User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
If you reopen it, will they come?
On April 16, the White House released federal guidelines for reopening American businesses – followed 3 days later by specific recommendations from the Centers for Medicare and Medicaid Services for .
Depending on where you live, you may have already reopened (or even never closed), or you may be awaiting the relaxation of restrictions in your state. (As I write this on June 10, the stay-at-home order in my state, New Jersey, is being rescinded.)
The big question, of course, is whether patients can be convinced that it is safe to leave their homes and come to your office. The answer may depend on how well you time your reopening and adhere to the appropriate federal, state, and independent guidelines.
The federal guidelines have three sections: criteria, which outline conditions each region or state should satisfy before reopening; preparedness, which lists how states should prepare for reopening; and phase guidelines, which detail responsibilities of individuals and employers during distinct reopening phases.
You should pay the most attention to the “criteria” section. The key question to ask: “Has my state or region satisfied the basic criteria for reopening?”
Those criteria are as follows:
- Symptoms reported within a 14-day period should be on a downward trajectory.
- Cases documented (or positive tests as a percentage of total tests) within a 14-day period should also be on a downward trajectory.
- Hospitals should be treating all patients without crisis care. They should also have a robust testing program in place for at-risk health care workers.
If your area meets these criteria, you can proceed to the CMS recommendations. They cover general advice related to personal protective equipment (PPE), workforce availability, facility considerations, sanitation protocols, supplies, and testing capacity.
The key takeaway: As long as your area has the resources to quickly respond to a surge of COVID-19 cases, you can start offering care to non-COVID patients. Keep seeing patients via telehealth as often as possible, and prioritize surgical/procedural care and high-complexity chronic disease management before moving on to preventive and cosmetic services.
The American Medical Association has issued its own checklist of criteria for reopening your practice to supplement the federal guidelines. Highlights include the following:
- Sit down with a calendar and pick an expected reopening day. Ideally, this should include a “soft reopening.” Make a plan to stock necessary PPE and write down plans for cleaning and staffing if an employee or patient is diagnosed with COVID-19 after visiting your office.
- Take a stepwise approach so you can identify challenges early and address them. It’s important to figure out which visits can continue via telehealth, and begin with just a few in-person visits each day. Plan out a schedule and clearly communicate it to patients, clinicians, and staff.
- Patient safety is your top concern. Encourage patients to visit without companions whenever possible, and of course, all individuals who visit the office should wear a cloth face covering.
- Screen employees for fevers and other symptoms of COVID-19; remember that those records are subject to HIPAA rules and must be kept confidential. Minimize contact between employees as much as possible.
- Do your best to screen patients before in-person visits, to verify they don’t have symptoms of COVID-19. Consider creating a script that office staff can use to contact patients 24 hours before they come in. Use this as a chance to ask about symptoms, and explain any reopening logistics they should know about.
- Contact your malpractice insurance carrier to discuss whether you need to make any changes to your coverage.
This would also be a great time to review your confidentiality, privacy, and data security protocols. COVID-19 presents new challenges for data privacy – for example, if you must inform coworkers or patients that they have come into contact with someone who tested positive. Make a plan that follows HIPAA guidelines during COVID-19. Also, make sure you have a plan for handling issues like paid sick leave or reporting COVID-19 cases to your local health department.
Another useful resource is the Medical Group Management Association’s COVID-19 Medical Practice Reopening Checklist. You can use it to confirm that you are addressing all the important items, and that you haven’t missed anything.
As for me, I am advising patients who are reluctant to seek treatment that many medical problems pose more risk than COVID-19, faster treatment means better outcomes, and because we maintain strict disinfection protocols, they are far less likely to be infected with COVID-19 in my office than, say, at a grocery store.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
On April 16, the White House released federal guidelines for reopening American businesses – followed 3 days later by specific recommendations from the Centers for Medicare and Medicaid Services for .
Depending on where you live, you may have already reopened (or even never closed), or you may be awaiting the relaxation of restrictions in your state. (As I write this on June 10, the stay-at-home order in my state, New Jersey, is being rescinded.)
The big question, of course, is whether patients can be convinced that it is safe to leave their homes and come to your office. The answer may depend on how well you time your reopening and adhere to the appropriate federal, state, and independent guidelines.
The federal guidelines have three sections: criteria, which outline conditions each region or state should satisfy before reopening; preparedness, which lists how states should prepare for reopening; and phase guidelines, which detail responsibilities of individuals and employers during distinct reopening phases.
You should pay the most attention to the “criteria” section. The key question to ask: “Has my state or region satisfied the basic criteria for reopening?”
Those criteria are as follows:
- Symptoms reported within a 14-day period should be on a downward trajectory.
- Cases documented (or positive tests as a percentage of total tests) within a 14-day period should also be on a downward trajectory.
- Hospitals should be treating all patients without crisis care. They should also have a robust testing program in place for at-risk health care workers.
If your area meets these criteria, you can proceed to the CMS recommendations. They cover general advice related to personal protective equipment (PPE), workforce availability, facility considerations, sanitation protocols, supplies, and testing capacity.
The key takeaway: As long as your area has the resources to quickly respond to a surge of COVID-19 cases, you can start offering care to non-COVID patients. Keep seeing patients via telehealth as often as possible, and prioritize surgical/procedural care and high-complexity chronic disease management before moving on to preventive and cosmetic services.
The American Medical Association has issued its own checklist of criteria for reopening your practice to supplement the federal guidelines. Highlights include the following:
- Sit down with a calendar and pick an expected reopening day. Ideally, this should include a “soft reopening.” Make a plan to stock necessary PPE and write down plans for cleaning and staffing if an employee or patient is diagnosed with COVID-19 after visiting your office.
- Take a stepwise approach so you can identify challenges early and address them. It’s important to figure out which visits can continue via telehealth, and begin with just a few in-person visits each day. Plan out a schedule and clearly communicate it to patients, clinicians, and staff.
- Patient safety is your top concern. Encourage patients to visit without companions whenever possible, and of course, all individuals who visit the office should wear a cloth face covering.
- Screen employees for fevers and other symptoms of COVID-19; remember that those records are subject to HIPAA rules and must be kept confidential. Minimize contact between employees as much as possible.
- Do your best to screen patients before in-person visits, to verify they don’t have symptoms of COVID-19. Consider creating a script that office staff can use to contact patients 24 hours before they come in. Use this as a chance to ask about symptoms, and explain any reopening logistics they should know about.
- Contact your malpractice insurance carrier to discuss whether you need to make any changes to your coverage.
This would also be a great time to review your confidentiality, privacy, and data security protocols. COVID-19 presents new challenges for data privacy – for example, if you must inform coworkers or patients that they have come into contact with someone who tested positive. Make a plan that follows HIPAA guidelines during COVID-19. Also, make sure you have a plan for handling issues like paid sick leave or reporting COVID-19 cases to your local health department.
Another useful resource is the Medical Group Management Association’s COVID-19 Medical Practice Reopening Checklist. You can use it to confirm that you are addressing all the important items, and that you haven’t missed anything.
As for me, I am advising patients who are reluctant to seek treatment that many medical problems pose more risk than COVID-19, faster treatment means better outcomes, and because we maintain strict disinfection protocols, they are far less likely to be infected with COVID-19 in my office than, say, at a grocery store.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
On April 16, the White House released federal guidelines for reopening American businesses – followed 3 days later by specific recommendations from the Centers for Medicare and Medicaid Services for .
Depending on where you live, you may have already reopened (or even never closed), or you may be awaiting the relaxation of restrictions in your state. (As I write this on June 10, the stay-at-home order in my state, New Jersey, is being rescinded.)
The big question, of course, is whether patients can be convinced that it is safe to leave their homes and come to your office. The answer may depend on how well you time your reopening and adhere to the appropriate federal, state, and independent guidelines.
The federal guidelines have three sections: criteria, which outline conditions each region or state should satisfy before reopening; preparedness, which lists how states should prepare for reopening; and phase guidelines, which detail responsibilities of individuals and employers during distinct reopening phases.
You should pay the most attention to the “criteria” section. The key question to ask: “Has my state or region satisfied the basic criteria for reopening?”
Those criteria are as follows:
- Symptoms reported within a 14-day period should be on a downward trajectory.
- Cases documented (or positive tests as a percentage of total tests) within a 14-day period should also be on a downward trajectory.
- Hospitals should be treating all patients without crisis care. They should also have a robust testing program in place for at-risk health care workers.
If your area meets these criteria, you can proceed to the CMS recommendations. They cover general advice related to personal protective equipment (PPE), workforce availability, facility considerations, sanitation protocols, supplies, and testing capacity.
The key takeaway: As long as your area has the resources to quickly respond to a surge of COVID-19 cases, you can start offering care to non-COVID patients. Keep seeing patients via telehealth as often as possible, and prioritize surgical/procedural care and high-complexity chronic disease management before moving on to preventive and cosmetic services.
The American Medical Association has issued its own checklist of criteria for reopening your practice to supplement the federal guidelines. Highlights include the following:
- Sit down with a calendar and pick an expected reopening day. Ideally, this should include a “soft reopening.” Make a plan to stock necessary PPE and write down plans for cleaning and staffing if an employee or patient is diagnosed with COVID-19 after visiting your office.
- Take a stepwise approach so you can identify challenges early and address them. It’s important to figure out which visits can continue via telehealth, and begin with just a few in-person visits each day. Plan out a schedule and clearly communicate it to patients, clinicians, and staff.
- Patient safety is your top concern. Encourage patients to visit without companions whenever possible, and of course, all individuals who visit the office should wear a cloth face covering.
- Screen employees for fevers and other symptoms of COVID-19; remember that those records are subject to HIPAA rules and must be kept confidential. Minimize contact between employees as much as possible.
- Do your best to screen patients before in-person visits, to verify they don’t have symptoms of COVID-19. Consider creating a script that office staff can use to contact patients 24 hours before they come in. Use this as a chance to ask about symptoms, and explain any reopening logistics they should know about.
- Contact your malpractice insurance carrier to discuss whether you need to make any changes to your coverage.
This would also be a great time to review your confidentiality, privacy, and data security protocols. COVID-19 presents new challenges for data privacy – for example, if you must inform coworkers or patients that they have come into contact with someone who tested positive. Make a plan that follows HIPAA guidelines during COVID-19. Also, make sure you have a plan for handling issues like paid sick leave or reporting COVID-19 cases to your local health department.
Another useful resource is the Medical Group Management Association’s COVID-19 Medical Practice Reopening Checklist. You can use it to confirm that you are addressing all the important items, and that you haven’t missed anything.
As for me, I am advising patients who are reluctant to seek treatment that many medical problems pose more risk than COVID-19, faster treatment means better outcomes, and because we maintain strict disinfection protocols, they are far less likely to be infected with COVID-19 in my office than, say, at a grocery store.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Racism joins COVID-19 at the primary care table
Primary care clinicians, already experiencing all-time high stress levels related to COVID-19, are now struggling to cope with the fallout from racism and the death of George Floyd, according to a survey conducted June 5-8.
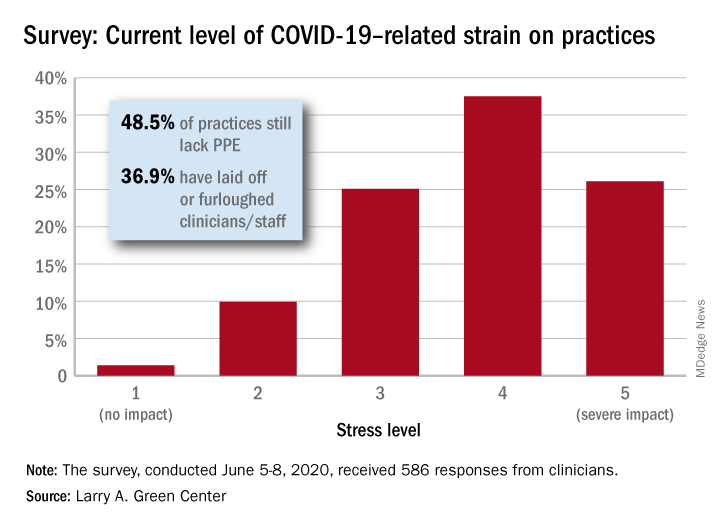
When asked how the situation has affected their practices, two-thirds of the survey’s 586 respondents said that George Floyd’s death and related events had been the subject of practice conversations and 12% “drew clear connections between the current racial unrest and the health of their patients,” the Larry A. Green Center said in a recent statement.
One-third of the clinicians also said that recent racism-related events have had a negative effect on their own well-being, and that connection was expressed even more strongly in a related survey of 1,111 patients that was conducted June 8.
In that survey, about 65% of patients said that racism affected emotional, psychological, and behavioral health, and 40% noted that George Floyd’s death had a negative impact on the well-being of friends, the Larry A. Green Center said in partnership with the Primary Care Collaborative and 3rd Conversation.
“The fact that so many patients and clinicians agree that racism is a driver of health points to the incredible role primary care plays in creating safe spaces to process deep societal and personal issues,” said Christine Bechtel, cofounder of 3rd Conversation, a community of patients and providers.
COVID-19, in the meantime, is still doing its thing. Almost two-thirds (63%) of respondents to the clinician survey said that stress levels at their practices had reached all-time-high levels in the last 4 weeks.
Just 1.4% of the primary care clinicians said that the pandemic had put no strain on their practices (see graph), and just 10% rated that strain as a 2 on a scale of 1-5, the center said. Among the stressors:
About 49% continue to lack PPE.
About 40% still have no or limited ability for testing.
About 37% of practice settings still report layoffs and furloughs.
About 31% report that clinician salaries are still being skipped or deferred.
“Both public and private policy makers must take immediate steps to stabilize primary care,” said Ann Greiner, president and CEO of the Primary Care Collaborative. “This financial support is necessary but not sufficient. Instead, we need wholesale reform of payment in order to achieve the kind of high-performing primary care that truly meets patient needs.”
Primary care clinicians, already experiencing all-time high stress levels related to COVID-19, are now struggling to cope with the fallout from racism and the death of George Floyd, according to a survey conducted June 5-8.

When asked how the situation has affected their practices, two-thirds of the survey’s 586 respondents said that George Floyd’s death and related events had been the subject of practice conversations and 12% “drew clear connections between the current racial unrest and the health of their patients,” the Larry A. Green Center said in a recent statement.
One-third of the clinicians also said that recent racism-related events have had a negative effect on their own well-being, and that connection was expressed even more strongly in a related survey of 1,111 patients that was conducted June 8.
In that survey, about 65% of patients said that racism affected emotional, psychological, and behavioral health, and 40% noted that George Floyd’s death had a negative impact on the well-being of friends, the Larry A. Green Center said in partnership with the Primary Care Collaborative and 3rd Conversation.
“The fact that so many patients and clinicians agree that racism is a driver of health points to the incredible role primary care plays in creating safe spaces to process deep societal and personal issues,” said Christine Bechtel, cofounder of 3rd Conversation, a community of patients and providers.
COVID-19, in the meantime, is still doing its thing. Almost two-thirds (63%) of respondents to the clinician survey said that stress levels at their practices had reached all-time-high levels in the last 4 weeks.
Just 1.4% of the primary care clinicians said that the pandemic had put no strain on their practices (see graph), and just 10% rated that strain as a 2 on a scale of 1-5, the center said. Among the stressors:
About 49% continue to lack PPE.
About 40% still have no or limited ability for testing.
About 37% of practice settings still report layoffs and furloughs.
About 31% report that clinician salaries are still being skipped or deferred.
“Both public and private policy makers must take immediate steps to stabilize primary care,” said Ann Greiner, president and CEO of the Primary Care Collaborative. “This financial support is necessary but not sufficient. Instead, we need wholesale reform of payment in order to achieve the kind of high-performing primary care that truly meets patient needs.”
Primary care clinicians, already experiencing all-time high stress levels related to COVID-19, are now struggling to cope with the fallout from racism and the death of George Floyd, according to a survey conducted June 5-8.

When asked how the situation has affected their practices, two-thirds of the survey’s 586 respondents said that George Floyd’s death and related events had been the subject of practice conversations and 12% “drew clear connections between the current racial unrest and the health of their patients,” the Larry A. Green Center said in a recent statement.
One-third of the clinicians also said that recent racism-related events have had a negative effect on their own well-being, and that connection was expressed even more strongly in a related survey of 1,111 patients that was conducted June 8.
In that survey, about 65% of patients said that racism affected emotional, psychological, and behavioral health, and 40% noted that George Floyd’s death had a negative impact on the well-being of friends, the Larry A. Green Center said in partnership with the Primary Care Collaborative and 3rd Conversation.
“The fact that so many patients and clinicians agree that racism is a driver of health points to the incredible role primary care plays in creating safe spaces to process deep societal and personal issues,” said Christine Bechtel, cofounder of 3rd Conversation, a community of patients and providers.
COVID-19, in the meantime, is still doing its thing. Almost two-thirds (63%) of respondents to the clinician survey said that stress levels at their practices had reached all-time-high levels in the last 4 weeks.
Just 1.4% of the primary care clinicians said that the pandemic had put no strain on their practices (see graph), and just 10% rated that strain as a 2 on a scale of 1-5, the center said. Among the stressors:
About 49% continue to lack PPE.
About 40% still have no or limited ability for testing.
About 37% of practice settings still report layoffs and furloughs.
About 31% report that clinician salaries are still being skipped or deferred.
“Both public and private policy makers must take immediate steps to stabilize primary care,” said Ann Greiner, president and CEO of the Primary Care Collaborative. “This financial support is necessary but not sufficient. Instead, we need wholesale reform of payment in order to achieve the kind of high-performing primary care that truly meets patient needs.”
New long-term data for antipsychotic in pediatric bipolar depression
The antipsychotic lurasidone (Latuda, Sunovion Pharmaceuticals) has long-term efficacy in the treatment of bipolar depression (BD) in children and adolescents, new research suggests.
In an open-label extension study involving patients aged 10-17 years, up to 2 years of treatment with lurasidone was associated with continued improvement in depressive symptoms. There were progressively higher rates of remission, recovery, and sustained remission.
Coinvestigator Manpreet K. Singh, MD, director of the Stanford Pediatric Mood Disorders Program, Stanford (Calif.) University, noted that early onset of BD is common. Although in pediatric populations, prevalence has been fairly stable at around 1.8%, these patients have “a very limited number of treatment options available for the depressed phases of BD,” which is often predominant and can be difficult to identify.
“A lot of youths who are experiencing depressive symptoms in the context of having had a manic episode will often have a relapsing and remitting course, even after the acute phase of treatment, so because kids can be on medications for long periods of time, a better understanding of what works ... is very important,” Dr. Singh said in an interview.
The findings were presented at the virtual American Society of Clinical Psychopharmacology (ASCP) 2020 annual meeting.
Long-term Efficacy
The Food and Drug Administration approved lurasidone as monotherapy for BD in children and adolescents in 2018. The aim of the current study was to evaluate the drug’s long-term efficacy in achieving response or remission in this population.
A total of 305 children who completed an initial 6-week double-blind study of lurasidone versus placebo entered the 2-year, open-label extension study. In the extension, they either continued taking lurasidone or were switched from placebo to lurasidone 20-80 mg/day. Of this group, 195 children completed 52 weeks of treatment, and 93 completed 104 weeks of treatment.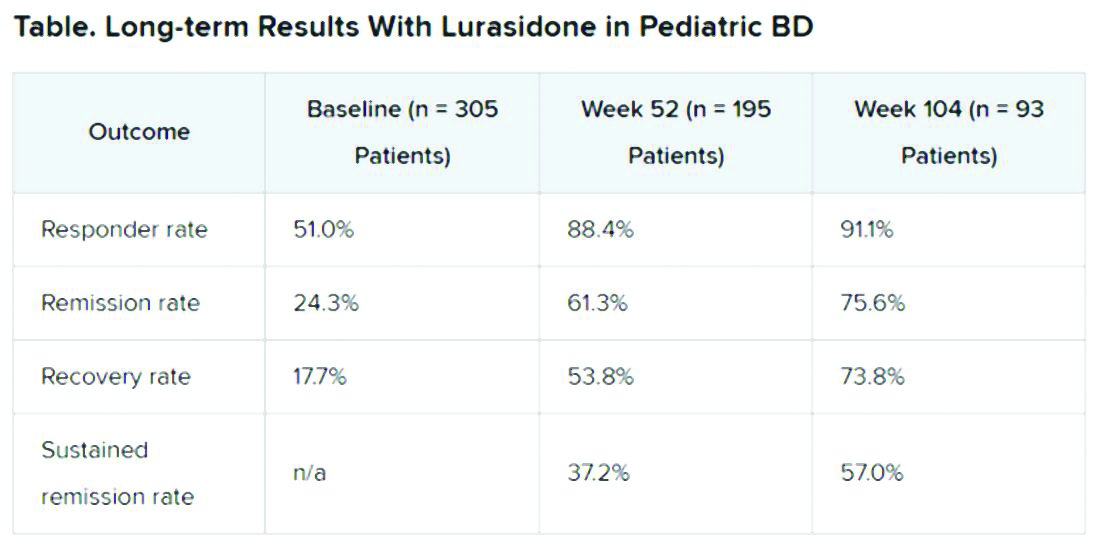
Efficacy was measured with the Children’s Depression Rating Scale, Revised (CDRS-R) and the Clinical Global Impression, Bipolar Depression Severity scale (CGI-BP-S). Functioning was evaluated with the clinician-rated Children’s Global Assessment Scale (CGAS); on that scale, a score of 70 or higher indicates no clinically meaningful functional impairment.
Remission criteria were met if a patient achieved a CDRS-R total score of 28 or less, a Young Mania Rating Scale (YMRS) total score of 8 or less, and a CGI-BP-S depression score of 3 or less.
Recovery criteria were met if a patient achieved remission and had a CGAS score of at least 70.
Sustained remission, a more stringent outcome, required that the patient meet remission criteria for at least 24 consecutive weeks.
In addition, there was a strong inverse correlation (r = –0.71) between depression severity, as measured by CDRS-R total score, and functioning, as measured by the CGAS.
“That’s the cool thing: As the depression symptoms and severity came down, the overall functioning in these kids improved,” Dr. Singh noted.
“This improvement in functioning ends up being much more clinically relevant and useful to clinicians than just showing an improvement in a set of symptoms because what brings a kid – or even an adult, for that matter – to see a clinician to get treatment is because something about their symptoms is causing significant functional impairment,” she said.
“So this is the take-home message: You can see that lurasidone ... demonstrates not just recovery from depressive symptoms but that this reduction in depressive symptoms corresponds to an improvement in functioning for these youths,” she added.
Potential Limitations
Commenting on the study, Christoph U. Correll, MD, professor of child and adolescent psychiatry, Charite Universitatsmedizin, Berlin, Germany, noted that BD is difficult to treat, especially for patients who are going through “a developmentally vulnerable phase of their lives.”
“Lurasidone is the only monotherapy approved for bipolar depression in youth and is fairly well tolerated,” said Dr. Correll, who was not part of the research. He added that the long-term effectiveness data on response and remission “add relevant information” to the field.
However, he noted that it is not clear whether the high and increasing rates of response and remission were based on the reporting of observed cases or on last-observation-carried-forward analyses. “Given the naturally high dropout rate in such a long-term study and the potential for a survival bias, this is a relevant methodological question that affects the interpretation of the data,” he said.
“Nevertheless, the very favorable results for cumulative response, remission, and sustained remission add to the evidence that lurasidone is an effective treatment for youth with bipolar depression. Since efficacy cannot be interpreted in isolation, data describing the tolerability, including long-term cardiometabolic effects, will be important complementary data to consider,” Dr. Correll said.
The study was funded by Sunovion Pharmaceuticals. Dr. Singh is on the advisory board for Sunovion, is a consultant for Google X and Limbix, and receives royalties from American Psychiatric Association Publishing. She has also received research support from Stanford’s Maternal Child Health Research Institute and Department of Psychiatry, the National Institute of Mental Health, the National Institute on Aging, Johnson and Johnson, Allergan, PCORI, and the Brain and Behavior Research Foundation. Dr. Correll has been a consultant or adviser to and has received honoraria from Sunovion, as well as Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Intra-Cellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Sumitomo Dainippon, Supernus, Takeda, and Teva.
A version of this article originally appeared on Medscape.com.
The antipsychotic lurasidone (Latuda, Sunovion Pharmaceuticals) has long-term efficacy in the treatment of bipolar depression (BD) in children and adolescents, new research suggests.
In an open-label extension study involving patients aged 10-17 years, up to 2 years of treatment with lurasidone was associated with continued improvement in depressive symptoms. There were progressively higher rates of remission, recovery, and sustained remission.
Coinvestigator Manpreet K. Singh, MD, director of the Stanford Pediatric Mood Disorders Program, Stanford (Calif.) University, noted that early onset of BD is common. Although in pediatric populations, prevalence has been fairly stable at around 1.8%, these patients have “a very limited number of treatment options available for the depressed phases of BD,” which is often predominant and can be difficult to identify.
“A lot of youths who are experiencing depressive symptoms in the context of having had a manic episode will often have a relapsing and remitting course, even after the acute phase of treatment, so because kids can be on medications for long periods of time, a better understanding of what works ... is very important,” Dr. Singh said in an interview.
The findings were presented at the virtual American Society of Clinical Psychopharmacology (ASCP) 2020 annual meeting.
Long-term Efficacy
The Food and Drug Administration approved lurasidone as monotherapy for BD in children and adolescents in 2018. The aim of the current study was to evaluate the drug’s long-term efficacy in achieving response or remission in this population.
A total of 305 children who completed an initial 6-week double-blind study of lurasidone versus placebo entered the 2-year, open-label extension study. In the extension, they either continued taking lurasidone or were switched from placebo to lurasidone 20-80 mg/day. Of this group, 195 children completed 52 weeks of treatment, and 93 completed 104 weeks of treatment.
Efficacy was measured with the Children’s Depression Rating Scale, Revised (CDRS-R) and the Clinical Global Impression, Bipolar Depression Severity scale (CGI-BP-S). Functioning was evaluated with the clinician-rated Children’s Global Assessment Scale (CGAS); on that scale, a score of 70 or higher indicates no clinically meaningful functional impairment.
Remission criteria were met if a patient achieved a CDRS-R total score of 28 or less, a Young Mania Rating Scale (YMRS) total score of 8 or less, and a CGI-BP-S depression score of 3 or less.
Recovery criteria were met if a patient achieved remission and had a CGAS score of at least 70.
Sustained remission, a more stringent outcome, required that the patient meet remission criteria for at least 24 consecutive weeks.
In addition, there was a strong inverse correlation (r = –0.71) between depression severity, as measured by CDRS-R total score, and functioning, as measured by the CGAS.
“That’s the cool thing: As the depression symptoms and severity came down, the overall functioning in these kids improved,” Dr. Singh noted.
“This improvement in functioning ends up being much more clinically relevant and useful to clinicians than just showing an improvement in a set of symptoms because what brings a kid – or even an adult, for that matter – to see a clinician to get treatment is because something about their symptoms is causing significant functional impairment,” she said.
“So this is the take-home message: You can see that lurasidone ... demonstrates not just recovery from depressive symptoms but that this reduction in depressive symptoms corresponds to an improvement in functioning for these youths,” she added.
Potential Limitations
Commenting on the study, Christoph U. Correll, MD, professor of child and adolescent psychiatry, Charite Universitatsmedizin, Berlin, Germany, noted that BD is difficult to treat, especially for patients who are going through “a developmentally vulnerable phase of their lives.”
“Lurasidone is the only monotherapy approved for bipolar depression in youth and is fairly well tolerated,” said Dr. Correll, who was not part of the research. He added that the long-term effectiveness data on response and remission “add relevant information” to the field.
However, he noted that it is not clear whether the high and increasing rates of response and remission were based on the reporting of observed cases or on last-observation-carried-forward analyses. “Given the naturally high dropout rate in such a long-term study and the potential for a survival bias, this is a relevant methodological question that affects the interpretation of the data,” he said.
“Nevertheless, the very favorable results for cumulative response, remission, and sustained remission add to the evidence that lurasidone is an effective treatment for youth with bipolar depression. Since efficacy cannot be interpreted in isolation, data describing the tolerability, including long-term cardiometabolic effects, will be important complementary data to consider,” Dr. Correll said.
The study was funded by Sunovion Pharmaceuticals. Dr. Singh is on the advisory board for Sunovion, is a consultant for Google X and Limbix, and receives royalties from American Psychiatric Association Publishing. She has also received research support from Stanford’s Maternal Child Health Research Institute and Department of Psychiatry, the National Institute of Mental Health, the National Institute on Aging, Johnson and Johnson, Allergan, PCORI, and the Brain and Behavior Research Foundation. Dr. Correll has been a consultant or adviser to and has received honoraria from Sunovion, as well as Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Intra-Cellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Sumitomo Dainippon, Supernus, Takeda, and Teva.
A version of this article originally appeared on Medscape.com.
The antipsychotic lurasidone (Latuda, Sunovion Pharmaceuticals) has long-term efficacy in the treatment of bipolar depression (BD) in children and adolescents, new research suggests.
In an open-label extension study involving patients aged 10-17 years, up to 2 years of treatment with lurasidone was associated with continued improvement in depressive symptoms. There were progressively higher rates of remission, recovery, and sustained remission.
Coinvestigator Manpreet K. Singh, MD, director of the Stanford Pediatric Mood Disorders Program, Stanford (Calif.) University, noted that early onset of BD is common. Although in pediatric populations, prevalence has been fairly stable at around 1.8%, these patients have “a very limited number of treatment options available for the depressed phases of BD,” which is often predominant and can be difficult to identify.
“A lot of youths who are experiencing depressive symptoms in the context of having had a manic episode will often have a relapsing and remitting course, even after the acute phase of treatment, so because kids can be on medications for long periods of time, a better understanding of what works ... is very important,” Dr. Singh said in an interview.
The findings were presented at the virtual American Society of Clinical Psychopharmacology (ASCP) 2020 annual meeting.
Long-term Efficacy
The Food and Drug Administration approved lurasidone as monotherapy for BD in children and adolescents in 2018. The aim of the current study was to evaluate the drug’s long-term efficacy in achieving response or remission in this population.
A total of 305 children who completed an initial 6-week double-blind study of lurasidone versus placebo entered the 2-year, open-label extension study. In the extension, they either continued taking lurasidone or were switched from placebo to lurasidone 20-80 mg/day. Of this group, 195 children completed 52 weeks of treatment, and 93 completed 104 weeks of treatment.
Efficacy was measured with the Children’s Depression Rating Scale, Revised (CDRS-R) and the Clinical Global Impression, Bipolar Depression Severity scale (CGI-BP-S). Functioning was evaluated with the clinician-rated Children’s Global Assessment Scale (CGAS); on that scale, a score of 70 or higher indicates no clinically meaningful functional impairment.
Remission criteria were met if a patient achieved a CDRS-R total score of 28 or less, a Young Mania Rating Scale (YMRS) total score of 8 or less, and a CGI-BP-S depression score of 3 or less.
Recovery criteria were met if a patient achieved remission and had a CGAS score of at least 70.
Sustained remission, a more stringent outcome, required that the patient meet remission criteria for at least 24 consecutive weeks.
In addition, there was a strong inverse correlation (r = –0.71) between depression severity, as measured by CDRS-R total score, and functioning, as measured by the CGAS.
“That’s the cool thing: As the depression symptoms and severity came down, the overall functioning in these kids improved,” Dr. Singh noted.
“This improvement in functioning ends up being much more clinically relevant and useful to clinicians than just showing an improvement in a set of symptoms because what brings a kid – or even an adult, for that matter – to see a clinician to get treatment is because something about their symptoms is causing significant functional impairment,” she said.
“So this is the take-home message: You can see that lurasidone ... demonstrates not just recovery from depressive symptoms but that this reduction in depressive symptoms corresponds to an improvement in functioning for these youths,” she added.
Potential Limitations
Commenting on the study, Christoph U. Correll, MD, professor of child and adolescent psychiatry, Charite Universitatsmedizin, Berlin, Germany, noted that BD is difficult to treat, especially for patients who are going through “a developmentally vulnerable phase of their lives.”
“Lurasidone is the only monotherapy approved for bipolar depression in youth and is fairly well tolerated,” said Dr. Correll, who was not part of the research. He added that the long-term effectiveness data on response and remission “add relevant information” to the field.
However, he noted that it is not clear whether the high and increasing rates of response and remission were based on the reporting of observed cases or on last-observation-carried-forward analyses. “Given the naturally high dropout rate in such a long-term study and the potential for a survival bias, this is a relevant methodological question that affects the interpretation of the data,” he said.
“Nevertheless, the very favorable results for cumulative response, remission, and sustained remission add to the evidence that lurasidone is an effective treatment for youth with bipolar depression. Since efficacy cannot be interpreted in isolation, data describing the tolerability, including long-term cardiometabolic effects, will be important complementary data to consider,” Dr. Correll said.
The study was funded by Sunovion Pharmaceuticals. Dr. Singh is on the advisory board for Sunovion, is a consultant for Google X and Limbix, and receives royalties from American Psychiatric Association Publishing. She has also received research support from Stanford’s Maternal Child Health Research Institute and Department of Psychiatry, the National Institute of Mental Health, the National Institute on Aging, Johnson and Johnson, Allergan, PCORI, and the Brain and Behavior Research Foundation. Dr. Correll has been a consultant or adviser to and has received honoraria from Sunovion, as well as Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Intra-Cellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Sumitomo Dainippon, Supernus, Takeda, and Teva.
A version of this article originally appeared on Medscape.com.
FROM ASCP 2020
Liposomal bupivacaine excreted in breast milk, but levels appear safe
based on a prospective cohort study.
Over the course of 4 days, relative neonatal dosages of bupivacaine were less than 1%, remaining below the 10% threshold of concern, reported Hiba J. Mustafa, MD, of the University of Minnesota, Minneapolis, and colleagues.
Liposomal bupivacaine can achieve up to 4 days of postcesarean pain control, which is significantly longer than the 8 hours provided by standard bupivacaine, the investigators wrote in Obstetrics & Gynecology. But usage of the liposomal formulation has not been widespread, they noted, partly because of a lack of clinical studies evaluating breast milk transfer and neonatal safety.
To address this knowledge gap, Dr. Mustafa and colleagues enrolled 30 healthy pregnant women scheduled to undergo cesarean birth at full term. All patients were aged 18-40 years, with an American Society of Anesthesiologists physical status of I or II. Exclusion criteria included a number of maternal and neonatal health concerns, such as sensitivity to local anesthetics, metabolic disorders, fetal anomaly, fetal growth restriction, and others.
The day of surgery, before the procedure, maternal blood samples were collected and used for baseline measurements.
Each woman received a spinal anesthetic including 150 mcg of morphine, 15 mcg of intrathecal fentanyl, and 1.4-1.6 mL of 0.75% hyperbaric bupivacaine hydrochloride. Within 30 minutes after birth, a bilateral transversus abdominus plane block was performed using 266 mg of 1.3% liposomal bupivacaine and 52 mg of 0.25% bupivacaine hydrochloride.
Using the block as time point zero, maternal blood and breast milk samples were collected at hour 2, 6, 12, 24, 48, 72, and 96. Sparse sampling was employed, such that participants were randomly assigned in a 1:1 ratio to provide paired blood and milk samples at hour 2, 12, and 48; or hour 6, 24, 72, and 96. Bupivacaine was quantified in samples by liquid chromatography–tandem mass spectrometry.
Using these data, the investigators determined bupivacaine concentrations in plasma and milk, milk/plasma area under the curve (AUC) ratios, neonatal dosage, and relative neonatal dosage. In addition, adverse events in both mothers and neonates were recorded for 2 weeks post partum.
Mean bupivacaine concentrations peaked in breast milk at 6 hours, at 58 ng/mL. This peak was followed by a steady reduction to an “almost undetectable” level of 5.2 ng/mL at 96 hours. Maternal plasma levels peaked first at hour 6 (155.9 ng/mL), then again at hour 48 (225.8 ng/mL), followed by a steady decline until hour 96, when the level reached 80.6 ng/mL.
Relative mean concentrations of milk to plasma were 44%, 36%, 28%, and 18% at hour 2, 6, 12, and 24, respectively. AUC ratios were used to represent exposure across various time intervals. For instance, the AUC ratio for milk/plasma from hour 0 to hour 2 was 0.45. The AUC findings declined steadily until the final ratio, which spanned hour 0 to hour 96, at 0.15.
These AUC ratios allowed for calculation of neonatal dosage and relative neonatal dosage using an average daily milk intake of 150 mL/kg per day. For the longest range, spanning from hour 0 to hour 96, the neonatal dosage was 15,155.4 ng/kg, which translated to a relative neonatal dosage of 0.396%.
No mothers or neonates experienced adverse events.
“Bupivacaine was transferred into mother’s milk such that an exclusively breastfeeding neonate would ingest less than 1% (relative neonatal dosage) of the maternal dose,” the investigators wrote, noting that this falls safely below the acceptable threshold of 10%.
“Because bupivacaine is metabolized primarily in the liver, a neonate’s absorption will likely be even lower [than modeled] given the first-pass effect,” they added.
Based on these findings, Dr. Mustafa and colleagues concluded that “the level of bupivacaine ingested by the sucking neonate is acceptable and compatible with breastfeeding.”
Michael G. Ross MD, MPH, Distinguished Professor of Obstetrics and Gynecology and Public Health at Geffen School of Medicine at the University of California, Los Angeles, commented that, this study adds to the literature of drug excretion into breast milk. “For the vast majority of drugs with passive transfer from maternal plasma to breast milk, the effective dosages of exclusive breastfeeding neonates are approximately 5% of the maternal (oral) dose. In the present study, the authors demonstrated a relative neonatal dosage of less than 1%. This low value results from consequences of minimal maternal plasma absorption (in the present case from transversus abdominis injection), maternal volume of distribution, transfer into breast milk, and the volume of milk ingestion. These results should provide reassurance for the safety of breastfeeding term infants under the conditions of the study.
“There are a number of study concerns, including the inability to differentiate absorption of the spinal bupivacaine from the liposomal bupivacaine, the lack of paired maternal plasma and breast milk sample, and the lack of detail as to how much milk was expressed for each sample. Importantly, breast milk composition varies from foremilk to hindmilk. Thus, a single sample may not accurately reflect the composition ingested by the infant. The suggestion of two peaks in maternal plasma concentration was not demonstrated statistically and may be an artifact of the timing of spinal and liposomal injections, or the fact that different patients were studied at each time period.
“Most importantly, despite the demonstrated safety, the authors acknowledge conflicting results of clinical benefits of liposomal bupivacaine injection. As such, I recommend that postcesarean transversus abdominis blocks be performed only under institutional review board-approved study protocols,” said Dr. Ross, codirector of the Institute for Women’ and Children’s Health at the Lundquist Institute, Torrance, Calif.*
The study was funded by the Thrasher Research Fund. The investigators reported no conflicts of interest. Dr. Ross had no relevant financial disclosures.
SOURCE: Mustafa et al. Obstet Gynecol. 2020 Jun 6. doi: 10.1097/AOG.0000000000003886.
*This article was updated 6/16/2020.
based on a prospective cohort study.
Over the course of 4 days, relative neonatal dosages of bupivacaine were less than 1%, remaining below the 10% threshold of concern, reported Hiba J. Mustafa, MD, of the University of Minnesota, Minneapolis, and colleagues.
Liposomal bupivacaine can achieve up to 4 days of postcesarean pain control, which is significantly longer than the 8 hours provided by standard bupivacaine, the investigators wrote in Obstetrics & Gynecology. But usage of the liposomal formulation has not been widespread, they noted, partly because of a lack of clinical studies evaluating breast milk transfer and neonatal safety.
To address this knowledge gap, Dr. Mustafa and colleagues enrolled 30 healthy pregnant women scheduled to undergo cesarean birth at full term. All patients were aged 18-40 years, with an American Society of Anesthesiologists physical status of I or II. Exclusion criteria included a number of maternal and neonatal health concerns, such as sensitivity to local anesthetics, metabolic disorders, fetal anomaly, fetal growth restriction, and others.
The day of surgery, before the procedure, maternal blood samples were collected and used for baseline measurements.
Each woman received a spinal anesthetic including 150 mcg of morphine, 15 mcg of intrathecal fentanyl, and 1.4-1.6 mL of 0.75% hyperbaric bupivacaine hydrochloride. Within 30 minutes after birth, a bilateral transversus abdominus plane block was performed using 266 mg of 1.3% liposomal bupivacaine and 52 mg of 0.25% bupivacaine hydrochloride.
Using the block as time point zero, maternal blood and breast milk samples were collected at hour 2, 6, 12, 24, 48, 72, and 96. Sparse sampling was employed, such that participants were randomly assigned in a 1:1 ratio to provide paired blood and milk samples at hour 2, 12, and 48; or hour 6, 24, 72, and 96. Bupivacaine was quantified in samples by liquid chromatography–tandem mass spectrometry.
Using these data, the investigators determined bupivacaine concentrations in plasma and milk, milk/plasma area under the curve (AUC) ratios, neonatal dosage, and relative neonatal dosage. In addition, adverse events in both mothers and neonates were recorded for 2 weeks post partum.
Mean bupivacaine concentrations peaked in breast milk at 6 hours, at 58 ng/mL. This peak was followed by a steady reduction to an “almost undetectable” level of 5.2 ng/mL at 96 hours. Maternal plasma levels peaked first at hour 6 (155.9 ng/mL), then again at hour 48 (225.8 ng/mL), followed by a steady decline until hour 96, when the level reached 80.6 ng/mL.
Relative mean concentrations of milk to plasma were 44%, 36%, 28%, and 18% at hour 2, 6, 12, and 24, respectively. AUC ratios were used to represent exposure across various time intervals. For instance, the AUC ratio for milk/plasma from hour 0 to hour 2 was 0.45. The AUC findings declined steadily until the final ratio, which spanned hour 0 to hour 96, at 0.15.
These AUC ratios allowed for calculation of neonatal dosage and relative neonatal dosage using an average daily milk intake of 150 mL/kg per day. For the longest range, spanning from hour 0 to hour 96, the neonatal dosage was 15,155.4 ng/kg, which translated to a relative neonatal dosage of 0.396%.
No mothers or neonates experienced adverse events.
“Bupivacaine was transferred into mother’s milk such that an exclusively breastfeeding neonate would ingest less than 1% (relative neonatal dosage) of the maternal dose,” the investigators wrote, noting that this falls safely below the acceptable threshold of 10%.
“Because bupivacaine is metabolized primarily in the liver, a neonate’s absorption will likely be even lower [than modeled] given the first-pass effect,” they added.
Based on these findings, Dr. Mustafa and colleagues concluded that “the level of bupivacaine ingested by the sucking neonate is acceptable and compatible with breastfeeding.”
Michael G. Ross MD, MPH, Distinguished Professor of Obstetrics and Gynecology and Public Health at Geffen School of Medicine at the University of California, Los Angeles, commented that, this study adds to the literature of drug excretion into breast milk. “For the vast majority of drugs with passive transfer from maternal plasma to breast milk, the effective dosages of exclusive breastfeeding neonates are approximately 5% of the maternal (oral) dose. In the present study, the authors demonstrated a relative neonatal dosage of less than 1%. This low value results from consequences of minimal maternal plasma absorption (in the present case from transversus abdominis injection), maternal volume of distribution, transfer into breast milk, and the volume of milk ingestion. These results should provide reassurance for the safety of breastfeeding term infants under the conditions of the study.
“There are a number of study concerns, including the inability to differentiate absorption of the spinal bupivacaine from the liposomal bupivacaine, the lack of paired maternal plasma and breast milk sample, and the lack of detail as to how much milk was expressed for each sample. Importantly, breast milk composition varies from foremilk to hindmilk. Thus, a single sample may not accurately reflect the composition ingested by the infant. The suggestion of two peaks in maternal plasma concentration was not demonstrated statistically and may be an artifact of the timing of spinal and liposomal injections, or the fact that different patients were studied at each time period.
“Most importantly, despite the demonstrated safety, the authors acknowledge conflicting results of clinical benefits of liposomal bupivacaine injection. As such, I recommend that postcesarean transversus abdominis blocks be performed only under institutional review board-approved study protocols,” said Dr. Ross, codirector of the Institute for Women’ and Children’s Health at the Lundquist Institute, Torrance, Calif.*
The study was funded by the Thrasher Research Fund. The investigators reported no conflicts of interest. Dr. Ross had no relevant financial disclosures.
SOURCE: Mustafa et al. Obstet Gynecol. 2020 Jun 6. doi: 10.1097/AOG.0000000000003886.
*This article was updated 6/16/2020.
based on a prospective cohort study.
Over the course of 4 days, relative neonatal dosages of bupivacaine were less than 1%, remaining below the 10% threshold of concern, reported Hiba J. Mustafa, MD, of the University of Minnesota, Minneapolis, and colleagues.
Liposomal bupivacaine can achieve up to 4 days of postcesarean pain control, which is significantly longer than the 8 hours provided by standard bupivacaine, the investigators wrote in Obstetrics & Gynecology. But usage of the liposomal formulation has not been widespread, they noted, partly because of a lack of clinical studies evaluating breast milk transfer and neonatal safety.
To address this knowledge gap, Dr. Mustafa and colleagues enrolled 30 healthy pregnant women scheduled to undergo cesarean birth at full term. All patients were aged 18-40 years, with an American Society of Anesthesiologists physical status of I or II. Exclusion criteria included a number of maternal and neonatal health concerns, such as sensitivity to local anesthetics, metabolic disorders, fetal anomaly, fetal growth restriction, and others.
The day of surgery, before the procedure, maternal blood samples were collected and used for baseline measurements.
Each woman received a spinal anesthetic including 150 mcg of morphine, 15 mcg of intrathecal fentanyl, and 1.4-1.6 mL of 0.75% hyperbaric bupivacaine hydrochloride. Within 30 minutes after birth, a bilateral transversus abdominus plane block was performed using 266 mg of 1.3% liposomal bupivacaine and 52 mg of 0.25% bupivacaine hydrochloride.
Using the block as time point zero, maternal blood and breast milk samples were collected at hour 2, 6, 12, 24, 48, 72, and 96. Sparse sampling was employed, such that participants were randomly assigned in a 1:1 ratio to provide paired blood and milk samples at hour 2, 12, and 48; or hour 6, 24, 72, and 96. Bupivacaine was quantified in samples by liquid chromatography–tandem mass spectrometry.
Using these data, the investigators determined bupivacaine concentrations in plasma and milk, milk/plasma area under the curve (AUC) ratios, neonatal dosage, and relative neonatal dosage. In addition, adverse events in both mothers and neonates were recorded for 2 weeks post partum.
Mean bupivacaine concentrations peaked in breast milk at 6 hours, at 58 ng/mL. This peak was followed by a steady reduction to an “almost undetectable” level of 5.2 ng/mL at 96 hours. Maternal plasma levels peaked first at hour 6 (155.9 ng/mL), then again at hour 48 (225.8 ng/mL), followed by a steady decline until hour 96, when the level reached 80.6 ng/mL.
Relative mean concentrations of milk to plasma were 44%, 36%, 28%, and 18% at hour 2, 6, 12, and 24, respectively. AUC ratios were used to represent exposure across various time intervals. For instance, the AUC ratio for milk/plasma from hour 0 to hour 2 was 0.45. The AUC findings declined steadily until the final ratio, which spanned hour 0 to hour 96, at 0.15.
These AUC ratios allowed for calculation of neonatal dosage and relative neonatal dosage using an average daily milk intake of 150 mL/kg per day. For the longest range, spanning from hour 0 to hour 96, the neonatal dosage was 15,155.4 ng/kg, which translated to a relative neonatal dosage of 0.396%.
No mothers or neonates experienced adverse events.
“Bupivacaine was transferred into mother’s milk such that an exclusively breastfeeding neonate would ingest less than 1% (relative neonatal dosage) of the maternal dose,” the investigators wrote, noting that this falls safely below the acceptable threshold of 10%.
“Because bupivacaine is metabolized primarily in the liver, a neonate’s absorption will likely be even lower [than modeled] given the first-pass effect,” they added.
Based on these findings, Dr. Mustafa and colleagues concluded that “the level of bupivacaine ingested by the sucking neonate is acceptable and compatible with breastfeeding.”
Michael G. Ross MD, MPH, Distinguished Professor of Obstetrics and Gynecology and Public Health at Geffen School of Medicine at the University of California, Los Angeles, commented that, this study adds to the literature of drug excretion into breast milk. “For the vast majority of drugs with passive transfer from maternal plasma to breast milk, the effective dosages of exclusive breastfeeding neonates are approximately 5% of the maternal (oral) dose. In the present study, the authors demonstrated a relative neonatal dosage of less than 1%. This low value results from consequences of minimal maternal plasma absorption (in the present case from transversus abdominis injection), maternal volume of distribution, transfer into breast milk, and the volume of milk ingestion. These results should provide reassurance for the safety of breastfeeding term infants under the conditions of the study.
“There are a number of study concerns, including the inability to differentiate absorption of the spinal bupivacaine from the liposomal bupivacaine, the lack of paired maternal plasma and breast milk sample, and the lack of detail as to how much milk was expressed for each sample. Importantly, breast milk composition varies from foremilk to hindmilk. Thus, a single sample may not accurately reflect the composition ingested by the infant. The suggestion of two peaks in maternal plasma concentration was not demonstrated statistically and may be an artifact of the timing of spinal and liposomal injections, or the fact that different patients were studied at each time period.
“Most importantly, despite the demonstrated safety, the authors acknowledge conflicting results of clinical benefits of liposomal bupivacaine injection. As such, I recommend that postcesarean transversus abdominis blocks be performed only under institutional review board-approved study protocols,” said Dr. Ross, codirector of the Institute for Women’ and Children’s Health at the Lundquist Institute, Torrance, Calif.*
The study was funded by the Thrasher Research Fund. The investigators reported no conflicts of interest. Dr. Ross had no relevant financial disclosures.
SOURCE: Mustafa et al. Obstet Gynecol. 2020 Jun 6. doi: 10.1097/AOG.0000000000003886.
*This article was updated 6/16/2020.
FROM OBSTETRICS & GYNECOLOGY
Daily Recap: Feds seek COVID-19 info through app, hospitalists take on new roles
Here are the stories our MDedge editors across specialties think you need to know about today:
FDA seeks COVID-19 info through CURE ID
Federal health officials are asking clinicians to use the free CURE ID mobile app and web platform as a tool to collect information on the treatment of patients with COVID-19. CURE ID is an Internet-based data repository first developed in 2013 as a collaboration between the Food and Drug Administration and the National Center for Advancing Translational Sciences, part of the National Institutes of Health. It provides licensed clinicians worldwide with an opportunity to report novel uses of existing drugs for patients with difficult-to-treat infectious diseases, including COVID-19, through a website, a smartphone, or other mobile device. “By utilizing the CURE ID platform now for COVID-19 case collection – in conjunction with data gathered from other registries, EHR systems, and clinical trials – data collected during an outbreak can be improved and coordinated,” said Heather A. Stone, MPH, a health science policy analyst in the office of medical policy at the FDA’s Center for Drug Evaluation and Research. “This may allow us to find possible treatments to help ease this pandemic, and prepare us better to fight the next one.” Read more.
Hospitalists take on new roles in COVID era
Whether it’s working shifts in the ICU, caring for ventilator patients, or reporting to postanesthesia care units and post-acute or step-down units, hospitalists are stepping into a variety of new roles as part of their frontline response to the COVID-19 pandemic. Valerie Vaughn, MD, a hospitalist with Michigan Medicine and assistant professor of medicine at the University of Michigan in Ann Arbor, was doing research on how to reduce overuse of antibiotics in hospitals when the COVID-19 crisis hit and dramatically redefined her job. “We were afraid that we might have 3,000 to 5,000 hospitalized COVID patients by now, based on predictive modeling done while the pandemic was still growing exponentially,” she explained. Although Michigan continues to have high COVID-19 infection rates, centered on nearby Detroit, “things are a lot better today than they were 4 weeks ago.” Dr. Vaughn helped to mobilize a team of 25 hospitalists, along with other health care professionals, who volunteered to manage COVID-19 patients in the ICU and other hospital units. Read more.
COVID-19 recommendations for rheumatic disease treatment
The European League Against Rheumatism (EULAR) issued provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2. Contrary to earlier expectations, there is no indication that patients with rheumatic and musculoskeletal diseases have a higher risk of contracting the virus or have a worse course if they do, according to the task force that worked on the recommendations. The task force also pointed out that rheumatology drugs are being used to treat COVID-19 patients who don’t have rheumatic diseases, raising the possibility of a shortage of disease-modifying antirheumatic drugs. Read more.
Mental health visits are 19% of ED costs
Mental and substance use disorders represented 19% of all emergency department visits in 2017 and cost $14.6 billion, according to figures from the Agency for Healthcare Research and Quality. The most costly mental and substance use disorder diagnosis was anxiety and fear-related disorders, accounting for $5.6 billion worth of visits, following by depressive disorders and alcohol-related disorders. Read more.
Food deserts linked to health issues in pregnancy
Living in a neighborhood lacking adequate access to affordable, high-quality food is associated with a somewhat greater risk of developing pregnancy morbidity, according to an observational study. Researchers found that women who lived in a food desert had a 1.6 times greater odds of pregnancy comorbidity than if they did not. “An additional, albeit less obvious factor that may be unique to patients suffering disproportionately from obstetric morbidity is exposure to toxic elements,” the researchers reported in Obstetrics & Gynecology. “It has been shown in a previous study that low-income, predominantly black communities of pregnant women may suffer disproportionately from lead or arsenic exposure.” Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
FDA seeks COVID-19 info through CURE ID
Federal health officials are asking clinicians to use the free CURE ID mobile app and web platform as a tool to collect information on the treatment of patients with COVID-19. CURE ID is an Internet-based data repository first developed in 2013 as a collaboration between the Food and Drug Administration and the National Center for Advancing Translational Sciences, part of the National Institutes of Health. It provides licensed clinicians worldwide with an opportunity to report novel uses of existing drugs for patients with difficult-to-treat infectious diseases, including COVID-19, through a website, a smartphone, or other mobile device. “By utilizing the CURE ID platform now for COVID-19 case collection – in conjunction with data gathered from other registries, EHR systems, and clinical trials – data collected during an outbreak can be improved and coordinated,” said Heather A. Stone, MPH, a health science policy analyst in the office of medical policy at the FDA’s Center for Drug Evaluation and Research. “This may allow us to find possible treatments to help ease this pandemic, and prepare us better to fight the next one.” Read more.
Hospitalists take on new roles in COVID era
Whether it’s working shifts in the ICU, caring for ventilator patients, or reporting to postanesthesia care units and post-acute or step-down units, hospitalists are stepping into a variety of new roles as part of their frontline response to the COVID-19 pandemic. Valerie Vaughn, MD, a hospitalist with Michigan Medicine and assistant professor of medicine at the University of Michigan in Ann Arbor, was doing research on how to reduce overuse of antibiotics in hospitals when the COVID-19 crisis hit and dramatically redefined her job. “We were afraid that we might have 3,000 to 5,000 hospitalized COVID patients by now, based on predictive modeling done while the pandemic was still growing exponentially,” she explained. Although Michigan continues to have high COVID-19 infection rates, centered on nearby Detroit, “things are a lot better today than they were 4 weeks ago.” Dr. Vaughn helped to mobilize a team of 25 hospitalists, along with other health care professionals, who volunteered to manage COVID-19 patients in the ICU and other hospital units. Read more.
COVID-19 recommendations for rheumatic disease treatment
The European League Against Rheumatism (EULAR) issued provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2. Contrary to earlier expectations, there is no indication that patients with rheumatic and musculoskeletal diseases have a higher risk of contracting the virus or have a worse course if they do, according to the task force that worked on the recommendations. The task force also pointed out that rheumatology drugs are being used to treat COVID-19 patients who don’t have rheumatic diseases, raising the possibility of a shortage of disease-modifying antirheumatic drugs. Read more.
Mental health visits are 19% of ED costs
Mental and substance use disorders represented 19% of all emergency department visits in 2017 and cost $14.6 billion, according to figures from the Agency for Healthcare Research and Quality. The most costly mental and substance use disorder diagnosis was anxiety and fear-related disorders, accounting for $5.6 billion worth of visits, following by depressive disorders and alcohol-related disorders. Read more.
Food deserts linked to health issues in pregnancy
Living in a neighborhood lacking adequate access to affordable, high-quality food is associated with a somewhat greater risk of developing pregnancy morbidity, according to an observational study. Researchers found that women who lived in a food desert had a 1.6 times greater odds of pregnancy comorbidity than if they did not. “An additional, albeit less obvious factor that may be unique to patients suffering disproportionately from obstetric morbidity is exposure to toxic elements,” the researchers reported in Obstetrics & Gynecology. “It has been shown in a previous study that low-income, predominantly black communities of pregnant women may suffer disproportionately from lead or arsenic exposure.” Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
FDA seeks COVID-19 info through CURE ID
Federal health officials are asking clinicians to use the free CURE ID mobile app and web platform as a tool to collect information on the treatment of patients with COVID-19. CURE ID is an Internet-based data repository first developed in 2013 as a collaboration between the Food and Drug Administration and the National Center for Advancing Translational Sciences, part of the National Institutes of Health. It provides licensed clinicians worldwide with an opportunity to report novel uses of existing drugs for patients with difficult-to-treat infectious diseases, including COVID-19, through a website, a smartphone, or other mobile device. “By utilizing the CURE ID platform now for COVID-19 case collection – in conjunction with data gathered from other registries, EHR systems, and clinical trials – data collected during an outbreak can be improved and coordinated,” said Heather A. Stone, MPH, a health science policy analyst in the office of medical policy at the FDA’s Center for Drug Evaluation and Research. “This may allow us to find possible treatments to help ease this pandemic, and prepare us better to fight the next one.” Read more.
Hospitalists take on new roles in COVID era
Whether it’s working shifts in the ICU, caring for ventilator patients, or reporting to postanesthesia care units and post-acute or step-down units, hospitalists are stepping into a variety of new roles as part of their frontline response to the COVID-19 pandemic. Valerie Vaughn, MD, a hospitalist with Michigan Medicine and assistant professor of medicine at the University of Michigan in Ann Arbor, was doing research on how to reduce overuse of antibiotics in hospitals when the COVID-19 crisis hit and dramatically redefined her job. “We were afraid that we might have 3,000 to 5,000 hospitalized COVID patients by now, based on predictive modeling done while the pandemic was still growing exponentially,” she explained. Although Michigan continues to have high COVID-19 infection rates, centered on nearby Detroit, “things are a lot better today than they were 4 weeks ago.” Dr. Vaughn helped to mobilize a team of 25 hospitalists, along with other health care professionals, who volunteered to manage COVID-19 patients in the ICU and other hospital units. Read more.
COVID-19 recommendations for rheumatic disease treatment
The European League Against Rheumatism (EULAR) issued provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2. Contrary to earlier expectations, there is no indication that patients with rheumatic and musculoskeletal diseases have a higher risk of contracting the virus or have a worse course if they do, according to the task force that worked on the recommendations. The task force also pointed out that rheumatology drugs are being used to treat COVID-19 patients who don’t have rheumatic diseases, raising the possibility of a shortage of disease-modifying antirheumatic drugs. Read more.
Mental health visits are 19% of ED costs
Mental and substance use disorders represented 19% of all emergency department visits in 2017 and cost $14.6 billion, according to figures from the Agency for Healthcare Research and Quality. The most costly mental and substance use disorder diagnosis was anxiety and fear-related disorders, accounting for $5.6 billion worth of visits, following by depressive disorders and alcohol-related disorders. Read more.
Food deserts linked to health issues in pregnancy
Living in a neighborhood lacking adequate access to affordable, high-quality food is associated with a somewhat greater risk of developing pregnancy morbidity, according to an observational study. Researchers found that women who lived in a food desert had a 1.6 times greater odds of pregnancy comorbidity than if they did not. “An additional, albeit less obvious factor that may be unique to patients suffering disproportionately from obstetric morbidity is exposure to toxic elements,” the researchers reported in Obstetrics & Gynecology. “It has been shown in a previous study that low-income, predominantly black communities of pregnant women may suffer disproportionately from lead or arsenic exposure.” Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Hospitalists stretch into new roles on COVID-19 front lines
‘Every single day is different’
In the midst of the COVID-19 pandemic, health systems, hospitals, and hospitalists – especially in hot spots like New York, Detroit, or Boston – have been challenged to stretch limits, redefine roles, and redeploy critical staff in response to rapidly changing needs on the ground.
Many hospitalists are working above and beyond their normal duties, sometimes beyond their training, specialty, or comfort zone and are rising to the occasion in ways they never imagined. These include doing shifts in ICUs, working with ventilator patients, and reporting to other atypical sites of care like postanesthesia care units and post-acute or step-down units.
Valerie Vaughn, MD, MSc, a hospitalist with Michigan Medicine and assistant professor of medicine at the University of Michigan in Ann Arbor, was doing research on how to reduce overuse of antibiotics in hospitals when the COVID-19 crisis hit and dramatically redefined her job. “We were afraid that we might have 3,000 to 5,000 hospitalized COVID patients by now, based on predictive modeling done while the pandemic was still growing exponentially,” she explained. Although Michigan continues to have high COVID-19 infection rates, centered on nearby Detroit, “things are a lot better today than they were 4 weeks ago.”
Dr. Vaughn helped to mobilize a team of 25 hospitalists, along with other health care providers, who volunteered to manage COVID-19 patients in the ICU and other hospital units. She was asked to help develop an all-COVID unit called the Regional Infectious Containment Unit or RICU, which opened March 16. Then, when the RICU became full, it was supplemented by two COVID-19 Moderate Care Units staffed by hospitalists who had “learned the ropes” in the RICU.
Both of these new models were defined in relation to the ICUs at Michigan Medicine – which were doubling in capacity, up to 200 beds at last count – and to the provision of intensive-level and long-term ventilator care for the sickest patients. The moderate care units are for patients who are not on ventilators but still very sick, for example, those receiving massive high-flow oxygen, often with a medical do-not-resuscitate/do-not-intubate order. “We established these units to do everything (medically) short of vents,” Dr. Vaughn said.
“We are having in-depth conversations about goals of care with patients soon after they arrive at the hospital. We know outcomes from ventilators are worse for COVID-positive patients who have comorbidities, and we’re using that information to inform these conversations. We’ve given scripts to clinicians to help guide them in leading these conversations. We can do other things than `use ventilators to manage their symptoms. But these are still difficult conversations,” Dr. Vaughn said.

“We also engaged palliative care early on and asked them to round with us on every [COVID] patient – until demand got too high.” The bottleneck has been the number of ICU beds available, she explained. “If you want your patient to come in and take that bed, make sure you’ve talked to the family about it.”
The COVID-19 team developed guidelines printed on pocket cards addressing critical care issues such as a refresher on how to treat acute respiratory distress syndrome and how to use vasopressors. (See the COVID-19 Continuing Medical Education Portal for web-accessible educational resources developed by Michigan Health).
It’s amazing how quickly patients can become very sick with COVID-19, Dr. Vaughn said. “One of the good things to happen from the beginning with our RICU is that a group of doctors became COVID care experts very quickly. We joined four to five hospitalists and their teams with each intensivist, so one critical care expert is there to do teaching and answer clinicians’ questions. The hospitalists coordinate the COVID care and talk to the families.”
Working on the front lines of this crisis, Dr. Vaughn said, has generated a powerful sense of purpose and camaraderie, creating bonds like in war time. “All of us on our days off feel a twinge of guilt for not being there in the hospital. The sense of gratitude we get from patients and families has been enormous, even when we were telling them bad news. That just brings us to tears.”
One of the hardest things for the doctors practicing above their typical scope of practice is that, when something bad happens, they can’t know whether it was a mistake on their part or not, she noted. “But I’ve never been so proud of our group or to be a hospitalist. No one has complained or pushed back. Everyone has responded by saying: ‘What can I do to help?’ ”
Enough work in hospital medicine
Hospitalists had not been deployed to care for ICU patients at Beth Israel Deaconess Medical Center (BIDMC) in Boston, a major hot spot for COVID-19, said Joseph Ming Wah Li, MD, SFHM, director of the hospital medicine program at BIDMC, when he spoke to The Hospitalist in mid-May. That’s because there were plenty of hospital medicine assignments to keep them busy. Dr. Li leads a service of 120 hospitalists practicing at four hospitals.
“As we speak today, we have 300 patients with COVID, with 70 or 80 of them in our ICU. I’m taking care of 17 patients today, 15 of them COVID-positive, and the other two placed in a former radiology holding suite adapted for COVID-negative patients. Our postanesthesia care unit is now an ICU filled with COVID patients,” he said.
“Half of my day is seeing patients and the other half I’m on Zoom calls. I’m also one of the resource allocation officers for BIDMC,” Dr. Li said. He helped to create a standard of care for the hospital, addressing what to do if there weren’t enough ICU beds or ventilators. “We’ve never actualized it and probably won’t, but it was important to go through this exercise, with a lot of discussion up front.”
Haki Laho, MD, an orthopedic hospitalist at New England Baptist Hospital (NEBH), also in Boston, has been redeployed to care for a different population of patients as his system tries to bunch patients. “All of a sudden – within hours and days – at the beginning of the pandemic and based on the recommendations, our whole system decided to stop all elective procedures and devote the resources to COVID,” he said.
NEBH is Beth Israel Lahey Health’s 141-bed orthopedic and surgical hospital, and the system has tried to keep the specialty facility COVID-19–free as much as possible, with the COVID-19 patients grouped together at BIDMC. Dr. Laho’s orthopedic hospitalist group, just five doctors, has been managing the influx of medical patients with multiple comorbidities – not COVID-19–infected but still a different kind of patient than they are used to.
“So far, so good. We’re dealing with it,” he said. “But if one of us got sick, the others would have to step up and do more shifts. We are physicians, internal medicine trained, but since my residency I hadn’t had to deal with these kinds of issues on a daily basis, such as setting up IV lines. I feel like I am back in residency mode.”
Convention Center medicine
Another Boston hospitalist, Amy Baughman, MD, who practices at Massachusetts General Hospital, is using her skills in a new setting, serving as a co-medical director at Boston Hope Medical Center, a 1,000-bed field hospital for patients with COVID-19. Open since April 10 and housed in the Boston Convention and Exhibition Center, it is a four-way collaboration between the Commonwealth of Massachusetts, the City of Boston, Partners HealthCare, and the Boston Health Care for the Homeless Program.
Boston Hope is divided into a post-acute care section for recovering COVID-19 patients and a respite section for undomiciled patients with COVID-19 who need a place to safely quarantine. Built for a maximum of 1,000 beds, it is currently using fewer, with 83 patients on the post-acute side and 73 on the respite side as of May 12. A total of 370 and 315, respectively, had been admitted through May 12.
The team had 5 days to put the field hospital together with the help of the Army National Guard. “During that first week I was installing hand sanitizer dispensers and making [personal protective equipment] signs. Everyone here has had to do things like that,” Dr. Baughman said. “We’ve had to be incredibly creative in our staffing, using doctors from primary care and subspecialties including dermatology, radiology, and orthopedics. We had to fast-track trainings on how to use EPIC and to provide post-acute COVID care. How do you simultaneously build a medical facility and lead teams to provide high quality care?”
Dr. Baughman still works hospitalist shifts half-time at Massachusetts General. Her prior experience providing post-acute care in the VA system was helpful in creating the post-acute level of care at Boston Hope.
“My medical director role involves supervising, staffing, and scheduling. My co-medical director, Dr. Kerri Palamara, and I also supervise the clinical care,” she said. “There are a lot of systems issues, like ordering labs or prescriptions, with couriers going back and forth. And we developed clinical pathways, such as for [deep vein thrombosis] prophylaxis or for COVID retesting to determine when it is safe to end a quarantine. We’re just now rolling out virtual specialist consultations,” she noted.
“It has gone incredibly well. So much of it has been about our ability and willingness to work hard, and take feedback and go forward. We don’t have time to harp on things. We have to be very solution oriented. At the same time, honestly, it’s been fun. Every single day is different,” Dr. Baughman said.
“It’s been an opportunity to use my skills in a totally new setting, and at a level of responsibility I haven’t had before, although that’s probably a common theme with COVID-19. I was put on this team because I am a hospitalist,” she said. “I think hospitalists have been the backbone of the response to COVID in this country. It’s been an opportunity for our specialty to shine. We need to embrace the opportunity.”
Balancing expertise and supervision
Mount Sinai Hospital (MSH) in Manhattan is in the New York epicenter of the COVID-19 crisis and has mobilized large numbers of pulmonary critical care and anesthesia physicians to staff up multiple ICUs for COVID-19 patients, said Andrew Dunn, MD, chief of the division of hospital medicine at Mount Sinai School of Medicine.
“My hospitalist group is covering many step-down units, medical wards, and atypical locations, providing advanced oxygen therapies, [bilevel positive airway pressure], high-flow nasal cannulas, and managing some patients on ventilators,” he said.
MSH has teaching services with house staff and nonteaching services. “We combined them into a unified service with house staff dispersed across all of the teams. We drafted a lot of nonhospitalists from different specialties to be attendings, and that has given us a tiered model, with a hospitalist supervising three or four nonhospitalist-led teams. Although the supervising hospitalists carry no patient caseloads of their own, this is primarily a clinical rather than an administrative role.”
At the peak, there were 40 rounding teams at MSH, each with a typical census of 15 patients or more, which meant that 10 supervisory hospitalists were responsible for 300 to 400 patients. “What we learned first was the need to balance the level of expertise. For example, a team may include a postgraduate year 3 resident and a radiology intern,” Dr. Dunn said. As COVID-19 census has started coming down, supervisory hospitalists are returning to direct care attending roles, and some hospitalists have been shared across the Mount Sinai system’s hospitals.
Dr. Dunn’s advice for hospitalists filling a supervisory role like this in a tiered model: Make sure you talk to your team the night before the first day of a scheduling block and try to address as many of their questions as possible. “If you wait until the morning of the shift to connect with them, anxiety will be high. But after going through a couple of scheduling cycles, we find that things are getting better. I think we’ve paid a lot of attention to the risks of burnout by our physicians. We’re using a model of 4 days on/4 off.”
Another variation on these themes is Joshua Shatzkes, MD, assistant professor of medicine and cardiology at Mount Sinai, who practices outpatient cardiology at MSH and in several off-site offices in Brooklyn. He saw early on that COVID-19 would have a huge effect on his practice, so he volunteered to help out with inpatient care. “I made it known to my chief that I was available, and I was deployed in the first week, after a weekend of cramming webinars and lectures on critical care and pulling out critical concepts that I already knew.”
Dr. Shatzkes said his career path led him into outpatient cardiology 11 years ago, where he was quickly too busy to see his patients when they went into the hospital, even though he missed hospital medicine. Working as a temporary hospitalist with the arrival of COVID-19, he has been invigorated and mobilized by the experience and reminded of why he went to medical school in the first place. “Each day’s shift went quickly but felt long. At the end of the day, I was tired but not exhausted. When I walked out of a patient’s room, they could tell, ‘This is a doctor who cared for me,’ ” he said.
After Dr. Shatzkes volunteered, he got the call from his division chief. “I was officially deployed for a 4-day shift at Mount Sinai and then as a backup.” On his first morning as an inpatient doctor, he was still getting oriented when calls started coming from the nurses. “I had five patients struggling to breathe. Their degree of hypoxia was remarkable. I kept them out of the ICU, at least for that day.”
Since then, he has continued to follow some of those patients in the hospital, along with some from his outpatient practice who were hospitalized, and others referred by colleagues, while remaining available to his outpatients through telemedicine. When this is all over, Dr. Shatzkes said, he would love to find a way to incorporate a hospital practice in his job – depending on the realities of New York traffic.
“Joshua is not a hospitalist, but he went on service and felt so fulfilled and rewarded, he asked me if he could stay on service,” Dr. Dunn said. “I also got an email from the nurse manager on the unit. They want him back.”
‘Every single day is different’
‘Every single day is different’
In the midst of the COVID-19 pandemic, health systems, hospitals, and hospitalists – especially in hot spots like New York, Detroit, or Boston – have been challenged to stretch limits, redefine roles, and redeploy critical staff in response to rapidly changing needs on the ground.
Many hospitalists are working above and beyond their normal duties, sometimes beyond their training, specialty, or comfort zone and are rising to the occasion in ways they never imagined. These include doing shifts in ICUs, working with ventilator patients, and reporting to other atypical sites of care like postanesthesia care units and post-acute or step-down units.
Valerie Vaughn, MD, MSc, a hospitalist with Michigan Medicine and assistant professor of medicine at the University of Michigan in Ann Arbor, was doing research on how to reduce overuse of antibiotics in hospitals when the COVID-19 crisis hit and dramatically redefined her job. “We were afraid that we might have 3,000 to 5,000 hospitalized COVID patients by now, based on predictive modeling done while the pandemic was still growing exponentially,” she explained. Although Michigan continues to have high COVID-19 infection rates, centered on nearby Detroit, “things are a lot better today than they were 4 weeks ago.”
Dr. Vaughn helped to mobilize a team of 25 hospitalists, along with other health care providers, who volunteered to manage COVID-19 patients in the ICU and other hospital units. She was asked to help develop an all-COVID unit called the Regional Infectious Containment Unit or RICU, which opened March 16. Then, when the RICU became full, it was supplemented by two COVID-19 Moderate Care Units staffed by hospitalists who had “learned the ropes” in the RICU.
Both of these new models were defined in relation to the ICUs at Michigan Medicine – which were doubling in capacity, up to 200 beds at last count – and to the provision of intensive-level and long-term ventilator care for the sickest patients. The moderate care units are for patients who are not on ventilators but still very sick, for example, those receiving massive high-flow oxygen, often with a medical do-not-resuscitate/do-not-intubate order. “We established these units to do everything (medically) short of vents,” Dr. Vaughn said.
“We are having in-depth conversations about goals of care with patients soon after they arrive at the hospital. We know outcomes from ventilators are worse for COVID-positive patients who have comorbidities, and we’re using that information to inform these conversations. We’ve given scripts to clinicians to help guide them in leading these conversations. We can do other things than `use ventilators to manage their symptoms. But these are still difficult conversations,” Dr. Vaughn said.

“We also engaged palliative care early on and asked them to round with us on every [COVID] patient – until demand got too high.” The bottleneck has been the number of ICU beds available, she explained. “If you want your patient to come in and take that bed, make sure you’ve talked to the family about it.”
The COVID-19 team developed guidelines printed on pocket cards addressing critical care issues such as a refresher on how to treat acute respiratory distress syndrome and how to use vasopressors. (See the COVID-19 Continuing Medical Education Portal for web-accessible educational resources developed by Michigan Health).
It’s amazing how quickly patients can become very sick with COVID-19, Dr. Vaughn said. “One of the good things to happen from the beginning with our RICU is that a group of doctors became COVID care experts very quickly. We joined four to five hospitalists and their teams with each intensivist, so one critical care expert is there to do teaching and answer clinicians’ questions. The hospitalists coordinate the COVID care and talk to the families.”
Working on the front lines of this crisis, Dr. Vaughn said, has generated a powerful sense of purpose and camaraderie, creating bonds like in war time. “All of us on our days off feel a twinge of guilt for not being there in the hospital. The sense of gratitude we get from patients and families has been enormous, even when we were telling them bad news. That just brings us to tears.”
One of the hardest things for the doctors practicing above their typical scope of practice is that, when something bad happens, they can’t know whether it was a mistake on their part or not, she noted. “But I’ve never been so proud of our group or to be a hospitalist. No one has complained or pushed back. Everyone has responded by saying: ‘What can I do to help?’ ”
Enough work in hospital medicine
Hospitalists had not been deployed to care for ICU patients at Beth Israel Deaconess Medical Center (BIDMC) in Boston, a major hot spot for COVID-19, said Joseph Ming Wah Li, MD, SFHM, director of the hospital medicine program at BIDMC, when he spoke to The Hospitalist in mid-May. That’s because there were plenty of hospital medicine assignments to keep them busy. Dr. Li leads a service of 120 hospitalists practicing at four hospitals.
“As we speak today, we have 300 patients with COVID, with 70 or 80 of them in our ICU. I’m taking care of 17 patients today, 15 of them COVID-positive, and the other two placed in a former radiology holding suite adapted for COVID-negative patients. Our postanesthesia care unit is now an ICU filled with COVID patients,” he said.
“Half of my day is seeing patients and the other half I’m on Zoom calls. I’m also one of the resource allocation officers for BIDMC,” Dr. Li said. He helped to create a standard of care for the hospital, addressing what to do if there weren’t enough ICU beds or ventilators. “We’ve never actualized it and probably won’t, but it was important to go through this exercise, with a lot of discussion up front.”
Haki Laho, MD, an orthopedic hospitalist at New England Baptist Hospital (NEBH), also in Boston, has been redeployed to care for a different population of patients as his system tries to bunch patients. “All of a sudden – within hours and days – at the beginning of the pandemic and based on the recommendations, our whole system decided to stop all elective procedures and devote the resources to COVID,” he said.
NEBH is Beth Israel Lahey Health’s 141-bed orthopedic and surgical hospital, and the system has tried to keep the specialty facility COVID-19–free as much as possible, with the COVID-19 patients grouped together at BIDMC. Dr. Laho’s orthopedic hospitalist group, just five doctors, has been managing the influx of medical patients with multiple comorbidities – not COVID-19–infected but still a different kind of patient than they are used to.
“So far, so good. We’re dealing with it,” he said. “But if one of us got sick, the others would have to step up and do more shifts. We are physicians, internal medicine trained, but since my residency I hadn’t had to deal with these kinds of issues on a daily basis, such as setting up IV lines. I feel like I am back in residency mode.”
Convention Center medicine
Another Boston hospitalist, Amy Baughman, MD, who practices at Massachusetts General Hospital, is using her skills in a new setting, serving as a co-medical director at Boston Hope Medical Center, a 1,000-bed field hospital for patients with COVID-19. Open since April 10 and housed in the Boston Convention and Exhibition Center, it is a four-way collaboration between the Commonwealth of Massachusetts, the City of Boston, Partners HealthCare, and the Boston Health Care for the Homeless Program.
Boston Hope is divided into a post-acute care section for recovering COVID-19 patients and a respite section for undomiciled patients with COVID-19 who need a place to safely quarantine. Built for a maximum of 1,000 beds, it is currently using fewer, with 83 patients on the post-acute side and 73 on the respite side as of May 12. A total of 370 and 315, respectively, had been admitted through May 12.
The team had 5 days to put the field hospital together with the help of the Army National Guard. “During that first week I was installing hand sanitizer dispensers and making [personal protective equipment] signs. Everyone here has had to do things like that,” Dr. Baughman said. “We’ve had to be incredibly creative in our staffing, using doctors from primary care and subspecialties including dermatology, radiology, and orthopedics. We had to fast-track trainings on how to use EPIC and to provide post-acute COVID care. How do you simultaneously build a medical facility and lead teams to provide high quality care?”
Dr. Baughman still works hospitalist shifts half-time at Massachusetts General. Her prior experience providing post-acute care in the VA system was helpful in creating the post-acute level of care at Boston Hope.
“My medical director role involves supervising, staffing, and scheduling. My co-medical director, Dr. Kerri Palamara, and I also supervise the clinical care,” she said. “There are a lot of systems issues, like ordering labs or prescriptions, with couriers going back and forth. And we developed clinical pathways, such as for [deep vein thrombosis] prophylaxis or for COVID retesting to determine when it is safe to end a quarantine. We’re just now rolling out virtual specialist consultations,” she noted.
“It has gone incredibly well. So much of it has been about our ability and willingness to work hard, and take feedback and go forward. We don’t have time to harp on things. We have to be very solution oriented. At the same time, honestly, it’s been fun. Every single day is different,” Dr. Baughman said.
“It’s been an opportunity to use my skills in a totally new setting, and at a level of responsibility I haven’t had before, although that’s probably a common theme with COVID-19. I was put on this team because I am a hospitalist,” she said. “I think hospitalists have been the backbone of the response to COVID in this country. It’s been an opportunity for our specialty to shine. We need to embrace the opportunity.”
Balancing expertise and supervision
Mount Sinai Hospital (MSH) in Manhattan is in the New York epicenter of the COVID-19 crisis and has mobilized large numbers of pulmonary critical care and anesthesia physicians to staff up multiple ICUs for COVID-19 patients, said Andrew Dunn, MD, chief of the division of hospital medicine at Mount Sinai School of Medicine.
“My hospitalist group is covering many step-down units, medical wards, and atypical locations, providing advanced oxygen therapies, [bilevel positive airway pressure], high-flow nasal cannulas, and managing some patients on ventilators,” he said.
MSH has teaching services with house staff and nonteaching services. “We combined them into a unified service with house staff dispersed across all of the teams. We drafted a lot of nonhospitalists from different specialties to be attendings, and that has given us a tiered model, with a hospitalist supervising three or four nonhospitalist-led teams. Although the supervising hospitalists carry no patient caseloads of their own, this is primarily a clinical rather than an administrative role.”
At the peak, there were 40 rounding teams at MSH, each with a typical census of 15 patients or more, which meant that 10 supervisory hospitalists were responsible for 300 to 400 patients. “What we learned first was the need to balance the level of expertise. For example, a team may include a postgraduate year 3 resident and a radiology intern,” Dr. Dunn said. As COVID-19 census has started coming down, supervisory hospitalists are returning to direct care attending roles, and some hospitalists have been shared across the Mount Sinai system’s hospitals.
Dr. Dunn’s advice for hospitalists filling a supervisory role like this in a tiered model: Make sure you talk to your team the night before the first day of a scheduling block and try to address as many of their questions as possible. “If you wait until the morning of the shift to connect with them, anxiety will be high. But after going through a couple of scheduling cycles, we find that things are getting better. I think we’ve paid a lot of attention to the risks of burnout by our physicians. We’re using a model of 4 days on/4 off.”
Another variation on these themes is Joshua Shatzkes, MD, assistant professor of medicine and cardiology at Mount Sinai, who practices outpatient cardiology at MSH and in several off-site offices in Brooklyn. He saw early on that COVID-19 would have a huge effect on his practice, so he volunteered to help out with inpatient care. “I made it known to my chief that I was available, and I was deployed in the first week, after a weekend of cramming webinars and lectures on critical care and pulling out critical concepts that I already knew.”
Dr. Shatzkes said his career path led him into outpatient cardiology 11 years ago, where he was quickly too busy to see his patients when they went into the hospital, even though he missed hospital medicine. Working as a temporary hospitalist with the arrival of COVID-19, he has been invigorated and mobilized by the experience and reminded of why he went to medical school in the first place. “Each day’s shift went quickly but felt long. At the end of the day, I was tired but not exhausted. When I walked out of a patient’s room, they could tell, ‘This is a doctor who cared for me,’ ” he said.
After Dr. Shatzkes volunteered, he got the call from his division chief. “I was officially deployed for a 4-day shift at Mount Sinai and then as a backup.” On his first morning as an inpatient doctor, he was still getting oriented when calls started coming from the nurses. “I had five patients struggling to breathe. Their degree of hypoxia was remarkable. I kept them out of the ICU, at least for that day.”
Since then, he has continued to follow some of those patients in the hospital, along with some from his outpatient practice who were hospitalized, and others referred by colleagues, while remaining available to his outpatients through telemedicine. When this is all over, Dr. Shatzkes said, he would love to find a way to incorporate a hospital practice in his job – depending on the realities of New York traffic.
“Joshua is not a hospitalist, but he went on service and felt so fulfilled and rewarded, he asked me if he could stay on service,” Dr. Dunn said. “I also got an email from the nurse manager on the unit. They want him back.”
In the midst of the COVID-19 pandemic, health systems, hospitals, and hospitalists – especially in hot spots like New York, Detroit, or Boston – have been challenged to stretch limits, redefine roles, and redeploy critical staff in response to rapidly changing needs on the ground.
Many hospitalists are working above and beyond their normal duties, sometimes beyond their training, specialty, or comfort zone and are rising to the occasion in ways they never imagined. These include doing shifts in ICUs, working with ventilator patients, and reporting to other atypical sites of care like postanesthesia care units and post-acute or step-down units.
Valerie Vaughn, MD, MSc, a hospitalist with Michigan Medicine and assistant professor of medicine at the University of Michigan in Ann Arbor, was doing research on how to reduce overuse of antibiotics in hospitals when the COVID-19 crisis hit and dramatically redefined her job. “We were afraid that we might have 3,000 to 5,000 hospitalized COVID patients by now, based on predictive modeling done while the pandemic was still growing exponentially,” she explained. Although Michigan continues to have high COVID-19 infection rates, centered on nearby Detroit, “things are a lot better today than they were 4 weeks ago.”
Dr. Vaughn helped to mobilize a team of 25 hospitalists, along with other health care providers, who volunteered to manage COVID-19 patients in the ICU and other hospital units. She was asked to help develop an all-COVID unit called the Regional Infectious Containment Unit or RICU, which opened March 16. Then, when the RICU became full, it was supplemented by two COVID-19 Moderate Care Units staffed by hospitalists who had “learned the ropes” in the RICU.
Both of these new models were defined in relation to the ICUs at Michigan Medicine – which were doubling in capacity, up to 200 beds at last count – and to the provision of intensive-level and long-term ventilator care for the sickest patients. The moderate care units are for patients who are not on ventilators but still very sick, for example, those receiving massive high-flow oxygen, often with a medical do-not-resuscitate/do-not-intubate order. “We established these units to do everything (medically) short of vents,” Dr. Vaughn said.
“We are having in-depth conversations about goals of care with patients soon after they arrive at the hospital. We know outcomes from ventilators are worse for COVID-positive patients who have comorbidities, and we’re using that information to inform these conversations. We’ve given scripts to clinicians to help guide them in leading these conversations. We can do other things than `use ventilators to manage their symptoms. But these are still difficult conversations,” Dr. Vaughn said.

“We also engaged palliative care early on and asked them to round with us on every [COVID] patient – until demand got too high.” The bottleneck has been the number of ICU beds available, she explained. “If you want your patient to come in and take that bed, make sure you’ve talked to the family about it.”
The COVID-19 team developed guidelines printed on pocket cards addressing critical care issues such as a refresher on how to treat acute respiratory distress syndrome and how to use vasopressors. (See the COVID-19 Continuing Medical Education Portal for web-accessible educational resources developed by Michigan Health).
It’s amazing how quickly patients can become very sick with COVID-19, Dr. Vaughn said. “One of the good things to happen from the beginning with our RICU is that a group of doctors became COVID care experts very quickly. We joined four to five hospitalists and their teams with each intensivist, so one critical care expert is there to do teaching and answer clinicians’ questions. The hospitalists coordinate the COVID care and talk to the families.”
Working on the front lines of this crisis, Dr. Vaughn said, has generated a powerful sense of purpose and camaraderie, creating bonds like in war time. “All of us on our days off feel a twinge of guilt for not being there in the hospital. The sense of gratitude we get from patients and families has been enormous, even when we were telling them bad news. That just brings us to tears.”
One of the hardest things for the doctors practicing above their typical scope of practice is that, when something bad happens, they can’t know whether it was a mistake on their part or not, she noted. “But I’ve never been so proud of our group or to be a hospitalist. No one has complained or pushed back. Everyone has responded by saying: ‘What can I do to help?’ ”
Enough work in hospital medicine
Hospitalists had not been deployed to care for ICU patients at Beth Israel Deaconess Medical Center (BIDMC) in Boston, a major hot spot for COVID-19, said Joseph Ming Wah Li, MD, SFHM, director of the hospital medicine program at BIDMC, when he spoke to The Hospitalist in mid-May. That’s because there were plenty of hospital medicine assignments to keep them busy. Dr. Li leads a service of 120 hospitalists practicing at four hospitals.
“As we speak today, we have 300 patients with COVID, with 70 or 80 of them in our ICU. I’m taking care of 17 patients today, 15 of them COVID-positive, and the other two placed in a former radiology holding suite adapted for COVID-negative patients. Our postanesthesia care unit is now an ICU filled with COVID patients,” he said.
“Half of my day is seeing patients and the other half I’m on Zoom calls. I’m also one of the resource allocation officers for BIDMC,” Dr. Li said. He helped to create a standard of care for the hospital, addressing what to do if there weren’t enough ICU beds or ventilators. “We’ve never actualized it and probably won’t, but it was important to go through this exercise, with a lot of discussion up front.”
Haki Laho, MD, an orthopedic hospitalist at New England Baptist Hospital (NEBH), also in Boston, has been redeployed to care for a different population of patients as his system tries to bunch patients. “All of a sudden – within hours and days – at the beginning of the pandemic and based on the recommendations, our whole system decided to stop all elective procedures and devote the resources to COVID,” he said.
NEBH is Beth Israel Lahey Health’s 141-bed orthopedic and surgical hospital, and the system has tried to keep the specialty facility COVID-19–free as much as possible, with the COVID-19 patients grouped together at BIDMC. Dr. Laho’s orthopedic hospitalist group, just five doctors, has been managing the influx of medical patients with multiple comorbidities – not COVID-19–infected but still a different kind of patient than they are used to.
“So far, so good. We’re dealing with it,” he said. “But if one of us got sick, the others would have to step up and do more shifts. We are physicians, internal medicine trained, but since my residency I hadn’t had to deal with these kinds of issues on a daily basis, such as setting up IV lines. I feel like I am back in residency mode.”
Convention Center medicine
Another Boston hospitalist, Amy Baughman, MD, who practices at Massachusetts General Hospital, is using her skills in a new setting, serving as a co-medical director at Boston Hope Medical Center, a 1,000-bed field hospital for patients with COVID-19. Open since April 10 and housed in the Boston Convention and Exhibition Center, it is a four-way collaboration between the Commonwealth of Massachusetts, the City of Boston, Partners HealthCare, and the Boston Health Care for the Homeless Program.
Boston Hope is divided into a post-acute care section for recovering COVID-19 patients and a respite section for undomiciled patients with COVID-19 who need a place to safely quarantine. Built for a maximum of 1,000 beds, it is currently using fewer, with 83 patients on the post-acute side and 73 on the respite side as of May 12. A total of 370 and 315, respectively, had been admitted through May 12.
The team had 5 days to put the field hospital together with the help of the Army National Guard. “During that first week I was installing hand sanitizer dispensers and making [personal protective equipment] signs. Everyone here has had to do things like that,” Dr. Baughman said. “We’ve had to be incredibly creative in our staffing, using doctors from primary care and subspecialties including dermatology, radiology, and orthopedics. We had to fast-track trainings on how to use EPIC and to provide post-acute COVID care. How do you simultaneously build a medical facility and lead teams to provide high quality care?”
Dr. Baughman still works hospitalist shifts half-time at Massachusetts General. Her prior experience providing post-acute care in the VA system was helpful in creating the post-acute level of care at Boston Hope.
“My medical director role involves supervising, staffing, and scheduling. My co-medical director, Dr. Kerri Palamara, and I also supervise the clinical care,” she said. “There are a lot of systems issues, like ordering labs or prescriptions, with couriers going back and forth. And we developed clinical pathways, such as for [deep vein thrombosis] prophylaxis or for COVID retesting to determine when it is safe to end a quarantine. We’re just now rolling out virtual specialist consultations,” she noted.
“It has gone incredibly well. So much of it has been about our ability and willingness to work hard, and take feedback and go forward. We don’t have time to harp on things. We have to be very solution oriented. At the same time, honestly, it’s been fun. Every single day is different,” Dr. Baughman said.
“It’s been an opportunity to use my skills in a totally new setting, and at a level of responsibility I haven’t had before, although that’s probably a common theme with COVID-19. I was put on this team because I am a hospitalist,” she said. “I think hospitalists have been the backbone of the response to COVID in this country. It’s been an opportunity for our specialty to shine. We need to embrace the opportunity.”
Balancing expertise and supervision
Mount Sinai Hospital (MSH) in Manhattan is in the New York epicenter of the COVID-19 crisis and has mobilized large numbers of pulmonary critical care and anesthesia physicians to staff up multiple ICUs for COVID-19 patients, said Andrew Dunn, MD, chief of the division of hospital medicine at Mount Sinai School of Medicine.
“My hospitalist group is covering many step-down units, medical wards, and atypical locations, providing advanced oxygen therapies, [bilevel positive airway pressure], high-flow nasal cannulas, and managing some patients on ventilators,” he said.
MSH has teaching services with house staff and nonteaching services. “We combined them into a unified service with house staff dispersed across all of the teams. We drafted a lot of nonhospitalists from different specialties to be attendings, and that has given us a tiered model, with a hospitalist supervising three or four nonhospitalist-led teams. Although the supervising hospitalists carry no patient caseloads of their own, this is primarily a clinical rather than an administrative role.”
At the peak, there were 40 rounding teams at MSH, each with a typical census of 15 patients or more, which meant that 10 supervisory hospitalists were responsible for 300 to 400 patients. “What we learned first was the need to balance the level of expertise. For example, a team may include a postgraduate year 3 resident and a radiology intern,” Dr. Dunn said. As COVID-19 census has started coming down, supervisory hospitalists are returning to direct care attending roles, and some hospitalists have been shared across the Mount Sinai system’s hospitals.
Dr. Dunn’s advice for hospitalists filling a supervisory role like this in a tiered model: Make sure you talk to your team the night before the first day of a scheduling block and try to address as many of their questions as possible. “If you wait until the morning of the shift to connect with them, anxiety will be high. But after going through a couple of scheduling cycles, we find that things are getting better. I think we’ve paid a lot of attention to the risks of burnout by our physicians. We’re using a model of 4 days on/4 off.”
Another variation on these themes is Joshua Shatzkes, MD, assistant professor of medicine and cardiology at Mount Sinai, who practices outpatient cardiology at MSH and in several off-site offices in Brooklyn. He saw early on that COVID-19 would have a huge effect on his practice, so he volunteered to help out with inpatient care. “I made it known to my chief that I was available, and I was deployed in the first week, after a weekend of cramming webinars and lectures on critical care and pulling out critical concepts that I already knew.”
Dr. Shatzkes said his career path led him into outpatient cardiology 11 years ago, where he was quickly too busy to see his patients when they went into the hospital, even though he missed hospital medicine. Working as a temporary hospitalist with the arrival of COVID-19, he has been invigorated and mobilized by the experience and reminded of why he went to medical school in the first place. “Each day’s shift went quickly but felt long. At the end of the day, I was tired but not exhausted. When I walked out of a patient’s room, they could tell, ‘This is a doctor who cared for me,’ ” he said.
After Dr. Shatzkes volunteered, he got the call from his division chief. “I was officially deployed for a 4-day shift at Mount Sinai and then as a backup.” On his first morning as an inpatient doctor, he was still getting oriented when calls started coming from the nurses. “I had five patients struggling to breathe. Their degree of hypoxia was remarkable. I kept them out of the ICU, at least for that day.”
Since then, he has continued to follow some of those patients in the hospital, along with some from his outpatient practice who were hospitalized, and others referred by colleagues, while remaining available to his outpatients through telemedicine. When this is all over, Dr. Shatzkes said, he would love to find a way to incorporate a hospital practice in his job – depending on the realities of New York traffic.
“Joshua is not a hospitalist, but he went on service and felt so fulfilled and rewarded, he asked me if he could stay on service,” Dr. Dunn said. “I also got an email from the nurse manager on the unit. They want him back.”
Clinicians urged to use CURE ID to report COVID-19 cases
in conjunction with ongoing clinical trial efforts.
“By utilizing the CURE ID platform now for COVID-19 case collection – in conjunction with data gathered from other registries, EHR systems, and clinical trials – data collected during an outbreak can be improved and coordinated,” Heather A. Stone, MPH, said during a June 9 webinar sponsored by the Food and Drug Administration. “This may allow us to find possible treatments to help ease this pandemic, and prepare us better to fight the next one.”
During the hour-long webinar, Ms. Stone, a health science policy analyst in the office of medical policy at the FDA’s Center for Drug Evaluation and Research, demonstrated CURE ID, an Internet-based data repository first developed in 2013 as a collaboration between the FDA and the National Center for Advancing Translational Sciences, a part of the National Institutes of Health (NCATS/NIH). It provides licensed clinicians worldwide with an opportunity to report novel uses of existing drugs for patients with difficult-to-treat infectious diseases, including COVID-19, through a website, a smartphone, or other mobile device. The app can be downloaded for free at http://cure.ncats.io. It can also be downloaded from the Apple app store or the Google Play store by searching “CURE ID.”
According to Ms. Stone, the platform’s three main goals are to enhance the understanding of new uses of approved medical products, to facilitate clinical trials and drug development, and to serve as a resource for physicians to share information where no FDA-approved product (which has been proven to be safe and effective) exists for the new use. CURE ID enables users to report their own cases as well as read cases of neglected infectious diseases with no sufficient approved therapies from other clinicians around the world. “It also enables clinicians to engage directly with communities of disease experts around the world, breaking down geographic and specialty silos,” Ms. Stone said. “It also enables them to access information on approved therapies for each disease and as well on active clinical trials.”
To date, CURE-ID contains information on 325 infectious diseases, including 1,580 case reports and 18,907 clinical trials. Initial pilot priority diseases include COVID-19, mycetoma, atypical mycobacteria, drug-resistant gonorrhea, rare and resistant fungal infections, as well as multidrug resistant gram-negative bacteria.
As of June 9, COVID-19-related data on the platform includes 151 case reports that have been extracted from the published literature or entered by clinician users, 80 discussion posts, and links to 694 clinical trials, 303 journal articles, 212 news articles, and 34 events. A total of 65 repurposed drugs have been identified as potential treatments for the virus, including 15 drugs with 10 or more cases.
“This facilitates clinicians reporting their real-world experiences treating COVID-19 patients, when patients are unable to be enrolled in a clinical trial,” Ms. Stone said. “It includes an updated case report form tailored to COVID-19 and data fields that have been harmonized with other real-world data and clinical trial platforms.” She pointed out that voluntary submission of cases to CURE ID is not a substitute for filing information with regulatory and public health authorities, where required. The platform also enables data to be entered and adverse events to be automatically shared with the FDA’s MedWatch Adverse Reporting System.
Ms. Stone concluded the webinar by announcing the formation of a new private-public partnership between the Critical Path Institute and the FDA and NCATS/NIH known as the CURE Drug Repurposing Collaboratory. The effort will begin with a pilot project focused on furthering drug development for COVID-19 through use of the CURE ID platform. “The Collaboratory will demonstrate how data shared from clinicians in real-time can be used to inform ongoing and future clinical trials, and potentially drug labeling,” Ms. Stone said. She reported having no financial disclosures.
in conjunction with ongoing clinical trial efforts.
“By utilizing the CURE ID platform now for COVID-19 case collection – in conjunction with data gathered from other registries, EHR systems, and clinical trials – data collected during an outbreak can be improved and coordinated,” Heather A. Stone, MPH, said during a June 9 webinar sponsored by the Food and Drug Administration. “This may allow us to find possible treatments to help ease this pandemic, and prepare us better to fight the next one.”
During the hour-long webinar, Ms. Stone, a health science policy analyst in the office of medical policy at the FDA’s Center for Drug Evaluation and Research, demonstrated CURE ID, an Internet-based data repository first developed in 2013 as a collaboration between the FDA and the National Center for Advancing Translational Sciences, a part of the National Institutes of Health (NCATS/NIH). It provides licensed clinicians worldwide with an opportunity to report novel uses of existing drugs for patients with difficult-to-treat infectious diseases, including COVID-19, through a website, a smartphone, or other mobile device. The app can be downloaded for free at http://cure.ncats.io. It can also be downloaded from the Apple app store or the Google Play store by searching “CURE ID.”
According to Ms. Stone, the platform’s three main goals are to enhance the understanding of new uses of approved medical products, to facilitate clinical trials and drug development, and to serve as a resource for physicians to share information where no FDA-approved product (which has been proven to be safe and effective) exists for the new use. CURE ID enables users to report their own cases as well as read cases of neglected infectious diseases with no sufficient approved therapies from other clinicians around the world. “It also enables clinicians to engage directly with communities of disease experts around the world, breaking down geographic and specialty silos,” Ms. Stone said. “It also enables them to access information on approved therapies for each disease and as well on active clinical trials.”
To date, CURE-ID contains information on 325 infectious diseases, including 1,580 case reports and 18,907 clinical trials. Initial pilot priority diseases include COVID-19, mycetoma, atypical mycobacteria, drug-resistant gonorrhea, rare and resistant fungal infections, as well as multidrug resistant gram-negative bacteria.
As of June 9, COVID-19-related data on the platform includes 151 case reports that have been extracted from the published literature or entered by clinician users, 80 discussion posts, and links to 694 clinical trials, 303 journal articles, 212 news articles, and 34 events. A total of 65 repurposed drugs have been identified as potential treatments for the virus, including 15 drugs with 10 or more cases.
“This facilitates clinicians reporting their real-world experiences treating COVID-19 patients, when patients are unable to be enrolled in a clinical trial,” Ms. Stone said. “It includes an updated case report form tailored to COVID-19 and data fields that have been harmonized with other real-world data and clinical trial platforms.” She pointed out that voluntary submission of cases to CURE ID is not a substitute for filing information with regulatory and public health authorities, where required. The platform also enables data to be entered and adverse events to be automatically shared with the FDA’s MedWatch Adverse Reporting System.
Ms. Stone concluded the webinar by announcing the formation of a new private-public partnership between the Critical Path Institute and the FDA and NCATS/NIH known as the CURE Drug Repurposing Collaboratory. The effort will begin with a pilot project focused on furthering drug development for COVID-19 through use of the CURE ID platform. “The Collaboratory will demonstrate how data shared from clinicians in real-time can be used to inform ongoing and future clinical trials, and potentially drug labeling,” Ms. Stone said. She reported having no financial disclosures.
in conjunction with ongoing clinical trial efforts.
“By utilizing the CURE ID platform now for COVID-19 case collection – in conjunction with data gathered from other registries, EHR systems, and clinical trials – data collected during an outbreak can be improved and coordinated,” Heather A. Stone, MPH, said during a June 9 webinar sponsored by the Food and Drug Administration. “This may allow us to find possible treatments to help ease this pandemic, and prepare us better to fight the next one.”
During the hour-long webinar, Ms. Stone, a health science policy analyst in the office of medical policy at the FDA’s Center for Drug Evaluation and Research, demonstrated CURE ID, an Internet-based data repository first developed in 2013 as a collaboration between the FDA and the National Center for Advancing Translational Sciences, a part of the National Institutes of Health (NCATS/NIH). It provides licensed clinicians worldwide with an opportunity to report novel uses of existing drugs for patients with difficult-to-treat infectious diseases, including COVID-19, through a website, a smartphone, or other mobile device. The app can be downloaded for free at http://cure.ncats.io. It can also be downloaded from the Apple app store or the Google Play store by searching “CURE ID.”
According to Ms. Stone, the platform’s three main goals are to enhance the understanding of new uses of approved medical products, to facilitate clinical trials and drug development, and to serve as a resource for physicians to share information where no FDA-approved product (which has been proven to be safe and effective) exists for the new use. CURE ID enables users to report their own cases as well as read cases of neglected infectious diseases with no sufficient approved therapies from other clinicians around the world. “It also enables clinicians to engage directly with communities of disease experts around the world, breaking down geographic and specialty silos,” Ms. Stone said. “It also enables them to access information on approved therapies for each disease and as well on active clinical trials.”
To date, CURE-ID contains information on 325 infectious diseases, including 1,580 case reports and 18,907 clinical trials. Initial pilot priority diseases include COVID-19, mycetoma, atypical mycobacteria, drug-resistant gonorrhea, rare and resistant fungal infections, as well as multidrug resistant gram-negative bacteria.
As of June 9, COVID-19-related data on the platform includes 151 case reports that have been extracted from the published literature or entered by clinician users, 80 discussion posts, and links to 694 clinical trials, 303 journal articles, 212 news articles, and 34 events. A total of 65 repurposed drugs have been identified as potential treatments for the virus, including 15 drugs with 10 or more cases.
“This facilitates clinicians reporting their real-world experiences treating COVID-19 patients, when patients are unable to be enrolled in a clinical trial,” Ms. Stone said. “It includes an updated case report form tailored to COVID-19 and data fields that have been harmonized with other real-world data and clinical trial platforms.” She pointed out that voluntary submission of cases to CURE ID is not a substitute for filing information with regulatory and public health authorities, where required. The platform also enables data to be entered and adverse events to be automatically shared with the FDA’s MedWatch Adverse Reporting System.
Ms. Stone concluded the webinar by announcing the formation of a new private-public partnership between the Critical Path Institute and the FDA and NCATS/NIH known as the CURE Drug Repurposing Collaboratory. The effort will begin with a pilot project focused on furthering drug development for COVID-19 through use of the CURE ID platform. “The Collaboratory will demonstrate how data shared from clinicians in real-time can be used to inform ongoing and future clinical trials, and potentially drug labeling,” Ms. Stone said. She reported having no financial disclosures.
EULAR’s COVID-19 recommendations offer no surprises
As might be expected, the “EULAR [European League Against Rheumatism] provisional recommendations for the management of rheumatic and musculoskeletal diseases [RMDs] in the context of SARS-CoV-2” concur with much of the guidance already released on how best to manage patients during the current pandemic.
Highlights of the five overarching principles are that, contrary to earlier expectations, “there is no indication that patients with RMDs have an additional, or have a higher, risk of contracting the virus, or that they fare a worse course” than the general population, said the task force convener Robert Landewé, MD, PhD, professor of rheumatology at the University of Amsterdam.
“The second pertinent highlight is that, when it comes to managerial discussions, whether or not to stop or to start treatment for RMDs, rheumatologists should definitely be involved,” Dr. Landewé said during a live session at the annual European Congress of Rheumatology, held online this year due to COVID-19. “In practice, something that happens very often is that immunosuppressive drugs are stopped by medical specialists involved in the care of COVID but without any expertise in treating patients with rheumatic diseases. We should try to avoid that situation.”
The third highlight, something many rheumatologists may already be well aware of, is that rheumatology drugs are being used to treat COVID-19 patients without RMDs and a shortage of disease-modifying antirheumatic drugs (DMARDs) agents is a real possibility. As such, the fifth overarching highlight states that the availability of both synthetic and biologic DMARDs is “a delicate societal responsibility” and that “the off-label use of DMARDs in COVID-19 outside the context of clinical trials should be discouraged.”
The EULAR recommendation are now published online in Annals of the Rheumatic Diseases and they are “what you could call an unprecedented set of recommendations,” Dr. Landewé said. “We have never done this before,” he added, referring to the speed and way in which they had to be put together, remotely, and with little scientific evidence currently available. “Three months ago we hadn’t even heard about the virus.”
From the first patient being identified in the Hubei province of China in November 2019, to the first U.S. patient in the state of Washington on Jan. 20, 2020, and to the first European patient identified a little over 10 days later, the COVID-19 pandemic has taken the world by storm. It was only declared a pandemic on March 11, 2020, however, and Dr. Landewé noted that the response to the pandemic had been very variable – some countries locking down their borders early, while others took their time to make an appropriate response, if at all.
The rheumatology community was particularly concerned, Dr. Landewé said, because people with autoimmune diseases who were taking immunosuppressant drugs might be at higher risk for becoming infected with SARS-CoV-2, and may be at higher risk than others for a worse disease course. Thankfully, that seems not to be the case according to data that are emerging from new registries that have been set up, including EULAR’s own COVID-19 registry.
There are 13 recommendations that cover 4 themes: general measures and prevention of SARS-CoV-2 infection; the management of RMD patients during the pandemic; the management of RMD patients who have COVID-19; and the prevention of other pulmonary infections in RMD patients.
Highlighting the first three general recommendations, Dr. Landewé said: “Follow the regular guidelines in your country; if a patient with RMD does not have symptoms of COVID-19, simply continue RMD treatments,” albeit with a couple of exceptions.
The next four recommendation highlights are to avoid visits to the hospital or to the office; use remote monitoring via the telephone, for example; and if visits cannot be avoided, then take appropriate precautions. Finally, if you suspect a patient has COVID-19, do a test.
If patients test positive, then the next four recommendations cover what to do, such as continuing use of RMD treatments, but in the case of glucocorticoids this should be the lowest possible dose necessary. There is no consensus on what to do in cases of mild symptoms; the recommendation is to “decide on a case-by-case basis,” said Dr. Landewé. If a patient’s symptoms worsen, then “seek expert advice immediately and follow local treatment recommendations. The rheumatologist is not the expert to treat COVID-19,” he added. That responsibility lies with the pulmonologist, infectious disease specialist, or maybe the intensive care specialist, depending on local situations.
On the whole, the EULAR recommendations are pretty similar to those already released by the American College of Rheumatology, said Ted Mikuls, MD, of the University of Nebraska Medical Center, Omaha. The ACR recommendations are “slightly more prescriptive”, he suggested, with 25 final guidance statements. For example, general statements focused not only on the use of glucocorticoids, but also other medicines, such as antihypertensives.
“There’s really not a [lot of], I would say, major differences in the two efforts and that’s ... somewhat reassuring that we’re approaching the unknown from very different parts of the world, and driving in a very similar place,” commented Dr. Mikuls, who is a member of the ACR COVID-19 recommendations task force.
“I think one of the very important similarities that I would highlight is that, in the absence of known exposure, in the absence of COVID-19 infection, our panel felt very strongly about the importance of continuing rheumatic disease treatments,” Dr. Mikuls observed. The ACR guidelines also touch upon societal perspectives, including “some statements that were made very specific to lupus, and the use of antimalarials, given supply chain issues that we have encountered.”
Dr. Mikuls also said that the American recommendations emphasized that “you really have to manage active inflammatory rheumatic disease. Even in the context of the COVID-19 pandemic, given what we saw as the potential risk of unchecked inflammation and unchecked rheumatic disease.”
One notable difference, however, is that the European recommendations advise on immunizations and pneumonia prophylaxis, saying that all patients without COVID-19 symptoms should make sure they are up to date with any recommended vaccinations, “with a particular focus on pneumococcal and influenza vaccinations,” Dr. Landewé said.
Another difference is that the ACR recommendations are a living document and could potentially be updated monthly if the evidence arrives to allow that. In that sense, the American guidance is more agile, with EULAR expecting to update its recommendations every 3 months.
“The current evidence is extremely sparse and fragmented,” Dr. Landewé said. “We, as a task force are essentially flying blindly. We also have to cover many jurisdictions within Europe, with many conflicting opinions. So the last word to say is that updates are truly necessary, but we have to wait a while.”
SOURCE: Landewé RB et al. Ann Rheum Dis. 2020 Jun 5. doi: 10.1136/annrheumdis-2020-217877.
As might be expected, the “EULAR [European League Against Rheumatism] provisional recommendations for the management of rheumatic and musculoskeletal diseases [RMDs] in the context of SARS-CoV-2” concur with much of the guidance already released on how best to manage patients during the current pandemic.
Highlights of the five overarching principles are that, contrary to earlier expectations, “there is no indication that patients with RMDs have an additional, or have a higher, risk of contracting the virus, or that they fare a worse course” than the general population, said the task force convener Robert Landewé, MD, PhD, professor of rheumatology at the University of Amsterdam.
“The second pertinent highlight is that, when it comes to managerial discussions, whether or not to stop or to start treatment for RMDs, rheumatologists should definitely be involved,” Dr. Landewé said during a live session at the annual European Congress of Rheumatology, held online this year due to COVID-19. “In practice, something that happens very often is that immunosuppressive drugs are stopped by medical specialists involved in the care of COVID but without any expertise in treating patients with rheumatic diseases. We should try to avoid that situation.”
The third highlight, something many rheumatologists may already be well aware of, is that rheumatology drugs are being used to treat COVID-19 patients without RMDs and a shortage of disease-modifying antirheumatic drugs (DMARDs) agents is a real possibility. As such, the fifth overarching highlight states that the availability of both synthetic and biologic DMARDs is “a delicate societal responsibility” and that “the off-label use of DMARDs in COVID-19 outside the context of clinical trials should be discouraged.”
The EULAR recommendation are now published online in Annals of the Rheumatic Diseases and they are “what you could call an unprecedented set of recommendations,” Dr. Landewé said. “We have never done this before,” he added, referring to the speed and way in which they had to be put together, remotely, and with little scientific evidence currently available. “Three months ago we hadn’t even heard about the virus.”
From the first patient being identified in the Hubei province of China in November 2019, to the first U.S. patient in the state of Washington on Jan. 20, 2020, and to the first European patient identified a little over 10 days later, the COVID-19 pandemic has taken the world by storm. It was only declared a pandemic on March 11, 2020, however, and Dr. Landewé noted that the response to the pandemic had been very variable – some countries locking down their borders early, while others took their time to make an appropriate response, if at all.
The rheumatology community was particularly concerned, Dr. Landewé said, because people with autoimmune diseases who were taking immunosuppressant drugs might be at higher risk for becoming infected with SARS-CoV-2, and may be at higher risk than others for a worse disease course. Thankfully, that seems not to be the case according to data that are emerging from new registries that have been set up, including EULAR’s own COVID-19 registry.
There are 13 recommendations that cover 4 themes: general measures and prevention of SARS-CoV-2 infection; the management of RMD patients during the pandemic; the management of RMD patients who have COVID-19; and the prevention of other pulmonary infections in RMD patients.
Highlighting the first three general recommendations, Dr. Landewé said: “Follow the regular guidelines in your country; if a patient with RMD does not have symptoms of COVID-19, simply continue RMD treatments,” albeit with a couple of exceptions.
The next four recommendation highlights are to avoid visits to the hospital or to the office; use remote monitoring via the telephone, for example; and if visits cannot be avoided, then take appropriate precautions. Finally, if you suspect a patient has COVID-19, do a test.
If patients test positive, then the next four recommendations cover what to do, such as continuing use of RMD treatments, but in the case of glucocorticoids this should be the lowest possible dose necessary. There is no consensus on what to do in cases of mild symptoms; the recommendation is to “decide on a case-by-case basis,” said Dr. Landewé. If a patient’s symptoms worsen, then “seek expert advice immediately and follow local treatment recommendations. The rheumatologist is not the expert to treat COVID-19,” he added. That responsibility lies with the pulmonologist, infectious disease specialist, or maybe the intensive care specialist, depending on local situations.
On the whole, the EULAR recommendations are pretty similar to those already released by the American College of Rheumatology, said Ted Mikuls, MD, of the University of Nebraska Medical Center, Omaha. The ACR recommendations are “slightly more prescriptive”, he suggested, with 25 final guidance statements. For example, general statements focused not only on the use of glucocorticoids, but also other medicines, such as antihypertensives.
“There’s really not a [lot of], I would say, major differences in the two efforts and that’s ... somewhat reassuring that we’re approaching the unknown from very different parts of the world, and driving in a very similar place,” commented Dr. Mikuls, who is a member of the ACR COVID-19 recommendations task force.
“I think one of the very important similarities that I would highlight is that, in the absence of known exposure, in the absence of COVID-19 infection, our panel felt very strongly about the importance of continuing rheumatic disease treatments,” Dr. Mikuls observed. The ACR guidelines also touch upon societal perspectives, including “some statements that were made very specific to lupus, and the use of antimalarials, given supply chain issues that we have encountered.”
Dr. Mikuls also said that the American recommendations emphasized that “you really have to manage active inflammatory rheumatic disease. Even in the context of the COVID-19 pandemic, given what we saw as the potential risk of unchecked inflammation and unchecked rheumatic disease.”
One notable difference, however, is that the European recommendations advise on immunizations and pneumonia prophylaxis, saying that all patients without COVID-19 symptoms should make sure they are up to date with any recommended vaccinations, “with a particular focus on pneumococcal and influenza vaccinations,” Dr. Landewé said.
Another difference is that the ACR recommendations are a living document and could potentially be updated monthly if the evidence arrives to allow that. In that sense, the American guidance is more agile, with EULAR expecting to update its recommendations every 3 months.
“The current evidence is extremely sparse and fragmented,” Dr. Landewé said. “We, as a task force are essentially flying blindly. We also have to cover many jurisdictions within Europe, with many conflicting opinions. So the last word to say is that updates are truly necessary, but we have to wait a while.”
SOURCE: Landewé RB et al. Ann Rheum Dis. 2020 Jun 5. doi: 10.1136/annrheumdis-2020-217877.
As might be expected, the “EULAR [European League Against Rheumatism] provisional recommendations for the management of rheumatic and musculoskeletal diseases [RMDs] in the context of SARS-CoV-2” concur with much of the guidance already released on how best to manage patients during the current pandemic.
Highlights of the five overarching principles are that, contrary to earlier expectations, “there is no indication that patients with RMDs have an additional, or have a higher, risk of contracting the virus, or that they fare a worse course” than the general population, said the task force convener Robert Landewé, MD, PhD, professor of rheumatology at the University of Amsterdam.
“The second pertinent highlight is that, when it comes to managerial discussions, whether or not to stop or to start treatment for RMDs, rheumatologists should definitely be involved,” Dr. Landewé said during a live session at the annual European Congress of Rheumatology, held online this year due to COVID-19. “In practice, something that happens very often is that immunosuppressive drugs are stopped by medical specialists involved in the care of COVID but without any expertise in treating patients with rheumatic diseases. We should try to avoid that situation.”
The third highlight, something many rheumatologists may already be well aware of, is that rheumatology drugs are being used to treat COVID-19 patients without RMDs and a shortage of disease-modifying antirheumatic drugs (DMARDs) agents is a real possibility. As such, the fifth overarching highlight states that the availability of both synthetic and biologic DMARDs is “a delicate societal responsibility” and that “the off-label use of DMARDs in COVID-19 outside the context of clinical trials should be discouraged.”
The EULAR recommendation are now published online in Annals of the Rheumatic Diseases and they are “what you could call an unprecedented set of recommendations,” Dr. Landewé said. “We have never done this before,” he added, referring to the speed and way in which they had to be put together, remotely, and with little scientific evidence currently available. “Three months ago we hadn’t even heard about the virus.”
From the first patient being identified in the Hubei province of China in November 2019, to the first U.S. patient in the state of Washington on Jan. 20, 2020, and to the first European patient identified a little over 10 days later, the COVID-19 pandemic has taken the world by storm. It was only declared a pandemic on March 11, 2020, however, and Dr. Landewé noted that the response to the pandemic had been very variable – some countries locking down their borders early, while others took their time to make an appropriate response, if at all.
The rheumatology community was particularly concerned, Dr. Landewé said, because people with autoimmune diseases who were taking immunosuppressant drugs might be at higher risk for becoming infected with SARS-CoV-2, and may be at higher risk than others for a worse disease course. Thankfully, that seems not to be the case according to data that are emerging from new registries that have been set up, including EULAR’s own COVID-19 registry.
There are 13 recommendations that cover 4 themes: general measures and prevention of SARS-CoV-2 infection; the management of RMD patients during the pandemic; the management of RMD patients who have COVID-19; and the prevention of other pulmonary infections in RMD patients.
Highlighting the first three general recommendations, Dr. Landewé said: “Follow the regular guidelines in your country; if a patient with RMD does not have symptoms of COVID-19, simply continue RMD treatments,” albeit with a couple of exceptions.
The next four recommendation highlights are to avoid visits to the hospital or to the office; use remote monitoring via the telephone, for example; and if visits cannot be avoided, then take appropriate precautions. Finally, if you suspect a patient has COVID-19, do a test.
If patients test positive, then the next four recommendations cover what to do, such as continuing use of RMD treatments, but in the case of glucocorticoids this should be the lowest possible dose necessary. There is no consensus on what to do in cases of mild symptoms; the recommendation is to “decide on a case-by-case basis,” said Dr. Landewé. If a patient’s symptoms worsen, then “seek expert advice immediately and follow local treatment recommendations. The rheumatologist is not the expert to treat COVID-19,” he added. That responsibility lies with the pulmonologist, infectious disease specialist, or maybe the intensive care specialist, depending on local situations.
On the whole, the EULAR recommendations are pretty similar to those already released by the American College of Rheumatology, said Ted Mikuls, MD, of the University of Nebraska Medical Center, Omaha. The ACR recommendations are “slightly more prescriptive”, he suggested, with 25 final guidance statements. For example, general statements focused not only on the use of glucocorticoids, but also other medicines, such as antihypertensives.
“There’s really not a [lot of], I would say, major differences in the two efforts and that’s ... somewhat reassuring that we’re approaching the unknown from very different parts of the world, and driving in a very similar place,” commented Dr. Mikuls, who is a member of the ACR COVID-19 recommendations task force.
“I think one of the very important similarities that I would highlight is that, in the absence of known exposure, in the absence of COVID-19 infection, our panel felt very strongly about the importance of continuing rheumatic disease treatments,” Dr. Mikuls observed. The ACR guidelines also touch upon societal perspectives, including “some statements that were made very specific to lupus, and the use of antimalarials, given supply chain issues that we have encountered.”
Dr. Mikuls also said that the American recommendations emphasized that “you really have to manage active inflammatory rheumatic disease. Even in the context of the COVID-19 pandemic, given what we saw as the potential risk of unchecked inflammation and unchecked rheumatic disease.”
One notable difference, however, is that the European recommendations advise on immunizations and pneumonia prophylaxis, saying that all patients without COVID-19 symptoms should make sure they are up to date with any recommended vaccinations, “with a particular focus on pneumococcal and influenza vaccinations,” Dr. Landewé said.
Another difference is that the ACR recommendations are a living document and could potentially be updated monthly if the evidence arrives to allow that. In that sense, the American guidance is more agile, with EULAR expecting to update its recommendations every 3 months.
“The current evidence is extremely sparse and fragmented,” Dr. Landewé said. “We, as a task force are essentially flying blindly. We also have to cover many jurisdictions within Europe, with many conflicting opinions. So the last word to say is that updates are truly necessary, but we have to wait a while.”
SOURCE: Landewé RB et al. Ann Rheum Dis. 2020 Jun 5. doi: 10.1136/annrheumdis-2020-217877.
FROM THE EULAR 2020 E-CONGRESS
Baloxavir effective, well tolerated for influenza treatment in children
according to Jeffrey Baker, MD, of Clinical Research Prime, Idaho Falls, and associates.
In the double-blind, randomized, controlled MiniSTONE-2 phase 3 trial, the investigators randomized 112 children aged 1-12 years to baloxavir and 57 to oseltamivir. The predominant influenza A subtype was H3N2 for both groups, followed by H1N1pdm09. Demographics and baseline characteristics were similar between treatment groups, the investigators wrote in the Pediatric Infectious Disease Journal.
The time to alleviation of signs and symptoms was a median 138 hours for those receiving baloxavir and 150 hours for those receiving oseltamivir, a nonsignificant difference. Duration of fever and of all symptoms also were similar between groups, as was the time to return to normal health and activity.
A total of 122 adverse events were reported in 84 children, with 95% of adverse events being resolved or resolving by the end of the study. The incidence of adverse events was 46% in those receiving baloxavir and 53% in those receiving oseltamivir, a nonsignificant difference, with the most common adverse event in both groups being gastrointestinal disorders. No deaths, serious adverse events, or hospitalizations were reported, but two patients receiving oseltamivir discontinued because of adverse events.
The study was funded by F. Hoffmann-La Roche. Dr. Baker and a coauthor received funding through their institutions for the conduct of the study; several coauthors reported being employed by and owning stocks in F. Hoffmann–La Roche. One coauthor reported receiving consultancy fees from F. Hoffmann–La Roche and grants from Shionogi.
SOURCE: Baker J et al. Pediatr Infect Dis J. 2020 Jun 5. doi: 10.1097/INF.0000000000002747.
according to Jeffrey Baker, MD, of Clinical Research Prime, Idaho Falls, and associates.
In the double-blind, randomized, controlled MiniSTONE-2 phase 3 trial, the investigators randomized 112 children aged 1-12 years to baloxavir and 57 to oseltamivir. The predominant influenza A subtype was H3N2 for both groups, followed by H1N1pdm09. Demographics and baseline characteristics were similar between treatment groups, the investigators wrote in the Pediatric Infectious Disease Journal.
The time to alleviation of signs and symptoms was a median 138 hours for those receiving baloxavir and 150 hours for those receiving oseltamivir, a nonsignificant difference. Duration of fever and of all symptoms also were similar between groups, as was the time to return to normal health and activity.
A total of 122 adverse events were reported in 84 children, with 95% of adverse events being resolved or resolving by the end of the study. The incidence of adverse events was 46% in those receiving baloxavir and 53% in those receiving oseltamivir, a nonsignificant difference, with the most common adverse event in both groups being gastrointestinal disorders. No deaths, serious adverse events, or hospitalizations were reported, but two patients receiving oseltamivir discontinued because of adverse events.
The study was funded by F. Hoffmann-La Roche. Dr. Baker and a coauthor received funding through their institutions for the conduct of the study; several coauthors reported being employed by and owning stocks in F. Hoffmann–La Roche. One coauthor reported receiving consultancy fees from F. Hoffmann–La Roche and grants from Shionogi.
SOURCE: Baker J et al. Pediatr Infect Dis J. 2020 Jun 5. doi: 10.1097/INF.0000000000002747.
according to Jeffrey Baker, MD, of Clinical Research Prime, Idaho Falls, and associates.
In the double-blind, randomized, controlled MiniSTONE-2 phase 3 trial, the investigators randomized 112 children aged 1-12 years to baloxavir and 57 to oseltamivir. The predominant influenza A subtype was H3N2 for both groups, followed by H1N1pdm09. Demographics and baseline characteristics were similar between treatment groups, the investigators wrote in the Pediatric Infectious Disease Journal.
The time to alleviation of signs and symptoms was a median 138 hours for those receiving baloxavir and 150 hours for those receiving oseltamivir, a nonsignificant difference. Duration of fever and of all symptoms also were similar between groups, as was the time to return to normal health and activity.
A total of 122 adverse events were reported in 84 children, with 95% of adverse events being resolved or resolving by the end of the study. The incidence of adverse events was 46% in those receiving baloxavir and 53% in those receiving oseltamivir, a nonsignificant difference, with the most common adverse event in both groups being gastrointestinal disorders. No deaths, serious adverse events, or hospitalizations were reported, but two patients receiving oseltamivir discontinued because of adverse events.
The study was funded by F. Hoffmann-La Roche. Dr. Baker and a coauthor received funding through their institutions for the conduct of the study; several coauthors reported being employed by and owning stocks in F. Hoffmann–La Roche. One coauthor reported receiving consultancy fees from F. Hoffmann–La Roche and grants from Shionogi.
SOURCE: Baker J et al. Pediatr Infect Dis J. 2020 Jun 5. doi: 10.1097/INF.0000000000002747.
FROM THE PEDIATRIC INFECTIOUS DISEASE JOURNAL
COVID-19: Where doctors can get help for emotional distress
Nisha Mehta, MD, said her phone has been ringing with calls from tearful and shaken physicians who are distressed and unsettled about their work and home situation and don’t know what to do.
What’s more, many frontline physicians are living apart from family to protect them from infection. “So many physicians have called me crying. ... They can’t even come home and get a hug,” Dr. Mehta said. “What I’m hearing from a lot of people who are in New York and New Jersey is not just that they go to work all day and it’s this exhausting process throughout the entire day, not only physically but also emotionally.”
Physician burnout has held a steady spotlight since long before the COVID-19 crisis began, Dr. Mehta said. “The reason for that is multifold, but in part, it’s hard for physicians to find an appropriate way to be able to process a lot of the emotions related to their work,” she said. “A lot of that brews below the surface, but COVID-19 has really brought many of these issues above that surface.”
Frustrated that governments weren’t doing enough to support health care workers during the pandemic, Dr. Mehta, a radiologist in Charlotte, N.C., decided there needed to be change. On April 4, Dr. Mehta and two physician colleagues submitted to Congress the COVID-19 Pandemic Physician Protection Act, which ensures, among other provisions, mental health coverage for health care workers. An accompanying petition on change.org had received nearly 300,000 signatures as of May 29.
Don’t suffer in silence
A career in medicine comes with immense stress in the best of times, she notes, and managing a pandemic in an already strained system has taken those challenges to newer heights. “We need better support structures at baseline for physician mental health,” said Dr. Mehta.
“That’s something we’ve always been lacking because it’s been against the culture of medicine for so long to say, ‘I’m having a hard time.’ ”
If you’re hurting, the first thing to recognize is that you are not alone in facing these challenges. This is true with respect not only to medical care but also to all of the family, financial, and business concerns physicians are currently facing. “Having all of those things hanging over your head is a lot. We’ve got to find ways to help each other out,” Dr. Mehta said.
Where to find support
Fortunately, the medical community has created several pathways to help its own.
The following list represents a cross-section of opportunities for caregivers to receive care for themselves.
Crisis hotlines
- Physician Support Line. This free and confidential hotline was launched on March 30 by Mona Masood, DO, a Philadelphia-area psychiatrist and moderator of a Facebook forum called the COVID-19 Physicians Group. The PSL is run by more than 600 volunteer psychiatrists who take calls from U.S. physicians 7 days a week from 8:00 a.m. to 1:00 a.m., with no appointment necessary. The toll-free number is 888-409-0141.
- For the Frontlines. This 24/7 help line provides free crisis counseling for frontline workers. They can text FRONTLINE to 741741 in the United States (support is also available for residents of Canada, Ireland, and the United Kingdom).
Resources from professional groups
- Action Collaborative on Clinician Well-Being and Resilience. Created by the National Academy of Medicine in 2017, the Action Collaborative comprises more than 60 organizations committed to reversing trends in clinician burnout. In response to the pandemic, the group has compiled a list of strategies and resources to support the health and well-being of clinicians who are providing healthcare during the COVID-19 outbreak.
- American Medical Association. The AMA has created a resource center dedicated to providing care for caregivers during the COVID-19 pandemic. The website includes specific guidance for managing mental health during the pandemic.
- American College of Physicians. The professional society of internal medicine physicians has created a comprehensive guide for physicians specific to COVID-19, with a section dedicated to clinician well-being that includes information about hotlines, counseling services, grief support, and more.
- American Hospital Association. The AHA’s website now includes regularly updated resources for healthcare clinicians and staff, as well as a special section dedicated to protecting and enabling healthcare workers in the midst of the pandemic.
Virtual psychological counseling
Not unlike the way telemedicine has allowed some physicians to keep seeing their patients, many modalities enable participation in therapy through video, chat, phone call, or any combination thereof. Look for a service that is convenient, flexible, and HIPAA compliant.
Traditional in-office mental health therapy has quickly moved to telemedicine. Many if not most insurers that cover counseling visits are paying for telepsychiatry or telecounseling. If you don’t know of an appropriate therapist, check the American Psychiatric Association or its state chapters; the American Psychological Association; or look for a licensed mental health counselor.
Because financial constraints are a potential barrier to therapy, Project Parachute, in cooperation with Eleos Health, has organized a cadre of therapists willing to provide pro bono online therapy for health care workers. The amount of free therapy provided to qualified frontline workers is up to the individual therapists. Discuss these parameters with your therapists up front.
Similar services are offered from companies such as Talkspace and BetterHelp on a subscription basis. These services are typically less expensive than in-person sessions. Ask about discounts for healthcare workers. Talkspace, for example, announced in March, “Effective immediately, healthcare workers across the country can get access to a free month of our...online therapy that includes unlimited text, video, and audio messaging with a licensed therapist.”
Online support groups and social media
For more on-demand peer support, look for groups such as the COR Sharing Circle for Healthcare Workers on Facebook. The site’s search engine can point users to plenty of other groups, many of which are closed (meaning posts are visible to members only).
Dr. Mehta hosts her own Facebook group called Physician Community. “I would like to think (and genuinely feel) that we’ve been doing a great job of supporting each other there with daily threads on challenges, treatments, pick-me-ups, vent posts, advocacy, and more,” she said.
For anyone in need, PeerRxMed is a free, peer-to-peer program for physicians and other health care workers that is designed to provide support, connection, encouragement, resources, and skill-building to optimize well-being.
For those craving spiritual comfort during this crisis, a number of churches have begun offering that experience virtually, too. First Unitarian Church of Worcester, Massachusetts, for example, offers weekly services via YouTube. Similar online programming is being offered from all sorts of organizations across denominations.
Apps
For DIY or on-the-spot coping support, apps can help physicians get through the day. Apps and websites that offer guided meditations and other relaxation tools include Headspace, Calm, and Insight Timer. Before downloading, look for special discounts and promotions for healthcare workers.
Additionally, COVID Coach is a free, secure app designed by the U.S. Department of Veterans Affairs that includes tools to help you cope with stress and stay well, safe, healthy, and connected. It also offers advice on navigating parenting, care giving, and working from home while social distancing, quarantined, or sheltering in place.
For practicing daily gratitude, Delightful Journal is a free app that offers journaling prompts, themes, reminders, and unlimited private space to record one’s thoughts.
Adopt a ritual
Although self-care for physicians is more crucial now than ever, it can look different for every individual. Along the same lines as keeping a journal, wellness experts often recommend beginning a “gratitude practice” to help provide solace and perspective.
Tweak and personalize these activities to suit your own needs, but be sure to use them even when you’re feeling well, said Mohana Karlekar, MD, medical director of palliative care and assistant professor at Vanderbilt University Medical Center, Nashville, Tenn.
One exercise she recommends is known as Three Good Things. “Every day, at the end of the day, think about three good things that have happened,” she explained. “You can always find the joys. And the joys don’t have to be enormous. There is joy – there is hope – in everything,” Dr. Karlekar said.
A version of this article originally appeared on Medscape.com.
Nisha Mehta, MD, said her phone has been ringing with calls from tearful and shaken physicians who are distressed and unsettled about their work and home situation and don’t know what to do.
What’s more, many frontline physicians are living apart from family to protect them from infection. “So many physicians have called me crying. ... They can’t even come home and get a hug,” Dr. Mehta said. “What I’m hearing from a lot of people who are in New York and New Jersey is not just that they go to work all day and it’s this exhausting process throughout the entire day, not only physically but also emotionally.”
Physician burnout has held a steady spotlight since long before the COVID-19 crisis began, Dr. Mehta said. “The reason for that is multifold, but in part, it’s hard for physicians to find an appropriate way to be able to process a lot of the emotions related to their work,” she said. “A lot of that brews below the surface, but COVID-19 has really brought many of these issues above that surface.”
Frustrated that governments weren’t doing enough to support health care workers during the pandemic, Dr. Mehta, a radiologist in Charlotte, N.C., decided there needed to be change. On April 4, Dr. Mehta and two physician colleagues submitted to Congress the COVID-19 Pandemic Physician Protection Act, which ensures, among other provisions, mental health coverage for health care workers. An accompanying petition on change.org had received nearly 300,000 signatures as of May 29.
Don’t suffer in silence
A career in medicine comes with immense stress in the best of times, she notes, and managing a pandemic in an already strained system has taken those challenges to newer heights. “We need better support structures at baseline for physician mental health,” said Dr. Mehta.
“That’s something we’ve always been lacking because it’s been against the culture of medicine for so long to say, ‘I’m having a hard time.’ ”
If you’re hurting, the first thing to recognize is that you are not alone in facing these challenges. This is true with respect not only to medical care but also to all of the family, financial, and business concerns physicians are currently facing. “Having all of those things hanging over your head is a lot. We’ve got to find ways to help each other out,” Dr. Mehta said.
Where to find support
Fortunately, the medical community has created several pathways to help its own.
The following list represents a cross-section of opportunities for caregivers to receive care for themselves.
Crisis hotlines
- Physician Support Line. This free and confidential hotline was launched on March 30 by Mona Masood, DO, a Philadelphia-area psychiatrist and moderator of a Facebook forum called the COVID-19 Physicians Group. The PSL is run by more than 600 volunteer psychiatrists who take calls from U.S. physicians 7 days a week from 8:00 a.m. to 1:00 a.m., with no appointment necessary. The toll-free number is 888-409-0141.
- For the Frontlines. This 24/7 help line provides free crisis counseling for frontline workers. They can text FRONTLINE to 741741 in the United States (support is also available for residents of Canada, Ireland, and the United Kingdom).
Resources from professional groups
- Action Collaborative on Clinician Well-Being and Resilience. Created by the National Academy of Medicine in 2017, the Action Collaborative comprises more than 60 organizations committed to reversing trends in clinician burnout. In response to the pandemic, the group has compiled a list of strategies and resources to support the health and well-being of clinicians who are providing healthcare during the COVID-19 outbreak.
- American Medical Association. The AMA has created a resource center dedicated to providing care for caregivers during the COVID-19 pandemic. The website includes specific guidance for managing mental health during the pandemic.
- American College of Physicians. The professional society of internal medicine physicians has created a comprehensive guide for physicians specific to COVID-19, with a section dedicated to clinician well-being that includes information about hotlines, counseling services, grief support, and more.
- American Hospital Association. The AHA’s website now includes regularly updated resources for healthcare clinicians and staff, as well as a special section dedicated to protecting and enabling healthcare workers in the midst of the pandemic.
Virtual psychological counseling
Not unlike the way telemedicine has allowed some physicians to keep seeing their patients, many modalities enable participation in therapy through video, chat, phone call, or any combination thereof. Look for a service that is convenient, flexible, and HIPAA compliant.
Traditional in-office mental health therapy has quickly moved to telemedicine. Many if not most insurers that cover counseling visits are paying for telepsychiatry or telecounseling. If you don’t know of an appropriate therapist, check the American Psychiatric Association or its state chapters; the American Psychological Association; or look for a licensed mental health counselor.
Because financial constraints are a potential barrier to therapy, Project Parachute, in cooperation with Eleos Health, has organized a cadre of therapists willing to provide pro bono online therapy for health care workers. The amount of free therapy provided to qualified frontline workers is up to the individual therapists. Discuss these parameters with your therapists up front.
Similar services are offered from companies such as Talkspace and BetterHelp on a subscription basis. These services are typically less expensive than in-person sessions. Ask about discounts for healthcare workers. Talkspace, for example, announced in March, “Effective immediately, healthcare workers across the country can get access to a free month of our...online therapy that includes unlimited text, video, and audio messaging with a licensed therapist.”
Online support groups and social media
For more on-demand peer support, look for groups such as the COR Sharing Circle for Healthcare Workers on Facebook. The site’s search engine can point users to plenty of other groups, many of which are closed (meaning posts are visible to members only).
Dr. Mehta hosts her own Facebook group called Physician Community. “I would like to think (and genuinely feel) that we’ve been doing a great job of supporting each other there with daily threads on challenges, treatments, pick-me-ups, vent posts, advocacy, and more,” she said.
For anyone in need, PeerRxMed is a free, peer-to-peer program for physicians and other health care workers that is designed to provide support, connection, encouragement, resources, and skill-building to optimize well-being.
For those craving spiritual comfort during this crisis, a number of churches have begun offering that experience virtually, too. First Unitarian Church of Worcester, Massachusetts, for example, offers weekly services via YouTube. Similar online programming is being offered from all sorts of organizations across denominations.
Apps
For DIY or on-the-spot coping support, apps can help physicians get through the day. Apps and websites that offer guided meditations and other relaxation tools include Headspace, Calm, and Insight Timer. Before downloading, look for special discounts and promotions for healthcare workers.
Additionally, COVID Coach is a free, secure app designed by the U.S. Department of Veterans Affairs that includes tools to help you cope with stress and stay well, safe, healthy, and connected. It also offers advice on navigating parenting, care giving, and working from home while social distancing, quarantined, or sheltering in place.
For practicing daily gratitude, Delightful Journal is a free app that offers journaling prompts, themes, reminders, and unlimited private space to record one’s thoughts.
Adopt a ritual
Although self-care for physicians is more crucial now than ever, it can look different for every individual. Along the same lines as keeping a journal, wellness experts often recommend beginning a “gratitude practice” to help provide solace and perspective.
Tweak and personalize these activities to suit your own needs, but be sure to use them even when you’re feeling well, said Mohana Karlekar, MD, medical director of palliative care and assistant professor at Vanderbilt University Medical Center, Nashville, Tenn.
One exercise she recommends is known as Three Good Things. “Every day, at the end of the day, think about three good things that have happened,” she explained. “You can always find the joys. And the joys don’t have to be enormous. There is joy – there is hope – in everything,” Dr. Karlekar said.
A version of this article originally appeared on Medscape.com.
Nisha Mehta, MD, said her phone has been ringing with calls from tearful and shaken physicians who are distressed and unsettled about their work and home situation and don’t know what to do.
What’s more, many frontline physicians are living apart from family to protect them from infection. “So many physicians have called me crying. ... They can’t even come home and get a hug,” Dr. Mehta said. “What I’m hearing from a lot of people who are in New York and New Jersey is not just that they go to work all day and it’s this exhausting process throughout the entire day, not only physically but also emotionally.”
Physician burnout has held a steady spotlight since long before the COVID-19 crisis began, Dr. Mehta said. “The reason for that is multifold, but in part, it’s hard for physicians to find an appropriate way to be able to process a lot of the emotions related to their work,” she said. “A lot of that brews below the surface, but COVID-19 has really brought many of these issues above that surface.”
Frustrated that governments weren’t doing enough to support health care workers during the pandemic, Dr. Mehta, a radiologist in Charlotte, N.C., decided there needed to be change. On April 4, Dr. Mehta and two physician colleagues submitted to Congress the COVID-19 Pandemic Physician Protection Act, which ensures, among other provisions, mental health coverage for health care workers. An accompanying petition on change.org had received nearly 300,000 signatures as of May 29.
Don’t suffer in silence
A career in medicine comes with immense stress in the best of times, she notes, and managing a pandemic in an already strained system has taken those challenges to newer heights. “We need better support structures at baseline for physician mental health,” said Dr. Mehta.
“That’s something we’ve always been lacking because it’s been against the culture of medicine for so long to say, ‘I’m having a hard time.’ ”
If you’re hurting, the first thing to recognize is that you are not alone in facing these challenges. This is true with respect not only to medical care but also to all of the family, financial, and business concerns physicians are currently facing. “Having all of those things hanging over your head is a lot. We’ve got to find ways to help each other out,” Dr. Mehta said.
Where to find support
Fortunately, the medical community has created several pathways to help its own.
The following list represents a cross-section of opportunities for caregivers to receive care for themselves.
Crisis hotlines
- Physician Support Line. This free and confidential hotline was launched on March 30 by Mona Masood, DO, a Philadelphia-area psychiatrist and moderator of a Facebook forum called the COVID-19 Physicians Group. The PSL is run by more than 600 volunteer psychiatrists who take calls from U.S. physicians 7 days a week from 8:00 a.m. to 1:00 a.m., with no appointment necessary. The toll-free number is 888-409-0141.
- For the Frontlines. This 24/7 help line provides free crisis counseling for frontline workers. They can text FRONTLINE to 741741 in the United States (support is also available for residents of Canada, Ireland, and the United Kingdom).
Resources from professional groups
- Action Collaborative on Clinician Well-Being and Resilience. Created by the National Academy of Medicine in 2017, the Action Collaborative comprises more than 60 organizations committed to reversing trends in clinician burnout. In response to the pandemic, the group has compiled a list of strategies and resources to support the health and well-being of clinicians who are providing healthcare during the COVID-19 outbreak.
- American Medical Association. The AMA has created a resource center dedicated to providing care for caregivers during the COVID-19 pandemic. The website includes specific guidance for managing mental health during the pandemic.
- American College of Physicians. The professional society of internal medicine physicians has created a comprehensive guide for physicians specific to COVID-19, with a section dedicated to clinician well-being that includes information about hotlines, counseling services, grief support, and more.
- American Hospital Association. The AHA’s website now includes regularly updated resources for healthcare clinicians and staff, as well as a special section dedicated to protecting and enabling healthcare workers in the midst of the pandemic.
Virtual psychological counseling
Not unlike the way telemedicine has allowed some physicians to keep seeing their patients, many modalities enable participation in therapy through video, chat, phone call, or any combination thereof. Look for a service that is convenient, flexible, and HIPAA compliant.
Traditional in-office mental health therapy has quickly moved to telemedicine. Many if not most insurers that cover counseling visits are paying for telepsychiatry or telecounseling. If you don’t know of an appropriate therapist, check the American Psychiatric Association or its state chapters; the American Psychological Association; or look for a licensed mental health counselor.
Because financial constraints are a potential barrier to therapy, Project Parachute, in cooperation with Eleos Health, has organized a cadre of therapists willing to provide pro bono online therapy for health care workers. The amount of free therapy provided to qualified frontline workers is up to the individual therapists. Discuss these parameters with your therapists up front.
Similar services are offered from companies such as Talkspace and BetterHelp on a subscription basis. These services are typically less expensive than in-person sessions. Ask about discounts for healthcare workers. Talkspace, for example, announced in March, “Effective immediately, healthcare workers across the country can get access to a free month of our...online therapy that includes unlimited text, video, and audio messaging with a licensed therapist.”
Online support groups and social media
For more on-demand peer support, look for groups such as the COR Sharing Circle for Healthcare Workers on Facebook. The site’s search engine can point users to plenty of other groups, many of which are closed (meaning posts are visible to members only).
Dr. Mehta hosts her own Facebook group called Physician Community. “I would like to think (and genuinely feel) that we’ve been doing a great job of supporting each other there with daily threads on challenges, treatments, pick-me-ups, vent posts, advocacy, and more,” she said.
For anyone in need, PeerRxMed is a free, peer-to-peer program for physicians and other health care workers that is designed to provide support, connection, encouragement, resources, and skill-building to optimize well-being.
For those craving spiritual comfort during this crisis, a number of churches have begun offering that experience virtually, too. First Unitarian Church of Worcester, Massachusetts, for example, offers weekly services via YouTube. Similar online programming is being offered from all sorts of organizations across denominations.
Apps
For DIY or on-the-spot coping support, apps can help physicians get through the day. Apps and websites that offer guided meditations and other relaxation tools include Headspace, Calm, and Insight Timer. Before downloading, look for special discounts and promotions for healthcare workers.
Additionally, COVID Coach is a free, secure app designed by the U.S. Department of Veterans Affairs that includes tools to help you cope with stress and stay well, safe, healthy, and connected. It also offers advice on navigating parenting, care giving, and working from home while social distancing, quarantined, or sheltering in place.
For practicing daily gratitude, Delightful Journal is a free app that offers journaling prompts, themes, reminders, and unlimited private space to record one’s thoughts.
Adopt a ritual
Although self-care for physicians is more crucial now than ever, it can look different for every individual. Along the same lines as keeping a journal, wellness experts often recommend beginning a “gratitude practice” to help provide solace and perspective.
Tweak and personalize these activities to suit your own needs, but be sure to use them even when you’re feeling well, said Mohana Karlekar, MD, medical director of palliative care and assistant professor at Vanderbilt University Medical Center, Nashville, Tenn.
One exercise she recommends is known as Three Good Things. “Every day, at the end of the day, think about three good things that have happened,” she explained. “You can always find the joys. And the joys don’t have to be enormous. There is joy – there is hope – in everything,” Dr. Karlekar said.
A version of this article originally appeared on Medscape.com.













