User login
News and Views that Matter to Pediatricians
The leading independent newspaper covering news and commentary in pediatrics.
FDA OKs iPLEDGE change for gender-neutral language
The Food and Drug Administration has approved a modification to the isotretinoin risk-mitigation program to make it more inclusive for transgender patients.
Beginning on Dec. 13, 2021, Previously, there were three risk categories: females of reproductive potential, females not of reproductive potential, and males.
In recent years, dermatologists and others have advocated for the change, hoping to make the process more inclusive and less intrusive for their transgender patients.
Isotretinoin (Accutane, Absorica, Amnesteem, Claravis, others) has a high risk of severe birth defects, and has been linked with other health issues, making it crucial for those with the ability to become pregnant to take contraceptive precautions while on the medication. Under the iPLEDGE program, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The FDA had given notification in June 2018 that the REMS modification and labeling change would be required, replacing the gender-specific language with gender-neutral language, according to an FDA spokesperson. The change was based on feedback that the gender-specific language can be a barrier to access for some patients. The FDA approved the modification on Oct. 8.
Expert reactions
“This is an exciting and welcome change from the FDA on iPLEDGE that many dermatologists, myself included, have advocated for quite a few years,” Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview.
In a report on the dermatologic care for lesbian, gay, bisexual, and transgender persons published in the Journal of the American Academy of Dermatology, Dr. Yeung and his colleagues noted that more than 10 million lesbian, gay, bisexual and transgender people live in the United States and that improving their health is a public health priority.
“For cisgender patients, nothing has changed – patients will continue to receive appropriate educational material related to isotretinoin based on their pregnancy potential,” Dr. Yeung said. “For transgender and gender diverse patients, this is a huge step forward.”
Under the previous system, doctors were asked to register patients using gender binary categories, “which were confusing when they did not reflect reality” for these patients, Dr. Yeung said. The new system, Dr. Yeung added, “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”
“The new terminology is not just respectful, it also is simpler and makes more sense,” agreed Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery at Mount Sinai Health System and professor of medicine at the Icahn School of Medicine at Mount Sinai, New York. “As it stood, a transgender man with his uterus and ovaries in place might be missed in the pregnancy surveillance system because he could simply be labeled a man and not followed further. At the same time, both transgender women and cisgender women who were at no risk of pregnancy could be subject to more medical scrutiny that might have been consider intrusive.”
The change “validates the important point that pregnancy potential is not exclusively defined by sociocultural constructs of gender and allow dermatologists to focus purely on what matters when prescribing isotretinoin – whether an individual is able to become pregnant or not, regardless of their gender identity,” Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., and suburban Maryland, who has also advocated for the change, said in an interview.
FDA elaborates
The modification includes important changes for doctors, pharmacists, and patients alike, according to the FDA.
Health care providers must assign and confirm their currently enrolled patient’s risk category when they first log in to the IPLEDGE REMS website on or after Dec. 13, the effective date. They should be sure any patient whose prescription RMA (iPLEDGE authorization) expires on Dec. 11-12 is told to obtain their prescription before midnight, Eastern time, Dec. 10.
Pharmacists will be affected, too, since the iPLEDGE REMS changed to a new platform vendor and the current “switch” pharmacy management system will be removed as a method to verify authorization to dispense isotretinoin. With these changes, as of Dec. 13, pharmacists can’t use the switch system to obtain a predispense authorization, or RMA (risk management authorization). They will need to obtain an RMA online by accessing the iPLEDGE REMS website or via telephone to the PLEDGE REMS center, 866-495-0654, before dispensing the prescription.
Patients, beginning Dec. 13, will have the option of presenting a unique QR code at the pharmacy on their smartphone rather than providing the iPLEDGE identification number. The code can be accessed by logging into their account on the iPLEDGE REMS website.
Patients with an isotretinoin prescription RMA that expires Dec. 11-12, must obtain the prescription before 11:59 p.m. Eastern time on Dec. 10. If the RMA expires before the prescription is picked up, the patient must begin the authorization process all over again.
Dr. Safer, Dr. Yeung, and Dr. Peebles have no relevant disclosures.
More information on the update and the isotretinoin REMS program is available on the FDA website.
The Food and Drug Administration has approved a modification to the isotretinoin risk-mitigation program to make it more inclusive for transgender patients.
Beginning on Dec. 13, 2021, Previously, there were three risk categories: females of reproductive potential, females not of reproductive potential, and males.
In recent years, dermatologists and others have advocated for the change, hoping to make the process more inclusive and less intrusive for their transgender patients.
Isotretinoin (Accutane, Absorica, Amnesteem, Claravis, others) has a high risk of severe birth defects, and has been linked with other health issues, making it crucial for those with the ability to become pregnant to take contraceptive precautions while on the medication. Under the iPLEDGE program, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The FDA had given notification in June 2018 that the REMS modification and labeling change would be required, replacing the gender-specific language with gender-neutral language, according to an FDA spokesperson. The change was based on feedback that the gender-specific language can be a barrier to access for some patients. The FDA approved the modification on Oct. 8.
Expert reactions
“This is an exciting and welcome change from the FDA on iPLEDGE that many dermatologists, myself included, have advocated for quite a few years,” Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview.
In a report on the dermatologic care for lesbian, gay, bisexual, and transgender persons published in the Journal of the American Academy of Dermatology, Dr. Yeung and his colleagues noted that more than 10 million lesbian, gay, bisexual and transgender people live in the United States and that improving their health is a public health priority.
“For cisgender patients, nothing has changed – patients will continue to receive appropriate educational material related to isotretinoin based on their pregnancy potential,” Dr. Yeung said. “For transgender and gender diverse patients, this is a huge step forward.”
Under the previous system, doctors were asked to register patients using gender binary categories, “which were confusing when they did not reflect reality” for these patients, Dr. Yeung said. The new system, Dr. Yeung added, “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”
“The new terminology is not just respectful, it also is simpler and makes more sense,” agreed Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery at Mount Sinai Health System and professor of medicine at the Icahn School of Medicine at Mount Sinai, New York. “As it stood, a transgender man with his uterus and ovaries in place might be missed in the pregnancy surveillance system because he could simply be labeled a man and not followed further. At the same time, both transgender women and cisgender women who were at no risk of pregnancy could be subject to more medical scrutiny that might have been consider intrusive.”
The change “validates the important point that pregnancy potential is not exclusively defined by sociocultural constructs of gender and allow dermatologists to focus purely on what matters when prescribing isotretinoin – whether an individual is able to become pregnant or not, regardless of their gender identity,” Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., and suburban Maryland, who has also advocated for the change, said in an interview.
FDA elaborates
The modification includes important changes for doctors, pharmacists, and patients alike, according to the FDA.
Health care providers must assign and confirm their currently enrolled patient’s risk category when they first log in to the IPLEDGE REMS website on or after Dec. 13, the effective date. They should be sure any patient whose prescription RMA (iPLEDGE authorization) expires on Dec. 11-12 is told to obtain their prescription before midnight, Eastern time, Dec. 10.
Pharmacists will be affected, too, since the iPLEDGE REMS changed to a new platform vendor and the current “switch” pharmacy management system will be removed as a method to verify authorization to dispense isotretinoin. With these changes, as of Dec. 13, pharmacists can’t use the switch system to obtain a predispense authorization, or RMA (risk management authorization). They will need to obtain an RMA online by accessing the iPLEDGE REMS website or via telephone to the PLEDGE REMS center, 866-495-0654, before dispensing the prescription.
Patients, beginning Dec. 13, will have the option of presenting a unique QR code at the pharmacy on their smartphone rather than providing the iPLEDGE identification number. The code can be accessed by logging into their account on the iPLEDGE REMS website.
Patients with an isotretinoin prescription RMA that expires Dec. 11-12, must obtain the prescription before 11:59 p.m. Eastern time on Dec. 10. If the RMA expires before the prescription is picked up, the patient must begin the authorization process all over again.
Dr. Safer, Dr. Yeung, and Dr. Peebles have no relevant disclosures.
More information on the update and the isotretinoin REMS program is available on the FDA website.
The Food and Drug Administration has approved a modification to the isotretinoin risk-mitigation program to make it more inclusive for transgender patients.
Beginning on Dec. 13, 2021, Previously, there were three risk categories: females of reproductive potential, females not of reproductive potential, and males.
In recent years, dermatologists and others have advocated for the change, hoping to make the process more inclusive and less intrusive for their transgender patients.
Isotretinoin (Accutane, Absorica, Amnesteem, Claravis, others) has a high risk of severe birth defects, and has been linked with other health issues, making it crucial for those with the ability to become pregnant to take contraceptive precautions while on the medication. Under the iPLEDGE program, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The FDA had given notification in June 2018 that the REMS modification and labeling change would be required, replacing the gender-specific language with gender-neutral language, according to an FDA spokesperson. The change was based on feedback that the gender-specific language can be a barrier to access for some patients. The FDA approved the modification on Oct. 8.
Expert reactions
“This is an exciting and welcome change from the FDA on iPLEDGE that many dermatologists, myself included, have advocated for quite a few years,” Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview.
In a report on the dermatologic care for lesbian, gay, bisexual, and transgender persons published in the Journal of the American Academy of Dermatology, Dr. Yeung and his colleagues noted that more than 10 million lesbian, gay, bisexual and transgender people live in the United States and that improving their health is a public health priority.
“For cisgender patients, nothing has changed – patients will continue to receive appropriate educational material related to isotretinoin based on their pregnancy potential,” Dr. Yeung said. “For transgender and gender diverse patients, this is a huge step forward.”
Under the previous system, doctors were asked to register patients using gender binary categories, “which were confusing when they did not reflect reality” for these patients, Dr. Yeung said. The new system, Dr. Yeung added, “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”
“The new terminology is not just respectful, it also is simpler and makes more sense,” agreed Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery at Mount Sinai Health System and professor of medicine at the Icahn School of Medicine at Mount Sinai, New York. “As it stood, a transgender man with his uterus and ovaries in place might be missed in the pregnancy surveillance system because he could simply be labeled a man and not followed further. At the same time, both transgender women and cisgender women who were at no risk of pregnancy could be subject to more medical scrutiny that might have been consider intrusive.”
The change “validates the important point that pregnancy potential is not exclusively defined by sociocultural constructs of gender and allow dermatologists to focus purely on what matters when prescribing isotretinoin – whether an individual is able to become pregnant or not, regardless of their gender identity,” Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., and suburban Maryland, who has also advocated for the change, said in an interview.
FDA elaborates
The modification includes important changes for doctors, pharmacists, and patients alike, according to the FDA.
Health care providers must assign and confirm their currently enrolled patient’s risk category when they first log in to the IPLEDGE REMS website on or after Dec. 13, the effective date. They should be sure any patient whose prescription RMA (iPLEDGE authorization) expires on Dec. 11-12 is told to obtain their prescription before midnight, Eastern time, Dec. 10.
Pharmacists will be affected, too, since the iPLEDGE REMS changed to a new platform vendor and the current “switch” pharmacy management system will be removed as a method to verify authorization to dispense isotretinoin. With these changes, as of Dec. 13, pharmacists can’t use the switch system to obtain a predispense authorization, or RMA (risk management authorization). They will need to obtain an RMA online by accessing the iPLEDGE REMS website or via telephone to the PLEDGE REMS center, 866-495-0654, before dispensing the prescription.
Patients, beginning Dec. 13, will have the option of presenting a unique QR code at the pharmacy on their smartphone rather than providing the iPLEDGE identification number. The code can be accessed by logging into their account on the iPLEDGE REMS website.
Patients with an isotretinoin prescription RMA that expires Dec. 11-12, must obtain the prescription before 11:59 p.m. Eastern time on Dec. 10. If the RMA expires before the prescription is picked up, the patient must begin the authorization process all over again.
Dr. Safer, Dr. Yeung, and Dr. Peebles have no relevant disclosures.
More information on the update and the isotretinoin REMS program is available on the FDA website.
9-step ladder may kids with allergies return to eggs
For many children in the process of outgrowing egg allergy, the step-wise reintroduction of foods that contain eggs can be achieved at home using a nine-rung laddered approach, according to updated guidelines from the British Society for Allergy and Clinical Immunology (BSACI).
Attempts to reintroduce egg into the child’s diet can start at the age of 12 months or 6 months from the last reaction, as long as past reactions have been mild to moderate and the child does not have asthma, according to guidelines from the BSACI, which represents allergists, pediatricians, and other health care practitioners.
According to the guidelines, the reintroduction needs to be guided by a specialist allergy service for children who have had severe reactions to egg or who have asthma.
Susan C. Leech, MB BChir, DCH, first author of the guidelines and a consultant in pediatric allergy with the Department of Child Health at Kings College Hospital, London, told this news organization that home reintroduction should begin slowly with small amounts of baked egg, starting with a pea-sized piece of cake, and should proceed gradually.
“Parents can be reassured that it’s a relatively safe thing to do as long as it’s done with caution,” said Dr. Leech.
The expanded guidelines include a new nine-step reintroduction ladder. It builds on a three-stage classification of egg-containing foods that was first introduced in BSACI guidelines in 2010.
On the bottom four rungs, children work their way through small but increasing amounts of fairy cakes (cupcakes), biscuits (cookies), and other foods containing baked eggs.
The next three rungs involve hard-boiled eggs, quiche, and other well-cooked egg products.
At the eighth rung, children can have small mouthfuls of runny scrambled eggs, mayonnaise, and other less-cooked or raw egg-containing products. At the top rung, children can have increasing amounts of those products as well as licks of cake batter.
The guidelines were published online September 29 in Clinical and Experimental Allergy along with a supplement that includes a series of examples showing how the guidelines apply to specific patient cases.
“These are examples only,” the guideline authors caution in the appendix. “Clinical judgment of severity is important as risk assessment is not always easy.”
Anna Nowak-Wegrzyn, MD, PhD, a professor of pediatrics at NYU Grossman School of Medicine and chief of pediatric allergy and immunology for Hassenfeld Children’s Hospital at NYU Langone, who was not involved in the BSACI guidelines, described the egg ladder as a “proactive” strategy that deserves further study and consideration.
“I think that this may be a valid approach,” said Dr. Nowak-Wegrzyn in an interview. “Eggs have good nutritional value, and they are present in a lot of foods, so avoidance creates logistical challenges.”
Using the egg ladder for home-based reintroduction may be especially suited in resource-poor areas where access to an allergist may be difficult, she said. It may also be suited for families that can’t visit the office because of pandemic-related restrictions.
“If the child had a severe reaction or if they have asthma, then it’s a no-go,” she added, “but if you have a patient who has a really mild reaction and you think that overall the risk of a significant reaction or bad symptoms is low, then it may be worth doing.”
Dr. Leech and Dr. Nowak-Wegrzyn have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For many children in the process of outgrowing egg allergy, the step-wise reintroduction of foods that contain eggs can be achieved at home using a nine-rung laddered approach, according to updated guidelines from the British Society for Allergy and Clinical Immunology (BSACI).
Attempts to reintroduce egg into the child’s diet can start at the age of 12 months or 6 months from the last reaction, as long as past reactions have been mild to moderate and the child does not have asthma, according to guidelines from the BSACI, which represents allergists, pediatricians, and other health care practitioners.
According to the guidelines, the reintroduction needs to be guided by a specialist allergy service for children who have had severe reactions to egg or who have asthma.
Susan C. Leech, MB BChir, DCH, first author of the guidelines and a consultant in pediatric allergy with the Department of Child Health at Kings College Hospital, London, told this news organization that home reintroduction should begin slowly with small amounts of baked egg, starting with a pea-sized piece of cake, and should proceed gradually.
“Parents can be reassured that it’s a relatively safe thing to do as long as it’s done with caution,” said Dr. Leech.
The expanded guidelines include a new nine-step reintroduction ladder. It builds on a three-stage classification of egg-containing foods that was first introduced in BSACI guidelines in 2010.
On the bottom four rungs, children work their way through small but increasing amounts of fairy cakes (cupcakes), biscuits (cookies), and other foods containing baked eggs.
The next three rungs involve hard-boiled eggs, quiche, and other well-cooked egg products.
At the eighth rung, children can have small mouthfuls of runny scrambled eggs, mayonnaise, and other less-cooked or raw egg-containing products. At the top rung, children can have increasing amounts of those products as well as licks of cake batter.
The guidelines were published online September 29 in Clinical and Experimental Allergy along with a supplement that includes a series of examples showing how the guidelines apply to specific patient cases.
“These are examples only,” the guideline authors caution in the appendix. “Clinical judgment of severity is important as risk assessment is not always easy.”
Anna Nowak-Wegrzyn, MD, PhD, a professor of pediatrics at NYU Grossman School of Medicine and chief of pediatric allergy and immunology for Hassenfeld Children’s Hospital at NYU Langone, who was not involved in the BSACI guidelines, described the egg ladder as a “proactive” strategy that deserves further study and consideration.
“I think that this may be a valid approach,” said Dr. Nowak-Wegrzyn in an interview. “Eggs have good nutritional value, and they are present in a lot of foods, so avoidance creates logistical challenges.”
Using the egg ladder for home-based reintroduction may be especially suited in resource-poor areas where access to an allergist may be difficult, she said. It may also be suited for families that can’t visit the office because of pandemic-related restrictions.
“If the child had a severe reaction or if they have asthma, then it’s a no-go,” she added, “but if you have a patient who has a really mild reaction and you think that overall the risk of a significant reaction or bad symptoms is low, then it may be worth doing.”
Dr. Leech and Dr. Nowak-Wegrzyn have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For many children in the process of outgrowing egg allergy, the step-wise reintroduction of foods that contain eggs can be achieved at home using a nine-rung laddered approach, according to updated guidelines from the British Society for Allergy and Clinical Immunology (BSACI).
Attempts to reintroduce egg into the child’s diet can start at the age of 12 months or 6 months from the last reaction, as long as past reactions have been mild to moderate and the child does not have asthma, according to guidelines from the BSACI, which represents allergists, pediatricians, and other health care practitioners.
According to the guidelines, the reintroduction needs to be guided by a specialist allergy service for children who have had severe reactions to egg or who have asthma.
Susan C. Leech, MB BChir, DCH, first author of the guidelines and a consultant in pediatric allergy with the Department of Child Health at Kings College Hospital, London, told this news organization that home reintroduction should begin slowly with small amounts of baked egg, starting with a pea-sized piece of cake, and should proceed gradually.
“Parents can be reassured that it’s a relatively safe thing to do as long as it’s done with caution,” said Dr. Leech.
The expanded guidelines include a new nine-step reintroduction ladder. It builds on a three-stage classification of egg-containing foods that was first introduced in BSACI guidelines in 2010.
On the bottom four rungs, children work their way through small but increasing amounts of fairy cakes (cupcakes), biscuits (cookies), and other foods containing baked eggs.
The next three rungs involve hard-boiled eggs, quiche, and other well-cooked egg products.
At the eighth rung, children can have small mouthfuls of runny scrambled eggs, mayonnaise, and other less-cooked or raw egg-containing products. At the top rung, children can have increasing amounts of those products as well as licks of cake batter.
The guidelines were published online September 29 in Clinical and Experimental Allergy along with a supplement that includes a series of examples showing how the guidelines apply to specific patient cases.
“These are examples only,” the guideline authors caution in the appendix. “Clinical judgment of severity is important as risk assessment is not always easy.”
Anna Nowak-Wegrzyn, MD, PhD, a professor of pediatrics at NYU Grossman School of Medicine and chief of pediatric allergy and immunology for Hassenfeld Children’s Hospital at NYU Langone, who was not involved in the BSACI guidelines, described the egg ladder as a “proactive” strategy that deserves further study and consideration.
“I think that this may be a valid approach,” said Dr. Nowak-Wegrzyn in an interview. “Eggs have good nutritional value, and they are present in a lot of foods, so avoidance creates logistical challenges.”
Using the egg ladder for home-based reintroduction may be especially suited in resource-poor areas where access to an allergist may be difficult, she said. It may also be suited for families that can’t visit the office because of pandemic-related restrictions.
“If the child had a severe reaction or if they have asthma, then it’s a no-go,” she added, “but if you have a patient who has a really mild reaction and you think that overall the risk of a significant reaction or bad symptoms is low, then it may be worth doing.”
Dr. Leech and Dr. Nowak-Wegrzyn have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pandemic data challenges infection link to Guillain-Barré syndrome
While pediatric cases of various types of infections fell by 45%-95% during the early months of the pandemic, cases of acute inflammatory demyelinating polyneuropathy (AIDP), an inflammatory neuropathy belonging to the clinical spectrum of Guillain-Barré syndrome, only fell by about 32%-37%, a rate that’s similar to the 35.1% decline in overall hospital admissions over that time period, researchers found. There was also no apparent link between the appearance of COVID-19 and the number of reported AIDP cases.
“There was no clear association between respiratory or gastrointestinal infections and rates of AIDP. Further, we found that AIDP did not have the expected dramatic reduction when community-acquired infections decreased during the pandemic,” Children’s Hospital of Philadelphia neurologist Craig A. Press, MD, PhD, said in an interview.
Dr. Press and colleagues presented their findings in a poster at the 50th annual meeting of the Child Neurology Society.
According to Dr. Press, the cause of AIDP in most patients is unclear, although infections and vaccinations are often linked to cases. “However, the data supporting this link is often weak. Infections with Campylobacter jejuni [bacteria that causes food poisoning] are known to be associated with AIDP, while rates of AIDP in the general population and in those with influenza are similar.”
For the new multicenter, cross-sectional study, researchers tracked AIDP data from the 47 pediatric hospitals that provide statistics to the Pediatric Health Information System. They focused on the period from January 2017 to September 2020, which included the first months of the COVID-19 pandemic in the United States.
“Social distancing, masks, and increased hand hygiene decrease community-acquired infectious rates in a dramatic way,” Dr. Press said. “If these infections were causing AIDP, we hypothesized that the cases of AIDP would drop substantially as a result.”
But this didn’t appear to happen. Researchers found that the numbers of various types of infections declined from April to September 2020: Respiratory infections dipped by 73%-78%, gastrointestinal infections fell by 45%-61%, and influenza infections dipped by 88%-95%. But AIDP cases didn’t fall as precipitously. In fact, their levels were about the same as they were in April 2017, a month when rates of gastrointestinal, respiratory disease and influenza infections were at seasonally low – but not abnormal – ebbs.
“While we must be cautious interpreting the results,” Dr. Press said, “this makes the link between infections as the main driver of pediatric AIDP less likely.”
However, he said, “this study does not exclude the possibility that rare infections cause AIDP – the data supporting that some more rare infections like campylobacter have a connection to AIDP are more robust – or that common infections very rarely lead to AIDP. While we look for triggers causing inflammatory disorders, AIDP maybe an autoinflammatory disorder without a clear trigger.”
Going forward, Dr. Press said, “we hope to look at infectious data in a more granular way to identify if specific viral or bacterial infectious may be associated with this or other inflammatory disorders. We believe that the use of data like this and the natural experiment that COVID-19 provided may help us to explore the impact of infections on disorders thought to be postinfectious.”
No study funding is reported, and the authors report no relevant disclosures.
While pediatric cases of various types of infections fell by 45%-95% during the early months of the pandemic, cases of acute inflammatory demyelinating polyneuropathy (AIDP), an inflammatory neuropathy belonging to the clinical spectrum of Guillain-Barré syndrome, only fell by about 32%-37%, a rate that’s similar to the 35.1% decline in overall hospital admissions over that time period, researchers found. There was also no apparent link between the appearance of COVID-19 and the number of reported AIDP cases.
“There was no clear association between respiratory or gastrointestinal infections and rates of AIDP. Further, we found that AIDP did not have the expected dramatic reduction when community-acquired infections decreased during the pandemic,” Children’s Hospital of Philadelphia neurologist Craig A. Press, MD, PhD, said in an interview.
Dr. Press and colleagues presented their findings in a poster at the 50th annual meeting of the Child Neurology Society.
According to Dr. Press, the cause of AIDP in most patients is unclear, although infections and vaccinations are often linked to cases. “However, the data supporting this link is often weak. Infections with Campylobacter jejuni [bacteria that causes food poisoning] are known to be associated with AIDP, while rates of AIDP in the general population and in those with influenza are similar.”
For the new multicenter, cross-sectional study, researchers tracked AIDP data from the 47 pediatric hospitals that provide statistics to the Pediatric Health Information System. They focused on the period from January 2017 to September 2020, which included the first months of the COVID-19 pandemic in the United States.
“Social distancing, masks, and increased hand hygiene decrease community-acquired infectious rates in a dramatic way,” Dr. Press said. “If these infections were causing AIDP, we hypothesized that the cases of AIDP would drop substantially as a result.”
But this didn’t appear to happen. Researchers found that the numbers of various types of infections declined from April to September 2020: Respiratory infections dipped by 73%-78%, gastrointestinal infections fell by 45%-61%, and influenza infections dipped by 88%-95%. But AIDP cases didn’t fall as precipitously. In fact, their levels were about the same as they were in April 2017, a month when rates of gastrointestinal, respiratory disease and influenza infections were at seasonally low – but not abnormal – ebbs.
“While we must be cautious interpreting the results,” Dr. Press said, “this makes the link between infections as the main driver of pediatric AIDP less likely.”
However, he said, “this study does not exclude the possibility that rare infections cause AIDP – the data supporting that some more rare infections like campylobacter have a connection to AIDP are more robust – or that common infections very rarely lead to AIDP. While we look for triggers causing inflammatory disorders, AIDP maybe an autoinflammatory disorder without a clear trigger.”
Going forward, Dr. Press said, “we hope to look at infectious data in a more granular way to identify if specific viral or bacterial infectious may be associated with this or other inflammatory disorders. We believe that the use of data like this and the natural experiment that COVID-19 provided may help us to explore the impact of infections on disorders thought to be postinfectious.”
No study funding is reported, and the authors report no relevant disclosures.
While pediatric cases of various types of infections fell by 45%-95% during the early months of the pandemic, cases of acute inflammatory demyelinating polyneuropathy (AIDP), an inflammatory neuropathy belonging to the clinical spectrum of Guillain-Barré syndrome, only fell by about 32%-37%, a rate that’s similar to the 35.1% decline in overall hospital admissions over that time period, researchers found. There was also no apparent link between the appearance of COVID-19 and the number of reported AIDP cases.
“There was no clear association between respiratory or gastrointestinal infections and rates of AIDP. Further, we found that AIDP did not have the expected dramatic reduction when community-acquired infections decreased during the pandemic,” Children’s Hospital of Philadelphia neurologist Craig A. Press, MD, PhD, said in an interview.
Dr. Press and colleagues presented their findings in a poster at the 50th annual meeting of the Child Neurology Society.
According to Dr. Press, the cause of AIDP in most patients is unclear, although infections and vaccinations are often linked to cases. “However, the data supporting this link is often weak. Infections with Campylobacter jejuni [bacteria that causes food poisoning] are known to be associated with AIDP, while rates of AIDP in the general population and in those with influenza are similar.”
For the new multicenter, cross-sectional study, researchers tracked AIDP data from the 47 pediatric hospitals that provide statistics to the Pediatric Health Information System. They focused on the period from January 2017 to September 2020, which included the first months of the COVID-19 pandemic in the United States.
“Social distancing, masks, and increased hand hygiene decrease community-acquired infectious rates in a dramatic way,” Dr. Press said. “If these infections were causing AIDP, we hypothesized that the cases of AIDP would drop substantially as a result.”
But this didn’t appear to happen. Researchers found that the numbers of various types of infections declined from April to September 2020: Respiratory infections dipped by 73%-78%, gastrointestinal infections fell by 45%-61%, and influenza infections dipped by 88%-95%. But AIDP cases didn’t fall as precipitously. In fact, their levels were about the same as they were in April 2017, a month when rates of gastrointestinal, respiratory disease and influenza infections were at seasonally low – but not abnormal – ebbs.
“While we must be cautious interpreting the results,” Dr. Press said, “this makes the link between infections as the main driver of pediatric AIDP less likely.”
However, he said, “this study does not exclude the possibility that rare infections cause AIDP – the data supporting that some more rare infections like campylobacter have a connection to AIDP are more robust – or that common infections very rarely lead to AIDP. While we look for triggers causing inflammatory disorders, AIDP maybe an autoinflammatory disorder without a clear trigger.”
Going forward, Dr. Press said, “we hope to look at infectious data in a more granular way to identify if specific viral or bacterial infectious may be associated with this or other inflammatory disorders. We believe that the use of data like this and the natural experiment that COVID-19 provided may help us to explore the impact of infections on disorders thought to be postinfectious.”
No study funding is reported, and the authors report no relevant disclosures.
FROM CNS 2021
Epidiolex plus THC lowers seizures in pediatric epilepsy
the component of cannabis that makes people high in larger quantities, researchers reported.
“THC can contribute to seizure control and mitigation some of the side effects of CBD,” said study coauthor and Austin, Tex., child neurologist Karen Keough, MD, in an interview. Dr. Keough and colleagues presented their findings at the 50th annual meeting of the Child Neurology Society.
In a landmark move, the Food and Drug Administration approved Epidiolex in 2018 for the treatment of seizures in two rare forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome. The agency had never before approved a drug with a purified ingredient derived from marijuana.
CBD, the active ingredient in Epidiolex, is nonpsychoactive. The use in medicine of THC, the main driver of marijuana’s ability to make people stoned, is much more controversial.
Dr. Keough said she had treated 60-70 children with CBD, at the same strength as in Epidiolex (100 mg), and 5 mg of THC before the drug was approved. “I was seeing some very impressive results, and some became seizure free who’d always been refractory,” she said.
When the Epidiolex became available, she said, some patients transitioned to it and stopped taking THC. According to her, some patients fared well. But others immediately experienced worse seizures, she said, and some developed side effects to Epidiolex in the absence of THC, such as agitation and appetite suppression.
Combination therapy
For the new study, a retrospective, unblinded cohort analysis, Dr. Keough and colleagues tracked patients who received various doses of CBD, in some cases as Epidiolex, and various doses of THC prescribed by the Texas Original Compassionate Cultivation dispensary, where she serves as chief medical officer.
The initial number of patients was 212; 135 consented to review and 10 were excluded for various reasons leaving a total of 74 subjects in the study. The subjects, whose median age at the start of the study was 12 years (range, 2-25 years), were tracked from 2018 to2021. Just over half (55%) were male, and they remained on the regimen for a median of 805 days (range, 400-1,141).
Of the 74 subjects, 45.9% had a reduction of seizures of more than 75%, and 20.3% had a reduction of 50%-75%. Only 4.1% saw their seizures worsen.
The THC doses varied from none to more than 12 mg/day; CBD doses varied from none to more than 26 mg/kg per day. O the 74 patients, 18 saw their greatest seizure reduction from baseline when they received no THC; 12 saw their greatest seizure reduction from baseline when they received 0-2 mg/kg per day of CBD.
Still controversial
Did the patients get high? In some cases they did, Dr. Keough said. However, “a lot of these patients are either too young or too cognitively limited to describe whether they’re feeling intoxicated. That’s one of the many reasons why this is so controversial. You have to go into this with eyes wide open. We’re working in an environment with limited information as to what an intoxicating dose is for a small kid.”
However, she said, it seems clear that “THC can enhance the effect of CBD in children with epilepsy” and reduce CBD side effects. It’s not surprising that the substances work differently since they interact with brain cells in different ways, she said.
For neurologists, she said, “the challenge is to find a reliable source of THC that you can count on and verify so you aren’t overdosing the patients.”
University of Saskatchewan, Saskatoon, child neurologist and cannabinoid researcher Richard Huntsman, MD, who’s familiar with the study findings, said in an interview that they “provide another strong signal that the addition of THC provides benefit, at least in some patients.”
But it’s still unclear “why some children respond best in regards to seizure reduction and side effect profile with combination CBD:THC therapy, and others seemed to do better with CBD alone,” he said. Also unknown: “the ideal THC:CBD ratio that allows optimal seizure control while preventing the potential harmful effects of THC.”
As for the future, he said, “as we are just scratching the surface of our knowledge about the use of cannabis-based therapies in children with neurological disorders, I suspect that the use of these therapies will expand over time.”
No study funding is reported. Dr. Keough disclosed serving as chief medical officer of Texas Original Compassionate Cultivation. Dr. Huntsman disclosed serving as lead investigator of the Cannabidiol in Children with Refractory Epileptic Encephalopathy study and serving on the boards of the Cannabinoid Research Initiative of Saskatchewan (University of Saskatchewan) and Canadian Childhood Cannabinoid Clinical Trials Consortium. He is also cochair of Health Canada’s Scientific Advisory Committee on Cannabinoids for Health Purposes.
the component of cannabis that makes people high in larger quantities, researchers reported.
“THC can contribute to seizure control and mitigation some of the side effects of CBD,” said study coauthor and Austin, Tex., child neurologist Karen Keough, MD, in an interview. Dr. Keough and colleagues presented their findings at the 50th annual meeting of the Child Neurology Society.
In a landmark move, the Food and Drug Administration approved Epidiolex in 2018 for the treatment of seizures in two rare forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome. The agency had never before approved a drug with a purified ingredient derived from marijuana.
CBD, the active ingredient in Epidiolex, is nonpsychoactive. The use in medicine of THC, the main driver of marijuana’s ability to make people stoned, is much more controversial.
Dr. Keough said she had treated 60-70 children with CBD, at the same strength as in Epidiolex (100 mg), and 5 mg of THC before the drug was approved. “I was seeing some very impressive results, and some became seizure free who’d always been refractory,” she said.
When the Epidiolex became available, she said, some patients transitioned to it and stopped taking THC. According to her, some patients fared well. But others immediately experienced worse seizures, she said, and some developed side effects to Epidiolex in the absence of THC, such as agitation and appetite suppression.
Combination therapy
For the new study, a retrospective, unblinded cohort analysis, Dr. Keough and colleagues tracked patients who received various doses of CBD, in some cases as Epidiolex, and various doses of THC prescribed by the Texas Original Compassionate Cultivation dispensary, where she serves as chief medical officer.
The initial number of patients was 212; 135 consented to review and 10 were excluded for various reasons leaving a total of 74 subjects in the study. The subjects, whose median age at the start of the study was 12 years (range, 2-25 years), were tracked from 2018 to2021. Just over half (55%) were male, and they remained on the regimen for a median of 805 days (range, 400-1,141).
Of the 74 subjects, 45.9% had a reduction of seizures of more than 75%, and 20.3% had a reduction of 50%-75%. Only 4.1% saw their seizures worsen.
The THC doses varied from none to more than 12 mg/day; CBD doses varied from none to more than 26 mg/kg per day. O the 74 patients, 18 saw their greatest seizure reduction from baseline when they received no THC; 12 saw their greatest seizure reduction from baseline when they received 0-2 mg/kg per day of CBD.
Still controversial
Did the patients get high? In some cases they did, Dr. Keough said. However, “a lot of these patients are either too young or too cognitively limited to describe whether they’re feeling intoxicated. That’s one of the many reasons why this is so controversial. You have to go into this with eyes wide open. We’re working in an environment with limited information as to what an intoxicating dose is for a small kid.”
However, she said, it seems clear that “THC can enhance the effect of CBD in children with epilepsy” and reduce CBD side effects. It’s not surprising that the substances work differently since they interact with brain cells in different ways, she said.
For neurologists, she said, “the challenge is to find a reliable source of THC that you can count on and verify so you aren’t overdosing the patients.”
University of Saskatchewan, Saskatoon, child neurologist and cannabinoid researcher Richard Huntsman, MD, who’s familiar with the study findings, said in an interview that they “provide another strong signal that the addition of THC provides benefit, at least in some patients.”
But it’s still unclear “why some children respond best in regards to seizure reduction and side effect profile with combination CBD:THC therapy, and others seemed to do better with CBD alone,” he said. Also unknown: “the ideal THC:CBD ratio that allows optimal seizure control while preventing the potential harmful effects of THC.”
As for the future, he said, “as we are just scratching the surface of our knowledge about the use of cannabis-based therapies in children with neurological disorders, I suspect that the use of these therapies will expand over time.”
No study funding is reported. Dr. Keough disclosed serving as chief medical officer of Texas Original Compassionate Cultivation. Dr. Huntsman disclosed serving as lead investigator of the Cannabidiol in Children with Refractory Epileptic Encephalopathy study and serving on the boards of the Cannabinoid Research Initiative of Saskatchewan (University of Saskatchewan) and Canadian Childhood Cannabinoid Clinical Trials Consortium. He is also cochair of Health Canada’s Scientific Advisory Committee on Cannabinoids for Health Purposes.
the component of cannabis that makes people high in larger quantities, researchers reported.
“THC can contribute to seizure control and mitigation some of the side effects of CBD,” said study coauthor and Austin, Tex., child neurologist Karen Keough, MD, in an interview. Dr. Keough and colleagues presented their findings at the 50th annual meeting of the Child Neurology Society.
In a landmark move, the Food and Drug Administration approved Epidiolex in 2018 for the treatment of seizures in two rare forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome. The agency had never before approved a drug with a purified ingredient derived from marijuana.
CBD, the active ingredient in Epidiolex, is nonpsychoactive. The use in medicine of THC, the main driver of marijuana’s ability to make people stoned, is much more controversial.
Dr. Keough said she had treated 60-70 children with CBD, at the same strength as in Epidiolex (100 mg), and 5 mg of THC before the drug was approved. “I was seeing some very impressive results, and some became seizure free who’d always been refractory,” she said.
When the Epidiolex became available, she said, some patients transitioned to it and stopped taking THC. According to her, some patients fared well. But others immediately experienced worse seizures, she said, and some developed side effects to Epidiolex in the absence of THC, such as agitation and appetite suppression.
Combination therapy
For the new study, a retrospective, unblinded cohort analysis, Dr. Keough and colleagues tracked patients who received various doses of CBD, in some cases as Epidiolex, and various doses of THC prescribed by the Texas Original Compassionate Cultivation dispensary, where she serves as chief medical officer.
The initial number of patients was 212; 135 consented to review and 10 were excluded for various reasons leaving a total of 74 subjects in the study. The subjects, whose median age at the start of the study was 12 years (range, 2-25 years), were tracked from 2018 to2021. Just over half (55%) were male, and they remained on the regimen for a median of 805 days (range, 400-1,141).
Of the 74 subjects, 45.9% had a reduction of seizures of more than 75%, and 20.3% had a reduction of 50%-75%. Only 4.1% saw their seizures worsen.
The THC doses varied from none to more than 12 mg/day; CBD doses varied from none to more than 26 mg/kg per day. O the 74 patients, 18 saw their greatest seizure reduction from baseline when they received no THC; 12 saw their greatest seizure reduction from baseline when they received 0-2 mg/kg per day of CBD.
Still controversial
Did the patients get high? In some cases they did, Dr. Keough said. However, “a lot of these patients are either too young or too cognitively limited to describe whether they’re feeling intoxicated. That’s one of the many reasons why this is so controversial. You have to go into this with eyes wide open. We’re working in an environment with limited information as to what an intoxicating dose is for a small kid.”
However, she said, it seems clear that “THC can enhance the effect of CBD in children with epilepsy” and reduce CBD side effects. It’s not surprising that the substances work differently since they interact with brain cells in different ways, she said.
For neurologists, she said, “the challenge is to find a reliable source of THC that you can count on and verify so you aren’t overdosing the patients.”
University of Saskatchewan, Saskatoon, child neurologist and cannabinoid researcher Richard Huntsman, MD, who’s familiar with the study findings, said in an interview that they “provide another strong signal that the addition of THC provides benefit, at least in some patients.”
But it’s still unclear “why some children respond best in regards to seizure reduction and side effect profile with combination CBD:THC therapy, and others seemed to do better with CBD alone,” he said. Also unknown: “the ideal THC:CBD ratio that allows optimal seizure control while preventing the potential harmful effects of THC.”
As for the future, he said, “as we are just scratching the surface of our knowledge about the use of cannabis-based therapies in children with neurological disorders, I suspect that the use of these therapies will expand over time.”
No study funding is reported. Dr. Keough disclosed serving as chief medical officer of Texas Original Compassionate Cultivation. Dr. Huntsman disclosed serving as lead investigator of the Cannabidiol in Children with Refractory Epileptic Encephalopathy study and serving on the boards of the Cannabinoid Research Initiative of Saskatchewan (University of Saskatchewan) and Canadian Childhood Cannabinoid Clinical Trials Consortium. He is also cochair of Health Canada’s Scientific Advisory Committee on Cannabinoids for Health Purposes.
FROM CNS 2021
‘Baby-wearing’ poses serious injury risks for infants, ED data show
Baby-wearing – carrying a child against your body in a sling, soft carrier, or other device – is associated with benefits like reduced crying and increased breastfeeding, studies have shown.
But this practice also entails risks. Babies can fall out of carriers, or be injured when an adult carrying them falls, for example.
researchers estimated in a study presented at the annual meeting of the American Academy of Pediatrics.
To characterize the epidemiology of these injuries, Samantha J. Rowe, MD, chief resident physician at Walter Reed National Military Medical Center in Bethesda, Md., and colleagues analyzed data from the National Electronic Injury Surveillance System between 2011 and 2020.
They included in their analysis data from patients aged 5 years and younger who sustained an injury associated with a baby-wearing product. Baby harnesses, carriers, slings, framed baby carriers, and soft baby carriers were among the devices included in the study. The researchers used 601 cases to generate national estimates.
An estimated 14,024 patients presented to EDs because of baby-wearing injuries, and 52% of the injuries occurred when a patient fell from the product.
Most injuries (61%) occurred in children aged 5 months and younger; 19.3% of these infants required hospitalization, most often for head injuries.
The investigators found that about 22% of the injuries were associated with a caregiver falling, noted Rachel Y. Moon, MD, who was not involved in the study.
“Carrying a baby changes your center of gravity – and can also obscure your vision of where you’re walking, so adults who use these devices should be cognizant of this,” said Dr. Moon, with the University of Virginia, Charlottesville.
Dr. Rowe often practiced baby-wearing with her daughter, and found that it was beneficial. And studies have demonstrated various benefits of baby-wearing, including improved thermoregulation and glycemic control.
Still, the new analysis illustrates the potential for baby-wearing products “to cause serious injury, especially in infants 5 months and younger,” Dr. Rowe said. “We need to provide more education to caregivers on safe baby-wearing and continue to improve our safety standards for baby-wearing products.”
Study coauthor Patrick T. Reeves, MD, with the Naval Medical Center at San Diego, offered additional guidance in a news release: “Like when buying a new pair of shoes, parents must be educated on the proper sizing, selection, and wear of baby carriers to prevent injury to themselves and their child.”
Parents also need to ensure that the child’s nose and mouth are not obstructed, Dr. Moon
In a recent article discussing the possible benefits of baby-wearing in terms of helping with breastfeeding, Dr. Moon also pointed out further safety considerations: “No matter which carrier is used, for safety reasons, we need to remind parents that the baby should be positioned so that the head is upright and the nose and mouth are not obstructed.”
The researchers and Dr. Moon had no relevant financial disclosures.
Baby-wearing – carrying a child against your body in a sling, soft carrier, or other device – is associated with benefits like reduced crying and increased breastfeeding, studies have shown.
But this practice also entails risks. Babies can fall out of carriers, or be injured when an adult carrying them falls, for example.
researchers estimated in a study presented at the annual meeting of the American Academy of Pediatrics.
To characterize the epidemiology of these injuries, Samantha J. Rowe, MD, chief resident physician at Walter Reed National Military Medical Center in Bethesda, Md., and colleagues analyzed data from the National Electronic Injury Surveillance System between 2011 and 2020.
They included in their analysis data from patients aged 5 years and younger who sustained an injury associated with a baby-wearing product. Baby harnesses, carriers, slings, framed baby carriers, and soft baby carriers were among the devices included in the study. The researchers used 601 cases to generate national estimates.
An estimated 14,024 patients presented to EDs because of baby-wearing injuries, and 52% of the injuries occurred when a patient fell from the product.
Most injuries (61%) occurred in children aged 5 months and younger; 19.3% of these infants required hospitalization, most often for head injuries.
The investigators found that about 22% of the injuries were associated with a caregiver falling, noted Rachel Y. Moon, MD, who was not involved in the study.
“Carrying a baby changes your center of gravity – and can also obscure your vision of where you’re walking, so adults who use these devices should be cognizant of this,” said Dr. Moon, with the University of Virginia, Charlottesville.
Dr. Rowe often practiced baby-wearing with her daughter, and found that it was beneficial. And studies have demonstrated various benefits of baby-wearing, including improved thermoregulation and glycemic control.
Still, the new analysis illustrates the potential for baby-wearing products “to cause serious injury, especially in infants 5 months and younger,” Dr. Rowe said. “We need to provide more education to caregivers on safe baby-wearing and continue to improve our safety standards for baby-wearing products.”
Study coauthor Patrick T. Reeves, MD, with the Naval Medical Center at San Diego, offered additional guidance in a news release: “Like when buying a new pair of shoes, parents must be educated on the proper sizing, selection, and wear of baby carriers to prevent injury to themselves and their child.”
Parents also need to ensure that the child’s nose and mouth are not obstructed, Dr. Moon
In a recent article discussing the possible benefits of baby-wearing in terms of helping with breastfeeding, Dr. Moon also pointed out further safety considerations: “No matter which carrier is used, for safety reasons, we need to remind parents that the baby should be positioned so that the head is upright and the nose and mouth are not obstructed.”
The researchers and Dr. Moon had no relevant financial disclosures.
Baby-wearing – carrying a child against your body in a sling, soft carrier, or other device – is associated with benefits like reduced crying and increased breastfeeding, studies have shown.
But this practice also entails risks. Babies can fall out of carriers, or be injured when an adult carrying them falls, for example.
researchers estimated in a study presented at the annual meeting of the American Academy of Pediatrics.
To characterize the epidemiology of these injuries, Samantha J. Rowe, MD, chief resident physician at Walter Reed National Military Medical Center in Bethesda, Md., and colleagues analyzed data from the National Electronic Injury Surveillance System between 2011 and 2020.
They included in their analysis data from patients aged 5 years and younger who sustained an injury associated with a baby-wearing product. Baby harnesses, carriers, slings, framed baby carriers, and soft baby carriers were among the devices included in the study. The researchers used 601 cases to generate national estimates.
An estimated 14,024 patients presented to EDs because of baby-wearing injuries, and 52% of the injuries occurred when a patient fell from the product.
Most injuries (61%) occurred in children aged 5 months and younger; 19.3% of these infants required hospitalization, most often for head injuries.
The investigators found that about 22% of the injuries were associated with a caregiver falling, noted Rachel Y. Moon, MD, who was not involved in the study.
“Carrying a baby changes your center of gravity – and can also obscure your vision of where you’re walking, so adults who use these devices should be cognizant of this,” said Dr. Moon, with the University of Virginia, Charlottesville.
Dr. Rowe often practiced baby-wearing with her daughter, and found that it was beneficial. And studies have demonstrated various benefits of baby-wearing, including improved thermoregulation and glycemic control.
Still, the new analysis illustrates the potential for baby-wearing products “to cause serious injury, especially in infants 5 months and younger,” Dr. Rowe said. “We need to provide more education to caregivers on safe baby-wearing and continue to improve our safety standards for baby-wearing products.”
Study coauthor Patrick T. Reeves, MD, with the Naval Medical Center at San Diego, offered additional guidance in a news release: “Like when buying a new pair of shoes, parents must be educated on the proper sizing, selection, and wear of baby carriers to prevent injury to themselves and their child.”
Parents also need to ensure that the child’s nose and mouth are not obstructed, Dr. Moon
In a recent article discussing the possible benefits of baby-wearing in terms of helping with breastfeeding, Dr. Moon also pointed out further safety considerations: “No matter which carrier is used, for safety reasons, we need to remind parents that the baby should be positioned so that the head is upright and the nose and mouth are not obstructed.”
The researchers and Dr. Moon had no relevant financial disclosures.
FROM AAP 2021
Is genetic testing valuable in the clinical management of epilepsy?
, new research shows.
Results of a survey that included more than 400 patients showed that positive findings from genetic testing helped guide clinical management in 50% of cases and improved patient outcomes in 75%. In addition, the findings were applicable to both children and adults.
“Fifty percent of the time the physicians reported that, yes, receiving the genetic diagnosis did change how they managed the patients,” reported co-investigator Dianalee McKnight, PhD, director of medical affairs at Invitae, a medical genetic testing company headquartered in San Francisco. In 81.3% of cases, providers reported they changed clinical management within 3 months of receiving the genetic results, she added.
The findings were presented at the 2021 World Congress of Neurology (WCN).
Test results can be practice-changing
Nearly 50% of positive genetic test results in epilepsy patients can help guide clinical management, Dr. McKnight noted. However, information on how physicians use genetic information in decision-making has been limited, prompting her conduct the survey.
A total of 1,567 physicians with 3,572 patients who had a definitive diagnosis of epilepsy were contacted. A total of 170 (10.8%) clinicians provided completed and eligible surveys on 429 patients with epilepsy.
The patient cohort comprised mostly children, with nearly 50 adults, which Dr. McKnight said is typical of the population receiving genetic testing in clinical practice.
She reported that genetic testing results prompted clinicians to make medication changes about 50% of the time. Other changes included specialist referral or to a clinical trial, monitoring for other neurological disease, and recommendations for dietary change or for surgery.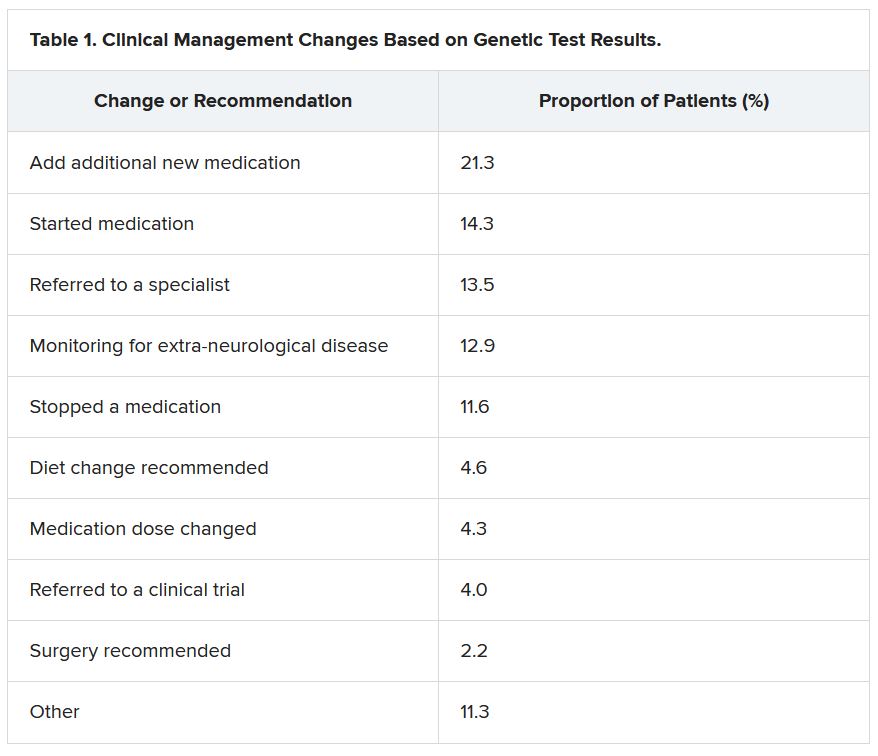
“Of the physicians who changed treatment, 75% reported there were positive outcomes for the patients,” Dr. McKnight told meeting attendees. “Most common was a reduction or a complete elimination of seizures, and that was reported in 65% of the cases.”
In many cases, the changes resulted in clinical improvements.
“There were 64 individuals who were having daily seizures before the genetic testing,” Dr. McKnight reported via email. “After receiving the genetic diagnosis and modifying their treatment, their physicians reported that 26% of individuals had complete seizure control and 46% of individuals had reduced seizure frequency to either weekly (20%), monthly (20%) or annually (6%).”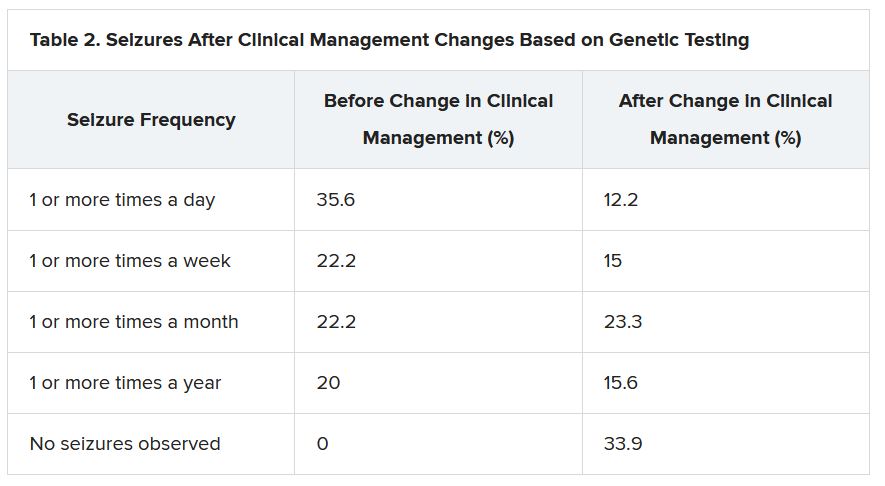
The best seizure control after modifying disease management occurred among children. Although the changes were not as dramatic for adults, they trended toward lower seizure frequency.
“It is still pretty significant that adults can receive genetic testing later in life and still have benefit in controlling their seizures,” Dr. McKnight said.
Twenty-three percent of patients showed improvement in behavior, development, academics, or movement issues, while 6% experienced reduced medication side effects.
Dr. McKnight also explored reasons for physicians not making changes to clinical management of patients based on the genetic results. The most common reason was that management was already consistent with the results (47.3%), followed by the results not being informative (26.1%), the results possibly being useful for future treatments in development (19.0%), or other or unknown reasons (7.6%).
Besides direct health and quality of life benefits from better seizure control, Dr. McKnight cited previous economic studies showing lower health care costs.
“It looked like an individual who has good seizure control will incur about 14,000 U.S. dollars a year compared with an individual with pretty poor seizure control, where it can be closer to 23,000 U.S. dollars a year,” Dr. McKnight said. This is mainly attributed to reduced hospitalizations and emergency department visits.
Dr. McKnight noted that currently there is no cost of genetic testing to the patient, the hospital, or insurers. Pharmaceutical companies, she said, sponsor the testing to potentially gather patients for clinical drug trials in development. However, patients remain completely anonymous.
Physicians who wish to have patient samples tested agree that the companies may contact them to ask if any of their patients with positive genetic test results would like to participate in a trial.
Dr. McKnight noted that genetic testing can be considered actionable in the clinic, helping to guide clinical decision-making and potentially leading to better outcomes. Going forward, she suggested performing large case-controlled studies “of individuals with the same genetic etiology ... to really find a true causation or correlation.”
Growing influence of genetic testing
Commenting on the findings, Jaysingh Singh, MD, co-director of the Epilepsy Surgery Center at the Ohio State University Wexner Medical Center in Columbus, noted that the study highlights the value of gene testing in improving outcomes in patients with epilepsy, particularly the pediatric population.
He said the findings make him optimistic about the potential of genetic testing in adult patients – with at least one caveat.
“The limitation is that if we do find some mutation, we don’t know what to do with that. That’s definitely one challenge. And we see that more often in the adult patient population,” said Dr. Singh, who was not involved with the research.
He noted that there is a small group of genetic mutations when, found in adults, may dramatically alter treatment.
For example, he noted that if there is a gene mutation related to mTOR pathways, that could provide a future target because there are already medications that target this pathway.
Genetic testing may also be useful in cases where patients have normal brain imaging and poor response to standard treatment or in cases where patients have congenital abnormalities such as intellectual impairment or facial dysmorphic features and a co-morbid seizure disorder, he said.
Dr. Singh noted that he has often found genetic testing impractical because “if I order DNA testing right now, it will take 4 months for me to get the results. I cannot wait 4 months for the results to come back” to adjust treatment.
Dr. McKnight is an employee of and a shareholder in Invitae, which funded the study. Dr. Singh has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
Results of a survey that included more than 400 patients showed that positive findings from genetic testing helped guide clinical management in 50% of cases and improved patient outcomes in 75%. In addition, the findings were applicable to both children and adults.
“Fifty percent of the time the physicians reported that, yes, receiving the genetic diagnosis did change how they managed the patients,” reported co-investigator Dianalee McKnight, PhD, director of medical affairs at Invitae, a medical genetic testing company headquartered in San Francisco. In 81.3% of cases, providers reported they changed clinical management within 3 months of receiving the genetic results, she added.
The findings were presented at the 2021 World Congress of Neurology (WCN).
Test results can be practice-changing
Nearly 50% of positive genetic test results in epilepsy patients can help guide clinical management, Dr. McKnight noted. However, information on how physicians use genetic information in decision-making has been limited, prompting her conduct the survey.
A total of 1,567 physicians with 3,572 patients who had a definitive diagnosis of epilepsy were contacted. A total of 170 (10.8%) clinicians provided completed and eligible surveys on 429 patients with epilepsy.
The patient cohort comprised mostly children, with nearly 50 adults, which Dr. McKnight said is typical of the population receiving genetic testing in clinical practice.
She reported that genetic testing results prompted clinicians to make medication changes about 50% of the time. Other changes included specialist referral or to a clinical trial, monitoring for other neurological disease, and recommendations for dietary change or for surgery.
“Of the physicians who changed treatment, 75% reported there were positive outcomes for the patients,” Dr. McKnight told meeting attendees. “Most common was a reduction or a complete elimination of seizures, and that was reported in 65% of the cases.”
In many cases, the changes resulted in clinical improvements.
“There were 64 individuals who were having daily seizures before the genetic testing,” Dr. McKnight reported via email. “After receiving the genetic diagnosis and modifying their treatment, their physicians reported that 26% of individuals had complete seizure control and 46% of individuals had reduced seizure frequency to either weekly (20%), monthly (20%) or annually (6%).”
The best seizure control after modifying disease management occurred among children. Although the changes were not as dramatic for adults, they trended toward lower seizure frequency.
“It is still pretty significant that adults can receive genetic testing later in life and still have benefit in controlling their seizures,” Dr. McKnight said.
Twenty-three percent of patients showed improvement in behavior, development, academics, or movement issues, while 6% experienced reduced medication side effects.
Dr. McKnight also explored reasons for physicians not making changes to clinical management of patients based on the genetic results. The most common reason was that management was already consistent with the results (47.3%), followed by the results not being informative (26.1%), the results possibly being useful for future treatments in development (19.0%), or other or unknown reasons (7.6%).
Besides direct health and quality of life benefits from better seizure control, Dr. McKnight cited previous economic studies showing lower health care costs.
“It looked like an individual who has good seizure control will incur about 14,000 U.S. dollars a year compared with an individual with pretty poor seizure control, where it can be closer to 23,000 U.S. dollars a year,” Dr. McKnight said. This is mainly attributed to reduced hospitalizations and emergency department visits.
Dr. McKnight noted that currently there is no cost of genetic testing to the patient, the hospital, or insurers. Pharmaceutical companies, she said, sponsor the testing to potentially gather patients for clinical drug trials in development. However, patients remain completely anonymous.
Physicians who wish to have patient samples tested agree that the companies may contact them to ask if any of their patients with positive genetic test results would like to participate in a trial.
Dr. McKnight noted that genetic testing can be considered actionable in the clinic, helping to guide clinical decision-making and potentially leading to better outcomes. Going forward, she suggested performing large case-controlled studies “of individuals with the same genetic etiology ... to really find a true causation or correlation.”
Growing influence of genetic testing
Commenting on the findings, Jaysingh Singh, MD, co-director of the Epilepsy Surgery Center at the Ohio State University Wexner Medical Center in Columbus, noted that the study highlights the value of gene testing in improving outcomes in patients with epilepsy, particularly the pediatric population.
He said the findings make him optimistic about the potential of genetic testing in adult patients – with at least one caveat.
“The limitation is that if we do find some mutation, we don’t know what to do with that. That’s definitely one challenge. And we see that more often in the adult patient population,” said Dr. Singh, who was not involved with the research.
He noted that there is a small group of genetic mutations when, found in adults, may dramatically alter treatment.
For example, he noted that if there is a gene mutation related to mTOR pathways, that could provide a future target because there are already medications that target this pathway.
Genetic testing may also be useful in cases where patients have normal brain imaging and poor response to standard treatment or in cases where patients have congenital abnormalities such as intellectual impairment or facial dysmorphic features and a co-morbid seizure disorder, he said.
Dr. Singh noted that he has often found genetic testing impractical because “if I order DNA testing right now, it will take 4 months for me to get the results. I cannot wait 4 months for the results to come back” to adjust treatment.
Dr. McKnight is an employee of and a shareholder in Invitae, which funded the study. Dr. Singh has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
Results of a survey that included more than 400 patients showed that positive findings from genetic testing helped guide clinical management in 50% of cases and improved patient outcomes in 75%. In addition, the findings were applicable to both children and adults.
“Fifty percent of the time the physicians reported that, yes, receiving the genetic diagnosis did change how they managed the patients,” reported co-investigator Dianalee McKnight, PhD, director of medical affairs at Invitae, a medical genetic testing company headquartered in San Francisco. In 81.3% of cases, providers reported they changed clinical management within 3 months of receiving the genetic results, she added.
The findings were presented at the 2021 World Congress of Neurology (WCN).
Test results can be practice-changing
Nearly 50% of positive genetic test results in epilepsy patients can help guide clinical management, Dr. McKnight noted. However, information on how physicians use genetic information in decision-making has been limited, prompting her conduct the survey.
A total of 1,567 physicians with 3,572 patients who had a definitive diagnosis of epilepsy were contacted. A total of 170 (10.8%) clinicians provided completed and eligible surveys on 429 patients with epilepsy.
The patient cohort comprised mostly children, with nearly 50 adults, which Dr. McKnight said is typical of the population receiving genetic testing in clinical practice.
She reported that genetic testing results prompted clinicians to make medication changes about 50% of the time. Other changes included specialist referral or to a clinical trial, monitoring for other neurological disease, and recommendations for dietary change or for surgery.
“Of the physicians who changed treatment, 75% reported there were positive outcomes for the patients,” Dr. McKnight told meeting attendees. “Most common was a reduction or a complete elimination of seizures, and that was reported in 65% of the cases.”
In many cases, the changes resulted in clinical improvements.
“There were 64 individuals who were having daily seizures before the genetic testing,” Dr. McKnight reported via email. “After receiving the genetic diagnosis and modifying their treatment, their physicians reported that 26% of individuals had complete seizure control and 46% of individuals had reduced seizure frequency to either weekly (20%), monthly (20%) or annually (6%).”
The best seizure control after modifying disease management occurred among children. Although the changes were not as dramatic for adults, they trended toward lower seizure frequency.
“It is still pretty significant that adults can receive genetic testing later in life and still have benefit in controlling their seizures,” Dr. McKnight said.
Twenty-three percent of patients showed improvement in behavior, development, academics, or movement issues, while 6% experienced reduced medication side effects.
Dr. McKnight also explored reasons for physicians not making changes to clinical management of patients based on the genetic results. The most common reason was that management was already consistent with the results (47.3%), followed by the results not being informative (26.1%), the results possibly being useful for future treatments in development (19.0%), or other or unknown reasons (7.6%).
Besides direct health and quality of life benefits from better seizure control, Dr. McKnight cited previous economic studies showing lower health care costs.
“It looked like an individual who has good seizure control will incur about 14,000 U.S. dollars a year compared with an individual with pretty poor seizure control, where it can be closer to 23,000 U.S. dollars a year,” Dr. McKnight said. This is mainly attributed to reduced hospitalizations and emergency department visits.
Dr. McKnight noted that currently there is no cost of genetic testing to the patient, the hospital, or insurers. Pharmaceutical companies, she said, sponsor the testing to potentially gather patients for clinical drug trials in development. However, patients remain completely anonymous.
Physicians who wish to have patient samples tested agree that the companies may contact them to ask if any of their patients with positive genetic test results would like to participate in a trial.
Dr. McKnight noted that genetic testing can be considered actionable in the clinic, helping to guide clinical decision-making and potentially leading to better outcomes. Going forward, she suggested performing large case-controlled studies “of individuals with the same genetic etiology ... to really find a true causation or correlation.”
Growing influence of genetic testing
Commenting on the findings, Jaysingh Singh, MD, co-director of the Epilepsy Surgery Center at the Ohio State University Wexner Medical Center in Columbus, noted that the study highlights the value of gene testing in improving outcomes in patients with epilepsy, particularly the pediatric population.
He said the findings make him optimistic about the potential of genetic testing in adult patients – with at least one caveat.
“The limitation is that if we do find some mutation, we don’t know what to do with that. That’s definitely one challenge. And we see that more often in the adult patient population,” said Dr. Singh, who was not involved with the research.
He noted that there is a small group of genetic mutations when, found in adults, may dramatically alter treatment.
For example, he noted that if there is a gene mutation related to mTOR pathways, that could provide a future target because there are already medications that target this pathway.
Genetic testing may also be useful in cases where patients have normal brain imaging and poor response to standard treatment or in cases where patients have congenital abnormalities such as intellectual impairment or facial dysmorphic features and a co-morbid seizure disorder, he said.
Dr. Singh noted that he has often found genetic testing impractical because “if I order DNA testing right now, it will take 4 months for me to get the results. I cannot wait 4 months for the results to come back” to adjust treatment.
Dr. McKnight is an employee of and a shareholder in Invitae, which funded the study. Dr. Singh has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From WCN 2021
Pediatricians can effectively promote gun safety
When pediatricians and other pediatric providers are given training and resource materials, levels of firearm screenings and anticipatory guidance about firearm safety increase significantly, according to two new studies presented at the annual meeting of the American Academy of Pediatrics.
“With the rise in firearm sales and injuries during the COVID-19 pandemic, it is more important than ever that pediatricians address the firearm epidemic,” said Alexandra Byrne, MD, a pediatric resident at the University of Florida in Gainesville, who presented one of the studies.
There were 4.3 million more firearms purchased from March through July 2020 than expected, a recent study estimates, and 4,075 more firearm injuries than expected from April through July 2020.
In states with more excess purchases, firearm injuries related to domestic violence increased in April (rate ratio, 2.60; 95% CI, 1.32-5.93) and May (RR, 1.79; 95% CI, 1.19-2.91) 2020. However, excess gun purchases had no effect on rates of firearm violence outside the home.
In addition to the link between firearms in the home and domestic violence, they are also linked to a three- to fourfold greater risk for teen suicide, and both depression and suicidal thoughts have risen in teens during the pandemic.
“The data are pretty clear that if you have an unlocked, loaded weapon in your home, and you have a kid who’s depressed or anxious or dysregulated or doing maladaptive things for the pandemic, they’re much more likely to inadvertently take their own or someone else’s life by grabbing [a gun],” said Cora Breuner, MD, MPH, professor of pediatrics at Seattle Children’s Hospital.
However, there is no difference in gun ownership or gun-safety measures between homes with and without at-risk children, previous research shows.
Training, guidance, and locks
Previous research has also shown that there has been a reluctance by pediatricians to conduct firearm screenings and counsel parents about gun safety in the home.
For their two-step program, Dr. Byrne’s team used a plan-do-study-act approach. They started by providing training on firearm safety, evidence-based recommendations for firearm screening, and anticipatory guidance regarding safe firearm storage to members of the general pediatrics division at the University of Florida. And they supplied clinics with free firearm locks.
Next they supplied clinics with posters and educational cards from the Be SMART campaign, an initiative of the Everytown for Gun Safety Support Fund, which provides materials for anyone, including physicians, to use.
During their study, the researchers sent three anonymous six-question online surveys – at baseline and 3 to 4 months after each of the two steps – to pediatric residents, physician assistants, advanced practice registered nurses, and attendings to assess the project. There were 52 responses to the first survey, for a response rate of 58.4%, 42 responses to the second survey, for a response rate of 47.2%, and 23 responses to the third survey, for a rate of response 25.8%.
The program nearly doubled screenings during well-child visits and dramatically increased the proportion of families who received a firearm lock when they told providers they had a firearm at home.
Previous research has shown “a significant increase in safe firearm storage when firearm locks were provided to families in clinic compared to verbal counseling alone,” Dr. Byrne said. “We know that safe firearm storage reduces injuries. Roughly one in three children in the United States lives in a home with a firearm. Individuals with a firearm are at two times the risk of homicide and three to four times the risk of suicide, so it is essential we further study how pediatricians can be most effective when it comes to firearm counseling.”
The difference in lock distribution as a result of the program is a “tremendous increase,” said Christopher S. Greeley, MD, MS, chief of the division of public health pediatrics at Texas Children’s Hospital and professor of pediatrics at Baylor College of Medicine in Houston, who was not involved in the research.
“Locks could go a long way to minimizing the risk,” he said in an interview, adding that nearly half of all teen suicide deaths that occurred over a decade in Houston involved a firearm.
Adding a social-history component
A program to increase firearm screening was also presented at the AAP conference.
After random review of medical records from 30 patients admitted to the hospital documented zero firearm screenings, Marjorie Farrington, MD, and Samantha Gunkelman, MD, from Akron Children’s Hospital in Ohio, implemented a program that they hope will increase firearm screenings during inpatient admissions to at least 50%.
They started their ongoing program in April 2020 by adding a social-history component to the history and physical (H&P) exam template and educating residents on how to screen and included guidance on safe firearm storage.
They also had physicians with firearm expertise give gun-safety lectures, and they plan to involve the Family Resource Center at their hospital in the creation of resources that can be incorporated into discharge instructions.
From April 2020 to June 2021, after the addition to the H&P template, 63% of the 5196 patients admitted to the hospital underwent a firearm screening. Of the 25% of patients who reported guns at home, 3% were not storing their firearms safely.
The pair used the “Store It Safe” Physician Handout provided by the Ohio chapter of the AAP.
Many pediatricians and pediatric trainees are not comfortable counseling on firearm safety, often a result of inadequate training on the topic.
The BulletPoints Project — developed by the Violence Prevention Research Program at the University of California, Davis — can also help physicians talk to patients about guns.
“Many pediatricians and pediatric trainees are not comfortable counseling on firearm safety, often a result of inadequate training on the topic,” Dr. Byrne said in an interview. “Additionally, it is a challenging topic that can often be met with resistance from patients and families. Lack of time during visits is also a huge barrier.”
Lack of training is an obstacle to greater firearm screenings, Dr. Greeley agreed, as are the feeling that guidance simply won’t make a difference and concerns about political pressure and divineness. The lack of research on firearm injuries and the impact of firearm screenings and anticipatory guidance is a challenge, he added, although that is starting to change.
Pediatricians need education on how to make a difference when it comes to firearm safety, and should follow AAP guidelines, Dr. Greeley said.
Counseling on firearm safety is in the same category as immunizations, seatbelts, substance use, helmets, and other public-health issues that are important to address at visits, regardless of how difficult it might be, Dr. Breuner told this news organization.
“It is our mission, as pediatricians, to provide every ounce of prevention in our well-child and anticipatory guidance visits,” she said. “It’s our job, so we shouldn’t shy away from it even though it’s hard.”
Doctors are more comfortable discussing firearm safety if they are firearm owners, previous research has shown, so she advises pediatricians who feel unqualified to discuss firearms to seek guidance from their peers on how to approach screenings and anticipatory guidance, she noted.
The firearm study being done in an academic center gives me great pause. The populations are often very different than private practice.
Both of these studies were conducted at single institutions and might not reflect what would work in private clinics.
“The firearm study being done in an academic center gives me great pause,” Dr. Greeley said. “The populations are often very different than private practice. I think that there is still a lot that remains unknown about decreasing household firearm injury and death.”
And the degree to which findings from these two gun-safety programs can be generalized to other academic centers or children’s hospitals is unclear.
“There are states where, I suspect, firearm screening is much more common. Some states have very pro-firearm cultures and others are anti-firearm,” Dr. Greeley said. “There are also likely differences within states,” particularly between urban and rural regions.
“Firearms are often a very personal issue for families, and pediatricians in ‘pro-firearm’ communities may have greater resistance to working on this,” he pointed out.
Nevertheless, Dr. Greeley said, “this is a promising strategy that could be part of a broad injury prevention initiative.”
Neither study noted any external funding. Dr. Byrne is a member of the Moms Demand Action Gainesville Chapter, which donated the firearm locks for the project. Dr. Breuner, Dr. Greeley, and Dr. Farrington have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When pediatricians and other pediatric providers are given training and resource materials, levels of firearm screenings and anticipatory guidance about firearm safety increase significantly, according to two new studies presented at the annual meeting of the American Academy of Pediatrics.
“With the rise in firearm sales and injuries during the COVID-19 pandemic, it is more important than ever that pediatricians address the firearm epidemic,” said Alexandra Byrne, MD, a pediatric resident at the University of Florida in Gainesville, who presented one of the studies.
There were 4.3 million more firearms purchased from March through July 2020 than expected, a recent study estimates, and 4,075 more firearm injuries than expected from April through July 2020.
In states with more excess purchases, firearm injuries related to domestic violence increased in April (rate ratio, 2.60; 95% CI, 1.32-5.93) and May (RR, 1.79; 95% CI, 1.19-2.91) 2020. However, excess gun purchases had no effect on rates of firearm violence outside the home.
In addition to the link between firearms in the home and domestic violence, they are also linked to a three- to fourfold greater risk for teen suicide, and both depression and suicidal thoughts have risen in teens during the pandemic.
“The data are pretty clear that if you have an unlocked, loaded weapon in your home, and you have a kid who’s depressed or anxious or dysregulated or doing maladaptive things for the pandemic, they’re much more likely to inadvertently take their own or someone else’s life by grabbing [a gun],” said Cora Breuner, MD, MPH, professor of pediatrics at Seattle Children’s Hospital.
However, there is no difference in gun ownership or gun-safety measures between homes with and without at-risk children, previous research shows.
Training, guidance, and locks
Previous research has also shown that there has been a reluctance by pediatricians to conduct firearm screenings and counsel parents about gun safety in the home.
For their two-step program, Dr. Byrne’s team used a plan-do-study-act approach. They started by providing training on firearm safety, evidence-based recommendations for firearm screening, and anticipatory guidance regarding safe firearm storage to members of the general pediatrics division at the University of Florida. And they supplied clinics with free firearm locks.
Next they supplied clinics with posters and educational cards from the Be SMART campaign, an initiative of the Everytown for Gun Safety Support Fund, which provides materials for anyone, including physicians, to use.
During their study, the researchers sent three anonymous six-question online surveys – at baseline and 3 to 4 months after each of the two steps – to pediatric residents, physician assistants, advanced practice registered nurses, and attendings to assess the project. There were 52 responses to the first survey, for a response rate of 58.4%, 42 responses to the second survey, for a response rate of 47.2%, and 23 responses to the third survey, for a rate of response 25.8%.
The program nearly doubled screenings during well-child visits and dramatically increased the proportion of families who received a firearm lock when they told providers they had a firearm at home.
Previous research has shown “a significant increase in safe firearm storage when firearm locks were provided to families in clinic compared to verbal counseling alone,” Dr. Byrne said. “We know that safe firearm storage reduces injuries. Roughly one in three children in the United States lives in a home with a firearm. Individuals with a firearm are at two times the risk of homicide and three to four times the risk of suicide, so it is essential we further study how pediatricians can be most effective when it comes to firearm counseling.”
The difference in lock distribution as a result of the program is a “tremendous increase,” said Christopher S. Greeley, MD, MS, chief of the division of public health pediatrics at Texas Children’s Hospital and professor of pediatrics at Baylor College of Medicine in Houston, who was not involved in the research.
“Locks could go a long way to minimizing the risk,” he said in an interview, adding that nearly half of all teen suicide deaths that occurred over a decade in Houston involved a firearm.
Adding a social-history component
A program to increase firearm screening was also presented at the AAP conference.
After random review of medical records from 30 patients admitted to the hospital documented zero firearm screenings, Marjorie Farrington, MD, and Samantha Gunkelman, MD, from Akron Children’s Hospital in Ohio, implemented a program that they hope will increase firearm screenings during inpatient admissions to at least 50%.
They started their ongoing program in April 2020 by adding a social-history component to the history and physical (H&P) exam template and educating residents on how to screen and included guidance on safe firearm storage.
They also had physicians with firearm expertise give gun-safety lectures, and they plan to involve the Family Resource Center at their hospital in the creation of resources that can be incorporated into discharge instructions.
From April 2020 to June 2021, after the addition to the H&P template, 63% of the 5196 patients admitted to the hospital underwent a firearm screening. Of the 25% of patients who reported guns at home, 3% were not storing their firearms safely.
The pair used the “Store It Safe” Physician Handout provided by the Ohio chapter of the AAP.
Many pediatricians and pediatric trainees are not comfortable counseling on firearm safety, often a result of inadequate training on the topic.
The BulletPoints Project — developed by the Violence Prevention Research Program at the University of California, Davis — can also help physicians talk to patients about guns.
“Many pediatricians and pediatric trainees are not comfortable counseling on firearm safety, often a result of inadequate training on the topic,” Dr. Byrne said in an interview. “Additionally, it is a challenging topic that can often be met with resistance from patients and families. Lack of time during visits is also a huge barrier.”
Lack of training is an obstacle to greater firearm screenings, Dr. Greeley agreed, as are the feeling that guidance simply won’t make a difference and concerns about political pressure and divineness. The lack of research on firearm injuries and the impact of firearm screenings and anticipatory guidance is a challenge, he added, although that is starting to change.
Pediatricians need education on how to make a difference when it comes to firearm safety, and should follow AAP guidelines, Dr. Greeley said.
Counseling on firearm safety is in the same category as immunizations, seatbelts, substance use, helmets, and other public-health issues that are important to address at visits, regardless of how difficult it might be, Dr. Breuner told this news organization.
“It is our mission, as pediatricians, to provide every ounce of prevention in our well-child and anticipatory guidance visits,” she said. “It’s our job, so we shouldn’t shy away from it even though it’s hard.”
Doctors are more comfortable discussing firearm safety if they are firearm owners, previous research has shown, so she advises pediatricians who feel unqualified to discuss firearms to seek guidance from their peers on how to approach screenings and anticipatory guidance, she noted.
The firearm study being done in an academic center gives me great pause. The populations are often very different than private practice.
Both of these studies were conducted at single institutions and might not reflect what would work in private clinics.
“The firearm study being done in an academic center gives me great pause,” Dr. Greeley said. “The populations are often very different than private practice. I think that there is still a lot that remains unknown about decreasing household firearm injury and death.”
And the degree to which findings from these two gun-safety programs can be generalized to other academic centers or children’s hospitals is unclear.
“There are states where, I suspect, firearm screening is much more common. Some states have very pro-firearm cultures and others are anti-firearm,” Dr. Greeley said. “There are also likely differences within states,” particularly between urban and rural regions.
“Firearms are often a very personal issue for families, and pediatricians in ‘pro-firearm’ communities may have greater resistance to working on this,” he pointed out.
Nevertheless, Dr. Greeley said, “this is a promising strategy that could be part of a broad injury prevention initiative.”
Neither study noted any external funding. Dr. Byrne is a member of the Moms Demand Action Gainesville Chapter, which donated the firearm locks for the project. Dr. Breuner, Dr. Greeley, and Dr. Farrington have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When pediatricians and other pediatric providers are given training and resource materials, levels of firearm screenings and anticipatory guidance about firearm safety increase significantly, according to two new studies presented at the annual meeting of the American Academy of Pediatrics.
“With the rise in firearm sales and injuries during the COVID-19 pandemic, it is more important than ever that pediatricians address the firearm epidemic,” said Alexandra Byrne, MD, a pediatric resident at the University of Florida in Gainesville, who presented one of the studies.
There were 4.3 million more firearms purchased from March through July 2020 than expected, a recent study estimates, and 4,075 more firearm injuries than expected from April through July 2020.
In states with more excess purchases, firearm injuries related to domestic violence increased in April (rate ratio, 2.60; 95% CI, 1.32-5.93) and May (RR, 1.79; 95% CI, 1.19-2.91) 2020. However, excess gun purchases had no effect on rates of firearm violence outside the home.
In addition to the link between firearms in the home and domestic violence, they are also linked to a three- to fourfold greater risk for teen suicide, and both depression and suicidal thoughts have risen in teens during the pandemic.
“The data are pretty clear that if you have an unlocked, loaded weapon in your home, and you have a kid who’s depressed or anxious or dysregulated or doing maladaptive things for the pandemic, they’re much more likely to inadvertently take their own or someone else’s life by grabbing [a gun],” said Cora Breuner, MD, MPH, professor of pediatrics at Seattle Children’s Hospital.
However, there is no difference in gun ownership or gun-safety measures between homes with and without at-risk children, previous research shows.
Training, guidance, and locks
Previous research has also shown that there has been a reluctance by pediatricians to conduct firearm screenings and counsel parents about gun safety in the home.
For their two-step program, Dr. Byrne’s team used a plan-do-study-act approach. They started by providing training on firearm safety, evidence-based recommendations for firearm screening, and anticipatory guidance regarding safe firearm storage to members of the general pediatrics division at the University of Florida. And they supplied clinics with free firearm locks.
Next they supplied clinics with posters and educational cards from the Be SMART campaign, an initiative of the Everytown for Gun Safety Support Fund, which provides materials for anyone, including physicians, to use.
During their study, the researchers sent three anonymous six-question online surveys – at baseline and 3 to 4 months after each of the two steps – to pediatric residents, physician assistants, advanced practice registered nurses, and attendings to assess the project. There were 52 responses to the first survey, for a response rate of 58.4%, 42 responses to the second survey, for a response rate of 47.2%, and 23 responses to the third survey, for a rate of response 25.8%.
The program nearly doubled screenings during well-child visits and dramatically increased the proportion of families who received a firearm lock when they told providers they had a firearm at home.
Previous research has shown “a significant increase in safe firearm storage when firearm locks were provided to families in clinic compared to verbal counseling alone,” Dr. Byrne said. “We know that safe firearm storage reduces injuries. Roughly one in three children in the United States lives in a home with a firearm. Individuals with a firearm are at two times the risk of homicide and three to four times the risk of suicide, so it is essential we further study how pediatricians can be most effective when it comes to firearm counseling.”
The difference in lock distribution as a result of the program is a “tremendous increase,” said Christopher S. Greeley, MD, MS, chief of the division of public health pediatrics at Texas Children’s Hospital and professor of pediatrics at Baylor College of Medicine in Houston, who was not involved in the research.
“Locks could go a long way to minimizing the risk,” he said in an interview, adding that nearly half of all teen suicide deaths that occurred over a decade in Houston involved a firearm.
Adding a social-history component
A program to increase firearm screening was also presented at the AAP conference.
After random review of medical records from 30 patients admitted to the hospital documented zero firearm screenings, Marjorie Farrington, MD, and Samantha Gunkelman, MD, from Akron Children’s Hospital in Ohio, implemented a program that they hope will increase firearm screenings during inpatient admissions to at least 50%.
They started their ongoing program in April 2020 by adding a social-history component to the history and physical (H&P) exam template and educating residents on how to screen and included guidance on safe firearm storage.
They also had physicians with firearm expertise give gun-safety lectures, and they plan to involve the Family Resource Center at their hospital in the creation of resources that can be incorporated into discharge instructions.
From April 2020 to June 2021, after the addition to the H&P template, 63% of the 5196 patients admitted to the hospital underwent a firearm screening. Of the 25% of patients who reported guns at home, 3% were not storing their firearms safely.
The pair used the “Store It Safe” Physician Handout provided by the Ohio chapter of the AAP.
Many pediatricians and pediatric trainees are not comfortable counseling on firearm safety, often a result of inadequate training on the topic.
The BulletPoints Project — developed by the Violence Prevention Research Program at the University of California, Davis — can also help physicians talk to patients about guns.
“Many pediatricians and pediatric trainees are not comfortable counseling on firearm safety, often a result of inadequate training on the topic,” Dr. Byrne said in an interview. “Additionally, it is a challenging topic that can often be met with resistance from patients and families. Lack of time during visits is also a huge barrier.”
Lack of training is an obstacle to greater firearm screenings, Dr. Greeley agreed, as are the feeling that guidance simply won’t make a difference and concerns about political pressure and divineness. The lack of research on firearm injuries and the impact of firearm screenings and anticipatory guidance is a challenge, he added, although that is starting to change.
Pediatricians need education on how to make a difference when it comes to firearm safety, and should follow AAP guidelines, Dr. Greeley said.
Counseling on firearm safety is in the same category as immunizations, seatbelts, substance use, helmets, and other public-health issues that are important to address at visits, regardless of how difficult it might be, Dr. Breuner told this news organization.
“It is our mission, as pediatricians, to provide every ounce of prevention in our well-child and anticipatory guidance visits,” she said. “It’s our job, so we shouldn’t shy away from it even though it’s hard.”
Doctors are more comfortable discussing firearm safety if they are firearm owners, previous research has shown, so she advises pediatricians who feel unqualified to discuss firearms to seek guidance from their peers on how to approach screenings and anticipatory guidance, she noted.
The firearm study being done in an academic center gives me great pause. The populations are often very different than private practice.
Both of these studies were conducted at single institutions and might not reflect what would work in private clinics.
“The firearm study being done in an academic center gives me great pause,” Dr. Greeley said. “The populations are often very different than private practice. I think that there is still a lot that remains unknown about decreasing household firearm injury and death.”
And the degree to which findings from these two gun-safety programs can be generalized to other academic centers or children’s hospitals is unclear.
“There are states where, I suspect, firearm screening is much more common. Some states have very pro-firearm cultures and others are anti-firearm,” Dr. Greeley said. “There are also likely differences within states,” particularly between urban and rural regions.
“Firearms are often a very personal issue for families, and pediatricians in ‘pro-firearm’ communities may have greater resistance to working on this,” he pointed out.
Nevertheless, Dr. Greeley said, “this is a promising strategy that could be part of a broad injury prevention initiative.”
Neither study noted any external funding. Dr. Byrne is a member of the Moms Demand Action Gainesville Chapter, which donated the firearm locks for the project. Dr. Breuner, Dr. Greeley, and Dr. Farrington have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAP 2021
You’ve been uneasy about the mother’s boyfriend: This may be why
The first patient of the afternoon is a 4-month-old in for his health maintenance visit. You’ve known his 20-year-old mother since she was a toddler. This infant has a 2-year-old sister. Also in the exam room is a young man you don’t recognize whom the mother introduces as Jason, her new boyfriend. He never makes eye contact and despite your best efforts you can’t get him to engage.
At the child’s next visit you are relieved to see the 6-month-old is alive and well and learn that your former patient and her two children have moved back in with her parents and Jason is no longer in the picture.
You don’t have to have been doing pediatrics very long to have learned that a “family” that includes an infant and a young adult male who is probably not the father is an environment in which the infant’s health and well-being is at significant risk. It is a situation in which child abuse even to the point of infanticide should be waving a red flag in your face.
Infanticide occurs in many animal species including our own. As abhorrent we may find the act, it occurs often enough to be, if not normal, at least not unexpected in certain circumstances. Theories abound as to what advantage the act of infanticide might convey to the success of a species. However, little if anything is known about any possible mechanisms that would allow it to occur.
Recently, a professor of molecular and cellular biology at Harvard University discovered a specific set of neurons in the mouse brain that controls aggressive behavior toward infants (Biological triggers for infant abuse, by Juan Siliezar, The Harvard Gazette, Sept 27, 2021). This same set of neurons also appears to trigger avoidance and neglect behaviors as well.
Research in other animal species has found that these antiparental behaviors occur in both virgins and sexually mature males who are strangers to the group. Interestingly, the behaviors switch off once individuals have their own offspring or have had the opportunity to familiarize themselves with infants. Not surprisingly, other studies have found that in some species environmental stress such as food shortage or threats of predation have triggered females to attack or ignore their offspring.
I think it is safe to assume a similar collection of neurons controlling aggressive behavior also exists in humans. One can imagine some well-read defense attorney dredging up this study and claiming that because his client had not yet fathered a child of his own that it was his nervous system’s normal response that made him toss his girlfriend’s baby against the wall.
The lead author of the study intends to study this collection of neurons in more depth to discover more about the process. It is conceivable that with more information her initial findings may help in the development of treatment and specific prevention strategies. Until that happens, we must rely on our intuition and keep our antennae tuned and alert for high-risk scenarios like the one I described at the opening of this letter.
We are left with leaning heavily on our community social work networks to keep close tabs on these high-risk families, offering both financial and emotional support. Parenting classes may be helpful, but some of this research leads me to suspect that immersing these young parents-to-be in hands-on child care situations might provide the best protection we can offer.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
The first patient of the afternoon is a 4-month-old in for his health maintenance visit. You’ve known his 20-year-old mother since she was a toddler. This infant has a 2-year-old sister. Also in the exam room is a young man you don’t recognize whom the mother introduces as Jason, her new boyfriend. He never makes eye contact and despite your best efforts you can’t get him to engage.
At the child’s next visit you are relieved to see the 6-month-old is alive and well and learn that your former patient and her two children have moved back in with her parents and Jason is no longer in the picture.
You don’t have to have been doing pediatrics very long to have learned that a “family” that includes an infant and a young adult male who is probably not the father is an environment in which the infant’s health and well-being is at significant risk. It is a situation in which child abuse even to the point of infanticide should be waving a red flag in your face.
Infanticide occurs in many animal species including our own. As abhorrent we may find the act, it occurs often enough to be, if not normal, at least not unexpected in certain circumstances. Theories abound as to what advantage the act of infanticide might convey to the success of a species. However, little if anything is known about any possible mechanisms that would allow it to occur.
Recently, a professor of molecular and cellular biology at Harvard University discovered a specific set of neurons in the mouse brain that controls aggressive behavior toward infants (Biological triggers for infant abuse, by Juan Siliezar, The Harvard Gazette, Sept 27, 2021). This same set of neurons also appears to trigger avoidance and neglect behaviors as well.
Research in other animal species has found that these antiparental behaviors occur in both virgins and sexually mature males who are strangers to the group. Interestingly, the behaviors switch off once individuals have their own offspring or have had the opportunity to familiarize themselves with infants. Not surprisingly, other studies have found that in some species environmental stress such as food shortage or threats of predation have triggered females to attack or ignore their offspring.
I think it is safe to assume a similar collection of neurons controlling aggressive behavior also exists in humans. One can imagine some well-read defense attorney dredging up this study and claiming that because his client had not yet fathered a child of his own that it was his nervous system’s normal response that made him toss his girlfriend’s baby against the wall.
The lead author of the study intends to study this collection of neurons in more depth to discover more about the process. It is conceivable that with more information her initial findings may help in the development of treatment and specific prevention strategies. Until that happens, we must rely on our intuition and keep our antennae tuned and alert for high-risk scenarios like the one I described at the opening of this letter.
We are left with leaning heavily on our community social work networks to keep close tabs on these high-risk families, offering both financial and emotional support. Parenting classes may be helpful, but some of this research leads me to suspect that immersing these young parents-to-be in hands-on child care situations might provide the best protection we can offer.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
The first patient of the afternoon is a 4-month-old in for his health maintenance visit. You’ve known his 20-year-old mother since she was a toddler. This infant has a 2-year-old sister. Also in the exam room is a young man you don’t recognize whom the mother introduces as Jason, her new boyfriend. He never makes eye contact and despite your best efforts you can’t get him to engage.
At the child’s next visit you are relieved to see the 6-month-old is alive and well and learn that your former patient and her two children have moved back in with her parents and Jason is no longer in the picture.
You don’t have to have been doing pediatrics very long to have learned that a “family” that includes an infant and a young adult male who is probably not the father is an environment in which the infant’s health and well-being is at significant risk. It is a situation in which child abuse even to the point of infanticide should be waving a red flag in your face.
Infanticide occurs in many animal species including our own. As abhorrent we may find the act, it occurs often enough to be, if not normal, at least not unexpected in certain circumstances. Theories abound as to what advantage the act of infanticide might convey to the success of a species. However, little if anything is known about any possible mechanisms that would allow it to occur.
Recently, a professor of molecular and cellular biology at Harvard University discovered a specific set of neurons in the mouse brain that controls aggressive behavior toward infants (Biological triggers for infant abuse, by Juan Siliezar, The Harvard Gazette, Sept 27, 2021). This same set of neurons also appears to trigger avoidance and neglect behaviors as well.
Research in other animal species has found that these antiparental behaviors occur in both virgins and sexually mature males who are strangers to the group. Interestingly, the behaviors switch off once individuals have their own offspring or have had the opportunity to familiarize themselves with infants. Not surprisingly, other studies have found that in some species environmental stress such as food shortage or threats of predation have triggered females to attack or ignore their offspring.
I think it is safe to assume a similar collection of neurons controlling aggressive behavior also exists in humans. One can imagine some well-read defense attorney dredging up this study and claiming that because his client had not yet fathered a child of his own that it was his nervous system’s normal response that made him toss his girlfriend’s baby against the wall.
The lead author of the study intends to study this collection of neurons in more depth to discover more about the process. It is conceivable that with more information her initial findings may help in the development of treatment and specific prevention strategies. Until that happens, we must rely on our intuition and keep our antennae tuned and alert for high-risk scenarios like the one I described at the opening of this letter.
We are left with leaning heavily on our community social work networks to keep close tabs on these high-risk families, offering both financial and emotional support. Parenting classes may be helpful, but some of this research leads me to suspect that immersing these young parents-to-be in hands-on child care situations might provide the best protection we can offer.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Synthetic chemical in consumer products linked to early death, study says
Daily exposure to phthalates, which are synthetic chemicals founds in many consumer products, may lead to hundreds of thousands of early deaths each year among older adults in the United States, according to a new study published Oct. 12, 2021, in the peer-reviewed journal Environmental Pollution.
The chemicals are found in hundreds of types of products, including children’s toys, food storage containers, makeup, perfume, and shampoo. In the study, those with the highest levels of phthalates had a greater risk of death from any cause, especially heart disease.
“This study adds to the growing database on the impact of plastics on the human body and bolsters public health and business cases for reducing or eliminating the use of plastics,” Leonardo Trasande, MD, the lead author and a professor of environmental medicine and population health at New York University Langone Health, told CNN.
Dr. Trasande and colleagues measured the urine concentration of phthalates in more than 5,000 adults aged 55-64 and compared the levels with the risk of early death over an average of 10 years. The research team controlled for preexisting heart diseases, diabetes, cancer, poor eating habits, physical activity, body mass, and other known hormone disruptors such as bisphenol A, or BPA, an industrial chemical that’s been used since the 1950s to make certain plastics and resins, according to the Mayo Clinic
The research team found that phthalates could contribute to 91,000-107,000 premature deaths per year in the United States. These early deaths could cost the nation $40 billion to $47 billion each year in lost economic productivity.
Phthalates interrupt the body’s endocrine system and hormone production. Previous studies have found that the chemicals are linked with developmental, reproductive, and immune system problems, according to NYU Langone Health. They’ve also been linked with asthma, childhood obesity, heart issues, and cancer.
“These chemicals have a rap sheet,” Dr. Trasande told CNN. “And the fact of the matter is that when you look at the entire body of evidence, it provides a haunting pattern of concern.”
Phthalates are often called “everywhere chemicals” because they are so common, CNN reported. Also called “plasticizers,” they are added to products to make them more durable, including PVC plumbing, vinyl flooring, medical tubing, garden hoses, food packaging, detergents, clothing, furniture, and automotive materials.
People are often exposed when they breathe contaminated air or consume food that comes into contact with the chemical, according to the Centers for Disease Control and Prevention. Children may be exposed by touching plastic items and putting their hands in their mouth.
Dr. Trasande told CNN that it’s possible to lessen exposure to phthalates and other endocrine disruptors such as BPA by using unscented lotions, laundry detergents, and cleaning supplies, as well as substituting glass, stainless steel, ceramic, and wood for plastic food storage.
“First, avoid plastics as much as you can. Never put plastic containers in the microwave or dishwasher, where the heat can break down the linings so they might be absorbed more readily,” he said. “In addition, cooking at home and reducing your use of processed foods can reduce the levels of the chemical exposures you come in contact with.”
A version of this article first appeared on WebMD.com.
Daily exposure to phthalates, which are synthetic chemicals founds in many consumer products, may lead to hundreds of thousands of early deaths each year among older adults in the United States, according to a new study published Oct. 12, 2021, in the peer-reviewed journal Environmental Pollution.
The chemicals are found in hundreds of types of products, including children’s toys, food storage containers, makeup, perfume, and shampoo. In the study, those with the highest levels of phthalates had a greater risk of death from any cause, especially heart disease.
“This study adds to the growing database on the impact of plastics on the human body and bolsters public health and business cases for reducing or eliminating the use of plastics,” Leonardo Trasande, MD, the lead author and a professor of environmental medicine and population health at New York University Langone Health, told CNN.
Dr. Trasande and colleagues measured the urine concentration of phthalates in more than 5,000 adults aged 55-64 and compared the levels with the risk of early death over an average of 10 years. The research team controlled for preexisting heart diseases, diabetes, cancer, poor eating habits, physical activity, body mass, and other known hormone disruptors such as bisphenol A, or BPA, an industrial chemical that’s been used since the 1950s to make certain plastics and resins, according to the Mayo Clinic
The research team found that phthalates could contribute to 91,000-107,000 premature deaths per year in the United States. These early deaths could cost the nation $40 billion to $47 billion each year in lost economic productivity.
Phthalates interrupt the body’s endocrine system and hormone production. Previous studies have found that the chemicals are linked with developmental, reproductive, and immune system problems, according to NYU Langone Health. They’ve also been linked with asthma, childhood obesity, heart issues, and cancer.
“These chemicals have a rap sheet,” Dr. Trasande told CNN. “And the fact of the matter is that when you look at the entire body of evidence, it provides a haunting pattern of concern.”
Phthalates are often called “everywhere chemicals” because they are so common, CNN reported. Also called “plasticizers,” they are added to products to make them more durable, including PVC plumbing, vinyl flooring, medical tubing, garden hoses, food packaging, detergents, clothing, furniture, and automotive materials.
People are often exposed when they breathe contaminated air or consume food that comes into contact with the chemical, according to the Centers for Disease Control and Prevention. Children may be exposed by touching plastic items and putting their hands in their mouth.
Dr. Trasande told CNN that it’s possible to lessen exposure to phthalates and other endocrine disruptors such as BPA by using unscented lotions, laundry detergents, and cleaning supplies, as well as substituting glass, stainless steel, ceramic, and wood for plastic food storage.
“First, avoid plastics as much as you can. Never put plastic containers in the microwave or dishwasher, where the heat can break down the linings so they might be absorbed more readily,” he said. “In addition, cooking at home and reducing your use of processed foods can reduce the levels of the chemical exposures you come in contact with.”
A version of this article first appeared on WebMD.com.
Daily exposure to phthalates, which are synthetic chemicals founds in many consumer products, may lead to hundreds of thousands of early deaths each year among older adults in the United States, according to a new study published Oct. 12, 2021, in the peer-reviewed journal Environmental Pollution.
The chemicals are found in hundreds of types of products, including children’s toys, food storage containers, makeup, perfume, and shampoo. In the study, those with the highest levels of phthalates had a greater risk of death from any cause, especially heart disease.
“This study adds to the growing database on the impact of plastics on the human body and bolsters public health and business cases for reducing or eliminating the use of plastics,” Leonardo Trasande, MD, the lead author and a professor of environmental medicine and population health at New York University Langone Health, told CNN.
Dr. Trasande and colleagues measured the urine concentration of phthalates in more than 5,000 adults aged 55-64 and compared the levels with the risk of early death over an average of 10 years. The research team controlled for preexisting heart diseases, diabetes, cancer, poor eating habits, physical activity, body mass, and other known hormone disruptors such as bisphenol A, or BPA, an industrial chemical that’s been used since the 1950s to make certain plastics and resins, according to the Mayo Clinic
The research team found that phthalates could contribute to 91,000-107,000 premature deaths per year in the United States. These early deaths could cost the nation $40 billion to $47 billion each year in lost economic productivity.
Phthalates interrupt the body’s endocrine system and hormone production. Previous studies have found that the chemicals are linked with developmental, reproductive, and immune system problems, according to NYU Langone Health. They’ve also been linked with asthma, childhood obesity, heart issues, and cancer.
“These chemicals have a rap sheet,” Dr. Trasande told CNN. “And the fact of the matter is that when you look at the entire body of evidence, it provides a haunting pattern of concern.”
Phthalates are often called “everywhere chemicals” because they are so common, CNN reported. Also called “plasticizers,” they are added to products to make them more durable, including PVC plumbing, vinyl flooring, medical tubing, garden hoses, food packaging, detergents, clothing, furniture, and automotive materials.
People are often exposed when they breathe contaminated air or consume food that comes into contact with the chemical, according to the Centers for Disease Control and Prevention. Children may be exposed by touching plastic items and putting their hands in their mouth.
Dr. Trasande told CNN that it’s possible to lessen exposure to phthalates and other endocrine disruptors such as BPA by using unscented lotions, laundry detergents, and cleaning supplies, as well as substituting glass, stainless steel, ceramic, and wood for plastic food storage.
“First, avoid plastics as much as you can. Never put plastic containers in the microwave or dishwasher, where the heat can break down the linings so they might be absorbed more readily,” he said. “In addition, cooking at home and reducing your use of processed foods can reduce the levels of the chemical exposures you come in contact with.”
A version of this article first appeared on WebMD.com.
Children and COVID-19: U.S. adds latest million cases in record time
The United States just passed the 6-million mark in COVID-19 cases among children, with the last million cases taking less time to record than any of the first five, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.

The five-millionth case was reported during the week of Aug. 27 to Sept. 2, and case number 6 million came during the week of Oct. 1-7, just 5 weeks later, compared with the 6 weeks it took to go from 1 million to 2 million last November and December, the AAP and CHA said in their weekly COVID-19 report.
New cases continued to drop, however, and that weekly count was down by 14.6% from the previous week and by 41.1% from the peak of almost 252,000 reached in early September, the two groups said while also noting limitations to the data, such as three states (Alabama, Nebraska, and Texas) that are no longer updating their COVID-19 dashboards.
Other metrics show similar drops in recent weeks. Among children aged 0-11 years, emergency department visits involving a COVID-19 diagnosis dropped from 4.1% of all ED visits in late August to 1.4% of ED visits on Oct. 6. ED visits with a COVID-19 diagnosis fell from a peak of 8.5% on Aug. 22 to 1.5% on Oct. 6 for 12- to 15-year-olds and from 8.5% to 1.5% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
The rate of new hospital admissions for children aged 0-17 years was down to 0.26 per 100,000 population on Oct. 9 after reaching 0.51 per 100,000 on Sept. 4. Hospitalizations in children totaled just over 64,000 from Aug. 1, 2020, to Oct. 9, 2021, which is just over 2% of all COVID-19–related admissions over that time period, the CDC said on its COVID Data Tracker.
That pattern, unfortunately, also applies to vaccinations. “The number of children receiving their first COVID-19 vaccine this week [Sept. 30 to Oct. 6], about 156,000, was the lowest number since vaccines were available,” the AAP said in a separate report on vaccination trends, adding that “the number of children receiving their first dose has steadily declined from 8 weeks ago when 586,000 children received their initial dose the week ending Aug. 11.”
The United States just passed the 6-million mark in COVID-19 cases among children, with the last million cases taking less time to record than any of the first five, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.

The five-millionth case was reported during the week of Aug. 27 to Sept. 2, and case number 6 million came during the week of Oct. 1-7, just 5 weeks later, compared with the 6 weeks it took to go from 1 million to 2 million last November and December, the AAP and CHA said in their weekly COVID-19 report.
New cases continued to drop, however, and that weekly count was down by 14.6% from the previous week and by 41.1% from the peak of almost 252,000 reached in early September, the two groups said while also noting limitations to the data, such as three states (Alabama, Nebraska, and Texas) that are no longer updating their COVID-19 dashboards.
Other metrics show similar drops in recent weeks. Among children aged 0-11 years, emergency department visits involving a COVID-19 diagnosis dropped from 4.1% of all ED visits in late August to 1.4% of ED visits on Oct. 6. ED visits with a COVID-19 diagnosis fell from a peak of 8.5% on Aug. 22 to 1.5% on Oct. 6 for 12- to 15-year-olds and from 8.5% to 1.5% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
The rate of new hospital admissions for children aged 0-17 years was down to 0.26 per 100,000 population on Oct. 9 after reaching 0.51 per 100,000 on Sept. 4. Hospitalizations in children totaled just over 64,000 from Aug. 1, 2020, to Oct. 9, 2021, which is just over 2% of all COVID-19–related admissions over that time period, the CDC said on its COVID Data Tracker.
That pattern, unfortunately, also applies to vaccinations. “The number of children receiving their first COVID-19 vaccine this week [Sept. 30 to Oct. 6], about 156,000, was the lowest number since vaccines were available,” the AAP said in a separate report on vaccination trends, adding that “the number of children receiving their first dose has steadily declined from 8 weeks ago when 586,000 children received their initial dose the week ending Aug. 11.”
The United States just passed the 6-million mark in COVID-19 cases among children, with the last million cases taking less time to record than any of the first five, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.

The five-millionth case was reported during the week of Aug. 27 to Sept. 2, and case number 6 million came during the week of Oct. 1-7, just 5 weeks later, compared with the 6 weeks it took to go from 1 million to 2 million last November and December, the AAP and CHA said in their weekly COVID-19 report.
New cases continued to drop, however, and that weekly count was down by 14.6% from the previous week and by 41.1% from the peak of almost 252,000 reached in early September, the two groups said while also noting limitations to the data, such as three states (Alabama, Nebraska, and Texas) that are no longer updating their COVID-19 dashboards.
Other metrics show similar drops in recent weeks. Among children aged 0-11 years, emergency department visits involving a COVID-19 diagnosis dropped from 4.1% of all ED visits in late August to 1.4% of ED visits on Oct. 6. ED visits with a COVID-19 diagnosis fell from a peak of 8.5% on Aug. 22 to 1.5% on Oct. 6 for 12- to 15-year-olds and from 8.5% to 1.5% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
The rate of new hospital admissions for children aged 0-17 years was down to 0.26 per 100,000 population on Oct. 9 after reaching 0.51 per 100,000 on Sept. 4. Hospitalizations in children totaled just over 64,000 from Aug. 1, 2020, to Oct. 9, 2021, which is just over 2% of all COVID-19–related admissions over that time period, the CDC said on its COVID Data Tracker.
That pattern, unfortunately, also applies to vaccinations. “The number of children receiving their first COVID-19 vaccine this week [Sept. 30 to Oct. 6], about 156,000, was the lowest number since vaccines were available,” the AAP said in a separate report on vaccination trends, adding that “the number of children receiving their first dose has steadily declined from 8 weeks ago when 586,000 children received their initial dose the week ending Aug. 11.”

