User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
The challenges of managing CMV infection during pregnancy
CASE Anomalous findings on fetal anatomic survey
A 27-year-old previously healthy primigravid woman is at 18 weeks’ gestation. She is a first-grade schoolteacher. On her fetal anatomic survey, the estimated fetal weight was in the eighth percentile. Echogenic bowel and a small amount of ascitic fluid were noted in the fetal abdomen. The lateral and third ventricles were mildly dilated, the head circumference was 2 standard deviations below normal, and the placenta was slightly thickened and edematous.
What is the most likely diagnosis?
What diagnostic tests are indicated?
What management options are available for this patient?
Cytomegalovirus (CMV) is the most common of the perinatally transmitted infections, affecting 1% to 4% of all pregnancies. Although the virus typically causes either asymptomatic infection or only mild illness in immunocompetent individuals, it can cause life-threatening disease in immunocompromised persons and in the developing fetus. In this article, we review the virology and epidemiology of CMV infection and then focus on the key methods to diagnose infection in the mother and fetus. We conclude by considering measures that may be of at least modest value in treating CMV in pregnancy.
Virology of CMV infection
Cytomegalovirus is a double-stranded DNA virus in the Herpesviridae family. This ubiquitous virus is present in virtually all secretions and excretions of an infected host, including blood, urine, saliva, breast milk, genital secretions, and tissues and organs used for donation. Infection is transmitted through direct contact with any of the substances listed; contact with infected urine or saliva is the most common mode of transmission. Disease occurrence does not show seasonal variation.
After exposure, an incubation period of 28 to 60 days ensues, followed by development of viremia and clinical symptoms. In the majority of exposed individuals, CMV establishes a lifelong latent infection, and recurrent episodes of illness can occur as a result of reactivation of latent virus (also known as secondary infection) or, more rarely, infection with a new viral strain. In fact, most CMV illness episodes in pregnancy represent a reactivation of a previous infection rather than a new infection.
Following initial infection, both IgM (immunoglobulin M) and IgG (immunoglobulin G) antibodies develop rapidly and can be detected in blood within 1 to 2 weeks. IgM levels typically wane within 30 to 60 days, although persistence for several months is not unusual, and levels also can increase with viral reactivation (secondary infection). IgG antibodies typically persist for many years after a primary infection.
Intrauterine CMV infection occurs through hematogenous transplacental passage during maternal viremia. The risk of transmission and severity of fetal effects depend on whether or not the infection is primary or secondary in nature as well as the gestational age at fetal exposure.1,2
Additionally, postnatal vertical transmission can occur through exposure to viral particles in genital secretions as well as breast milk. CMV acquired in the postnatal period rarely produces severe sequelae in a healthy term neonate, but it has been associated with an increased rate of complications in very low birth weight and premature newborns.3
Continue to: Who is at risk...
Who is at risk
Congenital CMV, which occurs in 2.1 to 7.7 per 10,000 live births in the United States, is both the most common congenital infection and the leading cause of nonhereditary congenital hearing loss in children.4,5 The main reservoir of CMV in the United States is young children in day care settings, with approximately 50% of this population showing evidence of viral shedding in saliva.1 Adult populations in North America have a high prevalence of CMV IgG antibodies indicative of prior infection, with rates reaching 50% to 80%. Among seronegative individuals aged 12 to 49, the rate of seroconversion is approximately 1 in 60 annually.6 Significant racial disparities have been noted in rates of seroprevalence and seroconversion, with higher rates of infection in non-Hispanic Black and Mexican American individuals.6 Overall, the rate of new CMV infection among pregnant women in the United States is 0.7% to 4%.7
Clinical manifestations
Manifestations of infection differ depending on whether or not infection is primary or recurrent (secondary) and whether or not the host is immunocompetent or has a compromised immune system. Unique manifestations develop in the fetus.
CMV infection in children and adults. Among individuals with a normal immune response, the typical course of CMV is either no symptoms or a mononucleosis-like illness. In symptomatic patients, the most common symptoms include malaise, fever, and night sweats, and the most common associated laboratory abnormalities are elevation in liver function tests and a decreased white blood cell count, with a predominance of lymphocytes.8
Immunocompromised individuals are at risk for significant morbidity and mortality resulting from CMV. Illness may be the result of reactivation of latent infection due to decreased immune function or may be acquired as a result of treatment such as transplantation of CMV-positive organs or tissues, including bone marrow. Virtually any organ system can be affected, with potential for permanent organ damage and death. Severe systemic infection also can occur.
CMV infection in the fetus and neonate. As noted previously, fetal infection develops as a result of transplacental passage coincident with maternal infection. The risk of CMV transmission to the fetus and the severity of fetal injury vary based on gestational age at fetal infection and whether or not maternal infection is primary or secondary.
In most studies, primary maternal infections are associated with higher rates of fetal infection and more severe fetal and neonatal disease manifestations.2,7,9,10 Primary infections carry an overall 30% to 40% risk of transmission to the fetus.7,11 The risk of fetal transmission is much lower with a recurrent infection and is usually less than 2%.11 Due to their greater overall incidence, secondary infections account for the majority of cases of fetal and neonatal CMV disease.7 Importantly, although secondary infections generally have been regarded as having a lower risk and lower severity of fetal and neonatal disease, several recent studies have demonstrated rates of complications similar to, and even exceeding, those of primary infections.12-15 The TABLE provides a summary of the risks of fetal transmission and symptomatic fetal infection based on trimester of pregnancy.2,11,16-18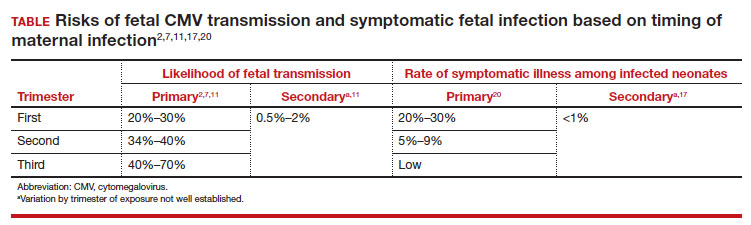
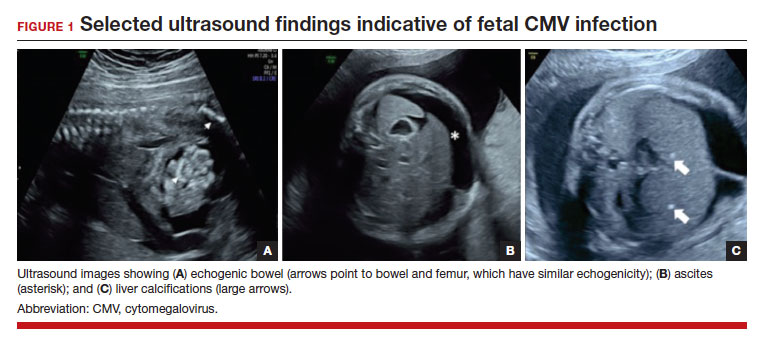
In the fetus, CMV may affect multiple organ systems. Among sonographic and magnetic resonance imaging (MRI) findings, central nervous system (CNS) anomalies are the most common.19,20 These can include microcephaly, ventriculomegaly, and periventricular calcifications. The gastrointestinal system also is frequently affected, and findings include echogenic bowel, hepatosplenomegaly, and liver calcifications. Lastly, isolated effusions, placentomegaly, fetal growth restriction, and even frank hydrops can develop. More favorable neurologic outcomes have been demonstrated in infants with no prenatal brain imaging abnormalities.20,21 However, the role of MRI in prenatal prognosis currently is not well defined.
FIGURE 1 illustrates selected sonographic findings associated with fetal CMV infection.
About 85% to 90% of infants with congenital CMV that results from primary maternal infection have no symptoms at birth. Among the 10% to 15% of infants that do have symptoms, petechial rash, jaundice, and hepatosplenomegaly are the most common manifestations (“blueberry muffin baby”). Approximately 10% to 20% of infants in this group have evidence of chorioretinitis on ophthalmologic examination, and 50% show either microcephaly or low birth weight.22Among survivors of symptomatic congenital CMV, more than 50% have long-term neurologic morbidities that may include sensorineural hearing loss, seizures, vision impairment, and developmental disabilities. Note that even when neonates appear asymptomatic at birth (regardless of whether infection is primary or secondary), 5% may develop microcephaly and motor deficits, 10% go on to develop sensorineural hearing loss, and the overall rate of neurologic morbidity reaches 13% to 15%.12,23 Some of the observed deficits manifest at several years of age, and, currently, no models exist for prediction of outcome.
Continue to: Diagnosing CMV infection...
Diagnosing CMV infection
Maternal infection
If maternal CMV infection is suspected based on a symptomatic illness or an abnormal fetal ultrasound exam, the first diagnostic test should be an assessment of IgM and IgG serology. If the former test results are positive and the latter negative, the diagnosis of acute CMV infection is confirmed. A positive serum CMV DNA polymerase chain reaction (PCR) test adds additional assurance that the diagnosis is correct. Primary infection, as noted above, poses the greatest risk of serious injury to the fetus.1
A frequent diagnostic dilemma arises when both the IgM and IgG antibody are positive. Remember that CMV IgM antibody can remain positive for 9 to 12 months after a primary infection and can reappear in the maternal serum in the face of a recurrent or reactivated infection. When confronted by both a positive IgM and positive IgG result, the clinician should then order IgG avidity testing. If the avidity is low to moderate, which reflects poor binding of antibody to the virus, the patient likely has an acute infection. If the avidity is high, which reflects enhanced binding of antibody to virus, the patient probably has a recurrent or reactivated infection; this scenario poses less danger to the developing fetus. The presence of CMV DNA in serum is also more consistent with acute infection, although viremia still can occur with recurrent infection. FIGURE 2 presents a suggested algorithm for the diagnosis of CMV in the pregnant patient.1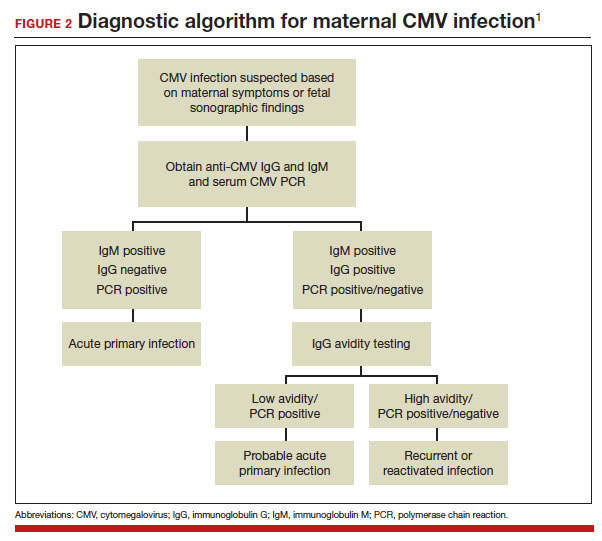
If a diagnosis of maternal CMV infection is confirmed, liver function tests should be obtained to determine if CMV hepatitis is present. If the liver function tests are abnormal, a coagulation profile also should be performed to identify the mother who might be at risk for peripartum hemorrhage.
Fetal infection
The single best test for confirmation of congenital CMV infection is detection of viral DNA and quantitation of viral load in the amniotic fluid by PCR. If the amniocentesis is performed prior to 20 weeks’ gestation and is negative, the test should be repeated in approximately 4 weeks.1,19,24
Detection of viral DNA indicates congenital infection. The ultimate task, however, is to determine if the infection has injured the fetus. Detailed ultrasound examination is the key to identifying fetal injury. As noted previously, the principal ultrasonographic findings that suggest congenital CMV infection include2,19,20,21,25:
- hydropic placenta
- fetal growth restriction
- microcephaly (head circumference more than 3 standard deviations below the mean)
- periventricular calcifications
- enlarged liver
- echogenic bowel
- ascites
- fetal hydrops.
Management: Evidence on CMV hyperimmune globulin, valacyclovir
If the immunocompetent mother has clinical manifestations of infection, she should receive symptomatic treatment. She should be encouraged to rest as much as possible, stay well hydrated, and use acetaminophen (1,000 mg every 6 to 8 hours) as needed for malaise and fever.
However, if the mother is immunocompromised and has signs of serious complications, such as chorioretinitis, hepatitis, or pneumonia, more aggressive therapy is indicated. Drugs used in this setting include foscarnet and ganciclovir and are best prescribed in consultation with a medical infectious disease specialist.
At this time, no consistently effective therapy for congenital infection is available. Therefore, if a patient has primary CMV infection in the first half of pregnancy, particularly in the first trimester, she should be counseled that the risk of fetal infection is approximately 40% and that approximately 5% to 15% of infants will be severely affected at birth. Given this information, some patients may opt for pregnancy termination.
In 2005, a report from Nigro and colleagues stimulated great hope that CMV-specific hyperimmune globulin (CytoGam) might be of value for both treatment and prophylaxis for congenital infection.26 These authors studied 157 women with confirmed primary CMV infection. One-hundred forty-eight women were asymptomatic and were identified by routine serologic screening, 8 had symptomatic infection, and 1 was identified because of abnormal fetal ultrasound findings. Forty-five women had CMV detected in amniotic fluid by PCR or culture more than 6 weeks before study enrollment. Thirty-one of these women were treated with intravenous hyperimmune globulin (200 U or 200 mg/kg maternal body weight); 14 declined treatment. Seven of the latter women had infants who were acutely symptomatic at the time of delivery; only 1 of the 31 treated women had an affected neonate (adjusted odds ratio [OR], 0.02; P<.001). In this same study, 84 women did not have a diagnostic amniocentesis because their infection occurred within 6 weeks of enrollment, their gestational age was less than 20 weeks, or they declined the procedure. Thirty-seven of these women received hyperimmune globulin (100 U or 100 mg/kg) every month until delivery, and 47 declined treatment. Six of the treated women delivered infected infants compared with 19 of the untreated women (adjusted OR, 0.32; P<.04).
Although these results were quite encouraging, several problems existed with the study’s design, as noted in an editorial that accompanied the study’s publication.27 First, the study was not randomized or placebo controlled. Second, patients were not stratified based on the severity of fetal ultrasound abnormalities. Third, the dosing of hyperimmune globulin varied; 9 of the 31 patients in the treatment group received additional infusions of drug into either the amniotic fluid or fetal umbilical vein. Moreover, patients in the prophylaxis group actually received a higher cumulative dose of hyperimmune globulin than patients in the treatment group.
Two subsequent investigations that were better designed were unable to verify the effectiveness of hyperimmune globulin. In 2014, Revello and colleagues reported the results of a prospective, randomized, placebo-controlled, double-blinded study of 124 women at 5 to 26 weeks’ gestation with confirmed primary CMV infection.28 The rate of congenital infection was 30% in the group treated with hyperimmune globulin and 44% in the placebo group (P=.13). There also was no significant difference in the concentration of serum CMV DNA in treated versus untreated mothers. Moreover, the number of adverse obstetric events (preterm delivery, fetal growth restriction, intrahepatic cholestasis of pregnancy, and postpartum preeclampsia) in the treatment group was higher than in the placebo group, 13% versus 2%.
In 2021, Hughes and colleagues published the results of a multicenter, double-blind trial in 399 women who had a diagnosis of primary CMV infection before 23 weeks’ gestation.29 The primary outcome was defined as a composite of congenital CMV infection or fetal/neonatal death. An adverse primary outcome occurred in 22.7% of the patients who received hyperimmune globulin and 19.4% of those who received placebo (relative risk, 1.17; 95% confidence interval [CI], 0.80–1.72; P=.42).
Continue to: Jacquemard and colleagues...
Jacquemard and colleagues then proposed a different approach.30 In a small pilot study of 20 patients, these authors used high doses of oral valacylovir (2 g 4 times daily) and documented therapeutic drug concentrations and a decline in CMV viral load in fetal serum. Patients were not stratified by severity of fetal injury at onset of treatment, so the authors were unable to define which fetuses were most likely to benefit from treatment.
In a follow-up investigation, Leruez-Ville and colleagues reported another small series in which high-dose oral valacyclovir (8 g daily) was used for treatment.31 They excluded fetuses with severe brain anomalies and fetuses with no sonographic evidence of injury. The median gestational age at diagnosis was 26 weeks. Thirty-four of 43 treated fetuses were free of injury at birth. In addition, the viral load in the neonate’s serum decreased significantly after treatment, and the platelet count increased. The authors then compared these outcomes to a historical cohort and confirmed that treatment increased the proportion of asymptomatic neonates from 43% without treatment to 82% with treatment (P<.05 with no overlapping confidence intervals).
We conclude from these investigations that hyperimmune globulin is unlikely to be of value in treating congenital CMV infection, especially if the fetus already has sonographic findings of severe injury. High-dose oral valacyclovir also is unlikely to be of value in severely affected fetuses, particularly those with evidence of CNS injury. However, antiviral therapy may be of modest value in situations when the fetus is less severely injured.
Preventive measures
Since no definitive treatment is available for congenital CMV infection, our efforts as clinicians should focus on measures that may prevent transmission of infection to the pregnant patient. These measures include:
- Encouraging patients to use careful handwashing techniques when handling infant diapers and toys.
- Encouraging patients to adopt safe sexual practices if not already engaged in a mutually faithful, monogamous relationship.
- Using CMV-negative blood when transfusing a pregnant woman or a fetus.
At the present time, unfortunately, a readily available and highly effective therapy for prevention of CMV infection is not available.
CASE Congenital infection diagnosed
The ultrasound findings are most consistent with congenital CMV infection, especially given the patient’s work as an elementary schoolteacher. The diagnosis of maternal infection is best established by conventional serology (positive IgM, negative IgM) and detection of viral DNA in maternal blood by PCR testing. The diagnosis of congenital infection is best confirmed by documentation of viral DNA in the amniotic fluid by PCR testing. Given that this fetus already has evidence of moderate to severe injury, no treatment is likely to be effective in reversing the abnormal ultrasound findings. Pregnancy termination may be an option, depending upon the patient’s desires and the legal restrictions prevalent in the patient’s geographic area. ●
- Cytomegalovirus infection is the most common of the perinatally transmitted infections.
- Maternal infection is often asymptomatic. When symptoms are present, they resemble those of an influenza-like illness. In immunocompromised persons, however, CMV may cause serious complications, including pneumonia, hepatitis, and chorioretinitis.
- The virus is transmitted by contact with contaminated body fluids, such as saliva, urine, blood, and genital secretions.
- The greatest risk of severe fetal injury results from primary maternal infection in the first trimester of pregnancy.
- Manifestations of severe congenital CMV infection include growth restriction, microcephaly, ventriculomegaly, hepatosplenomegaly, ascites, chorioretinitis, thrombocytopenia, purpura, and hydrops (“blueberry muffin baby”).
- Late manifestations of infection, which usually follow recurrent maternal infection, may appear as a child enters elementary school and include visual and auditory deficits, developmental delays, and learning disabilities.
- The diagnosis of maternal infection is confirmed by serology and detection of viral DNA in the serum by PCR testing.
- The diagnosis of fetal infection is best made by a combination of abnormal ultrasound findings and detection of CMV DNA in amniotic fluid. The characteristic ultrasound findings include placentomegaly, microcephaly, ventriculomegaly, growth restriction, echogenic bowel, and serous effusions/hydrops.
- Treatment of the mother with antiviral medications such as valacyclovir may be of modest value in reducing placental edema, decreasing viral load in the fetus, and hastening the resolution of some ultrasound findings, such as echogenic bowel.
- While initial studies seemed promising, the use of hyperimmune globulin has not proven to be consistently effective in treating congenital infection.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik’s Maternal Fetal Medicine: Principles and Practice. 8th ed. 2019:888-890.
- Chatzakis C, Ville Y, Makrydimas G, et al. Timing of primary maternal cytomegalovirus infection and rates of vertical transmission and fetal consequences. Am J Obstet Gynecol. 2020;223:870-883.e11. doi:10.1016/j.ajog.2020.05.038
- Kelly MS, Benjamin DK, Puopolo KM, et al. Postnatal cytomegalovirus infection and the risk for bronchopulmonary dysplasia. JAMA Pediatr. 2015;169:e153785. doi:10.1001 /jamapediatrics.2015.3785
- Messinger CJ, Lipsitch M, Bateman BT, et al. Association between congenital cytomegalovirus and the prevalence at birth of microcephaly in the United States. JAMA Pediatr. 2020;174:1159-1167. doi:10.1001/jamapediatrics.2020.3009
- De Cuyper E, Acke F, Keymeulen A, et al. Risk factors for hearing loss at birth in newborns with congenital cytomegalovirus infection. JAMA Otolaryngol Head Neck Surg. 2023;149:122-130. doi:10.1001/jamaoto.2022.4109
- Colugnati FA, Staras SA, Dollard SC, et al. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect Dis. 2007;7:71. doi:10.1186/1471-2334-7-71
- Stagno S, Pass RF, Cloud G, et al. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA. 1986;256:1904-1908.
- Wreghitt TG, Teare EL, Sule O, et al. Cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 2003;37:1603-1606. doi:10.1086/379711
- Fowler KB, Stagno S, Pass RF, et al. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med. 1992;326:663-667. doi:10.1056 /NEJM199203053261003
- Faure-Bardon V, Magny JF, Parodi M, et al. Sequelae of congenital cytomegalovirus following maternal primary infections are limited to those acquired in the first trimester of pregnancy. Clin Infect Dis. 2019;69:1526-1532. doi:10.1093/ cid/ciy1128
- Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17:253-276. doi:10.1002/ rmv.535
- Boppana SB, Pass RF, Britt WJ, et al. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J. 1992;11:93-99. doi:10.1097/00006454-199202000-00007
- Ross SA, Fowler KB, Ashrith G, et al. Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity. J Pediatr. 2006;148:332-336. doi:10.1016/j.jpeds.2005.09.003
- Zalel Y, Gilboa Y, Berkenshtat M, et al. Secondary cytomegalovirus infection can cause severe fetal sequelae despite maternal preconceptional immunity. Ultrasound Obstet Gynecol. 31:417-420. doi:10.1002/uog.5255
- Scaramuzzino F, Di Pastena M, Chiurchiu S, et al. Secondary cytomegalovirus infections: how much do we still not know? Comparison of children with symptomatic congenital cytomegalovirus born to mothers with primary and secondary infection. Front Pediatr. 2022;10:885926. doi:10.3389/fped.2022.885926
- Gindes L, Teperberg-Oikawa M, Sherman D, et al. Congenital cytomegalovirus infection following primary maternal infection in the third trimester. BJOG. 2008;115:830-835. doi:10.1111/j.1471-0528.2007.01651.x
- Hadar E, Dorfman E, Bardin R, et al. Symptomatic congenital cytomegalovirus disease following non-primary maternal infection: a retrospective cohort study. BMC Infect Dis. 2017;17:31. doi:10.1186/s12879-016-2161-3
- Elkan Miller T, Weisz B, Yinon Y, et al. Congenital cytomegalovirus infection following second and third trimester maternal infection is associated with mild childhood adverse outcome not predicted by prenatal imaging. J Pediatric Infect Dis Soc. 2021;10:562-568. doi:10.1093/jpids/ piaa154
- Lipitz S, Yinon Y, Malinger G, et al. Risk of cytomegalovirusassociated sequelae in relation to time of infection and findings on prenatal imaging. Ultrasound Obstet Gynecol. 2013;41:508-514. doi:10.1002/uog.12377
- Lipitz S, Elkan Miller T, Yinon Y, et al. Revisiting short- and long-term outcome after fetal first-trimester primary cytomegalovirus infection in relation to prenatal imaging findings. Ultrasound Obstet Gynecol. 2020;56:572-578. doi:10.1002/uog.21946
- Buca D, Di Mascio D, Rizzo G, et al. Outcome of fetuses with congenital cytomegalovirus infection and normal ultrasound at diagnosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2021;57:551-559. doi:10.1002/uog.23143
- Boppana SB, Ross SA, Fowler KB. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis. 2013;57 (suppl 4):S178-S181. doi:10.1093/cid/cit629
- Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17:355-363. doi:10.1002/rmv.544
- Hughes BL, Gyamfi-Bannerman C. Diagnosis and antenatal management of congenital cytomegalovirus infection. Am J Obstet Gynecol. 2016;214:B5-11. doi:10.1016 /j.ajog.2016.02.042
- Rouse DJ, Fette LM, Hughes BL, et al. Noninvasive prediction of congenital cytomegalovirus infection after maternal primary infection. Obstet Gynecol. 2022;139:400-406. doi:10.1097/AOG.0000000000004691
- Nigro G, Adler SP, La Torre R, et al; Congenital Cytomegalovirus Collaborating Group. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350-1362. doi:10.1056/NEJMoa043337
- Duff P. Immunotherapy for congenital cytomegalovirus infection. N Engl J Med. 2005;355:1402-1404. doi:10.1056 /NEJMe058172
- Revello MG, Lazzarotto T, Guerra B, et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med. 2014;370:1316-1326. doi:10.1056/NEJMoa1310214
- Hughes BL, Clifton RG, Rouse DJ, et al. A trial of hyperimmune globulin to prevent congenital cytomegalovirus infection. N Engl J Med. 2021;385:436-444. doi:10.1056/NEJMoa1913569
- Jacquemard F, Yamamoto M, Costa JM, et al. Maternal administration of valaciclovir in symptomatic intrauterine cytomegalovirus infection. BJOG. 2007;114:1113-1121. doi:10.1111/j.1471-0528.2007.01308.x
- Leruez-Ville M, Ghout I, Bussières L, et al. In utero treatment of congenital cytomegalovirus infection with valacyclovir in a multicenter, open-label, phase II study. Am J Obstet Gynecol. 2016;215:462.e1-462.e10. doi:10.1016/j.ajog.2016.04.003
CASE Anomalous findings on fetal anatomic survey
A 27-year-old previously healthy primigravid woman is at 18 weeks’ gestation. She is a first-grade schoolteacher. On her fetal anatomic survey, the estimated fetal weight was in the eighth percentile. Echogenic bowel and a small amount of ascitic fluid were noted in the fetal abdomen. The lateral and third ventricles were mildly dilated, the head circumference was 2 standard deviations below normal, and the placenta was slightly thickened and edematous.
What is the most likely diagnosis?
What diagnostic tests are indicated?
What management options are available for this patient?
Cytomegalovirus (CMV) is the most common of the perinatally transmitted infections, affecting 1% to 4% of all pregnancies. Although the virus typically causes either asymptomatic infection or only mild illness in immunocompetent individuals, it can cause life-threatening disease in immunocompromised persons and in the developing fetus. In this article, we review the virology and epidemiology of CMV infection and then focus on the key methods to diagnose infection in the mother and fetus. We conclude by considering measures that may be of at least modest value in treating CMV in pregnancy.
Virology of CMV infection
Cytomegalovirus is a double-stranded DNA virus in the Herpesviridae family. This ubiquitous virus is present in virtually all secretions and excretions of an infected host, including blood, urine, saliva, breast milk, genital secretions, and tissues and organs used for donation. Infection is transmitted through direct contact with any of the substances listed; contact with infected urine or saliva is the most common mode of transmission. Disease occurrence does not show seasonal variation.
After exposure, an incubation period of 28 to 60 days ensues, followed by development of viremia and clinical symptoms. In the majority of exposed individuals, CMV establishes a lifelong latent infection, and recurrent episodes of illness can occur as a result of reactivation of latent virus (also known as secondary infection) or, more rarely, infection with a new viral strain. In fact, most CMV illness episodes in pregnancy represent a reactivation of a previous infection rather than a new infection.
Following initial infection, both IgM (immunoglobulin M) and IgG (immunoglobulin G) antibodies develop rapidly and can be detected in blood within 1 to 2 weeks. IgM levels typically wane within 30 to 60 days, although persistence for several months is not unusual, and levels also can increase with viral reactivation (secondary infection). IgG antibodies typically persist for many years after a primary infection.
Intrauterine CMV infection occurs through hematogenous transplacental passage during maternal viremia. The risk of transmission and severity of fetal effects depend on whether or not the infection is primary or secondary in nature as well as the gestational age at fetal exposure.1,2
Additionally, postnatal vertical transmission can occur through exposure to viral particles in genital secretions as well as breast milk. CMV acquired in the postnatal period rarely produces severe sequelae in a healthy term neonate, but it has been associated with an increased rate of complications in very low birth weight and premature newborns.3
Continue to: Who is at risk...
Who is at risk
Congenital CMV, which occurs in 2.1 to 7.7 per 10,000 live births in the United States, is both the most common congenital infection and the leading cause of nonhereditary congenital hearing loss in children.4,5 The main reservoir of CMV in the United States is young children in day care settings, with approximately 50% of this population showing evidence of viral shedding in saliva.1 Adult populations in North America have a high prevalence of CMV IgG antibodies indicative of prior infection, with rates reaching 50% to 80%. Among seronegative individuals aged 12 to 49, the rate of seroconversion is approximately 1 in 60 annually.6 Significant racial disparities have been noted in rates of seroprevalence and seroconversion, with higher rates of infection in non-Hispanic Black and Mexican American individuals.6 Overall, the rate of new CMV infection among pregnant women in the United States is 0.7% to 4%.7
Clinical manifestations
Manifestations of infection differ depending on whether or not infection is primary or recurrent (secondary) and whether or not the host is immunocompetent or has a compromised immune system. Unique manifestations develop in the fetus.
CMV infection in children and adults. Among individuals with a normal immune response, the typical course of CMV is either no symptoms or a mononucleosis-like illness. In symptomatic patients, the most common symptoms include malaise, fever, and night sweats, and the most common associated laboratory abnormalities are elevation in liver function tests and a decreased white blood cell count, with a predominance of lymphocytes.8
Immunocompromised individuals are at risk for significant morbidity and mortality resulting from CMV. Illness may be the result of reactivation of latent infection due to decreased immune function or may be acquired as a result of treatment such as transplantation of CMV-positive organs or tissues, including bone marrow. Virtually any organ system can be affected, with potential for permanent organ damage and death. Severe systemic infection also can occur.
CMV infection in the fetus and neonate. As noted previously, fetal infection develops as a result of transplacental passage coincident with maternal infection. The risk of CMV transmission to the fetus and the severity of fetal injury vary based on gestational age at fetal infection and whether or not maternal infection is primary or secondary.
In most studies, primary maternal infections are associated with higher rates of fetal infection and more severe fetal and neonatal disease manifestations.2,7,9,10 Primary infections carry an overall 30% to 40% risk of transmission to the fetus.7,11 The risk of fetal transmission is much lower with a recurrent infection and is usually less than 2%.11 Due to their greater overall incidence, secondary infections account for the majority of cases of fetal and neonatal CMV disease.7 Importantly, although secondary infections generally have been regarded as having a lower risk and lower severity of fetal and neonatal disease, several recent studies have demonstrated rates of complications similar to, and even exceeding, those of primary infections.12-15 The TABLE provides a summary of the risks of fetal transmission and symptomatic fetal infection based on trimester of pregnancy.2,11,16-18
In the fetus, CMV may affect multiple organ systems. Among sonographic and magnetic resonance imaging (MRI) findings, central nervous system (CNS) anomalies are the most common.19,20 These can include microcephaly, ventriculomegaly, and periventricular calcifications. The gastrointestinal system also is frequently affected, and findings include echogenic bowel, hepatosplenomegaly, and liver calcifications. Lastly, isolated effusions, placentomegaly, fetal growth restriction, and even frank hydrops can develop. More favorable neurologic outcomes have been demonstrated in infants with no prenatal brain imaging abnormalities.20,21 However, the role of MRI in prenatal prognosis currently is not well defined.
FIGURE 1 illustrates selected sonographic findings associated with fetal CMV infection.

About 85% to 90% of infants with congenital CMV that results from primary maternal infection have no symptoms at birth. Among the 10% to 15% of infants that do have symptoms, petechial rash, jaundice, and hepatosplenomegaly are the most common manifestations (“blueberry muffin baby”). Approximately 10% to 20% of infants in this group have evidence of chorioretinitis on ophthalmologic examination, and 50% show either microcephaly or low birth weight.22Among survivors of symptomatic congenital CMV, more than 50% have long-term neurologic morbidities that may include sensorineural hearing loss, seizures, vision impairment, and developmental disabilities. Note that even when neonates appear asymptomatic at birth (regardless of whether infection is primary or secondary), 5% may develop microcephaly and motor deficits, 10% go on to develop sensorineural hearing loss, and the overall rate of neurologic morbidity reaches 13% to 15%.12,23 Some of the observed deficits manifest at several years of age, and, currently, no models exist for prediction of outcome.
Continue to: Diagnosing CMV infection...
Diagnosing CMV infection
Maternal infection
If maternal CMV infection is suspected based on a symptomatic illness or an abnormal fetal ultrasound exam, the first diagnostic test should be an assessment of IgM and IgG serology. If the former test results are positive and the latter negative, the diagnosis of acute CMV infection is confirmed. A positive serum CMV DNA polymerase chain reaction (PCR) test adds additional assurance that the diagnosis is correct. Primary infection, as noted above, poses the greatest risk of serious injury to the fetus.1
A frequent diagnostic dilemma arises when both the IgM and IgG antibody are positive. Remember that CMV IgM antibody can remain positive for 9 to 12 months after a primary infection and can reappear in the maternal serum in the face of a recurrent or reactivated infection. When confronted by both a positive IgM and positive IgG result, the clinician should then order IgG avidity testing. If the avidity is low to moderate, which reflects poor binding of antibody to the virus, the patient likely has an acute infection. If the avidity is high, which reflects enhanced binding of antibody to virus, the patient probably has a recurrent or reactivated infection; this scenario poses less danger to the developing fetus. The presence of CMV DNA in serum is also more consistent with acute infection, although viremia still can occur with recurrent infection. FIGURE 2 presents a suggested algorithm for the diagnosis of CMV in the pregnant patient.1
If a diagnosis of maternal CMV infection is confirmed, liver function tests should be obtained to determine if CMV hepatitis is present. If the liver function tests are abnormal, a coagulation profile also should be performed to identify the mother who might be at risk for peripartum hemorrhage.
Fetal infection
The single best test for confirmation of congenital CMV infection is detection of viral DNA and quantitation of viral load in the amniotic fluid by PCR. If the amniocentesis is performed prior to 20 weeks’ gestation and is negative, the test should be repeated in approximately 4 weeks.1,19,24
Detection of viral DNA indicates congenital infection. The ultimate task, however, is to determine if the infection has injured the fetus. Detailed ultrasound examination is the key to identifying fetal injury. As noted previously, the principal ultrasonographic findings that suggest congenital CMV infection include2,19,20,21,25:
- hydropic placenta
- fetal growth restriction
- microcephaly (head circumference more than 3 standard deviations below the mean)
- periventricular calcifications
- enlarged liver
- echogenic bowel
- ascites
- fetal hydrops.
Management: Evidence on CMV hyperimmune globulin, valacyclovir
If the immunocompetent mother has clinical manifestations of infection, she should receive symptomatic treatment. She should be encouraged to rest as much as possible, stay well hydrated, and use acetaminophen (1,000 mg every 6 to 8 hours) as needed for malaise and fever.
However, if the mother is immunocompromised and has signs of serious complications, such as chorioretinitis, hepatitis, or pneumonia, more aggressive therapy is indicated. Drugs used in this setting include foscarnet and ganciclovir and are best prescribed in consultation with a medical infectious disease specialist.
At this time, no consistently effective therapy for congenital infection is available. Therefore, if a patient has primary CMV infection in the first half of pregnancy, particularly in the first trimester, she should be counseled that the risk of fetal infection is approximately 40% and that approximately 5% to 15% of infants will be severely affected at birth. Given this information, some patients may opt for pregnancy termination.
In 2005, a report from Nigro and colleagues stimulated great hope that CMV-specific hyperimmune globulin (CytoGam) might be of value for both treatment and prophylaxis for congenital infection.26 These authors studied 157 women with confirmed primary CMV infection. One-hundred forty-eight women were asymptomatic and were identified by routine serologic screening, 8 had symptomatic infection, and 1 was identified because of abnormal fetal ultrasound findings. Forty-five women had CMV detected in amniotic fluid by PCR or culture more than 6 weeks before study enrollment. Thirty-one of these women were treated with intravenous hyperimmune globulin (200 U or 200 mg/kg maternal body weight); 14 declined treatment. Seven of the latter women had infants who were acutely symptomatic at the time of delivery; only 1 of the 31 treated women had an affected neonate (adjusted odds ratio [OR], 0.02; P<.001). In this same study, 84 women did not have a diagnostic amniocentesis because their infection occurred within 6 weeks of enrollment, their gestational age was less than 20 weeks, or they declined the procedure. Thirty-seven of these women received hyperimmune globulin (100 U or 100 mg/kg) every month until delivery, and 47 declined treatment. Six of the treated women delivered infected infants compared with 19 of the untreated women (adjusted OR, 0.32; P<.04).
Although these results were quite encouraging, several problems existed with the study’s design, as noted in an editorial that accompanied the study’s publication.27 First, the study was not randomized or placebo controlled. Second, patients were not stratified based on the severity of fetal ultrasound abnormalities. Third, the dosing of hyperimmune globulin varied; 9 of the 31 patients in the treatment group received additional infusions of drug into either the amniotic fluid or fetal umbilical vein. Moreover, patients in the prophylaxis group actually received a higher cumulative dose of hyperimmune globulin than patients in the treatment group.
Two subsequent investigations that were better designed were unable to verify the effectiveness of hyperimmune globulin. In 2014, Revello and colleagues reported the results of a prospective, randomized, placebo-controlled, double-blinded study of 124 women at 5 to 26 weeks’ gestation with confirmed primary CMV infection.28 The rate of congenital infection was 30% in the group treated with hyperimmune globulin and 44% in the placebo group (P=.13). There also was no significant difference in the concentration of serum CMV DNA in treated versus untreated mothers. Moreover, the number of adverse obstetric events (preterm delivery, fetal growth restriction, intrahepatic cholestasis of pregnancy, and postpartum preeclampsia) in the treatment group was higher than in the placebo group, 13% versus 2%.
In 2021, Hughes and colleagues published the results of a multicenter, double-blind trial in 399 women who had a diagnosis of primary CMV infection before 23 weeks’ gestation.29 The primary outcome was defined as a composite of congenital CMV infection or fetal/neonatal death. An adverse primary outcome occurred in 22.7% of the patients who received hyperimmune globulin and 19.4% of those who received placebo (relative risk, 1.17; 95% confidence interval [CI], 0.80–1.72; P=.42).
Continue to: Jacquemard and colleagues...
Jacquemard and colleagues then proposed a different approach.30 In a small pilot study of 20 patients, these authors used high doses of oral valacylovir (2 g 4 times daily) and documented therapeutic drug concentrations and a decline in CMV viral load in fetal serum. Patients were not stratified by severity of fetal injury at onset of treatment, so the authors were unable to define which fetuses were most likely to benefit from treatment.
In a follow-up investigation, Leruez-Ville and colleagues reported another small series in which high-dose oral valacyclovir (8 g daily) was used for treatment.31 They excluded fetuses with severe brain anomalies and fetuses with no sonographic evidence of injury. The median gestational age at diagnosis was 26 weeks. Thirty-four of 43 treated fetuses were free of injury at birth. In addition, the viral load in the neonate’s serum decreased significantly after treatment, and the platelet count increased. The authors then compared these outcomes to a historical cohort and confirmed that treatment increased the proportion of asymptomatic neonates from 43% without treatment to 82% with treatment (P<.05 with no overlapping confidence intervals).
We conclude from these investigations that hyperimmune globulin is unlikely to be of value in treating congenital CMV infection, especially if the fetus already has sonographic findings of severe injury. High-dose oral valacyclovir also is unlikely to be of value in severely affected fetuses, particularly those with evidence of CNS injury. However, antiviral therapy may be of modest value in situations when the fetus is less severely injured.
Preventive measures
Since no definitive treatment is available for congenital CMV infection, our efforts as clinicians should focus on measures that may prevent transmission of infection to the pregnant patient. These measures include:
- Encouraging patients to use careful handwashing techniques when handling infant diapers and toys.
- Encouraging patients to adopt safe sexual practices if not already engaged in a mutually faithful, monogamous relationship.
- Using CMV-negative blood when transfusing a pregnant woman or a fetus.
At the present time, unfortunately, a readily available and highly effective therapy for prevention of CMV infection is not available.
CASE Congenital infection diagnosed
The ultrasound findings are most consistent with congenital CMV infection, especially given the patient’s work as an elementary schoolteacher. The diagnosis of maternal infection is best established by conventional serology (positive IgM, negative IgM) and detection of viral DNA in maternal blood by PCR testing. The diagnosis of congenital infection is best confirmed by documentation of viral DNA in the amniotic fluid by PCR testing. Given that this fetus already has evidence of moderate to severe injury, no treatment is likely to be effective in reversing the abnormal ultrasound findings. Pregnancy termination may be an option, depending upon the patient’s desires and the legal restrictions prevalent in the patient’s geographic area. ●
- Cytomegalovirus infection is the most common of the perinatally transmitted infections.
- Maternal infection is often asymptomatic. When symptoms are present, they resemble those of an influenza-like illness. In immunocompromised persons, however, CMV may cause serious complications, including pneumonia, hepatitis, and chorioretinitis.
- The virus is transmitted by contact with contaminated body fluids, such as saliva, urine, blood, and genital secretions.
- The greatest risk of severe fetal injury results from primary maternal infection in the first trimester of pregnancy.
- Manifestations of severe congenital CMV infection include growth restriction, microcephaly, ventriculomegaly, hepatosplenomegaly, ascites, chorioretinitis, thrombocytopenia, purpura, and hydrops (“blueberry muffin baby”).
- Late manifestations of infection, which usually follow recurrent maternal infection, may appear as a child enters elementary school and include visual and auditory deficits, developmental delays, and learning disabilities.
- The diagnosis of maternal infection is confirmed by serology and detection of viral DNA in the serum by PCR testing.
- The diagnosis of fetal infection is best made by a combination of abnormal ultrasound findings and detection of CMV DNA in amniotic fluid. The characteristic ultrasound findings include placentomegaly, microcephaly, ventriculomegaly, growth restriction, echogenic bowel, and serous effusions/hydrops.
- Treatment of the mother with antiviral medications such as valacyclovir may be of modest value in reducing placental edema, decreasing viral load in the fetus, and hastening the resolution of some ultrasound findings, such as echogenic bowel.
- While initial studies seemed promising, the use of hyperimmune globulin has not proven to be consistently effective in treating congenital infection.
CASE Anomalous findings on fetal anatomic survey
A 27-year-old previously healthy primigravid woman is at 18 weeks’ gestation. She is a first-grade schoolteacher. On her fetal anatomic survey, the estimated fetal weight was in the eighth percentile. Echogenic bowel and a small amount of ascitic fluid were noted in the fetal abdomen. The lateral and third ventricles were mildly dilated, the head circumference was 2 standard deviations below normal, and the placenta was slightly thickened and edematous.
What is the most likely diagnosis?
What diagnostic tests are indicated?
What management options are available for this patient?
Cytomegalovirus (CMV) is the most common of the perinatally transmitted infections, affecting 1% to 4% of all pregnancies. Although the virus typically causes either asymptomatic infection or only mild illness in immunocompetent individuals, it can cause life-threatening disease in immunocompromised persons and in the developing fetus. In this article, we review the virology and epidemiology of CMV infection and then focus on the key methods to diagnose infection in the mother and fetus. We conclude by considering measures that may be of at least modest value in treating CMV in pregnancy.
Virology of CMV infection
Cytomegalovirus is a double-stranded DNA virus in the Herpesviridae family. This ubiquitous virus is present in virtually all secretions and excretions of an infected host, including blood, urine, saliva, breast milk, genital secretions, and tissues and organs used for donation. Infection is transmitted through direct contact with any of the substances listed; contact with infected urine or saliva is the most common mode of transmission. Disease occurrence does not show seasonal variation.
After exposure, an incubation period of 28 to 60 days ensues, followed by development of viremia and clinical symptoms. In the majority of exposed individuals, CMV establishes a lifelong latent infection, and recurrent episodes of illness can occur as a result of reactivation of latent virus (also known as secondary infection) or, more rarely, infection with a new viral strain. In fact, most CMV illness episodes in pregnancy represent a reactivation of a previous infection rather than a new infection.
Following initial infection, both IgM (immunoglobulin M) and IgG (immunoglobulin G) antibodies develop rapidly and can be detected in blood within 1 to 2 weeks. IgM levels typically wane within 30 to 60 days, although persistence for several months is not unusual, and levels also can increase with viral reactivation (secondary infection). IgG antibodies typically persist for many years after a primary infection.
Intrauterine CMV infection occurs through hematogenous transplacental passage during maternal viremia. The risk of transmission and severity of fetal effects depend on whether or not the infection is primary or secondary in nature as well as the gestational age at fetal exposure.1,2
Additionally, postnatal vertical transmission can occur through exposure to viral particles in genital secretions as well as breast milk. CMV acquired in the postnatal period rarely produces severe sequelae in a healthy term neonate, but it has been associated with an increased rate of complications in very low birth weight and premature newborns.3
Continue to: Who is at risk...
Who is at risk
Congenital CMV, which occurs in 2.1 to 7.7 per 10,000 live births in the United States, is both the most common congenital infection and the leading cause of nonhereditary congenital hearing loss in children.4,5 The main reservoir of CMV in the United States is young children in day care settings, with approximately 50% of this population showing evidence of viral shedding in saliva.1 Adult populations in North America have a high prevalence of CMV IgG antibodies indicative of prior infection, with rates reaching 50% to 80%. Among seronegative individuals aged 12 to 49, the rate of seroconversion is approximately 1 in 60 annually.6 Significant racial disparities have been noted in rates of seroprevalence and seroconversion, with higher rates of infection in non-Hispanic Black and Mexican American individuals.6 Overall, the rate of new CMV infection among pregnant women in the United States is 0.7% to 4%.7
Clinical manifestations
Manifestations of infection differ depending on whether or not infection is primary or recurrent (secondary) and whether or not the host is immunocompetent or has a compromised immune system. Unique manifestations develop in the fetus.
CMV infection in children and adults. Among individuals with a normal immune response, the typical course of CMV is either no symptoms or a mononucleosis-like illness. In symptomatic patients, the most common symptoms include malaise, fever, and night sweats, and the most common associated laboratory abnormalities are elevation in liver function tests and a decreased white blood cell count, with a predominance of lymphocytes.8
Immunocompromised individuals are at risk for significant morbidity and mortality resulting from CMV. Illness may be the result of reactivation of latent infection due to decreased immune function or may be acquired as a result of treatment such as transplantation of CMV-positive organs or tissues, including bone marrow. Virtually any organ system can be affected, with potential for permanent organ damage and death. Severe systemic infection also can occur.
CMV infection in the fetus and neonate. As noted previously, fetal infection develops as a result of transplacental passage coincident with maternal infection. The risk of CMV transmission to the fetus and the severity of fetal injury vary based on gestational age at fetal infection and whether or not maternal infection is primary or secondary.
In most studies, primary maternal infections are associated with higher rates of fetal infection and more severe fetal and neonatal disease manifestations.2,7,9,10 Primary infections carry an overall 30% to 40% risk of transmission to the fetus.7,11 The risk of fetal transmission is much lower with a recurrent infection and is usually less than 2%.11 Due to their greater overall incidence, secondary infections account for the majority of cases of fetal and neonatal CMV disease.7 Importantly, although secondary infections generally have been regarded as having a lower risk and lower severity of fetal and neonatal disease, several recent studies have demonstrated rates of complications similar to, and even exceeding, those of primary infections.12-15 The TABLE provides a summary of the risks of fetal transmission and symptomatic fetal infection based on trimester of pregnancy.2,11,16-18
In the fetus, CMV may affect multiple organ systems. Among sonographic and magnetic resonance imaging (MRI) findings, central nervous system (CNS) anomalies are the most common.19,20 These can include microcephaly, ventriculomegaly, and periventricular calcifications. The gastrointestinal system also is frequently affected, and findings include echogenic bowel, hepatosplenomegaly, and liver calcifications. Lastly, isolated effusions, placentomegaly, fetal growth restriction, and even frank hydrops can develop. More favorable neurologic outcomes have been demonstrated in infants with no prenatal brain imaging abnormalities.20,21 However, the role of MRI in prenatal prognosis currently is not well defined.
FIGURE 1 illustrates selected sonographic findings associated with fetal CMV infection.

About 85% to 90% of infants with congenital CMV that results from primary maternal infection have no symptoms at birth. Among the 10% to 15% of infants that do have symptoms, petechial rash, jaundice, and hepatosplenomegaly are the most common manifestations (“blueberry muffin baby”). Approximately 10% to 20% of infants in this group have evidence of chorioretinitis on ophthalmologic examination, and 50% show either microcephaly or low birth weight.22Among survivors of symptomatic congenital CMV, more than 50% have long-term neurologic morbidities that may include sensorineural hearing loss, seizures, vision impairment, and developmental disabilities. Note that even when neonates appear asymptomatic at birth (regardless of whether infection is primary or secondary), 5% may develop microcephaly and motor deficits, 10% go on to develop sensorineural hearing loss, and the overall rate of neurologic morbidity reaches 13% to 15%.12,23 Some of the observed deficits manifest at several years of age, and, currently, no models exist for prediction of outcome.
Continue to: Diagnosing CMV infection...
Diagnosing CMV infection
Maternal infection
If maternal CMV infection is suspected based on a symptomatic illness or an abnormal fetal ultrasound exam, the first diagnostic test should be an assessment of IgM and IgG serology. If the former test results are positive and the latter negative, the diagnosis of acute CMV infection is confirmed. A positive serum CMV DNA polymerase chain reaction (PCR) test adds additional assurance that the diagnosis is correct. Primary infection, as noted above, poses the greatest risk of serious injury to the fetus.1
A frequent diagnostic dilemma arises when both the IgM and IgG antibody are positive. Remember that CMV IgM antibody can remain positive for 9 to 12 months after a primary infection and can reappear in the maternal serum in the face of a recurrent or reactivated infection. When confronted by both a positive IgM and positive IgG result, the clinician should then order IgG avidity testing. If the avidity is low to moderate, which reflects poor binding of antibody to the virus, the patient likely has an acute infection. If the avidity is high, which reflects enhanced binding of antibody to virus, the patient probably has a recurrent or reactivated infection; this scenario poses less danger to the developing fetus. The presence of CMV DNA in serum is also more consistent with acute infection, although viremia still can occur with recurrent infection. FIGURE 2 presents a suggested algorithm for the diagnosis of CMV in the pregnant patient.1
If a diagnosis of maternal CMV infection is confirmed, liver function tests should be obtained to determine if CMV hepatitis is present. If the liver function tests are abnormal, a coagulation profile also should be performed to identify the mother who might be at risk for peripartum hemorrhage.
Fetal infection
The single best test for confirmation of congenital CMV infection is detection of viral DNA and quantitation of viral load in the amniotic fluid by PCR. If the amniocentesis is performed prior to 20 weeks’ gestation and is negative, the test should be repeated in approximately 4 weeks.1,19,24
Detection of viral DNA indicates congenital infection. The ultimate task, however, is to determine if the infection has injured the fetus. Detailed ultrasound examination is the key to identifying fetal injury. As noted previously, the principal ultrasonographic findings that suggest congenital CMV infection include2,19,20,21,25:
- hydropic placenta
- fetal growth restriction
- microcephaly (head circumference more than 3 standard deviations below the mean)
- periventricular calcifications
- enlarged liver
- echogenic bowel
- ascites
- fetal hydrops.
Management: Evidence on CMV hyperimmune globulin, valacyclovir
If the immunocompetent mother has clinical manifestations of infection, she should receive symptomatic treatment. She should be encouraged to rest as much as possible, stay well hydrated, and use acetaminophen (1,000 mg every 6 to 8 hours) as needed for malaise and fever.
However, if the mother is immunocompromised and has signs of serious complications, such as chorioretinitis, hepatitis, or pneumonia, more aggressive therapy is indicated. Drugs used in this setting include foscarnet and ganciclovir and are best prescribed in consultation with a medical infectious disease specialist.
At this time, no consistently effective therapy for congenital infection is available. Therefore, if a patient has primary CMV infection in the first half of pregnancy, particularly in the first trimester, she should be counseled that the risk of fetal infection is approximately 40% and that approximately 5% to 15% of infants will be severely affected at birth. Given this information, some patients may opt for pregnancy termination.
In 2005, a report from Nigro and colleagues stimulated great hope that CMV-specific hyperimmune globulin (CytoGam) might be of value for both treatment and prophylaxis for congenital infection.26 These authors studied 157 women with confirmed primary CMV infection. One-hundred forty-eight women were asymptomatic and were identified by routine serologic screening, 8 had symptomatic infection, and 1 was identified because of abnormal fetal ultrasound findings. Forty-five women had CMV detected in amniotic fluid by PCR or culture more than 6 weeks before study enrollment. Thirty-one of these women were treated with intravenous hyperimmune globulin (200 U or 200 mg/kg maternal body weight); 14 declined treatment. Seven of the latter women had infants who were acutely symptomatic at the time of delivery; only 1 of the 31 treated women had an affected neonate (adjusted odds ratio [OR], 0.02; P<.001). In this same study, 84 women did not have a diagnostic amniocentesis because their infection occurred within 6 weeks of enrollment, their gestational age was less than 20 weeks, or they declined the procedure. Thirty-seven of these women received hyperimmune globulin (100 U or 100 mg/kg) every month until delivery, and 47 declined treatment. Six of the treated women delivered infected infants compared with 19 of the untreated women (adjusted OR, 0.32; P<.04).
Although these results were quite encouraging, several problems existed with the study’s design, as noted in an editorial that accompanied the study’s publication.27 First, the study was not randomized or placebo controlled. Second, patients were not stratified based on the severity of fetal ultrasound abnormalities. Third, the dosing of hyperimmune globulin varied; 9 of the 31 patients in the treatment group received additional infusions of drug into either the amniotic fluid or fetal umbilical vein. Moreover, patients in the prophylaxis group actually received a higher cumulative dose of hyperimmune globulin than patients in the treatment group.
Two subsequent investigations that were better designed were unable to verify the effectiveness of hyperimmune globulin. In 2014, Revello and colleagues reported the results of a prospective, randomized, placebo-controlled, double-blinded study of 124 women at 5 to 26 weeks’ gestation with confirmed primary CMV infection.28 The rate of congenital infection was 30% in the group treated with hyperimmune globulin and 44% in the placebo group (P=.13). There also was no significant difference in the concentration of serum CMV DNA in treated versus untreated mothers. Moreover, the number of adverse obstetric events (preterm delivery, fetal growth restriction, intrahepatic cholestasis of pregnancy, and postpartum preeclampsia) in the treatment group was higher than in the placebo group, 13% versus 2%.
In 2021, Hughes and colleagues published the results of a multicenter, double-blind trial in 399 women who had a diagnosis of primary CMV infection before 23 weeks’ gestation.29 The primary outcome was defined as a composite of congenital CMV infection or fetal/neonatal death. An adverse primary outcome occurred in 22.7% of the patients who received hyperimmune globulin and 19.4% of those who received placebo (relative risk, 1.17; 95% confidence interval [CI], 0.80–1.72; P=.42).
Continue to: Jacquemard and colleagues...
Jacquemard and colleagues then proposed a different approach.30 In a small pilot study of 20 patients, these authors used high doses of oral valacylovir (2 g 4 times daily) and documented therapeutic drug concentrations and a decline in CMV viral load in fetal serum. Patients were not stratified by severity of fetal injury at onset of treatment, so the authors were unable to define which fetuses were most likely to benefit from treatment.
In a follow-up investigation, Leruez-Ville and colleagues reported another small series in which high-dose oral valacyclovir (8 g daily) was used for treatment.31 They excluded fetuses with severe brain anomalies and fetuses with no sonographic evidence of injury. The median gestational age at diagnosis was 26 weeks. Thirty-four of 43 treated fetuses were free of injury at birth. In addition, the viral load in the neonate’s serum decreased significantly after treatment, and the platelet count increased. The authors then compared these outcomes to a historical cohort and confirmed that treatment increased the proportion of asymptomatic neonates from 43% without treatment to 82% with treatment (P<.05 with no overlapping confidence intervals).
We conclude from these investigations that hyperimmune globulin is unlikely to be of value in treating congenital CMV infection, especially if the fetus already has sonographic findings of severe injury. High-dose oral valacyclovir also is unlikely to be of value in severely affected fetuses, particularly those with evidence of CNS injury. However, antiviral therapy may be of modest value in situations when the fetus is less severely injured.
Preventive measures
Since no definitive treatment is available for congenital CMV infection, our efforts as clinicians should focus on measures that may prevent transmission of infection to the pregnant patient. These measures include:
- Encouraging patients to use careful handwashing techniques when handling infant diapers and toys.
- Encouraging patients to adopt safe sexual practices if not already engaged in a mutually faithful, monogamous relationship.
- Using CMV-negative blood when transfusing a pregnant woman or a fetus.
At the present time, unfortunately, a readily available and highly effective therapy for prevention of CMV infection is not available.
CASE Congenital infection diagnosed
The ultrasound findings are most consistent with congenital CMV infection, especially given the patient’s work as an elementary schoolteacher. The diagnosis of maternal infection is best established by conventional serology (positive IgM, negative IgM) and detection of viral DNA in maternal blood by PCR testing. The diagnosis of congenital infection is best confirmed by documentation of viral DNA in the amniotic fluid by PCR testing. Given that this fetus already has evidence of moderate to severe injury, no treatment is likely to be effective in reversing the abnormal ultrasound findings. Pregnancy termination may be an option, depending upon the patient’s desires and the legal restrictions prevalent in the patient’s geographic area. ●
- Cytomegalovirus infection is the most common of the perinatally transmitted infections.
- Maternal infection is often asymptomatic. When symptoms are present, they resemble those of an influenza-like illness. In immunocompromised persons, however, CMV may cause serious complications, including pneumonia, hepatitis, and chorioretinitis.
- The virus is transmitted by contact with contaminated body fluids, such as saliva, urine, blood, and genital secretions.
- The greatest risk of severe fetal injury results from primary maternal infection in the first trimester of pregnancy.
- Manifestations of severe congenital CMV infection include growth restriction, microcephaly, ventriculomegaly, hepatosplenomegaly, ascites, chorioretinitis, thrombocytopenia, purpura, and hydrops (“blueberry muffin baby”).
- Late manifestations of infection, which usually follow recurrent maternal infection, may appear as a child enters elementary school and include visual and auditory deficits, developmental delays, and learning disabilities.
- The diagnosis of maternal infection is confirmed by serology and detection of viral DNA in the serum by PCR testing.
- The diagnosis of fetal infection is best made by a combination of abnormal ultrasound findings and detection of CMV DNA in amniotic fluid. The characteristic ultrasound findings include placentomegaly, microcephaly, ventriculomegaly, growth restriction, echogenic bowel, and serous effusions/hydrops.
- Treatment of the mother with antiviral medications such as valacyclovir may be of modest value in reducing placental edema, decreasing viral load in the fetus, and hastening the resolution of some ultrasound findings, such as echogenic bowel.
- While initial studies seemed promising, the use of hyperimmune globulin has not proven to be consistently effective in treating congenital infection.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik’s Maternal Fetal Medicine: Principles and Practice. 8th ed. 2019:888-890.
- Chatzakis C, Ville Y, Makrydimas G, et al. Timing of primary maternal cytomegalovirus infection and rates of vertical transmission and fetal consequences. Am J Obstet Gynecol. 2020;223:870-883.e11. doi:10.1016/j.ajog.2020.05.038
- Kelly MS, Benjamin DK, Puopolo KM, et al. Postnatal cytomegalovirus infection and the risk for bronchopulmonary dysplasia. JAMA Pediatr. 2015;169:e153785. doi:10.1001 /jamapediatrics.2015.3785
- Messinger CJ, Lipsitch M, Bateman BT, et al. Association between congenital cytomegalovirus and the prevalence at birth of microcephaly in the United States. JAMA Pediatr. 2020;174:1159-1167. doi:10.1001/jamapediatrics.2020.3009
- De Cuyper E, Acke F, Keymeulen A, et al. Risk factors for hearing loss at birth in newborns with congenital cytomegalovirus infection. JAMA Otolaryngol Head Neck Surg. 2023;149:122-130. doi:10.1001/jamaoto.2022.4109
- Colugnati FA, Staras SA, Dollard SC, et al. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect Dis. 2007;7:71. doi:10.1186/1471-2334-7-71
- Stagno S, Pass RF, Cloud G, et al. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA. 1986;256:1904-1908.
- Wreghitt TG, Teare EL, Sule O, et al. Cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 2003;37:1603-1606. doi:10.1086/379711
- Fowler KB, Stagno S, Pass RF, et al. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med. 1992;326:663-667. doi:10.1056 /NEJM199203053261003
- Faure-Bardon V, Magny JF, Parodi M, et al. Sequelae of congenital cytomegalovirus following maternal primary infections are limited to those acquired in the first trimester of pregnancy. Clin Infect Dis. 2019;69:1526-1532. doi:10.1093/ cid/ciy1128
- Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17:253-276. doi:10.1002/ rmv.535
- Boppana SB, Pass RF, Britt WJ, et al. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J. 1992;11:93-99. doi:10.1097/00006454-199202000-00007
- Ross SA, Fowler KB, Ashrith G, et al. Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity. J Pediatr. 2006;148:332-336. doi:10.1016/j.jpeds.2005.09.003
- Zalel Y, Gilboa Y, Berkenshtat M, et al. Secondary cytomegalovirus infection can cause severe fetal sequelae despite maternal preconceptional immunity. Ultrasound Obstet Gynecol. 31:417-420. doi:10.1002/uog.5255
- Scaramuzzino F, Di Pastena M, Chiurchiu S, et al. Secondary cytomegalovirus infections: how much do we still not know? Comparison of children with symptomatic congenital cytomegalovirus born to mothers with primary and secondary infection. Front Pediatr. 2022;10:885926. doi:10.3389/fped.2022.885926
- Gindes L, Teperberg-Oikawa M, Sherman D, et al. Congenital cytomegalovirus infection following primary maternal infection in the third trimester. BJOG. 2008;115:830-835. doi:10.1111/j.1471-0528.2007.01651.x
- Hadar E, Dorfman E, Bardin R, et al. Symptomatic congenital cytomegalovirus disease following non-primary maternal infection: a retrospective cohort study. BMC Infect Dis. 2017;17:31. doi:10.1186/s12879-016-2161-3
- Elkan Miller T, Weisz B, Yinon Y, et al. Congenital cytomegalovirus infection following second and third trimester maternal infection is associated with mild childhood adverse outcome not predicted by prenatal imaging. J Pediatric Infect Dis Soc. 2021;10:562-568. doi:10.1093/jpids/ piaa154
- Lipitz S, Yinon Y, Malinger G, et al. Risk of cytomegalovirusassociated sequelae in relation to time of infection and findings on prenatal imaging. Ultrasound Obstet Gynecol. 2013;41:508-514. doi:10.1002/uog.12377
- Lipitz S, Elkan Miller T, Yinon Y, et al. Revisiting short- and long-term outcome after fetal first-trimester primary cytomegalovirus infection in relation to prenatal imaging findings. Ultrasound Obstet Gynecol. 2020;56:572-578. doi:10.1002/uog.21946
- Buca D, Di Mascio D, Rizzo G, et al. Outcome of fetuses with congenital cytomegalovirus infection and normal ultrasound at diagnosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2021;57:551-559. doi:10.1002/uog.23143
- Boppana SB, Ross SA, Fowler KB. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis. 2013;57 (suppl 4):S178-S181. doi:10.1093/cid/cit629
- Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17:355-363. doi:10.1002/rmv.544
- Hughes BL, Gyamfi-Bannerman C. Diagnosis and antenatal management of congenital cytomegalovirus infection. Am J Obstet Gynecol. 2016;214:B5-11. doi:10.1016 /j.ajog.2016.02.042
- Rouse DJ, Fette LM, Hughes BL, et al. Noninvasive prediction of congenital cytomegalovirus infection after maternal primary infection. Obstet Gynecol. 2022;139:400-406. doi:10.1097/AOG.0000000000004691
- Nigro G, Adler SP, La Torre R, et al; Congenital Cytomegalovirus Collaborating Group. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350-1362. doi:10.1056/NEJMoa043337
- Duff P. Immunotherapy for congenital cytomegalovirus infection. N Engl J Med. 2005;355:1402-1404. doi:10.1056 /NEJMe058172
- Revello MG, Lazzarotto T, Guerra B, et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med. 2014;370:1316-1326. doi:10.1056/NEJMoa1310214
- Hughes BL, Clifton RG, Rouse DJ, et al. A trial of hyperimmune globulin to prevent congenital cytomegalovirus infection. N Engl J Med. 2021;385:436-444. doi:10.1056/NEJMoa1913569
- Jacquemard F, Yamamoto M, Costa JM, et al. Maternal administration of valaciclovir in symptomatic intrauterine cytomegalovirus infection. BJOG. 2007;114:1113-1121. doi:10.1111/j.1471-0528.2007.01308.x
- Leruez-Ville M, Ghout I, Bussières L, et al. In utero treatment of congenital cytomegalovirus infection with valacyclovir in a multicenter, open-label, phase II study. Am J Obstet Gynecol. 2016;215:462.e1-462.e10. doi:10.1016/j.ajog.2016.04.003
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik’s Maternal Fetal Medicine: Principles and Practice. 8th ed. 2019:888-890.
- Chatzakis C, Ville Y, Makrydimas G, et al. Timing of primary maternal cytomegalovirus infection and rates of vertical transmission and fetal consequences. Am J Obstet Gynecol. 2020;223:870-883.e11. doi:10.1016/j.ajog.2020.05.038
- Kelly MS, Benjamin DK, Puopolo KM, et al. Postnatal cytomegalovirus infection and the risk for bronchopulmonary dysplasia. JAMA Pediatr. 2015;169:e153785. doi:10.1001 /jamapediatrics.2015.3785
- Messinger CJ, Lipsitch M, Bateman BT, et al. Association between congenital cytomegalovirus and the prevalence at birth of microcephaly in the United States. JAMA Pediatr. 2020;174:1159-1167. doi:10.1001/jamapediatrics.2020.3009
- De Cuyper E, Acke F, Keymeulen A, et al. Risk factors for hearing loss at birth in newborns with congenital cytomegalovirus infection. JAMA Otolaryngol Head Neck Surg. 2023;149:122-130. doi:10.1001/jamaoto.2022.4109
- Colugnati FA, Staras SA, Dollard SC, et al. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect Dis. 2007;7:71. doi:10.1186/1471-2334-7-71
- Stagno S, Pass RF, Cloud G, et al. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA. 1986;256:1904-1908.
- Wreghitt TG, Teare EL, Sule O, et al. Cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 2003;37:1603-1606. doi:10.1086/379711
- Fowler KB, Stagno S, Pass RF, et al. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med. 1992;326:663-667. doi:10.1056 /NEJM199203053261003
- Faure-Bardon V, Magny JF, Parodi M, et al. Sequelae of congenital cytomegalovirus following maternal primary infections are limited to those acquired in the first trimester of pregnancy. Clin Infect Dis. 2019;69:1526-1532. doi:10.1093/ cid/ciy1128
- Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17:253-276. doi:10.1002/ rmv.535
- Boppana SB, Pass RF, Britt WJ, et al. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J. 1992;11:93-99. doi:10.1097/00006454-199202000-00007
- Ross SA, Fowler KB, Ashrith G, et al. Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity. J Pediatr. 2006;148:332-336. doi:10.1016/j.jpeds.2005.09.003
- Zalel Y, Gilboa Y, Berkenshtat M, et al. Secondary cytomegalovirus infection can cause severe fetal sequelae despite maternal preconceptional immunity. Ultrasound Obstet Gynecol. 31:417-420. doi:10.1002/uog.5255
- Scaramuzzino F, Di Pastena M, Chiurchiu S, et al. Secondary cytomegalovirus infections: how much do we still not know? Comparison of children with symptomatic congenital cytomegalovirus born to mothers with primary and secondary infection. Front Pediatr. 2022;10:885926. doi:10.3389/fped.2022.885926
- Gindes L, Teperberg-Oikawa M, Sherman D, et al. Congenital cytomegalovirus infection following primary maternal infection in the third trimester. BJOG. 2008;115:830-835. doi:10.1111/j.1471-0528.2007.01651.x
- Hadar E, Dorfman E, Bardin R, et al. Symptomatic congenital cytomegalovirus disease following non-primary maternal infection: a retrospective cohort study. BMC Infect Dis. 2017;17:31. doi:10.1186/s12879-016-2161-3
- Elkan Miller T, Weisz B, Yinon Y, et al. Congenital cytomegalovirus infection following second and third trimester maternal infection is associated with mild childhood adverse outcome not predicted by prenatal imaging. J Pediatric Infect Dis Soc. 2021;10:562-568. doi:10.1093/jpids/ piaa154
- Lipitz S, Yinon Y, Malinger G, et al. Risk of cytomegalovirusassociated sequelae in relation to time of infection and findings on prenatal imaging. Ultrasound Obstet Gynecol. 2013;41:508-514. doi:10.1002/uog.12377
- Lipitz S, Elkan Miller T, Yinon Y, et al. Revisiting short- and long-term outcome after fetal first-trimester primary cytomegalovirus infection in relation to prenatal imaging findings. Ultrasound Obstet Gynecol. 2020;56:572-578. doi:10.1002/uog.21946
- Buca D, Di Mascio D, Rizzo G, et al. Outcome of fetuses with congenital cytomegalovirus infection and normal ultrasound at diagnosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2021;57:551-559. doi:10.1002/uog.23143
- Boppana SB, Ross SA, Fowler KB. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis. 2013;57 (suppl 4):S178-S181. doi:10.1093/cid/cit629
- Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17:355-363. doi:10.1002/rmv.544
- Hughes BL, Gyamfi-Bannerman C. Diagnosis and antenatal management of congenital cytomegalovirus infection. Am J Obstet Gynecol. 2016;214:B5-11. doi:10.1016 /j.ajog.2016.02.042
- Rouse DJ, Fette LM, Hughes BL, et al. Noninvasive prediction of congenital cytomegalovirus infection after maternal primary infection. Obstet Gynecol. 2022;139:400-406. doi:10.1097/AOG.0000000000004691
- Nigro G, Adler SP, La Torre R, et al; Congenital Cytomegalovirus Collaborating Group. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350-1362. doi:10.1056/NEJMoa043337
- Duff P. Immunotherapy for congenital cytomegalovirus infection. N Engl J Med. 2005;355:1402-1404. doi:10.1056 /NEJMe058172
- Revello MG, Lazzarotto T, Guerra B, et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med. 2014;370:1316-1326. doi:10.1056/NEJMoa1310214
- Hughes BL, Clifton RG, Rouse DJ, et al. A trial of hyperimmune globulin to prevent congenital cytomegalovirus infection. N Engl J Med. 2021;385:436-444. doi:10.1056/NEJMoa1913569
- Jacquemard F, Yamamoto M, Costa JM, et al. Maternal administration of valaciclovir in symptomatic intrauterine cytomegalovirus infection. BJOG. 2007;114:1113-1121. doi:10.1111/j.1471-0528.2007.01308.x
- Leruez-Ville M, Ghout I, Bussières L, et al. In utero treatment of congenital cytomegalovirus infection with valacyclovir in a multicenter, open-label, phase II study. Am J Obstet Gynecol. 2016;215:462.e1-462.e10. doi:10.1016/j.ajog.2016.04.003
Second infection hikes long COVID risk: Expert Q&A
Those are two of the most striking findings of a comprehensive new research study of 138,000 veterans.
Lead researcher Ziyad Al-Aly, MD, chief of research at Veterans Affairs St. Louis Health Care and clinical epidemiologist at Washington University in St. Louis, spoke with this news organization about his team’s findings, what we know – and don’t – about long COVID, and what it means for physicians treating patients with the condition.
Excerpts of the interview follow.
Your research concluded that for those infected early in the pandemic, some long COVID symptoms declined over 2 years, but some did not. You have also concluded that long COVID is a chronic disease. Why?
We’ve been in this journey a little bit more than three and a half years. Some patients do experience some recovery. But that’s not the norm. Most people do not really fully recover. The health trajectory for people with long COVID is really very heterogeneous. There is no one-size-fits-all. There’s really no one line that I could give you that could cover all your patients. But it is very, very, very clear that a bunch of them experienced long COVID for sure; that’s really happening.
It happened in the pre-Delta era and in the Delta era, and with Omicron subvariants, even now. There are people who think, “This is a nothing-burger anymore,” or “It’s not an issue anymore.” It’s still happening with the current variants. Vaccines do reduce risk for long COVID, but do not completely eliminate the risk for long COVID.
You work with patients with long COVID in the clinic and also analyze data from thousands more. If long COVID does not go away, what should doctors look for in everyday practice that will help them recognize and help patients with long COVID?
Long COVID is not uncommon. We see it in the clinic in large numbers. Whatever clinic you’re running – if you’re running a cardiology clinic, or a nephrology clinic, or diabetes, or primary care – probably some of your people have it. You may not know about it. They may not tell you about it. You may not recognize it.
Not all long COVID is the same, and that’s really what makes it complex and makes it really hard to deal with in the clinic. But that’s the reality that we’re all dealing with. And it’s multisystemic; it’s not like it affects the heart only, the brain only, or the autonomic nervous system only. It does not behave in the same way in different individuals – they may have different manifestations, various health trajectories, and different outcomes. It’s important for doctors to get up to speed on long COVID as a multisystem illness.
Management at this point is really managing the symptoms. We don’t have a treatment for it; we don’t have a cure for it.
Some patients experience what you’ve described as partial recovery. What does that look like?
Some individuals do experience some recovery over time, but for most individuals, the recovery is long and arduous. Long COVID can last with them for many years. Some people may come back to the clinic and say, “I’m doing better,” but if you really flesh it out and dig deeper, they didn’t do better; they adjusted to a new baseline. They used to walk the dog three to four blocks, and now they walk the dog only half a block. They used to do an activity with their partner every Saturday or Sunday, and now they do half of that.
If you’re a physician, a primary care provider, or any other provider who is dealing with a patient with long COVID, know that this is really happening. It can happen even in vaccinated individuals. The presentation is heterogeneous. Some people may present to you with and say. “Well, before I had COVID I was mentally sharp and now having I’m having difficulty with memory, etc.” It can sometimes present as fatigue or postexertional malaise.
In some instances, it can present as sleep problems. It can present as what we call postural orthostatic tachycardia syndrome (POTS). Those people get a significant increase in heart rate with postural changes.
What the most important thing we can we learn from the emergence of long COVID?
This whole thing taught us that infections can cause chronic disease. That’s really the No. 1 lesson that I take from this pandemic – that infections can cause chronic disease.
Looking at only acute illness from COVID is really only looking at the tip of the iceberg. Beneath that tip of the iceberg lies this hidden toll of disease that we don’t really talk about that much.
This pandemic shone a very, very good light on the idea that there is really an intimate connection between infections and chronic disease. It was really hardwired into our medical training as doctors that most infections, when people get over the hump of the acute phase of the disease, it’s all behind them. I think long COVID has humbled us in many, many ways, but chief among those is the realization – the stark realization – that infections can cause chronic disease.
That’s really going back to your [first] question: What does it mean that some people are not recovering? They actually have chronic illness. I’m hoping that we will find a treatment, that we’ll start finding things that would help them get back to baseline. But at this point in time, what we’re dealing with is people with chronic illness or chronic disease that may continue to affect them for many years to come in the absence of a treatment or a cure.
A version of this article first appeared on Medscape.com.
Those are two of the most striking findings of a comprehensive new research study of 138,000 veterans.
Lead researcher Ziyad Al-Aly, MD, chief of research at Veterans Affairs St. Louis Health Care and clinical epidemiologist at Washington University in St. Louis, spoke with this news organization about his team’s findings, what we know – and don’t – about long COVID, and what it means for physicians treating patients with the condition.
Excerpts of the interview follow.
Your research concluded that for those infected early in the pandemic, some long COVID symptoms declined over 2 years, but some did not. You have also concluded that long COVID is a chronic disease. Why?
We’ve been in this journey a little bit more than three and a half years. Some patients do experience some recovery. But that’s not the norm. Most people do not really fully recover. The health trajectory for people with long COVID is really very heterogeneous. There is no one-size-fits-all. There’s really no one line that I could give you that could cover all your patients. But it is very, very, very clear that a bunch of them experienced long COVID for sure; that’s really happening.
It happened in the pre-Delta era and in the Delta era, and with Omicron subvariants, even now. There are people who think, “This is a nothing-burger anymore,” or “It’s not an issue anymore.” It’s still happening with the current variants. Vaccines do reduce risk for long COVID, but do not completely eliminate the risk for long COVID.
You work with patients with long COVID in the clinic and also analyze data from thousands more. If long COVID does not go away, what should doctors look for in everyday practice that will help them recognize and help patients with long COVID?
Long COVID is not uncommon. We see it in the clinic in large numbers. Whatever clinic you’re running – if you’re running a cardiology clinic, or a nephrology clinic, or diabetes, or primary care – probably some of your people have it. You may not know about it. They may not tell you about it. You may not recognize it.
Not all long COVID is the same, and that’s really what makes it complex and makes it really hard to deal with in the clinic. But that’s the reality that we’re all dealing with. And it’s multisystemic; it’s not like it affects the heart only, the brain only, or the autonomic nervous system only. It does not behave in the same way in different individuals – they may have different manifestations, various health trajectories, and different outcomes. It’s important for doctors to get up to speed on long COVID as a multisystem illness.
Management at this point is really managing the symptoms. We don’t have a treatment for it; we don’t have a cure for it.
Some patients experience what you’ve described as partial recovery. What does that look like?
Some individuals do experience some recovery over time, but for most individuals, the recovery is long and arduous. Long COVID can last with them for many years. Some people may come back to the clinic and say, “I’m doing better,” but if you really flesh it out and dig deeper, they didn’t do better; they adjusted to a new baseline. They used to walk the dog three to four blocks, and now they walk the dog only half a block. They used to do an activity with their partner every Saturday or Sunday, and now they do half of that.
If you’re a physician, a primary care provider, or any other provider who is dealing with a patient with long COVID, know that this is really happening. It can happen even in vaccinated individuals. The presentation is heterogeneous. Some people may present to you with and say. “Well, before I had COVID I was mentally sharp and now having I’m having difficulty with memory, etc.” It can sometimes present as fatigue or postexertional malaise.
In some instances, it can present as sleep problems. It can present as what we call postural orthostatic tachycardia syndrome (POTS). Those people get a significant increase in heart rate with postural changes.
What the most important thing we can we learn from the emergence of long COVID?
This whole thing taught us that infections can cause chronic disease. That’s really the No. 1 lesson that I take from this pandemic – that infections can cause chronic disease.
Looking at only acute illness from COVID is really only looking at the tip of the iceberg. Beneath that tip of the iceberg lies this hidden toll of disease that we don’t really talk about that much.
This pandemic shone a very, very good light on the idea that there is really an intimate connection between infections and chronic disease. It was really hardwired into our medical training as doctors that most infections, when people get over the hump of the acute phase of the disease, it’s all behind them. I think long COVID has humbled us in many, many ways, but chief among those is the realization – the stark realization – that infections can cause chronic disease.
That’s really going back to your [first] question: What does it mean that some people are not recovering? They actually have chronic illness. I’m hoping that we will find a treatment, that we’ll start finding things that would help them get back to baseline. But at this point in time, what we’re dealing with is people with chronic illness or chronic disease that may continue to affect them for many years to come in the absence of a treatment or a cure.
A version of this article first appeared on Medscape.com.
Those are two of the most striking findings of a comprehensive new research study of 138,000 veterans.
Lead researcher Ziyad Al-Aly, MD, chief of research at Veterans Affairs St. Louis Health Care and clinical epidemiologist at Washington University in St. Louis, spoke with this news organization about his team’s findings, what we know – and don’t – about long COVID, and what it means for physicians treating patients with the condition.
Excerpts of the interview follow.
Your research concluded that for those infected early in the pandemic, some long COVID symptoms declined over 2 years, but some did not. You have also concluded that long COVID is a chronic disease. Why?
We’ve been in this journey a little bit more than three and a half years. Some patients do experience some recovery. But that’s not the norm. Most people do not really fully recover. The health trajectory for people with long COVID is really very heterogeneous. There is no one-size-fits-all. There’s really no one line that I could give you that could cover all your patients. But it is very, very, very clear that a bunch of them experienced long COVID for sure; that’s really happening.
It happened in the pre-Delta era and in the Delta era, and with Omicron subvariants, even now. There are people who think, “This is a nothing-burger anymore,” or “It’s not an issue anymore.” It’s still happening with the current variants. Vaccines do reduce risk for long COVID, but do not completely eliminate the risk for long COVID.
You work with patients with long COVID in the clinic and also analyze data from thousands more. If long COVID does not go away, what should doctors look for in everyday practice that will help them recognize and help patients with long COVID?
Long COVID is not uncommon. We see it in the clinic in large numbers. Whatever clinic you’re running – if you’re running a cardiology clinic, or a nephrology clinic, or diabetes, or primary care – probably some of your people have it. You may not know about it. They may not tell you about it. You may not recognize it.
Not all long COVID is the same, and that’s really what makes it complex and makes it really hard to deal with in the clinic. But that’s the reality that we’re all dealing with. And it’s multisystemic; it’s not like it affects the heart only, the brain only, or the autonomic nervous system only. It does not behave in the same way in different individuals – they may have different manifestations, various health trajectories, and different outcomes. It’s important for doctors to get up to speed on long COVID as a multisystem illness.
Management at this point is really managing the symptoms. We don’t have a treatment for it; we don’t have a cure for it.
Some patients experience what you’ve described as partial recovery. What does that look like?
Some individuals do experience some recovery over time, but for most individuals, the recovery is long and arduous. Long COVID can last with them for many years. Some people may come back to the clinic and say, “I’m doing better,” but if you really flesh it out and dig deeper, they didn’t do better; they adjusted to a new baseline. They used to walk the dog three to four blocks, and now they walk the dog only half a block. They used to do an activity with their partner every Saturday or Sunday, and now they do half of that.
If you’re a physician, a primary care provider, or any other provider who is dealing with a patient with long COVID, know that this is really happening. It can happen even in vaccinated individuals. The presentation is heterogeneous. Some people may present to you with and say. “Well, before I had COVID I was mentally sharp and now having I’m having difficulty with memory, etc.” It can sometimes present as fatigue or postexertional malaise.
In some instances, it can present as sleep problems. It can present as what we call postural orthostatic tachycardia syndrome (POTS). Those people get a significant increase in heart rate with postural changes.
What the most important thing we can we learn from the emergence of long COVID?
This whole thing taught us that infections can cause chronic disease. That’s really the No. 1 lesson that I take from this pandemic – that infections can cause chronic disease.
Looking at only acute illness from COVID is really only looking at the tip of the iceberg. Beneath that tip of the iceberg lies this hidden toll of disease that we don’t really talk about that much.
This pandemic shone a very, very good light on the idea that there is really an intimate connection between infections and chronic disease. It was really hardwired into our medical training as doctors that most infections, when people get over the hump of the acute phase of the disease, it’s all behind them. I think long COVID has humbled us in many, many ways, but chief among those is the realization – the stark realization – that infections can cause chronic disease.
That’s really going back to your [first] question: What does it mean that some people are not recovering? They actually have chronic illness. I’m hoping that we will find a treatment, that we’ll start finding things that would help them get back to baseline. But at this point in time, what we’re dealing with is people with chronic illness or chronic disease that may continue to affect them for many years to come in the absence of a treatment or a cure.
A version of this article first appeared on Medscape.com.
Medicare 2024 base rate cut triggers calls for pay overhaul
Physicians in 2024 can expect a 3.4% drop in the conversion factor that determines their base Medicare pay, according to federal officials, but they also will receive more money for primary care and treating complex conditions.
The Centers for Medicare & Medicaid Services on Nov. 2 released its 2024 final physician fee schedule, triggering renewed concerns from doctors’ groups, who protested CMS’ cuts when they were first previewed earlier in 2023.
The 2024 conversion factor, or base rate for clinician pay, will be $32.74, a decrease of $1.15, or 3.4%, from 2023’s level. The pay cuts come as costs of providing health care are expected to rise as much as 4.6% in 2024, the American Medical Association said.
The new rule follows a 2% payment reduction in 2023, AMA president Jesse M. Ehrenfeld, MD, MPH, said in a statement.
“This is a recipe for financial instability,” Dr. Ehrenfeld said. “Patients and physicians will wonder why such thin gruel is being served.”
The AMA is among the many physician groups pressing Congress to change its approach to paying clinicians and consider inflation rates in determining future payments.
Medicare already includes automatic inflation adjusters in other payment rules, such as the ones for care provided in hospitals. But Congress in 2015 eliminated this feature for the physician fee schedule when it passed the Medicare Access and CHIP Reauthorization Act.
A pending House bill, the bipartisan Strengthening Medicare for Patients and Providers Act (H.R.2474), would return to permanently including a broader inflation adjuster in the Medicare physician fee schedule.
“This long-overdue change would not only help provide greater stability within the Medicare payment system, but it would also help physicians’ practices – many of whom operate as small business owners – more effectively navigate the ever-changing economic factors that impact their practices, including rising medical costs, workforce and labor challenges, administrative burdens, office rental prices and more,” Larry Bucshon, MD (R-Ind.), Ami Bera, MD (D-Calif.), Raul Ruiz, MD (D-Calif.), and Mariannette Miller-Meeks, MD (R-Iowa), wrote in an opinion article in the newspaper The Hill.
Major changes to determining Medicare physician pay remain unlikely in 2023. Still, Congress has softened or blocked slated cuts in physician pay in recent years, passing temporary “doc fixes” as add-ons to spending packages.
E/M add-on payment
“We’re encouraged to see that CMS listened to our concerns and extended telehealth flexibilities as well as implemented the G2211 code, which will help Medicare beneficiaries and their physicians better manage complex and chronic rheumatic diseases,” said Douglas White, MD, PhD, president of the ACR.
A version of this article first appeared on Medscape.com.
Physicians in 2024 can expect a 3.4% drop in the conversion factor that determines their base Medicare pay, according to federal officials, but they also will receive more money for primary care and treating complex conditions.
The Centers for Medicare & Medicaid Services on Nov. 2 released its 2024 final physician fee schedule, triggering renewed concerns from doctors’ groups, who protested CMS’ cuts when they were first previewed earlier in 2023.
The 2024 conversion factor, or base rate for clinician pay, will be $32.74, a decrease of $1.15, or 3.4%, from 2023’s level. The pay cuts come as costs of providing health care are expected to rise as much as 4.6% in 2024, the American Medical Association said.
The new rule follows a 2% payment reduction in 2023, AMA president Jesse M. Ehrenfeld, MD, MPH, said in a statement.
“This is a recipe for financial instability,” Dr. Ehrenfeld said. “Patients and physicians will wonder why such thin gruel is being served.”
The AMA is among the many physician groups pressing Congress to change its approach to paying clinicians and consider inflation rates in determining future payments.
Medicare already includes automatic inflation adjusters in other payment rules, such as the ones for care provided in hospitals. But Congress in 2015 eliminated this feature for the physician fee schedule when it passed the Medicare Access and CHIP Reauthorization Act.
A pending House bill, the bipartisan Strengthening Medicare for Patients and Providers Act (H.R.2474), would return to permanently including a broader inflation adjuster in the Medicare physician fee schedule.
“This long-overdue change would not only help provide greater stability within the Medicare payment system, but it would also help physicians’ practices – many of whom operate as small business owners – more effectively navigate the ever-changing economic factors that impact their practices, including rising medical costs, workforce and labor challenges, administrative burdens, office rental prices and more,” Larry Bucshon, MD (R-Ind.), Ami Bera, MD (D-Calif.), Raul Ruiz, MD (D-Calif.), and Mariannette Miller-Meeks, MD (R-Iowa), wrote in an opinion article in the newspaper The Hill.
Major changes to determining Medicare physician pay remain unlikely in 2023. Still, Congress has softened or blocked slated cuts in physician pay in recent years, passing temporary “doc fixes” as add-ons to spending packages.
E/M add-on payment
“We’re encouraged to see that CMS listened to our concerns and extended telehealth flexibilities as well as implemented the G2211 code, which will help Medicare beneficiaries and their physicians better manage complex and chronic rheumatic diseases,” said Douglas White, MD, PhD, president of the ACR.
A version of this article first appeared on Medscape.com.
Physicians in 2024 can expect a 3.4% drop in the conversion factor that determines their base Medicare pay, according to federal officials, but they also will receive more money for primary care and treating complex conditions.
The Centers for Medicare & Medicaid Services on Nov. 2 released its 2024 final physician fee schedule, triggering renewed concerns from doctors’ groups, who protested CMS’ cuts when they were first previewed earlier in 2023.
The 2024 conversion factor, or base rate for clinician pay, will be $32.74, a decrease of $1.15, or 3.4%, from 2023’s level. The pay cuts come as costs of providing health care are expected to rise as much as 4.6% in 2024, the American Medical Association said.
The new rule follows a 2% payment reduction in 2023, AMA president Jesse M. Ehrenfeld, MD, MPH, said in a statement.
“This is a recipe for financial instability,” Dr. Ehrenfeld said. “Patients and physicians will wonder why such thin gruel is being served.”
The AMA is among the many physician groups pressing Congress to change its approach to paying clinicians and consider inflation rates in determining future payments.
Medicare already includes automatic inflation adjusters in other payment rules, such as the ones for care provided in hospitals. But Congress in 2015 eliminated this feature for the physician fee schedule when it passed the Medicare Access and CHIP Reauthorization Act.
A pending House bill, the bipartisan Strengthening Medicare for Patients and Providers Act (H.R.2474), would return to permanently including a broader inflation adjuster in the Medicare physician fee schedule.
“This long-overdue change would not only help provide greater stability within the Medicare payment system, but it would also help physicians’ practices – many of whom operate as small business owners – more effectively navigate the ever-changing economic factors that impact their practices, including rising medical costs, workforce and labor challenges, administrative burdens, office rental prices and more,” Larry Bucshon, MD (R-Ind.), Ami Bera, MD (D-Calif.), Raul Ruiz, MD (D-Calif.), and Mariannette Miller-Meeks, MD (R-Iowa), wrote in an opinion article in the newspaper The Hill.
Major changes to determining Medicare physician pay remain unlikely in 2023. Still, Congress has softened or blocked slated cuts in physician pay in recent years, passing temporary “doc fixes” as add-ons to spending packages.
E/M add-on payment
“We’re encouraged to see that CMS listened to our concerns and extended telehealth flexibilities as well as implemented the G2211 code, which will help Medicare beneficiaries and their physicians better manage complex and chronic rheumatic diseases,” said Douglas White, MD, PhD, president of the ACR.
A version of this article first appeared on Medscape.com.
Patient contact time vs. admin: Is your contract fair?
What’s in a day’s work? For doctors, it’s typically a mix of seeing patients and completing paperwork and follow-up. Often it extends well past the standard workday.
Dennis Hursh, JD, managing partner of Physician Agreements Health Law, a Pennsylvania-based law firm that represents physicians, describes one overwhelmed ob.gyn. who recently consulted him for this problem.
“My client had accepted a position in a group practice where his contract stated he would be working during normal office hours, Monday through Friday, from 8 a.m. to 5 p.m. – in other words, a 40-hour workweek,” Mr. Hursh said.
But the distressed physician discovered that actually, he was working almost twice as many hours. “He’d get to work early to do charting, then see patients during the 40 hours, perhaps grabbing a quick sandwich for a few minutes – and then stay after 5 [p.m.] for a few more hours when he’d work on charts or other administrative tasks. Then he’d get something to eat, work on more charts, then go to bed, get up in the morning, and repeat.”
Mr. Hursh summarized the client’s life: “Eating, sleeping, practicing clinical medicine, and doing nonclinical tasks.”
It turned out that the 40-hour workweek included in the contract referred to patient-facing hours, not to all of the ancillary tasks that are part of practicing medicine in this day and age. “Unfortunately, this is far from an isolated story,” said Mr. Hursh.
Be aware of what’s in the contract
“The first draft of many standard physician employment contracts often omits mention of patient contact hour requirements and rather uses vague verbiage such as ‘full-time’ employment or ‘1.0 FTE’ – or full-time equivalent – without defining that term,” said Mr. Hursh. Typically, the 40 hours exclude call coverage, but most physicians understand that and, at least at first glance, it all sounds very reasonable.
But once charting, hours on the phone, arguing with managed care companies, sending in prescriptions, administrative meetings, and other tasks are thrown in, the work hours expand dramatically. Moreover, if your employer doesn’t utilize hospitalists, you may be expected to “round” outside of the 40 hours, which can be particularly burdensome if the employer admits patients to multiple hospitals.
Amanda Hill, JD, owner of Hill Health Law based in Austin, Texas, told this news organization that this predicament isn’t unique to physicians. Exempt employees who don’t clock in and out are often expected to work overtime – that is, to “work as long as it takes to get the job done.” It can affect NPs, PAs, and many others in the health care space. But the number of tasks that fall upon a doctor’s shoulders and the fact that patients’ health and lives are at stake up the ante and make the situation far more difficult for doctors than for employees in other industries.
So it’s important to nail down precise terms in the contract and, if possible, negotiate for a more humane schedule by specifying how the working hours will be used.
“It’s true that a 1.0 FTE definition is too vague,” Ms. Hill said. “I’ve negotiated a lot of contracts where we nail down in writing that the in-office schedule equals 34 hours per week, so the physician is guaranteed an additional 6 hours for administrative time.”
Mr. Hursh usually asks for 32 hours of patient contact per week, which leaves 1 full day per week to catch up on basic administrative tasks. “It’s important for employers to recognize that seeing patients isn’t the only thing a doctor does and there’s a lot of work in addition to face-to-face time,” he said.
But he hasn’t always been successful. One physician client was seeking a workweek consisting of 36 patient contact hours, “which is 90% of the usual FTE of a 40-hour week,” said Mr. Hursh. “But the employer called it ‘part-time,’ as if the doctor were planning to be lying in the sun for the other 4 hours.”
The client decided to accept a 10% pay cut and 10% less vacation to guarantee that she had those extra hours for administrative tasks. “She’s probably working way more than 36 hours a week, but maybe closer to 50 or 60 instead of 70 or more,” he said.
Clarify call coverage
Call coverage is typically not included in the hours a physician is contracted to work on a weekly basis. “Most contracts have call, and it’s usually evenly distributed among parties in a practice, but call can expand if another doctor is out sick, for example,” said Ms. Hill.
Sometimes the language in the contract is vague regarding call coverage. “I ask, how many shifts per year is the doctor is expected to work? Then, I try to negotiate extra pay if more shifts arise,” she said. “The hospital or practice may not demand extra call because they don’t want to pay extra money to the physician.”
On the other hand, some physicians may be eager to take extra call if it means extra income.
Ms. Hill stated that one of her clients was being paid as a “part-time, 2-day-a-week provider” but was asked to be on call and take night and weekend work. When you added it all up, she was putting in almost 30 hours a week.
“This is abusive to a provider that works so hard for patients,” Ms. Hill said. “We have to protect them through the contract language, so they have something hard and fast to point to when their administrator pushes them too hard. Doctors should get value for their time.”
Ms. Hill and her client pushed for more money, and the employer gave in. “All we had to do was to point out how many hours she was actually working. She didn’t mind all the extra call, but she wanted to be compensated.” The doctor’s salary was hiked by $25,000.
Differences in specialties and settings
There are some specialties where it might be easier to have more defined hours, while other specialties are more challenging. Anu Murthy, Esq., an attorney and associate contract review specialist at Contract Diagnostics (a national firm that reviews physician contracts) told this news organization that the work of hospitalists, intensivists, and emergency department physicians, for example, is done in shifts, which tend to be fixed hours.
“They need to get their charting completed so that whoever takes over on the next shift has access to the most recent notes about the patient,” she said. By contrast, surgeons can’t always account for how long a given surgery will take. “It could be as long as 9 hours,” she said. Notes need to be written immediately for the sake of the patient’s postsurgical care.
Dermatologists tend to deal with fewer emergencies, compared with other specialists, and it’s easier for their patients to be slotted into an organized schedule. On the other hand, primary care doctors – internists, family practice physicians, and pediatricians – may be seeing 40-50 patients a day, one every 15 minutes.
Practice setting also makes a difference, said Ms. Murthy. Veterans Administration (VA) hospitals or government-run clinics tend to have more rigidly defined hours, compared with other settings, so if you’re in a VA hospital or government-run clinic, work-life balance tends to be better.
Physicians who work remotely via telehealth also tend to have a better work-life balance, compared with those who see patients in person, Ms. Murthy said. But the difference may be in not having to spend extra time commuting to work or interacting with others in the work environment, since some research has suggested that telehealth physicians may actually spend more time engaged in charting after hours, compared with their in-person counterparts.
Using scribes to maximize your time
Elliott Trotter, MD, is an emergency medicine physician, associate clinical professor of emergency medicine at Texas Christian University Medical Schools, and founder of the ScribeNest, a Texas-based company that trains health care scribes. He told this news organization that there are ways to maximize one’s time during shifts so that much of the charting can be accomplished during working hours.
“About 28 years ago, I realized that the documentation load for physicians was enormous and at that time I developed the Modern Scribe, using premed students for ‘elbow support’ to help with the workload by documenting the ED encounters in real time during the encounter so I wouldn’t have to do so later.”
Over the years, as EHRs have become more ubiquitous and onerous, the role of the scribe has “evolved from a luxury to a necessity,” said Dr. Trotter. The scribes can actually record the encounter directly into the EHR so that the physician doesn’t have to do so later and doesn’t have to look at a computer screen but can look at the patient during the encounter.
“This enhances communication and has been shown to improve patient care,” he said.
Dr. Trotter said he rarely, if ever, needs to do documentation after hours. “But one of my physician colleagues had over 500 charts in his in-basket on a regular basis, which was overwhelming and untenable.”
The use of AI in health care is rapidly growing. Tools to help hasten the process of taking notes through use of AI-generated summaries is something appealing to many doctors. Ms. Hill warned physicians to “be careful not to rely so heavily on AI that you trust it over your own words.” She noted that it can make mistakes, and the liability always remains with the clinician.
Creating time-efficient strategies
Wilfrid Noel Raby, PhD, MD, a psychiatrist in private practice in Teaneck, N.J., was formerly a psychiatrist in the substance abuse unit at Montefiore Hospital, New York. He told this news organization that he developed a system whereby he rarely had to take work home with him. “I was working only 20 hours a week, but I was usually able to do my charting during those hours, as well as seeing patients,” he said. “I scheduled my appointments and structured a little ‘buffer time’ between them so that I had time to document the first appointment before moving on to the next one.”
There were days when this wasn’t possible because there were too many patients who needed to be seen back-to-back. “So I developed my own template where I could take rapid, very standardized notes that fit into the format of the EHR and met those expectations.” Then, when he had finished seeing patients, he could quickly enter the content of his notes into the EHR. If necessary, he completed his charting on a different day.
Viwek Bisen, DO, assistant professor of psychiatry, Hackensack (N.J.) University Medical Center, is a psychiatrist in the emergency department. “My contract is based on a traditional 40-hour workweek, with 80% of my time allotted to seeing patients and 20% of my time allotted to administration.”
But the way his time actually plays out is that he’s seeing patients during about half of the 32 hours. “The rest of the time, I’m charting, speaking to family members of patients, writing notes, engaging in team meetings, and dealing with insurance companies.” Dr. Bisen has developed his own system of completing his notes while still in the hospital. “I’ve learned to be efficient and manage my time better, so I no longer have to take work home with me.”
“At the end of the day, doctors are people,” Ms. Hill said. “They are not machines. Maybe in residency and fellowship they may grind out impossible shifts with little sleep, but this pace isn’t tenable for an entire career.”
A version of this article first appeared on Medscape.com.
What’s in a day’s work? For doctors, it’s typically a mix of seeing patients and completing paperwork and follow-up. Often it extends well past the standard workday.
Dennis Hursh, JD, managing partner of Physician Agreements Health Law, a Pennsylvania-based law firm that represents physicians, describes one overwhelmed ob.gyn. who recently consulted him for this problem.
“My client had accepted a position in a group practice where his contract stated he would be working during normal office hours, Monday through Friday, from 8 a.m. to 5 p.m. – in other words, a 40-hour workweek,” Mr. Hursh said.
But the distressed physician discovered that actually, he was working almost twice as many hours. “He’d get to work early to do charting, then see patients during the 40 hours, perhaps grabbing a quick sandwich for a few minutes – and then stay after 5 [p.m.] for a few more hours when he’d work on charts or other administrative tasks. Then he’d get something to eat, work on more charts, then go to bed, get up in the morning, and repeat.”
Mr. Hursh summarized the client’s life: “Eating, sleeping, practicing clinical medicine, and doing nonclinical tasks.”
It turned out that the 40-hour workweek included in the contract referred to patient-facing hours, not to all of the ancillary tasks that are part of practicing medicine in this day and age. “Unfortunately, this is far from an isolated story,” said Mr. Hursh.
Be aware of what’s in the contract
“The first draft of many standard physician employment contracts often omits mention of patient contact hour requirements and rather uses vague verbiage such as ‘full-time’ employment or ‘1.0 FTE’ – or full-time equivalent – without defining that term,” said Mr. Hursh. Typically, the 40 hours exclude call coverage, but most physicians understand that and, at least at first glance, it all sounds very reasonable.
But once charting, hours on the phone, arguing with managed care companies, sending in prescriptions, administrative meetings, and other tasks are thrown in, the work hours expand dramatically. Moreover, if your employer doesn’t utilize hospitalists, you may be expected to “round” outside of the 40 hours, which can be particularly burdensome if the employer admits patients to multiple hospitals.
Amanda Hill, JD, owner of Hill Health Law based in Austin, Texas, told this news organization that this predicament isn’t unique to physicians. Exempt employees who don’t clock in and out are often expected to work overtime – that is, to “work as long as it takes to get the job done.” It can affect NPs, PAs, and many others in the health care space. But the number of tasks that fall upon a doctor’s shoulders and the fact that patients’ health and lives are at stake up the ante and make the situation far more difficult for doctors than for employees in other industries.
So it’s important to nail down precise terms in the contract and, if possible, negotiate for a more humane schedule by specifying how the working hours will be used.
“It’s true that a 1.0 FTE definition is too vague,” Ms. Hill said. “I’ve negotiated a lot of contracts where we nail down in writing that the in-office schedule equals 34 hours per week, so the physician is guaranteed an additional 6 hours for administrative time.”
Mr. Hursh usually asks for 32 hours of patient contact per week, which leaves 1 full day per week to catch up on basic administrative tasks. “It’s important for employers to recognize that seeing patients isn’t the only thing a doctor does and there’s a lot of work in addition to face-to-face time,” he said.
But he hasn’t always been successful. One physician client was seeking a workweek consisting of 36 patient contact hours, “which is 90% of the usual FTE of a 40-hour week,” said Mr. Hursh. “But the employer called it ‘part-time,’ as if the doctor were planning to be lying in the sun for the other 4 hours.”
The client decided to accept a 10% pay cut and 10% less vacation to guarantee that she had those extra hours for administrative tasks. “She’s probably working way more than 36 hours a week, but maybe closer to 50 or 60 instead of 70 or more,” he said.
Clarify call coverage
Call coverage is typically not included in the hours a physician is contracted to work on a weekly basis. “Most contracts have call, and it’s usually evenly distributed among parties in a practice, but call can expand if another doctor is out sick, for example,” said Ms. Hill.
Sometimes the language in the contract is vague regarding call coverage. “I ask, how many shifts per year is the doctor is expected to work? Then, I try to negotiate extra pay if more shifts arise,” she said. “The hospital or practice may not demand extra call because they don’t want to pay extra money to the physician.”
On the other hand, some physicians may be eager to take extra call if it means extra income.
Ms. Hill stated that one of her clients was being paid as a “part-time, 2-day-a-week provider” but was asked to be on call and take night and weekend work. When you added it all up, she was putting in almost 30 hours a week.
“This is abusive to a provider that works so hard for patients,” Ms. Hill said. “We have to protect them through the contract language, so they have something hard and fast to point to when their administrator pushes them too hard. Doctors should get value for their time.”
Ms. Hill and her client pushed for more money, and the employer gave in. “All we had to do was to point out how many hours she was actually working. She didn’t mind all the extra call, but she wanted to be compensated.” The doctor’s salary was hiked by $25,000.
Differences in specialties and settings
There are some specialties where it might be easier to have more defined hours, while other specialties are more challenging. Anu Murthy, Esq., an attorney and associate contract review specialist at Contract Diagnostics (a national firm that reviews physician contracts) told this news organization that the work of hospitalists, intensivists, and emergency department physicians, for example, is done in shifts, which tend to be fixed hours.
“They need to get their charting completed so that whoever takes over on the next shift has access to the most recent notes about the patient,” she said. By contrast, surgeons can’t always account for how long a given surgery will take. “It could be as long as 9 hours,” she said. Notes need to be written immediately for the sake of the patient’s postsurgical care.
Dermatologists tend to deal with fewer emergencies, compared with other specialists, and it’s easier for their patients to be slotted into an organized schedule. On the other hand, primary care doctors – internists, family practice physicians, and pediatricians – may be seeing 40-50 patients a day, one every 15 minutes.
Practice setting also makes a difference, said Ms. Murthy. Veterans Administration (VA) hospitals or government-run clinics tend to have more rigidly defined hours, compared with other settings, so if you’re in a VA hospital or government-run clinic, work-life balance tends to be better.
Physicians who work remotely via telehealth also tend to have a better work-life balance, compared with those who see patients in person, Ms. Murthy said. But the difference may be in not having to spend extra time commuting to work or interacting with others in the work environment, since some research has suggested that telehealth physicians may actually spend more time engaged in charting after hours, compared with their in-person counterparts.
Using scribes to maximize your time
Elliott Trotter, MD, is an emergency medicine physician, associate clinical professor of emergency medicine at Texas Christian University Medical Schools, and founder of the ScribeNest, a Texas-based company that trains health care scribes. He told this news organization that there are ways to maximize one’s time during shifts so that much of the charting can be accomplished during working hours.
“About 28 years ago, I realized that the documentation load for physicians was enormous and at that time I developed the Modern Scribe, using premed students for ‘elbow support’ to help with the workload by documenting the ED encounters in real time during the encounter so I wouldn’t have to do so later.”
Over the years, as EHRs have become more ubiquitous and onerous, the role of the scribe has “evolved from a luxury to a necessity,” said Dr. Trotter. The scribes can actually record the encounter directly into the EHR so that the physician doesn’t have to do so later and doesn’t have to look at a computer screen but can look at the patient during the encounter.
“This enhances communication and has been shown to improve patient care,” he said.
Dr. Trotter said he rarely, if ever, needs to do documentation after hours. “But one of my physician colleagues had over 500 charts in his in-basket on a regular basis, which was overwhelming and untenable.”
The use of AI in health care is rapidly growing. Tools to help hasten the process of taking notes through use of AI-generated summaries is something appealing to many doctors. Ms. Hill warned physicians to “be careful not to rely so heavily on AI that you trust it over your own words.” She noted that it can make mistakes, and the liability always remains with the clinician.
Creating time-efficient strategies
Wilfrid Noel Raby, PhD, MD, a psychiatrist in private practice in Teaneck, N.J., was formerly a psychiatrist in the substance abuse unit at Montefiore Hospital, New York. He told this news organization that he developed a system whereby he rarely had to take work home with him. “I was working only 20 hours a week, but I was usually able to do my charting during those hours, as well as seeing patients,” he said. “I scheduled my appointments and structured a little ‘buffer time’ between them so that I had time to document the first appointment before moving on to the next one.”
There were days when this wasn’t possible because there were too many patients who needed to be seen back-to-back. “So I developed my own template where I could take rapid, very standardized notes that fit into the format of the EHR and met those expectations.” Then, when he had finished seeing patients, he could quickly enter the content of his notes into the EHR. If necessary, he completed his charting on a different day.
Viwek Bisen, DO, assistant professor of psychiatry, Hackensack (N.J.) University Medical Center, is a psychiatrist in the emergency department. “My contract is based on a traditional 40-hour workweek, with 80% of my time allotted to seeing patients and 20% of my time allotted to administration.”
But the way his time actually plays out is that he’s seeing patients during about half of the 32 hours. “The rest of the time, I’m charting, speaking to family members of patients, writing notes, engaging in team meetings, and dealing with insurance companies.” Dr. Bisen has developed his own system of completing his notes while still in the hospital. “I’ve learned to be efficient and manage my time better, so I no longer have to take work home with me.”
“At the end of the day, doctors are people,” Ms. Hill said. “They are not machines. Maybe in residency and fellowship they may grind out impossible shifts with little sleep, but this pace isn’t tenable for an entire career.”
A version of this article first appeared on Medscape.com.
What’s in a day’s work? For doctors, it’s typically a mix of seeing patients and completing paperwork and follow-up. Often it extends well past the standard workday.
Dennis Hursh, JD, managing partner of Physician Agreements Health Law, a Pennsylvania-based law firm that represents physicians, describes one overwhelmed ob.gyn. who recently consulted him for this problem.
“My client had accepted a position in a group practice where his contract stated he would be working during normal office hours, Monday through Friday, from 8 a.m. to 5 p.m. – in other words, a 40-hour workweek,” Mr. Hursh said.
But the distressed physician discovered that actually, he was working almost twice as many hours. “He’d get to work early to do charting, then see patients during the 40 hours, perhaps grabbing a quick sandwich for a few minutes – and then stay after 5 [p.m.] for a few more hours when he’d work on charts or other administrative tasks. Then he’d get something to eat, work on more charts, then go to bed, get up in the morning, and repeat.”
Mr. Hursh summarized the client’s life: “Eating, sleeping, practicing clinical medicine, and doing nonclinical tasks.”
It turned out that the 40-hour workweek included in the contract referred to patient-facing hours, not to all of the ancillary tasks that are part of practicing medicine in this day and age. “Unfortunately, this is far from an isolated story,” said Mr. Hursh.
Be aware of what’s in the contract
“The first draft of many standard physician employment contracts often omits mention of patient contact hour requirements and rather uses vague verbiage such as ‘full-time’ employment or ‘1.0 FTE’ – or full-time equivalent – without defining that term,” said Mr. Hursh. Typically, the 40 hours exclude call coverage, but most physicians understand that and, at least at first glance, it all sounds very reasonable.
But once charting, hours on the phone, arguing with managed care companies, sending in prescriptions, administrative meetings, and other tasks are thrown in, the work hours expand dramatically. Moreover, if your employer doesn’t utilize hospitalists, you may be expected to “round” outside of the 40 hours, which can be particularly burdensome if the employer admits patients to multiple hospitals.
Amanda Hill, JD, owner of Hill Health Law based in Austin, Texas, told this news organization that this predicament isn’t unique to physicians. Exempt employees who don’t clock in and out are often expected to work overtime – that is, to “work as long as it takes to get the job done.” It can affect NPs, PAs, and many others in the health care space. But the number of tasks that fall upon a doctor’s shoulders and the fact that patients’ health and lives are at stake up the ante and make the situation far more difficult for doctors than for employees in other industries.
So it’s important to nail down precise terms in the contract and, if possible, negotiate for a more humane schedule by specifying how the working hours will be used.
“It’s true that a 1.0 FTE definition is too vague,” Ms. Hill said. “I’ve negotiated a lot of contracts where we nail down in writing that the in-office schedule equals 34 hours per week, so the physician is guaranteed an additional 6 hours for administrative time.”
Mr. Hursh usually asks for 32 hours of patient contact per week, which leaves 1 full day per week to catch up on basic administrative tasks. “It’s important for employers to recognize that seeing patients isn’t the only thing a doctor does and there’s a lot of work in addition to face-to-face time,” he said.
But he hasn’t always been successful. One physician client was seeking a workweek consisting of 36 patient contact hours, “which is 90% of the usual FTE of a 40-hour week,” said Mr. Hursh. “But the employer called it ‘part-time,’ as if the doctor were planning to be lying in the sun for the other 4 hours.”
The client decided to accept a 10% pay cut and 10% less vacation to guarantee that she had those extra hours for administrative tasks. “She’s probably working way more than 36 hours a week, but maybe closer to 50 or 60 instead of 70 or more,” he said.
Clarify call coverage
Call coverage is typically not included in the hours a physician is contracted to work on a weekly basis. “Most contracts have call, and it’s usually evenly distributed among parties in a practice, but call can expand if another doctor is out sick, for example,” said Ms. Hill.
Sometimes the language in the contract is vague regarding call coverage. “I ask, how many shifts per year is the doctor is expected to work? Then, I try to negotiate extra pay if more shifts arise,” she said. “The hospital or practice may not demand extra call because they don’t want to pay extra money to the physician.”
On the other hand, some physicians may be eager to take extra call if it means extra income.
Ms. Hill stated that one of her clients was being paid as a “part-time, 2-day-a-week provider” but was asked to be on call and take night and weekend work. When you added it all up, she was putting in almost 30 hours a week.
“This is abusive to a provider that works so hard for patients,” Ms. Hill said. “We have to protect them through the contract language, so they have something hard and fast to point to when their administrator pushes them too hard. Doctors should get value for their time.”
Ms. Hill and her client pushed for more money, and the employer gave in. “All we had to do was to point out how many hours she was actually working. She didn’t mind all the extra call, but she wanted to be compensated.” The doctor’s salary was hiked by $25,000.
Differences in specialties and settings
There are some specialties where it might be easier to have more defined hours, while other specialties are more challenging. Anu Murthy, Esq., an attorney and associate contract review specialist at Contract Diagnostics (a national firm that reviews physician contracts) told this news organization that the work of hospitalists, intensivists, and emergency department physicians, for example, is done in shifts, which tend to be fixed hours.
“They need to get their charting completed so that whoever takes over on the next shift has access to the most recent notes about the patient,” she said. By contrast, surgeons can’t always account for how long a given surgery will take. “It could be as long as 9 hours,” she said. Notes need to be written immediately for the sake of the patient’s postsurgical care.
Dermatologists tend to deal with fewer emergencies, compared with other specialists, and it’s easier for their patients to be slotted into an organized schedule. On the other hand, primary care doctors – internists, family practice physicians, and pediatricians – may be seeing 40-50 patients a day, one every 15 minutes.
Practice setting also makes a difference, said Ms. Murthy. Veterans Administration (VA) hospitals or government-run clinics tend to have more rigidly defined hours, compared with other settings, so if you’re in a VA hospital or government-run clinic, work-life balance tends to be better.
Physicians who work remotely via telehealth also tend to have a better work-life balance, compared with those who see patients in person, Ms. Murthy said. But the difference may be in not having to spend extra time commuting to work or interacting with others in the work environment, since some research has suggested that telehealth physicians may actually spend more time engaged in charting after hours, compared with their in-person counterparts.
Using scribes to maximize your time
Elliott Trotter, MD, is an emergency medicine physician, associate clinical professor of emergency medicine at Texas Christian University Medical Schools, and founder of the ScribeNest, a Texas-based company that trains health care scribes. He told this news organization that there are ways to maximize one’s time during shifts so that much of the charting can be accomplished during working hours.
“About 28 years ago, I realized that the documentation load for physicians was enormous and at that time I developed the Modern Scribe, using premed students for ‘elbow support’ to help with the workload by documenting the ED encounters in real time during the encounter so I wouldn’t have to do so later.”
Over the years, as EHRs have become more ubiquitous and onerous, the role of the scribe has “evolved from a luxury to a necessity,” said Dr. Trotter. The scribes can actually record the encounter directly into the EHR so that the physician doesn’t have to do so later and doesn’t have to look at a computer screen but can look at the patient during the encounter.
“This enhances communication and has been shown to improve patient care,” he said.
Dr. Trotter said he rarely, if ever, needs to do documentation after hours. “But one of my physician colleagues had over 500 charts in his in-basket on a regular basis, which was overwhelming and untenable.”
The use of AI in health care is rapidly growing. Tools to help hasten the process of taking notes through use of AI-generated summaries is something appealing to many doctors. Ms. Hill warned physicians to “be careful not to rely so heavily on AI that you trust it over your own words.” She noted that it can make mistakes, and the liability always remains with the clinician.
Creating time-efficient strategies
Wilfrid Noel Raby, PhD, MD, a psychiatrist in private practice in Teaneck, N.J., was formerly a psychiatrist in the substance abuse unit at Montefiore Hospital, New York. He told this news organization that he developed a system whereby he rarely had to take work home with him. “I was working only 20 hours a week, but I was usually able to do my charting during those hours, as well as seeing patients,” he said. “I scheduled my appointments and structured a little ‘buffer time’ between them so that I had time to document the first appointment before moving on to the next one.”
There were days when this wasn’t possible because there were too many patients who needed to be seen back-to-back. “So I developed my own template where I could take rapid, very standardized notes that fit into the format of the EHR and met those expectations.” Then, when he had finished seeing patients, he could quickly enter the content of his notes into the EHR. If necessary, he completed his charting on a different day.
Viwek Bisen, DO, assistant professor of psychiatry, Hackensack (N.J.) University Medical Center, is a psychiatrist in the emergency department. “My contract is based on a traditional 40-hour workweek, with 80% of my time allotted to seeing patients and 20% of my time allotted to administration.”
But the way his time actually plays out is that he’s seeing patients during about half of the 32 hours. “The rest of the time, I’m charting, speaking to family members of patients, writing notes, engaging in team meetings, and dealing with insurance companies.” Dr. Bisen has developed his own system of completing his notes while still in the hospital. “I’ve learned to be efficient and manage my time better, so I no longer have to take work home with me.”
“At the end of the day, doctors are people,” Ms. Hill said. “They are not machines. Maybe in residency and fellowship they may grind out impossible shifts with little sleep, but this pace isn’t tenable for an entire career.”
A version of this article first appeared on Medscape.com.
Test all perinatally exposed infants for HCV: CDC
In utero–exposed infants should be tested at 2-6 months of life, much earlier than the current strategy of testing at 18 months.
HCV infection, which can lead to liver fibrosis and cirrhosis, liver failure, hepatic cancer, and transplant, will develop in 6%-7% of all perinatally exposed infants and children. Curative therapy with direct-acting antivirals can be administered starting at age 3, the CDC noted in Morbidity and Mortality Week Report (MMWR).
About 70% of children 18 months and older are not being tested with the current strategy of anti-HCV testing.
This current MMWR report supplements the 2020 CDC recommendations for adult HCV screening, which includes universal screening among pregnant persons during each pregnancy.
The new recommendations
- Perinatally exposed infants should receive a nucleic acid amplification test for HCV RNA at 2-6 months of age to identify those who might develop chronic HCV infection if not treated.
- Those with detectable HCV RNA should be managed in consultation with an expert in pediatric HCV.
- Infants with undetectable HCV RNA do not require further follow-up unless clinically warranted.
“Testing perinatally exposed infants beginning at age 2 months with a NAT for HCV RNA is cost-effective and allows for earlier linkage to care, appropriate evaluation, and the opportunity to provide curative, life-saving therapy,” the MMWR report said.
A growing problem
The CDC noted that rates of HCV infections during pregnancy are on the rise, corresponding with the ongoing opioid crisis and intravenous drug use.
Yet most perinatally exposed children are not tested for HCV infection and are not referred for hepatitis C care. Reasons might include lack of awareness of perinatal exposure by pediatric providers, lack of regular pediatric care among exposed children, and switching of health care providers before the former recommended testing age of 18 months.
The CDC’s testing recommendation is welcome news to Dawnette A. Lewis, MD, a maternal fetal medicine specialist at Northwell Health in New Hyde Park, N.Y. “As opposed to data for hep B and HIV, we have traditionally had little information and experience regarding the transmission and impact of hep C in pregnant women and their babies. We’ve been having that conversation about the lack of information for some time, and now there’s an opportunity to get evolving data on hep C and how it affects the baby, ” she said.
In her view, mothers will likely be quite accepting of testing for their infants. “It could be integrated into the routine newborn screening panel, so there should not be barriers to accessibility if they’re getting prenatal and neonatal care.”
Commenting on HCV testing for babies in an interview at his institution, Ravi R. Jhaveri, MD, division head of pediatric infectious diseases at Northwestern Medicine’s Ann & Robert H. Lurie Children’s Hospital of Chicago, said, “This is a terrific way to capitalize on the fact that infants already come to the doctor for many visits during the first months of life for their vaccines and their well-child check. And so this should be an easy way to streamline our testing strategy and hopefully lose many fewer patients.”
Northwestern Medicine is an innovative clinic offering HCV testing and treatment outside of clinical trials for pregnant women and their infants with the goal of preventing transmission from mother to child.
Northwestern is launching a clinical trial of treatment for HCV-positive pregnant patients during regular prenatal care. “With very simple treatments similar to taking a prenatal vitamin, it would be easy and seamless to fit into the existing schedule,” said Lyn Yee, MD, a Northwestern maternal-fetal medicine specialist.
Dr. Yee stressed that eliminating hepatitis C will likely be one of the most significant health advancements of the decade.
Dr. Lewis, Dr. Jhaveri, and Dr. Yee had no relevant conflicts of interest to declare with regard to their comments.
In utero–exposed infants should be tested at 2-6 months of life, much earlier than the current strategy of testing at 18 months.
HCV infection, which can lead to liver fibrosis and cirrhosis, liver failure, hepatic cancer, and transplant, will develop in 6%-7% of all perinatally exposed infants and children. Curative therapy with direct-acting antivirals can be administered starting at age 3, the CDC noted in Morbidity and Mortality Week Report (MMWR).
About 70% of children 18 months and older are not being tested with the current strategy of anti-HCV testing.
This current MMWR report supplements the 2020 CDC recommendations for adult HCV screening, which includes universal screening among pregnant persons during each pregnancy.
The new recommendations
- Perinatally exposed infants should receive a nucleic acid amplification test for HCV RNA at 2-6 months of age to identify those who might develop chronic HCV infection if not treated.
- Those with detectable HCV RNA should be managed in consultation with an expert in pediatric HCV.
- Infants with undetectable HCV RNA do not require further follow-up unless clinically warranted.
“Testing perinatally exposed infants beginning at age 2 months with a NAT for HCV RNA is cost-effective and allows for earlier linkage to care, appropriate evaluation, and the opportunity to provide curative, life-saving therapy,” the MMWR report said.
A growing problem
The CDC noted that rates of HCV infections during pregnancy are on the rise, corresponding with the ongoing opioid crisis and intravenous drug use.
Yet most perinatally exposed children are not tested for HCV infection and are not referred for hepatitis C care. Reasons might include lack of awareness of perinatal exposure by pediatric providers, lack of regular pediatric care among exposed children, and switching of health care providers before the former recommended testing age of 18 months.
The CDC’s testing recommendation is welcome news to Dawnette A. Lewis, MD, a maternal fetal medicine specialist at Northwell Health in New Hyde Park, N.Y. “As opposed to data for hep B and HIV, we have traditionally had little information and experience regarding the transmission and impact of hep C in pregnant women and their babies. We’ve been having that conversation about the lack of information for some time, and now there’s an opportunity to get evolving data on hep C and how it affects the baby, ” she said.
In her view, mothers will likely be quite accepting of testing for their infants. “It could be integrated into the routine newborn screening panel, so there should not be barriers to accessibility if they’re getting prenatal and neonatal care.”
Commenting on HCV testing for babies in an interview at his institution, Ravi R. Jhaveri, MD, division head of pediatric infectious diseases at Northwestern Medicine’s Ann & Robert H. Lurie Children’s Hospital of Chicago, said, “This is a terrific way to capitalize on the fact that infants already come to the doctor for many visits during the first months of life for their vaccines and their well-child check. And so this should be an easy way to streamline our testing strategy and hopefully lose many fewer patients.”
Northwestern Medicine is an innovative clinic offering HCV testing and treatment outside of clinical trials for pregnant women and their infants with the goal of preventing transmission from mother to child.
Northwestern is launching a clinical trial of treatment for HCV-positive pregnant patients during regular prenatal care. “With very simple treatments similar to taking a prenatal vitamin, it would be easy and seamless to fit into the existing schedule,” said Lyn Yee, MD, a Northwestern maternal-fetal medicine specialist.
Dr. Yee stressed that eliminating hepatitis C will likely be one of the most significant health advancements of the decade.
Dr. Lewis, Dr. Jhaveri, and Dr. Yee had no relevant conflicts of interest to declare with regard to their comments.
In utero–exposed infants should be tested at 2-6 months of life, much earlier than the current strategy of testing at 18 months.
HCV infection, which can lead to liver fibrosis and cirrhosis, liver failure, hepatic cancer, and transplant, will develop in 6%-7% of all perinatally exposed infants and children. Curative therapy with direct-acting antivirals can be administered starting at age 3, the CDC noted in Morbidity and Mortality Week Report (MMWR).
About 70% of children 18 months and older are not being tested with the current strategy of anti-HCV testing.
This current MMWR report supplements the 2020 CDC recommendations for adult HCV screening, which includes universal screening among pregnant persons during each pregnancy.
The new recommendations
- Perinatally exposed infants should receive a nucleic acid amplification test for HCV RNA at 2-6 months of age to identify those who might develop chronic HCV infection if not treated.
- Those with detectable HCV RNA should be managed in consultation with an expert in pediatric HCV.
- Infants with undetectable HCV RNA do not require further follow-up unless clinically warranted.
“Testing perinatally exposed infants beginning at age 2 months with a NAT for HCV RNA is cost-effective and allows for earlier linkage to care, appropriate evaluation, and the opportunity to provide curative, life-saving therapy,” the MMWR report said.
A growing problem
The CDC noted that rates of HCV infections during pregnancy are on the rise, corresponding with the ongoing opioid crisis and intravenous drug use.
Yet most perinatally exposed children are not tested for HCV infection and are not referred for hepatitis C care. Reasons might include lack of awareness of perinatal exposure by pediatric providers, lack of regular pediatric care among exposed children, and switching of health care providers before the former recommended testing age of 18 months.
The CDC’s testing recommendation is welcome news to Dawnette A. Lewis, MD, a maternal fetal medicine specialist at Northwell Health in New Hyde Park, N.Y. “As opposed to data for hep B and HIV, we have traditionally had little information and experience regarding the transmission and impact of hep C in pregnant women and their babies. We’ve been having that conversation about the lack of information for some time, and now there’s an opportunity to get evolving data on hep C and how it affects the baby, ” she said.
In her view, mothers will likely be quite accepting of testing for their infants. “It could be integrated into the routine newborn screening panel, so there should not be barriers to accessibility if they’re getting prenatal and neonatal care.”
Commenting on HCV testing for babies in an interview at his institution, Ravi R. Jhaveri, MD, division head of pediatric infectious diseases at Northwestern Medicine’s Ann & Robert H. Lurie Children’s Hospital of Chicago, said, “This is a terrific way to capitalize on the fact that infants already come to the doctor for many visits during the first months of life for their vaccines and their well-child check. And so this should be an easy way to streamline our testing strategy and hopefully lose many fewer patients.”
Northwestern Medicine is an innovative clinic offering HCV testing and treatment outside of clinical trials for pregnant women and their infants with the goal of preventing transmission from mother to child.
Northwestern is launching a clinical trial of treatment for HCV-positive pregnant patients during regular prenatal care. “With very simple treatments similar to taking a prenatal vitamin, it would be easy and seamless to fit into the existing schedule,” said Lyn Yee, MD, a Northwestern maternal-fetal medicine specialist.
Dr. Yee stressed that eliminating hepatitis C will likely be one of the most significant health advancements of the decade.
Dr. Lewis, Dr. Jhaveri, and Dr. Yee had no relevant conflicts of interest to declare with regard to their comments.
Adverse events related to embryo transfer catheters may be underreported to the FDA
, according to a new study presented at the American Society for Reproductive Medicine’s 2023 meeting.
ETCs are medical devices used routinely in assisted reproduction. The findings highlight the need for increased vigilance in tracking and reporting adverse events associated with these devices, according to the investigators.
“With hundreds of thousands of embryo transfers being performed per year, surveillance of the safety, performance, and quality of embryo transfer catheter devices is critical and should not be taken for granted,” said Anita Madison, MD, MPH, from the division of reproductive endocrinology and infertility at Johns Hopkins School of Medicine, Baltimore, who led the study. “There are a variety of transfer catheters with different indications, with little data on the superiority and safety of the brands compared to one another.”
Although the number of reported adverse events associated with ETCs is relatively small, the problems can significantly affect patient care, the researchers said.
Dr. Madison and her colleagues used the Manufacturer and User Facility Device Experience (MAUDE) database to identify adverse events associated with ETC devices. The MAUDE database is a voluntary reporting system that holds hundreds of thousands of medical device reports of suspected device-associated deaths, injuries, and malfunctions reported to the FDA annually.
For each adverse event in the database linked to an ECT, the researchers collected information related to the brand of the device, the nature of the event, and the nature of the reporter. The researchers omitted the device and manufacturer names from the presentation of the study findings, delineating them only as “Brand 1,” “Brand 2,” “Brand 3,” “Brand 4,” or “Other.”
Problems with devices included contamination, packaging problems, malfunction, mechanical flaws, and material separation. Patient-level adverse events included retaining of foreign body, trauma, malfunction, or failed embryo transfer.
Between 2014 and 2023, Dr. Madison and her colleagues identified 101 adverse events associated with ECTs in the database. About 25% of these occurred in 2018, with 27 cases reported. Contamination was the most prevalent problem, found in 68 reports; oil was the most common contaminant.
The distribution of types of adverse events varied, depending on ETC brand. A breakdown of occurrences revealed high numbers for Brand 2, with 52 adverse events. Although Brand 3 accounted for only 16 adverse events, the majority of these were related to device separation.
“That finding stood out,” Dr. Madison said.
Nearly 1 in 4 (22%) of all reported incidents led to overt patient harm. Retention of a foreign body was the prime type of injury, occurring in 12 cases. Malfunction and injury were found in four cases each, with two failed embryo transfers reported, Dr. Madison said.
Because the majority of these adverse event reports were submitted by manufacturers (87%) and were rarely submitted by end users (for example, physicians, lab staff), the researchers said their findings likely underestimate such problems.
“I’m surprised the [number of reported adverse events] is as low as it is,” said Kimball Pomeroy, PhD, scientific director at the World Egg and Sperm Bank, Scottsdale, Ariz., who was not part of the study team. “Laboratories are required to report failed devices; they have to have a plan for that.”
“It just comes down to underreporting,” added Valerie L. Baker, MD, director in the Division of Reproductive Endocrinology and Infertility at Johns Hopkins Medicine, Lutherville, Md., who was not affiliated with the study.
“In two of these reports, they failed to transfer the embryo; they actually lost the embryo,” Dr. Pomeroy added. “That’s drastic for those patients; it’s a serious problem that needs to be addressed.”
Citing these findings, the authors underscored the need for heightened surveillance of ETC devices and recommend further studies to assess the sensitivity of these procedures for attempting pregnancy. They urge physicians and lab staff involved in these procedures to exercise continued vigilance and to improve the reporting of problems with ETC devices.
Dr. Madison, Dr. Baker, and Dr. Pomeroy report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a new study presented at the American Society for Reproductive Medicine’s 2023 meeting.
ETCs are medical devices used routinely in assisted reproduction. The findings highlight the need for increased vigilance in tracking and reporting adverse events associated with these devices, according to the investigators.
“With hundreds of thousands of embryo transfers being performed per year, surveillance of the safety, performance, and quality of embryo transfer catheter devices is critical and should not be taken for granted,” said Anita Madison, MD, MPH, from the division of reproductive endocrinology and infertility at Johns Hopkins School of Medicine, Baltimore, who led the study. “There are a variety of transfer catheters with different indications, with little data on the superiority and safety of the brands compared to one another.”
Although the number of reported adverse events associated with ETCs is relatively small, the problems can significantly affect patient care, the researchers said.
Dr. Madison and her colleagues used the Manufacturer and User Facility Device Experience (MAUDE) database to identify adverse events associated with ETC devices. The MAUDE database is a voluntary reporting system that holds hundreds of thousands of medical device reports of suspected device-associated deaths, injuries, and malfunctions reported to the FDA annually.
For each adverse event in the database linked to an ECT, the researchers collected information related to the brand of the device, the nature of the event, and the nature of the reporter. The researchers omitted the device and manufacturer names from the presentation of the study findings, delineating them only as “Brand 1,” “Brand 2,” “Brand 3,” “Brand 4,” or “Other.”
Problems with devices included contamination, packaging problems, malfunction, mechanical flaws, and material separation. Patient-level adverse events included retaining of foreign body, trauma, malfunction, or failed embryo transfer.
Between 2014 and 2023, Dr. Madison and her colleagues identified 101 adverse events associated with ECTs in the database. About 25% of these occurred in 2018, with 27 cases reported. Contamination was the most prevalent problem, found in 68 reports; oil was the most common contaminant.
The distribution of types of adverse events varied, depending on ETC brand. A breakdown of occurrences revealed high numbers for Brand 2, with 52 adverse events. Although Brand 3 accounted for only 16 adverse events, the majority of these were related to device separation.
“That finding stood out,” Dr. Madison said.
Nearly 1 in 4 (22%) of all reported incidents led to overt patient harm. Retention of a foreign body was the prime type of injury, occurring in 12 cases. Malfunction and injury were found in four cases each, with two failed embryo transfers reported, Dr. Madison said.
Because the majority of these adverse event reports were submitted by manufacturers (87%) and were rarely submitted by end users (for example, physicians, lab staff), the researchers said their findings likely underestimate such problems.
“I’m surprised the [number of reported adverse events] is as low as it is,” said Kimball Pomeroy, PhD, scientific director at the World Egg and Sperm Bank, Scottsdale, Ariz., who was not part of the study team. “Laboratories are required to report failed devices; they have to have a plan for that.”
“It just comes down to underreporting,” added Valerie L. Baker, MD, director in the Division of Reproductive Endocrinology and Infertility at Johns Hopkins Medicine, Lutherville, Md., who was not affiliated with the study.
“In two of these reports, they failed to transfer the embryo; they actually lost the embryo,” Dr. Pomeroy added. “That’s drastic for those patients; it’s a serious problem that needs to be addressed.”
Citing these findings, the authors underscored the need for heightened surveillance of ETC devices and recommend further studies to assess the sensitivity of these procedures for attempting pregnancy. They urge physicians and lab staff involved in these procedures to exercise continued vigilance and to improve the reporting of problems with ETC devices.
Dr. Madison, Dr. Baker, and Dr. Pomeroy report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a new study presented at the American Society for Reproductive Medicine’s 2023 meeting.
ETCs are medical devices used routinely in assisted reproduction. The findings highlight the need for increased vigilance in tracking and reporting adverse events associated with these devices, according to the investigators.
“With hundreds of thousands of embryo transfers being performed per year, surveillance of the safety, performance, and quality of embryo transfer catheter devices is critical and should not be taken for granted,” said Anita Madison, MD, MPH, from the division of reproductive endocrinology and infertility at Johns Hopkins School of Medicine, Baltimore, who led the study. “There are a variety of transfer catheters with different indications, with little data on the superiority and safety of the brands compared to one another.”
Although the number of reported adverse events associated with ETCs is relatively small, the problems can significantly affect patient care, the researchers said.
Dr. Madison and her colleagues used the Manufacturer and User Facility Device Experience (MAUDE) database to identify adverse events associated with ETC devices. The MAUDE database is a voluntary reporting system that holds hundreds of thousands of medical device reports of suspected device-associated deaths, injuries, and malfunctions reported to the FDA annually.
For each adverse event in the database linked to an ECT, the researchers collected information related to the brand of the device, the nature of the event, and the nature of the reporter. The researchers omitted the device and manufacturer names from the presentation of the study findings, delineating them only as “Brand 1,” “Brand 2,” “Brand 3,” “Brand 4,” or “Other.”
Problems with devices included contamination, packaging problems, malfunction, mechanical flaws, and material separation. Patient-level adverse events included retaining of foreign body, trauma, malfunction, or failed embryo transfer.
Between 2014 and 2023, Dr. Madison and her colleagues identified 101 adverse events associated with ECTs in the database. About 25% of these occurred in 2018, with 27 cases reported. Contamination was the most prevalent problem, found in 68 reports; oil was the most common contaminant.
The distribution of types of adverse events varied, depending on ETC brand. A breakdown of occurrences revealed high numbers for Brand 2, with 52 adverse events. Although Brand 3 accounted for only 16 adverse events, the majority of these were related to device separation.
“That finding stood out,” Dr. Madison said.
Nearly 1 in 4 (22%) of all reported incidents led to overt patient harm. Retention of a foreign body was the prime type of injury, occurring in 12 cases. Malfunction and injury were found in four cases each, with two failed embryo transfers reported, Dr. Madison said.
Because the majority of these adverse event reports were submitted by manufacturers (87%) and were rarely submitted by end users (for example, physicians, lab staff), the researchers said their findings likely underestimate such problems.
“I’m surprised the [number of reported adverse events] is as low as it is,” said Kimball Pomeroy, PhD, scientific director at the World Egg and Sperm Bank, Scottsdale, Ariz., who was not part of the study team. “Laboratories are required to report failed devices; they have to have a plan for that.”
“It just comes down to underreporting,” added Valerie L. Baker, MD, director in the Division of Reproductive Endocrinology and Infertility at Johns Hopkins Medicine, Lutherville, Md., who was not affiliated with the study.
“In two of these reports, they failed to transfer the embryo; they actually lost the embryo,” Dr. Pomeroy added. “That’s drastic for those patients; it’s a serious problem that needs to be addressed.”
Citing these findings, the authors underscored the need for heightened surveillance of ETC devices and recommend further studies to assess the sensitivity of these procedures for attempting pregnancy. They urge physicians and lab staff involved in these procedures to exercise continued vigilance and to improve the reporting of problems with ETC devices.
Dr. Madison, Dr. Baker, and Dr. Pomeroy report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ASRM 2023
Digital tool clarifies menopause symptoms
GLASGOW – An interactive digital decision tool that individualizes menopause care received praise from primary care clinicians in the United Kingdom, who said it could improve patient care and streamline office visits.
“Access to hormone replacement therapy [HRT], as well as decision-making around treatment for menopausal symptoms, is often complicated by concerns around its safety, and there is still a knowledge and a confidence gap among health care professionals causing reluctance to prescribe HRT,” said Aini Kamal, MSc, from University College London. Ms. Kamal presented results of a survey about the tool at the annual meeting of the Royal College of General Practitioners.
For the study, Ms. Kamal, Daniel Reisel, MBBS, PhD, a gynecologist at UCL, and colleagues evaluated Wellspring with doctors, nurses, and pharmacists.
“Ensuring that women receive education around symptoms, so that they are empowered, is a key part of optimizing their care and sharing decision-making,” Dr. Reisel said in an interview. He added that U.K. primary care had seen an increase in cases of women presenting with symptoms associated with the perimenopause and menopause at a time when U.K. Members of Parliament are debating whether to make it mandatory for all women to have menopause check-up in their early 40s.
The online survey was completed by 280 participants, and respondents were primarily GPs with several years of relevant prescribing practice. Of those, 93% found information from national guidelines to be accurately presented in the tool, and 97% said they would recommend this decision aid to other health care professionals, Ms. Kamal reported.
Nearly all participants said they could see themselves using the tool with patients in the clinic or as an adjunct to virtual sessions. “This [finding] was particularly important because it demonstrates the clinical potential this tool has,” she said.
One consult, too many problems
Louise Newson, MBChB, who runs the U.K.’s largest menopause clinic, said primary care appointments are often time-pressured and follow a “’one problem-one consultation’” policy. As such, women are often thinking ‘Do I go with my joint pains, or my palpitations, tinnitus, or what?’ If a patient presents with tinnitus, a doctor might focus on the potential of an inner ear problem rather than a hormone deficiency, but I do know that if the woman is perimenopausal or menopausal, we often look to replace the missing hormones, and then if the tinnitus doesn’t improve we can revisit the ear problem.”
Dr. Newson noted that 17% of women in her clinics have had more than six GP visits in the year before she sees them, but in the year following, this figure drops to 1%. Acknowledging that a menopause consultation for a GP is time-consuming, Dr. Newson pointed out that taking time initially with the patient “means it will reduce the number of future consultations quickly, but more importantly, we also know that taking HRT reduces long-term risk of serious diseases, including heart disease and osteoporosis.”
The digital tool can be used by both doctors and patients to help women work through their symptoms and equip them with knowledge so their GP visits are more productive.
“When we see women who are empowered with knowledge [about menopause symptoms], then the consultations are quicker and essentially place the patient central to the discussion,” Dr. Newson said.
Ed Russell-Smith, MBChB, a GP in Scotland who moderated the session, said the tool “lays out a nicely structured approach and provides modern treatment options and resources for patients.”
However, he added “we also need to remember there are potential harms to be done from HRT too. It’s vitally important that while patients might see HRT as a panacea, doctors need to balance this with the risks involved for each individual. As a tool, I think Wellspring can help us in this respect to apply general principles to that patient and individualize treatment.”
Dr. Reisel, Dr. Newson, Ms. Kamal, and Dr. Russell-Smith disclosed no relevant financial relationships. The Wellspring Decision Aid was supported by UCL’s Institute for Women’s Health. The Newson Health clinic is fully private, but research is done via the nonprofit arm, which is supported by the clinic. There is no pharma involvement.
A version of this article first appeared on Medscape.com.
GLASGOW – An interactive digital decision tool that individualizes menopause care received praise from primary care clinicians in the United Kingdom, who said it could improve patient care and streamline office visits.
“Access to hormone replacement therapy [HRT], as well as decision-making around treatment for menopausal symptoms, is often complicated by concerns around its safety, and there is still a knowledge and a confidence gap among health care professionals causing reluctance to prescribe HRT,” said Aini Kamal, MSc, from University College London. Ms. Kamal presented results of a survey about the tool at the annual meeting of the Royal College of General Practitioners.
For the study, Ms. Kamal, Daniel Reisel, MBBS, PhD, a gynecologist at UCL, and colleagues evaluated Wellspring with doctors, nurses, and pharmacists.
“Ensuring that women receive education around symptoms, so that they are empowered, is a key part of optimizing their care and sharing decision-making,” Dr. Reisel said in an interview. He added that U.K. primary care had seen an increase in cases of women presenting with symptoms associated with the perimenopause and menopause at a time when U.K. Members of Parliament are debating whether to make it mandatory for all women to have menopause check-up in their early 40s.
The online survey was completed by 280 participants, and respondents were primarily GPs with several years of relevant prescribing practice. Of those, 93% found information from national guidelines to be accurately presented in the tool, and 97% said they would recommend this decision aid to other health care professionals, Ms. Kamal reported.
Nearly all participants said they could see themselves using the tool with patients in the clinic or as an adjunct to virtual sessions. “This [finding] was particularly important because it demonstrates the clinical potential this tool has,” she said.
One consult, too many problems
Louise Newson, MBChB, who runs the U.K.’s largest menopause clinic, said primary care appointments are often time-pressured and follow a “’one problem-one consultation’” policy. As such, women are often thinking ‘Do I go with my joint pains, or my palpitations, tinnitus, or what?’ If a patient presents with tinnitus, a doctor might focus on the potential of an inner ear problem rather than a hormone deficiency, but I do know that if the woman is perimenopausal or menopausal, we often look to replace the missing hormones, and then if the tinnitus doesn’t improve we can revisit the ear problem.”
Dr. Newson noted that 17% of women in her clinics have had more than six GP visits in the year before she sees them, but in the year following, this figure drops to 1%. Acknowledging that a menopause consultation for a GP is time-consuming, Dr. Newson pointed out that taking time initially with the patient “means it will reduce the number of future consultations quickly, but more importantly, we also know that taking HRT reduces long-term risk of serious diseases, including heart disease and osteoporosis.”
The digital tool can be used by both doctors and patients to help women work through their symptoms and equip them with knowledge so their GP visits are more productive.
“When we see women who are empowered with knowledge [about menopause symptoms], then the consultations are quicker and essentially place the patient central to the discussion,” Dr. Newson said.
Ed Russell-Smith, MBChB, a GP in Scotland who moderated the session, said the tool “lays out a nicely structured approach and provides modern treatment options and resources for patients.”
However, he added “we also need to remember there are potential harms to be done from HRT too. It’s vitally important that while patients might see HRT as a panacea, doctors need to balance this with the risks involved for each individual. As a tool, I think Wellspring can help us in this respect to apply general principles to that patient and individualize treatment.”
Dr. Reisel, Dr. Newson, Ms. Kamal, and Dr. Russell-Smith disclosed no relevant financial relationships. The Wellspring Decision Aid was supported by UCL’s Institute for Women’s Health. The Newson Health clinic is fully private, but research is done via the nonprofit arm, which is supported by the clinic. There is no pharma involvement.
A version of this article first appeared on Medscape.com.
GLASGOW – An interactive digital decision tool that individualizes menopause care received praise from primary care clinicians in the United Kingdom, who said it could improve patient care and streamline office visits.
“Access to hormone replacement therapy [HRT], as well as decision-making around treatment for menopausal symptoms, is often complicated by concerns around its safety, and there is still a knowledge and a confidence gap among health care professionals causing reluctance to prescribe HRT,” said Aini Kamal, MSc, from University College London. Ms. Kamal presented results of a survey about the tool at the annual meeting of the Royal College of General Practitioners.
For the study, Ms. Kamal, Daniel Reisel, MBBS, PhD, a gynecologist at UCL, and colleagues evaluated Wellspring with doctors, nurses, and pharmacists.
“Ensuring that women receive education around symptoms, so that they are empowered, is a key part of optimizing their care and sharing decision-making,” Dr. Reisel said in an interview. He added that U.K. primary care had seen an increase in cases of women presenting with symptoms associated with the perimenopause and menopause at a time when U.K. Members of Parliament are debating whether to make it mandatory for all women to have menopause check-up in their early 40s.
The online survey was completed by 280 participants, and respondents were primarily GPs with several years of relevant prescribing practice. Of those, 93% found information from national guidelines to be accurately presented in the tool, and 97% said they would recommend this decision aid to other health care professionals, Ms. Kamal reported.
Nearly all participants said they could see themselves using the tool with patients in the clinic or as an adjunct to virtual sessions. “This [finding] was particularly important because it demonstrates the clinical potential this tool has,” she said.
One consult, too many problems
Louise Newson, MBChB, who runs the U.K.’s largest menopause clinic, said primary care appointments are often time-pressured and follow a “’one problem-one consultation’” policy. As such, women are often thinking ‘Do I go with my joint pains, or my palpitations, tinnitus, or what?’ If a patient presents with tinnitus, a doctor might focus on the potential of an inner ear problem rather than a hormone deficiency, but I do know that if the woman is perimenopausal or menopausal, we often look to replace the missing hormones, and then if the tinnitus doesn’t improve we can revisit the ear problem.”
Dr. Newson noted that 17% of women in her clinics have had more than six GP visits in the year before she sees them, but in the year following, this figure drops to 1%. Acknowledging that a menopause consultation for a GP is time-consuming, Dr. Newson pointed out that taking time initially with the patient “means it will reduce the number of future consultations quickly, but more importantly, we also know that taking HRT reduces long-term risk of serious diseases, including heart disease and osteoporosis.”
The digital tool can be used by both doctors and patients to help women work through their symptoms and equip them with knowledge so their GP visits are more productive.
“When we see women who are empowered with knowledge [about menopause symptoms], then the consultations are quicker and essentially place the patient central to the discussion,” Dr. Newson said.
Ed Russell-Smith, MBChB, a GP in Scotland who moderated the session, said the tool “lays out a nicely structured approach and provides modern treatment options and resources for patients.”
However, he added “we also need to remember there are potential harms to be done from HRT too. It’s vitally important that while patients might see HRT as a panacea, doctors need to balance this with the risks involved for each individual. As a tool, I think Wellspring can help us in this respect to apply general principles to that patient and individualize treatment.”
Dr. Reisel, Dr. Newson, Ms. Kamal, and Dr. Russell-Smith disclosed no relevant financial relationships. The Wellspring Decision Aid was supported by UCL’s Institute for Women’s Health. The Newson Health clinic is fully private, but research is done via the nonprofit arm, which is supported by the clinic. There is no pharma involvement.
A version of this article first appeared on Medscape.com.
AT RCGP 2023
Why aren’t doctors managing pain during gynecologic procedures?
During a fellowship rotation in gynecology, Rebekah D. Fenton, MD, asked the attending physicians what pain management options they could offer patients for insertion of an intrauterine device (IUD). Their answer surprised her: None.
The research on the effectiveness of pain management techniques during the procedure were not strong enough to warrant providing potential relief.
But Dr. Fenton knew the attending physician was wrong: She’d received the drug lidocaine during a recent visit to her own ob.gyn. to get an IUD placed. The local anesthetic enabled her to avoid the experiences of many patients who often withstand debilitating cramping and pain during insertion, side effects that can last for hours after the procedure has ended.
By not teaching her how to administer pain treatment options such as lidocaine gel or injection, “they made the decision for me, whether I could give patients this option,” said Dr. Fenton, now an adolescent medicine specialist at Alivio Medical Center in Chicago.
As a result, patients undergoing IUD placements, biopsies, hysteroscopies, and pelvic exams are often subject to pain that could be mitigated.
Some research suggests simple numbing agents, including lidocaine, may induce less pain without the need for full anesthesia. But clinicians don’t always present these options.
During gynecologic procedures, the amount of pain a patient can expect is often downplayed by clinicians. Because every patient experiences the sensation differently, discussing options for pain management and the range of possible pain is paramount in building patient-clinician trust, and ultimately providing the best care for patients in the long run, according to Megan Wasson, DO, chair of the department of medical and surgical gynecology at Mayo Clinic Arizona in Phoenix.
“It comes down to shared decision-making so the patient is aware of the pain that should be expected and what avenue they want to go down,” Dr. Wasson said. “It’s not a one-size-fits-all.”
Lack of uniform protocols
The American College of Obstetricians and Gynecologists (ACOG) has clear guidelines for pain management during pregnancy and delivery but not for many routine gynecologic procedures. Some experts say not offering options for pain management based on lack of efficacy evidence can undermine a patient’s experience.
ACOG does have recommendations for reducing dilation pain during a hysteroscopy, including providing intravaginal misoprostol and estrogen. The organization also recommends performing a vaginoscopy instead if possible because the procedure is typically less painful than is a hysteroscopy.
For an IUD placement, ACOG states that the procedure “may cause temporary discomfort” and recommends that patients take over-the-counter pain relief before a procedure. The most recent clinical bulletin on the topic, published in 2016, states routine misoprostol is not recommended for IUD placement, although it may be considered with difficult insertions for management of pain.
A clinical inquiry published in 2020 outlined the efficacy of several pain options that practitioners can weigh with patients. The inquiry cited a 2019 meta-analysis of 38 studies that found lidocaine-prilocaine cream to be the most effective option for pain management during IUD placement, reducing insertion pain by nearly 30%. The inquiry concluded that a combination of 600 mcg of misoprostol and 4% lidocaine gel may be effective, while lower dosages of both drugs were not effective. A 2018 clinical trial cited in the analysis found that though a 20-cc 1% lidocaine paracervical block on its own did not reduce pain, the block mixed with sodium bicarbonate reduced pain during IUD insertion by 22%.
Some doctors make the decision to not use lidocaine without offering it to patients first, according to Dr. Fenton. Instead, clinicians should discuss any potential drawbacks, such as pain from administering the numbing agent with a needle or the procedure taking extra time while the patient waits for the lidocaine to kick in.
“That always felt unfair, to make that decision for [the patient],” Dr. Fenton said.
Often clinicians won’t know how a patient will respond to a procedure: A 2014 secondary analysis of a clinical trial compared how patients rated their pain after an IUD procedure to the amount of pain physicians perceived the procedure to cause. They found that the average pain scores patients reported were nearly twice as high as clinician expectations were.
ACOG’s guideline states that the evidence backing paracervical blocks and lidocaine to IUD insertion pain is controversial. The American College of Physicians also cites “low-quality evidence” to support patient reports of pain and discomfort during pelvic exams. Some studies have found up to 60% of women report these negative experiences.
The varying evidence highlights the need for a personalized approach – one that includes patients – to pain management for routine gynecological procedures.
“Usually patients are pretty good predictors,” said Lisa Bayer, MD, MPH, associate professor of obstetrics and gynecology at Oregon Health & Science University in Portland. “They can anticipate what different things are going to feel like based on previous experiences.”
Making patients part of the discussion
Clinicians should have open discussions with patients about their past experiences and current anxieties about a gynecologic procedure, according to Dr. Bayer.
“Part of it is just creating a really safe environment of trust as a medical provider,” she said.
A study published in 2016 of more than 800 patients undergoing oocyte retrieval, which has clear protocols for pain management, found that previous negative gynecologic experiences were significantly correlated to greater amounts of pain reported during the procedure.
If pain isn’t properly managed, patients may avoid care in the future, putting them at risk for unplanned pregnancies, skipped cancer screenings, and complications from undiagnosed conditions and infections, Dr. Bayer added. Clinician offices will not always have access to all pain management options, so making referrals to another physician who has access to the appropriate technique may be the best thing for the patient, Dr. Bayer said.
Downplaying the experience
Informing a patient that she will feel only a little discomfort during a procedure – when a clinician doesn’t know how exactly the patient will react – can also result in distrust.
When a clinician says, “ ’It’s only going to be a little cramp, it’s only going to be a little pinch,’ we know extreme pain is a possibility, we’ve seen it,” Dr. Fenton said. “But if we choose to disregard that [possibility], it feels invalidating for patients.”
Failing to fully explain the possible pain scale can also directly interfere with the procedure at hand.
“My first concern is if they aren’t anticipating the amount of pain they are going to experience, they may move; For biopsies and IUD insertions, we need them to be still,” Dr. Wasson said. “If they are unable to tolerate the procedure, we’ve put them through pain and not been able to accomplish the primary goal.”
Managing both pain and what patients can expect is even more crucial for adolescent and teenage patients who are often having their first gynecologic experience.
“We’re framing what these experiences look like,” Dr. Fenton said. “That means there are opportunities for creating a space that builds trust and security for the patients moving forward.”
A version of this article first appeared on Medscape.com.
During a fellowship rotation in gynecology, Rebekah D. Fenton, MD, asked the attending physicians what pain management options they could offer patients for insertion of an intrauterine device (IUD). Their answer surprised her: None.
The research on the effectiveness of pain management techniques during the procedure were not strong enough to warrant providing potential relief.
But Dr. Fenton knew the attending physician was wrong: She’d received the drug lidocaine during a recent visit to her own ob.gyn. to get an IUD placed. The local anesthetic enabled her to avoid the experiences of many patients who often withstand debilitating cramping and pain during insertion, side effects that can last for hours after the procedure has ended.
By not teaching her how to administer pain treatment options such as lidocaine gel or injection, “they made the decision for me, whether I could give patients this option,” said Dr. Fenton, now an adolescent medicine specialist at Alivio Medical Center in Chicago.
As a result, patients undergoing IUD placements, biopsies, hysteroscopies, and pelvic exams are often subject to pain that could be mitigated.
Some research suggests simple numbing agents, including lidocaine, may induce less pain without the need for full anesthesia. But clinicians don’t always present these options.
During gynecologic procedures, the amount of pain a patient can expect is often downplayed by clinicians. Because every patient experiences the sensation differently, discussing options for pain management and the range of possible pain is paramount in building patient-clinician trust, and ultimately providing the best care for patients in the long run, according to Megan Wasson, DO, chair of the department of medical and surgical gynecology at Mayo Clinic Arizona in Phoenix.
“It comes down to shared decision-making so the patient is aware of the pain that should be expected and what avenue they want to go down,” Dr. Wasson said. “It’s not a one-size-fits-all.”
Lack of uniform protocols
The American College of Obstetricians and Gynecologists (ACOG) has clear guidelines for pain management during pregnancy and delivery but not for many routine gynecologic procedures. Some experts say not offering options for pain management based on lack of efficacy evidence can undermine a patient’s experience.
ACOG does have recommendations for reducing dilation pain during a hysteroscopy, including providing intravaginal misoprostol and estrogen. The organization also recommends performing a vaginoscopy instead if possible because the procedure is typically less painful than is a hysteroscopy.
For an IUD placement, ACOG states that the procedure “may cause temporary discomfort” and recommends that patients take over-the-counter pain relief before a procedure. The most recent clinical bulletin on the topic, published in 2016, states routine misoprostol is not recommended for IUD placement, although it may be considered with difficult insertions for management of pain.
A clinical inquiry published in 2020 outlined the efficacy of several pain options that practitioners can weigh with patients. The inquiry cited a 2019 meta-analysis of 38 studies that found lidocaine-prilocaine cream to be the most effective option for pain management during IUD placement, reducing insertion pain by nearly 30%. The inquiry concluded that a combination of 600 mcg of misoprostol and 4% lidocaine gel may be effective, while lower dosages of both drugs were not effective. A 2018 clinical trial cited in the analysis found that though a 20-cc 1% lidocaine paracervical block on its own did not reduce pain, the block mixed with sodium bicarbonate reduced pain during IUD insertion by 22%.
Some doctors make the decision to not use lidocaine without offering it to patients first, according to Dr. Fenton. Instead, clinicians should discuss any potential drawbacks, such as pain from administering the numbing agent with a needle or the procedure taking extra time while the patient waits for the lidocaine to kick in.
“That always felt unfair, to make that decision for [the patient],” Dr. Fenton said.
Often clinicians won’t know how a patient will respond to a procedure: A 2014 secondary analysis of a clinical trial compared how patients rated their pain after an IUD procedure to the amount of pain physicians perceived the procedure to cause. They found that the average pain scores patients reported were nearly twice as high as clinician expectations were.
ACOG’s guideline states that the evidence backing paracervical blocks and lidocaine to IUD insertion pain is controversial. The American College of Physicians also cites “low-quality evidence” to support patient reports of pain and discomfort during pelvic exams. Some studies have found up to 60% of women report these negative experiences.
The varying evidence highlights the need for a personalized approach – one that includes patients – to pain management for routine gynecological procedures.
“Usually patients are pretty good predictors,” said Lisa Bayer, MD, MPH, associate professor of obstetrics and gynecology at Oregon Health & Science University in Portland. “They can anticipate what different things are going to feel like based on previous experiences.”
Making patients part of the discussion
Clinicians should have open discussions with patients about their past experiences and current anxieties about a gynecologic procedure, according to Dr. Bayer.
“Part of it is just creating a really safe environment of trust as a medical provider,” she said.
A study published in 2016 of more than 800 patients undergoing oocyte retrieval, which has clear protocols for pain management, found that previous negative gynecologic experiences were significantly correlated to greater amounts of pain reported during the procedure.
If pain isn’t properly managed, patients may avoid care in the future, putting them at risk for unplanned pregnancies, skipped cancer screenings, and complications from undiagnosed conditions and infections, Dr. Bayer added. Clinician offices will not always have access to all pain management options, so making referrals to another physician who has access to the appropriate technique may be the best thing for the patient, Dr. Bayer said.
Downplaying the experience
Informing a patient that she will feel only a little discomfort during a procedure – when a clinician doesn’t know how exactly the patient will react – can also result in distrust.
When a clinician says, “ ’It’s only going to be a little cramp, it’s only going to be a little pinch,’ we know extreme pain is a possibility, we’ve seen it,” Dr. Fenton said. “But if we choose to disregard that [possibility], it feels invalidating for patients.”
Failing to fully explain the possible pain scale can also directly interfere with the procedure at hand.
“My first concern is if they aren’t anticipating the amount of pain they are going to experience, they may move; For biopsies and IUD insertions, we need them to be still,” Dr. Wasson said. “If they are unable to tolerate the procedure, we’ve put them through pain and not been able to accomplish the primary goal.”
Managing both pain and what patients can expect is even more crucial for adolescent and teenage patients who are often having their first gynecologic experience.
“We’re framing what these experiences look like,” Dr. Fenton said. “That means there are opportunities for creating a space that builds trust and security for the patients moving forward.”
A version of this article first appeared on Medscape.com.
During a fellowship rotation in gynecology, Rebekah D. Fenton, MD, asked the attending physicians what pain management options they could offer patients for insertion of an intrauterine device (IUD). Their answer surprised her: None.
The research on the effectiveness of pain management techniques during the procedure were not strong enough to warrant providing potential relief.
But Dr. Fenton knew the attending physician was wrong: She’d received the drug lidocaine during a recent visit to her own ob.gyn. to get an IUD placed. The local anesthetic enabled her to avoid the experiences of many patients who often withstand debilitating cramping and pain during insertion, side effects that can last for hours after the procedure has ended.
By not teaching her how to administer pain treatment options such as lidocaine gel or injection, “they made the decision for me, whether I could give patients this option,” said Dr. Fenton, now an adolescent medicine specialist at Alivio Medical Center in Chicago.
As a result, patients undergoing IUD placements, biopsies, hysteroscopies, and pelvic exams are often subject to pain that could be mitigated.
Some research suggests simple numbing agents, including lidocaine, may induce less pain without the need for full anesthesia. But clinicians don’t always present these options.
During gynecologic procedures, the amount of pain a patient can expect is often downplayed by clinicians. Because every patient experiences the sensation differently, discussing options for pain management and the range of possible pain is paramount in building patient-clinician trust, and ultimately providing the best care for patients in the long run, according to Megan Wasson, DO, chair of the department of medical and surgical gynecology at Mayo Clinic Arizona in Phoenix.
“It comes down to shared decision-making so the patient is aware of the pain that should be expected and what avenue they want to go down,” Dr. Wasson said. “It’s not a one-size-fits-all.”
Lack of uniform protocols
The American College of Obstetricians and Gynecologists (ACOG) has clear guidelines for pain management during pregnancy and delivery but not for many routine gynecologic procedures. Some experts say not offering options for pain management based on lack of efficacy evidence can undermine a patient’s experience.
ACOG does have recommendations for reducing dilation pain during a hysteroscopy, including providing intravaginal misoprostol and estrogen. The organization also recommends performing a vaginoscopy instead if possible because the procedure is typically less painful than is a hysteroscopy.
For an IUD placement, ACOG states that the procedure “may cause temporary discomfort” and recommends that patients take over-the-counter pain relief before a procedure. The most recent clinical bulletin on the topic, published in 2016, states routine misoprostol is not recommended for IUD placement, although it may be considered with difficult insertions for management of pain.
A clinical inquiry published in 2020 outlined the efficacy of several pain options that practitioners can weigh with patients. The inquiry cited a 2019 meta-analysis of 38 studies that found lidocaine-prilocaine cream to be the most effective option for pain management during IUD placement, reducing insertion pain by nearly 30%. The inquiry concluded that a combination of 600 mcg of misoprostol and 4% lidocaine gel may be effective, while lower dosages of both drugs were not effective. A 2018 clinical trial cited in the analysis found that though a 20-cc 1% lidocaine paracervical block on its own did not reduce pain, the block mixed with sodium bicarbonate reduced pain during IUD insertion by 22%.
Some doctors make the decision to not use lidocaine without offering it to patients first, according to Dr. Fenton. Instead, clinicians should discuss any potential drawbacks, such as pain from administering the numbing agent with a needle or the procedure taking extra time while the patient waits for the lidocaine to kick in.
“That always felt unfair, to make that decision for [the patient],” Dr. Fenton said.
Often clinicians won’t know how a patient will respond to a procedure: A 2014 secondary analysis of a clinical trial compared how patients rated their pain after an IUD procedure to the amount of pain physicians perceived the procedure to cause. They found that the average pain scores patients reported were nearly twice as high as clinician expectations were.
ACOG’s guideline states that the evidence backing paracervical blocks and lidocaine to IUD insertion pain is controversial. The American College of Physicians also cites “low-quality evidence” to support patient reports of pain and discomfort during pelvic exams. Some studies have found up to 60% of women report these negative experiences.
The varying evidence highlights the need for a personalized approach – one that includes patients – to pain management for routine gynecological procedures.
“Usually patients are pretty good predictors,” said Lisa Bayer, MD, MPH, associate professor of obstetrics and gynecology at Oregon Health & Science University in Portland. “They can anticipate what different things are going to feel like based on previous experiences.”
Making patients part of the discussion
Clinicians should have open discussions with patients about their past experiences and current anxieties about a gynecologic procedure, according to Dr. Bayer.
“Part of it is just creating a really safe environment of trust as a medical provider,” she said.
A study published in 2016 of more than 800 patients undergoing oocyte retrieval, which has clear protocols for pain management, found that previous negative gynecologic experiences were significantly correlated to greater amounts of pain reported during the procedure.
If pain isn’t properly managed, patients may avoid care in the future, putting them at risk for unplanned pregnancies, skipped cancer screenings, and complications from undiagnosed conditions and infections, Dr. Bayer added. Clinician offices will not always have access to all pain management options, so making referrals to another physician who has access to the appropriate technique may be the best thing for the patient, Dr. Bayer said.
Downplaying the experience
Informing a patient that she will feel only a little discomfort during a procedure – when a clinician doesn’t know how exactly the patient will react – can also result in distrust.
When a clinician says, “ ’It’s only going to be a little cramp, it’s only going to be a little pinch,’ we know extreme pain is a possibility, we’ve seen it,” Dr. Fenton said. “But if we choose to disregard that [possibility], it feels invalidating for patients.”
Failing to fully explain the possible pain scale can also directly interfere with the procedure at hand.
“My first concern is if they aren’t anticipating the amount of pain they are going to experience, they may move; For biopsies and IUD insertions, we need them to be still,” Dr. Wasson said. “If they are unable to tolerate the procedure, we’ve put them through pain and not been able to accomplish the primary goal.”
Managing both pain and what patients can expect is even more crucial for adolescent and teenage patients who are often having their first gynecologic experience.
“We’re framing what these experiences look like,” Dr. Fenton said. “That means there are opportunities for creating a space that builds trust and security for the patients moving forward.”
A version of this article first appeared on Medscape.com.
Older adults at risk from inappropriate prescribing
Roughly 2% of prescriptions to older patients appear to be inappropriate – but the figure does not appear to differ between physicians and nurse practitioners, according to a study published in Annals of Internal Medicine.
Older adults are “especially vulnerable to adverse drug events from inappropriate prescribing due to comorbidities and aging-related physiological changes,” said Johnny Huynh, MA, doctoral candidate in economics at UCLA and lead author of the study. “Considering the volume of prescriptions for older adults, even a small percentage can translate to a big impact on adverse drug events and spending.”
In recent years, more states have granted prescriptive authority to NPs, while professional medical organizations have opposed the reforms and made claims about differences in quality of care.
The medical community must focus on the prescribing performance of individual clinicians rather than whether an NP has prescriptive authority, said David Studdert, LLB, ScD, MPH, professor of health policy at Stanford (Calif.) University and a co-author of the study.
“Don’t fixate on whether nurse practitioners have prescriptive authority or don’t,” said Mr. Studdert. “Just try to identify those practitioners who need to boost their performance.”
The investigators found that rates of potentially inappropriate prescribing were “virtually identical.” Adjusted rates were 1.66 per 100 prescriptions for NPs versus 1.68 per 100 prescriptions for physicians (adjusted odds ratio, 0.99; 95% confidence interval, 0.97-1.01).
“Older adults often have more than one chronic condition and are prescribed multiple medications to manage these conditions, putting them at risk for adverse events,” said Paula Rochon, MD, MPH, founding director of the Women’s Age Lab and professor in the Division of Geriatric Medicine at Dalla Lana School of Public Health in Toronto. “Furthermore, older women are more likely than men to have multiple medical problems and experience adverse drug events.”
Dr. Rochon led a 2021 research review on polypharmacy and inappropriate prescribing among older adults in both the United States and abroad. She and her team noted that while women are physiologically more susceptible to drug-related harm, rates of inappropriate prescribing also tend to be higher for women, such as in the case of senior U.S. veterans and older adults in Canada.
The researchers analyzed data over a 7-year period starting in 2013 from 23,669 primary care NPs and 50,060 physicians who wrote prescriptions for at least 100 patients with Medicare Part D coverage. Data from 29 states, which had all expanded prescriptive authority to NPs, was included.
Prescriptive quality was defined by the American Geriatrics Society’s Beers Criteria, a list of potentially inappropriate medications (PIMs) for adults ages 65 and over. Mr. Studdert said it’s important to note the nuance in the Beers Criteria.
“It’s not to say that there may not be certain clinical circumstances where it’s appropriate to” prescribe these drugs, Mr. Studdert said, “But generally, it’s not appropriate.”
Ten medications accounted for 99.5% of the PIMs prescribed, including drugs that were antidepressants, muscle relaxants, hypnotics, antihistamines (generation 1), antispasmodics, sulfonylureas, barbiturates, antineoplastics, thyroid medications, and nonsteroidal anti-inflammatory drugs.
The top three most frequently potentially inappropriately prescribed were antidepressants (0.393 NPs vs. 0.481 PCPs per 100 prescriptions), muscle relaxants (0.372 NPs vs. 0.305 PCPs per 100), and hypnotics (0.364 NPs vs. 0.440 PCPs per 100). Both antidepressants and hypnotics are associated with an increased risk for falls and fractures among older adults, while muscle relaxants have been shown to increase the risk for hospitalization in this population.
Despite the overall similar PIM rates, NPs were more present in the “tails,” or highest and lowest end of the quality bell curve. The higher variation among NPs means these patients are at a higher risk of receiving a prescription for an inappropriate medication, said David Chan, MD, PhD, associate professor of health policy at Stanford (Calif.) School of Medicine, and a co-author of the study.
Other studies have shown “high-intensity prescribers” were more likely to dispense drugs like benzodiazepines and opioids, which can be harmful to older patients.
According to Dr. Rochon, clinicians should use the Beers Criteria and STOPP/START Criteria to guide decision-making, along with the DRUGS framework, which follows a geriatric medicine approach that advises clinicians to discuss goals of care with their patients and conduct routine reviews of medications.
Prescribers should also avoid prescribing cascades, which “occur when a drug is prescribed, an adverse event occurs that is misinterpreted as a new medical condition, and a further drug is prescribed to treat that medical condition,” Dr. Rochon said.
To reduce cascades, “it’s important to document when a medication was started, why it was started, and who started it so that this information is available when evaluating if a medication continues to be needed,” she said.
The study was funded by grants from Robert Wood Johnson Foundation and National Science Foundation. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Roughly 2% of prescriptions to older patients appear to be inappropriate – but the figure does not appear to differ between physicians and nurse practitioners, according to a study published in Annals of Internal Medicine.
Older adults are “especially vulnerable to adverse drug events from inappropriate prescribing due to comorbidities and aging-related physiological changes,” said Johnny Huynh, MA, doctoral candidate in economics at UCLA and lead author of the study. “Considering the volume of prescriptions for older adults, even a small percentage can translate to a big impact on adverse drug events and spending.”
In recent years, more states have granted prescriptive authority to NPs, while professional medical organizations have opposed the reforms and made claims about differences in quality of care.
The medical community must focus on the prescribing performance of individual clinicians rather than whether an NP has prescriptive authority, said David Studdert, LLB, ScD, MPH, professor of health policy at Stanford (Calif.) University and a co-author of the study.
“Don’t fixate on whether nurse practitioners have prescriptive authority or don’t,” said Mr. Studdert. “Just try to identify those practitioners who need to boost their performance.”
The investigators found that rates of potentially inappropriate prescribing were “virtually identical.” Adjusted rates were 1.66 per 100 prescriptions for NPs versus 1.68 per 100 prescriptions for physicians (adjusted odds ratio, 0.99; 95% confidence interval, 0.97-1.01).
“Older adults often have more than one chronic condition and are prescribed multiple medications to manage these conditions, putting them at risk for adverse events,” said Paula Rochon, MD, MPH, founding director of the Women’s Age Lab and professor in the Division of Geriatric Medicine at Dalla Lana School of Public Health in Toronto. “Furthermore, older women are more likely than men to have multiple medical problems and experience adverse drug events.”
Dr. Rochon led a 2021 research review on polypharmacy and inappropriate prescribing among older adults in both the United States and abroad. She and her team noted that while women are physiologically more susceptible to drug-related harm, rates of inappropriate prescribing also tend to be higher for women, such as in the case of senior U.S. veterans and older adults in Canada.
The researchers analyzed data over a 7-year period starting in 2013 from 23,669 primary care NPs and 50,060 physicians who wrote prescriptions for at least 100 patients with Medicare Part D coverage. Data from 29 states, which had all expanded prescriptive authority to NPs, was included.
Prescriptive quality was defined by the American Geriatrics Society’s Beers Criteria, a list of potentially inappropriate medications (PIMs) for adults ages 65 and over. Mr. Studdert said it’s important to note the nuance in the Beers Criteria.
“It’s not to say that there may not be certain clinical circumstances where it’s appropriate to” prescribe these drugs, Mr. Studdert said, “But generally, it’s not appropriate.”
Ten medications accounted for 99.5% of the PIMs prescribed, including drugs that were antidepressants, muscle relaxants, hypnotics, antihistamines (generation 1), antispasmodics, sulfonylureas, barbiturates, antineoplastics, thyroid medications, and nonsteroidal anti-inflammatory drugs.
The top three most frequently potentially inappropriately prescribed were antidepressants (0.393 NPs vs. 0.481 PCPs per 100 prescriptions), muscle relaxants (0.372 NPs vs. 0.305 PCPs per 100), and hypnotics (0.364 NPs vs. 0.440 PCPs per 100). Both antidepressants and hypnotics are associated with an increased risk for falls and fractures among older adults, while muscle relaxants have been shown to increase the risk for hospitalization in this population.
Despite the overall similar PIM rates, NPs were more present in the “tails,” or highest and lowest end of the quality bell curve. The higher variation among NPs means these patients are at a higher risk of receiving a prescription for an inappropriate medication, said David Chan, MD, PhD, associate professor of health policy at Stanford (Calif.) School of Medicine, and a co-author of the study.
Other studies have shown “high-intensity prescribers” were more likely to dispense drugs like benzodiazepines and opioids, which can be harmful to older patients.
According to Dr. Rochon, clinicians should use the Beers Criteria and STOPP/START Criteria to guide decision-making, along with the DRUGS framework, which follows a geriatric medicine approach that advises clinicians to discuss goals of care with their patients and conduct routine reviews of medications.
Prescribers should also avoid prescribing cascades, which “occur when a drug is prescribed, an adverse event occurs that is misinterpreted as a new medical condition, and a further drug is prescribed to treat that medical condition,” Dr. Rochon said.
To reduce cascades, “it’s important to document when a medication was started, why it was started, and who started it so that this information is available when evaluating if a medication continues to be needed,” she said.
The study was funded by grants from Robert Wood Johnson Foundation and National Science Foundation. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Roughly 2% of prescriptions to older patients appear to be inappropriate – but the figure does not appear to differ between physicians and nurse practitioners, according to a study published in Annals of Internal Medicine.
Older adults are “especially vulnerable to adverse drug events from inappropriate prescribing due to comorbidities and aging-related physiological changes,” said Johnny Huynh, MA, doctoral candidate in economics at UCLA and lead author of the study. “Considering the volume of prescriptions for older adults, even a small percentage can translate to a big impact on adverse drug events and spending.”
In recent years, more states have granted prescriptive authority to NPs, while professional medical organizations have opposed the reforms and made claims about differences in quality of care.
The medical community must focus on the prescribing performance of individual clinicians rather than whether an NP has prescriptive authority, said David Studdert, LLB, ScD, MPH, professor of health policy at Stanford (Calif.) University and a co-author of the study.
“Don’t fixate on whether nurse practitioners have prescriptive authority or don’t,” said Mr. Studdert. “Just try to identify those practitioners who need to boost their performance.”
The investigators found that rates of potentially inappropriate prescribing were “virtually identical.” Adjusted rates were 1.66 per 100 prescriptions for NPs versus 1.68 per 100 prescriptions for physicians (adjusted odds ratio, 0.99; 95% confidence interval, 0.97-1.01).
“Older adults often have more than one chronic condition and are prescribed multiple medications to manage these conditions, putting them at risk for adverse events,” said Paula Rochon, MD, MPH, founding director of the Women’s Age Lab and professor in the Division of Geriatric Medicine at Dalla Lana School of Public Health in Toronto. “Furthermore, older women are more likely than men to have multiple medical problems and experience adverse drug events.”
Dr. Rochon led a 2021 research review on polypharmacy and inappropriate prescribing among older adults in both the United States and abroad. She and her team noted that while women are physiologically more susceptible to drug-related harm, rates of inappropriate prescribing also tend to be higher for women, such as in the case of senior U.S. veterans and older adults in Canada.
The researchers analyzed data over a 7-year period starting in 2013 from 23,669 primary care NPs and 50,060 physicians who wrote prescriptions for at least 100 patients with Medicare Part D coverage. Data from 29 states, which had all expanded prescriptive authority to NPs, was included.
Prescriptive quality was defined by the American Geriatrics Society’s Beers Criteria, a list of potentially inappropriate medications (PIMs) for adults ages 65 and over. Mr. Studdert said it’s important to note the nuance in the Beers Criteria.
“It’s not to say that there may not be certain clinical circumstances where it’s appropriate to” prescribe these drugs, Mr. Studdert said, “But generally, it’s not appropriate.”
Ten medications accounted for 99.5% of the PIMs prescribed, including drugs that were antidepressants, muscle relaxants, hypnotics, antihistamines (generation 1), antispasmodics, sulfonylureas, barbiturates, antineoplastics, thyroid medications, and nonsteroidal anti-inflammatory drugs.
The top three most frequently potentially inappropriately prescribed were antidepressants (0.393 NPs vs. 0.481 PCPs per 100 prescriptions), muscle relaxants (0.372 NPs vs. 0.305 PCPs per 100), and hypnotics (0.364 NPs vs. 0.440 PCPs per 100). Both antidepressants and hypnotics are associated with an increased risk for falls and fractures among older adults, while muscle relaxants have been shown to increase the risk for hospitalization in this population.
Despite the overall similar PIM rates, NPs were more present in the “tails,” or highest and lowest end of the quality bell curve. The higher variation among NPs means these patients are at a higher risk of receiving a prescription for an inappropriate medication, said David Chan, MD, PhD, associate professor of health policy at Stanford (Calif.) School of Medicine, and a co-author of the study.
Other studies have shown “high-intensity prescribers” were more likely to dispense drugs like benzodiazepines and opioids, which can be harmful to older patients.
According to Dr. Rochon, clinicians should use the Beers Criteria and STOPP/START Criteria to guide decision-making, along with the DRUGS framework, which follows a geriatric medicine approach that advises clinicians to discuss goals of care with their patients and conduct routine reviews of medications.
Prescribers should also avoid prescribing cascades, which “occur when a drug is prescribed, an adverse event occurs that is misinterpreted as a new medical condition, and a further drug is prescribed to treat that medical condition,” Dr. Rochon said.
To reduce cascades, “it’s important to document when a medication was started, why it was started, and who started it so that this information is available when evaluating if a medication continues to be needed,” she said.
The study was funded by grants from Robert Wood Johnson Foundation and National Science Foundation. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Perinatal depression rarely stands alone
Mental health conditions are the leading cause of pregnancy-related death in Illinois (40%) and across the United States (21%).1,2 Funding bodies, such as the Agency for Healthcare Research and Quality3 and the Health Resources and Service Administration,4 have spotlights on improving screening and access to care for depression and substance use disorders (SUDs). However, the needs of individuals with multiple mental health conditions still often go unrecognized and unaddressed in perinatal health settings.
The U.S. Preventive Services Task Force recommends that all adults be screened for depression, alcohol use, and drug use, and will be recommending screening for anxiety.5,6 The American College of Obstetrics and Gynecology recommends screening for perinatal mental health conditions including depression, anxiety, bipolar disorder, acute postpartum psychosis, and suicidality; however, despite these recommendations, screening and treatment for comorbid mental health disorders during pregnancy and the postpartum is not standard practice.7
Addressing perinatal mental health is critical because untreated mental health conditions during the perinatal period can cause long-term adverse psychiatric and medical outcomes for the birthing person, the baby, and the family.8 This commentary highlights the importance of recognizing and screening for perinatal mental health comorbidities, improving referral rates for mental health treatment, and raising awareness of the importance of addressing rural perinatal mental health.
Perinatal mental health comorbidities
Major depressive disorder is the most common mental health condition during the perinatal period9 and is often comorbid.10-12 In “Perinatal mental health in low-income urban and rural patients: The importance of screening for comorbidities,” Craemer et al.13 reported that nearly half of the perinatal patients who screened positive for MDD also screened positive for at least one other mental health condition, among them general anxiety disorder (GAD), SUD, posttraumatic stress disorder (PTSD), and suicidality.
Many (9%) of the perinatal patients with MDD had a severe comorbidity profile characterized by four diagnoses – MDD, GAD, SUD, and PTSD. In routine medical care these comorbidities often go undetected even though the risk to mothers and babies increases with more severe mental health symptoms.8
The high frequency of perinatal mental health comorbidities Craemer et al.13 found demonstrates a compelling need for comorbid mental health screening during the perinatal period, particularly for low-income Black, Hispanic, and rural birthing persons. Positive screens for perinatal mental health disorders may reflect the onset of these disorders in pregnancy or the postpartum, or preexisting disorders that have gone undetected or untreated before pregnancy.
For many patients, the perinatal period is the first time they are screened for any mental health disorder; typically, they are screened solely for depression. Screening alone can have a positive impact on perinatal mental health. In fact, the USPSTF found that programs to screen perinatal patients, with or without treatment-related support, resulted in a 2%-9% absolute reduction in depression prevalence.14 However, screening for MDD is too infrequent for many reasons, including the logistics of integrating screening into the clinic workflow and limited provider availability, time, and training in mental health.
We recommend screening perinatal patients for mental health comorbidities. This recommendation may seem impractical given the lack of screening tools for comorbid mental health conditions; however, the Computerized Adaptive Test for Mental Health (CAT-MH), the validated tool15-17 used in this study, is an ideal option. CAT-MH is uniquely capable of screening for MDD, GAD, PTSD, SUD, and suicidality in one platform and is routinely used in diverse settings including the Veterans Administration,18 foster care,19 and universities.20 The main limitation of this more comprehensive screening is that it takes about 10 minutes per patient. However, CAT-MH is self-administered and can be done in the waiting room or on a mobile device prior to a clinic visit.
CAT-MH can also be easily integrated into clinical workflow when added to the Electronic Medical Record21, and is a more comprehensive tool than existing perinatal depression tools such as the Perinatal Health Questionaire-9 (PHQ-9) and Edinburgh Perinatal Depression Scale (EPDS).22 Another limitation is cost – currently $5.00 per assessment – however, this is less than routine blood work.23 If CAT-MH is not an option, we recommend a stepped approach of screening for GAD when perinatal patients screen positive for MDD, as this is the most common comorbidity profile. The GAD-7 is a free and widely available tool.24
Barriers to care
In Craemer et al,13 nearly two-thirds (64.9%) of perinatal patients with a positive screen did not receive a referral to follow-up care or a medication prescription. These low referral rates may reflect a variety of widely recognized barriers to care, including lack of referral options, provider and/or patient reluctance to pursue referrals, barriers to insurance coverage, or inadequate behavioral health infrastructure to ensure referral and diagnostic follow-up.
Further, rural residing perinatal patients are an underserved population that need more resources and screening. Despite an on-site behavioral specialist at the rural clinic, Craemer et al13 found a stark disparity in referral rates: referrals to treatment for a positive diagnosis was over two times less at the rural clinic (23.9%), compared with the urban clinics (51.6%). The most common treatment offered at the rural clinic was a prescription for medication (17.4%), while referral to follow-up care was the most common at the urban clinics (35.5%). Rural areas not only have a shortage of health care providers, but community members seeking mental health care often encounter greater stigma, compared with urban residents.25,26
These data highlight an unmet need for referrals to treatment for patients in rural communities, particularly in Illinois where the pregnancy-related mortality ratio attributable to mental health conditions is three times greater in rural areas, compared with those residing in urban Cook County (Chicago).2 Increasing access and availability to mental health treatment and prevention resources in Illinois, especially in rural areas, is an opportunity to prevent pregnancy-related mortality attributable to mental health conditions.
Overall, there is a critical need for screening for perinatal mental health comorbidities, increased attention to low rates of referral to mental health treatment, and investing in rural perinatal mental health. Addressing perinatal mental health disorders is key to decreasing the burden of maternal mortality, particularly in Illinois.
Ms. Craemer and Ms. Sayah are senior research specialists at the Center for Research on Women & Gender, University of Illinois at Chicago. Dr. Duffecy is a professor of clinical psychiatry at the University of Illinois at Chicago. Dr. Geller is a professor of obstetrics & gynecology and director of the Center for Research on Women & Gender, University of Illinois at Chicago. Dr. Maki is a professor of psychiatry, psychology, and obstetrics & gynecology at the University of Illinois at Chicago.
References
1. Trost S et al. Pregnancy-related deaths: Data from maternal mortality review committees in 36 states, 2017-2019. Atlanta: Centers for Disease Control and Prevention, U.S. Department of Health & Human Services, 2022.
2. Illinois Department of Public Health. Illinois maternal morbidity and mortality report 2016-2017. 2021.
3. AHRQ. Funding opportunities to address opioid and other substance use disorders. Updated 2023.
4. HRSA. Screening and treatment for maternal mental health and substance use disorders.
5. U.S. Preventive Services Task Force. Recommendations for primary care practice. Accessed May 26, 2023.
6. U.S. Preventive Services Task Force. Draft recommendation statement: Anxiety in adults: Screening. 2022.
7. ACOG. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline. Number 4. 2023 June.
8. Meltzer-Brody S and Stuebe A. The long-term psychiatric and medical prognosis of perinatal mental illness. Best Pract Res Clin Obstet Gynaecol. 2014 Jan. doi: 10.1016/j.bpobgyn.2013.08.009.
9. Van Niel MS and Payne JL. Perinatal depression: A review. Cleve Clin J Med. 2020 May. doi: 10.3949/ccjm.87a.19054.
10. Wisner KL et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. 2013 May. doi: 10.1001/jamapsychiatry.2013.87.
11. Falah-Hassani K et al. The prevalence of antenatal and postnatal co-morbid anxiety and depression: A meta-analysis. Psychol Med. 2017 Sep. doi: 10.1017/S0033291717000617.
12. Pentecost R et al. Scoping review of the associations between perinatal substance use and perinatal depression and anxiety. J Obstet Gynecol Neonatal Nurs. 2021 Jul. doi: 10.1016/j.jogn.2021.02.008.
13. Craemer KA et al. Perinatal mental health in low-income urban and rural patients: The importance of screening for comorbidities. Gen Hosp Psychiatry. 2023 Jul-Aug. doi: 10.1016/j.genhosppsych.2023.05.007.
14. O’Connor E et al. Primary care screening for and treatment of depression in pregnant and postpartum women: Evidence report and systematic review for the U.S. Preventive Services Task Force. JAMA. 2016 Jan 26. doi: 10.1001/jama.2015.18948.
15. Kozhimannil KB et al. Racial and ethnic disparities in postpartum depression care among low-income women. Psychiatr Serv. 2011 Jun. doi: 10.1176/ps.62.6.pss6206_0619.
16. Wenzel ES et al. Depression and anxiety symptoms across pregnancy and the postpartum in low-income Black and Latina women. Arch Womens Ment Health. 2021 Dec. doi: 10.1007/s00737-021-01139-y.
17. Gibbons RD et al. Development of a computerized adaptive substance use disorder scale for screening and measurement: The CAT‐SUD. Addiction. 2020 Jul. doi: 10.1111/add.14938.
18. Brenner LA et al. Validation of a computerized adaptive test suicide scale (CAT-SS) among united states military veterans. PloS One. 2022 Jan 21. doi: 10.1371/journal.pone.0261920.
19. The Center for State Child Welfare Data. Using technology to diagnose and report on behavioral health challenges facing foster youth. 2018.
20. Kim JJ et al. The experience of depression, anxiety, and mania among perinatal women. Arch Womens Ment Health. 2016 Oct. doi: 10.1007/s00737-016-0632-6.
21. Tepper MC et al. Toward population health: Using a learning behavioral health system and measurement-based care to improve access, care, outcomes, and disparities. Community Ment Health J. 2022 Nov. doi: 10.1007/s10597-022-00957-3.
22. Wenzel E et al. Using computerised adaptive tests to screen for perinatal depression in underserved women of colour. Evid Based Ment Health. 2022 Feb. doi: 10.1136/ebmental-2021-300262.
23. Sanger-Katz M. They want it to be secret: How a common blood test can cost $11 or almost $1,000. New York Times. 2019 Apr 19.
24. Spitzer RL et al. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006 May 22. doi: 10.1001/archinte.166.10.1092.
25. Mollard E et al. An integrative review of postpartum depression in rural US communities. Arch Psychiatr Nurs. 2016 Jun. doi: 10.1016/j.apnu.2015.12.003.
26. Anglim AJ and Radke SM. Rural maternal health care outcomes, drivers, and patient perspectives. Clin Obstet Gynecol. 2022 Dec 1. doi: 10.1097/GRF.0000000000000753.
Mental health conditions are the leading cause of pregnancy-related death in Illinois (40%) and across the United States (21%).1,2 Funding bodies, such as the Agency for Healthcare Research and Quality3 and the Health Resources and Service Administration,4 have spotlights on improving screening and access to care for depression and substance use disorders (SUDs). However, the needs of individuals with multiple mental health conditions still often go unrecognized and unaddressed in perinatal health settings.
The U.S. Preventive Services Task Force recommends that all adults be screened for depression, alcohol use, and drug use, and will be recommending screening for anxiety.5,6 The American College of Obstetrics and Gynecology recommends screening for perinatal mental health conditions including depression, anxiety, bipolar disorder, acute postpartum psychosis, and suicidality; however, despite these recommendations, screening and treatment for comorbid mental health disorders during pregnancy and the postpartum is not standard practice.7
Addressing perinatal mental health is critical because untreated mental health conditions during the perinatal period can cause long-term adverse psychiatric and medical outcomes for the birthing person, the baby, and the family.8 This commentary highlights the importance of recognizing and screening for perinatal mental health comorbidities, improving referral rates for mental health treatment, and raising awareness of the importance of addressing rural perinatal mental health.
Perinatal mental health comorbidities
Major depressive disorder is the most common mental health condition during the perinatal period9 and is often comorbid.10-12 In “Perinatal mental health in low-income urban and rural patients: The importance of screening for comorbidities,” Craemer et al.13 reported that nearly half of the perinatal patients who screened positive for MDD also screened positive for at least one other mental health condition, among them general anxiety disorder (GAD), SUD, posttraumatic stress disorder (PTSD), and suicidality.
Many (9%) of the perinatal patients with MDD had a severe comorbidity profile characterized by four diagnoses – MDD, GAD, SUD, and PTSD. In routine medical care these comorbidities often go undetected even though the risk to mothers and babies increases with more severe mental health symptoms.8
The high frequency of perinatal mental health comorbidities Craemer et al.13 found demonstrates a compelling need for comorbid mental health screening during the perinatal period, particularly for low-income Black, Hispanic, and rural birthing persons. Positive screens for perinatal mental health disorders may reflect the onset of these disorders in pregnancy or the postpartum, or preexisting disorders that have gone undetected or untreated before pregnancy.
For many patients, the perinatal period is the first time they are screened for any mental health disorder; typically, they are screened solely for depression. Screening alone can have a positive impact on perinatal mental health. In fact, the USPSTF found that programs to screen perinatal patients, with or without treatment-related support, resulted in a 2%-9% absolute reduction in depression prevalence.14 However, screening for MDD is too infrequent for many reasons, including the logistics of integrating screening into the clinic workflow and limited provider availability, time, and training in mental health.
We recommend screening perinatal patients for mental health comorbidities. This recommendation may seem impractical given the lack of screening tools for comorbid mental health conditions; however, the Computerized Adaptive Test for Mental Health (CAT-MH), the validated tool15-17 used in this study, is an ideal option. CAT-MH is uniquely capable of screening for MDD, GAD, PTSD, SUD, and suicidality in one platform and is routinely used in diverse settings including the Veterans Administration,18 foster care,19 and universities.20 The main limitation of this more comprehensive screening is that it takes about 10 minutes per patient. However, CAT-MH is self-administered and can be done in the waiting room or on a mobile device prior to a clinic visit.
CAT-MH can also be easily integrated into clinical workflow when added to the Electronic Medical Record21, and is a more comprehensive tool than existing perinatal depression tools such as the Perinatal Health Questionaire-9 (PHQ-9) and Edinburgh Perinatal Depression Scale (EPDS).22 Another limitation is cost – currently $5.00 per assessment – however, this is less than routine blood work.23 If CAT-MH is not an option, we recommend a stepped approach of screening for GAD when perinatal patients screen positive for MDD, as this is the most common comorbidity profile. The GAD-7 is a free and widely available tool.24
Barriers to care
In Craemer et al,13 nearly two-thirds (64.9%) of perinatal patients with a positive screen did not receive a referral to follow-up care or a medication prescription. These low referral rates may reflect a variety of widely recognized barriers to care, including lack of referral options, provider and/or patient reluctance to pursue referrals, barriers to insurance coverage, or inadequate behavioral health infrastructure to ensure referral and diagnostic follow-up.
Further, rural residing perinatal patients are an underserved population that need more resources and screening. Despite an on-site behavioral specialist at the rural clinic, Craemer et al13 found a stark disparity in referral rates: referrals to treatment for a positive diagnosis was over two times less at the rural clinic (23.9%), compared with the urban clinics (51.6%). The most common treatment offered at the rural clinic was a prescription for medication (17.4%), while referral to follow-up care was the most common at the urban clinics (35.5%). Rural areas not only have a shortage of health care providers, but community members seeking mental health care often encounter greater stigma, compared with urban residents.25,26
These data highlight an unmet need for referrals to treatment for patients in rural communities, particularly in Illinois where the pregnancy-related mortality ratio attributable to mental health conditions is three times greater in rural areas, compared with those residing in urban Cook County (Chicago).2 Increasing access and availability to mental health treatment and prevention resources in Illinois, especially in rural areas, is an opportunity to prevent pregnancy-related mortality attributable to mental health conditions.
Overall, there is a critical need for screening for perinatal mental health comorbidities, increased attention to low rates of referral to mental health treatment, and investing in rural perinatal mental health. Addressing perinatal mental health disorders is key to decreasing the burden of maternal mortality, particularly in Illinois.
Ms. Craemer and Ms. Sayah are senior research specialists at the Center for Research on Women & Gender, University of Illinois at Chicago. Dr. Duffecy is a professor of clinical psychiatry at the University of Illinois at Chicago. Dr. Geller is a professor of obstetrics & gynecology and director of the Center for Research on Women & Gender, University of Illinois at Chicago. Dr. Maki is a professor of psychiatry, psychology, and obstetrics & gynecology at the University of Illinois at Chicago.
References
1. Trost S et al. Pregnancy-related deaths: Data from maternal mortality review committees in 36 states, 2017-2019. Atlanta: Centers for Disease Control and Prevention, U.S. Department of Health & Human Services, 2022.
2. Illinois Department of Public Health. Illinois maternal morbidity and mortality report 2016-2017. 2021.
3. AHRQ. Funding opportunities to address opioid and other substance use disorders. Updated 2023.
4. HRSA. Screening and treatment for maternal mental health and substance use disorders.
5. U.S. Preventive Services Task Force. Recommendations for primary care practice. Accessed May 26, 2023.
6. U.S. Preventive Services Task Force. Draft recommendation statement: Anxiety in adults: Screening. 2022.
7. ACOG. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline. Number 4. 2023 June.
8. Meltzer-Brody S and Stuebe A. The long-term psychiatric and medical prognosis of perinatal mental illness. Best Pract Res Clin Obstet Gynaecol. 2014 Jan. doi: 10.1016/j.bpobgyn.2013.08.009.
9. Van Niel MS and Payne JL. Perinatal depression: A review. Cleve Clin J Med. 2020 May. doi: 10.3949/ccjm.87a.19054.
10. Wisner KL et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. 2013 May. doi: 10.1001/jamapsychiatry.2013.87.
11. Falah-Hassani K et al. The prevalence of antenatal and postnatal co-morbid anxiety and depression: A meta-analysis. Psychol Med. 2017 Sep. doi: 10.1017/S0033291717000617.
12. Pentecost R et al. Scoping review of the associations between perinatal substance use and perinatal depression and anxiety. J Obstet Gynecol Neonatal Nurs. 2021 Jul. doi: 10.1016/j.jogn.2021.02.008.
13. Craemer KA et al. Perinatal mental health in low-income urban and rural patients: The importance of screening for comorbidities. Gen Hosp Psychiatry. 2023 Jul-Aug. doi: 10.1016/j.genhosppsych.2023.05.007.
14. O’Connor E et al. Primary care screening for and treatment of depression in pregnant and postpartum women: Evidence report and systematic review for the U.S. Preventive Services Task Force. JAMA. 2016 Jan 26. doi: 10.1001/jama.2015.18948.
15. Kozhimannil KB et al. Racial and ethnic disparities in postpartum depression care among low-income women. Psychiatr Serv. 2011 Jun. doi: 10.1176/ps.62.6.pss6206_0619.
16. Wenzel ES et al. Depression and anxiety symptoms across pregnancy and the postpartum in low-income Black and Latina women. Arch Womens Ment Health. 2021 Dec. doi: 10.1007/s00737-021-01139-y.
17. Gibbons RD et al. Development of a computerized adaptive substance use disorder scale for screening and measurement: The CAT‐SUD. Addiction. 2020 Jul. doi: 10.1111/add.14938.
18. Brenner LA et al. Validation of a computerized adaptive test suicide scale (CAT-SS) among united states military veterans. PloS One. 2022 Jan 21. doi: 10.1371/journal.pone.0261920.
19. The Center for State Child Welfare Data. Using technology to diagnose and report on behavioral health challenges facing foster youth. 2018.
20. Kim JJ et al. The experience of depression, anxiety, and mania among perinatal women. Arch Womens Ment Health. 2016 Oct. doi: 10.1007/s00737-016-0632-6.
21. Tepper MC et al. Toward population health: Using a learning behavioral health system and measurement-based care to improve access, care, outcomes, and disparities. Community Ment Health J. 2022 Nov. doi: 10.1007/s10597-022-00957-3.
22. Wenzel E et al. Using computerised adaptive tests to screen for perinatal depression in underserved women of colour. Evid Based Ment Health. 2022 Feb. doi: 10.1136/ebmental-2021-300262.
23. Sanger-Katz M. They want it to be secret: How a common blood test can cost $11 or almost $1,000. New York Times. 2019 Apr 19.
24. Spitzer RL et al. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006 May 22. doi: 10.1001/archinte.166.10.1092.
25. Mollard E et al. An integrative review of postpartum depression in rural US communities. Arch Psychiatr Nurs. 2016 Jun. doi: 10.1016/j.apnu.2015.12.003.
26. Anglim AJ and Radke SM. Rural maternal health care outcomes, drivers, and patient perspectives. Clin Obstet Gynecol. 2022 Dec 1. doi: 10.1097/GRF.0000000000000753.
Mental health conditions are the leading cause of pregnancy-related death in Illinois (40%) and across the United States (21%).1,2 Funding bodies, such as the Agency for Healthcare Research and Quality3 and the Health Resources and Service Administration,4 have spotlights on improving screening and access to care for depression and substance use disorders (SUDs). However, the needs of individuals with multiple mental health conditions still often go unrecognized and unaddressed in perinatal health settings.
The U.S. Preventive Services Task Force recommends that all adults be screened for depression, alcohol use, and drug use, and will be recommending screening for anxiety.5,6 The American College of Obstetrics and Gynecology recommends screening for perinatal mental health conditions including depression, anxiety, bipolar disorder, acute postpartum psychosis, and suicidality; however, despite these recommendations, screening and treatment for comorbid mental health disorders during pregnancy and the postpartum is not standard practice.7
Addressing perinatal mental health is critical because untreated mental health conditions during the perinatal period can cause long-term adverse psychiatric and medical outcomes for the birthing person, the baby, and the family.8 This commentary highlights the importance of recognizing and screening for perinatal mental health comorbidities, improving referral rates for mental health treatment, and raising awareness of the importance of addressing rural perinatal mental health.
Perinatal mental health comorbidities
Major depressive disorder is the most common mental health condition during the perinatal period9 and is often comorbid.10-12 In “Perinatal mental health in low-income urban and rural patients: The importance of screening for comorbidities,” Craemer et al.13 reported that nearly half of the perinatal patients who screened positive for MDD also screened positive for at least one other mental health condition, among them general anxiety disorder (GAD), SUD, posttraumatic stress disorder (PTSD), and suicidality.
Many (9%) of the perinatal patients with MDD had a severe comorbidity profile characterized by four diagnoses – MDD, GAD, SUD, and PTSD. In routine medical care these comorbidities often go undetected even though the risk to mothers and babies increases with more severe mental health symptoms.8
The high frequency of perinatal mental health comorbidities Craemer et al.13 found demonstrates a compelling need for comorbid mental health screening during the perinatal period, particularly for low-income Black, Hispanic, and rural birthing persons. Positive screens for perinatal mental health disorders may reflect the onset of these disorders in pregnancy or the postpartum, or preexisting disorders that have gone undetected or untreated before pregnancy.
For many patients, the perinatal period is the first time they are screened for any mental health disorder; typically, they are screened solely for depression. Screening alone can have a positive impact on perinatal mental health. In fact, the USPSTF found that programs to screen perinatal patients, with or without treatment-related support, resulted in a 2%-9% absolute reduction in depression prevalence.14 However, screening for MDD is too infrequent for many reasons, including the logistics of integrating screening into the clinic workflow and limited provider availability, time, and training in mental health.
We recommend screening perinatal patients for mental health comorbidities. This recommendation may seem impractical given the lack of screening tools for comorbid mental health conditions; however, the Computerized Adaptive Test for Mental Health (CAT-MH), the validated tool15-17 used in this study, is an ideal option. CAT-MH is uniquely capable of screening for MDD, GAD, PTSD, SUD, and suicidality in one platform and is routinely used in diverse settings including the Veterans Administration,18 foster care,19 and universities.20 The main limitation of this more comprehensive screening is that it takes about 10 minutes per patient. However, CAT-MH is self-administered and can be done in the waiting room or on a mobile device prior to a clinic visit.
CAT-MH can also be easily integrated into clinical workflow when added to the Electronic Medical Record21, and is a more comprehensive tool than existing perinatal depression tools such as the Perinatal Health Questionaire-9 (PHQ-9) and Edinburgh Perinatal Depression Scale (EPDS).22 Another limitation is cost – currently $5.00 per assessment – however, this is less than routine blood work.23 If CAT-MH is not an option, we recommend a stepped approach of screening for GAD when perinatal patients screen positive for MDD, as this is the most common comorbidity profile. The GAD-7 is a free and widely available tool.24
Barriers to care
In Craemer et al,13 nearly two-thirds (64.9%) of perinatal patients with a positive screen did not receive a referral to follow-up care or a medication prescription. These low referral rates may reflect a variety of widely recognized barriers to care, including lack of referral options, provider and/or patient reluctance to pursue referrals, barriers to insurance coverage, or inadequate behavioral health infrastructure to ensure referral and diagnostic follow-up.
Further, rural residing perinatal patients are an underserved population that need more resources and screening. Despite an on-site behavioral specialist at the rural clinic, Craemer et al13 found a stark disparity in referral rates: referrals to treatment for a positive diagnosis was over two times less at the rural clinic (23.9%), compared with the urban clinics (51.6%). The most common treatment offered at the rural clinic was a prescription for medication (17.4%), while referral to follow-up care was the most common at the urban clinics (35.5%). Rural areas not only have a shortage of health care providers, but community members seeking mental health care often encounter greater stigma, compared with urban residents.25,26
These data highlight an unmet need for referrals to treatment for patients in rural communities, particularly in Illinois where the pregnancy-related mortality ratio attributable to mental health conditions is three times greater in rural areas, compared with those residing in urban Cook County (Chicago).2 Increasing access and availability to mental health treatment and prevention resources in Illinois, especially in rural areas, is an opportunity to prevent pregnancy-related mortality attributable to mental health conditions.
Overall, there is a critical need for screening for perinatal mental health comorbidities, increased attention to low rates of referral to mental health treatment, and investing in rural perinatal mental health. Addressing perinatal mental health disorders is key to decreasing the burden of maternal mortality, particularly in Illinois.
Ms. Craemer and Ms. Sayah are senior research specialists at the Center for Research on Women & Gender, University of Illinois at Chicago. Dr. Duffecy is a professor of clinical psychiatry at the University of Illinois at Chicago. Dr. Geller is a professor of obstetrics & gynecology and director of the Center for Research on Women & Gender, University of Illinois at Chicago. Dr. Maki is a professor of psychiatry, psychology, and obstetrics & gynecology at the University of Illinois at Chicago.
References
1. Trost S et al. Pregnancy-related deaths: Data from maternal mortality review committees in 36 states, 2017-2019. Atlanta: Centers for Disease Control and Prevention, U.S. Department of Health & Human Services, 2022.
2. Illinois Department of Public Health. Illinois maternal morbidity and mortality report 2016-2017. 2021.
3. AHRQ. Funding opportunities to address opioid and other substance use disorders. Updated 2023.
4. HRSA. Screening and treatment for maternal mental health and substance use disorders.
5. U.S. Preventive Services Task Force. Recommendations for primary care practice. Accessed May 26, 2023.
6. U.S. Preventive Services Task Force. Draft recommendation statement: Anxiety in adults: Screening. 2022.
7. ACOG. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline. Number 4. 2023 June.
8. Meltzer-Brody S and Stuebe A. The long-term psychiatric and medical prognosis of perinatal mental illness. Best Pract Res Clin Obstet Gynaecol. 2014 Jan. doi: 10.1016/j.bpobgyn.2013.08.009.
9. Van Niel MS and Payne JL. Perinatal depression: A review. Cleve Clin J Med. 2020 May. doi: 10.3949/ccjm.87a.19054.
10. Wisner KL et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. 2013 May. doi: 10.1001/jamapsychiatry.2013.87.
11. Falah-Hassani K et al. The prevalence of antenatal and postnatal co-morbid anxiety and depression: A meta-analysis. Psychol Med. 2017 Sep. doi: 10.1017/S0033291717000617.
12. Pentecost R et al. Scoping review of the associations between perinatal substance use and perinatal depression and anxiety. J Obstet Gynecol Neonatal Nurs. 2021 Jul. doi: 10.1016/j.jogn.2021.02.008.
13. Craemer KA et al. Perinatal mental health in low-income urban and rural patients: The importance of screening for comorbidities. Gen Hosp Psychiatry. 2023 Jul-Aug. doi: 10.1016/j.genhosppsych.2023.05.007.
14. O’Connor E et al. Primary care screening for and treatment of depression in pregnant and postpartum women: Evidence report and systematic review for the U.S. Preventive Services Task Force. JAMA. 2016 Jan 26. doi: 10.1001/jama.2015.18948.
15. Kozhimannil KB et al. Racial and ethnic disparities in postpartum depression care among low-income women. Psychiatr Serv. 2011 Jun. doi: 10.1176/ps.62.6.pss6206_0619.
16. Wenzel ES et al. Depression and anxiety symptoms across pregnancy and the postpartum in low-income Black and Latina women. Arch Womens Ment Health. 2021 Dec. doi: 10.1007/s00737-021-01139-y.
17. Gibbons RD et al. Development of a computerized adaptive substance use disorder scale for screening and measurement: The CAT‐SUD. Addiction. 2020 Jul. doi: 10.1111/add.14938.
18. Brenner LA et al. Validation of a computerized adaptive test suicide scale (CAT-SS) among united states military veterans. PloS One. 2022 Jan 21. doi: 10.1371/journal.pone.0261920.
19. The Center for State Child Welfare Data. Using technology to diagnose and report on behavioral health challenges facing foster youth. 2018.
20. Kim JJ et al. The experience of depression, anxiety, and mania among perinatal women. Arch Womens Ment Health. 2016 Oct. doi: 10.1007/s00737-016-0632-6.
21. Tepper MC et al. Toward population health: Using a learning behavioral health system and measurement-based care to improve access, care, outcomes, and disparities. Community Ment Health J. 2022 Nov. doi: 10.1007/s10597-022-00957-3.
22. Wenzel E et al. Using computerised adaptive tests to screen for perinatal depression in underserved women of colour. Evid Based Ment Health. 2022 Feb. doi: 10.1136/ebmental-2021-300262.
23. Sanger-Katz M. They want it to be secret: How a common blood test can cost $11 or almost $1,000. New York Times. 2019 Apr 19.
24. Spitzer RL et al. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006 May 22. doi: 10.1001/archinte.166.10.1092.
25. Mollard E et al. An integrative review of postpartum depression in rural US communities. Arch Psychiatr Nurs. 2016 Jun. doi: 10.1016/j.apnu.2015.12.003.
26. Anglim AJ and Radke SM. Rural maternal health care outcomes, drivers, and patient perspectives. Clin Obstet Gynecol. 2022 Dec 1. doi: 10.1097/GRF.0000000000000753.







