User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Breakthroughs in the prevention of RSV disease among infants
Respiratory syncytial virus (RSV) is a negative-sense, single-stranded, ribonucleic acid (RNA) virus that is a member of Pneumoviridae family. Two subtypes, A and B, and multiple genotypes circulate during fall and winter seasonal outbreaks of RSV.1 RSV can cause severe lower respiratory tract disease including bronchiolitis, pneumonia, respiratory failure, and death. Each year, RSV disease causes the hospitalization of 1.5% to 2% of children younger than 6 months of age, resulting in 100 to 300 deaths.2 For infants younger than 1 year, RSV infection is the leading cause of hospitalization.3 In 2023, two new treatments have become available to prevent RSV disease: nirsevimab and RSVPreF vaccine.
Nirsevimab
Nirsevimab is an antibody to an RSV antigen. It has a long half-life and is approved for administration to infants, providing passive immunization. In contrast, administration of the RSVPreF vaccine to pregnant persons elicits active maternal immunity, resulting in the production of anti-RSV antibodies that are transferred to the fetus, resulting in passive immunity in the infant. Seasonal administration of nirsevimab and the RSV vaccine maximizes benefit to the infant and conserves limited health care resources. In temperate regions in the United States, the RSV infection season typically begins in October and peaks in December through mid-February and ends in April or May.4,5 In southern Florida, the RSV season often begins in August to September, peaks in November through December, and ends in March.4,5
This editorial reviews 3 strategies for prevention of RSV infection in infants, including:
- universal treatment of newborns with nirsevimab
- immunization of pregnant persons with an RSVpreF vaccine in the third trimester appropriately timed to occur just before the beginning or during RSV infection season
- prioritizing universal maternal RSV vaccination with reflex administration of nirsevimab to newborns when the pregnant person was not vaccinated.6
Of note, there are no studies that have evaluated the effectiveness of combining RSVpreF vaccine and nirsevimab. The Centers for Disease Control and Prevention (CDC) does not recommend combining both RSV vaccination of pregnant persons plus nirsevimab treatment of the infant, except in limited circumstances, such as for immunocompromised pregnant people with limited antibody production or newborns who have a massive transfusion, which dilutes antibody titres.6
RSV prevention strategy 1
Universal treatment of newborns and infants with nirsevimab
Nirsevimab (Beyfortus, Sanofi and AstraZeneca) is an IgG 1-kappa monoclonal antibody with a long half-life that targets the prefusion conformation of the RSV F-protein, resulting in passive immunity to infection.7 Passive immunization results in rapid protection against infection because it does not require activation of the immune system. Nirsevimab is long acting due to amino acid substitutions in the Fc region, increasing binding to the neonatal Fc receptor, which protects IgG antibodies from degradation, thereby extending the antibody half-life. The terminal halflife of nirsevimab is 71 days, and the duration of protection following a single dose is at least 5 months.
Nirsevimab is approved by the US Food and Drug Administration (FDA) for all neonates and infants born or entering their first RSV infection season and for children up to 24 months of age who are vulnerable to severe RSV during their second RSV infection season. For infants born outside the RSV infection season, nirsevimab should be administered once prior to the start of the next RSV infection season.7 Nirsevimab is administered as a single intramuscular injection at a dose of 50 mg for neonates and infants < 5 kg in weight and a dose of 100 mg for neonates and infants ≥ 5 kg in weight.7 The list average wholesale price for both doses is $594.8 Nirsevimab is contraindicated for patients with a serious hypersensitivity reaction to nirsevimab or its excipients.7 In clinical trials, adverse reactions including rash and injection site reaction were reported in 1.2% of participants.7 Some RSV variants may be resistant to neutralization with nirsevimab.7,9
In a randomized clinical trial, 1,490 infants born ≥ 35 weeks’ gestation, the rates of medically-attended RSV lower respiratory tract disease (MA RSV LRTD) through 150 days of follow-up in the placebo and nirsevimab groups were 5.0% and 1.2%, respectively (P < .001).7,10 Compared with placebo, nirsevimab reduced hospitalizations due to RSV LRTD by 60% through 150 days of follow up. In a randomized clinical trial enrolling 1,453 infants born between 29 weeks’ and < 35 weeks’ gestation, the rates of MA RSV LRTD through 150 days of follow up in the placebo and nirsevimab groups were 9.5% and 2.6%, respectively (P < .001). In this study of infants born preterm, compared with placebo, nirsevimab reduced hospitalization due to RSV LRTD by 70% through 150 days of follow up.7 Nirsevimab is thought to be cost-effective at the current price per dose, but more data are needed to precisely define the magnitude of the health care savings associated with universal nirsevimab administration.11-13 The CDC reports that the incremental cost-effectiveness ratio (ICER) per quality-adjusted life year (QALY) of nirsevimab administration to infants is approximately $250,000, given an estimated cost of $500 for one dose of vaccine.14
Universal passive vaccination of newborns is recommended by many state departments of public health, which can provide the vaccine without cost to clinicians and health care facilities participating in the children’s vaccination program.
Continue to: RSV prevention strategy 2...
RSV prevention strategy 2
Universal RSV vaccination of pregnant persons from September through January
The RSVpreF vaccine (Abryvso, Pfizer) is approved by the FDA for the active immunization of pregnant persons between 32 through 36 weeks’ gestation for the prevention of RSV LRTD in infants from birth through 6 months of age.15 Administration of the RSVpreF vaccine to pregnant people elicits the formation of antiRSV antibodies that are transferred transplacentally to the fetus, resulting in the protection of the infant from RSV during the first 6 months of life. The RSVpreF vaccine also is approved to prevent RSV LRTD in people aged ≥ 60 years.
The RSVpreF vaccine contains the prefusion form of the RSV fusion (F) protein responsible for viral entry into host cells. The vaccine contains 60 µg of both RSV preF A and preF B recombinant proteins. The vaccine is administered as a single intramuscular dose in a volume of 0.5 mL. The vaccine is provided in a vial in a lyophilized form and must be reconstituted prior to administration. The average wholesale price of RSVPreF vaccine is $354.16 The vaccine is contraindicated for people who have had an allergic reaction to any component of the vaccine. The most commonly reported adverse reaction is injection site pain (41%).15 The FDA reports a “numerical imbalance in preterm births in Abrysvo recipients compared to placebo recipients” (5.7% vs 4.7%), and “available data are insufficient to establish or exclude a causal relationship between preterm birth and Abrysvo.”15 In rabbits there is no evidence of developmental toxicity and congenital anomalies associated with the RSVpreF vaccine. In human studies, no differences in the rate of congenital anomalies or fetal deaths were noted between RSVpreF vaccine and placebo.
In a clinical trial, 6,975 pregnant participants 24 through 36 weeks’ gestation were randomly assigned to receive a placebo or the RSVpreF vaccine.15,17 After birth, follow-up of infants at 180 days, showed that the rates of MA RSV LRTD among the infants in the placebo and RSVpreF vaccine groups were 3.4% and 1.6%, respectively. At 180 days, the reported rates of severe RSV LRTD in the placebo and RSVpreF vaccine groups were 1.8% and 0.5%, respectively. In this study, among the subset of pregnant participants who received the RSVpreF vaccine (n = 1,572) or placebo (n = 1,539) at 32 through 36 weeks’ gestation, the rates of MA RSV LRTD among the infants in the placebo and RSVpreF vaccine groups were 3.6% and 1.5%, respectively. In the subset of pregnant participants vaccinated at 32 through 36 weeks’ gestation, at 180 days postvaccination, the reported rates of severe RSV LRTD in the placebo and RSVpreF vaccine groups were 1.6% and 0.4%, respectively.15
The CDC has recommended that the RSVpreF vaccine be administered to pregnant people 32 through 36 weeks’ gestation from September through the end of January in most of the continental United States to reduce the rate of RSV LRTD in infants.6 September was selected because it is 1 to 2 months before the start of the RSV season, and it takes at least 14 days for maternal vaccination to result in transplacental transfer of protective antibodies to the fetus. January was selected because it is 2 to 3 months before the anticipated end of the RSV season.6 The CDC also noted that, for regions with a different pattern of RSV seasonality, clinicians should follow the guidance of local public health officials. This applies to the states of Alaska, southern Florida, Hawaii, and Puerto Rico.6 The CDC recommended that infants born < 34 weeks’ gestation should receive nirsevimab.6
Maternal RSV vaccination is thought to be cost-effective for reducing RSV LRTD in infants. However, the cost-effectiveness analyses are sensitive to the pricing of the two main options: maternal RSV vaccination and nirsevimab.
It is estimated that nirsevimab may provide greater protection than maternal RSV vaccination from RSV LRTD, but the maternal RSVpreF vaccine is priced lower than nirsevimab.18 Focusing administration of RSVpreF vaccine from September through January of the RSV infection season is thought to maximize benefits to infants and reduce total cost of the vaccination program.19 With year-round RSVpreF vaccine dosing, the estimated ICER per quality-adjusted life-year (QALY) is approximately $400,000, whereas seasonal dosing reduces the cost to approximately $170,000.19
RSV prevention strategy 3
Vaccinate pregnant persons; reflex to newborn treatment with nirsevimab if maternal RSV vaccination did not occur
RSVpreF vaccination to all pregnant persons 32 through 36 weeks’ gestation during RSV infection season is not likely to result in 100% adherence. For instance, in a CDC-conducted survey only 47% of pregnant persons received an influenza vaccine.2 Newborns whose mothers did not receive an RSVpreF vaccine will need to be considered for treatment with nirsevimab. Collaboration and communication among obstetricians and pediatricians will be needed to avoid miscommunication and missed opportunities to treat newborns during the birth hospitalization. Enhancements in electronic health records, linking the mother’s vaccination record with the newborn’s medical record plus an added feature of electronic alerts when the mother did not receive an appropriately timed RSVpreF vaccine would improve the communication of important clinical information to the pediatrician.
Next steps for the upcoming peak RSV season
We are currently in the 2023–2024 RSV infection season and can expect a peak in cases of RSV between December 2023 and February 2024. The CDC recommends protecting all infants against RSV-associated LRTD. The options are to administer the maternal RSVpreF vaccine to pregnant persons or treating the infant with nirsevimab. The vaccine is just now becoming available for administration in regional pharmacies, physician practices, and health systems. Obstetrician-gynecologists should follow the recommendation of their state department of public health. As noted above, many state departments of public health are recommending that all newborns receive nirsevimab. For clinicians in those states, RSVPreF vaccination of pregnant persons is not a priority. ●
- Tramuto F, Massimo Maida C, Mazzucco W, et al. Molecular epidemiology and genetic diversity of human respiratory syncytial virus in Sicily during pre- and post-COVID-19 surveillance season. Pathogens. 2023;12:1099.
- Boudreau M, Vadlamudi NK, Bastien N, et al. Pediatric RSV-associated hospitalizations before and during the COVID-19 pandemic. JAMA Netw Open. 2023;6:e2336863.
- Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143(5 Suppl):S127-132.
- Hamid S, Winn A, Parikh R, et al. Seasonality of respiratory syncytial virus-United States 2017-2023. MMWR Morb Mortal Wkly Rep. 2023;72:355-361.
- Rose EB, Wheatley A, Langley G, et al. Respiratory syncytial virus seasonality-United States 2014-2017. MMWR Morb Mortal Wkly Rep. 2018;67:71-76.
- Fleming-Dutra KE, Jones JM, Roper LE, et al. Use of Pfizer respiratory syncytial virus vaccine during pregnancy for the prevention of respiratory syncytial virus associated lower respiratory tract disease in infants: recommendations of the Advisory Committee on Immunization Practices- United States 2023. MMWR Morb Mortal Wkly Rep. October 6, 2023. Accessed October 9, 2023. https://www.cdc.gov/mmwr/volumes/72/wr /mm7241e1.htm#print
- FDA package insert for Beyfortus. Accessed October 9, 2023. https://www.accessdata.fda.gov /drugsatfda_docs/label/2023/761328s000lbl.pdf
- Lexicomp. Nirsevimab: Drug information – UpToDate. Accessed October 9, 2023. https://www. wolterskluwer.com/en/solutions/lexicomp
- Ahani B, Tuffy KM, Aksyuk A, et al. Molecular and phenotypic characterization of RSV infections in infants during two nirsevimab randomized clinical trials. Nat Commun. 2023;14:4347.
- Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in late-preterm and term infants. N Engl J Med. 2022;386:837-846.
- Li X, Bilcke J, Vazquez-Fernandez L, et al. Costeffectiveness of respiratory syncytial virus disease protection strategies: maternal vaccine versus seasonal or year-round monoclonal antibody program in Norwegian children. J Infect Dis. 2022;226(Suppl 1):S95-S101.
- Hodgson D, Koltai M, Krauer F, et al. Optimal respiratory syncytial virus intervention programmes using nirsevimab in England and Wales. Vaccine. 2022;40:7151-7157.
- Yu T, Padula WV, Yieh L, et al. Cost-effectiveness of nirsevimab and palivizumab for respiratory syncytial virus prophylaxis in preterm infants 29-34 6/7 weeks’ gestation in the United States. Pediatr Neonatal. 2023;04:015.
- Jones J. Evidence to recommendations framework: nirsevimab in infants. Accessed October 27, 2023. https://www.cdc.gov/vaccines/acip/meet ings/downloads/slides-2023-02/slides-02-23/rsv -pediatric-04-jones-508.pdf
- Abrysvo [package insert]. Pfizer; New York, New York. August 2023.
- Lexicomp. Recombinant respiratory syncytial virus vaccine (RSVPreF) (Abrysvo): Drug information - UpToDate. Accessed October 9, 2023. https://www.wolterskluwer.com/en/solutions /lexicomp
- Kampmann B, Madhi SA, Munjal I, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388: 1451-1464.
- Baral R, Higgins D, Regan K, et al. Impact and costeffectiveness of potential interventions against infant respiratory syncytial virus (RSV) in 131 lowincome and middle-income countries using a static cohort model. BMJ Open. 2021;11:e046563.
- Fleming-Dutra KE. Evidence to recommendations framework updates: Pfizer maternal RSVpreF vaccine. June 22, 2023. Accessed October 27, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.cdc.gov/vaccines/acip /meetings/downloads/slides-2023-06-21-23/03 -RSV-Mat-Ped-Fleming-Dutra-508.pdf
- Razzaghi H, Kahn KE, Calhoun K, et al. Influenza, Tdap and COVID-19 vaccination coverage and hesitancy among pregnant women-United States, April 2023. MMWR Morb Mortal Wkly Rep.
Respiratory syncytial virus (RSV) is a negative-sense, single-stranded, ribonucleic acid (RNA) virus that is a member of Pneumoviridae family. Two subtypes, A and B, and multiple genotypes circulate during fall and winter seasonal outbreaks of RSV.1 RSV can cause severe lower respiratory tract disease including bronchiolitis, pneumonia, respiratory failure, and death. Each year, RSV disease causes the hospitalization of 1.5% to 2% of children younger than 6 months of age, resulting in 100 to 300 deaths.2 For infants younger than 1 year, RSV infection is the leading cause of hospitalization.3 In 2023, two new treatments have become available to prevent RSV disease: nirsevimab and RSVPreF vaccine.
Nirsevimab
Nirsevimab is an antibody to an RSV antigen. It has a long half-life and is approved for administration to infants, providing passive immunization. In contrast, administration of the RSVPreF vaccine to pregnant persons elicits active maternal immunity, resulting in the production of anti-RSV antibodies that are transferred to the fetus, resulting in passive immunity in the infant. Seasonal administration of nirsevimab and the RSV vaccine maximizes benefit to the infant and conserves limited health care resources. In temperate regions in the United States, the RSV infection season typically begins in October and peaks in December through mid-February and ends in April or May.4,5 In southern Florida, the RSV season often begins in August to September, peaks in November through December, and ends in March.4,5
This editorial reviews 3 strategies for prevention of RSV infection in infants, including:
- universal treatment of newborns with nirsevimab
- immunization of pregnant persons with an RSVpreF vaccine in the third trimester appropriately timed to occur just before the beginning or during RSV infection season
- prioritizing universal maternal RSV vaccination with reflex administration of nirsevimab to newborns when the pregnant person was not vaccinated.6
Of note, there are no studies that have evaluated the effectiveness of combining RSVpreF vaccine and nirsevimab. The Centers for Disease Control and Prevention (CDC) does not recommend combining both RSV vaccination of pregnant persons plus nirsevimab treatment of the infant, except in limited circumstances, such as for immunocompromised pregnant people with limited antibody production or newborns who have a massive transfusion, which dilutes antibody titres.6
RSV prevention strategy 1
Universal treatment of newborns and infants with nirsevimab
Nirsevimab (Beyfortus, Sanofi and AstraZeneca) is an IgG 1-kappa monoclonal antibody with a long half-life that targets the prefusion conformation of the RSV F-protein, resulting in passive immunity to infection.7 Passive immunization results in rapid protection against infection because it does not require activation of the immune system. Nirsevimab is long acting due to amino acid substitutions in the Fc region, increasing binding to the neonatal Fc receptor, which protects IgG antibodies from degradation, thereby extending the antibody half-life. The terminal halflife of nirsevimab is 71 days, and the duration of protection following a single dose is at least 5 months.
Nirsevimab is approved by the US Food and Drug Administration (FDA) for all neonates and infants born or entering their first RSV infection season and for children up to 24 months of age who are vulnerable to severe RSV during their second RSV infection season. For infants born outside the RSV infection season, nirsevimab should be administered once prior to the start of the next RSV infection season.7 Nirsevimab is administered as a single intramuscular injection at a dose of 50 mg for neonates and infants < 5 kg in weight and a dose of 100 mg for neonates and infants ≥ 5 kg in weight.7 The list average wholesale price for both doses is $594.8 Nirsevimab is contraindicated for patients with a serious hypersensitivity reaction to nirsevimab or its excipients.7 In clinical trials, adverse reactions including rash and injection site reaction were reported in 1.2% of participants.7 Some RSV variants may be resistant to neutralization with nirsevimab.7,9
In a randomized clinical trial, 1,490 infants born ≥ 35 weeks’ gestation, the rates of medically-attended RSV lower respiratory tract disease (MA RSV LRTD) through 150 days of follow-up in the placebo and nirsevimab groups were 5.0% and 1.2%, respectively (P < .001).7,10 Compared with placebo, nirsevimab reduced hospitalizations due to RSV LRTD by 60% through 150 days of follow up. In a randomized clinical trial enrolling 1,453 infants born between 29 weeks’ and < 35 weeks’ gestation, the rates of MA RSV LRTD through 150 days of follow up in the placebo and nirsevimab groups were 9.5% and 2.6%, respectively (P < .001). In this study of infants born preterm, compared with placebo, nirsevimab reduced hospitalization due to RSV LRTD by 70% through 150 days of follow up.7 Nirsevimab is thought to be cost-effective at the current price per dose, but more data are needed to precisely define the magnitude of the health care savings associated with universal nirsevimab administration.11-13 The CDC reports that the incremental cost-effectiveness ratio (ICER) per quality-adjusted life year (QALY) of nirsevimab administration to infants is approximately $250,000, given an estimated cost of $500 for one dose of vaccine.14
Universal passive vaccination of newborns is recommended by many state departments of public health, which can provide the vaccine without cost to clinicians and health care facilities participating in the children’s vaccination program.
Continue to: RSV prevention strategy 2...
RSV prevention strategy 2
Universal RSV vaccination of pregnant persons from September through January
The RSVpreF vaccine (Abryvso, Pfizer) is approved by the FDA for the active immunization of pregnant persons between 32 through 36 weeks’ gestation for the prevention of RSV LRTD in infants from birth through 6 months of age.15 Administration of the RSVpreF vaccine to pregnant people elicits the formation of antiRSV antibodies that are transferred transplacentally to the fetus, resulting in the protection of the infant from RSV during the first 6 months of life. The RSVpreF vaccine also is approved to prevent RSV LRTD in people aged ≥ 60 years.
The RSVpreF vaccine contains the prefusion form of the RSV fusion (F) protein responsible for viral entry into host cells. The vaccine contains 60 µg of both RSV preF A and preF B recombinant proteins. The vaccine is administered as a single intramuscular dose in a volume of 0.5 mL. The vaccine is provided in a vial in a lyophilized form and must be reconstituted prior to administration. The average wholesale price of RSVPreF vaccine is $354.16 The vaccine is contraindicated for people who have had an allergic reaction to any component of the vaccine. The most commonly reported adverse reaction is injection site pain (41%).15 The FDA reports a “numerical imbalance in preterm births in Abrysvo recipients compared to placebo recipients” (5.7% vs 4.7%), and “available data are insufficient to establish or exclude a causal relationship between preterm birth and Abrysvo.”15 In rabbits there is no evidence of developmental toxicity and congenital anomalies associated with the RSVpreF vaccine. In human studies, no differences in the rate of congenital anomalies or fetal deaths were noted between RSVpreF vaccine and placebo.
In a clinical trial, 6,975 pregnant participants 24 through 36 weeks’ gestation were randomly assigned to receive a placebo or the RSVpreF vaccine.15,17 After birth, follow-up of infants at 180 days, showed that the rates of MA RSV LRTD among the infants in the placebo and RSVpreF vaccine groups were 3.4% and 1.6%, respectively. At 180 days, the reported rates of severe RSV LRTD in the placebo and RSVpreF vaccine groups were 1.8% and 0.5%, respectively. In this study, among the subset of pregnant participants who received the RSVpreF vaccine (n = 1,572) or placebo (n = 1,539) at 32 through 36 weeks’ gestation, the rates of MA RSV LRTD among the infants in the placebo and RSVpreF vaccine groups were 3.6% and 1.5%, respectively. In the subset of pregnant participants vaccinated at 32 through 36 weeks’ gestation, at 180 days postvaccination, the reported rates of severe RSV LRTD in the placebo and RSVpreF vaccine groups were 1.6% and 0.4%, respectively.15
The CDC has recommended that the RSVpreF vaccine be administered to pregnant people 32 through 36 weeks’ gestation from September through the end of January in most of the continental United States to reduce the rate of RSV LRTD in infants.6 September was selected because it is 1 to 2 months before the start of the RSV season, and it takes at least 14 days for maternal vaccination to result in transplacental transfer of protective antibodies to the fetus. January was selected because it is 2 to 3 months before the anticipated end of the RSV season.6 The CDC also noted that, for regions with a different pattern of RSV seasonality, clinicians should follow the guidance of local public health officials. This applies to the states of Alaska, southern Florida, Hawaii, and Puerto Rico.6 The CDC recommended that infants born < 34 weeks’ gestation should receive nirsevimab.6
Maternal RSV vaccination is thought to be cost-effective for reducing RSV LRTD in infants. However, the cost-effectiveness analyses are sensitive to the pricing of the two main options: maternal RSV vaccination and nirsevimab.
It is estimated that nirsevimab may provide greater protection than maternal RSV vaccination from RSV LRTD, but the maternal RSVpreF vaccine is priced lower than nirsevimab.18 Focusing administration of RSVpreF vaccine from September through January of the RSV infection season is thought to maximize benefits to infants and reduce total cost of the vaccination program.19 With year-round RSVpreF vaccine dosing, the estimated ICER per quality-adjusted life-year (QALY) is approximately $400,000, whereas seasonal dosing reduces the cost to approximately $170,000.19
RSV prevention strategy 3
Vaccinate pregnant persons; reflex to newborn treatment with nirsevimab if maternal RSV vaccination did not occur
RSVpreF vaccination to all pregnant persons 32 through 36 weeks’ gestation during RSV infection season is not likely to result in 100% adherence. For instance, in a CDC-conducted survey only 47% of pregnant persons received an influenza vaccine.2 Newborns whose mothers did not receive an RSVpreF vaccine will need to be considered for treatment with nirsevimab. Collaboration and communication among obstetricians and pediatricians will be needed to avoid miscommunication and missed opportunities to treat newborns during the birth hospitalization. Enhancements in electronic health records, linking the mother’s vaccination record with the newborn’s medical record plus an added feature of electronic alerts when the mother did not receive an appropriately timed RSVpreF vaccine would improve the communication of important clinical information to the pediatrician.
Next steps for the upcoming peak RSV season
We are currently in the 2023–2024 RSV infection season and can expect a peak in cases of RSV between December 2023 and February 2024. The CDC recommends protecting all infants against RSV-associated LRTD. The options are to administer the maternal RSVpreF vaccine to pregnant persons or treating the infant with nirsevimab. The vaccine is just now becoming available for administration in regional pharmacies, physician practices, and health systems. Obstetrician-gynecologists should follow the recommendation of their state department of public health. As noted above, many state departments of public health are recommending that all newborns receive nirsevimab. For clinicians in those states, RSVPreF vaccination of pregnant persons is not a priority. ●
Respiratory syncytial virus (RSV) is a negative-sense, single-stranded, ribonucleic acid (RNA) virus that is a member of Pneumoviridae family. Two subtypes, A and B, and multiple genotypes circulate during fall and winter seasonal outbreaks of RSV.1 RSV can cause severe lower respiratory tract disease including bronchiolitis, pneumonia, respiratory failure, and death. Each year, RSV disease causes the hospitalization of 1.5% to 2% of children younger than 6 months of age, resulting in 100 to 300 deaths.2 For infants younger than 1 year, RSV infection is the leading cause of hospitalization.3 In 2023, two new treatments have become available to prevent RSV disease: nirsevimab and RSVPreF vaccine.
Nirsevimab
Nirsevimab is an antibody to an RSV antigen. It has a long half-life and is approved for administration to infants, providing passive immunization. In contrast, administration of the RSVPreF vaccine to pregnant persons elicits active maternal immunity, resulting in the production of anti-RSV antibodies that are transferred to the fetus, resulting in passive immunity in the infant. Seasonal administration of nirsevimab and the RSV vaccine maximizes benefit to the infant and conserves limited health care resources. In temperate regions in the United States, the RSV infection season typically begins in October and peaks in December through mid-February and ends in April or May.4,5 In southern Florida, the RSV season often begins in August to September, peaks in November through December, and ends in March.4,5
This editorial reviews 3 strategies for prevention of RSV infection in infants, including:
- universal treatment of newborns with nirsevimab
- immunization of pregnant persons with an RSVpreF vaccine in the third trimester appropriately timed to occur just before the beginning or during RSV infection season
- prioritizing universal maternal RSV vaccination with reflex administration of nirsevimab to newborns when the pregnant person was not vaccinated.6
Of note, there are no studies that have evaluated the effectiveness of combining RSVpreF vaccine and nirsevimab. The Centers for Disease Control and Prevention (CDC) does not recommend combining both RSV vaccination of pregnant persons plus nirsevimab treatment of the infant, except in limited circumstances, such as for immunocompromised pregnant people with limited antibody production or newborns who have a massive transfusion, which dilutes antibody titres.6
RSV prevention strategy 1
Universal treatment of newborns and infants with nirsevimab
Nirsevimab (Beyfortus, Sanofi and AstraZeneca) is an IgG 1-kappa monoclonal antibody with a long half-life that targets the prefusion conformation of the RSV F-protein, resulting in passive immunity to infection.7 Passive immunization results in rapid protection against infection because it does not require activation of the immune system. Nirsevimab is long acting due to amino acid substitutions in the Fc region, increasing binding to the neonatal Fc receptor, which protects IgG antibodies from degradation, thereby extending the antibody half-life. The terminal halflife of nirsevimab is 71 days, and the duration of protection following a single dose is at least 5 months.
Nirsevimab is approved by the US Food and Drug Administration (FDA) for all neonates and infants born or entering their first RSV infection season and for children up to 24 months of age who are vulnerable to severe RSV during their second RSV infection season. For infants born outside the RSV infection season, nirsevimab should be administered once prior to the start of the next RSV infection season.7 Nirsevimab is administered as a single intramuscular injection at a dose of 50 mg for neonates and infants < 5 kg in weight and a dose of 100 mg for neonates and infants ≥ 5 kg in weight.7 The list average wholesale price for both doses is $594.8 Nirsevimab is contraindicated for patients with a serious hypersensitivity reaction to nirsevimab or its excipients.7 In clinical trials, adverse reactions including rash and injection site reaction were reported in 1.2% of participants.7 Some RSV variants may be resistant to neutralization with nirsevimab.7,9
In a randomized clinical trial, 1,490 infants born ≥ 35 weeks’ gestation, the rates of medically-attended RSV lower respiratory tract disease (MA RSV LRTD) through 150 days of follow-up in the placebo and nirsevimab groups were 5.0% and 1.2%, respectively (P < .001).7,10 Compared with placebo, nirsevimab reduced hospitalizations due to RSV LRTD by 60% through 150 days of follow up. In a randomized clinical trial enrolling 1,453 infants born between 29 weeks’ and < 35 weeks’ gestation, the rates of MA RSV LRTD through 150 days of follow up in the placebo and nirsevimab groups were 9.5% and 2.6%, respectively (P < .001). In this study of infants born preterm, compared with placebo, nirsevimab reduced hospitalization due to RSV LRTD by 70% through 150 days of follow up.7 Nirsevimab is thought to be cost-effective at the current price per dose, but more data are needed to precisely define the magnitude of the health care savings associated with universal nirsevimab administration.11-13 The CDC reports that the incremental cost-effectiveness ratio (ICER) per quality-adjusted life year (QALY) of nirsevimab administration to infants is approximately $250,000, given an estimated cost of $500 for one dose of vaccine.14
Universal passive vaccination of newborns is recommended by many state departments of public health, which can provide the vaccine without cost to clinicians and health care facilities participating in the children’s vaccination program.
Continue to: RSV prevention strategy 2...
RSV prevention strategy 2
Universal RSV vaccination of pregnant persons from September through January
The RSVpreF vaccine (Abryvso, Pfizer) is approved by the FDA for the active immunization of pregnant persons between 32 through 36 weeks’ gestation for the prevention of RSV LRTD in infants from birth through 6 months of age.15 Administration of the RSVpreF vaccine to pregnant people elicits the formation of antiRSV antibodies that are transferred transplacentally to the fetus, resulting in the protection of the infant from RSV during the first 6 months of life. The RSVpreF vaccine also is approved to prevent RSV LRTD in people aged ≥ 60 years.
The RSVpreF vaccine contains the prefusion form of the RSV fusion (F) protein responsible for viral entry into host cells. The vaccine contains 60 µg of both RSV preF A and preF B recombinant proteins. The vaccine is administered as a single intramuscular dose in a volume of 0.5 mL. The vaccine is provided in a vial in a lyophilized form and must be reconstituted prior to administration. The average wholesale price of RSVPreF vaccine is $354.16 The vaccine is contraindicated for people who have had an allergic reaction to any component of the vaccine. The most commonly reported adverse reaction is injection site pain (41%).15 The FDA reports a “numerical imbalance in preterm births in Abrysvo recipients compared to placebo recipients” (5.7% vs 4.7%), and “available data are insufficient to establish or exclude a causal relationship between preterm birth and Abrysvo.”15 In rabbits there is no evidence of developmental toxicity and congenital anomalies associated with the RSVpreF vaccine. In human studies, no differences in the rate of congenital anomalies or fetal deaths were noted between RSVpreF vaccine and placebo.
In a clinical trial, 6,975 pregnant participants 24 through 36 weeks’ gestation were randomly assigned to receive a placebo or the RSVpreF vaccine.15,17 After birth, follow-up of infants at 180 days, showed that the rates of MA RSV LRTD among the infants in the placebo and RSVpreF vaccine groups were 3.4% and 1.6%, respectively. At 180 days, the reported rates of severe RSV LRTD in the placebo and RSVpreF vaccine groups were 1.8% and 0.5%, respectively. In this study, among the subset of pregnant participants who received the RSVpreF vaccine (n = 1,572) or placebo (n = 1,539) at 32 through 36 weeks’ gestation, the rates of MA RSV LRTD among the infants in the placebo and RSVpreF vaccine groups were 3.6% and 1.5%, respectively. In the subset of pregnant participants vaccinated at 32 through 36 weeks’ gestation, at 180 days postvaccination, the reported rates of severe RSV LRTD in the placebo and RSVpreF vaccine groups were 1.6% and 0.4%, respectively.15
The CDC has recommended that the RSVpreF vaccine be administered to pregnant people 32 through 36 weeks’ gestation from September through the end of January in most of the continental United States to reduce the rate of RSV LRTD in infants.6 September was selected because it is 1 to 2 months before the start of the RSV season, and it takes at least 14 days for maternal vaccination to result in transplacental transfer of protective antibodies to the fetus. January was selected because it is 2 to 3 months before the anticipated end of the RSV season.6 The CDC also noted that, for regions with a different pattern of RSV seasonality, clinicians should follow the guidance of local public health officials. This applies to the states of Alaska, southern Florida, Hawaii, and Puerto Rico.6 The CDC recommended that infants born < 34 weeks’ gestation should receive nirsevimab.6
Maternal RSV vaccination is thought to be cost-effective for reducing RSV LRTD in infants. However, the cost-effectiveness analyses are sensitive to the pricing of the two main options: maternal RSV vaccination and nirsevimab.
It is estimated that nirsevimab may provide greater protection than maternal RSV vaccination from RSV LRTD, but the maternal RSVpreF vaccine is priced lower than nirsevimab.18 Focusing administration of RSVpreF vaccine from September through January of the RSV infection season is thought to maximize benefits to infants and reduce total cost of the vaccination program.19 With year-round RSVpreF vaccine dosing, the estimated ICER per quality-adjusted life-year (QALY) is approximately $400,000, whereas seasonal dosing reduces the cost to approximately $170,000.19
RSV prevention strategy 3
Vaccinate pregnant persons; reflex to newborn treatment with nirsevimab if maternal RSV vaccination did not occur
RSVpreF vaccination to all pregnant persons 32 through 36 weeks’ gestation during RSV infection season is not likely to result in 100% adherence. For instance, in a CDC-conducted survey only 47% of pregnant persons received an influenza vaccine.2 Newborns whose mothers did not receive an RSVpreF vaccine will need to be considered for treatment with nirsevimab. Collaboration and communication among obstetricians and pediatricians will be needed to avoid miscommunication and missed opportunities to treat newborns during the birth hospitalization. Enhancements in electronic health records, linking the mother’s vaccination record with the newborn’s medical record plus an added feature of electronic alerts when the mother did not receive an appropriately timed RSVpreF vaccine would improve the communication of important clinical information to the pediatrician.
Next steps for the upcoming peak RSV season
We are currently in the 2023–2024 RSV infection season and can expect a peak in cases of RSV between December 2023 and February 2024. The CDC recommends protecting all infants against RSV-associated LRTD. The options are to administer the maternal RSVpreF vaccine to pregnant persons or treating the infant with nirsevimab. The vaccine is just now becoming available for administration in regional pharmacies, physician practices, and health systems. Obstetrician-gynecologists should follow the recommendation of their state department of public health. As noted above, many state departments of public health are recommending that all newborns receive nirsevimab. For clinicians in those states, RSVPreF vaccination of pregnant persons is not a priority. ●
- Tramuto F, Massimo Maida C, Mazzucco W, et al. Molecular epidemiology and genetic diversity of human respiratory syncytial virus in Sicily during pre- and post-COVID-19 surveillance season. Pathogens. 2023;12:1099.
- Boudreau M, Vadlamudi NK, Bastien N, et al. Pediatric RSV-associated hospitalizations before and during the COVID-19 pandemic. JAMA Netw Open. 2023;6:e2336863.
- Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143(5 Suppl):S127-132.
- Hamid S, Winn A, Parikh R, et al. Seasonality of respiratory syncytial virus-United States 2017-2023. MMWR Morb Mortal Wkly Rep. 2023;72:355-361.
- Rose EB, Wheatley A, Langley G, et al. Respiratory syncytial virus seasonality-United States 2014-2017. MMWR Morb Mortal Wkly Rep. 2018;67:71-76.
- Fleming-Dutra KE, Jones JM, Roper LE, et al. Use of Pfizer respiratory syncytial virus vaccine during pregnancy for the prevention of respiratory syncytial virus associated lower respiratory tract disease in infants: recommendations of the Advisory Committee on Immunization Practices- United States 2023. MMWR Morb Mortal Wkly Rep. October 6, 2023. Accessed October 9, 2023. https://www.cdc.gov/mmwr/volumes/72/wr /mm7241e1.htm#print
- FDA package insert for Beyfortus. Accessed October 9, 2023. https://www.accessdata.fda.gov /drugsatfda_docs/label/2023/761328s000lbl.pdf
- Lexicomp. Nirsevimab: Drug information – UpToDate. Accessed October 9, 2023. https://www. wolterskluwer.com/en/solutions/lexicomp
- Ahani B, Tuffy KM, Aksyuk A, et al. Molecular and phenotypic characterization of RSV infections in infants during two nirsevimab randomized clinical trials. Nat Commun. 2023;14:4347.
- Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in late-preterm and term infants. N Engl J Med. 2022;386:837-846.
- Li X, Bilcke J, Vazquez-Fernandez L, et al. Costeffectiveness of respiratory syncytial virus disease protection strategies: maternal vaccine versus seasonal or year-round monoclonal antibody program in Norwegian children. J Infect Dis. 2022;226(Suppl 1):S95-S101.
- Hodgson D, Koltai M, Krauer F, et al. Optimal respiratory syncytial virus intervention programmes using nirsevimab in England and Wales. Vaccine. 2022;40:7151-7157.
- Yu T, Padula WV, Yieh L, et al. Cost-effectiveness of nirsevimab and palivizumab for respiratory syncytial virus prophylaxis in preterm infants 29-34 6/7 weeks’ gestation in the United States. Pediatr Neonatal. 2023;04:015.
- Jones J. Evidence to recommendations framework: nirsevimab in infants. Accessed October 27, 2023. https://www.cdc.gov/vaccines/acip/meet ings/downloads/slides-2023-02/slides-02-23/rsv -pediatric-04-jones-508.pdf
- Abrysvo [package insert]. Pfizer; New York, New York. August 2023.
- Lexicomp. Recombinant respiratory syncytial virus vaccine (RSVPreF) (Abrysvo): Drug information - UpToDate. Accessed October 9, 2023. https://www.wolterskluwer.com/en/solutions /lexicomp
- Kampmann B, Madhi SA, Munjal I, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388: 1451-1464.
- Baral R, Higgins D, Regan K, et al. Impact and costeffectiveness of potential interventions against infant respiratory syncytial virus (RSV) in 131 lowincome and middle-income countries using a static cohort model. BMJ Open. 2021;11:e046563.
- Fleming-Dutra KE. Evidence to recommendations framework updates: Pfizer maternal RSVpreF vaccine. June 22, 2023. Accessed October 27, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.cdc.gov/vaccines/acip /meetings/downloads/slides-2023-06-21-23/03 -RSV-Mat-Ped-Fleming-Dutra-508.pdf
- Razzaghi H, Kahn KE, Calhoun K, et al. Influenza, Tdap and COVID-19 vaccination coverage and hesitancy among pregnant women-United States, April 2023. MMWR Morb Mortal Wkly Rep.
- Tramuto F, Massimo Maida C, Mazzucco W, et al. Molecular epidemiology and genetic diversity of human respiratory syncytial virus in Sicily during pre- and post-COVID-19 surveillance season. Pathogens. 2023;12:1099.
- Boudreau M, Vadlamudi NK, Bastien N, et al. Pediatric RSV-associated hospitalizations before and during the COVID-19 pandemic. JAMA Netw Open. 2023;6:e2336863.
- Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143(5 Suppl):S127-132.
- Hamid S, Winn A, Parikh R, et al. Seasonality of respiratory syncytial virus-United States 2017-2023. MMWR Morb Mortal Wkly Rep. 2023;72:355-361.
- Rose EB, Wheatley A, Langley G, et al. Respiratory syncytial virus seasonality-United States 2014-2017. MMWR Morb Mortal Wkly Rep. 2018;67:71-76.
- Fleming-Dutra KE, Jones JM, Roper LE, et al. Use of Pfizer respiratory syncytial virus vaccine during pregnancy for the prevention of respiratory syncytial virus associated lower respiratory tract disease in infants: recommendations of the Advisory Committee on Immunization Practices- United States 2023. MMWR Morb Mortal Wkly Rep. October 6, 2023. Accessed October 9, 2023. https://www.cdc.gov/mmwr/volumes/72/wr /mm7241e1.htm#print
- FDA package insert for Beyfortus. Accessed October 9, 2023. https://www.accessdata.fda.gov /drugsatfda_docs/label/2023/761328s000lbl.pdf
- Lexicomp. Nirsevimab: Drug information – UpToDate. Accessed October 9, 2023. https://www. wolterskluwer.com/en/solutions/lexicomp
- Ahani B, Tuffy KM, Aksyuk A, et al. Molecular and phenotypic characterization of RSV infections in infants during two nirsevimab randomized clinical trials. Nat Commun. 2023;14:4347.
- Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in late-preterm and term infants. N Engl J Med. 2022;386:837-846.
- Li X, Bilcke J, Vazquez-Fernandez L, et al. Costeffectiveness of respiratory syncytial virus disease protection strategies: maternal vaccine versus seasonal or year-round monoclonal antibody program in Norwegian children. J Infect Dis. 2022;226(Suppl 1):S95-S101.
- Hodgson D, Koltai M, Krauer F, et al. Optimal respiratory syncytial virus intervention programmes using nirsevimab in England and Wales. Vaccine. 2022;40:7151-7157.
- Yu T, Padula WV, Yieh L, et al. Cost-effectiveness of nirsevimab and palivizumab for respiratory syncytial virus prophylaxis in preterm infants 29-34 6/7 weeks’ gestation in the United States. Pediatr Neonatal. 2023;04:015.
- Jones J. Evidence to recommendations framework: nirsevimab in infants. Accessed October 27, 2023. https://www.cdc.gov/vaccines/acip/meet ings/downloads/slides-2023-02/slides-02-23/rsv -pediatric-04-jones-508.pdf
- Abrysvo [package insert]. Pfizer; New York, New York. August 2023.
- Lexicomp. Recombinant respiratory syncytial virus vaccine (RSVPreF) (Abrysvo): Drug information - UpToDate. Accessed October 9, 2023. https://www.wolterskluwer.com/en/solutions /lexicomp
- Kampmann B, Madhi SA, Munjal I, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388: 1451-1464.
- Baral R, Higgins D, Regan K, et al. Impact and costeffectiveness of potential interventions against infant respiratory syncytial virus (RSV) in 131 lowincome and middle-income countries using a static cohort model. BMJ Open. 2021;11:e046563.
- Fleming-Dutra KE. Evidence to recommendations framework updates: Pfizer maternal RSVpreF vaccine. June 22, 2023. Accessed October 27, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.cdc.gov/vaccines/acip /meetings/downloads/slides-2023-06-21-23/03 -RSV-Mat-Ped-Fleming-Dutra-508.pdf
- Razzaghi H, Kahn KE, Calhoun K, et al. Influenza, Tdap and COVID-19 vaccination coverage and hesitancy among pregnant women-United States, April 2023. MMWR Morb Mortal Wkly Rep.
2023 Update on cervical disease
Cervical cancer was the most common cancer killer of persons with a cervix in the early 1900s in the United States. Widespread adoption of the Pap test in the mid-20th century followed by large-scale outreach through programs such as the National Breast and Cervical Cancer Early Detection Program have dramatically reduced deaths from cervical cancer. The development of a highly effective vaccine that targets human papillomavirus (HPV), the virus implicated in all cervical cancers, has made prevention even more accessible and attainable. Primary prevention with HPV vaccination in conjunction with regular screening as recommended by current guidelines is the most effective way we can prevent cervical cancer.
Despite these advances, the incidence and death rates from cervical cancer have plateaued over the last decade.1 Additionally, many fear that due to the poor attendance at screening visits since the beginning of the COVID-19 pandemic, the incidence might further rise in the United States.2 Among those in the United States diagnosed with cervical cancer, more than 50% have not been screened in over 5 years or had their abnormal results not managed as recommended by current guidelines, suggesting that operational and access issues are contributors to incident cervical cancer. In addition, HPV vaccination rates have increased only slightly from year to year. According to the most recent data from the Centers for Disease Control and Prevention (CDC), coverage with 1 or more doses of HPV vaccine in 2021 increased only by 1.8% and has stagnated, with administration to about 75% of those for whom it is recommended.3 The plateauing will limit our ability to eradicate cervical cancer in the United States, permitting death from a largely preventable disease.
Establishing the framework for the eradication of cervical cancer
The World Health Organization (WHO) adopted a global strategy called the Cervical Cancer Elimination Initiative in August 2020. This initiative is a multipronged effort that focuses on vaccination (90% of girls fully vaccinated by age 15), screening (70% of women screened by age 35 with an effective test and again at age 45), and treatment (90% treatment of precancer and 90% management of women with invasive cancer).4
These are the numbers we need to achieve if all countries are to reach a cervical cancer incidence of less than 4 per 100,000 persons with a cervix. The WHO further suggests that each country should meet the “90-70-90” targets by 2030 if we are to achieve the low incidence by the turn of the century.4 To date, few regions of the world have achieved these goals, and sadly the United States is not among them.
In response to this call to action, many medical and policymaking organizations are taking inventory and implementing strategies to achieve the WHO 2030 targets for cervical cancer eradication. In the United States, the Society of Gynecologic Oncology (SGO; www.sgo.org), the American Society for Colposcopy and Cervical Pathology (ASCCP; www.ASCCP.org), the American College of Obstetricians and Gynecologists (ACOG; www.acog.org), the American Cancer Society (ACS; www.cancer.org), and many others have initiated programs in a collaborative esprit de corps with the aim of eradicating this deadly disease.
In this Update, we review several studies with evidence of screening and management strategies that show promise of accelerating the eradication of cervical cancer.
Continue to: Transitioning to primary HPV screening in the United States...
Transitioning to primary HPV screening in the United States
Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
The American Cancer Society released an updated cervical cancer screening guideline in July 2020 that recommended testing for HPV as the preferred strategy. Reasons behind the change, moving away from a Pap test as part of the initial screen, are:
- increased sensitivity of primary HPV testing when compared with conventional cervical cytology (Pap test)
- improved risk stratification to identify who is at risk for cervical cancer now and in the future
- improved efficiency in identifying those who need colposcopy, thus limiting unnecessary procedures without increasing the risk of false-negative tests, thereby missing cervical precancer or invasive cancer.
Some countries with organized screening programs have already made the switch. Self-sampling for HPV is currently being considered for an approved use in the United States, further improving access to screening for cervical cancer when the initial step can be completed by the patient at home or simplified in nontraditional health care settings.2
ACS initiative created to address barriers to primary HPV testing
Challenges to primary HPV testing remain, including laboratory implementation, payment, and operationalizing clinical workflow (for example, HPV testing with reflex cytology instead of cytology with reflex HPV testing).5 There are undoubtedly other unforeseen barriers in the current US health care environment.
In a recent commentary, Downs and colleagues described how the ACS has convened the Primary HPV Screening Initiative (PHSI), nested under the ACS National Roundtable on Cervical Cancer, which is charged with identifying critical barriers to, and opportunities for, transitioning to primary HPV screening.5 The deliverable will be a roadmap with tools and recommendations to support health systems, laboratories, providers, patients, and payers as they make this evolution.
Work groups will develop resources
Patients, particularly those who have had routine cervical cancer screening over their lifetime, also will be curious about the changes in recommendations. The Provider Needs Workgroup within the PHSI structure will develop tools and patient education materials regarding the data, workflow, benefits, and safety of this new paradigm for cervical cancer screening.
Laboratories that process and interpret tests likely will bear the heaviest load of changes. For example, not all commercially available HPV tests in the United States are approved by the US Food and Drug Administration (FDA) for primary HPV testing. Some sites will need to adapt their equipment to ensure adherence to FDA-approved tests. Laboratory workflows will need to be altered for aliquots to be tested for HPV first, and the remainder for cytology. Quality assurance and accreditation requirements for testing will need modifications, and further efforts will be needed to ensure sufficient numbers of trained cytopathologists, whose workforce is rapidly declining, for processing and reading cervical cytology.
In addition, payment for HPV testing alone, without the need for a Pap test, might not be supported by payers that support safety-net providers and sites, who arguably serve the most vulnerable patients and those most at risk for cervical cancer. Collaboration across medical professionals, societies, payers, and policymakers will provide a critical infrastructure to make the change in the most seamless fashion and limit the harm from missed opportunities for screening.
HPV testing as the primary screen for cervical cancer is now recommended in guidelines due to improved sensitivity and improved efficiency when compared with other methods of screening. Implementation of this new workflow for clinicians and labs will require collaboration across multiple stakeholders.
Continue to: The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN...
The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN
Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
One new technology that was recently FDA approved and recommended for management of abnormal cervical cancer screening testing is dual-stain (DS) testing. Dual-stain testing is a cytology-based test that evaluates the concurrent expression of p16, a tumor suppressor protein upregulated in HPV oncogenesis, and Ki-67, a cell proliferation marker.6,7 Two recent studies have showcased the outstanding clinical performance of DS testing and triage strategies that incorporate DS testing.
Higher specificity, fewer colposcopies needed with DS testing
Magkana and colleagues prospectively evaluated patients with atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL), or negative for intraepithelial lesion or malignancy (NILM) cytology referred for colposcopy, and they compared p16/Ki-67 DS testing with high-risk HPV (HR-HPV) testing for the detection of cervical intraepithelial neoplasia grade 2 or worse (CIN 2+); comparable sensitivities for CIN 2+ detection were seen (97.3% and 98.7%, respectively).8
Dual-stain testing exhibited higher specificity at 99.3% compared with HR-HPV testing at 52.2%. Incorporating DS testing into triage strategies also led to fewer colposcopies needed to detect CIN 2+ compared with current ASCCP guidelines that use traditional cervical cancer screening algorithms.
DS cytology strategy had the highest sensitivity for CIN 2+ detection
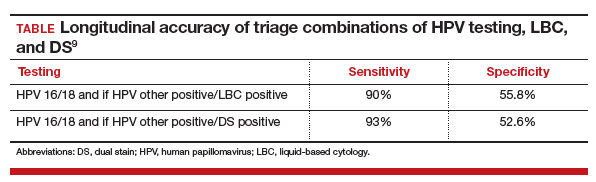
An additional study by Stanczuk and colleagues evaluated triage strategies in a cohort of HR-HPV positive patients who participated in the Scottish Papillomavirus Dumfries and Galloway study with HPV 16/18 genotyping (HPV 16/18), liquid-based cytology (LBC), and p16/Ki-67 DS cytology.9 Of these 3 triage strategies, DS cytology had the highest sensitivity for the detection of CIN 2+, at 77.7% (with a specificity of 74.2%), performance that is arguably better than cytology.
When evaluated in sequence as part of a triage strategy after HPV primary screening, HPV 16/18–positive patients reflexed to DS testing showed a similar sensitivity as those who would be triaged with LBC (TABLE).9
DS testing’s potential
These studies add to the growing body of literature that supports the use of DS testing in cervical cancer screening management guidelines and that are being incorporated into currently existing workflows. Furthermore, with advancements in digital imaging and machine learning, DS testing holds the potential for a high throughput, reproducible, and accurate risk stratification that can replace the current reliance on cytology, furthering the potential for a fully molecular Pap test.10,11
The introduction of p16/Ki-67 dual-stain testing has the potential to allow us to safely move away from a traditional Pap test for cervical cancer screening by allowing for more accurate and reliable identification of high-risk lesions with a molecular test that can be automated and have a high throughput.
Continue to: Cervical cancer screening in women older than age 65: Is there benefit?...
Cervical cancer screening in women older than age 65: Is there benefit?
Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
Current guidelines in the United States recommend that cervical cancer screening for all persons with a cervix end at age 65. These age restrictions were a change in guidelines updated in 2012 and endorsed by the US Preventive Services Task Force.12,13 Evidence suggests that because of high likelihood of regression and slow progression of disease, risks of screening prior to age 21 outweigh its benefits. With primary HPV testing, the age at screening debut is 25 for the same reasons.14 In people with a history of CIN 2+, active surveillance should continue for at least 25 years with HPV-based screening regardless of age. In the absence of a history of CIN 2+, however, the data to support discontinuation of screening after age 65 are less clear.
HPV positivity found to be most substantial risk for CIN 2+
In a study published this year in the Journal of Lower Genital Tract Disease, Firtina Tuncer and colleagues described their experience extending “routine screening” in patients older than 65 years.15 Data including cervical cytology, HPV test results, biopsy findings, and endocervical curettage results were collected, and abnormal findings were managed according to the 2012 and 2019 ASCCP guidelines.
When compared with negative HPV testing and normal cytology, the authors found that HPV positivity and abnormal cytology increased the risk of CIN 2+(odds ratio [OR], 136.1 and 13.1, respectively). Patients whose screening prior to age 65 had been insufficient or demonstrated CIN 2+ in the preceding 10 years were similarly more likely to have findings of CIN 2+ (OR, 9.7 when compared with HPV-negative controls).
The authors concluded that, among persons with a cervix older than age 65, previous screening and abnormal cytology were important in risk stratifications for CIN 2+; however, HPV positivity conferred the most substantial risk.
Study finds cervical dysplasia is prevalent in older populations
It has been suggested that screening for cervical cancer should continue beyond age 65 as cytology-based screening may have decreased sensitivity in older patients, which may contribute to the higher rates of advanced-stage diagnoses and cancer-related death in this population.16,17
Authors of an observational study conducted in Denmark invited persons with a cervix aged 69 and older to have one additional HPV-based screening test, and they referred them for colposcopy if HPV positive or in the presence of ASCUS or greater cytology.18 Among the 191 patients with HPV-positive results, 20% were found to have a diagnosis of CIN 2+, and 24.4% had CIN 2+ detected at another point in the study period. Notably, most patients diagnosed with CIN 2+ had no abnormalities visualized on colposcopy, and the majority of biopsies taken (65.8%) did not contain the transitional zone.
Biopsies underestimated CIN 2+ in 17.9% of cases compared with loop electrosurgical excision procedure (LEEP). These findings suggest both that high-grade cervical dysplasia is prevalent in an older population and that older populations may be susceptible to false-negative results. They also further support the use of HPV-based screening.
There are risk factors overscreening and underscreening that impact decision making regarding restricting screening to persons with a cervix younger than age 65. As more data become available, and as the population ages, it will be essential to closely examine the incidence of and trends in cervical cancer to determine appropriate patterns of screening.
Harnessing the immune system to improve survival rates in recurrent cervical cancer
Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Unfortunately, most clinical trials for recurrent or metastatic cervical cancer are negative trials or have results that show limited impact on disease outcomes. Currently, cervical cancer is treated with multiple agents, including platinum-based chemotherapy and bevacizumab, a medication that targets vascular growth. Despite these usually very effective drugs given in combination to cervical cancer patients, long-term survival remains low. Over the past few decades, many trials have been designed to help patients with this terrible disease, but few have shown significant promise.
Immune checkpoint inhibitors, such as pembrolizumab, have revolutionized care for many cancers. Checkpoint inhibitors block the proteins that cause a tumor to remain undetected by the immune system’s army of T cells. By blocking these proteins, the cancer cells can then be recognized by the immune system as foreign. Several studies have concluded that including immune checkpoint inhibitors in the comprehensive regimen for recurrent cervical cancer improves survival.
Addition of pembrolizumab increased survival
Investigators in the phase 3 double-blinded KEYNOTE-826 trial evaluated whether or not the addition of pembrolizumab to standard of care improved progression-free and overall survival in advanced, recurrent, or persistent cervical cancer.19 As part of the evaluation, the investigators measured the protein that turns off the immune system’s ability to recognize tumors, anti-programmed cell death protein-1 (PD-1).
Compared with placebo, the investigators found that, regardless of PD-1 status, the addition of pembrolizumab immunotherapy to the standard regimen increased progression-free survival and overall survival without any significantly increased adverse effects or safety concerns (FIGURE).19 At 1 year after treatment, more patients who received pembrolizumab were still alive regardless of PD-1 status, and their responses lasted longer. The most profound improvements were seen in patients whose tumors exhibited high expression of PD-L1, the target of pembrolizumab and many other immune checkpoint inhibitors.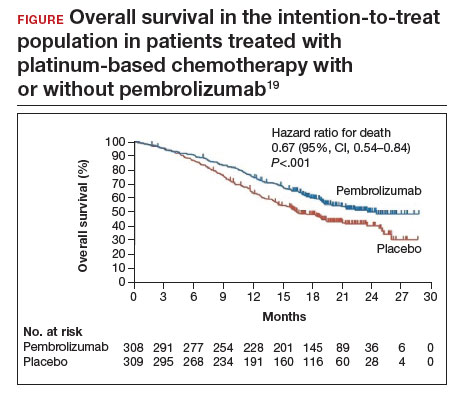
Despite these promising results, more studies are needed to find additional therapeutic targets and treatments. Using the immune system to fight cancer represents a promising step toward the ultimate goal of cervical cancer eradication. ●
Metastatic cervical cancer can be a devastating disease that cannot be treated surgically and therefore has limited treatment options that have curative intent. Immune checkpoint inhibition via pembrolizumab opens new avenues for treatment and is a huge step forward toward the goal of cervical cancer eradication.
- US Cancer Statistics Working Group. US Cancer Statistics Data Visualizations Tool, based on 2022 submission data (1999-2020). US Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. June 2023. Accessed October 9, 2023. https://gis.cdc.gov/Cancer/USCS/#/Trends/
- Einstein MH, Zhou N, Gabor L, et al. Primary human papillomavirus testing and other new technologies for cervical cancer screening. Obstet Gynecol. September 14, 2023. doi:10.1097/AOG.0000000000005393
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2020. MMWR Morbid Mortal Weekly Rep. 2021;70:1183-1190.
- Cervical cancer elimination initiative. World Health Organization. 2023. Accessed October 10, 2023. https ://www.who.int/initiatives/cervical-cancer-eliminationinitiative#cms
- Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
- Wentzensen N, Fetterman B, Castle PE, et al. p16/Ki-67 Dual stain cytology for detection of cervical precancer in HPV-positive women. J Natl Cancer Inst. 2015;107:djv257.
- Ikenberg H, Bergeron C, Schmidt D, et al; PALMS Study Group. Screening for cervical cancer precursors with p16 /Ki-67 dual-stained cytology: results of the PALMS study. J Natl Cancer Inst. 2013;105:1550-1557.
- Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
- Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
- Wright TC Jr, Stoler MH, Behrens CM, et al. Interlaboratory variation in the performance of liquid-based cytology: insights from the ATHENA trial. Int J Cancer. 2014;134: 1835-1843.
- Wentzensen N, Lahrmann B, Clarke MA, et al. Accuracy and efficiency of deep-learning-based automation of dual stain cytology in cervical cancer screening. J Natl Cancer Inst. 2021;113:72-79.
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121:829-846.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156: 880-891, W312.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
- Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
- Hammer A, Hee L, Blaakaer J, et al. Temporal patterns of cervical cancer screening among Danish women 55 years and older diagnosed with cervical cancer. J Low Genit Tract Dis. 2018;22:1-7.
- Hammer A, Soegaard V, Maimburg RD, et al. Cervical cancer screening history prior to a diagnosis of cervical cancer in Danish women aged 60 years and older—A national cohort study. Cancer Med. 2019;8:418-427.
- Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.

Cervical cancer was the most common cancer killer of persons with a cervix in the early 1900s in the United States. Widespread adoption of the Pap test in the mid-20th century followed by large-scale outreach through programs such as the National Breast and Cervical Cancer Early Detection Program have dramatically reduced deaths from cervical cancer. The development of a highly effective vaccine that targets human papillomavirus (HPV), the virus implicated in all cervical cancers, has made prevention even more accessible and attainable. Primary prevention with HPV vaccination in conjunction with regular screening as recommended by current guidelines is the most effective way we can prevent cervical cancer.
Despite these advances, the incidence and death rates from cervical cancer have plateaued over the last decade.1 Additionally, many fear that due to the poor attendance at screening visits since the beginning of the COVID-19 pandemic, the incidence might further rise in the United States.2 Among those in the United States diagnosed with cervical cancer, more than 50% have not been screened in over 5 years or had their abnormal results not managed as recommended by current guidelines, suggesting that operational and access issues are contributors to incident cervical cancer. In addition, HPV vaccination rates have increased only slightly from year to year. According to the most recent data from the Centers for Disease Control and Prevention (CDC), coverage with 1 or more doses of HPV vaccine in 2021 increased only by 1.8% and has stagnated, with administration to about 75% of those for whom it is recommended.3 The plateauing will limit our ability to eradicate cervical cancer in the United States, permitting death from a largely preventable disease.
Establishing the framework for the eradication of cervical cancer
The World Health Organization (WHO) adopted a global strategy called the Cervical Cancer Elimination Initiative in August 2020. This initiative is a multipronged effort that focuses on vaccination (90% of girls fully vaccinated by age 15), screening (70% of women screened by age 35 with an effective test and again at age 45), and treatment (90% treatment of precancer and 90% management of women with invasive cancer).4
These are the numbers we need to achieve if all countries are to reach a cervical cancer incidence of less than 4 per 100,000 persons with a cervix. The WHO further suggests that each country should meet the “90-70-90” targets by 2030 if we are to achieve the low incidence by the turn of the century.4 To date, few regions of the world have achieved these goals, and sadly the United States is not among them.
In response to this call to action, many medical and policymaking organizations are taking inventory and implementing strategies to achieve the WHO 2030 targets for cervical cancer eradication. In the United States, the Society of Gynecologic Oncology (SGO; www.sgo.org), the American Society for Colposcopy and Cervical Pathology (ASCCP; www.ASCCP.org), the American College of Obstetricians and Gynecologists (ACOG; www.acog.org), the American Cancer Society (ACS; www.cancer.org), and many others have initiated programs in a collaborative esprit de corps with the aim of eradicating this deadly disease.
In this Update, we review several studies with evidence of screening and management strategies that show promise of accelerating the eradication of cervical cancer.
Continue to: Transitioning to primary HPV screening in the United States...
Transitioning to primary HPV screening in the United States
Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
The American Cancer Society released an updated cervical cancer screening guideline in July 2020 that recommended testing for HPV as the preferred strategy. Reasons behind the change, moving away from a Pap test as part of the initial screen, are:
- increased sensitivity of primary HPV testing when compared with conventional cervical cytology (Pap test)
- improved risk stratification to identify who is at risk for cervical cancer now and in the future
- improved efficiency in identifying those who need colposcopy, thus limiting unnecessary procedures without increasing the risk of false-negative tests, thereby missing cervical precancer or invasive cancer.
Some countries with organized screening programs have already made the switch. Self-sampling for HPV is currently being considered for an approved use in the United States, further improving access to screening for cervical cancer when the initial step can be completed by the patient at home or simplified in nontraditional health care settings.2
ACS initiative created to address barriers to primary HPV testing
Challenges to primary HPV testing remain, including laboratory implementation, payment, and operationalizing clinical workflow (for example, HPV testing with reflex cytology instead of cytology with reflex HPV testing).5 There are undoubtedly other unforeseen barriers in the current US health care environment.
In a recent commentary, Downs and colleagues described how the ACS has convened the Primary HPV Screening Initiative (PHSI), nested under the ACS National Roundtable on Cervical Cancer, which is charged with identifying critical barriers to, and opportunities for, transitioning to primary HPV screening.5 The deliverable will be a roadmap with tools and recommendations to support health systems, laboratories, providers, patients, and payers as they make this evolution.
Work groups will develop resources
Patients, particularly those who have had routine cervical cancer screening over their lifetime, also will be curious about the changes in recommendations. The Provider Needs Workgroup within the PHSI structure will develop tools and patient education materials regarding the data, workflow, benefits, and safety of this new paradigm for cervical cancer screening.
Laboratories that process and interpret tests likely will bear the heaviest load of changes. For example, not all commercially available HPV tests in the United States are approved by the US Food and Drug Administration (FDA) for primary HPV testing. Some sites will need to adapt their equipment to ensure adherence to FDA-approved tests. Laboratory workflows will need to be altered for aliquots to be tested for HPV first, and the remainder for cytology. Quality assurance and accreditation requirements for testing will need modifications, and further efforts will be needed to ensure sufficient numbers of trained cytopathologists, whose workforce is rapidly declining, for processing and reading cervical cytology.
In addition, payment for HPV testing alone, without the need for a Pap test, might not be supported by payers that support safety-net providers and sites, who arguably serve the most vulnerable patients and those most at risk for cervical cancer. Collaboration across medical professionals, societies, payers, and policymakers will provide a critical infrastructure to make the change in the most seamless fashion and limit the harm from missed opportunities for screening.
HPV testing as the primary screen for cervical cancer is now recommended in guidelines due to improved sensitivity and improved efficiency when compared with other methods of screening. Implementation of this new workflow for clinicians and labs will require collaboration across multiple stakeholders.
Continue to: The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN...
The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN
Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
One new technology that was recently FDA approved and recommended for management of abnormal cervical cancer screening testing is dual-stain (DS) testing. Dual-stain testing is a cytology-based test that evaluates the concurrent expression of p16, a tumor suppressor protein upregulated in HPV oncogenesis, and Ki-67, a cell proliferation marker.6,7 Two recent studies have showcased the outstanding clinical performance of DS testing and triage strategies that incorporate DS testing.
Higher specificity, fewer colposcopies needed with DS testing
Magkana and colleagues prospectively evaluated patients with atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL), or negative for intraepithelial lesion or malignancy (NILM) cytology referred for colposcopy, and they compared p16/Ki-67 DS testing with high-risk HPV (HR-HPV) testing for the detection of cervical intraepithelial neoplasia grade 2 or worse (CIN 2+); comparable sensitivities for CIN 2+ detection were seen (97.3% and 98.7%, respectively).8
Dual-stain testing exhibited higher specificity at 99.3% compared with HR-HPV testing at 52.2%. Incorporating DS testing into triage strategies also led to fewer colposcopies needed to detect CIN 2+ compared with current ASCCP guidelines that use traditional cervical cancer screening algorithms.
DS cytology strategy had the highest sensitivity for CIN 2+ detection
An additional study by Stanczuk and colleagues evaluated triage strategies in a cohort of HR-HPV positive patients who participated in the Scottish Papillomavirus Dumfries and Galloway study with HPV 16/18 genotyping (HPV 16/18), liquid-based cytology (LBC), and p16/Ki-67 DS cytology.9 Of these 3 triage strategies, DS cytology had the highest sensitivity for the detection of CIN 2+, at 77.7% (with a specificity of 74.2%), performance that is arguably better than cytology.
When evaluated in sequence as part of a triage strategy after HPV primary screening, HPV 16/18–positive patients reflexed to DS testing showed a similar sensitivity as those who would be triaged with LBC (TABLE).9

DS testing’s potential
These studies add to the growing body of literature that supports the use of DS testing in cervical cancer screening management guidelines and that are being incorporated into currently existing workflows. Furthermore, with advancements in digital imaging and machine learning, DS testing holds the potential for a high throughput, reproducible, and accurate risk stratification that can replace the current reliance on cytology, furthering the potential for a fully molecular Pap test.10,11
The introduction of p16/Ki-67 dual-stain testing has the potential to allow us to safely move away from a traditional Pap test for cervical cancer screening by allowing for more accurate and reliable identification of high-risk lesions with a molecular test that can be automated and have a high throughput.
Continue to: Cervical cancer screening in women older than age 65: Is there benefit?...
Cervical cancer screening in women older than age 65: Is there benefit?
Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
Current guidelines in the United States recommend that cervical cancer screening for all persons with a cervix end at age 65. These age restrictions were a change in guidelines updated in 2012 and endorsed by the US Preventive Services Task Force.12,13 Evidence suggests that because of high likelihood of regression and slow progression of disease, risks of screening prior to age 21 outweigh its benefits. With primary HPV testing, the age at screening debut is 25 for the same reasons.14 In people with a history of CIN 2+, active surveillance should continue for at least 25 years with HPV-based screening regardless of age. In the absence of a history of CIN 2+, however, the data to support discontinuation of screening after age 65 are less clear.
HPV positivity found to be most substantial risk for CIN 2+
In a study published this year in the Journal of Lower Genital Tract Disease, Firtina Tuncer and colleagues described their experience extending “routine screening” in patients older than 65 years.15 Data including cervical cytology, HPV test results, biopsy findings, and endocervical curettage results were collected, and abnormal findings were managed according to the 2012 and 2019 ASCCP guidelines.
When compared with negative HPV testing and normal cytology, the authors found that HPV positivity and abnormal cytology increased the risk of CIN 2+(odds ratio [OR], 136.1 and 13.1, respectively). Patients whose screening prior to age 65 had been insufficient or demonstrated CIN 2+ in the preceding 10 years were similarly more likely to have findings of CIN 2+ (OR, 9.7 when compared with HPV-negative controls).
The authors concluded that, among persons with a cervix older than age 65, previous screening and abnormal cytology were important in risk stratifications for CIN 2+; however, HPV positivity conferred the most substantial risk.
Study finds cervical dysplasia is prevalent in older populations
It has been suggested that screening for cervical cancer should continue beyond age 65 as cytology-based screening may have decreased sensitivity in older patients, which may contribute to the higher rates of advanced-stage diagnoses and cancer-related death in this population.16,17
Authors of an observational study conducted in Denmark invited persons with a cervix aged 69 and older to have one additional HPV-based screening test, and they referred them for colposcopy if HPV positive or in the presence of ASCUS or greater cytology.18 Among the 191 patients with HPV-positive results, 20% were found to have a diagnosis of CIN 2+, and 24.4% had CIN 2+ detected at another point in the study period. Notably, most patients diagnosed with CIN 2+ had no abnormalities visualized on colposcopy, and the majority of biopsies taken (65.8%) did not contain the transitional zone.
Biopsies underestimated CIN 2+ in 17.9% of cases compared with loop electrosurgical excision procedure (LEEP). These findings suggest both that high-grade cervical dysplasia is prevalent in an older population and that older populations may be susceptible to false-negative results. They also further support the use of HPV-based screening.
There are risk factors overscreening and underscreening that impact decision making regarding restricting screening to persons with a cervix younger than age 65. As more data become available, and as the population ages, it will be essential to closely examine the incidence of and trends in cervical cancer to determine appropriate patterns of screening.
Harnessing the immune system to improve survival rates in recurrent cervical cancer
Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Unfortunately, most clinical trials for recurrent or metastatic cervical cancer are negative trials or have results that show limited impact on disease outcomes. Currently, cervical cancer is treated with multiple agents, including platinum-based chemotherapy and bevacizumab, a medication that targets vascular growth. Despite these usually very effective drugs given in combination to cervical cancer patients, long-term survival remains low. Over the past few decades, many trials have been designed to help patients with this terrible disease, but few have shown significant promise.
Immune checkpoint inhibitors, such as pembrolizumab, have revolutionized care for many cancers. Checkpoint inhibitors block the proteins that cause a tumor to remain undetected by the immune system’s army of T cells. By blocking these proteins, the cancer cells can then be recognized by the immune system as foreign. Several studies have concluded that including immune checkpoint inhibitors in the comprehensive regimen for recurrent cervical cancer improves survival.
Addition of pembrolizumab increased survival
Investigators in the phase 3 double-blinded KEYNOTE-826 trial evaluated whether or not the addition of pembrolizumab to standard of care improved progression-free and overall survival in advanced, recurrent, or persistent cervical cancer.19 As part of the evaluation, the investigators measured the protein that turns off the immune system’s ability to recognize tumors, anti-programmed cell death protein-1 (PD-1).
Compared with placebo, the investigators found that, regardless of PD-1 status, the addition of pembrolizumab immunotherapy to the standard regimen increased progression-free survival and overall survival without any significantly increased adverse effects or safety concerns (FIGURE).19 At 1 year after treatment, more patients who received pembrolizumab were still alive regardless of PD-1 status, and their responses lasted longer. The most profound improvements were seen in patients whose tumors exhibited high expression of PD-L1, the target of pembrolizumab and many other immune checkpoint inhibitors.
Despite these promising results, more studies are needed to find additional therapeutic targets and treatments. Using the immune system to fight cancer represents a promising step toward the ultimate goal of cervical cancer eradication. ●
Metastatic cervical cancer can be a devastating disease that cannot be treated surgically and therefore has limited treatment options that have curative intent. Immune checkpoint inhibition via pembrolizumab opens new avenues for treatment and is a huge step forward toward the goal of cervical cancer eradication.

Cervical cancer was the most common cancer killer of persons with a cervix in the early 1900s in the United States. Widespread adoption of the Pap test in the mid-20th century followed by large-scale outreach through programs such as the National Breast and Cervical Cancer Early Detection Program have dramatically reduced deaths from cervical cancer. The development of a highly effective vaccine that targets human papillomavirus (HPV), the virus implicated in all cervical cancers, has made prevention even more accessible and attainable. Primary prevention with HPV vaccination in conjunction with regular screening as recommended by current guidelines is the most effective way we can prevent cervical cancer.
Despite these advances, the incidence and death rates from cervical cancer have plateaued over the last decade.1 Additionally, many fear that due to the poor attendance at screening visits since the beginning of the COVID-19 pandemic, the incidence might further rise in the United States.2 Among those in the United States diagnosed with cervical cancer, more than 50% have not been screened in over 5 years or had their abnormal results not managed as recommended by current guidelines, suggesting that operational and access issues are contributors to incident cervical cancer. In addition, HPV vaccination rates have increased only slightly from year to year. According to the most recent data from the Centers for Disease Control and Prevention (CDC), coverage with 1 or more doses of HPV vaccine in 2021 increased only by 1.8% and has stagnated, with administration to about 75% of those for whom it is recommended.3 The plateauing will limit our ability to eradicate cervical cancer in the United States, permitting death from a largely preventable disease.
Establishing the framework for the eradication of cervical cancer
The World Health Organization (WHO) adopted a global strategy called the Cervical Cancer Elimination Initiative in August 2020. This initiative is a multipronged effort that focuses on vaccination (90% of girls fully vaccinated by age 15), screening (70% of women screened by age 35 with an effective test and again at age 45), and treatment (90% treatment of precancer and 90% management of women with invasive cancer).4
These are the numbers we need to achieve if all countries are to reach a cervical cancer incidence of less than 4 per 100,000 persons with a cervix. The WHO further suggests that each country should meet the “90-70-90” targets by 2030 if we are to achieve the low incidence by the turn of the century.4 To date, few regions of the world have achieved these goals, and sadly the United States is not among them.
In response to this call to action, many medical and policymaking organizations are taking inventory and implementing strategies to achieve the WHO 2030 targets for cervical cancer eradication. In the United States, the Society of Gynecologic Oncology (SGO; www.sgo.org), the American Society for Colposcopy and Cervical Pathology (ASCCP; www.ASCCP.org), the American College of Obstetricians and Gynecologists (ACOG; www.acog.org), the American Cancer Society (ACS; www.cancer.org), and many others have initiated programs in a collaborative esprit de corps with the aim of eradicating this deadly disease.
In this Update, we review several studies with evidence of screening and management strategies that show promise of accelerating the eradication of cervical cancer.
Continue to: Transitioning to primary HPV screening in the United States...
Transitioning to primary HPV screening in the United States
Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
The American Cancer Society released an updated cervical cancer screening guideline in July 2020 that recommended testing for HPV as the preferred strategy. Reasons behind the change, moving away from a Pap test as part of the initial screen, are:
- increased sensitivity of primary HPV testing when compared with conventional cervical cytology (Pap test)
- improved risk stratification to identify who is at risk for cervical cancer now and in the future
- improved efficiency in identifying those who need colposcopy, thus limiting unnecessary procedures without increasing the risk of false-negative tests, thereby missing cervical precancer or invasive cancer.
Some countries with organized screening programs have already made the switch. Self-sampling for HPV is currently being considered for an approved use in the United States, further improving access to screening for cervical cancer when the initial step can be completed by the patient at home or simplified in nontraditional health care settings.2
ACS initiative created to address barriers to primary HPV testing
Challenges to primary HPV testing remain, including laboratory implementation, payment, and operationalizing clinical workflow (for example, HPV testing with reflex cytology instead of cytology with reflex HPV testing).5 There are undoubtedly other unforeseen barriers in the current US health care environment.
In a recent commentary, Downs and colleagues described how the ACS has convened the Primary HPV Screening Initiative (PHSI), nested under the ACS National Roundtable on Cervical Cancer, which is charged with identifying critical barriers to, and opportunities for, transitioning to primary HPV screening.5 The deliverable will be a roadmap with tools and recommendations to support health systems, laboratories, providers, patients, and payers as they make this evolution.
Work groups will develop resources
Patients, particularly those who have had routine cervical cancer screening over their lifetime, also will be curious about the changes in recommendations. The Provider Needs Workgroup within the PHSI structure will develop tools and patient education materials regarding the data, workflow, benefits, and safety of this new paradigm for cervical cancer screening.
Laboratories that process and interpret tests likely will bear the heaviest load of changes. For example, not all commercially available HPV tests in the United States are approved by the US Food and Drug Administration (FDA) for primary HPV testing. Some sites will need to adapt their equipment to ensure adherence to FDA-approved tests. Laboratory workflows will need to be altered for aliquots to be tested for HPV first, and the remainder for cytology. Quality assurance and accreditation requirements for testing will need modifications, and further efforts will be needed to ensure sufficient numbers of trained cytopathologists, whose workforce is rapidly declining, for processing and reading cervical cytology.
In addition, payment for HPV testing alone, without the need for a Pap test, might not be supported by payers that support safety-net providers and sites, who arguably serve the most vulnerable patients and those most at risk for cervical cancer. Collaboration across medical professionals, societies, payers, and policymakers will provide a critical infrastructure to make the change in the most seamless fashion and limit the harm from missed opportunities for screening.
HPV testing as the primary screen for cervical cancer is now recommended in guidelines due to improved sensitivity and improved efficiency when compared with other methods of screening. Implementation of this new workflow for clinicians and labs will require collaboration across multiple stakeholders.
Continue to: The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN...
The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN
Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
One new technology that was recently FDA approved and recommended for management of abnormal cervical cancer screening testing is dual-stain (DS) testing. Dual-stain testing is a cytology-based test that evaluates the concurrent expression of p16, a tumor suppressor protein upregulated in HPV oncogenesis, and Ki-67, a cell proliferation marker.6,7 Two recent studies have showcased the outstanding clinical performance of DS testing and triage strategies that incorporate DS testing.
Higher specificity, fewer colposcopies needed with DS testing
Magkana and colleagues prospectively evaluated patients with atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL), or negative for intraepithelial lesion or malignancy (NILM) cytology referred for colposcopy, and they compared p16/Ki-67 DS testing with high-risk HPV (HR-HPV) testing for the detection of cervical intraepithelial neoplasia grade 2 or worse (CIN 2+); comparable sensitivities for CIN 2+ detection were seen (97.3% and 98.7%, respectively).8
Dual-stain testing exhibited higher specificity at 99.3% compared with HR-HPV testing at 52.2%. Incorporating DS testing into triage strategies also led to fewer colposcopies needed to detect CIN 2+ compared with current ASCCP guidelines that use traditional cervical cancer screening algorithms.
DS cytology strategy had the highest sensitivity for CIN 2+ detection
An additional study by Stanczuk and colleagues evaluated triage strategies in a cohort of HR-HPV positive patients who participated in the Scottish Papillomavirus Dumfries and Galloway study with HPV 16/18 genotyping (HPV 16/18), liquid-based cytology (LBC), and p16/Ki-67 DS cytology.9 Of these 3 triage strategies, DS cytology had the highest sensitivity for the detection of CIN 2+, at 77.7% (with a specificity of 74.2%), performance that is arguably better than cytology.
When evaluated in sequence as part of a triage strategy after HPV primary screening, HPV 16/18–positive patients reflexed to DS testing showed a similar sensitivity as those who would be triaged with LBC (TABLE).9

DS testing’s potential
These studies add to the growing body of literature that supports the use of DS testing in cervical cancer screening management guidelines and that are being incorporated into currently existing workflows. Furthermore, with advancements in digital imaging and machine learning, DS testing holds the potential for a high throughput, reproducible, and accurate risk stratification that can replace the current reliance on cytology, furthering the potential for a fully molecular Pap test.10,11
The introduction of p16/Ki-67 dual-stain testing has the potential to allow us to safely move away from a traditional Pap test for cervical cancer screening by allowing for more accurate and reliable identification of high-risk lesions with a molecular test that can be automated and have a high throughput.
Continue to: Cervical cancer screening in women older than age 65: Is there benefit?...
Cervical cancer screening in women older than age 65: Is there benefit?
Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
Current guidelines in the United States recommend that cervical cancer screening for all persons with a cervix end at age 65. These age restrictions were a change in guidelines updated in 2012 and endorsed by the US Preventive Services Task Force.12,13 Evidence suggests that because of high likelihood of regression and slow progression of disease, risks of screening prior to age 21 outweigh its benefits. With primary HPV testing, the age at screening debut is 25 for the same reasons.14 In people with a history of CIN 2+, active surveillance should continue for at least 25 years with HPV-based screening regardless of age. In the absence of a history of CIN 2+, however, the data to support discontinuation of screening after age 65 are less clear.
HPV positivity found to be most substantial risk for CIN 2+
In a study published this year in the Journal of Lower Genital Tract Disease, Firtina Tuncer and colleagues described their experience extending “routine screening” in patients older than 65 years.15 Data including cervical cytology, HPV test results, biopsy findings, and endocervical curettage results were collected, and abnormal findings were managed according to the 2012 and 2019 ASCCP guidelines.
When compared with negative HPV testing and normal cytology, the authors found that HPV positivity and abnormal cytology increased the risk of CIN 2+(odds ratio [OR], 136.1 and 13.1, respectively). Patients whose screening prior to age 65 had been insufficient or demonstrated CIN 2+ in the preceding 10 years were similarly more likely to have findings of CIN 2+ (OR, 9.7 when compared with HPV-negative controls).
The authors concluded that, among persons with a cervix older than age 65, previous screening and abnormal cytology were important in risk stratifications for CIN 2+; however, HPV positivity conferred the most substantial risk.
Study finds cervical dysplasia is prevalent in older populations
It has been suggested that screening for cervical cancer should continue beyond age 65 as cytology-based screening may have decreased sensitivity in older patients, which may contribute to the higher rates of advanced-stage diagnoses and cancer-related death in this population.16,17
Authors of an observational study conducted in Denmark invited persons with a cervix aged 69 and older to have one additional HPV-based screening test, and they referred them for colposcopy if HPV positive or in the presence of ASCUS or greater cytology.18 Among the 191 patients with HPV-positive results, 20% were found to have a diagnosis of CIN 2+, and 24.4% had CIN 2+ detected at another point in the study period. Notably, most patients diagnosed with CIN 2+ had no abnormalities visualized on colposcopy, and the majority of biopsies taken (65.8%) did not contain the transitional zone.
Biopsies underestimated CIN 2+ in 17.9% of cases compared with loop electrosurgical excision procedure (LEEP). These findings suggest both that high-grade cervical dysplasia is prevalent in an older population and that older populations may be susceptible to false-negative results. They also further support the use of HPV-based screening.
There are risk factors overscreening and underscreening that impact decision making regarding restricting screening to persons with a cervix younger than age 65. As more data become available, and as the population ages, it will be essential to closely examine the incidence of and trends in cervical cancer to determine appropriate patterns of screening.
Harnessing the immune system to improve survival rates in recurrent cervical cancer
Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Unfortunately, most clinical trials for recurrent or metastatic cervical cancer are negative trials or have results that show limited impact on disease outcomes. Currently, cervical cancer is treated with multiple agents, including platinum-based chemotherapy and bevacizumab, a medication that targets vascular growth. Despite these usually very effective drugs given in combination to cervical cancer patients, long-term survival remains low. Over the past few decades, many trials have been designed to help patients with this terrible disease, but few have shown significant promise.
Immune checkpoint inhibitors, such as pembrolizumab, have revolutionized care for many cancers. Checkpoint inhibitors block the proteins that cause a tumor to remain undetected by the immune system’s army of T cells. By blocking these proteins, the cancer cells can then be recognized by the immune system as foreign. Several studies have concluded that including immune checkpoint inhibitors in the comprehensive regimen for recurrent cervical cancer improves survival.
Addition of pembrolizumab increased survival
Investigators in the phase 3 double-blinded KEYNOTE-826 trial evaluated whether or not the addition of pembrolizumab to standard of care improved progression-free and overall survival in advanced, recurrent, or persistent cervical cancer.19 As part of the evaluation, the investigators measured the protein that turns off the immune system’s ability to recognize tumors, anti-programmed cell death protein-1 (PD-1).
Compared with placebo, the investigators found that, regardless of PD-1 status, the addition of pembrolizumab immunotherapy to the standard regimen increased progression-free survival and overall survival without any significantly increased adverse effects or safety concerns (FIGURE).19 At 1 year after treatment, more patients who received pembrolizumab were still alive regardless of PD-1 status, and their responses lasted longer. The most profound improvements were seen in patients whose tumors exhibited high expression of PD-L1, the target of pembrolizumab and many other immune checkpoint inhibitors.
Despite these promising results, more studies are needed to find additional therapeutic targets and treatments. Using the immune system to fight cancer represents a promising step toward the ultimate goal of cervical cancer eradication. ●
Metastatic cervical cancer can be a devastating disease that cannot be treated surgically and therefore has limited treatment options that have curative intent. Immune checkpoint inhibition via pembrolizumab opens new avenues for treatment and is a huge step forward toward the goal of cervical cancer eradication.
- US Cancer Statistics Working Group. US Cancer Statistics Data Visualizations Tool, based on 2022 submission data (1999-2020). US Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. June 2023. Accessed October 9, 2023. https://gis.cdc.gov/Cancer/USCS/#/Trends/
- Einstein MH, Zhou N, Gabor L, et al. Primary human papillomavirus testing and other new technologies for cervical cancer screening. Obstet Gynecol. September 14, 2023. doi:10.1097/AOG.0000000000005393
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2020. MMWR Morbid Mortal Weekly Rep. 2021;70:1183-1190.
- Cervical cancer elimination initiative. World Health Organization. 2023. Accessed October 10, 2023. https ://www.who.int/initiatives/cervical-cancer-eliminationinitiative#cms
- Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
- Wentzensen N, Fetterman B, Castle PE, et al. p16/Ki-67 Dual stain cytology for detection of cervical precancer in HPV-positive women. J Natl Cancer Inst. 2015;107:djv257.
- Ikenberg H, Bergeron C, Schmidt D, et al; PALMS Study Group. Screening for cervical cancer precursors with p16 /Ki-67 dual-stained cytology: results of the PALMS study. J Natl Cancer Inst. 2013;105:1550-1557.
- Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
- Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
- Wright TC Jr, Stoler MH, Behrens CM, et al. Interlaboratory variation in the performance of liquid-based cytology: insights from the ATHENA trial. Int J Cancer. 2014;134: 1835-1843.
- Wentzensen N, Lahrmann B, Clarke MA, et al. Accuracy and efficiency of deep-learning-based automation of dual stain cytology in cervical cancer screening. J Natl Cancer Inst. 2021;113:72-79.
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121:829-846.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156: 880-891, W312.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
- Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
- Hammer A, Hee L, Blaakaer J, et al. Temporal patterns of cervical cancer screening among Danish women 55 years and older diagnosed with cervical cancer. J Low Genit Tract Dis. 2018;22:1-7.
- Hammer A, Soegaard V, Maimburg RD, et al. Cervical cancer screening history prior to a diagnosis of cervical cancer in Danish women aged 60 years and older—A national cohort study. Cancer Med. 2019;8:418-427.
- Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
- US Cancer Statistics Working Group. US Cancer Statistics Data Visualizations Tool, based on 2022 submission data (1999-2020). US Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. June 2023. Accessed October 9, 2023. https://gis.cdc.gov/Cancer/USCS/#/Trends/
- Einstein MH, Zhou N, Gabor L, et al. Primary human papillomavirus testing and other new technologies for cervical cancer screening. Obstet Gynecol. September 14, 2023. doi:10.1097/AOG.0000000000005393
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2020. MMWR Morbid Mortal Weekly Rep. 2021;70:1183-1190.
- Cervical cancer elimination initiative. World Health Organization. 2023. Accessed October 10, 2023. https ://www.who.int/initiatives/cervical-cancer-eliminationinitiative#cms
- Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
- Wentzensen N, Fetterman B, Castle PE, et al. p16/Ki-67 Dual stain cytology for detection of cervical precancer in HPV-positive women. J Natl Cancer Inst. 2015;107:djv257.
- Ikenberg H, Bergeron C, Schmidt D, et al; PALMS Study Group. Screening for cervical cancer precursors with p16 /Ki-67 dual-stained cytology: results of the PALMS study. J Natl Cancer Inst. 2013;105:1550-1557.
- Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
- Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
- Wright TC Jr, Stoler MH, Behrens CM, et al. Interlaboratory variation in the performance of liquid-based cytology: insights from the ATHENA trial. Int J Cancer. 2014;134: 1835-1843.
- Wentzensen N, Lahrmann B, Clarke MA, et al. Accuracy and efficiency of deep-learning-based automation of dual stain cytology in cervical cancer screening. J Natl Cancer Inst. 2021;113:72-79.
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121:829-846.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156: 880-891, W312.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
- Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
- Hammer A, Hee L, Blaakaer J, et al. Temporal patterns of cervical cancer screening among Danish women 55 years and older diagnosed with cervical cancer. J Low Genit Tract Dis. 2018;22:1-7.
- Hammer A, Soegaard V, Maimburg RD, et al. Cervical cancer screening history prior to a diagnosis of cervical cancer in Danish women aged 60 years and older—A national cohort study. Cancer Med. 2019;8:418-427.
- Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Product Update
REVIEW
By James Greenberg, MD
Chief of Gynecology
Associate Professor, Harvard Medical School
Boston, Massachusetts
Guardenia: A “really great solution” for contained tissue extraction
The Guardenia Contained Extraction System, developed by Advanced Surgical Concepts (Wicklow, Ireland), offers a comprehensive approach to contained tissue extraction.
Background. Contained tissue extraction has been an integral part of laparoscopic procedures from at least the late 1980s and early 1990s. Bags of one sort or another are routinely used to remove from the abdomen all sorts of human tissues, including but not limited to ovaries, ectopic pregnancies, gallbladders, kidneys, spleens, and the list goes on. However, after the April 17, 2014, FDA Safety Communication discouraging the use of power morcellators with myomectomy or hysterectomy, the need for more robust contained tissue extraction systems has been ongoing, and there has yet to be a really good solution despite some innovative attempts.
Design/Functionality. Advanced Surgical Concepts’ Guardenia System is the latest attempt to provide a “really great solution” for surgeons to address my 3 “musts” for any system to gain traction in this niche.
- Must #1. An easy method for getting the device into the abdomen.
- Must #2. An easy method for getting the tissue into the bag.
- Must #3. An easy method for getting the tissue out of the body while still containing the cells within the system.
In my opinion based on my usage, Guardenia does a pretty good job addressing all my “musts.”
The Guardenia System is a sterile, single-use device with 3 main components—an introducer with a plunger that will fit through any standard 12-mm trocar, a polyurethane film bag supported by a nitinol ring, and an opening ring with expandable semi-rigid polyethylene “Guard Petals.” When I used it in the operating room to manually morcellate a 10-cm myoma through the umbilicus, I thought it was pretty spot on. Getting the bag into the abdomen is 100% intuitive but even has a nifty up-arrow built onto the tip to make sure the bag is opened in the proper direction. The bag material and the nitinol ring, in combination with the 17.5-cm opening ring, make the process of getting the specimen into the bag and exteriorized easier than any other system I have ever used. And, the opening ring with the Guard Petals yields a very large retraction area for the incision size while providing excellent protection to the surrounding tissues. Overall, Guardenia worked better than anything else I have previously used.
Innovation. Guardenia does not really introduce any fundamentally novel ideas, but it does combine a lot of standard technologies into a product whose sum is much larger than its parts. I would like to see it combined with an occlusive top piece to allow the retractor to be used for single-port laparoscopy as well, but that would just be the cherry on top.
Summary. I have been working on inventing a really good contained tissue extraction system for a long time, and I am a bit chagrined to see someone else outsmart me (low bar), but Advanced Surgical Concepts did, and I really like Guardenia. For pathology that is appropriate for contained morcellation, this device is definitely worth a try, and I suspect many surgeons will switch to it from whatever they are currently using.
FOR MORE INFORMATION, VISIT https://advancedsurgical.ie/guardenia-contained-extraction-system/
The views of the author are personal opinions and do not necessarily represent the views of
REVIEW
By James Greenberg, MD
Chief of Gynecology
Associate Professor, Harvard Medical School
Boston, Massachusetts
Guardenia: A “really great solution” for contained tissue extraction
The Guardenia Contained Extraction System, developed by Advanced Surgical Concepts (Wicklow, Ireland), offers a comprehensive approach to contained tissue extraction.
Background. Contained tissue extraction has been an integral part of laparoscopic procedures from at least the late 1980s and early 1990s. Bags of one sort or another are routinely used to remove from the abdomen all sorts of human tissues, including but not limited to ovaries, ectopic pregnancies, gallbladders, kidneys, spleens, and the list goes on. However, after the April 17, 2014, FDA Safety Communication discouraging the use of power morcellators with myomectomy or hysterectomy, the need for more robust contained tissue extraction systems has been ongoing, and there has yet to be a really good solution despite some innovative attempts.
Design/Functionality. Advanced Surgical Concepts’ Guardenia System is the latest attempt to provide a “really great solution” for surgeons to address my 3 “musts” for any system to gain traction in this niche.
- Must #1. An easy method for getting the device into the abdomen.
- Must #2. An easy method for getting the tissue into the bag.
- Must #3. An easy method for getting the tissue out of the body while still containing the cells within the system.
In my opinion based on my usage, Guardenia does a pretty good job addressing all my “musts.”
The Guardenia System is a sterile, single-use device with 3 main components—an introducer with a plunger that will fit through any standard 12-mm trocar, a polyurethane film bag supported by a nitinol ring, and an opening ring with expandable semi-rigid polyethylene “Guard Petals.” When I used it in the operating room to manually morcellate a 10-cm myoma through the umbilicus, I thought it was pretty spot on. Getting the bag into the abdomen is 100% intuitive but even has a nifty up-arrow built onto the tip to make sure the bag is opened in the proper direction. The bag material and the nitinol ring, in combination with the 17.5-cm opening ring, make the process of getting the specimen into the bag and exteriorized easier than any other system I have ever used. And, the opening ring with the Guard Petals yields a very large retraction area for the incision size while providing excellent protection to the surrounding tissues. Overall, Guardenia worked better than anything else I have previously used.
Innovation. Guardenia does not really introduce any fundamentally novel ideas, but it does combine a lot of standard technologies into a product whose sum is much larger than its parts. I would like to see it combined with an occlusive top piece to allow the retractor to be used for single-port laparoscopy as well, but that would just be the cherry on top.
Summary. I have been working on inventing a really good contained tissue extraction system for a long time, and I am a bit chagrined to see someone else outsmart me (low bar), but Advanced Surgical Concepts did, and I really like Guardenia. For pathology that is appropriate for contained morcellation, this device is definitely worth a try, and I suspect many surgeons will switch to it from whatever they are currently using.
FOR MORE INFORMATION, VISIT https://advancedsurgical.ie/guardenia-contained-extraction-system/
The views of the author are personal opinions and do not necessarily represent the views of
REVIEW
By James Greenberg, MD
Chief of Gynecology
Associate Professor, Harvard Medical School
Boston, Massachusetts
Guardenia: A “really great solution” for contained tissue extraction
The Guardenia Contained Extraction System, developed by Advanced Surgical Concepts (Wicklow, Ireland), offers a comprehensive approach to contained tissue extraction.
Background. Contained tissue extraction has been an integral part of laparoscopic procedures from at least the late 1980s and early 1990s. Bags of one sort or another are routinely used to remove from the abdomen all sorts of human tissues, including but not limited to ovaries, ectopic pregnancies, gallbladders, kidneys, spleens, and the list goes on. However, after the April 17, 2014, FDA Safety Communication discouraging the use of power morcellators with myomectomy or hysterectomy, the need for more robust contained tissue extraction systems has been ongoing, and there has yet to be a really good solution despite some innovative attempts.
Design/Functionality. Advanced Surgical Concepts’ Guardenia System is the latest attempt to provide a “really great solution” for surgeons to address my 3 “musts” for any system to gain traction in this niche.
- Must #1. An easy method for getting the device into the abdomen.
- Must #2. An easy method for getting the tissue into the bag.
- Must #3. An easy method for getting the tissue out of the body while still containing the cells within the system.
In my opinion based on my usage, Guardenia does a pretty good job addressing all my “musts.”
The Guardenia System is a sterile, single-use device with 3 main components—an introducer with a plunger that will fit through any standard 12-mm trocar, a polyurethane film bag supported by a nitinol ring, and an opening ring with expandable semi-rigid polyethylene “Guard Petals.” When I used it in the operating room to manually morcellate a 10-cm myoma through the umbilicus, I thought it was pretty spot on. Getting the bag into the abdomen is 100% intuitive but even has a nifty up-arrow built onto the tip to make sure the bag is opened in the proper direction. The bag material and the nitinol ring, in combination with the 17.5-cm opening ring, make the process of getting the specimen into the bag and exteriorized easier than any other system I have ever used. And, the opening ring with the Guard Petals yields a very large retraction area for the incision size while providing excellent protection to the surrounding tissues. Overall, Guardenia worked better than anything else I have previously used.
Innovation. Guardenia does not really introduce any fundamentally novel ideas, but it does combine a lot of standard technologies into a product whose sum is much larger than its parts. I would like to see it combined with an occlusive top piece to allow the retractor to be used for single-port laparoscopy as well, but that would just be the cherry on top.
Summary. I have been working on inventing a really good contained tissue extraction system for a long time, and I am a bit chagrined to see someone else outsmart me (low bar), but Advanced Surgical Concepts did, and I really like Guardenia. For pathology that is appropriate for contained morcellation, this device is definitely worth a try, and I suspect many surgeons will switch to it from whatever they are currently using.
FOR MORE INFORMATION, VISIT https://advancedsurgical.ie/guardenia-contained-extraction-system/
The views of the author are personal opinions and do not necessarily represent the views of
TNF blockers not associated with poorer pregnancy outcomes
SAN DIEGO – Continuing a tumor necrosis factor inhibitor (TNFi) during pregnancy does not increase risk of worse fetal or obstetric outcomes, according to new research presented at the annual meeting of the American College of Rheumatology.
Patients who continued a TNFi also had fewer severe infections requiring hospitalization, compared with those who stopped taking the medication during their pregnancy.
“The main message is that patients continuing were not doing worse than the patients stopping. It’s an important clinical message for rheumatologists who are not really confident in dealing with these drugs during pregnancy,” said Anna Moltó, MD, PhD, a rheumatologist at Cochin Hospital, Paris, who led the research. “It adds to the data that it seems to be safe,” she added in an interview.
Previous research, largely from pregnant patients with inflammatory bowel disease, suggests that taking a TNFi during pregnancy is safe, and 2020 ACR guidelines conditionally recommend continuing therapy prior to and during pregnancy; however, many people still stop taking the drugs during pregnancy for fear of potentially harming the fetus.
To better understand how TNFi use affected pregnancy outcomes, Dr. Moltó and colleagues analyzed data from a French nationwide health insurance database to identify adult women with chronic rheumatic inflammatory disease. All women included in the cohort had a singleton pregnancy between 2008 and 2017 and were taking a TNFi upon pregnancy diagnosis.
Patients who restarted TNFi after initially pausing because of pregnancy were included in the continuation group.
Researchers identified more than 2,000 pregnancies, including 1,503 in individuals with spondyloarthritis and 579 individuals with rheumatoid arthritis. Patients were, on average, 31 years old and were diagnosed with a rheumatic disease 4 years prior to their pregnancy.
About 72% (n = 1,497) discontinued TNFi after learning they were pregnant, and 584 individuals continued treatment. Dr. Moltó noted that data from more recent years might have captured lower discontinuation rates among pregnant individuals, but those data were not available for the study.
There was no difference in unfavorable obstetrical or infant outcomes, including spontaneous abortion, preeclampsia, gestational diabetes, major congenital malformation, and severe infection of the infant requiring hospitalization. Somewhat surprisingly, the data showed that women who discontinued a TNFi were more likely to be hospitalized for infection either during their pregnancy or up to 6 weeks after delivery, compared with those who continued therapy (1.3% vs. 0.2%, respectively).
Dr. Moltó is currently looking into what could be behind this counterintuitive result, but she hypothesizes that patients who had stopped TNFi may have been taking more glucocorticoids.
“At our institution, there is generally a comfort level with continuing TNF inhibitors during pregnancy, at least until about 36 weeks,” said Sara K. Tedeschi, MD, MPH, a rheumatologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Sometimes, there is concern for risk of infection to the infant, depending on the type of TNFi being used, she added during a press conference.
“I think that these are really informative and supportive data to let women know that they probably have a really good chance of doing very well during the pregnancy if they continue” their TNFi, said Dr. Tedeschi, who was not involved with the study.
TNF discontinuation on the decline
In a related study, researchers at McGill University, Montreal, found that TNFi discontinuation prior to pregnancy had decreased over time in individuals with chronic inflammatory diseases.
Using a database of U.S. insurance claims, they identified 3,372 women with RA, ankylosing spondylitis (AS), psoriasis/psoriatic arthritis (PsA), and/or inflammatory bowel disease (IBD) who previously used a TNFi and gave birth between 2011 and 2019. A patient was considered to have used a TNFi if she had filled a prescription or had an infusion procedure insurance claim within 12 weeks before the gestational period or anytime during pregnancy. Researchers did not have time-specific data to account for women who stopped treatment at pregnancy diagnosis.
Nearly half (47%) of all identified pregnancies were in individuals with IBD, and the rest included patients with RA (24%), psoriasis or PsA (16%), AS (3%), or more than one diagnosis (10%).
In total, 14% of women discontinued TNFi use in the 12 weeks before becoming pregnant and did not restart. From 2011 to 2013, 19% of patients stopped their TNFi, but this proportion decreased overtime, with 10% of patients stopping therapy from 2017 to 2019 (P < .0001).
This decline “possibly reflects the increase in real-world evidence about the safety of TNFi in pregnancy. That research, in turn, led to new guidelines recommending the continuation of TNFi during pregnancy,” first author Leah Flatman, a PhD candidate in epidemiology at McGill, said in an interview. “I think we can see this potentially as good news.”
More patients with RA, psoriasis/PsA, and AS discontinued TNFi therapy prior to conception (23%-25%), compared with those with IBD (5%).
Ms. Flatman noted that her study and Moltó’s study complement each other by providing data on individuals stopping TNFi prior to conception versus those stopping treatment after pregnancy diagnosis.
“These findings demonstrate that continuing TNFi during pregnancy appears not to be associated with an increase in adverse obstetrical or infant outcomes,” Ms. Flatman said of Dr. Moltó’s study. “As guidelines currently recommend continuing TNFi, studies like this help demonstrate that the guideline changes do not appear to be associated with an increase in adverse events.”
Dr. Moltó and Ms. Flatman disclosed no relevant financial relationships. Dr. Tedeschi has worked as a consultant for Novartis.
A version of this article appeared on Medscape.com.
SAN DIEGO – Continuing a tumor necrosis factor inhibitor (TNFi) during pregnancy does not increase risk of worse fetal or obstetric outcomes, according to new research presented at the annual meeting of the American College of Rheumatology.
Patients who continued a TNFi also had fewer severe infections requiring hospitalization, compared with those who stopped taking the medication during their pregnancy.
“The main message is that patients continuing were not doing worse than the patients stopping. It’s an important clinical message for rheumatologists who are not really confident in dealing with these drugs during pregnancy,” said Anna Moltó, MD, PhD, a rheumatologist at Cochin Hospital, Paris, who led the research. “It adds to the data that it seems to be safe,” she added in an interview.
Previous research, largely from pregnant patients with inflammatory bowel disease, suggests that taking a TNFi during pregnancy is safe, and 2020 ACR guidelines conditionally recommend continuing therapy prior to and during pregnancy; however, many people still stop taking the drugs during pregnancy for fear of potentially harming the fetus.
To better understand how TNFi use affected pregnancy outcomes, Dr. Moltó and colleagues analyzed data from a French nationwide health insurance database to identify adult women with chronic rheumatic inflammatory disease. All women included in the cohort had a singleton pregnancy between 2008 and 2017 and were taking a TNFi upon pregnancy diagnosis.
Patients who restarted TNFi after initially pausing because of pregnancy were included in the continuation group.
Researchers identified more than 2,000 pregnancies, including 1,503 in individuals with spondyloarthritis and 579 individuals with rheumatoid arthritis. Patients were, on average, 31 years old and were diagnosed with a rheumatic disease 4 years prior to their pregnancy.
About 72% (n = 1,497) discontinued TNFi after learning they were pregnant, and 584 individuals continued treatment. Dr. Moltó noted that data from more recent years might have captured lower discontinuation rates among pregnant individuals, but those data were not available for the study.
There was no difference in unfavorable obstetrical or infant outcomes, including spontaneous abortion, preeclampsia, gestational diabetes, major congenital malformation, and severe infection of the infant requiring hospitalization. Somewhat surprisingly, the data showed that women who discontinued a TNFi were more likely to be hospitalized for infection either during their pregnancy or up to 6 weeks after delivery, compared with those who continued therapy (1.3% vs. 0.2%, respectively).
Dr. Moltó is currently looking into what could be behind this counterintuitive result, but she hypothesizes that patients who had stopped TNFi may have been taking more glucocorticoids.
“At our institution, there is generally a comfort level with continuing TNF inhibitors during pregnancy, at least until about 36 weeks,” said Sara K. Tedeschi, MD, MPH, a rheumatologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Sometimes, there is concern for risk of infection to the infant, depending on the type of TNFi being used, she added during a press conference.
“I think that these are really informative and supportive data to let women know that they probably have a really good chance of doing very well during the pregnancy if they continue” their TNFi, said Dr. Tedeschi, who was not involved with the study.
TNF discontinuation on the decline
In a related study, researchers at McGill University, Montreal, found that TNFi discontinuation prior to pregnancy had decreased over time in individuals with chronic inflammatory diseases.
Using a database of U.S. insurance claims, they identified 3,372 women with RA, ankylosing spondylitis (AS), psoriasis/psoriatic arthritis (PsA), and/or inflammatory bowel disease (IBD) who previously used a TNFi and gave birth between 2011 and 2019. A patient was considered to have used a TNFi if she had filled a prescription or had an infusion procedure insurance claim within 12 weeks before the gestational period or anytime during pregnancy. Researchers did not have time-specific data to account for women who stopped treatment at pregnancy diagnosis.
Nearly half (47%) of all identified pregnancies were in individuals with IBD, and the rest included patients with RA (24%), psoriasis or PsA (16%), AS (3%), or more than one diagnosis (10%).
In total, 14% of women discontinued TNFi use in the 12 weeks before becoming pregnant and did not restart. From 2011 to 2013, 19% of patients stopped their TNFi, but this proportion decreased overtime, with 10% of patients stopping therapy from 2017 to 2019 (P < .0001).
This decline “possibly reflects the increase in real-world evidence about the safety of TNFi in pregnancy. That research, in turn, led to new guidelines recommending the continuation of TNFi during pregnancy,” first author Leah Flatman, a PhD candidate in epidemiology at McGill, said in an interview. “I think we can see this potentially as good news.”
More patients with RA, psoriasis/PsA, and AS discontinued TNFi therapy prior to conception (23%-25%), compared with those with IBD (5%).
Ms. Flatman noted that her study and Moltó’s study complement each other by providing data on individuals stopping TNFi prior to conception versus those stopping treatment after pregnancy diagnosis.
“These findings demonstrate that continuing TNFi during pregnancy appears not to be associated with an increase in adverse obstetrical or infant outcomes,” Ms. Flatman said of Dr. Moltó’s study. “As guidelines currently recommend continuing TNFi, studies like this help demonstrate that the guideline changes do not appear to be associated with an increase in adverse events.”
Dr. Moltó and Ms. Flatman disclosed no relevant financial relationships. Dr. Tedeschi has worked as a consultant for Novartis.
A version of this article appeared on Medscape.com.
SAN DIEGO – Continuing a tumor necrosis factor inhibitor (TNFi) during pregnancy does not increase risk of worse fetal or obstetric outcomes, according to new research presented at the annual meeting of the American College of Rheumatology.
Patients who continued a TNFi also had fewer severe infections requiring hospitalization, compared with those who stopped taking the medication during their pregnancy.
“The main message is that patients continuing were not doing worse than the patients stopping. It’s an important clinical message for rheumatologists who are not really confident in dealing with these drugs during pregnancy,” said Anna Moltó, MD, PhD, a rheumatologist at Cochin Hospital, Paris, who led the research. “It adds to the data that it seems to be safe,” she added in an interview.
Previous research, largely from pregnant patients with inflammatory bowel disease, suggests that taking a TNFi during pregnancy is safe, and 2020 ACR guidelines conditionally recommend continuing therapy prior to and during pregnancy; however, many people still stop taking the drugs during pregnancy for fear of potentially harming the fetus.
To better understand how TNFi use affected pregnancy outcomes, Dr. Moltó and colleagues analyzed data from a French nationwide health insurance database to identify adult women with chronic rheumatic inflammatory disease. All women included in the cohort had a singleton pregnancy between 2008 and 2017 and were taking a TNFi upon pregnancy diagnosis.
Patients who restarted TNFi after initially pausing because of pregnancy were included in the continuation group.
Researchers identified more than 2,000 pregnancies, including 1,503 in individuals with spondyloarthritis and 579 individuals with rheumatoid arthritis. Patients were, on average, 31 years old and were diagnosed with a rheumatic disease 4 years prior to their pregnancy.
About 72% (n = 1,497) discontinued TNFi after learning they were pregnant, and 584 individuals continued treatment. Dr. Moltó noted that data from more recent years might have captured lower discontinuation rates among pregnant individuals, but those data were not available for the study.
There was no difference in unfavorable obstetrical or infant outcomes, including spontaneous abortion, preeclampsia, gestational diabetes, major congenital malformation, and severe infection of the infant requiring hospitalization. Somewhat surprisingly, the data showed that women who discontinued a TNFi were more likely to be hospitalized for infection either during their pregnancy or up to 6 weeks after delivery, compared with those who continued therapy (1.3% vs. 0.2%, respectively).
Dr. Moltó is currently looking into what could be behind this counterintuitive result, but she hypothesizes that patients who had stopped TNFi may have been taking more glucocorticoids.
“At our institution, there is generally a comfort level with continuing TNF inhibitors during pregnancy, at least until about 36 weeks,” said Sara K. Tedeschi, MD, MPH, a rheumatologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Sometimes, there is concern for risk of infection to the infant, depending on the type of TNFi being used, she added during a press conference.
“I think that these are really informative and supportive data to let women know that they probably have a really good chance of doing very well during the pregnancy if they continue” their TNFi, said Dr. Tedeschi, who was not involved with the study.
TNF discontinuation on the decline
In a related study, researchers at McGill University, Montreal, found that TNFi discontinuation prior to pregnancy had decreased over time in individuals with chronic inflammatory diseases.
Using a database of U.S. insurance claims, they identified 3,372 women with RA, ankylosing spondylitis (AS), psoriasis/psoriatic arthritis (PsA), and/or inflammatory bowel disease (IBD) who previously used a TNFi and gave birth between 2011 and 2019. A patient was considered to have used a TNFi if she had filled a prescription or had an infusion procedure insurance claim within 12 weeks before the gestational period or anytime during pregnancy. Researchers did not have time-specific data to account for women who stopped treatment at pregnancy diagnosis.
Nearly half (47%) of all identified pregnancies were in individuals with IBD, and the rest included patients with RA (24%), psoriasis or PsA (16%), AS (3%), or more than one diagnosis (10%).
In total, 14% of women discontinued TNFi use in the 12 weeks before becoming pregnant and did not restart. From 2011 to 2013, 19% of patients stopped their TNFi, but this proportion decreased overtime, with 10% of patients stopping therapy from 2017 to 2019 (P < .0001).
This decline “possibly reflects the increase in real-world evidence about the safety of TNFi in pregnancy. That research, in turn, led to new guidelines recommending the continuation of TNFi during pregnancy,” first author Leah Flatman, a PhD candidate in epidemiology at McGill, said in an interview. “I think we can see this potentially as good news.”
More patients with RA, psoriasis/PsA, and AS discontinued TNFi therapy prior to conception (23%-25%), compared with those with IBD (5%).
Ms. Flatman noted that her study and Moltó’s study complement each other by providing data on individuals stopping TNFi prior to conception versus those stopping treatment after pregnancy diagnosis.
“These findings demonstrate that continuing TNFi during pregnancy appears not to be associated with an increase in adverse obstetrical or infant outcomes,” Ms. Flatman said of Dr. Moltó’s study. “As guidelines currently recommend continuing TNFi, studies like this help demonstrate that the guideline changes do not appear to be associated with an increase in adverse events.”
Dr. Moltó and Ms. Flatman disclosed no relevant financial relationships. Dr. Tedeschi has worked as a consultant for Novartis.
A version of this article appeared on Medscape.com.
AT ACR 2023
A nurse’s view: Women desperately need information about pelvic floor disorders
Pelvic floor disorders are embarrassing, annoying, painful, and extremely disruptive to a woman’s life, often resulting in depression, anxiety, and a poor self-image. According to a 2021 study, approximately 75% of peripartum women and 68% of postmenopausal women feel insufficiently informed about pelvic floor disorders.1
Consequently, a large majority of women are not seeking care for these disorders. This drives health care costs higher as women wait until their symptoms are unbearable until finally seeking help. Many of these women don’t know they have options.
Who is at risk?
To understand the scope of this growing problem, it is vital to see who is most at risk. Parity, age, body mass index, and race are significant factors, although any woman can have a pelvic floor disorder (PFD).
Urinary incontinence (UI), pelvic floor prolapses (POP), and fecal incontinence (FI) are three of the most common pelvic floor disorders. Pregnancy and childbirth, specifically a vaginal birth, greatly contribute to this population’s risk. In pregnancy, the increase in plasma volume and glomerular filtration rate, along with hormone changes impacting urethral pressure and the growing gravid uterus, cause urinary frequency and nocturia. This can result in urinary incontinence during and after pregnancy.
Indeed, 76% of women with urinary incontinence at 3 months postpartum report it 12 years later.1 Third- and fourth-degree lacerations during delivery are uncommon (3.3%), but can cause fecal incontinence, often requiring surgery.1 Independently, all of these symptoms have been correlated with sexual dysfunction and postpartum depression.
One-third of all women and 50% of women over the age of 55 are currently affected by a PFD. Contributing factors include hormone changes with menopause that affect the pelvic floor muscles and connective tissue, prior childbirth and pregnancy, constipation, heavy lifting, prior pelvic surgery, and obesity. These women are vulnerable to pelvic organ prolapse from the weakened pelvic floor muscles. They will often present with a vague complaint of “something is protruding out of my vagina.” These women also present with urinary incontinence or leakage, proclaiming they have to wear a diaper or a pad. Without proper knowledge, aging women think these issues are normal and nothing can be done.
The woman with a BMI above 30 may have damaged tissues supporting the uterus and bladder, weakening those organs, and causing a prolapse. Incontinence is a result of poor muscle and connective tissue of the vagina that support the urethra. Obese women can suffer from both urinary and bowel incontinence. By the year 2030, it is projected that one in two adults will be obese.2 This will greatly impact health care costs.
To date, there is little conclusive evidence on the impact of race on pelvic floor disorders. A study in Scientific Reports did find that Asian women have a significantly lower risk for any PFD.2 Some research has found that Black and Hispanic women have less risk for UI but are at higher risk for FI and other PFDs.3 Understandably, women of certain cultures and demographics may be less likely to report incontinence to their clinicians and may be less informed as well.
What can we do?
The American College of Obstetricians and Gynecologists (ACOG) has acknowledged the deficiencies and lack of standard care of pelvic health in pregnancy and postpartum.1 There are differences in definitions across clinical practice and in the medical literature. Inconsistent patient reporting of PFD symptoms occurs due to nonstandard methods (questionnaire, interview, physical exam). With the often-short time allotted for visits with health care providers, women may neglect to discuss their symptoms, especially if they have other more pressing matters to address.
At the first OB appointment, a pregnant woman should be given information on what are normal and abnormal symptoms, from the beginning through postpartum. At each visit, she should be given ample opportunity to discuss symptoms of pelvic health. Clinicians should continue assessing, questioning, and discussing treatment options as applicable. Women need to know that early recognition and treatment can have a positive affect on their pelvic health for years to come.
ACOG recommends all postpartum patients see an obstetric provider within 3 weeks of delivery.1 Most are seen at 6 weeks. Pelvic health should be discussed at this final postpartum appointment, including normal and abnormal symptoms within the next few months and beyond.
Regardless of pregnancy status, women need a safe and supportive place to describe their pelvic floor issues. There is a validated questionnaire tool available for postpartum, but one is desperately needed for all women, especially women at risk. A pelvic health assessment must be included in every annual exam.
Women need to know there are multiple treatment modalities including simple exercises, physical therapy, a variety of pessaries, medications, and surgery. Sometimes, all that is needed are a few lifestyle changes: avoiding pushing or straining while urinating or having a bowel movement, maintaining a healthy diet rich in high fiber foods, and drinking plenty of fluids.
The National Public Health Service in the United Kingdom recently announced a government-funded program for pelvic health services to begin in April 2024.4 This program will address the pelvic floor needs, assessment, education and treatment for women after childbirth.
There are multiple clinics in the United States focusing on women’s health that feature urogynecologists – specialists in pelvic floor disorders. These specialists do a thorough health and physical assessment, explain types of pelvic floor disorders, and suggest appropriate treatment options. Most importantly, urogynecologists listen and address a woman’s concerns and fears.
There is no reason for women to feel compromised at any age. We, as health care providers, just need to assess, educate, treat, and follow up.
Ms. Barnett is a registered nurse in the department of obstetrics, Mills-Peninsula Medical Center, Burlingame, Calif. She has disclosed no relevant financial relationships.
References
1. Madsen AM et al. Recognition and management of pelvic floor disorders in pregnancy and the postpartum period. Obstet Gynecol Clin North Am. 2021 Sep;48(3):571-84. doi: 10.1016/j.ogc.2021.05.009.
2. Kenne KA et al. Prevalence of pelvic floor disorders in adult women being seen in a primary care setting and associated risk factors. Sci Rep. 2022 June; (12):9878. doi: 10.1038/s41598-022-13501-w.
3. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311-6. doi: 10.1001/jama.300.11.1311.
4. United Kingdom Department of Health and Social Care. “National pelvic health service to support women.” 2023 Oct 19.
Pelvic floor disorders are embarrassing, annoying, painful, and extremely disruptive to a woman’s life, often resulting in depression, anxiety, and a poor self-image. According to a 2021 study, approximately 75% of peripartum women and 68% of postmenopausal women feel insufficiently informed about pelvic floor disorders.1
Consequently, a large majority of women are not seeking care for these disorders. This drives health care costs higher as women wait until their symptoms are unbearable until finally seeking help. Many of these women don’t know they have options.
Who is at risk?
To understand the scope of this growing problem, it is vital to see who is most at risk. Parity, age, body mass index, and race are significant factors, although any woman can have a pelvic floor disorder (PFD).
Urinary incontinence (UI), pelvic floor prolapses (POP), and fecal incontinence (FI) are three of the most common pelvic floor disorders. Pregnancy and childbirth, specifically a vaginal birth, greatly contribute to this population’s risk. In pregnancy, the increase in plasma volume and glomerular filtration rate, along with hormone changes impacting urethral pressure and the growing gravid uterus, cause urinary frequency and nocturia. This can result in urinary incontinence during and after pregnancy.
Indeed, 76% of women with urinary incontinence at 3 months postpartum report it 12 years later.1 Third- and fourth-degree lacerations during delivery are uncommon (3.3%), but can cause fecal incontinence, often requiring surgery.1 Independently, all of these symptoms have been correlated with sexual dysfunction and postpartum depression.
One-third of all women and 50% of women over the age of 55 are currently affected by a PFD. Contributing factors include hormone changes with menopause that affect the pelvic floor muscles and connective tissue, prior childbirth and pregnancy, constipation, heavy lifting, prior pelvic surgery, and obesity. These women are vulnerable to pelvic organ prolapse from the weakened pelvic floor muscles. They will often present with a vague complaint of “something is protruding out of my vagina.” These women also present with urinary incontinence or leakage, proclaiming they have to wear a diaper or a pad. Without proper knowledge, aging women think these issues are normal and nothing can be done.
The woman with a BMI above 30 may have damaged tissues supporting the uterus and bladder, weakening those organs, and causing a prolapse. Incontinence is a result of poor muscle and connective tissue of the vagina that support the urethra. Obese women can suffer from both urinary and bowel incontinence. By the year 2030, it is projected that one in two adults will be obese.2 This will greatly impact health care costs.
To date, there is little conclusive evidence on the impact of race on pelvic floor disorders. A study in Scientific Reports did find that Asian women have a significantly lower risk for any PFD.2 Some research has found that Black and Hispanic women have less risk for UI but are at higher risk for FI and other PFDs.3 Understandably, women of certain cultures and demographics may be less likely to report incontinence to their clinicians and may be less informed as well.
What can we do?
The American College of Obstetricians and Gynecologists (ACOG) has acknowledged the deficiencies and lack of standard care of pelvic health in pregnancy and postpartum.1 There are differences in definitions across clinical practice and in the medical literature. Inconsistent patient reporting of PFD symptoms occurs due to nonstandard methods (questionnaire, interview, physical exam). With the often-short time allotted for visits with health care providers, women may neglect to discuss their symptoms, especially if they have other more pressing matters to address.
At the first OB appointment, a pregnant woman should be given information on what are normal and abnormal symptoms, from the beginning through postpartum. At each visit, she should be given ample opportunity to discuss symptoms of pelvic health. Clinicians should continue assessing, questioning, and discussing treatment options as applicable. Women need to know that early recognition and treatment can have a positive affect on their pelvic health for years to come.
ACOG recommends all postpartum patients see an obstetric provider within 3 weeks of delivery.1 Most are seen at 6 weeks. Pelvic health should be discussed at this final postpartum appointment, including normal and abnormal symptoms within the next few months and beyond.
Regardless of pregnancy status, women need a safe and supportive place to describe their pelvic floor issues. There is a validated questionnaire tool available for postpartum, but one is desperately needed for all women, especially women at risk. A pelvic health assessment must be included in every annual exam.
Women need to know there are multiple treatment modalities including simple exercises, physical therapy, a variety of pessaries, medications, and surgery. Sometimes, all that is needed are a few lifestyle changes: avoiding pushing or straining while urinating or having a bowel movement, maintaining a healthy diet rich in high fiber foods, and drinking plenty of fluids.
The National Public Health Service in the United Kingdom recently announced a government-funded program for pelvic health services to begin in April 2024.4 This program will address the pelvic floor needs, assessment, education and treatment for women after childbirth.
There are multiple clinics in the United States focusing on women’s health that feature urogynecologists – specialists in pelvic floor disorders. These specialists do a thorough health and physical assessment, explain types of pelvic floor disorders, and suggest appropriate treatment options. Most importantly, urogynecologists listen and address a woman’s concerns and fears.
There is no reason for women to feel compromised at any age. We, as health care providers, just need to assess, educate, treat, and follow up.
Ms. Barnett is a registered nurse in the department of obstetrics, Mills-Peninsula Medical Center, Burlingame, Calif. She has disclosed no relevant financial relationships.
References
1. Madsen AM et al. Recognition and management of pelvic floor disorders in pregnancy and the postpartum period. Obstet Gynecol Clin North Am. 2021 Sep;48(3):571-84. doi: 10.1016/j.ogc.2021.05.009.
2. Kenne KA et al. Prevalence of pelvic floor disorders in adult women being seen in a primary care setting and associated risk factors. Sci Rep. 2022 June; (12):9878. doi: 10.1038/s41598-022-13501-w.
3. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311-6. doi: 10.1001/jama.300.11.1311.
4. United Kingdom Department of Health and Social Care. “National pelvic health service to support women.” 2023 Oct 19.
Pelvic floor disorders are embarrassing, annoying, painful, and extremely disruptive to a woman’s life, often resulting in depression, anxiety, and a poor self-image. According to a 2021 study, approximately 75% of peripartum women and 68% of postmenopausal women feel insufficiently informed about pelvic floor disorders.1
Consequently, a large majority of women are not seeking care for these disorders. This drives health care costs higher as women wait until their symptoms are unbearable until finally seeking help. Many of these women don’t know they have options.
Who is at risk?
To understand the scope of this growing problem, it is vital to see who is most at risk. Parity, age, body mass index, and race are significant factors, although any woman can have a pelvic floor disorder (PFD).
Urinary incontinence (UI), pelvic floor prolapses (POP), and fecal incontinence (FI) are three of the most common pelvic floor disorders. Pregnancy and childbirth, specifically a vaginal birth, greatly contribute to this population’s risk. In pregnancy, the increase in plasma volume and glomerular filtration rate, along with hormone changes impacting urethral pressure and the growing gravid uterus, cause urinary frequency and nocturia. This can result in urinary incontinence during and after pregnancy.
Indeed, 76% of women with urinary incontinence at 3 months postpartum report it 12 years later.1 Third- and fourth-degree lacerations during delivery are uncommon (3.3%), but can cause fecal incontinence, often requiring surgery.1 Independently, all of these symptoms have been correlated with sexual dysfunction and postpartum depression.
One-third of all women and 50% of women over the age of 55 are currently affected by a PFD. Contributing factors include hormone changes with menopause that affect the pelvic floor muscles and connective tissue, prior childbirth and pregnancy, constipation, heavy lifting, prior pelvic surgery, and obesity. These women are vulnerable to pelvic organ prolapse from the weakened pelvic floor muscles. They will often present with a vague complaint of “something is protruding out of my vagina.” These women also present with urinary incontinence or leakage, proclaiming they have to wear a diaper or a pad. Without proper knowledge, aging women think these issues are normal and nothing can be done.
The woman with a BMI above 30 may have damaged tissues supporting the uterus and bladder, weakening those organs, and causing a prolapse. Incontinence is a result of poor muscle and connective tissue of the vagina that support the urethra. Obese women can suffer from both urinary and bowel incontinence. By the year 2030, it is projected that one in two adults will be obese.2 This will greatly impact health care costs.
To date, there is little conclusive evidence on the impact of race on pelvic floor disorders. A study in Scientific Reports did find that Asian women have a significantly lower risk for any PFD.2 Some research has found that Black and Hispanic women have less risk for UI but are at higher risk for FI and other PFDs.3 Understandably, women of certain cultures and demographics may be less likely to report incontinence to their clinicians and may be less informed as well.
What can we do?
The American College of Obstetricians and Gynecologists (ACOG) has acknowledged the deficiencies and lack of standard care of pelvic health in pregnancy and postpartum.1 There are differences in definitions across clinical practice and in the medical literature. Inconsistent patient reporting of PFD symptoms occurs due to nonstandard methods (questionnaire, interview, physical exam). With the often-short time allotted for visits with health care providers, women may neglect to discuss their symptoms, especially if they have other more pressing matters to address.
At the first OB appointment, a pregnant woman should be given information on what are normal and abnormal symptoms, from the beginning through postpartum. At each visit, she should be given ample opportunity to discuss symptoms of pelvic health. Clinicians should continue assessing, questioning, and discussing treatment options as applicable. Women need to know that early recognition and treatment can have a positive affect on their pelvic health for years to come.
ACOG recommends all postpartum patients see an obstetric provider within 3 weeks of delivery.1 Most are seen at 6 weeks. Pelvic health should be discussed at this final postpartum appointment, including normal and abnormal symptoms within the next few months and beyond.
Regardless of pregnancy status, women need a safe and supportive place to describe their pelvic floor issues. There is a validated questionnaire tool available for postpartum, but one is desperately needed for all women, especially women at risk. A pelvic health assessment must be included in every annual exam.
Women need to know there are multiple treatment modalities including simple exercises, physical therapy, a variety of pessaries, medications, and surgery. Sometimes, all that is needed are a few lifestyle changes: avoiding pushing or straining while urinating or having a bowel movement, maintaining a healthy diet rich in high fiber foods, and drinking plenty of fluids.
The National Public Health Service in the United Kingdom recently announced a government-funded program for pelvic health services to begin in April 2024.4 This program will address the pelvic floor needs, assessment, education and treatment for women after childbirth.
There are multiple clinics in the United States focusing on women’s health that feature urogynecologists – specialists in pelvic floor disorders. These specialists do a thorough health and physical assessment, explain types of pelvic floor disorders, and suggest appropriate treatment options. Most importantly, urogynecologists listen and address a woman’s concerns and fears.
There is no reason for women to feel compromised at any age. We, as health care providers, just need to assess, educate, treat, and follow up.
Ms. Barnett is a registered nurse in the department of obstetrics, Mills-Peninsula Medical Center, Burlingame, Calif. She has disclosed no relevant financial relationships.
References
1. Madsen AM et al. Recognition and management of pelvic floor disorders in pregnancy and the postpartum period. Obstet Gynecol Clin North Am. 2021 Sep;48(3):571-84. doi: 10.1016/j.ogc.2021.05.009.
2. Kenne KA et al. Prevalence of pelvic floor disorders in adult women being seen in a primary care setting and associated risk factors. Sci Rep. 2022 June; (12):9878. doi: 10.1038/s41598-022-13501-w.
3. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311-6. doi: 10.1001/jama.300.11.1311.
4. United Kingdom Department of Health and Social Care. “National pelvic health service to support women.” 2023 Oct 19.
Pregnancy in rheumatic disease quadruples risk of cardiovascular events
SAN DIEGO – Pregnant individuals with autoimmune rheumatic diseases (ARDs) are at least four times more likely to experience an acute cardiovascular event (CVE) than are pregnant individuals without these conditions, according to new research presented at the annual meeting of the American College of Rheumatology. Pregnant individuals with primary antiphospholipid syndrome (APS) had a 15-fold increase in CVE risk.
Patients who experienced CVEs were also more likely to experience preterm birth and other adverse pregnancy outcomes (APOs).
Rashmi Dhital, MD, a rheumatology fellow at the University of California, San Diego, and colleagues examined the medical records of pregnant individuals in California who had delivered singleton live-born infants from 2005 to 2020. Using data from the Study of Outcomes in Mothers and Infants (SOMI) database, an administrative population-based birth cohort in California, they identified more than 7 million individuals, 19,340 with ARDs and 7,758 with APS.
They then analyzed how many patients experienced an acute CVE during pregnancy and up to 6 weeks after giving birth.
CVEs occurred in 2.0% of patients with ARDs, 6.9% of individuals with APS, and 0.4% of women without these conditions. CVE risk was four times higher in the ARDs group (adjusted relative risk, 4.1; 95% confidence interval, 3.7-4.5) and nearly 15 times higher in the APS group (aRR, 14.7; 95% CI, 13.5-16.0) than in the comparison group. Patients with systemic lupus erythematosus (SLE) had a sixfold higher risk of CVE, which was further exacerbated by concomitant APS (18-fold higher risk) or lupus nephritis (15-fold higher risk).
Dr. Dhital also classified CVEs as either venous thromboembolism and non-VTE events. Pregnant patients with APS had a high risk for VTE-only CVE (40-fold greater) and a 3.7-fold higher risk of non-VTE events, compared with pregnant patients without these conditions. Patients with SLE along with lupus nephritis had a 20-fold increased risk of VTE-only CVE and an 11-fold higher risk of non-VTE CVE.
Although the study grouped rheumatic diseases together, “lupus is generally driving these results,” Sharon Kolasinski, MD, of the University of Pennsylvania, Philadelphia, noted in an interview. She moderated the plenary session where the research was presented. “If you take out lupus, then what is the risk? That would be an interesting question.”
Between 25% and 30% of all CVEs occurred in the postpartum period, highlighting the importance of close monitoring of cardiovascular risks and events in women with ARDs or APS both during pregnancy and postpartum, Dr. Dhital noted.
Recognizing these risks “can sometimes be challenging due to a lower suspicion of CVE in younger patients, and also symptoms overlap with normal pregnancy,” Dr. Dhital said during her plenary presentation. Working with other clinical teams could help physicians detect these risks in patients.
“It’s important for us to remember that there’s increased risk of cardiovascular events in pregnancy in our patients. It’s uncommon, but it’s not zero,” added Dr. Kolasinski, and this study highlighted when physicians should be more focused about that risk.
Dr. Dhital noted there were some limitations to the study that are inherent in using administrative databases for research that relies on ICD codes, including “the availability of information on disease activity, medications, and labs, which may restrict clinical interpretation.”
SOMI data reinforced by National Inpatient Sample study
The findings were complemented by a study using the National Inpatient Sample database to explore CVE risk in pregnant individuals with various rheumatic diseases. Lead author Karun Shrestha, MD, a resident physician at St. Barnabas Hospital in New York, and colleagues identified delivery hospitalizations from 2016 to 2019 for individuals with SLE, RA, and systemic vasculitis and looked for CVEs including preeclampsia, peripartum cardiomyopathy (PPCM), heart failure, stroke, cardiac arrhythmias, and VTE.
Out of over 3.4 million delivery hospitalizations, researchers identified 5,900 individuals with SLE, 4,895 with RA, and 325 with vasculitis. After adjusting for confounding factors such as race, age, insurance, and other comorbidities, SLE was identified as an independent risk factor for preeclampsia (odds ratio, 1.5; 95% CI, 1.1-2.1), arrhythmia (OR, 3.17; 95% CI, 1.73-5.79), and venous thrombosis (OR, 8.4; 95% CI, 2.9-22.1). Vasculitis was tied to increased risk for preeclampsia (OR, 4.7; 95% CI, 2-11.3), stroke (OR, 513.3; 95% CI, 114-2,284), heart failure (OR, 24.17; 95% CI, 4.68-124.6), and PPCM (OR, 66.7; 95% CI, 8.7-509.4). RA was tied to an increased risk for preeclampsia (OR, 1.5; 95% CI, 1.05-2.1).
Patients with SLE or vasculitis had longer, more costly hospital stays, compared with those without these conditions, and they experienced higher rates of in-hospital mortality. While previous research has demonstrated that patients with SLE have higher risk of cardiac events, there is less literature on CVE risk in pregnancies for vasculitis, Dr. Shrestha said in an interview.
“It’s something to work on,” he said.
Adverse pregnancy outcomes higher with ARDs, APS
In a second abstract also led by Dr. Dhital using SOMI data, researchers found that pregnant individuals with ARDs or APS had a higher risk of experiencing an APO – preterm birth or small-for-gestational age – than individuals without these conditions. CVEs exacerbated that risk, regardless of underlying chronic health conditions.

Over half of patients with an ARD and a CVE during pregnancy experienced an APO – most commonly preterm birth. More than one in four pregnant individuals without ARD or APS who experienced a CVE also had an APO.
After differentiating CVEs as either VTE and non-VTE events, patients with ARD and a non-VTE CVE had a fivefold greater risk of early preterm birth (< 32 weeks) and a threefold higher risk of moderate preterm birth (32 to < 34 weeks).
“These findings highlight the need for close monitoring and management of pregnant women, not only for adverse outcomes, but also for cardiovascular risks and events, in order to identify those at the highest risk for adverse outcomes,” the authors wrote. “This need is particularly significant for individuals with ARDs, as 53.4% of our population with an ARD and CVE in pregnancy experienced an APO.”
Dr. Dhital, Dr. Kolasinski, and Dr. Shrestha disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant individuals with autoimmune rheumatic diseases (ARDs) are at least four times more likely to experience an acute cardiovascular event (CVE) than are pregnant individuals without these conditions, according to new research presented at the annual meeting of the American College of Rheumatology. Pregnant individuals with primary antiphospholipid syndrome (APS) had a 15-fold increase in CVE risk.
Patients who experienced CVEs were also more likely to experience preterm birth and other adverse pregnancy outcomes (APOs).
Rashmi Dhital, MD, a rheumatology fellow at the University of California, San Diego, and colleagues examined the medical records of pregnant individuals in California who had delivered singleton live-born infants from 2005 to 2020. Using data from the Study of Outcomes in Mothers and Infants (SOMI) database, an administrative population-based birth cohort in California, they identified more than 7 million individuals, 19,340 with ARDs and 7,758 with APS.
They then analyzed how many patients experienced an acute CVE during pregnancy and up to 6 weeks after giving birth.
CVEs occurred in 2.0% of patients with ARDs, 6.9% of individuals with APS, and 0.4% of women without these conditions. CVE risk was four times higher in the ARDs group (adjusted relative risk, 4.1; 95% confidence interval, 3.7-4.5) and nearly 15 times higher in the APS group (aRR, 14.7; 95% CI, 13.5-16.0) than in the comparison group. Patients with systemic lupus erythematosus (SLE) had a sixfold higher risk of CVE, which was further exacerbated by concomitant APS (18-fold higher risk) or lupus nephritis (15-fold higher risk).
Dr. Dhital also classified CVEs as either venous thromboembolism and non-VTE events. Pregnant patients with APS had a high risk for VTE-only CVE (40-fold greater) and a 3.7-fold higher risk of non-VTE events, compared with pregnant patients without these conditions. Patients with SLE along with lupus nephritis had a 20-fold increased risk of VTE-only CVE and an 11-fold higher risk of non-VTE CVE.
Although the study grouped rheumatic diseases together, “lupus is generally driving these results,” Sharon Kolasinski, MD, of the University of Pennsylvania, Philadelphia, noted in an interview. She moderated the plenary session where the research was presented. “If you take out lupus, then what is the risk? That would be an interesting question.”
Between 25% and 30% of all CVEs occurred in the postpartum period, highlighting the importance of close monitoring of cardiovascular risks and events in women with ARDs or APS both during pregnancy and postpartum, Dr. Dhital noted.
Recognizing these risks “can sometimes be challenging due to a lower suspicion of CVE in younger patients, and also symptoms overlap with normal pregnancy,” Dr. Dhital said during her plenary presentation. Working with other clinical teams could help physicians detect these risks in patients.
“It’s important for us to remember that there’s increased risk of cardiovascular events in pregnancy in our patients. It’s uncommon, but it’s not zero,” added Dr. Kolasinski, and this study highlighted when physicians should be more focused about that risk.
Dr. Dhital noted there were some limitations to the study that are inherent in using administrative databases for research that relies on ICD codes, including “the availability of information on disease activity, medications, and labs, which may restrict clinical interpretation.”
SOMI data reinforced by National Inpatient Sample study
The findings were complemented by a study using the National Inpatient Sample database to explore CVE risk in pregnant individuals with various rheumatic diseases. Lead author Karun Shrestha, MD, a resident physician at St. Barnabas Hospital in New York, and colleagues identified delivery hospitalizations from 2016 to 2019 for individuals with SLE, RA, and systemic vasculitis and looked for CVEs including preeclampsia, peripartum cardiomyopathy (PPCM), heart failure, stroke, cardiac arrhythmias, and VTE.
Out of over 3.4 million delivery hospitalizations, researchers identified 5,900 individuals with SLE, 4,895 with RA, and 325 with vasculitis. After adjusting for confounding factors such as race, age, insurance, and other comorbidities, SLE was identified as an independent risk factor for preeclampsia (odds ratio, 1.5; 95% CI, 1.1-2.1), arrhythmia (OR, 3.17; 95% CI, 1.73-5.79), and venous thrombosis (OR, 8.4; 95% CI, 2.9-22.1). Vasculitis was tied to increased risk for preeclampsia (OR, 4.7; 95% CI, 2-11.3), stroke (OR, 513.3; 95% CI, 114-2,284), heart failure (OR, 24.17; 95% CI, 4.68-124.6), and PPCM (OR, 66.7; 95% CI, 8.7-509.4). RA was tied to an increased risk for preeclampsia (OR, 1.5; 95% CI, 1.05-2.1).
Patients with SLE or vasculitis had longer, more costly hospital stays, compared with those without these conditions, and they experienced higher rates of in-hospital mortality. While previous research has demonstrated that patients with SLE have higher risk of cardiac events, there is less literature on CVE risk in pregnancies for vasculitis, Dr. Shrestha said in an interview.
“It’s something to work on,” he said.
Adverse pregnancy outcomes higher with ARDs, APS
In a second abstract also led by Dr. Dhital using SOMI data, researchers found that pregnant individuals with ARDs or APS had a higher risk of experiencing an APO – preterm birth or small-for-gestational age – than individuals without these conditions. CVEs exacerbated that risk, regardless of underlying chronic health conditions.

Over half of patients with an ARD and a CVE during pregnancy experienced an APO – most commonly preterm birth. More than one in four pregnant individuals without ARD or APS who experienced a CVE also had an APO.
After differentiating CVEs as either VTE and non-VTE events, patients with ARD and a non-VTE CVE had a fivefold greater risk of early preterm birth (< 32 weeks) and a threefold higher risk of moderate preterm birth (32 to < 34 weeks).
“These findings highlight the need for close monitoring and management of pregnant women, not only for adverse outcomes, but also for cardiovascular risks and events, in order to identify those at the highest risk for adverse outcomes,” the authors wrote. “This need is particularly significant for individuals with ARDs, as 53.4% of our population with an ARD and CVE in pregnancy experienced an APO.”
Dr. Dhital, Dr. Kolasinski, and Dr. Shrestha disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant individuals with autoimmune rheumatic diseases (ARDs) are at least four times more likely to experience an acute cardiovascular event (CVE) than are pregnant individuals without these conditions, according to new research presented at the annual meeting of the American College of Rheumatology. Pregnant individuals with primary antiphospholipid syndrome (APS) had a 15-fold increase in CVE risk.
Patients who experienced CVEs were also more likely to experience preterm birth and other adverse pregnancy outcomes (APOs).
Rashmi Dhital, MD, a rheumatology fellow at the University of California, San Diego, and colleagues examined the medical records of pregnant individuals in California who had delivered singleton live-born infants from 2005 to 2020. Using data from the Study of Outcomes in Mothers and Infants (SOMI) database, an administrative population-based birth cohort in California, they identified more than 7 million individuals, 19,340 with ARDs and 7,758 with APS.
They then analyzed how many patients experienced an acute CVE during pregnancy and up to 6 weeks after giving birth.
CVEs occurred in 2.0% of patients with ARDs, 6.9% of individuals with APS, and 0.4% of women without these conditions. CVE risk was four times higher in the ARDs group (adjusted relative risk, 4.1; 95% confidence interval, 3.7-4.5) and nearly 15 times higher in the APS group (aRR, 14.7; 95% CI, 13.5-16.0) than in the comparison group. Patients with systemic lupus erythematosus (SLE) had a sixfold higher risk of CVE, which was further exacerbated by concomitant APS (18-fold higher risk) or lupus nephritis (15-fold higher risk).
Dr. Dhital also classified CVEs as either venous thromboembolism and non-VTE events. Pregnant patients with APS had a high risk for VTE-only CVE (40-fold greater) and a 3.7-fold higher risk of non-VTE events, compared with pregnant patients without these conditions. Patients with SLE along with lupus nephritis had a 20-fold increased risk of VTE-only CVE and an 11-fold higher risk of non-VTE CVE.
Although the study grouped rheumatic diseases together, “lupus is generally driving these results,” Sharon Kolasinski, MD, of the University of Pennsylvania, Philadelphia, noted in an interview. She moderated the plenary session where the research was presented. “If you take out lupus, then what is the risk? That would be an interesting question.”
Between 25% and 30% of all CVEs occurred in the postpartum period, highlighting the importance of close monitoring of cardiovascular risks and events in women with ARDs or APS both during pregnancy and postpartum, Dr. Dhital noted.
Recognizing these risks “can sometimes be challenging due to a lower suspicion of CVE in younger patients, and also symptoms overlap with normal pregnancy,” Dr. Dhital said during her plenary presentation. Working with other clinical teams could help physicians detect these risks in patients.
“It’s important for us to remember that there’s increased risk of cardiovascular events in pregnancy in our patients. It’s uncommon, but it’s not zero,” added Dr. Kolasinski, and this study highlighted when physicians should be more focused about that risk.
Dr. Dhital noted there were some limitations to the study that are inherent in using administrative databases for research that relies on ICD codes, including “the availability of information on disease activity, medications, and labs, which may restrict clinical interpretation.”
SOMI data reinforced by National Inpatient Sample study
The findings were complemented by a study using the National Inpatient Sample database to explore CVE risk in pregnant individuals with various rheumatic diseases. Lead author Karun Shrestha, MD, a resident physician at St. Barnabas Hospital in New York, and colleagues identified delivery hospitalizations from 2016 to 2019 for individuals with SLE, RA, and systemic vasculitis and looked for CVEs including preeclampsia, peripartum cardiomyopathy (PPCM), heart failure, stroke, cardiac arrhythmias, and VTE.
Out of over 3.4 million delivery hospitalizations, researchers identified 5,900 individuals with SLE, 4,895 with RA, and 325 with vasculitis. After adjusting for confounding factors such as race, age, insurance, and other comorbidities, SLE was identified as an independent risk factor for preeclampsia (odds ratio, 1.5; 95% CI, 1.1-2.1), arrhythmia (OR, 3.17; 95% CI, 1.73-5.79), and venous thrombosis (OR, 8.4; 95% CI, 2.9-22.1). Vasculitis was tied to increased risk for preeclampsia (OR, 4.7; 95% CI, 2-11.3), stroke (OR, 513.3; 95% CI, 114-2,284), heart failure (OR, 24.17; 95% CI, 4.68-124.6), and PPCM (OR, 66.7; 95% CI, 8.7-509.4). RA was tied to an increased risk for preeclampsia (OR, 1.5; 95% CI, 1.05-2.1).
Patients with SLE or vasculitis had longer, more costly hospital stays, compared with those without these conditions, and they experienced higher rates of in-hospital mortality. While previous research has demonstrated that patients with SLE have higher risk of cardiac events, there is less literature on CVE risk in pregnancies for vasculitis, Dr. Shrestha said in an interview.
“It’s something to work on,” he said.
Adverse pregnancy outcomes higher with ARDs, APS
In a second abstract also led by Dr. Dhital using SOMI data, researchers found that pregnant individuals with ARDs or APS had a higher risk of experiencing an APO – preterm birth or small-for-gestational age – than individuals without these conditions. CVEs exacerbated that risk, regardless of underlying chronic health conditions.

Over half of patients with an ARD and a CVE during pregnancy experienced an APO – most commonly preterm birth. More than one in four pregnant individuals without ARD or APS who experienced a CVE also had an APO.
After differentiating CVEs as either VTE and non-VTE events, patients with ARD and a non-VTE CVE had a fivefold greater risk of early preterm birth (< 32 weeks) and a threefold higher risk of moderate preterm birth (32 to < 34 weeks).
“These findings highlight the need for close monitoring and management of pregnant women, not only for adverse outcomes, but also for cardiovascular risks and events, in order to identify those at the highest risk for adverse outcomes,” the authors wrote. “This need is particularly significant for individuals with ARDs, as 53.4% of our population with an ARD and CVE in pregnancy experienced an APO.”
Dr. Dhital, Dr. Kolasinski, and Dr. Shrestha disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACR 2023
Pregnancies with low anti-SSA/Ro autoantibody levels: Forgo fetal heart rhythm monitoring?
SAN DIEGO – Pregnant women with anti-SSA/Ro autoantibodies at titer levels of less than 1,000 ELISA units per mL are at minimal to no risk for fetal atrioventricular (AV) block and may be able to forgo traditional echocardiographic heart rhythm monitoring, results from an ongoing, prospective, multicenter trial demonstrated.
However, pregnant patients with higher titer antibodies seem to be at greatest risk for fetal AV block and may benefit from ambulatory fetal heart rhythm monitoring (FHRM), which can detect emergent AV block, according to the study findings. The findings were published online in Arthritis & Rheumatology and will be presented Nov. 13 at the American College of Rheumatology (ACR) 2023 Annual Meeting by Jill P. Buyon, MD, a rheumatologist who directs the division of rheumatology and the Lupus Center at NYU Langone Health in New York.
“While anti-Ro antibodies have been known to be associated with AV block for decades, it has become increasingly clear that antibody titers matter,” Dr. Buyon said in an interview.
For the investigation, which is the largest of its kind, researchers at 22 sites drew from the large multiracial national study of pregnant women, Surveillance To Prevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and use of frequent ambulatory FHRM on outcomes in women with no previously affected children and those at risk for recurrence. Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices.
These were followed up by weekly or biweekly echocardiograms, and ultrasound tests to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, the pregnant women texted the Doppler sound recordings in real time to a pediatric cardiologist, who immediately ordered an additional echocardiogram in cases of irregular or slowing fetal heart rates. If second-degree heart block was detected, drug therapy was initiated.
No AV block seen with low anti-Ro titers
Dr. Buyon, who led the study with Bettina Cuneo, MD, clinical scholar and professor of surgery and pediatrics at the University of Arizona in Tucson, presented findings from 413 pregnant subjects with a mean age of 33 years who finished monitoring surveillance: 152 women had low titers of both anti-Ro60 and –Ro52 (defined as < 1,000 ELISA units per mL), and 261 women with titers above the threshold for either antibody (defined as ≥ 1,000 ELISA units per mL). Of the 152 women with low titers of both anti-Ro60 and –Ro52, none of the pregnancies past 26 weeks resulted in AV block. Of the 261 women with titers above the threshold for either antibody, 10 of the pregnancies resulted in AV block (3.8%). The incidence of AV block increased with higher antibody titer levels, reaching 7.7% for those in the top quartile for anti–60-kD SSA/Ro; this increased to 27.3% in study participants with a previous child who had AV block, although numbers in this category were small.
Analysis of cumulative FHRM recordings between surveillance echocardiograms revealed that no case of second-degree or third-degree AV block was missed. In addition, 70% of AV blocks detected by FHRM were second-degree and all occurred less than 12 hours from normal FHRM and within another 45 minutes to 4.5 hours to echocardiogram. The one case of second/third-degree and two cases of third-degree AV block were diagnosed by urgent echocardiogram more than 17 to 72 hours from a previously normal FHRM episode.
Other factors besides high anti-Ro titer likely play a role
“STOP BLOQ nicely demonstrates that low titer is associated with a very low risk AV block, and intense monitoring may not be needed,” Dr. Buyon told this news organization. “However, high titer is not the whole answer since even women with the very highest titers can have healthy babies. This report also shows that titers stay constant through pregnancies in the same mother, whether there is the complication of AV block or not. This suggests other factors contribute to AV block.”
She added that FHRM can be easily performed by the mother, but at this time is still best interpreted by a cardiologist. “FHRM detected all cases of AV block, which can happen in hours,” she said. “FHRM should decrease the need for frequent echocardiograms. Some mothers do have more difficulty in deciding whether the baby’s heart is beating irregularly. We need [to improve our teaching] and for how best to have a cardiologist or trained listener interpret. FHRM can be done by the mother but needs interpretation by a cardiologist until we develop a device which can identify abnormalities.”
She acknowledged certain limitations of the study, including the fact that a commercial test for anti-SSA/Ro antibody levels is not available to all clinicians. “Try to find a lab that measures high titer anti-Ro antibodies, but if not, then use one of the common commercial tests such as the BioPlex 2000 autoimmune panels and consider decreased surveillance if titer is < 8,” Dr. Buyon advised.
Vaneet K. Sandhu, MD, a rheumatologist with Loma Linda (Calif.) Medical Center, who was asked to comment on the work, said that the study not only justifies the limited use of FHRM in those with high titer antibodies (followed by urgent fetal echocardiography where indicated), but also risk stratification for fetal AV block.
“For years, we have recommended frequent fetal echocardiography testing in pregnant women with positive anti-SSA/Ro,” Dr. Sandhu said. “This study tells us we need to look deeper. On one hand, recognizing that low titer anti-Ro antibodies do not confer a risk of AV block is cost effective. On the other hand, while the titer of the antibody appears to contribute to fetal AV block, we need to delve deeper into additional factors contributing to fetal AV block risk in order to better navigate our surveillance methods.”
The study was supported by NIH grants from the National Institute of Child Health and Human Development and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Sandhu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant women with anti-SSA/Ro autoantibodies at titer levels of less than 1,000 ELISA units per mL are at minimal to no risk for fetal atrioventricular (AV) block and may be able to forgo traditional echocardiographic heart rhythm monitoring, results from an ongoing, prospective, multicenter trial demonstrated.
However, pregnant patients with higher titer antibodies seem to be at greatest risk for fetal AV block and may benefit from ambulatory fetal heart rhythm monitoring (FHRM), which can detect emergent AV block, according to the study findings. The findings were published online in Arthritis & Rheumatology and will be presented Nov. 13 at the American College of Rheumatology (ACR) 2023 Annual Meeting by Jill P. Buyon, MD, a rheumatologist who directs the division of rheumatology and the Lupus Center at NYU Langone Health in New York.
“While anti-Ro antibodies have been known to be associated with AV block for decades, it has become increasingly clear that antibody titers matter,” Dr. Buyon said in an interview.
For the investigation, which is the largest of its kind, researchers at 22 sites drew from the large multiracial national study of pregnant women, Surveillance To Prevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and use of frequent ambulatory FHRM on outcomes in women with no previously affected children and those at risk for recurrence. Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices.
These were followed up by weekly or biweekly echocardiograms, and ultrasound tests to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, the pregnant women texted the Doppler sound recordings in real time to a pediatric cardiologist, who immediately ordered an additional echocardiogram in cases of irregular or slowing fetal heart rates. If second-degree heart block was detected, drug therapy was initiated.
No AV block seen with low anti-Ro titers
Dr. Buyon, who led the study with Bettina Cuneo, MD, clinical scholar and professor of surgery and pediatrics at the University of Arizona in Tucson, presented findings from 413 pregnant subjects with a mean age of 33 years who finished monitoring surveillance: 152 women had low titers of both anti-Ro60 and –Ro52 (defined as < 1,000 ELISA units per mL), and 261 women with titers above the threshold for either antibody (defined as ≥ 1,000 ELISA units per mL). Of the 152 women with low titers of both anti-Ro60 and –Ro52, none of the pregnancies past 26 weeks resulted in AV block. Of the 261 women with titers above the threshold for either antibody, 10 of the pregnancies resulted in AV block (3.8%). The incidence of AV block increased with higher antibody titer levels, reaching 7.7% for those in the top quartile for anti–60-kD SSA/Ro; this increased to 27.3% in study participants with a previous child who had AV block, although numbers in this category were small.
Analysis of cumulative FHRM recordings between surveillance echocardiograms revealed that no case of second-degree or third-degree AV block was missed. In addition, 70% of AV blocks detected by FHRM were second-degree and all occurred less than 12 hours from normal FHRM and within another 45 minutes to 4.5 hours to echocardiogram. The one case of second/third-degree and two cases of third-degree AV block were diagnosed by urgent echocardiogram more than 17 to 72 hours from a previously normal FHRM episode.
Other factors besides high anti-Ro titer likely play a role
“STOP BLOQ nicely demonstrates that low titer is associated with a very low risk AV block, and intense monitoring may not be needed,” Dr. Buyon told this news organization. “However, high titer is not the whole answer since even women with the very highest titers can have healthy babies. This report also shows that titers stay constant through pregnancies in the same mother, whether there is the complication of AV block or not. This suggests other factors contribute to AV block.”
She added that FHRM can be easily performed by the mother, but at this time is still best interpreted by a cardiologist. “FHRM detected all cases of AV block, which can happen in hours,” she said. “FHRM should decrease the need for frequent echocardiograms. Some mothers do have more difficulty in deciding whether the baby’s heart is beating irregularly. We need [to improve our teaching] and for how best to have a cardiologist or trained listener interpret. FHRM can be done by the mother but needs interpretation by a cardiologist until we develop a device which can identify abnormalities.”
She acknowledged certain limitations of the study, including the fact that a commercial test for anti-SSA/Ro antibody levels is not available to all clinicians. “Try to find a lab that measures high titer anti-Ro antibodies, but if not, then use one of the common commercial tests such as the BioPlex 2000 autoimmune panels and consider decreased surveillance if titer is < 8,” Dr. Buyon advised.
Vaneet K. Sandhu, MD, a rheumatologist with Loma Linda (Calif.) Medical Center, who was asked to comment on the work, said that the study not only justifies the limited use of FHRM in those with high titer antibodies (followed by urgent fetal echocardiography where indicated), but also risk stratification for fetal AV block.
“For years, we have recommended frequent fetal echocardiography testing in pregnant women with positive anti-SSA/Ro,” Dr. Sandhu said. “This study tells us we need to look deeper. On one hand, recognizing that low titer anti-Ro antibodies do not confer a risk of AV block is cost effective. On the other hand, while the titer of the antibody appears to contribute to fetal AV block, we need to delve deeper into additional factors contributing to fetal AV block risk in order to better navigate our surveillance methods.”
The study was supported by NIH grants from the National Institute of Child Health and Human Development and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Sandhu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant women with anti-SSA/Ro autoantibodies at titer levels of less than 1,000 ELISA units per mL are at minimal to no risk for fetal atrioventricular (AV) block and may be able to forgo traditional echocardiographic heart rhythm monitoring, results from an ongoing, prospective, multicenter trial demonstrated.
However, pregnant patients with higher titer antibodies seem to be at greatest risk for fetal AV block and may benefit from ambulatory fetal heart rhythm monitoring (FHRM), which can detect emergent AV block, according to the study findings. The findings were published online in Arthritis & Rheumatology and will be presented Nov. 13 at the American College of Rheumatology (ACR) 2023 Annual Meeting by Jill P. Buyon, MD, a rheumatologist who directs the division of rheumatology and the Lupus Center at NYU Langone Health in New York.
“While anti-Ro antibodies have been known to be associated with AV block for decades, it has become increasingly clear that antibody titers matter,” Dr. Buyon said in an interview.
For the investigation, which is the largest of its kind, researchers at 22 sites drew from the large multiracial national study of pregnant women, Surveillance To Prevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and use of frequent ambulatory FHRM on outcomes in women with no previously affected children and those at risk for recurrence. Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices.
These were followed up by weekly or biweekly echocardiograms, and ultrasound tests to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, the pregnant women texted the Doppler sound recordings in real time to a pediatric cardiologist, who immediately ordered an additional echocardiogram in cases of irregular or slowing fetal heart rates. If second-degree heart block was detected, drug therapy was initiated.
No AV block seen with low anti-Ro titers
Dr. Buyon, who led the study with Bettina Cuneo, MD, clinical scholar and professor of surgery and pediatrics at the University of Arizona in Tucson, presented findings from 413 pregnant subjects with a mean age of 33 years who finished monitoring surveillance: 152 women had low titers of both anti-Ro60 and –Ro52 (defined as < 1,000 ELISA units per mL), and 261 women with titers above the threshold for either antibody (defined as ≥ 1,000 ELISA units per mL). Of the 152 women with low titers of both anti-Ro60 and –Ro52, none of the pregnancies past 26 weeks resulted in AV block. Of the 261 women with titers above the threshold for either antibody, 10 of the pregnancies resulted in AV block (3.8%). The incidence of AV block increased with higher antibody titer levels, reaching 7.7% for those in the top quartile for anti–60-kD SSA/Ro; this increased to 27.3% in study participants with a previous child who had AV block, although numbers in this category were small.
Analysis of cumulative FHRM recordings between surveillance echocardiograms revealed that no case of second-degree or third-degree AV block was missed. In addition, 70% of AV blocks detected by FHRM were second-degree and all occurred less than 12 hours from normal FHRM and within another 45 minutes to 4.5 hours to echocardiogram. The one case of second/third-degree and two cases of third-degree AV block were diagnosed by urgent echocardiogram more than 17 to 72 hours from a previously normal FHRM episode.
Other factors besides high anti-Ro titer likely play a role
“STOP BLOQ nicely demonstrates that low titer is associated with a very low risk AV block, and intense monitoring may not be needed,” Dr. Buyon told this news organization. “However, high titer is not the whole answer since even women with the very highest titers can have healthy babies. This report also shows that titers stay constant through pregnancies in the same mother, whether there is the complication of AV block or not. This suggests other factors contribute to AV block.”
She added that FHRM can be easily performed by the mother, but at this time is still best interpreted by a cardiologist. “FHRM detected all cases of AV block, which can happen in hours,” she said. “FHRM should decrease the need for frequent echocardiograms. Some mothers do have more difficulty in deciding whether the baby’s heart is beating irregularly. We need [to improve our teaching] and for how best to have a cardiologist or trained listener interpret. FHRM can be done by the mother but needs interpretation by a cardiologist until we develop a device which can identify abnormalities.”
She acknowledged certain limitations of the study, including the fact that a commercial test for anti-SSA/Ro antibody levels is not available to all clinicians. “Try to find a lab that measures high titer anti-Ro antibodies, but if not, then use one of the common commercial tests such as the BioPlex 2000 autoimmune panels and consider decreased surveillance if titer is < 8,” Dr. Buyon advised.
Vaneet K. Sandhu, MD, a rheumatologist with Loma Linda (Calif.) Medical Center, who was asked to comment on the work, said that the study not only justifies the limited use of FHRM in those with high titer antibodies (followed by urgent fetal echocardiography where indicated), but also risk stratification for fetal AV block.
“For years, we have recommended frequent fetal echocardiography testing in pregnant women with positive anti-SSA/Ro,” Dr. Sandhu said. “This study tells us we need to look deeper. On one hand, recognizing that low titer anti-Ro antibodies do not confer a risk of AV block is cost effective. On the other hand, while the titer of the antibody appears to contribute to fetal AV block, we need to delve deeper into additional factors contributing to fetal AV block risk in order to better navigate our surveillance methods.”
The study was supported by NIH grants from the National Institute of Child Health and Human Development and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Sandhu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACR 2023
News & Perspectives from Ob.Gyn. News
CONFERENCE COVERAGE
MS, DMTs, and pregnancy: Beware of over-caution regarding treatment
MILAN—The news about multiple sclerosis (MS) and childbearing in women is largely good, a researcher told colleagues at the 9th Joint ECTRIMS-ACTRIMS Meeting. Evidence suggests that MS doesn’t disrupt fertility, pregnancy, birth, or lactation. However, there are still uncertainties about the timing of medical treatment for MS before, during, and after pregnancy.
Epidemiologist Emmanuelle Leray, PhD, of French School of Public Health in Rennes, urged neurologists to not be too eager to take women off medication—or too slow to put them back on it. “MS should not be undertreated due to a desire for pregnancy, as there are several options that are possible and compatible with pregnancy,” she said. As for after pregnancy, when women face a well-known high risk of MS rebound, “we can reasonably assume that women with active MS need to be advised to restart rapid, highly effective DMT [disease-modifying therapy] soon after delivery,” she said.
Women are more likely than men to develop MS, and they often do so during child-bearing years. Pregnancy among women with MS has become more common over the years: A 2018 Neurology study examined U.S. data from 2006 to 2014 and reported that the annual adjusted proportion of women with MS and pregnancy increased from 7.91% to 9.47%.
FEATURE
Employment vs. private practice: Who’s happier?
Alexandra Kharazi, MD, a California-based cardiothoracic surgeon, previously worked as an employed physician and is now in private practice. Though she appreciates that there are some trade-offs to working with her small group of three surgeons, Dr. Kharazi has no qualms about her choice.
“For me, it’s an issue of autonomy,” she said. “While I have to work a lot of hours, I don’t have to adhere to a strict schedule. I also don’t have to follow specific policies and rules.”
In contrast, Cassandra Boduch, MD, an employed psychiatrist with PsychPlus in Houston, is very satisfied with working as an employee. “I looked into private practice, but no one really prepares you for the complications that come with it,” she said. “There’s a lot more that goes into it than people realize.”
By hanging up her own shingle, Dr. Kharazi may be living a rapidly shrinking dream. According to the American Medical Association, between 2012 and 2022, the share of physicians working in private practice fell from 60% to 47%. The share of physicians working in hospitals as direct employees or contractors increased from about 6% to about 10% during the same time period.
Many factors contribute to these shifting trends, a major factor being economic stress stemming from payment cuts in Medicare. Add in rising practice costs and administrative burdens, and more doctors than ever are seeking employment, according to the AMA.
Though the traditional dream of owning your own practice may be slipping away, are employed physicians less happy than are their self-employed peers? By many measures, the answer is no.
In Medscape’s Employed Physicians Report 2023, doctors weighed in on the pros and cons of their jobs.
When asked what they like most about their jobs, employed physician respondents reported “not having to run a business” as their number-one benefit, followed closely by a stable income. The fact that employers pay for malpractice insurance ranked third, followed by work-life balance.
“We get no business classes in medical school or residency,” said one employed physician. “Having a good salary feels good,” said another. Yet another respondent chimed in: “Running a practice as a small business has become undoable over the past 10-12 years.”
And 50% of employed physicians said that they were “very satisfied/satisfied” with their degree of autonomy.
Continue to: LATEST NEWS...
LATEST NEWS
Three-quarters of menopausal women report unexpected symptoms
GLASGOW—Three-quarters of women going through perimenopause and menopause experience unexpected distressing, debilitating, and embarrassing symptoms but often fail to receive appropriate treatment, a large U.K.-based survey found.
“For too long, many people have thought of menopause as just hot flashes and vaginal dryness. But we know hormones work all over our body, so there are many symptoms beyond that,” said Daniel Reisel, MBBS, PhD, a gynecologist at University College London, who presented the survey findings at the 2023 annual meeting of the Royal College of General Practitioners.
Primary care physicians in the United Kingdom have seen an increase in cases of women presenting with symptoms associated with menopause at a time when the country’s Parliament is debating whether all women should have a menopause check-up in their early 40s, he said.
Still, only around 14% of menopausal women in the United Kingdom are prescribed hormone replacement therapy (HRT), despite national and international guidelines clearly stating the benefits of the treatment generally outweigh the risks.
Louise Newson, MBChB, who runs the U.K.’s largest menopause clinic, said many women with symptoms of menopause feel the medical system “gaslights” them—dismissing their concerns as trivial or even fabricated.
In her clinic, she typically sees many women with poor sleep, as well as muscle and joint pains. “Yet [when they visit their GPs], they are incorrectly told that it can’t be hormones because they’re still having periods,” she said.
Prescribed antidepressants often precede HRT
The new study sought to learn what women knew and experienced with respect to menopause symptoms and what they thought was important. Of the 5,744 women who responded to the survey, 79.4% were aged 40-60 years and 84.6% were White. “The survey respondents were not different from the distribution of ethnicities we see in NHS menopause care,” said Dr. Reisel, adding that “the barriers are greater for women in poorer areas and for those who are non-White.”
A total of 30.4% had two to five hospital consultations before the health care professional considered that symptoms were related to changing hormone levels; 38.5% were offered antidepressants before HRT. Nearly all (94.6%) said they had experienced negative mood changes and emotions since becoming perimenopausal or menopausal; of these, 19.1% were formally diagnosed with depression or a mood disorder.
“This all just highlights the frustrations I feel around menopause care,” Dr. Newson said. “Women are often not given the tools to properly understand what’s going on and then they don’t ask for the right treatment, and many are given antidepressants. It’s still medicalizing the menopause but in a different way.” ●
CONFERENCE COVERAGE
MS, DMTs, and pregnancy: Beware of over-caution regarding treatment
MILAN—The news about multiple sclerosis (MS) and childbearing in women is largely good, a researcher told colleagues at the 9th Joint ECTRIMS-ACTRIMS Meeting. Evidence suggests that MS doesn’t disrupt fertility, pregnancy, birth, or lactation. However, there are still uncertainties about the timing of medical treatment for MS before, during, and after pregnancy.
Epidemiologist Emmanuelle Leray, PhD, of French School of Public Health in Rennes, urged neurologists to not be too eager to take women off medication—or too slow to put them back on it. “MS should not be undertreated due to a desire for pregnancy, as there are several options that are possible and compatible with pregnancy,” she said. As for after pregnancy, when women face a well-known high risk of MS rebound, “we can reasonably assume that women with active MS need to be advised to restart rapid, highly effective DMT [disease-modifying therapy] soon after delivery,” she said.
Women are more likely than men to develop MS, and they often do so during child-bearing years. Pregnancy among women with MS has become more common over the years: A 2018 Neurology study examined U.S. data from 2006 to 2014 and reported that the annual adjusted proportion of women with MS and pregnancy increased from 7.91% to 9.47%.
FEATURE
Employment vs. private practice: Who’s happier?
Alexandra Kharazi, MD, a California-based cardiothoracic surgeon, previously worked as an employed physician and is now in private practice. Though she appreciates that there are some trade-offs to working with her small group of three surgeons, Dr. Kharazi has no qualms about her choice.
“For me, it’s an issue of autonomy,” she said. “While I have to work a lot of hours, I don’t have to adhere to a strict schedule. I also don’t have to follow specific policies and rules.”
In contrast, Cassandra Boduch, MD, an employed psychiatrist with PsychPlus in Houston, is very satisfied with working as an employee. “I looked into private practice, but no one really prepares you for the complications that come with it,” she said. “There’s a lot more that goes into it than people realize.”
By hanging up her own shingle, Dr. Kharazi may be living a rapidly shrinking dream. According to the American Medical Association, between 2012 and 2022, the share of physicians working in private practice fell from 60% to 47%. The share of physicians working in hospitals as direct employees or contractors increased from about 6% to about 10% during the same time period.
Many factors contribute to these shifting trends, a major factor being economic stress stemming from payment cuts in Medicare. Add in rising practice costs and administrative burdens, and more doctors than ever are seeking employment, according to the AMA.
Though the traditional dream of owning your own practice may be slipping away, are employed physicians less happy than are their self-employed peers? By many measures, the answer is no.
In Medscape’s Employed Physicians Report 2023, doctors weighed in on the pros and cons of their jobs.
When asked what they like most about their jobs, employed physician respondents reported “not having to run a business” as their number-one benefit, followed closely by a stable income. The fact that employers pay for malpractice insurance ranked third, followed by work-life balance.
“We get no business classes in medical school or residency,” said one employed physician. “Having a good salary feels good,” said another. Yet another respondent chimed in: “Running a practice as a small business has become undoable over the past 10-12 years.”
And 50% of employed physicians said that they were “very satisfied/satisfied” with their degree of autonomy.
Continue to: LATEST NEWS...
LATEST NEWS
Three-quarters of menopausal women report unexpected symptoms
GLASGOW—Three-quarters of women going through perimenopause and menopause experience unexpected distressing, debilitating, and embarrassing symptoms but often fail to receive appropriate treatment, a large U.K.-based survey found.
“For too long, many people have thought of menopause as just hot flashes and vaginal dryness. But we know hormones work all over our body, so there are many symptoms beyond that,” said Daniel Reisel, MBBS, PhD, a gynecologist at University College London, who presented the survey findings at the 2023 annual meeting of the Royal College of General Practitioners.
Primary care physicians in the United Kingdom have seen an increase in cases of women presenting with symptoms associated with menopause at a time when the country’s Parliament is debating whether all women should have a menopause check-up in their early 40s, he said.
Still, only around 14% of menopausal women in the United Kingdom are prescribed hormone replacement therapy (HRT), despite national and international guidelines clearly stating the benefits of the treatment generally outweigh the risks.
Louise Newson, MBChB, who runs the U.K.’s largest menopause clinic, said many women with symptoms of menopause feel the medical system “gaslights” them—dismissing their concerns as trivial or even fabricated.
In her clinic, she typically sees many women with poor sleep, as well as muscle and joint pains. “Yet [when they visit their GPs], they are incorrectly told that it can’t be hormones because they’re still having periods,” she said.
Prescribed antidepressants often precede HRT
The new study sought to learn what women knew and experienced with respect to menopause symptoms and what they thought was important. Of the 5,744 women who responded to the survey, 79.4% were aged 40-60 years and 84.6% were White. “The survey respondents were not different from the distribution of ethnicities we see in NHS menopause care,” said Dr. Reisel, adding that “the barriers are greater for women in poorer areas and for those who are non-White.”
A total of 30.4% had two to five hospital consultations before the health care professional considered that symptoms were related to changing hormone levels; 38.5% were offered antidepressants before HRT. Nearly all (94.6%) said they had experienced negative mood changes and emotions since becoming perimenopausal or menopausal; of these, 19.1% were formally diagnosed with depression or a mood disorder.
“This all just highlights the frustrations I feel around menopause care,” Dr. Newson said. “Women are often not given the tools to properly understand what’s going on and then they don’t ask for the right treatment, and many are given antidepressants. It’s still medicalizing the menopause but in a different way.” ●
CONFERENCE COVERAGE
MS, DMTs, and pregnancy: Beware of over-caution regarding treatment
MILAN—The news about multiple sclerosis (MS) and childbearing in women is largely good, a researcher told colleagues at the 9th Joint ECTRIMS-ACTRIMS Meeting. Evidence suggests that MS doesn’t disrupt fertility, pregnancy, birth, or lactation. However, there are still uncertainties about the timing of medical treatment for MS before, during, and after pregnancy.
Epidemiologist Emmanuelle Leray, PhD, of French School of Public Health in Rennes, urged neurologists to not be too eager to take women off medication—or too slow to put them back on it. “MS should not be undertreated due to a desire for pregnancy, as there are several options that are possible and compatible with pregnancy,” she said. As for after pregnancy, when women face a well-known high risk of MS rebound, “we can reasonably assume that women with active MS need to be advised to restart rapid, highly effective DMT [disease-modifying therapy] soon after delivery,” she said.
Women are more likely than men to develop MS, and they often do so during child-bearing years. Pregnancy among women with MS has become more common over the years: A 2018 Neurology study examined U.S. data from 2006 to 2014 and reported that the annual adjusted proportion of women with MS and pregnancy increased from 7.91% to 9.47%.
FEATURE
Employment vs. private practice: Who’s happier?
Alexandra Kharazi, MD, a California-based cardiothoracic surgeon, previously worked as an employed physician and is now in private practice. Though she appreciates that there are some trade-offs to working with her small group of three surgeons, Dr. Kharazi has no qualms about her choice.
“For me, it’s an issue of autonomy,” she said. “While I have to work a lot of hours, I don’t have to adhere to a strict schedule. I also don’t have to follow specific policies and rules.”
In contrast, Cassandra Boduch, MD, an employed psychiatrist with PsychPlus in Houston, is very satisfied with working as an employee. “I looked into private practice, but no one really prepares you for the complications that come with it,” she said. “There’s a lot more that goes into it than people realize.”
By hanging up her own shingle, Dr. Kharazi may be living a rapidly shrinking dream. According to the American Medical Association, between 2012 and 2022, the share of physicians working in private practice fell from 60% to 47%. The share of physicians working in hospitals as direct employees or contractors increased from about 6% to about 10% during the same time period.
Many factors contribute to these shifting trends, a major factor being economic stress stemming from payment cuts in Medicare. Add in rising practice costs and administrative burdens, and more doctors than ever are seeking employment, according to the AMA.
Though the traditional dream of owning your own practice may be slipping away, are employed physicians less happy than are their self-employed peers? By many measures, the answer is no.
In Medscape’s Employed Physicians Report 2023, doctors weighed in on the pros and cons of their jobs.
When asked what they like most about their jobs, employed physician respondents reported “not having to run a business” as their number-one benefit, followed closely by a stable income. The fact that employers pay for malpractice insurance ranked third, followed by work-life balance.
“We get no business classes in medical school or residency,” said one employed physician. “Having a good salary feels good,” said another. Yet another respondent chimed in: “Running a practice as a small business has become undoable over the past 10-12 years.”
And 50% of employed physicians said that they were “very satisfied/satisfied” with their degree of autonomy.
Continue to: LATEST NEWS...
LATEST NEWS
Three-quarters of menopausal women report unexpected symptoms
GLASGOW—Three-quarters of women going through perimenopause and menopause experience unexpected distressing, debilitating, and embarrassing symptoms but often fail to receive appropriate treatment, a large U.K.-based survey found.
“For too long, many people have thought of menopause as just hot flashes and vaginal dryness. But we know hormones work all over our body, so there are many symptoms beyond that,” said Daniel Reisel, MBBS, PhD, a gynecologist at University College London, who presented the survey findings at the 2023 annual meeting of the Royal College of General Practitioners.
Primary care physicians in the United Kingdom have seen an increase in cases of women presenting with symptoms associated with menopause at a time when the country’s Parliament is debating whether all women should have a menopause check-up in their early 40s, he said.
Still, only around 14% of menopausal women in the United Kingdom are prescribed hormone replacement therapy (HRT), despite national and international guidelines clearly stating the benefits of the treatment generally outweigh the risks.
Louise Newson, MBChB, who runs the U.K.’s largest menopause clinic, said many women with symptoms of menopause feel the medical system “gaslights” them—dismissing their concerns as trivial or even fabricated.
In her clinic, she typically sees many women with poor sleep, as well as muscle and joint pains. “Yet [when they visit their GPs], they are incorrectly told that it can’t be hormones because they’re still having periods,” she said.
Prescribed antidepressants often precede HRT
The new study sought to learn what women knew and experienced with respect to menopause symptoms and what they thought was important. Of the 5,744 women who responded to the survey, 79.4% were aged 40-60 years and 84.6% were White. “The survey respondents were not different from the distribution of ethnicities we see in NHS menopause care,” said Dr. Reisel, adding that “the barriers are greater for women in poorer areas and for those who are non-White.”
A total of 30.4% had two to five hospital consultations before the health care professional considered that symptoms were related to changing hormone levels; 38.5% were offered antidepressants before HRT. Nearly all (94.6%) said they had experienced negative mood changes and emotions since becoming perimenopausal or menopausal; of these, 19.1% were formally diagnosed with depression or a mood disorder.
“This all just highlights the frustrations I feel around menopause care,” Dr. Newson said. “Women are often not given the tools to properly understand what’s going on and then they don’t ask for the right treatment, and many are given antidepressants. It’s still medicalizing the menopause but in a different way.” ●
Does taking an NSAID while on hormonal contraception increase VTE risk?
Meaidi A, Mascolo A, Sessa M, et al. Venous thromboembolism with use of hormonal contraception and non-steroidal anti-inflammatory drugs: nationwide cohort study. BMJ. 2023;382:e074450. doi:10.1136/bmj-2022-074450
EXPERT COMMENTARY
Combination (estrogen plus progestin) hormonal contraceptives as well as non–aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) increase the risk of VTE events, including lower extremity clots and pulmonary embolism. Taking contraceptives formulated with ethinyl estradiol increases hepatic production of clotting factors on a dose-related basis. Newer progestins, including desogestrel and drospirenone, also may contribute to an elevated VTE risk, although this association is controversial.1 NSAIDs promote platelet aggregation, thereby activating the clotting system and formation of clots. Although studies that assessed the association between NSAID use and thrombosis have focused on arterial clots, a substantial literature suggests that NSAIDs, including older NSAIDs (such as ibuprofen, diclofenac, and naproxen), also increase VTE risk.2
Although combination contraceptives (oral contraceptives, patches, vaginal rings) and NSAIDs are both commonly used by reproductive-age women, little data have assessed the impact of concomitant use of these medications on VTE risk. Accordingly, investigators in Denmark, using national databases, conducted a retrospective cohort study to assess the impact that independent as well as concomitant use of these medications have on VTE risk.
Details of the study
Meaidi and colleagues included in the cohort reproductive-age women living in Denmark between 1996 and 2017 with no history of thrombosis, thrombophilia, cancer, tubal sterilization, hysterectomy, bilateral oophorectomy, or infertility treatment. National prescription data were used to assess exposure to hormonal contraception.
The investigators classified hormonal contraception into 3 VTE risk categories:
- high risk—estrogen-progestin patches and vaginal rings; oral contraceptives containing 50 µg of ethinyl estradiol; or the progestins desogestrel, drospirenone, gestodene, or cyproterone (with the latter 2 progestins not available in the United States)
- medium risk—all other combination oral contraceptives, including those formulated with the progestins norethindrone, norethindrone acetate, norgestrel, and levonorgestrel, as well as depot medroxyprogesterone acetate
- low/no risk—progestin-only pills, implants, and progestin-containing intrauterine devices (IUDs).
Because in Denmark NSAIDs are prescribed as a single package containing no more than 30 tablets, time exposed to non–aspirin NSAIDs was assumed to last 1 week from the prescription date.
The authors considered first-time diagnoses of lower limb venous thrombosis or pulmonary embolism that were made in hospitals to represent VTE. They also constructed a subgroup of VTE patients in whom the diagnosis was either confirmed with imaging or followed by prescription of an anticoagulant.
To address potential confounding, the authors adjusted their analysis based on age, calendar year, educational attainment, occurrence of pregnancy, surgery, hypertension, diabetes, polycystic ovary syndrome, endometriosis, migraine, systemic connective tissue diseases, inflammatory polyarthropathies, and use of tranexamic acid (a medication that may increase VTE risk). They also censored (temporarily excluded women from analysis) episodes associated with a transiently elevated risk of VTE: pregnancy and 6 months following delivery, 12 weeks after other pregnancy terminations, 8 weeks following any surgery involving hospital admission, and 8 weeks following prescription of tranexamic acid.
Continue to: VTEs associated with risk category of hormonal contraception used...
VTEs associated with risk category of hormonal contraception used
Results. The overall cohort included more than 2 million women who were followed for a median of 10 years. During 21.0 million person-years, 8,710 VTE events were diagnosed; almost one-third of these were pulmonary embolisms, with the remainder diagnosed as lower extremity VTE. Of these 8,710 women diagnosed with VTE, 7,043 (81%) were confirmed with either diagnostic imaging or prescription of an anticoagulant. Unfortunately, 228 women (2.6%) died within 30 days of the diagnosis of VTE.
The investigators identified concomitant use of hormonal contraception and NSAIDs in more than 500,000 women. Among women with such concomitant use, 58% were using contraceptives that were high risk while 23% used medium-risk and 19% used low/no-risk contraceptives. Ibuprofen (60%) was the most commonly used NSAID, followed by diclofenac (20%) and naproxen (6%). Between 97% and 98% of high-risk and medium-risk contraceptives were combination pills; 89% of low/no-risk contraceptives were progestin IUDs.
Compared with nonuse of both hormonal contraceptives and NSAIDs, incidence rate ratios of VTE adjusted for age, calendar year, and education were 8.1 (95% confidence interval [CI], 6.9–9.6) for use of NSAIDs only, 4.2 (95% CI, 4.0–4.4) for use of high-risk contraceptives only, 3.0 (95% CI, 2.8–3.2) for medium-risk contraceptive use, and 1.1 (95% CI, 1.0–1.3) for use of low/no-risk hormonal contraception. Risk of VTE was approximately twice as high with the use of diclofenac only compared with the risks associated with ibuprofen or naproxen use only.
With respect to concomitant use of NSAIDs and hormonal contraception, incidence rate ratios of VTE were 50.6 (95% CI, 44.2–57.8), 26.1 (95% CI, 19.6–34.7), and 5.7 (95% CI, 3.3–10.1), respectively, with use of high-risk, medium-risk, and low/no-risk hormonal contraceptives. Adjusting for time updated information on occurrences of migraine, connective tissue disorder, inflammatory polyarthropathies, endometriosis, polycystic ovary syndrome, hypertension, and diabetes did not materially affect these associations.
When analysis was limited to women without these occurring conditions, rate ratios were somewhat higher (5.7 and 4.1) for use of high-risk and medium-risk contraceptives only. Incidence rate ratios in this subcohort of healthier women were substantially higher for NSAID use only (15.0), and 111.7, 43.2, and 13.0, respectively, for concomitant use of NSAIDs with high-risk, medium-risk, and low/no-risk contraceptives. In this analysis of healthier women, diclofenac continued to be associated with substantially higher risks of VTE than ibuprofen or naproxen. When the stricter definition of VTE (confirmed cases) was used, adjusted rate ratios remained similar.
Absolute risks of VTE
Although some of the elevated rate ratios noted in this study might appear alarming, it is important to keep in mind that the baseline incidence of VTE in healthy reproductive-age women is low. Accordingly, as the authors pointed out, even among women who used NSAIDs concomitantly with high-risk combination hormonal contraceptives, the absolute risk of VTE was 2/10,000.
Study strengths and limitations
Strengths of this analysis by Meaidi and colleagues include the use of large, essentially all-inclusive national registries. In addition, nationwide Danish registry data that indicate a diagnosis of VTE have been found to have a high positive predictive value.3 Another strength is the large number of potentially confounding factors that the authors controlled for.
One potential limitation of their analysis is that the use of only prescribed NSAIDs was considered. Fortunately, however, the prevalence of over-the-counter ibuprofen use in Denmark is not high enough to materially affect the authors’ findings.4 Another potential limitation was that information on smoking and body mass index was not available for most of the women included in the study cohort. The authors countered this limitation by pointing out that, in Denmark, smoking and obesity are highly correlated with educational status, and that all analyses were adjusted for educational status. ●
It is important for clinicians and our patients to recognize that pregnancy—the condition prevented by hormonal contraception— is associated with far higher risks of VTE (10–14 VTE events per 10,000 deliveries) than the use of any modern hormonal contraceptive.5 Although concomitant use of combination contraceptives and NSAIDs increases VTE risk, the absolute risk is modest, particularly when the NSAID is ibuprofen or naproxen (these are the non–aspirin NSAIDs most commonly used in the United States6). Women who regularly take NSAIDs can minimize VTE risk by choosing hormonal contraceptives with little or no impact on the risk of VTE: the progestin implant, progestin IUDs, and progestinonly pills.
ANDREW M. KAUNITZ, MD, MSCP
- Reid RL. Oral hormonal contraception and venous thromboembolism (VTE). Contraception. 2014;89:235-236. doi:10.1016/j.contraception.2014.02.002
- Ungprasert P, Srivali N, Wijarnpreecha K, et al. Nonsteroidal anti-inflammatory drugs and risk of venous thromboembolism: a systematic review and meta-analysis. Rheumatology (Oxford). 2015;54:736-742. doi:10.1093 /rheumatology/keu408
- Sundbøll J, Adelborg K, Munch T, et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6:e012832. doi:10.1136/bmjopen-2016-012832
- Gaster N, Hallas J, Pottegård A, et al. The validity of Danish prescription data to measure use of aspirin and other nonsteroidal anti-inflammatory drugs and quantification of bias due to non-prescription drug use. Clin Epidemiol. 2021;13:569-579. doi:10.2147/CLEP.S311450
- Maughan BC, Marin M, Han J, et al. Venous thromboembolism during pregnancy and the postpartum period: risk factors, diagnostic testing, and treatment. Obstet Gynecol Surv. 2022;77:433-444. doi:10.1097/OGX.0000000000001043
- Chu A. Ibuprofen, naproxen, and more: the 8 most common NSAIDs. GoodRx. July 20, 2023. Accessed October 4, 2023. https://www.goodrx.com/classes/nsaids/nsaid-list
Meaidi A, Mascolo A, Sessa M, et al. Venous thromboembolism with use of hormonal contraception and non-steroidal anti-inflammatory drugs: nationwide cohort study. BMJ. 2023;382:e074450. doi:10.1136/bmj-2022-074450
EXPERT COMMENTARY
Combination (estrogen plus progestin) hormonal contraceptives as well as non–aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) increase the risk of VTE events, including lower extremity clots and pulmonary embolism. Taking contraceptives formulated with ethinyl estradiol increases hepatic production of clotting factors on a dose-related basis. Newer progestins, including desogestrel and drospirenone, also may contribute to an elevated VTE risk, although this association is controversial.1 NSAIDs promote platelet aggregation, thereby activating the clotting system and formation of clots. Although studies that assessed the association between NSAID use and thrombosis have focused on arterial clots, a substantial literature suggests that NSAIDs, including older NSAIDs (such as ibuprofen, diclofenac, and naproxen), also increase VTE risk.2
Although combination contraceptives (oral contraceptives, patches, vaginal rings) and NSAIDs are both commonly used by reproductive-age women, little data have assessed the impact of concomitant use of these medications on VTE risk. Accordingly, investigators in Denmark, using national databases, conducted a retrospective cohort study to assess the impact that independent as well as concomitant use of these medications have on VTE risk.
Details of the study
Meaidi and colleagues included in the cohort reproductive-age women living in Denmark between 1996 and 2017 with no history of thrombosis, thrombophilia, cancer, tubal sterilization, hysterectomy, bilateral oophorectomy, or infertility treatment. National prescription data were used to assess exposure to hormonal contraception.
The investigators classified hormonal contraception into 3 VTE risk categories:
- high risk—estrogen-progestin patches and vaginal rings; oral contraceptives containing 50 µg of ethinyl estradiol; or the progestins desogestrel, drospirenone, gestodene, or cyproterone (with the latter 2 progestins not available in the United States)
- medium risk—all other combination oral contraceptives, including those formulated with the progestins norethindrone, norethindrone acetate, norgestrel, and levonorgestrel, as well as depot medroxyprogesterone acetate
- low/no risk—progestin-only pills, implants, and progestin-containing intrauterine devices (IUDs).
Because in Denmark NSAIDs are prescribed as a single package containing no more than 30 tablets, time exposed to non–aspirin NSAIDs was assumed to last 1 week from the prescription date.
The authors considered first-time diagnoses of lower limb venous thrombosis or pulmonary embolism that were made in hospitals to represent VTE. They also constructed a subgroup of VTE patients in whom the diagnosis was either confirmed with imaging or followed by prescription of an anticoagulant.
To address potential confounding, the authors adjusted their analysis based on age, calendar year, educational attainment, occurrence of pregnancy, surgery, hypertension, diabetes, polycystic ovary syndrome, endometriosis, migraine, systemic connective tissue diseases, inflammatory polyarthropathies, and use of tranexamic acid (a medication that may increase VTE risk). They also censored (temporarily excluded women from analysis) episodes associated with a transiently elevated risk of VTE: pregnancy and 6 months following delivery, 12 weeks after other pregnancy terminations, 8 weeks following any surgery involving hospital admission, and 8 weeks following prescription of tranexamic acid.
Continue to: VTEs associated with risk category of hormonal contraception used...
VTEs associated with risk category of hormonal contraception used
Results. The overall cohort included more than 2 million women who were followed for a median of 10 years. During 21.0 million person-years, 8,710 VTE events were diagnosed; almost one-third of these were pulmonary embolisms, with the remainder diagnosed as lower extremity VTE. Of these 8,710 women diagnosed with VTE, 7,043 (81%) were confirmed with either diagnostic imaging or prescription of an anticoagulant. Unfortunately, 228 women (2.6%) died within 30 days of the diagnosis of VTE.
The investigators identified concomitant use of hormonal contraception and NSAIDs in more than 500,000 women. Among women with such concomitant use, 58% were using contraceptives that were high risk while 23% used medium-risk and 19% used low/no-risk contraceptives. Ibuprofen (60%) was the most commonly used NSAID, followed by diclofenac (20%) and naproxen (6%). Between 97% and 98% of high-risk and medium-risk contraceptives were combination pills; 89% of low/no-risk contraceptives were progestin IUDs.
Compared with nonuse of both hormonal contraceptives and NSAIDs, incidence rate ratios of VTE adjusted for age, calendar year, and education were 8.1 (95% confidence interval [CI], 6.9–9.6) for use of NSAIDs only, 4.2 (95% CI, 4.0–4.4) for use of high-risk contraceptives only, 3.0 (95% CI, 2.8–3.2) for medium-risk contraceptive use, and 1.1 (95% CI, 1.0–1.3) for use of low/no-risk hormonal contraception. Risk of VTE was approximately twice as high with the use of diclofenac only compared with the risks associated with ibuprofen or naproxen use only.
With respect to concomitant use of NSAIDs and hormonal contraception, incidence rate ratios of VTE were 50.6 (95% CI, 44.2–57.8), 26.1 (95% CI, 19.6–34.7), and 5.7 (95% CI, 3.3–10.1), respectively, with use of high-risk, medium-risk, and low/no-risk hormonal contraceptives. Adjusting for time updated information on occurrences of migraine, connective tissue disorder, inflammatory polyarthropathies, endometriosis, polycystic ovary syndrome, hypertension, and diabetes did not materially affect these associations.
When analysis was limited to women without these occurring conditions, rate ratios were somewhat higher (5.7 and 4.1) for use of high-risk and medium-risk contraceptives only. Incidence rate ratios in this subcohort of healthier women were substantially higher for NSAID use only (15.0), and 111.7, 43.2, and 13.0, respectively, for concomitant use of NSAIDs with high-risk, medium-risk, and low/no-risk contraceptives. In this analysis of healthier women, diclofenac continued to be associated with substantially higher risks of VTE than ibuprofen or naproxen. When the stricter definition of VTE (confirmed cases) was used, adjusted rate ratios remained similar.
Absolute risks of VTE
Although some of the elevated rate ratios noted in this study might appear alarming, it is important to keep in mind that the baseline incidence of VTE in healthy reproductive-age women is low. Accordingly, as the authors pointed out, even among women who used NSAIDs concomitantly with high-risk combination hormonal contraceptives, the absolute risk of VTE was 2/10,000.
Study strengths and limitations
Strengths of this analysis by Meaidi and colleagues include the use of large, essentially all-inclusive national registries. In addition, nationwide Danish registry data that indicate a diagnosis of VTE have been found to have a high positive predictive value.3 Another strength is the large number of potentially confounding factors that the authors controlled for.
One potential limitation of their analysis is that the use of only prescribed NSAIDs was considered. Fortunately, however, the prevalence of over-the-counter ibuprofen use in Denmark is not high enough to materially affect the authors’ findings.4 Another potential limitation was that information on smoking and body mass index was not available for most of the women included in the study cohort. The authors countered this limitation by pointing out that, in Denmark, smoking and obesity are highly correlated with educational status, and that all analyses were adjusted for educational status. ●
It is important for clinicians and our patients to recognize that pregnancy—the condition prevented by hormonal contraception— is associated with far higher risks of VTE (10–14 VTE events per 10,000 deliveries) than the use of any modern hormonal contraceptive.5 Although concomitant use of combination contraceptives and NSAIDs increases VTE risk, the absolute risk is modest, particularly when the NSAID is ibuprofen or naproxen (these are the non–aspirin NSAIDs most commonly used in the United States6). Women who regularly take NSAIDs can minimize VTE risk by choosing hormonal contraceptives with little or no impact on the risk of VTE: the progestin implant, progestin IUDs, and progestinonly pills.
ANDREW M. KAUNITZ, MD, MSCP
Meaidi A, Mascolo A, Sessa M, et al. Venous thromboembolism with use of hormonal contraception and non-steroidal anti-inflammatory drugs: nationwide cohort study. BMJ. 2023;382:e074450. doi:10.1136/bmj-2022-074450
EXPERT COMMENTARY
Combination (estrogen plus progestin) hormonal contraceptives as well as non–aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) increase the risk of VTE events, including lower extremity clots and pulmonary embolism. Taking contraceptives formulated with ethinyl estradiol increases hepatic production of clotting factors on a dose-related basis. Newer progestins, including desogestrel and drospirenone, also may contribute to an elevated VTE risk, although this association is controversial.1 NSAIDs promote platelet aggregation, thereby activating the clotting system and formation of clots. Although studies that assessed the association between NSAID use and thrombosis have focused on arterial clots, a substantial literature suggests that NSAIDs, including older NSAIDs (such as ibuprofen, diclofenac, and naproxen), also increase VTE risk.2
Although combination contraceptives (oral contraceptives, patches, vaginal rings) and NSAIDs are both commonly used by reproductive-age women, little data have assessed the impact of concomitant use of these medications on VTE risk. Accordingly, investigators in Denmark, using national databases, conducted a retrospective cohort study to assess the impact that independent as well as concomitant use of these medications have on VTE risk.
Details of the study
Meaidi and colleagues included in the cohort reproductive-age women living in Denmark between 1996 and 2017 with no history of thrombosis, thrombophilia, cancer, tubal sterilization, hysterectomy, bilateral oophorectomy, or infertility treatment. National prescription data were used to assess exposure to hormonal contraception.
The investigators classified hormonal contraception into 3 VTE risk categories:
- high risk—estrogen-progestin patches and vaginal rings; oral contraceptives containing 50 µg of ethinyl estradiol; or the progestins desogestrel, drospirenone, gestodene, or cyproterone (with the latter 2 progestins not available in the United States)
- medium risk—all other combination oral contraceptives, including those formulated with the progestins norethindrone, norethindrone acetate, norgestrel, and levonorgestrel, as well as depot medroxyprogesterone acetate
- low/no risk—progestin-only pills, implants, and progestin-containing intrauterine devices (IUDs).
Because in Denmark NSAIDs are prescribed as a single package containing no more than 30 tablets, time exposed to non–aspirin NSAIDs was assumed to last 1 week from the prescription date.
The authors considered first-time diagnoses of lower limb venous thrombosis or pulmonary embolism that were made in hospitals to represent VTE. They also constructed a subgroup of VTE patients in whom the diagnosis was either confirmed with imaging or followed by prescription of an anticoagulant.
To address potential confounding, the authors adjusted their analysis based on age, calendar year, educational attainment, occurrence of pregnancy, surgery, hypertension, diabetes, polycystic ovary syndrome, endometriosis, migraine, systemic connective tissue diseases, inflammatory polyarthropathies, and use of tranexamic acid (a medication that may increase VTE risk). They also censored (temporarily excluded women from analysis) episodes associated with a transiently elevated risk of VTE: pregnancy and 6 months following delivery, 12 weeks after other pregnancy terminations, 8 weeks following any surgery involving hospital admission, and 8 weeks following prescription of tranexamic acid.
Continue to: VTEs associated with risk category of hormonal contraception used...
VTEs associated with risk category of hormonal contraception used
Results. The overall cohort included more than 2 million women who were followed for a median of 10 years. During 21.0 million person-years, 8,710 VTE events were diagnosed; almost one-third of these were pulmonary embolisms, with the remainder diagnosed as lower extremity VTE. Of these 8,710 women diagnosed with VTE, 7,043 (81%) were confirmed with either diagnostic imaging or prescription of an anticoagulant. Unfortunately, 228 women (2.6%) died within 30 days of the diagnosis of VTE.
The investigators identified concomitant use of hormonal contraception and NSAIDs in more than 500,000 women. Among women with such concomitant use, 58% were using contraceptives that were high risk while 23% used medium-risk and 19% used low/no-risk contraceptives. Ibuprofen (60%) was the most commonly used NSAID, followed by diclofenac (20%) and naproxen (6%). Between 97% and 98% of high-risk and medium-risk contraceptives were combination pills; 89% of low/no-risk contraceptives were progestin IUDs.
Compared with nonuse of both hormonal contraceptives and NSAIDs, incidence rate ratios of VTE adjusted for age, calendar year, and education were 8.1 (95% confidence interval [CI], 6.9–9.6) for use of NSAIDs only, 4.2 (95% CI, 4.0–4.4) for use of high-risk contraceptives only, 3.0 (95% CI, 2.8–3.2) for medium-risk contraceptive use, and 1.1 (95% CI, 1.0–1.3) for use of low/no-risk hormonal contraception. Risk of VTE was approximately twice as high with the use of diclofenac only compared with the risks associated with ibuprofen or naproxen use only.
With respect to concomitant use of NSAIDs and hormonal contraception, incidence rate ratios of VTE were 50.6 (95% CI, 44.2–57.8), 26.1 (95% CI, 19.6–34.7), and 5.7 (95% CI, 3.3–10.1), respectively, with use of high-risk, medium-risk, and low/no-risk hormonal contraceptives. Adjusting for time updated information on occurrences of migraine, connective tissue disorder, inflammatory polyarthropathies, endometriosis, polycystic ovary syndrome, hypertension, and diabetes did not materially affect these associations.
When analysis was limited to women without these occurring conditions, rate ratios were somewhat higher (5.7 and 4.1) for use of high-risk and medium-risk contraceptives only. Incidence rate ratios in this subcohort of healthier women were substantially higher for NSAID use only (15.0), and 111.7, 43.2, and 13.0, respectively, for concomitant use of NSAIDs with high-risk, medium-risk, and low/no-risk contraceptives. In this analysis of healthier women, diclofenac continued to be associated with substantially higher risks of VTE than ibuprofen or naproxen. When the stricter definition of VTE (confirmed cases) was used, adjusted rate ratios remained similar.
Absolute risks of VTE
Although some of the elevated rate ratios noted in this study might appear alarming, it is important to keep in mind that the baseline incidence of VTE in healthy reproductive-age women is low. Accordingly, as the authors pointed out, even among women who used NSAIDs concomitantly with high-risk combination hormonal contraceptives, the absolute risk of VTE was 2/10,000.
Study strengths and limitations
Strengths of this analysis by Meaidi and colleagues include the use of large, essentially all-inclusive national registries. In addition, nationwide Danish registry data that indicate a diagnosis of VTE have been found to have a high positive predictive value.3 Another strength is the large number of potentially confounding factors that the authors controlled for.
One potential limitation of their analysis is that the use of only prescribed NSAIDs was considered. Fortunately, however, the prevalence of over-the-counter ibuprofen use in Denmark is not high enough to materially affect the authors’ findings.4 Another potential limitation was that information on smoking and body mass index was not available for most of the women included in the study cohort. The authors countered this limitation by pointing out that, in Denmark, smoking and obesity are highly correlated with educational status, and that all analyses were adjusted for educational status. ●
It is important for clinicians and our patients to recognize that pregnancy—the condition prevented by hormonal contraception— is associated with far higher risks of VTE (10–14 VTE events per 10,000 deliveries) than the use of any modern hormonal contraceptive.5 Although concomitant use of combination contraceptives and NSAIDs increases VTE risk, the absolute risk is modest, particularly when the NSAID is ibuprofen or naproxen (these are the non–aspirin NSAIDs most commonly used in the United States6). Women who regularly take NSAIDs can minimize VTE risk by choosing hormonal contraceptives with little or no impact on the risk of VTE: the progestin implant, progestin IUDs, and progestinonly pills.
ANDREW M. KAUNITZ, MD, MSCP
- Reid RL. Oral hormonal contraception and venous thromboembolism (VTE). Contraception. 2014;89:235-236. doi:10.1016/j.contraception.2014.02.002
- Ungprasert P, Srivali N, Wijarnpreecha K, et al. Nonsteroidal anti-inflammatory drugs and risk of venous thromboembolism: a systematic review and meta-analysis. Rheumatology (Oxford). 2015;54:736-742. doi:10.1093 /rheumatology/keu408
- Sundbøll J, Adelborg K, Munch T, et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6:e012832. doi:10.1136/bmjopen-2016-012832
- Gaster N, Hallas J, Pottegård A, et al. The validity of Danish prescription data to measure use of aspirin and other nonsteroidal anti-inflammatory drugs and quantification of bias due to non-prescription drug use. Clin Epidemiol. 2021;13:569-579. doi:10.2147/CLEP.S311450
- Maughan BC, Marin M, Han J, et al. Venous thromboembolism during pregnancy and the postpartum period: risk factors, diagnostic testing, and treatment. Obstet Gynecol Surv. 2022;77:433-444. doi:10.1097/OGX.0000000000001043
- Chu A. Ibuprofen, naproxen, and more: the 8 most common NSAIDs. GoodRx. July 20, 2023. Accessed October 4, 2023. https://www.goodrx.com/classes/nsaids/nsaid-list
- Reid RL. Oral hormonal contraception and venous thromboembolism (VTE). Contraception. 2014;89:235-236. doi:10.1016/j.contraception.2014.02.002
- Ungprasert P, Srivali N, Wijarnpreecha K, et al. Nonsteroidal anti-inflammatory drugs and risk of venous thromboembolism: a systematic review and meta-analysis. Rheumatology (Oxford). 2015;54:736-742. doi:10.1093 /rheumatology/keu408
- Sundbøll J, Adelborg K, Munch T, et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6:e012832. doi:10.1136/bmjopen-2016-012832
- Gaster N, Hallas J, Pottegård A, et al. The validity of Danish prescription data to measure use of aspirin and other nonsteroidal anti-inflammatory drugs and quantification of bias due to non-prescription drug use. Clin Epidemiol. 2021;13:569-579. doi:10.2147/CLEP.S311450
- Maughan BC, Marin M, Han J, et al. Venous thromboembolism during pregnancy and the postpartum period: risk factors, diagnostic testing, and treatment. Obstet Gynecol Surv. 2022;77:433-444. doi:10.1097/OGX.0000000000001043
- Chu A. Ibuprofen, naproxen, and more: the 8 most common NSAIDs. GoodRx. July 20, 2023. Accessed October 4, 2023. https://www.goodrx.com/classes/nsaids/nsaid-list
Is the 9-valent HPV vaccine safe and effective long term?
Restrepo J, Herrera T, Samakoses R, et al. Ten-year follow-up of 9-valent human papillomavirus vaccine: immunogenicity, effectiveness, and safety. Pediatrics. 2023;152:e2022060993. doi:10.1542/peds.2022-060993
EXPERT COMMENTARY
Infection with human papillomavirus (HPV) is associated with nearly all cases of cervical cancer. Long-term safety and efficacy of the bivalent (Cervarix) and quadrivalent (Gardasil) vaccines have been demonstrated for up to 10 to 14 years.1-6 It is estimated that the 9-valent vaccine (Gardasil 9), which was licensed in 2014 and protects against HPV 16/18/31/33/45/52/58 and HPV 6/11, could prevent up to 90% of cervical cancer cases. The bivalent and quadrivalent vaccines could ideally prevent 70% of cases of cervical cancer. In a recent study, authors compared the efficacy and safety of the newer 9-valent vaccine at 10 years with long-term outcomes of previous vaccine studies.7
Details of the study
Study V503-002 conducted by Luxembourg and colleagues originally enrolled 1,935 boys and girls from 66 sites in Africa, Asia, Europe, Latin America, and North America to receive 3 doses of the 9-valent HPV vaccine, with follow-up for 12 to 36 months to monitor safety and immunogenicity.8 In an extension of this investigation, Restrepo and colleagues revisited 40 of these sites in 13 countries to gather 10 years of long-term follow-up data.7
The final long-term follow-up cohort included 971 girls and 301 boys aged 9 to 15 at vaccination.
Results. At month 126, participants continued to have very high seropositive rates (81%–100%, depending on assay sensitivity and HPV type). There were no cases of high-grade cervical, vaginal, or vulvar dysplasia related to HPV strains covered in the vaccine. Rates of infection in women with the vaccine-targeted HPV types were very low—54.6 per 10,000 person-years—compared with 927.4 per 10,000 person-years for HPV types not included in the vaccine. No adverse events attributable to the vaccine were reported.
Study strengths and limitations
Strengths of this study included the use of rigorous end points similar to those used in the initial efficacy studies for easy comparison. Limitations included the relatively small size, which precluded a robust assessment of adverse events, as well as the lack of controls. Furthermore, this study looked at children receiving 3 doses of HPV vaccine prior to the age of 15 and may not be generalizable to people who receive the vaccine at an older age or in fewer doses. ●
Previous studies have shown that the 9-valent HPV vaccine is effective and yields immunological responses within 4 weeks of receiving 3 doses, with sustained immunogenicity up to 36 months. The study by Restrepo and colleagues provides long-term follow-up data that demonstrated sustained immunological responses at 10 years following immunization, with no cases of high-grade intraepithelial neoplasia related to the covered HPV types and no adverse events. These results compare favorably with those of prior studies of the bivalent and quadrivalent HPV vaccines. The 9-valent HPV vaccine can be recommended for use in children aged 9 to 15 with excellent confidence regarding its safety and sustained effectiveness for at least 10 years after vaccination.
DIANA MIAO, MD; SARAH FELDMAN, MD, MPH
- Naud PS, Roteli-Martins CM, De Carvalho NS, et al. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum Vaccin Immunother. 2014;10:2147-2162. doi:10.4161/hv.29532
- Schwarz TF, Galaj A, Spaczynski M, et al. Ten-year immune persistence and safety of the HPV-16/18 AS04-adjuvanted vaccine in females vaccinated at 15–55 years of age. Cancer Med. 2017;6:2723-2731. doi:10.1002/cam4.1155
- Ferris DG, Samakoses R, Block SL, et al. 4-valent human papillomavirus (4vHPV) vaccine in preadolescents and adolescents after 10 years. Pediatrics. 2017;140:e20163947. doi:10.1542/peds.2016-3947
- Kjaer SK, Nygård M, Sundström K, et al. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four Nordic countries. EClinicalMedicine. 2020;23:100401. doi:10.1016 /j.eclinm.2020.100401
- Porras C, Tsang SH, Herrero R, et al; Costa Rica Vaccine Trial Group. Efficacy of the bivalent HPV vaccine against HPV 16/18-associated precancer: long-term follow-up results from the Costa Rica Vaccine Trial. Lancet Oncol. 2020;21:16431652. doi:10.1016/S1470-2045(20)30524-6
- Van Damme P, Olsson SE, Block S, et al. Immunogenicity and safety of a 9-valent HPV vaccine. Pediatrics. 2015;136:e28-e39. doi:10.1542/peds.2014-3745
- Restrepo J, Herrera T, Samakoses R, et al. Ten-year follow-up of 9-valent human papillomavirus vaccine: immunogenicity, effectiveness, and safety. Pediatrics. 2023;152:e2022060993. doi:10.1542/peds.2022-060993
- Luxembourg A, Moreira Jr ED, Samakoses R, et al. Phase III, randomized controlled trial in girls 9-15 years old to evaluate lot consistency of a novel nine-valent human papillomavirus L1 virus-like particle vaccine. Hum Vaccin Immunother. 11:1306-1312. doi:10.1080/21645515.2015.1009819
Restrepo J, Herrera T, Samakoses R, et al. Ten-year follow-up of 9-valent human papillomavirus vaccine: immunogenicity, effectiveness, and safety. Pediatrics. 2023;152:e2022060993. doi:10.1542/peds.2022-060993
EXPERT COMMENTARY
Infection with human papillomavirus (HPV) is associated with nearly all cases of cervical cancer. Long-term safety and efficacy of the bivalent (Cervarix) and quadrivalent (Gardasil) vaccines have been demonstrated for up to 10 to 14 years.1-6 It is estimated that the 9-valent vaccine (Gardasil 9), which was licensed in 2014 and protects against HPV 16/18/31/33/45/52/58 and HPV 6/11, could prevent up to 90% of cervical cancer cases. The bivalent and quadrivalent vaccines could ideally prevent 70% of cases of cervical cancer. In a recent study, authors compared the efficacy and safety of the newer 9-valent vaccine at 10 years with long-term outcomes of previous vaccine studies.7
Details of the study
Study V503-002 conducted by Luxembourg and colleagues originally enrolled 1,935 boys and girls from 66 sites in Africa, Asia, Europe, Latin America, and North America to receive 3 doses of the 9-valent HPV vaccine, with follow-up for 12 to 36 months to monitor safety and immunogenicity.8 In an extension of this investigation, Restrepo and colleagues revisited 40 of these sites in 13 countries to gather 10 years of long-term follow-up data.7
The final long-term follow-up cohort included 971 girls and 301 boys aged 9 to 15 at vaccination.
Results. At month 126, participants continued to have very high seropositive rates (81%–100%, depending on assay sensitivity and HPV type). There were no cases of high-grade cervical, vaginal, or vulvar dysplasia related to HPV strains covered in the vaccine. Rates of infection in women with the vaccine-targeted HPV types were very low—54.6 per 10,000 person-years—compared with 927.4 per 10,000 person-years for HPV types not included in the vaccine. No adverse events attributable to the vaccine were reported.
Study strengths and limitations
Strengths of this study included the use of rigorous end points similar to those used in the initial efficacy studies for easy comparison. Limitations included the relatively small size, which precluded a robust assessment of adverse events, as well as the lack of controls. Furthermore, this study looked at children receiving 3 doses of HPV vaccine prior to the age of 15 and may not be generalizable to people who receive the vaccine at an older age or in fewer doses. ●
Previous studies have shown that the 9-valent HPV vaccine is effective and yields immunological responses within 4 weeks of receiving 3 doses, with sustained immunogenicity up to 36 months. The study by Restrepo and colleagues provides long-term follow-up data that demonstrated sustained immunological responses at 10 years following immunization, with no cases of high-grade intraepithelial neoplasia related to the covered HPV types and no adverse events. These results compare favorably with those of prior studies of the bivalent and quadrivalent HPV vaccines. The 9-valent HPV vaccine can be recommended for use in children aged 9 to 15 with excellent confidence regarding its safety and sustained effectiveness for at least 10 years after vaccination.
DIANA MIAO, MD; SARAH FELDMAN, MD, MPH
Restrepo J, Herrera T, Samakoses R, et al. Ten-year follow-up of 9-valent human papillomavirus vaccine: immunogenicity, effectiveness, and safety. Pediatrics. 2023;152:e2022060993. doi:10.1542/peds.2022-060993
EXPERT COMMENTARY
Infection with human papillomavirus (HPV) is associated with nearly all cases of cervical cancer. Long-term safety and efficacy of the bivalent (Cervarix) and quadrivalent (Gardasil) vaccines have been demonstrated for up to 10 to 14 years.1-6 It is estimated that the 9-valent vaccine (Gardasil 9), which was licensed in 2014 and protects against HPV 16/18/31/33/45/52/58 and HPV 6/11, could prevent up to 90% of cervical cancer cases. The bivalent and quadrivalent vaccines could ideally prevent 70% of cases of cervical cancer. In a recent study, authors compared the efficacy and safety of the newer 9-valent vaccine at 10 years with long-term outcomes of previous vaccine studies.7
Details of the study
Study V503-002 conducted by Luxembourg and colleagues originally enrolled 1,935 boys and girls from 66 sites in Africa, Asia, Europe, Latin America, and North America to receive 3 doses of the 9-valent HPV vaccine, with follow-up for 12 to 36 months to monitor safety and immunogenicity.8 In an extension of this investigation, Restrepo and colleagues revisited 40 of these sites in 13 countries to gather 10 years of long-term follow-up data.7
The final long-term follow-up cohort included 971 girls and 301 boys aged 9 to 15 at vaccination.
Results. At month 126, participants continued to have very high seropositive rates (81%–100%, depending on assay sensitivity and HPV type). There were no cases of high-grade cervical, vaginal, or vulvar dysplasia related to HPV strains covered in the vaccine. Rates of infection in women with the vaccine-targeted HPV types were very low—54.6 per 10,000 person-years—compared with 927.4 per 10,000 person-years for HPV types not included in the vaccine. No adverse events attributable to the vaccine were reported.
Study strengths and limitations
Strengths of this study included the use of rigorous end points similar to those used in the initial efficacy studies for easy comparison. Limitations included the relatively small size, which precluded a robust assessment of adverse events, as well as the lack of controls. Furthermore, this study looked at children receiving 3 doses of HPV vaccine prior to the age of 15 and may not be generalizable to people who receive the vaccine at an older age or in fewer doses. ●
Previous studies have shown that the 9-valent HPV vaccine is effective and yields immunological responses within 4 weeks of receiving 3 doses, with sustained immunogenicity up to 36 months. The study by Restrepo and colleagues provides long-term follow-up data that demonstrated sustained immunological responses at 10 years following immunization, with no cases of high-grade intraepithelial neoplasia related to the covered HPV types and no adverse events. These results compare favorably with those of prior studies of the bivalent and quadrivalent HPV vaccines. The 9-valent HPV vaccine can be recommended for use in children aged 9 to 15 with excellent confidence regarding its safety and sustained effectiveness for at least 10 years after vaccination.
DIANA MIAO, MD; SARAH FELDMAN, MD, MPH
- Naud PS, Roteli-Martins CM, De Carvalho NS, et al. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum Vaccin Immunother. 2014;10:2147-2162. doi:10.4161/hv.29532
- Schwarz TF, Galaj A, Spaczynski M, et al. Ten-year immune persistence and safety of the HPV-16/18 AS04-adjuvanted vaccine in females vaccinated at 15–55 years of age. Cancer Med. 2017;6:2723-2731. doi:10.1002/cam4.1155
- Ferris DG, Samakoses R, Block SL, et al. 4-valent human papillomavirus (4vHPV) vaccine in preadolescents and adolescents after 10 years. Pediatrics. 2017;140:e20163947. doi:10.1542/peds.2016-3947
- Kjaer SK, Nygård M, Sundström K, et al. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four Nordic countries. EClinicalMedicine. 2020;23:100401. doi:10.1016 /j.eclinm.2020.100401
- Porras C, Tsang SH, Herrero R, et al; Costa Rica Vaccine Trial Group. Efficacy of the bivalent HPV vaccine against HPV 16/18-associated precancer: long-term follow-up results from the Costa Rica Vaccine Trial. Lancet Oncol. 2020;21:16431652. doi:10.1016/S1470-2045(20)30524-6
- Van Damme P, Olsson SE, Block S, et al. Immunogenicity and safety of a 9-valent HPV vaccine. Pediatrics. 2015;136:e28-e39. doi:10.1542/peds.2014-3745
- Restrepo J, Herrera T, Samakoses R, et al. Ten-year follow-up of 9-valent human papillomavirus vaccine: immunogenicity, effectiveness, and safety. Pediatrics. 2023;152:e2022060993. doi:10.1542/peds.2022-060993
- Luxembourg A, Moreira Jr ED, Samakoses R, et al. Phase III, randomized controlled trial in girls 9-15 years old to evaluate lot consistency of a novel nine-valent human papillomavirus L1 virus-like particle vaccine. Hum Vaccin Immunother. 11:1306-1312. doi:10.1080/21645515.2015.1009819
- Naud PS, Roteli-Martins CM, De Carvalho NS, et al. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum Vaccin Immunother. 2014;10:2147-2162. doi:10.4161/hv.29532
- Schwarz TF, Galaj A, Spaczynski M, et al. Ten-year immune persistence and safety of the HPV-16/18 AS04-adjuvanted vaccine in females vaccinated at 15–55 years of age. Cancer Med. 2017;6:2723-2731. doi:10.1002/cam4.1155
- Ferris DG, Samakoses R, Block SL, et al. 4-valent human papillomavirus (4vHPV) vaccine in preadolescents and adolescents after 10 years. Pediatrics. 2017;140:e20163947. doi:10.1542/peds.2016-3947
- Kjaer SK, Nygård M, Sundström K, et al. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four Nordic countries. EClinicalMedicine. 2020;23:100401. doi:10.1016 /j.eclinm.2020.100401
- Porras C, Tsang SH, Herrero R, et al; Costa Rica Vaccine Trial Group. Efficacy of the bivalent HPV vaccine against HPV 16/18-associated precancer: long-term follow-up results from the Costa Rica Vaccine Trial. Lancet Oncol. 2020;21:16431652. doi:10.1016/S1470-2045(20)30524-6
- Van Damme P, Olsson SE, Block S, et al. Immunogenicity and safety of a 9-valent HPV vaccine. Pediatrics. 2015;136:e28-e39. doi:10.1542/peds.2014-3745
- Restrepo J, Herrera T, Samakoses R, et al. Ten-year follow-up of 9-valent human papillomavirus vaccine: immunogenicity, effectiveness, and safety. Pediatrics. 2023;152:e2022060993. doi:10.1542/peds.2022-060993
- Luxembourg A, Moreira Jr ED, Samakoses R, et al. Phase III, randomized controlled trial in girls 9-15 years old to evaluate lot consistency of a novel nine-valent human papillomavirus L1 virus-like particle vaccine. Hum Vaccin Immunother. 11:1306-1312. doi:10.1080/21645515.2015.1009819