User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
The right indoor relative humidity could ward off COVID
The “sweet spot” associated with reduced COVID-19 cases and deaths is 40%-60% indoor relative humidity, an MIT news release said. People who maintained indoor relative humidity outside those parameters had higher rates of catching COVID-19.
Most people are comfortable with 30%-50% relative humidity, researchers said. An airplane cabin has about 20% relative humidity.
Relative humidity is the amount of moisture in the air, compared with the total moisture the air can hold at a given temperature before saturating and forming condensation.
The study was published in The Journal of the Royal Society Interface. Researchers examined COVID-19 data and meteorological measurements from 121 countries from January 2020 through August 2020, before vaccines became available to the public.
“When outdoor temperatures were below the typical human comfort range, they assumed indoor spaces were heated to reach that comfort range. Based on the added heating, they calculated the associated drop in indoor relative humidity,” the MIT news release said.
The research teams found that when a region reported a rise in COVID-19 cases and deaths, the region’s estimated indoor relative humidity was either lower than 40% or higher than 60%, the release said.
“There’s potentially a protective effect of this intermediate indoor relative humidity,” said Connor Verheyen, the lead author and a PhD student in medical engineering and medical physics in the Harvard-MIT Program in Health Sciences and Technology.
Widespread use of the 40%-60% indoor humidity range could reduce the need for lockdowns and other widespread restrictions, the study concluded.
“Unlike measures that depend on individual compliance (for example, masking or hand-washing), indoor RH optimization would achieve high compliance because all occupants of a common indoor space would be exposed to similar ambient conditions,” the study said. “Compared to the long timelines and high costs of vaccine production and distribution, humidity control systems could potentially be implemented more quickly and cheaply in certain indoor settings.”
A version of this article first appeared on WebMD.com.
The “sweet spot” associated with reduced COVID-19 cases and deaths is 40%-60% indoor relative humidity, an MIT news release said. People who maintained indoor relative humidity outside those parameters had higher rates of catching COVID-19.
Most people are comfortable with 30%-50% relative humidity, researchers said. An airplane cabin has about 20% relative humidity.
Relative humidity is the amount of moisture in the air, compared with the total moisture the air can hold at a given temperature before saturating and forming condensation.
The study was published in The Journal of the Royal Society Interface. Researchers examined COVID-19 data and meteorological measurements from 121 countries from January 2020 through August 2020, before vaccines became available to the public.
“When outdoor temperatures were below the typical human comfort range, they assumed indoor spaces were heated to reach that comfort range. Based on the added heating, they calculated the associated drop in indoor relative humidity,” the MIT news release said.
The research teams found that when a region reported a rise in COVID-19 cases and deaths, the region’s estimated indoor relative humidity was either lower than 40% or higher than 60%, the release said.
“There’s potentially a protective effect of this intermediate indoor relative humidity,” said Connor Verheyen, the lead author and a PhD student in medical engineering and medical physics in the Harvard-MIT Program in Health Sciences and Technology.
Widespread use of the 40%-60% indoor humidity range could reduce the need for lockdowns and other widespread restrictions, the study concluded.
“Unlike measures that depend on individual compliance (for example, masking or hand-washing), indoor RH optimization would achieve high compliance because all occupants of a common indoor space would be exposed to similar ambient conditions,” the study said. “Compared to the long timelines and high costs of vaccine production and distribution, humidity control systems could potentially be implemented more quickly and cheaply in certain indoor settings.”
A version of this article first appeared on WebMD.com.
The “sweet spot” associated with reduced COVID-19 cases and deaths is 40%-60% indoor relative humidity, an MIT news release said. People who maintained indoor relative humidity outside those parameters had higher rates of catching COVID-19.
Most people are comfortable with 30%-50% relative humidity, researchers said. An airplane cabin has about 20% relative humidity.
Relative humidity is the amount of moisture in the air, compared with the total moisture the air can hold at a given temperature before saturating and forming condensation.
The study was published in The Journal of the Royal Society Interface. Researchers examined COVID-19 data and meteorological measurements from 121 countries from January 2020 through August 2020, before vaccines became available to the public.
“When outdoor temperatures were below the typical human comfort range, they assumed indoor spaces were heated to reach that comfort range. Based on the added heating, they calculated the associated drop in indoor relative humidity,” the MIT news release said.
The research teams found that when a region reported a rise in COVID-19 cases and deaths, the region’s estimated indoor relative humidity was either lower than 40% or higher than 60%, the release said.
“There’s potentially a protective effect of this intermediate indoor relative humidity,” said Connor Verheyen, the lead author and a PhD student in medical engineering and medical physics in the Harvard-MIT Program in Health Sciences and Technology.
Widespread use of the 40%-60% indoor humidity range could reduce the need for lockdowns and other widespread restrictions, the study concluded.
“Unlike measures that depend on individual compliance (for example, masking or hand-washing), indoor RH optimization would achieve high compliance because all occupants of a common indoor space would be exposed to similar ambient conditions,” the study said. “Compared to the long timelines and high costs of vaccine production and distribution, humidity control systems could potentially be implemented more quickly and cheaply in certain indoor settings.”
A version of this article first appeared on WebMD.com.
FROM THE JOURNAL OF THE ROYAL SOCIETY INTERFACE
Surgical management of early pregnancy loss
CASE Concern for surgical management after repeat miscarriage
A 34-year-old woman (G3P0030) with a history of recurrent pregnancy loss was recently diagnosed with a 7-week missed abortion. After her second miscarriage, she had an evaluation for recurrent pregnancy loss which was unremarkable. Both prior miscarriages were managed with dilation & curettage (D&C), but cytogenetic testing of the tissue did not yield a result in either case. The karyotype from the first pregnancy resulted as 46, XX but was confirmed to be due to maternal cell contamination, and the karyotype from the second pregnancy resulted in cell culture failure. The patient is interested in surgical management for her current missed abortion to help with tissue collection for cytogenetic testing, she but is concerned about her risk of intrauterine adhesions with repeated uterine instrumentation given 2 prior D&Cs, one of which was complicated by retained products of conception.
How do you approach the surgical management of this patient with recurrent pregnancy loss?
Approximately 1 in every 8 recognized pregnancies results in miscarriage. The risk of loss is lowest in women with no history of miscarriage (11%), and increases by about 10% for each additional miscarriage, reaching 42% in women with 3 or more previous losses. The population prevalence of women who have had 1 miscarriage is 11%, 2 miscarriages is 2%, and 3 or more is <1%.1 While 90% of miscarriages occur in the first trimester, their etiology can be quite varied.2 A woman’s age is the most strongly associated risk factor, with both very young (<20 years) and older age (>35 years) groups at highest risk. This association is largely attributed to an age-related increase in embryonic chromosomal aneuploidies, of which trisomies, particularly trisomy 16, are the most common.3 Maternal anatomic anomalies such as leiomyomas, intrauterine adhesions, Müllerian anomalies, and adenomyosis have been linked to an increased risk of miscarriage in addition to several lifestyle and environmental factor exposures.1
Regardless of the etiology, women with recurrent miscarriage are exposed to the potential for iatrogenic harm from the management of their pregnancy loss, including intrauterine adhesions and retained products, which may negatively impact future reproductive attempts. The management of patients with recurrent miscarriages demands special attention to reduce the risk of iatrogenic harm, maximize diagnostic evaluation of the products of conception, and improve future reproductive outcomes.
Management strategies
First trimester pregnancy loss may be managed expectantly, medically, or surgically. Approximately 76% of women who opt for expectant management will successfully pass pregnancy tissue, but for 1 out of every 6 women it may take longer than 14 days.4 For patients who prefer to expedite this process, medication abortion is a highly effective and safe option. According to Schreiber and colleagues, a combination of mifepristone and misoprostol together resulted in expulsion in approximately 91% of 148 patients, although 9% still required surgical intervention for incomplete passage of tissue.5 Both expectant management and medical management strategies are associated with the potential for retained products of conception requiring subsequent instrumentation as well as tissue that is often unsuitable or contaminated for cytogenetic analysis.
The most definitive treatment option is surgical management via manual or electric vacuum aspiration or curettage, with efficacy approaching 99.6% in some series.6 While highly effective, even ultrasound-guided evacuation carries with it procedure-related risks that are of particular consequence for patients of reproductive age, including adhesion formation and retained products of conception.
In 1997, Goldenberg and colleagues reported on the use of hysteroscopy for the management of retained products of conception as a strategy to minimize trauma to the uterus and maximize excision of retained tissue, both of which reduce potential for adhesion formation.7 Based on these data, several groups have extended the use of hysteroscopic resection for retained tissue to upfront evacuation following pregnancy loss, in lieu of D&C.8,9 This approach allows for the direct visualization of the focal removal of the implanted pregnancy tissue, which can:
- decrease the risk of intrauterine adhesion formation
- decrease the risk of retained products of conception
- allow for directed tissue sampling to improve the accuracy of cytogenetic testing
- allow for detection of embryo anatomic anomalies that often go undetected on traditional cytogenetic analysis.
For the remainder of this article, we will discuss the advantages of hysteroscopic management of a missed abortion in greater detail.
Continue to: Hysteroscopic management...
Hysteroscopic management
Like aspiration or curettage, hysteroscopic management may be offered once the diagnosis of fetal demise is confirmed on ultrasonography. The procedure may be accomplished in the office setting or in the operative room with either morcellation or resectoscopic instruments. Morcellation allows for improved visibility during the procedure given the ability of continuous suction to manage tissue fragments in the surgical field, while resectoscopic instruments offer the added benefit of electrosurgery should bleeding that is unresponsive to increased distention pressure be encountered. Use of the cold loop of the resectoscope to accomplish evacuation is advocated to avoid the thermal damage to the endometrium with electrosurgery. Regardless of the chosen instrument, there are several potential benefits for a hysteroscopic approach over the traditional ultrasound-guided or blind D&C.
Reducing risk of iatrogenic harm
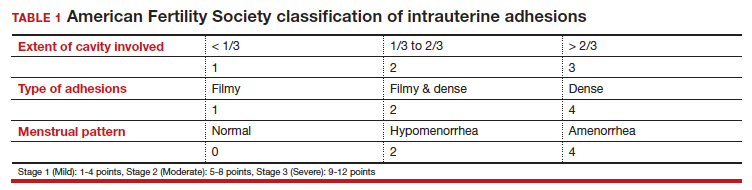
Intrauterine adhesions form secondary to trauma to the endometrial basalis layer, where a population of adult progenitor stem cells continuously work to regenerate the overlying functionalis layer. Once damaged, adhesions may form and range from thin, filmy adhesions to dense, cavity obliterating bands of scar tissue (FIGURE). The degree of severity and location of the adhesions account for the variable presentation that range from menstrual abnormalities to infertility and recurrent pregnancy loss. While several classification systems exist for scoring severity of adhesions, the American Fertility Society (now American Society for Reproductive Medicine) Classification system from 1988 is still commonly utilized (TABLE 1).
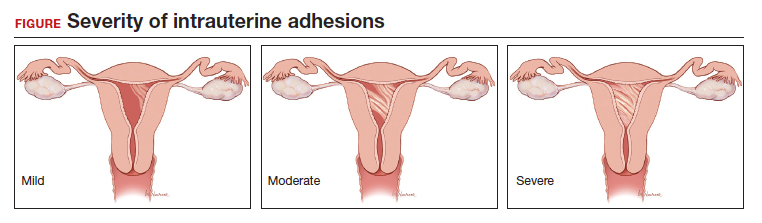
Intrauterine adhesions from D&C after pregnancy loss are not uncommon. A 2014 meta-analysis of 10 prospective studies including 912 women reported a pooled prevalence for intrauterine adhesions of 19.1% (95% confidence interval [CI], 12.8–27.5) on hysteroscopic evaluation within 12 months following curettage.10 Once formed, these adhesions are associated with long-term impairment in reproductive outcomes, regardless of if they were treated or not. In a long-term follow-up study of women with and without adhesions after recurrent D&C for miscarriage, women with treated adhesions reported lower live birth rates, longer time to pregnancy, higher rates of preterm birth and higher rates of peripartum complications compared with those without adhesions.11
Compared with curettage, hysteroscopy affords the surgeon complete visualization of the uterine cavity and tissue to be resected. This, in turn, minimizes trauma to the surrounding uterine cavity, minimizes the potential for post-procedural adhesion formation and their associated sequelae, and maximizes complete resection of tissue. Those treated with D&C appear to be significantly more likely to have adhesions than those treated via a hysteroscopic approach (30% vs 13%).12
Retained products of conception. Classically, a “gritty” sensation of the endometrium following evacuation of the uterus with a sharp curette has been used to indicate complete removal of tissue. The evolution from a nonvisualized procedure to ultrasound-guided vacuum aspiration of 1st trimester pregnancy tissue has been associated with a decreased risk of procedural complications and retained products of conception.13 However, even with intraoperative imaging, the risk of retained products of conception remains because it can be difficult to distinguish a small blood clot from retained pregnancy tissue on ultrasonography.
Retained pregnancy tissue can result in abnormal or heavy bleeding, require additional medical or surgical intervention, and is associated with endometrial inflammation and infection. Approximately 1 in every 4 women undergoing hysteroscopic resection of retained products are found to have evidence of endometritis in the resected tissue.14 This number is even higher in women with a diagnosis of recurrent pregnancy loss (62%).15
These complications from retained products of conception can be avoided with the hysteroscopic approach due to the direct visualization of the tissue removal. This benefit may be particularly beneficial in patients with known abnormal uterine cavities, such as those with Müllerian anomalies, uterine leiomyomas, preexisting adhesions, and history of placenta accreta spectrum disorder.
Continue to: Maximizing diagnostic yield...
Maximizing diagnostic yield
Many patients prefer surgical management of a missed abortion not for the procedural advantages, but to assist with tissue collection for cytogenetic testing of the pregnancy tissue. Given that embryonic chromosomal aneuploidy is implicated in 70% of miscarriages prior to 20 weeks’ gestation, genetic evaluation of the products of conception is commonly performed to identify a potential cause for the miscarriage.16 G-band karyotype is the most commonly performed genetic evaluation. Karyotype requires culturing of pregnancy tissue for 7-14 days to produce metaphase cells that are then chemically treated to arrest them at their maximally contracted stage. Cytogenetic evaluation is often curtailed when nonviable cells from products of conception fail to culture due to either time elapsed from diagnosis to demise or damage from tissue handling. Careful, directly observed tissue handling via a hysteroscopic approach may alleviate culture failure secondary to tissue damage.
Another concern with cultures of products of conception is the potential for maternal cell contamination. Early studies from the 1970s noted a significant skew toward 46, XX karyotype results in miscarried tissue as compared with 46, XY results. It was not until microsatellite analysis technology was available that it was determined that the result was due to analysis of maternal cells instead of products of conception.17 A 2014 study by Levy and colleagues and another by Lathi and colleagues that utilized single-nucleotide polymorphism (SNP) microarray found that maternal cell contamination affected 22% of all miscarriage samples analyzed and over half of karyotypes with a 46, XX result.18,19
Traditional “blind” suction and curettage may inadvertently collect maternal endometrial tissue and contaminate the culture of fetal cells, limiting the validity of karyotype for products of conception.20 The hysteroscopic approach may provide a higher diagnostic yield for karyotype analysis of fetal tissue by the nature of targeted tissue sampling under direct visualization, minimizing maternal cell contamination. One retrospective study by Cholkeri-Singh and colleagues evaluated rates of fetal chromosome detection without maternal contamination in a total of 264 patients undergoing either suction curettage or hysteroscopic resection. They found that fetal chromosomal detection without contamination was significantly higher in the hysteroscopy group compared with the suction curettage group (88.5 vs 64.8%, P< .001).21 Additionally, biopsies of tissue under direct visualization may enable the diagnosis of a true placental mosaicism and the study of the individual karyotype of each embryo in dizygotic twin missed abortions.
Finally, a hysteroscopic approach may afford the opportunity to also perform morphologic evaluation of the intact early fetus furthering the diagnostic utility of the procedure. With hysteroscopy, the gestational sac is identified and carefully entered, allowing for complete visualization of the early fetus and assessment of anatomic malformations that may provide insight into the pregnancy loss (ie, embryoscopy). In one series of 272 patients with missed abortions, while nearly 75% of conceptuses had abnormal karyotypes, 18% were found to have gross morphologic defects with a normal karyotype.22
Bottom line
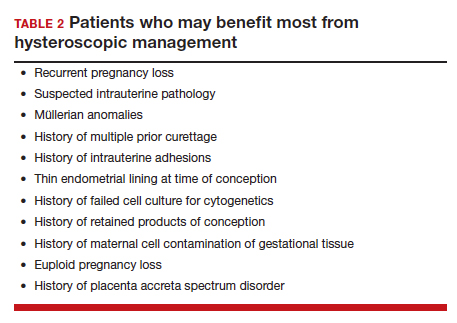
When faced with a patient with an early pregnancy loss, physicians should consider the decreased iatrogenic risks and improved diagnostic yield when deciding between D&C versus hysteroscopy for surgical management. There are certain patients with pre-existing risk factors that may stand to benefit the most (TABLE 2). Much like the opening case, those at risk for intrauterine adhesions, retained products of conception, or in whom a successful and accurate cytogenetic analysis is essential are the most likely to benefit from a hysteroscopic approach. The hysteroscopic approach also affords concurrent diagnosis and treatment of intrauterine pathology, such as leiomyomas and uterine septum, which are encountered approximately 12.5% of the time after one miscarriage and 29.4% of the time in patients with a history of more than one miscarriage.10 In the appropriately counseled patient and clinical setting, clinicians could also perform definitive surgical management during the same hysteroscopy. Finally, evaluation of the morphology of the demised fetus may provide additional information for patient counseling in those with euploid pregnancy losses.
CASE Resolved
Ultimately, our patient underwent complete hysteroscopic resection of the pregnancy tissue, which confirmed both a morphologically abnormal fetus and a 45, X karyotype of the products of conception. ●
- Quenby S, Gallos ID, Dhillon-Smith RK, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021;397:1658-1667.
- Kolte AM, Westergaard D, Lidegaard Ø, et al. Chance of live birth: a nationwide, registry-based cohort study. Hum Reprod Oxf Engl. 2021;36:1065-1073.
- Magnus MC, Wilcox AJ, Morken N-H, et al. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:869.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873-875.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Ireland LD, Gatter M, Chen AY. Medical compared with surgical abortion for effective pregnancy termination in the first trimester. Obstet Gynecol. 2015;126:22-28.
- Goldenberg M, Schiff E, Achiron R, et al. Managing residual trophoblastic tissue. Hysteroscopy for directing curettage. J Reprod Med. 1997;42:26-28.
- Weinberg S, Pansky M, Burshtein I, et al. A pilot study of guided conservative hysteroscopic evacuation of early miscarriage. J Minim Invasive Gynecol. 2021;28:1860-1867.
- Young S, Miller CE. Hysteroscopic resection for management of early pregnancy loss: a case report and literature review. FS Rep. 2022;3:163-167.
- Hooker AB, Lemmers M, Thurkow AL, et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update. 2014;20:262-278.
- Hooker AB, de Leeuw RA, Twisk JWR, et al. Reproductive performance of women with and without intrauterine adhesions following recurrent dilatation and curettage for miscarriage: long-term follow-up of a randomized controlled trial. Hum Reprod. 2021;36:70-81.
- Hooker AB, Aydin H, Brölmann HAM, et al. Longterm complications and reproductive outcome after the management of retained products of conception: a systematic review. Fertil Steril. 2016;105:156-164.e1-e2.
- Debby A, Malinger G, Harow E, et al. Transvaginal ultrasound after first-trimester uterine evacuation reduces the incidence of retained products of conception. Ultrasound Obstet Gynecol. 2006;27:61-64.
- Elder S, Bortoletto P, Romanski PA, et al. Chronic endometritis in women with suspected retained products of conception and their reproductive outcomes. Am J Reprod Immunol N Y N 1989. 2021;86:e13410.
- McQueen DB, Maniar KP, Hutchinson A, et al. Retained pregnancy tissue after miscarriage is associated with high rate of chronic endometritis. J Obstet Gynaecol J Inst Obstet Gynaecol. 2022;1-5.
- Soler A, Morales C, Mademont-Soler I, et al. Overview of chromosome abnormalities in first trimester miscarriages: a series of 1,011 consecutive chorionic villi sample karyotypes. Cytogenet Genome Res. 2017;152:81-89.
- Jarrett KL, Michaelis RC, Phelan MC, et al. Microsatellite analysis reveals a high incidence of maternal cell contamination in 46, XX products of conception consisting of villi or a combination of villi and membranous material. Am J Obstet Gynecol. 2001;185:198-203.
- Levy B, Sigurjonsson S, Pettersen B, et al. Genomic imbalance in products of conception: single-nucleotide polymorphism chromosomal microarray analysis. Obstet Gynecol. 2014;124:202-209.
- Lathi RB, Gustin SLF, Keller J, et al. Reliability of 46, XX results on miscarriage specimens: a review of 1,222 first-trimester miscarriage specimens. Fertil Steril. 2014;101:178-182.
- Chung JPW, Li Y, Law TSM, et al. Ultrasound-guided manual vacuum aspiration is an optimal method for obtaining products of conception from early pregnancy loss for cytogenetic testing. Int J Biochem Cell Biol. 2022;147:106226.
- Cholkeri-Singh A, Zamfirova I, Miller CE. Increased fetal chromosome detection with the use of operative hysteroscopy during evacuation of products of conception for diagnosed miscarriage. J Minim Invasive Gynecol. 2020;27:160-165.
- Philipp T, Philipp K, Reiner A, et al. Embryoscopic and cytogenetic analysis of 233 missed abortions: factors involved in the pathogenesis of developmental defects of early failed pregnancies. Hum Reprod. 2003;18:1724-1732.
CASE Concern for surgical management after repeat miscarriage
A 34-year-old woman (G3P0030) with a history of recurrent pregnancy loss was recently diagnosed with a 7-week missed abortion. After her second miscarriage, she had an evaluation for recurrent pregnancy loss which was unremarkable. Both prior miscarriages were managed with dilation & curettage (D&C), but cytogenetic testing of the tissue did not yield a result in either case. The karyotype from the first pregnancy resulted as 46, XX but was confirmed to be due to maternal cell contamination, and the karyotype from the second pregnancy resulted in cell culture failure. The patient is interested in surgical management for her current missed abortion to help with tissue collection for cytogenetic testing, she but is concerned about her risk of intrauterine adhesions with repeated uterine instrumentation given 2 prior D&Cs, one of which was complicated by retained products of conception.
How do you approach the surgical management of this patient with recurrent pregnancy loss?
Approximately 1 in every 8 recognized pregnancies results in miscarriage. The risk of loss is lowest in women with no history of miscarriage (11%), and increases by about 10% for each additional miscarriage, reaching 42% in women with 3 or more previous losses. The population prevalence of women who have had 1 miscarriage is 11%, 2 miscarriages is 2%, and 3 or more is <1%.1 While 90% of miscarriages occur in the first trimester, their etiology can be quite varied.2 A woman’s age is the most strongly associated risk factor, with both very young (<20 years) and older age (>35 years) groups at highest risk. This association is largely attributed to an age-related increase in embryonic chromosomal aneuploidies, of which trisomies, particularly trisomy 16, are the most common.3 Maternal anatomic anomalies such as leiomyomas, intrauterine adhesions, Müllerian anomalies, and adenomyosis have been linked to an increased risk of miscarriage in addition to several lifestyle and environmental factor exposures.1
Regardless of the etiology, women with recurrent miscarriage are exposed to the potential for iatrogenic harm from the management of their pregnancy loss, including intrauterine adhesions and retained products, which may negatively impact future reproductive attempts. The management of patients with recurrent miscarriages demands special attention to reduce the risk of iatrogenic harm, maximize diagnostic evaluation of the products of conception, and improve future reproductive outcomes.
Management strategies
First trimester pregnancy loss may be managed expectantly, medically, or surgically. Approximately 76% of women who opt for expectant management will successfully pass pregnancy tissue, but for 1 out of every 6 women it may take longer than 14 days.4 For patients who prefer to expedite this process, medication abortion is a highly effective and safe option. According to Schreiber and colleagues, a combination of mifepristone and misoprostol together resulted in expulsion in approximately 91% of 148 patients, although 9% still required surgical intervention for incomplete passage of tissue.5 Both expectant management and medical management strategies are associated with the potential for retained products of conception requiring subsequent instrumentation as well as tissue that is often unsuitable or contaminated for cytogenetic analysis.
The most definitive treatment option is surgical management via manual or electric vacuum aspiration or curettage, with efficacy approaching 99.6% in some series.6 While highly effective, even ultrasound-guided evacuation carries with it procedure-related risks that are of particular consequence for patients of reproductive age, including adhesion formation and retained products of conception.
In 1997, Goldenberg and colleagues reported on the use of hysteroscopy for the management of retained products of conception as a strategy to minimize trauma to the uterus and maximize excision of retained tissue, both of which reduce potential for adhesion formation.7 Based on these data, several groups have extended the use of hysteroscopic resection for retained tissue to upfront evacuation following pregnancy loss, in lieu of D&C.8,9 This approach allows for the direct visualization of the focal removal of the implanted pregnancy tissue, which can:
- decrease the risk of intrauterine adhesion formation
- decrease the risk of retained products of conception
- allow for directed tissue sampling to improve the accuracy of cytogenetic testing
- allow for detection of embryo anatomic anomalies that often go undetected on traditional cytogenetic analysis.
For the remainder of this article, we will discuss the advantages of hysteroscopic management of a missed abortion in greater detail.
Continue to: Hysteroscopic management...
Hysteroscopic management
Like aspiration or curettage, hysteroscopic management may be offered once the diagnosis of fetal demise is confirmed on ultrasonography. The procedure may be accomplished in the office setting or in the operative room with either morcellation or resectoscopic instruments. Morcellation allows for improved visibility during the procedure given the ability of continuous suction to manage tissue fragments in the surgical field, while resectoscopic instruments offer the added benefit of electrosurgery should bleeding that is unresponsive to increased distention pressure be encountered. Use of the cold loop of the resectoscope to accomplish evacuation is advocated to avoid the thermal damage to the endometrium with electrosurgery. Regardless of the chosen instrument, there are several potential benefits for a hysteroscopic approach over the traditional ultrasound-guided or blind D&C.
Reducing risk of iatrogenic harm
Intrauterine adhesions form secondary to trauma to the endometrial basalis layer, where a population of adult progenitor stem cells continuously work to regenerate the overlying functionalis layer. Once damaged, adhesions may form and range from thin, filmy adhesions to dense, cavity obliterating bands of scar tissue (FIGURE). The degree of severity and location of the adhesions account for the variable presentation that range from menstrual abnormalities to infertility and recurrent pregnancy loss. While several classification systems exist for scoring severity of adhesions, the American Fertility Society (now American Society for Reproductive Medicine) Classification system from 1988 is still commonly utilized (TABLE 1).


Intrauterine adhesions from D&C after pregnancy loss are not uncommon. A 2014 meta-analysis of 10 prospective studies including 912 women reported a pooled prevalence for intrauterine adhesions of 19.1% (95% confidence interval [CI], 12.8–27.5) on hysteroscopic evaluation within 12 months following curettage.10 Once formed, these adhesions are associated with long-term impairment in reproductive outcomes, regardless of if they were treated or not. In a long-term follow-up study of women with and without adhesions after recurrent D&C for miscarriage, women with treated adhesions reported lower live birth rates, longer time to pregnancy, higher rates of preterm birth and higher rates of peripartum complications compared with those without adhesions.11
Compared with curettage, hysteroscopy affords the surgeon complete visualization of the uterine cavity and tissue to be resected. This, in turn, minimizes trauma to the surrounding uterine cavity, minimizes the potential for post-procedural adhesion formation and their associated sequelae, and maximizes complete resection of tissue. Those treated with D&C appear to be significantly more likely to have adhesions than those treated via a hysteroscopic approach (30% vs 13%).12
Retained products of conception. Classically, a “gritty” sensation of the endometrium following evacuation of the uterus with a sharp curette has been used to indicate complete removal of tissue. The evolution from a nonvisualized procedure to ultrasound-guided vacuum aspiration of 1st trimester pregnancy tissue has been associated with a decreased risk of procedural complications and retained products of conception.13 However, even with intraoperative imaging, the risk of retained products of conception remains because it can be difficult to distinguish a small blood clot from retained pregnancy tissue on ultrasonography.
Retained pregnancy tissue can result in abnormal or heavy bleeding, require additional medical or surgical intervention, and is associated with endometrial inflammation and infection. Approximately 1 in every 4 women undergoing hysteroscopic resection of retained products are found to have evidence of endometritis in the resected tissue.14 This number is even higher in women with a diagnosis of recurrent pregnancy loss (62%).15
These complications from retained products of conception can be avoided with the hysteroscopic approach due to the direct visualization of the tissue removal. This benefit may be particularly beneficial in patients with known abnormal uterine cavities, such as those with Müllerian anomalies, uterine leiomyomas, preexisting adhesions, and history of placenta accreta spectrum disorder.
Continue to: Maximizing diagnostic yield...
Maximizing diagnostic yield
Many patients prefer surgical management of a missed abortion not for the procedural advantages, but to assist with tissue collection for cytogenetic testing of the pregnancy tissue. Given that embryonic chromosomal aneuploidy is implicated in 70% of miscarriages prior to 20 weeks’ gestation, genetic evaluation of the products of conception is commonly performed to identify a potential cause for the miscarriage.16 G-band karyotype is the most commonly performed genetic evaluation. Karyotype requires culturing of pregnancy tissue for 7-14 days to produce metaphase cells that are then chemically treated to arrest them at their maximally contracted stage. Cytogenetic evaluation is often curtailed when nonviable cells from products of conception fail to culture due to either time elapsed from diagnosis to demise or damage from tissue handling. Careful, directly observed tissue handling via a hysteroscopic approach may alleviate culture failure secondary to tissue damage.
Another concern with cultures of products of conception is the potential for maternal cell contamination. Early studies from the 1970s noted a significant skew toward 46, XX karyotype results in miscarried tissue as compared with 46, XY results. It was not until microsatellite analysis technology was available that it was determined that the result was due to analysis of maternal cells instead of products of conception.17 A 2014 study by Levy and colleagues and another by Lathi and colleagues that utilized single-nucleotide polymorphism (SNP) microarray found that maternal cell contamination affected 22% of all miscarriage samples analyzed and over half of karyotypes with a 46, XX result.18,19
Traditional “blind” suction and curettage may inadvertently collect maternal endometrial tissue and contaminate the culture of fetal cells, limiting the validity of karyotype for products of conception.20 The hysteroscopic approach may provide a higher diagnostic yield for karyotype analysis of fetal tissue by the nature of targeted tissue sampling under direct visualization, minimizing maternal cell contamination. One retrospective study by Cholkeri-Singh and colleagues evaluated rates of fetal chromosome detection without maternal contamination in a total of 264 patients undergoing either suction curettage or hysteroscopic resection. They found that fetal chromosomal detection without contamination was significantly higher in the hysteroscopy group compared with the suction curettage group (88.5 vs 64.8%, P< .001).21 Additionally, biopsies of tissue under direct visualization may enable the diagnosis of a true placental mosaicism and the study of the individual karyotype of each embryo in dizygotic twin missed abortions.
Finally, a hysteroscopic approach may afford the opportunity to also perform morphologic evaluation of the intact early fetus furthering the diagnostic utility of the procedure. With hysteroscopy, the gestational sac is identified and carefully entered, allowing for complete visualization of the early fetus and assessment of anatomic malformations that may provide insight into the pregnancy loss (ie, embryoscopy). In one series of 272 patients with missed abortions, while nearly 75% of conceptuses had abnormal karyotypes, 18% were found to have gross morphologic defects with a normal karyotype.22
Bottom line
When faced with a patient with an early pregnancy loss, physicians should consider the decreased iatrogenic risks and improved diagnostic yield when deciding between D&C versus hysteroscopy for surgical management. There are certain patients with pre-existing risk factors that may stand to benefit the most (TABLE 2). Much like the opening case, those at risk for intrauterine adhesions, retained products of conception, or in whom a successful and accurate cytogenetic analysis is essential are the most likely to benefit from a hysteroscopic approach. The hysteroscopic approach also affords concurrent diagnosis and treatment of intrauterine pathology, such as leiomyomas and uterine septum, which are encountered approximately 12.5% of the time after one miscarriage and 29.4% of the time in patients with a history of more than one miscarriage.10 In the appropriately counseled patient and clinical setting, clinicians could also perform definitive surgical management during the same hysteroscopy. Finally, evaluation of the morphology of the demised fetus may provide additional information for patient counseling in those with euploid pregnancy losses.

CASE Resolved
Ultimately, our patient underwent complete hysteroscopic resection of the pregnancy tissue, which confirmed both a morphologically abnormal fetus and a 45, X karyotype of the products of conception. ●
CASE Concern for surgical management after repeat miscarriage
A 34-year-old woman (G3P0030) with a history of recurrent pregnancy loss was recently diagnosed with a 7-week missed abortion. After her second miscarriage, she had an evaluation for recurrent pregnancy loss which was unremarkable. Both prior miscarriages were managed with dilation & curettage (D&C), but cytogenetic testing of the tissue did not yield a result in either case. The karyotype from the first pregnancy resulted as 46, XX but was confirmed to be due to maternal cell contamination, and the karyotype from the second pregnancy resulted in cell culture failure. The patient is interested in surgical management for her current missed abortion to help with tissue collection for cytogenetic testing, she but is concerned about her risk of intrauterine adhesions with repeated uterine instrumentation given 2 prior D&Cs, one of which was complicated by retained products of conception.
How do you approach the surgical management of this patient with recurrent pregnancy loss?
Approximately 1 in every 8 recognized pregnancies results in miscarriage. The risk of loss is lowest in women with no history of miscarriage (11%), and increases by about 10% for each additional miscarriage, reaching 42% in women with 3 or more previous losses. The population prevalence of women who have had 1 miscarriage is 11%, 2 miscarriages is 2%, and 3 or more is <1%.1 While 90% of miscarriages occur in the first trimester, their etiology can be quite varied.2 A woman’s age is the most strongly associated risk factor, with both very young (<20 years) and older age (>35 years) groups at highest risk. This association is largely attributed to an age-related increase in embryonic chromosomal aneuploidies, of which trisomies, particularly trisomy 16, are the most common.3 Maternal anatomic anomalies such as leiomyomas, intrauterine adhesions, Müllerian anomalies, and adenomyosis have been linked to an increased risk of miscarriage in addition to several lifestyle and environmental factor exposures.1
Regardless of the etiology, women with recurrent miscarriage are exposed to the potential for iatrogenic harm from the management of their pregnancy loss, including intrauterine adhesions and retained products, which may negatively impact future reproductive attempts. The management of patients with recurrent miscarriages demands special attention to reduce the risk of iatrogenic harm, maximize diagnostic evaluation of the products of conception, and improve future reproductive outcomes.
Management strategies
First trimester pregnancy loss may be managed expectantly, medically, or surgically. Approximately 76% of women who opt for expectant management will successfully pass pregnancy tissue, but for 1 out of every 6 women it may take longer than 14 days.4 For patients who prefer to expedite this process, medication abortion is a highly effective and safe option. According to Schreiber and colleagues, a combination of mifepristone and misoprostol together resulted in expulsion in approximately 91% of 148 patients, although 9% still required surgical intervention for incomplete passage of tissue.5 Both expectant management and medical management strategies are associated with the potential for retained products of conception requiring subsequent instrumentation as well as tissue that is often unsuitable or contaminated for cytogenetic analysis.
The most definitive treatment option is surgical management via manual or electric vacuum aspiration or curettage, with efficacy approaching 99.6% in some series.6 While highly effective, even ultrasound-guided evacuation carries with it procedure-related risks that are of particular consequence for patients of reproductive age, including adhesion formation and retained products of conception.
In 1997, Goldenberg and colleagues reported on the use of hysteroscopy for the management of retained products of conception as a strategy to minimize trauma to the uterus and maximize excision of retained tissue, both of which reduce potential for adhesion formation.7 Based on these data, several groups have extended the use of hysteroscopic resection for retained tissue to upfront evacuation following pregnancy loss, in lieu of D&C.8,9 This approach allows for the direct visualization of the focal removal of the implanted pregnancy tissue, which can:
- decrease the risk of intrauterine adhesion formation
- decrease the risk of retained products of conception
- allow for directed tissue sampling to improve the accuracy of cytogenetic testing
- allow for detection of embryo anatomic anomalies that often go undetected on traditional cytogenetic analysis.
For the remainder of this article, we will discuss the advantages of hysteroscopic management of a missed abortion in greater detail.
Continue to: Hysteroscopic management...
Hysteroscopic management
Like aspiration or curettage, hysteroscopic management may be offered once the diagnosis of fetal demise is confirmed on ultrasonography. The procedure may be accomplished in the office setting or in the operative room with either morcellation or resectoscopic instruments. Morcellation allows for improved visibility during the procedure given the ability of continuous suction to manage tissue fragments in the surgical field, while resectoscopic instruments offer the added benefit of electrosurgery should bleeding that is unresponsive to increased distention pressure be encountered. Use of the cold loop of the resectoscope to accomplish evacuation is advocated to avoid the thermal damage to the endometrium with electrosurgery. Regardless of the chosen instrument, there are several potential benefits for a hysteroscopic approach over the traditional ultrasound-guided or blind D&C.
Reducing risk of iatrogenic harm
Intrauterine adhesions form secondary to trauma to the endometrial basalis layer, where a population of adult progenitor stem cells continuously work to regenerate the overlying functionalis layer. Once damaged, adhesions may form and range from thin, filmy adhesions to dense, cavity obliterating bands of scar tissue (FIGURE). The degree of severity and location of the adhesions account for the variable presentation that range from menstrual abnormalities to infertility and recurrent pregnancy loss. While several classification systems exist for scoring severity of adhesions, the American Fertility Society (now American Society for Reproductive Medicine) Classification system from 1988 is still commonly utilized (TABLE 1).


Intrauterine adhesions from D&C after pregnancy loss are not uncommon. A 2014 meta-analysis of 10 prospective studies including 912 women reported a pooled prevalence for intrauterine adhesions of 19.1% (95% confidence interval [CI], 12.8–27.5) on hysteroscopic evaluation within 12 months following curettage.10 Once formed, these adhesions are associated with long-term impairment in reproductive outcomes, regardless of if they were treated or not. In a long-term follow-up study of women with and without adhesions after recurrent D&C for miscarriage, women with treated adhesions reported lower live birth rates, longer time to pregnancy, higher rates of preterm birth and higher rates of peripartum complications compared with those without adhesions.11
Compared with curettage, hysteroscopy affords the surgeon complete visualization of the uterine cavity and tissue to be resected. This, in turn, minimizes trauma to the surrounding uterine cavity, minimizes the potential for post-procedural adhesion formation and their associated sequelae, and maximizes complete resection of tissue. Those treated with D&C appear to be significantly more likely to have adhesions than those treated via a hysteroscopic approach (30% vs 13%).12
Retained products of conception. Classically, a “gritty” sensation of the endometrium following evacuation of the uterus with a sharp curette has been used to indicate complete removal of tissue. The evolution from a nonvisualized procedure to ultrasound-guided vacuum aspiration of 1st trimester pregnancy tissue has been associated with a decreased risk of procedural complications and retained products of conception.13 However, even with intraoperative imaging, the risk of retained products of conception remains because it can be difficult to distinguish a small blood clot from retained pregnancy tissue on ultrasonography.
Retained pregnancy tissue can result in abnormal or heavy bleeding, require additional medical or surgical intervention, and is associated with endometrial inflammation and infection. Approximately 1 in every 4 women undergoing hysteroscopic resection of retained products are found to have evidence of endometritis in the resected tissue.14 This number is even higher in women with a diagnosis of recurrent pregnancy loss (62%).15
These complications from retained products of conception can be avoided with the hysteroscopic approach due to the direct visualization of the tissue removal. This benefit may be particularly beneficial in patients with known abnormal uterine cavities, such as those with Müllerian anomalies, uterine leiomyomas, preexisting adhesions, and history of placenta accreta spectrum disorder.
Continue to: Maximizing diagnostic yield...
Maximizing diagnostic yield
Many patients prefer surgical management of a missed abortion not for the procedural advantages, but to assist with tissue collection for cytogenetic testing of the pregnancy tissue. Given that embryonic chromosomal aneuploidy is implicated in 70% of miscarriages prior to 20 weeks’ gestation, genetic evaluation of the products of conception is commonly performed to identify a potential cause for the miscarriage.16 G-band karyotype is the most commonly performed genetic evaluation. Karyotype requires culturing of pregnancy tissue for 7-14 days to produce metaphase cells that are then chemically treated to arrest them at their maximally contracted stage. Cytogenetic evaluation is often curtailed when nonviable cells from products of conception fail to culture due to either time elapsed from diagnosis to demise or damage from tissue handling. Careful, directly observed tissue handling via a hysteroscopic approach may alleviate culture failure secondary to tissue damage.
Another concern with cultures of products of conception is the potential for maternal cell contamination. Early studies from the 1970s noted a significant skew toward 46, XX karyotype results in miscarried tissue as compared with 46, XY results. It was not until microsatellite analysis technology was available that it was determined that the result was due to analysis of maternal cells instead of products of conception.17 A 2014 study by Levy and colleagues and another by Lathi and colleagues that utilized single-nucleotide polymorphism (SNP) microarray found that maternal cell contamination affected 22% of all miscarriage samples analyzed and over half of karyotypes with a 46, XX result.18,19
Traditional “blind” suction and curettage may inadvertently collect maternal endometrial tissue and contaminate the culture of fetal cells, limiting the validity of karyotype for products of conception.20 The hysteroscopic approach may provide a higher diagnostic yield for karyotype analysis of fetal tissue by the nature of targeted tissue sampling under direct visualization, minimizing maternal cell contamination. One retrospective study by Cholkeri-Singh and colleagues evaluated rates of fetal chromosome detection without maternal contamination in a total of 264 patients undergoing either suction curettage or hysteroscopic resection. They found that fetal chromosomal detection without contamination was significantly higher in the hysteroscopy group compared with the suction curettage group (88.5 vs 64.8%, P< .001).21 Additionally, biopsies of tissue under direct visualization may enable the diagnosis of a true placental mosaicism and the study of the individual karyotype of each embryo in dizygotic twin missed abortions.
Finally, a hysteroscopic approach may afford the opportunity to also perform morphologic evaluation of the intact early fetus furthering the diagnostic utility of the procedure. With hysteroscopy, the gestational sac is identified and carefully entered, allowing for complete visualization of the early fetus and assessment of anatomic malformations that may provide insight into the pregnancy loss (ie, embryoscopy). In one series of 272 patients with missed abortions, while nearly 75% of conceptuses had abnormal karyotypes, 18% were found to have gross morphologic defects with a normal karyotype.22
Bottom line
When faced with a patient with an early pregnancy loss, physicians should consider the decreased iatrogenic risks and improved diagnostic yield when deciding between D&C versus hysteroscopy for surgical management. There are certain patients with pre-existing risk factors that may stand to benefit the most (TABLE 2). Much like the opening case, those at risk for intrauterine adhesions, retained products of conception, or in whom a successful and accurate cytogenetic analysis is essential are the most likely to benefit from a hysteroscopic approach. The hysteroscopic approach also affords concurrent diagnosis and treatment of intrauterine pathology, such as leiomyomas and uterine septum, which are encountered approximately 12.5% of the time after one miscarriage and 29.4% of the time in patients with a history of more than one miscarriage.10 In the appropriately counseled patient and clinical setting, clinicians could also perform definitive surgical management during the same hysteroscopy. Finally, evaluation of the morphology of the demised fetus may provide additional information for patient counseling in those with euploid pregnancy losses.

CASE Resolved
Ultimately, our patient underwent complete hysteroscopic resection of the pregnancy tissue, which confirmed both a morphologically abnormal fetus and a 45, X karyotype of the products of conception. ●
- Quenby S, Gallos ID, Dhillon-Smith RK, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021;397:1658-1667.
- Kolte AM, Westergaard D, Lidegaard Ø, et al. Chance of live birth: a nationwide, registry-based cohort study. Hum Reprod Oxf Engl. 2021;36:1065-1073.
- Magnus MC, Wilcox AJ, Morken N-H, et al. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:869.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873-875.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Ireland LD, Gatter M, Chen AY. Medical compared with surgical abortion for effective pregnancy termination in the first trimester. Obstet Gynecol. 2015;126:22-28.
- Goldenberg M, Schiff E, Achiron R, et al. Managing residual trophoblastic tissue. Hysteroscopy for directing curettage. J Reprod Med. 1997;42:26-28.
- Weinberg S, Pansky M, Burshtein I, et al. A pilot study of guided conservative hysteroscopic evacuation of early miscarriage. J Minim Invasive Gynecol. 2021;28:1860-1867.
- Young S, Miller CE. Hysteroscopic resection for management of early pregnancy loss: a case report and literature review. FS Rep. 2022;3:163-167.
- Hooker AB, Lemmers M, Thurkow AL, et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update. 2014;20:262-278.
- Hooker AB, de Leeuw RA, Twisk JWR, et al. Reproductive performance of women with and without intrauterine adhesions following recurrent dilatation and curettage for miscarriage: long-term follow-up of a randomized controlled trial. Hum Reprod. 2021;36:70-81.
- Hooker AB, Aydin H, Brölmann HAM, et al. Longterm complications and reproductive outcome after the management of retained products of conception: a systematic review. Fertil Steril. 2016;105:156-164.e1-e2.
- Debby A, Malinger G, Harow E, et al. Transvaginal ultrasound after first-trimester uterine evacuation reduces the incidence of retained products of conception. Ultrasound Obstet Gynecol. 2006;27:61-64.
- Elder S, Bortoletto P, Romanski PA, et al. Chronic endometritis in women with suspected retained products of conception and their reproductive outcomes. Am J Reprod Immunol N Y N 1989. 2021;86:e13410.
- McQueen DB, Maniar KP, Hutchinson A, et al. Retained pregnancy tissue after miscarriage is associated with high rate of chronic endometritis. J Obstet Gynaecol J Inst Obstet Gynaecol. 2022;1-5.
- Soler A, Morales C, Mademont-Soler I, et al. Overview of chromosome abnormalities in first trimester miscarriages: a series of 1,011 consecutive chorionic villi sample karyotypes. Cytogenet Genome Res. 2017;152:81-89.
- Jarrett KL, Michaelis RC, Phelan MC, et al. Microsatellite analysis reveals a high incidence of maternal cell contamination in 46, XX products of conception consisting of villi or a combination of villi and membranous material. Am J Obstet Gynecol. 2001;185:198-203.
- Levy B, Sigurjonsson S, Pettersen B, et al. Genomic imbalance in products of conception: single-nucleotide polymorphism chromosomal microarray analysis. Obstet Gynecol. 2014;124:202-209.
- Lathi RB, Gustin SLF, Keller J, et al. Reliability of 46, XX results on miscarriage specimens: a review of 1,222 first-trimester miscarriage specimens. Fertil Steril. 2014;101:178-182.
- Chung JPW, Li Y, Law TSM, et al. Ultrasound-guided manual vacuum aspiration is an optimal method for obtaining products of conception from early pregnancy loss for cytogenetic testing. Int J Biochem Cell Biol. 2022;147:106226.
- Cholkeri-Singh A, Zamfirova I, Miller CE. Increased fetal chromosome detection with the use of operative hysteroscopy during evacuation of products of conception for diagnosed miscarriage. J Minim Invasive Gynecol. 2020;27:160-165.
- Philipp T, Philipp K, Reiner A, et al. Embryoscopic and cytogenetic analysis of 233 missed abortions: factors involved in the pathogenesis of developmental defects of early failed pregnancies. Hum Reprod. 2003;18:1724-1732.
- Quenby S, Gallos ID, Dhillon-Smith RK, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021;397:1658-1667.
- Kolte AM, Westergaard D, Lidegaard Ø, et al. Chance of live birth: a nationwide, registry-based cohort study. Hum Reprod Oxf Engl. 2021;36:1065-1073.
- Magnus MC, Wilcox AJ, Morken N-H, et al. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:869.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873-875.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Ireland LD, Gatter M, Chen AY. Medical compared with surgical abortion for effective pregnancy termination in the first trimester. Obstet Gynecol. 2015;126:22-28.
- Goldenberg M, Schiff E, Achiron R, et al. Managing residual trophoblastic tissue. Hysteroscopy for directing curettage. J Reprod Med. 1997;42:26-28.
- Weinberg S, Pansky M, Burshtein I, et al. A pilot study of guided conservative hysteroscopic evacuation of early miscarriage. J Minim Invasive Gynecol. 2021;28:1860-1867.
- Young S, Miller CE. Hysteroscopic resection for management of early pregnancy loss: a case report and literature review. FS Rep. 2022;3:163-167.
- Hooker AB, Lemmers M, Thurkow AL, et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update. 2014;20:262-278.
- Hooker AB, de Leeuw RA, Twisk JWR, et al. Reproductive performance of women with and without intrauterine adhesions following recurrent dilatation and curettage for miscarriage: long-term follow-up of a randomized controlled trial. Hum Reprod. 2021;36:70-81.
- Hooker AB, Aydin H, Brölmann HAM, et al. Longterm complications and reproductive outcome after the management of retained products of conception: a systematic review. Fertil Steril. 2016;105:156-164.e1-e2.
- Debby A, Malinger G, Harow E, et al. Transvaginal ultrasound after first-trimester uterine evacuation reduces the incidence of retained products of conception. Ultrasound Obstet Gynecol. 2006;27:61-64.
- Elder S, Bortoletto P, Romanski PA, et al. Chronic endometritis in women with suspected retained products of conception and their reproductive outcomes. Am J Reprod Immunol N Y N 1989. 2021;86:e13410.
- McQueen DB, Maniar KP, Hutchinson A, et al. Retained pregnancy tissue after miscarriage is associated with high rate of chronic endometritis. J Obstet Gynaecol J Inst Obstet Gynaecol. 2022;1-5.
- Soler A, Morales C, Mademont-Soler I, et al. Overview of chromosome abnormalities in first trimester miscarriages: a series of 1,011 consecutive chorionic villi sample karyotypes. Cytogenet Genome Res. 2017;152:81-89.
- Jarrett KL, Michaelis RC, Phelan MC, et al. Microsatellite analysis reveals a high incidence of maternal cell contamination in 46, XX products of conception consisting of villi or a combination of villi and membranous material. Am J Obstet Gynecol. 2001;185:198-203.
- Levy B, Sigurjonsson S, Pettersen B, et al. Genomic imbalance in products of conception: single-nucleotide polymorphism chromosomal microarray analysis. Obstet Gynecol. 2014;124:202-209.
- Lathi RB, Gustin SLF, Keller J, et al. Reliability of 46, XX results on miscarriage specimens: a review of 1,222 first-trimester miscarriage specimens. Fertil Steril. 2014;101:178-182.
- Chung JPW, Li Y, Law TSM, et al. Ultrasound-guided manual vacuum aspiration is an optimal method for obtaining products of conception from early pregnancy loss for cytogenetic testing. Int J Biochem Cell Biol. 2022;147:106226.
- Cholkeri-Singh A, Zamfirova I, Miller CE. Increased fetal chromosome detection with the use of operative hysteroscopy during evacuation of products of conception for diagnosed miscarriage. J Minim Invasive Gynecol. 2020;27:160-165.
- Philipp T, Philipp K, Reiner A, et al. Embryoscopic and cytogenetic analysis of 233 missed abortions: factors involved in the pathogenesis of developmental defects of early failed pregnancies. Hum Reprod. 2003;18:1724-1732.
Hormonal management of gender-diverse patients: SOC8 updates
In September, the World Professional Association for Transgender Health released its much-anticipated standards of care (SOC8). While this update has unfortunately received intense scrutiny for its guidance about gender-diverse adolescents and youth, the SOC8 is their most evidence-based version to date. Recommendations were developed based on data from independent systematic literature reviews, background reviews, and expert opinions.1 These guidelines also recognize knowledge deficits and are intended to be flexible to meet the individual needs of transgender patients. While the scope of this column will not delve into all 258 pages of these new standards, it will highlight pertinent information on hormonal management.
Ever since the original publication of the standards of care in 1979, gender-affirming hormone therapy (GAHT) has been considered medically necessary. The approach to GAHT depends on the patient’s goals and the age at which the patient is seeking to medically transition. Given the complexity of GAHT for transgender youth and adolescents, this article will focus primarily on adult patients.
There are a few pertinent differences in the management and monitoring of GAHT in adults. For patients assigned female at birth, testosterone is the primary modality by which patients can achieve masculinizing features. GAHT for patients assigned male at birth often consists of estrogen and an androgen-lowering medication. Like its predecessor, SOC8 recommends against prescribing ethinyl estradiol because of its marked association with thromboembolic events.
While the formulations of estrogen (oral, injectable, and patches) and hormone blockers (finasteride, spironolactone, gonadotropin-releasing hormone agonists, and bicalutamide) are discussed in prior standards of care, SOC8 further delineates their utilization. It suggests that transdermal estrogen should be considered in transgender women over the age of 45 who are at high risk for developing a venous thromboembolism or have a previous history of thromboembolism. Furthermore, SOC8 establishes spironolactone as the mainstay for androgen blockage and discourages routine usage of bicalutamide and finasteride because of a lack of safety data and questionable efficacy.1 Even though some patients anecdotally report increased breast growth with progesterone supplementation, there is insufficient evidence to regularly prescribe progesterone for breast development.1
Both WPATH and the Endocrine Society recommend checking serum levels of sex hormones every 3 months during the first year until stable levels are achieved, then once or twice a year thereafter.1 Hormone levels should be maintained at physiologic concentrations of the targeted gender. Some patients on feminizing GAHT often request evaluation of estrone/estradiol ratios as there was an assumption that higher ratios were associated with antagonistic effects on breast development. However, recent published evidence refutes this claim and estrone/estradiol ratios need not be measured.1
In addition to monitoring sex hormone levels, providers should check the metabolic effects that can be associated with GAHT. Both testosterone and estrogen can influence lipid panels: Testosterone can increase the red blood cell count, and spironolactone may cause hyperkalemia. While the SOC7 previously encouraged assessment of these laboratory values every 3 months, the new guidelines are more flexible in the frequency of testing of asymptomatic individuals as there is no strong evidence from published studies that supports these 3-month intervals.1
Providers are responsible for informing patients about the possible effects of GAHT on fertility. Estrogen often will cause a reduction in spermatogenesis, which may be irreversible. Patients who plan on taking estrogen should be counseled regarding sperm cryopreservation prior to starting GAHT. Even though testosterone inhibits ovulation and induces menstrual suppression, patients often regain their fertility after cessation of testosterone therapy. However, given the significant knowledge deficit about long-term fertility in transmasculine patients, providers should still offer oocyte or embryo cryopreservation.
Health care providers should collaborate with surgeons regarding preoperative and postoperative GAHT. To mitigate the risk of thromboembolism, many surgeons would stop hormones 1-4 weeks before and after gender-affirming surgery. Recent evidence does not support this practice, as studies indicate no increased risk for venous thromboembolism in individuals on GAHT undergoing surgery. These studies are consistent with other well-established guidelines on preoperative management of cisgender women taking estrogen or progestins. As exogenous sex steroids are necessary for bone health in patients who undergo gonadectomy, surgeons and other health care providers should educate patients on the importance of continuing GAHT.
There are many procedures available for gender-affirming surgery. Many of these surgeries involve three regions: the face, chest/breast, and/or genitalia (both internal and external). Prior to making a surgical referral, providers should be familiar with the surgeon’s scope of practice, performance measures, and surgical outcomes.1 For the first time, the SOC8 also addresses the surgical training of the providers who offer these procedures. While gender-affirming surgery can be performed by a variety of different specialists, training and documented supervision (often by an existing expert in gender-affirming surgery) is essential. Maintaining an active practice in these procedures, tracking surgical outcomes, and continuing education within the field of gender-affirming surgery are additional requirements for surgeons performing these complex operations.1
As their name implies, the SOC8 attempts to create a standardized guide to assist practitioners caring for gender-diverse patients. It’s important for providers to be familiar with updates while also recognizing the evolving nature of this rapidly growing field.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
Reference
1. World Professional Association for Transgender Health. Standards of care for the health of transgender and gender diverse people, Version 8. Int J Transgend Health. 2022 Sep 15. doi: 10.1080/26895269.2022.2100644.
In September, the World Professional Association for Transgender Health released its much-anticipated standards of care (SOC8). While this update has unfortunately received intense scrutiny for its guidance about gender-diverse adolescents and youth, the SOC8 is their most evidence-based version to date. Recommendations were developed based on data from independent systematic literature reviews, background reviews, and expert opinions.1 These guidelines also recognize knowledge deficits and are intended to be flexible to meet the individual needs of transgender patients. While the scope of this column will not delve into all 258 pages of these new standards, it will highlight pertinent information on hormonal management.
Ever since the original publication of the standards of care in 1979, gender-affirming hormone therapy (GAHT) has been considered medically necessary. The approach to GAHT depends on the patient’s goals and the age at which the patient is seeking to medically transition. Given the complexity of GAHT for transgender youth and adolescents, this article will focus primarily on adult patients.
There are a few pertinent differences in the management and monitoring of GAHT in adults. For patients assigned female at birth, testosterone is the primary modality by which patients can achieve masculinizing features. GAHT for patients assigned male at birth often consists of estrogen and an androgen-lowering medication. Like its predecessor, SOC8 recommends against prescribing ethinyl estradiol because of its marked association with thromboembolic events.
While the formulations of estrogen (oral, injectable, and patches) and hormone blockers (finasteride, spironolactone, gonadotropin-releasing hormone agonists, and bicalutamide) are discussed in prior standards of care, SOC8 further delineates their utilization. It suggests that transdermal estrogen should be considered in transgender women over the age of 45 who are at high risk for developing a venous thromboembolism or have a previous history of thromboembolism. Furthermore, SOC8 establishes spironolactone as the mainstay for androgen blockage and discourages routine usage of bicalutamide and finasteride because of a lack of safety data and questionable efficacy.1 Even though some patients anecdotally report increased breast growth with progesterone supplementation, there is insufficient evidence to regularly prescribe progesterone for breast development.1
Both WPATH and the Endocrine Society recommend checking serum levels of sex hormones every 3 months during the first year until stable levels are achieved, then once or twice a year thereafter.1 Hormone levels should be maintained at physiologic concentrations of the targeted gender. Some patients on feminizing GAHT often request evaluation of estrone/estradiol ratios as there was an assumption that higher ratios were associated with antagonistic effects on breast development. However, recent published evidence refutes this claim and estrone/estradiol ratios need not be measured.1
In addition to monitoring sex hormone levels, providers should check the metabolic effects that can be associated with GAHT. Both testosterone and estrogen can influence lipid panels: Testosterone can increase the red blood cell count, and spironolactone may cause hyperkalemia. While the SOC7 previously encouraged assessment of these laboratory values every 3 months, the new guidelines are more flexible in the frequency of testing of asymptomatic individuals as there is no strong evidence from published studies that supports these 3-month intervals.1
Providers are responsible for informing patients about the possible effects of GAHT on fertility. Estrogen often will cause a reduction in spermatogenesis, which may be irreversible. Patients who plan on taking estrogen should be counseled regarding sperm cryopreservation prior to starting GAHT. Even though testosterone inhibits ovulation and induces menstrual suppression, patients often regain their fertility after cessation of testosterone therapy. However, given the significant knowledge deficit about long-term fertility in transmasculine patients, providers should still offer oocyte or embryo cryopreservation.
Health care providers should collaborate with surgeons regarding preoperative and postoperative GAHT. To mitigate the risk of thromboembolism, many surgeons would stop hormones 1-4 weeks before and after gender-affirming surgery. Recent evidence does not support this practice, as studies indicate no increased risk for venous thromboembolism in individuals on GAHT undergoing surgery. These studies are consistent with other well-established guidelines on preoperative management of cisgender women taking estrogen or progestins. As exogenous sex steroids are necessary for bone health in patients who undergo gonadectomy, surgeons and other health care providers should educate patients on the importance of continuing GAHT.
There are many procedures available for gender-affirming surgery. Many of these surgeries involve three regions: the face, chest/breast, and/or genitalia (both internal and external). Prior to making a surgical referral, providers should be familiar with the surgeon’s scope of practice, performance measures, and surgical outcomes.1 For the first time, the SOC8 also addresses the surgical training of the providers who offer these procedures. While gender-affirming surgery can be performed by a variety of different specialists, training and documented supervision (often by an existing expert in gender-affirming surgery) is essential. Maintaining an active practice in these procedures, tracking surgical outcomes, and continuing education within the field of gender-affirming surgery are additional requirements for surgeons performing these complex operations.1
As their name implies, the SOC8 attempts to create a standardized guide to assist practitioners caring for gender-diverse patients. It’s important for providers to be familiar with updates while also recognizing the evolving nature of this rapidly growing field.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
Reference
1. World Professional Association for Transgender Health. Standards of care for the health of transgender and gender diverse people, Version 8. Int J Transgend Health. 2022 Sep 15. doi: 10.1080/26895269.2022.2100644.
In September, the World Professional Association for Transgender Health released its much-anticipated standards of care (SOC8). While this update has unfortunately received intense scrutiny for its guidance about gender-diverse adolescents and youth, the SOC8 is their most evidence-based version to date. Recommendations were developed based on data from independent systematic literature reviews, background reviews, and expert opinions.1 These guidelines also recognize knowledge deficits and are intended to be flexible to meet the individual needs of transgender patients. While the scope of this column will not delve into all 258 pages of these new standards, it will highlight pertinent information on hormonal management.
Ever since the original publication of the standards of care in 1979, gender-affirming hormone therapy (GAHT) has been considered medically necessary. The approach to GAHT depends on the patient’s goals and the age at which the patient is seeking to medically transition. Given the complexity of GAHT for transgender youth and adolescents, this article will focus primarily on adult patients.
There are a few pertinent differences in the management and monitoring of GAHT in adults. For patients assigned female at birth, testosterone is the primary modality by which patients can achieve masculinizing features. GAHT for patients assigned male at birth often consists of estrogen and an androgen-lowering medication. Like its predecessor, SOC8 recommends against prescribing ethinyl estradiol because of its marked association with thromboembolic events.
While the formulations of estrogen (oral, injectable, and patches) and hormone blockers (finasteride, spironolactone, gonadotropin-releasing hormone agonists, and bicalutamide) are discussed in prior standards of care, SOC8 further delineates their utilization. It suggests that transdermal estrogen should be considered in transgender women over the age of 45 who are at high risk for developing a venous thromboembolism or have a previous history of thromboembolism. Furthermore, SOC8 establishes spironolactone as the mainstay for androgen blockage and discourages routine usage of bicalutamide and finasteride because of a lack of safety data and questionable efficacy.1 Even though some patients anecdotally report increased breast growth with progesterone supplementation, there is insufficient evidence to regularly prescribe progesterone for breast development.1
Both WPATH and the Endocrine Society recommend checking serum levels of sex hormones every 3 months during the first year until stable levels are achieved, then once or twice a year thereafter.1 Hormone levels should be maintained at physiologic concentrations of the targeted gender. Some patients on feminizing GAHT often request evaluation of estrone/estradiol ratios as there was an assumption that higher ratios were associated with antagonistic effects on breast development. However, recent published evidence refutes this claim and estrone/estradiol ratios need not be measured.1
In addition to monitoring sex hormone levels, providers should check the metabolic effects that can be associated with GAHT. Both testosterone and estrogen can influence lipid panels: Testosterone can increase the red blood cell count, and spironolactone may cause hyperkalemia. While the SOC7 previously encouraged assessment of these laboratory values every 3 months, the new guidelines are more flexible in the frequency of testing of asymptomatic individuals as there is no strong evidence from published studies that supports these 3-month intervals.1
Providers are responsible for informing patients about the possible effects of GAHT on fertility. Estrogen often will cause a reduction in spermatogenesis, which may be irreversible. Patients who plan on taking estrogen should be counseled regarding sperm cryopreservation prior to starting GAHT. Even though testosterone inhibits ovulation and induces menstrual suppression, patients often regain their fertility after cessation of testosterone therapy. However, given the significant knowledge deficit about long-term fertility in transmasculine patients, providers should still offer oocyte or embryo cryopreservation.
Health care providers should collaborate with surgeons regarding preoperative and postoperative GAHT. To mitigate the risk of thromboembolism, many surgeons would stop hormones 1-4 weeks before and after gender-affirming surgery. Recent evidence does not support this practice, as studies indicate no increased risk for venous thromboembolism in individuals on GAHT undergoing surgery. These studies are consistent with other well-established guidelines on preoperative management of cisgender women taking estrogen or progestins. As exogenous sex steroids are necessary for bone health in patients who undergo gonadectomy, surgeons and other health care providers should educate patients on the importance of continuing GAHT.
There are many procedures available for gender-affirming surgery. Many of these surgeries involve three regions: the face, chest/breast, and/or genitalia (both internal and external). Prior to making a surgical referral, providers should be familiar with the surgeon’s scope of practice, performance measures, and surgical outcomes.1 For the first time, the SOC8 also addresses the surgical training of the providers who offer these procedures. While gender-affirming surgery can be performed by a variety of different specialists, training and documented supervision (often by an existing expert in gender-affirming surgery) is essential. Maintaining an active practice in these procedures, tracking surgical outcomes, and continuing education within the field of gender-affirming surgery are additional requirements for surgeons performing these complex operations.1
As their name implies, the SOC8 attempts to create a standardized guide to assist practitioners caring for gender-diverse patients. It’s important for providers to be familiar with updates while also recognizing the evolving nature of this rapidly growing field.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
Reference
1. World Professional Association for Transgender Health. Standards of care for the health of transgender and gender diverse people, Version 8. Int J Transgend Health. 2022 Sep 15. doi: 10.1080/26895269.2022.2100644.
Nurse practitioner fined $20k for advertising herself as ‘Doctor Sarah’
Last month, the San Luis Obispo County, California, District Attorney Dan Dow filed a complaint against Sarah Erny, RN, NP, citing unfair business practices and unprofessional conduct.
According to court documents, California’s Medical Practice Act does not permit individuals to refer to themselves as “doctor, physician, or any other terms or letters indicating or implying that he or she is a physician and surgeon ... without having ... a certificate as a physician and surgeon.”
Individuals who misrepresent themselves are subject to misdemeanor charges and civil penalties.
In addition to the fine, Ms. Erny agreed to refrain from referring to herself as a doctor in her practice and on social media. She has already deleted her Twitter account.
The case underscores tensions between physicians fighting to preserve their scope of practice and the allied professionals that U.S. lawmakers increasingly see as a less expensive way to improve access to health care.
The American Medical Association and specialty groups strongly oppose a new bill, the Improving Care and Access to Nurses Act, that would expand the scope of practice for nurse practitioners and physician assistants.
Court records show that Ms. Erny earned a doctor of nursing practice (DNP) degree from Vanderbilt University, Nashville, Tenn., and that she met the state requirements to obtain licensure as a registered nurse and nurse practitioner. In 2018, she opened a practice in Arroyo Grande, California, called Holistic Women’s Healing, where she provided medical services and drug supplements to patients.
She also entered a collaborative agreement with ob.gyn. Anika Moore, MD, for approximately 3 years. Dr. Moore’s medical practice was in another county and state, and the physician returned every 2 to 3 months to review a portion of Ms. Erny’s patient files.
Ms. Erny and Dr. Moore terminated the collaborative agreement in March, according to court documents.
However, Mr. Dow alleged that Ms. Erny regularly referred to herself as “Dr. Sarah” or “Dr. Sarah Erny” in her online advertising and social media accounts. Her patients “were so proud of her” that they called her doctor, and her supervising physician instructed staff to do the same.
Mr. Dow said Ms. Erny did not clearly advise the public that she was not a medical doctor and failed to identify her supervising physician. “Simply put, there is a great need for health care providers to state their level of training and licensing clearly and honestly in all of their advertising and marketing materials,” he said in a press release.
In California, nurse practitioners who have been certified by the Board of Registered Nursing may use the following titles: Advanced Practice Registered Nurse; Certified Nurse Practitioner; APRN-CNP; RN and NP; or a combination of other letters or words to identify specialization, such as adult nurse practitioner, pediatric nurse practitioner, obstetrical-gynecological nurse practitioner, and family nurse practitioner.
As educational requirements shift for advanced practice clinicians, similar cases will likely emerge, said Grant Martsolf, PhD, MPH, RN, FAAN, professor at the University of Pittsburgh School of Nursing.
“Scope of practice is governed by states, [so they] will have to figure [it] out as more professional disciplines move to clinical doctorates as the entry to practice. Pharma, [physical therapy], and [occupational therapy] have already done this, and advanced practice nursing is on its way. [Certified registered nurse anesthetists] are already required to get a DNP to sit for certification,” he said.
More guidance is needed, especially when considering other professions like dentists, clinical psychologists, and individuals with clinical or research doctorates who often call themselves doctors, Dr. Martsolf said.
“It seems that the honorific of ‘Dr.’ emerges from the degree, not from being a physician or surgeon,” he said.
Beyond the false advertising, Mr. Dow alleged that Ms. Erny did not file a fictitious business name statement for 2020 and 2021 – a requirement under the California Business and Professions Code to identify who is operating the business.
A version of this article first appeared on Medscape.com.
Last month, the San Luis Obispo County, California, District Attorney Dan Dow filed a complaint against Sarah Erny, RN, NP, citing unfair business practices and unprofessional conduct.
According to court documents, California’s Medical Practice Act does not permit individuals to refer to themselves as “doctor, physician, or any other terms or letters indicating or implying that he or she is a physician and surgeon ... without having ... a certificate as a physician and surgeon.”
Individuals who misrepresent themselves are subject to misdemeanor charges and civil penalties.
In addition to the fine, Ms. Erny agreed to refrain from referring to herself as a doctor in her practice and on social media. She has already deleted her Twitter account.
The case underscores tensions between physicians fighting to preserve their scope of practice and the allied professionals that U.S. lawmakers increasingly see as a less expensive way to improve access to health care.
The American Medical Association and specialty groups strongly oppose a new bill, the Improving Care and Access to Nurses Act, that would expand the scope of practice for nurse practitioners and physician assistants.
Court records show that Ms. Erny earned a doctor of nursing practice (DNP) degree from Vanderbilt University, Nashville, Tenn., and that she met the state requirements to obtain licensure as a registered nurse and nurse practitioner. In 2018, she opened a practice in Arroyo Grande, California, called Holistic Women’s Healing, where she provided medical services and drug supplements to patients.
She also entered a collaborative agreement with ob.gyn. Anika Moore, MD, for approximately 3 years. Dr. Moore’s medical practice was in another county and state, and the physician returned every 2 to 3 months to review a portion of Ms. Erny’s patient files.
Ms. Erny and Dr. Moore terminated the collaborative agreement in March, according to court documents.
However, Mr. Dow alleged that Ms. Erny regularly referred to herself as “Dr. Sarah” or “Dr. Sarah Erny” in her online advertising and social media accounts. Her patients “were so proud of her” that they called her doctor, and her supervising physician instructed staff to do the same.
Mr. Dow said Ms. Erny did not clearly advise the public that she was not a medical doctor and failed to identify her supervising physician. “Simply put, there is a great need for health care providers to state their level of training and licensing clearly and honestly in all of their advertising and marketing materials,” he said in a press release.
In California, nurse practitioners who have been certified by the Board of Registered Nursing may use the following titles: Advanced Practice Registered Nurse; Certified Nurse Practitioner; APRN-CNP; RN and NP; or a combination of other letters or words to identify specialization, such as adult nurse practitioner, pediatric nurse practitioner, obstetrical-gynecological nurse practitioner, and family nurse practitioner.
As educational requirements shift for advanced practice clinicians, similar cases will likely emerge, said Grant Martsolf, PhD, MPH, RN, FAAN, professor at the University of Pittsburgh School of Nursing.
“Scope of practice is governed by states, [so they] will have to figure [it] out as more professional disciplines move to clinical doctorates as the entry to practice. Pharma, [physical therapy], and [occupational therapy] have already done this, and advanced practice nursing is on its way. [Certified registered nurse anesthetists] are already required to get a DNP to sit for certification,” he said.
More guidance is needed, especially when considering other professions like dentists, clinical psychologists, and individuals with clinical or research doctorates who often call themselves doctors, Dr. Martsolf said.
“It seems that the honorific of ‘Dr.’ emerges from the degree, not from being a physician or surgeon,” he said.
Beyond the false advertising, Mr. Dow alleged that Ms. Erny did not file a fictitious business name statement for 2020 and 2021 – a requirement under the California Business and Professions Code to identify who is operating the business.
A version of this article first appeared on Medscape.com.
Last month, the San Luis Obispo County, California, District Attorney Dan Dow filed a complaint against Sarah Erny, RN, NP, citing unfair business practices and unprofessional conduct.
According to court documents, California’s Medical Practice Act does not permit individuals to refer to themselves as “doctor, physician, or any other terms or letters indicating or implying that he or she is a physician and surgeon ... without having ... a certificate as a physician and surgeon.”
Individuals who misrepresent themselves are subject to misdemeanor charges and civil penalties.
In addition to the fine, Ms. Erny agreed to refrain from referring to herself as a doctor in her practice and on social media. She has already deleted her Twitter account.
The case underscores tensions between physicians fighting to preserve their scope of practice and the allied professionals that U.S. lawmakers increasingly see as a less expensive way to improve access to health care.
The American Medical Association and specialty groups strongly oppose a new bill, the Improving Care and Access to Nurses Act, that would expand the scope of practice for nurse practitioners and physician assistants.
Court records show that Ms. Erny earned a doctor of nursing practice (DNP) degree from Vanderbilt University, Nashville, Tenn., and that she met the state requirements to obtain licensure as a registered nurse and nurse practitioner. In 2018, she opened a practice in Arroyo Grande, California, called Holistic Women’s Healing, where she provided medical services and drug supplements to patients.
She also entered a collaborative agreement with ob.gyn. Anika Moore, MD, for approximately 3 years. Dr. Moore’s medical practice was in another county and state, and the physician returned every 2 to 3 months to review a portion of Ms. Erny’s patient files.
Ms. Erny and Dr. Moore terminated the collaborative agreement in March, according to court documents.
However, Mr. Dow alleged that Ms. Erny regularly referred to herself as “Dr. Sarah” or “Dr. Sarah Erny” in her online advertising and social media accounts. Her patients “were so proud of her” that they called her doctor, and her supervising physician instructed staff to do the same.
Mr. Dow said Ms. Erny did not clearly advise the public that she was not a medical doctor and failed to identify her supervising physician. “Simply put, there is a great need for health care providers to state their level of training and licensing clearly and honestly in all of their advertising and marketing materials,” he said in a press release.
In California, nurse practitioners who have been certified by the Board of Registered Nursing may use the following titles: Advanced Practice Registered Nurse; Certified Nurse Practitioner; APRN-CNP; RN and NP; or a combination of other letters or words to identify specialization, such as adult nurse practitioner, pediatric nurse practitioner, obstetrical-gynecological nurse practitioner, and family nurse practitioner.
As educational requirements shift for advanced practice clinicians, similar cases will likely emerge, said Grant Martsolf, PhD, MPH, RN, FAAN, professor at the University of Pittsburgh School of Nursing.
“Scope of practice is governed by states, [so they] will have to figure [it] out as more professional disciplines move to clinical doctorates as the entry to practice. Pharma, [physical therapy], and [occupational therapy] have already done this, and advanced practice nursing is on its way. [Certified registered nurse anesthetists] are already required to get a DNP to sit for certification,” he said.
More guidance is needed, especially when considering other professions like dentists, clinical psychologists, and individuals with clinical or research doctorates who often call themselves doctors, Dr. Martsolf said.
“It seems that the honorific of ‘Dr.’ emerges from the degree, not from being a physician or surgeon,” he said.
Beyond the false advertising, Mr. Dow alleged that Ms. Erny did not file a fictitious business name statement for 2020 and 2021 – a requirement under the California Business and Professions Code to identify who is operating the business.
A version of this article first appeared on Medscape.com.
Twins born from embryos frozen 30 years ago
In what is believed to be a record, twins in Oregon were born this past Halloween from embryos that were frozen in 1992.
The National Embryo Donation Center says the twins, named Lydia and Timothy Ridgeway, are the longest frozen embryos to result in live birth, CNN reported.
Lydia was born at 5 pounds, 11 ounces. Timothy was born at 6 pounds, 7 ounces.
“There is something mind-boggling about it,” Philip Ridgeway told CNN as he and wife, Rachel Ridgeway, held their newborns. “I was 5 years old when God gave life to Lydia and Timothy, and he’s been preserving that life ever since.”
The babies were a result of embryo donation, usually from parents who have extra embryos after successfully having babies via in vitro fertilization (IVF).
In the case of newborns Lydia and Timothy, their donor parents are an anonymous married couple. The husband was in his early 50s at the time, and the couple used a 34-year-old egg donor, CNN reported.
After the embryos sat in storage on the West Coast from 1992 to 2007, the donor parents donated them to the National Embryo Donation Center in Knoxville, Tenn.
“In a sense, they’re our oldest children, even though they’re our smallest children,” said Philip Ridgeway.
The couple already had four other children, ages 8, 6, 3, and one that’s almost 2. None of their other children was conceived via IVF or donors.
“We’ve never had in our minds a set number of children we’d like to have,” Philip Ridgeway said. “We’ve always thought we’ll have as many as God wants to give us, and ... when we heard about embryo adoption, we thought that’s something we would like to do.”
In an article for Harvard Medical School, fertility expert Ellen S. Glazer said there are countless IVF-created embryos whose future path has five options.
“Those embarking on an IVF cycle are often laser-focused on the baby they long for,” wrote Ms. Glazer, a clinical social worker whose practice focuses on reproductive issues. “Most hope a cycle will yield several embryos, because it frequently takes more than one embryo transfer to achieve a successful full-term pregnancy. Any remaining embryos may offer the hope of future pregnancies and additional children.”
If the embryos are not used, the five options are:
- Discard the remaining embryos.
- Have another child anyway, even if a larger family wasn’t the original plan.
- Donate the embryos to science.
- Donate the embryos to another person or couple.
- Decide not to decide. (In this situation, clinics use the term “abandon” when a family avoids contact and stops paying storage fees.)
For the Ridgeways, when they were offered information to help them choose among donated embryos, they decided to focus on those with the lowest identification numbers on the list.
“We weren’t looking to get the embryos that have been frozen the longest in the world,” Philip Ridgeway said. “We just wanted the ones that had been waiting the longest.”
A version of this article first appeared on WebMD.com.
In what is believed to be a record, twins in Oregon were born this past Halloween from embryos that were frozen in 1992.
The National Embryo Donation Center says the twins, named Lydia and Timothy Ridgeway, are the longest frozen embryos to result in live birth, CNN reported.
Lydia was born at 5 pounds, 11 ounces. Timothy was born at 6 pounds, 7 ounces.
“There is something mind-boggling about it,” Philip Ridgeway told CNN as he and wife, Rachel Ridgeway, held their newborns. “I was 5 years old when God gave life to Lydia and Timothy, and he’s been preserving that life ever since.”
The babies were a result of embryo donation, usually from parents who have extra embryos after successfully having babies via in vitro fertilization (IVF).
In the case of newborns Lydia and Timothy, their donor parents are an anonymous married couple. The husband was in his early 50s at the time, and the couple used a 34-year-old egg donor, CNN reported.
After the embryos sat in storage on the West Coast from 1992 to 2007, the donor parents donated them to the National Embryo Donation Center in Knoxville, Tenn.
“In a sense, they’re our oldest children, even though they’re our smallest children,” said Philip Ridgeway.
The couple already had four other children, ages 8, 6, 3, and one that’s almost 2. None of their other children was conceived via IVF or donors.
“We’ve never had in our minds a set number of children we’d like to have,” Philip Ridgeway said. “We’ve always thought we’ll have as many as God wants to give us, and ... when we heard about embryo adoption, we thought that’s something we would like to do.”
In an article for Harvard Medical School, fertility expert Ellen S. Glazer said there are countless IVF-created embryos whose future path has five options.
“Those embarking on an IVF cycle are often laser-focused on the baby they long for,” wrote Ms. Glazer, a clinical social worker whose practice focuses on reproductive issues. “Most hope a cycle will yield several embryos, because it frequently takes more than one embryo transfer to achieve a successful full-term pregnancy. Any remaining embryos may offer the hope of future pregnancies and additional children.”
If the embryos are not used, the five options are:
- Discard the remaining embryos.
- Have another child anyway, even if a larger family wasn’t the original plan.
- Donate the embryos to science.
- Donate the embryos to another person or couple.
- Decide not to decide. (In this situation, clinics use the term “abandon” when a family avoids contact and stops paying storage fees.)
For the Ridgeways, when they were offered information to help them choose among donated embryos, they decided to focus on those with the lowest identification numbers on the list.
“We weren’t looking to get the embryos that have been frozen the longest in the world,” Philip Ridgeway said. “We just wanted the ones that had been waiting the longest.”
A version of this article first appeared on WebMD.com.
In what is believed to be a record, twins in Oregon were born this past Halloween from embryos that were frozen in 1992.
The National Embryo Donation Center says the twins, named Lydia and Timothy Ridgeway, are the longest frozen embryos to result in live birth, CNN reported.
Lydia was born at 5 pounds, 11 ounces. Timothy was born at 6 pounds, 7 ounces.
“There is something mind-boggling about it,” Philip Ridgeway told CNN as he and wife, Rachel Ridgeway, held their newborns. “I was 5 years old when God gave life to Lydia and Timothy, and he’s been preserving that life ever since.”
The babies were a result of embryo donation, usually from parents who have extra embryos after successfully having babies via in vitro fertilization (IVF).
In the case of newborns Lydia and Timothy, their donor parents are an anonymous married couple. The husband was in his early 50s at the time, and the couple used a 34-year-old egg donor, CNN reported.
After the embryos sat in storage on the West Coast from 1992 to 2007, the donor parents donated them to the National Embryo Donation Center in Knoxville, Tenn.
“In a sense, they’re our oldest children, even though they’re our smallest children,” said Philip Ridgeway.
The couple already had four other children, ages 8, 6, 3, and one that’s almost 2. None of their other children was conceived via IVF or donors.
“We’ve never had in our minds a set number of children we’d like to have,” Philip Ridgeway said. “We’ve always thought we’ll have as many as God wants to give us, and ... when we heard about embryo adoption, we thought that’s something we would like to do.”
In an article for Harvard Medical School, fertility expert Ellen S. Glazer said there are countless IVF-created embryos whose future path has five options.
“Those embarking on an IVF cycle are often laser-focused on the baby they long for,” wrote Ms. Glazer, a clinical social worker whose practice focuses on reproductive issues. “Most hope a cycle will yield several embryos, because it frequently takes more than one embryo transfer to achieve a successful full-term pregnancy. Any remaining embryos may offer the hope of future pregnancies and additional children.”
If the embryos are not used, the five options are:
- Discard the remaining embryos.
- Have another child anyway, even if a larger family wasn’t the original plan.
- Donate the embryos to science.
- Donate the embryos to another person or couple.
- Decide not to decide. (In this situation, clinics use the term “abandon” when a family avoids contact and stops paying storage fees.)
For the Ridgeways, when they were offered information to help them choose among donated embryos, they decided to focus on those with the lowest identification numbers on the list.
“We weren’t looking to get the embryos that have been frozen the longest in the world,” Philip Ridgeway said. “We just wanted the ones that had been waiting the longest.”
A version of this article first appeared on WebMD.com.
As STDs proliferate, companies rush to market at-home test kits. But are they reliable?
Among the more remarkable legacies of the COVID-19 pandemic is how quickly federal regulators, the health care industry, and consumers moved to make at-home testing a reliable tool for managing a public health crisis.
But that fast-track focus is missing from another, less publicized epidemic: an explosion in sexually transmitted diseases that can cause chronic pain and infertility among infected adults and disable or kill infected newborns. The disparity has amplified calls from researchers, public health advocates, and health care companies urging the federal government to greenlight at-home testing kits that could vastly multiply the number of Americans testing for STDs.
Online shoppers can already choose from more than a dozen self-testing kits, typically ranging in price from $69 to $500, depending on the brand and the variety of infections they can detect.
But, except for HIV tests, the Food and Drug Administration hasn’t approved STD test kits for use outside a medical setting. That leaves consumers unsure about their reliability even as at-home use grows dramatically.
The STD epidemic is “out of control,” said Amesh Adalja, MD, a senior scholar at the Johns Hopkins University Center for Health Security. “We know we are missing diagnoses. We know that contact tracing is happening late or not at all. If we’re really serious about tackling the STD crisis, we have to get more people diagnosed.”
Preliminary data for 2021 showed nearly 2.5 million reported cases of chlamydia, gonorrhea, and syphilis in the United States, according to the Centers for Disease Control and Prevention. Reported cases of syphilis and gonorrhea have been climbing for about a decade. In its most recent prevalence estimate, the agency said that on any given day, one in five Americans are infected with any of eight common STDs.
The push to make at-home testing for STDs as easy and commonplace as at-home COVID and pregnancy testing is coming from several sectors. Public health officials say their overextended staffers can’t handle the staggering need for testing and surveillance. Diagnostic and pharmaceutical companies see a business opportunity in the unmet demand.
The medical science underpinning STD testing is not particularly new or mysterious. Depending on the test, it may involve collecting a urine sample, pricking a finger for blood, or swabbing the mouth, genitals, or anus for discharge or cell samples. Medical centers and community health clinics have performed such testing for decades.
The issue for regulators is whether sampling kits can be reliably adapted for in-home use. Unlike rapid antigen tests for COVID, which produce results in 15-20 minutes, the home STD kits on the market require patients to collect their own samples, and then package and mail them to a lab for analysis.
In the past 3 years, as the pandemic prompted clinics that provide low-cost care to drastically curtail in-person services, a number of public health departments – among them state agencies in Alabama, Alaska, and Maryland – have started mailing free STD test kits to residents. Universities and nonprofits are also spearheading at-home testing efforts.
And dozens of commercial enterprises are jumping into or ramping up direct-to-consumer sales. Everly Health, a digital health company that sells a variety of lab tests online, reported sales for its suite of STD kits grew 120% in the first half of this year compared with the first half of 2021.
CVS Health began selling its own bundled STD kit in October, priced at $99.99. Unlike most home kits, CVS’ version is available in stores.
Hologic, Abbott, and Molecular Testing Labs are among the companies urgently developing tests. And Cue Health, which sells COVID tests, is poised to launch a clinical trial for a rapid home test for chlamydia and gonorrhea that would set a new bar, providing results in about 20 minutes.
Alberto Gutierrez, who formerly led the FDA office that oversees diagnostic tests, said agency officials have been concerned about the reliability of home tests for years. The FDA wants companies to prove that home collection kits are as accurate as those used in clinics, and that samples don’t degrade during shipping.
“The agency doesn’t believe these tests are legally marketed at this point,” said Mr. Gutierrez, a partner at NDA Partners, a consulting firm that advises companies seeking to bring health care products to market.
“CVS should not be selling that test,” he added.
In response to KHN questions, the FDA said it considers home collection kits, which can include swabs, lancets, transport tubes, and chemicals to stabilize the samples, to be devices that require agency review. The FDA “generally does not comment” on whether it plans to take action on any specific case, the statement said.
CVS spokesperson Mary Gattuso said the pharmacy chain is following the law. “We are committed to ensuring the products we offer are safe, work as intended, comply with regulations, and satisfy customers,” Ms. Gattuso said.
Everly Health and other companies described their kits as laboratory-developed tests, akin to the diagnostics some hospitals create for in-house use. And they contend their tests can be legally marketed because their labs have been certified by a different agency, the Centers for Medicare & Medicaid Services.
“The instruments and assays used by the laboratories we use are comparable to – and often the same as – those used by the labs a doctor’s office uses,” said Liz Kwo, MD, chief medical officer at Everly Health. “Our at-home sample collection methods, like dried blood spots and saliva, have been widely used for decades.”
Home collection kits appeal to Uxmal Caldera, 27, of Miami Beach, who prefers to test in the privacy of his home. Mr. Caldera, who doesn’t have a car, said home testing saves him the time and expense of getting to a clinic.
Mr. Caldera has been testing himself for HIV and other STDs every 3 months for more than a year, part of routine monitoring for people taking PrEP, a regimen of daily pills to prevent HIV infection.
“Doing it by yourself is not hard at all,” said Mr. Caldera, who is uninsured but receives the tests free through a community foundation. “The instructions are really clear. I get the results in maybe 4 days. For sure, I would recommend it to other people.”
Leandro Mena, MD, director of the CDC’s division of STD prevention, said he would like to see at-home STD testing become as routine as home pregnancy tests. An estimated 16 million–20 million tests for gonorrhea and chlamydia are performed in the United States each year, Dr. Mena said. Widespread use of at-home STD testing could double or triple that number.
He noted that doctors have years of experience using home collection kits.
The Johns Hopkins Center for Point-of-Care Technologies Research for Sexually Transmitted Diseases has distributed roughly 23,000 at-home STD kits since 2004, said Charlotte Gaydos, DrPH, a principal investigator with the center. The FDA generally allows such use if it’s part of research overseen by medical professionals. The center’s tests are now used by the Alaska health department, as well as Native American tribes in Arizona and Oklahoma.
Dr. Gaydos has published dozens of studies establishing that home collection kits for diseases such as chlamydia and gonorrhea are accurate and easy to use.
“There’s a huge amount of data showing that home testing works,” said Dr. Gaydos.
But Dr. Gaydos noted that her studies have been limited to small sample sizes. She said she doesn’t have the millions of dollars in funding it would take to run the sort of comprehensive trial the FDA typically requires for approval.
Jenny Mahn, director of clinical and sexual health at the National Coalition of STD Directors, said many public health labs are reluctant to handle home kits. “The public health labs won’t touch it without FDA’s blessing.”
Public health clinics often provide STD testing at little to no cost, while health insurance typically covers in-person testing at a private practice. But most consumers pay out-of-pocket for direct-to-consumer kits. Commercial pricing puts them out of reach for many people, particularly teens and young adults, who account for nearly half of STDs.
Adalja said the FDA has a history of moving slowly on home testing. The agency spent 7 years evaluating the first home HIV test it approved, which hit the market in 2012.
“Home testing is the way of the future,” said Laura Lindberg, PhD, a professor of public health at Rutgers University, Piscataway, N.J. “The pandemic opened the door to testing and treatment at home without traveling to a health care provider, and we aren’t going to be able to put the genie back in the bottle.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Among the more remarkable legacies of the COVID-19 pandemic is how quickly federal regulators, the health care industry, and consumers moved to make at-home testing a reliable tool for managing a public health crisis.
But that fast-track focus is missing from another, less publicized epidemic: an explosion in sexually transmitted diseases that can cause chronic pain and infertility among infected adults and disable or kill infected newborns. The disparity has amplified calls from researchers, public health advocates, and health care companies urging the federal government to greenlight at-home testing kits that could vastly multiply the number of Americans testing for STDs.
Online shoppers can already choose from more than a dozen self-testing kits, typically ranging in price from $69 to $500, depending on the brand and the variety of infections they can detect.
But, except for HIV tests, the Food and Drug Administration hasn’t approved STD test kits for use outside a medical setting. That leaves consumers unsure about their reliability even as at-home use grows dramatically.
The STD epidemic is “out of control,” said Amesh Adalja, MD, a senior scholar at the Johns Hopkins University Center for Health Security. “We know we are missing diagnoses. We know that contact tracing is happening late or not at all. If we’re really serious about tackling the STD crisis, we have to get more people diagnosed.”
Preliminary data for 2021 showed nearly 2.5 million reported cases of chlamydia, gonorrhea, and syphilis in the United States, according to the Centers for Disease Control and Prevention. Reported cases of syphilis and gonorrhea have been climbing for about a decade. In its most recent prevalence estimate, the agency said that on any given day, one in five Americans are infected with any of eight common STDs.
The push to make at-home testing for STDs as easy and commonplace as at-home COVID and pregnancy testing is coming from several sectors. Public health officials say their overextended staffers can’t handle the staggering need for testing and surveillance. Diagnostic and pharmaceutical companies see a business opportunity in the unmet demand.
The medical science underpinning STD testing is not particularly new or mysterious. Depending on the test, it may involve collecting a urine sample, pricking a finger for blood, or swabbing the mouth, genitals, or anus for discharge or cell samples. Medical centers and community health clinics have performed such testing for decades.
The issue for regulators is whether sampling kits can be reliably adapted for in-home use. Unlike rapid antigen tests for COVID, which produce results in 15-20 minutes, the home STD kits on the market require patients to collect their own samples, and then package and mail them to a lab for analysis.
In the past 3 years, as the pandemic prompted clinics that provide low-cost care to drastically curtail in-person services, a number of public health departments – among them state agencies in Alabama, Alaska, and Maryland – have started mailing free STD test kits to residents. Universities and nonprofits are also spearheading at-home testing efforts.
And dozens of commercial enterprises are jumping into or ramping up direct-to-consumer sales. Everly Health, a digital health company that sells a variety of lab tests online, reported sales for its suite of STD kits grew 120% in the first half of this year compared with the first half of 2021.
CVS Health began selling its own bundled STD kit in October, priced at $99.99. Unlike most home kits, CVS’ version is available in stores.
Hologic, Abbott, and Molecular Testing Labs are among the companies urgently developing tests. And Cue Health, which sells COVID tests, is poised to launch a clinical trial for a rapid home test for chlamydia and gonorrhea that would set a new bar, providing results in about 20 minutes.
Alberto Gutierrez, who formerly led the FDA office that oversees diagnostic tests, said agency officials have been concerned about the reliability of home tests for years. The FDA wants companies to prove that home collection kits are as accurate as those used in clinics, and that samples don’t degrade during shipping.
“The agency doesn’t believe these tests are legally marketed at this point,” said Mr. Gutierrez, a partner at NDA Partners, a consulting firm that advises companies seeking to bring health care products to market.
“CVS should not be selling that test,” he added.
In response to KHN questions, the FDA said it considers home collection kits, which can include swabs, lancets, transport tubes, and chemicals to stabilize the samples, to be devices that require agency review. The FDA “generally does not comment” on whether it plans to take action on any specific case, the statement said.
CVS spokesperson Mary Gattuso said the pharmacy chain is following the law. “We are committed to ensuring the products we offer are safe, work as intended, comply with regulations, and satisfy customers,” Ms. Gattuso said.
Everly Health and other companies described their kits as laboratory-developed tests, akin to the diagnostics some hospitals create for in-house use. And they contend their tests can be legally marketed because their labs have been certified by a different agency, the Centers for Medicare & Medicaid Services.
“The instruments and assays used by the laboratories we use are comparable to – and often the same as – those used by the labs a doctor’s office uses,” said Liz Kwo, MD, chief medical officer at Everly Health. “Our at-home sample collection methods, like dried blood spots and saliva, have been widely used for decades.”
Home collection kits appeal to Uxmal Caldera, 27, of Miami Beach, who prefers to test in the privacy of his home. Mr. Caldera, who doesn’t have a car, said home testing saves him the time and expense of getting to a clinic.
Mr. Caldera has been testing himself for HIV and other STDs every 3 months for more than a year, part of routine monitoring for people taking PrEP, a regimen of daily pills to prevent HIV infection.
“Doing it by yourself is not hard at all,” said Mr. Caldera, who is uninsured but receives the tests free through a community foundation. “The instructions are really clear. I get the results in maybe 4 days. For sure, I would recommend it to other people.”
Leandro Mena, MD, director of the CDC’s division of STD prevention, said he would like to see at-home STD testing become as routine as home pregnancy tests. An estimated 16 million–20 million tests for gonorrhea and chlamydia are performed in the United States each year, Dr. Mena said. Widespread use of at-home STD testing could double or triple that number.
He noted that doctors have years of experience using home collection kits.
The Johns Hopkins Center for Point-of-Care Technologies Research for Sexually Transmitted Diseases has distributed roughly 23,000 at-home STD kits since 2004, said Charlotte Gaydos, DrPH, a principal investigator with the center. The FDA generally allows such use if it’s part of research overseen by medical professionals. The center’s tests are now used by the Alaska health department, as well as Native American tribes in Arizona and Oklahoma.
Dr. Gaydos has published dozens of studies establishing that home collection kits for diseases such as chlamydia and gonorrhea are accurate and easy to use.
“There’s a huge amount of data showing that home testing works,” said Dr. Gaydos.
But Dr. Gaydos noted that her studies have been limited to small sample sizes. She said she doesn’t have the millions of dollars in funding it would take to run the sort of comprehensive trial the FDA typically requires for approval.
Jenny Mahn, director of clinical and sexual health at the National Coalition of STD Directors, said many public health labs are reluctant to handle home kits. “The public health labs won’t touch it without FDA’s blessing.”
Public health clinics often provide STD testing at little to no cost, while health insurance typically covers in-person testing at a private practice. But most consumers pay out-of-pocket for direct-to-consumer kits. Commercial pricing puts them out of reach for many people, particularly teens and young adults, who account for nearly half of STDs.
Adalja said the FDA has a history of moving slowly on home testing. The agency spent 7 years evaluating the first home HIV test it approved, which hit the market in 2012.
“Home testing is the way of the future,” said Laura Lindberg, PhD, a professor of public health at Rutgers University, Piscataway, N.J. “The pandemic opened the door to testing and treatment at home without traveling to a health care provider, and we aren’t going to be able to put the genie back in the bottle.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Among the more remarkable legacies of the COVID-19 pandemic is how quickly federal regulators, the health care industry, and consumers moved to make at-home testing a reliable tool for managing a public health crisis.
But that fast-track focus is missing from another, less publicized epidemic: an explosion in sexually transmitted diseases that can cause chronic pain and infertility among infected adults and disable or kill infected newborns. The disparity has amplified calls from researchers, public health advocates, and health care companies urging the federal government to greenlight at-home testing kits that could vastly multiply the number of Americans testing for STDs.
Online shoppers can already choose from more than a dozen self-testing kits, typically ranging in price from $69 to $500, depending on the brand and the variety of infections they can detect.
But, except for HIV tests, the Food and Drug Administration hasn’t approved STD test kits for use outside a medical setting. That leaves consumers unsure about their reliability even as at-home use grows dramatically.
The STD epidemic is “out of control,” said Amesh Adalja, MD, a senior scholar at the Johns Hopkins University Center for Health Security. “We know we are missing diagnoses. We know that contact tracing is happening late or not at all. If we’re really serious about tackling the STD crisis, we have to get more people diagnosed.”
Preliminary data for 2021 showed nearly 2.5 million reported cases of chlamydia, gonorrhea, and syphilis in the United States, according to the Centers for Disease Control and Prevention. Reported cases of syphilis and gonorrhea have been climbing for about a decade. In its most recent prevalence estimate, the agency said that on any given day, one in five Americans are infected with any of eight common STDs.
The push to make at-home testing for STDs as easy and commonplace as at-home COVID and pregnancy testing is coming from several sectors. Public health officials say their overextended staffers can’t handle the staggering need for testing and surveillance. Diagnostic and pharmaceutical companies see a business opportunity in the unmet demand.
The medical science underpinning STD testing is not particularly new or mysterious. Depending on the test, it may involve collecting a urine sample, pricking a finger for blood, or swabbing the mouth, genitals, or anus for discharge or cell samples. Medical centers and community health clinics have performed such testing for decades.
The issue for regulators is whether sampling kits can be reliably adapted for in-home use. Unlike rapid antigen tests for COVID, which produce results in 15-20 minutes, the home STD kits on the market require patients to collect their own samples, and then package and mail them to a lab for analysis.
In the past 3 years, as the pandemic prompted clinics that provide low-cost care to drastically curtail in-person services, a number of public health departments – among them state agencies in Alabama, Alaska, and Maryland – have started mailing free STD test kits to residents. Universities and nonprofits are also spearheading at-home testing efforts.
And dozens of commercial enterprises are jumping into or ramping up direct-to-consumer sales. Everly Health, a digital health company that sells a variety of lab tests online, reported sales for its suite of STD kits grew 120% in the first half of this year compared with the first half of 2021.
CVS Health began selling its own bundled STD kit in October, priced at $99.99. Unlike most home kits, CVS’ version is available in stores.
Hologic, Abbott, and Molecular Testing Labs are among the companies urgently developing tests. And Cue Health, which sells COVID tests, is poised to launch a clinical trial for a rapid home test for chlamydia and gonorrhea that would set a new bar, providing results in about 20 minutes.
Alberto Gutierrez, who formerly led the FDA office that oversees diagnostic tests, said agency officials have been concerned about the reliability of home tests for years. The FDA wants companies to prove that home collection kits are as accurate as those used in clinics, and that samples don’t degrade during shipping.
“The agency doesn’t believe these tests are legally marketed at this point,” said Mr. Gutierrez, a partner at NDA Partners, a consulting firm that advises companies seeking to bring health care products to market.
“CVS should not be selling that test,” he added.
In response to KHN questions, the FDA said it considers home collection kits, which can include swabs, lancets, transport tubes, and chemicals to stabilize the samples, to be devices that require agency review. The FDA “generally does not comment” on whether it plans to take action on any specific case, the statement said.
CVS spokesperson Mary Gattuso said the pharmacy chain is following the law. “We are committed to ensuring the products we offer are safe, work as intended, comply with regulations, and satisfy customers,” Ms. Gattuso said.
Everly Health and other companies described their kits as laboratory-developed tests, akin to the diagnostics some hospitals create for in-house use. And they contend their tests can be legally marketed because their labs have been certified by a different agency, the Centers for Medicare & Medicaid Services.
“The instruments and assays used by the laboratories we use are comparable to – and often the same as – those used by the labs a doctor’s office uses,” said Liz Kwo, MD, chief medical officer at Everly Health. “Our at-home sample collection methods, like dried blood spots and saliva, have been widely used for decades.”
Home collection kits appeal to Uxmal Caldera, 27, of Miami Beach, who prefers to test in the privacy of his home. Mr. Caldera, who doesn’t have a car, said home testing saves him the time and expense of getting to a clinic.
Mr. Caldera has been testing himself for HIV and other STDs every 3 months for more than a year, part of routine monitoring for people taking PrEP, a regimen of daily pills to prevent HIV infection.
“Doing it by yourself is not hard at all,” said Mr. Caldera, who is uninsured but receives the tests free through a community foundation. “The instructions are really clear. I get the results in maybe 4 days. For sure, I would recommend it to other people.”
Leandro Mena, MD, director of the CDC’s division of STD prevention, said he would like to see at-home STD testing become as routine as home pregnancy tests. An estimated 16 million–20 million tests for gonorrhea and chlamydia are performed in the United States each year, Dr. Mena said. Widespread use of at-home STD testing could double or triple that number.
He noted that doctors have years of experience using home collection kits.
The Johns Hopkins Center for Point-of-Care Technologies Research for Sexually Transmitted Diseases has distributed roughly 23,000 at-home STD kits since 2004, said Charlotte Gaydos, DrPH, a principal investigator with the center. The FDA generally allows such use if it’s part of research overseen by medical professionals. The center’s tests are now used by the Alaska health department, as well as Native American tribes in Arizona and Oklahoma.
Dr. Gaydos has published dozens of studies establishing that home collection kits for diseases such as chlamydia and gonorrhea are accurate and easy to use.
“There’s a huge amount of data showing that home testing works,” said Dr. Gaydos.
But Dr. Gaydos noted that her studies have been limited to small sample sizes. She said she doesn’t have the millions of dollars in funding it would take to run the sort of comprehensive trial the FDA typically requires for approval.
Jenny Mahn, director of clinical and sexual health at the National Coalition of STD Directors, said many public health labs are reluctant to handle home kits. “The public health labs won’t touch it without FDA’s blessing.”
Public health clinics often provide STD testing at little to no cost, while health insurance typically covers in-person testing at a private practice. But most consumers pay out-of-pocket for direct-to-consumer kits. Commercial pricing puts them out of reach for many people, particularly teens and young adults, who account for nearly half of STDs.
Adalja said the FDA has a history of moving slowly on home testing. The agency spent 7 years evaluating the first home HIV test it approved, which hit the market in 2012.
“Home testing is the way of the future,” said Laura Lindberg, PhD, a professor of public health at Rutgers University, Piscataway, N.J. “The pandemic opened the door to testing and treatment at home without traveling to a health care provider, and we aren’t going to be able to put the genie back in the bottle.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
At-home births rose during the pandemic, CDC reports
More women gave birth at home in America last year, continuing a pandemic trend and reaching the highest level in decades, according to figures released by the CDC.
The report said that almost 52,000 births occurred at home in 2021, out of 4 million total births in the country. This was an increase of 12% from 2020. The figure rose by 22% in 2020, when the COVID-19 pandemic hit, over 2019.
There were several possible reasons for the increase in home births. Infection rates and hospitalizations were high. Vaccinations were not available or were not widely used, and many people avoided going to hospitals or the doctor, said Elizabeth Gregory, the report’s lead author.
Also, some women didn’t have health insurance, lived far from a medical facility, or could not get to a hospital fast enough. About 25% of home births are not planned, the Associated Press reported.
Increases in home births occurred across all races, but home births were less common among Hispanics.
The AP reported that home births are riskier than hospital births, according to the American College of Obstetricians and Gynecologists. The organization advises against home births for women carrying multiple babies or who have previously had a cesarean section.
“Hospitals and accredited birth centers are the safest places to give birth, because although serious complications associated with labor and delivery are rare, they can be catastrophic,” said Jeffrey Ecker, M.D., chief of obstetrics and gynecology at Massachusetts General Hospital, Boston.
A version of this article first appeared on WebMD.com.
More women gave birth at home in America last year, continuing a pandemic trend and reaching the highest level in decades, according to figures released by the CDC.
The report said that almost 52,000 births occurred at home in 2021, out of 4 million total births in the country. This was an increase of 12% from 2020. The figure rose by 22% in 2020, when the COVID-19 pandemic hit, over 2019.
There were several possible reasons for the increase in home births. Infection rates and hospitalizations were high. Vaccinations were not available or were not widely used, and many people avoided going to hospitals or the doctor, said Elizabeth Gregory, the report’s lead author.
Also, some women didn’t have health insurance, lived far from a medical facility, or could not get to a hospital fast enough. About 25% of home births are not planned, the Associated Press reported.
Increases in home births occurred across all races, but home births were less common among Hispanics.
The AP reported that home births are riskier than hospital births, according to the American College of Obstetricians and Gynecologists. The organization advises against home births for women carrying multiple babies or who have previously had a cesarean section.
“Hospitals and accredited birth centers are the safest places to give birth, because although serious complications associated with labor and delivery are rare, they can be catastrophic,” said Jeffrey Ecker, M.D., chief of obstetrics and gynecology at Massachusetts General Hospital, Boston.
A version of this article first appeared on WebMD.com.
More women gave birth at home in America last year, continuing a pandemic trend and reaching the highest level in decades, according to figures released by the CDC.
The report said that almost 52,000 births occurred at home in 2021, out of 4 million total births in the country. This was an increase of 12% from 2020. The figure rose by 22% in 2020, when the COVID-19 pandemic hit, over 2019.
There were several possible reasons for the increase in home births. Infection rates and hospitalizations were high. Vaccinations were not available or were not widely used, and many people avoided going to hospitals or the doctor, said Elizabeth Gregory, the report’s lead author.
Also, some women didn’t have health insurance, lived far from a medical facility, or could not get to a hospital fast enough. About 25% of home births are not planned, the Associated Press reported.
Increases in home births occurred across all races, but home births were less common among Hispanics.
The AP reported that home births are riskier than hospital births, according to the American College of Obstetricians and Gynecologists. The organization advises against home births for women carrying multiple babies or who have previously had a cesarean section.
“Hospitals and accredited birth centers are the safest places to give birth, because although serious complications associated with labor and delivery are rare, they can be catastrophic,” said Jeffrey Ecker, M.D., chief of obstetrics and gynecology at Massachusetts General Hospital, Boston.
A version of this article first appeared on WebMD.com.
Long-term behavioral follow-up of children exposed to mood stabilizers and antidepressants: A look forward
Much of the focus of reproductive psychiatry over the last 1 to 2 decades has been on issues regarding risk of fetal exposure to psychiatric medications in the context of the specific risk for teratogenesis or organ malformation. Concerns and questions are mostly focused on exposure to any number of medications that women take during the first trimester, as it is during that period that the major organs are formed.
More recently, there has been appropriate interest in the effect of fetal exposure to psychiatric medications with respect to risk for obstetrical and neonatal complications. This particularly has been the case with respect to antidepressants where fetal exposure to these medications, which while associated with symptoms of transient jitteriness and irritability about 20% of the time, have not been associated with symptoms requiring frank clinical intervention.
Concerning mood stabilizers, the risk for organ dysgenesis following fetal exposure to sodium valproate has been very well established, and we’ve known for over a decade about the adverse effects of fetal exposure to sodium valproate on behavioral outcomes (Lancet Neurol. 2013 Mar;12[3]:244-52). We also now have ample data on lamotrigine, one of the most widely used medicines by reproductive-age women for treatment of bipolar disorder that supports the absence of a risk of organ malformation in first-trimester exposure.
Most recently, in a study of 292 children of women with epilepsy, an evaluation of women being treated with more modern anticonvulsants such as lamotrigine and levetiracetam alone or as polytherapy was performed. The results showed no difference in language, motor, cognitive, social, emotional, and general adaptive functioning in children exposed to either lamotrigine or levetiracetam relative to unexposed children of women with epilepsy. However, the researchers found an increase in anti-epileptic drug plasma level appeared to be associated with decreased motor and sensory function. These are reassuring data that really confirm earlier work, which failed to reveal a signal of concern for lamotrigine and now provide some of the first data on levetiracetam, which is widely used by reproductive-age women with epilepsy (JAMA Neurol. 2021 Aug 1;78[8]:927-936). While one caveat of the study is a short follow-up of 2 years, the absence of a signal of concern is reassuring. With more and more data demonstrating bipolar disorder is an illness that requires chronic treatment for many people, and that discontinuation is associated with high risk for relapse, it is an advance in the field to have data on risk for teratogenesis and data on longer-term neurobehavioral outcomes.
There is vast information regarding reproductive safety, organ malformation, and acute neonatal outcomes for antidepressants. The last decade has brought interest in and analysis of specific reports of increased risk of both autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) following fetal exposure to antidepressants. What can be said based on reviews of pooled meta-analyses is that the risk for ASD and ADHD has been put to rest for most clinicians and patients (J Clin Psychiatry. 2020 May 26;81[3]:20f13463). With other neurodevelopmental disorders, results have been somewhat inconclusive. Over the last 5-10 years, there have been sporadic reports of concerns about problems in a specific domain of neurodevelopment in offspring of women who have used antidepressants during pregnancy, whether it be speech, language, or motor functioning, but no signal of concern has been consistent.
In a previous column, I addressed a Danish study that showed no increased risk of longer-term sequelae after fetal exposure to antidepressants. Now, a new study has examined 1.93 million pregnancies in the Medicaid Analytic eXtract and 1.25 million pregnancies in the IBM MarketScan Research Database with follow-up up to 14 years of age where the specific interval for fetal exposure was from gestational age of 19 weeks to delivery, as that is the period that corresponds most to synaptogenesis in the brain. The researchers examined a spectrum of neurodevelopmental disorders such as developmental speech issues, ADHD, ASD, dyslexia, and learning disorders, among others. They found a twofold increased risk for neurodevelopmental disorders in the unadjusted models that flattened to no finding when factoring in environmental and genetic risk variables, highlighting the importance of dealing appropriately with confounders when performing these analyses. Those confounders examined include the mother’s use of alcohol and tobacco, and her body mass index and overall general health (JAMA Intern Med. 2022;182[11]:1149-60).
Given the consistency of these results with earlier data, patients can be increasingly comfortable as they weigh the benefits and risks of antidepressant use during pregnancy, factoring in the risk of fetal exposure with added data on long-term neurobehavioral sequelae. With that said, we need to remember the importance of initiatives to address alcohol consumption, poor nutrition, tobacco use, elevated BMI, and general health during pregnancy. These are modifiable risks that we as clinicians should focus on in order to optimize outcomes during pregnancy.
We have come so far in knowledge about fetal exposure to antidepressants relative to other classes of medications women take during pregnancy, about which, frankly, we are still starved for data. As use of psychiatric medications during pregnancy continues to grow, we can rest a bit more comfortably. But we should also address some of the other behaviors that have adverse effects on maternal and child well-being.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Much of the focus of reproductive psychiatry over the last 1 to 2 decades has been on issues regarding risk of fetal exposure to psychiatric medications in the context of the specific risk for teratogenesis or organ malformation. Concerns and questions are mostly focused on exposure to any number of medications that women take during the first trimester, as it is during that period that the major organs are formed.
More recently, there has been appropriate interest in the effect of fetal exposure to psychiatric medications with respect to risk for obstetrical and neonatal complications. This particularly has been the case with respect to antidepressants where fetal exposure to these medications, which while associated with symptoms of transient jitteriness and irritability about 20% of the time, have not been associated with symptoms requiring frank clinical intervention.
Concerning mood stabilizers, the risk for organ dysgenesis following fetal exposure to sodium valproate has been very well established, and we’ve known for over a decade about the adverse effects of fetal exposure to sodium valproate on behavioral outcomes (Lancet Neurol. 2013 Mar;12[3]:244-52). We also now have ample data on lamotrigine, one of the most widely used medicines by reproductive-age women for treatment of bipolar disorder that supports the absence of a risk of organ malformation in first-trimester exposure.
Most recently, in a study of 292 children of women with epilepsy, an evaluation of women being treated with more modern anticonvulsants such as lamotrigine and levetiracetam alone or as polytherapy was performed. The results showed no difference in language, motor, cognitive, social, emotional, and general adaptive functioning in children exposed to either lamotrigine or levetiracetam relative to unexposed children of women with epilepsy. However, the researchers found an increase in anti-epileptic drug plasma level appeared to be associated with decreased motor and sensory function. These are reassuring data that really confirm earlier work, which failed to reveal a signal of concern for lamotrigine and now provide some of the first data on levetiracetam, which is widely used by reproductive-age women with epilepsy (JAMA Neurol. 2021 Aug 1;78[8]:927-936). While one caveat of the study is a short follow-up of 2 years, the absence of a signal of concern is reassuring. With more and more data demonstrating bipolar disorder is an illness that requires chronic treatment for many people, and that discontinuation is associated with high risk for relapse, it is an advance in the field to have data on risk for teratogenesis and data on longer-term neurobehavioral outcomes.
There is vast information regarding reproductive safety, organ malformation, and acute neonatal outcomes for antidepressants. The last decade has brought interest in and analysis of specific reports of increased risk of both autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) following fetal exposure to antidepressants. What can be said based on reviews of pooled meta-analyses is that the risk for ASD and ADHD has been put to rest for most clinicians and patients (J Clin Psychiatry. 2020 May 26;81[3]:20f13463). With other neurodevelopmental disorders, results have been somewhat inconclusive. Over the last 5-10 years, there have been sporadic reports of concerns about problems in a specific domain of neurodevelopment in offspring of women who have used antidepressants during pregnancy, whether it be speech, language, or motor functioning, but no signal of concern has been consistent.
In a previous column, I addressed a Danish study that showed no increased risk of longer-term sequelae after fetal exposure to antidepressants. Now, a new study has examined 1.93 million pregnancies in the Medicaid Analytic eXtract and 1.25 million pregnancies in the IBM MarketScan Research Database with follow-up up to 14 years of age where the specific interval for fetal exposure was from gestational age of 19 weeks to delivery, as that is the period that corresponds most to synaptogenesis in the brain. The researchers examined a spectrum of neurodevelopmental disorders such as developmental speech issues, ADHD, ASD, dyslexia, and learning disorders, among others. They found a twofold increased risk for neurodevelopmental disorders in the unadjusted models that flattened to no finding when factoring in environmental and genetic risk variables, highlighting the importance of dealing appropriately with confounders when performing these analyses. Those confounders examined include the mother’s use of alcohol and tobacco, and her body mass index and overall general health (JAMA Intern Med. 2022;182[11]:1149-60).
Given the consistency of these results with earlier data, patients can be increasingly comfortable as they weigh the benefits and risks of antidepressant use during pregnancy, factoring in the risk of fetal exposure with added data on long-term neurobehavioral sequelae. With that said, we need to remember the importance of initiatives to address alcohol consumption, poor nutrition, tobacco use, elevated BMI, and general health during pregnancy. These are modifiable risks that we as clinicians should focus on in order to optimize outcomes during pregnancy.
We have come so far in knowledge about fetal exposure to antidepressants relative to other classes of medications women take during pregnancy, about which, frankly, we are still starved for data. As use of psychiatric medications during pregnancy continues to grow, we can rest a bit more comfortably. But we should also address some of the other behaviors that have adverse effects on maternal and child well-being.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Much of the focus of reproductive psychiatry over the last 1 to 2 decades has been on issues regarding risk of fetal exposure to psychiatric medications in the context of the specific risk for teratogenesis or organ malformation. Concerns and questions are mostly focused on exposure to any number of medications that women take during the first trimester, as it is during that period that the major organs are formed.
More recently, there has been appropriate interest in the effect of fetal exposure to psychiatric medications with respect to risk for obstetrical and neonatal complications. This particularly has been the case with respect to antidepressants where fetal exposure to these medications, which while associated with symptoms of transient jitteriness and irritability about 20% of the time, have not been associated with symptoms requiring frank clinical intervention.
Concerning mood stabilizers, the risk for organ dysgenesis following fetal exposure to sodium valproate has been very well established, and we’ve known for over a decade about the adverse effects of fetal exposure to sodium valproate on behavioral outcomes (Lancet Neurol. 2013 Mar;12[3]:244-52). We also now have ample data on lamotrigine, one of the most widely used medicines by reproductive-age women for treatment of bipolar disorder that supports the absence of a risk of organ malformation in first-trimester exposure.
Most recently, in a study of 292 children of women with epilepsy, an evaluation of women being treated with more modern anticonvulsants such as lamotrigine and levetiracetam alone or as polytherapy was performed. The results showed no difference in language, motor, cognitive, social, emotional, and general adaptive functioning in children exposed to either lamotrigine or levetiracetam relative to unexposed children of women with epilepsy. However, the researchers found an increase in anti-epileptic drug plasma level appeared to be associated with decreased motor and sensory function. These are reassuring data that really confirm earlier work, which failed to reveal a signal of concern for lamotrigine and now provide some of the first data on levetiracetam, which is widely used by reproductive-age women with epilepsy (JAMA Neurol. 2021 Aug 1;78[8]:927-936). While one caveat of the study is a short follow-up of 2 years, the absence of a signal of concern is reassuring. With more and more data demonstrating bipolar disorder is an illness that requires chronic treatment for many people, and that discontinuation is associated with high risk for relapse, it is an advance in the field to have data on risk for teratogenesis and data on longer-term neurobehavioral outcomes.
There is vast information regarding reproductive safety, organ malformation, and acute neonatal outcomes for antidepressants. The last decade has brought interest in and analysis of specific reports of increased risk of both autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) following fetal exposure to antidepressants. What can be said based on reviews of pooled meta-analyses is that the risk for ASD and ADHD has been put to rest for most clinicians and patients (J Clin Psychiatry. 2020 May 26;81[3]:20f13463). With other neurodevelopmental disorders, results have been somewhat inconclusive. Over the last 5-10 years, there have been sporadic reports of concerns about problems in a specific domain of neurodevelopment in offspring of women who have used antidepressants during pregnancy, whether it be speech, language, or motor functioning, but no signal of concern has been consistent.
In a previous column, I addressed a Danish study that showed no increased risk of longer-term sequelae after fetal exposure to antidepressants. Now, a new study has examined 1.93 million pregnancies in the Medicaid Analytic eXtract and 1.25 million pregnancies in the IBM MarketScan Research Database with follow-up up to 14 years of age where the specific interval for fetal exposure was from gestational age of 19 weeks to delivery, as that is the period that corresponds most to synaptogenesis in the brain. The researchers examined a spectrum of neurodevelopmental disorders such as developmental speech issues, ADHD, ASD, dyslexia, and learning disorders, among others. They found a twofold increased risk for neurodevelopmental disorders in the unadjusted models that flattened to no finding when factoring in environmental and genetic risk variables, highlighting the importance of dealing appropriately with confounders when performing these analyses. Those confounders examined include the mother’s use of alcohol and tobacco, and her body mass index and overall general health (JAMA Intern Med. 2022;182[11]:1149-60).
Given the consistency of these results with earlier data, patients can be increasingly comfortable as they weigh the benefits and risks of antidepressant use during pregnancy, factoring in the risk of fetal exposure with added data on long-term neurobehavioral sequelae. With that said, we need to remember the importance of initiatives to address alcohol consumption, poor nutrition, tobacco use, elevated BMI, and general health during pregnancy. These are modifiable risks that we as clinicians should focus on in order to optimize outcomes during pregnancy.
We have come so far in knowledge about fetal exposure to antidepressants relative to other classes of medications women take during pregnancy, about which, frankly, we are still starved for data. As use of psychiatric medications during pregnancy continues to grow, we can rest a bit more comfortably. But we should also address some of the other behaviors that have adverse effects on maternal and child well-being.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Top 10 unproven infertility tests and treatments
In 2019, a New York Times opinion piece titled, “The Big IVF Add-On Racket – This is no way to treat patients desperate for a baby”1 alleged exploitation of infertility patients based on a Fertility and Sterility article, “Do à la carte menus serve infertility patients? The ethics and regulation of in vitro fertility add-ons.”2 The desperation of infertility patients combined with their financial burden, caused by inconsistent insurance coverage, has resulted in a perfect storm of frustration and overzealous recommendations for a successful outcome. Since the inception of in vitro fertilization (IVF) itself, infertility patients have been subjected to many unproven tests and procedures that enter the mainstream of care before unequivocal efficacy and safety have been shown.
From ovarian stimulation with intrauterine insemination (IUI) or IVF along with intracytoplasmic sperm injection (ICSI), assisted hatching, and preimplantation genetic testing for aneuploidy (PGT-A), a multitude of options with varying success can overwhelm fertility patients as they walk the tightrope of wanting “the kitchen sink” of treatment while experiencing sticker shock. This month’s article examines the top 10 infertility add-ons that have yet to be shown to improve pregnancy outcomes.
1. Blood testing: Prolactin and FSH
In a woman with ovulatory monthly menstrual cycles, a serum prolactin level provides no elucidation of the cause of infertility. If obtained following ovulation, prolactin can often be physiologically elevated, thereby compelling a repeat blood level, which is ideally performed during the early proliferative phase. False elevations of prolactin can be caused by an early morning blood sample, eating, and stress – which may result from worry caused by having to repeat the unnecessary initial blood test!
Follicle-stimulating hormone (FSH) was a first-line hormone test to assess for ovarian age. For nearly 15 years now, FSH has been replaced by anti-Müllerian hormone as a more reliable and earlier test for diminished ovarian reserve. However, FSH is still the hormone test of choice to diagnose primary ovarian insufficiency. Note that the use of ovarian age testing in a woman without infertility can result in both unnecessary patient anxiety and additional testing.
2. Endometrial scratch
The concept was understandable, that is, induce endometrial trauma by a biopsy or “scratch,” that results in an inflammatory and immunologic response to increase implantation. Endometrial sampling was recommended to be performed during the month prior to the embryo transfer cycle. While the procedure is brief, the pain response of women varies from minimal to severe. Unfortunately, a randomized controlled trial of over 1,300 patients did not show any improvement in the IVF live birth rate from the scratch procedure.3
3. Diagnostic laparoscopy
In years past, a diagnosis of unexplained infertility was not accepted until a laparoscopy was performed that revealed a normal pelvis. This approach subjected many women to an unindicated and a potentially risky surgery that has not shown benefit. The American Society for Reproductive Medicine’s ReproductiveFacts.org website states: “Routine diagnostic laparoscopy should not be performed unless there is a suspicion of pelvic pathology based on clinical history, an abnormal pelvic exam, or abnormalities identified with less invasive testing. In patients with a normal hysterosalpingogram or the presence of a unilaterally patent tube, diagnostic laparoscopy typically will not change the initial recommendation for treatment.”
4. Prescribing clomiphene citrate without IUI
Ovulation dysfunction is found in 40% of female factors for fertility. Provided testing reveals a reasonably normal sperm analysis and hysterosalpingogram, ovulation induction medication with ultrasound monitoring along with an hCG trigger is appropriate. In women who ovulate with unexplained infertility and/or mild male factor, the use of clomiphene citrate or letrozole with timed intercourse is often prescribed, particularly in clinics when IUI preparation is not available. Unfortunately, without including IUI, the use of oral ovarian stimulation has been shown by good evidence to be no more effective than natural cycle attempts at conception.4
5. Thrombophilia testing
Recurrent miscarriage, defined by the spontaneous loss of two or more pregnancies (often during the first trimester but may include up to 20 weeks estimated gestational age), has remained an ill-defined problem that lacks a consensus on the most optimal evaluation and treatment. In 2006, an international consensus statement provided guidance on laboratory testing for antiphospholipid syndrome limited to lupus anticoagulant, anticardiolipin IgG and IgM, and IgG and IgM anti–beta2-glycoprotein I assays.5 ASRM does not recommend additional thrombophilia tests as they are unproven causative factors of recurrent miscarriage.
6. Screening hysteroscopy
A standard infertility evaluation includes ovulation testing, assessment of fallopian tube patency, and a sperm analysis. In a subfertile women with a normal ultrasound or hysterosalpingogram in the basic fertility work‐up, a Cochrane data review concluded there is no definitive evidence for improved outcome with a screening hysteroscopy prior to IUI or IVF.6,7 Two large trials included in the Cochrane review, confirmed similar live birth rates whether or not hysteroscopy was performed before IVF. There may value in screening patients with recurrent implantation failure.
7. PGT-A for all
As the efficacy of the first generation of embryo preimplantation genetic testing, i.e., FISH (fluorescence in situ hybridization) was disproven, so has the same result been determined for PGT-A, specifically in women younger than 35.8 In an elegant randomized prospective trial, Munne and colleagues showed no improvement in the ongoing pregnancy rate (OPR) of study patients of all ages who were enrolled with the intention to treat. However, a subanalysis of patients aged 35-40 who completed the protocol did show an improved OPR and lower miscarriage rate per embryo transfer. While there is no evidence to support improved outcomes with the universal application of PGT-A, there may be some benefit in women older than 35 as well as in certain individual patient circumstances.
8. ICSI for nonmale factor infertility; assisted hatching
In an effort to reduce the risk of fertilization failure, programs have broadened the use of ICSI to nonmale factor infertility. While it has been used in PGT to reduce the risk of DNA contamination, particularly in PGT-M (monogenic disorder) and PGT-SR (structural rearrangement) cases, ICSI has not been shown to improve outcomes when there is a normal sperm analysis.9 During IVF embryo development, assisted hatching involves the thinning and/or opening of the zona pellucida either by chemical, mechanical, or laser means around the embryo before transfer with the intention of facilitating implantation. The routine use of assisted hatching is not recommended based on the lack of increase in live birth rates and because it may increase multiple pregnancy and monozygotic twinning rates.10
9. Acupuncture
Four meta-analyses showed no evidence of the overall benefit of acupuncture for improving live birth rates regardless of whether acupuncture was performed around the time of oocyte retrieval or around the day of embryo transfer. Consequently, acupuncture cannot be recommended routinely to improve IVF outcomes.11
10. Immunologic tests/treatments
Given the “foreign” genetic nature of a fetus, attempts to suppress the maternal immunologic response to sustain the pregnancy have been made for decades, especially for recurrent miscarriage and recurrent implantation failure with IVF. Testing has included natural killer (NK) cells, human leukocyte antigen (HLA) genotypes, and cytokines. While NK cells can be examined by endometrial biopsy, levels fluctuate based on the cycle phase, and no correlation between peripheral blood testing and uterine NK cell levels has been shown. Further, no consensus has been reached on reliable normal reference ranges in uterine NK cells.12
Several treatments have been proposed to somehow modulate the immune system during the implantation process thereby improving implantation and live birth, including lipid emulsion (intralipid) infusion, intravenous immunoglobulin, leukocyte immunization therapy, tacrolimus, anti–tumor necrosis factor agents, and granulocyte colony-stimulating factor. A recent systematic review and meta-analysis cited low-quality studies and did not recommend the use of any of these immune treatments.13 Further, immunomodulation has many known side effects, some of which are serious (including hepatosplenomegaly, thrombocytopenia, leukopenia, renal failure, thromboembolism, and anaphylactic reactions). Excluding women with autoimmune disease, taking glucocorticoids or other immune treatments to improve fertility has not been proven.13
Conclusion
To quote the New York Times opinion piece, “IVF remains an under-regulated arena, and entrepreneurial doctors and pharmaceutical and life science companies are eager to find new ways to cash in on a growing global market that is projected to be as large as $40 billion by 2024.” While this bold statement compels a huge “Ouch!”, it reminds us of our obligation to provide evidence-based medicine and to include emotional and financial harm to our oath of Primum non nocere.
References
1. The News York Times. 2019 Dec 12. Opinion.
2. Wilkinson J et al. Fertil Steril. 2019;112(6):973-7.
3. Lensen S et al. N Engl J Med. 2019 Jan 24;380(4):325-34.
4. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2020;113(2):305-22.
5. Miyakis S et al. J Thromb Haemost. 2006;4(2):295-306.
6. Kamath MS et al. Cochrane Database Syst Rev. 2019 Apr 16;4(4):CD012856.
7. Bosteels J et al. Cochrane Database Syst Rev. 2013 Jan 31;(1):CD009461.
8. Munne S et al. Fertil Steril. 2019;112(6):1071-9.
9. Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Fertil Steril. 2020;114(2):239-45.
10. Lacey L et al. Cochrane Database Syst Rev. March 7 2021;3:2199.
11. Coyle ME et al. Acupunct Med. 2021;39(1):20-9.
12. Von Woon E et al. Hum Reprod Update. 2022;30;28(4):548-82.
13. Achilli C et al. Fertil Steril. 2018;110(6):1089-100.
In 2019, a New York Times opinion piece titled, “The Big IVF Add-On Racket – This is no way to treat patients desperate for a baby”1 alleged exploitation of infertility patients based on a Fertility and Sterility article, “Do à la carte menus serve infertility patients? The ethics and regulation of in vitro fertility add-ons.”2 The desperation of infertility patients combined with their financial burden, caused by inconsistent insurance coverage, has resulted in a perfect storm of frustration and overzealous recommendations for a successful outcome. Since the inception of in vitro fertilization (IVF) itself, infertility patients have been subjected to many unproven tests and procedures that enter the mainstream of care before unequivocal efficacy and safety have been shown.
From ovarian stimulation with intrauterine insemination (IUI) or IVF along with intracytoplasmic sperm injection (ICSI), assisted hatching, and preimplantation genetic testing for aneuploidy (PGT-A), a multitude of options with varying success can overwhelm fertility patients as they walk the tightrope of wanting “the kitchen sink” of treatment while experiencing sticker shock. This month’s article examines the top 10 infertility add-ons that have yet to be shown to improve pregnancy outcomes.
1. Blood testing: Prolactin and FSH
In a woman with ovulatory monthly menstrual cycles, a serum prolactin level provides no elucidation of the cause of infertility. If obtained following ovulation, prolactin can often be physiologically elevated, thereby compelling a repeat blood level, which is ideally performed during the early proliferative phase. False elevations of prolactin can be caused by an early morning blood sample, eating, and stress – which may result from worry caused by having to repeat the unnecessary initial blood test!
Follicle-stimulating hormone (FSH) was a first-line hormone test to assess for ovarian age. For nearly 15 years now, FSH has been replaced by anti-Müllerian hormone as a more reliable and earlier test for diminished ovarian reserve. However, FSH is still the hormone test of choice to diagnose primary ovarian insufficiency. Note that the use of ovarian age testing in a woman without infertility can result in both unnecessary patient anxiety and additional testing.
2. Endometrial scratch
The concept was understandable, that is, induce endometrial trauma by a biopsy or “scratch,” that results in an inflammatory and immunologic response to increase implantation. Endometrial sampling was recommended to be performed during the month prior to the embryo transfer cycle. While the procedure is brief, the pain response of women varies from minimal to severe. Unfortunately, a randomized controlled trial of over 1,300 patients did not show any improvement in the IVF live birth rate from the scratch procedure.3
3. Diagnostic laparoscopy
In years past, a diagnosis of unexplained infertility was not accepted until a laparoscopy was performed that revealed a normal pelvis. This approach subjected many women to an unindicated and a potentially risky surgery that has not shown benefit. The American Society for Reproductive Medicine’s ReproductiveFacts.org website states: “Routine diagnostic laparoscopy should not be performed unless there is a suspicion of pelvic pathology based on clinical history, an abnormal pelvic exam, or abnormalities identified with less invasive testing. In patients with a normal hysterosalpingogram or the presence of a unilaterally patent tube, diagnostic laparoscopy typically will not change the initial recommendation for treatment.”
4. Prescribing clomiphene citrate without IUI
Ovulation dysfunction is found in 40% of female factors for fertility. Provided testing reveals a reasonably normal sperm analysis and hysterosalpingogram, ovulation induction medication with ultrasound monitoring along with an hCG trigger is appropriate. In women who ovulate with unexplained infertility and/or mild male factor, the use of clomiphene citrate or letrozole with timed intercourse is often prescribed, particularly in clinics when IUI preparation is not available. Unfortunately, without including IUI, the use of oral ovarian stimulation has been shown by good evidence to be no more effective than natural cycle attempts at conception.4
5. Thrombophilia testing
Recurrent miscarriage, defined by the spontaneous loss of two or more pregnancies (often during the first trimester but may include up to 20 weeks estimated gestational age), has remained an ill-defined problem that lacks a consensus on the most optimal evaluation and treatment. In 2006, an international consensus statement provided guidance on laboratory testing for antiphospholipid syndrome limited to lupus anticoagulant, anticardiolipin IgG and IgM, and IgG and IgM anti–beta2-glycoprotein I assays.5 ASRM does not recommend additional thrombophilia tests as they are unproven causative factors of recurrent miscarriage.
6. Screening hysteroscopy
A standard infertility evaluation includes ovulation testing, assessment of fallopian tube patency, and a sperm analysis. In a subfertile women with a normal ultrasound or hysterosalpingogram in the basic fertility work‐up, a Cochrane data review concluded there is no definitive evidence for improved outcome with a screening hysteroscopy prior to IUI or IVF.6,7 Two large trials included in the Cochrane review, confirmed similar live birth rates whether or not hysteroscopy was performed before IVF. There may value in screening patients with recurrent implantation failure.
7. PGT-A for all
As the efficacy of the first generation of embryo preimplantation genetic testing, i.e., FISH (fluorescence in situ hybridization) was disproven, so has the same result been determined for PGT-A, specifically in women younger than 35.8 In an elegant randomized prospective trial, Munne and colleagues showed no improvement in the ongoing pregnancy rate (OPR) of study patients of all ages who were enrolled with the intention to treat. However, a subanalysis of patients aged 35-40 who completed the protocol did show an improved OPR and lower miscarriage rate per embryo transfer. While there is no evidence to support improved outcomes with the universal application of PGT-A, there may be some benefit in women older than 35 as well as in certain individual patient circumstances.
8. ICSI for nonmale factor infertility; assisted hatching
In an effort to reduce the risk of fertilization failure, programs have broadened the use of ICSI to nonmale factor infertility. While it has been used in PGT to reduce the risk of DNA contamination, particularly in PGT-M (monogenic disorder) and PGT-SR (structural rearrangement) cases, ICSI has not been shown to improve outcomes when there is a normal sperm analysis.9 During IVF embryo development, assisted hatching involves the thinning and/or opening of the zona pellucida either by chemical, mechanical, or laser means around the embryo before transfer with the intention of facilitating implantation. The routine use of assisted hatching is not recommended based on the lack of increase in live birth rates and because it may increase multiple pregnancy and monozygotic twinning rates.10
9. Acupuncture
Four meta-analyses showed no evidence of the overall benefit of acupuncture for improving live birth rates regardless of whether acupuncture was performed around the time of oocyte retrieval or around the day of embryo transfer. Consequently, acupuncture cannot be recommended routinely to improve IVF outcomes.11
10. Immunologic tests/treatments
Given the “foreign” genetic nature of a fetus, attempts to suppress the maternal immunologic response to sustain the pregnancy have been made for decades, especially for recurrent miscarriage and recurrent implantation failure with IVF. Testing has included natural killer (NK) cells, human leukocyte antigen (HLA) genotypes, and cytokines. While NK cells can be examined by endometrial biopsy, levels fluctuate based on the cycle phase, and no correlation between peripheral blood testing and uterine NK cell levels has been shown. Further, no consensus has been reached on reliable normal reference ranges in uterine NK cells.12
Several treatments have been proposed to somehow modulate the immune system during the implantation process thereby improving implantation and live birth, including lipid emulsion (intralipid) infusion, intravenous immunoglobulin, leukocyte immunization therapy, tacrolimus, anti–tumor necrosis factor agents, and granulocyte colony-stimulating factor. A recent systematic review and meta-analysis cited low-quality studies and did not recommend the use of any of these immune treatments.13 Further, immunomodulation has many known side effects, some of which are serious (including hepatosplenomegaly, thrombocytopenia, leukopenia, renal failure, thromboembolism, and anaphylactic reactions). Excluding women with autoimmune disease, taking glucocorticoids or other immune treatments to improve fertility has not been proven.13
Conclusion
To quote the New York Times opinion piece, “IVF remains an under-regulated arena, and entrepreneurial doctors and pharmaceutical and life science companies are eager to find new ways to cash in on a growing global market that is projected to be as large as $40 billion by 2024.” While this bold statement compels a huge “Ouch!”, it reminds us of our obligation to provide evidence-based medicine and to include emotional and financial harm to our oath of Primum non nocere.
References
1. The News York Times. 2019 Dec 12. Opinion.
2. Wilkinson J et al. Fertil Steril. 2019;112(6):973-7.
3. Lensen S et al. N Engl J Med. 2019 Jan 24;380(4):325-34.
4. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2020;113(2):305-22.
5. Miyakis S et al. J Thromb Haemost. 2006;4(2):295-306.
6. Kamath MS et al. Cochrane Database Syst Rev. 2019 Apr 16;4(4):CD012856.
7. Bosteels J et al. Cochrane Database Syst Rev. 2013 Jan 31;(1):CD009461.
8. Munne S et al. Fertil Steril. 2019;112(6):1071-9.
9. Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Fertil Steril. 2020;114(2):239-45.
10. Lacey L et al. Cochrane Database Syst Rev. March 7 2021;3:2199.
11. Coyle ME et al. Acupunct Med. 2021;39(1):20-9.
12. Von Woon E et al. Hum Reprod Update. 2022;30;28(4):548-82.
13. Achilli C et al. Fertil Steril. 2018;110(6):1089-100.
In 2019, a New York Times opinion piece titled, “The Big IVF Add-On Racket – This is no way to treat patients desperate for a baby”1 alleged exploitation of infertility patients based on a Fertility and Sterility article, “Do à la carte menus serve infertility patients? The ethics and regulation of in vitro fertility add-ons.”2 The desperation of infertility patients combined with their financial burden, caused by inconsistent insurance coverage, has resulted in a perfect storm of frustration and overzealous recommendations for a successful outcome. Since the inception of in vitro fertilization (IVF) itself, infertility patients have been subjected to many unproven tests and procedures that enter the mainstream of care before unequivocal efficacy and safety have been shown.
From ovarian stimulation with intrauterine insemination (IUI) or IVF along with intracytoplasmic sperm injection (ICSI), assisted hatching, and preimplantation genetic testing for aneuploidy (PGT-A), a multitude of options with varying success can overwhelm fertility patients as they walk the tightrope of wanting “the kitchen sink” of treatment while experiencing sticker shock. This month’s article examines the top 10 infertility add-ons that have yet to be shown to improve pregnancy outcomes.
1. Blood testing: Prolactin and FSH
In a woman with ovulatory monthly menstrual cycles, a serum prolactin level provides no elucidation of the cause of infertility. If obtained following ovulation, prolactin can often be physiologically elevated, thereby compelling a repeat blood level, which is ideally performed during the early proliferative phase. False elevations of prolactin can be caused by an early morning blood sample, eating, and stress – which may result from worry caused by having to repeat the unnecessary initial blood test!
Follicle-stimulating hormone (FSH) was a first-line hormone test to assess for ovarian age. For nearly 15 years now, FSH has been replaced by anti-Müllerian hormone as a more reliable and earlier test for diminished ovarian reserve. However, FSH is still the hormone test of choice to diagnose primary ovarian insufficiency. Note that the use of ovarian age testing in a woman without infertility can result in both unnecessary patient anxiety and additional testing.
2. Endometrial scratch
The concept was understandable, that is, induce endometrial trauma by a biopsy or “scratch,” that results in an inflammatory and immunologic response to increase implantation. Endometrial sampling was recommended to be performed during the month prior to the embryo transfer cycle. While the procedure is brief, the pain response of women varies from minimal to severe. Unfortunately, a randomized controlled trial of over 1,300 patients did not show any improvement in the IVF live birth rate from the scratch procedure.3
3. Diagnostic laparoscopy
In years past, a diagnosis of unexplained infertility was not accepted until a laparoscopy was performed that revealed a normal pelvis. This approach subjected many women to an unindicated and a potentially risky surgery that has not shown benefit. The American Society for Reproductive Medicine’s ReproductiveFacts.org website states: “Routine diagnostic laparoscopy should not be performed unless there is a suspicion of pelvic pathology based on clinical history, an abnormal pelvic exam, or abnormalities identified with less invasive testing. In patients with a normal hysterosalpingogram or the presence of a unilaterally patent tube, diagnostic laparoscopy typically will not change the initial recommendation for treatment.”
4. Prescribing clomiphene citrate without IUI
Ovulation dysfunction is found in 40% of female factors for fertility. Provided testing reveals a reasonably normal sperm analysis and hysterosalpingogram, ovulation induction medication with ultrasound monitoring along with an hCG trigger is appropriate. In women who ovulate with unexplained infertility and/or mild male factor, the use of clomiphene citrate or letrozole with timed intercourse is often prescribed, particularly in clinics when IUI preparation is not available. Unfortunately, without including IUI, the use of oral ovarian stimulation has been shown by good evidence to be no more effective than natural cycle attempts at conception.4
5. Thrombophilia testing
Recurrent miscarriage, defined by the spontaneous loss of two or more pregnancies (often during the first trimester but may include up to 20 weeks estimated gestational age), has remained an ill-defined problem that lacks a consensus on the most optimal evaluation and treatment. In 2006, an international consensus statement provided guidance on laboratory testing for antiphospholipid syndrome limited to lupus anticoagulant, anticardiolipin IgG and IgM, and IgG and IgM anti–beta2-glycoprotein I assays.5 ASRM does not recommend additional thrombophilia tests as they are unproven causative factors of recurrent miscarriage.
6. Screening hysteroscopy
A standard infertility evaluation includes ovulation testing, assessment of fallopian tube patency, and a sperm analysis. In a subfertile women with a normal ultrasound or hysterosalpingogram in the basic fertility work‐up, a Cochrane data review concluded there is no definitive evidence for improved outcome with a screening hysteroscopy prior to IUI or IVF.6,7 Two large trials included in the Cochrane review, confirmed similar live birth rates whether or not hysteroscopy was performed before IVF. There may value in screening patients with recurrent implantation failure.
7. PGT-A for all
As the efficacy of the first generation of embryo preimplantation genetic testing, i.e., FISH (fluorescence in situ hybridization) was disproven, so has the same result been determined for PGT-A, specifically in women younger than 35.8 In an elegant randomized prospective trial, Munne and colleagues showed no improvement in the ongoing pregnancy rate (OPR) of study patients of all ages who were enrolled with the intention to treat. However, a subanalysis of patients aged 35-40 who completed the protocol did show an improved OPR and lower miscarriage rate per embryo transfer. While there is no evidence to support improved outcomes with the universal application of PGT-A, there may be some benefit in women older than 35 as well as in certain individual patient circumstances.
8. ICSI for nonmale factor infertility; assisted hatching
In an effort to reduce the risk of fertilization failure, programs have broadened the use of ICSI to nonmale factor infertility. While it has been used in PGT to reduce the risk of DNA contamination, particularly in PGT-M (monogenic disorder) and PGT-SR (structural rearrangement) cases, ICSI has not been shown to improve outcomes when there is a normal sperm analysis.9 During IVF embryo development, assisted hatching involves the thinning and/or opening of the zona pellucida either by chemical, mechanical, or laser means around the embryo before transfer with the intention of facilitating implantation. The routine use of assisted hatching is not recommended based on the lack of increase in live birth rates and because it may increase multiple pregnancy and monozygotic twinning rates.10
9. Acupuncture
Four meta-analyses showed no evidence of the overall benefit of acupuncture for improving live birth rates regardless of whether acupuncture was performed around the time of oocyte retrieval or around the day of embryo transfer. Consequently, acupuncture cannot be recommended routinely to improve IVF outcomes.11
10. Immunologic tests/treatments
Given the “foreign” genetic nature of a fetus, attempts to suppress the maternal immunologic response to sustain the pregnancy have been made for decades, especially for recurrent miscarriage and recurrent implantation failure with IVF. Testing has included natural killer (NK) cells, human leukocyte antigen (HLA) genotypes, and cytokines. While NK cells can be examined by endometrial biopsy, levels fluctuate based on the cycle phase, and no correlation between peripheral blood testing and uterine NK cell levels has been shown. Further, no consensus has been reached on reliable normal reference ranges in uterine NK cells.12
Several treatments have been proposed to somehow modulate the immune system during the implantation process thereby improving implantation and live birth, including lipid emulsion (intralipid) infusion, intravenous immunoglobulin, leukocyte immunization therapy, tacrolimus, anti–tumor necrosis factor agents, and granulocyte colony-stimulating factor. A recent systematic review and meta-analysis cited low-quality studies and did not recommend the use of any of these immune treatments.13 Further, immunomodulation has many known side effects, some of which are serious (including hepatosplenomegaly, thrombocytopenia, leukopenia, renal failure, thromboembolism, and anaphylactic reactions). Excluding women with autoimmune disease, taking glucocorticoids or other immune treatments to improve fertility has not been proven.13
Conclusion
To quote the New York Times opinion piece, “IVF remains an under-regulated arena, and entrepreneurial doctors and pharmaceutical and life science companies are eager to find new ways to cash in on a growing global market that is projected to be as large as $40 billion by 2024.” While this bold statement compels a huge “Ouch!”, it reminds us of our obligation to provide evidence-based medicine and to include emotional and financial harm to our oath of Primum non nocere.
References
1. The News York Times. 2019 Dec 12. Opinion.
2. Wilkinson J et al. Fertil Steril. 2019;112(6):973-7.
3. Lensen S et al. N Engl J Med. 2019 Jan 24;380(4):325-34.
4. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2020;113(2):305-22.
5. Miyakis S et al. J Thromb Haemost. 2006;4(2):295-306.
6. Kamath MS et al. Cochrane Database Syst Rev. 2019 Apr 16;4(4):CD012856.
7. Bosteels J et al. Cochrane Database Syst Rev. 2013 Jan 31;(1):CD009461.
8. Munne S et al. Fertil Steril. 2019;112(6):1071-9.
9. Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Fertil Steril. 2020;114(2):239-45.
10. Lacey L et al. Cochrane Database Syst Rev. March 7 2021;3:2199.
11. Coyle ME et al. Acupunct Med. 2021;39(1):20-9.
12. Von Woon E et al. Hum Reprod Update. 2022;30;28(4):548-82.
13. Achilli C et al. Fertil Steril. 2018;110(6):1089-100.
Why your professional persona may be considered unprofessional
On one of the first days of medical school, Adaira Landry, MD, applied her favorite dark shade of lipstick and headed to her orientation. She was eager to learn about program expectations and connect with fellow aspiring physicians. But when Dr. Landry got there, one of her brand-new peers turned to her and asked, “Why do you wear your lipstick like an angry Black woman?”
“Imagine hearing that,” Dr. Landry, now an emergency medical physician in Boston, says. “It was so hurtful.”
So, what is a “standard-issue doctor” expected to look like? Physicians manage their appearances in myriad ways: through clothes, accessories, hair style, makeup; through a social media presence or lack thereof; in the rhythms and nuances of their interactions with patients and colleagues. These things add up to a professional “persona” – the Latin word for “mask,” or the face on display for the world to see.
While the health care field itself is diversifying, its guidelines for professionalism appear slower to change, often excluding or frowning upon expressions of individual personality or identity.
“Medicine is run primarily by men. It’s an objective truth,” Dr. Landry says. “Currently and historically, the standard of professionalism, especially in the physical sense, was set by them. As we increase diversity and welcome people bringing their authentic self to work, the prior definitions of professionalism are obviously in need of change.”
Split social media personalities
In August 2020, the Journal of Vascular Surgery published a study on the “prevalence of unprofessional social media content among young vascular surgeons.” The content that was deemed “unprofessional” included opinions on political issues like abortion and gun control. Photos of physicians holding alcoholic drinks or wearing “inappropriate/offensive attire,” including underwear, “provocative Halloween costumes,” and “bikinis/swimwear” were also censured. Six men and one woman worked on the study, and three of the male researchers took on the task of seeking out the “unprofessional” photos on social media. The resulting paper was reviewed by an all-male editorial board.
The study sparked immediate backlash and prompted hundreds of health care professionals to post photos of themselves in bathing suits with the hashtag “#medbikini.” The journal then retracted the study and issued an apology on Twitter, recognizing “errors in the design of the study with regards to conscious and unconscious bias.”
The researchers’ original definition of professionalism suggests that physicians should manage their personae even outside of work hours. “I think medicine in general is a very conservative and hierarchical field of study and of work, to say the least,” says Sarah Fraser, MD, a family medicine physician in Nova Scotia, Canada. “There’s this view that we have to have completely separate personal and professional lives, like church and state.”
The #medbikini controversy inspired Dr. Fraser to write an op-ed for the British Medical Journal blog about the flaws of requiring physicians to keep their personal and professional selves separate. The piece referenced Robert Louis Stevenson’s 1886 Gothic novella “The Strange Case of Dr. Jekyll and Mr. Hyde,” in which the respected scientist Dr. Jekyll creates an alter ego so he can express his evil urges without experiencing guilt, punishment, or loss of livelihood. Dr. Fraser likened this story to the pressure physicians feel to shrink or split themselves to squeeze into a narrow definition of professionalism.
But Dr. Landry points out that some elements of expression seen as unprofessional cannot be entirely separated from a physician’s fundamental identity. “For Black women, our daily behaviors and forms of expression that are deemed ‘unprofessional’ are much more subtle than being able to wear a bikini on social media,” she says. “The way we wear our hair, the tone of our voice, the color of our lipstick, the way we wear scrub caps are parts of us that are called into question.”
Keeping up appearances
The stereotype of what a doctor should look like starts to shape physicians’ professional personae in medical school. When Jennifer Caputo-Seidler, MD, started medical school in 2008, the dress code requirements for male students were simple: pants, a button-down shirt, a tie. But then there were the rules for women: Hair should be tied back. Minimal makeup. No flashy jewelry. Nothing without sleeves. Neutral colors. High necklines. Low hemlines. “The message I got was that we need to dress like the men in order to be taken seriously and to be seen as professional,” says Dr. Caputo-Seidler, now an assistant professor of medicine at the University of South Florida, Tampa, “and so that’s what I did.”
A 2018 analysis of 78 “draw-a-scientist” studies found that children have overwhelmingly associated scientific fields with men for the last 50 years. Overall, children drew 73% of scientists as men. The drawings grew more gender diverse over time, but even as more women entered scientific fields, both boys and girls continued to draw significantly more male than female scientists.
Not everyone at Dr. Caputo-Seidler’s medical school adhered to the environment’s gendered expectations. One resident she worked with often wore voluminous hairstyles, lipstick, and high heels. Dr. Caputo-Seidler overheard her peers as they gossiped behind the resident’s back, ridiculing the way she looked.
“She was good at her job,” Dr. Caputo-Seidler says. “She knew her patients. She had things down. She was, by all measures, very competent. But when people saw her dressing outside the norm and being forward with her femininity, there was definitely a lot of chatter about it.”
While expectations for a conservative appearance may disproportionately affect women, and particularly women of color, they also affect men who deviate from the norm. “As an LGBTQ+ person working as a ‘professional,’ I have countless stories and moments where I had my professionalism questioned,” Blair Peters, MD, a plastic surgeon and assistant professor at Oregon Health & Science University, Portland, wrote on Twitter. “Why is it ‘unprofessional’ to have colored hair? Why is it ‘unprofessional’ to have a visible tattoo? Why is it ‘unprofessional’ to wear bright colors and patterns?”
Dr. Fraser remembers a fellow medical student who had full-sleeve tattoos on both of his arms. A preceptor made a comment about it to Dr. Fraser, and then instructed the student to cover up his tattoos. “I think that there are scenarios when having tattoos or having different-colored hair or expressing your individual personality could help you even better bond with your patients,” Dr. Fraser says, “especially if you’re, for example, working with youth.”
Unmasking health care
Beyond the facets of dress codes and social media posts, the issue of professional personae speaks to the deeper issue of inclusion in medicine. As the field grows increasingly diverse, health care institutions and those they serve may need to expand their definitions of professionalism to include more truthful expressions of who contemporary health care professionals are as people.
Dr. Fraser suggests that the benefits of physicians embracing self-expression – rather than assimilating to an outdated model of professionalism – extend beyond the individual.
“Whether it comes to what you choose to wear to the clinic on a day-to-day basis, or what you choose to share on a social media account, as long as it’s not harming others, then I think that it’s a positive thing to be able to be yourself and express yourself,” she says. “I feel like doctors are expected to have a different personality when we’re at the clinic, and usually it’s more conservative or objective or aloof. But I think that by being open about who we are, we’ll actually help build a trusting relationship with both patients and society.”
A version of this article first appeared on Medscape.com.
On one of the first days of medical school, Adaira Landry, MD, applied her favorite dark shade of lipstick and headed to her orientation. She was eager to learn about program expectations and connect with fellow aspiring physicians. But when Dr. Landry got there, one of her brand-new peers turned to her and asked, “Why do you wear your lipstick like an angry Black woman?”
“Imagine hearing that,” Dr. Landry, now an emergency medical physician in Boston, says. “It was so hurtful.”
So, what is a “standard-issue doctor” expected to look like? Physicians manage their appearances in myriad ways: through clothes, accessories, hair style, makeup; through a social media presence or lack thereof; in the rhythms and nuances of their interactions with patients and colleagues. These things add up to a professional “persona” – the Latin word for “mask,” or the face on display for the world to see.
While the health care field itself is diversifying, its guidelines for professionalism appear slower to change, often excluding or frowning upon expressions of individual personality or identity.
“Medicine is run primarily by men. It’s an objective truth,” Dr. Landry says. “Currently and historically, the standard of professionalism, especially in the physical sense, was set by them. As we increase diversity and welcome people bringing their authentic self to work, the prior definitions of professionalism are obviously in need of change.”
Split social media personalities
In August 2020, the Journal of Vascular Surgery published a study on the “prevalence of unprofessional social media content among young vascular surgeons.” The content that was deemed “unprofessional” included opinions on political issues like abortion and gun control. Photos of physicians holding alcoholic drinks or wearing “inappropriate/offensive attire,” including underwear, “provocative Halloween costumes,” and “bikinis/swimwear” were also censured. Six men and one woman worked on the study, and three of the male researchers took on the task of seeking out the “unprofessional” photos on social media. The resulting paper was reviewed by an all-male editorial board.
The study sparked immediate backlash and prompted hundreds of health care professionals to post photos of themselves in bathing suits with the hashtag “#medbikini.” The journal then retracted the study and issued an apology on Twitter, recognizing “errors in the design of the study with regards to conscious and unconscious bias.”
The researchers’ original definition of professionalism suggests that physicians should manage their personae even outside of work hours. “I think medicine in general is a very conservative and hierarchical field of study and of work, to say the least,” says Sarah Fraser, MD, a family medicine physician in Nova Scotia, Canada. “There’s this view that we have to have completely separate personal and professional lives, like church and state.”
The #medbikini controversy inspired Dr. Fraser to write an op-ed for the British Medical Journal blog about the flaws of requiring physicians to keep their personal and professional selves separate. The piece referenced Robert Louis Stevenson’s 1886 Gothic novella “The Strange Case of Dr. Jekyll and Mr. Hyde,” in which the respected scientist Dr. Jekyll creates an alter ego so he can express his evil urges without experiencing guilt, punishment, or loss of livelihood. Dr. Fraser likened this story to the pressure physicians feel to shrink or split themselves to squeeze into a narrow definition of professionalism.
But Dr. Landry points out that some elements of expression seen as unprofessional cannot be entirely separated from a physician’s fundamental identity. “For Black women, our daily behaviors and forms of expression that are deemed ‘unprofessional’ are much more subtle than being able to wear a bikini on social media,” she says. “The way we wear our hair, the tone of our voice, the color of our lipstick, the way we wear scrub caps are parts of us that are called into question.”
Keeping up appearances
The stereotype of what a doctor should look like starts to shape physicians’ professional personae in medical school. When Jennifer Caputo-Seidler, MD, started medical school in 2008, the dress code requirements for male students were simple: pants, a button-down shirt, a tie. But then there were the rules for women: Hair should be tied back. Minimal makeup. No flashy jewelry. Nothing without sleeves. Neutral colors. High necklines. Low hemlines. “The message I got was that we need to dress like the men in order to be taken seriously and to be seen as professional,” says Dr. Caputo-Seidler, now an assistant professor of medicine at the University of South Florida, Tampa, “and so that’s what I did.”
A 2018 analysis of 78 “draw-a-scientist” studies found that children have overwhelmingly associated scientific fields with men for the last 50 years. Overall, children drew 73% of scientists as men. The drawings grew more gender diverse over time, but even as more women entered scientific fields, both boys and girls continued to draw significantly more male than female scientists.
Not everyone at Dr. Caputo-Seidler’s medical school adhered to the environment’s gendered expectations. One resident she worked with often wore voluminous hairstyles, lipstick, and high heels. Dr. Caputo-Seidler overheard her peers as they gossiped behind the resident’s back, ridiculing the way she looked.
“She was good at her job,” Dr. Caputo-Seidler says. “She knew her patients. She had things down. She was, by all measures, very competent. But when people saw her dressing outside the norm and being forward with her femininity, there was definitely a lot of chatter about it.”
While expectations for a conservative appearance may disproportionately affect women, and particularly women of color, they also affect men who deviate from the norm. “As an LGBTQ+ person working as a ‘professional,’ I have countless stories and moments where I had my professionalism questioned,” Blair Peters, MD, a plastic surgeon and assistant professor at Oregon Health & Science University, Portland, wrote on Twitter. “Why is it ‘unprofessional’ to have colored hair? Why is it ‘unprofessional’ to have a visible tattoo? Why is it ‘unprofessional’ to wear bright colors and patterns?”
Dr. Fraser remembers a fellow medical student who had full-sleeve tattoos on both of his arms. A preceptor made a comment about it to Dr. Fraser, and then instructed the student to cover up his tattoos. “I think that there are scenarios when having tattoos or having different-colored hair or expressing your individual personality could help you even better bond with your patients,” Dr. Fraser says, “especially if you’re, for example, working with youth.”
Unmasking health care
Beyond the facets of dress codes and social media posts, the issue of professional personae speaks to the deeper issue of inclusion in medicine. As the field grows increasingly diverse, health care institutions and those they serve may need to expand their definitions of professionalism to include more truthful expressions of who contemporary health care professionals are as people.
Dr. Fraser suggests that the benefits of physicians embracing self-expression – rather than assimilating to an outdated model of professionalism – extend beyond the individual.
“Whether it comes to what you choose to wear to the clinic on a day-to-day basis, or what you choose to share on a social media account, as long as it’s not harming others, then I think that it’s a positive thing to be able to be yourself and express yourself,” she says. “I feel like doctors are expected to have a different personality when we’re at the clinic, and usually it’s more conservative or objective or aloof. But I think that by being open about who we are, we’ll actually help build a trusting relationship with both patients and society.”
A version of this article first appeared on Medscape.com.
On one of the first days of medical school, Adaira Landry, MD, applied her favorite dark shade of lipstick and headed to her orientation. She was eager to learn about program expectations and connect with fellow aspiring physicians. But when Dr. Landry got there, one of her brand-new peers turned to her and asked, “Why do you wear your lipstick like an angry Black woman?”
“Imagine hearing that,” Dr. Landry, now an emergency medical physician in Boston, says. “It was so hurtful.”
So, what is a “standard-issue doctor” expected to look like? Physicians manage their appearances in myriad ways: through clothes, accessories, hair style, makeup; through a social media presence or lack thereof; in the rhythms and nuances of their interactions with patients and colleagues. These things add up to a professional “persona” – the Latin word for “mask,” or the face on display for the world to see.
While the health care field itself is diversifying, its guidelines for professionalism appear slower to change, often excluding or frowning upon expressions of individual personality or identity.
“Medicine is run primarily by men. It’s an objective truth,” Dr. Landry says. “Currently and historically, the standard of professionalism, especially in the physical sense, was set by them. As we increase diversity and welcome people bringing their authentic self to work, the prior definitions of professionalism are obviously in need of change.”
Split social media personalities
In August 2020, the Journal of Vascular Surgery published a study on the “prevalence of unprofessional social media content among young vascular surgeons.” The content that was deemed “unprofessional” included opinions on political issues like abortion and gun control. Photos of physicians holding alcoholic drinks or wearing “inappropriate/offensive attire,” including underwear, “provocative Halloween costumes,” and “bikinis/swimwear” were also censured. Six men and one woman worked on the study, and three of the male researchers took on the task of seeking out the “unprofessional” photos on social media. The resulting paper was reviewed by an all-male editorial board.
The study sparked immediate backlash and prompted hundreds of health care professionals to post photos of themselves in bathing suits with the hashtag “#medbikini.” The journal then retracted the study and issued an apology on Twitter, recognizing “errors in the design of the study with regards to conscious and unconscious bias.”
The researchers’ original definition of professionalism suggests that physicians should manage their personae even outside of work hours. “I think medicine in general is a very conservative and hierarchical field of study and of work, to say the least,” says Sarah Fraser, MD, a family medicine physician in Nova Scotia, Canada. “There’s this view that we have to have completely separate personal and professional lives, like church and state.”
The #medbikini controversy inspired Dr. Fraser to write an op-ed for the British Medical Journal blog about the flaws of requiring physicians to keep their personal and professional selves separate. The piece referenced Robert Louis Stevenson’s 1886 Gothic novella “The Strange Case of Dr. Jekyll and Mr. Hyde,” in which the respected scientist Dr. Jekyll creates an alter ego so he can express his evil urges without experiencing guilt, punishment, or loss of livelihood. Dr. Fraser likened this story to the pressure physicians feel to shrink or split themselves to squeeze into a narrow definition of professionalism.
But Dr. Landry points out that some elements of expression seen as unprofessional cannot be entirely separated from a physician’s fundamental identity. “For Black women, our daily behaviors and forms of expression that are deemed ‘unprofessional’ are much more subtle than being able to wear a bikini on social media,” she says. “The way we wear our hair, the tone of our voice, the color of our lipstick, the way we wear scrub caps are parts of us that are called into question.”
Keeping up appearances
The stereotype of what a doctor should look like starts to shape physicians’ professional personae in medical school. When Jennifer Caputo-Seidler, MD, started medical school in 2008, the dress code requirements for male students were simple: pants, a button-down shirt, a tie. But then there were the rules for women: Hair should be tied back. Minimal makeup. No flashy jewelry. Nothing without sleeves. Neutral colors. High necklines. Low hemlines. “The message I got was that we need to dress like the men in order to be taken seriously and to be seen as professional,” says Dr. Caputo-Seidler, now an assistant professor of medicine at the University of South Florida, Tampa, “and so that’s what I did.”
A 2018 analysis of 78 “draw-a-scientist” studies found that children have overwhelmingly associated scientific fields with men for the last 50 years. Overall, children drew 73% of scientists as men. The drawings grew more gender diverse over time, but even as more women entered scientific fields, both boys and girls continued to draw significantly more male than female scientists.
Not everyone at Dr. Caputo-Seidler’s medical school adhered to the environment’s gendered expectations. One resident she worked with often wore voluminous hairstyles, lipstick, and high heels. Dr. Caputo-Seidler overheard her peers as they gossiped behind the resident’s back, ridiculing the way she looked.
“She was good at her job,” Dr. Caputo-Seidler says. “She knew her patients. She had things down. She was, by all measures, very competent. But when people saw her dressing outside the norm and being forward with her femininity, there was definitely a lot of chatter about it.”
While expectations for a conservative appearance may disproportionately affect women, and particularly women of color, they also affect men who deviate from the norm. “As an LGBTQ+ person working as a ‘professional,’ I have countless stories and moments where I had my professionalism questioned,” Blair Peters, MD, a plastic surgeon and assistant professor at Oregon Health & Science University, Portland, wrote on Twitter. “Why is it ‘unprofessional’ to have colored hair? Why is it ‘unprofessional’ to have a visible tattoo? Why is it ‘unprofessional’ to wear bright colors and patterns?”
Dr. Fraser remembers a fellow medical student who had full-sleeve tattoos on both of his arms. A preceptor made a comment about it to Dr. Fraser, and then instructed the student to cover up his tattoos. “I think that there are scenarios when having tattoos or having different-colored hair or expressing your individual personality could help you even better bond with your patients,” Dr. Fraser says, “especially if you’re, for example, working with youth.”
Unmasking health care
Beyond the facets of dress codes and social media posts, the issue of professional personae speaks to the deeper issue of inclusion in medicine. As the field grows increasingly diverse, health care institutions and those they serve may need to expand their definitions of professionalism to include more truthful expressions of who contemporary health care professionals are as people.
Dr. Fraser suggests that the benefits of physicians embracing self-expression – rather than assimilating to an outdated model of professionalism – extend beyond the individual.
“Whether it comes to what you choose to wear to the clinic on a day-to-day basis, or what you choose to share on a social media account, as long as it’s not harming others, then I think that it’s a positive thing to be able to be yourself and express yourself,” she says. “I feel like doctors are expected to have a different personality when we’re at the clinic, and usually it’s more conservative or objective or aloof. But I think that by being open about who we are, we’ll actually help build a trusting relationship with both patients and society.”
A version of this article first appeared on Medscape.com.






