension program, which had been in place for almost a decade, led to delayed recall, especially among women, supporting “the mental retirement hypothesis that decreased mental activity results in worsening cognitive skills,” the investigators said in a written statement.
There also was a drop in social engagement, with lower rates of volunteering and social interaction than people who didn’t receive the pension. Some behaviors, like regular alcohol consumption, did improve over the previous year, as did total health in general, but “the adverse effects of early retirement on mental and social engagement significantly outweigh the program’s protective effect on various health behaviors,” Plamen Nikolov, PhD, said about his research.
So if you’re looking to retire early, don’t skimp on the crosswords and the bingo nights. Stay busy in a good way. Your brain will thank you.
Indiana Jones and the First Smallpox Ancestor
Smallpox was, not that long ago, one of the most devastating diseases known to humanity, killing 300 million people in the 20th century alone. Eradicating it has to be one of medicine’s crowning achievements. Now it can only be found in museums, which is where it belongs.
Here’s the thing with smallpox though: For all it did to us, we know frustratingly little about where it came from. Until very recently, the best available genetic evidence placed its emergence in the 17th century, which clashes with historical data. You know what that means, right? It’s time to dig out the fedora and whip, cue the music, and dig into a recently published study spanning continents in search of the mythical smallpox origin story.
We pick up in 2020, when genetic evidence definitively showed smallpox in a Viking burial site, moving the disease’s emergence a thousand years earlier. Which is all well and good, but there’s solid visual evidence that Egyptian pharaohs were dying of smallpox, as their bodies show the signature scarring. Historians were pretty sure smallpox went back about 4,000 years, but there was no genetic material to prove it.
Since there aren’t any 4,000-year-old smallpox germs laying around, the researchers chose to attack the problem another way – by burning down a Venetian catacomb, er, conducting a analysis of historical smallpox genetics to find the virus’s origin. By analyzing the genomes of various strains at different periods of time, they were able to determine that the variola virus had a definitive common ancestor. Some of the genetic components in the Viking-age sample, for example, persisted until the 18th century.
Armed with this information, the scientists determined that the first smallpox ancestor emerged about 3,800 years ago. That’s very close to the historians’ estimate for the disease’s emergence. Proof at last of smallpox’s truly ancient origin. One might even say the researchers chose wisely.
The only hall of fame that really matters
LOTME loves the holiday season – the food, the gifts, the radio stations that play nothing but Christmas music – but for us the most wonderful time of the year comes just a bit later. No, it’s not our annual Golden Globes slap bet. Nope, not even the “excitement” of the College Football Playoff National Championship. It’s time for the National Inventors Hall of Fame to announce its latest inductees, and we could hardly sleep last night after putting cookies out for Thomas Edison. Fasten your seatbelts!
- Robert G. Bryant is a NASA chemist who developed Langley Research Center-Soluble Imide (yes, that’s the actual name) a polymer used as an insulation material for leads in implantable cardiac resynchronization therapy devices.
- Rory Cooper is a biomedical engineer who was paralyzed in a bicycle accident. His work has improved manual and electric wheelchairs and advanced the health, mobility, and social inclusion of people with disabilities and older adults. He is also the first NIHF inductee named Rory.
- Katalin Karikó, a biochemist, and Drew Weissman, an immunologist, “discovered how to enable messenger ribonucleic acid (mRNA) to enter cells without triggering the body’s immune system,” NIHF said, and that laid the foundation for the mRNA COVID-19 vaccines developed by Pfizer-BioNTech and Moderna. That, of course, led to the antivax movement, which has provided so much LOTME fodder over the years.
- Angela Hartley Brodie was a biochemist who discovered and developed a class of drugs called aromatase inhibitors, which can stop the production of hormones that fuel cancer cell growth and are used to treat breast cancer in 500,000 women worldwide each year.
We can’t mention all of the inductees for 2023 (our editor made that very clear), but we would like to offer a special shout-out to brothers Cyril (the first Cyril in the NIHF, by the way) and Louis Keller, who invented the world’s first compact loader, which eventually became the Bobcat skid-steer loader. Not really medical, you’re probably thinking, but we’re sure that someone, somewhere, at some time, used one to build a hospital, landscape a hospital, or clean up after the demolition of a hospital.
The ‘scheme’ in the name should have been a clue
Retirement. The shiny reward to a lifetime’s worth of working and saving. We’re all literally working to get there, some of us more to get there early, but current research reveals that early retirement isn’t the relaxing finish line we dream about, cognitively speaking.
Researchers at Binghamton (N.Y.) University set out to examine just how retirement plans affect cognitive performance. They started off with China’s New Rural Pension Scheme (scheme probably has a less negative connotation in Chinese), a plan that financially aids the growing rural retirement-age population in the country. Then they looked at data from the Chinese Health and Retirement Longitudinal Survey, which tests cognition with a focus on episodic memory and parts of intact mental status.
What they found was the opposite of what you would expect out of retirees with nothing but time on their hands.
The pension program, which had been in place for almost a decade, led to delayed recall, especially among women, supporting “the mental retirement hypothesis that decreased mental activity results in worsening cognitive skills,” the investigators said in a written statement.
There also was a drop in social engagement, with lower rates of volunteering and social interaction than people who didn’t receive the pension. Some behaviors, like regular alcohol consumption, did improve over the previous year, as did total health in general, but “the adverse effects of early retirement on mental and social engagement significantly outweigh the program’s protective effect on various health behaviors,” Plamen Nikolov, PhD, said about his research.
So if you’re looking to retire early, don’t skimp on the crosswords and the bingo nights. Stay busy in a good way. Your brain will thank you.
Indiana Jones and the First Smallpox Ancestor
Smallpox was, not that long ago, one of the most devastating diseases known to humanity, killing 300 million people in the 20th century alone. Eradicating it has to be one of medicine’s crowning achievements. Now it can only be found in museums, which is where it belongs.
Here’s the thing with smallpox though: For all it did to us, we know frustratingly little about where it came from. Until very recently, the best available genetic evidence placed its emergence in the 17th century, which clashes with historical data. You know what that means, right? It’s time to dig out the fedora and whip, cue the music, and dig into a recently published study spanning continents in search of the mythical smallpox origin story.
We pick up in 2020, when genetic evidence definitively showed smallpox in a Viking burial site, moving the disease’s emergence a thousand years earlier. Which is all well and good, but there’s solid visual evidence that Egyptian pharaohs were dying of smallpox, as their bodies show the signature scarring. Historians were pretty sure smallpox went back about 4,000 years, but there was no genetic material to prove it.
Since there aren’t any 4,000-year-old smallpox germs laying around, the researchers chose to attack the problem another way – by burning down a Venetian catacomb, er, conducting a analysis of historical smallpox genetics to find the virus’s origin. By analyzing the genomes of various strains at different periods of time, they were able to determine that the variola virus had a definitive common ancestor. Some of the genetic components in the Viking-age sample, for example, persisted until the 18th century.
Armed with this information, the scientists determined that the first smallpox ancestor emerged about 3,800 years ago. That’s very close to the historians’ estimate for the disease’s emergence. Proof at last of smallpox’s truly ancient origin. One might even say the researchers chose wisely.
The only hall of fame that really matters
LOTME loves the holiday season – the food, the gifts, the radio stations that play nothing but Christmas music – but for us the most wonderful time of the year comes just a bit later. No, it’s not our annual Golden Globes slap bet. Nope, not even the “excitement” of the College Football Playoff National Championship. It’s time for the National Inventors Hall of Fame to announce its latest inductees, and we could hardly sleep last night after putting cookies out for Thomas Edison. Fasten your seatbelts!
- Robert G. Bryant is a NASA chemist who developed Langley Research Center-Soluble Imide (yes, that’s the actual name) a polymer used as an insulation material for leads in implantable cardiac resynchronization therapy devices.
- Rory Cooper is a biomedical engineer who was paralyzed in a bicycle accident. His work has improved manual and electric wheelchairs and advanced the health, mobility, and social inclusion of people with disabilities and older adults. He is also the first NIHF inductee named Rory.
- Katalin Karikó, a biochemist, and Drew Weissman, an immunologist, “discovered how to enable messenger ribonucleic acid (mRNA) to enter cells without triggering the body’s immune system,” NIHF said, and that laid the foundation for the mRNA COVID-19 vaccines developed by Pfizer-BioNTech and Moderna. That, of course, led to the antivax movement, which has provided so much LOTME fodder over the years.
- Angela Hartley Brodie was a biochemist who discovered and developed a class of drugs called aromatase inhibitors, which can stop the production of hormones that fuel cancer cell growth and are used to treat breast cancer in 500,000 women worldwide each year.
We can’t mention all of the inductees for 2023 (our editor made that very clear), but we would like to offer a special shout-out to brothers Cyril (the first Cyril in the NIHF, by the way) and Louis Keller, who invented the world’s first compact loader, which eventually became the Bobcat skid-steer loader. Not really medical, you’re probably thinking, but we’re sure that someone, somewhere, at some time, used one to build a hospital, landscape a hospital, or clean up after the demolition of a hospital.
The ‘scheme’ in the name should have been a clue
Retirement. The shiny reward to a lifetime’s worth of working and saving. We’re all literally working to get there, some of us more to get there early, but current research reveals that early retirement isn’t the relaxing finish line we dream about, cognitively speaking.
Researchers at Binghamton (N.Y.) University set out to examine just how retirement plans affect cognitive performance. They started off with China’s New Rural Pension Scheme (scheme probably has a less negative connotation in Chinese), a plan that financially aids the growing rural retirement-age population in the country. Then they looked at data from the Chinese Health and Retirement Longitudinal Survey, which tests cognition with a focus on episodic memory and parts of intact mental status.
What they found was the opposite of what you would expect out of retirees with nothing but time on their hands.
The pension program, which had been in place for almost a decade, led to delayed recall, especially among women, supporting “the mental retirement hypothesis that decreased mental activity results in worsening cognitive skills,” the investigators said in a written statement.
There also was a drop in social engagement, with lower rates of volunteering and social interaction than people who didn’t receive the pension. Some behaviors, like regular alcohol consumption, did improve over the previous year, as did total health in general, but “the adverse effects of early retirement on mental and social engagement significantly outweigh the program’s protective effect on various health behaviors,” Plamen Nikolov, PhD, said about his research.
So if you’re looking to retire early, don’t skimp on the crosswords and the bingo nights. Stay busy in a good way. Your brain will thank you.
Indiana Jones and the First Smallpox Ancestor
Smallpox was, not that long ago, one of the most devastating diseases known to humanity, killing 300 million people in the 20th century alone. Eradicating it has to be one of medicine’s crowning achievements. Now it can only be found in museums, which is where it belongs.
Here’s the thing with smallpox though: For all it did to us, we know frustratingly little about where it came from. Until very recently, the best available genetic evidence placed its emergence in the 17th century, which clashes with historical data. You know what that means, right? It’s time to dig out the fedora and whip, cue the music, and dig into a recently published study spanning continents in search of the mythical smallpox origin story.
We pick up in 2020, when genetic evidence definitively showed smallpox in a Viking burial site, moving the disease’s emergence a thousand years earlier. Which is all well and good, but there’s solid visual evidence that Egyptian pharaohs were dying of smallpox, as their bodies show the signature scarring. Historians were pretty sure smallpox went back about 4,000 years, but there was no genetic material to prove it.
Since there aren’t any 4,000-year-old smallpox germs laying around, the researchers chose to attack the problem another way – by burning down a Venetian catacomb, er, conducting a analysis of historical smallpox genetics to find the virus’s origin. By analyzing the genomes of various strains at different periods of time, they were able to determine that the variola virus had a definitive common ancestor. Some of the genetic components in the Viking-age sample, for example, persisted until the 18th century.
Armed with this information, the scientists determined that the first smallpox ancestor emerged about 3,800 years ago. That’s very close to the historians’ estimate for the disease’s emergence. Proof at last of smallpox’s truly ancient origin. One might even say the researchers chose wisely.
The only hall of fame that really matters
LOTME loves the holiday season – the food, the gifts, the radio stations that play nothing but Christmas music – but for us the most wonderful time of the year comes just a bit later. No, it’s not our annual Golden Globes slap bet. Nope, not even the “excitement” of the College Football Playoff National Championship. It’s time for the National Inventors Hall of Fame to announce its latest inductees, and we could hardly sleep last night after putting cookies out for Thomas Edison. Fasten your seatbelts!
- Robert G. Bryant is a NASA chemist who developed Langley Research Center-Soluble Imide (yes, that’s the actual name) a polymer used as an insulation material for leads in implantable cardiac resynchronization therapy devices.
- Rory Cooper is a biomedical engineer who was paralyzed in a bicycle accident. His work has improved manual and electric wheelchairs and advanced the health, mobility, and social inclusion of people with disabilities and older adults. He is also the first NIHF inductee named Rory.
- Katalin Karikó, a biochemist, and Drew Weissman, an immunologist, “discovered how to enable messenger ribonucleic acid (mRNA) to enter cells without triggering the body’s immune system,” NIHF said, and that laid the foundation for the mRNA COVID-19 vaccines developed by Pfizer-BioNTech and Moderna. That, of course, led to the antivax movement, which has provided so much LOTME fodder over the years.
- Angela Hartley Brodie was a biochemist who discovered and developed a class of drugs called aromatase inhibitors, which can stop the production of hormones that fuel cancer cell growth and are used to treat breast cancer in 500,000 women worldwide each year.
We can’t mention all of the inductees for 2023 (our editor made that very clear), but we would like to offer a special shout-out to brothers Cyril (the first Cyril in the NIHF, by the way) and Louis Keller, who invented the world’s first compact loader, which eventually became the Bobcat skid-steer loader. Not really medical, you’re probably thinking, but we’re sure that someone, somewhere, at some time, used one to build a hospital, landscape a hospital, or clean up after the demolition of a hospital.