User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Fetuses suffer the effects of poverty in the womb
Poverty is known to be associated with poor health outcomes throughout life. Now, new research has shown that, from as early as the second trimester of pregnancy, fetuses are already feeling the effects of poverty.
“There is a well-recognized health inequality where quality and duration of life are lower among the most poor. This divide is present both within and between countries,” said Steve Turner, who led the study.
Given the association of poverty and low birth weight, the authors of the new multi-national study, published in the Journal of Epidemiology and Community Health, hypothesized that “individuals from highest household income compared to those with lowest household income will have increased fetal size in the second and third trimester and birth.”
For their study, researchers from the University of Aberdeen gathered details of ante-natal and birth size – second and third trimester fetal ultrasound measurements of estimated fetal weight, biparietal diameter, and femur length, as well as birth measurements of weight, occipitofrontal circumference, and crown heel length – from eight cohorts that included 21,714 individuals from nations including Scotland, England, Saudi Arabia, the U.S., Netherlands, Spain, Norway, Sweden, and France.
They then related these to household income, taking into account other factors, including mother’s age, height, number of other children, and smoking, analyzing the data using cross-sectional two-stage individual patient data analyses and a longitudinal one-stage individual patient data analysis.
Household income closely related to birth size
The authors found that higher household income was associated with larger fetal head size and weight but not length, from the second half of pregnancy, compared with lowest household income. They said that their results argue for “a relationship where household income is closely related to birth size.”
The results showed that, across the countries studied, babies were smaller at birth if they came from a lower income household, and this discrepancy in size was already apparent at 20 weeks gestation.
“This is the first time that size differences have been found at such an early stage of development,” the authors said, “and also the first time it has been compared across continents.”
Professor Turner pointed out that “what this study shows is that the inequality, as seen by reduced size in fetal life, is present long before birth, and this poverty gap widens between twenty weeks gestation and birth.”
He added: “Basically, regardless of whether you live in Saudi, the U.S., or Europe, and accounting for things that might affect fetal growth, if your parents are poor, you will be smaller before birth and at birth compared to if your parents were not poor.”
Increase engagement with pregnant mothers living in poverty
He emphasized how this was problematic, as small size before and after birth puts an individual at “increased risk for many serious illnesses in later life.”
The authors hope that this study will encourage health care providers to recognize the health risks associated with lower income for mothers and their unborn children and to provide more support and guidance to mitigate the risks.
They said, “interventions aimed at softening the impact of poverty on pregnant mothers could reduce incidence of small for gestational age and the associated burden of excessive morbidity and mortality throughout the life course.”
Professor Turner described how the mechanisms that drive this inequity may be explained by pregnant mothers from poor households having difficulty in accessing or engaging with antenatal care.
“We would like to see health care providers around the world strive to increase engagement with pregnant mothers living in poverty,” he said. “This engagement will reward all of society by putting unborn children on a trajectory to longer and healthier lives.”
A version of this article first appeared on Medscape UK.
Poverty is known to be associated with poor health outcomes throughout life. Now, new research has shown that, from as early as the second trimester of pregnancy, fetuses are already feeling the effects of poverty.
“There is a well-recognized health inequality where quality and duration of life are lower among the most poor. This divide is present both within and between countries,” said Steve Turner, who led the study.
Given the association of poverty and low birth weight, the authors of the new multi-national study, published in the Journal of Epidemiology and Community Health, hypothesized that “individuals from highest household income compared to those with lowest household income will have increased fetal size in the second and third trimester and birth.”
For their study, researchers from the University of Aberdeen gathered details of ante-natal and birth size – second and third trimester fetal ultrasound measurements of estimated fetal weight, biparietal diameter, and femur length, as well as birth measurements of weight, occipitofrontal circumference, and crown heel length – from eight cohorts that included 21,714 individuals from nations including Scotland, England, Saudi Arabia, the U.S., Netherlands, Spain, Norway, Sweden, and France.
They then related these to household income, taking into account other factors, including mother’s age, height, number of other children, and smoking, analyzing the data using cross-sectional two-stage individual patient data analyses and a longitudinal one-stage individual patient data analysis.
Household income closely related to birth size
The authors found that higher household income was associated with larger fetal head size and weight but not length, from the second half of pregnancy, compared with lowest household income. They said that their results argue for “a relationship where household income is closely related to birth size.”
The results showed that, across the countries studied, babies were smaller at birth if they came from a lower income household, and this discrepancy in size was already apparent at 20 weeks gestation.
“This is the first time that size differences have been found at such an early stage of development,” the authors said, “and also the first time it has been compared across continents.”
Professor Turner pointed out that “what this study shows is that the inequality, as seen by reduced size in fetal life, is present long before birth, and this poverty gap widens between twenty weeks gestation and birth.”
He added: “Basically, regardless of whether you live in Saudi, the U.S., or Europe, and accounting for things that might affect fetal growth, if your parents are poor, you will be smaller before birth and at birth compared to if your parents were not poor.”
Increase engagement with pregnant mothers living in poverty
He emphasized how this was problematic, as small size before and after birth puts an individual at “increased risk for many serious illnesses in later life.”
The authors hope that this study will encourage health care providers to recognize the health risks associated with lower income for mothers and their unborn children and to provide more support and guidance to mitigate the risks.
They said, “interventions aimed at softening the impact of poverty on pregnant mothers could reduce incidence of small for gestational age and the associated burden of excessive morbidity and mortality throughout the life course.”
Professor Turner described how the mechanisms that drive this inequity may be explained by pregnant mothers from poor households having difficulty in accessing or engaging with antenatal care.
“We would like to see health care providers around the world strive to increase engagement with pregnant mothers living in poverty,” he said. “This engagement will reward all of society by putting unborn children on a trajectory to longer and healthier lives.”
A version of this article first appeared on Medscape UK.
Poverty is known to be associated with poor health outcomes throughout life. Now, new research has shown that, from as early as the second trimester of pregnancy, fetuses are already feeling the effects of poverty.
“There is a well-recognized health inequality where quality and duration of life are lower among the most poor. This divide is present both within and between countries,” said Steve Turner, who led the study.
Given the association of poverty and low birth weight, the authors of the new multi-national study, published in the Journal of Epidemiology and Community Health, hypothesized that “individuals from highest household income compared to those with lowest household income will have increased fetal size in the second and third trimester and birth.”
For their study, researchers from the University of Aberdeen gathered details of ante-natal and birth size – second and third trimester fetal ultrasound measurements of estimated fetal weight, biparietal diameter, and femur length, as well as birth measurements of weight, occipitofrontal circumference, and crown heel length – from eight cohorts that included 21,714 individuals from nations including Scotland, England, Saudi Arabia, the U.S., Netherlands, Spain, Norway, Sweden, and France.
They then related these to household income, taking into account other factors, including mother’s age, height, number of other children, and smoking, analyzing the data using cross-sectional two-stage individual patient data analyses and a longitudinal one-stage individual patient data analysis.
Household income closely related to birth size
The authors found that higher household income was associated with larger fetal head size and weight but not length, from the second half of pregnancy, compared with lowest household income. They said that their results argue for “a relationship where household income is closely related to birth size.”
The results showed that, across the countries studied, babies were smaller at birth if they came from a lower income household, and this discrepancy in size was already apparent at 20 weeks gestation.
“This is the first time that size differences have been found at such an early stage of development,” the authors said, “and also the first time it has been compared across continents.”
Professor Turner pointed out that “what this study shows is that the inequality, as seen by reduced size in fetal life, is present long before birth, and this poverty gap widens between twenty weeks gestation and birth.”
He added: “Basically, regardless of whether you live in Saudi, the U.S., or Europe, and accounting for things that might affect fetal growth, if your parents are poor, you will be smaller before birth and at birth compared to if your parents were not poor.”
Increase engagement with pregnant mothers living in poverty
He emphasized how this was problematic, as small size before and after birth puts an individual at “increased risk for many serious illnesses in later life.”
The authors hope that this study will encourage health care providers to recognize the health risks associated with lower income for mothers and their unborn children and to provide more support and guidance to mitigate the risks.
They said, “interventions aimed at softening the impact of poverty on pregnant mothers could reduce incidence of small for gestational age and the associated burden of excessive morbidity and mortality throughout the life course.”
Professor Turner described how the mechanisms that drive this inequity may be explained by pregnant mothers from poor households having difficulty in accessing or engaging with antenatal care.
“We would like to see health care providers around the world strive to increase engagement with pregnant mothers living in poverty,” he said. “This engagement will reward all of society by putting unborn children on a trajectory to longer and healthier lives.”
A version of this article first appeared on Medscape UK.
FROM THE JOURNAL OF EPIDEMIOLOGY AND COMMUNITY HEALTH
Emerging tick-borne pathogen has spread to state of Georgia
Heartland virus (HRTV), an emerging infection first detected in lone star ticks in Missouri in 2009, has spread to lone star ticks in Georgia, a study published in Emerging Infectious Diseases reports.
HRTV disease is transmitted by the bite of an infected Amblyomma americanum tick, named “lone star” because of the silver-white spot on the female scutum (back).
“By … sampling … in an area with reported exposure to HRTV in wildlife and humans and testing for infection in thousands of ticks from multiple sites and physiologic stages, we confirmed the presence of HRTV in Georgia,” the authors write.
“This information about the expanding geographic range of lone star ticks, combined with increased human presence in tick-infested habitats, can be used to improve strategies for preventing tick bites and to alert physicians about this emerging tickborne virus infection,” a press release by the Centers for Disease Control and Prevention notes.
Persistent field and lab work led to HRTV discovery in Georgia
The search for infected lone star ticks began after a retroactive analysis confirmed that a person who died in Georgia in 2005 from an unidentified illness was infected with HRTV. A subsequent analysis of serum samples collected earlier from local white-tailed deer showed that the deer had been exposed to HRTV since at least 2001, according to a press release by Emory University.
These discoveries prompted local researchers to investigate whether lone star ticks in rural, woodsy central Georgia were carrying HRTV.
Lead study author Yamila Romer, MD, an infectious disease clinician and microbiologist in the department of environmental sciences at Emory University in Atlanta, and her colleagues collected samples of ticks in 2018 at 26 sites near the location of the patient who died and the seropositive deer. In 2019, they focused their collections on the two sites that had provided the most ticks in 2018.
From April to October in both years, the research team visited sites weekly to swish white flannel flags through underbrush. They picked off adult and nymph Amblyomma americanum ticks, placed them into vials, and transported them to their lab. They sorted 9,294 ticks by sex, life stage, and collection site. Then they crushed the ticks and extracted their RNA.
To confirm viral infection, the team tested RNA extracted from cell culture supernatants using a real-time polymerase chain reaction test specific for HRTV.
In the three pools of ticks that tested positive for HRTV, the researchers found a minimum infection rate of 0.46/1,000 ticks, suggesting that about 1 of every 2,000 ticks carried HRTV. They sequenced the genome of the three isolates and found that the genomes were similar to one another but were very different from the genomes from HRTV samples taken outside Georgia.
Catherine A. Hill, PhD, a professor of entomology and vector biology and the interim head of the department of entomology at Purdue University in West Lafayette, Ind., was impressed with the researchers’ discovery.
“Heartland virus is difficult to detect,” she said in an email. “The prevalence of human cases is low, and the virus appears to be present at very low levels in populations of lone star tick. The investigators went to some lengths to survey for the virus, collect, and process thousands of ticks – and they found the needle in the haystack.” Dr. Hill was not involved in the study.
Georgia data help researchers monitor HRTV spread
HRTV was first identified in 2009 in Missouri in two people hospitalized with fever, muscle pain, diarrhea, and low white blood cell and platelet counts. Researchers traced the infections to lone star ticks, and they found antibodies to the virus in blood samples from deer and other wild mammals.
According to the CDC, U.S. cases of tick-borne diseases more than doubled between 2004 and 2016. As of January 2021, more than 50 human cases of HRTV disease had been reported in 11 Midwestern and Southeastern states: Arkansas, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Missouri, North Carolina, Oklahoma, and Tennessee.
Precautions, signs, symptoms, testing, and treatment
“The lone star tick is aggressive and will actively seek out a human host to bite,” Dr. Hill noted.
She recommends that health care providers advise patients to avoid tick habitat, wear protective clothing, apply repellants, know the signs and symptoms of tick-borne disease, and seek immediate medical care if they become ill.
Common symptoms of HRTV disease include fatigue, fever, nausea, diarrhea, and anorexia. Treatment is supportive. Many patients have been hospitalized, and some with comorbidities have died.
HRTV infection is rarely tested for, and the disease burden is unknown. With no commercial tests available in the United States, the CDC performs molecular and serologic testing for HRTV infection. The agency advises doctors to contact their state health department if they suspect a patient may have HRTV disease.
Further research is needed
Samantha M. Wisely, PhD, a professor of wildlife ecology and the director of the Cervidae Health Research Initiative at the University of Florida in Gainesville, was not surprised by the study finding.
“The more we look for heartland virus, the more places we find it,” Dr. Wisely told this news organization in an email.
“Little is known about which wildlife play a role in maintaining the virus on the landscape,” said Dr. Wisely, who was not involved in the study. “White-tailed deer have been shown to produce antibodies, meaning they have been exposed to the virus, but no one has actually found the virus in a wildlife species.”
The whole-genome sequencing of the virus was particularly important, Dr. Wisely explained. “Whole-genome data allow researchers to better understand viral evolution, pathogenicity, and viral dynamics across space and time – how it is evolving.”
The study was supported by a grant from the Emory University Research Council. The authors, Dr. Wisely, and Dr. Hill have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Heartland virus (HRTV), an emerging infection first detected in lone star ticks in Missouri in 2009, has spread to lone star ticks in Georgia, a study published in Emerging Infectious Diseases reports.
HRTV disease is transmitted by the bite of an infected Amblyomma americanum tick, named “lone star” because of the silver-white spot on the female scutum (back).
“By … sampling … in an area with reported exposure to HRTV in wildlife and humans and testing for infection in thousands of ticks from multiple sites and physiologic stages, we confirmed the presence of HRTV in Georgia,” the authors write.
“This information about the expanding geographic range of lone star ticks, combined with increased human presence in tick-infested habitats, can be used to improve strategies for preventing tick bites and to alert physicians about this emerging tickborne virus infection,” a press release by the Centers for Disease Control and Prevention notes.
Persistent field and lab work led to HRTV discovery in Georgia
The search for infected lone star ticks began after a retroactive analysis confirmed that a person who died in Georgia in 2005 from an unidentified illness was infected with HRTV. A subsequent analysis of serum samples collected earlier from local white-tailed deer showed that the deer had been exposed to HRTV since at least 2001, according to a press release by Emory University.
These discoveries prompted local researchers to investigate whether lone star ticks in rural, woodsy central Georgia were carrying HRTV.
Lead study author Yamila Romer, MD, an infectious disease clinician and microbiologist in the department of environmental sciences at Emory University in Atlanta, and her colleagues collected samples of ticks in 2018 at 26 sites near the location of the patient who died and the seropositive deer. In 2019, they focused their collections on the two sites that had provided the most ticks in 2018.
From April to October in both years, the research team visited sites weekly to swish white flannel flags through underbrush. They picked off adult and nymph Amblyomma americanum ticks, placed them into vials, and transported them to their lab. They sorted 9,294 ticks by sex, life stage, and collection site. Then they crushed the ticks and extracted their RNA.
To confirm viral infection, the team tested RNA extracted from cell culture supernatants using a real-time polymerase chain reaction test specific for HRTV.
In the three pools of ticks that tested positive for HRTV, the researchers found a minimum infection rate of 0.46/1,000 ticks, suggesting that about 1 of every 2,000 ticks carried HRTV. They sequenced the genome of the three isolates and found that the genomes were similar to one another but were very different from the genomes from HRTV samples taken outside Georgia.
Catherine A. Hill, PhD, a professor of entomology and vector biology and the interim head of the department of entomology at Purdue University in West Lafayette, Ind., was impressed with the researchers’ discovery.
“Heartland virus is difficult to detect,” she said in an email. “The prevalence of human cases is low, and the virus appears to be present at very low levels in populations of lone star tick. The investigators went to some lengths to survey for the virus, collect, and process thousands of ticks – and they found the needle in the haystack.” Dr. Hill was not involved in the study.
Georgia data help researchers monitor HRTV spread
HRTV was first identified in 2009 in Missouri in two people hospitalized with fever, muscle pain, diarrhea, and low white blood cell and platelet counts. Researchers traced the infections to lone star ticks, and they found antibodies to the virus in blood samples from deer and other wild mammals.
According to the CDC, U.S. cases of tick-borne diseases more than doubled between 2004 and 2016. As of January 2021, more than 50 human cases of HRTV disease had been reported in 11 Midwestern and Southeastern states: Arkansas, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Missouri, North Carolina, Oklahoma, and Tennessee.
Precautions, signs, symptoms, testing, and treatment
“The lone star tick is aggressive and will actively seek out a human host to bite,” Dr. Hill noted.
She recommends that health care providers advise patients to avoid tick habitat, wear protective clothing, apply repellants, know the signs and symptoms of tick-borne disease, and seek immediate medical care if they become ill.
Common symptoms of HRTV disease include fatigue, fever, nausea, diarrhea, and anorexia. Treatment is supportive. Many patients have been hospitalized, and some with comorbidities have died.
HRTV infection is rarely tested for, and the disease burden is unknown. With no commercial tests available in the United States, the CDC performs molecular and serologic testing for HRTV infection. The agency advises doctors to contact their state health department if they suspect a patient may have HRTV disease.
Further research is needed
Samantha M. Wisely, PhD, a professor of wildlife ecology and the director of the Cervidae Health Research Initiative at the University of Florida in Gainesville, was not surprised by the study finding.
“The more we look for heartland virus, the more places we find it,” Dr. Wisely told this news organization in an email.
“Little is known about which wildlife play a role in maintaining the virus on the landscape,” said Dr. Wisely, who was not involved in the study. “White-tailed deer have been shown to produce antibodies, meaning they have been exposed to the virus, but no one has actually found the virus in a wildlife species.”
The whole-genome sequencing of the virus was particularly important, Dr. Wisely explained. “Whole-genome data allow researchers to better understand viral evolution, pathogenicity, and viral dynamics across space and time – how it is evolving.”
The study was supported by a grant from the Emory University Research Council. The authors, Dr. Wisely, and Dr. Hill have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Heartland virus (HRTV), an emerging infection first detected in lone star ticks in Missouri in 2009, has spread to lone star ticks in Georgia, a study published in Emerging Infectious Diseases reports.
HRTV disease is transmitted by the bite of an infected Amblyomma americanum tick, named “lone star” because of the silver-white spot on the female scutum (back).
“By … sampling … in an area with reported exposure to HRTV in wildlife and humans and testing for infection in thousands of ticks from multiple sites and physiologic stages, we confirmed the presence of HRTV in Georgia,” the authors write.
“This information about the expanding geographic range of lone star ticks, combined with increased human presence in tick-infested habitats, can be used to improve strategies for preventing tick bites and to alert physicians about this emerging tickborne virus infection,” a press release by the Centers for Disease Control and Prevention notes.
Persistent field and lab work led to HRTV discovery in Georgia
The search for infected lone star ticks began after a retroactive analysis confirmed that a person who died in Georgia in 2005 from an unidentified illness was infected with HRTV. A subsequent analysis of serum samples collected earlier from local white-tailed deer showed that the deer had been exposed to HRTV since at least 2001, according to a press release by Emory University.
These discoveries prompted local researchers to investigate whether lone star ticks in rural, woodsy central Georgia were carrying HRTV.
Lead study author Yamila Romer, MD, an infectious disease clinician and microbiologist in the department of environmental sciences at Emory University in Atlanta, and her colleagues collected samples of ticks in 2018 at 26 sites near the location of the patient who died and the seropositive deer. In 2019, they focused their collections on the two sites that had provided the most ticks in 2018.
From April to October in both years, the research team visited sites weekly to swish white flannel flags through underbrush. They picked off adult and nymph Amblyomma americanum ticks, placed them into vials, and transported them to their lab. They sorted 9,294 ticks by sex, life stage, and collection site. Then they crushed the ticks and extracted their RNA.
To confirm viral infection, the team tested RNA extracted from cell culture supernatants using a real-time polymerase chain reaction test specific for HRTV.
In the three pools of ticks that tested positive for HRTV, the researchers found a minimum infection rate of 0.46/1,000 ticks, suggesting that about 1 of every 2,000 ticks carried HRTV. They sequenced the genome of the three isolates and found that the genomes were similar to one another but were very different from the genomes from HRTV samples taken outside Georgia.
Catherine A. Hill, PhD, a professor of entomology and vector biology and the interim head of the department of entomology at Purdue University in West Lafayette, Ind., was impressed with the researchers’ discovery.
“Heartland virus is difficult to detect,” she said in an email. “The prevalence of human cases is low, and the virus appears to be present at very low levels in populations of lone star tick. The investigators went to some lengths to survey for the virus, collect, and process thousands of ticks – and they found the needle in the haystack.” Dr. Hill was not involved in the study.
Georgia data help researchers monitor HRTV spread
HRTV was first identified in 2009 in Missouri in two people hospitalized with fever, muscle pain, diarrhea, and low white blood cell and platelet counts. Researchers traced the infections to lone star ticks, and they found antibodies to the virus in blood samples from deer and other wild mammals.
According to the CDC, U.S. cases of tick-borne diseases more than doubled between 2004 and 2016. As of January 2021, more than 50 human cases of HRTV disease had been reported in 11 Midwestern and Southeastern states: Arkansas, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Missouri, North Carolina, Oklahoma, and Tennessee.
Precautions, signs, symptoms, testing, and treatment
“The lone star tick is aggressive and will actively seek out a human host to bite,” Dr. Hill noted.
She recommends that health care providers advise patients to avoid tick habitat, wear protective clothing, apply repellants, know the signs and symptoms of tick-borne disease, and seek immediate medical care if they become ill.
Common symptoms of HRTV disease include fatigue, fever, nausea, diarrhea, and anorexia. Treatment is supportive. Many patients have been hospitalized, and some with comorbidities have died.
HRTV infection is rarely tested for, and the disease burden is unknown. With no commercial tests available in the United States, the CDC performs molecular and serologic testing for HRTV infection. The agency advises doctors to contact their state health department if they suspect a patient may have HRTV disease.
Further research is needed
Samantha M. Wisely, PhD, a professor of wildlife ecology and the director of the Cervidae Health Research Initiative at the University of Florida in Gainesville, was not surprised by the study finding.
“The more we look for heartland virus, the more places we find it,” Dr. Wisely told this news organization in an email.
“Little is known about which wildlife play a role in maintaining the virus on the landscape,” said Dr. Wisely, who was not involved in the study. “White-tailed deer have been shown to produce antibodies, meaning they have been exposed to the virus, but no one has actually found the virus in a wildlife species.”
The whole-genome sequencing of the virus was particularly important, Dr. Wisely explained. “Whole-genome data allow researchers to better understand viral evolution, pathogenicity, and viral dynamics across space and time – how it is evolving.”
The study was supported by a grant from the Emory University Research Council. The authors, Dr. Wisely, and Dr. Hill have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EMERGING INFECTIOUS DISEASES
Michigan COVID cases possibly the first from animals in U.S.
The cluster, which previously included three cases, marks the first known instance of likely animal-to-human “spillover” of the virus in the United States, according to the New York Times. All four people fully recovered.
Two of the infected people were employees of a mink farm in Michigan that had an outbreak in October 2020. The other two people didn’t have known links to the farm, which may mean that the coronavirus variant among mink may have been circulating more widely among residents in that area during that time.
Virus samples from all four people contained two mutations that may show signs of an adaptation to mink. The mutations have also been documented in farmed mink in Europe and people with connections to those farms.
“This, in addition to the mink farmworkers testing positive for COVID-19 after the mink herd had begun experiencing illness and increased mortality, suggests that the most likely hypothesis is that the workers were infected after contact with mink on the farm,” Casey Barton Behravesh, DVM, who directs the Centers for Disease Control and Prevention’s One Health Office, told the newspaper.
But researchers are unable to prove the cause, she noted.
“Because there are few genetic sequences available from the communities around the farm, it is impossible to know for sure whether the mutations came from mink on the farm or were already circulating in the community,” she said.
In August 2020, the U.S. Department of Agriculture announced the first confirmed COVID-19 case in mink at farms in Utah, followed by a case in Wisconsin. Worldwide, the coronavirus has been detected in mink on farms in the Netherlands, Denmark, Poland, and Spain.
In early October 2020, Michigan officials announced that the coronavirus had been detected in mink on a local farm. Several of the animals had died. The CDC helped to investigate the outbreak by collecting samples from animals, farmworkers, and residents in the community.
By March 2021, the CDC had updated its website to note that a “small number of people” had contracted a coronavirus variant that “contained unique mink-related mutations.”
In April 2021, the Detroit Free Press and the Documenting COVID-19 project first reported on the first three cases – two farmworkers and a taxidermist who didn’t have a connection to the mink farm. This week, the news outlets reported an update that the fourth case was the taxidermist’s wife.
Earlier this month, National Geographic first reported on the fourth human case based on government documents about the mink farm outbreak.
Overall, animal-to-human transmission is rare, but the CDC is continuing to monitor potential coronavirus cases in wildlife, livestock, and zoo animals for new variants and virus reservoirs, the Times reported.
“These results highlight the importance of routinely studying the genetic material of SARS-CoV-2 in susceptible animal populations like mink, as well as in people,” the CDC wrote.
A version of this article first appeared on WebMD.com.
The cluster, which previously included three cases, marks the first known instance of likely animal-to-human “spillover” of the virus in the United States, according to the New York Times. All four people fully recovered.
Two of the infected people were employees of a mink farm in Michigan that had an outbreak in October 2020. The other two people didn’t have known links to the farm, which may mean that the coronavirus variant among mink may have been circulating more widely among residents in that area during that time.
Virus samples from all four people contained two mutations that may show signs of an adaptation to mink. The mutations have also been documented in farmed mink in Europe and people with connections to those farms.
“This, in addition to the mink farmworkers testing positive for COVID-19 after the mink herd had begun experiencing illness and increased mortality, suggests that the most likely hypothesis is that the workers were infected after contact with mink on the farm,” Casey Barton Behravesh, DVM, who directs the Centers for Disease Control and Prevention’s One Health Office, told the newspaper.
But researchers are unable to prove the cause, she noted.
“Because there are few genetic sequences available from the communities around the farm, it is impossible to know for sure whether the mutations came from mink on the farm or were already circulating in the community,” she said.
In August 2020, the U.S. Department of Agriculture announced the first confirmed COVID-19 case in mink at farms in Utah, followed by a case in Wisconsin. Worldwide, the coronavirus has been detected in mink on farms in the Netherlands, Denmark, Poland, and Spain.
In early October 2020, Michigan officials announced that the coronavirus had been detected in mink on a local farm. Several of the animals had died. The CDC helped to investigate the outbreak by collecting samples from animals, farmworkers, and residents in the community.
By March 2021, the CDC had updated its website to note that a “small number of people” had contracted a coronavirus variant that “contained unique mink-related mutations.”
In April 2021, the Detroit Free Press and the Documenting COVID-19 project first reported on the first three cases – two farmworkers and a taxidermist who didn’t have a connection to the mink farm. This week, the news outlets reported an update that the fourth case was the taxidermist’s wife.
Earlier this month, National Geographic first reported on the fourth human case based on government documents about the mink farm outbreak.
Overall, animal-to-human transmission is rare, but the CDC is continuing to monitor potential coronavirus cases in wildlife, livestock, and zoo animals for new variants and virus reservoirs, the Times reported.
“These results highlight the importance of routinely studying the genetic material of SARS-CoV-2 in susceptible animal populations like mink, as well as in people,” the CDC wrote.
A version of this article first appeared on WebMD.com.
The cluster, which previously included three cases, marks the first known instance of likely animal-to-human “spillover” of the virus in the United States, according to the New York Times. All four people fully recovered.
Two of the infected people were employees of a mink farm in Michigan that had an outbreak in October 2020. The other two people didn’t have known links to the farm, which may mean that the coronavirus variant among mink may have been circulating more widely among residents in that area during that time.
Virus samples from all four people contained two mutations that may show signs of an adaptation to mink. The mutations have also been documented in farmed mink in Europe and people with connections to those farms.
“This, in addition to the mink farmworkers testing positive for COVID-19 after the mink herd had begun experiencing illness and increased mortality, suggests that the most likely hypothesis is that the workers were infected after contact with mink on the farm,” Casey Barton Behravesh, DVM, who directs the Centers for Disease Control and Prevention’s One Health Office, told the newspaper.
But researchers are unable to prove the cause, she noted.
“Because there are few genetic sequences available from the communities around the farm, it is impossible to know for sure whether the mutations came from mink on the farm or were already circulating in the community,” she said.
In August 2020, the U.S. Department of Agriculture announced the first confirmed COVID-19 case in mink at farms in Utah, followed by a case in Wisconsin. Worldwide, the coronavirus has been detected in mink on farms in the Netherlands, Denmark, Poland, and Spain.
In early October 2020, Michigan officials announced that the coronavirus had been detected in mink on a local farm. Several of the animals had died. The CDC helped to investigate the outbreak by collecting samples from animals, farmworkers, and residents in the community.
By March 2021, the CDC had updated its website to note that a “small number of people” had contracted a coronavirus variant that “contained unique mink-related mutations.”
In April 2021, the Detroit Free Press and the Documenting COVID-19 project first reported on the first three cases – two farmworkers and a taxidermist who didn’t have a connection to the mink farm. This week, the news outlets reported an update that the fourth case was the taxidermist’s wife.
Earlier this month, National Geographic first reported on the fourth human case based on government documents about the mink farm outbreak.
Overall, animal-to-human transmission is rare, but the CDC is continuing to monitor potential coronavirus cases in wildlife, livestock, and zoo animals for new variants and virus reservoirs, the Times reported.
“These results highlight the importance of routinely studying the genetic material of SARS-CoV-2 in susceptible animal populations like mink, as well as in people,” the CDC wrote.
A version of this article first appeared on WebMD.com.
Children and COVID: Decline in new cases comes to an end
It was a good run while it lasted.
according to the American Academy of Pediatrics and the Children’s Hospital Association.
The number of reported pediatric cases for the week was 33,146, and the actual increase from the previous week was just 7,231 cases, the AAP and CHA said, but some reports suggest that the new COVID variants and subvariants are starting to have an effect on incidence in some areas while mask mandates continue to fall.
Data from the Centers for Disease Control and Prevention show that, over the last week or two, the 7-day average for percentage of emergency department visits with diagnosed COVID has risen from 0.5% to 0.6% in children aged 0-11 years, from 0.3% to 0.5% among 12- to 15-year-olds, and from 0.3% to 0.4% in 16- and 17-year-olds. Small increases, to be sure, but increases nonetheless.
A somewhat similar scenario is playing out for new admissions of children aged 0-17, which have leveled out after dropping from a high of 1.25 per 100,000 population in mid-January to 0.13 per 100,000 in early April. Over the last 2 weeks, the rate has been alternating between 0.13 and 0.14 per 100,000, the CDC said on its COVID Data Tracker.
The latest news on the vaccination front came from Pfizer and BIoNTech, which announced that a third dose of its COVID-19 vaccine boosted immune protection in children aged 5-11 years in a phase 2/3 trial. Protection against the Omicron strain was 36 times higher than the two previous doses, the companies said, adding that they plan to submit a request for emergency use authorization of a booster dose in the near future.
The ongoing vaccination effort, however, produced mixed results in the last week. Initial vaccinations among children aged 5-11 years fell 14.5% to another new low while initial doses were up 9.3% for those aged 12-17, the AAP said. Overall, just 28.2% of the country’s 5- to 11-year-olds are fully vaccinated, compared with 58.7% of those aged 12-17, the CDC reported.
It was a good run while it lasted.
according to the American Academy of Pediatrics and the Children’s Hospital Association.
The number of reported pediatric cases for the week was 33,146, and the actual increase from the previous week was just 7,231 cases, the AAP and CHA said, but some reports suggest that the new COVID variants and subvariants are starting to have an effect on incidence in some areas while mask mandates continue to fall.
Data from the Centers for Disease Control and Prevention show that, over the last week or two, the 7-day average for percentage of emergency department visits with diagnosed COVID has risen from 0.5% to 0.6% in children aged 0-11 years, from 0.3% to 0.5% among 12- to 15-year-olds, and from 0.3% to 0.4% in 16- and 17-year-olds. Small increases, to be sure, but increases nonetheless.
A somewhat similar scenario is playing out for new admissions of children aged 0-17, which have leveled out after dropping from a high of 1.25 per 100,000 population in mid-January to 0.13 per 100,000 in early April. Over the last 2 weeks, the rate has been alternating between 0.13 and 0.14 per 100,000, the CDC said on its COVID Data Tracker.
The latest news on the vaccination front came from Pfizer and BIoNTech, which announced that a third dose of its COVID-19 vaccine boosted immune protection in children aged 5-11 years in a phase 2/3 trial. Protection against the Omicron strain was 36 times higher than the two previous doses, the companies said, adding that they plan to submit a request for emergency use authorization of a booster dose in the near future.
The ongoing vaccination effort, however, produced mixed results in the last week. Initial vaccinations among children aged 5-11 years fell 14.5% to another new low while initial doses were up 9.3% for those aged 12-17, the AAP said. Overall, just 28.2% of the country’s 5- to 11-year-olds are fully vaccinated, compared with 58.7% of those aged 12-17, the CDC reported.
It was a good run while it lasted.
according to the American Academy of Pediatrics and the Children’s Hospital Association.
The number of reported pediatric cases for the week was 33,146, and the actual increase from the previous week was just 7,231 cases, the AAP and CHA said, but some reports suggest that the new COVID variants and subvariants are starting to have an effect on incidence in some areas while mask mandates continue to fall.
Data from the Centers for Disease Control and Prevention show that, over the last week or two, the 7-day average for percentage of emergency department visits with diagnosed COVID has risen from 0.5% to 0.6% in children aged 0-11 years, from 0.3% to 0.5% among 12- to 15-year-olds, and from 0.3% to 0.4% in 16- and 17-year-olds. Small increases, to be sure, but increases nonetheless.
A somewhat similar scenario is playing out for new admissions of children aged 0-17, which have leveled out after dropping from a high of 1.25 per 100,000 population in mid-January to 0.13 per 100,000 in early April. Over the last 2 weeks, the rate has been alternating between 0.13 and 0.14 per 100,000, the CDC said on its COVID Data Tracker.
The latest news on the vaccination front came from Pfizer and BIoNTech, which announced that a third dose of its COVID-19 vaccine boosted immune protection in children aged 5-11 years in a phase 2/3 trial. Protection against the Omicron strain was 36 times higher than the two previous doses, the companies said, adding that they plan to submit a request for emergency use authorization of a booster dose in the near future.
The ongoing vaccination effort, however, produced mixed results in the last week. Initial vaccinations among children aged 5-11 years fell 14.5% to another new low while initial doses were up 9.3% for those aged 12-17, the AAP said. Overall, just 28.2% of the country’s 5- to 11-year-olds are fully vaccinated, compared with 58.7% of those aged 12-17, the CDC reported.
Childhood abuse may increase risk of MS in women
, according to the first prospective cohort study of its kind.
More research is needed to uncover underlying mechanisms of action, according to lead author Karine Eid, MD, a PhD candidate at Haukeland University Hospital, Bergen, Norway, and colleagues.
“Trauma and stressful life events have been associated with an increased risk of autoimmune disorders,” the investigators wrote in the Journal Of Neurology, Neurosurgery, & Psychiatry. “Whether adverse events in childhood can have an impact on MS susceptibility is not known.”
The present study recruited participants from the Norwegian Mother, Father and Child cohort, a population consisting of Norwegian women who were pregnant from 1999 to 2008. Of the 77,997 participating women, 14,477 reported emotional, sexual, and/or physical abuse in childhood, while the remaining 63,520 women reported no abuse. After a mean follow-up of 13 years, 300 women were diagnosed with MS, among whom 24% reported a history of childhood abuse, compared with 19% among women who did not develop MS.
To look for associations between childhood abuse and risk of MS, the investigators used a Cox model adjusted for confounders and mediators, including smoking, obesity, adult socioeconomic factors, and childhood social status. The model revealed that emotional abuse increased the risk of MS by 40% (hazard ratio [HR] 1.40; 95% confidence interval [CI], 1.03-1.90), and sexual abuse increased the risk of MS by 65% (HR 1.65; 95% CI, 1.13-2.39).
Although physical abuse alone did not significantly increase risk of MS (HR 1.31; 95% CI, 0.83-2.06), it did contribute to a dose-response relationship when women were exposed to more than one type of childhood abuse. Women exposed to two out of three abuse categories had a 66% increased risk of MS (HR 1.66; 95% CI, 1.04-2.67), whereas women exposed to all three types of abuse had the highest risk of MS, at 93% (HR 1.93; 95% CI, 1.02-3.67).
Dr. Eid and colleagues noted that their findings are supported by previous retrospective research, and discussed possible mechanisms of action.
“The increased risk of MS after exposure to childhood sexual and emotional abuse may have a biological explanation,” they wrote. “Childhood abuse can cause dysregulation of the hypothalamic-pituitary-adrenal axis, lead to oxidative stress, and induce a proinflammatory state decades into adulthood. Psychological stress has been shown to disrupt the blood-brain barrier and cause epigenetic changes that may increase the risk of neurodegenerative disorders, including MS.
“The underlying mechanisms behind this association should be investigated further,” they concluded.
Study findings should guide interventions
Commenting on the research, Ruth Ann Marrie, MD, PhD, professor of medicine and community health sciences and director of the multiple sclerosis clinic at Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, said that the present study “has several strengths compared to prior studies – including that it is prospective and the sample size.”
Dr. Marrie, who was not involved in the study, advised clinicians in the field to take note of the findings, as patients with a history of abuse may need unique interventions.
“Providers need to recognize the higher prevalence of childhood maltreatment in people with MS,” Dr. Marrie said in an interview. “These findings dovetail with others that suggest that adverse childhood experiences are associated with increased mental health concerns and pain catastrophizing in people with MS. Affected individuals may benefit from additional psychological supports and trauma-informed care.”
Tiffany Joy Braley, MD, associate professor of neurology, and Carri Polick, RN and PhD candidate at the school of nursing, University of Michigan, Ann Arbor, who published a case report last year highlighting the importance of evaluating stress exposure in MS, suggested that the findings should guide interventions at both a system and patient level.
“Although a cause-and-effect relationship cannot be established by the current study, these and related findings should be considered in the context of system level and policy interventions that address links between environment and health care disparities,” they said in a joint, written comment. “Given recent impetus to provide trauma-informed health care, these data could be particularly informative in neurological conditions which are associated with high mental health comorbidity. Traumatic stress screening practices could lead to referrals for appropriate support services and more personalized health care.”
While several mechanisms have been proposed to explain the link between traumatic stress and MS, more work is needed in this area, they added.
This knowledge gap was acknowledged by Dr. Marrie.
“Our understanding of the etiology of MS remains incomplete,” Dr. Marrie said. “We still need a better understanding of mechanisms by which adverse childhood experiences lead to MS, how they interact with other risk factors for MS (beyond smoking and obesity), and whether there are any interventions that can mitigate the risk of developing MS that is associated with adverse childhood experiences.”
The investigators disclosed relationships with Novartis, Biogen, Merck, and others. Dr. Marrie receives research support from the Canadian Institutes of Health Research, the National Multiple Sclerosis Society, MS Society of Canada, the Consortium of Multiple Sclerosis Centers, Crohn’s and Colitis Canada, Research Manitoba, and the Arthritis Society; she has no pharmaceutical support. Dr. Braley and Ms. Polick reported no conflicts of interest.
, according to the first prospective cohort study of its kind.
More research is needed to uncover underlying mechanisms of action, according to lead author Karine Eid, MD, a PhD candidate at Haukeland University Hospital, Bergen, Norway, and colleagues.
“Trauma and stressful life events have been associated with an increased risk of autoimmune disorders,” the investigators wrote in the Journal Of Neurology, Neurosurgery, & Psychiatry. “Whether adverse events in childhood can have an impact on MS susceptibility is not known.”
The present study recruited participants from the Norwegian Mother, Father and Child cohort, a population consisting of Norwegian women who were pregnant from 1999 to 2008. Of the 77,997 participating women, 14,477 reported emotional, sexual, and/or physical abuse in childhood, while the remaining 63,520 women reported no abuse. After a mean follow-up of 13 years, 300 women were diagnosed with MS, among whom 24% reported a history of childhood abuse, compared with 19% among women who did not develop MS.
To look for associations between childhood abuse and risk of MS, the investigators used a Cox model adjusted for confounders and mediators, including smoking, obesity, adult socioeconomic factors, and childhood social status. The model revealed that emotional abuse increased the risk of MS by 40% (hazard ratio [HR] 1.40; 95% confidence interval [CI], 1.03-1.90), and sexual abuse increased the risk of MS by 65% (HR 1.65; 95% CI, 1.13-2.39).
Although physical abuse alone did not significantly increase risk of MS (HR 1.31; 95% CI, 0.83-2.06), it did contribute to a dose-response relationship when women were exposed to more than one type of childhood abuse. Women exposed to two out of three abuse categories had a 66% increased risk of MS (HR 1.66; 95% CI, 1.04-2.67), whereas women exposed to all three types of abuse had the highest risk of MS, at 93% (HR 1.93; 95% CI, 1.02-3.67).
Dr. Eid and colleagues noted that their findings are supported by previous retrospective research, and discussed possible mechanisms of action.
“The increased risk of MS after exposure to childhood sexual and emotional abuse may have a biological explanation,” they wrote. “Childhood abuse can cause dysregulation of the hypothalamic-pituitary-adrenal axis, lead to oxidative stress, and induce a proinflammatory state decades into adulthood. Psychological stress has been shown to disrupt the blood-brain barrier and cause epigenetic changes that may increase the risk of neurodegenerative disorders, including MS.
“The underlying mechanisms behind this association should be investigated further,” they concluded.
Study findings should guide interventions
Commenting on the research, Ruth Ann Marrie, MD, PhD, professor of medicine and community health sciences and director of the multiple sclerosis clinic at Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, said that the present study “has several strengths compared to prior studies – including that it is prospective and the sample size.”
Dr. Marrie, who was not involved in the study, advised clinicians in the field to take note of the findings, as patients with a history of abuse may need unique interventions.
“Providers need to recognize the higher prevalence of childhood maltreatment in people with MS,” Dr. Marrie said in an interview. “These findings dovetail with others that suggest that adverse childhood experiences are associated with increased mental health concerns and pain catastrophizing in people with MS. Affected individuals may benefit from additional psychological supports and trauma-informed care.”
Tiffany Joy Braley, MD, associate professor of neurology, and Carri Polick, RN and PhD candidate at the school of nursing, University of Michigan, Ann Arbor, who published a case report last year highlighting the importance of evaluating stress exposure in MS, suggested that the findings should guide interventions at both a system and patient level.
“Although a cause-and-effect relationship cannot be established by the current study, these and related findings should be considered in the context of system level and policy interventions that address links between environment and health care disparities,” they said in a joint, written comment. “Given recent impetus to provide trauma-informed health care, these data could be particularly informative in neurological conditions which are associated with high mental health comorbidity. Traumatic stress screening practices could lead to referrals for appropriate support services and more personalized health care.”
While several mechanisms have been proposed to explain the link between traumatic stress and MS, more work is needed in this area, they added.
This knowledge gap was acknowledged by Dr. Marrie.
“Our understanding of the etiology of MS remains incomplete,” Dr. Marrie said. “We still need a better understanding of mechanisms by which adverse childhood experiences lead to MS, how they interact with other risk factors for MS (beyond smoking and obesity), and whether there are any interventions that can mitigate the risk of developing MS that is associated with adverse childhood experiences.”
The investigators disclosed relationships with Novartis, Biogen, Merck, and others. Dr. Marrie receives research support from the Canadian Institutes of Health Research, the National Multiple Sclerosis Society, MS Society of Canada, the Consortium of Multiple Sclerosis Centers, Crohn’s and Colitis Canada, Research Manitoba, and the Arthritis Society; she has no pharmaceutical support. Dr. Braley and Ms. Polick reported no conflicts of interest.
, according to the first prospective cohort study of its kind.
More research is needed to uncover underlying mechanisms of action, according to lead author Karine Eid, MD, a PhD candidate at Haukeland University Hospital, Bergen, Norway, and colleagues.
“Trauma and stressful life events have been associated with an increased risk of autoimmune disorders,” the investigators wrote in the Journal Of Neurology, Neurosurgery, & Psychiatry. “Whether adverse events in childhood can have an impact on MS susceptibility is not known.”
The present study recruited participants from the Norwegian Mother, Father and Child cohort, a population consisting of Norwegian women who were pregnant from 1999 to 2008. Of the 77,997 participating women, 14,477 reported emotional, sexual, and/or physical abuse in childhood, while the remaining 63,520 women reported no abuse. After a mean follow-up of 13 years, 300 women were diagnosed with MS, among whom 24% reported a history of childhood abuse, compared with 19% among women who did not develop MS.
To look for associations between childhood abuse and risk of MS, the investigators used a Cox model adjusted for confounders and mediators, including smoking, obesity, adult socioeconomic factors, and childhood social status. The model revealed that emotional abuse increased the risk of MS by 40% (hazard ratio [HR] 1.40; 95% confidence interval [CI], 1.03-1.90), and sexual abuse increased the risk of MS by 65% (HR 1.65; 95% CI, 1.13-2.39).
Although physical abuse alone did not significantly increase risk of MS (HR 1.31; 95% CI, 0.83-2.06), it did contribute to a dose-response relationship when women were exposed to more than one type of childhood abuse. Women exposed to two out of three abuse categories had a 66% increased risk of MS (HR 1.66; 95% CI, 1.04-2.67), whereas women exposed to all three types of abuse had the highest risk of MS, at 93% (HR 1.93; 95% CI, 1.02-3.67).
Dr. Eid and colleagues noted that their findings are supported by previous retrospective research, and discussed possible mechanisms of action.
“The increased risk of MS after exposure to childhood sexual and emotional abuse may have a biological explanation,” they wrote. “Childhood abuse can cause dysregulation of the hypothalamic-pituitary-adrenal axis, lead to oxidative stress, and induce a proinflammatory state decades into adulthood. Psychological stress has been shown to disrupt the blood-brain barrier and cause epigenetic changes that may increase the risk of neurodegenerative disorders, including MS.
“The underlying mechanisms behind this association should be investigated further,” they concluded.
Study findings should guide interventions
Commenting on the research, Ruth Ann Marrie, MD, PhD, professor of medicine and community health sciences and director of the multiple sclerosis clinic at Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, said that the present study “has several strengths compared to prior studies – including that it is prospective and the sample size.”
Dr. Marrie, who was not involved in the study, advised clinicians in the field to take note of the findings, as patients with a history of abuse may need unique interventions.
“Providers need to recognize the higher prevalence of childhood maltreatment in people with MS,” Dr. Marrie said in an interview. “These findings dovetail with others that suggest that adverse childhood experiences are associated with increased mental health concerns and pain catastrophizing in people with MS. Affected individuals may benefit from additional psychological supports and trauma-informed care.”
Tiffany Joy Braley, MD, associate professor of neurology, and Carri Polick, RN and PhD candidate at the school of nursing, University of Michigan, Ann Arbor, who published a case report last year highlighting the importance of evaluating stress exposure in MS, suggested that the findings should guide interventions at both a system and patient level.
“Although a cause-and-effect relationship cannot be established by the current study, these and related findings should be considered in the context of system level and policy interventions that address links between environment and health care disparities,” they said in a joint, written comment. “Given recent impetus to provide trauma-informed health care, these data could be particularly informative in neurological conditions which are associated with high mental health comorbidity. Traumatic stress screening practices could lead to referrals for appropriate support services and more personalized health care.”
While several mechanisms have been proposed to explain the link between traumatic stress and MS, more work is needed in this area, they added.
This knowledge gap was acknowledged by Dr. Marrie.
“Our understanding of the etiology of MS remains incomplete,” Dr. Marrie said. “We still need a better understanding of mechanisms by which adverse childhood experiences lead to MS, how they interact with other risk factors for MS (beyond smoking and obesity), and whether there are any interventions that can mitigate the risk of developing MS that is associated with adverse childhood experiences.”
The investigators disclosed relationships with Novartis, Biogen, Merck, and others. Dr. Marrie receives research support from the Canadian Institutes of Health Research, the National Multiple Sclerosis Society, MS Society of Canada, the Consortium of Multiple Sclerosis Centers, Crohn’s and Colitis Canada, Research Manitoba, and the Arthritis Society; she has no pharmaceutical support. Dr. Braley and Ms. Polick reported no conflicts of interest.
FROM THE JOURNAL OF NEUROLOGY, NEUROSURGERY, & PSYCHIATRY
Med school to pay $1.2 million to students in refunds and debt cancellation in FTC settlement
Although it disputed the allegations, The complaint referenced the school’s medical license exam test pass rate and residency matches along with violations of rules that protect consumers, including those dealing with credit contracts.
The school, based in the Caribbean with operations in Illinois, agreed to pay $1.2 million toward refunds and debt cancellation for students harmed by the marketing in the past 5 years.
“While we strongly disagree with the FTC’s approach to this matter, we did not want a lengthy legal process to distract from our mission of providing a quality medical education at an affordable cost,” Kaushik Guha, executive vice president of the parent of the school, Human Resources Development Services, said in a YouTube statement posted on the school’s website.
“Saint James lured students by lying about their chances of success,” Samuel Levine, director of the FTC’s Bureau of Consumer Protection, said in a press release. The settlement agreement was with HRDS, which bills itself as providing students from “non-traditional backgrounds the opportunity to pursue a medical degree and practice in the U.S. or Canada,” according to the school’s statement.
The complaint alleges that, since at least April 2018, the school, HRDS, and its operator Mr. Guha has lured students using “phony claims about the standardized test pass rate and students’ residency or job prospects. They lured consumers with false guarantees of student success at passing a critical medical school standardized test, the United States Medical Licensing Examination Step 1 Exam.”
For example, a brochure distributed at open houses claimed a first-time Step 1 pass rate of about 96.8%. The brochure further claimed: “Saint James is the first and only medical school to offer a USMLE Step 1 Pass Guarantee,” according to the FTC complaint.
The FTC said the USMLE rate is lower than touted and lower than reported by other U.S. and Canadian medical schools. “Since 2017, only 35% of Saint James students who have completed the necessary coursework to take the USMLE Step 1 exam passed the test.”
The school also misrepresented the residency match rate as “the same” as American medical schools, according to the complaint. For example, the school instructed telemarketers to tell consumers that the match rate for the school’s students was 85%-90%. The school stated on its website that the residency match rate for Saint James students was 83%. “In fact, the match rate for SJSM students is lower than touted and lower than that reported by U.S. medical schools. Since 2018, defendants’ average match rate has been 63%.”
The FTC also claims the school used illegal credit contracts when marketing financing for tuition and living expenses for students. “The financing contracts contained language attempting to waive consumers’ rights under federal law and omit legally mandated disclosures.”
Saint James’ tuition ranges from about $6,650 to $9,859 per trimester, depending on campus and course study, the complaint states. Between 2016 and 2020, about 1,300 students were enrolled each year in Saint James’ schools. Students who attended the schools between 2016 and 2022 are eligible for a refund under the settlement.
Saint James is required to notify consumers whose debts are being canceled through Delta Financial Solutions, Saint James’ financing partner. The debt will also be deleted from consumers’ credit reports.
“We have chosen to settle with the FTC over its allegations that disclosures on our website and in Delta’s loan agreements were insufficient,” Mr. Guha stated on the school website. “However, we have added additional language and clarifications any time the USMLE pass rate and placement rates are mentioned.”
He said he hopes the school will be “an industry leader for transparency and accountability” and that the school’s “efforts will lead to lasting change throughout the for-profit educational industry.”
Mr. Guha added that more than 600 of the school’s alumni are serving as doctors, including many “working to bridge the health equity gap in underserved areas in North America.”
The FTC has been cracking down on deceptive practices by for-profit institutions. In October, the FTC put 70 for-profit colleges on notice that it would investigate false promises the schools make about their graduates’ job prospects, expected earnings, and other educational outcomes and would levy significant financial penalties against violators. Saint James was not on that list, which included several of the largest for-profit universities in the nation, including Capella University, DeVry University, Strayer University, and Walden University.
A version of this article first appeared on Medscape.com.
Although it disputed the allegations, The complaint referenced the school’s medical license exam test pass rate and residency matches along with violations of rules that protect consumers, including those dealing with credit contracts.
The school, based in the Caribbean with operations in Illinois, agreed to pay $1.2 million toward refunds and debt cancellation for students harmed by the marketing in the past 5 years.
“While we strongly disagree with the FTC’s approach to this matter, we did not want a lengthy legal process to distract from our mission of providing a quality medical education at an affordable cost,” Kaushik Guha, executive vice president of the parent of the school, Human Resources Development Services, said in a YouTube statement posted on the school’s website.
“Saint James lured students by lying about their chances of success,” Samuel Levine, director of the FTC’s Bureau of Consumer Protection, said in a press release. The settlement agreement was with HRDS, which bills itself as providing students from “non-traditional backgrounds the opportunity to pursue a medical degree and practice in the U.S. or Canada,” according to the school’s statement.
The complaint alleges that, since at least April 2018, the school, HRDS, and its operator Mr. Guha has lured students using “phony claims about the standardized test pass rate and students’ residency or job prospects. They lured consumers with false guarantees of student success at passing a critical medical school standardized test, the United States Medical Licensing Examination Step 1 Exam.”
For example, a brochure distributed at open houses claimed a first-time Step 1 pass rate of about 96.8%. The brochure further claimed: “Saint James is the first and only medical school to offer a USMLE Step 1 Pass Guarantee,” according to the FTC complaint.
The FTC said the USMLE rate is lower than touted and lower than reported by other U.S. and Canadian medical schools. “Since 2017, only 35% of Saint James students who have completed the necessary coursework to take the USMLE Step 1 exam passed the test.”
The school also misrepresented the residency match rate as “the same” as American medical schools, according to the complaint. For example, the school instructed telemarketers to tell consumers that the match rate for the school’s students was 85%-90%. The school stated on its website that the residency match rate for Saint James students was 83%. “In fact, the match rate for SJSM students is lower than touted and lower than that reported by U.S. medical schools. Since 2018, defendants’ average match rate has been 63%.”
The FTC also claims the school used illegal credit contracts when marketing financing for tuition and living expenses for students. “The financing contracts contained language attempting to waive consumers’ rights under federal law and omit legally mandated disclosures.”
Saint James’ tuition ranges from about $6,650 to $9,859 per trimester, depending on campus and course study, the complaint states. Between 2016 and 2020, about 1,300 students were enrolled each year in Saint James’ schools. Students who attended the schools between 2016 and 2022 are eligible for a refund under the settlement.
Saint James is required to notify consumers whose debts are being canceled through Delta Financial Solutions, Saint James’ financing partner. The debt will also be deleted from consumers’ credit reports.
“We have chosen to settle with the FTC over its allegations that disclosures on our website and in Delta’s loan agreements were insufficient,” Mr. Guha stated on the school website. “However, we have added additional language and clarifications any time the USMLE pass rate and placement rates are mentioned.”
He said he hopes the school will be “an industry leader for transparency and accountability” and that the school’s “efforts will lead to lasting change throughout the for-profit educational industry.”
Mr. Guha added that more than 600 of the school’s alumni are serving as doctors, including many “working to bridge the health equity gap in underserved areas in North America.”
The FTC has been cracking down on deceptive practices by for-profit institutions. In October, the FTC put 70 for-profit colleges on notice that it would investigate false promises the schools make about their graduates’ job prospects, expected earnings, and other educational outcomes and would levy significant financial penalties against violators. Saint James was not on that list, which included several of the largest for-profit universities in the nation, including Capella University, DeVry University, Strayer University, and Walden University.
A version of this article first appeared on Medscape.com.
Although it disputed the allegations, The complaint referenced the school’s medical license exam test pass rate and residency matches along with violations of rules that protect consumers, including those dealing with credit contracts.
The school, based in the Caribbean with operations in Illinois, agreed to pay $1.2 million toward refunds and debt cancellation for students harmed by the marketing in the past 5 years.
“While we strongly disagree with the FTC’s approach to this matter, we did not want a lengthy legal process to distract from our mission of providing a quality medical education at an affordable cost,” Kaushik Guha, executive vice president of the parent of the school, Human Resources Development Services, said in a YouTube statement posted on the school’s website.
“Saint James lured students by lying about their chances of success,” Samuel Levine, director of the FTC’s Bureau of Consumer Protection, said in a press release. The settlement agreement was with HRDS, which bills itself as providing students from “non-traditional backgrounds the opportunity to pursue a medical degree and practice in the U.S. or Canada,” according to the school’s statement.
The complaint alleges that, since at least April 2018, the school, HRDS, and its operator Mr. Guha has lured students using “phony claims about the standardized test pass rate and students’ residency or job prospects. They lured consumers with false guarantees of student success at passing a critical medical school standardized test, the United States Medical Licensing Examination Step 1 Exam.”
For example, a brochure distributed at open houses claimed a first-time Step 1 pass rate of about 96.8%. The brochure further claimed: “Saint James is the first and only medical school to offer a USMLE Step 1 Pass Guarantee,” according to the FTC complaint.
The FTC said the USMLE rate is lower than touted and lower than reported by other U.S. and Canadian medical schools. “Since 2017, only 35% of Saint James students who have completed the necessary coursework to take the USMLE Step 1 exam passed the test.”
The school also misrepresented the residency match rate as “the same” as American medical schools, according to the complaint. For example, the school instructed telemarketers to tell consumers that the match rate for the school’s students was 85%-90%. The school stated on its website that the residency match rate for Saint James students was 83%. “In fact, the match rate for SJSM students is lower than touted and lower than that reported by U.S. medical schools. Since 2018, defendants’ average match rate has been 63%.”
The FTC also claims the school used illegal credit contracts when marketing financing for tuition and living expenses for students. “The financing contracts contained language attempting to waive consumers’ rights under federal law and omit legally mandated disclosures.”
Saint James’ tuition ranges from about $6,650 to $9,859 per trimester, depending on campus and course study, the complaint states. Between 2016 and 2020, about 1,300 students were enrolled each year in Saint James’ schools. Students who attended the schools between 2016 and 2022 are eligible for a refund under the settlement.
Saint James is required to notify consumers whose debts are being canceled through Delta Financial Solutions, Saint James’ financing partner. The debt will also be deleted from consumers’ credit reports.
“We have chosen to settle with the FTC over its allegations that disclosures on our website and in Delta’s loan agreements were insufficient,” Mr. Guha stated on the school website. “However, we have added additional language and clarifications any time the USMLE pass rate and placement rates are mentioned.”
He said he hopes the school will be “an industry leader for transparency and accountability” and that the school’s “efforts will lead to lasting change throughout the for-profit educational industry.”
Mr. Guha added that more than 600 of the school’s alumni are serving as doctors, including many “working to bridge the health equity gap in underserved areas in North America.”
The FTC has been cracking down on deceptive practices by for-profit institutions. In October, the FTC put 70 for-profit colleges on notice that it would investigate false promises the schools make about their graduates’ job prospects, expected earnings, and other educational outcomes and would levy significant financial penalties against violators. Saint James was not on that list, which included several of the largest for-profit universities in the nation, including Capella University, DeVry University, Strayer University, and Walden University.
A version of this article first appeared on Medscape.com.
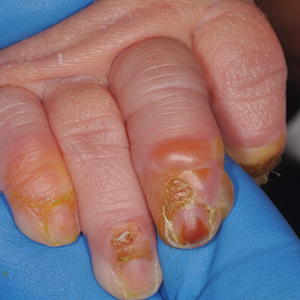
Blistering Lesions in a Newborn
The Diagnosis: Epidermolysis Bullosa
Our patient was found to have epidermolysis bullosa (EB), a rare genetic disease in which the superficial layers of the skin separate to form vesicles or bullae due to a mutation in the keratin 14 gene, KRT14. Separation of the skin occurs due to cleavage of various proteins that connect the epidermis to the dermis. A genetic mutation in KRT14, one of the more common genetic mutations associated with EB, results in cleavage at the basal epidermal protein keratin 14. The skin of individuals with EB typically is fragile and cannot tolerate friction or manipulation due to the risk for new bullae formation.1 Epidermolysis bullosa is rare, affecting approximately 20 children per 1 million births in the United States, and is not commonly seen by most general adult dermatologists.2
In our patient, the differential diagnoses included staphylococcal scalded skin syndrome (SSSS), Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), herpes simplex virus (HSV), and bullous pemphigoid (BP). Symptoms of SSSS can range from mild and localized to full-body exfoliation of the skin. Although SSSS can resemble other bullous disorders, its etiology arises from the Staphylococcus exotoxin targeting desmoglein in the stratum granulosum— the layer of the epidermis between the stratum corneum and stratum spinosum.3 Lesions start on the face, neck, and body folds, which was consistent with our patient’s presentation. However, bullae continued to develop in our patient despite antibiotic therapy, which reduced the likelihood of SSSS. Stevens-Johnson syndrome/toxic epidermal necrolysis develops rapidly and often involves the mucosa, which our patient initially did not have. In children, SJS/TEN can develop secondary to infection, whereas in adults it more commonly is associated with medication administration.4 Although the mother tested negative for HSV, the infant was started on acyclovir, which ultimately was discontinued due to low clinical suspicion. The clinical presentation of HSV (ie, clustered vesicles) was not consistent with our patient’s presentation. Bullous pemphigoid is a subepithelial blistering disease seen in older adults. Tense, fluidfilled blisters primarily are seen on the trunk and flexures. Although infantile BP can occur, it usually does not present in the neonatal period but rather at approximately 3 to 5 months of age.5
High clinical suspicion for EB due to the common characteristics of bullae location and formation following skin manipulation led to genetic testing in our patient. Mild forms of EB simplex typically appear on the upper and lower extremities with sparing of the trunk. In more severe cases of EB simplex, truncal and mucosal involvement may occur.6 In our case, the infant had a classic distribution of arm and leg blisters with truncal sparing. Epidermolysis bullosa may not be diagnosed in the neonatal period because of its similarities to other more common diseases, such as HSV or bullous impetigo, or other genetic blistering diseases, such as epidermolytic ichthyosis and incontinentia pigmenti.6
Epidermolysis bullosa can be inherited in an autosomal-dominant or autosomal-recessive fashion or with de novo mutations and is classified based on the location of cleavage in the skin. The 4 classical subtypes— simplex, junctional, dystrophic, and Kindler—have now been further subclassified. Epidermolysis bullosa simplex (intraepidermal split) is now separated into basal and suprabasal, with further subclassification including the distribution of blisters (generalized or localized) and the severity of cutaneous or extracutaneous involvement.7
In our case, the infant was found to have intraepidermal EB (simplex) due to a KRT14 mutation (missense mutation).6 KRT14 (17q21.2) and KRT5 (12q13.3) are the 2 most common mutations causing cleavage at the basal intraepidermal layer. Thickening of the palms, soles, and nails can be seen; however, blisters heal well without scarring, as seen in our patient. Junctional EB due to cleavage at the intralamina lucida often involves mutations in laminin 332, plectin, and α6β4 integrin. Infants with junctional EB often die from severe infection, dehydration, or malnutrition due to mucosal involvement. Dystrophic EB occurs due to a collagen VII mutation in the dermis, leading to blisters at the sublamina densa and more severe symptoms in the recessive form.7
Newborn management for infants with EB differs from normal newborn care due to increased skin fragility with physical manipulation. Minimal skin manipulation and proper wound care are essential from the first day of life. For new bullae formation, bullae should be ruptured with a needle at the base of the blister and drained. The remaining skin overlying the wound should remain in place as a natural wound barrier. Patients with EB should not have tape or adhesive bandages applied directly to the skin. Instead, nonadhesive dressings can be placed directly on wounds and covered in soft wraps circumferentially. Dressings can be taped together without involving the skin. The cost for supplies for families to manage bullae is expensive. Fortunately, there are resources available for supplies and support for families, including the EB Research Partnership (https://www.ebresearch.org/) and DEBRA of America (https://www.debra.org/).
Currently, there is no cure for EB. Current treatment involves wound care, prevention, and symptomatic relief. Prevention includes avoiding activities that may result in increased friction of the skin and ensuring careful manipulation. Children with EB may have pain or itching from their blisters, which can be treated with oral acetaminophen or ibuprofen and diphenhydramine, respectively. Other complications of EB include anemia, dehydration, constipation, infection, and malnutrition. In more severe forms of EB, complications including eye problems, mucosal strictures, and skin cancer may occur.8 Future treatment directions include gene therapy, bone marrow transplantation, protein replacement therapies, and cell-based therapies. Prognosis for infants with EB due to KRT14 mutation is good, as it is a milder subtype of EB with a full life expectancy and improvement of blistering skin with age. The most at-risk time for early death is during infancy due to increased risk for infection.8 In this case, our patient showed full healing with no scar formation, which suggested a reassuring prognosis.
- Fine JD, Bruckner-Tuderman L, Eady RAJ, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103-1126.
- Wolff K, Johnson RA, Saavedra AP, et al. Hereditary epidermolysis bullosa. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017:94-99.
- Ross A, Shoff HW. Staphylococcus scalded skin syndrome. In: StatPearls. StatPearls Publishing; 2020:1-20.
- Alerhand S, Cassella C, Koyfman A. Steven-Johnson syndrome and toxic epidermal necrolysis in the pediatric population. Pediatr Emerg Care. 2016;32:472-476.
- Schwieger-Briel A, Moellmann C, Mattulat B, et al. Bullous pemphigoid in infants: characteristics, diagnosis and treatment. Orphanet J Rare Dis. 2014;9:185.
- Gonzalez ME. Evaluation and treatment of the newborn with epidermolysis bullosa. Semin Perinatol. 2013;37:32-39.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627.
- Watkins J. Diagnosis, treatment and management of epidermolysis bullosa. Br J Nurs. 2016;25:428-431.
The Diagnosis: Epidermolysis Bullosa
Our patient was found to have epidermolysis bullosa (EB), a rare genetic disease in which the superficial layers of the skin separate to form vesicles or bullae due to a mutation in the keratin 14 gene, KRT14. Separation of the skin occurs due to cleavage of various proteins that connect the epidermis to the dermis. A genetic mutation in KRT14, one of the more common genetic mutations associated with EB, results in cleavage at the basal epidermal protein keratin 14. The skin of individuals with EB typically is fragile and cannot tolerate friction or manipulation due to the risk for new bullae formation.1 Epidermolysis bullosa is rare, affecting approximately 20 children per 1 million births in the United States, and is not commonly seen by most general adult dermatologists.2
In our patient, the differential diagnoses included staphylococcal scalded skin syndrome (SSSS), Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), herpes simplex virus (HSV), and bullous pemphigoid (BP). Symptoms of SSSS can range from mild and localized to full-body exfoliation of the skin. Although SSSS can resemble other bullous disorders, its etiology arises from the Staphylococcus exotoxin targeting desmoglein in the stratum granulosum— the layer of the epidermis between the stratum corneum and stratum spinosum.3 Lesions start on the face, neck, and body folds, which was consistent with our patient’s presentation. However, bullae continued to develop in our patient despite antibiotic therapy, which reduced the likelihood of SSSS. Stevens-Johnson syndrome/toxic epidermal necrolysis develops rapidly and often involves the mucosa, which our patient initially did not have. In children, SJS/TEN can develop secondary to infection, whereas in adults it more commonly is associated with medication administration.4 Although the mother tested negative for HSV, the infant was started on acyclovir, which ultimately was discontinued due to low clinical suspicion. The clinical presentation of HSV (ie, clustered vesicles) was not consistent with our patient’s presentation. Bullous pemphigoid is a subepithelial blistering disease seen in older adults. Tense, fluidfilled blisters primarily are seen on the trunk and flexures. Although infantile BP can occur, it usually does not present in the neonatal period but rather at approximately 3 to 5 months of age.5
High clinical suspicion for EB due to the common characteristics of bullae location and formation following skin manipulation led to genetic testing in our patient. Mild forms of EB simplex typically appear on the upper and lower extremities with sparing of the trunk. In more severe cases of EB simplex, truncal and mucosal involvement may occur.6 In our case, the infant had a classic distribution of arm and leg blisters with truncal sparing. Epidermolysis bullosa may not be diagnosed in the neonatal period because of its similarities to other more common diseases, such as HSV or bullous impetigo, or other genetic blistering diseases, such as epidermolytic ichthyosis and incontinentia pigmenti.6
Epidermolysis bullosa can be inherited in an autosomal-dominant or autosomal-recessive fashion or with de novo mutations and is classified based on the location of cleavage in the skin. The 4 classical subtypes— simplex, junctional, dystrophic, and Kindler—have now been further subclassified. Epidermolysis bullosa simplex (intraepidermal split) is now separated into basal and suprabasal, with further subclassification including the distribution of blisters (generalized or localized) and the severity of cutaneous or extracutaneous involvement.7
In our case, the infant was found to have intraepidermal EB (simplex) due to a KRT14 mutation (missense mutation).6 KRT14 (17q21.2) and KRT5 (12q13.3) are the 2 most common mutations causing cleavage at the basal intraepidermal layer. Thickening of the palms, soles, and nails can be seen; however, blisters heal well without scarring, as seen in our patient. Junctional EB due to cleavage at the intralamina lucida often involves mutations in laminin 332, plectin, and α6β4 integrin. Infants with junctional EB often die from severe infection, dehydration, or malnutrition due to mucosal involvement. Dystrophic EB occurs due to a collagen VII mutation in the dermis, leading to blisters at the sublamina densa and more severe symptoms in the recessive form.7
Newborn management for infants with EB differs from normal newborn care due to increased skin fragility with physical manipulation. Minimal skin manipulation and proper wound care are essential from the first day of life. For new bullae formation, bullae should be ruptured with a needle at the base of the blister and drained. The remaining skin overlying the wound should remain in place as a natural wound barrier. Patients with EB should not have tape or adhesive bandages applied directly to the skin. Instead, nonadhesive dressings can be placed directly on wounds and covered in soft wraps circumferentially. Dressings can be taped together without involving the skin. The cost for supplies for families to manage bullae is expensive. Fortunately, there are resources available for supplies and support for families, including the EB Research Partnership (https://www.ebresearch.org/) and DEBRA of America (https://www.debra.org/).
Currently, there is no cure for EB. Current treatment involves wound care, prevention, and symptomatic relief. Prevention includes avoiding activities that may result in increased friction of the skin and ensuring careful manipulation. Children with EB may have pain or itching from their blisters, which can be treated with oral acetaminophen or ibuprofen and diphenhydramine, respectively. Other complications of EB include anemia, dehydration, constipation, infection, and malnutrition. In more severe forms of EB, complications including eye problems, mucosal strictures, and skin cancer may occur.8 Future treatment directions include gene therapy, bone marrow transplantation, protein replacement therapies, and cell-based therapies. Prognosis for infants with EB due to KRT14 mutation is good, as it is a milder subtype of EB with a full life expectancy and improvement of blistering skin with age. The most at-risk time for early death is during infancy due to increased risk for infection.8 In this case, our patient showed full healing with no scar formation, which suggested a reassuring prognosis.
The Diagnosis: Epidermolysis Bullosa
Our patient was found to have epidermolysis bullosa (EB), a rare genetic disease in which the superficial layers of the skin separate to form vesicles or bullae due to a mutation in the keratin 14 gene, KRT14. Separation of the skin occurs due to cleavage of various proteins that connect the epidermis to the dermis. A genetic mutation in KRT14, one of the more common genetic mutations associated with EB, results in cleavage at the basal epidermal protein keratin 14. The skin of individuals with EB typically is fragile and cannot tolerate friction or manipulation due to the risk for new bullae formation.1 Epidermolysis bullosa is rare, affecting approximately 20 children per 1 million births in the United States, and is not commonly seen by most general adult dermatologists.2
In our patient, the differential diagnoses included staphylococcal scalded skin syndrome (SSSS), Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), herpes simplex virus (HSV), and bullous pemphigoid (BP). Symptoms of SSSS can range from mild and localized to full-body exfoliation of the skin. Although SSSS can resemble other bullous disorders, its etiology arises from the Staphylococcus exotoxin targeting desmoglein in the stratum granulosum— the layer of the epidermis between the stratum corneum and stratum spinosum.3 Lesions start on the face, neck, and body folds, which was consistent with our patient’s presentation. However, bullae continued to develop in our patient despite antibiotic therapy, which reduced the likelihood of SSSS. Stevens-Johnson syndrome/toxic epidermal necrolysis develops rapidly and often involves the mucosa, which our patient initially did not have. In children, SJS/TEN can develop secondary to infection, whereas in adults it more commonly is associated with medication administration.4 Although the mother tested negative for HSV, the infant was started on acyclovir, which ultimately was discontinued due to low clinical suspicion. The clinical presentation of HSV (ie, clustered vesicles) was not consistent with our patient’s presentation. Bullous pemphigoid is a subepithelial blistering disease seen in older adults. Tense, fluidfilled blisters primarily are seen on the trunk and flexures. Although infantile BP can occur, it usually does not present in the neonatal period but rather at approximately 3 to 5 months of age.5
High clinical suspicion for EB due to the common characteristics of bullae location and formation following skin manipulation led to genetic testing in our patient. Mild forms of EB simplex typically appear on the upper and lower extremities with sparing of the trunk. In more severe cases of EB simplex, truncal and mucosal involvement may occur.6 In our case, the infant had a classic distribution of arm and leg blisters with truncal sparing. Epidermolysis bullosa may not be diagnosed in the neonatal period because of its similarities to other more common diseases, such as HSV or bullous impetigo, or other genetic blistering diseases, such as epidermolytic ichthyosis and incontinentia pigmenti.6
Epidermolysis bullosa can be inherited in an autosomal-dominant or autosomal-recessive fashion or with de novo mutations and is classified based on the location of cleavage in the skin. The 4 classical subtypes— simplex, junctional, dystrophic, and Kindler—have now been further subclassified. Epidermolysis bullosa simplex (intraepidermal split) is now separated into basal and suprabasal, with further subclassification including the distribution of blisters (generalized or localized) and the severity of cutaneous or extracutaneous involvement.7
In our case, the infant was found to have intraepidermal EB (simplex) due to a KRT14 mutation (missense mutation).6 KRT14 (17q21.2) and KRT5 (12q13.3) are the 2 most common mutations causing cleavage at the basal intraepidermal layer. Thickening of the palms, soles, and nails can be seen; however, blisters heal well without scarring, as seen in our patient. Junctional EB due to cleavage at the intralamina lucida often involves mutations in laminin 332, plectin, and α6β4 integrin. Infants with junctional EB often die from severe infection, dehydration, or malnutrition due to mucosal involvement. Dystrophic EB occurs due to a collagen VII mutation in the dermis, leading to blisters at the sublamina densa and more severe symptoms in the recessive form.7
Newborn management for infants with EB differs from normal newborn care due to increased skin fragility with physical manipulation. Minimal skin manipulation and proper wound care are essential from the first day of life. For new bullae formation, bullae should be ruptured with a needle at the base of the blister and drained. The remaining skin overlying the wound should remain in place as a natural wound barrier. Patients with EB should not have tape or adhesive bandages applied directly to the skin. Instead, nonadhesive dressings can be placed directly on wounds and covered in soft wraps circumferentially. Dressings can be taped together without involving the skin. The cost for supplies for families to manage bullae is expensive. Fortunately, there are resources available for supplies and support for families, including the EB Research Partnership (https://www.ebresearch.org/) and DEBRA of America (https://www.debra.org/).
Currently, there is no cure for EB. Current treatment involves wound care, prevention, and symptomatic relief. Prevention includes avoiding activities that may result in increased friction of the skin and ensuring careful manipulation. Children with EB may have pain or itching from their blisters, which can be treated with oral acetaminophen or ibuprofen and diphenhydramine, respectively. Other complications of EB include anemia, dehydration, constipation, infection, and malnutrition. In more severe forms of EB, complications including eye problems, mucosal strictures, and skin cancer may occur.8 Future treatment directions include gene therapy, bone marrow transplantation, protein replacement therapies, and cell-based therapies. Prognosis for infants with EB due to KRT14 mutation is good, as it is a milder subtype of EB with a full life expectancy and improvement of blistering skin with age. The most at-risk time for early death is during infancy due to increased risk for infection.8 In this case, our patient showed full healing with no scar formation, which suggested a reassuring prognosis.
- Fine JD, Bruckner-Tuderman L, Eady RAJ, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103-1126.
- Wolff K, Johnson RA, Saavedra AP, et al. Hereditary epidermolysis bullosa. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017:94-99.
- Ross A, Shoff HW. Staphylococcus scalded skin syndrome. In: StatPearls. StatPearls Publishing; 2020:1-20.
- Alerhand S, Cassella C, Koyfman A. Steven-Johnson syndrome and toxic epidermal necrolysis in the pediatric population. Pediatr Emerg Care. 2016;32:472-476.
- Schwieger-Briel A, Moellmann C, Mattulat B, et al. Bullous pemphigoid in infants: characteristics, diagnosis and treatment. Orphanet J Rare Dis. 2014;9:185.
- Gonzalez ME. Evaluation and treatment of the newborn with epidermolysis bullosa. Semin Perinatol. 2013;37:32-39.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627.
- Watkins J. Diagnosis, treatment and management of epidermolysis bullosa. Br J Nurs. 2016;25:428-431.
- Fine JD, Bruckner-Tuderman L, Eady RAJ, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103-1126.
- Wolff K, Johnson RA, Saavedra AP, et al. Hereditary epidermolysis bullosa. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017:94-99.
- Ross A, Shoff HW. Staphylococcus scalded skin syndrome. In: StatPearls. StatPearls Publishing; 2020:1-20.
- Alerhand S, Cassella C, Koyfman A. Steven-Johnson syndrome and toxic epidermal necrolysis in the pediatric population. Pediatr Emerg Care. 2016;32:472-476.
- Schwieger-Briel A, Moellmann C, Mattulat B, et al. Bullous pemphigoid in infants: characteristics, diagnosis and treatment. Orphanet J Rare Dis. 2014;9:185.
- Gonzalez ME. Evaluation and treatment of the newborn with epidermolysis bullosa. Semin Perinatol. 2013;37:32-39.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627.
- Watkins J. Diagnosis, treatment and management of epidermolysis bullosa. Br J Nurs. 2016;25:428-431.
A 4-day-old infant boy presented with blisters on the skin. He was born at 36 weeks’ gestation by cesarean delivery to a nulliparous mother who received appropriate prenatal care. On day 2 of life, the patient developed bullae with breakdown of the skin on the bilateral heels and on the skin surrounding intravenous injection sites. Similar blisters subsequently developed on the fingers (top), thighs, groin, and toes (bottom), sparing the oral mucosa and trunk. He remained afebrile and stable and was started on ampicillin, gentamicin, and acyclovir with continued development of blisters. Two weeks later he developed painful ulcers on the tongue that bled upon scraping.
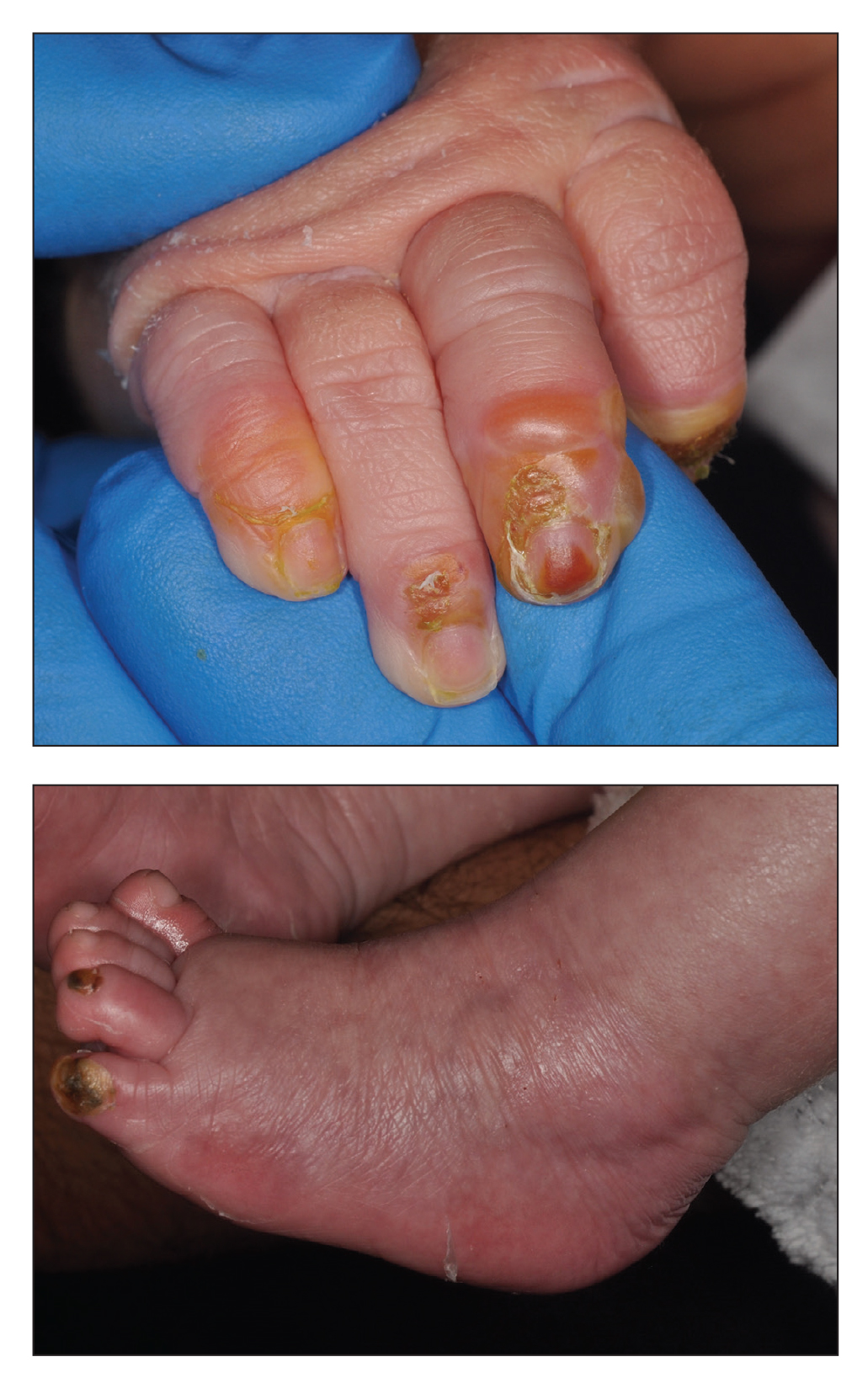
Age and ferritin levels may predict MIS-C severity
, according to a Canadian multicenter cohort study.
The adjusted absolute risk for admission to an intensive care unit was 43.6% among children aged 6 years and older and 46.2% in children aged 13 to 17 years, compared with 18.4% in children aged 5 years or younger.
“We do not understand why teens get more severe MIS-C than younger children,” senior author Joan Robinson, MD, of the University of Alberta, Edmonton, told this news organization. “It is possible that more exposures to other coronaviruses in the past result in them having a more robust immune response to SARS-CoV-2, which results in more inflammation.”
The data were published in the Canadian Medical Association Journal.
A multinational study
The study included data on 232 children admitted with probable or confirmed MIS-C at 15 hospitals in Canada, Iran, and Costa Rica between March 1, 2020, and March 7, 2021. The median age of the children was 5.8 years, 56.0% were boys, and 21.6% had comorbidities.
Although cardiac involvement was common (58.6%), and almost one-third of the cohort (31.5%) was admitted to an ICU, “recovery was typically rapid, with 85% of patients discharged within 10 days,” said Dr. Robinson, for the Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC).
Older age as a risk
The results suggest that older age is associated with increased risk of severe MIS-C. “However, one would then predict that adults would be at even higher risk than teens, whereas the same syndrome in adults (MIS-A) is very, very rare,” said Dr. Robinson.
The study also found that children admitted with ferritin levels greater than 500 μg/L, signaling greater inflammation, also had an increased risk for ICU admission, compared with those with lower levels (adjusted risk difference, 18.4%; relative risk, 1.69). “This is presumably because the more inflammation that the child has, the more likely they are to have inflammation of the heart, which can lead to low blood pressure,” said Dr. Robinson.
Features of MIS-C
Among all patients with MIS-C, gastrointestinal involvement was common (89.2%), as were mucocutaneous findings (84.5%). Children with MIS-C had fever for a median duration of 6 days. “Clinicians who see children in their practice commonly have to determine why a child is febrile. Our study shows that one mainly has to consider MIS-C if febrile children have a rash and one or more of vomiting, diarrhea, or abdominal pain,” said Dr. Robinson.
The study also found that patients with MIS-C who were admitted to the hospital in the latter part of the study period (Nov. 1, 2020, to March 7, 2021) were slightly more likely to require ICU admission, compared with those admitted between March 1 and Oct. 31, 2020. “We cannot provide a clear explanation [for this],” the authors noted. “The features of severe MIS-C were widely publicized by May 2020, so it seems unlikely that severe cases were missed early in the study period. SARS-CoV-2 variants of concern have replaced the wild-type virus. It is possible that the immune response to circulating variants alters the severity of COVID-19 and MIS-C, when compared with wild-type virus.”
Despite initial concerns that pediatric COVID-19 vaccines might cause MIS-C, Dr. Robinson says data suggest this is rarely, if ever, the case, and that vaccines actually prevent the syndrome. She says further studies will be needed to assess MIS-C risk following reinfection with SARS-CoV-2. “I am an optimistic person, and it is my hope that MIS-C following reinfection is rare,” she said. “If this is the case, perhaps we will see very few cases once almost all children have been immunized and/or had SARS-CoV-2 infection.”
‘Differences across countries’
Adrienne Randolph, MD, a pediatrician at Harvard Medical School, Boston, and senior author of a large case series of patients with MIS-C, said that the Canadian study is valuable because it includes children from three countries. “It’s very interesting that there are differences across countries,” she said. “The patients in Iran had the highest percentage (58.7%) going into the ICU, whereas Costa Rica had the lowest percentage (9.2%), and the percentage going to the ICU in Canada (34.7%) was less than the percentages we see in the U.S. – which is pretty consistently about 60% to 70% of MIS-C patients going into the ICU.” Dr. Randolph was not involved in the current study.
Reasons for differences in the rates of ICU visits will be important to explore in the effort to standardize diagnostic criteria, stratification of severity, and recommendations for treatment of MIS-C, said Dr. Randolph.
“What is consistent is that the younger kids, zero to 5 years, in general are less ill,” she said. “That’s been consistent across multiple countries.” It’s unclear whether the cause of this difference is that parents observe younger patients more closely than they do teenagers, or whether other aspects of adolescence, such as prevalence of obesity and attendant inflammation, are at work, said Dr. Randolph.
What is also unclear is why hospitalized patients with MIS-C had higher percentages of ICU admission in the latter part of the study period, compared with the earlier period. “Did the patients change, or did practice change as we got to understand the disease process?” asked Dr. Randolph. “It could be that they got better at the diagnosis and were weeding out some of the patients who they realized didn’t need to be hospitalized. At the very beginning, we had a very low threshold to admit patients, because we didn’t know, and then, over time, people understood what was going on and felt more comfortable monitoring them as outpatients.”
This study was partially funded by a Janeway Foundation Research Grant to support data collection. Dr. Robinson disclosed no conflicts of interest. Dr. Randolph reported receiving royalties from UpToDate and personal fees from the La Jolla Pharmaceutical Company.
A version of this article first appeared on Medscape.com.
, according to a Canadian multicenter cohort study.
The adjusted absolute risk for admission to an intensive care unit was 43.6% among children aged 6 years and older and 46.2% in children aged 13 to 17 years, compared with 18.4% in children aged 5 years or younger.
“We do not understand why teens get more severe MIS-C than younger children,” senior author Joan Robinson, MD, of the University of Alberta, Edmonton, told this news organization. “It is possible that more exposures to other coronaviruses in the past result in them having a more robust immune response to SARS-CoV-2, which results in more inflammation.”
The data were published in the Canadian Medical Association Journal.
A multinational study
The study included data on 232 children admitted with probable or confirmed MIS-C at 15 hospitals in Canada, Iran, and Costa Rica between March 1, 2020, and March 7, 2021. The median age of the children was 5.8 years, 56.0% were boys, and 21.6% had comorbidities.
Although cardiac involvement was common (58.6%), and almost one-third of the cohort (31.5%) was admitted to an ICU, “recovery was typically rapid, with 85% of patients discharged within 10 days,” said Dr. Robinson, for the Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC).
Older age as a risk
The results suggest that older age is associated with increased risk of severe MIS-C. “However, one would then predict that adults would be at even higher risk than teens, whereas the same syndrome in adults (MIS-A) is very, very rare,” said Dr. Robinson.
The study also found that children admitted with ferritin levels greater than 500 μg/L, signaling greater inflammation, also had an increased risk for ICU admission, compared with those with lower levels (adjusted risk difference, 18.4%; relative risk, 1.69). “This is presumably because the more inflammation that the child has, the more likely they are to have inflammation of the heart, which can lead to low blood pressure,” said Dr. Robinson.
Features of MIS-C
Among all patients with MIS-C, gastrointestinal involvement was common (89.2%), as were mucocutaneous findings (84.5%). Children with MIS-C had fever for a median duration of 6 days. “Clinicians who see children in their practice commonly have to determine why a child is febrile. Our study shows that one mainly has to consider MIS-C if febrile children have a rash and one or more of vomiting, diarrhea, or abdominal pain,” said Dr. Robinson.
The study also found that patients with MIS-C who were admitted to the hospital in the latter part of the study period (Nov. 1, 2020, to March 7, 2021) were slightly more likely to require ICU admission, compared with those admitted between March 1 and Oct. 31, 2020. “We cannot provide a clear explanation [for this],” the authors noted. “The features of severe MIS-C were widely publicized by May 2020, so it seems unlikely that severe cases were missed early in the study period. SARS-CoV-2 variants of concern have replaced the wild-type virus. It is possible that the immune response to circulating variants alters the severity of COVID-19 and MIS-C, when compared with wild-type virus.”
Despite initial concerns that pediatric COVID-19 vaccines might cause MIS-C, Dr. Robinson says data suggest this is rarely, if ever, the case, and that vaccines actually prevent the syndrome. She says further studies will be needed to assess MIS-C risk following reinfection with SARS-CoV-2. “I am an optimistic person, and it is my hope that MIS-C following reinfection is rare,” she said. “If this is the case, perhaps we will see very few cases once almost all children have been immunized and/or had SARS-CoV-2 infection.”
‘Differences across countries’
Adrienne Randolph, MD, a pediatrician at Harvard Medical School, Boston, and senior author of a large case series of patients with MIS-C, said that the Canadian study is valuable because it includes children from three countries. “It’s very interesting that there are differences across countries,” she said. “The patients in Iran had the highest percentage (58.7%) going into the ICU, whereas Costa Rica had the lowest percentage (9.2%), and the percentage going to the ICU in Canada (34.7%) was less than the percentages we see in the U.S. – which is pretty consistently about 60% to 70% of MIS-C patients going into the ICU.” Dr. Randolph was not involved in the current study.
Reasons for differences in the rates of ICU visits will be important to explore in the effort to standardize diagnostic criteria, stratification of severity, and recommendations for treatment of MIS-C, said Dr. Randolph.
“What is consistent is that the younger kids, zero to 5 years, in general are less ill,” she said. “That’s been consistent across multiple countries.” It’s unclear whether the cause of this difference is that parents observe younger patients more closely than they do teenagers, or whether other aspects of adolescence, such as prevalence of obesity and attendant inflammation, are at work, said Dr. Randolph.
What is also unclear is why hospitalized patients with MIS-C had higher percentages of ICU admission in the latter part of the study period, compared with the earlier period. “Did the patients change, or did practice change as we got to understand the disease process?” asked Dr. Randolph. “It could be that they got better at the diagnosis and were weeding out some of the patients who they realized didn’t need to be hospitalized. At the very beginning, we had a very low threshold to admit patients, because we didn’t know, and then, over time, people understood what was going on and felt more comfortable monitoring them as outpatients.”
This study was partially funded by a Janeway Foundation Research Grant to support data collection. Dr. Robinson disclosed no conflicts of interest. Dr. Randolph reported receiving royalties from UpToDate and personal fees from the La Jolla Pharmaceutical Company.
A version of this article first appeared on Medscape.com.
, according to a Canadian multicenter cohort study.
The adjusted absolute risk for admission to an intensive care unit was 43.6% among children aged 6 years and older and 46.2% in children aged 13 to 17 years, compared with 18.4% in children aged 5 years or younger.
“We do not understand why teens get more severe MIS-C than younger children,” senior author Joan Robinson, MD, of the University of Alberta, Edmonton, told this news organization. “It is possible that more exposures to other coronaviruses in the past result in them having a more robust immune response to SARS-CoV-2, which results in more inflammation.”
The data were published in the Canadian Medical Association Journal.
A multinational study
The study included data on 232 children admitted with probable or confirmed MIS-C at 15 hospitals in Canada, Iran, and Costa Rica between March 1, 2020, and March 7, 2021. The median age of the children was 5.8 years, 56.0% were boys, and 21.6% had comorbidities.
Although cardiac involvement was common (58.6%), and almost one-third of the cohort (31.5%) was admitted to an ICU, “recovery was typically rapid, with 85% of patients discharged within 10 days,” said Dr. Robinson, for the Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC).
Older age as a risk
The results suggest that older age is associated with increased risk of severe MIS-C. “However, one would then predict that adults would be at even higher risk than teens, whereas the same syndrome in adults (MIS-A) is very, very rare,” said Dr. Robinson.
The study also found that children admitted with ferritin levels greater than 500 μg/L, signaling greater inflammation, also had an increased risk for ICU admission, compared with those with lower levels (adjusted risk difference, 18.4%; relative risk, 1.69). “This is presumably because the more inflammation that the child has, the more likely they are to have inflammation of the heart, which can lead to low blood pressure,” said Dr. Robinson.
Features of MIS-C
Among all patients with MIS-C, gastrointestinal involvement was common (89.2%), as were mucocutaneous findings (84.5%). Children with MIS-C had fever for a median duration of 6 days. “Clinicians who see children in their practice commonly have to determine why a child is febrile. Our study shows that one mainly has to consider MIS-C if febrile children have a rash and one or more of vomiting, diarrhea, or abdominal pain,” said Dr. Robinson.
The study also found that patients with MIS-C who were admitted to the hospital in the latter part of the study period (Nov. 1, 2020, to March 7, 2021) were slightly more likely to require ICU admission, compared with those admitted between March 1 and Oct. 31, 2020. “We cannot provide a clear explanation [for this],” the authors noted. “The features of severe MIS-C were widely publicized by May 2020, so it seems unlikely that severe cases were missed early in the study period. SARS-CoV-2 variants of concern have replaced the wild-type virus. It is possible that the immune response to circulating variants alters the severity of COVID-19 and MIS-C, when compared with wild-type virus.”
Despite initial concerns that pediatric COVID-19 vaccines might cause MIS-C, Dr. Robinson says data suggest this is rarely, if ever, the case, and that vaccines actually prevent the syndrome. She says further studies will be needed to assess MIS-C risk following reinfection with SARS-CoV-2. “I am an optimistic person, and it is my hope that MIS-C following reinfection is rare,” she said. “If this is the case, perhaps we will see very few cases once almost all children have been immunized and/or had SARS-CoV-2 infection.”
‘Differences across countries’
Adrienne Randolph, MD, a pediatrician at Harvard Medical School, Boston, and senior author of a large case series of patients with MIS-C, said that the Canadian study is valuable because it includes children from three countries. “It’s very interesting that there are differences across countries,” she said. “The patients in Iran had the highest percentage (58.7%) going into the ICU, whereas Costa Rica had the lowest percentage (9.2%), and the percentage going to the ICU in Canada (34.7%) was less than the percentages we see in the U.S. – which is pretty consistently about 60% to 70% of MIS-C patients going into the ICU.” Dr. Randolph was not involved in the current study.
Reasons for differences in the rates of ICU visits will be important to explore in the effort to standardize diagnostic criteria, stratification of severity, and recommendations for treatment of MIS-C, said Dr. Randolph.
“What is consistent is that the younger kids, zero to 5 years, in general are less ill,” she said. “That’s been consistent across multiple countries.” It’s unclear whether the cause of this difference is that parents observe younger patients more closely than they do teenagers, or whether other aspects of adolescence, such as prevalence of obesity and attendant inflammation, are at work, said Dr. Randolph.
What is also unclear is why hospitalized patients with MIS-C had higher percentages of ICU admission in the latter part of the study period, compared with the earlier period. “Did the patients change, or did practice change as we got to understand the disease process?” asked Dr. Randolph. “It could be that they got better at the diagnosis and were weeding out some of the patients who they realized didn’t need to be hospitalized. At the very beginning, we had a very low threshold to admit patients, because we didn’t know, and then, over time, people understood what was going on and felt more comfortable monitoring them as outpatients.”
This study was partially funded by a Janeway Foundation Research Grant to support data collection. Dr. Robinson disclosed no conflicts of interest. Dr. Randolph reported receiving royalties from UpToDate and personal fees from the La Jolla Pharmaceutical Company.
A version of this article first appeared on Medscape.com.
Judge strikes down Biden mask mandate for planes, transit
The mandate, enacted in February 2021, is unconstitutional because Congress never granted the Centers for Disease Control and Prevention the power to create such a requirement, U.S. District Judge Kathryn Kimball Mizelle said in her order issued April 18.
“Congress addressed whether the CDC may enact preventative measures that condition the interstate travel of an entire population to CDC dictates. It may not,” the order says.
While the government argued that the definition of “sanitation” in federal law allows it to create travel restrictions like the use of masks, Judge Mizelle disagreed.
“A power to improve ‘sanitation’ would easily extend to requiring vaccinations against COVID-19, the seasonal flu, or other diseases. Or to mandatory social distancing, coughing-into-elbows, and daily multivitamins,” she wrote.
The Biden administration has extended the mask mandate several times since it was first announced. Most recently, the mandate was extended last week and was set to end May 3.
The rule has been alternately praised and criticized by airlines, pilots, and flight attendants. Lawsuits have been filed over the mandate, but Judge Mizelle ruled in favor of two people and the Health Freedom Defense Fund, who filed suit in July 2021.
It is not yet clear if the Biden administration will appeal the decision.
A version of this article first appeared on WebMD.com.
The mandate, enacted in February 2021, is unconstitutional because Congress never granted the Centers for Disease Control and Prevention the power to create such a requirement, U.S. District Judge Kathryn Kimball Mizelle said in her order issued April 18.
“Congress addressed whether the CDC may enact preventative measures that condition the interstate travel of an entire population to CDC dictates. It may not,” the order says.
While the government argued that the definition of “sanitation” in federal law allows it to create travel restrictions like the use of masks, Judge Mizelle disagreed.
“A power to improve ‘sanitation’ would easily extend to requiring vaccinations against COVID-19, the seasonal flu, or other diseases. Or to mandatory social distancing, coughing-into-elbows, and daily multivitamins,” she wrote.
The Biden administration has extended the mask mandate several times since it was first announced. Most recently, the mandate was extended last week and was set to end May 3.
The rule has been alternately praised and criticized by airlines, pilots, and flight attendants. Lawsuits have been filed over the mandate, but Judge Mizelle ruled in favor of two people and the Health Freedom Defense Fund, who filed suit in July 2021.
It is not yet clear if the Biden administration will appeal the decision.
A version of this article first appeared on WebMD.com.
The mandate, enacted in February 2021, is unconstitutional because Congress never granted the Centers for Disease Control and Prevention the power to create such a requirement, U.S. District Judge Kathryn Kimball Mizelle said in her order issued April 18.
“Congress addressed whether the CDC may enact preventative measures that condition the interstate travel of an entire population to CDC dictates. It may not,” the order says.
While the government argued that the definition of “sanitation” in federal law allows it to create travel restrictions like the use of masks, Judge Mizelle disagreed.
“A power to improve ‘sanitation’ would easily extend to requiring vaccinations against COVID-19, the seasonal flu, or other diseases. Or to mandatory social distancing, coughing-into-elbows, and daily multivitamins,” she wrote.
The Biden administration has extended the mask mandate several times since it was first announced. Most recently, the mandate was extended last week and was set to end May 3.
The rule has been alternately praised and criticized by airlines, pilots, and flight attendants. Lawsuits have been filed over the mandate, but Judge Mizelle ruled in favor of two people and the Health Freedom Defense Fund, who filed suit in July 2021.
It is not yet clear if the Biden administration will appeal the decision.
A version of this article first appeared on WebMD.com.
Pediatric hepatitis cases may be linked to adenovirus, CDC says
Internationally, 108 cases have been reported in the United Kingdom, with 79 cases occurring in England. There are three documented cases in Spain, and similar cases are being reported in Denmark and the Netherlands, according to an article in Science. In the United Kingdom, cases have been reported in children up to 16 years old, but most affected children are between 2 and 5 years old. Eight children in the United Kingdom have required liver transplants.
On April 14, the CDC said that nine cases have been recorded in Alabama since the fall of 2021. All of these cases have been in children between 1 and 6 years old, and two children have needed liver transplants. Two additional cases have been reported in North Carolina, according to Stat News, and both children have since recovered.
Hepatitis A, B, C, D, and E viruses—common causes of hepatitis—have been ruled out in the U.K. and Spanish cases. More than three-fourths (77%) of the children sickened in the United Kingdom and all nine cases in Alabama have tested positive for a form of the adenovirus. While adenovirus can cause hepatitis in children, it is usually in those who are immunocompromised.
The CDC health alert advises clinicians who have cases of unexplained hepatitis in children to test for adenovirus and report these cases to the CDC as well as state public health authorities. The agency recommends nucleic acid amplification testing to detect adenovirus using respiratory swabs, stool samples or rectal swabs, or blood.
Officials are exploring whether these cases are linked to a version of the virus called adenovirus 41, which is associated with gut inflammation. The most recent case in Alabama was reported in February, and five of the nine children in the state with these puzzling cases of hepatitis have tested positive for adenovirus 41.
There have yet to be any links among the cases in Alabama or North Carolina, and investigators in the United Kingdom have also not found any connections in their cases, STAT News reports.
“CDC is working with state health departments to see if there are additional U.S. cases and what may be causing these cases,” said Kristen Nordlund, a CDC spokesperson, in a statement to STAT News. “At this time, adenovirus may be the cause for these, but investigators are still learning more – including ruling out the more common causes of hepatitis.”
Looking for other explanations
None of the children in the United States with hepatitis had COVID-19, but a few children in the United Kingdom have tested positive for the virus; none of these children have received the COVID-19 vaccine.
While the U.K. Health Security Agency says their investigation “continues to point toward a link to adenovirus infection,” they are also considering other contributing factors such as an environmental cause or COVID-19.
“COVID has been consistently shown to increase liver test numbers,” Nancy Reau, MD, the section chief of hepatology at Rush University in Chicago, said in an interview with this news organization. “It has been shown to cause other organ involvement besides just pulmonary symptoms and respiratory failure. As this virus evolves, it might be that in children, it is more able to present as hepatitis.”
A version of this article first appeared on Medscape.com.
This article was updated 4/22/22.
Internationally, 108 cases have been reported in the United Kingdom, with 79 cases occurring in England. There are three documented cases in Spain, and similar cases are being reported in Denmark and the Netherlands, according to an article in Science. In the United Kingdom, cases have been reported in children up to 16 years old, but most affected children are between 2 and 5 years old. Eight children in the United Kingdom have required liver transplants.
On April 14, the CDC said that nine cases have been recorded in Alabama since the fall of 2021. All of these cases have been in children between 1 and 6 years old, and two children have needed liver transplants. Two additional cases have been reported in North Carolina, according to Stat News, and both children have since recovered.
Hepatitis A, B, C, D, and E viruses—common causes of hepatitis—have been ruled out in the U.K. and Spanish cases. More than three-fourths (77%) of the children sickened in the United Kingdom and all nine cases in Alabama have tested positive for a form of the adenovirus. While adenovirus can cause hepatitis in children, it is usually in those who are immunocompromised.
The CDC health alert advises clinicians who have cases of unexplained hepatitis in children to test for adenovirus and report these cases to the CDC as well as state public health authorities. The agency recommends nucleic acid amplification testing to detect adenovirus using respiratory swabs, stool samples or rectal swabs, or blood.
Officials are exploring whether these cases are linked to a version of the virus called adenovirus 41, which is associated with gut inflammation. The most recent case in Alabama was reported in February, and five of the nine children in the state with these puzzling cases of hepatitis have tested positive for adenovirus 41.
There have yet to be any links among the cases in Alabama or North Carolina, and investigators in the United Kingdom have also not found any connections in their cases, STAT News reports.
“CDC is working with state health departments to see if there are additional U.S. cases and what may be causing these cases,” said Kristen Nordlund, a CDC spokesperson, in a statement to STAT News. “At this time, adenovirus may be the cause for these, but investigators are still learning more – including ruling out the more common causes of hepatitis.”
Looking for other explanations
None of the children in the United States with hepatitis had COVID-19, but a few children in the United Kingdom have tested positive for the virus; none of these children have received the COVID-19 vaccine.
While the U.K. Health Security Agency says their investigation “continues to point toward a link to adenovirus infection,” they are also considering other contributing factors such as an environmental cause or COVID-19.
“COVID has been consistently shown to increase liver test numbers,” Nancy Reau, MD, the section chief of hepatology at Rush University in Chicago, said in an interview with this news organization. “It has been shown to cause other organ involvement besides just pulmonary symptoms and respiratory failure. As this virus evolves, it might be that in children, it is more able to present as hepatitis.”
A version of this article first appeared on Medscape.com.
This article was updated 4/22/22.
Internationally, 108 cases have been reported in the United Kingdom, with 79 cases occurring in England. There are three documented cases in Spain, and similar cases are being reported in Denmark and the Netherlands, according to an article in Science. In the United Kingdom, cases have been reported in children up to 16 years old, but most affected children are between 2 and 5 years old. Eight children in the United Kingdom have required liver transplants.
On April 14, the CDC said that nine cases have been recorded in Alabama since the fall of 2021. All of these cases have been in children between 1 and 6 years old, and two children have needed liver transplants. Two additional cases have been reported in North Carolina, according to Stat News, and both children have since recovered.
Hepatitis A, B, C, D, and E viruses—common causes of hepatitis—have been ruled out in the U.K. and Spanish cases. More than three-fourths (77%) of the children sickened in the United Kingdom and all nine cases in Alabama have tested positive for a form of the adenovirus. While adenovirus can cause hepatitis in children, it is usually in those who are immunocompromised.
The CDC health alert advises clinicians who have cases of unexplained hepatitis in children to test for adenovirus and report these cases to the CDC as well as state public health authorities. The agency recommends nucleic acid amplification testing to detect adenovirus using respiratory swabs, stool samples or rectal swabs, or blood.
Officials are exploring whether these cases are linked to a version of the virus called adenovirus 41, which is associated with gut inflammation. The most recent case in Alabama was reported in February, and five of the nine children in the state with these puzzling cases of hepatitis have tested positive for adenovirus 41.
There have yet to be any links among the cases in Alabama or North Carolina, and investigators in the United Kingdom have also not found any connections in their cases, STAT News reports.
“CDC is working with state health departments to see if there are additional U.S. cases and what may be causing these cases,” said Kristen Nordlund, a CDC spokesperson, in a statement to STAT News. “At this time, adenovirus may be the cause for these, but investigators are still learning more – including ruling out the more common causes of hepatitis.”
Looking for other explanations
None of the children in the United States with hepatitis had COVID-19, but a few children in the United Kingdom have tested positive for the virus; none of these children have received the COVID-19 vaccine.
While the U.K. Health Security Agency says their investigation “continues to point toward a link to adenovirus infection,” they are also considering other contributing factors such as an environmental cause or COVID-19.
“COVID has been consistently shown to increase liver test numbers,” Nancy Reau, MD, the section chief of hepatology at Rush University in Chicago, said in an interview with this news organization. “It has been shown to cause other organ involvement besides just pulmonary symptoms and respiratory failure. As this virus evolves, it might be that in children, it is more able to present as hepatitis.”
A version of this article first appeared on Medscape.com.
This article was updated 4/22/22.