User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Doctors are disappearing from emergency departments as hospitals look to cut costs
She didn’t know much about miscarriage, but this seemed like one.
In the emergency department, she was examined then sent home, she said. She went back when her cramping became excruciating. Then home again. It ultimately took three trips to the ED on 3 consecutive days, generating three separate bills, before she saw a doctor who looked at her blood work and confirmed her fears.
“At the time I wasn’t thinking, ‘Oh, I need to see a doctor,’ ” Ms. Valle recalled. “But when you think about it, it’s like, ‘Well, dang – why didn’t I see a doctor?’ ” It’s unclear whether the repeat visits were due to delays in seeing a physician, but the experience worried her. And she’s still paying the bills.
The hospital declined to discuss Ms. Valle’s care, citing patient privacy. But 17 months before her 3-day ordeal, Tennova had outsourced its emergency departments to American Physician Partners, a medical staffing company owned by private equity investors. APP employs fewer doctors in its EDs as one of its cost-saving initiatives to increase earnings, according to a confidential company document obtained by KHN and NPR.
This staffing strategy has permeated hospitals, and particularly emergency departments, that seek to reduce their top expense: physician labor. While diagnosing and treating patients was once their domain, doctors are increasingly being replaced by nurse practitioners and physician assistants, collectively known as “midlevel practitioners,” who can perform many of the same duties and generate much of the same revenue for less than half of the pay.
“APP has numerous cost saving initiatives underway as part of the Company’s continual focus on cost optimization,” the document says, including a “shift of staffing” between doctors and midlevel practitioners.
In a statement to KHN, American Physician Partners said this strategy is a way to ensure all EDs remain fully staffed, calling it a “blended model” that allows doctors, nurse practitioners, and physician assistants “to provide care to their fullest potential.”
Critics of this strategy say the quest to save money results in treatment meted out by someone with far less training than a physician, leaving patients vulnerable to misdiagnoses, higher medical bills, and inadequate care. And these fears are bolstered by evidence that suggests dropping doctors from EDs may not be good for patients.
A working paper, published in October by the National Bureau of Economic Research, analyzed roughly 1.1 million visits to 44 EDs throughout the Veterans Health Administration, where nurse practitioners can treat patients without oversight from doctors.
Researchers found that treatment by a nurse practitioner resulted on average in a 7% increase in cost of care and an 11% increase in length of stay, extending patients’ time in the ED by minutes for minor visits and hours for longer ones. These gaps widened among patients with more severe diagnoses, the study said, but could be somewhat mitigated by nurse practitioners with more experience.
The study also found that ED patients treated by a nurse practitioner were 20% more likely to be readmitted to the hospital for a preventable reason within 30 days, although the overall risk of readmission remained very small.
Yiqun Chen, PhD, who is an assistant professor of economics at the University of Illinois at Chicago and coauthored the study, said these findings are not an indictment of nurse practitioners in the ED. Instead, she said, she hopes the study will guide how to best deploy nurse practitioners: in treatment of simpler patients or circumstances when no doctor is available.
“It’s not just a simple question of if we can substitute physicians with nurse practitioners or not,” Dr. Chen said. “It depends on how we use them. If we just use them as independent providers, especially ... for relatively complicated patients, it doesn’t seem to be a very good use.”
Dr. Chen’s research echoes smaller studies, like one from The Harvey L. Neiman Health Policy Institute that found nonphysician practitioners in EDs were associated with a 5.3% increase in imaging, which could unnecessarily increase bills for patients. Separately, a study at the Hattiesburg Clinic in Mississippi found that midlevel practitioners in primary care – not in the emergency department – increased the out-of-pocket costs to patients while also leading to worse performance on 9 of 10 quality-of-care metrics, including cancer screenings and vaccination rates.
But definitive evidence remains elusive that replacing ER doctors with nonphysicians has a negative impact on patients, said Cameron Gettel, MD, an assistant professor of emergency medicine at Yale University, New Haven, Conn. Private equity investment and the use of midlevel practitioners rose in lockstep in the ED, Dr. Gettel said, and in the absence of game-changing research, the pattern will likely continue.
“Worse patient outcomes haven’t really been shown across the board,” he said. “And I think until that is shown, then they will continue to play an increasing role.”
For private equity, dropping ED docs is a “simple equation”
Private equity companies pool money from wealthy investors to buy their way into various industries, often slashing spending and seeking to flip businesses in 3 to 7 years. While this business model is a proven moneymaker on Wall Street, it raises concerns in health care, where critics worry the pressure to turn big profits will influence life-or-death decisions that were once left solely to medical professionals.
Nearly $1 trillion in private equity funds have gone into almost 8,000 health care transactions over the past decade, according to industry tracker PitchBook, including buying into medical staffing companies that many hospitals hire to manage their emergency departments.
Two firms dominate the ED staffing industry: TeamHealth, bought by private equity firm Blackstone in 2016, and Envision Healthcare, bought by KKR in 2018. Trying to undercut these staffing giants is American Physician Partners, a rapidly expanding company that runs EDs in at least 17 states and is 50% owned by private equity firm BBH Capital Partners.
These staffing companies have been among the most aggressive in replacing doctors to cut costs, said Robert McNamara, MD, a founder of the American Academy of Emergency Medicine and chair of emergency medicine at Temple University, Philadelphia.
“It’s a relatively simple equation,” Dr. McNamara said. “Their No. 1 expense is the board-certified emergency physician. So they are going to want to keep that expense as low as possible.”
Not everyone sees the trend of private equity in ED staffing in a negative light. Jennifer Orozco, president of the American Academy of Physician Associates, which represents physician assistants, said even if the change – to use more nonphysician providers – is driven by the staffing firms’ desire to make more money, patients are still well served by a team approach that includes nurse practitioners and physician assistants.
“Though I see that shift, it’s not about profits at the end of the day,” Ms. Orozco said. “It’s about the patient.”
The “shift” is nearly invisible to patients because hospitals rarely promote branding from their ED staffing firms and there is little public documentation of private equity investments.
Arthur Smolensky, MD, a Tennessee emergency medicine specialist attempting to measure private equity’s intrusion into EDs, said his review of hospital job postings and employment contracts in 14 major metropolitan areas found that 43% of ED patients were seen in EDs staffed by companies with nonphysician owners, nearly all of whom are private equity investors.
Dr. Smolensky hopes to publish his full study, expanding to 55 metro areas, later this year. But this research will merely quantify what many doctors already know: The ED has changed. Demoralized by an increased focus on profit, and wary of a looming surplus of emergency medicine residents because there are fewer jobs to fill, many experienced doctors are leaving the ED on their own, he said.
“Most of us didn’t go into medicine to supervise an army of people that are not as well trained as we are,” Dr. Smolensky said. “We want to take care of patients.”
“I guess we’re the first guinea pigs for our ER”
Joshua Allen, a nurse practitioner at a small Kentucky hospital, snaked a rubber hose through a rack of pork ribs to practice inserting a chest tube to fix a collapsed lung.
It was 2020, and American Physician Partners was restructuring the ED where Mr. Allen worked, reducing shifts from two doctors to one. Once Mr. Allen had placed 10 tubes under a doctor’s supervision, he would be allowed to do it on his own.
“I guess we’re the first guinea pigs for our ER,” he said. “If we do have a major trauma and multiple victims come in, there’s only one doctor there. ... We need to be prepared.”
Mr. Allen is one of many midlevel practitioners finding work in emergency departments. Nurse practitioners and physician assistants are among the fastest-growing occupations in the nation, according to the U.S. Bureau of Labor Statistics.
Generally, they have master’s degrees and receive several years of specialized schooling but have significantly less training than doctors. Many are permitted to diagnose patients and prescribe medication with little or no supervision from a doctor, although limitations vary by state.
The Neiman Institute found that the share of ED visits in which a midlevel practitioner was the main clinician increased by more than 172% between 2005 and 2020. Another study, in the Journal of Emergency Medicine, reported that if trends continue there may be equal numbers of midlevel practitioners and doctors in EDs by 2030.
There is little mystery as to why. Federal data shows emergency medicine doctors are paid about $310,000 a year on average, while nurse practitioners and physician assistants earn less than $120,000. Generally, hospitals can bill for care by a midlevel practitioner at 85% the rate of a doctor while paying them less than half as much.
Private equity can make millions in the gap.
For example, Envision once encouraged EDs to employ “the least expensive resource” and treat up to 35% of patients with midlevel practitioners, according to a 2017 PowerPoint presentation. The presentation drew scorn on social media and disappeared from Envision’s website.
Envision declined a request for a phone interview. In a written statement to KHN, spokesperson Aliese Polk said the company does not direct its physician leaders on how to care for patients and called the presentation a “concept guide” that does not represent current views.
American Physician Partners touted roughly the same staffing strategy in 2021 in response to the No Surprises Act, which threatened the company’s profits by outlawing surprise medical bills. In its confidential pitch to lenders, the company estimated it could cut almost $6 million by shifting more staffing from physicians to midlevel practitioners.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
She didn’t know much about miscarriage, but this seemed like one.
In the emergency department, she was examined then sent home, she said. She went back when her cramping became excruciating. Then home again. It ultimately took three trips to the ED on 3 consecutive days, generating three separate bills, before she saw a doctor who looked at her blood work and confirmed her fears.
“At the time I wasn’t thinking, ‘Oh, I need to see a doctor,’ ” Ms. Valle recalled. “But when you think about it, it’s like, ‘Well, dang – why didn’t I see a doctor?’ ” It’s unclear whether the repeat visits were due to delays in seeing a physician, but the experience worried her. And she’s still paying the bills.
The hospital declined to discuss Ms. Valle’s care, citing patient privacy. But 17 months before her 3-day ordeal, Tennova had outsourced its emergency departments to American Physician Partners, a medical staffing company owned by private equity investors. APP employs fewer doctors in its EDs as one of its cost-saving initiatives to increase earnings, according to a confidential company document obtained by KHN and NPR.
This staffing strategy has permeated hospitals, and particularly emergency departments, that seek to reduce their top expense: physician labor. While diagnosing and treating patients was once their domain, doctors are increasingly being replaced by nurse practitioners and physician assistants, collectively known as “midlevel practitioners,” who can perform many of the same duties and generate much of the same revenue for less than half of the pay.
“APP has numerous cost saving initiatives underway as part of the Company’s continual focus on cost optimization,” the document says, including a “shift of staffing” between doctors and midlevel practitioners.
In a statement to KHN, American Physician Partners said this strategy is a way to ensure all EDs remain fully staffed, calling it a “blended model” that allows doctors, nurse practitioners, and physician assistants “to provide care to their fullest potential.”
Critics of this strategy say the quest to save money results in treatment meted out by someone with far less training than a physician, leaving patients vulnerable to misdiagnoses, higher medical bills, and inadequate care. And these fears are bolstered by evidence that suggests dropping doctors from EDs may not be good for patients.
A working paper, published in October by the National Bureau of Economic Research, analyzed roughly 1.1 million visits to 44 EDs throughout the Veterans Health Administration, where nurse practitioners can treat patients without oversight from doctors.
Researchers found that treatment by a nurse practitioner resulted on average in a 7% increase in cost of care and an 11% increase in length of stay, extending patients’ time in the ED by minutes for minor visits and hours for longer ones. These gaps widened among patients with more severe diagnoses, the study said, but could be somewhat mitigated by nurse practitioners with more experience.
The study also found that ED patients treated by a nurse practitioner were 20% more likely to be readmitted to the hospital for a preventable reason within 30 days, although the overall risk of readmission remained very small.
Yiqun Chen, PhD, who is an assistant professor of economics at the University of Illinois at Chicago and coauthored the study, said these findings are not an indictment of nurse practitioners in the ED. Instead, she said, she hopes the study will guide how to best deploy nurse practitioners: in treatment of simpler patients or circumstances when no doctor is available.
“It’s not just a simple question of if we can substitute physicians with nurse practitioners or not,” Dr. Chen said. “It depends on how we use them. If we just use them as independent providers, especially ... for relatively complicated patients, it doesn’t seem to be a very good use.”
Dr. Chen’s research echoes smaller studies, like one from The Harvey L. Neiman Health Policy Institute that found nonphysician practitioners in EDs were associated with a 5.3% increase in imaging, which could unnecessarily increase bills for patients. Separately, a study at the Hattiesburg Clinic in Mississippi found that midlevel practitioners in primary care – not in the emergency department – increased the out-of-pocket costs to patients while also leading to worse performance on 9 of 10 quality-of-care metrics, including cancer screenings and vaccination rates.
But definitive evidence remains elusive that replacing ER doctors with nonphysicians has a negative impact on patients, said Cameron Gettel, MD, an assistant professor of emergency medicine at Yale University, New Haven, Conn. Private equity investment and the use of midlevel practitioners rose in lockstep in the ED, Dr. Gettel said, and in the absence of game-changing research, the pattern will likely continue.
“Worse patient outcomes haven’t really been shown across the board,” he said. “And I think until that is shown, then they will continue to play an increasing role.”
For private equity, dropping ED docs is a “simple equation”
Private equity companies pool money from wealthy investors to buy their way into various industries, often slashing spending and seeking to flip businesses in 3 to 7 years. While this business model is a proven moneymaker on Wall Street, it raises concerns in health care, where critics worry the pressure to turn big profits will influence life-or-death decisions that were once left solely to medical professionals.
Nearly $1 trillion in private equity funds have gone into almost 8,000 health care transactions over the past decade, according to industry tracker PitchBook, including buying into medical staffing companies that many hospitals hire to manage their emergency departments.
Two firms dominate the ED staffing industry: TeamHealth, bought by private equity firm Blackstone in 2016, and Envision Healthcare, bought by KKR in 2018. Trying to undercut these staffing giants is American Physician Partners, a rapidly expanding company that runs EDs in at least 17 states and is 50% owned by private equity firm BBH Capital Partners.
These staffing companies have been among the most aggressive in replacing doctors to cut costs, said Robert McNamara, MD, a founder of the American Academy of Emergency Medicine and chair of emergency medicine at Temple University, Philadelphia.
“It’s a relatively simple equation,” Dr. McNamara said. “Their No. 1 expense is the board-certified emergency physician. So they are going to want to keep that expense as low as possible.”
Not everyone sees the trend of private equity in ED staffing in a negative light. Jennifer Orozco, president of the American Academy of Physician Associates, which represents physician assistants, said even if the change – to use more nonphysician providers – is driven by the staffing firms’ desire to make more money, patients are still well served by a team approach that includes nurse practitioners and physician assistants.
“Though I see that shift, it’s not about profits at the end of the day,” Ms. Orozco said. “It’s about the patient.”
The “shift” is nearly invisible to patients because hospitals rarely promote branding from their ED staffing firms and there is little public documentation of private equity investments.
Arthur Smolensky, MD, a Tennessee emergency medicine specialist attempting to measure private equity’s intrusion into EDs, said his review of hospital job postings and employment contracts in 14 major metropolitan areas found that 43% of ED patients were seen in EDs staffed by companies with nonphysician owners, nearly all of whom are private equity investors.
Dr. Smolensky hopes to publish his full study, expanding to 55 metro areas, later this year. But this research will merely quantify what many doctors already know: The ED has changed. Demoralized by an increased focus on profit, and wary of a looming surplus of emergency medicine residents because there are fewer jobs to fill, many experienced doctors are leaving the ED on their own, he said.
“Most of us didn’t go into medicine to supervise an army of people that are not as well trained as we are,” Dr. Smolensky said. “We want to take care of patients.”
“I guess we’re the first guinea pigs for our ER”
Joshua Allen, a nurse practitioner at a small Kentucky hospital, snaked a rubber hose through a rack of pork ribs to practice inserting a chest tube to fix a collapsed lung.
It was 2020, and American Physician Partners was restructuring the ED where Mr. Allen worked, reducing shifts from two doctors to one. Once Mr. Allen had placed 10 tubes under a doctor’s supervision, he would be allowed to do it on his own.
“I guess we’re the first guinea pigs for our ER,” he said. “If we do have a major trauma and multiple victims come in, there’s only one doctor there. ... We need to be prepared.”
Mr. Allen is one of many midlevel practitioners finding work in emergency departments. Nurse practitioners and physician assistants are among the fastest-growing occupations in the nation, according to the U.S. Bureau of Labor Statistics.
Generally, they have master’s degrees and receive several years of specialized schooling but have significantly less training than doctors. Many are permitted to diagnose patients and prescribe medication with little or no supervision from a doctor, although limitations vary by state.
The Neiman Institute found that the share of ED visits in which a midlevel practitioner was the main clinician increased by more than 172% between 2005 and 2020. Another study, in the Journal of Emergency Medicine, reported that if trends continue there may be equal numbers of midlevel practitioners and doctors in EDs by 2030.
There is little mystery as to why. Federal data shows emergency medicine doctors are paid about $310,000 a year on average, while nurse practitioners and physician assistants earn less than $120,000. Generally, hospitals can bill for care by a midlevel practitioner at 85% the rate of a doctor while paying them less than half as much.
Private equity can make millions in the gap.
For example, Envision once encouraged EDs to employ “the least expensive resource” and treat up to 35% of patients with midlevel practitioners, according to a 2017 PowerPoint presentation. The presentation drew scorn on social media and disappeared from Envision’s website.
Envision declined a request for a phone interview. In a written statement to KHN, spokesperson Aliese Polk said the company does not direct its physician leaders on how to care for patients and called the presentation a “concept guide” that does not represent current views.
American Physician Partners touted roughly the same staffing strategy in 2021 in response to the No Surprises Act, which threatened the company’s profits by outlawing surprise medical bills. In its confidential pitch to lenders, the company estimated it could cut almost $6 million by shifting more staffing from physicians to midlevel practitioners.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
She didn’t know much about miscarriage, but this seemed like one.
In the emergency department, she was examined then sent home, she said. She went back when her cramping became excruciating. Then home again. It ultimately took three trips to the ED on 3 consecutive days, generating three separate bills, before she saw a doctor who looked at her blood work and confirmed her fears.
“At the time I wasn’t thinking, ‘Oh, I need to see a doctor,’ ” Ms. Valle recalled. “But when you think about it, it’s like, ‘Well, dang – why didn’t I see a doctor?’ ” It’s unclear whether the repeat visits were due to delays in seeing a physician, but the experience worried her. And she’s still paying the bills.
The hospital declined to discuss Ms. Valle’s care, citing patient privacy. But 17 months before her 3-day ordeal, Tennova had outsourced its emergency departments to American Physician Partners, a medical staffing company owned by private equity investors. APP employs fewer doctors in its EDs as one of its cost-saving initiatives to increase earnings, according to a confidential company document obtained by KHN and NPR.
This staffing strategy has permeated hospitals, and particularly emergency departments, that seek to reduce their top expense: physician labor. While diagnosing and treating patients was once their domain, doctors are increasingly being replaced by nurse practitioners and physician assistants, collectively known as “midlevel practitioners,” who can perform many of the same duties and generate much of the same revenue for less than half of the pay.
“APP has numerous cost saving initiatives underway as part of the Company’s continual focus on cost optimization,” the document says, including a “shift of staffing” between doctors and midlevel practitioners.
In a statement to KHN, American Physician Partners said this strategy is a way to ensure all EDs remain fully staffed, calling it a “blended model” that allows doctors, nurse practitioners, and physician assistants “to provide care to their fullest potential.”
Critics of this strategy say the quest to save money results in treatment meted out by someone with far less training than a physician, leaving patients vulnerable to misdiagnoses, higher medical bills, and inadequate care. And these fears are bolstered by evidence that suggests dropping doctors from EDs may not be good for patients.
A working paper, published in October by the National Bureau of Economic Research, analyzed roughly 1.1 million visits to 44 EDs throughout the Veterans Health Administration, where nurse practitioners can treat patients without oversight from doctors.
Researchers found that treatment by a nurse practitioner resulted on average in a 7% increase in cost of care and an 11% increase in length of stay, extending patients’ time in the ED by minutes for minor visits and hours for longer ones. These gaps widened among patients with more severe diagnoses, the study said, but could be somewhat mitigated by nurse practitioners with more experience.
The study also found that ED patients treated by a nurse practitioner were 20% more likely to be readmitted to the hospital for a preventable reason within 30 days, although the overall risk of readmission remained very small.
Yiqun Chen, PhD, who is an assistant professor of economics at the University of Illinois at Chicago and coauthored the study, said these findings are not an indictment of nurse practitioners in the ED. Instead, she said, she hopes the study will guide how to best deploy nurse practitioners: in treatment of simpler patients or circumstances when no doctor is available.
“It’s not just a simple question of if we can substitute physicians with nurse practitioners or not,” Dr. Chen said. “It depends on how we use them. If we just use them as independent providers, especially ... for relatively complicated patients, it doesn’t seem to be a very good use.”
Dr. Chen’s research echoes smaller studies, like one from The Harvey L. Neiman Health Policy Institute that found nonphysician practitioners in EDs were associated with a 5.3% increase in imaging, which could unnecessarily increase bills for patients. Separately, a study at the Hattiesburg Clinic in Mississippi found that midlevel practitioners in primary care – not in the emergency department – increased the out-of-pocket costs to patients while also leading to worse performance on 9 of 10 quality-of-care metrics, including cancer screenings and vaccination rates.
But definitive evidence remains elusive that replacing ER doctors with nonphysicians has a negative impact on patients, said Cameron Gettel, MD, an assistant professor of emergency medicine at Yale University, New Haven, Conn. Private equity investment and the use of midlevel practitioners rose in lockstep in the ED, Dr. Gettel said, and in the absence of game-changing research, the pattern will likely continue.
“Worse patient outcomes haven’t really been shown across the board,” he said. “And I think until that is shown, then they will continue to play an increasing role.”
For private equity, dropping ED docs is a “simple equation”
Private equity companies pool money from wealthy investors to buy their way into various industries, often slashing spending and seeking to flip businesses in 3 to 7 years. While this business model is a proven moneymaker on Wall Street, it raises concerns in health care, where critics worry the pressure to turn big profits will influence life-or-death decisions that were once left solely to medical professionals.
Nearly $1 trillion in private equity funds have gone into almost 8,000 health care transactions over the past decade, according to industry tracker PitchBook, including buying into medical staffing companies that many hospitals hire to manage their emergency departments.
Two firms dominate the ED staffing industry: TeamHealth, bought by private equity firm Blackstone in 2016, and Envision Healthcare, bought by KKR in 2018. Trying to undercut these staffing giants is American Physician Partners, a rapidly expanding company that runs EDs in at least 17 states and is 50% owned by private equity firm BBH Capital Partners.
These staffing companies have been among the most aggressive in replacing doctors to cut costs, said Robert McNamara, MD, a founder of the American Academy of Emergency Medicine and chair of emergency medicine at Temple University, Philadelphia.
“It’s a relatively simple equation,” Dr. McNamara said. “Their No. 1 expense is the board-certified emergency physician. So they are going to want to keep that expense as low as possible.”
Not everyone sees the trend of private equity in ED staffing in a negative light. Jennifer Orozco, president of the American Academy of Physician Associates, which represents physician assistants, said even if the change – to use more nonphysician providers – is driven by the staffing firms’ desire to make more money, patients are still well served by a team approach that includes nurse practitioners and physician assistants.
“Though I see that shift, it’s not about profits at the end of the day,” Ms. Orozco said. “It’s about the patient.”
The “shift” is nearly invisible to patients because hospitals rarely promote branding from their ED staffing firms and there is little public documentation of private equity investments.
Arthur Smolensky, MD, a Tennessee emergency medicine specialist attempting to measure private equity’s intrusion into EDs, said his review of hospital job postings and employment contracts in 14 major metropolitan areas found that 43% of ED patients were seen in EDs staffed by companies with nonphysician owners, nearly all of whom are private equity investors.
Dr. Smolensky hopes to publish his full study, expanding to 55 metro areas, later this year. But this research will merely quantify what many doctors already know: The ED has changed. Demoralized by an increased focus on profit, and wary of a looming surplus of emergency medicine residents because there are fewer jobs to fill, many experienced doctors are leaving the ED on their own, he said.
“Most of us didn’t go into medicine to supervise an army of people that are not as well trained as we are,” Dr. Smolensky said. “We want to take care of patients.”
“I guess we’re the first guinea pigs for our ER”
Joshua Allen, a nurse practitioner at a small Kentucky hospital, snaked a rubber hose through a rack of pork ribs to practice inserting a chest tube to fix a collapsed lung.
It was 2020, and American Physician Partners was restructuring the ED where Mr. Allen worked, reducing shifts from two doctors to one. Once Mr. Allen had placed 10 tubes under a doctor’s supervision, he would be allowed to do it on his own.
“I guess we’re the first guinea pigs for our ER,” he said. “If we do have a major trauma and multiple victims come in, there’s only one doctor there. ... We need to be prepared.”
Mr. Allen is one of many midlevel practitioners finding work in emergency departments. Nurse practitioners and physician assistants are among the fastest-growing occupations in the nation, according to the U.S. Bureau of Labor Statistics.
Generally, they have master’s degrees and receive several years of specialized schooling but have significantly less training than doctors. Many are permitted to diagnose patients and prescribe medication with little or no supervision from a doctor, although limitations vary by state.
The Neiman Institute found that the share of ED visits in which a midlevel practitioner was the main clinician increased by more than 172% between 2005 and 2020. Another study, in the Journal of Emergency Medicine, reported that if trends continue there may be equal numbers of midlevel practitioners and doctors in EDs by 2030.
There is little mystery as to why. Federal data shows emergency medicine doctors are paid about $310,000 a year on average, while nurse practitioners and physician assistants earn less than $120,000. Generally, hospitals can bill for care by a midlevel practitioner at 85% the rate of a doctor while paying them less than half as much.
Private equity can make millions in the gap.
For example, Envision once encouraged EDs to employ “the least expensive resource” and treat up to 35% of patients with midlevel practitioners, according to a 2017 PowerPoint presentation. The presentation drew scorn on social media and disappeared from Envision’s website.
Envision declined a request for a phone interview. In a written statement to KHN, spokesperson Aliese Polk said the company does not direct its physician leaders on how to care for patients and called the presentation a “concept guide” that does not represent current views.
American Physician Partners touted roughly the same staffing strategy in 2021 in response to the No Surprises Act, which threatened the company’s profits by outlawing surprise medical bills. In its confidential pitch to lenders, the company estimated it could cut almost $6 million by shifting more staffing from physicians to midlevel practitioners.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
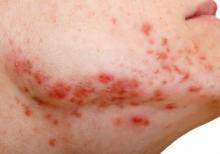
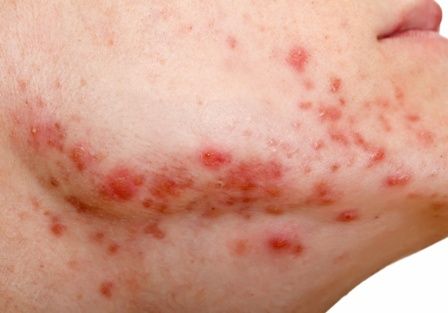
USPSTF recommends against routine herpes screening for asymptomatic teens and adults
Asymptomatic adults, teens, and pregnant women with no known history or symptoms of herpes infection need not undergo routine screening, according to the latest recommendation from the U.S. Preventive Services Task Force.
The 2023 recommendation reaffirms the conclusion from 2016, wrote Carol M. Mangione, MD, of the University of California, Los Angeles, and members of the task force.
“Currently, routine serologic screening for genital herpes is limited by the low predictive value of the widely available serologic screening tests and the expected high rate of false-positive results likely to occur with routine screening of asymptomatic persons in the U.S.,” the authors said.
In the recommendation, published in JAMA, the authors affirmed with moderate certainty and a grade D recommendation that the risks of routine screening for herpes simplex virus (HSV) in asymptomatic individuals outweigh the benefits.
The task force found no new evidence on the accuracy of serologic screening tests, the benefits of early detection and treatment, or on the harms of screening and treatment since the 2016 review of 17 studies in 19 publications, with data from more than 9,000 individuals.
Studies of the accuracy of serologic screening for herpes simplex virus-2 in the 2016 report mainly reflect populations with higher HSV-2 prevalence and are of limited applicability to the U.S. primary care population, the authors wrote. Evidence from the 2016 review also showed limited and inconsistent support for the early identification and treatment of HSV-2 in asymptomatic individuals, including those who were pregnant.
No new evidence has emerged since 2016 regarding harms of screening or treating genital herpes in asymptomatic individuals, the authors noted. “Based on previous evidence, the USPSTF estimated that using the widely available serologic tests for HSV-2, nearly 1 of every 2 diagnoses in the general U.S. primary care population could be false,” they said. The task force also concluded that the low accuracy of the current tests could prompt unnecessary treatment for individuals with false-positive diagnoses, as well as social and emotional harm for these individuals.
During a period of public comment from Aug. 16, 2022, to Sept. 12, 2022, individuals expressed concerns that the recommendation against routine screening showed a disinclination to take herpes seriously, and concerns that asymptomatic individuals could transmit the infection to sexual partners, the authors said. However, the estimated seroprevalence of HSV-1 and HSV-2 has declined in recent decades, and other comments supported the USPSTF’s analysis of the evidence and noted their consistency with current clinical practice.
The task force noted that research gaps remain and recognized the need to improve screening and treatment of genital herpes to prevent symptomatic episodes and transmission. Specifically, the USPSTF recommendation calls for more research to assess the accuracy of screening tests, to enroll more study participants from populations disproportionately affected by HSV, to examine the effect of behavioral counseling, and to clarify associations between HSV and pregnancy outcomes. In addition, the task force called for research to create an effective vaccine to prevent genital HSV infection and to develop a cure.
Targeted screening makes sense for now
“Given the frequency and severity of the range of diseases seen with HSV and the large proportion of persons who are asymptomatic, identifying carriers through type-specific serologic screening has long been considered a plausible strategy,” Mark D. Pearlman, MD, of the University of Michigan, Ann Arbor, wrote in an accompanying editorial.
However, accuracy of the currently available serology screening tests is low, and the adverse social and psychological effects and the impact on relationships for many asymptomatic individuals who test positive and may be incorrectly identified as infected remains a concern, said Dr. Pearlman.
Although some may be disagree about the value of routine serotesting for HSV-2 in asymptomatic individuals, other strategies can reduce the spread of infection and help those infected, he said.
Many experts continue to recommend targeted serotesting to high-risk populations, such as pregnant women whose nonpregnant partner is known to have genital or oral herpes and whose own infection status or serostatus is uncertain, said Dr. Pearlman. Other targeted strategies include screening individuals with recurrent or atypical genital symptoms and negative polymerase chain reaction assay or culture results, a clinical herpes diagnosis without laboratory confirmation, or those at increased risk because of a high number of sexual partners or a history of HIV infection, he said.
“Of note, the current CDC STI guidelines and ACOG both concur with the USPSTF that routine screening in the general population or routine screening during pregnancy are not recommended,” Dr. Pearlman said. Meanwhile, research efforts continue to help reduce the impact of HSV disease and development of a more effective testing methodology “might tip the balance in favor of routine screening” in the future, he emphasized.
The recommendations were supported by the Agency for Healthcare Research and Quality. The members of the task force received reimbursement for travel and an honorarium but had no other relevant financial conflicts to disclose. Dr. Pearlman had no financial conflicts to disclose.
Asymptomatic adults, teens, and pregnant women with no known history or symptoms of herpes infection need not undergo routine screening, according to the latest recommendation from the U.S. Preventive Services Task Force.
The 2023 recommendation reaffirms the conclusion from 2016, wrote Carol M. Mangione, MD, of the University of California, Los Angeles, and members of the task force.
“Currently, routine serologic screening for genital herpes is limited by the low predictive value of the widely available serologic screening tests and the expected high rate of false-positive results likely to occur with routine screening of asymptomatic persons in the U.S.,” the authors said.
In the recommendation, published in JAMA, the authors affirmed with moderate certainty and a grade D recommendation that the risks of routine screening for herpes simplex virus (HSV) in asymptomatic individuals outweigh the benefits.
The task force found no new evidence on the accuracy of serologic screening tests, the benefits of early detection and treatment, or on the harms of screening and treatment since the 2016 review of 17 studies in 19 publications, with data from more than 9,000 individuals.
Studies of the accuracy of serologic screening for herpes simplex virus-2 in the 2016 report mainly reflect populations with higher HSV-2 prevalence and are of limited applicability to the U.S. primary care population, the authors wrote. Evidence from the 2016 review also showed limited and inconsistent support for the early identification and treatment of HSV-2 in asymptomatic individuals, including those who were pregnant.
No new evidence has emerged since 2016 regarding harms of screening or treating genital herpes in asymptomatic individuals, the authors noted. “Based on previous evidence, the USPSTF estimated that using the widely available serologic tests for HSV-2, nearly 1 of every 2 diagnoses in the general U.S. primary care population could be false,” they said. The task force also concluded that the low accuracy of the current tests could prompt unnecessary treatment for individuals with false-positive diagnoses, as well as social and emotional harm for these individuals.
During a period of public comment from Aug. 16, 2022, to Sept. 12, 2022, individuals expressed concerns that the recommendation against routine screening showed a disinclination to take herpes seriously, and concerns that asymptomatic individuals could transmit the infection to sexual partners, the authors said. However, the estimated seroprevalence of HSV-1 and HSV-2 has declined in recent decades, and other comments supported the USPSTF’s analysis of the evidence and noted their consistency with current clinical practice.
The task force noted that research gaps remain and recognized the need to improve screening and treatment of genital herpes to prevent symptomatic episodes and transmission. Specifically, the USPSTF recommendation calls for more research to assess the accuracy of screening tests, to enroll more study participants from populations disproportionately affected by HSV, to examine the effect of behavioral counseling, and to clarify associations between HSV and pregnancy outcomes. In addition, the task force called for research to create an effective vaccine to prevent genital HSV infection and to develop a cure.
Targeted screening makes sense for now
“Given the frequency and severity of the range of diseases seen with HSV and the large proportion of persons who are asymptomatic, identifying carriers through type-specific serologic screening has long been considered a plausible strategy,” Mark D. Pearlman, MD, of the University of Michigan, Ann Arbor, wrote in an accompanying editorial.
However, accuracy of the currently available serology screening tests is low, and the adverse social and psychological effects and the impact on relationships for many asymptomatic individuals who test positive and may be incorrectly identified as infected remains a concern, said Dr. Pearlman.
Although some may be disagree about the value of routine serotesting for HSV-2 in asymptomatic individuals, other strategies can reduce the spread of infection and help those infected, he said.
Many experts continue to recommend targeted serotesting to high-risk populations, such as pregnant women whose nonpregnant partner is known to have genital or oral herpes and whose own infection status or serostatus is uncertain, said Dr. Pearlman. Other targeted strategies include screening individuals with recurrent or atypical genital symptoms and negative polymerase chain reaction assay or culture results, a clinical herpes diagnosis without laboratory confirmation, or those at increased risk because of a high number of sexual partners or a history of HIV infection, he said.
“Of note, the current CDC STI guidelines and ACOG both concur with the USPSTF that routine screening in the general population or routine screening during pregnancy are not recommended,” Dr. Pearlman said. Meanwhile, research efforts continue to help reduce the impact of HSV disease and development of a more effective testing methodology “might tip the balance in favor of routine screening” in the future, he emphasized.
The recommendations were supported by the Agency for Healthcare Research and Quality. The members of the task force received reimbursement for travel and an honorarium but had no other relevant financial conflicts to disclose. Dr. Pearlman had no financial conflicts to disclose.
Asymptomatic adults, teens, and pregnant women with no known history or symptoms of herpes infection need not undergo routine screening, according to the latest recommendation from the U.S. Preventive Services Task Force.
The 2023 recommendation reaffirms the conclusion from 2016, wrote Carol M. Mangione, MD, of the University of California, Los Angeles, and members of the task force.
“Currently, routine serologic screening for genital herpes is limited by the low predictive value of the widely available serologic screening tests and the expected high rate of false-positive results likely to occur with routine screening of asymptomatic persons in the U.S.,” the authors said.
In the recommendation, published in JAMA, the authors affirmed with moderate certainty and a grade D recommendation that the risks of routine screening for herpes simplex virus (HSV) in asymptomatic individuals outweigh the benefits.
The task force found no new evidence on the accuracy of serologic screening tests, the benefits of early detection and treatment, or on the harms of screening and treatment since the 2016 review of 17 studies in 19 publications, with data from more than 9,000 individuals.
Studies of the accuracy of serologic screening for herpes simplex virus-2 in the 2016 report mainly reflect populations with higher HSV-2 prevalence and are of limited applicability to the U.S. primary care population, the authors wrote. Evidence from the 2016 review also showed limited and inconsistent support for the early identification and treatment of HSV-2 in asymptomatic individuals, including those who were pregnant.
No new evidence has emerged since 2016 regarding harms of screening or treating genital herpes in asymptomatic individuals, the authors noted. “Based on previous evidence, the USPSTF estimated that using the widely available serologic tests for HSV-2, nearly 1 of every 2 diagnoses in the general U.S. primary care population could be false,” they said. The task force also concluded that the low accuracy of the current tests could prompt unnecessary treatment for individuals with false-positive diagnoses, as well as social and emotional harm for these individuals.
During a period of public comment from Aug. 16, 2022, to Sept. 12, 2022, individuals expressed concerns that the recommendation against routine screening showed a disinclination to take herpes seriously, and concerns that asymptomatic individuals could transmit the infection to sexual partners, the authors said. However, the estimated seroprevalence of HSV-1 and HSV-2 has declined in recent decades, and other comments supported the USPSTF’s analysis of the evidence and noted their consistency with current clinical practice.
The task force noted that research gaps remain and recognized the need to improve screening and treatment of genital herpes to prevent symptomatic episodes and transmission. Specifically, the USPSTF recommendation calls for more research to assess the accuracy of screening tests, to enroll more study participants from populations disproportionately affected by HSV, to examine the effect of behavioral counseling, and to clarify associations between HSV and pregnancy outcomes. In addition, the task force called for research to create an effective vaccine to prevent genital HSV infection and to develop a cure.
Targeted screening makes sense for now
“Given the frequency and severity of the range of diseases seen with HSV and the large proportion of persons who are asymptomatic, identifying carriers through type-specific serologic screening has long been considered a plausible strategy,” Mark D. Pearlman, MD, of the University of Michigan, Ann Arbor, wrote in an accompanying editorial.
However, accuracy of the currently available serology screening tests is low, and the adverse social and psychological effects and the impact on relationships for many asymptomatic individuals who test positive and may be incorrectly identified as infected remains a concern, said Dr. Pearlman.
Although some may be disagree about the value of routine serotesting for HSV-2 in asymptomatic individuals, other strategies can reduce the spread of infection and help those infected, he said.
Many experts continue to recommend targeted serotesting to high-risk populations, such as pregnant women whose nonpregnant partner is known to have genital or oral herpes and whose own infection status or serostatus is uncertain, said Dr. Pearlman. Other targeted strategies include screening individuals with recurrent or atypical genital symptoms and negative polymerase chain reaction assay or culture results, a clinical herpes diagnosis without laboratory confirmation, or those at increased risk because of a high number of sexual partners or a history of HIV infection, he said.
“Of note, the current CDC STI guidelines and ACOG both concur with the USPSTF that routine screening in the general population or routine screening during pregnancy are not recommended,” Dr. Pearlman said. Meanwhile, research efforts continue to help reduce the impact of HSV disease and development of a more effective testing methodology “might tip the balance in favor of routine screening” in the future, he emphasized.
The recommendations were supported by the Agency for Healthcare Research and Quality. The members of the task force received reimbursement for travel and an honorarium but had no other relevant financial conflicts to disclose. Dr. Pearlman had no financial conflicts to disclose.
FROM JAMA
Antenatal corticosteroids: Fresh answers to old questions
Giving corticosteroids to pregnant women at risk for preterm birth before 34 weeks of gestational age has been the standard of care since the 1990s, but a few scenarios for their use remain up for debate. Two studies presented this week at the 2023 meeting sponsored by the Society for Maternal–Fetal Medicine provided some fresh insight into the practice that could help clinicians better manage pregnant patients.
Neurodevelopmental outcomes in late preterm
First, should antenatal corticosteroids (ACS) be given to mothers who present with late preterm labor, defined as 34-36 weeks’ gestational age?
A landmark randomized clinical trial published in 2016 demonstrated that use of ACS in mothers in late preterm labor reduced severe respiratory complications. That practice has largely been adopted by clinicians. The only downside, according to the researchers, was that infants whose mothers received steroid therapy were more likely to develop hypoglycemia. The condition is self-limiting, but studies have raised concern about the potential long-term risk of neurocognitive or psychological outcomes in infants with hypoglycemia.
Cynthia Gyamfi-Bannerman, MD, MSc, endowed chair and professor of obstetrics, gynecology, and reproductive sciences at the University of California, San Diego, led the 2016 study. Her team was unable to secure funding for their originally planned follow-up study of the infants 2 years later. But once the American College of Obstetricians and Gynecologists endorsed the practice and more women received ACS in the late preterm period, Dr. Gyamfi-Bannerman and her colleagues felt the need to “follow up the infants just to see what the outcomes are from a neurodevelopmental standpoint,” she said.
Dr. Gyamfi-Bannerman and colleagues recruited children older than age 6 from the original trial whose parents were willing to have them participate in a follow-up study. A total of 949 from the initial 2,831 cohort completed cognitive testing and received assessments for cerebral palsy, social impairment within the autism spectrum, and behavioral and emotional problems.
At the SMFM conference, Dr. Gyamfi-Bannerman reported no differences in the primary outcome of cognitive function between those whose mothers had received a single course of betamethasone and those who did not, or any differences in rates of the other outcomes.
Kathy Zhang-Rutledge, MD, a maternal-fetal medicine specialist who practices with Obstetrix Maternal Fetal Medicine Group of Houston, part of Pediatrix Medical Group, said she was glad to see a study that addressed the potential long-term adverse events associated with ACS in the late preterm period.
“Having this pretty large study – with really good neurological testing results – should help reassure clinicians that this is something they should consider adopting in their practice,” Dr. Zhang-Rutledge said.
Are boosters better?
The second unresolved question was if a repeat course of ACS should be administered when a woman at risk for preterm birth receives a course of steroids but does not deliver in the following 7 days.
Any benefits to the initial course of ACS wear off after a week. As a result, clinicians often give booster courses 7 days after the first dose if the infant is likely to be delivered in the following week. A 2009 study showed this approach may protect infants from respiratory problems, but data on long-term outcomes have been weak.
ACOG guidelines say to “consider” a booster dose in women who are less than 34 weeks’ gestation at risk for preterm delivery within 7 days.
The exception is when the mother already has experienced preterm prelabor rupture of membranes (PPROM), because ACS may increase the risk for infection for both mother and child. ACOG doesn’t take a stand on use of booster doses for PPROM, citing a lack of data to show that potential benefits outweigh the potential risks of this approach.
A recent multicenter, double-blinded, randomized clinical trial attempted to fill that void in knowledge. Between 2016 and 2022, 194 women with PPROM and gestational age less than 32 weeks who had received an initial ACS course at least 7 days prior to randomization received a booster course of ACS or saline placebo.
“Our primary outcome was designed to be like the prior rescue study (in 2009) that we did with patients with intact membranes,” said Andrew Combs, MD, PhD, a maternal-fetal medicine specialist at Pediatrix Medical Group in Sunrise, Fla., who participated in the earlier study. “It was a composite of neonatal morbidity that was any one of a variety of outcomes including respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, and neonatal death.”
The primary outcome occurred in 64% of women who received booster ACS and 66% with placebo (odds ratio, 0.82; 95% confidence interval, 0.43-1.57), according to Dr. Combs, who presented the findings at SMFM.
Although the study was not powered to detect significant differences in specific outcomes, the rate of neonatal sepsis was not higher in the ACS group, suggesting that ACS may be safe if membranes have ruptured, the researchers reported. But because the booster course of ACS did not prevent respiratory morbidity, clinicians may wonder what to do with the findings.
Niraj Chavan, MD, an associate professor in the department of obstetrics, gynecology, and women’s health at Saint Louis University, said he was unsure how the study would affect clinical practice.
The relatively small sample number of patients prevented analysis of specific outcomes and subgroup analyses of important variables such as race, ethnicity, gestational age, and other comorbid conditions in the mothers, he said. So clinicians still must weigh potential risks and benefits on a case-by-case basis.
“You have to think about it in buckets,” he said, “One is conditions that would increase the risk for neonatal morbidity. The other is the risk for infection, both for the mom and the baby.”
But for Dr. Combs, the interpretation of the findings was simpler: “We concluded that there’s no indication to give a booster course of steroids after a week has elapsed in patients with ruptured membranes.”
The study presented by Dr. Gyamfi-Bannerman was funded by the National Institute of Child Health and Human Development. The study presented by Dr. Combs was funded by MEDNAX Center for Research, Education, and Quality, which in 2022 was renamed Pediatrix Center for Research, Education,and Quality. Dr. Combs is an employee of Pediatrix Medical Group but has no conflicts of interest. Dr. Gyamfi-Bannerman, Dr. Zhang-Rutledge, and Dr. Chavan report no relevant financial relationships.
Ann Thomas is a pediatrician and epidemiologist in Portland, Ore.
A version of this article originally appeared on Medscape.com.
Giving corticosteroids to pregnant women at risk for preterm birth before 34 weeks of gestational age has been the standard of care since the 1990s, but a few scenarios for their use remain up for debate. Two studies presented this week at the 2023 meeting sponsored by the Society for Maternal–Fetal Medicine provided some fresh insight into the practice that could help clinicians better manage pregnant patients.
Neurodevelopmental outcomes in late preterm
First, should antenatal corticosteroids (ACS) be given to mothers who present with late preterm labor, defined as 34-36 weeks’ gestational age?
A landmark randomized clinical trial published in 2016 demonstrated that use of ACS in mothers in late preterm labor reduced severe respiratory complications. That practice has largely been adopted by clinicians. The only downside, according to the researchers, was that infants whose mothers received steroid therapy were more likely to develop hypoglycemia. The condition is self-limiting, but studies have raised concern about the potential long-term risk of neurocognitive or psychological outcomes in infants with hypoglycemia.
Cynthia Gyamfi-Bannerman, MD, MSc, endowed chair and professor of obstetrics, gynecology, and reproductive sciences at the University of California, San Diego, led the 2016 study. Her team was unable to secure funding for their originally planned follow-up study of the infants 2 years later. But once the American College of Obstetricians and Gynecologists endorsed the practice and more women received ACS in the late preterm period, Dr. Gyamfi-Bannerman and her colleagues felt the need to “follow up the infants just to see what the outcomes are from a neurodevelopmental standpoint,” she said.
Dr. Gyamfi-Bannerman and colleagues recruited children older than age 6 from the original trial whose parents were willing to have them participate in a follow-up study. A total of 949 from the initial 2,831 cohort completed cognitive testing and received assessments for cerebral palsy, social impairment within the autism spectrum, and behavioral and emotional problems.
At the SMFM conference, Dr. Gyamfi-Bannerman reported no differences in the primary outcome of cognitive function between those whose mothers had received a single course of betamethasone and those who did not, or any differences in rates of the other outcomes.
Kathy Zhang-Rutledge, MD, a maternal-fetal medicine specialist who practices with Obstetrix Maternal Fetal Medicine Group of Houston, part of Pediatrix Medical Group, said she was glad to see a study that addressed the potential long-term adverse events associated with ACS in the late preterm period.
“Having this pretty large study – with really good neurological testing results – should help reassure clinicians that this is something they should consider adopting in their practice,” Dr. Zhang-Rutledge said.
Are boosters better?
The second unresolved question was if a repeat course of ACS should be administered when a woman at risk for preterm birth receives a course of steroids but does not deliver in the following 7 days.
Any benefits to the initial course of ACS wear off after a week. As a result, clinicians often give booster courses 7 days after the first dose if the infant is likely to be delivered in the following week. A 2009 study showed this approach may protect infants from respiratory problems, but data on long-term outcomes have been weak.
ACOG guidelines say to “consider” a booster dose in women who are less than 34 weeks’ gestation at risk for preterm delivery within 7 days.
The exception is when the mother already has experienced preterm prelabor rupture of membranes (PPROM), because ACS may increase the risk for infection for both mother and child. ACOG doesn’t take a stand on use of booster doses for PPROM, citing a lack of data to show that potential benefits outweigh the potential risks of this approach.
A recent multicenter, double-blinded, randomized clinical trial attempted to fill that void in knowledge. Between 2016 and 2022, 194 women with PPROM and gestational age less than 32 weeks who had received an initial ACS course at least 7 days prior to randomization received a booster course of ACS or saline placebo.
“Our primary outcome was designed to be like the prior rescue study (in 2009) that we did with patients with intact membranes,” said Andrew Combs, MD, PhD, a maternal-fetal medicine specialist at Pediatrix Medical Group in Sunrise, Fla., who participated in the earlier study. “It was a composite of neonatal morbidity that was any one of a variety of outcomes including respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, and neonatal death.”
The primary outcome occurred in 64% of women who received booster ACS and 66% with placebo (odds ratio, 0.82; 95% confidence interval, 0.43-1.57), according to Dr. Combs, who presented the findings at SMFM.
Although the study was not powered to detect significant differences in specific outcomes, the rate of neonatal sepsis was not higher in the ACS group, suggesting that ACS may be safe if membranes have ruptured, the researchers reported. But because the booster course of ACS did not prevent respiratory morbidity, clinicians may wonder what to do with the findings.
Niraj Chavan, MD, an associate professor in the department of obstetrics, gynecology, and women’s health at Saint Louis University, said he was unsure how the study would affect clinical practice.
The relatively small sample number of patients prevented analysis of specific outcomes and subgroup analyses of important variables such as race, ethnicity, gestational age, and other comorbid conditions in the mothers, he said. So clinicians still must weigh potential risks and benefits on a case-by-case basis.
“You have to think about it in buckets,” he said, “One is conditions that would increase the risk for neonatal morbidity. The other is the risk for infection, both for the mom and the baby.”
But for Dr. Combs, the interpretation of the findings was simpler: “We concluded that there’s no indication to give a booster course of steroids after a week has elapsed in patients with ruptured membranes.”
The study presented by Dr. Gyamfi-Bannerman was funded by the National Institute of Child Health and Human Development. The study presented by Dr. Combs was funded by MEDNAX Center for Research, Education, and Quality, which in 2022 was renamed Pediatrix Center for Research, Education,and Quality. Dr. Combs is an employee of Pediatrix Medical Group but has no conflicts of interest. Dr. Gyamfi-Bannerman, Dr. Zhang-Rutledge, and Dr. Chavan report no relevant financial relationships.
Ann Thomas is a pediatrician and epidemiologist in Portland, Ore.
A version of this article originally appeared on Medscape.com.
Giving corticosteroids to pregnant women at risk for preterm birth before 34 weeks of gestational age has been the standard of care since the 1990s, but a few scenarios for their use remain up for debate. Two studies presented this week at the 2023 meeting sponsored by the Society for Maternal–Fetal Medicine provided some fresh insight into the practice that could help clinicians better manage pregnant patients.
Neurodevelopmental outcomes in late preterm
First, should antenatal corticosteroids (ACS) be given to mothers who present with late preterm labor, defined as 34-36 weeks’ gestational age?
A landmark randomized clinical trial published in 2016 demonstrated that use of ACS in mothers in late preterm labor reduced severe respiratory complications. That practice has largely been adopted by clinicians. The only downside, according to the researchers, was that infants whose mothers received steroid therapy were more likely to develop hypoglycemia. The condition is self-limiting, but studies have raised concern about the potential long-term risk of neurocognitive or psychological outcomes in infants with hypoglycemia.
Cynthia Gyamfi-Bannerman, MD, MSc, endowed chair and professor of obstetrics, gynecology, and reproductive sciences at the University of California, San Diego, led the 2016 study. Her team was unable to secure funding for their originally planned follow-up study of the infants 2 years later. But once the American College of Obstetricians and Gynecologists endorsed the practice and more women received ACS in the late preterm period, Dr. Gyamfi-Bannerman and her colleagues felt the need to “follow up the infants just to see what the outcomes are from a neurodevelopmental standpoint,” she said.
Dr. Gyamfi-Bannerman and colleagues recruited children older than age 6 from the original trial whose parents were willing to have them participate in a follow-up study. A total of 949 from the initial 2,831 cohort completed cognitive testing and received assessments for cerebral palsy, social impairment within the autism spectrum, and behavioral and emotional problems.
At the SMFM conference, Dr. Gyamfi-Bannerman reported no differences in the primary outcome of cognitive function between those whose mothers had received a single course of betamethasone and those who did not, or any differences in rates of the other outcomes.
Kathy Zhang-Rutledge, MD, a maternal-fetal medicine specialist who practices with Obstetrix Maternal Fetal Medicine Group of Houston, part of Pediatrix Medical Group, said she was glad to see a study that addressed the potential long-term adverse events associated with ACS in the late preterm period.
“Having this pretty large study – with really good neurological testing results – should help reassure clinicians that this is something they should consider adopting in their practice,” Dr. Zhang-Rutledge said.
Are boosters better?
The second unresolved question was if a repeat course of ACS should be administered when a woman at risk for preterm birth receives a course of steroids but does not deliver in the following 7 days.
Any benefits to the initial course of ACS wear off after a week. As a result, clinicians often give booster courses 7 days after the first dose if the infant is likely to be delivered in the following week. A 2009 study showed this approach may protect infants from respiratory problems, but data on long-term outcomes have been weak.
ACOG guidelines say to “consider” a booster dose in women who are less than 34 weeks’ gestation at risk for preterm delivery within 7 days.
The exception is when the mother already has experienced preterm prelabor rupture of membranes (PPROM), because ACS may increase the risk for infection for both mother and child. ACOG doesn’t take a stand on use of booster doses for PPROM, citing a lack of data to show that potential benefits outweigh the potential risks of this approach.
A recent multicenter, double-blinded, randomized clinical trial attempted to fill that void in knowledge. Between 2016 and 2022, 194 women with PPROM and gestational age less than 32 weeks who had received an initial ACS course at least 7 days prior to randomization received a booster course of ACS or saline placebo.
“Our primary outcome was designed to be like the prior rescue study (in 2009) that we did with patients with intact membranes,” said Andrew Combs, MD, PhD, a maternal-fetal medicine specialist at Pediatrix Medical Group in Sunrise, Fla., who participated in the earlier study. “It was a composite of neonatal morbidity that was any one of a variety of outcomes including respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, and neonatal death.”
The primary outcome occurred in 64% of women who received booster ACS and 66% with placebo (odds ratio, 0.82; 95% confidence interval, 0.43-1.57), according to Dr. Combs, who presented the findings at SMFM.
Although the study was not powered to detect significant differences in specific outcomes, the rate of neonatal sepsis was not higher in the ACS group, suggesting that ACS may be safe if membranes have ruptured, the researchers reported. But because the booster course of ACS did not prevent respiratory morbidity, clinicians may wonder what to do with the findings.
Niraj Chavan, MD, an associate professor in the department of obstetrics, gynecology, and women’s health at Saint Louis University, said he was unsure how the study would affect clinical practice.
The relatively small sample number of patients prevented analysis of specific outcomes and subgroup analyses of important variables such as race, ethnicity, gestational age, and other comorbid conditions in the mothers, he said. So clinicians still must weigh potential risks and benefits on a case-by-case basis.
“You have to think about it in buckets,” he said, “One is conditions that would increase the risk for neonatal morbidity. The other is the risk for infection, both for the mom and the baby.”
But for Dr. Combs, the interpretation of the findings was simpler: “We concluded that there’s no indication to give a booster course of steroids after a week has elapsed in patients with ruptured membranes.”
The study presented by Dr. Gyamfi-Bannerman was funded by the National Institute of Child Health and Human Development. The study presented by Dr. Combs was funded by MEDNAX Center for Research, Education, and Quality, which in 2022 was renamed Pediatrix Center for Research, Education,and Quality. Dr. Combs is an employee of Pediatrix Medical Group but has no conflicts of interest. Dr. Gyamfi-Bannerman, Dr. Zhang-Rutledge, and Dr. Chavan report no relevant financial relationships.
Ann Thomas is a pediatrician and epidemiologist in Portland, Ore.
A version of this article originally appeared on Medscape.com.
FROM THE PREGNANCY MEETING
Doctors and dating: There’s an app (or three) for that
Pounding heart, sweating, insomnia. Surges of dopamine, norepinephrine, and adrenaline. All symptoms of a very common yet frustrating condition: Falling in love.
The prognosis is vague. A prescription pad and knowledge of biochemistry aren’t helpful when it comes to relationships.
Medical training can consume decades when others are exploring relationships and starting families. There are few recent data on this, but
But there is hope! By age 36, the number of doctors in long-term relationships had overtaken everyone else by more than 10% for women and 20% for men. The Medscape 2022 Physician Happiness & Lifestyle Report found that 83% were in committed relationships, and even better, happy ones. At least three-quarters of doctors in every specialty described their partnerships as “very good” or “good.”
How should a single medical student, resident, or attending physician find happiness ever after in 2023? Sometimes Mr./Ms. Right can be found in the anatomy lab or hospital, with sparks flying between students or colleagues. But for many in health care, along with millions of others looking for love, the solution is dating apps.
When ‘MD’ is a turnoff
Dr. M, a psychiatry resident in California who prefers not to give her name, hadn’t found a life partner during college, grad school, or medical school. When she passed her final Step 3 board exam, she decided it was time to take the plunge. She signed up for popular dating apps like Hinge, Bumble, and Coffee Meets Bagel, but her dates seemed to follow a disappointing pattern.
“I met lots of guys, but it was incredibly rare to find another physician,” said Dr. M. “I found myself always wanting to talk about my life as a resident. More often than not, the guys would give me this blank stare as I complained about being on call or spoke about spending 12 hours a day studying for a board exam, or even the process of The Match and how I ended up in California.”
Both of Dr. M’s parents are physicians, and she grew up watching how they supported each other through residency, exams, and exhausting schedules. A relationship with another physician, her parents told her, would give both partners the best chance to understand each other’s lives. The problem was how to find one.
That was when Dr. M saw an ad for a dating app with a cute medical name: DownToDate, a play on the clinical evidence resource UpToDate. “I thought it was a meme,” she said. “It was this doctors-only app. I remember thinking, ‘this has to be a joke,’ but then it was very real.”
She signed up and was required to provide a photo of her ID and her NPI number. Immediately, men began “requesting a consult,” the app’s form of “liking” her profile, and sending her “pages” (messages).
DownToDate was created by another physician, Robin Boyer, MD, MBA, a pediatrics resident in Loma Linda, Calif. The inspiration came in 2020 during the initial COVID crisis. Exhausted from long and often heartbreaking shifts, Dr. Boyer was grateful for her husband’s unwavering support. But many of her coresidents weren’t so lucky. The women in particular talked about their dating struggles, and there was a recurring theme. They didn’t feel confident putting “physician” on a dating site profile.
“If you’re male and you tell people you’re a doctor, it seems like it really attracts people,” Dr. Boyer said. “But if you’re female, it brings up a lot of stereotypes where you’re perceived as too intimidating either as the breadwinner, being more educated, or having a [demanding] career. It does make it more difficult.”
Dr. Boyer met her husband in high school, and she had never used a dating app. She convinced a coresident, Celestine Odigwe, MD, to pursue the idea as partners. They began researching the market within their network and heard from over a thousand interested physicians, both men and women, heterosexual and LGBTQ+. They even created fake accounts on other sites to gauge how easy it is to falsify a profile. From these insights, the app took shape. It launched in 2021 and currently has more than 5000 verified users.
Branches from the same tree
Around the same time that DownToDate began, Shivani Shah, DO, a pediatric neurology resident at Duke University, Durham, N.C., and her brother, Sagar Shah, an entrepreneur, had a similar idea.
At the time, Dr. Shah was a fourth-year medical student about to move from New Jersey to North Carolina. Friends who were internal medicine residents described the grueling reality of the early COVID pandemic.
“It was just horrible,” said Dr. Shah. “You were isolated from your family, your support system, everything. ... I think the pandemic really pushed us into realizing that this is a very important need, and sometimes it feels like community is lacking in the health care field.”
The sibling duo developed ForeverX, an app for health care workers to find meaningful and long-term romantic connections. It launched in 2021.
Concerned that the medical field was “siloed,” the Shahs chose to open the app to physicians, dentists, nurses, physical therapists, and other health care professionals. “Opening up the doors to more communication” between the health care branches was a priority.
To prevent catfishing, the app uses a twofold vetting system. Each user submits a photo of their driver’s license and a selfie that must match. There is also health care verification through an NPI number, nurse’s ID, or a manual process for those without either. None of the information is stored.
Through personal experience with dating apps, Dr. Shah hopes ForeverX can improve on some of their flaws, particularly the problem of matches being overly filtered by preferences. The “natural way” of meeting people is not filtered. And while most people have a dating checklist in mind, meeting someone face to face might send some of those prerequisites “out the window.”
“You can’t really put into words how you feel with someone ... the vibe,” Dr. Shah said. That is why her goal is to get people off the app and on an actual date IRL. “Something we’ve discussed internally is, how do we make this experience that’s virtual more human?”
She acknowledged that certain requirements, like a desire for children, might be crucial to some users. Many female doctors in their 30’s feel the “time crunch” of a ticking biological clock.
Optimize your date-ability
“I think people either love or hate dating apps, and I love them,” said Kevin Jubbal, MD. “I get to meet cool people and schedule dates from the comfort of my home.”
Dr. Jubbal, a former plastic surgery resident who left medicine to become an entrepreneur, is the founder of Med School Insiders, a tutoring and advising resource for premeds, medical students, and residents. His YouTube channel has more than 1.5 million subscribers, and he often receives questions about whether dating is feasible in medical school and how to balance a personal and academic/professional life.
Those who hate dating apps or receive few matches would do well to look inward instead of blaming the process, he said. It helps to view the experience as a learning tool that provides feedback very quickly.
“If you want to find a really amazing person, then you need to be what you want to find,” said Dr. Jubbal. “If you want to find someone who’s fit and intelligent and well read and well traveled, you need to be that. Otherwise, you’re probably not going to attract that person.”
An app designed to help single female MDs
Ifie Williams, MD, a psychiatrist in Washington, D.C., believes a wider dating pool is key – provided everyone understands the situation up front. When Dr. Williams started residency in 2014, she was “as single as can be.” She tried many dating apps, but they were extremely time consuming. Even when she set specific preferences, she found herself sifting through “matches” that didn’t fit her criteria.
“Dating nowadays has become almost like a second job,” said Dr. Williams. “Just the amount of time that people are having to spend on apps, swiping left and right and then meeting people. You think they’re interested and then you deal with all these games.”
By 2017, Dr. Williams had invented Miss Doctor, a dating app that would connect female physicians and other doctoral-level professionals with men or women on a similar achievement level.
By definition, these people would not be intimidated by ambitious, busy women. They would be heavily screened and vetted. And one other proviso: they would have to pay for “likes.”
Most dating apps charge a subscription fee. Users are allowed to “like” numerous profiles and perhaps not bother responding to many matches. By contrast, Miss Doctor accounts are free and include a limited number of “likes” to indicate interest. Beyond that, there’s a price.
“We wanted to find a way to make people a little more intentional with how they like people on the app, so they give a little more thought to it,” Dr. Williams said. “So, we monetize it and use that to change behavior.”
After an initial launch in 2017, the app had to take a back seat while Dr. Williams started her psychiatry practice and got married herself. She plans to relaunch it in spring 2023.
Male or female, there is general agreement that finding time to date as a young physician isn’t easy. While DownToDate has had “doctor meets doctor” success stories, many users are still searching for “the one.”
Dr. Boyer believes that career challenges are not a reason to give up. “There are so many single and available people out there,” she said. “And everyone’s deserving of love. Even if you only have an hour a week.”
A version of this article first appeared on Medscape.com.
Pounding heart, sweating, insomnia. Surges of dopamine, norepinephrine, and adrenaline. All symptoms of a very common yet frustrating condition: Falling in love.
The prognosis is vague. A prescription pad and knowledge of biochemistry aren’t helpful when it comes to relationships.
Medical training can consume decades when others are exploring relationships and starting families. There are few recent data on this, but
But there is hope! By age 36, the number of doctors in long-term relationships had overtaken everyone else by more than 10% for women and 20% for men. The Medscape 2022 Physician Happiness & Lifestyle Report found that 83% were in committed relationships, and even better, happy ones. At least three-quarters of doctors in every specialty described their partnerships as “very good” or “good.”
How should a single medical student, resident, or attending physician find happiness ever after in 2023? Sometimes Mr./Ms. Right can be found in the anatomy lab or hospital, with sparks flying between students or colleagues. But for many in health care, along with millions of others looking for love, the solution is dating apps.
When ‘MD’ is a turnoff
Dr. M, a psychiatry resident in California who prefers not to give her name, hadn’t found a life partner during college, grad school, or medical school. When she passed her final Step 3 board exam, she decided it was time to take the plunge. She signed up for popular dating apps like Hinge, Bumble, and Coffee Meets Bagel, but her dates seemed to follow a disappointing pattern.
“I met lots of guys, but it was incredibly rare to find another physician,” said Dr. M. “I found myself always wanting to talk about my life as a resident. More often than not, the guys would give me this blank stare as I complained about being on call or spoke about spending 12 hours a day studying for a board exam, or even the process of The Match and how I ended up in California.”
Both of Dr. M’s parents are physicians, and she grew up watching how they supported each other through residency, exams, and exhausting schedules. A relationship with another physician, her parents told her, would give both partners the best chance to understand each other’s lives. The problem was how to find one.
That was when Dr. M saw an ad for a dating app with a cute medical name: DownToDate, a play on the clinical evidence resource UpToDate. “I thought it was a meme,” she said. “It was this doctors-only app. I remember thinking, ‘this has to be a joke,’ but then it was very real.”
She signed up and was required to provide a photo of her ID and her NPI number. Immediately, men began “requesting a consult,” the app’s form of “liking” her profile, and sending her “pages” (messages).
DownToDate was created by another physician, Robin Boyer, MD, MBA, a pediatrics resident in Loma Linda, Calif. The inspiration came in 2020 during the initial COVID crisis. Exhausted from long and often heartbreaking shifts, Dr. Boyer was grateful for her husband’s unwavering support. But many of her coresidents weren’t so lucky. The women in particular talked about their dating struggles, and there was a recurring theme. They didn’t feel confident putting “physician” on a dating site profile.
“If you’re male and you tell people you’re a doctor, it seems like it really attracts people,” Dr. Boyer said. “But if you’re female, it brings up a lot of stereotypes where you’re perceived as too intimidating either as the breadwinner, being more educated, or having a [demanding] career. It does make it more difficult.”
Dr. Boyer met her husband in high school, and she had never used a dating app. She convinced a coresident, Celestine Odigwe, MD, to pursue the idea as partners. They began researching the market within their network and heard from over a thousand interested physicians, both men and women, heterosexual and LGBTQ+. They even created fake accounts on other sites to gauge how easy it is to falsify a profile. From these insights, the app took shape. It launched in 2021 and currently has more than 5000 verified users.
Branches from the same tree
Around the same time that DownToDate began, Shivani Shah, DO, a pediatric neurology resident at Duke University, Durham, N.C., and her brother, Sagar Shah, an entrepreneur, had a similar idea.
At the time, Dr. Shah was a fourth-year medical student about to move from New Jersey to North Carolina. Friends who were internal medicine residents described the grueling reality of the early COVID pandemic.
“It was just horrible,” said Dr. Shah. “You were isolated from your family, your support system, everything. ... I think the pandemic really pushed us into realizing that this is a very important need, and sometimes it feels like community is lacking in the health care field.”
The sibling duo developed ForeverX, an app for health care workers to find meaningful and long-term romantic connections. It launched in 2021.
Concerned that the medical field was “siloed,” the Shahs chose to open the app to physicians, dentists, nurses, physical therapists, and other health care professionals. “Opening up the doors to more communication” between the health care branches was a priority.
To prevent catfishing, the app uses a twofold vetting system. Each user submits a photo of their driver’s license and a selfie that must match. There is also health care verification through an NPI number, nurse’s ID, or a manual process for those without either. None of the information is stored.
Through personal experience with dating apps, Dr. Shah hopes ForeverX can improve on some of their flaws, particularly the problem of matches being overly filtered by preferences. The “natural way” of meeting people is not filtered. And while most people have a dating checklist in mind, meeting someone face to face might send some of those prerequisites “out the window.”
“You can’t really put into words how you feel with someone ... the vibe,” Dr. Shah said. That is why her goal is to get people off the app and on an actual date IRL. “Something we’ve discussed internally is, how do we make this experience that’s virtual more human?”
She acknowledged that certain requirements, like a desire for children, might be crucial to some users. Many female doctors in their 30’s feel the “time crunch” of a ticking biological clock.
Optimize your date-ability
“I think people either love or hate dating apps, and I love them,” said Kevin Jubbal, MD. “I get to meet cool people and schedule dates from the comfort of my home.”
Dr. Jubbal, a former plastic surgery resident who left medicine to become an entrepreneur, is the founder of Med School Insiders, a tutoring and advising resource for premeds, medical students, and residents. His YouTube channel has more than 1.5 million subscribers, and he often receives questions about whether dating is feasible in medical school and how to balance a personal and academic/professional life.
Those who hate dating apps or receive few matches would do well to look inward instead of blaming the process, he said. It helps to view the experience as a learning tool that provides feedback very quickly.
“If you want to find a really amazing person, then you need to be what you want to find,” said Dr. Jubbal. “If you want to find someone who’s fit and intelligent and well read and well traveled, you need to be that. Otherwise, you’re probably not going to attract that person.”
An app designed to help single female MDs
Ifie Williams, MD, a psychiatrist in Washington, D.C., believes a wider dating pool is key – provided everyone understands the situation up front. When Dr. Williams started residency in 2014, she was “as single as can be.” She tried many dating apps, but they were extremely time consuming. Even when she set specific preferences, she found herself sifting through “matches” that didn’t fit her criteria.
“Dating nowadays has become almost like a second job,” said Dr. Williams. “Just the amount of time that people are having to spend on apps, swiping left and right and then meeting people. You think they’re interested and then you deal with all these games.”
By 2017, Dr. Williams had invented Miss Doctor, a dating app that would connect female physicians and other doctoral-level professionals with men or women on a similar achievement level.
By definition, these people would not be intimidated by ambitious, busy women. They would be heavily screened and vetted. And one other proviso: they would have to pay for “likes.”
Most dating apps charge a subscription fee. Users are allowed to “like” numerous profiles and perhaps not bother responding to many matches. By contrast, Miss Doctor accounts are free and include a limited number of “likes” to indicate interest. Beyond that, there’s a price.
“We wanted to find a way to make people a little more intentional with how they like people on the app, so they give a little more thought to it,” Dr. Williams said. “So, we monetize it and use that to change behavior.”
After an initial launch in 2017, the app had to take a back seat while Dr. Williams started her psychiatry practice and got married herself. She plans to relaunch it in spring 2023.
Male or female, there is general agreement that finding time to date as a young physician isn’t easy. While DownToDate has had “doctor meets doctor” success stories, many users are still searching for “the one.”
Dr. Boyer believes that career challenges are not a reason to give up. “There are so many single and available people out there,” she said. “And everyone’s deserving of love. Even if you only have an hour a week.”
A version of this article first appeared on Medscape.com.
Pounding heart, sweating, insomnia. Surges of dopamine, norepinephrine, and adrenaline. All symptoms of a very common yet frustrating condition: Falling in love.
The prognosis is vague. A prescription pad and knowledge of biochemistry aren’t helpful when it comes to relationships.
Medical training can consume decades when others are exploring relationships and starting families. There are few recent data on this, but
But there is hope! By age 36, the number of doctors in long-term relationships had overtaken everyone else by more than 10% for women and 20% for men. The Medscape 2022 Physician Happiness & Lifestyle Report found that 83% were in committed relationships, and even better, happy ones. At least three-quarters of doctors in every specialty described their partnerships as “very good” or “good.”
How should a single medical student, resident, or attending physician find happiness ever after in 2023? Sometimes Mr./Ms. Right can be found in the anatomy lab or hospital, with sparks flying between students or colleagues. But for many in health care, along with millions of others looking for love, the solution is dating apps.
When ‘MD’ is a turnoff
Dr. M, a psychiatry resident in California who prefers not to give her name, hadn’t found a life partner during college, grad school, or medical school. When she passed her final Step 3 board exam, she decided it was time to take the plunge. She signed up for popular dating apps like Hinge, Bumble, and Coffee Meets Bagel, but her dates seemed to follow a disappointing pattern.
“I met lots of guys, but it was incredibly rare to find another physician,” said Dr. M. “I found myself always wanting to talk about my life as a resident. More often than not, the guys would give me this blank stare as I complained about being on call or spoke about spending 12 hours a day studying for a board exam, or even the process of The Match and how I ended up in California.”
Both of Dr. M’s parents are physicians, and she grew up watching how they supported each other through residency, exams, and exhausting schedules. A relationship with another physician, her parents told her, would give both partners the best chance to understand each other’s lives. The problem was how to find one.
That was when Dr. M saw an ad for a dating app with a cute medical name: DownToDate, a play on the clinical evidence resource UpToDate. “I thought it was a meme,” she said. “It was this doctors-only app. I remember thinking, ‘this has to be a joke,’ but then it was very real.”
She signed up and was required to provide a photo of her ID and her NPI number. Immediately, men began “requesting a consult,” the app’s form of “liking” her profile, and sending her “pages” (messages).
DownToDate was created by another physician, Robin Boyer, MD, MBA, a pediatrics resident in Loma Linda, Calif. The inspiration came in 2020 during the initial COVID crisis. Exhausted from long and often heartbreaking shifts, Dr. Boyer was grateful for her husband’s unwavering support. But many of her coresidents weren’t so lucky. The women in particular talked about their dating struggles, and there was a recurring theme. They didn’t feel confident putting “physician” on a dating site profile.
“If you’re male and you tell people you’re a doctor, it seems like it really attracts people,” Dr. Boyer said. “But if you’re female, it brings up a lot of stereotypes where you’re perceived as too intimidating either as the breadwinner, being more educated, or having a [demanding] career. It does make it more difficult.”
Dr. Boyer met her husband in high school, and she had never used a dating app. She convinced a coresident, Celestine Odigwe, MD, to pursue the idea as partners. They began researching the market within their network and heard from over a thousand interested physicians, both men and women, heterosexual and LGBTQ+. They even created fake accounts on other sites to gauge how easy it is to falsify a profile. From these insights, the app took shape. It launched in 2021 and currently has more than 5000 verified users.
Branches from the same tree
Around the same time that DownToDate began, Shivani Shah, DO, a pediatric neurology resident at Duke University, Durham, N.C., and her brother, Sagar Shah, an entrepreneur, had a similar idea.
At the time, Dr. Shah was a fourth-year medical student about to move from New Jersey to North Carolina. Friends who were internal medicine residents described the grueling reality of the early COVID pandemic.
“It was just horrible,” said Dr. Shah. “You were isolated from your family, your support system, everything. ... I think the pandemic really pushed us into realizing that this is a very important need, and sometimes it feels like community is lacking in the health care field.”
The sibling duo developed ForeverX, an app for health care workers to find meaningful and long-term romantic connections. It launched in 2021.
Concerned that the medical field was “siloed,” the Shahs chose to open the app to physicians, dentists, nurses, physical therapists, and other health care professionals. “Opening up the doors to more communication” between the health care branches was a priority.
To prevent catfishing, the app uses a twofold vetting system. Each user submits a photo of their driver’s license and a selfie that must match. There is also health care verification through an NPI number, nurse’s ID, or a manual process for those without either. None of the information is stored.
Through personal experience with dating apps, Dr. Shah hopes ForeverX can improve on some of their flaws, particularly the problem of matches being overly filtered by preferences. The “natural way” of meeting people is not filtered. And while most people have a dating checklist in mind, meeting someone face to face might send some of those prerequisites “out the window.”
“You can’t really put into words how you feel with someone ... the vibe,” Dr. Shah said. That is why her goal is to get people off the app and on an actual date IRL. “Something we’ve discussed internally is, how do we make this experience that’s virtual more human?”
She acknowledged that certain requirements, like a desire for children, might be crucial to some users. Many female doctors in their 30’s feel the “time crunch” of a ticking biological clock.
Optimize your date-ability
“I think people either love or hate dating apps, and I love them,” said Kevin Jubbal, MD. “I get to meet cool people and schedule dates from the comfort of my home.”
Dr. Jubbal, a former plastic surgery resident who left medicine to become an entrepreneur, is the founder of Med School Insiders, a tutoring and advising resource for premeds, medical students, and residents. His YouTube channel has more than 1.5 million subscribers, and he often receives questions about whether dating is feasible in medical school and how to balance a personal and academic/professional life.
Those who hate dating apps or receive few matches would do well to look inward instead of blaming the process, he said. It helps to view the experience as a learning tool that provides feedback very quickly.
“If you want to find a really amazing person, then you need to be what you want to find,” said Dr. Jubbal. “If you want to find someone who’s fit and intelligent and well read and well traveled, you need to be that. Otherwise, you’re probably not going to attract that person.”
An app designed to help single female MDs
Ifie Williams, MD, a psychiatrist in Washington, D.C., believes a wider dating pool is key – provided everyone understands the situation up front. When Dr. Williams started residency in 2014, she was “as single as can be.” She tried many dating apps, but they were extremely time consuming. Even when she set specific preferences, she found herself sifting through “matches” that didn’t fit her criteria.
“Dating nowadays has become almost like a second job,” said Dr. Williams. “Just the amount of time that people are having to spend on apps, swiping left and right and then meeting people. You think they’re interested and then you deal with all these games.”
By 2017, Dr. Williams had invented Miss Doctor, a dating app that would connect female physicians and other doctoral-level professionals with men or women on a similar achievement level.
By definition, these people would not be intimidated by ambitious, busy women. They would be heavily screened and vetted. And one other proviso: they would have to pay for “likes.”
Most dating apps charge a subscription fee. Users are allowed to “like” numerous profiles and perhaps not bother responding to many matches. By contrast, Miss Doctor accounts are free and include a limited number of “likes” to indicate interest. Beyond that, there’s a price.
“We wanted to find a way to make people a little more intentional with how they like people on the app, so they give a little more thought to it,” Dr. Williams said. “So, we monetize it and use that to change behavior.”
After an initial launch in 2017, the app had to take a back seat while Dr. Williams started her psychiatry practice and got married herself. She plans to relaunch it in spring 2023.
Male or female, there is general agreement that finding time to date as a young physician isn’t easy. While DownToDate has had “doctor meets doctor” success stories, many users are still searching for “the one.”
Dr. Boyer believes that career challenges are not a reason to give up. “There are so many single and available people out there,” she said. “And everyone’s deserving of love. Even if you only have an hour a week.”
A version of this article first appeared on Medscape.com.
Vibrating pill can help treat constipation
The drug-free solution is designed for daily use. In a trial, the pill produced at least one additional weekly bowel movement for 41% of participants, compared with at least one additional bowel movement for 23% of participants who took a placebo pill.
Vibrant was approved by the Food and Drug Administration in August but is just now becoming available for doctors to prescribe, the company announced Wednesday.
Because it is not a drug, Vibrant is considered a class 2 medical device by the FDA, which is the same class as contact lenses.
Here’s how it works: Around bedtime, the pill is inserted in a pod to activate it, then swallowed. It travels the digestive tract and reaches the large intestine about 14 hours later.
“Then it goes to work,” the company explained in a news release. “After it’s swallowed, it is active for about 2 hours, goes quiet for around 6, hours and then activates again for another 2 hours.”
“There are little vibrations for 3 seconds on, 3 seconds off,” said Cathy Collis, chief commercial officer for Israel-based Vibrant Gastro, in a statement.
The vibrations help trigger peristalsis, the wave-like muscle contractions that move food through the gastrointestinal tract, the company said. Decreased peristalsis is a cause of constipation, which is defined as having less than three bowel movements per week, according to the Cleveland Clinic.
About 2.5 million people see their doctor each year for constipation. The pills are made of what the company called “medical-grade material” that is the same as what’s used to make gastroenterology cameras.
In the trial, most people did not report feeling the pill inside of them.
“A minority could feel it,” said Eamonn Quigley, MD, chief of gastroenterology at Houston Methodist Hospital, in a statement. “None of them felt it was being uncomfortable. And none of them stopped taking it because of that.”
Dr. Quigley helped test the capsules and does not have a financial stake in the company, according to Vibrant.
The pills do not dissolve inside a person’s body. Rather, “after they’ve done their job, the person’s body poops them out, and they’re flushed away,” the company said.
A version of this article first appeared on WebMD.com.
The drug-free solution is designed for daily use. In a trial, the pill produced at least one additional weekly bowel movement for 41% of participants, compared with at least one additional bowel movement for 23% of participants who took a placebo pill.
Vibrant was approved by the Food and Drug Administration in August but is just now becoming available for doctors to prescribe, the company announced Wednesday.
Because it is not a drug, Vibrant is considered a class 2 medical device by the FDA, which is the same class as contact lenses.
Here’s how it works: Around bedtime, the pill is inserted in a pod to activate it, then swallowed. It travels the digestive tract and reaches the large intestine about 14 hours later.
“Then it goes to work,” the company explained in a news release. “After it’s swallowed, it is active for about 2 hours, goes quiet for around 6, hours and then activates again for another 2 hours.”
“There are little vibrations for 3 seconds on, 3 seconds off,” said Cathy Collis, chief commercial officer for Israel-based Vibrant Gastro, in a statement.
The vibrations help trigger peristalsis, the wave-like muscle contractions that move food through the gastrointestinal tract, the company said. Decreased peristalsis is a cause of constipation, which is defined as having less than three bowel movements per week, according to the Cleveland Clinic.
About 2.5 million people see their doctor each year for constipation. The pills are made of what the company called “medical-grade material” that is the same as what’s used to make gastroenterology cameras.
In the trial, most people did not report feeling the pill inside of them.
“A minority could feel it,” said Eamonn Quigley, MD, chief of gastroenterology at Houston Methodist Hospital, in a statement. “None of them felt it was being uncomfortable. And none of them stopped taking it because of that.”
Dr. Quigley helped test the capsules and does not have a financial stake in the company, according to Vibrant.
The pills do not dissolve inside a person’s body. Rather, “after they’ve done their job, the person’s body poops them out, and they’re flushed away,” the company said.
A version of this article first appeared on WebMD.com.
The drug-free solution is designed for daily use. In a trial, the pill produced at least one additional weekly bowel movement for 41% of participants, compared with at least one additional bowel movement for 23% of participants who took a placebo pill.
Vibrant was approved by the Food and Drug Administration in August but is just now becoming available for doctors to prescribe, the company announced Wednesday.
Because it is not a drug, Vibrant is considered a class 2 medical device by the FDA, which is the same class as contact lenses.
Here’s how it works: Around bedtime, the pill is inserted in a pod to activate it, then swallowed. It travels the digestive tract and reaches the large intestine about 14 hours later.
“Then it goes to work,” the company explained in a news release. “After it’s swallowed, it is active for about 2 hours, goes quiet for around 6, hours and then activates again for another 2 hours.”
“There are little vibrations for 3 seconds on, 3 seconds off,” said Cathy Collis, chief commercial officer for Israel-based Vibrant Gastro, in a statement.
The vibrations help trigger peristalsis, the wave-like muscle contractions that move food through the gastrointestinal tract, the company said. Decreased peristalsis is a cause of constipation, which is defined as having less than three bowel movements per week, according to the Cleveland Clinic.
About 2.5 million people see their doctor each year for constipation. The pills are made of what the company called “medical-grade material” that is the same as what’s used to make gastroenterology cameras.
In the trial, most people did not report feeling the pill inside of them.
“A minority could feel it,” said Eamonn Quigley, MD, chief of gastroenterology at Houston Methodist Hospital, in a statement. “None of them felt it was being uncomfortable. And none of them stopped taking it because of that.”
Dr. Quigley helped test the capsules and does not have a financial stake in the company, according to Vibrant.
The pills do not dissolve inside a person’s body. Rather, “after they’ve done their job, the person’s body poops them out, and they’re flushed away,” the company said.
A version of this article first appeared on WebMD.com.
NICU use up, birth weights down in babies of mothers with HCV
Infants born to women infected with the hepatitis C virus (HCV) faced twice the risk of stays in the neonatal ICU (NICU) and 2.7 times the risk of low birth weight, a new analysis finds, even when researchers adjusted their data to control for injectable drug use and maternal medical comorbidity.
Clinicians should be “aware that the infants of pregnant people with HCV may have a high rate of need for higher-level pediatric care,” said Brenna L. Hughes, MD, MSc, chief of maternal fetal medicine at Duke University Medical Center, Durham, N.C. She spoke in an interview about the findings, which were presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
As Dr. Hughes noted, “HCV remains a serious problem in pregnancy because it often goes undiagnosed and/or untreated prior to pregnancy. It can be passed to infants, and this can cause significant health-related outcomes for children as they age.”
For the multicenter U.S. study, researchers identified 249 pregnant mothers with HCV from a 2012-2018 cohort and matched them by gestational age to controls (n = 486). The average age was 28; 71.1% of the cases were non-Hispanic White versus 41.6% of the controls; 8.4% of cases were non-Hispanic Black versus 32.1% of controls (P < .001 for race/ethnicity analysis); and 73% of cases were smokers versus 18% of controls (P < .001). More than 19% of cases reported injectable drug use during pregnancy versus 0.2% of controls (P < .001).
The researchers adjusted their findings for maternal age, body mass index, injectable drug use, and maternal comorbidity.
An earlier analysis of the study data found that 6% of pregnant women with HCV passed it on to their infants, especially those with high levels of virus in their systems. For the new study, researchers focused on various outcomes to test the assumption that “adverse pregnancy outcomes associated with HCV are related to prematurity or to ongoing use of injection drugs,” Dr. Hughes said.
There was no increase in rates of preterm birth or adverse maternal outcomes in the HCV cases. However, infants born to women with HCV were more likely than the controls to require a stay in the NICU (45% vs. 19%; adjusted relative risk, 1.99; 95% confidence interval, 1.54-2.58). They were also more likely to have lower birth weights (small for gestational age < 5th percentile) (10.6% vs. 3.1%; ARR, 2.72; 95% CI, 1.38-5.34).
No difference in outcomes was seen when HCV cases with viremia (33%) were excluded.
“The most surprising finding was that the need for higher-level pediatric care was so high even though there wasn’t an increased risk of prematurity,” Dr. Hughes said.
She added it’s not clear why NICU stays and low birth weights were more common in infants of women with HCV. “It is possible that the higher risk of need for higher-level pediatric care was related to a need for observation or treatment due to use of opioid replacement therapies with opioid agonists.” As for lower birth weight, “there may be other unmeasured risk factors.”
Tatyana Kushner, MD, MSCE, of the division of liver diseases at Icahn School of Medicine at Mount Sinai, New York, said in an interview that the study adds to limited data about HCV in pregnancy. “These findings have been demonstrated in prior studies, and it would be important to tease apart whether [low birth weight] is related to the virus itself or more related to other confounding associated factors such as maternal substance use as well as other associated social determinants of health among women with HCV.”
As for the study’s message, Dr. Kushner said it makes it clear that “hepatitis C adversely impacts outcomes of pregnancy and it is important to identify women of childbearing age for treatment early, ideally prior to pregnancy, in order to improve their pregnancy outcomes. In addition, treatment of hepatitis C during pregnancy should be explored further to determine if treatment during pregnancy can improve outcomes.”
At the moment, she said, “there are ongoing studies to delineate the safety and efficacy of hepatitis C treatment during pregnancy. Given that we are screening for hepatitis C during pregnancy, we need clear recommendations on the use of direct-acting antivirals in people who screen positive.”
The study was funded by the National Institute of Child Health and Human Development. The authors have no disclosures. Dr. Kushner disclosed research support (Gilead) and advisory board service (Gilead, AbbVie, Bausch, GlaxoSmithKline, and Eiger).
Infants born to women infected with the hepatitis C virus (HCV) faced twice the risk of stays in the neonatal ICU (NICU) and 2.7 times the risk of low birth weight, a new analysis finds, even when researchers adjusted their data to control for injectable drug use and maternal medical comorbidity.
Clinicians should be “aware that the infants of pregnant people with HCV may have a high rate of need for higher-level pediatric care,” said Brenna L. Hughes, MD, MSc, chief of maternal fetal medicine at Duke University Medical Center, Durham, N.C. She spoke in an interview about the findings, which were presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
As Dr. Hughes noted, “HCV remains a serious problem in pregnancy because it often goes undiagnosed and/or untreated prior to pregnancy. It can be passed to infants, and this can cause significant health-related outcomes for children as they age.”
For the multicenter U.S. study, researchers identified 249 pregnant mothers with HCV from a 2012-2018 cohort and matched them by gestational age to controls (n = 486). The average age was 28; 71.1% of the cases were non-Hispanic White versus 41.6% of the controls; 8.4% of cases were non-Hispanic Black versus 32.1% of controls (P < .001 for race/ethnicity analysis); and 73% of cases were smokers versus 18% of controls (P < .001). More than 19% of cases reported injectable drug use during pregnancy versus 0.2% of controls (P < .001).
The researchers adjusted their findings for maternal age, body mass index, injectable drug use, and maternal comorbidity.
An earlier analysis of the study data found that 6% of pregnant women with HCV passed it on to their infants, especially those with high levels of virus in their systems. For the new study, researchers focused on various outcomes to test the assumption that “adverse pregnancy outcomes associated with HCV are related to prematurity or to ongoing use of injection drugs,” Dr. Hughes said.
There was no increase in rates of preterm birth or adverse maternal outcomes in the HCV cases. However, infants born to women with HCV were more likely than the controls to require a stay in the NICU (45% vs. 19%; adjusted relative risk, 1.99; 95% confidence interval, 1.54-2.58). They were also more likely to have lower birth weights (small for gestational age < 5th percentile) (10.6% vs. 3.1%; ARR, 2.72; 95% CI, 1.38-5.34).
No difference in outcomes was seen when HCV cases with viremia (33%) were excluded.
“The most surprising finding was that the need for higher-level pediatric care was so high even though there wasn’t an increased risk of prematurity,” Dr. Hughes said.
She added it’s not clear why NICU stays and low birth weights were more common in infants of women with HCV. “It is possible that the higher risk of need for higher-level pediatric care was related to a need for observation or treatment due to use of opioid replacement therapies with opioid agonists.” As for lower birth weight, “there may be other unmeasured risk factors.”
Tatyana Kushner, MD, MSCE, of the division of liver diseases at Icahn School of Medicine at Mount Sinai, New York, said in an interview that the study adds to limited data about HCV in pregnancy. “These findings have been demonstrated in prior studies, and it would be important to tease apart whether [low birth weight] is related to the virus itself or more related to other confounding associated factors such as maternal substance use as well as other associated social determinants of health among women with HCV.”
As for the study’s message, Dr. Kushner said it makes it clear that “hepatitis C adversely impacts outcomes of pregnancy and it is important to identify women of childbearing age for treatment early, ideally prior to pregnancy, in order to improve their pregnancy outcomes. In addition, treatment of hepatitis C during pregnancy should be explored further to determine if treatment during pregnancy can improve outcomes.”
At the moment, she said, “there are ongoing studies to delineate the safety and efficacy of hepatitis C treatment during pregnancy. Given that we are screening for hepatitis C during pregnancy, we need clear recommendations on the use of direct-acting antivirals in people who screen positive.”
The study was funded by the National Institute of Child Health and Human Development. The authors have no disclosures. Dr. Kushner disclosed research support (Gilead) and advisory board service (Gilead, AbbVie, Bausch, GlaxoSmithKline, and Eiger).
Infants born to women infected with the hepatitis C virus (HCV) faced twice the risk of stays in the neonatal ICU (NICU) and 2.7 times the risk of low birth weight, a new analysis finds, even when researchers adjusted their data to control for injectable drug use and maternal medical comorbidity.
Clinicians should be “aware that the infants of pregnant people with HCV may have a high rate of need for higher-level pediatric care,” said Brenna L. Hughes, MD, MSc, chief of maternal fetal medicine at Duke University Medical Center, Durham, N.C. She spoke in an interview about the findings, which were presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
As Dr. Hughes noted, “HCV remains a serious problem in pregnancy because it often goes undiagnosed and/or untreated prior to pregnancy. It can be passed to infants, and this can cause significant health-related outcomes for children as they age.”
For the multicenter U.S. study, researchers identified 249 pregnant mothers with HCV from a 2012-2018 cohort and matched them by gestational age to controls (n = 486). The average age was 28; 71.1% of the cases were non-Hispanic White versus 41.6% of the controls; 8.4% of cases were non-Hispanic Black versus 32.1% of controls (P < .001 for race/ethnicity analysis); and 73% of cases were smokers versus 18% of controls (P < .001). More than 19% of cases reported injectable drug use during pregnancy versus 0.2% of controls (P < .001).
The researchers adjusted their findings for maternal age, body mass index, injectable drug use, and maternal comorbidity.
An earlier analysis of the study data found that 6% of pregnant women with HCV passed it on to their infants, especially those with high levels of virus in their systems. For the new study, researchers focused on various outcomes to test the assumption that “adverse pregnancy outcomes associated with HCV are related to prematurity or to ongoing use of injection drugs,” Dr. Hughes said.
There was no increase in rates of preterm birth or adverse maternal outcomes in the HCV cases. However, infants born to women with HCV were more likely than the controls to require a stay in the NICU (45% vs. 19%; adjusted relative risk, 1.99; 95% confidence interval, 1.54-2.58). They were also more likely to have lower birth weights (small for gestational age < 5th percentile) (10.6% vs. 3.1%; ARR, 2.72; 95% CI, 1.38-5.34).
No difference in outcomes was seen when HCV cases with viremia (33%) were excluded.
“The most surprising finding was that the need for higher-level pediatric care was so high even though there wasn’t an increased risk of prematurity,” Dr. Hughes said.
She added it’s not clear why NICU stays and low birth weights were more common in infants of women with HCV. “It is possible that the higher risk of need for higher-level pediatric care was related to a need for observation or treatment due to use of opioid replacement therapies with opioid agonists.” As for lower birth weight, “there may be other unmeasured risk factors.”
Tatyana Kushner, MD, MSCE, of the division of liver diseases at Icahn School of Medicine at Mount Sinai, New York, said in an interview that the study adds to limited data about HCV in pregnancy. “These findings have been demonstrated in prior studies, and it would be important to tease apart whether [low birth weight] is related to the virus itself or more related to other confounding associated factors such as maternal substance use as well as other associated social determinants of health among women with HCV.”
As for the study’s message, Dr. Kushner said it makes it clear that “hepatitis C adversely impacts outcomes of pregnancy and it is important to identify women of childbearing age for treatment early, ideally prior to pregnancy, in order to improve their pregnancy outcomes. In addition, treatment of hepatitis C during pregnancy should be explored further to determine if treatment during pregnancy can improve outcomes.”
At the moment, she said, “there are ongoing studies to delineate the safety and efficacy of hepatitis C treatment during pregnancy. Given that we are screening for hepatitis C during pregnancy, we need clear recommendations on the use of direct-acting antivirals in people who screen positive.”
The study was funded by the National Institute of Child Health and Human Development. The authors have no disclosures. Dr. Kushner disclosed research support (Gilead) and advisory board service (Gilead, AbbVie, Bausch, GlaxoSmithKline, and Eiger).
FROM THE PREGNANCY MEETING
Embattled iPLEDGE program: Changes ahead?
In December 2021, major changes took effect in the iPLEDGE program, the Food and Drug Administration–required safety program for managing the risks of isotretinoin’s teratogenicity and preventing exposure during pregnancy. Now, more modifications may be coming to the acne drug’s safety program.
The risk evaluation and mitigation strategy (REMS) requirements. The aim, according to the FDA meeting announcement, is “to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of isotretinoin oral capsules for patients.”
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane. Its former brand name was Accutane.
Problems began to surface days after a new, gender-neutral approach to the risk mitigation program was launched on Dec. 13, 2021. That program had been approved earlier by the FDA.
However, the problems that were encountered were a result of glitches in changes in the platform that had been planned, and were not related to the gender-neutral changes. The iPLEDGE program had transitioned to the new platform, and the rollout was far from smooth. Dermatologists, pharmacists, patients, parents of patients, and others were frustrated and angry that they could not access the new platform and obtain the medication promptly. Reaching the help line to sort out problems was another exercise in frustration. Wait times while on hold were unbearably long, or problems were not resolved over the phone.
(The new gender-neutral approach, which advocates said was needed to preserve inclusiveness of their patients, including transgender patients, places potential patients into two categories: those who can become pregnant, and those who cannot. Previously, there were three categories into which patients were classified: females who have reproductive potential, females who do not have reproductive potential, and males.)
Before pharmacists can fill a prescription for isotretinoin, a medical provider must confirm a patient’s negative pregnancy test and inform a patient with reproductive potential of the risks of the medication.
In January 2022, to deal with the chaotic launch and subsequent problems, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve the problems reported by clinicians, pharmacists, and patients.
The American Academy of Dermatology Association formed an iPLEDGE work group to address the issues and suggest solutions. It has made several requests of and suggestions for the IPMG, which manages the program, according to Andrea L. Zaenglein, MD, professor of dermatology and pediatrics at Penn State Hershey (Pa.) Medical Center, and a member of the work group.
“We are asking them to eliminate the monthly attestation for patients who can’t get pregnant and to review and modify restrictive and punitive waiting and lockout periods for all patients,” she told this news organization.
As of February 2023, most of the platform glitches had been smoothed out, Dr. Zaenglein said. Still, “improvements to the design of the website could improve the user interface,” she added.
The FDA has established a docket for the public to submit comments before the meeting. The docket number is FDA-2022-N-3071. The electronic filing system will accept comments until 11:59 p.m. Eastern time on March 27. Background material and a link to the live webcast of the panel meeting will be available to the public no later than 2 days before the meeting and will be posted on the FDA web page or at the time of the meeting.
A version of this article first appeared on Medscape.com.
In December 2021, major changes took effect in the iPLEDGE program, the Food and Drug Administration–required safety program for managing the risks of isotretinoin’s teratogenicity and preventing exposure during pregnancy. Now, more modifications may be coming to the acne drug’s safety program.
The risk evaluation and mitigation strategy (REMS) requirements. The aim, according to the FDA meeting announcement, is “to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of isotretinoin oral capsules for patients.”
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane. Its former brand name was Accutane.
Problems began to surface days after a new, gender-neutral approach to the risk mitigation program was launched on Dec. 13, 2021. That program had been approved earlier by the FDA.
However, the problems that were encountered were a result of glitches in changes in the platform that had been planned, and were not related to the gender-neutral changes. The iPLEDGE program had transitioned to the new platform, and the rollout was far from smooth. Dermatologists, pharmacists, patients, parents of patients, and others were frustrated and angry that they could not access the new platform and obtain the medication promptly. Reaching the help line to sort out problems was another exercise in frustration. Wait times while on hold were unbearably long, or problems were not resolved over the phone.
(The new gender-neutral approach, which advocates said was needed to preserve inclusiveness of their patients, including transgender patients, places potential patients into two categories: those who can become pregnant, and those who cannot. Previously, there were three categories into which patients were classified: females who have reproductive potential, females who do not have reproductive potential, and males.)
Before pharmacists can fill a prescription for isotretinoin, a medical provider must confirm a patient’s negative pregnancy test and inform a patient with reproductive potential of the risks of the medication.
In January 2022, to deal with the chaotic launch and subsequent problems, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve the problems reported by clinicians, pharmacists, and patients.
The American Academy of Dermatology Association formed an iPLEDGE work group to address the issues and suggest solutions. It has made several requests of and suggestions for the IPMG, which manages the program, according to Andrea L. Zaenglein, MD, professor of dermatology and pediatrics at Penn State Hershey (Pa.) Medical Center, and a member of the work group.
“We are asking them to eliminate the monthly attestation for patients who can’t get pregnant and to review and modify restrictive and punitive waiting and lockout periods for all patients,” she told this news organization.
As of February 2023, most of the platform glitches had been smoothed out, Dr. Zaenglein said. Still, “improvements to the design of the website could improve the user interface,” she added.
The FDA has established a docket for the public to submit comments before the meeting. The docket number is FDA-2022-N-3071. The electronic filing system will accept comments until 11:59 p.m. Eastern time on March 27. Background material and a link to the live webcast of the panel meeting will be available to the public no later than 2 days before the meeting and will be posted on the FDA web page or at the time of the meeting.
A version of this article first appeared on Medscape.com.
In December 2021, major changes took effect in the iPLEDGE program, the Food and Drug Administration–required safety program for managing the risks of isotretinoin’s teratogenicity and preventing exposure during pregnancy. Now, more modifications may be coming to the acne drug’s safety program.
The risk evaluation and mitigation strategy (REMS) requirements. The aim, according to the FDA meeting announcement, is “to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of isotretinoin oral capsules for patients.”
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane. Its former brand name was Accutane.
Problems began to surface days after a new, gender-neutral approach to the risk mitigation program was launched on Dec. 13, 2021. That program had been approved earlier by the FDA.
However, the problems that were encountered were a result of glitches in changes in the platform that had been planned, and were not related to the gender-neutral changes. The iPLEDGE program had transitioned to the new platform, and the rollout was far from smooth. Dermatologists, pharmacists, patients, parents of patients, and others were frustrated and angry that they could not access the new platform and obtain the medication promptly. Reaching the help line to sort out problems was another exercise in frustration. Wait times while on hold were unbearably long, or problems were not resolved over the phone.
(The new gender-neutral approach, which advocates said was needed to preserve inclusiveness of their patients, including transgender patients, places potential patients into two categories: those who can become pregnant, and those who cannot. Previously, there were three categories into which patients were classified: females who have reproductive potential, females who do not have reproductive potential, and males.)
Before pharmacists can fill a prescription for isotretinoin, a medical provider must confirm a patient’s negative pregnancy test and inform a patient with reproductive potential of the risks of the medication.
In January 2022, to deal with the chaotic launch and subsequent problems, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve the problems reported by clinicians, pharmacists, and patients.
The American Academy of Dermatology Association formed an iPLEDGE work group to address the issues and suggest solutions. It has made several requests of and suggestions for the IPMG, which manages the program, according to Andrea L. Zaenglein, MD, professor of dermatology and pediatrics at Penn State Hershey (Pa.) Medical Center, and a member of the work group.
“We are asking them to eliminate the monthly attestation for patients who can’t get pregnant and to review and modify restrictive and punitive waiting and lockout periods for all patients,” she told this news organization.
As of February 2023, most of the platform glitches had been smoothed out, Dr. Zaenglein said. Still, “improvements to the design of the website could improve the user interface,” she added.
The FDA has established a docket for the public to submit comments before the meeting. The docket number is FDA-2022-N-3071. The electronic filing system will accept comments until 11:59 p.m. Eastern time on March 27. Background material and a link to the live webcast of the panel meeting will be available to the public no later than 2 days before the meeting and will be posted on the FDA web page or at the time of the meeting.
A version of this article first appeared on Medscape.com.
‘Forever chemicals’ up type 2 diabetes risk in midlife White women
Middle-aged White women who had higher levels of some breakdown products of phthalates – a class of endocrine disrupting chemicals (EDCs), or “forever chemicals,” that act as plasticizers – had a significantly greater risk of developing type 2 diabetes over a 6-year period compared with other similar women.
However, this association was not seen among Black or Asian middle-aged women.
These findings from the Study of Women’s Health Across the Nation – Multipollutant Study (SWAN-MPS), by Mia Q. Peng, PhD, MPH, and colleagues, have been published online in the Journal of Clinical Endocrinology & Metabolism.
“Overall, our study has added some evidence to support the potential diabetogenic effects of phthalates, but it also highlights that much is still unknown about the metabolic effects of these chemicals,” the group noted.
“The apparent racial/ethnic differences in the associations between phthalates and incident diabetes should be investigated in future studies,” they cautioned.
Recruiting younger participants and observing them longer, they suggested, “will also help us understand the effects of phthalates on different stages of the diabetogenic process, including whether body fat gain is an important mediator.”
Phthalates are all around us
Low-molecular-weight phthalates are frequently added to personal care products, such as fragrance, nail polish, and some feminine hygiene products, as solvents, plasticizers, and fixatives, the researchers explained.
And high-molecular-weight phthalates are frequently added to polyvinyl chloride plastic products, such as plastic food packaging, clothing, and vinyl flooring, as plasticizers.
Phthalates have been hypothesized to contribute to the development of diabetes, but longitudinal evidence in humans was limited.
“Given widespread exposure to phthalates and the enormous costs of diabetes to individuals and societies, ongoing investments in the research on phthalates’ metabolic effects are warranted,” the researchers concluded.
Racial differences in phthalates and incident diabetes
“A new finding is that we observed some phthalates are associated with a higher risk of diabetes development, especially in White women [that] were not seen in Black or Asian women,” senior author Sung Kyun Park, ScD, MPH, of the University of Michigan, Ann Arbor, told this news organization.
“We were surprised to see the racial/ethnic differences,” added Dr. Peng, formerly of the University of Michigan and now at Lifecourse Epidemiology of Adiposity and Diabetes Center, University of Colorado Anschutz Medical Campus.
A possible explanation is that “compared to White women, Black women develop diabetes at a younger age and are exposed to higher levels of several phthalates,” and this study excluded women who already had diabetes by midlife, she noted.
“Although our study was conducted in a cohort of women,” Dr. Park stressed, “we hope that our findings are not interpreted that only women should be concerned of phthalates. Our findings add to the current literature that phthalates may be a potential risk factor for type 2 diabetes.
“Certain phthalates are prohibited in children’s toys and child care articles,” Dr. Peng noted, as explained by the U.S. Consumer Product Safety Commission. In addition, a bill has been introduced in Congress to ban phthalates in food contact substances.
“If phthalates are removed from plastics and other consumer products,” she cautioned, “we do have to be careful in the process to avoid replacing them with some other potentially harmful chemicals.”
A well-known example of this type of “regrettable substitution,” Dr. Park added, “is ‘BPA-free’ plastics that replaced bisphenol A with other bisphenols such as bisphenol-F (BPF) or bisphenol-S (BPS). The product has a label of ‘BPA-free’, but those replaced chemicals turned out to be equally toxic. Science is slow to determine if a new chemical introduced to the market is safe and can replace a regulated chemical.”
And studies have shown that a diet rich in meat, fat, and ultraprocessed foods is associated with increased exposures to some phthalates, especially when the foods are obtained away from home, such as fast foods, Dr. Peng observed. In addition, some phthalates are added to personal care products such as fragrance.
“As a first step,” she said, “I think reducing consumption of ultraprocessed foods packaged in plastics may help reduce phthalate exposure.”
A 2020 report from the Endocrine Society and the International Pollutants Elimination Network (IPEN), titled, “Plastics, EDCs, and Health,” summarizes research on bisphenol A, per- and polyfluoroalkyl substances (PFAS), phthalates, and other EDCs that leach from plastics. The Endocrine Society website also has a link to a 2-page summary.
Levels of 12 phthalate metabolites
Previously, the researchers reported how another class of “forever chemicals,” PFAS, were associated with risk of hypertension in a 17-year follow-up of middle-aged women in the SWAN study.
In the current study, they analyzed data from 1,308 women in SWAN-MPS who had been recruited at five study sites (Oakland, Calif; Los Angeles; Detroit; Pittsburgh; and Boston).
The women were between ages 42 and 52 years in 1996-1997 and self-identified as White, Black, Chinese, or Japanese.
They did not have diabetes in 1999-2000 and had sufficient urine samples for phthalate assessment then and midway through a 6-year follow-up.
The women were a median age of 49 years in 1999-2000. About half were White, 20% were Black, 13% were Chinese, and 15% were Japanese.
Researchers analyzed levels of 12 metabolites, chosen because their parent phthalates have been widely used in industry and commerce, and exposure to these phthalates is a national biomonitoring priority.
The measured phthalates were:
Three metabolites of low-molecular-weight phthalates:
- mono-ethyl phthalate (MEP)
- mono-n-butyl phthalate (MnBP)
- mono-isobutyl phthalate (MiBP)
Four metabolites of the high-molecular-weight phthalate di(2-ethylhexyl) phthalate (DEHP), which is of particular public health interest:
- mono(2-ethylhexyl) phthalate (MEHP)
- mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP)
- mono(2-ethyl-5-oxohexyl) phthalate (MEOHP)
- mono(2-ethyl-5-carboxypentyl) phthalate (MECPP)
Five metabolites of other high-molecular-weight phthalates:
- monobenzyl phthalate (MBzP)
- monoisononyl phthalate (MiNP)
- mono-carboxyoctyl phthalate (MCOP)
- mono-carboxy-isononyl phthalate (MCNP)
- mono(3-carboxypropyl) phthalate (MCPP)
The researchers excluded MiNP from all analyses because it was detected in less than 1% of urine samples.
The different phthalate metabolites were detected in 84.8% of samples (MEHP) to 100% of samples (MnBP and MECPP).
Women who were younger, Black, current smokers, or obese generally had higher concentrations of phthalate metabolites.
Over 6 years, 61 women developed diabetes (an incidence rate of 8.1 per 1000 person-years).
Compared with other women, those with incident diabetes had significantly higher concentrations of all phthalate metabolites except DEHP metabolites and MCPP.
Phthalates were not associated with incident diabetes in Black or Asian women.
However, among White women, each doubling of the concentrations of MiBP, MBzP, MCOP, MCNP, and MCCP was associated with a 30% to 63% higher incidence of diabetes (HR 1.30 for MCNP; HR 1.63 for MiBP).
The SWAN study was supported by the National Institutes of Health, Department of Health & Human Services, National Institute on Aging, National Institute of Nursing Research, NIH Office of Research on Women’s Health, and SWAN Repository. The current study was supported by the National Center for Research Resources, National Center for Advancing Translational Sciences, NIH, National Institute of Environmental Health, and Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. Dr. Peng was supported by an Interdisciplinary Research Training on Health and Aging grant from the NIA. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Middle-aged White women who had higher levels of some breakdown products of phthalates – a class of endocrine disrupting chemicals (EDCs), or “forever chemicals,” that act as plasticizers – had a significantly greater risk of developing type 2 diabetes over a 6-year period compared with other similar women.
However, this association was not seen among Black or Asian middle-aged women.
These findings from the Study of Women’s Health Across the Nation – Multipollutant Study (SWAN-MPS), by Mia Q. Peng, PhD, MPH, and colleagues, have been published online in the Journal of Clinical Endocrinology & Metabolism.
“Overall, our study has added some evidence to support the potential diabetogenic effects of phthalates, but it also highlights that much is still unknown about the metabolic effects of these chemicals,” the group noted.
“The apparent racial/ethnic differences in the associations between phthalates and incident diabetes should be investigated in future studies,” they cautioned.
Recruiting younger participants and observing them longer, they suggested, “will also help us understand the effects of phthalates on different stages of the diabetogenic process, including whether body fat gain is an important mediator.”
Phthalates are all around us
Low-molecular-weight phthalates are frequently added to personal care products, such as fragrance, nail polish, and some feminine hygiene products, as solvents, plasticizers, and fixatives, the researchers explained.
And high-molecular-weight phthalates are frequently added to polyvinyl chloride plastic products, such as plastic food packaging, clothing, and vinyl flooring, as plasticizers.
Phthalates have been hypothesized to contribute to the development of diabetes, but longitudinal evidence in humans was limited.
“Given widespread exposure to phthalates and the enormous costs of diabetes to individuals and societies, ongoing investments in the research on phthalates’ metabolic effects are warranted,” the researchers concluded.
Racial differences in phthalates and incident diabetes
“A new finding is that we observed some phthalates are associated with a higher risk of diabetes development, especially in White women [that] were not seen in Black or Asian women,” senior author Sung Kyun Park, ScD, MPH, of the University of Michigan, Ann Arbor, told this news organization.
“We were surprised to see the racial/ethnic differences,” added Dr. Peng, formerly of the University of Michigan and now at Lifecourse Epidemiology of Adiposity and Diabetes Center, University of Colorado Anschutz Medical Campus.
A possible explanation is that “compared to White women, Black women develop diabetes at a younger age and are exposed to higher levels of several phthalates,” and this study excluded women who already had diabetes by midlife, she noted.
“Although our study was conducted in a cohort of women,” Dr. Park stressed, “we hope that our findings are not interpreted that only women should be concerned of phthalates. Our findings add to the current literature that phthalates may be a potential risk factor for type 2 diabetes.
“Certain phthalates are prohibited in children’s toys and child care articles,” Dr. Peng noted, as explained by the U.S. Consumer Product Safety Commission. In addition, a bill has been introduced in Congress to ban phthalates in food contact substances.
“If phthalates are removed from plastics and other consumer products,” she cautioned, “we do have to be careful in the process to avoid replacing them with some other potentially harmful chemicals.”
A well-known example of this type of “regrettable substitution,” Dr. Park added, “is ‘BPA-free’ plastics that replaced bisphenol A with other bisphenols such as bisphenol-F (BPF) or bisphenol-S (BPS). The product has a label of ‘BPA-free’, but those replaced chemicals turned out to be equally toxic. Science is slow to determine if a new chemical introduced to the market is safe and can replace a regulated chemical.”
And studies have shown that a diet rich in meat, fat, and ultraprocessed foods is associated with increased exposures to some phthalates, especially when the foods are obtained away from home, such as fast foods, Dr. Peng observed. In addition, some phthalates are added to personal care products such as fragrance.
“As a first step,” she said, “I think reducing consumption of ultraprocessed foods packaged in plastics may help reduce phthalate exposure.”
A 2020 report from the Endocrine Society and the International Pollutants Elimination Network (IPEN), titled, “Plastics, EDCs, and Health,” summarizes research on bisphenol A, per- and polyfluoroalkyl substances (PFAS), phthalates, and other EDCs that leach from plastics. The Endocrine Society website also has a link to a 2-page summary.
Levels of 12 phthalate metabolites
Previously, the researchers reported how another class of “forever chemicals,” PFAS, were associated with risk of hypertension in a 17-year follow-up of middle-aged women in the SWAN study.
In the current study, they analyzed data from 1,308 women in SWAN-MPS who had been recruited at five study sites (Oakland, Calif; Los Angeles; Detroit; Pittsburgh; and Boston).
The women were between ages 42 and 52 years in 1996-1997 and self-identified as White, Black, Chinese, or Japanese.
They did not have diabetes in 1999-2000 and had sufficient urine samples for phthalate assessment then and midway through a 6-year follow-up.
The women were a median age of 49 years in 1999-2000. About half were White, 20% were Black, 13% were Chinese, and 15% were Japanese.
Researchers analyzed levels of 12 metabolites, chosen because their parent phthalates have been widely used in industry and commerce, and exposure to these phthalates is a national biomonitoring priority.
The measured phthalates were:
Three metabolites of low-molecular-weight phthalates:
- mono-ethyl phthalate (MEP)
- mono-n-butyl phthalate (MnBP)
- mono-isobutyl phthalate (MiBP)
Four metabolites of the high-molecular-weight phthalate di(2-ethylhexyl) phthalate (DEHP), which is of particular public health interest:
- mono(2-ethylhexyl) phthalate (MEHP)
- mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP)
- mono(2-ethyl-5-oxohexyl) phthalate (MEOHP)
- mono(2-ethyl-5-carboxypentyl) phthalate (MECPP)
Five metabolites of other high-molecular-weight phthalates:
- monobenzyl phthalate (MBzP)
- monoisononyl phthalate (MiNP)
- mono-carboxyoctyl phthalate (MCOP)
- mono-carboxy-isononyl phthalate (MCNP)
- mono(3-carboxypropyl) phthalate (MCPP)
The researchers excluded MiNP from all analyses because it was detected in less than 1% of urine samples.
The different phthalate metabolites were detected in 84.8% of samples (MEHP) to 100% of samples (MnBP and MECPP).
Women who were younger, Black, current smokers, or obese generally had higher concentrations of phthalate metabolites.
Over 6 years, 61 women developed diabetes (an incidence rate of 8.1 per 1000 person-years).
Compared with other women, those with incident diabetes had significantly higher concentrations of all phthalate metabolites except DEHP metabolites and MCPP.
Phthalates were not associated with incident diabetes in Black or Asian women.
However, among White women, each doubling of the concentrations of MiBP, MBzP, MCOP, MCNP, and MCCP was associated with a 30% to 63% higher incidence of diabetes (HR 1.30 for MCNP; HR 1.63 for MiBP).
The SWAN study was supported by the National Institutes of Health, Department of Health & Human Services, National Institute on Aging, National Institute of Nursing Research, NIH Office of Research on Women’s Health, and SWAN Repository. The current study was supported by the National Center for Research Resources, National Center for Advancing Translational Sciences, NIH, National Institute of Environmental Health, and Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. Dr. Peng was supported by an Interdisciplinary Research Training on Health and Aging grant from the NIA. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Middle-aged White women who had higher levels of some breakdown products of phthalates – a class of endocrine disrupting chemicals (EDCs), or “forever chemicals,” that act as plasticizers – had a significantly greater risk of developing type 2 diabetes over a 6-year period compared with other similar women.
However, this association was not seen among Black or Asian middle-aged women.
These findings from the Study of Women’s Health Across the Nation – Multipollutant Study (SWAN-MPS), by Mia Q. Peng, PhD, MPH, and colleagues, have been published online in the Journal of Clinical Endocrinology & Metabolism.
“Overall, our study has added some evidence to support the potential diabetogenic effects of phthalates, but it also highlights that much is still unknown about the metabolic effects of these chemicals,” the group noted.
“The apparent racial/ethnic differences in the associations between phthalates and incident diabetes should be investigated in future studies,” they cautioned.
Recruiting younger participants and observing them longer, they suggested, “will also help us understand the effects of phthalates on different stages of the diabetogenic process, including whether body fat gain is an important mediator.”
Phthalates are all around us
Low-molecular-weight phthalates are frequently added to personal care products, such as fragrance, nail polish, and some feminine hygiene products, as solvents, plasticizers, and fixatives, the researchers explained.
And high-molecular-weight phthalates are frequently added to polyvinyl chloride plastic products, such as plastic food packaging, clothing, and vinyl flooring, as plasticizers.
Phthalates have been hypothesized to contribute to the development of diabetes, but longitudinal evidence in humans was limited.
“Given widespread exposure to phthalates and the enormous costs of diabetes to individuals and societies, ongoing investments in the research on phthalates’ metabolic effects are warranted,” the researchers concluded.
Racial differences in phthalates and incident diabetes
“A new finding is that we observed some phthalates are associated with a higher risk of diabetes development, especially in White women [that] were not seen in Black or Asian women,” senior author Sung Kyun Park, ScD, MPH, of the University of Michigan, Ann Arbor, told this news organization.
“We were surprised to see the racial/ethnic differences,” added Dr. Peng, formerly of the University of Michigan and now at Lifecourse Epidemiology of Adiposity and Diabetes Center, University of Colorado Anschutz Medical Campus.
A possible explanation is that “compared to White women, Black women develop diabetes at a younger age and are exposed to higher levels of several phthalates,” and this study excluded women who already had diabetes by midlife, she noted.
“Although our study was conducted in a cohort of women,” Dr. Park stressed, “we hope that our findings are not interpreted that only women should be concerned of phthalates. Our findings add to the current literature that phthalates may be a potential risk factor for type 2 diabetes.
“Certain phthalates are prohibited in children’s toys and child care articles,” Dr. Peng noted, as explained by the U.S. Consumer Product Safety Commission. In addition, a bill has been introduced in Congress to ban phthalates in food contact substances.
“If phthalates are removed from plastics and other consumer products,” she cautioned, “we do have to be careful in the process to avoid replacing them with some other potentially harmful chemicals.”
A well-known example of this type of “regrettable substitution,” Dr. Park added, “is ‘BPA-free’ plastics that replaced bisphenol A with other bisphenols such as bisphenol-F (BPF) or bisphenol-S (BPS). The product has a label of ‘BPA-free’, but those replaced chemicals turned out to be equally toxic. Science is slow to determine if a new chemical introduced to the market is safe and can replace a regulated chemical.”
And studies have shown that a diet rich in meat, fat, and ultraprocessed foods is associated with increased exposures to some phthalates, especially when the foods are obtained away from home, such as fast foods, Dr. Peng observed. In addition, some phthalates are added to personal care products such as fragrance.
“As a first step,” she said, “I think reducing consumption of ultraprocessed foods packaged in plastics may help reduce phthalate exposure.”
A 2020 report from the Endocrine Society and the International Pollutants Elimination Network (IPEN), titled, “Plastics, EDCs, and Health,” summarizes research on bisphenol A, per- and polyfluoroalkyl substances (PFAS), phthalates, and other EDCs that leach from plastics. The Endocrine Society website also has a link to a 2-page summary.
Levels of 12 phthalate metabolites
Previously, the researchers reported how another class of “forever chemicals,” PFAS, were associated with risk of hypertension in a 17-year follow-up of middle-aged women in the SWAN study.
In the current study, they analyzed data from 1,308 women in SWAN-MPS who had been recruited at five study sites (Oakland, Calif; Los Angeles; Detroit; Pittsburgh; and Boston).
The women were between ages 42 and 52 years in 1996-1997 and self-identified as White, Black, Chinese, or Japanese.
They did not have diabetes in 1999-2000 and had sufficient urine samples for phthalate assessment then and midway through a 6-year follow-up.
The women were a median age of 49 years in 1999-2000. About half were White, 20% were Black, 13% were Chinese, and 15% were Japanese.
Researchers analyzed levels of 12 metabolites, chosen because their parent phthalates have been widely used in industry and commerce, and exposure to these phthalates is a national biomonitoring priority.
The measured phthalates were:
Three metabolites of low-molecular-weight phthalates:
- mono-ethyl phthalate (MEP)
- mono-n-butyl phthalate (MnBP)
- mono-isobutyl phthalate (MiBP)
Four metabolites of the high-molecular-weight phthalate di(2-ethylhexyl) phthalate (DEHP), which is of particular public health interest:
- mono(2-ethylhexyl) phthalate (MEHP)
- mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP)
- mono(2-ethyl-5-oxohexyl) phthalate (MEOHP)
- mono(2-ethyl-5-carboxypentyl) phthalate (MECPP)
Five metabolites of other high-molecular-weight phthalates:
- monobenzyl phthalate (MBzP)
- monoisononyl phthalate (MiNP)
- mono-carboxyoctyl phthalate (MCOP)
- mono-carboxy-isononyl phthalate (MCNP)
- mono(3-carboxypropyl) phthalate (MCPP)
The researchers excluded MiNP from all analyses because it was detected in less than 1% of urine samples.
The different phthalate metabolites were detected in 84.8% of samples (MEHP) to 100% of samples (MnBP and MECPP).
Women who were younger, Black, current smokers, or obese generally had higher concentrations of phthalate metabolites.
Over 6 years, 61 women developed diabetes (an incidence rate of 8.1 per 1000 person-years).
Compared with other women, those with incident diabetes had significantly higher concentrations of all phthalate metabolites except DEHP metabolites and MCPP.
Phthalates were not associated with incident diabetes in Black or Asian women.
However, among White women, each doubling of the concentrations of MiBP, MBzP, MCOP, MCNP, and MCCP was associated with a 30% to 63% higher incidence of diabetes (HR 1.30 for MCNP; HR 1.63 for MiBP).
The SWAN study was supported by the National Institutes of Health, Department of Health & Human Services, National Institute on Aging, National Institute of Nursing Research, NIH Office of Research on Women’s Health, and SWAN Repository. The current study was supported by the National Center for Research Resources, National Center for Advancing Translational Sciences, NIH, National Institute of Environmental Health, and Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. Dr. Peng was supported by an Interdisciplinary Research Training on Health and Aging grant from the NIA. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
‘Valid option’ for partial breast irradiation in breast cancer
The study covered in this summary was published on researchsquare.com as a preprint and has not yet been peer reviewed.
Key takeaway
Why this matters
- According to numerous guidelines, partial breast irradiation after lumpectomy is a sound approach for early-stage breast cancer, but there is a lack of consensus about treatment schedules.
- The investigators suggest that 30 Gy in five daily fractions is a “valid option” for these patients in a field that lacks consensus.
Study design
- The team reviewed 381 women with early breast cancer treated with this approach (30 Gy in five daily fractions) at their center from 2013 to 2022.
- Half of patients had left-sided tumors, 94.5% had invasive ductal carcinomas, 96.6% had grade 1 or grade 2 disease, and tumors were luminal like in 99.2% of patients.
- Following lumpectomy, women underwent partial breast irradiation to the tumor bed plus 15 mm of isometric expansion beyond it.
- Follow-up was a median of 28 months.
Key results
- Seven patients (2%) had a local recurrence, of which two were in the treatment field.
- Three-year local control, disease-free survival, and overall survival were high (97.5%, 95.7%, and 96.9%, respectively).
- Nearly 90% of patients and 97% of physicians reported good or excellent cosmesis.
- Ten patients (2.9%) had grade 2 late toxicities, including edema, asthenia, and fibrosis; there were no grade 3 or higher adverse events.
- Five patients (1.5%) had late cardiac major events, four of whom were treated on the right breast; three patients (0.9%) had late pulmonary fibrosis.
- The safety and efficacy outcomes are in line with previous reports, including those that used different dosage and/or fractionation schedules.
Limitations
- The study was retrospective, with a relatively short follow-up.
- Quality of life was not assessed.
- There was no objective baseline measure of cosmesis against which to compare cosmetic results.
Disclosures
- There was no funding for the study, and the investigators didn’t have any conflicts of interest to report.
This is a summary of a preprint research study, “One-Week External Beam Partial Breast Irradiation: Survival and Toxicity Outcomes,” led by Riccardo Ray Colciago from the Fondazione IRCCS Istituto Nazionale dei Tumori, Milan. The study has not been peer reviewed. The full text can be found at researchsquare.com.
A version of this article first appeared on Medscape.com.
The study covered in this summary was published on researchsquare.com as a preprint and has not yet been peer reviewed.
Key takeaway
Why this matters
- According to numerous guidelines, partial breast irradiation after lumpectomy is a sound approach for early-stage breast cancer, but there is a lack of consensus about treatment schedules.
- The investigators suggest that 30 Gy in five daily fractions is a “valid option” for these patients in a field that lacks consensus.
Study design
- The team reviewed 381 women with early breast cancer treated with this approach (30 Gy in five daily fractions) at their center from 2013 to 2022.
- Half of patients had left-sided tumors, 94.5% had invasive ductal carcinomas, 96.6% had grade 1 or grade 2 disease, and tumors were luminal like in 99.2% of patients.
- Following lumpectomy, women underwent partial breast irradiation to the tumor bed plus 15 mm of isometric expansion beyond it.
- Follow-up was a median of 28 months.
Key results
- Seven patients (2%) had a local recurrence, of which two were in the treatment field.
- Three-year local control, disease-free survival, and overall survival were high (97.5%, 95.7%, and 96.9%, respectively).
- Nearly 90% of patients and 97% of physicians reported good or excellent cosmesis.
- Ten patients (2.9%) had grade 2 late toxicities, including edema, asthenia, and fibrosis; there were no grade 3 or higher adverse events.
- Five patients (1.5%) had late cardiac major events, four of whom were treated on the right breast; three patients (0.9%) had late pulmonary fibrosis.
- The safety and efficacy outcomes are in line with previous reports, including those that used different dosage and/or fractionation schedules.
Limitations
- The study was retrospective, with a relatively short follow-up.
- Quality of life was not assessed.
- There was no objective baseline measure of cosmesis against which to compare cosmetic results.
Disclosures
- There was no funding for the study, and the investigators didn’t have any conflicts of interest to report.
This is a summary of a preprint research study, “One-Week External Beam Partial Breast Irradiation: Survival and Toxicity Outcomes,” led by Riccardo Ray Colciago from the Fondazione IRCCS Istituto Nazionale dei Tumori, Milan. The study has not been peer reviewed. The full text can be found at researchsquare.com.
A version of this article first appeared on Medscape.com.
The study covered in this summary was published on researchsquare.com as a preprint and has not yet been peer reviewed.
Key takeaway
Why this matters
- According to numerous guidelines, partial breast irradiation after lumpectomy is a sound approach for early-stage breast cancer, but there is a lack of consensus about treatment schedules.
- The investigators suggest that 30 Gy in five daily fractions is a “valid option” for these patients in a field that lacks consensus.
Study design
- The team reviewed 381 women with early breast cancer treated with this approach (30 Gy in five daily fractions) at their center from 2013 to 2022.
- Half of patients had left-sided tumors, 94.5% had invasive ductal carcinomas, 96.6% had grade 1 or grade 2 disease, and tumors were luminal like in 99.2% of patients.
- Following lumpectomy, women underwent partial breast irradiation to the tumor bed plus 15 mm of isometric expansion beyond it.
- Follow-up was a median of 28 months.
Key results
- Seven patients (2%) had a local recurrence, of which two were in the treatment field.
- Three-year local control, disease-free survival, and overall survival were high (97.5%, 95.7%, and 96.9%, respectively).
- Nearly 90% of patients and 97% of physicians reported good or excellent cosmesis.
- Ten patients (2.9%) had grade 2 late toxicities, including edema, asthenia, and fibrosis; there were no grade 3 or higher adverse events.
- Five patients (1.5%) had late cardiac major events, four of whom were treated on the right breast; three patients (0.9%) had late pulmonary fibrosis.
- The safety and efficacy outcomes are in line with previous reports, including those that used different dosage and/or fractionation schedules.
Limitations
- The study was retrospective, with a relatively short follow-up.
- Quality of life was not assessed.
- There was no objective baseline measure of cosmesis against which to compare cosmetic results.
Disclosures
- There was no funding for the study, and the investigators didn’t have any conflicts of interest to report.
This is a summary of a preprint research study, “One-Week External Beam Partial Breast Irradiation: Survival and Toxicity Outcomes,” led by Riccardo Ray Colciago from the Fondazione IRCCS Istituto Nazionale dei Tumori, Milan. The study has not been peer reviewed. The full text can be found at researchsquare.com.
A version of this article first appeared on Medscape.com.
Radiotherapy for early breast cancer: Sharp cutoff at age 70
say researchers reporting new data showing a sharp cut-off at age 70.
“In our study, one of the most significant variables in determining whether breast cancer patients who are close their 70th birthday are recommended standard-of-care radiation or de-escalated treatment is whether they show up a few months before or a few months after that 70th birthday,” commented study author Wesley J. Talcott, MD, of the department of therapeutic radiology at the Yale School of Medicine, New Haven, Conn.
The results show a trend in which radiation therapy is 50% less likely to be prescribed for patients age 70 and older with early-stage breast cancer, even when controlling for population size, patient demographics, and disease specific variables.
This suggests that oncologists are weighing the variable of age too heavily when deciding on adjuvant treatments, the authors suggest.
“In certain circumstances, breast cancer oncology providers are treating age like a binary categorical variable when selecting patients for treatments or diagnostic procedures, rather than the continuous variable that it is,” Dr. Talcott commented.
The study was published online in the International Journal of Radiation Oncology: Biology, Physics.
Approached for comment, Casey Chollet-Lipscomb, MD, radiation oncologist with Tennessee Oncology, Nashville, who was not associated with the study, agreed with its main finding.
“The study helps emphasize the importance of individualized care,” she said. “Increasing age is the most common risk factor for breast cancer, but breast cancer is an incredibly diverse disease. While you can observe trends based on age, every patient is unique, and they can’t be lumped into one bucket and prescribed treatment based on a strict age cutoff.”
The retrospective study included two cohorts of women identified in the National Cancer Data Base (2004-2017) all of whom underwent lumpectomy for early-stage breast cancer. All patients had “strong indications” for adjuvant treatment.
Patients in cohort 1 (n = 160,990) included women with estrogen-receptor negative cancer, tumor size greater than 3 cm, who were determined to be “appropriate” for radiation therapy.
Patients in cohort 2 (n = 394,946) had hormone-receptor positive cancer, tumor size greater than 5 mm, and were considered to be “appropriate” candidates for endocrine therapy.
Multivariable analysis was performed to control for comorbidity burden (measured by the Charlson-Deyo Comorbidity Index), race and ethnicity, insurance status, academic versus non-academic treatment center, median annual income of a patient’s area of residence, distance from the site of treatment, and pathology variables including number of lymph nodes sampled, histologic grade, and genomic risk score.
In cohort 1, radiation was recommended for 90%-92% of patients between the ages of 50-69; this dropped to 81% for those aged 70.
After MVA, it was determined that age difference was an independent predictor for adjuvant radiation recommendation only at age 70 versus 69 (odds ratio, 0.47; 95% confidence interval 0.39-0.57, P < .001).
For cohort 2, year-over-year age difference predicted endocrine therapy recommendation only at the juncture between age 70 versus 69 (OR, 0.86, 95% CI 0.74-0.99, P = .001).
“Our results don’t say that we should be increasing the amount of treatment for patients over the age [of] 70 or decreasing that patient treatment for patients younger than age 70. What we believe is that we need to be assessing physiologic age of our patients when treating patients,” Dr. Talcott said.
“We would do this by looking at not just how many years a patient has been on this Earth but also what their current health status is, how many good quality-of-life years they might have after treatment or without it, and what the patient wants in terms of burden of treatment. This is a much more valuable way to approach the allocation of treatments than using age alone,” he added.
Both Dr. Talcott and Dr. Chollet-Lipscomb agreed that a limitation of the study was a lack of data on how physicians decided on a specific treatment in each individual case, but they agree that even without this information the results were “significant.”
Dr. Chollet-Lipscomb also highlighted the factors other than age she would use to determine the best adjuvant treatment for a patient with early stage breast cancer, including the individual features of the tumor, how aggressive it looks under the microscope, what the receptor status is, and a patient’s overall performance status and comorbidities.
Dr. Talcott and Dr. Chollet-Lipscomb report no relevant financial relationships. The authors had no acknowledgement of research support for this study.
A version of this article first appeared on Medscape.com.
say researchers reporting new data showing a sharp cut-off at age 70.
“In our study, one of the most significant variables in determining whether breast cancer patients who are close their 70th birthday are recommended standard-of-care radiation or de-escalated treatment is whether they show up a few months before or a few months after that 70th birthday,” commented study author Wesley J. Talcott, MD, of the department of therapeutic radiology at the Yale School of Medicine, New Haven, Conn.
The results show a trend in which radiation therapy is 50% less likely to be prescribed for patients age 70 and older with early-stage breast cancer, even when controlling for population size, patient demographics, and disease specific variables.
This suggests that oncologists are weighing the variable of age too heavily when deciding on adjuvant treatments, the authors suggest.
“In certain circumstances, breast cancer oncology providers are treating age like a binary categorical variable when selecting patients for treatments or diagnostic procedures, rather than the continuous variable that it is,” Dr. Talcott commented.
The study was published online in the International Journal of Radiation Oncology: Biology, Physics.
Approached for comment, Casey Chollet-Lipscomb, MD, radiation oncologist with Tennessee Oncology, Nashville, who was not associated with the study, agreed with its main finding.
“The study helps emphasize the importance of individualized care,” she said. “Increasing age is the most common risk factor for breast cancer, but breast cancer is an incredibly diverse disease. While you can observe trends based on age, every patient is unique, and they can’t be lumped into one bucket and prescribed treatment based on a strict age cutoff.”
The retrospective study included two cohorts of women identified in the National Cancer Data Base (2004-2017) all of whom underwent lumpectomy for early-stage breast cancer. All patients had “strong indications” for adjuvant treatment.
Patients in cohort 1 (n = 160,990) included women with estrogen-receptor negative cancer, tumor size greater than 3 cm, who were determined to be “appropriate” for radiation therapy.
Patients in cohort 2 (n = 394,946) had hormone-receptor positive cancer, tumor size greater than 5 mm, and were considered to be “appropriate” candidates for endocrine therapy.
Multivariable analysis was performed to control for comorbidity burden (measured by the Charlson-Deyo Comorbidity Index), race and ethnicity, insurance status, academic versus non-academic treatment center, median annual income of a patient’s area of residence, distance from the site of treatment, and pathology variables including number of lymph nodes sampled, histologic grade, and genomic risk score.
In cohort 1, radiation was recommended for 90%-92% of patients between the ages of 50-69; this dropped to 81% for those aged 70.
After MVA, it was determined that age difference was an independent predictor for adjuvant radiation recommendation only at age 70 versus 69 (odds ratio, 0.47; 95% confidence interval 0.39-0.57, P < .001).
For cohort 2, year-over-year age difference predicted endocrine therapy recommendation only at the juncture between age 70 versus 69 (OR, 0.86, 95% CI 0.74-0.99, P = .001).
“Our results don’t say that we should be increasing the amount of treatment for patients over the age [of] 70 or decreasing that patient treatment for patients younger than age 70. What we believe is that we need to be assessing physiologic age of our patients when treating patients,” Dr. Talcott said.
“We would do this by looking at not just how many years a patient has been on this Earth but also what their current health status is, how many good quality-of-life years they might have after treatment or without it, and what the patient wants in terms of burden of treatment. This is a much more valuable way to approach the allocation of treatments than using age alone,” he added.
Both Dr. Talcott and Dr. Chollet-Lipscomb agreed that a limitation of the study was a lack of data on how physicians decided on a specific treatment in each individual case, but they agree that even without this information the results were “significant.”
Dr. Chollet-Lipscomb also highlighted the factors other than age she would use to determine the best adjuvant treatment for a patient with early stage breast cancer, including the individual features of the tumor, how aggressive it looks under the microscope, what the receptor status is, and a patient’s overall performance status and comorbidities.
Dr. Talcott and Dr. Chollet-Lipscomb report no relevant financial relationships. The authors had no acknowledgement of research support for this study.
A version of this article first appeared on Medscape.com.
say researchers reporting new data showing a sharp cut-off at age 70.
“In our study, one of the most significant variables in determining whether breast cancer patients who are close their 70th birthday are recommended standard-of-care radiation or de-escalated treatment is whether they show up a few months before or a few months after that 70th birthday,” commented study author Wesley J. Talcott, MD, of the department of therapeutic radiology at the Yale School of Medicine, New Haven, Conn.
The results show a trend in which radiation therapy is 50% less likely to be prescribed for patients age 70 and older with early-stage breast cancer, even when controlling for population size, patient demographics, and disease specific variables.
This suggests that oncologists are weighing the variable of age too heavily when deciding on adjuvant treatments, the authors suggest.
“In certain circumstances, breast cancer oncology providers are treating age like a binary categorical variable when selecting patients for treatments or diagnostic procedures, rather than the continuous variable that it is,” Dr. Talcott commented.
The study was published online in the International Journal of Radiation Oncology: Biology, Physics.
Approached for comment, Casey Chollet-Lipscomb, MD, radiation oncologist with Tennessee Oncology, Nashville, who was not associated with the study, agreed with its main finding.
“The study helps emphasize the importance of individualized care,” she said. “Increasing age is the most common risk factor for breast cancer, but breast cancer is an incredibly diverse disease. While you can observe trends based on age, every patient is unique, and they can’t be lumped into one bucket and prescribed treatment based on a strict age cutoff.”
The retrospective study included two cohorts of women identified in the National Cancer Data Base (2004-2017) all of whom underwent lumpectomy for early-stage breast cancer. All patients had “strong indications” for adjuvant treatment.
Patients in cohort 1 (n = 160,990) included women with estrogen-receptor negative cancer, tumor size greater than 3 cm, who were determined to be “appropriate” for radiation therapy.
Patients in cohort 2 (n = 394,946) had hormone-receptor positive cancer, tumor size greater than 5 mm, and were considered to be “appropriate” candidates for endocrine therapy.
Multivariable analysis was performed to control for comorbidity burden (measured by the Charlson-Deyo Comorbidity Index), race and ethnicity, insurance status, academic versus non-academic treatment center, median annual income of a patient’s area of residence, distance from the site of treatment, and pathology variables including number of lymph nodes sampled, histologic grade, and genomic risk score.
In cohort 1, radiation was recommended for 90%-92% of patients between the ages of 50-69; this dropped to 81% for those aged 70.
After MVA, it was determined that age difference was an independent predictor for adjuvant radiation recommendation only at age 70 versus 69 (odds ratio, 0.47; 95% confidence interval 0.39-0.57, P < .001).
For cohort 2, year-over-year age difference predicted endocrine therapy recommendation only at the juncture between age 70 versus 69 (OR, 0.86, 95% CI 0.74-0.99, P = .001).
“Our results don’t say that we should be increasing the amount of treatment for patients over the age [of] 70 or decreasing that patient treatment for patients younger than age 70. What we believe is that we need to be assessing physiologic age of our patients when treating patients,” Dr. Talcott said.
“We would do this by looking at not just how many years a patient has been on this Earth but also what their current health status is, how many good quality-of-life years they might have after treatment or without it, and what the patient wants in terms of burden of treatment. This is a much more valuable way to approach the allocation of treatments than using age alone,” he added.
Both Dr. Talcott and Dr. Chollet-Lipscomb agreed that a limitation of the study was a lack of data on how physicians decided on a specific treatment in each individual case, but they agree that even without this information the results were “significant.”
Dr. Chollet-Lipscomb also highlighted the factors other than age she would use to determine the best adjuvant treatment for a patient with early stage breast cancer, including the individual features of the tumor, how aggressive it looks under the microscope, what the receptor status is, and a patient’s overall performance status and comorbidities.
Dr. Talcott and Dr. Chollet-Lipscomb report no relevant financial relationships. The authors had no acknowledgement of research support for this study.
A version of this article first appeared on Medscape.com.
FROM THE INTERNATIONAL JOURNAL OF RADIATION ONCOLOGY: BIOLOGY, PHYSICS