User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
HHS okays first U.S. pilot to mandate coverage of gender-affirming care
The approval means transgender-related care must be included as part of the essential benefits offered on the state’s Affordable Care Act marketplace, which includes private individual and small group insurance plans. The coverage will start Jan. 1, 2023. Colorado is the first state in the United States to require such coverage.
The HHS notes that gender-affirming treatments to be covered include eye and lid modifications, face tightening, facial bone remodeling for facial feminization, breast/chest construction and reductions, and laser hair removal.
“I am proud to stand with Colorado to remove barriers that have historically made it difficult for transgender people to access health coverage and medical care,” said HHS Secretary Xavier Becerra in a statement.
“Colorado’s expansion of their essential health benefits to include gender-affirming surgery and other treatments is a model for other states to follow, and we invite other states to follow suit,” said Centers for Medicare & Medicaid Services Administrator Chiquita Brooks-LaSure in the statement.
Medicaid already covers comprehensive transgender care in Colorado.
The LGBTQ+ advocacy group One Colorado estimated that, thanks to the Affordable Care Act, only 5% of the state’s LGBTQ+ community was uninsured in 2019, compared to 10% in 2011.
However, 34% of transgender respondents to a One Colorado poll in 2018 said they had been denied coverage for an LGBTQ-specific medical service, such as gender-affirming care. Sixty-two percent said that a lack of insurance or limited insurance was a barrier to care; 84% said another barrier was the lack of adequately trained mental and behavioral health professionals.
Mental health also covered
The Colorado plan requires individual and small group plans to cover an annual 45- to 60-minute mental health wellness exam with a qualified mental health care practitioner. The visit can include behavioral health screening, education and consultation about healthy lifestyle changes, referrals to mental health treatment, and discussion of potential medication options.
The plans also must cover an additional 15 medications as alternatives to opioids and up to six acupuncture visits annually.
“This plan expands access to mental health services for Coloradans while helping those fighting substance abuse to overcome their addiction,” said Governor Jared Polis in a statement.
“This improves care for Coloradans and ensures that even more Coloradans have access to help when they need it,” he said.
A version of this article first appeared on Medscape.com.
The approval means transgender-related care must be included as part of the essential benefits offered on the state’s Affordable Care Act marketplace, which includes private individual and small group insurance plans. The coverage will start Jan. 1, 2023. Colorado is the first state in the United States to require such coverage.
The HHS notes that gender-affirming treatments to be covered include eye and lid modifications, face tightening, facial bone remodeling for facial feminization, breast/chest construction and reductions, and laser hair removal.
“I am proud to stand with Colorado to remove barriers that have historically made it difficult for transgender people to access health coverage and medical care,” said HHS Secretary Xavier Becerra in a statement.
“Colorado’s expansion of their essential health benefits to include gender-affirming surgery and other treatments is a model for other states to follow, and we invite other states to follow suit,” said Centers for Medicare & Medicaid Services Administrator Chiquita Brooks-LaSure in the statement.
Medicaid already covers comprehensive transgender care in Colorado.
The LGBTQ+ advocacy group One Colorado estimated that, thanks to the Affordable Care Act, only 5% of the state’s LGBTQ+ community was uninsured in 2019, compared to 10% in 2011.
However, 34% of transgender respondents to a One Colorado poll in 2018 said they had been denied coverage for an LGBTQ-specific medical service, such as gender-affirming care. Sixty-two percent said that a lack of insurance or limited insurance was a barrier to care; 84% said another barrier was the lack of adequately trained mental and behavioral health professionals.
Mental health also covered
The Colorado plan requires individual and small group plans to cover an annual 45- to 60-minute mental health wellness exam with a qualified mental health care practitioner. The visit can include behavioral health screening, education and consultation about healthy lifestyle changes, referrals to mental health treatment, and discussion of potential medication options.
The plans also must cover an additional 15 medications as alternatives to opioids and up to six acupuncture visits annually.
“This plan expands access to mental health services for Coloradans while helping those fighting substance abuse to overcome their addiction,” said Governor Jared Polis in a statement.
“This improves care for Coloradans and ensures that even more Coloradans have access to help when they need it,” he said.
A version of this article first appeared on Medscape.com.
The approval means transgender-related care must be included as part of the essential benefits offered on the state’s Affordable Care Act marketplace, which includes private individual and small group insurance plans. The coverage will start Jan. 1, 2023. Colorado is the first state in the United States to require such coverage.
The HHS notes that gender-affirming treatments to be covered include eye and lid modifications, face tightening, facial bone remodeling for facial feminization, breast/chest construction and reductions, and laser hair removal.
“I am proud to stand with Colorado to remove barriers that have historically made it difficult for transgender people to access health coverage and medical care,” said HHS Secretary Xavier Becerra in a statement.
“Colorado’s expansion of their essential health benefits to include gender-affirming surgery and other treatments is a model for other states to follow, and we invite other states to follow suit,” said Centers for Medicare & Medicaid Services Administrator Chiquita Brooks-LaSure in the statement.
Medicaid already covers comprehensive transgender care in Colorado.
The LGBTQ+ advocacy group One Colorado estimated that, thanks to the Affordable Care Act, only 5% of the state’s LGBTQ+ community was uninsured in 2019, compared to 10% in 2011.
However, 34% of transgender respondents to a One Colorado poll in 2018 said they had been denied coverage for an LGBTQ-specific medical service, such as gender-affirming care. Sixty-two percent said that a lack of insurance or limited insurance was a barrier to care; 84% said another barrier was the lack of adequately trained mental and behavioral health professionals.
Mental health also covered
The Colorado plan requires individual and small group plans to cover an annual 45- to 60-minute mental health wellness exam with a qualified mental health care practitioner. The visit can include behavioral health screening, education and consultation about healthy lifestyle changes, referrals to mental health treatment, and discussion of potential medication options.
The plans also must cover an additional 15 medications as alternatives to opioids and up to six acupuncture visits annually.
“This plan expands access to mental health services for Coloradans while helping those fighting substance abuse to overcome their addiction,” said Governor Jared Polis in a statement.
“This improves care for Coloradans and ensures that even more Coloradans have access to help when they need it,” he said.
A version of this article first appeared on Medscape.com.
Stay tuned for CSI: Olive oil
Cracking down on food fraud
How do you know the olive oil in your pantry is from Greece? Or that the avocados on your toast are from Mexico? The label, right? Well, maybe not. False claims of origin are a huge problem in the food industry, costing over $30 billion in economic damage annually.
Fear not, citizens, because botanists are on the job, and they’ve found a cheaper and more efficient way to expose that non-Greek olive oil.
How? Florian Cueni, PhD, of the University of Basel, Switzerland, and associates developed a new model to simulate oxygen isotope ratios in plants from a specific region, based on the temperature, precipitation, growing season information, and humidity data. Previously, botanists had to collect reference data from the claimed origin country and from other regions to validate where the product actually came from.
“With minor adjustments to the parameters, our model can be used to determine all plant products,” said senior investigator Ansgar Kahmen. This can open up the door for even more plant forensics, including drug confiscations and illegal timber logging, with information that will hold up in court.
Why pay Greek-olive prices for olives from California?
Fear leads to anger, anger leads to unhelpful online reviews
And reading angry online reviews leads to hate and suffering. We may have co-opted Master Yoda’s wise words ever so slightly, but anyone who’s done any shopping online (so everyone) knows that the review section of any product can be downright villainous. Do these reviews affect what we buy?
The angry online product review was the subject of a recent study published in MIS Quarterly. In a series of experiments, participants were shown a series of realistic online reviews with varying amounts of anger but with similar amounts of information. After reading the reviews, participants rated helpfulness, their personal opinion of the product/retailer, and whether or not they would buy the product.
Participants overwhelmingly rated calmly written reviews as more helpful than angrily written ones. One would expect, then, that those unhelpful angry reviews would have little effect on the participant’s view or willingness to buy a product, but the study investigators found the opposite. Reading angry reviews made the participants more likely to reject the product, even though they didn’t think the angry review was useful. And when you think about it, it does make sense. Anger means drama, and we can’t resist a juicy bit of drama.
So while we should all aspire to be Yoda and rise above anger and hatred, in reality we seem to be channeling Emperor Palpatine. We let the hate flow through us, and in our anger, we ignore perfectly good products. On the plus side, now we can shoot lightning out of our hands, so that’s pretty cool.
Health care is heading to the hall of fame
We couldn’t be happier here at LOTME because it’s that time of year again.
No, we’re not talking about Healthcare Security and Safety Week or National Metric Week, although those are both kind of important. Hmm, maybe we should talk about health care security or the metric system. After all, in this country, medicine is one of the metric system’s biggest customers. And who doesn’t love picograms? They’re the unit-of-measurement equivalent of a koala.
So we’re doing the metric system, then? Nah.
We’re excited because the 2022 inductees to the National Inventors Hall of Fame were just announced, and, as usual, the world of health care is well represented.
First up is the surprisingly relevant (thanks to the party guest that won’t leave, SARS-CoV-2) pair of Katalin Karikó, PhD, and Drew Weissman, MD, who worked together in the early 2000s to modify mRNA “so it could avoid immediate immune detection, remain active longer and efficiently instruct cells to create antigens to protect against severe disease.” Their discoveries eventually led to the use of modified mRNA in the COVID-19 vaccines.
The second, albeit posthumous, physician-inductee is Patricia Bath, MD, who was the first Black female physician to receive a U.S. patent for a medical invention. The laserphaco device and technique to remove cataracts “performed all steps of cataract removal: making the incision, destroying the lens, and vacuuming out the fractured pieces.”
Two other inductees have somewhat tenuous connections to medical care. Lonnie Johnson invented the Super Soaker, a powerful squirt gun that has been criticized by psychologists for encouraging violence, and Carl Benz invented the automobile, which sort of means he invented the ambulance, so there you go.
The induction ceremony takes place on May 5, 2022, in Washington, DC. If you’re attending the black-tie dinner at The Anthem, let us know and we’ll split an Uber. It’s our only night to be fancy.
Cracking down on food fraud
How do you know the olive oil in your pantry is from Greece? Or that the avocados on your toast are from Mexico? The label, right? Well, maybe not. False claims of origin are a huge problem in the food industry, costing over $30 billion in economic damage annually.
Fear not, citizens, because botanists are on the job, and they’ve found a cheaper and more efficient way to expose that non-Greek olive oil.
How? Florian Cueni, PhD, of the University of Basel, Switzerland, and associates developed a new model to simulate oxygen isotope ratios in plants from a specific region, based on the temperature, precipitation, growing season information, and humidity data. Previously, botanists had to collect reference data from the claimed origin country and from other regions to validate where the product actually came from.
“With minor adjustments to the parameters, our model can be used to determine all plant products,” said senior investigator Ansgar Kahmen. This can open up the door for even more plant forensics, including drug confiscations and illegal timber logging, with information that will hold up in court.
Why pay Greek-olive prices for olives from California?
Fear leads to anger, anger leads to unhelpful online reviews
And reading angry online reviews leads to hate and suffering. We may have co-opted Master Yoda’s wise words ever so slightly, but anyone who’s done any shopping online (so everyone) knows that the review section of any product can be downright villainous. Do these reviews affect what we buy?
The angry online product review was the subject of a recent study published in MIS Quarterly. In a series of experiments, participants were shown a series of realistic online reviews with varying amounts of anger but with similar amounts of information. After reading the reviews, participants rated helpfulness, their personal opinion of the product/retailer, and whether or not they would buy the product.
Participants overwhelmingly rated calmly written reviews as more helpful than angrily written ones. One would expect, then, that those unhelpful angry reviews would have little effect on the participant’s view or willingness to buy a product, but the study investigators found the opposite. Reading angry reviews made the participants more likely to reject the product, even though they didn’t think the angry review was useful. And when you think about it, it does make sense. Anger means drama, and we can’t resist a juicy bit of drama.
So while we should all aspire to be Yoda and rise above anger and hatred, in reality we seem to be channeling Emperor Palpatine. We let the hate flow through us, and in our anger, we ignore perfectly good products. On the plus side, now we can shoot lightning out of our hands, so that’s pretty cool.
Health care is heading to the hall of fame
We couldn’t be happier here at LOTME because it’s that time of year again.
No, we’re not talking about Healthcare Security and Safety Week or National Metric Week, although those are both kind of important. Hmm, maybe we should talk about health care security or the metric system. After all, in this country, medicine is one of the metric system’s biggest customers. And who doesn’t love picograms? They’re the unit-of-measurement equivalent of a koala.
So we’re doing the metric system, then? Nah.
We’re excited because the 2022 inductees to the National Inventors Hall of Fame were just announced, and, as usual, the world of health care is well represented.
First up is the surprisingly relevant (thanks to the party guest that won’t leave, SARS-CoV-2) pair of Katalin Karikó, PhD, and Drew Weissman, MD, who worked together in the early 2000s to modify mRNA “so it could avoid immediate immune detection, remain active longer and efficiently instruct cells to create antigens to protect against severe disease.” Their discoveries eventually led to the use of modified mRNA in the COVID-19 vaccines.
The second, albeit posthumous, physician-inductee is Patricia Bath, MD, who was the first Black female physician to receive a U.S. patent for a medical invention. The laserphaco device and technique to remove cataracts “performed all steps of cataract removal: making the incision, destroying the lens, and vacuuming out the fractured pieces.”
Two other inductees have somewhat tenuous connections to medical care. Lonnie Johnson invented the Super Soaker, a powerful squirt gun that has been criticized by psychologists for encouraging violence, and Carl Benz invented the automobile, which sort of means he invented the ambulance, so there you go.
The induction ceremony takes place on May 5, 2022, in Washington, DC. If you’re attending the black-tie dinner at The Anthem, let us know and we’ll split an Uber. It’s our only night to be fancy.
Cracking down on food fraud
How do you know the olive oil in your pantry is from Greece? Or that the avocados on your toast are from Mexico? The label, right? Well, maybe not. False claims of origin are a huge problem in the food industry, costing over $30 billion in economic damage annually.
Fear not, citizens, because botanists are on the job, and they’ve found a cheaper and more efficient way to expose that non-Greek olive oil.
How? Florian Cueni, PhD, of the University of Basel, Switzerland, and associates developed a new model to simulate oxygen isotope ratios in plants from a specific region, based on the temperature, precipitation, growing season information, and humidity data. Previously, botanists had to collect reference data from the claimed origin country and from other regions to validate where the product actually came from.
“With minor adjustments to the parameters, our model can be used to determine all plant products,” said senior investigator Ansgar Kahmen. This can open up the door for even more plant forensics, including drug confiscations and illegal timber logging, with information that will hold up in court.
Why pay Greek-olive prices for olives from California?
Fear leads to anger, anger leads to unhelpful online reviews
And reading angry online reviews leads to hate and suffering. We may have co-opted Master Yoda’s wise words ever so slightly, but anyone who’s done any shopping online (so everyone) knows that the review section of any product can be downright villainous. Do these reviews affect what we buy?
The angry online product review was the subject of a recent study published in MIS Quarterly. In a series of experiments, participants were shown a series of realistic online reviews with varying amounts of anger but with similar amounts of information. After reading the reviews, participants rated helpfulness, their personal opinion of the product/retailer, and whether or not they would buy the product.
Participants overwhelmingly rated calmly written reviews as more helpful than angrily written ones. One would expect, then, that those unhelpful angry reviews would have little effect on the participant’s view or willingness to buy a product, but the study investigators found the opposite. Reading angry reviews made the participants more likely to reject the product, even though they didn’t think the angry review was useful. And when you think about it, it does make sense. Anger means drama, and we can’t resist a juicy bit of drama.
So while we should all aspire to be Yoda and rise above anger and hatred, in reality we seem to be channeling Emperor Palpatine. We let the hate flow through us, and in our anger, we ignore perfectly good products. On the plus side, now we can shoot lightning out of our hands, so that’s pretty cool.
Health care is heading to the hall of fame
We couldn’t be happier here at LOTME because it’s that time of year again.
No, we’re not talking about Healthcare Security and Safety Week or National Metric Week, although those are both kind of important. Hmm, maybe we should talk about health care security or the metric system. After all, in this country, medicine is one of the metric system’s biggest customers. And who doesn’t love picograms? They’re the unit-of-measurement equivalent of a koala.
So we’re doing the metric system, then? Nah.
We’re excited because the 2022 inductees to the National Inventors Hall of Fame were just announced, and, as usual, the world of health care is well represented.
First up is the surprisingly relevant (thanks to the party guest that won’t leave, SARS-CoV-2) pair of Katalin Karikó, PhD, and Drew Weissman, MD, who worked together in the early 2000s to modify mRNA “so it could avoid immediate immune detection, remain active longer and efficiently instruct cells to create antigens to protect against severe disease.” Their discoveries eventually led to the use of modified mRNA in the COVID-19 vaccines.
The second, albeit posthumous, physician-inductee is Patricia Bath, MD, who was the first Black female physician to receive a U.S. patent for a medical invention. The laserphaco device and technique to remove cataracts “performed all steps of cataract removal: making the incision, destroying the lens, and vacuuming out the fractured pieces.”
Two other inductees have somewhat tenuous connections to medical care. Lonnie Johnson invented the Super Soaker, a powerful squirt gun that has been criticized by psychologists for encouraging violence, and Carl Benz invented the automobile, which sort of means he invented the ambulance, so there you go.
The induction ceremony takes place on May 5, 2022, in Washington, DC. If you’re attending the black-tie dinner at The Anthem, let us know and we’ll split an Uber. It’s our only night to be fancy.
Uncomplicated pediatric chest infection: Antibiotics don’t help
Unless pneumonia is suspected, clinicians should not prescribe antibiotics for most children with chest infections, according to findings of the ARTIC-PC randomized controlled trial, published in The Lancet.
“Prescribing for children with uncomplicated chest infections is still common in most countries,” said lead author Paul Little, MD, professor of primary care research at the University of Southampton, England, in an interview.
But there are barriers to stopping this practice, he said. “If you prescribe an antibiotic and the child gets better, even if the antibiotic was not doing that much, the parents then think that it was the antibiotic that was responsible for the recovery and so expect antibiotics the next time. So, physician prescribing of antibiotics in effect medicalizes illness and keeps the cycle of expectations, reconsultations, and prescriptions going.”
The study included 432 children aged 6 months to 12 years (median age, 3.2 years) who presented at 56 general practices in England with acute, uncomplicated lower respiratory tract infection (LRTI) of less than 21 days’ duration and in whom pneumonia was not suspected clinically. The children were randomly assigned to undergo 7 days of treatment with either amoxicillin 50 mg/kg or placebo. The primary outcome was duration of symptoms rated moderately bad or worse.
For up to 4 weeks, parents scored symptoms – including cough, phlegm, shortness of breath, wheeze, blocked or runny nose, disturbed sleep, feeling generally unwell, fever, and interference with normal activities – in a daily diary. The secondary outcome was symptom severity. Prespecified analyses were made for key clinical subgroups of patients for whom clinicians commonly prescribe (those with chest signs, fever, physician rating of unwell, sputum or chest rattle, and shortness of breath).
There was no significant difference in outcome between children treated with antibiotics and those treated with placebo. The median duration of moderately bad or worse symptoms was similar between the antibiotics group and the placebo group (5 vs. 6 days; hazard ratio, 1.13), as was the median time until symptoms were rated absent or as causing very little problem (7 vs. 8 days; HR, 1.09). There was a small significant difference between the groups in symptom severity score on days 2-4 after seeing the doctor (1.8 in the antibiotics group vs. 2.1 in the placebo group), “which was equivalent to less than one child in three rating symptoms a slight problem rather than very little problem,” the study authors report. “The treatment effects for all outcomes were similar for most subgroups ... but the effect of antibiotics was slightly, but not significantly, greater among those with fever or those who were unwell,” they add.
The investigators conclude that “similar to adults, antibiotics are unlikely to make a clinically important difference to the symptom burden for uncomplicated lower respiratory tract infections in children – both overall, and for the key clinical subgroups where antibiotic prescribing is most common.” They recommend that clinicians provide “safety-netting advice” to parents, such as explaining what illness course to expect and when a return visit would be necessary.
The findings provide “more evidence to do less,” wrote Rianne Oostenbrink, MD, PhD, from Erasmus MC-Sophia, in Rotterdam, the Netherlands, and Lina Jankauskaite, MD, PhD, from Lithuanian University of Health Sciences, Kaunas, in an accompanying comment.
“Overtesting and overtreatment of children are especially prominent in infectious diseases, when fever or other symptoms such as cough can be unspecific and can be of viral or bacterial origin,” they write.
The commenters note that despite antibiotics, most children did have moderately bad or worse symptoms on day 3, and symptoms had improved in about 75% of children in both groups at day 14. “A notable finding of this study is that only a few children had moderately bad or worse symptoms by day 14, and antibiotics did not alleviate the symptoms compared with placebo. Additionally, this trial aligns with other studies that have shown that reducing antibiotic treatment for LRTI is not associated with prolonged morbidity or higher incidence of complications.”
The study was funded by the UK National Institute for Health Research. Dr. Little, Dr. Jankauskaite, and Dr. Oostenbrink have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Unless pneumonia is suspected, clinicians should not prescribe antibiotics for most children with chest infections, according to findings of the ARTIC-PC randomized controlled trial, published in The Lancet.
“Prescribing for children with uncomplicated chest infections is still common in most countries,” said lead author Paul Little, MD, professor of primary care research at the University of Southampton, England, in an interview.
But there are barriers to stopping this practice, he said. “If you prescribe an antibiotic and the child gets better, even if the antibiotic was not doing that much, the parents then think that it was the antibiotic that was responsible for the recovery and so expect antibiotics the next time. So, physician prescribing of antibiotics in effect medicalizes illness and keeps the cycle of expectations, reconsultations, and prescriptions going.”
The study included 432 children aged 6 months to 12 years (median age, 3.2 years) who presented at 56 general practices in England with acute, uncomplicated lower respiratory tract infection (LRTI) of less than 21 days’ duration and in whom pneumonia was not suspected clinically. The children were randomly assigned to undergo 7 days of treatment with either amoxicillin 50 mg/kg or placebo. The primary outcome was duration of symptoms rated moderately bad or worse.
For up to 4 weeks, parents scored symptoms – including cough, phlegm, shortness of breath, wheeze, blocked or runny nose, disturbed sleep, feeling generally unwell, fever, and interference with normal activities – in a daily diary. The secondary outcome was symptom severity. Prespecified analyses were made for key clinical subgroups of patients for whom clinicians commonly prescribe (those with chest signs, fever, physician rating of unwell, sputum or chest rattle, and shortness of breath).
There was no significant difference in outcome between children treated with antibiotics and those treated with placebo. The median duration of moderately bad or worse symptoms was similar between the antibiotics group and the placebo group (5 vs. 6 days; hazard ratio, 1.13), as was the median time until symptoms were rated absent or as causing very little problem (7 vs. 8 days; HR, 1.09). There was a small significant difference between the groups in symptom severity score on days 2-4 after seeing the doctor (1.8 in the antibiotics group vs. 2.1 in the placebo group), “which was equivalent to less than one child in three rating symptoms a slight problem rather than very little problem,” the study authors report. “The treatment effects for all outcomes were similar for most subgroups ... but the effect of antibiotics was slightly, but not significantly, greater among those with fever or those who were unwell,” they add.
The investigators conclude that “similar to adults, antibiotics are unlikely to make a clinically important difference to the symptom burden for uncomplicated lower respiratory tract infections in children – both overall, and for the key clinical subgroups where antibiotic prescribing is most common.” They recommend that clinicians provide “safety-netting advice” to parents, such as explaining what illness course to expect and when a return visit would be necessary.
The findings provide “more evidence to do less,” wrote Rianne Oostenbrink, MD, PhD, from Erasmus MC-Sophia, in Rotterdam, the Netherlands, and Lina Jankauskaite, MD, PhD, from Lithuanian University of Health Sciences, Kaunas, in an accompanying comment.
“Overtesting and overtreatment of children are especially prominent in infectious diseases, when fever or other symptoms such as cough can be unspecific and can be of viral or bacterial origin,” they write.
The commenters note that despite antibiotics, most children did have moderately bad or worse symptoms on day 3, and symptoms had improved in about 75% of children in both groups at day 14. “A notable finding of this study is that only a few children had moderately bad or worse symptoms by day 14, and antibiotics did not alleviate the symptoms compared with placebo. Additionally, this trial aligns with other studies that have shown that reducing antibiotic treatment for LRTI is not associated with prolonged morbidity or higher incidence of complications.”
The study was funded by the UK National Institute for Health Research. Dr. Little, Dr. Jankauskaite, and Dr. Oostenbrink have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Unless pneumonia is suspected, clinicians should not prescribe antibiotics for most children with chest infections, according to findings of the ARTIC-PC randomized controlled trial, published in The Lancet.
“Prescribing for children with uncomplicated chest infections is still common in most countries,” said lead author Paul Little, MD, professor of primary care research at the University of Southampton, England, in an interview.
But there are barriers to stopping this practice, he said. “If you prescribe an antibiotic and the child gets better, even if the antibiotic was not doing that much, the parents then think that it was the antibiotic that was responsible for the recovery and so expect antibiotics the next time. So, physician prescribing of antibiotics in effect medicalizes illness and keeps the cycle of expectations, reconsultations, and prescriptions going.”
The study included 432 children aged 6 months to 12 years (median age, 3.2 years) who presented at 56 general practices in England with acute, uncomplicated lower respiratory tract infection (LRTI) of less than 21 days’ duration and in whom pneumonia was not suspected clinically. The children were randomly assigned to undergo 7 days of treatment with either amoxicillin 50 mg/kg or placebo. The primary outcome was duration of symptoms rated moderately bad or worse.
For up to 4 weeks, parents scored symptoms – including cough, phlegm, shortness of breath, wheeze, blocked or runny nose, disturbed sleep, feeling generally unwell, fever, and interference with normal activities – in a daily diary. The secondary outcome was symptom severity. Prespecified analyses were made for key clinical subgroups of patients for whom clinicians commonly prescribe (those with chest signs, fever, physician rating of unwell, sputum or chest rattle, and shortness of breath).
There was no significant difference in outcome between children treated with antibiotics and those treated with placebo. The median duration of moderately bad or worse symptoms was similar between the antibiotics group and the placebo group (5 vs. 6 days; hazard ratio, 1.13), as was the median time until symptoms were rated absent or as causing very little problem (7 vs. 8 days; HR, 1.09). There was a small significant difference between the groups in symptom severity score on days 2-4 after seeing the doctor (1.8 in the antibiotics group vs. 2.1 in the placebo group), “which was equivalent to less than one child in three rating symptoms a slight problem rather than very little problem,” the study authors report. “The treatment effects for all outcomes were similar for most subgroups ... but the effect of antibiotics was slightly, but not significantly, greater among those with fever or those who were unwell,” they add.
The investigators conclude that “similar to adults, antibiotics are unlikely to make a clinically important difference to the symptom burden for uncomplicated lower respiratory tract infections in children – both overall, and for the key clinical subgroups where antibiotic prescribing is most common.” They recommend that clinicians provide “safety-netting advice” to parents, such as explaining what illness course to expect and when a return visit would be necessary.
The findings provide “more evidence to do less,” wrote Rianne Oostenbrink, MD, PhD, from Erasmus MC-Sophia, in Rotterdam, the Netherlands, and Lina Jankauskaite, MD, PhD, from Lithuanian University of Health Sciences, Kaunas, in an accompanying comment.
“Overtesting and overtreatment of children are especially prominent in infectious diseases, when fever or other symptoms such as cough can be unspecific and can be of viral or bacterial origin,” they write.
The commenters note that despite antibiotics, most children did have moderately bad or worse symptoms on day 3, and symptoms had improved in about 75% of children in both groups at day 14. “A notable finding of this study is that only a few children had moderately bad or worse symptoms by day 14, and antibiotics did not alleviate the symptoms compared with placebo. Additionally, this trial aligns with other studies that have shown that reducing antibiotic treatment for LRTI is not associated with prolonged morbidity or higher incidence of complications.”
The study was funded by the UK National Institute for Health Research. Dr. Little, Dr. Jankauskaite, and Dr. Oostenbrink have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CrossFit enters primary care with fitness-minded docs, data
with the launch of its newest service, CrossFit Precision Care.
Developed by family medicine physician Julie Foucher, MD, and other CrossFit-trained doctors, the new service aims to help CrossFit members build plans to protect and improve their health, according to a statement by the company.
CrossFit Precision Care plans to meet this goal through utilizing doctors who understand the CrossFit philosophy, individualized care, data-driven recommendations, proactive lifestyle changes, and continual health optimization. Informing these plans and changes are CrossFit Precision Care’s analysis through a few different methods.
CrossFit’s partner in the endeavor, Wild Health, will provide genomic testing to determine a patient’s genetic predispositions to help optimize the health plans. Blood testing reveals many things that may affect a person’s health, such as hormone status, lipid levels, thyroid function, and cardiovascular risks. An overall lifestyle review includes exercise routines, eating habits, social life, and other patterns or behaviors.
Connecting with doctors who understand CrossFit
Dr. Foucher is no stranger to CrossFit. She has competed in the CrossFit Games four times and discusses the sport regularly on Twitter and Instagram. Now, she works directly with CrossFit to help it provide users with individualized data-driven plans.
“I met Eric Roza last July,” Dr. Foucher says of CrossFit’s CEO. “We talked and saw a lot of potential for CrossFit and health care providers to work together, so we started brainstorming.”
When Dr. Foucher and Mr. Roza got to know Wild Health, specifically, two of its physician cofounders, it was a natural fit, she said. Dr. Foucher says that many who train in CrossFit or go to CrossFit-affiliated gyms feel a disconnect with their family doctors: “[CrossFit is] a pretty polarizing topic, but there are also a lot of doctors who know that people are having health improvements with these programs,” she said.
Through use of Wild Health’s precision services and algorithms, CrossFit Precision Care plans to connect its users with CrossFit-trained health care practitioners. This personalized approach allows health care practitioners to build closer relationships with users of the program, who may feel more comfortable working with doctors who understand their lifestyle. Wild Health’s precision medicine approach, with trackable data such as biomarker status and risk scores, gives doctors a more complete picture of a patient’s needs and history, according to a statement on the partnership.
A better use of data
“To me,” Dr. Foucher says of family medicine, “that was the best option coming out of residency. It was consistent with my morals.” She says much of the current health care system is algorithm based. If a patient is experiencing certain symptoms, treatment is recommended on the basis of whatever yields the best results from the data – but this doesn’t always factor in a patient’s full history and genetics. It can be difficult for doctors to build trusting and personal relationships with patients. “In our current system, there’s not a lot of time or great tools to do that,” she says.
With the approach Wild Health and CrossFit Precision Care both use, however, Dr. Foucher says she sees a huge opportunity for optimizing patient and health care practitioner relationships.
“I see huge potential here, and I really think that this should be the standard for primary care going forwards,” Dr. Foucher explains. “The nice thing about [this approach] is that it has a really quick learning curve and is relatively easy to implement with patients. Before Wild Health optimized it, the tech and data would take about 10 hours per patient to put together. But now, we can incorporate things that work with wearable tech and track results over time and allow the patient and doctor to use this platform to create relationships. And this is something that can scale to many more patients.”
According to its website, CrossFit Precision Care is currently launching an invite-only beta test version of the program in eight states ahead of an expected national release.
A version of this article first appeared on Medscape.com.
with the launch of its newest service, CrossFit Precision Care.
Developed by family medicine physician Julie Foucher, MD, and other CrossFit-trained doctors, the new service aims to help CrossFit members build plans to protect and improve their health, according to a statement by the company.
CrossFit Precision Care plans to meet this goal through utilizing doctors who understand the CrossFit philosophy, individualized care, data-driven recommendations, proactive lifestyle changes, and continual health optimization. Informing these plans and changes are CrossFit Precision Care’s analysis through a few different methods.
CrossFit’s partner in the endeavor, Wild Health, will provide genomic testing to determine a patient’s genetic predispositions to help optimize the health plans. Blood testing reveals many things that may affect a person’s health, such as hormone status, lipid levels, thyroid function, and cardiovascular risks. An overall lifestyle review includes exercise routines, eating habits, social life, and other patterns or behaviors.
Connecting with doctors who understand CrossFit
Dr. Foucher is no stranger to CrossFit. She has competed in the CrossFit Games four times and discusses the sport regularly on Twitter and Instagram. Now, she works directly with CrossFit to help it provide users with individualized data-driven plans.
“I met Eric Roza last July,” Dr. Foucher says of CrossFit’s CEO. “We talked and saw a lot of potential for CrossFit and health care providers to work together, so we started brainstorming.”
When Dr. Foucher and Mr. Roza got to know Wild Health, specifically, two of its physician cofounders, it was a natural fit, she said. Dr. Foucher says that many who train in CrossFit or go to CrossFit-affiliated gyms feel a disconnect with their family doctors: “[CrossFit is] a pretty polarizing topic, but there are also a lot of doctors who know that people are having health improvements with these programs,” she said.
Through use of Wild Health’s precision services and algorithms, CrossFit Precision Care plans to connect its users with CrossFit-trained health care practitioners. This personalized approach allows health care practitioners to build closer relationships with users of the program, who may feel more comfortable working with doctors who understand their lifestyle. Wild Health’s precision medicine approach, with trackable data such as biomarker status and risk scores, gives doctors a more complete picture of a patient’s needs and history, according to a statement on the partnership.
A better use of data
“To me,” Dr. Foucher says of family medicine, “that was the best option coming out of residency. It was consistent with my morals.” She says much of the current health care system is algorithm based. If a patient is experiencing certain symptoms, treatment is recommended on the basis of whatever yields the best results from the data – but this doesn’t always factor in a patient’s full history and genetics. It can be difficult for doctors to build trusting and personal relationships with patients. “In our current system, there’s not a lot of time or great tools to do that,” she says.
With the approach Wild Health and CrossFit Precision Care both use, however, Dr. Foucher says she sees a huge opportunity for optimizing patient and health care practitioner relationships.
“I see huge potential here, and I really think that this should be the standard for primary care going forwards,” Dr. Foucher explains. “The nice thing about [this approach] is that it has a really quick learning curve and is relatively easy to implement with patients. Before Wild Health optimized it, the tech and data would take about 10 hours per patient to put together. But now, we can incorporate things that work with wearable tech and track results over time and allow the patient and doctor to use this platform to create relationships. And this is something that can scale to many more patients.”
According to its website, CrossFit Precision Care is currently launching an invite-only beta test version of the program in eight states ahead of an expected national release.
A version of this article first appeared on Medscape.com.
with the launch of its newest service, CrossFit Precision Care.
Developed by family medicine physician Julie Foucher, MD, and other CrossFit-trained doctors, the new service aims to help CrossFit members build plans to protect and improve their health, according to a statement by the company.
CrossFit Precision Care plans to meet this goal through utilizing doctors who understand the CrossFit philosophy, individualized care, data-driven recommendations, proactive lifestyle changes, and continual health optimization. Informing these plans and changes are CrossFit Precision Care’s analysis through a few different methods.
CrossFit’s partner in the endeavor, Wild Health, will provide genomic testing to determine a patient’s genetic predispositions to help optimize the health plans. Blood testing reveals many things that may affect a person’s health, such as hormone status, lipid levels, thyroid function, and cardiovascular risks. An overall lifestyle review includes exercise routines, eating habits, social life, and other patterns or behaviors.
Connecting with doctors who understand CrossFit
Dr. Foucher is no stranger to CrossFit. She has competed in the CrossFit Games four times and discusses the sport regularly on Twitter and Instagram. Now, she works directly with CrossFit to help it provide users with individualized data-driven plans.
“I met Eric Roza last July,” Dr. Foucher says of CrossFit’s CEO. “We talked and saw a lot of potential for CrossFit and health care providers to work together, so we started brainstorming.”
When Dr. Foucher and Mr. Roza got to know Wild Health, specifically, two of its physician cofounders, it was a natural fit, she said. Dr. Foucher says that many who train in CrossFit or go to CrossFit-affiliated gyms feel a disconnect with their family doctors: “[CrossFit is] a pretty polarizing topic, but there are also a lot of doctors who know that people are having health improvements with these programs,” she said.
Through use of Wild Health’s precision services and algorithms, CrossFit Precision Care plans to connect its users with CrossFit-trained health care practitioners. This personalized approach allows health care practitioners to build closer relationships with users of the program, who may feel more comfortable working with doctors who understand their lifestyle. Wild Health’s precision medicine approach, with trackable data such as biomarker status and risk scores, gives doctors a more complete picture of a patient’s needs and history, according to a statement on the partnership.
A better use of data
“To me,” Dr. Foucher says of family medicine, “that was the best option coming out of residency. It was consistent with my morals.” She says much of the current health care system is algorithm based. If a patient is experiencing certain symptoms, treatment is recommended on the basis of whatever yields the best results from the data – but this doesn’t always factor in a patient’s full history and genetics. It can be difficult for doctors to build trusting and personal relationships with patients. “In our current system, there’s not a lot of time or great tools to do that,” she says.
With the approach Wild Health and CrossFit Precision Care both use, however, Dr. Foucher says she sees a huge opportunity for optimizing patient and health care practitioner relationships.
“I see huge potential here, and I really think that this should be the standard for primary care going forwards,” Dr. Foucher explains. “The nice thing about [this approach] is that it has a really quick learning curve and is relatively easy to implement with patients. Before Wild Health optimized it, the tech and data would take about 10 hours per patient to put together. But now, we can incorporate things that work with wearable tech and track results over time and allow the patient and doctor to use this platform to create relationships. And this is something that can scale to many more patients.”
According to its website, CrossFit Precision Care is currently launching an invite-only beta test version of the program in eight states ahead of an expected national release.
A version of this article first appeared on Medscape.com.
FDA OKs iPLEDGE change for gender-neutral language
The Food and Drug Administration has approved a modification to the isotretinoin risk-mitigation program to make it more inclusive for transgender patients.
Beginning on Dec. 13, 2021, Previously, there were three risk categories: females of reproductive potential, females not of reproductive potential, and males.
In recent years, dermatologists and others have advocated for the change, hoping to make the process more inclusive and less intrusive for their transgender patients.
Isotretinoin (Accutane, Absorica, Amnesteem, Claravis, others) has a high risk of severe birth defects, and has been linked with other health issues, making it crucial for those with the ability to become pregnant to take contraceptive precautions while on the medication. Under the iPLEDGE program, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The FDA had given notification in June 2018 that the REMS modification and labeling change would be required, replacing the gender-specific language with gender-neutral language, according to an FDA spokesperson. The change was based on feedback that the gender-specific language can be a barrier to access for some patients. The FDA approved the modification on Oct. 8.
Expert reactions
“This is an exciting and welcome change from the FDA on iPLEDGE that many dermatologists, myself included, have advocated for quite a few years,” Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview.
In a report on the dermatologic care for lesbian, gay, bisexual, and transgender persons published in the Journal of the American Academy of Dermatology, Dr. Yeung and his colleagues noted that more than 10 million lesbian, gay, bisexual and transgender people live in the United States and that improving their health is a public health priority.
“For cisgender patients, nothing has changed – patients will continue to receive appropriate educational material related to isotretinoin based on their pregnancy potential,” Dr. Yeung said. “For transgender and gender diverse patients, this is a huge step forward.”
Under the previous system, doctors were asked to register patients using gender binary categories, “which were confusing when they did not reflect reality” for these patients, Dr. Yeung said. The new system, Dr. Yeung added, “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”
“The new terminology is not just respectful, it also is simpler and makes more sense,” agreed Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery at Mount Sinai Health System and professor of medicine at the Icahn School of Medicine at Mount Sinai, New York. “As it stood, a transgender man with his uterus and ovaries in place might be missed in the pregnancy surveillance system because he could simply be labeled a man and not followed further. At the same time, both transgender women and cisgender women who were at no risk of pregnancy could be subject to more medical scrutiny that might have been consider intrusive.”
The change “validates the important point that pregnancy potential is not exclusively defined by sociocultural constructs of gender and allow dermatologists to focus purely on what matters when prescribing isotretinoin – whether an individual is able to become pregnant or not, regardless of their gender identity,” Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., and suburban Maryland, who has also advocated for the change, said in an interview.
FDA elaborates
The modification includes important changes for doctors, pharmacists, and patients alike, according to the FDA.
Health care providers must assign and confirm their currently enrolled patient’s risk category when they first log in to the IPLEDGE REMS website on or after Dec. 13, the effective date. They should be sure any patient whose prescription RMA (iPLEDGE authorization) expires on Dec. 11-12 is told to obtain their prescription before midnight, Eastern time, Dec. 10.
Pharmacists will be affected, too, since the iPLEDGE REMS changed to a new platform vendor and the current “switch” pharmacy management system will be removed as a method to verify authorization to dispense isotretinoin. With these changes, as of Dec. 13, pharmacists can’t use the switch system to obtain a predispense authorization, or RMA (risk management authorization). They will need to obtain an RMA online by accessing the iPLEDGE REMS website or via telephone to the PLEDGE REMS center, 866-495-0654, before dispensing the prescription.
Patients, beginning Dec. 13, will have the option of presenting a unique QR code at the pharmacy on their smartphone rather than providing the iPLEDGE identification number. The code can be accessed by logging into their account on the iPLEDGE REMS website.
Patients with an isotretinoin prescription RMA that expires Dec. 11-12, must obtain the prescription before 11:59 p.m. Eastern time on Dec. 10. If the RMA expires before the prescription is picked up, the patient must begin the authorization process all over again.
Dr. Safer, Dr. Yeung, and Dr. Peebles have no relevant disclosures.
More information on the update and the isotretinoin REMS program is available on the FDA website.
The Food and Drug Administration has approved a modification to the isotretinoin risk-mitigation program to make it more inclusive for transgender patients.
Beginning on Dec. 13, 2021, Previously, there were three risk categories: females of reproductive potential, females not of reproductive potential, and males.
In recent years, dermatologists and others have advocated for the change, hoping to make the process more inclusive and less intrusive for their transgender patients.
Isotretinoin (Accutane, Absorica, Amnesteem, Claravis, others) has a high risk of severe birth defects, and has been linked with other health issues, making it crucial for those with the ability to become pregnant to take contraceptive precautions while on the medication. Under the iPLEDGE program, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The FDA had given notification in June 2018 that the REMS modification and labeling change would be required, replacing the gender-specific language with gender-neutral language, according to an FDA spokesperson. The change was based on feedback that the gender-specific language can be a barrier to access for some patients. The FDA approved the modification on Oct. 8.
Expert reactions
“This is an exciting and welcome change from the FDA on iPLEDGE that many dermatologists, myself included, have advocated for quite a few years,” Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview.
In a report on the dermatologic care for lesbian, gay, bisexual, and transgender persons published in the Journal of the American Academy of Dermatology, Dr. Yeung and his colleagues noted that more than 10 million lesbian, gay, bisexual and transgender people live in the United States and that improving their health is a public health priority.
“For cisgender patients, nothing has changed – patients will continue to receive appropriate educational material related to isotretinoin based on their pregnancy potential,” Dr. Yeung said. “For transgender and gender diverse patients, this is a huge step forward.”
Under the previous system, doctors were asked to register patients using gender binary categories, “which were confusing when they did not reflect reality” for these patients, Dr. Yeung said. The new system, Dr. Yeung added, “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”
“The new terminology is not just respectful, it also is simpler and makes more sense,” agreed Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery at Mount Sinai Health System and professor of medicine at the Icahn School of Medicine at Mount Sinai, New York. “As it stood, a transgender man with his uterus and ovaries in place might be missed in the pregnancy surveillance system because he could simply be labeled a man and not followed further. At the same time, both transgender women and cisgender women who were at no risk of pregnancy could be subject to more medical scrutiny that might have been consider intrusive.”
The change “validates the important point that pregnancy potential is not exclusively defined by sociocultural constructs of gender and allow dermatologists to focus purely on what matters when prescribing isotretinoin – whether an individual is able to become pregnant or not, regardless of their gender identity,” Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., and suburban Maryland, who has also advocated for the change, said in an interview.
FDA elaborates
The modification includes important changes for doctors, pharmacists, and patients alike, according to the FDA.
Health care providers must assign and confirm their currently enrolled patient’s risk category when they first log in to the IPLEDGE REMS website on or after Dec. 13, the effective date. They should be sure any patient whose prescription RMA (iPLEDGE authorization) expires on Dec. 11-12 is told to obtain their prescription before midnight, Eastern time, Dec. 10.
Pharmacists will be affected, too, since the iPLEDGE REMS changed to a new platform vendor and the current “switch” pharmacy management system will be removed as a method to verify authorization to dispense isotretinoin. With these changes, as of Dec. 13, pharmacists can’t use the switch system to obtain a predispense authorization, or RMA (risk management authorization). They will need to obtain an RMA online by accessing the iPLEDGE REMS website or via telephone to the PLEDGE REMS center, 866-495-0654, before dispensing the prescription.
Patients, beginning Dec. 13, will have the option of presenting a unique QR code at the pharmacy on their smartphone rather than providing the iPLEDGE identification number. The code can be accessed by logging into their account on the iPLEDGE REMS website.
Patients with an isotretinoin prescription RMA that expires Dec. 11-12, must obtain the prescription before 11:59 p.m. Eastern time on Dec. 10. If the RMA expires before the prescription is picked up, the patient must begin the authorization process all over again.
Dr. Safer, Dr. Yeung, and Dr. Peebles have no relevant disclosures.
More information on the update and the isotretinoin REMS program is available on the FDA website.
The Food and Drug Administration has approved a modification to the isotretinoin risk-mitigation program to make it more inclusive for transgender patients.
Beginning on Dec. 13, 2021, Previously, there were three risk categories: females of reproductive potential, females not of reproductive potential, and males.
In recent years, dermatologists and others have advocated for the change, hoping to make the process more inclusive and less intrusive for their transgender patients.
Isotretinoin (Accutane, Absorica, Amnesteem, Claravis, others) has a high risk of severe birth defects, and has been linked with other health issues, making it crucial for those with the ability to become pregnant to take contraceptive precautions while on the medication. Under the iPLEDGE program, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The FDA had given notification in June 2018 that the REMS modification and labeling change would be required, replacing the gender-specific language with gender-neutral language, according to an FDA spokesperson. The change was based on feedback that the gender-specific language can be a barrier to access for some patients. The FDA approved the modification on Oct. 8.
Expert reactions
“This is an exciting and welcome change from the FDA on iPLEDGE that many dermatologists, myself included, have advocated for quite a few years,” Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview.
In a report on the dermatologic care for lesbian, gay, bisexual, and transgender persons published in the Journal of the American Academy of Dermatology, Dr. Yeung and his colleagues noted that more than 10 million lesbian, gay, bisexual and transgender people live in the United States and that improving their health is a public health priority.
“For cisgender patients, nothing has changed – patients will continue to receive appropriate educational material related to isotretinoin based on their pregnancy potential,” Dr. Yeung said. “For transgender and gender diverse patients, this is a huge step forward.”
Under the previous system, doctors were asked to register patients using gender binary categories, “which were confusing when they did not reflect reality” for these patients, Dr. Yeung said. The new system, Dr. Yeung added, “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”
“The new terminology is not just respectful, it also is simpler and makes more sense,” agreed Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery at Mount Sinai Health System and professor of medicine at the Icahn School of Medicine at Mount Sinai, New York. “As it stood, a transgender man with his uterus and ovaries in place might be missed in the pregnancy surveillance system because he could simply be labeled a man and not followed further. At the same time, both transgender women and cisgender women who were at no risk of pregnancy could be subject to more medical scrutiny that might have been consider intrusive.”
The change “validates the important point that pregnancy potential is not exclusively defined by sociocultural constructs of gender and allow dermatologists to focus purely on what matters when prescribing isotretinoin – whether an individual is able to become pregnant or not, regardless of their gender identity,” Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., and suburban Maryland, who has also advocated for the change, said in an interview.
FDA elaborates
The modification includes important changes for doctors, pharmacists, and patients alike, according to the FDA.
Health care providers must assign and confirm their currently enrolled patient’s risk category when they first log in to the IPLEDGE REMS website on or after Dec. 13, the effective date. They should be sure any patient whose prescription RMA (iPLEDGE authorization) expires on Dec. 11-12 is told to obtain their prescription before midnight, Eastern time, Dec. 10.
Pharmacists will be affected, too, since the iPLEDGE REMS changed to a new platform vendor and the current “switch” pharmacy management system will be removed as a method to verify authorization to dispense isotretinoin. With these changes, as of Dec. 13, pharmacists can’t use the switch system to obtain a predispense authorization, or RMA (risk management authorization). They will need to obtain an RMA online by accessing the iPLEDGE REMS website or via telephone to the PLEDGE REMS center, 866-495-0654, before dispensing the prescription.
Patients, beginning Dec. 13, will have the option of presenting a unique QR code at the pharmacy on their smartphone rather than providing the iPLEDGE identification number. The code can be accessed by logging into their account on the iPLEDGE REMS website.
Patients with an isotretinoin prescription RMA that expires Dec. 11-12, must obtain the prescription before 11:59 p.m. Eastern time on Dec. 10. If the RMA expires before the prescription is picked up, the patient must begin the authorization process all over again.
Dr. Safer, Dr. Yeung, and Dr. Peebles have no relevant disclosures.
More information on the update and the isotretinoin REMS program is available on the FDA website.
9-step ladder may kids with allergies return to eggs
For many children in the process of outgrowing egg allergy, the step-wise reintroduction of foods that contain eggs can be achieved at home using a nine-rung laddered approach, according to updated guidelines from the British Society for Allergy and Clinical Immunology (BSACI).
Attempts to reintroduce egg into the child’s diet can start at the age of 12 months or 6 months from the last reaction, as long as past reactions have been mild to moderate and the child does not have asthma, according to guidelines from the BSACI, which represents allergists, pediatricians, and other health care practitioners.
According to the guidelines, the reintroduction needs to be guided by a specialist allergy service for children who have had severe reactions to egg or who have asthma.
Susan C. Leech, MB BChir, DCH, first author of the guidelines and a consultant in pediatric allergy with the Department of Child Health at Kings College Hospital, London, told this news organization that home reintroduction should begin slowly with small amounts of baked egg, starting with a pea-sized piece of cake, and should proceed gradually.
“Parents can be reassured that it’s a relatively safe thing to do as long as it’s done with caution,” said Dr. Leech.
The expanded guidelines include a new nine-step reintroduction ladder. It builds on a three-stage classification of egg-containing foods that was first introduced in BSACI guidelines in 2010.
On the bottom four rungs, children work their way through small but increasing amounts of fairy cakes (cupcakes), biscuits (cookies), and other foods containing baked eggs.
The next three rungs involve hard-boiled eggs, quiche, and other well-cooked egg products.
At the eighth rung, children can have small mouthfuls of runny scrambled eggs, mayonnaise, and other less-cooked or raw egg-containing products. At the top rung, children can have increasing amounts of those products as well as licks of cake batter.
The guidelines were published online September 29 in Clinical and Experimental Allergy along with a supplement that includes a series of examples showing how the guidelines apply to specific patient cases.
“These are examples only,” the guideline authors caution in the appendix. “Clinical judgment of severity is important as risk assessment is not always easy.”
Anna Nowak-Wegrzyn, MD, PhD, a professor of pediatrics at NYU Grossman School of Medicine and chief of pediatric allergy and immunology for Hassenfeld Children’s Hospital at NYU Langone, who was not involved in the BSACI guidelines, described the egg ladder as a “proactive” strategy that deserves further study and consideration.
“I think that this may be a valid approach,” said Dr. Nowak-Wegrzyn in an interview. “Eggs have good nutritional value, and they are present in a lot of foods, so avoidance creates logistical challenges.”
Using the egg ladder for home-based reintroduction may be especially suited in resource-poor areas where access to an allergist may be difficult, she said. It may also be suited for families that can’t visit the office because of pandemic-related restrictions.
“If the child had a severe reaction or if they have asthma, then it’s a no-go,” she added, “but if you have a patient who has a really mild reaction and you think that overall the risk of a significant reaction or bad symptoms is low, then it may be worth doing.”
Dr. Leech and Dr. Nowak-Wegrzyn have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For many children in the process of outgrowing egg allergy, the step-wise reintroduction of foods that contain eggs can be achieved at home using a nine-rung laddered approach, according to updated guidelines from the British Society for Allergy and Clinical Immunology (BSACI).
Attempts to reintroduce egg into the child’s diet can start at the age of 12 months or 6 months from the last reaction, as long as past reactions have been mild to moderate and the child does not have asthma, according to guidelines from the BSACI, which represents allergists, pediatricians, and other health care practitioners.
According to the guidelines, the reintroduction needs to be guided by a specialist allergy service for children who have had severe reactions to egg or who have asthma.
Susan C. Leech, MB BChir, DCH, first author of the guidelines and a consultant in pediatric allergy with the Department of Child Health at Kings College Hospital, London, told this news organization that home reintroduction should begin slowly with small amounts of baked egg, starting with a pea-sized piece of cake, and should proceed gradually.
“Parents can be reassured that it’s a relatively safe thing to do as long as it’s done with caution,” said Dr. Leech.
The expanded guidelines include a new nine-step reintroduction ladder. It builds on a three-stage classification of egg-containing foods that was first introduced in BSACI guidelines in 2010.
On the bottom four rungs, children work their way through small but increasing amounts of fairy cakes (cupcakes), biscuits (cookies), and other foods containing baked eggs.
The next three rungs involve hard-boiled eggs, quiche, and other well-cooked egg products.
At the eighth rung, children can have small mouthfuls of runny scrambled eggs, mayonnaise, and other less-cooked or raw egg-containing products. At the top rung, children can have increasing amounts of those products as well as licks of cake batter.
The guidelines were published online September 29 in Clinical and Experimental Allergy along with a supplement that includes a series of examples showing how the guidelines apply to specific patient cases.
“These are examples only,” the guideline authors caution in the appendix. “Clinical judgment of severity is important as risk assessment is not always easy.”
Anna Nowak-Wegrzyn, MD, PhD, a professor of pediatrics at NYU Grossman School of Medicine and chief of pediatric allergy and immunology for Hassenfeld Children’s Hospital at NYU Langone, who was not involved in the BSACI guidelines, described the egg ladder as a “proactive” strategy that deserves further study and consideration.
“I think that this may be a valid approach,” said Dr. Nowak-Wegrzyn in an interview. “Eggs have good nutritional value, and they are present in a lot of foods, so avoidance creates logistical challenges.”
Using the egg ladder for home-based reintroduction may be especially suited in resource-poor areas where access to an allergist may be difficult, she said. It may also be suited for families that can’t visit the office because of pandemic-related restrictions.
“If the child had a severe reaction or if they have asthma, then it’s a no-go,” she added, “but if you have a patient who has a really mild reaction and you think that overall the risk of a significant reaction or bad symptoms is low, then it may be worth doing.”
Dr. Leech and Dr. Nowak-Wegrzyn have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For many children in the process of outgrowing egg allergy, the step-wise reintroduction of foods that contain eggs can be achieved at home using a nine-rung laddered approach, according to updated guidelines from the British Society for Allergy and Clinical Immunology (BSACI).
Attempts to reintroduce egg into the child’s diet can start at the age of 12 months or 6 months from the last reaction, as long as past reactions have been mild to moderate and the child does not have asthma, according to guidelines from the BSACI, which represents allergists, pediatricians, and other health care practitioners.
According to the guidelines, the reintroduction needs to be guided by a specialist allergy service for children who have had severe reactions to egg or who have asthma.
Susan C. Leech, MB BChir, DCH, first author of the guidelines and a consultant in pediatric allergy with the Department of Child Health at Kings College Hospital, London, told this news organization that home reintroduction should begin slowly with small amounts of baked egg, starting with a pea-sized piece of cake, and should proceed gradually.
“Parents can be reassured that it’s a relatively safe thing to do as long as it’s done with caution,” said Dr. Leech.
The expanded guidelines include a new nine-step reintroduction ladder. It builds on a three-stage classification of egg-containing foods that was first introduced in BSACI guidelines in 2010.
On the bottom four rungs, children work their way through small but increasing amounts of fairy cakes (cupcakes), biscuits (cookies), and other foods containing baked eggs.
The next three rungs involve hard-boiled eggs, quiche, and other well-cooked egg products.
At the eighth rung, children can have small mouthfuls of runny scrambled eggs, mayonnaise, and other less-cooked or raw egg-containing products. At the top rung, children can have increasing amounts of those products as well as licks of cake batter.
The guidelines were published online September 29 in Clinical and Experimental Allergy along with a supplement that includes a series of examples showing how the guidelines apply to specific patient cases.
“These are examples only,” the guideline authors caution in the appendix. “Clinical judgment of severity is important as risk assessment is not always easy.”
Anna Nowak-Wegrzyn, MD, PhD, a professor of pediatrics at NYU Grossman School of Medicine and chief of pediatric allergy and immunology for Hassenfeld Children’s Hospital at NYU Langone, who was not involved in the BSACI guidelines, described the egg ladder as a “proactive” strategy that deserves further study and consideration.
“I think that this may be a valid approach,” said Dr. Nowak-Wegrzyn in an interview. “Eggs have good nutritional value, and they are present in a lot of foods, so avoidance creates logistical challenges.”
Using the egg ladder for home-based reintroduction may be especially suited in resource-poor areas where access to an allergist may be difficult, she said. It may also be suited for families that can’t visit the office because of pandemic-related restrictions.
“If the child had a severe reaction or if they have asthma, then it’s a no-go,” she added, “but if you have a patient who has a really mild reaction and you think that overall the risk of a significant reaction or bad symptoms is low, then it may be worth doing.”
Dr. Leech and Dr. Nowak-Wegrzyn have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pandemic data challenges infection link to Guillain-Barré syndrome
While pediatric cases of various types of infections fell by 45%-95% during the early months of the pandemic, cases of acute inflammatory demyelinating polyneuropathy (AIDP), an inflammatory neuropathy belonging to the clinical spectrum of Guillain-Barré syndrome, only fell by about 32%-37%, a rate that’s similar to the 35.1% decline in overall hospital admissions over that time period, researchers found. There was also no apparent link between the appearance of COVID-19 and the number of reported AIDP cases.
“There was no clear association between respiratory or gastrointestinal infections and rates of AIDP. Further, we found that AIDP did not have the expected dramatic reduction when community-acquired infections decreased during the pandemic,” Children’s Hospital of Philadelphia neurologist Craig A. Press, MD, PhD, said in an interview.
Dr. Press and colleagues presented their findings in a poster at the 50th annual meeting of the Child Neurology Society.
According to Dr. Press, the cause of AIDP in most patients is unclear, although infections and vaccinations are often linked to cases. “However, the data supporting this link is often weak. Infections with Campylobacter jejuni [bacteria that causes food poisoning] are known to be associated with AIDP, while rates of AIDP in the general population and in those with influenza are similar.”
For the new multicenter, cross-sectional study, researchers tracked AIDP data from the 47 pediatric hospitals that provide statistics to the Pediatric Health Information System. They focused on the period from January 2017 to September 2020, which included the first months of the COVID-19 pandemic in the United States.
“Social distancing, masks, and increased hand hygiene decrease community-acquired infectious rates in a dramatic way,” Dr. Press said. “If these infections were causing AIDP, we hypothesized that the cases of AIDP would drop substantially as a result.”
But this didn’t appear to happen. Researchers found that the numbers of various types of infections declined from April to September 2020: Respiratory infections dipped by 73%-78%, gastrointestinal infections fell by 45%-61%, and influenza infections dipped by 88%-95%. But AIDP cases didn’t fall as precipitously. In fact, their levels were about the same as they were in April 2017, a month when rates of gastrointestinal, respiratory disease and influenza infections were at seasonally low – but not abnormal – ebbs.
“While we must be cautious interpreting the results,” Dr. Press said, “this makes the link between infections as the main driver of pediatric AIDP less likely.”
However, he said, “this study does not exclude the possibility that rare infections cause AIDP – the data supporting that some more rare infections like campylobacter have a connection to AIDP are more robust – or that common infections very rarely lead to AIDP. While we look for triggers causing inflammatory disorders, AIDP maybe an autoinflammatory disorder without a clear trigger.”
Going forward, Dr. Press said, “we hope to look at infectious data in a more granular way to identify if specific viral or bacterial infectious may be associated with this or other inflammatory disorders. We believe that the use of data like this and the natural experiment that COVID-19 provided may help us to explore the impact of infections on disorders thought to be postinfectious.”
No study funding is reported, and the authors report no relevant disclosures.
While pediatric cases of various types of infections fell by 45%-95% during the early months of the pandemic, cases of acute inflammatory demyelinating polyneuropathy (AIDP), an inflammatory neuropathy belonging to the clinical spectrum of Guillain-Barré syndrome, only fell by about 32%-37%, a rate that’s similar to the 35.1% decline in overall hospital admissions over that time period, researchers found. There was also no apparent link between the appearance of COVID-19 and the number of reported AIDP cases.
“There was no clear association between respiratory or gastrointestinal infections and rates of AIDP. Further, we found that AIDP did not have the expected dramatic reduction when community-acquired infections decreased during the pandemic,” Children’s Hospital of Philadelphia neurologist Craig A. Press, MD, PhD, said in an interview.
Dr. Press and colleagues presented their findings in a poster at the 50th annual meeting of the Child Neurology Society.
According to Dr. Press, the cause of AIDP in most patients is unclear, although infections and vaccinations are often linked to cases. “However, the data supporting this link is often weak. Infections with Campylobacter jejuni [bacteria that causes food poisoning] are known to be associated with AIDP, while rates of AIDP in the general population and in those with influenza are similar.”
For the new multicenter, cross-sectional study, researchers tracked AIDP data from the 47 pediatric hospitals that provide statistics to the Pediatric Health Information System. They focused on the period from January 2017 to September 2020, which included the first months of the COVID-19 pandemic in the United States.
“Social distancing, masks, and increased hand hygiene decrease community-acquired infectious rates in a dramatic way,” Dr. Press said. “If these infections were causing AIDP, we hypothesized that the cases of AIDP would drop substantially as a result.”
But this didn’t appear to happen. Researchers found that the numbers of various types of infections declined from April to September 2020: Respiratory infections dipped by 73%-78%, gastrointestinal infections fell by 45%-61%, and influenza infections dipped by 88%-95%. But AIDP cases didn’t fall as precipitously. In fact, their levels were about the same as they were in April 2017, a month when rates of gastrointestinal, respiratory disease and influenza infections were at seasonally low – but not abnormal – ebbs.
“While we must be cautious interpreting the results,” Dr. Press said, “this makes the link between infections as the main driver of pediatric AIDP less likely.”
However, he said, “this study does not exclude the possibility that rare infections cause AIDP – the data supporting that some more rare infections like campylobacter have a connection to AIDP are more robust – or that common infections very rarely lead to AIDP. While we look for triggers causing inflammatory disorders, AIDP maybe an autoinflammatory disorder without a clear trigger.”
Going forward, Dr. Press said, “we hope to look at infectious data in a more granular way to identify if specific viral or bacterial infectious may be associated with this or other inflammatory disorders. We believe that the use of data like this and the natural experiment that COVID-19 provided may help us to explore the impact of infections on disorders thought to be postinfectious.”
No study funding is reported, and the authors report no relevant disclosures.
While pediatric cases of various types of infections fell by 45%-95% during the early months of the pandemic, cases of acute inflammatory demyelinating polyneuropathy (AIDP), an inflammatory neuropathy belonging to the clinical spectrum of Guillain-Barré syndrome, only fell by about 32%-37%, a rate that’s similar to the 35.1% decline in overall hospital admissions over that time period, researchers found. There was also no apparent link between the appearance of COVID-19 and the number of reported AIDP cases.
“There was no clear association between respiratory or gastrointestinal infections and rates of AIDP. Further, we found that AIDP did not have the expected dramatic reduction when community-acquired infections decreased during the pandemic,” Children’s Hospital of Philadelphia neurologist Craig A. Press, MD, PhD, said in an interview.
Dr. Press and colleagues presented their findings in a poster at the 50th annual meeting of the Child Neurology Society.
According to Dr. Press, the cause of AIDP in most patients is unclear, although infections and vaccinations are often linked to cases. “However, the data supporting this link is often weak. Infections with Campylobacter jejuni [bacteria that causes food poisoning] are known to be associated with AIDP, while rates of AIDP in the general population and in those with influenza are similar.”
For the new multicenter, cross-sectional study, researchers tracked AIDP data from the 47 pediatric hospitals that provide statistics to the Pediatric Health Information System. They focused on the period from January 2017 to September 2020, which included the first months of the COVID-19 pandemic in the United States.
“Social distancing, masks, and increased hand hygiene decrease community-acquired infectious rates in a dramatic way,” Dr. Press said. “If these infections were causing AIDP, we hypothesized that the cases of AIDP would drop substantially as a result.”
But this didn’t appear to happen. Researchers found that the numbers of various types of infections declined from April to September 2020: Respiratory infections dipped by 73%-78%, gastrointestinal infections fell by 45%-61%, and influenza infections dipped by 88%-95%. But AIDP cases didn’t fall as precipitously. In fact, their levels were about the same as they were in April 2017, a month when rates of gastrointestinal, respiratory disease and influenza infections were at seasonally low – but not abnormal – ebbs.
“While we must be cautious interpreting the results,” Dr. Press said, “this makes the link between infections as the main driver of pediatric AIDP less likely.”
However, he said, “this study does not exclude the possibility that rare infections cause AIDP – the data supporting that some more rare infections like campylobacter have a connection to AIDP are more robust – or that common infections very rarely lead to AIDP. While we look for triggers causing inflammatory disorders, AIDP maybe an autoinflammatory disorder without a clear trigger.”
Going forward, Dr. Press said, “we hope to look at infectious data in a more granular way to identify if specific viral or bacterial infectious may be associated with this or other inflammatory disorders. We believe that the use of data like this and the natural experiment that COVID-19 provided may help us to explore the impact of infections on disorders thought to be postinfectious.”
No study funding is reported, and the authors report no relevant disclosures.
FROM CNS 2021
Epidiolex plus THC lowers seizures in pediatric epilepsy
the component of cannabis that makes people high in larger quantities, researchers reported.
“THC can contribute to seizure control and mitigation some of the side effects of CBD,” said study coauthor and Austin, Tex., child neurologist Karen Keough, MD, in an interview. Dr. Keough and colleagues presented their findings at the 50th annual meeting of the Child Neurology Society.
In a landmark move, the Food and Drug Administration approved Epidiolex in 2018 for the treatment of seizures in two rare forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome. The agency had never before approved a drug with a purified ingredient derived from marijuana.
CBD, the active ingredient in Epidiolex, is nonpsychoactive. The use in medicine of THC, the main driver of marijuana’s ability to make people stoned, is much more controversial.
Dr. Keough said she had treated 60-70 children with CBD, at the same strength as in Epidiolex (100 mg), and 5 mg of THC before the drug was approved. “I was seeing some very impressive results, and some became seizure free who’d always been refractory,” she said.
When the Epidiolex became available, she said, some patients transitioned to it and stopped taking THC. According to her, some patients fared well. But others immediately experienced worse seizures, she said, and some developed side effects to Epidiolex in the absence of THC, such as agitation and appetite suppression.
Combination therapy
For the new study, a retrospective, unblinded cohort analysis, Dr. Keough and colleagues tracked patients who received various doses of CBD, in some cases as Epidiolex, and various doses of THC prescribed by the Texas Original Compassionate Cultivation dispensary, where she serves as chief medical officer.
The initial number of patients was 212; 135 consented to review and 10 were excluded for various reasons leaving a total of 74 subjects in the study. The subjects, whose median age at the start of the study was 12 years (range, 2-25 years), were tracked from 2018 to2021. Just over half (55%) were male, and they remained on the regimen for a median of 805 days (range, 400-1,141).
Of the 74 subjects, 45.9% had a reduction of seizures of more than 75%, and 20.3% had a reduction of 50%-75%. Only 4.1% saw their seizures worsen.
The THC doses varied from none to more than 12 mg/day; CBD doses varied from none to more than 26 mg/kg per day. O the 74 patients, 18 saw their greatest seizure reduction from baseline when they received no THC; 12 saw their greatest seizure reduction from baseline when they received 0-2 mg/kg per day of CBD.
Still controversial
Did the patients get high? In some cases they did, Dr. Keough said. However, “a lot of these patients are either too young or too cognitively limited to describe whether they’re feeling intoxicated. That’s one of the many reasons why this is so controversial. You have to go into this with eyes wide open. We’re working in an environment with limited information as to what an intoxicating dose is for a small kid.”
However, she said, it seems clear that “THC can enhance the effect of CBD in children with epilepsy” and reduce CBD side effects. It’s not surprising that the substances work differently since they interact with brain cells in different ways, she said.
For neurologists, she said, “the challenge is to find a reliable source of THC that you can count on and verify so you aren’t overdosing the patients.”
University of Saskatchewan, Saskatoon, child neurologist and cannabinoid researcher Richard Huntsman, MD, who’s familiar with the study findings, said in an interview that they “provide another strong signal that the addition of THC provides benefit, at least in some patients.”
But it’s still unclear “why some children respond best in regards to seizure reduction and side effect profile with combination CBD:THC therapy, and others seemed to do better with CBD alone,” he said. Also unknown: “the ideal THC:CBD ratio that allows optimal seizure control while preventing the potential harmful effects of THC.”
As for the future, he said, “as we are just scratching the surface of our knowledge about the use of cannabis-based therapies in children with neurological disorders, I suspect that the use of these therapies will expand over time.”
No study funding is reported. Dr. Keough disclosed serving as chief medical officer of Texas Original Compassionate Cultivation. Dr. Huntsman disclosed serving as lead investigator of the Cannabidiol in Children with Refractory Epileptic Encephalopathy study and serving on the boards of the Cannabinoid Research Initiative of Saskatchewan (University of Saskatchewan) and Canadian Childhood Cannabinoid Clinical Trials Consortium. He is also cochair of Health Canada’s Scientific Advisory Committee on Cannabinoids for Health Purposes.
the component of cannabis that makes people high in larger quantities, researchers reported.
“THC can contribute to seizure control and mitigation some of the side effects of CBD,” said study coauthor and Austin, Tex., child neurologist Karen Keough, MD, in an interview. Dr. Keough and colleagues presented their findings at the 50th annual meeting of the Child Neurology Society.
In a landmark move, the Food and Drug Administration approved Epidiolex in 2018 for the treatment of seizures in two rare forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome. The agency had never before approved a drug with a purified ingredient derived from marijuana.
CBD, the active ingredient in Epidiolex, is nonpsychoactive. The use in medicine of THC, the main driver of marijuana’s ability to make people stoned, is much more controversial.
Dr. Keough said she had treated 60-70 children with CBD, at the same strength as in Epidiolex (100 mg), and 5 mg of THC before the drug was approved. “I was seeing some very impressive results, and some became seizure free who’d always been refractory,” she said.
When the Epidiolex became available, she said, some patients transitioned to it and stopped taking THC. According to her, some patients fared well. But others immediately experienced worse seizures, she said, and some developed side effects to Epidiolex in the absence of THC, such as agitation and appetite suppression.
Combination therapy
For the new study, a retrospective, unblinded cohort analysis, Dr. Keough and colleagues tracked patients who received various doses of CBD, in some cases as Epidiolex, and various doses of THC prescribed by the Texas Original Compassionate Cultivation dispensary, where she serves as chief medical officer.
The initial number of patients was 212; 135 consented to review and 10 were excluded for various reasons leaving a total of 74 subjects in the study. The subjects, whose median age at the start of the study was 12 years (range, 2-25 years), were tracked from 2018 to2021. Just over half (55%) were male, and they remained on the regimen for a median of 805 days (range, 400-1,141).
Of the 74 subjects, 45.9% had a reduction of seizures of more than 75%, and 20.3% had a reduction of 50%-75%. Only 4.1% saw their seizures worsen.
The THC doses varied from none to more than 12 mg/day; CBD doses varied from none to more than 26 mg/kg per day. O the 74 patients, 18 saw their greatest seizure reduction from baseline when they received no THC; 12 saw their greatest seizure reduction from baseline when they received 0-2 mg/kg per day of CBD.
Still controversial
Did the patients get high? In some cases they did, Dr. Keough said. However, “a lot of these patients are either too young or too cognitively limited to describe whether they’re feeling intoxicated. That’s one of the many reasons why this is so controversial. You have to go into this with eyes wide open. We’re working in an environment with limited information as to what an intoxicating dose is for a small kid.”
However, she said, it seems clear that “THC can enhance the effect of CBD in children with epilepsy” and reduce CBD side effects. It’s not surprising that the substances work differently since they interact with brain cells in different ways, she said.
For neurologists, she said, “the challenge is to find a reliable source of THC that you can count on and verify so you aren’t overdosing the patients.”
University of Saskatchewan, Saskatoon, child neurologist and cannabinoid researcher Richard Huntsman, MD, who’s familiar with the study findings, said in an interview that they “provide another strong signal that the addition of THC provides benefit, at least in some patients.”
But it’s still unclear “why some children respond best in regards to seizure reduction and side effect profile with combination CBD:THC therapy, and others seemed to do better with CBD alone,” he said. Also unknown: “the ideal THC:CBD ratio that allows optimal seizure control while preventing the potential harmful effects of THC.”
As for the future, he said, “as we are just scratching the surface of our knowledge about the use of cannabis-based therapies in children with neurological disorders, I suspect that the use of these therapies will expand over time.”
No study funding is reported. Dr. Keough disclosed serving as chief medical officer of Texas Original Compassionate Cultivation. Dr. Huntsman disclosed serving as lead investigator of the Cannabidiol in Children with Refractory Epileptic Encephalopathy study and serving on the boards of the Cannabinoid Research Initiative of Saskatchewan (University of Saskatchewan) and Canadian Childhood Cannabinoid Clinical Trials Consortium. He is also cochair of Health Canada’s Scientific Advisory Committee on Cannabinoids for Health Purposes.
the component of cannabis that makes people high in larger quantities, researchers reported.
“THC can contribute to seizure control and mitigation some of the side effects of CBD,” said study coauthor and Austin, Tex., child neurologist Karen Keough, MD, in an interview. Dr. Keough and colleagues presented their findings at the 50th annual meeting of the Child Neurology Society.
In a landmark move, the Food and Drug Administration approved Epidiolex in 2018 for the treatment of seizures in two rare forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome. The agency had never before approved a drug with a purified ingredient derived from marijuana.
CBD, the active ingredient in Epidiolex, is nonpsychoactive. The use in medicine of THC, the main driver of marijuana’s ability to make people stoned, is much more controversial.
Dr. Keough said she had treated 60-70 children with CBD, at the same strength as in Epidiolex (100 mg), and 5 mg of THC before the drug was approved. “I was seeing some very impressive results, and some became seizure free who’d always been refractory,” she said.
When the Epidiolex became available, she said, some patients transitioned to it and stopped taking THC. According to her, some patients fared well. But others immediately experienced worse seizures, she said, and some developed side effects to Epidiolex in the absence of THC, such as agitation and appetite suppression.
Combination therapy
For the new study, a retrospective, unblinded cohort analysis, Dr. Keough and colleagues tracked patients who received various doses of CBD, in some cases as Epidiolex, and various doses of THC prescribed by the Texas Original Compassionate Cultivation dispensary, where she serves as chief medical officer.
The initial number of patients was 212; 135 consented to review and 10 were excluded for various reasons leaving a total of 74 subjects in the study. The subjects, whose median age at the start of the study was 12 years (range, 2-25 years), were tracked from 2018 to2021. Just over half (55%) were male, and they remained on the regimen for a median of 805 days (range, 400-1,141).
Of the 74 subjects, 45.9% had a reduction of seizures of more than 75%, and 20.3% had a reduction of 50%-75%. Only 4.1% saw their seizures worsen.
The THC doses varied from none to more than 12 mg/day; CBD doses varied from none to more than 26 mg/kg per day. O the 74 patients, 18 saw their greatest seizure reduction from baseline when they received no THC; 12 saw their greatest seizure reduction from baseline when they received 0-2 mg/kg per day of CBD.
Still controversial
Did the patients get high? In some cases they did, Dr. Keough said. However, “a lot of these patients are either too young or too cognitively limited to describe whether they’re feeling intoxicated. That’s one of the many reasons why this is so controversial. You have to go into this with eyes wide open. We’re working in an environment with limited information as to what an intoxicating dose is for a small kid.”
However, she said, it seems clear that “THC can enhance the effect of CBD in children with epilepsy” and reduce CBD side effects. It’s not surprising that the substances work differently since they interact with brain cells in different ways, she said.
For neurologists, she said, “the challenge is to find a reliable source of THC that you can count on and verify so you aren’t overdosing the patients.”
University of Saskatchewan, Saskatoon, child neurologist and cannabinoid researcher Richard Huntsman, MD, who’s familiar with the study findings, said in an interview that they “provide another strong signal that the addition of THC provides benefit, at least in some patients.”
But it’s still unclear “why some children respond best in regards to seizure reduction and side effect profile with combination CBD:THC therapy, and others seemed to do better with CBD alone,” he said. Also unknown: “the ideal THC:CBD ratio that allows optimal seizure control while preventing the potential harmful effects of THC.”
As for the future, he said, “as we are just scratching the surface of our knowledge about the use of cannabis-based therapies in children with neurological disorders, I suspect that the use of these therapies will expand over time.”
No study funding is reported. Dr. Keough disclosed serving as chief medical officer of Texas Original Compassionate Cultivation. Dr. Huntsman disclosed serving as lead investigator of the Cannabidiol in Children with Refractory Epileptic Encephalopathy study and serving on the boards of the Cannabinoid Research Initiative of Saskatchewan (University of Saskatchewan) and Canadian Childhood Cannabinoid Clinical Trials Consortium. He is also cochair of Health Canada’s Scientific Advisory Committee on Cannabinoids for Health Purposes.
FROM CNS 2021
‘Baby-wearing’ poses serious injury risks for infants, ED data show
Baby-wearing – carrying a child against your body in a sling, soft carrier, or other device – is associated with benefits like reduced crying and increased breastfeeding, studies have shown.
But this practice also entails risks. Babies can fall out of carriers, or be injured when an adult carrying them falls, for example.
researchers estimated in a study presented at the annual meeting of the American Academy of Pediatrics.
To characterize the epidemiology of these injuries, Samantha J. Rowe, MD, chief resident physician at Walter Reed National Military Medical Center in Bethesda, Md., and colleagues analyzed data from the National Electronic Injury Surveillance System between 2011 and 2020.
They included in their analysis data from patients aged 5 years and younger who sustained an injury associated with a baby-wearing product. Baby harnesses, carriers, slings, framed baby carriers, and soft baby carriers were among the devices included in the study. The researchers used 601 cases to generate national estimates.
An estimated 14,024 patients presented to EDs because of baby-wearing injuries, and 52% of the injuries occurred when a patient fell from the product.
Most injuries (61%) occurred in children aged 5 months and younger; 19.3% of these infants required hospitalization, most often for head injuries.
The investigators found that about 22% of the injuries were associated with a caregiver falling, noted Rachel Y. Moon, MD, who was not involved in the study.
“Carrying a baby changes your center of gravity – and can also obscure your vision of where you’re walking, so adults who use these devices should be cognizant of this,” said Dr. Moon, with the University of Virginia, Charlottesville.
Dr. Rowe often practiced baby-wearing with her daughter, and found that it was beneficial. And studies have demonstrated various benefits of baby-wearing, including improved thermoregulation and glycemic control.
Still, the new analysis illustrates the potential for baby-wearing products “to cause serious injury, especially in infants 5 months and younger,” Dr. Rowe said. “We need to provide more education to caregivers on safe baby-wearing and continue to improve our safety standards for baby-wearing products.”
Study coauthor Patrick T. Reeves, MD, with the Naval Medical Center at San Diego, offered additional guidance in a news release: “Like when buying a new pair of shoes, parents must be educated on the proper sizing, selection, and wear of baby carriers to prevent injury to themselves and their child.”
Parents also need to ensure that the child’s nose and mouth are not obstructed, Dr. Moon
In a recent article discussing the possible benefits of baby-wearing in terms of helping with breastfeeding, Dr. Moon also pointed out further safety considerations: “No matter which carrier is used, for safety reasons, we need to remind parents that the baby should be positioned so that the head is upright and the nose and mouth are not obstructed.”
The researchers and Dr. Moon had no relevant financial disclosures.
Baby-wearing – carrying a child against your body in a sling, soft carrier, or other device – is associated with benefits like reduced crying and increased breastfeeding, studies have shown.
But this practice also entails risks. Babies can fall out of carriers, or be injured when an adult carrying them falls, for example.
researchers estimated in a study presented at the annual meeting of the American Academy of Pediatrics.
To characterize the epidemiology of these injuries, Samantha J. Rowe, MD, chief resident physician at Walter Reed National Military Medical Center in Bethesda, Md., and colleagues analyzed data from the National Electronic Injury Surveillance System between 2011 and 2020.
They included in their analysis data from patients aged 5 years and younger who sustained an injury associated with a baby-wearing product. Baby harnesses, carriers, slings, framed baby carriers, and soft baby carriers were among the devices included in the study. The researchers used 601 cases to generate national estimates.
An estimated 14,024 patients presented to EDs because of baby-wearing injuries, and 52% of the injuries occurred when a patient fell from the product.
Most injuries (61%) occurred in children aged 5 months and younger; 19.3% of these infants required hospitalization, most often for head injuries.
The investigators found that about 22% of the injuries were associated with a caregiver falling, noted Rachel Y. Moon, MD, who was not involved in the study.
“Carrying a baby changes your center of gravity – and can also obscure your vision of where you’re walking, so adults who use these devices should be cognizant of this,” said Dr. Moon, with the University of Virginia, Charlottesville.
Dr. Rowe often practiced baby-wearing with her daughter, and found that it was beneficial. And studies have demonstrated various benefits of baby-wearing, including improved thermoregulation and glycemic control.
Still, the new analysis illustrates the potential for baby-wearing products “to cause serious injury, especially in infants 5 months and younger,” Dr. Rowe said. “We need to provide more education to caregivers on safe baby-wearing and continue to improve our safety standards for baby-wearing products.”
Study coauthor Patrick T. Reeves, MD, with the Naval Medical Center at San Diego, offered additional guidance in a news release: “Like when buying a new pair of shoes, parents must be educated on the proper sizing, selection, and wear of baby carriers to prevent injury to themselves and their child.”
Parents also need to ensure that the child’s nose and mouth are not obstructed, Dr. Moon
In a recent article discussing the possible benefits of baby-wearing in terms of helping with breastfeeding, Dr. Moon also pointed out further safety considerations: “No matter which carrier is used, for safety reasons, we need to remind parents that the baby should be positioned so that the head is upright and the nose and mouth are not obstructed.”
The researchers and Dr. Moon had no relevant financial disclosures.
Baby-wearing – carrying a child against your body in a sling, soft carrier, or other device – is associated with benefits like reduced crying and increased breastfeeding, studies have shown.
But this practice also entails risks. Babies can fall out of carriers, or be injured when an adult carrying them falls, for example.
researchers estimated in a study presented at the annual meeting of the American Academy of Pediatrics.
To characterize the epidemiology of these injuries, Samantha J. Rowe, MD, chief resident physician at Walter Reed National Military Medical Center in Bethesda, Md., and colleagues analyzed data from the National Electronic Injury Surveillance System between 2011 and 2020.
They included in their analysis data from patients aged 5 years and younger who sustained an injury associated with a baby-wearing product. Baby harnesses, carriers, slings, framed baby carriers, and soft baby carriers were among the devices included in the study. The researchers used 601 cases to generate national estimates.
An estimated 14,024 patients presented to EDs because of baby-wearing injuries, and 52% of the injuries occurred when a patient fell from the product.
Most injuries (61%) occurred in children aged 5 months and younger; 19.3% of these infants required hospitalization, most often for head injuries.
The investigators found that about 22% of the injuries were associated with a caregiver falling, noted Rachel Y. Moon, MD, who was not involved in the study.
“Carrying a baby changes your center of gravity – and can also obscure your vision of where you’re walking, so adults who use these devices should be cognizant of this,” said Dr. Moon, with the University of Virginia, Charlottesville.
Dr. Rowe often practiced baby-wearing with her daughter, and found that it was beneficial. And studies have demonstrated various benefits of baby-wearing, including improved thermoregulation and glycemic control.
Still, the new analysis illustrates the potential for baby-wearing products “to cause serious injury, especially in infants 5 months and younger,” Dr. Rowe said. “We need to provide more education to caregivers on safe baby-wearing and continue to improve our safety standards for baby-wearing products.”
Study coauthor Patrick T. Reeves, MD, with the Naval Medical Center at San Diego, offered additional guidance in a news release: “Like when buying a new pair of shoes, parents must be educated on the proper sizing, selection, and wear of baby carriers to prevent injury to themselves and their child.”
Parents also need to ensure that the child’s nose and mouth are not obstructed, Dr. Moon
In a recent article discussing the possible benefits of baby-wearing in terms of helping with breastfeeding, Dr. Moon also pointed out further safety considerations: “No matter which carrier is used, for safety reasons, we need to remind parents that the baby should be positioned so that the head is upright and the nose and mouth are not obstructed.”
The researchers and Dr. Moon had no relevant financial disclosures.
FROM AAP 2021
Is genetic testing valuable in the clinical management of epilepsy?
, new research shows.
Results of a survey that included more than 400 patients showed that positive findings from genetic testing helped guide clinical management in 50% of cases and improved patient outcomes in 75%. In addition, the findings were applicable to both children and adults.
“Fifty percent of the time the physicians reported that, yes, receiving the genetic diagnosis did change how they managed the patients,” reported co-investigator Dianalee McKnight, PhD, director of medical affairs at Invitae, a medical genetic testing company headquartered in San Francisco. In 81.3% of cases, providers reported they changed clinical management within 3 months of receiving the genetic results, she added.
The findings were presented at the 2021 World Congress of Neurology (WCN).
Test results can be practice-changing
Nearly 50% of positive genetic test results in epilepsy patients can help guide clinical management, Dr. McKnight noted. However, information on how physicians use genetic information in decision-making has been limited, prompting her conduct the survey.
A total of 1,567 physicians with 3,572 patients who had a definitive diagnosis of epilepsy were contacted. A total of 170 (10.8%) clinicians provided completed and eligible surveys on 429 patients with epilepsy.
The patient cohort comprised mostly children, with nearly 50 adults, which Dr. McKnight said is typical of the population receiving genetic testing in clinical practice.
She reported that genetic testing results prompted clinicians to make medication changes about 50% of the time. Other changes included specialist referral or to a clinical trial, monitoring for other neurological disease, and recommendations for dietary change or for surgery.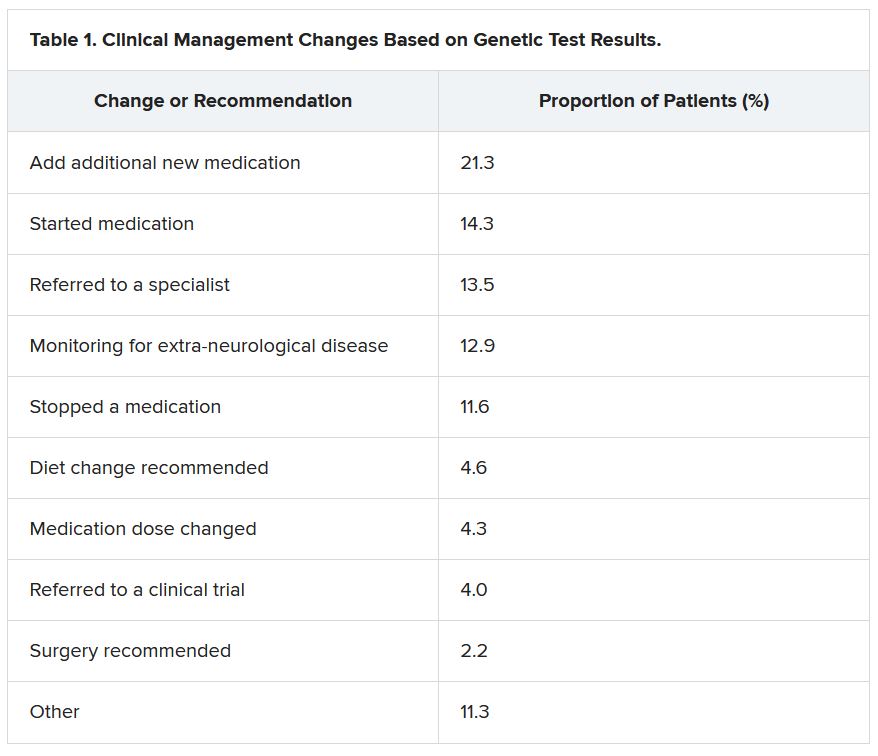
“Of the physicians who changed treatment, 75% reported there were positive outcomes for the patients,” Dr. McKnight told meeting attendees. “Most common was a reduction or a complete elimination of seizures, and that was reported in 65% of the cases.”
In many cases, the changes resulted in clinical improvements.
“There were 64 individuals who were having daily seizures before the genetic testing,” Dr. McKnight reported via email. “After receiving the genetic diagnosis and modifying their treatment, their physicians reported that 26% of individuals had complete seizure control and 46% of individuals had reduced seizure frequency to either weekly (20%), monthly (20%) or annually (6%).”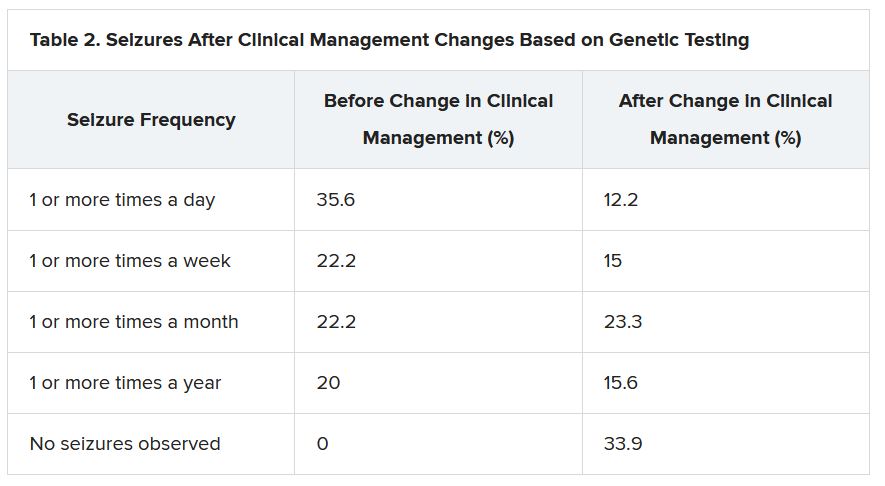
The best seizure control after modifying disease management occurred among children. Although the changes were not as dramatic for adults, they trended toward lower seizure frequency.
“It is still pretty significant that adults can receive genetic testing later in life and still have benefit in controlling their seizures,” Dr. McKnight said.
Twenty-three percent of patients showed improvement in behavior, development, academics, or movement issues, while 6% experienced reduced medication side effects.
Dr. McKnight also explored reasons for physicians not making changes to clinical management of patients based on the genetic results. The most common reason was that management was already consistent with the results (47.3%), followed by the results not being informative (26.1%), the results possibly being useful for future treatments in development (19.0%), or other or unknown reasons (7.6%).
Besides direct health and quality of life benefits from better seizure control, Dr. McKnight cited previous economic studies showing lower health care costs.
“It looked like an individual who has good seizure control will incur about 14,000 U.S. dollars a year compared with an individual with pretty poor seizure control, where it can be closer to 23,000 U.S. dollars a year,” Dr. McKnight said. This is mainly attributed to reduced hospitalizations and emergency department visits.
Dr. McKnight noted that currently there is no cost of genetic testing to the patient, the hospital, or insurers. Pharmaceutical companies, she said, sponsor the testing to potentially gather patients for clinical drug trials in development. However, patients remain completely anonymous.
Physicians who wish to have patient samples tested agree that the companies may contact them to ask if any of their patients with positive genetic test results would like to participate in a trial.
Dr. McKnight noted that genetic testing can be considered actionable in the clinic, helping to guide clinical decision-making and potentially leading to better outcomes. Going forward, she suggested performing large case-controlled studies “of individuals with the same genetic etiology ... to really find a true causation or correlation.”
Growing influence of genetic testing
Commenting on the findings, Jaysingh Singh, MD, co-director of the Epilepsy Surgery Center at the Ohio State University Wexner Medical Center in Columbus, noted that the study highlights the value of gene testing in improving outcomes in patients with epilepsy, particularly the pediatric population.
He said the findings make him optimistic about the potential of genetic testing in adult patients – with at least one caveat.
“The limitation is that if we do find some mutation, we don’t know what to do with that. That’s definitely one challenge. And we see that more often in the adult patient population,” said Dr. Singh, who was not involved with the research.
He noted that there is a small group of genetic mutations when, found in adults, may dramatically alter treatment.
For example, he noted that if there is a gene mutation related to mTOR pathways, that could provide a future target because there are already medications that target this pathway.
Genetic testing may also be useful in cases where patients have normal brain imaging and poor response to standard treatment or in cases where patients have congenital abnormalities such as intellectual impairment or facial dysmorphic features and a co-morbid seizure disorder, he said.
Dr. Singh noted that he has often found genetic testing impractical because “if I order DNA testing right now, it will take 4 months for me to get the results. I cannot wait 4 months for the results to come back” to adjust treatment.
Dr. McKnight is an employee of and a shareholder in Invitae, which funded the study. Dr. Singh has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
Results of a survey that included more than 400 patients showed that positive findings from genetic testing helped guide clinical management in 50% of cases and improved patient outcomes in 75%. In addition, the findings were applicable to both children and adults.
“Fifty percent of the time the physicians reported that, yes, receiving the genetic diagnosis did change how they managed the patients,” reported co-investigator Dianalee McKnight, PhD, director of medical affairs at Invitae, a medical genetic testing company headquartered in San Francisco. In 81.3% of cases, providers reported they changed clinical management within 3 months of receiving the genetic results, she added.
The findings were presented at the 2021 World Congress of Neurology (WCN).
Test results can be practice-changing
Nearly 50% of positive genetic test results in epilepsy patients can help guide clinical management, Dr. McKnight noted. However, information on how physicians use genetic information in decision-making has been limited, prompting her conduct the survey.
A total of 1,567 physicians with 3,572 patients who had a definitive diagnosis of epilepsy were contacted. A total of 170 (10.8%) clinicians provided completed and eligible surveys on 429 patients with epilepsy.
The patient cohort comprised mostly children, with nearly 50 adults, which Dr. McKnight said is typical of the population receiving genetic testing in clinical practice.
She reported that genetic testing results prompted clinicians to make medication changes about 50% of the time. Other changes included specialist referral or to a clinical trial, monitoring for other neurological disease, and recommendations for dietary change or for surgery.
“Of the physicians who changed treatment, 75% reported there were positive outcomes for the patients,” Dr. McKnight told meeting attendees. “Most common was a reduction or a complete elimination of seizures, and that was reported in 65% of the cases.”
In many cases, the changes resulted in clinical improvements.
“There were 64 individuals who were having daily seizures before the genetic testing,” Dr. McKnight reported via email. “After receiving the genetic diagnosis and modifying their treatment, their physicians reported that 26% of individuals had complete seizure control and 46% of individuals had reduced seizure frequency to either weekly (20%), monthly (20%) or annually (6%).”
The best seizure control after modifying disease management occurred among children. Although the changes were not as dramatic for adults, they trended toward lower seizure frequency.
“It is still pretty significant that adults can receive genetic testing later in life and still have benefit in controlling their seizures,” Dr. McKnight said.
Twenty-three percent of patients showed improvement in behavior, development, academics, or movement issues, while 6% experienced reduced medication side effects.
Dr. McKnight also explored reasons for physicians not making changes to clinical management of patients based on the genetic results. The most common reason was that management was already consistent with the results (47.3%), followed by the results not being informative (26.1%), the results possibly being useful for future treatments in development (19.0%), or other or unknown reasons (7.6%).
Besides direct health and quality of life benefits from better seizure control, Dr. McKnight cited previous economic studies showing lower health care costs.
“It looked like an individual who has good seizure control will incur about 14,000 U.S. dollars a year compared with an individual with pretty poor seizure control, where it can be closer to 23,000 U.S. dollars a year,” Dr. McKnight said. This is mainly attributed to reduced hospitalizations and emergency department visits.
Dr. McKnight noted that currently there is no cost of genetic testing to the patient, the hospital, or insurers. Pharmaceutical companies, she said, sponsor the testing to potentially gather patients for clinical drug trials in development. However, patients remain completely anonymous.
Physicians who wish to have patient samples tested agree that the companies may contact them to ask if any of their patients with positive genetic test results would like to participate in a trial.
Dr. McKnight noted that genetic testing can be considered actionable in the clinic, helping to guide clinical decision-making and potentially leading to better outcomes. Going forward, she suggested performing large case-controlled studies “of individuals with the same genetic etiology ... to really find a true causation or correlation.”
Growing influence of genetic testing
Commenting on the findings, Jaysingh Singh, MD, co-director of the Epilepsy Surgery Center at the Ohio State University Wexner Medical Center in Columbus, noted that the study highlights the value of gene testing in improving outcomes in patients with epilepsy, particularly the pediatric population.
He said the findings make him optimistic about the potential of genetic testing in adult patients – with at least one caveat.
“The limitation is that if we do find some mutation, we don’t know what to do with that. That’s definitely one challenge. And we see that more often in the adult patient population,” said Dr. Singh, who was not involved with the research.
He noted that there is a small group of genetic mutations when, found in adults, may dramatically alter treatment.
For example, he noted that if there is a gene mutation related to mTOR pathways, that could provide a future target because there are already medications that target this pathway.
Genetic testing may also be useful in cases where patients have normal brain imaging and poor response to standard treatment or in cases where patients have congenital abnormalities such as intellectual impairment or facial dysmorphic features and a co-morbid seizure disorder, he said.
Dr. Singh noted that he has often found genetic testing impractical because “if I order DNA testing right now, it will take 4 months for me to get the results. I cannot wait 4 months for the results to come back” to adjust treatment.
Dr. McKnight is an employee of and a shareholder in Invitae, which funded the study. Dr. Singh has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
Results of a survey that included more than 400 patients showed that positive findings from genetic testing helped guide clinical management in 50% of cases and improved patient outcomes in 75%. In addition, the findings were applicable to both children and adults.
“Fifty percent of the time the physicians reported that, yes, receiving the genetic diagnosis did change how they managed the patients,” reported co-investigator Dianalee McKnight, PhD, director of medical affairs at Invitae, a medical genetic testing company headquartered in San Francisco. In 81.3% of cases, providers reported they changed clinical management within 3 months of receiving the genetic results, she added.
The findings were presented at the 2021 World Congress of Neurology (WCN).
Test results can be practice-changing
Nearly 50% of positive genetic test results in epilepsy patients can help guide clinical management, Dr. McKnight noted. However, information on how physicians use genetic information in decision-making has been limited, prompting her conduct the survey.
A total of 1,567 physicians with 3,572 patients who had a definitive diagnosis of epilepsy were contacted. A total of 170 (10.8%) clinicians provided completed and eligible surveys on 429 patients with epilepsy.
The patient cohort comprised mostly children, with nearly 50 adults, which Dr. McKnight said is typical of the population receiving genetic testing in clinical practice.
She reported that genetic testing results prompted clinicians to make medication changes about 50% of the time. Other changes included specialist referral or to a clinical trial, monitoring for other neurological disease, and recommendations for dietary change or for surgery.
“Of the physicians who changed treatment, 75% reported there were positive outcomes for the patients,” Dr. McKnight told meeting attendees. “Most common was a reduction or a complete elimination of seizures, and that was reported in 65% of the cases.”
In many cases, the changes resulted in clinical improvements.
“There were 64 individuals who were having daily seizures before the genetic testing,” Dr. McKnight reported via email. “After receiving the genetic diagnosis and modifying their treatment, their physicians reported that 26% of individuals had complete seizure control and 46% of individuals had reduced seizure frequency to either weekly (20%), monthly (20%) or annually (6%).”
The best seizure control after modifying disease management occurred among children. Although the changes were not as dramatic for adults, they trended toward lower seizure frequency.
“It is still pretty significant that adults can receive genetic testing later in life and still have benefit in controlling their seizures,” Dr. McKnight said.
Twenty-three percent of patients showed improvement in behavior, development, academics, or movement issues, while 6% experienced reduced medication side effects.
Dr. McKnight also explored reasons for physicians not making changes to clinical management of patients based on the genetic results. The most common reason was that management was already consistent with the results (47.3%), followed by the results not being informative (26.1%), the results possibly being useful for future treatments in development (19.0%), or other or unknown reasons (7.6%).
Besides direct health and quality of life benefits from better seizure control, Dr. McKnight cited previous economic studies showing lower health care costs.
“It looked like an individual who has good seizure control will incur about 14,000 U.S. dollars a year compared with an individual with pretty poor seizure control, where it can be closer to 23,000 U.S. dollars a year,” Dr. McKnight said. This is mainly attributed to reduced hospitalizations and emergency department visits.
Dr. McKnight noted that currently there is no cost of genetic testing to the patient, the hospital, or insurers. Pharmaceutical companies, she said, sponsor the testing to potentially gather patients for clinical drug trials in development. However, patients remain completely anonymous.
Physicians who wish to have patient samples tested agree that the companies may contact them to ask if any of their patients with positive genetic test results would like to participate in a trial.
Dr. McKnight noted that genetic testing can be considered actionable in the clinic, helping to guide clinical decision-making and potentially leading to better outcomes. Going forward, she suggested performing large case-controlled studies “of individuals with the same genetic etiology ... to really find a true causation or correlation.”
Growing influence of genetic testing
Commenting on the findings, Jaysingh Singh, MD, co-director of the Epilepsy Surgery Center at the Ohio State University Wexner Medical Center in Columbus, noted that the study highlights the value of gene testing in improving outcomes in patients with epilepsy, particularly the pediatric population.
He said the findings make him optimistic about the potential of genetic testing in adult patients – with at least one caveat.
“The limitation is that if we do find some mutation, we don’t know what to do with that. That’s definitely one challenge. And we see that more often in the adult patient population,” said Dr. Singh, who was not involved with the research.
He noted that there is a small group of genetic mutations when, found in adults, may dramatically alter treatment.
For example, he noted that if there is a gene mutation related to mTOR pathways, that could provide a future target because there are already medications that target this pathway.
Genetic testing may also be useful in cases where patients have normal brain imaging and poor response to standard treatment or in cases where patients have congenital abnormalities such as intellectual impairment or facial dysmorphic features and a co-morbid seizure disorder, he said.
Dr. Singh noted that he has often found genetic testing impractical because “if I order DNA testing right now, it will take 4 months for me to get the results. I cannot wait 4 months for the results to come back” to adjust treatment.
Dr. McKnight is an employee of and a shareholder in Invitae, which funded the study. Dr. Singh has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From WCN 2021



