User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
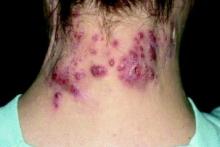
Study eyes characteristics of pediatric patients with hidradenitis suppurativa
INDIANAPOLIS – in a study presented at the annual meeting of the Society for Pediatric Dermatology.
In addition, 44% presented with scarring, which suggests that HS may be underdiagnosed in this patient population. Those are the key findings from the study, a single-center retrospective chart review presented by Stephanie Sanchez during a poster session at the meeting.
“There is limited research on HS within the pediatric population,” said Ms. Sanchez, a fourth-year medical student at Boston University. “It’s not very well defined or characterized.” The “unusually high number of pediatric patients with HS” at Boston Medical Center provided “a unique opportunity to study this topic.”
Working with her mentor, Lisa Shen, MD, associate medical director of pediatric dermatology at Boston University, Ms. Sanchez and colleagues retrospectively reviewed the medical records of 303 patients aged 4-18 years who were diagnosed with HS at Boston Medical Center from 2012 to 2021. Boston Medical Center is the largest safety net hospital in New England. All data points and outcome measures were collected within 6 months of the patient’s HS diagnosis date.
Of the 303 patients with HS, 84% were female and 16% were male. Complete information about race was available in 286 patients. Of these, 65% were Black/African American, 11% were White, and the rest were from other racial groups. The mean age at symptom onset was 13 years, while the mean age at diagnosis was 15 years, and the mean delay to diagnosis was 2 years. A family history of HS was reported in 36% of patients.
The most common clinical features in these HS patients were pain/tenderness (90%), pustules/papules (65%), discharge/drainage (62%), and deep-seated nodules (51%). Scarring was present in 44% of patients at the time of diagnosis. The three most common sites of involvement were the axillary area (79%), the pubic area (36%), and the inguinal folds/inner thighs (34%).
Obesity was the most common comorbidity at the time of diagnosis, with 64% of patients affected. The next most common comorbidities were acne vulgaris (36%), acanthosis nigricans (25%), depression (18%), being overweight (17%), polycystic ovary syndrome (16%) and anxiety (13%). None had type 1 diabetes or metabolic syndrome.
Referring to the large population of underserved minority patients at Boston Medical Center, Dr. Shen noted, “we have to make sure not to underestimate the prevalence of obesity in this population as they get older. We need to start from a younger age to incorporate multidisciplinary care such as weight management, nutrition, and working with our pediatric surgery colleagues in trying to tackle [HS] because there is data to suggest that the earlier we intervene, the better outcomes they have. That makes sense.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the findings, said that the study “highlights the impressive and concerning gap and delays in diagnosis, not too dissimilar to what the literature shows in adult HS patients, which unfortunately has tremendous ramifications, both physically and emotionally/psychosocially.”
While this single-center study identified potential risk factors, such as obesity and self-identifying as Black, he said, “it is important to note that this condition does not discriminate and therefore it is important not to miss the cases that don’t follow the textbook nor stigmatize this condition as one that only impacts certain demographics.”
The researchers reported having no financial disclosures. Dr. Friedman, who was not involved with the study, reported that he serves as a consultant and/or advisor to numerous pharmaceutical companies. He is a speaker for companies including, Regeneron, Sanofi, AbbVie, Janssen, Incyte, and Brickell Biotech, and has received grants from Pfizer, the Dermatology Foundation, Almirall, Incyte, Galderma, and Janssen.
INDIANAPOLIS – in a study presented at the annual meeting of the Society for Pediatric Dermatology.
In addition, 44% presented with scarring, which suggests that HS may be underdiagnosed in this patient population. Those are the key findings from the study, a single-center retrospective chart review presented by Stephanie Sanchez during a poster session at the meeting.
“There is limited research on HS within the pediatric population,” said Ms. Sanchez, a fourth-year medical student at Boston University. “It’s not very well defined or characterized.” The “unusually high number of pediatric patients with HS” at Boston Medical Center provided “a unique opportunity to study this topic.”
Working with her mentor, Lisa Shen, MD, associate medical director of pediatric dermatology at Boston University, Ms. Sanchez and colleagues retrospectively reviewed the medical records of 303 patients aged 4-18 years who were diagnosed with HS at Boston Medical Center from 2012 to 2021. Boston Medical Center is the largest safety net hospital in New England. All data points and outcome measures were collected within 6 months of the patient’s HS diagnosis date.
Of the 303 patients with HS, 84% were female and 16% were male. Complete information about race was available in 286 patients. Of these, 65% were Black/African American, 11% were White, and the rest were from other racial groups. The mean age at symptom onset was 13 years, while the mean age at diagnosis was 15 years, and the mean delay to diagnosis was 2 years. A family history of HS was reported in 36% of patients.
The most common clinical features in these HS patients were pain/tenderness (90%), pustules/papules (65%), discharge/drainage (62%), and deep-seated nodules (51%). Scarring was present in 44% of patients at the time of diagnosis. The three most common sites of involvement were the axillary area (79%), the pubic area (36%), and the inguinal folds/inner thighs (34%).
Obesity was the most common comorbidity at the time of diagnosis, with 64% of patients affected. The next most common comorbidities were acne vulgaris (36%), acanthosis nigricans (25%), depression (18%), being overweight (17%), polycystic ovary syndrome (16%) and anxiety (13%). None had type 1 diabetes or metabolic syndrome.
Referring to the large population of underserved minority patients at Boston Medical Center, Dr. Shen noted, “we have to make sure not to underestimate the prevalence of obesity in this population as they get older. We need to start from a younger age to incorporate multidisciplinary care such as weight management, nutrition, and working with our pediatric surgery colleagues in trying to tackle [HS] because there is data to suggest that the earlier we intervene, the better outcomes they have. That makes sense.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the findings, said that the study “highlights the impressive and concerning gap and delays in diagnosis, not too dissimilar to what the literature shows in adult HS patients, which unfortunately has tremendous ramifications, both physically and emotionally/psychosocially.”
While this single-center study identified potential risk factors, such as obesity and self-identifying as Black, he said, “it is important to note that this condition does not discriminate and therefore it is important not to miss the cases that don’t follow the textbook nor stigmatize this condition as one that only impacts certain demographics.”
The researchers reported having no financial disclosures. Dr. Friedman, who was not involved with the study, reported that he serves as a consultant and/or advisor to numerous pharmaceutical companies. He is a speaker for companies including, Regeneron, Sanofi, AbbVie, Janssen, Incyte, and Brickell Biotech, and has received grants from Pfizer, the Dermatology Foundation, Almirall, Incyte, Galderma, and Janssen.
INDIANAPOLIS – in a study presented at the annual meeting of the Society for Pediatric Dermatology.
In addition, 44% presented with scarring, which suggests that HS may be underdiagnosed in this patient population. Those are the key findings from the study, a single-center retrospective chart review presented by Stephanie Sanchez during a poster session at the meeting.
“There is limited research on HS within the pediatric population,” said Ms. Sanchez, a fourth-year medical student at Boston University. “It’s not very well defined or characterized.” The “unusually high number of pediatric patients with HS” at Boston Medical Center provided “a unique opportunity to study this topic.”
Working with her mentor, Lisa Shen, MD, associate medical director of pediatric dermatology at Boston University, Ms. Sanchez and colleagues retrospectively reviewed the medical records of 303 patients aged 4-18 years who were diagnosed with HS at Boston Medical Center from 2012 to 2021. Boston Medical Center is the largest safety net hospital in New England. All data points and outcome measures were collected within 6 months of the patient’s HS diagnosis date.
Of the 303 patients with HS, 84% were female and 16% were male. Complete information about race was available in 286 patients. Of these, 65% were Black/African American, 11% were White, and the rest were from other racial groups. The mean age at symptom onset was 13 years, while the mean age at diagnosis was 15 years, and the mean delay to diagnosis was 2 years. A family history of HS was reported in 36% of patients.
The most common clinical features in these HS patients were pain/tenderness (90%), pustules/papules (65%), discharge/drainage (62%), and deep-seated nodules (51%). Scarring was present in 44% of patients at the time of diagnosis. The three most common sites of involvement were the axillary area (79%), the pubic area (36%), and the inguinal folds/inner thighs (34%).
Obesity was the most common comorbidity at the time of diagnosis, with 64% of patients affected. The next most common comorbidities were acne vulgaris (36%), acanthosis nigricans (25%), depression (18%), being overweight (17%), polycystic ovary syndrome (16%) and anxiety (13%). None had type 1 diabetes or metabolic syndrome.
Referring to the large population of underserved minority patients at Boston Medical Center, Dr. Shen noted, “we have to make sure not to underestimate the prevalence of obesity in this population as they get older. We need to start from a younger age to incorporate multidisciplinary care such as weight management, nutrition, and working with our pediatric surgery colleagues in trying to tackle [HS] because there is data to suggest that the earlier we intervene, the better outcomes they have. That makes sense.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the findings, said that the study “highlights the impressive and concerning gap and delays in diagnosis, not too dissimilar to what the literature shows in adult HS patients, which unfortunately has tremendous ramifications, both physically and emotionally/psychosocially.”
While this single-center study identified potential risk factors, such as obesity and self-identifying as Black, he said, “it is important to note that this condition does not discriminate and therefore it is important not to miss the cases that don’t follow the textbook nor stigmatize this condition as one that only impacts certain demographics.”
The researchers reported having no financial disclosures. Dr. Friedman, who was not involved with the study, reported that he serves as a consultant and/or advisor to numerous pharmaceutical companies. He is a speaker for companies including, Regeneron, Sanofi, AbbVie, Janssen, Incyte, and Brickell Biotech, and has received grants from Pfizer, the Dermatology Foundation, Almirall, Incyte, Galderma, and Janssen.
AT SPD 2022
Shift schedule today could worsen that stroke tomorrow
Body clocks and the shifting risks of stroke
Health care professionals, we’re sure, are no strangers to rotating shifts. And, as practitioners of the shiftly arts, you should know new research shows that working those kinds of hours can have lasting effects on your health. And it’s all based on your sleep-wake cycle.
In a study published in Neurobiology of Sleep and Circadian Rhythms, investigators at Texas A&M University looked at the effects of working these kinds of shifts for a long period of time and then returning to a regular 24-hour cycle later in life. The study piggybacks on a previous study, which showed that rats on shift schedules had more severe stroke outcomes than those who were on a 24-hour cycle.
The current study demonstrates that working rotating shifts does have a lasting effect, by way of messing with the sleep-wake cycle. Based on the research, the rats that performed those kinds of shifts never got back to a normal schedule. When strokes occurred, outcomes were much worse, and the females had a higher mortality rate and more severe functional deficits than the males.
Now for the “good” news: Even if you’re among those who haven’t worked a rotating shift, you may not be safe either.
People who have regular working hours have a tendency to take work home and stay up late, especially with so many moving to a remote-work model. And if you’re staying up late on the weekends you’re producing what lead author David J. Earnest, PhD, called “social jet lag,” which messes with your circadian rhythm to wind you down for sleep. All of these things can lead to the same kind of effects that working rotating shifts has on your health, he said in a written statement.
How do you combat this? Dr. Earnest recommended creating a sleep schedule and setting regular mealtimes. Also ease up on high-fat foods, drinking, and smoking. The connection between your brain and gut also could play a part in how severe a stroke can be.
So continue to work hard, but not too hard.
Got 3 minutes? You got time for culture
Much like a Krabby Patty, art is good for your soul. Seriously, staring at a 500-year-old painting may not seem like much, but research has proven time and again that going to a museum and looking at paintings by long-dead artists you probably know better as pizza-eating superhero turtles improves mood, stress, and well-being.
A couple of years ago, however, museums and art galleries ran into a big virus-shaped problem. You may have heard of it. All of a sudden it became a very bad idea for people to gather together in one building and huddle around the Mona Lisa, which, by the way, is a lot smaller in person than you might expect. But, rather than sit around with a bunch of priceless art for an indeterminate amount of time, museums brought their exhibits to the Internet so that people from all over the world could see great works from their couches.
This is absolutely a good thing for public access, but do these virtual art exhibits provide the same health benefits as going to a museum in person? That’s what a group of European researchers aimed to find out, and in a study published in Frontiers of Psychology, that’s exactly what they found.
Their directive to the 84 study participants was simple: Take a well-being survey, engage with either of a pair of online exhibits (a Monet painting and a display of Japanese culinary traditions) for just 3 minutes, then take another well-being assessment. The results were quite clear: Even just a couple of minutes of viewing art online improved all the well-being categories on the survey, such as lowering anxiety, negative mood, and loneliness, as well as increasing subjective well-being. Also, the more beautiful or meaningful a person found the art, the more their mood and well-being improved.
The researchers noted that these results could help access in places where access to art is limited, such as waiting rooms, hospitals, and rural areas. Let’s just hope it sticks to that, and that big businesses don’t take notice. Just imagine them plastering ads with classic Renaissance artworks. After all, art makes you feel good, and you know what else feels good on a hot summer day? An ice-cold Coca-Cola! By the way, we’re taking offers, advertising agencies. The LOTME staff can absolutely be bought.
Appetite for etymology
Today on “It’s a Thing,” we examine various states of hunger and what they should be called. Our first guest is that historically hungry royal person, King Henry VIII of England. Your majesty, have you ever been “hangry?”
KH8: First, let me thank you for inviting me on the show, Maurice. I’m a huge fan. A recent study done in the United Kingdom and Austria showed that “hunger is associated with greater levels of anger and irritability, as well as lower levels of pleasure,” according to a Eurekalert statement. So, yes, I have been “hangry.”
Maurice: Now to our next guest. Martha Stewart, can you add anything about that study?
Martha: Happy to, Maurice. The 64 participants used a smartphone app to record their hunger levels and emotional states five times a day for 21 days. It’s the first time that “hanger” was studied outside a lab, and it showed that hunger “was associated with 37% of the variance in irritability, 34% of the variance in anger, and 38% of the variance in pleasure recorded by the participants,” the investigators said in that statement.
Maurice: It’s official, then. Hangry is a thing, and we don’t need to put it in quotes anymore. Now let’s meet our third and final guest, Betty Crocker. Betty, I’m told you have a study to plug.
Betty: That’s right, Mo. Researchers at Tel Aviv University looked at survey data from almost 3,000 men and women and found that men ate 17% more food during the warmer months (March to September) than they did the rest of the year. Among women, however, caloric intake did not change.
KH8: I saw that study. Didn’t they put 27 people out in the sun and then take blood samples?
Betty: Indeed they did, Hank. After 25 minutes of sun exposure, the 13 men felt hungrier than before, but the 14 women did not. The men also had higher levels of ghrelin, an appetite-stimulating hormone, than the women.
Maurice: To sum all this up, then, we’ve got angry and hungry officially combining to make hangry, and now it looks like the sun is causing hunger in men, which makes them … sungry?
Martha: It’s a thing.
Chicken cutlets with a side of COVID
You stopped at the drive through at McDonald’s on the way home from work, and while you’re looking for something sweet in the refrigerator for dessert, you see that chicken breast that expires today.
Freezing meat that’s about to expire might be your go-to so it doesn’t go to waste, but it’s been found that SARS-CoV-2 can live in meat that’s been in the refrigerator or freezer for more than a month.
Researchers exposed chicken, beef, pork, and salmon to surrogate viruses that are similar to COVID but not as harmful and stored them in freezers at –4° F and in the refrigerator at 39.2° F. “We even found that the viruses could be cultured after [being frozen for] that length of time,” lead author Emily Bailey, PhD, of Campbell University in Buies Creek, N.C., said in Study Finds.
The team began its research after hearing of COVID-19 outbreaks where there were no reports of community transmission, such as in Southeast Asia. Tracing eventually led to packaged meats as the culprits in those cases. SARS-CoV-2 is able to replicate in the gut, as well as the respiratory tract, so it could affect the gut before respiratory symptoms start. It is crucial to ensure cross contamination doesn’t occur, and inadequate sanitation prior to packaging needs to be addressed, the investigators said.
Honestly, we didn’t think anything could survive in a freezer for that long, but SARS-CoV-2 is a fighter.
Body clocks and the shifting risks of stroke
Health care professionals, we’re sure, are no strangers to rotating shifts. And, as practitioners of the shiftly arts, you should know new research shows that working those kinds of hours can have lasting effects on your health. And it’s all based on your sleep-wake cycle.
In a study published in Neurobiology of Sleep and Circadian Rhythms, investigators at Texas A&M University looked at the effects of working these kinds of shifts for a long period of time and then returning to a regular 24-hour cycle later in life. The study piggybacks on a previous study, which showed that rats on shift schedules had more severe stroke outcomes than those who were on a 24-hour cycle.
The current study demonstrates that working rotating shifts does have a lasting effect, by way of messing with the sleep-wake cycle. Based on the research, the rats that performed those kinds of shifts never got back to a normal schedule. When strokes occurred, outcomes were much worse, and the females had a higher mortality rate and more severe functional deficits than the males.
Now for the “good” news: Even if you’re among those who haven’t worked a rotating shift, you may not be safe either.
People who have regular working hours have a tendency to take work home and stay up late, especially with so many moving to a remote-work model. And if you’re staying up late on the weekends you’re producing what lead author David J. Earnest, PhD, called “social jet lag,” which messes with your circadian rhythm to wind you down for sleep. All of these things can lead to the same kind of effects that working rotating shifts has on your health, he said in a written statement.
How do you combat this? Dr. Earnest recommended creating a sleep schedule and setting regular mealtimes. Also ease up on high-fat foods, drinking, and smoking. The connection between your brain and gut also could play a part in how severe a stroke can be.
So continue to work hard, but not too hard.
Got 3 minutes? You got time for culture
Much like a Krabby Patty, art is good for your soul. Seriously, staring at a 500-year-old painting may not seem like much, but research has proven time and again that going to a museum and looking at paintings by long-dead artists you probably know better as pizza-eating superhero turtles improves mood, stress, and well-being.
A couple of years ago, however, museums and art galleries ran into a big virus-shaped problem. You may have heard of it. All of a sudden it became a very bad idea for people to gather together in one building and huddle around the Mona Lisa, which, by the way, is a lot smaller in person than you might expect. But, rather than sit around with a bunch of priceless art for an indeterminate amount of time, museums brought their exhibits to the Internet so that people from all over the world could see great works from their couches.
This is absolutely a good thing for public access, but do these virtual art exhibits provide the same health benefits as going to a museum in person? That’s what a group of European researchers aimed to find out, and in a study published in Frontiers of Psychology, that’s exactly what they found.
Their directive to the 84 study participants was simple: Take a well-being survey, engage with either of a pair of online exhibits (a Monet painting and a display of Japanese culinary traditions) for just 3 minutes, then take another well-being assessment. The results were quite clear: Even just a couple of minutes of viewing art online improved all the well-being categories on the survey, such as lowering anxiety, negative mood, and loneliness, as well as increasing subjective well-being. Also, the more beautiful or meaningful a person found the art, the more their mood and well-being improved.
The researchers noted that these results could help access in places where access to art is limited, such as waiting rooms, hospitals, and rural areas. Let’s just hope it sticks to that, and that big businesses don’t take notice. Just imagine them plastering ads with classic Renaissance artworks. After all, art makes you feel good, and you know what else feels good on a hot summer day? An ice-cold Coca-Cola! By the way, we’re taking offers, advertising agencies. The LOTME staff can absolutely be bought.
Appetite for etymology
Today on “It’s a Thing,” we examine various states of hunger and what they should be called. Our first guest is that historically hungry royal person, King Henry VIII of England. Your majesty, have you ever been “hangry?”
KH8: First, let me thank you for inviting me on the show, Maurice. I’m a huge fan. A recent study done in the United Kingdom and Austria showed that “hunger is associated with greater levels of anger and irritability, as well as lower levels of pleasure,” according to a Eurekalert statement. So, yes, I have been “hangry.”
Maurice: Now to our next guest. Martha Stewart, can you add anything about that study?
Martha: Happy to, Maurice. The 64 participants used a smartphone app to record their hunger levels and emotional states five times a day for 21 days. It’s the first time that “hanger” was studied outside a lab, and it showed that hunger “was associated with 37% of the variance in irritability, 34% of the variance in anger, and 38% of the variance in pleasure recorded by the participants,” the investigators said in that statement.
Maurice: It’s official, then. Hangry is a thing, and we don’t need to put it in quotes anymore. Now let’s meet our third and final guest, Betty Crocker. Betty, I’m told you have a study to plug.
Betty: That’s right, Mo. Researchers at Tel Aviv University looked at survey data from almost 3,000 men and women and found that men ate 17% more food during the warmer months (March to September) than they did the rest of the year. Among women, however, caloric intake did not change.
KH8: I saw that study. Didn’t they put 27 people out in the sun and then take blood samples?
Betty: Indeed they did, Hank. After 25 minutes of sun exposure, the 13 men felt hungrier than before, but the 14 women did not. The men also had higher levels of ghrelin, an appetite-stimulating hormone, than the women.
Maurice: To sum all this up, then, we’ve got angry and hungry officially combining to make hangry, and now it looks like the sun is causing hunger in men, which makes them … sungry?
Martha: It’s a thing.
Chicken cutlets with a side of COVID
You stopped at the drive through at McDonald’s on the way home from work, and while you’re looking for something sweet in the refrigerator for dessert, you see that chicken breast that expires today.
Freezing meat that’s about to expire might be your go-to so it doesn’t go to waste, but it’s been found that SARS-CoV-2 can live in meat that’s been in the refrigerator or freezer for more than a month.
Researchers exposed chicken, beef, pork, and salmon to surrogate viruses that are similar to COVID but not as harmful and stored them in freezers at –4° F and in the refrigerator at 39.2° F. “We even found that the viruses could be cultured after [being frozen for] that length of time,” lead author Emily Bailey, PhD, of Campbell University in Buies Creek, N.C., said in Study Finds.
The team began its research after hearing of COVID-19 outbreaks where there were no reports of community transmission, such as in Southeast Asia. Tracing eventually led to packaged meats as the culprits in those cases. SARS-CoV-2 is able to replicate in the gut, as well as the respiratory tract, so it could affect the gut before respiratory symptoms start. It is crucial to ensure cross contamination doesn’t occur, and inadequate sanitation prior to packaging needs to be addressed, the investigators said.
Honestly, we didn’t think anything could survive in a freezer for that long, but SARS-CoV-2 is a fighter.
Body clocks and the shifting risks of stroke
Health care professionals, we’re sure, are no strangers to rotating shifts. And, as practitioners of the shiftly arts, you should know new research shows that working those kinds of hours can have lasting effects on your health. And it’s all based on your sleep-wake cycle.
In a study published in Neurobiology of Sleep and Circadian Rhythms, investigators at Texas A&M University looked at the effects of working these kinds of shifts for a long period of time and then returning to a regular 24-hour cycle later in life. The study piggybacks on a previous study, which showed that rats on shift schedules had more severe stroke outcomes than those who were on a 24-hour cycle.
The current study demonstrates that working rotating shifts does have a lasting effect, by way of messing with the sleep-wake cycle. Based on the research, the rats that performed those kinds of shifts never got back to a normal schedule. When strokes occurred, outcomes were much worse, and the females had a higher mortality rate and more severe functional deficits than the males.
Now for the “good” news: Even if you’re among those who haven’t worked a rotating shift, you may not be safe either.
People who have regular working hours have a tendency to take work home and stay up late, especially with so many moving to a remote-work model. And if you’re staying up late on the weekends you’re producing what lead author David J. Earnest, PhD, called “social jet lag,” which messes with your circadian rhythm to wind you down for sleep. All of these things can lead to the same kind of effects that working rotating shifts has on your health, he said in a written statement.
How do you combat this? Dr. Earnest recommended creating a sleep schedule and setting regular mealtimes. Also ease up on high-fat foods, drinking, and smoking. The connection between your brain and gut also could play a part in how severe a stroke can be.
So continue to work hard, but not too hard.
Got 3 minutes? You got time for culture
Much like a Krabby Patty, art is good for your soul. Seriously, staring at a 500-year-old painting may not seem like much, but research has proven time and again that going to a museum and looking at paintings by long-dead artists you probably know better as pizza-eating superhero turtles improves mood, stress, and well-being.
A couple of years ago, however, museums and art galleries ran into a big virus-shaped problem. You may have heard of it. All of a sudden it became a very bad idea for people to gather together in one building and huddle around the Mona Lisa, which, by the way, is a lot smaller in person than you might expect. But, rather than sit around with a bunch of priceless art for an indeterminate amount of time, museums brought their exhibits to the Internet so that people from all over the world could see great works from their couches.
This is absolutely a good thing for public access, but do these virtual art exhibits provide the same health benefits as going to a museum in person? That’s what a group of European researchers aimed to find out, and in a study published in Frontiers of Psychology, that’s exactly what they found.
Their directive to the 84 study participants was simple: Take a well-being survey, engage with either of a pair of online exhibits (a Monet painting and a display of Japanese culinary traditions) for just 3 minutes, then take another well-being assessment. The results were quite clear: Even just a couple of minutes of viewing art online improved all the well-being categories on the survey, such as lowering anxiety, negative mood, and loneliness, as well as increasing subjective well-being. Also, the more beautiful or meaningful a person found the art, the more their mood and well-being improved.
The researchers noted that these results could help access in places where access to art is limited, such as waiting rooms, hospitals, and rural areas. Let’s just hope it sticks to that, and that big businesses don’t take notice. Just imagine them plastering ads with classic Renaissance artworks. After all, art makes you feel good, and you know what else feels good on a hot summer day? An ice-cold Coca-Cola! By the way, we’re taking offers, advertising agencies. The LOTME staff can absolutely be bought.
Appetite for etymology
Today on “It’s a Thing,” we examine various states of hunger and what they should be called. Our first guest is that historically hungry royal person, King Henry VIII of England. Your majesty, have you ever been “hangry?”
KH8: First, let me thank you for inviting me on the show, Maurice. I’m a huge fan. A recent study done in the United Kingdom and Austria showed that “hunger is associated with greater levels of anger and irritability, as well as lower levels of pleasure,” according to a Eurekalert statement. So, yes, I have been “hangry.”
Maurice: Now to our next guest. Martha Stewart, can you add anything about that study?
Martha: Happy to, Maurice. The 64 participants used a smartphone app to record their hunger levels and emotional states five times a day for 21 days. It’s the first time that “hanger” was studied outside a lab, and it showed that hunger “was associated with 37% of the variance in irritability, 34% of the variance in anger, and 38% of the variance in pleasure recorded by the participants,” the investigators said in that statement.
Maurice: It’s official, then. Hangry is a thing, and we don’t need to put it in quotes anymore. Now let’s meet our third and final guest, Betty Crocker. Betty, I’m told you have a study to plug.
Betty: That’s right, Mo. Researchers at Tel Aviv University looked at survey data from almost 3,000 men and women and found that men ate 17% more food during the warmer months (March to September) than they did the rest of the year. Among women, however, caloric intake did not change.
KH8: I saw that study. Didn’t they put 27 people out in the sun and then take blood samples?
Betty: Indeed they did, Hank. After 25 minutes of sun exposure, the 13 men felt hungrier than before, but the 14 women did not. The men also had higher levels of ghrelin, an appetite-stimulating hormone, than the women.
Maurice: To sum all this up, then, we’ve got angry and hungry officially combining to make hangry, and now it looks like the sun is causing hunger in men, which makes them … sungry?
Martha: It’s a thing.
Chicken cutlets with a side of COVID
You stopped at the drive through at McDonald’s on the way home from work, and while you’re looking for something sweet in the refrigerator for dessert, you see that chicken breast that expires today.
Freezing meat that’s about to expire might be your go-to so it doesn’t go to waste, but it’s been found that SARS-CoV-2 can live in meat that’s been in the refrigerator or freezer for more than a month.
Researchers exposed chicken, beef, pork, and salmon to surrogate viruses that are similar to COVID but not as harmful and stored them in freezers at –4° F and in the refrigerator at 39.2° F. “We even found that the viruses could be cultured after [being frozen for] that length of time,” lead author Emily Bailey, PhD, of Campbell University in Buies Creek, N.C., said in Study Finds.
The team began its research after hearing of COVID-19 outbreaks where there were no reports of community transmission, such as in Southeast Asia. Tracing eventually led to packaged meats as the culprits in those cases. SARS-CoV-2 is able to replicate in the gut, as well as the respiratory tract, so it could affect the gut before respiratory symptoms start. It is crucial to ensure cross contamination doesn’t occur, and inadequate sanitation prior to packaging needs to be addressed, the investigators said.
Honestly, we didn’t think anything could survive in a freezer for that long, but SARS-CoV-2 is a fighter.
In the quest for a cure for type 1 diabetes, two companies merge
The $320 million cash purchase “will accelerate our goal of transforming, if not curing, type 1 diabetes by expanding our capabilities and bringing additional tools, technologies, and assets to our current stem cell-based programs,” said Vertex Chief Executive Officer and President Reshma Kewalramani, MD, in a company statement.
Last month, Vertex reported on a phase 1/2 multicenter clinical trial for two patients with type 1 diabetes who experienced improved blood glucose control with half doses of the company’s investigational allogeneic stem cell-derived islets (VX-880).
The first person to receive the product remained completely insulin-independent at 9 months post-transplant. A third patient has received the full targeted dose, but the data for this participant have yet to be reported.
For Viacyte’s part, last week the company announced that a clinical hold placed by the U.S. Food and Drug Administration on the trial has been lifted, allowing it to move forward with a planned total enrollment of 17 patients.
“The FDA requested additional information on the program, which we provided expeditiously. We are pleased that the hold has been lifted and look forward to continuing the Phase 1/2 trial in the U.S.,” a Vertex spokesperson told this news organization.
And a company official for ViaCyte presented results for three patients who received pancreatic precursor (PEC-01) cells derived from the company’s proprietary pluripotent stem cell line at the annual meeting of the Endocrine Society held in June. The cells are housed in an open delivery device implanted into a patient’s forearm. All three participants experienced improved blood glucose levels.
That presentation followed ViaCyte’s announcement in February that the first patient with type 1 diabetes had been dosed in a Phase 1 clinical trial of its investigational allogeneic, gene-edited, stem cell-derived product, VCTX210, developed in collaboration with CRISPR Therapeutics’ gene-editing technology. The aim is to generate islet cells that will produce insulin while avoiding recognition by the immune system, thus rendering immunosuppressive drugs unnecessary.
According to Vertex’s announcement, “The acquisition of ViaCyte provides Vertex with complementary assets, capabilities, and technologies, including additional human stem cell lines, intellectual property around stem cell differentiation, and Good Manufacturing Practice ... facilities for cell-based therapies that could accelerate Vertex’s ongoing type 1 diabetes programs. The acquisition also provides access to novel hypoimmune stem cell assets via the ViaCyte collaboration with CRISPR Therapeutics.”
In response to the announcement, the type 1 diabetes advocacy organization JDRF, which has funded the work of both companies, said in a statement that the acquisition “represents a significant stride in cures research for the type 1 diabetes community.”
“The coming together of two leaders in the cell-derived therapies field will undoubtedly accelerate the development of VX-880 by combining their resources, technologies, intellectual property, and more,” it added.
A third company developing stem cell–derived islet cell therapies, Sernova, said in a statement provided to this news organization: “We are very confident that bringing important game-changing technologies together, as we are seeing across the industry, will result in several viable technologies for the millions of people with type 1 diabetes ... We are thrilled that there are several technologies under development using different approaches that have the potential to provide a ‘functional cure’ for this disease.”
Vertex anticipates the acquisition will close later in 2022.
A version of this article first appeared on Medscape.com.
The $320 million cash purchase “will accelerate our goal of transforming, if not curing, type 1 diabetes by expanding our capabilities and bringing additional tools, technologies, and assets to our current stem cell-based programs,” said Vertex Chief Executive Officer and President Reshma Kewalramani, MD, in a company statement.
Last month, Vertex reported on a phase 1/2 multicenter clinical trial for two patients with type 1 diabetes who experienced improved blood glucose control with half doses of the company’s investigational allogeneic stem cell-derived islets (VX-880).
The first person to receive the product remained completely insulin-independent at 9 months post-transplant. A third patient has received the full targeted dose, but the data for this participant have yet to be reported.
For Viacyte’s part, last week the company announced that a clinical hold placed by the U.S. Food and Drug Administration on the trial has been lifted, allowing it to move forward with a planned total enrollment of 17 patients.
“The FDA requested additional information on the program, which we provided expeditiously. We are pleased that the hold has been lifted and look forward to continuing the Phase 1/2 trial in the U.S.,” a Vertex spokesperson told this news organization.
And a company official for ViaCyte presented results for three patients who received pancreatic precursor (PEC-01) cells derived from the company’s proprietary pluripotent stem cell line at the annual meeting of the Endocrine Society held in June. The cells are housed in an open delivery device implanted into a patient’s forearm. All three participants experienced improved blood glucose levels.
That presentation followed ViaCyte’s announcement in February that the first patient with type 1 diabetes had been dosed in a Phase 1 clinical trial of its investigational allogeneic, gene-edited, stem cell-derived product, VCTX210, developed in collaboration with CRISPR Therapeutics’ gene-editing technology. The aim is to generate islet cells that will produce insulin while avoiding recognition by the immune system, thus rendering immunosuppressive drugs unnecessary.
According to Vertex’s announcement, “The acquisition of ViaCyte provides Vertex with complementary assets, capabilities, and technologies, including additional human stem cell lines, intellectual property around stem cell differentiation, and Good Manufacturing Practice ... facilities for cell-based therapies that could accelerate Vertex’s ongoing type 1 diabetes programs. The acquisition also provides access to novel hypoimmune stem cell assets via the ViaCyte collaboration with CRISPR Therapeutics.”
In response to the announcement, the type 1 diabetes advocacy organization JDRF, which has funded the work of both companies, said in a statement that the acquisition “represents a significant stride in cures research for the type 1 diabetes community.”
“The coming together of two leaders in the cell-derived therapies field will undoubtedly accelerate the development of VX-880 by combining their resources, technologies, intellectual property, and more,” it added.
A third company developing stem cell–derived islet cell therapies, Sernova, said in a statement provided to this news organization: “We are very confident that bringing important game-changing technologies together, as we are seeing across the industry, will result in several viable technologies for the millions of people with type 1 diabetes ... We are thrilled that there are several technologies under development using different approaches that have the potential to provide a ‘functional cure’ for this disease.”
Vertex anticipates the acquisition will close later in 2022.
A version of this article first appeared on Medscape.com.
The $320 million cash purchase “will accelerate our goal of transforming, if not curing, type 1 diabetes by expanding our capabilities and bringing additional tools, technologies, and assets to our current stem cell-based programs,” said Vertex Chief Executive Officer and President Reshma Kewalramani, MD, in a company statement.
Last month, Vertex reported on a phase 1/2 multicenter clinical trial for two patients with type 1 diabetes who experienced improved blood glucose control with half doses of the company’s investigational allogeneic stem cell-derived islets (VX-880).
The first person to receive the product remained completely insulin-independent at 9 months post-transplant. A third patient has received the full targeted dose, but the data for this participant have yet to be reported.
For Viacyte’s part, last week the company announced that a clinical hold placed by the U.S. Food and Drug Administration on the trial has been lifted, allowing it to move forward with a planned total enrollment of 17 patients.
“The FDA requested additional information on the program, which we provided expeditiously. We are pleased that the hold has been lifted and look forward to continuing the Phase 1/2 trial in the U.S.,” a Vertex spokesperson told this news organization.
And a company official for ViaCyte presented results for three patients who received pancreatic precursor (PEC-01) cells derived from the company’s proprietary pluripotent stem cell line at the annual meeting of the Endocrine Society held in June. The cells are housed in an open delivery device implanted into a patient’s forearm. All three participants experienced improved blood glucose levels.
That presentation followed ViaCyte’s announcement in February that the first patient with type 1 diabetes had been dosed in a Phase 1 clinical trial of its investigational allogeneic, gene-edited, stem cell-derived product, VCTX210, developed in collaboration with CRISPR Therapeutics’ gene-editing technology. The aim is to generate islet cells that will produce insulin while avoiding recognition by the immune system, thus rendering immunosuppressive drugs unnecessary.
According to Vertex’s announcement, “The acquisition of ViaCyte provides Vertex with complementary assets, capabilities, and technologies, including additional human stem cell lines, intellectual property around stem cell differentiation, and Good Manufacturing Practice ... facilities for cell-based therapies that could accelerate Vertex’s ongoing type 1 diabetes programs. The acquisition also provides access to novel hypoimmune stem cell assets via the ViaCyte collaboration with CRISPR Therapeutics.”
In response to the announcement, the type 1 diabetes advocacy organization JDRF, which has funded the work of both companies, said in a statement that the acquisition “represents a significant stride in cures research for the type 1 diabetes community.”
“The coming together of two leaders in the cell-derived therapies field will undoubtedly accelerate the development of VX-880 by combining their resources, technologies, intellectual property, and more,” it added.
A third company developing stem cell–derived islet cell therapies, Sernova, said in a statement provided to this news organization: “We are very confident that bringing important game-changing technologies together, as we are seeing across the industry, will result in several viable technologies for the millions of people with type 1 diabetes ... We are thrilled that there are several technologies under development using different approaches that have the potential to provide a ‘functional cure’ for this disease.”
Vertex anticipates the acquisition will close later in 2022.
A version of this article first appeared on Medscape.com.
Justice Department task force to fight abortion ban overreach
Department officials announced July 12 that the task force formalizes an existing work group and recent efforts to protect access to reproductive health care considering the Supreme Court’s decision to overturn Roe v. Wade.
The task force will monitor state and local legislation and consider legal action against states that ban abortion medication, out-of-state travel for an abortion, and other measures that try to prevent reproductive health services that are authorized by federal law.
“The Supreme Court’s Dobbs decision is a devastating blow to reproductive freedom in the United States,” Associate Attorney General Vanita Gupta, the task force chair, said in a statement.
“The Court abandoned 50 years of precedent and took away the constitutional right to abortion, preventing women all over the country from being able to make critical decisions about our bodies, our health, and our futures,” she said. “The Justice Department is committed to protecting access to reproductive services.”
The task force includes representatives from the Justice Department’s Civil Division, Civil Rights Division, U.S. attorneys’ offices, Office of the Solicitor General, Office for Access to Justice, Office of Legal Counsel, Office of Legal Policy, Office of Legislative Affairs, Office of the Associate Attorney General, Office of the Deputy Attorney General, and Office of the Attorney General.
The task force is charged with coordinating federal government responses, including proactive and defensive legal action, the department said. Task force members will work with agencies across the federal government to support their work on issues related to reproductive rights and access to reproductive health care.
The Justice Department will also continue to work with external groups, such as reproductive services providers, advocates, and state attorneys general offices. It will also work with the Office of Counsel to the President to hold a meeting with private pro bono attorneys, bar associations, and public interest groups to encourage lawyers to represent patients, providers, and others in reproductive health services cases.
“Recognizing that the best way to protect reproductive freedom is through congressional action, the task force will also coordinate providing technical assistance to Congress in connection with federal legislation to codify reproductive rights and ensure access to comprehensive reproductive services,” the department wrote. “It will also coordinate the provision of technical assistance concerning federal constitutional protections to states seeking to afford legal protection to out-of-state patients and providers who offer legal reproductive health care.”
The announcement comes as some activists and lawmakers have expressed frustration about the White House’s response to changes in abortion law in recent weeks, according to The Washington Post. They’ve called on the Biden administration to do more in the wake of the Supreme Court ruling.
On July 8, President Joe Biden signed an executive order to direct his administration to pursue a variety of measures aimed at protecting abortion access, reproductive health care services, and patient privacy.
On July 11, the Department of Health & Human Services issued guidance to remind hospitals of their duty to comply with the Emergency Medical Treatment and Labor Act (EMTALA), which stands “irrespective of any state laws or mandates that apply to specific procedures.” The law requires health care personnel to provide medical screening and stabilizing treatment to patients in emergency medical situations. In the case of pregnancy, emergencies may include ectopic pregnancy, complications of pregnancy loss, or severe hypertensive disorders. Doctors must terminate a pregnancy if it’s necessary to stabilize the patient.
“When a state law prohibits abortion and does not include an exception for the life and health of the pregnant person – or draws the exception more narrowly than EMTALA’s emergency medical condition definition – that state law is preempted,” the department wrote.
Since the Supreme Court’s ruling to overturn Roe, more than a dozen states have moved to ban or severely restrict abortions, according to a state tracker by The Washington Post. Some of the laws have been temporarily blocked by courts in Kentucky, Louisiana, and Utah.
At the same time, some Republican-led states have moved to ban other reproductive health care services, such as abortion medication and telehealth visits, the newspaper reported. The Food and Drug Administration approved mifepristone in 2000, saying the pill is safe and effective for use during the first 10 weeks of pregnancy.
The Justice Department task force said it will monitor legislation that seeks to ban mifepristone, as well as block people’s ability to inform each other about reproductive care available across the country.
“We’re seeing the intimidation already in states that are making people afraid to share information about legal abortion services in other states,” Nancy Northup, president and chief executive of the Center for Reproductive Rights, told the newspaper.
The center served as the legal counsel for the Jackson Women’s Health Organization in the case that overturned Roe. Ms. Northup said the group is already involved in more than three dozen lawsuits and has filed several more since the Supreme Court’s ruling.
“It is a really frightening time,” she said.
A version of this article first appeared on WebMD.com.
Department officials announced July 12 that the task force formalizes an existing work group and recent efforts to protect access to reproductive health care considering the Supreme Court’s decision to overturn Roe v. Wade.
The task force will monitor state and local legislation and consider legal action against states that ban abortion medication, out-of-state travel for an abortion, and other measures that try to prevent reproductive health services that are authorized by federal law.
“The Supreme Court’s Dobbs decision is a devastating blow to reproductive freedom in the United States,” Associate Attorney General Vanita Gupta, the task force chair, said in a statement.
“The Court abandoned 50 years of precedent and took away the constitutional right to abortion, preventing women all over the country from being able to make critical decisions about our bodies, our health, and our futures,” she said. “The Justice Department is committed to protecting access to reproductive services.”
The task force includes representatives from the Justice Department’s Civil Division, Civil Rights Division, U.S. attorneys’ offices, Office of the Solicitor General, Office for Access to Justice, Office of Legal Counsel, Office of Legal Policy, Office of Legislative Affairs, Office of the Associate Attorney General, Office of the Deputy Attorney General, and Office of the Attorney General.
The task force is charged with coordinating federal government responses, including proactive and defensive legal action, the department said. Task force members will work with agencies across the federal government to support their work on issues related to reproductive rights and access to reproductive health care.
The Justice Department will also continue to work with external groups, such as reproductive services providers, advocates, and state attorneys general offices. It will also work with the Office of Counsel to the President to hold a meeting with private pro bono attorneys, bar associations, and public interest groups to encourage lawyers to represent patients, providers, and others in reproductive health services cases.
“Recognizing that the best way to protect reproductive freedom is through congressional action, the task force will also coordinate providing technical assistance to Congress in connection with federal legislation to codify reproductive rights and ensure access to comprehensive reproductive services,” the department wrote. “It will also coordinate the provision of technical assistance concerning federal constitutional protections to states seeking to afford legal protection to out-of-state patients and providers who offer legal reproductive health care.”
The announcement comes as some activists and lawmakers have expressed frustration about the White House’s response to changes in abortion law in recent weeks, according to The Washington Post. They’ve called on the Biden administration to do more in the wake of the Supreme Court ruling.
On July 8, President Joe Biden signed an executive order to direct his administration to pursue a variety of measures aimed at protecting abortion access, reproductive health care services, and patient privacy.
On July 11, the Department of Health & Human Services issued guidance to remind hospitals of their duty to comply with the Emergency Medical Treatment and Labor Act (EMTALA), which stands “irrespective of any state laws or mandates that apply to specific procedures.” The law requires health care personnel to provide medical screening and stabilizing treatment to patients in emergency medical situations. In the case of pregnancy, emergencies may include ectopic pregnancy, complications of pregnancy loss, or severe hypertensive disorders. Doctors must terminate a pregnancy if it’s necessary to stabilize the patient.
“When a state law prohibits abortion and does not include an exception for the life and health of the pregnant person – or draws the exception more narrowly than EMTALA’s emergency medical condition definition – that state law is preempted,” the department wrote.
Since the Supreme Court’s ruling to overturn Roe, more than a dozen states have moved to ban or severely restrict abortions, according to a state tracker by The Washington Post. Some of the laws have been temporarily blocked by courts in Kentucky, Louisiana, and Utah.
At the same time, some Republican-led states have moved to ban other reproductive health care services, such as abortion medication and telehealth visits, the newspaper reported. The Food and Drug Administration approved mifepristone in 2000, saying the pill is safe and effective for use during the first 10 weeks of pregnancy.
The Justice Department task force said it will monitor legislation that seeks to ban mifepristone, as well as block people’s ability to inform each other about reproductive care available across the country.
“We’re seeing the intimidation already in states that are making people afraid to share information about legal abortion services in other states,” Nancy Northup, president and chief executive of the Center for Reproductive Rights, told the newspaper.
The center served as the legal counsel for the Jackson Women’s Health Organization in the case that overturned Roe. Ms. Northup said the group is already involved in more than three dozen lawsuits and has filed several more since the Supreme Court’s ruling.
“It is a really frightening time,” she said.
A version of this article first appeared on WebMD.com.
Department officials announced July 12 that the task force formalizes an existing work group and recent efforts to protect access to reproductive health care considering the Supreme Court’s decision to overturn Roe v. Wade.
The task force will monitor state and local legislation and consider legal action against states that ban abortion medication, out-of-state travel for an abortion, and other measures that try to prevent reproductive health services that are authorized by federal law.
“The Supreme Court’s Dobbs decision is a devastating blow to reproductive freedom in the United States,” Associate Attorney General Vanita Gupta, the task force chair, said in a statement.
“The Court abandoned 50 years of precedent and took away the constitutional right to abortion, preventing women all over the country from being able to make critical decisions about our bodies, our health, and our futures,” she said. “The Justice Department is committed to protecting access to reproductive services.”
The task force includes representatives from the Justice Department’s Civil Division, Civil Rights Division, U.S. attorneys’ offices, Office of the Solicitor General, Office for Access to Justice, Office of Legal Counsel, Office of Legal Policy, Office of Legislative Affairs, Office of the Associate Attorney General, Office of the Deputy Attorney General, and Office of the Attorney General.
The task force is charged with coordinating federal government responses, including proactive and defensive legal action, the department said. Task force members will work with agencies across the federal government to support their work on issues related to reproductive rights and access to reproductive health care.
The Justice Department will also continue to work with external groups, such as reproductive services providers, advocates, and state attorneys general offices. It will also work with the Office of Counsel to the President to hold a meeting with private pro bono attorneys, bar associations, and public interest groups to encourage lawyers to represent patients, providers, and others in reproductive health services cases.
“Recognizing that the best way to protect reproductive freedom is through congressional action, the task force will also coordinate providing technical assistance to Congress in connection with federal legislation to codify reproductive rights and ensure access to comprehensive reproductive services,” the department wrote. “It will also coordinate the provision of technical assistance concerning federal constitutional protections to states seeking to afford legal protection to out-of-state patients and providers who offer legal reproductive health care.”
The announcement comes as some activists and lawmakers have expressed frustration about the White House’s response to changes in abortion law in recent weeks, according to The Washington Post. They’ve called on the Biden administration to do more in the wake of the Supreme Court ruling.
On July 8, President Joe Biden signed an executive order to direct his administration to pursue a variety of measures aimed at protecting abortion access, reproductive health care services, and patient privacy.
On July 11, the Department of Health & Human Services issued guidance to remind hospitals of their duty to comply with the Emergency Medical Treatment and Labor Act (EMTALA), which stands “irrespective of any state laws or mandates that apply to specific procedures.” The law requires health care personnel to provide medical screening and stabilizing treatment to patients in emergency medical situations. In the case of pregnancy, emergencies may include ectopic pregnancy, complications of pregnancy loss, or severe hypertensive disorders. Doctors must terminate a pregnancy if it’s necessary to stabilize the patient.
“When a state law prohibits abortion and does not include an exception for the life and health of the pregnant person – or draws the exception more narrowly than EMTALA’s emergency medical condition definition – that state law is preempted,” the department wrote.
Since the Supreme Court’s ruling to overturn Roe, more than a dozen states have moved to ban or severely restrict abortions, according to a state tracker by The Washington Post. Some of the laws have been temporarily blocked by courts in Kentucky, Louisiana, and Utah.
At the same time, some Republican-led states have moved to ban other reproductive health care services, such as abortion medication and telehealth visits, the newspaper reported. The Food and Drug Administration approved mifepristone in 2000, saying the pill is safe and effective for use during the first 10 weeks of pregnancy.
The Justice Department task force said it will monitor legislation that seeks to ban mifepristone, as well as block people’s ability to inform each other about reproductive care available across the country.
“We’re seeing the intimidation already in states that are making people afraid to share information about legal abortion services in other states,” Nancy Northup, president and chief executive of the Center for Reproductive Rights, told the newspaper.
The center served as the legal counsel for the Jackson Women’s Health Organization in the case that overturned Roe. Ms. Northup said the group is already involved in more than three dozen lawsuits and has filed several more since the Supreme Court’s ruling.
“It is a really frightening time,” she said.
A version of this article first appeared on WebMD.com.
What influences a trainee’s decision to choose pediatric dermatology as a career?
INDIANAPOLIS – Three during and after fellowship.
Those are key findings from a survey of current and prior pediatric dermatology fellows, which sought to investigate what factors influence their career decisions.
According to the study’s principal investigator, Lucia Z. Diaz, MD, pediatric dermatology suffers from workforce shortages and geographic maldistribution as a subspecialty in the United States. She also noted that, from 2016 to 2021, 100% of pediatric dermatology applicants matched, yet about 15 of every 31 positions remained unfilled during each of those years. This suggests that there may be a lack of trainee mentorship secondary to a lack of available pediatric dermatologists.
“Somewhere along the way, we lose trainees to general dermatology, or they may go through a pediatric dermatology fellowship but not actually see children upon completion of their training,” Dr. Diaz, chief of pediatric dermatology at the University of Texas at Austin, said in an interview at the annual meeting of the Society for Pediatric Dermatology, where the study was presented during a poster session. “We wanted to find out factors influencing this.”
For the study, Dr. Diaz, Courtney N. Haller, MD, a first-year dermatology resident at the University of Texas at Austin, and their colleagues emailed a 37-item survey to 59 current and prior pediatric dermatology fellows who trained in the United States in the past 4 years (classes of 2019-2022). Current fellows were asked to share their future plans, and past fellows were asked to share details about their current practice situation including practice type (such as academics, private practice, and a mix of adult and pediatrics), and the researchers used descriptive statistics and chi-square analyses to evaluate qualitative data.
In all, 41 survey participants gave complete responses, and 3 gave partial responses. Of these, 8 were current fellows, 36 were past fellows, and 38 were female. The researchers found that 67% of survey respondents first became interested in pediatric dermatology in medical school, while the decision to pursue a fellowship occurred then (33%) or during their third year of dermatology residency (33%). Early exposure to pediatric dermatology, from medical school through dermatology PGY-2, was significantly associated with an early decision to pursue a pediatric dermatology career (P = .004).
In addition, respondents at institutions with two or more pediatric dermatology faculty were significantly more likely to cite home institution mentorship as an influencing factor in their career decision (P = .035).
“I thought that the interest in pediatric dermatology would peak early on during dermatology residency, but it primarily happens during medical school,” said Dr. Diaz, who is also associate director of the dermatology residency program at the medical school. “Mentorship and early exposure to pediatric dermatology during medical school are really important.”
The top three factors that discouraged respondents from pursuing a pediatric dermatology fellowship included a lack of salary benefit with additional training (83%), additional time required to complete training (73%), and geographic relocation (20%). After fellowship, 51% of respondents said they plan to or currently work in academic settings, while 88% said they plan to work full time or currently were working full time.
Interestingly, fellows with additional pediatric training such as an internship or residency were not more likely to see a greater percentage of pediatric patients in practice than those without this training (P = .14). The top 3 reasons for not seeing pediatric patients 100% of the clinical time were interest in seeing adult patients (67%), financial factors (56%), and interest in performing more procedures (56%).
In other findings, the top three factors in deciding practice location were proximity to extended family (63%), practice type (59%), and income (51%).
Adelaide A. Hebert, MD, who was asked to comment on the study, said that the lack of salary benefit from additional training is a sticking point for many fellows. “The market trends of supply and demand do not work in pediatric dermatology,” said Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. “You would think that, because there are fewer of us, we should be paid more, but it does not work that way.”
She characterized the overall study findings as “a real testament to what the challenges are” in recruiting trainees to pediatric dermatology. “The influence of mentors resonates in this assessment, but influences that are somewhat beyond our control also play a role, such as lack of salary benefit from additional training, interest in seeing adult patients, and financial factors.”
Neither the researchers nor Dr. Hebert reported having relevant financial disclosures.
INDIANAPOLIS – Three during and after fellowship.
Those are key findings from a survey of current and prior pediatric dermatology fellows, which sought to investigate what factors influence their career decisions.
According to the study’s principal investigator, Lucia Z. Diaz, MD, pediatric dermatology suffers from workforce shortages and geographic maldistribution as a subspecialty in the United States. She also noted that, from 2016 to 2021, 100% of pediatric dermatology applicants matched, yet about 15 of every 31 positions remained unfilled during each of those years. This suggests that there may be a lack of trainee mentorship secondary to a lack of available pediatric dermatologists.
“Somewhere along the way, we lose trainees to general dermatology, or they may go through a pediatric dermatology fellowship but not actually see children upon completion of their training,” Dr. Diaz, chief of pediatric dermatology at the University of Texas at Austin, said in an interview at the annual meeting of the Society for Pediatric Dermatology, where the study was presented during a poster session. “We wanted to find out factors influencing this.”
For the study, Dr. Diaz, Courtney N. Haller, MD, a first-year dermatology resident at the University of Texas at Austin, and their colleagues emailed a 37-item survey to 59 current and prior pediatric dermatology fellows who trained in the United States in the past 4 years (classes of 2019-2022). Current fellows were asked to share their future plans, and past fellows were asked to share details about their current practice situation including practice type (such as academics, private practice, and a mix of adult and pediatrics), and the researchers used descriptive statistics and chi-square analyses to evaluate qualitative data.
In all, 41 survey participants gave complete responses, and 3 gave partial responses. Of these, 8 were current fellows, 36 were past fellows, and 38 were female. The researchers found that 67% of survey respondents first became interested in pediatric dermatology in medical school, while the decision to pursue a fellowship occurred then (33%) or during their third year of dermatology residency (33%). Early exposure to pediatric dermatology, from medical school through dermatology PGY-2, was significantly associated with an early decision to pursue a pediatric dermatology career (P = .004).
In addition, respondents at institutions with two or more pediatric dermatology faculty were significantly more likely to cite home institution mentorship as an influencing factor in their career decision (P = .035).
“I thought that the interest in pediatric dermatology would peak early on during dermatology residency, but it primarily happens during medical school,” said Dr. Diaz, who is also associate director of the dermatology residency program at the medical school. “Mentorship and early exposure to pediatric dermatology during medical school are really important.”
The top three factors that discouraged respondents from pursuing a pediatric dermatology fellowship included a lack of salary benefit with additional training (83%), additional time required to complete training (73%), and geographic relocation (20%). After fellowship, 51% of respondents said they plan to or currently work in academic settings, while 88% said they plan to work full time or currently were working full time.
Interestingly, fellows with additional pediatric training such as an internship or residency were not more likely to see a greater percentage of pediatric patients in practice than those without this training (P = .14). The top 3 reasons for not seeing pediatric patients 100% of the clinical time were interest in seeing adult patients (67%), financial factors (56%), and interest in performing more procedures (56%).
In other findings, the top three factors in deciding practice location were proximity to extended family (63%), practice type (59%), and income (51%).
Adelaide A. Hebert, MD, who was asked to comment on the study, said that the lack of salary benefit from additional training is a sticking point for many fellows. “The market trends of supply and demand do not work in pediatric dermatology,” said Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. “You would think that, because there are fewer of us, we should be paid more, but it does not work that way.”
She characterized the overall study findings as “a real testament to what the challenges are” in recruiting trainees to pediatric dermatology. “The influence of mentors resonates in this assessment, but influences that are somewhat beyond our control also play a role, such as lack of salary benefit from additional training, interest in seeing adult patients, and financial factors.”
Neither the researchers nor Dr. Hebert reported having relevant financial disclosures.
INDIANAPOLIS – Three during and after fellowship.
Those are key findings from a survey of current and prior pediatric dermatology fellows, which sought to investigate what factors influence their career decisions.
According to the study’s principal investigator, Lucia Z. Diaz, MD, pediatric dermatology suffers from workforce shortages and geographic maldistribution as a subspecialty in the United States. She also noted that, from 2016 to 2021, 100% of pediatric dermatology applicants matched, yet about 15 of every 31 positions remained unfilled during each of those years. This suggests that there may be a lack of trainee mentorship secondary to a lack of available pediatric dermatologists.
“Somewhere along the way, we lose trainees to general dermatology, or they may go through a pediatric dermatology fellowship but not actually see children upon completion of their training,” Dr. Diaz, chief of pediatric dermatology at the University of Texas at Austin, said in an interview at the annual meeting of the Society for Pediatric Dermatology, where the study was presented during a poster session. “We wanted to find out factors influencing this.”
For the study, Dr. Diaz, Courtney N. Haller, MD, a first-year dermatology resident at the University of Texas at Austin, and their colleagues emailed a 37-item survey to 59 current and prior pediatric dermatology fellows who trained in the United States in the past 4 years (classes of 2019-2022). Current fellows were asked to share their future plans, and past fellows were asked to share details about their current practice situation including practice type (such as academics, private practice, and a mix of adult and pediatrics), and the researchers used descriptive statistics and chi-square analyses to evaluate qualitative data.
In all, 41 survey participants gave complete responses, and 3 gave partial responses. Of these, 8 were current fellows, 36 were past fellows, and 38 were female. The researchers found that 67% of survey respondents first became interested in pediatric dermatology in medical school, while the decision to pursue a fellowship occurred then (33%) or during their third year of dermatology residency (33%). Early exposure to pediatric dermatology, from medical school through dermatology PGY-2, was significantly associated with an early decision to pursue a pediatric dermatology career (P = .004).
In addition, respondents at institutions with two or more pediatric dermatology faculty were significantly more likely to cite home institution mentorship as an influencing factor in their career decision (P = .035).
“I thought that the interest in pediatric dermatology would peak early on during dermatology residency, but it primarily happens during medical school,” said Dr. Diaz, who is also associate director of the dermatology residency program at the medical school. “Mentorship and early exposure to pediatric dermatology during medical school are really important.”
The top three factors that discouraged respondents from pursuing a pediatric dermatology fellowship included a lack of salary benefit with additional training (83%), additional time required to complete training (73%), and geographic relocation (20%). After fellowship, 51% of respondents said they plan to or currently work in academic settings, while 88% said they plan to work full time or currently were working full time.
Interestingly, fellows with additional pediatric training such as an internship or residency were not more likely to see a greater percentage of pediatric patients in practice than those without this training (P = .14). The top 3 reasons for not seeing pediatric patients 100% of the clinical time were interest in seeing adult patients (67%), financial factors (56%), and interest in performing more procedures (56%).
In other findings, the top three factors in deciding practice location were proximity to extended family (63%), practice type (59%), and income (51%).
Adelaide A. Hebert, MD, who was asked to comment on the study, said that the lack of salary benefit from additional training is a sticking point for many fellows. “The market trends of supply and demand do not work in pediatric dermatology,” said Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. “You would think that, because there are fewer of us, we should be paid more, but it does not work that way.”
She characterized the overall study findings as “a real testament to what the challenges are” in recruiting trainees to pediatric dermatology. “The influence of mentors resonates in this assessment, but influences that are somewhat beyond our control also play a role, such as lack of salary benefit from additional training, interest in seeing adult patients, and financial factors.”
Neither the researchers nor Dr. Hebert reported having relevant financial disclosures.
AT SPD 2022
Uveitis in juvenile arthritis patients persists into midlife
Active uveitis remained in 43.4% of juvenile idiopathic arthritis (JIA) patients up to 40 years after a diagnosis, based on data from 30 individuals.
Uveitis occurs in approximately 10%-20% of patients with JIA, but data on the long-term activity and prevalence are limited, although previous studies suggest that uveitis can persist into adulthood, wrote Dr. Angelika Skarin of Skåne University in Lund, Sweden, and colleagues.
In a study published in Pediatric Rheumatology, the researchers reviewed ophthalmic records from 30 JIA patients at a mean of 40.7 years after uveitis onset. They compared these records to data collected from the same patient population at a mean of 7.2 and 24.0 years after onset. In the previous follow-up studies, 49% of the patients had active uveitis at 24 years, and the prevalence of cataracts and glaucoma increased between the 7-year and 24-year assessments.
In the current study, 43.4% of the population had active uveitis at the 40-year follow-up, which corresponded to 23.6% of the original study cohort. The mean age of the participants overall was 46.9 years, the mean duration of joint disease was 42.99 years, and the mean time from onset of uveitis was 40.7 years.
In addition, 66.6% of the patients in the current study had cataracts or had undergone cataract surgery in one or both eyes, and 40.0% had glaucoma.
By the time of the current study, of the original cohort of 55 individuals, 11 were deceased; rheumatic disease was declared the main cause in four patients and a contributing factor in three others.
Potential drivers of the earliest cases of glaucoma and ocular hypertension (G/OH) include increased intraocular pressure as a result of topical corticosteroid treatment, the researchers noted in their discussion. However, G/OH occurring later than the 7-year follow-up was “more likely to be the type observed in many patients with long-standing chronic uveitis, where a gradual increase in intraocular pressure is assumed to be caused by impaired aqueous outflow,” they said.
Only 4 of the 30 patients did not have regular ophthalmology visits, which suggests a study population with ocular symptoms or concerns about their eyesight, the researchers wrote. “The fact that 13% of our original cohort were reported to have severe visual impairment or worse in both eyes at any of the three follow-ups is noteworthy,” compared to reports of visual impairment of less than 0.5% in a German study in the general population for similar ages.
The findings were limited by several factors, including the retrospective design, small study population, and lack of data on 25 of the original 55-member study cohort, which may reduce the reliability of the current study, the researchers noted. However, the results reflect data from previous studies and support the need for JIA patients to continue regular ophthalmic checkups throughout life, they concluded.
The study was supported by Stiftelsen för Synskadade i f.d. Malmöhus län, Sweden, Skånes Universitetssjukhus Stiftelser och Donationer, Ögonfonden, and the Swedish Society of Medicine. The researchers had no financial conflicts to disclose.
Active uveitis remained in 43.4% of juvenile idiopathic arthritis (JIA) patients up to 40 years after a diagnosis, based on data from 30 individuals.
Uveitis occurs in approximately 10%-20% of patients with JIA, but data on the long-term activity and prevalence are limited, although previous studies suggest that uveitis can persist into adulthood, wrote Dr. Angelika Skarin of Skåne University in Lund, Sweden, and colleagues.
In a study published in Pediatric Rheumatology, the researchers reviewed ophthalmic records from 30 JIA patients at a mean of 40.7 years after uveitis onset. They compared these records to data collected from the same patient population at a mean of 7.2 and 24.0 years after onset. In the previous follow-up studies, 49% of the patients had active uveitis at 24 years, and the prevalence of cataracts and glaucoma increased between the 7-year and 24-year assessments.
In the current study, 43.4% of the population had active uveitis at the 40-year follow-up, which corresponded to 23.6% of the original study cohort. The mean age of the participants overall was 46.9 years, the mean duration of joint disease was 42.99 years, and the mean time from onset of uveitis was 40.7 years.
In addition, 66.6% of the patients in the current study had cataracts or had undergone cataract surgery in one or both eyes, and 40.0% had glaucoma.
By the time of the current study, of the original cohort of 55 individuals, 11 were deceased; rheumatic disease was declared the main cause in four patients and a contributing factor in three others.
Potential drivers of the earliest cases of glaucoma and ocular hypertension (G/OH) include increased intraocular pressure as a result of topical corticosteroid treatment, the researchers noted in their discussion. However, G/OH occurring later than the 7-year follow-up was “more likely to be the type observed in many patients with long-standing chronic uveitis, where a gradual increase in intraocular pressure is assumed to be caused by impaired aqueous outflow,” they said.
Only 4 of the 30 patients did not have regular ophthalmology visits, which suggests a study population with ocular symptoms or concerns about their eyesight, the researchers wrote. “The fact that 13% of our original cohort were reported to have severe visual impairment or worse in both eyes at any of the three follow-ups is noteworthy,” compared to reports of visual impairment of less than 0.5% in a German study in the general population for similar ages.
The findings were limited by several factors, including the retrospective design, small study population, and lack of data on 25 of the original 55-member study cohort, which may reduce the reliability of the current study, the researchers noted. However, the results reflect data from previous studies and support the need for JIA patients to continue regular ophthalmic checkups throughout life, they concluded.
The study was supported by Stiftelsen för Synskadade i f.d. Malmöhus län, Sweden, Skånes Universitetssjukhus Stiftelser och Donationer, Ögonfonden, and the Swedish Society of Medicine. The researchers had no financial conflicts to disclose.
Active uveitis remained in 43.4% of juvenile idiopathic arthritis (JIA) patients up to 40 years after a diagnosis, based on data from 30 individuals.
Uveitis occurs in approximately 10%-20% of patients with JIA, but data on the long-term activity and prevalence are limited, although previous studies suggest that uveitis can persist into adulthood, wrote Dr. Angelika Skarin of Skåne University in Lund, Sweden, and colleagues.
In a study published in Pediatric Rheumatology, the researchers reviewed ophthalmic records from 30 JIA patients at a mean of 40.7 years after uveitis onset. They compared these records to data collected from the same patient population at a mean of 7.2 and 24.0 years after onset. In the previous follow-up studies, 49% of the patients had active uveitis at 24 years, and the prevalence of cataracts and glaucoma increased between the 7-year and 24-year assessments.
In the current study, 43.4% of the population had active uveitis at the 40-year follow-up, which corresponded to 23.6% of the original study cohort. The mean age of the participants overall was 46.9 years, the mean duration of joint disease was 42.99 years, and the mean time from onset of uveitis was 40.7 years.
In addition, 66.6% of the patients in the current study had cataracts or had undergone cataract surgery in one or both eyes, and 40.0% had glaucoma.
By the time of the current study, of the original cohort of 55 individuals, 11 were deceased; rheumatic disease was declared the main cause in four patients and a contributing factor in three others.
Potential drivers of the earliest cases of glaucoma and ocular hypertension (G/OH) include increased intraocular pressure as a result of topical corticosteroid treatment, the researchers noted in their discussion. However, G/OH occurring later than the 7-year follow-up was “more likely to be the type observed in many patients with long-standing chronic uveitis, where a gradual increase in intraocular pressure is assumed to be caused by impaired aqueous outflow,” they said.
Only 4 of the 30 patients did not have regular ophthalmology visits, which suggests a study population with ocular symptoms or concerns about their eyesight, the researchers wrote. “The fact that 13% of our original cohort were reported to have severe visual impairment or worse in both eyes at any of the three follow-ups is noteworthy,” compared to reports of visual impairment of less than 0.5% in a German study in the general population for similar ages.
The findings were limited by several factors, including the retrospective design, small study population, and lack of data on 25 of the original 55-member study cohort, which may reduce the reliability of the current study, the researchers noted. However, the results reflect data from previous studies and support the need for JIA patients to continue regular ophthalmic checkups throughout life, they concluded.
The study was supported by Stiftelsen för Synskadade i f.d. Malmöhus län, Sweden, Skånes Universitetssjukhus Stiftelser och Donationer, Ögonfonden, and the Swedish Society of Medicine. The researchers had no financial conflicts to disclose.
FROM PEDIATRIC RHEUMATOLOGY
Pulse oximeters lead to less oxygen supplementation for people of color
The new research suggests that skin color–related differences in pulse oximeter readings are in fact impacting clinical decision-making, lead author Eric R. Gottlieb, MD, of Brigham and Women’s Hospital and Massachusetts Institute of Technology, both in Boston, and colleagues wrote. This suggests that technology needs to updated to improve health equity, they continued, in their paper published in JAMA Internal Medicine.
“It has been known for decades that these readings are affected by various surface pigmentations, including nail polish and skin melanin, which may affect light absorption and scattering,” the investigators wrote. “This increases the risk of hidden hypoxemia [among patients with darker skin], in which patients have falsely elevated SpO2 readings, usually defined as 92% or greater, with a blood hemoglobin oxygen saturation less than 88%.”
Although published reports on this phenomenon date back to the 1980s, clinical significance has been largely discounted, they said, citing a 2008 paper on the topic, which stated that “oximetry need not have exact accuracy” to determine if a patient needs oxygen supplementation.
‘We’re not providing equal care’
Questioning the validity of this statement, Dr. Gottlieb and colleagues conducted a retrospective cohort study involving 3,069 patients admitted to intensive care at the Beth Israel Deaconess Medical Center in Boston between 2008 and 2019, thereby excluding patients treated during the COVID-19 pandemic. The population consisted of four races/ethnicities: White (87%), Black (7%), Hispanic (4%), and Asian (3%).
Aligning with previous studies, multivariable linear regression analyses showed that Asian, Black, and Hispanic patients had significantly higher SpO2 readings than White patients in relation to hemoglobin oxygen saturation values, suggesting falsely elevated readings.
Further modeling showed that these same patient groups also received lower oxygen delivery rates, which were not explained directly by race/ethnicity, but instead were mediated by the discrepancy between SpO2 and hemoglobin oxygen saturation values. In other words, physicians were responding consistently to pulse oximetry readings, rather than exhibiting a direct racial/ethnic bias in their clinical decision-making.
“We’re not providing equal care,” Dr. Gottlieb said in an interview. “It’s not that the patients are sicker, or have other socioeconomic explanations for why this happens to them. It’s us. It’s our technology. And that’s something that really has to be fixed.”
The investigators offered a cautionary view of corrective algorithms, as these “have exacerbated disparities and are subject to ethical concerns;” for example, with glomerular filtration rate estimations in Black patients.
Dr. Gottlieb also cautioned against action by individual physicians, who may now be inclined to change how they interpret pulse oximeter readings based on a patient’s race or ethnicity.
“I don’t think that we can expect physicians, every time they see a patient, to be second guessing whether the number basically reflects the truth,” he said.
Instead, Dr. Gottlieb suggested that the burden of change rests upon the shoulders of institutions, including hospitals and device manufacturers, both of which “really need to take the responsibility” for making sure that pulse oximeters are “equitable and have similar performance across races.”
While Dr. Gottlieb said that skin color likely plays the greatest role in measurement discrepancies, he encouraged stakeholders “to think broadly about this, and not just assume that it’s entirely skin color,” noting a small amount of evidence indicating that blood chemistry may also play a role. Still, he predicted that colorimetry – the direct measurement of skin color – will probably be incorporated into pulse oximeters of the future.
Black patients 3X more likely to have hidden hypoxia than White patients
Michael Sjoding, MD, of the University of Michigan, Ann Arbor, was one of the first to raise awareness of skin color–related issues with pulse oximeters during the throes of the COVID-19 pandemic. His study, which involved more than 10,000 patients, showed that Black patients were threefold more likely to have hidden hypoxia than White patients.
The present study shows that such discrepancies are indeed clinically significant, Dr. Sjoding said in an interview. And these data are needed, he added, to bring about change.
“What is being asked is potentially a big deal,” Dr. Sjoding said. “Pulse oximeters are everywhere, and it would be a big undertaking to redesign pulse oximeters and purchase new pulse oximeters. You need a compelling body of evidence to do that. I think it’s there now, clearly. So I’m hopeful that we’re going to finally move forward, towards having devices that we are confident work accurately in everyone.”
Why it has taken so long to gather this evidence, however, is a thornier topic, considering race-related discrepancies in pulse oximeter readings were first documented more than 3 decades ago.
“We sort of rediscovered something that had been known and had been described in the past,” Dr. Sjoding said. He explained how he and many of his colleagues had completed pulmonary fellowships, yet none of them knew of these potential issues with pulse oximeters until they began to observe differences in their own patients during the pandemic.
“I’ll give previous generations of researchers the benefit of the doubt,” Dr. Sjoding said, pointing out that techniques in data gathering and analysis have advanced considerably over the years. “The types of studies that were done before were very different than what we did.”
Yet Dr. Sjoding entertained the possibility that other factors may have been at play.
“I think definitely there’s a social commentary on prioritization of research,” he said.
The study was supported by grants from the National Institutes of Health. The investigators and Dr. Sjoding reported no conflicts of interest.
The new research suggests that skin color–related differences in pulse oximeter readings are in fact impacting clinical decision-making, lead author Eric R. Gottlieb, MD, of Brigham and Women’s Hospital and Massachusetts Institute of Technology, both in Boston, and colleagues wrote. This suggests that technology needs to updated to improve health equity, they continued, in their paper published in JAMA Internal Medicine.
“It has been known for decades that these readings are affected by various surface pigmentations, including nail polish and skin melanin, which may affect light absorption and scattering,” the investigators wrote. “This increases the risk of hidden hypoxemia [among patients with darker skin], in which patients have falsely elevated SpO2 readings, usually defined as 92% or greater, with a blood hemoglobin oxygen saturation less than 88%.”
Although published reports on this phenomenon date back to the 1980s, clinical significance has been largely discounted, they said, citing a 2008 paper on the topic, which stated that “oximetry need not have exact accuracy” to determine if a patient needs oxygen supplementation.
‘We’re not providing equal care’
Questioning the validity of this statement, Dr. Gottlieb and colleagues conducted a retrospective cohort study involving 3,069 patients admitted to intensive care at the Beth Israel Deaconess Medical Center in Boston between 2008 and 2019, thereby excluding patients treated during the COVID-19 pandemic. The population consisted of four races/ethnicities: White (87%), Black (7%), Hispanic (4%), and Asian (3%).
Aligning with previous studies, multivariable linear regression analyses showed that Asian, Black, and Hispanic patients had significantly higher SpO2 readings than White patients in relation to hemoglobin oxygen saturation values, suggesting falsely elevated readings.
Further modeling showed that these same patient groups also received lower oxygen delivery rates, which were not explained directly by race/ethnicity, but instead were mediated by the discrepancy between SpO2 and hemoglobin oxygen saturation values. In other words, physicians were responding consistently to pulse oximetry readings, rather than exhibiting a direct racial/ethnic bias in their clinical decision-making.
“We’re not providing equal care,” Dr. Gottlieb said in an interview. “It’s not that the patients are sicker, or have other socioeconomic explanations for why this happens to them. It’s us. It’s our technology. And that’s something that really has to be fixed.”
The investigators offered a cautionary view of corrective algorithms, as these “have exacerbated disparities and are subject to ethical concerns;” for example, with glomerular filtration rate estimations in Black patients.
Dr. Gottlieb also cautioned against action by individual physicians, who may now be inclined to change how they interpret pulse oximeter readings based on a patient’s race or ethnicity.
“I don’t think that we can expect physicians, every time they see a patient, to be second guessing whether the number basically reflects the truth,” he said.
Instead, Dr. Gottlieb suggested that the burden of change rests upon the shoulders of institutions, including hospitals and device manufacturers, both of which “really need to take the responsibility” for making sure that pulse oximeters are “equitable and have similar performance across races.”
While Dr. Gottlieb said that skin color likely plays the greatest role in measurement discrepancies, he encouraged stakeholders “to think broadly about this, and not just assume that it’s entirely skin color,” noting a small amount of evidence indicating that blood chemistry may also play a role. Still, he predicted that colorimetry – the direct measurement of skin color – will probably be incorporated into pulse oximeters of the future.
Black patients 3X more likely to have hidden hypoxia than White patients
Michael Sjoding, MD, of the University of Michigan, Ann Arbor, was one of the first to raise awareness of skin color–related issues with pulse oximeters during the throes of the COVID-19 pandemic. His study, which involved more than 10,000 patients, showed that Black patients were threefold more likely to have hidden hypoxia than White patients.
The present study shows that such discrepancies are indeed clinically significant, Dr. Sjoding said in an interview. And these data are needed, he added, to bring about change.
“What is being asked is potentially a big deal,” Dr. Sjoding said. “Pulse oximeters are everywhere, and it would be a big undertaking to redesign pulse oximeters and purchase new pulse oximeters. You need a compelling body of evidence to do that. I think it’s there now, clearly. So I’m hopeful that we’re going to finally move forward, towards having devices that we are confident work accurately in everyone.”
Why it has taken so long to gather this evidence, however, is a thornier topic, considering race-related discrepancies in pulse oximeter readings were first documented more than 3 decades ago.
“We sort of rediscovered something that had been known and had been described in the past,” Dr. Sjoding said. He explained how he and many of his colleagues had completed pulmonary fellowships, yet none of them knew of these potential issues with pulse oximeters until they began to observe differences in their own patients during the pandemic.
“I’ll give previous generations of researchers the benefit of the doubt,” Dr. Sjoding said, pointing out that techniques in data gathering and analysis have advanced considerably over the years. “The types of studies that were done before were very different than what we did.”
Yet Dr. Sjoding entertained the possibility that other factors may have been at play.
“I think definitely there’s a social commentary on prioritization of research,” he said.
The study was supported by grants from the National Institutes of Health. The investigators and Dr. Sjoding reported no conflicts of interest.
The new research suggests that skin color–related differences in pulse oximeter readings are in fact impacting clinical decision-making, lead author Eric R. Gottlieb, MD, of Brigham and Women’s Hospital and Massachusetts Institute of Technology, both in Boston, and colleagues wrote. This suggests that technology needs to updated to improve health equity, they continued, in their paper published in JAMA Internal Medicine.
“It has been known for decades that these readings are affected by various surface pigmentations, including nail polish and skin melanin, which may affect light absorption and scattering,” the investigators wrote. “This increases the risk of hidden hypoxemia [among patients with darker skin], in which patients have falsely elevated SpO2 readings, usually defined as 92% or greater, with a blood hemoglobin oxygen saturation less than 88%.”
Although published reports on this phenomenon date back to the 1980s, clinical significance has been largely discounted, they said, citing a 2008 paper on the topic, which stated that “oximetry need not have exact accuracy” to determine if a patient needs oxygen supplementation.
‘We’re not providing equal care’
Questioning the validity of this statement, Dr. Gottlieb and colleagues conducted a retrospective cohort study involving 3,069 patients admitted to intensive care at the Beth Israel Deaconess Medical Center in Boston between 2008 and 2019, thereby excluding patients treated during the COVID-19 pandemic. The population consisted of four races/ethnicities: White (87%), Black (7%), Hispanic (4%), and Asian (3%).
Aligning with previous studies, multivariable linear regression analyses showed that Asian, Black, and Hispanic patients had significantly higher SpO2 readings than White patients in relation to hemoglobin oxygen saturation values, suggesting falsely elevated readings.
Further modeling showed that these same patient groups also received lower oxygen delivery rates, which were not explained directly by race/ethnicity, but instead were mediated by the discrepancy between SpO2 and hemoglobin oxygen saturation values. In other words, physicians were responding consistently to pulse oximetry readings, rather than exhibiting a direct racial/ethnic bias in their clinical decision-making.
“We’re not providing equal care,” Dr. Gottlieb said in an interview. “It’s not that the patients are sicker, or have other socioeconomic explanations for why this happens to them. It’s us. It’s our technology. And that’s something that really has to be fixed.”
The investigators offered a cautionary view of corrective algorithms, as these “have exacerbated disparities and are subject to ethical concerns;” for example, with glomerular filtration rate estimations in Black patients.
Dr. Gottlieb also cautioned against action by individual physicians, who may now be inclined to change how they interpret pulse oximeter readings based on a patient’s race or ethnicity.
“I don’t think that we can expect physicians, every time they see a patient, to be second guessing whether the number basically reflects the truth,” he said.
Instead, Dr. Gottlieb suggested that the burden of change rests upon the shoulders of institutions, including hospitals and device manufacturers, both of which “really need to take the responsibility” for making sure that pulse oximeters are “equitable and have similar performance across races.”
While Dr. Gottlieb said that skin color likely plays the greatest role in measurement discrepancies, he encouraged stakeholders “to think broadly about this, and not just assume that it’s entirely skin color,” noting a small amount of evidence indicating that blood chemistry may also play a role. Still, he predicted that colorimetry – the direct measurement of skin color – will probably be incorporated into pulse oximeters of the future.
Black patients 3X more likely to have hidden hypoxia than White patients
Michael Sjoding, MD, of the University of Michigan, Ann Arbor, was one of the first to raise awareness of skin color–related issues with pulse oximeters during the throes of the COVID-19 pandemic. His study, which involved more than 10,000 patients, showed that Black patients were threefold more likely to have hidden hypoxia than White patients.
The present study shows that such discrepancies are indeed clinically significant, Dr. Sjoding said in an interview. And these data are needed, he added, to bring about change.
“What is being asked is potentially a big deal,” Dr. Sjoding said. “Pulse oximeters are everywhere, and it would be a big undertaking to redesign pulse oximeters and purchase new pulse oximeters. You need a compelling body of evidence to do that. I think it’s there now, clearly. So I’m hopeful that we’re going to finally move forward, towards having devices that we are confident work accurately in everyone.”
Why it has taken so long to gather this evidence, however, is a thornier topic, considering race-related discrepancies in pulse oximeter readings were first documented more than 3 decades ago.
“We sort of rediscovered something that had been known and had been described in the past,” Dr. Sjoding said. He explained how he and many of his colleagues had completed pulmonary fellowships, yet none of them knew of these potential issues with pulse oximeters until they began to observe differences in their own patients during the pandemic.
“I’ll give previous generations of researchers the benefit of the doubt,” Dr. Sjoding said, pointing out that techniques in data gathering and analysis have advanced considerably over the years. “The types of studies that were done before were very different than what we did.”
Yet Dr. Sjoding entertained the possibility that other factors may have been at play.
“I think definitely there’s a social commentary on prioritization of research,” he said.
The study was supported by grants from the National Institutes of Health. The investigators and Dr. Sjoding reported no conflicts of interest.
FROM JAMA INTERNAL MEDICINE
Addressing posttraumatic stress disorder in children and adolescents
Luke is a 12-year-old who presents for a well-child visit accompanied by his foster mother. He appears more solemn and taciturn than at previous visits. He is not interested in talking about any topics, including things he enjoys. His foster mother states that he has been more irritable, oppositional, and behaviorally dysregulated over the past 2 months. She also notes that his sleep has been poor. He reports this is because of nightmares and trouble falling asleep. Luke states that he will at times remember seeing his mother being struck by his father and – even when he does not want to – will have thoughts about hiding from his dad after being hit. You learn from the foster mother that he has been residing with her for the past 2 months and that he is now in state custody following significant parental home substance use, witnessing domestic violence, and being physically abused by his father.
The above narrative may sound all too familiar to those in pediatric primary care. You may wonder if there is a potential posttraumatic response to the witnessed trauma, but does the patient meet criteria for a trauma-related disorder? If so, what are the best next steps?
Prevalence of posttraumatic stress disorder in the general pediatric population
According to the 2020 National Survey of Children’s Health, approximately 40% of children age 17 and under report experiencing at least one adverse childhood experience. Within the 12-17 age range, it rises to over 50%.1 Adverse childhood experiences (ACEs) are potentially traumatic events and include items such as experiencing violence/abuse/neglect, witnessing violence in the home or community, having a family member attempt or die by suicide, and other adverse household and environmental situations. The accumulation of these ACEs can lead to long-term adverse emotional, physical, and behavioral outcomes.2
However, adverse childhood experiences do not always translate into PTSD. According to one national survey of 13- to 18-year-olds, the lifetime prevalence of PTSD is notably lower than exposure rates to ACEs and is estimated at 5% of adolescents, with higher rates among females (8%) versus males (2.3%).3
There are various risk factors for the development of PTSD that may play a role including genetic vulnerability, length of the trauma (for example, a one-time event versus repeated trauma for years), characteristics specific to the trauma, and the aftermath of the trauma. Again, it is important to note that not all youth exposed to a traumatic event will develop PTSD. Those who do make up a small percentage of at-risk children.4
Diagnosing PTSD in a child or adolescent
For a pediatric patient to be diagnosed with PTSD according to the DSM-5 criteria, they must experience a potentially traumatic event and meet criteria from four categories of symptoms. Trauma is defined as direct or indirect exposure to actual or threatened death, serious injury, or sexual violence. The four symptom categories are re-experiencing, avoidance, hyperarousal, and negative alteration in cognition and mood. The number of symptoms needed from each category varies based on the child’s age, with differing cutoffs based on whether the child is younger or older than 6 years old. Moreover, symptoms must be present for at least 1 month.5
Trauma can be assessed in the office by using a focused interview that includes the full DSM diagnostic criteria. There are additional trauma rating screeners and assessment tools that can be used including the Child PTSD Symptom Scale, Child Trauma Screening Questionnaire, UCLA Posttraumatic Stress Disorder Reaction Index, and the Trauma Symptom Checklist for Children, to name a few. Many of these allow for multiple informants, including the child/adolescent, thereby allowing for varying perspectives regarding trauma reactions.
Treatment options
Familiarity with evidence-based treatment for trauma may be useful to ensure that referral is targeted for the patient/family. There are no Food and Drug Administrations–approved medications for children with PTSD, though medications can be used to target specific PTSD symptoms (e.g. prazosin for trauma-related nightmares) as well as commonly comorbid conditions such as depression. Becoming familiar with the available therapeutic modalities offered in your area is recommended.
Highlighting trauma-focused cognitive behavioral therapy (TF-CBT)
The treatment with the most research evidence for traumatized children is trauma-focused cognitive behavioral therapy (TF-CBT), which is a 12- to 25-session therapeutic intervention for patients 3-18 years old (with some evidence for young adults as well) with PTSD and/or trauma-related behaviors. TF-CBT uses a components-based treatment model encompassed by the PRACTICE acronym/mnemonic.6,7
- P – Psychoeducation and parenting skills.
- R – Relaxation techniques: Focused breathing, progressive muscle relaxation, and teaching the child to control their thoughts (thought stopping).
- A – Affective expression and regulation (feeling identification): To help the child and parent learn to control their emotional reaction to reminders by expanding their emotional vocabulary, enhancing their skills in identification and expression of emotions, and encouraging self-soothing activities
- C – Cognitive coping and processing: Through this component, the child learns to understand the relationships between thoughts, feelings, and behaviors and think in new and healthier ways.
- T – Trauma narrative and processing: Gradual exposure exercises including verbal, written, and/or symbolic recounting of traumatic event(s) so the child learns to be able to discuss the events when they choose to in ways that do not produce overwhelming emotions. Following the completion of the narrative, clients are supported in identifying, challenging, and correcting cognitive distortions and dysfunctional beliefs.
- I – In vivo exposure: Encourage the gradual exposure to innocuous trauma reminders in the child’s environment so the child learns they can control their emotional reactions to things that remind them of the trauma, starting with nonthreatening examples of reminders.
- C – Conjoint parent/child sessions: Sessions generally deal with psycho-education, sharing the trauma narrative, anxiety management, and correction of cognitive distortions. The family works to enhance communication and create opportunities for therapeutic discussion regarding the trauma.
- E – Enhancing personal safety and future growth: Provide training and education with respect to personal safety skills and healthy sexuality and interpersonal relationships; encourage the utilization of skills learned in managing future stressors and/or trauma reminders.
Of note, some elements of this therapy that could possibly be easily incorporated into a primary care office visit include relaxation techniques and focus on coping skills/strategies.
Summary
Children and adolescents often present with trauma-related symptoms to the primary care office. Having increasing familiarity with PTSD diagnostic criteria and treatment modalities will likely lead to increased confidence and comfort recognizing symptoms and when placing a referral. This may also lead to shorter wait times for receiving targeted treatment and ultimately should lead to better outcomes for affected children and families.
Dr. Abdul-Kareem is at the University of Vermont, Burlington.
References
1. National Survey of Children’s Health (2016 - present). https://nschdata.org/browse/survey.
2. Adverse Childhood Experiences (ACEs). Centers for Disease Control and Prevention. https://www.cdc.gov/violenceprevention/aces/index.html].
3. Post-Traumatic Stress Disorder (PTSD). National Institute of Mental Health. https://www.nimh.nih.gov/health/statistics/post-traumatic-stress-disorder-ptsd,
4. Martin A et al. Lewis’s Child and Adolescent Psychiatry (5th edition). Lippincott Williams & Wilkins: Philadelphia, 2017.
5. American Psychiatric Association. Neurodevelopmental disorders. In: DSM-5. 2013.
6. Trauma-Focused Cognitive Behavioral Therapy. The National Child Traumatic Stress Network. https://www.nctsn.org/interventions/trauma-focused-cognitive-behavioral-therapy.
7. Trauma-Focused Cognitive-Behavioral Therapy (TF-CBT). California Evidence-Based Clearinghouse for Child Welfare. https://www.cebc4cw.org/program/trauma-focused-cognitive-behavioral-therapy/.
Luke is a 12-year-old who presents for a well-child visit accompanied by his foster mother. He appears more solemn and taciturn than at previous visits. He is not interested in talking about any topics, including things he enjoys. His foster mother states that he has been more irritable, oppositional, and behaviorally dysregulated over the past 2 months. She also notes that his sleep has been poor. He reports this is because of nightmares and trouble falling asleep. Luke states that he will at times remember seeing his mother being struck by his father and – even when he does not want to – will have thoughts about hiding from his dad after being hit. You learn from the foster mother that he has been residing with her for the past 2 months and that he is now in state custody following significant parental home substance use, witnessing domestic violence, and being physically abused by his father.
The above narrative may sound all too familiar to those in pediatric primary care. You may wonder if there is a potential posttraumatic response to the witnessed trauma, but does the patient meet criteria for a trauma-related disorder? If so, what are the best next steps?
Prevalence of posttraumatic stress disorder in the general pediatric population
According to the 2020 National Survey of Children’s Health, approximately 40% of children age 17 and under report experiencing at least one adverse childhood experience. Within the 12-17 age range, it rises to over 50%.1 Adverse childhood experiences (ACEs) are potentially traumatic events and include items such as experiencing violence/abuse/neglect, witnessing violence in the home or community, having a family member attempt or die by suicide, and other adverse household and environmental situations. The accumulation of these ACEs can lead to long-term adverse emotional, physical, and behavioral outcomes.2
However, adverse childhood experiences do not always translate into PTSD. According to one national survey of 13- to 18-year-olds, the lifetime prevalence of PTSD is notably lower than exposure rates to ACEs and is estimated at 5% of adolescents, with higher rates among females (8%) versus males (2.3%).3
There are various risk factors for the development of PTSD that may play a role including genetic vulnerability, length of the trauma (for example, a one-time event versus repeated trauma for years), characteristics specific to the trauma, and the aftermath of the trauma. Again, it is important to note that not all youth exposed to a traumatic event will develop PTSD. Those who do make up a small percentage of at-risk children.4
Diagnosing PTSD in a child or adolescent
For a pediatric patient to be diagnosed with PTSD according to the DSM-5 criteria, they must experience a potentially traumatic event and meet criteria from four categories of symptoms. Trauma is defined as direct or indirect exposure to actual or threatened death, serious injury, or sexual violence. The four symptom categories are re-experiencing, avoidance, hyperarousal, and negative alteration in cognition and mood. The number of symptoms needed from each category varies based on the child’s age, with differing cutoffs based on whether the child is younger or older than 6 years old. Moreover, symptoms must be present for at least 1 month.5
Trauma can be assessed in the office by using a focused interview that includes the full DSM diagnostic criteria. There are additional trauma rating screeners and assessment tools that can be used including the Child PTSD Symptom Scale, Child Trauma Screening Questionnaire, UCLA Posttraumatic Stress Disorder Reaction Index, and the Trauma Symptom Checklist for Children, to name a few. Many of these allow for multiple informants, including the child/adolescent, thereby allowing for varying perspectives regarding trauma reactions.
Treatment options
Familiarity with evidence-based treatment for trauma may be useful to ensure that referral is targeted for the patient/family. There are no Food and Drug Administrations–approved medications for children with PTSD, though medications can be used to target specific PTSD symptoms (e.g. prazosin for trauma-related nightmares) as well as commonly comorbid conditions such as depression. Becoming familiar with the available therapeutic modalities offered in your area is recommended.
Highlighting trauma-focused cognitive behavioral therapy (TF-CBT)
The treatment with the most research evidence for traumatized children is trauma-focused cognitive behavioral therapy (TF-CBT), which is a 12- to 25-session therapeutic intervention for patients 3-18 years old (with some evidence for young adults as well) with PTSD and/or trauma-related behaviors. TF-CBT uses a components-based treatment model encompassed by the PRACTICE acronym/mnemonic.6,7
- P – Psychoeducation and parenting skills.
- R – Relaxation techniques: Focused breathing, progressive muscle relaxation, and teaching the child to control their thoughts (thought stopping).
- A – Affective expression and regulation (feeling identification): To help the child and parent learn to control their emotional reaction to reminders by expanding their emotional vocabulary, enhancing their skills in identification and expression of emotions, and encouraging self-soothing activities
- C – Cognitive coping and processing: Through this component, the child learns to understand the relationships between thoughts, feelings, and behaviors and think in new and healthier ways.
- T – Trauma narrative and processing: Gradual exposure exercises including verbal, written, and/or symbolic recounting of traumatic event(s) so the child learns to be able to discuss the events when they choose to in ways that do not produce overwhelming emotions. Following the completion of the narrative, clients are supported in identifying, challenging, and correcting cognitive distortions and dysfunctional beliefs.
- I – In vivo exposure: Encourage the gradual exposure to innocuous trauma reminders in the child’s environment so the child learns they can control their emotional reactions to things that remind them of the trauma, starting with nonthreatening examples of reminders.
- C – Conjoint parent/child sessions: Sessions generally deal with psycho-education, sharing the trauma narrative, anxiety management, and correction of cognitive distortions. The family works to enhance communication and create opportunities for therapeutic discussion regarding the trauma.
- E – Enhancing personal safety and future growth: Provide training and education with respect to personal safety skills and healthy sexuality and interpersonal relationships; encourage the utilization of skills learned in managing future stressors and/or trauma reminders.
Of note, some elements of this therapy that could possibly be easily incorporated into a primary care office visit include relaxation techniques and focus on coping skills/strategies.
Summary
Children and adolescents often present with trauma-related symptoms to the primary care office. Having increasing familiarity with PTSD diagnostic criteria and treatment modalities will likely lead to increased confidence and comfort recognizing symptoms and when placing a referral. This may also lead to shorter wait times for receiving targeted treatment and ultimately should lead to better outcomes for affected children and families.
Dr. Abdul-Kareem is at the University of Vermont, Burlington.
References
1. National Survey of Children’s Health (2016 - present). https://nschdata.org/browse/survey.
2. Adverse Childhood Experiences (ACEs). Centers for Disease Control and Prevention. https://www.cdc.gov/violenceprevention/aces/index.html].
3. Post-Traumatic Stress Disorder (PTSD). National Institute of Mental Health. https://www.nimh.nih.gov/health/statistics/post-traumatic-stress-disorder-ptsd,
4. Martin A et al. Lewis’s Child and Adolescent Psychiatry (5th edition). Lippincott Williams & Wilkins: Philadelphia, 2017.
5. American Psychiatric Association. Neurodevelopmental disorders. In: DSM-5. 2013.
6. Trauma-Focused Cognitive Behavioral Therapy. The National Child Traumatic Stress Network. https://www.nctsn.org/interventions/trauma-focused-cognitive-behavioral-therapy.
7. Trauma-Focused Cognitive-Behavioral Therapy (TF-CBT). California Evidence-Based Clearinghouse for Child Welfare. https://www.cebc4cw.org/program/trauma-focused-cognitive-behavioral-therapy/.
Luke is a 12-year-old who presents for a well-child visit accompanied by his foster mother. He appears more solemn and taciturn than at previous visits. He is not interested in talking about any topics, including things he enjoys. His foster mother states that he has been more irritable, oppositional, and behaviorally dysregulated over the past 2 months. She also notes that his sleep has been poor. He reports this is because of nightmares and trouble falling asleep. Luke states that he will at times remember seeing his mother being struck by his father and – even when he does not want to – will have thoughts about hiding from his dad after being hit. You learn from the foster mother that he has been residing with her for the past 2 months and that he is now in state custody following significant parental home substance use, witnessing domestic violence, and being physically abused by his father.
The above narrative may sound all too familiar to those in pediatric primary care. You may wonder if there is a potential posttraumatic response to the witnessed trauma, but does the patient meet criteria for a trauma-related disorder? If so, what are the best next steps?
Prevalence of posttraumatic stress disorder in the general pediatric population
According to the 2020 National Survey of Children’s Health, approximately 40% of children age 17 and under report experiencing at least one adverse childhood experience. Within the 12-17 age range, it rises to over 50%.1 Adverse childhood experiences (ACEs) are potentially traumatic events and include items such as experiencing violence/abuse/neglect, witnessing violence in the home or community, having a family member attempt or die by suicide, and other adverse household and environmental situations. The accumulation of these ACEs can lead to long-term adverse emotional, physical, and behavioral outcomes.2
However, adverse childhood experiences do not always translate into PTSD. According to one national survey of 13- to 18-year-olds, the lifetime prevalence of PTSD is notably lower than exposure rates to ACEs and is estimated at 5% of adolescents, with higher rates among females (8%) versus males (2.3%).3
There are various risk factors for the development of PTSD that may play a role including genetic vulnerability, length of the trauma (for example, a one-time event versus repeated trauma for years), characteristics specific to the trauma, and the aftermath of the trauma. Again, it is important to note that not all youth exposed to a traumatic event will develop PTSD. Those who do make up a small percentage of at-risk children.4
Diagnosing PTSD in a child or adolescent
For a pediatric patient to be diagnosed with PTSD according to the DSM-5 criteria, they must experience a potentially traumatic event and meet criteria from four categories of symptoms. Trauma is defined as direct or indirect exposure to actual or threatened death, serious injury, or sexual violence. The four symptom categories are re-experiencing, avoidance, hyperarousal, and negative alteration in cognition and mood. The number of symptoms needed from each category varies based on the child’s age, with differing cutoffs based on whether the child is younger or older than 6 years old. Moreover, symptoms must be present for at least 1 month.5
Trauma can be assessed in the office by using a focused interview that includes the full DSM diagnostic criteria. There are additional trauma rating screeners and assessment tools that can be used including the Child PTSD Symptom Scale, Child Trauma Screening Questionnaire, UCLA Posttraumatic Stress Disorder Reaction Index, and the Trauma Symptom Checklist for Children, to name a few. Many of these allow for multiple informants, including the child/adolescent, thereby allowing for varying perspectives regarding trauma reactions.
Treatment options
Familiarity with evidence-based treatment for trauma may be useful to ensure that referral is targeted for the patient/family. There are no Food and Drug Administrations–approved medications for children with PTSD, though medications can be used to target specific PTSD symptoms (e.g. prazosin for trauma-related nightmares) as well as commonly comorbid conditions such as depression. Becoming familiar with the available therapeutic modalities offered in your area is recommended.
Highlighting trauma-focused cognitive behavioral therapy (TF-CBT)
The treatment with the most research evidence for traumatized children is trauma-focused cognitive behavioral therapy (TF-CBT), which is a 12- to 25-session therapeutic intervention for patients 3-18 years old (with some evidence for young adults as well) with PTSD and/or trauma-related behaviors. TF-CBT uses a components-based treatment model encompassed by the PRACTICE acronym/mnemonic.6,7
- P – Psychoeducation and parenting skills.
- R – Relaxation techniques: Focused breathing, progressive muscle relaxation, and teaching the child to control their thoughts (thought stopping).
- A – Affective expression and regulation (feeling identification): To help the child and parent learn to control their emotional reaction to reminders by expanding their emotional vocabulary, enhancing their skills in identification and expression of emotions, and encouraging self-soothing activities
- C – Cognitive coping and processing: Through this component, the child learns to understand the relationships between thoughts, feelings, and behaviors and think in new and healthier ways.
- T – Trauma narrative and processing: Gradual exposure exercises including verbal, written, and/or symbolic recounting of traumatic event(s) so the child learns to be able to discuss the events when they choose to in ways that do not produce overwhelming emotions. Following the completion of the narrative, clients are supported in identifying, challenging, and correcting cognitive distortions and dysfunctional beliefs.
- I – In vivo exposure: Encourage the gradual exposure to innocuous trauma reminders in the child’s environment so the child learns they can control their emotional reactions to things that remind them of the trauma, starting with nonthreatening examples of reminders.
- C – Conjoint parent/child sessions: Sessions generally deal with psycho-education, sharing the trauma narrative, anxiety management, and correction of cognitive distortions. The family works to enhance communication and create opportunities for therapeutic discussion regarding the trauma.
- E – Enhancing personal safety and future growth: Provide training and education with respect to personal safety skills and healthy sexuality and interpersonal relationships; encourage the utilization of skills learned in managing future stressors and/or trauma reminders.
Of note, some elements of this therapy that could possibly be easily incorporated into a primary care office visit include relaxation techniques and focus on coping skills/strategies.
Summary
Children and adolescents often present with trauma-related symptoms to the primary care office. Having increasing familiarity with PTSD diagnostic criteria and treatment modalities will likely lead to increased confidence and comfort recognizing symptoms and when placing a referral. This may also lead to shorter wait times for receiving targeted treatment and ultimately should lead to better outcomes for affected children and families.
Dr. Abdul-Kareem is at the University of Vermont, Burlington.
References
1. National Survey of Children’s Health (2016 - present). https://nschdata.org/browse/survey.
2. Adverse Childhood Experiences (ACEs). Centers for Disease Control and Prevention. https://www.cdc.gov/violenceprevention/aces/index.html].
3. Post-Traumatic Stress Disorder (PTSD). National Institute of Mental Health. https://www.nimh.nih.gov/health/statistics/post-traumatic-stress-disorder-ptsd,
4. Martin A et al. Lewis’s Child and Adolescent Psychiatry (5th edition). Lippincott Williams & Wilkins: Philadelphia, 2017.
5. American Psychiatric Association. Neurodevelopmental disorders. In: DSM-5. 2013.
6. Trauma-Focused Cognitive Behavioral Therapy. The National Child Traumatic Stress Network. https://www.nctsn.org/interventions/trauma-focused-cognitive-behavioral-therapy.
7. Trauma-Focused Cognitive-Behavioral Therapy (TF-CBT). California Evidence-Based Clearinghouse for Child Welfare. https://www.cebc4cw.org/program/trauma-focused-cognitive-behavioral-therapy/.
Topical gel for epidermolysis bullosa shows ongoing benefit
GLASGOW, Scotland – the phase 3 safety and efficacy study of the treatment.
Over 200 patients from the trial, including 105 who began treatment with a control gel, continued taking oleogel-S10 after 90 days. The current interim analysis at 12 months indicates there was a 55% reduction in the proportion of the body affected, compared with baseline.
Moreover, reductions in skin activity scores seen in the double-blind phase of the trial were maintained during the open-label extension. About 6% of patients experienced adverse events that led to withdrawal from the study.
The results show that oleogel-S10 was associated with “accelerated wound healing,” said study presenter Tracey Cunningham, MD, chief medical officer, Amryt Pharmaceuticals DAC, Dublin, which is developing the topical agent. “There were no new safety signals with this longer exposure to oleogel-S10, and patients had sustained improvement in wound burden,” she added.
The research was presented at the British Association of Dermatologists (BAD) 2022 Annual Meeting on July 6.
In April, European Medicines Agency recommended approval of oleogel-S10 for the treatment of partial-thickness skin wounds associated with dystrophic and junctional EB for patients aged 6 months and older.
However, just a month earlier, the U.S. Food and Drug Administration declined to approve the topical agent for use in EB, even after it extended its review by 3 months to include additional analyses of data previously submitted by the company.
In the post-presentation discussion, Dr. Cunningham said that the FDA had “not been satisfied at this point with the information that we have given them,” adding, “We don’t agree with the decision, and we will be appealing.”
Raman K. Madan, MD, a dermatologist at Northwell Health, Huntington, New York, who was not involved in the study, said that the reductions in wound healing seen in the study are “meaningful” and that the numbers represent a “big breakthrough.”
He told this news organization that there are “very few products on the market” for EB and that having an option for patients “would be amazing.”
“The big issue here would be cost and coverage for patients,” he said. If approved, “hopefully” it will be affordable, he added.
Dr. Madan noted that from his perspective, the majority of the reactions to the topical gel were “mild,” and there are “a lot of confounding factors” underlying the number of serious adverse events. “These patients with epidermolysis are prone to some of these issues regardless of treatment,” he said.
During her presentation, Dr. Cunningham noted that EB is a rare, debilitating condition that is characterized by varying degrees of skin fragility, blisters, and impaired wound healing that in turn lead to serious complications that affect quality of life.
While wound management is a “fundamental priority” for patients living with EB, she said, there is a “high, unmet” clinical need.
To those ends, EASE was the largest randomized controlled phase 3 efficacy and safety study in EB. In the study, 252 patients were allocated to receive oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing.
The double-blind phase of the trial met its primary endpoint: A higher proportion of patients who were given oleogel-S10 achieved first complete closure of the EB target wound by day 45, compared with patients who were given control gel, at 41.3% versus 28.9%. This equated to a relative risk of wound closure by day 45 of 1.44, or an odds ratio of 1.84 (P = .013).
However, as reported at the time by this news organization, the difference in time to wound healing by day 90 between the two patient groups was not statistically significant (P = .302), with 50.5% of oleogel-S10 patients achieving wound closure, versus 43.9% of those in the control group.
Dr. Cunningham discussed the open-label extension, which involved 205 patients from the double-blind phase (mean age, of 16.3 years) treated with oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing for 24 months.
In presenting the results of the first 12 months of the open-label extension, she said that oleogel-S10 led to “consistent” reductions in the body surface area percentage (BSAP) affected by EB. The overall reduction from baseline was 55% after receiving treatment for 15 months.
Between day 90 and month 12 of the open-label extension, the absolute BSAP was reduced from 7.4% to 5.4% for patients who had received oleogel-S10 from the start of the study. For those who started in the control group and then switched to the oleogel-S10 arm during the open-label extension, the reduction was from 8.3% to 6.4%.
Dr. Cunningham pointed out that a 1% reduction in BSAP equates approximately to the palmar surface of the hand.
Scores on the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI) Skin activity subscale indicated that the reductions achieved in the double-blind phase of the trial were maintained.
Among patients who received oleogel-S10 from the start of the trial, EBDASI Skin scores were reduced from 19.6 at baseline to 13.5 at 12 months’ follow-up in the open-label extension. The reduction was from 19.6 to 13.5 for those who began the trial taking control gel.
Dr. Cunningham showed that adverse events of any grade were seen in 72.0% of patients who began taking oleogel-S10 at the start of the trial and in 69.5% of those who began the trial taking control gel.
Serious adverse events were recorded in 23.0% and 20.0% of patients, respectively, while 6.0% of those who initially received oleogel-S10 and 6.7% of those initially assigned to control gel experienced adverse events that led to study withdrawal during the open-label phase.
The most frequently reported adverse events in the open-label extension were wound complications, seen in 39.5% of patients; anemia, seen in 14.1%; wound infection, seen in 9.3%; pyrexia, seen in 8.3%; and pruritus, seen in 5.9%. No more details regarding adverse events were provided.
The study was funded by Amryt Pharmaceuticals DAC. Dr. Cunningham is an employee of Amryt Pharmaceuticals. No other relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
GLASGOW, Scotland – the phase 3 safety and efficacy study of the treatment.
Over 200 patients from the trial, including 105 who began treatment with a control gel, continued taking oleogel-S10 after 90 days. The current interim analysis at 12 months indicates there was a 55% reduction in the proportion of the body affected, compared with baseline.
Moreover, reductions in skin activity scores seen in the double-blind phase of the trial were maintained during the open-label extension. About 6% of patients experienced adverse events that led to withdrawal from the study.
The results show that oleogel-S10 was associated with “accelerated wound healing,” said study presenter Tracey Cunningham, MD, chief medical officer, Amryt Pharmaceuticals DAC, Dublin, which is developing the topical agent. “There were no new safety signals with this longer exposure to oleogel-S10, and patients had sustained improvement in wound burden,” she added.
The research was presented at the British Association of Dermatologists (BAD) 2022 Annual Meeting on July 6.
In April, European Medicines Agency recommended approval of oleogel-S10 for the treatment of partial-thickness skin wounds associated with dystrophic and junctional EB for patients aged 6 months and older.
However, just a month earlier, the U.S. Food and Drug Administration declined to approve the topical agent for use in EB, even after it extended its review by 3 months to include additional analyses of data previously submitted by the company.
In the post-presentation discussion, Dr. Cunningham said that the FDA had “not been satisfied at this point with the information that we have given them,” adding, “We don’t agree with the decision, and we will be appealing.”
Raman K. Madan, MD, a dermatologist at Northwell Health, Huntington, New York, who was not involved in the study, said that the reductions in wound healing seen in the study are “meaningful” and that the numbers represent a “big breakthrough.”
He told this news organization that there are “very few products on the market” for EB and that having an option for patients “would be amazing.”
“The big issue here would be cost and coverage for patients,” he said. If approved, “hopefully” it will be affordable, he added.
Dr. Madan noted that from his perspective, the majority of the reactions to the topical gel were “mild,” and there are “a lot of confounding factors” underlying the number of serious adverse events. “These patients with epidermolysis are prone to some of these issues regardless of treatment,” he said.
During her presentation, Dr. Cunningham noted that EB is a rare, debilitating condition that is characterized by varying degrees of skin fragility, blisters, and impaired wound healing that in turn lead to serious complications that affect quality of life.
While wound management is a “fundamental priority” for patients living with EB, she said, there is a “high, unmet” clinical need.
To those ends, EASE was the largest randomized controlled phase 3 efficacy and safety study in EB. In the study, 252 patients were allocated to receive oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing.
The double-blind phase of the trial met its primary endpoint: A higher proportion of patients who were given oleogel-S10 achieved first complete closure of the EB target wound by day 45, compared with patients who were given control gel, at 41.3% versus 28.9%. This equated to a relative risk of wound closure by day 45 of 1.44, or an odds ratio of 1.84 (P = .013).
However, as reported at the time by this news organization, the difference in time to wound healing by day 90 between the two patient groups was not statistically significant (P = .302), with 50.5% of oleogel-S10 patients achieving wound closure, versus 43.9% of those in the control group.
Dr. Cunningham discussed the open-label extension, which involved 205 patients from the double-blind phase (mean age, of 16.3 years) treated with oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing for 24 months.
In presenting the results of the first 12 months of the open-label extension, she said that oleogel-S10 led to “consistent” reductions in the body surface area percentage (BSAP) affected by EB. The overall reduction from baseline was 55% after receiving treatment for 15 months.
Between day 90 and month 12 of the open-label extension, the absolute BSAP was reduced from 7.4% to 5.4% for patients who had received oleogel-S10 from the start of the study. For those who started in the control group and then switched to the oleogel-S10 arm during the open-label extension, the reduction was from 8.3% to 6.4%.
Dr. Cunningham pointed out that a 1% reduction in BSAP equates approximately to the palmar surface of the hand.
Scores on the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI) Skin activity subscale indicated that the reductions achieved in the double-blind phase of the trial were maintained.
Among patients who received oleogel-S10 from the start of the trial, EBDASI Skin scores were reduced from 19.6 at baseline to 13.5 at 12 months’ follow-up in the open-label extension. The reduction was from 19.6 to 13.5 for those who began the trial taking control gel.
Dr. Cunningham showed that adverse events of any grade were seen in 72.0% of patients who began taking oleogel-S10 at the start of the trial and in 69.5% of those who began the trial taking control gel.
Serious adverse events were recorded in 23.0% and 20.0% of patients, respectively, while 6.0% of those who initially received oleogel-S10 and 6.7% of those initially assigned to control gel experienced adverse events that led to study withdrawal during the open-label phase.
The most frequently reported adverse events in the open-label extension were wound complications, seen in 39.5% of patients; anemia, seen in 14.1%; wound infection, seen in 9.3%; pyrexia, seen in 8.3%; and pruritus, seen in 5.9%. No more details regarding adverse events were provided.
The study was funded by Amryt Pharmaceuticals DAC. Dr. Cunningham is an employee of Amryt Pharmaceuticals. No other relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
GLASGOW, Scotland – the phase 3 safety and efficacy study of the treatment.
Over 200 patients from the trial, including 105 who began treatment with a control gel, continued taking oleogel-S10 after 90 days. The current interim analysis at 12 months indicates there was a 55% reduction in the proportion of the body affected, compared with baseline.
Moreover, reductions in skin activity scores seen in the double-blind phase of the trial were maintained during the open-label extension. About 6% of patients experienced adverse events that led to withdrawal from the study.
The results show that oleogel-S10 was associated with “accelerated wound healing,” said study presenter Tracey Cunningham, MD, chief medical officer, Amryt Pharmaceuticals DAC, Dublin, which is developing the topical agent. “There were no new safety signals with this longer exposure to oleogel-S10, and patients had sustained improvement in wound burden,” she added.
The research was presented at the British Association of Dermatologists (BAD) 2022 Annual Meeting on July 6.
In April, European Medicines Agency recommended approval of oleogel-S10 for the treatment of partial-thickness skin wounds associated with dystrophic and junctional EB for patients aged 6 months and older.
However, just a month earlier, the U.S. Food and Drug Administration declined to approve the topical agent for use in EB, even after it extended its review by 3 months to include additional analyses of data previously submitted by the company.
In the post-presentation discussion, Dr. Cunningham said that the FDA had “not been satisfied at this point with the information that we have given them,” adding, “We don’t agree with the decision, and we will be appealing.”
Raman K. Madan, MD, a dermatologist at Northwell Health, Huntington, New York, who was not involved in the study, said that the reductions in wound healing seen in the study are “meaningful” and that the numbers represent a “big breakthrough.”
He told this news organization that there are “very few products on the market” for EB and that having an option for patients “would be amazing.”
“The big issue here would be cost and coverage for patients,” he said. If approved, “hopefully” it will be affordable, he added.
Dr. Madan noted that from his perspective, the majority of the reactions to the topical gel were “mild,” and there are “a lot of confounding factors” underlying the number of serious adverse events. “These patients with epidermolysis are prone to some of these issues regardless of treatment,” he said.
During her presentation, Dr. Cunningham noted that EB is a rare, debilitating condition that is characterized by varying degrees of skin fragility, blisters, and impaired wound healing that in turn lead to serious complications that affect quality of life.
While wound management is a “fundamental priority” for patients living with EB, she said, there is a “high, unmet” clinical need.
To those ends, EASE was the largest randomized controlled phase 3 efficacy and safety study in EB. In the study, 252 patients were allocated to receive oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing.
The double-blind phase of the trial met its primary endpoint: A higher proportion of patients who were given oleogel-S10 achieved first complete closure of the EB target wound by day 45, compared with patients who were given control gel, at 41.3% versus 28.9%. This equated to a relative risk of wound closure by day 45 of 1.44, or an odds ratio of 1.84 (P = .013).
However, as reported at the time by this news organization, the difference in time to wound healing by day 90 between the two patient groups was not statistically significant (P = .302), with 50.5% of oleogel-S10 patients achieving wound closure, versus 43.9% of those in the control group.
Dr. Cunningham discussed the open-label extension, which involved 205 patients from the double-blind phase (mean age, of 16.3 years) treated with oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing for 24 months.
In presenting the results of the first 12 months of the open-label extension, she said that oleogel-S10 led to “consistent” reductions in the body surface area percentage (BSAP) affected by EB. The overall reduction from baseline was 55% after receiving treatment for 15 months.
Between day 90 and month 12 of the open-label extension, the absolute BSAP was reduced from 7.4% to 5.4% for patients who had received oleogel-S10 from the start of the study. For those who started in the control group and then switched to the oleogel-S10 arm during the open-label extension, the reduction was from 8.3% to 6.4%.
Dr. Cunningham pointed out that a 1% reduction in BSAP equates approximately to the palmar surface of the hand.
Scores on the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI) Skin activity subscale indicated that the reductions achieved in the double-blind phase of the trial were maintained.
Among patients who received oleogel-S10 from the start of the trial, EBDASI Skin scores were reduced from 19.6 at baseline to 13.5 at 12 months’ follow-up in the open-label extension. The reduction was from 19.6 to 13.5 for those who began the trial taking control gel.
Dr. Cunningham showed that adverse events of any grade were seen in 72.0% of patients who began taking oleogel-S10 at the start of the trial and in 69.5% of those who began the trial taking control gel.
Serious adverse events were recorded in 23.0% and 20.0% of patients, respectively, while 6.0% of those who initially received oleogel-S10 and 6.7% of those initially assigned to control gel experienced adverse events that led to study withdrawal during the open-label phase.
The most frequently reported adverse events in the open-label extension were wound complications, seen in 39.5% of patients; anemia, seen in 14.1%; wound infection, seen in 9.3%; pyrexia, seen in 8.3%; and pruritus, seen in 5.9%. No more details regarding adverse events were provided.
The study was funded by Amryt Pharmaceuticals DAC. Dr. Cunningham is an employee of Amryt Pharmaceuticals. No other relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
Children and COVID: Vaccination a harder sell in the summer
The COVID-19 vaccination effort in the youngest children has begun much more slowly than the most recent rollout for older children, according to the Centers for Disease Control and Prevention.
in early November of 2021, based on CDC data last updated on July 7.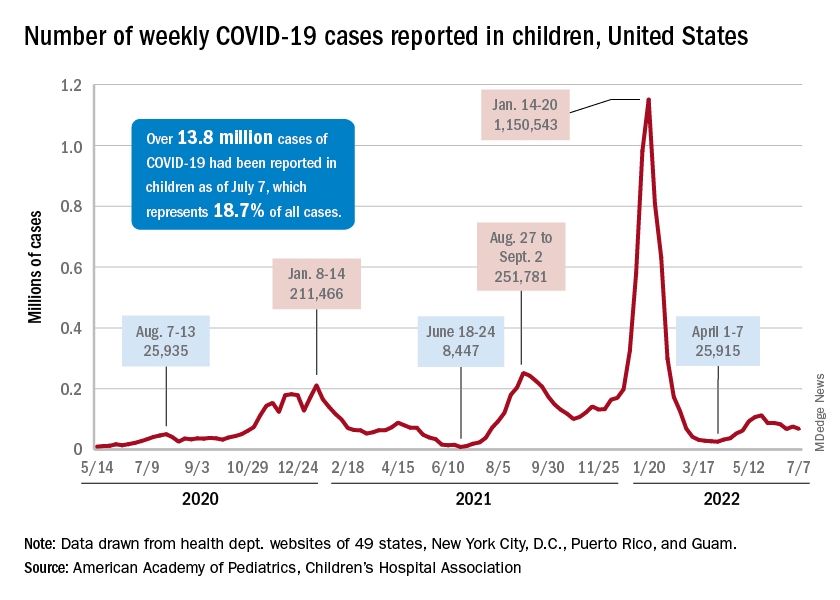
That approval, of course, came between the Delta and Omicron surges, when awareness was higher. The low initial uptake among those under age 5, however, was not unexpected by the Biden administration. “That number in and of itself is very much in line with our expectation, and we’re eager to continue working closely with partners to build on this start,” a senior administration official told ABC News.
With approval of the vaccine occurring after the school year was over, parents’ thoughts have been focused more on vacations and less on vaccinations. “Even before these vaccines officially became available, this was going to be a different rollout; it was going to take more time,” the official explained.
Incidence measures continue on different paths
New COVID-19 cases dropped during the latest reporting week (July 1-7), returning to the downward trend that began in late May and then stopped for 1 week (June 24-30), when cases were up by 12.4%, according to the American Academy of Pediatrics and the Children’s Hospital Association.
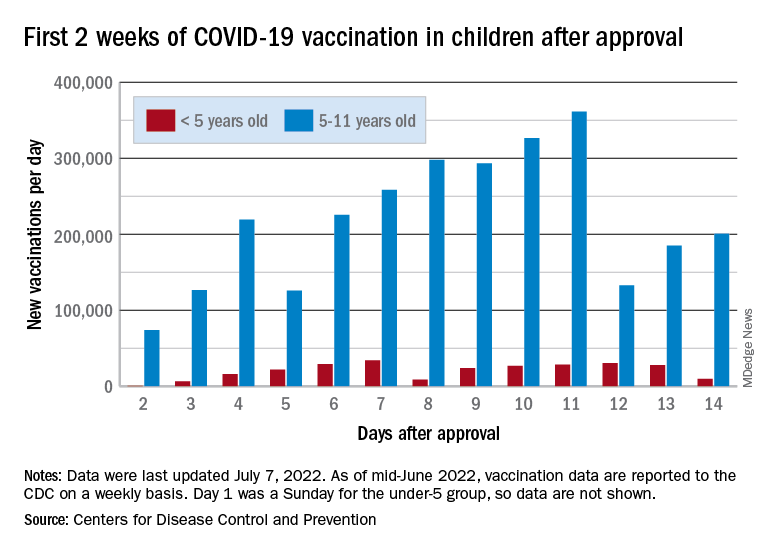
Children also represent a smaller share of cases, probably because of underreporting. “There has been a notable decline in the portion of reported weekly COVID-19 cases that are children,” the two groups said in their weekly COVID report. Although “cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases.”
Other measures, however, have been rising slowly but steadily since the spring. New admissions of patients aged 0-17 years with confirmed COVID, which were down to 0.13 per 100,000 population in early April, had climbed to 0.39 per 100,000 by July 7, the CDC said on its COVID Data Tracker.
Emergency department visits continue to show the same upward trend, despite a small decline in early June. A COVID diagnosis was involved in just 0.5% of ED visits in children aged 0-11 years on March 26, but by July 6 the rate was 4.7%. Increases were not as high among older children: From 0.3% on March 26 to 2.5% on July 6 for those aged 12-15 and from 0.3% to 2.4% for 16- and 17-year-olds, according to the CDC.
The COVID-19 vaccination effort in the youngest children has begun much more slowly than the most recent rollout for older children, according to the Centers for Disease Control and Prevention.
in early November of 2021, based on CDC data last updated on July 7.
That approval, of course, came between the Delta and Omicron surges, when awareness was higher. The low initial uptake among those under age 5, however, was not unexpected by the Biden administration. “That number in and of itself is very much in line with our expectation, and we’re eager to continue working closely with partners to build on this start,” a senior administration official told ABC News.
With approval of the vaccine occurring after the school year was over, parents’ thoughts have been focused more on vacations and less on vaccinations. “Even before these vaccines officially became available, this was going to be a different rollout; it was going to take more time,” the official explained.
Incidence measures continue on different paths
New COVID-19 cases dropped during the latest reporting week (July 1-7), returning to the downward trend that began in late May and then stopped for 1 week (June 24-30), when cases were up by 12.4%, according to the American Academy of Pediatrics and the Children’s Hospital Association.

Children also represent a smaller share of cases, probably because of underreporting. “There has been a notable decline in the portion of reported weekly COVID-19 cases that are children,” the two groups said in their weekly COVID report. Although “cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases.”
Other measures, however, have been rising slowly but steadily since the spring. New admissions of patients aged 0-17 years with confirmed COVID, which were down to 0.13 per 100,000 population in early April, had climbed to 0.39 per 100,000 by July 7, the CDC said on its COVID Data Tracker.
Emergency department visits continue to show the same upward trend, despite a small decline in early June. A COVID diagnosis was involved in just 0.5% of ED visits in children aged 0-11 years on March 26, but by July 6 the rate was 4.7%. Increases were not as high among older children: From 0.3% on March 26 to 2.5% on July 6 for those aged 12-15 and from 0.3% to 2.4% for 16- and 17-year-olds, according to the CDC.
The COVID-19 vaccination effort in the youngest children has begun much more slowly than the most recent rollout for older children, according to the Centers for Disease Control and Prevention.
in early November of 2021, based on CDC data last updated on July 7.
That approval, of course, came between the Delta and Omicron surges, when awareness was higher. The low initial uptake among those under age 5, however, was not unexpected by the Biden administration. “That number in and of itself is very much in line with our expectation, and we’re eager to continue working closely with partners to build on this start,” a senior administration official told ABC News.
With approval of the vaccine occurring after the school year was over, parents’ thoughts have been focused more on vacations and less on vaccinations. “Even before these vaccines officially became available, this was going to be a different rollout; it was going to take more time,” the official explained.
Incidence measures continue on different paths
New COVID-19 cases dropped during the latest reporting week (July 1-7), returning to the downward trend that began in late May and then stopped for 1 week (June 24-30), when cases were up by 12.4%, according to the American Academy of Pediatrics and the Children’s Hospital Association.

Children also represent a smaller share of cases, probably because of underreporting. “There has been a notable decline in the portion of reported weekly COVID-19 cases that are children,” the two groups said in their weekly COVID report. Although “cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases.”
Other measures, however, have been rising slowly but steadily since the spring. New admissions of patients aged 0-17 years with confirmed COVID, which were down to 0.13 per 100,000 population in early April, had climbed to 0.39 per 100,000 by July 7, the CDC said on its COVID Data Tracker.
Emergency department visits continue to show the same upward trend, despite a small decline in early June. A COVID diagnosis was involved in just 0.5% of ED visits in children aged 0-11 years on March 26, but by July 6 the rate was 4.7%. Increases were not as high among older children: From 0.3% on March 26 to 2.5% on July 6 for those aged 12-15 and from 0.3% to 2.4% for 16- and 17-year-olds, according to the CDC.