User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Tranq-contaminated fentanyl now in 48 states, DEA warns
The DEA warning comes on the heels of a Food and Drug Administration announcement that it would begin more closely monitoring imports of the raw materials and bulk shipments of xylazine, also known as “tranq” and “zombie drug.”
Xylazine was first approved by the FDA in 1972 as a sedative and analgesic for use only in animals, but is increasingly being detected in illicit street drugs, and is often mixed with fentanyl, cocaine, and methamphetamine.
The FDA warned in November that naloxone (Narcan) would not reverse xylazine-related overdoses because the tranquilizer is not an opioid. It does suppress respiration and repeated exposures may lead to dependence and withdrawal, said the agency. Users are also experiencing severe necrosis at injection sites.
“Xylazine is making the deadliest drug threat our country has ever faced, fentanyl, even deadlier,” said DEA Administrator Anne Milgram in a statement. “The DEA Laboratory System is reporting that in 2022 approximately 23% of fentanyl powder and 7% of fentanyl pills seized by the DEA contained xylazine.”
Xylazine use has spread quickly, from its start in the Philadelphia area to the Northeast, the South, and most recently the West.
Citing data from the Centers for Disease Control and Prevention, the DEA said that 66% of the 107,735 overdose deaths for the year ending August 2022 involved synthetic opioids such as fentanyl. The DEA said that the Sinaloa Cartel and Jalisco Cartel in Mexico, using chemicals sourced from China, are primarily responsible for trafficking fentanyl in the United States.
A version of this article originally appeared on Medscape.com.
The DEA warning comes on the heels of a Food and Drug Administration announcement that it would begin more closely monitoring imports of the raw materials and bulk shipments of xylazine, also known as “tranq” and “zombie drug.”
Xylazine was first approved by the FDA in 1972 as a sedative and analgesic for use only in animals, but is increasingly being detected in illicit street drugs, and is often mixed with fentanyl, cocaine, and methamphetamine.
The FDA warned in November that naloxone (Narcan) would not reverse xylazine-related overdoses because the tranquilizer is not an opioid. It does suppress respiration and repeated exposures may lead to dependence and withdrawal, said the agency. Users are also experiencing severe necrosis at injection sites.
“Xylazine is making the deadliest drug threat our country has ever faced, fentanyl, even deadlier,” said DEA Administrator Anne Milgram in a statement. “The DEA Laboratory System is reporting that in 2022 approximately 23% of fentanyl powder and 7% of fentanyl pills seized by the DEA contained xylazine.”
Xylazine use has spread quickly, from its start in the Philadelphia area to the Northeast, the South, and most recently the West.
Citing data from the Centers for Disease Control and Prevention, the DEA said that 66% of the 107,735 overdose deaths for the year ending August 2022 involved synthetic opioids such as fentanyl. The DEA said that the Sinaloa Cartel and Jalisco Cartel in Mexico, using chemicals sourced from China, are primarily responsible for trafficking fentanyl in the United States.
A version of this article originally appeared on Medscape.com.
The DEA warning comes on the heels of a Food and Drug Administration announcement that it would begin more closely monitoring imports of the raw materials and bulk shipments of xylazine, also known as “tranq” and “zombie drug.”
Xylazine was first approved by the FDA in 1972 as a sedative and analgesic for use only in animals, but is increasingly being detected in illicit street drugs, and is often mixed with fentanyl, cocaine, and methamphetamine.
The FDA warned in November that naloxone (Narcan) would not reverse xylazine-related overdoses because the tranquilizer is not an opioid. It does suppress respiration and repeated exposures may lead to dependence and withdrawal, said the agency. Users are also experiencing severe necrosis at injection sites.
“Xylazine is making the deadliest drug threat our country has ever faced, fentanyl, even deadlier,” said DEA Administrator Anne Milgram in a statement. “The DEA Laboratory System is reporting that in 2022 approximately 23% of fentanyl powder and 7% of fentanyl pills seized by the DEA contained xylazine.”
Xylazine use has spread quickly, from its start in the Philadelphia area to the Northeast, the South, and most recently the West.
Citing data from the Centers for Disease Control and Prevention, the DEA said that 66% of the 107,735 overdose deaths for the year ending August 2022 involved synthetic opioids such as fentanyl. The DEA said that the Sinaloa Cartel and Jalisco Cartel in Mexico, using chemicals sourced from China, are primarily responsible for trafficking fentanyl in the United States.
A version of this article originally appeared on Medscape.com.
Sweaty treatment for social anxiety could pass the sniff test
Getting sweet on sweat
Are you the sort of person who struggles in social situations? Have the past 3 years been a secret respite from the terror and exhaustion of meeting new people? We understand your plight. People kind of suck. And you don’t have to look far to be reminded of it.
Unfortunately, on occasion we all have to interact with other human beings. If you suffer from social anxiety, this is not a fun thing to do. But new research indicates that there may be a way to alleviate the stress for those with social anxiety: armpits.
Specifically, sweat from the armpits of other people. Yes, this means a group of scientists gathered up some volunteers and collected their armpit sweat while the volunteers watched a variety of movies (horror, comedy, romance, etc.). Our condolences to the poor unpaid interns tasked with gathering the sweat.
Once they had their precious new medicine, the researchers took a group of women and administered a round of mindfulness therapy. Some of the participants then received the various sweats, while the rest were forced to smell only clean air. (The horror!) Lo and behold, the sweat groups had their anxiety scores reduced by about 40% after their therapy, compared with just 17% in the control group.
The researchers also found that the source of the sweat didn’t matter. Their study subjects responded the same to sweat excreted during a scary movie as they did to sweat from a comedy, a result that surprised the researchers. They suggested chemosignals in the sweat may affect the treatment response and advised further research. Which means more sweat collection! They plan on testing emotionally neutral movies next time, and if we can make a humble suggestion, they also should try the sweatiest movies.
Before the Food and Drug Administration can approve armpit sweat as a treatment for social anxiety, we have some advice for those shut-in introverts out there. Next time you have to interact with rabid extroverts, instead of shaking their hands, walk up to them and take a deep whiff of their armpits. Establish dominance. Someone will feel awkward, and science has proved it won’t be you.
The puff that vaccinates
Ever been shot with a Nerf gun or hit with a foam pool tube? More annoying than painful, right? If we asked if you’d rather get pelted with one of those than receive a traditional vaccine injection, you would choose the former. Maybe someday you actually will.
During the boredom of the early pandemic lockdown, Jeremiah Gassensmith, PhD, of the department of chemistry and biochemistry at the University of Texas, Dallas, ordered a compressed gas–powered jet injection system to fool around with at home. Hey, who didn’t? Anyway, when it was time to go back to the lab he handed it over to one of his grad students, Yalini Wijesundara, and asked her to see what could be done with it.
In her tinkering she found that the jet injector could deliver metal-organic frameworks (MOFs) that can hold a bunch of different materials, like proteins and nucleic acids, through the skin.
Thus the “MOF-Jet” was born!
Jet injectors are nothing new, but they hurt. The MOF-Jet, however, is practically painless and cheaper than the gene guns that veterinarians use to inject biological cargo attached to the surface of a metal microparticle.
Changing the carrier gas also changes the time needed to break down the MOF and thus alters delivery of the drug inside. “If you shoot it with carbon dioxide, it will release its cargo faster within cells; if you use regular air, it will take 4 or 5 days,” Ms. Wijesundara explained in a written statement. That means the same drug could be released over different timescales without changing its formulation.
While testing on onion cells and mice, Ms. Wijesundara noted that it was as easy as “pointing and shooting” to distribute the puff of gas into the cells. A saving grace to those with needle anxiety. Not that we would know anything about needle anxiety.
More testing needs to be done before bringing this technology to human use, obviously, but we’re looking forward to saying goodbye to that dreaded prick and hello to a puff.
Your hippocampus is showing
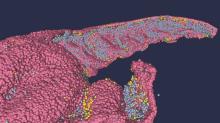
Brain anatomy is one of the many, many things that’s not really our thing, but we do know a cool picture when we see one. Case in point: The image just below, which happens to be a full-scale, single-cell resolution model of the CA1 region of the hippocampus that “replicates the structure and architecture of the area, along with the position and relative connectivity of the neurons,” according to a statement from the Human Brain Project.
“We have performed a data mining operation on high resolution images of the human hippocampus, obtained from the BigBrain database. The position of individual neurons has been derived from a detailed analysis of these images,” said senior author Michele Migliore, PhD, of the Italian National Research Council’s Institute of Biophysics in Palermo.
Yes, he did say BigBrain database. BigBrain is – we checked and it’s definitely not this – a 3D model of a brain that was sectioned into 7,404 slices just 20 micrometers thick and then scanned by MRI. Digital reconstruction of those slices was done by supercomputer and the results are now available for analysis.
Dr. Migliore and his associates developed an image-processing algorithm to obtain neuronal positioning distribution and an algorithm to generate neuronal connectivity by approximating the shapes of dendrites and axons. (Our brains are starting to hurt just trying to write this.) “Some fit into narrow cones, others have a broad complex extension that can be approximated by dedicated geometrical volumes, and the connectivity to nearby neurons changes accordingly,” explained lead author Daniela Gandolfi of the University of Modena (Italy) and Reggio Emilia.
The investigators have made their dataset and the extraction methodology available on the EBRAINS platform and through the Human Brain Project and are moving on to other brain regions. And then, once everyone can find their way in and around the old gray matter, it should bring an end to conversations like this, which no doubt occur between male and female neuroscientists every day:
“Arnold, I think we’re lost.”
“Don’t worry, Bev, I know where I’m going.”
“Stop and ask this lady for directions.”
“I said I can find it.”
“Just ask her.”
“Fine. Excuse me, ma’am, can you tell us how to get to the corpora quadrigemina from here?
Getting sweet on sweat
Are you the sort of person who struggles in social situations? Have the past 3 years been a secret respite from the terror and exhaustion of meeting new people? We understand your plight. People kind of suck. And you don’t have to look far to be reminded of it.
Unfortunately, on occasion we all have to interact with other human beings. If you suffer from social anxiety, this is not a fun thing to do. But new research indicates that there may be a way to alleviate the stress for those with social anxiety: armpits.
Specifically, sweat from the armpits of other people. Yes, this means a group of scientists gathered up some volunteers and collected their armpit sweat while the volunteers watched a variety of movies (horror, comedy, romance, etc.). Our condolences to the poor unpaid interns tasked with gathering the sweat.
Once they had their precious new medicine, the researchers took a group of women and administered a round of mindfulness therapy. Some of the participants then received the various sweats, while the rest were forced to smell only clean air. (The horror!) Lo and behold, the sweat groups had their anxiety scores reduced by about 40% after their therapy, compared with just 17% in the control group.
The researchers also found that the source of the sweat didn’t matter. Their study subjects responded the same to sweat excreted during a scary movie as they did to sweat from a comedy, a result that surprised the researchers. They suggested chemosignals in the sweat may affect the treatment response and advised further research. Which means more sweat collection! They plan on testing emotionally neutral movies next time, and if we can make a humble suggestion, they also should try the sweatiest movies.
Before the Food and Drug Administration can approve armpit sweat as a treatment for social anxiety, we have some advice for those shut-in introverts out there. Next time you have to interact with rabid extroverts, instead of shaking their hands, walk up to them and take a deep whiff of their armpits. Establish dominance. Someone will feel awkward, and science has proved it won’t be you.
The puff that vaccinates
Ever been shot with a Nerf gun or hit with a foam pool tube? More annoying than painful, right? If we asked if you’d rather get pelted with one of those than receive a traditional vaccine injection, you would choose the former. Maybe someday you actually will.
During the boredom of the early pandemic lockdown, Jeremiah Gassensmith, PhD, of the department of chemistry and biochemistry at the University of Texas, Dallas, ordered a compressed gas–powered jet injection system to fool around with at home. Hey, who didn’t? Anyway, when it was time to go back to the lab he handed it over to one of his grad students, Yalini Wijesundara, and asked her to see what could be done with it.
In her tinkering she found that the jet injector could deliver metal-organic frameworks (MOFs) that can hold a bunch of different materials, like proteins and nucleic acids, through the skin.
Thus the “MOF-Jet” was born!
Jet injectors are nothing new, but they hurt. The MOF-Jet, however, is practically painless and cheaper than the gene guns that veterinarians use to inject biological cargo attached to the surface of a metal microparticle.
Changing the carrier gas also changes the time needed to break down the MOF and thus alters delivery of the drug inside. “If you shoot it with carbon dioxide, it will release its cargo faster within cells; if you use regular air, it will take 4 or 5 days,” Ms. Wijesundara explained in a written statement. That means the same drug could be released over different timescales without changing its formulation.
While testing on onion cells and mice, Ms. Wijesundara noted that it was as easy as “pointing and shooting” to distribute the puff of gas into the cells. A saving grace to those with needle anxiety. Not that we would know anything about needle anxiety.
More testing needs to be done before bringing this technology to human use, obviously, but we’re looking forward to saying goodbye to that dreaded prick and hello to a puff.
Your hippocampus is showing
Brain anatomy is one of the many, many things that’s not really our thing, but we do know a cool picture when we see one. Case in point: The image just below, which happens to be a full-scale, single-cell resolution model of the CA1 region of the hippocampus that “replicates the structure and architecture of the area, along with the position and relative connectivity of the neurons,” according to a statement from the Human Brain Project.
“We have performed a data mining operation on high resolution images of the human hippocampus, obtained from the BigBrain database. The position of individual neurons has been derived from a detailed analysis of these images,” said senior author Michele Migliore, PhD, of the Italian National Research Council’s Institute of Biophysics in Palermo.
Yes, he did say BigBrain database. BigBrain is – we checked and it’s definitely not this – a 3D model of a brain that was sectioned into 7,404 slices just 20 micrometers thick and then scanned by MRI. Digital reconstruction of those slices was done by supercomputer and the results are now available for analysis.
Dr. Migliore and his associates developed an image-processing algorithm to obtain neuronal positioning distribution and an algorithm to generate neuronal connectivity by approximating the shapes of dendrites and axons. (Our brains are starting to hurt just trying to write this.) “Some fit into narrow cones, others have a broad complex extension that can be approximated by dedicated geometrical volumes, and the connectivity to nearby neurons changes accordingly,” explained lead author Daniela Gandolfi of the University of Modena (Italy) and Reggio Emilia.
The investigators have made their dataset and the extraction methodology available on the EBRAINS platform and through the Human Brain Project and are moving on to other brain regions. And then, once everyone can find their way in and around the old gray matter, it should bring an end to conversations like this, which no doubt occur between male and female neuroscientists every day:
“Arnold, I think we’re lost.”
“Don’t worry, Bev, I know where I’m going.”
“Stop and ask this lady for directions.”
“I said I can find it.”
“Just ask her.”
“Fine. Excuse me, ma’am, can you tell us how to get to the corpora quadrigemina from here?
Getting sweet on sweat
Are you the sort of person who struggles in social situations? Have the past 3 years been a secret respite from the terror and exhaustion of meeting new people? We understand your plight. People kind of suck. And you don’t have to look far to be reminded of it.
Unfortunately, on occasion we all have to interact with other human beings. If you suffer from social anxiety, this is not a fun thing to do. But new research indicates that there may be a way to alleviate the stress for those with social anxiety: armpits.
Specifically, sweat from the armpits of other people. Yes, this means a group of scientists gathered up some volunteers and collected their armpit sweat while the volunteers watched a variety of movies (horror, comedy, romance, etc.). Our condolences to the poor unpaid interns tasked with gathering the sweat.
Once they had their precious new medicine, the researchers took a group of women and administered a round of mindfulness therapy. Some of the participants then received the various sweats, while the rest were forced to smell only clean air. (The horror!) Lo and behold, the sweat groups had their anxiety scores reduced by about 40% after their therapy, compared with just 17% in the control group.
The researchers also found that the source of the sweat didn’t matter. Their study subjects responded the same to sweat excreted during a scary movie as they did to sweat from a comedy, a result that surprised the researchers. They suggested chemosignals in the sweat may affect the treatment response and advised further research. Which means more sweat collection! They plan on testing emotionally neutral movies next time, and if we can make a humble suggestion, they also should try the sweatiest movies.
Before the Food and Drug Administration can approve armpit sweat as a treatment for social anxiety, we have some advice for those shut-in introverts out there. Next time you have to interact with rabid extroverts, instead of shaking their hands, walk up to them and take a deep whiff of their armpits. Establish dominance. Someone will feel awkward, and science has proved it won’t be you.
The puff that vaccinates
Ever been shot with a Nerf gun or hit with a foam pool tube? More annoying than painful, right? If we asked if you’d rather get pelted with one of those than receive a traditional vaccine injection, you would choose the former. Maybe someday you actually will.
During the boredom of the early pandemic lockdown, Jeremiah Gassensmith, PhD, of the department of chemistry and biochemistry at the University of Texas, Dallas, ordered a compressed gas–powered jet injection system to fool around with at home. Hey, who didn’t? Anyway, when it was time to go back to the lab he handed it over to one of his grad students, Yalini Wijesundara, and asked her to see what could be done with it.
In her tinkering she found that the jet injector could deliver metal-organic frameworks (MOFs) that can hold a bunch of different materials, like proteins and nucleic acids, through the skin.
Thus the “MOF-Jet” was born!
Jet injectors are nothing new, but they hurt. The MOF-Jet, however, is practically painless and cheaper than the gene guns that veterinarians use to inject biological cargo attached to the surface of a metal microparticle.
Changing the carrier gas also changes the time needed to break down the MOF and thus alters delivery of the drug inside. “If you shoot it with carbon dioxide, it will release its cargo faster within cells; if you use regular air, it will take 4 or 5 days,” Ms. Wijesundara explained in a written statement. That means the same drug could be released over different timescales without changing its formulation.
While testing on onion cells and mice, Ms. Wijesundara noted that it was as easy as “pointing and shooting” to distribute the puff of gas into the cells. A saving grace to those with needle anxiety. Not that we would know anything about needle anxiety.
More testing needs to be done before bringing this technology to human use, obviously, but we’re looking forward to saying goodbye to that dreaded prick and hello to a puff.
Your hippocampus is showing
Brain anatomy is one of the many, many things that’s not really our thing, but we do know a cool picture when we see one. Case in point: The image just below, which happens to be a full-scale, single-cell resolution model of the CA1 region of the hippocampus that “replicates the structure and architecture of the area, along with the position and relative connectivity of the neurons,” according to a statement from the Human Brain Project.
“We have performed a data mining operation on high resolution images of the human hippocampus, obtained from the BigBrain database. The position of individual neurons has been derived from a detailed analysis of these images,” said senior author Michele Migliore, PhD, of the Italian National Research Council’s Institute of Biophysics in Palermo.
Yes, he did say BigBrain database. BigBrain is – we checked and it’s definitely not this – a 3D model of a brain that was sectioned into 7,404 slices just 20 micrometers thick and then scanned by MRI. Digital reconstruction of those slices was done by supercomputer and the results are now available for analysis.
Dr. Migliore and his associates developed an image-processing algorithm to obtain neuronal positioning distribution and an algorithm to generate neuronal connectivity by approximating the shapes of dendrites and axons. (Our brains are starting to hurt just trying to write this.) “Some fit into narrow cones, others have a broad complex extension that can be approximated by dedicated geometrical volumes, and the connectivity to nearby neurons changes accordingly,” explained lead author Daniela Gandolfi of the University of Modena (Italy) and Reggio Emilia.
The investigators have made their dataset and the extraction methodology available on the EBRAINS platform and through the Human Brain Project and are moving on to other brain regions. And then, once everyone can find their way in and around the old gray matter, it should bring an end to conversations like this, which no doubt occur between male and female neuroscientists every day:
“Arnold, I think we’re lost.”
“Don’t worry, Bev, I know where I’m going.”
“Stop and ask this lady for directions.”
“I said I can find it.”
“Just ask her.”
“Fine. Excuse me, ma’am, can you tell us how to get to the corpora quadrigemina from here?
Stutz: The psychiatrist as movie star
For as long as I can remember, psychiatrists have talked about what the appropriate boundaries are for self-disclosure about personal issues with patients. There is obviously no exact answer as to what is acceptable to disclose; this depends on the doctor, the patient, the “brand” of psychotherapy, the patient’s issues, the nature of what is being disclosed, and maybe the alignment of the stars on that particular day. “Stutz,” the Netflix documentary that Oscar-nominated actor/director Jonah Hill has made about his psychiatrist, Phil Stutz, MD, adds a whole new chapter to the discussion.
“Okay, entertain me,” Dr. Stutz says as his patient takes a seat. The therapeutic relationship and the paradigm Dr. Stutz has created to help his patients has been healing for Jonah Hill. The very serious and intimate dialogue that follows unfolds with moments of humor, warmth, and open affection. Hill candidly tells us why he is making this documentary – to share what he has learned and to honor his therapist – but we don’t know why Dr. Stutz has agreed to the endeavor and we’re left to our own inferences.
Dr. Stutz is the coauthor, with Barry Michels, of a best-selling self-help book, “The Tools: 5 Tools to Help You Find Courage, Creativity, and Willpower – and Inspire You to Live Life in Forward Motion.” He talks about his restlessness with the psychodynamic method during his training as a resident in New York – he wanted to offer his patients more immediate relief and a supervisor told him, “Don’t you dare!”
In the film, he talks about giving patients hope and direction. And Hill makes the comment, “In traditional therapy, you’re paying this person and you save all your problems for them, and they just listen, and your friends – who are idiots – give you advice, unsolicited, and you want your friends just to listen, and you want your therapist to give you advice!” Dr. Stutz gives advice and he is like no other therapist Jonah has ever had.
The premise of the film is that we are watching a single therapy session and Dr. Stutz will discuss the use of his tools and techniques that Hill has found helpful. Jonah is the interviewer, and when the doctor suggests it would be helpful if Jonah talked about his life, the patient/director rebuffs him; this documentary is about the psychiatrist.
Early in the film an alarm goes off, Dr. Stutz does not hear it, and Jonah has to remind him that it’s time for him to take his pills. The psychiatrist has Parkinson’s disease and how it has affected him becomes one focal point for the film. We later learn that he lost a younger brother as a child (something Hill did not know before they started filming) and grew up in the shadow of that loss. His extroverted father made it clear that medicine was the only acceptable career path for his son, and his introverted and depressed mother spent her days proclaiming that all men were as awful as her own abusive father.
About a third of the way through the film, the focus shifts. Jonah suddenly confesses that he is feeling stuck with regard to the movie, that he is troubled by the fact that he has not been able to share his distress with Dr. Stutz during their real-life, unfilmed therapy sessions, and the viewers learn that the single-session concept was disingenuous – they have been filming this documentary for two years, against a green screen and not in an office, always wearing the same clothes, and Jonah pulls off a wig that he wears to disguise the fact that he changed his hairstyle months earlier.
It’s a bit unnerving as they throw the wig around, and Jonah agrees to be more open about the issues he has struggled with. He acknowledges that this has been difficult, and he says, “I just keep asking myself, like, was this a f***ing horrible idea for a patient to make a movie about his therapist?” From my perspective as a psychiatrist-viewer, it’s a good question to ask!
Dr. Stutz reassures Jonah that it is okay to be vulnerable. “Failure, weakness, vulnerability – it’s like a connector, it connects you to the rest of the world.” A super-sized cardboard cutout of an obese 14-year-old Jonah now joins the room, and we learn that he continues to struggle with his self-image. Things get more real.
Peppered throughout the film, there are lessons from Dr. Stutz about his “tools,” constructs he uses to help people restructure their worlds and take action to move forward. One such construct he calls “the maze,” which occurs when one person in an interpersonal relationship is waiting for fairness and becomes preoccupied with feeling injured.
Jonah inquires about Dr. Stutz’s romantic life and the therapist replies with a transparency that overrides our usual professional boundaries. We all learn that Dr. Stutz is not in a relationship, he’s never been married, but there is a woman he has had some involvement with on and off for 40 years. Jonah’s line of questioning rivals that of any therapist. “How do you think it affects you, having your mom hate men and you being a man?” Dr. Stutz admits that he can never feel safe with women. “Did you ever override that wall you built with your mom and get close to a woman?” When Jonah professes, “I don’t feel anything but love for you and I just want you to be happy,” my own feeling was that the tables had turned too far, that the therapist’s failed romantic life risked being a burden to the patient.
Still, there is something about the relationship between the two men that is touching and beautiful. Dr. Stutz as a therapist is charismatic, caring, self-assured, and optimistic, and he radiates hope and certainty. He mixes an intense intimacy with humor in a way that is both authentic and entertaining. The interspersed jokes break the intensity, but they don’t diminish his wisdom and the healing he imparts.
Dr. Stutz is a psychiatrist, and his strength is clearly as a psychotherapist, yet there is not a single mention of psychotropic medications – there is a banter about recreational drugs and medications for Parkinson’s disease. If Hill is taking medication for depression or anxiety, and if prescribing is part of Dr. Stutz’s arsenal, the viewer is not made aware of this.
Dr. Stutz eschews the slow, detached, and “neutral” pace of psychodynamic therapy and the whole concept of the therapist as a blank wall for the transference to play out on, but here the transference screams: Jonah loves him, he respect and honors him, he wants him to be happy, and he is afraid of losing him.
“Stutz” is a movie about a larger-than-life psychiatrist, one whose warmth and inspiration are healing. I imagine his tools are helpful, but his personality is what carries the load. If a viewer has not had experience with psychiatry, and this film inspires him to begin therapy, there may be a good deal of disappointment. In this case, the patient is a successful actor, and one might wonder if that, together with the entire years-long project of filming, has altered the relationship well beyond the usual therapeutic hour.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University in Baltimore.
For as long as I can remember, psychiatrists have talked about what the appropriate boundaries are for self-disclosure about personal issues with patients. There is obviously no exact answer as to what is acceptable to disclose; this depends on the doctor, the patient, the “brand” of psychotherapy, the patient’s issues, the nature of what is being disclosed, and maybe the alignment of the stars on that particular day. “Stutz,” the Netflix documentary that Oscar-nominated actor/director Jonah Hill has made about his psychiatrist, Phil Stutz, MD, adds a whole new chapter to the discussion.
“Okay, entertain me,” Dr. Stutz says as his patient takes a seat. The therapeutic relationship and the paradigm Dr. Stutz has created to help his patients has been healing for Jonah Hill. The very serious and intimate dialogue that follows unfolds with moments of humor, warmth, and open affection. Hill candidly tells us why he is making this documentary – to share what he has learned and to honor his therapist – but we don’t know why Dr. Stutz has agreed to the endeavor and we’re left to our own inferences.
Dr. Stutz is the coauthor, with Barry Michels, of a best-selling self-help book, “The Tools: 5 Tools to Help You Find Courage, Creativity, and Willpower – and Inspire You to Live Life in Forward Motion.” He talks about his restlessness with the psychodynamic method during his training as a resident in New York – he wanted to offer his patients more immediate relief and a supervisor told him, “Don’t you dare!”
In the film, he talks about giving patients hope and direction. And Hill makes the comment, “In traditional therapy, you’re paying this person and you save all your problems for them, and they just listen, and your friends – who are idiots – give you advice, unsolicited, and you want your friends just to listen, and you want your therapist to give you advice!” Dr. Stutz gives advice and he is like no other therapist Jonah has ever had.
The premise of the film is that we are watching a single therapy session and Dr. Stutz will discuss the use of his tools and techniques that Hill has found helpful. Jonah is the interviewer, and when the doctor suggests it would be helpful if Jonah talked about his life, the patient/director rebuffs him; this documentary is about the psychiatrist.
Early in the film an alarm goes off, Dr. Stutz does not hear it, and Jonah has to remind him that it’s time for him to take his pills. The psychiatrist has Parkinson’s disease and how it has affected him becomes one focal point for the film. We later learn that he lost a younger brother as a child (something Hill did not know before they started filming) and grew up in the shadow of that loss. His extroverted father made it clear that medicine was the only acceptable career path for his son, and his introverted and depressed mother spent her days proclaiming that all men were as awful as her own abusive father.
About a third of the way through the film, the focus shifts. Jonah suddenly confesses that he is feeling stuck with regard to the movie, that he is troubled by the fact that he has not been able to share his distress with Dr. Stutz during their real-life, unfilmed therapy sessions, and the viewers learn that the single-session concept was disingenuous – they have been filming this documentary for two years, against a green screen and not in an office, always wearing the same clothes, and Jonah pulls off a wig that he wears to disguise the fact that he changed his hairstyle months earlier.
It’s a bit unnerving as they throw the wig around, and Jonah agrees to be more open about the issues he has struggled with. He acknowledges that this has been difficult, and he says, “I just keep asking myself, like, was this a f***ing horrible idea for a patient to make a movie about his therapist?” From my perspective as a psychiatrist-viewer, it’s a good question to ask!
Dr. Stutz reassures Jonah that it is okay to be vulnerable. “Failure, weakness, vulnerability – it’s like a connector, it connects you to the rest of the world.” A super-sized cardboard cutout of an obese 14-year-old Jonah now joins the room, and we learn that he continues to struggle with his self-image. Things get more real.
Peppered throughout the film, there are lessons from Dr. Stutz about his “tools,” constructs he uses to help people restructure their worlds and take action to move forward. One such construct he calls “the maze,” which occurs when one person in an interpersonal relationship is waiting for fairness and becomes preoccupied with feeling injured.
Jonah inquires about Dr. Stutz’s romantic life and the therapist replies with a transparency that overrides our usual professional boundaries. We all learn that Dr. Stutz is not in a relationship, he’s never been married, but there is a woman he has had some involvement with on and off for 40 years. Jonah’s line of questioning rivals that of any therapist. “How do you think it affects you, having your mom hate men and you being a man?” Dr. Stutz admits that he can never feel safe with women. “Did you ever override that wall you built with your mom and get close to a woman?” When Jonah professes, “I don’t feel anything but love for you and I just want you to be happy,” my own feeling was that the tables had turned too far, that the therapist’s failed romantic life risked being a burden to the patient.
Still, there is something about the relationship between the two men that is touching and beautiful. Dr. Stutz as a therapist is charismatic, caring, self-assured, and optimistic, and he radiates hope and certainty. He mixes an intense intimacy with humor in a way that is both authentic and entertaining. The interspersed jokes break the intensity, but they don’t diminish his wisdom and the healing he imparts.
Dr. Stutz is a psychiatrist, and his strength is clearly as a psychotherapist, yet there is not a single mention of psychotropic medications – there is a banter about recreational drugs and medications for Parkinson’s disease. If Hill is taking medication for depression or anxiety, and if prescribing is part of Dr. Stutz’s arsenal, the viewer is not made aware of this.
Dr. Stutz eschews the slow, detached, and “neutral” pace of psychodynamic therapy and the whole concept of the therapist as a blank wall for the transference to play out on, but here the transference screams: Jonah loves him, he respect and honors him, he wants him to be happy, and he is afraid of losing him.
“Stutz” is a movie about a larger-than-life psychiatrist, one whose warmth and inspiration are healing. I imagine his tools are helpful, but his personality is what carries the load. If a viewer has not had experience with psychiatry, and this film inspires him to begin therapy, there may be a good deal of disappointment. In this case, the patient is a successful actor, and one might wonder if that, together with the entire years-long project of filming, has altered the relationship well beyond the usual therapeutic hour.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University in Baltimore.
For as long as I can remember, psychiatrists have talked about what the appropriate boundaries are for self-disclosure about personal issues with patients. There is obviously no exact answer as to what is acceptable to disclose; this depends on the doctor, the patient, the “brand” of psychotherapy, the patient’s issues, the nature of what is being disclosed, and maybe the alignment of the stars on that particular day. “Stutz,” the Netflix documentary that Oscar-nominated actor/director Jonah Hill has made about his psychiatrist, Phil Stutz, MD, adds a whole new chapter to the discussion.
“Okay, entertain me,” Dr. Stutz says as his patient takes a seat. The therapeutic relationship and the paradigm Dr. Stutz has created to help his patients has been healing for Jonah Hill. The very serious and intimate dialogue that follows unfolds with moments of humor, warmth, and open affection. Hill candidly tells us why he is making this documentary – to share what he has learned and to honor his therapist – but we don’t know why Dr. Stutz has agreed to the endeavor and we’re left to our own inferences.
Dr. Stutz is the coauthor, with Barry Michels, of a best-selling self-help book, “The Tools: 5 Tools to Help You Find Courage, Creativity, and Willpower – and Inspire You to Live Life in Forward Motion.” He talks about his restlessness with the psychodynamic method during his training as a resident in New York – he wanted to offer his patients more immediate relief and a supervisor told him, “Don’t you dare!”
In the film, he talks about giving patients hope and direction. And Hill makes the comment, “In traditional therapy, you’re paying this person and you save all your problems for them, and they just listen, and your friends – who are idiots – give you advice, unsolicited, and you want your friends just to listen, and you want your therapist to give you advice!” Dr. Stutz gives advice and he is like no other therapist Jonah has ever had.
The premise of the film is that we are watching a single therapy session and Dr. Stutz will discuss the use of his tools and techniques that Hill has found helpful. Jonah is the interviewer, and when the doctor suggests it would be helpful if Jonah talked about his life, the patient/director rebuffs him; this documentary is about the psychiatrist.
Early in the film an alarm goes off, Dr. Stutz does not hear it, and Jonah has to remind him that it’s time for him to take his pills. The psychiatrist has Parkinson’s disease and how it has affected him becomes one focal point for the film. We later learn that he lost a younger brother as a child (something Hill did not know before they started filming) and grew up in the shadow of that loss. His extroverted father made it clear that medicine was the only acceptable career path for his son, and his introverted and depressed mother spent her days proclaiming that all men were as awful as her own abusive father.
About a third of the way through the film, the focus shifts. Jonah suddenly confesses that he is feeling stuck with regard to the movie, that he is troubled by the fact that he has not been able to share his distress with Dr. Stutz during their real-life, unfilmed therapy sessions, and the viewers learn that the single-session concept was disingenuous – they have been filming this documentary for two years, against a green screen and not in an office, always wearing the same clothes, and Jonah pulls off a wig that he wears to disguise the fact that he changed his hairstyle months earlier.
It’s a bit unnerving as they throw the wig around, and Jonah agrees to be more open about the issues he has struggled with. He acknowledges that this has been difficult, and he says, “I just keep asking myself, like, was this a f***ing horrible idea for a patient to make a movie about his therapist?” From my perspective as a psychiatrist-viewer, it’s a good question to ask!
Dr. Stutz reassures Jonah that it is okay to be vulnerable. “Failure, weakness, vulnerability – it’s like a connector, it connects you to the rest of the world.” A super-sized cardboard cutout of an obese 14-year-old Jonah now joins the room, and we learn that he continues to struggle with his self-image. Things get more real.
Peppered throughout the film, there are lessons from Dr. Stutz about his “tools,” constructs he uses to help people restructure their worlds and take action to move forward. One such construct he calls “the maze,” which occurs when one person in an interpersonal relationship is waiting for fairness and becomes preoccupied with feeling injured.
Jonah inquires about Dr. Stutz’s romantic life and the therapist replies with a transparency that overrides our usual professional boundaries. We all learn that Dr. Stutz is not in a relationship, he’s never been married, but there is a woman he has had some involvement with on and off for 40 years. Jonah’s line of questioning rivals that of any therapist. “How do you think it affects you, having your mom hate men and you being a man?” Dr. Stutz admits that he can never feel safe with women. “Did you ever override that wall you built with your mom and get close to a woman?” When Jonah professes, “I don’t feel anything but love for you and I just want you to be happy,” my own feeling was that the tables had turned too far, that the therapist’s failed romantic life risked being a burden to the patient.
Still, there is something about the relationship between the two men that is touching and beautiful. Dr. Stutz as a therapist is charismatic, caring, self-assured, and optimistic, and he radiates hope and certainty. He mixes an intense intimacy with humor in a way that is both authentic and entertaining. The interspersed jokes break the intensity, but they don’t diminish his wisdom and the healing he imparts.
Dr. Stutz is a psychiatrist, and his strength is clearly as a psychotherapist, yet there is not a single mention of psychotropic medications – there is a banter about recreational drugs and medications for Parkinson’s disease. If Hill is taking medication for depression or anxiety, and if prescribing is part of Dr. Stutz’s arsenal, the viewer is not made aware of this.
Dr. Stutz eschews the slow, detached, and “neutral” pace of psychodynamic therapy and the whole concept of the therapist as a blank wall for the transference to play out on, but here the transference screams: Jonah loves him, he respect and honors him, he wants him to be happy, and he is afraid of losing him.
“Stutz” is a movie about a larger-than-life psychiatrist, one whose warmth and inspiration are healing. I imagine his tools are helpful, but his personality is what carries the load. If a viewer has not had experience with psychiatry, and this film inspires him to begin therapy, there may be a good deal of disappointment. In this case, the patient is a successful actor, and one might wonder if that, together with the entire years-long project of filming, has altered the relationship well beyond the usual therapeutic hour.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University in Baltimore.
FDA approves OTC naloxone, but will cost be a barrier?
Greater access to the drug should mean more lives saved. However, it’s unclear how much the nasal spray will cost and whether pharmacies will stock the product openly on shelves.
Currently, major pharmacy chains such as CVS and Walgreens make naloxone available without prescription, but consumers have to ask a pharmacist to dispense the drug.
“The major question is what is it going to cost,” Brian Hurley, MD, MBA, president-elect of the American Society of Addiction Medicine, said in an interview. “In order for people to access it they have to be able to afford it.”
“We won’t accomplish much if people can’t afford to buy Narcan,” said Chuck Ingoglia, president and CEO of the National Council for Mental Wellbeing, in a statement. Still, he applauded the FDA.
“No single approach will end overdose deaths but making Narcan easy to obtain and widely available likely will save countless lives annually,” he said.
“The timeline for availability and price of this OTC product is determined by the manufacturer,” the FDA said in a statement.
Commissioner Robert M. Califf, MD, called for the drug’s manufacturer to “make accessibility to the product a priority by making it available as soon as possible and at an affordable price.”
Emergent BioSolutions did not comment on cost. It said in a statement that the spray “will be available on U.S. shelves and at online retailers by the late summer,” after it has adapted Narcan for direct-to-consumer use, including more consumer-oriented packaging.
Naloxone’s cost varies, depending on geographic location and whether it is generic. According to GoodRX, a box containing two doses of generic naloxone costs $31-$100, depending on location and coupon availability.
A two-dose box of Narcan costs $135-$140. Emergent reported a 14% decline in naloxone sales in 2022 – to $373.7 million – blaming it in part on the introduction of generic formulations.
Dr. Hurley said he expects those who purchase Narcan at a drug store will primarily already be shopping there. It may or may not be those who most often experience overdose, such as people leaving incarceration or experiencing homelessness.
Having Narcan available over-the-counter “is an important supplement but it doesn’t replace the existing array of naloxone distribution programs,” Dr. Hurley said.
The FDA has encouraged naloxone manufacturers to seek OTC approval for the medication since at least 2019, when it designed a model label for a theoretical OTC product.
In November, the agency said it had determined that some naloxone products had the potential to be safe and effective for OTC use and again urged drugmakers to seek such an approval.
Emergent BioSolutions was the first to pursue OTC approval, but another manufacturer – the nonprofit Harm Reduction Therapeutics – is awaiting approval of its application to sell its spray directly to consumers.
Scott Gottlieb, MD, who was the FDA commissioner from 2017 to 2019, said in a tweet that more work needed to be done.
“This regulatory move should be followed by a strong push by elected officials to support wider deployment of Narcan, getting more doses into the hands of at risk households and frontline workers,” he tweeted.
Mr. Ingoglia said that “Narcan represents a second chance. By giving people a second chance, we also give them an opportunity to enter treatment if they so choose. You can’t recover if you’re dead, and we shouldn’t turn our backs on those who may choose a pathway to recovery that includes treatment.”
A version of this article first appeared on Medscape.com.
Greater access to the drug should mean more lives saved. However, it’s unclear how much the nasal spray will cost and whether pharmacies will stock the product openly on shelves.
Currently, major pharmacy chains such as CVS and Walgreens make naloxone available without prescription, but consumers have to ask a pharmacist to dispense the drug.
“The major question is what is it going to cost,” Brian Hurley, MD, MBA, president-elect of the American Society of Addiction Medicine, said in an interview. “In order for people to access it they have to be able to afford it.”
“We won’t accomplish much if people can’t afford to buy Narcan,” said Chuck Ingoglia, president and CEO of the National Council for Mental Wellbeing, in a statement. Still, he applauded the FDA.
“No single approach will end overdose deaths but making Narcan easy to obtain and widely available likely will save countless lives annually,” he said.
“The timeline for availability and price of this OTC product is determined by the manufacturer,” the FDA said in a statement.
Commissioner Robert M. Califf, MD, called for the drug’s manufacturer to “make accessibility to the product a priority by making it available as soon as possible and at an affordable price.”
Emergent BioSolutions did not comment on cost. It said in a statement that the spray “will be available on U.S. shelves and at online retailers by the late summer,” after it has adapted Narcan for direct-to-consumer use, including more consumer-oriented packaging.
Naloxone’s cost varies, depending on geographic location and whether it is generic. According to GoodRX, a box containing two doses of generic naloxone costs $31-$100, depending on location and coupon availability.
A two-dose box of Narcan costs $135-$140. Emergent reported a 14% decline in naloxone sales in 2022 – to $373.7 million – blaming it in part on the introduction of generic formulations.
Dr. Hurley said he expects those who purchase Narcan at a drug store will primarily already be shopping there. It may or may not be those who most often experience overdose, such as people leaving incarceration or experiencing homelessness.
Having Narcan available over-the-counter “is an important supplement but it doesn’t replace the existing array of naloxone distribution programs,” Dr. Hurley said.
The FDA has encouraged naloxone manufacturers to seek OTC approval for the medication since at least 2019, when it designed a model label for a theoretical OTC product.
In November, the agency said it had determined that some naloxone products had the potential to be safe and effective for OTC use and again urged drugmakers to seek such an approval.
Emergent BioSolutions was the first to pursue OTC approval, but another manufacturer – the nonprofit Harm Reduction Therapeutics – is awaiting approval of its application to sell its spray directly to consumers.
Scott Gottlieb, MD, who was the FDA commissioner from 2017 to 2019, said in a tweet that more work needed to be done.
“This regulatory move should be followed by a strong push by elected officials to support wider deployment of Narcan, getting more doses into the hands of at risk households and frontline workers,” he tweeted.
Mr. Ingoglia said that “Narcan represents a second chance. By giving people a second chance, we also give them an opportunity to enter treatment if they so choose. You can’t recover if you’re dead, and we shouldn’t turn our backs on those who may choose a pathway to recovery that includes treatment.”
A version of this article first appeared on Medscape.com.
Greater access to the drug should mean more lives saved. However, it’s unclear how much the nasal spray will cost and whether pharmacies will stock the product openly on shelves.
Currently, major pharmacy chains such as CVS and Walgreens make naloxone available without prescription, but consumers have to ask a pharmacist to dispense the drug.
“The major question is what is it going to cost,” Brian Hurley, MD, MBA, president-elect of the American Society of Addiction Medicine, said in an interview. “In order for people to access it they have to be able to afford it.”
“We won’t accomplish much if people can’t afford to buy Narcan,” said Chuck Ingoglia, president and CEO of the National Council for Mental Wellbeing, in a statement. Still, he applauded the FDA.
“No single approach will end overdose deaths but making Narcan easy to obtain and widely available likely will save countless lives annually,” he said.
“The timeline for availability and price of this OTC product is determined by the manufacturer,” the FDA said in a statement.
Commissioner Robert M. Califf, MD, called for the drug’s manufacturer to “make accessibility to the product a priority by making it available as soon as possible and at an affordable price.”
Emergent BioSolutions did not comment on cost. It said in a statement that the spray “will be available on U.S. shelves and at online retailers by the late summer,” after it has adapted Narcan for direct-to-consumer use, including more consumer-oriented packaging.
Naloxone’s cost varies, depending on geographic location and whether it is generic. According to GoodRX, a box containing two doses of generic naloxone costs $31-$100, depending on location and coupon availability.
A two-dose box of Narcan costs $135-$140. Emergent reported a 14% decline in naloxone sales in 2022 – to $373.7 million – blaming it in part on the introduction of generic formulations.
Dr. Hurley said he expects those who purchase Narcan at a drug store will primarily already be shopping there. It may or may not be those who most often experience overdose, such as people leaving incarceration or experiencing homelessness.
Having Narcan available over-the-counter “is an important supplement but it doesn’t replace the existing array of naloxone distribution programs,” Dr. Hurley said.
The FDA has encouraged naloxone manufacturers to seek OTC approval for the medication since at least 2019, when it designed a model label for a theoretical OTC product.
In November, the agency said it had determined that some naloxone products had the potential to be safe and effective for OTC use and again urged drugmakers to seek such an approval.
Emergent BioSolutions was the first to pursue OTC approval, but another manufacturer – the nonprofit Harm Reduction Therapeutics – is awaiting approval of its application to sell its spray directly to consumers.
Scott Gottlieb, MD, who was the FDA commissioner from 2017 to 2019, said in a tweet that more work needed to be done.
“This regulatory move should be followed by a strong push by elected officials to support wider deployment of Narcan, getting more doses into the hands of at risk households and frontline workers,” he tweeted.
Mr. Ingoglia said that “Narcan represents a second chance. By giving people a second chance, we also give them an opportunity to enter treatment if they so choose. You can’t recover if you’re dead, and we shouldn’t turn our backs on those who may choose a pathway to recovery that includes treatment.”
A version of this article first appeared on Medscape.com.
Melatonin: A new way to reduce self-harm?
. However, at least one expert has some concerns about the strength of the evidence.
The results suggest improving sleep hygiene in this population may reduce self-injury, study investigator Sarah E. Bergen, PhD, associate professor, department of medical epidemiology and biostatistics, Karolinska Institute, Stockholm, said in an interview.
In addition, she noted, for “pediatric patients who are experiencing sleep problems, melatonin is a safe and effective way to help them.”
Dr. Bergen believes clinicians should recommend melatonin to all teens because “there’s little harm that could come from it and possibly a lot of benefit.”
The findings were published online in the Journal of Child Psychology and Psychiatry.
Few treatments available
Research shows sleep disorders like insomnia are common in youth, particularly among those with psychiatric disorders. Sleep disorders can significantly affect daytime functioning, cognition, emotional regulation, and behavior, and can be a risk factor for unintentional injuries such as falls and vehicular accidents, as well as for intentional self-harm.
The lifetime prevalence of self-harm in youth is estimated to be 17%, but this varies across study designs. There are few treatments for self-harm in youth, although psychosocial treatments appear promising.
Melatonin is a naturally occurring hormone secreted primarily by the pineal gland in response to darkness. It helps promote and maintain the normal sleep-wake cycle and is involved in other biological functions.
In Sweden, melatonin is the most commonly prescribed drug for sleep disturbances in children and adolescents. Prior to 2020, during the course of the study, it was only available by prescription.
The study, which used linked national databases, included 25,575 children and adolescents, 58.2% of them male, who initiated a melatonin treatment between the ages of 6 and 18 years.
Researchers estimated the risks of self-harm, including poisoning (57%) and cutting (34%). The fact that poisoning was more common than cutting was somewhat surprising, said Dr. Bergen. “I would have thought the opposite would be true; that cutting was more prevalent.”
The study examined the risk of self-harm in individual participants by comparing the last unmedicated month with the 12 months after initiating melatonin treatment. In this way, they accounted for potential confounders such as genetics, sleep disorder severity, and psychiatric disorders.
The median age at first melatonin prescription was 13 years for males and 15 years for females.
While there were no statistically significant changes in relative risk for body injuries, falls, and transport accidents, the relative risk for self-injury was statistically significantly lower during the months following melatonin treatment initiation.
The incidence rate ratio in the month following treatment was 0.58 (95% confidence interval, 0.46-0.73) for self-harm and 0.59 (95% CI, 0.45-0.78) for poisoning.
Higher risks in females
The relative risk of self-harm was higher in females than males. This, said Dr. Bergen, is possibly because self-harm is more common in adolescence than in childhood. Female study participants were older than their male counterparts.
Melatonin may help male teens, too, she said. “It’s just that the problem is not that great in males to begin with, so a decrease is not very dramatic after melatonin initiation.”
About 87.2% of participants treated with melatonin were diagnosed with at least one psychiatric disorder. Attention-deficit hyperactivity disorder, the most common comorbidity, was diagnosed in more than 50% of new melatonin users. This isn’t surprising, because sleep disturbances are associated with this psychiatric condition and are frequent side effects of ADHD medications.
After ADHD, anxiety and depression were the next most common psychiatric disorders among study subjects. The analysis found risks for self-harm and poisoning were largely driven by patients suffering from one or both of these disorders, particularly among females.
The IRR in the month following melatonin treatment initiation was 0.46 (95% CI, 0.27-0.76] among adolescent females with psychiatric disorders, after excluding antidepressant users.
Melatonin may reduce the risk of self-harm by treating sleep problems related to psychiatric comorbidities, especially anxiety and depression. It could also decrease pain sensitivity experienced by adolescents who self-harm.
Other factors could play a role in treating sleep problems and/or preventing self-harm in these patients. For example, increased clinician awareness and monitoring, behavioral interventions, a placebo effect, and concurrent use of other medications.
When researchers ran an analysis that excluded individuals taking an antidepressant, “surprisingly, there wasn’t much difference,” said Dr. Bergen. “We thought antidepressants might be causing some of the effect we observed, but when we removed antidepressant users, we saw a very similar pattern of intentional self-harm rates following melatonin use, which suggests melatonin is causal, but we can’t prove that.”
Other sleep medications such as sedatives could also affect self-harm rates by improving sleep. However, these are not typically prescribed to children because of their side effects and overdose potential, said Dr. Bergen.
“Melatonin is extremely safe and side effects are rare; it’s impossible to overdose, and people really can’t hurt themselves with it.”
More research needed
Adrian Jacques Ambrose, MD, medical director, Columbia University Irving Medical Center, and assistant professor of psychiatry, Columbia University, New York, pointed out some evidence in the study is relatively weak.
“When the authors separated out the on- and off-melatonin groups, it looks like there wasn’t a statistically significant difference [in IRRs] between the two groups – for example, in any injury, self-harm, or poisoning – and this weakens their argument that melatonin is associated with self-harm and poisoning.”
Given the current youth mental health crisis, more research “would absolutely be indicated” to better explore possible additional variables, said Dr. Ambrose.
“For example, some additional follow-up studies may add on covariates in conjunction with melatonin usage, such as the number of medical appointments, the presence of psychotherapeutic interventions, dosage of melatonin, or even the sleepiness scale, to evaluate whether the symptoms of sleep disturbances are more directly correlated with the self-harm behaviors.”
The study was supported by the European Union’s Horizon 2020 Research and Innovation Programme. Dr. Bergen and Dr. Ambrose report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
. However, at least one expert has some concerns about the strength of the evidence.
The results suggest improving sleep hygiene in this population may reduce self-injury, study investigator Sarah E. Bergen, PhD, associate professor, department of medical epidemiology and biostatistics, Karolinska Institute, Stockholm, said in an interview.
In addition, she noted, for “pediatric patients who are experiencing sleep problems, melatonin is a safe and effective way to help them.”
Dr. Bergen believes clinicians should recommend melatonin to all teens because “there’s little harm that could come from it and possibly a lot of benefit.”
The findings were published online in the Journal of Child Psychology and Psychiatry.
Few treatments available
Research shows sleep disorders like insomnia are common in youth, particularly among those with psychiatric disorders. Sleep disorders can significantly affect daytime functioning, cognition, emotional regulation, and behavior, and can be a risk factor for unintentional injuries such as falls and vehicular accidents, as well as for intentional self-harm.
The lifetime prevalence of self-harm in youth is estimated to be 17%, but this varies across study designs. There are few treatments for self-harm in youth, although psychosocial treatments appear promising.
Melatonin is a naturally occurring hormone secreted primarily by the pineal gland in response to darkness. It helps promote and maintain the normal sleep-wake cycle and is involved in other biological functions.
In Sweden, melatonin is the most commonly prescribed drug for sleep disturbances in children and adolescents. Prior to 2020, during the course of the study, it was only available by prescription.
The study, which used linked national databases, included 25,575 children and adolescents, 58.2% of them male, who initiated a melatonin treatment between the ages of 6 and 18 years.
Researchers estimated the risks of self-harm, including poisoning (57%) and cutting (34%). The fact that poisoning was more common than cutting was somewhat surprising, said Dr. Bergen. “I would have thought the opposite would be true; that cutting was more prevalent.”
The study examined the risk of self-harm in individual participants by comparing the last unmedicated month with the 12 months after initiating melatonin treatment. In this way, they accounted for potential confounders such as genetics, sleep disorder severity, and psychiatric disorders.
The median age at first melatonin prescription was 13 years for males and 15 years for females.
While there were no statistically significant changes in relative risk for body injuries, falls, and transport accidents, the relative risk for self-injury was statistically significantly lower during the months following melatonin treatment initiation.
The incidence rate ratio in the month following treatment was 0.58 (95% confidence interval, 0.46-0.73) for self-harm and 0.59 (95% CI, 0.45-0.78) for poisoning.
Higher risks in females
The relative risk of self-harm was higher in females than males. This, said Dr. Bergen, is possibly because self-harm is more common in adolescence than in childhood. Female study participants were older than their male counterparts.
Melatonin may help male teens, too, she said. “It’s just that the problem is not that great in males to begin with, so a decrease is not very dramatic after melatonin initiation.”
About 87.2% of participants treated with melatonin were diagnosed with at least one psychiatric disorder. Attention-deficit hyperactivity disorder, the most common comorbidity, was diagnosed in more than 50% of new melatonin users. This isn’t surprising, because sleep disturbances are associated with this psychiatric condition and are frequent side effects of ADHD medications.
After ADHD, anxiety and depression were the next most common psychiatric disorders among study subjects. The analysis found risks for self-harm and poisoning were largely driven by patients suffering from one or both of these disorders, particularly among females.
The IRR in the month following melatonin treatment initiation was 0.46 (95% CI, 0.27-0.76] among adolescent females with psychiatric disorders, after excluding antidepressant users.
Melatonin may reduce the risk of self-harm by treating sleep problems related to psychiatric comorbidities, especially anxiety and depression. It could also decrease pain sensitivity experienced by adolescents who self-harm.
Other factors could play a role in treating sleep problems and/or preventing self-harm in these patients. For example, increased clinician awareness and monitoring, behavioral interventions, a placebo effect, and concurrent use of other medications.
When researchers ran an analysis that excluded individuals taking an antidepressant, “surprisingly, there wasn’t much difference,” said Dr. Bergen. “We thought antidepressants might be causing some of the effect we observed, but when we removed antidepressant users, we saw a very similar pattern of intentional self-harm rates following melatonin use, which suggests melatonin is causal, but we can’t prove that.”
Other sleep medications such as sedatives could also affect self-harm rates by improving sleep. However, these are not typically prescribed to children because of their side effects and overdose potential, said Dr. Bergen.
“Melatonin is extremely safe and side effects are rare; it’s impossible to overdose, and people really can’t hurt themselves with it.”
More research needed
Adrian Jacques Ambrose, MD, medical director, Columbia University Irving Medical Center, and assistant professor of psychiatry, Columbia University, New York, pointed out some evidence in the study is relatively weak.
“When the authors separated out the on- and off-melatonin groups, it looks like there wasn’t a statistically significant difference [in IRRs] between the two groups – for example, in any injury, self-harm, or poisoning – and this weakens their argument that melatonin is associated with self-harm and poisoning.”
Given the current youth mental health crisis, more research “would absolutely be indicated” to better explore possible additional variables, said Dr. Ambrose.
“For example, some additional follow-up studies may add on covariates in conjunction with melatonin usage, such as the number of medical appointments, the presence of psychotherapeutic interventions, dosage of melatonin, or even the sleepiness scale, to evaluate whether the symptoms of sleep disturbances are more directly correlated with the self-harm behaviors.”
The study was supported by the European Union’s Horizon 2020 Research and Innovation Programme. Dr. Bergen and Dr. Ambrose report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
. However, at least one expert has some concerns about the strength of the evidence.
The results suggest improving sleep hygiene in this population may reduce self-injury, study investigator Sarah E. Bergen, PhD, associate professor, department of medical epidemiology and biostatistics, Karolinska Institute, Stockholm, said in an interview.
In addition, she noted, for “pediatric patients who are experiencing sleep problems, melatonin is a safe and effective way to help them.”
Dr. Bergen believes clinicians should recommend melatonin to all teens because “there’s little harm that could come from it and possibly a lot of benefit.”
The findings were published online in the Journal of Child Psychology and Psychiatry.
Few treatments available
Research shows sleep disorders like insomnia are common in youth, particularly among those with psychiatric disorders. Sleep disorders can significantly affect daytime functioning, cognition, emotional regulation, and behavior, and can be a risk factor for unintentional injuries such as falls and vehicular accidents, as well as for intentional self-harm.
The lifetime prevalence of self-harm in youth is estimated to be 17%, but this varies across study designs. There are few treatments for self-harm in youth, although psychosocial treatments appear promising.
Melatonin is a naturally occurring hormone secreted primarily by the pineal gland in response to darkness. It helps promote and maintain the normal sleep-wake cycle and is involved in other biological functions.
In Sweden, melatonin is the most commonly prescribed drug for sleep disturbances in children and adolescents. Prior to 2020, during the course of the study, it was only available by prescription.
The study, which used linked national databases, included 25,575 children and adolescents, 58.2% of them male, who initiated a melatonin treatment between the ages of 6 and 18 years.
Researchers estimated the risks of self-harm, including poisoning (57%) and cutting (34%). The fact that poisoning was more common than cutting was somewhat surprising, said Dr. Bergen. “I would have thought the opposite would be true; that cutting was more prevalent.”
The study examined the risk of self-harm in individual participants by comparing the last unmedicated month with the 12 months after initiating melatonin treatment. In this way, they accounted for potential confounders such as genetics, sleep disorder severity, and psychiatric disorders.
The median age at first melatonin prescription was 13 years for males and 15 years for females.
While there were no statistically significant changes in relative risk for body injuries, falls, and transport accidents, the relative risk for self-injury was statistically significantly lower during the months following melatonin treatment initiation.
The incidence rate ratio in the month following treatment was 0.58 (95% confidence interval, 0.46-0.73) for self-harm and 0.59 (95% CI, 0.45-0.78) for poisoning.
Higher risks in females
The relative risk of self-harm was higher in females than males. This, said Dr. Bergen, is possibly because self-harm is more common in adolescence than in childhood. Female study participants were older than their male counterparts.
Melatonin may help male teens, too, she said. “It’s just that the problem is not that great in males to begin with, so a decrease is not very dramatic after melatonin initiation.”
About 87.2% of participants treated with melatonin were diagnosed with at least one psychiatric disorder. Attention-deficit hyperactivity disorder, the most common comorbidity, was diagnosed in more than 50% of new melatonin users. This isn’t surprising, because sleep disturbances are associated with this psychiatric condition and are frequent side effects of ADHD medications.
After ADHD, anxiety and depression were the next most common psychiatric disorders among study subjects. The analysis found risks for self-harm and poisoning were largely driven by patients suffering from one or both of these disorders, particularly among females.
The IRR in the month following melatonin treatment initiation was 0.46 (95% CI, 0.27-0.76] among adolescent females with psychiatric disorders, after excluding antidepressant users.
Melatonin may reduce the risk of self-harm by treating sleep problems related to psychiatric comorbidities, especially anxiety and depression. It could also decrease pain sensitivity experienced by adolescents who self-harm.
Other factors could play a role in treating sleep problems and/or preventing self-harm in these patients. For example, increased clinician awareness and monitoring, behavioral interventions, a placebo effect, and concurrent use of other medications.
When researchers ran an analysis that excluded individuals taking an antidepressant, “surprisingly, there wasn’t much difference,” said Dr. Bergen. “We thought antidepressants might be causing some of the effect we observed, but when we removed antidepressant users, we saw a very similar pattern of intentional self-harm rates following melatonin use, which suggests melatonin is causal, but we can’t prove that.”
Other sleep medications such as sedatives could also affect self-harm rates by improving sleep. However, these are not typically prescribed to children because of their side effects and overdose potential, said Dr. Bergen.
“Melatonin is extremely safe and side effects are rare; it’s impossible to overdose, and people really can’t hurt themselves with it.”
More research needed
Adrian Jacques Ambrose, MD, medical director, Columbia University Irving Medical Center, and assistant professor of psychiatry, Columbia University, New York, pointed out some evidence in the study is relatively weak.
“When the authors separated out the on- and off-melatonin groups, it looks like there wasn’t a statistically significant difference [in IRRs] between the two groups – for example, in any injury, self-harm, or poisoning – and this weakens their argument that melatonin is associated with self-harm and poisoning.”
Given the current youth mental health crisis, more research “would absolutely be indicated” to better explore possible additional variables, said Dr. Ambrose.
“For example, some additional follow-up studies may add on covariates in conjunction with melatonin usage, such as the number of medical appointments, the presence of psychotherapeutic interventions, dosage of melatonin, or even the sleepiness scale, to evaluate whether the symptoms of sleep disturbances are more directly correlated with the self-harm behaviors.”
The study was supported by the European Union’s Horizon 2020 Research and Innovation Programme. Dr. Bergen and Dr. Ambrose report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CHILD PSYCHOLOGY AND PSYCHIATRY
Luxe vacations, private jets: Medical device maker, surgeon to pay $46 million penalty in kickback scheme
according to experts familiar with the federal Anti-Kickback Statute.
Historically, enforcement actions have primarily focused on the person or organization offering the perks – and not necessarily the physicians accepting it, Steven W. Ortquist, founder and principal of Arete Compliance Solutions, LLC, in Phoenix, told this news organization.
But that’s changing.
“In recent years, we are seeing a trend toward holding physicians and others on the receiving end of the inducement accountable as well,” said Mr. Ortquist, who is a past board member and president of the Health Care Compliance Association. He noted that authorities usually pursue the inducing company first before moving on to individual clinicians or practices.
The Department of Justice followed a similar pattern in a recently announced kickback settlement that ensnared an intraocular lens distributor, an ophthalmology equipment supplier, two CEOs, and a surgeon. Precision Lens must pay more than $43 million for offering high-end vacations and other expensive perks to surgeons who used its cataract products.
The verdict marks the end of a 6-week civil jury trial, where evidence emerged that Paul Ehlen, owner of Precision Lens and its parent company, Cameron-Ehlen Group, maintained a secret “slush fund” for paying kickbacks to ophthalmic surgeons. The inducement scheme netted the Minnesota-based company millions in sales and led to the submission of 64,575 false Medicare claims from 2006 to 2015, a violation of the Anti-Kickback Statute and the False Claims Act.
According to court documents, physicians received luxury travel and entertainment packages, including skiing, fishing, and golfing excursions at exclusive destinations, often traveling via private jet to attend Broadway musicals and major sporting events. Mr. Ehlen and company representatives also sold frequent flyer miles to physicians at a steep discount, allowing them to take personal and business trips below fair market value.
Federal authorities initially announced an investigation into the business practices of Precision Lens in 2017 after receiving a whistleblower complaint from Kipp Fesenmaier, a former executive at Sightpath Medical, an ophthalmology supplier and “corporate partner” of Precision Lens. Mr. Fesenmaier alleged that both companies were involved in an inducement scheme.
Sightpath Medical and its CEO, James Tiffany, agreed to a $12 million settlement to resolve the kickback allegations.
The Department of Justice subsequently investigated Jitendra Swarup, MD, an ophthalmologist and cataract surgeon who allegedly received “unlawful remuneration from Sightpath, Precision, and Ehlen” and filed false insurance claims. In addition to accepting expensive hunting and fishing trips from the medical device companies, Dr. Swarup was paid more than $100,000 per year for consulting services he did not fully render.
Dr. Swarup agreed to a nearly $3 million settlement and participation in a 3-year corporate integrity agreement with the Office of Inspector General. In exchange for compliance with such contracts, the OIG permits physicians to continue participating in Medicare, Medicaid, and other federal health care programs.
In a statement from attorneys, Precision Lens and Mr. Ehlen pledged to appeal the verdict and “defend ... our wholly appropriate actions” while remaining focused on their commitment to health care clinicians and manufacturers.
‘Endless’ opportunities for inducement
Unfortunately, opportunities for inducement are “endless,” experts say. Extravagant trips, dinners, and gifts can trigger a violation, but so can nearly anything of value.
Just last year, Biotronik reached a $12.95 million settlement amid allegations that company representatives wined and dined physicians to induce their use of its pacemakers and defibrillators. To date, no physicians have been charged.
But after a record-breaking number of whistleblower judgments last fiscal year totaling more than $2 billion, physicians should take note, Radha Bhatnagar, Esq, director of compliance at The CM Group, told the news organization.
“When manufacturers offer physicians kickbacks with the added element of fraudulent Medicare or Medicaid reimbursements, that is typically when manufacturers and individuals face civil and criminal liability,” said Ms. Bhatnagar, something the Department of Justice alluded to when announcing a settlement involving 15 Texas physicians last year.
In another case, Kingsley R. Chin, an orthopedic surgeon and designer of a spinal implant, was indicted in 2021 for paying millions of dollars in sham consulting fees to physicians who used his products. At least six surgeons who accepted money from Dr. Chin were later named in a civil case and ordered to pay $3.3 million in penalties.
Jason Montone, DO, an orthopedic surgeon who accepted the illicit payments, agreed to a plea deal with a reduced prison sentence, 1 year of supervised release, and a fine of $379,000.
Although Dr. Chin’s sentencing hasn’t been announced, violating kickback laws can result in a sentence of up to 10 years.
A version of this article originally appeared on Medscape.com.
according to experts familiar with the federal Anti-Kickback Statute.
Historically, enforcement actions have primarily focused on the person or organization offering the perks – and not necessarily the physicians accepting it, Steven W. Ortquist, founder and principal of Arete Compliance Solutions, LLC, in Phoenix, told this news organization.
But that’s changing.
“In recent years, we are seeing a trend toward holding physicians and others on the receiving end of the inducement accountable as well,” said Mr. Ortquist, who is a past board member and president of the Health Care Compliance Association. He noted that authorities usually pursue the inducing company first before moving on to individual clinicians or practices.
The Department of Justice followed a similar pattern in a recently announced kickback settlement that ensnared an intraocular lens distributor, an ophthalmology equipment supplier, two CEOs, and a surgeon. Precision Lens must pay more than $43 million for offering high-end vacations and other expensive perks to surgeons who used its cataract products.
The verdict marks the end of a 6-week civil jury trial, where evidence emerged that Paul Ehlen, owner of Precision Lens and its parent company, Cameron-Ehlen Group, maintained a secret “slush fund” for paying kickbacks to ophthalmic surgeons. The inducement scheme netted the Minnesota-based company millions in sales and led to the submission of 64,575 false Medicare claims from 2006 to 2015, a violation of the Anti-Kickback Statute and the False Claims Act.
According to court documents, physicians received luxury travel and entertainment packages, including skiing, fishing, and golfing excursions at exclusive destinations, often traveling via private jet to attend Broadway musicals and major sporting events. Mr. Ehlen and company representatives also sold frequent flyer miles to physicians at a steep discount, allowing them to take personal and business trips below fair market value.
Federal authorities initially announced an investigation into the business practices of Precision Lens in 2017 after receiving a whistleblower complaint from Kipp Fesenmaier, a former executive at Sightpath Medical, an ophthalmology supplier and “corporate partner” of Precision Lens. Mr. Fesenmaier alleged that both companies were involved in an inducement scheme.
Sightpath Medical and its CEO, James Tiffany, agreed to a $12 million settlement to resolve the kickback allegations.
The Department of Justice subsequently investigated Jitendra Swarup, MD, an ophthalmologist and cataract surgeon who allegedly received “unlawful remuneration from Sightpath, Precision, and Ehlen” and filed false insurance claims. In addition to accepting expensive hunting and fishing trips from the medical device companies, Dr. Swarup was paid more than $100,000 per year for consulting services he did not fully render.
Dr. Swarup agreed to a nearly $3 million settlement and participation in a 3-year corporate integrity agreement with the Office of Inspector General. In exchange for compliance with such contracts, the OIG permits physicians to continue participating in Medicare, Medicaid, and other federal health care programs.
In a statement from attorneys, Precision Lens and Mr. Ehlen pledged to appeal the verdict and “defend ... our wholly appropriate actions” while remaining focused on their commitment to health care clinicians and manufacturers.
‘Endless’ opportunities for inducement
Unfortunately, opportunities for inducement are “endless,” experts say. Extravagant trips, dinners, and gifts can trigger a violation, but so can nearly anything of value.
Just last year, Biotronik reached a $12.95 million settlement amid allegations that company representatives wined and dined physicians to induce their use of its pacemakers and defibrillators. To date, no physicians have been charged.
But after a record-breaking number of whistleblower judgments last fiscal year totaling more than $2 billion, physicians should take note, Radha Bhatnagar, Esq, director of compliance at The CM Group, told the news organization.
“When manufacturers offer physicians kickbacks with the added element of fraudulent Medicare or Medicaid reimbursements, that is typically when manufacturers and individuals face civil and criminal liability,” said Ms. Bhatnagar, something the Department of Justice alluded to when announcing a settlement involving 15 Texas physicians last year.
In another case, Kingsley R. Chin, an orthopedic surgeon and designer of a spinal implant, was indicted in 2021 for paying millions of dollars in sham consulting fees to physicians who used his products. At least six surgeons who accepted money from Dr. Chin were later named in a civil case and ordered to pay $3.3 million in penalties.
Jason Montone, DO, an orthopedic surgeon who accepted the illicit payments, agreed to a plea deal with a reduced prison sentence, 1 year of supervised release, and a fine of $379,000.
Although Dr. Chin’s sentencing hasn’t been announced, violating kickback laws can result in a sentence of up to 10 years.
A version of this article originally appeared on Medscape.com.
according to experts familiar with the federal Anti-Kickback Statute.
Historically, enforcement actions have primarily focused on the person or organization offering the perks – and not necessarily the physicians accepting it, Steven W. Ortquist, founder and principal of Arete Compliance Solutions, LLC, in Phoenix, told this news organization.
But that’s changing.
“In recent years, we are seeing a trend toward holding physicians and others on the receiving end of the inducement accountable as well,” said Mr. Ortquist, who is a past board member and president of the Health Care Compliance Association. He noted that authorities usually pursue the inducing company first before moving on to individual clinicians or practices.
The Department of Justice followed a similar pattern in a recently announced kickback settlement that ensnared an intraocular lens distributor, an ophthalmology equipment supplier, two CEOs, and a surgeon. Precision Lens must pay more than $43 million for offering high-end vacations and other expensive perks to surgeons who used its cataract products.
The verdict marks the end of a 6-week civil jury trial, where evidence emerged that Paul Ehlen, owner of Precision Lens and its parent company, Cameron-Ehlen Group, maintained a secret “slush fund” for paying kickbacks to ophthalmic surgeons. The inducement scheme netted the Minnesota-based company millions in sales and led to the submission of 64,575 false Medicare claims from 2006 to 2015, a violation of the Anti-Kickback Statute and the False Claims Act.
According to court documents, physicians received luxury travel and entertainment packages, including skiing, fishing, and golfing excursions at exclusive destinations, often traveling via private jet to attend Broadway musicals and major sporting events. Mr. Ehlen and company representatives also sold frequent flyer miles to physicians at a steep discount, allowing them to take personal and business trips below fair market value.
Federal authorities initially announced an investigation into the business practices of Precision Lens in 2017 after receiving a whistleblower complaint from Kipp Fesenmaier, a former executive at Sightpath Medical, an ophthalmology supplier and “corporate partner” of Precision Lens. Mr. Fesenmaier alleged that both companies were involved in an inducement scheme.
Sightpath Medical and its CEO, James Tiffany, agreed to a $12 million settlement to resolve the kickback allegations.
The Department of Justice subsequently investigated Jitendra Swarup, MD, an ophthalmologist and cataract surgeon who allegedly received “unlawful remuneration from Sightpath, Precision, and Ehlen” and filed false insurance claims. In addition to accepting expensive hunting and fishing trips from the medical device companies, Dr. Swarup was paid more than $100,000 per year for consulting services he did not fully render.
Dr. Swarup agreed to a nearly $3 million settlement and participation in a 3-year corporate integrity agreement with the Office of Inspector General. In exchange for compliance with such contracts, the OIG permits physicians to continue participating in Medicare, Medicaid, and other federal health care programs.
In a statement from attorneys, Precision Lens and Mr. Ehlen pledged to appeal the verdict and “defend ... our wholly appropriate actions” while remaining focused on their commitment to health care clinicians and manufacturers.
‘Endless’ opportunities for inducement
Unfortunately, opportunities for inducement are “endless,” experts say. Extravagant trips, dinners, and gifts can trigger a violation, but so can nearly anything of value.
Just last year, Biotronik reached a $12.95 million settlement amid allegations that company representatives wined and dined physicians to induce their use of its pacemakers and defibrillators. To date, no physicians have been charged.
But after a record-breaking number of whistleblower judgments last fiscal year totaling more than $2 billion, physicians should take note, Radha Bhatnagar, Esq, director of compliance at The CM Group, told the news organization.
“When manufacturers offer physicians kickbacks with the added element of fraudulent Medicare or Medicaid reimbursements, that is typically when manufacturers and individuals face civil and criminal liability,” said Ms. Bhatnagar, something the Department of Justice alluded to when announcing a settlement involving 15 Texas physicians last year.
In another case, Kingsley R. Chin, an orthopedic surgeon and designer of a spinal implant, was indicted in 2021 for paying millions of dollars in sham consulting fees to physicians who used his products. At least six surgeons who accepted money from Dr. Chin were later named in a civil case and ordered to pay $3.3 million in penalties.
Jason Montone, DO, an orthopedic surgeon who accepted the illicit payments, agreed to a plea deal with a reduced prison sentence, 1 year of supervised release, and a fine of $379,000.
Although Dr. Chin’s sentencing hasn’t been announced, violating kickback laws can result in a sentence of up to 10 years.
A version of this article originally appeared on Medscape.com.
Longer telomeres tied to better brain health
, new research suggests.
“This is the largest and most systematic investigation of telomere length and brain structure and function,” said Anya Topiwala, of the University of Oxford (England). “We found that longer telomeres associated with protection against dementia. The links with brain structure, we think, offer a possible mechanism for this protection. The hope is, by understanding the mechanism, new treatment targets could be uncovered,” Dr. Topiwala said.
The study was published online in PLOS ONE.
UK Biobank cohort
Telomeres form protective caps at the ends of chromosomes, and they progressively shorten with age, which may increase susceptibility to age-related diseases including Alzheimer’s disease. The mechanism underlying this risk is unclear and may involve changes in brain structure and function. However, the relationship between telomere length and neuroimaging markers is poorly characterized.
Dr. Topiwala and colleagues compared telomere length in white blood cells to brain MRI and health record data in 31,661 middle-aged and older adults in UK Biobank. They found that longer leucocyte telomere length (LTL) was associated with a larger volume of global and subcortical grey matter and a larger hippocampus – both of which shrink in patients with Alzheimer’s disease. Longer telomeres were also associated with a thicker cerebral cortex, which thins as Alzheimer’s disease progresses.
Longer LTL was also associated with reduced incidence of dementia during follow-up (hazard ratio, 0.93; 95% confidence interval, 0.91-0.96).
Dr. Topiwala noted that many of the factors related to telomere shortening, such as age, genetics, and sex, can’t be changed. However, in a previous study, her team found that drinking alcohol may shorten telomere length. “So by this logic, reducing your alcohol intake could curb the shortening,” Dr. Topiwala said.
She said that a limitation of the study is that telomere length was measured in blood rather than brain and that it’s not clear at present how closely the two relate. Also, UK Biobank participants are generally more healthy than is the general population. Also, though telomere length and brain measures were associated, “we cannot from this study prove one is causing the other,” she added.
Need for more research
Commenting on the research, Percy Griffin, PhD, Alzheimer’s Association director of scientific engagement, said that it’s been “known for some time that shortened telomeres – the caps at the end of DNA – are associated with increased aging.”
This new study is “interesting,” said Dr. Percy, in that it shows an association between longer telomere length in white blood cells and healthier brain structures in the areas associated with Alzheimer’s disease. The longer telomeres were also associated with lower incidence of all-cause dementia.
But echoing Dr. Topiwala, “association does not mean causation,” Dr. Griffin said. “More research is needed to understand how diverse mechanisms contributing to Alzheimer’s and other dementia can be targeted.”
“The Alzheimer’s Association is accelerating the discovery of novel therapies through its Part the Cloud funding program, which has invested more than $65 million to accelerate the development of 65 drug development programs,” Dr. Griffin said.
The study had no specific funding. Dr. Topiwala and Dr. Griffin report no relevant disclosures.
A version of this article first appeared on Medscape.com.
, new research suggests.
“This is the largest and most systematic investigation of telomere length and brain structure and function,” said Anya Topiwala, of the University of Oxford (England). “We found that longer telomeres associated with protection against dementia. The links with brain structure, we think, offer a possible mechanism for this protection. The hope is, by understanding the mechanism, new treatment targets could be uncovered,” Dr. Topiwala said.
The study was published online in PLOS ONE.
UK Biobank cohort
Telomeres form protective caps at the ends of chromosomes, and they progressively shorten with age, which may increase susceptibility to age-related diseases including Alzheimer’s disease. The mechanism underlying this risk is unclear and may involve changes in brain structure and function. However, the relationship between telomere length and neuroimaging markers is poorly characterized.
Dr. Topiwala and colleagues compared telomere length in white blood cells to brain MRI and health record data in 31,661 middle-aged and older adults in UK Biobank. They found that longer leucocyte telomere length (LTL) was associated with a larger volume of global and subcortical grey matter and a larger hippocampus – both of which shrink in patients with Alzheimer’s disease. Longer telomeres were also associated with a thicker cerebral cortex, which thins as Alzheimer’s disease progresses.
Longer LTL was also associated with reduced incidence of dementia during follow-up (hazard ratio, 0.93; 95% confidence interval, 0.91-0.96).
Dr. Topiwala noted that many of the factors related to telomere shortening, such as age, genetics, and sex, can’t be changed. However, in a previous study, her team found that drinking alcohol may shorten telomere length. “So by this logic, reducing your alcohol intake could curb the shortening,” Dr. Topiwala said.
She said that a limitation of the study is that telomere length was measured in blood rather than brain and that it’s not clear at present how closely the two relate. Also, UK Biobank participants are generally more healthy than is the general population. Also, though telomere length and brain measures were associated, “we cannot from this study prove one is causing the other,” she added.
Need for more research
Commenting on the research, Percy Griffin, PhD, Alzheimer’s Association director of scientific engagement, said that it’s been “known for some time that shortened telomeres – the caps at the end of DNA – are associated with increased aging.”
This new study is “interesting,” said Dr. Percy, in that it shows an association between longer telomere length in white blood cells and healthier brain structures in the areas associated with Alzheimer’s disease. The longer telomeres were also associated with lower incidence of all-cause dementia.
But echoing Dr. Topiwala, “association does not mean causation,” Dr. Griffin said. “More research is needed to understand how diverse mechanisms contributing to Alzheimer’s and other dementia can be targeted.”
“The Alzheimer’s Association is accelerating the discovery of novel therapies through its Part the Cloud funding program, which has invested more than $65 million to accelerate the development of 65 drug development programs,” Dr. Griffin said.
The study had no specific funding. Dr. Topiwala and Dr. Griffin report no relevant disclosures.
A version of this article first appeared on Medscape.com.
, new research suggests.
“This is the largest and most systematic investigation of telomere length and brain structure and function,” said Anya Topiwala, of the University of Oxford (England). “We found that longer telomeres associated with protection against dementia. The links with brain structure, we think, offer a possible mechanism for this protection. The hope is, by understanding the mechanism, new treatment targets could be uncovered,” Dr. Topiwala said.
The study was published online in PLOS ONE.
UK Biobank cohort
Telomeres form protective caps at the ends of chromosomes, and they progressively shorten with age, which may increase susceptibility to age-related diseases including Alzheimer’s disease. The mechanism underlying this risk is unclear and may involve changes in brain structure and function. However, the relationship between telomere length and neuroimaging markers is poorly characterized.
Dr. Topiwala and colleagues compared telomere length in white blood cells to brain MRI and health record data in 31,661 middle-aged and older adults in UK Biobank. They found that longer leucocyte telomere length (LTL) was associated with a larger volume of global and subcortical grey matter and a larger hippocampus – both of which shrink in patients with Alzheimer’s disease. Longer telomeres were also associated with a thicker cerebral cortex, which thins as Alzheimer’s disease progresses.
Longer LTL was also associated with reduced incidence of dementia during follow-up (hazard ratio, 0.93; 95% confidence interval, 0.91-0.96).
Dr. Topiwala noted that many of the factors related to telomere shortening, such as age, genetics, and sex, can’t be changed. However, in a previous study, her team found that drinking alcohol may shorten telomere length. “So by this logic, reducing your alcohol intake could curb the shortening,” Dr. Topiwala said.
She said that a limitation of the study is that telomere length was measured in blood rather than brain and that it’s not clear at present how closely the two relate. Also, UK Biobank participants are generally more healthy than is the general population. Also, though telomere length and brain measures were associated, “we cannot from this study prove one is causing the other,” she added.
Need for more research
Commenting on the research, Percy Griffin, PhD, Alzheimer’s Association director of scientific engagement, said that it’s been “known for some time that shortened telomeres – the caps at the end of DNA – are associated with increased aging.”
This new study is “interesting,” said Dr. Percy, in that it shows an association between longer telomere length in white blood cells and healthier brain structures in the areas associated with Alzheimer’s disease. The longer telomeres were also associated with lower incidence of all-cause dementia.
But echoing Dr. Topiwala, “association does not mean causation,” Dr. Griffin said. “More research is needed to understand how diverse mechanisms contributing to Alzheimer’s and other dementia can be targeted.”
“The Alzheimer’s Association is accelerating the discovery of novel therapies through its Part the Cloud funding program, which has invested more than $65 million to accelerate the development of 65 drug development programs,” Dr. Griffin said.
The study had no specific funding. Dr. Topiwala and Dr. Griffin report no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM PLOS ONE
New schizophrenia genes identified
The genes were identified through a meta-analysis comparing gene sequences of 35,828 people with schizophrenia to 107,877 people without the condition.
The study builds on a report published last year that identified 10 genes with rare variants that are directly tied to schizophrenia risk. But that study, like most prior genetic analyses on psychiatric illnesses, was done on the DNA from people of European ancestry.
About 40% of the genetic samples included in this new work came from people of non-European ancestry, which researchers say makes it the most ethnically diverse schizophrenia genetics study to date.
Based on the findings, researchers concluded that the schizophrenia risk conferred by the rare genetic variants found on the new genes they discovered and on those previously identified is conserved across ethnicities.
The new genes, SRRM2 and AKAP11, contain rare protein-truncating variants (PTVs) that investigators say could be the cause of schizophrenia in some patients. The results could have significant implications for drug development.

“It’s not curing the illness, but it is taking us a step closer so that we’re able to say that this may be the cause of the illness in a particular patient,” senior investigator Alexander Charney, MD, PhD, associate professor of psychiatry, genetics and genomic sciences, neuroscience, and neurosurgery, at Icahn School of Medicine at Mount Sinai, New York, said in an interview.
The findings were published online in Nature Genetics.
Schizophrenia’s genetic architecture
Prior studies suggest the genetic architecture of schizophrenia may be influenced by common single-nucleotide polymorphisms, copy number variants and rare PTVs.
Investigators note that rare PTVs are important because they can link disease risk directly to individual genes. But identifying the PTVs and the genes that harbor them requires large patient cohorts, far bigger than any single institution can provide.
Dr. Charney and other researchers are part of the Psychiatric Genomics Consortium, a collaboration of researchers from hundreds of institutions around the world established in 2007 to create large cohorts for genetic studies of psychiatric disease.
For this study, investigators sequenced a new cohort of 11,580 schizophrenia cases and 10,555 controls of diverse ancestries. The analysis showed that the findings previously established in predominantly European cohorts extended to non-European populations.
They then conducted a meta-analysis of the new cohort combined with datasets from earlier studies, creating a pooled sample of 35,828 cases and 107,877 controls.
This meta-analysis revealed two new genes linked to schizophrenia, SRRM2 and AKAP11. The third gene flagged in the study, PCLO, was previously implicated in schizophrenia but is now identified as having a shared risk for schizophrenia and autism.
The rare PVTs on the 12 genes identified so far through this type of study are probably only involved in a small fraction of schizophrenia cases, Dr. Charney acknowledged. However, the discovery could lead to new treatments that could benefit all patients with the disease, he added.
“There are multiple pathways to psychosis and there’s also multiple pathways to treat psychosis,” Dr. Charney said. “There’s reason to believe if you can find a mechanism by which a human being could develop a psychosis, then reversing that mechanism could help a lot of people who have psychosis for another reason.”
Importance of diverse cohorts
Commenting on the findings, Jennifer Gladys Mulle, MHS, PhD, associate professor of psychiatry at the Robert Wood Johnson Medical School at Rutgers University, Piscataway, N.J., noted that while genetic discoveries have led to new therapies in other medical conditions, that has not been the case with schizophrenia.
“In other disorders, having genetic findings have really opened a window into the molecular mechanisms, which has allowed us to develop pharmaceuticals and understand the disease process better,” said Dr. Mulle, who was not part of this study. “But because we haven’t had that in schizophrenia, it’s really held us back. Having genetic variants associated with schizophrenia may really help us understand the mechanism.”
The inclusion of diverse populations is also a key contribution of this study, Dr. Mulle added.
“So far a lot of the work we’ve done in genetics has been on people of European ancestry,” Dr. Mulle said. “The fact that they have found results that are generalizable across multiple ethnicities really suggests that if we develop pharmaceutical agents based on these findings, it will help many people.”
More attention has been paid recently to a growing problem in the study of genetics of psychiatric disorders: More than 95% of participants in genome-wide association studies that seek to identify gene variants linked to disease are of European ancestry.
Dr. Charney and his colleagues had that in mind when they designed the study.
“We can’t get to a place where genetics is clinically useful if we don’t know the extent to which a particular observation that’s found in one population is also true for other populations,” Dr. Charney said.
The study was funded by the National Institutes of Health. Dr. Charney and Dr. Mulle report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The genes were identified through a meta-analysis comparing gene sequences of 35,828 people with schizophrenia to 107,877 people without the condition.
The study builds on a report published last year that identified 10 genes with rare variants that are directly tied to schizophrenia risk. But that study, like most prior genetic analyses on psychiatric illnesses, was done on the DNA from people of European ancestry.
About 40% of the genetic samples included in this new work came from people of non-European ancestry, which researchers say makes it the most ethnically diverse schizophrenia genetics study to date.
Based on the findings, researchers concluded that the schizophrenia risk conferred by the rare genetic variants found on the new genes they discovered and on those previously identified is conserved across ethnicities.
The new genes, SRRM2 and AKAP11, contain rare protein-truncating variants (PTVs) that investigators say could be the cause of schizophrenia in some patients. The results could have significant implications for drug development.

“It’s not curing the illness, but it is taking us a step closer so that we’re able to say that this may be the cause of the illness in a particular patient,” senior investigator Alexander Charney, MD, PhD, associate professor of psychiatry, genetics and genomic sciences, neuroscience, and neurosurgery, at Icahn School of Medicine at Mount Sinai, New York, said in an interview.
The findings were published online in Nature Genetics.
Schizophrenia’s genetic architecture
Prior studies suggest the genetic architecture of schizophrenia may be influenced by common single-nucleotide polymorphisms, copy number variants and rare PTVs.
Investigators note that rare PTVs are important because they can link disease risk directly to individual genes. But identifying the PTVs and the genes that harbor them requires large patient cohorts, far bigger than any single institution can provide.
Dr. Charney and other researchers are part of the Psychiatric Genomics Consortium, a collaboration of researchers from hundreds of institutions around the world established in 2007 to create large cohorts for genetic studies of psychiatric disease.
For this study, investigators sequenced a new cohort of 11,580 schizophrenia cases and 10,555 controls of diverse ancestries. The analysis showed that the findings previously established in predominantly European cohorts extended to non-European populations.
They then conducted a meta-analysis of the new cohort combined with datasets from earlier studies, creating a pooled sample of 35,828 cases and 107,877 controls.
This meta-analysis revealed two new genes linked to schizophrenia, SRRM2 and AKAP11. The third gene flagged in the study, PCLO, was previously implicated in schizophrenia but is now identified as having a shared risk for schizophrenia and autism.
The rare PVTs on the 12 genes identified so far through this type of study are probably only involved in a small fraction of schizophrenia cases, Dr. Charney acknowledged. However, the discovery could lead to new treatments that could benefit all patients with the disease, he added.
“There are multiple pathways to psychosis and there’s also multiple pathways to treat psychosis,” Dr. Charney said. “There’s reason to believe if you can find a mechanism by which a human being could develop a psychosis, then reversing that mechanism could help a lot of people who have psychosis for another reason.”
Importance of diverse cohorts
Commenting on the findings, Jennifer Gladys Mulle, MHS, PhD, associate professor of psychiatry at the Robert Wood Johnson Medical School at Rutgers University, Piscataway, N.J., noted that while genetic discoveries have led to new therapies in other medical conditions, that has not been the case with schizophrenia.
“In other disorders, having genetic findings have really opened a window into the molecular mechanisms, which has allowed us to develop pharmaceuticals and understand the disease process better,” said Dr. Mulle, who was not part of this study. “But because we haven’t had that in schizophrenia, it’s really held us back. Having genetic variants associated with schizophrenia may really help us understand the mechanism.”
The inclusion of diverse populations is also a key contribution of this study, Dr. Mulle added.
“So far a lot of the work we’ve done in genetics has been on people of European ancestry,” Dr. Mulle said. “The fact that they have found results that are generalizable across multiple ethnicities really suggests that if we develop pharmaceutical agents based on these findings, it will help many people.”
More attention has been paid recently to a growing problem in the study of genetics of psychiatric disorders: More than 95% of participants in genome-wide association studies that seek to identify gene variants linked to disease are of European ancestry.
Dr. Charney and his colleagues had that in mind when they designed the study.
“We can’t get to a place where genetics is clinically useful if we don’t know the extent to which a particular observation that’s found in one population is also true for other populations,” Dr. Charney said.
The study was funded by the National Institutes of Health. Dr. Charney and Dr. Mulle report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The genes were identified through a meta-analysis comparing gene sequences of 35,828 people with schizophrenia to 107,877 people without the condition.
The study builds on a report published last year that identified 10 genes with rare variants that are directly tied to schizophrenia risk. But that study, like most prior genetic analyses on psychiatric illnesses, was done on the DNA from people of European ancestry.
About 40% of the genetic samples included in this new work came from people of non-European ancestry, which researchers say makes it the most ethnically diverse schizophrenia genetics study to date.
Based on the findings, researchers concluded that the schizophrenia risk conferred by the rare genetic variants found on the new genes they discovered and on those previously identified is conserved across ethnicities.
The new genes, SRRM2 and AKAP11, contain rare protein-truncating variants (PTVs) that investigators say could be the cause of schizophrenia in some patients. The results could have significant implications for drug development.

“It’s not curing the illness, but it is taking us a step closer so that we’re able to say that this may be the cause of the illness in a particular patient,” senior investigator Alexander Charney, MD, PhD, associate professor of psychiatry, genetics and genomic sciences, neuroscience, and neurosurgery, at Icahn School of Medicine at Mount Sinai, New York, said in an interview.
The findings were published online in Nature Genetics.
Schizophrenia’s genetic architecture
Prior studies suggest the genetic architecture of schizophrenia may be influenced by common single-nucleotide polymorphisms, copy number variants and rare PTVs.
Investigators note that rare PTVs are important because they can link disease risk directly to individual genes. But identifying the PTVs and the genes that harbor them requires large patient cohorts, far bigger than any single institution can provide.
Dr. Charney and other researchers are part of the Psychiatric Genomics Consortium, a collaboration of researchers from hundreds of institutions around the world established in 2007 to create large cohorts for genetic studies of psychiatric disease.
For this study, investigators sequenced a new cohort of 11,580 schizophrenia cases and 10,555 controls of diverse ancestries. The analysis showed that the findings previously established in predominantly European cohorts extended to non-European populations.
They then conducted a meta-analysis of the new cohort combined with datasets from earlier studies, creating a pooled sample of 35,828 cases and 107,877 controls.
This meta-analysis revealed two new genes linked to schizophrenia, SRRM2 and AKAP11. The third gene flagged in the study, PCLO, was previously implicated in schizophrenia but is now identified as having a shared risk for schizophrenia and autism.
The rare PVTs on the 12 genes identified so far through this type of study are probably only involved in a small fraction of schizophrenia cases, Dr. Charney acknowledged. However, the discovery could lead to new treatments that could benefit all patients with the disease, he added.
“There are multiple pathways to psychosis and there’s also multiple pathways to treat psychosis,” Dr. Charney said. “There’s reason to believe if you can find a mechanism by which a human being could develop a psychosis, then reversing that mechanism could help a lot of people who have psychosis for another reason.”
Importance of diverse cohorts
Commenting on the findings, Jennifer Gladys Mulle, MHS, PhD, associate professor of psychiatry at the Robert Wood Johnson Medical School at Rutgers University, Piscataway, N.J., noted that while genetic discoveries have led to new therapies in other medical conditions, that has not been the case with schizophrenia.
“In other disorders, having genetic findings have really opened a window into the molecular mechanisms, which has allowed us to develop pharmaceuticals and understand the disease process better,” said Dr. Mulle, who was not part of this study. “But because we haven’t had that in schizophrenia, it’s really held us back. Having genetic variants associated with schizophrenia may really help us understand the mechanism.”
The inclusion of diverse populations is also a key contribution of this study, Dr. Mulle added.
“So far a lot of the work we’ve done in genetics has been on people of European ancestry,” Dr. Mulle said. “The fact that they have found results that are generalizable across multiple ethnicities really suggests that if we develop pharmaceutical agents based on these findings, it will help many people.”
More attention has been paid recently to a growing problem in the study of genetics of psychiatric disorders: More than 95% of participants in genome-wide association studies that seek to identify gene variants linked to disease are of European ancestry.
Dr. Charney and his colleagues had that in mind when they designed the study.
“We can’t get to a place where genetics is clinically useful if we don’t know the extent to which a particular observation that’s found in one population is also true for other populations,” Dr. Charney said.
The study was funded by the National Institutes of Health. Dr. Charney and Dr. Mulle report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE GENETICS
Tooth loss and diabetes together hasten mental decline
most specifically in those 65-74 years of age, new findings suggest.
The data come from a 12-year follow-up of older adults in a nationally representative U.S. survey.
“From a clinical perspective, our study demonstrates the importance of improving access to dental health care and integrating primary dental and medical care. Health care professionals and family caregivers should pay close attention to the cognitive status of diabetic older adults with poor oral health status,” lead author Bei Wu, PhD, of New York University, said in an interview. Dr. Wu is the Dean’s Professor in Global Health and codirector of the NYU Aging Incubator.
Moreover, said Dr. Wu: “For individuals with both poor oral health and diabetes, regular dental visits should be encouraged in addition to adherence to the diabetes self-care protocol.”
Diabetes has long been recognized as a risk factor for cognitive decline, but the findings have been inconsistent for different age groups. Tooth loss has also been linked to cognitive decline and dementia, as well as diabetes.
The mechanisms aren’t entirely clear, but “co-occurring diabetes and poor oral health may increase the risk for dementia, possibly via the potentially interrelated pathways of chronic inflammation and cardiovascular risk factors,” Dr. Wu said.
The new study, published in the Journal of Dental Research, is the first to examine the relationships between all three conditions by age group.
Diabetes, edentulism, and cognitive decline
The data came from a total of 9,948 participants in the Health and Retirement Study (HRS) from 2006 to 2018. At baseline, 5,440 participants were aged 65-74 years, 3,300 were aged 75-84, and 1,208 were aged 85 years or older.
They were assessed every 2 years using the 35-point Telephone Survey for Cognitive Status, which included tests of immediate and delayed word recall, repeated subtracting by 7, counting backward from 20, naming objects, and naming the president and vice president of the U.S. As might be expected, the youngest group scored the highest, averaging 23 points, while the oldest group scored lowest, at 18.5 points.
Participants were also asked if they had ever been told by a doctor that they have diabetes. Another question was: “Have you lost all of your upper and lower natural permanent teeth?”
The condition of having no teeth is known as edentulism.
The percentages of participants who reported having both diabetes and edentulism were 6.0%, 6.7%, and 5.0% for those aged 65-74 years, 75-84 years, and 85 years or older, respectively. The proportions with neither of those conditions were 63.5%, 60.4%, and 58.3% in those three age groups, respectively (P < .001).
Compared with their counterparts with neither diabetes nor edentulism at baseline, older adults with both conditions aged 65-74 years (P < .001) and aged 75-84 years had worse cognitive function (P < .001).
In terms of the rate of cognitive decline, compared with those with neither condition from the same age cohort, older adults aged 65-74 years with both conditions declined at a higher rate (P < .001).
Having diabetes alone led to accelerated cognitive decline in older adults aged 65-74 years (P < .001). Having edentulism alone led to accelerated decline in older adults aged 65-74 years (P < .001) and older adults aged 75-84 years (P < 0.01).
“Our study finds the co-occurrence of diabetes and edentulism led to a worse cognitive function and a faster cognitive decline in older adults aged 65-74 years,” say Wu and colleagues.
Study limitations: Better data needed
The study has several limitations, most of them due to the data source. For example, while the HRS collects detailed information on cognitive status, edentulism is its only measure of oral health. There were no data on whether individuals had replacements such as dentures or implants that would affect their ability to eat, which could influence other health factors.
“I have made repeated appeals for federal funding to collect more oral health-related information in large national surveys,” Dr. Wu told this news organization.
Similarly, assessments of diabetes status such as hemoglobin A1c were only available for small subsets and not sufficient to demonstrate statistical significance, she explained.
Dr. Wu suggested that both oral health and cognitive screening might be included in the “Welcome to Medicare” preventive visit. In addition, “Oral hygiene practice should also be highlighted to improve cognitive health. Developing dental care interventions and programs are needed for reducing the societal cost of dementia.”
The study was partially supported by the National Institutes of Health. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
most specifically in those 65-74 years of age, new findings suggest.
The data come from a 12-year follow-up of older adults in a nationally representative U.S. survey.
“From a clinical perspective, our study demonstrates the importance of improving access to dental health care and integrating primary dental and medical care. Health care professionals and family caregivers should pay close attention to the cognitive status of diabetic older adults with poor oral health status,” lead author Bei Wu, PhD, of New York University, said in an interview. Dr. Wu is the Dean’s Professor in Global Health and codirector of the NYU Aging Incubator.
Moreover, said Dr. Wu: “For individuals with both poor oral health and diabetes, regular dental visits should be encouraged in addition to adherence to the diabetes self-care protocol.”
Diabetes has long been recognized as a risk factor for cognitive decline, but the findings have been inconsistent for different age groups. Tooth loss has also been linked to cognitive decline and dementia, as well as diabetes.
The mechanisms aren’t entirely clear, but “co-occurring diabetes and poor oral health may increase the risk for dementia, possibly via the potentially interrelated pathways of chronic inflammation and cardiovascular risk factors,” Dr. Wu said.
The new study, published in the Journal of Dental Research, is the first to examine the relationships between all three conditions by age group.
Diabetes, edentulism, and cognitive decline
The data came from a total of 9,948 participants in the Health and Retirement Study (HRS) from 2006 to 2018. At baseline, 5,440 participants were aged 65-74 years, 3,300 were aged 75-84, and 1,208 were aged 85 years or older.
They were assessed every 2 years using the 35-point Telephone Survey for Cognitive Status, which included tests of immediate and delayed word recall, repeated subtracting by 7, counting backward from 20, naming objects, and naming the president and vice president of the U.S. As might be expected, the youngest group scored the highest, averaging 23 points, while the oldest group scored lowest, at 18.5 points.
Participants were also asked if they had ever been told by a doctor that they have diabetes. Another question was: “Have you lost all of your upper and lower natural permanent teeth?”
The condition of having no teeth is known as edentulism.
The percentages of participants who reported having both diabetes and edentulism were 6.0%, 6.7%, and 5.0% for those aged 65-74 years, 75-84 years, and 85 years or older, respectively. The proportions with neither of those conditions were 63.5%, 60.4%, and 58.3% in those three age groups, respectively (P < .001).
Compared with their counterparts with neither diabetes nor edentulism at baseline, older adults with both conditions aged 65-74 years (P < .001) and aged 75-84 years had worse cognitive function (P < .001).
In terms of the rate of cognitive decline, compared with those with neither condition from the same age cohort, older adults aged 65-74 years with both conditions declined at a higher rate (P < .001).
Having diabetes alone led to accelerated cognitive decline in older adults aged 65-74 years (P < .001). Having edentulism alone led to accelerated decline in older adults aged 65-74 years (P < .001) and older adults aged 75-84 years (P < 0.01).
“Our study finds the co-occurrence of diabetes and edentulism led to a worse cognitive function and a faster cognitive decline in older adults aged 65-74 years,” say Wu and colleagues.
Study limitations: Better data needed
The study has several limitations, most of them due to the data source. For example, while the HRS collects detailed information on cognitive status, edentulism is its only measure of oral health. There were no data on whether individuals had replacements such as dentures or implants that would affect their ability to eat, which could influence other health factors.
“I have made repeated appeals for federal funding to collect more oral health-related information in large national surveys,” Dr. Wu told this news organization.
Similarly, assessments of diabetes status such as hemoglobin A1c were only available for small subsets and not sufficient to demonstrate statistical significance, she explained.
Dr. Wu suggested that both oral health and cognitive screening might be included in the “Welcome to Medicare” preventive visit. In addition, “Oral hygiene practice should also be highlighted to improve cognitive health. Developing dental care interventions and programs are needed for reducing the societal cost of dementia.”
The study was partially supported by the National Institutes of Health. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
most specifically in those 65-74 years of age, new findings suggest.
The data come from a 12-year follow-up of older adults in a nationally representative U.S. survey.
“From a clinical perspective, our study demonstrates the importance of improving access to dental health care and integrating primary dental and medical care. Health care professionals and family caregivers should pay close attention to the cognitive status of diabetic older adults with poor oral health status,” lead author Bei Wu, PhD, of New York University, said in an interview. Dr. Wu is the Dean’s Professor in Global Health and codirector of the NYU Aging Incubator.
Moreover, said Dr. Wu: “For individuals with both poor oral health and diabetes, regular dental visits should be encouraged in addition to adherence to the diabetes self-care protocol.”
Diabetes has long been recognized as a risk factor for cognitive decline, but the findings have been inconsistent for different age groups. Tooth loss has also been linked to cognitive decline and dementia, as well as diabetes.
The mechanisms aren’t entirely clear, but “co-occurring diabetes and poor oral health may increase the risk for dementia, possibly via the potentially interrelated pathways of chronic inflammation and cardiovascular risk factors,” Dr. Wu said.
The new study, published in the Journal of Dental Research, is the first to examine the relationships between all three conditions by age group.
Diabetes, edentulism, and cognitive decline
The data came from a total of 9,948 participants in the Health and Retirement Study (HRS) from 2006 to 2018. At baseline, 5,440 participants were aged 65-74 years, 3,300 were aged 75-84, and 1,208 were aged 85 years or older.
They were assessed every 2 years using the 35-point Telephone Survey for Cognitive Status, which included tests of immediate and delayed word recall, repeated subtracting by 7, counting backward from 20, naming objects, and naming the president and vice president of the U.S. As might be expected, the youngest group scored the highest, averaging 23 points, while the oldest group scored lowest, at 18.5 points.
Participants were also asked if they had ever been told by a doctor that they have diabetes. Another question was: “Have you lost all of your upper and lower natural permanent teeth?”
The condition of having no teeth is known as edentulism.
The percentages of participants who reported having both diabetes and edentulism were 6.0%, 6.7%, and 5.0% for those aged 65-74 years, 75-84 years, and 85 years or older, respectively. The proportions with neither of those conditions were 63.5%, 60.4%, and 58.3% in those three age groups, respectively (P < .001).
Compared with their counterparts with neither diabetes nor edentulism at baseline, older adults with both conditions aged 65-74 years (P < .001) and aged 75-84 years had worse cognitive function (P < .001).
In terms of the rate of cognitive decline, compared with those with neither condition from the same age cohort, older adults aged 65-74 years with both conditions declined at a higher rate (P < .001).
Having diabetes alone led to accelerated cognitive decline in older adults aged 65-74 years (P < .001). Having edentulism alone led to accelerated decline in older adults aged 65-74 years (P < .001) and older adults aged 75-84 years (P < 0.01).
“Our study finds the co-occurrence of diabetes and edentulism led to a worse cognitive function and a faster cognitive decline in older adults aged 65-74 years,” say Wu and colleagues.
Study limitations: Better data needed
The study has several limitations, most of them due to the data source. For example, while the HRS collects detailed information on cognitive status, edentulism is its only measure of oral health. There were no data on whether individuals had replacements such as dentures or implants that would affect their ability to eat, which could influence other health factors.
“I have made repeated appeals for federal funding to collect more oral health-related information in large national surveys,” Dr. Wu told this news organization.
Similarly, assessments of diabetes status such as hemoglobin A1c were only available for small subsets and not sufficient to demonstrate statistical significance, she explained.
Dr. Wu suggested that both oral health and cognitive screening might be included in the “Welcome to Medicare” preventive visit. In addition, “Oral hygiene practice should also be highlighted to improve cognitive health. Developing dental care interventions and programs are needed for reducing the societal cost of dementia.”
The study was partially supported by the National Institutes of Health. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF DENTAL RESEARCH
Watch for buprenorphine ‘spiking’ in urine drug tests
Urine drug testing can be valuable for monitoring patients undergoing treatment with buprenorphine for opioid use disorder (OUD). However, some patients alter their test results by adding buprenorphine directly to their urine sample to imply adherence, a new study shows.
“I anticipate a much-needed increase” in the number of people gaining access to buprenorphine therapy, given elimination of the X waiver, first author Jarratt D. Pytell, MD, with University of Colorado at Denver, Aurora, said in a statement.
“New prescribers of buprenorphine will need to learn how to conduct the increasingly complex initiation of treatment and then gauge whether it is successful or not,” added Dr. Pytell, a general internist and addiction medicine specialist.
“Spiking suggests that treatment is not working – especially in patients continuing illicit drug use. Detecting spiking allows clinicians to adjust or intensify the treatment plan,” Dr. Pytell said in an interview.
The study was published online in JAMA Psychiatry.
A sign of elevated patient risk
In a cross-sectional study using Millennium Health’s proprietary urine drug test (UDT) database, researchers analyzed 507,735 urine specimens from 58,476 OUD patients collected between January 2017 and April 2022.
A total of 9546 (1.9%) specimens from 4,550 patients (7.6%) were suggestive of spiking.
UDT specimens suggestive of spiking had two times the odds of being positive for other opioids (fentanyl or heroin), compared with opioid negative samples.
UDT specimens obtained from primary care clinics, from patients aged 35-44 years, and from patients living in the South Atlantic region of the United States were also more likely to be suggestive of buprenorphine spiking.
“Our study demonstrated that a buprenorphine to norbuprenorphine ratio of less than 0.02 indicates the possibility of spiking,” Dr. Pytell said in an interview.
“Nevertheless, it is important to note that this cutoff is not a definitive standard and further controlled studies are necessary to determine its predictive value for spiking. But recognizing possible spiking is very important since it demonstrates a point of elevated risk for the patient and the treatment approach should be reconsidered,” Dr. Pytell said.
“At Millennium Health, we have been tracking the enormity of the drug use crisis. This study suggests that spiking is an important patient safety issue, and it is not uncommon,” study coauthor Eric Dawson, PharmD, vice president of clinical affairs, Millennium Health, said in a statement.
“Detection of spiking requires definitive drug testing. Immunoassay-based, point-of-care tests cannot detect spiking because they are generally incapable of quantitative analysis and differentiating buprenorphine from norbuprenorphine,” Dr. Dawson said.
Best practices?
“We need to develop best practices specific for this situation where a patient has added buprenorphine to the urine drug test specimen,” said Dr. Pytell.
“As with all unexpected findings, it is crucial for clinicians to approach this finding in a nonjudgmental manner and work with the patient to understand what might have motivated them to alter their urine specimen,” he added.
Dr. Pytell said a common reaction for clinicians might be to discontinue treatment. However, this is actually a time to try and engage with the patient.
“Clinicians should work collaboratively with patients to identify potential reasons for spiking and determine what changes may need to be made to better support the patient’s recovery,” Dr. Pytell said.
“This could include more frequent monitoring or referral to a higher level of care. In addition, clinicians should be aware that patients who engage in spiking may be experiencing other challenges that impact their ability to adhere to treatment, such as inadequate housing, mental health issues, or financial strain. Addressing these underlying issues may help patients overcome barriers to treatment adherence and reduce the likelihood of future spiking,” Dr. Pytell said.
This study was supported by Millennium Health. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Urine drug testing can be valuable for monitoring patients undergoing treatment with buprenorphine for opioid use disorder (OUD). However, some patients alter their test results by adding buprenorphine directly to their urine sample to imply adherence, a new study shows.
“I anticipate a much-needed increase” in the number of people gaining access to buprenorphine therapy, given elimination of the X waiver, first author Jarratt D. Pytell, MD, with University of Colorado at Denver, Aurora, said in a statement.
“New prescribers of buprenorphine will need to learn how to conduct the increasingly complex initiation of treatment and then gauge whether it is successful or not,” added Dr. Pytell, a general internist and addiction medicine specialist.
“Spiking suggests that treatment is not working – especially in patients continuing illicit drug use. Detecting spiking allows clinicians to adjust or intensify the treatment plan,” Dr. Pytell said in an interview.
The study was published online in JAMA Psychiatry.
A sign of elevated patient risk
In a cross-sectional study using Millennium Health’s proprietary urine drug test (UDT) database, researchers analyzed 507,735 urine specimens from 58,476 OUD patients collected between January 2017 and April 2022.
A total of 9546 (1.9%) specimens from 4,550 patients (7.6%) were suggestive of spiking.
UDT specimens suggestive of spiking had two times the odds of being positive for other opioids (fentanyl or heroin), compared with opioid negative samples.
UDT specimens obtained from primary care clinics, from patients aged 35-44 years, and from patients living in the South Atlantic region of the United States were also more likely to be suggestive of buprenorphine spiking.
“Our study demonstrated that a buprenorphine to norbuprenorphine ratio of less than 0.02 indicates the possibility of spiking,” Dr. Pytell said in an interview.
“Nevertheless, it is important to note that this cutoff is not a definitive standard and further controlled studies are necessary to determine its predictive value for spiking. But recognizing possible spiking is very important since it demonstrates a point of elevated risk for the patient and the treatment approach should be reconsidered,” Dr. Pytell said.
“At Millennium Health, we have been tracking the enormity of the drug use crisis. This study suggests that spiking is an important patient safety issue, and it is not uncommon,” study coauthor Eric Dawson, PharmD, vice president of clinical affairs, Millennium Health, said in a statement.
“Detection of spiking requires definitive drug testing. Immunoassay-based, point-of-care tests cannot detect spiking because they are generally incapable of quantitative analysis and differentiating buprenorphine from norbuprenorphine,” Dr. Dawson said.
Best practices?
“We need to develop best practices specific for this situation where a patient has added buprenorphine to the urine drug test specimen,” said Dr. Pytell.
“As with all unexpected findings, it is crucial for clinicians to approach this finding in a nonjudgmental manner and work with the patient to understand what might have motivated them to alter their urine specimen,” he added.
Dr. Pytell said a common reaction for clinicians might be to discontinue treatment. However, this is actually a time to try and engage with the patient.
“Clinicians should work collaboratively with patients to identify potential reasons for spiking and determine what changes may need to be made to better support the patient’s recovery,” Dr. Pytell said.
“This could include more frequent monitoring or referral to a higher level of care. In addition, clinicians should be aware that patients who engage in spiking may be experiencing other challenges that impact their ability to adhere to treatment, such as inadequate housing, mental health issues, or financial strain. Addressing these underlying issues may help patients overcome barriers to treatment adherence and reduce the likelihood of future spiking,” Dr. Pytell said.
This study was supported by Millennium Health. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Urine drug testing can be valuable for monitoring patients undergoing treatment with buprenorphine for opioid use disorder (OUD). However, some patients alter their test results by adding buprenorphine directly to their urine sample to imply adherence, a new study shows.
“I anticipate a much-needed increase” in the number of people gaining access to buprenorphine therapy, given elimination of the X waiver, first author Jarratt D. Pytell, MD, with University of Colorado at Denver, Aurora, said in a statement.
“New prescribers of buprenorphine will need to learn how to conduct the increasingly complex initiation of treatment and then gauge whether it is successful or not,” added Dr. Pytell, a general internist and addiction medicine specialist.
“Spiking suggests that treatment is not working – especially in patients continuing illicit drug use. Detecting spiking allows clinicians to adjust or intensify the treatment plan,” Dr. Pytell said in an interview.
The study was published online in JAMA Psychiatry.
A sign of elevated patient risk
In a cross-sectional study using Millennium Health’s proprietary urine drug test (UDT) database, researchers analyzed 507,735 urine specimens from 58,476 OUD patients collected between January 2017 and April 2022.
A total of 9546 (1.9%) specimens from 4,550 patients (7.6%) were suggestive of spiking.
UDT specimens suggestive of spiking had two times the odds of being positive for other opioids (fentanyl or heroin), compared with opioid negative samples.
UDT specimens obtained from primary care clinics, from patients aged 35-44 years, and from patients living in the South Atlantic region of the United States were also more likely to be suggestive of buprenorphine spiking.
“Our study demonstrated that a buprenorphine to norbuprenorphine ratio of less than 0.02 indicates the possibility of spiking,” Dr. Pytell said in an interview.
“Nevertheless, it is important to note that this cutoff is not a definitive standard and further controlled studies are necessary to determine its predictive value for spiking. But recognizing possible spiking is very important since it demonstrates a point of elevated risk for the patient and the treatment approach should be reconsidered,” Dr. Pytell said.
“At Millennium Health, we have been tracking the enormity of the drug use crisis. This study suggests that spiking is an important patient safety issue, and it is not uncommon,” study coauthor Eric Dawson, PharmD, vice president of clinical affairs, Millennium Health, said in a statement.
“Detection of spiking requires definitive drug testing. Immunoassay-based, point-of-care tests cannot detect spiking because they are generally incapable of quantitative analysis and differentiating buprenorphine from norbuprenorphine,” Dr. Dawson said.
Best practices?
“We need to develop best practices specific for this situation where a patient has added buprenorphine to the urine drug test specimen,” said Dr. Pytell.
“As with all unexpected findings, it is crucial for clinicians to approach this finding in a nonjudgmental manner and work with the patient to understand what might have motivated them to alter their urine specimen,” he added.
Dr. Pytell said a common reaction for clinicians might be to discontinue treatment. However, this is actually a time to try and engage with the patient.
“Clinicians should work collaboratively with patients to identify potential reasons for spiking and determine what changes may need to be made to better support the patient’s recovery,” Dr. Pytell said.
“This could include more frequent monitoring or referral to a higher level of care. In addition, clinicians should be aware that patients who engage in spiking may be experiencing other challenges that impact their ability to adhere to treatment, such as inadequate housing, mental health issues, or financial strain. Addressing these underlying issues may help patients overcome barriers to treatment adherence and reduce the likelihood of future spiking,” Dr. Pytell said.
This study was supported by Millennium Health. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY