User login
News and Views that Matter to Pediatricians
The leading independent newspaper covering news and commentary in pediatrics.
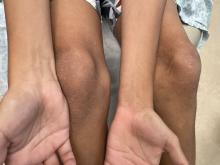
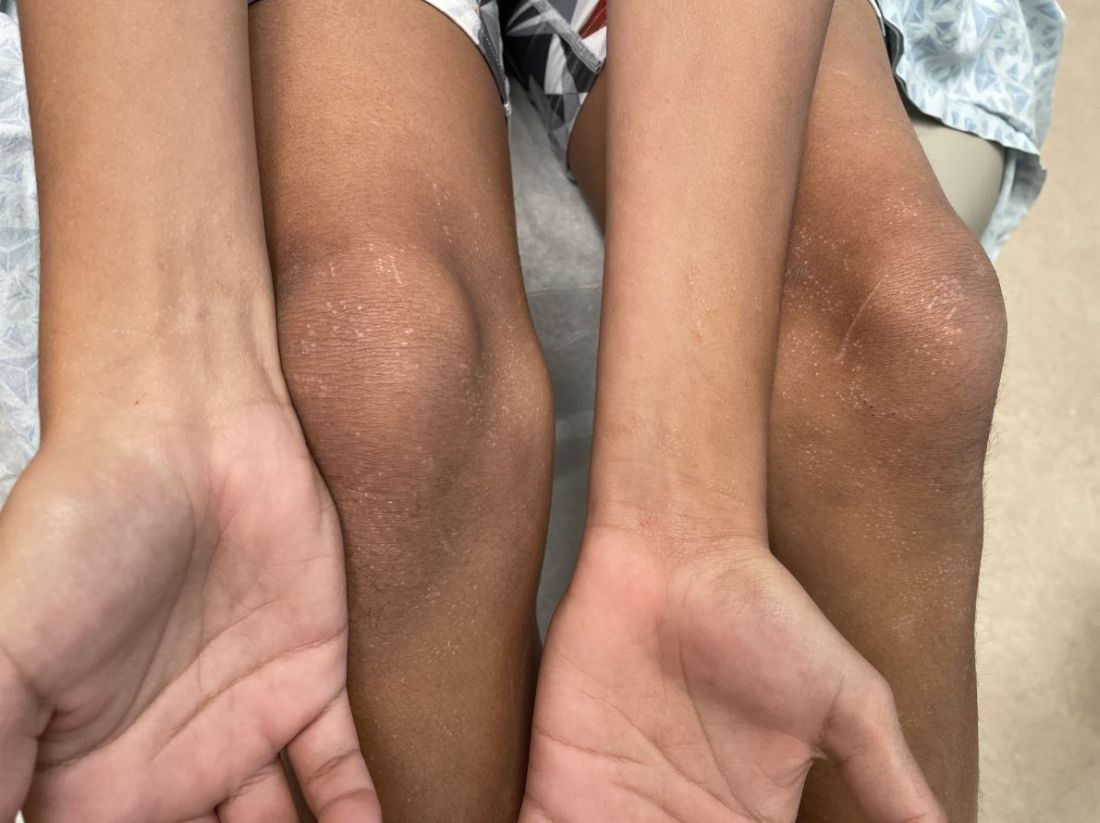
An 11-year-old boy presents with small itchy bumps on the wrists, face, arms, and legs
The patient was diagnosed with lichen nitidus, given the characteristic clinical presentation.
Lichen nitidus is a rare chronic inflammatory condition of the skin that most commonly presents in children and young adults and does not seem to be restricted to any sex or race. The classic lesions are described as asymptomatic to slightly pruritic, small (1 mm), skin-colored to hypopigmented flat-topped papules.
Koebner phenomenon is usually seen in which the skin lesions appear in areas of traumatized healthy skin. The extremities, abdomen, chest, and penis are common locations for the lesions to occur. Rarely, the oral mucosa or nails can be involved. It has been described in patients with a diagnosis of Crohn’s disease, Niemann-Pick disease, Down syndrome, and HIV. The rare, generalized purpuric variant has been reported in a few cases associated with interferon and ribavirin treatment for hepatitis C infection and nivolumab treatment for cancer. The pathophysiology of lichen nitidus is unknown.
Lichen nitidus can occur in the presence of other skin conditions like lichen planus, atopic dermatitis, vitiligo, erythema nodosum, and lichen spinulosus. Histopathologic characteristics of lichen nitidus are described as a “ball and claw” of epidermal rete around a lymphohistiocytic infiltrate. Parakeratosis overlying epidermal atrophy and focal basal liquefaction degeneration is also seen.
The differential diagnosis of lichen nitidus includes flat warts, which can present as clusters of small flat-topped papules that can show a pseudo-Koebner phenomenon (where the virus is seeded in traumatized skin). The morphological difference between the condition is that lichen nitidus lesions are usually monomorphic, compared with flat warts, which usually present with different sizes and shapes.
Patients with a history of allergic contact dermatitis may present with a generalized monomorphic eruption of skin-colored papules (known as ID reaction) that can sometimes be very similar to lichen nitidus. Allergic contact dermatitis tends to respond fairly quickly to topical or systemic corticosteroids, unlike lichen nitidus. There are a few reports that consider lichen nitidus to be a variant of lichen planus, although they have different histopathologic findings. Lichen planus lesions are described as polygonal, pruritic, purple to pink papules most commonly seen on the wrists, lower back, and ankles. Lichen planus can be seen in patients with hepatitis C and may also occur secondary to medication.
Milia are small keratin cysts on the skin that are commonly seen in babies as primary milia and can be seen in older children secondary to trauma (commonly on the eyelids) or medications. Given their size and monomorphic appearance, they can sometimes be confused with lichen nitidus.
Lichen nitidus is often asymptomatic and the lesions resolve within a few months to years. Topical corticosteroids can be helpful to alleviate the symptoms in patients who present with pruritus. In more persistent and generalized cases, phototherapy, systemic corticosteroids, acitretin, isotretinoin, or cyclosporine can be considered.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Chu J and Lam JM. CMAJ. 2014 Dec 9;186(18):E688.
Lestringant G et al. Dermatology 1996;192:171-3.
Peterson JA et al. Proc (Bayl Univ Med Cent). 2021 Aug 25;35(1):70-2.
Schwartz C and Goodman MB. “Lichen nitidus,” in StatPearls. Treasure Island, Fla.: StatPearls Publishing, 2022.
The patient was diagnosed with lichen nitidus, given the characteristic clinical presentation.
Lichen nitidus is a rare chronic inflammatory condition of the skin that most commonly presents in children and young adults and does not seem to be restricted to any sex or race. The classic lesions are described as asymptomatic to slightly pruritic, small (1 mm), skin-colored to hypopigmented flat-topped papules.
Koebner phenomenon is usually seen in which the skin lesions appear in areas of traumatized healthy skin. The extremities, abdomen, chest, and penis are common locations for the lesions to occur. Rarely, the oral mucosa or nails can be involved. It has been described in patients with a diagnosis of Crohn’s disease, Niemann-Pick disease, Down syndrome, and HIV. The rare, generalized purpuric variant has been reported in a few cases associated with interferon and ribavirin treatment for hepatitis C infection and nivolumab treatment for cancer. The pathophysiology of lichen nitidus is unknown.
Lichen nitidus can occur in the presence of other skin conditions like lichen planus, atopic dermatitis, vitiligo, erythema nodosum, and lichen spinulosus. Histopathologic characteristics of lichen nitidus are described as a “ball and claw” of epidermal rete around a lymphohistiocytic infiltrate. Parakeratosis overlying epidermal atrophy and focal basal liquefaction degeneration is also seen.
The differential diagnosis of lichen nitidus includes flat warts, which can present as clusters of small flat-topped papules that can show a pseudo-Koebner phenomenon (where the virus is seeded in traumatized skin). The morphological difference between the condition is that lichen nitidus lesions are usually monomorphic, compared with flat warts, which usually present with different sizes and shapes.
Patients with a history of allergic contact dermatitis may present with a generalized monomorphic eruption of skin-colored papules (known as ID reaction) that can sometimes be very similar to lichen nitidus. Allergic contact dermatitis tends to respond fairly quickly to topical or systemic corticosteroids, unlike lichen nitidus. There are a few reports that consider lichen nitidus to be a variant of lichen planus, although they have different histopathologic findings. Lichen planus lesions are described as polygonal, pruritic, purple to pink papules most commonly seen on the wrists, lower back, and ankles. Lichen planus can be seen in patients with hepatitis C and may also occur secondary to medication.
Milia are small keratin cysts on the skin that are commonly seen in babies as primary milia and can be seen in older children secondary to trauma (commonly on the eyelids) or medications. Given their size and monomorphic appearance, they can sometimes be confused with lichen nitidus.
Lichen nitidus is often asymptomatic and the lesions resolve within a few months to years. Topical corticosteroids can be helpful to alleviate the symptoms in patients who present with pruritus. In more persistent and generalized cases, phototherapy, systemic corticosteroids, acitretin, isotretinoin, or cyclosporine can be considered.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Chu J and Lam JM. CMAJ. 2014 Dec 9;186(18):E688.
Lestringant G et al. Dermatology 1996;192:171-3.
Peterson JA et al. Proc (Bayl Univ Med Cent). 2021 Aug 25;35(1):70-2.
Schwartz C and Goodman MB. “Lichen nitidus,” in StatPearls. Treasure Island, Fla.: StatPearls Publishing, 2022.
The patient was diagnosed with lichen nitidus, given the characteristic clinical presentation.
Lichen nitidus is a rare chronic inflammatory condition of the skin that most commonly presents in children and young adults and does not seem to be restricted to any sex or race. The classic lesions are described as asymptomatic to slightly pruritic, small (1 mm), skin-colored to hypopigmented flat-topped papules.
Koebner phenomenon is usually seen in which the skin lesions appear in areas of traumatized healthy skin. The extremities, abdomen, chest, and penis are common locations for the lesions to occur. Rarely, the oral mucosa or nails can be involved. It has been described in patients with a diagnosis of Crohn’s disease, Niemann-Pick disease, Down syndrome, and HIV. The rare, generalized purpuric variant has been reported in a few cases associated with interferon and ribavirin treatment for hepatitis C infection and nivolumab treatment for cancer. The pathophysiology of lichen nitidus is unknown.
Lichen nitidus can occur in the presence of other skin conditions like lichen planus, atopic dermatitis, vitiligo, erythema nodosum, and lichen spinulosus. Histopathologic characteristics of lichen nitidus are described as a “ball and claw” of epidermal rete around a lymphohistiocytic infiltrate. Parakeratosis overlying epidermal atrophy and focal basal liquefaction degeneration is also seen.
The differential diagnosis of lichen nitidus includes flat warts, which can present as clusters of small flat-topped papules that can show a pseudo-Koebner phenomenon (where the virus is seeded in traumatized skin). The morphological difference between the condition is that lichen nitidus lesions are usually monomorphic, compared with flat warts, which usually present with different sizes and shapes.
Patients with a history of allergic contact dermatitis may present with a generalized monomorphic eruption of skin-colored papules (known as ID reaction) that can sometimes be very similar to lichen nitidus. Allergic contact dermatitis tends to respond fairly quickly to topical or systemic corticosteroids, unlike lichen nitidus. There are a few reports that consider lichen nitidus to be a variant of lichen planus, although they have different histopathologic findings. Lichen planus lesions are described as polygonal, pruritic, purple to pink papules most commonly seen on the wrists, lower back, and ankles. Lichen planus can be seen in patients with hepatitis C and may also occur secondary to medication.
Milia are small keratin cysts on the skin that are commonly seen in babies as primary milia and can be seen in older children secondary to trauma (commonly on the eyelids) or medications. Given their size and monomorphic appearance, they can sometimes be confused with lichen nitidus.
Lichen nitidus is often asymptomatic and the lesions resolve within a few months to years. Topical corticosteroids can be helpful to alleviate the symptoms in patients who present with pruritus. In more persistent and generalized cases, phototherapy, systemic corticosteroids, acitretin, isotretinoin, or cyclosporine can be considered.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Chu J and Lam JM. CMAJ. 2014 Dec 9;186(18):E688.
Lestringant G et al. Dermatology 1996;192:171-3.
Peterson JA et al. Proc (Bayl Univ Med Cent). 2021 Aug 25;35(1):70-2.
Schwartz C and Goodman MB. “Lichen nitidus,” in StatPearls. Treasure Island, Fla.: StatPearls Publishing, 2022.
An 11-year-old male with a prior history of atopic dermatitis as a young child, presents with 6 months of slightly itchy, small bumps on the wrists, face, arms, and legs. Has been treated with fluocinolone oil and hydrocortisone 2.5% for a month with no change in the lesions. Besides the use of topical corticosteroids, he has not been taking any other medications.
On physical examination he has multiple skin-colored, flat-topped papules that coalesce into plaques on the arms, legs, chest, and back (Photo 1). Koebner phenomenon was also seen on the knees and arms. There were no lesions in the mouth or on the nails.
Doctors and dating: There’s an app (or three) for that
Pounding heart, sweating, insomnia. Surges of dopamine, norepinephrine, and adrenaline. All symptoms of a very common yet frustrating condition: Falling in love.
The prognosis is vague. A prescription pad and knowledge of biochemistry aren’t helpful when it comes to relationships.
Medical training can consume decades when others are exploring relationships and starting families. There are few recent data on this, but
But there is hope! By age 36, the number of doctors in long-term relationships had overtaken everyone else by more than 10% for women and 20% for men. The Medscape 2022 Physician Happiness & Lifestyle Report found that 83% were in committed relationships, and even better, happy ones. At least three-quarters of doctors in every specialty described their partnerships as “very good” or “good.”
How should a single medical student, resident, or attending physician find happiness ever after in 2023? Sometimes Mr./Ms. Right can be found in the anatomy lab or hospital, with sparks flying between students or colleagues. But for many in health care, along with millions of others looking for love, the solution is dating apps.
When ‘MD’ is a turnoff
Dr. M, a psychiatry resident in California who prefers not to give her name, hadn’t found a life partner during college, grad school, or medical school. When she passed her final Step 3 board exam, she decided it was time to take the plunge. She signed up for popular dating apps like Hinge, Bumble, and Coffee Meets Bagel, but her dates seemed to follow a disappointing pattern.
“I met lots of guys, but it was incredibly rare to find another physician,” said Dr. M. “I found myself always wanting to talk about my life as a resident. More often than not, the guys would give me this blank stare as I complained about being on call or spoke about spending 12 hours a day studying for a board exam, or even the process of The Match and how I ended up in California.”
Both of Dr. M’s parents are physicians, and she grew up watching how they supported each other through residency, exams, and exhausting schedules. A relationship with another physician, her parents told her, would give both partners the best chance to understand each other’s lives. The problem was how to find one.
That was when Dr. M saw an ad for a dating app with a cute medical name: DownToDate, a play on the clinical evidence resource UpToDate. “I thought it was a meme,” she said. “It was this doctors-only app. I remember thinking, ‘this has to be a joke,’ but then it was very real.”
She signed up and was required to provide a photo of her ID and her NPI number. Immediately, men began “requesting a consult,” the app’s form of “liking” her profile, and sending her “pages” (messages).
DownToDate was created by another physician, Robin Boyer, MD, MBA, a pediatrics resident in Loma Linda, Calif. The inspiration came in 2020 during the initial COVID crisis. Exhausted from long and often heartbreaking shifts, Dr. Boyer was grateful for her husband’s unwavering support. But many of her coresidents weren’t so lucky. The women in particular talked about their dating struggles, and there was a recurring theme. They didn’t feel confident putting “physician” on a dating site profile.
“If you’re male and you tell people you’re a doctor, it seems like it really attracts people,” Dr. Boyer said. “But if you’re female, it brings up a lot of stereotypes where you’re perceived as too intimidating either as the breadwinner, being more educated, or having a [demanding] career. It does make it more difficult.”
Dr. Boyer met her husband in high school, and she had never used a dating app. She convinced a coresident, Celestine Odigwe, MD, to pursue the idea as partners. They began researching the market within their network and heard from over a thousand interested physicians, both men and women, heterosexual and LGBTQ+. They even created fake accounts on other sites to gauge how easy it is to falsify a profile. From these insights, the app took shape. It launched in 2021 and currently has more than 5000 verified users.
Branches from the same tree
Around the same time that DownToDate began, Shivani Shah, DO, a pediatric neurology resident at Duke University, Durham, N.C., and her brother, Sagar Shah, an entrepreneur, had a similar idea.
At the time, Dr. Shah was a fourth-year medical student about to move from New Jersey to North Carolina. Friends who were internal medicine residents described the grueling reality of the early COVID pandemic.
“It was just horrible,” said Dr. Shah. “You were isolated from your family, your support system, everything. ... I think the pandemic really pushed us into realizing that this is a very important need, and sometimes it feels like community is lacking in the health care field.”
The sibling duo developed ForeverX, an app for health care workers to find meaningful and long-term romantic connections. It launched in 2021.
Concerned that the medical field was “siloed,” the Shahs chose to open the app to physicians, dentists, nurses, physical therapists, and other health care professionals. “Opening up the doors to more communication” between the health care branches was a priority.
To prevent catfishing, the app uses a twofold vetting system. Each user submits a photo of their driver’s license and a selfie that must match. There is also health care verification through an NPI number, nurse’s ID, or a manual process for those without either. None of the information is stored.
Through personal experience with dating apps, Dr. Shah hopes ForeverX can improve on some of their flaws, particularly the problem of matches being overly filtered by preferences. The “natural way” of meeting people is not filtered. And while most people have a dating checklist in mind, meeting someone face to face might send some of those prerequisites “out the window.”
“You can’t really put into words how you feel with someone ... the vibe,” Dr. Shah said. That is why her goal is to get people off the app and on an actual date IRL. “Something we’ve discussed internally is, how do we make this experience that’s virtual more human?”
She acknowledged that certain requirements, like a desire for children, might be crucial to some users. Many female doctors in their 30’s feel the “time crunch” of a ticking biological clock.
Optimize your date-ability
“I think people either love or hate dating apps, and I love them,” said Kevin Jubbal, MD. “I get to meet cool people and schedule dates from the comfort of my home.”
Dr. Jubbal, a former plastic surgery resident who left medicine to become an entrepreneur, is the founder of Med School Insiders, a tutoring and advising resource for premeds, medical students, and residents. His YouTube channel has more than 1.5 million subscribers, and he often receives questions about whether dating is feasible in medical school and how to balance a personal and academic/professional life.
Those who hate dating apps or receive few matches would do well to look inward instead of blaming the process, he said. It helps to view the experience as a learning tool that provides feedback very quickly.
“If you want to find a really amazing person, then you need to be what you want to find,” said Dr. Jubbal. “If you want to find someone who’s fit and intelligent and well read and well traveled, you need to be that. Otherwise, you’re probably not going to attract that person.”
An app designed to help single female MDs
Ifie Williams, MD, a psychiatrist in Washington, D.C., believes a wider dating pool is key – provided everyone understands the situation up front. When Dr. Williams started residency in 2014, she was “as single as can be.” She tried many dating apps, but they were extremely time consuming. Even when she set specific preferences, she found herself sifting through “matches” that didn’t fit her criteria.
“Dating nowadays has become almost like a second job,” said Dr. Williams. “Just the amount of time that people are having to spend on apps, swiping left and right and then meeting people. You think they’re interested and then you deal with all these games.”
By 2017, Dr. Williams had invented Miss Doctor, a dating app that would connect female physicians and other doctoral-level professionals with men or women on a similar achievement level.
By definition, these people would not be intimidated by ambitious, busy women. They would be heavily screened and vetted. And one other proviso: they would have to pay for “likes.”
Most dating apps charge a subscription fee. Users are allowed to “like” numerous profiles and perhaps not bother responding to many matches. By contrast, Miss Doctor accounts are free and include a limited number of “likes” to indicate interest. Beyond that, there’s a price.
“We wanted to find a way to make people a little more intentional with how they like people on the app, so they give a little more thought to it,” Dr. Williams said. “So, we monetize it and use that to change behavior.”
After an initial launch in 2017, the app had to take a back seat while Dr. Williams started her psychiatry practice and got married herself. She plans to relaunch it in spring 2023.
Male or female, there is general agreement that finding time to date as a young physician isn’t easy. While DownToDate has had “doctor meets doctor” success stories, many users are still searching for “the one.”
Dr. Boyer believes that career challenges are not a reason to give up. “There are so many single and available people out there,” she said. “And everyone’s deserving of love. Even if you only have an hour a week.”
A version of this article first appeared on Medscape.com.
Pounding heart, sweating, insomnia. Surges of dopamine, norepinephrine, and adrenaline. All symptoms of a very common yet frustrating condition: Falling in love.
The prognosis is vague. A prescription pad and knowledge of biochemistry aren’t helpful when it comes to relationships.
Medical training can consume decades when others are exploring relationships and starting families. There are few recent data on this, but
But there is hope! By age 36, the number of doctors in long-term relationships had overtaken everyone else by more than 10% for women and 20% for men. The Medscape 2022 Physician Happiness & Lifestyle Report found that 83% were in committed relationships, and even better, happy ones. At least three-quarters of doctors in every specialty described their partnerships as “very good” or “good.”
How should a single medical student, resident, or attending physician find happiness ever after in 2023? Sometimes Mr./Ms. Right can be found in the anatomy lab or hospital, with sparks flying between students or colleagues. But for many in health care, along with millions of others looking for love, the solution is dating apps.
When ‘MD’ is a turnoff
Dr. M, a psychiatry resident in California who prefers not to give her name, hadn’t found a life partner during college, grad school, or medical school. When she passed her final Step 3 board exam, she decided it was time to take the plunge. She signed up for popular dating apps like Hinge, Bumble, and Coffee Meets Bagel, but her dates seemed to follow a disappointing pattern.
“I met lots of guys, but it was incredibly rare to find another physician,” said Dr. M. “I found myself always wanting to talk about my life as a resident. More often than not, the guys would give me this blank stare as I complained about being on call or spoke about spending 12 hours a day studying for a board exam, or even the process of The Match and how I ended up in California.”
Both of Dr. M’s parents are physicians, and she grew up watching how they supported each other through residency, exams, and exhausting schedules. A relationship with another physician, her parents told her, would give both partners the best chance to understand each other’s lives. The problem was how to find one.
That was when Dr. M saw an ad for a dating app with a cute medical name: DownToDate, a play on the clinical evidence resource UpToDate. “I thought it was a meme,” she said. “It was this doctors-only app. I remember thinking, ‘this has to be a joke,’ but then it was very real.”
She signed up and was required to provide a photo of her ID and her NPI number. Immediately, men began “requesting a consult,” the app’s form of “liking” her profile, and sending her “pages” (messages).
DownToDate was created by another physician, Robin Boyer, MD, MBA, a pediatrics resident in Loma Linda, Calif. The inspiration came in 2020 during the initial COVID crisis. Exhausted from long and often heartbreaking shifts, Dr. Boyer was grateful for her husband’s unwavering support. But many of her coresidents weren’t so lucky. The women in particular talked about their dating struggles, and there was a recurring theme. They didn’t feel confident putting “physician” on a dating site profile.
“If you’re male and you tell people you’re a doctor, it seems like it really attracts people,” Dr. Boyer said. “But if you’re female, it brings up a lot of stereotypes where you’re perceived as too intimidating either as the breadwinner, being more educated, or having a [demanding] career. It does make it more difficult.”
Dr. Boyer met her husband in high school, and she had never used a dating app. She convinced a coresident, Celestine Odigwe, MD, to pursue the idea as partners. They began researching the market within their network and heard from over a thousand interested physicians, both men and women, heterosexual and LGBTQ+. They even created fake accounts on other sites to gauge how easy it is to falsify a profile. From these insights, the app took shape. It launched in 2021 and currently has more than 5000 verified users.
Branches from the same tree
Around the same time that DownToDate began, Shivani Shah, DO, a pediatric neurology resident at Duke University, Durham, N.C., and her brother, Sagar Shah, an entrepreneur, had a similar idea.
At the time, Dr. Shah was a fourth-year medical student about to move from New Jersey to North Carolina. Friends who were internal medicine residents described the grueling reality of the early COVID pandemic.
“It was just horrible,” said Dr. Shah. “You were isolated from your family, your support system, everything. ... I think the pandemic really pushed us into realizing that this is a very important need, and sometimes it feels like community is lacking in the health care field.”
The sibling duo developed ForeverX, an app for health care workers to find meaningful and long-term romantic connections. It launched in 2021.
Concerned that the medical field was “siloed,” the Shahs chose to open the app to physicians, dentists, nurses, physical therapists, and other health care professionals. “Opening up the doors to more communication” between the health care branches was a priority.
To prevent catfishing, the app uses a twofold vetting system. Each user submits a photo of their driver’s license and a selfie that must match. There is also health care verification through an NPI number, nurse’s ID, or a manual process for those without either. None of the information is stored.
Through personal experience with dating apps, Dr. Shah hopes ForeverX can improve on some of their flaws, particularly the problem of matches being overly filtered by preferences. The “natural way” of meeting people is not filtered. And while most people have a dating checklist in mind, meeting someone face to face might send some of those prerequisites “out the window.”
“You can’t really put into words how you feel with someone ... the vibe,” Dr. Shah said. That is why her goal is to get people off the app and on an actual date IRL. “Something we’ve discussed internally is, how do we make this experience that’s virtual more human?”
She acknowledged that certain requirements, like a desire for children, might be crucial to some users. Many female doctors in their 30’s feel the “time crunch” of a ticking biological clock.
Optimize your date-ability
“I think people either love or hate dating apps, and I love them,” said Kevin Jubbal, MD. “I get to meet cool people and schedule dates from the comfort of my home.”
Dr. Jubbal, a former plastic surgery resident who left medicine to become an entrepreneur, is the founder of Med School Insiders, a tutoring and advising resource for premeds, medical students, and residents. His YouTube channel has more than 1.5 million subscribers, and he often receives questions about whether dating is feasible in medical school and how to balance a personal and academic/professional life.
Those who hate dating apps or receive few matches would do well to look inward instead of blaming the process, he said. It helps to view the experience as a learning tool that provides feedback very quickly.
“If you want to find a really amazing person, then you need to be what you want to find,” said Dr. Jubbal. “If you want to find someone who’s fit and intelligent and well read and well traveled, you need to be that. Otherwise, you’re probably not going to attract that person.”
An app designed to help single female MDs
Ifie Williams, MD, a psychiatrist in Washington, D.C., believes a wider dating pool is key – provided everyone understands the situation up front. When Dr. Williams started residency in 2014, she was “as single as can be.” She tried many dating apps, but they were extremely time consuming. Even when she set specific preferences, she found herself sifting through “matches” that didn’t fit her criteria.
“Dating nowadays has become almost like a second job,” said Dr. Williams. “Just the amount of time that people are having to spend on apps, swiping left and right and then meeting people. You think they’re interested and then you deal with all these games.”
By 2017, Dr. Williams had invented Miss Doctor, a dating app that would connect female physicians and other doctoral-level professionals with men or women on a similar achievement level.
By definition, these people would not be intimidated by ambitious, busy women. They would be heavily screened and vetted. And one other proviso: they would have to pay for “likes.”
Most dating apps charge a subscription fee. Users are allowed to “like” numerous profiles and perhaps not bother responding to many matches. By contrast, Miss Doctor accounts are free and include a limited number of “likes” to indicate interest. Beyond that, there’s a price.
“We wanted to find a way to make people a little more intentional with how they like people on the app, so they give a little more thought to it,” Dr. Williams said. “So, we monetize it and use that to change behavior.”
After an initial launch in 2017, the app had to take a back seat while Dr. Williams started her psychiatry practice and got married herself. She plans to relaunch it in spring 2023.
Male or female, there is general agreement that finding time to date as a young physician isn’t easy. While DownToDate has had “doctor meets doctor” success stories, many users are still searching for “the one.”
Dr. Boyer believes that career challenges are not a reason to give up. “There are so many single and available people out there,” she said. “And everyone’s deserving of love. Even if you only have an hour a week.”
A version of this article first appeared on Medscape.com.
Pounding heart, sweating, insomnia. Surges of dopamine, norepinephrine, and adrenaline. All symptoms of a very common yet frustrating condition: Falling in love.
The prognosis is vague. A prescription pad and knowledge of biochemistry aren’t helpful when it comes to relationships.
Medical training can consume decades when others are exploring relationships and starting families. There are few recent data on this, but
But there is hope! By age 36, the number of doctors in long-term relationships had overtaken everyone else by more than 10% for women and 20% for men. The Medscape 2022 Physician Happiness & Lifestyle Report found that 83% were in committed relationships, and even better, happy ones. At least three-quarters of doctors in every specialty described their partnerships as “very good” or “good.”
How should a single medical student, resident, or attending physician find happiness ever after in 2023? Sometimes Mr./Ms. Right can be found in the anatomy lab or hospital, with sparks flying between students or colleagues. But for many in health care, along with millions of others looking for love, the solution is dating apps.
When ‘MD’ is a turnoff
Dr. M, a psychiatry resident in California who prefers not to give her name, hadn’t found a life partner during college, grad school, or medical school. When she passed her final Step 3 board exam, she decided it was time to take the plunge. She signed up for popular dating apps like Hinge, Bumble, and Coffee Meets Bagel, but her dates seemed to follow a disappointing pattern.
“I met lots of guys, but it was incredibly rare to find another physician,” said Dr. M. “I found myself always wanting to talk about my life as a resident. More often than not, the guys would give me this blank stare as I complained about being on call or spoke about spending 12 hours a day studying for a board exam, or even the process of The Match and how I ended up in California.”
Both of Dr. M’s parents are physicians, and she grew up watching how they supported each other through residency, exams, and exhausting schedules. A relationship with another physician, her parents told her, would give both partners the best chance to understand each other’s lives. The problem was how to find one.
That was when Dr. M saw an ad for a dating app with a cute medical name: DownToDate, a play on the clinical evidence resource UpToDate. “I thought it was a meme,” she said. “It was this doctors-only app. I remember thinking, ‘this has to be a joke,’ but then it was very real.”
She signed up and was required to provide a photo of her ID and her NPI number. Immediately, men began “requesting a consult,” the app’s form of “liking” her profile, and sending her “pages” (messages).
DownToDate was created by another physician, Robin Boyer, MD, MBA, a pediatrics resident in Loma Linda, Calif. The inspiration came in 2020 during the initial COVID crisis. Exhausted from long and often heartbreaking shifts, Dr. Boyer was grateful for her husband’s unwavering support. But many of her coresidents weren’t so lucky. The women in particular talked about their dating struggles, and there was a recurring theme. They didn’t feel confident putting “physician” on a dating site profile.
“If you’re male and you tell people you’re a doctor, it seems like it really attracts people,” Dr. Boyer said. “But if you’re female, it brings up a lot of stereotypes where you’re perceived as too intimidating either as the breadwinner, being more educated, or having a [demanding] career. It does make it more difficult.”
Dr. Boyer met her husband in high school, and she had never used a dating app. She convinced a coresident, Celestine Odigwe, MD, to pursue the idea as partners. They began researching the market within their network and heard from over a thousand interested physicians, both men and women, heterosexual and LGBTQ+. They even created fake accounts on other sites to gauge how easy it is to falsify a profile. From these insights, the app took shape. It launched in 2021 and currently has more than 5000 verified users.
Branches from the same tree
Around the same time that DownToDate began, Shivani Shah, DO, a pediatric neurology resident at Duke University, Durham, N.C., and her brother, Sagar Shah, an entrepreneur, had a similar idea.
At the time, Dr. Shah was a fourth-year medical student about to move from New Jersey to North Carolina. Friends who were internal medicine residents described the grueling reality of the early COVID pandemic.
“It was just horrible,” said Dr. Shah. “You were isolated from your family, your support system, everything. ... I think the pandemic really pushed us into realizing that this is a very important need, and sometimes it feels like community is lacking in the health care field.”
The sibling duo developed ForeverX, an app for health care workers to find meaningful and long-term romantic connections. It launched in 2021.
Concerned that the medical field was “siloed,” the Shahs chose to open the app to physicians, dentists, nurses, physical therapists, and other health care professionals. “Opening up the doors to more communication” between the health care branches was a priority.
To prevent catfishing, the app uses a twofold vetting system. Each user submits a photo of their driver’s license and a selfie that must match. There is also health care verification through an NPI number, nurse’s ID, or a manual process for those without either. None of the information is stored.
Through personal experience with dating apps, Dr. Shah hopes ForeverX can improve on some of their flaws, particularly the problem of matches being overly filtered by preferences. The “natural way” of meeting people is not filtered. And while most people have a dating checklist in mind, meeting someone face to face might send some of those prerequisites “out the window.”
“You can’t really put into words how you feel with someone ... the vibe,” Dr. Shah said. That is why her goal is to get people off the app and on an actual date IRL. “Something we’ve discussed internally is, how do we make this experience that’s virtual more human?”
She acknowledged that certain requirements, like a desire for children, might be crucial to some users. Many female doctors in their 30’s feel the “time crunch” of a ticking biological clock.
Optimize your date-ability
“I think people either love or hate dating apps, and I love them,” said Kevin Jubbal, MD. “I get to meet cool people and schedule dates from the comfort of my home.”
Dr. Jubbal, a former plastic surgery resident who left medicine to become an entrepreneur, is the founder of Med School Insiders, a tutoring and advising resource for premeds, medical students, and residents. His YouTube channel has more than 1.5 million subscribers, and he often receives questions about whether dating is feasible in medical school and how to balance a personal and academic/professional life.
Those who hate dating apps or receive few matches would do well to look inward instead of blaming the process, he said. It helps to view the experience as a learning tool that provides feedback very quickly.
“If you want to find a really amazing person, then you need to be what you want to find,” said Dr. Jubbal. “If you want to find someone who’s fit and intelligent and well read and well traveled, you need to be that. Otherwise, you’re probably not going to attract that person.”
An app designed to help single female MDs
Ifie Williams, MD, a psychiatrist in Washington, D.C., believes a wider dating pool is key – provided everyone understands the situation up front. When Dr. Williams started residency in 2014, she was “as single as can be.” She tried many dating apps, but they were extremely time consuming. Even when she set specific preferences, she found herself sifting through “matches” that didn’t fit her criteria.
“Dating nowadays has become almost like a second job,” said Dr. Williams. “Just the amount of time that people are having to spend on apps, swiping left and right and then meeting people. You think they’re interested and then you deal with all these games.”
By 2017, Dr. Williams had invented Miss Doctor, a dating app that would connect female physicians and other doctoral-level professionals with men or women on a similar achievement level.
By definition, these people would not be intimidated by ambitious, busy women. They would be heavily screened and vetted. And one other proviso: they would have to pay for “likes.”
Most dating apps charge a subscription fee. Users are allowed to “like” numerous profiles and perhaps not bother responding to many matches. By contrast, Miss Doctor accounts are free and include a limited number of “likes” to indicate interest. Beyond that, there’s a price.
“We wanted to find a way to make people a little more intentional with how they like people on the app, so they give a little more thought to it,” Dr. Williams said. “So, we monetize it and use that to change behavior.”
After an initial launch in 2017, the app had to take a back seat while Dr. Williams started her psychiatry practice and got married herself. She plans to relaunch it in spring 2023.
Male or female, there is general agreement that finding time to date as a young physician isn’t easy. While DownToDate has had “doctor meets doctor” success stories, many users are still searching for “the one.”
Dr. Boyer believes that career challenges are not a reason to give up. “There are so many single and available people out there,” she said. “And everyone’s deserving of love. Even if you only have an hour a week.”
A version of this article first appeared on Medscape.com.
NICU use up, birth weights down in babies of mothers with HCV
Infants born to women infected with the hepatitis C virus (HCV) faced twice the risk of stays in the neonatal ICU (NICU) and 2.7 times the risk of low birth weight, a new analysis finds, even when researchers adjusted their data to control for injectable drug use and maternal medical comorbidity.
Clinicians should be “aware that the infants of pregnant people with HCV may have a high rate of need for higher-level pediatric care,” said Brenna L. Hughes, MD, MSc, chief of maternal fetal medicine at Duke University Medical Center, Durham, N.C. She spoke in an interview about the findings, which were presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
As Dr. Hughes noted, “HCV remains a serious problem in pregnancy because it often goes undiagnosed and/or untreated prior to pregnancy. It can be passed to infants, and this can cause significant health-related outcomes for children as they age.”
For the multicenter U.S. study, researchers identified 249 pregnant mothers with HCV from a 2012-2018 cohort and matched them by gestational age to controls (n = 486). The average age was 28; 71.1% of the cases were non-Hispanic White versus 41.6% of the controls; 8.4% of cases were non-Hispanic Black versus 32.1% of controls (P < .001 for race/ethnicity analysis); and 73% of cases were smokers versus 18% of controls (P < .001). More than 19% of cases reported injectable drug use during pregnancy versus 0.2% of controls (P < .001).
The researchers adjusted their findings for maternal age, body mass index, injectable drug use, and maternal comorbidity.
An earlier analysis of the study data found that 6% of pregnant women with HCV passed it on to their infants, especially those with high levels of virus in their systems. For the new study, researchers focused on various outcomes to test the assumption that “adverse pregnancy outcomes associated with HCV are related to prematurity or to ongoing use of injection drugs,” Dr. Hughes said.
There was no increase in rates of preterm birth or adverse maternal outcomes in the HCV cases. However, infants born to women with HCV were more likely than the controls to require a stay in the NICU (45% vs. 19%; adjusted relative risk, 1.99; 95% confidence interval, 1.54-2.58). They were also more likely to have lower birth weights (small for gestational age < 5th percentile) (10.6% vs. 3.1%; ARR, 2.72; 95% CI, 1.38-5.34).
No difference in outcomes was seen when HCV cases with viremia (33%) were excluded.
“The most surprising finding was that the need for higher-level pediatric care was so high even though there wasn’t an increased risk of prematurity,” Dr. Hughes said.
She added it’s not clear why NICU stays and low birth weights were more common in infants of women with HCV. “It is possible that the higher risk of need for higher-level pediatric care was related to a need for observation or treatment due to use of opioid replacement therapies with opioid agonists.” As for lower birth weight, “there may be other unmeasured risk factors.”
Tatyana Kushner, MD, MSCE, of the division of liver diseases at Icahn School of Medicine at Mount Sinai, New York, said in an interview that the study adds to limited data about HCV in pregnancy. “These findings have been demonstrated in prior studies, and it would be important to tease apart whether [low birth weight] is related to the virus itself or more related to other confounding associated factors such as maternal substance use as well as other associated social determinants of health among women with HCV.”
As for the study’s message, Dr. Kushner said it makes it clear that “hepatitis C adversely impacts outcomes of pregnancy and it is important to identify women of childbearing age for treatment early, ideally prior to pregnancy, in order to improve their pregnancy outcomes. In addition, treatment of hepatitis C during pregnancy should be explored further to determine if treatment during pregnancy can improve outcomes.”
At the moment, she said, “there are ongoing studies to delineate the safety and efficacy of hepatitis C treatment during pregnancy. Given that we are screening for hepatitis C during pregnancy, we need clear recommendations on the use of direct-acting antivirals in people who screen positive.”
The study was funded by the National Institute of Child Health and Human Development. The authors have no disclosures. Dr. Kushner disclosed research support (Gilead) and advisory board service (Gilead, AbbVie, Bausch, GlaxoSmithKline, and Eiger).
Infants born to women infected with the hepatitis C virus (HCV) faced twice the risk of stays in the neonatal ICU (NICU) and 2.7 times the risk of low birth weight, a new analysis finds, even when researchers adjusted their data to control for injectable drug use and maternal medical comorbidity.
Clinicians should be “aware that the infants of pregnant people with HCV may have a high rate of need for higher-level pediatric care,” said Brenna L. Hughes, MD, MSc, chief of maternal fetal medicine at Duke University Medical Center, Durham, N.C. She spoke in an interview about the findings, which were presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
As Dr. Hughes noted, “HCV remains a serious problem in pregnancy because it often goes undiagnosed and/or untreated prior to pregnancy. It can be passed to infants, and this can cause significant health-related outcomes for children as they age.”
For the multicenter U.S. study, researchers identified 249 pregnant mothers with HCV from a 2012-2018 cohort and matched them by gestational age to controls (n = 486). The average age was 28; 71.1% of the cases were non-Hispanic White versus 41.6% of the controls; 8.4% of cases were non-Hispanic Black versus 32.1% of controls (P < .001 for race/ethnicity analysis); and 73% of cases were smokers versus 18% of controls (P < .001). More than 19% of cases reported injectable drug use during pregnancy versus 0.2% of controls (P < .001).
The researchers adjusted their findings for maternal age, body mass index, injectable drug use, and maternal comorbidity.
An earlier analysis of the study data found that 6% of pregnant women with HCV passed it on to their infants, especially those with high levels of virus in their systems. For the new study, researchers focused on various outcomes to test the assumption that “adverse pregnancy outcomes associated with HCV are related to prematurity or to ongoing use of injection drugs,” Dr. Hughes said.
There was no increase in rates of preterm birth or adverse maternal outcomes in the HCV cases. However, infants born to women with HCV were more likely than the controls to require a stay in the NICU (45% vs. 19%; adjusted relative risk, 1.99; 95% confidence interval, 1.54-2.58). They were also more likely to have lower birth weights (small for gestational age < 5th percentile) (10.6% vs. 3.1%; ARR, 2.72; 95% CI, 1.38-5.34).
No difference in outcomes was seen when HCV cases with viremia (33%) were excluded.
“The most surprising finding was that the need for higher-level pediatric care was so high even though there wasn’t an increased risk of prematurity,” Dr. Hughes said.
She added it’s not clear why NICU stays and low birth weights were more common in infants of women with HCV. “It is possible that the higher risk of need for higher-level pediatric care was related to a need for observation or treatment due to use of opioid replacement therapies with opioid agonists.” As for lower birth weight, “there may be other unmeasured risk factors.”
Tatyana Kushner, MD, MSCE, of the division of liver diseases at Icahn School of Medicine at Mount Sinai, New York, said in an interview that the study adds to limited data about HCV in pregnancy. “These findings have been demonstrated in prior studies, and it would be important to tease apart whether [low birth weight] is related to the virus itself or more related to other confounding associated factors such as maternal substance use as well as other associated social determinants of health among women with HCV.”
As for the study’s message, Dr. Kushner said it makes it clear that “hepatitis C adversely impacts outcomes of pregnancy and it is important to identify women of childbearing age for treatment early, ideally prior to pregnancy, in order to improve their pregnancy outcomes. In addition, treatment of hepatitis C during pregnancy should be explored further to determine if treatment during pregnancy can improve outcomes.”
At the moment, she said, “there are ongoing studies to delineate the safety and efficacy of hepatitis C treatment during pregnancy. Given that we are screening for hepatitis C during pregnancy, we need clear recommendations on the use of direct-acting antivirals in people who screen positive.”
The study was funded by the National Institute of Child Health and Human Development. The authors have no disclosures. Dr. Kushner disclosed research support (Gilead) and advisory board service (Gilead, AbbVie, Bausch, GlaxoSmithKline, and Eiger).
Infants born to women infected with the hepatitis C virus (HCV) faced twice the risk of stays in the neonatal ICU (NICU) and 2.7 times the risk of low birth weight, a new analysis finds, even when researchers adjusted their data to control for injectable drug use and maternal medical comorbidity.
Clinicians should be “aware that the infants of pregnant people with HCV may have a high rate of need for higher-level pediatric care,” said Brenna L. Hughes, MD, MSc, chief of maternal fetal medicine at Duke University Medical Center, Durham, N.C. She spoke in an interview about the findings, which were presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
As Dr. Hughes noted, “HCV remains a serious problem in pregnancy because it often goes undiagnosed and/or untreated prior to pregnancy. It can be passed to infants, and this can cause significant health-related outcomes for children as they age.”
For the multicenter U.S. study, researchers identified 249 pregnant mothers with HCV from a 2012-2018 cohort and matched them by gestational age to controls (n = 486). The average age was 28; 71.1% of the cases were non-Hispanic White versus 41.6% of the controls; 8.4% of cases were non-Hispanic Black versus 32.1% of controls (P < .001 for race/ethnicity analysis); and 73% of cases were smokers versus 18% of controls (P < .001). More than 19% of cases reported injectable drug use during pregnancy versus 0.2% of controls (P < .001).
The researchers adjusted their findings for maternal age, body mass index, injectable drug use, and maternal comorbidity.
An earlier analysis of the study data found that 6% of pregnant women with HCV passed it on to their infants, especially those with high levels of virus in their systems. For the new study, researchers focused on various outcomes to test the assumption that “adverse pregnancy outcomes associated with HCV are related to prematurity or to ongoing use of injection drugs,” Dr. Hughes said.
There was no increase in rates of preterm birth or adverse maternal outcomes in the HCV cases. However, infants born to women with HCV were more likely than the controls to require a stay in the NICU (45% vs. 19%; adjusted relative risk, 1.99; 95% confidence interval, 1.54-2.58). They were also more likely to have lower birth weights (small for gestational age < 5th percentile) (10.6% vs. 3.1%; ARR, 2.72; 95% CI, 1.38-5.34).
No difference in outcomes was seen when HCV cases with viremia (33%) were excluded.
“The most surprising finding was that the need for higher-level pediatric care was so high even though there wasn’t an increased risk of prematurity,” Dr. Hughes said.
She added it’s not clear why NICU stays and low birth weights were more common in infants of women with HCV. “It is possible that the higher risk of need for higher-level pediatric care was related to a need for observation or treatment due to use of opioid replacement therapies with opioid agonists.” As for lower birth weight, “there may be other unmeasured risk factors.”
Tatyana Kushner, MD, MSCE, of the division of liver diseases at Icahn School of Medicine at Mount Sinai, New York, said in an interview that the study adds to limited data about HCV in pregnancy. “These findings have been demonstrated in prior studies, and it would be important to tease apart whether [low birth weight] is related to the virus itself or more related to other confounding associated factors such as maternal substance use as well as other associated social determinants of health among women with HCV.”
As for the study’s message, Dr. Kushner said it makes it clear that “hepatitis C adversely impacts outcomes of pregnancy and it is important to identify women of childbearing age for treatment early, ideally prior to pregnancy, in order to improve their pregnancy outcomes. In addition, treatment of hepatitis C during pregnancy should be explored further to determine if treatment during pregnancy can improve outcomes.”
At the moment, she said, “there are ongoing studies to delineate the safety and efficacy of hepatitis C treatment during pregnancy. Given that we are screening for hepatitis C during pregnancy, we need clear recommendations on the use of direct-acting antivirals in people who screen positive.”
The study was funded by the National Institute of Child Health and Human Development. The authors have no disclosures. Dr. Kushner disclosed research support (Gilead) and advisory board service (Gilead, AbbVie, Bausch, GlaxoSmithKline, and Eiger).
FROM THE PREGNANCY MEETING
Embattled iPLEDGE program: Changes ahead?
In December 2021, major changes took effect in the iPLEDGE program, the Food and Drug Administration–required safety program for managing the risks of isotretinoin’s teratogenicity and preventing exposure during pregnancy. Now, more modifications may be coming to the acne drug’s safety program.
The risk evaluation and mitigation strategy (REMS) requirements. The aim, according to the FDA meeting announcement, is “to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of isotretinoin oral capsules for patients.”
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane. Its former brand name was Accutane.
Problems began to surface days after a new, gender-neutral approach to the risk mitigation program was launched on Dec. 13, 2021. That program had been approved earlier by the FDA.
However, the problems that were encountered were a result of glitches in changes in the platform that had been planned, and were not related to the gender-neutral changes. The iPLEDGE program had transitioned to the new platform, and the rollout was far from smooth. Dermatologists, pharmacists, patients, parents of patients, and others were frustrated and angry that they could not access the new platform and obtain the medication promptly. Reaching the help line to sort out problems was another exercise in frustration. Wait times while on hold were unbearably long, or problems were not resolved over the phone.
(The new gender-neutral approach, which advocates said was needed to preserve inclusiveness of their patients, including transgender patients, places potential patients into two categories: those who can become pregnant, and those who cannot. Previously, there were three categories into which patients were classified: females who have reproductive potential, females who do not have reproductive potential, and males.)
Before pharmacists can fill a prescription for isotretinoin, a medical provider must confirm a patient’s negative pregnancy test and inform a patient with reproductive potential of the risks of the medication.
In January 2022, to deal with the chaotic launch and subsequent problems, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve the problems reported by clinicians, pharmacists, and patients.
The American Academy of Dermatology Association formed an iPLEDGE work group to address the issues and suggest solutions. It has made several requests of and suggestions for the IPMG, which manages the program, according to Andrea L. Zaenglein, MD, professor of dermatology and pediatrics at Penn State Hershey (Pa.) Medical Center, and a member of the work group.
“We are asking them to eliminate the monthly attestation for patients who can’t get pregnant and to review and modify restrictive and punitive waiting and lockout periods for all patients,” she told this news organization.
As of February 2023, most of the platform glitches had been smoothed out, Dr. Zaenglein said. Still, “improvements to the design of the website could improve the user interface,” she added.
The FDA has established a docket for the public to submit comments before the meeting. The docket number is FDA-2022-N-3071. The electronic filing system will accept comments until 11:59 p.m. Eastern time on March 27. Background material and a link to the live webcast of the panel meeting will be available to the public no later than 2 days before the meeting and will be posted on the FDA web page or at the time of the meeting.
A version of this article first appeared on Medscape.com.
In December 2021, major changes took effect in the iPLEDGE program, the Food and Drug Administration–required safety program for managing the risks of isotretinoin’s teratogenicity and preventing exposure during pregnancy. Now, more modifications may be coming to the acne drug’s safety program.
The risk evaluation and mitigation strategy (REMS) requirements. The aim, according to the FDA meeting announcement, is “to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of isotretinoin oral capsules for patients.”
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane. Its former brand name was Accutane.
Problems began to surface days after a new, gender-neutral approach to the risk mitigation program was launched on Dec. 13, 2021. That program had been approved earlier by the FDA.
However, the problems that were encountered were a result of glitches in changes in the platform that had been planned, and were not related to the gender-neutral changes. The iPLEDGE program had transitioned to the new platform, and the rollout was far from smooth. Dermatologists, pharmacists, patients, parents of patients, and others were frustrated and angry that they could not access the new platform and obtain the medication promptly. Reaching the help line to sort out problems was another exercise in frustration. Wait times while on hold were unbearably long, or problems were not resolved over the phone.
(The new gender-neutral approach, which advocates said was needed to preserve inclusiveness of their patients, including transgender patients, places potential patients into two categories: those who can become pregnant, and those who cannot. Previously, there were three categories into which patients were classified: females who have reproductive potential, females who do not have reproductive potential, and males.)
Before pharmacists can fill a prescription for isotretinoin, a medical provider must confirm a patient’s negative pregnancy test and inform a patient with reproductive potential of the risks of the medication.
In January 2022, to deal with the chaotic launch and subsequent problems, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve the problems reported by clinicians, pharmacists, and patients.
The American Academy of Dermatology Association formed an iPLEDGE work group to address the issues and suggest solutions. It has made several requests of and suggestions for the IPMG, which manages the program, according to Andrea L. Zaenglein, MD, professor of dermatology and pediatrics at Penn State Hershey (Pa.) Medical Center, and a member of the work group.
“We are asking them to eliminate the monthly attestation for patients who can’t get pregnant and to review and modify restrictive and punitive waiting and lockout periods for all patients,” she told this news organization.
As of February 2023, most of the platform glitches had been smoothed out, Dr. Zaenglein said. Still, “improvements to the design of the website could improve the user interface,” she added.
The FDA has established a docket for the public to submit comments before the meeting. The docket number is FDA-2022-N-3071. The electronic filing system will accept comments until 11:59 p.m. Eastern time on March 27. Background material and a link to the live webcast of the panel meeting will be available to the public no later than 2 days before the meeting and will be posted on the FDA web page or at the time of the meeting.
A version of this article first appeared on Medscape.com.
In December 2021, major changes took effect in the iPLEDGE program, the Food and Drug Administration–required safety program for managing the risks of isotretinoin’s teratogenicity and preventing exposure during pregnancy. Now, more modifications may be coming to the acne drug’s safety program.
The risk evaluation and mitigation strategy (REMS) requirements. The aim, according to the FDA meeting announcement, is “to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of isotretinoin oral capsules for patients.”
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane. Its former brand name was Accutane.
Problems began to surface days after a new, gender-neutral approach to the risk mitigation program was launched on Dec. 13, 2021. That program had been approved earlier by the FDA.
However, the problems that were encountered were a result of glitches in changes in the platform that had been planned, and were not related to the gender-neutral changes. The iPLEDGE program had transitioned to the new platform, and the rollout was far from smooth. Dermatologists, pharmacists, patients, parents of patients, and others were frustrated and angry that they could not access the new platform and obtain the medication promptly. Reaching the help line to sort out problems was another exercise in frustration. Wait times while on hold were unbearably long, or problems were not resolved over the phone.
(The new gender-neutral approach, which advocates said was needed to preserve inclusiveness of their patients, including transgender patients, places potential patients into two categories: those who can become pregnant, and those who cannot. Previously, there were three categories into which patients were classified: females who have reproductive potential, females who do not have reproductive potential, and males.)
Before pharmacists can fill a prescription for isotretinoin, a medical provider must confirm a patient’s negative pregnancy test and inform a patient with reproductive potential of the risks of the medication.
In January 2022, to deal with the chaotic launch and subsequent problems, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve the problems reported by clinicians, pharmacists, and patients.
The American Academy of Dermatology Association formed an iPLEDGE work group to address the issues and suggest solutions. It has made several requests of and suggestions for the IPMG, which manages the program, according to Andrea L. Zaenglein, MD, professor of dermatology and pediatrics at Penn State Hershey (Pa.) Medical Center, and a member of the work group.
“We are asking them to eliminate the monthly attestation for patients who can’t get pregnant and to review and modify restrictive and punitive waiting and lockout periods for all patients,” she told this news organization.
As of February 2023, most of the platform glitches had been smoothed out, Dr. Zaenglein said. Still, “improvements to the design of the website could improve the user interface,” she added.
The FDA has established a docket for the public to submit comments before the meeting. The docket number is FDA-2022-N-3071. The electronic filing system will accept comments until 11:59 p.m. Eastern time on March 27. Background material and a link to the live webcast of the panel meeting will be available to the public no later than 2 days before the meeting and will be posted on the FDA web page or at the time of the meeting.
A version of this article first appeared on Medscape.com.
FDA expands oral JAK abrocitinib to adolescents with AD
Abrocitinib, taken once daily, previously was approved only for treating adults aged 18 and older.
It joins upadacitinib (Rinvoq), previously the only oral JAK inhibitor to be approved for use by adolescents aged 12 through 17 with refractory moderate to severe AD.
The indication has been expanded for teens whose disease is not adequately controlled with other systemic drugs, including biologics, or those for whom use of those drugs is not advised.
Prescribing information was updated to reflect data from JADE TEEN, a phase 3, randomized, placebo-controlled trial that supported the indication for adolescents. That trial evaluated both the 100-mg and 200-mg doses of abrocitinib in comparison with placebo in 285 adolescents aged 12-18 who had moderate to severe AD and who were also receiving background therapy with topical medications.
The most common toxicities that were reported in at least 1% of patients treated with abrocitinib for up to 16 weeks included nasopharyngitis, nausea, and headache.
Efficacy measures included improvements in itch, skin clearance, and disease severity using the Investigator Global Assessment (IGA), the Peak Pruritus Numerical Rating Scale (PP-NRS), and the Eczema Area and Severity Index (EASI), according to the Pfizer statement announcing the expanded approval.
Select JADE TEEN findings include the following:
- IGA response rate of 0 or 1 at week 12: 39% with abrocitinib 100 mg; 46% with abrocitinib 200 mg; and 24% with placebo.
- EASI-75 response rate at week 12: 64%, 71%, and 41%, respectively.
- Proportion of participants achieving PP-NRS with at least a 4-point decrease from baseline at week 2: 13%, 25%, and 8%, respectively.
Data included in the prescribing information now encompass five randomized, placebo-controlled clinical trials and a long-term extension study with more than 1,600 patients treated with abrocitinib, according to the statement from Pfizer.
In a 2021 story, when JADE TEEN trial results were presented, Lawrence Eichenfield, MD, professor of dermatology and pediatrics, University of California, San Diego, and Rady Children’s Hospital, San Diego, told this news organization that he welcomed oral JAKs as a weapon against atopic dermatitis.
He noted that moderate to severe AD can have a tremendous impact on adolescents. “Traditionally, we have treated it with intermittent topical corticosteroids, but this has left a significant percentage of patients without long-term disease control,” he said.
Abrocitinib is not recommended for use with other JAK inhibitors, biologic immunomodulators, or other immunosuppressants.
AD, one of the most common inflammatory skin diseases, affects approximately 5%-10% of adults in the United States and approximately 11% of children. About one in three adults and one in three children and adolescents aged 17 and younger with AD have moderate to severe disease.
JAK inhibition is thought to modulate multiple cytokines involved in AD, including interleukin (IL)–4, IL-13, IL-31, IL-22, and thymic stromal lymphopoietin.
Prescribing information includes a warning that use of abrocitinib should be avoided by patients with an active, serious infection, including localized infections. A boxed warning is included in the labels of JAK inhibitors regarding the risk of serious infections, mortality, major cardiovascular events, and thrombosis.
Treatment risks and benefits should be carefully considered for patients with chronic or recurrent infections or those who have lived in or traveled in areas of endemic tuberculosis or endemic mycoses, the information states.
A version of this article first appeared on Medscape.com.
Abrocitinib, taken once daily, previously was approved only for treating adults aged 18 and older.
It joins upadacitinib (Rinvoq), previously the only oral JAK inhibitor to be approved for use by adolescents aged 12 through 17 with refractory moderate to severe AD.
The indication has been expanded for teens whose disease is not adequately controlled with other systemic drugs, including biologics, or those for whom use of those drugs is not advised.
Prescribing information was updated to reflect data from JADE TEEN, a phase 3, randomized, placebo-controlled trial that supported the indication for adolescents. That trial evaluated both the 100-mg and 200-mg doses of abrocitinib in comparison with placebo in 285 adolescents aged 12-18 who had moderate to severe AD and who were also receiving background therapy with topical medications.
The most common toxicities that were reported in at least 1% of patients treated with abrocitinib for up to 16 weeks included nasopharyngitis, nausea, and headache.
Efficacy measures included improvements in itch, skin clearance, and disease severity using the Investigator Global Assessment (IGA), the Peak Pruritus Numerical Rating Scale (PP-NRS), and the Eczema Area and Severity Index (EASI), according to the Pfizer statement announcing the expanded approval.
Select JADE TEEN findings include the following:
- IGA response rate of 0 or 1 at week 12: 39% with abrocitinib 100 mg; 46% with abrocitinib 200 mg; and 24% with placebo.
- EASI-75 response rate at week 12: 64%, 71%, and 41%, respectively.
- Proportion of participants achieving PP-NRS with at least a 4-point decrease from baseline at week 2: 13%, 25%, and 8%, respectively.
Data included in the prescribing information now encompass five randomized, placebo-controlled clinical trials and a long-term extension study with more than 1,600 patients treated with abrocitinib, according to the statement from Pfizer.
In a 2021 story, when JADE TEEN trial results were presented, Lawrence Eichenfield, MD, professor of dermatology and pediatrics, University of California, San Diego, and Rady Children’s Hospital, San Diego, told this news organization that he welcomed oral JAKs as a weapon against atopic dermatitis.
He noted that moderate to severe AD can have a tremendous impact on adolescents. “Traditionally, we have treated it with intermittent topical corticosteroids, but this has left a significant percentage of patients without long-term disease control,” he said.
Abrocitinib is not recommended for use with other JAK inhibitors, biologic immunomodulators, or other immunosuppressants.
AD, one of the most common inflammatory skin diseases, affects approximately 5%-10% of adults in the United States and approximately 11% of children. About one in three adults and one in three children and adolescents aged 17 and younger with AD have moderate to severe disease.
JAK inhibition is thought to modulate multiple cytokines involved in AD, including interleukin (IL)–4, IL-13, IL-31, IL-22, and thymic stromal lymphopoietin.
Prescribing information includes a warning that use of abrocitinib should be avoided by patients with an active, serious infection, including localized infections. A boxed warning is included in the labels of JAK inhibitors regarding the risk of serious infections, mortality, major cardiovascular events, and thrombosis.
Treatment risks and benefits should be carefully considered for patients with chronic or recurrent infections or those who have lived in or traveled in areas of endemic tuberculosis or endemic mycoses, the information states.
A version of this article first appeared on Medscape.com.
Abrocitinib, taken once daily, previously was approved only for treating adults aged 18 and older.
It joins upadacitinib (Rinvoq), previously the only oral JAK inhibitor to be approved for use by adolescents aged 12 through 17 with refractory moderate to severe AD.
The indication has been expanded for teens whose disease is not adequately controlled with other systemic drugs, including biologics, or those for whom use of those drugs is not advised.
Prescribing information was updated to reflect data from JADE TEEN, a phase 3, randomized, placebo-controlled trial that supported the indication for adolescents. That trial evaluated both the 100-mg and 200-mg doses of abrocitinib in comparison with placebo in 285 adolescents aged 12-18 who had moderate to severe AD and who were also receiving background therapy with topical medications.
The most common toxicities that were reported in at least 1% of patients treated with abrocitinib for up to 16 weeks included nasopharyngitis, nausea, and headache.
Efficacy measures included improvements in itch, skin clearance, and disease severity using the Investigator Global Assessment (IGA), the Peak Pruritus Numerical Rating Scale (PP-NRS), and the Eczema Area and Severity Index (EASI), according to the Pfizer statement announcing the expanded approval.
Select JADE TEEN findings include the following:
- IGA response rate of 0 or 1 at week 12: 39% with abrocitinib 100 mg; 46% with abrocitinib 200 mg; and 24% with placebo.
- EASI-75 response rate at week 12: 64%, 71%, and 41%, respectively.
- Proportion of participants achieving PP-NRS with at least a 4-point decrease from baseline at week 2: 13%, 25%, and 8%, respectively.
Data included in the prescribing information now encompass five randomized, placebo-controlled clinical trials and a long-term extension study with more than 1,600 patients treated with abrocitinib, according to the statement from Pfizer.
In a 2021 story, when JADE TEEN trial results were presented, Lawrence Eichenfield, MD, professor of dermatology and pediatrics, University of California, San Diego, and Rady Children’s Hospital, San Diego, told this news organization that he welcomed oral JAKs as a weapon against atopic dermatitis.
He noted that moderate to severe AD can have a tremendous impact on adolescents. “Traditionally, we have treated it with intermittent topical corticosteroids, but this has left a significant percentage of patients without long-term disease control,” he said.
Abrocitinib is not recommended for use with other JAK inhibitors, biologic immunomodulators, or other immunosuppressants.
AD, one of the most common inflammatory skin diseases, affects approximately 5%-10% of adults in the United States and approximately 11% of children. About one in three adults and one in three children and adolescents aged 17 and younger with AD have moderate to severe disease.
JAK inhibition is thought to modulate multiple cytokines involved in AD, including interleukin (IL)–4, IL-13, IL-31, IL-22, and thymic stromal lymphopoietin.
Prescribing information includes a warning that use of abrocitinib should be avoided by patients with an active, serious infection, including localized infections. A boxed warning is included in the labels of JAK inhibitors regarding the risk of serious infections, mortality, major cardiovascular events, and thrombosis.
Treatment risks and benefits should be carefully considered for patients with chronic or recurrent infections or those who have lived in or traveled in areas of endemic tuberculosis or endemic mycoses, the information states.
A version of this article first appeared on Medscape.com.
Differences in brain structure linked to social disadvantage
Brain volume disparities among young children of different races may be attributable to adverse childhood experiences related to socioeconomic conditions and structural racism, new research suggests.
Investigators from the Belmont, Mass.–based McLean Hospital, an affiliate of Mass General Brigham, found that 9- and 10-year-old children of different racial and socioeconomic backgrounds have subtle neurobiological differences in gray matter volume in certain brain regions associated with trauma and stress.
Lead investigator Nathaniel Harnett, PhD, of the department of psychiatry at Harvard Medical School, Boston, believes this research shows evidence that “structural racism” – broad socioeconomic disadvantages that lead to poverty and emotional trauma – may affect brain structures and growth and ultimately may lead to psychiatric illness.
“For clinicians, I think the take-home message is that we really need to be more aware about the ways in which the disproportionate burden of stress might impact some groups,” Dr. Harnett told this news organization.
“This in turn can affect the way they respond either to later stress or maybe even treatment outcomes.” He added that other brain regions and compensatory mechanisms are likely to be involved, and more work needs to explore these connections.
The study was published online in the American Journal of Psychiatry.
‘Toxic stressor’
Dr. Harnett and colleagues used MRI and survey data from the 2019 Adolescent Brain Cognitive Development (ABCD) study involving over 12,000 children from 21 sites across the United States.
Participating children provided information about emotional and physical conflicts in the household. The ABCD study also surveyed the parents about their race and ethnicity, parental education, employment, and family income. Another factor in the analysis was neighborhood disadvantage, based on the Area Deprivation Index utilizing 17 socioeconomic indicators from the U.S. Census, including poverty and housing.
Comparing brain MRI findings from approximately 7,300 White children and 1,800 Black children in the ABCD study, Dr. Harnett’s group found that Black children had lower gray matter volume in the amygdala, hippocampus, and other subregions of the prefrontal cortex.
Experience of adversity was the “sole factor” explaining brain volume differences, with household income being the predominant factor.
Compared with White children, Black children were three times less likely to have parents who were currently employed. In addition, White parents were more likely than Black parents to have higher education at 75.2% versus 40.6%. Black families had significantly lower household income than White families and experienced more family conflict, material hardship, neighborhood disadvantage, and traumatic events.
The researchers analyzed race-related differences in posttraumatic stress disorder symptoms and the relationship with adversity and found that Black children had significantly greater PTSD symptom severity, and that symptom severity was “further predicted by adversity.”
“Taken together, early-life adversity may act as a toxic stressor that disproportionately impacts Black children as a result of their significantly greater exposure to adversity and contributes to differential neural development of key threat-processing regions,” the investigators write.
“These parts of the brain are involved in what we typically call threat learning,” Dr. Harnett explained. “Threat learning is basically learning to recognize potential dangers in our environment and selecting behaviors to keep us safe, whether we’re going to run away from a danger or face it head on. When you have chronic exposure to things that can be dangerous or can make you feel unsafe, that might have an impact on how these brain regions develop, with potential implications for how these regions function later on in life.”
A consequence of toxic stress
This study is part of a growing body of work on the influence of “toxic stress” and other forms of PTSD on brain architecture. The authors note that prolonged exposure to adverse experiences leads to excessive activation of stress-response systems and accumulation of stress hormones. This disrupts immune and metabolic regulatory systems that influence the developing structures of the brain.
The study helps to contradict the “pseudoscientific falsehood” of biologic race-related differences in brain volume, instead emphasizing the role of adversity brought on by structural racism, the authors add.
In an accompanying editor’s note, the publication’s Editor-in-Chief Ned H. Kalin, MD, called childhood adversity, maltreatment, and stress, “significant risk factors for the development of psychopathology.”
These findings are “critically important, as they speak to the need for psychiatry as a field to be outspoken about the detrimental psychological impacts of race-related disparities in childhood adversity, to call out the fact that these disparities stem from structural racism, and to vigorously support rectifying efforts by pursuing policy changes,” he stated in a news release.
Social construct?
Joan Luby, MD, coauthor of an accompanying editorial, said she and her coauthor “really appreciate the study and think the findings are overall very consistent with the emerging literature, increasing the confidence [in the findings].”
Dr. Luby, a professor of child psychiatry and director of the Early Emotional Development Program, Washington University, St. Louis, noted that she “takes issue” with the fact that the study “makes inferences regarding race, when we think those inferences aren’t well justified, are misinterpretations, and could be misleading.”
Race is a “social construct” and there are many sources of adversity that the authors didn’t measure in the study and are likely the source of any remaining variance they found, including experiences of structural racism and discrimination,” said Dr. Luby, who was not involved in the study.
“How people look doesn’t have any bearing on their inherent biological characteristics, and more [needs to be studied] on how they experience the psychosocial environment and how the psychosocial environment rejects or reacts to them.”
These psychosocial issues “have to be taken into account and measured in a very comprehensive way,” she added.
The ABCD study was supported by the National Institutes of Health and additional federal partners. Dr. Harnett reports no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Luby receives royalties from Guilford Press. Her coauthor reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Brain volume disparities among young children of different races may be attributable to adverse childhood experiences related to socioeconomic conditions and structural racism, new research suggests.
Investigators from the Belmont, Mass.–based McLean Hospital, an affiliate of Mass General Brigham, found that 9- and 10-year-old children of different racial and socioeconomic backgrounds have subtle neurobiological differences in gray matter volume in certain brain regions associated with trauma and stress.
Lead investigator Nathaniel Harnett, PhD, of the department of psychiatry at Harvard Medical School, Boston, believes this research shows evidence that “structural racism” – broad socioeconomic disadvantages that lead to poverty and emotional trauma – may affect brain structures and growth and ultimately may lead to psychiatric illness.
“For clinicians, I think the take-home message is that we really need to be more aware about the ways in which the disproportionate burden of stress might impact some groups,” Dr. Harnett told this news organization.
“This in turn can affect the way they respond either to later stress or maybe even treatment outcomes.” He added that other brain regions and compensatory mechanisms are likely to be involved, and more work needs to explore these connections.
The study was published online in the American Journal of Psychiatry.
‘Toxic stressor’
Dr. Harnett and colleagues used MRI and survey data from the 2019 Adolescent Brain Cognitive Development (ABCD) study involving over 12,000 children from 21 sites across the United States.
Participating children provided information about emotional and physical conflicts in the household. The ABCD study also surveyed the parents about their race and ethnicity, parental education, employment, and family income. Another factor in the analysis was neighborhood disadvantage, based on the Area Deprivation Index utilizing 17 socioeconomic indicators from the U.S. Census, including poverty and housing.
Comparing brain MRI findings from approximately 7,300 White children and 1,800 Black children in the ABCD study, Dr. Harnett’s group found that Black children had lower gray matter volume in the amygdala, hippocampus, and other subregions of the prefrontal cortex.
Experience of adversity was the “sole factor” explaining brain volume differences, with household income being the predominant factor.
Compared with White children, Black children were three times less likely to have parents who were currently employed. In addition, White parents were more likely than Black parents to have higher education at 75.2% versus 40.6%. Black families had significantly lower household income than White families and experienced more family conflict, material hardship, neighborhood disadvantage, and traumatic events.
The researchers analyzed race-related differences in posttraumatic stress disorder symptoms and the relationship with adversity and found that Black children had significantly greater PTSD symptom severity, and that symptom severity was “further predicted by adversity.”
“Taken together, early-life adversity may act as a toxic stressor that disproportionately impacts Black children as a result of their significantly greater exposure to adversity and contributes to differential neural development of key threat-processing regions,” the investigators write.
“These parts of the brain are involved in what we typically call threat learning,” Dr. Harnett explained. “Threat learning is basically learning to recognize potential dangers in our environment and selecting behaviors to keep us safe, whether we’re going to run away from a danger or face it head on. When you have chronic exposure to things that can be dangerous or can make you feel unsafe, that might have an impact on how these brain regions develop, with potential implications for how these regions function later on in life.”
A consequence of toxic stress
This study is part of a growing body of work on the influence of “toxic stress” and other forms of PTSD on brain architecture. The authors note that prolonged exposure to adverse experiences leads to excessive activation of stress-response systems and accumulation of stress hormones. This disrupts immune and metabolic regulatory systems that influence the developing structures of the brain.
The study helps to contradict the “pseudoscientific falsehood” of biologic race-related differences in brain volume, instead emphasizing the role of adversity brought on by structural racism, the authors add.
In an accompanying editor’s note, the publication’s Editor-in-Chief Ned H. Kalin, MD, called childhood adversity, maltreatment, and stress, “significant risk factors for the development of psychopathology.”
These findings are “critically important, as they speak to the need for psychiatry as a field to be outspoken about the detrimental psychological impacts of race-related disparities in childhood adversity, to call out the fact that these disparities stem from structural racism, and to vigorously support rectifying efforts by pursuing policy changes,” he stated in a news release.
Social construct?
Joan Luby, MD, coauthor of an accompanying editorial, said she and her coauthor “really appreciate the study and think the findings are overall very consistent with the emerging literature, increasing the confidence [in the findings].”
Dr. Luby, a professor of child psychiatry and director of the Early Emotional Development Program, Washington University, St. Louis, noted that she “takes issue” with the fact that the study “makes inferences regarding race, when we think those inferences aren’t well justified, are misinterpretations, and could be misleading.”
Race is a “social construct” and there are many sources of adversity that the authors didn’t measure in the study and are likely the source of any remaining variance they found, including experiences of structural racism and discrimination,” said Dr. Luby, who was not involved in the study.
“How people look doesn’t have any bearing on their inherent biological characteristics, and more [needs to be studied] on how they experience the psychosocial environment and how the psychosocial environment rejects or reacts to them.”
These psychosocial issues “have to be taken into account and measured in a very comprehensive way,” she added.
The ABCD study was supported by the National Institutes of Health and additional federal partners. Dr. Harnett reports no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Luby receives royalties from Guilford Press. Her coauthor reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Brain volume disparities among young children of different races may be attributable to adverse childhood experiences related to socioeconomic conditions and structural racism, new research suggests.
Investigators from the Belmont, Mass.–based McLean Hospital, an affiliate of Mass General Brigham, found that 9- and 10-year-old children of different racial and socioeconomic backgrounds have subtle neurobiological differences in gray matter volume in certain brain regions associated with trauma and stress.
Lead investigator Nathaniel Harnett, PhD, of the department of psychiatry at Harvard Medical School, Boston, believes this research shows evidence that “structural racism” – broad socioeconomic disadvantages that lead to poverty and emotional trauma – may affect brain structures and growth and ultimately may lead to psychiatric illness.
“For clinicians, I think the take-home message is that we really need to be more aware about the ways in which the disproportionate burden of stress might impact some groups,” Dr. Harnett told this news organization.
“This in turn can affect the way they respond either to later stress or maybe even treatment outcomes.” He added that other brain regions and compensatory mechanisms are likely to be involved, and more work needs to explore these connections.
The study was published online in the American Journal of Psychiatry.
‘Toxic stressor’
Dr. Harnett and colleagues used MRI and survey data from the 2019 Adolescent Brain Cognitive Development (ABCD) study involving over 12,000 children from 21 sites across the United States.
Participating children provided information about emotional and physical conflicts in the household. The ABCD study also surveyed the parents about their race and ethnicity, parental education, employment, and family income. Another factor in the analysis was neighborhood disadvantage, based on the Area Deprivation Index utilizing 17 socioeconomic indicators from the U.S. Census, including poverty and housing.
Comparing brain MRI findings from approximately 7,300 White children and 1,800 Black children in the ABCD study, Dr. Harnett’s group found that Black children had lower gray matter volume in the amygdala, hippocampus, and other subregions of the prefrontal cortex.
Experience of adversity was the “sole factor” explaining brain volume differences, with household income being the predominant factor.
Compared with White children, Black children were three times less likely to have parents who were currently employed. In addition, White parents were more likely than Black parents to have higher education at 75.2% versus 40.6%. Black families had significantly lower household income than White families and experienced more family conflict, material hardship, neighborhood disadvantage, and traumatic events.
The researchers analyzed race-related differences in posttraumatic stress disorder symptoms and the relationship with adversity and found that Black children had significantly greater PTSD symptom severity, and that symptom severity was “further predicted by adversity.”
“Taken together, early-life adversity may act as a toxic stressor that disproportionately impacts Black children as a result of their significantly greater exposure to adversity and contributes to differential neural development of key threat-processing regions,” the investigators write.
“These parts of the brain are involved in what we typically call threat learning,” Dr. Harnett explained. “Threat learning is basically learning to recognize potential dangers in our environment and selecting behaviors to keep us safe, whether we’re going to run away from a danger or face it head on. When you have chronic exposure to things that can be dangerous or can make you feel unsafe, that might have an impact on how these brain regions develop, with potential implications for how these regions function later on in life.”
A consequence of toxic stress
This study is part of a growing body of work on the influence of “toxic stress” and other forms of PTSD on brain architecture. The authors note that prolonged exposure to adverse experiences leads to excessive activation of stress-response systems and accumulation of stress hormones. This disrupts immune and metabolic regulatory systems that influence the developing structures of the brain.
The study helps to contradict the “pseudoscientific falsehood” of biologic race-related differences in brain volume, instead emphasizing the role of adversity brought on by structural racism, the authors add.
In an accompanying editor’s note, the publication’s Editor-in-Chief Ned H. Kalin, MD, called childhood adversity, maltreatment, and stress, “significant risk factors for the development of psychopathology.”
These findings are “critically important, as they speak to the need for psychiatry as a field to be outspoken about the detrimental psychological impacts of race-related disparities in childhood adversity, to call out the fact that these disparities stem from structural racism, and to vigorously support rectifying efforts by pursuing policy changes,” he stated in a news release.
Social construct?
Joan Luby, MD, coauthor of an accompanying editorial, said she and her coauthor “really appreciate the study and think the findings are overall very consistent with the emerging literature, increasing the confidence [in the findings].”
Dr. Luby, a professor of child psychiatry and director of the Early Emotional Development Program, Washington University, St. Louis, noted that she “takes issue” with the fact that the study “makes inferences regarding race, when we think those inferences aren’t well justified, are misinterpretations, and could be misleading.”
Race is a “social construct” and there are many sources of adversity that the authors didn’t measure in the study and are likely the source of any remaining variance they found, including experiences of structural racism and discrimination,” said Dr. Luby, who was not involved in the study.
“How people look doesn’t have any bearing on their inherent biological characteristics, and more [needs to be studied] on how they experience the psychosocial environment and how the psychosocial environment rejects or reacts to them.”
These psychosocial issues “have to be taken into account and measured in a very comprehensive way,” she added.
The ABCD study was supported by the National Institutes of Health and additional federal partners. Dr. Harnett reports no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Luby receives royalties from Guilford Press. Her coauthor reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Algorithm can spot signs of autism in babies, study says
, a study from Duke University, Durham, N.C., says.
“We can use the first 30 days of a child’s health care experience to say, ‘This child is really at risk,’ ” said David Mandell, DSc, a professor of psychiatry at the University of Pennsylvania, Philadelphia, in USA Today. He was not involved in the research.
Researchers analyzed electronic medical records of 45,000 children treated in the Duke University Health System as infants between 2006 and 2020. They created an algorithm that could predict which babies later developed autism. These babies were more likely to have been to an ophthalmologist or neurologist; had stomach or gastrointestinal issues; or received physical therapy.
“A huge number of factors across the infant’s entire health profile” went into the models, said study coauthor Matthew Engelhard, MD, an assistant professor of biostatistics and bioinformatics at Duke University. “Each one of those factors contributes incrementally.”
USA Today said the team “paid particular attention to how the model performed in groups of children who are often overlooked by traditional screening methods and, therefore, miss the advantages of early diagnosis, including girls, children of color, and children with combined diagnoses of autism and ADHD,” according to Dr. Engelhard.
The study could lead to the algorithm being used with other tools to diagnose and help children earlier, said study author Geraldine Dawson, PhD, who directs the Duke Center for Autism and Brain Development.
“We need to be thinking about autism as not only a behavioral health condition but also a condition that involves physical health,” she said. “This is one way to take advantage of that information: in doing a better job at early detection.”
Autism is a complicated condition that includes communication and behavior challenges involving a range of symptoms and skills. It can be minor or a disability that requires full-time care.
A version of this article first appeared on WebMD.com.
, a study from Duke University, Durham, N.C., says.
“We can use the first 30 days of a child’s health care experience to say, ‘This child is really at risk,’ ” said David Mandell, DSc, a professor of psychiatry at the University of Pennsylvania, Philadelphia, in USA Today. He was not involved in the research.
Researchers analyzed electronic medical records of 45,000 children treated in the Duke University Health System as infants between 2006 and 2020. They created an algorithm that could predict which babies later developed autism. These babies were more likely to have been to an ophthalmologist or neurologist; had stomach or gastrointestinal issues; or received physical therapy.
“A huge number of factors across the infant’s entire health profile” went into the models, said study coauthor Matthew Engelhard, MD, an assistant professor of biostatistics and bioinformatics at Duke University. “Each one of those factors contributes incrementally.”
USA Today said the team “paid particular attention to how the model performed in groups of children who are often overlooked by traditional screening methods and, therefore, miss the advantages of early diagnosis, including girls, children of color, and children with combined diagnoses of autism and ADHD,” according to Dr. Engelhard.
The study could lead to the algorithm being used with other tools to diagnose and help children earlier, said study author Geraldine Dawson, PhD, who directs the Duke Center for Autism and Brain Development.
“We need to be thinking about autism as not only a behavioral health condition but also a condition that involves physical health,” she said. “This is one way to take advantage of that information: in doing a better job at early detection.”
Autism is a complicated condition that includes communication and behavior challenges involving a range of symptoms and skills. It can be minor or a disability that requires full-time care.
A version of this article first appeared on WebMD.com.
, a study from Duke University, Durham, N.C., says.
“We can use the first 30 days of a child’s health care experience to say, ‘This child is really at risk,’ ” said David Mandell, DSc, a professor of psychiatry at the University of Pennsylvania, Philadelphia, in USA Today. He was not involved in the research.
Researchers analyzed electronic medical records of 45,000 children treated in the Duke University Health System as infants between 2006 and 2020. They created an algorithm that could predict which babies later developed autism. These babies were more likely to have been to an ophthalmologist or neurologist; had stomach or gastrointestinal issues; or received physical therapy.
“A huge number of factors across the infant’s entire health profile” went into the models, said study coauthor Matthew Engelhard, MD, an assistant professor of biostatistics and bioinformatics at Duke University. “Each one of those factors contributes incrementally.”
USA Today said the team “paid particular attention to how the model performed in groups of children who are often overlooked by traditional screening methods and, therefore, miss the advantages of early diagnosis, including girls, children of color, and children with combined diagnoses of autism and ADHD,” according to Dr. Engelhard.
The study could lead to the algorithm being used with other tools to diagnose and help children earlier, said study author Geraldine Dawson, PhD, who directs the Duke Center for Autism and Brain Development.
“We need to be thinking about autism as not only a behavioral health condition but also a condition that involves physical health,” she said. “This is one way to take advantage of that information: in doing a better job at early detection.”
Autism is a complicated condition that includes communication and behavior challenges involving a range of symptoms and skills. It can be minor or a disability that requires full-time care.
A version of this article first appeared on WebMD.com.
FROM JAMA NETWORK OPEN
Risk of infections low among kids receiving systemic meds for psoriasis, study finds
.
Those are key findings from what is believed to be the largest cohort study of its kind to estimate the 6-month rate of infections among children with psoriasis who started treatment with ustekinumab, etanercept, or methotrexate.
“Clinical trials have demonstrated high efficacy of new immunomodulatory agents in treating children with psoriasis,” lead author Maria C. Schneeweiss, MD, of the division of pharmacoepidemiology in the departments of medicine and dermatology at Brigham and Women’s Hospital and Harvard Medical School, Boston, and colleagues wrote in the article, which was published online in JAMA Dermatology. “However, the risk of infections in clinical practice has not been fully characterized by comparing these medications against each other in pairwise comparisons.”
Drawing from two large U.S. insurance claims databases, the researchers identified 2,338 patients aged 17 years and younger who were receiving treatment with a topical medication for psoriasis and started new treatment with ustekinumab, etanercept, or methotrexate. They stratified their analysis by the time before pediatric labeling (2009-2015) and after pediatric approval (2016-2021), and their follow-up of patients started 1 day after initiating treatment and ended at 6 months.
Of the 2,338 patients, 1,368 (58%) were girls. From 2009 through 2021, 379 patients began treatment with ustekinumab, 779 patients began treatment with etanercept, and 1,180 patients began treatment with methotrexate. The propensity score–adjusted incidence rate of serious infection was 18.4 per 1,000 person-years (3 events) for those who used ustekinumab, 25.6 per 1,000 person-years (9 events) for those who used etanercept, and 14.9 per 1,000 person-years (8 events) for those who used methotrexate. The adjusted rate of outpatient infections was 254.9 per 1,000 person-years (39 events) for those who used ustekinumab, 435.7 per 1,000 person-years (139 events) for those who used etanercept, and 433.6 per 1,000 person-years (209 events) for those who used methotrexate. Meanwhile, the adjusted rate ratio of outpatient infections was 0.58 for ustekinumab vs. etanercept, 0.66 for ustekinumab vs. methotrexate, and 0.95 for etanercept vs. methotrexate. The researchers found that ratios were similar during the off-label use era and after pediatric labeling.
Anna L. Grossberg, MD, director of pediatric dermatology at the Johns Hopkins Children’s Center, Baltimore, who was asked to comment on the work, told this news organization that the data on outpatient infections in ustekinumab users “demonstrated that they may have a decreased risk of infection compared to pediatric psoriasis patients treated with methotrexate or the TNF-alpha inhibitor etanercept. This is previously unreported and reflects my personal experience with this medication in my own pediatric psoriasis patients.” She added the study’s overall findings lend further support to the safety of biologic medications and nonbiologic systemic immunomodulatory treatments for management of psoriasis. “This study will help guide pediatric dermatologists in counseling patients and their families about these risks [of infection], and in general providing reassurance that these risks appear to be quite low,” Dr. Grossberg said. “In particular, ustekinumab, a newer biologic medication that was recently FDA-approved for children 6 years and older for pediatric psoriasis, was not associated with higher infection rates than the other agents analyzed in this study, and in fact appears to carry a reduced risk compared to both etanercept and methotrexate.”
She noted certain limitations of the study, including its reliance on insurance claims data, “which can be limiting because information on possible confounding variables may not be known,” she said. “For example, the authors point out that environmental and behavioral risk factors for serious infection could not be evaluated or adjusted for, nor could the severity of the patients’ psoriasis. Additionally, this study only reported on outpatient infections that resulted in an antibiotic or other medications being prescribed and filled. It therefore may have missed children who presented with certain viral infections (examples could include the common cold and uncomplicated ear infections), which often will not require a prescription medication. Furthermore, it would fail to capture those who may have been seen for an infection but failed to fill the intended prescription.”
Dr. Schneeweiss reported receiving grants from AbbVie and UCB to Brigham and Women’s Hospital unrelated to the topic of this study and outside the submitted work. The study was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Grossberg reported having no financial disclosures.
.
Those are key findings from what is believed to be the largest cohort study of its kind to estimate the 6-month rate of infections among children with psoriasis who started treatment with ustekinumab, etanercept, or methotrexate.
“Clinical trials have demonstrated high efficacy of new immunomodulatory agents in treating children with psoriasis,” lead author Maria C. Schneeweiss, MD, of the division of pharmacoepidemiology in the departments of medicine and dermatology at Brigham and Women’s Hospital and Harvard Medical School, Boston, and colleagues wrote in the article, which was published online in JAMA Dermatology. “However, the risk of infections in clinical practice has not been fully characterized by comparing these medications against each other in pairwise comparisons.”
Drawing from two large U.S. insurance claims databases, the researchers identified 2,338 patients aged 17 years and younger who were receiving treatment with a topical medication for psoriasis and started new treatment with ustekinumab, etanercept, or methotrexate. They stratified their analysis by the time before pediatric labeling (2009-2015) and after pediatric approval (2016-2021), and their follow-up of patients started 1 day after initiating treatment and ended at 6 months.
Of the 2,338 patients, 1,368 (58%) were girls. From 2009 through 2021, 379 patients began treatment with ustekinumab, 779 patients began treatment with etanercept, and 1,180 patients began treatment with methotrexate. The propensity score–adjusted incidence rate of serious infection was 18.4 per 1,000 person-years (3 events) for those who used ustekinumab, 25.6 per 1,000 person-years (9 events) for those who used etanercept, and 14.9 per 1,000 person-years (8 events) for those who used methotrexate. The adjusted rate of outpatient infections was 254.9 per 1,000 person-years (39 events) for those who used ustekinumab, 435.7 per 1,000 person-years (139 events) for those who used etanercept, and 433.6 per 1,000 person-years (209 events) for those who used methotrexate. Meanwhile, the adjusted rate ratio of outpatient infections was 0.58 for ustekinumab vs. etanercept, 0.66 for ustekinumab vs. methotrexate, and 0.95 for etanercept vs. methotrexate. The researchers found that ratios were similar during the off-label use era and after pediatric labeling.
Anna L. Grossberg, MD, director of pediatric dermatology at the Johns Hopkins Children’s Center, Baltimore, who was asked to comment on the work, told this news organization that the data on outpatient infections in ustekinumab users “demonstrated that they may have a decreased risk of infection compared to pediatric psoriasis patients treated with methotrexate or the TNF-alpha inhibitor etanercept. This is previously unreported and reflects my personal experience with this medication in my own pediatric psoriasis patients.” She added the study’s overall findings lend further support to the safety of biologic medications and nonbiologic systemic immunomodulatory treatments for management of psoriasis. “This study will help guide pediatric dermatologists in counseling patients and their families about these risks [of infection], and in general providing reassurance that these risks appear to be quite low,” Dr. Grossberg said. “In particular, ustekinumab, a newer biologic medication that was recently FDA-approved for children 6 years and older for pediatric psoriasis, was not associated with higher infection rates than the other agents analyzed in this study, and in fact appears to carry a reduced risk compared to both etanercept and methotrexate.”
She noted certain limitations of the study, including its reliance on insurance claims data, “which can be limiting because information on possible confounding variables may not be known,” she said. “For example, the authors point out that environmental and behavioral risk factors for serious infection could not be evaluated or adjusted for, nor could the severity of the patients’ psoriasis. Additionally, this study only reported on outpatient infections that resulted in an antibiotic or other medications being prescribed and filled. It therefore may have missed children who presented with certain viral infections (examples could include the common cold and uncomplicated ear infections), which often will not require a prescription medication. Furthermore, it would fail to capture those who may have been seen for an infection but failed to fill the intended prescription.”
Dr. Schneeweiss reported receiving grants from AbbVie and UCB to Brigham and Women’s Hospital unrelated to the topic of this study and outside the submitted work. The study was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Grossberg reported having no financial disclosures.
.
Those are key findings from what is believed to be the largest cohort study of its kind to estimate the 6-month rate of infections among children with psoriasis who started treatment with ustekinumab, etanercept, or methotrexate.
“Clinical trials have demonstrated high efficacy of new immunomodulatory agents in treating children with psoriasis,” lead author Maria C. Schneeweiss, MD, of the division of pharmacoepidemiology in the departments of medicine and dermatology at Brigham and Women’s Hospital and Harvard Medical School, Boston, and colleagues wrote in the article, which was published online in JAMA Dermatology. “However, the risk of infections in clinical practice has not been fully characterized by comparing these medications against each other in pairwise comparisons.”
Drawing from two large U.S. insurance claims databases, the researchers identified 2,338 patients aged 17 years and younger who were receiving treatment with a topical medication for psoriasis and started new treatment with ustekinumab, etanercept, or methotrexate. They stratified their analysis by the time before pediatric labeling (2009-2015) and after pediatric approval (2016-2021), and their follow-up of patients started 1 day after initiating treatment and ended at 6 months.
Of the 2,338 patients, 1,368 (58%) were girls. From 2009 through 2021, 379 patients began treatment with ustekinumab, 779 patients began treatment with etanercept, and 1,180 patients began treatment with methotrexate. The propensity score–adjusted incidence rate of serious infection was 18.4 per 1,000 person-years (3 events) for those who used ustekinumab, 25.6 per 1,000 person-years (9 events) for those who used etanercept, and 14.9 per 1,000 person-years (8 events) for those who used methotrexate. The adjusted rate of outpatient infections was 254.9 per 1,000 person-years (39 events) for those who used ustekinumab, 435.7 per 1,000 person-years (139 events) for those who used etanercept, and 433.6 per 1,000 person-years (209 events) for those who used methotrexate. Meanwhile, the adjusted rate ratio of outpatient infections was 0.58 for ustekinumab vs. etanercept, 0.66 for ustekinumab vs. methotrexate, and 0.95 for etanercept vs. methotrexate. The researchers found that ratios were similar during the off-label use era and after pediatric labeling.
Anna L. Grossberg, MD, director of pediatric dermatology at the Johns Hopkins Children’s Center, Baltimore, who was asked to comment on the work, told this news organization that the data on outpatient infections in ustekinumab users “demonstrated that they may have a decreased risk of infection compared to pediatric psoriasis patients treated with methotrexate or the TNF-alpha inhibitor etanercept. This is previously unreported and reflects my personal experience with this medication in my own pediatric psoriasis patients.” She added the study’s overall findings lend further support to the safety of biologic medications and nonbiologic systemic immunomodulatory treatments for management of psoriasis. “This study will help guide pediatric dermatologists in counseling patients and their families about these risks [of infection], and in general providing reassurance that these risks appear to be quite low,” Dr. Grossberg said. “In particular, ustekinumab, a newer biologic medication that was recently FDA-approved for children 6 years and older for pediatric psoriasis, was not associated with higher infection rates than the other agents analyzed in this study, and in fact appears to carry a reduced risk compared to both etanercept and methotrexate.”
She noted certain limitations of the study, including its reliance on insurance claims data, “which can be limiting because information on possible confounding variables may not be known,” she said. “For example, the authors point out that environmental and behavioral risk factors for serious infection could not be evaluated or adjusted for, nor could the severity of the patients’ psoriasis. Additionally, this study only reported on outpatient infections that resulted in an antibiotic or other medications being prescribed and filled. It therefore may have missed children who presented with certain viral infections (examples could include the common cold and uncomplicated ear infections), which often will not require a prescription medication. Furthermore, it would fail to capture those who may have been seen for an infection but failed to fill the intended prescription.”
Dr. Schneeweiss reported receiving grants from AbbVie and UCB to Brigham and Women’s Hospital unrelated to the topic of this study and outside the submitted work. The study was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Grossberg reported having no financial disclosures.
FROM JAMA DERMATOLOGY
Novel neuroprotective agent promising in stroke
preliminary results of a first-in-human study show.
The findings illustrate that it is possible to improve outcomes for stroke patients “not only with reperfusion therapy but with neuroprotectants,” study author Macarena Hernandez, PhD, associate professor, University Complutense, Madrid, told this news organization.
Dr. Hernandez said she hopes these positive results will spur investigation into other neuroprotective agents.
The findings were presented at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Best doses
The study investigated ApTOLL, which blocks the TOLL-like receptor 4 (TLR4) that induces inflammation after a stroke. Previous studies found that ApTOLL protected brain tissue in animal models of stroke.
The phase 1B part of the study found no safety issues and determined the best two doses to be used in phase 2A were 0.05 mg/kg and 0.2 mg/kg.
The analysis included 139 patients at 14 centers in Spain and France (mean age, about 70 years; 42% women) who had a large-vessel occlusion and were eligible for endovascular therapy.
“Our aim was to have a very homogeneous population” to try to replicate in humans what had worked in animals, another study author, Marc Ribó, MD, interventional neurologist, Hospital Vall d’Hebron, Barcelona, told this news organization.
Study participants had an Alberta Stroke Program Early CT Score (ASPECTS) of 5-10, and estimated infarct core volume on CT-perfusion was 5-70 mL. All were treated within 6 hours of stroke onset.
Researchers randomly assigned patients to receive the low dose of the drug, the high dose of the drug, or placebo. The drug was administered intravenously over a 30-minute period just prior to the groin puncture for the thrombectomy procedure.
“So, the drug had already started to work when they underwent the usual standard practice, the thrombectomy,” said Dr. Ribó.
Those who were eligible also received tissue plasminogen activator.
The primary endpoint was safety, including death, symptomatic intracranial hemorrhage (SICH), and recurrent stroke.
Lower mortality
At 90 days, there was a statistically significant lower mortality rate in the high-dose group, compared with the group that received placebo (4.76% vs. 18.18%).
The mortality rate was 26.19% in the low-dose group, but Dr. Ribó stressed that this dose was a quarter of the higher dose and so performed “much more like placebo.”
The higher dose also yielded a better SICH outcome (4.76% of patients vs. 7.27% for placebo and 7.14% for the lower dose). And it was superior in terms of brain edema (2.4% of the population vs. 7.3% for the placebo and 4.8% for the low-dose groups).
About 7.1% of the high-dose group, 3.7% of the placebo group, and 4.8% of the low-dose group had a recurrent transient ischemic attack or stroke.
A secondary efficacy endpoint was infarct volume on MRI at 72 hours. Here, for the higher-dose group, mean infarct volume was reduced, compared with the patients who received placebo (–29.31 cc; 90% confidence interval, –49.28 to –9.34).
This higher dose was also superior for the secondary outcome of National Institutes of Health Stroke Scale score at 72 hours and for the disability outcome on the modified Rankin Score (mRS).
Clear shift in disability
“There was a clear shift toward less disability across levels of the mRS score in the high-dose group at 90 days,” said Dr. Ribó.
He added that he and his colleagues are “very happy” with these results, as they reflect “a consistency” of outcomes.
“We observed that the infarct volumes were lower in the high-dose group, and that led to a significant lower NIH score, meaning less clinical neurological symptoms at 72 hours, and finally, this led to less disability at 90 days.”
These results are “very exciting,” Dr. Hernandez added. “This is the first neuroprotectant that has demonstrated this acute effect in reducing deaths, in reducing the infarct volume and improving functionality long-term in patients treated with the higher dose.”
Dr. Ribó noted the treatment would eventually be used in addition to reperfusion therapy. “It’s not competing with reperfusion treatment; it’s an additional layer” of treatment.
Although it would initially be offered only to patients eligible for thrombectomy, researchers will explore the drug’s effectiveness for other stroke patients, said Dr. Ribó. “We wanted to secure this indication, and from there, progressively expand to other profiles of stroke patients, and even to patients with intracranial hemorrhage.”
The study confirmed the safety of the drug. “There were no safety issues at all,” said Dr. Ribó. “We were initially concerned that an anti-inflammatory in these patients could lead to higher rates of infections, but this was absolutely not the case.”
The next step is to confirm the effects in a larger, multicenter study, which is planned to launch at the end of this year, said Dr. Hernandez.
‘Very robust results’
In a comment, Philip B. Gorelick, MD, professor of neurology, Northwestern University, Chicago, said that, while this was a small early-phase study, the results are “very robust.”
“The authors demonstrated proof of a neuroprotective effect; they showed at 90 days that the death rates were substantially reduced by about four times – 4% vs. 18% – and the size of the damaged tissue at about 72 hours was reduced by 40%,” said Dr. Gorelick, who did not participate in the study.
He also noted that the disability was “less pronounced” at 90 days in the 0.2 mg/kg group.
“So overall, these are very encouraging results,” said Dr. Gorelick. “We have had a lot of difficulty finding neuroprotectant drugs that work, and this drug, in combination with endovascular therapy, seems to be very promising.”
However, he stressed the drug “is not ready for prime-time practice.”
“The proof in the pudding will be in the large-scale main phase 3 trials,” he added.
The study was funded by aptaTargets. Dr. Hernandez is chief scientific officer at aptaTargets. Dr. Ribó is an adviser at AptaTargets; a consultant at Medtronic; has ownership interest in Anaconda and NoraHealth; is a consultant for Cerenovus and Philips; and has stock options at Methink. Dr. Gorelick has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
preliminary results of a first-in-human study show.
The findings illustrate that it is possible to improve outcomes for stroke patients “not only with reperfusion therapy but with neuroprotectants,” study author Macarena Hernandez, PhD, associate professor, University Complutense, Madrid, told this news organization.
Dr. Hernandez said she hopes these positive results will spur investigation into other neuroprotective agents.
The findings were presented at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Best doses
The study investigated ApTOLL, which blocks the TOLL-like receptor 4 (TLR4) that induces inflammation after a stroke. Previous studies found that ApTOLL protected brain tissue in animal models of stroke.
The phase 1B part of the study found no safety issues and determined the best two doses to be used in phase 2A were 0.05 mg/kg and 0.2 mg/kg.
The analysis included 139 patients at 14 centers in Spain and France (mean age, about 70 years; 42% women) who had a large-vessel occlusion and were eligible for endovascular therapy.
“Our aim was to have a very homogeneous population” to try to replicate in humans what had worked in animals, another study author, Marc Ribó, MD, interventional neurologist, Hospital Vall d’Hebron, Barcelona, told this news organization.
Study participants had an Alberta Stroke Program Early CT Score (ASPECTS) of 5-10, and estimated infarct core volume on CT-perfusion was 5-70 mL. All were treated within 6 hours of stroke onset.
Researchers randomly assigned patients to receive the low dose of the drug, the high dose of the drug, or placebo. The drug was administered intravenously over a 30-minute period just prior to the groin puncture for the thrombectomy procedure.
“So, the drug had already started to work when they underwent the usual standard practice, the thrombectomy,” said Dr. Ribó.
Those who were eligible also received tissue plasminogen activator.
The primary endpoint was safety, including death, symptomatic intracranial hemorrhage (SICH), and recurrent stroke.
Lower mortality
At 90 days, there was a statistically significant lower mortality rate in the high-dose group, compared with the group that received placebo (4.76% vs. 18.18%).
The mortality rate was 26.19% in the low-dose group, but Dr. Ribó stressed that this dose was a quarter of the higher dose and so performed “much more like placebo.”
The higher dose also yielded a better SICH outcome (4.76% of patients vs. 7.27% for placebo and 7.14% for the lower dose). And it was superior in terms of brain edema (2.4% of the population vs. 7.3% for the placebo and 4.8% for the low-dose groups).
About 7.1% of the high-dose group, 3.7% of the placebo group, and 4.8% of the low-dose group had a recurrent transient ischemic attack or stroke.
A secondary efficacy endpoint was infarct volume on MRI at 72 hours. Here, for the higher-dose group, mean infarct volume was reduced, compared with the patients who received placebo (–29.31 cc; 90% confidence interval, –49.28 to –9.34).
This higher dose was also superior for the secondary outcome of National Institutes of Health Stroke Scale score at 72 hours and for the disability outcome on the modified Rankin Score (mRS).
Clear shift in disability
“There was a clear shift toward less disability across levels of the mRS score in the high-dose group at 90 days,” said Dr. Ribó.
He added that he and his colleagues are “very happy” with these results, as they reflect “a consistency” of outcomes.
“We observed that the infarct volumes were lower in the high-dose group, and that led to a significant lower NIH score, meaning less clinical neurological symptoms at 72 hours, and finally, this led to less disability at 90 days.”
These results are “very exciting,” Dr. Hernandez added. “This is the first neuroprotectant that has demonstrated this acute effect in reducing deaths, in reducing the infarct volume and improving functionality long-term in patients treated with the higher dose.”
Dr. Ribó noted the treatment would eventually be used in addition to reperfusion therapy. “It’s not competing with reperfusion treatment; it’s an additional layer” of treatment.
Although it would initially be offered only to patients eligible for thrombectomy, researchers will explore the drug’s effectiveness for other stroke patients, said Dr. Ribó. “We wanted to secure this indication, and from there, progressively expand to other profiles of stroke patients, and even to patients with intracranial hemorrhage.”
The study confirmed the safety of the drug. “There were no safety issues at all,” said Dr. Ribó. “We were initially concerned that an anti-inflammatory in these patients could lead to higher rates of infections, but this was absolutely not the case.”
The next step is to confirm the effects in a larger, multicenter study, which is planned to launch at the end of this year, said Dr. Hernandez.
‘Very robust results’
In a comment, Philip B. Gorelick, MD, professor of neurology, Northwestern University, Chicago, said that, while this was a small early-phase study, the results are “very robust.”
“The authors demonstrated proof of a neuroprotective effect; they showed at 90 days that the death rates were substantially reduced by about four times – 4% vs. 18% – and the size of the damaged tissue at about 72 hours was reduced by 40%,” said Dr. Gorelick, who did not participate in the study.
He also noted that the disability was “less pronounced” at 90 days in the 0.2 mg/kg group.
“So overall, these are very encouraging results,” said Dr. Gorelick. “We have had a lot of difficulty finding neuroprotectant drugs that work, and this drug, in combination with endovascular therapy, seems to be very promising.”
However, he stressed the drug “is not ready for prime-time practice.”
“The proof in the pudding will be in the large-scale main phase 3 trials,” he added.
The study was funded by aptaTargets. Dr. Hernandez is chief scientific officer at aptaTargets. Dr. Ribó is an adviser at AptaTargets; a consultant at Medtronic; has ownership interest in Anaconda and NoraHealth; is a consultant for Cerenovus and Philips; and has stock options at Methink. Dr. Gorelick has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
preliminary results of a first-in-human study show.
The findings illustrate that it is possible to improve outcomes for stroke patients “not only with reperfusion therapy but with neuroprotectants,” study author Macarena Hernandez, PhD, associate professor, University Complutense, Madrid, told this news organization.
Dr. Hernandez said she hopes these positive results will spur investigation into other neuroprotective agents.
The findings were presented at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Best doses
The study investigated ApTOLL, which blocks the TOLL-like receptor 4 (TLR4) that induces inflammation after a stroke. Previous studies found that ApTOLL protected brain tissue in animal models of stroke.
The phase 1B part of the study found no safety issues and determined the best two doses to be used in phase 2A were 0.05 mg/kg and 0.2 mg/kg.
The analysis included 139 patients at 14 centers in Spain and France (mean age, about 70 years; 42% women) who had a large-vessel occlusion and were eligible for endovascular therapy.
“Our aim was to have a very homogeneous population” to try to replicate in humans what had worked in animals, another study author, Marc Ribó, MD, interventional neurologist, Hospital Vall d’Hebron, Barcelona, told this news organization.
Study participants had an Alberta Stroke Program Early CT Score (ASPECTS) of 5-10, and estimated infarct core volume on CT-perfusion was 5-70 mL. All were treated within 6 hours of stroke onset.
Researchers randomly assigned patients to receive the low dose of the drug, the high dose of the drug, or placebo. The drug was administered intravenously over a 30-minute period just prior to the groin puncture for the thrombectomy procedure.
“So, the drug had already started to work when they underwent the usual standard practice, the thrombectomy,” said Dr. Ribó.
Those who were eligible also received tissue plasminogen activator.
The primary endpoint was safety, including death, symptomatic intracranial hemorrhage (SICH), and recurrent stroke.
Lower mortality
At 90 days, there was a statistically significant lower mortality rate in the high-dose group, compared with the group that received placebo (4.76% vs. 18.18%).
The mortality rate was 26.19% in the low-dose group, but Dr. Ribó stressed that this dose was a quarter of the higher dose and so performed “much more like placebo.”
The higher dose also yielded a better SICH outcome (4.76% of patients vs. 7.27% for placebo and 7.14% for the lower dose). And it was superior in terms of brain edema (2.4% of the population vs. 7.3% for the placebo and 4.8% for the low-dose groups).
About 7.1% of the high-dose group, 3.7% of the placebo group, and 4.8% of the low-dose group had a recurrent transient ischemic attack or stroke.
A secondary efficacy endpoint was infarct volume on MRI at 72 hours. Here, for the higher-dose group, mean infarct volume was reduced, compared with the patients who received placebo (–29.31 cc; 90% confidence interval, –49.28 to –9.34).
This higher dose was also superior for the secondary outcome of National Institutes of Health Stroke Scale score at 72 hours and for the disability outcome on the modified Rankin Score (mRS).
Clear shift in disability
“There was a clear shift toward less disability across levels of the mRS score in the high-dose group at 90 days,” said Dr. Ribó.
He added that he and his colleagues are “very happy” with these results, as they reflect “a consistency” of outcomes.
“We observed that the infarct volumes were lower in the high-dose group, and that led to a significant lower NIH score, meaning less clinical neurological symptoms at 72 hours, and finally, this led to less disability at 90 days.”
These results are “very exciting,” Dr. Hernandez added. “This is the first neuroprotectant that has demonstrated this acute effect in reducing deaths, in reducing the infarct volume and improving functionality long-term in patients treated with the higher dose.”
Dr. Ribó noted the treatment would eventually be used in addition to reperfusion therapy. “It’s not competing with reperfusion treatment; it’s an additional layer” of treatment.
Although it would initially be offered only to patients eligible for thrombectomy, researchers will explore the drug’s effectiveness for other stroke patients, said Dr. Ribó. “We wanted to secure this indication, and from there, progressively expand to other profiles of stroke patients, and even to patients with intracranial hemorrhage.”
The study confirmed the safety of the drug. “There were no safety issues at all,” said Dr. Ribó. “We were initially concerned that an anti-inflammatory in these patients could lead to higher rates of infections, but this was absolutely not the case.”
The next step is to confirm the effects in a larger, multicenter study, which is planned to launch at the end of this year, said Dr. Hernandez.
‘Very robust results’
In a comment, Philip B. Gorelick, MD, professor of neurology, Northwestern University, Chicago, said that, while this was a small early-phase study, the results are “very robust.”
“The authors demonstrated proof of a neuroprotective effect; they showed at 90 days that the death rates were substantially reduced by about four times – 4% vs. 18% – and the size of the damaged tissue at about 72 hours was reduced by 40%,” said Dr. Gorelick, who did not participate in the study.
He also noted that the disability was “less pronounced” at 90 days in the 0.2 mg/kg group.
“So overall, these are very encouraging results,” said Dr. Gorelick. “We have had a lot of difficulty finding neuroprotectant drugs that work, and this drug, in combination with endovascular therapy, seems to be very promising.”
However, he stressed the drug “is not ready for prime-time practice.”
“The proof in the pudding will be in the large-scale main phase 3 trials,” he added.
The study was funded by aptaTargets. Dr. Hernandez is chief scientific officer at aptaTargets. Dr. Ribó is an adviser at AptaTargets; a consultant at Medtronic; has ownership interest in Anaconda and NoraHealth; is a consultant for Cerenovus and Philips; and has stock options at Methink. Dr. Gorelick has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ISC 2023
How a concussion led a former football player/WWE star to a pioneering neuroscience career
On Oct. 5, 2022, at 10:24 a.m., Chris Nowinski, PhD, cofounder of the Boston-based Concussion Legacy Foundation (CLF), was in his home office when the email came through.
“I pounded my desk, shouted YES! and went to find my wife so I could pick her up and give her a big hug,” he recalled. “It was the culmination of 15 years of research and hard work.”
Robert Cantu, MD, who has been studying head trauma for 50+ years and has published more than 500 papers about it, compares the announcement to the 1964 Surgeon General’s report that linked cigarette smoking with lung cancer and heart disease. With the NIH and the Centers of Disease Control and Prevention (CDC) now in agreement about the risks of participating in impact sports and activities, he said, “We’ve reached a tipping point that should finally prompt deniers such as the NHL, NCAA, FIFA, World Rugby, the International Olympic Committee, and other [sports organizations] to remove all unnecessary head trauma from their sports.”
“A lot of the credit for this must go to Chris,” added Dr. Cantu, medical director and director of clinical research at the Cantu Concussion Center at Emerson Hospital in Concord, Mass. “Clinicians like myself can reach only so many people by writing papers and giving speeches at medical conferences. For this to happen, the message needed to get out to parents, athletes, and society in general. And Chris was the vehicle for doing that.”
Dr. Nowinski didn’t set out to be the messenger. He played football at Harvard in the late 1990s, making second-team All-Ivy as a defensive tackle his senior year. In 2000, he enrolled in Killer Kowalski’s Wrestling Institute and eventually joined Vince McMahon’s World Wrestling Entertainment (WWE).
There he played the role of 295-pound villain “Chris Harvard,” an intellectual snob who dressed in crimson tights and insulted the crowd’s IQ. “Roses are red. Violets are blue. The reason I’m talking so slowly is because no one in [insert name of town he was appearing in] has passed grade 2!”
“I’d often apply my education during a match,” he wrote in his book, “Head Games: Football’s Concussion Crisis.“ In a match in Bridgeport, Conn., I assaulted [my opponent] with a human skeleton, ripped off the skull, got down on bended knee, and began reciting Hamlet. Those were good times.”
Those good times ended abruptly, however, during a match with Bubba Ray Dudley at the Hartford Civic Center in Connecticut in 2003. Even though pro wrestling matches are rehearsed, and the blows aren’t real, accidents happen. Mr. Dudley mistakenly kicked Dr. Nowinski in the jaw with enough force to put him on his back and make the whole ring shake.
“Holy shit, kid! You okay?” asked the referee. Before a foggy Dr. Nowinski could reply, 300-pound Mr. Dudley crashed down on him, hooked his leg, and the ref began counting, “One! Two! …” Dr. Nowinski instinctively kicked out but had forgotten the rest of the script. He managed to finish the match and stagger backstage.
His coherence and awareness gradually returned, but a “throbbing headache” persisted. A locker room doctor said he might have a concussion and recommended he wait to see how he felt before wrestling in Albany, N.Y., the next evening.
The following day the headache had subsided, but he still felt “a little strange.” Nonetheless, he told the doctor he was fine and strutted out to again battle Bubba Ray, this time in a match where he eventually got thrown through a ringside table and suffered the Dudley Death Drop. Afterward, “I crawled backstage and laid down. The headache was much, much worse.”
An event and a process
Dr. Nowinski continued to insist he was “fine” and wrestled a few more matches in the following days before finally acknowledging something was wrong. He’d had his bell rung numerous times in football, but this was different. Even more worrisome, none of the doctors he consulted could give him any definitive answers. He finally found his way to Emerson Hospital, where Dr. Cantu was the chief of neurosurgery.
“I remember that day vividly,” said Dr. Cantu. “Chris was this big, strapping, handsome guy – a hell of an athlete whose star was rising. He didn’t realize that he’d suffered a series of concussions and that trying to push through them was the worst thing he could be doing.”
Concussions and their effects were misunderstood by many athletes, coaches, and even physicians back then. It was assumed that the quarter inch of bone surrounding the adult brain provided adequate protection from common sports impacts and that any aftereffects were temporary. A common treatment was smelling salts and a pat on the back as the athlete returned to action.
However, the brain floats inside the skull in a bath of cerebral fluid. Any significant impact causes it to slosh violently from side to side, damaging tissue, synapses, and cells resulting in inflammation that can manifest as confusion and brain fog.
“A concussion is actually not defined by a physical injury,” explained Dr. Nowinski, “but by a loss of brain function that is induced by trauma. Concussion is not just an event, but also a process.” It’s almost as if the person has suffered a small seizure.
Fortunately, most concussion symptoms resolve within 2 weeks, but in some cases, especially if there’s been additional head trauma, they can persist, causing anxiety, depression, anger, and/or sleep disorders. Known as postconcussion syndrome (PCS), this is what Dr. Nowinski was unknowingly suffering from when he consulted Dr. Cantu.
In fact, one night it an Indianapolis hotel, weeks after his initial concussion, he awoke to find himself on the floor and the room in shambles. His girlfriend was yelling his name and shaking him. She told him he’d been having a nightmare and had suddenly started screaming and tearing up the room. “I didn’t remember any of it,” he said.
Dr. Cantu eventually advised Dr. Nowinski against ever returning to the ring or any activity with the risk for head injury. Research shows that sustaining a single significant concussion increases the risk of subsequent more-severe brain injuries.
“My diagnosis could have sent Chris off the deep end because he could no longer do what he wanted to do with this life,” said Dr. Cantu. “But instead, he used it as a tool to find meaning for his life.”
Dr. Nowinski decided to use his experience as a teaching opportunity, not just for other athletes but also for sports organizations and the medical community.
His book, which focused on the NFL’s “tobacco-industry-like refusal to acknowledge the depths of the problem,” was published in 2006. A year later, Dr. Nowinski partnered with Dr. Cantu to found the Sports Legacy Institute, which eventually became the Concussion Legacy Foundation (CLF).
Cold calling for brain donations
Robert Stern, PhD, is another highly respected authority in the study of neurodegenerative disease. In 2007, he was directing the clinical core of Boston University’s Alzheimer’s Disease Center. After giving a lecture to a group of financial planners and elder-law attorneys one morning, he got a request for a private meeting from a fellow named Chris Nowinski.
“I’d never heard of him, but I agreed,” recalled Dr. Stern, a professor of neurology, neurosurgery, anatomy, and neurobiology at Boston University. “A few days later, this larger-than-life guy walked into our conference room at the BU School of Medicine, exuding a great deal of passion, intellect, and determination. He told me his story and then started talking about the long-term consequences of concussions in sports.”
Dr. Stern had seen patients with dementia pugilistica, the old-school term for CTE. These were mostly boxers with cognitive and behavioral impairment. “But I had not heard about football players,” he said. “I hadn’t put the two together. And as I was listening to Chris, I realized if what he was saying was true then it was not only a potentially huge public health issue, but it was also a potentially huge scientific issue in the field of neurodegenerative disease.”
Dr. Nowinski introduced Dr. Stern to Dr. Cantu, and together with Ann McKee, MD, professor of neurology and pathology at BU, they cofounded the Center for the Study of Traumatic Encephalopathy (CSTE) in 2008. It was the first center of its kind devoted to the study of CTE in the world.
One of Dr. Nowinski’s first jobs at the CSTE was soliciting and procuring brain donations. Since CTE is generally a progressive condition that can take decades to manifest, autopsy was the only way to detect it.
The brains of two former Pittsburgh Steelers, Mike Webster and Terry Long, had been examined after their untimely deaths. After immunostaining, investigators found both former NFL players had “protein misfolds” characteristic of CTE.
This finding drew a lot of public and scientific attention, given that Mr. Long died by suicide and Mr. Webster was homeless when he died of a heart attack. But more scientific evidence was needed to prove a causal link between the head trauma and CTE.
Dr. Nowinski scoured obituaries looking for potential brains to study. When he found one, he would cold call the family and try to convince them to donate it to science. The first brain he secured for the center belonged to John Grimsley, a former NFL linebacker who in 2008 died at age 45 of an accidental gunshot wound. Often, Dr. Nowinski would even be the courier, traveling to pick up the brain after it had been harvested.
Over the next 10 years, Dr. Nowinski and his research team secured 500 brain donations. The research that resulted was staggering. In the beginning only 45 cases of CTE had been identified in the world, but in the first 111 NFL players who were autopsied, 110 had the disorder.
Of the first 53 college football players autopsied, 48 had CTE. Although Dr. Nowinski’s initial focus was football, evidence of CTE was soon detected among athletes in boxing, hockey, soccer, and rugby, as well as in combat veterans. However, the National Football League and other governing sports bodies initially denied any connection between sport-related head trauma and CTE.
Cumulative damage
In 2017, after 7 years of study, Dr. Nowinski earned a PhD in neurology. As the scientific evidence continued to accumulate, two shifts occurred that Dr. Stern said represent Dr. Nowinski’s greatest contributions. First, concussion is now widely recognized as an acute brain injury with symptoms that need to be immediately diagnosed and addressed.
“This is a completely different story from where things were just 10 years ago,” said Dr. Stern, “and Chris played a central role, if not the central role, in raising awareness about that.”
All 50 states and the District of Columbia now have laws regarding sports-related concussion. And there are brain banks in Australia, Canada, New Zealand, Brazil, and the United Kingdom studying CTE. More than 2,500 athletes in a variety of sports, including NASCAR’s Dale Earnhardt Jr. and NFL hall of famer Nick Buoniconti, have publicly pledged to donate their brains to science after their deaths.
Second, said Dr. Stern, we now know that although concussions can contribute to CTE, they are not the sole cause. It’s repetitive subconcussive trauma, without symptoms of concussion, that do the most damage.
“These happen during every practice and in every game,” said Dr. Stern. In fact, it’s estimated that pro football players suffer thousands of subconcussive incidents over the course of their careers. So, a player doesn’t have to see stars or lose consciousness to suffer brain damage; small impacts can accumulate over time.
Understanding this point is crucial for making youth sports safer. “Chris has played a critical role in raising awareness here, too,” said Dr. Stern. “Allowing our kids to get hit in the head over and over can put them at greater risk for later problems, plus it just doesn’t make common sense.”
“The biggest misconception surrounding head trauma in sports,” said Dr. Nowinski, “is the belief among players, coaches, and even the medical and scientific communities that if you get hit in the head and don’t have any symptoms then you’re okay and there hasn’t been any damage. That couldn’t be further from the truth. We now know that people are suffering serious brain injuries due to the accumulated effect of subconcussive impacts, and we need to get the word out about that.”
A major initiative from the Concussion Legacy Foundation called “Stop Hitting Kids in the Head” has the goal of convincing every sport to eliminate repetitive head impacts in players under age 14 – the time when the skull and brain are still developing and most vulnerable – by 2026. In fact, Dr. Nowinski wrote that “there could be a lot of kids who are misdiagnosed and medicated for various behavioral or emotional problems that may actually be head injury–related.”
Starting in 2009, the NFL adopted a series of rule changes designed to better protect its players against repeated head trauma. Among them is a ban on spearing or leading with the helmet, penalties for hitting defenseless players, and more stringent return-to-play guidelines, including concussion protocols.
The NFL has also put more emphasis on flag football options for youngsters and, for the first time, showcased this alternative in the 2023 Pro Bowl. But Dr. Nowinski is pressuring the league to go further. “While acknowledging that the game causes CTE, the NFL still underwrites recruiting 5-year-olds to play tackle football,” he said. “In my opinion, that’s unethical, and it needs to be addressed.”
WWE one of the most responsive organizations
Dr. Nowinski said WWE has been one of the most responsive sports organizations for protecting athletes. A doctor is now ringside at every match as is an observer who knows the script, thereby allowing for instant medical intervention if something goes wrong. “Since everyone is trying to look like they have a concussion all the time, it takes a deep understanding of the business to recognize a real one,” he said.
But this hasn’t been the case with other sports. “I am eternally disappointed in the response of the professional sports industry to the knowledge of CTE and long-term concussion symptoms,” said Dr. Nowinski.
“For example, FIFA [international soccer’s governing body] still doesn’t allow doctors to evaluate [potentially concussed] players on the sidelines and put them back in the game with a free substitution [if they’re deemed okay]. Not giving players proper medical care for a brain injury is unethical,” he said. BU’s Center for the Study of Traumatic Encephalopathy diagnosed the first CTE case in soccer in 2012, and in 2015 Dr. Nowinski successfully lobbied U.S. Soccer to ban heading the ball before age 11.
“Unfortunately, many governing bodies have circled the wagons in denying their sport causes CTE,” he continued. “FIFA, World Rugby, the NHL, even the NCAA and International Olympic Committee refuse to acknowledge it and, therefore, aren’t taking any steps to prevent it. They see it as a threat to their business model. Hopefully, now that the NIH and CDC are aligned about the risks of head impact in sports, this will begin to change.”
Meanwhile, research is continuing. Scientists are getting closer to being able to diagnose CTE in living humans, with ongoing studies using PET scans, blood markers, and spinal fluid markers. In 2019, researchers identified tau proteins specific to CTE that they believe are distinct from those of Alzheimer’s and other neurodegenerative diseases. Next step would be developing a drug to slow the development of CTE once detected.
Nonetheless, athletes at all levels in impact sports still don’t fully appreciate the risks of repeated head trauma and especially subconcussive blows. “I talk to former NFL and college players every week,” said Dr. Stern. “Some tell me, ‘I love the sport, it gave me so much, and I would do it again, but I’m not letting my grandchildren play.’ But others say, ‘As long as they know the risks, they can make their own decision.’ “
Dr. Nowinski has a daughter who is 4 and a son who’s 2. Both play soccer but, thanks to dad, heading isn’t allowed in their age groups. If they continue playing sports, Dr. Nowinski said he’ll make sure they understand the risks and how to protect themselves. This is a conversation all parents should have with their kids at every level to make sure they play safe, he added.
Those in the medical community can also volunteer their time to explain head trauma to athletes, coaches, and school administrators to be sure they understand its seriousness and are doing everything to protect players.
As you watch this year’s Super Bowl, Dr. Nowinski and his team would like you to keep something in mind. Those young men on the field for your entertainment are receiving mild brain trauma repeatedly throughout the game.
Even if it’s not a huge hit that gets replayed and makes everyone gasp, even if no one gets ushered into the little sideline tent for a concussion screening, even if no one loses consciousness, brain damage is still occurring. Watch the heads of the players during every play and think about what’s going on inside their skulls regardless of how big and strong those helmets look.
A version of this article first appeared on Medscape.com.
On Oct. 5, 2022, at 10:24 a.m., Chris Nowinski, PhD, cofounder of the Boston-based Concussion Legacy Foundation (CLF), was in his home office when the email came through.
“I pounded my desk, shouted YES! and went to find my wife so I could pick her up and give her a big hug,” he recalled. “It was the culmination of 15 years of research and hard work.”
Robert Cantu, MD, who has been studying head trauma for 50+ years and has published more than 500 papers about it, compares the announcement to the 1964 Surgeon General’s report that linked cigarette smoking with lung cancer and heart disease. With the NIH and the Centers of Disease Control and Prevention (CDC) now in agreement about the risks of participating in impact sports and activities, he said, “We’ve reached a tipping point that should finally prompt deniers such as the NHL, NCAA, FIFA, World Rugby, the International Olympic Committee, and other [sports organizations] to remove all unnecessary head trauma from their sports.”
“A lot of the credit for this must go to Chris,” added Dr. Cantu, medical director and director of clinical research at the Cantu Concussion Center at Emerson Hospital in Concord, Mass. “Clinicians like myself can reach only so many people by writing papers and giving speeches at medical conferences. For this to happen, the message needed to get out to parents, athletes, and society in general. And Chris was the vehicle for doing that.”
Dr. Nowinski didn’t set out to be the messenger. He played football at Harvard in the late 1990s, making second-team All-Ivy as a defensive tackle his senior year. In 2000, he enrolled in Killer Kowalski’s Wrestling Institute and eventually joined Vince McMahon’s World Wrestling Entertainment (WWE).
There he played the role of 295-pound villain “Chris Harvard,” an intellectual snob who dressed in crimson tights and insulted the crowd’s IQ. “Roses are red. Violets are blue. The reason I’m talking so slowly is because no one in [insert name of town he was appearing in] has passed grade 2!”
“I’d often apply my education during a match,” he wrote in his book, “Head Games: Football’s Concussion Crisis.“ In a match in Bridgeport, Conn., I assaulted [my opponent] with a human skeleton, ripped off the skull, got down on bended knee, and began reciting Hamlet. Those were good times.”
Those good times ended abruptly, however, during a match with Bubba Ray Dudley at the Hartford Civic Center in Connecticut in 2003. Even though pro wrestling matches are rehearsed, and the blows aren’t real, accidents happen. Mr. Dudley mistakenly kicked Dr. Nowinski in the jaw with enough force to put him on his back and make the whole ring shake.
“Holy shit, kid! You okay?” asked the referee. Before a foggy Dr. Nowinski could reply, 300-pound Mr. Dudley crashed down on him, hooked his leg, and the ref began counting, “One! Two! …” Dr. Nowinski instinctively kicked out but had forgotten the rest of the script. He managed to finish the match and stagger backstage.
His coherence and awareness gradually returned, but a “throbbing headache” persisted. A locker room doctor said he might have a concussion and recommended he wait to see how he felt before wrestling in Albany, N.Y., the next evening.
The following day the headache had subsided, but he still felt “a little strange.” Nonetheless, he told the doctor he was fine and strutted out to again battle Bubba Ray, this time in a match where he eventually got thrown through a ringside table and suffered the Dudley Death Drop. Afterward, “I crawled backstage and laid down. The headache was much, much worse.”
An event and a process
Dr. Nowinski continued to insist he was “fine” and wrestled a few more matches in the following days before finally acknowledging something was wrong. He’d had his bell rung numerous times in football, but this was different. Even more worrisome, none of the doctors he consulted could give him any definitive answers. He finally found his way to Emerson Hospital, where Dr. Cantu was the chief of neurosurgery.
“I remember that day vividly,” said Dr. Cantu. “Chris was this big, strapping, handsome guy – a hell of an athlete whose star was rising. He didn’t realize that he’d suffered a series of concussions and that trying to push through them was the worst thing he could be doing.”
Concussions and their effects were misunderstood by many athletes, coaches, and even physicians back then. It was assumed that the quarter inch of bone surrounding the adult brain provided adequate protection from common sports impacts and that any aftereffects were temporary. A common treatment was smelling salts and a pat on the back as the athlete returned to action.
However, the brain floats inside the skull in a bath of cerebral fluid. Any significant impact causes it to slosh violently from side to side, damaging tissue, synapses, and cells resulting in inflammation that can manifest as confusion and brain fog.
“A concussion is actually not defined by a physical injury,” explained Dr. Nowinski, “but by a loss of brain function that is induced by trauma. Concussion is not just an event, but also a process.” It’s almost as if the person has suffered a small seizure.
Fortunately, most concussion symptoms resolve within 2 weeks, but in some cases, especially if there’s been additional head trauma, they can persist, causing anxiety, depression, anger, and/or sleep disorders. Known as postconcussion syndrome (PCS), this is what Dr. Nowinski was unknowingly suffering from when he consulted Dr. Cantu.
In fact, one night it an Indianapolis hotel, weeks after his initial concussion, he awoke to find himself on the floor and the room in shambles. His girlfriend was yelling his name and shaking him. She told him he’d been having a nightmare and had suddenly started screaming and tearing up the room. “I didn’t remember any of it,” he said.
Dr. Cantu eventually advised Dr. Nowinski against ever returning to the ring or any activity with the risk for head injury. Research shows that sustaining a single significant concussion increases the risk of subsequent more-severe brain injuries.
“My diagnosis could have sent Chris off the deep end because he could no longer do what he wanted to do with this life,” said Dr. Cantu. “But instead, he used it as a tool to find meaning for his life.”
Dr. Nowinski decided to use his experience as a teaching opportunity, not just for other athletes but also for sports organizations and the medical community.
His book, which focused on the NFL’s “tobacco-industry-like refusal to acknowledge the depths of the problem,” was published in 2006. A year later, Dr. Nowinski partnered with Dr. Cantu to found the Sports Legacy Institute, which eventually became the Concussion Legacy Foundation (CLF).
Cold calling for brain donations
Robert Stern, PhD, is another highly respected authority in the study of neurodegenerative disease. In 2007, he was directing the clinical core of Boston University’s Alzheimer’s Disease Center. After giving a lecture to a group of financial planners and elder-law attorneys one morning, he got a request for a private meeting from a fellow named Chris Nowinski.
“I’d never heard of him, but I agreed,” recalled Dr. Stern, a professor of neurology, neurosurgery, anatomy, and neurobiology at Boston University. “A few days later, this larger-than-life guy walked into our conference room at the BU School of Medicine, exuding a great deal of passion, intellect, and determination. He told me his story and then started talking about the long-term consequences of concussions in sports.”
Dr. Stern had seen patients with dementia pugilistica, the old-school term for CTE. These were mostly boxers with cognitive and behavioral impairment. “But I had not heard about football players,” he said. “I hadn’t put the two together. And as I was listening to Chris, I realized if what he was saying was true then it was not only a potentially huge public health issue, but it was also a potentially huge scientific issue in the field of neurodegenerative disease.”
Dr. Nowinski introduced Dr. Stern to Dr. Cantu, and together with Ann McKee, MD, professor of neurology and pathology at BU, they cofounded the Center for the Study of Traumatic Encephalopathy (CSTE) in 2008. It was the first center of its kind devoted to the study of CTE in the world.
One of Dr. Nowinski’s first jobs at the CSTE was soliciting and procuring brain donations. Since CTE is generally a progressive condition that can take decades to manifest, autopsy was the only way to detect it.
The brains of two former Pittsburgh Steelers, Mike Webster and Terry Long, had been examined after their untimely deaths. After immunostaining, investigators found both former NFL players had “protein misfolds” characteristic of CTE.
This finding drew a lot of public and scientific attention, given that Mr. Long died by suicide and Mr. Webster was homeless when he died of a heart attack. But more scientific evidence was needed to prove a causal link between the head trauma and CTE.
Dr. Nowinski scoured obituaries looking for potential brains to study. When he found one, he would cold call the family and try to convince them to donate it to science. The first brain he secured for the center belonged to John Grimsley, a former NFL linebacker who in 2008 died at age 45 of an accidental gunshot wound. Often, Dr. Nowinski would even be the courier, traveling to pick up the brain after it had been harvested.
Over the next 10 years, Dr. Nowinski and his research team secured 500 brain donations. The research that resulted was staggering. In the beginning only 45 cases of CTE had been identified in the world, but in the first 111 NFL players who were autopsied, 110 had the disorder.
Of the first 53 college football players autopsied, 48 had CTE. Although Dr. Nowinski’s initial focus was football, evidence of CTE was soon detected among athletes in boxing, hockey, soccer, and rugby, as well as in combat veterans. However, the National Football League and other governing sports bodies initially denied any connection between sport-related head trauma and CTE.
Cumulative damage
In 2017, after 7 years of study, Dr. Nowinski earned a PhD in neurology. As the scientific evidence continued to accumulate, two shifts occurred that Dr. Stern said represent Dr. Nowinski’s greatest contributions. First, concussion is now widely recognized as an acute brain injury with symptoms that need to be immediately diagnosed and addressed.
“This is a completely different story from where things were just 10 years ago,” said Dr. Stern, “and Chris played a central role, if not the central role, in raising awareness about that.”
All 50 states and the District of Columbia now have laws regarding sports-related concussion. And there are brain banks in Australia, Canada, New Zealand, Brazil, and the United Kingdom studying CTE. More than 2,500 athletes in a variety of sports, including NASCAR’s Dale Earnhardt Jr. and NFL hall of famer Nick Buoniconti, have publicly pledged to donate their brains to science after their deaths.
Second, said Dr. Stern, we now know that although concussions can contribute to CTE, they are not the sole cause. It’s repetitive subconcussive trauma, without symptoms of concussion, that do the most damage.
“These happen during every practice and in every game,” said Dr. Stern. In fact, it’s estimated that pro football players suffer thousands of subconcussive incidents over the course of their careers. So, a player doesn’t have to see stars or lose consciousness to suffer brain damage; small impacts can accumulate over time.
Understanding this point is crucial for making youth sports safer. “Chris has played a critical role in raising awareness here, too,” said Dr. Stern. “Allowing our kids to get hit in the head over and over can put them at greater risk for later problems, plus it just doesn’t make common sense.”
“The biggest misconception surrounding head trauma in sports,” said Dr. Nowinski, “is the belief among players, coaches, and even the medical and scientific communities that if you get hit in the head and don’t have any symptoms then you’re okay and there hasn’t been any damage. That couldn’t be further from the truth. We now know that people are suffering serious brain injuries due to the accumulated effect of subconcussive impacts, and we need to get the word out about that.”
A major initiative from the Concussion Legacy Foundation called “Stop Hitting Kids in the Head” has the goal of convincing every sport to eliminate repetitive head impacts in players under age 14 – the time when the skull and brain are still developing and most vulnerable – by 2026. In fact, Dr. Nowinski wrote that “there could be a lot of kids who are misdiagnosed and medicated for various behavioral or emotional problems that may actually be head injury–related.”
Starting in 2009, the NFL adopted a series of rule changes designed to better protect its players against repeated head trauma. Among them is a ban on spearing or leading with the helmet, penalties for hitting defenseless players, and more stringent return-to-play guidelines, including concussion protocols.
The NFL has also put more emphasis on flag football options for youngsters and, for the first time, showcased this alternative in the 2023 Pro Bowl. But Dr. Nowinski is pressuring the league to go further. “While acknowledging that the game causes CTE, the NFL still underwrites recruiting 5-year-olds to play tackle football,” he said. “In my opinion, that’s unethical, and it needs to be addressed.”
WWE one of the most responsive organizations
Dr. Nowinski said WWE has been one of the most responsive sports organizations for protecting athletes. A doctor is now ringside at every match as is an observer who knows the script, thereby allowing for instant medical intervention if something goes wrong. “Since everyone is trying to look like they have a concussion all the time, it takes a deep understanding of the business to recognize a real one,” he said.
But this hasn’t been the case with other sports. “I am eternally disappointed in the response of the professional sports industry to the knowledge of CTE and long-term concussion symptoms,” said Dr. Nowinski.
“For example, FIFA [international soccer’s governing body] still doesn’t allow doctors to evaluate [potentially concussed] players on the sidelines and put them back in the game with a free substitution [if they’re deemed okay]. Not giving players proper medical care for a brain injury is unethical,” he said. BU’s Center for the Study of Traumatic Encephalopathy diagnosed the first CTE case in soccer in 2012, and in 2015 Dr. Nowinski successfully lobbied U.S. Soccer to ban heading the ball before age 11.
“Unfortunately, many governing bodies have circled the wagons in denying their sport causes CTE,” he continued. “FIFA, World Rugby, the NHL, even the NCAA and International Olympic Committee refuse to acknowledge it and, therefore, aren’t taking any steps to prevent it. They see it as a threat to their business model. Hopefully, now that the NIH and CDC are aligned about the risks of head impact in sports, this will begin to change.”
Meanwhile, research is continuing. Scientists are getting closer to being able to diagnose CTE in living humans, with ongoing studies using PET scans, blood markers, and spinal fluid markers. In 2019, researchers identified tau proteins specific to CTE that they believe are distinct from those of Alzheimer’s and other neurodegenerative diseases. Next step would be developing a drug to slow the development of CTE once detected.
Nonetheless, athletes at all levels in impact sports still don’t fully appreciate the risks of repeated head trauma and especially subconcussive blows. “I talk to former NFL and college players every week,” said Dr. Stern. “Some tell me, ‘I love the sport, it gave me so much, and I would do it again, but I’m not letting my grandchildren play.’ But others say, ‘As long as they know the risks, they can make their own decision.’ “
Dr. Nowinski has a daughter who is 4 and a son who’s 2. Both play soccer but, thanks to dad, heading isn’t allowed in their age groups. If they continue playing sports, Dr. Nowinski said he’ll make sure they understand the risks and how to protect themselves. This is a conversation all parents should have with their kids at every level to make sure they play safe, he added.
Those in the medical community can also volunteer their time to explain head trauma to athletes, coaches, and school administrators to be sure they understand its seriousness and are doing everything to protect players.
As you watch this year’s Super Bowl, Dr. Nowinski and his team would like you to keep something in mind. Those young men on the field for your entertainment are receiving mild brain trauma repeatedly throughout the game.
Even if it’s not a huge hit that gets replayed and makes everyone gasp, even if no one gets ushered into the little sideline tent for a concussion screening, even if no one loses consciousness, brain damage is still occurring. Watch the heads of the players during every play and think about what’s going on inside their skulls regardless of how big and strong those helmets look.
A version of this article first appeared on Medscape.com.
On Oct. 5, 2022, at 10:24 a.m., Chris Nowinski, PhD, cofounder of the Boston-based Concussion Legacy Foundation (CLF), was in his home office when the email came through.
“I pounded my desk, shouted YES! and went to find my wife so I could pick her up and give her a big hug,” he recalled. “It was the culmination of 15 years of research and hard work.”
Robert Cantu, MD, who has been studying head trauma for 50+ years and has published more than 500 papers about it, compares the announcement to the 1964 Surgeon General’s report that linked cigarette smoking with lung cancer and heart disease. With the NIH and the Centers of Disease Control and Prevention (CDC) now in agreement about the risks of participating in impact sports and activities, he said, “We’ve reached a tipping point that should finally prompt deniers such as the NHL, NCAA, FIFA, World Rugby, the International Olympic Committee, and other [sports organizations] to remove all unnecessary head trauma from their sports.”
“A lot of the credit for this must go to Chris,” added Dr. Cantu, medical director and director of clinical research at the Cantu Concussion Center at Emerson Hospital in Concord, Mass. “Clinicians like myself can reach only so many people by writing papers and giving speeches at medical conferences. For this to happen, the message needed to get out to parents, athletes, and society in general. And Chris was the vehicle for doing that.”
Dr. Nowinski didn’t set out to be the messenger. He played football at Harvard in the late 1990s, making second-team All-Ivy as a defensive tackle his senior year. In 2000, he enrolled in Killer Kowalski’s Wrestling Institute and eventually joined Vince McMahon’s World Wrestling Entertainment (WWE).
There he played the role of 295-pound villain “Chris Harvard,” an intellectual snob who dressed in crimson tights and insulted the crowd’s IQ. “Roses are red. Violets are blue. The reason I’m talking so slowly is because no one in [insert name of town he was appearing in] has passed grade 2!”
“I’d often apply my education during a match,” he wrote in his book, “Head Games: Football’s Concussion Crisis.“ In a match in Bridgeport, Conn., I assaulted [my opponent] with a human skeleton, ripped off the skull, got down on bended knee, and began reciting Hamlet. Those were good times.”
Those good times ended abruptly, however, during a match with Bubba Ray Dudley at the Hartford Civic Center in Connecticut in 2003. Even though pro wrestling matches are rehearsed, and the blows aren’t real, accidents happen. Mr. Dudley mistakenly kicked Dr. Nowinski in the jaw with enough force to put him on his back and make the whole ring shake.
“Holy shit, kid! You okay?” asked the referee. Before a foggy Dr. Nowinski could reply, 300-pound Mr. Dudley crashed down on him, hooked his leg, and the ref began counting, “One! Two! …” Dr. Nowinski instinctively kicked out but had forgotten the rest of the script. He managed to finish the match and stagger backstage.
His coherence and awareness gradually returned, but a “throbbing headache” persisted. A locker room doctor said he might have a concussion and recommended he wait to see how he felt before wrestling in Albany, N.Y., the next evening.
The following day the headache had subsided, but he still felt “a little strange.” Nonetheless, he told the doctor he was fine and strutted out to again battle Bubba Ray, this time in a match where he eventually got thrown through a ringside table and suffered the Dudley Death Drop. Afterward, “I crawled backstage and laid down. The headache was much, much worse.”
An event and a process
Dr. Nowinski continued to insist he was “fine” and wrestled a few more matches in the following days before finally acknowledging something was wrong. He’d had his bell rung numerous times in football, but this was different. Even more worrisome, none of the doctors he consulted could give him any definitive answers. He finally found his way to Emerson Hospital, where Dr. Cantu was the chief of neurosurgery.
“I remember that day vividly,” said Dr. Cantu. “Chris was this big, strapping, handsome guy – a hell of an athlete whose star was rising. He didn’t realize that he’d suffered a series of concussions and that trying to push through them was the worst thing he could be doing.”
Concussions and their effects were misunderstood by many athletes, coaches, and even physicians back then. It was assumed that the quarter inch of bone surrounding the adult brain provided adequate protection from common sports impacts and that any aftereffects were temporary. A common treatment was smelling salts and a pat on the back as the athlete returned to action.
However, the brain floats inside the skull in a bath of cerebral fluid. Any significant impact causes it to slosh violently from side to side, damaging tissue, synapses, and cells resulting in inflammation that can manifest as confusion and brain fog.
“A concussion is actually not defined by a physical injury,” explained Dr. Nowinski, “but by a loss of brain function that is induced by trauma. Concussion is not just an event, but also a process.” It’s almost as if the person has suffered a small seizure.
Fortunately, most concussion symptoms resolve within 2 weeks, but in some cases, especially if there’s been additional head trauma, they can persist, causing anxiety, depression, anger, and/or sleep disorders. Known as postconcussion syndrome (PCS), this is what Dr. Nowinski was unknowingly suffering from when he consulted Dr. Cantu.
In fact, one night it an Indianapolis hotel, weeks after his initial concussion, he awoke to find himself on the floor and the room in shambles. His girlfriend was yelling his name and shaking him. She told him he’d been having a nightmare and had suddenly started screaming and tearing up the room. “I didn’t remember any of it,” he said.
Dr. Cantu eventually advised Dr. Nowinski against ever returning to the ring or any activity with the risk for head injury. Research shows that sustaining a single significant concussion increases the risk of subsequent more-severe brain injuries.
“My diagnosis could have sent Chris off the deep end because he could no longer do what he wanted to do with this life,” said Dr. Cantu. “But instead, he used it as a tool to find meaning for his life.”
Dr. Nowinski decided to use his experience as a teaching opportunity, not just for other athletes but also for sports organizations and the medical community.
His book, which focused on the NFL’s “tobacco-industry-like refusal to acknowledge the depths of the problem,” was published in 2006. A year later, Dr. Nowinski partnered with Dr. Cantu to found the Sports Legacy Institute, which eventually became the Concussion Legacy Foundation (CLF).
Cold calling for brain donations
Robert Stern, PhD, is another highly respected authority in the study of neurodegenerative disease. In 2007, he was directing the clinical core of Boston University’s Alzheimer’s Disease Center. After giving a lecture to a group of financial planners and elder-law attorneys one morning, he got a request for a private meeting from a fellow named Chris Nowinski.
“I’d never heard of him, but I agreed,” recalled Dr. Stern, a professor of neurology, neurosurgery, anatomy, and neurobiology at Boston University. “A few days later, this larger-than-life guy walked into our conference room at the BU School of Medicine, exuding a great deal of passion, intellect, and determination. He told me his story and then started talking about the long-term consequences of concussions in sports.”
Dr. Stern had seen patients with dementia pugilistica, the old-school term for CTE. These were mostly boxers with cognitive and behavioral impairment. “But I had not heard about football players,” he said. “I hadn’t put the two together. And as I was listening to Chris, I realized if what he was saying was true then it was not only a potentially huge public health issue, but it was also a potentially huge scientific issue in the field of neurodegenerative disease.”
Dr. Nowinski introduced Dr. Stern to Dr. Cantu, and together with Ann McKee, MD, professor of neurology and pathology at BU, they cofounded the Center for the Study of Traumatic Encephalopathy (CSTE) in 2008. It was the first center of its kind devoted to the study of CTE in the world.
One of Dr. Nowinski’s first jobs at the CSTE was soliciting and procuring brain donations. Since CTE is generally a progressive condition that can take decades to manifest, autopsy was the only way to detect it.
The brains of two former Pittsburgh Steelers, Mike Webster and Terry Long, had been examined after their untimely deaths. After immunostaining, investigators found both former NFL players had “protein misfolds” characteristic of CTE.
This finding drew a lot of public and scientific attention, given that Mr. Long died by suicide and Mr. Webster was homeless when he died of a heart attack. But more scientific evidence was needed to prove a causal link between the head trauma and CTE.
Dr. Nowinski scoured obituaries looking for potential brains to study. When he found one, he would cold call the family and try to convince them to donate it to science. The first brain he secured for the center belonged to John Grimsley, a former NFL linebacker who in 2008 died at age 45 of an accidental gunshot wound. Often, Dr. Nowinski would even be the courier, traveling to pick up the brain after it had been harvested.
Over the next 10 years, Dr. Nowinski and his research team secured 500 brain donations. The research that resulted was staggering. In the beginning only 45 cases of CTE had been identified in the world, but in the first 111 NFL players who were autopsied, 110 had the disorder.
Of the first 53 college football players autopsied, 48 had CTE. Although Dr. Nowinski’s initial focus was football, evidence of CTE was soon detected among athletes in boxing, hockey, soccer, and rugby, as well as in combat veterans. However, the National Football League and other governing sports bodies initially denied any connection between sport-related head trauma and CTE.
Cumulative damage
In 2017, after 7 years of study, Dr. Nowinski earned a PhD in neurology. As the scientific evidence continued to accumulate, two shifts occurred that Dr. Stern said represent Dr. Nowinski’s greatest contributions. First, concussion is now widely recognized as an acute brain injury with symptoms that need to be immediately diagnosed and addressed.
“This is a completely different story from where things were just 10 years ago,” said Dr. Stern, “and Chris played a central role, if not the central role, in raising awareness about that.”
All 50 states and the District of Columbia now have laws regarding sports-related concussion. And there are brain banks in Australia, Canada, New Zealand, Brazil, and the United Kingdom studying CTE. More than 2,500 athletes in a variety of sports, including NASCAR’s Dale Earnhardt Jr. and NFL hall of famer Nick Buoniconti, have publicly pledged to donate their brains to science after their deaths.
Second, said Dr. Stern, we now know that although concussions can contribute to CTE, they are not the sole cause. It’s repetitive subconcussive trauma, without symptoms of concussion, that do the most damage.
“These happen during every practice and in every game,” said Dr. Stern. In fact, it’s estimated that pro football players suffer thousands of subconcussive incidents over the course of their careers. So, a player doesn’t have to see stars or lose consciousness to suffer brain damage; small impacts can accumulate over time.
Understanding this point is crucial for making youth sports safer. “Chris has played a critical role in raising awareness here, too,” said Dr. Stern. “Allowing our kids to get hit in the head over and over can put them at greater risk for later problems, plus it just doesn’t make common sense.”
“The biggest misconception surrounding head trauma in sports,” said Dr. Nowinski, “is the belief among players, coaches, and even the medical and scientific communities that if you get hit in the head and don’t have any symptoms then you’re okay and there hasn’t been any damage. That couldn’t be further from the truth. We now know that people are suffering serious brain injuries due to the accumulated effect of subconcussive impacts, and we need to get the word out about that.”
A major initiative from the Concussion Legacy Foundation called “Stop Hitting Kids in the Head” has the goal of convincing every sport to eliminate repetitive head impacts in players under age 14 – the time when the skull and brain are still developing and most vulnerable – by 2026. In fact, Dr. Nowinski wrote that “there could be a lot of kids who are misdiagnosed and medicated for various behavioral or emotional problems that may actually be head injury–related.”
Starting in 2009, the NFL adopted a series of rule changes designed to better protect its players against repeated head trauma. Among them is a ban on spearing or leading with the helmet, penalties for hitting defenseless players, and more stringent return-to-play guidelines, including concussion protocols.
The NFL has also put more emphasis on flag football options for youngsters and, for the first time, showcased this alternative in the 2023 Pro Bowl. But Dr. Nowinski is pressuring the league to go further. “While acknowledging that the game causes CTE, the NFL still underwrites recruiting 5-year-olds to play tackle football,” he said. “In my opinion, that’s unethical, and it needs to be addressed.”
WWE one of the most responsive organizations
Dr. Nowinski said WWE has been one of the most responsive sports organizations for protecting athletes. A doctor is now ringside at every match as is an observer who knows the script, thereby allowing for instant medical intervention if something goes wrong. “Since everyone is trying to look like they have a concussion all the time, it takes a deep understanding of the business to recognize a real one,” he said.
But this hasn’t been the case with other sports. “I am eternally disappointed in the response of the professional sports industry to the knowledge of CTE and long-term concussion symptoms,” said Dr. Nowinski.
“For example, FIFA [international soccer’s governing body] still doesn’t allow doctors to evaluate [potentially concussed] players on the sidelines and put them back in the game with a free substitution [if they’re deemed okay]. Not giving players proper medical care for a brain injury is unethical,” he said. BU’s Center for the Study of Traumatic Encephalopathy diagnosed the first CTE case in soccer in 2012, and in 2015 Dr. Nowinski successfully lobbied U.S. Soccer to ban heading the ball before age 11.
“Unfortunately, many governing bodies have circled the wagons in denying their sport causes CTE,” he continued. “FIFA, World Rugby, the NHL, even the NCAA and International Olympic Committee refuse to acknowledge it and, therefore, aren’t taking any steps to prevent it. They see it as a threat to their business model. Hopefully, now that the NIH and CDC are aligned about the risks of head impact in sports, this will begin to change.”
Meanwhile, research is continuing. Scientists are getting closer to being able to diagnose CTE in living humans, with ongoing studies using PET scans, blood markers, and spinal fluid markers. In 2019, researchers identified tau proteins specific to CTE that they believe are distinct from those of Alzheimer’s and other neurodegenerative diseases. Next step would be developing a drug to slow the development of CTE once detected.
Nonetheless, athletes at all levels in impact sports still don’t fully appreciate the risks of repeated head trauma and especially subconcussive blows. “I talk to former NFL and college players every week,” said Dr. Stern. “Some tell me, ‘I love the sport, it gave me so much, and I would do it again, but I’m not letting my grandchildren play.’ But others say, ‘As long as they know the risks, they can make their own decision.’ “
Dr. Nowinski has a daughter who is 4 and a son who’s 2. Both play soccer but, thanks to dad, heading isn’t allowed in their age groups. If they continue playing sports, Dr. Nowinski said he’ll make sure they understand the risks and how to protect themselves. This is a conversation all parents should have with their kids at every level to make sure they play safe, he added.
Those in the medical community can also volunteer their time to explain head trauma to athletes, coaches, and school administrators to be sure they understand its seriousness and are doing everything to protect players.
As you watch this year’s Super Bowl, Dr. Nowinski and his team would like you to keep something in mind. Those young men on the field for your entertainment are receiving mild brain trauma repeatedly throughout the game.
Even if it’s not a huge hit that gets replayed and makes everyone gasp, even if no one gets ushered into the little sideline tent for a concussion screening, even if no one loses consciousness, brain damage is still occurring. Watch the heads of the players during every play and think about what’s going on inside their skulls regardless of how big and strong those helmets look.
A version of this article first appeared on Medscape.com.