User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Physicians react: Should docs lose their licenses for spreading false COVID information?
Doctors providing “fraudulent” COVID-19 information became a hot-button issue for physicians responding to Medscape’s recent article, "Shouldn’t Doctors Who Spread False COVID-19 Information Lose Their Licenses?”
COVID-19 safety recommendations are set by mainstream medical organizations as new information becomes available, but some doctors consistently oppose advice from the Centers for Disease Control and Prevention and other medical authorities. These physicians often promote off-label, unapproved use of medications for COVID-19 and/or contradict mainstream safety guidelines such as vaccines, masks, and social distancing.
Some medical organizations are concerned that these doctors are hampering efforts to control the highly contagious coronavirus and are, at worst, placing lives in danger with their contrarian views that can spread like wildfire on social media sites. Their words are often used by those who refuse to be vaccinated or wear masks.
State licensing boards have mostly refused to discipline these doctors for making false and/or misleading claims, but as the virus spreads, there are calls to take action against them. However, others worry that such actions would violate free speech and critical thought.
Yes, those doctors are doing wrong
Several physicians took a strong stand against their fellow doctors who are spreading misinformation about COVID-19.
One doctor endorsed the idea of removing licenses for spreading misinformation and called for criminal prosecution: “It should certainly be grounds for cancellation of all licensing (after appropriate examination to rule out acute psychotic episodes, dementia, tumor, etc.) and very likely [include] a charge of manslaughter.”
Another health care provider said, “A person who does not accept science should not, of course, be allowed to practice medicine. One who argues publicly that vaccines and masks don’t work should be prosecuted for crimes ranging from reckless endangerment to attempted murder.”
One reader framed COVID-19 misinformers in stark terms: “These men and women are medical prostitutes. Their medical and surgical colleges [should] have a panel to track in-court testimony and the disinformation they spread ...”
“This is malpractice of the worst kind,” said a clinician. “Public health officials and science are quite clear on [the] best practices for safety during a pandemic, which is killing millions. This is a standard of care.”
“Medical Boards should suspend licenses and give the physician a chance to testify [about] the scientific basis for his comments,” added a health care provider. “Boards involve themselves in all kinds of perceived disciplinary infractions. We are in the midst of a lethal pandemic. I would think that would take precedence over many other issues?”
“I do believe that physicians have the responsibility to speak the truth and have scientifically displayed minds,” said a reader. “Not [to] promulgate misleading, false, and/or unverified information.”
“Any physician, who holds a license, should abide [by] government and state regulation,” asserted a doctor. “He should be disciplined by the board for spreading medical/public misinformation since he is creating potential harm to the population.”
One specialist insisted that “state boards do not do enough to restrict/limit the practice of physicians touting questionable therapies.”
“Any doctor who spreads false information about Covid is hurting our country, our individuals, and our economy and leading to needless deaths,” asserted a physician. “However, there are uncertainties, and where those exist, physicians [should] simply say ‘it is unknown.’”
No, those physicians have a right to speak their beliefs
However, many physicians worried that science and controversial thought were being muzzled.
“Absolutely no,” a doctor stated. “Who judges what is misinformation in this age where debate is canceled? Science advances with challenge, and it’s not about an authority dictating the allowable opinion.”
Another clinician claimed the “truth is very difficult to discern from less-than-truth in a country running on a profit-oriented economic ideology.”
One specialist warned that if disinformation doctors are held responsible, then “that means a lot of doctors” will be “gone” because “almost anything that is written or said about COVID can be contested.”
Another physician warned his colleagues about suppressing new ideas: “To condemn what we didn’t try, or purposefully ignore a different approach because [it] doesn’t agree with our opinion is suppression of information.”
Some doctors insisted the issue extended beyond medicine and into Constitutional freedoms. They also expressed their mistrust in the government to regulate physicians.
“There is a First Amendment in this country,” said one reader. “What you think is false may not be so. The people can listen to whoever they want to and make their own medical decisions. We do not need one iota more of politicizing medicine. Having an MD or DO does not mean you relinquish your First Amendment rights.”
“One of the fundamental problems with a system that allows government to ‘license’ physicians, or any other profession, is that politics inevitably turn to cronyism, and big businesses and wealthy people start controlling the government,” argued a doctor.
One clinician suggested enforcement against health food, drug company commercials, and talk shows: “What about all the [misinformation] at the health food stores and the like. Doctors of natural-whatever? Those info-commercials on tv. How many faxes do I get to ‘approve’ because ‘patients request’ braces and pain-treating expensive compounds advertised on TV? We tolerate those ... What about Dr. Oz and the docs on talk shows claiming BS?”
And the debate goes even further
Some physicians questioned the very notion of claiming “truth.”
“Nobody should be certain that they have the ‘absolute truth,’” said one reader. “In fact, the best clinical insights exceed so-called knowledge by at least one step.”
“Who can determine exactly what is truth?” asked another clinician. “For sure, the ‘Federal Government,’ who ‘is here to help you,’ is not qualified to make such determinations, and who are you to make such a suggestion as to remove someone’s license because they disagree with you? Give me a break!”
Another physician echoed that sentiment: “What’s true and false is often and certainly currently debatable. There are well-qualified physicians (with credentials such as the development of mRNA technology), virologists, and biostatisticians that have valid thoughts on this but do not necessarily agree with the drug company-sponsored journals and news channels (most of them). Their voices should be heard, and they should not lose their licenses. They are doing their work in good conscience.”
One reader commented that he wanted his “freedom of speech,” and offered this defiant advice: “You can take this license and shove it.”
Finally, a physician noted that the political climate has influenced medical directives: “If someone in a leadership role knowingly, and with intent, spread false information, that is wrong. However, during this global pandemic the active and the politics have combined. Red state no mandate, blue state mandate – what does that tell you about American leadership?”
A version of this article first appeared on Medscape.com.
Doctors providing “fraudulent” COVID-19 information became a hot-button issue for physicians responding to Medscape’s recent article, "Shouldn’t Doctors Who Spread False COVID-19 Information Lose Their Licenses?”
COVID-19 safety recommendations are set by mainstream medical organizations as new information becomes available, but some doctors consistently oppose advice from the Centers for Disease Control and Prevention and other medical authorities. These physicians often promote off-label, unapproved use of medications for COVID-19 and/or contradict mainstream safety guidelines such as vaccines, masks, and social distancing.
Some medical organizations are concerned that these doctors are hampering efforts to control the highly contagious coronavirus and are, at worst, placing lives in danger with their contrarian views that can spread like wildfire on social media sites. Their words are often used by those who refuse to be vaccinated or wear masks.
State licensing boards have mostly refused to discipline these doctors for making false and/or misleading claims, but as the virus spreads, there are calls to take action against them. However, others worry that such actions would violate free speech and critical thought.
Yes, those doctors are doing wrong
Several physicians took a strong stand against their fellow doctors who are spreading misinformation about COVID-19.
One doctor endorsed the idea of removing licenses for spreading misinformation and called for criminal prosecution: “It should certainly be grounds for cancellation of all licensing (after appropriate examination to rule out acute psychotic episodes, dementia, tumor, etc.) and very likely [include] a charge of manslaughter.”
Another health care provider said, “A person who does not accept science should not, of course, be allowed to practice medicine. One who argues publicly that vaccines and masks don’t work should be prosecuted for crimes ranging from reckless endangerment to attempted murder.”
One reader framed COVID-19 misinformers in stark terms: “These men and women are medical prostitutes. Their medical and surgical colleges [should] have a panel to track in-court testimony and the disinformation they spread ...”
“This is malpractice of the worst kind,” said a clinician. “Public health officials and science are quite clear on [the] best practices for safety during a pandemic, which is killing millions. This is a standard of care.”
“Medical Boards should suspend licenses and give the physician a chance to testify [about] the scientific basis for his comments,” added a health care provider. “Boards involve themselves in all kinds of perceived disciplinary infractions. We are in the midst of a lethal pandemic. I would think that would take precedence over many other issues?”
“I do believe that physicians have the responsibility to speak the truth and have scientifically displayed minds,” said a reader. “Not [to] promulgate misleading, false, and/or unverified information.”
“Any physician, who holds a license, should abide [by] government and state regulation,” asserted a doctor. “He should be disciplined by the board for spreading medical/public misinformation since he is creating potential harm to the population.”
One specialist insisted that “state boards do not do enough to restrict/limit the practice of physicians touting questionable therapies.”
“Any doctor who spreads false information about Covid is hurting our country, our individuals, and our economy and leading to needless deaths,” asserted a physician. “However, there are uncertainties, and where those exist, physicians [should] simply say ‘it is unknown.’”
No, those physicians have a right to speak their beliefs
However, many physicians worried that science and controversial thought were being muzzled.
“Absolutely no,” a doctor stated. “Who judges what is misinformation in this age where debate is canceled? Science advances with challenge, and it’s not about an authority dictating the allowable opinion.”
Another clinician claimed the “truth is very difficult to discern from less-than-truth in a country running on a profit-oriented economic ideology.”
One specialist warned that if disinformation doctors are held responsible, then “that means a lot of doctors” will be “gone” because “almost anything that is written or said about COVID can be contested.”
Another physician warned his colleagues about suppressing new ideas: “To condemn what we didn’t try, or purposefully ignore a different approach because [it] doesn’t agree with our opinion is suppression of information.”
Some doctors insisted the issue extended beyond medicine and into Constitutional freedoms. They also expressed their mistrust in the government to regulate physicians.
“There is a First Amendment in this country,” said one reader. “What you think is false may not be so. The people can listen to whoever they want to and make their own medical decisions. We do not need one iota more of politicizing medicine. Having an MD or DO does not mean you relinquish your First Amendment rights.”
“One of the fundamental problems with a system that allows government to ‘license’ physicians, or any other profession, is that politics inevitably turn to cronyism, and big businesses and wealthy people start controlling the government,” argued a doctor.
One clinician suggested enforcement against health food, drug company commercials, and talk shows: “What about all the [misinformation] at the health food stores and the like. Doctors of natural-whatever? Those info-commercials on tv. How many faxes do I get to ‘approve’ because ‘patients request’ braces and pain-treating expensive compounds advertised on TV? We tolerate those ... What about Dr. Oz and the docs on talk shows claiming BS?”
And the debate goes even further
Some physicians questioned the very notion of claiming “truth.”
“Nobody should be certain that they have the ‘absolute truth,’” said one reader. “In fact, the best clinical insights exceed so-called knowledge by at least one step.”
“Who can determine exactly what is truth?” asked another clinician. “For sure, the ‘Federal Government,’ who ‘is here to help you,’ is not qualified to make such determinations, and who are you to make such a suggestion as to remove someone’s license because they disagree with you? Give me a break!”
Another physician echoed that sentiment: “What’s true and false is often and certainly currently debatable. There are well-qualified physicians (with credentials such as the development of mRNA technology), virologists, and biostatisticians that have valid thoughts on this but do not necessarily agree with the drug company-sponsored journals and news channels (most of them). Their voices should be heard, and they should not lose their licenses. They are doing their work in good conscience.”
One reader commented that he wanted his “freedom of speech,” and offered this defiant advice: “You can take this license and shove it.”
Finally, a physician noted that the political climate has influenced medical directives: “If someone in a leadership role knowingly, and with intent, spread false information, that is wrong. However, during this global pandemic the active and the politics have combined. Red state no mandate, blue state mandate – what does that tell you about American leadership?”
A version of this article first appeared on Medscape.com.
Doctors providing “fraudulent” COVID-19 information became a hot-button issue for physicians responding to Medscape’s recent article, "Shouldn’t Doctors Who Spread False COVID-19 Information Lose Their Licenses?”
COVID-19 safety recommendations are set by mainstream medical organizations as new information becomes available, but some doctors consistently oppose advice from the Centers for Disease Control and Prevention and other medical authorities. These physicians often promote off-label, unapproved use of medications for COVID-19 and/or contradict mainstream safety guidelines such as vaccines, masks, and social distancing.
Some medical organizations are concerned that these doctors are hampering efforts to control the highly contagious coronavirus and are, at worst, placing lives in danger with their contrarian views that can spread like wildfire on social media sites. Their words are often used by those who refuse to be vaccinated or wear masks.
State licensing boards have mostly refused to discipline these doctors for making false and/or misleading claims, but as the virus spreads, there are calls to take action against them. However, others worry that such actions would violate free speech and critical thought.
Yes, those doctors are doing wrong
Several physicians took a strong stand against their fellow doctors who are spreading misinformation about COVID-19.
One doctor endorsed the idea of removing licenses for spreading misinformation and called for criminal prosecution: “It should certainly be grounds for cancellation of all licensing (after appropriate examination to rule out acute psychotic episodes, dementia, tumor, etc.) and very likely [include] a charge of manslaughter.”
Another health care provider said, “A person who does not accept science should not, of course, be allowed to practice medicine. One who argues publicly that vaccines and masks don’t work should be prosecuted for crimes ranging from reckless endangerment to attempted murder.”
One reader framed COVID-19 misinformers in stark terms: “These men and women are medical prostitutes. Their medical and surgical colleges [should] have a panel to track in-court testimony and the disinformation they spread ...”
“This is malpractice of the worst kind,” said a clinician. “Public health officials and science are quite clear on [the] best practices for safety during a pandemic, which is killing millions. This is a standard of care.”
“Medical Boards should suspend licenses and give the physician a chance to testify [about] the scientific basis for his comments,” added a health care provider. “Boards involve themselves in all kinds of perceived disciplinary infractions. We are in the midst of a lethal pandemic. I would think that would take precedence over many other issues?”
“I do believe that physicians have the responsibility to speak the truth and have scientifically displayed minds,” said a reader. “Not [to] promulgate misleading, false, and/or unverified information.”
“Any physician, who holds a license, should abide [by] government and state regulation,” asserted a doctor. “He should be disciplined by the board for spreading medical/public misinformation since he is creating potential harm to the population.”
One specialist insisted that “state boards do not do enough to restrict/limit the practice of physicians touting questionable therapies.”
“Any doctor who spreads false information about Covid is hurting our country, our individuals, and our economy and leading to needless deaths,” asserted a physician. “However, there are uncertainties, and where those exist, physicians [should] simply say ‘it is unknown.’”
No, those physicians have a right to speak their beliefs
However, many physicians worried that science and controversial thought were being muzzled.
“Absolutely no,” a doctor stated. “Who judges what is misinformation in this age where debate is canceled? Science advances with challenge, and it’s not about an authority dictating the allowable opinion.”
Another clinician claimed the “truth is very difficult to discern from less-than-truth in a country running on a profit-oriented economic ideology.”
One specialist warned that if disinformation doctors are held responsible, then “that means a lot of doctors” will be “gone” because “almost anything that is written or said about COVID can be contested.”
Another physician warned his colleagues about suppressing new ideas: “To condemn what we didn’t try, or purposefully ignore a different approach because [it] doesn’t agree with our opinion is suppression of information.”
Some doctors insisted the issue extended beyond medicine and into Constitutional freedoms. They also expressed their mistrust in the government to regulate physicians.
“There is a First Amendment in this country,” said one reader. “What you think is false may not be so. The people can listen to whoever they want to and make their own medical decisions. We do not need one iota more of politicizing medicine. Having an MD or DO does not mean you relinquish your First Amendment rights.”
“One of the fundamental problems with a system that allows government to ‘license’ physicians, or any other profession, is that politics inevitably turn to cronyism, and big businesses and wealthy people start controlling the government,” argued a doctor.
One clinician suggested enforcement against health food, drug company commercials, and talk shows: “What about all the [misinformation] at the health food stores and the like. Doctors of natural-whatever? Those info-commercials on tv. How many faxes do I get to ‘approve’ because ‘patients request’ braces and pain-treating expensive compounds advertised on TV? We tolerate those ... What about Dr. Oz and the docs on talk shows claiming BS?”
And the debate goes even further
Some physicians questioned the very notion of claiming “truth.”
“Nobody should be certain that they have the ‘absolute truth,’” said one reader. “In fact, the best clinical insights exceed so-called knowledge by at least one step.”
“Who can determine exactly what is truth?” asked another clinician. “For sure, the ‘Federal Government,’ who ‘is here to help you,’ is not qualified to make such determinations, and who are you to make such a suggestion as to remove someone’s license because they disagree with you? Give me a break!”
Another physician echoed that sentiment: “What’s true and false is often and certainly currently debatable. There are well-qualified physicians (with credentials such as the development of mRNA technology), virologists, and biostatisticians that have valid thoughts on this but do not necessarily agree with the drug company-sponsored journals and news channels (most of them). Their voices should be heard, and they should not lose their licenses. They are doing their work in good conscience.”
One reader commented that he wanted his “freedom of speech,” and offered this defiant advice: “You can take this license and shove it.”
Finally, a physician noted that the political climate has influenced medical directives: “If someone in a leadership role knowingly, and with intent, spread false information, that is wrong. However, during this global pandemic the active and the politics have combined. Red state no mandate, blue state mandate – what does that tell you about American leadership?”
A version of this article first appeared on Medscape.com.
Much lower risk of false-positive breast screen in Norway versus U.S.
Nearly 1 in 5 women who receive the recommended 10 biennial screening rounds for breast cancer in Norway will get a false positive result, and 1 in 20 women will receive a false positive result that leads to an invasive procedure, a new analysis shows.
While the risk may seem high, it is actually much lower than what researchers have reported in the U.S., the study authors note in their paper, published online Dec. 21 in Cancer.
“I am proud about the low rate of recalls we have in Norway and Europe – and hope we can keep it that low for the future,” said senior author Solveig Hofvind, PhD, head of BreastScreen Norway, a nationwide screening program that invites women aged 50 to 69 to mammographic screening every other year.
“The double reading in Europe is probably the main reason for the lower rate in Europe compared to the U.S., where single reading is used,” she said in an interview.
Until now, Dr. Hofvind and her colleagues say, no studies have been performed using exclusively empirical data to describe the cumulative risk of experiencing a false positive screening result in Europe because of the need for long-term follow-up and complete data registration.
For their study, the researchers turned to the Cancer Registry of Norway, which administers BreastScreen Norway. They focused on data from 1995 to 2019 on women aged 50 to 69 years who had attended one or more screening rounds and could potentially attend all 10 screening examinations over the 20-year period.
Women were excluded if they were diagnosed with breast cancer before attending screening, participated in interventional research, self-referred for screening, were recalled due to self-reported symptoms or technically inadequate mammograms, or declined follow-up after a positive screen.
Among more than 421,000 women who underwent nearly 1.9 million screening examinations, 11.3% experienced at least one false positive result and 3.3% experienced at least one false positive involving an invasive procedure, such as fine-needle aspiration cytology, core-needle biopsy, or open biopsy.
The cumulative risk of experiencing a first false positive screen was 18.0% and that of experiencing a false positive that involved an invasive procedure was 5.01%. Adjusting for irregular attendance, age at screening, or the number of screens attended had little effect on the estimates.
The results closely match earlier findings from Norway that have been based on assumptions rather than exclusively empirical data. However, these findings differ from results reported in U.S. studies, which have relied largely on data from the Breast Cancer Surveillance Consortium, the researchers say.
“The latter have indicated that, for women who initiate biennial screening at the age of 50 years, the cumulative risk after 10 years is 42% for experiencing at least one false-positive screening result and 6.4% for experiencing at least one false-positive screening result involving an invasive procedure,” Dr. Hofvind and her colleagues write.
Several principal investigators with the Breast Cancer Surveillance Consortium did not respond or were unavailable for comment when contacted by this news organization.
However, the study authors highlighted several factors that could help explain the discrepancy between the U.S. and European results.
In addition to double mammogram reading, “European guidelines recommend that breast radiologists read 3,500 to 11,000 mammograms annually, whereas 960 every 2 years are required by the U.S. Mammography Quality Standards Act,” the researchers note. They also point out that previous screening mammograms are readily available in Norway, whereas this is not always the case in the U.S.
“False-positive screening results are a part of the screening for breast cancer – and the women need to be informed about the risk,” Dr. Hofvind concluded. “The screening programs should aim to keep the rate as low as possible for the women [given] the costs.”
The study was supported by the Dam Foundation via the Norwegian Breast Cancer Society.
A version of this article first appeared on Medscape.com.
Nearly 1 in 5 women who receive the recommended 10 biennial screening rounds for breast cancer in Norway will get a false positive result, and 1 in 20 women will receive a false positive result that leads to an invasive procedure, a new analysis shows.
While the risk may seem high, it is actually much lower than what researchers have reported in the U.S., the study authors note in their paper, published online Dec. 21 in Cancer.
“I am proud about the low rate of recalls we have in Norway and Europe – and hope we can keep it that low for the future,” said senior author Solveig Hofvind, PhD, head of BreastScreen Norway, a nationwide screening program that invites women aged 50 to 69 to mammographic screening every other year.
“The double reading in Europe is probably the main reason for the lower rate in Europe compared to the U.S., where single reading is used,” she said in an interview.
Until now, Dr. Hofvind and her colleagues say, no studies have been performed using exclusively empirical data to describe the cumulative risk of experiencing a false positive screening result in Europe because of the need for long-term follow-up and complete data registration.
For their study, the researchers turned to the Cancer Registry of Norway, which administers BreastScreen Norway. They focused on data from 1995 to 2019 on women aged 50 to 69 years who had attended one or more screening rounds and could potentially attend all 10 screening examinations over the 20-year period.
Women were excluded if they were diagnosed with breast cancer before attending screening, participated in interventional research, self-referred for screening, were recalled due to self-reported symptoms or technically inadequate mammograms, or declined follow-up after a positive screen.
Among more than 421,000 women who underwent nearly 1.9 million screening examinations, 11.3% experienced at least one false positive result and 3.3% experienced at least one false positive involving an invasive procedure, such as fine-needle aspiration cytology, core-needle biopsy, or open biopsy.
The cumulative risk of experiencing a first false positive screen was 18.0% and that of experiencing a false positive that involved an invasive procedure was 5.01%. Adjusting for irregular attendance, age at screening, or the number of screens attended had little effect on the estimates.
The results closely match earlier findings from Norway that have been based on assumptions rather than exclusively empirical data. However, these findings differ from results reported in U.S. studies, which have relied largely on data from the Breast Cancer Surveillance Consortium, the researchers say.
“The latter have indicated that, for women who initiate biennial screening at the age of 50 years, the cumulative risk after 10 years is 42% for experiencing at least one false-positive screening result and 6.4% for experiencing at least one false-positive screening result involving an invasive procedure,” Dr. Hofvind and her colleagues write.
Several principal investigators with the Breast Cancer Surveillance Consortium did not respond or were unavailable for comment when contacted by this news organization.
However, the study authors highlighted several factors that could help explain the discrepancy between the U.S. and European results.
In addition to double mammogram reading, “European guidelines recommend that breast radiologists read 3,500 to 11,000 mammograms annually, whereas 960 every 2 years are required by the U.S. Mammography Quality Standards Act,” the researchers note. They also point out that previous screening mammograms are readily available in Norway, whereas this is not always the case in the U.S.
“False-positive screening results are a part of the screening for breast cancer – and the women need to be informed about the risk,” Dr. Hofvind concluded. “The screening programs should aim to keep the rate as low as possible for the women [given] the costs.”
The study was supported by the Dam Foundation via the Norwegian Breast Cancer Society.
A version of this article first appeared on Medscape.com.
Nearly 1 in 5 women who receive the recommended 10 biennial screening rounds for breast cancer in Norway will get a false positive result, and 1 in 20 women will receive a false positive result that leads to an invasive procedure, a new analysis shows.
While the risk may seem high, it is actually much lower than what researchers have reported in the U.S., the study authors note in their paper, published online Dec. 21 in Cancer.
“I am proud about the low rate of recalls we have in Norway and Europe – and hope we can keep it that low for the future,” said senior author Solveig Hofvind, PhD, head of BreastScreen Norway, a nationwide screening program that invites women aged 50 to 69 to mammographic screening every other year.
“The double reading in Europe is probably the main reason for the lower rate in Europe compared to the U.S., where single reading is used,” she said in an interview.
Until now, Dr. Hofvind and her colleagues say, no studies have been performed using exclusively empirical data to describe the cumulative risk of experiencing a false positive screening result in Europe because of the need for long-term follow-up and complete data registration.
For their study, the researchers turned to the Cancer Registry of Norway, which administers BreastScreen Norway. They focused on data from 1995 to 2019 on women aged 50 to 69 years who had attended one or more screening rounds and could potentially attend all 10 screening examinations over the 20-year period.
Women were excluded if they were diagnosed with breast cancer before attending screening, participated in interventional research, self-referred for screening, were recalled due to self-reported symptoms or technically inadequate mammograms, or declined follow-up after a positive screen.
Among more than 421,000 women who underwent nearly 1.9 million screening examinations, 11.3% experienced at least one false positive result and 3.3% experienced at least one false positive involving an invasive procedure, such as fine-needle aspiration cytology, core-needle biopsy, or open biopsy.
The cumulative risk of experiencing a first false positive screen was 18.0% and that of experiencing a false positive that involved an invasive procedure was 5.01%. Adjusting for irregular attendance, age at screening, or the number of screens attended had little effect on the estimates.
The results closely match earlier findings from Norway that have been based on assumptions rather than exclusively empirical data. However, these findings differ from results reported in U.S. studies, which have relied largely on data from the Breast Cancer Surveillance Consortium, the researchers say.
“The latter have indicated that, for women who initiate biennial screening at the age of 50 years, the cumulative risk after 10 years is 42% for experiencing at least one false-positive screening result and 6.4% for experiencing at least one false-positive screening result involving an invasive procedure,” Dr. Hofvind and her colleagues write.
Several principal investigators with the Breast Cancer Surveillance Consortium did not respond or were unavailable for comment when contacted by this news organization.
However, the study authors highlighted several factors that could help explain the discrepancy between the U.S. and European results.
In addition to double mammogram reading, “European guidelines recommend that breast radiologists read 3,500 to 11,000 mammograms annually, whereas 960 every 2 years are required by the U.S. Mammography Quality Standards Act,” the researchers note. They also point out that previous screening mammograms are readily available in Norway, whereas this is not always the case in the U.S.
“False-positive screening results are a part of the screening for breast cancer – and the women need to be informed about the risk,” Dr. Hofvind concluded. “The screening programs should aim to keep the rate as low as possible for the women [given] the costs.”
The study was supported by the Dam Foundation via the Norwegian Breast Cancer Society.
A version of this article first appeared on Medscape.com.
U.S. reports record-breaking 1.35 million new COVID cases in a day
The United States reported 1.35 million new COVID-19 cases on Jan. 10, logging the highest daily total for any country in the world during the pandemic.
The United States set the previous record of 1 million cases on Jan. 3. (A large number of cases are reported on Mondays, since many states don’t provide updates over the weekend, according to Reuters.)
Still, the 7-day average for new cases has surpassed 700,000, tripling in 2 weeks as the contagious Omicron variant continues to spread across the country.
The daily record of new cases came a day after the United States crossed the grim milestone of 60 million COVID-19 cases during the pandemic, according to the latest data from Johns Hopkins University. More than 11 million new cases were reported in the past 28 days, with 5 million reported since Jan. 2.
Globally, more than 310 million cases have been reported, resulting in nearly 5.5 million COVID-19 deaths. Almost 40 million cases have been confirmed worldwide during the past month, with the United States accounting for 28% of those.
Texas became the second state to report more than 5 million cases since the pandemic began, behind California’s total of 6 million cases. Florida has reported more than 4.6 million, while New York has reported more than 4.1 million.
The United States has also hit an all-time high for hospitalizations, with nearly 146,000 COVID-19 patients in hospitals across the country, according to the latest data from the U.S. Department of Health and Human Services. The previous record was 142,000 hospitalizations in January 2021.
Jan. 11’s hospitalizations are more than twice as many as 2 weeks ago, according to CNN. About 78% of inpatient beds are in use nationwide, and 21% are being used for COVID-19 patients.
Deaths are averaging about 1,700 per day, Reuters reported, which is up from 1,400 in recent days but not much higher than earlier this winter. The peak average was 3,400 daily deaths in mid-January 2021.
The surging numbers of cases and hospitalizations across the country are straining hospitals. On Jan. 10, Virginia Gov. Ralph Northam declared a state of emergency after the number of intensive care unit hospitalizations more than doubled since Dec. 1, CNN reported. The order allows hospitals to expand bed capacity, use telehealth options, and be more flexible with staffing.
Texas is hiring at least 2,700 medical staff to help with the surge, CNN reported, and Kentucky has mobilized the National Guard to provide support.
“Omicron continues to burn through the commonwealth, growing at levels we have never seen before. Omicron is significantly more contagious than even the Delta variant,” Kentucky Gov. Andy Beshear said during a news briefing Jan. 10.
Kentucky reported its highest weekly total of cases last week and has its highest rate of positive tests, at 26%. Mr. Beshear said the state is down to 134 available adult ICU beds.
“If it spreads at the rate we are seeing, it is certainly going to fill up our hospitals,” he said.
A version of this article first appeared on WebMD.com.
The United States reported 1.35 million new COVID-19 cases on Jan. 10, logging the highest daily total for any country in the world during the pandemic.
The United States set the previous record of 1 million cases on Jan. 3. (A large number of cases are reported on Mondays, since many states don’t provide updates over the weekend, according to Reuters.)
Still, the 7-day average for new cases has surpassed 700,000, tripling in 2 weeks as the contagious Omicron variant continues to spread across the country.
The daily record of new cases came a day after the United States crossed the grim milestone of 60 million COVID-19 cases during the pandemic, according to the latest data from Johns Hopkins University. More than 11 million new cases were reported in the past 28 days, with 5 million reported since Jan. 2.
Globally, more than 310 million cases have been reported, resulting in nearly 5.5 million COVID-19 deaths. Almost 40 million cases have been confirmed worldwide during the past month, with the United States accounting for 28% of those.
Texas became the second state to report more than 5 million cases since the pandemic began, behind California’s total of 6 million cases. Florida has reported more than 4.6 million, while New York has reported more than 4.1 million.
The United States has also hit an all-time high for hospitalizations, with nearly 146,000 COVID-19 patients in hospitals across the country, according to the latest data from the U.S. Department of Health and Human Services. The previous record was 142,000 hospitalizations in January 2021.
Jan. 11’s hospitalizations are more than twice as many as 2 weeks ago, according to CNN. About 78% of inpatient beds are in use nationwide, and 21% are being used for COVID-19 patients.
Deaths are averaging about 1,700 per day, Reuters reported, which is up from 1,400 in recent days but not much higher than earlier this winter. The peak average was 3,400 daily deaths in mid-January 2021.
The surging numbers of cases and hospitalizations across the country are straining hospitals. On Jan. 10, Virginia Gov. Ralph Northam declared a state of emergency after the number of intensive care unit hospitalizations more than doubled since Dec. 1, CNN reported. The order allows hospitals to expand bed capacity, use telehealth options, and be more flexible with staffing.
Texas is hiring at least 2,700 medical staff to help with the surge, CNN reported, and Kentucky has mobilized the National Guard to provide support.
“Omicron continues to burn through the commonwealth, growing at levels we have never seen before. Omicron is significantly more contagious than even the Delta variant,” Kentucky Gov. Andy Beshear said during a news briefing Jan. 10.
Kentucky reported its highest weekly total of cases last week and has its highest rate of positive tests, at 26%. Mr. Beshear said the state is down to 134 available adult ICU beds.
“If it spreads at the rate we are seeing, it is certainly going to fill up our hospitals,” he said.
A version of this article first appeared on WebMD.com.
The United States reported 1.35 million new COVID-19 cases on Jan. 10, logging the highest daily total for any country in the world during the pandemic.
The United States set the previous record of 1 million cases on Jan. 3. (A large number of cases are reported on Mondays, since many states don’t provide updates over the weekend, according to Reuters.)
Still, the 7-day average for new cases has surpassed 700,000, tripling in 2 weeks as the contagious Omicron variant continues to spread across the country.
The daily record of new cases came a day after the United States crossed the grim milestone of 60 million COVID-19 cases during the pandemic, according to the latest data from Johns Hopkins University. More than 11 million new cases were reported in the past 28 days, with 5 million reported since Jan. 2.
Globally, more than 310 million cases have been reported, resulting in nearly 5.5 million COVID-19 deaths. Almost 40 million cases have been confirmed worldwide during the past month, with the United States accounting for 28% of those.
Texas became the second state to report more than 5 million cases since the pandemic began, behind California’s total of 6 million cases. Florida has reported more than 4.6 million, while New York has reported more than 4.1 million.
The United States has also hit an all-time high for hospitalizations, with nearly 146,000 COVID-19 patients in hospitals across the country, according to the latest data from the U.S. Department of Health and Human Services. The previous record was 142,000 hospitalizations in January 2021.
Jan. 11’s hospitalizations are more than twice as many as 2 weeks ago, according to CNN. About 78% of inpatient beds are in use nationwide, and 21% are being used for COVID-19 patients.
Deaths are averaging about 1,700 per day, Reuters reported, which is up from 1,400 in recent days but not much higher than earlier this winter. The peak average was 3,400 daily deaths in mid-January 2021.
The surging numbers of cases and hospitalizations across the country are straining hospitals. On Jan. 10, Virginia Gov. Ralph Northam declared a state of emergency after the number of intensive care unit hospitalizations more than doubled since Dec. 1, CNN reported. The order allows hospitals to expand bed capacity, use telehealth options, and be more flexible with staffing.
Texas is hiring at least 2,700 medical staff to help with the surge, CNN reported, and Kentucky has mobilized the National Guard to provide support.
“Omicron continues to burn through the commonwealth, growing at levels we have never seen before. Omicron is significantly more contagious than even the Delta variant,” Kentucky Gov. Andy Beshear said during a news briefing Jan. 10.
Kentucky reported its highest weekly total of cases last week and has its highest rate of positive tests, at 26%. Mr. Beshear said the state is down to 134 available adult ICU beds.
“If it spreads at the rate we are seeing, it is certainly going to fill up our hospitals,” he said.
A version of this article first appeared on WebMD.com.
HPV testing plus cytology catches two times more cervical lesions
, according to a new study.
The study, which analyzed data from Mexico’s population-based hrHPV screening program over 6 years, confirms the importance of HPV screening for catching high-grade cervical lesions early.
“Our results provide evidence that hrHPV testing is the best strategy for a timely diagnosis of CIN2+ lesions while avoiding overtreatment of young women,” the study authors write. “Many countries now use hrHPV testing as the primary screening method, given it has higher sensitivity and detects more cervical cancer precursor lesions, such as CIN2+.”
According to Erik Jansen, MSc, the analysis supports recent updates to U.S. screening standards and confirms findings from previous trials, which show that HPV testing significantly improves prevention of cervical cancer.
“The significance of this paper is that the data reported is from a long follow-up in a country that implemented HPV screening on a large scale,” Mr. Jansen, PhD candidate in the Department of Public Health, Erasmus University Medical Center, Rotterdam, the Netherlands, told this news organization.
The study, conducted by Mexico’s National Institute of Public Health, analyzed screening data from the country’s public cervical cancer prevention program from 2010 to 2015. More than 2 million women aged 34 to 65 who had hrHPV-based screening tests followed by cytologic triage if they were HPV positive were included, as were 2.8 million women of the same age who received cytologic testing alone.
In the hrHPV group, 1.2% of women (n = 24,276) received referrals to colposcopy versus 3.1% of women (n = 90,980) in the cytology group. And among all women, only 0.8% who had abnormal results (n = 16,459) in the HPV went for a colposcopy versus 1.5% (n = 43,638) in the cytology group.
Overall, the authors found that 13.3 colposcopies were required to detect a single CIN2+ case in the cytology group compared to 5.7 colposcopies in the hrHPV with cytologic triage group.
The authors also note that the cost of colposcopies was three times lower in the HPV testing group and that the positive predictive value of hrHPV testing with cytologic triage was 17.5% versus 7.5% for cytology alone.
“The positive predictive value did not change for either screening strategy whether or not women lost to follow-up were taken into account,” the authors write.
Although Mr. Jansen noted that the findings are important, he also pointed to several limitations – namely, the significant loss to follow-up in the HPV group.
The HPV testing and cytologic triage happened in separate visits, and under the two-visit protocol, more than 50% of women who tested positive for HPV didn’t return for cytology. Such a significant loss to follow-up may call some of the findings into question, Mr. Jansen noted.
For instance, the rate of colposcopy referrals does not account for HPV-positive women who skipped their cytology screening. Assuming the same HPV risk for women who received cytology and those who did not, Mr. Jansen calculated that without any loss to follow-up, the colposcopy referral rate would have increased from the reported 1.2% to 2.6%, which is much closer to the 3.1% of the women referred in the cytology arm.
The lower colposcopy costs in the HPV group were also likely due, in part, to the loss to follow-up, which is not necessarily a good thing, Mr. Jansen said.
Still, “this study does confirm the finding that a primary HPV screening program is more effective than cytology [alone],” Mr. Jansen said.
Co-author Eduardo Franco reported receiving grants and personal fees from MSD and has a pending patent, “Methylation Markers in Cervical Cancer.” All other authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
, according to a new study.
The study, which analyzed data from Mexico’s population-based hrHPV screening program over 6 years, confirms the importance of HPV screening for catching high-grade cervical lesions early.
“Our results provide evidence that hrHPV testing is the best strategy for a timely diagnosis of CIN2+ lesions while avoiding overtreatment of young women,” the study authors write. “Many countries now use hrHPV testing as the primary screening method, given it has higher sensitivity and detects more cervical cancer precursor lesions, such as CIN2+.”
According to Erik Jansen, MSc, the analysis supports recent updates to U.S. screening standards and confirms findings from previous trials, which show that HPV testing significantly improves prevention of cervical cancer.
“The significance of this paper is that the data reported is from a long follow-up in a country that implemented HPV screening on a large scale,” Mr. Jansen, PhD candidate in the Department of Public Health, Erasmus University Medical Center, Rotterdam, the Netherlands, told this news organization.
The study, conducted by Mexico’s National Institute of Public Health, analyzed screening data from the country’s public cervical cancer prevention program from 2010 to 2015. More than 2 million women aged 34 to 65 who had hrHPV-based screening tests followed by cytologic triage if they were HPV positive were included, as were 2.8 million women of the same age who received cytologic testing alone.
In the hrHPV group, 1.2% of women (n = 24,276) received referrals to colposcopy versus 3.1% of women (n = 90,980) in the cytology group. And among all women, only 0.8% who had abnormal results (n = 16,459) in the HPV went for a colposcopy versus 1.5% (n = 43,638) in the cytology group.
Overall, the authors found that 13.3 colposcopies were required to detect a single CIN2+ case in the cytology group compared to 5.7 colposcopies in the hrHPV with cytologic triage group.
The authors also note that the cost of colposcopies was three times lower in the HPV testing group and that the positive predictive value of hrHPV testing with cytologic triage was 17.5% versus 7.5% for cytology alone.
“The positive predictive value did not change for either screening strategy whether or not women lost to follow-up were taken into account,” the authors write.
Although Mr. Jansen noted that the findings are important, he also pointed to several limitations – namely, the significant loss to follow-up in the HPV group.
The HPV testing and cytologic triage happened in separate visits, and under the two-visit protocol, more than 50% of women who tested positive for HPV didn’t return for cytology. Such a significant loss to follow-up may call some of the findings into question, Mr. Jansen noted.
For instance, the rate of colposcopy referrals does not account for HPV-positive women who skipped their cytology screening. Assuming the same HPV risk for women who received cytology and those who did not, Mr. Jansen calculated that without any loss to follow-up, the colposcopy referral rate would have increased from the reported 1.2% to 2.6%, which is much closer to the 3.1% of the women referred in the cytology arm.
The lower colposcopy costs in the HPV group were also likely due, in part, to the loss to follow-up, which is not necessarily a good thing, Mr. Jansen said.
Still, “this study does confirm the finding that a primary HPV screening program is more effective than cytology [alone],” Mr. Jansen said.
Co-author Eduardo Franco reported receiving grants and personal fees from MSD and has a pending patent, “Methylation Markers in Cervical Cancer.” All other authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
, according to a new study.
The study, which analyzed data from Mexico’s population-based hrHPV screening program over 6 years, confirms the importance of HPV screening for catching high-grade cervical lesions early.
“Our results provide evidence that hrHPV testing is the best strategy for a timely diagnosis of CIN2+ lesions while avoiding overtreatment of young women,” the study authors write. “Many countries now use hrHPV testing as the primary screening method, given it has higher sensitivity and detects more cervical cancer precursor lesions, such as CIN2+.”
According to Erik Jansen, MSc, the analysis supports recent updates to U.S. screening standards and confirms findings from previous trials, which show that HPV testing significantly improves prevention of cervical cancer.
“The significance of this paper is that the data reported is from a long follow-up in a country that implemented HPV screening on a large scale,” Mr. Jansen, PhD candidate in the Department of Public Health, Erasmus University Medical Center, Rotterdam, the Netherlands, told this news organization.
The study, conducted by Mexico’s National Institute of Public Health, analyzed screening data from the country’s public cervical cancer prevention program from 2010 to 2015. More than 2 million women aged 34 to 65 who had hrHPV-based screening tests followed by cytologic triage if they were HPV positive were included, as were 2.8 million women of the same age who received cytologic testing alone.
In the hrHPV group, 1.2% of women (n = 24,276) received referrals to colposcopy versus 3.1% of women (n = 90,980) in the cytology group. And among all women, only 0.8% who had abnormal results (n = 16,459) in the HPV went for a colposcopy versus 1.5% (n = 43,638) in the cytology group.
Overall, the authors found that 13.3 colposcopies were required to detect a single CIN2+ case in the cytology group compared to 5.7 colposcopies in the hrHPV with cytologic triage group.
The authors also note that the cost of colposcopies was three times lower in the HPV testing group and that the positive predictive value of hrHPV testing with cytologic triage was 17.5% versus 7.5% for cytology alone.
“The positive predictive value did not change for either screening strategy whether or not women lost to follow-up were taken into account,” the authors write.
Although Mr. Jansen noted that the findings are important, he also pointed to several limitations – namely, the significant loss to follow-up in the HPV group.
The HPV testing and cytologic triage happened in separate visits, and under the two-visit protocol, more than 50% of women who tested positive for HPV didn’t return for cytology. Such a significant loss to follow-up may call some of the findings into question, Mr. Jansen noted.
For instance, the rate of colposcopy referrals does not account for HPV-positive women who skipped their cytology screening. Assuming the same HPV risk for women who received cytology and those who did not, Mr. Jansen calculated that without any loss to follow-up, the colposcopy referral rate would have increased from the reported 1.2% to 2.6%, which is much closer to the 3.1% of the women referred in the cytology arm.
The lower colposcopy costs in the HPV group were also likely due, in part, to the loss to follow-up, which is not necessarily a good thing, Mr. Jansen said.
Still, “this study does confirm the finding that a primary HPV screening program is more effective than cytology [alone],” Mr. Jansen said.
Co-author Eduardo Franco reported receiving grants and personal fees from MSD and has a pending patent, “Methylation Markers in Cervical Cancer.” All other authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Are there perinatal benefits to pregnant patients after bariatric surgery?
Getahun D, Fassett MJ, Jacobsen SJ, et al. Perinatal outcomes after bariatric surgery. Am J Obstet Gynecol. 2021;S0002-9378(21)00771-7. doi: 10.1016/j.ajog.2021 .06.087.
EXPERT COMMENTARY
Prepregnancy obesity continues to rise in the United States, with a prevalence of 29% among reproductive-age women in 2019, an 11% increase from 2016.1 Pregnant patients with obesity are at increased risk for multiple adverse perinatal outcomes, including gestational diabetes and preeclampsia. Bariatric surgery is effective for weight loss and has been shown to improve comorbidities associated with obesity,2 and it may have potential benefits for pregnancy outcomes, such as reducing rates of gestational diabetes and preeclampsia.3-5 However, little was known about other outcomes as well as other potential factors before a recent study in which investigators examined perinatal outcomes after bariatric surgery.
Details of the study
Getahun and colleagues conducted a population-based, retrospective study of pregnant patients who were eligible for bariatric surgery (body mass index [BMI] ≥40 kg/m2 with no comorbidities or a BMI between 35 and 40 kg/m2 with obesity-related comorbidities, such as diabetes). They aimed to evaluate the association of bariatric surgery with adverse perinatal outcomes.
Results. In a large sample of pregnant patients eligible for bariatric surgery (N = 20,213), the authors found that patients who had bariatric surgery (n = 1,886) had a reduced risk of macrosomia (aOR, 0.24), preeclampsia (aOR, 0.53), gestational diabetes (aOR, 0.60), and cesarean delivery (aOR, 0.65) compared with those who did not have bariatric surgery (n = 18,327). They also found that patients who had bariatric surgery had an increased risk of small-for-gestational age neonates (aOR, 2.46) and postpartum hemorrhage (aOR, 1.79).
These results remained after adjusting for other potential confounders. The authors evaluated the outcomes based on the timing of surgery and the patients’ pregnancy (<1 year, 1-1.5 years, 1.5-2 years, >2 years). The outcomes were more favorable among the patients who had the bariatric surgery regardless of the time interval of surgery to pregnancy than those who did not have the surgery. In addition, the benefits of bariatric surgery did not differ between the 2 most common types of bariatric surgery (Roux-en-Y gastric bypass and vertical sleeve gastrectomy) performed in this study, and both had better outcomes than those who did not have the surgery. Finally, patients with chronic hypertension and pregestational diabetes who had bariatric surgery also had lower risks of adverse outcomes than those without bariatric surgery.
Study strengths and limitations
Given the study’s retrospective design, uncertainties and important confounders could not be addressed, such as why certain eligible patients had the surgery and others did not. However, with its large sample size and an appropriate comparison group, the study findings further support the perinatal benefits of bariatric surgery in obese patients. Of note, this study also had a large sample of Black and Hispanic patients, populations known to have higher rates of obesity1 and pregnancy complications. Subgroup analyses within each racial/ethnic group revealed that those who had the surgery had lower risks of adverse perinatal outcomes than those who did not.
Patients who had the bariatric surgery had an increased risk of postpartum hemorrhage; however, there is no physiologic basis or theory to explain this finding, so further studies are needed. Lastly, although patients who had bariatric surgery had an increased risk of small-for-gestational-age babies and the study was not powered for the risk of stillbirth, the patients who had the surgery had a reduced risk of neonates admitted to the neonatal intensive care unit. More data would have been beneficial to assess if these small-for-gestational-age babies were healthy. In general, obese patients tend to have larger and unhealthy babies; thus, healthier babies, even if small for gestational age, would not be an adverse outcome.
Benefits of bariatric surgery extend to perinatal outcomes
This study reinforces current practice that includes eligible patients being counseled about the health-related benefits of bariatric surgery, which now includes more perinatal outcomes. The finding of the increased risk of small-for-gestational-age fetuses supports the practice of a screening growth ultrasound exam in patients who had bariatric surgery. ●
An important, modifiable risk factor for adverse perinatal outcomes is the patient’s prepregnancy BMI at the time of pregnancy. Bariatric surgery is an effective procedure for weight loss. There are many perinatal benefits for eligible patients who have bariatric surgery before pregnancy. Clinicians should counsel their obese patients who are considering or planning pregnancy about the benefits of bariatric surgery.
RODNEY A. MCLAREN, JR, MD, AND VINCENZO BERGHELLA, MD
- Driscoll AK, Gregory ECW. Increases in prepregnancy obesity: United States, 2016-2019. NCHS Data Brief. 2020 Nov;(392):1-8.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724- 1737. doi: 10.1001/jama.292.14.1724.
- Maggard MA, Yermilov I, Li Z, et al. Pregnancy and fertility following bariatric surgery: a systematic review. JAMA. 2008;300:2286-2296. doi: 10.1001/jama.2008.641.
- Watanabe A, Seki Y, Haruta H, et al. Maternal impacts and perinatal outcomes after three types of bariatric surgery at a single institution. Arch Gynecol Obstet. 2019;300:145-152. doi: 10.1007/s00404-019-05195-9.
- Balestrin B, Urbanetz AA, Barbieri MM, et al. Pregnancy after bariatric surgery: a comparative study of post-bariatric pregnant women versus non-bariatric obese pregnant women. Obes Surg. 2019;29:3142-3148. doi: 10.1007/s11695- 019-03961-x.
Getahun D, Fassett MJ, Jacobsen SJ, et al. Perinatal outcomes after bariatric surgery. Am J Obstet Gynecol. 2021;S0002-9378(21)00771-7. doi: 10.1016/j.ajog.2021 .06.087.
EXPERT COMMENTARY
Prepregnancy obesity continues to rise in the United States, with a prevalence of 29% among reproductive-age women in 2019, an 11% increase from 2016.1 Pregnant patients with obesity are at increased risk for multiple adverse perinatal outcomes, including gestational diabetes and preeclampsia. Bariatric surgery is effective for weight loss and has been shown to improve comorbidities associated with obesity,2 and it may have potential benefits for pregnancy outcomes, such as reducing rates of gestational diabetes and preeclampsia.3-5 However, little was known about other outcomes as well as other potential factors before a recent study in which investigators examined perinatal outcomes after bariatric surgery.
Details of the study
Getahun and colleagues conducted a population-based, retrospective study of pregnant patients who were eligible for bariatric surgery (body mass index [BMI] ≥40 kg/m2 with no comorbidities or a BMI between 35 and 40 kg/m2 with obesity-related comorbidities, such as diabetes). They aimed to evaluate the association of bariatric surgery with adverse perinatal outcomes.
Results. In a large sample of pregnant patients eligible for bariatric surgery (N = 20,213), the authors found that patients who had bariatric surgery (n = 1,886) had a reduced risk of macrosomia (aOR, 0.24), preeclampsia (aOR, 0.53), gestational diabetes (aOR, 0.60), and cesarean delivery (aOR, 0.65) compared with those who did not have bariatric surgery (n = 18,327). They also found that patients who had bariatric surgery had an increased risk of small-for-gestational age neonates (aOR, 2.46) and postpartum hemorrhage (aOR, 1.79).
These results remained after adjusting for other potential confounders. The authors evaluated the outcomes based on the timing of surgery and the patients’ pregnancy (<1 year, 1-1.5 years, 1.5-2 years, >2 years). The outcomes were more favorable among the patients who had the bariatric surgery regardless of the time interval of surgery to pregnancy than those who did not have the surgery. In addition, the benefits of bariatric surgery did not differ between the 2 most common types of bariatric surgery (Roux-en-Y gastric bypass and vertical sleeve gastrectomy) performed in this study, and both had better outcomes than those who did not have the surgery. Finally, patients with chronic hypertension and pregestational diabetes who had bariatric surgery also had lower risks of adverse outcomes than those without bariatric surgery.
Study strengths and limitations
Given the study’s retrospective design, uncertainties and important confounders could not be addressed, such as why certain eligible patients had the surgery and others did not. However, with its large sample size and an appropriate comparison group, the study findings further support the perinatal benefits of bariatric surgery in obese patients. Of note, this study also had a large sample of Black and Hispanic patients, populations known to have higher rates of obesity1 and pregnancy complications. Subgroup analyses within each racial/ethnic group revealed that those who had the surgery had lower risks of adverse perinatal outcomes than those who did not.
Patients who had the bariatric surgery had an increased risk of postpartum hemorrhage; however, there is no physiologic basis or theory to explain this finding, so further studies are needed. Lastly, although patients who had bariatric surgery had an increased risk of small-for-gestational-age babies and the study was not powered for the risk of stillbirth, the patients who had the surgery had a reduced risk of neonates admitted to the neonatal intensive care unit. More data would have been beneficial to assess if these small-for-gestational-age babies were healthy. In general, obese patients tend to have larger and unhealthy babies; thus, healthier babies, even if small for gestational age, would not be an adverse outcome.
Benefits of bariatric surgery extend to perinatal outcomes
This study reinforces current practice that includes eligible patients being counseled about the health-related benefits of bariatric surgery, which now includes more perinatal outcomes. The finding of the increased risk of small-for-gestational-age fetuses supports the practice of a screening growth ultrasound exam in patients who had bariatric surgery. ●
An important, modifiable risk factor for adverse perinatal outcomes is the patient’s prepregnancy BMI at the time of pregnancy. Bariatric surgery is an effective procedure for weight loss. There are many perinatal benefits for eligible patients who have bariatric surgery before pregnancy. Clinicians should counsel their obese patients who are considering or planning pregnancy about the benefits of bariatric surgery.
RODNEY A. MCLAREN, JR, MD, AND VINCENZO BERGHELLA, MD
Getahun D, Fassett MJ, Jacobsen SJ, et al. Perinatal outcomes after bariatric surgery. Am J Obstet Gynecol. 2021;S0002-9378(21)00771-7. doi: 10.1016/j.ajog.2021 .06.087.
EXPERT COMMENTARY
Prepregnancy obesity continues to rise in the United States, with a prevalence of 29% among reproductive-age women in 2019, an 11% increase from 2016.1 Pregnant patients with obesity are at increased risk for multiple adverse perinatal outcomes, including gestational diabetes and preeclampsia. Bariatric surgery is effective for weight loss and has been shown to improve comorbidities associated with obesity,2 and it may have potential benefits for pregnancy outcomes, such as reducing rates of gestational diabetes and preeclampsia.3-5 However, little was known about other outcomes as well as other potential factors before a recent study in which investigators examined perinatal outcomes after bariatric surgery.
Details of the study
Getahun and colleagues conducted a population-based, retrospective study of pregnant patients who were eligible for bariatric surgery (body mass index [BMI] ≥40 kg/m2 with no comorbidities or a BMI between 35 and 40 kg/m2 with obesity-related comorbidities, such as diabetes). They aimed to evaluate the association of bariatric surgery with adverse perinatal outcomes.
Results. In a large sample of pregnant patients eligible for bariatric surgery (N = 20,213), the authors found that patients who had bariatric surgery (n = 1,886) had a reduced risk of macrosomia (aOR, 0.24), preeclampsia (aOR, 0.53), gestational diabetes (aOR, 0.60), and cesarean delivery (aOR, 0.65) compared with those who did not have bariatric surgery (n = 18,327). They also found that patients who had bariatric surgery had an increased risk of small-for-gestational age neonates (aOR, 2.46) and postpartum hemorrhage (aOR, 1.79).
These results remained after adjusting for other potential confounders. The authors evaluated the outcomes based on the timing of surgery and the patients’ pregnancy (<1 year, 1-1.5 years, 1.5-2 years, >2 years). The outcomes were more favorable among the patients who had the bariatric surgery regardless of the time interval of surgery to pregnancy than those who did not have the surgery. In addition, the benefits of bariatric surgery did not differ between the 2 most common types of bariatric surgery (Roux-en-Y gastric bypass and vertical sleeve gastrectomy) performed in this study, and both had better outcomes than those who did not have the surgery. Finally, patients with chronic hypertension and pregestational diabetes who had bariatric surgery also had lower risks of adverse outcomes than those without bariatric surgery.
Study strengths and limitations
Given the study’s retrospective design, uncertainties and important confounders could not be addressed, such as why certain eligible patients had the surgery and others did not. However, with its large sample size and an appropriate comparison group, the study findings further support the perinatal benefits of bariatric surgery in obese patients. Of note, this study also had a large sample of Black and Hispanic patients, populations known to have higher rates of obesity1 and pregnancy complications. Subgroup analyses within each racial/ethnic group revealed that those who had the surgery had lower risks of adverse perinatal outcomes than those who did not.
Patients who had the bariatric surgery had an increased risk of postpartum hemorrhage; however, there is no physiologic basis or theory to explain this finding, so further studies are needed. Lastly, although patients who had bariatric surgery had an increased risk of small-for-gestational-age babies and the study was not powered for the risk of stillbirth, the patients who had the surgery had a reduced risk of neonates admitted to the neonatal intensive care unit. More data would have been beneficial to assess if these small-for-gestational-age babies were healthy. In general, obese patients tend to have larger and unhealthy babies; thus, healthier babies, even if small for gestational age, would not be an adverse outcome.
Benefits of bariatric surgery extend to perinatal outcomes
This study reinforces current practice that includes eligible patients being counseled about the health-related benefits of bariatric surgery, which now includes more perinatal outcomes. The finding of the increased risk of small-for-gestational-age fetuses supports the practice of a screening growth ultrasound exam in patients who had bariatric surgery. ●
An important, modifiable risk factor for adverse perinatal outcomes is the patient’s prepregnancy BMI at the time of pregnancy. Bariatric surgery is an effective procedure for weight loss. There are many perinatal benefits for eligible patients who have bariatric surgery before pregnancy. Clinicians should counsel their obese patients who are considering or planning pregnancy about the benefits of bariatric surgery.
RODNEY A. MCLAREN, JR, MD, AND VINCENZO BERGHELLA, MD
- Driscoll AK, Gregory ECW. Increases in prepregnancy obesity: United States, 2016-2019. NCHS Data Brief. 2020 Nov;(392):1-8.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724- 1737. doi: 10.1001/jama.292.14.1724.
- Maggard MA, Yermilov I, Li Z, et al. Pregnancy and fertility following bariatric surgery: a systematic review. JAMA. 2008;300:2286-2296. doi: 10.1001/jama.2008.641.
- Watanabe A, Seki Y, Haruta H, et al. Maternal impacts and perinatal outcomes after three types of bariatric surgery at a single institution. Arch Gynecol Obstet. 2019;300:145-152. doi: 10.1007/s00404-019-05195-9.
- Balestrin B, Urbanetz AA, Barbieri MM, et al. Pregnancy after bariatric surgery: a comparative study of post-bariatric pregnant women versus non-bariatric obese pregnant women. Obes Surg. 2019;29:3142-3148. doi: 10.1007/s11695- 019-03961-x.
- Driscoll AK, Gregory ECW. Increases in prepregnancy obesity: United States, 2016-2019. NCHS Data Brief. 2020 Nov;(392):1-8.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724- 1737. doi: 10.1001/jama.292.14.1724.
- Maggard MA, Yermilov I, Li Z, et al. Pregnancy and fertility following bariatric surgery: a systematic review. JAMA. 2008;300:2286-2296. doi: 10.1001/jama.2008.641.
- Watanabe A, Seki Y, Haruta H, et al. Maternal impacts and perinatal outcomes after three types of bariatric surgery at a single institution. Arch Gynecol Obstet. 2019;300:145-152. doi: 10.1007/s00404-019-05195-9.
- Balestrin B, Urbanetz AA, Barbieri MM, et al. Pregnancy after bariatric surgery: a comparative study of post-bariatric pregnant women versus non-bariatric obese pregnant women. Obes Surg. 2019;29:3142-3148. doi: 10.1007/s11695- 019-03961-x.
Heavy snoring in early pregnancy linked to increased insulin resistance
Severe maternal sleep-disordered breathing (SDB) is a known risk factor for gestational diabetes, which is commonly diagnosed in the second or third trimester of pregnancy.
Now, a new study suggests that increases in insulin resistance, a precursor for gestational diabetes, may take place as early as the first trimester of pregnancy in women with risk factors for obstructive sleep apnea (OSA), such as overweight and habitual snoring.
This finding could potentially provide physicians with a window of opportunity to improve outcomes by screening at-risk women early in pregnancy or even prior to conception, Laura Sanapo, MD, assistant professor of medicine (research) at Brown University, Providence, R.I., and colleagues wrote in Sleep.
“Further studies are needed to investigate the association and its impact on the development of gestational diabetes, and to establish whether early-gestation or pregestational treatment of SDB would improve glucose metabolic outcomes in pregnancy,” they wrote.
”What this paper demonstrates is that the changes that predate gestational diabetes are seen much earlier in pregnancy,” senior study author Ghada Bourjeily, MD, professor of medicine at Brown University, said in an interview. Women should be screened for SDB rather than insulin resistance in early pregnancy since continuous positive airway pressure therapy (CPAP) is a highly effective intervention.
Waiting until midpregnancy to screen for OSA “is too late to make significant changes in the care of these women,” said Dr. Bourjeily, who is also director of research and training at the Women’s Medicine Collaborative at The Miriam Hospital in Providence, R.I. “By the time you diagnose gestational diabetes, the cat is out of the bag.”
For the study, women with early singleton pregnancies and risk factors for OSA such as habitual snoring and a median body mass index (BMI) of at least 27 kg/m2 were recruited from two prospective clinical trial studies enriched for OSA positivity. Women with a history of pregestational diabetes and those using CPAP or receiving chronic steroid therapy were excluded from the current study.
A total of 192 study participants underwent in-home sleep study (HSAT) and homeostatic model assessment (HOMA) between 11 and 15 gestational weeks, respectively. The association between continuous measures of SDB as a respiratory-event index as well as oxygen-desaturation index and glucose metabolism parameters such as insulin resistance (HOMA-IR) were analyzed after adjusting for gestational age, maternal age, BMI, ethnicity, race, and parity.
In all, 61 women (32%) were diagnosed with OSA based on respiratory event index values greater than or equal to five events per hour. These participants were more likely to be older, to have a high BMI, and to be multipara, compared with women who didn’t have a diagnosis of OSA. Women with a diagnosis of OSA exhibited higher glucose and C-peptide values and a higher degree of insulin resistance, compared with women without OSA, the researchers found. An increase of 0.3 in HOMA-IR related to maternal SDB in early pregnancy may significantly affect glucose metabolism.
Although the findings of the current study cannot be extrapolated to women who don’t have overweight or obesity, some women with normal-range BMI (18.5-24.9) are also at increased risk of glucose metabolism changes, Dr. Bourjeily pointed out. This includes those of Southeast Asian descent. “We found that the association of SDB parameters with insulin resistance was actually happening independently of BMI and other factors.”
Ideally, screening for SDB would begin prior to pregnancy, Dr. Bourjeily said. A BMI greater than 25 should be taken into account and patients asked if they snore and if so, whether it’s loud enough to wake their partner. They should also be asked about experiencing daytime sleepiness.
“Based on these answers, especially in women screened prior to pregnancy, there will be time to make the diagnosis of sleep apnea and get the patient on CPAP,” Dr. Bourjeily said.
“This is an interesting study and one of the rare ones looking at early pregnancy and some of the mechanisms that could possibly be contributing to gestational diabetes,” commented Grenye O’Malley, MD, assistant professor in the division of endocrinology, diabetes, and bone disease at the Icahn School of Medicine at Mount Sinai, New York. Dr. O’Malley was not involved in the study.
“It confirms our suspicions that there’s probably a lot of things happening earlier in pregnancy before a diagnosis of gestational diabetes. It also confirms that some of the mechanisms are probably very similar to those involved in the association between disordered sleep and the development of type 2 diabetes.”
However, it’s too early to determine whether screening for SDB and the use of CPAP will prevent glycemic changes, Dr. O’Malley said in an interview. “Whenever we screen, we ask whether we have an intervention that changes outcomes and we don’t know that yet.”
Some of the symptoms of SDB are also common in early pregnancy, such as a BMI greater than 25 and daytime sleepiness, Dr. O’Malley pointed out. It was unclear whether the study participants had a propensity to develop type 2 diabetes or whether they were at risk of gestational diabetes.
This study was funded by the National Heart, Lung, and Blood Institute; the National Institute for Child Health; and the National Institute of General Medical Sciences. Dr. Bourjeily and colleagues, as well as Dr. O’Malley, reported having no potential financial conflicts of interest.
Severe maternal sleep-disordered breathing (SDB) is a known risk factor for gestational diabetes, which is commonly diagnosed in the second or third trimester of pregnancy.
Now, a new study suggests that increases in insulin resistance, a precursor for gestational diabetes, may take place as early as the first trimester of pregnancy in women with risk factors for obstructive sleep apnea (OSA), such as overweight and habitual snoring.
This finding could potentially provide physicians with a window of opportunity to improve outcomes by screening at-risk women early in pregnancy or even prior to conception, Laura Sanapo, MD, assistant professor of medicine (research) at Brown University, Providence, R.I., and colleagues wrote in Sleep.
“Further studies are needed to investigate the association and its impact on the development of gestational diabetes, and to establish whether early-gestation or pregestational treatment of SDB would improve glucose metabolic outcomes in pregnancy,” they wrote.
”What this paper demonstrates is that the changes that predate gestational diabetes are seen much earlier in pregnancy,” senior study author Ghada Bourjeily, MD, professor of medicine at Brown University, said in an interview. Women should be screened for SDB rather than insulin resistance in early pregnancy since continuous positive airway pressure therapy (CPAP) is a highly effective intervention.
Waiting until midpregnancy to screen for OSA “is too late to make significant changes in the care of these women,” said Dr. Bourjeily, who is also director of research and training at the Women’s Medicine Collaborative at The Miriam Hospital in Providence, R.I. “By the time you diagnose gestational diabetes, the cat is out of the bag.”
For the study, women with early singleton pregnancies and risk factors for OSA such as habitual snoring and a median body mass index (BMI) of at least 27 kg/m2 were recruited from two prospective clinical trial studies enriched for OSA positivity. Women with a history of pregestational diabetes and those using CPAP or receiving chronic steroid therapy were excluded from the current study.
A total of 192 study participants underwent in-home sleep study (HSAT) and homeostatic model assessment (HOMA) between 11 and 15 gestational weeks, respectively. The association between continuous measures of SDB as a respiratory-event index as well as oxygen-desaturation index and glucose metabolism parameters such as insulin resistance (HOMA-IR) were analyzed after adjusting for gestational age, maternal age, BMI, ethnicity, race, and parity.
In all, 61 women (32%) were diagnosed with OSA based on respiratory event index values greater than or equal to five events per hour. These participants were more likely to be older, to have a high BMI, and to be multipara, compared with women who didn’t have a diagnosis of OSA. Women with a diagnosis of OSA exhibited higher glucose and C-peptide values and a higher degree of insulin resistance, compared with women without OSA, the researchers found. An increase of 0.3 in HOMA-IR related to maternal SDB in early pregnancy may significantly affect glucose metabolism.
Although the findings of the current study cannot be extrapolated to women who don’t have overweight or obesity, some women with normal-range BMI (18.5-24.9) are also at increased risk of glucose metabolism changes, Dr. Bourjeily pointed out. This includes those of Southeast Asian descent. “We found that the association of SDB parameters with insulin resistance was actually happening independently of BMI and other factors.”
Ideally, screening for SDB would begin prior to pregnancy, Dr. Bourjeily said. A BMI greater than 25 should be taken into account and patients asked if they snore and if so, whether it’s loud enough to wake their partner. They should also be asked about experiencing daytime sleepiness.
“Based on these answers, especially in women screened prior to pregnancy, there will be time to make the diagnosis of sleep apnea and get the patient on CPAP,” Dr. Bourjeily said.
“This is an interesting study and one of the rare ones looking at early pregnancy and some of the mechanisms that could possibly be contributing to gestational diabetes,” commented Grenye O’Malley, MD, assistant professor in the division of endocrinology, diabetes, and bone disease at the Icahn School of Medicine at Mount Sinai, New York. Dr. O’Malley was not involved in the study.
“It confirms our suspicions that there’s probably a lot of things happening earlier in pregnancy before a diagnosis of gestational diabetes. It also confirms that some of the mechanisms are probably very similar to those involved in the association between disordered sleep and the development of type 2 diabetes.”
However, it’s too early to determine whether screening for SDB and the use of CPAP will prevent glycemic changes, Dr. O’Malley said in an interview. “Whenever we screen, we ask whether we have an intervention that changes outcomes and we don’t know that yet.”
Some of the symptoms of SDB are also common in early pregnancy, such as a BMI greater than 25 and daytime sleepiness, Dr. O’Malley pointed out. It was unclear whether the study participants had a propensity to develop type 2 diabetes or whether they were at risk of gestational diabetes.
This study was funded by the National Heart, Lung, and Blood Institute; the National Institute for Child Health; and the National Institute of General Medical Sciences. Dr. Bourjeily and colleagues, as well as Dr. O’Malley, reported having no potential financial conflicts of interest.
Severe maternal sleep-disordered breathing (SDB) is a known risk factor for gestational diabetes, which is commonly diagnosed in the second or third trimester of pregnancy.
Now, a new study suggests that increases in insulin resistance, a precursor for gestational diabetes, may take place as early as the first trimester of pregnancy in women with risk factors for obstructive sleep apnea (OSA), such as overweight and habitual snoring.
This finding could potentially provide physicians with a window of opportunity to improve outcomes by screening at-risk women early in pregnancy or even prior to conception, Laura Sanapo, MD, assistant professor of medicine (research) at Brown University, Providence, R.I., and colleagues wrote in Sleep.
“Further studies are needed to investigate the association and its impact on the development of gestational diabetes, and to establish whether early-gestation or pregestational treatment of SDB would improve glucose metabolic outcomes in pregnancy,” they wrote.
”What this paper demonstrates is that the changes that predate gestational diabetes are seen much earlier in pregnancy,” senior study author Ghada Bourjeily, MD, professor of medicine at Brown University, said in an interview. Women should be screened for SDB rather than insulin resistance in early pregnancy since continuous positive airway pressure therapy (CPAP) is a highly effective intervention.
Waiting until midpregnancy to screen for OSA “is too late to make significant changes in the care of these women,” said Dr. Bourjeily, who is also director of research and training at the Women’s Medicine Collaborative at The Miriam Hospital in Providence, R.I. “By the time you diagnose gestational diabetes, the cat is out of the bag.”
For the study, women with early singleton pregnancies and risk factors for OSA such as habitual snoring and a median body mass index (BMI) of at least 27 kg/m2 were recruited from two prospective clinical trial studies enriched for OSA positivity. Women with a history of pregestational diabetes and those using CPAP or receiving chronic steroid therapy were excluded from the current study.
A total of 192 study participants underwent in-home sleep study (HSAT) and homeostatic model assessment (HOMA) between 11 and 15 gestational weeks, respectively. The association between continuous measures of SDB as a respiratory-event index as well as oxygen-desaturation index and glucose metabolism parameters such as insulin resistance (HOMA-IR) were analyzed after adjusting for gestational age, maternal age, BMI, ethnicity, race, and parity.
In all, 61 women (32%) were diagnosed with OSA based on respiratory event index values greater than or equal to five events per hour. These participants were more likely to be older, to have a high BMI, and to be multipara, compared with women who didn’t have a diagnosis of OSA. Women with a diagnosis of OSA exhibited higher glucose and C-peptide values and a higher degree of insulin resistance, compared with women without OSA, the researchers found. An increase of 0.3 in HOMA-IR related to maternal SDB in early pregnancy may significantly affect glucose metabolism.
Although the findings of the current study cannot be extrapolated to women who don’t have overweight or obesity, some women with normal-range BMI (18.5-24.9) are also at increased risk of glucose metabolism changes, Dr. Bourjeily pointed out. This includes those of Southeast Asian descent. “We found that the association of SDB parameters with insulin resistance was actually happening independently of BMI and other factors.”
Ideally, screening for SDB would begin prior to pregnancy, Dr. Bourjeily said. A BMI greater than 25 should be taken into account and patients asked if they snore and if so, whether it’s loud enough to wake their partner. They should also be asked about experiencing daytime sleepiness.
“Based on these answers, especially in women screened prior to pregnancy, there will be time to make the diagnosis of sleep apnea and get the patient on CPAP,” Dr. Bourjeily said.
“This is an interesting study and one of the rare ones looking at early pregnancy and some of the mechanisms that could possibly be contributing to gestational diabetes,” commented Grenye O’Malley, MD, assistant professor in the division of endocrinology, diabetes, and bone disease at the Icahn School of Medicine at Mount Sinai, New York. Dr. O’Malley was not involved in the study.
“It confirms our suspicions that there’s probably a lot of things happening earlier in pregnancy before a diagnosis of gestational diabetes. It also confirms that some of the mechanisms are probably very similar to those involved in the association between disordered sleep and the development of type 2 diabetes.”
However, it’s too early to determine whether screening for SDB and the use of CPAP will prevent glycemic changes, Dr. O’Malley said in an interview. “Whenever we screen, we ask whether we have an intervention that changes outcomes and we don’t know that yet.”
Some of the symptoms of SDB are also common in early pregnancy, such as a BMI greater than 25 and daytime sleepiness, Dr. O’Malley pointed out. It was unclear whether the study participants had a propensity to develop type 2 diabetes or whether they were at risk of gestational diabetes.
This study was funded by the National Heart, Lung, and Blood Institute; the National Institute for Child Health; and the National Institute of General Medical Sciences. Dr. Bourjeily and colleagues, as well as Dr. O’Malley, reported having no potential financial conflicts of interest.
FROM SLEEP
PA name change bad for patients and the profession
Physician assistants (PAs) are angry with me, and with good reason. I had the audacity to lump them together with nurse practitioners (NPs) in my book “Patients at Risk,” an act which one highly placed PA leader called “distasteful” in a private conversation with me.
I will admit that PAs have reason to be upset. With competitive acceptance rates including a requirement for extensive health care experience before PA school, standardized training, and at least 2,000 hours of clinical experience before graduation, the profession is a stark contrast to the haphazard training and 500 clinical hours required of NPs today. Further, unlike NPs, who have sought independent practice since the 1980s, PAs have traditionally been close allies with physicians, generally working in a 1:1 supervision model.
The truth is that it hurt to include PAs with NPs in my book. I’ve had my own close relationships with PAs over the years and found the PAs I worked with to be outstanding clinicians. Unfortunately, the profession has given me no choice. Following a model set by the NP profession,
Their efforts began with a change in terminology. “Optimal team practice” (OTP) was supposed to give PAs more flexibility, allowing them to work for hospitals or physician groups rather than under the responsibility of one physician. Not surprisingly, corporations and even academic centers have been quick to take advantage, hiring PAs and placing them in positions without adequate physician support. OTP paved the way for independent practice, as PAs sought and gained independence from any physician supervision in North Dakota, the first state to grant them that right.
Most recently, PAs have determined to change their name entirely, calling themselves physician associates. This move by the American Academy of Physician Assistants is the culmination of a years-long marketing study on how to increase the relevance and improve patient perception of the PA profession. The AAPA decision is expected to galvanize state and local PA organizations to lobby legislators for legal and regulatory changes that allow the use of the “physician associate” title, which is not currently a legal representation of PA licensure.
PAs’ latest attempt at title and branding reform follows years of advocacy to not be referred to as physician extenders or midlevel providers. For example, to gain more public acceptance of the PA model, the profession launched the public relations campaign “Your PA Can,” closely mirroring the “We Choose NPs” media blitz. PAs have also followed other dangerous precedents set by NPs, including 100% online training and a new “Doctor of Medical Science” degree, allowing PAs, as well as NPs, to now be called “doctors.”
I can understand PA reasoning even if I don’t agree with it. PAs are frustrated to be treated as second-class citizens compared with NPs, who have been granted independent practice in half the states in the union despite having a fraction of PA training. Frankly, it’s unfair that NPs are being hired preferentially over PAs simply because of looser legal requirements for physician oversight. The bottom line is that NPs have been more successful at persuading legislators to allow them independence – but that doesn’t make it right for either group.
While PAs have more clinical training upon graduation than NPs, they still have far less than physicians. PAs generally attend a 2-year master’s degree program after college which includes 2,000 hours of hands-on clinical work. By comparison, the average medical student spends 4 years and receives 5,000-6,000 hours of supervised clinical training upon graduation. But this isn’t considered enough for a graduate medical student to practice medicine independently.
Physicians must complete at least 3 years of postgraduate residency training in most states to receive a medical license, and by the time a physician is permitted to practice medicine unsupervised, they will have attained no fewer than 15,000-20,000 hours of supervised clinical practice, with years of specialty-specific training.
Patients want and deserve access to truly physician-led care, but in many parts of the country, physicians are being replaced by nonphysician practitioners to boost corporate profits. In many cases, patients are kept in the dark about the differences in training between the medical professionals now in charge of their care. The American Medical Association and other critics have expressed concern that the proposed title of “physician associate” is likely to further obscure the training and roles of medical professionals, already a source of confusion to patients.
One specific criticism is that a physician associate has historically referred to a physician (MD or DO) in a private practice group who has not yet achieved the status of partner. These physician associates are fully licensed medical doctors who have completed medical school and residency training and are in the process of completing a partnership track with their group to participate fully in financial and administrative processes. This nomenclature is similar to that of attorneys on a partnership track. Thus, the use of the term “physician associate” for someone other than a medical doctor is seen as misleading, particularly to patients who cannot be expected to have familiarity with the differences in training.
Efforts to separate the PA profession from a close-working relationship with a physician are bad not only for patients but for PAs as well. Many PAs who desire physician involvement may find themselves hung out to dry, hired by companies and expected to perform outside of their comfort level. The profession also risks ostracizing physician allies, many of whom have preferentially sought to work with PAs.
My sincere hope is that the PA profession will return to its traditional roots of a physician-PA relationship, a model that has been demonstrated to result in high-quality patient care. When that day comes, I will happily re-title my book. But as long as the AAPA continues to work to remove physicians from the equation, patients are indeed at risk.
Rebekah Bernard, MD, is a family physician in Fort Myers, Florida, and president of Physicians for Patient Protection. She is the coauthor of Patients at Risk: The Rise of the Nurse Practitioner and Physician Assistant in Healthcare (Irvine, Calif.: Universal Publishers, 2020). She had no relevant financial disclosures. A version of this article first appeared on Medscape.com.
Physician assistants (PAs) are angry with me, and with good reason. I had the audacity to lump them together with nurse practitioners (NPs) in my book “Patients at Risk,” an act which one highly placed PA leader called “distasteful” in a private conversation with me.
I will admit that PAs have reason to be upset. With competitive acceptance rates including a requirement for extensive health care experience before PA school, standardized training, and at least 2,000 hours of clinical experience before graduation, the profession is a stark contrast to the haphazard training and 500 clinical hours required of NPs today. Further, unlike NPs, who have sought independent practice since the 1980s, PAs have traditionally been close allies with physicians, generally working in a 1:1 supervision model.
The truth is that it hurt to include PAs with NPs in my book. I’ve had my own close relationships with PAs over the years and found the PAs I worked with to be outstanding clinicians. Unfortunately, the profession has given me no choice. Following a model set by the NP profession,
Their efforts began with a change in terminology. “Optimal team practice” (OTP) was supposed to give PAs more flexibility, allowing them to work for hospitals or physician groups rather than under the responsibility of one physician. Not surprisingly, corporations and even academic centers have been quick to take advantage, hiring PAs and placing them in positions without adequate physician support. OTP paved the way for independent practice, as PAs sought and gained independence from any physician supervision in North Dakota, the first state to grant them that right.
Most recently, PAs have determined to change their name entirely, calling themselves physician associates. This move by the American Academy of Physician Assistants is the culmination of a years-long marketing study on how to increase the relevance and improve patient perception of the PA profession. The AAPA decision is expected to galvanize state and local PA organizations to lobby legislators for legal and regulatory changes that allow the use of the “physician associate” title, which is not currently a legal representation of PA licensure.
PAs’ latest attempt at title and branding reform follows years of advocacy to not be referred to as physician extenders or midlevel providers. For example, to gain more public acceptance of the PA model, the profession launched the public relations campaign “Your PA Can,” closely mirroring the “We Choose NPs” media blitz. PAs have also followed other dangerous precedents set by NPs, including 100% online training and a new “Doctor of Medical Science” degree, allowing PAs, as well as NPs, to now be called “doctors.”
I can understand PA reasoning even if I don’t agree with it. PAs are frustrated to be treated as second-class citizens compared with NPs, who have been granted independent practice in half the states in the union despite having a fraction of PA training. Frankly, it’s unfair that NPs are being hired preferentially over PAs simply because of looser legal requirements for physician oversight. The bottom line is that NPs have been more successful at persuading legislators to allow them independence – but that doesn’t make it right for either group.
While PAs have more clinical training upon graduation than NPs, they still have far less than physicians. PAs generally attend a 2-year master’s degree program after college which includes 2,000 hours of hands-on clinical work. By comparison, the average medical student spends 4 years and receives 5,000-6,000 hours of supervised clinical training upon graduation. But this isn’t considered enough for a graduate medical student to practice medicine independently.
Physicians must complete at least 3 years of postgraduate residency training in most states to receive a medical license, and by the time a physician is permitted to practice medicine unsupervised, they will have attained no fewer than 15,000-20,000 hours of supervised clinical practice, with years of specialty-specific training.
Patients want and deserve access to truly physician-led care, but in many parts of the country, physicians are being replaced by nonphysician practitioners to boost corporate profits. In many cases, patients are kept in the dark about the differences in training between the medical professionals now in charge of their care. The American Medical Association and other critics have expressed concern that the proposed title of “physician associate” is likely to further obscure the training and roles of medical professionals, already a source of confusion to patients.
One specific criticism is that a physician associate has historically referred to a physician (MD or DO) in a private practice group who has not yet achieved the status of partner. These physician associates are fully licensed medical doctors who have completed medical school and residency training and are in the process of completing a partnership track with their group to participate fully in financial and administrative processes. This nomenclature is similar to that of attorneys on a partnership track. Thus, the use of the term “physician associate” for someone other than a medical doctor is seen as misleading, particularly to patients who cannot be expected to have familiarity with the differences in training.
Efforts to separate the PA profession from a close-working relationship with a physician are bad not only for patients but for PAs as well. Many PAs who desire physician involvement may find themselves hung out to dry, hired by companies and expected to perform outside of their comfort level. The profession also risks ostracizing physician allies, many of whom have preferentially sought to work with PAs.
My sincere hope is that the PA profession will return to its traditional roots of a physician-PA relationship, a model that has been demonstrated to result in high-quality patient care. When that day comes, I will happily re-title my book. But as long as the AAPA continues to work to remove physicians from the equation, patients are indeed at risk.
Rebekah Bernard, MD, is a family physician in Fort Myers, Florida, and president of Physicians for Patient Protection. She is the coauthor of Patients at Risk: The Rise of the Nurse Practitioner and Physician Assistant in Healthcare (Irvine, Calif.: Universal Publishers, 2020). She had no relevant financial disclosures. A version of this article first appeared on Medscape.com.
Physician assistants (PAs) are angry with me, and with good reason. I had the audacity to lump them together with nurse practitioners (NPs) in my book “Patients at Risk,” an act which one highly placed PA leader called “distasteful” in a private conversation with me.
I will admit that PAs have reason to be upset. With competitive acceptance rates including a requirement for extensive health care experience before PA school, standardized training, and at least 2,000 hours of clinical experience before graduation, the profession is a stark contrast to the haphazard training and 500 clinical hours required of NPs today. Further, unlike NPs, who have sought independent practice since the 1980s, PAs have traditionally been close allies with physicians, generally working in a 1:1 supervision model.
The truth is that it hurt to include PAs with NPs in my book. I’ve had my own close relationships with PAs over the years and found the PAs I worked with to be outstanding clinicians. Unfortunately, the profession has given me no choice. Following a model set by the NP profession,
Their efforts began with a change in terminology. “Optimal team practice” (OTP) was supposed to give PAs more flexibility, allowing them to work for hospitals or physician groups rather than under the responsibility of one physician. Not surprisingly, corporations and even academic centers have been quick to take advantage, hiring PAs and placing them in positions without adequate physician support. OTP paved the way for independent practice, as PAs sought and gained independence from any physician supervision in North Dakota, the first state to grant them that right.
Most recently, PAs have determined to change their name entirely, calling themselves physician associates. This move by the American Academy of Physician Assistants is the culmination of a years-long marketing study on how to increase the relevance and improve patient perception of the PA profession. The AAPA decision is expected to galvanize state and local PA organizations to lobby legislators for legal and regulatory changes that allow the use of the “physician associate” title, which is not currently a legal representation of PA licensure.
PAs’ latest attempt at title and branding reform follows years of advocacy to not be referred to as physician extenders or midlevel providers. For example, to gain more public acceptance of the PA model, the profession launched the public relations campaign “Your PA Can,” closely mirroring the “We Choose NPs” media blitz. PAs have also followed other dangerous precedents set by NPs, including 100% online training and a new “Doctor of Medical Science” degree, allowing PAs, as well as NPs, to now be called “doctors.”
I can understand PA reasoning even if I don’t agree with it. PAs are frustrated to be treated as second-class citizens compared with NPs, who have been granted independent practice in half the states in the union despite having a fraction of PA training. Frankly, it’s unfair that NPs are being hired preferentially over PAs simply because of looser legal requirements for physician oversight. The bottom line is that NPs have been more successful at persuading legislators to allow them independence – but that doesn’t make it right for either group.
While PAs have more clinical training upon graduation than NPs, they still have far less than physicians. PAs generally attend a 2-year master’s degree program after college which includes 2,000 hours of hands-on clinical work. By comparison, the average medical student spends 4 years and receives 5,000-6,000 hours of supervised clinical training upon graduation. But this isn’t considered enough for a graduate medical student to practice medicine independently.
Physicians must complete at least 3 years of postgraduate residency training in most states to receive a medical license, and by the time a physician is permitted to practice medicine unsupervised, they will have attained no fewer than 15,000-20,000 hours of supervised clinical practice, with years of specialty-specific training.
Patients want and deserve access to truly physician-led care, but in many parts of the country, physicians are being replaced by nonphysician practitioners to boost corporate profits. In many cases, patients are kept in the dark about the differences in training between the medical professionals now in charge of their care. The American Medical Association and other critics have expressed concern that the proposed title of “physician associate” is likely to further obscure the training and roles of medical professionals, already a source of confusion to patients.
One specific criticism is that a physician associate has historically referred to a physician (MD or DO) in a private practice group who has not yet achieved the status of partner. These physician associates are fully licensed medical doctors who have completed medical school and residency training and are in the process of completing a partnership track with their group to participate fully in financial and administrative processes. This nomenclature is similar to that of attorneys on a partnership track. Thus, the use of the term “physician associate” for someone other than a medical doctor is seen as misleading, particularly to patients who cannot be expected to have familiarity with the differences in training.
Efforts to separate the PA profession from a close-working relationship with a physician are bad not only for patients but for PAs as well. Many PAs who desire physician involvement may find themselves hung out to dry, hired by companies and expected to perform outside of their comfort level. The profession also risks ostracizing physician allies, many of whom have preferentially sought to work with PAs.
My sincere hope is that the PA profession will return to its traditional roots of a physician-PA relationship, a model that has been demonstrated to result in high-quality patient care. When that day comes, I will happily re-title my book. But as long as the AAPA continues to work to remove physicians from the equation, patients are indeed at risk.
Rebekah Bernard, MD, is a family physician in Fort Myers, Florida, and president of Physicians for Patient Protection. She is the coauthor of Patients at Risk: The Rise of the Nurse Practitioner and Physician Assistant in Healthcare (Irvine, Calif.: Universal Publishers, 2020). She had no relevant financial disclosures. A version of this article first appeared on Medscape.com.
At-risk Americans become eligible for fourth COVID shot this week
The Centers for Disease Control and Prevention endorsed a third dose of the Pfizer or Moderna vaccines for moderately and severely immunocompromised people on Aug. 13, which is considered part of their first immunization series rather than a booster shot.
In October, the CDC said moderately and severely immunocompromised people could receive a booster shot, or a fourth dose of the vaccine , 6 months after their third dose.
But the CDC last week shortened the timeline to 5 months for a booster shot of the Pfizer or Moderna vaccines. That means immunocompromised people could begin signing up for a fourth shot later this week, the New York Times reported.
About 2.7% of U.S. adults, or about 7 million adults, are considered immunocompromised, according to the CDC. They’re more likely to contract severe COVID-19, have a higher risk for long COVID, have lower antibody levels after vaccination, and develop serious breakthrough infections. About 40% of hospitalized breakthrough cases are among immunocompromised people.
According to CDC guidance, people are considered to be “moderately or severely immunocompromised” if they have:
- Active cancer treatment for tumors or cancers of the blood
- Had an organ transplant and are taking medicine to suppress the immune system
- Had a stem cell transplant in the last 2 years and are taking medicine to suppress the immune system
- Advanced or untreated HIV infection
- Moderate or severe primary immunodeficiency, such as DiGeorge syndrome or Wiskott-Aldrich syndrome
- Active treatment with high-dose corticosteroids or other drugs that suppress the immune response
So far, only moderately and severely immunocompromised Americans are eligible for a fourth shot. Israel has begun offering fourth doses to high-risk groups, including older adults, but the Biden administration hasn’t yet said whether the United States will follow, the Times reported.
Overall, the focus remains on getting third shots to Americans who are eligible for boosters, Rochelle Walensky, MD, the CDC director, told reporters Jan. 7. U.S. officials will remain in touch with Israel to follow their data on fourth shots.
“We will be following our own data carefully as well, to see how these boosters are working in terms of waning effectiveness, not just for infection but, importantly, for severe disease,” she said.
A version of this article first appeared on WebMD.com .
The Centers for Disease Control and Prevention endorsed a third dose of the Pfizer or Moderna vaccines for moderately and severely immunocompromised people on Aug. 13, which is considered part of their first immunization series rather than a booster shot.
In October, the CDC said moderately and severely immunocompromised people could receive a booster shot, or a fourth dose of the vaccine , 6 months after their third dose.
But the CDC last week shortened the timeline to 5 months for a booster shot of the Pfizer or Moderna vaccines. That means immunocompromised people could begin signing up for a fourth shot later this week, the New York Times reported.
About 2.7% of U.S. adults, or about 7 million adults, are considered immunocompromised, according to the CDC. They’re more likely to contract severe COVID-19, have a higher risk for long COVID, have lower antibody levels after vaccination, and develop serious breakthrough infections. About 40% of hospitalized breakthrough cases are among immunocompromised people.
According to CDC guidance, people are considered to be “moderately or severely immunocompromised” if they have:
- Active cancer treatment for tumors or cancers of the blood
- Had an organ transplant and are taking medicine to suppress the immune system
- Had a stem cell transplant in the last 2 years and are taking medicine to suppress the immune system
- Advanced or untreated HIV infection
- Moderate or severe primary immunodeficiency, such as DiGeorge syndrome or Wiskott-Aldrich syndrome
- Active treatment with high-dose corticosteroids or other drugs that suppress the immune response
So far, only moderately and severely immunocompromised Americans are eligible for a fourth shot. Israel has begun offering fourth doses to high-risk groups, including older adults, but the Biden administration hasn’t yet said whether the United States will follow, the Times reported.
Overall, the focus remains on getting third shots to Americans who are eligible for boosters, Rochelle Walensky, MD, the CDC director, told reporters Jan. 7. U.S. officials will remain in touch with Israel to follow their data on fourth shots.
“We will be following our own data carefully as well, to see how these boosters are working in terms of waning effectiveness, not just for infection but, importantly, for severe disease,” she said.
A version of this article first appeared on WebMD.com .
The Centers for Disease Control and Prevention endorsed a third dose of the Pfizer or Moderna vaccines for moderately and severely immunocompromised people on Aug. 13, which is considered part of their first immunization series rather than a booster shot.
In October, the CDC said moderately and severely immunocompromised people could receive a booster shot, or a fourth dose of the vaccine , 6 months after their third dose.
But the CDC last week shortened the timeline to 5 months for a booster shot of the Pfizer or Moderna vaccines. That means immunocompromised people could begin signing up for a fourth shot later this week, the New York Times reported.
About 2.7% of U.S. adults, or about 7 million adults, are considered immunocompromised, according to the CDC. They’re more likely to contract severe COVID-19, have a higher risk for long COVID, have lower antibody levels after vaccination, and develop serious breakthrough infections. About 40% of hospitalized breakthrough cases are among immunocompromised people.
According to CDC guidance, people are considered to be “moderately or severely immunocompromised” if they have:
- Active cancer treatment for tumors or cancers of the blood
- Had an organ transplant and are taking medicine to suppress the immune system
- Had a stem cell transplant in the last 2 years and are taking medicine to suppress the immune system
- Advanced or untreated HIV infection
- Moderate or severe primary immunodeficiency, such as DiGeorge syndrome or Wiskott-Aldrich syndrome
- Active treatment with high-dose corticosteroids or other drugs that suppress the immune response
So far, only moderately and severely immunocompromised Americans are eligible for a fourth shot. Israel has begun offering fourth doses to high-risk groups, including older adults, but the Biden administration hasn’t yet said whether the United States will follow, the Times reported.
Overall, the focus remains on getting third shots to Americans who are eligible for boosters, Rochelle Walensky, MD, the CDC director, told reporters Jan. 7. U.S. officials will remain in touch with Israel to follow their data on fourth shots.
“We will be following our own data carefully as well, to see how these boosters are working in terms of waning effectiveness, not just for infection but, importantly, for severe disease,” she said.
A version of this article first appeared on WebMD.com .
Individualize the duration of postpartum magnesium treatment for patients with preeclampsia to best balance the benefits and harms of treatment
Preeclampsia complicates 3% to 8% of pregnancies.1-3 The incidence of preeclampsia is influenced by the clinical characteristics of the pregnant population, including the prevalence of overweight, obesity, chronic hypertension, diabetes, nulliparity, advanced maternal age, multiple gestations, kidney disease, and a history of preeclampsia in a prior pregnancy.4
Magnesium treatment reduces the rate of eclampsia among patients with preeclampsia
For patients with preeclampsia, magnesium treatment reduces the risk of seizure. In the Magpie trial, 9,992 pregnant patients were treated for 24 hours with magnesium or placebo.5 The magnesium treatment regimen was either a 4-g IV bolus over 10 to 15 minutes followed by a continuous infusion of 1 g/hr or an intramuscular regimen (10-g intramuscular loading dose followed by 5 g IM every 4 hours). Eclamptic seizures occurred in 0.8% and 1.9% of patients treated with magnesium or placebo, respectively (relative risk [RR], 0.42; 95% confidence interval [CI], 0.29 to 0.60). Among patients with a multiple gestation, the rate of eclampsia was 2% and 6% in the patients treated with magnesium or placebo, respectively. The number of patients who needed to be treated to prevent one eclamptic event was 63 and 109 for patients with preeclampsia with and without severe features, respectively. Intrapartum treatment with magnesium also reduced the risk of placental abruption from 3.2% for the patients receiving placebo to 2.0% among the patients treated with magnesium (RR, 0.67; 99% CI, 0.45- 0.89). Maternal death was reduced with magnesium treatment compared with placebo (0.2% vs 0.4%), but the difference was not statistically significant.
In the Magpie trial, side effects were reported by 24% and 5% of patients treated with magnesium and placebo, respectively. The most common side effects were flushing, nausea, vomiting, and muscle weakness. Of note, magnesium treatment is contraindicated in patients with myasthenia gravis because it can cause muscle weakness and hypoventilation.6 For patients with preeclampsia and myasthenia gravis, levetiracetam may be utilized to reduce the risk of seizure.6
Duration of postpartum magnesium treatment
There are no studies with a sufficient number of participants to definitively determine the optimal duration of postpartum magnesium therapy. A properly powered study would likely require more than 16,000 to 20,000 participants to identify clinically meaningful differences in the rate of postpartum eclampsia among patients treated with magnesium for 12 or 24 hours.7,8 It is unlikely that such a study will be completed. Hence, the duration of postpartum magnesium must be based on clinical judgment, balancing the risks and benefits of treatment.
The American College of Obstetricians and Gynecologists (ACOG) recommends continuing magnesium treatment for 24 hours postpartum. They advise, “For patients requiring cesarean delivery (before the onset of labor), the infusion should ideally begin before surgery and continue during surgery, as well as 24 hours afterwards. For patients who deliver vaginally, the infusion should continue for 24 hours after delivery.”9
Multiple randomized trials have reported on the outcomes associated with 12 hours versus 24 hours of postpartum magnesium therapy (TABLE). Because the rate of postpartum eclamptic seizure is very low, none of the studies were sufficiently powered to provide a definitive answer to the benefits and harms of the shorter versus longer time frame of magnesium therapy.10-15
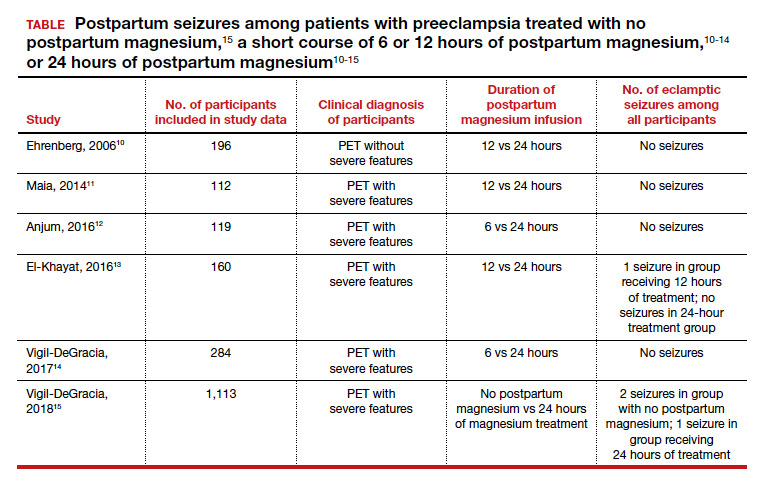
Continue to: The harms of prolonged postpartum magnesium infusion...
The harms of prolonged postpartum magnesium infusion
The harms of prolonging treatment with postpartum magnesium infusion are generally not emphasized in the medical literature. However, side effects that can occur are flushing, nausea, vomiting, and muscle weakness, delayed early ambulation, delayed return to full diet, delayed discontinuation of a bladder catheter, and delayed initiation of breastfeeding.5,15 In one large clinical trial, 1,113 patients with preeclampsia with severe features who received intrapartum magnesium for ≥8 hours were randomized after birth to immediate discontinuation of magnesium or continuation of magnesium for 24 hours.15 There was 1 seizure in the group of 555 patients who received 24 hours of postpartum magnesium and 2 seizures in the group of 558 patients who received no magnesium after birth. In this trial, continuation of magnesium postpartum resulted in delayed initiation of breastfeeding and delayed ambulation.15
Balancing the benefits and harms of postpartum magnesium infusion
An important clinical point is that magnesium treatment will not prevent all seizures associated with preeclampsia; in the Magpie trial, among the 5,055 patients with preeclampsia treated with magnesium there were 40 (0.8%) seizures.5 Magnesium treatment will reduce but not eliminate the risk of seizure. Clinicians should have a plan to treat seizures that occur while a woman is being treated with magnesium.
In the absence of high-quality data to guide the duration of postpartum magnesium therapy it is best to use clinical parameters to balance the benefits and harms of postpartum magnesium treatment.16-18 Patients may want to participate in the decision about the duration of postpartum magnesium treatment after receiving counseling about the benefits and harms.
For patients with preeclampsia without severe features, many clinicians are no longer ordering intrapartum magnesium for prevention of seizures because they believe the risk of seizure in patients without severe disease is very low. Hence, these patients will not receive postpartum magnesium treatment unless they evolve to preeclampsia with severe features or develop a “red flag” warning postpartum (see below).
For patients with preeclampsia without severe features who received intrapartum magnesium, after birth, the magnesium infusion could be stopped immediately or within 12 hours of birth. For patients with preeclampsia without severe features, early termination of the magnesium infusion best balances the benefit of seizure reduction with the harms of delayed early ambulation, return to full diet, discontinuation of the bladder catheter, and initiation of breastfeeding.
For patients with preeclampsia with severe features, 24 hours of magnesium may best balance the benefits and harms of treatment. However, if the patient continues to have “red flag” findings, continued magnesium treatment beyond 24 hours may be warranted.
Red flag findings include: an eclamptic seizure before or after birth, ongoing or recurring severe headaches, visual scotomata, nausea, vomiting, epigastric pain, severe hypertension, oliguria, rising creatinine, or liver transaminases and declining platelet count.
The hypertensive diseases of pregnancy, including preeclampsia often appear suddenly and may evolve rapidly, threatening the health of both mother and fetus. A high level of suspicion that a hypertensive disease might be the cause of vague symptoms such as epigastric discomfort or headache may accelerate early diagnosis. Rapid treatment of severe hypertension with intravenous labetalol and hydralazine, and intrapartum plus postpartum administration of magnesium to prevent placental abruption and eclampsia will optimize patient outcomes. No patient, patient’s family members, or clinician, wants to experience the grief of a preventable maternal, fetal, or newborn death due to hypertension.19 Obstetricians, midwives, labor nurses, obstetrical anesthesiologists and doulas play key roles in preventing maternal, fetal, and newborn morbidity and death from hypertensive diseases of pregnancy. As a team we are the last line of defense protecting the health of our patients. ●
- World Health Organization. WHO International Collaborative Study of Hypertensive Disorders of Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. Am J Obstet Gynecol. 1988;158:80-83.
- Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209:544.e1-e12. doi: 10.1016 /j.ajog.2013.08.019.
- Mayrink K, Souza RT, Feitosa FE, et al. Incidence and risk factors for preeclampsia in a cohort of healthy nulliparous patients: a nested casecontrol study. Sci Rep. 2019;9:9517. doi: 10.1038 /s41598-019-46011-3.
- Bartsch E, Medcalf KE, Park AL, et al. High risk of pre-eclampsia identification group. BMJ. 2016;353:i1753. doi: 10.1136/bmj.i1753.
- Altman D, Carroli G, Duley L; The Magpie Trial Collaborative Group. Do patients with preeclampsia, and their babies, benefit from magnesium sulfate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877- 1890. doi: 10.1016/s0140-6736(02)08778-0.
- Lake AJ, Al Hkabbaz A, Keeney R. Severe preeclampsia in the setting of myasthenia gravis. Case Rep Obstet Gynecol. 2017;9204930. doi: 10.1155/2017/9204930.
- Hurd WW, Ventolini G, Stolfi A. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;102: 196-197. doi: 10.1016/s0029-7844(03)00471-x.
- Scott JR. Safety of eliminating postpartum magnesium sulphate: intriguing but not yet proven. BJOG. 2018;125:1312. doi: 10.1111/1471 -0528.15317.
- Gestational hypertension and preeclampsia. ACOG Practice Bulletin No. 222. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2020;135:e237-e260. doi: 10.1097/AOG .0000000000003891.
- Ehrenberg H, Mercer BM. Abbreviated postpartum magnesium sulfate therapy for patients with mild preeclampsia: a randomized controlled trial. Obstet Gynecol. 2006;108:833-888. doi: 10.1097 /01.AOG.0000236493.35347.d8.
- Maia SB, Katz L, Neto CN, et al. Abbreviated (12- hour) versus traditional (24-hour) postpartum magnesium sulfate therapy in severe pre-eclampsia. Int J Gynaecol Obstet. 2014;126:260-264. doi: 10.1016/j.ijgo.2014.03.024.
- Anjum S, Rajaram GP, Bano I. Short-course (6-h) magnesium sulfate therapy in severe preeclampsia. Arch Gynecol Obstet. 2016;293:983-986. doi: 10.1007/s00404-015-3903-y.
- El-Khayat W, Atef A, Abdelatty S, et al. A novel protocol for postpartum magnesium sulphate in severe pre-eclampsia: a randomized controlled pilot trial. J Matern Fetal Neonatal Med. 2016;29: 154-158. doi: 10.3109/14767058.2014.991915.
- Vigil-De Gracia P, Ramirez R, Duran Y, et al. Magnesium sulfate for 6 vs 24 hours post-delivery in patients who received magnesium sulfate for less than 8 hours before birth: a randomized clinical trial. BMC Pregnancy Childbirth. 2017;17:241. doi: 10.1186/s12884-017-1424-3.
- Vigil-DeGracia P, Ludmir J, Ng J, et al. Is there benefit to continue magnesium sulphate postpartum in patients receiving magnesium sulphate before delivery? A randomized controlled study. BJOG. 2018;125:1304-1311. doi: 10.1111/1471 -0528.15320.
- Ascarelli MH, Johnson V, May WL, et al. Individually determined postpartum magnesium sulfate therapy with clinical parameters to safety and cost-effectively shorten treatment for preeclampsia. Am J Obstet Gynecol. 1998;179:952-956. doi: 10.1016/s0002-9378(98)70195-4.
- Isler CM, Barrilleaux PS, Rinehart BK, et al. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;101:66-69. doi: 10.1016/s0029 -7844(02)02317-7.
- Fontenot MT, Lewis DF, Frederick JB, et al. A prospective randomized trial of magnesium sulfate in severe preeclampsia: use of diuresis as a clinical parameter to determine the duration of postpartum therapy. Am J Obstet Gynecol. 2005;192:1788- 1793. doi: 10.1016/j.ajog.2004.12.056.
- Tsigas EZ. The Preeclampsia Foundation: the voice and views of the patient and family. Am J Obstet Gynecol. Epub August 23, 2021. doi: 10.1016/j.ajog.2020.10.053.
Preeclampsia complicates 3% to 8% of pregnancies.1-3 The incidence of preeclampsia is influenced by the clinical characteristics of the pregnant population, including the prevalence of overweight, obesity, chronic hypertension, diabetes, nulliparity, advanced maternal age, multiple gestations, kidney disease, and a history of preeclampsia in a prior pregnancy.4
Magnesium treatment reduces the rate of eclampsia among patients with preeclampsia
For patients with preeclampsia, magnesium treatment reduces the risk of seizure. In the Magpie trial, 9,992 pregnant patients were treated for 24 hours with magnesium or placebo.5 The magnesium treatment regimen was either a 4-g IV bolus over 10 to 15 minutes followed by a continuous infusion of 1 g/hr or an intramuscular regimen (10-g intramuscular loading dose followed by 5 g IM every 4 hours). Eclamptic seizures occurred in 0.8% and 1.9% of patients treated with magnesium or placebo, respectively (relative risk [RR], 0.42; 95% confidence interval [CI], 0.29 to 0.60). Among patients with a multiple gestation, the rate of eclampsia was 2% and 6% in the patients treated with magnesium or placebo, respectively. The number of patients who needed to be treated to prevent one eclamptic event was 63 and 109 for patients with preeclampsia with and without severe features, respectively. Intrapartum treatment with magnesium also reduced the risk of placental abruption from 3.2% for the patients receiving placebo to 2.0% among the patients treated with magnesium (RR, 0.67; 99% CI, 0.45- 0.89). Maternal death was reduced with magnesium treatment compared with placebo (0.2% vs 0.4%), but the difference was not statistically significant.
In the Magpie trial, side effects were reported by 24% and 5% of patients treated with magnesium and placebo, respectively. The most common side effects were flushing, nausea, vomiting, and muscle weakness. Of note, magnesium treatment is contraindicated in patients with myasthenia gravis because it can cause muscle weakness and hypoventilation.6 For patients with preeclampsia and myasthenia gravis, levetiracetam may be utilized to reduce the risk of seizure.6
Duration of postpartum magnesium treatment
There are no studies with a sufficient number of participants to definitively determine the optimal duration of postpartum magnesium therapy. A properly powered study would likely require more than 16,000 to 20,000 participants to identify clinically meaningful differences in the rate of postpartum eclampsia among patients treated with magnesium for 12 or 24 hours.7,8 It is unlikely that such a study will be completed. Hence, the duration of postpartum magnesium must be based on clinical judgment, balancing the risks and benefits of treatment.
The American College of Obstetricians and Gynecologists (ACOG) recommends continuing magnesium treatment for 24 hours postpartum. They advise, “For patients requiring cesarean delivery (before the onset of labor), the infusion should ideally begin before surgery and continue during surgery, as well as 24 hours afterwards. For patients who deliver vaginally, the infusion should continue for 24 hours after delivery.”9
Multiple randomized trials have reported on the outcomes associated with 12 hours versus 24 hours of postpartum magnesium therapy (TABLE). Because the rate of postpartum eclamptic seizure is very low, none of the studies were sufficiently powered to provide a definitive answer to the benefits and harms of the shorter versus longer time frame of magnesium therapy.10-15

Continue to: The harms of prolonged postpartum magnesium infusion...
The harms of prolonged postpartum magnesium infusion
The harms of prolonging treatment with postpartum magnesium infusion are generally not emphasized in the medical literature. However, side effects that can occur are flushing, nausea, vomiting, and muscle weakness, delayed early ambulation, delayed return to full diet, delayed discontinuation of a bladder catheter, and delayed initiation of breastfeeding.5,15 In one large clinical trial, 1,113 patients with preeclampsia with severe features who received intrapartum magnesium for ≥8 hours were randomized after birth to immediate discontinuation of magnesium or continuation of magnesium for 24 hours.15 There was 1 seizure in the group of 555 patients who received 24 hours of postpartum magnesium and 2 seizures in the group of 558 patients who received no magnesium after birth. In this trial, continuation of magnesium postpartum resulted in delayed initiation of breastfeeding and delayed ambulation.15
Balancing the benefits and harms of postpartum magnesium infusion
An important clinical point is that magnesium treatment will not prevent all seizures associated with preeclampsia; in the Magpie trial, among the 5,055 patients with preeclampsia treated with magnesium there were 40 (0.8%) seizures.5 Magnesium treatment will reduce but not eliminate the risk of seizure. Clinicians should have a plan to treat seizures that occur while a woman is being treated with magnesium.
In the absence of high-quality data to guide the duration of postpartum magnesium therapy it is best to use clinical parameters to balance the benefits and harms of postpartum magnesium treatment.16-18 Patients may want to participate in the decision about the duration of postpartum magnesium treatment after receiving counseling about the benefits and harms.
For patients with preeclampsia without severe features, many clinicians are no longer ordering intrapartum magnesium for prevention of seizures because they believe the risk of seizure in patients without severe disease is very low. Hence, these patients will not receive postpartum magnesium treatment unless they evolve to preeclampsia with severe features or develop a “red flag” warning postpartum (see below).
For patients with preeclampsia without severe features who received intrapartum magnesium, after birth, the magnesium infusion could be stopped immediately or within 12 hours of birth. For patients with preeclampsia without severe features, early termination of the magnesium infusion best balances the benefit of seizure reduction with the harms of delayed early ambulation, return to full diet, discontinuation of the bladder catheter, and initiation of breastfeeding.
For patients with preeclampsia with severe features, 24 hours of magnesium may best balance the benefits and harms of treatment. However, if the patient continues to have “red flag” findings, continued magnesium treatment beyond 24 hours may be warranted.
Red flag findings include: an eclamptic seizure before or after birth, ongoing or recurring severe headaches, visual scotomata, nausea, vomiting, epigastric pain, severe hypertension, oliguria, rising creatinine, or liver transaminases and declining platelet count.
The hypertensive diseases of pregnancy, including preeclampsia often appear suddenly and may evolve rapidly, threatening the health of both mother and fetus. A high level of suspicion that a hypertensive disease might be the cause of vague symptoms such as epigastric discomfort or headache may accelerate early diagnosis. Rapid treatment of severe hypertension with intravenous labetalol and hydralazine, and intrapartum plus postpartum administration of magnesium to prevent placental abruption and eclampsia will optimize patient outcomes. No patient, patient’s family members, or clinician, wants to experience the grief of a preventable maternal, fetal, or newborn death due to hypertension.19 Obstetricians, midwives, labor nurses, obstetrical anesthesiologists and doulas play key roles in preventing maternal, fetal, and newborn morbidity and death from hypertensive diseases of pregnancy. As a team we are the last line of defense protecting the health of our patients. ●
Preeclampsia complicates 3% to 8% of pregnancies.1-3 The incidence of preeclampsia is influenced by the clinical characteristics of the pregnant population, including the prevalence of overweight, obesity, chronic hypertension, diabetes, nulliparity, advanced maternal age, multiple gestations, kidney disease, and a history of preeclampsia in a prior pregnancy.4
Magnesium treatment reduces the rate of eclampsia among patients with preeclampsia
For patients with preeclampsia, magnesium treatment reduces the risk of seizure. In the Magpie trial, 9,992 pregnant patients were treated for 24 hours with magnesium or placebo.5 The magnesium treatment regimen was either a 4-g IV bolus over 10 to 15 minutes followed by a continuous infusion of 1 g/hr or an intramuscular regimen (10-g intramuscular loading dose followed by 5 g IM every 4 hours). Eclamptic seizures occurred in 0.8% and 1.9% of patients treated with magnesium or placebo, respectively (relative risk [RR], 0.42; 95% confidence interval [CI], 0.29 to 0.60). Among patients with a multiple gestation, the rate of eclampsia was 2% and 6% in the patients treated with magnesium or placebo, respectively. The number of patients who needed to be treated to prevent one eclamptic event was 63 and 109 for patients with preeclampsia with and without severe features, respectively. Intrapartum treatment with magnesium also reduced the risk of placental abruption from 3.2% for the patients receiving placebo to 2.0% among the patients treated with magnesium (RR, 0.67; 99% CI, 0.45- 0.89). Maternal death was reduced with magnesium treatment compared with placebo (0.2% vs 0.4%), but the difference was not statistically significant.
In the Magpie trial, side effects were reported by 24% and 5% of patients treated with magnesium and placebo, respectively. The most common side effects were flushing, nausea, vomiting, and muscle weakness. Of note, magnesium treatment is contraindicated in patients with myasthenia gravis because it can cause muscle weakness and hypoventilation.6 For patients with preeclampsia and myasthenia gravis, levetiracetam may be utilized to reduce the risk of seizure.6
Duration of postpartum magnesium treatment
There are no studies with a sufficient number of participants to definitively determine the optimal duration of postpartum magnesium therapy. A properly powered study would likely require more than 16,000 to 20,000 participants to identify clinically meaningful differences in the rate of postpartum eclampsia among patients treated with magnesium for 12 or 24 hours.7,8 It is unlikely that such a study will be completed. Hence, the duration of postpartum magnesium must be based on clinical judgment, balancing the risks and benefits of treatment.
The American College of Obstetricians and Gynecologists (ACOG) recommends continuing magnesium treatment for 24 hours postpartum. They advise, “For patients requiring cesarean delivery (before the onset of labor), the infusion should ideally begin before surgery and continue during surgery, as well as 24 hours afterwards. For patients who deliver vaginally, the infusion should continue for 24 hours after delivery.”9
Multiple randomized trials have reported on the outcomes associated with 12 hours versus 24 hours of postpartum magnesium therapy (TABLE). Because the rate of postpartum eclamptic seizure is very low, none of the studies were sufficiently powered to provide a definitive answer to the benefits and harms of the shorter versus longer time frame of magnesium therapy.10-15

Continue to: The harms of prolonged postpartum magnesium infusion...
The harms of prolonged postpartum magnesium infusion
The harms of prolonging treatment with postpartum magnesium infusion are generally not emphasized in the medical literature. However, side effects that can occur are flushing, nausea, vomiting, and muscle weakness, delayed early ambulation, delayed return to full diet, delayed discontinuation of a bladder catheter, and delayed initiation of breastfeeding.5,15 In one large clinical trial, 1,113 patients with preeclampsia with severe features who received intrapartum magnesium for ≥8 hours were randomized after birth to immediate discontinuation of magnesium or continuation of magnesium for 24 hours.15 There was 1 seizure in the group of 555 patients who received 24 hours of postpartum magnesium and 2 seizures in the group of 558 patients who received no magnesium after birth. In this trial, continuation of magnesium postpartum resulted in delayed initiation of breastfeeding and delayed ambulation.15
Balancing the benefits and harms of postpartum magnesium infusion
An important clinical point is that magnesium treatment will not prevent all seizures associated with preeclampsia; in the Magpie trial, among the 5,055 patients with preeclampsia treated with magnesium there were 40 (0.8%) seizures.5 Magnesium treatment will reduce but not eliminate the risk of seizure. Clinicians should have a plan to treat seizures that occur while a woman is being treated with magnesium.
In the absence of high-quality data to guide the duration of postpartum magnesium therapy it is best to use clinical parameters to balance the benefits and harms of postpartum magnesium treatment.16-18 Patients may want to participate in the decision about the duration of postpartum magnesium treatment after receiving counseling about the benefits and harms.
For patients with preeclampsia without severe features, many clinicians are no longer ordering intrapartum magnesium for prevention of seizures because they believe the risk of seizure in patients without severe disease is very low. Hence, these patients will not receive postpartum magnesium treatment unless they evolve to preeclampsia with severe features or develop a “red flag” warning postpartum (see below).
For patients with preeclampsia without severe features who received intrapartum magnesium, after birth, the magnesium infusion could be stopped immediately or within 12 hours of birth. For patients with preeclampsia without severe features, early termination of the magnesium infusion best balances the benefit of seizure reduction with the harms of delayed early ambulation, return to full diet, discontinuation of the bladder catheter, and initiation of breastfeeding.
For patients with preeclampsia with severe features, 24 hours of magnesium may best balance the benefits and harms of treatment. However, if the patient continues to have “red flag” findings, continued magnesium treatment beyond 24 hours may be warranted.
Red flag findings include: an eclamptic seizure before or after birth, ongoing or recurring severe headaches, visual scotomata, nausea, vomiting, epigastric pain, severe hypertension, oliguria, rising creatinine, or liver transaminases and declining platelet count.
The hypertensive diseases of pregnancy, including preeclampsia often appear suddenly and may evolve rapidly, threatening the health of both mother and fetus. A high level of suspicion that a hypertensive disease might be the cause of vague symptoms such as epigastric discomfort or headache may accelerate early diagnosis. Rapid treatment of severe hypertension with intravenous labetalol and hydralazine, and intrapartum plus postpartum administration of magnesium to prevent placental abruption and eclampsia will optimize patient outcomes. No patient, patient’s family members, or clinician, wants to experience the grief of a preventable maternal, fetal, or newborn death due to hypertension.19 Obstetricians, midwives, labor nurses, obstetrical anesthesiologists and doulas play key roles in preventing maternal, fetal, and newborn morbidity and death from hypertensive diseases of pregnancy. As a team we are the last line of defense protecting the health of our patients. ●
- World Health Organization. WHO International Collaborative Study of Hypertensive Disorders of Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. Am J Obstet Gynecol. 1988;158:80-83.
- Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209:544.e1-e12. doi: 10.1016 /j.ajog.2013.08.019.
- Mayrink K, Souza RT, Feitosa FE, et al. Incidence and risk factors for preeclampsia in a cohort of healthy nulliparous patients: a nested casecontrol study. Sci Rep. 2019;9:9517. doi: 10.1038 /s41598-019-46011-3.
- Bartsch E, Medcalf KE, Park AL, et al. High risk of pre-eclampsia identification group. BMJ. 2016;353:i1753. doi: 10.1136/bmj.i1753.
- Altman D, Carroli G, Duley L; The Magpie Trial Collaborative Group. Do patients with preeclampsia, and their babies, benefit from magnesium sulfate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877- 1890. doi: 10.1016/s0140-6736(02)08778-0.
- Lake AJ, Al Hkabbaz A, Keeney R. Severe preeclampsia in the setting of myasthenia gravis. Case Rep Obstet Gynecol. 2017;9204930. doi: 10.1155/2017/9204930.
- Hurd WW, Ventolini G, Stolfi A. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;102: 196-197. doi: 10.1016/s0029-7844(03)00471-x.
- Scott JR. Safety of eliminating postpartum magnesium sulphate: intriguing but not yet proven. BJOG. 2018;125:1312. doi: 10.1111/1471 -0528.15317.
- Gestational hypertension and preeclampsia. ACOG Practice Bulletin No. 222. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2020;135:e237-e260. doi: 10.1097/AOG .0000000000003891.
- Ehrenberg H, Mercer BM. Abbreviated postpartum magnesium sulfate therapy for patients with mild preeclampsia: a randomized controlled trial. Obstet Gynecol. 2006;108:833-888. doi: 10.1097 /01.AOG.0000236493.35347.d8.
- Maia SB, Katz L, Neto CN, et al. Abbreviated (12- hour) versus traditional (24-hour) postpartum magnesium sulfate therapy in severe pre-eclampsia. Int J Gynaecol Obstet. 2014;126:260-264. doi: 10.1016/j.ijgo.2014.03.024.
- Anjum S, Rajaram GP, Bano I. Short-course (6-h) magnesium sulfate therapy in severe preeclampsia. Arch Gynecol Obstet. 2016;293:983-986. doi: 10.1007/s00404-015-3903-y.
- El-Khayat W, Atef A, Abdelatty S, et al. A novel protocol for postpartum magnesium sulphate in severe pre-eclampsia: a randomized controlled pilot trial. J Matern Fetal Neonatal Med. 2016;29: 154-158. doi: 10.3109/14767058.2014.991915.
- Vigil-De Gracia P, Ramirez R, Duran Y, et al. Magnesium sulfate for 6 vs 24 hours post-delivery in patients who received magnesium sulfate for less than 8 hours before birth: a randomized clinical trial. BMC Pregnancy Childbirth. 2017;17:241. doi: 10.1186/s12884-017-1424-3.
- Vigil-DeGracia P, Ludmir J, Ng J, et al. Is there benefit to continue magnesium sulphate postpartum in patients receiving magnesium sulphate before delivery? A randomized controlled study. BJOG. 2018;125:1304-1311. doi: 10.1111/1471 -0528.15320.
- Ascarelli MH, Johnson V, May WL, et al. Individually determined postpartum magnesium sulfate therapy with clinical parameters to safety and cost-effectively shorten treatment for preeclampsia. Am J Obstet Gynecol. 1998;179:952-956. doi: 10.1016/s0002-9378(98)70195-4.
- Isler CM, Barrilleaux PS, Rinehart BK, et al. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;101:66-69. doi: 10.1016/s0029 -7844(02)02317-7.
- Fontenot MT, Lewis DF, Frederick JB, et al. A prospective randomized trial of magnesium sulfate in severe preeclampsia: use of diuresis as a clinical parameter to determine the duration of postpartum therapy. Am J Obstet Gynecol. 2005;192:1788- 1793. doi: 10.1016/j.ajog.2004.12.056.
- Tsigas EZ. The Preeclampsia Foundation: the voice and views of the patient and family. Am J Obstet Gynecol. Epub August 23, 2021. doi: 10.1016/j.ajog.2020.10.053.
- World Health Organization. WHO International Collaborative Study of Hypertensive Disorders of Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. Am J Obstet Gynecol. 1988;158:80-83.
- Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209:544.e1-e12. doi: 10.1016 /j.ajog.2013.08.019.
- Mayrink K, Souza RT, Feitosa FE, et al. Incidence and risk factors for preeclampsia in a cohort of healthy nulliparous patients: a nested casecontrol study. Sci Rep. 2019;9:9517. doi: 10.1038 /s41598-019-46011-3.
- Bartsch E, Medcalf KE, Park AL, et al. High risk of pre-eclampsia identification group. BMJ. 2016;353:i1753. doi: 10.1136/bmj.i1753.
- Altman D, Carroli G, Duley L; The Magpie Trial Collaborative Group. Do patients with preeclampsia, and their babies, benefit from magnesium sulfate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877- 1890. doi: 10.1016/s0140-6736(02)08778-0.
- Lake AJ, Al Hkabbaz A, Keeney R. Severe preeclampsia in the setting of myasthenia gravis. Case Rep Obstet Gynecol. 2017;9204930. doi: 10.1155/2017/9204930.
- Hurd WW, Ventolini G, Stolfi A. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;102: 196-197. doi: 10.1016/s0029-7844(03)00471-x.
- Scott JR. Safety of eliminating postpartum magnesium sulphate: intriguing but not yet proven. BJOG. 2018;125:1312. doi: 10.1111/1471 -0528.15317.
- Gestational hypertension and preeclampsia. ACOG Practice Bulletin No. 222. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2020;135:e237-e260. doi: 10.1097/AOG .0000000000003891.
- Ehrenberg H, Mercer BM. Abbreviated postpartum magnesium sulfate therapy for patients with mild preeclampsia: a randomized controlled trial. Obstet Gynecol. 2006;108:833-888. doi: 10.1097 /01.AOG.0000236493.35347.d8.
- Maia SB, Katz L, Neto CN, et al. Abbreviated (12- hour) versus traditional (24-hour) postpartum magnesium sulfate therapy in severe pre-eclampsia. Int J Gynaecol Obstet. 2014;126:260-264. doi: 10.1016/j.ijgo.2014.03.024.
- Anjum S, Rajaram GP, Bano I. Short-course (6-h) magnesium sulfate therapy in severe preeclampsia. Arch Gynecol Obstet. 2016;293:983-986. doi: 10.1007/s00404-015-3903-y.
- El-Khayat W, Atef A, Abdelatty S, et al. A novel protocol for postpartum magnesium sulphate in severe pre-eclampsia: a randomized controlled pilot trial. J Matern Fetal Neonatal Med. 2016;29: 154-158. doi: 10.3109/14767058.2014.991915.
- Vigil-De Gracia P, Ramirez R, Duran Y, et al. Magnesium sulfate for 6 vs 24 hours post-delivery in patients who received magnesium sulfate for less than 8 hours before birth: a randomized clinical trial. BMC Pregnancy Childbirth. 2017;17:241. doi: 10.1186/s12884-017-1424-3.
- Vigil-DeGracia P, Ludmir J, Ng J, et al. Is there benefit to continue magnesium sulphate postpartum in patients receiving magnesium sulphate before delivery? A randomized controlled study. BJOG. 2018;125:1304-1311. doi: 10.1111/1471 -0528.15320.
- Ascarelli MH, Johnson V, May WL, et al. Individually determined postpartum magnesium sulfate therapy with clinical parameters to safety and cost-effectively shorten treatment for preeclampsia. Am J Obstet Gynecol. 1998;179:952-956. doi: 10.1016/s0002-9378(98)70195-4.
- Isler CM, Barrilleaux PS, Rinehart BK, et al. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;101:66-69. doi: 10.1016/s0029 -7844(02)02317-7.
- Fontenot MT, Lewis DF, Frederick JB, et al. A prospective randomized trial of magnesium sulfate in severe preeclampsia: use of diuresis as a clinical parameter to determine the duration of postpartum therapy. Am J Obstet Gynecol. 2005;192:1788- 1793. doi: 10.1016/j.ajog.2004.12.056.
- Tsigas EZ. The Preeclampsia Foundation: the voice and views of the patient and family. Am J Obstet Gynecol. Epub August 23, 2021. doi: 10.1016/j.ajog.2020.10.053.
Lack of high school education vaccine hesitancy predictor
Lack of a high school education is a predictor of whether a person will be resistant to getting the COVID-19 vaccine, a new study shows.
Researchers from the University of North Carolina looked at vaccination rates in 3,142 counties in the U.S. They compared them to population characteristics based on the CDC Social Vulnerability Index.
They found that more than half of the unvaccinated adults in the U.S. with strong vaccine hesitancy had a high school education or less. Vaccine hesitancy was defined as refusal to be vaccinated even if the COVID-19 vaccine was available.
The other main predictor for vaccine hesitancy was concern about vaccine availability and distribution, the researchers said.
“Our study suggests that low education levels are a major contributor to vaccine hesitancy and ultimately vaccination levels,” the authors wrote. The study was published in the American Journal of Infection Control. “Specifically, low vaccination levels were found in communities with a less educated population and with more concern about vaccine uptake capacity, suggesting that education is an ongoing challenge.”
“Our findings suggest that policy makers and community leaders should tailor vaccine information and efforts to those with limited education and specifically address knowledge concerns that are prevalent and likely more modifiable.”
The study was based on data gathered months ago. It says that as of May 9, 2021, 34.7% of the U.S. population was fully vaccinated and that 8% reported a strong unwillingness to get vaccinated.
At press time, the Centers for Disease Control and Prevention’s COVID Data Tracker showed that 62.5% of the U.S. population was fully vaccinated.
According to the study, other consistent characteristics of people who are vaccine hesitant are that they belong to a racial minority, are 65 or older, live in a household with children 18 or younger, or are unemployed.
When asked why they were vaccine hesitant, people gave these reasons: Lack of trust in COVID-19 vaccines (55%), concerns about side effects (48%), and lack of trust in government (46%).
Lack of access to vaccines, often cited in previous studies about resistance to other vaccines, was not cited as a reason for not getting the COVID-19 vaccine.
“COVID-19 vaccine hesitancy is a public health threat,” the researchers concluded. “Since education levels are not easily modifiable, our results suggest that policymakers would be best served by closing knowledge gaps to overcome negative perceptions of the vaccine through tailored interventions.”
A version of this article first appeared on WebMD.com.
Lack of a high school education is a predictor of whether a person will be resistant to getting the COVID-19 vaccine, a new study shows.
Researchers from the University of North Carolina looked at vaccination rates in 3,142 counties in the U.S. They compared them to population characteristics based on the CDC Social Vulnerability Index.
They found that more than half of the unvaccinated adults in the U.S. with strong vaccine hesitancy had a high school education or less. Vaccine hesitancy was defined as refusal to be vaccinated even if the COVID-19 vaccine was available.
The other main predictor for vaccine hesitancy was concern about vaccine availability and distribution, the researchers said.
“Our study suggests that low education levels are a major contributor to vaccine hesitancy and ultimately vaccination levels,” the authors wrote. The study was published in the American Journal of Infection Control. “Specifically, low vaccination levels were found in communities with a less educated population and with more concern about vaccine uptake capacity, suggesting that education is an ongoing challenge.”
“Our findings suggest that policy makers and community leaders should tailor vaccine information and efforts to those with limited education and specifically address knowledge concerns that are prevalent and likely more modifiable.”
The study was based on data gathered months ago. It says that as of May 9, 2021, 34.7% of the U.S. population was fully vaccinated and that 8% reported a strong unwillingness to get vaccinated.
At press time, the Centers for Disease Control and Prevention’s COVID Data Tracker showed that 62.5% of the U.S. population was fully vaccinated.
According to the study, other consistent characteristics of people who are vaccine hesitant are that they belong to a racial minority, are 65 or older, live in a household with children 18 or younger, or are unemployed.
When asked why they were vaccine hesitant, people gave these reasons: Lack of trust in COVID-19 vaccines (55%), concerns about side effects (48%), and lack of trust in government (46%).
Lack of access to vaccines, often cited in previous studies about resistance to other vaccines, was not cited as a reason for not getting the COVID-19 vaccine.
“COVID-19 vaccine hesitancy is a public health threat,” the researchers concluded. “Since education levels are not easily modifiable, our results suggest that policymakers would be best served by closing knowledge gaps to overcome negative perceptions of the vaccine through tailored interventions.”
A version of this article first appeared on WebMD.com.
Lack of a high school education is a predictor of whether a person will be resistant to getting the COVID-19 vaccine, a new study shows.
Researchers from the University of North Carolina looked at vaccination rates in 3,142 counties in the U.S. They compared them to population characteristics based on the CDC Social Vulnerability Index.
They found that more than half of the unvaccinated adults in the U.S. with strong vaccine hesitancy had a high school education or less. Vaccine hesitancy was defined as refusal to be vaccinated even if the COVID-19 vaccine was available.
The other main predictor for vaccine hesitancy was concern about vaccine availability and distribution, the researchers said.
“Our study suggests that low education levels are a major contributor to vaccine hesitancy and ultimately vaccination levels,” the authors wrote. The study was published in the American Journal of Infection Control. “Specifically, low vaccination levels were found in communities with a less educated population and with more concern about vaccine uptake capacity, suggesting that education is an ongoing challenge.”
“Our findings suggest that policy makers and community leaders should tailor vaccine information and efforts to those with limited education and specifically address knowledge concerns that are prevalent and likely more modifiable.”
The study was based on data gathered months ago. It says that as of May 9, 2021, 34.7% of the U.S. population was fully vaccinated and that 8% reported a strong unwillingness to get vaccinated.
At press time, the Centers for Disease Control and Prevention’s COVID Data Tracker showed that 62.5% of the U.S. population was fully vaccinated.
According to the study, other consistent characteristics of people who are vaccine hesitant are that they belong to a racial minority, are 65 or older, live in a household with children 18 or younger, or are unemployed.
When asked why they were vaccine hesitant, people gave these reasons: Lack of trust in COVID-19 vaccines (55%), concerns about side effects (48%), and lack of trust in government (46%).
Lack of access to vaccines, often cited in previous studies about resistance to other vaccines, was not cited as a reason for not getting the COVID-19 vaccine.
“COVID-19 vaccine hesitancy is a public health threat,” the researchers concluded. “Since education levels are not easily modifiable, our results suggest that policymakers would be best served by closing knowledge gaps to overcome negative perceptions of the vaccine through tailored interventions.”
A version of this article first appeared on WebMD.com.
FROM THE AMERICAN JOURNAL OF INFECTION CONTROL