User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Long COVID and mental illness: New guidance
The consensus guidance statement on the assessment and treatment of mental health symptoms in patients with post-acute sequelae of SARS-CoV-2 infection (PASC), also known as long COVID, was published online in Physical Medicine and Rehabilitation, the journal of the American Academy of Physical Medicine and Rehabilitation (AAPM&R).
The statement was developed by a task force that included experts from physical medicine, neurology, neuropsychiatry, neuropsychology, rehabilitation psychology, and primary care. It is the eighth guidance statement on long COVID published by AAPM&R).
“Many of our patients have reported experiences in which their symptoms of long COVID have been dismissed either by loved ones in the community, or also amongst health care providers, and they’ve been told their symptoms are in their head or due to a mental health condition, but that’s simply not true,” Abby L. Cheng, MD, a physiatrist at Barnes Jewish Hospital in St. Louis and a coauthor of the new guidance, said in a press briefing.
“Long COVID is real, and mental health conditions do not cause long COVID,” Dr. Cheng added.
Millions of Americans affected
Anxiety and depression have been reported as the second and third most common symptoms of long COVID, according to the guidance statement.
There is some evidence that the body’s inflammatory response – specifically, circulating cytokines – may contribute to the worsening of mental health symptoms or may bring on new symptoms of anxiety or depression, said Dr. Cheng. Cytokines may also affect levels of brain chemicals, such as serotonin, she said.
Researchers are also exploring whether the persistence of virus in the body, miniature blood clots in the body and brain, and changes to the gut microbiome affect the mental health of people with long COVID.
Some mental health symptoms – such as fatigue, brain fog, sleep disturbances, and tachycardia – can mimic long COVID symptoms, said Dr. Cheng.
The treatment is the same for someone with or without long COVID who has anxiety, depression, posttraumatic stress disorder, or other mental health conditions and includes treatment of coexisting medical conditions, supportive therapy and cognitive-behavioral therapy, and pharmacologic interventions, she said.
“Group therapy may have a particular role in the long COVID population because it really provides that social connection and awareness of additional resources in addition to validation of their experiences,” Dr. Cheng said.
The guidance suggests that primary care practitioners – if it’s within their comfort zone and they have the training – can be the first line for managing mental health symptoms.
But for patients whose symptoms are interfering with functioning and their ability to interact with the community, the guidance urges primary care clinicians to refer the patient to a specialist.
“It leaves the door open to them to practice within their scope but also gives guidance as to how, why, and who should be referred to the next level of care,” said Dr. Cheng.
Coauthor Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine at UT Health San Antonio, Texas, said that although fewer people are now getting long COVID, “it’s still an impactful number.”
The Centers for Disease Control and Prevention recently estimated that about 7% of American adults (18 million) and 1.3% of children had experienced long COVID.
Dr. Gutierrez said that it’s an evolving number, as some patients who have a second or third or fourth SARS-CoV-2 infection experience exacerbations of previous bouts of long COVID or develop long COVID for the first time.
“We are still getting new patients on a regular basis with long COVID,” said AAPM&R President Steven R. Flanagan, MD, a physical medicine specialist.
“This is a problem that really is not going away. It is still real and still ever-present,” said Dr. Flanagan, chair of rehabilitation medicine at NYU Langone Health.
A version of this article first appeared on Medscape.com.
The consensus guidance statement on the assessment and treatment of mental health symptoms in patients with post-acute sequelae of SARS-CoV-2 infection (PASC), also known as long COVID, was published online in Physical Medicine and Rehabilitation, the journal of the American Academy of Physical Medicine and Rehabilitation (AAPM&R).
The statement was developed by a task force that included experts from physical medicine, neurology, neuropsychiatry, neuropsychology, rehabilitation psychology, and primary care. It is the eighth guidance statement on long COVID published by AAPM&R).
“Many of our patients have reported experiences in which their symptoms of long COVID have been dismissed either by loved ones in the community, or also amongst health care providers, and they’ve been told their symptoms are in their head or due to a mental health condition, but that’s simply not true,” Abby L. Cheng, MD, a physiatrist at Barnes Jewish Hospital in St. Louis and a coauthor of the new guidance, said in a press briefing.
“Long COVID is real, and mental health conditions do not cause long COVID,” Dr. Cheng added.
Millions of Americans affected
Anxiety and depression have been reported as the second and third most common symptoms of long COVID, according to the guidance statement.
There is some evidence that the body’s inflammatory response – specifically, circulating cytokines – may contribute to the worsening of mental health symptoms or may bring on new symptoms of anxiety or depression, said Dr. Cheng. Cytokines may also affect levels of brain chemicals, such as serotonin, she said.
Researchers are also exploring whether the persistence of virus in the body, miniature blood clots in the body and brain, and changes to the gut microbiome affect the mental health of people with long COVID.
Some mental health symptoms – such as fatigue, brain fog, sleep disturbances, and tachycardia – can mimic long COVID symptoms, said Dr. Cheng.
The treatment is the same for someone with or without long COVID who has anxiety, depression, posttraumatic stress disorder, or other mental health conditions and includes treatment of coexisting medical conditions, supportive therapy and cognitive-behavioral therapy, and pharmacologic interventions, she said.
“Group therapy may have a particular role in the long COVID population because it really provides that social connection and awareness of additional resources in addition to validation of their experiences,” Dr. Cheng said.
The guidance suggests that primary care practitioners – if it’s within their comfort zone and they have the training – can be the first line for managing mental health symptoms.
But for patients whose symptoms are interfering with functioning and their ability to interact with the community, the guidance urges primary care clinicians to refer the patient to a specialist.
“It leaves the door open to them to practice within their scope but also gives guidance as to how, why, and who should be referred to the next level of care,” said Dr. Cheng.
Coauthor Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine at UT Health San Antonio, Texas, said that although fewer people are now getting long COVID, “it’s still an impactful number.”
The Centers for Disease Control and Prevention recently estimated that about 7% of American adults (18 million) and 1.3% of children had experienced long COVID.
Dr. Gutierrez said that it’s an evolving number, as some patients who have a second or third or fourth SARS-CoV-2 infection experience exacerbations of previous bouts of long COVID or develop long COVID for the first time.
“We are still getting new patients on a regular basis with long COVID,” said AAPM&R President Steven R. Flanagan, MD, a physical medicine specialist.
“This is a problem that really is not going away. It is still real and still ever-present,” said Dr. Flanagan, chair of rehabilitation medicine at NYU Langone Health.
A version of this article first appeared on Medscape.com.
The consensus guidance statement on the assessment and treatment of mental health symptoms in patients with post-acute sequelae of SARS-CoV-2 infection (PASC), also known as long COVID, was published online in Physical Medicine and Rehabilitation, the journal of the American Academy of Physical Medicine and Rehabilitation (AAPM&R).
The statement was developed by a task force that included experts from physical medicine, neurology, neuropsychiatry, neuropsychology, rehabilitation psychology, and primary care. It is the eighth guidance statement on long COVID published by AAPM&R).
“Many of our patients have reported experiences in which their symptoms of long COVID have been dismissed either by loved ones in the community, or also amongst health care providers, and they’ve been told their symptoms are in their head or due to a mental health condition, but that’s simply not true,” Abby L. Cheng, MD, a physiatrist at Barnes Jewish Hospital in St. Louis and a coauthor of the new guidance, said in a press briefing.
“Long COVID is real, and mental health conditions do not cause long COVID,” Dr. Cheng added.
Millions of Americans affected
Anxiety and depression have been reported as the second and third most common symptoms of long COVID, according to the guidance statement.
There is some evidence that the body’s inflammatory response – specifically, circulating cytokines – may contribute to the worsening of mental health symptoms or may bring on new symptoms of anxiety or depression, said Dr. Cheng. Cytokines may also affect levels of brain chemicals, such as serotonin, she said.
Researchers are also exploring whether the persistence of virus in the body, miniature blood clots in the body and brain, and changes to the gut microbiome affect the mental health of people with long COVID.
Some mental health symptoms – such as fatigue, brain fog, sleep disturbances, and tachycardia – can mimic long COVID symptoms, said Dr. Cheng.
The treatment is the same for someone with or without long COVID who has anxiety, depression, posttraumatic stress disorder, or other mental health conditions and includes treatment of coexisting medical conditions, supportive therapy and cognitive-behavioral therapy, and pharmacologic interventions, she said.
“Group therapy may have a particular role in the long COVID population because it really provides that social connection and awareness of additional resources in addition to validation of their experiences,” Dr. Cheng said.
The guidance suggests that primary care practitioners – if it’s within their comfort zone and they have the training – can be the first line for managing mental health symptoms.
But for patients whose symptoms are interfering with functioning and their ability to interact with the community, the guidance urges primary care clinicians to refer the patient to a specialist.
“It leaves the door open to them to practice within their scope but also gives guidance as to how, why, and who should be referred to the next level of care,” said Dr. Cheng.
Coauthor Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine at UT Health San Antonio, Texas, said that although fewer people are now getting long COVID, “it’s still an impactful number.”
The Centers for Disease Control and Prevention recently estimated that about 7% of American adults (18 million) and 1.3% of children had experienced long COVID.
Dr. Gutierrez said that it’s an evolving number, as some patients who have a second or third or fourth SARS-CoV-2 infection experience exacerbations of previous bouts of long COVID or develop long COVID for the first time.
“We are still getting new patients on a regular basis with long COVID,” said AAPM&R President Steven R. Flanagan, MD, a physical medicine specialist.
“This is a problem that really is not going away. It is still real and still ever-present,” said Dr. Flanagan, chair of rehabilitation medicine at NYU Langone Health.
A version of this article first appeared on Medscape.com.
FROM PHYSICAL MEDICINE AND REHABILITATION
Two biomarkers promising for preeclampsia prediction
Two biomarkers – pregnancy-associated plasma protein A2 (PAPP-A2) and activin A – when added to relevant clinical information have a better positive predictive value than and a comparable negative predictive value to the currently used ratio of soluble fms-like tyrosine kinase 1 (sFlt-1) to placental growth factor (PlGF), new research suggests.
The third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia. By contrast, , according to the authors.
Preeclampsia has “potentially devastating maternal and fetal complications, [including] significantly increased cardiovascular risk for affected women later in life,” study author Stella S. Daskalopoulou, MD, PhD, associate professor of medicine at McGill University Health Centre in Montreal, said in an interview.
“A more accurate prediction of preeclampsia is expected to improve risk stratification and clinical care and shape clinical practice guidelines,” she said.
The study was published online in the Canadian Journal of Cardiology.
Better predictive value
For a prospective cohort study, the investigators recruited 192 women with first-trimester high-risk singleton pregnancies from tertiary obstetric clinics in Montreal.
At baseline, they collected clinical information, including height, prepregnancy weight, personal and family medical history, and medication use.
At each trimester, blood pressure was measured, and blood samples were collected to quantify sFlt-1, PlGF, PAPP-A2, PAPP-A, activin A, inhibin A, follistatin, and glycosylated fibronectin. For the sFlt-1:PlGF ratio, the researchers used a cutoff point of 38, based on prior evidence. Because there are no agreed-upon cutoff points for the other biomarkers, they chose cutoff points that maximized sensitivity and specificity.
Pregnancies were considered high risk if the mother had any of the following conditions: prepregnancy BMI ≥ 25, maternal age ≥ 35 years, chronic hypertension, diabetes, renal disease, conception via in vitro fertilization, or maternal or first-degree family history of preeclampsia.
The primary outcome was preeclampsia, which was defined according to the Society of Obstetrics and Gynecology guidelines as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure of ≥ mm Hg together with either proteinuria or maternal end-organ dysfunction.
A total of 18 women (9.38%) developed preeclampsia. Those women had higher blood pressure at baseline (although it was within normal limits) and were more likely to have preexisting diabetes or a previous pregnancy with preeclampsia. They were also more likely to report Black race. Serum levels of PAPP-A, PAPP-A2, activin A, and inhibin A were significantly different between patients who developed preeclampsia and those who did not. These levels were increased throughout pregnancy.
Alongside the sFlt-1:PlGF ratio, two biomarkers, PAPP-A2 (odds ratio, 1.78) and activin A (OR, 1.84), were significantly associated with the primary outcome after adjustment for age, prepregnancy BMI, race, and mean arterial pressure.
When added to a model that included those clinical factors, a positive third-trimester result for both PAPP-A2 and activin A had a better positive predictive value than the sFlt-1:PlGF ratio added to the clinical model (91.67% vs. 66.67%). The two biomarkers also had a negative predictive value that was comparable to that of the sFlt-1:PlGF ratio (97.69% vs. 96%).
Study limitations include the small sample size and missing covariates for some participants. Furthermore, the findings cannot be generalized to low-risk populations.
“Whereas the third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia, PAPP-A2 and activin A had both high positive and negative predictive values and thus could serve as biomarkers to predict the occurrence (and absence) of preeclampsia; these findings will be validated in future studies,” the authors concluded.
Dr. Daskalopoulou said that her group is currently performing a large multinational study, PULSE, “which will be the ideal platform to validate and extend our findings. The aim of the study is to predict preeclampsia using a multimodal approach that includes arterial stiffness measurements and blood biomarkers.”
She expanded on the potential benefits of this research. “Finding an accurate predictive tool would not only help design appropriate early care plans for truly high-risk pregnant women, including monitoring and delivery planning, but also facilitate the development of novel strategies for the prevention and treatment of preeclampsia, improving the life of millions of young mothers and their offspring around the world.”
Promising biomarkers
Commenting on the study, Nieca Goldberg, MD, clinical associate professor of medicine at NYU Langone Health and medical director of Atria, both in New York, said, “These biomarkers are promising, as the current biomarker, sFlt-1:PlGF, is good at ruling out preeclampsia in the short term, while the new biomarkers show that they are better at ruling in preeclampsia” as well as ruling it out. Dr. Goldberg was not involved in the research.
“The current study is small, some participant data points are missing, and the researchers only studied high-risk pregnancies,” she added. “We need larger studies of all the risk markers, in both high- and low-risk pregnancies that are followed throughout pregnancy.”
This work was supported by the Fonds de recherche du Québec Santé (FRQS), Heart and Stroke Foundation of Canada, McGill University Department of Obstetrics and Gynecology Academic Enrichment Fund, and Canadian Foundation for Women›s Health. Dr. Daskalopoulou is a senior clinician-scientist supported by a FRQS Clinician Scientist-Senior salary award. Dr. Daskalopoulou and Dr. Goldberg disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
Two biomarkers – pregnancy-associated plasma protein A2 (PAPP-A2) and activin A – when added to relevant clinical information have a better positive predictive value than and a comparable negative predictive value to the currently used ratio of soluble fms-like tyrosine kinase 1 (sFlt-1) to placental growth factor (PlGF), new research suggests.
The third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia. By contrast, , according to the authors.
Preeclampsia has “potentially devastating maternal and fetal complications, [including] significantly increased cardiovascular risk for affected women later in life,” study author Stella S. Daskalopoulou, MD, PhD, associate professor of medicine at McGill University Health Centre in Montreal, said in an interview.
“A more accurate prediction of preeclampsia is expected to improve risk stratification and clinical care and shape clinical practice guidelines,” she said.
The study was published online in the Canadian Journal of Cardiology.
Better predictive value
For a prospective cohort study, the investigators recruited 192 women with first-trimester high-risk singleton pregnancies from tertiary obstetric clinics in Montreal.
At baseline, they collected clinical information, including height, prepregnancy weight, personal and family medical history, and medication use.
At each trimester, blood pressure was measured, and blood samples were collected to quantify sFlt-1, PlGF, PAPP-A2, PAPP-A, activin A, inhibin A, follistatin, and glycosylated fibronectin. For the sFlt-1:PlGF ratio, the researchers used a cutoff point of 38, based on prior evidence. Because there are no agreed-upon cutoff points for the other biomarkers, they chose cutoff points that maximized sensitivity and specificity.
Pregnancies were considered high risk if the mother had any of the following conditions: prepregnancy BMI ≥ 25, maternal age ≥ 35 years, chronic hypertension, diabetes, renal disease, conception via in vitro fertilization, or maternal or first-degree family history of preeclampsia.
The primary outcome was preeclampsia, which was defined according to the Society of Obstetrics and Gynecology guidelines as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure of ≥ mm Hg together with either proteinuria or maternal end-organ dysfunction.
A total of 18 women (9.38%) developed preeclampsia. Those women had higher blood pressure at baseline (although it was within normal limits) and were more likely to have preexisting diabetes or a previous pregnancy with preeclampsia. They were also more likely to report Black race. Serum levels of PAPP-A, PAPP-A2, activin A, and inhibin A were significantly different between patients who developed preeclampsia and those who did not. These levels were increased throughout pregnancy.
Alongside the sFlt-1:PlGF ratio, two biomarkers, PAPP-A2 (odds ratio, 1.78) and activin A (OR, 1.84), were significantly associated with the primary outcome after adjustment for age, prepregnancy BMI, race, and mean arterial pressure.
When added to a model that included those clinical factors, a positive third-trimester result for both PAPP-A2 and activin A had a better positive predictive value than the sFlt-1:PlGF ratio added to the clinical model (91.67% vs. 66.67%). The two biomarkers also had a negative predictive value that was comparable to that of the sFlt-1:PlGF ratio (97.69% vs. 96%).
Study limitations include the small sample size and missing covariates for some participants. Furthermore, the findings cannot be generalized to low-risk populations.
“Whereas the third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia, PAPP-A2 and activin A had both high positive and negative predictive values and thus could serve as biomarkers to predict the occurrence (and absence) of preeclampsia; these findings will be validated in future studies,” the authors concluded.
Dr. Daskalopoulou said that her group is currently performing a large multinational study, PULSE, “which will be the ideal platform to validate and extend our findings. The aim of the study is to predict preeclampsia using a multimodal approach that includes arterial stiffness measurements and blood biomarkers.”
She expanded on the potential benefits of this research. “Finding an accurate predictive tool would not only help design appropriate early care plans for truly high-risk pregnant women, including monitoring and delivery planning, but also facilitate the development of novel strategies for the prevention and treatment of preeclampsia, improving the life of millions of young mothers and their offspring around the world.”
Promising biomarkers
Commenting on the study, Nieca Goldberg, MD, clinical associate professor of medicine at NYU Langone Health and medical director of Atria, both in New York, said, “These biomarkers are promising, as the current biomarker, sFlt-1:PlGF, is good at ruling out preeclampsia in the short term, while the new biomarkers show that they are better at ruling in preeclampsia” as well as ruling it out. Dr. Goldberg was not involved in the research.
“The current study is small, some participant data points are missing, and the researchers only studied high-risk pregnancies,” she added. “We need larger studies of all the risk markers, in both high- and low-risk pregnancies that are followed throughout pregnancy.”
This work was supported by the Fonds de recherche du Québec Santé (FRQS), Heart and Stroke Foundation of Canada, McGill University Department of Obstetrics and Gynecology Academic Enrichment Fund, and Canadian Foundation for Women›s Health. Dr. Daskalopoulou is a senior clinician-scientist supported by a FRQS Clinician Scientist-Senior salary award. Dr. Daskalopoulou and Dr. Goldberg disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
Two biomarkers – pregnancy-associated plasma protein A2 (PAPP-A2) and activin A – when added to relevant clinical information have a better positive predictive value than and a comparable negative predictive value to the currently used ratio of soluble fms-like tyrosine kinase 1 (sFlt-1) to placental growth factor (PlGF), new research suggests.
The third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia. By contrast, , according to the authors.
Preeclampsia has “potentially devastating maternal and fetal complications, [including] significantly increased cardiovascular risk for affected women later in life,” study author Stella S. Daskalopoulou, MD, PhD, associate professor of medicine at McGill University Health Centre in Montreal, said in an interview.
“A more accurate prediction of preeclampsia is expected to improve risk stratification and clinical care and shape clinical practice guidelines,” she said.
The study was published online in the Canadian Journal of Cardiology.
Better predictive value
For a prospective cohort study, the investigators recruited 192 women with first-trimester high-risk singleton pregnancies from tertiary obstetric clinics in Montreal.
At baseline, they collected clinical information, including height, prepregnancy weight, personal and family medical history, and medication use.
At each trimester, blood pressure was measured, and blood samples were collected to quantify sFlt-1, PlGF, PAPP-A2, PAPP-A, activin A, inhibin A, follistatin, and glycosylated fibronectin. For the sFlt-1:PlGF ratio, the researchers used a cutoff point of 38, based on prior evidence. Because there are no agreed-upon cutoff points for the other biomarkers, they chose cutoff points that maximized sensitivity and specificity.
Pregnancies were considered high risk if the mother had any of the following conditions: prepregnancy BMI ≥ 25, maternal age ≥ 35 years, chronic hypertension, diabetes, renal disease, conception via in vitro fertilization, or maternal or first-degree family history of preeclampsia.
The primary outcome was preeclampsia, which was defined according to the Society of Obstetrics and Gynecology guidelines as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure of ≥ mm Hg together with either proteinuria or maternal end-organ dysfunction.
A total of 18 women (9.38%) developed preeclampsia. Those women had higher blood pressure at baseline (although it was within normal limits) and were more likely to have preexisting diabetes or a previous pregnancy with preeclampsia. They were also more likely to report Black race. Serum levels of PAPP-A, PAPP-A2, activin A, and inhibin A were significantly different between patients who developed preeclampsia and those who did not. These levels were increased throughout pregnancy.
Alongside the sFlt-1:PlGF ratio, two biomarkers, PAPP-A2 (odds ratio, 1.78) and activin A (OR, 1.84), were significantly associated with the primary outcome after adjustment for age, prepregnancy BMI, race, and mean arterial pressure.
When added to a model that included those clinical factors, a positive third-trimester result for both PAPP-A2 and activin A had a better positive predictive value than the sFlt-1:PlGF ratio added to the clinical model (91.67% vs. 66.67%). The two biomarkers also had a negative predictive value that was comparable to that of the sFlt-1:PlGF ratio (97.69% vs. 96%).
Study limitations include the small sample size and missing covariates for some participants. Furthermore, the findings cannot be generalized to low-risk populations.
“Whereas the third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia, PAPP-A2 and activin A had both high positive and negative predictive values and thus could serve as biomarkers to predict the occurrence (and absence) of preeclampsia; these findings will be validated in future studies,” the authors concluded.
Dr. Daskalopoulou said that her group is currently performing a large multinational study, PULSE, “which will be the ideal platform to validate and extend our findings. The aim of the study is to predict preeclampsia using a multimodal approach that includes arterial stiffness measurements and blood biomarkers.”
She expanded on the potential benefits of this research. “Finding an accurate predictive tool would not only help design appropriate early care plans for truly high-risk pregnant women, including monitoring and delivery planning, but also facilitate the development of novel strategies for the prevention and treatment of preeclampsia, improving the life of millions of young mothers and their offspring around the world.”
Promising biomarkers
Commenting on the study, Nieca Goldberg, MD, clinical associate professor of medicine at NYU Langone Health and medical director of Atria, both in New York, said, “These biomarkers are promising, as the current biomarker, sFlt-1:PlGF, is good at ruling out preeclampsia in the short term, while the new biomarkers show that they are better at ruling in preeclampsia” as well as ruling it out. Dr. Goldberg was not involved in the research.
“The current study is small, some participant data points are missing, and the researchers only studied high-risk pregnancies,” she added. “We need larger studies of all the risk markers, in both high- and low-risk pregnancies that are followed throughout pregnancy.”
This work was supported by the Fonds de recherche du Québec Santé (FRQS), Heart and Stroke Foundation of Canada, McGill University Department of Obstetrics and Gynecology Academic Enrichment Fund, and Canadian Foundation for Women›s Health. Dr. Daskalopoulou is a senior clinician-scientist supported by a FRQS Clinician Scientist-Senior salary award. Dr. Daskalopoulou and Dr. Goldberg disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
FROM THE CANADIAN JOURNAL OF CARDIOLOGY
U.S. infant mortality rates rise for first time in 2 decades
The overall mortality rate and the rate for neonatal infants, those younger than 28 days old, rose by 3% from 2021 to 2022, says the Centers for Disease Control and Prevention’s National Center for Health Statistics. The mortality rate for infants older than 28 days rose by 4%.
Meanwhile, infant deaths caused by maternal complications rose by 8% and those caused by bacterial sepsis rose by 14%, the report says.
“We live in a country with significant resources, so the infant mortality rate and the increase are shockingly high,” wrote Sandy Chung, MD, of the American Academy of Pediatrics, to CNN. “As pediatricians who help children grow into healthy adults, any death of any child is one too many. The infant mortality rate in this country in unacceptable.”
Experts say the increase could be a sign of an underlying health care issue, an unusual occurrence, or partly related to the COVID-19 pandemic.
The infant mortality rate rose among mothers aged 25-29 years; for preterm babies; for boys; and in Georgia, Iowa, Missouri, and Texas. The rate declined in Nevada.
“Mortality rates increased significantly among infants of American Indian and Alaska Native non-Hispanic ... and White non-Hispanic women,” the report says.
“Mortality rates for infants of Black women did not increase by much, the report found, but Black infants experienced the highest overall rates of infant mortality: nearly 11 deaths per 1,000 births, or over double the mortality rate of White infants,” CNN wrote.
“We know that for people who live in or near poverty and for certain racial and ethnic groups there are significant challenges with getting access to a doctor or getting treatments,” Dr. Chung wrote. “This can lead to moms and babies showing up for care when they are sicker and more likely have serious outcomes, even death.”
A version of this article first appeared on WebMD.com.
The overall mortality rate and the rate for neonatal infants, those younger than 28 days old, rose by 3% from 2021 to 2022, says the Centers for Disease Control and Prevention’s National Center for Health Statistics. The mortality rate for infants older than 28 days rose by 4%.
Meanwhile, infant deaths caused by maternal complications rose by 8% and those caused by bacterial sepsis rose by 14%, the report says.
“We live in a country with significant resources, so the infant mortality rate and the increase are shockingly high,” wrote Sandy Chung, MD, of the American Academy of Pediatrics, to CNN. “As pediatricians who help children grow into healthy adults, any death of any child is one too many. The infant mortality rate in this country in unacceptable.”
Experts say the increase could be a sign of an underlying health care issue, an unusual occurrence, or partly related to the COVID-19 pandemic.
The infant mortality rate rose among mothers aged 25-29 years; for preterm babies; for boys; and in Georgia, Iowa, Missouri, and Texas. The rate declined in Nevada.
“Mortality rates increased significantly among infants of American Indian and Alaska Native non-Hispanic ... and White non-Hispanic women,” the report says.
“Mortality rates for infants of Black women did not increase by much, the report found, but Black infants experienced the highest overall rates of infant mortality: nearly 11 deaths per 1,000 births, or over double the mortality rate of White infants,” CNN wrote.
“We know that for people who live in or near poverty and for certain racial and ethnic groups there are significant challenges with getting access to a doctor or getting treatments,” Dr. Chung wrote. “This can lead to moms and babies showing up for care when they are sicker and more likely have serious outcomes, even death.”
A version of this article first appeared on WebMD.com.
The overall mortality rate and the rate for neonatal infants, those younger than 28 days old, rose by 3% from 2021 to 2022, says the Centers for Disease Control and Prevention’s National Center for Health Statistics. The mortality rate for infants older than 28 days rose by 4%.
Meanwhile, infant deaths caused by maternal complications rose by 8% and those caused by bacterial sepsis rose by 14%, the report says.
“We live in a country with significant resources, so the infant mortality rate and the increase are shockingly high,” wrote Sandy Chung, MD, of the American Academy of Pediatrics, to CNN. “As pediatricians who help children grow into healthy adults, any death of any child is one too many. The infant mortality rate in this country in unacceptable.”
Experts say the increase could be a sign of an underlying health care issue, an unusual occurrence, or partly related to the COVID-19 pandemic.
The infant mortality rate rose among mothers aged 25-29 years; for preterm babies; for boys; and in Georgia, Iowa, Missouri, and Texas. The rate declined in Nevada.
“Mortality rates increased significantly among infants of American Indian and Alaska Native non-Hispanic ... and White non-Hispanic women,” the report says.
“Mortality rates for infants of Black women did not increase by much, the report found, but Black infants experienced the highest overall rates of infant mortality: nearly 11 deaths per 1,000 births, or over double the mortality rate of White infants,” CNN wrote.
“We know that for people who live in or near poverty and for certain racial and ethnic groups there are significant challenges with getting access to a doctor or getting treatments,” Dr. Chung wrote. “This can lead to moms and babies showing up for care when they are sicker and more likely have serious outcomes, even death.”
A version of this article first appeared on WebMD.com.
AI algorithm aids egg retrieval date during fertility treatment cycles
According to the researchers, such an algorithm is needed due to the increased demand for fertility treatments, as well as the high day-to-day variability in lab workload.
According to the study investigators, predicting retrieval dates in advance for ongoing cycles is of major importance for both patients and clinicians.
“The population requiring fertility treatments, including genetic testing and fertility preservation, has massively increased, and this causes many more cycles and a high day-to-day variability in IVF activity, especially in the lab workload,” said Rohi Hourvitz, MBA, from FertilAI, an Israeli health care company focused on developing technologies that improve fertility treatments.
“We also need to accommodate and reschedule for non-working days, which causes a big issue with managing the workload in many clinics around the world,” added Mr. Hourvitz, who presented the research highlighting AI’s growing role in reproductive medicine.
In addition, AI has recently emerged as an effective tool for assisting in clinical decision-making in assisted reproductive technology, prompting further research in this space, he said.
The new study used a dataset of 9,550 predictable antagonist cycles (defined as having all necessary data) gathered from one lab with over 50 physicians between August 2018 and October 2022. The data were split into two subsets: one for training the AI model and the other for prospective testing.
To train and test the AI model, data from nearly 6,000 predictable antagonist cycles were used. Key factors used for each cycle included estrogen levels, mean follicle size, primary follicle size, and various patient demographics. Other features were considered, but Mr. Hourvitz noted that primary follicle size influenced the algorithm most, “because that is what most of us use when we want to trigger.”
Mr. Hourvitz explained that these patient data were run through an algorithm that produced a graph predicting the most probable date for a cycle retrieval.
“We could accurately predict when those ‘peak days’ were going to be happening in the clinic, and we could also give a pretty good estimate on how many cycles you’re going to have every day,” Mr. Hourvitz said, explaining that this information could help clinics more efficiently allocate resources and manage patients.
According to Mr. Hourvitz, the predictions derived from this study could improve various aspects of fertility treatments and related procedures, including better staff planning and caseload management in IVF labs, as well as higher-quality eggs at retrieval. Patients would have a clearer timeline for their treatment cycles.
Nikica Zaninovic, PhD, MS, director of the embryology lab at Weill Cornell Medical College, New York City, cautioned that the new findings are not yet ready for clinical application but emphasized the importance of more AI research focusing on the quality of oocytes, not only embryos.
“We’re so focused on the end of the process: the embryo,” Dr. Zaninovic, who was not involved in the research, said in an interview. “I think the focus should be on the beginning – the quality of eggs and sperm, not just the quantity – because that’s what the embryos will depend on.”
He noted the increasing numbers of young women in the United States undergoing egg freezing.
“Cornell is the largest academic IVF center in the United States; 20%-30% of all of the patients that we treat are actually freezing their eggs,” he said. “It’s a huge population.”
“When they come to us, they ask how many eggs they’ll need to guarantee one or two children in the future,” Dr. Zaninovic continued. “We don’t have that answer, so we always tell them [we’ll retrieve] as many as we can. That’s not the answer; we need to be more precise. We’re still lacking these tools, and I think that’s where the research will go.”
The study was funded by FertilAI. Mr. Hourvitz is a shareholder and CEO of FertilAI. Dr. Zaninovic is president of the AI Fertility Society.
A version of this article appeared on Medscape.com.
According to the researchers, such an algorithm is needed due to the increased demand for fertility treatments, as well as the high day-to-day variability in lab workload.
According to the study investigators, predicting retrieval dates in advance for ongoing cycles is of major importance for both patients and clinicians.
“The population requiring fertility treatments, including genetic testing and fertility preservation, has massively increased, and this causes many more cycles and a high day-to-day variability in IVF activity, especially in the lab workload,” said Rohi Hourvitz, MBA, from FertilAI, an Israeli health care company focused on developing technologies that improve fertility treatments.
“We also need to accommodate and reschedule for non-working days, which causes a big issue with managing the workload in many clinics around the world,” added Mr. Hourvitz, who presented the research highlighting AI’s growing role in reproductive medicine.
In addition, AI has recently emerged as an effective tool for assisting in clinical decision-making in assisted reproductive technology, prompting further research in this space, he said.
The new study used a dataset of 9,550 predictable antagonist cycles (defined as having all necessary data) gathered from one lab with over 50 physicians between August 2018 and October 2022. The data were split into two subsets: one for training the AI model and the other for prospective testing.
To train and test the AI model, data from nearly 6,000 predictable antagonist cycles were used. Key factors used for each cycle included estrogen levels, mean follicle size, primary follicle size, and various patient demographics. Other features were considered, but Mr. Hourvitz noted that primary follicle size influenced the algorithm most, “because that is what most of us use when we want to trigger.”
Mr. Hourvitz explained that these patient data were run through an algorithm that produced a graph predicting the most probable date for a cycle retrieval.
“We could accurately predict when those ‘peak days’ were going to be happening in the clinic, and we could also give a pretty good estimate on how many cycles you’re going to have every day,” Mr. Hourvitz said, explaining that this information could help clinics more efficiently allocate resources and manage patients.
According to Mr. Hourvitz, the predictions derived from this study could improve various aspects of fertility treatments and related procedures, including better staff planning and caseload management in IVF labs, as well as higher-quality eggs at retrieval. Patients would have a clearer timeline for their treatment cycles.
Nikica Zaninovic, PhD, MS, director of the embryology lab at Weill Cornell Medical College, New York City, cautioned that the new findings are not yet ready for clinical application but emphasized the importance of more AI research focusing on the quality of oocytes, not only embryos.
“We’re so focused on the end of the process: the embryo,” Dr. Zaninovic, who was not involved in the research, said in an interview. “I think the focus should be on the beginning – the quality of eggs and sperm, not just the quantity – because that’s what the embryos will depend on.”
He noted the increasing numbers of young women in the United States undergoing egg freezing.
“Cornell is the largest academic IVF center in the United States; 20%-30% of all of the patients that we treat are actually freezing their eggs,” he said. “It’s a huge population.”
“When they come to us, they ask how many eggs they’ll need to guarantee one or two children in the future,” Dr. Zaninovic continued. “We don’t have that answer, so we always tell them [we’ll retrieve] as many as we can. That’s not the answer; we need to be more precise. We’re still lacking these tools, and I think that’s where the research will go.”
The study was funded by FertilAI. Mr. Hourvitz is a shareholder and CEO of FertilAI. Dr. Zaninovic is president of the AI Fertility Society.
A version of this article appeared on Medscape.com.
According to the researchers, such an algorithm is needed due to the increased demand for fertility treatments, as well as the high day-to-day variability in lab workload.
According to the study investigators, predicting retrieval dates in advance for ongoing cycles is of major importance for both patients and clinicians.
“The population requiring fertility treatments, including genetic testing and fertility preservation, has massively increased, and this causes many more cycles and a high day-to-day variability in IVF activity, especially in the lab workload,” said Rohi Hourvitz, MBA, from FertilAI, an Israeli health care company focused on developing technologies that improve fertility treatments.
“We also need to accommodate and reschedule for non-working days, which causes a big issue with managing the workload in many clinics around the world,” added Mr. Hourvitz, who presented the research highlighting AI’s growing role in reproductive medicine.
In addition, AI has recently emerged as an effective tool for assisting in clinical decision-making in assisted reproductive technology, prompting further research in this space, he said.
The new study used a dataset of 9,550 predictable antagonist cycles (defined as having all necessary data) gathered from one lab with over 50 physicians between August 2018 and October 2022. The data were split into two subsets: one for training the AI model and the other for prospective testing.
To train and test the AI model, data from nearly 6,000 predictable antagonist cycles were used. Key factors used for each cycle included estrogen levels, mean follicle size, primary follicle size, and various patient demographics. Other features were considered, but Mr. Hourvitz noted that primary follicle size influenced the algorithm most, “because that is what most of us use when we want to trigger.”
Mr. Hourvitz explained that these patient data were run through an algorithm that produced a graph predicting the most probable date for a cycle retrieval.
“We could accurately predict when those ‘peak days’ were going to be happening in the clinic, and we could also give a pretty good estimate on how many cycles you’re going to have every day,” Mr. Hourvitz said, explaining that this information could help clinics more efficiently allocate resources and manage patients.
According to Mr. Hourvitz, the predictions derived from this study could improve various aspects of fertility treatments and related procedures, including better staff planning and caseload management in IVF labs, as well as higher-quality eggs at retrieval. Patients would have a clearer timeline for their treatment cycles.
Nikica Zaninovic, PhD, MS, director of the embryology lab at Weill Cornell Medical College, New York City, cautioned that the new findings are not yet ready for clinical application but emphasized the importance of more AI research focusing on the quality of oocytes, not only embryos.
“We’re so focused on the end of the process: the embryo,” Dr. Zaninovic, who was not involved in the research, said in an interview. “I think the focus should be on the beginning – the quality of eggs and sperm, not just the quantity – because that’s what the embryos will depend on.”
He noted the increasing numbers of young women in the United States undergoing egg freezing.
“Cornell is the largest academic IVF center in the United States; 20%-30% of all of the patients that we treat are actually freezing their eggs,” he said. “It’s a huge population.”
“When they come to us, they ask how many eggs they’ll need to guarantee one or two children in the future,” Dr. Zaninovic continued. “We don’t have that answer, so we always tell them [we’ll retrieve] as many as we can. That’s not the answer; we need to be more precise. We’re still lacking these tools, and I think that’s where the research will go.”
The study was funded by FertilAI. Mr. Hourvitz is a shareholder and CEO of FertilAI. Dr. Zaninovic is president of the AI Fertility Society.
A version of this article appeared on Medscape.com.
FROM ASRM 2023
Particulate pollution increases the risk for breast cancer
MADRID – according to a new analysis of the XENAIR study presented at the European Society of Medical Oncology (ESMO) Congress 2023. Béatrice Fervers, MD, PhD, head of the environmental cancer prevention department at the Léon Bérard Center, Lyon, France, presented her findings.
“To our knowledge, this study is the first to examine the risk of breast cancer associated with long-term exposure of subjects to atmospheric pollution both at home and in the workplace, estimated using a very small spatial resolution [statistical] model,” said the researchers.
“Our data showed a statistically significant association between long-term exposure to fine particulate matter air pollution, at home and at work, and risk of breast cancer. This [finding] contrasts with previous research that looked only at fine particulate exposure where women were living and showed small or no effects on breast cancer risk,” said Dr. Fervers in a press release issued before the Congress.
The XENAIR study carried out on the prospective, longitudinal E3N cohort a year ago showed an increased risk for breast cancer after exposure to five atmospheric pollutants. Notably, it showed an increased risk in women exposed to BaP and PCB153, two pollutants classed as endocrine-disrupting chemicals, during perimenopause.
Increased linear risk
In this new analysis, exposure to PM2.5, PM10, and NO2 pollution at home and in the workplace of 2,419 women with breast cancer was compared with that of 2,984 women without breast cancer during the period from 1990 to 2011.
This was a case-control study in which participants were matched by department of residence in France, age (± 1 year), date (± 3 months), and menopausal status at the time of the blood draw.
Breast cancer risk increased by 28% when exposure to fine particulate (PM2.5) air pollution increased by 10 mcg/m3. The increment is approximately equivalent to the difference in PM2.5 particulate concentration typically seen in rural versus urban areas of Europe.
Smaller increases in breast cancer risk were also recorded in women exposed to high levels of larger particulate air pollution (PM10 and NO2).
No change in effect was seen according to menopausal status. Analyses that examined hormone receptor status showed a positive but not significant association for PM2.5 in cases of estrogen receptor positive breast cancer.
Dr. Fervers and colleagues plan to investigate the effects of pollution exposure during the commute to get a complete picture of effects on breast cancer risk.
Regulators respond
Charles Swanton, PhD, a clinician scientist at the Francis Crick Institute, London, emphasized the importance of these new results for breast cancer. At last year’s ESMO Congress, he explained how particulate matter air pollution caused tumor proliferation in patients with a certain type of genetic mutation.
“Fine particle pollutants can penetrate deep into the lungs, enter the bloodstream, and be absorbed into breast and other tissue. There is already evidence that air pollutants can change the architecture of the breast. It will be important to test if pollutants allow cells in breast tissue with pre-existing mutations to expand and drive tumor promotion, possibly through inflammatory processes, similar to our observations in nonsmokers with lung cancer,” said Dr. Swanton in the ESMO press release.
“It is very concerning that small pollutant particles in the air and indeed microplastic particles of similar size are getting into the environment when we don’t yet understand their potential to promote cancer. There is an urgent need to set up laboratory studies to investigate the effects of these small air pollutant particles on the latency, grade, aggression, and progression of breast tumors,” he added.
“There is now strong epidemiological and biological evidence for the link between PM2.5 particulate exposure and cancer, and there are good clinical and economic reasons for reducing pollution to prevent cancers,” said Jean-Yves Blay, MD, PhD, director of public policy for ESMO.
Following a proposal from the European Commission in October 2022 to reduce the limit for PM2.5 particulates in the air from the current 25 mcg/m3 to 10 mcg/m3 by 2030, ESMO urged a further reduction in the PM2.5 limit to 5 mcg/m3, in line with the World Health Organization’s air quality guidance, according to the press release.
“Reducing PM2.5 particles in the air to the WHO recommended level is critical because of their association with a variety of tumor types, including breast cancer,” Dr. Blay added.
In September 2023, the European Parliament adopted in a plenary session its report on the ongoing revision of the EU Ambient Air Quality Directives, which reflects ESMO’s recommendations to set the annual limit value for PM2.5 at 5 mcg/m³. This adoption opens interinstitutional negotiations between the legislators (the European Parliament, European Commission, and EU Council) to agree on the final text of the directive.
“By supporting our requests with solid scientific evidence, we are offering a new dimension to health public policy. The work is not over, and change will not happen overnight, but we are moving in the right direction,” concluded Dr. Blay.
The new analysis of the XENAIR study was funded by ARC Foundation for cancer research; the French Agency for Food, Environmental, and Occupational Health and Safety; French National League against Cancer; and Fondation de France, an independent private organization, recognized as being in the public interest. The authors report no relevant financial relationships.
This article was translated from the Medscape French edition and a version appeared on Medscape.com.
MADRID – according to a new analysis of the XENAIR study presented at the European Society of Medical Oncology (ESMO) Congress 2023. Béatrice Fervers, MD, PhD, head of the environmental cancer prevention department at the Léon Bérard Center, Lyon, France, presented her findings.
“To our knowledge, this study is the first to examine the risk of breast cancer associated with long-term exposure of subjects to atmospheric pollution both at home and in the workplace, estimated using a very small spatial resolution [statistical] model,” said the researchers.
“Our data showed a statistically significant association between long-term exposure to fine particulate matter air pollution, at home and at work, and risk of breast cancer. This [finding] contrasts with previous research that looked only at fine particulate exposure where women were living and showed small or no effects on breast cancer risk,” said Dr. Fervers in a press release issued before the Congress.
The XENAIR study carried out on the prospective, longitudinal E3N cohort a year ago showed an increased risk for breast cancer after exposure to five atmospheric pollutants. Notably, it showed an increased risk in women exposed to BaP and PCB153, two pollutants classed as endocrine-disrupting chemicals, during perimenopause.
Increased linear risk
In this new analysis, exposure to PM2.5, PM10, and NO2 pollution at home and in the workplace of 2,419 women with breast cancer was compared with that of 2,984 women without breast cancer during the period from 1990 to 2011.
This was a case-control study in which participants were matched by department of residence in France, age (± 1 year), date (± 3 months), and menopausal status at the time of the blood draw.
Breast cancer risk increased by 28% when exposure to fine particulate (PM2.5) air pollution increased by 10 mcg/m3. The increment is approximately equivalent to the difference in PM2.5 particulate concentration typically seen in rural versus urban areas of Europe.
Smaller increases in breast cancer risk were also recorded in women exposed to high levels of larger particulate air pollution (PM10 and NO2).
No change in effect was seen according to menopausal status. Analyses that examined hormone receptor status showed a positive but not significant association for PM2.5 in cases of estrogen receptor positive breast cancer.
Dr. Fervers and colleagues plan to investigate the effects of pollution exposure during the commute to get a complete picture of effects on breast cancer risk.
Regulators respond
Charles Swanton, PhD, a clinician scientist at the Francis Crick Institute, London, emphasized the importance of these new results for breast cancer. At last year’s ESMO Congress, he explained how particulate matter air pollution caused tumor proliferation in patients with a certain type of genetic mutation.
“Fine particle pollutants can penetrate deep into the lungs, enter the bloodstream, and be absorbed into breast and other tissue. There is already evidence that air pollutants can change the architecture of the breast. It will be important to test if pollutants allow cells in breast tissue with pre-existing mutations to expand and drive tumor promotion, possibly through inflammatory processes, similar to our observations in nonsmokers with lung cancer,” said Dr. Swanton in the ESMO press release.
“It is very concerning that small pollutant particles in the air and indeed microplastic particles of similar size are getting into the environment when we don’t yet understand their potential to promote cancer. There is an urgent need to set up laboratory studies to investigate the effects of these small air pollutant particles on the latency, grade, aggression, and progression of breast tumors,” he added.
“There is now strong epidemiological and biological evidence for the link between PM2.5 particulate exposure and cancer, and there are good clinical and economic reasons for reducing pollution to prevent cancers,” said Jean-Yves Blay, MD, PhD, director of public policy for ESMO.
Following a proposal from the European Commission in October 2022 to reduce the limit for PM2.5 particulates in the air from the current 25 mcg/m3 to 10 mcg/m3 by 2030, ESMO urged a further reduction in the PM2.5 limit to 5 mcg/m3, in line with the World Health Organization’s air quality guidance, according to the press release.
“Reducing PM2.5 particles in the air to the WHO recommended level is critical because of their association with a variety of tumor types, including breast cancer,” Dr. Blay added.
In September 2023, the European Parliament adopted in a plenary session its report on the ongoing revision of the EU Ambient Air Quality Directives, which reflects ESMO’s recommendations to set the annual limit value for PM2.5 at 5 mcg/m³. This adoption opens interinstitutional negotiations between the legislators (the European Parliament, European Commission, and EU Council) to agree on the final text of the directive.
“By supporting our requests with solid scientific evidence, we are offering a new dimension to health public policy. The work is not over, and change will not happen overnight, but we are moving in the right direction,” concluded Dr. Blay.
The new analysis of the XENAIR study was funded by ARC Foundation for cancer research; the French Agency for Food, Environmental, and Occupational Health and Safety; French National League against Cancer; and Fondation de France, an independent private organization, recognized as being in the public interest. The authors report no relevant financial relationships.
This article was translated from the Medscape French edition and a version appeared on Medscape.com.
MADRID – according to a new analysis of the XENAIR study presented at the European Society of Medical Oncology (ESMO) Congress 2023. Béatrice Fervers, MD, PhD, head of the environmental cancer prevention department at the Léon Bérard Center, Lyon, France, presented her findings.
“To our knowledge, this study is the first to examine the risk of breast cancer associated with long-term exposure of subjects to atmospheric pollution both at home and in the workplace, estimated using a very small spatial resolution [statistical] model,” said the researchers.
“Our data showed a statistically significant association between long-term exposure to fine particulate matter air pollution, at home and at work, and risk of breast cancer. This [finding] contrasts with previous research that looked only at fine particulate exposure where women were living and showed small or no effects on breast cancer risk,” said Dr. Fervers in a press release issued before the Congress.
The XENAIR study carried out on the prospective, longitudinal E3N cohort a year ago showed an increased risk for breast cancer after exposure to five atmospheric pollutants. Notably, it showed an increased risk in women exposed to BaP and PCB153, two pollutants classed as endocrine-disrupting chemicals, during perimenopause.
Increased linear risk
In this new analysis, exposure to PM2.5, PM10, and NO2 pollution at home and in the workplace of 2,419 women with breast cancer was compared with that of 2,984 women without breast cancer during the period from 1990 to 2011.
This was a case-control study in which participants were matched by department of residence in France, age (± 1 year), date (± 3 months), and menopausal status at the time of the blood draw.
Breast cancer risk increased by 28% when exposure to fine particulate (PM2.5) air pollution increased by 10 mcg/m3. The increment is approximately equivalent to the difference in PM2.5 particulate concentration typically seen in rural versus urban areas of Europe.
Smaller increases in breast cancer risk were also recorded in women exposed to high levels of larger particulate air pollution (PM10 and NO2).
No change in effect was seen according to menopausal status. Analyses that examined hormone receptor status showed a positive but not significant association for PM2.5 in cases of estrogen receptor positive breast cancer.
Dr. Fervers and colleagues plan to investigate the effects of pollution exposure during the commute to get a complete picture of effects on breast cancer risk.
Regulators respond
Charles Swanton, PhD, a clinician scientist at the Francis Crick Institute, London, emphasized the importance of these new results for breast cancer. At last year’s ESMO Congress, he explained how particulate matter air pollution caused tumor proliferation in patients with a certain type of genetic mutation.
“Fine particle pollutants can penetrate deep into the lungs, enter the bloodstream, and be absorbed into breast and other tissue. There is already evidence that air pollutants can change the architecture of the breast. It will be important to test if pollutants allow cells in breast tissue with pre-existing mutations to expand and drive tumor promotion, possibly through inflammatory processes, similar to our observations in nonsmokers with lung cancer,” said Dr. Swanton in the ESMO press release.
“It is very concerning that small pollutant particles in the air and indeed microplastic particles of similar size are getting into the environment when we don’t yet understand their potential to promote cancer. There is an urgent need to set up laboratory studies to investigate the effects of these small air pollutant particles on the latency, grade, aggression, and progression of breast tumors,” he added.
“There is now strong epidemiological and biological evidence for the link between PM2.5 particulate exposure and cancer, and there are good clinical and economic reasons for reducing pollution to prevent cancers,” said Jean-Yves Blay, MD, PhD, director of public policy for ESMO.
Following a proposal from the European Commission in October 2022 to reduce the limit for PM2.5 particulates in the air from the current 25 mcg/m3 to 10 mcg/m3 by 2030, ESMO urged a further reduction in the PM2.5 limit to 5 mcg/m3, in line with the World Health Organization’s air quality guidance, according to the press release.
“Reducing PM2.5 particles in the air to the WHO recommended level is critical because of their association with a variety of tumor types, including breast cancer,” Dr. Blay added.
In September 2023, the European Parliament adopted in a plenary session its report on the ongoing revision of the EU Ambient Air Quality Directives, which reflects ESMO’s recommendations to set the annual limit value for PM2.5 at 5 mcg/m³. This adoption opens interinstitutional negotiations between the legislators (the European Parliament, European Commission, and EU Council) to agree on the final text of the directive.
“By supporting our requests with solid scientific evidence, we are offering a new dimension to health public policy. The work is not over, and change will not happen overnight, but we are moving in the right direction,” concluded Dr. Blay.
The new analysis of the XENAIR study was funded by ARC Foundation for cancer research; the French Agency for Food, Environmental, and Occupational Health and Safety; French National League against Cancer; and Fondation de France, an independent private organization, recognized as being in the public interest. The authors report no relevant financial relationships.
This article was translated from the Medscape French edition and a version appeared on Medscape.com.
AT ESMO 2023
Standing BP measures improve hypertension diagnosis
TOPLINE:
results of a new study suggest.
METHODOLOGY:
- The study included 125 adults, mean age 49 years and 62% female, who were free of cardiovascular disease and had no previous history of hypertension.
- Researchers collected data on 24-hour ambulatory blood pressure monitoring (ABPM), and three BP measurements in the seated position, then three in the standing position.
- They assessed overall diagnostic accuracy of seated and standing BP using the area under the receiver operating characteristic (AUROC) curve and considered a Bayes factor (BF) of 3 or greater as significant.
- They defined the presence of hypertension (HTN) by the 2017 American College of Cardiology/American Heart Association and 2023 European Society of Hypertension HTN guidelines based on ABPM.
- Sensitivity and specificity of standing BP was determined using cutoffs derived from Youden index, while sensitivity and specificity of seated BP was determined using the cutoff of 130/80 mm Hg and by 140/90 mm Hg.
TAKEAWAY:
- The AUROC for standing office systolic blood pressure (SBP; 0.81; 0.71-0.92) was significantly higher than for seated office SBP (0.70; 0.49-0.91) in diagnosing HTN when defined as an average 24-hour SBP ≥ 125 mm Hg (BF = 11.8), and significantly higher for seated versus standing office diastolic blood pressure (DBP; 0.65; 0.49-0.82) in diagnosing HTN when defined as an average 24-hour DBP ≥ 75 mm Hg (BF = 4.9).
- The AUROCs for adding standing office BP to seated office BP improved the accuracy of detecting HTN, compared with seated office BP alone when HTN was defined as an average 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg, or when defined as an average 24-hour SBP/DBP ≥ 130/80 mm Hg or daytime SBP/DBP ≥ 135/85 mm Hg (all BFs > 3).
- Sensitivity of standing SBP was 71%, compared with 43% for seated SBP.
IN PRACTICE:
The “excellent diagnostic performance” for standing BP measures revealed by the study “highlights that standing office BP has acceptable discriminative capabilities in identifying the presence of hypertension in adults,” the authors write.
SOURCE:
The study was conducted by John M. Giacona, Hypertension Section, department of internal medicine, University of Texas Southwestern Medical Center, Dallas, and colleagues. It was published online in Scientific Reports.
LIMITATIONS:
As the study enrolled only adults free of comorbidities who were not taking antihypertensive medications, the results may not be applicable to other patients. The study design was retrospective, and the order of BP measurements was not randomized (standing BP measurements were obtained only after seated BP).
DISCLOSURES:
The study was supported by the National Institutes of Health. The authors have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
results of a new study suggest.
METHODOLOGY:
- The study included 125 adults, mean age 49 years and 62% female, who were free of cardiovascular disease and had no previous history of hypertension.
- Researchers collected data on 24-hour ambulatory blood pressure monitoring (ABPM), and three BP measurements in the seated position, then three in the standing position.
- They assessed overall diagnostic accuracy of seated and standing BP using the area under the receiver operating characteristic (AUROC) curve and considered a Bayes factor (BF) of 3 or greater as significant.
- They defined the presence of hypertension (HTN) by the 2017 American College of Cardiology/American Heart Association and 2023 European Society of Hypertension HTN guidelines based on ABPM.
- Sensitivity and specificity of standing BP was determined using cutoffs derived from Youden index, while sensitivity and specificity of seated BP was determined using the cutoff of 130/80 mm Hg and by 140/90 mm Hg.
TAKEAWAY:
- The AUROC for standing office systolic blood pressure (SBP; 0.81; 0.71-0.92) was significantly higher than for seated office SBP (0.70; 0.49-0.91) in diagnosing HTN when defined as an average 24-hour SBP ≥ 125 mm Hg (BF = 11.8), and significantly higher for seated versus standing office diastolic blood pressure (DBP; 0.65; 0.49-0.82) in diagnosing HTN when defined as an average 24-hour DBP ≥ 75 mm Hg (BF = 4.9).
- The AUROCs for adding standing office BP to seated office BP improved the accuracy of detecting HTN, compared with seated office BP alone when HTN was defined as an average 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg, or when defined as an average 24-hour SBP/DBP ≥ 130/80 mm Hg or daytime SBP/DBP ≥ 135/85 mm Hg (all BFs > 3).
- Sensitivity of standing SBP was 71%, compared with 43% for seated SBP.
IN PRACTICE:
The “excellent diagnostic performance” for standing BP measures revealed by the study “highlights that standing office BP has acceptable discriminative capabilities in identifying the presence of hypertension in adults,” the authors write.
SOURCE:
The study was conducted by John M. Giacona, Hypertension Section, department of internal medicine, University of Texas Southwestern Medical Center, Dallas, and colleagues. It was published online in Scientific Reports.
LIMITATIONS:
As the study enrolled only adults free of comorbidities who were not taking antihypertensive medications, the results may not be applicable to other patients. The study design was retrospective, and the order of BP measurements was not randomized (standing BP measurements were obtained only after seated BP).
DISCLOSURES:
The study was supported by the National Institutes of Health. The authors have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
results of a new study suggest.
METHODOLOGY:
- The study included 125 adults, mean age 49 years and 62% female, who were free of cardiovascular disease and had no previous history of hypertension.
- Researchers collected data on 24-hour ambulatory blood pressure monitoring (ABPM), and three BP measurements in the seated position, then three in the standing position.
- They assessed overall diagnostic accuracy of seated and standing BP using the area under the receiver operating characteristic (AUROC) curve and considered a Bayes factor (BF) of 3 or greater as significant.
- They defined the presence of hypertension (HTN) by the 2017 American College of Cardiology/American Heart Association and 2023 European Society of Hypertension HTN guidelines based on ABPM.
- Sensitivity and specificity of standing BP was determined using cutoffs derived from Youden index, while sensitivity and specificity of seated BP was determined using the cutoff of 130/80 mm Hg and by 140/90 mm Hg.
TAKEAWAY:
- The AUROC for standing office systolic blood pressure (SBP; 0.81; 0.71-0.92) was significantly higher than for seated office SBP (0.70; 0.49-0.91) in diagnosing HTN when defined as an average 24-hour SBP ≥ 125 mm Hg (BF = 11.8), and significantly higher for seated versus standing office diastolic blood pressure (DBP; 0.65; 0.49-0.82) in diagnosing HTN when defined as an average 24-hour DBP ≥ 75 mm Hg (BF = 4.9).
- The AUROCs for adding standing office BP to seated office BP improved the accuracy of detecting HTN, compared with seated office BP alone when HTN was defined as an average 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg, or when defined as an average 24-hour SBP/DBP ≥ 130/80 mm Hg or daytime SBP/DBP ≥ 135/85 mm Hg (all BFs > 3).
- Sensitivity of standing SBP was 71%, compared with 43% for seated SBP.
IN PRACTICE:
The “excellent diagnostic performance” for standing BP measures revealed by the study “highlights that standing office BP has acceptable discriminative capabilities in identifying the presence of hypertension in adults,” the authors write.
SOURCE:
The study was conducted by John M. Giacona, Hypertension Section, department of internal medicine, University of Texas Southwestern Medical Center, Dallas, and colleagues. It was published online in Scientific Reports.
LIMITATIONS:
As the study enrolled only adults free of comorbidities who were not taking antihypertensive medications, the results may not be applicable to other patients. The study design was retrospective, and the order of BP measurements was not randomized (standing BP measurements were obtained only after seated BP).
DISCLOSURES:
The study was supported by the National Institutes of Health. The authors have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Case Q: How soon after taking emergency contraception can a patient begin hormonal contraception?
Individuals spend close to half of their lives preventing, or planning for, pregnancy. As such, contraception plays a major role in patient-provider interactions. Contraception counseling and management is a common scenario encountered in the general gynecologist’s practice. Luckily, we have two evidence-based guidelines developed by the US Centers for Disease Control and Prevention (CDC) that support the provision of contraceptive care:
- US Medical Eligibility for Contraceptive Use (US-MEC),1 which provides guidance on which patients can safely use a method
- US Selected Practice Recommendations for Contraceptive Use (US-SPR),2 which provides method-specific guidance on how to use a method (including how to: initiate or start a method; manage adherence issues, such as a missed pill, etc; and manage common issues like breakthrough bleeding). Both of these guidelines are updated routinely and are publicly available online or for free, through smartphone applications.
While most contraceptive care is straightforward, there are circumstances that require additional consideration. In this 3-part series we review 3 clinical cases, existing evidence to guide management decisions, and our recommendations. In part 1, we focus on restarting hormonal contraception after ulipristal acetate administration. In parts 2 and 3, we will discuss removal of a nonpalpable contraceptive implant and the consideration of a levonorgestrel-releasing intrauterine device (LNG-IUD) for emergency contraception.
- After using ulipristal acetate for emergency contraception, advise patients to wait at least 5 days to initiate hormonal contraception and about the importance of abstaining or using a back-up method for another 7 days with the start of their hormonal contraceptive method
CASE Meeting emergency and follow-up contraception needs
A 27-year-old woman (G0) presents to you after having unprotected intercourse 4 days ago. She does not formally track her menstrual cycles and is unsure when her last menstrual period was. She is not using contraception but is interested in starting a method. After counseling, she elects to take a dose of oral ulipristal acetate (UPA; Ella) now for emergency contraception and would like to start a combined oral contraceptive (COC) pill moving forward.
How soon after taking UPA should you tell her to start the combined hormonal pill?
Effectiveness of hormonal contraception following UPA
UPA does not appear to decrease the efficacy of COCs when started around the same time. However, immediately starting a hormonal contraceptive can decrease the effectiveness of UPA, and as such, it is recommended to take UPA and then abstain or use a backup method for 7 days before initiating a hormonal contraceptive method.1 By obtaining some additional information from your patient and with the use of shared decision making, though, your patient may be able to start their contraceptive method earlier than 5 days after UPA.
What is UPA
UPA is a progesterone receptor modulator used for emergency contraception intenhded to prevent pregnancy after unprotected intercourse or contraceptive failure.3 It works by delaying follicular rupture at least 5 days, if taken before the peak of the luteinizing hormone (LH) surge. If taken after that timeframe, it does not work. Since UPA competes for the progesterone receptor, there is a concern that the effectiveness of UPA may be decreased if a progestin-containing form of contraception is started immediately after taking UPA, or vice versa.4 Several studies have now specifically looked at the interaction between UPA and progestin-containing contraceptives, including at how UPA is impacted by the contraceptive method, and conversely, how the contraceptive method is impacted by UPA.5-8
Data on types of hormonal contraception. Brache and colleagues demonstrated that UPA users who started a desogestrel progestin-only pill (DSG POP) the next day had higher rates of ovulation within 5 days of taking UPA (45%), compared with those who the next day started a placebo pill (3%).6 This type of progestin-only pill is not available in the United States.
A study by Edelman and colleagues demonstrated similar findings in those starting a COC pill containing estrogen and progestin. When taking a COC two days after UPA use, more participants had evidence of follicular rupture in less than 5 days.5 It should be noted that these studies focused on ovulation, which—while necessary for conception to occur—is a surrogate biomarker for pregnancy risk. Additional studies have looked at the impact of UPA on the COC and have not found that UPA impacts ovulation suppression of the COC with its initiation or use.8
Considering unprotected intercourse and UPA timing. Of course, the risk of pregnancy is reliant on cycle timing plus the presence of viable sperm in the reproductive tract. Sperm have been shown to only be viable in the reproductive tract for 5 days, which could result in fertilization and subsequent pregnancy. Longevity of an egg is much shorter, at 12 to 24 hours after ovulation. For this patient, her exposure was 4 days ago, but sperm are only viable for approximately 5 days—she could consider taking the UPA now and then starting a COC earlier than 5 days since she only needs an extra day or two of protection from the UPA from the sperm in her reproductive tract. Your patient’s involvement in this decision making is paramount, as only they can prioritize their desire to avoid pregnancy from their recent act of unprotected intercourse versus their immediate needs for starting their method of contraception. It is important that individuals abstain from sexual activity or use an additional back-up method during the first 7 days of starting their method of contraception.
Continue to: Counseling considerations for the case patient...
Counseling considerations for the case patient
For a patient planning to start or resume a hormonal contraceptive method after taking UPA, the waiting period recommended by the CDC (5 days) is most beneficial for patients who are uncertain about their menstrual cycle timing in relation to the act of unprotected intercourse that already occurred and need to prioritize maximum effectiveness of emergency contraception.
Patients with unsure cycle-sex timing planning to self-start or resume a short-term hormonal contraceptive method (eg, pills, patches, or rings), should be counseled to wait 5 days after the most recent act of unprotected sex, before taking their hormonal contraceptive method.7 Patients with unsure cycle-sex timing planning to use provider-dependent hormonal contraceptive methods (eg, those requiring a prescription, including a progestin-contraceptive implant or depot medroxyprogesterone acetate) should also be counseled to wait. Timing of levonorgestrel and copper intrauterine devices are addressed in part 3 of this series.
However, if your patient has a good understanding of their menstrual cycle, and the primary concern is exposure from subsequent sexual encounters and not the recent unprotected intercourse, it is advisable to provide UPA and immediately initiate a contraceptive method. One of the primary reasons for emergency contraception failure is that its effectiveness is limited to the most recent act of unprotected sexual intercourse and does not extend to subsequent acts throughout the month.
For these patients with sure cycle-sex timing who are planning to start or resume short-or long-term contraceptive methods, and whose primary concern is to prevent pregnancy risk from subsequent sexual encounters, immediately initiating a contraceptive method is advisable. For provider-dependent methods, we must weigh the risk of unintended pregnancy from the act of intercourse that already occurred (and the potential to increase that risk by initiating a method that could compromise UPA efficacy) versus the future risk of pregnancy if the patient cannot return for a contraception visit.7
In short, starting the contraceptive method at the time of UPA use can be considered after shared decision making with the patient and understanding what their primary concerns are.
Important point
Counsel on using backup barrier contraception after UPA
Oral emergency contraception only covers that one act of unprotected intercourse and does not continue to protect a patient from pregnancy for the rest of their cycle. When taken before ovulation, UPA works by delaying follicular development and rupture for at least 5 days. Patients who continue to have unprotected intercourse after taking UPA are at a high risk of an unintended pregnancy from this ‘stalled’ follicle that will eventually ovulate. Follicular maturation resumes after UPA’s effects wane, and the patient is primed for ovulation (and therefore unintended pregnancy) if ongoing unprotected intercourse occurs for the rest of their cycle.
Therefore, it is important to counsel patients on the need, if they do not desire a pregnancy, to abstain or start a method of contraception.
Final question
What about starting or resuming non–hormonal contraceptive methods?
Non-hormonal contraceptive methods can be started immediately with UPA use.1
CASE Resolved
After shared decision making, the patient decides to start using the COC pill. You prescribe her both UPA for emergency contraception and a combined hormonal contraceptive pill. Given her unsure cycle-sex timing, she expresses to you that her most important priority is preventing unintended pregnancy. You counsel her to set a reminder on her phone to start taking the pill 5 days from her most recent act of unprotected intercourse. You also counsel her to use a back-up barrier method of contraception for 7 days after starting her COC pill. ●
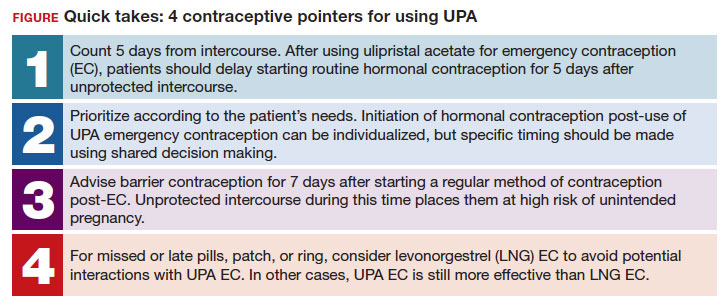
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. Morb Mortal Wkly Rep. 2016;65:1-66. https://doi .org/10.15585/mmwr.rr6504a1
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. US Selected Practice Recommendations for Contraceptive Use (US-SPR). Accessed October 11, 2023. https://www.cdc.gov/reproductivehealth /contraception/mmwr/spr/summary.html
- Ella [package insert]. Charleston, SC; Afaxys, Inc. 2014.
- Salcedo J, Rodriguez MI, Curtis KM, et al. When can a woman resume or initiate contraception after taking emergency contraceptive pills? A systematic review. Contraception. 2013;87:602-604. https://doi.org/10.1016 /j.contraception.2012.08.013
- Edelman AB, Jensen JT, McCrimmon S, et al. Combined oral contraceptive interference with the ability of ulipristal acetate to delay ovulation: a prospective cohort study. Contraception. 2018;98:463-466. doi: 10.1016/j.contraception.2018.08.003
- Brache V, Cochon L, Duijkers IJM, et al. A prospective, randomized, pharmacodynamic study of quick-starting a desogestrel progestin-only pill following ulipristal acetate for emergency contraception. Hum Reprod Oxf Engl. 2015;30:2785-2793. https://doi.org/10.1093/humrep /dev241
- Cameron ST, Berger C, Michie L, et al. The effects on ovarian activity of ulipristal acetate when ‘quickstarting’ a combined oral contraceptive pill: a prospective, randomized, doubleblind parallel-arm, placebo-controlled study. Hum Reprod. 2015;30:1566-1572. doi: 10.1093/humrep/dev115
- Banh C, Rautenberg T, Diujkers I, et al. The effects on ovarian activity of delaying versus immediately restarting combined oral contraception after missing three pills and taking ulipristal acetate 30 mg. Contraception. 2020;102:145-151. doi: 10.1016/j.contraception.2020.05.013
- American Society for Emergency Contraception. Providing ongoing hormonal contraception after use of emergency contraceptive pills. September 2016. Accessed October 11, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj /https://www.americansocietyforec.org/_files/ugd/7f2e0b _ff1bc90bea204644ba28d1b0e6a6a6a8.pdf
Individuals spend close to half of their lives preventing, or planning for, pregnancy. As such, contraception plays a major role in patient-provider interactions. Contraception counseling and management is a common scenario encountered in the general gynecologist’s practice. Luckily, we have two evidence-based guidelines developed by the US Centers for Disease Control and Prevention (CDC) that support the provision of contraceptive care:
- US Medical Eligibility for Contraceptive Use (US-MEC),1 which provides guidance on which patients can safely use a method
- US Selected Practice Recommendations for Contraceptive Use (US-SPR),2 which provides method-specific guidance on how to use a method (including how to: initiate or start a method; manage adherence issues, such as a missed pill, etc; and manage common issues like breakthrough bleeding). Both of these guidelines are updated routinely and are publicly available online or for free, through smartphone applications.
While most contraceptive care is straightforward, there are circumstances that require additional consideration. In this 3-part series we review 3 clinical cases, existing evidence to guide management decisions, and our recommendations. In part 1, we focus on restarting hormonal contraception after ulipristal acetate administration. In parts 2 and 3, we will discuss removal of a nonpalpable contraceptive implant and the consideration of a levonorgestrel-releasing intrauterine device (LNG-IUD) for emergency contraception.
- After using ulipristal acetate for emergency contraception, advise patients to wait at least 5 days to initiate hormonal contraception and about the importance of abstaining or using a back-up method for another 7 days with the start of their hormonal contraceptive method
CASE Meeting emergency and follow-up contraception needs
A 27-year-old woman (G0) presents to you after having unprotected intercourse 4 days ago. She does not formally track her menstrual cycles and is unsure when her last menstrual period was. She is not using contraception but is interested in starting a method. After counseling, she elects to take a dose of oral ulipristal acetate (UPA; Ella) now for emergency contraception and would like to start a combined oral contraceptive (COC) pill moving forward.
How soon after taking UPA should you tell her to start the combined hormonal pill?
Effectiveness of hormonal contraception following UPA
UPA does not appear to decrease the efficacy of COCs when started around the same time. However, immediately starting a hormonal contraceptive can decrease the effectiveness of UPA, and as such, it is recommended to take UPA and then abstain or use a backup method for 7 days before initiating a hormonal contraceptive method.1 By obtaining some additional information from your patient and with the use of shared decision making, though, your patient may be able to start their contraceptive method earlier than 5 days after UPA.
What is UPA
UPA is a progesterone receptor modulator used for emergency contraception intenhded to prevent pregnancy after unprotected intercourse or contraceptive failure.3 It works by delaying follicular rupture at least 5 days, if taken before the peak of the luteinizing hormone (LH) surge. If taken after that timeframe, it does not work. Since UPA competes for the progesterone receptor, there is a concern that the effectiveness of UPA may be decreased if a progestin-containing form of contraception is started immediately after taking UPA, or vice versa.4 Several studies have now specifically looked at the interaction between UPA and progestin-containing contraceptives, including at how UPA is impacted by the contraceptive method, and conversely, how the contraceptive method is impacted by UPA.5-8
Data on types of hormonal contraception. Brache and colleagues demonstrated that UPA users who started a desogestrel progestin-only pill (DSG POP) the next day had higher rates of ovulation within 5 days of taking UPA (45%), compared with those who the next day started a placebo pill (3%).6 This type of progestin-only pill is not available in the United States.
A study by Edelman and colleagues demonstrated similar findings in those starting a COC pill containing estrogen and progestin. When taking a COC two days after UPA use, more participants had evidence of follicular rupture in less than 5 days.5 It should be noted that these studies focused on ovulation, which—while necessary for conception to occur—is a surrogate biomarker for pregnancy risk. Additional studies have looked at the impact of UPA on the COC and have not found that UPA impacts ovulation suppression of the COC with its initiation or use.8
Considering unprotected intercourse and UPA timing. Of course, the risk of pregnancy is reliant on cycle timing plus the presence of viable sperm in the reproductive tract. Sperm have been shown to only be viable in the reproductive tract for 5 days, which could result in fertilization and subsequent pregnancy. Longevity of an egg is much shorter, at 12 to 24 hours after ovulation. For this patient, her exposure was 4 days ago, but sperm are only viable for approximately 5 days—she could consider taking the UPA now and then starting a COC earlier than 5 days since she only needs an extra day or two of protection from the UPA from the sperm in her reproductive tract. Your patient’s involvement in this decision making is paramount, as only they can prioritize their desire to avoid pregnancy from their recent act of unprotected intercourse versus their immediate needs for starting their method of contraception. It is important that individuals abstain from sexual activity or use an additional back-up method during the first 7 days of starting their method of contraception.
Continue to: Counseling considerations for the case patient...
Counseling considerations for the case patient
For a patient planning to start or resume a hormonal contraceptive method after taking UPA, the waiting period recommended by the CDC (5 days) is most beneficial for patients who are uncertain about their menstrual cycle timing in relation to the act of unprotected intercourse that already occurred and need to prioritize maximum effectiveness of emergency contraception.
Patients with unsure cycle-sex timing planning to self-start or resume a short-term hormonal contraceptive method (eg, pills, patches, or rings), should be counseled to wait 5 days after the most recent act of unprotected sex, before taking their hormonal contraceptive method.7 Patients with unsure cycle-sex timing planning to use provider-dependent hormonal contraceptive methods (eg, those requiring a prescription, including a progestin-contraceptive implant or depot medroxyprogesterone acetate) should also be counseled to wait. Timing of levonorgestrel and copper intrauterine devices are addressed in part 3 of this series.
However, if your patient has a good understanding of their menstrual cycle, and the primary concern is exposure from subsequent sexual encounters and not the recent unprotected intercourse, it is advisable to provide UPA and immediately initiate a contraceptive method. One of the primary reasons for emergency contraception failure is that its effectiveness is limited to the most recent act of unprotected sexual intercourse and does not extend to subsequent acts throughout the month.
For these patients with sure cycle-sex timing who are planning to start or resume short-or long-term contraceptive methods, and whose primary concern is to prevent pregnancy risk from subsequent sexual encounters, immediately initiating a contraceptive method is advisable. For provider-dependent methods, we must weigh the risk of unintended pregnancy from the act of intercourse that already occurred (and the potential to increase that risk by initiating a method that could compromise UPA efficacy) versus the future risk of pregnancy if the patient cannot return for a contraception visit.7
In short, starting the contraceptive method at the time of UPA use can be considered after shared decision making with the patient and understanding what their primary concerns are.
Important point
Counsel on using backup barrier contraception after UPA
Oral emergency contraception only covers that one act of unprotected intercourse and does not continue to protect a patient from pregnancy for the rest of their cycle. When taken before ovulation, UPA works by delaying follicular development and rupture for at least 5 days. Patients who continue to have unprotected intercourse after taking UPA are at a high risk of an unintended pregnancy from this ‘stalled’ follicle that will eventually ovulate. Follicular maturation resumes after UPA’s effects wane, and the patient is primed for ovulation (and therefore unintended pregnancy) if ongoing unprotected intercourse occurs for the rest of their cycle.
Therefore, it is important to counsel patients on the need, if they do not desire a pregnancy, to abstain or start a method of contraception.
Final question
What about starting or resuming non–hormonal contraceptive methods?
Non-hormonal contraceptive methods can be started immediately with UPA use.1
CASE Resolved
After shared decision making, the patient decides to start using the COC pill. You prescribe her both UPA for emergency contraception and a combined hormonal contraceptive pill. Given her unsure cycle-sex timing, she expresses to you that her most important priority is preventing unintended pregnancy. You counsel her to set a reminder on her phone to start taking the pill 5 days from her most recent act of unprotected intercourse. You also counsel her to use a back-up barrier method of contraception for 7 days after starting her COC pill. ●

Individuals spend close to half of their lives preventing, or planning for, pregnancy. As such, contraception plays a major role in patient-provider interactions. Contraception counseling and management is a common scenario encountered in the general gynecologist’s practice. Luckily, we have two evidence-based guidelines developed by the US Centers for Disease Control and Prevention (CDC) that support the provision of contraceptive care:
- US Medical Eligibility for Contraceptive Use (US-MEC),1 which provides guidance on which patients can safely use a method
- US Selected Practice Recommendations for Contraceptive Use (US-SPR),2 which provides method-specific guidance on how to use a method (including how to: initiate or start a method; manage adherence issues, such as a missed pill, etc; and manage common issues like breakthrough bleeding). Both of these guidelines are updated routinely and are publicly available online or for free, through smartphone applications.
While most contraceptive care is straightforward, there are circumstances that require additional consideration. In this 3-part series we review 3 clinical cases, existing evidence to guide management decisions, and our recommendations. In part 1, we focus on restarting hormonal contraception after ulipristal acetate administration. In parts 2 and 3, we will discuss removal of a nonpalpable contraceptive implant and the consideration of a levonorgestrel-releasing intrauterine device (LNG-IUD) for emergency contraception.
- After using ulipristal acetate for emergency contraception, advise patients to wait at least 5 days to initiate hormonal contraception and about the importance of abstaining or using a back-up method for another 7 days with the start of their hormonal contraceptive method
CASE Meeting emergency and follow-up contraception needs
A 27-year-old woman (G0) presents to you after having unprotected intercourse 4 days ago. She does not formally track her menstrual cycles and is unsure when her last menstrual period was. She is not using contraception but is interested in starting a method. After counseling, she elects to take a dose of oral ulipristal acetate (UPA; Ella) now for emergency contraception and would like to start a combined oral contraceptive (COC) pill moving forward.
How soon after taking UPA should you tell her to start the combined hormonal pill?
Effectiveness of hormonal contraception following UPA
UPA does not appear to decrease the efficacy of COCs when started around the same time. However, immediately starting a hormonal contraceptive can decrease the effectiveness of UPA, and as such, it is recommended to take UPA and then abstain or use a backup method for 7 days before initiating a hormonal contraceptive method.1 By obtaining some additional information from your patient and with the use of shared decision making, though, your patient may be able to start their contraceptive method earlier than 5 days after UPA.
What is UPA
UPA is a progesterone receptor modulator used for emergency contraception intenhded to prevent pregnancy after unprotected intercourse or contraceptive failure.3 It works by delaying follicular rupture at least 5 days, if taken before the peak of the luteinizing hormone (LH) surge. If taken after that timeframe, it does not work. Since UPA competes for the progesterone receptor, there is a concern that the effectiveness of UPA may be decreased if a progestin-containing form of contraception is started immediately after taking UPA, or vice versa.4 Several studies have now specifically looked at the interaction between UPA and progestin-containing contraceptives, including at how UPA is impacted by the contraceptive method, and conversely, how the contraceptive method is impacted by UPA.5-8
Data on types of hormonal contraception. Brache and colleagues demonstrated that UPA users who started a desogestrel progestin-only pill (DSG POP) the next day had higher rates of ovulation within 5 days of taking UPA (45%), compared with those who the next day started a placebo pill (3%).6 This type of progestin-only pill is not available in the United States.
A study by Edelman and colleagues demonstrated similar findings in those starting a COC pill containing estrogen and progestin. When taking a COC two days after UPA use, more participants had evidence of follicular rupture in less than 5 days.5 It should be noted that these studies focused on ovulation, which—while necessary for conception to occur—is a surrogate biomarker for pregnancy risk. Additional studies have looked at the impact of UPA on the COC and have not found that UPA impacts ovulation suppression of the COC with its initiation or use.8
Considering unprotected intercourse and UPA timing. Of course, the risk of pregnancy is reliant on cycle timing plus the presence of viable sperm in the reproductive tract. Sperm have been shown to only be viable in the reproductive tract for 5 days, which could result in fertilization and subsequent pregnancy. Longevity of an egg is much shorter, at 12 to 24 hours after ovulation. For this patient, her exposure was 4 days ago, but sperm are only viable for approximately 5 days—she could consider taking the UPA now and then starting a COC earlier than 5 days since she only needs an extra day or two of protection from the UPA from the sperm in her reproductive tract. Your patient’s involvement in this decision making is paramount, as only they can prioritize their desire to avoid pregnancy from their recent act of unprotected intercourse versus their immediate needs for starting their method of contraception. It is important that individuals abstain from sexual activity or use an additional back-up method during the first 7 days of starting their method of contraception.
Continue to: Counseling considerations for the case patient...
Counseling considerations for the case patient
For a patient planning to start or resume a hormonal contraceptive method after taking UPA, the waiting period recommended by the CDC (5 days) is most beneficial for patients who are uncertain about their menstrual cycle timing in relation to the act of unprotected intercourse that already occurred and need to prioritize maximum effectiveness of emergency contraception.
Patients with unsure cycle-sex timing planning to self-start or resume a short-term hormonal contraceptive method (eg, pills, patches, or rings), should be counseled to wait 5 days after the most recent act of unprotected sex, before taking their hormonal contraceptive method.7 Patients with unsure cycle-sex timing planning to use provider-dependent hormonal contraceptive methods (eg, those requiring a prescription, including a progestin-contraceptive implant or depot medroxyprogesterone acetate) should also be counseled to wait. Timing of levonorgestrel and copper intrauterine devices are addressed in part 3 of this series.
However, if your patient has a good understanding of their menstrual cycle, and the primary concern is exposure from subsequent sexual encounters and not the recent unprotected intercourse, it is advisable to provide UPA and immediately initiate a contraceptive method. One of the primary reasons for emergency contraception failure is that its effectiveness is limited to the most recent act of unprotected sexual intercourse and does not extend to subsequent acts throughout the month.
For these patients with sure cycle-sex timing who are planning to start or resume short-or long-term contraceptive methods, and whose primary concern is to prevent pregnancy risk from subsequent sexual encounters, immediately initiating a contraceptive method is advisable. For provider-dependent methods, we must weigh the risk of unintended pregnancy from the act of intercourse that already occurred (and the potential to increase that risk by initiating a method that could compromise UPA efficacy) versus the future risk of pregnancy if the patient cannot return for a contraception visit.7
In short, starting the contraceptive method at the time of UPA use can be considered after shared decision making with the patient and understanding what their primary concerns are.
Important point
Counsel on using backup barrier contraception after UPA
Oral emergency contraception only covers that one act of unprotected intercourse and does not continue to protect a patient from pregnancy for the rest of their cycle. When taken before ovulation, UPA works by delaying follicular development and rupture for at least 5 days. Patients who continue to have unprotected intercourse after taking UPA are at a high risk of an unintended pregnancy from this ‘stalled’ follicle that will eventually ovulate. Follicular maturation resumes after UPA’s effects wane, and the patient is primed for ovulation (and therefore unintended pregnancy) if ongoing unprotected intercourse occurs for the rest of their cycle.
Therefore, it is important to counsel patients on the need, if they do not desire a pregnancy, to abstain or start a method of contraception.
Final question
What about starting or resuming non–hormonal contraceptive methods?
Non-hormonal contraceptive methods can be started immediately with UPA use.1
CASE Resolved
After shared decision making, the patient decides to start using the COC pill. You prescribe her both UPA for emergency contraception and a combined hormonal contraceptive pill. Given her unsure cycle-sex timing, she expresses to you that her most important priority is preventing unintended pregnancy. You counsel her to set a reminder on her phone to start taking the pill 5 days from her most recent act of unprotected intercourse. You also counsel her to use a back-up barrier method of contraception for 7 days after starting her COC pill. ●

- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. Morb Mortal Wkly Rep. 2016;65:1-66. https://doi .org/10.15585/mmwr.rr6504a1
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. US Selected Practice Recommendations for Contraceptive Use (US-SPR). Accessed October 11, 2023. https://www.cdc.gov/reproductivehealth /contraception/mmwr/spr/summary.html
- Ella [package insert]. Charleston, SC; Afaxys, Inc. 2014.
- Salcedo J, Rodriguez MI, Curtis KM, et al. When can a woman resume or initiate contraception after taking emergency contraceptive pills? A systematic review. Contraception. 2013;87:602-604. https://doi.org/10.1016 /j.contraception.2012.08.013
- Edelman AB, Jensen JT, McCrimmon S, et al. Combined oral contraceptive interference with the ability of ulipristal acetate to delay ovulation: a prospective cohort study. Contraception. 2018;98:463-466. doi: 10.1016/j.contraception.2018.08.003
- Brache V, Cochon L, Duijkers IJM, et al. A prospective, randomized, pharmacodynamic study of quick-starting a desogestrel progestin-only pill following ulipristal acetate for emergency contraception. Hum Reprod Oxf Engl. 2015;30:2785-2793. https://doi.org/10.1093/humrep /dev241
- Cameron ST, Berger C, Michie L, et al. The effects on ovarian activity of ulipristal acetate when ‘quickstarting’ a combined oral contraceptive pill: a prospective, randomized, doubleblind parallel-arm, placebo-controlled study. Hum Reprod. 2015;30:1566-1572. doi: 10.1093/humrep/dev115
- Banh C, Rautenberg T, Diujkers I, et al. The effects on ovarian activity of delaying versus immediately restarting combined oral contraception after missing three pills and taking ulipristal acetate 30 mg. Contraception. 2020;102:145-151. doi: 10.1016/j.contraception.2020.05.013
- American Society for Emergency Contraception. Providing ongoing hormonal contraception after use of emergency contraceptive pills. September 2016. Accessed October 11, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj /https://www.americansocietyforec.org/_files/ugd/7f2e0b _ff1bc90bea204644ba28d1b0e6a6a6a8.pdf
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. Morb Mortal Wkly Rep. 2016;65:1-66. https://doi .org/10.15585/mmwr.rr6504a1
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. US Selected Practice Recommendations for Contraceptive Use (US-SPR). Accessed October 11, 2023. https://www.cdc.gov/reproductivehealth /contraception/mmwr/spr/summary.html
- Ella [package insert]. Charleston, SC; Afaxys, Inc. 2014.
- Salcedo J, Rodriguez MI, Curtis KM, et al. When can a woman resume or initiate contraception after taking emergency contraceptive pills? A systematic review. Contraception. 2013;87:602-604. https://doi.org/10.1016 /j.contraception.2012.08.013
- Edelman AB, Jensen JT, McCrimmon S, et al. Combined oral contraceptive interference with the ability of ulipristal acetate to delay ovulation: a prospective cohort study. Contraception. 2018;98:463-466. doi: 10.1016/j.contraception.2018.08.003
- Brache V, Cochon L, Duijkers IJM, et al. A prospective, randomized, pharmacodynamic study of quick-starting a desogestrel progestin-only pill following ulipristal acetate for emergency contraception. Hum Reprod Oxf Engl. 2015;30:2785-2793. https://doi.org/10.1093/humrep /dev241
- Cameron ST, Berger C, Michie L, et al. The effects on ovarian activity of ulipristal acetate when ‘quickstarting’ a combined oral contraceptive pill: a prospective, randomized, doubleblind parallel-arm, placebo-controlled study. Hum Reprod. 2015;30:1566-1572. doi: 10.1093/humrep/dev115
- Banh C, Rautenberg T, Diujkers I, et al. The effects on ovarian activity of delaying versus immediately restarting combined oral contraception after missing three pills and taking ulipristal acetate 30 mg. Contraception. 2020;102:145-151. doi: 10.1016/j.contraception.2020.05.013
- American Society for Emergency Contraception. Providing ongoing hormonal contraception after use of emergency contraceptive pills. September 2016. Accessed October 11, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj /https://www.americansocietyforec.org/_files/ugd/7f2e0b _ff1bc90bea204644ba28d1b0e6a6a6a8.pdf
RSV vaccination during pregnancy: Finally ready for prime time
A 28-year-old primigravid woman at 30 weeks’ gestation inquires about the new vaccine to protect her newborn baby against respiratory syncytial virus infection (RSV). Her neighbor’s daughter recently was hospitalized for the treatment of RSV, and she is understandably concerned about her own newborn. The patient is healthy, and she has never had any serious respiratory infection. She is taking no medications other than prenatal vitamins.
What advice should you give her?
If you decide to administer this vaccine, what is the appropriate timing of administration?
Are there any maternal or fetal safety concerns related to use of this vaccine in pregnancy?
Respiratory syncytial virus (RSV) is a member of the Paramyxoviridae family. It is an enveloped, single-stranded RNA virus that is 150-300 nm in size. The virus codes for 10 virus-specific proteins. The 2 most important are the G protein, which enables the virus to attach to host cells, and the F protein, which facilitates the entry of the virus into the host cell by fusing the host and viral membranes. Two distinct subtypes exist: A and B. There is genetic variation within each subtype and between subtypes. These subtle genetic variations create the potential for reinfections, and hence, research has focused on development of a vaccine that covers both subtypes.1
RSV is the most common cause of acute lower respiratory tract infection in infants younger than 6 months of age. In these children, RSV is one of the most prominent causes of death, with mortality particularly marked in low- and middle-resource countries as well as in children who were born premature and/or who are immunocompromised. RSV has its greatest impact during winter epidemics in temperate climates and during the rainy seasons in tropical climates. The virus rarely is encountered in the summer.1 Among young children, RSV primarily is transmitted via close contact with contaminated fingers or fomites and by self-inoculation of the conjunctiva or anterior nares. The incubation period of the infection is 4 to 6 days, and viral shedding may persist for 2 weeks or longer. Most patients gradually recover within 1 to 2 weeks.1 Adults who contract RSV usually have symptoms suggestive of a common cold; however, in older adults or those who have comorbidities, serious and potentially life-threatening lower respiratory tract infections may develop.
Recently, there have been 2 main approaches to the prevention and treatment of RSV in infants. One has been the development of monoclonal antibodies such as motavizumab, palivizumab, and nirsevimab. The other has been the development of a vaccine that could be administered to pregnant women and which could provide protection for the neonate in the early months of life.2,3
In late August 2023, the US Food and Drug Administration (FDA) announced the approval of a new bivalent RSV prefusion F vaccine (ABRYSVO, Pfizer) intended for administration to pregnant women.4 Of note, previous efforts to develop whole-virus vaccines either have been ineffective or have potentiated the disease in infants who became infected; development of an effective vaccine had eluded scientists and clinicians for nearly 50 years.2 Thus, the new vaccine that targets the F protein of the virus represents a major and welcomed breakthrough.
This article reviews the 3 most recent investigations that preceded the ultimate approval of this vaccine and discusses specific logistical issues related to vaccine administration.
Continue to: First step toward vaccine approval...
First step toward vaccine approval
Madhi and colleagues5 were among the first to conduct a large well-designed study to evaluate the effectiveness of maternal vaccination in preventing neonatal infection in the first few months of life. The authors enrolled more than 4,500 healthy pregnant women at 28 to 36 weeks of gestation and assigned them to receive either a single intramuscular dose of an RSV fusion (F) protein vaccine or placebo in a ratio of 2:1. The primary end point was a “medically significant lower respiratory tract infection” within the first 90 days of life. The percentage of infants who met the primary end point was low in both groups: 1.5% in the vaccine group and 2.4% in the placebo group (efficacy 39.4%). The efficacy of the vaccine in preventing lower respiratory tract infection with severe hypoxemia was 48.3% and 44.4% in preventing hospitalization. Although there were differences between the 2 groups, they did not meet the prespecified success criterion for efficacy. Vaccine recipients had more local injection site reactions (40.7% vs 9.9%); however, there was no difference in the frequency of other adverse effects.
Intermediate step: Continued assessment of vaccine safety and immunogenicity
The next important step in the development of the RSV vaccine was a study by Simoes et al,6 who conducted a phase 2b trial to determine the safety and immunogenicity of the RSVpreF vaccine. The authors randomly assigned pregnant women at 24 to 36 weeks of gestation to receive either 120 or 240 µg of RSVpreF vaccine or placebo. The key endpoints were the following: maternal and infant safety; the maternal-to-infant transplacental transfer ratio; and the presence of RSV A, B, and combined A/B neutralizing antibody in maternal serum and umbilical cord blood at delivery. The authors conducted a planned interim analysis that included 327 mothers who received the vaccine. The incidence of adverse effects was similar in mothers and infants in the vaccine compared with the placebo group. None of the adverse effects were judged to be serious. The transplacental neutralizing antibody transfer ratios ranged from 1.4 to 2.1 across a range of gestational ages. The vaccine elicited meaningful neutralizing titers of antibody in maternal serum even up to 7 weeks after immunization. The levels of neutralizing antibodies in umbilical cord blood did not vary substantially with respect to gestational age. A post hoc analysis showed that the transferred antibodies prevented medically-attended RSV-associated lower respiratory tract illnesses in the infants.
Final step: Convincing proof of efficacy
The most recent of the 3 studies, and the one that had the greatest impact in convincing the FDA to approve the vaccine, was the report by Kampmann and colleagues.7 The authors conducted a phase 3 prospective, randomized, double-blind trial in 18 different countries over 4 RSV seasons: 2 in the northern hemisphere and 2 in the southern hemisphere. They enrolled healthy pregnant women with singleton gestations at 24 to 36 weeks of gestation and assigned them in a 1:1 ratio to a single intramuscular injection of 120 µg of a bivalent RSV prefusion F protein-based (RSVpreF) vaccine or placebo. They excluded patients with any recognized risk factor for an adverse pregnancy outcome, including preterm labor. The 2 primary efficacy endpoints were a medically-attended severe RSV–lower respiratory tract infection and any medically attended RSV-associated lower respiratory tract illness in infants within 90, 120, 150, and 180 days after birth.
The efficacy of the vaccine in preventing severe lower respiratory tract illness within 90 days of delivery was 81.8% (99.5% confidence interval [CI], 40.6–96.3). The efficacy within 180 days of delivery was 69.4% (97.58% CI, 44.3–84.1). These differences reached the study’s pre-established statistical criteria for success. The overall rate of lower respiratory tract infections was not significantly different. The frequencies of adverse effects in mothers and infants were similar in the vaccine and placebo groups. In particular, the frequency of preterm delivery in the vaccine group was 0.8%, compared with 0.6% in the placebo group (P = NS).
In previous reports to the FDA,4 the frequency rate of preterm delivery in RSV vaccine recipients was slightly increased in vaccine recipients compared with patients who received placebo. The difference among the groups was too small to infer a causal relationship; however, as a condition of vaccine approval, the FDA has required Pfizer to conduct a postmarketing study to be certain that administration of the vaccine does not increase the risk for preterm delivery.
Practical details
The new vaccine is a bivalent recombinant vaccine that elicits a robust antibody response against the F (fusion) protein of the virus. In addition to the F antigen, the vaccine contains the following buffer ingredients: tromethamine, sucrose, mannitol, polysorbate, and sodium chloride.8 There are no preservatives in the vaccine.
The vaccine should be administered in a single, 0.5 mL, intramuscular injection at 32 to 36 weeks of gestation. Patients who are allergic to any of the components of the vaccine should not be vaccinated. Patients with a mild upper respiratory tract infection may receive the vaccine. Administration should be delayed in patients who are moderately to severely ill. The vaccine may be administered at the same time as other vaccines, such as influenza or Tdap.
The most common side effects of the vaccine are local injection site reactions, such as pain, redness, or swelling. Some patients may experience mild systemic manifestations, including fatigue, fever, headache, nausea, diarrhea, arthralgias, and myalgias. According to the Centers for Disease Control and Prevention, the approximate wholesale acquisition cost of the vaccine is $320 for 1 injection.
CASE Resolution
This patient is healthy and has no contraindication to the new RSV vaccine. According to the FDA, the optimal time for administration of the vaccine is 32 to 36 weeks of gestation. The patient should anticipate very few side effects following the vaccination, and the vaccine has approximately 80% efficacy in preventing severe lower respiratory tract infection in her neonate. ●
- RSV is the most common cause of acute lower respiratory tract infection in infants younger than 6 months of age.
- In low- and middle-resource countries, RSV is a leading cause of infant death.
- In late August 2023, the FDA approved the first RSV vaccine that can be administered to pregnant women to provide protection for the infant in the first few months of life.
- The vaccine specifically targets the F protein of the virus, a protein which is essential for facilitating fusion between the viral and host cell membranes, resulting in penetration of the virus into the host cell.
- The vaccine should be administered as a single intramuscular injection at 32 to 36 weeks’ gestation.
- The vaccine is approximately 82% effective in preventing severe lower respiratory tract infection in infants within the first 6 months of life.
- To exercise an abundance of caution, because of a possible association between administration of the vaccine and an increased risk for preterm delivery, vaccination should be delayed until 36 weeks in patients clearly identified as at-risk for preterm delivery.
- Dolin R. Common viral respiratory infections. In, Isselbacher KJ, Braunwald E, Wilson JD, et al, eds. Harrison’s Principles of Internal Medicine. 13th ed. McGraw-Hill; 1994:805-806.
- Mazur N, Terstappen J, Baral R, et al. Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. Lancet Infect Dis. 2023;23:E2-E21.
- Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386:837-846.
- US Food and Drug Administration News Release. August 21, 2023. Accessed October 26, 2023. https://www.fda.gov/news -events/press-announcements/fda-approves-first-vaccine -pregnant-individuals-prevent-rsv-infants
- Madhi SA, Polack FP, Piedra PA, et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med. 2020;383:426-439.
- Simoes EAF, Center KJ, Tita ATN, et al. Prefusion F proteinbased respiratory syncytial virus immunization in pregnancy. N Eng J Med. 2022;386:1615-1626.
- Kampmann B, Madhi SA, Munjal I, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388:1451-1464.
- Centers for Disease Control and Prevention. Vaccine Information Statement. Respiratory Syncytial Virus (RSV) Vaccine VIS. October 19, 2023. Accessed October 26, 2023. https://www. cdc.gov/vaccines/hcp/vis/vis-statements/rsv.html
A 28-year-old primigravid woman at 30 weeks’ gestation inquires about the new vaccine to protect her newborn baby against respiratory syncytial virus infection (RSV). Her neighbor’s daughter recently was hospitalized for the treatment of RSV, and she is understandably concerned about her own newborn. The patient is healthy, and she has never had any serious respiratory infection. She is taking no medications other than prenatal vitamins.
What advice should you give her?
If you decide to administer this vaccine, what is the appropriate timing of administration?
Are there any maternal or fetal safety concerns related to use of this vaccine in pregnancy?
Respiratory syncytial virus (RSV) is a member of the Paramyxoviridae family. It is an enveloped, single-stranded RNA virus that is 150-300 nm in size. The virus codes for 10 virus-specific proteins. The 2 most important are the G protein, which enables the virus to attach to host cells, and the F protein, which facilitates the entry of the virus into the host cell by fusing the host and viral membranes. Two distinct subtypes exist: A and B. There is genetic variation within each subtype and between subtypes. These subtle genetic variations create the potential for reinfections, and hence, research has focused on development of a vaccine that covers both subtypes.1
RSV is the most common cause of acute lower respiratory tract infection in infants younger than 6 months of age. In these children, RSV is one of the most prominent causes of death, with mortality particularly marked in low- and middle-resource countries as well as in children who were born premature and/or who are immunocompromised. RSV has its greatest impact during winter epidemics in temperate climates and during the rainy seasons in tropical climates. The virus rarely is encountered in the summer.1 Among young children, RSV primarily is transmitted via close contact with contaminated fingers or fomites and by self-inoculation of the conjunctiva or anterior nares. The incubation period of the infection is 4 to 6 days, and viral shedding may persist for 2 weeks or longer. Most patients gradually recover within 1 to 2 weeks.1 Adults who contract RSV usually have symptoms suggestive of a common cold; however, in older adults or those who have comorbidities, serious and potentially life-threatening lower respiratory tract infections may develop.
Recently, there have been 2 main approaches to the prevention and treatment of RSV in infants. One has been the development of monoclonal antibodies such as motavizumab, palivizumab, and nirsevimab. The other has been the development of a vaccine that could be administered to pregnant women and which could provide protection for the neonate in the early months of life.2,3
In late August 2023, the US Food and Drug Administration (FDA) announced the approval of a new bivalent RSV prefusion F vaccine (ABRYSVO, Pfizer) intended for administration to pregnant women.4 Of note, previous efforts to develop whole-virus vaccines either have been ineffective or have potentiated the disease in infants who became infected; development of an effective vaccine had eluded scientists and clinicians for nearly 50 years.2 Thus, the new vaccine that targets the F protein of the virus represents a major and welcomed breakthrough.
This article reviews the 3 most recent investigations that preceded the ultimate approval of this vaccine and discusses specific logistical issues related to vaccine administration.
Continue to: First step toward vaccine approval...
First step toward vaccine approval
Madhi and colleagues5 were among the first to conduct a large well-designed study to evaluate the effectiveness of maternal vaccination in preventing neonatal infection in the first few months of life. The authors enrolled more than 4,500 healthy pregnant women at 28 to 36 weeks of gestation and assigned them to receive either a single intramuscular dose of an RSV fusion (F) protein vaccine or placebo in a ratio of 2:1. The primary end point was a “medically significant lower respiratory tract infection” within the first 90 days of life. The percentage of infants who met the primary end point was low in both groups: 1.5% in the vaccine group and 2.4% in the placebo group (efficacy 39.4%). The efficacy of the vaccine in preventing lower respiratory tract infection with severe hypoxemia was 48.3% and 44.4% in preventing hospitalization. Although there were differences between the 2 groups, they did not meet the prespecified success criterion for efficacy. Vaccine recipients had more local injection site reactions (40.7% vs 9.9%); however, there was no difference in the frequency of other adverse effects.
Intermediate step: Continued assessment of vaccine safety and immunogenicity
The next important step in the development of the RSV vaccine was a study by Simoes et al,6 who conducted a phase 2b trial to determine the safety and immunogenicity of the RSVpreF vaccine. The authors randomly assigned pregnant women at 24 to 36 weeks of gestation to receive either 120 or 240 µg of RSVpreF vaccine or placebo. The key endpoints were the following: maternal and infant safety; the maternal-to-infant transplacental transfer ratio; and the presence of RSV A, B, and combined A/B neutralizing antibody in maternal serum and umbilical cord blood at delivery. The authors conducted a planned interim analysis that included 327 mothers who received the vaccine. The incidence of adverse effects was similar in mothers and infants in the vaccine compared with the placebo group. None of the adverse effects were judged to be serious. The transplacental neutralizing antibody transfer ratios ranged from 1.4 to 2.1 across a range of gestational ages. The vaccine elicited meaningful neutralizing titers of antibody in maternal serum even up to 7 weeks after immunization. The levels of neutralizing antibodies in umbilical cord blood did not vary substantially with respect to gestational age. A post hoc analysis showed that the transferred antibodies prevented medically-attended RSV-associated lower respiratory tract illnesses in the infants.
Final step: Convincing proof of efficacy
The most recent of the 3 studies, and the one that had the greatest impact in convincing the FDA to approve the vaccine, was the report by Kampmann and colleagues.7 The authors conducted a phase 3 prospective, randomized, double-blind trial in 18 different countries over 4 RSV seasons: 2 in the northern hemisphere and 2 in the southern hemisphere. They enrolled healthy pregnant women with singleton gestations at 24 to 36 weeks of gestation and assigned them in a 1:1 ratio to a single intramuscular injection of 120 µg of a bivalent RSV prefusion F protein-based (RSVpreF) vaccine or placebo. They excluded patients with any recognized risk factor for an adverse pregnancy outcome, including preterm labor. The 2 primary efficacy endpoints were a medically-attended severe RSV–lower respiratory tract infection and any medically attended RSV-associated lower respiratory tract illness in infants within 90, 120, 150, and 180 days after birth.
The efficacy of the vaccine in preventing severe lower respiratory tract illness within 90 days of delivery was 81.8% (99.5% confidence interval [CI], 40.6–96.3). The efficacy within 180 days of delivery was 69.4% (97.58% CI, 44.3–84.1). These differences reached the study’s pre-established statistical criteria for success. The overall rate of lower respiratory tract infections was not significantly different. The frequencies of adverse effects in mothers and infants were similar in the vaccine and placebo groups. In particular, the frequency of preterm delivery in the vaccine group was 0.8%, compared with 0.6% in the placebo group (P = NS).
In previous reports to the FDA,4 the frequency rate of preterm delivery in RSV vaccine recipients was slightly increased in vaccine recipients compared with patients who received placebo. The difference among the groups was too small to infer a causal relationship; however, as a condition of vaccine approval, the FDA has required Pfizer to conduct a postmarketing study to be certain that administration of the vaccine does not increase the risk for preterm delivery.
Practical details
The new vaccine is a bivalent recombinant vaccine that elicits a robust antibody response against the F (fusion) protein of the virus. In addition to the F antigen, the vaccine contains the following buffer ingredients: tromethamine, sucrose, mannitol, polysorbate, and sodium chloride.8 There are no preservatives in the vaccine.
The vaccine should be administered in a single, 0.5 mL, intramuscular injection at 32 to 36 weeks of gestation. Patients who are allergic to any of the components of the vaccine should not be vaccinated. Patients with a mild upper respiratory tract infection may receive the vaccine. Administration should be delayed in patients who are moderately to severely ill. The vaccine may be administered at the same time as other vaccines, such as influenza or Tdap.
The most common side effects of the vaccine are local injection site reactions, such as pain, redness, or swelling. Some patients may experience mild systemic manifestations, including fatigue, fever, headache, nausea, diarrhea, arthralgias, and myalgias. According to the Centers for Disease Control and Prevention, the approximate wholesale acquisition cost of the vaccine is $320 for 1 injection.
CASE Resolution
This patient is healthy and has no contraindication to the new RSV vaccine. According to the FDA, the optimal time for administration of the vaccine is 32 to 36 weeks of gestation. The patient should anticipate very few side effects following the vaccination, and the vaccine has approximately 80% efficacy in preventing severe lower respiratory tract infection in her neonate. ●
- RSV is the most common cause of acute lower respiratory tract infection in infants younger than 6 months of age.
- In low- and middle-resource countries, RSV is a leading cause of infant death.
- In late August 2023, the FDA approved the first RSV vaccine that can be administered to pregnant women to provide protection for the infant in the first few months of life.
- The vaccine specifically targets the F protein of the virus, a protein which is essential for facilitating fusion between the viral and host cell membranes, resulting in penetration of the virus into the host cell.
- The vaccine should be administered as a single intramuscular injection at 32 to 36 weeks’ gestation.
- The vaccine is approximately 82% effective in preventing severe lower respiratory tract infection in infants within the first 6 months of life.
- To exercise an abundance of caution, because of a possible association between administration of the vaccine and an increased risk for preterm delivery, vaccination should be delayed until 36 weeks in patients clearly identified as at-risk for preterm delivery.
A 28-year-old primigravid woman at 30 weeks’ gestation inquires about the new vaccine to protect her newborn baby against respiratory syncytial virus infection (RSV). Her neighbor’s daughter recently was hospitalized for the treatment of RSV, and she is understandably concerned about her own newborn. The patient is healthy, and she has never had any serious respiratory infection. She is taking no medications other than prenatal vitamins.
What advice should you give her?
If you decide to administer this vaccine, what is the appropriate timing of administration?
Are there any maternal or fetal safety concerns related to use of this vaccine in pregnancy?
Respiratory syncytial virus (RSV) is a member of the Paramyxoviridae family. It is an enveloped, single-stranded RNA virus that is 150-300 nm in size. The virus codes for 10 virus-specific proteins. The 2 most important are the G protein, which enables the virus to attach to host cells, and the F protein, which facilitates the entry of the virus into the host cell by fusing the host and viral membranes. Two distinct subtypes exist: A and B. There is genetic variation within each subtype and between subtypes. These subtle genetic variations create the potential for reinfections, and hence, research has focused on development of a vaccine that covers both subtypes.1
RSV is the most common cause of acute lower respiratory tract infection in infants younger than 6 months of age. In these children, RSV is one of the most prominent causes of death, with mortality particularly marked in low- and middle-resource countries as well as in children who were born premature and/or who are immunocompromised. RSV has its greatest impact during winter epidemics in temperate climates and during the rainy seasons in tropical climates. The virus rarely is encountered in the summer.1 Among young children, RSV primarily is transmitted via close contact with contaminated fingers or fomites and by self-inoculation of the conjunctiva or anterior nares. The incubation period of the infection is 4 to 6 days, and viral shedding may persist for 2 weeks or longer. Most patients gradually recover within 1 to 2 weeks.1 Adults who contract RSV usually have symptoms suggestive of a common cold; however, in older adults or those who have comorbidities, serious and potentially life-threatening lower respiratory tract infections may develop.
Recently, there have been 2 main approaches to the prevention and treatment of RSV in infants. One has been the development of monoclonal antibodies such as motavizumab, palivizumab, and nirsevimab. The other has been the development of a vaccine that could be administered to pregnant women and which could provide protection for the neonate in the early months of life.2,3
In late August 2023, the US Food and Drug Administration (FDA) announced the approval of a new bivalent RSV prefusion F vaccine (ABRYSVO, Pfizer) intended for administration to pregnant women.4 Of note, previous efforts to develop whole-virus vaccines either have been ineffective or have potentiated the disease in infants who became infected; development of an effective vaccine had eluded scientists and clinicians for nearly 50 years.2 Thus, the new vaccine that targets the F protein of the virus represents a major and welcomed breakthrough.
This article reviews the 3 most recent investigations that preceded the ultimate approval of this vaccine and discusses specific logistical issues related to vaccine administration.
Continue to: First step toward vaccine approval...
First step toward vaccine approval
Madhi and colleagues5 were among the first to conduct a large well-designed study to evaluate the effectiveness of maternal vaccination in preventing neonatal infection in the first few months of life. The authors enrolled more than 4,500 healthy pregnant women at 28 to 36 weeks of gestation and assigned them to receive either a single intramuscular dose of an RSV fusion (F) protein vaccine or placebo in a ratio of 2:1. The primary end point was a “medically significant lower respiratory tract infection” within the first 90 days of life. The percentage of infants who met the primary end point was low in both groups: 1.5% in the vaccine group and 2.4% in the placebo group (efficacy 39.4%). The efficacy of the vaccine in preventing lower respiratory tract infection with severe hypoxemia was 48.3% and 44.4% in preventing hospitalization. Although there were differences between the 2 groups, they did not meet the prespecified success criterion for efficacy. Vaccine recipients had more local injection site reactions (40.7% vs 9.9%); however, there was no difference in the frequency of other adverse effects.
Intermediate step: Continued assessment of vaccine safety and immunogenicity
The next important step in the development of the RSV vaccine was a study by Simoes et al,6 who conducted a phase 2b trial to determine the safety and immunogenicity of the RSVpreF vaccine. The authors randomly assigned pregnant women at 24 to 36 weeks of gestation to receive either 120 or 240 µg of RSVpreF vaccine or placebo. The key endpoints were the following: maternal and infant safety; the maternal-to-infant transplacental transfer ratio; and the presence of RSV A, B, and combined A/B neutralizing antibody in maternal serum and umbilical cord blood at delivery. The authors conducted a planned interim analysis that included 327 mothers who received the vaccine. The incidence of adverse effects was similar in mothers and infants in the vaccine compared with the placebo group. None of the adverse effects were judged to be serious. The transplacental neutralizing antibody transfer ratios ranged from 1.4 to 2.1 across a range of gestational ages. The vaccine elicited meaningful neutralizing titers of antibody in maternal serum even up to 7 weeks after immunization. The levels of neutralizing antibodies in umbilical cord blood did not vary substantially with respect to gestational age. A post hoc analysis showed that the transferred antibodies prevented medically-attended RSV-associated lower respiratory tract illnesses in the infants.
Final step: Convincing proof of efficacy
The most recent of the 3 studies, and the one that had the greatest impact in convincing the FDA to approve the vaccine, was the report by Kampmann and colleagues.7 The authors conducted a phase 3 prospective, randomized, double-blind trial in 18 different countries over 4 RSV seasons: 2 in the northern hemisphere and 2 in the southern hemisphere. They enrolled healthy pregnant women with singleton gestations at 24 to 36 weeks of gestation and assigned them in a 1:1 ratio to a single intramuscular injection of 120 µg of a bivalent RSV prefusion F protein-based (RSVpreF) vaccine or placebo. They excluded patients with any recognized risk factor for an adverse pregnancy outcome, including preterm labor. The 2 primary efficacy endpoints were a medically-attended severe RSV–lower respiratory tract infection and any medically attended RSV-associated lower respiratory tract illness in infants within 90, 120, 150, and 180 days after birth.
The efficacy of the vaccine in preventing severe lower respiratory tract illness within 90 days of delivery was 81.8% (99.5% confidence interval [CI], 40.6–96.3). The efficacy within 180 days of delivery was 69.4% (97.58% CI, 44.3–84.1). These differences reached the study’s pre-established statistical criteria for success. The overall rate of lower respiratory tract infections was not significantly different. The frequencies of adverse effects in mothers and infants were similar in the vaccine and placebo groups. In particular, the frequency of preterm delivery in the vaccine group was 0.8%, compared with 0.6% in the placebo group (P = NS).
In previous reports to the FDA,4 the frequency rate of preterm delivery in RSV vaccine recipients was slightly increased in vaccine recipients compared with patients who received placebo. The difference among the groups was too small to infer a causal relationship; however, as a condition of vaccine approval, the FDA has required Pfizer to conduct a postmarketing study to be certain that administration of the vaccine does not increase the risk for preterm delivery.
Practical details
The new vaccine is a bivalent recombinant vaccine that elicits a robust antibody response against the F (fusion) protein of the virus. In addition to the F antigen, the vaccine contains the following buffer ingredients: tromethamine, sucrose, mannitol, polysorbate, and sodium chloride.8 There are no preservatives in the vaccine.
The vaccine should be administered in a single, 0.5 mL, intramuscular injection at 32 to 36 weeks of gestation. Patients who are allergic to any of the components of the vaccine should not be vaccinated. Patients with a mild upper respiratory tract infection may receive the vaccine. Administration should be delayed in patients who are moderately to severely ill. The vaccine may be administered at the same time as other vaccines, such as influenza or Tdap.
The most common side effects of the vaccine are local injection site reactions, such as pain, redness, or swelling. Some patients may experience mild systemic manifestations, including fatigue, fever, headache, nausea, diarrhea, arthralgias, and myalgias. According to the Centers for Disease Control and Prevention, the approximate wholesale acquisition cost of the vaccine is $320 for 1 injection.
CASE Resolution
This patient is healthy and has no contraindication to the new RSV vaccine. According to the FDA, the optimal time for administration of the vaccine is 32 to 36 weeks of gestation. The patient should anticipate very few side effects following the vaccination, and the vaccine has approximately 80% efficacy in preventing severe lower respiratory tract infection in her neonate. ●
- RSV is the most common cause of acute lower respiratory tract infection in infants younger than 6 months of age.
- In low- and middle-resource countries, RSV is a leading cause of infant death.
- In late August 2023, the FDA approved the first RSV vaccine that can be administered to pregnant women to provide protection for the infant in the first few months of life.
- The vaccine specifically targets the F protein of the virus, a protein which is essential for facilitating fusion between the viral and host cell membranes, resulting in penetration of the virus into the host cell.
- The vaccine should be administered as a single intramuscular injection at 32 to 36 weeks’ gestation.
- The vaccine is approximately 82% effective in preventing severe lower respiratory tract infection in infants within the first 6 months of life.
- To exercise an abundance of caution, because of a possible association between administration of the vaccine and an increased risk for preterm delivery, vaccination should be delayed until 36 weeks in patients clearly identified as at-risk for preterm delivery.
- Dolin R. Common viral respiratory infections. In, Isselbacher KJ, Braunwald E, Wilson JD, et al, eds. Harrison’s Principles of Internal Medicine. 13th ed. McGraw-Hill; 1994:805-806.
- Mazur N, Terstappen J, Baral R, et al. Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. Lancet Infect Dis. 2023;23:E2-E21.
- Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386:837-846.
- US Food and Drug Administration News Release. August 21, 2023. Accessed October 26, 2023. https://www.fda.gov/news -events/press-announcements/fda-approves-first-vaccine -pregnant-individuals-prevent-rsv-infants
- Madhi SA, Polack FP, Piedra PA, et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med. 2020;383:426-439.
- Simoes EAF, Center KJ, Tita ATN, et al. Prefusion F proteinbased respiratory syncytial virus immunization in pregnancy. N Eng J Med. 2022;386:1615-1626.
- Kampmann B, Madhi SA, Munjal I, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388:1451-1464.
- Centers for Disease Control and Prevention. Vaccine Information Statement. Respiratory Syncytial Virus (RSV) Vaccine VIS. October 19, 2023. Accessed October 26, 2023. https://www. cdc.gov/vaccines/hcp/vis/vis-statements/rsv.html
- Dolin R. Common viral respiratory infections. In, Isselbacher KJ, Braunwald E, Wilson JD, et al, eds. Harrison’s Principles of Internal Medicine. 13th ed. McGraw-Hill; 1994:805-806.
- Mazur N, Terstappen J, Baral R, et al. Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. Lancet Infect Dis. 2023;23:E2-E21.
- Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386:837-846.
- US Food and Drug Administration News Release. August 21, 2023. Accessed October 26, 2023. https://www.fda.gov/news -events/press-announcements/fda-approves-first-vaccine -pregnant-individuals-prevent-rsv-infants
- Madhi SA, Polack FP, Piedra PA, et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med. 2020;383:426-439.
- Simoes EAF, Center KJ, Tita ATN, et al. Prefusion F proteinbased respiratory syncytial virus immunization in pregnancy. N Eng J Med. 2022;386:1615-1626.
- Kampmann B, Madhi SA, Munjal I, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388:1451-1464.
- Centers for Disease Control and Prevention. Vaccine Information Statement. Respiratory Syncytial Virus (RSV) Vaccine VIS. October 19, 2023. Accessed October 26, 2023. https://www. cdc.gov/vaccines/hcp/vis/vis-statements/rsv.html
The multiple meanings of sex
Knowing the sex of a developing fetus is a common question many expectant parents ask at their prenatal appointments. While the sex of a fetus has minimal clinical significance to obstetrician/gynecologists, technology has made ascertaining the answer to this question much more accessible.
In addition to detecting certain genetic abnormalities, both noninvasive prenatal testing (NIPT) and preimplantation genetic testing (PGT) can discern the chromosomal sex of a fetus prior to birth. At the 20-week anatomy scan, the ultrasonographer can detect the presence of external genitalia to determine the sex. In fact, when a baby is first born, obstetrician/gynecologists are consistently asked “do I have a boy or a girl?” Assigning the sex of a newborn is one of the many tasks we complete in the delivery room. However, some of you reading this article would disagree.
“You cannot assign sex at birth.” “Sex is fixed, you cannot change biology.” These are examples of statements that frequent the comments section of my medical articles and plague professionals who treat gender diverse patients. I would argue, as would many biologists, scientists, and physicians, that these statements oversimplify biologic reality.
The term “sex” has multiple meanings: It can allude to the act of reproduction itself, but in the context of sexual determination and sexual differentiation, it can refer to the biologic and structural composition of a developing human. Within this paradigm, there exist three definitions for sex: chromosomal, gonadal, and phenotypic.
Chromosomal sex refers to the genetic makeup of a human, typically XX or XY chromosomes. There are also variations within this seemingly binary system. Embryos can have an extra sex chromosome, as seen in Klinefelter syndrome, which is characterized by XXY karyotype. Embryos can also be devoid of a sex chromosome, as observed in Turner’s syndrome, which is characterized by an XO karyotype. These variations can impact fertility and expression of secondary sexual characteristics as the type of sex chromosomes present results in primary sex determination, or the development of gonads.
Most often, individuals with a chromosomal makeup of XX are considered female and will subsequently develop ovaries that produce oocytes (eggs). Individuals with XY chromosomes are deemed male and will go on to develop testes, which are responsible for spermatogenesis (sperm production).
Gonadal sex is the presence of either testes or ovaries. The primary function of testes is to produce sperm for reproduction and to secrete testosterone, the primary male sex hormone. Similarly, ovaries produce oocytes and secrete estrogen as the primary female sex hormone. Gonads can be surgically removed either via orchiectomy (the removal of testes), or oophorectomy (the removal of ovaries) for a variety of reasons. There is no current medical technology that can replace the function of these structures, although patients can be placed on hormone replacement to counter the negative physiologic consequences resulting from their removal.
Secondary sex determination, or sexual differentiation, is the development of external genitalia and internal genital tracts because of the hormones produced from the gonads. At puberty, further differentiation occurs with the development of pubic and axillary hair and breast growth. This process determines phenotypic sex – the visible distinction between male and female.
When opponents of gender affirming care state that individuals cannot change sex, are they correct or false? The answer to this question is entirely dependent on which definition of sex they are using. Chromosomal? Gonadal? Phenotypic? It is an immutable fact that humans cannot change chromosomal sex. No one in the transgender community, either provider or patient, would dispute this. However, we can remove gonadal structures and alter phenotypic sex.
In fact, many cisgender individuals also revise their phenotypic sex when they undergo augmentation mammaplasty, penile enlargement, or vulvoplasty procedures for the exact same reason.
Circling back to the debate about whether we can “assign sex at birth,” it all depends on what definition of sex you are referencing. At birth, obstetrician/gynecologists most often look at the phenotypic sex and make assumptions about the genetic and gonadal sex based on the secondary sexual characteristics. So yes, we can, and we do assign sex at birth. However, in the case of intersex individuals, these physical characteristics may not align with their gonadal and chromosomal composition.
In the case of an infant that has a known XY karyotype prior to birth but a female phenotype at birth (as seen in a condition called complete androgen insensitivity syndrome), what sex should be assigned to that baby? Should the infant be raised male or female? A lot of unintended but significant harm has resulted from providers and parents trying to answer that very question. The mistreatment of intersex patients through forced and coercive medical and surgical treatments, often in infancy, should serve as a dark reminder that sex and gender are not as biologically binary as we would like to believe.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa. She has no relevant disclosures.
References
Moore KL and Persaud TVN. The urogenital system. In: Before we are born: essentials of embryology and birth defects. 7th ed. Philadelphia: Saunders Elsevier;2008:163-89.
Standring S. Development of the urogenital system. In: Gray’s Anatomy, 42nd ed. Philadelphia: Elsevier;2021:341-64.
Escobar O et al. Pediatric endocrinology. In: Zitelli BJ, ed. Zitelli and Davis’ atlas of pediatric physical diagnosis 8th edition. Philadelphia: Elsevier;2023:342-81.
Knowing the sex of a developing fetus is a common question many expectant parents ask at their prenatal appointments. While the sex of a fetus has minimal clinical significance to obstetrician/gynecologists, technology has made ascertaining the answer to this question much more accessible.
In addition to detecting certain genetic abnormalities, both noninvasive prenatal testing (NIPT) and preimplantation genetic testing (PGT) can discern the chromosomal sex of a fetus prior to birth. At the 20-week anatomy scan, the ultrasonographer can detect the presence of external genitalia to determine the sex. In fact, when a baby is first born, obstetrician/gynecologists are consistently asked “do I have a boy or a girl?” Assigning the sex of a newborn is one of the many tasks we complete in the delivery room. However, some of you reading this article would disagree.
“You cannot assign sex at birth.” “Sex is fixed, you cannot change biology.” These are examples of statements that frequent the comments section of my medical articles and plague professionals who treat gender diverse patients. I would argue, as would many biologists, scientists, and physicians, that these statements oversimplify biologic reality.
The term “sex” has multiple meanings: It can allude to the act of reproduction itself, but in the context of sexual determination and sexual differentiation, it can refer to the biologic and structural composition of a developing human. Within this paradigm, there exist three definitions for sex: chromosomal, gonadal, and phenotypic.
Chromosomal sex refers to the genetic makeup of a human, typically XX or XY chromosomes. There are also variations within this seemingly binary system. Embryos can have an extra sex chromosome, as seen in Klinefelter syndrome, which is characterized by XXY karyotype. Embryos can also be devoid of a sex chromosome, as observed in Turner’s syndrome, which is characterized by an XO karyotype. These variations can impact fertility and expression of secondary sexual characteristics as the type of sex chromosomes present results in primary sex determination, or the development of gonads.
Most often, individuals with a chromosomal makeup of XX are considered female and will subsequently develop ovaries that produce oocytes (eggs). Individuals with XY chromosomes are deemed male and will go on to develop testes, which are responsible for spermatogenesis (sperm production).
Gonadal sex is the presence of either testes or ovaries. The primary function of testes is to produce sperm for reproduction and to secrete testosterone, the primary male sex hormone. Similarly, ovaries produce oocytes and secrete estrogen as the primary female sex hormone. Gonads can be surgically removed either via orchiectomy (the removal of testes), or oophorectomy (the removal of ovaries) for a variety of reasons. There is no current medical technology that can replace the function of these structures, although patients can be placed on hormone replacement to counter the negative physiologic consequences resulting from their removal.
Secondary sex determination, or sexual differentiation, is the development of external genitalia and internal genital tracts because of the hormones produced from the gonads. At puberty, further differentiation occurs with the development of pubic and axillary hair and breast growth. This process determines phenotypic sex – the visible distinction between male and female.
When opponents of gender affirming care state that individuals cannot change sex, are they correct or false? The answer to this question is entirely dependent on which definition of sex they are using. Chromosomal? Gonadal? Phenotypic? It is an immutable fact that humans cannot change chromosomal sex. No one in the transgender community, either provider or patient, would dispute this. However, we can remove gonadal structures and alter phenotypic sex.
In fact, many cisgender individuals also revise their phenotypic sex when they undergo augmentation mammaplasty, penile enlargement, or vulvoplasty procedures for the exact same reason.
Circling back to the debate about whether we can “assign sex at birth,” it all depends on what definition of sex you are referencing. At birth, obstetrician/gynecologists most often look at the phenotypic sex and make assumptions about the genetic and gonadal sex based on the secondary sexual characteristics. So yes, we can, and we do assign sex at birth. However, in the case of intersex individuals, these physical characteristics may not align with their gonadal and chromosomal composition.
In the case of an infant that has a known XY karyotype prior to birth but a female phenotype at birth (as seen in a condition called complete androgen insensitivity syndrome), what sex should be assigned to that baby? Should the infant be raised male or female? A lot of unintended but significant harm has resulted from providers and parents trying to answer that very question. The mistreatment of intersex patients through forced and coercive medical and surgical treatments, often in infancy, should serve as a dark reminder that sex and gender are not as biologically binary as we would like to believe.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa. She has no relevant disclosures.
References
Moore KL and Persaud TVN. The urogenital system. In: Before we are born: essentials of embryology and birth defects. 7th ed. Philadelphia: Saunders Elsevier;2008:163-89.
Standring S. Development of the urogenital system. In: Gray’s Anatomy, 42nd ed. Philadelphia: Elsevier;2021:341-64.
Escobar O et al. Pediatric endocrinology. In: Zitelli BJ, ed. Zitelli and Davis’ atlas of pediatric physical diagnosis 8th edition. Philadelphia: Elsevier;2023:342-81.
Knowing the sex of a developing fetus is a common question many expectant parents ask at their prenatal appointments. While the sex of a fetus has minimal clinical significance to obstetrician/gynecologists, technology has made ascertaining the answer to this question much more accessible.
In addition to detecting certain genetic abnormalities, both noninvasive prenatal testing (NIPT) and preimplantation genetic testing (PGT) can discern the chromosomal sex of a fetus prior to birth. At the 20-week anatomy scan, the ultrasonographer can detect the presence of external genitalia to determine the sex. In fact, when a baby is first born, obstetrician/gynecologists are consistently asked “do I have a boy or a girl?” Assigning the sex of a newborn is one of the many tasks we complete in the delivery room. However, some of you reading this article would disagree.
“You cannot assign sex at birth.” “Sex is fixed, you cannot change biology.” These are examples of statements that frequent the comments section of my medical articles and plague professionals who treat gender diverse patients. I would argue, as would many biologists, scientists, and physicians, that these statements oversimplify biologic reality.
The term “sex” has multiple meanings: It can allude to the act of reproduction itself, but in the context of sexual determination and sexual differentiation, it can refer to the biologic and structural composition of a developing human. Within this paradigm, there exist three definitions for sex: chromosomal, gonadal, and phenotypic.
Chromosomal sex refers to the genetic makeup of a human, typically XX or XY chromosomes. There are also variations within this seemingly binary system. Embryos can have an extra sex chromosome, as seen in Klinefelter syndrome, which is characterized by XXY karyotype. Embryos can also be devoid of a sex chromosome, as observed in Turner’s syndrome, which is characterized by an XO karyotype. These variations can impact fertility and expression of secondary sexual characteristics as the type of sex chromosomes present results in primary sex determination, or the development of gonads.
Most often, individuals with a chromosomal makeup of XX are considered female and will subsequently develop ovaries that produce oocytes (eggs). Individuals with XY chromosomes are deemed male and will go on to develop testes, which are responsible for spermatogenesis (sperm production).
Gonadal sex is the presence of either testes or ovaries. The primary function of testes is to produce sperm for reproduction and to secrete testosterone, the primary male sex hormone. Similarly, ovaries produce oocytes and secrete estrogen as the primary female sex hormone. Gonads can be surgically removed either via orchiectomy (the removal of testes), or oophorectomy (the removal of ovaries) for a variety of reasons. There is no current medical technology that can replace the function of these structures, although patients can be placed on hormone replacement to counter the negative physiologic consequences resulting from their removal.
Secondary sex determination, or sexual differentiation, is the development of external genitalia and internal genital tracts because of the hormones produced from the gonads. At puberty, further differentiation occurs with the development of pubic and axillary hair and breast growth. This process determines phenotypic sex – the visible distinction between male and female.
When opponents of gender affirming care state that individuals cannot change sex, are they correct or false? The answer to this question is entirely dependent on which definition of sex they are using. Chromosomal? Gonadal? Phenotypic? It is an immutable fact that humans cannot change chromosomal sex. No one in the transgender community, either provider or patient, would dispute this. However, we can remove gonadal structures and alter phenotypic sex.
In fact, many cisgender individuals also revise their phenotypic sex when they undergo augmentation mammaplasty, penile enlargement, or vulvoplasty procedures for the exact same reason.
Circling back to the debate about whether we can “assign sex at birth,” it all depends on what definition of sex you are referencing. At birth, obstetrician/gynecologists most often look at the phenotypic sex and make assumptions about the genetic and gonadal sex based on the secondary sexual characteristics. So yes, we can, and we do assign sex at birth. However, in the case of intersex individuals, these physical characteristics may not align with their gonadal and chromosomal composition.
In the case of an infant that has a known XY karyotype prior to birth but a female phenotype at birth (as seen in a condition called complete androgen insensitivity syndrome), what sex should be assigned to that baby? Should the infant be raised male or female? A lot of unintended but significant harm has resulted from providers and parents trying to answer that very question. The mistreatment of intersex patients through forced and coercive medical and surgical treatments, often in infancy, should serve as a dark reminder that sex and gender are not as biologically binary as we would like to believe.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa. She has no relevant disclosures.
References
Moore KL and Persaud TVN. The urogenital system. In: Before we are born: essentials of embryology and birth defects. 7th ed. Philadelphia: Saunders Elsevier;2008:163-89.
Standring S. Development of the urogenital system. In: Gray’s Anatomy, 42nd ed. Philadelphia: Elsevier;2021:341-64.
Escobar O et al. Pediatric endocrinology. In: Zitelli BJ, ed. Zitelli and Davis’ atlas of pediatric physical diagnosis 8th edition. Philadelphia: Elsevier;2023:342-81.
Hypertensive disorders of pregnancy and high stroke risk in Black women
I’d like to talk with you about a recent report from the large-scale Black Women’s Health Study, published in the new journal NEJM Evidence.
This study looked at the association between hypertensive disorders of pregnancy, including preeclampsia and gestational hypertension, and the risk for stroke over the next 20 (median, 22) years. Previous studies have linked hypertensive disorders of pregnancy with an increased risk for stroke. However, most of these studies have been done in White women of European ancestry, and evidence in Black women has been very limited, despite a disproportionately high risk of having a hypertensive disorder of pregnancy and also of stroke.
– overall, a 66% increased risk, an 80% increased risk with gestational hypertension, and about a 50% increased risk with preeclampsia.
We know that pregnancy itself can lead to some remodeling of the vascular system, but we don’t know whether a direct causal relationship exists between preeclampsia or gestational hypertension and subsequent stroke. Another potential explanation is that these complications of pregnancy serve as a window into a woman’s future cardiometabolic health and a marker of her cardiovascular risk.
Regardless, the clinical implications are the same. First, we would want to prevent these complications of pregnancy whenever possible. Some women will be candidates for the use of aspirin if they are at high risk for preeclampsia, and certainly for monitoring blood pressure very closely during pregnancy. It will also be important to maintain blood pressure control in the postpartum period and during the subsequent years of adulthood to minimize risk for stroke, because hypertension is such a powerful risk factor for stroke.
It will also be tremendously important to intensify lifestyle modifications such as increasing physical activity and having a heart-healthy diet. These complications of pregnancy have also been linked in other studies to an increased risk for subsequent coronary heart disease events and heart failure.
This transcript has been edited for clarity.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, and chief of the division of preventive medicine, Brigham and Women’s Hospital, both in Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
I’d like to talk with you about a recent report from the large-scale Black Women’s Health Study, published in the new journal NEJM Evidence.
This study looked at the association between hypertensive disorders of pregnancy, including preeclampsia and gestational hypertension, and the risk for stroke over the next 20 (median, 22) years. Previous studies have linked hypertensive disorders of pregnancy with an increased risk for stroke. However, most of these studies have been done in White women of European ancestry, and evidence in Black women has been very limited, despite a disproportionately high risk of having a hypertensive disorder of pregnancy and also of stroke.
– overall, a 66% increased risk, an 80% increased risk with gestational hypertension, and about a 50% increased risk with preeclampsia.
We know that pregnancy itself can lead to some remodeling of the vascular system, but we don’t know whether a direct causal relationship exists between preeclampsia or gestational hypertension and subsequent stroke. Another potential explanation is that these complications of pregnancy serve as a window into a woman’s future cardiometabolic health and a marker of her cardiovascular risk.
Regardless, the clinical implications are the same. First, we would want to prevent these complications of pregnancy whenever possible. Some women will be candidates for the use of aspirin if they are at high risk for preeclampsia, and certainly for monitoring blood pressure very closely during pregnancy. It will also be important to maintain blood pressure control in the postpartum period and during the subsequent years of adulthood to minimize risk for stroke, because hypertension is such a powerful risk factor for stroke.
It will also be tremendously important to intensify lifestyle modifications such as increasing physical activity and having a heart-healthy diet. These complications of pregnancy have also been linked in other studies to an increased risk for subsequent coronary heart disease events and heart failure.
This transcript has been edited for clarity.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, and chief of the division of preventive medicine, Brigham and Women’s Hospital, both in Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
I’d like to talk with you about a recent report from the large-scale Black Women’s Health Study, published in the new journal NEJM Evidence.
This study looked at the association between hypertensive disorders of pregnancy, including preeclampsia and gestational hypertension, and the risk for stroke over the next 20 (median, 22) years. Previous studies have linked hypertensive disorders of pregnancy with an increased risk for stroke. However, most of these studies have been done in White women of European ancestry, and evidence in Black women has been very limited, despite a disproportionately high risk of having a hypertensive disorder of pregnancy and also of stroke.
– overall, a 66% increased risk, an 80% increased risk with gestational hypertension, and about a 50% increased risk with preeclampsia.
We know that pregnancy itself can lead to some remodeling of the vascular system, but we don’t know whether a direct causal relationship exists between preeclampsia or gestational hypertension and subsequent stroke. Another potential explanation is that these complications of pregnancy serve as a window into a woman’s future cardiometabolic health and a marker of her cardiovascular risk.
Regardless, the clinical implications are the same. First, we would want to prevent these complications of pregnancy whenever possible. Some women will be candidates for the use of aspirin if they are at high risk for preeclampsia, and certainly for monitoring blood pressure very closely during pregnancy. It will also be important to maintain blood pressure control in the postpartum period and during the subsequent years of adulthood to minimize risk for stroke, because hypertension is such a powerful risk factor for stroke.
It will also be tremendously important to intensify lifestyle modifications such as increasing physical activity and having a heart-healthy diet. These complications of pregnancy have also been linked in other studies to an increased risk for subsequent coronary heart disease events and heart failure.
This transcript has been edited for clarity.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, and chief of the division of preventive medicine, Brigham and Women’s Hospital, both in Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.





