User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Novel agent shows promise against cat allergy
One dose of the novel agent, REGN1908-1909 (Regeneron Pharmaceuticals) resulted in a rapid and durable reduction in cat-allergen-induced bronchoconstriction in cat-allergic subjects with mild asthma.
The finding, from a phase 2 randomized placebo-controlled study, is good news for the millions of people who are plagued by cat allergies, the investigators say.
The study, which was sponsored by Regeneron, was presented in a late breaking oral abstract session at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“REGN1908-1909 contains antibodies against Fel d 1, the major cat allergen, and here we show that it quickly and lastingly reduces acute bronchoconstriction in people with cat allergy,” lead author Frederic J. de Blay, MD, Strasbourg University Hospital, France, said in an interview.
Dr. de Blay admitted he is “quite excited” about the results.
“This study was performed in an environmental exposure chamber, and we clearly demonstrate that these antibodies decrease the asthmatic response to cat allergen within 8 days, and that these effects last 3 months. I never saw that in my life. I was a little bit skeptical at first, so to obtain such robust results after just 8 days, after just one injection, I was very surprised,” he said.
Dr. de Blay and his team screened potential participants to make sure they were cat allergic by exposing them to cat allergen for up to 2 hours while they were in the environmental exposure chamber. To be eligible for the study, participants had to show an early asthmatic response (EAR), defined as a reduction in forced expiratory volume in 1 second (FEV1) of at least 20% from baseline.
The participants were then randomized to receive either single-dose REGN1908-1909, 600 mg, subcutaneously (n = 29 patients) or placebo (n = 27 patients) prior to cat-allergen exposure in the controlled environmental chamber.
Dr. de Blay developed the chamber used in the study: the ALYATEC environmental exposure chamber.
“The chamber is 60 meters square, or 150 cubic meters, and can accommodate 20 patients. We are able to nebulize cat allergen, mice allergen, or whatever we wish to study so we can standardize the exposure. We can control the particle size and the amount so we know the exact amount of allergen that the patient has been exposed to,” he explained.
To test the efficacy of REGN1908-1909 in reducing acute bronchoconstriction, or EAR, the researchers measured FEV1 at baseline, and on days 8, 29, 57, and 85 in both groups. During each exposure, measurements were taken every 10 minutes for periods that lasted up to 4 hours.
They found that the probability of remaining in the chamber with no asthmatic response was substantially elevated in the group treated with REGN1908-1909.
Compared with placebo, REGN1908-1909 significantly increased the median time to EAR, from 51 minutes at baseline to more than 4 hours on day 8, (hazard ratio [HR], 0.36; P < .0083), day 29 (HR, 0.24; P < .0001), day 57 (HR, 0.45; P = .0222), and day 85 (HR, 0.27; P = .0003).
The FEV1 area under the curve (AUC) was also better with REGN1908-1909 than with placebo at day 8 (15.2% vs. 1.6%; P < .001). And a single dose reduced skin-test reactivity to cat allergen at 1 week, which persisted for up to 4 months.
In addition, participants who received REGN1908-1909 were able to tolerate a threefold higher amount of the cat allergen than those who received placebo (P = .003).
“We initially gave 40 nanograms of cat allergen, and then 8 days later they were able to stay longer in the chamber and inhale more of the allergen, to almost triple the amount they had originally been given. That 40 nanograms is very close to real world exposure,” Dr. de Blay noted.
Regeneron plans to start a phase 3 trial soon, he reported.
Promising results
“The study is well designed and shows a reduction in drop of FEV1 in response to cat allergen provocation and a decreased AUC in cat SPT response over 4 months,” Jonathan A. Bernstein, MD, professor of medicine at the University of Cincinnati, said in an interview.
“These are very promising results, which show that REGN1908-1909 can be a novel treatment for cat-induced asthma, which is often the only sensitization patients have. And they love their cats – one-third of the U.S. population has a cat and one-third has a dog, and 50% have both,” noted Dr. Bernstein, who was not involved with the study.
“This novel study used our scientific knowledge of the cat allergen itself to design a targeted antibody-based treatment that demonstrates significant benefit even after the first shot,” added Edwin H. Kim, MD, director of the UNC Food Allergy Initiative at the University of North Carolina at Chapel Hill.
“This strategy has the potential to revolutionize not only our treatment of common environmental allergies but also other allergic diseases with well-described triggers, such as food and drug allergy,” Dr. Kim, who was not part of the study, said in an interview.
Dr. de Blay reported a financial relationship with Regeneron Pharmaceuticals, which sponsored the study. Dr. Bernstein and Dr. Kim have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One dose of the novel agent, REGN1908-1909 (Regeneron Pharmaceuticals) resulted in a rapid and durable reduction in cat-allergen-induced bronchoconstriction in cat-allergic subjects with mild asthma.
The finding, from a phase 2 randomized placebo-controlled study, is good news for the millions of people who are plagued by cat allergies, the investigators say.
The study, which was sponsored by Regeneron, was presented in a late breaking oral abstract session at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“REGN1908-1909 contains antibodies against Fel d 1, the major cat allergen, and here we show that it quickly and lastingly reduces acute bronchoconstriction in people with cat allergy,” lead author Frederic J. de Blay, MD, Strasbourg University Hospital, France, said in an interview.
Dr. de Blay admitted he is “quite excited” about the results.
“This study was performed in an environmental exposure chamber, and we clearly demonstrate that these antibodies decrease the asthmatic response to cat allergen within 8 days, and that these effects last 3 months. I never saw that in my life. I was a little bit skeptical at first, so to obtain such robust results after just 8 days, after just one injection, I was very surprised,” he said.
Dr. de Blay and his team screened potential participants to make sure they were cat allergic by exposing them to cat allergen for up to 2 hours while they were in the environmental exposure chamber. To be eligible for the study, participants had to show an early asthmatic response (EAR), defined as a reduction in forced expiratory volume in 1 second (FEV1) of at least 20% from baseline.
The participants were then randomized to receive either single-dose REGN1908-1909, 600 mg, subcutaneously (n = 29 patients) or placebo (n = 27 patients) prior to cat-allergen exposure in the controlled environmental chamber.
Dr. de Blay developed the chamber used in the study: the ALYATEC environmental exposure chamber.
“The chamber is 60 meters square, or 150 cubic meters, and can accommodate 20 patients. We are able to nebulize cat allergen, mice allergen, or whatever we wish to study so we can standardize the exposure. We can control the particle size and the amount so we know the exact amount of allergen that the patient has been exposed to,” he explained.
To test the efficacy of REGN1908-1909 in reducing acute bronchoconstriction, or EAR, the researchers measured FEV1 at baseline, and on days 8, 29, 57, and 85 in both groups. During each exposure, measurements were taken every 10 minutes for periods that lasted up to 4 hours.
They found that the probability of remaining in the chamber with no asthmatic response was substantially elevated in the group treated with REGN1908-1909.
Compared with placebo, REGN1908-1909 significantly increased the median time to EAR, from 51 minutes at baseline to more than 4 hours on day 8, (hazard ratio [HR], 0.36; P < .0083), day 29 (HR, 0.24; P < .0001), day 57 (HR, 0.45; P = .0222), and day 85 (HR, 0.27; P = .0003).
The FEV1 area under the curve (AUC) was also better with REGN1908-1909 than with placebo at day 8 (15.2% vs. 1.6%; P < .001). And a single dose reduced skin-test reactivity to cat allergen at 1 week, which persisted for up to 4 months.
In addition, participants who received REGN1908-1909 were able to tolerate a threefold higher amount of the cat allergen than those who received placebo (P = .003).
“We initially gave 40 nanograms of cat allergen, and then 8 days later they were able to stay longer in the chamber and inhale more of the allergen, to almost triple the amount they had originally been given. That 40 nanograms is very close to real world exposure,” Dr. de Blay noted.
Regeneron plans to start a phase 3 trial soon, he reported.
Promising results
“The study is well designed and shows a reduction in drop of FEV1 in response to cat allergen provocation and a decreased AUC in cat SPT response over 4 months,” Jonathan A. Bernstein, MD, professor of medicine at the University of Cincinnati, said in an interview.
“These are very promising results, which show that REGN1908-1909 can be a novel treatment for cat-induced asthma, which is often the only sensitization patients have. And they love their cats – one-third of the U.S. population has a cat and one-third has a dog, and 50% have both,” noted Dr. Bernstein, who was not involved with the study.
“This novel study used our scientific knowledge of the cat allergen itself to design a targeted antibody-based treatment that demonstrates significant benefit even after the first shot,” added Edwin H. Kim, MD, director of the UNC Food Allergy Initiative at the University of North Carolina at Chapel Hill.
“This strategy has the potential to revolutionize not only our treatment of common environmental allergies but also other allergic diseases with well-described triggers, such as food and drug allergy,” Dr. Kim, who was not part of the study, said in an interview.
Dr. de Blay reported a financial relationship with Regeneron Pharmaceuticals, which sponsored the study. Dr. Bernstein and Dr. Kim have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One dose of the novel agent, REGN1908-1909 (Regeneron Pharmaceuticals) resulted in a rapid and durable reduction in cat-allergen-induced bronchoconstriction in cat-allergic subjects with mild asthma.
The finding, from a phase 2 randomized placebo-controlled study, is good news for the millions of people who are plagued by cat allergies, the investigators say.
The study, which was sponsored by Regeneron, was presented in a late breaking oral abstract session at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“REGN1908-1909 contains antibodies against Fel d 1, the major cat allergen, and here we show that it quickly and lastingly reduces acute bronchoconstriction in people with cat allergy,” lead author Frederic J. de Blay, MD, Strasbourg University Hospital, France, said in an interview.
Dr. de Blay admitted he is “quite excited” about the results.
“This study was performed in an environmental exposure chamber, and we clearly demonstrate that these antibodies decrease the asthmatic response to cat allergen within 8 days, and that these effects last 3 months. I never saw that in my life. I was a little bit skeptical at first, so to obtain such robust results after just 8 days, after just one injection, I was very surprised,” he said.
Dr. de Blay and his team screened potential participants to make sure they were cat allergic by exposing them to cat allergen for up to 2 hours while they were in the environmental exposure chamber. To be eligible for the study, participants had to show an early asthmatic response (EAR), defined as a reduction in forced expiratory volume in 1 second (FEV1) of at least 20% from baseline.
The participants were then randomized to receive either single-dose REGN1908-1909, 600 mg, subcutaneously (n = 29 patients) or placebo (n = 27 patients) prior to cat-allergen exposure in the controlled environmental chamber.
Dr. de Blay developed the chamber used in the study: the ALYATEC environmental exposure chamber.
“The chamber is 60 meters square, or 150 cubic meters, and can accommodate 20 patients. We are able to nebulize cat allergen, mice allergen, or whatever we wish to study so we can standardize the exposure. We can control the particle size and the amount so we know the exact amount of allergen that the patient has been exposed to,” he explained.
To test the efficacy of REGN1908-1909 in reducing acute bronchoconstriction, or EAR, the researchers measured FEV1 at baseline, and on days 8, 29, 57, and 85 in both groups. During each exposure, measurements were taken every 10 minutes for periods that lasted up to 4 hours.
They found that the probability of remaining in the chamber with no asthmatic response was substantially elevated in the group treated with REGN1908-1909.
Compared with placebo, REGN1908-1909 significantly increased the median time to EAR, from 51 minutes at baseline to more than 4 hours on day 8, (hazard ratio [HR], 0.36; P < .0083), day 29 (HR, 0.24; P < .0001), day 57 (HR, 0.45; P = .0222), and day 85 (HR, 0.27; P = .0003).
The FEV1 area under the curve (AUC) was also better with REGN1908-1909 than with placebo at day 8 (15.2% vs. 1.6%; P < .001). And a single dose reduced skin-test reactivity to cat allergen at 1 week, which persisted for up to 4 months.
In addition, participants who received REGN1908-1909 were able to tolerate a threefold higher amount of the cat allergen than those who received placebo (P = .003).
“We initially gave 40 nanograms of cat allergen, and then 8 days later they were able to stay longer in the chamber and inhale more of the allergen, to almost triple the amount they had originally been given. That 40 nanograms is very close to real world exposure,” Dr. de Blay noted.
Regeneron plans to start a phase 3 trial soon, he reported.
Promising results
“The study is well designed and shows a reduction in drop of FEV1 in response to cat allergen provocation and a decreased AUC in cat SPT response over 4 months,” Jonathan A. Bernstein, MD, professor of medicine at the University of Cincinnati, said in an interview.
“These are very promising results, which show that REGN1908-1909 can be a novel treatment for cat-induced asthma, which is often the only sensitization patients have. And they love their cats – one-third of the U.S. population has a cat and one-third has a dog, and 50% have both,” noted Dr. Bernstein, who was not involved with the study.
“This novel study used our scientific knowledge of the cat allergen itself to design a targeted antibody-based treatment that demonstrates significant benefit even after the first shot,” added Edwin H. Kim, MD, director of the UNC Food Allergy Initiative at the University of North Carolina at Chapel Hill.
“This strategy has the potential to revolutionize not only our treatment of common environmental allergies but also other allergic diseases with well-described triggers, such as food and drug allergy,” Dr. Kim, who was not part of the study, said in an interview.
Dr. de Blay reported a financial relationship with Regeneron Pharmaceuticals, which sponsored the study. Dr. Bernstein and Dr. Kim have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAAAI
Adalimumab earns FDA approval for ulcerative colitis in children
Adalimumab has received approval from the Food and Drug Administration for use in pediatric patients aged 5 years and older with moderately to severely active ulcerative colitis (UC), according to a press release from manufacturer AbbVie.
Approval was based on data from the phase 3 ENVISION 1 randomized, double-blind study (NCT02065557) in which 60% of patients receiving the higher induction dose showed clinical remission according to Partial May Score (PMS) at 8 weeks, 45% of whom were in remission according to Full Mayo Score (FMS) at 1 year. The approval means that children with UC and their families have the option of a subcutaneous biologic that can be administered at home.
In the ENVISION 1 study, clinical remission was defined as a PMS (based on stool frequency, rectal bleeding, and physician’s global assessment) ranging from 0 to 3 at the end of the 8-week induction period or FMS (which adds endoscopy) at the end of 52 weeks as less than or equal to 2 points total, with no individual subscore greater than 1. The study included children aged 4-17 years with active UC who were randomized to a low-dose or high-dose group.
“Through week 8, patients in both dosage groups received 2.4 mg/kg (maximum of 160 mg) at week 0, 1.2 mg/kg (maximum of 80 mg) at week 2, and 0.6 mg/kg (maximum of 40 mg) at weeks 4 and 6. The higher-dosage group also received an additional dosage of 2.4 mg/kg (maximum of 160 mg) at week 1,” according to the company press release. Between weeks 8 and 52, patients received double-blind placebo or 0.6 mg/kg adalimumab (maximum of 40 mg) every other week (maintenance standard dose) or every week (maintenance high dose).
No new adalimumab safety signals were noted in the study; headache and worsening UC were the most frequently reported treatment-emergent adverse events, and 22.6% of patients experienced a serious adverse event. “No deaths, malignancies, active tuberculosis or demyelinating disease were observed in this study,” according to the company. Full prescribing information can be found on the FDA website.
Approval expands options
“The number of FDA-approved biologics to treat pediatric UC has been limited,” said Atsushi Sakuraba, MD, PhD, an inflammatory bowel disease specialist at the University of Chicago, in an interview. “Infliximab is approved for pediatric UC and CD [Crohn’s disease], but adalimumab was only approved for CD in pediatric patients, and vedolizumab and ustekinumab are approved for neither UC nor CD in pediatric patients,” he said. “Thus, the addition of adalimumab as a treatment option for pediatric UC is of great benefit for pediatricians and patients.”
The approval will impact clinical practice in several ways, Dr. Sakuraba said. “Adalimumab may be used for those who lose response to infliximab, but may also be used as a first-line biologic because it is self-injectable,” he emphasized. Given that adalimumab is already in use for pediatric patients with Crohn’s disease, Dr. Sakuraba said he does not see any barriers to implementation of its use for children with UC. In addition, “the potential for better disease control, reduction of disease-related complications, and improved quality of life outweighs the risk of adverse reactions,” Dr. Sakuraba said. “The news of no new safety concern in the 52-week study period further supports the safety of biologic treatment in pediatric patients,” he added.
As for additional research: “It remains to be determined whether combination therapy with an immunomodulator would be more effective than monotherapy, and also whether there are any additional risks of adverse events with the addition of immunomodulators such as long-term risk of malignancy, especially lymphoproliferative disorders,” Dr. Sakuraba noted. “Therapeutic options for pediatric [inflammatory bowel disease] are limited and lagging, compared to adult patients, so studies of non-TNF [tumor necrosis factor] biologics such vedolizumab and ustekinumab are also awaited,” he said.
Dr. Sakuraba had no financial conflicts to disclose.
This article was updated March 3, 2021.
Adalimumab has received approval from the Food and Drug Administration for use in pediatric patients aged 5 years and older with moderately to severely active ulcerative colitis (UC), according to a press release from manufacturer AbbVie.
Approval was based on data from the phase 3 ENVISION 1 randomized, double-blind study (NCT02065557) in which 60% of patients receiving the higher induction dose showed clinical remission according to Partial May Score (PMS) at 8 weeks, 45% of whom were in remission according to Full Mayo Score (FMS) at 1 year. The approval means that children with UC and their families have the option of a subcutaneous biologic that can be administered at home.
In the ENVISION 1 study, clinical remission was defined as a PMS (based on stool frequency, rectal bleeding, and physician’s global assessment) ranging from 0 to 3 at the end of the 8-week induction period or FMS (which adds endoscopy) at the end of 52 weeks as less than or equal to 2 points total, with no individual subscore greater than 1. The study included children aged 4-17 years with active UC who were randomized to a low-dose or high-dose group.
“Through week 8, patients in both dosage groups received 2.4 mg/kg (maximum of 160 mg) at week 0, 1.2 mg/kg (maximum of 80 mg) at week 2, and 0.6 mg/kg (maximum of 40 mg) at weeks 4 and 6. The higher-dosage group also received an additional dosage of 2.4 mg/kg (maximum of 160 mg) at week 1,” according to the company press release. Between weeks 8 and 52, patients received double-blind placebo or 0.6 mg/kg adalimumab (maximum of 40 mg) every other week (maintenance standard dose) or every week (maintenance high dose).
No new adalimumab safety signals were noted in the study; headache and worsening UC were the most frequently reported treatment-emergent adverse events, and 22.6% of patients experienced a serious adverse event. “No deaths, malignancies, active tuberculosis or demyelinating disease were observed in this study,” according to the company. Full prescribing information can be found on the FDA website.
Approval expands options
“The number of FDA-approved biologics to treat pediatric UC has been limited,” said Atsushi Sakuraba, MD, PhD, an inflammatory bowel disease specialist at the University of Chicago, in an interview. “Infliximab is approved for pediatric UC and CD [Crohn’s disease], but adalimumab was only approved for CD in pediatric patients, and vedolizumab and ustekinumab are approved for neither UC nor CD in pediatric patients,” he said. “Thus, the addition of adalimumab as a treatment option for pediatric UC is of great benefit for pediatricians and patients.”
The approval will impact clinical practice in several ways, Dr. Sakuraba said. “Adalimumab may be used for those who lose response to infliximab, but may also be used as a first-line biologic because it is self-injectable,” he emphasized. Given that adalimumab is already in use for pediatric patients with Crohn’s disease, Dr. Sakuraba said he does not see any barriers to implementation of its use for children with UC. In addition, “the potential for better disease control, reduction of disease-related complications, and improved quality of life outweighs the risk of adverse reactions,” Dr. Sakuraba said. “The news of no new safety concern in the 52-week study period further supports the safety of biologic treatment in pediatric patients,” he added.
As for additional research: “It remains to be determined whether combination therapy with an immunomodulator would be more effective than monotherapy, and also whether there are any additional risks of adverse events with the addition of immunomodulators such as long-term risk of malignancy, especially lymphoproliferative disorders,” Dr. Sakuraba noted. “Therapeutic options for pediatric [inflammatory bowel disease] are limited and lagging, compared to adult patients, so studies of non-TNF [tumor necrosis factor] biologics such vedolizumab and ustekinumab are also awaited,” he said.
Dr. Sakuraba had no financial conflicts to disclose.
This article was updated March 3, 2021.
Adalimumab has received approval from the Food and Drug Administration for use in pediatric patients aged 5 years and older with moderately to severely active ulcerative colitis (UC), according to a press release from manufacturer AbbVie.
Approval was based on data from the phase 3 ENVISION 1 randomized, double-blind study (NCT02065557) in which 60% of patients receiving the higher induction dose showed clinical remission according to Partial May Score (PMS) at 8 weeks, 45% of whom were in remission according to Full Mayo Score (FMS) at 1 year. The approval means that children with UC and their families have the option of a subcutaneous biologic that can be administered at home.
In the ENVISION 1 study, clinical remission was defined as a PMS (based on stool frequency, rectal bleeding, and physician’s global assessment) ranging from 0 to 3 at the end of the 8-week induction period or FMS (which adds endoscopy) at the end of 52 weeks as less than or equal to 2 points total, with no individual subscore greater than 1. The study included children aged 4-17 years with active UC who were randomized to a low-dose or high-dose group.
“Through week 8, patients in both dosage groups received 2.4 mg/kg (maximum of 160 mg) at week 0, 1.2 mg/kg (maximum of 80 mg) at week 2, and 0.6 mg/kg (maximum of 40 mg) at weeks 4 and 6. The higher-dosage group also received an additional dosage of 2.4 mg/kg (maximum of 160 mg) at week 1,” according to the company press release. Between weeks 8 and 52, patients received double-blind placebo or 0.6 mg/kg adalimumab (maximum of 40 mg) every other week (maintenance standard dose) or every week (maintenance high dose).
No new adalimumab safety signals were noted in the study; headache and worsening UC were the most frequently reported treatment-emergent adverse events, and 22.6% of patients experienced a serious adverse event. “No deaths, malignancies, active tuberculosis or demyelinating disease were observed in this study,” according to the company. Full prescribing information can be found on the FDA website.
Approval expands options
“The number of FDA-approved biologics to treat pediatric UC has been limited,” said Atsushi Sakuraba, MD, PhD, an inflammatory bowel disease specialist at the University of Chicago, in an interview. “Infliximab is approved for pediatric UC and CD [Crohn’s disease], but adalimumab was only approved for CD in pediatric patients, and vedolizumab and ustekinumab are approved for neither UC nor CD in pediatric patients,” he said. “Thus, the addition of adalimumab as a treatment option for pediatric UC is of great benefit for pediatricians and patients.”
The approval will impact clinical practice in several ways, Dr. Sakuraba said. “Adalimumab may be used for those who lose response to infliximab, but may also be used as a first-line biologic because it is self-injectable,” he emphasized. Given that adalimumab is already in use for pediatric patients with Crohn’s disease, Dr. Sakuraba said he does not see any barriers to implementation of its use for children with UC. In addition, “the potential for better disease control, reduction of disease-related complications, and improved quality of life outweighs the risk of adverse reactions,” Dr. Sakuraba said. “The news of no new safety concern in the 52-week study period further supports the safety of biologic treatment in pediatric patients,” he added.
As for additional research: “It remains to be determined whether combination therapy with an immunomodulator would be more effective than monotherapy, and also whether there are any additional risks of adverse events with the addition of immunomodulators such as long-term risk of malignancy, especially lymphoproliferative disorders,” Dr. Sakuraba noted. “Therapeutic options for pediatric [inflammatory bowel disease] are limited and lagging, compared to adult patients, so studies of non-TNF [tumor necrosis factor] biologics such vedolizumab and ustekinumab are also awaited,” he said.
Dr. Sakuraba had no financial conflicts to disclose.
This article was updated March 3, 2021.
Decline in children’s COVID-19 cases slows
The number of new COVID-19 cases in children declined for the sixth consecutive week, but the drop was the smallest yet, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
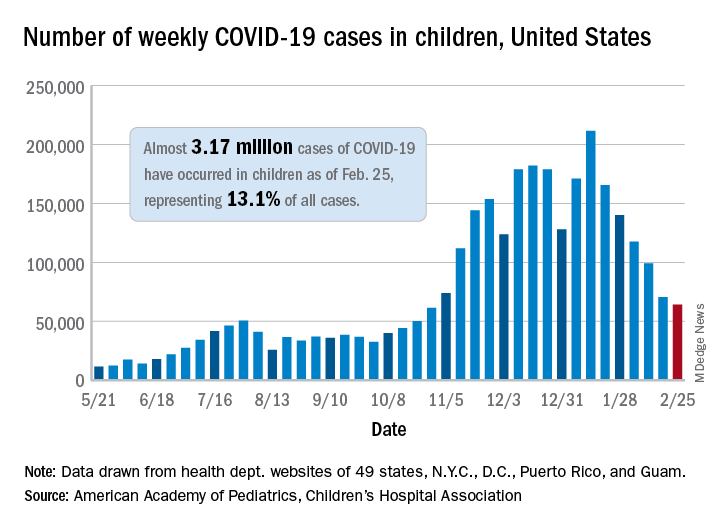
That drop of almost 6,400 cases, or 9.0%, falls short of the declines recorded in any the previous 5 weeks, which ranged from 18,000 to 46,000 cases and 15.3% to 28.7%, based on data from the heath departments of 49 states (excluding New York), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of children infected with SARS-CoV-2 is up to almost 3.17 million, which represents 13.1% of cases among all age groups. That cumulative proportion was unchanged from the previous week, which has occurred only three other times over the course of the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Despite the 6-week decline in new cases, however, the cumulative rate continued to climb, rising from 4,124 cases per 100,000 children to 4,209 for the week of Feb. 19-25. The states, not surprisingly, fall on both sides of that national tally. The lowest rates can be found in Hawaii (1,040 per 100,000 children), Vermont (2,111 per 100,000), and Maine (2,394), while the highest rates were recorded in North Dakota (8,580), Tennessee (7,851), and Rhode Island (7,223), the AAP and CHA said.
The number of new child deaths, nine, stayed in single digits for a second consecutive week, although it was up from six deaths reported a week earlier. Total COVID-19–related deaths in children now number 256, which represents just 0.06% of coronavirus deaths for all ages among the 43 states (along with New York City and Guam) reporting such data.
Among those jurisdictions, Texas (40), Arizona (27), and New York City (23) have reported the most deaths in children, while nine states and the District of Columbia have reported no deaths yet, the AAP and CHA noted.
The number of new COVID-19 cases in children declined for the sixth consecutive week, but the drop was the smallest yet, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

That drop of almost 6,400 cases, or 9.0%, falls short of the declines recorded in any the previous 5 weeks, which ranged from 18,000 to 46,000 cases and 15.3% to 28.7%, based on data from the heath departments of 49 states (excluding New York), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of children infected with SARS-CoV-2 is up to almost 3.17 million, which represents 13.1% of cases among all age groups. That cumulative proportion was unchanged from the previous week, which has occurred only three other times over the course of the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Despite the 6-week decline in new cases, however, the cumulative rate continued to climb, rising from 4,124 cases per 100,000 children to 4,209 for the week of Feb. 19-25. The states, not surprisingly, fall on both sides of that national tally. The lowest rates can be found in Hawaii (1,040 per 100,000 children), Vermont (2,111 per 100,000), and Maine (2,394), while the highest rates were recorded in North Dakota (8,580), Tennessee (7,851), and Rhode Island (7,223), the AAP and CHA said.
The number of new child deaths, nine, stayed in single digits for a second consecutive week, although it was up from six deaths reported a week earlier. Total COVID-19–related deaths in children now number 256, which represents just 0.06% of coronavirus deaths for all ages among the 43 states (along with New York City and Guam) reporting such data.
Among those jurisdictions, Texas (40), Arizona (27), and New York City (23) have reported the most deaths in children, while nine states and the District of Columbia have reported no deaths yet, the AAP and CHA noted.
The number of new COVID-19 cases in children declined for the sixth consecutive week, but the drop was the smallest yet, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

That drop of almost 6,400 cases, or 9.0%, falls short of the declines recorded in any the previous 5 weeks, which ranged from 18,000 to 46,000 cases and 15.3% to 28.7%, based on data from the heath departments of 49 states (excluding New York), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of children infected with SARS-CoV-2 is up to almost 3.17 million, which represents 13.1% of cases among all age groups. That cumulative proportion was unchanged from the previous week, which has occurred only three other times over the course of the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Despite the 6-week decline in new cases, however, the cumulative rate continued to climb, rising from 4,124 cases per 100,000 children to 4,209 for the week of Feb. 19-25. The states, not surprisingly, fall on both sides of that national tally. The lowest rates can be found in Hawaii (1,040 per 100,000 children), Vermont (2,111 per 100,000), and Maine (2,394), while the highest rates were recorded in North Dakota (8,580), Tennessee (7,851), and Rhode Island (7,223), the AAP and CHA said.
The number of new child deaths, nine, stayed in single digits for a second consecutive week, although it was up from six deaths reported a week earlier. Total COVID-19–related deaths in children now number 256, which represents just 0.06% of coronavirus deaths for all ages among the 43 states (along with New York City and Guam) reporting such data.
Among those jurisdictions, Texas (40), Arizona (27), and New York City (23) have reported the most deaths in children, while nine states and the District of Columbia have reported no deaths yet, the AAP and CHA noted.
Fired for good judgment a sign of physicians’ lost respect
What happened to Hasan Gokal, MD, should stick painfully in the craws of all physicians. It should serve as a call to action, because Dr. Gokal is sitting at home today without a job and under threat of further legal action while we continue about our day.
Dr. Gokal’s “crime” is that he vaccinated 10 strangers and acquaintances with soon-to-expire doses of the Moderna COVID-19 vaccine. He drove to the homes of some in the dark of night and injected others on his Sugar Land, Texas, lawn. He spent hours in a frantic search for willing recipients to beat the expiration clock. With minutes to spare, he gave the last dose to his at-risk wife, who has symptomatic pulmonary sarcoidosis, but whose age meant she did not fall into a vaccine priority tier.
According to the New York Times, Dr. Gokal’s wife was hesitant, afraid he might get into trouble. But why would she be hesitant? He wasn’t doing anything immoral. Perhaps she knew how far physicians have fallen and how bitterly they both could suffer.
In Barren County, Ky., where I live, a state of emergency was declared by our judge executive because of inclement weather. This directive allows our emergency management to “waive procedures and formalities otherwise required by the law.” It’s too bad that the same courtesy was not afforded to Dr. Gokal in Texas. It’s a shame that ice and snow didn’t drive his actions. Perhaps that would have protected him against the harsh criticism. Rather, it was his oath to patients and dedication to his fellow humans that motivated him, and for that, he was made to suffer.
Dr. Gokal was right to think that pouring the last 10 vaccine doses down the toilet would be an egregious act. But he was wrong in thinking his decision to find takers for the vaccine would be viewed as expedient. Instead, he was accused of graft and even nepotism. And there is the rub. That he was fired and charged with the theft of $137 worth of vaccines says everything about how physicians are treated in the year 2021. Dr. Gokal’s lawyer says the charge carried a maximum penalty of 1 year in prison and a fine of nearly $4,000.
Thank God a sage judge threw out the case and “rebuked” the office of District Attorney Kim Ogg. That hasn’t stopped her from threatening to bring the case to a grand jury. That threat invites anyone faced with the same scenario to flush the extra vaccine doses into the septic system. It encourages us to choose the toilet handle to avoid a mug shot.
And we can’t ignore the racial slant to this story. The Times reported that Dr. Gokal asked the officials, “Are you suggesting that there were too many Indian names in this group?”
“Exactly” was the answer. Let that sink in.
None of this would have happened 20 years ago. Back then, no one would have questioned the wisdom a physician gains from all our years of training and residency. In an age when anyone who conducts an office visit is now called “doctor,” respect for the letters “MD” has been leveled. We physicians have lost our autonomy and been cowed into submission.
But whatever his profession, Hasan Gokal was fired for being a good human. Today, the sun rose on 10 individuals who now enjoy better protection against a deadly pandemic. They include a bed-bound nonagenarian. A woman in her 80s with dementia. A mother with a child who uses a ventilator. All now have antibodies against SARS-CoV2 because of the tireless actions of Dr. Gokal.
Yet Dr. Gokal’s future is uncertain. Will we help him, or will we leave him to the wolves? In an email exchange with his lawyer’s office, I learned that Dr. Gokal has received offers of employment but is unable to entertain them because the actions by the Harris County District Attorney triggered an automatic review by the Texas Medical Board. A GoFundMe page was launched, but an appreciative Dr. Gokal stated publicly that he’d rather the money go to a needy charity.
In the last paragraph of the Times article, Dr. Gokal asks, “How can I take it back?” referencing stories about “the Pakistani doctor in Houston who stole all those vaccines.”
Let’s help him take back his story. In helping him, perhaps we can take back a little control. We could start with letters of support that could be mailed to his lawyer, Paul Doyle, Esq., of Houston, or tweet, respectfully of course, to the district attorney @Kimoggforda.
We can also let the Harris County Public Health Department in Houston know what we think of their actions.
On Martin Luther King Day, Kim Ogg, the district attorney who charged Dr. Gokal, tweeted MLK’s famous quote: “Injustice anywhere is a threat to justice everywhere.”
Let that motivate us to action.
Melissa Walton-Shirley, MD, is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. In addition to opinion writing, she enjoys spending time with her husband, daughters and parents, and sidelines as a backing vocalist for local rock bands. A version of this article first appeared on Medscape.com.
What happened to Hasan Gokal, MD, should stick painfully in the craws of all physicians. It should serve as a call to action, because Dr. Gokal is sitting at home today without a job and under threat of further legal action while we continue about our day.
Dr. Gokal’s “crime” is that he vaccinated 10 strangers and acquaintances with soon-to-expire doses of the Moderna COVID-19 vaccine. He drove to the homes of some in the dark of night and injected others on his Sugar Land, Texas, lawn. He spent hours in a frantic search for willing recipients to beat the expiration clock. With minutes to spare, he gave the last dose to his at-risk wife, who has symptomatic pulmonary sarcoidosis, but whose age meant she did not fall into a vaccine priority tier.
According to the New York Times, Dr. Gokal’s wife was hesitant, afraid he might get into trouble. But why would she be hesitant? He wasn’t doing anything immoral. Perhaps she knew how far physicians have fallen and how bitterly they both could suffer.
In Barren County, Ky., where I live, a state of emergency was declared by our judge executive because of inclement weather. This directive allows our emergency management to “waive procedures and formalities otherwise required by the law.” It’s too bad that the same courtesy was not afforded to Dr. Gokal in Texas. It’s a shame that ice and snow didn’t drive his actions. Perhaps that would have protected him against the harsh criticism. Rather, it was his oath to patients and dedication to his fellow humans that motivated him, and for that, he was made to suffer.
Dr. Gokal was right to think that pouring the last 10 vaccine doses down the toilet would be an egregious act. But he was wrong in thinking his decision to find takers for the vaccine would be viewed as expedient. Instead, he was accused of graft and even nepotism. And there is the rub. That he was fired and charged with the theft of $137 worth of vaccines says everything about how physicians are treated in the year 2021. Dr. Gokal’s lawyer says the charge carried a maximum penalty of 1 year in prison and a fine of nearly $4,000.
Thank God a sage judge threw out the case and “rebuked” the office of District Attorney Kim Ogg. That hasn’t stopped her from threatening to bring the case to a grand jury. That threat invites anyone faced with the same scenario to flush the extra vaccine doses into the septic system. It encourages us to choose the toilet handle to avoid a mug shot.
And we can’t ignore the racial slant to this story. The Times reported that Dr. Gokal asked the officials, “Are you suggesting that there were too many Indian names in this group?”
“Exactly” was the answer. Let that sink in.
None of this would have happened 20 years ago. Back then, no one would have questioned the wisdom a physician gains from all our years of training and residency. In an age when anyone who conducts an office visit is now called “doctor,” respect for the letters “MD” has been leveled. We physicians have lost our autonomy and been cowed into submission.
But whatever his profession, Hasan Gokal was fired for being a good human. Today, the sun rose on 10 individuals who now enjoy better protection against a deadly pandemic. They include a bed-bound nonagenarian. A woman in her 80s with dementia. A mother with a child who uses a ventilator. All now have antibodies against SARS-CoV2 because of the tireless actions of Dr. Gokal.
Yet Dr. Gokal’s future is uncertain. Will we help him, or will we leave him to the wolves? In an email exchange with his lawyer’s office, I learned that Dr. Gokal has received offers of employment but is unable to entertain them because the actions by the Harris County District Attorney triggered an automatic review by the Texas Medical Board. A GoFundMe page was launched, but an appreciative Dr. Gokal stated publicly that he’d rather the money go to a needy charity.
In the last paragraph of the Times article, Dr. Gokal asks, “How can I take it back?” referencing stories about “the Pakistani doctor in Houston who stole all those vaccines.”
Let’s help him take back his story. In helping him, perhaps we can take back a little control. We could start with letters of support that could be mailed to his lawyer, Paul Doyle, Esq., of Houston, or tweet, respectfully of course, to the district attorney @Kimoggforda.
We can also let the Harris County Public Health Department in Houston know what we think of their actions.
On Martin Luther King Day, Kim Ogg, the district attorney who charged Dr. Gokal, tweeted MLK’s famous quote: “Injustice anywhere is a threat to justice everywhere.”
Let that motivate us to action.
Melissa Walton-Shirley, MD, is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. In addition to opinion writing, she enjoys spending time with her husband, daughters and parents, and sidelines as a backing vocalist for local rock bands. A version of this article first appeared on Medscape.com.
What happened to Hasan Gokal, MD, should stick painfully in the craws of all physicians. It should serve as a call to action, because Dr. Gokal is sitting at home today without a job and under threat of further legal action while we continue about our day.
Dr. Gokal’s “crime” is that he vaccinated 10 strangers and acquaintances with soon-to-expire doses of the Moderna COVID-19 vaccine. He drove to the homes of some in the dark of night and injected others on his Sugar Land, Texas, lawn. He spent hours in a frantic search for willing recipients to beat the expiration clock. With minutes to spare, he gave the last dose to his at-risk wife, who has symptomatic pulmonary sarcoidosis, but whose age meant she did not fall into a vaccine priority tier.
According to the New York Times, Dr. Gokal’s wife was hesitant, afraid he might get into trouble. But why would she be hesitant? He wasn’t doing anything immoral. Perhaps she knew how far physicians have fallen and how bitterly they both could suffer.
In Barren County, Ky., where I live, a state of emergency was declared by our judge executive because of inclement weather. This directive allows our emergency management to “waive procedures and formalities otherwise required by the law.” It’s too bad that the same courtesy was not afforded to Dr. Gokal in Texas. It’s a shame that ice and snow didn’t drive his actions. Perhaps that would have protected him against the harsh criticism. Rather, it was his oath to patients and dedication to his fellow humans that motivated him, and for that, he was made to suffer.
Dr. Gokal was right to think that pouring the last 10 vaccine doses down the toilet would be an egregious act. But he was wrong in thinking his decision to find takers for the vaccine would be viewed as expedient. Instead, he was accused of graft and even nepotism. And there is the rub. That he was fired and charged with the theft of $137 worth of vaccines says everything about how physicians are treated in the year 2021. Dr. Gokal’s lawyer says the charge carried a maximum penalty of 1 year in prison and a fine of nearly $4,000.
Thank God a sage judge threw out the case and “rebuked” the office of District Attorney Kim Ogg. That hasn’t stopped her from threatening to bring the case to a grand jury. That threat invites anyone faced with the same scenario to flush the extra vaccine doses into the septic system. It encourages us to choose the toilet handle to avoid a mug shot.
And we can’t ignore the racial slant to this story. The Times reported that Dr. Gokal asked the officials, “Are you suggesting that there were too many Indian names in this group?”
“Exactly” was the answer. Let that sink in.
None of this would have happened 20 years ago. Back then, no one would have questioned the wisdom a physician gains from all our years of training and residency. In an age when anyone who conducts an office visit is now called “doctor,” respect for the letters “MD” has been leveled. We physicians have lost our autonomy and been cowed into submission.
But whatever his profession, Hasan Gokal was fired for being a good human. Today, the sun rose on 10 individuals who now enjoy better protection against a deadly pandemic. They include a bed-bound nonagenarian. A woman in her 80s with dementia. A mother with a child who uses a ventilator. All now have antibodies against SARS-CoV2 because of the tireless actions of Dr. Gokal.
Yet Dr. Gokal’s future is uncertain. Will we help him, or will we leave him to the wolves? In an email exchange with his lawyer’s office, I learned that Dr. Gokal has received offers of employment but is unable to entertain them because the actions by the Harris County District Attorney triggered an automatic review by the Texas Medical Board. A GoFundMe page was launched, but an appreciative Dr. Gokal stated publicly that he’d rather the money go to a needy charity.
In the last paragraph of the Times article, Dr. Gokal asks, “How can I take it back?” referencing stories about “the Pakistani doctor in Houston who stole all those vaccines.”
Let’s help him take back his story. In helping him, perhaps we can take back a little control. We could start with letters of support that could be mailed to his lawyer, Paul Doyle, Esq., of Houston, or tweet, respectfully of course, to the district attorney @Kimoggforda.
We can also let the Harris County Public Health Department in Houston know what we think of their actions.
On Martin Luther King Day, Kim Ogg, the district attorney who charged Dr. Gokal, tweeted MLK’s famous quote: “Injustice anywhere is a threat to justice everywhere.”
Let that motivate us to action.
Melissa Walton-Shirley, MD, is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. In addition to opinion writing, she enjoys spending time with her husband, daughters and parents, and sidelines as a backing vocalist for local rock bands. A version of this article first appeared on Medscape.com.
Patients with asthma say most doctors don’t ask about cannabis use
Among individuals with asthma and allergies who use cannabis, more than half said they aren’t willing to discuss their use of cannabis with their doctor and their doctor doesn’t ask, according to recent research at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
In an online survey of respondents with asthma and allergies in the Allergy & Asthma Network, 88 of 489 (18.0%) reported cannabis use. Of these respondents, 37.5% said they wanted to discuss their cannabis use with their doctor, 51.1% said they would not want to, and 11.4% reported they were unsure. In addition, 40.9% of respondents said their doctor inquired about cannabis use, while 51.1% said their doctor did not bring up cannabis use at all, either through a verbal discussion or on an intake form.
To date, there has not been much research on use of cannabis among patients with allergies and asthma, Joanna S. Zeiger, MS, PhD, of the Canna Research Foundation in Boulder, Colo., said in her presentation. “This is a group with whom route of administration could have broad adverse effects. Smoking or vaping cannabis in this population could lead to increased symptoms of cough and wheeze, as well as increased use of asthma medications and exacerbations of their disease.”
Dr. Zeiger and colleagues recruited 489 respondents for the AAN Pain, Exercise, and Cannabis Experience Survey study through social media channels between May 2020 and September 2020. In the survey, the researchers asked questions about the nature of the respondent’s cannabis use (medical, recreational, or both), the types of cannabinoids used (tetrahydrocannabinol [THC], cannabidiol [CBD], or both), the route of administration (capsule, edible, oil/tincture, smoke, spray, topical, or vaporizer), and subjective effects. Most of the respondents reported using both THC and CBD, with smoking, edibles, and vaping being the most comment route of administration.
Of the 88 respondents who said they currently used cannabis, 60.2% were aged less than 50 years, 72.4% were women, and 71.6% were White. A majority of respondents had been using cannabis for 3 or more years (54.5%) , used it less than one time per day (60.2%), and used it for pain (68.2%). Current asthma was reported in 51 respondents (58.0%), and 39.2% had uncontrolled asthma. Half of those respondents with uncontrolled asthma reported smoking cannabis, and 25.0% reported coughing because of cannabis. Both THC and CBD were used by 47.7% of respondents; 33% reported THC use alone, while 19.3% used CBD alone.
Reported effects of cannabis use
The most common positive effects of using cannabis reported among respondents were that it helped with sleep (66 respondents), calmed them down (60 respondents), reduced pain (60 respondents), or decreased anxiety (59 respondents). Many respondents who reported positive effects were using both THC and CBD. For example, respondents who reported using cannabinoids for calming, 46.7% reported using both, compared with 36.7% who used THC only and 16.7% who used CBD only. Among respondents who reported that cannabis helped them sleep, 51.5% used both THC and CBD.
Regarding adverse effects, there were no significant differences based on use of THC or CBD, but 31.9% of respondents who said they smoked cannabis and 4.9% of respondents who used cannabis through a route of administration that wasn’t smoking reported they coughed with their cannabis use (P < .001). No respondents reported anaphyalaxis, although, among individuals who did not use cannabis, 2.5% reported a cannabis allergy.
‘Cannabis allergy is real’
Commenting on the research, Gordon L. Sussman MD, allergist, clinical immunologist, and clinical professor of medicine at the University of Toronto, said the survey is a thorough questionnaire that is likely representative of attitudes about cannabis in the United States and countries where cannabis is not broadly legalized.
Cannabis allergy, however, is not uncommon, and “is something that people should be aware of,” he said. “Cannabis IgE allergy is real, is probably fairly common, and is something that [clinicians] should be asking about routinely.”
One limitation of the research was not knowing the number of people who declined to answer the survey, as there may be a bias in the results toward people who want to answer the questions, compared with those who did not want to answer. “When you do a survey, only a certain number of people are going to answer, and [you also want input from] people that don’t answer,” Dr. Sussman said.
Dr. Sussman acknowledged it can be difficult to get patients to admit cannabis use, even in countries like Canada where it is legal. Surveys like the one administered by Dr. Zeiger and colleagues are “the first step” to getting updated assessments of cannabis attitudes and recommendations. “The next step is doing an international survey, so you get different countries’ viewpoints and perspectives,” he said.
This study was supported by the Allergy & Asthma Network and the Canna Research Foundation. Three authors are affiliated with the Canna Research Foundation. Dr. Sussman reported no financial conflicts of interest. Dr. Sussman participates in the International Cannabis Allergy KAP Collaboration, a group founded by one of the coauthors, William Silvers, MD, but Dr. Sussman was not involved with this study.
Among individuals with asthma and allergies who use cannabis, more than half said they aren’t willing to discuss their use of cannabis with their doctor and their doctor doesn’t ask, according to recent research at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
In an online survey of respondents with asthma and allergies in the Allergy & Asthma Network, 88 of 489 (18.0%) reported cannabis use. Of these respondents, 37.5% said they wanted to discuss their cannabis use with their doctor, 51.1% said they would not want to, and 11.4% reported they were unsure. In addition, 40.9% of respondents said their doctor inquired about cannabis use, while 51.1% said their doctor did not bring up cannabis use at all, either through a verbal discussion or on an intake form.
To date, there has not been much research on use of cannabis among patients with allergies and asthma, Joanna S. Zeiger, MS, PhD, of the Canna Research Foundation in Boulder, Colo., said in her presentation. “This is a group with whom route of administration could have broad adverse effects. Smoking or vaping cannabis in this population could lead to increased symptoms of cough and wheeze, as well as increased use of asthma medications and exacerbations of their disease.”
Dr. Zeiger and colleagues recruited 489 respondents for the AAN Pain, Exercise, and Cannabis Experience Survey study through social media channels between May 2020 and September 2020. In the survey, the researchers asked questions about the nature of the respondent’s cannabis use (medical, recreational, or both), the types of cannabinoids used (tetrahydrocannabinol [THC], cannabidiol [CBD], or both), the route of administration (capsule, edible, oil/tincture, smoke, spray, topical, or vaporizer), and subjective effects. Most of the respondents reported using both THC and CBD, with smoking, edibles, and vaping being the most comment route of administration.
Of the 88 respondents who said they currently used cannabis, 60.2% were aged less than 50 years, 72.4% were women, and 71.6% were White. A majority of respondents had been using cannabis for 3 or more years (54.5%) , used it less than one time per day (60.2%), and used it for pain (68.2%). Current asthma was reported in 51 respondents (58.0%), and 39.2% had uncontrolled asthma. Half of those respondents with uncontrolled asthma reported smoking cannabis, and 25.0% reported coughing because of cannabis. Both THC and CBD were used by 47.7% of respondents; 33% reported THC use alone, while 19.3% used CBD alone.
Reported effects of cannabis use
The most common positive effects of using cannabis reported among respondents were that it helped with sleep (66 respondents), calmed them down (60 respondents), reduced pain (60 respondents), or decreased anxiety (59 respondents). Many respondents who reported positive effects were using both THC and CBD. For example, respondents who reported using cannabinoids for calming, 46.7% reported using both, compared with 36.7% who used THC only and 16.7% who used CBD only. Among respondents who reported that cannabis helped them sleep, 51.5% used both THC and CBD.
Regarding adverse effects, there were no significant differences based on use of THC or CBD, but 31.9% of respondents who said they smoked cannabis and 4.9% of respondents who used cannabis through a route of administration that wasn’t smoking reported they coughed with their cannabis use (P < .001). No respondents reported anaphyalaxis, although, among individuals who did not use cannabis, 2.5% reported a cannabis allergy.
‘Cannabis allergy is real’
Commenting on the research, Gordon L. Sussman MD, allergist, clinical immunologist, and clinical professor of medicine at the University of Toronto, said the survey is a thorough questionnaire that is likely representative of attitudes about cannabis in the United States and countries where cannabis is not broadly legalized.
Cannabis allergy, however, is not uncommon, and “is something that people should be aware of,” he said. “Cannabis IgE allergy is real, is probably fairly common, and is something that [clinicians] should be asking about routinely.”
One limitation of the research was not knowing the number of people who declined to answer the survey, as there may be a bias in the results toward people who want to answer the questions, compared with those who did not want to answer. “When you do a survey, only a certain number of people are going to answer, and [you also want input from] people that don’t answer,” Dr. Sussman said.
Dr. Sussman acknowledged it can be difficult to get patients to admit cannabis use, even in countries like Canada where it is legal. Surveys like the one administered by Dr. Zeiger and colleagues are “the first step” to getting updated assessments of cannabis attitudes and recommendations. “The next step is doing an international survey, so you get different countries’ viewpoints and perspectives,” he said.
This study was supported by the Allergy & Asthma Network and the Canna Research Foundation. Three authors are affiliated with the Canna Research Foundation. Dr. Sussman reported no financial conflicts of interest. Dr. Sussman participates in the International Cannabis Allergy KAP Collaboration, a group founded by one of the coauthors, William Silvers, MD, but Dr. Sussman was not involved with this study.
Among individuals with asthma and allergies who use cannabis, more than half said they aren’t willing to discuss their use of cannabis with their doctor and their doctor doesn’t ask, according to recent research at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
In an online survey of respondents with asthma and allergies in the Allergy & Asthma Network, 88 of 489 (18.0%) reported cannabis use. Of these respondents, 37.5% said they wanted to discuss their cannabis use with their doctor, 51.1% said they would not want to, and 11.4% reported they were unsure. In addition, 40.9% of respondents said their doctor inquired about cannabis use, while 51.1% said their doctor did not bring up cannabis use at all, either through a verbal discussion or on an intake form.
To date, there has not been much research on use of cannabis among patients with allergies and asthma, Joanna S. Zeiger, MS, PhD, of the Canna Research Foundation in Boulder, Colo., said in her presentation. “This is a group with whom route of administration could have broad adverse effects. Smoking or vaping cannabis in this population could lead to increased symptoms of cough and wheeze, as well as increased use of asthma medications and exacerbations of their disease.”
Dr. Zeiger and colleagues recruited 489 respondents for the AAN Pain, Exercise, and Cannabis Experience Survey study through social media channels between May 2020 and September 2020. In the survey, the researchers asked questions about the nature of the respondent’s cannabis use (medical, recreational, or both), the types of cannabinoids used (tetrahydrocannabinol [THC], cannabidiol [CBD], or both), the route of administration (capsule, edible, oil/tincture, smoke, spray, topical, or vaporizer), and subjective effects. Most of the respondents reported using both THC and CBD, with smoking, edibles, and vaping being the most comment route of administration.
Of the 88 respondents who said they currently used cannabis, 60.2% were aged less than 50 years, 72.4% were women, and 71.6% were White. A majority of respondents had been using cannabis for 3 or more years (54.5%) , used it less than one time per day (60.2%), and used it for pain (68.2%). Current asthma was reported in 51 respondents (58.0%), and 39.2% had uncontrolled asthma. Half of those respondents with uncontrolled asthma reported smoking cannabis, and 25.0% reported coughing because of cannabis. Both THC and CBD were used by 47.7% of respondents; 33% reported THC use alone, while 19.3% used CBD alone.
Reported effects of cannabis use
The most common positive effects of using cannabis reported among respondents were that it helped with sleep (66 respondents), calmed them down (60 respondents), reduced pain (60 respondents), or decreased anxiety (59 respondents). Many respondents who reported positive effects were using both THC and CBD. For example, respondents who reported using cannabinoids for calming, 46.7% reported using both, compared with 36.7% who used THC only and 16.7% who used CBD only. Among respondents who reported that cannabis helped them sleep, 51.5% used both THC and CBD.
Regarding adverse effects, there were no significant differences based on use of THC or CBD, but 31.9% of respondents who said they smoked cannabis and 4.9% of respondents who used cannabis through a route of administration that wasn’t smoking reported they coughed with their cannabis use (P < .001). No respondents reported anaphyalaxis, although, among individuals who did not use cannabis, 2.5% reported a cannabis allergy.
‘Cannabis allergy is real’
Commenting on the research, Gordon L. Sussman MD, allergist, clinical immunologist, and clinical professor of medicine at the University of Toronto, said the survey is a thorough questionnaire that is likely representative of attitudes about cannabis in the United States and countries where cannabis is not broadly legalized.
Cannabis allergy, however, is not uncommon, and “is something that people should be aware of,” he said. “Cannabis IgE allergy is real, is probably fairly common, and is something that [clinicians] should be asking about routinely.”
One limitation of the research was not knowing the number of people who declined to answer the survey, as there may be a bias in the results toward people who want to answer the questions, compared with those who did not want to answer. “When you do a survey, only a certain number of people are going to answer, and [you also want input from] people that don’t answer,” Dr. Sussman said.
Dr. Sussman acknowledged it can be difficult to get patients to admit cannabis use, even in countries like Canada where it is legal. Surveys like the one administered by Dr. Zeiger and colleagues are “the first step” to getting updated assessments of cannabis attitudes and recommendations. “The next step is doing an international survey, so you get different countries’ viewpoints and perspectives,” he said.
This study was supported by the Allergy & Asthma Network and the Canna Research Foundation. Three authors are affiliated with the Canna Research Foundation. Dr. Sussman reported no financial conflicts of interest. Dr. Sussman participates in the International Cannabis Allergy KAP Collaboration, a group founded by one of the coauthors, William Silvers, MD, but Dr. Sussman was not involved with this study.
FROM AAAAI 2021
COVID-19 vaccination linked to less mechanical ventilation
new evidence reveals.
Compared with residents younger than 50 – so far vaccinated at lower rates than those of the higher-risk older people – Israelis 70 and older were 67% less likely to require mechanical ventilation for SARS-CoV-2 infection in February 2021 compared with October-December 2020.
“This study provides preliminary evidence at the population level for the reduction in risk for severe COVID-19, as manifested by need for mechanical ventilation, after vaccination with the Pfizer-BioNTech COVID-19 vaccine,” wrote lead author Ehud Rinott, department of public health, faculty of health sciences, Ben-Gurion University of the Negev in Beer-Sheva, Israel, and colleagues.
The study was published online Feb. 26, 2021, in Morbidity and Mortality Weekly Report.
The progress of COVID-19 vaccination across Israel presents researchers with a unique opportunity to study effectiveness on a population level. In this study, 84% of residents 70 and older received two-dose vaccinations. In contrast, only 10% of people in Israel younger than 50 received the same vaccine coverage.
Along with senior author Yair Lewis, MD, PhD, and coauthor Ilan Youngster, MD, Mr. Rinott compared mechanical ventilation rates between Oct. 2, 2020, and Feb. 9, 2021. They found that the ratio of people 70 and older compared with those younger than 50 requiring mechanical ventilation changed from 5.8:1 to 1.9:1 between these periods. This translates to the 67% decrease.
The study offers a “real-world” look at vaccination effectiveness, adding to more controlled evidence from clinical trials. “Achieving high vaccination coverage through intensive vaccination campaigns has the potential to substantially reduce COVID-19-associated morbidity and mortality,” the researchers wrote.
Israel started a national vaccination program on Dec. 20, 2020, targeting high-risk residents including people 60 and older, health care workers, and those with relevant comorbidities. At the same time, in addition to immunization, Israel has used strategies like stay-at-home orders, school closures, mask mandates, and more.
Potential limitations include a limited ability to account for the effect of the stay-at-home orders, spread of virus variants, and other concomitant factors; a potential for a delayed reporting of cases; and variability in mitigation measures by age group.
Dr. Youngster reported receipt of consulting fees from MyBiotix Ltd.
A version of this article first appeared on Medscape.com.
new evidence reveals.
Compared with residents younger than 50 – so far vaccinated at lower rates than those of the higher-risk older people – Israelis 70 and older were 67% less likely to require mechanical ventilation for SARS-CoV-2 infection in February 2021 compared with October-December 2020.
“This study provides preliminary evidence at the population level for the reduction in risk for severe COVID-19, as manifested by need for mechanical ventilation, after vaccination with the Pfizer-BioNTech COVID-19 vaccine,” wrote lead author Ehud Rinott, department of public health, faculty of health sciences, Ben-Gurion University of the Negev in Beer-Sheva, Israel, and colleagues.
The study was published online Feb. 26, 2021, in Morbidity and Mortality Weekly Report.
The progress of COVID-19 vaccination across Israel presents researchers with a unique opportunity to study effectiveness on a population level. In this study, 84% of residents 70 and older received two-dose vaccinations. In contrast, only 10% of people in Israel younger than 50 received the same vaccine coverage.
Along with senior author Yair Lewis, MD, PhD, and coauthor Ilan Youngster, MD, Mr. Rinott compared mechanical ventilation rates between Oct. 2, 2020, and Feb. 9, 2021. They found that the ratio of people 70 and older compared with those younger than 50 requiring mechanical ventilation changed from 5.8:1 to 1.9:1 between these periods. This translates to the 67% decrease.
The study offers a “real-world” look at vaccination effectiveness, adding to more controlled evidence from clinical trials. “Achieving high vaccination coverage through intensive vaccination campaigns has the potential to substantially reduce COVID-19-associated morbidity and mortality,” the researchers wrote.
Israel started a national vaccination program on Dec. 20, 2020, targeting high-risk residents including people 60 and older, health care workers, and those with relevant comorbidities. At the same time, in addition to immunization, Israel has used strategies like stay-at-home orders, school closures, mask mandates, and more.
Potential limitations include a limited ability to account for the effect of the stay-at-home orders, spread of virus variants, and other concomitant factors; a potential for a delayed reporting of cases; and variability in mitigation measures by age group.
Dr. Youngster reported receipt of consulting fees from MyBiotix Ltd.
A version of this article first appeared on Medscape.com.
new evidence reveals.
Compared with residents younger than 50 – so far vaccinated at lower rates than those of the higher-risk older people – Israelis 70 and older were 67% less likely to require mechanical ventilation for SARS-CoV-2 infection in February 2021 compared with October-December 2020.
“This study provides preliminary evidence at the population level for the reduction in risk for severe COVID-19, as manifested by need for mechanical ventilation, after vaccination with the Pfizer-BioNTech COVID-19 vaccine,” wrote lead author Ehud Rinott, department of public health, faculty of health sciences, Ben-Gurion University of the Negev in Beer-Sheva, Israel, and colleagues.
The study was published online Feb. 26, 2021, in Morbidity and Mortality Weekly Report.
The progress of COVID-19 vaccination across Israel presents researchers with a unique opportunity to study effectiveness on a population level. In this study, 84% of residents 70 and older received two-dose vaccinations. In contrast, only 10% of people in Israel younger than 50 received the same vaccine coverage.
Along with senior author Yair Lewis, MD, PhD, and coauthor Ilan Youngster, MD, Mr. Rinott compared mechanical ventilation rates between Oct. 2, 2020, and Feb. 9, 2021. They found that the ratio of people 70 and older compared with those younger than 50 requiring mechanical ventilation changed from 5.8:1 to 1.9:1 between these periods. This translates to the 67% decrease.
The study offers a “real-world” look at vaccination effectiveness, adding to more controlled evidence from clinical trials. “Achieving high vaccination coverage through intensive vaccination campaigns has the potential to substantially reduce COVID-19-associated morbidity and mortality,” the researchers wrote.
Israel started a national vaccination program on Dec. 20, 2020, targeting high-risk residents including people 60 and older, health care workers, and those with relevant comorbidities. At the same time, in addition to immunization, Israel has used strategies like stay-at-home orders, school closures, mask mandates, and more.
Potential limitations include a limited ability to account for the effect of the stay-at-home orders, spread of virus variants, and other concomitant factors; a potential for a delayed reporting of cases; and variability in mitigation measures by age group.
Dr. Youngster reported receipt of consulting fees from MyBiotix Ltd.
A version of this article first appeared on Medscape.com.
Peanut sublingual immunotherapy feasible and effective in toddlers
Sublingual immunotherapy for the treatment of peanut allergy is safe and effective, even in children as young as age 1 year.
In a double-blind, placebo-controlled, food challenge (DBPCFC) of some 36 peanut-allergic children (mean age 2.2 years, range 1-4 years), those who were randomly assigned to receive peanut sublingual immunotherapy (PNSLIT) showed significant desensitization compared with those who received placebo.
In addition, there was a “strong potential” for sustained unresponsiveness at 3 months for the toddlers who received the active treatment.
The findings were presented in a late breaking oral abstract session at the 2021 American Academy of Allergy, Asthma & Immunology virtual annual meeting (Abstract L2).
“A year ago, the Food and Drug Administration approved the oral agent Palforzia (peanut allergen powder) for the treatment of peanut allergy in children 4 and older, and it is a great option, but I think what we have learned over time is that this approach is not for everybody,” Edwin H. Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill, said in an interview.
Palforzia is a powder that is mixed in food like yogurt or pudding which the child then eats daily, according to a rigorous schedule. But Palforzia treatment presents some difficulties.
“Palforzia requires getting the powder dose, mixing it with food, like pudding or apple sauce, then eating it, which can take up to 30 minutes depending on age and kids’ cooperation. It tastes and smells like peanut which can cause aversion. Kids have to refrain from exercise or strenuous activity for at least 30 minutes before and after dosing and have to be observed for up to 2 hours post dose for symptoms,” Dr. Kim said.
“It’s a great drug, but the treatment could be overly difficult for certain families to be able to do, and in some cases the side effects may be more than certain patients are able or willing to handle, so there is a real urgent need for alternative approaches,” Dr. Kim said. “SLIT is several drops under the tongue, held for 2 minutes, swallowed and done.”
In the current placebo-controlled study, he and his group tested the feasibility, efficacy, and safety of the sublingual approach to peanut allergy in children age 4 years and younger.
Both groups were similar with regard to gender, race, ethnicity, atopic history, peanut skin prick test, and qualifying DBPCFC, and all children were previously allergic with positive blood and skin tests, with a positive reaction during baseline food challenge, thus proving the allergy and establishing the baseline threshold.
“We have learned from some studies, for instance the DEVIL and LEAP studies, that strongly suggest that the immune systems in younger patients may be more amenable to change, and there may be some justification for early intervention,” he said.
“Based on both of those ideas, we wanted to take our sublingual approach, which we have shown to have a pretty good efficacy in older children, and bring it down to this younger group and see if it still could have the same efficacy and also maintain what seems to be a very good safety signal.”
The researchers randomly assigned the children to receive PNSLIT at a daily maintenance dose of 4 mg peanut protein (n = 19) or to receive placebo (n = 17) for 36 months.
“There was a 5- to 6-month buildup period where the SLIT dose was increased every 1-2 weeks up to the target dose of 4 mg, and then the final dose of 4 mg was continued through to the end of the study,” Dr. Kim noted.
Over a total of 20,593 potential dosing days, the children took 91.2% of SLIT doses and 93.5% of placebo doses.
At the end of the 3-year study period, the children were challenged by DBPCFC with up to 4,333 mg of peanut protein.
Sustained unresponsiveness was assessed by an identical DBPCFC after discontinuation of the immunotherapy for 3 months.
Cumulative tolerated dose increased from a median of 143 mg to 4,443 mg in the PNSLIT group, compared with a median of 43 mg to 143 mg in the placebo group (P < .0001).
Fourteen of the children receiving PNSLIT, and none of the children receiving placebo, passed the desensitization food challenge. Twelve of the children receiving PNSLIT and two of the children receiving placebo passed the sustained unresponsiveness challenge.
Children who underwent the immunotherapy saw a decrease in their peanut skin prick test from 10 mm to 3.25 mm, compared to an increase from 11.5 mm to 12 mm with placebo (P < .0001).
The most common side effect reported was itching or irritation in the mouth. Most side effects resolved on their own, although some patients used an antihistamine. Getting children as young as 1 to hold the dose under their tongue was a challenge in some instances, but it eventually worked out, Dr. Kim said.
“It took a lot of work from the parents as well as from our research coordinators in trying to train these young kids to, first of all, allow us to put the peanut medication in the mouth and then to try as best as possible to keep it in their mouth for up to 2 minutes, but the families involved in our study were very dedicated and so we were able to get through that,” he said.
Study merits larger numbers
“Among the 36 who completed the 3 years of therapy, the authors report significant rates of desensitization among treated children compared with those receiving placebo. Furthermore, this effect was persistent for at least 3 months after stopping therapy in a subgroup of the children,” said Leonard B. Bacharier, MD, director of the Center for Pediatric Asthma, Monroe Carell Jr. Children’s Hospital at Vanderbilt, Nashville, Tenn.
“Overall, these findings suggest the promise of peanut SLIT, which should be studied in larger numbers of preschool children,” Dr. Bacharier, who was not part of the study, said in an interview.
Jonathan A. Bernstein, MD, professor of medicine, University of Cincinnati, agreed.
“It’s a well-designed study, it’s small, but it’s promising,” Dr. Bernstein, who was not involved with the study, said in an interview.
“They did show that most of the patients who got the sublingual therapy were able to get to the target dose and develop tolerance, so I think it’s promising. We know that this stuff works. This is just more data from a well-controlled study in a younger population,” he said.
“We do OIT [oral immunotherapy] and sublingual but we don’t do it in such young children in our practice. The youngest is 3 years old, because they have to understand what is going on and cooperate. If they don’t cooperate it’s not possible.”
Dr. Kim reported financial relationships with DBV Technologies, Kenota Health, Ukko, Aimmune Therapeutics, ALK, AllerGenis, Belhaven Pharma, Duke Clinical Research Institute, Nutricia, NIH/NIAID, NIH/NCCIH, NIH/Immune Tolerance Network, FARE, and the Wallace Foundation. Dr. Bacharier and Dr. Bernstein have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sublingual immunotherapy for the treatment of peanut allergy is safe and effective, even in children as young as age 1 year.
In a double-blind, placebo-controlled, food challenge (DBPCFC) of some 36 peanut-allergic children (mean age 2.2 years, range 1-4 years), those who were randomly assigned to receive peanut sublingual immunotherapy (PNSLIT) showed significant desensitization compared with those who received placebo.
In addition, there was a “strong potential” for sustained unresponsiveness at 3 months for the toddlers who received the active treatment.
The findings were presented in a late breaking oral abstract session at the 2021 American Academy of Allergy, Asthma & Immunology virtual annual meeting (Abstract L2).
“A year ago, the Food and Drug Administration approved the oral agent Palforzia (peanut allergen powder) for the treatment of peanut allergy in children 4 and older, and it is a great option, but I think what we have learned over time is that this approach is not for everybody,” Edwin H. Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill, said in an interview.
Palforzia is a powder that is mixed in food like yogurt or pudding which the child then eats daily, according to a rigorous schedule. But Palforzia treatment presents some difficulties.
“Palforzia requires getting the powder dose, mixing it with food, like pudding or apple sauce, then eating it, which can take up to 30 minutes depending on age and kids’ cooperation. It tastes and smells like peanut which can cause aversion. Kids have to refrain from exercise or strenuous activity for at least 30 minutes before and after dosing and have to be observed for up to 2 hours post dose for symptoms,” Dr. Kim said.
“It’s a great drug, but the treatment could be overly difficult for certain families to be able to do, and in some cases the side effects may be more than certain patients are able or willing to handle, so there is a real urgent need for alternative approaches,” Dr. Kim said. “SLIT is several drops under the tongue, held for 2 minutes, swallowed and done.”
In the current placebo-controlled study, he and his group tested the feasibility, efficacy, and safety of the sublingual approach to peanut allergy in children age 4 years and younger.
Both groups were similar with regard to gender, race, ethnicity, atopic history, peanut skin prick test, and qualifying DBPCFC, and all children were previously allergic with positive blood and skin tests, with a positive reaction during baseline food challenge, thus proving the allergy and establishing the baseline threshold.
“We have learned from some studies, for instance the DEVIL and LEAP studies, that strongly suggest that the immune systems in younger patients may be more amenable to change, and there may be some justification for early intervention,” he said.
“Based on both of those ideas, we wanted to take our sublingual approach, which we have shown to have a pretty good efficacy in older children, and bring it down to this younger group and see if it still could have the same efficacy and also maintain what seems to be a very good safety signal.”
The researchers randomly assigned the children to receive PNSLIT at a daily maintenance dose of 4 mg peanut protein (n = 19) or to receive placebo (n = 17) for 36 months.
“There was a 5- to 6-month buildup period where the SLIT dose was increased every 1-2 weeks up to the target dose of 4 mg, and then the final dose of 4 mg was continued through to the end of the study,” Dr. Kim noted.
Over a total of 20,593 potential dosing days, the children took 91.2% of SLIT doses and 93.5% of placebo doses.
At the end of the 3-year study period, the children were challenged by DBPCFC with up to 4,333 mg of peanut protein.
Sustained unresponsiveness was assessed by an identical DBPCFC after discontinuation of the immunotherapy for 3 months.
Cumulative tolerated dose increased from a median of 143 mg to 4,443 mg in the PNSLIT group, compared with a median of 43 mg to 143 mg in the placebo group (P < .0001).
Fourteen of the children receiving PNSLIT, and none of the children receiving placebo, passed the desensitization food challenge. Twelve of the children receiving PNSLIT and two of the children receiving placebo passed the sustained unresponsiveness challenge.
Children who underwent the immunotherapy saw a decrease in their peanut skin prick test from 10 mm to 3.25 mm, compared to an increase from 11.5 mm to 12 mm with placebo (P < .0001).
The most common side effect reported was itching or irritation in the mouth. Most side effects resolved on their own, although some patients used an antihistamine. Getting children as young as 1 to hold the dose under their tongue was a challenge in some instances, but it eventually worked out, Dr. Kim said.
“It took a lot of work from the parents as well as from our research coordinators in trying to train these young kids to, first of all, allow us to put the peanut medication in the mouth and then to try as best as possible to keep it in their mouth for up to 2 minutes, but the families involved in our study were very dedicated and so we were able to get through that,” he said.
Study merits larger numbers
“Among the 36 who completed the 3 years of therapy, the authors report significant rates of desensitization among treated children compared with those receiving placebo. Furthermore, this effect was persistent for at least 3 months after stopping therapy in a subgroup of the children,” said Leonard B. Bacharier, MD, director of the Center for Pediatric Asthma, Monroe Carell Jr. Children’s Hospital at Vanderbilt, Nashville, Tenn.
“Overall, these findings suggest the promise of peanut SLIT, which should be studied in larger numbers of preschool children,” Dr. Bacharier, who was not part of the study, said in an interview.
Jonathan A. Bernstein, MD, professor of medicine, University of Cincinnati, agreed.
“It’s a well-designed study, it’s small, but it’s promising,” Dr. Bernstein, who was not involved with the study, said in an interview.
“They did show that most of the patients who got the sublingual therapy were able to get to the target dose and develop tolerance, so I think it’s promising. We know that this stuff works. This is just more data from a well-controlled study in a younger population,” he said.
“We do OIT [oral immunotherapy] and sublingual but we don’t do it in such young children in our practice. The youngest is 3 years old, because they have to understand what is going on and cooperate. If they don’t cooperate it’s not possible.”
Dr. Kim reported financial relationships with DBV Technologies, Kenota Health, Ukko, Aimmune Therapeutics, ALK, AllerGenis, Belhaven Pharma, Duke Clinical Research Institute, Nutricia, NIH/NIAID, NIH/NCCIH, NIH/Immune Tolerance Network, FARE, and the Wallace Foundation. Dr. Bacharier and Dr. Bernstein have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sublingual immunotherapy for the treatment of peanut allergy is safe and effective, even in children as young as age 1 year.
In a double-blind, placebo-controlled, food challenge (DBPCFC) of some 36 peanut-allergic children (mean age 2.2 years, range 1-4 years), those who were randomly assigned to receive peanut sublingual immunotherapy (PNSLIT) showed significant desensitization compared with those who received placebo.
In addition, there was a “strong potential” for sustained unresponsiveness at 3 months for the toddlers who received the active treatment.
The findings were presented in a late breaking oral abstract session at the 2021 American Academy of Allergy, Asthma & Immunology virtual annual meeting (Abstract L2).
“A year ago, the Food and Drug Administration approved the oral agent Palforzia (peanut allergen powder) for the treatment of peanut allergy in children 4 and older, and it is a great option, but I think what we have learned over time is that this approach is not for everybody,” Edwin H. Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill, said in an interview.
Palforzia is a powder that is mixed in food like yogurt or pudding which the child then eats daily, according to a rigorous schedule. But Palforzia treatment presents some difficulties.
“Palforzia requires getting the powder dose, mixing it with food, like pudding or apple sauce, then eating it, which can take up to 30 minutes depending on age and kids’ cooperation. It tastes and smells like peanut which can cause aversion. Kids have to refrain from exercise or strenuous activity for at least 30 minutes before and after dosing and have to be observed for up to 2 hours post dose for symptoms,” Dr. Kim said.
“It’s a great drug, but the treatment could be overly difficult for certain families to be able to do, and in some cases the side effects may be more than certain patients are able or willing to handle, so there is a real urgent need for alternative approaches,” Dr. Kim said. “SLIT is several drops under the tongue, held for 2 minutes, swallowed and done.”
In the current placebo-controlled study, he and his group tested the feasibility, efficacy, and safety of the sublingual approach to peanut allergy in children age 4 years and younger.
Both groups were similar with regard to gender, race, ethnicity, atopic history, peanut skin prick test, and qualifying DBPCFC, and all children were previously allergic with positive blood and skin tests, with a positive reaction during baseline food challenge, thus proving the allergy and establishing the baseline threshold.
“We have learned from some studies, for instance the DEVIL and LEAP studies, that strongly suggest that the immune systems in younger patients may be more amenable to change, and there may be some justification for early intervention,” he said.
“Based on both of those ideas, we wanted to take our sublingual approach, which we have shown to have a pretty good efficacy in older children, and bring it down to this younger group and see if it still could have the same efficacy and also maintain what seems to be a very good safety signal.”
The researchers randomly assigned the children to receive PNSLIT at a daily maintenance dose of 4 mg peanut protein (n = 19) or to receive placebo (n = 17) for 36 months.
“There was a 5- to 6-month buildup period where the SLIT dose was increased every 1-2 weeks up to the target dose of 4 mg, and then the final dose of 4 mg was continued through to the end of the study,” Dr. Kim noted.
Over a total of 20,593 potential dosing days, the children took 91.2% of SLIT doses and 93.5% of placebo doses.
At the end of the 3-year study period, the children were challenged by DBPCFC with up to 4,333 mg of peanut protein.
Sustained unresponsiveness was assessed by an identical DBPCFC after discontinuation of the immunotherapy for 3 months.
Cumulative tolerated dose increased from a median of 143 mg to 4,443 mg in the PNSLIT group, compared with a median of 43 mg to 143 mg in the placebo group (P < .0001).
Fourteen of the children receiving PNSLIT, and none of the children receiving placebo, passed the desensitization food challenge. Twelve of the children receiving PNSLIT and two of the children receiving placebo passed the sustained unresponsiveness challenge.
Children who underwent the immunotherapy saw a decrease in their peanut skin prick test from 10 mm to 3.25 mm, compared to an increase from 11.5 mm to 12 mm with placebo (P < .0001).
The most common side effect reported was itching or irritation in the mouth. Most side effects resolved on their own, although some patients used an antihistamine. Getting children as young as 1 to hold the dose under their tongue was a challenge in some instances, but it eventually worked out, Dr. Kim said.
“It took a lot of work from the parents as well as from our research coordinators in trying to train these young kids to, first of all, allow us to put the peanut medication in the mouth and then to try as best as possible to keep it in their mouth for up to 2 minutes, but the families involved in our study were very dedicated and so we were able to get through that,” he said.
Study merits larger numbers
“Among the 36 who completed the 3 years of therapy, the authors report significant rates of desensitization among treated children compared with those receiving placebo. Furthermore, this effect was persistent for at least 3 months after stopping therapy in a subgroup of the children,” said Leonard B. Bacharier, MD, director of the Center for Pediatric Asthma, Monroe Carell Jr. Children’s Hospital at Vanderbilt, Nashville, Tenn.
“Overall, these findings suggest the promise of peanut SLIT, which should be studied in larger numbers of preschool children,” Dr. Bacharier, who was not part of the study, said in an interview.
Jonathan A. Bernstein, MD, professor of medicine, University of Cincinnati, agreed.
“It’s a well-designed study, it’s small, but it’s promising,” Dr. Bernstein, who was not involved with the study, said in an interview.
“They did show that most of the patients who got the sublingual therapy were able to get to the target dose and develop tolerance, so I think it’s promising. We know that this stuff works. This is just more data from a well-controlled study in a younger population,” he said.
“We do OIT [oral immunotherapy] and sublingual but we don’t do it in such young children in our practice. The youngest is 3 years old, because they have to understand what is going on and cooperate. If they don’t cooperate it’s not possible.”
Dr. Kim reported financial relationships with DBV Technologies, Kenota Health, Ukko, Aimmune Therapeutics, ALK, AllerGenis, Belhaven Pharma, Duke Clinical Research Institute, Nutricia, NIH/NIAID, NIH/NCCIH, NIH/Immune Tolerance Network, FARE, and the Wallace Foundation. Dr. Bacharier and Dr. Bernstein have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAAAI
Heavier girls hit hormonal puberty earlier, but develop breasts later
Girls with more body fat experienced earlier menarche and hormone changes, but later full breast development, compared with those with normal weight, according to longitudinal data from 90 girls aged 8-15 years.
A link between obesity and early puberty has been observed among U.S. girls for decades, but more recent studies suggest that “girls with greater childhood adiposity have earlier thelarche and progress through puberty at a faster rate than normal weight girls,” wrote Madison T. Ortega, MD, of the National Institute of Environmental Health Sciences, Durham, N.C., and colleagues. However, studies involving hormone levels have yielded mixed results, they said.
In a study published in the Journal of Clinical Endocrinology & Metabolism , the researchers followed 36 girls with overweight or obesity and 54 girls with normal weight for 4 years; normal weight was defined as body mass index in the 5th to 85th percentile, overweight was defined as BMI in the 85th to 95th percentile, and obese was defined as greater than 95th percentile. Overweight and obese were combined into one category for comparison with normal weight girls.
Participants had an average of 2.8 study visits during this period and provided additional information by phone and online. Visits included measurement of total body fat using dual-energy x-ray absorptiometry (DXA), Tanner staging, breast ultrasound for morphological staging (BMORPH; A-E), pelvic ultrasound, hormone tests, and menarchal status assessment.
Overall, girls with overweight/obesity (OW/OB) had significantly more advanced breast development at baseline than did those with normal weight (NW), but these girls progressed through BMORPH stage D later than did NW girls. Early-stage breast development was not affected by total body fat. However, “an increase of 5 percentage points in mean total body fat, for example, was associated with a 26% decrease in the transition rate out of stage D,” the researchers noted.
Hormone levels were similar at baseline for follicle-stimulating hormone, inhibin B, estrone (E1), total and free testosterone, and androstenedione. However, these levels increased more quickly after 1 year for girls with OW/OB, while they plateaued in girls with NW and dropped among girls with lower total body fat. Total body fat had no apparent effect on other reproductive hormones including luteinizing hormone, modified vaginal maturation index, and estradiol 2.
The average age of menarche was 12.4 years across all participants, but girls with higher total body fat at baseline were more likely to reach menarche at a younger age. “For every 1-unit increase in visit one total body fat, the chance of achieving menarche at any given time point was 3% higher,” the researchers said. No interaction appeared between race and total body fat with regard to menarche.
Several surprising findings
The study is important because “there have been no longitudinal studies in U.S. girls to examine how total body fat affects serum reproductive hormones or the development of the breast and ovaries using ultrasound imaging,” corresponding author Natalie Shaw, MD, of the National Institute of Environmental Health Sciences, said in an interview.
Dr. Shaw said she was surprised by several of the study findings. “Others have reported increased male-like hormones (androgens) in overweight/obese girls in cross-sectional studies; however, we were surprised to find that FSH and inhibin B were also elevated in girls with excess body fat,” she said. “We also found, unexpectedly, that even though the breast bud appears earlier in overweight/obese girls (thelarche), which signals the onset of puberty, the breast matured more slowly during the course of puberty in overweight/obese girls compared with normal weight girls,” she noted.
“The main take-home message is that puberty looks different in girls with excess body fat; they develop breast tissue earlier, yet take longer to achieve a fully mature breast, and they undergo menarche earlier,” Dr. Shaw said. Clinicians should be aware of the hormonal differences based on body fat, Dr. Shaw emphasized. “Girls with greater body fat had higher levels of FSH (a pituitary hormone), inhibin B (an ovarian hormone), and male-like reproductive hormones (e.g., testosterone) that are made by the adrenal glands and the ovaries in the late stages of puberty,” she said.
Potential implications for adulthood
“The findings in this study contribute to better understanding how total body fat impacts hormonal findings of puberty,” M. Susan Jay, MD, of the Medical College of Wisconsin and Children’s Hospital of Wisconsin, Milwaukee, said in an interview. “Prior studies have linked weight gain as a factor that contributes to pubertal development, but this study is attempting to longitudinally investigate how body weight may affect clinical and biochemical pubertal markers in girls,” she noted.
“The take-home message is that this study and other earlier studies have illustrated that puberty is not a fixed pattern in all individual girls,” Dr. Jay emphasized. “Rather, there are environmental factors which can impact pubertal course,” she said. “In effect, there are pathways through puberty in individual adolescents that require greater ongoing studies to further identify the arc of puberty and the impact of how the length in various stages may affect exposure to estrogen and other neurohormonal factors,” she explained. These factors impact not only adolescence but also future health in adulthood, she said.
“Ongoing prospective studies are needed to identify how factors such as body weight can affect adolescent pubertal development and the possible impact long after adolescence for health issues such as breast cancer,” Dr. Jay added.
The study findings were limited by several factors including the available data from only two completed study visits for most participants, as well as the racial differences among body weight groups and lack of standardized timing for blood draws, the researchers noted.
The study was supported in part by the National Institute of Environmental Health Sciences, and corresponding author Dr. Shaw disclosed support as a Lasker Clinical Research Scholar. The other researchers, as well as Dr. Jay, had no disclosures.
Girls with more body fat experienced earlier menarche and hormone changes, but later full breast development, compared with those with normal weight, according to longitudinal data from 90 girls aged 8-15 years.
A link between obesity and early puberty has been observed among U.S. girls for decades, but more recent studies suggest that “girls with greater childhood adiposity have earlier thelarche and progress through puberty at a faster rate than normal weight girls,” wrote Madison T. Ortega, MD, of the National Institute of Environmental Health Sciences, Durham, N.C., and colleagues. However, studies involving hormone levels have yielded mixed results, they said.
In a study published in the Journal of Clinical Endocrinology & Metabolism , the researchers followed 36 girls with overweight or obesity and 54 girls with normal weight for 4 years; normal weight was defined as body mass index in the 5th to 85th percentile, overweight was defined as BMI in the 85th to 95th percentile, and obese was defined as greater than 95th percentile. Overweight and obese were combined into one category for comparison with normal weight girls.
Participants had an average of 2.8 study visits during this period and provided additional information by phone and online. Visits included measurement of total body fat using dual-energy x-ray absorptiometry (DXA), Tanner staging, breast ultrasound for morphological staging (BMORPH; A-E), pelvic ultrasound, hormone tests, and menarchal status assessment.
Overall, girls with overweight/obesity (OW/OB) had significantly more advanced breast development at baseline than did those with normal weight (NW), but these girls progressed through BMORPH stage D later than did NW girls. Early-stage breast development was not affected by total body fat. However, “an increase of 5 percentage points in mean total body fat, for example, was associated with a 26% decrease in the transition rate out of stage D,” the researchers noted.
Hormone levels were similar at baseline for follicle-stimulating hormone, inhibin B, estrone (E1), total and free testosterone, and androstenedione. However, these levels increased more quickly after 1 year for girls with OW/OB, while they plateaued in girls with NW and dropped among girls with lower total body fat. Total body fat had no apparent effect on other reproductive hormones including luteinizing hormone, modified vaginal maturation index, and estradiol 2.
The average age of menarche was 12.4 years across all participants, but girls with higher total body fat at baseline were more likely to reach menarche at a younger age. “For every 1-unit increase in visit one total body fat, the chance of achieving menarche at any given time point was 3% higher,” the researchers said. No interaction appeared between race and total body fat with regard to menarche.
Several surprising findings
The study is important because “there have been no longitudinal studies in U.S. girls to examine how total body fat affects serum reproductive hormones or the development of the breast and ovaries using ultrasound imaging,” corresponding author Natalie Shaw, MD, of the National Institute of Environmental Health Sciences, said in an interview.
Dr. Shaw said she was surprised by several of the study findings. “Others have reported increased male-like hormones (androgens) in overweight/obese girls in cross-sectional studies; however, we were surprised to find that FSH and inhibin B were also elevated in girls with excess body fat,” she said. “We also found, unexpectedly, that even though the breast bud appears earlier in overweight/obese girls (thelarche), which signals the onset of puberty, the breast matured more slowly during the course of puberty in overweight/obese girls compared with normal weight girls,” she noted.
“The main take-home message is that puberty looks different in girls with excess body fat; they develop breast tissue earlier, yet take longer to achieve a fully mature breast, and they undergo menarche earlier,” Dr. Shaw said. Clinicians should be aware of the hormonal differences based on body fat, Dr. Shaw emphasized. “Girls with greater body fat had higher levels of FSH (a pituitary hormone), inhibin B (an ovarian hormone), and male-like reproductive hormones (e.g., testosterone) that are made by the adrenal glands and the ovaries in the late stages of puberty,” she said.
Potential implications for adulthood
“The findings in this study contribute to better understanding how total body fat impacts hormonal findings of puberty,” M. Susan Jay, MD, of the Medical College of Wisconsin and Children’s Hospital of Wisconsin, Milwaukee, said in an interview. “Prior studies have linked weight gain as a factor that contributes to pubertal development, but this study is attempting to longitudinally investigate how body weight may affect clinical and biochemical pubertal markers in girls,” she noted.
“The take-home message is that this study and other earlier studies have illustrated that puberty is not a fixed pattern in all individual girls,” Dr. Jay emphasized. “Rather, there are environmental factors which can impact pubertal course,” she said. “In effect, there are pathways through puberty in individual adolescents that require greater ongoing studies to further identify the arc of puberty and the impact of how the length in various stages may affect exposure to estrogen and other neurohormonal factors,” she explained. These factors impact not only adolescence but also future health in adulthood, she said.
“Ongoing prospective studies are needed to identify how factors such as body weight can affect adolescent pubertal development and the possible impact long after adolescence for health issues such as breast cancer,” Dr. Jay added.
The study findings were limited by several factors including the available data from only two completed study visits for most participants, as well as the racial differences among body weight groups and lack of standardized timing for blood draws, the researchers noted.
The study was supported in part by the National Institute of Environmental Health Sciences, and corresponding author Dr. Shaw disclosed support as a Lasker Clinical Research Scholar. The other researchers, as well as Dr. Jay, had no disclosures.
Girls with more body fat experienced earlier menarche and hormone changes, but later full breast development, compared with those with normal weight, according to longitudinal data from 90 girls aged 8-15 years.
A link between obesity and early puberty has been observed among U.S. girls for decades, but more recent studies suggest that “girls with greater childhood adiposity have earlier thelarche and progress through puberty at a faster rate than normal weight girls,” wrote Madison T. Ortega, MD, of the National Institute of Environmental Health Sciences, Durham, N.C., and colleagues. However, studies involving hormone levels have yielded mixed results, they said.
In a study published in the Journal of Clinical Endocrinology & Metabolism , the researchers followed 36 girls with overweight or obesity and 54 girls with normal weight for 4 years; normal weight was defined as body mass index in the 5th to 85th percentile, overweight was defined as BMI in the 85th to 95th percentile, and obese was defined as greater than 95th percentile. Overweight and obese were combined into one category for comparison with normal weight girls.
Participants had an average of 2.8 study visits during this period and provided additional information by phone and online. Visits included measurement of total body fat using dual-energy x-ray absorptiometry (DXA), Tanner staging, breast ultrasound for morphological staging (BMORPH; A-E), pelvic ultrasound, hormone tests, and menarchal status assessment.
Overall, girls with overweight/obesity (OW/OB) had significantly more advanced breast development at baseline than did those with normal weight (NW), but these girls progressed through BMORPH stage D later than did NW girls. Early-stage breast development was not affected by total body fat. However, “an increase of 5 percentage points in mean total body fat, for example, was associated with a 26% decrease in the transition rate out of stage D,” the researchers noted.
Hormone levels were similar at baseline for follicle-stimulating hormone, inhibin B, estrone (E1), total and free testosterone, and androstenedione. However, these levels increased more quickly after 1 year for girls with OW/OB, while they plateaued in girls with NW and dropped among girls with lower total body fat. Total body fat had no apparent effect on other reproductive hormones including luteinizing hormone, modified vaginal maturation index, and estradiol 2.
The average age of menarche was 12.4 years across all participants, but girls with higher total body fat at baseline were more likely to reach menarche at a younger age. “For every 1-unit increase in visit one total body fat, the chance of achieving menarche at any given time point was 3% higher,” the researchers said. No interaction appeared between race and total body fat with regard to menarche.
Several surprising findings
The study is important because “there have been no longitudinal studies in U.S. girls to examine how total body fat affects serum reproductive hormones or the development of the breast and ovaries using ultrasound imaging,” corresponding author Natalie Shaw, MD, of the National Institute of Environmental Health Sciences, said in an interview.
Dr. Shaw said she was surprised by several of the study findings. “Others have reported increased male-like hormones (androgens) in overweight/obese girls in cross-sectional studies; however, we were surprised to find that FSH and inhibin B were also elevated in girls with excess body fat,” she said. “We also found, unexpectedly, that even though the breast bud appears earlier in overweight/obese girls (thelarche), which signals the onset of puberty, the breast matured more slowly during the course of puberty in overweight/obese girls compared with normal weight girls,” she noted.
“The main take-home message is that puberty looks different in girls with excess body fat; they develop breast tissue earlier, yet take longer to achieve a fully mature breast, and they undergo menarche earlier,” Dr. Shaw said. Clinicians should be aware of the hormonal differences based on body fat, Dr. Shaw emphasized. “Girls with greater body fat had higher levels of FSH (a pituitary hormone), inhibin B (an ovarian hormone), and male-like reproductive hormones (e.g., testosterone) that are made by the adrenal glands and the ovaries in the late stages of puberty,” she said.
Potential implications for adulthood
“The findings in this study contribute to better understanding how total body fat impacts hormonal findings of puberty,” M. Susan Jay, MD, of the Medical College of Wisconsin and Children’s Hospital of Wisconsin, Milwaukee, said in an interview. “Prior studies have linked weight gain as a factor that contributes to pubertal development, but this study is attempting to longitudinally investigate how body weight may affect clinical and biochemical pubertal markers in girls,” she noted.
“The take-home message is that this study and other earlier studies have illustrated that puberty is not a fixed pattern in all individual girls,” Dr. Jay emphasized. “Rather, there are environmental factors which can impact pubertal course,” she said. “In effect, there are pathways through puberty in individual adolescents that require greater ongoing studies to further identify the arc of puberty and the impact of how the length in various stages may affect exposure to estrogen and other neurohormonal factors,” she explained. These factors impact not only adolescence but also future health in adulthood, she said.
“Ongoing prospective studies are needed to identify how factors such as body weight can affect adolescent pubertal development and the possible impact long after adolescence for health issues such as breast cancer,” Dr. Jay added.
The study findings were limited by several factors including the available data from only two completed study visits for most participants, as well as the racial differences among body weight groups and lack of standardized timing for blood draws, the researchers noted.
The study was supported in part by the National Institute of Environmental Health Sciences, and corresponding author Dr. Shaw disclosed support as a Lasker Clinical Research Scholar. The other researchers, as well as Dr. Jay, had no disclosures.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Asthma not an independent risk factor for severe COVID-19, hospitalization
Asthma is not an independent risk factor for more severe disease or hospitalization due to COVID-19, according to recent research presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“In our cohort of patients tested for SARS-CoV-2 at Stanford between March and September, asthma was not an independent risk factor in and of itself for hospitalization or more severe disease from COVID,” Lauren E. Eggert, MD, of the Sean N. Parker Center for Allergy and Asthma Research at Stanford (Calif.) University, said in a poster presentation at the meeting. “What’s more, allergic asthma actually decreased the risk of hospitalization by nearly half.”
Dr. Eggert noted that there have been conflicting data on whether comorbid asthma is or is not a risk factor for more severe COVID-19. “The general thought at the beginning of the pandemic was that because COVID-19 is predominantly a viral respiratory illness, and viral illnesses are known to cause asthma exacerbations, that patients with asthma may be at higher risk if they got COVID infection,” she explained. “But some of the data also showed that Th2 inflammation downregulates ACE2 receptor [expression], which has been shown to be the port of entry for the SARS-CoV-2 virus, so maybe allergy might have a protective effect.”
The researchers at Stanford University identified 168,190 patients at Stanford Health Care who had a positive real-time reverse transcriptase polymerase chain reaction (RT-PCR) test for SARS-CoV-2 between March and September 2020 and collected data from their electronic medical records on their history of asthma, if they were hospitalized, comorbid conditions, and laboratory values. Patients who had no other data available except for a positive SARS-CoV-2 result, or were younger than 28 days, were excluded from the study. Dr. Eggert and colleagues used COVID-19 treatment guidelines from the National Institutes of Health to assess disease severity, which grades COVID-19 severity as asymptomatic or presymptomatic infection, mild illness, moderate illness, severe illness, and critical illness.
In total, the researchers analyzed 5,596 patients who were SARS-CoV-2 positive, with 605 patients (10.8%) hospitalized within 14 days of receiving a positive test. Of these, 100 patients (16.5%) were patients with asthma. There were no significant differences between groups hospitalized and not hospitalized due to COVID-19 in patients with asthma and with no asthma.
Among patients with asthma and COVID-19, 28.0% had asymptomatic illness, 19.0% had moderate disease, 33.0% had severe disease, and 20.0% had critical COVID-19, compared with 36.0% of patients without asthma who had asymptomatic illness, 12.0% with moderate disease, 30.0% with severe disease, and 21.0% with critical COVID-19. Dr. Eggert and colleagues performed a univariate analysis, which showed a significant association between asthma and COVID-19 related hospitalization (odds ratio, 1.53; 95% confidence interval, 1.2-1.93; P < .001), but when adjusting for factors such as diabetes, obesity coronary heart disease, and hypertension, they found there was not a significant association between asthma and hospitalization due to COVID-19 (OR, 1.12; 95% CI, 0.86-1.45; P < .40).
In a univariate analysis, asthma was associated with more severe disease in patients hospitalized for COVID-19, but the results were not significant (OR, 1.21; 95% CI, 0.8-1.85; P = .37). When analyzing allergic asthma alone in a univariate analysis, the researchers found a significant association between allergic asthma and lower hospitalization risk, compared with patients who had nonallergic asthma (OR, 0.55; 95% CI, 0.31-0.92; P = .029), and this association remained after they performed a multivariate analysis as well.
“When we stratified by allergic asthma versus nonallergic asthma, we found that having a diagnosis of allergic asthma actually conferred a protective effect, and there was almost half the risk of hospitalization in asthmatics with allergic asthma as compared to others, which we thought was very interesting,” Dr. Eggert said.
“Eosinophil levels during hospitalization, even when adjusted for systemic steroid use – and we followed patients out through September, when dexamethasone was standard of care – also correlated with better outcomes,” she explained. “This is independent of asthmatic status.”
The researchers noted that confirmation of these results are needed through large, multicenter cohort studies, particularly with regard to how allergic asthma might have a protective effect against SARS-CoV-2 infection. “I think going forward, these findings are very interesting and need to be looked at further to explain the mechanism behind them better,” Dr. Eggert said.
“I think there is also a lot of interest in how this might affect our patients on biologics, which deplete the eosinophils and get rid of that allergic phenotype,” she added. “Does that have any effect on disease severity? Unfortunately, the number of patents on biologics was very small in our cohort, but I do think this is an interesting area for exploration.”
This study was funded in part by the Sean N. Parker Center for Allergy & Asthma Research, Stanford University, Sunshine Foundation, Crown Foundation, and the Parker Foundation.
Asthma is not an independent risk factor for more severe disease or hospitalization due to COVID-19, according to recent research presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“In our cohort of patients tested for SARS-CoV-2 at Stanford between March and September, asthma was not an independent risk factor in and of itself for hospitalization or more severe disease from COVID,” Lauren E. Eggert, MD, of the Sean N. Parker Center for Allergy and Asthma Research at Stanford (Calif.) University, said in a poster presentation at the meeting. “What’s more, allergic asthma actually decreased the risk of hospitalization by nearly half.”
Dr. Eggert noted that there have been conflicting data on whether comorbid asthma is or is not a risk factor for more severe COVID-19. “The general thought at the beginning of the pandemic was that because COVID-19 is predominantly a viral respiratory illness, and viral illnesses are known to cause asthma exacerbations, that patients with asthma may be at higher risk if they got COVID infection,” she explained. “But some of the data also showed that Th2 inflammation downregulates ACE2 receptor [expression], which has been shown to be the port of entry for the SARS-CoV-2 virus, so maybe allergy might have a protective effect.”
The researchers at Stanford University identified 168,190 patients at Stanford Health Care who had a positive real-time reverse transcriptase polymerase chain reaction (RT-PCR) test for SARS-CoV-2 between March and September 2020 and collected data from their electronic medical records on their history of asthma, if they were hospitalized, comorbid conditions, and laboratory values. Patients who had no other data available except for a positive SARS-CoV-2 result, or were younger than 28 days, were excluded from the study. Dr. Eggert and colleagues used COVID-19 treatment guidelines from the National Institutes of Health to assess disease severity, which grades COVID-19 severity as asymptomatic or presymptomatic infection, mild illness, moderate illness, severe illness, and critical illness.
In total, the researchers analyzed 5,596 patients who were SARS-CoV-2 positive, with 605 patients (10.8%) hospitalized within 14 days of receiving a positive test. Of these, 100 patients (16.5%) were patients with asthma. There were no significant differences between groups hospitalized and not hospitalized due to COVID-19 in patients with asthma and with no asthma.
Among patients with asthma and COVID-19, 28.0% had asymptomatic illness, 19.0% had moderate disease, 33.0% had severe disease, and 20.0% had critical COVID-19, compared with 36.0% of patients without asthma who had asymptomatic illness, 12.0% with moderate disease, 30.0% with severe disease, and 21.0% with critical COVID-19. Dr. Eggert and colleagues performed a univariate analysis, which showed a significant association between asthma and COVID-19 related hospitalization (odds ratio, 1.53; 95% confidence interval, 1.2-1.93; P < .001), but when adjusting for factors such as diabetes, obesity coronary heart disease, and hypertension, they found there was not a significant association between asthma and hospitalization due to COVID-19 (OR, 1.12; 95% CI, 0.86-1.45; P < .40).
In a univariate analysis, asthma was associated with more severe disease in patients hospitalized for COVID-19, but the results were not significant (OR, 1.21; 95% CI, 0.8-1.85; P = .37). When analyzing allergic asthma alone in a univariate analysis, the researchers found a significant association between allergic asthma and lower hospitalization risk, compared with patients who had nonallergic asthma (OR, 0.55; 95% CI, 0.31-0.92; P = .029), and this association remained after they performed a multivariate analysis as well.
“When we stratified by allergic asthma versus nonallergic asthma, we found that having a diagnosis of allergic asthma actually conferred a protective effect, and there was almost half the risk of hospitalization in asthmatics with allergic asthma as compared to others, which we thought was very interesting,” Dr. Eggert said.
“Eosinophil levels during hospitalization, even when adjusted for systemic steroid use – and we followed patients out through September, when dexamethasone was standard of care – also correlated with better outcomes,” she explained. “This is independent of asthmatic status.”
The researchers noted that confirmation of these results are needed through large, multicenter cohort studies, particularly with regard to how allergic asthma might have a protective effect against SARS-CoV-2 infection. “I think going forward, these findings are very interesting and need to be looked at further to explain the mechanism behind them better,” Dr. Eggert said.
“I think there is also a lot of interest in how this might affect our patients on biologics, which deplete the eosinophils and get rid of that allergic phenotype,” she added. “Does that have any effect on disease severity? Unfortunately, the number of patents on biologics was very small in our cohort, but I do think this is an interesting area for exploration.”
This study was funded in part by the Sean N. Parker Center for Allergy & Asthma Research, Stanford University, Sunshine Foundation, Crown Foundation, and the Parker Foundation.
Asthma is not an independent risk factor for more severe disease or hospitalization due to COVID-19, according to recent research presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“In our cohort of patients tested for SARS-CoV-2 at Stanford between March and September, asthma was not an independent risk factor in and of itself for hospitalization or more severe disease from COVID,” Lauren E. Eggert, MD, of the Sean N. Parker Center for Allergy and Asthma Research at Stanford (Calif.) University, said in a poster presentation at the meeting. “What’s more, allergic asthma actually decreased the risk of hospitalization by nearly half.”
Dr. Eggert noted that there have been conflicting data on whether comorbid asthma is or is not a risk factor for more severe COVID-19. “The general thought at the beginning of the pandemic was that because COVID-19 is predominantly a viral respiratory illness, and viral illnesses are known to cause asthma exacerbations, that patients with asthma may be at higher risk if they got COVID infection,” she explained. “But some of the data also showed that Th2 inflammation downregulates ACE2 receptor [expression], which has been shown to be the port of entry for the SARS-CoV-2 virus, so maybe allergy might have a protective effect.”
The researchers at Stanford University identified 168,190 patients at Stanford Health Care who had a positive real-time reverse transcriptase polymerase chain reaction (RT-PCR) test for SARS-CoV-2 between March and September 2020 and collected data from their electronic medical records on their history of asthma, if they were hospitalized, comorbid conditions, and laboratory values. Patients who had no other data available except for a positive SARS-CoV-2 result, or were younger than 28 days, were excluded from the study. Dr. Eggert and colleagues used COVID-19 treatment guidelines from the National Institutes of Health to assess disease severity, which grades COVID-19 severity as asymptomatic or presymptomatic infection, mild illness, moderate illness, severe illness, and critical illness.
In total, the researchers analyzed 5,596 patients who were SARS-CoV-2 positive, with 605 patients (10.8%) hospitalized within 14 days of receiving a positive test. Of these, 100 patients (16.5%) were patients with asthma. There were no significant differences between groups hospitalized and not hospitalized due to COVID-19 in patients with asthma and with no asthma.
Among patients with asthma and COVID-19, 28.0% had asymptomatic illness, 19.0% had moderate disease, 33.0% had severe disease, and 20.0% had critical COVID-19, compared with 36.0% of patients without asthma who had asymptomatic illness, 12.0% with moderate disease, 30.0% with severe disease, and 21.0% with critical COVID-19. Dr. Eggert and colleagues performed a univariate analysis, which showed a significant association between asthma and COVID-19 related hospitalization (odds ratio, 1.53; 95% confidence interval, 1.2-1.93; P < .001), but when adjusting for factors such as diabetes, obesity coronary heart disease, and hypertension, they found there was not a significant association between asthma and hospitalization due to COVID-19 (OR, 1.12; 95% CI, 0.86-1.45; P < .40).
In a univariate analysis, asthma was associated with more severe disease in patients hospitalized for COVID-19, but the results were not significant (OR, 1.21; 95% CI, 0.8-1.85; P = .37). When analyzing allergic asthma alone in a univariate analysis, the researchers found a significant association between allergic asthma and lower hospitalization risk, compared with patients who had nonallergic asthma (OR, 0.55; 95% CI, 0.31-0.92; P = .029), and this association remained after they performed a multivariate analysis as well.
“When we stratified by allergic asthma versus nonallergic asthma, we found that having a diagnosis of allergic asthma actually conferred a protective effect, and there was almost half the risk of hospitalization in asthmatics with allergic asthma as compared to others, which we thought was very interesting,” Dr. Eggert said.
“Eosinophil levels during hospitalization, even when adjusted for systemic steroid use – and we followed patients out through September, when dexamethasone was standard of care – also correlated with better outcomes,” she explained. “This is independent of asthmatic status.”
The researchers noted that confirmation of these results are needed through large, multicenter cohort studies, particularly with regard to how allergic asthma might have a protective effect against SARS-CoV-2 infection. “I think going forward, these findings are very interesting and need to be looked at further to explain the mechanism behind them better,” Dr. Eggert said.
“I think there is also a lot of interest in how this might affect our patients on biologics, which deplete the eosinophils and get rid of that allergic phenotype,” she added. “Does that have any effect on disease severity? Unfortunately, the number of patents on biologics was very small in our cohort, but I do think this is an interesting area for exploration.”
This study was funded in part by the Sean N. Parker Center for Allergy & Asthma Research, Stanford University, Sunshine Foundation, Crown Foundation, and the Parker Foundation.
FROM AAAAI
Frequent medication refills show some patients not achieving asthma control
While most commercially insured patients with asthma have good disease control, some patients may not, according to a recent review of U.S. administrative claims data.
The results of the retrospective analysis, presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year, showed some patients with asthma had two or more refills for prescribed systemic corticosteroids (SCS) or short-acting beta agonists (SABA) within a period of 12 months.
“,” Randall Brown, MD, MPH, pulmonologist and senior director of Global Respiratory Medical Affairs at Teva Pharmaceuticals in West Chester, Penn., said in a presentation at the meeting. “Understanding the extent of systemic steroid and SABA prescriptions among patients with asthma and the distribution of those prescriptions across disease severity can be useful in determining the degree of disease control.”
Global Initiative for Asthma (GINA) guidelines consider factors such as symptom control and risk of exacerbation when determining asthma severity, but uncontrolled asthma can still be difficult to assess. Dr. Brown and colleagues set out to determine the prevalence of uncontrolled asthma for patients in the IBM/Watson MarketScan U.S. claims database as well as the rate of uncontrolled asthma by GINA classification. In total, 597,955 patients who had an asthma diagnosis between 12 months before or up to 3 months after the index data of filling a SABA prescription were included for analysis. Patients were at least 12 years old with commercial insurance for at least 12 months, and had no other respiratory diseases other than asthma during the 12 months prior to the index date and during the study period.
The researchers then measured each patient’s 2018 GINA classification of asthma severity based on the number of SCS and SABA prescription claims made between January and December 2017. Overall, 54.3% patients were GINA Step 1, 14.6% were Step 2, 10.2% were Step 3, 19.8% were Step 4, and 1.1% were Step 5.
Dr. Brown and colleagues found that, regardless of GINA disease severity, 18.8% of patients filled two or more SCS prescriptions in 1 year, 27.4% filled three or more SABA prescriptions in 1 year, and 38.7% filled two or more SCS and/or three or more SABA prescriptions in 1 year. “[A] large proportion of these patients did not meet the GINA goal of disease control,” Dr. Brown said.
The researchers found 13% of patients with uncontrolled asthma categorized as GINA Step 1, 20% of patients categorized as GINA Step 2, 19% of patients who were GINA Step 3, 31% of patients who were GINA Step 4, and 54% of patients categorized as GINA Step 5 filled two or more two or more SCS prescriptions per year.
The proportion of patients with uncontrolled asthma who filled three or more SABA prescriptions per year included 19% in GINA Step 1, 29% in GINA Step 2, 35% in GINA Step 3, 44% in GINA Step 4, and 57% in GINA Step 5 groups. For patients who filled both two or more SCS and/or three or more SABA prescriptions per year, the proportion of patients with uncontrolled asthma by GINA category was 29% in GINA Step 1, 42% in GINA Step 2, 46% in GINA Step 3, 58% in GINA Step 4, and 76% of patients in GINA Step 5.
While “poor control was seen across all of the GINA disease severity classifications, the greatest proportion of uncontrolled disease was seen at the highest disease severity, which was also true when we used a stricter definition of uncontrolled disease,” Dr. Brown said. When the researchers applied stricter criteria for patients categorized as GINA Step 5, 39% of patients filled three or more SCS, 41% filled four or more SABA, and 60% filled three or more SCS and/or four or more SABA prescriptions over 12 months.
Dr. Brown said that the analysis “highlights the need for improved asthma management strategies within each of the asthma GINA classification steps.”
“While this population that was studied may be reflective of the wider insured U.S. population, the proportions of uncontrolled asthma may be even greater in non–commercially insured patients within the United States,” he said. “Updates to GINA guidelines incorporate recent consensus [and] recent scientific information and therapies, but many patients in the U.S. are not meeting the GINA goal of disease control. Newer paradigms for systemic corticosteroid-free asthma control as a target of disease ‘remission’ are becoming more commonplace. Such changes and goals may lead to improved asthma management strategies and advancement in treatment.”
This study was funded in part by Teva Branded Pharmaceutical Products R&D, which also provided funding for medical writing assistance from Ashfield MedComms. The authors report being employees of Teva Pharmaceuticals.
While most commercially insured patients with asthma have good disease control, some patients may not, according to a recent review of U.S. administrative claims data.
The results of the retrospective analysis, presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year, showed some patients with asthma had two or more refills for prescribed systemic corticosteroids (SCS) or short-acting beta agonists (SABA) within a period of 12 months.
“,” Randall Brown, MD, MPH, pulmonologist and senior director of Global Respiratory Medical Affairs at Teva Pharmaceuticals in West Chester, Penn., said in a presentation at the meeting. “Understanding the extent of systemic steroid and SABA prescriptions among patients with asthma and the distribution of those prescriptions across disease severity can be useful in determining the degree of disease control.”
Global Initiative for Asthma (GINA) guidelines consider factors such as symptom control and risk of exacerbation when determining asthma severity, but uncontrolled asthma can still be difficult to assess. Dr. Brown and colleagues set out to determine the prevalence of uncontrolled asthma for patients in the IBM/Watson MarketScan U.S. claims database as well as the rate of uncontrolled asthma by GINA classification. In total, 597,955 patients who had an asthma diagnosis between 12 months before or up to 3 months after the index data of filling a SABA prescription were included for analysis. Patients were at least 12 years old with commercial insurance for at least 12 months, and had no other respiratory diseases other than asthma during the 12 months prior to the index date and during the study period.
The researchers then measured each patient’s 2018 GINA classification of asthma severity based on the number of SCS and SABA prescription claims made between January and December 2017. Overall, 54.3% patients were GINA Step 1, 14.6% were Step 2, 10.2% were Step 3, 19.8% were Step 4, and 1.1% were Step 5.
Dr. Brown and colleagues found that, regardless of GINA disease severity, 18.8% of patients filled two or more SCS prescriptions in 1 year, 27.4% filled three or more SABA prescriptions in 1 year, and 38.7% filled two or more SCS and/or three or more SABA prescriptions in 1 year. “[A] large proportion of these patients did not meet the GINA goal of disease control,” Dr. Brown said.
The researchers found 13% of patients with uncontrolled asthma categorized as GINA Step 1, 20% of patients categorized as GINA Step 2, 19% of patients who were GINA Step 3, 31% of patients who were GINA Step 4, and 54% of patients categorized as GINA Step 5 filled two or more two or more SCS prescriptions per year.
The proportion of patients with uncontrolled asthma who filled three or more SABA prescriptions per year included 19% in GINA Step 1, 29% in GINA Step 2, 35% in GINA Step 3, 44% in GINA Step 4, and 57% in GINA Step 5 groups. For patients who filled both two or more SCS and/or three or more SABA prescriptions per year, the proportion of patients with uncontrolled asthma by GINA category was 29% in GINA Step 1, 42% in GINA Step 2, 46% in GINA Step 3, 58% in GINA Step 4, and 76% of patients in GINA Step 5.
While “poor control was seen across all of the GINA disease severity classifications, the greatest proportion of uncontrolled disease was seen at the highest disease severity, which was also true when we used a stricter definition of uncontrolled disease,” Dr. Brown said. When the researchers applied stricter criteria for patients categorized as GINA Step 5, 39% of patients filled three or more SCS, 41% filled four or more SABA, and 60% filled three or more SCS and/or four or more SABA prescriptions over 12 months.
Dr. Brown said that the analysis “highlights the need for improved asthma management strategies within each of the asthma GINA classification steps.”
“While this population that was studied may be reflective of the wider insured U.S. population, the proportions of uncontrolled asthma may be even greater in non–commercially insured patients within the United States,” he said. “Updates to GINA guidelines incorporate recent consensus [and] recent scientific information and therapies, but many patients in the U.S. are not meeting the GINA goal of disease control. Newer paradigms for systemic corticosteroid-free asthma control as a target of disease ‘remission’ are becoming more commonplace. Such changes and goals may lead to improved asthma management strategies and advancement in treatment.”
This study was funded in part by Teva Branded Pharmaceutical Products R&D, which also provided funding for medical writing assistance from Ashfield MedComms. The authors report being employees of Teva Pharmaceuticals.
While most commercially insured patients with asthma have good disease control, some patients may not, according to a recent review of U.S. administrative claims data.
The results of the retrospective analysis, presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year, showed some patients with asthma had two or more refills for prescribed systemic corticosteroids (SCS) or short-acting beta agonists (SABA) within a period of 12 months.
“,” Randall Brown, MD, MPH, pulmonologist and senior director of Global Respiratory Medical Affairs at Teva Pharmaceuticals in West Chester, Penn., said in a presentation at the meeting. “Understanding the extent of systemic steroid and SABA prescriptions among patients with asthma and the distribution of those prescriptions across disease severity can be useful in determining the degree of disease control.”
Global Initiative for Asthma (GINA) guidelines consider factors such as symptom control and risk of exacerbation when determining asthma severity, but uncontrolled asthma can still be difficult to assess. Dr. Brown and colleagues set out to determine the prevalence of uncontrolled asthma for patients in the IBM/Watson MarketScan U.S. claims database as well as the rate of uncontrolled asthma by GINA classification. In total, 597,955 patients who had an asthma diagnosis between 12 months before or up to 3 months after the index data of filling a SABA prescription were included for analysis. Patients were at least 12 years old with commercial insurance for at least 12 months, and had no other respiratory diseases other than asthma during the 12 months prior to the index date and during the study period.
The researchers then measured each patient’s 2018 GINA classification of asthma severity based on the number of SCS and SABA prescription claims made between January and December 2017. Overall, 54.3% patients were GINA Step 1, 14.6% were Step 2, 10.2% were Step 3, 19.8% were Step 4, and 1.1% were Step 5.
Dr. Brown and colleagues found that, regardless of GINA disease severity, 18.8% of patients filled two or more SCS prescriptions in 1 year, 27.4% filled three or more SABA prescriptions in 1 year, and 38.7% filled two or more SCS and/or three or more SABA prescriptions in 1 year. “[A] large proportion of these patients did not meet the GINA goal of disease control,” Dr. Brown said.
The researchers found 13% of patients with uncontrolled asthma categorized as GINA Step 1, 20% of patients categorized as GINA Step 2, 19% of patients who were GINA Step 3, 31% of patients who were GINA Step 4, and 54% of patients categorized as GINA Step 5 filled two or more two or more SCS prescriptions per year.
The proportion of patients with uncontrolled asthma who filled three or more SABA prescriptions per year included 19% in GINA Step 1, 29% in GINA Step 2, 35% in GINA Step 3, 44% in GINA Step 4, and 57% in GINA Step 5 groups. For patients who filled both two or more SCS and/or three or more SABA prescriptions per year, the proportion of patients with uncontrolled asthma by GINA category was 29% in GINA Step 1, 42% in GINA Step 2, 46% in GINA Step 3, 58% in GINA Step 4, and 76% of patients in GINA Step 5.
While “poor control was seen across all of the GINA disease severity classifications, the greatest proportion of uncontrolled disease was seen at the highest disease severity, which was also true when we used a stricter definition of uncontrolled disease,” Dr. Brown said. When the researchers applied stricter criteria for patients categorized as GINA Step 5, 39% of patients filled three or more SCS, 41% filled four or more SABA, and 60% filled three or more SCS and/or four or more SABA prescriptions over 12 months.
Dr. Brown said that the analysis “highlights the need for improved asthma management strategies within each of the asthma GINA classification steps.”
“While this population that was studied may be reflective of the wider insured U.S. population, the proportions of uncontrolled asthma may be even greater in non–commercially insured patients within the United States,” he said. “Updates to GINA guidelines incorporate recent consensus [and] recent scientific information and therapies, but many patients in the U.S. are not meeting the GINA goal of disease control. Newer paradigms for systemic corticosteroid-free asthma control as a target of disease ‘remission’ are becoming more commonplace. Such changes and goals may lead to improved asthma management strategies and advancement in treatment.”
This study was funded in part by Teva Branded Pharmaceutical Products R&D, which also provided funding for medical writing assistance from Ashfield MedComms. The authors report being employees of Teva Pharmaceuticals.
FROM AAAAI







