User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
The devil in the (masking) details
The Devil’s own face covering?
It’s been over a year and a half since the COVID-19 emergency was declared in the United States, and we’ve been starting to wonder what our good friend SARS-CoV-2 has left to give. The collective cynic/optimist in us figures that the insanity can’t last forever, right?
Maybe not forever, but …
A group of parents is suing the Central Bucks (Pa.) School District over school mask mandates, suggesting that the district has no legal authority to enforce such measures. Most of their arguments, Philadelphia Magazine says, are pretty standard stuff: Masks are causing depression, anxiety, and discomfort in their children; masks are a violation of their constitutional rights; and “masks are being used as a control mechanism over the population.”
There are some unusual claims, though. One of the parents, Shannon Harris, said that “wearing masks interferes with their religious duty to spread the word of God and forces them to participate in a satanic ritual,” according to the Philadelphia Inquirer.
Philadelphia Magazine decided to check on that “satanic ritual” claim by asking an expert, in this case a spokesperson for the Church of Satan. The Reverend Raul Antony said that “simply ‘wearing a mask’ is not a Satanic ritual, and anyone that genuinely thinks otherwise is a blithering idiot,” adding that the group’s rituals were available on its website.
COVID, you never let us down.
You’re the (hurricane) wind beneath my wings
Marriage isn’t easy. From finances to everyday stressors like work and children, maintaining a solid relationship is tough. Then a natural disaster shows up on top of everything else, and marriages actually improve, researchers found.
In a study published by Psychological Science, researchers surveyed 231 newlywed couples about the satisfaction of their marriage before and after Hurricane Harvey in 2017. They found after the hurricane couples had a “significant boost” in the satisfaction of their relationship.
One would think something like this would create what researchers call a “stress spillover,” creating a decrease in relationship satisfaction. Destruction to your home or even displacement after a natural disaster seems pretty stressful. But, “a natural disaster can really put things in perspective. People realize how important their partner is to them when they are jolted out of the day-to-day stress of life,” said Hannah Williamson, PhD, the lead author of the study.
And although everyone saw an increase, the biggest jumps in relationship satisfaction belonged to the people who were most unhappy before the hurricane. Unfortunately, the researchers also found that the effects were only temporary and the dissatisfaction came back within a year.
Dr. Williamson thinks there may be something to these findings that can be beneficial from a therapy standpoint where “couples can shift their perspective in a similar way without having to go through a natural disaster.”
Let’s hope she’s right, because the alternative is to seek out a rampaging hurricane every time your relationship is on the rocks, and that just seems impractical after the second or third year.
Not-so-essential oils
Many people use essential oils as a way to unwind and relax. Stressed? Can’t sleep? There’s probably an essential oil for that. However, it seems like these days a lot of things we love and/or think are good for us have a side that’s not so.
According to the Centers for Disease Control and Prevention, a woman from Georgia died from a rare bacteria called Burkholderia pseudomallei. There have been three previous infections in Kansas, Minnesota, and Texas throughout 2021; two of the four infections were in children. Melioidosis, the disease caused by B. pseudomallei, is usually found in southeast Asia and isn’t obvious or easy to diagnose, especially in places like decidedly untropical Minnesota.
The Georgia case was the real break in this medical mystery, as the infection was traced back to a Walmart product called “Better Homes and Gardens Essential Oil Infused Aromatherapy Room Spray with Gemstones” (a very pithy name). The bacteria were in the lavender and chamomile scent. The CDC is investigating all other product scents, and Walmart has recalled all lots of the product.
If you’ve got that particular essential oil, it’s probably for the best that you stop using it. Don’t worry, we’re sure there’s plenty of other essential oil–infused aromatherapy room sprays with gemstones out there for your scent-based needs.
Welcome to the Ministry of Sleep-Deprived Walks
Walking is simple, right? You put one foot in front of the other, and soon you’re walking out the door. Little kids can do it. Even zombies can walk, and they don’t even have brains.
Research from MIT and the University of São Paulo has shown that walking is a little trickier than we might think. One researcher in particular noticed that student volunteers tended to perform worse toward the end of semesters, as project deadlines and multiple exams crashed over their heads and they were deprived of solid sleep schedules.
In a study published in Scientific Reports, our intrepid walking researchers had a collection of students monitor their sleep patterns for 2 weeks; on average, the students got 6 hours per night, though some were able to compensate on weekends. On the final day of a 14-day period, some students pulled all-nighters while the rest were allowed to sleep as usual. Then all students performed a walking test involving keeping time with a metronome.
To absolutely no one’s surprise, the students who performed all-nighters before being tested walked the worst, but between the other students, the ones who compensated for sleep deprivation on weekends did better than those who got 6 hours every night, despite getting a similar amount of sleep overall. This effect persisted even when the compensating students performed their walking tests late in the week, just before they got their weekend beauty sleep.
The moral of the story? Sleep is good, and you should get more of it. But if you can’t, sleep in on weekends. Science has given you permission. All those suburban dads looking to get their teenagers up at 8 in the morning must be sweating right now.
The Devil’s own face covering?
It’s been over a year and a half since the COVID-19 emergency was declared in the United States, and we’ve been starting to wonder what our good friend SARS-CoV-2 has left to give. The collective cynic/optimist in us figures that the insanity can’t last forever, right?
Maybe not forever, but …
A group of parents is suing the Central Bucks (Pa.) School District over school mask mandates, suggesting that the district has no legal authority to enforce such measures. Most of their arguments, Philadelphia Magazine says, are pretty standard stuff: Masks are causing depression, anxiety, and discomfort in their children; masks are a violation of their constitutional rights; and “masks are being used as a control mechanism over the population.”
There are some unusual claims, though. One of the parents, Shannon Harris, said that “wearing masks interferes with their religious duty to spread the word of God and forces them to participate in a satanic ritual,” according to the Philadelphia Inquirer.
Philadelphia Magazine decided to check on that “satanic ritual” claim by asking an expert, in this case a spokesperson for the Church of Satan. The Reverend Raul Antony said that “simply ‘wearing a mask’ is not a Satanic ritual, and anyone that genuinely thinks otherwise is a blithering idiot,” adding that the group’s rituals were available on its website.
COVID, you never let us down.
You’re the (hurricane) wind beneath my wings
Marriage isn’t easy. From finances to everyday stressors like work and children, maintaining a solid relationship is tough. Then a natural disaster shows up on top of everything else, and marriages actually improve, researchers found.
In a study published by Psychological Science, researchers surveyed 231 newlywed couples about the satisfaction of their marriage before and after Hurricane Harvey in 2017. They found after the hurricane couples had a “significant boost” in the satisfaction of their relationship.
One would think something like this would create what researchers call a “stress spillover,” creating a decrease in relationship satisfaction. Destruction to your home or even displacement after a natural disaster seems pretty stressful. But, “a natural disaster can really put things in perspective. People realize how important their partner is to them when they are jolted out of the day-to-day stress of life,” said Hannah Williamson, PhD, the lead author of the study.
And although everyone saw an increase, the biggest jumps in relationship satisfaction belonged to the people who were most unhappy before the hurricane. Unfortunately, the researchers also found that the effects were only temporary and the dissatisfaction came back within a year.
Dr. Williamson thinks there may be something to these findings that can be beneficial from a therapy standpoint where “couples can shift their perspective in a similar way without having to go through a natural disaster.”
Let’s hope she’s right, because the alternative is to seek out a rampaging hurricane every time your relationship is on the rocks, and that just seems impractical after the second or third year.
Not-so-essential oils
Many people use essential oils as a way to unwind and relax. Stressed? Can’t sleep? There’s probably an essential oil for that. However, it seems like these days a lot of things we love and/or think are good for us have a side that’s not so.
According to the Centers for Disease Control and Prevention, a woman from Georgia died from a rare bacteria called Burkholderia pseudomallei. There have been three previous infections in Kansas, Minnesota, and Texas throughout 2021; two of the four infections were in children. Melioidosis, the disease caused by B. pseudomallei, is usually found in southeast Asia and isn’t obvious or easy to diagnose, especially in places like decidedly untropical Minnesota.
The Georgia case was the real break in this medical mystery, as the infection was traced back to a Walmart product called “Better Homes and Gardens Essential Oil Infused Aromatherapy Room Spray with Gemstones” (a very pithy name). The bacteria were in the lavender and chamomile scent. The CDC is investigating all other product scents, and Walmart has recalled all lots of the product.
If you’ve got that particular essential oil, it’s probably for the best that you stop using it. Don’t worry, we’re sure there’s plenty of other essential oil–infused aromatherapy room sprays with gemstones out there for your scent-based needs.
Welcome to the Ministry of Sleep-Deprived Walks
Walking is simple, right? You put one foot in front of the other, and soon you’re walking out the door. Little kids can do it. Even zombies can walk, and they don’t even have brains.
Research from MIT and the University of São Paulo has shown that walking is a little trickier than we might think. One researcher in particular noticed that student volunteers tended to perform worse toward the end of semesters, as project deadlines and multiple exams crashed over their heads and they were deprived of solid sleep schedules.
In a study published in Scientific Reports, our intrepid walking researchers had a collection of students monitor their sleep patterns for 2 weeks; on average, the students got 6 hours per night, though some were able to compensate on weekends. On the final day of a 14-day period, some students pulled all-nighters while the rest were allowed to sleep as usual. Then all students performed a walking test involving keeping time with a metronome.
To absolutely no one’s surprise, the students who performed all-nighters before being tested walked the worst, but between the other students, the ones who compensated for sleep deprivation on weekends did better than those who got 6 hours every night, despite getting a similar amount of sleep overall. This effect persisted even when the compensating students performed their walking tests late in the week, just before they got their weekend beauty sleep.
The moral of the story? Sleep is good, and you should get more of it. But if you can’t, sleep in on weekends. Science has given you permission. All those suburban dads looking to get their teenagers up at 8 in the morning must be sweating right now.
The Devil’s own face covering?
It’s been over a year and a half since the COVID-19 emergency was declared in the United States, and we’ve been starting to wonder what our good friend SARS-CoV-2 has left to give. The collective cynic/optimist in us figures that the insanity can’t last forever, right?
Maybe not forever, but …
A group of parents is suing the Central Bucks (Pa.) School District over school mask mandates, suggesting that the district has no legal authority to enforce such measures. Most of their arguments, Philadelphia Magazine says, are pretty standard stuff: Masks are causing depression, anxiety, and discomfort in their children; masks are a violation of their constitutional rights; and “masks are being used as a control mechanism over the population.”
There are some unusual claims, though. One of the parents, Shannon Harris, said that “wearing masks interferes with their religious duty to spread the word of God and forces them to participate in a satanic ritual,” according to the Philadelphia Inquirer.
Philadelphia Magazine decided to check on that “satanic ritual” claim by asking an expert, in this case a spokesperson for the Church of Satan. The Reverend Raul Antony said that “simply ‘wearing a mask’ is not a Satanic ritual, and anyone that genuinely thinks otherwise is a blithering idiot,” adding that the group’s rituals were available on its website.
COVID, you never let us down.
You’re the (hurricane) wind beneath my wings
Marriage isn’t easy. From finances to everyday stressors like work and children, maintaining a solid relationship is tough. Then a natural disaster shows up on top of everything else, and marriages actually improve, researchers found.
In a study published by Psychological Science, researchers surveyed 231 newlywed couples about the satisfaction of their marriage before and after Hurricane Harvey in 2017. They found after the hurricane couples had a “significant boost” in the satisfaction of their relationship.
One would think something like this would create what researchers call a “stress spillover,” creating a decrease in relationship satisfaction. Destruction to your home or even displacement after a natural disaster seems pretty stressful. But, “a natural disaster can really put things in perspective. People realize how important their partner is to them when they are jolted out of the day-to-day stress of life,” said Hannah Williamson, PhD, the lead author of the study.
And although everyone saw an increase, the biggest jumps in relationship satisfaction belonged to the people who were most unhappy before the hurricane. Unfortunately, the researchers also found that the effects were only temporary and the dissatisfaction came back within a year.
Dr. Williamson thinks there may be something to these findings that can be beneficial from a therapy standpoint where “couples can shift their perspective in a similar way without having to go through a natural disaster.”
Let’s hope she’s right, because the alternative is to seek out a rampaging hurricane every time your relationship is on the rocks, and that just seems impractical after the second or third year.
Not-so-essential oils
Many people use essential oils as a way to unwind and relax. Stressed? Can’t sleep? There’s probably an essential oil for that. However, it seems like these days a lot of things we love and/or think are good for us have a side that’s not so.
According to the Centers for Disease Control and Prevention, a woman from Georgia died from a rare bacteria called Burkholderia pseudomallei. There have been three previous infections in Kansas, Minnesota, and Texas throughout 2021; two of the four infections were in children. Melioidosis, the disease caused by B. pseudomallei, is usually found in southeast Asia and isn’t obvious or easy to diagnose, especially in places like decidedly untropical Minnesota.
The Georgia case was the real break in this medical mystery, as the infection was traced back to a Walmart product called “Better Homes and Gardens Essential Oil Infused Aromatherapy Room Spray with Gemstones” (a very pithy name). The bacteria were in the lavender and chamomile scent. The CDC is investigating all other product scents, and Walmart has recalled all lots of the product.
If you’ve got that particular essential oil, it’s probably for the best that you stop using it. Don’t worry, we’re sure there’s plenty of other essential oil–infused aromatherapy room sprays with gemstones out there for your scent-based needs.
Welcome to the Ministry of Sleep-Deprived Walks
Walking is simple, right? You put one foot in front of the other, and soon you’re walking out the door. Little kids can do it. Even zombies can walk, and they don’t even have brains.
Research from MIT and the University of São Paulo has shown that walking is a little trickier than we might think. One researcher in particular noticed that student volunteers tended to perform worse toward the end of semesters, as project deadlines and multiple exams crashed over their heads and they were deprived of solid sleep schedules.
In a study published in Scientific Reports, our intrepid walking researchers had a collection of students monitor their sleep patterns for 2 weeks; on average, the students got 6 hours per night, though some were able to compensate on weekends. On the final day of a 14-day period, some students pulled all-nighters while the rest were allowed to sleep as usual. Then all students performed a walking test involving keeping time with a metronome.
To absolutely no one’s surprise, the students who performed all-nighters before being tested walked the worst, but between the other students, the ones who compensated for sleep deprivation on weekends did better than those who got 6 hours every night, despite getting a similar amount of sleep overall. This effect persisted even when the compensating students performed their walking tests late in the week, just before they got their weekend beauty sleep.
The moral of the story? Sleep is good, and you should get more of it. But if you can’t, sleep in on weekends. Science has given you permission. All those suburban dads looking to get their teenagers up at 8 in the morning must be sweating right now.
Cervical cancer mortality stagnates despite screening
Approximately 12,000 new cases of cervical cancer are diagnosed in women in the United States each year, based on data from the Centers for Disease Control and Prevention, said B.J. Rimel, MD, of Cedars-Sinai Medical Center, Los Angeles, in a presentation at the virtual Advancing NIH Research on the Health of Women conference sponsored by the National Institutes of Health.
Despite increased cervical cancer prevention and screening efforts, the incidence of, and mortality from, cervical cancer has remained stable for the past 2 decades, said Dr. Rimel.
Cervical cancer is the only cancer that can be prevented by vaccination, Dr. Rimel noted. It is essential to identify the women who are dying from cervical cancer, as well as who gets screened, who gets vaccinated, and who ends up in clinical trials, she said.
Novel agents for treating cervical cancer suggest that improvement in stagnant mortality rates is possible, said Dr. Rimel. She noted recent studies of cemiplimab, tisotumab vedotin, and a combination therapy involving pembrolizumab and platinum/paclitaxel, with and without bevacizumab.
Dr. Rimel suggested several opportunities to improve the identification and treatment of cervical cancer: Treat it like a rare disease; address structural racism through clinical trials; create opportunities for low–socioeconomic status patients to be involved in research; and develop solutions according to location (urban vs. rural), she said.
Compared with other cancers, cervical cancer is relatively rare in the United States, Dr. Rimel said. However, “It is important that those with cervical cancer can get treated and get healed from the disease,” she said. To better identify the women with cervical cancer who need treatment and to get them into clinical trials, she suggested using strategies employed by rare disease groups, such as seeking out patient support groups and registries.
Significant racial and ethnic disparities persist in cervical cancer, Dr. Rimel emphasized. Data from the CDC show that Black and Hispanic women in the United States are diagnosed with cervical cancer more frequently than women of other races and ethnicities and are less likely to survive.
“Reimagine cervical cancer as a disease of patients who are historically underrepresented due to race, language, poverty, and location,” she said.
Improving equity in cervical cancer care involves structural and trial-specific issues, said Dr. Rimel. Structural issues start with addressing how women enter into the health care system, she said. Consider where women receive care, and whether women have the opportunity to be vaccinated, and later screened, she said. Consider barriers to cervical cancer trials in centers with larger underserved populations, not only cost or insurance, but also issues of language and trust between patients and health care providers, she noted.
To improve the equity of cervical cancer clinical trials, consider potential barriers to enrollment, she added.
“Low English fluency is a barrier to trial enrollment,” said Dr. Rimel. In-person translation is essential for consent to participate in a trial, and “clinical trial budgets must reflect this requirement,” she added. Patient-reported outcomes need to be in the patient’s preferred language, “this includes online content,” Dr. Rimel said.
Dr. Rimel presented other strategies for clinical trial designs to improve equity.
“Compensate patients for their travel, or provide them with tech to allow for off-site monitoring,” she proposed. Patients of lower socioeconomic status in rural and urban areas have different barriers to enrollment, but virtual visits might be an option for those able to access the Internet when given a device. For others, smaller trial sites closer to home, combined with compensation for travel or missed work, might create more opportunities to participate, Dr. Rimel said. Finally, researchers should consider potential roles for smaller or broader studies that involve less travel and testing that would be feasible for more patients who might not otherwise participate in a clinical trial, she concluded.
Dr. Rimel had no financial conflicts to disclose.
Approximately 12,000 new cases of cervical cancer are diagnosed in women in the United States each year, based on data from the Centers for Disease Control and Prevention, said B.J. Rimel, MD, of Cedars-Sinai Medical Center, Los Angeles, in a presentation at the virtual Advancing NIH Research on the Health of Women conference sponsored by the National Institutes of Health.
Despite increased cervical cancer prevention and screening efforts, the incidence of, and mortality from, cervical cancer has remained stable for the past 2 decades, said Dr. Rimel.
Cervical cancer is the only cancer that can be prevented by vaccination, Dr. Rimel noted. It is essential to identify the women who are dying from cervical cancer, as well as who gets screened, who gets vaccinated, and who ends up in clinical trials, she said.
Novel agents for treating cervical cancer suggest that improvement in stagnant mortality rates is possible, said Dr. Rimel. She noted recent studies of cemiplimab, tisotumab vedotin, and a combination therapy involving pembrolizumab and platinum/paclitaxel, with and without bevacizumab.
Dr. Rimel suggested several opportunities to improve the identification and treatment of cervical cancer: Treat it like a rare disease; address structural racism through clinical trials; create opportunities for low–socioeconomic status patients to be involved in research; and develop solutions according to location (urban vs. rural), she said.
Compared with other cancers, cervical cancer is relatively rare in the United States, Dr. Rimel said. However, “It is important that those with cervical cancer can get treated and get healed from the disease,” she said. To better identify the women with cervical cancer who need treatment and to get them into clinical trials, she suggested using strategies employed by rare disease groups, such as seeking out patient support groups and registries.
Significant racial and ethnic disparities persist in cervical cancer, Dr. Rimel emphasized. Data from the CDC show that Black and Hispanic women in the United States are diagnosed with cervical cancer more frequently than women of other races and ethnicities and are less likely to survive.
“Reimagine cervical cancer as a disease of patients who are historically underrepresented due to race, language, poverty, and location,” she said.
Improving equity in cervical cancer care involves structural and trial-specific issues, said Dr. Rimel. Structural issues start with addressing how women enter into the health care system, she said. Consider where women receive care, and whether women have the opportunity to be vaccinated, and later screened, she said. Consider barriers to cervical cancer trials in centers with larger underserved populations, not only cost or insurance, but also issues of language and trust between patients and health care providers, she noted.
To improve the equity of cervical cancer clinical trials, consider potential barriers to enrollment, she added.
“Low English fluency is a barrier to trial enrollment,” said Dr. Rimel. In-person translation is essential for consent to participate in a trial, and “clinical trial budgets must reflect this requirement,” she added. Patient-reported outcomes need to be in the patient’s preferred language, “this includes online content,” Dr. Rimel said.
Dr. Rimel presented other strategies for clinical trial designs to improve equity.
“Compensate patients for their travel, or provide them with tech to allow for off-site monitoring,” she proposed. Patients of lower socioeconomic status in rural and urban areas have different barriers to enrollment, but virtual visits might be an option for those able to access the Internet when given a device. For others, smaller trial sites closer to home, combined with compensation for travel or missed work, might create more opportunities to participate, Dr. Rimel said. Finally, researchers should consider potential roles for smaller or broader studies that involve less travel and testing that would be feasible for more patients who might not otherwise participate in a clinical trial, she concluded.
Dr. Rimel had no financial conflicts to disclose.
Approximately 12,000 new cases of cervical cancer are diagnosed in women in the United States each year, based on data from the Centers for Disease Control and Prevention, said B.J. Rimel, MD, of Cedars-Sinai Medical Center, Los Angeles, in a presentation at the virtual Advancing NIH Research on the Health of Women conference sponsored by the National Institutes of Health.
Despite increased cervical cancer prevention and screening efforts, the incidence of, and mortality from, cervical cancer has remained stable for the past 2 decades, said Dr. Rimel.
Cervical cancer is the only cancer that can be prevented by vaccination, Dr. Rimel noted. It is essential to identify the women who are dying from cervical cancer, as well as who gets screened, who gets vaccinated, and who ends up in clinical trials, she said.
Novel agents for treating cervical cancer suggest that improvement in stagnant mortality rates is possible, said Dr. Rimel. She noted recent studies of cemiplimab, tisotumab vedotin, and a combination therapy involving pembrolizumab and platinum/paclitaxel, with and without bevacizumab.
Dr. Rimel suggested several opportunities to improve the identification and treatment of cervical cancer: Treat it like a rare disease; address structural racism through clinical trials; create opportunities for low–socioeconomic status patients to be involved in research; and develop solutions according to location (urban vs. rural), she said.
Compared with other cancers, cervical cancer is relatively rare in the United States, Dr. Rimel said. However, “It is important that those with cervical cancer can get treated and get healed from the disease,” she said. To better identify the women with cervical cancer who need treatment and to get them into clinical trials, she suggested using strategies employed by rare disease groups, such as seeking out patient support groups and registries.
Significant racial and ethnic disparities persist in cervical cancer, Dr. Rimel emphasized. Data from the CDC show that Black and Hispanic women in the United States are diagnosed with cervical cancer more frequently than women of other races and ethnicities and are less likely to survive.
“Reimagine cervical cancer as a disease of patients who are historically underrepresented due to race, language, poverty, and location,” she said.
Improving equity in cervical cancer care involves structural and trial-specific issues, said Dr. Rimel. Structural issues start with addressing how women enter into the health care system, she said. Consider where women receive care, and whether women have the opportunity to be vaccinated, and later screened, she said. Consider barriers to cervical cancer trials in centers with larger underserved populations, not only cost or insurance, but also issues of language and trust between patients and health care providers, she noted.
To improve the equity of cervical cancer clinical trials, consider potential barriers to enrollment, she added.
“Low English fluency is a barrier to trial enrollment,” said Dr. Rimel. In-person translation is essential for consent to participate in a trial, and “clinical trial budgets must reflect this requirement,” she added. Patient-reported outcomes need to be in the patient’s preferred language, “this includes online content,” Dr. Rimel said.
Dr. Rimel presented other strategies for clinical trial designs to improve equity.
“Compensate patients for their travel, or provide them with tech to allow for off-site monitoring,” she proposed. Patients of lower socioeconomic status in rural and urban areas have different barriers to enrollment, but virtual visits might be an option for those able to access the Internet when given a device. For others, smaller trial sites closer to home, combined with compensation for travel or missed work, might create more opportunities to participate, Dr. Rimel said. Finally, researchers should consider potential roles for smaller or broader studies that involve less travel and testing that would be feasible for more patients who might not otherwise participate in a clinical trial, she concluded.
Dr. Rimel had no financial conflicts to disclose.
FROM ADVANCING NIH RESEARCH ON THE HEALTH OF WOMEN
Which specialties get the biggest markups over Medicare rates?
Anesthesiologists charge private insurers more than 300% above Medicare rates, a markup that is higher than that of 16 other specialties, according to a study released by the Urban Institute.
The Washington-based nonprofit institute found that the lowest markups were in psychiatry, ophthalmology, ob.gyn., family medicine, gastroenterology, and internal medicine, at 110%-120% of Medicare rates. .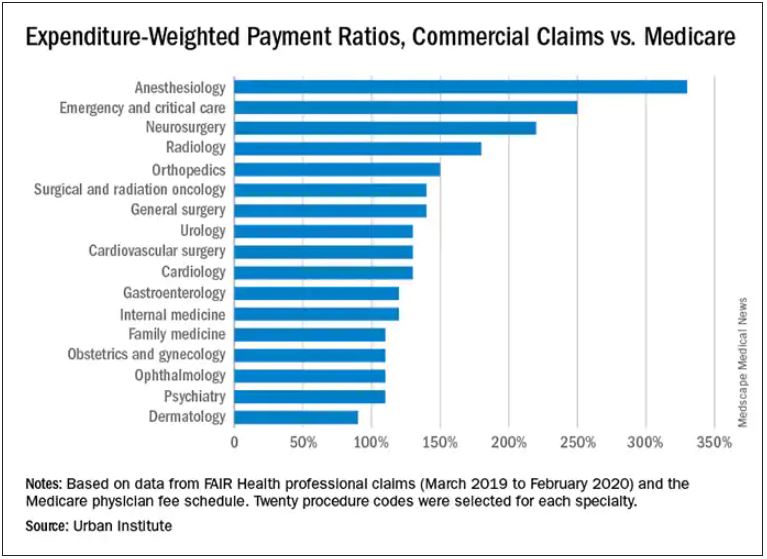
In the middle are cardiology and cardiovascular surgery (130%), urology (130%), general surgery, surgical and radiation oncology (all at 140%), and orthopedics (150%).
At the top end were radiology (180%), neurosurgery (220%), emergency and critical care (250%), and anesthesiology (330%).
The wide variation in payments could be cited in support of the idea of applying Medicare rates across all physician specialties, say the study authors. Although lowering practitioner payments might lead to savings, it “will also create more pushback from providers, especially if these rates are introduced in the employer market,” write researchers Stacey McMorrow, PhD, Robert A. Berenson, MD, and John Holahan, PhD.
It is not known whether lowering commercial payment rates might decrease patient access, they write.
The authors also note that specialties in which the potential for a fee reduction was greatest were also the specialties for which baseline compensation was highest – from $350,000 annually for emergency physicians to $800,000 a year for neurosurgeons. Annual compensation for ob.gyns., dermatologists, and opthalmologists is about $350,000 a year, which suggests that “these specialties are similarly well compensated by both Medicare and commercial insurers,” the authors write.
The investigators assessed the top 20 procedure codes by expenditure in each of 17 physician specialties. They estimated the commercial-to-Medicare payment ratio for each service and constructed weighted averages across services for each specialty at the national level and for 12 states for which data for all the specialties and services were available.
The researchers analyzed claims from the FAIR Health database between March 2019 and March 2020. That database represents 60 insurers covering 150 million people.
Pediatric and geriatric specialties, nonphysician practitioners, out-of-network clinicians, and ambulatory surgery center claims were excluded. Codes with modifiers, J codes, and clinical laboratory services were also not included.
The charges used in the study were not the actual contracted rates. The authors instead used “imputed allowed amounts” for each claim line. That method was used to protect the confidentiality of the negotiated rates.
With regard to all specialties, the lowest compensated services were procedures, evaluation and management, and tests, which received 140%-150% of the Medicare rate. Treatments and imaging were marked up 160%. Anesthesia was reimbursed at a rate 330% higher than the rate Medicare would pay.
The authors also assessed geographic variation for the 12 states for which they had data.
Similar to findings in other studies, the researchers found that the markup was lowest in Pennsylvania (120%) and highest in Wisconsin (260%). The U.S. average was 160%. California and Missouri were at 150%; Michigan was right at the average.
For physicians in Illinois, Louisiana, Colorado, Texas, and New York, markups were 170%-180% over the Medicare rate. Markups for clinicians in New Jersey (190%) and Arizona (200%) were closest to the Wisconsin rate.
The authors note some study limitations, including the fact that they excluded out-of-network practitioners, “and such payments may disproportionately affect certain specialties.”
A version of this article first appeared on Medscape.com.
Anesthesiologists charge private insurers more than 300% above Medicare rates, a markup that is higher than that of 16 other specialties, according to a study released by the Urban Institute.
The Washington-based nonprofit institute found that the lowest markups were in psychiatry, ophthalmology, ob.gyn., family medicine, gastroenterology, and internal medicine, at 110%-120% of Medicare rates. .
In the middle are cardiology and cardiovascular surgery (130%), urology (130%), general surgery, surgical and radiation oncology (all at 140%), and orthopedics (150%).
At the top end were radiology (180%), neurosurgery (220%), emergency and critical care (250%), and anesthesiology (330%).
The wide variation in payments could be cited in support of the idea of applying Medicare rates across all physician specialties, say the study authors. Although lowering practitioner payments might lead to savings, it “will also create more pushback from providers, especially if these rates are introduced in the employer market,” write researchers Stacey McMorrow, PhD, Robert A. Berenson, MD, and John Holahan, PhD.
It is not known whether lowering commercial payment rates might decrease patient access, they write.
The authors also note that specialties in which the potential for a fee reduction was greatest were also the specialties for which baseline compensation was highest – from $350,000 annually for emergency physicians to $800,000 a year for neurosurgeons. Annual compensation for ob.gyns., dermatologists, and opthalmologists is about $350,000 a year, which suggests that “these specialties are similarly well compensated by both Medicare and commercial insurers,” the authors write.
The investigators assessed the top 20 procedure codes by expenditure in each of 17 physician specialties. They estimated the commercial-to-Medicare payment ratio for each service and constructed weighted averages across services for each specialty at the national level and for 12 states for which data for all the specialties and services were available.
The researchers analyzed claims from the FAIR Health database between March 2019 and March 2020. That database represents 60 insurers covering 150 million people.
Pediatric and geriatric specialties, nonphysician practitioners, out-of-network clinicians, and ambulatory surgery center claims were excluded. Codes with modifiers, J codes, and clinical laboratory services were also not included.
The charges used in the study were not the actual contracted rates. The authors instead used “imputed allowed amounts” for each claim line. That method was used to protect the confidentiality of the negotiated rates.
With regard to all specialties, the lowest compensated services were procedures, evaluation and management, and tests, which received 140%-150% of the Medicare rate. Treatments and imaging were marked up 160%. Anesthesia was reimbursed at a rate 330% higher than the rate Medicare would pay.
The authors also assessed geographic variation for the 12 states for which they had data.
Similar to findings in other studies, the researchers found that the markup was lowest in Pennsylvania (120%) and highest in Wisconsin (260%). The U.S. average was 160%. California and Missouri were at 150%; Michigan was right at the average.
For physicians in Illinois, Louisiana, Colorado, Texas, and New York, markups were 170%-180% over the Medicare rate. Markups for clinicians in New Jersey (190%) and Arizona (200%) were closest to the Wisconsin rate.
The authors note some study limitations, including the fact that they excluded out-of-network practitioners, “and such payments may disproportionately affect certain specialties.”
A version of this article first appeared on Medscape.com.
Anesthesiologists charge private insurers more than 300% above Medicare rates, a markup that is higher than that of 16 other specialties, according to a study released by the Urban Institute.
The Washington-based nonprofit institute found that the lowest markups were in psychiatry, ophthalmology, ob.gyn., family medicine, gastroenterology, and internal medicine, at 110%-120% of Medicare rates. .
In the middle are cardiology and cardiovascular surgery (130%), urology (130%), general surgery, surgical and radiation oncology (all at 140%), and orthopedics (150%).
At the top end were radiology (180%), neurosurgery (220%), emergency and critical care (250%), and anesthesiology (330%).
The wide variation in payments could be cited in support of the idea of applying Medicare rates across all physician specialties, say the study authors. Although lowering practitioner payments might lead to savings, it “will also create more pushback from providers, especially if these rates are introduced in the employer market,” write researchers Stacey McMorrow, PhD, Robert A. Berenson, MD, and John Holahan, PhD.
It is not known whether lowering commercial payment rates might decrease patient access, they write.
The authors also note that specialties in which the potential for a fee reduction was greatest were also the specialties for which baseline compensation was highest – from $350,000 annually for emergency physicians to $800,000 a year for neurosurgeons. Annual compensation for ob.gyns., dermatologists, and opthalmologists is about $350,000 a year, which suggests that “these specialties are similarly well compensated by both Medicare and commercial insurers,” the authors write.
The investigators assessed the top 20 procedure codes by expenditure in each of 17 physician specialties. They estimated the commercial-to-Medicare payment ratio for each service and constructed weighted averages across services for each specialty at the national level and for 12 states for which data for all the specialties and services were available.
The researchers analyzed claims from the FAIR Health database between March 2019 and March 2020. That database represents 60 insurers covering 150 million people.
Pediatric and geriatric specialties, nonphysician practitioners, out-of-network clinicians, and ambulatory surgery center claims were excluded. Codes with modifiers, J codes, and clinical laboratory services were also not included.
The charges used in the study were not the actual contracted rates. The authors instead used “imputed allowed amounts” for each claim line. That method was used to protect the confidentiality of the negotiated rates.
With regard to all specialties, the lowest compensated services were procedures, evaluation and management, and tests, which received 140%-150% of the Medicare rate. Treatments and imaging were marked up 160%. Anesthesia was reimbursed at a rate 330% higher than the rate Medicare would pay.
The authors also assessed geographic variation for the 12 states for which they had data.
Similar to findings in other studies, the researchers found that the markup was lowest in Pennsylvania (120%) and highest in Wisconsin (260%). The U.S. average was 160%. California and Missouri were at 150%; Michigan was right at the average.
For physicians in Illinois, Louisiana, Colorado, Texas, and New York, markups were 170%-180% over the Medicare rate. Markups for clinicians in New Jersey (190%) and Arizona (200%) were closest to the Wisconsin rate.
The authors note some study limitations, including the fact that they excluded out-of-network practitioners, “and such payments may disproportionately affect certain specialties.”
A version of this article first appeared on Medscape.com.
Ohio records more deaths than births for first time
In 2020, around 143,661 Ohioans died and 129,313 Ohioans were born, according to The Columbus Dispatch. The trend appears to have continued so far this year, with 107,462 deaths and 100,781 births reported to date.
Deaths haven’t surpassed births in the 112 years since the state began compiling data in 1909, the newspaper reported. The state’s birth rate has been declining for years while the number of deaths has risen, though data shows that the COVID-19 pandemic accelerated the shift.
“It doesn’t surprise me at all,” Joseph Gastaldo, MD, the medical director of infectious diseases for OhioHealth, told the newspaper.
Ohio’s birth rate fell by 4% in 2020, which defied public expectations about a pandemic birth boom. In addition, the state reported 13,927 COVID-19 deaths throughout the year.
“It’s COVID, clearly,” he noted.
Alabama also recorded more deaths than births for the first time last year, according to The New York Times. The state reported 64,714 deaths and 57,641 births in 2020.
“Our state literally shrunk in 2020,” Scott Harris, MD, the state health officer for Alabama, said at a news conference in September.
The state had never recorded a gap that large, even during World War I, World War II, and the 1918 flu pandemic, he said. Alabama has kept records on the numbers since 1900.
“We’ve never had a time when deaths exceeded births,” Dr. Harris said.
In fact, about half of U.S. states reported death rates higher than birth rates in 2020, according to a recent study from researchers at the University of New Hampshire. In 2019, only five states --Maine, New Hampshire, Rhode Island, Vermont, and West Virginia -- reported more deaths than births.
In 2020, the United States reported a record of nearly 3.4 million deaths, which was 18% more than in 2019, the researchers found. COVID-19 was the primary reason for the increase in deaths, accounting for about 11% of total deaths. Meanwhile, births dropped by 4% to about 3.6 million.
The surplus of births over deaths added 229,000 people to the U.S. population in 2020, as compared to 892,000 in 2019, which means the country’s population growth slowed last year. The decline, paired with lower immigration rates during the pandemic, led to the smallest annual percentage population gain in at least 100 years.
“Deaths will likely exceed births again in many states in 2021,” Kenneth Johnson, PhD, a senior demographer and study author, wrote in a statement.
“How large or protracted these fertility declines and mortality increases will be remains to be seen, but they have already dramatically reduced population growth in the United States,” he said.
A version of this article first appeared on WebMD.com.
In 2020, around 143,661 Ohioans died and 129,313 Ohioans were born, according to The Columbus Dispatch. The trend appears to have continued so far this year, with 107,462 deaths and 100,781 births reported to date.
Deaths haven’t surpassed births in the 112 years since the state began compiling data in 1909, the newspaper reported. The state’s birth rate has been declining for years while the number of deaths has risen, though data shows that the COVID-19 pandemic accelerated the shift.
“It doesn’t surprise me at all,” Joseph Gastaldo, MD, the medical director of infectious diseases for OhioHealth, told the newspaper.
Ohio’s birth rate fell by 4% in 2020, which defied public expectations about a pandemic birth boom. In addition, the state reported 13,927 COVID-19 deaths throughout the year.
“It’s COVID, clearly,” he noted.
Alabama also recorded more deaths than births for the first time last year, according to The New York Times. The state reported 64,714 deaths and 57,641 births in 2020.
“Our state literally shrunk in 2020,” Scott Harris, MD, the state health officer for Alabama, said at a news conference in September.
The state had never recorded a gap that large, even during World War I, World War II, and the 1918 flu pandemic, he said. Alabama has kept records on the numbers since 1900.
“We’ve never had a time when deaths exceeded births,” Dr. Harris said.
In fact, about half of U.S. states reported death rates higher than birth rates in 2020, according to a recent study from researchers at the University of New Hampshire. In 2019, only five states --Maine, New Hampshire, Rhode Island, Vermont, and West Virginia -- reported more deaths than births.
In 2020, the United States reported a record of nearly 3.4 million deaths, which was 18% more than in 2019, the researchers found. COVID-19 was the primary reason for the increase in deaths, accounting for about 11% of total deaths. Meanwhile, births dropped by 4% to about 3.6 million.
The surplus of births over deaths added 229,000 people to the U.S. population in 2020, as compared to 892,000 in 2019, which means the country’s population growth slowed last year. The decline, paired with lower immigration rates during the pandemic, led to the smallest annual percentage population gain in at least 100 years.
“Deaths will likely exceed births again in many states in 2021,” Kenneth Johnson, PhD, a senior demographer and study author, wrote in a statement.
“How large or protracted these fertility declines and mortality increases will be remains to be seen, but they have already dramatically reduced population growth in the United States,” he said.
A version of this article first appeared on WebMD.com.
In 2020, around 143,661 Ohioans died and 129,313 Ohioans were born, according to The Columbus Dispatch. The trend appears to have continued so far this year, with 107,462 deaths and 100,781 births reported to date.
Deaths haven’t surpassed births in the 112 years since the state began compiling data in 1909, the newspaper reported. The state’s birth rate has been declining for years while the number of deaths has risen, though data shows that the COVID-19 pandemic accelerated the shift.
“It doesn’t surprise me at all,” Joseph Gastaldo, MD, the medical director of infectious diseases for OhioHealth, told the newspaper.
Ohio’s birth rate fell by 4% in 2020, which defied public expectations about a pandemic birth boom. In addition, the state reported 13,927 COVID-19 deaths throughout the year.
“It’s COVID, clearly,” he noted.
Alabama also recorded more deaths than births for the first time last year, according to The New York Times. The state reported 64,714 deaths and 57,641 births in 2020.
“Our state literally shrunk in 2020,” Scott Harris, MD, the state health officer for Alabama, said at a news conference in September.
The state had never recorded a gap that large, even during World War I, World War II, and the 1918 flu pandemic, he said. Alabama has kept records on the numbers since 1900.
“We’ve never had a time when deaths exceeded births,” Dr. Harris said.
In fact, about half of U.S. states reported death rates higher than birth rates in 2020, according to a recent study from researchers at the University of New Hampshire. In 2019, only five states --Maine, New Hampshire, Rhode Island, Vermont, and West Virginia -- reported more deaths than births.
In 2020, the United States reported a record of nearly 3.4 million deaths, which was 18% more than in 2019, the researchers found. COVID-19 was the primary reason for the increase in deaths, accounting for about 11% of total deaths. Meanwhile, births dropped by 4% to about 3.6 million.
The surplus of births over deaths added 229,000 people to the U.S. population in 2020, as compared to 892,000 in 2019, which means the country’s population growth slowed last year. The decline, paired with lower immigration rates during the pandemic, led to the smallest annual percentage population gain in at least 100 years.
“Deaths will likely exceed births again in many states in 2021,” Kenneth Johnson, PhD, a senior demographer and study author, wrote in a statement.
“How large or protracted these fertility declines and mortality increases will be remains to be seen, but they have already dramatically reduced population growth in the United States,” he said.
A version of this article first appeared on WebMD.com.
Hot temperatures in outdoor lockboxes increase sample errors
, according to results from a recent study published in the American Journal of Clinical Pathology.
“Our findings indicate that samples (centrifuged or not centrifuged) were impacted by extreme summer temperatures when stored for short periods of time inside commonly used steel lockboxes,” Joseph R. Wiencek, PhD, medical director of clinical chemistry, Vanderbilt University School of Medicine Core Laboratory in Nashville, said in an interview.
Dr. Wiencek and colleagues picked two dates during the summer of 2019 in a mid-Atlantic state to place two courier lockboxes (LabLocker-KF300) outside in hot temperatures (32º C) starting at 11 a.m., with one lockbox containing two 24-oz cold packs (Nordic NI24) and the other containing no cold packs. The researchers monitored the temperatures of each lockbox over the course of 4 hours.
Overall, eight participants had seven samples in lithium heparin drawn for two studies evaluating centrifuged or not centrifuged samples. In the first study, four participants had seven samples drawn, with one centrifuged sample serving as a control for each patient. The other six samples were wrapped in paper towels, placed in resealable plastic bags, and distributed evenly in the warm and cold lockboxes. The samples did not directly touch the cold packs in the cold lockbox. At 1 hour, 2 hours, and 4 hours, a participant’s sample was removed from each lockbox and centrifuged.
In the second study, another four participants had seven samples drawn. As in the first study, all samples were centrifuged and placed in the lockboxes. For both studies, when samples were centrifuged, plasma from samples was left on the gel barrier when analyzed for concentrations of C-reactive protein, a comprehensive metabolic panel, lactate dehydrogenase (LDH), a lipid panel, magnesium, and phosphorus (Abbott Architect c16000).
In the study of uncentrifuged samples, Dr. Wiencek and colleagues found that when the temperature outside ranged from 28.2º to 44.0º C (mean 40.4º C), the temperature of the cold lockbox was between 16.5º to 22.3º C (mean 22.3º C). The temperature ranged between 34.4º to 46.9º C (mean 42.6º C) in the warm lockbox. For centrifuged samples, the cold lockbox temperature was between 12.2º to 23.0º C (mean 18.0º C) and the warm lockbox was between 25. to 40.8º C (mean 35.2º C) when the outdoor temperature ranged from 27.2º to 46.3º C (mean 37.9º C).
The researchers also calculated the significant change limit (SCL) for each analyte in each sample, finding that aspartate aminotransferase, glucose, LDH, and potassium significantly exceeded the SCL in both the centrifuged and uncentrifuged samples, with the greatest changes seen at the 4-hour timepoint for samples in the warm lockbox (P < .05 for all).
Lockbox instructions are “consistently inconsistent”
In viewing instructions for lockboxes across institutions, Dr. Wiencek said the “outdoor courier lockbox instructions among private, academic and reference laboratories were consistently inconsistent.” For example, no laboratories cited time restrictions for samples in lockboxes, and their descriptions on the number of cold packs a laboratory should use and where the lockbox should be placed varied. The inconsistencies “highlighted the emergent need for standardization and guidance documents for institutions to implement,” Dr. Wiencek said.
One unanswered question is how widespread the problem is. It is unclear how many outdoor courier lockboxes are currently in use in the United States or globally; however, experts agreed it was a common occurrence, with some of the largest laboratory service providers offering outdoor courier lockboxes to their clients.
“Courier lockboxes are everywhere. All you need to do is walk around your clinics that are at your hospitals or clinics located around your grocery store to find them,” Dr. Wiencek said. “Some hang on doors, while others can be found on the ground in direct sunlight on a hot summer day.”
What’s more, institutions may not realize how leaving samples outdoors for extended periods can affect results. “Care teams are commonly unaware that samples placed in these poorly designed lockboxes can experience extreme summer or winter temperatures that may lead to incorrect results,” Dr. Wiencek said. “Healthcare providers need to understand the hidden dangers courier lockboxes have on the quality of their patient’s test results.”
Amy L. Pyle-Eilola, PhD, clinical chemistry director at Nationwide Children’s Hospital in Columbus, Ohio, said a major strength of the study by Dr. Wiencek and colleagues “is just that it was done at all.”
“I appreciate the real-world nature of this study and that it provides a snapshot of what conditions are really like in a lockbox in the summer,” she said in an interview.
In the clinical lab, receiving samples that had been sitting in a courier lockbox “is not uncommon,” Dr. Pyle-Eilola said.
“When I have encountered these situations, I have struggled to decide if it is still appropriate to run the tests. I always look to the medical literature for assistance with these situations, but there has been a paucity of information available on the impact of lockbox storage,” she explained.
The study by Dr. Wiencek and colleagues “provides some much-needed evidence for what is acceptable for lockbox storage conditions,” she said.
Areas of future research
Rodney E. Rohde, PhD, university distinguished chair and professor of the Clinical Laboratory Science (CLS) Program at Texas State University in San Marcos, said in an interview that the study “does a nice job of looking at multiple analytes and controlling for several variables,” but the sample size is small and the results may be difficult to generalize.
Dr. Pyle-Eilola highlighted another limitation — “a common shortcoming of these kinds of studies” — in the use of healthy donors for patient samples, which narrows the range of assay results.
“It is possible that more significant variation in results may be observed in additional analytes if the samples had higher concentrations of those analytes,” she said. “Moreover, this is clinically relevant as the samples stored in such lockboxes are not always from healthy individuals and have abnormal concentrations of analytes.”
Mario Plebani, MD, professor of clinical biochemistry and clinical molecular biology and chief of the department of laboratory medicine at University Hospital of Padova in Padova, Italy, agreed with that assessment.
“[T]he risks for errors and patient safety are higher for values near to the upper or lower reference value, and in general for samples collected in patients with particular diseases and clinical conditions,” he said in an interview.
“This paper deserves a commenting editorial to better highlight the urgent need for further studies on the same issue and in general on the risk in the pre-pre-analytical phase, including sample storage and transportation,” he noted.
Another area of future research is studying patient samples exposed to hotter or colder temperatures in outdoor courier lockboxes outside the mid-Atlantic area. “Here in Texas, temperatures can reach extreme heat levels,” Dr. Rohde said, who added that use of outdoor lockboxes is “very common in my region.”
Dr. Wiencek disclosed he has been a consultant on this research topic for Roche Diagnostics and received an honorarium for speaking on the subject from the American Association for Clinical Chemistry and American Society of Clinical Pathology. The other authors have no relevant conflict of interest. Dr. Pyle-Eilola, Dr. Rohde, and Dr. Plebani have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to results from a recent study published in the American Journal of Clinical Pathology.
“Our findings indicate that samples (centrifuged or not centrifuged) were impacted by extreme summer temperatures when stored for short periods of time inside commonly used steel lockboxes,” Joseph R. Wiencek, PhD, medical director of clinical chemistry, Vanderbilt University School of Medicine Core Laboratory in Nashville, said in an interview.
Dr. Wiencek and colleagues picked two dates during the summer of 2019 in a mid-Atlantic state to place two courier lockboxes (LabLocker-KF300) outside in hot temperatures (32º C) starting at 11 a.m., with one lockbox containing two 24-oz cold packs (Nordic NI24) and the other containing no cold packs. The researchers monitored the temperatures of each lockbox over the course of 4 hours.
Overall, eight participants had seven samples in lithium heparin drawn for two studies evaluating centrifuged or not centrifuged samples. In the first study, four participants had seven samples drawn, with one centrifuged sample serving as a control for each patient. The other six samples were wrapped in paper towels, placed in resealable plastic bags, and distributed evenly in the warm and cold lockboxes. The samples did not directly touch the cold packs in the cold lockbox. At 1 hour, 2 hours, and 4 hours, a participant’s sample was removed from each lockbox and centrifuged.
In the second study, another four participants had seven samples drawn. As in the first study, all samples were centrifuged and placed in the lockboxes. For both studies, when samples were centrifuged, plasma from samples was left on the gel barrier when analyzed for concentrations of C-reactive protein, a comprehensive metabolic panel, lactate dehydrogenase (LDH), a lipid panel, magnesium, and phosphorus (Abbott Architect c16000).
In the study of uncentrifuged samples, Dr. Wiencek and colleagues found that when the temperature outside ranged from 28.2º to 44.0º C (mean 40.4º C), the temperature of the cold lockbox was between 16.5º to 22.3º C (mean 22.3º C). The temperature ranged between 34.4º to 46.9º C (mean 42.6º C) in the warm lockbox. For centrifuged samples, the cold lockbox temperature was between 12.2º to 23.0º C (mean 18.0º C) and the warm lockbox was between 25. to 40.8º C (mean 35.2º C) when the outdoor temperature ranged from 27.2º to 46.3º C (mean 37.9º C).
The researchers also calculated the significant change limit (SCL) for each analyte in each sample, finding that aspartate aminotransferase, glucose, LDH, and potassium significantly exceeded the SCL in both the centrifuged and uncentrifuged samples, with the greatest changes seen at the 4-hour timepoint for samples in the warm lockbox (P < .05 for all).
Lockbox instructions are “consistently inconsistent”
In viewing instructions for lockboxes across institutions, Dr. Wiencek said the “outdoor courier lockbox instructions among private, academic and reference laboratories were consistently inconsistent.” For example, no laboratories cited time restrictions for samples in lockboxes, and their descriptions on the number of cold packs a laboratory should use and where the lockbox should be placed varied. The inconsistencies “highlighted the emergent need for standardization and guidance documents for institutions to implement,” Dr. Wiencek said.
One unanswered question is how widespread the problem is. It is unclear how many outdoor courier lockboxes are currently in use in the United States or globally; however, experts agreed it was a common occurrence, with some of the largest laboratory service providers offering outdoor courier lockboxes to their clients.
“Courier lockboxes are everywhere. All you need to do is walk around your clinics that are at your hospitals or clinics located around your grocery store to find them,” Dr. Wiencek said. “Some hang on doors, while others can be found on the ground in direct sunlight on a hot summer day.”
What’s more, institutions may not realize how leaving samples outdoors for extended periods can affect results. “Care teams are commonly unaware that samples placed in these poorly designed lockboxes can experience extreme summer or winter temperatures that may lead to incorrect results,” Dr. Wiencek said. “Healthcare providers need to understand the hidden dangers courier lockboxes have on the quality of their patient’s test results.”
Amy L. Pyle-Eilola, PhD, clinical chemistry director at Nationwide Children’s Hospital in Columbus, Ohio, said a major strength of the study by Dr. Wiencek and colleagues “is just that it was done at all.”
“I appreciate the real-world nature of this study and that it provides a snapshot of what conditions are really like in a lockbox in the summer,” she said in an interview.
In the clinical lab, receiving samples that had been sitting in a courier lockbox “is not uncommon,” Dr. Pyle-Eilola said.
“When I have encountered these situations, I have struggled to decide if it is still appropriate to run the tests. I always look to the medical literature for assistance with these situations, but there has been a paucity of information available on the impact of lockbox storage,” she explained.
The study by Dr. Wiencek and colleagues “provides some much-needed evidence for what is acceptable for lockbox storage conditions,” she said.
Areas of future research
Rodney E. Rohde, PhD, university distinguished chair and professor of the Clinical Laboratory Science (CLS) Program at Texas State University in San Marcos, said in an interview that the study “does a nice job of looking at multiple analytes and controlling for several variables,” but the sample size is small and the results may be difficult to generalize.
Dr. Pyle-Eilola highlighted another limitation — “a common shortcoming of these kinds of studies” — in the use of healthy donors for patient samples, which narrows the range of assay results.
“It is possible that more significant variation in results may be observed in additional analytes if the samples had higher concentrations of those analytes,” she said. “Moreover, this is clinically relevant as the samples stored in such lockboxes are not always from healthy individuals and have abnormal concentrations of analytes.”
Mario Plebani, MD, professor of clinical biochemistry and clinical molecular biology and chief of the department of laboratory medicine at University Hospital of Padova in Padova, Italy, agreed with that assessment.
“[T]he risks for errors and patient safety are higher for values near to the upper or lower reference value, and in general for samples collected in patients with particular diseases and clinical conditions,” he said in an interview.
“This paper deserves a commenting editorial to better highlight the urgent need for further studies on the same issue and in general on the risk in the pre-pre-analytical phase, including sample storage and transportation,” he noted.
Another area of future research is studying patient samples exposed to hotter or colder temperatures in outdoor courier lockboxes outside the mid-Atlantic area. “Here in Texas, temperatures can reach extreme heat levels,” Dr. Rohde said, who added that use of outdoor lockboxes is “very common in my region.”
Dr. Wiencek disclosed he has been a consultant on this research topic for Roche Diagnostics and received an honorarium for speaking on the subject from the American Association for Clinical Chemistry and American Society of Clinical Pathology. The other authors have no relevant conflict of interest. Dr. Pyle-Eilola, Dr. Rohde, and Dr. Plebani have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to results from a recent study published in the American Journal of Clinical Pathology.
“Our findings indicate that samples (centrifuged or not centrifuged) were impacted by extreme summer temperatures when stored for short periods of time inside commonly used steel lockboxes,” Joseph R. Wiencek, PhD, medical director of clinical chemistry, Vanderbilt University School of Medicine Core Laboratory in Nashville, said in an interview.
Dr. Wiencek and colleagues picked two dates during the summer of 2019 in a mid-Atlantic state to place two courier lockboxes (LabLocker-KF300) outside in hot temperatures (32º C) starting at 11 a.m., with one lockbox containing two 24-oz cold packs (Nordic NI24) and the other containing no cold packs. The researchers monitored the temperatures of each lockbox over the course of 4 hours.
Overall, eight participants had seven samples in lithium heparin drawn for two studies evaluating centrifuged or not centrifuged samples. In the first study, four participants had seven samples drawn, with one centrifuged sample serving as a control for each patient. The other six samples were wrapped in paper towels, placed in resealable plastic bags, and distributed evenly in the warm and cold lockboxes. The samples did not directly touch the cold packs in the cold lockbox. At 1 hour, 2 hours, and 4 hours, a participant’s sample was removed from each lockbox and centrifuged.
In the second study, another four participants had seven samples drawn. As in the first study, all samples were centrifuged and placed in the lockboxes. For both studies, when samples were centrifuged, plasma from samples was left on the gel barrier when analyzed for concentrations of C-reactive protein, a comprehensive metabolic panel, lactate dehydrogenase (LDH), a lipid panel, magnesium, and phosphorus (Abbott Architect c16000).
In the study of uncentrifuged samples, Dr. Wiencek and colleagues found that when the temperature outside ranged from 28.2º to 44.0º C (mean 40.4º C), the temperature of the cold lockbox was between 16.5º to 22.3º C (mean 22.3º C). The temperature ranged between 34.4º to 46.9º C (mean 42.6º C) in the warm lockbox. For centrifuged samples, the cold lockbox temperature was between 12.2º to 23.0º C (mean 18.0º C) and the warm lockbox was between 25. to 40.8º C (mean 35.2º C) when the outdoor temperature ranged from 27.2º to 46.3º C (mean 37.9º C).
The researchers also calculated the significant change limit (SCL) for each analyte in each sample, finding that aspartate aminotransferase, glucose, LDH, and potassium significantly exceeded the SCL in both the centrifuged and uncentrifuged samples, with the greatest changes seen at the 4-hour timepoint for samples in the warm lockbox (P < .05 for all).
Lockbox instructions are “consistently inconsistent”
In viewing instructions for lockboxes across institutions, Dr. Wiencek said the “outdoor courier lockbox instructions among private, academic and reference laboratories were consistently inconsistent.” For example, no laboratories cited time restrictions for samples in lockboxes, and their descriptions on the number of cold packs a laboratory should use and where the lockbox should be placed varied. The inconsistencies “highlighted the emergent need for standardization and guidance documents for institutions to implement,” Dr. Wiencek said.
One unanswered question is how widespread the problem is. It is unclear how many outdoor courier lockboxes are currently in use in the United States or globally; however, experts agreed it was a common occurrence, with some of the largest laboratory service providers offering outdoor courier lockboxes to their clients.
“Courier lockboxes are everywhere. All you need to do is walk around your clinics that are at your hospitals or clinics located around your grocery store to find them,” Dr. Wiencek said. “Some hang on doors, while others can be found on the ground in direct sunlight on a hot summer day.”
What’s more, institutions may not realize how leaving samples outdoors for extended periods can affect results. “Care teams are commonly unaware that samples placed in these poorly designed lockboxes can experience extreme summer or winter temperatures that may lead to incorrect results,” Dr. Wiencek said. “Healthcare providers need to understand the hidden dangers courier lockboxes have on the quality of their patient’s test results.”
Amy L. Pyle-Eilola, PhD, clinical chemistry director at Nationwide Children’s Hospital in Columbus, Ohio, said a major strength of the study by Dr. Wiencek and colleagues “is just that it was done at all.”
“I appreciate the real-world nature of this study and that it provides a snapshot of what conditions are really like in a lockbox in the summer,” she said in an interview.
In the clinical lab, receiving samples that had been sitting in a courier lockbox “is not uncommon,” Dr. Pyle-Eilola said.
“When I have encountered these situations, I have struggled to decide if it is still appropriate to run the tests. I always look to the medical literature for assistance with these situations, but there has been a paucity of information available on the impact of lockbox storage,” she explained.
The study by Dr. Wiencek and colleagues “provides some much-needed evidence for what is acceptable for lockbox storage conditions,” she said.
Areas of future research
Rodney E. Rohde, PhD, university distinguished chair and professor of the Clinical Laboratory Science (CLS) Program at Texas State University in San Marcos, said in an interview that the study “does a nice job of looking at multiple analytes and controlling for several variables,” but the sample size is small and the results may be difficult to generalize.
Dr. Pyle-Eilola highlighted another limitation — “a common shortcoming of these kinds of studies” — in the use of healthy donors for patient samples, which narrows the range of assay results.
“It is possible that more significant variation in results may be observed in additional analytes if the samples had higher concentrations of those analytes,” she said. “Moreover, this is clinically relevant as the samples stored in such lockboxes are not always from healthy individuals and have abnormal concentrations of analytes.”
Mario Plebani, MD, professor of clinical biochemistry and clinical molecular biology and chief of the department of laboratory medicine at University Hospital of Padova in Padova, Italy, agreed with that assessment.
“[T]he risks for errors and patient safety are higher for values near to the upper or lower reference value, and in general for samples collected in patients with particular diseases and clinical conditions,” he said in an interview.
“This paper deserves a commenting editorial to better highlight the urgent need for further studies on the same issue and in general on the risk in the pre-pre-analytical phase, including sample storage and transportation,” he noted.
Another area of future research is studying patient samples exposed to hotter or colder temperatures in outdoor courier lockboxes outside the mid-Atlantic area. “Here in Texas, temperatures can reach extreme heat levels,” Dr. Rohde said, who added that use of outdoor lockboxes is “very common in my region.”
Dr. Wiencek disclosed he has been a consultant on this research topic for Roche Diagnostics and received an honorarium for speaking on the subject from the American Association for Clinical Chemistry and American Society of Clinical Pathology. The other authors have no relevant conflict of interest. Dr. Pyle-Eilola, Dr. Rohde, and Dr. Plebani have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Unvaccinated pregnant women have more severe COVID
An increasing number of people who are unvaccinated and pregnant are being hospitalized for COVID-19, report investigators who saw hospital admissions double in a single year.
“With the surge, we had expected to begin treating patients who developed severe or critical illness again in pregnancy,” says Emily Adhikari, MD, from the University of Texas Southwestern Medical Center in Dallas. “But we did not expect the level of respiratory illness that we began to see in our patients. That was a surprise and an alarming finding that we felt was really important to get out there.”
The researchers followed more than 1,500 pregnant women diagnosed with COVID-19 who received care from Parkland Health and Hospital System in Dallas County, one of the nation’s busiest for deliveries. After the emergence of the Delta variant, the number of pregnant women hospitalized with COVID-19 more than doubled over the previous year.
And 82 pregnant women went on to develop severe or critical COVID, they report in their study, published online in the American Journal of Obstetrics and Gynecology. All but 1 of these patients were unvaccinated, 10 needed a ventilator, and two died.
The proportion of cases that were critical was about 5% in 2020. However, in April 2021, even though the number of total cases remained low, the number of severe illnesses started to rise. After the Delta variant became dominant, both the number and severity of cases increased, and after August 2021, more than 25% of pregnant people diagnosed with COVID-19 required hospitalization.
Hospitalizations Double
“We need to focus and really act urgently to recommend vaccination in pregnancy because that is the primary prevention tool that we have,” says Dr. Adhikari. “We do not have a proven cure for this illness, and that is important to know.”
These findings, which focus on a vulnerable population, are especially important given the elevated prevalence of COVID-19 in pregnant people of lower economic status, said Lissette Tanner, MD, MPH, from Emory University in Atlanta, who was not involved with the study.
“There are higher rates of hospitalization and death among Black, Hispanic, and Native American communities,” she reported. “It is essential to know how the virus is affecting those most affected and often most disadvantaged to deal with the pandemic.”
Vaccination rates are low in this population; just 19.2% of pregnant women receive at least one dose during pregnancy, according to the CDC. But pregnancy confers a higher risk for severe COVID-19 illness and for adverse outcomes, such as preterm birth and stillbirth.
Of the 665 people in the study cohort who were pregnant or had given birth when the vaccines were available, only 21.4% received at least one dose of a COVID-19 vaccine.
Given the increased risk for COVID-19 during pregnancy, the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the CDC recommend vaccination for people who are pregnant, breastfeeding, or trying to get pregnant.
According to ACOG, pregnant women who are fully vaccinated can follow the same guidelines as everyone else who is fully vaccinated; however, to prevent breakthrough infections, they might want to continue wearing a mask. ACOG also recommends that those not fully vaccinated follow physical-distancing guidelines and limit contact with people as much as possible to avoid infection.
A version of this article first appeared on WebMD.com.
An increasing number of people who are unvaccinated and pregnant are being hospitalized for COVID-19, report investigators who saw hospital admissions double in a single year.
“With the surge, we had expected to begin treating patients who developed severe or critical illness again in pregnancy,” says Emily Adhikari, MD, from the University of Texas Southwestern Medical Center in Dallas. “But we did not expect the level of respiratory illness that we began to see in our patients. That was a surprise and an alarming finding that we felt was really important to get out there.”
The researchers followed more than 1,500 pregnant women diagnosed with COVID-19 who received care from Parkland Health and Hospital System in Dallas County, one of the nation’s busiest for deliveries. After the emergence of the Delta variant, the number of pregnant women hospitalized with COVID-19 more than doubled over the previous year.
And 82 pregnant women went on to develop severe or critical COVID, they report in their study, published online in the American Journal of Obstetrics and Gynecology. All but 1 of these patients were unvaccinated, 10 needed a ventilator, and two died.
The proportion of cases that were critical was about 5% in 2020. However, in April 2021, even though the number of total cases remained low, the number of severe illnesses started to rise. After the Delta variant became dominant, both the number and severity of cases increased, and after August 2021, more than 25% of pregnant people diagnosed with COVID-19 required hospitalization.
Hospitalizations Double
“We need to focus and really act urgently to recommend vaccination in pregnancy because that is the primary prevention tool that we have,” says Dr. Adhikari. “We do not have a proven cure for this illness, and that is important to know.”
These findings, which focus on a vulnerable population, are especially important given the elevated prevalence of COVID-19 in pregnant people of lower economic status, said Lissette Tanner, MD, MPH, from Emory University in Atlanta, who was not involved with the study.
“There are higher rates of hospitalization and death among Black, Hispanic, and Native American communities,” she reported. “It is essential to know how the virus is affecting those most affected and often most disadvantaged to deal with the pandemic.”
Vaccination rates are low in this population; just 19.2% of pregnant women receive at least one dose during pregnancy, according to the CDC. But pregnancy confers a higher risk for severe COVID-19 illness and for adverse outcomes, such as preterm birth and stillbirth.
Of the 665 people in the study cohort who were pregnant or had given birth when the vaccines were available, only 21.4% received at least one dose of a COVID-19 vaccine.
Given the increased risk for COVID-19 during pregnancy, the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the CDC recommend vaccination for people who are pregnant, breastfeeding, or trying to get pregnant.
According to ACOG, pregnant women who are fully vaccinated can follow the same guidelines as everyone else who is fully vaccinated; however, to prevent breakthrough infections, they might want to continue wearing a mask. ACOG also recommends that those not fully vaccinated follow physical-distancing guidelines and limit contact with people as much as possible to avoid infection.
A version of this article first appeared on WebMD.com.
An increasing number of people who are unvaccinated and pregnant are being hospitalized for COVID-19, report investigators who saw hospital admissions double in a single year.
“With the surge, we had expected to begin treating patients who developed severe or critical illness again in pregnancy,” says Emily Adhikari, MD, from the University of Texas Southwestern Medical Center in Dallas. “But we did not expect the level of respiratory illness that we began to see in our patients. That was a surprise and an alarming finding that we felt was really important to get out there.”
The researchers followed more than 1,500 pregnant women diagnosed with COVID-19 who received care from Parkland Health and Hospital System in Dallas County, one of the nation’s busiest for deliveries. After the emergence of the Delta variant, the number of pregnant women hospitalized with COVID-19 more than doubled over the previous year.
And 82 pregnant women went on to develop severe or critical COVID, they report in their study, published online in the American Journal of Obstetrics and Gynecology. All but 1 of these patients were unvaccinated, 10 needed a ventilator, and two died.
The proportion of cases that were critical was about 5% in 2020. However, in April 2021, even though the number of total cases remained low, the number of severe illnesses started to rise. After the Delta variant became dominant, both the number and severity of cases increased, and after August 2021, more than 25% of pregnant people diagnosed with COVID-19 required hospitalization.
Hospitalizations Double
“We need to focus and really act urgently to recommend vaccination in pregnancy because that is the primary prevention tool that we have,” says Dr. Adhikari. “We do not have a proven cure for this illness, and that is important to know.”
These findings, which focus on a vulnerable population, are especially important given the elevated prevalence of COVID-19 in pregnant people of lower economic status, said Lissette Tanner, MD, MPH, from Emory University in Atlanta, who was not involved with the study.
“There are higher rates of hospitalization and death among Black, Hispanic, and Native American communities,” she reported. “It is essential to know how the virus is affecting those most affected and often most disadvantaged to deal with the pandemic.”
Vaccination rates are low in this population; just 19.2% of pregnant women receive at least one dose during pregnancy, according to the CDC. But pregnancy confers a higher risk for severe COVID-19 illness and for adverse outcomes, such as preterm birth and stillbirth.
Of the 665 people in the study cohort who were pregnant or had given birth when the vaccines were available, only 21.4% received at least one dose of a COVID-19 vaccine.
Given the increased risk for COVID-19 during pregnancy, the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the CDC recommend vaccination for people who are pregnant, breastfeeding, or trying to get pregnant.
According to ACOG, pregnant women who are fully vaccinated can follow the same guidelines as everyone else who is fully vaccinated; however, to prevent breakthrough infections, they might want to continue wearing a mask. ACOG also recommends that those not fully vaccinated follow physical-distancing guidelines and limit contact with people as much as possible to avoid infection.
A version of this article first appeared on WebMD.com.
FDA panel votes to approve Pfizer’s vaccine for children
Seventeen of the 18 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Oct. 26 voted to recommend the 10-microgram shot for kids, which is one-third the dose given to adults.
One member, Michael Kurilla, MD, director of the division of clinical innovation at the National Institutes of Health, Bethesda, Md., abstained from voting.
If the FDA follows the recommendation, as it typically does, and issues an Emergency Use Authorization for the vaccine, the shots could be available within days.
After the FDA’s final decision, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet to make specific recommendations for its use. The CDC committee must stick closely to the conditions for use spelled out in the EUA, so their recommendations are likely to be similar to those made by the FDA. Their next meeting is scheduled for Nov. 2 and 3.
In the end, some on the panel felt uneasy with their decision.
“I voted yes primarily because I wanted to make sure that children who really need this vaccine, the Black and brown children of our country, get the vaccine,” said James Hildreth, MD, PhD, president and CEO of Meharry Medical College in Nashville.
“But to be honest, the best way to protect the health of some children will be to do nothing because they will be just fine,” he said.
Others said they were surprised by how difficult the decision had been.
“This is a much tougher one than we had expected going into it,” said committee member Eric Rubin, MD, editor and chief of the New England Journal of Medicine, during the FDA advisory committee’s meeting.
Ahead of the vote, the committee heard presentations outlining the expected benefits of vaccinating children along with potential risks.
“Children have been greatly impacted by the pandemic,” said Fiona Havers, MD, a medical officer with the CDC in Atlanta who reviewed the epidemiology of COVID-19 in kids.
In the second year of the pandemic, as more seniors have been vaccinated against the virus, COVID cases have largely shifted from older to younger age groups.
So far, there have been more than 1.9 million COVID-19 cases in children ages 5 through 11 in the United States.. Cases in kids saw a big jump in July and August with summer travel, schools reopening, and the dominance of the Delta variant.
And those are just the cases reported to the CDC. Regular testing of anonymous blood samples collected at sites across the United States indicates that 6 times as many kids have had COVID than what is reflected in official counts.
Last winter, blood sample testing showed about 13% of children had antibodies against the virus, suggesting they’d been infected. By this summer, that number had risen to 42%.
That figure clearly made an impression on many members of the committee who asked the FDA’s vaccine reviewers if they had tried to account for immunity from past infections in their modeling. They had not.
Some felt that even with a highly effective vaccine — new data presented by Pfizer showed the children’s dose was 90% effective at preventing symptomatic infections in kids — caution was warranted as much is still unknown about myocarditis, a rare side effect of the mRNA vaccines.
Myocarditis has been more common in younger age groups. It usually goes away over time but requires hospital care. It’s not known if myocarditis could have lingering effects for those who experience it.
There were no cases of myocarditis seen in Pfizer’s studies of the vaccine in children, and no other serious events were seen. Vaccine side effects reported in the Pfizer studies were mostly mild and included fatigue, headache, and pain at the injection site.
“We think we have optimized the immune response and minimized our reactions,” said William Gruber, MD, senior vice president vaccine research and clinical development at Pfizer.
But the studies didn’t include enough participants to pick up rare, but serious adverse events like myocarditis.
“We’re worried about a side effect that we can’t measure yet, but it’s probably real, and we see a benefit that isn’t the same as it is in older age groups,” said Dr. Rubin.
Benefits vs. risks
FDA modeled the benefits and risks for children under a variety of scenarios. The benefits of the vaccines to children very much depend on the amount of transmission in the community.
When transmission is high, the benefits to children — in terms of infections, hospitalizations, ICU admissions — clearly outweigh its risks.
But when COVID-19 rates are low in the community, as they were in June, FDA analysts predicted the vaccines might send more children to the hospital for myocarditis than the virus would.
The FDA noted that kids who are hospitalized for myocarditis tend not to be as ill as children with COVID-19, however.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” Dr. Hildreth said.
But others warned against complacency.
“Thinking that this is going to be the end of the wave permanently may be a little overly optimistic,” said committee chairman Arnold Monto, MD, a professor of public health and epidemiology at the University of Michigan, Ann Arbor.
The majority of COVID-19 cases in children are mild. Only about 1% of kids are hospitalized for their infections, according to CDC data. But the rates of hospitalizations in kids are about 3 times higher for people of color — including Blacks, Hispanics, and Native Americans, as compared to Whites and Asian Americans.
Since the start of the pandemic, 94 children ages 5 to 11 have died, making it the eighth leading cause of death for kids this age last year.
More than 5,200 children have developed a delayed complication from their infections called Multi-System Inflammatory Syndrome (MIS-C).
MIS-C can be severe and require hospital care and can lead to myocarditis. Children ages 5 to 11 are the age group at greatest risk for this complication.
Kids can also get long COVID. There’s not a lot of data on how often this happens, though it appears to be less frequent in children than in adults.
But a survey in the United Kingdom found that 7%-8% of kids have symptoms from their infections that last longer than 12 weeks, Dr. Havers said. Symptoms that can linger for kids include fatigue, cough, muscle and joint pain, headaches, and insomnia.
More than 1 million children have been impacted by school closures so far this year, and quarantines have had lasting impacts on learning, social development, and mental health.
Even though kids aren’t usually COVID superspreaders, they can still pass the infection on to others.
“What is clear is that secondary transmission from children, both to other children and to adults, does occur,” Dr. Havers said.
For that reason, they can continue the spread of the virus and give it opportunities to mutate and become more dangerous.
Safety monitoring to continue
Some committee members referenced thousands of letters they had received within the past few days urging them to vote against the vaccine.
Jay Portnoy, MD, a professor of pediatrics at Children’s Mercy Hospital in Kansas City, Mo., said he had personally received about 4,000 emails.
“But I feel like I need to also represent the consumers, the parents that I see every day in the clinic who are terrified of sending their children to school because they’re not protected against COVID,” he said, explaining his vote to recommend authorization.
“Our kids are going to be dealing with this virus for many years to come. It’s going to come repeatedly. Getting this vaccine is just the first step that they can take to protect themselves from having bad outcomes,” Dr. Portnoy said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, reminded members of the committee that there were several government surveillance systems in place to catch any potential safety issues in near real time.
“I really appreciate very much the concern here. The safety monitoring of this vaccine will continue,” Dr. Marks said. “I do view this as one of our greatest responsibilities.”
“I really am so grateful that we had this discussion and voted to approve,” said Capt. Amanda Cohn, MD, chief medical officer at the National Center for Immunization and Respiratory Diseases.
“I think the benefits in this age group really are super important even if they are lower than for other age groups.”
This article was updated 10/27/21.
A version of this article first appeared on WebMD.com.
Seventeen of the 18 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Oct. 26 voted to recommend the 10-microgram shot for kids, which is one-third the dose given to adults.
One member, Michael Kurilla, MD, director of the division of clinical innovation at the National Institutes of Health, Bethesda, Md., abstained from voting.
If the FDA follows the recommendation, as it typically does, and issues an Emergency Use Authorization for the vaccine, the shots could be available within days.
After the FDA’s final decision, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet to make specific recommendations for its use. The CDC committee must stick closely to the conditions for use spelled out in the EUA, so their recommendations are likely to be similar to those made by the FDA. Their next meeting is scheduled for Nov. 2 and 3.
In the end, some on the panel felt uneasy with their decision.
“I voted yes primarily because I wanted to make sure that children who really need this vaccine, the Black and brown children of our country, get the vaccine,” said James Hildreth, MD, PhD, president and CEO of Meharry Medical College in Nashville.
“But to be honest, the best way to protect the health of some children will be to do nothing because they will be just fine,” he said.
Others said they were surprised by how difficult the decision had been.
“This is a much tougher one than we had expected going into it,” said committee member Eric Rubin, MD, editor and chief of the New England Journal of Medicine, during the FDA advisory committee’s meeting.
Ahead of the vote, the committee heard presentations outlining the expected benefits of vaccinating children along with potential risks.
“Children have been greatly impacted by the pandemic,” said Fiona Havers, MD, a medical officer with the CDC in Atlanta who reviewed the epidemiology of COVID-19 in kids.
In the second year of the pandemic, as more seniors have been vaccinated against the virus, COVID cases have largely shifted from older to younger age groups.
So far, there have been more than 1.9 million COVID-19 cases in children ages 5 through 11 in the United States.. Cases in kids saw a big jump in July and August with summer travel, schools reopening, and the dominance of the Delta variant.
And those are just the cases reported to the CDC. Regular testing of anonymous blood samples collected at sites across the United States indicates that 6 times as many kids have had COVID than what is reflected in official counts.
Last winter, blood sample testing showed about 13% of children had antibodies against the virus, suggesting they’d been infected. By this summer, that number had risen to 42%.
That figure clearly made an impression on many members of the committee who asked the FDA’s vaccine reviewers if they had tried to account for immunity from past infections in their modeling. They had not.
Some felt that even with a highly effective vaccine — new data presented by Pfizer showed the children’s dose was 90% effective at preventing symptomatic infections in kids — caution was warranted as much is still unknown about myocarditis, a rare side effect of the mRNA vaccines.
Myocarditis has been more common in younger age groups. It usually goes away over time but requires hospital care. It’s not known if myocarditis could have lingering effects for those who experience it.
There were no cases of myocarditis seen in Pfizer’s studies of the vaccine in children, and no other serious events were seen. Vaccine side effects reported in the Pfizer studies were mostly mild and included fatigue, headache, and pain at the injection site.
“We think we have optimized the immune response and minimized our reactions,” said William Gruber, MD, senior vice president vaccine research and clinical development at Pfizer.
But the studies didn’t include enough participants to pick up rare, but serious adverse events like myocarditis.
“We’re worried about a side effect that we can’t measure yet, but it’s probably real, and we see a benefit that isn’t the same as it is in older age groups,” said Dr. Rubin.
Benefits vs. risks
FDA modeled the benefits and risks for children under a variety of scenarios. The benefits of the vaccines to children very much depend on the amount of transmission in the community.
When transmission is high, the benefits to children — in terms of infections, hospitalizations, ICU admissions — clearly outweigh its risks.
But when COVID-19 rates are low in the community, as they were in June, FDA analysts predicted the vaccines might send more children to the hospital for myocarditis than the virus would.
The FDA noted that kids who are hospitalized for myocarditis tend not to be as ill as children with COVID-19, however.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” Dr. Hildreth said.
But others warned against complacency.
“Thinking that this is going to be the end of the wave permanently may be a little overly optimistic,” said committee chairman Arnold Monto, MD, a professor of public health and epidemiology at the University of Michigan, Ann Arbor.
The majority of COVID-19 cases in children are mild. Only about 1% of kids are hospitalized for their infections, according to CDC data. But the rates of hospitalizations in kids are about 3 times higher for people of color — including Blacks, Hispanics, and Native Americans, as compared to Whites and Asian Americans.
Since the start of the pandemic, 94 children ages 5 to 11 have died, making it the eighth leading cause of death for kids this age last year.
More than 5,200 children have developed a delayed complication from their infections called Multi-System Inflammatory Syndrome (MIS-C).
MIS-C can be severe and require hospital care and can lead to myocarditis. Children ages 5 to 11 are the age group at greatest risk for this complication.
Kids can also get long COVID. There’s not a lot of data on how often this happens, though it appears to be less frequent in children than in adults.
But a survey in the United Kingdom found that 7%-8% of kids have symptoms from their infections that last longer than 12 weeks, Dr. Havers said. Symptoms that can linger for kids include fatigue, cough, muscle and joint pain, headaches, and insomnia.
More than 1 million children have been impacted by school closures so far this year, and quarantines have had lasting impacts on learning, social development, and mental health.
Even though kids aren’t usually COVID superspreaders, they can still pass the infection on to others.
“What is clear is that secondary transmission from children, both to other children and to adults, does occur,” Dr. Havers said.
For that reason, they can continue the spread of the virus and give it opportunities to mutate and become more dangerous.
Safety monitoring to continue
Some committee members referenced thousands of letters they had received within the past few days urging them to vote against the vaccine.
Jay Portnoy, MD, a professor of pediatrics at Children’s Mercy Hospital in Kansas City, Mo., said he had personally received about 4,000 emails.
“But I feel like I need to also represent the consumers, the parents that I see every day in the clinic who are terrified of sending their children to school because they’re not protected against COVID,” he said, explaining his vote to recommend authorization.
“Our kids are going to be dealing with this virus for many years to come. It’s going to come repeatedly. Getting this vaccine is just the first step that they can take to protect themselves from having bad outcomes,” Dr. Portnoy said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, reminded members of the committee that there were several government surveillance systems in place to catch any potential safety issues in near real time.
“I really appreciate very much the concern here. The safety monitoring of this vaccine will continue,” Dr. Marks said. “I do view this as one of our greatest responsibilities.”
“I really am so grateful that we had this discussion and voted to approve,” said Capt. Amanda Cohn, MD, chief medical officer at the National Center for Immunization and Respiratory Diseases.
“I think the benefits in this age group really are super important even if they are lower than for other age groups.”
This article was updated 10/27/21.
A version of this article first appeared on WebMD.com.
Seventeen of the 18 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Oct. 26 voted to recommend the 10-microgram shot for kids, which is one-third the dose given to adults.
One member, Michael Kurilla, MD, director of the division of clinical innovation at the National Institutes of Health, Bethesda, Md., abstained from voting.
If the FDA follows the recommendation, as it typically does, and issues an Emergency Use Authorization for the vaccine, the shots could be available within days.
After the FDA’s final decision, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet to make specific recommendations for its use. The CDC committee must stick closely to the conditions for use spelled out in the EUA, so their recommendations are likely to be similar to those made by the FDA. Their next meeting is scheduled for Nov. 2 and 3.
In the end, some on the panel felt uneasy with their decision.
“I voted yes primarily because I wanted to make sure that children who really need this vaccine, the Black and brown children of our country, get the vaccine,” said James Hildreth, MD, PhD, president and CEO of Meharry Medical College in Nashville.
“But to be honest, the best way to protect the health of some children will be to do nothing because they will be just fine,” he said.
Others said they were surprised by how difficult the decision had been.
“This is a much tougher one than we had expected going into it,” said committee member Eric Rubin, MD, editor and chief of the New England Journal of Medicine, during the FDA advisory committee’s meeting.
Ahead of the vote, the committee heard presentations outlining the expected benefits of vaccinating children along with potential risks.
“Children have been greatly impacted by the pandemic,” said Fiona Havers, MD, a medical officer with the CDC in Atlanta who reviewed the epidemiology of COVID-19 in kids.
In the second year of the pandemic, as more seniors have been vaccinated against the virus, COVID cases have largely shifted from older to younger age groups.
So far, there have been more than 1.9 million COVID-19 cases in children ages 5 through 11 in the United States.. Cases in kids saw a big jump in July and August with summer travel, schools reopening, and the dominance of the Delta variant.
And those are just the cases reported to the CDC. Regular testing of anonymous blood samples collected at sites across the United States indicates that 6 times as many kids have had COVID than what is reflected in official counts.
Last winter, blood sample testing showed about 13% of children had antibodies against the virus, suggesting they’d been infected. By this summer, that number had risen to 42%.
That figure clearly made an impression on many members of the committee who asked the FDA’s vaccine reviewers if they had tried to account for immunity from past infections in their modeling. They had not.
Some felt that even with a highly effective vaccine — new data presented by Pfizer showed the children’s dose was 90% effective at preventing symptomatic infections in kids — caution was warranted as much is still unknown about myocarditis, a rare side effect of the mRNA vaccines.
Myocarditis has been more common in younger age groups. It usually goes away over time but requires hospital care. It’s not known if myocarditis could have lingering effects for those who experience it.
There were no cases of myocarditis seen in Pfizer’s studies of the vaccine in children, and no other serious events were seen. Vaccine side effects reported in the Pfizer studies were mostly mild and included fatigue, headache, and pain at the injection site.
“We think we have optimized the immune response and minimized our reactions,” said William Gruber, MD, senior vice president vaccine research and clinical development at Pfizer.
But the studies didn’t include enough participants to pick up rare, but serious adverse events like myocarditis.
“We’re worried about a side effect that we can’t measure yet, but it’s probably real, and we see a benefit that isn’t the same as it is in older age groups,” said Dr. Rubin.
Benefits vs. risks
FDA modeled the benefits and risks for children under a variety of scenarios. The benefits of the vaccines to children very much depend on the amount of transmission in the community.
When transmission is high, the benefits to children — in terms of infections, hospitalizations, ICU admissions — clearly outweigh its risks.
But when COVID-19 rates are low in the community, as they were in June, FDA analysts predicted the vaccines might send more children to the hospital for myocarditis than the virus would.
The FDA noted that kids who are hospitalized for myocarditis tend not to be as ill as children with COVID-19, however.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” Dr. Hildreth said.
But others warned against complacency.
“Thinking that this is going to be the end of the wave permanently may be a little overly optimistic,” said committee chairman Arnold Monto, MD, a professor of public health and epidemiology at the University of Michigan, Ann Arbor.
The majority of COVID-19 cases in children are mild. Only about 1% of kids are hospitalized for their infections, according to CDC data. But the rates of hospitalizations in kids are about 3 times higher for people of color — including Blacks, Hispanics, and Native Americans, as compared to Whites and Asian Americans.
Since the start of the pandemic, 94 children ages 5 to 11 have died, making it the eighth leading cause of death for kids this age last year.
More than 5,200 children have developed a delayed complication from their infections called Multi-System Inflammatory Syndrome (MIS-C).
MIS-C can be severe and require hospital care and can lead to myocarditis. Children ages 5 to 11 are the age group at greatest risk for this complication.
Kids can also get long COVID. There’s not a lot of data on how often this happens, though it appears to be less frequent in children than in adults.
But a survey in the United Kingdom found that 7%-8% of kids have symptoms from their infections that last longer than 12 weeks, Dr. Havers said. Symptoms that can linger for kids include fatigue, cough, muscle and joint pain, headaches, and insomnia.
More than 1 million children have been impacted by school closures so far this year, and quarantines have had lasting impacts on learning, social development, and mental health.
Even though kids aren’t usually COVID superspreaders, they can still pass the infection on to others.
“What is clear is that secondary transmission from children, both to other children and to adults, does occur,” Dr. Havers said.
For that reason, they can continue the spread of the virus and give it opportunities to mutate and become more dangerous.
Safety monitoring to continue
Some committee members referenced thousands of letters they had received within the past few days urging them to vote against the vaccine.
Jay Portnoy, MD, a professor of pediatrics at Children’s Mercy Hospital in Kansas City, Mo., said he had personally received about 4,000 emails.
“But I feel like I need to also represent the consumers, the parents that I see every day in the clinic who are terrified of sending their children to school because they’re not protected against COVID,” he said, explaining his vote to recommend authorization.
“Our kids are going to be dealing with this virus for many years to come. It’s going to come repeatedly. Getting this vaccine is just the first step that they can take to protect themselves from having bad outcomes,” Dr. Portnoy said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, reminded members of the committee that there were several government surveillance systems in place to catch any potential safety issues in near real time.
“I really appreciate very much the concern here. The safety monitoring of this vaccine will continue,” Dr. Marks said. “I do view this as one of our greatest responsibilities.”
“I really am so grateful that we had this discussion and voted to approve,” said Capt. Amanda Cohn, MD, chief medical officer at the National Center for Immunization and Respiratory Diseases.
“I think the benefits in this age group really are super important even if they are lower than for other age groups.”
This article was updated 10/27/21.
A version of this article first appeared on WebMD.com.
Risk-based antenatal type-and-screen blood testing safe and economical
Implementing a selective type-and-screen blood testing policy in the labor and delivery unit was associated with projected annual savings of close to $200,000, a large single-center study found. Furthermore, there was no evidence of increased maternal morbidity in the university-based facility performing more than 4,400 deliveries per year, according to Ashley E. Benson, MD, MA, of the department of obstetrics and gynecology at the University of Utah, Salt Lake City, and colleagues.
The study, published in Obstetrics & Gynecology, evaluated patient safety, resource utilization, and transfusion-related costs after a policy change from universal type and screen to selective, risk-based type and screen on admission to labor and delivery.
“There had been some national interest in moving toward decreased resource utilization, and findings that universal screening was not cost effective,” Dr. Benson, who has since relocated to Oregon Health & Science University, Portland, said in an interview. An earlier cost-effective modeling study at her center had suggested that universal test and screen was not cost effective and likely not safer either. “So based on that data we felt an implementation study was warranted.”
The switch to a selective policy was made in 2018, after which her group compared outcomes from October 2017 to September 2019, looking those both 1 year preimplementation and 1 year post implementation.
One year post implementation, the following outcomes emerged, compared with preimplementation:
- Overall projected saving of $181,000 a year in the maternity unit
- Lower mean monthly type- and screen-related costs, such as those for ABO typing, antibody screen, and antibody workup. cross-matches, hold clots, and transfused products: $9,753 vs. $20,676 in the preimplementation year (P < .001)
- A lower mean monthly cost of total transfusion preparedness: $25,090 vs. $39,211 (P < .001)
- No differences in emergency-release transfusion events (four vs. three, P = .99),the study’s primary safety outcome
- Fewer emergency-release red blood cell units transfused (9 vs. 24, P = .002) and O-negative RBC units transfused (8 vs. 18, P = .016)
- No differences in hysterectomies (0.05% vs. 0.1%, P = .44) and ICU admissions (0.45% vs. 0.51%, P = .43)
“In a year of selective type and screen, we saw a 51% reduction in costs related to type and screen, and a 38% reduction in overall transfusion-related costs,” the authors wrote. “This study supports other literature suggesting that more judicious use of type and screen may be safe and cost effective.”
Dr. Benson said the results were positively received when presented a meeting 2 years ago but the published version has yet to prompt feedback.
The study
Antepartum patients underwent transfusion preparedness tests according to the center’s standard antenatal admission order sets and were risk stratified in alignment with California Maternal Quality Care Collaborative recommendations. The mean maternal age of patients in both time periods was similar at just over 29 years and the mean gestational age at delivery was just under 38 weeks.
Under the new policy, a “hold clot” is obtained for women stratified as low or medium risk on admission. In this instance, a tube of patient blood is held in the blood bank but processed only if needed, as in the event of active hemorrhage or an order for transfusion. A blood cross-match is obtained on all women stratified as high risk or having a prior positive antibody screen.
Relevant costs were the direct costs of transfusion-related testing in the labor and delivery unit from a health system perspective.
Obstetric hemorrhage is the leading cause of maternal death worldwide, the authors pointed out. While transfusion in obstetric patients occurs in only 1% or 2% of all deliveries it is nevertheless difficult to predict which patients will need transfusion, with only 2%-8% of those stratified as high risk ultimately requiring transfusion. Although obstetric hemorrhage safety bundles recommend risk stratification on admission to labor and delivery with selective type and screen for higher-risk individuals, for safety and simplicity’s sake, many labor and delivery units perform universal type and screen.
The authors cautioned that these results occurred in an academic tertiary care center with systems fine-tuned to deal with active hemorrhage and deliver timely transfusable blood. “At the moment we don’t have enough data to say whether the selective approach would be safe in hospitals with more limited blood bank capacity and access and fewer transfusion specialists in a setting optimized to respond to emergent needs, Dr. Benson said.
Katayoun F. M. Fomani, MD, a transfusion medicine specialist and medical director of blood bank and transfusion services at Long Island Jewish Medical Center, New York, agreed. “This approach only works in a controlled environment such as in this study where eligible women were assessed antenatally at the same center, but it would not work at every institution,” she said in an interview. “In addition, all patients were assessed according to the California Collaborative guideline, which itself increases the safety level but is not followed everywhere.”
The obstetric division at her hospital in New York adheres to the universal type and screen. “We have patients coming in from outside whose antenatal testing was not done at our hospital,” she said. “For this selective approach to work you need a controlled population and the electronic resources and personnel to follow each patient carefully.”
The authors indicated no specific funding for this study and disclosed no potential conflicts of interest. Dr. Fomani had no potential competing interests to declare.
Implementing a selective type-and-screen blood testing policy in the labor and delivery unit was associated with projected annual savings of close to $200,000, a large single-center study found. Furthermore, there was no evidence of increased maternal morbidity in the university-based facility performing more than 4,400 deliveries per year, according to Ashley E. Benson, MD, MA, of the department of obstetrics and gynecology at the University of Utah, Salt Lake City, and colleagues.
The study, published in Obstetrics & Gynecology, evaluated patient safety, resource utilization, and transfusion-related costs after a policy change from universal type and screen to selective, risk-based type and screen on admission to labor and delivery.
“There had been some national interest in moving toward decreased resource utilization, and findings that universal screening was not cost effective,” Dr. Benson, who has since relocated to Oregon Health & Science University, Portland, said in an interview. An earlier cost-effective modeling study at her center had suggested that universal test and screen was not cost effective and likely not safer either. “So based on that data we felt an implementation study was warranted.”
The switch to a selective policy was made in 2018, after which her group compared outcomes from October 2017 to September 2019, looking those both 1 year preimplementation and 1 year post implementation.
One year post implementation, the following outcomes emerged, compared with preimplementation:
- Overall projected saving of $181,000 a year in the maternity unit
- Lower mean monthly type- and screen-related costs, such as those for ABO typing, antibody screen, and antibody workup. cross-matches, hold clots, and transfused products: $9,753 vs. $20,676 in the preimplementation year (P < .001)
- A lower mean monthly cost of total transfusion preparedness: $25,090 vs. $39,211 (P < .001)
- No differences in emergency-release transfusion events (four vs. three, P = .99),the study’s primary safety outcome
- Fewer emergency-release red blood cell units transfused (9 vs. 24, P = .002) and O-negative RBC units transfused (8 vs. 18, P = .016)
- No differences in hysterectomies (0.05% vs. 0.1%, P = .44) and ICU admissions (0.45% vs. 0.51%, P = .43)
“In a year of selective type and screen, we saw a 51% reduction in costs related to type and screen, and a 38% reduction in overall transfusion-related costs,” the authors wrote. “This study supports other literature suggesting that more judicious use of type and screen may be safe and cost effective.”
Dr. Benson said the results were positively received when presented a meeting 2 years ago but the published version has yet to prompt feedback.
The study
Antepartum patients underwent transfusion preparedness tests according to the center’s standard antenatal admission order sets and were risk stratified in alignment with California Maternal Quality Care Collaborative recommendations. The mean maternal age of patients in both time periods was similar at just over 29 years and the mean gestational age at delivery was just under 38 weeks.
Under the new policy, a “hold clot” is obtained for women stratified as low or medium risk on admission. In this instance, a tube of patient blood is held in the blood bank but processed only if needed, as in the event of active hemorrhage or an order for transfusion. A blood cross-match is obtained on all women stratified as high risk or having a prior positive antibody screen.
Relevant costs were the direct costs of transfusion-related testing in the labor and delivery unit from a health system perspective.
Obstetric hemorrhage is the leading cause of maternal death worldwide, the authors pointed out. While transfusion in obstetric patients occurs in only 1% or 2% of all deliveries it is nevertheless difficult to predict which patients will need transfusion, with only 2%-8% of those stratified as high risk ultimately requiring transfusion. Although obstetric hemorrhage safety bundles recommend risk stratification on admission to labor and delivery with selective type and screen for higher-risk individuals, for safety and simplicity’s sake, many labor and delivery units perform universal type and screen.
The authors cautioned that these results occurred in an academic tertiary care center with systems fine-tuned to deal with active hemorrhage and deliver timely transfusable blood. “At the moment we don’t have enough data to say whether the selective approach would be safe in hospitals with more limited blood bank capacity and access and fewer transfusion specialists in a setting optimized to respond to emergent needs, Dr. Benson said.
Katayoun F. M. Fomani, MD, a transfusion medicine specialist and medical director of blood bank and transfusion services at Long Island Jewish Medical Center, New York, agreed. “This approach only works in a controlled environment such as in this study where eligible women were assessed antenatally at the same center, but it would not work at every institution,” she said in an interview. “In addition, all patients were assessed according to the California Collaborative guideline, which itself increases the safety level but is not followed everywhere.”
The obstetric division at her hospital in New York adheres to the universal type and screen. “We have patients coming in from outside whose antenatal testing was not done at our hospital,” she said. “For this selective approach to work you need a controlled population and the electronic resources and personnel to follow each patient carefully.”
The authors indicated no specific funding for this study and disclosed no potential conflicts of interest. Dr. Fomani had no potential competing interests to declare.
Implementing a selective type-and-screen blood testing policy in the labor and delivery unit was associated with projected annual savings of close to $200,000, a large single-center study found. Furthermore, there was no evidence of increased maternal morbidity in the university-based facility performing more than 4,400 deliveries per year, according to Ashley E. Benson, MD, MA, of the department of obstetrics and gynecology at the University of Utah, Salt Lake City, and colleagues.
The study, published in Obstetrics & Gynecology, evaluated patient safety, resource utilization, and transfusion-related costs after a policy change from universal type and screen to selective, risk-based type and screen on admission to labor and delivery.
“There had been some national interest in moving toward decreased resource utilization, and findings that universal screening was not cost effective,” Dr. Benson, who has since relocated to Oregon Health & Science University, Portland, said in an interview. An earlier cost-effective modeling study at her center had suggested that universal test and screen was not cost effective and likely not safer either. “So based on that data we felt an implementation study was warranted.”
The switch to a selective policy was made in 2018, after which her group compared outcomes from October 2017 to September 2019, looking those both 1 year preimplementation and 1 year post implementation.
One year post implementation, the following outcomes emerged, compared with preimplementation:
- Overall projected saving of $181,000 a year in the maternity unit
- Lower mean monthly type- and screen-related costs, such as those for ABO typing, antibody screen, and antibody workup. cross-matches, hold clots, and transfused products: $9,753 vs. $20,676 in the preimplementation year (P < .001)
- A lower mean monthly cost of total transfusion preparedness: $25,090 vs. $39,211 (P < .001)
- No differences in emergency-release transfusion events (four vs. three, P = .99),the study’s primary safety outcome
- Fewer emergency-release red blood cell units transfused (9 vs. 24, P = .002) and O-negative RBC units transfused (8 vs. 18, P = .016)
- No differences in hysterectomies (0.05% vs. 0.1%, P = .44) and ICU admissions (0.45% vs. 0.51%, P = .43)
“In a year of selective type and screen, we saw a 51% reduction in costs related to type and screen, and a 38% reduction in overall transfusion-related costs,” the authors wrote. “This study supports other literature suggesting that more judicious use of type and screen may be safe and cost effective.”
Dr. Benson said the results were positively received when presented a meeting 2 years ago but the published version has yet to prompt feedback.
The study
Antepartum patients underwent transfusion preparedness tests according to the center’s standard antenatal admission order sets and were risk stratified in alignment with California Maternal Quality Care Collaborative recommendations. The mean maternal age of patients in both time periods was similar at just over 29 years and the mean gestational age at delivery was just under 38 weeks.
Under the new policy, a “hold clot” is obtained for women stratified as low or medium risk on admission. In this instance, a tube of patient blood is held in the blood bank but processed only if needed, as in the event of active hemorrhage or an order for transfusion. A blood cross-match is obtained on all women stratified as high risk or having a prior positive antibody screen.
Relevant costs were the direct costs of transfusion-related testing in the labor and delivery unit from a health system perspective.
Obstetric hemorrhage is the leading cause of maternal death worldwide, the authors pointed out. While transfusion in obstetric patients occurs in only 1% or 2% of all deliveries it is nevertheless difficult to predict which patients will need transfusion, with only 2%-8% of those stratified as high risk ultimately requiring transfusion. Although obstetric hemorrhage safety bundles recommend risk stratification on admission to labor and delivery with selective type and screen for higher-risk individuals, for safety and simplicity’s sake, many labor and delivery units perform universal type and screen.
The authors cautioned that these results occurred in an academic tertiary care center with systems fine-tuned to deal with active hemorrhage and deliver timely transfusable blood. “At the moment we don’t have enough data to say whether the selective approach would be safe in hospitals with more limited blood bank capacity and access and fewer transfusion specialists in a setting optimized to respond to emergent needs, Dr. Benson said.
Katayoun F. M. Fomani, MD, a transfusion medicine specialist and medical director of blood bank and transfusion services at Long Island Jewish Medical Center, New York, agreed. “This approach only works in a controlled environment such as in this study where eligible women were assessed antenatally at the same center, but it would not work at every institution,” she said in an interview. “In addition, all patients were assessed according to the California Collaborative guideline, which itself increases the safety level but is not followed everywhere.”
The obstetric division at her hospital in New York adheres to the universal type and screen. “We have patients coming in from outside whose antenatal testing was not done at our hospital,” she said. “For this selective approach to work you need a controlled population and the electronic resources and personnel to follow each patient carefully.”
The authors indicated no specific funding for this study and disclosed no potential conflicts of interest. Dr. Fomani had no potential competing interests to declare.
FROM OBSTETRICS & GYNECOLOGY
Unvaccinated people likely to catch COVID repeatedly
according to a recent study published in The Lancet Microbe.
Since COVID-19 hasn’t existed for long enough to perform a long-term study, researchers at Yale University and the University of North Carolina at Charlotte looked at reinfection data for six other human-infecting coronaviruses, including SARS and MERS.
“Reinfection can reasonably happen in three months or less,” Jeffrey Townsend, PhD, lead study author and a biostatistics professor at the Yale School of Public Health, said in a statement.
“Therefore, those who have been naturally infected should get vaccinated,” he said. “Previous infection alone can offer very little long-term protection against subsequent infections.”
The research team looked at post-infection data for six coronaviruses between 1984-2020 and found reinfection ranged from 128 days to 28 years. They calculated that reinfection with COVID-19 would likely occur between 3 months to 5 years after peak antibody response, with an average of 16 months. This is less than half the duration seen for other coronaviruses that circulate among humans.
The risk of COVID-19 reinfection is about 5% at three months, which jumps to 50% after 17 months, the research team found. Reinfection could become increasingly common as immunity wanes and new variants develop, they said.
“We tend to think about immunity as being immune or not immune. Our study cautions that we instead should be more focused on the risk of reinfection through time,” Alex Dornburg, PhD, senior study author and assistant professor of bioinformatics and genomics at UNC, said in the statement.
“As new variants arise, previous immune responses become less effective at combating the virus,” he said. “Those who were naturally infected early in the pandemic are increasingly likely to become reinfected in the near future.”
Study estimates are based on average times of declining immunity across different coronaviruses, the researchers told the Yale Daily News. At the individual level, people have different levels of immunity, which can provide shorter or longer duration of protection based on immune status, immunity within a community, age, underlying health conditions, environmental exposure, and other factors.
The research team said that preventive health measures and global distribution of vaccines will be “critical” in minimizing reinfection and COVID-19 deaths. In areas with low vaccination rates, for instance, unvaccinated people should continue safety practices such as social distancing, wearing masks, and proper indoor ventilation to avoid reinfection.
“We need to be very aware of the fact that this disease is likely to be circulating over the long term and that we don’t have this long-term immunity that many people seem to be hoping to rely on in order to protect them from disease,” Dr. Townsend told the newspaper.
A version of this article first appeared on WebMD.com.
according to a recent study published in The Lancet Microbe.
Since COVID-19 hasn’t existed for long enough to perform a long-term study, researchers at Yale University and the University of North Carolina at Charlotte looked at reinfection data for six other human-infecting coronaviruses, including SARS and MERS.
“Reinfection can reasonably happen in three months or less,” Jeffrey Townsend, PhD, lead study author and a biostatistics professor at the Yale School of Public Health, said in a statement.
“Therefore, those who have been naturally infected should get vaccinated,” he said. “Previous infection alone can offer very little long-term protection against subsequent infections.”
The research team looked at post-infection data for six coronaviruses between 1984-2020 and found reinfection ranged from 128 days to 28 years. They calculated that reinfection with COVID-19 would likely occur between 3 months to 5 years after peak antibody response, with an average of 16 months. This is less than half the duration seen for other coronaviruses that circulate among humans.
The risk of COVID-19 reinfection is about 5% at three months, which jumps to 50% after 17 months, the research team found. Reinfection could become increasingly common as immunity wanes and new variants develop, they said.
“We tend to think about immunity as being immune or not immune. Our study cautions that we instead should be more focused on the risk of reinfection through time,” Alex Dornburg, PhD, senior study author and assistant professor of bioinformatics and genomics at UNC, said in the statement.
“As new variants arise, previous immune responses become less effective at combating the virus,” he said. “Those who were naturally infected early in the pandemic are increasingly likely to become reinfected in the near future.”
Study estimates are based on average times of declining immunity across different coronaviruses, the researchers told the Yale Daily News. At the individual level, people have different levels of immunity, which can provide shorter or longer duration of protection based on immune status, immunity within a community, age, underlying health conditions, environmental exposure, and other factors.
The research team said that preventive health measures and global distribution of vaccines will be “critical” in minimizing reinfection and COVID-19 deaths. In areas with low vaccination rates, for instance, unvaccinated people should continue safety practices such as social distancing, wearing masks, and proper indoor ventilation to avoid reinfection.
“We need to be very aware of the fact that this disease is likely to be circulating over the long term and that we don’t have this long-term immunity that many people seem to be hoping to rely on in order to protect them from disease,” Dr. Townsend told the newspaper.
A version of this article first appeared on WebMD.com.
according to a recent study published in The Lancet Microbe.
Since COVID-19 hasn’t existed for long enough to perform a long-term study, researchers at Yale University and the University of North Carolina at Charlotte looked at reinfection data for six other human-infecting coronaviruses, including SARS and MERS.
“Reinfection can reasonably happen in three months or less,” Jeffrey Townsend, PhD, lead study author and a biostatistics professor at the Yale School of Public Health, said in a statement.
“Therefore, those who have been naturally infected should get vaccinated,” he said. “Previous infection alone can offer very little long-term protection against subsequent infections.”
The research team looked at post-infection data for six coronaviruses between 1984-2020 and found reinfection ranged from 128 days to 28 years. They calculated that reinfection with COVID-19 would likely occur between 3 months to 5 years after peak antibody response, with an average of 16 months. This is less than half the duration seen for other coronaviruses that circulate among humans.
The risk of COVID-19 reinfection is about 5% at three months, which jumps to 50% after 17 months, the research team found. Reinfection could become increasingly common as immunity wanes and new variants develop, they said.
“We tend to think about immunity as being immune or not immune. Our study cautions that we instead should be more focused on the risk of reinfection through time,” Alex Dornburg, PhD, senior study author and assistant professor of bioinformatics and genomics at UNC, said in the statement.
“As new variants arise, previous immune responses become less effective at combating the virus,” he said. “Those who were naturally infected early in the pandemic are increasingly likely to become reinfected in the near future.”
Study estimates are based on average times of declining immunity across different coronaviruses, the researchers told the Yale Daily News. At the individual level, people have different levels of immunity, which can provide shorter or longer duration of protection based on immune status, immunity within a community, age, underlying health conditions, environmental exposure, and other factors.
The research team said that preventive health measures and global distribution of vaccines will be “critical” in minimizing reinfection and COVID-19 deaths. In areas with low vaccination rates, for instance, unvaccinated people should continue safety practices such as social distancing, wearing masks, and proper indoor ventilation to avoid reinfection.
“We need to be very aware of the fact that this disease is likely to be circulating over the long term and that we don’t have this long-term immunity that many people seem to be hoping to rely on in order to protect them from disease,” Dr. Townsend told the newspaper.
A version of this article first appeared on WebMD.com.
Identify patient and hospital factors to reduce maternal mortality
Maternal mortality is a public health crisis for all women, said Elizabeth A. Howell, MD, of the University of Pennsylvania, Philadelphia, in a presentation at the virtual Advancing NIH Research on the Health of Women conference sponsored by the National Institutes of Health.
The maternal mortality rate in the United States in 2018 was 17.4 maternal deaths per 100,000 live births, according to data from the Centers for Disease Control and Prevention, Dr. Howell said. Maternal mortality is defined as death during pregnancy or within 42 days of delivery; pregnancy-related mortality includes death during pregnancy or within 1 year of pregnancy, from pregnancy or as a result of any cause related to, or aggravated by, pregnancy, according to the CDC.
However, “Black women are two to three times more likely than White women to die from a pregnancy-related cause,” Dr. Howell said. These disparities are even more marked in some cities; data show that Black women in New York City are eight times more likely than White women to die from a pregnancy-related cause, she noted.
Pregnancy-related mortality persists regardless of education level, and remains significantly higher in Black women, compared with White women with at least a college degree, Dr. Howell added.
In her presentation, Dr. Howell reviewed some top causes of maternal mortality overall, and potential factors driving disparities. Data from the CDC show cardiomyopathy, cardiovascular conditions, and preeclampsia/eclampsia as the top three underlying causes of pregnancy-related deaths among non-Hispanic Black women, compared with mental health conditions, cardiovascular conditions, and hemorrhage in non-Hispanic White women, Dr. Howell said.
To help prevent maternal mortality across all populations, “It is important for us to think about the timing of deaths so we can better understand the causes,” said Dr. Howell.
CDC Vital Signs data show that approximately one-third of pregnancy-related deaths occur during pregnancy, but approximately 20% occur between 43 and 365 days postpartum, she said.
Although cardiovascular conditions top the list of clinical causes of pregnancy-related maternal mortality, maternal self-harm should not be discounted, and is likely underreported, Dr. Howell said. Data show that the peak incidence of maternal suicide occurs between 9 and 12 months’ postpartum, and risk factors include major depression, substance use disorder, and intimate partner violence, she noted.
Dr. Howell then shared the results of studies she conducted in 2020 and 2016 on racial disparities, hospital quality, and maternal mortality. One of her key findings in the 2020 study, presented at this year’s virtual meeting of the American College of Obstetricians and Gynecologists, showed that women delivering in the lowest-ranked hospitals had six times the rate of severe maternal morbidity, and an accompanying simulation/thought exercise showed that the hospital of delivery accounted for approximately half of the disparity in severe maternal morbidity between Black and White women. An earlier study she published in 2016 of between-hospital differences in New York City showed that Black and Latina women were significantly more likely than White women to deliver in hospitals with higher rates of severe maternal mortality.
These findings illustrate that “racial segregation in neighborhoods is also part of the story,” of maternal mortality, Dr. Howell said.
Dr. Howell outlined ways the health care community can reduce severe maternal morbidity and mortality for all women, including promoting contraception and preconception health, improving postpartum management, eliminating bias, and using patient navigators as needed to enhance communication among the care team,
“Think about ways to engage the community,” in support of women’s pregnancy health, Dr. Howell said. She also emphasized the need to enroll more pregnant women in clinical trials.
Don’t exclude pregnant women from trials
In a follow-up session, Cynthia Gyamfi-Bannerman, MD, of the University of California, San Diego, expanded on opportunities to include pregnant women in clinical research.
Clinical trials for pregnant people fall into two categories, she noted; those studying interventions to improve pregnancy outcomes and those studying interventions for common medical conditions that coexist with pregnancy. These trials are either initiated by the investigators, conducted under contract, or federally funded, Dr. Gyamfi-Bannerman said. Currently, the only obstetric clinical trials research network is the Maternal-Fetal Medicine Units Network, established in 1986 by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The MFMU has conducted significant and life-saving research, but “we need more networks to focus on researching pregnancy complications,” Dr. Gyamfi-Bannerman said. Once the infrastructure exists in multiple settings, the ability to conduct trials will improve, she said.
Dr. Gyamfi-Bannerman stressed the need to engage and involve community-based physicians in clinical trials; using those relationships to enroll a more diverse population for whom working with their local physician would be more feasible than traveling to a larger clinical trial center.
She also commented on the need to include pregnant women in nonobstetric clinical trials. The exclusion of pregnant women from COVID-19 vaccine trials left clinicians with no information for guiding pregnant patients, she said. “It is important to think about why we are excluding pregnant women,” she said.
Finally, Dr. Gyamfi-Bannerman recommended a national effort to coordinate and leverage EHR data, which could have an effect on reducing maternal morbidity by facilitating the study of nonobstetric interventions in pregnancy, such as behavior interventions and mental health care.
Dr. Howell and Dr. Gyamfi-Bannerman had no financial conflicts to disclose.
Maternal mortality is a public health crisis for all women, said Elizabeth A. Howell, MD, of the University of Pennsylvania, Philadelphia, in a presentation at the virtual Advancing NIH Research on the Health of Women conference sponsored by the National Institutes of Health.
The maternal mortality rate in the United States in 2018 was 17.4 maternal deaths per 100,000 live births, according to data from the Centers for Disease Control and Prevention, Dr. Howell said. Maternal mortality is defined as death during pregnancy or within 42 days of delivery; pregnancy-related mortality includes death during pregnancy or within 1 year of pregnancy, from pregnancy or as a result of any cause related to, or aggravated by, pregnancy, according to the CDC.
However, “Black women are two to three times more likely than White women to die from a pregnancy-related cause,” Dr. Howell said. These disparities are even more marked in some cities; data show that Black women in New York City are eight times more likely than White women to die from a pregnancy-related cause, she noted.
Pregnancy-related mortality persists regardless of education level, and remains significantly higher in Black women, compared with White women with at least a college degree, Dr. Howell added.
In her presentation, Dr. Howell reviewed some top causes of maternal mortality overall, and potential factors driving disparities. Data from the CDC show cardiomyopathy, cardiovascular conditions, and preeclampsia/eclampsia as the top three underlying causes of pregnancy-related deaths among non-Hispanic Black women, compared with mental health conditions, cardiovascular conditions, and hemorrhage in non-Hispanic White women, Dr. Howell said.
To help prevent maternal mortality across all populations, “It is important for us to think about the timing of deaths so we can better understand the causes,” said Dr. Howell.
CDC Vital Signs data show that approximately one-third of pregnancy-related deaths occur during pregnancy, but approximately 20% occur between 43 and 365 days postpartum, she said.
Although cardiovascular conditions top the list of clinical causes of pregnancy-related maternal mortality, maternal self-harm should not be discounted, and is likely underreported, Dr. Howell said. Data show that the peak incidence of maternal suicide occurs between 9 and 12 months’ postpartum, and risk factors include major depression, substance use disorder, and intimate partner violence, she noted.
Dr. Howell then shared the results of studies she conducted in 2020 and 2016 on racial disparities, hospital quality, and maternal mortality. One of her key findings in the 2020 study, presented at this year’s virtual meeting of the American College of Obstetricians and Gynecologists, showed that women delivering in the lowest-ranked hospitals had six times the rate of severe maternal morbidity, and an accompanying simulation/thought exercise showed that the hospital of delivery accounted for approximately half of the disparity in severe maternal morbidity between Black and White women. An earlier study she published in 2016 of between-hospital differences in New York City showed that Black and Latina women were significantly more likely than White women to deliver in hospitals with higher rates of severe maternal mortality.
These findings illustrate that “racial segregation in neighborhoods is also part of the story,” of maternal mortality, Dr. Howell said.
Dr. Howell outlined ways the health care community can reduce severe maternal morbidity and mortality for all women, including promoting contraception and preconception health, improving postpartum management, eliminating bias, and using patient navigators as needed to enhance communication among the care team,
“Think about ways to engage the community,” in support of women’s pregnancy health, Dr. Howell said. She also emphasized the need to enroll more pregnant women in clinical trials.
Don’t exclude pregnant women from trials
In a follow-up session, Cynthia Gyamfi-Bannerman, MD, of the University of California, San Diego, expanded on opportunities to include pregnant women in clinical research.
Clinical trials for pregnant people fall into two categories, she noted; those studying interventions to improve pregnancy outcomes and those studying interventions for common medical conditions that coexist with pregnancy. These trials are either initiated by the investigators, conducted under contract, or federally funded, Dr. Gyamfi-Bannerman said. Currently, the only obstetric clinical trials research network is the Maternal-Fetal Medicine Units Network, established in 1986 by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The MFMU has conducted significant and life-saving research, but “we need more networks to focus on researching pregnancy complications,” Dr. Gyamfi-Bannerman said. Once the infrastructure exists in multiple settings, the ability to conduct trials will improve, she said.
Dr. Gyamfi-Bannerman stressed the need to engage and involve community-based physicians in clinical trials; using those relationships to enroll a more diverse population for whom working with their local physician would be more feasible than traveling to a larger clinical trial center.
She also commented on the need to include pregnant women in nonobstetric clinical trials. The exclusion of pregnant women from COVID-19 vaccine trials left clinicians with no information for guiding pregnant patients, she said. “It is important to think about why we are excluding pregnant women,” she said.
Finally, Dr. Gyamfi-Bannerman recommended a national effort to coordinate and leverage EHR data, which could have an effect on reducing maternal morbidity by facilitating the study of nonobstetric interventions in pregnancy, such as behavior interventions and mental health care.
Dr. Howell and Dr. Gyamfi-Bannerman had no financial conflicts to disclose.
Maternal mortality is a public health crisis for all women, said Elizabeth A. Howell, MD, of the University of Pennsylvania, Philadelphia, in a presentation at the virtual Advancing NIH Research on the Health of Women conference sponsored by the National Institutes of Health.
The maternal mortality rate in the United States in 2018 was 17.4 maternal deaths per 100,000 live births, according to data from the Centers for Disease Control and Prevention, Dr. Howell said. Maternal mortality is defined as death during pregnancy or within 42 days of delivery; pregnancy-related mortality includes death during pregnancy or within 1 year of pregnancy, from pregnancy or as a result of any cause related to, or aggravated by, pregnancy, according to the CDC.
However, “Black women are two to three times more likely than White women to die from a pregnancy-related cause,” Dr. Howell said. These disparities are even more marked in some cities; data show that Black women in New York City are eight times more likely than White women to die from a pregnancy-related cause, she noted.
Pregnancy-related mortality persists regardless of education level, and remains significantly higher in Black women, compared with White women with at least a college degree, Dr. Howell added.
In her presentation, Dr. Howell reviewed some top causes of maternal mortality overall, and potential factors driving disparities. Data from the CDC show cardiomyopathy, cardiovascular conditions, and preeclampsia/eclampsia as the top three underlying causes of pregnancy-related deaths among non-Hispanic Black women, compared with mental health conditions, cardiovascular conditions, and hemorrhage in non-Hispanic White women, Dr. Howell said.
To help prevent maternal mortality across all populations, “It is important for us to think about the timing of deaths so we can better understand the causes,” said Dr. Howell.
CDC Vital Signs data show that approximately one-third of pregnancy-related deaths occur during pregnancy, but approximately 20% occur between 43 and 365 days postpartum, she said.
Although cardiovascular conditions top the list of clinical causes of pregnancy-related maternal mortality, maternal self-harm should not be discounted, and is likely underreported, Dr. Howell said. Data show that the peak incidence of maternal suicide occurs between 9 and 12 months’ postpartum, and risk factors include major depression, substance use disorder, and intimate partner violence, she noted.
Dr. Howell then shared the results of studies she conducted in 2020 and 2016 on racial disparities, hospital quality, and maternal mortality. One of her key findings in the 2020 study, presented at this year’s virtual meeting of the American College of Obstetricians and Gynecologists, showed that women delivering in the lowest-ranked hospitals had six times the rate of severe maternal morbidity, and an accompanying simulation/thought exercise showed that the hospital of delivery accounted for approximately half of the disparity in severe maternal morbidity between Black and White women. An earlier study she published in 2016 of between-hospital differences in New York City showed that Black and Latina women were significantly more likely than White women to deliver in hospitals with higher rates of severe maternal mortality.
These findings illustrate that “racial segregation in neighborhoods is also part of the story,” of maternal mortality, Dr. Howell said.
Dr. Howell outlined ways the health care community can reduce severe maternal morbidity and mortality for all women, including promoting contraception and preconception health, improving postpartum management, eliminating bias, and using patient navigators as needed to enhance communication among the care team,
“Think about ways to engage the community,” in support of women’s pregnancy health, Dr. Howell said. She also emphasized the need to enroll more pregnant women in clinical trials.
Don’t exclude pregnant women from trials
In a follow-up session, Cynthia Gyamfi-Bannerman, MD, of the University of California, San Diego, expanded on opportunities to include pregnant women in clinical research.
Clinical trials for pregnant people fall into two categories, she noted; those studying interventions to improve pregnancy outcomes and those studying interventions for common medical conditions that coexist with pregnancy. These trials are either initiated by the investigators, conducted under contract, or federally funded, Dr. Gyamfi-Bannerman said. Currently, the only obstetric clinical trials research network is the Maternal-Fetal Medicine Units Network, established in 1986 by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The MFMU has conducted significant and life-saving research, but “we need more networks to focus on researching pregnancy complications,” Dr. Gyamfi-Bannerman said. Once the infrastructure exists in multiple settings, the ability to conduct trials will improve, she said.
Dr. Gyamfi-Bannerman stressed the need to engage and involve community-based physicians in clinical trials; using those relationships to enroll a more diverse population for whom working with their local physician would be more feasible than traveling to a larger clinical trial center.
She also commented on the need to include pregnant women in nonobstetric clinical trials. The exclusion of pregnant women from COVID-19 vaccine trials left clinicians with no information for guiding pregnant patients, she said. “It is important to think about why we are excluding pregnant women,” she said.
Finally, Dr. Gyamfi-Bannerman recommended a national effort to coordinate and leverage EHR data, which could have an effect on reducing maternal morbidity by facilitating the study of nonobstetric interventions in pregnancy, such as behavior interventions and mental health care.
Dr. Howell and Dr. Gyamfi-Bannerman had no financial conflicts to disclose.
FROM ADVANCING NIH RESEARCH ON THE HEALTH OF WOMEN




