User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
I’m a physician battling long COVID. I can assure you it’s real
One in 5. It almost seems unimaginable that this is the real number of people who are struggling with long COVID, especially considering how many people in the United States have had COVID-19 at this point (more than 96 million).
Even more unimaginable at this time is that it’s happening to me. I’ve experienced not only the disabling effects of long COVID, but I’ve also seen, firsthand, the frustration of navigating diagnosis and treatment. It’s given me a taste of what millions of other patients are going through.
Vaxxed, masked, and (too) relaxed
I caught COVID-19 (probably Omicron BA.5) that presented as sniffles, making me think it was probably just allergies. However, my resting heart rate was up on my Garmin watch, so of course I got tested and was positive.
With my symptoms virtually nonexistent, it seemed, at the time, merely an inconvenience, because I was forced to isolate away from family and friends, who all stayed negative.
But 2 weeks later, I began to have urticaria – hives – after physical exertion. Did that mean my mast cells were angry? There’s some evidence these immune cells become overactivated in some patients with COVID. Next, I began to experience lightheadedness and the rapid heartbeat of tachycardia. The tachycardia was especially bad any time I physically exerted myself, including on a walk. Imagine me – a lover of all bargain shopping – cutting short a trip to the outlet mall on a particularly bad day when my heart rate was 140 after taking just a few steps. This was orthostatic intolerance.
Then came the severe worsening of my migraines – which are often vestibular, making me nauseated and dizzy on top of the throbbing.
I was of course familiar with these symptoms, as professor and chair of the department of rehabilitation medicine at the Joe R. and Teresa Lozano Long School of Medicine at University of Texas Health Science Center, San Antonio. I developed a post-COVID recovery clinic to help patients.
So I knew about postexertional malaise (PEM) and postexertional symptom exacerbation (PESE), but I was now experiencing these distressing symptoms firsthand.
Clinicians really need to look for this cardinal sign of long COVID as well as evidence of myalgic encephalomyelitis or chronic fatigue syndrome (ME/CFS). ME/CFS is marked by exacerbation of fatigue or symptoms after an activity that could previously be done without these aftereffects. In my case, as an All-American Masters miler with several marathons under my belt, running 5 miles is a walk in the park. But now, I pay for those 5 miles for the rest of the day on the couch or with palpitations, dizziness, and fatigue the following day. Busy clinic day full of procedures? I would have to be sitting by the end of it. Bed by 9 PM was not always early enough.
Becoming a statistic
Here I am, one of the leading experts in the country on caring for people with long COVID, featured in the national news and having testified in front of Congress, and now I am part of that lived experience. Me – a healthy athlete, with no comorbidities, a normal BMI, vaccinated and boosted, and after an almost asymptomatic bout of COVID-19, a victim to long COVID.
You just never know how your body is going to react. Neuroinflammation occurred in studies with mice with mild respiratory COVID and could be happening to me. I did not want a chronic immune-mediated vasculopathy.
So, I did what any other hyperaware physician-researcher would do. I enrolled in the RECOVER trial – a study my own institution is taking part in and one that I recommend to my own patients.
I also decided that I need to access care and not just ignore my symptoms or try to treat them myself.
That’s when things got difficult. There was a wait of at least a month to see my primary care provider – but I was able to use my privileged position as a physician to get in sooner.
My provider said that she had limited knowledge of long COVID, and she hesitated to order some of the tests and treatments that I recommended because they were not yet considered standard of care. I can understand the hesitation. It is engrained in medical education to follow evidence based on the highest-quality research studies. We are slowly learning more about long COVID, but acknowledging the learning curve offers little to patients who need help now.
This has made me realize that we cannot wait on an evidence-based approach – which can take decades to develop – while people are suffering. And it’s important that everyone on the front line learn about some of the manifestations and disease management of long COVID.
I left this first physician visit feeling more defeated than anything and decided to try to push through. That, I quickly realized, was not the right thing to do.
So again, after a couple of significant crashes and days of severe migraines, I phoned a friend: Ratna Bhavaraju-Sanka, MD, the amazing neurologist who treats patients with long COVID alongside me. She squeezed me in on a non-clinic day. Again, I had the privilege to see a specialist most people wait half a year to see. I was diagnosed with both autonomic dysfunction and intractable migraine.
She ordered some intravenous fluids and IV magnesium that would probably help both. But then another obstacle arose. My institution’s infusion center is focused on patients with cancer, and I was unable to schedule treatments there.
Luckily, I knew about the concierge mobile IV hydration therapy companies that come to your house – mostly offering a hangover treatment service. And I am thankful that I had the health literacy and financial ability to pay for some fluids at home.
On another particularly bad day, I phoned other friends – higher-ups at the hospital – who expedited a slot at the hospital infusion center and approval for the IV magnesium.
Thanks to my access, knowledge, and other privileges, I got fairly quick if imperfect care, enrolled in a research trial, and received medications. I knew to pace myself. The vast majority of others with long COVID lack these advantages.
The patient with long COVID
Things I have learned that others can learn, too:
- Acknowledge and recognize that long COVID is a disease that is affecting 1 in 5 Americans who catch COVID. Many look completely “normal on the outside.” Please listen to your patients.
- Autonomic dysfunction is a common manifestation of long COVID. A 10-minute stand test goes a long way in diagnosing this condition, from the American Academy of Physical Medicine and Rehabilitation. It is not just anxiety.
- “That’s only in research” is dismissive and harmful. Think outside the box. Follow guidelines. Consider encouraging patients to sign up for trials.
- Screen for PEM/PESE and teach your patients to pace themselves, because pushing through it or doing graded exercises will be harmful.
- We need to train more physicians to treat postacute sequelae of SARS-CoV-2 infection () and other postinfectious conditions, such as ME/CFS.
If long COVID is hard for physicians to understand and deal with, imagine how difficult it is for patients with no expertise in this area.
It is exponentially harder for those with fewer resources, time, and health literacy. My lived experience with long COVID has shown me that being a patient is never easy. You put your body and fate into the hands of trusted professionals and expect validation and assistance, not gaslighting or gatekeeping.
Along with millions of others, I am tired of waiting.
Dr. Gutierrez is Professor and Distinguished Chair, department of rehabilitation medicine, University of Texas Health Science Center at San Antonio. She reported receiving honoraria for lecturing on long COVID and receiving a research grant from Co-PI for the NIH RECOVER trial.
A version of this article first appeared on Medscape.com.
One in 5. It almost seems unimaginable that this is the real number of people who are struggling with long COVID, especially considering how many people in the United States have had COVID-19 at this point (more than 96 million).
Even more unimaginable at this time is that it’s happening to me. I’ve experienced not only the disabling effects of long COVID, but I’ve also seen, firsthand, the frustration of navigating diagnosis and treatment. It’s given me a taste of what millions of other patients are going through.
Vaxxed, masked, and (too) relaxed
I caught COVID-19 (probably Omicron BA.5) that presented as sniffles, making me think it was probably just allergies. However, my resting heart rate was up on my Garmin watch, so of course I got tested and was positive.
With my symptoms virtually nonexistent, it seemed, at the time, merely an inconvenience, because I was forced to isolate away from family and friends, who all stayed negative.
But 2 weeks later, I began to have urticaria – hives – after physical exertion. Did that mean my mast cells were angry? There’s some evidence these immune cells become overactivated in some patients with COVID. Next, I began to experience lightheadedness and the rapid heartbeat of tachycardia. The tachycardia was especially bad any time I physically exerted myself, including on a walk. Imagine me – a lover of all bargain shopping – cutting short a trip to the outlet mall on a particularly bad day when my heart rate was 140 after taking just a few steps. This was orthostatic intolerance.
Then came the severe worsening of my migraines – which are often vestibular, making me nauseated and dizzy on top of the throbbing.
I was of course familiar with these symptoms, as professor and chair of the department of rehabilitation medicine at the Joe R. and Teresa Lozano Long School of Medicine at University of Texas Health Science Center, San Antonio. I developed a post-COVID recovery clinic to help patients.
So I knew about postexertional malaise (PEM) and postexertional symptom exacerbation (PESE), but I was now experiencing these distressing symptoms firsthand.
Clinicians really need to look for this cardinal sign of long COVID as well as evidence of myalgic encephalomyelitis or chronic fatigue syndrome (ME/CFS). ME/CFS is marked by exacerbation of fatigue or symptoms after an activity that could previously be done without these aftereffects. In my case, as an All-American Masters miler with several marathons under my belt, running 5 miles is a walk in the park. But now, I pay for those 5 miles for the rest of the day on the couch or with palpitations, dizziness, and fatigue the following day. Busy clinic day full of procedures? I would have to be sitting by the end of it. Bed by 9 PM was not always early enough.
Becoming a statistic
Here I am, one of the leading experts in the country on caring for people with long COVID, featured in the national news and having testified in front of Congress, and now I am part of that lived experience. Me – a healthy athlete, with no comorbidities, a normal BMI, vaccinated and boosted, and after an almost asymptomatic bout of COVID-19, a victim to long COVID.
You just never know how your body is going to react. Neuroinflammation occurred in studies with mice with mild respiratory COVID and could be happening to me. I did not want a chronic immune-mediated vasculopathy.
So, I did what any other hyperaware physician-researcher would do. I enrolled in the RECOVER trial – a study my own institution is taking part in and one that I recommend to my own patients.
I also decided that I need to access care and not just ignore my symptoms or try to treat them myself.
That’s when things got difficult. There was a wait of at least a month to see my primary care provider – but I was able to use my privileged position as a physician to get in sooner.
My provider said that she had limited knowledge of long COVID, and she hesitated to order some of the tests and treatments that I recommended because they were not yet considered standard of care. I can understand the hesitation. It is engrained in medical education to follow evidence based on the highest-quality research studies. We are slowly learning more about long COVID, but acknowledging the learning curve offers little to patients who need help now.
This has made me realize that we cannot wait on an evidence-based approach – which can take decades to develop – while people are suffering. And it’s important that everyone on the front line learn about some of the manifestations and disease management of long COVID.
I left this first physician visit feeling more defeated than anything and decided to try to push through. That, I quickly realized, was not the right thing to do.
So again, after a couple of significant crashes and days of severe migraines, I phoned a friend: Ratna Bhavaraju-Sanka, MD, the amazing neurologist who treats patients with long COVID alongside me. She squeezed me in on a non-clinic day. Again, I had the privilege to see a specialist most people wait half a year to see. I was diagnosed with both autonomic dysfunction and intractable migraine.
She ordered some intravenous fluids and IV magnesium that would probably help both. But then another obstacle arose. My institution’s infusion center is focused on patients with cancer, and I was unable to schedule treatments there.
Luckily, I knew about the concierge mobile IV hydration therapy companies that come to your house – mostly offering a hangover treatment service. And I am thankful that I had the health literacy and financial ability to pay for some fluids at home.
On another particularly bad day, I phoned other friends – higher-ups at the hospital – who expedited a slot at the hospital infusion center and approval for the IV magnesium.
Thanks to my access, knowledge, and other privileges, I got fairly quick if imperfect care, enrolled in a research trial, and received medications. I knew to pace myself. The vast majority of others with long COVID lack these advantages.
The patient with long COVID
Things I have learned that others can learn, too:
- Acknowledge and recognize that long COVID is a disease that is affecting 1 in 5 Americans who catch COVID. Many look completely “normal on the outside.” Please listen to your patients.
- Autonomic dysfunction is a common manifestation of long COVID. A 10-minute stand test goes a long way in diagnosing this condition, from the American Academy of Physical Medicine and Rehabilitation. It is not just anxiety.
- “That’s only in research” is dismissive and harmful. Think outside the box. Follow guidelines. Consider encouraging patients to sign up for trials.
- Screen for PEM/PESE and teach your patients to pace themselves, because pushing through it or doing graded exercises will be harmful.
- We need to train more physicians to treat postacute sequelae of SARS-CoV-2 infection () and other postinfectious conditions, such as ME/CFS.
If long COVID is hard for physicians to understand and deal with, imagine how difficult it is for patients with no expertise in this area.
It is exponentially harder for those with fewer resources, time, and health literacy. My lived experience with long COVID has shown me that being a patient is never easy. You put your body and fate into the hands of trusted professionals and expect validation and assistance, not gaslighting or gatekeeping.
Along with millions of others, I am tired of waiting.
Dr. Gutierrez is Professor and Distinguished Chair, department of rehabilitation medicine, University of Texas Health Science Center at San Antonio. She reported receiving honoraria for lecturing on long COVID and receiving a research grant from Co-PI for the NIH RECOVER trial.
A version of this article first appeared on Medscape.com.
One in 5. It almost seems unimaginable that this is the real number of people who are struggling with long COVID, especially considering how many people in the United States have had COVID-19 at this point (more than 96 million).
Even more unimaginable at this time is that it’s happening to me. I’ve experienced not only the disabling effects of long COVID, but I’ve also seen, firsthand, the frustration of navigating diagnosis and treatment. It’s given me a taste of what millions of other patients are going through.
Vaxxed, masked, and (too) relaxed
I caught COVID-19 (probably Omicron BA.5) that presented as sniffles, making me think it was probably just allergies. However, my resting heart rate was up on my Garmin watch, so of course I got tested and was positive.
With my symptoms virtually nonexistent, it seemed, at the time, merely an inconvenience, because I was forced to isolate away from family and friends, who all stayed negative.
But 2 weeks later, I began to have urticaria – hives – after physical exertion. Did that mean my mast cells were angry? There’s some evidence these immune cells become overactivated in some patients with COVID. Next, I began to experience lightheadedness and the rapid heartbeat of tachycardia. The tachycardia was especially bad any time I physically exerted myself, including on a walk. Imagine me – a lover of all bargain shopping – cutting short a trip to the outlet mall on a particularly bad day when my heart rate was 140 after taking just a few steps. This was orthostatic intolerance.
Then came the severe worsening of my migraines – which are often vestibular, making me nauseated and dizzy on top of the throbbing.
I was of course familiar with these symptoms, as professor and chair of the department of rehabilitation medicine at the Joe R. and Teresa Lozano Long School of Medicine at University of Texas Health Science Center, San Antonio. I developed a post-COVID recovery clinic to help patients.
So I knew about postexertional malaise (PEM) and postexertional symptom exacerbation (PESE), but I was now experiencing these distressing symptoms firsthand.
Clinicians really need to look for this cardinal sign of long COVID as well as evidence of myalgic encephalomyelitis or chronic fatigue syndrome (ME/CFS). ME/CFS is marked by exacerbation of fatigue or symptoms after an activity that could previously be done without these aftereffects. In my case, as an All-American Masters miler with several marathons under my belt, running 5 miles is a walk in the park. But now, I pay for those 5 miles for the rest of the day on the couch or with palpitations, dizziness, and fatigue the following day. Busy clinic day full of procedures? I would have to be sitting by the end of it. Bed by 9 PM was not always early enough.
Becoming a statistic
Here I am, one of the leading experts in the country on caring for people with long COVID, featured in the national news and having testified in front of Congress, and now I am part of that lived experience. Me – a healthy athlete, with no comorbidities, a normal BMI, vaccinated and boosted, and after an almost asymptomatic bout of COVID-19, a victim to long COVID.
You just never know how your body is going to react. Neuroinflammation occurred in studies with mice with mild respiratory COVID and could be happening to me. I did not want a chronic immune-mediated vasculopathy.
So, I did what any other hyperaware physician-researcher would do. I enrolled in the RECOVER trial – a study my own institution is taking part in and one that I recommend to my own patients.
I also decided that I need to access care and not just ignore my symptoms or try to treat them myself.
That’s when things got difficult. There was a wait of at least a month to see my primary care provider – but I was able to use my privileged position as a physician to get in sooner.
My provider said that she had limited knowledge of long COVID, and she hesitated to order some of the tests and treatments that I recommended because they were not yet considered standard of care. I can understand the hesitation. It is engrained in medical education to follow evidence based on the highest-quality research studies. We are slowly learning more about long COVID, but acknowledging the learning curve offers little to patients who need help now.
This has made me realize that we cannot wait on an evidence-based approach – which can take decades to develop – while people are suffering. And it’s important that everyone on the front line learn about some of the manifestations and disease management of long COVID.
I left this first physician visit feeling more defeated than anything and decided to try to push through. That, I quickly realized, was not the right thing to do.
So again, after a couple of significant crashes and days of severe migraines, I phoned a friend: Ratna Bhavaraju-Sanka, MD, the amazing neurologist who treats patients with long COVID alongside me. She squeezed me in on a non-clinic day. Again, I had the privilege to see a specialist most people wait half a year to see. I was diagnosed with both autonomic dysfunction and intractable migraine.
She ordered some intravenous fluids and IV magnesium that would probably help both. But then another obstacle arose. My institution’s infusion center is focused on patients with cancer, and I was unable to schedule treatments there.
Luckily, I knew about the concierge mobile IV hydration therapy companies that come to your house – mostly offering a hangover treatment service. And I am thankful that I had the health literacy and financial ability to pay for some fluids at home.
On another particularly bad day, I phoned other friends – higher-ups at the hospital – who expedited a slot at the hospital infusion center and approval for the IV magnesium.
Thanks to my access, knowledge, and other privileges, I got fairly quick if imperfect care, enrolled in a research trial, and received medications. I knew to pace myself. The vast majority of others with long COVID lack these advantages.
The patient with long COVID
Things I have learned that others can learn, too:
- Acknowledge and recognize that long COVID is a disease that is affecting 1 in 5 Americans who catch COVID. Many look completely “normal on the outside.” Please listen to your patients.
- Autonomic dysfunction is a common manifestation of long COVID. A 10-minute stand test goes a long way in diagnosing this condition, from the American Academy of Physical Medicine and Rehabilitation. It is not just anxiety.
- “That’s only in research” is dismissive and harmful. Think outside the box. Follow guidelines. Consider encouraging patients to sign up for trials.
- Screen for PEM/PESE and teach your patients to pace themselves, because pushing through it or doing graded exercises will be harmful.
- We need to train more physicians to treat postacute sequelae of SARS-CoV-2 infection () and other postinfectious conditions, such as ME/CFS.
If long COVID is hard for physicians to understand and deal with, imagine how difficult it is for patients with no expertise in this area.
It is exponentially harder for those with fewer resources, time, and health literacy. My lived experience with long COVID has shown me that being a patient is never easy. You put your body and fate into the hands of trusted professionals and expect validation and assistance, not gaslighting or gatekeeping.
Along with millions of others, I am tired of waiting.
Dr. Gutierrez is Professor and Distinguished Chair, department of rehabilitation medicine, University of Texas Health Science Center at San Antonio. She reported receiving honoraria for lecturing on long COVID and receiving a research grant from Co-PI for the NIH RECOVER trial.
A version of this article first appeared on Medscape.com.
BMI and reproduction – weighing the evidence
Arguably, no topic during an infertility consultation generates more of an emotional reaction than discussing body mass index (BMI), particularly when it is high. Patients have become increasingly sensitive to weight discussions with their physicians because of concerns about body shaming. Among patients with an elevated BMI, criticism on social media of health care professionals’ counseling and a preemptive presentation of “Don’t Weigh Me” cards have become popular responses. Despite the medical evidence on impaired reproduction with an abnormal BMI, patients are choosing to forgo the topic. Research has demonstrated “extensive evidence [of] strong weight bias” in a wide range of health staff.1 A “viral” TikTok study revealed that medical “gaslighting” founded in weight stigma and bias is harmful, as reported on KevinMD.com.2 This month, we review the effect of abnormal BMI, both high and low, on reproduction and pregnancy.
A method to assess relative weight was first described in 1832 as its ratio in kilograms divided by the square of the height in meters, or the Quetelet Index. The search for a functional assessment of relative body weight began after World War II when reports by actuaries noted the increased mortality of overweight policyholders. The relationship between weight and cardiovascular disease was further revealed in epidemiologic studies. The Quetelet Index became the BMI in 1972.3
Weight measurement is a mainstay in the assessment of a patient’s vital signs along with blood pressure, pulse rate, respiration rate, and temperature. Weight is vital to the calculation of medication dosage – for instance, administration of conscious sedative drugs, methotrexate, and gonadotropins. Some state boards of medicine, such as Florida, have a limitation on patient BMI at office-based surgery centers (40 kg/m2).
Obesity is a disease
As reported by the World Health Organization in 2022, the disease of obesity is an epidemic afflicting more than 1 billion people worldwide, or 1 in 8 individuals globally.4 The health implications of an elevated BMI include increased mortality, diabetes, heart disease, and stroke, physical limitations to activities of daily living, and complications affecting reproduction.
Female obesity is related to poorer outcomes in natural and assisted conception, including an increased risk of miscarriage. Compared with normal-weight women, those with obesity are three times more likely to have ovulatory dysfunction,5 infertility,6 a lower chance for conception,7 higher rate of miscarriage, and low birth weight.8,9During pregnancy, women with obesity have three to four times higher rates of gestational diabetes and preeclampsia,10 as well as likelihood of delivering preterm,11 having a fetus with macrosomia and birth defects, and a 1.3- to 2.1-times higher risk of stillbirth.12
Obesity is present in 40%-80% of women with polycystic ovary syndrome,13 the most common cause of ovulatory dysfunction from dysregulation of the hypothalamic-pituitary-ovarian axis. While PCOS is associated with reproductive and metabolic consequences, even in regularly ovulating women, increasing obesity appears to be associated with decreasing spontaneous pregnancy rates and increased time to pregnancy.14
Obesity and IVF
Women with obesity have reduced success with assisted reproductive technology, an increased number of canceled cycles, and poorer quality oocytes retrieved. A prospective cohort study of nearly 2,000 women reported that every 5 kg of body weight increase (from the patient’s baseline weight at age 18) was associated with a 5% increase in the mean duration of time required for conception (95% confidence interval, 3%-7%).15 Given that approximately 90% of these women had regular menstrual cycles, ovulatory dysfunction was not the suspected pathophysiology.
A meta-analysis of 21 cohort studies reported a lower likelihood of live birth following in vitro fertilization for women with obesity, compared with normal-weight women (risk ratio, 0.85; 95% CI, 0.82-0.87).16 A further subgroup analysis that evaluated only women with PCOS showed a reduction in the live birth rate following IVF for individuals with obesity, compared with normal-weight individuals (RR, 0.78; 95% CI, 0.74-0.82).
In a retrospective study of almost 500,000 fresh autologous IVF cycles, women with obesity had a 6% reduction in pregnancy rates and a 13% reduction in live birth rates, compared with normal-weight women. Both high and low BMI were associated with an increased risk of low birth weight and preterm delivery.17 The live birth rates per transfer for normal-weight and higher-weight women were 38% and 33%, respectively.
Contrarily, a randomized controlled trial showed that an intensive weight-reduction program resulted in a large weight loss but did not substantially affect live birth rates in women with obesity scheduled for IVF.18
Low BMI
A noteworthy cause of low BMI is functional hypothalamic amenorrhea (FHA), a disorder with low energy availability either from decreased caloric intake and/or excessive energy expenditure associated with eating disorders, excessive exercise, and stress. Consequently, a reduced GnRH drive results in a decreased pulse frequency and amplitude leading to low levels of follicle-stimulating hormone and luteinizing hormone, resulting in anovulation. Correction of lifestyle behaviors related to FHA can restore menstrual cycles. After normal weight is achieved, it appears unlikely that fertility is affected.19 In 47% of adolescent patients with anorexia, menses spontaneously returned within the first 12 months after admission, with an improved prognosis in secondary over primary amenorrhea.20,21 Interestingly, mildly and significantly underweight infertile women have pregnancy and live birth rates similar to normal-weight patients after IVF treatment.22
Pregnancy is complicated in underweight women, resulting in an increased risk of anemia, fetal growth retardation, and low birth weight, as well as preterm birth.21
Take-home message
The extremes of BMI both impair natural reproduction. Elevated BMI reduces success with IVF but rapid weight loss prior to IVF does not improve outcomes. A normal BMI is the goal for optimal reproductive and pregnancy health.
Dr. Trolice is director of the IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Talumaa B et al. Obesity Rev. 2022;23:e13494.
2. https://bit.ly/3rHCivE.
3. Eknoyan G. Nephrol Dial Transplant. 2008;23:47-51.
4. Wells JCK. Dis Models Mech. 2012;5:595-607.
5. Brewer CJ and Balen AH. Reproduction. 2010;140:347-64.
6. Silvestris E et al. Reprod Biol Endocrinol. 2018;16:22.
7. Wise LA et al. Hum Reprod. 2010;25:253-64.
8. Bellver J. Curr Opin Obstet Gynecol. 2022;34:114-21.
9. Dickey RP et al. Am J Obstet Gynecol. 2013;209:349.e1.
10. Alwash SM et al. Obes Res Clin Pract. 2021;15:425-30.
11. Cnattingius S et al. JAMA. 2013;309:2362-70.
12. Aune D et al. JAMA. 2014;311:1536-46.
13. Sam S. Obes Manag. 2007;3:69-73.
14. van der Steeg JW et al. Hum Reprod. 2008;23:324-8.
15. Gaskins AJ et al. Obstet Gynecol. 2015;126:850-8.
16. Sermondade N et al. Hum Reprod Update. 2019;25:439-519.
17. Kawwass JF et al. Fertil Steril. 2016;106[7]:1742-50.
18. Einarsson S et al. Hum Reprod. 2017;32:1621-30.
19. Chaer R et al. Diseases. 2020;8:46.
20. Dempfle A et al. Psychiatry. 2013;13:308.
21. Verma A and Shrimali L. J Clin Diagn Res. 2012;6:1531-3.
22. Romanski PA et al. Reprod Biomed Online. 2020;42:366-74.
Arguably, no topic during an infertility consultation generates more of an emotional reaction than discussing body mass index (BMI), particularly when it is high. Patients have become increasingly sensitive to weight discussions with their physicians because of concerns about body shaming. Among patients with an elevated BMI, criticism on social media of health care professionals’ counseling and a preemptive presentation of “Don’t Weigh Me” cards have become popular responses. Despite the medical evidence on impaired reproduction with an abnormal BMI, patients are choosing to forgo the topic. Research has demonstrated “extensive evidence [of] strong weight bias” in a wide range of health staff.1 A “viral” TikTok study revealed that medical “gaslighting” founded in weight stigma and bias is harmful, as reported on KevinMD.com.2 This month, we review the effect of abnormal BMI, both high and low, on reproduction and pregnancy.
A method to assess relative weight was first described in 1832 as its ratio in kilograms divided by the square of the height in meters, or the Quetelet Index. The search for a functional assessment of relative body weight began after World War II when reports by actuaries noted the increased mortality of overweight policyholders. The relationship between weight and cardiovascular disease was further revealed in epidemiologic studies. The Quetelet Index became the BMI in 1972.3
Weight measurement is a mainstay in the assessment of a patient’s vital signs along with blood pressure, pulse rate, respiration rate, and temperature. Weight is vital to the calculation of medication dosage – for instance, administration of conscious sedative drugs, methotrexate, and gonadotropins. Some state boards of medicine, such as Florida, have a limitation on patient BMI at office-based surgery centers (40 kg/m2).
Obesity is a disease
As reported by the World Health Organization in 2022, the disease of obesity is an epidemic afflicting more than 1 billion people worldwide, or 1 in 8 individuals globally.4 The health implications of an elevated BMI include increased mortality, diabetes, heart disease, and stroke, physical limitations to activities of daily living, and complications affecting reproduction.
Female obesity is related to poorer outcomes in natural and assisted conception, including an increased risk of miscarriage. Compared with normal-weight women, those with obesity are three times more likely to have ovulatory dysfunction,5 infertility,6 a lower chance for conception,7 higher rate of miscarriage, and low birth weight.8,9During pregnancy, women with obesity have three to four times higher rates of gestational diabetes and preeclampsia,10 as well as likelihood of delivering preterm,11 having a fetus with macrosomia and birth defects, and a 1.3- to 2.1-times higher risk of stillbirth.12
Obesity is present in 40%-80% of women with polycystic ovary syndrome,13 the most common cause of ovulatory dysfunction from dysregulation of the hypothalamic-pituitary-ovarian axis. While PCOS is associated with reproductive and metabolic consequences, even in regularly ovulating women, increasing obesity appears to be associated with decreasing spontaneous pregnancy rates and increased time to pregnancy.14
Obesity and IVF
Women with obesity have reduced success with assisted reproductive technology, an increased number of canceled cycles, and poorer quality oocytes retrieved. A prospective cohort study of nearly 2,000 women reported that every 5 kg of body weight increase (from the patient’s baseline weight at age 18) was associated with a 5% increase in the mean duration of time required for conception (95% confidence interval, 3%-7%).15 Given that approximately 90% of these women had regular menstrual cycles, ovulatory dysfunction was not the suspected pathophysiology.
A meta-analysis of 21 cohort studies reported a lower likelihood of live birth following in vitro fertilization for women with obesity, compared with normal-weight women (risk ratio, 0.85; 95% CI, 0.82-0.87).16 A further subgroup analysis that evaluated only women with PCOS showed a reduction in the live birth rate following IVF for individuals with obesity, compared with normal-weight individuals (RR, 0.78; 95% CI, 0.74-0.82).
In a retrospective study of almost 500,000 fresh autologous IVF cycles, women with obesity had a 6% reduction in pregnancy rates and a 13% reduction in live birth rates, compared with normal-weight women. Both high and low BMI were associated with an increased risk of low birth weight and preterm delivery.17 The live birth rates per transfer for normal-weight and higher-weight women were 38% and 33%, respectively.
Contrarily, a randomized controlled trial showed that an intensive weight-reduction program resulted in a large weight loss but did not substantially affect live birth rates in women with obesity scheduled for IVF.18
Low BMI
A noteworthy cause of low BMI is functional hypothalamic amenorrhea (FHA), a disorder with low energy availability either from decreased caloric intake and/or excessive energy expenditure associated with eating disorders, excessive exercise, and stress. Consequently, a reduced GnRH drive results in a decreased pulse frequency and amplitude leading to low levels of follicle-stimulating hormone and luteinizing hormone, resulting in anovulation. Correction of lifestyle behaviors related to FHA can restore menstrual cycles. After normal weight is achieved, it appears unlikely that fertility is affected.19 In 47% of adolescent patients with anorexia, menses spontaneously returned within the first 12 months after admission, with an improved prognosis in secondary over primary amenorrhea.20,21 Interestingly, mildly and significantly underweight infertile women have pregnancy and live birth rates similar to normal-weight patients after IVF treatment.22
Pregnancy is complicated in underweight women, resulting in an increased risk of anemia, fetal growth retardation, and low birth weight, as well as preterm birth.21
Take-home message
The extremes of BMI both impair natural reproduction. Elevated BMI reduces success with IVF but rapid weight loss prior to IVF does not improve outcomes. A normal BMI is the goal for optimal reproductive and pregnancy health.
Dr. Trolice is director of the IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Talumaa B et al. Obesity Rev. 2022;23:e13494.
2. https://bit.ly/3rHCivE.
3. Eknoyan G. Nephrol Dial Transplant. 2008;23:47-51.
4. Wells JCK. Dis Models Mech. 2012;5:595-607.
5. Brewer CJ and Balen AH. Reproduction. 2010;140:347-64.
6. Silvestris E et al. Reprod Biol Endocrinol. 2018;16:22.
7. Wise LA et al. Hum Reprod. 2010;25:253-64.
8. Bellver J. Curr Opin Obstet Gynecol. 2022;34:114-21.
9. Dickey RP et al. Am J Obstet Gynecol. 2013;209:349.e1.
10. Alwash SM et al. Obes Res Clin Pract. 2021;15:425-30.
11. Cnattingius S et al. JAMA. 2013;309:2362-70.
12. Aune D et al. JAMA. 2014;311:1536-46.
13. Sam S. Obes Manag. 2007;3:69-73.
14. van der Steeg JW et al. Hum Reprod. 2008;23:324-8.
15. Gaskins AJ et al. Obstet Gynecol. 2015;126:850-8.
16. Sermondade N et al. Hum Reprod Update. 2019;25:439-519.
17. Kawwass JF et al. Fertil Steril. 2016;106[7]:1742-50.
18. Einarsson S et al. Hum Reprod. 2017;32:1621-30.
19. Chaer R et al. Diseases. 2020;8:46.
20. Dempfle A et al. Psychiatry. 2013;13:308.
21. Verma A and Shrimali L. J Clin Diagn Res. 2012;6:1531-3.
22. Romanski PA et al. Reprod Biomed Online. 2020;42:366-74.
Arguably, no topic during an infertility consultation generates more of an emotional reaction than discussing body mass index (BMI), particularly when it is high. Patients have become increasingly sensitive to weight discussions with their physicians because of concerns about body shaming. Among patients with an elevated BMI, criticism on social media of health care professionals’ counseling and a preemptive presentation of “Don’t Weigh Me” cards have become popular responses. Despite the medical evidence on impaired reproduction with an abnormal BMI, patients are choosing to forgo the topic. Research has demonstrated “extensive evidence [of] strong weight bias” in a wide range of health staff.1 A “viral” TikTok study revealed that medical “gaslighting” founded in weight stigma and bias is harmful, as reported on KevinMD.com.2 This month, we review the effect of abnormal BMI, both high and low, on reproduction and pregnancy.
A method to assess relative weight was first described in 1832 as its ratio in kilograms divided by the square of the height in meters, or the Quetelet Index. The search for a functional assessment of relative body weight began after World War II when reports by actuaries noted the increased mortality of overweight policyholders. The relationship between weight and cardiovascular disease was further revealed in epidemiologic studies. The Quetelet Index became the BMI in 1972.3
Weight measurement is a mainstay in the assessment of a patient’s vital signs along with blood pressure, pulse rate, respiration rate, and temperature. Weight is vital to the calculation of medication dosage – for instance, administration of conscious sedative drugs, methotrexate, and gonadotropins. Some state boards of medicine, such as Florida, have a limitation on patient BMI at office-based surgery centers (40 kg/m2).
Obesity is a disease
As reported by the World Health Organization in 2022, the disease of obesity is an epidemic afflicting more than 1 billion people worldwide, or 1 in 8 individuals globally.4 The health implications of an elevated BMI include increased mortality, diabetes, heart disease, and stroke, physical limitations to activities of daily living, and complications affecting reproduction.
Female obesity is related to poorer outcomes in natural and assisted conception, including an increased risk of miscarriage. Compared with normal-weight women, those with obesity are three times more likely to have ovulatory dysfunction,5 infertility,6 a lower chance for conception,7 higher rate of miscarriage, and low birth weight.8,9During pregnancy, women with obesity have three to four times higher rates of gestational diabetes and preeclampsia,10 as well as likelihood of delivering preterm,11 having a fetus with macrosomia and birth defects, and a 1.3- to 2.1-times higher risk of stillbirth.12
Obesity is present in 40%-80% of women with polycystic ovary syndrome,13 the most common cause of ovulatory dysfunction from dysregulation of the hypothalamic-pituitary-ovarian axis. While PCOS is associated with reproductive and metabolic consequences, even in regularly ovulating women, increasing obesity appears to be associated with decreasing spontaneous pregnancy rates and increased time to pregnancy.14
Obesity and IVF
Women with obesity have reduced success with assisted reproductive technology, an increased number of canceled cycles, and poorer quality oocytes retrieved. A prospective cohort study of nearly 2,000 women reported that every 5 kg of body weight increase (from the patient’s baseline weight at age 18) was associated with a 5% increase in the mean duration of time required for conception (95% confidence interval, 3%-7%).15 Given that approximately 90% of these women had regular menstrual cycles, ovulatory dysfunction was not the suspected pathophysiology.
A meta-analysis of 21 cohort studies reported a lower likelihood of live birth following in vitro fertilization for women with obesity, compared with normal-weight women (risk ratio, 0.85; 95% CI, 0.82-0.87).16 A further subgroup analysis that evaluated only women with PCOS showed a reduction in the live birth rate following IVF for individuals with obesity, compared with normal-weight individuals (RR, 0.78; 95% CI, 0.74-0.82).
In a retrospective study of almost 500,000 fresh autologous IVF cycles, women with obesity had a 6% reduction in pregnancy rates and a 13% reduction in live birth rates, compared with normal-weight women. Both high and low BMI were associated with an increased risk of low birth weight and preterm delivery.17 The live birth rates per transfer for normal-weight and higher-weight women were 38% and 33%, respectively.
Contrarily, a randomized controlled trial showed that an intensive weight-reduction program resulted in a large weight loss but did not substantially affect live birth rates in women with obesity scheduled for IVF.18
Low BMI
A noteworthy cause of low BMI is functional hypothalamic amenorrhea (FHA), a disorder with low energy availability either from decreased caloric intake and/or excessive energy expenditure associated with eating disorders, excessive exercise, and stress. Consequently, a reduced GnRH drive results in a decreased pulse frequency and amplitude leading to low levels of follicle-stimulating hormone and luteinizing hormone, resulting in anovulation. Correction of lifestyle behaviors related to FHA can restore menstrual cycles. After normal weight is achieved, it appears unlikely that fertility is affected.19 In 47% of adolescent patients with anorexia, menses spontaneously returned within the first 12 months after admission, with an improved prognosis in secondary over primary amenorrhea.20,21 Interestingly, mildly and significantly underweight infertile women have pregnancy and live birth rates similar to normal-weight patients after IVF treatment.22
Pregnancy is complicated in underweight women, resulting in an increased risk of anemia, fetal growth retardation, and low birth weight, as well as preterm birth.21
Take-home message
The extremes of BMI both impair natural reproduction. Elevated BMI reduces success with IVF but rapid weight loss prior to IVF does not improve outcomes. A normal BMI is the goal for optimal reproductive and pregnancy health.
Dr. Trolice is director of the IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Talumaa B et al. Obesity Rev. 2022;23:e13494.
2. https://bit.ly/3rHCivE.
3. Eknoyan G. Nephrol Dial Transplant. 2008;23:47-51.
4. Wells JCK. Dis Models Mech. 2012;5:595-607.
5. Brewer CJ and Balen AH. Reproduction. 2010;140:347-64.
6. Silvestris E et al. Reprod Biol Endocrinol. 2018;16:22.
7. Wise LA et al. Hum Reprod. 2010;25:253-64.
8. Bellver J. Curr Opin Obstet Gynecol. 2022;34:114-21.
9. Dickey RP et al. Am J Obstet Gynecol. 2013;209:349.e1.
10. Alwash SM et al. Obes Res Clin Pract. 2021;15:425-30.
11. Cnattingius S et al. JAMA. 2013;309:2362-70.
12. Aune D et al. JAMA. 2014;311:1536-46.
13. Sam S. Obes Manag. 2007;3:69-73.
14. van der Steeg JW et al. Hum Reprod. 2008;23:324-8.
15. Gaskins AJ et al. Obstet Gynecol. 2015;126:850-8.
16. Sermondade N et al. Hum Reprod Update. 2019;25:439-519.
17. Kawwass JF et al. Fertil Steril. 2016;106[7]:1742-50.
18. Einarsson S et al. Hum Reprod. 2017;32:1621-30.
19. Chaer R et al. Diseases. 2020;8:46.
20. Dempfle A et al. Psychiatry. 2013;13:308.
21. Verma A and Shrimali L. J Clin Diagn Res. 2012;6:1531-3.
22. Romanski PA et al. Reprod Biomed Online. 2020;42:366-74.
Would a national provider directory save docs’ time, help patients?
When a consumer uses a health plan provider directory to look up a physician, there’s a high probability that the entry for that doctor is incomplete or inaccurate. The Centers for Medicare & Medicaid Services would like to change that by creating a National Directory of Healthcare Providers and Services, which the agency believes would be more valuable to consumers.
In asking for public comments on whether and how it should establish the directory, CMS argues that this data repository would help patients locate physicians and could help with care coordination, health information exchange, and public health data reporting.
However, it’s not clear that such a directory would be any better than current insurance company listings or that people would use it. But a national directory could benefit physician practices by reducing their administrative work, according to observers.
In requesting public comment on the proposed national directory, CMS explains that provider organizations face “redundant and burdensome reporting requirements to multiple databases.” The directory could greatly reduce this challenge by requiring health care organizations to report provider information to a single database. Currently, physician practices have to submit these data to an average of 20 payers each, according to CMS.
“Right now, [physicians are] inundated with requests, and it takes a lot of time to update this stuff,” said David Zetter, a practice management consultant in Mechanicsburg, Pa.. “If there were one national repository of this information, that would be a good move.”
CMS envisions the National Directory as a central hub from which payers could obtain the latest provider data, which would be updated through a standardized application programming interface (API). Consequently, the insurers would no longer need to have providers submit this information to them separately.
CMS is soliciting input on what should be included in the directory. It notes that in addition to contact information, insurer directories also include a physicians’ specialties, health plan affiliations, and whether they accept new patients.
CMS’ 60-day public comment period ends Dec. 6. After that, the agency will decide what steps to take if it is decided that CMS has the legal authority to create the directory.
Terrible track record
In its annual reviews of health plan directories, CMS found that, from 2017 to 2022, only 47% of provider entries were complete. Only 73% of the providers could be matched to published directories. And only 28% of the provider names, addresses, and specialties in the directories matched those in the National Provider Identifier (NPI) registry.
Many of the mistakes in provider directories stem from errors made by practice staff, who have many other duties besides updating directory data. Yet an astonishing amount of time and effort is devoted to this task. A 2019 survey found that physician practices spend $2.76 billion annually on directory maintenance, or nearly $1000 per month per practice, on average.
The Council for Affordable Quality Healthcare, which conducted the survey, estimated that placing all directory data collection on a single platform could save the average practice $4,746 per year. For all practices in the United States, that works out to about $1.1 billion annually, CAQH said.
Pros and cons of national directory
For all the money spent on maintaining provider directories, consumers don’t use them very much. According to a 2021 Press Ganey survey, fewer than 5% of consumers seeking a primary care doctor get their information from an insurer or a benefits manager. About half search the internet first, and 24% seek a referral from a physician.
A national provider directory would be useful only if it were done right, Mr. Zetter said. Citing the inaccuracy and incompleteness of health plan directories, he said it was likely that a national directory would have similar problems. Data entered by practice staff would have to be automatically validated, perhaps through use of some kind of AI algorithm.
Effect on coordination of care
Mr. Zetter doubts the directory could improve care coordination, because primary care doctors usually refer patients to specialists they already know.
But Julia Adler-Milstein, PhD, professor of medicine and director of the Center for Clinical Informatics at the University of California, San Francisco, said that a national directory could improve communications among providers when patients select specialists outside of their primary care physician’s referral network.
“Especially if it’s not an established referral relationship, that’s where a national directory would be helpful, not only to locate the physicians but also to understand their preferences in how they’d like to receive information,” she said in an interview.
Dr. Adler-Milstein worries less than Mr. Zetter does about the challenge of ensuring the accuracy of data in the directory. She pointed out that the National Plan and Provider Enumeration System, which includes the NPI registry, has done a good job of validating provider name, address, and specialty information.
Dr. Adler-Milstein is more concerned about whether the proposed directory would address physician preferences as to how they wish to receive information. For example, while some physicians may prefer to be contacted directly, others may prefer or are required to communicate through their practices or health systems.
Efficiency in data exchange
The API used by the proposed directory would be based on the Fast Health Interoperability Resources standard that all electronic health record vendors must now include in their products. That raises the question of whether communications using contact information from the directory would be sent through a secure email system or through integrated EHR systems, Dr. Adler-Milstein said.
“I’m not sure whether the directory could support that [integration],” she said. “If it focuses on the concept of secure email exchange, that’s a relatively inefficient way of doing it,” because providers want clinical messages to pop up in their EHR workflow rather than their inboxes.
Nevertheless, Dr. Milstein-Adler added, the directory “would clearly take a lot of today’s manual work out of the system. I think organizations like UCSF would be very motivated to support the directory, knowing that people were going to a single source to find the updated information, including preferences in how we’d like people to communicate with us. There would be a lot of efficiency reasons for organizations to use this national directory.”
A version of this article first appeared on Medscape.com.
When a consumer uses a health plan provider directory to look up a physician, there’s a high probability that the entry for that doctor is incomplete or inaccurate. The Centers for Medicare & Medicaid Services would like to change that by creating a National Directory of Healthcare Providers and Services, which the agency believes would be more valuable to consumers.
In asking for public comments on whether and how it should establish the directory, CMS argues that this data repository would help patients locate physicians and could help with care coordination, health information exchange, and public health data reporting.
However, it’s not clear that such a directory would be any better than current insurance company listings or that people would use it. But a national directory could benefit physician practices by reducing their administrative work, according to observers.
In requesting public comment on the proposed national directory, CMS explains that provider organizations face “redundant and burdensome reporting requirements to multiple databases.” The directory could greatly reduce this challenge by requiring health care organizations to report provider information to a single database. Currently, physician practices have to submit these data to an average of 20 payers each, according to CMS.
“Right now, [physicians are] inundated with requests, and it takes a lot of time to update this stuff,” said David Zetter, a practice management consultant in Mechanicsburg, Pa.. “If there were one national repository of this information, that would be a good move.”
CMS envisions the National Directory as a central hub from which payers could obtain the latest provider data, which would be updated through a standardized application programming interface (API). Consequently, the insurers would no longer need to have providers submit this information to them separately.
CMS is soliciting input on what should be included in the directory. It notes that in addition to contact information, insurer directories also include a physicians’ specialties, health plan affiliations, and whether they accept new patients.
CMS’ 60-day public comment period ends Dec. 6. After that, the agency will decide what steps to take if it is decided that CMS has the legal authority to create the directory.
Terrible track record
In its annual reviews of health plan directories, CMS found that, from 2017 to 2022, only 47% of provider entries were complete. Only 73% of the providers could be matched to published directories. And only 28% of the provider names, addresses, and specialties in the directories matched those in the National Provider Identifier (NPI) registry.
Many of the mistakes in provider directories stem from errors made by practice staff, who have many other duties besides updating directory data. Yet an astonishing amount of time and effort is devoted to this task. A 2019 survey found that physician practices spend $2.76 billion annually on directory maintenance, or nearly $1000 per month per practice, on average.
The Council for Affordable Quality Healthcare, which conducted the survey, estimated that placing all directory data collection on a single platform could save the average practice $4,746 per year. For all practices in the United States, that works out to about $1.1 billion annually, CAQH said.
Pros and cons of national directory
For all the money spent on maintaining provider directories, consumers don’t use them very much. According to a 2021 Press Ganey survey, fewer than 5% of consumers seeking a primary care doctor get their information from an insurer or a benefits manager. About half search the internet first, and 24% seek a referral from a physician.
A national provider directory would be useful only if it were done right, Mr. Zetter said. Citing the inaccuracy and incompleteness of health plan directories, he said it was likely that a national directory would have similar problems. Data entered by practice staff would have to be automatically validated, perhaps through use of some kind of AI algorithm.
Effect on coordination of care
Mr. Zetter doubts the directory could improve care coordination, because primary care doctors usually refer patients to specialists they already know.
But Julia Adler-Milstein, PhD, professor of medicine and director of the Center for Clinical Informatics at the University of California, San Francisco, said that a national directory could improve communications among providers when patients select specialists outside of their primary care physician’s referral network.
“Especially if it’s not an established referral relationship, that’s where a national directory would be helpful, not only to locate the physicians but also to understand their preferences in how they’d like to receive information,” she said in an interview.
Dr. Adler-Milstein worries less than Mr. Zetter does about the challenge of ensuring the accuracy of data in the directory. She pointed out that the National Plan and Provider Enumeration System, which includes the NPI registry, has done a good job of validating provider name, address, and specialty information.
Dr. Adler-Milstein is more concerned about whether the proposed directory would address physician preferences as to how they wish to receive information. For example, while some physicians may prefer to be contacted directly, others may prefer or are required to communicate through their practices or health systems.
Efficiency in data exchange
The API used by the proposed directory would be based on the Fast Health Interoperability Resources standard that all electronic health record vendors must now include in their products. That raises the question of whether communications using contact information from the directory would be sent through a secure email system or through integrated EHR systems, Dr. Adler-Milstein said.
“I’m not sure whether the directory could support that [integration],” she said. “If it focuses on the concept of secure email exchange, that’s a relatively inefficient way of doing it,” because providers want clinical messages to pop up in their EHR workflow rather than their inboxes.
Nevertheless, Dr. Milstein-Adler added, the directory “would clearly take a lot of today’s manual work out of the system. I think organizations like UCSF would be very motivated to support the directory, knowing that people were going to a single source to find the updated information, including preferences in how we’d like people to communicate with us. There would be a lot of efficiency reasons for organizations to use this national directory.”
A version of this article first appeared on Medscape.com.
When a consumer uses a health plan provider directory to look up a physician, there’s a high probability that the entry for that doctor is incomplete or inaccurate. The Centers for Medicare & Medicaid Services would like to change that by creating a National Directory of Healthcare Providers and Services, which the agency believes would be more valuable to consumers.
In asking for public comments on whether and how it should establish the directory, CMS argues that this data repository would help patients locate physicians and could help with care coordination, health information exchange, and public health data reporting.
However, it’s not clear that such a directory would be any better than current insurance company listings or that people would use it. But a national directory could benefit physician practices by reducing their administrative work, according to observers.
In requesting public comment on the proposed national directory, CMS explains that provider organizations face “redundant and burdensome reporting requirements to multiple databases.” The directory could greatly reduce this challenge by requiring health care organizations to report provider information to a single database. Currently, physician practices have to submit these data to an average of 20 payers each, according to CMS.
“Right now, [physicians are] inundated with requests, and it takes a lot of time to update this stuff,” said David Zetter, a practice management consultant in Mechanicsburg, Pa.. “If there were one national repository of this information, that would be a good move.”
CMS envisions the National Directory as a central hub from which payers could obtain the latest provider data, which would be updated through a standardized application programming interface (API). Consequently, the insurers would no longer need to have providers submit this information to them separately.
CMS is soliciting input on what should be included in the directory. It notes that in addition to contact information, insurer directories also include a physicians’ specialties, health plan affiliations, and whether they accept new patients.
CMS’ 60-day public comment period ends Dec. 6. After that, the agency will decide what steps to take if it is decided that CMS has the legal authority to create the directory.
Terrible track record
In its annual reviews of health plan directories, CMS found that, from 2017 to 2022, only 47% of provider entries were complete. Only 73% of the providers could be matched to published directories. And only 28% of the provider names, addresses, and specialties in the directories matched those in the National Provider Identifier (NPI) registry.
Many of the mistakes in provider directories stem from errors made by practice staff, who have many other duties besides updating directory data. Yet an astonishing amount of time and effort is devoted to this task. A 2019 survey found that physician practices spend $2.76 billion annually on directory maintenance, or nearly $1000 per month per practice, on average.
The Council for Affordable Quality Healthcare, which conducted the survey, estimated that placing all directory data collection on a single platform could save the average practice $4,746 per year. For all practices in the United States, that works out to about $1.1 billion annually, CAQH said.
Pros and cons of national directory
For all the money spent on maintaining provider directories, consumers don’t use them very much. According to a 2021 Press Ganey survey, fewer than 5% of consumers seeking a primary care doctor get their information from an insurer or a benefits manager. About half search the internet first, and 24% seek a referral from a physician.
A national provider directory would be useful only if it were done right, Mr. Zetter said. Citing the inaccuracy and incompleteness of health plan directories, he said it was likely that a national directory would have similar problems. Data entered by practice staff would have to be automatically validated, perhaps through use of some kind of AI algorithm.
Effect on coordination of care
Mr. Zetter doubts the directory could improve care coordination, because primary care doctors usually refer patients to specialists they already know.
But Julia Adler-Milstein, PhD, professor of medicine and director of the Center for Clinical Informatics at the University of California, San Francisco, said that a national directory could improve communications among providers when patients select specialists outside of their primary care physician’s referral network.
“Especially if it’s not an established referral relationship, that’s where a national directory would be helpful, not only to locate the physicians but also to understand their preferences in how they’d like to receive information,” she said in an interview.
Dr. Adler-Milstein worries less than Mr. Zetter does about the challenge of ensuring the accuracy of data in the directory. She pointed out that the National Plan and Provider Enumeration System, which includes the NPI registry, has done a good job of validating provider name, address, and specialty information.
Dr. Adler-Milstein is more concerned about whether the proposed directory would address physician preferences as to how they wish to receive information. For example, while some physicians may prefer to be contacted directly, others may prefer or are required to communicate through their practices or health systems.
Efficiency in data exchange
The API used by the proposed directory would be based on the Fast Health Interoperability Resources standard that all electronic health record vendors must now include in their products. That raises the question of whether communications using contact information from the directory would be sent through a secure email system or through integrated EHR systems, Dr. Adler-Milstein said.
“I’m not sure whether the directory could support that [integration],” she said. “If it focuses on the concept of secure email exchange, that’s a relatively inefficient way of doing it,” because providers want clinical messages to pop up in their EHR workflow rather than their inboxes.
Nevertheless, Dr. Milstein-Adler added, the directory “would clearly take a lot of today’s manual work out of the system. I think organizations like UCSF would be very motivated to support the directory, knowing that people were going to a single source to find the updated information, including preferences in how we’d like people to communicate with us. There would be a lot of efficiency reasons for organizations to use this national directory.”
A version of this article first appeared on Medscape.com.
Plant-based diet cut hot flashes 78%: WAVS study
Women eating a reduced-fat vegan diet combined with a daily serving of soybeans experienced a 78% reduction in frequency of menopausal hot flashes over 12 weeks, in a small, nonblinded, randomized-controlled trial.
“We do not fully understand yet why this combination works, but it seems that these three elements are key: avoiding animal products, reducing fat, and adding a serving of soybeans,” lead researcher Neal Barnard, MD, explained in a press release. “These new results suggest that a diet change should be considered as a first-line treatment for troublesome vasomotor symptoms, including night sweats and hot flashes,” added Dr. Barnard, who is president of the Physicians Committee for Responsible Medicine, and adjunct professor at George Washington University, Washington.
But, while “the findings from this very small study complement everything we know about the benefits of an excellent diet and the health benefits of soy,” they should be interpreted with some caution, commented Susan Reed, MD, president of the North American Menopause Society, and associate program director of the women’s reproductive research program at the University of Washington, Seattle.
For the trial, called WAVS (Women’s Study for the Alleviation of Vasomotor Symptoms), the researchers randomized 84 postmenopausal women with at least two moderate to severe hot flashes daily to either the intervention or usual diet, with a total of 71 subjects completing the 12-week study, published in Menopause. Criteria for exclusion included any cause of vasomotor symptoms other than natural menopause, current use of a low-fat, vegan diet that includes daily soy products, soy allergy, and body mass index < 18.5 kg/m2.
Participants in the intervention group were asked to avoid animal-derived foods, minimize their use of oils and fatty foods such as nuts and avocados, and include half a cup (86 g) of cooked soybeans daily in their diets. They were also offered 1-hour virtual group meetings each week, in which a registered dietitian or research staff provided information on food preparation and managing common dietary challenges.
Control group participants were asked to continue their usual diets and attend four 1-hour group sessions.
At baseline and then after the 12-week study period, dietary intake was self-recorded for 2 weekdays and 1 weekend day, hot flash frequency and severity was recorded for 1 week using a mobile app, and the effect of menopausal symptoms on quality of life was measured using the vasomotor, psychosocial, physical, and sexual domains of the Menopause-Specific Quality of Life (MENQOL) questionnaire.
Equol production was also assessed in a subset of 15 intervention and 12 control participants who had urinary isoflavone concentrations measured after eating half a cup (86 g) of soybeans twice daily for 3 days. This was based on the theory that diets such as the intervention in this study “seem to foster the growth of gut bacteria capable of converting daidzein to equol,” noted the authors. The ability to produce equol is detected more frequently in individuals following vegetarian diets than in omnivores and … has been proposed as a factor in soy’s apparent health benefits.”
The study found that total hot flash frequency decreased by 78% in the intervention group (P < .001) and 39% (P < .001) in the control group (between-group P = .003), and moderate to severe hot flashes decreased by 88% versus 34%, respectively (from 5.0 to 0.6 per day, P < .001 vs. from 4.4 to 2.9 per day, P < .001; between-group P < .001). Among participants with at least seven moderate to severe hot flashes per day at baseline, moderate to severe hot flashes decreased by 93% (from 10.6 to 0.7 per day) in the intervention group (P < .001) and 36% (from 9.0 to 5.8 per day) in the control group (P = .01, between-group P < .001). The changes in hot flashes were paralleled by changes in the MENQOL findings, with significant between-group differences in the vasomotor (P = 0.004), physical (P = 0.01), and sexual (P = 0.03) domains.
Changes in frequency of severe hot flashes correlated directly with changes in fat intake, and inversely with changes in carbohydrate and fiber intake, such that “the greater the reduction in fat intake and the greater the increases in carbohydrate and fiber consumption, the greater the reduction in severe hot flashes,” noted the researchers. Mean body weight also decreased by 3.6 kg in the intervention group and 0.2 kg in the control group (P < .001). “Equol-production status had no apparent effect on hot flashes,” they added.
The study is the second phase of WAVS, which comprises two parts, the first of which showed similar results, but was conducted in the fall, raising questions about whether cooler temperatures were partly responsible for the results. Phase 2 of WAVS enrolled participants in the spring “ruling out the effect of outside temperature,” noted the authors.
“Eating a healthy diet at midlife is so important for long-term health and a sense of well-being for peri- and postmenopausal women,” said Dr Reed, but she urged caution in interpreting the findings. “This was an unblinded study,” she told this news organization. “Women were recruited to this study anticipating that they would be in a study on a soy diet. Individuals who sign up for a study are hoping for benefit from the intervention. The controls who don’t get the soy diet are often disappointed, so there is no benefit from a nonblinded control arm for their hot flashes. And that is exactly what we saw here. Blinded studies can hide what you are getting, so everyone in the study (intervention and controls) has the same anticipated benefit. But you cannot blind a soy diet.”
Dr. Reed also noted that, while the biologic mechanism of benefit should be equol production, this was not shown – given that both equol producers and nonproducers in the soy intervention reported marked symptom reduction.
“Only prior studies with estrogen interventions have observed reductions of hot flashes of the amount reported here,” she concluded. “Hopefully future large studies will clarify the role of soy diet for decreasing hot flashes.”
Dr. Barnard writes books and articles and gives lectures related to nutrition and health and has received royalties and honoraria from these sources. Dr. Reed has no relevant disclosures.
Women eating a reduced-fat vegan diet combined with a daily serving of soybeans experienced a 78% reduction in frequency of menopausal hot flashes over 12 weeks, in a small, nonblinded, randomized-controlled trial.
“We do not fully understand yet why this combination works, but it seems that these three elements are key: avoiding animal products, reducing fat, and adding a serving of soybeans,” lead researcher Neal Barnard, MD, explained in a press release. “These new results suggest that a diet change should be considered as a first-line treatment for troublesome vasomotor symptoms, including night sweats and hot flashes,” added Dr. Barnard, who is president of the Physicians Committee for Responsible Medicine, and adjunct professor at George Washington University, Washington.
But, while “the findings from this very small study complement everything we know about the benefits of an excellent diet and the health benefits of soy,” they should be interpreted with some caution, commented Susan Reed, MD, president of the North American Menopause Society, and associate program director of the women’s reproductive research program at the University of Washington, Seattle.
For the trial, called WAVS (Women’s Study for the Alleviation of Vasomotor Symptoms), the researchers randomized 84 postmenopausal women with at least two moderate to severe hot flashes daily to either the intervention or usual diet, with a total of 71 subjects completing the 12-week study, published in Menopause. Criteria for exclusion included any cause of vasomotor symptoms other than natural menopause, current use of a low-fat, vegan diet that includes daily soy products, soy allergy, and body mass index < 18.5 kg/m2.
Participants in the intervention group were asked to avoid animal-derived foods, minimize their use of oils and fatty foods such as nuts and avocados, and include half a cup (86 g) of cooked soybeans daily in their diets. They were also offered 1-hour virtual group meetings each week, in which a registered dietitian or research staff provided information on food preparation and managing common dietary challenges.
Control group participants were asked to continue their usual diets and attend four 1-hour group sessions.
At baseline and then after the 12-week study period, dietary intake was self-recorded for 2 weekdays and 1 weekend day, hot flash frequency and severity was recorded for 1 week using a mobile app, and the effect of menopausal symptoms on quality of life was measured using the vasomotor, psychosocial, physical, and sexual domains of the Menopause-Specific Quality of Life (MENQOL) questionnaire.
Equol production was also assessed in a subset of 15 intervention and 12 control participants who had urinary isoflavone concentrations measured after eating half a cup (86 g) of soybeans twice daily for 3 days. This was based on the theory that diets such as the intervention in this study “seem to foster the growth of gut bacteria capable of converting daidzein to equol,” noted the authors. The ability to produce equol is detected more frequently in individuals following vegetarian diets than in omnivores and … has been proposed as a factor in soy’s apparent health benefits.”
The study found that total hot flash frequency decreased by 78% in the intervention group (P < .001) and 39% (P < .001) in the control group (between-group P = .003), and moderate to severe hot flashes decreased by 88% versus 34%, respectively (from 5.0 to 0.6 per day, P < .001 vs. from 4.4 to 2.9 per day, P < .001; between-group P < .001). Among participants with at least seven moderate to severe hot flashes per day at baseline, moderate to severe hot flashes decreased by 93% (from 10.6 to 0.7 per day) in the intervention group (P < .001) and 36% (from 9.0 to 5.8 per day) in the control group (P = .01, between-group P < .001). The changes in hot flashes were paralleled by changes in the MENQOL findings, with significant between-group differences in the vasomotor (P = 0.004), physical (P = 0.01), and sexual (P = 0.03) domains.
Changes in frequency of severe hot flashes correlated directly with changes in fat intake, and inversely with changes in carbohydrate and fiber intake, such that “the greater the reduction in fat intake and the greater the increases in carbohydrate and fiber consumption, the greater the reduction in severe hot flashes,” noted the researchers. Mean body weight also decreased by 3.6 kg in the intervention group and 0.2 kg in the control group (P < .001). “Equol-production status had no apparent effect on hot flashes,” they added.
The study is the second phase of WAVS, which comprises two parts, the first of which showed similar results, but was conducted in the fall, raising questions about whether cooler temperatures were partly responsible for the results. Phase 2 of WAVS enrolled participants in the spring “ruling out the effect of outside temperature,” noted the authors.
“Eating a healthy diet at midlife is so important for long-term health and a sense of well-being for peri- and postmenopausal women,” said Dr Reed, but she urged caution in interpreting the findings. “This was an unblinded study,” she told this news organization. “Women were recruited to this study anticipating that they would be in a study on a soy diet. Individuals who sign up for a study are hoping for benefit from the intervention. The controls who don’t get the soy diet are often disappointed, so there is no benefit from a nonblinded control arm for their hot flashes. And that is exactly what we saw here. Blinded studies can hide what you are getting, so everyone in the study (intervention and controls) has the same anticipated benefit. But you cannot blind a soy diet.”
Dr. Reed also noted that, while the biologic mechanism of benefit should be equol production, this was not shown – given that both equol producers and nonproducers in the soy intervention reported marked symptom reduction.
“Only prior studies with estrogen interventions have observed reductions of hot flashes of the amount reported here,” she concluded. “Hopefully future large studies will clarify the role of soy diet for decreasing hot flashes.”
Dr. Barnard writes books and articles and gives lectures related to nutrition and health and has received royalties and honoraria from these sources. Dr. Reed has no relevant disclosures.
Women eating a reduced-fat vegan diet combined with a daily serving of soybeans experienced a 78% reduction in frequency of menopausal hot flashes over 12 weeks, in a small, nonblinded, randomized-controlled trial.
“We do not fully understand yet why this combination works, but it seems that these three elements are key: avoiding animal products, reducing fat, and adding a serving of soybeans,” lead researcher Neal Barnard, MD, explained in a press release. “These new results suggest that a diet change should be considered as a first-line treatment for troublesome vasomotor symptoms, including night sweats and hot flashes,” added Dr. Barnard, who is president of the Physicians Committee for Responsible Medicine, and adjunct professor at George Washington University, Washington.
But, while “the findings from this very small study complement everything we know about the benefits of an excellent diet and the health benefits of soy,” they should be interpreted with some caution, commented Susan Reed, MD, president of the North American Menopause Society, and associate program director of the women’s reproductive research program at the University of Washington, Seattle.
For the trial, called WAVS (Women’s Study for the Alleviation of Vasomotor Symptoms), the researchers randomized 84 postmenopausal women with at least two moderate to severe hot flashes daily to either the intervention or usual diet, with a total of 71 subjects completing the 12-week study, published in Menopause. Criteria for exclusion included any cause of vasomotor symptoms other than natural menopause, current use of a low-fat, vegan diet that includes daily soy products, soy allergy, and body mass index < 18.5 kg/m2.
Participants in the intervention group were asked to avoid animal-derived foods, minimize their use of oils and fatty foods such as nuts and avocados, and include half a cup (86 g) of cooked soybeans daily in their diets. They were also offered 1-hour virtual group meetings each week, in which a registered dietitian or research staff provided information on food preparation and managing common dietary challenges.
Control group participants were asked to continue their usual diets and attend four 1-hour group sessions.
At baseline and then after the 12-week study period, dietary intake was self-recorded for 2 weekdays and 1 weekend day, hot flash frequency and severity was recorded for 1 week using a mobile app, and the effect of menopausal symptoms on quality of life was measured using the vasomotor, psychosocial, physical, and sexual domains of the Menopause-Specific Quality of Life (MENQOL) questionnaire.
Equol production was also assessed in a subset of 15 intervention and 12 control participants who had urinary isoflavone concentrations measured after eating half a cup (86 g) of soybeans twice daily for 3 days. This was based on the theory that diets such as the intervention in this study “seem to foster the growth of gut bacteria capable of converting daidzein to equol,” noted the authors. The ability to produce equol is detected more frequently in individuals following vegetarian diets than in omnivores and … has been proposed as a factor in soy’s apparent health benefits.”
The study found that total hot flash frequency decreased by 78% in the intervention group (P < .001) and 39% (P < .001) in the control group (between-group P = .003), and moderate to severe hot flashes decreased by 88% versus 34%, respectively (from 5.0 to 0.6 per day, P < .001 vs. from 4.4 to 2.9 per day, P < .001; between-group P < .001). Among participants with at least seven moderate to severe hot flashes per day at baseline, moderate to severe hot flashes decreased by 93% (from 10.6 to 0.7 per day) in the intervention group (P < .001) and 36% (from 9.0 to 5.8 per day) in the control group (P = .01, between-group P < .001). The changes in hot flashes were paralleled by changes in the MENQOL findings, with significant between-group differences in the vasomotor (P = 0.004), physical (P = 0.01), and sexual (P = 0.03) domains.
Changes in frequency of severe hot flashes correlated directly with changes in fat intake, and inversely with changes in carbohydrate and fiber intake, such that “the greater the reduction in fat intake and the greater the increases in carbohydrate and fiber consumption, the greater the reduction in severe hot flashes,” noted the researchers. Mean body weight also decreased by 3.6 kg in the intervention group and 0.2 kg in the control group (P < .001). “Equol-production status had no apparent effect on hot flashes,” they added.
The study is the second phase of WAVS, which comprises two parts, the first of which showed similar results, but was conducted in the fall, raising questions about whether cooler temperatures were partly responsible for the results. Phase 2 of WAVS enrolled participants in the spring “ruling out the effect of outside temperature,” noted the authors.
“Eating a healthy diet at midlife is so important for long-term health and a sense of well-being for peri- and postmenopausal women,” said Dr Reed, but she urged caution in interpreting the findings. “This was an unblinded study,” she told this news organization. “Women were recruited to this study anticipating that they would be in a study on a soy diet. Individuals who sign up for a study are hoping for benefit from the intervention. The controls who don’t get the soy diet are often disappointed, so there is no benefit from a nonblinded control arm for their hot flashes. And that is exactly what we saw here. Blinded studies can hide what you are getting, so everyone in the study (intervention and controls) has the same anticipated benefit. But you cannot blind a soy diet.”
Dr. Reed also noted that, while the biologic mechanism of benefit should be equol production, this was not shown – given that both equol producers and nonproducers in the soy intervention reported marked symptom reduction.
“Only prior studies with estrogen interventions have observed reductions of hot flashes of the amount reported here,” she concluded. “Hopefully future large studies will clarify the role of soy diet for decreasing hot flashes.”
Dr. Barnard writes books and articles and gives lectures related to nutrition and health and has received royalties and honoraria from these sources. Dr. Reed has no relevant disclosures.
From Frankenstein to Lecter: Hollywood’s baddest docs
Masks can be scary on Halloween, but more so when they come with scrubs, scalpels, and God complexes. In March, Medscape readers chose their favorite characters and performers in the Hollywood health care system. from a deep bench (and no, Dr Evil didn’t go to medical school; neither did Dr No, for that matter). Before you see these folks who’d rather haunt than heal, we urge you to seek a second opinion.
George Harris (Richard Widmark, “Coma,” 1978)
“Medicine is now a great social force,” says Dr. George Harris (Richard Widmark), chief of surgery at Boston Memorial. Because the public trusts doctors, “we’ll make the hard decisions” – like choosing which young, healthy patients to put into an irreversible coma to harvest their organs. Harris’ audience of one here is Dr. Susan Wheeler (Genevieve Bujold), the upstart who has uncovered his plot, and whom Harris has just drugged to prepare her as his next unintentional donor. “Coma” was based on a bestseller by Robin Cook and directed by Michael Crichton, who left Harvard Medical School for a career in popular books and films, including “The Andromeda Strain” and “Jurassic Park.” Although Dr. Harris starts out as a reassuring friend and mentor to Dr. Wheeler, older moviegoers won’t forget that he launched to stardom by tossing a woman in a wheelchair down the stairs in 1947’s “Kiss of Death.”
Christian Szell (Laurence Olivier, “Marathon Man,” 1976)
He may look harmless, but Christian Szell (Laurence Olivier) is a sadist with a secret, a stash, and throat-slitting skills. Szell, a dentist known as the White Angel of Auschwitz for his war crimes, stops at nothing to protect the diamonds he stole from his victims in the camps. In one of Hollywood’s most infamous torture scenes, Szell tries to extract information from Babe Levy (Dustin Hoffman), an innocent grad student, plying the tools of his trade. When Szell asks, “Is it safe?” he’s not curious about whether Babe’s insurance covers anesthesia.
Orin Scrivello (Steve Martin, “Little Shop of Horrors,” 1986)
Sticking with deranged dentists, Orin Scrivello, DDS, (Steve Martin) sings and dances his way into your nightmares buoyed by copious helpings of nitrous oxide. Orin’s too-encouraging momma told him to parlay his sadistic tendencies into a career “where people will pay you to be inhumane.” Sonny listened. Moviegoers were treated to screeching sound effects of a tooth getting yanked during an Elvis-like musical number shot in part from inside a patient’s mouth. Martin makes a creepy scene more fun than a long, slow root canal.
Henry Frankenstein (Colin Clive, “Frankenstein,” 1931)
His alarming need for fresh corpses forced Henry Frankenstein (Colin Clive) to leave medical school and experiment solo in a castle. He insists to his betrothed that he hasn’t gone mad when she arrives as he is bringing a dead body back to life during a raging lightning storm. When she and Henry’s mentor, Dr Waldman, witness him succeed, Waldman warns Henry that the former owner of the purloined brain was a notorious criminal. When Henry exclaims: “It’s alive, it’s alive !” little did he know that he created the face (Boris Karloff) that would launch a thousand sequels, a spectacular satire, and untold Halloween masks.
Dr. Gogol (Peter Lorre, “Mad Love,” 1935)
A few years after playing doctor Frankenstein, Colin Clive became the patient of a mad medic himself. A concert pianist whose hands have been mangled in a train wreck, Clive’s wife turns to Dr. Gogol (Peter Lorre, in his Hollywood debut), who promises to surgically reattach the musician’s hands. Unfortunately, Gogol is so obsessed with the wife, a star of gory stage shows, that he has created a wax figure of her. He schemes to win her in the flesh by attaching a murderer’s hands to Clive, then frame him for committing murder with those hands. Gogol utters the madman’s lament: “I have conquered science. Why can’t I conquer love?” A modern remake would surely have him asking, “Why do they swipe left?
Hannibal Lecter (Anthony Hopkins, “Silence of the Lambs,” 1991)
The FBI, hunting for a serial killer, sends trainee Clarice Starling (Jodie Foster) to seek insight into the murderer from the imprisoned Dr. Hannibal Lecter (Anthony Hopkins), a brilliant psychiatrist with a penchant for murder — and a taste for the flesh of his victims. Lecter proves to be a menace from their first meeting; the bars and glass surrounding his cell offer Clarice no protection from his gaze and ability to read her mind. In his own way, the urbane, pathologically charming Lecter takes a shine to Clarice, helping with the case while embarking on another murderous spree against men who recently wronged her. When he escapes, his plans do not include dinner with – or of – Clarice, but others, well, they’re not so lucky.
Henry Jekyll (Fredric March, “Dr. Jekyll and Mr. Hyde,” 1931)
Henry Jekyll (Fredric March) is a jumble of personalities. By day, he’s a kindly doctor in Victorian London with an American accent. But he is so determined to split good and evil personalities that he devises a potion to outsource his id. As he watches himself morph into Mr. Hyde – a hairy, cone-headed dude in serious need of an orthodontist – he exclaims, “Free! Free at last!” Free, that is, for his simian side to engage in debauchery, abuse, self-hatred, intimations of rape, and ultimately murder – all of which are explored in this pre-Code film, the first talkie version of Robert Louis Stevenson’s story.
Dr. Moreau (Charles Laughton, “Island of Lost Souls,” 1932)
“Strange-looking natives you have here,” shipwreck victim Edward Parker (Richard Arlen) tells his host, the white-suited, whip-wielding Dr Moreau. Before long, we learn that Moreau’s evil veterinary talents have created an island population of human/beast hybrids who are forced to follow his laws – especially one forbidding them from eating meat or walking on all fours. Lawbreakers get taken to the House of Pain, a medical setting which, as its name suggests, lacks adequate analgesia. Burt Lancaster and Marlon Brando took on the Moreau role in later versions, but Laughton is the creepiest when he asks, “Do you know what it means to feel like God?” The film was banned for years in Britain, and H.G. Wells despised this take on his antivivisection tale.
Charles Nichols (Jeroen Krabbé, “The Fugitive,” 1993)
Richard Kimble, a Chicago vascular surgeon, arrives home to find that a man just brutally murdered his loving wife. The killer escapes, and Kimble falls into the frame-up. Convicted for the murder and headed to prison, Kimble breaks free in an epic escape scene. He spends the rest of the movie all but giving his right arm to find the murderer, while being pursued by a dogged U.S. Marshal played with gusto by Tommy Lee Jones. Kimble eventually discovers that his colleague, Dr. Charles Nichols (Jeroen Krabbé), is not quite the best friend a man could have – or the most ethical of clinical investigators.
Elliot and Beverly Mantle (Jeremy Irons, “Dead Ringers,” 1988)
“You’ve got to try the movie star,” fertility specialist Elliot Mantle (Jeremy Irons) implores to his identical but meek twin brother, Beverly (also Jeremy Irons), talking about an actress-patient (Genevieve Bujold) as if she were a menu item. Beverly shares a practice with Elliot, along with a soul and an easily satisfied drug addiction. Beverly is unaware that Elliot seduces patients before passing them off to his brother, including the actress. Beverly is in love with the actress, which upsets the equilibrium of their shared soul. He aims to fix this, but not without some trauma involving freakish and unsanitary operating implements.
Dean Armitage (Bradley Whitford, “Get Out,” 2017)
Neurosurgeon Dean Armitage (Bradley Whitford) was such a fan of President Obama that he would have voted for him a third time if he could. At least, that’s how he portrays himself to Chris (Daniel Kaluuya), an African American photographer and the new boyfriend of Armitage’s White daughter. The Armitage estate has plenty of people of color – on staff, anyway – but Chris finds them odd and distant. It turns out that a gathering of rich White people is in fact an auction for his eyesight. Horror ensues. The main message from this film is not unlike that of Russian operatives who fall out of favor with the Kremlin: Don’t drink the tea.
A version of this article first appeared on Medscape.com.
Masks can be scary on Halloween, but more so when they come with scrubs, scalpels, and God complexes. In March, Medscape readers chose their favorite characters and performers in the Hollywood health care system. from a deep bench (and no, Dr Evil didn’t go to medical school; neither did Dr No, for that matter). Before you see these folks who’d rather haunt than heal, we urge you to seek a second opinion.
George Harris (Richard Widmark, “Coma,” 1978)
“Medicine is now a great social force,” says Dr. George Harris (Richard Widmark), chief of surgery at Boston Memorial. Because the public trusts doctors, “we’ll make the hard decisions” – like choosing which young, healthy patients to put into an irreversible coma to harvest their organs. Harris’ audience of one here is Dr. Susan Wheeler (Genevieve Bujold), the upstart who has uncovered his plot, and whom Harris has just drugged to prepare her as his next unintentional donor. “Coma” was based on a bestseller by Robin Cook and directed by Michael Crichton, who left Harvard Medical School for a career in popular books and films, including “The Andromeda Strain” and “Jurassic Park.” Although Dr. Harris starts out as a reassuring friend and mentor to Dr. Wheeler, older moviegoers won’t forget that he launched to stardom by tossing a woman in a wheelchair down the stairs in 1947’s “Kiss of Death.”
Christian Szell (Laurence Olivier, “Marathon Man,” 1976)
He may look harmless, but Christian Szell (Laurence Olivier) is a sadist with a secret, a stash, and throat-slitting skills. Szell, a dentist known as the White Angel of Auschwitz for his war crimes, stops at nothing to protect the diamonds he stole from his victims in the camps. In one of Hollywood’s most infamous torture scenes, Szell tries to extract information from Babe Levy (Dustin Hoffman), an innocent grad student, plying the tools of his trade. When Szell asks, “Is it safe?” he’s not curious about whether Babe’s insurance covers anesthesia.
Orin Scrivello (Steve Martin, “Little Shop of Horrors,” 1986)
Sticking with deranged dentists, Orin Scrivello, DDS, (Steve Martin) sings and dances his way into your nightmares buoyed by copious helpings of nitrous oxide. Orin’s too-encouraging momma told him to parlay his sadistic tendencies into a career “where people will pay you to be inhumane.” Sonny listened. Moviegoers were treated to screeching sound effects of a tooth getting yanked during an Elvis-like musical number shot in part from inside a patient’s mouth. Martin makes a creepy scene more fun than a long, slow root canal.
Henry Frankenstein (Colin Clive, “Frankenstein,” 1931)
His alarming need for fresh corpses forced Henry Frankenstein (Colin Clive) to leave medical school and experiment solo in a castle. He insists to his betrothed that he hasn’t gone mad when she arrives as he is bringing a dead body back to life during a raging lightning storm. When she and Henry’s mentor, Dr Waldman, witness him succeed, Waldman warns Henry that the former owner of the purloined brain was a notorious criminal. When Henry exclaims: “It’s alive, it’s alive !” little did he know that he created the face (Boris Karloff) that would launch a thousand sequels, a spectacular satire, and untold Halloween masks.
Dr. Gogol (Peter Lorre, “Mad Love,” 1935)
A few years after playing doctor Frankenstein, Colin Clive became the patient of a mad medic himself. A concert pianist whose hands have been mangled in a train wreck, Clive’s wife turns to Dr. Gogol (Peter Lorre, in his Hollywood debut), who promises to surgically reattach the musician’s hands. Unfortunately, Gogol is so obsessed with the wife, a star of gory stage shows, that he has created a wax figure of her. He schemes to win her in the flesh by attaching a murderer’s hands to Clive, then frame him for committing murder with those hands. Gogol utters the madman’s lament: “I have conquered science. Why can’t I conquer love?” A modern remake would surely have him asking, “Why do they swipe left?
Hannibal Lecter (Anthony Hopkins, “Silence of the Lambs,” 1991)
The FBI, hunting for a serial killer, sends trainee Clarice Starling (Jodie Foster) to seek insight into the murderer from the imprisoned Dr. Hannibal Lecter (Anthony Hopkins), a brilliant psychiatrist with a penchant for murder — and a taste for the flesh of his victims. Lecter proves to be a menace from their first meeting; the bars and glass surrounding his cell offer Clarice no protection from his gaze and ability to read her mind. In his own way, the urbane, pathologically charming Lecter takes a shine to Clarice, helping with the case while embarking on another murderous spree against men who recently wronged her. When he escapes, his plans do not include dinner with – or of – Clarice, but others, well, they’re not so lucky.
Henry Jekyll (Fredric March, “Dr. Jekyll and Mr. Hyde,” 1931)
Henry Jekyll (Fredric March) is a jumble of personalities. By day, he’s a kindly doctor in Victorian London with an American accent. But he is so determined to split good and evil personalities that he devises a potion to outsource his id. As he watches himself morph into Mr. Hyde – a hairy, cone-headed dude in serious need of an orthodontist – he exclaims, “Free! Free at last!” Free, that is, for his simian side to engage in debauchery, abuse, self-hatred, intimations of rape, and ultimately murder – all of which are explored in this pre-Code film, the first talkie version of Robert Louis Stevenson’s story.
Dr. Moreau (Charles Laughton, “Island of Lost Souls,” 1932)
“Strange-looking natives you have here,” shipwreck victim Edward Parker (Richard Arlen) tells his host, the white-suited, whip-wielding Dr Moreau. Before long, we learn that Moreau’s evil veterinary talents have created an island population of human/beast hybrids who are forced to follow his laws – especially one forbidding them from eating meat or walking on all fours. Lawbreakers get taken to the House of Pain, a medical setting which, as its name suggests, lacks adequate analgesia. Burt Lancaster and Marlon Brando took on the Moreau role in later versions, but Laughton is the creepiest when he asks, “Do you know what it means to feel like God?” The film was banned for years in Britain, and H.G. Wells despised this take on his antivivisection tale.
Charles Nichols (Jeroen Krabbé, “The Fugitive,” 1993)
Richard Kimble, a Chicago vascular surgeon, arrives home to find that a man just brutally murdered his loving wife. The killer escapes, and Kimble falls into the frame-up. Convicted for the murder and headed to prison, Kimble breaks free in an epic escape scene. He spends the rest of the movie all but giving his right arm to find the murderer, while being pursued by a dogged U.S. Marshal played with gusto by Tommy Lee Jones. Kimble eventually discovers that his colleague, Dr. Charles Nichols (Jeroen Krabbé), is not quite the best friend a man could have – or the most ethical of clinical investigators.
Elliot and Beverly Mantle (Jeremy Irons, “Dead Ringers,” 1988)
“You’ve got to try the movie star,” fertility specialist Elliot Mantle (Jeremy Irons) implores to his identical but meek twin brother, Beverly (also Jeremy Irons), talking about an actress-patient (Genevieve Bujold) as if she were a menu item. Beverly shares a practice with Elliot, along with a soul and an easily satisfied drug addiction. Beverly is unaware that Elliot seduces patients before passing them off to his brother, including the actress. Beverly is in love with the actress, which upsets the equilibrium of their shared soul. He aims to fix this, but not without some trauma involving freakish and unsanitary operating implements.
Dean Armitage (Bradley Whitford, “Get Out,” 2017)
Neurosurgeon Dean Armitage (Bradley Whitford) was such a fan of President Obama that he would have voted for him a third time if he could. At least, that’s how he portrays himself to Chris (Daniel Kaluuya), an African American photographer and the new boyfriend of Armitage’s White daughter. The Armitage estate has plenty of people of color – on staff, anyway – but Chris finds them odd and distant. It turns out that a gathering of rich White people is in fact an auction for his eyesight. Horror ensues. The main message from this film is not unlike that of Russian operatives who fall out of favor with the Kremlin: Don’t drink the tea.
A version of this article first appeared on Medscape.com.
Masks can be scary on Halloween, but more so when they come with scrubs, scalpels, and God complexes. In March, Medscape readers chose their favorite characters and performers in the Hollywood health care system. from a deep bench (and no, Dr Evil didn’t go to medical school; neither did Dr No, for that matter). Before you see these folks who’d rather haunt than heal, we urge you to seek a second opinion.
George Harris (Richard Widmark, “Coma,” 1978)
“Medicine is now a great social force,” says Dr. George Harris (Richard Widmark), chief of surgery at Boston Memorial. Because the public trusts doctors, “we’ll make the hard decisions” – like choosing which young, healthy patients to put into an irreversible coma to harvest their organs. Harris’ audience of one here is Dr. Susan Wheeler (Genevieve Bujold), the upstart who has uncovered his plot, and whom Harris has just drugged to prepare her as his next unintentional donor. “Coma” was based on a bestseller by Robin Cook and directed by Michael Crichton, who left Harvard Medical School for a career in popular books and films, including “The Andromeda Strain” and “Jurassic Park.” Although Dr. Harris starts out as a reassuring friend and mentor to Dr. Wheeler, older moviegoers won’t forget that he launched to stardom by tossing a woman in a wheelchair down the stairs in 1947’s “Kiss of Death.”
Christian Szell (Laurence Olivier, “Marathon Man,” 1976)
He may look harmless, but Christian Szell (Laurence Olivier) is a sadist with a secret, a stash, and throat-slitting skills. Szell, a dentist known as the White Angel of Auschwitz for his war crimes, stops at nothing to protect the diamonds he stole from his victims in the camps. In one of Hollywood’s most infamous torture scenes, Szell tries to extract information from Babe Levy (Dustin Hoffman), an innocent grad student, plying the tools of his trade. When Szell asks, “Is it safe?” he’s not curious about whether Babe’s insurance covers anesthesia.
Orin Scrivello (Steve Martin, “Little Shop of Horrors,” 1986)
Sticking with deranged dentists, Orin Scrivello, DDS, (Steve Martin) sings and dances his way into your nightmares buoyed by copious helpings of nitrous oxide. Orin’s too-encouraging momma told him to parlay his sadistic tendencies into a career “where people will pay you to be inhumane.” Sonny listened. Moviegoers were treated to screeching sound effects of a tooth getting yanked during an Elvis-like musical number shot in part from inside a patient’s mouth. Martin makes a creepy scene more fun than a long, slow root canal.
Henry Frankenstein (Colin Clive, “Frankenstein,” 1931)
His alarming need for fresh corpses forced Henry Frankenstein (Colin Clive) to leave medical school and experiment solo in a castle. He insists to his betrothed that he hasn’t gone mad when she arrives as he is bringing a dead body back to life during a raging lightning storm. When she and Henry’s mentor, Dr Waldman, witness him succeed, Waldman warns Henry that the former owner of the purloined brain was a notorious criminal. When Henry exclaims: “It’s alive, it’s alive !” little did he know that he created the face (Boris Karloff) that would launch a thousand sequels, a spectacular satire, and untold Halloween masks.
Dr. Gogol (Peter Lorre, “Mad Love,” 1935)
A few years after playing doctor Frankenstein, Colin Clive became the patient of a mad medic himself. A concert pianist whose hands have been mangled in a train wreck, Clive’s wife turns to Dr. Gogol (Peter Lorre, in his Hollywood debut), who promises to surgically reattach the musician’s hands. Unfortunately, Gogol is so obsessed with the wife, a star of gory stage shows, that he has created a wax figure of her. He schemes to win her in the flesh by attaching a murderer’s hands to Clive, then frame him for committing murder with those hands. Gogol utters the madman’s lament: “I have conquered science. Why can’t I conquer love?” A modern remake would surely have him asking, “Why do they swipe left?
Hannibal Lecter (Anthony Hopkins, “Silence of the Lambs,” 1991)
The FBI, hunting for a serial killer, sends trainee Clarice Starling (Jodie Foster) to seek insight into the murderer from the imprisoned Dr. Hannibal Lecter (Anthony Hopkins), a brilliant psychiatrist with a penchant for murder — and a taste for the flesh of his victims. Lecter proves to be a menace from their first meeting; the bars and glass surrounding his cell offer Clarice no protection from his gaze and ability to read her mind. In his own way, the urbane, pathologically charming Lecter takes a shine to Clarice, helping with the case while embarking on another murderous spree against men who recently wronged her. When he escapes, his plans do not include dinner with – or of – Clarice, but others, well, they’re not so lucky.
Henry Jekyll (Fredric March, “Dr. Jekyll and Mr. Hyde,” 1931)
Henry Jekyll (Fredric March) is a jumble of personalities. By day, he’s a kindly doctor in Victorian London with an American accent. But he is so determined to split good and evil personalities that he devises a potion to outsource his id. As he watches himself morph into Mr. Hyde – a hairy, cone-headed dude in serious need of an orthodontist – he exclaims, “Free! Free at last!” Free, that is, for his simian side to engage in debauchery, abuse, self-hatred, intimations of rape, and ultimately murder – all of which are explored in this pre-Code film, the first talkie version of Robert Louis Stevenson’s story.
Dr. Moreau (Charles Laughton, “Island of Lost Souls,” 1932)
“Strange-looking natives you have here,” shipwreck victim Edward Parker (Richard Arlen) tells his host, the white-suited, whip-wielding Dr Moreau. Before long, we learn that Moreau’s evil veterinary talents have created an island population of human/beast hybrids who are forced to follow his laws – especially one forbidding them from eating meat or walking on all fours. Lawbreakers get taken to the House of Pain, a medical setting which, as its name suggests, lacks adequate analgesia. Burt Lancaster and Marlon Brando took on the Moreau role in later versions, but Laughton is the creepiest when he asks, “Do you know what it means to feel like God?” The film was banned for years in Britain, and H.G. Wells despised this take on his antivivisection tale.
Charles Nichols (Jeroen Krabbé, “The Fugitive,” 1993)
Richard Kimble, a Chicago vascular surgeon, arrives home to find that a man just brutally murdered his loving wife. The killer escapes, and Kimble falls into the frame-up. Convicted for the murder and headed to prison, Kimble breaks free in an epic escape scene. He spends the rest of the movie all but giving his right arm to find the murderer, while being pursued by a dogged U.S. Marshal played with gusto by Tommy Lee Jones. Kimble eventually discovers that his colleague, Dr. Charles Nichols (Jeroen Krabbé), is not quite the best friend a man could have – or the most ethical of clinical investigators.
Elliot and Beverly Mantle (Jeremy Irons, “Dead Ringers,” 1988)
“You’ve got to try the movie star,” fertility specialist Elliot Mantle (Jeremy Irons) implores to his identical but meek twin brother, Beverly (also Jeremy Irons), talking about an actress-patient (Genevieve Bujold) as if she were a menu item. Beverly shares a practice with Elliot, along with a soul and an easily satisfied drug addiction. Beverly is unaware that Elliot seduces patients before passing them off to his brother, including the actress. Beverly is in love with the actress, which upsets the equilibrium of their shared soul. He aims to fix this, but not without some trauma involving freakish and unsanitary operating implements.
Dean Armitage (Bradley Whitford, “Get Out,” 2017)
Neurosurgeon Dean Armitage (Bradley Whitford) was such a fan of President Obama that he would have voted for him a third time if he could. At least, that’s how he portrays himself to Chris (Daniel Kaluuya), an African American photographer and the new boyfriend of Armitage’s White daughter. The Armitage estate has plenty of people of color – on staff, anyway – but Chris finds them odd and distant. It turns out that a gathering of rich White people is in fact an auction for his eyesight. Horror ensues. The main message from this film is not unlike that of Russian operatives who fall out of favor with the Kremlin: Don’t drink the tea.
A version of this article first appeared on Medscape.com.
Fertility physicians say they lack access to miscarriage drugs
In a survey taken before the Supreme Court’s Dobbs ruling regarding abortion rights, two-thirds of assisted reproduction technology (ART) physicians who don’t offer mifepristone/misoprostol to patients with early pregnancy loss (EPL) reported that they lack access to the drugs.
The numbers are likely higher now. In the wake of the court ruling, some physicians in states with new abortion restrictions fear they won’t be able to properly treat women with miscarriages. Access to mifepristone, a component of medication abortions along with misoprostol, is at the center of their concerns.
“These restrictions that were put in place to restrict abortion care have far-reaching implications regarding miscarriages and early pregnancy loss and the assisted reproduction community is not immune,” obstetrics and gynecology specialist Zachary Anderson, MD, a resident physician at the University of Southern California, Los Angeles, said in an interview. He presented the findings at the American Society for Reproductive Medicine’s 2022 meeting.
Early pregnancy loss – defined as a miscarriage within 12 weeks and 6 days of conception – is common in all pregnancies and affects an estimated 15% of those who rely on in vitro fertilization (IVF). In women who conceive through intrauterine insemination or IVF, “an abnormal karyotype embryo/fetus is the cause of miscarriage in more than two-thirds of cases,” Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, Orlando, said in an interview. “The options of management are observation – with no ability to determine when passage of the products of conception will occur – vs. mifepristone/misoprostol or suction D&C.”
Dr. Trolice added that “most woman select the medical treatment protocol, which is 200 mg mifepristone orally followed by 800 mcg misoprostol vaginally 24 hours later. If no signs of heavy bleeding occur after 3 hours following misoprostol, the patient should repeat the dose of 800 micrograms vaginally.”
According to the Reuters news service, some abortion bans target mifepristone. In October 2022, the American College of Obstetricians and Gynecologists asked the Food and Drug Administration to approve mifepristone for use in miscarriage management; such use is now off label, although it is approved to end early pregnancies in conjunction with misoprostol.
For the new study, researchers sent anonymous surveys to 826 members of the Society of Reproductive Endocrinology and Infertility and received 101 responses (12% response rate, 51% women, 86% non-Hispanic White, average age 52, 52% urban, and 51% in private practice).
More than two-thirds (70%) said they diagnosed early pregnancy loss at least once a week; 47% prefer treatment with misoprostol alone, 18% surgery in an operating room, 15% expectant management (monitoring a miscarriage as it occurs without medical intervention), 10% surgery in the office, and 3% mifepristone-misoprostol.
Of those who don’t offer mifepristone-misoprostol, 68% said they lack access, and 26% said they lack familiarity with the treatment.
Study coauthor Brian T. Nguyen, MD, MSc, assistant professor of obstetrics and gynecology at USC, said in an interview that mifepristone, a highly effective drug, is treated differently from other medications “for no good reason.”
Dr. Anderson, who led the study, urged colleagues to get the appropriate certification to prescribe mifepristone. “Providers overestimate how difficult it is to become certified to prescribe it,” he said.
Dr. Trolice, who is familiar with the study findings, said the response rate is low, and the results might be biased because those with preconceived opinions may be more likely to respond.
However, he said, “The results are not surprising in that medication is more commonly preferred (nearly 50%) given the devastation of a miscarriage and the desire to expedite resolution. Approximately one-third prefer surgical management, which would allow for genetic testing of the embryo/fetus to potentially determine a cause of the pregnancy loss.”
As for the medications used to treat early pregnancy loss, many ART physicians “treat pregnancy loss with misoprostol both pre- and post Dobbs,” he said. “The difficulty in obtaining mifepristone remains.”
The study authors and Dr. Trolice report no disclosures.
In a survey taken before the Supreme Court’s Dobbs ruling regarding abortion rights, two-thirds of assisted reproduction technology (ART) physicians who don’t offer mifepristone/misoprostol to patients with early pregnancy loss (EPL) reported that they lack access to the drugs.
The numbers are likely higher now. In the wake of the court ruling, some physicians in states with new abortion restrictions fear they won’t be able to properly treat women with miscarriages. Access to mifepristone, a component of medication abortions along with misoprostol, is at the center of their concerns.
“These restrictions that were put in place to restrict abortion care have far-reaching implications regarding miscarriages and early pregnancy loss and the assisted reproduction community is not immune,” obstetrics and gynecology specialist Zachary Anderson, MD, a resident physician at the University of Southern California, Los Angeles, said in an interview. He presented the findings at the American Society for Reproductive Medicine’s 2022 meeting.
Early pregnancy loss – defined as a miscarriage within 12 weeks and 6 days of conception – is common in all pregnancies and affects an estimated 15% of those who rely on in vitro fertilization (IVF). In women who conceive through intrauterine insemination or IVF, “an abnormal karyotype embryo/fetus is the cause of miscarriage in more than two-thirds of cases,” Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, Orlando, said in an interview. “The options of management are observation – with no ability to determine when passage of the products of conception will occur – vs. mifepristone/misoprostol or suction D&C.”
Dr. Trolice added that “most woman select the medical treatment protocol, which is 200 mg mifepristone orally followed by 800 mcg misoprostol vaginally 24 hours later. If no signs of heavy bleeding occur after 3 hours following misoprostol, the patient should repeat the dose of 800 micrograms vaginally.”
According to the Reuters news service, some abortion bans target mifepristone. In October 2022, the American College of Obstetricians and Gynecologists asked the Food and Drug Administration to approve mifepristone for use in miscarriage management; such use is now off label, although it is approved to end early pregnancies in conjunction with misoprostol.
For the new study, researchers sent anonymous surveys to 826 members of the Society of Reproductive Endocrinology and Infertility and received 101 responses (12% response rate, 51% women, 86% non-Hispanic White, average age 52, 52% urban, and 51% in private practice).
More than two-thirds (70%) said they diagnosed early pregnancy loss at least once a week; 47% prefer treatment with misoprostol alone, 18% surgery in an operating room, 15% expectant management (monitoring a miscarriage as it occurs without medical intervention), 10% surgery in the office, and 3% mifepristone-misoprostol.
Of those who don’t offer mifepristone-misoprostol, 68% said they lack access, and 26% said they lack familiarity with the treatment.
Study coauthor Brian T. Nguyen, MD, MSc, assistant professor of obstetrics and gynecology at USC, said in an interview that mifepristone, a highly effective drug, is treated differently from other medications “for no good reason.”
Dr. Anderson, who led the study, urged colleagues to get the appropriate certification to prescribe mifepristone. “Providers overestimate how difficult it is to become certified to prescribe it,” he said.
Dr. Trolice, who is familiar with the study findings, said the response rate is low, and the results might be biased because those with preconceived opinions may be more likely to respond.
However, he said, “The results are not surprising in that medication is more commonly preferred (nearly 50%) given the devastation of a miscarriage and the desire to expedite resolution. Approximately one-third prefer surgical management, which would allow for genetic testing of the embryo/fetus to potentially determine a cause of the pregnancy loss.”
As for the medications used to treat early pregnancy loss, many ART physicians “treat pregnancy loss with misoprostol both pre- and post Dobbs,” he said. “The difficulty in obtaining mifepristone remains.”
The study authors and Dr. Trolice report no disclosures.
In a survey taken before the Supreme Court’s Dobbs ruling regarding abortion rights, two-thirds of assisted reproduction technology (ART) physicians who don’t offer mifepristone/misoprostol to patients with early pregnancy loss (EPL) reported that they lack access to the drugs.
The numbers are likely higher now. In the wake of the court ruling, some physicians in states with new abortion restrictions fear they won’t be able to properly treat women with miscarriages. Access to mifepristone, a component of medication abortions along with misoprostol, is at the center of their concerns.
“These restrictions that were put in place to restrict abortion care have far-reaching implications regarding miscarriages and early pregnancy loss and the assisted reproduction community is not immune,” obstetrics and gynecology specialist Zachary Anderson, MD, a resident physician at the University of Southern California, Los Angeles, said in an interview. He presented the findings at the American Society for Reproductive Medicine’s 2022 meeting.
Early pregnancy loss – defined as a miscarriage within 12 weeks and 6 days of conception – is common in all pregnancies and affects an estimated 15% of those who rely on in vitro fertilization (IVF). In women who conceive through intrauterine insemination or IVF, “an abnormal karyotype embryo/fetus is the cause of miscarriage in more than two-thirds of cases,” Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, Orlando, said in an interview. “The options of management are observation – with no ability to determine when passage of the products of conception will occur – vs. mifepristone/misoprostol or suction D&C.”
Dr. Trolice added that “most woman select the medical treatment protocol, which is 200 mg mifepristone orally followed by 800 mcg misoprostol vaginally 24 hours later. If no signs of heavy bleeding occur after 3 hours following misoprostol, the patient should repeat the dose of 800 micrograms vaginally.”
According to the Reuters news service, some abortion bans target mifepristone. In October 2022, the American College of Obstetricians and Gynecologists asked the Food and Drug Administration to approve mifepristone for use in miscarriage management; such use is now off label, although it is approved to end early pregnancies in conjunction with misoprostol.
For the new study, researchers sent anonymous surveys to 826 members of the Society of Reproductive Endocrinology and Infertility and received 101 responses (12% response rate, 51% women, 86% non-Hispanic White, average age 52, 52% urban, and 51% in private practice).
More than two-thirds (70%) said they diagnosed early pregnancy loss at least once a week; 47% prefer treatment with misoprostol alone, 18% surgery in an operating room, 15% expectant management (monitoring a miscarriage as it occurs without medical intervention), 10% surgery in the office, and 3% mifepristone-misoprostol.
Of those who don’t offer mifepristone-misoprostol, 68% said they lack access, and 26% said they lack familiarity with the treatment.
Study coauthor Brian T. Nguyen, MD, MSc, assistant professor of obstetrics and gynecology at USC, said in an interview that mifepristone, a highly effective drug, is treated differently from other medications “for no good reason.”
Dr. Anderson, who led the study, urged colleagues to get the appropriate certification to prescribe mifepristone. “Providers overestimate how difficult it is to become certified to prescribe it,” he said.
Dr. Trolice, who is familiar with the study findings, said the response rate is low, and the results might be biased because those with preconceived opinions may be more likely to respond.
However, he said, “The results are not surprising in that medication is more commonly preferred (nearly 50%) given the devastation of a miscarriage and the desire to expedite resolution. Approximately one-third prefer surgical management, which would allow for genetic testing of the embryo/fetus to potentially determine a cause of the pregnancy loss.”
As for the medications used to treat early pregnancy loss, many ART physicians “treat pregnancy loss with misoprostol both pre- and post Dobbs,” he said. “The difficulty in obtaining mifepristone remains.”
The study authors and Dr. Trolice report no disclosures.
FROM ASRM 2022
Is it flu, RSV, or COVID? Experts fear the ‘tripledemic’
Just when we thought this holiday season, finally, would be the back-to-normal one, some infectious disease experts are warning that a so-called “tripledemic” – influenza, COVID-19, and RSV – may be in the forecast.
The warning isn’t without basis.
The flu season has gotten an early start. As of Oct. 21, early increases in seasonal flu activity have been reported in most of the country, the Centers for Disease Control and Prevention said, with the southeast and south-central areas having the highest activity levels.
Children’s hospitals and EDs are seeing a surge in children with RSV.
COVID-19 cases are trending down, according to the CDC, but epidemiologists – scientists who study disease outbreaks – always have their eyes on emerging variants.
said Justin Lessler, PhD, a professor of epidemiology at the University of North Carolina at Chapel Hill. Dr. Lessler is on the coordinating team for the COVID-19 Scenario Modeling Hub, which aims to predict the course COVID-19, and the Flu Scenario Modeling Hub, which does the same for influenza.
For COVID-19, some models are predicting some spikes before Christmas, he said, and others see a new wave in 2023. For the flu, the model is predicting an earlier-than-usual start, as the CDC has reported.
While flu activity is relatively low, the CDC said, the season is off to an early start. For the week ending Oct. 21, 1,674 patients were hospitalized for flu, higher than in the summer months but fewer than the 2,675 hospitalizations for the week of May 15, 2022.
As of Oct. 20, COVID-19 cases have declined 12% over the last 2 weeks, nationwide. But hospitalizations are up 10% in much of the Northeast, The New York Times reports, and the improvement in cases and deaths has been slowing down.
As of Oct. 15, 15% of RSV tests reported nationwide were positive, compared with about 11% at that time in 2021, the CDC said. The surveillance collects information from 75 counties in 12 states.
Experts point out that the viruses – all three are respiratory viruses – are simply playing catchup.
“They spread the same way and along with lots of other viruses, and you tend to see an increase in them during the cold months,” said Timothy Brewer, MD, professor of medicine and epidemiology at UCLA.
The increase in all three viruses “is almost predictable at this point in the pandemic,” said Dean Blumberg, MD, a professor and chief of pediatric infectious diseases at the University of California Davis Health. “All the respiratory viruses are out of whack.”
Last year, RSV cases were up, too, and began to appear very early, he said, in the summer instead of in the cooler months. Flu also appeared early in 2021, as it has in 2022.
That contrasts with the flu season of 2020-2021, when COVID precautions were nearly universal, and cases were down. At UC Davis, “we didn’t have one pediatric admission due to influenza in the 2020-2021 [flu] season,” Dr. Blumberg said.
The number of pediatric flu deaths usually range from 37 to 199 per year, according to CDC records. But in the 2020-2021 season, the CDC recorded one pediatric flu death in the U.S.
Both children and adults have had less contact with others the past two seasons, Dr. Blumberg said, “and they don’t get the immunity they got with those infections [previously]. That’s why we are seeing out-of-season, early season [viruses].”
Eventually, he said, the cases of flu and RSV will return to previous levels. “It could be as soon as next year,” Dr. Blumberg said. And COVID-19, hopefully, will become like influenza, he said.
“RSV has always come around in the fall and winter,” said Elizabeth Murray, DO, a pediatric emergency medicine doctor at the University of Rochester (N.Y.) Medical Center and a spokesperson for the American Academy of Pediatrics. In 2022, children are back in school and for the most part not masking. “It’s a perfect storm for all the germs to spread now. They’ve just been waiting for their opportunity to come back.”
Self-care vs. not
RSV can pose a risk for anyone, but most at risk are children under age 5, especially infants under age 1, and adults over age 65. There is no vaccine for it. Symptoms include a runny nose, decreased appetite, coughing, sneezing, fever, and wheezing. But in young infants, there may only be decreased activity, crankiness, and breathing issues, the CDC said.
Keep an eye on the breathing if RSV is suspected, Dr. Murray tells parents. If your child can’t breathe easily, is unable to lie down comfortably, can’t speak clearly, or is sucking in the chest muscles to breathe, get medical help. Most kids with RSV can stay home and recover, she said, but often will need to be checked by a medical professional.
She advises against getting an oximeter to measure oxygen levels for home use. “They are often not accurate,” she said. If in doubt about how serious your child’s symptoms are, “don’t wait it out,” and don’t hesitate to call 911.
Symptoms of flu, COVID, and RSV can overlap. But each can involve breathing problems, which can be an emergency.
“It’s important to seek medical attention for any concerning symptoms, but especially severe shortness of breath or difficulty breathing, as these could signal the need for supplemental oxygen or other emergency interventions,” said Mandy De Vries, a respiratory therapist and director of education at the American Association for Respiratory Care. Inhalation treatment or mechanical ventilation may be needed for severe respiratory issues.
Precautions
To avoid the tripledemic – or any single infection – Timothy Brewer, MD, a professor of medicine and epidemiology at the University of California, Los Angeles, suggests some familiar measures: “Stay home if you’re feeling sick. Make sure you are up to date on your vaccinations. Wear a mask indoors.”
A version of this article first appeared on Medscape.com.
Just when we thought this holiday season, finally, would be the back-to-normal one, some infectious disease experts are warning that a so-called “tripledemic” – influenza, COVID-19, and RSV – may be in the forecast.
The warning isn’t without basis.
The flu season has gotten an early start. As of Oct. 21, early increases in seasonal flu activity have been reported in most of the country, the Centers for Disease Control and Prevention said, with the southeast and south-central areas having the highest activity levels.
Children’s hospitals and EDs are seeing a surge in children with RSV.
COVID-19 cases are trending down, according to the CDC, but epidemiologists – scientists who study disease outbreaks – always have their eyes on emerging variants.
said Justin Lessler, PhD, a professor of epidemiology at the University of North Carolina at Chapel Hill. Dr. Lessler is on the coordinating team for the COVID-19 Scenario Modeling Hub, which aims to predict the course COVID-19, and the Flu Scenario Modeling Hub, which does the same for influenza.
For COVID-19, some models are predicting some spikes before Christmas, he said, and others see a new wave in 2023. For the flu, the model is predicting an earlier-than-usual start, as the CDC has reported.
While flu activity is relatively low, the CDC said, the season is off to an early start. For the week ending Oct. 21, 1,674 patients were hospitalized for flu, higher than in the summer months but fewer than the 2,675 hospitalizations for the week of May 15, 2022.
As of Oct. 20, COVID-19 cases have declined 12% over the last 2 weeks, nationwide. But hospitalizations are up 10% in much of the Northeast, The New York Times reports, and the improvement in cases and deaths has been slowing down.
As of Oct. 15, 15% of RSV tests reported nationwide were positive, compared with about 11% at that time in 2021, the CDC said. The surveillance collects information from 75 counties in 12 states.
Experts point out that the viruses – all three are respiratory viruses – are simply playing catchup.
“They spread the same way and along with lots of other viruses, and you tend to see an increase in them during the cold months,” said Timothy Brewer, MD, professor of medicine and epidemiology at UCLA.
The increase in all three viruses “is almost predictable at this point in the pandemic,” said Dean Blumberg, MD, a professor and chief of pediatric infectious diseases at the University of California Davis Health. “All the respiratory viruses are out of whack.”
Last year, RSV cases were up, too, and began to appear very early, he said, in the summer instead of in the cooler months. Flu also appeared early in 2021, as it has in 2022.
That contrasts with the flu season of 2020-2021, when COVID precautions were nearly universal, and cases were down. At UC Davis, “we didn’t have one pediatric admission due to influenza in the 2020-2021 [flu] season,” Dr. Blumberg said.
The number of pediatric flu deaths usually range from 37 to 199 per year, according to CDC records. But in the 2020-2021 season, the CDC recorded one pediatric flu death in the U.S.
Both children and adults have had less contact with others the past two seasons, Dr. Blumberg said, “and they don’t get the immunity they got with those infections [previously]. That’s why we are seeing out-of-season, early season [viruses].”
Eventually, he said, the cases of flu and RSV will return to previous levels. “It could be as soon as next year,” Dr. Blumberg said. And COVID-19, hopefully, will become like influenza, he said.
“RSV has always come around in the fall and winter,” said Elizabeth Murray, DO, a pediatric emergency medicine doctor at the University of Rochester (N.Y.) Medical Center and a spokesperson for the American Academy of Pediatrics. In 2022, children are back in school and for the most part not masking. “It’s a perfect storm for all the germs to spread now. They’ve just been waiting for their opportunity to come back.”
Self-care vs. not
RSV can pose a risk for anyone, but most at risk are children under age 5, especially infants under age 1, and adults over age 65. There is no vaccine for it. Symptoms include a runny nose, decreased appetite, coughing, sneezing, fever, and wheezing. But in young infants, there may only be decreased activity, crankiness, and breathing issues, the CDC said.
Keep an eye on the breathing if RSV is suspected, Dr. Murray tells parents. If your child can’t breathe easily, is unable to lie down comfortably, can’t speak clearly, or is sucking in the chest muscles to breathe, get medical help. Most kids with RSV can stay home and recover, she said, but often will need to be checked by a medical professional.
She advises against getting an oximeter to measure oxygen levels for home use. “They are often not accurate,” she said. If in doubt about how serious your child’s symptoms are, “don’t wait it out,” and don’t hesitate to call 911.
Symptoms of flu, COVID, and RSV can overlap. But each can involve breathing problems, which can be an emergency.
“It’s important to seek medical attention for any concerning symptoms, but especially severe shortness of breath or difficulty breathing, as these could signal the need for supplemental oxygen or other emergency interventions,” said Mandy De Vries, a respiratory therapist and director of education at the American Association for Respiratory Care. Inhalation treatment or mechanical ventilation may be needed for severe respiratory issues.
Precautions
To avoid the tripledemic – or any single infection – Timothy Brewer, MD, a professor of medicine and epidemiology at the University of California, Los Angeles, suggests some familiar measures: “Stay home if you’re feeling sick. Make sure you are up to date on your vaccinations. Wear a mask indoors.”
A version of this article first appeared on Medscape.com.
Just when we thought this holiday season, finally, would be the back-to-normal one, some infectious disease experts are warning that a so-called “tripledemic” – influenza, COVID-19, and RSV – may be in the forecast.
The warning isn’t without basis.
The flu season has gotten an early start. As of Oct. 21, early increases in seasonal flu activity have been reported in most of the country, the Centers for Disease Control and Prevention said, with the southeast and south-central areas having the highest activity levels.
Children’s hospitals and EDs are seeing a surge in children with RSV.
COVID-19 cases are trending down, according to the CDC, but epidemiologists – scientists who study disease outbreaks – always have their eyes on emerging variants.
said Justin Lessler, PhD, a professor of epidemiology at the University of North Carolina at Chapel Hill. Dr. Lessler is on the coordinating team for the COVID-19 Scenario Modeling Hub, which aims to predict the course COVID-19, and the Flu Scenario Modeling Hub, which does the same for influenza.
For COVID-19, some models are predicting some spikes before Christmas, he said, and others see a new wave in 2023. For the flu, the model is predicting an earlier-than-usual start, as the CDC has reported.
While flu activity is relatively low, the CDC said, the season is off to an early start. For the week ending Oct. 21, 1,674 patients were hospitalized for flu, higher than in the summer months but fewer than the 2,675 hospitalizations for the week of May 15, 2022.
As of Oct. 20, COVID-19 cases have declined 12% over the last 2 weeks, nationwide. But hospitalizations are up 10% in much of the Northeast, The New York Times reports, and the improvement in cases and deaths has been slowing down.
As of Oct. 15, 15% of RSV tests reported nationwide were positive, compared with about 11% at that time in 2021, the CDC said. The surveillance collects information from 75 counties in 12 states.
Experts point out that the viruses – all three are respiratory viruses – are simply playing catchup.
“They spread the same way and along with lots of other viruses, and you tend to see an increase in them during the cold months,” said Timothy Brewer, MD, professor of medicine and epidemiology at UCLA.
The increase in all three viruses “is almost predictable at this point in the pandemic,” said Dean Blumberg, MD, a professor and chief of pediatric infectious diseases at the University of California Davis Health. “All the respiratory viruses are out of whack.”
Last year, RSV cases were up, too, and began to appear very early, he said, in the summer instead of in the cooler months. Flu also appeared early in 2021, as it has in 2022.
That contrasts with the flu season of 2020-2021, when COVID precautions were nearly universal, and cases were down. At UC Davis, “we didn’t have one pediatric admission due to influenza in the 2020-2021 [flu] season,” Dr. Blumberg said.
The number of pediatric flu deaths usually range from 37 to 199 per year, according to CDC records. But in the 2020-2021 season, the CDC recorded one pediatric flu death in the U.S.
Both children and adults have had less contact with others the past two seasons, Dr. Blumberg said, “and they don’t get the immunity they got with those infections [previously]. That’s why we are seeing out-of-season, early season [viruses].”
Eventually, he said, the cases of flu and RSV will return to previous levels. “It could be as soon as next year,” Dr. Blumberg said. And COVID-19, hopefully, will become like influenza, he said.
“RSV has always come around in the fall and winter,” said Elizabeth Murray, DO, a pediatric emergency medicine doctor at the University of Rochester (N.Y.) Medical Center and a spokesperson for the American Academy of Pediatrics. In 2022, children are back in school and for the most part not masking. “It’s a perfect storm for all the germs to spread now. They’ve just been waiting for their opportunity to come back.”
Self-care vs. not
RSV can pose a risk for anyone, but most at risk are children under age 5, especially infants under age 1, and adults over age 65. There is no vaccine for it. Symptoms include a runny nose, decreased appetite, coughing, sneezing, fever, and wheezing. But in young infants, there may only be decreased activity, crankiness, and breathing issues, the CDC said.
Keep an eye on the breathing if RSV is suspected, Dr. Murray tells parents. If your child can’t breathe easily, is unable to lie down comfortably, can’t speak clearly, or is sucking in the chest muscles to breathe, get medical help. Most kids with RSV can stay home and recover, she said, but often will need to be checked by a medical professional.
She advises against getting an oximeter to measure oxygen levels for home use. “They are often not accurate,” she said. If in doubt about how serious your child’s symptoms are, “don’t wait it out,” and don’t hesitate to call 911.
Symptoms of flu, COVID, and RSV can overlap. But each can involve breathing problems, which can be an emergency.
“It’s important to seek medical attention for any concerning symptoms, but especially severe shortness of breath or difficulty breathing, as these could signal the need for supplemental oxygen or other emergency interventions,” said Mandy De Vries, a respiratory therapist and director of education at the American Association for Respiratory Care. Inhalation treatment or mechanical ventilation may be needed for severe respiratory issues.
Precautions
To avoid the tripledemic – or any single infection – Timothy Brewer, MD, a professor of medicine and epidemiology at the University of California, Los Angeles, suggests some familiar measures: “Stay home if you’re feeling sick. Make sure you are up to date on your vaccinations. Wear a mask indoors.”
A version of this article first appeared on Medscape.com.
HPV-positive women who undergo IVF don’t have worse outcomes
A new study provides more evidence that HPV infection doesn’t raise the risk of poor outcomes in women who undergo fertility treatment via in vitro fertilization with fresh embryos. In fact, HPV-positive women were somewhat more likely than HPV-negative women to become pregnant (relative risk, 1.20; 95% confidence interval, 1.03-1.39) and have live births (RR, 1.39; 95% CI, 1.13-1.70), researchers reported Oct. 24 at the American Society for Reproductive Medicine’s 2022 meeting .
“This evidence should reassure women that being HPV positive will not affect live birth rates after a fresh embryo transfer cycle,” said study coauthor and ob.gyn. Nina Vyas, MD, a clinical fellow at Weill Cornell Medicine, New York, in an interview.
According to Dr. Vyas, previous studies have offered conflicting results about whether HPV affects pregnancy outcomes. In 2006, for example, her group performed a pilot study (Fertil Steril. Jun 16. doi: 10.1016/j.fertnstert.2006.01.051) that linked lower pregnancy rates to HPV-positive tests on the day of egg retrieval.
“We sought to reevaluate this finding in a retrospective manner,” Dr. Vyas said. “You’re taking eggs out of their home, injecting with sperm, and putting them back. There’s so much that we don’t know, and we want to make sure there’s no extra risk.”
Also, she added, “prior studies had a relatively low sample size. We sought to use our patient volume to address this question on a larger scale. Our current study benefits from a large sample size and using the clinically meaningful endpoint of live birth as our primary outcome.”
For the new study, researchers retrospectively analyzed 1,333 patients (of 2,209 screened) who received first fresh embryo transfers from 2017 to 2019. All had cytology or HPV status documented per cervical cancer screening guidelines within 6 months before embryos were transferred.
The researchers looked at only fresh embryo transfers “so we could account for pregnancy outcomes closest to the documented HPV status at the time of egg retrieval,” Dr. Vyas said.
Ten percent (133) of patients were HPV positive. Of those, 60.1% became pregnant, and 43.6% of them had live births. Of the HPV-negative women (90% of subjects, n = 1,200), 52.2% became pregnant and 33.5% had live births. The researchers didn’t calculate P values, but Dr. Vyas said an analysis determined that the differences between HPV-positive and HPV-negative women were statistically significant.
The study size doesn’t allow researchers to determine whether HPV actually has a protective effect on pregnancy/live birth rates in IVF, Dr. Vyas said. Even if it did, the virus is dangerous.
What else could explain the discrepancy? “Some elements driving this could the smaller sample size of the HPV-positive group, differences in HPV prevalence between the general population and our population,” she said, “or other confounding factors we were not able to appreciate due to the limitations of the retrospective study.”
Researchers also reported that they found “no significant difference in biochemical or spontaneous abortion rates” between HPV-positive and HPV-negative women.
What is the message of the study? “Women with HPV can rest assured that they won’t have worse outcomes than their non-HPV [infected] counterparts after a fresh embryo transfer cycle,” Dr. Vyas said.
In an interview, McGill University, Montreal, epidemiologist Helen Trottier, PhD, MSc, noted that she recently coauthored a study that linked persistent HPV infection in pregnancy to premature births. The findings appear convincing, she said: “I think we can say that HPV is associated with preterm birth.”
She praised the new study but noted “the relative risks that are reported need to be adjusted for race and possibly other factors.”
Dr. Vyas said that kind of adjustment will occur in a future study that’s in progress. “We are now prospectively enrolling patients and collecting cytology data to understand whether there might be a difference for women with higher malignancy potential/different types of HPV genotypes.”
The study authors have no disclosures. Disclosure information for Dr. Trottier was unavailable.
A new study provides more evidence that HPV infection doesn’t raise the risk of poor outcomes in women who undergo fertility treatment via in vitro fertilization with fresh embryos. In fact, HPV-positive women were somewhat more likely than HPV-negative women to become pregnant (relative risk, 1.20; 95% confidence interval, 1.03-1.39) and have live births (RR, 1.39; 95% CI, 1.13-1.70), researchers reported Oct. 24 at the American Society for Reproductive Medicine’s 2022 meeting .
“This evidence should reassure women that being HPV positive will not affect live birth rates after a fresh embryo transfer cycle,” said study coauthor and ob.gyn. Nina Vyas, MD, a clinical fellow at Weill Cornell Medicine, New York, in an interview.
According to Dr. Vyas, previous studies have offered conflicting results about whether HPV affects pregnancy outcomes. In 2006, for example, her group performed a pilot study (Fertil Steril. Jun 16. doi: 10.1016/j.fertnstert.2006.01.051) that linked lower pregnancy rates to HPV-positive tests on the day of egg retrieval.
“We sought to reevaluate this finding in a retrospective manner,” Dr. Vyas said. “You’re taking eggs out of their home, injecting with sperm, and putting them back. There’s so much that we don’t know, and we want to make sure there’s no extra risk.”
Also, she added, “prior studies had a relatively low sample size. We sought to use our patient volume to address this question on a larger scale. Our current study benefits from a large sample size and using the clinically meaningful endpoint of live birth as our primary outcome.”
For the new study, researchers retrospectively analyzed 1,333 patients (of 2,209 screened) who received first fresh embryo transfers from 2017 to 2019. All had cytology or HPV status documented per cervical cancer screening guidelines within 6 months before embryos were transferred.
The researchers looked at only fresh embryo transfers “so we could account for pregnancy outcomes closest to the documented HPV status at the time of egg retrieval,” Dr. Vyas said.
Ten percent (133) of patients were HPV positive. Of those, 60.1% became pregnant, and 43.6% of them had live births. Of the HPV-negative women (90% of subjects, n = 1,200), 52.2% became pregnant and 33.5% had live births. The researchers didn’t calculate P values, but Dr. Vyas said an analysis determined that the differences between HPV-positive and HPV-negative women were statistically significant.
The study size doesn’t allow researchers to determine whether HPV actually has a protective effect on pregnancy/live birth rates in IVF, Dr. Vyas said. Even if it did, the virus is dangerous.
What else could explain the discrepancy? “Some elements driving this could the smaller sample size of the HPV-positive group, differences in HPV prevalence between the general population and our population,” she said, “or other confounding factors we were not able to appreciate due to the limitations of the retrospective study.”
Researchers also reported that they found “no significant difference in biochemical or spontaneous abortion rates” between HPV-positive and HPV-negative women.
What is the message of the study? “Women with HPV can rest assured that they won’t have worse outcomes than their non-HPV [infected] counterparts after a fresh embryo transfer cycle,” Dr. Vyas said.
In an interview, McGill University, Montreal, epidemiologist Helen Trottier, PhD, MSc, noted that she recently coauthored a study that linked persistent HPV infection in pregnancy to premature births. The findings appear convincing, she said: “I think we can say that HPV is associated with preterm birth.”
She praised the new study but noted “the relative risks that are reported need to be adjusted for race and possibly other factors.”
Dr. Vyas said that kind of adjustment will occur in a future study that’s in progress. “We are now prospectively enrolling patients and collecting cytology data to understand whether there might be a difference for women with higher malignancy potential/different types of HPV genotypes.”
The study authors have no disclosures. Disclosure information for Dr. Trottier was unavailable.
A new study provides more evidence that HPV infection doesn’t raise the risk of poor outcomes in women who undergo fertility treatment via in vitro fertilization with fresh embryos. In fact, HPV-positive women were somewhat more likely than HPV-negative women to become pregnant (relative risk, 1.20; 95% confidence interval, 1.03-1.39) and have live births (RR, 1.39; 95% CI, 1.13-1.70), researchers reported Oct. 24 at the American Society for Reproductive Medicine’s 2022 meeting .
“This evidence should reassure women that being HPV positive will not affect live birth rates after a fresh embryo transfer cycle,” said study coauthor and ob.gyn. Nina Vyas, MD, a clinical fellow at Weill Cornell Medicine, New York, in an interview.
According to Dr. Vyas, previous studies have offered conflicting results about whether HPV affects pregnancy outcomes. In 2006, for example, her group performed a pilot study (Fertil Steril. Jun 16. doi: 10.1016/j.fertnstert.2006.01.051) that linked lower pregnancy rates to HPV-positive tests on the day of egg retrieval.
“We sought to reevaluate this finding in a retrospective manner,” Dr. Vyas said. “You’re taking eggs out of their home, injecting with sperm, and putting them back. There’s so much that we don’t know, and we want to make sure there’s no extra risk.”
Also, she added, “prior studies had a relatively low sample size. We sought to use our patient volume to address this question on a larger scale. Our current study benefits from a large sample size and using the clinically meaningful endpoint of live birth as our primary outcome.”
For the new study, researchers retrospectively analyzed 1,333 patients (of 2,209 screened) who received first fresh embryo transfers from 2017 to 2019. All had cytology or HPV status documented per cervical cancer screening guidelines within 6 months before embryos were transferred.
The researchers looked at only fresh embryo transfers “so we could account for pregnancy outcomes closest to the documented HPV status at the time of egg retrieval,” Dr. Vyas said.
Ten percent (133) of patients were HPV positive. Of those, 60.1% became pregnant, and 43.6% of them had live births. Of the HPV-negative women (90% of subjects, n = 1,200), 52.2% became pregnant and 33.5% had live births. The researchers didn’t calculate P values, but Dr. Vyas said an analysis determined that the differences between HPV-positive and HPV-negative women were statistically significant.
The study size doesn’t allow researchers to determine whether HPV actually has a protective effect on pregnancy/live birth rates in IVF, Dr. Vyas said. Even if it did, the virus is dangerous.
What else could explain the discrepancy? “Some elements driving this could the smaller sample size of the HPV-positive group, differences in HPV prevalence between the general population and our population,” she said, “or other confounding factors we were not able to appreciate due to the limitations of the retrospective study.”
Researchers also reported that they found “no significant difference in biochemical or spontaneous abortion rates” between HPV-positive and HPV-negative women.
What is the message of the study? “Women with HPV can rest assured that they won’t have worse outcomes than their non-HPV [infected] counterparts after a fresh embryo transfer cycle,” Dr. Vyas said.
In an interview, McGill University, Montreal, epidemiologist Helen Trottier, PhD, MSc, noted that she recently coauthored a study that linked persistent HPV infection in pregnancy to premature births. The findings appear convincing, she said: “I think we can say that HPV is associated with preterm birth.”
She praised the new study but noted “the relative risks that are reported need to be adjusted for race and possibly other factors.”
Dr. Vyas said that kind of adjustment will occur in a future study that’s in progress. “We are now prospectively enrolling patients and collecting cytology data to understand whether there might be a difference for women with higher malignancy potential/different types of HPV genotypes.”
The study authors have no disclosures. Disclosure information for Dr. Trottier was unavailable.
FROM ASRM 2022
Decoding mechanisms of diabetic embryopathy suggests therapeutic targets
Before the introduction of insulin, there were few reported cases of pregnancy complicated by diabetes because women with the disease too often did not live to childbearing age, and when they did, they were often counseled to terminate their pregnancies. Perinatal and maternal mortality in the limited number of reported pregnancies were 70% and 40%, respectively,1 making the risks of continuing the pregnancy quite high.
After insulin became available, maternal mortality dropped dramatically, down to a few percent. Perinatal mortality also declined, but it took several decades to achieve a similar magnitude of reduction.2 Today, with insulin therapy and tight glucose control as well as improved perinatal care, almost all women with diabetes can contemplate pregnancy with greater hope for normal outcomes.
Problems persist, however. Maternal diabetes continues to cause a variety of adverse outcomes, including infants large for gestational age, prematurity, and structural birth defects. Birth defects and prematurity, in fact, are the top causes of the unacceptably high infant mortality rate in the United States – a rate that is about 70% higher than the average in comparable developed countries.3
Infant mortality is considered an indicator of population health and of the development of a country; to reduce its rate, we must address these two areas.
Women with type 1 and type 2 diabetes are five times more likely to have a child with birth defects than are nondiabetic women.4 Up to 10% of women with preexisting diabetes will have fetuses with a major congenital malformation.5
Over the years we have been striving in our Center for Birth Defects Research to understand the pathomechanisms and the molecular and epigenetic alterations behind the high rates of birth defects in the offspring of women with preexisting diabetes. We have focused on heart defects and neural tube defects (particularly the latter), which together cause significant mortality, morbidity, disability, and human suffering.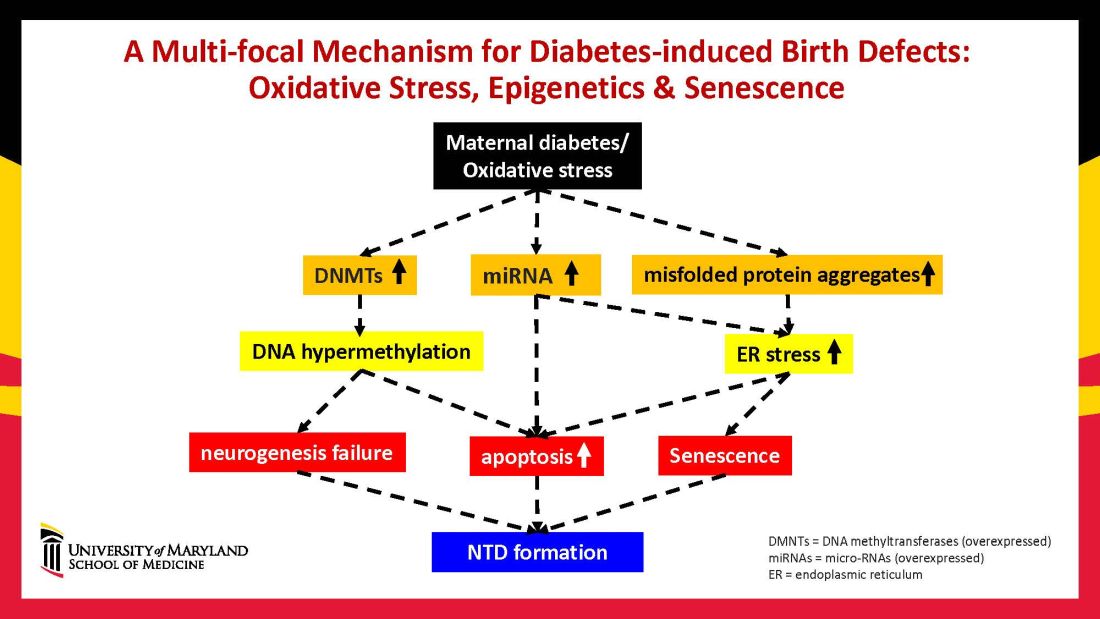
Using animal models that mimic human diabetic pregnancy, we have made significant strides in our understanding of the mechanisms, uncovering molecular pathways involving oxidative stress, senescence/premature cellular aging, and epigenetic modifications (Figure 1). Understanding these pathways is providing us, in turn, with potential therapeutic targets and approaches that may be used in the future to prevent birth defects in women who enter pregnancy with type 1 or type 2 diabetes.
Unraveling the role of oxidative stress
Our mouse models accurately reflect the human conditions of diabetes in pregnancy and diabetic embryopathy. Offspring of mice with type 1 and type 2 diabetes have a similarly higher rate of neural tube defects and congenital heart disease, compared to mice without diabetes. We observe a similar incidence of anencephaly and spina bifida, and of cardiac septation defects in the mouse embryo hearts, for instance.
A primary mechanism and causal event of diabetic embryopathy is hyperglycemia-induced apoptosis in embryonic cells. Excessive cell death in the neural epithelium or in the developing heart leads to abnormal organogenesis and dysfunctional developmental events that cause birth defects. We have identified pathways leading to apoptosis, and have found that many of these pathways crosstalk with each other.
Hyperglycemia induces oxidative stress – one of these pathways – by causing sustained generation of reactive oxygen species. The cells’ mitochondrial function is significantly impaired by the hyperglycemia response, and this diabetes-induced mitochondrial dysfunction further increases the production of reactive oxygen species and a weakening of the endogenous cellular antioxidant systems, both of which then exacerbate oxidative stress.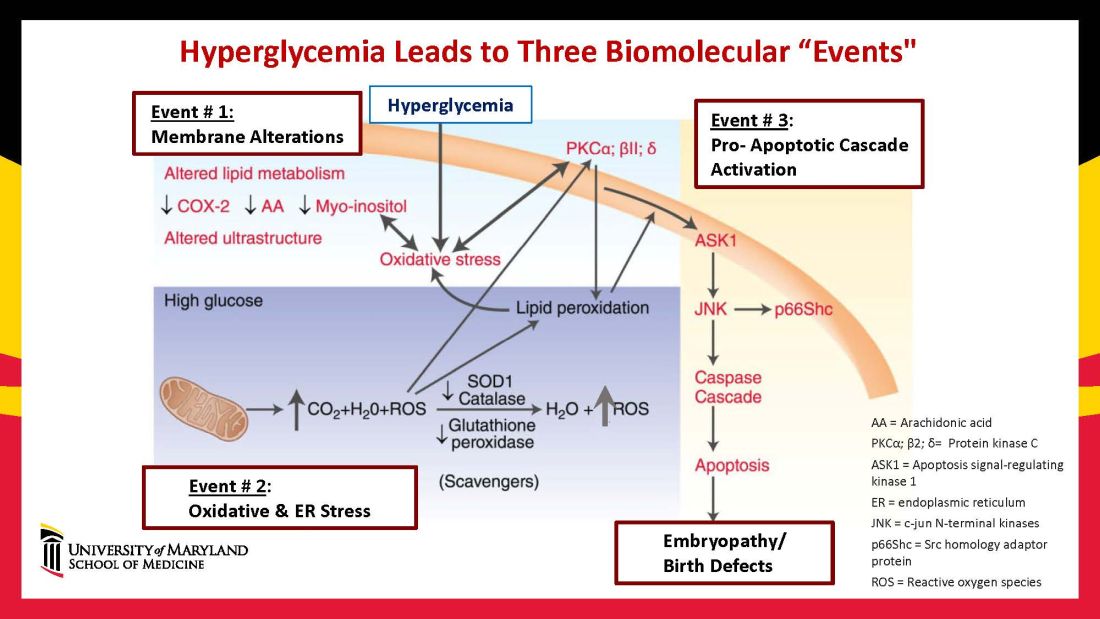
Our research has detailed what happens downstream. We’ve learned that oxidative stress in embryos exposed to maternal diabetes activates a cascade of proapoptotic kinase signaling molecules – for example, protein kinase C isoforms such as PKCalpha; apoptosis signal-regulating kinase 1; and c-Jun-N-terminal kinases – that ultimately lead to abnormal cell death in the neuroepithelium before neural tube closure (Figure 2).5
Hyperglycemia also alters membrane biochemistry in the developing embryo, suppressing lipids including arachidonic acid and myoinositol, and induces the elevation of other molecules that cause newly synthesized proteins to be misfolded. A build-up of misfolded/unfolded proteins triggers or exacerbates endoplasmic reticulum stress, which, like oxidative stress, plays a role in the activation of proapoptotic kinase signaling and apoptosis.6
When we’ve deleted genes for some of the proapoptotic kinase–signaling intermediates, or otherwise inhibited oxidative and endoplasmic reticulum stresses, we’ve been able to ameliorate neural cell apoptosis and the formation of neural tube defects. Studying the processes both forward and backward gives us confidence that the pathways are real and important, and that altering the pathways can alter the outcomes.
Reduced autophagy and induction of cellular senescence
Just as mitochondria are negatively affected by hyperglycemic conditions, so are autophagosomes – organelles that play a key role in removing abnormal or damaged stem cells and cellular components (including unfolded protein aggregates) and in maintaining cellular homeostasis. A high level of autophagy is essential for neural tube closure as well as cardiac morphogenesis.
In our models, maternal diabetes significantly suppressed the process of autophagy in neuroepithelial cells. We have identified responsible molecular intermediates and a key regulating gene for autophagy impairment and have found that deletion of the gene restores autophagy and reduces the development of neural tube defects.4 Administration of a naturally occurring compound, trehalose, which reactivates autophagy, had a similar effect.7Exposure to hyperglycemia not only causes cell death and suppresses autophagy, it also impairs other aspects of cellular function. More recently, we have shown that cells in the neuroepithelium become quiescent and cease proliferating. The quiescent cells, those cells with premature aging markers, also produce cytokines that influence the functioning and development of neighboring cells, causing additional cell death.
All told, premature senescence in the neuroepithelium adversely affects the neurulation process, leading to neural tube defects. In our mouse model, the senomorphic agent rapamycin suppressed cellular senescence, reduced the number of apoptotic neuroepithelial cells, and reduced the formation of neural tube defects.8
The role of epigenetics, future interventions
Epigenetics – the process by which gene expression and function can be modified by environmental conditions without modification of the DNA sequence – has become an additional area of focus in diabetic embryopathy. Our lab has studied the overexpression of both DNA methyltransferases (DNMTs) that cause DNA hypermethylation, and of microRNAs (miRNAs) that can suppress gene expression at the posttranscriptional level. Both are considered to be primary epigenetic mechanisms involved in human diseases and it appears that they are influential in the incidence of birth defects in diabetic mothers.
In our mouse models, maternal diabetes induces DNA hypermethylation via the increase of DNMTs, leading to the silencing of genes essential for neural tube closure and formation of the developing heart. MiRNAs also play a role; in addition to finding altered DNMT activity in the neural epithelium and other tissues of diabetes-exposed embryos, we also found altered miRNA expression. By deleting miRNA genes or by inhibiting DNMT activity through treatment with antioxidants, we saw significant reductions in birth defects.
In one study of the green tea polyphenol epigallocatechin gallate (EGCG), we demonstrated inhibition of diabetes-elevated DNMT expression and activity and suppression of DNA hypermethylation. The expression of genes essential for neural tube closure was restored, with a subsequent reduction in neural tube defects from 29.5% to 2% in embryos treated with EGCG.9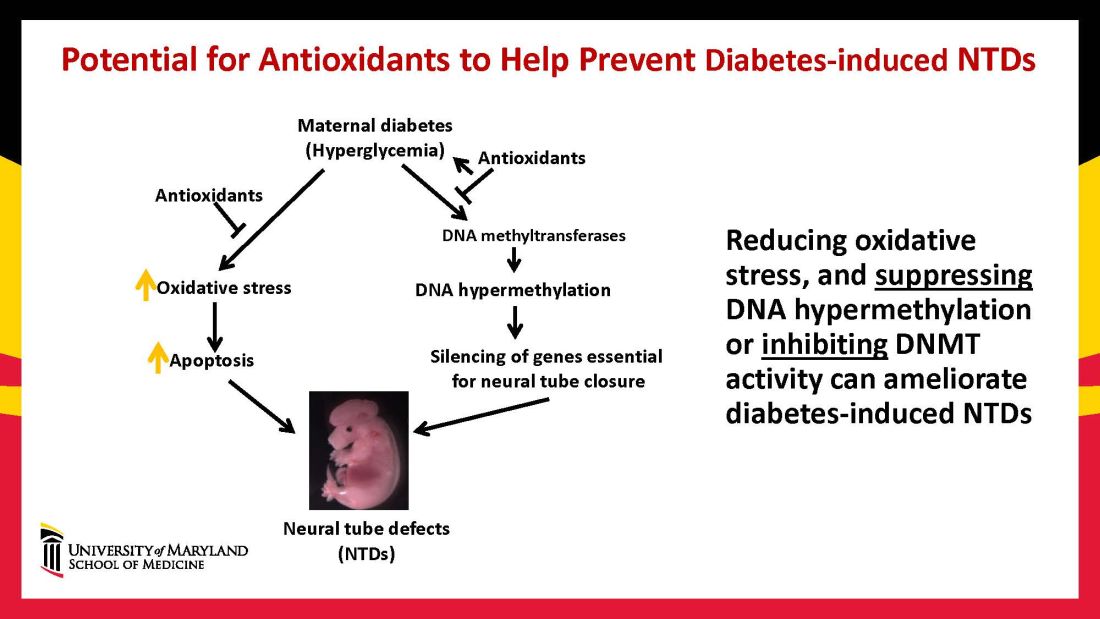
Our interventions to reverse or alter the mechanisms and pathways leading to birth defects have not only helped prove causation, but have given us hope for the future. Antioxidants are among the compounds that could be used as dietary supplements during pregnancy to prevent structural birth defects (Figure 3). Other compounds could activate the process of autophagy (for example, trehalose) and antisenescence compounds similar to rapamycin could be used to reduce numbers of senescent cells in the neuroepithelium or the developing heart.
Dr. Reece and Dr. Yang reported no relevant disclosures.
Dr. Reece, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president, endowed professor and director of CARTI, and codirector of the Center for Birth Defects.
*This story was updated on Nov. 3, 2022
References
1. Z Zhiyong and Reece EA. Clin Lab Med. 2013;33(2)207-33.
2. Reece EA and Coustan DR. Diabetes and obesity in women. Wolters Kluwer: 2019. 4th ed. (https://www.amazon.com/Diabetes-Obesity-Women-Albert-Reece/dp/1496390547).
3. The Peterson-KFF Health System Tracker. www.healthsystemtracker.org.
4. Wang F et al. Nat. Commun. 2017;8:15182.
5. Yang P et al. Am J Obstet Gynecol. 2015;212(5):569-79.
6. Li X et al. Diabetes. 2013 Feb;62(2):599-608.
7. Xu C et al. Am J Physiol Endocrinol Metab. 2013 Sep 1;305(5):E667-78.
8. Xu C et al. Sci Adv. 2021;7(27):eabf5089.
9. Zhong J et al. Am J Obstet Gynecol. 2016 Sep;215(3):368.e1-10.
Before the introduction of insulin, there were few reported cases of pregnancy complicated by diabetes because women with the disease too often did not live to childbearing age, and when they did, they were often counseled to terminate their pregnancies. Perinatal and maternal mortality in the limited number of reported pregnancies were 70% and 40%, respectively,1 making the risks of continuing the pregnancy quite high.
After insulin became available, maternal mortality dropped dramatically, down to a few percent. Perinatal mortality also declined, but it took several decades to achieve a similar magnitude of reduction.2 Today, with insulin therapy and tight glucose control as well as improved perinatal care, almost all women with diabetes can contemplate pregnancy with greater hope for normal outcomes.
Problems persist, however. Maternal diabetes continues to cause a variety of adverse outcomes, including infants large for gestational age, prematurity, and structural birth defects. Birth defects and prematurity, in fact, are the top causes of the unacceptably high infant mortality rate in the United States – a rate that is about 70% higher than the average in comparable developed countries.3
Infant mortality is considered an indicator of population health and of the development of a country; to reduce its rate, we must address these two areas.
Women with type 1 and type 2 diabetes are five times more likely to have a child with birth defects than are nondiabetic women.4 Up to 10% of women with preexisting diabetes will have fetuses with a major congenital malformation.5
Over the years we have been striving in our Center for Birth Defects Research to understand the pathomechanisms and the molecular and epigenetic alterations behind the high rates of birth defects in the offspring of women with preexisting diabetes. We have focused on heart defects and neural tube defects (particularly the latter), which together cause significant mortality, morbidity, disability, and human suffering.
Using animal models that mimic human diabetic pregnancy, we have made significant strides in our understanding of the mechanisms, uncovering molecular pathways involving oxidative stress, senescence/premature cellular aging, and epigenetic modifications (Figure 1). Understanding these pathways is providing us, in turn, with potential therapeutic targets and approaches that may be used in the future to prevent birth defects in women who enter pregnancy with type 1 or type 2 diabetes.
Unraveling the role of oxidative stress
Our mouse models accurately reflect the human conditions of diabetes in pregnancy and diabetic embryopathy. Offspring of mice with type 1 and type 2 diabetes have a similarly higher rate of neural tube defects and congenital heart disease, compared to mice without diabetes. We observe a similar incidence of anencephaly and spina bifida, and of cardiac septation defects in the mouse embryo hearts, for instance.
A primary mechanism and causal event of diabetic embryopathy is hyperglycemia-induced apoptosis in embryonic cells. Excessive cell death in the neural epithelium or in the developing heart leads to abnormal organogenesis and dysfunctional developmental events that cause birth defects. We have identified pathways leading to apoptosis, and have found that many of these pathways crosstalk with each other.
Hyperglycemia induces oxidative stress – one of these pathways – by causing sustained generation of reactive oxygen species. The cells’ mitochondrial function is significantly impaired by the hyperglycemia response, and this diabetes-induced mitochondrial dysfunction further increases the production of reactive oxygen species and a weakening of the endogenous cellular antioxidant systems, both of which then exacerbate oxidative stress.
Our research has detailed what happens downstream. We’ve learned that oxidative stress in embryos exposed to maternal diabetes activates a cascade of proapoptotic kinase signaling molecules – for example, protein kinase C isoforms such as PKCalpha; apoptosis signal-regulating kinase 1; and c-Jun-N-terminal kinases – that ultimately lead to abnormal cell death in the neuroepithelium before neural tube closure (Figure 2).5
Hyperglycemia also alters membrane biochemistry in the developing embryo, suppressing lipids including arachidonic acid and myoinositol, and induces the elevation of other molecules that cause newly synthesized proteins to be misfolded. A build-up of misfolded/unfolded proteins triggers or exacerbates endoplasmic reticulum stress, which, like oxidative stress, plays a role in the activation of proapoptotic kinase signaling and apoptosis.6
When we’ve deleted genes for some of the proapoptotic kinase–signaling intermediates, or otherwise inhibited oxidative and endoplasmic reticulum stresses, we’ve been able to ameliorate neural cell apoptosis and the formation of neural tube defects. Studying the processes both forward and backward gives us confidence that the pathways are real and important, and that altering the pathways can alter the outcomes.
Reduced autophagy and induction of cellular senescence
Just as mitochondria are negatively affected by hyperglycemic conditions, so are autophagosomes – organelles that play a key role in removing abnormal or damaged stem cells and cellular components (including unfolded protein aggregates) and in maintaining cellular homeostasis. A high level of autophagy is essential for neural tube closure as well as cardiac morphogenesis.
In our models, maternal diabetes significantly suppressed the process of autophagy in neuroepithelial cells. We have identified responsible molecular intermediates and a key regulating gene for autophagy impairment and have found that deletion of the gene restores autophagy and reduces the development of neural tube defects.4 Administration of a naturally occurring compound, trehalose, which reactivates autophagy, had a similar effect.7Exposure to hyperglycemia not only causes cell death and suppresses autophagy, it also impairs other aspects of cellular function. More recently, we have shown that cells in the neuroepithelium become quiescent and cease proliferating. The quiescent cells, those cells with premature aging markers, also produce cytokines that influence the functioning and development of neighboring cells, causing additional cell death.
All told, premature senescence in the neuroepithelium adversely affects the neurulation process, leading to neural tube defects. In our mouse model, the senomorphic agent rapamycin suppressed cellular senescence, reduced the number of apoptotic neuroepithelial cells, and reduced the formation of neural tube defects.8
The role of epigenetics, future interventions
Epigenetics – the process by which gene expression and function can be modified by environmental conditions without modification of the DNA sequence – has become an additional area of focus in diabetic embryopathy. Our lab has studied the overexpression of both DNA methyltransferases (DNMTs) that cause DNA hypermethylation, and of microRNAs (miRNAs) that can suppress gene expression at the posttranscriptional level. Both are considered to be primary epigenetic mechanisms involved in human diseases and it appears that they are influential in the incidence of birth defects in diabetic mothers.
In our mouse models, maternal diabetes induces DNA hypermethylation via the increase of DNMTs, leading to the silencing of genes essential for neural tube closure and formation of the developing heart. MiRNAs also play a role; in addition to finding altered DNMT activity in the neural epithelium and other tissues of diabetes-exposed embryos, we also found altered miRNA expression. By deleting miRNA genes or by inhibiting DNMT activity through treatment with antioxidants, we saw significant reductions in birth defects.
In one study of the green tea polyphenol epigallocatechin gallate (EGCG), we demonstrated inhibition of diabetes-elevated DNMT expression and activity and suppression of DNA hypermethylation. The expression of genes essential for neural tube closure was restored, with a subsequent reduction in neural tube defects from 29.5% to 2% in embryos treated with EGCG.9
Our interventions to reverse or alter the mechanisms and pathways leading to birth defects have not only helped prove causation, but have given us hope for the future. Antioxidants are among the compounds that could be used as dietary supplements during pregnancy to prevent structural birth defects (Figure 3). Other compounds could activate the process of autophagy (for example, trehalose) and antisenescence compounds similar to rapamycin could be used to reduce numbers of senescent cells in the neuroepithelium or the developing heart.
Dr. Reece and Dr. Yang reported no relevant disclosures.
Dr. Reece, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president, endowed professor and director of CARTI, and codirector of the Center for Birth Defects.
*This story was updated on Nov. 3, 2022
References
1. Z Zhiyong and Reece EA. Clin Lab Med. 2013;33(2)207-33.
2. Reece EA and Coustan DR. Diabetes and obesity in women. Wolters Kluwer: 2019. 4th ed. (https://www.amazon.com/Diabetes-Obesity-Women-Albert-Reece/dp/1496390547).
3. The Peterson-KFF Health System Tracker. www.healthsystemtracker.org.
4. Wang F et al. Nat. Commun. 2017;8:15182.
5. Yang P et al. Am J Obstet Gynecol. 2015;212(5):569-79.
6. Li X et al. Diabetes. 2013 Feb;62(2):599-608.
7. Xu C et al. Am J Physiol Endocrinol Metab. 2013 Sep 1;305(5):E667-78.
8. Xu C et al. Sci Adv. 2021;7(27):eabf5089.
9. Zhong J et al. Am J Obstet Gynecol. 2016 Sep;215(3):368.e1-10.
Before the introduction of insulin, there were few reported cases of pregnancy complicated by diabetes because women with the disease too often did not live to childbearing age, and when they did, they were often counseled to terminate their pregnancies. Perinatal and maternal mortality in the limited number of reported pregnancies were 70% and 40%, respectively,1 making the risks of continuing the pregnancy quite high.
After insulin became available, maternal mortality dropped dramatically, down to a few percent. Perinatal mortality also declined, but it took several decades to achieve a similar magnitude of reduction.2 Today, with insulin therapy and tight glucose control as well as improved perinatal care, almost all women with diabetes can contemplate pregnancy with greater hope for normal outcomes.
Problems persist, however. Maternal diabetes continues to cause a variety of adverse outcomes, including infants large for gestational age, prematurity, and structural birth defects. Birth defects and prematurity, in fact, are the top causes of the unacceptably high infant mortality rate in the United States – a rate that is about 70% higher than the average in comparable developed countries.3
Infant mortality is considered an indicator of population health and of the development of a country; to reduce its rate, we must address these two areas.
Women with type 1 and type 2 diabetes are five times more likely to have a child with birth defects than are nondiabetic women.4 Up to 10% of women with preexisting diabetes will have fetuses with a major congenital malformation.5
Over the years we have been striving in our Center for Birth Defects Research to understand the pathomechanisms and the molecular and epigenetic alterations behind the high rates of birth defects in the offspring of women with preexisting diabetes. We have focused on heart defects and neural tube defects (particularly the latter), which together cause significant mortality, morbidity, disability, and human suffering.
Using animal models that mimic human diabetic pregnancy, we have made significant strides in our understanding of the mechanisms, uncovering molecular pathways involving oxidative stress, senescence/premature cellular aging, and epigenetic modifications (Figure 1). Understanding these pathways is providing us, in turn, with potential therapeutic targets and approaches that may be used in the future to prevent birth defects in women who enter pregnancy with type 1 or type 2 diabetes.
Unraveling the role of oxidative stress
Our mouse models accurately reflect the human conditions of diabetes in pregnancy and diabetic embryopathy. Offspring of mice with type 1 and type 2 diabetes have a similarly higher rate of neural tube defects and congenital heart disease, compared to mice without diabetes. We observe a similar incidence of anencephaly and spina bifida, and of cardiac septation defects in the mouse embryo hearts, for instance.
A primary mechanism and causal event of diabetic embryopathy is hyperglycemia-induced apoptosis in embryonic cells. Excessive cell death in the neural epithelium or in the developing heart leads to abnormal organogenesis and dysfunctional developmental events that cause birth defects. We have identified pathways leading to apoptosis, and have found that many of these pathways crosstalk with each other.
Hyperglycemia induces oxidative stress – one of these pathways – by causing sustained generation of reactive oxygen species. The cells’ mitochondrial function is significantly impaired by the hyperglycemia response, and this diabetes-induced mitochondrial dysfunction further increases the production of reactive oxygen species and a weakening of the endogenous cellular antioxidant systems, both of which then exacerbate oxidative stress.
Our research has detailed what happens downstream. We’ve learned that oxidative stress in embryos exposed to maternal diabetes activates a cascade of proapoptotic kinase signaling molecules – for example, protein kinase C isoforms such as PKCalpha; apoptosis signal-regulating kinase 1; and c-Jun-N-terminal kinases – that ultimately lead to abnormal cell death in the neuroepithelium before neural tube closure (Figure 2).5
Hyperglycemia also alters membrane biochemistry in the developing embryo, suppressing lipids including arachidonic acid and myoinositol, and induces the elevation of other molecules that cause newly synthesized proteins to be misfolded. A build-up of misfolded/unfolded proteins triggers or exacerbates endoplasmic reticulum stress, which, like oxidative stress, plays a role in the activation of proapoptotic kinase signaling and apoptosis.6
When we’ve deleted genes for some of the proapoptotic kinase–signaling intermediates, or otherwise inhibited oxidative and endoplasmic reticulum stresses, we’ve been able to ameliorate neural cell apoptosis and the formation of neural tube defects. Studying the processes both forward and backward gives us confidence that the pathways are real and important, and that altering the pathways can alter the outcomes.
Reduced autophagy and induction of cellular senescence
Just as mitochondria are negatively affected by hyperglycemic conditions, so are autophagosomes – organelles that play a key role in removing abnormal or damaged stem cells and cellular components (including unfolded protein aggregates) and in maintaining cellular homeostasis. A high level of autophagy is essential for neural tube closure as well as cardiac morphogenesis.
In our models, maternal diabetes significantly suppressed the process of autophagy in neuroepithelial cells. We have identified responsible molecular intermediates and a key regulating gene for autophagy impairment and have found that deletion of the gene restores autophagy and reduces the development of neural tube defects.4 Administration of a naturally occurring compound, trehalose, which reactivates autophagy, had a similar effect.7Exposure to hyperglycemia not only causes cell death and suppresses autophagy, it also impairs other aspects of cellular function. More recently, we have shown that cells in the neuroepithelium become quiescent and cease proliferating. The quiescent cells, those cells with premature aging markers, also produce cytokines that influence the functioning and development of neighboring cells, causing additional cell death.
All told, premature senescence in the neuroepithelium adversely affects the neurulation process, leading to neural tube defects. In our mouse model, the senomorphic agent rapamycin suppressed cellular senescence, reduced the number of apoptotic neuroepithelial cells, and reduced the formation of neural tube defects.8
The role of epigenetics, future interventions
Epigenetics – the process by which gene expression and function can be modified by environmental conditions without modification of the DNA sequence – has become an additional area of focus in diabetic embryopathy. Our lab has studied the overexpression of both DNA methyltransferases (DNMTs) that cause DNA hypermethylation, and of microRNAs (miRNAs) that can suppress gene expression at the posttranscriptional level. Both are considered to be primary epigenetic mechanisms involved in human diseases and it appears that they are influential in the incidence of birth defects in diabetic mothers.
In our mouse models, maternal diabetes induces DNA hypermethylation via the increase of DNMTs, leading to the silencing of genes essential for neural tube closure and formation of the developing heart. MiRNAs also play a role; in addition to finding altered DNMT activity in the neural epithelium and other tissues of diabetes-exposed embryos, we also found altered miRNA expression. By deleting miRNA genes or by inhibiting DNMT activity through treatment with antioxidants, we saw significant reductions in birth defects.
In one study of the green tea polyphenol epigallocatechin gallate (EGCG), we demonstrated inhibition of diabetes-elevated DNMT expression and activity and suppression of DNA hypermethylation. The expression of genes essential for neural tube closure was restored, with a subsequent reduction in neural tube defects from 29.5% to 2% in embryos treated with EGCG.9
Our interventions to reverse or alter the mechanisms and pathways leading to birth defects have not only helped prove causation, but have given us hope for the future. Antioxidants are among the compounds that could be used as dietary supplements during pregnancy to prevent structural birth defects (Figure 3). Other compounds could activate the process of autophagy (for example, trehalose) and antisenescence compounds similar to rapamycin could be used to reduce numbers of senescent cells in the neuroepithelium or the developing heart.
Dr. Reece and Dr. Yang reported no relevant disclosures.
Dr. Reece, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president, endowed professor and director of CARTI, and codirector of the Center for Birth Defects.
*This story was updated on Nov. 3, 2022
References
1. Z Zhiyong and Reece EA. Clin Lab Med. 2013;33(2)207-33.
2. Reece EA and Coustan DR. Diabetes and obesity in women. Wolters Kluwer: 2019. 4th ed. (https://www.amazon.com/Diabetes-Obesity-Women-Albert-Reece/dp/1496390547).
3. The Peterson-KFF Health System Tracker. www.healthsystemtracker.org.
4. Wang F et al. Nat. Commun. 2017;8:15182.
5. Yang P et al. Am J Obstet Gynecol. 2015;212(5):569-79.
6. Li X et al. Diabetes. 2013 Feb;62(2):599-608.
7. Xu C et al. Am J Physiol Endocrinol Metab. 2013 Sep 1;305(5):E667-78.
8. Xu C et al. Sci Adv. 2021;7(27):eabf5089.
9. Zhong J et al. Am J Obstet Gynecol. 2016 Sep;215(3):368.e1-10.
Discoveries in diabetic embryogenesis
Many issues surrounding pregnancy care of women with preexisting diabetes remain challenging, especially in light of the relentless increase in maternal morbidity and mortality in the United States and globally. Rising rates of death and severe morbidity in diabetic women have continued despite significant advances in insulin pharmacology and administration technology.
However, despite these advances in glucose monitoring and insulin administration, fetal mortality and childhood morbidity rates continue to climb. This is because critical fetal structural anomalies arise from developmental errors occurring in the embryonic period – between 2 and 13 weeks of gestation – a time when most women with preexisting diabetes are just entering into prenatal care, often with suboptimal glycemic control.
Thus, significant future progress in reducing fetal mortality and childhood disability in infants of diabetic mothers will depend upon effective interventions in the first trimester while embryogenesis and critical organ formation are underway.
In this issue of Ob.Gyn. News, the editor of Master Class in Obstetrics, E. Albert Reece MD, PhD, MBA, steps into the role of coauthor. He and his research colleague Peixin Yang, PhD, present exciting insights into the cellular mechanisms underlying structural birth defects in infants of diabetic mothers – especially cardiac and neural tube defects – and also provide a glimpse into some potentially effective maternal pharmacologic interventions. After appropriate human trials, these interventions could be effectively applied from the time of a positive pregnancy test with potentially dramatic results.
Dr. Reece and Dr. Yang, who lead the Center for the Study of Birth Defects at the University of Maryland School of Medicine, share their impressive accumulation of data from embryos of pregnant diabetic rodents. They demonstrate convincingly that, in first-trimester rodent embryos, maternal hyperglycemia induces excessive apoptosis, which in turn leads to structural defects in critical fetal organs. They further found that maternal hyperglycemia reduces embryonic autophagosomes – the developmentally essential organelles that remove abnormal or damaged cells during embryo formation.
These investigators also identified reactivators of these organelles which, when administered maternally in the first trimester, significantly reduced the incidence of neural tube defects. Thus, for optimal development of diabetes-affected embryos, first-trimester administration of reactivators of autophagy could offer a significant, life-changing intervention in the foreseeable future.
Dr. Moore is professor emeritus of maternal-fetal medicine and chair emeritus in the department of obstetrics, gynecology, and reproductive sciences at UC San Diego Health. He reported no disclosures.
*This story was updated on Nov. 3, 2022.
Many issues surrounding pregnancy care of women with preexisting diabetes remain challenging, especially in light of the relentless increase in maternal morbidity and mortality in the United States and globally. Rising rates of death and severe morbidity in diabetic women have continued despite significant advances in insulin pharmacology and administration technology.
However, despite these advances in glucose monitoring and insulin administration, fetal mortality and childhood morbidity rates continue to climb. This is because critical fetal structural anomalies arise from developmental errors occurring in the embryonic period – between 2 and 13 weeks of gestation – a time when most women with preexisting diabetes are just entering into prenatal care, often with suboptimal glycemic control.
Thus, significant future progress in reducing fetal mortality and childhood disability in infants of diabetic mothers will depend upon effective interventions in the first trimester while embryogenesis and critical organ formation are underway.
In this issue of Ob.Gyn. News, the editor of Master Class in Obstetrics, E. Albert Reece MD, PhD, MBA, steps into the role of coauthor. He and his research colleague Peixin Yang, PhD, present exciting insights into the cellular mechanisms underlying structural birth defects in infants of diabetic mothers – especially cardiac and neural tube defects – and also provide a glimpse into some potentially effective maternal pharmacologic interventions. After appropriate human trials, these interventions could be effectively applied from the time of a positive pregnancy test with potentially dramatic results.
Dr. Reece and Dr. Yang, who lead the Center for the Study of Birth Defects at the University of Maryland School of Medicine, share their impressive accumulation of data from embryos of pregnant diabetic rodents. They demonstrate convincingly that, in first-trimester rodent embryos, maternal hyperglycemia induces excessive apoptosis, which in turn leads to structural defects in critical fetal organs. They further found that maternal hyperglycemia reduces embryonic autophagosomes – the developmentally essential organelles that remove abnormal or damaged cells during embryo formation.
These investigators also identified reactivators of these organelles which, when administered maternally in the first trimester, significantly reduced the incidence of neural tube defects. Thus, for optimal development of diabetes-affected embryos, first-trimester administration of reactivators of autophagy could offer a significant, life-changing intervention in the foreseeable future.
Dr. Moore is professor emeritus of maternal-fetal medicine and chair emeritus in the department of obstetrics, gynecology, and reproductive sciences at UC San Diego Health. He reported no disclosures.
*This story was updated on Nov. 3, 2022.
Many issues surrounding pregnancy care of women with preexisting diabetes remain challenging, especially in light of the relentless increase in maternal morbidity and mortality in the United States and globally. Rising rates of death and severe morbidity in diabetic women have continued despite significant advances in insulin pharmacology and administration technology.
However, despite these advances in glucose monitoring and insulin administration, fetal mortality and childhood morbidity rates continue to climb. This is because critical fetal structural anomalies arise from developmental errors occurring in the embryonic period – between 2 and 13 weeks of gestation – a time when most women with preexisting diabetes are just entering into prenatal care, often with suboptimal glycemic control.
Thus, significant future progress in reducing fetal mortality and childhood disability in infants of diabetic mothers will depend upon effective interventions in the first trimester while embryogenesis and critical organ formation are underway.
In this issue of Ob.Gyn. News, the editor of Master Class in Obstetrics, E. Albert Reece MD, PhD, MBA, steps into the role of coauthor. He and his research colleague Peixin Yang, PhD, present exciting insights into the cellular mechanisms underlying structural birth defects in infants of diabetic mothers – especially cardiac and neural tube defects – and also provide a glimpse into some potentially effective maternal pharmacologic interventions. After appropriate human trials, these interventions could be effectively applied from the time of a positive pregnancy test with potentially dramatic results.
Dr. Reece and Dr. Yang, who lead the Center for the Study of Birth Defects at the University of Maryland School of Medicine, share their impressive accumulation of data from embryos of pregnant diabetic rodents. They demonstrate convincingly that, in first-trimester rodent embryos, maternal hyperglycemia induces excessive apoptosis, which in turn leads to structural defects in critical fetal organs. They further found that maternal hyperglycemia reduces embryonic autophagosomes – the developmentally essential organelles that remove abnormal or damaged cells during embryo formation.
These investigators also identified reactivators of these organelles which, when administered maternally in the first trimester, significantly reduced the incidence of neural tube defects. Thus, for optimal development of diabetes-affected embryos, first-trimester administration of reactivators of autophagy could offer a significant, life-changing intervention in the foreseeable future.
Dr. Moore is professor emeritus of maternal-fetal medicine and chair emeritus in the department of obstetrics, gynecology, and reproductive sciences at UC San Diego Health. He reported no disclosures.
*This story was updated on Nov. 3, 2022.








