User login
News and Views that Matter to Pediatricians
The leading independent newspaper covering news and commentary in pediatrics.
Genetic tests prompt therapy adjustments in children with epilepsy
Physicians at a Boston hospital adjusted medical management for nearly three-quarters of patients with infantile- or childhood-onset epilepsy who were diagnosed with genetic epilepsy, researchers reported at the annual meeting of the American Epilepsy Society. The findings provide new insight into the usefulness of genetic tests in children with epilepsy of unknown cause.
“. Genetic testing should be included as part of the standard evaluation of individuals with unexplained pediatric epilepsy as a means of achieving diagnostic precision and informing clinical management,” study lead author Isabel Haviland, MD, a neurologist with Boston Children’s Hospital/Harvard Medical School, said in an interview.
According to Dr. Haviland, the causes of epilepsy are unexplained in an estimated two-thirds of pediatric epilepsy cases. “Increasingly, when genetic testing is available, previously unexplained cases of pediatric epilepsy are being found to have single-gene etiologies,” she said. “Though a genetic diagnosis in this population has implications for medical care, the direct impact on medical management in a clinical setting has not been measured. We aimed to describe the impact of genetic diagnosis on medical management in a cohort of individuals with pediatric epilepsy.”
Researchers tracked 602 patients at Boston Children’s Hospital who received next-generation gene sequencing testing from 2012 to 2019. Of those, Dr. Haviland said, 152 (25%) had a positive result that indicated genetic epilepsy (46% female, median age of onset = 6 months [2-15 months]). These patients were included in the study.
“We documented an impact on medical management in nearly three-fourths of participants (72%),” Dr. Haviland said. “A genetic diagnosis affected at least one of four categories of medical management, including care coordination (48%), treatment (45%), counseling about a change in prognosis (28%), and change in diagnosis for a few individuals who had a prior established diagnosis (1%).”
As examples, she mentioned three cases:
- Testing revealed that a subject has a disease-causing genetic variant in a gene called PRRT2. “This gene is involved in the release of neurotransmitters in the brain,” Dr. Haviland said. “Thanks to his diagnosis, he was treated with the antiseizure medication oxcarbazepine, which is often effective for epilepsy caused by variants in this gene. He had excellent response to the medication and later became seizure free.”
- A subject had a variation in the SCN1A gene that causes types of epilepsy. “At the time of his diagnosis, there was a trial for a medication called fenfluramine being offered for individuals with SCN1A variants, and his family elected to participate,” she said. “This medication was later approved by the [Food and Drug Administration] for SCN1A-related epilepsy.”
- Testing identified disease-causing variant in the GRIN2A gene in another subject. “This gene is involved in brain cell communication,” Dr. Haviland said. “This individual was treated with memantine, which acts on the specific biological pathway affected by the gene. This treatment would not have been considered without the genetic diagnosis as it is currently only approved for Alzheimer’s disease.”
In addition, Dr. Haviland said, researchers found that “there was impact on medical management both in those with earlier age of epilepsy onset (under 2 years) and those with later age of onset, as well as both in those with developmental disorders (such as autism spectrum disorder, intellectual disability and developmental delay) and those with normal development.
As for the cost of genetic tests, Dr. Haviland pointed to a 2019 study that she said estimated epilepsy panel testing runs from $1,500 to $7,500, and the whole exome sequencing from $4,500 to $7,000. “Insurers sometimes cover testing, but not always,” she said. “In some cases, insurance will only cover testing if it is documented that results will directly alter medical management, which highlights the importance of our findings.”
No study funding was reported. Dr. Haviland and several other authors report no disclosures. One author reports consulting fees from Takeda, Zogenix, Marinus, and FOXG1 Research Foundation. Another author reports research support from the International Foundation for CDKL5 Research.
Physicians at a Boston hospital adjusted medical management for nearly three-quarters of patients with infantile- or childhood-onset epilepsy who were diagnosed with genetic epilepsy, researchers reported at the annual meeting of the American Epilepsy Society. The findings provide new insight into the usefulness of genetic tests in children with epilepsy of unknown cause.
“. Genetic testing should be included as part of the standard evaluation of individuals with unexplained pediatric epilepsy as a means of achieving diagnostic precision and informing clinical management,” study lead author Isabel Haviland, MD, a neurologist with Boston Children’s Hospital/Harvard Medical School, said in an interview.
According to Dr. Haviland, the causes of epilepsy are unexplained in an estimated two-thirds of pediatric epilepsy cases. “Increasingly, when genetic testing is available, previously unexplained cases of pediatric epilepsy are being found to have single-gene etiologies,” she said. “Though a genetic diagnosis in this population has implications for medical care, the direct impact on medical management in a clinical setting has not been measured. We aimed to describe the impact of genetic diagnosis on medical management in a cohort of individuals with pediatric epilepsy.”
Researchers tracked 602 patients at Boston Children’s Hospital who received next-generation gene sequencing testing from 2012 to 2019. Of those, Dr. Haviland said, 152 (25%) had a positive result that indicated genetic epilepsy (46% female, median age of onset = 6 months [2-15 months]). These patients were included in the study.
“We documented an impact on medical management in nearly three-fourths of participants (72%),” Dr. Haviland said. “A genetic diagnosis affected at least one of four categories of medical management, including care coordination (48%), treatment (45%), counseling about a change in prognosis (28%), and change in diagnosis for a few individuals who had a prior established diagnosis (1%).”
As examples, she mentioned three cases:
- Testing revealed that a subject has a disease-causing genetic variant in a gene called PRRT2. “This gene is involved in the release of neurotransmitters in the brain,” Dr. Haviland said. “Thanks to his diagnosis, he was treated with the antiseizure medication oxcarbazepine, which is often effective for epilepsy caused by variants in this gene. He had excellent response to the medication and later became seizure free.”
- A subject had a variation in the SCN1A gene that causes types of epilepsy. “At the time of his diagnosis, there was a trial for a medication called fenfluramine being offered for individuals with SCN1A variants, and his family elected to participate,” she said. “This medication was later approved by the [Food and Drug Administration] for SCN1A-related epilepsy.”
- Testing identified disease-causing variant in the GRIN2A gene in another subject. “This gene is involved in brain cell communication,” Dr. Haviland said. “This individual was treated with memantine, which acts on the specific biological pathway affected by the gene. This treatment would not have been considered without the genetic diagnosis as it is currently only approved for Alzheimer’s disease.”
In addition, Dr. Haviland said, researchers found that “there was impact on medical management both in those with earlier age of epilepsy onset (under 2 years) and those with later age of onset, as well as both in those with developmental disorders (such as autism spectrum disorder, intellectual disability and developmental delay) and those with normal development.
As for the cost of genetic tests, Dr. Haviland pointed to a 2019 study that she said estimated epilepsy panel testing runs from $1,500 to $7,500, and the whole exome sequencing from $4,500 to $7,000. “Insurers sometimes cover testing, but not always,” she said. “In some cases, insurance will only cover testing if it is documented that results will directly alter medical management, which highlights the importance of our findings.”
No study funding was reported. Dr. Haviland and several other authors report no disclosures. One author reports consulting fees from Takeda, Zogenix, Marinus, and FOXG1 Research Foundation. Another author reports research support from the International Foundation for CDKL5 Research.
Physicians at a Boston hospital adjusted medical management for nearly three-quarters of patients with infantile- or childhood-onset epilepsy who were diagnosed with genetic epilepsy, researchers reported at the annual meeting of the American Epilepsy Society. The findings provide new insight into the usefulness of genetic tests in children with epilepsy of unknown cause.
“. Genetic testing should be included as part of the standard evaluation of individuals with unexplained pediatric epilepsy as a means of achieving diagnostic precision and informing clinical management,” study lead author Isabel Haviland, MD, a neurologist with Boston Children’s Hospital/Harvard Medical School, said in an interview.
According to Dr. Haviland, the causes of epilepsy are unexplained in an estimated two-thirds of pediatric epilepsy cases. “Increasingly, when genetic testing is available, previously unexplained cases of pediatric epilepsy are being found to have single-gene etiologies,” she said. “Though a genetic diagnosis in this population has implications for medical care, the direct impact on medical management in a clinical setting has not been measured. We aimed to describe the impact of genetic diagnosis on medical management in a cohort of individuals with pediatric epilepsy.”
Researchers tracked 602 patients at Boston Children’s Hospital who received next-generation gene sequencing testing from 2012 to 2019. Of those, Dr. Haviland said, 152 (25%) had a positive result that indicated genetic epilepsy (46% female, median age of onset = 6 months [2-15 months]). These patients were included in the study.
“We documented an impact on medical management in nearly three-fourths of participants (72%),” Dr. Haviland said. “A genetic diagnosis affected at least one of four categories of medical management, including care coordination (48%), treatment (45%), counseling about a change in prognosis (28%), and change in diagnosis for a few individuals who had a prior established diagnosis (1%).”
As examples, she mentioned three cases:
- Testing revealed that a subject has a disease-causing genetic variant in a gene called PRRT2. “This gene is involved in the release of neurotransmitters in the brain,” Dr. Haviland said. “Thanks to his diagnosis, he was treated with the antiseizure medication oxcarbazepine, which is often effective for epilepsy caused by variants in this gene. He had excellent response to the medication and later became seizure free.”
- A subject had a variation in the SCN1A gene that causes types of epilepsy. “At the time of his diagnosis, there was a trial for a medication called fenfluramine being offered for individuals with SCN1A variants, and his family elected to participate,” she said. “This medication was later approved by the [Food and Drug Administration] for SCN1A-related epilepsy.”
- Testing identified disease-causing variant in the GRIN2A gene in another subject. “This gene is involved in brain cell communication,” Dr. Haviland said. “This individual was treated with memantine, which acts on the specific biological pathway affected by the gene. This treatment would not have been considered without the genetic diagnosis as it is currently only approved for Alzheimer’s disease.”
In addition, Dr. Haviland said, researchers found that “there was impact on medical management both in those with earlier age of epilepsy onset (under 2 years) and those with later age of onset, as well as both in those with developmental disorders (such as autism spectrum disorder, intellectual disability and developmental delay) and those with normal development.
As for the cost of genetic tests, Dr. Haviland pointed to a 2019 study that she said estimated epilepsy panel testing runs from $1,500 to $7,500, and the whole exome sequencing from $4,500 to $7,000. “Insurers sometimes cover testing, but not always,” she said. “In some cases, insurance will only cover testing if it is documented that results will directly alter medical management, which highlights the importance of our findings.”
No study funding was reported. Dr. Haviland and several other authors report no disclosures. One author reports consulting fees from Takeda, Zogenix, Marinus, and FOXG1 Research Foundation. Another author reports research support from the International Foundation for CDKL5 Research.
FROM AES 2021
WPATH draft on gender dysphoria ‘skewed and misses urgent issues’
New draft guidance from the World Professional Association for Transgender Health (WPATH) is raising serious concerns among professionals caring for people with gender dysphoria, prompting claims that WPATH is an organization “captured by activists.”
Experts in adolescent and child psychology, as well as pediatric health, have expressed dismay that the WPATH Standards of Care (SOC) 8 appear to miss some of the most urgent issues in the field of transgender medicine and are considered to express a radical and unreserved leaning towards “gender-affirmation.”
The WPATH SOC 8 document is available for view and comment until Dec. 16 until 11.59 PM EST, after which time revisions will be made and the final version published.
Despite repeated attempts by this news organization to seek clarification on certain aspects of the guidance from members of the WPATH SOC 8 committee, requests were declined “until the guidance is finalized.”
According to the WPATH website, the SOC 8 aims to provide “clinical guidance for health professionals to assist transgender and gender diverse people with safe and effective pathways” to manage their gender dysphoria and potentially transition.
Such pathways may relate to primary care, gynecologic and urologic care, reproductive options, voice and communication therapy, mental health services, and hormonal or surgical treatments, among others.
WPATH adds that it was felt necessary to revise the existing SOC 7 (published in 2012) because of recent “globally unprecedented increase and visibility of transgender and gender-diverse people seeking support and gender-affirming medical treatment.”
Gender-affirming medical treatment means different things at different ages. In the case of kids with gender dysphoria who have not yet entered puberty associated with their birth sex, this might include prescribing so-called “puberty blockers” to delay natural puberty – gonadotrophin-releasing hormone analogs that are licensed for use in precocious puberty in children. Such agents have not been licensed for use in children with gender dysphoria, however, so any use for this purpose is off-label.
Following puberty blockade – or in cases where adolescents have already undergone natural puberty – the next step is to begin cross-sex hormones. So, for a female patient who wants to transition to male (FTM), that would be lifelong testosterone, and for a male who wants to be female (MTF), it involves lifelong estrogen. Again, use of such hormones in transgender individuals is entirely off-label.
Just last month, two of America’s leading experts on transgender medicine, both psychologists – including one who is transgender – told this news organization they were concerned that the quality of the evaluations of youth with gender dysphoria are being stifled by activists who are worried that open discussions will further stigmatize trans individuals.
They subsequently wrote an op-ed on the topic entitled, “The mental health establishment is failing trans kids,” which was finally published in the Washington Post on Nov. 24, after numerous other mainstream U.S. media outlets had rejected it.
New SOC 8 ‘is not evidence based,’ should not be new ‘gold standard’
One expert says the draft SOC 8 lacks balance and does not address certain issues, while paying undue attention to others that detract from real questions facing the field of transgender medicine, both in the United States and around the world.
Julia Mason, MD, is a pediatrician based in Gresham, Oregon, with a special interest in children and adolescents experiencing gender dysphoria. “The SOC 8 shows us that WPATH remains captured by activists,” she asserts.
Dr. Mason questions the integrity of WPATH based on what she has read in the draft SOC 8.
“We need a serious organization to take a sober look at the evidence, and that is why we have established the Society for Evidence-Based Gender Medicine [SEGM],” she noted. “This is what we do – we are looking at all of the evidence.”
Dr. Mason is a clinical advisor to SEGM, an organization set-up to evaluate current interventions and evidence on gender dysphoria.
The pediatrician has particular concerns regarding the child and adolescent chapters in the draft SOC 8. The adolescent chapter states: “Guidelines are meant to provide a gold standard based on the available evidence at this moment of time.”
Dr. Mason disputes this assertion. “This document should not be the new gold standard going forward, primarily because it is not evidence based.”
In an interview, Dr. Mason explained that WPATH say they used the “Delphi consensus process” to determine their recommendations, but “this process is designed for use with a panel of experts when evidence is lacking. I would say they didn’t have a panel of experts. They largely had a panel of activists, with a few experts.”
There is no mention, for example, of England’s National Institute for Health and Care Excellence (NICE) evidence reviews on puberty blockers and cross-sex hormones from earlier this year. These reviews determined that no studies have compared cross-sex hormones or puberty blockers with a control group and all follow-up periods for cross-sex hormones were relatively short.
This disappoints Dr. Mason: “These are significant; they are important documents.”
And much of the evidence quoted comes from the well-known and often-quoted “Dutch-protocol” study of 2011, in which the children studied were much younger at the time of their gender dysphoria, compared with the many adolescents who make up the current surge in presentation at gender clinics worldwide, she adds.
Rapid-onset GD: adolescents presenting late with little history
Dr. Mason also stresses that the SOC 8 does not address the most urgent issues in transgender medicine today, mainly because it does not address rapid-onset gender dysphoria (ROGD): “This is the dilemma of the 21st century; it’s new.”
ROGD – a term first coined in 2018 by researcher Lisa Littman, MD, MPH, now president of the Institute for Comprehensive Gender Dysphoria Research (ICGDR) – refers to the phenomena of adolescents expressing a desire to transition from their birth sex after little or no apparent previous indication.
However, the SOC 8 does make reference to aspects of adolescent development that might impact their decision-making processes around gender identity during teen years. The chapter on adolescents reads: “... adolescence is also often associated with increased risk-taking behaviors. Along with these notable changes ... individuation from parents ... [there is] often a heightened focus on peer relationships, which can be both positive and detrimental.”
The guidance goes on to point out that “it is critical to understand how all of these aspects of development may impact the decision-making for a given young person within their specific cultural context.”
Desistance and detransitioning not adequately addressed
Dr. Mason also says there is little mention “about detransitioning in this SOC [8], and ‘gender dysphoria’ and ‘trans’ are terms that are not defined.”
Likewise, there is no mention of desistance, she highlights, which is when individuals naturally resolve their dysphoria around their birth sex as they grow older.
The most recent published data seen by this news organization relates to a study from March 2021 that showed nearly 88% of boys who struggled with gender identity in childhood (approximate mean age 8 years and follow-up at approximate mean age 20 years) desisted. It reads: “Of the 139 participants, 17 (12.2%) were classified as ‘persisters’ and the remaining 122 (87.8%) were classified as desisters.”
“Most children with gender dysphoria will desist and lose their concept of themselves as being the opposite gender,” Dr. Mason explains. “This is the safest path for a child – desistance.”
“Transition can turn a healthy young person into a lifelong medical patient and has significant health risks,” she emphasizes, stressing that transition has not been shown to decrease the probability of suicide, or attempts at suicide, despite myriad claims saying otherwise.
“Before we were routinely transitioning kids at school, the vast majority of children grew out of their gender dysphoria. This history is not recognized at all in these SOC [8],” she maintains.
Ken Zucker, PhD, CPsych, an author of the study of desistance in boys, says the terms desistence and persistence of gender dysphoria have caused some consternation in certain circles.
An editor of the Archives of Sexual Behavior and professor in the department of psychiatry, University of Toronto, Dr. Zucker has published widely on the topic.
He told this news organization: “The terms persistence and desistance have become verboten among the WPATH cognoscenti. Perhaps the contributors to SOC 8 have come up with alternative descriptors.”
“The term ‘desistance’ is particularly annoying to some of the gender-affirming clinicians, because they don’t believe that desistance is bona fide,” Dr. Zucker points out.
“The desistance resisters are like anti-vaxxers – nothing one can provide as evidence for the efficacy of vaccines is sufficient. There will always be a new objection.”
Other mental health issues, in particular ADHD and autism
It is also widely acknowledged that there is a higher rate of neurodevelopmental and psychiatric diagnoses in individuals with gender dysphoria. For example, one 2020 study found that transgender people were three to six times as likely to be autistic as cisgender people (those whose gender is aligned with their birth sex).
Statement one in the chapter on adolescents in draft WPATH SOC 8 does give a nod to this, pointing out that health professionals working with gender diverse adolescents “should receive training and develop expertise in autism spectrum disorders and other neurodiversity conditions.”
It also notes that in some cases “a more extended assessment process may be useful, such as for youth with more complex presentations (e.g., complicated mental health histories, co-occurring autism spectrum characteristics in particular) and an absence of experienced childhood gender incongruence.”
However, Dr. Mason stresses that underlying mental health issues are central to addressing how to manage a significant number of these patients.
“If a young person has ADHD or autism, they are not ready to make decisions about the rest of their life at age 18. Even a neurotypical young person is still developing their frontal cortex in their early 20s, and it takes longer for those with ADHD or on the autism spectrum.”
She firmly believes that the guidance does not give sufficient consideration to comorbidities in people over the age of 18.
According to their [SOC 8] guidelines, “once someone is 18 they are ready for anything,” says Dr. Mason.
Offering some explanation for the increased prevalence of ADHD and autism in those with gender dysphoria, Dr. Mason notes that children can have “hyperfocus” and those with autism will fixate on a particular area of interest. “If a child is unhappy in their life, and this can be more likely if someone is neuro-atypical, then it is likely that the individual might go online and find this one solution [for example, a transgender identity] that seems to fix everything.”
Perceptions of femininity and masculinity can also be extra challenging for a child with autism, Dr. Mason says. “It is relatively easy for an autistic girl to feel like she should be a boy because the rules of femininity are composed of nonverbal, subtle behaviors that can be difficult to pick up on,” she points out. “An autistic child who isn’t particularly good at nonverbal communication might not pick up on these and thus feel they are not very ‘female.’”
“There’s a whole lot of grass-is-greener-type thinking. Girls think boys have an easier life, and boys think girls have an easier life. I know some detransitioners who have spoken eloquently about realizing their mistake on this,” she adds.
Other parts of the SOC 8 that Dr. Mason disagrees with include the recommendation in the adolescent chapter that 14-year-olds are mature enough to start cross-sex hormones, that is, giving testosterone to a female who wants to transition to male or estrogen to a male who wishes to transition to female. “I think that’s far too young,” she asserts.
And she points out that the document states 17-year-olds are ready for genital reassignment surgery. Again, she believes this is far too young.
“Also, the SOC 8 document does not clarify who is appropriate for surgery. Whenever surgery is discussed, it becomes very vague,” she said.
A version of this article first appeared on Medscape.com.
New draft guidance from the World Professional Association for Transgender Health (WPATH) is raising serious concerns among professionals caring for people with gender dysphoria, prompting claims that WPATH is an organization “captured by activists.”
Experts in adolescent and child psychology, as well as pediatric health, have expressed dismay that the WPATH Standards of Care (SOC) 8 appear to miss some of the most urgent issues in the field of transgender medicine and are considered to express a radical and unreserved leaning towards “gender-affirmation.”
The WPATH SOC 8 document is available for view and comment until Dec. 16 until 11.59 PM EST, after which time revisions will be made and the final version published.
Despite repeated attempts by this news organization to seek clarification on certain aspects of the guidance from members of the WPATH SOC 8 committee, requests were declined “until the guidance is finalized.”
According to the WPATH website, the SOC 8 aims to provide “clinical guidance for health professionals to assist transgender and gender diverse people with safe and effective pathways” to manage their gender dysphoria and potentially transition.
Such pathways may relate to primary care, gynecologic and urologic care, reproductive options, voice and communication therapy, mental health services, and hormonal or surgical treatments, among others.
WPATH adds that it was felt necessary to revise the existing SOC 7 (published in 2012) because of recent “globally unprecedented increase and visibility of transgender and gender-diverse people seeking support and gender-affirming medical treatment.”
Gender-affirming medical treatment means different things at different ages. In the case of kids with gender dysphoria who have not yet entered puberty associated with their birth sex, this might include prescribing so-called “puberty blockers” to delay natural puberty – gonadotrophin-releasing hormone analogs that are licensed for use in precocious puberty in children. Such agents have not been licensed for use in children with gender dysphoria, however, so any use for this purpose is off-label.
Following puberty blockade – or in cases where adolescents have already undergone natural puberty – the next step is to begin cross-sex hormones. So, for a female patient who wants to transition to male (FTM), that would be lifelong testosterone, and for a male who wants to be female (MTF), it involves lifelong estrogen. Again, use of such hormones in transgender individuals is entirely off-label.
Just last month, two of America’s leading experts on transgender medicine, both psychologists – including one who is transgender – told this news organization they were concerned that the quality of the evaluations of youth with gender dysphoria are being stifled by activists who are worried that open discussions will further stigmatize trans individuals.
They subsequently wrote an op-ed on the topic entitled, “The mental health establishment is failing trans kids,” which was finally published in the Washington Post on Nov. 24, after numerous other mainstream U.S. media outlets had rejected it.
New SOC 8 ‘is not evidence based,’ should not be new ‘gold standard’
One expert says the draft SOC 8 lacks balance and does not address certain issues, while paying undue attention to others that detract from real questions facing the field of transgender medicine, both in the United States and around the world.
Julia Mason, MD, is a pediatrician based in Gresham, Oregon, with a special interest in children and adolescents experiencing gender dysphoria. “The SOC 8 shows us that WPATH remains captured by activists,” she asserts.
Dr. Mason questions the integrity of WPATH based on what she has read in the draft SOC 8.
“We need a serious organization to take a sober look at the evidence, and that is why we have established the Society for Evidence-Based Gender Medicine [SEGM],” she noted. “This is what we do – we are looking at all of the evidence.”
Dr. Mason is a clinical advisor to SEGM, an organization set-up to evaluate current interventions and evidence on gender dysphoria.
The pediatrician has particular concerns regarding the child and adolescent chapters in the draft SOC 8. The adolescent chapter states: “Guidelines are meant to provide a gold standard based on the available evidence at this moment of time.”
Dr. Mason disputes this assertion. “This document should not be the new gold standard going forward, primarily because it is not evidence based.”
In an interview, Dr. Mason explained that WPATH say they used the “Delphi consensus process” to determine their recommendations, but “this process is designed for use with a panel of experts when evidence is lacking. I would say they didn’t have a panel of experts. They largely had a panel of activists, with a few experts.”
There is no mention, for example, of England’s National Institute for Health and Care Excellence (NICE) evidence reviews on puberty blockers and cross-sex hormones from earlier this year. These reviews determined that no studies have compared cross-sex hormones or puberty blockers with a control group and all follow-up periods for cross-sex hormones were relatively short.
This disappoints Dr. Mason: “These are significant; they are important documents.”
And much of the evidence quoted comes from the well-known and often-quoted “Dutch-protocol” study of 2011, in which the children studied were much younger at the time of their gender dysphoria, compared with the many adolescents who make up the current surge in presentation at gender clinics worldwide, she adds.
Rapid-onset GD: adolescents presenting late with little history
Dr. Mason also stresses that the SOC 8 does not address the most urgent issues in transgender medicine today, mainly because it does not address rapid-onset gender dysphoria (ROGD): “This is the dilemma of the 21st century; it’s new.”
ROGD – a term first coined in 2018 by researcher Lisa Littman, MD, MPH, now president of the Institute for Comprehensive Gender Dysphoria Research (ICGDR) – refers to the phenomena of adolescents expressing a desire to transition from their birth sex after little or no apparent previous indication.
However, the SOC 8 does make reference to aspects of adolescent development that might impact their decision-making processes around gender identity during teen years. The chapter on adolescents reads: “... adolescence is also often associated with increased risk-taking behaviors. Along with these notable changes ... individuation from parents ... [there is] often a heightened focus on peer relationships, which can be both positive and detrimental.”
The guidance goes on to point out that “it is critical to understand how all of these aspects of development may impact the decision-making for a given young person within their specific cultural context.”
Desistance and detransitioning not adequately addressed
Dr. Mason also says there is little mention “about detransitioning in this SOC [8], and ‘gender dysphoria’ and ‘trans’ are terms that are not defined.”
Likewise, there is no mention of desistance, she highlights, which is when individuals naturally resolve their dysphoria around their birth sex as they grow older.
The most recent published data seen by this news organization relates to a study from March 2021 that showed nearly 88% of boys who struggled with gender identity in childhood (approximate mean age 8 years and follow-up at approximate mean age 20 years) desisted. It reads: “Of the 139 participants, 17 (12.2%) were classified as ‘persisters’ and the remaining 122 (87.8%) were classified as desisters.”
“Most children with gender dysphoria will desist and lose their concept of themselves as being the opposite gender,” Dr. Mason explains. “This is the safest path for a child – desistance.”
“Transition can turn a healthy young person into a lifelong medical patient and has significant health risks,” she emphasizes, stressing that transition has not been shown to decrease the probability of suicide, or attempts at suicide, despite myriad claims saying otherwise.
“Before we were routinely transitioning kids at school, the vast majority of children grew out of their gender dysphoria. This history is not recognized at all in these SOC [8],” she maintains.
Ken Zucker, PhD, CPsych, an author of the study of desistance in boys, says the terms desistence and persistence of gender dysphoria have caused some consternation in certain circles.
An editor of the Archives of Sexual Behavior and professor in the department of psychiatry, University of Toronto, Dr. Zucker has published widely on the topic.
He told this news organization: “The terms persistence and desistance have become verboten among the WPATH cognoscenti. Perhaps the contributors to SOC 8 have come up with alternative descriptors.”
“The term ‘desistance’ is particularly annoying to some of the gender-affirming clinicians, because they don’t believe that desistance is bona fide,” Dr. Zucker points out.
“The desistance resisters are like anti-vaxxers – nothing one can provide as evidence for the efficacy of vaccines is sufficient. There will always be a new objection.”
Other mental health issues, in particular ADHD and autism
It is also widely acknowledged that there is a higher rate of neurodevelopmental and psychiatric diagnoses in individuals with gender dysphoria. For example, one 2020 study found that transgender people were three to six times as likely to be autistic as cisgender people (those whose gender is aligned with their birth sex).
Statement one in the chapter on adolescents in draft WPATH SOC 8 does give a nod to this, pointing out that health professionals working with gender diverse adolescents “should receive training and develop expertise in autism spectrum disorders and other neurodiversity conditions.”
It also notes that in some cases “a more extended assessment process may be useful, such as for youth with more complex presentations (e.g., complicated mental health histories, co-occurring autism spectrum characteristics in particular) and an absence of experienced childhood gender incongruence.”
However, Dr. Mason stresses that underlying mental health issues are central to addressing how to manage a significant number of these patients.
“If a young person has ADHD or autism, they are not ready to make decisions about the rest of their life at age 18. Even a neurotypical young person is still developing their frontal cortex in their early 20s, and it takes longer for those with ADHD or on the autism spectrum.”
She firmly believes that the guidance does not give sufficient consideration to comorbidities in people over the age of 18.
According to their [SOC 8] guidelines, “once someone is 18 they are ready for anything,” says Dr. Mason.
Offering some explanation for the increased prevalence of ADHD and autism in those with gender dysphoria, Dr. Mason notes that children can have “hyperfocus” and those with autism will fixate on a particular area of interest. “If a child is unhappy in their life, and this can be more likely if someone is neuro-atypical, then it is likely that the individual might go online and find this one solution [for example, a transgender identity] that seems to fix everything.”
Perceptions of femininity and masculinity can also be extra challenging for a child with autism, Dr. Mason says. “It is relatively easy for an autistic girl to feel like she should be a boy because the rules of femininity are composed of nonverbal, subtle behaviors that can be difficult to pick up on,” she points out. “An autistic child who isn’t particularly good at nonverbal communication might not pick up on these and thus feel they are not very ‘female.’”
“There’s a whole lot of grass-is-greener-type thinking. Girls think boys have an easier life, and boys think girls have an easier life. I know some detransitioners who have spoken eloquently about realizing their mistake on this,” she adds.
Other parts of the SOC 8 that Dr. Mason disagrees with include the recommendation in the adolescent chapter that 14-year-olds are mature enough to start cross-sex hormones, that is, giving testosterone to a female who wants to transition to male or estrogen to a male who wishes to transition to female. “I think that’s far too young,” she asserts.
And she points out that the document states 17-year-olds are ready for genital reassignment surgery. Again, she believes this is far too young.
“Also, the SOC 8 document does not clarify who is appropriate for surgery. Whenever surgery is discussed, it becomes very vague,” she said.
A version of this article first appeared on Medscape.com.
New draft guidance from the World Professional Association for Transgender Health (WPATH) is raising serious concerns among professionals caring for people with gender dysphoria, prompting claims that WPATH is an organization “captured by activists.”
Experts in adolescent and child psychology, as well as pediatric health, have expressed dismay that the WPATH Standards of Care (SOC) 8 appear to miss some of the most urgent issues in the field of transgender medicine and are considered to express a radical and unreserved leaning towards “gender-affirmation.”
The WPATH SOC 8 document is available for view and comment until Dec. 16 until 11.59 PM EST, after which time revisions will be made and the final version published.
Despite repeated attempts by this news organization to seek clarification on certain aspects of the guidance from members of the WPATH SOC 8 committee, requests were declined “until the guidance is finalized.”
According to the WPATH website, the SOC 8 aims to provide “clinical guidance for health professionals to assist transgender and gender diverse people with safe and effective pathways” to manage their gender dysphoria and potentially transition.
Such pathways may relate to primary care, gynecologic and urologic care, reproductive options, voice and communication therapy, mental health services, and hormonal or surgical treatments, among others.
WPATH adds that it was felt necessary to revise the existing SOC 7 (published in 2012) because of recent “globally unprecedented increase and visibility of transgender and gender-diverse people seeking support and gender-affirming medical treatment.”
Gender-affirming medical treatment means different things at different ages. In the case of kids with gender dysphoria who have not yet entered puberty associated with their birth sex, this might include prescribing so-called “puberty blockers” to delay natural puberty – gonadotrophin-releasing hormone analogs that are licensed for use in precocious puberty in children. Such agents have not been licensed for use in children with gender dysphoria, however, so any use for this purpose is off-label.
Following puberty blockade – or in cases where adolescents have already undergone natural puberty – the next step is to begin cross-sex hormones. So, for a female patient who wants to transition to male (FTM), that would be lifelong testosterone, and for a male who wants to be female (MTF), it involves lifelong estrogen. Again, use of such hormones in transgender individuals is entirely off-label.
Just last month, two of America’s leading experts on transgender medicine, both psychologists – including one who is transgender – told this news organization they were concerned that the quality of the evaluations of youth with gender dysphoria are being stifled by activists who are worried that open discussions will further stigmatize trans individuals.
They subsequently wrote an op-ed on the topic entitled, “The mental health establishment is failing trans kids,” which was finally published in the Washington Post on Nov. 24, after numerous other mainstream U.S. media outlets had rejected it.
New SOC 8 ‘is not evidence based,’ should not be new ‘gold standard’
One expert says the draft SOC 8 lacks balance and does not address certain issues, while paying undue attention to others that detract from real questions facing the field of transgender medicine, both in the United States and around the world.
Julia Mason, MD, is a pediatrician based in Gresham, Oregon, with a special interest in children and adolescents experiencing gender dysphoria. “The SOC 8 shows us that WPATH remains captured by activists,” she asserts.
Dr. Mason questions the integrity of WPATH based on what she has read in the draft SOC 8.
“We need a serious organization to take a sober look at the evidence, and that is why we have established the Society for Evidence-Based Gender Medicine [SEGM],” she noted. “This is what we do – we are looking at all of the evidence.”
Dr. Mason is a clinical advisor to SEGM, an organization set-up to evaluate current interventions and evidence on gender dysphoria.
The pediatrician has particular concerns regarding the child and adolescent chapters in the draft SOC 8. The adolescent chapter states: “Guidelines are meant to provide a gold standard based on the available evidence at this moment of time.”
Dr. Mason disputes this assertion. “This document should not be the new gold standard going forward, primarily because it is not evidence based.”
In an interview, Dr. Mason explained that WPATH say they used the “Delphi consensus process” to determine their recommendations, but “this process is designed for use with a panel of experts when evidence is lacking. I would say they didn’t have a panel of experts. They largely had a panel of activists, with a few experts.”
There is no mention, for example, of England’s National Institute for Health and Care Excellence (NICE) evidence reviews on puberty blockers and cross-sex hormones from earlier this year. These reviews determined that no studies have compared cross-sex hormones or puberty blockers with a control group and all follow-up periods for cross-sex hormones were relatively short.
This disappoints Dr. Mason: “These are significant; they are important documents.”
And much of the evidence quoted comes from the well-known and often-quoted “Dutch-protocol” study of 2011, in which the children studied were much younger at the time of their gender dysphoria, compared with the many adolescents who make up the current surge in presentation at gender clinics worldwide, she adds.
Rapid-onset GD: adolescents presenting late with little history
Dr. Mason also stresses that the SOC 8 does not address the most urgent issues in transgender medicine today, mainly because it does not address rapid-onset gender dysphoria (ROGD): “This is the dilemma of the 21st century; it’s new.”
ROGD – a term first coined in 2018 by researcher Lisa Littman, MD, MPH, now president of the Institute for Comprehensive Gender Dysphoria Research (ICGDR) – refers to the phenomena of adolescents expressing a desire to transition from their birth sex after little or no apparent previous indication.
However, the SOC 8 does make reference to aspects of adolescent development that might impact their decision-making processes around gender identity during teen years. The chapter on adolescents reads: “... adolescence is also often associated with increased risk-taking behaviors. Along with these notable changes ... individuation from parents ... [there is] often a heightened focus on peer relationships, which can be both positive and detrimental.”
The guidance goes on to point out that “it is critical to understand how all of these aspects of development may impact the decision-making for a given young person within their specific cultural context.”
Desistance and detransitioning not adequately addressed
Dr. Mason also says there is little mention “about detransitioning in this SOC [8], and ‘gender dysphoria’ and ‘trans’ are terms that are not defined.”
Likewise, there is no mention of desistance, she highlights, which is when individuals naturally resolve their dysphoria around their birth sex as they grow older.
The most recent published data seen by this news organization relates to a study from March 2021 that showed nearly 88% of boys who struggled with gender identity in childhood (approximate mean age 8 years and follow-up at approximate mean age 20 years) desisted. It reads: “Of the 139 participants, 17 (12.2%) were classified as ‘persisters’ and the remaining 122 (87.8%) were classified as desisters.”
“Most children with gender dysphoria will desist and lose their concept of themselves as being the opposite gender,” Dr. Mason explains. “This is the safest path for a child – desistance.”
“Transition can turn a healthy young person into a lifelong medical patient and has significant health risks,” she emphasizes, stressing that transition has not been shown to decrease the probability of suicide, or attempts at suicide, despite myriad claims saying otherwise.
“Before we were routinely transitioning kids at school, the vast majority of children grew out of their gender dysphoria. This history is not recognized at all in these SOC [8],” she maintains.
Ken Zucker, PhD, CPsych, an author of the study of desistance in boys, says the terms desistence and persistence of gender dysphoria have caused some consternation in certain circles.
An editor of the Archives of Sexual Behavior and professor in the department of psychiatry, University of Toronto, Dr. Zucker has published widely on the topic.
He told this news organization: “The terms persistence and desistance have become verboten among the WPATH cognoscenti. Perhaps the contributors to SOC 8 have come up with alternative descriptors.”
“The term ‘desistance’ is particularly annoying to some of the gender-affirming clinicians, because they don’t believe that desistance is bona fide,” Dr. Zucker points out.
“The desistance resisters are like anti-vaxxers – nothing one can provide as evidence for the efficacy of vaccines is sufficient. There will always be a new objection.”
Other mental health issues, in particular ADHD and autism
It is also widely acknowledged that there is a higher rate of neurodevelopmental and psychiatric diagnoses in individuals with gender dysphoria. For example, one 2020 study found that transgender people were three to six times as likely to be autistic as cisgender people (those whose gender is aligned with their birth sex).
Statement one in the chapter on adolescents in draft WPATH SOC 8 does give a nod to this, pointing out that health professionals working with gender diverse adolescents “should receive training and develop expertise in autism spectrum disorders and other neurodiversity conditions.”
It also notes that in some cases “a more extended assessment process may be useful, such as for youth with more complex presentations (e.g., complicated mental health histories, co-occurring autism spectrum characteristics in particular) and an absence of experienced childhood gender incongruence.”
However, Dr. Mason stresses that underlying mental health issues are central to addressing how to manage a significant number of these patients.
“If a young person has ADHD or autism, they are not ready to make decisions about the rest of their life at age 18. Even a neurotypical young person is still developing their frontal cortex in their early 20s, and it takes longer for those with ADHD or on the autism spectrum.”
She firmly believes that the guidance does not give sufficient consideration to comorbidities in people over the age of 18.
According to their [SOC 8] guidelines, “once someone is 18 they are ready for anything,” says Dr. Mason.
Offering some explanation for the increased prevalence of ADHD and autism in those with gender dysphoria, Dr. Mason notes that children can have “hyperfocus” and those with autism will fixate on a particular area of interest. “If a child is unhappy in their life, and this can be more likely if someone is neuro-atypical, then it is likely that the individual might go online and find this one solution [for example, a transgender identity] that seems to fix everything.”
Perceptions of femininity and masculinity can also be extra challenging for a child with autism, Dr. Mason says. “It is relatively easy for an autistic girl to feel like she should be a boy because the rules of femininity are composed of nonverbal, subtle behaviors that can be difficult to pick up on,” she points out. “An autistic child who isn’t particularly good at nonverbal communication might not pick up on these and thus feel they are not very ‘female.’”
“There’s a whole lot of grass-is-greener-type thinking. Girls think boys have an easier life, and boys think girls have an easier life. I know some detransitioners who have spoken eloquently about realizing their mistake on this,” she adds.
Other parts of the SOC 8 that Dr. Mason disagrees with include the recommendation in the adolescent chapter that 14-year-olds are mature enough to start cross-sex hormones, that is, giving testosterone to a female who wants to transition to male or estrogen to a male who wishes to transition to female. “I think that’s far too young,” she asserts.
And she points out that the document states 17-year-olds are ready for genital reassignment surgery. Again, she believes this is far too young.
“Also, the SOC 8 document does not clarify who is appropriate for surgery. Whenever surgery is discussed, it becomes very vague,” she said.
A version of this article first appeared on Medscape.com.
Advisory on youth mental health crisis gets mixed reviews
The advisory on youth mental health from Surgeon General Vivek Murthy, MD, casts a necessary spotlight on the crisis, clinical psychiatrists say. But some think it could have produced more specifics about funding and payment parity for reimbursement.
The 53-page advisory says that about one in five U.S. children and adolescents aged 3-17 suffer from a mental, emotional, developmental, or behavioral disorder. In the decade before COVID, feelings of sadness and hopelessness, as well as suicidal behaviors, were on the rise. The pandemic has exacerbated symptoms of anxiety, depression, and other mental health issues in young people. Compared with 2019, ED visits in early 2021 for suspected suicide attempts rose 51% in adolescent girls and 4% in boys. “Depressive and anxiety symptoms doubled during the pandemic,” the advisory said.
Scope of the advisory
The advisory, released Dec. 7, covers all sectors and considers all social and policy factors that might be contributing to this crisis, said Jessica (Jessi) Gold, MD, MS, an assistant professor in the department of psychiatry at Washington University, St. Louis.
“It is always possible to reimagine health care to be more patient centered and mental health forward.” But changes of this magnitude take time, Dr. Gold, also director of wellness, engagement, and outreach at the university, said in an interview.
She has seen the impact of the pandemic firsthand in her clinic among students and frontline health care workers aged 18-30. People in that age group “feel everything deeply,” Dr. Gold said. Emotions tied to COVID-19 are just a part of it. Confounding factors, such as climate change, racism, and school shootings all contribute to their overall mental health.
Some children and adolescents with social anxiety have fared better during the pandemic, but those who are part of demographic groups such as racial and ethnic minorities, LGBTQ individuals, low-income youth, and those involved in juvenile justice or welfare systems face a higher risk of mental health challenges, the pandemic notwithstanding.
In her work with schools, Denese Shervington, MD, MPH, has witnessed more mental health challenges related to isolation and separation. “There’s an overall worry about the loss of what used to be, the seeming predictability and certainty of prepandemic life,” said Dr. Shervington, clinical professor of psychiatry at Tulane University, and president and CEO of the Institute for Women and Ethnic Studies, both in New Orleans.
A systems of care plan
The advisory lists actionable items for health care and 10 other industry sectors to improve mental health of children and young adults.
Health care organizations and professionals were advised to take the following six steps:
- Implement trauma-informed care principles and other prevention strategies. This may involve referring patients to resources such as economic and legal supports, school enrichment programs, and educating families on healthy child development in the clinic.
- Routinely screen children for mental health challenges and risk factors such as adverse childhood experiences during primary care well-visits or annual physicals, or at schools or EDs. Primary care physicians should use principles of trauma-informed care to conduct these screenings.
- Screen parents, caregivers, and other family members for depression, intimate partner violence, substance use, and other challenges. These can be done in tandem with broader assessments of social determinants of health such as food or housing insecurity.
- Combine efforts of clinical staff with trusted community partners and child welfare and juvenile justice. Hospital-based violence intervention programs, for example, identify patients at risk of repeat violent injury and refer them to hospital- and community-based resources.
- Build multidisciplinary teams, enlisting children and families to develop services that are tailored to their needs for screening and treatment. Such services should reflect cultural diversity and offered in multiple languages.
- Support the well-being of mental health workers and community leaders to foster their ability to help youth and their families.
Dr. Murthy is talking about a “systems of care” approach, in which all sectors that touch children and youth – not just health care – must work together and do their jobs effectively but collaboratively to address this public health crisis, said Aradhana (Bela) Sood MD, MSHA, FAACAP, senior professor of child mental health policy at Virginia Commonwealth University, Richmond. “An investment in infrastructure support of positive mental health in early childhood, be it in schools, communities, or family well-being will lead to a future where illness is not the result of major preventable societal factors, such as a lack of social supports and trauma.”
Changes will ‘take a lot of buy-in’
The recommendations are actionable in the real world – but there are a lot of them, said Dr. Gold. Dr. Murthy doesn’t specify what the plan is to accomplish these metrics or fund them, she added. He “has money and funders like foundations as steps, but foundations have also suffered in the pandemic, so it is not that simple.” Many of these changes are wide in scope and will take a lot of buy-in.
Dr. Shervington would like to have seen more of a focus on educator well-being, given that young people spend a lot of time in educational settings.
“My organization just completed a study in New Orleans that showed teachers having elevated levels of trauma-based conditions since the pandemic,” she said. Schools are indeed a key place to support holistic mental health by focusing on school climate, Dr. Sood added. “If school administrators became uniformly consistent with recognizing the importance of psychological wellness as a prerequisite of good learning, they will create environments where teachers are keenly aware of a child’s mental wellness and make reduction of bullying, wellness check-ins, [and] school-based mental health clinics a priority.
“These are ways nonmedical, community-based supports can enhance student well-being, and reduce depression and other mental health conditions,” Dr. Sood added.
Child psychiatrists stretched ‘even thinner’
Despite mental health parity rules, health plans have not been held accountable. That failure, combined with excessive demands for prior authorization for mental health treatments “have led to dangerous shortages of psychiatrists able to accept insurance,” said Paul S. Nestadt, MD, an assistant professor and public mental health researcher at Johns Hopkins University, Baltimore.
“This is particularly true for child psychiatrists, who are stretched even thinner than those of us in general practice,” Dr. Nestadt said.
While he doesn’t address it head on, Dr. Murthy uses classic parity language when he states that “mental health is no less important than physical health,” said Dr. Nestadt, who consulted with the surgeon general on developing this advisory. “While many of us would have liked to see parity highlighted more directly, this advisory was designed to be an overview.”
Highlighting social media, gun violence
Dr. Nestadt said he was pleased that the advisory emphasized the importance of restricting access to lethal means in preventing youth suicide.
“With youth suicide rates rising faster than in other age groups, and suicide mortality tied so closely to method availability, the surgeon general made the right choice in highlighting the role of guns in suicide,” he said.
The advisory also discussed the role of media and social media companies in addressing the crisis, which is important, said Dr. Gold.
“I believe very strongly that the way we talk about and portray mental health in the media matters,” she said. “I have seen it matter in the clinic with patients. They’ll wonder if someone will think they are now violent if they are diagnosed with a mental illness. Stories change the narrative.”
While the advisory isn’t perfect, the state of youth mental health “will only get worse if we don’t do something,” noted Dr. Gold. “It is critical that this is validated and discussed at the highest level and messages like Dr. Murthy’s get heard.”
Dr. Gold, Dr. Shervington, and Dr. Sood had no disclosures. Dr. Nestadt disclosed serving as a consultant to the surgeon general advisory.
The advisory on youth mental health from Surgeon General Vivek Murthy, MD, casts a necessary spotlight on the crisis, clinical psychiatrists say. But some think it could have produced more specifics about funding and payment parity for reimbursement.
The 53-page advisory says that about one in five U.S. children and adolescents aged 3-17 suffer from a mental, emotional, developmental, or behavioral disorder. In the decade before COVID, feelings of sadness and hopelessness, as well as suicidal behaviors, were on the rise. The pandemic has exacerbated symptoms of anxiety, depression, and other mental health issues in young people. Compared with 2019, ED visits in early 2021 for suspected suicide attempts rose 51% in adolescent girls and 4% in boys. “Depressive and anxiety symptoms doubled during the pandemic,” the advisory said.
Scope of the advisory
The advisory, released Dec. 7, covers all sectors and considers all social and policy factors that might be contributing to this crisis, said Jessica (Jessi) Gold, MD, MS, an assistant professor in the department of psychiatry at Washington University, St. Louis.
“It is always possible to reimagine health care to be more patient centered and mental health forward.” But changes of this magnitude take time, Dr. Gold, also director of wellness, engagement, and outreach at the university, said in an interview.
She has seen the impact of the pandemic firsthand in her clinic among students and frontline health care workers aged 18-30. People in that age group “feel everything deeply,” Dr. Gold said. Emotions tied to COVID-19 are just a part of it. Confounding factors, such as climate change, racism, and school shootings all contribute to their overall mental health.
Some children and adolescents with social anxiety have fared better during the pandemic, but those who are part of demographic groups such as racial and ethnic minorities, LGBTQ individuals, low-income youth, and those involved in juvenile justice or welfare systems face a higher risk of mental health challenges, the pandemic notwithstanding.
In her work with schools, Denese Shervington, MD, MPH, has witnessed more mental health challenges related to isolation and separation. “There’s an overall worry about the loss of what used to be, the seeming predictability and certainty of prepandemic life,” said Dr. Shervington, clinical professor of psychiatry at Tulane University, and president and CEO of the Institute for Women and Ethnic Studies, both in New Orleans.
A systems of care plan
The advisory lists actionable items for health care and 10 other industry sectors to improve mental health of children and young adults.
Health care organizations and professionals were advised to take the following six steps:
- Implement trauma-informed care principles and other prevention strategies. This may involve referring patients to resources such as economic and legal supports, school enrichment programs, and educating families on healthy child development in the clinic.
- Routinely screen children for mental health challenges and risk factors such as adverse childhood experiences during primary care well-visits or annual physicals, or at schools or EDs. Primary care physicians should use principles of trauma-informed care to conduct these screenings.
- Screen parents, caregivers, and other family members for depression, intimate partner violence, substance use, and other challenges. These can be done in tandem with broader assessments of social determinants of health such as food or housing insecurity.
- Combine efforts of clinical staff with trusted community partners and child welfare and juvenile justice. Hospital-based violence intervention programs, for example, identify patients at risk of repeat violent injury and refer them to hospital- and community-based resources.
- Build multidisciplinary teams, enlisting children and families to develop services that are tailored to their needs for screening and treatment. Such services should reflect cultural diversity and offered in multiple languages.
- Support the well-being of mental health workers and community leaders to foster their ability to help youth and their families.
Dr. Murthy is talking about a “systems of care” approach, in which all sectors that touch children and youth – not just health care – must work together and do their jobs effectively but collaboratively to address this public health crisis, said Aradhana (Bela) Sood MD, MSHA, FAACAP, senior professor of child mental health policy at Virginia Commonwealth University, Richmond. “An investment in infrastructure support of positive mental health in early childhood, be it in schools, communities, or family well-being will lead to a future where illness is not the result of major preventable societal factors, such as a lack of social supports and trauma.”
Changes will ‘take a lot of buy-in’
The recommendations are actionable in the real world – but there are a lot of them, said Dr. Gold. Dr. Murthy doesn’t specify what the plan is to accomplish these metrics or fund them, she added. He “has money and funders like foundations as steps, but foundations have also suffered in the pandemic, so it is not that simple.” Many of these changes are wide in scope and will take a lot of buy-in.
Dr. Shervington would like to have seen more of a focus on educator well-being, given that young people spend a lot of time in educational settings.
“My organization just completed a study in New Orleans that showed teachers having elevated levels of trauma-based conditions since the pandemic,” she said. Schools are indeed a key place to support holistic mental health by focusing on school climate, Dr. Sood added. “If school administrators became uniformly consistent with recognizing the importance of psychological wellness as a prerequisite of good learning, they will create environments where teachers are keenly aware of a child’s mental wellness and make reduction of bullying, wellness check-ins, [and] school-based mental health clinics a priority.
“These are ways nonmedical, community-based supports can enhance student well-being, and reduce depression and other mental health conditions,” Dr. Sood added.
Child psychiatrists stretched ‘even thinner’
Despite mental health parity rules, health plans have not been held accountable. That failure, combined with excessive demands for prior authorization for mental health treatments “have led to dangerous shortages of psychiatrists able to accept insurance,” said Paul S. Nestadt, MD, an assistant professor and public mental health researcher at Johns Hopkins University, Baltimore.
“This is particularly true for child psychiatrists, who are stretched even thinner than those of us in general practice,” Dr. Nestadt said.
While he doesn’t address it head on, Dr. Murthy uses classic parity language when he states that “mental health is no less important than physical health,” said Dr. Nestadt, who consulted with the surgeon general on developing this advisory. “While many of us would have liked to see parity highlighted more directly, this advisory was designed to be an overview.”
Highlighting social media, gun violence
Dr. Nestadt said he was pleased that the advisory emphasized the importance of restricting access to lethal means in preventing youth suicide.
“With youth suicide rates rising faster than in other age groups, and suicide mortality tied so closely to method availability, the surgeon general made the right choice in highlighting the role of guns in suicide,” he said.
The advisory also discussed the role of media and social media companies in addressing the crisis, which is important, said Dr. Gold.
“I believe very strongly that the way we talk about and portray mental health in the media matters,” she said. “I have seen it matter in the clinic with patients. They’ll wonder if someone will think they are now violent if they are diagnosed with a mental illness. Stories change the narrative.”
While the advisory isn’t perfect, the state of youth mental health “will only get worse if we don’t do something,” noted Dr. Gold. “It is critical that this is validated and discussed at the highest level and messages like Dr. Murthy’s get heard.”
Dr. Gold, Dr. Shervington, and Dr. Sood had no disclosures. Dr. Nestadt disclosed serving as a consultant to the surgeon general advisory.
The advisory on youth mental health from Surgeon General Vivek Murthy, MD, casts a necessary spotlight on the crisis, clinical psychiatrists say. But some think it could have produced more specifics about funding and payment parity for reimbursement.
The 53-page advisory says that about one in five U.S. children and adolescents aged 3-17 suffer from a mental, emotional, developmental, or behavioral disorder. In the decade before COVID, feelings of sadness and hopelessness, as well as suicidal behaviors, were on the rise. The pandemic has exacerbated symptoms of anxiety, depression, and other mental health issues in young people. Compared with 2019, ED visits in early 2021 for suspected suicide attempts rose 51% in adolescent girls and 4% in boys. “Depressive and anxiety symptoms doubled during the pandemic,” the advisory said.
Scope of the advisory
The advisory, released Dec. 7, covers all sectors and considers all social and policy factors that might be contributing to this crisis, said Jessica (Jessi) Gold, MD, MS, an assistant professor in the department of psychiatry at Washington University, St. Louis.
“It is always possible to reimagine health care to be more patient centered and mental health forward.” But changes of this magnitude take time, Dr. Gold, also director of wellness, engagement, and outreach at the university, said in an interview.
She has seen the impact of the pandemic firsthand in her clinic among students and frontline health care workers aged 18-30. People in that age group “feel everything deeply,” Dr. Gold said. Emotions tied to COVID-19 are just a part of it. Confounding factors, such as climate change, racism, and school shootings all contribute to their overall mental health.
Some children and adolescents with social anxiety have fared better during the pandemic, but those who are part of demographic groups such as racial and ethnic minorities, LGBTQ individuals, low-income youth, and those involved in juvenile justice or welfare systems face a higher risk of mental health challenges, the pandemic notwithstanding.
In her work with schools, Denese Shervington, MD, MPH, has witnessed more mental health challenges related to isolation and separation. “There’s an overall worry about the loss of what used to be, the seeming predictability and certainty of prepandemic life,” said Dr. Shervington, clinical professor of psychiatry at Tulane University, and president and CEO of the Institute for Women and Ethnic Studies, both in New Orleans.
A systems of care plan
The advisory lists actionable items for health care and 10 other industry sectors to improve mental health of children and young adults.
Health care organizations and professionals were advised to take the following six steps:
- Implement trauma-informed care principles and other prevention strategies. This may involve referring patients to resources such as economic and legal supports, school enrichment programs, and educating families on healthy child development in the clinic.
- Routinely screen children for mental health challenges and risk factors such as adverse childhood experiences during primary care well-visits or annual physicals, or at schools or EDs. Primary care physicians should use principles of trauma-informed care to conduct these screenings.
- Screen parents, caregivers, and other family members for depression, intimate partner violence, substance use, and other challenges. These can be done in tandem with broader assessments of social determinants of health such as food or housing insecurity.
- Combine efforts of clinical staff with trusted community partners and child welfare and juvenile justice. Hospital-based violence intervention programs, for example, identify patients at risk of repeat violent injury and refer them to hospital- and community-based resources.
- Build multidisciplinary teams, enlisting children and families to develop services that are tailored to their needs for screening and treatment. Such services should reflect cultural diversity and offered in multiple languages.
- Support the well-being of mental health workers and community leaders to foster their ability to help youth and their families.
Dr. Murthy is talking about a “systems of care” approach, in which all sectors that touch children and youth – not just health care – must work together and do their jobs effectively but collaboratively to address this public health crisis, said Aradhana (Bela) Sood MD, MSHA, FAACAP, senior professor of child mental health policy at Virginia Commonwealth University, Richmond. “An investment in infrastructure support of positive mental health in early childhood, be it in schools, communities, or family well-being will lead to a future where illness is not the result of major preventable societal factors, such as a lack of social supports and trauma.”
Changes will ‘take a lot of buy-in’
The recommendations are actionable in the real world – but there are a lot of them, said Dr. Gold. Dr. Murthy doesn’t specify what the plan is to accomplish these metrics or fund them, she added. He “has money and funders like foundations as steps, but foundations have also suffered in the pandemic, so it is not that simple.” Many of these changes are wide in scope and will take a lot of buy-in.
Dr. Shervington would like to have seen more of a focus on educator well-being, given that young people spend a lot of time in educational settings.
“My organization just completed a study in New Orleans that showed teachers having elevated levels of trauma-based conditions since the pandemic,” she said. Schools are indeed a key place to support holistic mental health by focusing on school climate, Dr. Sood added. “If school administrators became uniformly consistent with recognizing the importance of psychological wellness as a prerequisite of good learning, they will create environments where teachers are keenly aware of a child’s mental wellness and make reduction of bullying, wellness check-ins, [and] school-based mental health clinics a priority.
“These are ways nonmedical, community-based supports can enhance student well-being, and reduce depression and other mental health conditions,” Dr. Sood added.
Child psychiatrists stretched ‘even thinner’
Despite mental health parity rules, health plans have not been held accountable. That failure, combined with excessive demands for prior authorization for mental health treatments “have led to dangerous shortages of psychiatrists able to accept insurance,” said Paul S. Nestadt, MD, an assistant professor and public mental health researcher at Johns Hopkins University, Baltimore.
“This is particularly true for child psychiatrists, who are stretched even thinner than those of us in general practice,” Dr. Nestadt said.
While he doesn’t address it head on, Dr. Murthy uses classic parity language when he states that “mental health is no less important than physical health,” said Dr. Nestadt, who consulted with the surgeon general on developing this advisory. “While many of us would have liked to see parity highlighted more directly, this advisory was designed to be an overview.”
Highlighting social media, gun violence
Dr. Nestadt said he was pleased that the advisory emphasized the importance of restricting access to lethal means in preventing youth suicide.
“With youth suicide rates rising faster than in other age groups, and suicide mortality tied so closely to method availability, the surgeon general made the right choice in highlighting the role of guns in suicide,” he said.
The advisory also discussed the role of media and social media companies in addressing the crisis, which is important, said Dr. Gold.
“I believe very strongly that the way we talk about and portray mental health in the media matters,” she said. “I have seen it matter in the clinic with patients. They’ll wonder if someone will think they are now violent if they are diagnosed with a mental illness. Stories change the narrative.”
While the advisory isn’t perfect, the state of youth mental health “will only get worse if we don’t do something,” noted Dr. Gold. “It is critical that this is validated and discussed at the highest level and messages like Dr. Murthy’s get heard.”
Dr. Gold, Dr. Shervington, and Dr. Sood had no disclosures. Dr. Nestadt disclosed serving as a consultant to the surgeon general advisory.
CDC panel backs mRNA COVID vaccines over J&J because of clot risk
because the Johnson & Johnson shot carries the risk of a rare but potentially fatal side effect that causes blood clots and bleeding in the brain.
In an emergency meeting on December 16, the CDC’s Advisory Committee on Immunization Practices, or ACIP, voted unanimously (15-0) to state a preference for the mRNA vaccines over the Johnson & Johnson shot. The vote came after the panel heard a safety update on cases of thrombosis with thrombocytopenia syndrome, or TTS, a condition that causes large clots that deplete the blood of platelets, resulting in uncontrolled bleeding.
The move brings the United States in line with other wealthy countries. In May, Denmark dropped the Johnson & Johnson shot from its vaccination program because of this risk. Australia and Greece have limited the use of a similar vaccine, made by AstraZeneca, in younger people because of the TTS risk. Both vaccines use the envelope of a different kind of virus, called an adenovirus, to sneak the vaccine instructions into cells. On Dec. 16, health officials said they had determined that TTS was likely due to a class effect, meaning it happens with all adenovirus vector vaccines.
The risk of dying from TTS after a Johnson & Johnson shot is extremely rare. There is an estimated 1 death for every 2 million doses of the vaccine given in the general population. That risk is higher for women ages 30 to 49, rising to about 2 deaths for every 1 million doses given in this age group. There’s no question that the Johnson & Johnson shot has saved many more lives than it has taken, experts said
Still, the committee previously paused the use of the Johnson & Johnson vaccine in April after the first cases of TTS came to light. That pause was lifted just 10 days later, after a new warning was added to the vaccine’s label to raise awareness of the risk.
In updating the safety information on Johnson & Johnson, the panel noted that the warning label had not sufficiently lowered the risk of death from TTS. Doctors seem to be aware of the condition because none of the patients who had developed TTS had been treated with the blood thinner heparin, which can make the syndrome worse. But patients continued to die even after the label was added, the panel noted, because TTS can progress so quickly that doctors simply don’t have time to treat it.
For that reason, and because there are other, safer vaccines available, the panel decided to make what’s called a preferential statement, saying the Pfizer and Moderna mRNA vaccines should be preferred over Johnson & Johnson.
The statement leaves the J&J vaccine on the market and available to patients who are at risk of a severe allergic reaction to the mRNA vaccines. It also means that people can still choose the J&J vaccine if they still want it after being informed about the risks.
About 17 million first doses and 900,000 second doses of the Johnson & Johnson vaccine have been given in the United States. Through the end of August, 54 cases of thrombosis with thrombocytopenia syndrome (TTS) have occurred after the J&J shots in the United States. Nearly half of those were in women ages 30 to 49. There have been nine deaths from TTS after Johnson & Johnson shots.
A version of this article first appeared on WebMD.com.
because the Johnson & Johnson shot carries the risk of a rare but potentially fatal side effect that causes blood clots and bleeding in the brain.
In an emergency meeting on December 16, the CDC’s Advisory Committee on Immunization Practices, or ACIP, voted unanimously (15-0) to state a preference for the mRNA vaccines over the Johnson & Johnson shot. The vote came after the panel heard a safety update on cases of thrombosis with thrombocytopenia syndrome, or TTS, a condition that causes large clots that deplete the blood of platelets, resulting in uncontrolled bleeding.
The move brings the United States in line with other wealthy countries. In May, Denmark dropped the Johnson & Johnson shot from its vaccination program because of this risk. Australia and Greece have limited the use of a similar vaccine, made by AstraZeneca, in younger people because of the TTS risk. Both vaccines use the envelope of a different kind of virus, called an adenovirus, to sneak the vaccine instructions into cells. On Dec. 16, health officials said they had determined that TTS was likely due to a class effect, meaning it happens with all adenovirus vector vaccines.
The risk of dying from TTS after a Johnson & Johnson shot is extremely rare. There is an estimated 1 death for every 2 million doses of the vaccine given in the general population. That risk is higher for women ages 30 to 49, rising to about 2 deaths for every 1 million doses given in this age group. There’s no question that the Johnson & Johnson shot has saved many more lives than it has taken, experts said
Still, the committee previously paused the use of the Johnson & Johnson vaccine in April after the first cases of TTS came to light. That pause was lifted just 10 days later, after a new warning was added to the vaccine’s label to raise awareness of the risk.
In updating the safety information on Johnson & Johnson, the panel noted that the warning label had not sufficiently lowered the risk of death from TTS. Doctors seem to be aware of the condition because none of the patients who had developed TTS had been treated with the blood thinner heparin, which can make the syndrome worse. But patients continued to die even after the label was added, the panel noted, because TTS can progress so quickly that doctors simply don’t have time to treat it.
For that reason, and because there are other, safer vaccines available, the panel decided to make what’s called a preferential statement, saying the Pfizer and Moderna mRNA vaccines should be preferred over Johnson & Johnson.
The statement leaves the J&J vaccine on the market and available to patients who are at risk of a severe allergic reaction to the mRNA vaccines. It also means that people can still choose the J&J vaccine if they still want it after being informed about the risks.
About 17 million first doses and 900,000 second doses of the Johnson & Johnson vaccine have been given in the United States. Through the end of August, 54 cases of thrombosis with thrombocytopenia syndrome (TTS) have occurred after the J&J shots in the United States. Nearly half of those were in women ages 30 to 49. There have been nine deaths from TTS after Johnson & Johnson shots.
A version of this article first appeared on WebMD.com.
because the Johnson & Johnson shot carries the risk of a rare but potentially fatal side effect that causes blood clots and bleeding in the brain.
In an emergency meeting on December 16, the CDC’s Advisory Committee on Immunization Practices, or ACIP, voted unanimously (15-0) to state a preference for the mRNA vaccines over the Johnson & Johnson shot. The vote came after the panel heard a safety update on cases of thrombosis with thrombocytopenia syndrome, or TTS, a condition that causes large clots that deplete the blood of platelets, resulting in uncontrolled bleeding.
The move brings the United States in line with other wealthy countries. In May, Denmark dropped the Johnson & Johnson shot from its vaccination program because of this risk. Australia and Greece have limited the use of a similar vaccine, made by AstraZeneca, in younger people because of the TTS risk. Both vaccines use the envelope of a different kind of virus, called an adenovirus, to sneak the vaccine instructions into cells. On Dec. 16, health officials said they had determined that TTS was likely due to a class effect, meaning it happens with all adenovirus vector vaccines.
The risk of dying from TTS after a Johnson & Johnson shot is extremely rare. There is an estimated 1 death for every 2 million doses of the vaccine given in the general population. That risk is higher for women ages 30 to 49, rising to about 2 deaths for every 1 million doses given in this age group. There’s no question that the Johnson & Johnson shot has saved many more lives than it has taken, experts said
Still, the committee previously paused the use of the Johnson & Johnson vaccine in April after the first cases of TTS came to light. That pause was lifted just 10 days later, after a new warning was added to the vaccine’s label to raise awareness of the risk.
In updating the safety information on Johnson & Johnson, the panel noted that the warning label had not sufficiently lowered the risk of death from TTS. Doctors seem to be aware of the condition because none of the patients who had developed TTS had been treated with the blood thinner heparin, which can make the syndrome worse. But patients continued to die even after the label was added, the panel noted, because TTS can progress so quickly that doctors simply don’t have time to treat it.
For that reason, and because there are other, safer vaccines available, the panel decided to make what’s called a preferential statement, saying the Pfizer and Moderna mRNA vaccines should be preferred over Johnson & Johnson.
The statement leaves the J&J vaccine on the market and available to patients who are at risk of a severe allergic reaction to the mRNA vaccines. It also means that people can still choose the J&J vaccine if they still want it after being informed about the risks.
About 17 million first doses and 900,000 second doses of the Johnson & Johnson vaccine have been given in the United States. Through the end of August, 54 cases of thrombosis with thrombocytopenia syndrome (TTS) have occurred after the J&J shots in the United States. Nearly half of those were in women ages 30 to 49. There have been nine deaths from TTS after Johnson & Johnson shots.
A version of this article first appeared on WebMD.com.
Telemedicine helps SCD patients survive COVID, but more need access
ATLANTA – , according to an investigator at the annual meeting of the American Society of Hematology.
During the first COVID-19 wave in the summer of 2020, Atlanta’s Grady Sickle Cell Center, the nation’s largest adult sickle cell center, recorded two deaths among the 20 COVID-19_infected patients seen there, said Fuad El Rassi, MD, of Emory University, Atlanta.
Virtual visits, launched to deliver health care needs in the wake of a Georgia’s 2020 statewide shelter-in-place order, helped protect patients from COVID-19 infection, Dr. El Rassi said in a press conference at the meeting.
“The patients’ diligence and awareness to stay home during the pandemic have proven crucial to reducing morbidity and mortality in this vulnerable population,” he said. “The option of having virtual visits for health care delivery was key and should be utilized further in sickle cell care.”
However, virtual visits and other best practices to prevent and treat COVID-19 in patients with sickle cell disease can be challenging to implement outside of large, specialized centers such as Grady.
“The majority of sickle cell patients in major metropolitan areas are not plugged into dedicated sickle cell centers, and that’s a key issue,” said Dr. El Rassi.
“There’s a huge shortage of such clinics around major metropolitan areas, and that restricts things for the general population, unfortunately.”
COVID-19 prevention remains a challenge, no matter where patients are treated. Only about 50% of the center’s sickle cell disease patients are immunized, according to Dr. El Rassi, who added that assessment of vaccine response among those patients is ongoing.
Ifeyinwa (Ify) Osunkwo, MD, MPH, a sickle cell disease specialist, said long-term sustainability of virtual visits depends greatly on states’ continuation of laws or policies that facilitate access to telemedicine. A total of 22 states changed laws or policies during the pandemic to promote access to telemedicine, according to the Commonwealth Fund.
Virtual care is more challenging in states where expanded telemedicine coverage is not available or is ended, said Dr. Osunkwo, director of the Sickle Cell Enterprise at Levine Cancer Institute. The institute is part of Atrium Health, a large health system that operates in four states.
“We are no longer able to do virtual visits for our South Carolinian patients, even though across the border in North Carolina, you can still provide virtual care,” Dr. Osunkwo said in an interview.
“Sickle cell patients suffer from social determinants [of health], so getting to their doctor when they have a regular outpatient visit is kind of hard,” she added. “And having that virtual option actually makes them more adherent, and they have better access to care overall.”
In the study presented at the ASH meeting by Dr. El Rassi and colleagues, there were a total of 55 patients with COVID-19 among the 1,343 sickle cell disease patients they tracked. Of the 55 patients with COVID-19, 28 were female and 27 were male, and 35% were on hydroxyurea for disease modification.
Among these 55 patients with COVID-19, 44 (80%) were hospitalized, and the hospitalizations of 15 (27%) were deemed related to COVID-19 signs and symptoms, Dr. El Rassi said. Twelve of the 55 patients (22%) had emergency visits, including 5 (9%) because of COVID-19 symptoms, he added.
The two deaths from COVID-19 occurred in June and July 2020, said Dr. El Rassi, adding that those patients were among 20 total cases diagnosed from March to September of 2020.
Over the second reported wave of COVID-19, from October 2020 to March 2021, there were no deaths seen among 35 total COVID-19 cases, according to the report at the ASH meeting.
In an interview, Kaitlin Strumph, MD, a sickle cell disease specialist at the Children’s Hospital at Montefiore in New York, noted that patients with sickle cell disease who contract COVID-19 are considered at high risk for morbidity and mortality.
“Patients and providers should not let down their guard,” Dr. Strumph said in an interview. “The best way to protect people from COVID-19 right now is prevention, and vaccinations are the key to further improving outcomes.”
Virtual visits can help bridge gaps in care for patients with sickle cell disease, said Dr. Strumph, given that limited access to care is a large driver of health disparities in this population.
“Telemedicine allows patients to stay home and limit their exposure to COVID-19 out in the community and at the hospital,” she said. “I think most providers feel confident that virtual visits are a huge benefit for the community, and we hope they are here to stay.”
Dr. El Rassi reported disclosures related to Cyclerion, Novartis, Pfizer, Global Blood Therapeutics and bluebird bio.
ATLANTA – , according to an investigator at the annual meeting of the American Society of Hematology.
During the first COVID-19 wave in the summer of 2020, Atlanta’s Grady Sickle Cell Center, the nation’s largest adult sickle cell center, recorded two deaths among the 20 COVID-19_infected patients seen there, said Fuad El Rassi, MD, of Emory University, Atlanta.
Virtual visits, launched to deliver health care needs in the wake of a Georgia’s 2020 statewide shelter-in-place order, helped protect patients from COVID-19 infection, Dr. El Rassi said in a press conference at the meeting.
“The patients’ diligence and awareness to stay home during the pandemic have proven crucial to reducing morbidity and mortality in this vulnerable population,” he said. “The option of having virtual visits for health care delivery was key and should be utilized further in sickle cell care.”
However, virtual visits and other best practices to prevent and treat COVID-19 in patients with sickle cell disease can be challenging to implement outside of large, specialized centers such as Grady.
“The majority of sickle cell patients in major metropolitan areas are not plugged into dedicated sickle cell centers, and that’s a key issue,” said Dr. El Rassi.
“There’s a huge shortage of such clinics around major metropolitan areas, and that restricts things for the general population, unfortunately.”
COVID-19 prevention remains a challenge, no matter where patients are treated. Only about 50% of the center’s sickle cell disease patients are immunized, according to Dr. El Rassi, who added that assessment of vaccine response among those patients is ongoing.
Ifeyinwa (Ify) Osunkwo, MD, MPH, a sickle cell disease specialist, said long-term sustainability of virtual visits depends greatly on states’ continuation of laws or policies that facilitate access to telemedicine. A total of 22 states changed laws or policies during the pandemic to promote access to telemedicine, according to the Commonwealth Fund.
Virtual care is more challenging in states where expanded telemedicine coverage is not available or is ended, said Dr. Osunkwo, director of the Sickle Cell Enterprise at Levine Cancer Institute. The institute is part of Atrium Health, a large health system that operates in four states.
“We are no longer able to do virtual visits for our South Carolinian patients, even though across the border in North Carolina, you can still provide virtual care,” Dr. Osunkwo said in an interview.
“Sickle cell patients suffer from social determinants [of health], so getting to their doctor when they have a regular outpatient visit is kind of hard,” she added. “And having that virtual option actually makes them more adherent, and they have better access to care overall.”
In the study presented at the ASH meeting by Dr. El Rassi and colleagues, there were a total of 55 patients with COVID-19 among the 1,343 sickle cell disease patients they tracked. Of the 55 patients with COVID-19, 28 were female and 27 were male, and 35% were on hydroxyurea for disease modification.
Among these 55 patients with COVID-19, 44 (80%) were hospitalized, and the hospitalizations of 15 (27%) were deemed related to COVID-19 signs and symptoms, Dr. El Rassi said. Twelve of the 55 patients (22%) had emergency visits, including 5 (9%) because of COVID-19 symptoms, he added.
The two deaths from COVID-19 occurred in June and July 2020, said Dr. El Rassi, adding that those patients were among 20 total cases diagnosed from March to September of 2020.
Over the second reported wave of COVID-19, from October 2020 to March 2021, there were no deaths seen among 35 total COVID-19 cases, according to the report at the ASH meeting.
In an interview, Kaitlin Strumph, MD, a sickle cell disease specialist at the Children’s Hospital at Montefiore in New York, noted that patients with sickle cell disease who contract COVID-19 are considered at high risk for morbidity and mortality.
“Patients and providers should not let down their guard,” Dr. Strumph said in an interview. “The best way to protect people from COVID-19 right now is prevention, and vaccinations are the key to further improving outcomes.”
Virtual visits can help bridge gaps in care for patients with sickle cell disease, said Dr. Strumph, given that limited access to care is a large driver of health disparities in this population.
“Telemedicine allows patients to stay home and limit their exposure to COVID-19 out in the community and at the hospital,” she said. “I think most providers feel confident that virtual visits are a huge benefit for the community, and we hope they are here to stay.”
Dr. El Rassi reported disclosures related to Cyclerion, Novartis, Pfizer, Global Blood Therapeutics and bluebird bio.
ATLANTA – , according to an investigator at the annual meeting of the American Society of Hematology.
During the first COVID-19 wave in the summer of 2020, Atlanta’s Grady Sickle Cell Center, the nation’s largest adult sickle cell center, recorded two deaths among the 20 COVID-19_infected patients seen there, said Fuad El Rassi, MD, of Emory University, Atlanta.
Virtual visits, launched to deliver health care needs in the wake of a Georgia’s 2020 statewide shelter-in-place order, helped protect patients from COVID-19 infection, Dr. El Rassi said in a press conference at the meeting.
“The patients’ diligence and awareness to stay home during the pandemic have proven crucial to reducing morbidity and mortality in this vulnerable population,” he said. “The option of having virtual visits for health care delivery was key and should be utilized further in sickle cell care.”
However, virtual visits and other best practices to prevent and treat COVID-19 in patients with sickle cell disease can be challenging to implement outside of large, specialized centers such as Grady.
“The majority of sickle cell patients in major metropolitan areas are not plugged into dedicated sickle cell centers, and that’s a key issue,” said Dr. El Rassi.
“There’s a huge shortage of such clinics around major metropolitan areas, and that restricts things for the general population, unfortunately.”
COVID-19 prevention remains a challenge, no matter where patients are treated. Only about 50% of the center’s sickle cell disease patients are immunized, according to Dr. El Rassi, who added that assessment of vaccine response among those patients is ongoing.
Ifeyinwa (Ify) Osunkwo, MD, MPH, a sickle cell disease specialist, said long-term sustainability of virtual visits depends greatly on states’ continuation of laws or policies that facilitate access to telemedicine. A total of 22 states changed laws or policies during the pandemic to promote access to telemedicine, according to the Commonwealth Fund.
Virtual care is more challenging in states where expanded telemedicine coverage is not available or is ended, said Dr. Osunkwo, director of the Sickle Cell Enterprise at Levine Cancer Institute. The institute is part of Atrium Health, a large health system that operates in four states.
“We are no longer able to do virtual visits for our South Carolinian patients, even though across the border in North Carolina, you can still provide virtual care,” Dr. Osunkwo said in an interview.
“Sickle cell patients suffer from social determinants [of health], so getting to their doctor when they have a regular outpatient visit is kind of hard,” she added. “And having that virtual option actually makes them more adherent, and they have better access to care overall.”
In the study presented at the ASH meeting by Dr. El Rassi and colleagues, there were a total of 55 patients with COVID-19 among the 1,343 sickle cell disease patients they tracked. Of the 55 patients with COVID-19, 28 were female and 27 were male, and 35% were on hydroxyurea for disease modification.
Among these 55 patients with COVID-19, 44 (80%) were hospitalized, and the hospitalizations of 15 (27%) were deemed related to COVID-19 signs and symptoms, Dr. El Rassi said. Twelve of the 55 patients (22%) had emergency visits, including 5 (9%) because of COVID-19 symptoms, he added.
The two deaths from COVID-19 occurred in June and July 2020, said Dr. El Rassi, adding that those patients were among 20 total cases diagnosed from March to September of 2020.
Over the second reported wave of COVID-19, from October 2020 to March 2021, there were no deaths seen among 35 total COVID-19 cases, according to the report at the ASH meeting.
In an interview, Kaitlin Strumph, MD, a sickle cell disease specialist at the Children’s Hospital at Montefiore in New York, noted that patients with sickle cell disease who contract COVID-19 are considered at high risk for morbidity and mortality.
“Patients and providers should not let down their guard,” Dr. Strumph said in an interview. “The best way to protect people from COVID-19 right now is prevention, and vaccinations are the key to further improving outcomes.”
Virtual visits can help bridge gaps in care for patients with sickle cell disease, said Dr. Strumph, given that limited access to care is a large driver of health disparities in this population.
“Telemedicine allows patients to stay home and limit their exposure to COVID-19 out in the community and at the hospital,” she said. “I think most providers feel confident that virtual visits are a huge benefit for the community, and we hope they are here to stay.”
Dr. El Rassi reported disclosures related to Cyclerion, Novartis, Pfizer, Global Blood Therapeutics and bluebird bio.
FROM ASH 2021
Califf plans work on opioids, accelerated approvals on return to FDA
Robert M. Califf, MD, plans to take a close look at federal policies on opioid prescriptions in his expected second turn as the top U.S. regulator of medical products, as well as keep closer tabs on the performance of drugs cleared with accelerated approvals.
Dr. Califf on Tuesday fielded questions at a Senate hearing about his nomination by President Joe Biden to serve as administrator of the U.S. Food and Drug Administration, a role in which he served in the Obama administration. He also spoke about the need to bolster the nation’s ability to maintain an adequate supply of key medical products, including drugs.
Members of the Senate Health, Education, Labor and Pensions Committee, which is handling Dr. Califf’s nomination, were largely cordial and supportive during the hearing. Sen. Patty Murray (D-Wash.), the committee chair, and the panel’s top Republican, Sen. Richard Burr of North Carolina, addressed Dr. Califf during the hearing as if he would soon serve again as the FDA’s leader. Both were among the senators who voted 89-4 to confirm Dr. Califf in a February 2016 vote.
Dr. Califf “was previously confirmed to lead FDA in an overwhelming bipartisan vote, and I look forward to working with him again to ensure FDA continues to protect families across the country, uphold the gold standard of safety and effectiveness, and put science and data first,” Sen. Murray said.
Less enthusiastic about Dr. Califf was Sen. Bernie Sanders (I-VT), who was among the seven senators who did not vote on Dr. Califf’s nomination in 2016.
Sen. Sanders objected in 2016 to Dr. Califf’s ties to the pharmaceutical industry, and he did so again Tuesday. A noted leader in conducting clinical trials, Dr. Califf has worked with many drugmakers. But at the hearing, Dr. Califf said he concurs with Sen. Sanders on an idea strongly opposed by the pharmaceutical industry.
In response to Sen. Sanders’ question, Dr. Califf said he already is “on record as being in favor of Medicare negotiating with the industry on prices.”
The FDA would not take direct part in negotiations, as this work would be handled by the Centers for Medicare & Medicaid Services. Democrats want to give Medicare some negotiating authority through their sweeping Build Back Better Act.
People in the United States are dismayed over both the cost of prescription drugs and the widespread distribution of prescription painkillers that helped fuel the current opioid epidemic, Sen. Sanders told Dr. Califf. Many people will be concerned about an FDA commissioner who has benefited from close ties to the industry, Sen. Sanders said.
“How are they going to believe that you’re going to be an independent and strong voice against this enormously powerful, special interest?” Sen. Sanders asked.
“I’m totally with you on the concept that the price of pharmaceuticals is way too high in this country,” Dr. Califf said in reply.
Dr. Califf was paid $2.7 million in salary and bonus by Verily Life Sciences, the biomedical research organization operated by Alphabet, parent company of Google, according to his federal financial disclosure. He also reported holding board positions with pharmaceutical companies AmyriAD and Centessa Pharmaceuticals.
Bloomberg Government reported that Dr. Califf has ties to about 16 other research organizations and biotech companies. Bloomberg Government also said that, in his earlier FDA service, Dr. Califf kept a whiteboard in his office that listed all the activities and projects that required his recusal, citing as a source Howard Sklamberg, who was a deputy commissioner under Dr. Califf.
“He was very, very, very careful,” Mr. Sklamberg, who’s now an attorney at Arnold & Porter LLP, told Bloomberg Government.
‘Work to do’ on opioids
Senators looped back repeatedly to the topic of opioids during Dr. Califf’s hearing, reflecting deep concerns about the FDA’s efforts to warn of the risks of prescription painkillers.
There were an estimated 100,306 drug overdose deaths in the United States in the 12 months ending in April, an increase of 28.5% from the 78,056 deaths during the same period the year before, according to the Centers for Disease Control and Prevention.
Dr. Califf said he plans to focus on what information the FDA conveys to the public about the risks of prescription painkillers, including a look at what the labels for these products say.
“I am committed to do a comprehensive review of the status of opioids, early in my tenure,” Dr. Califf said.
Dr. Califf indicated that physicians are still too quick to provide excess doses of these medicines, despite years of efforts to restrain their use. He said he knows relatives who were given 30-day prescriptions for opioids after minor surgery.
“So I know we have work to do,” Dr. Califf said.
Concerns about the FDA’s previous work in managing opioids has led to protests from a few Democratic senators about the prospect of President Biden nominating the acting FDA commissioner, Janet Woodcock, MD, for the permanent post.
At the hearing, Sen. Ben Ray Luján (D-NM) raised the case of the FDA’s approval of the powerful Zohydro painkiller. The agency approved that drug despite an 11-2 vote against it by the FDA’s Anesthetic and Analgesic Drug Products Advisory Committee.
Sen. Luján asked Dr. Califf what he would do if an FDA advisory committee voted “overwhelmingly” against recommending approval of a medicine, as happened in the Zohydro case.
While not mentioned by Sen. Luján in this exchange during the hearing with Dr. Califf, the FDA staff’s rejection of recommendations of advisory committees has been a growing concern among researchers.
The agency last year approved aducanumab (Aduhelm, Biogen), a drug for Alzheimer’s disease, dismissing the advice of its Peripheral and Central Nervous System Drugs Advisory Committee. That decision triggered the resignation of several members of the panel. The FDA staff also earlier rejected the conclusion the majority of members of the same advisory committee offered in 2016 on eteplirsen (Exondys 51, Sarepta), a drug for Duchenne muscular dystrophy.
Dr. Califf told Sen. Luján he had done recent research into how often the FDA staff does not concur with the recommendations of an advisory committee. He said the FDA takes a different course of action in about 25% of cases. In about three-quarters of those cases, the FDA staff opts for a “more stringent” approach regarding allowing the public access to the drug, as opposed to a more generous one as seen in the Zohydro, Aduhelm, and Exondys 51 cases.
Still, Dr. Califf said that when there’s an 11-2 advisory committee vote against recommendation of a product, “the leaders at FDA really need to take a close look” at what’s happening.
Question on accelerated approvals
The FDA’s approval of aducanumab drew attention to a debate already underway about conditional clearances known as accelerated approvals.
The FDA has used this path since the 1990s to speed access to drugs for serious conditions. The trade-off for early access is that the agency sometimes makes the wrong call based on initial findings, and clears a medicine later found not to benefit patients as expected.
The FDA’s cancer division is in the midst of public efforts to address cases where drugmakers have not been able to deliver studies that support accelerated approvals of their oncology drugs. In addition, the Office of Inspector General of the U.S. Department of Health & Human Services announced in August that it is reviewing the FDA’s handling of the accelerated approval process.
At Tuesday’s hearing, Sen. Burr grilled Dr. Califf about how he would respond to calls to change how the FDA handles the accelerated-approval process.
“Can you commit to me and to patients who may rely on cutting-edge treatments that you will not support efforts to narrow this pathway or raise the bar for drugs to be approved under those pathways?” Burr asked Califf.
Dr. Califf responded by saying he was “a fan of accelerated approval – for the right conditions.”
Earlier, in his opening statement, Dr. Califf had said his mother benefited directly from the accelerated approval of new drugs for multiple myeloma. Dr. Califf told Sen. Burr that he had spent “countless hours with patient groups” and understands the need to speed the approval of medicines for serious diseases.
But the FDA also has to make sure it holds up its end of the bargain struck with accelerated approvals. This involves checking on how these medicines work once they are marketed.
“We’re accepting that there’s more uncertainty,” Dr. Califf said. “That means we’ve got to have a better system to evaluate these products as they’re used on the market. And I think there are ways that we can do that now. Technology is making this possible in ways that it just was not possible before.”
Worries about the medical supply chain
Sen. Susan Collins (R-Maine) asked Dr. Califf about the vulnerability of the U.S. medical system to disruptions of the supply chain. She raised concerns about China’s dominance in antibiotic manufacturing as an example. She asked if Congress could do more to encourage domestic manufacturing of medical supplies, such as by offering tax incentives.
Dr. Califf told Sen. Collins he shared her concern about the U.S. manufacturing of ingredients used in both branded and generic drugs. He said he recently has served on a committee of the National Academy of Medicine that is examining supply chain issues.
This committee will soon release a report with specific recommendations, Dr. Califf said.
“We don’t have enough competitive entities in what’s become sort of a commodity business” of drug manufacturing, Dr. Califf said. “So we need a number of steps to make the system more resilient.”
A version of this article first appeared on Medscape.com.
Robert M. Califf, MD, plans to take a close look at federal policies on opioid prescriptions in his expected second turn as the top U.S. regulator of medical products, as well as keep closer tabs on the performance of drugs cleared with accelerated approvals.
Dr. Califf on Tuesday fielded questions at a Senate hearing about his nomination by President Joe Biden to serve as administrator of the U.S. Food and Drug Administration, a role in which he served in the Obama administration. He also spoke about the need to bolster the nation’s ability to maintain an adequate supply of key medical products, including drugs.
Members of the Senate Health, Education, Labor and Pensions Committee, which is handling Dr. Califf’s nomination, were largely cordial and supportive during the hearing. Sen. Patty Murray (D-Wash.), the committee chair, and the panel’s top Republican, Sen. Richard Burr of North Carolina, addressed Dr. Califf during the hearing as if he would soon serve again as the FDA’s leader. Both were among the senators who voted 89-4 to confirm Dr. Califf in a February 2016 vote.
Dr. Califf “was previously confirmed to lead FDA in an overwhelming bipartisan vote, and I look forward to working with him again to ensure FDA continues to protect families across the country, uphold the gold standard of safety and effectiveness, and put science and data first,” Sen. Murray said.
Less enthusiastic about Dr. Califf was Sen. Bernie Sanders (I-VT), who was among the seven senators who did not vote on Dr. Califf’s nomination in 2016.
Sen. Sanders objected in 2016 to Dr. Califf’s ties to the pharmaceutical industry, and he did so again Tuesday. A noted leader in conducting clinical trials, Dr. Califf has worked with many drugmakers. But at the hearing, Dr. Califf said he concurs with Sen. Sanders on an idea strongly opposed by the pharmaceutical industry.
In response to Sen. Sanders’ question, Dr. Califf said he already is “on record as being in favor of Medicare negotiating with the industry on prices.”
The FDA would not take direct part in negotiations, as this work would be handled by the Centers for Medicare & Medicaid Services. Democrats want to give Medicare some negotiating authority through their sweeping Build Back Better Act.
People in the United States are dismayed over both the cost of prescription drugs and the widespread distribution of prescription painkillers that helped fuel the current opioid epidemic, Sen. Sanders told Dr. Califf. Many people will be concerned about an FDA commissioner who has benefited from close ties to the industry, Sen. Sanders said.
“How are they going to believe that you’re going to be an independent and strong voice against this enormously powerful, special interest?” Sen. Sanders asked.
“I’m totally with you on the concept that the price of pharmaceuticals is way too high in this country,” Dr. Califf said in reply.
Dr. Califf was paid $2.7 million in salary and bonus by Verily Life Sciences, the biomedical research organization operated by Alphabet, parent company of Google, according to his federal financial disclosure. He also reported holding board positions with pharmaceutical companies AmyriAD and Centessa Pharmaceuticals.
Bloomberg Government reported that Dr. Califf has ties to about 16 other research organizations and biotech companies. Bloomberg Government also said that, in his earlier FDA service, Dr. Califf kept a whiteboard in his office that listed all the activities and projects that required his recusal, citing as a source Howard Sklamberg, who was a deputy commissioner under Dr. Califf.
“He was very, very, very careful,” Mr. Sklamberg, who’s now an attorney at Arnold & Porter LLP, told Bloomberg Government.
‘Work to do’ on opioids
Senators looped back repeatedly to the topic of opioids during Dr. Califf’s hearing, reflecting deep concerns about the FDA’s efforts to warn of the risks of prescription painkillers.
There were an estimated 100,306 drug overdose deaths in the United States in the 12 months ending in April, an increase of 28.5% from the 78,056 deaths during the same period the year before, according to the Centers for Disease Control and Prevention.
Dr. Califf said he plans to focus on what information the FDA conveys to the public about the risks of prescription painkillers, including a look at what the labels for these products say.
“I am committed to do a comprehensive review of the status of opioids, early in my tenure,” Dr. Califf said.
Dr. Califf indicated that physicians are still too quick to provide excess doses of these medicines, despite years of efforts to restrain their use. He said he knows relatives who were given 30-day prescriptions for opioids after minor surgery.
“So I know we have work to do,” Dr. Califf said.
Concerns about the FDA’s previous work in managing opioids has led to protests from a few Democratic senators about the prospect of President Biden nominating the acting FDA commissioner, Janet Woodcock, MD, for the permanent post.
At the hearing, Sen. Ben Ray Luján (D-NM) raised the case of the FDA’s approval of the powerful Zohydro painkiller. The agency approved that drug despite an 11-2 vote against it by the FDA’s Anesthetic and Analgesic Drug Products Advisory Committee.
Sen. Luján asked Dr. Califf what he would do if an FDA advisory committee voted “overwhelmingly” against recommending approval of a medicine, as happened in the Zohydro case.
While not mentioned by Sen. Luján in this exchange during the hearing with Dr. Califf, the FDA staff’s rejection of recommendations of advisory committees has been a growing concern among researchers.
The agency last year approved aducanumab (Aduhelm, Biogen), a drug for Alzheimer’s disease, dismissing the advice of its Peripheral and Central Nervous System Drugs Advisory Committee. That decision triggered the resignation of several members of the panel. The FDA staff also earlier rejected the conclusion the majority of members of the same advisory committee offered in 2016 on eteplirsen (Exondys 51, Sarepta), a drug for Duchenne muscular dystrophy.
Dr. Califf told Sen. Luján he had done recent research into how often the FDA staff does not concur with the recommendations of an advisory committee. He said the FDA takes a different course of action in about 25% of cases. In about three-quarters of those cases, the FDA staff opts for a “more stringent” approach regarding allowing the public access to the drug, as opposed to a more generous one as seen in the Zohydro, Aduhelm, and Exondys 51 cases.
Still, Dr. Califf said that when there’s an 11-2 advisory committee vote against recommendation of a product, “the leaders at FDA really need to take a close look” at what’s happening.
Question on accelerated approvals
The FDA’s approval of aducanumab drew attention to a debate already underway about conditional clearances known as accelerated approvals.
The FDA has used this path since the 1990s to speed access to drugs for serious conditions. The trade-off for early access is that the agency sometimes makes the wrong call based on initial findings, and clears a medicine later found not to benefit patients as expected.
The FDA’s cancer division is in the midst of public efforts to address cases where drugmakers have not been able to deliver studies that support accelerated approvals of their oncology drugs. In addition, the Office of Inspector General of the U.S. Department of Health & Human Services announced in August that it is reviewing the FDA’s handling of the accelerated approval process.
At Tuesday’s hearing, Sen. Burr grilled Dr. Califf about how he would respond to calls to change how the FDA handles the accelerated-approval process.
“Can you commit to me and to patients who may rely on cutting-edge treatments that you will not support efforts to narrow this pathway or raise the bar for drugs to be approved under those pathways?” Burr asked Califf.
Dr. Califf responded by saying he was “a fan of accelerated approval – for the right conditions.”
Earlier, in his opening statement, Dr. Califf had said his mother benefited directly from the accelerated approval of new drugs for multiple myeloma. Dr. Califf told Sen. Burr that he had spent “countless hours with patient groups” and understands the need to speed the approval of medicines for serious diseases.
But the FDA also has to make sure it holds up its end of the bargain struck with accelerated approvals. This involves checking on how these medicines work once they are marketed.
“We’re accepting that there’s more uncertainty,” Dr. Califf said. “That means we’ve got to have a better system to evaluate these products as they’re used on the market. And I think there are ways that we can do that now. Technology is making this possible in ways that it just was not possible before.”
Worries about the medical supply chain
Sen. Susan Collins (R-Maine) asked Dr. Califf about the vulnerability of the U.S. medical system to disruptions of the supply chain. She raised concerns about China’s dominance in antibiotic manufacturing as an example. She asked if Congress could do more to encourage domestic manufacturing of medical supplies, such as by offering tax incentives.
Dr. Califf told Sen. Collins he shared her concern about the U.S. manufacturing of ingredients used in both branded and generic drugs. He said he recently has served on a committee of the National Academy of Medicine that is examining supply chain issues.
This committee will soon release a report with specific recommendations, Dr. Califf said.
“We don’t have enough competitive entities in what’s become sort of a commodity business” of drug manufacturing, Dr. Califf said. “So we need a number of steps to make the system more resilient.”
A version of this article first appeared on Medscape.com.
Robert M. Califf, MD, plans to take a close look at federal policies on opioid prescriptions in his expected second turn as the top U.S. regulator of medical products, as well as keep closer tabs on the performance of drugs cleared with accelerated approvals.
Dr. Califf on Tuesday fielded questions at a Senate hearing about his nomination by President Joe Biden to serve as administrator of the U.S. Food and Drug Administration, a role in which he served in the Obama administration. He also spoke about the need to bolster the nation’s ability to maintain an adequate supply of key medical products, including drugs.
Members of the Senate Health, Education, Labor and Pensions Committee, which is handling Dr. Califf’s nomination, were largely cordial and supportive during the hearing. Sen. Patty Murray (D-Wash.), the committee chair, and the panel’s top Republican, Sen. Richard Burr of North Carolina, addressed Dr. Califf during the hearing as if he would soon serve again as the FDA’s leader. Both were among the senators who voted 89-4 to confirm Dr. Califf in a February 2016 vote.
Dr. Califf “was previously confirmed to lead FDA in an overwhelming bipartisan vote, and I look forward to working with him again to ensure FDA continues to protect families across the country, uphold the gold standard of safety and effectiveness, and put science and data first,” Sen. Murray said.
Less enthusiastic about Dr. Califf was Sen. Bernie Sanders (I-VT), who was among the seven senators who did not vote on Dr. Califf’s nomination in 2016.
Sen. Sanders objected in 2016 to Dr. Califf’s ties to the pharmaceutical industry, and he did so again Tuesday. A noted leader in conducting clinical trials, Dr. Califf has worked with many drugmakers. But at the hearing, Dr. Califf said he concurs with Sen. Sanders on an idea strongly opposed by the pharmaceutical industry.
In response to Sen. Sanders’ question, Dr. Califf said he already is “on record as being in favor of Medicare negotiating with the industry on prices.”
The FDA would not take direct part in negotiations, as this work would be handled by the Centers for Medicare & Medicaid Services. Democrats want to give Medicare some negotiating authority through their sweeping Build Back Better Act.
People in the United States are dismayed over both the cost of prescription drugs and the widespread distribution of prescription painkillers that helped fuel the current opioid epidemic, Sen. Sanders told Dr. Califf. Many people will be concerned about an FDA commissioner who has benefited from close ties to the industry, Sen. Sanders said.
“How are they going to believe that you’re going to be an independent and strong voice against this enormously powerful, special interest?” Sen. Sanders asked.
“I’m totally with you on the concept that the price of pharmaceuticals is way too high in this country,” Dr. Califf said in reply.
Dr. Califf was paid $2.7 million in salary and bonus by Verily Life Sciences, the biomedical research organization operated by Alphabet, parent company of Google, according to his federal financial disclosure. He also reported holding board positions with pharmaceutical companies AmyriAD and Centessa Pharmaceuticals.
Bloomberg Government reported that Dr. Califf has ties to about 16 other research organizations and biotech companies. Bloomberg Government also said that, in his earlier FDA service, Dr. Califf kept a whiteboard in his office that listed all the activities and projects that required his recusal, citing as a source Howard Sklamberg, who was a deputy commissioner under Dr. Califf.
“He was very, very, very careful,” Mr. Sklamberg, who’s now an attorney at Arnold & Porter LLP, told Bloomberg Government.
‘Work to do’ on opioids
Senators looped back repeatedly to the topic of opioids during Dr. Califf’s hearing, reflecting deep concerns about the FDA’s efforts to warn of the risks of prescription painkillers.
There were an estimated 100,306 drug overdose deaths in the United States in the 12 months ending in April, an increase of 28.5% from the 78,056 deaths during the same period the year before, according to the Centers for Disease Control and Prevention.
Dr. Califf said he plans to focus on what information the FDA conveys to the public about the risks of prescription painkillers, including a look at what the labels for these products say.
“I am committed to do a comprehensive review of the status of opioids, early in my tenure,” Dr. Califf said.
Dr. Califf indicated that physicians are still too quick to provide excess doses of these medicines, despite years of efforts to restrain their use. He said he knows relatives who were given 30-day prescriptions for opioids after minor surgery.
“So I know we have work to do,” Dr. Califf said.
Concerns about the FDA’s previous work in managing opioids has led to protests from a few Democratic senators about the prospect of President Biden nominating the acting FDA commissioner, Janet Woodcock, MD, for the permanent post.
At the hearing, Sen. Ben Ray Luján (D-NM) raised the case of the FDA’s approval of the powerful Zohydro painkiller. The agency approved that drug despite an 11-2 vote against it by the FDA’s Anesthetic and Analgesic Drug Products Advisory Committee.
Sen. Luján asked Dr. Califf what he would do if an FDA advisory committee voted “overwhelmingly” against recommending approval of a medicine, as happened in the Zohydro case.
While not mentioned by Sen. Luján in this exchange during the hearing with Dr. Califf, the FDA staff’s rejection of recommendations of advisory committees has been a growing concern among researchers.
The agency last year approved aducanumab (Aduhelm, Biogen), a drug for Alzheimer’s disease, dismissing the advice of its Peripheral and Central Nervous System Drugs Advisory Committee. That decision triggered the resignation of several members of the panel. The FDA staff also earlier rejected the conclusion the majority of members of the same advisory committee offered in 2016 on eteplirsen (Exondys 51, Sarepta), a drug for Duchenne muscular dystrophy.
Dr. Califf told Sen. Luján he had done recent research into how often the FDA staff does not concur with the recommendations of an advisory committee. He said the FDA takes a different course of action in about 25% of cases. In about three-quarters of those cases, the FDA staff opts for a “more stringent” approach regarding allowing the public access to the drug, as opposed to a more generous one as seen in the Zohydro, Aduhelm, and Exondys 51 cases.
Still, Dr. Califf said that when there’s an 11-2 advisory committee vote against recommendation of a product, “the leaders at FDA really need to take a close look” at what’s happening.
Question on accelerated approvals
The FDA’s approval of aducanumab drew attention to a debate already underway about conditional clearances known as accelerated approvals.
The FDA has used this path since the 1990s to speed access to drugs for serious conditions. The trade-off for early access is that the agency sometimes makes the wrong call based on initial findings, and clears a medicine later found not to benefit patients as expected.
The FDA’s cancer division is in the midst of public efforts to address cases where drugmakers have not been able to deliver studies that support accelerated approvals of their oncology drugs. In addition, the Office of Inspector General of the U.S. Department of Health & Human Services announced in August that it is reviewing the FDA’s handling of the accelerated approval process.
At Tuesday’s hearing, Sen. Burr grilled Dr. Califf about how he would respond to calls to change how the FDA handles the accelerated-approval process.
“Can you commit to me and to patients who may rely on cutting-edge treatments that you will not support efforts to narrow this pathway or raise the bar for drugs to be approved under those pathways?” Burr asked Califf.
Dr. Califf responded by saying he was “a fan of accelerated approval – for the right conditions.”
Earlier, in his opening statement, Dr. Califf had said his mother benefited directly from the accelerated approval of new drugs for multiple myeloma. Dr. Califf told Sen. Burr that he had spent “countless hours with patient groups” and understands the need to speed the approval of medicines for serious diseases.
But the FDA also has to make sure it holds up its end of the bargain struck with accelerated approvals. This involves checking on how these medicines work once they are marketed.
“We’re accepting that there’s more uncertainty,” Dr. Califf said. “That means we’ve got to have a better system to evaluate these products as they’re used on the market. And I think there are ways that we can do that now. Technology is making this possible in ways that it just was not possible before.”
Worries about the medical supply chain
Sen. Susan Collins (R-Maine) asked Dr. Califf about the vulnerability of the U.S. medical system to disruptions of the supply chain. She raised concerns about China’s dominance in antibiotic manufacturing as an example. She asked if Congress could do more to encourage domestic manufacturing of medical supplies, such as by offering tax incentives.
Dr. Califf told Sen. Collins he shared her concern about the U.S. manufacturing of ingredients used in both branded and generic drugs. He said he recently has served on a committee of the National Academy of Medicine that is examining supply chain issues.
This committee will soon release a report with specific recommendations, Dr. Califf said.
“We don’t have enough competitive entities in what’s become sort of a commodity business” of drug manufacturing, Dr. Califf said. “So we need a number of steps to make the system more resilient.”
A version of this article first appeared on Medscape.com.
iPLEDGE rollout described as a failure, chaotic, and a disaster
The that launched on Dec. 13, and what can be done to fix it.
By most accounts, the rollout was disastrous, chaotic, and a failure. Dermatologists on Twitter and elsewhere are angry and frustrated, with some calling for a temporary halt to the program until the bugs can be ironed out.
On Twitter Dec. 15, the Academy posted: “Due to the unacceptable situation with #iPLEDGE, the @US_FDA has convened an emergency meeting with AADA representatives tomorrow, December 16.”
The switch to a new platform was met with frustration from physicians, pharmacists, and patients alike. The new website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they attempted to follow instructions to enter information. Calls to obtain support from a live person often required hours on hold, several said.
The new approach to the isotretinoin risk-mitigation program itself isn’t under fire. It was welcomed by dermatologists and others who had long requested the change. Instead of three risk categories (females of reproductive potential, females not of reproductive potential, and males), there are now two (those who can get pregnant and those who cannot). Advocates for the change said it will make the experience more inclusive for transgender patients. The previous categories, some contended, were a barrier to access to care.
Because isotretinoin (Absorica, Amnesteem, Claravis, others), an oral retinoid used to treat severe forms of acne, is teratogenic, with a high risk of birth defects, and has also been associated with other health issues, those who take the medication who are able to get pregnant must take contraceptive precautions. The risk evaluation and mitigation program (REMS), mandated by the FDA, stipulates that physicians, patients, and pharmacists prescribing, using, or dispensing the drug must all be registered with requirements that include the use of two forms of an effective contraceptive and regular pregnancy tests by those capable of becoming pregnant.
A day of frustration
Before navigating the new website, a new log-on name was needed, said Ilona J. Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco. “They made you create a month-day-year date of personal significance.” When she tried to log on, she got locked out, she said in an interview.
The transition from the old website to the new, which Dr. Frieden said is now administered by a different vendor, was done quickly. The previous website shut down Dec. 10, and the new one launched Dec. 13, the first day for the new approach.
“A slower rollout would have helped,” Dr. Frieden said. While she and other dermatologists said they offered input previously on how to make the transition go more smoothly, no one seemed to want that help. “We did have a listening session with the FDA,” Dr. Frieden said. That was before the scheduled meeting of Dec. 16.
Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, also was frustrated with the rollout. “The week before the transition, one of my staff had to call iPLEDGE. They had a 177-minute wait to get to a human.
“They want us to register patients online now instead of signing forms in the office, but the links to view, download, or print don’t work,” Dr. Goldberg said in an interview.
This was after receiving information from the iPLEDGE REMS program, which stated, “The iPLEDGE REMS website will be updated to a modernized platform. All program materials and educational tools will be now available to you at the click of a button.’’
Dr. Goldberg also received calls from three patients who reported that they couldn’t complete the quiz that is required of patients capable of reproducing to demonstrate their comprehension about risk. Without the completed quiz, required monthly, the prescription can’t be refilled.
“It’s chaotic,” said Howa Yeung, MD, assistant professor of dermatology at Emory University, Atlanta. “The change is sudden, it’s a major change in the workflow. The process of reverification [required] is not that hard, but a lot of people have trouble even logging into the platform.”
What would help? To have a human on the phone to help navigate the system, Dr. Yeung said.
The glitches are delaying prescriptions for established patients and new ones as well, Dr. Yeung said. Existing patients who can get pregnant have 7 days after their negative pregnancy test to get their prescription filled. “And over the weekend the website was down,” he said, so that was a 2-day delay.
“The information we have and were told to use doesn’t match what is in their database,” said Mitesh Patel, PharmD, owner of Sunshine Pharmacy in White Plains, N.Y., who said pharmacists are experiencing issues with the new platform similar to those of doctors.
Twitter users had a lot to say, as well. Jack Resneck Jr., MD, professor of dermatology at the University of California, San Francisco, tweeted: “#Accutane has basically been pulled from market by utter incompetence of @SyneosHealth hired by @US_FDA to administer risk mgmt program.”
Dr. Resneck, president-elect of the American Medical Association, noted the crashed website, help line with 6-hour hold times, and patients unable to get the drug.
Adewole Adamson, MD, a dermatologist at the University of Texas, Austin, tweeted, “Dermatologists around the US are BIG mad about the current accutane debacle brought on by @SyneosHealth and @US_FDA. What a disaster for patient care!”
Several called for the FDA to immediately halt the program and let physicians manage the risk until the platform could be improved.
Are fixes in sight?
On Tuesday, Dec. 14, AADA President Kenneth J. Tomecki, MD, issued a statement expressing disappointment about the transition.
“In advance of this transition, the AADA engaged the FDA and the iPLEDGE administrator, Syneos Health, about the numerous workflow concerns raised by dermatologists and how the impending changes would threaten patient access to necessary medication. Those concerns have become a reality across the country and we’re working to ensure patients can maintain safe and appropriate access to the treatment they need.”
The AADA, the statement continues, supports efforts to streamline the program while keeping patient safety and incorporating input from physicians.
“We are very aware of the problems with the implementation of the iPLEDGE program,” FDA spokesperson Charlie Kohler said in an email. “We are continuing to work closely with the isotretinoin manufacturers to ensure that they implement a smoothly functioning iPLEDGE REMS program and that patient care is not interrupted.”
“Syneos Health appreciates the concern about iPLEDGE,” said Gary Gatyas, a spokesperson for Syneos Health. “While Syneos Health does not maintain the iPLEDGE system or contact center, we are doing what we can to help the responsible parties with a resolution.” Meanwhile, he recommended that people contact the call center.
He did not respond immediately to questions about who is responsible for maintaining the system and call center.
Dr. Goldberg, Dr. Frieden, and Dr. Yeung have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The that launched on Dec. 13, and what can be done to fix it.
By most accounts, the rollout was disastrous, chaotic, and a failure. Dermatologists on Twitter and elsewhere are angry and frustrated, with some calling for a temporary halt to the program until the bugs can be ironed out.
On Twitter Dec. 15, the Academy posted: “Due to the unacceptable situation with #iPLEDGE, the @US_FDA has convened an emergency meeting with AADA representatives tomorrow, December 16.”
The switch to a new platform was met with frustration from physicians, pharmacists, and patients alike. The new website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they attempted to follow instructions to enter information. Calls to obtain support from a live person often required hours on hold, several said.
The new approach to the isotretinoin risk-mitigation program itself isn’t under fire. It was welcomed by dermatologists and others who had long requested the change. Instead of three risk categories (females of reproductive potential, females not of reproductive potential, and males), there are now two (those who can get pregnant and those who cannot). Advocates for the change said it will make the experience more inclusive for transgender patients. The previous categories, some contended, were a barrier to access to care.
Because isotretinoin (Absorica, Amnesteem, Claravis, others), an oral retinoid used to treat severe forms of acne, is teratogenic, with a high risk of birth defects, and has also been associated with other health issues, those who take the medication who are able to get pregnant must take contraceptive precautions. The risk evaluation and mitigation program (REMS), mandated by the FDA, stipulates that physicians, patients, and pharmacists prescribing, using, or dispensing the drug must all be registered with requirements that include the use of two forms of an effective contraceptive and regular pregnancy tests by those capable of becoming pregnant.
A day of frustration
Before navigating the new website, a new log-on name was needed, said Ilona J. Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco. “They made you create a month-day-year date of personal significance.” When she tried to log on, she got locked out, she said in an interview.
The transition from the old website to the new, which Dr. Frieden said is now administered by a different vendor, was done quickly. The previous website shut down Dec. 10, and the new one launched Dec. 13, the first day for the new approach.
“A slower rollout would have helped,” Dr. Frieden said. While she and other dermatologists said they offered input previously on how to make the transition go more smoothly, no one seemed to want that help. “We did have a listening session with the FDA,” Dr. Frieden said. That was before the scheduled meeting of Dec. 16.
Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, also was frustrated with the rollout. “The week before the transition, one of my staff had to call iPLEDGE. They had a 177-minute wait to get to a human.
“They want us to register patients online now instead of signing forms in the office, but the links to view, download, or print don’t work,” Dr. Goldberg said in an interview.
This was after receiving information from the iPLEDGE REMS program, which stated, “The iPLEDGE REMS website will be updated to a modernized platform. All program materials and educational tools will be now available to you at the click of a button.’’
Dr. Goldberg also received calls from three patients who reported that they couldn’t complete the quiz that is required of patients capable of reproducing to demonstrate their comprehension about risk. Without the completed quiz, required monthly, the prescription can’t be refilled.
“It’s chaotic,” said Howa Yeung, MD, assistant professor of dermatology at Emory University, Atlanta. “The change is sudden, it’s a major change in the workflow. The process of reverification [required] is not that hard, but a lot of people have trouble even logging into the platform.”
What would help? To have a human on the phone to help navigate the system, Dr. Yeung said.
The glitches are delaying prescriptions for established patients and new ones as well, Dr. Yeung said. Existing patients who can get pregnant have 7 days after their negative pregnancy test to get their prescription filled. “And over the weekend the website was down,” he said, so that was a 2-day delay.
“The information we have and were told to use doesn’t match what is in their database,” said Mitesh Patel, PharmD, owner of Sunshine Pharmacy in White Plains, N.Y., who said pharmacists are experiencing issues with the new platform similar to those of doctors.
Twitter users had a lot to say, as well. Jack Resneck Jr., MD, professor of dermatology at the University of California, San Francisco, tweeted: “#Accutane has basically been pulled from market by utter incompetence of @SyneosHealth hired by @US_FDA to administer risk mgmt program.”
Dr. Resneck, president-elect of the American Medical Association, noted the crashed website, help line with 6-hour hold times, and patients unable to get the drug.
Adewole Adamson, MD, a dermatologist at the University of Texas, Austin, tweeted, “Dermatologists around the US are BIG mad about the current accutane debacle brought on by @SyneosHealth and @US_FDA. What a disaster for patient care!”
Several called for the FDA to immediately halt the program and let physicians manage the risk until the platform could be improved.
Are fixes in sight?
On Tuesday, Dec. 14, AADA President Kenneth J. Tomecki, MD, issued a statement expressing disappointment about the transition.
“In advance of this transition, the AADA engaged the FDA and the iPLEDGE administrator, Syneos Health, about the numerous workflow concerns raised by dermatologists and how the impending changes would threaten patient access to necessary medication. Those concerns have become a reality across the country and we’re working to ensure patients can maintain safe and appropriate access to the treatment they need.”
The AADA, the statement continues, supports efforts to streamline the program while keeping patient safety and incorporating input from physicians.
“We are very aware of the problems with the implementation of the iPLEDGE program,” FDA spokesperson Charlie Kohler said in an email. “We are continuing to work closely with the isotretinoin manufacturers to ensure that they implement a smoothly functioning iPLEDGE REMS program and that patient care is not interrupted.”
“Syneos Health appreciates the concern about iPLEDGE,” said Gary Gatyas, a spokesperson for Syneos Health. “While Syneos Health does not maintain the iPLEDGE system or contact center, we are doing what we can to help the responsible parties with a resolution.” Meanwhile, he recommended that people contact the call center.
He did not respond immediately to questions about who is responsible for maintaining the system and call center.
Dr. Goldberg, Dr. Frieden, and Dr. Yeung have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The that launched on Dec. 13, and what can be done to fix it.
By most accounts, the rollout was disastrous, chaotic, and a failure. Dermatologists on Twitter and elsewhere are angry and frustrated, with some calling for a temporary halt to the program until the bugs can be ironed out.
On Twitter Dec. 15, the Academy posted: “Due to the unacceptable situation with #iPLEDGE, the @US_FDA has convened an emergency meeting with AADA representatives tomorrow, December 16.”
The switch to a new platform was met with frustration from physicians, pharmacists, and patients alike. The new website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they attempted to follow instructions to enter information. Calls to obtain support from a live person often required hours on hold, several said.
The new approach to the isotretinoin risk-mitigation program itself isn’t under fire. It was welcomed by dermatologists and others who had long requested the change. Instead of three risk categories (females of reproductive potential, females not of reproductive potential, and males), there are now two (those who can get pregnant and those who cannot). Advocates for the change said it will make the experience more inclusive for transgender patients. The previous categories, some contended, were a barrier to access to care.
Because isotretinoin (Absorica, Amnesteem, Claravis, others), an oral retinoid used to treat severe forms of acne, is teratogenic, with a high risk of birth defects, and has also been associated with other health issues, those who take the medication who are able to get pregnant must take contraceptive precautions. The risk evaluation and mitigation program (REMS), mandated by the FDA, stipulates that physicians, patients, and pharmacists prescribing, using, or dispensing the drug must all be registered with requirements that include the use of two forms of an effective contraceptive and regular pregnancy tests by those capable of becoming pregnant.
A day of frustration
Before navigating the new website, a new log-on name was needed, said Ilona J. Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco. “They made you create a month-day-year date of personal significance.” When she tried to log on, she got locked out, she said in an interview.
The transition from the old website to the new, which Dr. Frieden said is now administered by a different vendor, was done quickly. The previous website shut down Dec. 10, and the new one launched Dec. 13, the first day for the new approach.
“A slower rollout would have helped,” Dr. Frieden said. While she and other dermatologists said they offered input previously on how to make the transition go more smoothly, no one seemed to want that help. “We did have a listening session with the FDA,” Dr. Frieden said. That was before the scheduled meeting of Dec. 16.
Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, also was frustrated with the rollout. “The week before the transition, one of my staff had to call iPLEDGE. They had a 177-minute wait to get to a human.
“They want us to register patients online now instead of signing forms in the office, but the links to view, download, or print don’t work,” Dr. Goldberg said in an interview.
This was after receiving information from the iPLEDGE REMS program, which stated, “The iPLEDGE REMS website will be updated to a modernized platform. All program materials and educational tools will be now available to you at the click of a button.’’
Dr. Goldberg also received calls from three patients who reported that they couldn’t complete the quiz that is required of patients capable of reproducing to demonstrate their comprehension about risk. Without the completed quiz, required monthly, the prescription can’t be refilled.
“It’s chaotic,” said Howa Yeung, MD, assistant professor of dermatology at Emory University, Atlanta. “The change is sudden, it’s a major change in the workflow. The process of reverification [required] is not that hard, but a lot of people have trouble even logging into the platform.”
What would help? To have a human on the phone to help navigate the system, Dr. Yeung said.
The glitches are delaying prescriptions for established patients and new ones as well, Dr. Yeung said. Existing patients who can get pregnant have 7 days after their negative pregnancy test to get their prescription filled. “And over the weekend the website was down,” he said, so that was a 2-day delay.
“The information we have and were told to use doesn’t match what is in their database,” said Mitesh Patel, PharmD, owner of Sunshine Pharmacy in White Plains, N.Y., who said pharmacists are experiencing issues with the new platform similar to those of doctors.
Twitter users had a lot to say, as well. Jack Resneck Jr., MD, professor of dermatology at the University of California, San Francisco, tweeted: “#Accutane has basically been pulled from market by utter incompetence of @SyneosHealth hired by @US_FDA to administer risk mgmt program.”
Dr. Resneck, president-elect of the American Medical Association, noted the crashed website, help line with 6-hour hold times, and patients unable to get the drug.
Adewole Adamson, MD, a dermatologist at the University of Texas, Austin, tweeted, “Dermatologists around the US are BIG mad about the current accutane debacle brought on by @SyneosHealth and @US_FDA. What a disaster for patient care!”
Several called for the FDA to immediately halt the program and let physicians manage the risk until the platform could be improved.
Are fixes in sight?
On Tuesday, Dec. 14, AADA President Kenneth J. Tomecki, MD, issued a statement expressing disappointment about the transition.
“In advance of this transition, the AADA engaged the FDA and the iPLEDGE administrator, Syneos Health, about the numerous workflow concerns raised by dermatologists and how the impending changes would threaten patient access to necessary medication. Those concerns have become a reality across the country and we’re working to ensure patients can maintain safe and appropriate access to the treatment they need.”
The AADA, the statement continues, supports efforts to streamline the program while keeping patient safety and incorporating input from physicians.
“We are very aware of the problems with the implementation of the iPLEDGE program,” FDA spokesperson Charlie Kohler said in an email. “We are continuing to work closely with the isotretinoin manufacturers to ensure that they implement a smoothly functioning iPLEDGE REMS program and that patient care is not interrupted.”
“Syneos Health appreciates the concern about iPLEDGE,” said Gary Gatyas, a spokesperson for Syneos Health. “While Syneos Health does not maintain the iPLEDGE system or contact center, we are doing what we can to help the responsible parties with a resolution.” Meanwhile, he recommended that people contact the call center.
He did not respond immediately to questions about who is responsible for maintaining the system and call center.
Dr. Goldberg, Dr. Frieden, and Dr. Yeung have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA updates risks, cautions for clotting-bleeding disorder on Janssen COVID-19 vaccine
Updated Janssen/Johnson & Johnson COVID-19 vaccine fact sheets for health care professionals and the general public now include a contraindication to its use in persons with a history of thrombosis with thrombocytopenia after receiving it “or any other adenovirus-vectored COVID-19 vaccine,” the U.S. Food and Drug Administration has announced.
Thrombosis with thrombocytopenia syndrome (TTS) – thrombocytopenia and increased bleeding risk along with documented thrombosis – after administration of the Janssen Ad26.COV2.S vaccine remains rare. But over all age groups, about one in seven cases have been fatal, said the agency.
the provider fact sheet states.
Although TTS associated with the Janssen COVID-19 vaccine has been reported in men and women aged 18 and older, the highest reported rate has been for women aged 30-49, the agency states. The rate in that group has been about 1 case per 100,000 doses administered.
Symptoms of TTS may occur 1-2 weeks after administration of the Janssen COVID-19 vaccine, the FDA says, based on data from the Vaccine Adverse Events Reporting System (VAERS).
Its clinical course shares features with autoimmune heparin-induced thrombocytopenia. In individuals with suspected TTS following receipt of the Janssen COVID-19 vaccine, the agency cautions, the use of heparin “may be harmful and alternative treatments may be needed. Consultation with hematology specialists is strongly recommended.”
The apparent excess risk of TTS remains under investigation, but “the FDA continues to find that the known and potential benefits of the Janssen COVID-19 vaccine outweigh its known and potential risks in individuals 18 years of age and older,” the agency states.
A version of this article first appeared on Medscape.com.
Updated Janssen/Johnson & Johnson COVID-19 vaccine fact sheets for health care professionals and the general public now include a contraindication to its use in persons with a history of thrombosis with thrombocytopenia after receiving it “or any other adenovirus-vectored COVID-19 vaccine,” the U.S. Food and Drug Administration has announced.
Thrombosis with thrombocytopenia syndrome (TTS) – thrombocytopenia and increased bleeding risk along with documented thrombosis – after administration of the Janssen Ad26.COV2.S vaccine remains rare. But over all age groups, about one in seven cases have been fatal, said the agency.
the provider fact sheet states.
Although TTS associated with the Janssen COVID-19 vaccine has been reported in men and women aged 18 and older, the highest reported rate has been for women aged 30-49, the agency states. The rate in that group has been about 1 case per 100,000 doses administered.
Symptoms of TTS may occur 1-2 weeks after administration of the Janssen COVID-19 vaccine, the FDA says, based on data from the Vaccine Adverse Events Reporting System (VAERS).
Its clinical course shares features with autoimmune heparin-induced thrombocytopenia. In individuals with suspected TTS following receipt of the Janssen COVID-19 vaccine, the agency cautions, the use of heparin “may be harmful and alternative treatments may be needed. Consultation with hematology specialists is strongly recommended.”
The apparent excess risk of TTS remains under investigation, but “the FDA continues to find that the known and potential benefits of the Janssen COVID-19 vaccine outweigh its known and potential risks in individuals 18 years of age and older,” the agency states.
A version of this article first appeared on Medscape.com.
Updated Janssen/Johnson & Johnson COVID-19 vaccine fact sheets for health care professionals and the general public now include a contraindication to its use in persons with a history of thrombosis with thrombocytopenia after receiving it “or any other adenovirus-vectored COVID-19 vaccine,” the U.S. Food and Drug Administration has announced.
Thrombosis with thrombocytopenia syndrome (TTS) – thrombocytopenia and increased bleeding risk along with documented thrombosis – after administration of the Janssen Ad26.COV2.S vaccine remains rare. But over all age groups, about one in seven cases have been fatal, said the agency.
the provider fact sheet states.
Although TTS associated with the Janssen COVID-19 vaccine has been reported in men and women aged 18 and older, the highest reported rate has been for women aged 30-49, the agency states. The rate in that group has been about 1 case per 100,000 doses administered.
Symptoms of TTS may occur 1-2 weeks after administration of the Janssen COVID-19 vaccine, the FDA says, based on data from the Vaccine Adverse Events Reporting System (VAERS).
Its clinical course shares features with autoimmune heparin-induced thrombocytopenia. In individuals with suspected TTS following receipt of the Janssen COVID-19 vaccine, the agency cautions, the use of heparin “may be harmful and alternative treatments may be needed. Consultation with hematology specialists is strongly recommended.”
The apparent excess risk of TTS remains under investigation, but “the FDA continues to find that the known and potential benefits of the Janssen COVID-19 vaccine outweigh its known and potential risks in individuals 18 years of age and older,” the agency states.
A version of this article first appeared on Medscape.com.
A pandemic silver lining? Dramatic drop in teen drug use
Illicit drug use among U.S. teenagers dropped sharply in 2021, likely because of stay-at-home orders and other restrictions on social activities due to the COVID-19 pandemic.
The latest findings, from the Monitoring the Future survey, represent the largest 1-year decrease in overall illicit drug use reported since the survey began in 1975.
“We have never seen such dramatic decreases in drug use among teens in just a 1-year period,” Nora Volkow, MD, director of the National Institute on Drug Abuse (NIDA), said in a news release.
“These data are unprecedented and highlight one unexpected potential consequence of the COVID-19 pandemic, which caused seismic shifts in the day-to-day lives of adolescents,” said Dr. Volkow.
The annual Monitoring the Future survey is conducted by researchers at the University of Michigan, Ann Arbor, and funded by NIDA, to assess drug and alcohol use and related attitudes among adolescent students across the United States.
This year’s self-reported survey included 32,260 students in grades 8, 10, and 12 across 319 public and private schools.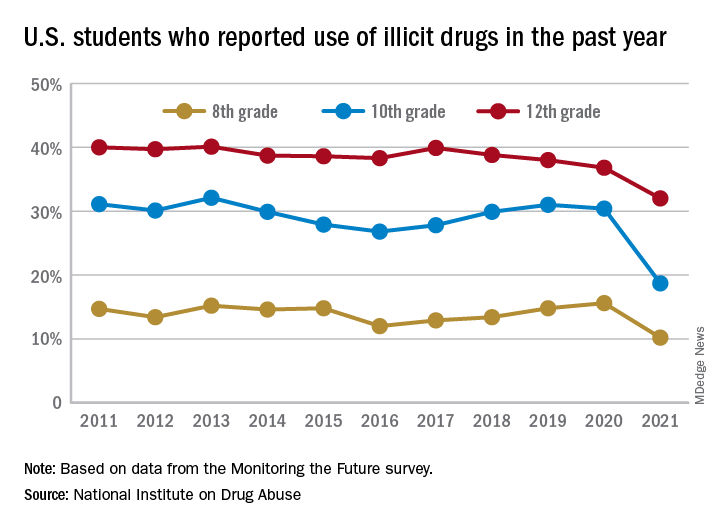
Compared with 2020, the percentage of students reporting any illicit drug use (other than marijuana) in 2021 decreased significantly for 8th graders (down 5.4%), 10th graders (down 11.7%), and 12th graders (down 4.8%).
For alcohol, about 47% of 12th graders and 29% of 10th graders said they drank alcohol in 2021, down significantly from 55% and 41%, respectively, in 2020. The percentage of 8th graders who said they drank alcohol remained stable (17% in 2021 and 20% in 2020).
For teen vaping, about 27% of 12th graders and 20% of 10th graders said they had vaped nicotine in 2021, down significantly from nearly 35% and 31%, respectively, in 2020. Fewer 8th graders also vaped nicotine in 2021 compared with 2020 (12% vs. 17%).
For marijuana, use dropped significantly for all three grades in 2021 compared with 2020. About 31% of 12th graders and 17% of 10th graders said they used marijuana in 2021, down from 35% and 28% in 2020. Among 8th graders, 7% used marijuana in 2021, down from 11% in 2020.
The latest survey also shows significant declines in use of a range of other drugs for many of the age cohorts, including cocaine, hallucinogens, and nonmedical use of amphetamines, tranquilizers, and prescription opioids.
“We knew that this year’s data would illuminate how the COVID-19 pandemic may have impacted substance use among young people, and in the coming years, we will find out whether those impacts are long-lasting as we continue tracking the drug use patterns of these unique cohorts of adolescents,” Richard A. Miech, PhD, who heads the Monitoring the Future study at the University of Michigan, said in the news release.
“Moving forward, it will be crucial to identify the pivotal elements of this past year that contributed to decreased drug use – whether related to drug availability, family involvement, differences in peer pressure, or other factors – and harness them to inform future prevention efforts,” Dr. Volkow added.
In 2021, students across all age groups reported moderate increases in feelings of boredom, anxiety, depression, loneliness, worry, difficulty sleeping, and other negative mental health indicators since the beginning of the pandemic.
A version of this article first appeared on Medscape.com.
Illicit drug use among U.S. teenagers dropped sharply in 2021, likely because of stay-at-home orders and other restrictions on social activities due to the COVID-19 pandemic.
The latest findings, from the Monitoring the Future survey, represent the largest 1-year decrease in overall illicit drug use reported since the survey began in 1975.
“We have never seen such dramatic decreases in drug use among teens in just a 1-year period,” Nora Volkow, MD, director of the National Institute on Drug Abuse (NIDA), said in a news release.
“These data are unprecedented and highlight one unexpected potential consequence of the COVID-19 pandemic, which caused seismic shifts in the day-to-day lives of adolescents,” said Dr. Volkow.
The annual Monitoring the Future survey is conducted by researchers at the University of Michigan, Ann Arbor, and funded by NIDA, to assess drug and alcohol use and related attitudes among adolescent students across the United States.
This year’s self-reported survey included 32,260 students in grades 8, 10, and 12 across 319 public and private schools.
Compared with 2020, the percentage of students reporting any illicit drug use (other than marijuana) in 2021 decreased significantly for 8th graders (down 5.4%), 10th graders (down 11.7%), and 12th graders (down 4.8%).
For alcohol, about 47% of 12th graders and 29% of 10th graders said they drank alcohol in 2021, down significantly from 55% and 41%, respectively, in 2020. The percentage of 8th graders who said they drank alcohol remained stable (17% in 2021 and 20% in 2020).
For teen vaping, about 27% of 12th graders and 20% of 10th graders said they had vaped nicotine in 2021, down significantly from nearly 35% and 31%, respectively, in 2020. Fewer 8th graders also vaped nicotine in 2021 compared with 2020 (12% vs. 17%).
For marijuana, use dropped significantly for all three grades in 2021 compared with 2020. About 31% of 12th graders and 17% of 10th graders said they used marijuana in 2021, down from 35% and 28% in 2020. Among 8th graders, 7% used marijuana in 2021, down from 11% in 2020.
The latest survey also shows significant declines in use of a range of other drugs for many of the age cohorts, including cocaine, hallucinogens, and nonmedical use of amphetamines, tranquilizers, and prescription opioids.
“We knew that this year’s data would illuminate how the COVID-19 pandemic may have impacted substance use among young people, and in the coming years, we will find out whether those impacts are long-lasting as we continue tracking the drug use patterns of these unique cohorts of adolescents,” Richard A. Miech, PhD, who heads the Monitoring the Future study at the University of Michigan, said in the news release.
“Moving forward, it will be crucial to identify the pivotal elements of this past year that contributed to decreased drug use – whether related to drug availability, family involvement, differences in peer pressure, or other factors – and harness them to inform future prevention efforts,” Dr. Volkow added.
In 2021, students across all age groups reported moderate increases in feelings of boredom, anxiety, depression, loneliness, worry, difficulty sleeping, and other negative mental health indicators since the beginning of the pandemic.
A version of this article first appeared on Medscape.com.
Illicit drug use among U.S. teenagers dropped sharply in 2021, likely because of stay-at-home orders and other restrictions on social activities due to the COVID-19 pandemic.
The latest findings, from the Monitoring the Future survey, represent the largest 1-year decrease in overall illicit drug use reported since the survey began in 1975.
“We have never seen such dramatic decreases in drug use among teens in just a 1-year period,” Nora Volkow, MD, director of the National Institute on Drug Abuse (NIDA), said in a news release.
“These data are unprecedented and highlight one unexpected potential consequence of the COVID-19 pandemic, which caused seismic shifts in the day-to-day lives of adolescents,” said Dr. Volkow.
The annual Monitoring the Future survey is conducted by researchers at the University of Michigan, Ann Arbor, and funded by NIDA, to assess drug and alcohol use and related attitudes among adolescent students across the United States.
This year’s self-reported survey included 32,260 students in grades 8, 10, and 12 across 319 public and private schools.
Compared with 2020, the percentage of students reporting any illicit drug use (other than marijuana) in 2021 decreased significantly for 8th graders (down 5.4%), 10th graders (down 11.7%), and 12th graders (down 4.8%).
For alcohol, about 47% of 12th graders and 29% of 10th graders said they drank alcohol in 2021, down significantly from 55% and 41%, respectively, in 2020. The percentage of 8th graders who said they drank alcohol remained stable (17% in 2021 and 20% in 2020).
For teen vaping, about 27% of 12th graders and 20% of 10th graders said they had vaped nicotine in 2021, down significantly from nearly 35% and 31%, respectively, in 2020. Fewer 8th graders also vaped nicotine in 2021 compared with 2020 (12% vs. 17%).
For marijuana, use dropped significantly for all three grades in 2021 compared with 2020. About 31% of 12th graders and 17% of 10th graders said they used marijuana in 2021, down from 35% and 28% in 2020. Among 8th graders, 7% used marijuana in 2021, down from 11% in 2020.
The latest survey also shows significant declines in use of a range of other drugs for many of the age cohorts, including cocaine, hallucinogens, and nonmedical use of amphetamines, tranquilizers, and prescription opioids.
“We knew that this year’s data would illuminate how the COVID-19 pandemic may have impacted substance use among young people, and in the coming years, we will find out whether those impacts are long-lasting as we continue tracking the drug use patterns of these unique cohorts of adolescents,” Richard A. Miech, PhD, who heads the Monitoring the Future study at the University of Michigan, said in the news release.
“Moving forward, it will be crucial to identify the pivotal elements of this past year that contributed to decreased drug use – whether related to drug availability, family involvement, differences in peer pressure, or other factors – and harness them to inform future prevention efforts,” Dr. Volkow added.
In 2021, students across all age groups reported moderate increases in feelings of boredom, anxiety, depression, loneliness, worry, difficulty sleeping, and other negative mental health indicators since the beginning of the pandemic.
A version of this article first appeared on Medscape.com.
Medicare insulin negotiations seen saving $17 billion
Medicare could have saved more than $16.7 billion on three kinds of insulin products from 2011 to 2017 if it had secured the same discounts other federal health programs get through negotiations, House Democrats argue in a new report.
On Dec. 10, Democrats on the House Committee on Oversight and Reform released a final majority staff report, which they say is the culmination of an almost 3-year investigation into pharmaceutical pricing and business practices. The report draws from 1.5 million pages of internal company documents, the committee says.
Documents from insulin makers Eli Lilly, Novo Nordisk, and Sanofi indicate these firms “raised their prices in lockstep in order to maintain ‘pricing parity’,” with senior executives encouraging the practice, the committee staff writes in the report.
“In a discussion among Novo Nordisk employees about an Eli Lilly price increase for a different diabetes product on Dec. 24, 2015, a Novo Nordisk pricing analyst remarked, ‘[M]aybe Sanofi will wait until tomorrow morning to announce their price increase ... that’s all I want for Christmas,’” the report states.
House Democrats are seeking to use the report findings to aid their Senate colleagues’ attempt to pass the sweeping Build Back Better bill, which includes many provisions addressing drug costs.
It’s still unclear when the Senate will act on the measure. The House passed the Build Back Better bill, 220-213, in November. It includes a provision that would allow Medicare to negotiate the prices of certain drugs covered by Part D pharmacy plans.
That would mark a reversal of the stance taken when Congress created the pharmacy benefit in a 2003 law, which left negotiations to insurers that cover Part D plans.
Republicans have long argued insurers get the best deals on drugs for people on Medicare. Democrats say this approach sacrifices much of Medicare’s bargaining clout, scattering it among plans.
“This fight has been going on since the Medicare Part D legislation which gave away the store” to drugmakers, said Speaker Nancy Pelosi (D-CA) at a Dec. 10 press conference about the House Oversight report. “And they got used to having the store to themselves.”
The Endocrine Society is urging the Senate to protect the insulin affordability provisions included in the Build Back Better Act and move quickly to pass this crucial legislation.
“We implore all Senators to ensure these provisions are not scaled back. The Build Back Better Act represents the best opportunity to address the price of insulin. Millions of Americans cannot wait any longer for a solution,” it said in a statement issued Dec. 14.
Better deals for military, medicaid programs
Medicare is unusual among federal programs in that it doesn’t directly leverage its clout to lower drug costs.
Total Part D expenditures were approximately $105 billion last year, according to Medicare’s board of trustees. This spending is divided among the many insurers that run Part D plans, which then make a myriad of decisions about formularies and other factors that affect pricing.
For drugs administered by clinicians, and thus covered by Medicare Part B, the program pays a premium of the reported average sales price. Part B drug spending was $39 billion in 2019, an increase of about 11.6% from the previous year, according to the Medicare Payment Advisory Commission.
In contrast, federal law calls for steep reductions in drug prices for people on Medicaid.
The Department of Veterans Affairs (VA) and the Defense Department (DoD)’s Tricare program use several bargaining strategies to lower prices. To control costs, VA and DoD often use formularies of preferred drugs, steer patients to lower-cost drugs, and buy drugs in large volumes, “all of which increase their leverage with drug manufacturers,” the staff of the Congressional Budget Office (CBO) wrote in a Feb. 2021 report.
The CBO report examines how those different federal agencies’ approaches played out in terms of prices, net of applicable rebates, and discounts of 176 top-selling brand-name drugs in Medicare Part D.
The average price for this group of drugs was $118 in Medicaid. And for VA and DoD, the average prices were $190 and $184, respectively, for drugs dispensed at the agencies’ medical facilities or by mail.
But for Medicare Part D, the average price was $343, CBO said in the report, which was one of the sources consulted by House Oversight staff when developing their report released on Dec. 10.
Insulin still of interest, 100 years after its discovery
The House Oversight report runs to almost 270 pages. It addresses several issues with drug prices, including strategies pharmaceutical companies have used to thwart generic competition. On Monday, the trade group America’s Health Insurance Plans separately released its own report looking at patents and delays to the introduction of generic drugs.
Yet, much of the debate on drug prices has focused on one of the oldest widely produced prescription drugs, insulin.
Even with the allowance of generic competition for the essential medicine, branded versions of insulin have been some of the costliest products for Medicare in recent years. Eli Lilly, Novo Nordisk, and Sanofi dominate the insulin market.
Medicare Part D spent about $2.5 billion in 2019 on Sanofi’s Lantus Solostar insulin, or about $2,585 per person in the program using it. The program also paid about $1.1 billion for another form of Lantus, or about $2,746 per patient.
Medicare Part D also spent about $1.84 billion in 2019 on Novo Nordisk’s NovoLog FlexPen, or about $3,063 per person.
Medicare Part D’s drug spending dashboard also lists eight versions of Lilly’s Humalog, with combined 2019 spending of more than $2 billion. The cost per patient in Medicare Part D ranges from $5,619 to $1,462.
“Over the past 20 years, they have repeatedly and dramatically raised the list prices of their rapid-acting and long-acting insulins and reaped billions of dollars in revenues,” write the House Oversight staff in their report.
Republicans on the House Oversight and Reform Committee disagree with their Democratic colleagues on many points in the debate on drug prices, but they also looked at insulin as a cause for concern.
GOP members of the committee released a separate report on Dec. 10. They call for greater clarity into the role middlemen in the drug-supply chain – known as pharmaceutical benefit managers – may play in the rising costs of medicines. The GOP report notes that there are bills pending in the House that would seek to steer any discounts offered on insulin within the supply chain toward consumers (Insulin Price Reduction Act H.R. 4906, Insulin Cost Reduction Act H.R. 5623).
Democratic staff in the committee’s report seek to draw attention to how manufacturers priced their insulin products, including the comment by the Novo Nordisk employee about wishing for a price hike for a competitor’s product.
In a statement provided to this news organization, Novo Nordisk said the committee’s report reflects “a limited picture of the efforts put forth by our company and other companies to manage formulary access.”
“This glimpse into the complexity of pricing, formularies, and the health care system demonstrates why Novo Nordisk continues to advocate for comprehensive solutions,” Denmark’s Novo Nordisk said in the statement.
$35 a month for insulin?
Paris-based Sanofi said it makes insulin-pricing decisions independently from competitors. Sanofi said the net price of its insulins has declined by 53% since 2012, arguing the high prices charged to patients reflect decisions made elsewhere in the supply chain.
“Over the same period, the net price for commercial and Medicare Part D plans of Lantus has fallen 44.9%, while average out-of-pocket costs for patients with commercial insurance and Medicare Part D has risen approximately 82%,” Sanofi said.
“For all the focus on the growth of list prices, today, the average net price of Lantus is below 2006 levels. That is why we support policy reforms to require health plans to share negotiated savings with patients by requiring patient cost-sharing be tied to the net prices.”
Indianapolis-based Lilly offered a similar response in a statement to this news organization.
“Lilly, like other companies, monitors competitor list-price changes that are available through publicly available services,” the company said. “However, any changes we make to our list prices are independent decisions, and to the extent they consider competitors they are informed only through publicly available data.”
Despite rising insurance deductibles, the average monthly out-of-pocket cost for Lilly insulin has dropped 27% to $28.05 over the past 4 years, the company said in an interview. Lilly also noted that there are “several affordability options now available” allowing people to purchase their monthly prescription of its insulin for $35, “whether they are uninsured or use commercial insurance, Medicaid, or a participating Medicare Part D plan.”
In 2020, Lilly had announced that people with commercial insurance and those without insurance would be able to get monthly prescriptions of Lilly insulin for $35.
The Build Back Better Act would require insurers, including Medicare Part D plans and private group or individual health plans, to charge patient cost-sharing of no more than $35 per month for insulin products, said the staff of the nonprofit Kaiser Family Foundation (KFF) in a review of the bill.
“Private group or individual plans would not be required to cover all insulin products, just one of each dosage form (vial, pen) and insulin type (rapid-acting, short-acting, intermediate-acting, and long-acting), for no more than $35,” the KFF staff state in the report.
People enrolled in Medicare can already choose to enroll in a Part D plan participating in a federal test program that can secure certain insulin products for them at a monthly copayment of $35. In 2022, a total of 2,159 Part D plans will participate in this model, a 32% increase in participating plans since 2021, KFF said.
A version of this article first appeared on Medscape.com.
Medicare could have saved more than $16.7 billion on three kinds of insulin products from 2011 to 2017 if it had secured the same discounts other federal health programs get through negotiations, House Democrats argue in a new report.
On Dec. 10, Democrats on the House Committee on Oversight and Reform released a final majority staff report, which they say is the culmination of an almost 3-year investigation into pharmaceutical pricing and business practices. The report draws from 1.5 million pages of internal company documents, the committee says.
Documents from insulin makers Eli Lilly, Novo Nordisk, and Sanofi indicate these firms “raised their prices in lockstep in order to maintain ‘pricing parity’,” with senior executives encouraging the practice, the committee staff writes in the report.
“In a discussion among Novo Nordisk employees about an Eli Lilly price increase for a different diabetes product on Dec. 24, 2015, a Novo Nordisk pricing analyst remarked, ‘[M]aybe Sanofi will wait until tomorrow morning to announce their price increase ... that’s all I want for Christmas,’” the report states.
House Democrats are seeking to use the report findings to aid their Senate colleagues’ attempt to pass the sweeping Build Back Better bill, which includes many provisions addressing drug costs.
It’s still unclear when the Senate will act on the measure. The House passed the Build Back Better bill, 220-213, in November. It includes a provision that would allow Medicare to negotiate the prices of certain drugs covered by Part D pharmacy plans.
That would mark a reversal of the stance taken when Congress created the pharmacy benefit in a 2003 law, which left negotiations to insurers that cover Part D plans.
Republicans have long argued insurers get the best deals on drugs for people on Medicare. Democrats say this approach sacrifices much of Medicare’s bargaining clout, scattering it among plans.
“This fight has been going on since the Medicare Part D legislation which gave away the store” to drugmakers, said Speaker Nancy Pelosi (D-CA) at a Dec. 10 press conference about the House Oversight report. “And they got used to having the store to themselves.”
The Endocrine Society is urging the Senate to protect the insulin affordability provisions included in the Build Back Better Act and move quickly to pass this crucial legislation.
“We implore all Senators to ensure these provisions are not scaled back. The Build Back Better Act represents the best opportunity to address the price of insulin. Millions of Americans cannot wait any longer for a solution,” it said in a statement issued Dec. 14.
Better deals for military, medicaid programs
Medicare is unusual among federal programs in that it doesn’t directly leverage its clout to lower drug costs.
Total Part D expenditures were approximately $105 billion last year, according to Medicare’s board of trustees. This spending is divided among the many insurers that run Part D plans, which then make a myriad of decisions about formularies and other factors that affect pricing.
For drugs administered by clinicians, and thus covered by Medicare Part B, the program pays a premium of the reported average sales price. Part B drug spending was $39 billion in 2019, an increase of about 11.6% from the previous year, according to the Medicare Payment Advisory Commission.
In contrast, federal law calls for steep reductions in drug prices for people on Medicaid.
The Department of Veterans Affairs (VA) and the Defense Department (DoD)’s Tricare program use several bargaining strategies to lower prices. To control costs, VA and DoD often use formularies of preferred drugs, steer patients to lower-cost drugs, and buy drugs in large volumes, “all of which increase their leverage with drug manufacturers,” the staff of the Congressional Budget Office (CBO) wrote in a Feb. 2021 report.
The CBO report examines how those different federal agencies’ approaches played out in terms of prices, net of applicable rebates, and discounts of 176 top-selling brand-name drugs in Medicare Part D.
The average price for this group of drugs was $118 in Medicaid. And for VA and DoD, the average prices were $190 and $184, respectively, for drugs dispensed at the agencies’ medical facilities or by mail.
But for Medicare Part D, the average price was $343, CBO said in the report, which was one of the sources consulted by House Oversight staff when developing their report released on Dec. 10.
Insulin still of interest, 100 years after its discovery
The House Oversight report runs to almost 270 pages. It addresses several issues with drug prices, including strategies pharmaceutical companies have used to thwart generic competition. On Monday, the trade group America’s Health Insurance Plans separately released its own report looking at patents and delays to the introduction of generic drugs.
Yet, much of the debate on drug prices has focused on one of the oldest widely produced prescription drugs, insulin.
Even with the allowance of generic competition for the essential medicine, branded versions of insulin have been some of the costliest products for Medicare in recent years. Eli Lilly, Novo Nordisk, and Sanofi dominate the insulin market.
Medicare Part D spent about $2.5 billion in 2019 on Sanofi’s Lantus Solostar insulin, or about $2,585 per person in the program using it. The program also paid about $1.1 billion for another form of Lantus, or about $2,746 per patient.
Medicare Part D also spent about $1.84 billion in 2019 on Novo Nordisk’s NovoLog FlexPen, or about $3,063 per person.
Medicare Part D’s drug spending dashboard also lists eight versions of Lilly’s Humalog, with combined 2019 spending of more than $2 billion. The cost per patient in Medicare Part D ranges from $5,619 to $1,462.
“Over the past 20 years, they have repeatedly and dramatically raised the list prices of their rapid-acting and long-acting insulins and reaped billions of dollars in revenues,” write the House Oversight staff in their report.
Republicans on the House Oversight and Reform Committee disagree with their Democratic colleagues on many points in the debate on drug prices, but they also looked at insulin as a cause for concern.
GOP members of the committee released a separate report on Dec. 10. They call for greater clarity into the role middlemen in the drug-supply chain – known as pharmaceutical benefit managers – may play in the rising costs of medicines. The GOP report notes that there are bills pending in the House that would seek to steer any discounts offered on insulin within the supply chain toward consumers (Insulin Price Reduction Act H.R. 4906, Insulin Cost Reduction Act H.R. 5623).
Democratic staff in the committee’s report seek to draw attention to how manufacturers priced their insulin products, including the comment by the Novo Nordisk employee about wishing for a price hike for a competitor’s product.
In a statement provided to this news organization, Novo Nordisk said the committee’s report reflects “a limited picture of the efforts put forth by our company and other companies to manage formulary access.”
“This glimpse into the complexity of pricing, formularies, and the health care system demonstrates why Novo Nordisk continues to advocate for comprehensive solutions,” Denmark’s Novo Nordisk said in the statement.
$35 a month for insulin?
Paris-based Sanofi said it makes insulin-pricing decisions independently from competitors. Sanofi said the net price of its insulins has declined by 53% since 2012, arguing the high prices charged to patients reflect decisions made elsewhere in the supply chain.
“Over the same period, the net price for commercial and Medicare Part D plans of Lantus has fallen 44.9%, while average out-of-pocket costs for patients with commercial insurance and Medicare Part D has risen approximately 82%,” Sanofi said.
“For all the focus on the growth of list prices, today, the average net price of Lantus is below 2006 levels. That is why we support policy reforms to require health plans to share negotiated savings with patients by requiring patient cost-sharing be tied to the net prices.”
Indianapolis-based Lilly offered a similar response in a statement to this news organization.
“Lilly, like other companies, monitors competitor list-price changes that are available through publicly available services,” the company said. “However, any changes we make to our list prices are independent decisions, and to the extent they consider competitors they are informed only through publicly available data.”
Despite rising insurance deductibles, the average monthly out-of-pocket cost for Lilly insulin has dropped 27% to $28.05 over the past 4 years, the company said in an interview. Lilly also noted that there are “several affordability options now available” allowing people to purchase their monthly prescription of its insulin for $35, “whether they are uninsured or use commercial insurance, Medicaid, or a participating Medicare Part D plan.”
In 2020, Lilly had announced that people with commercial insurance and those without insurance would be able to get monthly prescriptions of Lilly insulin for $35.
The Build Back Better Act would require insurers, including Medicare Part D plans and private group or individual health plans, to charge patient cost-sharing of no more than $35 per month for insulin products, said the staff of the nonprofit Kaiser Family Foundation (KFF) in a review of the bill.
“Private group or individual plans would not be required to cover all insulin products, just one of each dosage form (vial, pen) and insulin type (rapid-acting, short-acting, intermediate-acting, and long-acting), for no more than $35,” the KFF staff state in the report.
People enrolled in Medicare can already choose to enroll in a Part D plan participating in a federal test program that can secure certain insulin products for them at a monthly copayment of $35. In 2022, a total of 2,159 Part D plans will participate in this model, a 32% increase in participating plans since 2021, KFF said.
A version of this article first appeared on Medscape.com.
Medicare could have saved more than $16.7 billion on three kinds of insulin products from 2011 to 2017 if it had secured the same discounts other federal health programs get through negotiations, House Democrats argue in a new report.
On Dec. 10, Democrats on the House Committee on Oversight and Reform released a final majority staff report, which they say is the culmination of an almost 3-year investigation into pharmaceutical pricing and business practices. The report draws from 1.5 million pages of internal company documents, the committee says.
Documents from insulin makers Eli Lilly, Novo Nordisk, and Sanofi indicate these firms “raised their prices in lockstep in order to maintain ‘pricing parity’,” with senior executives encouraging the practice, the committee staff writes in the report.
“In a discussion among Novo Nordisk employees about an Eli Lilly price increase for a different diabetes product on Dec. 24, 2015, a Novo Nordisk pricing analyst remarked, ‘[M]aybe Sanofi will wait until tomorrow morning to announce their price increase ... that’s all I want for Christmas,’” the report states.
House Democrats are seeking to use the report findings to aid their Senate colleagues’ attempt to pass the sweeping Build Back Better bill, which includes many provisions addressing drug costs.
It’s still unclear when the Senate will act on the measure. The House passed the Build Back Better bill, 220-213, in November. It includes a provision that would allow Medicare to negotiate the prices of certain drugs covered by Part D pharmacy plans.
That would mark a reversal of the stance taken when Congress created the pharmacy benefit in a 2003 law, which left negotiations to insurers that cover Part D plans.
Republicans have long argued insurers get the best deals on drugs for people on Medicare. Democrats say this approach sacrifices much of Medicare’s bargaining clout, scattering it among plans.
“This fight has been going on since the Medicare Part D legislation which gave away the store” to drugmakers, said Speaker Nancy Pelosi (D-CA) at a Dec. 10 press conference about the House Oversight report. “And they got used to having the store to themselves.”
The Endocrine Society is urging the Senate to protect the insulin affordability provisions included in the Build Back Better Act and move quickly to pass this crucial legislation.
“We implore all Senators to ensure these provisions are not scaled back. The Build Back Better Act represents the best opportunity to address the price of insulin. Millions of Americans cannot wait any longer for a solution,” it said in a statement issued Dec. 14.
Better deals for military, medicaid programs
Medicare is unusual among federal programs in that it doesn’t directly leverage its clout to lower drug costs.
Total Part D expenditures were approximately $105 billion last year, according to Medicare’s board of trustees. This spending is divided among the many insurers that run Part D plans, which then make a myriad of decisions about formularies and other factors that affect pricing.
For drugs administered by clinicians, and thus covered by Medicare Part B, the program pays a premium of the reported average sales price. Part B drug spending was $39 billion in 2019, an increase of about 11.6% from the previous year, according to the Medicare Payment Advisory Commission.
In contrast, federal law calls for steep reductions in drug prices for people on Medicaid.
The Department of Veterans Affairs (VA) and the Defense Department (DoD)’s Tricare program use several bargaining strategies to lower prices. To control costs, VA and DoD often use formularies of preferred drugs, steer patients to lower-cost drugs, and buy drugs in large volumes, “all of which increase their leverage with drug manufacturers,” the staff of the Congressional Budget Office (CBO) wrote in a Feb. 2021 report.
The CBO report examines how those different federal agencies’ approaches played out in terms of prices, net of applicable rebates, and discounts of 176 top-selling brand-name drugs in Medicare Part D.
The average price for this group of drugs was $118 in Medicaid. And for VA and DoD, the average prices were $190 and $184, respectively, for drugs dispensed at the agencies’ medical facilities or by mail.
But for Medicare Part D, the average price was $343, CBO said in the report, which was one of the sources consulted by House Oversight staff when developing their report released on Dec. 10.
Insulin still of interest, 100 years after its discovery
The House Oversight report runs to almost 270 pages. It addresses several issues with drug prices, including strategies pharmaceutical companies have used to thwart generic competition. On Monday, the trade group America’s Health Insurance Plans separately released its own report looking at patents and delays to the introduction of generic drugs.
Yet, much of the debate on drug prices has focused on one of the oldest widely produced prescription drugs, insulin.
Even with the allowance of generic competition for the essential medicine, branded versions of insulin have been some of the costliest products for Medicare in recent years. Eli Lilly, Novo Nordisk, and Sanofi dominate the insulin market.
Medicare Part D spent about $2.5 billion in 2019 on Sanofi’s Lantus Solostar insulin, or about $2,585 per person in the program using it. The program also paid about $1.1 billion for another form of Lantus, or about $2,746 per patient.
Medicare Part D also spent about $1.84 billion in 2019 on Novo Nordisk’s NovoLog FlexPen, or about $3,063 per person.
Medicare Part D’s drug spending dashboard also lists eight versions of Lilly’s Humalog, with combined 2019 spending of more than $2 billion. The cost per patient in Medicare Part D ranges from $5,619 to $1,462.
“Over the past 20 years, they have repeatedly and dramatically raised the list prices of their rapid-acting and long-acting insulins and reaped billions of dollars in revenues,” write the House Oversight staff in their report.
Republicans on the House Oversight and Reform Committee disagree with their Democratic colleagues on many points in the debate on drug prices, but they also looked at insulin as a cause for concern.
GOP members of the committee released a separate report on Dec. 10. They call for greater clarity into the role middlemen in the drug-supply chain – known as pharmaceutical benefit managers – may play in the rising costs of medicines. The GOP report notes that there are bills pending in the House that would seek to steer any discounts offered on insulin within the supply chain toward consumers (Insulin Price Reduction Act H.R. 4906, Insulin Cost Reduction Act H.R. 5623).
Democratic staff in the committee’s report seek to draw attention to how manufacturers priced their insulin products, including the comment by the Novo Nordisk employee about wishing for a price hike for a competitor’s product.
In a statement provided to this news organization, Novo Nordisk said the committee’s report reflects “a limited picture of the efforts put forth by our company and other companies to manage formulary access.”
“This glimpse into the complexity of pricing, formularies, and the health care system demonstrates why Novo Nordisk continues to advocate for comprehensive solutions,” Denmark’s Novo Nordisk said in the statement.
$35 a month for insulin?
Paris-based Sanofi said it makes insulin-pricing decisions independently from competitors. Sanofi said the net price of its insulins has declined by 53% since 2012, arguing the high prices charged to patients reflect decisions made elsewhere in the supply chain.
“Over the same period, the net price for commercial and Medicare Part D plans of Lantus has fallen 44.9%, while average out-of-pocket costs for patients with commercial insurance and Medicare Part D has risen approximately 82%,” Sanofi said.
“For all the focus on the growth of list prices, today, the average net price of Lantus is below 2006 levels. That is why we support policy reforms to require health plans to share negotiated savings with patients by requiring patient cost-sharing be tied to the net prices.”
Indianapolis-based Lilly offered a similar response in a statement to this news organization.
“Lilly, like other companies, monitors competitor list-price changes that are available through publicly available services,” the company said. “However, any changes we make to our list prices are independent decisions, and to the extent they consider competitors they are informed only through publicly available data.”
Despite rising insurance deductibles, the average monthly out-of-pocket cost for Lilly insulin has dropped 27% to $28.05 over the past 4 years, the company said in an interview. Lilly also noted that there are “several affordability options now available” allowing people to purchase their monthly prescription of its insulin for $35, “whether they are uninsured or use commercial insurance, Medicaid, or a participating Medicare Part D plan.”
In 2020, Lilly had announced that people with commercial insurance and those without insurance would be able to get monthly prescriptions of Lilly insulin for $35.
The Build Back Better Act would require insurers, including Medicare Part D plans and private group or individual health plans, to charge patient cost-sharing of no more than $35 per month for insulin products, said the staff of the nonprofit Kaiser Family Foundation (KFF) in a review of the bill.
“Private group or individual plans would not be required to cover all insulin products, just one of each dosage form (vial, pen) and insulin type (rapid-acting, short-acting, intermediate-acting, and long-acting), for no more than $35,” the KFF staff state in the report.
People enrolled in Medicare can already choose to enroll in a Part D plan participating in a federal test program that can secure certain insulin products for them at a monthly copayment of $35. In 2022, a total of 2,159 Part D plans will participate in this model, a 32% increase in participating plans since 2021, KFF said.
A version of this article first appeared on Medscape.com.