User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
UTIs in pregnancy: Managing urethritis, asymptomatic bacteriuria, cystitis, and pyelonephritis
CASE Pregnant woman with dysuria and suprapubic pain
A 25-year-old primigravid woman at 18 weeks of gestation requests evaluation because of the acute onset of frequent urination, dysuria, urination hesitancy, and suprapubic pain on the morning following intercourse. She has not experienced similar symptoms in the past. On physical examination, her temperature is 38 °C, pulse is 96 beats per minute, respirations are 18 per minute, and blood pressure is 100/76 mm Hg. She has no urethral discharge but is tender to palpation in the suprapubic area.
- What is the most likely diagnosis?
- What tests would be of greatest value in confirming the diagnosis?
- What is the most appropriate treatment for this patient?
Meet our perpetrator
Urinary tract infections (UTIs) are among the most common infections that occur during pregnancy. They may take one of 4 forms: acute urethritis, asymptomatic bacteriuria, acute cystitis, and acute pyelonephritis.1 The first 3 conditions usually are straightforward to diagnose and treat, and they usually do not cause major problems for the mother or fetus. The latter, however, can cause serious complications that pose risk to the mother and fetus.
This article will review the microbiology, clinical manifestations, diagnosis, and treatment of these 4 disorders in pregnancy. Particular emphasis will be placed on measures to avoid adverse effects on the mother and fetus from antimicrobial agents.
Acute urethritis
Acute urethritis can be caused by low concentrations of coliform organisms in the lower urinary tract, but it usually is secondary to infection by Neisseria gonorrhea or Chlamydia trachomatis. In most prenatal populations, the prevalence of one or the other of these 2 infections is approximately 5%.1
The most common clinical manifestations of acute urethritis are a purulent urethral discharge, increased frequency of urination, dysuria, and urination hesitancy. The diagnosis most easily is confirmed by obtaining a specimen of urethral discharge or urine and evaluating the sample by a nucleic acid amplification test for both gonorrhea and chlamydia.
Antibiotic therapy is preferred. The current recommended treatment for gonococcal urethritis is a single intramuscular injection of ceftriaxone 500 mg. If the patient prefers oral therapy, she can receive cefixime 800 mg in a single dose. For patients with an allergy to beta-lactam antibiotics, the alternative regimen is gentamicin 240 mg in a single intramuscular injection, combined with oral azithromycin in a single 2,000-mg dose. For treatment of chlamydia in pregnancy, the recommended therapy is azithromycin 1,000 mg in a single oral dose.2 A test of cure should be performed within 4 weeks of treatment.
Asymptomatic bacteriuria
Asymptomatic bacteriuria (ASB) is the most common UTI in women. Approximately 5% to 8% of all sexually active women have ASB. The condition is not unique to pregnancy; it is associated primarily with the assumption of coital activity. In point of fact, the ASB typically precedes pregnancy by several years.1
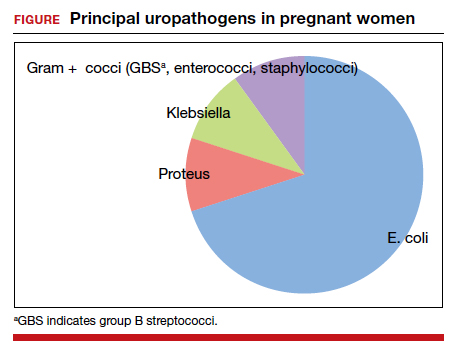
The principal pathogens responsible for ASB are shown in the FIGURE. Over 80% of first-time infections are secondary to Escherichia coli. As more recurrent episodes occur, the other Gram-negative bacilli (ie, Klebsiella pneumoniae, Proteus species) become more prevalent. The 3 major Gram-positive cocci that cause UTIs are enterococci, Staphylococcus saprophyticus, and group B streptococci.1,3,4
In nonpregnant women, ASB usually remains completely asymptomatic, and ascending infections only rarely occur. In pregnancy, however, urinary stasis is present due to the effects of progesterone on ureteral peristalsis and because of pressure on the ureters (particularly the right) by the expanding gravid uterus. As a result, ascending infection may occur in approximately 20% of patients if the lower tract infection is not identified and treated adequately.1
Clean-catch specimens and treatment
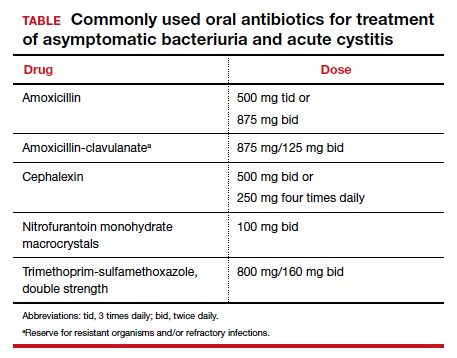
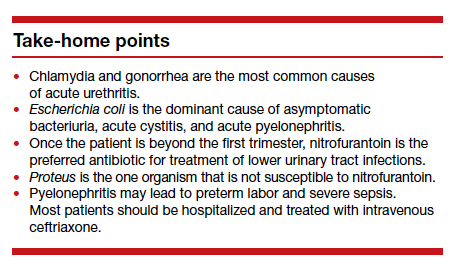
The standard criterion for the diagnosis of ASB is a urine culture that shows greater than 100,000 colonies/mL of a recognized uropathogen.1 The urine usually is obtained as a midstream, clean-catch specimen, and the test should be performed at the time of the first prenatal appointment. Once identified, ASB should be treated promptly with one of the antibiotics listed in the TABLE. Nitrofurantoin monohydrate macrocrystals (nitrofurantoin) and trimethoprim-sulfamethoxazole should be avoided in the first trimester unless no other drug is active against the identified microorganism.
Both have been linked to teratogenic fetal effects.5-8 The defects associated with the former drug include eye abnormalities, heart defects, and facial clefts. The abnormalities associated with the latter include neural tube defects, heart defects, choanal atresia, and diaphragmatic hernia. In the first trimester, amoxicillin and cephalexin are reasonable choices for treatment. As a general rule, a 3-day course of antibiotics will be effective for eradicating the initial episode of ASB. For recurrent infections, a 7- to 10-day course is indicated.1,3,4
Acute cystitis
The microbiology of acute cystitis essentially is identical to that of asymptomatic bacteriuria. The usual clinical manifestations include increased frequency of urination, dysuria, and urination hesitancy in association with suprapubic discomfort and a lowgrade fever.
The diagnosis can be quickly confirmed by testing the urine pH and assessing for leukocyte esterase and nitrites. If both the leukocyte esterase and nitrite test are positive, the probability of a positive urine culture is high; however, the nitrite test can be falsely negative if the urine has been incubating in the bladder for only a short period of time or if a Gram-positive organism is responsible for the infection. The urine pH is particularly helpful if it is elevated in the range of 8. This finding strongly is associated with a Proteus infection.1,9-11
Continue to: In-out catheterization is ideal...
In-out catheterization is ideal
I recommend that the urine sample be obtained by an in-out catheterization in symptomatic patients. This technique eliminates any concern about contamination of the specimen by vaginal organisms and provides a “pure” sample for culture. When urine is obtained by this method, the criterion for a positive culture result is greater than 100 colonies/mL.3 If the urine is obtained by the midstream clean-catch method, the cutoff for a positive culture remains greater than 100,000 colonies/mL.1,3
Unless the clinician is working in a low resource environment, the culture should always be obtained even though the patient will be empirically treated prior to the culture result being available. The culture can be helpful in guiding changes in antibiotic therapy if the initial response to treatment is poor.
In the first trimester, empiric treatment should be with amoxicillin or cephalexin. Beyond the first trimester, nitrofurantoin should be the drug of choice. This antibiotic is inexpensive and well-tolerated. It has limited effect on the bowel or vaginal flora and is unlikely to cause a secondary yeast infection or diarrhea. If a Proteus infection is suspected, however, trimethoprim-sulfamethoxazole double strength (800 mg/ 160 mg) should be used because this organism is not susceptible to nitrofurantoin. The duration of therapy should be a minimum of 3 days with the first infection and 7 to 10 days with recurrent infections.1,3,4
Acute pyelonephritis
Pyelonephritis may develop de novo or may result from inadequate treatment of lower urinary tract infection. The right kidney is affected in approximately 75% of cases because the right ureter is more subject to compression by the gravid uterus. The principal pathogen is E coli, although Klebsiella pneumoniae and Proteus species also are of importance. Other aerobic Gram-negative bacilli, such as Pseudomonas and Serratia species, are much less common unless the patient is immunosuppressed or has an indwelling catheter.1
The characteristic clinical manifestations of acute pyelonephritis are high fever (>39 °C), shaking chills, nausea and vomiting, and flank pain and tenderness. Increased frequency of urination and dysuria also may be present. In addition, pyelonephritis may be accompanied by preterm labor, sepsis, and acute respiratory distress syndrome (ARDS). The diagnosis is established by clinical findings, urinalysis, and urine culture. The urine specimen should be obtained by an in-out catheterization, analyzed initially by dipstick for nitrites and leukocyte esterase, and submitted for culture and sensitivity. Blood cultures should be obtained, and chest radiography should be performed if ARDS is suspected.
Empiric treatment should be started as soon as these initial diagnostic tests are completed. Many women in the first 20 weeks of pregnancy will not be seriously ill and may be treated as outpatients. I recommend an initial dose of intramuscular ceftriaxone 2 g followed by oral amoxicillin-clavulanate (875 mg twice daily) for a total of 10 days. If the patient is allergic to beta-lactam antibiotics, oral trimethoprim-sulfamethoxazole double strength (800 mg/160 mg) twice daily would be an excellent alternative.1,12
Treating seriously ill patients
Patients who are more seriously ill, particularly in the second half of pregnancy when preterm labor is more likely, should be hospitalized for supportive care (intravenous [IV] fluids, antipyretics, anti-emetics) and treatment with parenteral antibiotics.1,12 At our medical center, IV ceftriaxone (2 g every 24 hours), is the agent of choice. It has a convenient dosing schedule and covers almost all of the potential uropathogens. If an unusually drug-resistant organism is suspected, gentamicin or aztreonam can be combined with ceftriaxone to ensure complete coverage (see dosage recommendations below).
If the patient is allergic to beta-lactam antibiotics, alternative IV agents include:
- trimethoprim-sulfamethoxazole (8–10 mg/kg/d in 2 divided doses)
- gentamicin (5 mg/kg of ideal body weight every 24 hours)
- aztreonam (2 g every 8 hours)
Parenteral antibiotics should be administered until the patient has been afebrile and asymptomatic for 24 hours. At this point, oral antibiotics, based on sensitivity testing, can be started, and the patient can be discharged to complete a 10-day course of therapy.
Continue to: Treatment failure...
Treatment failure
Obstetric patients with pyelonephritis usually respond promptly to antibiotics. More than 75% will be afebrile within 48 hours, and more than 90% will be afebrile within 72 hours. When patients fail to respond promptly, 2 major causes should be considered. The first is antibiotic resistance, and this problem can be corrected on the basis of the sensitivity studies. The second is ureteral obstruction, secondary either to the effect of the gravid uterus or a urinary stone. If obstruction is suspected, renal ultrasonography should be performed. Depending upon the cause of the obstruction, a procedure such as a percutaneous nephrostomy or cystoscopic removal of the stone may be necessary.
Recurrence is possible. Following an initial episode of pyelonephritis, approximately 20% of patients will experience a recurrent lower or upper tract infection.1 Because of this recurrence rate, I recommend that these patients receive suppressive doses of antibiotics for the remainder of pregnancy. An ideal agent for suppression is nitrofurantoin (100 mg at bedtime). An alternative agent is trimethoprim-sulfamethoxazole double strength (800 mg/160 mg) once daily. Amoxicillin and cephalexin are less desirable for prophylaxis because of their adverse effects on vaginal and bowel flora and their propensity for precipitating yeast infection and/or diarrhea.
CASE Resolved
The most likely diagnosis in this patient is acute cystitis. An in-out catheterization should be performed to obtain an uncontaminated urine specimen. A portion of the specimen should be forwarded to the laboratory for urine culture and sensitivity. Another portion should be used for assessment by dipstick. If the nitrite and leukocyte tests are positive, the diagnosis of acute cystitis is confirmed. Since this infection is the patient’s first episode, a reasonable antibiotic regimen would be oral nitrofurantoin (100 mg twice daily) for 3 days. The course should be extended to 7 days if symptoms persist at the end of 3 days. ●
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862- 919.
- St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s Treatment Guidelines for Gonococcal Infection, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1911-1916.
- Hooton TM. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
- Finn SD. Acute uncomplicated urinary tract infection in women. N Engl J Med. 2003;349:259-266.
- Crider KS, Cleves MA, Reefhuis J, et al. Antibacterial medication use during pregnancy and risk of birth defects. Arch Pediatr Adolesc Med. 2009;163:978-985.
- Ailes EC, Gilboa SM, Gill SK, et al. Association between antibiotic use among pregnant women with urinary tract infections in the first trimester and birth defects, National Birth Defects Prevention Study 1997 to 2011. Birth Defects Res A Clin Mol Teratol. 2016;106:940-949.
- ACOG Committee Opinion No. 717 summary: sulfonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol. 2017;130:666-667.
- Duff P. Which antibiotics should be used with caution in pregnant women with UTI? OBG Manag. 2018;30:14-17.
- Hooton TM, Roberts PL, Cox ME, et al. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med. 2013;369:1883-1891.
- Mignini L, Carroli G, Abalos E, et al. Accuracy of diagnostic tests to detect asymptomatic bacteriuria during pregnancy. Obstet Gynecol. 2009;113:346-352.
- Schneeberger C, van den Heuvel ER, Erwich JJHM, et al. Contamination rates of three urine-sampling methods to assess bacteriuria in pregnant women. Obstet Gynecol. 2013;121:299-305.
- Duff P. Antibiotic selection in obstetrics: making cost-effective choices. Clin Obstet Gynecol. 2002;45:59-72.
CASE Pregnant woman with dysuria and suprapubic pain
A 25-year-old primigravid woman at 18 weeks of gestation requests evaluation because of the acute onset of frequent urination, dysuria, urination hesitancy, and suprapubic pain on the morning following intercourse. She has not experienced similar symptoms in the past. On physical examination, her temperature is 38 °C, pulse is 96 beats per minute, respirations are 18 per minute, and blood pressure is 100/76 mm Hg. She has no urethral discharge but is tender to palpation in the suprapubic area.
- What is the most likely diagnosis?
- What tests would be of greatest value in confirming the diagnosis?
- What is the most appropriate treatment for this patient?
Meet our perpetrator
Urinary tract infections (UTIs) are among the most common infections that occur during pregnancy. They may take one of 4 forms: acute urethritis, asymptomatic bacteriuria, acute cystitis, and acute pyelonephritis.1 The first 3 conditions usually are straightforward to diagnose and treat, and they usually do not cause major problems for the mother or fetus. The latter, however, can cause serious complications that pose risk to the mother and fetus.
This article will review the microbiology, clinical manifestations, diagnosis, and treatment of these 4 disorders in pregnancy. Particular emphasis will be placed on measures to avoid adverse effects on the mother and fetus from antimicrobial agents.
Acute urethritis
Acute urethritis can be caused by low concentrations of coliform organisms in the lower urinary tract, but it usually is secondary to infection by Neisseria gonorrhea or Chlamydia trachomatis. In most prenatal populations, the prevalence of one or the other of these 2 infections is approximately 5%.1
The most common clinical manifestations of acute urethritis are a purulent urethral discharge, increased frequency of urination, dysuria, and urination hesitancy. The diagnosis most easily is confirmed by obtaining a specimen of urethral discharge or urine and evaluating the sample by a nucleic acid amplification test for both gonorrhea and chlamydia.
Antibiotic therapy is preferred. The current recommended treatment for gonococcal urethritis is a single intramuscular injection of ceftriaxone 500 mg. If the patient prefers oral therapy, she can receive cefixime 800 mg in a single dose. For patients with an allergy to beta-lactam antibiotics, the alternative regimen is gentamicin 240 mg in a single intramuscular injection, combined with oral azithromycin in a single 2,000-mg dose. For treatment of chlamydia in pregnancy, the recommended therapy is azithromycin 1,000 mg in a single oral dose.2 A test of cure should be performed within 4 weeks of treatment.
Asymptomatic bacteriuria
Asymptomatic bacteriuria (ASB) is the most common UTI in women. Approximately 5% to 8% of all sexually active women have ASB. The condition is not unique to pregnancy; it is associated primarily with the assumption of coital activity. In point of fact, the ASB typically precedes pregnancy by several years.1
The principal pathogens responsible for ASB are shown in the FIGURE. Over 80% of first-time infections are secondary to Escherichia coli. As more recurrent episodes occur, the other Gram-negative bacilli (ie, Klebsiella pneumoniae, Proteus species) become more prevalent. The 3 major Gram-positive cocci that cause UTIs are enterococci, Staphylococcus saprophyticus, and group B streptococci.1,3,4

In nonpregnant women, ASB usually remains completely asymptomatic, and ascending infections only rarely occur. In pregnancy, however, urinary stasis is present due to the effects of progesterone on ureteral peristalsis and because of pressure on the ureters (particularly the right) by the expanding gravid uterus. As a result, ascending infection may occur in approximately 20% of patients if the lower tract infection is not identified and treated adequately.1
Clean-catch specimens and treatment
The standard criterion for the diagnosis of ASB is a urine culture that shows greater than 100,000 colonies/mL of a recognized uropathogen.1 The urine usually is obtained as a midstream, clean-catch specimen, and the test should be performed at the time of the first prenatal appointment. Once identified, ASB should be treated promptly with one of the antibiotics listed in the TABLE. Nitrofurantoin monohydrate macrocrystals (nitrofurantoin) and trimethoprim-sulfamethoxazole should be avoided in the first trimester unless no other drug is active against the identified microorganism.

Both have been linked to teratogenic fetal effects.5-8 The defects associated with the former drug include eye abnormalities, heart defects, and facial clefts. The abnormalities associated with the latter include neural tube defects, heart defects, choanal atresia, and diaphragmatic hernia. In the first trimester, amoxicillin and cephalexin are reasonable choices for treatment. As a general rule, a 3-day course of antibiotics will be effective for eradicating the initial episode of ASB. For recurrent infections, a 7- to 10-day course is indicated.1,3,4
Acute cystitis
The microbiology of acute cystitis essentially is identical to that of asymptomatic bacteriuria. The usual clinical manifestations include increased frequency of urination, dysuria, and urination hesitancy in association with suprapubic discomfort and a lowgrade fever.
The diagnosis can be quickly confirmed by testing the urine pH and assessing for leukocyte esterase and nitrites. If both the leukocyte esterase and nitrite test are positive, the probability of a positive urine culture is high; however, the nitrite test can be falsely negative if the urine has been incubating in the bladder for only a short period of time or if a Gram-positive organism is responsible for the infection. The urine pH is particularly helpful if it is elevated in the range of 8. This finding strongly is associated with a Proteus infection.1,9-11
Continue to: In-out catheterization is ideal...
In-out catheterization is ideal
I recommend that the urine sample be obtained by an in-out catheterization in symptomatic patients. This technique eliminates any concern about contamination of the specimen by vaginal organisms and provides a “pure” sample for culture. When urine is obtained by this method, the criterion for a positive culture result is greater than 100 colonies/mL.3 If the urine is obtained by the midstream clean-catch method, the cutoff for a positive culture remains greater than 100,000 colonies/mL.1,3
Unless the clinician is working in a low resource environment, the culture should always be obtained even though the patient will be empirically treated prior to the culture result being available. The culture can be helpful in guiding changes in antibiotic therapy if the initial response to treatment is poor.
In the first trimester, empiric treatment should be with amoxicillin or cephalexin. Beyond the first trimester, nitrofurantoin should be the drug of choice. This antibiotic is inexpensive and well-tolerated. It has limited effect on the bowel or vaginal flora and is unlikely to cause a secondary yeast infection or diarrhea. If a Proteus infection is suspected, however, trimethoprim-sulfamethoxazole double strength (800 mg/ 160 mg) should be used because this organism is not susceptible to nitrofurantoin. The duration of therapy should be a minimum of 3 days with the first infection and 7 to 10 days with recurrent infections.1,3,4
Acute pyelonephritis
Pyelonephritis may develop de novo or may result from inadequate treatment of lower urinary tract infection. The right kidney is affected in approximately 75% of cases because the right ureter is more subject to compression by the gravid uterus. The principal pathogen is E coli, although Klebsiella pneumoniae and Proteus species also are of importance. Other aerobic Gram-negative bacilli, such as Pseudomonas and Serratia species, are much less common unless the patient is immunosuppressed or has an indwelling catheter.1
The characteristic clinical manifestations of acute pyelonephritis are high fever (>39 °C), shaking chills, nausea and vomiting, and flank pain and tenderness. Increased frequency of urination and dysuria also may be present. In addition, pyelonephritis may be accompanied by preterm labor, sepsis, and acute respiratory distress syndrome (ARDS). The diagnosis is established by clinical findings, urinalysis, and urine culture. The urine specimen should be obtained by an in-out catheterization, analyzed initially by dipstick for nitrites and leukocyte esterase, and submitted for culture and sensitivity. Blood cultures should be obtained, and chest radiography should be performed if ARDS is suspected.
Empiric treatment should be started as soon as these initial diagnostic tests are completed. Many women in the first 20 weeks of pregnancy will not be seriously ill and may be treated as outpatients. I recommend an initial dose of intramuscular ceftriaxone 2 g followed by oral amoxicillin-clavulanate (875 mg twice daily) for a total of 10 days. If the patient is allergic to beta-lactam antibiotics, oral trimethoprim-sulfamethoxazole double strength (800 mg/160 mg) twice daily would be an excellent alternative.1,12
Treating seriously ill patients
Patients who are more seriously ill, particularly in the second half of pregnancy when preterm labor is more likely, should be hospitalized for supportive care (intravenous [IV] fluids, antipyretics, anti-emetics) and treatment with parenteral antibiotics.1,12 At our medical center, IV ceftriaxone (2 g every 24 hours), is the agent of choice. It has a convenient dosing schedule and covers almost all of the potential uropathogens. If an unusually drug-resistant organism is suspected, gentamicin or aztreonam can be combined with ceftriaxone to ensure complete coverage (see dosage recommendations below).
If the patient is allergic to beta-lactam antibiotics, alternative IV agents include:
- trimethoprim-sulfamethoxazole (8–10 mg/kg/d in 2 divided doses)
- gentamicin (5 mg/kg of ideal body weight every 24 hours)
- aztreonam (2 g every 8 hours)
Parenteral antibiotics should be administered until the patient has been afebrile and asymptomatic for 24 hours. At this point, oral antibiotics, based on sensitivity testing, can be started, and the patient can be discharged to complete a 10-day course of therapy.
Continue to: Treatment failure...
Treatment failure
Obstetric patients with pyelonephritis usually respond promptly to antibiotics. More than 75% will be afebrile within 48 hours, and more than 90% will be afebrile within 72 hours. When patients fail to respond promptly, 2 major causes should be considered. The first is antibiotic resistance, and this problem can be corrected on the basis of the sensitivity studies. The second is ureteral obstruction, secondary either to the effect of the gravid uterus or a urinary stone. If obstruction is suspected, renal ultrasonography should be performed. Depending upon the cause of the obstruction, a procedure such as a percutaneous nephrostomy or cystoscopic removal of the stone may be necessary.
Recurrence is possible. Following an initial episode of pyelonephritis, approximately 20% of patients will experience a recurrent lower or upper tract infection.1 Because of this recurrence rate, I recommend that these patients receive suppressive doses of antibiotics for the remainder of pregnancy. An ideal agent for suppression is nitrofurantoin (100 mg at bedtime). An alternative agent is trimethoprim-sulfamethoxazole double strength (800 mg/160 mg) once daily. Amoxicillin and cephalexin are less desirable for prophylaxis because of their adverse effects on vaginal and bowel flora and their propensity for precipitating yeast infection and/or diarrhea.
CASE Resolved
The most likely diagnosis in this patient is acute cystitis. An in-out catheterization should be performed to obtain an uncontaminated urine specimen. A portion of the specimen should be forwarded to the laboratory for urine culture and sensitivity. Another portion should be used for assessment by dipstick. If the nitrite and leukocyte tests are positive, the diagnosis of acute cystitis is confirmed. Since this infection is the patient’s first episode, a reasonable antibiotic regimen would be oral nitrofurantoin (100 mg twice daily) for 3 days. The course should be extended to 7 days if symptoms persist at the end of 3 days. ●

CASE Pregnant woman with dysuria and suprapubic pain
A 25-year-old primigravid woman at 18 weeks of gestation requests evaluation because of the acute onset of frequent urination, dysuria, urination hesitancy, and suprapubic pain on the morning following intercourse. She has not experienced similar symptoms in the past. On physical examination, her temperature is 38 °C, pulse is 96 beats per minute, respirations are 18 per minute, and blood pressure is 100/76 mm Hg. She has no urethral discharge but is tender to palpation in the suprapubic area.
- What is the most likely diagnosis?
- What tests would be of greatest value in confirming the diagnosis?
- What is the most appropriate treatment for this patient?
Meet our perpetrator
Urinary tract infections (UTIs) are among the most common infections that occur during pregnancy. They may take one of 4 forms: acute urethritis, asymptomatic bacteriuria, acute cystitis, and acute pyelonephritis.1 The first 3 conditions usually are straightforward to diagnose and treat, and they usually do not cause major problems for the mother or fetus. The latter, however, can cause serious complications that pose risk to the mother and fetus.
This article will review the microbiology, clinical manifestations, diagnosis, and treatment of these 4 disorders in pregnancy. Particular emphasis will be placed on measures to avoid adverse effects on the mother and fetus from antimicrobial agents.
Acute urethritis
Acute urethritis can be caused by low concentrations of coliform organisms in the lower urinary tract, but it usually is secondary to infection by Neisseria gonorrhea or Chlamydia trachomatis. In most prenatal populations, the prevalence of one or the other of these 2 infections is approximately 5%.1
The most common clinical manifestations of acute urethritis are a purulent urethral discharge, increased frequency of urination, dysuria, and urination hesitancy. The diagnosis most easily is confirmed by obtaining a specimen of urethral discharge or urine and evaluating the sample by a nucleic acid amplification test for both gonorrhea and chlamydia.
Antibiotic therapy is preferred. The current recommended treatment for gonococcal urethritis is a single intramuscular injection of ceftriaxone 500 mg. If the patient prefers oral therapy, she can receive cefixime 800 mg in a single dose. For patients with an allergy to beta-lactam antibiotics, the alternative regimen is gentamicin 240 mg in a single intramuscular injection, combined with oral azithromycin in a single 2,000-mg dose. For treatment of chlamydia in pregnancy, the recommended therapy is azithromycin 1,000 mg in a single oral dose.2 A test of cure should be performed within 4 weeks of treatment.
Asymptomatic bacteriuria
Asymptomatic bacteriuria (ASB) is the most common UTI in women. Approximately 5% to 8% of all sexually active women have ASB. The condition is not unique to pregnancy; it is associated primarily with the assumption of coital activity. In point of fact, the ASB typically precedes pregnancy by several years.1
The principal pathogens responsible for ASB are shown in the FIGURE. Over 80% of first-time infections are secondary to Escherichia coli. As more recurrent episodes occur, the other Gram-negative bacilli (ie, Klebsiella pneumoniae, Proteus species) become more prevalent. The 3 major Gram-positive cocci that cause UTIs are enterococci, Staphylococcus saprophyticus, and group B streptococci.1,3,4

In nonpregnant women, ASB usually remains completely asymptomatic, and ascending infections only rarely occur. In pregnancy, however, urinary stasis is present due to the effects of progesterone on ureteral peristalsis and because of pressure on the ureters (particularly the right) by the expanding gravid uterus. As a result, ascending infection may occur in approximately 20% of patients if the lower tract infection is not identified and treated adequately.1
Clean-catch specimens and treatment
The standard criterion for the diagnosis of ASB is a urine culture that shows greater than 100,000 colonies/mL of a recognized uropathogen.1 The urine usually is obtained as a midstream, clean-catch specimen, and the test should be performed at the time of the first prenatal appointment. Once identified, ASB should be treated promptly with one of the antibiotics listed in the TABLE. Nitrofurantoin monohydrate macrocrystals (nitrofurantoin) and trimethoprim-sulfamethoxazole should be avoided in the first trimester unless no other drug is active against the identified microorganism.

Both have been linked to teratogenic fetal effects.5-8 The defects associated with the former drug include eye abnormalities, heart defects, and facial clefts. The abnormalities associated with the latter include neural tube defects, heart defects, choanal atresia, and diaphragmatic hernia. In the first trimester, amoxicillin and cephalexin are reasonable choices for treatment. As a general rule, a 3-day course of antibiotics will be effective for eradicating the initial episode of ASB. For recurrent infections, a 7- to 10-day course is indicated.1,3,4
Acute cystitis
The microbiology of acute cystitis essentially is identical to that of asymptomatic bacteriuria. The usual clinical manifestations include increased frequency of urination, dysuria, and urination hesitancy in association with suprapubic discomfort and a lowgrade fever.
The diagnosis can be quickly confirmed by testing the urine pH and assessing for leukocyte esterase and nitrites. If both the leukocyte esterase and nitrite test are positive, the probability of a positive urine culture is high; however, the nitrite test can be falsely negative if the urine has been incubating in the bladder for only a short period of time or if a Gram-positive organism is responsible for the infection. The urine pH is particularly helpful if it is elevated in the range of 8. This finding strongly is associated with a Proteus infection.1,9-11
Continue to: In-out catheterization is ideal...
In-out catheterization is ideal
I recommend that the urine sample be obtained by an in-out catheterization in symptomatic patients. This technique eliminates any concern about contamination of the specimen by vaginal organisms and provides a “pure” sample for culture. When urine is obtained by this method, the criterion for a positive culture result is greater than 100 colonies/mL.3 If the urine is obtained by the midstream clean-catch method, the cutoff for a positive culture remains greater than 100,000 colonies/mL.1,3
Unless the clinician is working in a low resource environment, the culture should always be obtained even though the patient will be empirically treated prior to the culture result being available. The culture can be helpful in guiding changes in antibiotic therapy if the initial response to treatment is poor.
In the first trimester, empiric treatment should be with amoxicillin or cephalexin. Beyond the first trimester, nitrofurantoin should be the drug of choice. This antibiotic is inexpensive and well-tolerated. It has limited effect on the bowel or vaginal flora and is unlikely to cause a secondary yeast infection or diarrhea. If a Proteus infection is suspected, however, trimethoprim-sulfamethoxazole double strength (800 mg/ 160 mg) should be used because this organism is not susceptible to nitrofurantoin. The duration of therapy should be a minimum of 3 days with the first infection and 7 to 10 days with recurrent infections.1,3,4
Acute pyelonephritis
Pyelonephritis may develop de novo or may result from inadequate treatment of lower urinary tract infection. The right kidney is affected in approximately 75% of cases because the right ureter is more subject to compression by the gravid uterus. The principal pathogen is E coli, although Klebsiella pneumoniae and Proteus species also are of importance. Other aerobic Gram-negative bacilli, such as Pseudomonas and Serratia species, are much less common unless the patient is immunosuppressed or has an indwelling catheter.1
The characteristic clinical manifestations of acute pyelonephritis are high fever (>39 °C), shaking chills, nausea and vomiting, and flank pain and tenderness. Increased frequency of urination and dysuria also may be present. In addition, pyelonephritis may be accompanied by preterm labor, sepsis, and acute respiratory distress syndrome (ARDS). The diagnosis is established by clinical findings, urinalysis, and urine culture. The urine specimen should be obtained by an in-out catheterization, analyzed initially by dipstick for nitrites and leukocyte esterase, and submitted for culture and sensitivity. Blood cultures should be obtained, and chest radiography should be performed if ARDS is suspected.
Empiric treatment should be started as soon as these initial diagnostic tests are completed. Many women in the first 20 weeks of pregnancy will not be seriously ill and may be treated as outpatients. I recommend an initial dose of intramuscular ceftriaxone 2 g followed by oral amoxicillin-clavulanate (875 mg twice daily) for a total of 10 days. If the patient is allergic to beta-lactam antibiotics, oral trimethoprim-sulfamethoxazole double strength (800 mg/160 mg) twice daily would be an excellent alternative.1,12
Treating seriously ill patients
Patients who are more seriously ill, particularly in the second half of pregnancy when preterm labor is more likely, should be hospitalized for supportive care (intravenous [IV] fluids, antipyretics, anti-emetics) and treatment with parenteral antibiotics.1,12 At our medical center, IV ceftriaxone (2 g every 24 hours), is the agent of choice. It has a convenient dosing schedule and covers almost all of the potential uropathogens. If an unusually drug-resistant organism is suspected, gentamicin or aztreonam can be combined with ceftriaxone to ensure complete coverage (see dosage recommendations below).
If the patient is allergic to beta-lactam antibiotics, alternative IV agents include:
- trimethoprim-sulfamethoxazole (8–10 mg/kg/d in 2 divided doses)
- gentamicin (5 mg/kg of ideal body weight every 24 hours)
- aztreonam (2 g every 8 hours)
Parenteral antibiotics should be administered until the patient has been afebrile and asymptomatic for 24 hours. At this point, oral antibiotics, based on sensitivity testing, can be started, and the patient can be discharged to complete a 10-day course of therapy.
Continue to: Treatment failure...
Treatment failure
Obstetric patients with pyelonephritis usually respond promptly to antibiotics. More than 75% will be afebrile within 48 hours, and more than 90% will be afebrile within 72 hours. When patients fail to respond promptly, 2 major causes should be considered. The first is antibiotic resistance, and this problem can be corrected on the basis of the sensitivity studies. The second is ureteral obstruction, secondary either to the effect of the gravid uterus or a urinary stone. If obstruction is suspected, renal ultrasonography should be performed. Depending upon the cause of the obstruction, a procedure such as a percutaneous nephrostomy or cystoscopic removal of the stone may be necessary.
Recurrence is possible. Following an initial episode of pyelonephritis, approximately 20% of patients will experience a recurrent lower or upper tract infection.1 Because of this recurrence rate, I recommend that these patients receive suppressive doses of antibiotics for the remainder of pregnancy. An ideal agent for suppression is nitrofurantoin (100 mg at bedtime). An alternative agent is trimethoprim-sulfamethoxazole double strength (800 mg/160 mg) once daily. Amoxicillin and cephalexin are less desirable for prophylaxis because of their adverse effects on vaginal and bowel flora and their propensity for precipitating yeast infection and/or diarrhea.
CASE Resolved
The most likely diagnosis in this patient is acute cystitis. An in-out catheterization should be performed to obtain an uncontaminated urine specimen. A portion of the specimen should be forwarded to the laboratory for urine culture and sensitivity. Another portion should be used for assessment by dipstick. If the nitrite and leukocyte tests are positive, the diagnosis of acute cystitis is confirmed. Since this infection is the patient’s first episode, a reasonable antibiotic regimen would be oral nitrofurantoin (100 mg twice daily) for 3 days. The course should be extended to 7 days if symptoms persist at the end of 3 days. ●

- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862- 919.
- St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s Treatment Guidelines for Gonococcal Infection, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1911-1916.
- Hooton TM. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
- Finn SD. Acute uncomplicated urinary tract infection in women. N Engl J Med. 2003;349:259-266.
- Crider KS, Cleves MA, Reefhuis J, et al. Antibacterial medication use during pregnancy and risk of birth defects. Arch Pediatr Adolesc Med. 2009;163:978-985.
- Ailes EC, Gilboa SM, Gill SK, et al. Association between antibiotic use among pregnant women with urinary tract infections in the first trimester and birth defects, National Birth Defects Prevention Study 1997 to 2011. Birth Defects Res A Clin Mol Teratol. 2016;106:940-949.
- ACOG Committee Opinion No. 717 summary: sulfonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol. 2017;130:666-667.
- Duff P. Which antibiotics should be used with caution in pregnant women with UTI? OBG Manag. 2018;30:14-17.
- Hooton TM, Roberts PL, Cox ME, et al. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med. 2013;369:1883-1891.
- Mignini L, Carroli G, Abalos E, et al. Accuracy of diagnostic tests to detect asymptomatic bacteriuria during pregnancy. Obstet Gynecol. 2009;113:346-352.
- Schneeberger C, van den Heuvel ER, Erwich JJHM, et al. Contamination rates of three urine-sampling methods to assess bacteriuria in pregnant women. Obstet Gynecol. 2013;121:299-305.
- Duff P. Antibiotic selection in obstetrics: making cost-effective choices. Clin Obstet Gynecol. 2002;45:59-72.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862- 919.
- St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s Treatment Guidelines for Gonococcal Infection, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1911-1916.
- Hooton TM. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
- Finn SD. Acute uncomplicated urinary tract infection in women. N Engl J Med. 2003;349:259-266.
- Crider KS, Cleves MA, Reefhuis J, et al. Antibacterial medication use during pregnancy and risk of birth defects. Arch Pediatr Adolesc Med. 2009;163:978-985.
- Ailes EC, Gilboa SM, Gill SK, et al. Association between antibiotic use among pregnant women with urinary tract infections in the first trimester and birth defects, National Birth Defects Prevention Study 1997 to 2011. Birth Defects Res A Clin Mol Teratol. 2016;106:940-949.
- ACOG Committee Opinion No. 717 summary: sulfonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol. 2017;130:666-667.
- Duff P. Which antibiotics should be used with caution in pregnant women with UTI? OBG Manag. 2018;30:14-17.
- Hooton TM, Roberts PL, Cox ME, et al. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med. 2013;369:1883-1891.
- Mignini L, Carroli G, Abalos E, et al. Accuracy of diagnostic tests to detect asymptomatic bacteriuria during pregnancy. Obstet Gynecol. 2009;113:346-352.
- Schneeberger C, van den Heuvel ER, Erwich JJHM, et al. Contamination rates of three urine-sampling methods to assess bacteriuria in pregnant women. Obstet Gynecol. 2013;121:299-305.
- Duff P. Antibiotic selection in obstetrics: making cost-effective choices. Clin Obstet Gynecol. 2002;45:59-72.
The Supreme Court and reproductive rights
There is now great interest in the Supreme Court’s handling of cases that involve a woman’s ability to have an abortion. Recent decisions, and those planned in the next few months will be the source of intense scrutiny. But the Court’s involvement in reproductive rights did not begin with abortion. In fact, the Supreme Court has a long history of controversial decisions dealing with reproductive rights.
Involuntary sterilization
A notable, even infamous, case was Buck v Bell (1927)—later discredited—in which the Court reviewed a state law that provided for the involuntary sterilization of the “feeble minded.”1 The 8-1 decision was that the state could choose to have such a law to protect the so-called genetic health of the state. The law was based on a theory of eugenics. The opinion by the highly respected Justice Oliver Wendell Holmes included the unfortunate conclusion, “Three generations of imbeciles are enough.”2 As mentioned, the law has since been thoroughly discredited. In 1942, the Court did come to a different result, holding in Skinner v Oklahoma that it was unconstitutional for a state to involuntarily sterilize “habitual criminals.”3
Contraception
Forty years after Buck, in Griswold v Connecticut, the Court reviewed a state law that prohibited the distribution of any drug or device used for contraception (even for married couples).4 In a 7-2 decision, the Supreme Court struck down the state law as violating a marital right of privacy. Beyond its specific holding, Griswold was important in several ways. First, a physician was raising the rights of patients (not specifically his own rights). This is notable because, ordinarily in court, litigants may argue their own rights, not the rights of others. This has been important in later reproductive rights cases because often it has been physicians raising and arguing the rights of patients.
A second interesting part of Griswold was the source of this constitutional right of privacy. The Constitution contains no express privacy provision. In Griswold, the Court found that the 1st, 3rd, 4th, and 9th Amendments create the right to privacy in marital relations. Writing for the majority, Justice Douglas found that “emanations” from these amendments have “penumbras” that create a right of marital privacy.
Although Griswold was based on marital privacy, a few years later, in 1972, the Court essentially converted that right to one of reproductive privacy (“the decision whether to bear or beget a child.”) In Eisenstadt v Baird, the Court held that it was a violation of equal protection (the 14th Amendment) for a state to allow contraception to the married but deny it to an unmarried person.5
Continue to: Abortion...
Abortion
In 1971, the Court had heard arguments in 2 cases that raised issues regarding whether state laws prohibiting abortion were constitutional. The first oral argument in Roe v Wade is widely considered one of the worst oral arguments in modern history, and for several reasons the Court set the case for rehearing the following Term (October 1972). In January 1973, the Court decided Roe v Wade.6 The 7-2 decision was written by Justice Blackmun, who had at one point been the attorney for the Mayo Clinic and might be considered one of the first “health lawyers.” The Court held that the Constitution (perhaps in the 14th or 9th Amendment) includes a right of privacy that includes the right of a woman to choose to have, or not to have, an abortion. In implementing the right, the Court held that a state may impose only modest medical safeguards for the mother (eg, requiring that abortions be performed by a licensed physician). In the second trimester, to the point of viability, a state could impose only limitations on abortion that were reasonably directed to ensure the health of the mother. After a fetus was viable (could live outside the mother’s body), the state was free to regulate or prohibit abortions and protect the fetus. At the time, viability was approximately the beginning of the third trimester.6
The clear majority of the Court in Roe (7-2) may have suggested that there was not strong opposition to the decision. That, of course, was not the case. Legal and political conflict surrounding the case has been, and remains, intense. Since 1973, the Court has been called upon to decide many abortion cases, and each case seems to beget more controversy and still more cases.
Some of the legal objections to Roe and other abortion decisions are that the constitutional basis for the decision remains unclear—a specific right of privacy is not contained in the text of the Constitution. Several locations of a possible right of privacy have been mentioned by various justices, but “substantive due process” became the common constitutional basis for the right. Critics note that “substantive” due process (as opposed to procedural due process) is not mentioned in the Constitution, and it is short on clear guiding principles. Beyond those jurisprudential issues, of course, there were strong religious and philosophical objections to abortion. What followed Roe has been a long series of efforts to limit or discourage access to abortion, and the Supreme Court has had to decide a great many abortion cases (and a few contraception cases) over the last 50 years. Most years (except from 2008‒2013) the Court has heard, on average, at least one abortion case.
By way of examples, here are some of the issues related to abortion that the Court has decided:
- Payment and facilities. States and the federal government are not required to pay for abortions for women who cannot afford them or to provide facilities for abortion.7-10
- Informed consent. Some states’ special informed consent requirements for abortion were upheld, but complex consents that required the father’s participation were not.
- Ability to advertise. Prohibitions on advertisement of abortion services were struck down.
- Location. Requirements for hospital-only abortions (or similar regulations) were struck down.
- Anti-abortion protests. Several cases addressed guidelines involving demonstrations near abortion clinics.
Of particular importance was the case of Planned Parenthood of Southeastern Pennsylvania v Casey—“Casey.”11 In 1992 that case reaffirmed the “essential” holding of Roe v Wade. A plurality in that case de-emphasized the trimester framework and applied an “undue burden” test on limitations on abortion. In the more recent cases argued before the Court, Casey is frequently referred to as specifically reaffirming, and therefore solidifying, Roe.
Consent for minors
There have been several cases since 1973 that involved contraceptives or abortions and “minors” (generally, adolescents aged <18 years, although there are some state-defined exceptions). These cases typically involve 2 issues: the right of minors to consent to treatment and the obligation of the physician to provide information to parents about treatment to their minor daughter. In 1977 the Court struck down a New York law that prohibited the distribution of contraceptives to minors.12 However, abortion issues involving minors have been more complicated. While the Court has struck down “2-parent” consent statutes,13 it has generally upheld 1-parent consent statutes, but only if those statutes contain a “judicial bypass” provision and an emergency medical provision.11,14,15 (This bypass allows a minor to “bypass” parental consent to abortion in some circumstances, and instead seek judicial authorization for an abortion.) Generally, the Court has upheld parental notification for abortions, with exceptions where it would be harmful to the minor who is seeking the abortion.16-19
Continue to: Who can perform an abortion...
Who can perform an abortion
Over the years there have also been several cases raising questions about the professionals who can perform abortions, their hospital privileges, and what facilities can perform abortions. Two of those cases in recent years have, for example, seen the Court strike down state statutes that required the physicians who perform abortions to have admitting privileges at least in 1 nearby hospital.20,21 The basis for these decisions is that the admitting qualification is an “undue burden” because it serves almost no health purpose, while significantly limiting the number of professionals who can perform abortions.
Cases this Term
The current Term of the Court (officially the “October 2021 Term”) may be one of the most significant for reproductive rights in recent history. The Court accepted 6 abortion-related cases to hear. It dismissed 3 of those cases, which had become “moot” because the Biden administration changed the rules that had been legally challenged.22-24 It has heard arguments in the 3 (technically 4) remaining cases, in which decisions will be announced over the next several months.
The first of these cases (involving the Texas Heartbeat Act) raises very important, but vexing, procedural issues about a Texas abortion law. The second (Whole Woman’s Health v Jackson) is a direct challenge to Roe v Wade. The third case (Cameron v EMW Women’s Surgical Center) involves the narrow question of whether a state attorney general can intervene in a case to uphold a state abortion law when another state official refuses to defend the law.25 It is worthwhile taking a look at the first 2 of these cases.
Texas Heartbeat Act
In the first case (technically, it is 2 cases, as we will see), the Texas legislature adopted a law that prohibits abortions after there is a discernable heartbeat (around 6 weeks of pregnancy). The law precludes state officials from enforcing the law. Instead, it allows almost any private citizen to seek monetary damages ($10,000 plus fees) from anyone who performs an abortion or “aids and abets” an abortion. (This is in some ways similar to “private attorney general” actions found in the False Claims Act, and in some civil rights and labor laws.) This statute is clearly inconsistent with Roe in that it prohibits abortions before the end of the second trimester. If it were a usual law—a Texas law being enforced by state officials—federal courts would issue injunctions to state officials against enforcing the law. The difficulty with the Texas law (and its very purpose) is that there are procedural limitations in federal law that make it very difficult to find a path for federal courts to review the Texas statute quickly. For example, would federal courts enjoin every private citizen of the state? There is a longstanding Constitutional doctrine that precludes federal courts from enjoining state courts.26 Therefore, it is difficult to challenge the law before someone performing or aiding an abortion has been ordered to pay the private citizen who is enforcing it. In the interim, which could be months or even years, health care providers face uncertainty about continuing to provide abortion services. Some providers would stop providing abortion services, reducing the availability of those services.
Two cases challenge this Texas procedure. In the first, Whole Woman’s Health v Jackson, 27 abortion providers seek to find some way through the procedural thicket to allow an immediate challenge to the statute. It is important because this technique of exclusive private enforcement could be used in any number of ways by the state to chill important constitutional rights (beyond abortion—to speech, to bear arms). In the second case involving the Texas law, U.S. v Texas, the federal government seeks to intervene in the case, which is another unusual procedure.28 The Court found these questions so important and difficult that it allowed 3 hours of argument (and 4 sets of lawyers). It seems likely that the Court will find a mechanism for allowing some early federal court review of individual enforcement of state laws, while minimizing harm to the state-national federalism that is at the heart of the Constitution.
For the recent procedural decisions in the Texas cases, see the “Current Court Decisions” box below.
On December 10, 2021, the Court handed down two decisions in reproductive freedom (abortion) cases, both involving the Texas abortion law (which prohibits most abortions after a fetal heartbeat can be detected and allows only private individuals to enforce the law). The more significant of the two cases, Whole Woman’s Health v Jackson,1 was the request of abortion providers (and others) to allow them to challenge the constitutionality of the Texas law by suing various state officials or a private individual, before the enforcement of the new Texas law.
The decision of the Court was somewhat complex because of the split among justices. Overall:
- The Court held 8-1 that before the law is enforced, providers have the ability to sue executives of medical licensing boards. This was based on the possibility that there could be licensure discipline for professionals who violate the new abortion law. Only Justice Thomas dissented from this part of the decision, which was written by Justice Gorsuch.
- The Court unanimously held that state-court judges could not be sued to stop enforcement of the law, and dismissed them from the suit.
- In a 5-4 split the Court held that state court clerks (and the state attorney general) could not be brought into federal court as a way of challenging the law. This was based on the 11th Amendment, sovereign immunity, and an important precedent from 1908.2 Chief Justice Roberts wrote from the justices who were essentially in dissent (Justices Bryer, Kagan, and Sotomayor). Justice Sotomayor also wrote a dissent (joined by Justices Breyer and Kagan) urging that there should be some way for providers to test the constitutionality of the law before enforcement. Allowing an action against state court clerks would be a good way to do that. She also expressly noted the problem of the Texas law approach being used by other states to attack any number of constitutional rights.
- The Court unanimously dismissed (for lack of standing) the one private citizen who had been sued. He had signed a sworn statement that he did not intend to seek the damages against abortion providers under the Texas law.
- The Court declined again to stay the Texas law while it is being challenged. That is, it left standing the 5th Circuit order allowing the law to go into effect.
- In a second, related case, the Court dismissed, without deciding, the Biden administration’s request to become a party in the Texas abortion case.3
References
- Whole Woman’s Health v Jackson, No. 21–463 (Dec. 10, 2021). https://www.supremecourt.gov /opinions/21pdf/21-463_new_8o6b.pdf.
- Ex parte Young, 209 U.S. 123 (1908).
- U.S. v Texas, 21-588 (Dec. 10, 2021). https://www.supremecourt.gov/opinions/21pdf/21-588 _c07d.pdf
Continue to: Re-evaluating the viability standard...
Re-evaluating the viability standard
The substantive abortion issue in Dobbs v Jackson Women’s Health Organization is the constitutionality of the Mississippi Gestational Age Act, which allows abortions after 15 weeks of pregnancy only for medical emergencies or severe fetal abnormality.29 This case is likely the most watched and controversial case of the Term. Many medical organizations have filed amicus curiae briefs, including the American College of Obstetricians and Gynecologists joined by the American Medical Association,30 the International Federation of Gynecology and Obstetrics,31 and the American Association of Pro-Life Obstetricians and Gynecologists.32 The reason for all this attention is that the Court has accepted to resolve “whether all pre-viability prohibitions on elective abortions are unconstitutional.” Thus, it represents a direct challenge to the trimester/viability structure of Roe.
It appears that there are 3 justices ready to outright overrule Roe, 3 that would uphold it as is, and 3 who are not in favor of Roe, but feel bound by precedent or are not in favor of a traumatic move. For that reason, there may be a narrow decision in this case. For example, the Court might find a procedural way to avoid directly deciding the abortion issue in this case, or it might uphold the right to abortion but change the viability standard. It is also true that predicting what the Court will do in controversial cases is a fool’s errand.
The complexity of reproductive rights and the ObGyn practice
These cases and policies affect the day-to-day practice of obstetrics. It is the most legally complex area of medical practice for several reasons. The law varies considerably from state to state. The clinician who practices both in California and across the border in Arizona will face substantially different laws, especially regarding the treatment of adolescents. And the reproductive rights laws in many states are a complicated mix of state statutes and state court decisions, with an overlay of federal statutes and court decisions, and a series of both state and federal regulations. This article demonstrates an additional complexity for practitioners—the continuous change in the law surrounding reproductive rights—and practice involving adolescent patients is especially difficult.
There are some good state-by-state reviews of laws related to abortion and contraception. We find the Guttmacher Institute particularly helpful. (See “State-by-state reviews of laws related to abortion and contraception”.) Although these are good resources, they are not the basis for legal practice with the current law in a state. The complexity and ever-changing nature of reproductive rights is one of the reasons we believe that it is important that anyone in active ObGyn practice maintain an ongoing professional relationship with a lawyer with expertise in this area of practice. This relationship should establish and update policies and procedures consistent with local law, consent and other forms, reporting of possible child abuse, and the like. An annual legal checkup may be as important for physicians as a physical checkup is for their patients.

Future outcomes
At the end of the Term, we will review the outcome of the cases noted above—and the possibility of follow-on cases. Whatever the Court does this Term, it will not be the end of the legal and political struggles over abortion and other reproductive issues. These questions deeply divide our society, and the cases and controversies reflect that continuing division. ●
- Buck v Bell, 274 U.S. 200 (1927).
- Id. at 207.
- Skinner v State of Oklahoma, ex rel. Williamson, 316 U.S. 535 (1942).
- Griswold v Connecticut, 381 U.S. 479 (1965)
- Eisenstadt v Baird, 405 U.S. 438 (1972).
- Roe v Wade, 410 U.S. 113 (1973).
- Harris v McRae, 448 U.S. 297 (1980).
- Williams v Zbaraz, 448 U.S 358 (1980).
- Webster v Reproductive Health Services, 492 U.S. 490 (1989).
- Rust v Sullivan, 500 U.S. 173 (1991).
- Planned Parenthood of Southeastern Pennsylvania v Casey, 505 U.S. 833 (1992).
- Carey v Population Services, 431 U.S. 678 (1977).
- Bellotti v Baird, 443 U.S. 622 (1979).
- Planned Parenthood of Kansas City v Ashcroft, 462 U.S. 476 (1983).
- Planned Parenthood of Northern New England v Ayotte, 546 U.S. 320 (2006).
- H.L. v Matheson, 450 U.S. 398 (1981).
- Hodgson v Minnesota, 497 U.S. 417 (1990).
- Ohio v Akron Center for Reproductive Health, 497 U.S. 502 (1990).
- Lambert v Wicklund, 520 U.S. 292 (1997).
- June Medical Services v Russo, 591 U.S. ___ (2020), https:// www.supremecourt.gov/opinions/19pdf/18-1323_c07d.pdf.
- Whole Woman’s Health v Hellerstedt, 579 U.S. 582 (2016).
- AMA v Becerra, dismissed May 17, 2021, https://www .scotusblog.com/case-files/cases/american-medical -association-v-cochran.
- Becerra v Baltimore, dismissed May 17, 2021, https://www .scotusblog.com/case-files/cases/cochran-v-mayor-and-city-council-of-baltimore.
- Oregon v Becerra, dismissed May 17, 2021, https://www .scotusblog.com/case-files/cases/oregon-v-cochran.
- Cameron v EMW Women’s Surgical Center, 20-601. https:// www.scotusblog.com/case-files/cases/cameron-v-emw -womens-surgical-center-p-s-c.
- Ex parte Young, 209 U.S. 123 (1908).
- Whole Woman’s Health v Jackson, 21-463. https://www .scotusblog.com/case-files/cases/whole-womans-health-v -jackson.
- U.S. v Texas, 21-588. https://www.scotusblog.com/case-files /cases/united-states-v-texas-3.
- Dobbs v Jackson Women’s Health Organization, 19-1392. https://www.scotusblog.com/case-files/cases/dobbs-v -jackson-womens-health-organization.
- Brief of Amici Curiae American College of Obstetricians and Gynecologists et al., Dobbs v Jackson Women’s Health Organization (Sep. 2021). https://www.acog.org/ -/media/project/acog/acogorg/files/advocacy/amicus -briefs/2021/20210920-dobbs-v-jwho-amicus-brief.pdf?la=e n&hash=717DFDD07A03B93A04490E66835BB8C5.
- Brief Amicus Curiae of International Federation of Gynecology and Obstetrics, Dobbs v Jackson Women’s Health Organization (Sep. 20, 2021). https://www.supremecourt. gov/DocketPDF/19/19-1392/193019/20210920155508744 _41426%20pdf%20Chen.pdf.
- Brief Amicus Curiae of American Association of Pro-Life Obstetricians and Gynecologists. Dobbs v Jackson Women’s Health Organization (Sep. 2021). https://www.supremecourt .gov/DocketPDF/19/19-1392/185350/20210729163532595 _No.%2019-1392%20-%20American%20Association %20of%20Pro-Life%20Obstetricians%20and%20 Gynecologists%20-%20Amicus%20Brief%20in%20Support %20of%20Petitioner%20-%207-29-21.pdf.

There is now great interest in the Supreme Court’s handling of cases that involve a woman’s ability to have an abortion. Recent decisions, and those planned in the next few months will be the source of intense scrutiny. But the Court’s involvement in reproductive rights did not begin with abortion. In fact, the Supreme Court has a long history of controversial decisions dealing with reproductive rights.
Involuntary sterilization
A notable, even infamous, case was Buck v Bell (1927)—later discredited—in which the Court reviewed a state law that provided for the involuntary sterilization of the “feeble minded.”1 The 8-1 decision was that the state could choose to have such a law to protect the so-called genetic health of the state. The law was based on a theory of eugenics. The opinion by the highly respected Justice Oliver Wendell Holmes included the unfortunate conclusion, “Three generations of imbeciles are enough.”2 As mentioned, the law has since been thoroughly discredited. In 1942, the Court did come to a different result, holding in Skinner v Oklahoma that it was unconstitutional for a state to involuntarily sterilize “habitual criminals.”3
Contraception
Forty years after Buck, in Griswold v Connecticut, the Court reviewed a state law that prohibited the distribution of any drug or device used for contraception (even for married couples).4 In a 7-2 decision, the Supreme Court struck down the state law as violating a marital right of privacy. Beyond its specific holding, Griswold was important in several ways. First, a physician was raising the rights of patients (not specifically his own rights). This is notable because, ordinarily in court, litigants may argue their own rights, not the rights of others. This has been important in later reproductive rights cases because often it has been physicians raising and arguing the rights of patients.
A second interesting part of Griswold was the source of this constitutional right of privacy. The Constitution contains no express privacy provision. In Griswold, the Court found that the 1st, 3rd, 4th, and 9th Amendments create the right to privacy in marital relations. Writing for the majority, Justice Douglas found that “emanations” from these amendments have “penumbras” that create a right of marital privacy.
Although Griswold was based on marital privacy, a few years later, in 1972, the Court essentially converted that right to one of reproductive privacy (“the decision whether to bear or beget a child.”) In Eisenstadt v Baird, the Court held that it was a violation of equal protection (the 14th Amendment) for a state to allow contraception to the married but deny it to an unmarried person.5
Continue to: Abortion...
Abortion
In 1971, the Court had heard arguments in 2 cases that raised issues regarding whether state laws prohibiting abortion were constitutional. The first oral argument in Roe v Wade is widely considered one of the worst oral arguments in modern history, and for several reasons the Court set the case for rehearing the following Term (October 1972). In January 1973, the Court decided Roe v Wade.6 The 7-2 decision was written by Justice Blackmun, who had at one point been the attorney for the Mayo Clinic and might be considered one of the first “health lawyers.” The Court held that the Constitution (perhaps in the 14th or 9th Amendment) includes a right of privacy that includes the right of a woman to choose to have, or not to have, an abortion. In implementing the right, the Court held that a state may impose only modest medical safeguards for the mother (eg, requiring that abortions be performed by a licensed physician). In the second trimester, to the point of viability, a state could impose only limitations on abortion that were reasonably directed to ensure the health of the mother. After a fetus was viable (could live outside the mother’s body), the state was free to regulate or prohibit abortions and protect the fetus. At the time, viability was approximately the beginning of the third trimester.6
The clear majority of the Court in Roe (7-2) may have suggested that there was not strong opposition to the decision. That, of course, was not the case. Legal and political conflict surrounding the case has been, and remains, intense. Since 1973, the Court has been called upon to decide many abortion cases, and each case seems to beget more controversy and still more cases.
Some of the legal objections to Roe and other abortion decisions are that the constitutional basis for the decision remains unclear—a specific right of privacy is not contained in the text of the Constitution. Several locations of a possible right of privacy have been mentioned by various justices, but “substantive due process” became the common constitutional basis for the right. Critics note that “substantive” due process (as opposed to procedural due process) is not mentioned in the Constitution, and it is short on clear guiding principles. Beyond those jurisprudential issues, of course, there were strong religious and philosophical objections to abortion. What followed Roe has been a long series of efforts to limit or discourage access to abortion, and the Supreme Court has had to decide a great many abortion cases (and a few contraception cases) over the last 50 years. Most years (except from 2008‒2013) the Court has heard, on average, at least one abortion case.
By way of examples, here are some of the issues related to abortion that the Court has decided:
- Payment and facilities. States and the federal government are not required to pay for abortions for women who cannot afford them or to provide facilities for abortion.7-10
- Informed consent. Some states’ special informed consent requirements for abortion were upheld, but complex consents that required the father’s participation were not.
- Ability to advertise. Prohibitions on advertisement of abortion services were struck down.
- Location. Requirements for hospital-only abortions (or similar regulations) were struck down.
- Anti-abortion protests. Several cases addressed guidelines involving demonstrations near abortion clinics.
Of particular importance was the case of Planned Parenthood of Southeastern Pennsylvania v Casey—“Casey.”11 In 1992 that case reaffirmed the “essential” holding of Roe v Wade. A plurality in that case de-emphasized the trimester framework and applied an “undue burden” test on limitations on abortion. In the more recent cases argued before the Court, Casey is frequently referred to as specifically reaffirming, and therefore solidifying, Roe.
Consent for minors
There have been several cases since 1973 that involved contraceptives or abortions and “minors” (generally, adolescents aged <18 years, although there are some state-defined exceptions). These cases typically involve 2 issues: the right of minors to consent to treatment and the obligation of the physician to provide information to parents about treatment to their minor daughter. In 1977 the Court struck down a New York law that prohibited the distribution of contraceptives to minors.12 However, abortion issues involving minors have been more complicated. While the Court has struck down “2-parent” consent statutes,13 it has generally upheld 1-parent consent statutes, but only if those statutes contain a “judicial bypass” provision and an emergency medical provision.11,14,15 (This bypass allows a minor to “bypass” parental consent to abortion in some circumstances, and instead seek judicial authorization for an abortion.) Generally, the Court has upheld parental notification for abortions, with exceptions where it would be harmful to the minor who is seeking the abortion.16-19
Continue to: Who can perform an abortion...
Who can perform an abortion
Over the years there have also been several cases raising questions about the professionals who can perform abortions, their hospital privileges, and what facilities can perform abortions. Two of those cases in recent years have, for example, seen the Court strike down state statutes that required the physicians who perform abortions to have admitting privileges at least in 1 nearby hospital.20,21 The basis for these decisions is that the admitting qualification is an “undue burden” because it serves almost no health purpose, while significantly limiting the number of professionals who can perform abortions.
Cases this Term
The current Term of the Court (officially the “October 2021 Term”) may be one of the most significant for reproductive rights in recent history. The Court accepted 6 abortion-related cases to hear. It dismissed 3 of those cases, which had become “moot” because the Biden administration changed the rules that had been legally challenged.22-24 It has heard arguments in the 3 (technically 4) remaining cases, in which decisions will be announced over the next several months.
The first of these cases (involving the Texas Heartbeat Act) raises very important, but vexing, procedural issues about a Texas abortion law. The second (Whole Woman’s Health v Jackson) is a direct challenge to Roe v Wade. The third case (Cameron v EMW Women’s Surgical Center) involves the narrow question of whether a state attorney general can intervene in a case to uphold a state abortion law when another state official refuses to defend the law.25 It is worthwhile taking a look at the first 2 of these cases.
Texas Heartbeat Act
In the first case (technically, it is 2 cases, as we will see), the Texas legislature adopted a law that prohibits abortions after there is a discernable heartbeat (around 6 weeks of pregnancy). The law precludes state officials from enforcing the law. Instead, it allows almost any private citizen to seek monetary damages ($10,000 plus fees) from anyone who performs an abortion or “aids and abets” an abortion. (This is in some ways similar to “private attorney general” actions found in the False Claims Act, and in some civil rights and labor laws.) This statute is clearly inconsistent with Roe in that it prohibits abortions before the end of the second trimester. If it were a usual law—a Texas law being enforced by state officials—federal courts would issue injunctions to state officials against enforcing the law. The difficulty with the Texas law (and its very purpose) is that there are procedural limitations in federal law that make it very difficult to find a path for federal courts to review the Texas statute quickly. For example, would federal courts enjoin every private citizen of the state? There is a longstanding Constitutional doctrine that precludes federal courts from enjoining state courts.26 Therefore, it is difficult to challenge the law before someone performing or aiding an abortion has been ordered to pay the private citizen who is enforcing it. In the interim, which could be months or even years, health care providers face uncertainty about continuing to provide abortion services. Some providers would stop providing abortion services, reducing the availability of those services.
Two cases challenge this Texas procedure. In the first, Whole Woman’s Health v Jackson, 27 abortion providers seek to find some way through the procedural thicket to allow an immediate challenge to the statute. It is important because this technique of exclusive private enforcement could be used in any number of ways by the state to chill important constitutional rights (beyond abortion—to speech, to bear arms). In the second case involving the Texas law, U.S. v Texas, the federal government seeks to intervene in the case, which is another unusual procedure.28 The Court found these questions so important and difficult that it allowed 3 hours of argument (and 4 sets of lawyers). It seems likely that the Court will find a mechanism for allowing some early federal court review of individual enforcement of state laws, while minimizing harm to the state-national federalism that is at the heart of the Constitution.
For the recent procedural decisions in the Texas cases, see the “Current Court Decisions” box below.
On December 10, 2021, the Court handed down two decisions in reproductive freedom (abortion) cases, both involving the Texas abortion law (which prohibits most abortions after a fetal heartbeat can be detected and allows only private individuals to enforce the law). The more significant of the two cases, Whole Woman’s Health v Jackson,1 was the request of abortion providers (and others) to allow them to challenge the constitutionality of the Texas law by suing various state officials or a private individual, before the enforcement of the new Texas law.
The decision of the Court was somewhat complex because of the split among justices. Overall:
- The Court held 8-1 that before the law is enforced, providers have the ability to sue executives of medical licensing boards. This was based on the possibility that there could be licensure discipline for professionals who violate the new abortion law. Only Justice Thomas dissented from this part of the decision, which was written by Justice Gorsuch.
- The Court unanimously held that state-court judges could not be sued to stop enforcement of the law, and dismissed them from the suit.
- In a 5-4 split the Court held that state court clerks (and the state attorney general) could not be brought into federal court as a way of challenging the law. This was based on the 11th Amendment, sovereign immunity, and an important precedent from 1908.2 Chief Justice Roberts wrote from the justices who were essentially in dissent (Justices Bryer, Kagan, and Sotomayor). Justice Sotomayor also wrote a dissent (joined by Justices Breyer and Kagan) urging that there should be some way for providers to test the constitutionality of the law before enforcement. Allowing an action against state court clerks would be a good way to do that. She also expressly noted the problem of the Texas law approach being used by other states to attack any number of constitutional rights.
- The Court unanimously dismissed (for lack of standing) the one private citizen who had been sued. He had signed a sworn statement that he did not intend to seek the damages against abortion providers under the Texas law.
- The Court declined again to stay the Texas law while it is being challenged. That is, it left standing the 5th Circuit order allowing the law to go into effect.
- In a second, related case, the Court dismissed, without deciding, the Biden administration’s request to become a party in the Texas abortion case.3
References
- Whole Woman’s Health v Jackson, No. 21–463 (Dec. 10, 2021). https://www.supremecourt.gov /opinions/21pdf/21-463_new_8o6b.pdf.
- Ex parte Young, 209 U.S. 123 (1908).
- U.S. v Texas, 21-588 (Dec. 10, 2021). https://www.supremecourt.gov/opinions/21pdf/21-588 _c07d.pdf
Continue to: Re-evaluating the viability standard...
Re-evaluating the viability standard
The substantive abortion issue in Dobbs v Jackson Women’s Health Organization is the constitutionality of the Mississippi Gestational Age Act, which allows abortions after 15 weeks of pregnancy only for medical emergencies or severe fetal abnormality.29 This case is likely the most watched and controversial case of the Term. Many medical organizations have filed amicus curiae briefs, including the American College of Obstetricians and Gynecologists joined by the American Medical Association,30 the International Federation of Gynecology and Obstetrics,31 and the American Association of Pro-Life Obstetricians and Gynecologists.32 The reason for all this attention is that the Court has accepted to resolve “whether all pre-viability prohibitions on elective abortions are unconstitutional.” Thus, it represents a direct challenge to the trimester/viability structure of Roe.
It appears that there are 3 justices ready to outright overrule Roe, 3 that would uphold it as is, and 3 who are not in favor of Roe, but feel bound by precedent or are not in favor of a traumatic move. For that reason, there may be a narrow decision in this case. For example, the Court might find a procedural way to avoid directly deciding the abortion issue in this case, or it might uphold the right to abortion but change the viability standard. It is also true that predicting what the Court will do in controversial cases is a fool’s errand.
The complexity of reproductive rights and the ObGyn practice
These cases and policies affect the day-to-day practice of obstetrics. It is the most legally complex area of medical practice for several reasons. The law varies considerably from state to state. The clinician who practices both in California and across the border in Arizona will face substantially different laws, especially regarding the treatment of adolescents. And the reproductive rights laws in many states are a complicated mix of state statutes and state court decisions, with an overlay of federal statutes and court decisions, and a series of both state and federal regulations. This article demonstrates an additional complexity for practitioners—the continuous change in the law surrounding reproductive rights—and practice involving adolescent patients is especially difficult.
There are some good state-by-state reviews of laws related to abortion and contraception. We find the Guttmacher Institute particularly helpful. (See “State-by-state reviews of laws related to abortion and contraception”.) Although these are good resources, they are not the basis for legal practice with the current law in a state. The complexity and ever-changing nature of reproductive rights is one of the reasons we believe that it is important that anyone in active ObGyn practice maintain an ongoing professional relationship with a lawyer with expertise in this area of practice. This relationship should establish and update policies and procedures consistent with local law, consent and other forms, reporting of possible child abuse, and the like. An annual legal checkup may be as important for physicians as a physical checkup is for their patients.

Future outcomes
At the end of the Term, we will review the outcome of the cases noted above—and the possibility of follow-on cases. Whatever the Court does this Term, it will not be the end of the legal and political struggles over abortion and other reproductive issues. These questions deeply divide our society, and the cases and controversies reflect that continuing division. ●

There is now great interest in the Supreme Court’s handling of cases that involve a woman’s ability to have an abortion. Recent decisions, and those planned in the next few months will be the source of intense scrutiny. But the Court’s involvement in reproductive rights did not begin with abortion. In fact, the Supreme Court has a long history of controversial decisions dealing with reproductive rights.
Involuntary sterilization
A notable, even infamous, case was Buck v Bell (1927)—later discredited—in which the Court reviewed a state law that provided for the involuntary sterilization of the “feeble minded.”1 The 8-1 decision was that the state could choose to have such a law to protect the so-called genetic health of the state. The law was based on a theory of eugenics. The opinion by the highly respected Justice Oliver Wendell Holmes included the unfortunate conclusion, “Three generations of imbeciles are enough.”2 As mentioned, the law has since been thoroughly discredited. In 1942, the Court did come to a different result, holding in Skinner v Oklahoma that it was unconstitutional for a state to involuntarily sterilize “habitual criminals.”3
Contraception
Forty years after Buck, in Griswold v Connecticut, the Court reviewed a state law that prohibited the distribution of any drug or device used for contraception (even for married couples).4 In a 7-2 decision, the Supreme Court struck down the state law as violating a marital right of privacy. Beyond its specific holding, Griswold was important in several ways. First, a physician was raising the rights of patients (not specifically his own rights). This is notable because, ordinarily in court, litigants may argue their own rights, not the rights of others. This has been important in later reproductive rights cases because often it has been physicians raising and arguing the rights of patients.
A second interesting part of Griswold was the source of this constitutional right of privacy. The Constitution contains no express privacy provision. In Griswold, the Court found that the 1st, 3rd, 4th, and 9th Amendments create the right to privacy in marital relations. Writing for the majority, Justice Douglas found that “emanations” from these amendments have “penumbras” that create a right of marital privacy.
Although Griswold was based on marital privacy, a few years later, in 1972, the Court essentially converted that right to one of reproductive privacy (“the decision whether to bear or beget a child.”) In Eisenstadt v Baird, the Court held that it was a violation of equal protection (the 14th Amendment) for a state to allow contraception to the married but deny it to an unmarried person.5
Continue to: Abortion...
Abortion
In 1971, the Court had heard arguments in 2 cases that raised issues regarding whether state laws prohibiting abortion were constitutional. The first oral argument in Roe v Wade is widely considered one of the worst oral arguments in modern history, and for several reasons the Court set the case for rehearing the following Term (October 1972). In January 1973, the Court decided Roe v Wade.6 The 7-2 decision was written by Justice Blackmun, who had at one point been the attorney for the Mayo Clinic and might be considered one of the first “health lawyers.” The Court held that the Constitution (perhaps in the 14th or 9th Amendment) includes a right of privacy that includes the right of a woman to choose to have, or not to have, an abortion. In implementing the right, the Court held that a state may impose only modest medical safeguards for the mother (eg, requiring that abortions be performed by a licensed physician). In the second trimester, to the point of viability, a state could impose only limitations on abortion that were reasonably directed to ensure the health of the mother. After a fetus was viable (could live outside the mother’s body), the state was free to regulate or prohibit abortions and protect the fetus. At the time, viability was approximately the beginning of the third trimester.6
The clear majority of the Court in Roe (7-2) may have suggested that there was not strong opposition to the decision. That, of course, was not the case. Legal and political conflict surrounding the case has been, and remains, intense. Since 1973, the Court has been called upon to decide many abortion cases, and each case seems to beget more controversy and still more cases.
Some of the legal objections to Roe and other abortion decisions are that the constitutional basis for the decision remains unclear—a specific right of privacy is not contained in the text of the Constitution. Several locations of a possible right of privacy have been mentioned by various justices, but “substantive due process” became the common constitutional basis for the right. Critics note that “substantive” due process (as opposed to procedural due process) is not mentioned in the Constitution, and it is short on clear guiding principles. Beyond those jurisprudential issues, of course, there were strong religious and philosophical objections to abortion. What followed Roe has been a long series of efforts to limit or discourage access to abortion, and the Supreme Court has had to decide a great many abortion cases (and a few contraception cases) over the last 50 years. Most years (except from 2008‒2013) the Court has heard, on average, at least one abortion case.
By way of examples, here are some of the issues related to abortion that the Court has decided:
- Payment and facilities. States and the federal government are not required to pay for abortions for women who cannot afford them or to provide facilities for abortion.7-10
- Informed consent. Some states’ special informed consent requirements for abortion were upheld, but complex consents that required the father’s participation were not.
- Ability to advertise. Prohibitions on advertisement of abortion services were struck down.
- Location. Requirements for hospital-only abortions (or similar regulations) were struck down.
- Anti-abortion protests. Several cases addressed guidelines involving demonstrations near abortion clinics.
Of particular importance was the case of Planned Parenthood of Southeastern Pennsylvania v Casey—“Casey.”11 In 1992 that case reaffirmed the “essential” holding of Roe v Wade. A plurality in that case de-emphasized the trimester framework and applied an “undue burden” test on limitations on abortion. In the more recent cases argued before the Court, Casey is frequently referred to as specifically reaffirming, and therefore solidifying, Roe.
Consent for minors
There have been several cases since 1973 that involved contraceptives or abortions and “minors” (generally, adolescents aged <18 years, although there are some state-defined exceptions). These cases typically involve 2 issues: the right of minors to consent to treatment and the obligation of the physician to provide information to parents about treatment to their minor daughter. In 1977 the Court struck down a New York law that prohibited the distribution of contraceptives to minors.12 However, abortion issues involving minors have been more complicated. While the Court has struck down “2-parent” consent statutes,13 it has generally upheld 1-parent consent statutes, but only if those statutes contain a “judicial bypass” provision and an emergency medical provision.11,14,15 (This bypass allows a minor to “bypass” parental consent to abortion in some circumstances, and instead seek judicial authorization for an abortion.) Generally, the Court has upheld parental notification for abortions, with exceptions where it would be harmful to the minor who is seeking the abortion.16-19
Continue to: Who can perform an abortion...
Who can perform an abortion
Over the years there have also been several cases raising questions about the professionals who can perform abortions, their hospital privileges, and what facilities can perform abortions. Two of those cases in recent years have, for example, seen the Court strike down state statutes that required the physicians who perform abortions to have admitting privileges at least in 1 nearby hospital.20,21 The basis for these decisions is that the admitting qualification is an “undue burden” because it serves almost no health purpose, while significantly limiting the number of professionals who can perform abortions.
Cases this Term
The current Term of the Court (officially the “October 2021 Term”) may be one of the most significant for reproductive rights in recent history. The Court accepted 6 abortion-related cases to hear. It dismissed 3 of those cases, which had become “moot” because the Biden administration changed the rules that had been legally challenged.22-24 It has heard arguments in the 3 (technically 4) remaining cases, in which decisions will be announced over the next several months.
The first of these cases (involving the Texas Heartbeat Act) raises very important, but vexing, procedural issues about a Texas abortion law. The second (Whole Woman’s Health v Jackson) is a direct challenge to Roe v Wade. The third case (Cameron v EMW Women’s Surgical Center) involves the narrow question of whether a state attorney general can intervene in a case to uphold a state abortion law when another state official refuses to defend the law.25 It is worthwhile taking a look at the first 2 of these cases.
Texas Heartbeat Act
In the first case (technically, it is 2 cases, as we will see), the Texas legislature adopted a law that prohibits abortions after there is a discernable heartbeat (around 6 weeks of pregnancy). The law precludes state officials from enforcing the law. Instead, it allows almost any private citizen to seek monetary damages ($10,000 plus fees) from anyone who performs an abortion or “aids and abets” an abortion. (This is in some ways similar to “private attorney general” actions found in the False Claims Act, and in some civil rights and labor laws.) This statute is clearly inconsistent with Roe in that it prohibits abortions before the end of the second trimester. If it were a usual law—a Texas law being enforced by state officials—federal courts would issue injunctions to state officials against enforcing the law. The difficulty with the Texas law (and its very purpose) is that there are procedural limitations in federal law that make it very difficult to find a path for federal courts to review the Texas statute quickly. For example, would federal courts enjoin every private citizen of the state? There is a longstanding Constitutional doctrine that precludes federal courts from enjoining state courts.26 Therefore, it is difficult to challenge the law before someone performing or aiding an abortion has been ordered to pay the private citizen who is enforcing it. In the interim, which could be months or even years, health care providers face uncertainty about continuing to provide abortion services. Some providers would stop providing abortion services, reducing the availability of those services.
Two cases challenge this Texas procedure. In the first, Whole Woman’s Health v Jackson, 27 abortion providers seek to find some way through the procedural thicket to allow an immediate challenge to the statute. It is important because this technique of exclusive private enforcement could be used in any number of ways by the state to chill important constitutional rights (beyond abortion—to speech, to bear arms). In the second case involving the Texas law, U.S. v Texas, the federal government seeks to intervene in the case, which is another unusual procedure.28 The Court found these questions so important and difficult that it allowed 3 hours of argument (and 4 sets of lawyers). It seems likely that the Court will find a mechanism for allowing some early federal court review of individual enforcement of state laws, while minimizing harm to the state-national federalism that is at the heart of the Constitution.
For the recent procedural decisions in the Texas cases, see the “Current Court Decisions” box below.
On December 10, 2021, the Court handed down two decisions in reproductive freedom (abortion) cases, both involving the Texas abortion law (which prohibits most abortions after a fetal heartbeat can be detected and allows only private individuals to enforce the law). The more significant of the two cases, Whole Woman’s Health v Jackson,1 was the request of abortion providers (and others) to allow them to challenge the constitutionality of the Texas law by suing various state officials or a private individual, before the enforcement of the new Texas law.
The decision of the Court was somewhat complex because of the split among justices. Overall:
- The Court held 8-1 that before the law is enforced, providers have the ability to sue executives of medical licensing boards. This was based on the possibility that there could be licensure discipline for professionals who violate the new abortion law. Only Justice Thomas dissented from this part of the decision, which was written by Justice Gorsuch.
- The Court unanimously held that state-court judges could not be sued to stop enforcement of the law, and dismissed them from the suit.
- In a 5-4 split the Court held that state court clerks (and the state attorney general) could not be brought into federal court as a way of challenging the law. This was based on the 11th Amendment, sovereign immunity, and an important precedent from 1908.2 Chief Justice Roberts wrote from the justices who were essentially in dissent (Justices Bryer, Kagan, and Sotomayor). Justice Sotomayor also wrote a dissent (joined by Justices Breyer and Kagan) urging that there should be some way for providers to test the constitutionality of the law before enforcement. Allowing an action against state court clerks would be a good way to do that. She also expressly noted the problem of the Texas law approach being used by other states to attack any number of constitutional rights.
- The Court unanimously dismissed (for lack of standing) the one private citizen who had been sued. He had signed a sworn statement that he did not intend to seek the damages against abortion providers under the Texas law.
- The Court declined again to stay the Texas law while it is being challenged. That is, it left standing the 5th Circuit order allowing the law to go into effect.
- In a second, related case, the Court dismissed, without deciding, the Biden administration’s request to become a party in the Texas abortion case.3
References
- Whole Woman’s Health v Jackson, No. 21–463 (Dec. 10, 2021). https://www.supremecourt.gov /opinions/21pdf/21-463_new_8o6b.pdf.
- Ex parte Young, 209 U.S. 123 (1908).
- U.S. v Texas, 21-588 (Dec. 10, 2021). https://www.supremecourt.gov/opinions/21pdf/21-588 _c07d.pdf
Continue to: Re-evaluating the viability standard...
Re-evaluating the viability standard
The substantive abortion issue in Dobbs v Jackson Women’s Health Organization is the constitutionality of the Mississippi Gestational Age Act, which allows abortions after 15 weeks of pregnancy only for medical emergencies or severe fetal abnormality.29 This case is likely the most watched and controversial case of the Term. Many medical organizations have filed amicus curiae briefs, including the American College of Obstetricians and Gynecologists joined by the American Medical Association,30 the International Federation of Gynecology and Obstetrics,31 and the American Association of Pro-Life Obstetricians and Gynecologists.32 The reason for all this attention is that the Court has accepted to resolve “whether all pre-viability prohibitions on elective abortions are unconstitutional.” Thus, it represents a direct challenge to the trimester/viability structure of Roe.
It appears that there are 3 justices ready to outright overrule Roe, 3 that would uphold it as is, and 3 who are not in favor of Roe, but feel bound by precedent or are not in favor of a traumatic move. For that reason, there may be a narrow decision in this case. For example, the Court might find a procedural way to avoid directly deciding the abortion issue in this case, or it might uphold the right to abortion but change the viability standard. It is also true that predicting what the Court will do in controversial cases is a fool’s errand.
The complexity of reproductive rights and the ObGyn practice
These cases and policies affect the day-to-day practice of obstetrics. It is the most legally complex area of medical practice for several reasons. The law varies considerably from state to state. The clinician who practices both in California and across the border in Arizona will face substantially different laws, especially regarding the treatment of adolescents. And the reproductive rights laws in many states are a complicated mix of state statutes and state court decisions, with an overlay of federal statutes and court decisions, and a series of both state and federal regulations. This article demonstrates an additional complexity for practitioners—the continuous change in the law surrounding reproductive rights—and practice involving adolescent patients is especially difficult.
There are some good state-by-state reviews of laws related to abortion and contraception. We find the Guttmacher Institute particularly helpful. (See “State-by-state reviews of laws related to abortion and contraception”.) Although these are good resources, they are not the basis for legal practice with the current law in a state. The complexity and ever-changing nature of reproductive rights is one of the reasons we believe that it is important that anyone in active ObGyn practice maintain an ongoing professional relationship with a lawyer with expertise in this area of practice. This relationship should establish and update policies and procedures consistent with local law, consent and other forms, reporting of possible child abuse, and the like. An annual legal checkup may be as important for physicians as a physical checkup is for their patients.

Future outcomes
At the end of the Term, we will review the outcome of the cases noted above—and the possibility of follow-on cases. Whatever the Court does this Term, it will not be the end of the legal and political struggles over abortion and other reproductive issues. These questions deeply divide our society, and the cases and controversies reflect that continuing division. ●
- Buck v Bell, 274 U.S. 200 (1927).
- Id. at 207.
- Skinner v State of Oklahoma, ex rel. Williamson, 316 U.S. 535 (1942).
- Griswold v Connecticut, 381 U.S. 479 (1965)
- Eisenstadt v Baird, 405 U.S. 438 (1972).
- Roe v Wade, 410 U.S. 113 (1973).
- Harris v McRae, 448 U.S. 297 (1980).
- Williams v Zbaraz, 448 U.S 358 (1980).
- Webster v Reproductive Health Services, 492 U.S. 490 (1989).
- Rust v Sullivan, 500 U.S. 173 (1991).
- Planned Parenthood of Southeastern Pennsylvania v Casey, 505 U.S. 833 (1992).
- Carey v Population Services, 431 U.S. 678 (1977).
- Bellotti v Baird, 443 U.S. 622 (1979).
- Planned Parenthood of Kansas City v Ashcroft, 462 U.S. 476 (1983).
- Planned Parenthood of Northern New England v Ayotte, 546 U.S. 320 (2006).
- H.L. v Matheson, 450 U.S. 398 (1981).
- Hodgson v Minnesota, 497 U.S. 417 (1990).
- Ohio v Akron Center for Reproductive Health, 497 U.S. 502 (1990).
- Lambert v Wicklund, 520 U.S. 292 (1997).
- June Medical Services v Russo, 591 U.S. ___ (2020), https:// www.supremecourt.gov/opinions/19pdf/18-1323_c07d.pdf.
- Whole Woman’s Health v Hellerstedt, 579 U.S. 582 (2016).
- AMA v Becerra, dismissed May 17, 2021, https://www .scotusblog.com/case-files/cases/american-medical -association-v-cochran.
- Becerra v Baltimore, dismissed May 17, 2021, https://www .scotusblog.com/case-files/cases/cochran-v-mayor-and-city-council-of-baltimore.
- Oregon v Becerra, dismissed May 17, 2021, https://www .scotusblog.com/case-files/cases/oregon-v-cochran.
- Cameron v EMW Women’s Surgical Center, 20-601. https:// www.scotusblog.com/case-files/cases/cameron-v-emw -womens-surgical-center-p-s-c.
- Ex parte Young, 209 U.S. 123 (1908).
- Whole Woman’s Health v Jackson, 21-463. https://www .scotusblog.com/case-files/cases/whole-womans-health-v -jackson.
- U.S. v Texas, 21-588. https://www.scotusblog.com/case-files /cases/united-states-v-texas-3.
- Dobbs v Jackson Women’s Health Organization, 19-1392. https://www.scotusblog.com/case-files/cases/dobbs-v -jackson-womens-health-organization.
- Brief of Amici Curiae American College of Obstetricians and Gynecologists et al., Dobbs v Jackson Women’s Health Organization (Sep. 2021). https://www.acog.org/ -/media/project/acog/acogorg/files/advocacy/amicus -briefs/2021/20210920-dobbs-v-jwho-amicus-brief.pdf?la=e n&hash=717DFDD07A03B93A04490E66835BB8C5.
- Brief Amicus Curiae of International Federation of Gynecology and Obstetrics, Dobbs v Jackson Women’s Health Organization (Sep. 20, 2021). https://www.supremecourt. gov/DocketPDF/19/19-1392/193019/20210920155508744 _41426%20pdf%20Chen.pdf.
- Brief Amicus Curiae of American Association of Pro-Life Obstetricians and Gynecologists. Dobbs v Jackson Women’s Health Organization (Sep. 2021). https://www.supremecourt .gov/DocketPDF/19/19-1392/185350/20210729163532595 _No.%2019-1392%20-%20American%20Association %20of%20Pro-Life%20Obstetricians%20and%20 Gynecologists%20-%20Amicus%20Brief%20in%20Support %20of%20Petitioner%20-%207-29-21.pdf.
- Buck v Bell, 274 U.S. 200 (1927).
- Id. at 207.
- Skinner v State of Oklahoma, ex rel. Williamson, 316 U.S. 535 (1942).
- Griswold v Connecticut, 381 U.S. 479 (1965)
- Eisenstadt v Baird, 405 U.S. 438 (1972).
- Roe v Wade, 410 U.S. 113 (1973).
- Harris v McRae, 448 U.S. 297 (1980).
- Williams v Zbaraz, 448 U.S 358 (1980).
- Webster v Reproductive Health Services, 492 U.S. 490 (1989).
- Rust v Sullivan, 500 U.S. 173 (1991).
- Planned Parenthood of Southeastern Pennsylvania v Casey, 505 U.S. 833 (1992).
- Carey v Population Services, 431 U.S. 678 (1977).
- Bellotti v Baird, 443 U.S. 622 (1979).
- Planned Parenthood of Kansas City v Ashcroft, 462 U.S. 476 (1983).
- Planned Parenthood of Northern New England v Ayotte, 546 U.S. 320 (2006).
- H.L. v Matheson, 450 U.S. 398 (1981).
- Hodgson v Minnesota, 497 U.S. 417 (1990).
- Ohio v Akron Center for Reproductive Health, 497 U.S. 502 (1990).
- Lambert v Wicklund, 520 U.S. 292 (1997).
- June Medical Services v Russo, 591 U.S. ___ (2020), https:// www.supremecourt.gov/opinions/19pdf/18-1323_c07d.pdf.
- Whole Woman’s Health v Hellerstedt, 579 U.S. 582 (2016).
- AMA v Becerra, dismissed May 17, 2021, https://www .scotusblog.com/case-files/cases/american-medical -association-v-cochran.
- Becerra v Baltimore, dismissed May 17, 2021, https://www .scotusblog.com/case-files/cases/cochran-v-mayor-and-city-council-of-baltimore.
- Oregon v Becerra, dismissed May 17, 2021, https://www .scotusblog.com/case-files/cases/oregon-v-cochran.
- Cameron v EMW Women’s Surgical Center, 20-601. https:// www.scotusblog.com/case-files/cases/cameron-v-emw -womens-surgical-center-p-s-c.
- Ex parte Young, 209 U.S. 123 (1908).
- Whole Woman’s Health v Jackson, 21-463. https://www .scotusblog.com/case-files/cases/whole-womans-health-v -jackson.
- U.S. v Texas, 21-588. https://www.scotusblog.com/case-files /cases/united-states-v-texas-3.
- Dobbs v Jackson Women’s Health Organization, 19-1392. https://www.scotusblog.com/case-files/cases/dobbs-v -jackson-womens-health-organization.
- Brief of Amici Curiae American College of Obstetricians and Gynecologists et al., Dobbs v Jackson Women’s Health Organization (Sep. 2021). https://www.acog.org/ -/media/project/acog/acogorg/files/advocacy/amicus -briefs/2021/20210920-dobbs-v-jwho-amicus-brief.pdf?la=e n&hash=717DFDD07A03B93A04490E66835BB8C5.
- Brief Amicus Curiae of International Federation of Gynecology and Obstetrics, Dobbs v Jackson Women’s Health Organization (Sep. 20, 2021). https://www.supremecourt. gov/DocketPDF/19/19-1392/193019/20210920155508744 _41426%20pdf%20Chen.pdf.
- Brief Amicus Curiae of American Association of Pro-Life Obstetricians and Gynecologists. Dobbs v Jackson Women’s Health Organization (Sep. 2021). https://www.supremecourt .gov/DocketPDF/19/19-1392/185350/20210729163532595 _No.%2019-1392%20-%20American%20Association %20of%20Pro-Life%20Obstetricians%20and%20 Gynecologists%20-%20Amicus%20Brief%20in%20Support %20of%20Petitioner%20-%207-29-21.pdf.
Quebec plans to fine unvaccinated adults
The amount hasn’t been decided yet, but it will be “significant” and more than $100. More details will be released at a later date, The Associated Press reported.
“Those who refuse to get their first doses in the coming weeks will have to pay a new health contribution,” Premier Francois Legault said during a news conference.
Not getting vaccinated burdens the health care system, and not all residents should pay for it, he said. About 10% of adults in Quebec are unvaccinated, but they represent about 50% of intensive care patients.
“I think it’s reasonable a majority of the population is asking that there be consequences,” he said. “It’s a question of fairness for the 90% of the population that have made some sacrifices. We owe them.”
The fine will apply to those who don’t qualify for a medical exemption, Mr. Legault said.
Provinces across Canada have reported a surge in COVID-19 cases due to the Omicron variant, with Quebec being one of the hardest-hit, according to Reuters. The province is regularly recording the highest daily case count across the country.
Quebec also has announced a 10 p.m. to 5 a.m. curfew, the AP reported. Starting Jan. 18, liquor and cannabis stores in the province will require proof of vaccination, and shopping malls and hair salons could soon require them as well.
About a quarter of all Canadians live in Quebec, according to CNN. The province was one of the first in Canada to require proof of vaccination for residents to eat in restaurants, go to the gym, or attend sporting events.
Some European countries have announced fees for unvaccinated residents, the AP reported, but Quebec is the first in Canada to announce a financial penalty for those who don’t get a shot.
In Greece, people older than 60 have until Jan. 16 to receive the first dose, or they will be fined 100 euros for every month they remain unvaccinated, the AP reported.
Austria will impose fines up to 3,600 euros for those who don’t follow the vaccine mandate for ages 14 and older, which is slated to start in February.
In Italy, residents who are 50 and older are required to be vaccinated. In mid-February, those who are unvaccinated could be fined up to 1,600 euros if they enter their workplaces, the AP reported.
A version of this article first appeared on WebMD.com.
The amount hasn’t been decided yet, but it will be “significant” and more than $100. More details will be released at a later date, The Associated Press reported.
“Those who refuse to get their first doses in the coming weeks will have to pay a new health contribution,” Premier Francois Legault said during a news conference.
Not getting vaccinated burdens the health care system, and not all residents should pay for it, he said. About 10% of adults in Quebec are unvaccinated, but they represent about 50% of intensive care patients.
“I think it’s reasonable a majority of the population is asking that there be consequences,” he said. “It’s a question of fairness for the 90% of the population that have made some sacrifices. We owe them.”
The fine will apply to those who don’t qualify for a medical exemption, Mr. Legault said.
Provinces across Canada have reported a surge in COVID-19 cases due to the Omicron variant, with Quebec being one of the hardest-hit, according to Reuters. The province is regularly recording the highest daily case count across the country.
Quebec also has announced a 10 p.m. to 5 a.m. curfew, the AP reported. Starting Jan. 18, liquor and cannabis stores in the province will require proof of vaccination, and shopping malls and hair salons could soon require them as well.
About a quarter of all Canadians live in Quebec, according to CNN. The province was one of the first in Canada to require proof of vaccination for residents to eat in restaurants, go to the gym, or attend sporting events.
Some European countries have announced fees for unvaccinated residents, the AP reported, but Quebec is the first in Canada to announce a financial penalty for those who don’t get a shot.
In Greece, people older than 60 have until Jan. 16 to receive the first dose, or they will be fined 100 euros for every month they remain unvaccinated, the AP reported.
Austria will impose fines up to 3,600 euros for those who don’t follow the vaccine mandate for ages 14 and older, which is slated to start in February.
In Italy, residents who are 50 and older are required to be vaccinated. In mid-February, those who are unvaccinated could be fined up to 1,600 euros if they enter their workplaces, the AP reported.
A version of this article first appeared on WebMD.com.
The amount hasn’t been decided yet, but it will be “significant” and more than $100. More details will be released at a later date, The Associated Press reported.
“Those who refuse to get their first doses in the coming weeks will have to pay a new health contribution,” Premier Francois Legault said during a news conference.
Not getting vaccinated burdens the health care system, and not all residents should pay for it, he said. About 10% of adults in Quebec are unvaccinated, but they represent about 50% of intensive care patients.
“I think it’s reasonable a majority of the population is asking that there be consequences,” he said. “It’s a question of fairness for the 90% of the population that have made some sacrifices. We owe them.”
The fine will apply to those who don’t qualify for a medical exemption, Mr. Legault said.
Provinces across Canada have reported a surge in COVID-19 cases due to the Omicron variant, with Quebec being one of the hardest-hit, according to Reuters. The province is regularly recording the highest daily case count across the country.
Quebec also has announced a 10 p.m. to 5 a.m. curfew, the AP reported. Starting Jan. 18, liquor and cannabis stores in the province will require proof of vaccination, and shopping malls and hair salons could soon require them as well.
About a quarter of all Canadians live in Quebec, according to CNN. The province was one of the first in Canada to require proof of vaccination for residents to eat in restaurants, go to the gym, or attend sporting events.
Some European countries have announced fees for unvaccinated residents, the AP reported, but Quebec is the first in Canada to announce a financial penalty for those who don’t get a shot.
In Greece, people older than 60 have until Jan. 16 to receive the first dose, or they will be fined 100 euros for every month they remain unvaccinated, the AP reported.
Austria will impose fines up to 3,600 euros for those who don’t follow the vaccine mandate for ages 14 and older, which is slated to start in February.
In Italy, residents who are 50 and older are required to be vaccinated. In mid-February, those who are unvaccinated could be fined up to 1,600 euros if they enter their workplaces, the AP reported.
A version of this article first appeared on WebMD.com.
CDC to update mask recommendations as Omicron spreads
Director Rochelle Walensky, MD, said on Jan. 12.
“We are preparing an update to the info on our mask website to best reflect the options that are available to people and the different levels of protection different masks provide, and we want to provide Americans the best and most updated information to choose what mask is going to be right for them,” she said at a White House news briefing.
While the higher-quality masks provide better protection, they can be uncomfortable to wear, expensive, and harder to find. That’s why Dr. Walensky added an important caveat.
“Any mask is better than no mask, and we do encourage all Americans to wear a well-fitting mask to protect themselves and prevent the spread of COVID-19. That recommendation is not going to change,” she said.
“Most importantly, the best mask that you wear is the one you will wear and the one you can keep on all day long and tolerate in public indoor settings.”
Meanwhile, the World Health Organization was more focused on vaccines.
WHO officials stressed on Jan. 12 that global vaccine distribution is first priority in defeating the highly contagious Omicron variant, as well as other variants that may evolve.
The WHO’s Technical Advisory Group on COVID-19 Vaccine Composition – a group of experts assessing how COVID-19 vaccines perform against Omicron and other emerging variants – says there is an “urgent need” for broader access to vaccines, along with reviewing and updating current vaccines as needed to ensure protection.
The WHO also disputed the idea that COVID-19 could become endemic in one largely vaccinated nation, while the rest of the world remains unprotected.
“It is up to us how this pandemic unfolds,” Maria Van Kerkhove, PhD, the WHO’s technical lead on COVID-19 response, said at a news briefing.
The WHO has a goal of vaccinating 70% of the population of every country by the middle of the year.
But right now, 90 countries have yet to reach 40% vaccination rates, and 36 of those countries have less than 10% of their populations vaccinated, according to WHO Director General Tedros Adhanom Ghebreyesus, PhD.
A staggering 85% of the African population has not received a first dose.
But progress is being made, Dr. Ghebreyesus said at the briefing.
The WHO said there were over 15 million COVID-19 cases reported last week – the most ever in a single week – and this is likely an underestimate.
The Omicron variant, first identified in South Africa 2 months ago and now found on all seven continents, is “rapidly replacing Delta in almost all countries,” Dr. Ghebreyesus said.
Dr. Walensky said this week’s U.S. daily average COVID-19 case count was 751,000, an increase of 47% from last week. The average daily hospital admissions this week is 19,800, an increase of 33%. Deaths are up 40%, reaching 1,600 per day.
But she also reported new data that supports other research showing Omicron may produce less severe disease. Kaiser Permanente Southern California released a study on Jan. 11 showing that, compared with Delta infections, Omicron was associated with a 53% reduction in hospitalizations, a 74% reduction in intensive care unit admissions, and a 91% lower risk of death.
In the study, no patients with Omicron required mechanical ventilation. The strain now accounts for 98% of cases nationwide.
But Dr. Walensky warned the lower disease severity is not enough to make up for the sheer number of cases that continue to overwhelm hospital systems.
“While we are seeing early evidence that Omicron is less severe than Delta and that those infected are less likely to require hospitalization, it’s important to note that Omicron continues to be much more transmissible than Delta,” she said. “The sudden rise in cases due to Omicron is resulting in unprecedented daily case counts, sickness, absenteeism, and strains on our health care system.”
A version of this article first appeared on WebMD.com.
Director Rochelle Walensky, MD, said on Jan. 12.
“We are preparing an update to the info on our mask website to best reflect the options that are available to people and the different levels of protection different masks provide, and we want to provide Americans the best and most updated information to choose what mask is going to be right for them,” she said at a White House news briefing.
While the higher-quality masks provide better protection, they can be uncomfortable to wear, expensive, and harder to find. That’s why Dr. Walensky added an important caveat.
“Any mask is better than no mask, and we do encourage all Americans to wear a well-fitting mask to protect themselves and prevent the spread of COVID-19. That recommendation is not going to change,” she said.
“Most importantly, the best mask that you wear is the one you will wear and the one you can keep on all day long and tolerate in public indoor settings.”
Meanwhile, the World Health Organization was more focused on vaccines.
WHO officials stressed on Jan. 12 that global vaccine distribution is first priority in defeating the highly contagious Omicron variant, as well as other variants that may evolve.
The WHO’s Technical Advisory Group on COVID-19 Vaccine Composition – a group of experts assessing how COVID-19 vaccines perform against Omicron and other emerging variants – says there is an “urgent need” for broader access to vaccines, along with reviewing and updating current vaccines as needed to ensure protection.
The WHO also disputed the idea that COVID-19 could become endemic in one largely vaccinated nation, while the rest of the world remains unprotected.
“It is up to us how this pandemic unfolds,” Maria Van Kerkhove, PhD, the WHO’s technical lead on COVID-19 response, said at a news briefing.
The WHO has a goal of vaccinating 70% of the population of every country by the middle of the year.
But right now, 90 countries have yet to reach 40% vaccination rates, and 36 of those countries have less than 10% of their populations vaccinated, according to WHO Director General Tedros Adhanom Ghebreyesus, PhD.
A staggering 85% of the African population has not received a first dose.
But progress is being made, Dr. Ghebreyesus said at the briefing.
The WHO said there were over 15 million COVID-19 cases reported last week – the most ever in a single week – and this is likely an underestimate.
The Omicron variant, first identified in South Africa 2 months ago and now found on all seven continents, is “rapidly replacing Delta in almost all countries,” Dr. Ghebreyesus said.
Dr. Walensky said this week’s U.S. daily average COVID-19 case count was 751,000, an increase of 47% from last week. The average daily hospital admissions this week is 19,800, an increase of 33%. Deaths are up 40%, reaching 1,600 per day.
But she also reported new data that supports other research showing Omicron may produce less severe disease. Kaiser Permanente Southern California released a study on Jan. 11 showing that, compared with Delta infections, Omicron was associated with a 53% reduction in hospitalizations, a 74% reduction in intensive care unit admissions, and a 91% lower risk of death.
In the study, no patients with Omicron required mechanical ventilation. The strain now accounts for 98% of cases nationwide.
But Dr. Walensky warned the lower disease severity is not enough to make up for the sheer number of cases that continue to overwhelm hospital systems.
“While we are seeing early evidence that Omicron is less severe than Delta and that those infected are less likely to require hospitalization, it’s important to note that Omicron continues to be much more transmissible than Delta,” she said. “The sudden rise in cases due to Omicron is resulting in unprecedented daily case counts, sickness, absenteeism, and strains on our health care system.”
A version of this article first appeared on WebMD.com.
Director Rochelle Walensky, MD, said on Jan. 12.
“We are preparing an update to the info on our mask website to best reflect the options that are available to people and the different levels of protection different masks provide, and we want to provide Americans the best and most updated information to choose what mask is going to be right for them,” she said at a White House news briefing.
While the higher-quality masks provide better protection, they can be uncomfortable to wear, expensive, and harder to find. That’s why Dr. Walensky added an important caveat.
“Any mask is better than no mask, and we do encourage all Americans to wear a well-fitting mask to protect themselves and prevent the spread of COVID-19. That recommendation is not going to change,” she said.
“Most importantly, the best mask that you wear is the one you will wear and the one you can keep on all day long and tolerate in public indoor settings.”
Meanwhile, the World Health Organization was more focused on vaccines.
WHO officials stressed on Jan. 12 that global vaccine distribution is first priority in defeating the highly contagious Omicron variant, as well as other variants that may evolve.
The WHO’s Technical Advisory Group on COVID-19 Vaccine Composition – a group of experts assessing how COVID-19 vaccines perform against Omicron and other emerging variants – says there is an “urgent need” for broader access to vaccines, along with reviewing and updating current vaccines as needed to ensure protection.
The WHO also disputed the idea that COVID-19 could become endemic in one largely vaccinated nation, while the rest of the world remains unprotected.
“It is up to us how this pandemic unfolds,” Maria Van Kerkhove, PhD, the WHO’s technical lead on COVID-19 response, said at a news briefing.
The WHO has a goal of vaccinating 70% of the population of every country by the middle of the year.
But right now, 90 countries have yet to reach 40% vaccination rates, and 36 of those countries have less than 10% of their populations vaccinated, according to WHO Director General Tedros Adhanom Ghebreyesus, PhD.
A staggering 85% of the African population has not received a first dose.
But progress is being made, Dr. Ghebreyesus said at the briefing.
The WHO said there were over 15 million COVID-19 cases reported last week – the most ever in a single week – and this is likely an underestimate.
The Omicron variant, first identified in South Africa 2 months ago and now found on all seven continents, is “rapidly replacing Delta in almost all countries,” Dr. Ghebreyesus said.
Dr. Walensky said this week’s U.S. daily average COVID-19 case count was 751,000, an increase of 47% from last week. The average daily hospital admissions this week is 19,800, an increase of 33%. Deaths are up 40%, reaching 1,600 per day.
But she also reported new data that supports other research showing Omicron may produce less severe disease. Kaiser Permanente Southern California released a study on Jan. 11 showing that, compared with Delta infections, Omicron was associated with a 53% reduction in hospitalizations, a 74% reduction in intensive care unit admissions, and a 91% lower risk of death.
In the study, no patients with Omicron required mechanical ventilation. The strain now accounts for 98% of cases nationwide.
But Dr. Walensky warned the lower disease severity is not enough to make up for the sheer number of cases that continue to overwhelm hospital systems.
“While we are seeing early evidence that Omicron is less severe than Delta and that those infected are less likely to require hospitalization, it’s important to note that Omicron continues to be much more transmissible than Delta,” she said. “The sudden rise in cases due to Omicron is resulting in unprecedented daily case counts, sickness, absenteeism, and strains on our health care system.”
A version of this article first appeared on WebMD.com.
Urine for a new vaccine alternative
Urine for a new vaccine alternative
Yep, you read that right: Another vaccine alternative. Urine sounds disgusting, but you’ve got to admit, it’s resourceful at least.
Christopher Key, the leader of a group of antivaxxers known as the “Vaccine Police,” is now claiming that you should do “urine therapy,” when means drinking your own pee to ward off COVID-19. According to My. Key, “tons and tons of research” shows the benefits of drinking urine to fight COVID-19, the Guardian reported.
He doesn’t seem like the best source of information, especially since he’s been arrested in the past for refusing to wear a mask in a store. Not wanting to wear a mask in a store doesn’t seem like much, but he also believes that those who administer the COVID-19 vaccine should be “executed” and he tried to impersonate a law official toattempt to arrest a Democratic governor for vaccine mandates.
The overwhelming amount of COVID-19 misinformation has been stressful, yet sometimes laugh-worthy. Urine is not the first “cure” and probably won’t be the last. If you heard something works in a sketchy group on Facebook, it’s probably safe to assume that it absolutely does not. Please don’t recycle your urine.
Vaccine or beer? You must now choose
As the COVID-19 pandemic drags on toward its third year, the large subset of the population who refuse to get vaccinated has proved nearly intractable. Governments have tried numerous incentives to boost vaccination rates, ranging from free beer to million dollar lotteries. Needless to say, beyond their ability to generate LOTME stories, these incentives have been less than effective.
As the frankly unfairly contagious Omicron variant makes it way through the world, our friends in the Great White North have decided enough is enough. If the carrot doesn’t work, the people of Quebec are going to get the stick. Starting on Jan. 18, vaccination cards will be required to enter stores that sell alcohol or cannabis, better known as the things that have gotten us all through this pandemic.
And you know what? Cutting off the booze supply seems to be working. Christian Dubé, Quebec’s health minister, said that the number of vaccination appointments had quadrupled in the new year, rising from 1,500 per day to 6,000 per day, according to the CTV News report. Now, those aren’t massive numbers, but this is big empty Canada we’re talking about, and the unvaccinated make up about 10% of Quebec’s population, so 6,000 a day is quite impressive.
Mr. Dubé added that additional nonessential businesses could be added to the restriction list in the coming weeks, but we’re not sure it’ll be necessary. Those middle-aged soccer moms will do anything to secure their daily merlot. Also, alcohol and cannabis nonessential? The LOTME staff is appalled and offended at this insinuation.
All I need is the polyester that I breathe
When you do laundry, you’re probably thinking more of how to get that ketchup stain out of your white shirt than the effect it has on the environment. Well, research shows it actually has some significance.
That significance comes in the form of microfibers, which are released from natural fabrics such as cotton and from synthetic fabrics such as polyester, which are also considered to be microplastics.
The microfibers that get released in the water when we wash clothes are filtered out eventually, but the dryer is the real culprit, according to a study in Environmental Science & Technology Letters. We’re talking a discharge of up to 120 million microfiber fragments directly into the air annually from just one dryer!
Dryers, they found, emitted between 1.4-40 times more microfibers than did washing machines in previous studies. And polyester fabrics produced more fragments when load sizes increased, while fragment production from cotton fabrics remained constant.
Recent findings suggest that inhaling these microfibers can cause lung inflammation, increase cancer risk, and induce asthma attacks. The authors of the current study suggested additional filtration should be done on dryer vents to reduce the amount of pollutants emitted into the air.
Who would have thought just drying your sheets could be such a dangerous act?
It’s always in the last place you look
At least a million times every morning in this country, a million children yell something like this as they get ready for school: “Mom, have you seen my ...?”
Well, thanks to Defector.com, now we know what Mom should yell back: “Look in your weird cousin Mortimer!”
We will explain ... again.
When they’re not dealing with COVID-19, the folks who work in emergency departments spend a lot of their time removing things that are stuck in people’s bodily orifices. The U.S. Consumer Product Safety Commission even keeps track of them.
So if you’re looking for the number 8 button from the TV remote, or maybe a bullet, check Mortimer’s nose. Maybe you’re missing a lollipop, a hairpin, or some espresso beans. Mortimer’s friend Beulah might have put them in her ear.
Has an earbud gone missing? Another friend of Mortimer’s went to the ED with something stuck in his throat and said that he had a “pill in one hand and his earbud in the other hand, got distracted and took the earbud instead.” Yes, that is an actual quote (via Defector) from the CPSC database.
What about that old saying that someone’s lost his marbles? Well, the ED found one of Mortimer’s marbles ... in his penis. Also a spork, and a bread twist tie, and a chopstick. No, not all at the same time. As for Beulah, a barbell and a Spider-Man action figure somehow found their way – not at the same time, thank goodness – into her vagina.
And have you ever heard someone say that they’re “not going to stand for this”? Mortimer has, so he sat down ... on a light bulb, and a rolling pin, and a billiard ball. Yup, the ED had to remove these items from his rectum.
But not all at the same time, thank goodness.
Urine for a new vaccine alternative
Yep, you read that right: Another vaccine alternative. Urine sounds disgusting, but you’ve got to admit, it’s resourceful at least.
Christopher Key, the leader of a group of antivaxxers known as the “Vaccine Police,” is now claiming that you should do “urine therapy,” when means drinking your own pee to ward off COVID-19. According to My. Key, “tons and tons of research” shows the benefits of drinking urine to fight COVID-19, the Guardian reported.
He doesn’t seem like the best source of information, especially since he’s been arrested in the past for refusing to wear a mask in a store. Not wanting to wear a mask in a store doesn’t seem like much, but he also believes that those who administer the COVID-19 vaccine should be “executed” and he tried to impersonate a law official toattempt to arrest a Democratic governor for vaccine mandates.
The overwhelming amount of COVID-19 misinformation has been stressful, yet sometimes laugh-worthy. Urine is not the first “cure” and probably won’t be the last. If you heard something works in a sketchy group on Facebook, it’s probably safe to assume that it absolutely does not. Please don’t recycle your urine.
Vaccine or beer? You must now choose
As the COVID-19 pandemic drags on toward its third year, the large subset of the population who refuse to get vaccinated has proved nearly intractable. Governments have tried numerous incentives to boost vaccination rates, ranging from free beer to million dollar lotteries. Needless to say, beyond their ability to generate LOTME stories, these incentives have been less than effective.
As the frankly unfairly contagious Omicron variant makes it way through the world, our friends in the Great White North have decided enough is enough. If the carrot doesn’t work, the people of Quebec are going to get the stick. Starting on Jan. 18, vaccination cards will be required to enter stores that sell alcohol or cannabis, better known as the things that have gotten us all through this pandemic.
And you know what? Cutting off the booze supply seems to be working. Christian Dubé, Quebec’s health minister, said that the number of vaccination appointments had quadrupled in the new year, rising from 1,500 per day to 6,000 per day, according to the CTV News report. Now, those aren’t massive numbers, but this is big empty Canada we’re talking about, and the unvaccinated make up about 10% of Quebec’s population, so 6,000 a day is quite impressive.
Mr. Dubé added that additional nonessential businesses could be added to the restriction list in the coming weeks, but we’re not sure it’ll be necessary. Those middle-aged soccer moms will do anything to secure their daily merlot. Also, alcohol and cannabis nonessential? The LOTME staff is appalled and offended at this insinuation.
All I need is the polyester that I breathe
When you do laundry, you’re probably thinking more of how to get that ketchup stain out of your white shirt than the effect it has on the environment. Well, research shows it actually has some significance.
That significance comes in the form of microfibers, which are released from natural fabrics such as cotton and from synthetic fabrics such as polyester, which are also considered to be microplastics.
The microfibers that get released in the water when we wash clothes are filtered out eventually, but the dryer is the real culprit, according to a study in Environmental Science & Technology Letters. We’re talking a discharge of up to 120 million microfiber fragments directly into the air annually from just one dryer!
Dryers, they found, emitted between 1.4-40 times more microfibers than did washing machines in previous studies. And polyester fabrics produced more fragments when load sizes increased, while fragment production from cotton fabrics remained constant.
Recent findings suggest that inhaling these microfibers can cause lung inflammation, increase cancer risk, and induce asthma attacks. The authors of the current study suggested additional filtration should be done on dryer vents to reduce the amount of pollutants emitted into the air.
Who would have thought just drying your sheets could be such a dangerous act?
It’s always in the last place you look
At least a million times every morning in this country, a million children yell something like this as they get ready for school: “Mom, have you seen my ...?”
Well, thanks to Defector.com, now we know what Mom should yell back: “Look in your weird cousin Mortimer!”
We will explain ... again.
When they’re not dealing with COVID-19, the folks who work in emergency departments spend a lot of their time removing things that are stuck in people’s bodily orifices. The U.S. Consumer Product Safety Commission even keeps track of them.
So if you’re looking for the number 8 button from the TV remote, or maybe a bullet, check Mortimer’s nose. Maybe you’re missing a lollipop, a hairpin, or some espresso beans. Mortimer’s friend Beulah might have put them in her ear.
Has an earbud gone missing? Another friend of Mortimer’s went to the ED with something stuck in his throat and said that he had a “pill in one hand and his earbud in the other hand, got distracted and took the earbud instead.” Yes, that is an actual quote (via Defector) from the CPSC database.
What about that old saying that someone’s lost his marbles? Well, the ED found one of Mortimer’s marbles ... in his penis. Also a spork, and a bread twist tie, and a chopstick. No, not all at the same time. As for Beulah, a barbell and a Spider-Man action figure somehow found their way – not at the same time, thank goodness – into her vagina.
And have you ever heard someone say that they’re “not going to stand for this”? Mortimer has, so he sat down ... on a light bulb, and a rolling pin, and a billiard ball. Yup, the ED had to remove these items from his rectum.
But not all at the same time, thank goodness.
Urine for a new vaccine alternative
Yep, you read that right: Another vaccine alternative. Urine sounds disgusting, but you’ve got to admit, it’s resourceful at least.
Christopher Key, the leader of a group of antivaxxers known as the “Vaccine Police,” is now claiming that you should do “urine therapy,” when means drinking your own pee to ward off COVID-19. According to My. Key, “tons and tons of research” shows the benefits of drinking urine to fight COVID-19, the Guardian reported.
He doesn’t seem like the best source of information, especially since he’s been arrested in the past for refusing to wear a mask in a store. Not wanting to wear a mask in a store doesn’t seem like much, but he also believes that those who administer the COVID-19 vaccine should be “executed” and he tried to impersonate a law official toattempt to arrest a Democratic governor for vaccine mandates.
The overwhelming amount of COVID-19 misinformation has been stressful, yet sometimes laugh-worthy. Urine is not the first “cure” and probably won’t be the last. If you heard something works in a sketchy group on Facebook, it’s probably safe to assume that it absolutely does not. Please don’t recycle your urine.
Vaccine or beer? You must now choose
As the COVID-19 pandemic drags on toward its third year, the large subset of the population who refuse to get vaccinated has proved nearly intractable. Governments have tried numerous incentives to boost vaccination rates, ranging from free beer to million dollar lotteries. Needless to say, beyond their ability to generate LOTME stories, these incentives have been less than effective.
As the frankly unfairly contagious Omicron variant makes it way through the world, our friends in the Great White North have decided enough is enough. If the carrot doesn’t work, the people of Quebec are going to get the stick. Starting on Jan. 18, vaccination cards will be required to enter stores that sell alcohol or cannabis, better known as the things that have gotten us all through this pandemic.
And you know what? Cutting off the booze supply seems to be working. Christian Dubé, Quebec’s health minister, said that the number of vaccination appointments had quadrupled in the new year, rising from 1,500 per day to 6,000 per day, according to the CTV News report. Now, those aren’t massive numbers, but this is big empty Canada we’re talking about, and the unvaccinated make up about 10% of Quebec’s population, so 6,000 a day is quite impressive.
Mr. Dubé added that additional nonessential businesses could be added to the restriction list in the coming weeks, but we’re not sure it’ll be necessary. Those middle-aged soccer moms will do anything to secure their daily merlot. Also, alcohol and cannabis nonessential? The LOTME staff is appalled and offended at this insinuation.
All I need is the polyester that I breathe
When you do laundry, you’re probably thinking more of how to get that ketchup stain out of your white shirt than the effect it has on the environment. Well, research shows it actually has some significance.
That significance comes in the form of microfibers, which are released from natural fabrics such as cotton and from synthetic fabrics such as polyester, which are also considered to be microplastics.
The microfibers that get released in the water when we wash clothes are filtered out eventually, but the dryer is the real culprit, according to a study in Environmental Science & Technology Letters. We’re talking a discharge of up to 120 million microfiber fragments directly into the air annually from just one dryer!
Dryers, they found, emitted between 1.4-40 times more microfibers than did washing machines in previous studies. And polyester fabrics produced more fragments when load sizes increased, while fragment production from cotton fabrics remained constant.
Recent findings suggest that inhaling these microfibers can cause lung inflammation, increase cancer risk, and induce asthma attacks. The authors of the current study suggested additional filtration should be done on dryer vents to reduce the amount of pollutants emitted into the air.
Who would have thought just drying your sheets could be such a dangerous act?
It’s always in the last place you look
At least a million times every morning in this country, a million children yell something like this as they get ready for school: “Mom, have you seen my ...?”
Well, thanks to Defector.com, now we know what Mom should yell back: “Look in your weird cousin Mortimer!”
We will explain ... again.
When they’re not dealing with COVID-19, the folks who work in emergency departments spend a lot of their time removing things that are stuck in people’s bodily orifices. The U.S. Consumer Product Safety Commission even keeps track of them.
So if you’re looking for the number 8 button from the TV remote, or maybe a bullet, check Mortimer’s nose. Maybe you’re missing a lollipop, a hairpin, or some espresso beans. Mortimer’s friend Beulah might have put them in her ear.
Has an earbud gone missing? Another friend of Mortimer’s went to the ED with something stuck in his throat and said that he had a “pill in one hand and his earbud in the other hand, got distracted and took the earbud instead.” Yes, that is an actual quote (via Defector) from the CPSC database.
What about that old saying that someone’s lost his marbles? Well, the ED found one of Mortimer’s marbles ... in his penis. Also a spork, and a bread twist tie, and a chopstick. No, not all at the same time. As for Beulah, a barbell and a Spider-Man action figure somehow found their way – not at the same time, thank goodness – into her vagina.
And have you ever heard someone say that they’re “not going to stand for this”? Mortimer has, so he sat down ... on a light bulb, and a rolling pin, and a billiard ball. Yup, the ED had to remove these items from his rectum.
But not all at the same time, thank goodness.
The troubling trend of repackaging feminine hygiene products for the next generation
Feminine hygiene products have been commercially available for decades. They are commonly marketed to reduce odor or clean vaginal discharge and menses. Multiple formulas are available as topical washes, wipes, creams, sprays, powders, deodorants, and douches.1 Products on the market range from those used externally on the vulva, such as wipes and sprays, to liquid solutions used intravaginally, such as washes and douches.
Who uses feminine hygiene products?
According to a 2006 study, the majority of women who use douches started using them between age 15 and 19 years, but some women initiate this practice habit as early as age 10 to 14.1 Predictably, women who douche are more likely to perceive douche products as safe.1
Demographic data on douche utilization are mixed: Some studies show that there are no significant racial differences in douching practices,2 while others have found that Black and African American women are more likely to practice douching than White and Hispanic women.1,3 Studies have shown a significant difference in attitudes toward douching and knowledge of normal vaginal symptoms among US racial demographics, although this must be examined through the historical context of racism and the lens of medical anthropology.4
Women cite that common reasons they use feminine hygiene products are to feel clean, to control odor, and to use after menses and intercourse.1,2
Modern marketing approaches
From wipes to soaps to douches, feminine hygiene products often are advertised to promote “funk-free periods”5 and “freshness,” fostering an environment in which women and men develop unrealistic standards for what is considered normal genital odor and resulting in poor body image.6
Recently, Vagisil (Combe Incorporated) marketing efforts faced backlash from the ObGyn community for targeting younger populations with a specific product line for adolescents called OMV! In addition, attention has been drawn to VCF vaginal odor eliminating film (Apothecus Pharmaceutical Corp), small stamp-sized dissolving films that are placed in the vaginal canal in contact with the epithelium. This product has entered the market of feminine hygiene products accompanied by slogans of eliminating “feminine odor” and providing “confidence for women to be intimate.”
Continue to: Effects of feminine hygiene products on the vaginal microbiome...
Effects of feminine hygiene products on the vaginal microbiome
Frequent use of feminine hygiene products has been associated with recurrent vaginitis, bacterial vaginosis, and general irritation/itch,7,8 which can cause more discharge and odor. Ironically, this may result in women using the product more frequently since they often seek out these products to eliminate odor and discharge.1,2
The pH of the vagina changes during a woman’s lifetime, but in the reproductive years, the normal pH range is typically 3.8 to 4.4.9 This range allows for a normal vaginal flora to form with bacteria such as Lactobacillus species and Gardnerella vaginalis, while feminine hygiene products have a wide range of pH.9,10
Regardless of the formulation, most feminine hygiene products contain ingredients and compositions that potentially are detrimental to the health of the vulva and vagina. Many products contain acidic ingredients, such as citric acid, lactic acid, and dehydroacetic acid, that can alter the vaginal pH and weaken the vaginal barrier by wiping out normal vaginal flora10 despite being advertised for use on “sensitive areas” (TABLE). Lactic acid also has been found to increase diverse anaerobic bacteria in the vaginal microbiome.11 Some feminine hygiene products have been shown to suppress Lactobacillus growth at 2 hours after use and to kill all lactobacilli at 24 hours.10 Shifts in microbiota numbers often occur when the vaginal pH has been altered, as is frequently the case with feminine hygiene products. In the absence of microbiome bacteria, the presence of vaginal hygiene products has been shown to increase interleukin-8 (IL-8), suggesting a proinflammatory reaction.10
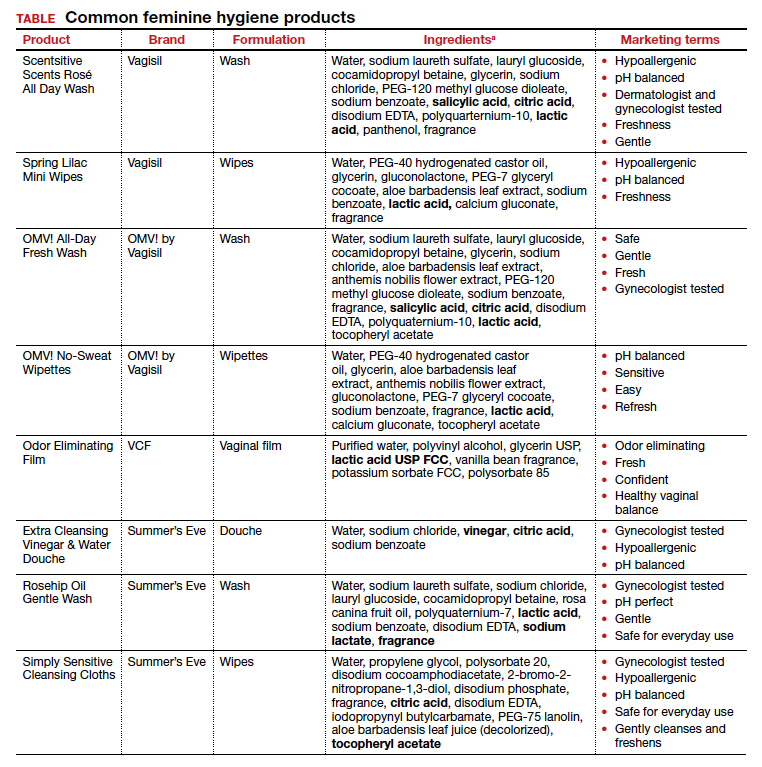
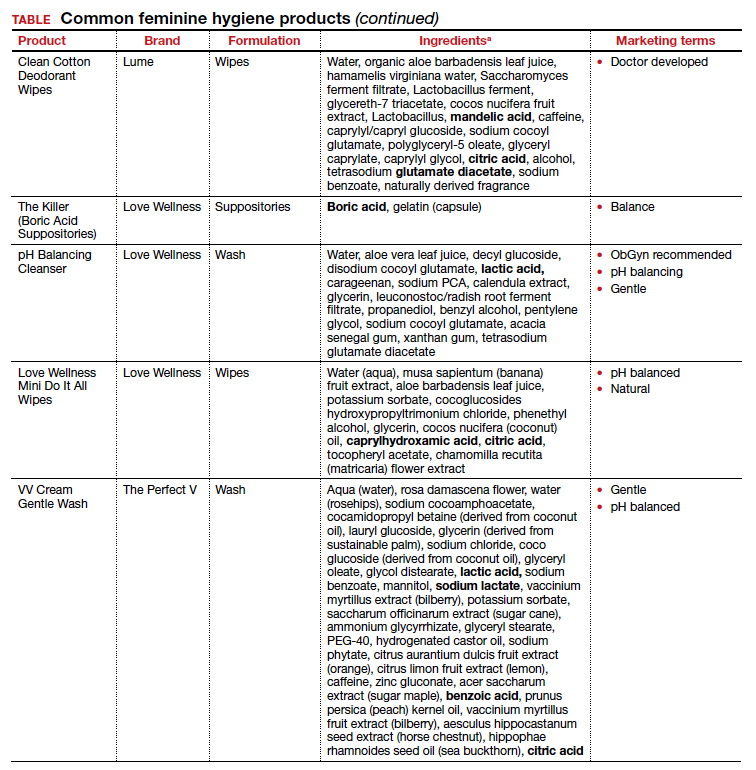
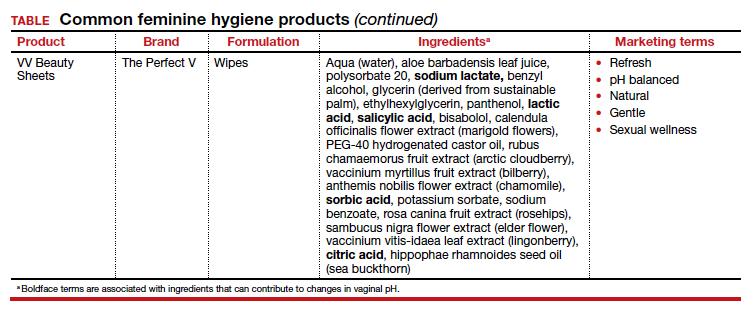
A study in the United Kingdom found that women who used bubble bath, antiseptics, or douche products had a higher incidence of bacterial vaginosis compared with women who did not use such products.7 Women in Canada who used feminine hygiene products were more likely to report adverse conditions, including yeast infections, bacterial vaginosis, urinary tract infections, and sexually transmitted diseases.8 Furthermore, a significant association exists between vaginal douching and endometrial infection by bacterial vaginosis–associated organisms.12
Additionally, a study that analyzed volatile organic compound levels in the blood with the use of feminine hygiene products revealed a significant positive dose-exposure relationship between the frequency of vaginal douching in the last 6 months and concentrations of 1,4-dichloromethane, one of the volatile organic compounds.3 This points to the issue of not only disruption of pH and vaginal flora but also to the introduction of harmful substances that can further disrupt the vaginal barrier.
Understand the products to help educate patients
Use of feminine hygiene products is common among women. While women depend on the market to filter out products that are considered unsafe or may have harmful side effects,1 unfortunately that is not necessarily the case. With increasingly more feminine products on the market and the target demographic becoming younger, women of all ages are susceptible to misinformation that could affect their vaginal health long term.
It is vital that clinicians understand the topical effects of these products in order to properly educate and counsel patients. Ultimately, research on feminine hygiene products is limited and, as more products come to market, we must continue to reassess the effects of topical products on the vaginal epithelium and vulvar tissues. ●
- Grimley DM, Annang L, Foushee HR, et al. Vaginal douches and other feminine hygiene products: women’s practices and perceptions of product safety. Matern Child Health J. 2006;10:303-310. doi: 10.1007/s10995-005-0054-y.
- Foch BJ, McDaniel ND, Chacko MR. Racial differences in vaginal douching knowledge, attitude, and practices among sexually active adolescents. J Pediatr Adolesc Gynecol. 2001;14:29-33. doi: 10.1016/S1083-3188(00)00080-2.
- Lin N, Ding N, Meza-Wilson E, et al. Volatile organic compounds in feminine hygiene products sold in the US market: a survey of products and health risks. Environ Int. 2020;144:105740. doi: 10.1016/j.envint.2020.105740.
- Wayne State University Digital Commons. Guy-Lee AK. Rituals reproducing race: African American women’s feminine hygiene practices, shared experiences, and power. 2017. http://digitalcommons.wayne.edu/oa_dissertations/1806. Accessed December 13, 2021.
- YouTube. OMV! by Vagisil—Intimate care products designed by teens. July 10, 2020. www.youtube.com/ watch?v=VkVsCagrAw0. Accessed December 13, 2021.
- Jenkins A, O’Doherty KC. The clean vagina, the healthy vagina, and the dirty vagina: exploring women’s portrayals of the vagina in relation to vaginal cleansing product use. Fem Psychol. 2021;31:192-211. doi: 10.1177/0959353520944144.
- Rajamanoharan S, Low N, Jones SB, et al. Bacterial vaginosis, ethnicity, and the use of genital cleansing agents: a case control study. Sex Transm Dis. 1999;26:404-409.
- Crann SE, Cunningham S, Albert A, et al. Vaginal health and hygiene practices and product use in Canada: a national cross-sectional survey. BMC Womens Health. 2018;18:52. doi: 10.1186/s12905-018-0543-y.
- Chen Y, Bruning E, Rubino J, et al. Role of female intimate hygiene in vulvovaginal health: global hygiene practices and product usage. Womens Health (London). 2017;13:58-67. doi: 10.1177/1745505717731011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd. v24i0.19703.
- Van der Veer C, Bruisten SM, Van Houdt R, et al. Effects of an over-the-counter lactic-acid containing intra-vaginal douching product on the vaginal microbiota. BMC Microbiol. 2019;19:168. doi: 10.1186/s12866-019-1545-0.
- Gondwe T, Ness R, Totten PA, et al. Novel bacterial vaginosis-associated organisms mediate the relationship between vaginal douching and pelvic inflammatory disease. Sex Transm Infect. 2020;96:439-444. doi: 10.1136/ sextrans-2019-054191.
Feminine hygiene products have been commercially available for decades. They are commonly marketed to reduce odor or clean vaginal discharge and menses. Multiple formulas are available as topical washes, wipes, creams, sprays, powders, deodorants, and douches.1 Products on the market range from those used externally on the vulva, such as wipes and sprays, to liquid solutions used intravaginally, such as washes and douches.
Who uses feminine hygiene products?
According to a 2006 study, the majority of women who use douches started using them between age 15 and 19 years, but some women initiate this practice habit as early as age 10 to 14.1 Predictably, women who douche are more likely to perceive douche products as safe.1
Demographic data on douche utilization are mixed: Some studies show that there are no significant racial differences in douching practices,2 while others have found that Black and African American women are more likely to practice douching than White and Hispanic women.1,3 Studies have shown a significant difference in attitudes toward douching and knowledge of normal vaginal symptoms among US racial demographics, although this must be examined through the historical context of racism and the lens of medical anthropology.4
Women cite that common reasons they use feminine hygiene products are to feel clean, to control odor, and to use after menses and intercourse.1,2
Modern marketing approaches
From wipes to soaps to douches, feminine hygiene products often are advertised to promote “funk-free periods”5 and “freshness,” fostering an environment in which women and men develop unrealistic standards for what is considered normal genital odor and resulting in poor body image.6
Recently, Vagisil (Combe Incorporated) marketing efforts faced backlash from the ObGyn community for targeting younger populations with a specific product line for adolescents called OMV! In addition, attention has been drawn to VCF vaginal odor eliminating film (Apothecus Pharmaceutical Corp), small stamp-sized dissolving films that are placed in the vaginal canal in contact with the epithelium. This product has entered the market of feminine hygiene products accompanied by slogans of eliminating “feminine odor” and providing “confidence for women to be intimate.”
Continue to: Effects of feminine hygiene products on the vaginal microbiome...
Effects of feminine hygiene products on the vaginal microbiome
Frequent use of feminine hygiene products has been associated with recurrent vaginitis, bacterial vaginosis, and general irritation/itch,7,8 which can cause more discharge and odor. Ironically, this may result in women using the product more frequently since they often seek out these products to eliminate odor and discharge.1,2
The pH of the vagina changes during a woman’s lifetime, but in the reproductive years, the normal pH range is typically 3.8 to 4.4.9 This range allows for a normal vaginal flora to form with bacteria such as Lactobacillus species and Gardnerella vaginalis, while feminine hygiene products have a wide range of pH.9,10
Regardless of the formulation, most feminine hygiene products contain ingredients and compositions that potentially are detrimental to the health of the vulva and vagina. Many products contain acidic ingredients, such as citric acid, lactic acid, and dehydroacetic acid, that can alter the vaginal pH and weaken the vaginal barrier by wiping out normal vaginal flora10 despite being advertised for use on “sensitive areas” (TABLE). Lactic acid also has been found to increase diverse anaerobic bacteria in the vaginal microbiome.11 Some feminine hygiene products have been shown to suppress Lactobacillus growth at 2 hours after use and to kill all lactobacilli at 24 hours.10 Shifts in microbiota numbers often occur when the vaginal pH has been altered, as is frequently the case with feminine hygiene products. In the absence of microbiome bacteria, the presence of vaginal hygiene products has been shown to increase interleukin-8 (IL-8), suggesting a proinflammatory reaction.10



A study in the United Kingdom found that women who used bubble bath, antiseptics, or douche products had a higher incidence of bacterial vaginosis compared with women who did not use such products.7 Women in Canada who used feminine hygiene products were more likely to report adverse conditions, including yeast infections, bacterial vaginosis, urinary tract infections, and sexually transmitted diseases.8 Furthermore, a significant association exists between vaginal douching and endometrial infection by bacterial vaginosis–associated organisms.12
Additionally, a study that analyzed volatile organic compound levels in the blood with the use of feminine hygiene products revealed a significant positive dose-exposure relationship between the frequency of vaginal douching in the last 6 months and concentrations of 1,4-dichloromethane, one of the volatile organic compounds.3 This points to the issue of not only disruption of pH and vaginal flora but also to the introduction of harmful substances that can further disrupt the vaginal barrier.
Understand the products to help educate patients
Use of feminine hygiene products is common among women. While women depend on the market to filter out products that are considered unsafe or may have harmful side effects,1 unfortunately that is not necessarily the case. With increasingly more feminine products on the market and the target demographic becoming younger, women of all ages are susceptible to misinformation that could affect their vaginal health long term.
It is vital that clinicians understand the topical effects of these products in order to properly educate and counsel patients. Ultimately, research on feminine hygiene products is limited and, as more products come to market, we must continue to reassess the effects of topical products on the vaginal epithelium and vulvar tissues. ●
Feminine hygiene products have been commercially available for decades. They are commonly marketed to reduce odor or clean vaginal discharge and menses. Multiple formulas are available as topical washes, wipes, creams, sprays, powders, deodorants, and douches.1 Products on the market range from those used externally on the vulva, such as wipes and sprays, to liquid solutions used intravaginally, such as washes and douches.
Who uses feminine hygiene products?
According to a 2006 study, the majority of women who use douches started using them between age 15 and 19 years, but some women initiate this practice habit as early as age 10 to 14.1 Predictably, women who douche are more likely to perceive douche products as safe.1
Demographic data on douche utilization are mixed: Some studies show that there are no significant racial differences in douching practices,2 while others have found that Black and African American women are more likely to practice douching than White and Hispanic women.1,3 Studies have shown a significant difference in attitudes toward douching and knowledge of normal vaginal symptoms among US racial demographics, although this must be examined through the historical context of racism and the lens of medical anthropology.4
Women cite that common reasons they use feminine hygiene products are to feel clean, to control odor, and to use after menses and intercourse.1,2
Modern marketing approaches
From wipes to soaps to douches, feminine hygiene products often are advertised to promote “funk-free periods”5 and “freshness,” fostering an environment in which women and men develop unrealistic standards for what is considered normal genital odor and resulting in poor body image.6
Recently, Vagisil (Combe Incorporated) marketing efforts faced backlash from the ObGyn community for targeting younger populations with a specific product line for adolescents called OMV! In addition, attention has been drawn to VCF vaginal odor eliminating film (Apothecus Pharmaceutical Corp), small stamp-sized dissolving films that are placed in the vaginal canal in contact with the epithelium. This product has entered the market of feminine hygiene products accompanied by slogans of eliminating “feminine odor” and providing “confidence for women to be intimate.”
Continue to: Effects of feminine hygiene products on the vaginal microbiome...
Effects of feminine hygiene products on the vaginal microbiome
Frequent use of feminine hygiene products has been associated with recurrent vaginitis, bacterial vaginosis, and general irritation/itch,7,8 which can cause more discharge and odor. Ironically, this may result in women using the product more frequently since they often seek out these products to eliminate odor and discharge.1,2
The pH of the vagina changes during a woman’s lifetime, but in the reproductive years, the normal pH range is typically 3.8 to 4.4.9 This range allows for a normal vaginal flora to form with bacteria such as Lactobacillus species and Gardnerella vaginalis, while feminine hygiene products have a wide range of pH.9,10
Regardless of the formulation, most feminine hygiene products contain ingredients and compositions that potentially are detrimental to the health of the vulva and vagina. Many products contain acidic ingredients, such as citric acid, lactic acid, and dehydroacetic acid, that can alter the vaginal pH and weaken the vaginal barrier by wiping out normal vaginal flora10 despite being advertised for use on “sensitive areas” (TABLE). Lactic acid also has been found to increase diverse anaerobic bacteria in the vaginal microbiome.11 Some feminine hygiene products have been shown to suppress Lactobacillus growth at 2 hours after use and to kill all lactobacilli at 24 hours.10 Shifts in microbiota numbers often occur when the vaginal pH has been altered, as is frequently the case with feminine hygiene products. In the absence of microbiome bacteria, the presence of vaginal hygiene products has been shown to increase interleukin-8 (IL-8), suggesting a proinflammatory reaction.10



A study in the United Kingdom found that women who used bubble bath, antiseptics, or douche products had a higher incidence of bacterial vaginosis compared with women who did not use such products.7 Women in Canada who used feminine hygiene products were more likely to report adverse conditions, including yeast infections, bacterial vaginosis, urinary tract infections, and sexually transmitted diseases.8 Furthermore, a significant association exists between vaginal douching and endometrial infection by bacterial vaginosis–associated organisms.12
Additionally, a study that analyzed volatile organic compound levels in the blood with the use of feminine hygiene products revealed a significant positive dose-exposure relationship between the frequency of vaginal douching in the last 6 months and concentrations of 1,4-dichloromethane, one of the volatile organic compounds.3 This points to the issue of not only disruption of pH and vaginal flora but also to the introduction of harmful substances that can further disrupt the vaginal barrier.
Understand the products to help educate patients
Use of feminine hygiene products is common among women. While women depend on the market to filter out products that are considered unsafe or may have harmful side effects,1 unfortunately that is not necessarily the case. With increasingly more feminine products on the market and the target demographic becoming younger, women of all ages are susceptible to misinformation that could affect their vaginal health long term.
It is vital that clinicians understand the topical effects of these products in order to properly educate and counsel patients. Ultimately, research on feminine hygiene products is limited and, as more products come to market, we must continue to reassess the effects of topical products on the vaginal epithelium and vulvar tissues. ●
- Grimley DM, Annang L, Foushee HR, et al. Vaginal douches and other feminine hygiene products: women’s practices and perceptions of product safety. Matern Child Health J. 2006;10:303-310. doi: 10.1007/s10995-005-0054-y.
- Foch BJ, McDaniel ND, Chacko MR. Racial differences in vaginal douching knowledge, attitude, and practices among sexually active adolescents. J Pediatr Adolesc Gynecol. 2001;14:29-33. doi: 10.1016/S1083-3188(00)00080-2.
- Lin N, Ding N, Meza-Wilson E, et al. Volatile organic compounds in feminine hygiene products sold in the US market: a survey of products and health risks. Environ Int. 2020;144:105740. doi: 10.1016/j.envint.2020.105740.
- Wayne State University Digital Commons. Guy-Lee AK. Rituals reproducing race: African American women’s feminine hygiene practices, shared experiences, and power. 2017. http://digitalcommons.wayne.edu/oa_dissertations/1806. Accessed December 13, 2021.
- YouTube. OMV! by Vagisil—Intimate care products designed by teens. July 10, 2020. www.youtube.com/ watch?v=VkVsCagrAw0. Accessed December 13, 2021.
- Jenkins A, O’Doherty KC. The clean vagina, the healthy vagina, and the dirty vagina: exploring women’s portrayals of the vagina in relation to vaginal cleansing product use. Fem Psychol. 2021;31:192-211. doi: 10.1177/0959353520944144.
- Rajamanoharan S, Low N, Jones SB, et al. Bacterial vaginosis, ethnicity, and the use of genital cleansing agents: a case control study. Sex Transm Dis. 1999;26:404-409.
- Crann SE, Cunningham S, Albert A, et al. Vaginal health and hygiene practices and product use in Canada: a national cross-sectional survey. BMC Womens Health. 2018;18:52. doi: 10.1186/s12905-018-0543-y.
- Chen Y, Bruning E, Rubino J, et al. Role of female intimate hygiene in vulvovaginal health: global hygiene practices and product usage. Womens Health (London). 2017;13:58-67. doi: 10.1177/1745505717731011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd. v24i0.19703.
- Van der Veer C, Bruisten SM, Van Houdt R, et al. Effects of an over-the-counter lactic-acid containing intra-vaginal douching product on the vaginal microbiota. BMC Microbiol. 2019;19:168. doi: 10.1186/s12866-019-1545-0.
- Gondwe T, Ness R, Totten PA, et al. Novel bacterial vaginosis-associated organisms mediate the relationship between vaginal douching and pelvic inflammatory disease. Sex Transm Infect. 2020;96:439-444. doi: 10.1136/ sextrans-2019-054191.
- Grimley DM, Annang L, Foushee HR, et al. Vaginal douches and other feminine hygiene products: women’s practices and perceptions of product safety. Matern Child Health J. 2006;10:303-310. doi: 10.1007/s10995-005-0054-y.
- Foch BJ, McDaniel ND, Chacko MR. Racial differences in vaginal douching knowledge, attitude, and practices among sexually active adolescents. J Pediatr Adolesc Gynecol. 2001;14:29-33. doi: 10.1016/S1083-3188(00)00080-2.
- Lin N, Ding N, Meza-Wilson E, et al. Volatile organic compounds in feminine hygiene products sold in the US market: a survey of products and health risks. Environ Int. 2020;144:105740. doi: 10.1016/j.envint.2020.105740.
- Wayne State University Digital Commons. Guy-Lee AK. Rituals reproducing race: African American women’s feminine hygiene practices, shared experiences, and power. 2017. http://digitalcommons.wayne.edu/oa_dissertations/1806. Accessed December 13, 2021.
- YouTube. OMV! by Vagisil—Intimate care products designed by teens. July 10, 2020. www.youtube.com/ watch?v=VkVsCagrAw0. Accessed December 13, 2021.
- Jenkins A, O’Doherty KC. The clean vagina, the healthy vagina, and the dirty vagina: exploring women’s portrayals of the vagina in relation to vaginal cleansing product use. Fem Psychol. 2021;31:192-211. doi: 10.1177/0959353520944144.
- Rajamanoharan S, Low N, Jones SB, et al. Bacterial vaginosis, ethnicity, and the use of genital cleansing agents: a case control study. Sex Transm Dis. 1999;26:404-409.
- Crann SE, Cunningham S, Albert A, et al. Vaginal health and hygiene practices and product use in Canada: a national cross-sectional survey. BMC Womens Health. 2018;18:52. doi: 10.1186/s12905-018-0543-y.
- Chen Y, Bruning E, Rubino J, et al. Role of female intimate hygiene in vulvovaginal health: global hygiene practices and product usage. Womens Health (London). 2017;13:58-67. doi: 10.1177/1745505717731011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd. v24i0.19703.
- Van der Veer C, Bruisten SM, Van Houdt R, et al. Effects of an over-the-counter lactic-acid containing intra-vaginal douching product on the vaginal microbiota. BMC Microbiol. 2019;19:168. doi: 10.1186/s12866-019-1545-0.
- Gondwe T, Ness R, Totten PA, et al. Novel bacterial vaginosis-associated organisms mediate the relationship between vaginal douching and pelvic inflammatory disease. Sex Transm Infect. 2020;96:439-444. doi: 10.1136/ sextrans-2019-054191.
Increased access to LARC may improve birth outcomes
Policies increasing access to immediate postpartum long-acting reversible contraception (LARC) were associated with reductions in preterm birth and low birth weight, based on data from South Carolina’s Medicaid program.
Preterm birth and low birth weight represent the second-leading cause of infant mortality in the United States, wrote Maria W. Steenland, SD, of Brown University, Providence, R.I., and colleagues. Previous policy interventions to reduce preterm birth and low birth weight have focused on services before and during pregnancy, they said. LARC is a safe and effective postpartum intervention, but cost has been a limiting factor, they noted.
In 2012, the Medicaid program in South Carolina began reimbursing hospitals for immediate postpartum LARC independent of global maternity payments. In a previous study, the researchers found that the implementation of this policy had reduced the number of short-interval births among adolescents.
The goal of the current study, published in JAMA Pediatrics, was to analyze the association between South Carolina’s policy change and rates of preterm birth and low birth weight among individuals with Medicaid coverage during childbirth. The researchers analyzed data from 186,953 Medicaid-paid births between January 2009 and December 2015 in South Carolina. Of these, 46,414 births (24.8%) occurred in hospitals that provided immediate postpartum LARC in response to the policy change. Overall, the implementing hospitals had more annual births paid for by Medicaid compared to nonimplementing hospitals (1,105 vs. 511) and were less likely to be rural (33.3% vs. 46.8%) and had a greater share of preterm births (15.5% vs. 9.5%). Prior to the policy change, the probability of a preterm birth in the next 4 years was 4.4% for patients at implementing hospitals and 3.5% for those in nonimplementing hospitals, and the probability of a low-birth-weight birth was 3.6% and 2.9%, respectively.
The policy change was associated with a decrease of 0.4 percentage points for preterm birth and 0.3 percentage points for subsequent low-birth-weight birth.
When the results were stratified based on race and ethnicity, the policy change was associated with a decrease of 0.5 percentage points in the probability of preterm birth in both non-Hispanic Blacks and non-Hispanic Whites. No significant differences appeared in the association between the policy change and rates of preterm birth or low-birth-weight birth between non-Hispanic Black and non-Hispanic White individuals.
However, the policy was associated with a significant decrease of 0.6 percentage points in the probability of short-interval birth among non-Hispanic Blacks, and a decrease of 1.6 percentage points in the probability of another birth within 4 years overall. The policy change also was associated with a significant increase of 27 days between births among non-Hispanic Blacks, but not with any significant change among non-Hispanic Whites or the study population overall.
“In addition, although our data cannot speak to this, the policy may have affected the intendedness of subsequent pregnancies, leading to healthier behaviors before and during pregnancy, such as early initiation of prenatal care,” the researchers wrote in their discussion of the findings.
The study findings were limited by several factors including the lack of data on pregnancy intention or abortion, and the lack of data on patient-reported outcomes, notably the provision of patient-centered counseling and whether such counseling was biased, the researchers noted. Other limitations included a lack of data on infant mortality and potential confounding from risk profiles of patients in implementing vs. nonimplementing hospitals, they wrote.
Also, the study provides population-level data, which does not guide clinical decision-making about intervals between childbirth and subsequent pregnancy, the researchers emphasized.
Although the data support the value of postpartum contraception in improving birth outcomes, “it is imperative that efforts to expand access focus on assuring comprehensive access to all forms of contraception without coercion,” the researchers concluded. “Additional policy solutions are needed to improve infant health, including those that directly address structural and interpersonal racism to reduce racial disparities in infant health,” they said.
The study is important because, although immediate postpartum LARC policies were first implemented almost a decade ago in the United States, population-level evidence on the effects of these policies remains scarce, Dr. Steenland said in an interview.
Existing barriers to improving access to immediate postpartum LARC include health professional training and logistics within hospitals, as well as ensuring correct billing and timely reimbursements, Dr. Steenland said. “Simple and clear billing procedures, and advanced reimbursement so that hospitals can have devices stocked would make it easier to provide this service,” she noted.
“This service has gone from being almost completely unavailable, to available in some hospitals, mainly those that are urban, teaching, and high volume,” said Dr. Steenland. “Additional research is needed to determine how health systems can make this service available to all birthing persons,” she said. “Also, critically, additional research is needed to identify strategies to ensure that counseling for immediate postpartum LARC, and family planning more generally, is patient-centered, so that the availability of immediate postpartum LARC increases, rather than restricts, choice,” she added. “Finally, additional research is needed to determine whether postpartum people have affordable and accessible access to LARC removal services,” Dr. Steenland emphasized.
Immediate post partum is critical period
The immediate postpartum period is a critical time for access to contraception because many women do not return for postpartum visits after hospital discharge, Tracey A. Wilkinson, MD, and Jeffrey F. Peipert, MD, of Indiana University, Indianapolis, wrote in an accompanying editorial. “The focus on contraception access during the postpartum period prior to hospital discharge is important because of the potential sequelae of a subsequent unintended pregnancy or short interpregnancy intervals,” they noted. These issues may be more acute in marginalized communities, and policies to expand immediate postpartum LARC are in place in a majority of states, the editorialists said.
However, they agreed with the authors’ statements that implementation of LARC must be done in a manner that supports patient choice and avoids coercion. Given the baseline disparities of the infant outcomes studied, increased access to immediate postpartum LARC must be provided in a way that does not exacerbate these disparities, they said. “This ultimately means that plans to increase access to contraception should emphasize availability while avoiding coercion, and if a patient ultimately decides to discontinue a method, enable that to occur easily and seamlessly, including LARC device removal,” they explained.
“Future studies examining patient centeredness of these postpartum LARC implementation efforts would be an important element to augment these data and show the impact in additional spheres beyond infant outcomes,” they added.
Overcome trust barriers and offer options
“In a time of restrictive access to abortion and contraception in many states, any additional increase in access can potentially be meaningful,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. “Additionally, given the significantly higher rates of infant and maternal morbidity and mortality among the non-Hispanic Black population, seeing an intervention that can improve outcomes for both mothers and babies is also potentially very positive,” she said.
Dr. Prager said she was not surprised by the study findings, as immediate LARC is much more common in other countries and has shown similar outcomes. “Additionally, I am reassured by the fact that the increased number of days until the next pregnancy is not higher, as this indirectly indicates that patients were able to get their LARC removed when they desired another pregnancy,” she noted.
Barriers to improving access to immediate postpartum LARC in the Medicaid population may include mistrust for any long-acting contraception, “especially if they perceive that cessation of the method will be difficult to achieve,” Dr. Prager noted. “Certainly, counseling about LARC removal should be an element of counseling prior to any initiation, and lack of access to removal of an IUD or implant can be categorized as a form of reproductive coercion,” she said. Dr. Prager said that such counseling might be more effective if it occurred during prenatal visits, “so if providers are not talking about this during routine OB visits and patients only hear about immediate postpartum LARC when they are in the hospital for delivery, they may be less likely to accept the practice,” she said. “Finally, although Medicaid will cover the cost of immediate postpartum LARC, private insurers do not do so consistently in all states, so some hospitals may find this process too difficult to navigate and therefore not offer immediate postpartum LARC,” Dr. Prager emphasized.
As for additional research, Dr. Prager said she would like to see more studies in an overall United States population of pregnant people, both Medicaid patients and others, on whether the immediate postpartum timing of LARC is desired.
“I would like to couple that with patients’ impressions or experiences of their ability to access contraception outside of the immediate postpartum time period, and also their impressions or experience with ability to have LARC removed, since they are the only contraceptives not necessarily within personal control for initiation or cessation,” Dr. Prager said.
The study was supported by the National Institute for Child Health and Development, and lead author Dr. Steenland received support from other National Institutes of Health grants. The researchers had no financial conflicts to disclose. The editorial was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Peipert disclosed serving on advisory boards for Bayer and CooperSurgical, and receiving research support from Merck, Bayer, and CooperSurgical/Teva. Dr. Wilkinson had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn. News.
Policies increasing access to immediate postpartum long-acting reversible contraception (LARC) were associated with reductions in preterm birth and low birth weight, based on data from South Carolina’s Medicaid program.
Preterm birth and low birth weight represent the second-leading cause of infant mortality in the United States, wrote Maria W. Steenland, SD, of Brown University, Providence, R.I., and colleagues. Previous policy interventions to reduce preterm birth and low birth weight have focused on services before and during pregnancy, they said. LARC is a safe and effective postpartum intervention, but cost has been a limiting factor, they noted.
In 2012, the Medicaid program in South Carolina began reimbursing hospitals for immediate postpartum LARC independent of global maternity payments. In a previous study, the researchers found that the implementation of this policy had reduced the number of short-interval births among adolescents.
The goal of the current study, published in JAMA Pediatrics, was to analyze the association between South Carolina’s policy change and rates of preterm birth and low birth weight among individuals with Medicaid coverage during childbirth. The researchers analyzed data from 186,953 Medicaid-paid births between January 2009 and December 2015 in South Carolina. Of these, 46,414 births (24.8%) occurred in hospitals that provided immediate postpartum LARC in response to the policy change. Overall, the implementing hospitals had more annual births paid for by Medicaid compared to nonimplementing hospitals (1,105 vs. 511) and were less likely to be rural (33.3% vs. 46.8%) and had a greater share of preterm births (15.5% vs. 9.5%). Prior to the policy change, the probability of a preterm birth in the next 4 years was 4.4% for patients at implementing hospitals and 3.5% for those in nonimplementing hospitals, and the probability of a low-birth-weight birth was 3.6% and 2.9%, respectively.
The policy change was associated with a decrease of 0.4 percentage points for preterm birth and 0.3 percentage points for subsequent low-birth-weight birth.
When the results were stratified based on race and ethnicity, the policy change was associated with a decrease of 0.5 percentage points in the probability of preterm birth in both non-Hispanic Blacks and non-Hispanic Whites. No significant differences appeared in the association between the policy change and rates of preterm birth or low-birth-weight birth between non-Hispanic Black and non-Hispanic White individuals.
However, the policy was associated with a significant decrease of 0.6 percentage points in the probability of short-interval birth among non-Hispanic Blacks, and a decrease of 1.6 percentage points in the probability of another birth within 4 years overall. The policy change also was associated with a significant increase of 27 days between births among non-Hispanic Blacks, but not with any significant change among non-Hispanic Whites or the study population overall.
“In addition, although our data cannot speak to this, the policy may have affected the intendedness of subsequent pregnancies, leading to healthier behaviors before and during pregnancy, such as early initiation of prenatal care,” the researchers wrote in their discussion of the findings.
The study findings were limited by several factors including the lack of data on pregnancy intention or abortion, and the lack of data on patient-reported outcomes, notably the provision of patient-centered counseling and whether such counseling was biased, the researchers noted. Other limitations included a lack of data on infant mortality and potential confounding from risk profiles of patients in implementing vs. nonimplementing hospitals, they wrote.
Also, the study provides population-level data, which does not guide clinical decision-making about intervals between childbirth and subsequent pregnancy, the researchers emphasized.
Although the data support the value of postpartum contraception in improving birth outcomes, “it is imperative that efforts to expand access focus on assuring comprehensive access to all forms of contraception without coercion,” the researchers concluded. “Additional policy solutions are needed to improve infant health, including those that directly address structural and interpersonal racism to reduce racial disparities in infant health,” they said.
The study is important because, although immediate postpartum LARC policies were first implemented almost a decade ago in the United States, population-level evidence on the effects of these policies remains scarce, Dr. Steenland said in an interview.
Existing barriers to improving access to immediate postpartum LARC include health professional training and logistics within hospitals, as well as ensuring correct billing and timely reimbursements, Dr. Steenland said. “Simple and clear billing procedures, and advanced reimbursement so that hospitals can have devices stocked would make it easier to provide this service,” she noted.
“This service has gone from being almost completely unavailable, to available in some hospitals, mainly those that are urban, teaching, and high volume,” said Dr. Steenland. “Additional research is needed to determine how health systems can make this service available to all birthing persons,” she said. “Also, critically, additional research is needed to identify strategies to ensure that counseling for immediate postpartum LARC, and family planning more generally, is patient-centered, so that the availability of immediate postpartum LARC increases, rather than restricts, choice,” she added. “Finally, additional research is needed to determine whether postpartum people have affordable and accessible access to LARC removal services,” Dr. Steenland emphasized.
Immediate post partum is critical period
The immediate postpartum period is a critical time for access to contraception because many women do not return for postpartum visits after hospital discharge, Tracey A. Wilkinson, MD, and Jeffrey F. Peipert, MD, of Indiana University, Indianapolis, wrote in an accompanying editorial. “The focus on contraception access during the postpartum period prior to hospital discharge is important because of the potential sequelae of a subsequent unintended pregnancy or short interpregnancy intervals,” they noted. These issues may be more acute in marginalized communities, and policies to expand immediate postpartum LARC are in place in a majority of states, the editorialists said.
However, they agreed with the authors’ statements that implementation of LARC must be done in a manner that supports patient choice and avoids coercion. Given the baseline disparities of the infant outcomes studied, increased access to immediate postpartum LARC must be provided in a way that does not exacerbate these disparities, they said. “This ultimately means that plans to increase access to contraception should emphasize availability while avoiding coercion, and if a patient ultimately decides to discontinue a method, enable that to occur easily and seamlessly, including LARC device removal,” they explained.
“Future studies examining patient centeredness of these postpartum LARC implementation efforts would be an important element to augment these data and show the impact in additional spheres beyond infant outcomes,” they added.
Overcome trust barriers and offer options
“In a time of restrictive access to abortion and contraception in many states, any additional increase in access can potentially be meaningful,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. “Additionally, given the significantly higher rates of infant and maternal morbidity and mortality among the non-Hispanic Black population, seeing an intervention that can improve outcomes for both mothers and babies is also potentially very positive,” she said.
Dr. Prager said she was not surprised by the study findings, as immediate LARC is much more common in other countries and has shown similar outcomes. “Additionally, I am reassured by the fact that the increased number of days until the next pregnancy is not higher, as this indirectly indicates that patients were able to get their LARC removed when they desired another pregnancy,” she noted.
Barriers to improving access to immediate postpartum LARC in the Medicaid population may include mistrust for any long-acting contraception, “especially if they perceive that cessation of the method will be difficult to achieve,” Dr. Prager noted. “Certainly, counseling about LARC removal should be an element of counseling prior to any initiation, and lack of access to removal of an IUD or implant can be categorized as a form of reproductive coercion,” she said. Dr. Prager said that such counseling might be more effective if it occurred during prenatal visits, “so if providers are not talking about this during routine OB visits and patients only hear about immediate postpartum LARC when they are in the hospital for delivery, they may be less likely to accept the practice,” she said. “Finally, although Medicaid will cover the cost of immediate postpartum LARC, private insurers do not do so consistently in all states, so some hospitals may find this process too difficult to navigate and therefore not offer immediate postpartum LARC,” Dr. Prager emphasized.
As for additional research, Dr. Prager said she would like to see more studies in an overall United States population of pregnant people, both Medicaid patients and others, on whether the immediate postpartum timing of LARC is desired.
“I would like to couple that with patients’ impressions or experiences of their ability to access contraception outside of the immediate postpartum time period, and also their impressions or experience with ability to have LARC removed, since they are the only contraceptives not necessarily within personal control for initiation or cessation,” Dr. Prager said.
The study was supported by the National Institute for Child Health and Development, and lead author Dr. Steenland received support from other National Institutes of Health grants. The researchers had no financial conflicts to disclose. The editorial was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Peipert disclosed serving on advisory boards for Bayer and CooperSurgical, and receiving research support from Merck, Bayer, and CooperSurgical/Teva. Dr. Wilkinson had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn. News.
Policies increasing access to immediate postpartum long-acting reversible contraception (LARC) were associated with reductions in preterm birth and low birth weight, based on data from South Carolina’s Medicaid program.
Preterm birth and low birth weight represent the second-leading cause of infant mortality in the United States, wrote Maria W. Steenland, SD, of Brown University, Providence, R.I., and colleagues. Previous policy interventions to reduce preterm birth and low birth weight have focused on services before and during pregnancy, they said. LARC is a safe and effective postpartum intervention, but cost has been a limiting factor, they noted.
In 2012, the Medicaid program in South Carolina began reimbursing hospitals for immediate postpartum LARC independent of global maternity payments. In a previous study, the researchers found that the implementation of this policy had reduced the number of short-interval births among adolescents.
The goal of the current study, published in JAMA Pediatrics, was to analyze the association between South Carolina’s policy change and rates of preterm birth and low birth weight among individuals with Medicaid coverage during childbirth. The researchers analyzed data from 186,953 Medicaid-paid births between January 2009 and December 2015 in South Carolina. Of these, 46,414 births (24.8%) occurred in hospitals that provided immediate postpartum LARC in response to the policy change. Overall, the implementing hospitals had more annual births paid for by Medicaid compared to nonimplementing hospitals (1,105 vs. 511) and were less likely to be rural (33.3% vs. 46.8%) and had a greater share of preterm births (15.5% vs. 9.5%). Prior to the policy change, the probability of a preterm birth in the next 4 years was 4.4% for patients at implementing hospitals and 3.5% for those in nonimplementing hospitals, and the probability of a low-birth-weight birth was 3.6% and 2.9%, respectively.
The policy change was associated with a decrease of 0.4 percentage points for preterm birth and 0.3 percentage points for subsequent low-birth-weight birth.
When the results were stratified based on race and ethnicity, the policy change was associated with a decrease of 0.5 percentage points in the probability of preterm birth in both non-Hispanic Blacks and non-Hispanic Whites. No significant differences appeared in the association between the policy change and rates of preterm birth or low-birth-weight birth between non-Hispanic Black and non-Hispanic White individuals.
However, the policy was associated with a significant decrease of 0.6 percentage points in the probability of short-interval birth among non-Hispanic Blacks, and a decrease of 1.6 percentage points in the probability of another birth within 4 years overall. The policy change also was associated with a significant increase of 27 days between births among non-Hispanic Blacks, but not with any significant change among non-Hispanic Whites or the study population overall.
“In addition, although our data cannot speak to this, the policy may have affected the intendedness of subsequent pregnancies, leading to healthier behaviors before and during pregnancy, such as early initiation of prenatal care,” the researchers wrote in their discussion of the findings.
The study findings were limited by several factors including the lack of data on pregnancy intention or abortion, and the lack of data on patient-reported outcomes, notably the provision of patient-centered counseling and whether such counseling was biased, the researchers noted. Other limitations included a lack of data on infant mortality and potential confounding from risk profiles of patients in implementing vs. nonimplementing hospitals, they wrote.
Also, the study provides population-level data, which does not guide clinical decision-making about intervals between childbirth and subsequent pregnancy, the researchers emphasized.
Although the data support the value of postpartum contraception in improving birth outcomes, “it is imperative that efforts to expand access focus on assuring comprehensive access to all forms of contraception without coercion,” the researchers concluded. “Additional policy solutions are needed to improve infant health, including those that directly address structural and interpersonal racism to reduce racial disparities in infant health,” they said.
The study is important because, although immediate postpartum LARC policies were first implemented almost a decade ago in the United States, population-level evidence on the effects of these policies remains scarce, Dr. Steenland said in an interview.
Existing barriers to improving access to immediate postpartum LARC include health professional training and logistics within hospitals, as well as ensuring correct billing and timely reimbursements, Dr. Steenland said. “Simple and clear billing procedures, and advanced reimbursement so that hospitals can have devices stocked would make it easier to provide this service,” she noted.
“This service has gone from being almost completely unavailable, to available in some hospitals, mainly those that are urban, teaching, and high volume,” said Dr. Steenland. “Additional research is needed to determine how health systems can make this service available to all birthing persons,” she said. “Also, critically, additional research is needed to identify strategies to ensure that counseling for immediate postpartum LARC, and family planning more generally, is patient-centered, so that the availability of immediate postpartum LARC increases, rather than restricts, choice,” she added. “Finally, additional research is needed to determine whether postpartum people have affordable and accessible access to LARC removal services,” Dr. Steenland emphasized.
Immediate post partum is critical period
The immediate postpartum period is a critical time for access to contraception because many women do not return for postpartum visits after hospital discharge, Tracey A. Wilkinson, MD, and Jeffrey F. Peipert, MD, of Indiana University, Indianapolis, wrote in an accompanying editorial. “The focus on contraception access during the postpartum period prior to hospital discharge is important because of the potential sequelae of a subsequent unintended pregnancy or short interpregnancy intervals,” they noted. These issues may be more acute in marginalized communities, and policies to expand immediate postpartum LARC are in place in a majority of states, the editorialists said.
However, they agreed with the authors’ statements that implementation of LARC must be done in a manner that supports patient choice and avoids coercion. Given the baseline disparities of the infant outcomes studied, increased access to immediate postpartum LARC must be provided in a way that does not exacerbate these disparities, they said. “This ultimately means that plans to increase access to contraception should emphasize availability while avoiding coercion, and if a patient ultimately decides to discontinue a method, enable that to occur easily and seamlessly, including LARC device removal,” they explained.
“Future studies examining patient centeredness of these postpartum LARC implementation efforts would be an important element to augment these data and show the impact in additional spheres beyond infant outcomes,” they added.
Overcome trust barriers and offer options
“In a time of restrictive access to abortion and contraception in many states, any additional increase in access can potentially be meaningful,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. “Additionally, given the significantly higher rates of infant and maternal morbidity and mortality among the non-Hispanic Black population, seeing an intervention that can improve outcomes for both mothers and babies is also potentially very positive,” she said.
Dr. Prager said she was not surprised by the study findings, as immediate LARC is much more common in other countries and has shown similar outcomes. “Additionally, I am reassured by the fact that the increased number of days until the next pregnancy is not higher, as this indirectly indicates that patients were able to get their LARC removed when they desired another pregnancy,” she noted.
Barriers to improving access to immediate postpartum LARC in the Medicaid population may include mistrust for any long-acting contraception, “especially if they perceive that cessation of the method will be difficult to achieve,” Dr. Prager noted. “Certainly, counseling about LARC removal should be an element of counseling prior to any initiation, and lack of access to removal of an IUD or implant can be categorized as a form of reproductive coercion,” she said. Dr. Prager said that such counseling might be more effective if it occurred during prenatal visits, “so if providers are not talking about this during routine OB visits and patients only hear about immediate postpartum LARC when they are in the hospital for delivery, they may be less likely to accept the practice,” she said. “Finally, although Medicaid will cover the cost of immediate postpartum LARC, private insurers do not do so consistently in all states, so some hospitals may find this process too difficult to navigate and therefore not offer immediate postpartum LARC,” Dr. Prager emphasized.
As for additional research, Dr. Prager said she would like to see more studies in an overall United States population of pregnant people, both Medicaid patients and others, on whether the immediate postpartum timing of LARC is desired.
“I would like to couple that with patients’ impressions or experiences of their ability to access contraception outside of the immediate postpartum time period, and also their impressions or experience with ability to have LARC removed, since they are the only contraceptives not necessarily within personal control for initiation or cessation,” Dr. Prager said.
The study was supported by the National Institute for Child Health and Development, and lead author Dr. Steenland received support from other National Institutes of Health grants. The researchers had no financial conflicts to disclose. The editorial was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Peipert disclosed serving on advisory boards for Bayer and CooperSurgical, and receiving research support from Merck, Bayer, and CooperSurgical/Teva. Dr. Wilkinson had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn. News.
FROM JAMA PEDIATRICS
Symptoms common in high-risk, early-stage ovarian cancer
A new study offers fresh insight into early indications of high-risk, early-stage, epithelial ovarian cancer: More than 70% have at least one symptom such as abdominal/pelvic pain or increased girth/fullness, and women with larger tumors have more symptoms.
“Even in early-stage disease, ovarian cancer is not necessarily a silent disease,” said lead author and gynecologic oncologist/surgeon John K. Chan, MD, of Palo Alto Medical Foundation/California Pacific/Sutter Research Institute.
The study appeared online Jan. 6, in the journal Obstetrics & Gynecology.*
According to Dr. Chan, most previous studies of symptoms in ovarian cancer have focused on those with advanced disease since that’s when it’s typically diagnosed. “Given these gaps in knowledge from prior reports, we performed this analysis to evaluate the presentation and characteristic symptoms of early-stage ovarian cancer and to attempt to identify the relationship between these symptoms with respect to clinicopathologic characteristics and prognosis in early-stage disease.”
Dr. Chan and colleagues retrospectively tracked 419 patients who were subjects in a clinical trial of chemotherapy doses. The patients all had high-risk, early-stage epithelial ovarian cancer (stage IA-IB and grade 3, any clear cell, stage IC or II).
Of the patients, 40% presented with one symptom, while 32% had multiple symptoms. The other 28% had no symptoms, and their masses were diagnosed upon discovery during physical examination. “Other investigators have found that nearly 95% of patients with ovarian cancer were symptomatic,” Dr. Chan said. “The lower percentage of symptomatic patients in our study may be because all 419 patients had early-stage disease as opposed to advanced-stage disease.”
The most common symptoms were abdominal or pelvic pain (31%; 95% confidence interval, 27%-36%), fullness or increased abdominal girth (27%; 95% CI, 22%-31%), abnormal vaginal bleeding (13%; 95% CI, 10%-17%), urinary problems (10%; 95% CI, 8%-14%), and gastrointestinal problems (6%; 95% CI, 4%-8%).
There was no statistically significant link between number of symptoms and age (younger than 60 or 60 or older), cancer stage, or histologic subtype. However, patients with the largest tumors (>15 cm) were more likely to have multiple symptoms than those with the smallest tumors (10 cm or smaller): 46% vs. 21% (P < .001).
Also, 79% of those with the largest tumors (>15 cm) had at least one symptom, compared with 65% of those with the smallest tumors (10 cm or smaller, P < .001)
Unlike other studies, this report didn’t find a link between the number of symptoms and mortality. This finding surprised the researchers, Dr. Chan said, as did the lack of connections between symptoms and age, stage, or histologic subtype. “We were expecting that the younger patients may have more symptoms given the association with endometriosis and clear cell cancers,” he said. “We also thought that those who are less symptomatic may have more stage I and low-grade indolent tumors with better survival, but we did not find that.”
The researchers noted limitations such as the lack of standardization in the patient data.
In the big picture, Dr. Chan said, “patients and health care professionals need to have a higher index of suspicion in symptomatic ovarian cancer patients to increase early detection and potentially improve cures. Ovarian cancer does not always kill. In fact, up to 80% of our early-stage disease patients are cured.”
He called for “additional research to evaluate symptom awareness in early-stage cancers and possibly incorporating novel serum biomarkers and wearable monitoring devices. Wearables may be able to assess for frequency or duration of symptoms, which may be an important factor in distinguishing symptoms that are more concerning for ovarian cancer.”
In an adjoining commentary, Barbara A. Goff, MD, chair of obstetrics and gynecology at the University of Washington, Seattle, noted that, while ovarian cancers diagnosed early have a high survival rate, prospective randomized trials of transvaginal ultrasonography and tumor marker screening strategies have failed to reduce mortality. There’s currently no recommended screening test for women at average risk.
There are other challenges, she wrote. For one, “many health care professionals are seemingly unaware of the symptoms typically associated with ovarian cancer, so misdiagnosis remains common.” And “one of the concerns about the symptoms of ovarian cancer is that they can be vague and commonly present in the general population.”
Dr. Goff praised the study, called for more education about the symptoms of ovarian cancer, and wrote that “symptom recognition with appropriate diagnostic testing remains very important in our efforts to improve outcomes.”
The National Institutes of Health funded the study. Several study authors, including Dr. Chan, reported various disclosures.
Correction, 1/31/22: An earlier version of this article misstated the date of publication.
A new study offers fresh insight into early indications of high-risk, early-stage, epithelial ovarian cancer: More than 70% have at least one symptom such as abdominal/pelvic pain or increased girth/fullness, and women with larger tumors have more symptoms.
“Even in early-stage disease, ovarian cancer is not necessarily a silent disease,” said lead author and gynecologic oncologist/surgeon John K. Chan, MD, of Palo Alto Medical Foundation/California Pacific/Sutter Research Institute.
The study appeared online Jan. 6, in the journal Obstetrics & Gynecology.*
According to Dr. Chan, most previous studies of symptoms in ovarian cancer have focused on those with advanced disease since that’s when it’s typically diagnosed. “Given these gaps in knowledge from prior reports, we performed this analysis to evaluate the presentation and characteristic symptoms of early-stage ovarian cancer and to attempt to identify the relationship between these symptoms with respect to clinicopathologic characteristics and prognosis in early-stage disease.”
Dr. Chan and colleagues retrospectively tracked 419 patients who were subjects in a clinical trial of chemotherapy doses. The patients all had high-risk, early-stage epithelial ovarian cancer (stage IA-IB and grade 3, any clear cell, stage IC or II).
Of the patients, 40% presented with one symptom, while 32% had multiple symptoms. The other 28% had no symptoms, and their masses were diagnosed upon discovery during physical examination. “Other investigators have found that nearly 95% of patients with ovarian cancer were symptomatic,” Dr. Chan said. “The lower percentage of symptomatic patients in our study may be because all 419 patients had early-stage disease as opposed to advanced-stage disease.”
The most common symptoms were abdominal or pelvic pain (31%; 95% confidence interval, 27%-36%), fullness or increased abdominal girth (27%; 95% CI, 22%-31%), abnormal vaginal bleeding (13%; 95% CI, 10%-17%), urinary problems (10%; 95% CI, 8%-14%), and gastrointestinal problems (6%; 95% CI, 4%-8%).
There was no statistically significant link between number of symptoms and age (younger than 60 or 60 or older), cancer stage, or histologic subtype. However, patients with the largest tumors (>15 cm) were more likely to have multiple symptoms than those with the smallest tumors (10 cm or smaller): 46% vs. 21% (P < .001).
Also, 79% of those with the largest tumors (>15 cm) had at least one symptom, compared with 65% of those with the smallest tumors (10 cm or smaller, P < .001)
Unlike other studies, this report didn’t find a link between the number of symptoms and mortality. This finding surprised the researchers, Dr. Chan said, as did the lack of connections between symptoms and age, stage, or histologic subtype. “We were expecting that the younger patients may have more symptoms given the association with endometriosis and clear cell cancers,” he said. “We also thought that those who are less symptomatic may have more stage I and low-grade indolent tumors with better survival, but we did not find that.”
The researchers noted limitations such as the lack of standardization in the patient data.
In the big picture, Dr. Chan said, “patients and health care professionals need to have a higher index of suspicion in symptomatic ovarian cancer patients to increase early detection and potentially improve cures. Ovarian cancer does not always kill. In fact, up to 80% of our early-stage disease patients are cured.”
He called for “additional research to evaluate symptom awareness in early-stage cancers and possibly incorporating novel serum biomarkers and wearable monitoring devices. Wearables may be able to assess for frequency or duration of symptoms, which may be an important factor in distinguishing symptoms that are more concerning for ovarian cancer.”
In an adjoining commentary, Barbara A. Goff, MD, chair of obstetrics and gynecology at the University of Washington, Seattle, noted that, while ovarian cancers diagnosed early have a high survival rate, prospective randomized trials of transvaginal ultrasonography and tumor marker screening strategies have failed to reduce mortality. There’s currently no recommended screening test for women at average risk.
There are other challenges, she wrote. For one, “many health care professionals are seemingly unaware of the symptoms typically associated with ovarian cancer, so misdiagnosis remains common.” And “one of the concerns about the symptoms of ovarian cancer is that they can be vague and commonly present in the general population.”
Dr. Goff praised the study, called for more education about the symptoms of ovarian cancer, and wrote that “symptom recognition with appropriate diagnostic testing remains very important in our efforts to improve outcomes.”
The National Institutes of Health funded the study. Several study authors, including Dr. Chan, reported various disclosures.
Correction, 1/31/22: An earlier version of this article misstated the date of publication.
A new study offers fresh insight into early indications of high-risk, early-stage, epithelial ovarian cancer: More than 70% have at least one symptom such as abdominal/pelvic pain or increased girth/fullness, and women with larger tumors have more symptoms.
“Even in early-stage disease, ovarian cancer is not necessarily a silent disease,” said lead author and gynecologic oncologist/surgeon John K. Chan, MD, of Palo Alto Medical Foundation/California Pacific/Sutter Research Institute.
The study appeared online Jan. 6, in the journal Obstetrics & Gynecology.*
According to Dr. Chan, most previous studies of symptoms in ovarian cancer have focused on those with advanced disease since that’s when it’s typically diagnosed. “Given these gaps in knowledge from prior reports, we performed this analysis to evaluate the presentation and characteristic symptoms of early-stage ovarian cancer and to attempt to identify the relationship between these symptoms with respect to clinicopathologic characteristics and prognosis in early-stage disease.”
Dr. Chan and colleagues retrospectively tracked 419 patients who were subjects in a clinical trial of chemotherapy doses. The patients all had high-risk, early-stage epithelial ovarian cancer (stage IA-IB and grade 3, any clear cell, stage IC or II).
Of the patients, 40% presented with one symptom, while 32% had multiple symptoms. The other 28% had no symptoms, and their masses were diagnosed upon discovery during physical examination. “Other investigators have found that nearly 95% of patients with ovarian cancer were symptomatic,” Dr. Chan said. “The lower percentage of symptomatic patients in our study may be because all 419 patients had early-stage disease as opposed to advanced-stage disease.”
The most common symptoms were abdominal or pelvic pain (31%; 95% confidence interval, 27%-36%), fullness or increased abdominal girth (27%; 95% CI, 22%-31%), abnormal vaginal bleeding (13%; 95% CI, 10%-17%), urinary problems (10%; 95% CI, 8%-14%), and gastrointestinal problems (6%; 95% CI, 4%-8%).
There was no statistically significant link between number of symptoms and age (younger than 60 or 60 or older), cancer stage, or histologic subtype. However, patients with the largest tumors (>15 cm) were more likely to have multiple symptoms than those with the smallest tumors (10 cm or smaller): 46% vs. 21% (P < .001).
Also, 79% of those with the largest tumors (>15 cm) had at least one symptom, compared with 65% of those with the smallest tumors (10 cm or smaller, P < .001)
Unlike other studies, this report didn’t find a link between the number of symptoms and mortality. This finding surprised the researchers, Dr. Chan said, as did the lack of connections between symptoms and age, stage, or histologic subtype. “We were expecting that the younger patients may have more symptoms given the association with endometriosis and clear cell cancers,” he said. “We also thought that those who are less symptomatic may have more stage I and low-grade indolent tumors with better survival, but we did not find that.”
The researchers noted limitations such as the lack of standardization in the patient data.
In the big picture, Dr. Chan said, “patients and health care professionals need to have a higher index of suspicion in symptomatic ovarian cancer patients to increase early detection and potentially improve cures. Ovarian cancer does not always kill. In fact, up to 80% of our early-stage disease patients are cured.”
He called for “additional research to evaluate symptom awareness in early-stage cancers and possibly incorporating novel serum biomarkers and wearable monitoring devices. Wearables may be able to assess for frequency or duration of symptoms, which may be an important factor in distinguishing symptoms that are more concerning for ovarian cancer.”
In an adjoining commentary, Barbara A. Goff, MD, chair of obstetrics and gynecology at the University of Washington, Seattle, noted that, while ovarian cancers diagnosed early have a high survival rate, prospective randomized trials of transvaginal ultrasonography and tumor marker screening strategies have failed to reduce mortality. There’s currently no recommended screening test for women at average risk.
There are other challenges, she wrote. For one, “many health care professionals are seemingly unaware of the symptoms typically associated with ovarian cancer, so misdiagnosis remains common.” And “one of the concerns about the symptoms of ovarian cancer is that they can be vague and commonly present in the general population.”
Dr. Goff praised the study, called for more education about the symptoms of ovarian cancer, and wrote that “symptom recognition with appropriate diagnostic testing remains very important in our efforts to improve outcomes.”
The National Institutes of Health funded the study. Several study authors, including Dr. Chan, reported various disclosures.
Correction, 1/31/22: An earlier version of this article misstated the date of publication.
FROM OBSTETRICS & GYNECOLOGY
Prior authorization abuse: It’s time for health insurance CEOs and their proxies to cease and desist the practice once and for all!
Before reading this editorial and concluding that the author (me) has lost his grip on reality, I would ask that you consider the facts I provide below and the ramifications incurred by your patients and practices, due to the misbehaviors adopted by the health insurance industry.
- Two of the most common issues discussed in today’s health care environment are revenue generation and provider/staff burnout.
While these issues are impacted by several factors, one of the most common denominators is increasing administrative workloads driven by non–revenue-generating activities. Consider this:
- A recent American Medical Association survey pointed out that during the course of the average workweek, a physician completes an average of 37 prior authorization requests. Physicians and their staff spend an average of 16.4 hours per week completing prior authorization requirements for patient medicines, procedures, and medical services that they may need.1
- While physicians report that about 65% of prior authorizations take only 1 day, they report that 26% take 3 or more days.2
The potential significance of the generated delays
While this may not seem like a long time (other than the impact it has on staff workload), consider the impact this can have on the patient if the medication being requested is: PrEP, the morning after pill, or other contraceptives? The consequences of the delay or denial could be a lifetime living with HIV, or an unintended pregnancy. This is to say nothing on the larger impact to family, partners, and the potential social stigma faced by all.
Beyond the personal costs and costs within your practice associated with the additional workload, consider the financial costs. The average cost to complete a prior authorization remains the single highest cost for the health care industry at $13.40 per manual transaction, and $7.19 per partially electronic web portal transaction,3 meaning that if I did only one prescription per week, I probably would not mind, but at $13.40 per prior authorization, this burden amounts to millions, actually $767 million by recent estimates.3 Additionally, if you factor in the number of denials and potential follow-ups, this creates a significant amount of waste and spending.
Ultimately, in my experience, I have found that most prior authorizations are simply unnecessary. Here, I’ve picked key examples from just my own recent experiences:
- My patient was denied access to a particular birth control pill she had been on successfully before, and my office was told she needed to try and fail on 5 different generic pills before she could be approved. However, the Affordable Care Act’s (ACA; aka Obamacare) Contraceptive Mandate requires coverage of all contraceptives determined to be most appropriate between a patient and their provider (see below).
- A menopausal patient was denied coverage twice (electronically) for generic micronized progesterone, and I was asked to write a letter of appeal because the insurance company wanted me to use medroxyprogesterone acetate instead. Polling my nearby retail independent pharmacy, the total cost difference per year was $19.96 savings/year ($47.01 ‒ $27.05 = $19.96). My pharmacist did note it could have been a different amount at a large chain pharmacy. Really? I had to write a letter, following two denials, to save less than $20, for a full year!
- A 78-year-old patient using Prolia for severe osteoporosis and preexisting fractures was delayed in getting her next Prolia injection due to a prior authorization snafu. She ended up with multiple additional fractures, a well-described effect of the increase in bone turnover when stopping or delaying this medication. She is now disabled.
- A 94-year-old patient was sent an email reminder to get the medical practice to authorize a refill of ileostomy bags. The email went to spam, and the patient ran out of bags prior to a holiday weekend. I got them in 2 days on Amazon Prime. But who emails a 94-year-old? And ileostomy bags! When does anyone stop needing ileostomy bags?
- I requested a prior authorization for Orilissa (clearly off label) because a severely progestogen-sensitive patient (augmented depression) with severe premenstrual dysphoric disorder requiring hospitalization was thought by her psychiatrist to be better off without menstrual periods. I completed the proper paperwork, two electronic appeals, and a letter of explanation including available references on the use of gonadotropin-releasing hormone analogues for such patients. I was then told I would need to have a peer-to-peer discussion, so I filled out that paperwork, which clearly noted that I am a board-certified reproductive endocrinologist. I got a phone call a few days later by a pleasant, young-sounding pediatric rheumatologist. Our interaction did not go well for him. This was not peer-to-peer!
Let us be clear, prior authorizations have nothing to do with patient care. In fact, they are solely about the money. We in ObGyn have mostly inexpensive and generic products, but even that fact has not lowered the excessive burden of the prior authorization process. In the case of contraception, whether you like the ACA or not it is the law, and it contains specific provisions regarding contraception. With the goals of providing broad access to patients and incentives to developers for new and novel contraceptive methods, these provisions require insurers to cover, without cost-sharing, women’s preventive services including the full range of FDA-approved contraceptives (currently 18 different method categories), and additional methods identified by the FDA as they become available. Further, providers must have an easily accessible, transparent, and sufficiently expedient exceptions process that is not unduly burdensome on the individual or a provider (or other individuals acting as a patient’s authorized representative).
And while I can regale you with chapter and verse and citations of the legal precedent and language, it boils down to this:
- The AMA reported that medical practices spend an average of 2 business days a week per physician to comply with health plans’ inefficient and overused prior-authorization protocols.4 To keep up with the administrative burden, 2 out of 5 physicians (40%) employ staff members who work exclusively on tasks associated with prior authorization.4
- About 86% of practices reported an increased burden of prior authorizations in the last 5 years.5
Continue to: What is to be done?
What is to be done?
I do have suggested solutions. Given the insurance industry’s complete lack of progress in voluntarily reducing the burdens of prior authorizations agreed to in their consensus statement with the AMA, American Hospital Association, America’s Health Insurance Plans, American Pharmacists Association, Blue Cross Blue Shield Association, and the Medical Group Management Association, I say, why not fine them? The AMA is calling on Congress to pass legislation that would codify much of the agreement, in which the above parties had already agreed that reforms were needed to reduce prior authorization burdens and enhance patient-centered care.6
A good model for enforcement via fines could be based on the old “incident to” rules of Medicare. These state that a physician needs to be “in the space” when advanced practice nurses or physician assistants see Medicare recipients. If they are not actually “in the space” they are subject to a fine. As a completely theoretical example, let’s say the claim was for $100. The practitioner would have to pay it back plus triple that amount in damages, or $400. They can also be fined up to $11,000 per claim and kick you out of Medicare and Medicaid. Take my example of Prolia from above…a single shot of Prolia is about $1,000. The insurer would theoretically have to pay $14,000/claim (the claim + triple damages + $11,000) if it was determined that the prior authorization was unnecessary. Seems about right to me. Or we could just sit the health insurance CEOs and their proxies in the corner on 2-foot-tall plastic Little Tikes® chairs for a “timeout” (dunce cap optional), like the outset of the article says.
Until the detrimental prior authorization process is challenged at all levels, we will continue to see and feel the effects of the harm it causes. Being able to drive change through advocacy and education is the best way we as clinicians can impact not just the future of health care but provide for the daily care of our patients who depend on and trust us to provide for their medical needs. We must be the impactors of change for ourselves, colleagues, staff, and profession if we are to really make advancements into the future.
Oh…and health insurance CEOs and their proxies, to get out of their “time-out” would still be entitled to one phone call to beg forgiveness from their mommies/daddies, priest/ rabbi/pastor, psychologist/psychiatrist/mystic healer, etc., but alas, the average wait time is an hour, and if anyone answers the phone, they have a grade school education used in following an irrelevant algorithm. ●
- Corder JC. Streamlining the insurance prior authorization debacle. Mo Med. 2018;115:312-314.
- Prior authorization hurdles have led to serious adverse events. American Medical Association website. February 5, 2019. https://www.ama-assn .org/press-center/press-releases/prior-author ization-hurdles-have-led-serious-adverse -events. Accessed November 29, 2021.
- Council for Affordable Quality Healthcare. 2020 CAQH INDEX. https://www.caqh.org/sites /default/files/explorations/index/2020-caqh -index.pdf. Accessed November 22, 2021.
- Most physicians had little relief from prior authorization as COVID cases soared. American Medical Association website. April 7, 2021. https:// www.ama-assn.org/press-center/press-releases /most-physicians-had-little-relief-prior-author ization-covid-cases. Accessed November 29, 2021.
- Robeznieks A. 1 in 4 doctors say prior authorization has led to a serious adverse event. American Medical Association website. February 5, 2019. https://www .ama-assn.org/practice-management/sustainability /1-4-doctors-say-prior-authorization-has-led-serious -adverse. Accessed November 29, 2021.
- Physicians call on Congress to address prior authorization reform. American Medical Association website. May 14, 2021. https://www .ama-assn.org/press-center/press-releases /physicians-call-congress-address-prior-author ization-reform. Accessed November 29, 2021.
Before reading this editorial and concluding that the author (me) has lost his grip on reality, I would ask that you consider the facts I provide below and the ramifications incurred by your patients and practices, due to the misbehaviors adopted by the health insurance industry.
- Two of the most common issues discussed in today’s health care environment are revenue generation and provider/staff burnout.
While these issues are impacted by several factors, one of the most common denominators is increasing administrative workloads driven by non–revenue-generating activities. Consider this:
- A recent American Medical Association survey pointed out that during the course of the average workweek, a physician completes an average of 37 prior authorization requests. Physicians and their staff spend an average of 16.4 hours per week completing prior authorization requirements for patient medicines, procedures, and medical services that they may need.1
- While physicians report that about 65% of prior authorizations take only 1 day, they report that 26% take 3 or more days.2
The potential significance of the generated delays
While this may not seem like a long time (other than the impact it has on staff workload), consider the impact this can have on the patient if the medication being requested is: PrEP, the morning after pill, or other contraceptives? The consequences of the delay or denial could be a lifetime living with HIV, or an unintended pregnancy. This is to say nothing on the larger impact to family, partners, and the potential social stigma faced by all.
Beyond the personal costs and costs within your practice associated with the additional workload, consider the financial costs. The average cost to complete a prior authorization remains the single highest cost for the health care industry at $13.40 per manual transaction, and $7.19 per partially electronic web portal transaction,3 meaning that if I did only one prescription per week, I probably would not mind, but at $13.40 per prior authorization, this burden amounts to millions, actually $767 million by recent estimates.3 Additionally, if you factor in the number of denials and potential follow-ups, this creates a significant amount of waste and spending.
Ultimately, in my experience, I have found that most prior authorizations are simply unnecessary. Here, I’ve picked key examples from just my own recent experiences:
- My patient was denied access to a particular birth control pill she had been on successfully before, and my office was told she needed to try and fail on 5 different generic pills before she could be approved. However, the Affordable Care Act’s (ACA; aka Obamacare) Contraceptive Mandate requires coverage of all contraceptives determined to be most appropriate between a patient and their provider (see below).
- A menopausal patient was denied coverage twice (electronically) for generic micronized progesterone, and I was asked to write a letter of appeal because the insurance company wanted me to use medroxyprogesterone acetate instead. Polling my nearby retail independent pharmacy, the total cost difference per year was $19.96 savings/year ($47.01 ‒ $27.05 = $19.96). My pharmacist did note it could have been a different amount at a large chain pharmacy. Really? I had to write a letter, following two denials, to save less than $20, for a full year!
- A 78-year-old patient using Prolia for severe osteoporosis and preexisting fractures was delayed in getting her next Prolia injection due to a prior authorization snafu. She ended up with multiple additional fractures, a well-described effect of the increase in bone turnover when stopping or delaying this medication. She is now disabled.
- A 94-year-old patient was sent an email reminder to get the medical practice to authorize a refill of ileostomy bags. The email went to spam, and the patient ran out of bags prior to a holiday weekend. I got them in 2 days on Amazon Prime. But who emails a 94-year-old? And ileostomy bags! When does anyone stop needing ileostomy bags?
- I requested a prior authorization for Orilissa (clearly off label) because a severely progestogen-sensitive patient (augmented depression) with severe premenstrual dysphoric disorder requiring hospitalization was thought by her psychiatrist to be better off without menstrual periods. I completed the proper paperwork, two electronic appeals, and a letter of explanation including available references on the use of gonadotropin-releasing hormone analogues for such patients. I was then told I would need to have a peer-to-peer discussion, so I filled out that paperwork, which clearly noted that I am a board-certified reproductive endocrinologist. I got a phone call a few days later by a pleasant, young-sounding pediatric rheumatologist. Our interaction did not go well for him. This was not peer-to-peer!
Let us be clear, prior authorizations have nothing to do with patient care. In fact, they are solely about the money. We in ObGyn have mostly inexpensive and generic products, but even that fact has not lowered the excessive burden of the prior authorization process. In the case of contraception, whether you like the ACA or not it is the law, and it contains specific provisions regarding contraception. With the goals of providing broad access to patients and incentives to developers for new and novel contraceptive methods, these provisions require insurers to cover, without cost-sharing, women’s preventive services including the full range of FDA-approved contraceptives (currently 18 different method categories), and additional methods identified by the FDA as they become available. Further, providers must have an easily accessible, transparent, and sufficiently expedient exceptions process that is not unduly burdensome on the individual or a provider (or other individuals acting as a patient’s authorized representative).
And while I can regale you with chapter and verse and citations of the legal precedent and language, it boils down to this:
- The AMA reported that medical practices spend an average of 2 business days a week per physician to comply with health plans’ inefficient and overused prior-authorization protocols.4 To keep up with the administrative burden, 2 out of 5 physicians (40%) employ staff members who work exclusively on tasks associated with prior authorization.4
- About 86% of practices reported an increased burden of prior authorizations in the last 5 years.5
Continue to: What is to be done?
What is to be done?
I do have suggested solutions. Given the insurance industry’s complete lack of progress in voluntarily reducing the burdens of prior authorizations agreed to in their consensus statement with the AMA, American Hospital Association, America’s Health Insurance Plans, American Pharmacists Association, Blue Cross Blue Shield Association, and the Medical Group Management Association, I say, why not fine them? The AMA is calling on Congress to pass legislation that would codify much of the agreement, in which the above parties had already agreed that reforms were needed to reduce prior authorization burdens and enhance patient-centered care.6
A good model for enforcement via fines could be based on the old “incident to” rules of Medicare. These state that a physician needs to be “in the space” when advanced practice nurses or physician assistants see Medicare recipients. If they are not actually “in the space” they are subject to a fine. As a completely theoretical example, let’s say the claim was for $100. The practitioner would have to pay it back plus triple that amount in damages, or $400. They can also be fined up to $11,000 per claim and kick you out of Medicare and Medicaid. Take my example of Prolia from above…a single shot of Prolia is about $1,000. The insurer would theoretically have to pay $14,000/claim (the claim + triple damages + $11,000) if it was determined that the prior authorization was unnecessary. Seems about right to me. Or we could just sit the health insurance CEOs and their proxies in the corner on 2-foot-tall plastic Little Tikes® chairs for a “timeout” (dunce cap optional), like the outset of the article says.
Until the detrimental prior authorization process is challenged at all levels, we will continue to see and feel the effects of the harm it causes. Being able to drive change through advocacy and education is the best way we as clinicians can impact not just the future of health care but provide for the daily care of our patients who depend on and trust us to provide for their medical needs. We must be the impactors of change for ourselves, colleagues, staff, and profession if we are to really make advancements into the future.
Oh…and health insurance CEOs and their proxies, to get out of their “time-out” would still be entitled to one phone call to beg forgiveness from their mommies/daddies, priest/ rabbi/pastor, psychologist/psychiatrist/mystic healer, etc., but alas, the average wait time is an hour, and if anyone answers the phone, they have a grade school education used in following an irrelevant algorithm. ●
Before reading this editorial and concluding that the author (me) has lost his grip on reality, I would ask that you consider the facts I provide below and the ramifications incurred by your patients and practices, due to the misbehaviors adopted by the health insurance industry.
- Two of the most common issues discussed in today’s health care environment are revenue generation and provider/staff burnout.
While these issues are impacted by several factors, one of the most common denominators is increasing administrative workloads driven by non–revenue-generating activities. Consider this:
- A recent American Medical Association survey pointed out that during the course of the average workweek, a physician completes an average of 37 prior authorization requests. Physicians and their staff spend an average of 16.4 hours per week completing prior authorization requirements for patient medicines, procedures, and medical services that they may need.1
- While physicians report that about 65% of prior authorizations take only 1 day, they report that 26% take 3 or more days.2
The potential significance of the generated delays
While this may not seem like a long time (other than the impact it has on staff workload), consider the impact this can have on the patient if the medication being requested is: PrEP, the morning after pill, or other contraceptives? The consequences of the delay or denial could be a lifetime living with HIV, or an unintended pregnancy. This is to say nothing on the larger impact to family, partners, and the potential social stigma faced by all.
Beyond the personal costs and costs within your practice associated with the additional workload, consider the financial costs. The average cost to complete a prior authorization remains the single highest cost for the health care industry at $13.40 per manual transaction, and $7.19 per partially electronic web portal transaction,3 meaning that if I did only one prescription per week, I probably would not mind, but at $13.40 per prior authorization, this burden amounts to millions, actually $767 million by recent estimates.3 Additionally, if you factor in the number of denials and potential follow-ups, this creates a significant amount of waste and spending.
Ultimately, in my experience, I have found that most prior authorizations are simply unnecessary. Here, I’ve picked key examples from just my own recent experiences:
- My patient was denied access to a particular birth control pill she had been on successfully before, and my office was told she needed to try and fail on 5 different generic pills before she could be approved. However, the Affordable Care Act’s (ACA; aka Obamacare) Contraceptive Mandate requires coverage of all contraceptives determined to be most appropriate between a patient and their provider (see below).
- A menopausal patient was denied coverage twice (electronically) for generic micronized progesterone, and I was asked to write a letter of appeal because the insurance company wanted me to use medroxyprogesterone acetate instead. Polling my nearby retail independent pharmacy, the total cost difference per year was $19.96 savings/year ($47.01 ‒ $27.05 = $19.96). My pharmacist did note it could have been a different amount at a large chain pharmacy. Really? I had to write a letter, following two denials, to save less than $20, for a full year!
- A 78-year-old patient using Prolia for severe osteoporosis and preexisting fractures was delayed in getting her next Prolia injection due to a prior authorization snafu. She ended up with multiple additional fractures, a well-described effect of the increase in bone turnover when stopping or delaying this medication. She is now disabled.
- A 94-year-old patient was sent an email reminder to get the medical practice to authorize a refill of ileostomy bags. The email went to spam, and the patient ran out of bags prior to a holiday weekend. I got them in 2 days on Amazon Prime. But who emails a 94-year-old? And ileostomy bags! When does anyone stop needing ileostomy bags?
- I requested a prior authorization for Orilissa (clearly off label) because a severely progestogen-sensitive patient (augmented depression) with severe premenstrual dysphoric disorder requiring hospitalization was thought by her psychiatrist to be better off without menstrual periods. I completed the proper paperwork, two electronic appeals, and a letter of explanation including available references on the use of gonadotropin-releasing hormone analogues for such patients. I was then told I would need to have a peer-to-peer discussion, so I filled out that paperwork, which clearly noted that I am a board-certified reproductive endocrinologist. I got a phone call a few days later by a pleasant, young-sounding pediatric rheumatologist. Our interaction did not go well for him. This was not peer-to-peer!
Let us be clear, prior authorizations have nothing to do with patient care. In fact, they are solely about the money. We in ObGyn have mostly inexpensive and generic products, but even that fact has not lowered the excessive burden of the prior authorization process. In the case of contraception, whether you like the ACA or not it is the law, and it contains specific provisions regarding contraception. With the goals of providing broad access to patients and incentives to developers for new and novel contraceptive methods, these provisions require insurers to cover, without cost-sharing, women’s preventive services including the full range of FDA-approved contraceptives (currently 18 different method categories), and additional methods identified by the FDA as they become available. Further, providers must have an easily accessible, transparent, and sufficiently expedient exceptions process that is not unduly burdensome on the individual or a provider (or other individuals acting as a patient’s authorized representative).
And while I can regale you with chapter and verse and citations of the legal precedent and language, it boils down to this:
- The AMA reported that medical practices spend an average of 2 business days a week per physician to comply with health plans’ inefficient and overused prior-authorization protocols.4 To keep up with the administrative burden, 2 out of 5 physicians (40%) employ staff members who work exclusively on tasks associated with prior authorization.4
- About 86% of practices reported an increased burden of prior authorizations in the last 5 years.5
Continue to: What is to be done?
What is to be done?
I do have suggested solutions. Given the insurance industry’s complete lack of progress in voluntarily reducing the burdens of prior authorizations agreed to in their consensus statement with the AMA, American Hospital Association, America’s Health Insurance Plans, American Pharmacists Association, Blue Cross Blue Shield Association, and the Medical Group Management Association, I say, why not fine them? The AMA is calling on Congress to pass legislation that would codify much of the agreement, in which the above parties had already agreed that reforms were needed to reduce prior authorization burdens and enhance patient-centered care.6
A good model for enforcement via fines could be based on the old “incident to” rules of Medicare. These state that a physician needs to be “in the space” when advanced practice nurses or physician assistants see Medicare recipients. If they are not actually “in the space” they are subject to a fine. As a completely theoretical example, let’s say the claim was for $100. The practitioner would have to pay it back plus triple that amount in damages, or $400. They can also be fined up to $11,000 per claim and kick you out of Medicare and Medicaid. Take my example of Prolia from above…a single shot of Prolia is about $1,000. The insurer would theoretically have to pay $14,000/claim (the claim + triple damages + $11,000) if it was determined that the prior authorization was unnecessary. Seems about right to me. Or we could just sit the health insurance CEOs and their proxies in the corner on 2-foot-tall plastic Little Tikes® chairs for a “timeout” (dunce cap optional), like the outset of the article says.
Until the detrimental prior authorization process is challenged at all levels, we will continue to see and feel the effects of the harm it causes. Being able to drive change through advocacy and education is the best way we as clinicians can impact not just the future of health care but provide for the daily care of our patients who depend on and trust us to provide for their medical needs. We must be the impactors of change for ourselves, colleagues, staff, and profession if we are to really make advancements into the future.
Oh…and health insurance CEOs and their proxies, to get out of their “time-out” would still be entitled to one phone call to beg forgiveness from their mommies/daddies, priest/ rabbi/pastor, psychologist/psychiatrist/mystic healer, etc., but alas, the average wait time is an hour, and if anyone answers the phone, they have a grade school education used in following an irrelevant algorithm. ●
- Corder JC. Streamlining the insurance prior authorization debacle. Mo Med. 2018;115:312-314.
- Prior authorization hurdles have led to serious adverse events. American Medical Association website. February 5, 2019. https://www.ama-assn .org/press-center/press-releases/prior-author ization-hurdles-have-led-serious-adverse -events. Accessed November 29, 2021.
- Council for Affordable Quality Healthcare. 2020 CAQH INDEX. https://www.caqh.org/sites /default/files/explorations/index/2020-caqh -index.pdf. Accessed November 22, 2021.
- Most physicians had little relief from prior authorization as COVID cases soared. American Medical Association website. April 7, 2021. https:// www.ama-assn.org/press-center/press-releases /most-physicians-had-little-relief-prior-author ization-covid-cases. Accessed November 29, 2021.
- Robeznieks A. 1 in 4 doctors say prior authorization has led to a serious adverse event. American Medical Association website. February 5, 2019. https://www .ama-assn.org/practice-management/sustainability /1-4-doctors-say-prior-authorization-has-led-serious -adverse. Accessed November 29, 2021.
- Physicians call on Congress to address prior authorization reform. American Medical Association website. May 14, 2021. https://www .ama-assn.org/press-center/press-releases /physicians-call-congress-address-prior-author ization-reform. Accessed November 29, 2021.
- Corder JC. Streamlining the insurance prior authorization debacle. Mo Med. 2018;115:312-314.
- Prior authorization hurdles have led to serious adverse events. American Medical Association website. February 5, 2019. https://www.ama-assn .org/press-center/press-releases/prior-author ization-hurdles-have-led-serious-adverse -events. Accessed November 29, 2021.
- Council for Affordable Quality Healthcare. 2020 CAQH INDEX. https://www.caqh.org/sites /default/files/explorations/index/2020-caqh -index.pdf. Accessed November 22, 2021.
- Most physicians had little relief from prior authorization as COVID cases soared. American Medical Association website. April 7, 2021. https:// www.ama-assn.org/press-center/press-releases /most-physicians-had-little-relief-prior-author ization-covid-cases. Accessed November 29, 2021.
- Robeznieks A. 1 in 4 doctors say prior authorization has led to a serious adverse event. American Medical Association website. February 5, 2019. https://www .ama-assn.org/practice-management/sustainability /1-4-doctors-say-prior-authorization-has-led-serious -adverse. Accessed November 29, 2021.
- Physicians call on Congress to address prior authorization reform. American Medical Association website. May 14, 2021. https://www .ama-assn.org/press-center/press-releases /physicians-call-congress-address-prior-author ization-reform. Accessed November 29, 2021.
Common cold could protect against COVID-19, study says
, according to a small study published Jan. 10 in Nature Communications.
Previous studies have shown that T cells created from other coronaviruses can recognize SARS-CoV-2, the virus that causes COVID-19. In the new study, researchers at Imperial College London found that the presence of these T cells at the time of COVID-19 exposure could reduce the chance of getting infected.
The findings could provide a blueprint for a second-generation, universal vaccine to prevent infection from COVID-19 variants, including Omicron and ones that crop up later.
“Being exposed to SARS-CoV-2 virus doesn’t always result in infection, and we’ve been keen to understand why,” Rhia Kundu, PhD, the lead study author from Imperial’s National Heart and Lung Institute, said in a statement.
People with higher levels of T cells from the common cold were less likely to become infected with COVID-19, the researchers found.
“While this is an important discovery, it is only one form of protection, and I would stress that no one should rely on this alone,” Dr. Kundu said. “Instead, the best way to protect yourself against COVID-19 is to be fully vaccinated, including getting your booster dose.”
For the study, Dr. Kundu and colleagues analyzed blood samples from 52 people who lived with someone with confirmed COVID-19 in September 2020. Among the 26 people who didn’t contract COVID-19, there were “significantly higher levels” of preexisting T cells from common cold coronaviruses, as compared with the 26 people who did become infected.
The T cells researched in the study are considered “cross-reactive” and can recognize the proteins of SARS-CoV-2. They offer protection by targeting proteins inside the SARS-CoV-2 virus, rather than the spike proteins on the surface that allow the virus to invade cells.
The current COVID-19 vaccines target the spike proteins, which are more likely to mutate than internal proteins, the researchers wrote. The Omicron variant, for instance, has numerous mutations on spike proteins that may allow it to evade vaccines.
The data suggest that the next step of COVID-19 vaccine development could focus on internal proteins, the researchers said, which could provide lasting protection because T-cell responses persist longer than antibody responses that fade within a few months of vaccination.
“New vaccines that include these conserved, internal proteins would therefore induce broadly protective T-cell responses that should protect against current and future SARS-CoV-2 variants,” Ajit Lalvani, MD, the senior study author and director of Imperial’s respiratory infections health protection research unit, said in the statement.
But more research is needed, the authors said, noting that the study had a small sample size and lacked ethnic diversity, which puts limits on the research.
A version of this article first appeared on WebMD.com
, according to a small study published Jan. 10 in Nature Communications.
Previous studies have shown that T cells created from other coronaviruses can recognize SARS-CoV-2, the virus that causes COVID-19. In the new study, researchers at Imperial College London found that the presence of these T cells at the time of COVID-19 exposure could reduce the chance of getting infected.
The findings could provide a blueprint for a second-generation, universal vaccine to prevent infection from COVID-19 variants, including Omicron and ones that crop up later.
“Being exposed to SARS-CoV-2 virus doesn’t always result in infection, and we’ve been keen to understand why,” Rhia Kundu, PhD, the lead study author from Imperial’s National Heart and Lung Institute, said in a statement.
People with higher levels of T cells from the common cold were less likely to become infected with COVID-19, the researchers found.
“While this is an important discovery, it is only one form of protection, and I would stress that no one should rely on this alone,” Dr. Kundu said. “Instead, the best way to protect yourself against COVID-19 is to be fully vaccinated, including getting your booster dose.”
For the study, Dr. Kundu and colleagues analyzed blood samples from 52 people who lived with someone with confirmed COVID-19 in September 2020. Among the 26 people who didn’t contract COVID-19, there were “significantly higher levels” of preexisting T cells from common cold coronaviruses, as compared with the 26 people who did become infected.
The T cells researched in the study are considered “cross-reactive” and can recognize the proteins of SARS-CoV-2. They offer protection by targeting proteins inside the SARS-CoV-2 virus, rather than the spike proteins on the surface that allow the virus to invade cells.
The current COVID-19 vaccines target the spike proteins, which are more likely to mutate than internal proteins, the researchers wrote. The Omicron variant, for instance, has numerous mutations on spike proteins that may allow it to evade vaccines.
The data suggest that the next step of COVID-19 vaccine development could focus on internal proteins, the researchers said, which could provide lasting protection because T-cell responses persist longer than antibody responses that fade within a few months of vaccination.
“New vaccines that include these conserved, internal proteins would therefore induce broadly protective T-cell responses that should protect against current and future SARS-CoV-2 variants,” Ajit Lalvani, MD, the senior study author and director of Imperial’s respiratory infections health protection research unit, said in the statement.
But more research is needed, the authors said, noting that the study had a small sample size and lacked ethnic diversity, which puts limits on the research.
A version of this article first appeared on WebMD.com
, according to a small study published Jan. 10 in Nature Communications.
Previous studies have shown that T cells created from other coronaviruses can recognize SARS-CoV-2, the virus that causes COVID-19. In the new study, researchers at Imperial College London found that the presence of these T cells at the time of COVID-19 exposure could reduce the chance of getting infected.
The findings could provide a blueprint for a second-generation, universal vaccine to prevent infection from COVID-19 variants, including Omicron and ones that crop up later.
“Being exposed to SARS-CoV-2 virus doesn’t always result in infection, and we’ve been keen to understand why,” Rhia Kundu, PhD, the lead study author from Imperial’s National Heart and Lung Institute, said in a statement.
People with higher levels of T cells from the common cold were less likely to become infected with COVID-19, the researchers found.
“While this is an important discovery, it is only one form of protection, and I would stress that no one should rely on this alone,” Dr. Kundu said. “Instead, the best way to protect yourself against COVID-19 is to be fully vaccinated, including getting your booster dose.”
For the study, Dr. Kundu and colleagues analyzed blood samples from 52 people who lived with someone with confirmed COVID-19 in September 2020. Among the 26 people who didn’t contract COVID-19, there were “significantly higher levels” of preexisting T cells from common cold coronaviruses, as compared with the 26 people who did become infected.
The T cells researched in the study are considered “cross-reactive” and can recognize the proteins of SARS-CoV-2. They offer protection by targeting proteins inside the SARS-CoV-2 virus, rather than the spike proteins on the surface that allow the virus to invade cells.
The current COVID-19 vaccines target the spike proteins, which are more likely to mutate than internal proteins, the researchers wrote. The Omicron variant, for instance, has numerous mutations on spike proteins that may allow it to evade vaccines.
The data suggest that the next step of COVID-19 vaccine development could focus on internal proteins, the researchers said, which could provide lasting protection because T-cell responses persist longer than antibody responses that fade within a few months of vaccination.
“New vaccines that include these conserved, internal proteins would therefore induce broadly protective T-cell responses that should protect against current and future SARS-CoV-2 variants,” Ajit Lalvani, MD, the senior study author and director of Imperial’s respiratory infections health protection research unit, said in the statement.
But more research is needed, the authors said, noting that the study had a small sample size and lacked ethnic diversity, which puts limits on the research.
A version of this article first appeared on WebMD.com